
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
ENVIRONMENTAL HEALTH CRITERIA 34
CHLORDANE
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization.
Published under the joint sponsorship of
the United Nations Environment Programme,
the International Labour Organisation,
and the World Health Organization
World Health Orgnization
Geneva, 1984
The International Programme on Chemical Safety (IPCS) is a
joint venture of the United Nations Environment Programme, the
International Labour Organisation, and the World Health
Organization. The main objective of the IPCS is to carry out and
disseminate evaluations of the effects of chemicals on human health
and the quality of the environment. Supporting activities include
the development of epidemiological, experimental laboratory, and
risk-assessment methods that could produce internationally
comparable results, and the development of manpower in the field of
toxicology. Other activities carried out by the IPCS include the
development of know-how for coping with chemical accidents,
coordination of laboratory testing and epidemiological studies, and
promotion of research on the mechanisms of the biological action of
chemicals.
ISBN 92 4 154094 X
The World Health Organization welcomes requests for permission
to reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made
to the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 1984
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved.
The designations employed and the presentation of the material
in this publication do not imply the expression of any opinion
whatsoever on the part of the Secretariat of the World Health
Organization concerning the legal status of any country, territory,
city or area or of its authorities, or concerning the delimitation
of its frontiers or boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar
nature that are not mentioned. Errors and omissions excepted, the
names of proprietary products are distinguished by initial capital
letters.
CONTENTS
ENVIRONMENTAL HEALTH CRITERIA FOR CHLORDANE
1. SUMMARY AND RECOMMENDATIONS
1.1. Summary
1.1.1. Identity and analytical methods
1.1.2. Use and sources of exposure
1.1.3. Environmental concentrations, exposures,
and effects
1.1.4. Kinetics and metabolism
1.1.5. Studies on experimental animals
1.1.6. Effects on man
1.2. Recommendations
2. IDENTITY, PROPERTIES AND ANALYTICAL METHODS
2.1. Identity
2.2. Properties and analytical methods
2.2.1. Physical and chemical properties
2.2.2. Analytical methods
3. SOURCES OF ENVIRONMENTAL POLLUTION, ENVIRONMENTAL
TRANSPORT AND DISTRIBUTION
3.1. Sources of environmental pollution
3.1.1. Industrial production and uses
3.2. Environmental transport and distribution
3.2.1. Air
3.2.2. Water
3.2.3. Soil
3.2.4. Abiotic degradation
3.2.5. Biodegradation
4. ENVIRONMENTAL LEVELS AND EXPOSURES
4.1. Environmental levels
4.1.1. Air
4.1.2. Water
4.1.3. Soil
4.1.4. Crops and wildlife
4.1.5. Food
4.1.6. Human milk
4.2. General population exposure
4.3. Occupational exposure
5. KINETICS AND METABOLISM
5.1. Absorption
5.2. Distribution and storage
5.3. Biotransformation
5.4. Elimination and excretion
6. STUDIES ON EXPERIMENTAL ANIMALS
6.1. Short-term exposures
6.1.1. Oral exposure
6.1.2. Dermal exposure
6.1.3. Parenteral exposure
6.2. Long-term exposures
6.2.1. Oral exposure
6.2.2. Dermal exposure
6.3. Reproduction studies and teratogenicity
6.4. Mutagenicity
6.5. Carcinogenicity
6.6. Behavioural studies
6.7. Other studies
6.8. Factors influencing toxicity
7. EFFECTS ON MAN: EPIDEMIOLOGICAL AND CLINICAL STUDIES
7.1. Poisoning incidents
7.2. Occupational and epidemiological studies
7.3. Treatment of poisoning
8. EFFECTS ON THE ENVIRONMENT
8.1. Toxicity for aquatic organisms
8.2. Toxicity for terrestrial organisms
8.3. Toxicity for microorganisms
8.4. Bioaccumulation and biomagnification
8.5. Population and community effects
8.6. Effects on the abiotic environment
8.7. Appraisal
9. PREVIOUS EVALUATIONS OF CHLORDANE BY INTERNATIONAL BODIES
10. EVALUATION OF HEALTH RISKS FOR MAN AND EFFECTS ON THE ENVIRONMENT
10.1. Chlordane toxicity
10.2. Exposure to chlordane
10.3. Evaluation of overall environmental effects
10.4. Evaluation of risks for human health
and the environment
REFERENCES
TASK GROUP MEETING ON ENVIRONMENTAL HEALTH CRITERIA FOR
ORGANOCHLORINE PESTICIDES OTHER THAN DDT (CHLORDANE,
HEPTACHLOR, MIREX, CHLORDECONE, KELEVAN, CAMPHECHLOR)
Members
Dr Z. Adamis, National Institute of Occupational Health,
Budapest, Hungary
Dr D.A. Akintonwa, Department of Biochemistry, Faculty of
Medicine, University of Calabar, Calabar, Nigeriaa
Dr R. Goulding, Chairman of the Scientific Sub-committee, UK
Pesticides Safety Precautions Scheme, Ministry of
Agriculture, Fisheries & Food, London, England (Chairman)
Dr S.K. Kashyap, National Institute of Occupational Health
(Indian Council of Medical Research), Meghaninager,
Ahmedabad, India
Dr D.C. Villeneuve, Environmental Contaminants Section,
Environmental Health Centre, Tunney's Pasture, Ottawa,
Ontario, Canada (Rapporteur)
Dr D. Wassermann, Department of Occupational Health, The
Hebrew University, Haddassah Medical School, Jerusalem,
Israel (Vice-Chairman)
Representatives of Other Organizations
Dr C.J. Calo, European Chemical Industry Ecology and
Toxicology Centre (ECETOC)
Mme van der Venne, Commission of the European Communities (CEC),
Health and Safety Directorate, Luxembourg
Dr D.M. Whitacre, International Group of National Associations
of Agrochemical Manufacturers (GIFAP)
Secretariat
Dr M. Gilbert, International Register for Potentially Toxic
Chemicals, United Nations Environment Programme, Geneva,
Switzerland
Mme B. Goelzer, Division of Noncommunicable Diseases, Office
of Occupational Health, World Health Organization, Geneva,
Switzerland
-------------------------------------------------------------------
a Unable to attend.
Secretariat (contd.)
Dr Y. Hasegawa, Division of Environmental Health,
Environmental Hazards and Food Protection, World Health
Organization, Geneva, Switzerland
Dr K.W. Jager, Division of Environmental Health, International
Programme on Chemical Safety, World Health Organization,
Geneva, Switzerland (Secretary)
Mr B. Labarthe, International Register for Potentially Toxic
Chemicals, United Nations Environment Programme, Geneva,
Switzerland
Dr I.M. Lindquist, International Labour Organization, Geneva,
Switzerland
Dr M. Vandekar, Division of Vector Biology and Control,
Pesticides Development and Safe Use Unit, World Health
Organization, Geneva, Switzerland
Mr J.D. Wilbourn, Unit of Carcinogen Identification and
Evaluation, International Agency for Research on Cancer,
Lyons, France
NOTE TO READERS OF THE CRITERIA DOCUMENTS
While every effort has been made to present information in the
criteria documents as accurately as possible without unduly
delaying their publication, mistakes might have occurred and are
likely to occur in the future. In the interest of all users of the
environmental health criteria documents, readers are kindly
requested to communicate any errors found to the Manager of the
International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland, in order that they may be
included in corrigenda, which will appear in subsequent volumes.
In addition, experts in any particular field dealt with in the
criteria documents are kindly requested to make available to the
WHO Secretariat any important published information that may have
inadvertently been omitted and which may change the evaluation of
health risks from exposure to the environmental agent under
examination, so that the information may be considered in the event
of updating and re-evaluation of the conlcusions contained in the
criteria documents.
* * *
A detailed data profile and a legal file can be obtained from
the International Register of Pontentially Toxic Chemicals, Palais
des Nations, 1211 Geneva 10, Switzerland ( Telephone no. 988400 -
985850)
ENVIRONMENTAL HEALTH CRITERIA FOR CHLORDANE
Following the recommendations of the United Nations Conference
on the Human Environment held in Stockholm in 1972, and in response
to a number of World Health Resolutions (WHA23.60, WHA24.47,
WHA25.58, WHA26.68), and the recommendation of the Governing
Council of the United Nations Environment Programme, (UNEP/GC/10,
3 July 1973), a programme on the integrated assessment of the
health effects of environmental pollution was initiated in 1973.
The programme, known as the WHO Environmental Health Criteria
Programme, has been implemented with the support of the Environment
Fund of the United Nations Environment Programme. In 1980, the
Environmental Health Criteria Programme was incorporated into the
International Programme on Chemical Safety (IPCS). The result of
the Environmental Health Criteria Programme is a series of criteria
documents.
A WHO Task Group on Environmental Health Criteria for
organochlorine pesticides other than DDT met in Geneva from
28 November - 2 December, 1984. Dr K.W. Jager opened the meeting
on behalf of the Director-General. The Task Group reviewed and
revised the draft criteria document on chlordane and made an
evaluation of the health risks of exposure to chlordane.
The first drafts of the document were prepared by
Dr D.C. Villeneuve of Canada, and Dr S. Dobson of the United
Kingdom.
The efforts of all who helped in the preparation and
finalization of the document are gratefully acknowledged.
* * *
Partial financial support for the publication of this criteria
document was kindly provided by the United States Department of
Health and Human Services, through a contract from the National
Institute of Environmental Health Sciences, Research Triangle Park,
North Carolina, USA - a WHO Collaborating Centre for Environmental
Health Effects.
1. SUMMARY AND RECOMMENDATIONS
1.1. Summary
1.1.1. Identity and analytical methods
Chlordane is a viscous, light yellow to amber-coloured liquid.
Technical chlordane is a mixture of at least 26 different
components and up to 14 distinct chromatographic components have
been described. Its main components are alpha- and gamma-
chlordane.
Analysis is difficult because of the complex nature of
chlordane. The principal method for its qualitative and
quantitative determination is gas-liquid chromatography with
electron capture detection.
1.1.2. Use and sources of exposure
Chlordane has been used for more than 35 years as a broad-
spectrum contact insecticide, mainly on non-agricultural crops and
on animals. In its country of origin, the USA, its use is now
restricted to underground termite control. In several other
countries, approved uses have been gradually withdrawn.
The main source of exposure of the general population is
through residues in food. This is not a significant problem since
chlordane is not normally used on food crops, and residues in food
of animal origin are usually below accepted residue levels in
various countries. Under normal circumstances, chlordane intake
from air and water is insignificant. Chlordane has, however, been
detected in the air of buildings where the compound has been used
for termite and other insect control.
Under occupational exposure conditions, both inhalation and
skin contact are relevant, if adequate preventive and protection
measures are lacking.
1.1.3. Environmental concentrations, exposures, and effects
Chlordane is stable to light under normal conditions. It is
readily adsorbed on soil particles and therefore there is no
significant migration through the soil profile or leaching into
ground water. Some volatilisation into air from treated soils, and
some run-off into surface waters can take place.
Chlordane is fairly persistent in soil and sediments,
especially in the form of its alpha- and gamma-isomers, which are,
to a certain extent, translocated into crops grown on the soil.
Limited bioaccumulation in the adipose tissue of terrestrial
and aquatic organisms can take place. In general, concentration
factors in mammals are less than 1.
Chlordane is highly toxic to earthworms, which may present its
greatest long-term hazard for the environment.
1.1.4. Kinetics and metabolism
In experimental animals, chlordane is readily absorbed via the
skin and through oral ingestion, and probably also following
inhalation. It is readily distributed in the body, the highest
levels being found in adipose tissue, followed by the liver. The
distribution was found to be similar in the rat and the rabbit.
The metabolism of chlordane, which is a complex mixture, has been
largely elucidated. Several metabolites have been identified and
species differences have been found. Oxychlordane is the most
relevant animal metabolite, being more persistant and toxic than
the parent compound.
Following a single, oral dose, elimination of chlordane was
almost complete after 7 days in the rat. After long-term exposure,
elimination from the body was slower.
1.1.5. Studies on experimental animals
Chlordane is moderately toxic according to the scale of Hodge &
Sterner (1956) (acute oral LD50 for rat: 200 - 590 mg/kg body
weight). WHO (1984) classified the technical product as moderately
hazardous. Most of its metabolites are slightly to moderately
toxic, with the exception of oxychlordane, which is highly toxic
(acute oral LD50 for rat: 19.1 mg/kg body weight).
Signs of poisoning in various animal species are neurotoxic
manifestations such as disorientation, tremors, and convulsions.
Death may follow respiratory failure. On continuous exposure, a
certain degree of accumulation may occur in the body, mainly in
adipose tissue and to a lesser extent in the liver. The induction
of hepatic microsomal enzyme activity is one of the most sensitive
parameters for long-term, low-level chlordane exposure. At higher
levels, liver hypertrophy with histopathological and functional
changes may occur.
At high dosages (50 - 320 mg/kg diet), chlordane decreased the
fertility of rats and mice and the viability of the offspring.
There were no indications for teratogenicity.
Chlordane is not generally active in short-term tests for
genetic activity.
It induces hepatocellular carcinomas in mice.
1.1.6. Effects on man
Cases of accidental and suicidal poisoning with chlordane have
been reported. With the exception of suicide cases, recovery was
generally complete. The acute lethal dose for man is estimated to
be 25 - 50 mg/kg body weight. No adverse effects have been
reported in occupationally-exposed workers. Epidemiological data
are insufficient to judge the potential carcinogenicity of
chlordane for man.
1.2. Recommendations
(a) Figures relating to the current production and use of
chlordane should be made available;
(b) More information on human exposure from sources other
than food, such as its used in termite control, are
required;
(c) Further research is required in order to better
assess the significance for man of the carcinogenic
findings in mice;
(d) Epidemiological studies on workers who, in the past,
have been exposed to chlordane, should continue.
2. IDENTITY, PROPERTIES AND ANALYTICAL METHODS
2.1. Identity
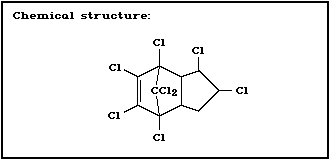 Molecular formula: C10H6C18
CAS chemical name: 1,2,4,5,6,7,8,8-octachloro-2,3,3a,4,
7,7a-hexahydro-4,7-methano-1H-indene
Common trade names: Aspon, Belt, CD 68, Chlorindan,
Chlorkil, Chlordane, Corodan,
Cortilan-neu, Dowchlor, HCS 3260,
Kypchlor, M140, Niran, Octachlor,
Octaterr, Ortho-Klor, Synklor, Tat
Chlor 4, Topichlor, Toxichlor,
Velsicol-1068
CAS registry number: 57-47-9
Relative molecular mass: 409.8
2.2. Properties and Analytical Methods
2.2.1. Physical and chemical properties
Chlordane is a viscous, light yellow to amber-coloured liquid
(IARC, 1979) with a melting point of 106 - 107 °C for the alpha-
isomer and 104 - 105 °C for the gamma-isomer. It has a density of
1.59 - 1.63 g/ml and a vapour pressure of 1 x 10-5 mm Hg at 25 °C.
It is insoluble in water but soluble in most organic solvents.
The major isomers of chlordane have the endo-endo-configuration
on the carbon skeleton (US EPA, 1976a,b). However, the term
chlordane actually refers to a complex mixture of chlordane
isomers, other chlorinated hydrocarbons and by-products. According
to the Canada National Research Council (1974), the technical
product is described as follows:
"Technical chlordane is a mixture of insecticidal
components, including chlorinated addition and substitution
derivatives of 4,7-methano-3a,4,7,7a-tetrahydro-indane.
The chlorine content is 64-67%. The principal components
are alpha- and gamma-chlordane (C10H6 x Cl8), heptachlor
(C10H5Cl7) and non-achlor (C10H5Cl9). Technical chlordane
conforms to the biological, chemical and physical properties
of reference technical chlordane".
The production of technical chlordane is strictly controlled
and its composition varies within narrow limits (Canada, National
Research Council, 1974). Technical chlordane is a mixture of at
least 26 different components, mainly however alpha- and gamma-
chlordane. Up to 14 distinct chromatographic components have been
described (Cochrane et al., 1975; Cochrane & Greenhalgh, 1976; US
EPA, 1976a,b; Gaeb et al., 1977; Sovocool et al., 1977; Kadam et
al., 1978; Parlar et al., 1979). The composition has been
essentially, but not completely, worked out. Chlordane is
available in the USA in five basic formulations (von Rumker et al.,
1974, IARC, 1979), including 5% granules, oil solutions containing
chlordane at 2 - 200 g/litre, and emulsifiable concentrates
containing chlordane at 400 - 800 g/litre.
2.2.2. Analytical methods
Determination of chlordane residues is difficult because
of the complex nature of the components and the fact that each
component degrades independently. Resulting residues may bear
little relation to the proportions in the technical product
(Council for Agriculture Science and Technology, 1975).
Separation from interfering materials can be carried out by thin-
layer chromatography or other partition and clean-up methods (US
EPA, 1976a,b). Extraction from crops, other plant products, dairy
products, plants, and oils was achieved with an 80 - 110%
efficiency using acetonitrile for extraction, petroleum ether for
partitioning, and clean-up on a Florisil column (Canada, National
Research Council, 1974). Gel-permeation chromatography can also be
used for clean-up, particularly with human adipose tissue (Wright
et al., 1978).
The principal method for the qualitative and quantitative
estimation of chlordane isomers is gas-liquid chromatography with
electron capture detection (US EPA, 1976a,b). This method has a
high sensitivity and specificity (Canada, National Research
Council, 1974; Cochrane et al., 1975). According to Atallah et al.
(1977), the highly sensitive electron capture detector can,
however, lead to the incorrect identification of residues of
chlordane and its metabolites. Confirmation of gas-chromatographic
analysis can be carried out with GLC-Mass spectrometry, a method
that can also give a better determination of some of the components
such as heptachlorepoxide (US EPA, 1976a,b). Other methods of
detection include bioassay, carbon-skeleton chromatography,
colorimetric, and total chlorine methods (US EPA, 1976a,b).
Analysis for total organically-bound chlorine (Canada, National
Research Council, 1974) remains the preferred method for the
determination of technical chlordane and the active ingredient
(chlordane) in formulations.
3. SOURCES OF ENVIRONMENTAL POLLUTION, ENVIRONMENTAL
TRANSPORT AND DISTRIBUTION
3.1. Sources of Environmental Pollution
3.1.1. Industrial production and uses
Chlordane was first prepared in the 1940s by exhaustive
chlorination of the cyclopentadiene-hexachlorocyclopentadiene
adduct (IARC, 1979). It was first described as an insecticide in
1945 by Kearns (Spencer, 1973).
Chlordane is produced commercially by reacting hexachloro-
cyclopentadiene with cyclopentadiene to form chlordene, which is
then chlorinated to produce chlordane (IARC, 1979). Chlordane was
first produced commercially in the USA in 1947 (IARC, 1979).
Production in the USA, in 1974, amounted to 9.5 million kg (IARC,
1979). Chlordane is not produced in Europe nor has it ever been
manufactured in Japan (IARC, 1979). In Japan, the only permitted
use of the compound is for the control of termites. It is also
used against wood-boring beetles and in ant baits. Both the
amounts of chlordane produced and used have decreased considerably
in recent years (WHO, 1982).
Chlordane has been used as an insecticide for more than 35
years. It is a versatile, broad spectrum, contact insecticide and
is used mainly for non-agricultural purposes (primarily for the
protection of structures, but also on lawn and turf, ornamental
trees, and drainage ditches) (von Rumker et al., 1974). Further-
more, it is used on corn, potatoes, and livestock. In 1978, a US
EPA cancellation proceeding led to a settlement on contested uses.
This settlement allowed for limited usage by crop, location, amount
allowed, and maximum time interval for use.
Since 1 July 1983, the only use of chlordane approved in the
USA is for the control of underground termites (IARC, 1979). In
Canada, the use of chlordane is controlled under the Pest Control
Products Act and it is used for the protection of structures,
ornamental plants, lawns, and various crops. Accepted uses vary
from province to provide (Canada, National Research Council, 1974).
3.2. Environmental Transport and Distribution
3.2.1. Air
Entry into the atmosphere occurs mainly through aerial
applications of dusts and sprays, soil erosion by the wind, and
volatilization from soil and water (Canada, National Research
Council, 1974).
3.2.2. Water
Few data are available on the routes of entry or the behaviour
and fate of chlordane in aquatic systems. It can be assumed that
not much originates from ground water since there is little
leaching of chlordane. One possible source is surface run-off, but
no studies have tested the extent of this assumption. Another
source is rain; however, in two studies, chlordane levels did not
exceed 2 - 3 ng/litre rain water (Bevenue et al., 1972a; US EPA,
1976a,b).
One important aspect of chlordane residues is that they
accumulate in sediment. The fate and behaviour of chlordane was
investigated in an isolated fresh water lake, previously free from
pesticide residues (Oloffs et al., 1978). The lake was treated
with technical chlordane at 10 µg/litre, and sediment samples were
analysed for chlordane residues 7, 24, 52, 279, and 421 days after
treatment. It was found that water residue concentrations declined
rapidly. After 7 days, only 46.1% of the chlordane residue
remained. After 421 days, residues were still detectable, but all
levels were below 0.01% of the initial concentration. It was
observed that chlordane residues moved quickly to the bottom
sediment and persisted there. Mean residue levels in sediment were
35.29 µg/kg wet weight after 7 days and 10.31 µg/kg after 421 days.
3.2.3. Soil
Chlordane is used almost exclusively as a soil insecticide to
control soil pests such as termites (Canada, National Research
Council, 1974). Thus, residues of chlordane are mainly present in
this environmental compartment. In most temperate climates, only
the two chlordane isomers generally persist (Canada, National
Research Council, 1974). For example, in Nova Scotia, chlordane
was applied at 5 kg/ha per year to sandy loam soil for 3 years.
Fifteen years later, approximately 15% of the residues remained,
the alpha and gammma isomers being the major components (US EPA,
1976a,b).
The components of technical chlordane are relatively insoluble
in water and are readily adsorbed onto soil particles. As a
result, one of the characteristics of soil residues is that they do
not migrate readily through the soil profile (Canada, National
Research Council, 1974; von Rumker et al., 1974). In general, not
more than 15% of the residues migrate below the cultivated layer
(Canada, National Research Council, 1974). As a result, residues
are not likely to become a serious contaminant of the lower soil
strata or deep water sources (Canada, National Research Council,
1974). The organic matter and moisture contents of the soil can
affect the volatilization of chlordane components (Stauffer, 1977).
The organic matter causes greater adsorption and thus reduces
volatilization while soil moisture increases volatilization
(Stauffer, 1977). Also, liquid formulations are more volatile than
granular (Atallah et al., 1979).
3.2.4. Abiotic degradation
Chlordane is stable to light under normal conditions. When it
is exposed to photosensitizers such as rotenone or benzophenone and
short irradiation exposure at wavelengths above 300 nm, some
components will isomerize (Canada, National Research Council,
1974). No detectable degradation products were formed on plant
foliage in the absence of a photosensitizer (Ivie et al., 1972).
3.2.5. Biodegradation
Three conversion products of gamma-chlordane were found in
white cabbage and carrots, 4 weeks after application. One of the
two metabolites isolated from white cabbage (35% of the total), was
given the chlordene chlorohydrin structure. The other isolated
metabolite (15% of the total) was assigned the dihydroxy-beta-
dihydroheptachlor structure. The third metabolite was not
identified. 1,2-Dichlorochlordene, oxychlordane, and photo-alpha-
chlordane, as well as the parent chlordane compounds, were found in
alfalfa after treatment of the soil with chlordane (Canada,
National Research Council, 1974).
Oxychlordane (or 1,2-dichlorochlordene epoxide) is the common
metabolite derived from both alpha- and gamma-chlordane. It has
been found in the fat of pigs fed either of the isomers and in the
milk and cheese from cows fed alfalfa treated with technical
chlordane. According to some authors, alpha- and gamma-chlordane
give rise to oxychlordane via the intermediate 1,2-dichloro-
chlordene (Canada, National Research Council, 1974).
4. ENVIRONMENTAL LEVELS AND EXPOSURES
4.1. Environmental Levels
4.1.1. Air
Generally, atmospheric concentrations of chlordane appear to be
insignificant. However, chlordane has been detected in the air of
buildings where the compound has been used for termite or other
insect control (US EPA, 1976a,b). Insufficient information is
available on this.
4.1.2. Water
Data from several studies indicate that contamination of water
with chlordane is not a widespread problem (US EPA, 1976a,b), and
that, generally, water residue levels are non-measureable or very
low (Canada, National Research Council, 1974).
In a study of the bottom material of 26 tributary streams in
San Fransisco Bay, chlordane was found to be ubiquitous at
concentrations ranging from a trace to 800 µg/kg (Oloffs et al.,
1978).
No chlordane was detected in 188 samples of surface water from
southern Florida but it was detected in 30% of 214 sediment samples
(Mattraw, 1975). In a study in Hawaii in 1970-71 (Bevenue et
al., 1972b), chlordane was found in drinking-water in 9% of samples
at a mean level of 1.0 ng/litre. Chlordane was detected in non-
potable waters from canals at levels ranging from 3.7 - 9.1
ng/litre. Again, sediments showed much higher levels of 190 - 378
µg/kg. Chlordane was found to occur in the lower Mississippi river
almost continuously throughout 1974 at values ranging from 1.3 to
2.9 ng/litre (Brodtmann, 1976). In a study in the lower
Mississippi (Barthel et al., 1969), during 1964-67, chlordane
residues ranged from 0.80 to 2.80 mg/kg in river bed material
samples. In tributaries of the river, values ranged from 0.56 -
6.44 mg/kg, in 13 out of 348 samples. No chlordane was detected in
any water or sediment samples taken from the upper Great Lakes in
1974 (Glooschenko et al., 1976). In one study in 1976 (Harrington
et al., 1978), chlordane contamination of a municipal water system
was reported, concentrations of chlordane accidently rising to 1.2
g/litre.
4.1.3. Soil
One study has shown that the alpha- and gamma-isomers of
chlordane are less persistent in mineral sand soil than in organic
mucky soil (Harris & Sans, 1976). Data from the National Soils
Monitoring Program in 1970 showed that chlordane occurred in 0.07%
of 1346 sites in 35 States. The range of residues was 0.01 - 13.34
mg/kg dry weight with a mean of 0.08 mg/kg (Crockett et al., 1974).
Monitoring of the corn belt region in the USA (12 States) in 1970
showed chlordane to be one of the most commonly detected
insecticides with values of ND - 0.20 mg/kg (Carey et al., 1973).
Data from 9 States in 1971 detected chlordane in one soil sample at
0.04 mg/kg dry weight (Gowen et al., 1976). Monitoring in 1973
showed residues ranging from 0.001 - 0.020 mg/kg with generally
higher values in urban areas. When urban soils were tested in 14
cities in the USA in 1970, values ranged from 0.01 - 1.27 mg/kg.
In the Atlantic provinces of Canada, chlordane was detected in less
than 10% of agricultural lands at concentrations below 1 mg/kg dry
weight (Duffy & Wong, 1967). In another study on chlordane in
Saskatchewan soils (Saha & Sumner, 1971), 7 out of 41 samples
contained chlordane with residue values ranging from 0.01 to 3.91
mg/kg dry weight. The duration of soil contamination has been
studied by several investigators. Using bioassay techniques, it
was found in one study that 15% of active ingredients remained in
turf soils in Wisconsin after 12 years (Lichtenstein & Poliuka,
1959). In a study in 1970 (Lichtenstein, 1970), it was found that
10 years after application of chlordane at 8.5 kg /ha,
approximately 18 - 20% remained.
4.1.4. Crops and wildlife
Since chlordane residues are present predominantly in the soil,
translocation into plants is an important factor. In most
temperate climates, alpha- and gamma-chlordane are the principal
plant residues. In the Canadian climate, the composition of plant
residues resembles that of technical chlordane (Canada, National
Research Council, 1974). In a field study on soil/plant
relationships, it was found that the relationship between residues
in soil and those in crops was not consistent and consequently not
predictable (Boyd, 1971).
In a three-year study, Onsager et al. (1970) monitored
chlordane residues in sugar beets cultivated on loam soil treated
once at 6 different application rates ranging from 1.4 - 22.4
kg/ha. In the first growing season, only sugar beets treated at
the two lowest rates (1.4 and 2.8 mg/kg) showed residues below 0.3
mg/kg dry weight. In the last two seasons, beets from soil treated
at all rates contained residues below this value. In another
study, uptake by root crops was shown to be related to the soil
type (Stewart, 1975). When chlordane was applied to sandy loam
soil with 12% clay at 15 kg/ha, residues in beets, carrots,
parsnips, potatoes, and rutabagas were 0.03, 0.26, 0.24, 0.04, and
0.01 mg/kg, respectively. In sandy loam containing 28% clay,
values were 0.01, 0.07, 0.12, 0.15, and 0.01 mg/kg, respectively.
A study of residues in alfalfa following applications of high
purity chlordane showed that during the first 4 months following
treatment, oxychlordane and photo-alpha-chlordane accounted for 16%
and 17% of the residues, respectively (Wilson & Oloffs, 1973a).
Generally, no detectable residues of chlordane were found in
wildlife such as birds (Canada, National Research Council, 1974;
Fitzhugh & Fairchild, 1976; Clark & Krynitsky, 1978). However,
in one study (Clark & Prouty, 1976), mean total residues of
oxychlordane ranging from 0.11 to 6.63 µg found in the carcasses
of bats from Maryland and Virginia were attributed to chlordane
use.
In extensive surveys, residues in fish have generally been low.
