
PESTICIDE RESIDUES IN FOOD - 1982
Sponsored jointly by FAO and WHO
EVALUATIONS 1982
Data and recommendations of the joint meeting
of the FAO Panel of Experts on Pesticide Residues
in Food and the Environment and the
WHO Expert Group on Pesticide Residues
Rome, 23 November - 2 December 1982
Food and Agriculture Organization of the United Nations
Rome 1983
CHLORDANE
 Explanation
Chlordane was evaluated in the Joint Meetings of 1963, 1965,
1967, 1969, 1970, 1972, 1974 and 1977 (FAO/WHO, 1964, 1965, 1968,
1970, 1971, 1973, 1975 and 1978)1/. Several acute oral studies and a
90-day subchronic feeding study in rats, of Industrial Bio-Test
Laboratories (IBT) origin, have been identified, which have not been
validated. However, the no-effect level(s) (NEL) and ADI were not
taken from the results of these data.
Additional data pertaining to metabolism, absorption,
distribution and excretion have been made available and are reviewed
in this addendum.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, Distribution and Excretion
More than 90% of single oral doses of high purity chlordane
(98% + of a 3:1 mixture of cis- and trans-chlordane), cis-chlordane
and trans-chlordane were eliminated from rats in the excreta within
7 days after treatment, with faeces being the major route of
elimination for both sexes. Single oral doses of oxychlordane resulted
in only 21% of the administered dose appearing in rat excreta after 7
days, demonstrating a greater potential for this metabolite of
chlordane to accumulate in animals than the cis- and trans-chlordane
isomers. Continuous feeding of cis- and trans-chlordane separately in
the diet of rats for 14 days demonstrated preferential elimination of
the cis-isomer. After 14 days of treatment, 75% of the total cis- and
65% for the trans-chlordane were eliminated which indicates that in
long-term exposures the trans-isomer would contribute a relatively
greater amount to the body burden of the exposed animal than would the
cis-isomer (Barnett and Dorough 1974).
1/ See Annex 2 for WHO and FAO documentation.
In a separate study, administration of cis-chlordane or trans-
chlordane in single oral doses to rats via stomach intubation resulted
in 86% of the total cis- and 66% of the total trans-chlordane being
eliminated within 7 days after treatment. Furthermore, the rate of
excretion of cis-chlordane (59% in 24 h) was more rapid than that of
trans-chlordane (27%) (Tashiro and Matsumura 1977). There were no
apparent sex differences with respect to the rate of dissipation, nor
did males and females differ in the nature and relative concentrations
of metabolites (Barnett and Dorough 1974).
Biotransformation
The metabolism of chlordane, per se, in rats resulted in the
formation of dichlorochlordene via dehydrogenation followed by
epoxidation to oxychlordane and subsequent hydroxylation. There was
also direct hydroxylation of both the cis and trans-isomers to
1-exo-hydroxydihydrochlordene with excretion in the faeces and urine
as the glucuronide (Tashiro and Matsumara 1977).
The level of residues in tissues of rats was generally low,
except in fat. Metabolism of trans-chlordane resulted in higher tissue
residues than cis-chlordane, primarily because of the formation of
1,2-dichlorochlordane, which was favoured for the trans-isomer, thus
leading to higher levels of oxychlordane. Feeding female rats high
purity chlordane at levels up to 25 ppm for 56 days resulted in
chlordane-14C equivalent in the fat at levels 3-4 times that in the
diet. These levels did not plateau at the end of the treatment period.
However, after 4 weeks removal from the diet, tissue levels were
reduced by 60%. The levels of residues in tissues (other than fat)
after 56 days of treatment were as follows (as the fraction of the
administered dose): liver (1/8) > kidney (1/10) > brain (1/25)
> muscle (1/50). Oxychlordane was the major component of all tissues
and, after removing chlordane from the diet, contributed most of the
14C residue in tissues (Barnett and Dorough 1974; Tashiro and
Matsumura 1977; Street and Blau 1972).
The metabolic intermediates of cis- and trans-chlordane, as well
as oxychlordane, were further converted to two major metabolites,
1-exo-hydroxy-2-chlorochlordene and 1-exo-hydroxy-2-chloro-2,3
epoxychlordene. These latter metabolites were not readily degraded
further in rats and may accumulate as terminal residues in the animal.