In 1976, residues in several species from Lake Erie and Lake Saint
Claire in Canada were found to range from non-detectable to 0.046
mg/kg fresh weight (Frank et al., 1978a,b). Residues in Canadian
commercially caught fish in 1970 were not detectable (Reinke et
al., 1972). The National Pesticide Monitoring Program from 1967-68
found chlordane residues in 128 out of 590 fish samples at levels
generally less than 0.5 mg/kg (Wilson & Oloffs, 1973b). From
1972-76, chlordane residues were found in only 3% of the samples of
estuarine fish in the USA (Butler & Schutzmann, 1978). Chlordane
residues were also not detectable in fish and fishery products from
the Northwestern Atlantic (Meith-Avcin et al., 1973; Sims et al.,
1977).
4.1.5. Food
There have been many studies in Canada, Great Britain, the USA,
and other countries on the occurrence of pesticide residues in
food. Generally, the results of these studies showed that residues
of chlordane seldom occur and uptake by man is negligible (Canada,
National Research Council, 1974). Residue tolerances for chlordane
have been established at the following levels: Belgium, Luxembourg,
and the Netherlands, 0.1 mg/kg, Canada, 0.3 mg/kg, European
Economic Community, 0.2 mg/kg and the USA, 0.3 mg/kg. These levels
are for a wide variety of foods (US EPA, 1976a,b). A temporary
acceptable daily intake (ADI) for human beings for the sum of the
alpha- and gamma-isomers of chlordane and oxychlordane of 0 - 0.001
mg/kg body weight was advised by the Joint Meeting on Pesticide
Residues (FAO/WHO, 1983). Chlordane is rarely present in market
basket surveys, and then only at low levels. It is not among the
top 10 chlorinated pesticides usually found as residues in food (US
EPA, 1976a,b). For example, in a survey in the USA from 1963 to
1969, chlordane residues were found in less than 1% of the samples
and ranged from 1 - 5 µg/kg.
It has already been shown that crops will translocate chlordane
residues from the soil. Generally, the amounts in crops are low.
Residues tend to accumulate in the crude oils of oil-seed crops at
levels higher than those in the original seed and in the oil-seed
meal. However, these levels are reduced by refining processes.
Chlordane residues were found in meat, milk, and eggs. Residues in
feed crops or from direct applications to cattle and poultry were
shown to result in significant residues in milk, meat, and eggs (US
EPA, 1976a,b). In a study on eggs in Canada, gamma-chlordane was
found in 78% of the samples with a mean value of 2 µg/kg fresh
weight and alpha-chlordane in 81% of the eggs with a mean value of
1 µg/kg (Mes et al., 1974). In another study (Herrick et al.,
1969), no residues were found in the eggs of chickens fed chlordane
in their diet at 0.08 mg/kg for a week.
In a study on samples of cow's milk analysed in the USA, 87%
were positive for chlordane with levels ranging from 0.02 to 0.06
mg/litre (IARC, 1979). In another study (US EPA, 1976a,b), the
milk of cows grazing on pastures with chlordane applied at 0.55
kg/ha contained an average chlordane concentration of 0.03
mg/litre. No residues were found at lower treatment levels.
Chlordane was also found in Canadian meat samples at levels ranging
from 0 to 106 µg/kg in beef, 0 to 32 µg/kg in pork, and 0 to 70
µg/kg in fowl (Saschenbrecker, 1976).
4.1.6. Human milk
Several studies on pesticide residues in human breast milk did
not reveal any residues of chlordane, but oxychlordane, trans-
nonachlor, and heptachlor epoxide were found, which may be related
to chlordane exposure. In a study on 54 women in Arkansas and
Mississippi from 1973-74, breast milk contained oxychlordane at
0.005 mg/litre, heptachlor epoxide at 0.004 mg/litre, and trans-
nonachlor at 0.001 mg/litre (Strassman & Kutz, 1977). In another
study on 34 samples of breast milk in Northern Mississippi from
1973-75, oxychlordane levels were found of 0.005 mg/litre in high-
pesticide-usage areas and 0.002 mg/litre in low-usage areas
(Barnett et al., 1979). In a survey involving 1436 lactating
mothers in the USA, the mean levels of oxychlordane in the milk
ranged from 75.4 - 116 µg/litre on an adjusted fat basis (Savage,
1976). In a study of Canadian human milk samples in 1974,
oxychlordane was found in 77% of the samples, trans-nonachlor in
68%, and heptachlor epoxide in 69%, each at a mean level of 1
mg/litre whole milk (Mes & Davies, 1978).
Jensen (1983) recently reviewed the levels of chlordane
and oxychlordane in human milk, and his data, including the
above-mentioned studies as well as more recent data, are
reproduced in Table 1.
4.2. General Population Exposure
Oxychlordane was found together with other organochlorine
pesticides in human fat samples at levels ranging from 0.03 to
0.4 mg/kg wet weight (mean 0.14 mg/kg) in residents of the USA
(Biros & Enos, 1973). Sovocool & Lewis (1975) also reported
the identification of oxychlordane in human fat. As indicated
by Biros & Enos (1973), the occurrence of oxychlordane
residues in human adipose tissue in the general population may
reflect previous exposure to chlordane and/or oxychlordane.
This organochlorine compound is included in the human tissue
residue monitoring program (Kutz et al., 1976).
4.3. Occupational Exposure
Permissible levels of exposure to chlordane in the workplace
air have been adopted in different countries (ILO, 1980). Examples
include: 0.5 mg/m3 as a time-weighted average concentration in
Belgium, Finland, Japan, the Netherlands, and the USA (both OSHA
and ACGIH), 0.3 mg/m3 as a time-weighted average and 0.6 mg/m3
as a ceiling concentration in Romania, and 0.01 mg/m3 as maximum
allowable concentration in the USSR.
People primarily exposed are those employed in the application
of chlordane for the control of insects and pests (IARC, 1979).
Chlordane has been found in household dust in the homes of farmers
(mean level 5.79 mg/kg air-dried dust) and pesticide formulators
(mean level 23.11 mg/kg) (Starr et al., 1974).
Table 1. Chlordane and oxychlordane in human milka
------------------------------------------------------------------------------------------------------
Oxychlordane and
chlordane content inb
---------------------
No. of samples Fat Whole milk Milk fat
Area, year (% positive) % (mean) (mg/litre) (mg/kg) References
------------------------------------------------------------------------------------------------------
Canada (1975) 100 2.2 1 - Mes & Davies (1978)
(< 2)
Japan
Tokyo (1978) 11 - 0.5 - Miyazaki et al. (1980)
(0.1 - 1.0)
Tokyo (1979) 12 - 0.5 - Miyazaki et al. (1980)
(0.3 - 1.1)
Mexico (1976) 620 - - 0.40 (median) FAO/WHO (1981)
Spain (1979) 45 (17.8%) - 0.3c 0.026c Lora et al. (1979)
(0 - 0.72)
USA
Arkansas/Mississippi 57 (46%) 3.0 12/10 - Strassman & Kutz (1977);
(1973-4) (0 - 20) FAO/WHO (1981)
------------------------------------------------------------------------------------------------------
Table 1. (contd.)
------------------------------------------------------------------------------------------------------
Oxychlordane and
chlordane content inb
---------------------
No. of samples Fat Whole milk Milk fat
Area, year (% positive) % (mean) (mg/litre) (mg/kg) References
------------------------------------------------------------------------------------------------------
Hawaii (1979-80) 50 (100%) 3.2 - 0.059 Takahashi et al. (1981)
(0.01 - 0.16)
Mississippi (1973-5) 34 (100%) - 5 0.13 Barnett et al. (1979)
(pesticide area) (1 - 22) (0.03 - 0.70)
Mississippi (1973-5) 6 (68%) - 2 0.05 Barnett et al. (1979)
(non-pesticide area) (0 - 4) (0 - 0.12)
USA-NE (1975) 233 - - 0.08 ± 0.05 Savage (1976);
Savage et al. (1981)
USA-SE (1975) 288 - - 0.12 ± 0.15 Savage (1976);
Savage et al. (1981)
USA-MW (1975) 378 - - 0.08 ± 0.05 Savage (1976);
Savage et al. (1981)
USA-SW (1975) 388 - - 0.11 ± 0.35 Savage (1976);
Savage et al. (1981)
USA-NW (1975) 149 - - 0.08 ± 0.05 Savage (1976);
Savage et al. (1981)
USA (total) (1975) 1 436 (74%) - 2 (median) 0.096 ± 0.195 Savage (1976);
(0.013 - 0.57) Savage et al. (1981)
FAO/WHO (1981)
------------------------------------------------------------------------------------------------------
a From: Jensen (1983).
b Results are expressed as means ± S.D. Ranges are listed in parentheses below.
c Chlordane.
5. KINETICS AND METABOLISM
5.1. Absorption
In studies on 4 male rabbits, a combination of 14C-alpha and
gamma-chlordane (approximately 1700 mg of each, administered orally
in 4 doses at 4-day intervals), was well absorbed (Balba & Saha,
1978). Brief exposure of dogs to topically applied chlordane
solutions (3.2 g/litre) resulted in a significant and long-lasting
decrease in the biological half-life of orally-administered
warfarin (Bachmann & Burkman, 1974).
5.2. Distribution and Storage
Studies using radio-labelled chlordane showed that after oral
administration, the radioactivity was well distributed in tissues
of rats (Barnett & Dorough, 1974) and rabbits (Balba & Saha, 1978).
Rats, whether being treated with single oral doses of chlordane or
fed diets containing this compound, retained the highest levels of
residues in adipose tissue, followed by the liver, kidney, brain,
and muscle. More of the gamma-isomers was retained than of the
alpha-isomer. Residues in the fat of rats fed radiolabelled
chlordane (3:1 alpha- and gamma-chlordane) at 1, 5, and 25 mg/kg
diet for 56 days were approximately 3 times higher than those in
the diets. Oxychlordane was the most persistent residue in the
tissues of these rats after chlordane was removed from the diet
(Barnett & Dorough, 1974). The tissue distribution of chlordane in
rabbits was found to be similar to that in rats (Poonawalla &
Korte, 1971; Balba & Saha, 1978).
5.3. Biotransformation
Poonawalla & Korte (1971) showed that 70% of gamma-chlordane
fed to rabbits was excreted in the urine in the form of
metabolities, a.o., gamma-1-hydroxy-2-chlorodihydrochlordene and
1,2-dihydroxydichlordene.
Oxychlordane has been isolated from the fat of dogs, rats
(Polen et al., 1971), pigs (Schwemmer et al., 1970), and cattle
(Lawrence et al., 1970). It has also been isolated from human fat
(Biros & Enos, 1973). Barnett & Dorough (1974) indicated that the
faecal extracts of rats fed 14C-chlordane showed the presence of
eight radioactive areas on the TLC plate. Although the structures
of the metabolites were not fully elucidated, they were tentatively
identified as mono-, di-, and tri-hydroxylated products of
chlordane.
The major route of metabolism for both alpha- and gamma-
chlordane was via dichlorochlordene and oxychlordane (Tashiro &
Matsumura, 1977). The results of these studies were in general
agreement with the proposal of Street & Blau (1972) and the results
of in vitro metabolism studies of Brimfield et al. (1978).
Tashiro & Matsumura (1977) were able to isolate 1-exo-hydroxy-2-
endo-chloro-2,3-exoepoxychlordene, and found another major
metabolic route for alpha-chlordane that involved a more direct
hydroxylation reaction to form 1-exo-hydroxy-dihydrochlordenes and
1,2-gamma-dihydroxydihydrochlordene. Both Brimfield et al. (1978)
and Tashiro & Matsumura (1977) indicated that oxychlordane and, to
a lesser extent, heptachlor were metabolites of chlordane.
However, they did not agree as to whether these two metabolites
would be terminal residues or intermediates in the metabolic
pathways of chlordane. Recent investigations have indicated that
other metabolites were present in the urine of rabbits fed
chlordane. Thus, alpha-chlordane gave rise to 1-hydroxy-2-
chlorochlordene, 1-hydroxychlordene, and gamma-chlordene
chlorhydrin. Administration of the gamma-isomer resulted in
excretion in the urine of the rabbits of 1,2-dichlorochlordene,
1-hydroxy-2-chlorochlordene, gamma-chlordene chlorhydrin, and
3-hydroxychlordane (Balba & Saha, 1978).
In vitro metabolism studies have been summarized by Brimfield
& Street (1979). By incubation of alpha- and gamma-chlordane with
rat liver postmitochondrial supernatant, dichlorchlordene and
oxychlordane were isolated, a result that was similar to those from
in vivo studies. Hart et al. (1963) and Hart & Fouts (1965)
reported that chlordane induced non-specific microsomal enzyme
activity in the rat, resembling, from this point of view,
phenobarbital.
5.4. Elimination and Excretion
Elimination of radiolabelled chlordane (3:1 alpha- and gamma-
chlordane) and the individual isomers was studied in rats. Single
oral doses of 0.05, 0.2, and 1 mg/kg body weight in corn oil were
almost completely eliminated after 7 days; 24 h after administra-
tion, 70% of alpha-chlordane and 60% of the gamma-isomer were
excreted. Female rats excreted more of the dose in the urine than
the males (Barnett & Dorough, 1974).
6. STUDIES ON EXPERIMENTAL ANIMALS
The toxicity and the residue data on chlordane including some
unpublished studies have been reviewed several times by
international bodies such as FAO/WHO (1968, 1973, 1978, 1981,
1983), CEC (1981), and IARC (1979). For their conclusions, refer
to section 9.
6.1. Short-term Exposures
6.1.1. Oral exposure
The acute toxicity of chlordane in several animal species is
shown in Table 2.
The signs associated with acute chlordane poisoning include
ataxia, convulsions, respiratory failure, and cyanosis followed by
death (US EPA, 1976a,b). Correlation between respiratory
difficulty and EEG patterns suggest that respiratory failure is a
contributing factor in chlordane-induced mortality (Hyde &
Falkenberg, 1976). Pathological manifestations include haemorrhage
in the gastrointestinal tract, kidneys, lung, and heart as well as
pulmonary congestion and oedema, and degenerative changes in the
central nervous system (US EPA, 1976a,b).
Seven dogs were given chlordane in single oral doses ranging
from 200 - 700 mg/kg body weight. Convulsions were seen in one dog
at 200 mg/kg (lowest dose) but 700 mg/kg (highest dose) did not
induce any effects (Batte & Turk, 1948). Four groups of 2 - 4 dogs
were given chlordane orally in doses of 5 - 200 mg/kg body weight.
All of the dogs died within 25 days to 93 weeks (Lehman, 1952b).
Chlordane administered by stomach-tube to sheep at 500 mg/kg
body weight induced toxic symptoms (incoordination, partial
blindness). Full recovery occurred in 5 - 6 days. A dose of 1000
mg/kg body weight induced severe respiratory and nervous symptoms
16 h after treatment and death after 48 h (Welch, 1948).
When a diet containing 1000 mg chlordane/kg was fed to 12 male
rats, all of them died within 10 days (Stohlman et al., 1950). At
500 mg/kg all 12 died within 70 days and, at 300 mg/kg, 9 animals
out of 12 were alive after 100 days. Daily oral doses of 6.25 - 25
mg/kg body weight administered to 5 rats for 15 days did not induce
tremors or convulsions, but daily doses of 50 mg/kg body weight
induced toxic symptoms, and 2 of the animals died (Ambrose et al.,
1953). Cytoplasmic bodies in the liver cells were observed in all
groups and were dose-related.
Table 2. Acute toxicity of chlordane in experimental animals
---------------------------------------------------------------------------
Species Sex Route Vehicle LD50 Reference
(mg/kg)
---------------------------------------------------------------------------
Rat F dermal xylene 530 Gaines (1969)
Rat M dermal xylene 205 Gaines (1969a)
Rabbit NS dermal "early" < 780 Ingle (1965b)
chlordane
Rabbit NS dermal "later" 1100 - 1200 Ingle (1965b)
chlordane
(more purified)
Rat M oral peanut oil 335 Gaines (1969)
Rat F oral peanut oil 430 Gaines (1969)
Rat NS oral variety 200 - 590a Ambrose et al. (1953);
Ingle (1965a)
Rat NS oral NS 283 Buck et al. (1973)
Rat NS oral NS 350 Truhaut et al. (1974)
Rabbit NS oral NS 100 - 300a Stohlman et al. (1950)
Rabbit NS oral NS 20 - 40a Ingle (unpublished
data, 1955)
Hamster NS oral NS 1720 Truhaut et al. (1974)
Goat NS oral NS 180 Welch (1948)
Sheep NS oral NS 500 - 1000 Welch (1948)
Chicken NS oral NS 220 - 230 FAO/WHO (1968)
Mallard NS oral NS 1200 Buck et al. (1973)
Cow NS oral NS 25 - 90 Buck et al. (1973)
---------------------------------------------------------------------------
a The wide range is explained by the use of different solvents and the
fact that chlordane produced before 1950 contained a considerable
amount of hexachlorocyclopentadiene.
NS - Not specified.
6.1.2. Dermal exposure
The single-dose dermal LD50 of "early" chlordane in rabbits
was reported to be less than 780 mg/kg body weight (Ingle, 1965b),
and it was noted that this concentration caused severe skin
irritation, tremors, and convulsions (Lehman, 1952a). The dermal
LD50 of the later, more purified chlordane was 1100 - 1200 mg/kg
(Ingle, 1965b).
6.1.3. Parenteral exposure
Male gerbils were dosed intramuscularly with chlordane at 2.5
mg/kg body weight every 3 days for 45 days. Treatment induced
hyperproteinemia, hyperglycemia, and enhanced serum alkaline and
acid phosphatase activities (Karel & Saxena, 1976).
6.2. Long-term Exposures
6.2.1. Oral exposure
Groups of 4 - 7 male and 4 - 7 female dogs were fed dietary
levels of 0, 0.3, 3, 15, or 30 mg chlordane/kg for 2 years.
Abnormalities in the results of clinical liver function tests were
seen in the 15 and 30 mg/kg groups. In animals selected for
necropsy at the end of the first year, increased relative liver
weights and associated hepatocellular changes were found at 30
mg/kg; at the end of two years, dose-related increases in relative
liver weights were found at 15 and 30 mg/kg, with non-dose-related
hepatocellular changes. There was no difference between the
severity of the liver lesions of the 30 mg/kg animals and those of
four animals withdrawn from 30 mg/kg treatment for eight months
prior to sacrifice. Liver biopsies on two animals of the 30 mg/kg
group at 1, 3, and 6 months showed hepatocellular changes at 6
months but not at 1 or 3 months. No adverse effects were seen on
behaviour, appearance, survival, weight gain, blood picture, or the
results of periodic physical examinations, at any level (Wazeter,
1967).
Twenty-four rats, 12 of each sex, were fed dietary levels of
2.5, 25, or 75 mg chlordane/kg for 2 years (Lehman, 1952b). The
two higher levels caused moderate to severe signs of toxicity. The
lowest level caused histological liver changes. Rats were fed
technical chlordane (early production) for two years at levels in
the diet of 0, 5, 10, 30, 150 or 300 mg/kg (Ingle, 1952; 1965a).
Convulsions and tremors were observed in animals receiving 150
mg/kg or more. Hepatocellular alterations consisting of
hypertrophy, cytoplasmic oxyphilia and hyalinization, karyorrhexis,
karyolysis, and cell necrosis were obvious at 150 and 300 mg/kg,
slight at 30 mg/kg, minimal at 10 mg/kg and absent at 5 mg/kg.
Growth was retarded and liver weight and mortality rate increased
at 150 and 300 mg/kg. In a subsequent study on rats (Ingle,
1965a), technical chlordane of later production containing fewer
by-products was administered at levels of 2.5 - 300 mg/kg diet for
2 years. Changes in food consumption, growth, and mortality rate
were seen only at the highest dose. Cellular alterations were seen
at 50 mg/kg and higher.
Rats were fed chlordane at levels ranging from 10 to 1280 mg/kg
diet for 407 days (Ambrose et al., 1953). The animals in the two
highest dose groups died early; liver weight was increased at 320
mg/kg; fatty infiltration and cytoplasmic margination were seen in
the liver parenchymatous cells in males at 40 mg/kg and above, and
in females at 80 mg/kg and above.
Groups, each comprising 20 male and 20 female rats, were fed
dietary levels of 0, 5, 15, 25, or 35 mg/kg of alpha-chlordane, 15,
25, 35, or 75 mg/kg of gamma-chlordane or 5, 15, 25, 35 or 50 mg/kg
of a 1:1 mixture of alpha- and gamma-chlordane (Ingle, 1969). In
the group fed alpha-chlordane, growth retardation became apparent
in the rats fed 35 mg/kg after 4 months in males and after 5 months
in females; with gamma-chlordane, the 75 mg/kg group of males only
displayed growth retardation after 8 months. With the mixture,
growth retardation was evident in both sexes fed 50 mg/kg,
beginning earlier in males than in females. Growth retardation was
not evident in any group fed lower doses of either isomer. Food
consumption bore a relationship to growth. Increased mortality
rates for both sexes became significant in the groups fed alpha-
chlordane at 35 mg/kg, gamma-chlordane at 75 mg/kg, or the alpha-
gamma mixture at 50 mg/kg. Haematocrit was normal for all test
groups. Autopsy did not reveal any gross pathological lesions.
There was no evidence of tumours. Histological examination did not
show any changes from feeding chlordane in any organ except the
liver. Compression of sinusoids due to slight hepatic cell
hypertrophy in the centrolobular region and minimal bile duct
proliferation were evident with administration of alpha-chlordane
at 35 mg/kg. The same changes were noted, but were minimal with
the same isomer at 25 mg/kg. Slight to moderate cytoplasmic
homogeneity of the hepatic cells in the centrolobular region,
minimal cytoplasmic margination, and minimal cell hypertrophy with
compressed sinusoids were noted with administration of gamma-
chlordane at 75 mg/kg. Slight cytoplasmic homogeneity of hepatic
cells in the centrolobular region and occasional cytoplasmic
margination were observed with the alpha-gamma mixture at 50 mg/kg.
The above alterations were minimal with the same mixture at 35
mg/kg. No liver changes were evident after feeding lower levels of
the chlordane isomers.
Groups of 6 female and 6 male rats were fed 2.5 mg or 25 mg of
a sample of technical chlordane containing 60 - 75% chlordane and
25 - 40% unrelated products per kg diet for up to 9 months (Ortega
et al., 1957). Centrolobular cell hypertrophy, cytoplasmic
margination, and cytoplasmic bodies were observed in the liver in 1
male fed 2.5 mg/kg and in 5 males fed 25 mg/kg. No changes were
seen in females.
In a two-year feeding study, pure-bred male and female beagle
dogs were fed chlordane at levels of 0, 0.3, 3.0, 15, or 30 mg/kg
diet. No adverse treatment-related alterations were observed in
behaviour, appearance, eye examination, body weight, food
consumption, haematology, or plasma biochemistry. Some liver
enzyme activities were altered throughout the study at the 15 and
30 mg/kg levels. Relative liver weights were slightly increased
after two years in the two highest groups. Treatment-related
microscopic changes, observed in dogs fed the two highest levels,
consisted of enlargement of centrolobular hepatocytes with
margination of coarse cytoplasmic granules (IRDC, 1967).
The Joint Meeting on Pesticide Residues (JMPR) reviewed the
toxicity data on chlordane at its 1977 meeting (FAO/WHO 1978) and
decided on the following "no-observed-adverse-effect levels":
- rat: 5 mg/kg in the diet, equivalent to 0.25 mg/kg
body weight; and
- dog: 3 mg/kg in the diet, equivalent to 0.075 mg/kg
body weight.
These "no-observed-adverse-effect levels" were confirmed by the
1982 JMPR (FAO/WHO, 1983).
6.2.2. Dermal exposure
When male guinea-pigs were exposed to chlordane at 67 mg/kg
body weight/day, through dermal painting for 90 days, mild
degenerative changes in the skin and testis were evident (Datta et
al., 1975).
The ninety-day repeated daily dose LD50 for rabbits was
reported in the paper by Lehman (1952a) (section 6.1.2) to be from
20 - 40 mg/kg body weight. Ingle (1965b) reviewed the dermal
toxicity of chlordane and attributed the toxicity of early
technical chlordane to the significant content of hexachloro-
cyclopentadiene (HCPD). A more pure product, which did not contain
significant quantities of HCPD, was only half as toxic to rabbits
as the earlier chlordane and did not cause any skin irritation or
damage to mucous membranes.
6.3. Reproduction Studies and Teratogenicity
Rats, maintained from weaning on a diet containing a chlordane
level of 320 mg/kg, showed reduced rates of mating, of viable
litters, and an increased rate of death of progeny prior to
weaning. It was concluded that, at this dosage, chlordane
interfered with both fertility and litter survival (Ambrose et al.,
1953). Groups of 10 male and 20 female rats were used in a 3-
generation study at dietary levels of technical chlordane of 0,
0.3, 3, 15, 30, and 60 mg/kg (Ingle, unpublished data, 1967). Two
litters in each filial generation were studied. Levels up to and
including 30 mg/kg did not have any effect on fertility, number of
offspring, or weight, growth, or mortality rate of the young
animals to weaning age. Autopsy of animals after weaning did not
reveal any gross or microscopic differences between the groups. At
60 mg/kg, there was a high (10.6%) mortality rate in the second F3
generation litters during the latter part of the nursing period;
these animals showed gross and microscopic pathological changes,
comparable with those characteristic for chlordane intoxication.
However, survivors of this generation did not show any tissue
changes at all. A third set of F3 litters at 60 mg/kg suffered 17%
mortality during the nursing period, with symptomatology and gross
and microscopic tissue changes characteristic of chlordane
intoxication. Third F3 generation litters from dams removed from
the 60 mg/kg group and placed on chlordane-free diets for 30 days
prior to remating showed no differences in any respect from control
litters. No evidence of teratogenicity was found in this study.
Hens and cocks fed up to 0.3 mg chlordane/kg diet did not show
any toxic symptoms or any adverse effects on egg weight,
hatchability, or growth of chicks (Biotox, unpublished data, 1969).
Mice fed diets containing chlordane at 25 - 100 mg/kg for 6
generations showed decreased viability in the first and second
generations at 100 mg/kg; in the third generation at this level, no
offspring were produced (Keplinger et al., 1968). At 50 mg/kg,
viability was reduced in the fourth and fifth generations, and at
25 mg/kg no statistically significant effects were observed, even
after 6 generations.
Chlordane was administered to rabbits orally at levels of 1.0,
5.0, and 15 mg/kg body weight per day on the 6th - 18th days of
gestation. A control group and a positive control group were used.
No changes were seen in behaviour, appearance, or body weight.
Miscarriages were seen in 3 rabbits at the 1.0 mg/kg level and one
rabbit at 15 mg/kg dose level. No effects on any of the maternal
or fetal parameters were noted. No teratogenic effects were noted
(IRDC, 1972).
6.4. Mutagenicity
Alpha-Chlordane, gamma-chlordane, and chlordene were tested in
the Ames Salmonella microsome assay and were not mutagenic (Simmon
et al., 1977). Chlordane was not mutagenic when tested using 5
different strains of Salmonella typhimurium in the Ames assay
(Ercegovich & Rashid, 1977). Chlordane was shown to enhance the
number of ouabain-resistant mutants in Chinese hamster V79 cells
and was considered weakly mutagenic (Ahmed et al., 1977b).
Chlordane induced unscheduled DNA synthesis in SV-40 human
cells in culture without activation (Ahmed et al., 1977a). It was
established that chlordane-treated cells did not (for the most
part) re-enter mitosis. They were, instead, arrested somewhere
between the G1 and G2 phases of the cell cycle. Studies involving
DNA synthesis were undertaken to determine more precisely at which
phase (G1, S, G2) the cells are blocked. The data showed that the
treated cells were as competant in DNA replication as the control
cells. In both control and treated cultures, 25 - 30% of total DNA
persisted as light-density material indicating that some of the
pre-existing DNA never engaged in DNA synthesis. Either a large
fraction of cells failed to complete DNA synthesis or 25 - 30% of
the cells did not enter phase S. In any case, treated and control
cells behaved the same in terms of DNA synthesis, indicating that
treatment of the cells with chlordane blocked the cells at the G2
stage of the cell cycle (Brubaker et al., 1970).
Chlordane induced gene conversions in Saccharomyces cerevisiae
strain D4 (Chambers & Dutta, 1976).
Neither alpha-chlordane (42, 58, and 290 mg/kg body weight
single ip doses or 5 daily oral doses of 75 mg/kg body weight) nor
the gamma-isomer (5 daily oral doses of 50 mg/kg body weight) had a
significant effect in a dominant lethal assay on mice (Epstein et
al., 1972). Technical chlordane at dose levels of 50 or 100 mg/kg
body weight in a dominant lethal study using mice failed to induce
any dominant lethal changes (Arnold et al., 1977).
More recent studies on animal and human cells in culture have
shown that chlordane is not mutagenic or is only weakly mutagenic
(Williams, 1979; Maslansky & Williams, 1981; Tong et al., 1981).
Further work by Telang et al. (1982) showed that chlordane was not
mutagenic to an adult rat liver cell line but inhibited cell to
cell communication in a rat liver 6-thioguanine resistant/sensitive
cell line. Telang et al. proposed that chlordane was exhibiting
properties exerted by many promoting agents.
6.5. Carcinogenicity
Epstein (1976) reported a previously unpublished study by the
International Research and Development Corporation, carried out in
1973, in which groups of 100 male and 100 female Charles River CD-1
mice, 6 weeks of age, were fed technical-grade chlordane (purity
not given) at 5, 25, and 50 mg/kg diet, for 18 months. Excluding
10 animals sacrificed from each group for interim study at 6
months, mortality rates at 18 months ranged from 27 - 49%, except
in males and females receiving the 50 mg/kg diet, in which the
mortality rates were 86 and 75%, respectively. A relatively large
number of the deceased animals was lost due to autolysis. A dose-
related increased incidence of liver hyperplastic nodules was
reported in the 25 and 50 mg/kg diet test groups and a dose-related
increased incidence of liver cell hypertrophy was found in all test
groups. A significant incidence of hepatocellular carcinomas
compared with controls was also reported. In the males receiving
chlordane at 0, 5, 25, or 50 mg/kg diet, hepatocellular carcinomas
were found in 3/33, 5/55, 41/52, and 32/39 animals, respectively;
in females, the respective incidences were 0/45, 0/61, 32/50, and
26/37.
Groups of 50 male and 50 female B6C3F1 hybrid mice, 5 weeks of
age, were fed analytical-grade chlordane, consisting of 94.8%
chlordane (71.7% alpha-chlordane and 23.1% gamma-chlordane), 0.3%
heptachlor, 0.6% nonachlor, 1.1% hexachlorocyclopentadiene, 0.25%
chlordene isomers, and other chlorinated compounds for 80 weeks
(NCI, 1977). Males received initial levels of 20 or 40 mg/kg diet,
and females 40 and 80 mg/kg diet; time-weighted average dietary
concentrations were 30 and 56 mg/kg for males and 30 and 64 mg/kg
diet for females. There were 20 male and 10 female matched
controls and 100 male and 80 female pooled controls. Survival in
all groups was relatively high, i.e., over 60% in treated males,
over 80% in treated females, and over 90% in male and female
controls. A dose-related increase in the incidence of
hepatocellular carcinomas was found in males and females. The
incidences were 43/49 and 34/49 in high-dose males and females,
respectively, and 16/48 and 3/47 in low-dose males and females,
respectively, compared with 2/18 and 0/19 in male and female
matched controls, respectively.
Groups of 50 male and 50 female, 5-week-old Osborne-Mendel rats
were given analytical-grade chlordane in the diet for 80 weeks, at
initial levels of 400 and 800 mg/kg for males and 200 and 400 mg/kg
for females (NCI, 1977). The levels had to be reduced during the
study because of adverse toxic effects; the time-weighted average
dietary concentrations were 407 and 203 mg/kg for males and 241 and
121 mg/kg for females. There were 10 male and 10 female matched
controls and 60 male and 60 female pooled controls. Survivors were
killed at 80 weeks, at which time approximately 50% of treated and
control males and 60% of treated females and 90% of control females
were still alive. In all treated animals combined, there was an
excess incidence of follicular-cell thyroid neoplasms (10/75 in
treated females and 7/65 in treated males versus 0/10, 3/58, 0/6,
and 4/51 in matched and pooled female and male controls); there was
an excess of malignant fibrous histiocytomas in the treated male
groups (8/88 versus 0/8 and 2/58 in matched and pooled male
controls).
A committee of the National Academy of Sciences (NAS) in the
USA was asked to review all available carcinogenicity data on
chlordane as part of the cancellation hearings. Chlordane was not
found to be carcinogenic in rats and the only target organ site for
carcinogenic response in certain strains of mice was the liver.
The committee concluded that "there are no adequate data to show
that these compounds are carcinogenic in humans, but because of
their carcinogenicity in certain mouse strains and the extensive
similarity of the carcinogenic action of chemicals in animals and
in humans, the committee concluded that chlordane, heptachlor
and/or their metabolites may be carcinogenic in humans. Although
the magnitude of risk is greater than if no carcinogenicity had
been found in certain mouse strains, in the opinion of the
committee the magnitude of risk cannot be reliably estimated
because of the uncertainties in the available data and in the
extrapolation of carcinogenicity data from laboratory animals to
humans" (US NAS, 1977).
IARC (1979), in its evaluation of the carcinogenic risk of
chlordane, concluded: "There is sufficient evidence that chlordane
is carcinogenic in mice." In 1982, another IARC Working Group
reviewed existing data on chlordane and concluded that there was
limited evidence for the carcinogenicity of chlordane for
experimental animals (IARC, 1982). The group of Williams (Telang
et al., 1982) suggested that chlordane had the properties of many
promoting agents.
6.6. Behavioural Studies
Offspring of chlordane-treated mice (1 or 2.5 mg/kg body weight
for 7 consecutive days) made fewer conditioned avoidance responses
than controls (Al-Hachim & Al-Baker, 1973). In addition, progeny
of mothers receiving the higher dose were more active than the
controls.
6.7. Other Studies
Chlordane induces hepatic mixed-function oxidase enzymes in
rats (Fouts, 1963; Hart et al., 1963; Hart & Fouts, 1965;
Villeneuve et al., 1972; den Tonkelaar et al., 1974; Madhukar &
Matsumura, 1979) and enhances estrone metabolism in rats and mice
(Welch et al., 1971). Chlordane has been shown to inhibit skin 7-
ethoxycoumarin de-ethylase activity (7-EC) (EC 1.14.13) in mice at
doses which induced hepatic 7-EC activity (Pohl & Fouts, 1977).
Several studies were carried out in which rats were fed chlordane
at levels of 2, 5, 10, 20, or 50 mg/kg diet during two weeks (den
Tonkelaar et al., 1974). At the end of this period, the liver
microsomal enzymes hexobarbital oxidase (EC 1.1), aminopyrine
demethylase (EC 1.5.3), and aniline hydroxylase (EC 1.14.14) were
determined. A no-observed-adverse-effect level of 5 mg/kg was
found for chlordane. Chlordane inhibition of rat brain ATPase
activity (Folmar, 1978) and bovine carbonic anhydrase (EC 4.2.1.1)
(Maguire & Watkin, 1975) has been demonstrated in in vitro
systems. The in vivo inhibition of rat brain ATPase has also
been reported (Drummond et al., 1980).
Three female and 3 male baboons were fed atherogenic diets to
which chlordane was added at 0.1 or 1 mg/kg body weight per day for
two years. At the higher dose level, chlordane increased
cytochrome P-450 activity significantly, otherwise no adverse
effects on general health or on any major organ systems were found
(McGill, 1979).
Acute intramuscular doses (50 mg/kg body weight) of chlordane
have been shown to increase the alkaline phosphatase (EC 3.1.3.1)
activity in gerbils temporarily (Karel, 1976) and stimulate
gluconeogenetic enzymes in the liver and kidney cortex of rats
(Kacew & Singhal, 1973a). Oral administration of 200 mg alpha-
chlordane/kg body weight to mature Wistar rats also induced
elevated serum levels of glucose and urea with a concomitant
decrease in liver glycogen at sacrifice, 1 h later (Kacew &
Singhal, 1973b). The chlordane-induced alterations have been
attributed to the enhanced ability of these organs to synthesize
cyclic AMP (Kacew & Singhal, 1973b, 1974; Singhal & Kacew, 1973,
1976; Kraybill, 1977).
Rats were administered chlordane ip daily for 42 days at levels
of 0.15, 1.75, or 25.0 mg/kg body weight. Results revealed "dose-
dependent alterations of brain potentials without behavioural signs
of chronic toxicity" (Hyde & Falkenberg, 1976, Hyde et al., 1978).
Chlordane has also been shown to influence brain biogenic amines
including acetylcholine (Hrdina et al., 1973).
Prenatal exposure to 0.16 mg chlordane/kg body weight, in
peanut butter, on each day of gestation, resulted in increased
plasma corticosterone levels in adult male mice (Cranmer et al.,
1978). When neonatal mice were treated with 0.075 mg alpha- or
gamma-chlordane on days 2 - 4 after birth, growth rates were
depressed and eye and vaginal opening were delayed (Talamantes &
Jang, 1977).
Female Balb C mice were mated and treated with chlordane at
0.16 or 8 mg/kg body weight throughout gestation. Decreased cell-
mediated immune competence was found in offspring of high-dose-
treated females, at 101 days of age, challenged with oxazolone
(Spyker-Cranmer et al., 1982).
Chlordane added to the medium at a concentration of
1 mg/kg inhibited the growth of Streptococcus viridans in
vitro by more than 50%. Total growth inhibition occurred at a
chlordane concentration of 3 mg/kg (Goes et al., 1978).
6.8. Factors Influencing Toxicity
Metabolites
The acute oral toxicity of chlordane isomers and their
metabolites is summarized in Table 3.
Table 3. Toxicity of chlordane isomers and metabolites
-----------------------------------------------------------------------
Compound Species LD50 (mg/kg Reference
(sex) body weight)
-----------------------------------------------------------------------
alpha-chlordane rat (M) 392 Wazeter et al. (1968)
gamma-chlordane rat (M) 327 Wazeter et al. (1968)
alpha + gamma- rat (M) 371 Wazeter et al. (1968)
chlordane (1:1 ratio)
oxychlordane rat (M,F) 19.1 Mastri et al. (1969a)
chlordene rat (M,F) over 4600 Mastri et al. (1969b)
3-chlorchlordene rat (M,F) over 4600 Mastri et al. (1969b)
1-hydroxychlordene rat (M,F) over 4600 Mastri et al. (1969b)
chlordene epoxide rat (M,F) over 4600 Mastri et al. (1969c)
1-hydroxy, 2,3-epoxy rat (F) over 4600 Mastri et al. (1969c)
chlordene
2-chlorchlordene rat (F) over 10 200 Mastri et al. (1969c)
-----------------------------------------------------------------------
Only oxychlordane was more toxic than the parent compounds.
Two male and 2 female rats were given the chlordane metabolite
oxychlordane in their diet, at a level of 2.0 mg/kg body weight
(Plank et al., 1970). Following 90 days feeding, the surviving
animals were sacrificed. Body-weight gain, food consumption,
behaviour, mortality rate, organ weights and organ to body weight
ratios, and the results of haematological and blood chemistry tests
and urological studies were considered to be within the normal
range for the strain of rat used. No gross pathological
abnormalities were evident, and no histopathological lesions could
be attributed to oxychlordane.
Groups of 25 male and 25 female rats were fed dietary levels of
1-hydroxychlordene of 0, 100, 250, 500, 1000, and 2000 mg/kg for up
to 224 days (Ingle, 1965a). After 110 days, 3 females from each
feeding level were mated with males at all levels. The mortality
rate in all groups was low, and no statistically significant
differences existed. No gross abnormalities were revealed at
autopsy after 224 days. The histopathological study of the
visceral organs did not show any pathological effects. Slight to
moderate hyperplasia of the smooth endoplasmatic reticulum and
cytoplasmic margination of a few liver cells were noted at the 1000
and 2000 mg/kg levels.
Interactions
Chlordane has been shown to exert a protective effect against
several organophosphorous and carbamate insecticides (Williams et
al., 1967; Street et al., 1969; Williams & Casterline, 1970; US
EPA, 1976a,b).
Protein deficiency has been shown to double the acute toxicity
of chlordane in rats (Boyd, 1972).
Chlordane has also been shown to increase the hepatotoxic
effects of carbon tetrachloride in the rat (Stenger et al., 1975;
Mahon, 1977; Mahon & Oloffs, 1979; Mahon et al., 1979).
7. EFFECTS ON MAN: EPIDEMIOLOGICAL AND CLINICAL STUDIES
7.1. Poisoning Incidents
A 15-month-old girl ingested a mouthful of chlordane suspension
and, after 3 h, displayed tremors and incoordination (Lensky &
Evans, 1952). Repeated seizures developed and she was treated with
ethyl chloride, amobarbital, and gastric lavage with magnesium
sulfate. The child recovered completely and ataxia and
excitability disappeared after 2 - 3 weeks. At 26 years of age,
she was in excellent health and appeared not to suffer any
consequences from the childhood episode (Taylor et al., 1979).
A 2-year-old child had drunk an unknown amount of a 74%
formulation of chlordane (Curley & Garrettson, 1969). Vomiting
preceeded convulsions, which were controlled by phenobarbital; the
EEG pattern was normal within 40 h and the child recovered.
A similar poisoning incident was observed with a 4-year-old
child (Aldrich & Holmes, 1969). Convulsions were treated with
phenobarbital. As with the previous case, the individual
recovered.
Two other cases of chlordane poisoning were reported in 1955
(Derbes et al., 1955). One was caused by the absorption of
accidentally-spilled chlordane and the other was a suicide attempt
where the individual (female) swallowed 6 g of chlordane (104 mg/kg
body weight) and died 9 1/2 days after the incident (Derbes et al.,
1955).
When a section of a municipal water system in Chattanooga,
Tennessee, USA was contaminated with chlordane in concentrations of
up to 1.2 g/litre in 1976, 13 persons showed gastrointestinal
and/or neurological symptoms (Harrington et al., 1978).
7.2. Occupational and Epidemiological Studies
No deleterious effects associated with occupational exposure to
chlordane have been reported. Twenty-two men, who had been
occupationally exposed to chlordane during its manufacture for
periods of 1 - 3 years, did not show any evidence of intoxication
(Princi & Spurbeck, 1951). Other clinical studies have been
reported on men engaged in the manufacture of chlordane (Alvarez &
Hyman, 1953; Fishbein et al., 1964; Morgan & Roan, 1969).
Infante et al. (1978) reviewed 25 previously reported cases of
blood dyscrasia together with a small number of newly identified
cases of aplastic anaemia, leukaemia, or neuroblastoma in children
in relation to their possible association with pre- and post-natal
chlordane or heptachlor exposure and reported an anecdotal
relationship. However, in a case-control study, no association was
found between blood dyscrasias and occupational exposure to a
number of pesticides including chlordane (Wang & Gruffenman, 1981).
Wang & MacMahon (1979 a,b) studied a cohort of workers engaged
in the manufacture of chlordane, heptachlor, and endrin and another
cohort of 16 000 pesticide-spraying personnel, including termite-
control workers. Both studies showed a deficit of deaths from all
cancers and slight excesses of lung, skin, or bladder cancer that
were not statistically significant.
In 1982, an IARC Working Group concluded that the above studies
were inadequate to evaluate the carcinogenicity of chlordane for
human beings (IARC, 1982).
Shindell & Associates (1981) studied the mortality experience
of 783 workers engaged in the manufacture of chlordane and
heptachlor. Workers had been employed for a minimum of 3 months 5,
10, 15, or 20 years ago. SMRs for cancer were not increased among
124 deaths.
In a retrospective cohort study of workers involved in the
production of chlorinated hydrocarbon pesticides, Ditraglia et al.
(1981) studied the workers in a chlordane-manufacturing plant; the
same workers were also studied by Wang & MacMahon (1979a). SMRs
for all cancer deaths were lower than expected; a slight excess of
stomach cancer (3 vs 0.99 expected), which was observed, was not
statistically significant. The number of workers studied was small
and further follow-up of the cohort was recommended by the authors.
MacMahon & Wang (1982) carried out a second follow-up study of
mortality rates in a cohort of workers employed in spraying
pesticides, including termite-control workers. Among 540 deaths
for which the cause was ascertainable, small excesses of bladder
cancer in termite-control operators and of skin and lung cancer in
other operators were observed, but these were not statistically
significant.
7.3. Treatment of Poisoning
In case of overexposure, medical advice should be sought
immediately.
Treatment before person is seen by a physician
The person should stop work immediately, remove contaminated
clothing and wash the affected skin with soap and water, if
available, flushing the area with large quantities of water. If
swallowed, vomiting should be induced, if the person is conscious
(FAO/WHO, 1978).
Medical Treatment
If the pesticide has been ingested, gastric lavage should be
Performed with 2 - 4 litres of water, followed by saline
purgatives. Barbiturates (preferably phenobarbitone or
pentobarbitone) or diazepam should be given intramuscularly or
intravenously in sufficient dosage to control restlessness or
convulsions. Mechanical respiratory assistance with oxygen may be
required. Calcium gluconate, 10% in 10 ml, injected
intramuscularly at 4-h intervals, may be helpful. Contraindicated
are oily purgatives, epinephrine, and other adrenergic drugs and
central stimulants of all types (FAO/WHO, 1978).
8. EFFECTS ON THE ENVIRONMENT
8.1. Toxicity for Aquatic Organisms
Data on the toxicity of chlordane for aquatic organisms are
given in Table 4. A more comprehensive table, listing different
conditions and exposure times is available on request from IRPTC,
Geneva, Switzerland. It is of importance for the interpretation of
these data to note that a change in the purity of technical of
chlordane occurred in the early 1950s.
Studies of the effects of chlordane on fish began with the
application of the original material to rainbow trout by Cope et
al. (1947). They determined minimum disabling 24-h doses of
chlordane in a xylene emulsion, an acetone solution, a fuel oil
solution, and a Velsicol AR-50 solution, and found this to be
higher than 6 mg chlordane/litre. An application of 1.12 kg/ha of
a field formulation of chlordane to a small pond killed 87% of
bluegills present (Surber, 1948), application of 0.56 kg/ha killed
some bluegills whilst at 0.28 kg/ha all fish survived (Linduska &
Surber, 1948). In a study by Lawrence (1950) on bluegill
fingerlings, large-mouth black bass fingerlings, and juvenile
goldfish in aquaria, no deaths occurred at 100 µg chlordane/litre
(original formulation), whilst at 200 µg/litre, a 30-h exposure
killed bass, and an 87-h exposure killed bluegills; goldfish were
not affected. In earthen ponds, large-mouth black bass fingerlings
were killed by a concentration of 200 µg/litre, but bluegills and
fingerlings and juvenile goldfish survived.
Studies using the current formulation of chlordane are
summarised in Table 4. A definite temperature effect demonstrated
by Macek et al. (1969) during acute exposures, i.e., fish showed a
greater susceptibility at higher temperatures, was not present in
96-h exposure studies. Temperature effects were also noted in
toxicity tests on tubificid worms, Branchuria sowerbyi (Naqvi,
1973). In static tests, 500 µg chlordane/litre caused 100%
mortality at 4.4° and 32 °C, but no mortality at 21 °C. Nutrition
has been shown to affect chlordane toxicity in rainbow trout, with
96-h LC50s ranging from 8.2 to 47 µg/litre, depending on the
composition of the diet given to the fish (Merhle et al., 1974).
Recent in vitro studies on bluegills (Koch et al., 1971)
and on rainbow trout (Davis et al., 1972) have shown that chlordane
acts as an inhibitor of ATPase systems.
8.2. Toxicity for Terrestrial Organisms
Studies of the effects of chlordane on soil microfauna have
been limited to work on nematodes. Populations of plant-feeding
nematodes were reduced by an insecticide mixture containing
chlordane (as well as DDT, diazinon, and zinophos) in July,
following application in March, but at no other time during the
study period (Corbett & Webb, 1968). Nematodes are generally
unaffected by most soil insecticides (Edwards, 1965a).
Table 4. Toxicity of chlordane for aquatic organisms
---------------------------------------------------------------------------------------------------------
Organism Age/ Temp pH Flow/ Grade Hard- alk sal End Para- Concen- Reference
size (° C) stat ness (mg/ o/oo point meter tration
(mg/ litre) (µg/
litre) litre)
---------------------------------------------------------------------------------------------------------
Eastern oyster 29-53 31.6 flow tech 27.3 % reduc- 96-h 6.2 Parrish et
(Crassostrea mm tion shell EC50 al. (1976)
virginica) deposition
Annelid stat sea 288-h 220 McLeese et
(Nereis virens) water LC50 al. (1982)
Scud 5 mm 16.7 7.9 flow tech 148 152 immobili- 168-h 97.1 Cardwell et
(Hyalella juv. sation EC50 al. (1977)
azteca)
Cladoceran 1st 15.5 7.4- stat immobili- 48-h 29 Sanders &
( Daphnia pulex) instar 7.8 sation EC50 Cope (1966)
Pink shrimp 50-65 28.4 flow tech 21.8 96-h 0.4 Parrish et
(Penaeus mm LC50 al. (1976)
duorarum)
Dungeness crab adult 13 stat tech 25 96-h 220 Caldwell
(Cancer LC50 (1977)
magister) zoeal 13 stat tech 25 immobili- 96-h 1.3
sation EC50
Backswimmer 5-6 mm 18-24 stat 25% EM 168-h 0.79 Konar (1968)
(Notonecta) sp LC50
Water scorpion 24-28 18-24 stat 25% EM 168-h 182 Konar (1968)
(Nepa) sp mm LC50
Bluegill 38-84 25 7.1 flow 100% 20 18 96-h 22 Henderson et
(Lepomis mm LC50 al. (1959)
macrochirus)
---------------------------------------------------------------------------------------------------------
Table 4. (contd.)
---------------------------------------------------------------------------------------------------------
Organism Age/ Temp pH Flow/ Grade Hard- alk sal End Para- Concen- Reference
size (° C) stat ness (mg/ o/oo point meter tration
(mg/ litre) (µg/
litre) litre)
---------------------------------------------------------------------------------------------------------
Fathead minnow 1 day 21/25 7.7 flow tech 156 166 major 11-mo Cardwell et.
(Pimephales chronic lowest al. (1977)
promelas) effects dose
life
cycle
tests
38-84 25 7.1 flow 100% 20 18 96-h 52 Henderson et
mm LC50 al. (1959)
38-84 25 7.1 flow 75% 20 18 96-h 170 Henderson et
mm EM LC50 al. (1959)
Rainbow trout 0.9 g 13 tech 96-h 7.8 Cope (1965)
(Salmo gairdneri) LC50
Murrel 24-26 18-24 stat 25% EM 168-h 0.51 Konar (1968)
(Channa mm LC50
punctatus)
48-50 18-24 stat 25% EM 168-h 3 Konar (1968)
mm LC50
100- 18-24 stat 25% EM 168-h 25.5 Konar (1968)
105 mm LC50
Tropical fish juv. 18-24 stat 25% EM 168-h 0.7- Konar (1968)
LC50 3.7
Channel catfish finger 25 stat tech 96-h 500 Clemens &
(Ictalurus ling LC50 Sneed (1959)
punctatus)
Common toad tad- 18-20 stat 48-h 2000 Ludemann &
(Bufo bufo) pole LC50 Neumann
(1962)
---------------------------------------------------------------------------------------------------------
Soil fauna populations (mainly arthropods with a small
percentage of earthworms and nematodes) were reduced to very low
levels by the normal application rate (112 kg/ha per year) of a
commercial formulation of chlordane (Gould & Hampstead, 1951).
However, Long et al. (1967) did not find any significant reductions
in most soil arthropod populations following application of
technical chlordane to sugar cane at 2.24 kg/ha, although numbers
of Diplura and Pauropoda were reduced. In a summary of the effects
of pesticides on soil invertebrates, Edwards (1965b) stated that
chlordane caused a large reduction in the numbers of Coleoptera,
Diptera, hemiedaphic Collembola, and non-predatory mites, but
little reduction in the numbers of edaphic Collembola or predatory
mites at application rates of 1.12 - 2.24 kg/ha. He also stated
that chlordane was lethal for fly and beetle larvae. Fox (1958)
produced a few data indicating that carabid and staphylinid beetle
populations returned to normal 3 years after a field application
of chlordane (as a 40% wettable powder) at 8.96 or 11.2 kg/ha.
Chlordane is toxic both to earthworms and to enchytraeid worms
at application rates well within the range of normal usage (Hopkins
& Kirk, 1957; Doane, 1962). The toxicity of chlordane for earth-
worms is given in Table 5. Legg (1968) made a single application
of 25% EC chlordane at 9.0, 13.4, or 20.2 kg/ha on closely-mown
turf and counted worm casts for up to 13 months. After 19 days,
there were reductions of 52, 72, and 98%, respectively, for the 3
doses compared with control plots; after 13 months, the reductions
were 89, 95, and 97%, respectively. Long et al. (1967) reported a
significant reduction in earthworm populations, 6 - 11 months after
an application of chlordane at 2.2 kg/ha. A reduction in worm
casts to zero, 1 year after an application of chlordane at 11.2
kg/ha, either as granules or in spray formulation was shown by
Doane (1962). Lidgate (1966) applied chlordane, either as 20%
granules or 75% EC diluted with water, at rates between 13.4 and
35.2 kg/ha, to a putting green and measured worm activity by the
number of worm casts. The first count of casts, 18 days after
treatment, showed that worm activity was significantly depressed by
granular applications of 17.6 and 35.2 kg/ha but not by spray
formulations of 13.4 or 26.4 kg/ha. Activity became depressed on
sprayed plots about 8 weeks after treatment. There were still
significantly fewer worm casts on treated plots, 5 years later.
Table 5. Toxicity of chlordane for earthworms
-----------------------------------------------------------------------------------------
Organism Grade Application Concen- Effect Reference
method tration
(kg/ha)
-----------------------------------------------------------------------------------------
Earthworm 25% EC spray 9 52% reduction in worm Legg (1968)
(Lumbricus casts at 19 days
terrestris)
25% EC spray 13.4 72% reduction in worm Legg (1968)
casts at 19 days
25% EC spray 20.2 98% reduction in worm Legg (1968)
casts at 19 days
25% EC spray 9 89% reduction in worm Legg (1968)
casts at 1 year
25% EC spray 13.4 95% reduction in worm Legg (1968)
casts at 1 year
25% EC spray 20.2 97% reduction in worm Legg (1968)
casts at 1 year
Red earth- 5% dust soil 35 0% mortality in Hopkins & Kirk (1957)
worm incorpor- 4 days
(Eisenia ation
sp)
5% dust soil 70 46% mortality in Hopkins & Kirk (1957)
incorpor- 4 days
ation
5% dust soil 141 40% mortality in Hopkins & Kirk (1957)
incorpor- 4 days
ation
5% dust soil 282 79% mortality in Hopkins & Kirk (1957)
incorpor- 4 days
ation
5% dust soil 100 96-h LC50 Hopkins & Kirk (1957)
incorpor-
ation
-----------------------------------------------------------------------------------------
The toxicity of chlordane for birds, when given in the diet, is
summarised in Table 6. LC50 values as mg/kg diet ranged from 170
to 858 in studies where chlordane was given for between 5 days and
100 weeks. When chlordane was applied to marshland at 1.12 kg/ha,
the fecundity of marsh birds was affected (Hanson, 1952); blue-
winged teal and shovelers did not produce any young, and coot and
redwinged-blackbirds produced 60% fewer young. It was postulated
that chlordane had caused disruption in food cycles in the marsh
and that this was the probable cause of reproductive failure in the
birds.
8.3. Toxicity for Microorganisms
Some effects of chlordane on microorganisms are summarised in
Table 7. Its effects may be due, at least in part, to inhibition
of enzyme activity (Nakas, 1977). Some work has been reported on
the effects of chlordane on soil microorganisms. Gram-positive
bacteria appear to be more sensitive to chlordane than gram-
negative bacteria, since the growth of gram-positives was inhibited
whilst that of gram-negatives was unaffected (Trudgill et al.,
1971). When Bacillus subtilis cultures were treated with technical
chlordane at 20 mg/litre, they ceased to grow. Viable count and
respiration rate fell to zero after about 3 h of exposure. The
actual concentration experienced by the bacteria is not known, but
it is likely to be less than the 20 mg/litre added because of the
poor solubility of chlordane in aqueous solution. Langlois & Sides
(1972) investigated the effects of constituents of technical
chlordane on the growth of Staphylococcus aureus. The viability
of the culture, the length of the lag-phase, and the generation
time were affected by the amount of chlordane and gamma-chlordane
applied.
8.4. Bioaccumulation and Biomagnification
Grimes & Morrison (1975) examined the uptake of chlordane by 13
types of bacteria and found that although the uptake of alpha- and
beta-isomers of chlordane was the same for any one species, the
concentration factors (CF) differed greatly between species. The
CFs ranged from a few hundred to several thousand, with 3 species
giving much higher values. The highest CF was 53 000 for
Caulobacter vibrioides. Caulobacter cells were found to contain 4
distinct lipid-containing materials, and this was offered as an
explanation of the high CF. Sanborn et al. (1976) used unlabelled
chlordane and labelled 14C-chlordane on filamentous Oedogonium
alga and obtained CFs of 49 500 and 98 386. The lower figure may
be due to uncertainties in determining chlordane in solution and in
the alga. Moore et al. (1977), using the planktonic alga
Ankistrodesmus amalloides, obtained a very much lower CF of 5560,
but even this species shows accumulation potential.
Table 6. Toxicity of chlordane for birds
--------------------------------------------------------------------------------
Species Age Route Parameter Concen- Reference
trationa
(mg/kg)
--------------------------------------------------------------------------------
Mallard 4 - 5 mo oral acute LD50 1200 Tucker & Crabtree (1970)
10 days diet 5-day LC50b 858 Hill et al. (1975)
Bobwhite 14 weeks diet 10-week LC0 10 Ludke (1976)
quail 17 days diet 5-day LC50b 331 Hill et al. (1975)
young diet 100-day LC50 100 DeWitt et al. (1963)
young diet 10-day LC50 250 DeWitt et al. (1963)
adult diet 100-day LC50 250 DeWitt et al. (1963)
Japanese 7 days diet 5-day LC50b 350 Hill et al. (1975)
quail
Ring-necked 15 days diet 5-day LC50b 430 Hill et al. (1975)
pheasant young diet 10-day LC50 500 DeWitt et al. (1963)
young diet 100-day LC50 50 DeWitt et al. (1963)
adult diet 100-day LC50 200 DeWitt et al. (1963)
Cowbird adult diet 30-week LC50 500 DeWitt et al. (1963)
--------------------------------------------------------------------------------
a Concentration in mg/kg body weight for oral dosing; concentration in mg/kg
diet for dietary dosing.
b 5 days of treated diet followed by 3 days of clean diet. Mortality rate
determined on day 8.