Neither appeared to be more toxic than the cis- or trans-isomers, but
this has not been verified in mammals (Tashiro and Matsumura 1977).
Rat and human in vitro liver preparations displayed almost
identical degradation abilities for trans-chlordane, but not for
trans-nonachlor, which accumulated in humans but not in rats. Trans-
nonachlor is a major constituent (7%) of technical chlordane. Both
single oral dosing (0.05 µ Ci) and four-week dietary administration
(100 ppm) of trans-nonachlor to rats have demonstrated the overall
metabolic pattern and rate of excretion to be the same as for trans-
chlordane. The fact that trans-nonachlor was not metabolized
efficiently by human liver cells may be due to the inability to form
trans-chlordane, which represents a significant difference from the
rat (Tashiro and Matsumura 1978).
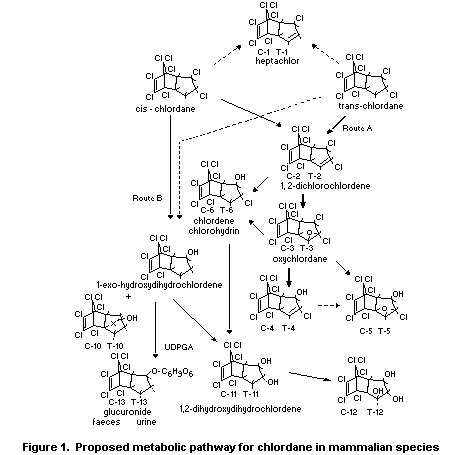
A proposed metabolic pathway for chlordane in mammalian species
is shown in Figure 1.
TOXICOLOGICAL STUDIES
Long-Term Study
Rat
The data submitted to the 1982 Meeting for evaluation of long-
term effects were not sufficient in many critical areas of data
content and were, therefore, inadequate for inclusion in this
monograph.
COMMENTS
The ADI and long-term studies considered in support of the ADI
were evaluated by the JMPR in 1967 and 1977 (FAO/WHO 1968 and 1978).
Additional data on metabolism have been received.
Significant differences in the metabolism of chlordane between
rats and humans have been identified. Specifically, trans-nonachlor, a
major constituent of technical chlordane, is poorly metabolized in
humans. The Meeting was concerned about the accumulation of trans-
nonachlor in humans. However, this concern was partly alleviated by
the information that only very low levels (0.1 ppm on milk fat basis)
appear to have been found in human milk samples.
The Meeting was informed that studies pertaining to chlordane
toxicology were in progress at the Research Institute for Animal
Science in Biochemistry and Toxicology in Japan. The Meeting hopes
that this data will eventually be made available to it.
Pending receipt of a study on oxychlordane of at least 90 days
duration in the rat, the Meeting assigned a temporary ADI to
chlordane.
TOXICOLOGICAL EVALUATION
Level Causing no Toxicological Effect
Rat: 5 mg/kg in the diet, equivalent to 0.25 mg/kg bw
Dog: 3 mg/kg in the diet, equivalent to 0.075 mg/kg bw
Explanation
Chlordane was evaluated in the Joint Meetings of 1963, 1965,
1967, 1969, 1970, 1972, 1974 and 1977 (FAO/WHO, 1964, 1965, 1968,
1970, 1971, 1973, 1975 and 1978)1/. Several acute oral studies and a
90-day subchronic feeding study in rats, of Industrial Bio-Test
Laboratories (IBT) origin, have been identified, which have not been
validated. However, the no-effect level(s) (NEL) and ADI were not
taken from the results of these data.
Additional data pertaining to metabolism, absorption,
distribution and excretion have been made available and are reviewed
in this addendum.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, Distribution and Excretion
More than 90% of single oral doses of high purity chlordane
(98% + of a 3:1 mixture of cis- and trans-chlordane), cis-chlordane
and trans-chlordane were eliminated from rats in the excreta within
7 days after treatment, with faeces being the major route of
elimination for both sexes. Single oral doses of oxychlordane resulted
in only 21% of the administered dose appearing in rat excreta after 7
days, demonstrating a greater potential for this metabolite of
chlordane to accumulate in animals than the cis- and trans-chlordane
isomers. Continuous feeding of cis- and trans-chlordane separately in
the diet of rats for 14 days demonstrated preferential elimination of
the cis-isomer. After 14 days of treatment, 75% of the total cis- and
65% for the trans-chlordane were eliminated which indicates that in
long-term exposures the trans-isomer would contribute a relatively
greater amount to the body burden of the exposed animal than would the
cis-isomer (Barnett and Dorough 1974).