Table 7. Toxicity of chlordane for microorganisms
----------------------------------------------------------------------------------------
Organism F/M/ Temp Grade Solvent Endpoint Time Concen- Reference
S (° C) tration
(µg/
litre)a
----------------------------------------------------------------------------------------
Scenedesmus F 23 tech acetone stimulation of 5 - 7 0.1- Glooschenko &
quadricauda cell division days 100 Lott (1977)
F 23 tech acetone inhibition of 1 - 5 50 & Glooschenko &
photosynthesis days 100 Lott (1977)
Chlamydomonas S 23 tech acetone stimulation of 7 - 11 0.1- Glooschenko &
sp cell division days 50 Lott (1977)
S 23 tech acetone stimulation of 3 - 4 0.1- Glooschenko &
respiration h 100 Lott (1977)
S 23 tech acetone inhibition of 7 - 11 100 Glooschenko &
cell division days Lott (1977)
F 23- 60% acetone 50% reduction 3 days 100 000 Clegg &
25 ATPase Koevenig
activity; (1974)
no effect on
cell density
Volvox sp F 18- 20% none 100% mortality 7 days 1 Konar (1968)
Pandorina sp 24 EM
Closterium sp
Chlorella F 23- 60% acetone reduced ATPase 3 days 100 000 Clegg &
ellipsoidea 25 levels; Koevenig
Euglena no effect on (1974)
elastica cell density
Exuviella M 60% methanol virtual cessa- 7 days 50 Magnani et
baltica tion of growth al. (1978)
Natural M 60% acetone 94% decrease 4 h 1000 Butler (1963)
estuarine in product-
phytoplankton ivity
Estuarine M 7-14 60% methanol no effect 5 days 5 Biggs et al.
phytoplankton M 7-14 60% methanol growth and 14C 5 days 10 (1978)
uptake reduced
in laboratory
Bacillus S tech acetone growth ceased; 3 h 20 000 Trudgill et
subtilis decline in al. (1971)
viable count;
respiration
to zero
----------------------------------------------------------------------------------------
a Solubility of chlordane: 6 - 9 µg/litre. F = freshwater; M = marine; S = soil.
Accumulation of alpha- and and gamma-isomers of chlordane and
nonachlor, and chlordenes was studied by Cardwell et al. (1977) in
3 species of freshwater invertebrates. Chironomus larvae did not
show any detectable accumulation. After one week's exposure to 1.7 -
21.6 µg/litre, Daphnia magna gave CFs ranging from 15 000 to
175 000. After 65 days of exposure to 1.4 - 11.5 µ/litre, Hyallela
azteca showed CFs ranging from 41 000 to 144 000. After a 24-h
exposure to 0.5 µg alpha- and gamma-chlordane/litre, Daphnia pulex
gave a CF of 24 000 (Moore et al., 1977). Sanborn et al. (1976)
found a CF of 6132 for insect larvae. A fresh-water gastropod
Physa sp., concentrated chlordane 132 613 times (Sanborn et al.,
1976). Little bioaccumulation has been observed in the marine
invertebrate species studied. Wilson (1965) exposed oysters to
0.01 mg chlordane/litre and determined a CF of 7300, which is
considerably lower than that for fresh-water gastropods. Water and
oyster samples from Galveston Bay, Texas were analysed following an
extensive mosquito-control programme (Casper, 1967). Oysters
sampled did not contain any detectable chlordane and only 2 out of
9 water samples gave a positive result of less than 0.001 µg/litre.
Chlordane was detected by Bugg et al. (1967) in 20 out of 133
oyster samples taken from the South Atlantic and the Gulf of
Mexico, but 19 of these gave values of less than 0.01 mg/kg drained
weight. Clams, which were living in water containing chlordane at
0.01 µg/litre for 106 days, showed CFs of 1000 or less (Godsil &
Johnson, 1968). Parrish et al. (1976) reported that chlordane was
concentrated in the tissues of the estuarine pink and grass shrimps
at 1000 - 2300 times levels measured in water.
Few data are available on soil invertebrates. One report on
earthworms has been published by Gish (1970), who measured the
levels of gamma-chlordane in the soil and earthworms. CFs of 0.37,
7.1, 10.6, and 152 were obtained for 4 worms in agricultural soils.
Several studies are available on fresh-water fish. Henderson et
al. (1969) found that fish from Atlantic-coast streams, which gave
positive samples, contained between 0.1 and 7.29 mg chlordane/kg
(whole fish wet weight), fish from the Great Lake drainage areas
contained between 0.01 and 0.39 mg/kg, and fish from the
Mississippi River system contained between 0.01 and 0.72 mg/kg.
Fish from other systems (Hudson Bay, Colorado River, Interior
basins, Californian streams, Columbia River, Pacific coast and
Alaskan streams) contained less than 0.01 mg/kg. A further study
by Henderson et al. (1971) yielded 16 positive samples out of 666
fish taken from 50 sites, giving chlordane levels of between 0.09
and 13.5 mg/kg (whole fish wet weight). Working on chlordane
accumulation in sucker-fish, Roberts et al. (1977) showed that
accumulation from food was directly proportional to the lipid
levels of the fish. Chlordane was given to the northern redhorse
sucker, Moxostoma macrolepidotum, in the feed at 45 g/kg dry
feed for 5 consecutive days and to the white sucker, Catostomus
commersoni, directly to the stomach in a single dose of 340 mg
in corn oil. Both tests gave CF values of less than, or equal, to
0.52. CF values obtained in fish by uptake from the food were
lower than those obtained by uptake from water (goldfish at a CF of
162 with chlordane in diet (Moore et al., 1977); mosquito fish at a
CF of 8258 with chlordane in diet and in water (Sanborn et al.,
1976)). This suggests that chlordane is taken up directly from
water (bioaccumulated) more than it is from ingested food
(biomagnified).
Schimmel et al. (1976a,b) reported that CFs for two species of
marine fish were similar to those found in fresh-water species.
Spot and sheepshead minnow concentrated gamma-chlordane 3700 - 14
800 and 9000 - 16 800 times, respectively, in 4 days, and 3300 -
5100 and 10 300 times, respectively, in 24 days. Veith et al.
(1979) exposed fathead minnows to 5.9 µg chlordane/litre for 32
days and obtained a CF of 37 800 for the whole body. Parrish et
al. (1978) similarly reported 16 000 for whole body in the
sheepshead minnow after exposure for 189 days. They also
determined the CF after only 28 days exposure and found a
comparable whole-body value of 15 300. A range of CFs between 9000
and 16 786 was reported by Schimmel et al. (1976a) when sheepshead
minnows were exposed to gamma-chlordane (in technical heptachlor)
at 1.1 - 2.8 µg/litre for 96 h. In a field study, long-term
exposure (209 days or less) of large-mouth bass to between 0.01 and
0.1 µg/litre chlordane gave concentration factors of between 157
and 3308 (Godsil & Johnson, 1968).
Food chain magnification is unlikely in terrestrial organisms.
Species of birds and mammals studied show little bioaccumulation,
probably because chlordane is rapidly broken down in homoiotherms.
There are very few data on birds. One report (Foster et al., 1972)
refers to a study on the accumulation of chlordane in laying hens
fed 0.1 mg/kg diet. CF values were maximal after 7 - 9 weeks, diet
to fat was between 0.01 and 3.3, and diet to eggs was between 0.01
and 2. After 3 weeks on an untreated diet, chlordane was not
detectable. McCaskey et al. (1968) dosed hens with the equivalent
of a diet containing 10 - 15 mg 60% technical chlordane/kg for 5
days and obtained a maximum CF value in eggs of 0.38 on day 6.
8.5. Population and Community Effects
The fate of 14C-chlordane was investigated in a terrestrial-
aquatic model ecosystem composed of an alga (Oedogonium), snails
(Physa), mosquito larvae (Culex), and fish (Gambusia)
(Sanborn et al., 1976). Accumulation of chlordane residues was
evident in all organisms in the ecosystem, but no toxic effects
were reported.
Exposure of a natural phytoplankton population to 5 and 10 µg
chlordane/litre for 5 days had virtually no effect on its species
composition (Biggs et al., 1978).
8.6. Effects on the Abiotic Environment
No data are available on abiotic effects.
8.7. Appraisal
Data on aquatic toxicity require interpretation. Although the
solubility of chlordane in water has been measured at between 6
and 9 µg/litre, in many studies on its aquatic toxicity, chlordane
has been applied at much higher nominal concentrations. Either the
chlordane has not been in solution or was added with a solvent.
Actual levels of chlordane in natural waters rarely exceed 250
ng/litre with most levels below 20 ng/litre. Test levels are
therefore unrealistically high. The data should thus be critically
examined unless the actual concentrations experienced by the test
organisms were measured.
Few long-term studies of the chronic effects of chlordane are
available. Data do not define threshold levels, although they
suggest levels at which effects might be expected. Data seldom
provide quantitative dose-response relationships.
Effects of chlordane on primary producers in the aquatic food
chain are largely unknown because studies have used unrealistically
high concentrations. Some data on lethal doses for aquatic
organisms are available, but data on sub-lethal effects on
reproduction or behaviour are not. There are data on the acute
toxicity of chlordane for fish at concentrations approaching the
water solubility, but few on long-term exposure at lower doses.
Most terrestrial studies have been on soil organisms. Effects
here might be due to the heptachlor in technical chlordane or to a
combination of heptachlor and chlordane. Only very high
application rates of chlordane affected arthropods. Field studies
do not indicate direct toxicity but a combination of toxicity and
avoidance of the compound.
Of major importance is the clear toxicity of chlordane for
earthworms with implications for soil fertility. Molluscs also
appear to be particularly sensitive to chlordane.
9. PREVIOUS EVALUATIONS OF CHLORDANE BY INTERNATIONAL BODIES
An IARC Working Group (IARC, 1982) concluded that the available
epidemiological studies on chlordane were inadequate for the
evaluation of the cancer risk for man and that there was limited
evidence of the carcinogenicity of chlordane for experimental
animals.
WHO has recommended a guideline value of 0.3 µg/litre for
chlordane (total isomers) in drinking-water (WHO, 1982).
The Joint Meeting on Pesticide Residues (JMPR) reviewed
residues and toxicity data on chlordane on several occasions in the
past (1965, 1967, 1970). In November 1972, it re-established
residue tolerences ranging from 0.02 - 0.5 mg/kg for the sum of
alpha- and gamma-isomers of chlordane and oxychlordane (FAO/WHO,
1973). The acceptable daily intake (ADI) for human beings of
0 - 0.001 mg/kg body weight was confirmed in December 1977
(FAO/WHO, 1978). This was based on no-observed-adverse-effect-
levels of:
- 5 mg/kg in the diet, equivalent to 0.25 mg/kg body
weight in the rat; and
- 3 mg/kg in the diet, equivalent to 0.075 mg/kg body
weight in the dog.
Both "no-observed-adverse-effect-levels" and the ADI were
reviewed by the 1982 JMPR (FAO/WHO, 1983). The ADI was given
"temporary" status pending the results of toxicology studies still
in progress.
WHO (1984), in its "Guidelines to the Use of the WHO
Recommended Classification of Pesticides by Hazard", classified
technical chlordane as moderately hazardous.
The WHO/FAO (1978) has issued practical advice in its "Data
Sheet on Pesticides" including one on Chlordane (No. 36) dealing
with labelling, safe-handling, transport, storage, disposal,
decontamination, training and medical supervision of workers, first
aid and medical treatment.
Regulatory standards established by national bodies in 12
different countries (Argentina, Brazil, Czechoslovakia, the Federal
Republic of Germany, India, Japan, Kenya, Mexico, Sweden, the
United Kingdom, the USA, and the USSR) and the EEC is available
from the IRPTC (International Register of Potentially Toxic
Chemicals) Legal file (IRPTC, 1983).
The CEC reviewed the data available on chlordane in 1981.
10. EVALUATION OF HEALTH RISKS FOR MAN AND EFFECTS ON THE
ENVIRONMENT
10.1. Chlordane Toxicity
Chlordane is readily absorbed in both animals and man via the
skin, via ingestion, and probably also by inhalation. Some
accumulation occurs in the body on repeated exposure - mainly in
adipose tissue. Elimination from the body is fairly slow. The
half-life in various species, including man, is of the order of a
few weeks.
The oral LD50 values of chlordane in the rat range from
200 - 590 mg/kg body weight. Thus, chlordane is moderately toxic
in acute exposures.
Acute poisoning in man and animals is characterized by
manifestations of central nervous system stimulation such as
disorientation, tremors, and convulsions. Death may follow
respiratory failure.
In experimental animals (rats and dogs), prolonged exposure
to levels in the diet exceeding 3 - 5 mg/kg resulted in the
induction of hepatic microsomal enzymes and, at a later stage,
liver hypertrophy with histological changes. At higher levels
(i.e., > 15 mg/kg body weight per day), chlordane is hepatotoxic.
For no-observed-adverse-effect levels see section 6.1.1.
At dosages above 30 mg/kg diet, chlordane interferes with
reproduction in rats and mice, but this was reversible after
exposure ceased. There are no indications for teratogenicity in
the rabbit at 15 mg/kg body weight per day.
Chlordane produces hepatocellular carcinomas in mice. It
is not generally active in short-term tests designed to measure
genetic activity. Chlordane can interfere with cell to cell
communication in vitro, a characteristic of many promoting agents.
10.2. Exposure to Chlordane
Food is the major source of exposure of the general population
to chlordane, but the use of chlordane on food crops has decreased
and residues in food from animal origin are low. Some chlordane
exposure can occur in buildings where chlordane has been used for
termite or other insect control.
No adverse health effects have been reported in workers engaged
in the manufacture of chlordane or in pest-control operations,
where exposure could be quite high. However, several cases of
accidental and suicidal poisoning in man have been reported
resulting in the symptoms described in section 7.1.
10.3. Evaluation of Overall Environmental Effects
Chlordane is used primarily to control soil pests. Technical
chlordane is a mixture of chlorinated hydrocarbons and contains
heptachlor, which might contribute significantly to the
insecticidal properties of the technical formulation.
About half of the chlordane applied to soil disappears in the
first season, presumably by volatalisation or by "run-off" into
surface waters. Remaining residues persist for several seasons.
If chlordane is applied annually for several successive seasons,
residues accumulate in the soil. Most chlordane persists in the
cultivated levels, since there is little leaching into subsoil.
The high rate of metabolism of chlordane in warm-blooded
animals means that there is little possibility of accumulation in
these animals or magnification in food chains at this level.
Concentration factors are generally modest in aquatic organisms;
this combined with its low solubility in water means that chlordane
presents a limited hazard for aquatic vertebrates. Long-term
effects are not sufficiently well-documented to say that there is
not a potential hazard for fish, but this seems unlikely from the
information available, as far as temperate areas are concerned.
The compound shows a higher toxicity at higher temperatures.
Significant mortality in tropical species of fish at concentrations
well within the solubility of the compound, suggest that chlordane
may be a greater aquatic hazard at lower latitudes.
The high toxicity of chlordane for earthworms may constitute
its greatest potential hazard. The long-term effects of reduced
numbers of earthworms in the soil cannot be readily assessed
because the ecology of the animal is still poorly understood.
10.4. Evaluation of Risks for Human Health and the Environment
Although there is no evidence that incriminates chlordane as a
human carcinogen, the suspicion principally arising from the mouse
carcinogenicity studies cannot be entirely put aside. Further
research is required to elucidate this problem. Nevertheless, in
the present state of knowledge, it is concluded that:
1. As long as occupational hygiene procedures are maintained
to keep exposure levels to a minimum, whether or not by the
imposition of maximum allowable concentrations, there is
little reason to believe that workers will be at risk from
their handling, or contacts, with chlordane.
2. For the general population, consumers should suffer no
adverse effects from chlordane as food residues, provided
that the intake is kept within the temporary ADI set by the
Joint FAO/WHO Meeting.
In certain regions of the world, the exposure of the
general population to chlordane may be augmented by its
use as a termiticide in buildings.
3. Apart from the possible long-term adverse effects on
aquatic organisms in tropical areas and the depleted soil
fertility that may arise, in time, from the suppression of
the earthworm population, chlordane seems to cause little
environmental concern in its normal use as a termiticide
and in other non-agricultural applications.
REFERENCES
AHMED, F.E., HART, R.W., & LEWIS, N.J. (1977a) Pesticide
induced DNA damage and its repair in cultured human cells.
Mutat. Res., 42: 161-174.
AHMED, F.E., LEWIS, N.J., & HART, R.W. (1977b) Pesticide
induced ouabain resistant mutants in Chinese hamster. Chem.
Biol. Interactions, 19: 369-374.
ALDRICH, F.D. & HOLMES, J.H. (1969) Acute chlordane
intoxication in a child. Case report with toxicological
data. Arch. environ. Health, 19: 129-132.
AL-HACHIM, G.M. & AL-BAKER, A. (1973) Effects of chlordane
on conditioned avoidance response, brain seizure threshold and
open-field performance of prenatally-treated mice. Br. J.
Pharmacol., 49: 311-315.
ALVAREZ, W.C. & HYMAN, S. (1953) Absence of toxic
manifestations in workers exposed to chlordane. Arch. ind.
Hyg. occup. Med., 7: 197-210.
AMBROSE, A.M., CHRISTENSEN, H.E., ROBBINS, D.J., & RATHER,
L.J. (1953) Toxicology and pharmacological studies on
chlordane. Arch. ind. Hyg. occup. Med., 1: 197-210.
ARNOLD, D.W., KENNEDY, G.L., KEPLINGER, M.L., CALANDRA, J.C.,
& CALO, C.J. (1977) Dominant lethal studies with technical
chlordane, HCS-3260, and heptachlor: Heptachlor epoxide.
J. Toxicol. environ. Health, 2: 547-555.
ATALLAH, Y.H., WHITACRE, D.M., & POLEN, P.B. (1977) Artifacts in
monitoring - related analysis of human adipose tissue for some
organochlorine pesticides. Chemosphere, 6: 17-20.
ATALLAH, Y.H., WHITACRE, D.M., & HOO, B.L. (1979)
Comparative volatility of liquid and granular formations of
chlordane and heptachlor from soil. Bull. environ. Contam.
Toxicol., 22: 570-574.
BACHMANN, K.A. & BURKMAN, A.M. (1974) Topical application of
a chlordane containing ectoparasiticide: Effect on plasma
half-life of warfarin in dogs. Pharmacologist, 16: 284.
BALBA, H.M. & SAHA, J.G. (1978) Studies on the distribution,
excretion, and metabolism of alpha- and gamma-isomers of 14C
chlordane in rabbits. J. environ. Sci. Health, B13: 211-233.
BARNETT, R.W. & DOROUGH, H.W. (1974) Metabolism of chlordane
in rats. J. agric. food Chem., 22: 612-619.
BARNETT, R.W., D'ERCOLE, A.J., CAIN, J.D., & ARTHUR, R.D.
(1979) Organochlorine pesticide residues in human milk
samples from women living in north-west and north-east
Mississippi, 1973-1975. Pestic. Monit. J., 13: 47-51.
BARTHEL, W.F., HAWTHORNE, J.C., FORD, J.H., BOLTON, G.C.,
McDOWELL, L.L., GRISSINGER, E.H., & PARSONS, D.A. (1969)
Pesticide residues in sediments of the Lower Mississippi River
and its tributaries. Pestic. Monit. J., 3: 8-34.
BATTE, E.G. & TURK, R.D. (1948) Toxicity of some synthetic
insecticides to dogs. J. econ. Entomol., 41: 102-103.
BEVENUE, A., OGATA, J.N., & HYLIN, J.W. (1972a) Organo-
chlorine pesticides in rain water, Oahu, Hawaii, 1971-1972.
Bull. environ. Contam. Toxicol., 8: 238-241.
BEVENUE, A., HYLIN, J.W., KAWANO, Y., & KELLEY, T.W. (1972b)
Organochlorine pesticide residues in water, sediment, algae,
and fish, Hawaii, 1970-71. Pestic. Monit. J., 6: 56-64.
BIGGS, D.C., ROWLAND, R.G., O'CONNORS, H.B., POWERS, C.D., &
WURSTER, C.F. (l978) A comparison of the effects of
chlordane and PCB on the growth, photosynthesis and cell size
of estuarine phytoplankton. Environ. Pollut., 15: 253-263.
BIROS, F.J. & ENOS, H.F. (1973) Oxychlordane residues in
human adipose tissue. Bull. environ. Contam. Toxicol., 10:
257-260.
BOYD, J.C. (1971) Field study of a chlordane residue
problem: Soil and plant relationships. Bull. environ. Contam.
Toxicol., 6: 177-182.
BOYD, E.M. (1972) Chlordane. In: Protein deficiency and
pesticide toxicity, Springfield, Ill., Charles C. Thomas,
468 pp.
BRIMFIELD, A.A. & STREET, J.C. (1979) Mammalian
biotransformation of chlordane: in vivo and primary hepatic
comparisons. Ann. New York Acad. Sci., 320: 247-256.
BRIMFIELD, A.A., STREET, J.C., FUTRELL, J., & CHATFIELD, D.A.
(1978) Identification of products arising from the metabolism
of cis- and trans-chlordane in rat liver microsomes in vitro:
Outline of a possible metabolic pathway. Pestic. Biochem.
Physiol., 9: 84-95.
BRODTMANN, N.V., Jr (1976) Continuous analysis of
chlorinated hydrocarbon pesticides in the lower Mississippi
river. Bull. environ. Contam. Toxicol., 15: 33-39.
BRUBAKER, P.E., FLAMM, W.G., & BERNHEIM, N.J. (1970) Effect
of gamma-chlordane on synchronized lymphoma cells and
inhibition of cell division. Nature (Lond.), 226: 548-549.
BUCK, W.B., OSWEILER, G.D., & VAN GELDER, G.A. (1973)
Clinical and diagnostic veterinary toxicology, Dubuque, Iowa,
Kendall Hunt.
BUGG, J.C., HIGGINS, J.E., & ROBERTSON, E.A. (l967) Residues
in fish, wildlife and estuaries: chlorinated pesticide levels
in the eastern oyster (Crassostrea virginica) from selected
areas of the South Atlantic and Gulf of Mexico. Pestic. Monit.
J., 1: 9-12.
BUTLER, P.A. (l963) Commercial fishery investigations,
Washington DC, US Department of the Interior, Fish and
Wildlife Service, pp. 5-28 (Circular 199).
BUTLER, P.A. & SCHUTZMANN, R.L. (1978) Residues in
pesticides and PCBs in estuarine fish, 1972-1976; National
Pesticide Monitoring Program. Pestic. Monit. J., 12: 51-59.
CALDWELL, R.S. (l977) Biological effects of pesticides on
the Dungeness crab, Washington DC, US Environment Protection
Agency (Report No. EPA 600/3-77-131).
CANADA, NATIONAL RESEARCH COUNCIL (1974) Chlordane: Its
effects on Canadian ecosystems and its chemistry, Canada, NRC
(Report Monograph Non Serials 189).
CARDWELL, R.D., FOREMAN, D.G., PAYNE, T.R., & WILBUR, D.J.
(l977) Acute and chronic toxicity of chlordane to fish and
invertebrates, Washington DC, US Environment Protection
Agency, 126 pp (Report No. EPA 600/3-77-019).
CAREY, A.E., WIERSMA, G.B., TAI, H., & MITCHELL, W.G. (1973)
Organochlorine pesticide residues in soils and crops of the
Corn Belt region, United States, 1970. Pestic. Monit. J., 6:
369-376.
CASPER, V.L. (l967) Galveston Bay pesticide study - water
and oyster samples analysed for pesticide residues following
mosquito control programme. Pestic. Monit. J., 1: 13-15.
CEC (1981) Criteria (dose-effects relationship) for organo-
chlorine pesticides, Oxford, CEC, Pergamon Press.
CHAMBERS, C. & DUTTA, S.K. (1976) Mutagenic tests of
chlordane on different microbial tester strains. Genetics,
83: S13.
CLARK, D.R., Jr & KRYNITSKY, A. (1978) Organochlorine
residues and reproduction in the little brown bat, Laurel,
Maryland, June 1976. Pestic. Monit. J., 12: 113-116.
CLARK, D.R., Jr & PROUTY, R.M. (1976) Organochlorine
residues in three bat species from four localities in Maryland
and West Virginia, 1973. Pestic. Monit. J., 10: 44-53.
CLEGG, T.J. & KOEVENIG, J.L. (l974) The effect of four
chlorinated hydrocarbon pesticides and one organophosphate
pesticide on ATP levels in three species of photosynthesizing
fresh-water algae. Bot. Gaz., 135: 368-372.
CLEMENS, H.P. & SNEED, K.E. (l959) Lethal doses of several
commercial chemicals for fingerling channel catfish,
Washington DC, US Department of the Interior, Fish and
Wildlife Service, 10 pp (Special Scientific Report on
Fisheries No. 316).
COCHRANE, W.P. & GREENHALGH, R. (1976) Chemical composition
of technical chlordane. J. Assoc. Off. Anal. Chem., 59:
696-702.
COCHRANE, W.P., PARLAR, H., GAEB, S., & KORTE, F. (1975)
Structural elucidation of the chlordene isomer constituents of
technical chlordane. J. agric. food Chem., 23: 882-886.
COPE, O.B. (l965) Sport fishery investigations, Washington
DC, US Department of the Interior, Fish and Wildlife Service
(Circular 226).
COPE, O.B., GJULLIN, C.M., & STORM, A. (l947) Effects of
some insecticides on trout and salmon in Alaska, with
reference to black-fly control. Trans. Am. Fish. Soc., 77:
160-177.
CORBETT, D.C.M. & WEBB, R.M. (l968) Effect of herbicides in
minimum tillage, Suffolk, England, Richard Clay (The Chaucer
Press) Ltd., pp. 158-159 (Report of the Rothamsted
Experimental Station for l967).
COUNCIL FOR AGRICULTURAL SCIENCE & TECHNOLOGY (1975)
Chlordane and heptachlor, Ames, Iowa, Iowa State University,
Department of Agronomy, pp. 1-71 (Report No. 47, Oct. 3).
CRANMER, J.S., AVERY, D.L., GRADY, R.R., & KITAY, J.I. (1978)
Postnatal endocrine dysfunction resulting from prenatal
exposure to carbofuran diazinon or chlordane. J. environ.
Pathol. Toxicol., 2: 357-370.
CROCKETT, A.B., WIERSMA, G.B., TAI, H., MITCHELL, W.G., SAND,
P.F., & CAREY, A.E. (1974) Pesticide residue levels in soils
and crops, FY-70: National soils monitoring program II.
Pestic. Monit. J., 8: 69-97.
CURLEY, A. & GARRETTSON, L.K. (1969) Acute chlordane
poisoning: clinical and chemical studies. Arch. environ.
Health, 18: 211-215.
DATTA, K.K., GUPTA, P.C., & DIKSHITH, T.S.S. (1975) Effect
of chlordane on the skin of male guinea pigs. In: Zaidi,
S.H., ed. Environmental pollution and human health, Lucknow,
India, Industrial Toxicology Research Centre, pp. 608-611.
DAVIS, R.W., FRIEDHOFF, J.M., & WEDENEYER, G.A. (l972)
Organochlorine insecticide, herbicide and polychlorinated
biphenyl (PCB) inhibition of NaK-ATPase in rainbow trout.
Bull. environ. Contam. Toxicol., 8: 69-72.
DEN TONKELAAR, E.M. & VAN ESCH, G.J. (1974) No-effect levels
of organochlorine pesticides based on induction of microsomal
liver enzymes in short-term toxicity experiments. Toxicology,
2: 371-380.
DERBES, J.V., DENT, H.J., FOREST, W.W., & JOHNSON, M.F.
(1955) Fatal chlordane poisoning. J. Am. Med. Assoc.,
158(15): 1367-1369.
DEWITT, J.B., STICKEL, W.H., & SPRINGER, P.F. (1963)
Wildlife studies, Patuxent Wildlife Research Centre l961-l962,
Washington DC, US Department of the Interior, Fish and
Wildlife Service, pp. 74-96 (Circular 167).
DITRAGLIA, D., BROWN, D.P., NAMEKATA, T., & IVERSON N.
(1981) Mortality study of workers employed at organochlorine
pesticide manufacturing plants. Scand. J. Work Environ.
Health, 7(Suppl. 4): 140-146.
DOANE, C.C. (l962) Effects of certain insecticides on
earthworms. J. econ. Entomol., 55: 416-418.
DRUMMOND, L., CHETTY, K.N., BAILEY, M., & DESAIAH, D. (1980)
in vivo effect of chlordane on rat brain ATP-ase system. Fed.
Proc., 39: 998.
DUFFY, J.R. & WONG, N. (1967) Residues of organochlorine
insecticides and their metabolites in soil in the Atlantic
Provinces of Canada. J. agric. food Chem., 15(3): 457-464.
EDWARDS, C.A. (l965a) Some side-effects resulting from the
use of persistent insecticides. Ann. appl. Biol., 55: 329-331.
EDWARDS, C.A. (l965b) Effects of pesticide residues on soil
invertebrates and plants. In: Goodman, G.T., Edwards, R.W., &
Lambert, J.M., ed. Ecology and the industrial society,
Reading, England, British Ecological Society, Symposium No. 5,
pp. 239-261.
EPSTEIN, S.S. (1976) Carcinogenicity of heptachlor and
chlordane. Sci. total Environ., 6: 103-154.
EPSTEIN, S.S., ARNOLD, E., ANDREA, J., BASS, W., & BISHOP, Y.
(1972) Detection of chemical mutagens by the dominant lethal
assay in the mouse. Toxicol. appl. Pharmacol., 23: 288-335.
ERCEGOVICH, C.D. & RASHID, K.A. (1977) Mutagenesis induced
in mutant strains of Salmonella typhimurium by pesticides.
Soc. Abstr. Pap., 174: 43.
FAO/WHO (1968) 1967 Evaluations of some pesticide residues
in food, Rome, Food and Agriculture Organization of the United
Nations (The Monographs).
FAO/WHO (1973) 1972 Evaluations of some pesticide residues
in food, Rome, Food and Agriculture Organization of the United
Nations.
FAO/WHO (1978) 1977 Evaluations of some pesticide residues
in food, Rome, Food and Agriculture Organization of the United
Nations.
FAO/WHO (1981) Joint FAO/WHO food and animal feed
contamination monitoring programme. Summary of data received
from collaborating centres - 1977-1980. Part B - Contaminants,
Geneva, World Health Organization.
FAO/WHO (1983) Pesticide residues in food - 1982, Rome, Food
and Agriculture Organization of the United Nations, pp. 16-17
(FAO Plant Production and Protection Paper 46).
FISHBEIN, W.I., WHITE, J.V., & ISAACS, H.J. (1964) Survey of
workers exposed to chlordane. Ind. Med. Surg., 33: 726-727.
FITZHUGH, O.G. & FAIRCHILD, H.E. (1976) Pesticidal aspects
of chlordane in relation to man and the environment,
Washington DC, US Environmental Protection Agency, US NTIS,
114 pp (PB Rep., ISS PB- 257107).
FOLMAR, L.C. (1978) In vitro inhibition of rat brain
ATPase, pNPPase, and ATP-32p exchange by chlorinated-diphenyl
ethanes and cyclodiene insecticides. Bull. environ. Contam.
Toxicol., 19: 481-488.
FOSTER, T.S., MORLEY, H.V., PURKAYASTHA, R., GREENHALGH, R., &
HUNT, J.R. (l972) Residues in eggs and tissues of hens fed a
ration containing low levels of pesticides with and without
charcoal. J. econ. Entomol., 65: 982-988.
FOUTS, J.R. (1963) Factors influencing the metabolism of
drugs in liver microsomes. Ann. New York Acad. Sci., 104:
875-880.
FOX, C.J.S. (l958) Some effects of insecticides on the
wireworms and vegetation of grassland in Nova Scotia. In:
Proceedings of the Tenth Congress on Entomology, Canada,
Vol. 3, pp. 297-300.
FRANK, R., BRAUN, H.E., HOLDRINET, M., DODGE, D.P., & NAPSZY,
S.J. (1978a) Residues of organochlorine insecticides and
polychlorinated biphenyls in fish from lakes Saint Clair and
Erie, Canada, 1968-1976. Pestic. Monit. J., 12: 69-80.
FRANK, R., HOLDRINET, M., BRAUN, H.E., DODGE, D.P., &
SPRANGLER, G.E. (1978b) Residues of organochlorine insecticides
and polychlorinated biphenyls in fish from lakes Huron and
Superior, Canada, 1968-1976. Pestic. Monit. J., 12: 60-68.
GAEB, S., BORN, L., PARLAR, H., & KORTE, F. (1977)
Structural elucidation of an octachloro component of technical
chlordane (Compound K) by spectroscopic and X-ray methods.
J. agric. food Chem., 25: 1365-1371.
GAINES, T.B. (1969) Acute toxicity of pesticides. Toxicol.
appl. Pharmacol., 14: 525-534.
GISH, C.D. (l970) Pesticides in soil. Organochlorine
insecticide residues in soils and soil invertebrates from
agricultural land. Pestic. Monit. J., 3: 241-252.
GLOOSCHENKO, V. & LOTT, J.N.A. (l977) The effects of
chlordane on the green algae, Scenedesmus quadricauda and
Chlamydomonas sp. Can. J. Bot., 55: 2866-2872.
GLOOSCHENKO, W.A., STRACHAN, W.M.J., & SAMPSON, R.C.J.
(1976) Residues in water: distribution of pesticides and
polychlorinated biphenyls in water, sediments, and seston of
the Upper Great Lakes - 1974. Pestic. Monit. J., 10: 61-67.
GODSIL, P.J. & JOHNSON, W.C. (l968) Residues in fish,
wildlife and estuaries. Pesticide monitoring of the aquatic
biota at the Tule Lake National Wildlife Refuge. Pestic.
Monit. J., 1: 21-26.
GOES, T.R., SAVAGE, E.P., & BOYD, W.L. (1978) In vitro
inhibition of oral viridans streptococci by chlordane. Arch.
environ. Contam. Toxicol., 7: 449-456.
GOULD, E. & HAMPSTEAD, E.O. (l95l) The toxicity of
cumulative spray residues in soils. J. econ. Entomol., 44:
713-717.
GOWEN, J.A., WIERSMA, G.B., TAI, H., & MITCHELL, W.G. (1976)
Pesticide levels in hay and soils from nine states, 1971.
Pestic. Monit. J., 10: 114-116.
GRIMES, D.J. & MORRISON, S.M. (1975) Bacterial
bioconcentration of chlorinated hydrocarbon insecticides from
aqueous systems. Microbiol. Ecol., 2: 43-59.
HANSON, W.R. (1952) Effects of some herbicides and
insecticides on the biota of North Dakota marshes. J. Wildl.
Manage., 16: 299-308.
HARRINGTON, J.M., BAKER, E.L., Jr, FOLLAND, D.S., SAUCIER,
J.W., & SANDIFER, S.H. (1978) Chlordane contamination of a
municipal water system. Environ. Res., 15: 155-159.
HARRIS, C.R. & SANS, W.W. (1976) Persistence of velsicol
HCS-3260 (AG-chlordane) in mineral and organic soil. Proc.
Entomol. Soc. Ontario, 106: 34-38.
HART, L.G. & FOUTS, J.R. (1965) Studies of the possible
mechanisms by which chlordane stimulates hepatic microsomal
drug metabolism in the rat. Biochem. Pharmacol., 14: 263-272.
HART, L.G., SHULTICE, R.W., & FOUTS, J.R. (1963) Stimulatory
effects of chlordane on hepatic microsomal drug metabolism in
the rat. Toxicol. appl. Pharmacol., 5: 371-386.
HENDERSON, C., PICKERING, Q.H., & TARZWELL, C.M. (1959) The
relative toxicity of ten chlorinated hydrocarbon insecticides
to four species of fish. Trans. Am. Fish. Soc., 88: 23-32.
HENDERSON, C., JOHNSON, W.L., & INGLIS, A. (1969)
Organochlorine insecticide residues in fish. Pestic. Monit.
J., 3: 145-171.
HENDERSON, C., INGLIS, A., & JOHNSON, W.L. (1971)
Organochlorine insecticide residues in fish - Fall 1969.
National pesticide monitoring program. Pestic. Monit. J., 5:
1-11.
HERRICK, G.M., FRY, J.I., FONG, W.G., & GOLDEN, D.C. (1969)
Insecticide residues in eggs resulting from the dusting and
short-term feeding of low levels of chlorinated hydrocarbon
insecticides to hens. J. agric. food Chem., 17: 291-295.
HILL, E.F., HEATH, R.G., SPANN, J.W., & WILLIAMS, J.D.
(1975) Lethal dietary toxicities of environmental pollutants
to birds, Washington DC, US Department of the Interior, Fish
and Wildlife Service, 61 pp (Special Scientific Report No.
191).
HODGE, H.C. & STERNER, J.H. (1956) Combine and tabulation of
toxicity classes. In: Spector, W.B., ed. Handbook of
toxicology, Philadelphia, Pennsylvania, W.B. Saunders Company,
Vol. 10.
HOPKINS, A.R. & KIRK, V.M. (1957) Effect of several
insecticides on the English Red Worm. J. econ. Entomol., 50:
699-700.
HRDINA, P.D., PETERS, D.A.V., & SINGHAL, R.L. (1973) Acute
neurotoxic effects of alpha-chlordane and alterations in brain
biogenic amines. Proc. Can. Fed. Biol. Soc., 16: 103.
HYDE, K.M. & FALKENBERG, R.L. (1976) Neuroelectrical
disturbance as indicator of chronic chlordane toxicity.
Toxicol. appl. Pharmacol., 37: 499-515.
HYDE, K.M., CRANDALL, J.C., KORTMAN, K.E., & MCCOY, W.K.
(1978) EEG, ECG, and respiratory response to acute
insecticide exposure. Bull. environ. Contam. Toxicol., 19:
47-55.
IARC (1979) Some halogenated hydrocarbons. Chlordane, Lyons,
International Agency for Research on Cancer, pp. 45-65
(Monographs on the Evaluation of the Carcinogenic Risk of
Chemicals to Humans, No. 20).
IARC (1982) Chemicals and industrial processes associated
with cancer in humans, Lyons, International Agency for
Research on Cancer, pp. 80-83 (Monographs on the Evaluation of
the Carcinogenic Risk of Chemicals to Humans, Suppl. 4).
ILO (1980) Occupational exposure limits for airborne toxic
substances, 2nd (revised) ed., Geneva, International Labour
Office (Occupational Safety and Health Series No. 37).
INFANTE, P.F., EPSTEIN, S.S., & NEWTON, W.A., Jr (1978)
Blood dyscrasias and childhood tumors and exposure to
chlordane and heptachlor. Scand. J. Work Environ. Health, 4:
137-150.
INGLE, L. (1952) Chronic oral toxicity of chlordane to
rats. Arch. ind. Hyg. occup. Med., 6: 357-367.
INGLE, L. (1965a) Effects of 1-hydroxychlordene when
incorporated into the diets of rats for 224 days, Urbana,
Illinois, University of Illinois, Department of Zoology
(Report for Velsicol Chemical Corporation).
INGLE, L. (1965b) Monograph on chlordane - toxicological &
pharmacological properties, Urbana, Illinois, University of
Illinois, Food & Drug Library (Library of Congress, Card
No. 65-28686A).
INGLE, L. (1969) Albino rats subjected to alpha and gamma
chlordane incorporated into the daily diet for 78 weeks
(Report from the University of Illinois).
INRS (1983) Valeurs limites pour les concentrations des
substances dangereuses dans l'air des locaux de travail,
Paris, France, Institut National de Recherche et de Sécurité
pour la Préventions des Accidents du Travail et des Maladies
Professionelles (Cahiers de notes documentaires, No. 110).
IRDC (1967) Chlordane, two-year chronic feeding study in the
beagle dog, Mattawan, Michigan, International Research and
Development Corporation (Report 163-001, sponsored by Velsicol
Chemical Corporation).
IRDC (1972) Chlordane, teratology study in rabbits,
Mattawan, Michigan, International Research and Development
Corporation (Report 163-106, sponsored by Velsicol Chemical
Corporation).
IRPTC (1983) IRPTC Legal file 1983, Geneva, International
Register of Potentially Toxic Chemicals, United Nations
Environment Programme (UNEP), Vols. I & II.
IVIE, G.W., KNOW, J.R., KHALIFA, S., YAMAMOTO, I., & CASIDA,
J.E. (1972) Novel photoproducts of heptachlor epoxide,
trans-chlordane, and trans-nonachlor. Bull. environ. Contam.
Toxicol., 7: 376-382.
JENSEN, A.A. (1983) Chemical contaminants in human milk.
Residue Rev., 89: 1-128.
KACEW, S. & SINGHAL, R.L. (1973a) Metabolic alterations after
chronic exposure to alpha-chlordane. Toxicol. appl.
Pharmacol., 24: 539-544.
KACEW, S. & SINGHAL, R.L. (1973b) The influence of
p,p'-DDT, alpha-chlordane, heptachlor and endrin on hepatic
and renal carbohydrate metabolism and cyclic AMP-adenyl
cyclase system. Life Sci., 13: 1363-1371.
KADAM, A.N., BENDRE, S.B., & GHATGE, B.B. (1978) Gas
chromatography of technical chlordane and identification of
some of the components. Pesticides, 12: 13-15.
KAREL, A.K. (1976) Acute chlordane toxicity on the serum
alkaline phosphatase activity of Meriones hurricanae, Jerdon
(gerbil). Arch. int. Physiol. Biochem., 84: 63-68.
KAREL, A.K. & SAXENA, S.C. (1976) Chronic chlordane
toxicity: Effect on blood biochemistry of Meriones hurricanae,
Jerdon, the Indian desert gerbil. Pestic. Biochem. Physiol.,
6: 111-114.
KEPLINGER, M.L., DEICHMANN, W.B., & SALA, F. (1968) Effects
of pesticides on reproduction in mice. Ind. Med. Surg., 37:
525.
KOCH, R.B., CUTCOMB, L.K., & YAP, H.H. (1971) Inhibition of
oligomycin sensitive and insensitive fish adenosine
triphosphatase activity by chlorinated hydrocarbon
insecticides. Biochem. Pharmacol., 20: 3243-3245.
KONAR, S.K. (1968) Experimental use of chlordane in fishery
management. Prog. Fish. Cult., 30: 96-99.
KRAYBILL, H.F.T. (1977a) The determination of carcinogenesis
induced by trace contaminants in potable water. In: Borchardt,
J.A., Cleland, J.K., Redman, W.J., & Olivier, J., ed. Viruses
and trace contaminants in water and wastewater, Ann Arbor,
Michigan, Ann Arbor Science Publishers, Inc., pp. 109-123
(Seminar, Ann Arbor, Michigan, January 26-28, 1977. XIV 249P).
KUTZ, F.W., YOBS, A.R., STRASSMAN, S.C. (1976) Organochlorine
pesticide residues in human adipose tissue. Bull. Soc.
Pharmacol. Environ. Pathol., 4(1): 17-19.
LANGLOIS, B.E. & SIDES, K.G. (1972) Effect of heptachlor and
related compounds on growth of Staphylococcus aureus. Bull
environ. Contam. Toxicol., 8: 158-164.
LAWRENCE, J.M. (1950) Toxicity of some new insecticides to
several species of pondfish. Prog. Fish. Cult., 12: 141-146.
LAWRENCE, J.H., BARRON, R.P., CHEN, J.Y.T., LOMBARDO, P., &
BENSON, W.R. (1970) Note on identification of a chlordane
metabolite found in milk and cheese. J. Assoc. Off. Agric.
Chem., 53: 261-262.
LEGG, D.C. (1968) Comparison of various worm killing
chemicals. J. Sports Turf Res. Inst., 44: 47-48.
LEHMAN, A.J. (1952a) Chemicals in foods: A report to the
Association of Food & Drug Officials on current developments.
Part II. Pesticides Section II: Dermal Toxicity. Assoc. Food
Drug Off. Q. Bull., 16: 3-9.
LEHMAN, A.J. (1952b) A report to the Association of Food and
Drug Officials on current developments. Section III: Subacute
and chronic toxicity. Assoc. Food Drug Off. Q. Bull., 16:
47-53.
LENSKY, P. & EVANS, H.L. (1952) Human poisoning by
chlordane. Report of a case. J. Am. Med. Assoc., 121: 826-827.
LICHTENSTEIN, E.P. & POLIUKA, J.B. (1959) Persistence of
some chlorinated hydrocarbon insecticides in turf soils.
J. econ. Entomol., 52(2): 280-293.
LICHTENSTEIN, E.P., SCHULTZ, K.K., FUHREMANN, T.W., & LIANG,
T.T. (l970) Degradation of aldrin and heptachlor in field
soils during a ten-year period translocation into crops.
J. agric. food Chem., 18: 100-106.
LIDGATE, H.J. (1966) Earthworm control with chlordane.
J. Sports Turf Res. Inst., 42: 5-8.
LINDUSKA, J.P. & SURBER, E.W. (1948) Effect of DDT and other
insecticides on fish and wildlife. Summary of investigations
during 1947, Washington DC, US Department of the Interior,
Fish and Wildlife Service, 19 pp (Circular 15).
LONG, W.H., ANDERSON, H.L., ISA, A.L., & KYLE, M.L. (1967)
Sugarcane growth responses to chlordane and microarthropods
and effects of chlordane on soil fauna. J. econ. Entomol., 60:
623-629.
LORA, R.P., MARTEACHE, A.H., VILLAR, L.M.P., GIMENEZ, R.L.,
VILLAREJO, M.J., & PEREZ, J.I. (l979) [Organochlorine
pesticides in the milk of Spanish humans.] Rev. Esp. pediatr.,
35: 93 (in Spanish).
LUDEMANN, D. & NEUMANN, H. (1962) [On the effects of the
latest contact insecticide on fresh water animals.] Anz.
Schaelingskd., 35: 5-9 (in German).
LUDKE, J.L. (1976) Organochlorine pesticide residues
associated with mortality: additivity of chlordane and endrin.
Bull. environ. Contam. Toxicol., 16: 253-260.
MACEK, K.J., HUTCHINSON, C., & COPE, O.B. (1969) The effects
of temperature on the susceptibility of bluegills and rainbow
trout to selected pesticides. Bull. environ. Contam. Toxicol.,
4: 174-183.
MACMAHON, B. & WANG, H.H. (1982) A second follow-up of
mortality in a cohort of pesticide applicators, Cambridge,
Massachusetts, Harvard School of Public Health, Department of
Epidemiology.
MADHUKAR, B.V. & MATSUMURA, F. (1979) Comparison of
induction patterns of rat hepatic microsomal mixed-function
oxidases by pesticides and related chemicals. Pestic.
Biochem. Physiol., 11: 301-308.
MAGNANI, B., POWERS, C.D., WURSTER, C.F., & O'CONNORS, H.B.
(1978) Effects of chlordane and heptachlor on the marine dino
flagellate Exuviella baltica Lohmann. Bull. environ. Contam.
Toxicol., 20: 1-8.
MAGUIRE, J. & WATKIN, N. (1975) Carbonic anhydrase
inhibition. Bull. environ. Contam. Toxicol., 13: 625-629.
MAHON, D.C. (1977) Interactions, in rats, between carbon
tetrachloride-induced liver cirrhosis and chronic treatment
with the insecticide chlordane. Diss. Abstr. Int., 38: 4012B.
MAHON, D.C. & OLOFFS, P.C. (1979) Effects of sub-chronic
low-level dietary intake of chlordane on rats with cirrhosis
of the liver. J. environ. Sci. Health, B14: 227-246.
MAHON, D.C., NAIR, K.K., & OLOFFS, P.C. (1979) DNA in rat
hepatocyte nuclei: Effects of treatment with low levels of
carbon tetrachloride and/or chlordane. Can. J. Zool., 57:
1003-1009.
MASLANSKY, C.J. & WILLIAMS, G.M. (1981) Evidence for an
epigenetic mode of action in organochlorine pesticide
hepatocarcinogenicity: A lack of genotoxicity in rat, mouse
and hamster hepatocytes. J. Toxicol. environ. Health, 8:
121-130.
MASTRI, C., KEPLINGER, M.L., & FANCHER, O.E. (1969a) Acute
oral toxicity study on synthetic (X) in male and female
albino rats, Illinois, Industrial Bio-Test Laboratories
(Report for Velsicol Chemical Corporation).
MASTRI, C., KEPLINGER, M.L., & FANCHER, O.E. (1969b) Acute
oral toxicity study on 4 chlordenes in albino rats, Illinois,
Industrial Bio-Test Laboratories (Report for Velsicol Chemical
Corporation).
MASTRI, C., KEPLINGER, M.L., & FANCHER, O.E. (1969c) Acute
oral toxicity study on 2 chlordenes in female albino rats,
Illinois, Industrial Bio-Test Laboratories (Report for
Velsicol Chemical Corporation).
MATTRAW, H.C., Jr (1975) Occurrence of chlorinated hydro-
carbon insecticides, Southern Florida, 1968-1972. Pestic.
Monit. J., 9: 106-114.
MCCASKEY, T.A., STENP, A.R., LISKA, B.J., & STADELMAN, W.J.
(1968) Residues in egg yolks and raw and cooked tissues from
laying hens administered selected chlorinated hydrocarbon
insecticides. Poult. Sci., 47: 564-569.
MCCLEESE, D.W., BURRIDGE, L.E., & VAN DINTER, J. (1982)
Toxicities of five organochlorine compounds in water and
sediment to Nereis virens. Bull. environ. Contam. Toxicol.,
28: 216-220.
MCGILL, H.C. (1979) Pilot study of the effects of pesticides
on blood lipoproteins, arteries, and cardiac muscle of
baboons, Washington DC, US Environmental Protection Agency.
MEHRLE, P.M., JOHNSON, W.W., & MEYER, F.L. (1974)
Nutritional effects of chlordane toxicity to rainbow trout.
Bull. environ. Contam. Toxicol., 12: 513-517.
MEITH-AVCIN, N., WARLEN, S.M., & BARBER, R.T. (1973)
Organochlorine insecticide residues in a Bathyl-Demersal Fish
from 2500 metres. Environ. Lett., 5: 215-221.
MES, J. & DAVIES, D.J. (1978) Variation in the
polychlorinated biphenyl and organochlor pesticide residues
during human breastfeeding and its diurnal pattern.
Chemosphere, 7: 699-706.
MES, J., COFFIN, D.E., & CAMPBELL, D.T. (1974)
Polychlorinated biphenyl and organochlorine pesticide residues
in Canadian chicken eggs. Pestic. Monit. J., 8: 8-11.
MIYAZAKI, T., AKIYAMA, K., KANEKO, S., HORII, S., & YAMAGISHI,
T. (1980) Chlordane residues in human milk. Bull. environ.
Contam. Toxicol., 25: 518.
MOORE, R., TORO, E., STANTON, M., & KHAN, M.A.Q. (1977)
Absorption and elimination of 14C-alpha and gamma-chlordane by
a fresh-water alga, daphnid, and goldfish. Arch. environ.
Contam. Toxicol., 6: 411-420.
MORGAN, D.P. & ROAN, C.C. (1969) Renal function in persons
occupationally exposed to pesticides. Arch. environ. Health
19: 633-636.
NAKAS, J.P. (1977) Chlordane. Inhibition of endopeptidase
activity in the marine bacterium, Aeromonas proteolytica, New
Jersey, Rutgers State University, 123 pp (Dissertation).
NAQVI, S.M. (1973) Toxicity of twenty-three insecticides to
a tubificid worm Branchiura sowerbyi from the Mississippi
Delta. J. econ. Entomol., 66: 70-74.
NCI (1977) Bioassay of chlordane for possible
carcinogenicity, Bethesda, Maryland, National Cancer Institute
(CAS NO. 57-74-9).
OLOFFS, P.C., ALBRIGHT, L.J., & SZETO, S.Y. (1978)
Persistence of residues in water and sediment of a fresh-water
lake after surface application of technical chlordane.
J. environ. Sci. Health, B13: 47-58.
ONSAGER, J.A., RUSK, H.W., & BUTLER, L.I. (1970) Residues of
aldrin, dieldrin, chlordane in soil and sugar beets. J. econ.
Entomol., 63: 1143-1146.
ORTEGA, P., HAYES, W.J., & DURHAM, W.F. (1957) Pathologic
changes in the liver of rats after feeding low levels of
various insecticides. Am. Med. Assoc. Arch. Pathol., 64:
614-622.
PARLAR, H., HUSTERT, K., GAEB, S., & KORTE, F. (1979)
Isolation, identification, and chromatographic characterization
of some chlorinated C10 hydrocarbons in technical chlordane.
J. agric. food Chem., 27: 278-283.
PARRISH, P.R., SCHIMMEL, S.C., HANSEN, D.J., PATRICK, J.M., &
FORESTER, J. (1976) Chlordane: effects on several estuarine
organisms. J. Toxicol. environ. Health, 1: 485-494.
PARRISH, P.R., DYAR, E.A., ENOS, J.M., & WILSON, W.G. (1978)
Chronic toxicity of chlordane, trifluralin, and pentachlorophenol
to sheepshead minnows (Cyprinodon variegatus), Washington DC,
US Environmental Protection Agency (Report No. EPA-600/3-78-010).
PLANK, J., KEPLINGER, M.L., FANCHER, O.E., & RICHTER, W.R.
(1970) 90-day subacute oral toxicity of synthetic "X"
(oxychlordane) in albino rats, Illinois, Industrial Bio-Test
Laboratories (Report for Velsicol Chemical Corporation).
POHL, R.J. & FOUTS, J.R. (1977) Xenobiotic metabolism in
skin of hairless mice exposed to ultraviolet radiation,
aroclor 1260, or chlordane. Environ. Health Perspect., 20:
230.
POLEN, P.B., HESTER, M., & BENZIGER, J. (1971)
Characterization of oxychlordane, animal metabolite of
chlordane. Bull. environ. Contam. Toxicol., 5: 521-528.
POONAWALLA, N.H. & KORTE, F. (1971) Metabolism of trans-
chlordane-14C and isolation and identification of its
metabolites from the urine of rabbits. J. agric. food Chem.,
19: 467.
PRINCI, F. & SPURBECK, G.H. (1951) A study of workers
exposed to the insecticides chlordane, aldrin and dieldrin.
Arch. ind. Hyg. occup. Med., 3: 64-72.
REINKE, J., UTHE, J.F., & JAMIESON, D. (1972) Organochlorine
pesticide residues in commercially caught fish in Canada,
1970. Pestic. Monit. J., 6: 43.
ROBERTS, J.R., DEFREITAS, A.S.W., & GIDNEY, M.A.J. (1977)
Influence of lipid pool size on bioaccumulation of the
insecticide chlordane by northern redhorse suckers ( Moxostoma
macrolepidotum). J. Fish. Res. Board Can., 34: 89-97.
SAHA, J.A. & SUMNER, A.K. (1971) Organochlorine insecticide
residues in soil from vegetable farms in Saskatchewan.
Pestic. Monit. J., 5(1): 28-31.
SANBORN, J.R., METCALF, R.L., BRUCE, W.N., & LU, P.-Y.
(1976) The fate of chlordane and toxaphene in a
terrestrial-aquatic model ecosystem. Environ. Entomol., 5:
533-538.
SANDERS, H.O. & COPE, O.B. (1966) Toxicities of several
pesticides to two species of cladocerans. Trans. Am. Fish.
Soc., 95: 165-169.
SASCHENBRECKER, P.W. (1976) Levels of terminal pesticide
residues in Canadian meat. Can. vet. J., 17: 158-163.
SAVAGE, E.P. (1976) National study to determine levels of
chlorinated hydrocarbon insecticides in human milk: 1975-76,
Washington DC, US Environmental Protection Agency (Report,
Iss. EPA/540/9-78/005, Order no. PB284393).
SAVAGE, E.P., KEEFE, T.J., TESSARI, J.D., WHEELER, H.W.,
APPLEHANS, F.M., GOES, E.A., & FORD, S.A. (1981) National
study of chlorinated hydrocarbon insecticide residues in human
milk. Am. J. Epidemiol., 113: 413.
SCHIMMEL, S.C., PATRICK, J.M., & FORESTER, J. (1976a)
Heptachlor: toxicity to and uptake by several estuarine
organisms. J. Toxicol. environ. Health, 1: 955-965.
SCHIMMEL, S.C., PATRICK, J.M., & FORESTER, J. (1976b)
Heptachalor: uptake, depuration, retention, and metabolism by
spot Leiostomus xanthurus. J. Toxicol. environ. Health, 2:
169-178.
SCHWEMMER, B., COCHRANE, W.P., & POLAN, P.B. (1970)
Oxychlordane, animal metabolite of chlordane, isolation and
synthesis. Science, 169: 1087.
SHINDELL & ASSOCIATES (1981) Report of epidemiologic study
of the employees of Velsicol Chemical Corporation Plant,
Memphis, Tennessee, January 1952 - December 1979, Milwaukee,
Wisconsin (Report of a study sponsored by Velsicol Chemical
Coporation).
SIMMON, V.F., KAUHANEN, K., & TARDIFF, R.G. (1977) Mutagenic
activity of chemicals identified in drinking water. Dev.
Toxicol. environ. Sci., 2: 249-258.
SIMS, G.G., CAMPBELL, J.R., ZEMLYAK, F., & GRAHAM, J.M.
(1977) Organochlorine residues in fish and fishery products
from the Northwest Atlantic. Bull. environ. Contam. Toxicol.,
18: 697-705.
SINGHAL, R.L. & KACEW, S. (1973) Evidence for the role of
cyclic AMP in the mechanism of action of organochlorine
pesticides. J. Cell Biol., 59: 321.
SINGHAL, R.L. & KACEW, S. (1976) The role of cyclic AMP in
chlorinated hydrocarbon-induced toxicity. Fed. Proc., 35:
2618-2663.
SOVOCOOL, G.W. & LEWIS, R.G. (1975) The identification of
trace levels of organic pollutants in human tissue compounds
related to chlordane/heptachlor exposure. Environ. Health, 9:
265-279.
SOVOCOOL, G.W., LEWIS, R.G., HARLESS, R.L., WILSON, N.K., &
ZEHR, R.D. (1977) Analysis of technical chlordane by gas
chromatography mass spectrometry. Anal. Chem., 49: 734-740.
SPENCER, E.Y. (1973) Guide to the chemicals used in crop
protection, 6th ed., Ontario, Research Branch Agriculture
Canada, pp. 94-95 (Publication No. 1093).
SPYKER-CRANMER, J.M., BARRETT, J.B., AVERY, D.L., & CRANMER,
M.F. (1982) Immunoteratology of chlordane: Cell-mediated and
humoral immune responses in adult mice expressed in utero.
Toxicol. appl. Pharmacol., 62: 402-408.
STARR, H.G., Jr, ALDRICH, F.D., MCDOUGAL, W.D. III, & MOUNCE,
L.M. (1974) Contribution of household dust to the human
exposure to pesticides. Pestic. Monit. J., 8: 209-212.
STAUFFER, T.B. (1977) Chlordane volatility, Florida, Civil
and Environmental Engineering Development Office, pp. 1-29
(CEEDO-TR-77-9).