1/ See Annex 2 for WHO and FAO documentation.
In a separate study, administration of cis-chlordane or trans-
chlordane in single oral doses to rats via stomach intubation resulted
in 86% of the total cis- and 66% of the total trans-chlordane being
eliminated within 7 days after treatment. Furthermore, the rate of
excretion of cis-chlordane (59% in 24 h) was more rapid than that of
trans-chlordane (27%) (Tashiro and Matsumura 1977). There were no
apparent sex differences with respect to the rate of dissipation, nor
did males and females differ in the nature and relative concentrations
of metabolites (Barnett and Dorough 1974).
Biotransformation
The metabolism of chlordane, per se, in rats resulted in the
formation of dichlorochlordene via dehydrogenation followed by
epoxidation to oxychlordane and subsequent hydroxylation. There was
also direct hydroxylation of both the cis and trans-isomers to
1-exo-hydroxydihydrochlordene with excretion in the faeces and urine
as the glucuronide (Tashiro and Matsumara 1977).
The level of residues in tissues of rats was generally low,
except in fat. Metabolism of trans-chlordane resulted in higher tissue
residues than cis-chlordane, primarily because of the formation of
1,2-dichlorochlordane, which was favoured for the trans-isomer, thus
leading to higher levels of oxychlordane. Feeding female rats high
purity chlordane at levels up to 25 ppm for 56 days resulted in
chlordane-14C equivalent in the fat at levels 3-4 times that in the
diet. These levels did not plateau at the end of the treatment period.
However, after 4 weeks removal from the diet, tissue levels were
reduced by 60%. The levels of residues in tissues (other than fat)
after 56 days of treatment were as follows (as the fraction of the
administered dose): liver (1/8) > kidney (1/10) > brain (1/25)
> muscle (1/50). Oxychlordane was the major component of all tissues
and, after removing chlordane from the diet, contributed most of the
14C residue in tissues (Barnett and Dorough 1974; Tashiro and
Matsumura 1977; Street and Blau 1972).
The metabolic intermediates of cis- and trans-chlordane, as well
as oxychlordane, were further converted to two major metabolites,
1-exo-hydroxy-2-chlorochlordene and 1-exo-hydroxy-2-chloro-2,3
epoxychlordene. These latter metabolites were not readily degraded
further in rats and may accumulate as terminal residues in the animal.
Neither appeared to be more toxic than the cis- or trans-isomers, but
this has not been verified in mammals (Tashiro and Matsumura 1977).
Rat and human in vitro liver preparations displayed almost
identical degradation abilities for trans-chlordane, but not for
trans-nonachlor, which accumulated in humans but not in rats. Trans-
nonachlor is a major constituent (7%) of technical chlordane. Both
single oral dosing (0.05 µ Ci) and four-week dietary administration
(100 ppm) of trans-nonachlor to rats have demonstrated the overall
metabolic pattern and rate of excretion to be the same as for trans-
chlordane. The fact that trans-nonachlor was not metabolized
efficiently by human liver cells may be due to the inability to form
trans-chlordane, which represents a significant difference from the
rat (Tashiro and Matsumura 1978).
A proposed metabolic pathway for chlordane in mammalian species
is shown in Figure 1.
TOXICOLOGICAL STUDIES
Long-Term Study
Rat
The data submitted to the 1982 Meeting for evaluation of long-
term effects were not sufficient in many critical areas of data
content and were, therefore, inadequate for inclusion in this
monograph.
COMMENTS
The ADI and long-term studies considered in support of the ADI
were evaluated by the JMPR in 1967 and 1977 (FAO/WHO 1968 and 1978).
Additional data on metabolism have been received.
Significant differences in the metabolism of chlordane between
rats and humans have been identified. Specifically, trans-nonachlor, a
major constituent of technical chlordane, is poorly metabolized in
humans. The Meeting was concerned about the accumulation of trans-
nonachlor in humans. However, this concern was partly alleviated by
the information that only very low levels (0.1 ppm on milk fat basis)
appear to have been found in human milk samples.