STENGER, R.J., PORWAY, M., JOHNSON, J.E.A., & DATTA, R.K.
(1975) Effects of chlordane pretreatment on the
hepatotoxicity of carbon tetrachloride. Exp. mol. Pathol.,
23: 144-153.
STEWART, D.K.R. (1975) Chlordane uptake from soil by root
crops. Environ. Entomol., 4: 254-256.
STOHLMAN, E.F., THORP, W.T.S, & SMITH, M.I. (1950) Toxic
action of chlordane. J. ind. Hyg. occup. Med., 1: 13-19.
STRASSMAN, S.C. & KUTZ, F.W. (1977) Insecticide residues in
human milk from Arkansas and Mississippi, 1973-74. Pestic.
Monit. J., 10: 130-133.
STREET, J.C. & BLAU, S.E. (1972) Oxychlordane - accumulation
in rat adipose tissue on feeding chlordane isomers or
technical chlordane. J. agric. food Chem., 20: 395-397.
STREET, J.C., MAYER, F.L., & WAGSTAFF, J. (1969) Ecological
significance of pesticide interactions. Ind. Med. Surg., 38:
409-414.
SURBER, E.W. (1948) Chemical control agents and their
effects on fish. Prog. Fish. Cult., 10: 125-131.
TALAMANTES, F. & JANG, H. (1977) Effects of chlordane
isomers administered to female mice during the neonatal
period. J. Toxicol. environ. Health, 3: 713-720.
TASHIRO, S. & MATSUMURA, F. (1977) Metabolic routes of cis-
and trans-chlordane in rats. J. agric. food Chem., 25:
872-880.
TAYLOR, J.R., CALABRESE, V.P., & BLANKE, R.V. (1979)
Organochlorine and other insecticides. In: Vinken, P.J. &
Bruyn, G.W., ed. Handbook of clinical neurology, Vol. 36.
Intoxications of the nervous system, Amsterdam, New York,
Elsevier/North-Holland Biomedical Press, Part 1.
TELANG, S., TONG, C., & WILLIAMS, G.M. (1982) Epigenetic
membrane effects of a possible tumour promoting type on
cultured liver cells by the non-genotoxic organochlorine
pesticides chlordane and heptachlor. Carcinogenesis, 3:
1175-1178.
TONG, C., FAZIO, M., & WILLIAMS, G.M. (1981) Rat
hepatocyte-mediated mutagenesis of human cells by carcinogenic
polycyclic aromatic hydrocarbons but not organochlorine
pesticides. Proc. Soc. Exp. Biol. Med., 167: 572-575.
TRUDGILL, P.W., WIDDUS, R., & REES, J.S. (1971) Effects of
organochlorine insecticides on bacterial growth, respiration,
and viability. J. gen. Microbiol., 69: 1-13.
TRUHAUT, R., GAK, J.C., & GRAILLOT, C. (1974) Study of the
modalities and action mechanisms of organochlorine
insecticides. I. Comparative study of the acute toxicity in
hamster and rat. J. Eur. Toxicol., 7: 159-166.
TUCKER, R.K. & CRABTREE, D.G. (1970) Handbook of toxicity to
wildlife, Washington DC, US Department of the Interior, Bureau
of Sport Fishing and Wildlife Research (Publication No. 84).
US EPA (1976a) Pesticidal aspects of chlordane in relation
to man and the environment, Washington DC, US Environmental
Protection Agency (US Department of Commerce, National
Technical Information Service, publication No. PB-2577107).
US EPA (1976b) Pesticidal aspects of chlordane and
heptachlor in relation to man and the environment. A further
review 1972-1976, Washington DC, US Environmental Protection
Agency, p. 33 (US Department of Commerce, National Technical
Information Service, publication No. PB-258339).
US NAS (1977) An evaluation of the carcinogenicity of
chlordane and heptachlor. Natl Acad. Sci. J., October,
Washington, DC.
VEITH, G.D., DEFOE, D.L., & BERGSTEDT, B.V. (1979) Measuring
and estimating the bioconcentration factor of chemicals in
fish. J. Fish. Res. Board Can., 36: 1040-1048.
VILLENEUVE, D.C., GRANT, D.L., & PHILLIPS, W.E. (1972)
Modification of pentobarbital sleeping times in rats following
chronic polychlorinated biphenyl ingestion. Bull. environ.
Contam. Toxicol., 7: 264-269.
VON RUMKER, R., LAWLESS, E.W., MEINERS, A.F., LAWRENCE, K.A.,
KELSO, G.L., & HORAY, F. (1974) Production, distribution,
use and environmental impact potential of selected pesticides.
Natl Tech. Inf. Serv. (PB-236), 795: 439.
WANG, H.H. & GRUFFENMAN, S. (1981) Aplastic anemia and
occupational pesticide exposure: A case-control study.
J. occup. Med., 23: 364-366.
WANG, H.H. & MACMAHON, B. (1979a) Mortality of workers
employed in the manufacture of chlordane and heptachlor.
J. occup. Med., 26: 745-748.
WANG, H.H. & MACMAHON, B. (1979b) Mortality of pesticide
applicators. J. occup. Med., 26: 741-744.
WAZETER, F.X. (1967) Two-year chronic feeding study in the
beagle dog, Mattawan, Michigan, International Research and
Development Corporation (Report for Velsicol Chemical
Corporation).
WAZETER, F.X., BUTLER, R.H., GEIL, R.G., & REHKAMPER, J.A.
(1968) Alpha-chlordane, gamma-chlordane, alpha gamma-
chlordane. Comparative acute oral toxicity (LD50) in male
albino rats, Mattawan, Michigan, International Research and
Development Corporation (Report for Velsicol Chemical
Corporation.
WELCH, R.M. (1948) Tests of the toxicity to sheep and cattle
of certain of the newer insecticides. J. econ. Entomol., 41:
36-39.
WELCH, R.M., LEVIN, W., KUNTZMAN, R., JACOBSON, M., & CONNEY,
A.H. (1971) Effect of halogenated hydrocarbon insecticides
on the metabolism and uterotropic action of estrogens in rats
and mice. Toxicol. appl. Pharmacol., 19: 234-246.
WHO (1982) Guidelines for drinking water quality, Vol. 1,
Recommendations, Geneva, World Health Organization, 82 pp
(EFP/82.39).
WHO (1984) The use of WHO recommended classification of
pesticides by hazard, Geneva, World Health Organization
(Unpublished Report VBC/84.2).
WHO/FAO (1978) Chlordane, Geneva, World Health Organization
(Data Sheets on pesticides No. 36).
WILLIAMS, G.M. (1979) Liver cell culture systems for the
study of hepatocarcinogenesis. In: Margison, G.P., ed.
Advances in medical oncology research and education, Oxford,
Pergamon Press, Vol. 1, pp. 273-280.
WILLIAMS, C.H. & CASTERLINE, J.L., Jr (1970) Effects on
toxicity and on enzyme activity of the interactions between
aldrin, chlordane, piperonyl butoxide and banol in rats.
Proc. Soc. Exp. Biol. Med., 135: 46-50.
WILLIAMS, C.H., CASTERLINE, J.L., & JACOBSON, K.H. (1967)
Studies of toxicity and enzyme activity from interaction
between chlorinated hydrocarbon and carbamate insecticides.
Toxicol. appl. Pharmacol., 11: 302-307.
WILSON, A.J. (1965) Chemical assays. In: Annual report for
the fiscal year ending June 30, 1965, Gulfbreeze, Florida,
Bureau of Community Fisheries, Biology Laboratory, 247 pp
(Circular 6-7).
WILSON, D.M. & OLOFFS, P.C. (1973a) Residues in alfalfa
following soil treatment with high purity chlordane (Velsicol
HCS-3260). Bull. environ. Contam. Toxicol., 9: 337-344.
WILSON, D.M. & OLOFFS, P.C. (1973b) Persistence and movement
of a and q chlordane in soils following treatment with
high-purity chlordane (Velsicol HCS-3260). Can. J. Soil Sci.,
53: 465-472.
WRIGHT, L.H., LEWIS, R.G., CRIST, H.L., SOVOCOOL, G.W., &
SIMPSON, J.M. (1978) The identification of polychlorinated
terphenyls at trace levels in human adipose tissue by gas
chromatography/mass spectrometry. J. anal. Toxicol., 2: 76-79.
Molecular formula: C10H6C18
CAS chemical name: 1,2,4,5,6,7,8,8-octachloro-2,3,3a,4,
7,7a-hexahydro-4,7-methano-1H-indene
Common trade names: Aspon, Belt, CD 68, Chlorindan,
Chlorkil, Chlordane, Corodan,
Cortilan-neu, Dowchlor, HCS 3260,
Kypchlor, M140, Niran, Octachlor,
Octaterr, Ortho-Klor, Synklor, Tat
Chlor 4, Topichlor, Toxichlor,
Velsicol-1068
CAS registry number: 57-47-9
Relative molecular mass: 409.8
2.2. Properties and Analytical Methods
2.2.1. Physical and chemical properties
Chlordane is a viscous, light yellow to amber-coloured liquid
(IARC, 1979) with a melting point of 106 - 107 °C for the alpha-
isomer and 104 - 105 °C for the gamma-isomer. It has a density of
1.59 - 1.63 g/ml and a vapour pressure of 1 x 10-5 mm Hg at 25 °C.
It is insoluble in water but soluble in most organic solvents.
The major isomers of chlordane have the endo-endo-configuration
on the carbon skeleton (US EPA, 1976a,b). However, the term
chlordane actually refers to a complex mixture of chlordane
isomers, other chlorinated hydrocarbons and by-products. According
to the Canada National Research Council (1974), the technical
product is described as follows:
"Technical chlordane is a mixture of insecticidal
components, including chlorinated addition and substitution
derivatives of 4,7-methano-3a,4,7,7a-tetrahydro-indane.
The chlorine content is 64-67%. The principal components
are alpha- and gamma-chlordane (C10H6 x Cl8), heptachlor
(C10H5Cl7) and non-achlor (C10H5Cl9). Technical chlordane
conforms to the biological, chemical and physical properties
of reference technical chlordane".
The production of technical chlordane is strictly controlled
and its composition varies within narrow limits (Canada, National
Research Council, 1974). Technical chlordane is a mixture of at
least 26 different components, mainly however alpha- and gamma-
chlordane. Up to 14 distinct chromatographic components have been
described (Cochrane et al., 1975; Cochrane & Greenhalgh, 1976; US
EPA, 1976a,b; Gaeb et al., 1977; Sovocool et al., 1977; Kadam et
al., 1978; Parlar et al., 1979). The composition has been
essentially, but not completely, worked out. Chlordane is
available in the USA in five basic formulations (von Rumker et al.,
1974, IARC, 1979), including 5% granules, oil solutions containing
chlordane at 2 - 200 g/litre, and emulsifiable concentrates
containing chlordane at 400 - 800 g/litre.
2.2.2. Analytical methods
Determination of chlordane residues is difficult because
of the complex nature of the components and the fact that each
component degrades independently. Resulting residues may bear
little relation to the proportions in the technical product
(Council for Agriculture Science and Technology, 1975).
Separation from interfering materials can be carried out by thin-
layer chromatography or other partition and clean-up methods (US
EPA, 1976a,b). Extraction from crops, other plant products, dairy
products, plants, and oils was achieved with an 80 - 110%
efficiency using acetonitrile for extraction, petroleum ether for
partitioning, and clean-up on a Florisil column (Canada, National
Research Council, 1974). Gel-permeation chromatography can also be
used for clean-up, particularly with human adipose tissue (Wright
et al., 1978).
The principal method for the qualitative and quantitative
estimation of chlordane isomers is gas-liquid chromatography with
electron capture detection (US EPA, 1976a,b). This method has a
high sensitivity and specificity (Canada, National Research
Council, 1974; Cochrane et al., 1975). According to Atallah et al.
(1977), the highly sensitive electron capture detector can,
however, lead to the incorrect identification of residues of
chlordane and its metabolites. Confirmation of gas-chromatographic
analysis can be carried out with GLC-Mass spectrometry, a method
that can also give a better determination of some of the components
such as heptachlorepoxide (US EPA, 1976a,b). Other methods of
detection include bioassay, carbon-skeleton chromatography,
colorimetric, and total chlorine methods (US EPA, 1976a,b).
Analysis for total organically-bound chlorine (Canada, National
Research Council, 1974) remains the preferred method for the
determination of technical chlordane and the active ingredient
(chlordane) in formulations.
3. SOURCES OF ENVIRONMENTAL POLLUTION, ENVIRONMENTAL
TRANSPORT AND DISTRIBUTION
3.1. Sources of Environmental Pollution
3.1.1. Industrial production and uses
Chlordane was first prepared in the 1940s by exhaustive
chlorination of the cyclopentadiene-hexachlorocyclopentadiene
adduct (IARC, 1979). It was first described as an insecticide in
1945 by Kearns (Spencer, 1973).
Chlordane is produced commercially by reacting hexachloro-
cyclopentadiene with cyclopentadiene to form chlordene, which is
then chlorinated to produce chlordane (IARC, 1979). Chlordane was
first produced commercially in the USA in 1947 (IARC, 1979).
Production in the USA, in 1974, amounted to 9.5 million kg (IARC,
1979). Chlordane is not produced in Europe nor has it ever been
manufactured in Japan (IARC, 1979). In Japan, the only permitted
use of the compound is for the control of termites. It is also
used against wood-boring beetles and in ant baits. Both the
amounts of chlordane produced and used have decreased considerably
in recent years (WHO, 1982).
Chlordane has been used as an insecticide for more than 35
years. It is a versatile, broad spectrum, contact insecticide and
is used mainly for non-agricultural purposes (primarily for the
protection of structures, but also on lawn and turf, ornamental
trees, and drainage ditches) (von Rumker et al., 1974). Further-
more, it is used on corn, potatoes, and livestock. In 1978, a US
EPA cancellation proceeding led to a settlement on contested uses.
This settlement allowed for limited usage by crop, location, amount
allowed, and maximum time interval for use.
Since 1 July 1983, the only use of chlordane approved in the
USA is for the control of underground termites (IARC, 1979). In
Canada, the use of chlordane is controlled under the Pest Control
Products Act and it is used for the protection of structures,
ornamental plants, lawns, and various crops. Accepted uses vary
from province to provide (Canada, National Research Council, 1974).
3.2. Environmental Transport and Distribution
3.2.1. Air
Entry into the atmosphere occurs mainly through aerial
applications of dusts and sprays, soil erosion by the wind, and
volatilization from soil and water (Canada, National Research
Council, 1974).
3.2.2. Water
Few data are available on the routes of entry or the behaviour
and fate of chlordane in aquatic systems. It can be assumed that
not much originates from ground water since there is little
leaching of chlordane. One possible source is surface run-off, but
no studies have tested the extent of this assumption. Another
source is rain; however, in two studies, chlordane levels did not
exceed 2 - 3 ng/litre rain water (Bevenue et al., 1972a; US EPA,
1976a,b).
One important aspect of chlordane residues is that they
accumulate in sediment. The fate and behaviour of chlordane was
investigated in an isolated fresh water lake, previously free from
pesticide residues (Oloffs et al., 1978). The lake was treated
with technical chlordane at 10 µg/litre, and sediment samples were
analysed for chlordane residues 7, 24, 52, 279, and 421 days after
treatment. It was found that water residue concentrations declined
rapidly. After 7 days, only 46.1% of the chlordane residue
remained. After 421 days, residues were still detectable, but all
levels were below 0.01% of the initial concentration. It was
observed that chlordane residues moved quickly to the bottom
sediment and persisted there. Mean residue levels in sediment were
35.29 µg/kg wet weight after 7 days and 10.31 µg/kg after 421 days.
3.2.3. Soil
Chlordane is used almost exclusively as a soil insecticide to
control soil pests such as termites (Canada, National Research
Council, 1974). Thus, residues of chlordane are mainly present in
this environmental compartment. In most temperate climates, only
the two chlordane isomers generally persist (Canada, National
Research Council, 1974). For example, in Nova Scotia, chlordane
was applied at 5 kg/ha per year to sandy loam soil for 3 years.
Fifteen years later, approximately 15% of the residues remained,
the alpha and gammma isomers being the major components (US EPA,
1976a,b).
The components of technical chlordane are relatively insoluble
in water and are readily adsorbed onto soil particles. As a
result, one of the characteristics of soil residues is that they do
not migrate readily through the soil profile (Canada, National
Research Council, 1974; von Rumker et al., 1974). In general, not
more than 15% of the residues migrate below the cultivated layer
(Canada, National Research Council, 1974). As a result, residues
are not likely to become a serious contaminant of the lower soil
strata or deep water sources (Canada, National Research Council,
1974). The organic matter and moisture contents of the soil can
affect the volatilization of chlordane components (Stauffer, 1977).
The organic matter causes greater adsorption and thus reduces
volatilization while soil moisture increases volatilization
(Stauffer, 1977). Also, liquid formulations are more volatile than
granular (Atallah et al., 1979).
3.2.4. Abiotic degradation
Chlordane is stable to light under normal conditions. When it
is exposed to photosensitizers such as rotenone or benzophenone and
short irradiation exposure at wavelengths above 300 nm, some
components will isomerize (Canada, National Research Council,
1974). No detectable degradation products were formed on plant
foliage in the absence of a photosensitizer (Ivie et al., 1972).
3.2.5. Biodegradation
Three conversion products of gamma-chlordane were found in
white cabbage and carrots, 4 weeks after application. One of the
two metabolites isolated from white cabbage (35% of the total), was
given the chlordene chlorohydrin structure. The other isolated
metabolite (15% of the total) was assigned the dihydroxy-beta-
dihydroheptachlor structure. The third metabolite was not
identified. 1,2-Dichlorochlordene, oxychlordane, and photo-alpha-
chlordane, as well as the parent chlordane compounds, were found in
alfalfa after treatment of the soil with chlordane (Canada,
National Research Council, 1974).
Oxychlordane (or 1,2-dichlorochlordene epoxide) is the common
metabolite derived from both alpha- and gamma-chlordane. It has
been found in the fat of pigs fed either of the isomers and in the
milk and cheese from cows fed alfalfa treated with technical
chlordane. According to some authors, alpha- and gamma-chlordane
give rise to oxychlordane via the intermediate 1,2-dichloro-
chlordene (Canada, National Research Council, 1974).
4. ENVIRONMENTAL LEVELS AND EXPOSURES
4.1. Environmental Levels
4.1.1. Air
Generally, atmospheric concentrations of chlordane appear to be
insignificant. However, chlordane has been detected in the air of
buildings where the compound has been used for termite or other
insect control (US EPA, 1976a,b). Insufficient information is
available on this.
4.1.2. Water
Data from several studies indicate that contamination of water
with chlordane is not a widespread problem (US EPA, 1976a,b), and
that, generally, water residue levels are non-measureable or very
low (Canada, National Research Council, 1974).
In a study of the bottom material of 26 tributary streams in
San Fransisco Bay, chlordane was found to be ubiquitous at
concentrations ranging from a trace to 800 µg/kg (Oloffs et al.,
1978).
No chlordane was detected in 188 samples of surface water from
southern Florida but it was detected in 30% of 214 sediment samples
(Mattraw, 1975). In a study in Hawaii in 1970-71 (Bevenue et
al., 1972b), chlordane was found in drinking-water in 9% of samples
at a mean level of 1.0 ng/litre. Chlordane was detected in non-
potable waters from canals at levels ranging from 3.7 - 9.1
ng/litre. Again, sediments showed much higher levels of 190 - 378
µg/kg. Chlordane was found to occur in the lower Mississippi river
almost continuously throughout 1974 at values ranging from 1.3 to
2.9 ng/litre (Brodtmann, 1976). In a study in the lower
Mississippi (Barthel et al., 1969), during 1964-67, chlordane
residues ranged from 0.80 to 2.80 mg/kg in river bed material
samples. In tributaries of the river, values ranged from 0.56 -
6.44 mg/kg, in 13 out of 348 samples. No chlordane was detected in
any water or sediment samples taken from the upper Great Lakes in
1974 (Glooschenko et al., 1976). In one study in 1976 (Harrington
et al., 1978), chlordane contamination of a municipal water system
was reported, concentrations of chlordane accidently rising to 1.2
g/litre.
4.1.3. Soil
One study has shown that the alpha- and gamma-isomers of
chlordane are less persistent in mineral sand soil than in organic
mucky soil (Harris & Sans, 1976). Data from the National Soils
Monitoring Program in 1970 showed that chlordane occurred in 0.07%
of 1346 sites in 35 States. The range of residues was 0.01 - 13.34
mg/kg dry weight with a mean of 0.08 mg/kg (Crockett et al., 1974).
Monitoring of the corn belt region in the USA (12 States) in 1970
showed chlordane to be one of the most commonly detected
insecticides with values of ND - 0.20 mg/kg (Carey et al., 1973).
Data from 9 States in 1971 detected chlordane in one soil sample at
0.04 mg/kg dry weight (Gowen et al., 1976). Monitoring in 1973
showed residues ranging from 0.001 - 0.020 mg/kg with generally
higher values in urban areas. When urban soils were tested in 14
cities in the USA in 1970, values ranged from 0.01 - 1.27 mg/kg.
In the Atlantic provinces of Canada, chlordane was detected in less
than 10% of agricultural lands at concentrations below 1 mg/kg dry
weight (Duffy & Wong, 1967). In another study on chlordane in
Saskatchewan soils (Saha & Sumner, 1971), 7 out of 41 samples
contained chlordane with residue values ranging from 0.01 to 3.91
mg/kg dry weight. The duration of soil contamination has been
studied by several investigators. Using bioassay techniques, it
was found in one study that 15% of active ingredients remained in
turf soils in Wisconsin after 12 years (Lichtenstein & Poliuka,
1959). In a study in 1970 (Lichtenstein, 1970), it was found that
10 years after application of chlordane at 8.5 kg /ha,
approximately 18 - 20% remained.
4.1.4. Crops and wildlife
Since chlordane residues are present predominantly in the soil,
translocation into plants is an important factor. In most
temperate climates, alpha- and gamma-chlordane are the principal
plant residues. In the Canadian climate, the composition of plant
residues resembles that of technical chlordane (Canada, National
Research Council, 1974). In a field study on soil/plant
relationships, it was found that the relationship between residues
in soil and those in crops was not consistent and consequently not
predictable (Boyd, 1971).
In a three-year study, Onsager et al. (1970) monitored
chlordane residues in sugar beets cultivated on loam soil treated
once at 6 different application rates ranging from 1.4 - 22.4
kg/ha. In the first growing season, only sugar beets treated at
the two lowest rates (1.4 and 2.8 mg/kg) showed residues below 0.3
mg/kg dry weight. In the last two seasons, beets from soil treated
at all rates contained residues below this value. In another
study, uptake by root crops was shown to be related to the soil
type (Stewart, 1975). When chlordane was applied to sandy loam
soil with 12% clay at 15 kg/ha, residues in beets, carrots,
parsnips, potatoes, and rutabagas were 0.03, 0.26, 0.24, 0.04, and
0.01 mg/kg, respectively. In sandy loam containing 28% clay,
values were 0.01, 0.07, 0.12, 0.15, and 0.01 mg/kg, respectively.
A study of residues in alfalfa following applications of high
purity chlordane showed that during the first 4 months following
treatment, oxychlordane and photo-alpha-chlordane accounted for 16%
and 17% of the residues, respectively (Wilson & Oloffs, 1973a).
Generally, no detectable residues of chlordane were found in
wildlife such as birds (Canada, National Research Council, 1974;
Fitzhugh & Fairchild, 1976; Clark & Krynitsky, 1978). However,
in one study (Clark & Prouty, 1976), mean total residues of
oxychlordane ranging from 0.11 to 6.63 µg found in the carcasses
of bats from Maryland and Virginia were attributed to chlordane
use.
In extensive surveys, residues in fish have generally been low.
In 1976, residues in several species from Lake Erie and Lake Saint
Claire in Canada were found to range from non-detectable to 0.046
mg/kg fresh weight (Frank et al., 1978a,b). Residues in Canadian
commercially caught fish in 1970 were not detectable (Reinke et
al., 1972). The National Pesticide Monitoring Program from 1967-68
found chlordane residues in 128 out of 590 fish samples at levels
generally less than 0.5 mg/kg (Wilson & Oloffs, 1973b). From
1972-76, chlordane residues were found in only 3% of the samples of
estuarine fish in the USA (Butler & Schutzmann, 1978). Chlordane
residues were also not detectable in fish and fishery products from
the Northwestern Atlantic (Meith-Avcin et al., 1973; Sims et al.,
1977).
4.1.5. Food
There have been many studies in Canada, Great Britain, the USA,
and other countries on the occurrence of pesticide residues in
food. Generally, the results of these studies showed that residues
of chlordane seldom occur and uptake by man is negligible (Canada,
National Research Council, 1974). Residue tolerances for chlordane
have been established at the following levels: Belgium, Luxembourg,
and the Netherlands, 0.1 mg/kg, Canada, 0.3 mg/kg, European
Economic Community, 0.2 mg/kg and the USA, 0.3 mg/kg. These levels
are for a wide variety of foods (US EPA, 1976a,b). A temporary
acceptable daily intake (ADI) for human beings for the sum of the
alpha- and gamma-isomers of chlordane and oxychlordane of 0 - 0.001
mg/kg body weight was advised by the Joint Meeting on Pesticide
Residues (FAO/WHO, 1983). Chlordane is rarely present in market
basket surveys, and then only at low levels. It is not among the
top 10 chlorinated pesticides usually found as residues in food (US
EPA, 1976a,b). For example, in a survey in the USA from 1963 to
1969, chlordane residues were found in less than 1% of the samples
and ranged from 1 - 5 µg/kg.
It has already been shown that crops will translocate chlordane
residues from the soil. Generally, the amounts in crops are low.
Residues tend to accumulate in the crude oils of oil-seed crops at
levels higher than those in the original seed and in the oil-seed
meal. However, these levels are reduced by refining processes.
Chlordane residues were found in meat, milk, and eggs. Residues in
feed crops or from direct applications to cattle and poultry were
shown to result in significant residues in milk, meat, and eggs (US
EPA, 1976a,b). In a study on eggs in Canada, gamma-chlordane was
found in 78% of the samples with a mean value of 2 µg/kg fresh
weight and alpha-chlordane in 81% of the eggs with a mean value of
1 µg/kg (Mes et al., 1974). In another study (Herrick et al.,
1969), no residues were found in the eggs of chickens fed chlordane
in their diet at 0.08 mg/kg for a week.
In a study on samples of cow's milk analysed in the USA, 87%
were positive for chlordane with levels ranging from 0.02 to 0.06
mg/litre (IARC, 1979). In another study (US EPA, 1976a,b), the
milk of cows grazing on pastures with chlordane applied at 0.55
kg/ha contained an average chlordane concentration of 0.03
mg/litre. No residues were found at lower treatment levels.
Chlordane was also found in Canadian meat samples at levels ranging
from 0 to 106 µg/kg in beef, 0 to 32 µg/kg in pork, and 0 to 70
µg/kg in fowl (Saschenbrecker, 1976).
4.1.6. Human milk
Several studies on pesticide residues in human breast milk did
not reveal any residues of chlordane, but oxychlordane, trans-
nonachlor, and heptachlor epoxide were found, which may be related
to chlordane exposure. In a study on 54 women in Arkansas and
Mississippi from 1973-74, breast milk contained oxychlordane at
0.005 mg/litre, heptachlor epoxide at 0.004 mg/litre, and trans-
nonachlor at 0.001 mg/litre (Strassman & Kutz, 1977). In another
study on 34 samples of breast milk in Northern Mississippi from
1973-75, oxychlordane levels were found of 0.005 mg/litre in high-
pesticide-usage areas and 0.002 mg/litre in low-usage areas
(Barnett et al., 1979). In a survey involving 1436 lactating
mothers in the USA, the mean levels of oxychlordane in the milk
ranged from 75.4 - 116 µg/litre on an adjusted fat basis (Savage,
1976). In a study of Canadian human milk samples in 1974,
oxychlordane was found in 77% of the samples, trans-nonachlor in
68%, and heptachlor epoxide in 69%, each at a mean level of 1
mg/litre whole milk (Mes & Davies, 1978).
Jensen (1983) recently reviewed the levels of chlordane
and oxychlordane in human milk, and his data, including the
above-mentioned studies as well as more recent data, are
reproduced in Table 1.
4.2. General Population Exposure
Oxychlordane was found together with other organochlorine
pesticides in human fat samples at levels ranging from 0.03 to
0.4 mg/kg wet weight (mean 0.14 mg/kg) in residents of the USA
(Biros & Enos, 1973). Sovocool & Lewis (1975) also reported
the identification of oxychlordane in human fat. As indicated
by Biros & Enos (1973), the occurrence of oxychlordane
residues in human adipose tissue in the general population may
reflect previous exposure to chlordane and/or oxychlordane.
This organochlorine compound is included in the human tissue
residue monitoring program (Kutz et al., 1976).
4.3. Occupational Exposure
Permissible levels of exposure to chlordane in the workplace
air have been adopted in different countries (ILO, 1980). Examples
include: 0.5 mg/m3 as a time-weighted average concentration in
Belgium, Finland, Japan, the Netherlands, and the USA (both OSHA
and ACGIH), 0.3 mg/m3 as a time-weighted average and 0.6 mg/m3
as a ceiling concentration in Romania, and 0.01 mg/m3 as maximum
allowable concentration in the USSR.
People primarily exposed are those employed in the application
of chlordane for the control of insects and pests (IARC, 1979).
Chlordane has been found in household dust in the homes of farmers
(mean level 5.79 mg/kg air-dried dust) and pesticide formulators
(mean level 23.11 mg/kg) (Starr et al., 1974).
Table 1. Chlordane and oxychlordane in human milka
------------------------------------------------------------------------------------------------------
Oxychlordane and
chlordane content inb
---------------------
No. of samples Fat Whole milk Milk fat
Area, year (% positive) % (mean) (mg/litre) (mg/kg) References
------------------------------------------------------------------------------------------------------
Canada (1975) 100 2.2 1 - Mes & Davies (1978)
(< 2)
Japan
Tokyo (1978) 11 - 0.5 - Miyazaki et al. (1980)
(0.1 - 1.0)
Tokyo (1979) 12 - 0.5 - Miyazaki et al. (1980)
(0.3 - 1.1)
Mexico (1976) 620 - - 0.40 (median) FAO/WHO (1981)
Spain (1979) 45 (17.8%) - 0.3c 0.026c Lora et al. (1979)
(0 - 0.72)
USA
Arkansas/Mississippi 57 (46%) 3.0 12/10 - Strassman & Kutz (1977);
(1973-4) (0 - 20) FAO/WHO (1981)
------------------------------------------------------------------------------------------------------
Table 1. (contd.)
------------------------------------------------------------------------------------------------------
Oxychlordane and
chlordane content inb
---------------------
No. of samples Fat Whole milk Milk fat
Area, year (% positive) % (mean) (mg/litre) (mg/kg) References
------------------------------------------------------------------------------------------------------
Hawaii (1979-80) 50 (100%) 3.2 - 0.059 Takahashi et al. (1981)
(0.01 - 0.16)
Mississippi (1973-5) 34 (100%) - 5 0.13 Barnett et al. (1979)
(pesticide area) (1 - 22) (0.03 - 0.70)
Mississippi (1973-5) 6 (68%) - 2 0.05 Barnett et al. (1979)
(non-pesticide area) (0 - 4) (0 - 0.12)
USA-NE (1975) 233 - - 0.08 ± 0.05 Savage (1976);
Savage et al. (1981)
USA-SE (1975) 288 - - 0.12 ± 0.15 Savage (1976);
Savage et al. (1981)
USA-MW (1975) 378 - - 0.08 ± 0.05 Savage (1976);
Savage et al. (1981)
USA-SW (1975) 388 - - 0.11 ± 0.35 Savage (1976);
Savage et al. (1981)
USA-NW (1975) 149 - - 0.08 ± 0.05 Savage (1976);
Savage et al. (1981)
USA (total) (1975) 1 436 (74%) - 2 (median) 0.096 ± 0.195 Savage (1976);
(0.013 - 0.57) Savage et al. (1981)
FAO/WHO (1981)
------------------------------------------------------------------------------------------------------
a From: Jensen (1983).
b Results are expressed as means ± S.D. Ranges are listed in parentheses below.
c Chlordane.
5. KINETICS AND METABOLISM
5.1. Absorption
In studies on 4 male rabbits, a combination of 14C-alpha and
gamma-chlordane (approximately 1700 mg of each, administered orally
in 4 doses at 4-day intervals), was well absorbed (Balba & Saha,
1978). Brief exposure of dogs to topically applied chlordane
solutions (3.2 g/litre) resulted in a significant and long-lasting
decrease in the biological half-life of orally-administered
warfarin (Bachmann & Burkman, 1974).
5.2. Distribution and Storage
Studies using radio-labelled chlordane showed that after oral
administration, the radioactivity was well distributed in tissues
of rats (Barnett & Dorough, 1974) and rabbits (Balba & Saha, 1978).
Rats, whether being treated with single oral doses of chlordane or
fed diets containing this compound, retained the highest levels of
residues in adipose tissue, followed by the liver, kidney, brain,
and muscle. More of the gamma-isomers was retained than of the
alpha-isomer. Residues in the fat of rats fed radiolabelled
chlordane (3:1 alpha- and gamma-chlordane) at 1, 5, and 25 mg/kg
diet for 56 days were approximately 3 times higher than those in
the diets. Oxychlordane was the most persistent residue in the
tissues of these rats after chlordane was removed from the diet
(Barnett & Dorough, 1974). The tissue distribution of chlordane in
rabbits was found to be similar to that in rats (Poonawalla &
Korte, 1971; Balba & Saha, 1978).
5.3. Biotransformation
Poonawalla & Korte (1971) showed that 70% of gamma-chlordane
fed to rabbits was excreted in the urine in the form of
metabolities, a.o., gamma-1-hydroxy-2-chlorodihydrochlordene and
1,2-dihydroxydichlordene.
Oxychlordane has been isolated from the fat of dogs, rats
(Polen et al., 1971), pigs (Schwemmer et al., 1970), and cattle
(Lawrence et al., 1970). It has also been isolated from human fat
(Biros & Enos, 1973). Barnett & Dorough (1974) indicated that the
faecal extracts of rats fed 14C-chlordane showed the presence of
eight radioactive areas on the TLC plate. Although the structures
of the metabolites were not fully elucidated, they were tentatively
identified as mono-, di-, and tri-hydroxylated products of
chlordane.
The major route of metabolism for both alpha- and gamma-
chlordane was via dichlorochlordene and oxychlordane (Tashiro &
Matsumura, 1977). The results of these studies were in general
agreement with the proposal of Street & Blau (1972) and the results
of in vitro metabolism studies of Brimfield et al. (1978).
Tashiro & Matsumura (1977) were able to isolate 1-exo-hydroxy-2-
endo-chloro-2,3-exoepoxychlordene, and found another major
metabolic route for alpha-chlordane that involved a more direct
hydroxylation reaction to form 1-exo-hydroxy-dihydrochlordenes and
1,2-gamma-dihydroxydihydrochlordene. Both Brimfield et al. (1978)
and Tashiro & Matsumura (1977) indicated that oxychlordane and, to
a lesser extent, heptachlor were metabolites of chlordane.
However, they did not agree as to whether these two metabolites
would be terminal residues or intermediates in the metabolic
pathways of chlordane. Recent investigations have indicated that
other metabolites were present in the urine of rabbits fed
chlordane. Thus, alpha-chlordane gave rise to 1-hydroxy-2-
chlorochlordene, 1-hydroxychlordene, and gamma-chlordene
chlorhydrin. Administration of the gamma-isomer resulted in
excretion in the urine of the rabbits of 1,2-dichlorochlordene,
1-hydroxy-2-chlorochlordene, gamma-chlordene chlorhydrin, and
3-hydroxychlordane (Balba & Saha, 1978).
In vitro metabolism studies have been summarized by Brimfield
& Street (1979). By incubation of alpha- and gamma-chlordane with
rat liver postmitochondrial supernatant, dichlorchlordene and
oxychlordane were isolated, a result that was similar to those from
in vivo studies. Hart et al. (1963) and Hart & Fouts (1965)
reported that chlordane induced non-specific microsomal enzyme
activity in the rat, resembling, from this point of view,
phenobarbital.
5.4. Elimination and Excretion
Elimination of radiolabelled chlordane (3:1 alpha- and gamma-
chlordane) and the individual isomers was studied in rats. Single
oral doses of 0.05, 0.2, and 1 mg/kg body weight in corn oil were
almost completely eliminated after 7 days; 24 h after administra-
tion, 70% of alpha-chlordane and 60% of the gamma-isomer were
excreted. Female rats excreted more of the dose in the urine than
the males (Barnett & Dorough, 1974).
6. STUDIES ON EXPERIMENTAL ANIMALS
The toxicity and the residue data on chlordane including some
unpublished studies have been reviewed several times by
international bodies such as FAO/WHO (1968, 1973, 1978, 1981,
1983), CEC (1981), and IARC (1979). For their conclusions, refer
to section 9.
6.1. Short-term Exposures
6.1.1. Oral exposure
The acute toxicity of chlordane in several animal species is
shown in Table 2.
The signs associated with acute chlordane poisoning include
ataxia, convulsions, respiratory failure, and cyanosis followed by
death (US EPA, 1976a,b). Correlation between respiratory
difficulty and EEG patterns suggest that respiratory failure is a
contributing factor in chlordane-induced mortality (Hyde &
Falkenberg, 1976). Pathological manifestations include haemorrhage
in the gastrointestinal tract, kidneys, lung, and heart as well as
pulmonary congestion and oedema, and degenerative changes in the
central nervous system (US EPA, 1976a,b).
Seven dogs were given chlordane in single oral doses ranging
from 200 - 700 mg/kg body weight. Convulsions were seen in one dog
at 200 mg/kg (lowest dose) but 700 mg/kg (highest dose) did not
induce any effects (Batte & Turk, 1948). Four groups of 2 - 4 dogs
were given chlordane orally in doses of 5 - 200 mg/kg body weight.
All of the dogs died within 25 days to 93 weeks (Lehman, 1952b).
Chlordane administered by stomach-tube to sheep at 500 mg/kg
body weight induced toxic symptoms (incoordination, partial
blindness). Full recovery occurred in 5 - 6 days. A dose of 1000
mg/kg body weight induced severe respiratory and nervous symptoms
16 h after treatment and death after 48 h (Welch, 1948).
When a diet containing 1000 mg chlordane/kg was fed to 12 male
rats, all of them died within 10 days (Stohlman et al., 1950). At
500 mg/kg all 12 died within 70 days and, at 300 mg/kg, 9 animals
out of 12 were alive after 100 days. Daily oral doses of 6.25 - 25
mg/kg body weight administered to 5 rats for 15 days did not induce
tremors or convulsions, but daily doses of 50 mg/kg body weight
induced toxic symptoms, and 2 of the animals died (Ambrose et al.,
1953). Cytoplasmic bodies in the liver cells were observed in all
groups and were dose-related.
Table 2. Acute toxicity of chlordane in experimental animals
---------------------------------------------------------------------------
Species Sex Route Vehicle LD50 Reference
(mg/kg)
---------------------------------------------------------------------------
Rat F dermal xylene 530 Gaines (1969)
Rat M dermal xylene 205 Gaines (1969a)
Rabbit NS dermal "early" < 780 Ingle (1965b)
chlordane
Rabbit NS dermal "later" 1100 - 1200 Ingle (1965b)
chlordane
(more purified)
Rat M oral peanut oil 335 Gaines (1969)
Rat F oral peanut oil 430 Gaines (1969)
Rat NS oral variety 200 - 590a Ambrose et al. (1953);
Ingle (1965a)
Rat NS oral NS 283 Buck et al. (1973)
Rat NS oral NS 350 Truhaut et al. (1974)
Rabbit NS oral NS 100 - 300a Stohlman et al. (1950)
Rabbit NS oral NS 20 - 40a Ingle (unpublished
data, 1955)
Hamster NS oral NS 1720 Truhaut et al. (1974)
Goat NS oral NS 180 Welch (1948)
Sheep NS oral NS 500 - 1000 Welch (1948)
Chicken NS oral NS 220 - 230 FAO/WHO (1968)
Mallard NS oral NS 1200 Buck et al. (1973)
Cow NS oral NS 25 - 90 Buck et al. (1973)
---------------------------------------------------------------------------
a The wide range is explained by the use of different solvents and the
fact that chlordane produced before 1950 contained a considerable
amount of hexachlorocyclopentadiene.
NS - Not specified.
6.1.2. Dermal exposure
The single-dose dermal LD50 of "early" chlordane in rabbits
was reported to be less than 780 mg/kg body weight (Ingle, 1965b),
and it was noted that this concentration caused severe skin
irritation, tremors, and convulsions (Lehman, 1952a). The dermal
LD50 of the later, more purified chlordane was 1100 - 1200 mg/kg
(Ingle, 1965b).
6.1.3. Parenteral exposure
Male gerbils were dosed intramuscularly with chlordane at 2.5
mg/kg body weight every 3 days for 45 days. Treatment induced
hyperproteinemia, hyperglycemia, and enhanced serum alkaline and
acid phosphatase activities (Karel & Saxena, 1976).
6.2. Long-term Exposures
6.2.1. Oral exposure
Groups of 4 - 7 male and 4 - 7 female dogs were fed dietary
levels of 0, 0.3, 3, 15, or 30 mg chlordane/kg for 2 years.
Abnormalities in the results of clinical liver function tests were
seen in the 15 and 30 mg/kg groups. In animals selected for
necropsy at the end of the first year, increased relative liver
weights and associated hepatocellular changes were found at 30
mg/kg; at the end of two years, dose-related increases in relative
liver weights were found at 15 and 30 mg/kg, with non-dose-related
hepatocellular changes. There was no difference between the
severity of the liver lesions of the 30 mg/kg animals and those of
four animals withdrawn from 30 mg/kg treatment for eight months
prior to sacrifice. Liver biopsies on two animals of the 30 mg/kg
group at 1, 3, and 6 months showed hepatocellular changes at 6
months but not at 1 or 3 months. No adverse effects were seen on
behaviour, appearance, survival, weight gain, blood picture, or the
results of periodic physical examinations, at any level (Wazeter,
1967).
Twenty-four rats, 12 of each sex, were fed dietary levels of
2.5, 25, or 75 mg chlordane/kg for 2 years (Lehman, 1952b). The
two higher levels caused moderate to severe signs of toxicity. The
lowest level caused histological liver changes. Rats were fed
technical chlordane (early production) for two years at levels in
the diet of 0, 5, 10, 30, 150 or 300 mg/kg (Ingle, 1952; 1965a).
Convulsions and tremors were observed in animals receiving 150
mg/kg or more. Hepatocellular alterations consisting of
hypertrophy, cytoplasmic oxyphilia and hyalinization, karyorrhexis,
karyolysis, and cell necrosis were obvious at 150 and 300 mg/kg,
slight at 30 mg/kg, minimal at 10 mg/kg and absent at 5 mg/kg.
Growth was retarded and liver weight and mortality rate increased
at 150 and 300 mg/kg. In a subsequent study on rats (Ingle,
1965a), technical chlordane of later production containing fewer
by-products was administered at levels of 2.5 - 300 mg/kg diet for
2 years. Changes in food consumption, growth, and mortality rate
were seen only at the highest dose. Cellular alterations were seen
at 50 mg/kg and higher.
Rats were fed chlordane at levels ranging from 10 to 1280 mg/kg
diet for 407 days (Ambrose et al., 1953). The animals in the two
highest dose groups died early; liver weight was increased at 320
mg/kg; fatty infiltration and cytoplasmic margination were seen in
the liver parenchymatous cells in males at 40 mg/kg and above, and
in females at 80 mg/kg and above.
Groups, each comprising 20 male and 20 female rats, were fed
dietary levels of 0, 5, 15, 25, or 35 mg/kg of alpha-chlordane, 15,
25, 35, or 75 mg/kg of gamma-chlordane or 5, 15, 25, 35 or 50 mg/kg
of a 1:1 mixture of alpha- and gamma-chlordane (Ingle, 1969). In
the group fed alpha-chlordane, growth retardation became apparent
in the rats fed 35 mg/kg after 4 months in males and after 5 months
in females; with gamma-chlordane, the 75 mg/kg group of males only
displayed growth retardation after 8 months. With the mixture,
growth retardation was evident in both sexes fed 50 mg/kg,
beginning earlier in males than in females. Growth retardation was
not evident in any group fed lower doses of either isomer. Food
consumption bore a relationship to growth. Increased mortality
rates for both sexes became significant in the groups fed alpha-
chlordane at 35 mg/kg, gamma-chlordane at 75 mg/kg, or the alpha-
gamma mixture at 50 mg/kg. Haematocrit was normal for all test
groups. Autopsy did not reveal any gross pathological lesions.
There was no evidence of tumours. Histological examination did not
show any changes from feeding chlordane in any organ except the
liver. Compression of sinusoids due to slight hepatic cell
hypertrophy in the centrolobular region and minimal bile duct
proliferation were evident with administration of alpha-chlordane
at 35 mg/kg. The same changes were noted, but were minimal with
the same isomer at 25 mg/kg. Slight to moderate cytoplasmic
homogeneity of the hepatic cells in the centrolobular region,
minimal cytoplasmic margination, and minimal cell hypertrophy with
compressed sinusoids were noted with administration of gamma-
chlordane at 75 mg/kg. Slight cytoplasmic homogeneity of hepatic
cells in the centrolobular region and occasional cytoplasmic
margination were observed with the alpha-gamma mixture at 50 mg/kg.
The above alterations were minimal with the same mixture at 35
mg/kg. No liver changes were evident after feeding lower levels of
the chlordane isomers.
Groups of 6 female and 6 male rats were fed 2.5 mg or 25 mg of
a sample of technical chlordane containing 60 - 75% chlordane and
25 - 40% unrelated products per kg diet for up to 9 months (Ortega
et al., 1957). Centrolobular cell hypertrophy, cytoplasmic
margination, and cytoplasmic bodies were observed in the liver in 1
male fed 2.5 mg/kg and in 5 males fed 25 mg/kg. No changes were
seen in females.
In a two-year feeding study, pure-bred male and female beagle
dogs were fed chlordane at levels of 0, 0.3, 3.0, 15, or 30 mg/kg
diet. No adverse treatment-related alterations were observed in
behaviour, appearance, eye examination, body weight, food
consumption, haematology, or plasma biochemistry. Some liver
enzyme activities were altered throughout the study at the 15 and
30 mg/kg levels. Relative liver weights were slightly increased
after two years in the two highest groups. Treatment-related
microscopic changes, observed in dogs fed the two highest levels,
consisted of enlargement of centrolobular hepatocytes with
margination of coarse cytoplasmic granules (IRDC, 1967).
The Joint Meeting on Pesticide Residues (JMPR) reviewed the
toxicity data on chlordane at its 1977 meeting (FAO/WHO 1978) and
decided on the following "no-observed-adverse-effect levels":
- rat: 5 mg/kg in the diet, equivalent to 0.25 mg/kg
body weight; and
- dog: 3 mg/kg in the diet, equivalent to 0.075 mg/kg
body weight.
These "no-observed-adverse-effect levels" were confirmed by the
1982 JMPR (FAO/WHO, 1983).
6.2.2. Dermal exposure
When male guinea-pigs were exposed to chlordane at 67 mg/kg
body weight/day, through dermal painting for 90 days, mild
degenerative changes in the skin and testis were evident (Datta et
al., 1975).
The ninety-day repeated daily dose LD50 for rabbits was
reported in the paper by Lehman (1952a) (section 6.1.2) to be from
20 - 40 mg/kg body weight. Ingle (1965b) reviewed the dermal
toxicity of chlordane and attributed the toxicity of early
technical chlordane to the significant content of hexachloro-
cyclopentadiene (HCPD). A more pure product, which did not contain
significant quantities of HCPD, was only half as toxic to rabbits
as the earlier chlordane and did not cause any skin irritation or
damage to mucous membranes.
6.3. Reproduction Studies and Teratogenicity
Rats, maintained from weaning on a diet containing a chlordane
level of 320 mg/kg, showed reduced rates of mating, of viable
litters, and an increased rate of death of progeny prior to
weaning. It was concluded that, at this dosage, chlordane
interfered with both fertility and litter survival (Ambrose et al.,
1953). Groups of 10 male and 20 female rats were used in a 3-
generation study at dietary levels of technical chlordane of 0,
0.3, 3, 15, 30, and 60 mg/kg (Ingle, unpublished data, 1967). Two
litters in each filial generation were studied. Levels up to and
including 30 mg/kg did not have any effect on fertility, number of
offspring, or weight, growth, or mortality rate of the young
animals to weaning age. Autopsy of animals after weaning did not
reveal any gross or microscopic differences between the groups. At
60 mg/kg, there was a high (10.6%) mortality rate in the second F3
generation litters during the latter part of the nursing period;
these animals showed gross and microscopic pathological changes,
comparable with those characteristic for chlordane intoxication.
However, survivors of this generation did not show any tissue
changes at all. A third set of F3 litters at 60 mg/kg suffered 17%
mortality during the nursing period, with symptomatology and gross
and microscopic tissue changes characteristic of chlordane
intoxication. Third F3 generation litters from dams removed from
the 60 mg/kg group and placed on chlordane-free diets for 30 days
prior to remating showed no differences in any respect from control
litters. No evidence of teratogenicity was found in this study.
Hens and cocks fed up to 0.3 mg chlordane/kg diet did not show
any toxic symptoms or any adverse effects on egg weight,
hatchability, or growth of chicks (Biotox, unpublished data, 1969).
Mice fed diets containing chlordane at 25 - 100 mg/kg for 6
generations showed decreased viability in the first and second
generations at 100 mg/kg; in the third generation at this level, no
offspring were produced (Keplinger et al., 1968). At 50 mg/kg,
viability was reduced in the fourth and fifth generations, and at
25 mg/kg no statistically significant effects were observed, even
after 6 generations.
Chlordane was administered to rabbits orally at levels of 1.0,
5.0, and 15 mg/kg body weight per day on the 6th - 18th days of
gestation. A control group and a positive control group were used.
No changes were seen in behaviour, appearance, or body weight.
Miscarriages were seen in 3 rabbits at the 1.0 mg/kg level and one
rabbit at 15 mg/kg dose level. No effects on any of the maternal
or fetal parameters were noted. No teratogenic effects were noted
(IRDC, 1972).
6.4. Mutagenicity
Alpha-Chlordane, gamma-chlordane, and chlordene were tested in
the Ames Salmonella microsome assay and were not mutagenic (Simmon
et al., 1977). Chlordane was not mutagenic when tested using 5
different strains of Salmonella typhimurium in the Ames assay
(Ercegovich & Rashid, 1977). Chlordane was shown to enhance the
number of ouabain-resistant mutants in Chinese hamster V79 cells
and was considered weakly mutagenic (Ahmed et al., 1977b).
Chlordane induced unscheduled DNA synthesis in SV-40 human
cells in culture without activation (Ahmed et al., 1977a). It was
established that chlordane-treated cells did not (for the most
part) re-enter mitosis. They were, instead, arrested somewhere
between the G1 and G2 phases of the cell cycle. Studies involving
DNA synthesis were undertaken to determine more precisely at which
phase (G1, S, G2) the cells are blocked. The data showed that the
treated cells were as competant in DNA replication as the control
cells. In both control and treated cultures, 25 - 30% of total DNA
persisted as light-density material indicating that some of the
pre-existing DNA never engaged in DNA synthesis. Either a large
fraction of cells failed to complete DNA synthesis or 25 - 30% of
the cells did not enter phase S. In any case, treated and control
cells behaved the same in terms of DNA synthesis, indicating that
treatment of the cells with chlordane blocked the cells at the G2
stage of the cell cycle (Brubaker et al., 1970).
Chlordane induced gene conversions in Saccharomyces cerevisiae
strain D4 (Chambers & Dutta, 1976).
Neither alpha-chlordane (42, 58, and 290 mg/kg body weight
single ip doses or 5 daily oral doses of 75 mg/kg body weight) nor
the gamma-isomer (5 daily oral doses of 50 mg/kg body weight) had a
significant effect in a dominant lethal assay on mice (Epstein et
al., 1972). Technical chlordane at dose levels of 50 or 100 mg/kg
body weight in a dominant lethal study using mice failed to induce
any dominant lethal changes (Arnold et al., 1977).
More recent studies on animal and human cells in culture have
shown that chlordane is not mutagenic or is only weakly mutagenic
(Williams, 1979; Maslansky & Williams, 1981; Tong et al., 1981).
Further work by Telang et al. (1982) showed that chlordane was not
mutagenic to an adult rat liver cell line but inhibited cell to
cell communication in a rat liver 6-thioguanine resistant/sensitive
cell line. Telang et al. proposed that chlordane was exhibiting
properties exerted by many promoting agents.
6.5. Carcinogenicity
Epstein (1976) reported a previously unpublished study by the
International Research and Development Corporation, carried out in
1973, in which groups of 100 male and 100 female Charles River CD-1
mice, 6 weeks of age, were fed technical-grade chlordane (purity
not given) at 5, 25, and 50 mg/kg diet, for 18 months. Excluding
10 animals sacrificed from each group for interim study at 6
months, mortality rates at 18 months ranged from 27 - 49%, except
in males and females receiving the 50 mg/kg diet, in which the
mortality rates were 86 and 75%, respectively. A relatively large
number of the deceased animals was lost due to autolysis. A dose-
related increased incidence of liver hyperplastic nodules was
reported in the 25 and 50 mg/kg diet test groups and a dose-related
increased incidence of liver cell hypertrophy was found in all test
groups. A significant incidence of hepatocellular carcinomas
compared with controls was also reported. In the males receiving
chlordane at 0, 5, 25, or 50 mg/kg diet, hepatocellular carcinomas
were found in 3/33, 5/55, 41/52, and 32/39 animals, respectively;
in females, the respective incidences were 0/45, 0/61, 32/50, and
26/37.
Groups of 50 male and 50 female B6C3F1 hybrid mice, 5 weeks of
age, were fed analytical-grade chlordane, consisting of 94.8%
chlordane (71.7% alpha-chlordane and 23.1% gamma-chlordane), 0.3%
heptachlor, 0.6% nonachlor, 1.1% hexachlorocyclopentadiene, 0.25%
chlordene isomers, and other chlorinated compounds for 80 weeks
(NCI, 1977). Males received initial levels of 20 or 40 mg/kg diet,
and females 40 and 80 mg/kg diet; time-weighted average dietary
concentrations were 30 and 56 mg/kg for males and 30 and 64 mg/kg
diet for females. There were 20 male and 10 female matched
controls and 100 male and 80 female pooled controls. Survival in
all groups was relatively high, i.e., over 60% in treated males,
over 80% in treated females, and over 90% in male and female
controls. A dose-related increase in the incidence of
hepatocellular carcinomas was found in males and females. The
incidences were 43/49 and 34/49 in high-dose males and females,
respectively, and 16/48 and 3/47 in low-dose males and females,
respectively, compared with 2/18 and 0/19 in male and female
matched controls, respectively.
Groups of 50 male and 50 female, 5-week-old Osborne-Mendel rats
were given analytical-grade chlordane in the diet for 80 weeks, at
initial levels of 400 and 800 mg/kg for males and 200 and 400 mg/kg
for females (NCI, 1977). The levels had to be reduced during the
study because of adverse toxic effects; the time-weighted average
dietary concentrations were 407 and 203 mg/kg for males and 241 and
121 mg/kg for females. There were 10 male and 10 female matched
controls and 60 male and 60 female pooled controls. Survivors were
killed at 80 weeks, at which time approximately 50% of treated and
control males and 60% of treated females and 90% of control females
were still alive. In all treated animals combined, there was an
excess incidence of follicular-cell thyroid neoplasms (10/75 in
treated females and 7/65 in treated males versus 0/10, 3/58, 0/6,
and 4/51 in matched and pooled female and male controls); there was
an excess of malignant fibrous histiocytomas in the treated male
groups (8/88 versus 0/8 and 2/58 in matched and pooled male
controls).
A committee of the National Academy of Sciences (NAS) in the
USA was asked to review all available carcinogenicity data on
chlordane as part of the cancellation hearings. Chlordane was not
found to be carcinogenic in rats and the only target organ site for
carcinogenic response in certain strains of mice was the liver.
The committee concluded that "there are no adequate data to show
that these compounds are carcinogenic in humans, but because of
their carcinogenicity in certain mouse strains and the extensive
similarity of the carcinogenic action of chemicals in animals and
in humans, the committee concluded that chlordane, heptachlor
and/or their metabolites may be carcinogenic in humans. Although
the magnitude of risk is greater than if no carcinogenicity had
been found in certain mouse strains, in the opinion of the
committee the magnitude of risk cannot be reliably estimated
because of the uncertainties in the available data and in the
extrapolation of carcinogenicity data from laboratory animals to
humans" (US NAS, 1977).
IARC (1979), in its evaluation of the carcinogenic risk of
chlordane, concluded: "There is sufficient evidence that chlordane
is carcinogenic in mice." In 1982, another IARC Working Group
reviewed existing data on chlordane and concluded that there was
limited evidence for the carcinogenicity of chlordane for
experimental animals (IARC, 1982). The group of Williams (Telang
et al., 1982) suggested that chlordane had the properties of many
promoting agents.
6.6. Behavioural Studies
Offspring of chlordane-treated mice (1 or 2.5 mg/kg body weight
for 7 consecutive days) made fewer conditioned avoidance responses
than controls (Al-Hachim & Al-Baker, 1973). In addition, progeny
of mothers receiving the higher dose were more active than the
controls.
6.7. Other Studies
Chlordane induces hepatic mixed-function oxidase enzymes in
rats (Fouts, 1963; Hart et al., 1963; Hart & Fouts, 1965;
Villeneuve et al., 1972; den Tonkelaar et al., 1974; Madhukar &
Matsumura, 1979) and enhances estrone metabolism in rats and mice
(Welch et al., 1971). Chlordane has been shown to inhibit skin 7-
ethoxycoumarin de-ethylase activity (7-EC) (EC 1.14.13) in mice at
doses which induced hepatic 7-EC activity (Pohl & Fouts, 1977).
Several studies were carried out in which rats were fed chlordane
at levels of 2, 5, 10, 20, or 50 mg/kg diet during two weeks (den
Tonkelaar et al., 1974). At the end of this period, the liver
microsomal enzymes hexobarbital oxidase (EC 1.1), aminopyrine
demethylase (EC 1.5.3), and aniline hydroxylase (EC 1.14.14) were
determined. A no-observed-adverse-effect level of 5 mg/kg was
found for chlordane. Chlordane inhibition of rat brain ATPase
activity (Folmar, 1978) and bovine carbonic anhydrase (EC 4.2.1.1)
(Maguire & Watkin, 1975) has been demonstrated in in vitro
systems. The in vivo inhibition of rat brain ATPase has also
been reported (Drummond et al., 1980).