The Meeting was informed that studies pertaining to chlordane
toxicology were in progress at the Research Institute for Animal
Science in Biochemistry and Toxicology in Japan. The Meeting hopes
that this data will eventually be made available to it.
Pending receipt of a study on oxychlordane of at least 90 days
duration in the rat, the Meeting assigned a temporary ADI to
chlordane.
TOXICOLOGICAL EVALUATION
Level Causing no Toxicological Effect
Rat: 5 mg/kg in the diet, equivalent to 0.25 mg/kg bw
Dog: 3 mg/kg in the diet, equivalent to 0.075 mg/kg bw
 Estimate of Temporary Acceptable Daily Intake in Man
0 - 0.001 mg/kg bw
FURTHER WORK OR INFORMATION
Required (by 1984)
A study of at least 90 days' duration in rats using oxychlordane.
Desirable
1. Submission of the ongoing studies in Japan.
2. Submission of monitoring data pertinent to the oxychlordane and
trans-nonachlor levels found in humans.
REFERENCES
Barnett, J.R. and Dorough, H.W. Metabolism of chlordane in rats, J.
1974 Agric. Food Chem. 22:612-619.
Street, J.C. and Blau, S.E. Oxychlordane: accumulation in rat
1972 adipose tissue on feeding chlordane isomers of technical
chlordane. J. Agric. Food Chem. 20:395-397.
Tashiro, S. and Matsumura, F. Metabolic routes of cis- and trans-
1977 chlordane in rats. J. Agric. Food Chem. 25:872-880.
1978 Metabolic routes of trans-nonachlor and related chlordane
components in rat and man. Arch. Environm. Contam. Toxicol.
7:113-127.
Estimate of Temporary Acceptable Daily Intake in Man
0 - 0.001 mg/kg bw
FURTHER WORK OR INFORMATION
Required (by 1984)
A study of at least 90 days' duration in rats using oxychlordane.
Desirable
1. Submission of the ongoing studies in Japan.
2. Submission of monitoring data pertinent to the oxychlordane and
trans-nonachlor levels found in humans.
REFERENCES
Barnett, J.R. and Dorough, H.W. Metabolism of chlordane in rats, J.
1974 Agric. Food Chem. 22:612-619.
Street, J.C. and Blau, S.E. Oxychlordane: accumulation in rat
1972 adipose tissue on feeding chlordane isomers of technical
chlordane. J. Agric. Food Chem. 20:395-397.
Tashiro, S. and Matsumura, F. Metabolic routes of cis- and trans-
1977 chlordane in rats. J. Agric. Food Chem. 25:872-880.
1978 Metabolic routes of trans-nonachlor and related chlordane
components in rat and man. Arch. Environm. Contam. Toxicol.
7:113-127.
 Explanation
Chlordane was evaluated in the Joint Meetings of 1963, 1965,
1967, 1969, 1970, 1972, 1974 and 1977 (FAO/WHO, 1964, 1965, 1968,
1970, 1971, 1973, 1975 and 1978)1/. Several acute oral studies and a
90-day subchronic feeding study in rats, of Industrial Bio-Test
Laboratories (IBT) origin, have been identified, which have not been
validated. However, the no-effect level(s) (NEL) and ADI were not
taken from the results of these data.
Additional data pertaining to metabolism, absorption,
distribution and excretion have been made available and are reviewed
in this addendum.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, Distribution and Excretion
More than 90% of single oral doses of high purity chlordane
(98% + of a 3:1 mixture of cis- and trans-chlordane), cis-chlordane
and trans-chlordane were eliminated from rats in the excreta within
7 days after treatment, with faeces being the major route of
elimination for both sexes. Single oral doses of oxychlordane resulted
in only 21% of the administered dose appearing in rat excreta after 7
days, demonstrating a greater potential for this metabolite of
chlordane to accumulate in animals than the cis- and trans-chlordane
isomers. Continuous feeding of cis- and trans-chlordane separately in
the diet of rats for 14 days demonstrated preferential elimination of
the cis-isomer. After 14 days of treatment, 75% of the total cis- and
65% for the trans-chlordane were eliminated which indicates that in
long-term exposures the trans-isomer would contribute a relatively
greater amount to the body burden of the exposed animal than would the
cis-isomer (Barnett and Dorough 1974).
1/ See Annex 2 for WHO and FAO documentation.
In a separate study, administration of cis-chlordane or trans-
chlordane in single oral doses to rats via stomach intubation resulted
in 86% of the total cis- and 66% of the total trans-chlordane being
eliminated within 7 days after treatment. Furthermore, the rate of
excretion of cis-chlordane (59% in 24 h) was more rapid than that of
trans-chlordane (27%) (Tashiro and Matsumura 1977). There were no
apparent sex differences with respect to the rate of dissipation, nor
did males and females differ in the nature and relative concentrations
of metabolites (Barnett and Dorough 1974).
Biotransformation
The metabolism of chlordane, per se, in rats resulted in the
formation of dichlorochlordene via dehydrogenation followed by
epoxidation to oxychlordane and subsequent hydroxylation. There was
also direct hydroxylation of both the cis and trans-isomers to
1-exo-hydroxydihydrochlordene with excretion in the faeces and urine
as the glucuronide (Tashiro and Matsumara 1977).
The level of residues in tissues of rats was generally low,
except in fat. Metabolism of trans-chlordane resulted in higher tissue
residues than cis-chlordane, primarily because of the formation of
1,2-dichlorochlordane, which was favoured for the trans-isomer, thus
leading to higher levels of oxychlordane. Feeding female rats high
purity chlordane at levels up to 25 ppm for 56 days resulted in
chlordane-14C equivalent in the fat at levels 3-4 times that in the
diet. These levels did not plateau at the end of the treatment period.
However, after 4 weeks removal from the diet, tissue levels were
reduced by 60%. The levels of residues in tissues (other than fat)
after 56 days of treatment were as follows (as the fraction of the
administered dose): liver (1/8) > kidney (1/10) > brain (1/25)
> muscle (1/50). Oxychlordane was the major component of all tissues
and, after removing chlordane from the diet, contributed most of the
14C residue in tissues (Barnett and Dorough 1974; Tashiro and
Matsumura 1977; Street and Blau 1972).
The metabolic intermediates of cis- and trans-chlordane, as well
as oxychlordane, were further converted to two major metabolites,
1-exo-hydroxy-2-chlorochlordene and 1-exo-hydroxy-2-chloro-2,3
epoxychlordene. These latter metabolites were not readily degraded
further in rats and may accumulate as terminal residues in the animal.
Neither appeared to be more toxic than the cis- or trans-isomers, but
this has not been verified in mammals (Tashiro and Matsumura 1977).
Rat and human in vitro liver preparations displayed almost
identical degradation abilities for trans-chlordane, but not for
trans-nonachlor, which accumulated in humans but not in rats. Trans-
nonachlor is a major constituent (7%) of technical chlordane. Both
single oral dosing (0.05 µ Ci) and four-week dietary administration
(100 ppm) of trans-nonachlor to rats have demonstrated the overall
metabolic pattern and rate of excretion to be the same as for trans-
chlordane. The fact that trans-nonachlor was not metabolized
efficiently by human liver cells may be due to the inability to form
trans-chlordane, which represents a significant difference from the
rat (Tashiro and Matsumura 1978).
A proposed metabolic pathway for chlordane in mammalian species
is shown in Figure 1.
TOXICOLOGICAL STUDIES
Long-Term Study
Rat
The data submitted to the 1982 Meeting for evaluation of long-
term effects were not sufficient in many critical areas of data
content and were, therefore, inadequate for inclusion in this
monograph.
COMMENTS
The ADI and long-term studies considered in support of the ADI
were evaluated by the JMPR in 1967 and 1977 (FAO/WHO 1968 and 1978).
Additional data on metabolism have been received.
Significant differences in the metabolism of chlordane between
rats and humans have been identified. Specifically, trans-nonachlor, a
major constituent of technical chlordane, is poorly metabolized in
humans. The Meeting was concerned about the accumulation of trans-
nonachlor in humans. However, this concern was partly alleviated by
the information that only very low levels (0.1 ppm on milk fat basis)
appear to have been found in human milk samples.
The Meeting was informed that studies pertaining to chlordane
toxicology were in progress at the Research Institute for Animal
Science in Biochemistry and Toxicology in Japan. The Meeting hopes
that this data will eventually be made available to it.
Pending receipt of a study on oxychlordane of at least 90 days
duration in the rat, the Meeting assigned a temporary ADI to
chlordane.
TOXICOLOGICAL EVALUATION
Level Causing no Toxicological Effect
Rat: 5 mg/kg in the diet, equivalent to 0.25 mg/kg bw
Dog: 3 mg/kg in the diet, equivalent to 0.075 mg/kg bw
Explanation
Chlordane was evaluated in the Joint Meetings of 1963, 1965,
1967, 1969, 1970, 1972, 1974 and 1977 (FAO/WHO, 1964, 1965, 1968,
1970, 1971, 1973, 1975 and 1978)1/. Several acute oral studies and a
90-day subchronic feeding study in rats, of Industrial Bio-Test
Laboratories (IBT) origin, have been identified, which have not been
validated. However, the no-effect level(s) (NEL) and ADI were not
taken from the results of these data.
Additional data pertaining to metabolism, absorption,
distribution and excretion have been made available and are reviewed
in this addendum.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, Distribution and Excretion
More than 90% of single oral doses of high purity chlordane
(98% + of a 3:1 mixture of cis- and trans-chlordane), cis-chlordane
and trans-chlordane were eliminated from rats in the excreta within
7 days after treatment, with faeces being the major route of
elimination for both sexes. Single oral doses of oxychlordane resulted
in only 21% of the administered dose appearing in rat excreta after 7
days, demonstrating a greater potential for this metabolite of
chlordane to accumulate in animals than the cis- and trans-chlordane
isomers. Continuous feeding of cis- and trans-chlordane separately in
the diet of rats for 14 days demonstrated preferential elimination of
the cis-isomer. After 14 days of treatment, 75% of the total cis- and
65% for the trans-chlordane were eliminated which indicates that in
long-term exposures the trans-isomer would contribute a relatively
greater amount to the body burden of the exposed animal than would the
cis-isomer (Barnett and Dorough 1974).
1/ See Annex 2 for WHO and FAO documentation.
In a separate study, administration of cis-chlordane or trans-
chlordane in single oral doses to rats via stomach intubation resulted
in 86% of the total cis- and 66% of the total trans-chlordane being
eliminated within 7 days after treatment. Furthermore, the rate of
excretion of cis-chlordane (59% in 24 h) was more rapid than that of
trans-chlordane (27%) (Tashiro and Matsumura 1977). There were no
apparent sex differences with respect to the rate of dissipation, nor
did males and females differ in the nature and relative concentrations
of metabolites (Barnett and Dorough 1974).
Biotransformation
The metabolism of chlordane, per se, in rats resulted in the
formation of dichlorochlordene via dehydrogenation followed by
epoxidation to oxychlordane and subsequent hydroxylation. There was
also direct hydroxylation of both the cis and trans-isomers to
1-exo-hydroxydihydrochlordene with excretion in the faeces and urine
as the glucuronide (Tashiro and Matsumara 1977).
The level of residues in tissues of rats was generally low,
except in fat. Metabolism of trans-chlordane resulted in higher tissue
residues than cis-chlordane, primarily because of the formation of
1,2-dichlorochlordane, which was favoured for the trans-isomer, thus
leading to higher levels of oxychlordane. Feeding female rats high
purity chlordane at levels up to 25 ppm for 56 days resulted in
chlordane-14C equivalent in the fat at levels 3-4 times that in the
diet. These levels did not plateau at the end of the treatment period.
However, after 4 weeks removal from the diet, tissue levels were
reduced by 60%. The levels of residues in tissues (other than fat)
after 56 days of treatment were as follows (as the fraction of the
administered dose): liver (1/8) > kidney (1/10) > brain (1/25)
> muscle (1/50). Oxychlordane was the major component of all tissues
and, after removing chlordane from the diet, contributed most of the
14C residue in tissues (Barnett and Dorough 1974; Tashiro and
Matsumura 1977; Street and Blau 1972).
The metabolic intermediates of cis- and trans-chlordane, as well
as oxychlordane, were further converted to two major metabolites,
1-exo-hydroxy-2-chlorochlordene and 1-exo-hydroxy-2-chloro-2,3
epoxychlordene. These latter metabolites were not readily degraded
further in rats and may accumulate as terminal residues in the animal.
Neither appeared to be more toxic than the cis- or trans-isomers, but
this has not been verified in mammals (Tashiro and Matsumura 1977).
Rat and human in vitro liver preparations displayed almost
identical degradation abilities for trans-chlordane, but not for
trans-nonachlor, which accumulated in humans but not in rats. Trans-
nonachlor is a major constituent (7%) of technical chlordane. Both
single oral dosing (0.05 µ Ci) and four-week dietary administration
(100 ppm) of trans-nonachlor to rats have demonstrated the overall
metabolic pattern and rate of excretion to be the same as for trans-
chlordane. The fact that trans-nonachlor was not metabolized
efficiently by human liver cells may be due to the inability to form
trans-chlordane, which represents a significant difference from the
rat (Tashiro and Matsumura 1978).
A proposed metabolic pathway for chlordane in mammalian species
is shown in Figure 1.
TOXICOLOGICAL STUDIES
Long-Term Study
Rat
The data submitted to the 1982 Meeting for evaluation of long-
term effects were not sufficient in many critical areas of data
content and were, therefore, inadequate for inclusion in this
monograph.
COMMENTS
The ADI and long-term studies considered in support of the ADI
were evaluated by the JMPR in 1967 and 1977 (FAO/WHO 1968 and 1978).
Additional data on metabolism have been received.
Significant differences in the metabolism of chlordane between
rats and humans have been identified. Specifically, trans-nonachlor, a
major constituent of technical chlordane, is poorly metabolized in
humans. The Meeting was concerned about the accumulation of trans-
nonachlor in humans. However, this concern was partly alleviated by
the information that only very low levels (0.1 ppm on milk fat basis)
appear to have been found in human milk samples.
The Meeting was informed that studies pertaining to chlordane
toxicology were in progress at the Research Institute for Animal
Science in Biochemistry and Toxicology in Japan. The Meeting hopes
that this data will eventually be made available to it.
Pending receipt of a study on oxychlordane of at least 90 days
duration in the rat, the Meeting assigned a temporary ADI to
chlordane.
TOXICOLOGICAL EVALUATION
Level Causing no Toxicological Effect
Rat: 5 mg/kg in the diet, equivalent to 0.25 mg/kg bw
Dog: 3 mg/kg in the diet, equivalent to 0.075 mg/kg bw
 Estimate of Temporary Acceptable Daily Intake in Man
0 - 0.001 mg/kg bw
FURTHER WORK OR INFORMATION
Required (by 1984)
A study of at least 90 days' duration in rats using oxychlordane.
Desirable
1. Submission of the ongoing studies in Japan.
2. Submission of monitoring data pertinent to the oxychlordane and
trans-nonachlor levels found in humans.
REFERENCES
Barnett, J.R. and Dorough, H.W. Metabolism of chlordane in rats, J.
1974 Agric. Food Chem. 22:612-619.
Street, J.C. and Blau, S.E. Oxychlordane: accumulation in rat
1972 adipose tissue on feeding chlordane isomers of technical
chlordane. J. Agric. Food Chem. 20:395-397.
Tashiro, S. and Matsumura, F. Metabolic routes of cis- and trans-
1977 chlordane in rats. J. Agric. Food Chem. 25:872-880.
1978 Metabolic routes of trans-nonachlor and related chlordane
components in rat and man. Arch. Environm. Contam. Toxicol.
7:113-127.
Estimate of Temporary Acceptable Daily Intake in Man
0 - 0.001 mg/kg bw
FURTHER WORK OR INFORMATION
Required (by 1984)
A study of at least 90 days' duration in rats using oxychlordane.
Desirable
1. Submission of the ongoing studies in Japan.
2. Submission of monitoring data pertinent to the oxychlordane and
trans-nonachlor levels found in humans.
REFERENCES
Barnett, J.R. and Dorough, H.W. Metabolism of chlordane in rats, J.
1974 Agric. Food Chem. 22:612-619.
Street, J.C. and Blau, S.E. Oxychlordane: accumulation in rat
1972 adipose tissue on feeding chlordane isomers of technical
chlordane. J. Agric. Food Chem. 20:395-397.
Tashiro, S. and Matsumura, F. Metabolic routes of cis- and trans-
1977 chlordane in rats. J. Agric. Food Chem. 25:872-880.
1978 Metabolic routes of trans-nonachlor and related chlordane
components in rat and man. Arch. Environm. Contam. Toxicol.
7:113-127.