Three female and 3 male baboons were fed atherogenic diets to
which chlordane was added at 0.1 or 1 mg/kg body weight per day for
two years. At the higher dose level, chlordane increased
cytochrome P-450 activity significantly, otherwise no adverse
effects on general health or on any major organ systems were found
(McGill, 1979).
Acute intramuscular doses (50 mg/kg body weight) of chlordane
have been shown to increase the alkaline phosphatase (EC 3.1.3.1)
activity in gerbils temporarily (Karel, 1976) and stimulate
gluconeogenetic enzymes in the liver and kidney cortex of rats
(Kacew & Singhal, 1973a). Oral administration of 200 mg alpha-
chlordane/kg body weight to mature Wistar rats also induced
elevated serum levels of glucose and urea with a concomitant
decrease in liver glycogen at sacrifice, 1 h later (Kacew &
Singhal, 1973b). The chlordane-induced alterations have been
attributed to the enhanced ability of these organs to synthesize
cyclic AMP (Kacew & Singhal, 1973b, 1974; Singhal & Kacew, 1973,
1976; Kraybill, 1977).
Rats were administered chlordane ip daily for 42 days at levels
of 0.15, 1.75, or 25.0 mg/kg body weight. Results revealed "dose-
dependent alterations of brain potentials without behavioural signs
of chronic toxicity" (Hyde & Falkenberg, 1976, Hyde et al., 1978).
Chlordane has also been shown to influence brain biogenic amines
including acetylcholine (Hrdina et al., 1973).
Prenatal exposure to 0.16 mg chlordane/kg body weight, in
peanut butter, on each day of gestation, resulted in increased
plasma corticosterone levels in adult male mice (Cranmer et al.,
1978). When neonatal mice were treated with 0.075 mg alpha- or
gamma-chlordane on days 2 - 4 after birth, growth rates were
depressed and eye and vaginal opening were delayed (Talamantes &
Jang, 1977).
Female Balb C mice were mated and treated with chlordane at
0.16 or 8 mg/kg body weight throughout gestation. Decreased cell-
mediated immune competence was found in offspring of high-dose-
treated females, at 101 days of age, challenged with oxazolone
(Spyker-Cranmer et al., 1982).
Chlordane added to the medium at a concentration of
1 mg/kg inhibited the growth of Streptococcus viridans in
vitro by more than 50%. Total growth inhibition occurred at a
chlordane concentration of 3 mg/kg (Goes et al., 1978).
6.8. Factors Influencing Toxicity
Metabolites
The acute oral toxicity of chlordane isomers and their
metabolites is summarized in Table 3.
Table 3. Toxicity of chlordane isomers and metabolites
-----------------------------------------------------------------------
Compound Species LD50 (mg/kg Reference
(sex) body weight)
-----------------------------------------------------------------------
alpha-chlordane rat (M) 392 Wazeter et al. (1968)
gamma-chlordane rat (M) 327 Wazeter et al. (1968)
alpha + gamma- rat (M) 371 Wazeter et al. (1968)
chlordane (1:1 ratio)
oxychlordane rat (M,F) 19.1 Mastri et al. (1969a)
chlordene rat (M,F) over 4600 Mastri et al. (1969b)
3-chlorchlordene rat (M,F) over 4600 Mastri et al. (1969b)
1-hydroxychlordene rat (M,F) over 4600 Mastri et al. (1969b)
chlordene epoxide rat (M,F) over 4600 Mastri et al. (1969c)
1-hydroxy, 2,3-epoxy rat (F) over 4600 Mastri et al. (1969c)
chlordene
2-chlorchlordene rat (F) over 10 200 Mastri et al. (1969c)
-----------------------------------------------------------------------
Only oxychlordane was more toxic than the parent compounds.
Two male and 2 female rats were given the chlordane metabolite
oxychlordane in their diet, at a level of 2.0 mg/kg body weight
(Plank et al., 1970). Following 90 days feeding, the surviving
animals were sacrificed. Body-weight gain, food consumption,
behaviour, mortality rate, organ weights and organ to body weight
ratios, and the results of haematological and blood chemistry tests
and urological studies were considered to be within the normal
range for the strain of rat used. No gross pathological
abnormalities were evident, and no histopathological lesions could
be attributed to oxychlordane.
Groups of 25 male and 25 female rats were fed dietary levels of
1-hydroxychlordene of 0, 100, 250, 500, 1000, and 2000 mg/kg for up
to 224 days (Ingle, 1965a). After 110 days, 3 females from each
feeding level were mated with males at all levels. The mortality
rate in all groups was low, and no statistically significant
differences existed. No gross abnormalities were revealed at
autopsy after 224 days. The histopathological study of the
visceral organs did not show any pathological effects. Slight to
moderate hyperplasia of the smooth endoplasmatic reticulum and
cytoplasmic margination of a few liver cells were noted at the 1000
and 2000 mg/kg levels.
Interactions
Chlordane has been shown to exert a protective effect against
several organophosphorous and carbamate insecticides (Williams et
al., 1967; Street et al., 1969; Williams & Casterline, 1970; US
EPA, 1976a,b).
Protein deficiency has been shown to double the acute toxicity
of chlordane in rats (Boyd, 1972).
Chlordane has also been shown to increase the hepatotoxic
effects of carbon tetrachloride in the rat (Stenger et al., 1975;
Mahon, 1977; Mahon & Oloffs, 1979; Mahon et al., 1979).
7. EFFECTS ON MAN: EPIDEMIOLOGICAL AND CLINICAL STUDIES
7.1. Poisoning Incidents
A 15-month-old girl ingested a mouthful of chlordane suspension
and, after 3 h, displayed tremors and incoordination (Lensky &
Evans, 1952). Repeated seizures developed and she was treated with
ethyl chloride, amobarbital, and gastric lavage with magnesium
sulfate. The child recovered completely and ataxia and
excitability disappeared after 2 - 3 weeks. At 26 years of age,
she was in excellent health and appeared not to suffer any
consequences from the childhood episode (Taylor et al., 1979).
A 2-year-old child had drunk an unknown amount of a 74%
formulation of chlordane (Curley & Garrettson, 1969). Vomiting
preceeded convulsions, which were controlled by phenobarbital; the
EEG pattern was normal within 40 h and the child recovered.
A similar poisoning incident was observed with a 4-year-old
child (Aldrich & Holmes, 1969). Convulsions were treated with
phenobarbital. As with the previous case, the individual
recovered.
Two other cases of chlordane poisoning were reported in 1955
(Derbes et al., 1955). One was caused by the absorption of
accidentally-spilled chlordane and the other was a suicide attempt
where the individual (female) swallowed 6 g of chlordane (104 mg/kg
body weight) and died 9 1/2 days after the incident (Derbes et al.,
1955).
When a section of a municipal water system in Chattanooga,
Tennessee, USA was contaminated with chlordane in concentrations of
up to 1.2 g/litre in 1976, 13 persons showed gastrointestinal
and/or neurological symptoms (Harrington et al., 1978).
7.2. Occupational and Epidemiological Studies
No deleterious effects associated with occupational exposure to
chlordane have been reported. Twenty-two men, who had been
occupationally exposed to chlordane during its manufacture for
periods of 1 - 3 years, did not show any evidence of intoxication
(Princi & Spurbeck, 1951). Other clinical studies have been
reported on men engaged in the manufacture of chlordane (Alvarez &
Hyman, 1953; Fishbein et al., 1964; Morgan & Roan, 1969).
Infante et al. (1978) reviewed 25 previously reported cases of
blood dyscrasia together with a small number of newly identified
cases of aplastic anaemia, leukaemia, or neuroblastoma in children
in relation to their possible association with pre- and post-natal
chlordane or heptachlor exposure and reported an anecdotal
relationship. However, in a case-control study, no association was
found between blood dyscrasias and occupational exposure to a
number of pesticides including chlordane (Wang & Gruffenman, 1981).
Wang & MacMahon (1979 a,b) studied a cohort of workers engaged
in the manufacture of chlordane, heptachlor, and endrin and another
cohort of 16 000 pesticide-spraying personnel, including termite-
control workers. Both studies showed a deficit of deaths from all
cancers and slight excesses of lung, skin, or bladder cancer that
were not statistically significant.
In 1982, an IARC Working Group concluded that the above studies
were inadequate to evaluate the carcinogenicity of chlordane for
human beings (IARC, 1982).
Shindell & Associates (1981) studied the mortality experience
of 783 workers engaged in the manufacture of chlordane and
heptachlor. Workers had been employed for a minimum of 3 months 5,
10, 15, or 20 years ago. SMRs for cancer were not increased among
124 deaths.
In a retrospective cohort study of workers involved in the
production of chlorinated hydrocarbon pesticides, Ditraglia et al.
(1981) studied the workers in a chlordane-manufacturing plant; the
same workers were also studied by Wang & MacMahon (1979a). SMRs
for all cancer deaths were lower than expected; a slight excess of
stomach cancer (3 vs 0.99 expected), which was observed, was not
statistically significant. The number of workers studied was small
and further follow-up of the cohort was recommended by the authors.
MacMahon & Wang (1982) carried out a second follow-up study of
mortality rates in a cohort of workers employed in spraying
pesticides, including termite-control workers. Among 540 deaths
for which the cause was ascertainable, small excesses of bladder
cancer in termite-control operators and of skin and lung cancer in
other operators were observed, but these were not statistically
significant.
7.3. Treatment of Poisoning
In case of overexposure, medical advice should be sought
immediately.
Treatment before person is seen by a physician
The person should stop work immediately, remove contaminated
clothing and wash the affected skin with soap and water, if
available, flushing the area with large quantities of water. If
swallowed, vomiting should be induced, if the person is conscious
(FAO/WHO, 1978).
Medical Treatment
If the pesticide has been ingested, gastric lavage should be
Performed with 2 - 4 litres of water, followed by saline
purgatives. Barbiturates (preferably phenobarbitone or
pentobarbitone) or diazepam should be given intramuscularly or
intravenously in sufficient dosage to control restlessness or
convulsions. Mechanical respiratory assistance with oxygen may be
required. Calcium gluconate, 10% in 10 ml, injected
intramuscularly at 4-h intervals, may be helpful. Contraindicated
are oily purgatives, epinephrine, and other adrenergic drugs and
central stimulants of all types (FAO/WHO, 1978).
8. EFFECTS ON THE ENVIRONMENT
8.1. Toxicity for Aquatic Organisms
Data on the toxicity of chlordane for aquatic organisms are
given in Table 4. A more comprehensive table, listing different
conditions and exposure times is available on request from IRPTC,
Geneva, Switzerland. It is of importance for the interpretation of
these data to note that a change in the purity of technical of
chlordane occurred in the early 1950s.
Studies of the effects of chlordane on fish began with the
application of the original material to rainbow trout by Cope et
al. (1947). They determined minimum disabling 24-h doses of
chlordane in a xylene emulsion, an acetone solution, a fuel oil
solution, and a Velsicol AR-50 solution, and found this to be
higher than 6 mg chlordane/litre. An application of 1.12 kg/ha of
a field formulation of chlordane to a small pond killed 87% of
bluegills present (Surber, 1948), application of 0.56 kg/ha killed
some bluegills whilst at 0.28 kg/ha all fish survived (Linduska &
Surber, 1948). In a study by Lawrence (1950) on bluegill
fingerlings, large-mouth black bass fingerlings, and juvenile
goldfish in aquaria, no deaths occurred at 100 µg chlordane/litre
(original formulation), whilst at 200 µg/litre, a 30-h exposure
killed bass, and an 87-h exposure killed bluegills; goldfish were
not affected. In earthen ponds, large-mouth black bass fingerlings
were killed by a concentration of 200 µg/litre, but bluegills and
fingerlings and juvenile goldfish survived.
Studies using the current formulation of chlordane are
summarised in Table 4. A definite temperature effect demonstrated
by Macek et al. (1969) during acute exposures, i.e., fish showed a
greater susceptibility at higher temperatures, was not present in
96-h exposure studies. Temperature effects were also noted in
toxicity tests on tubificid worms, Branchuria sowerbyi (Naqvi,
1973). In static tests, 500 µg chlordane/litre caused 100%
mortality at 4.4° and 32 °C, but no mortality at 21 °C. Nutrition
has been shown to affect chlordane toxicity in rainbow trout, with
96-h LC50s ranging from 8.2 to 47 µg/litre, depending on the
composition of the diet given to the fish (Merhle et al., 1974).
Recent in vitro studies on bluegills (Koch et al., 1971)
and on rainbow trout (Davis et al., 1972) have shown that chlordane
acts as an inhibitor of ATPase systems.
8.2. Toxicity for Terrestrial Organisms
Studies of the effects of chlordane on soil microfauna have
been limited to work on nematodes. Populations of plant-feeding
nematodes were reduced by an insecticide mixture containing
chlordane (as well as DDT, diazinon, and zinophos) in July,
following application in March, but at no other time during the
study period (Corbett & Webb, 1968). Nematodes are generally
unaffected by most soil insecticides (Edwards, 1965a).
Table 4. Toxicity of chlordane for aquatic organisms
---------------------------------------------------------------------------------------------------------
Organism Age/ Temp pH Flow/ Grade Hard- alk sal End Para- Concen- Reference
size (° C) stat ness (mg/ o/oo point meter tration
(mg/ litre) (µg/
litre) litre)
---------------------------------------------------------------------------------------------------------
Eastern oyster 29-53 31.6 flow tech 27.3 % reduc- 96-h 6.2 Parrish et
(Crassostrea mm tion shell EC50 al. (1976)
virginica) deposition
Annelid stat sea 288-h 220 McLeese et
(Nereis virens) water LC50 al. (1982)
Scud 5 mm 16.7 7.9 flow tech 148 152 immobili- 168-h 97.1 Cardwell et
(Hyalella juv. sation EC50 al. (1977)
azteca)
Cladoceran 1st 15.5 7.4- stat immobili- 48-h 29 Sanders &
( Daphnia pulex) instar 7.8 sation EC50 Cope (1966)
Pink shrimp 50-65 28.4 flow tech 21.8 96-h 0.4 Parrish et
(Penaeus mm LC50 al. (1976)
duorarum)
Dungeness crab adult 13 stat tech 25 96-h 220 Caldwell
(Cancer LC50 (1977)
magister) zoeal 13 stat tech 25 immobili- 96-h 1.3
sation EC50
Backswimmer 5-6 mm 18-24 stat 25% EM 168-h 0.79 Konar (1968)
(Notonecta) sp LC50
Water scorpion 24-28 18-24 stat 25% EM 168-h 182 Konar (1968)
(Nepa) sp mm LC50
Bluegill 38-84 25 7.1 flow 100% 20 18 96-h 22 Henderson et
(Lepomis mm LC50 al. (1959)
macrochirus)
---------------------------------------------------------------------------------------------------------
Table 4. (contd.)
---------------------------------------------------------------------------------------------------------
Organism Age/ Temp pH Flow/ Grade Hard- alk sal End Para- Concen- Reference
size (° C) stat ness (mg/ o/oo point meter tration
(mg/ litre) (µg/
litre) litre)
---------------------------------------------------------------------------------------------------------
Fathead minnow 1 day 21/25 7.7 flow tech 156 166 major 11-mo Cardwell et.
(Pimephales chronic lowest al. (1977)
promelas) effects dose
life
cycle
tests
38-84 25 7.1 flow 100% 20 18 96-h 52 Henderson et
mm LC50 al. (1959)
38-84 25 7.1 flow 75% 20 18 96-h 170 Henderson et
mm EM LC50 al. (1959)
Rainbow trout 0.9 g 13 tech 96-h 7.8 Cope (1965)
(Salmo gairdneri) LC50
Murrel 24-26 18-24 stat 25% EM 168-h 0.51 Konar (1968)
(Channa mm LC50
punctatus)
48-50 18-24 stat 25% EM 168-h 3 Konar (1968)
mm LC50
100- 18-24 stat 25% EM 168-h 25.5 Konar (1968)
105 mm LC50
Tropical fish juv. 18-24 stat 25% EM 168-h 0.7- Konar (1968)
LC50 3.7
Channel catfish finger 25 stat tech 96-h 500 Clemens &
(Ictalurus ling LC50 Sneed (1959)
punctatus)
Common toad tad- 18-20 stat 48-h 2000 Ludemann &
(Bufo bufo) pole LC50 Neumann
(1962)
---------------------------------------------------------------------------------------------------------
Soil fauna populations (mainly arthropods with a small
percentage of earthworms and nematodes) were reduced to very low
levels by the normal application rate (112 kg/ha per year) of a
commercial formulation of chlordane (Gould & Hampstead, 1951).
However, Long et al. (1967) did not find any significant reductions
in most soil arthropod populations following application of
technical chlordane to sugar cane at 2.24 kg/ha, although numbers
of Diplura and Pauropoda were reduced. In a summary of the effects
of pesticides on soil invertebrates, Edwards (1965b) stated that
chlordane caused a large reduction in the numbers of Coleoptera,
Diptera, hemiedaphic Collembola, and non-predatory mites, but
little reduction in the numbers of edaphic Collembola or predatory
mites at application rates of 1.12 - 2.24 kg/ha. He also stated
that chlordane was lethal for fly and beetle larvae. Fox (1958)
produced a few data indicating that carabid and staphylinid beetle
populations returned to normal 3 years after a field application
of chlordane (as a 40% wettable powder) at 8.96 or 11.2 kg/ha.
Chlordane is toxic both to earthworms and to enchytraeid worms
at application rates well within the range of normal usage (Hopkins
& Kirk, 1957; Doane, 1962). The toxicity of chlordane for earth-
worms is given in Table 5. Legg (1968) made a single application
of 25% EC chlordane at 9.0, 13.4, or 20.2 kg/ha on closely-mown
turf and counted worm casts for up to 13 months. After 19 days,
there were reductions of 52, 72, and 98%, respectively, for the 3
doses compared with control plots; after 13 months, the reductions
were 89, 95, and 97%, respectively. Long et al. (1967) reported a
significant reduction in earthworm populations, 6 - 11 months after
an application of chlordane at 2.2 kg/ha. A reduction in worm
casts to zero, 1 year after an application of chlordane at 11.2
kg/ha, either as granules or in spray formulation was shown by
Doane (1962). Lidgate (1966) applied chlordane, either as 20%
granules or 75% EC diluted with water, at rates between 13.4 and
35.2 kg/ha, to a putting green and measured worm activity by the
number of worm casts. The first count of casts, 18 days after
treatment, showed that worm activity was significantly depressed by
granular applications of 17.6 and 35.2 kg/ha but not by spray
formulations of 13.4 or 26.4 kg/ha. Activity became depressed on
sprayed plots about 8 weeks after treatment. There were still
significantly fewer worm casts on treated plots, 5 years later.
Table 5. Toxicity of chlordane for earthworms
-----------------------------------------------------------------------------------------
Organism Grade Application Concen- Effect Reference
method tration
(kg/ha)
-----------------------------------------------------------------------------------------
Earthworm 25% EC spray 9 52% reduction in worm Legg (1968)
(Lumbricus casts at 19 days
terrestris)
25% EC spray 13.4 72% reduction in worm Legg (1968)
casts at 19 days
25% EC spray 20.2 98% reduction in worm Legg (1968)
casts at 19 days
25% EC spray 9 89% reduction in worm Legg (1968)
casts at 1 year
25% EC spray 13.4 95% reduction in worm Legg (1968)
casts at 1 year
25% EC spray 20.2 97% reduction in worm Legg (1968)
casts at 1 year
Red earth- 5% dust soil 35 0% mortality in Hopkins & Kirk (1957)
worm incorpor- 4 days
(Eisenia ation
sp)
5% dust soil 70 46% mortality in Hopkins & Kirk (1957)
incorpor- 4 days
ation
5% dust soil 141 40% mortality in Hopkins & Kirk (1957)
incorpor- 4 days
ation
5% dust soil 282 79% mortality in Hopkins & Kirk (1957)
incorpor- 4 days
ation
5% dust soil 100 96-h LC50 Hopkins & Kirk (1957)
incorpor-
ation
-----------------------------------------------------------------------------------------
The toxicity of chlordane for birds, when given in the diet, is
summarised in Table 6. LC50 values as mg/kg diet ranged from 170
to 858 in studies where chlordane was given for between 5 days and
100 weeks. When chlordane was applied to marshland at 1.12 kg/ha,
the fecundity of marsh birds was affected (Hanson, 1952); blue-
winged teal and shovelers did not produce any young, and coot and
redwinged-blackbirds produced 60% fewer young. It was postulated
that chlordane had caused disruption in food cycles in the marsh
and that this was the probable cause of reproductive failure in the
birds.
8.3. Toxicity for Microorganisms
Some effects of chlordane on microorganisms are summarised in
Table 7. Its effects may be due, at least in part, to inhibition
of enzyme activity (Nakas, 1977). Some work has been reported on
the effects of chlordane on soil microorganisms. Gram-positive
bacteria appear to be more sensitive to chlordane than gram-
negative bacteria, since the growth of gram-positives was inhibited
whilst that of gram-negatives was unaffected (Trudgill et al.,
1971). When Bacillus subtilis cultures were treated with technical
chlordane at 20 mg/litre, they ceased to grow. Viable count and
respiration rate fell to zero after about 3 h of exposure. The
actual concentration experienced by the bacteria is not known, but
it is likely to be less than the 20 mg/litre added because of the
poor solubility of chlordane in aqueous solution. Langlois & Sides
(1972) investigated the effects of constituents of technical
chlordane on the growth of Staphylococcus aureus. The viability
of the culture, the length of the lag-phase, and the generation
time were affected by the amount of chlordane and gamma-chlordane
applied.
8.4. Bioaccumulation and Biomagnification
Grimes & Morrison (1975) examined the uptake of chlordane by 13
types of bacteria and found that although the uptake of alpha- and
beta-isomers of chlordane was the same for any one species, the
concentration factors (CF) differed greatly between species. The
CFs ranged from a few hundred to several thousand, with 3 species
giving much higher values. The highest CF was 53 000 for
Caulobacter vibrioides. Caulobacter cells were found to contain 4
distinct lipid-containing materials, and this was offered as an
explanation of the high CF. Sanborn et al. (1976) used unlabelled
chlordane and labelled 14C-chlordane on filamentous Oedogonium
alga and obtained CFs of 49 500 and 98 386. The lower figure may
be due to uncertainties in determining chlordane in solution and in
the alga. Moore et al. (1977), using the planktonic alga
Ankistrodesmus amalloides, obtained a very much lower CF of 5560,
but even this species shows accumulation potential.
Table 6. Toxicity of chlordane for birds
--------------------------------------------------------------------------------
Species Age Route Parameter Concen- Reference
trationa
(mg/kg)
--------------------------------------------------------------------------------
Mallard 4 - 5 mo oral acute LD50 1200 Tucker & Crabtree (1970)
10 days diet 5-day LC50b 858 Hill et al. (1975)
Bobwhite 14 weeks diet 10-week LC0 10 Ludke (1976)
quail 17 days diet 5-day LC50b 331 Hill et al. (1975)
young diet 100-day LC50 100 DeWitt et al. (1963)
young diet 10-day LC50 250 DeWitt et al. (1963)
adult diet 100-day LC50 250 DeWitt et al. (1963)
Japanese 7 days diet 5-day LC50b 350 Hill et al. (1975)
quail
Ring-necked 15 days diet 5-day LC50b 430 Hill et al. (1975)
pheasant young diet 10-day LC50 500 DeWitt et al. (1963)
young diet 100-day LC50 50 DeWitt et al. (1963)
adult diet 100-day LC50 200 DeWitt et al. (1963)
Cowbird adult diet 30-week LC50 500 DeWitt et al. (1963)
--------------------------------------------------------------------------------
a Concentration in mg/kg body weight for oral dosing; concentration in mg/kg
diet for dietary dosing.
b 5 days of treated diet followed by 3 days of clean diet. Mortality rate
determined on day 8.
Table 7. Toxicity of chlordane for microorganisms
----------------------------------------------------------------------------------------
Organism F/M/ Temp Grade Solvent Endpoint Time Concen- Reference
S (° C) tration
(µg/
litre)a
----------------------------------------------------------------------------------------
Scenedesmus F 23 tech acetone stimulation of 5 - 7 0.1- Glooschenko &
quadricauda cell division days 100 Lott (1977)
F 23 tech acetone inhibition of 1 - 5 50 & Glooschenko &
photosynthesis days 100 Lott (1977)
Chlamydomonas S 23 tech acetone stimulation of 7 - 11 0.1- Glooschenko &
sp cell division days 50 Lott (1977)
S 23 tech acetone stimulation of 3 - 4 0.1- Glooschenko &
respiration h 100 Lott (1977)
S 23 tech acetone inhibition of 7 - 11 100 Glooschenko &
cell division days Lott (1977)
F 23- 60% acetone 50% reduction 3 days 100 000 Clegg &
25 ATPase Koevenig
activity; (1974)
no effect on
cell density
Volvox sp F 18- 20% none 100% mortality 7 days 1 Konar (1968)
Pandorina sp 24 EM
Closterium sp
Chlorella F 23- 60% acetone reduced ATPase 3 days 100 000 Clegg &
ellipsoidea 25 levels; Koevenig
Euglena no effect on (1974)
elastica cell density
Exuviella M 60% methanol virtual cessa- 7 days 50 Magnani et
baltica tion of growth al. (1978)
Natural M 60% acetone 94% decrease 4 h 1000 Butler (1963)
estuarine in product-
phytoplankton ivity
Estuarine M 7-14 60% methanol no effect 5 days 5 Biggs et al.
phytoplankton M 7-14 60% methanol growth and 14C 5 days 10 (1978)
uptake reduced
in laboratory
Bacillus S tech acetone growth ceased; 3 h 20 000 Trudgill et
subtilis decline in al. (1971)
viable count;
respiration
to zero
----------------------------------------------------------------------------------------
a Solubility of chlordane: 6 - 9 µg/litre. F = freshwater; M = marine; S = soil.
Accumulation of alpha- and and gamma-isomers of chlordane and
nonachlor, and chlordenes was studied by Cardwell et al. (1977) in
3 species of freshwater invertebrates. Chironomus larvae did not
show any detectable accumulation. After one week's exposure to 1.7 -
21.6 µg/litre, Daphnia magna gave CFs ranging from 15 000 to
175 000. After 65 days of exposure to 1.4 - 11.5 µ/litre, Hyallela
azteca showed CFs ranging from 41 000 to 144 000. After a 24-h
exposure to 0.5 µg alpha- and gamma-chlordane/litre, Daphnia pulex
gave a CF of 24 000 (Moore et al., 1977). Sanborn et al. (1976)
found a CF of 6132 for insect larvae. A fresh-water gastropod
Physa sp., concentrated chlordane 132 613 times (Sanborn et al.,
1976). Little bioaccumulation has been observed in the marine
invertebrate species studied. Wilson (1965) exposed oysters to
0.01 mg chlordane/litre and determined a CF of 7300, which is
considerably lower than that for fresh-water gastropods. Water and
oyster samples from Galveston Bay, Texas were analysed following an
extensive mosquito-control programme (Casper, 1967). Oysters
sampled did not contain any detectable chlordane and only 2 out of
9 water samples gave a positive result of less than 0.001 µg/litre.
Chlordane was detected by Bugg et al. (1967) in 20 out of 133
oyster samples taken from the South Atlantic and the Gulf of
Mexico, but 19 of these gave values of less than 0.01 mg/kg drained
weight. Clams, which were living in water containing chlordane at
0.01 µg/litre for 106 days, showed CFs of 1000 or less (Godsil &
Johnson, 1968). Parrish et al. (1976) reported that chlordane was
concentrated in the tissues of the estuarine pink and grass shrimps
at 1000 - 2300 times levels measured in water.
Few data are available on soil invertebrates. One report on
earthworms has been published by Gish (1970), who measured the
levels of gamma-chlordane in the soil and earthworms. CFs of 0.37,
7.1, 10.6, and 152 were obtained for 4 worms in agricultural soils.
Several studies are available on fresh-water fish. Henderson et
al. (1969) found that fish from Atlantic-coast streams, which gave
positive samples, contained between 0.1 and 7.29 mg chlordane/kg
(whole fish wet weight), fish from the Great Lake drainage areas
contained between 0.01 and 0.39 mg/kg, and fish from the
Mississippi River system contained between 0.01 and 0.72 mg/kg.
Fish from other systems (Hudson Bay, Colorado River, Interior
basins, Californian streams, Columbia River, Pacific coast and
Alaskan streams) contained less than 0.01 mg/kg. A further study
by Henderson et al. (1971) yielded 16 positive samples out of 666
fish taken from 50 sites, giving chlordane levels of between 0.09
and 13.5 mg/kg (whole fish wet weight). Working on chlordane
accumulation in sucker-fish, Roberts et al. (1977) showed that
accumulation from food was directly proportional to the lipid
levels of the fish. Chlordane was given to the northern redhorse
sucker, Moxostoma macrolepidotum, in the feed at 45 g/kg dry
feed for 5 consecutive days and to the white sucker, Catostomus
commersoni, directly to the stomach in a single dose of 340 mg
in corn oil. Both tests gave CF values of less than, or equal, to
0.52. CF values obtained in fish by uptake from the food were
lower than those obtained by uptake from water (goldfish at a CF of
162 with chlordane in diet (Moore et al., 1977); mosquito fish at a
CF of 8258 with chlordane in diet and in water (Sanborn et al.,
1976)). This suggests that chlordane is taken up directly from
water (bioaccumulated) more than it is from ingested food
(biomagnified).
Schimmel et al. (1976a,b) reported that CFs for two species of
marine fish were similar to those found in fresh-water species.
Spot and sheepshead minnow concentrated gamma-chlordane 3700 - 14
800 and 9000 - 16 800 times, respectively, in 4 days, and 3300 -
5100 and 10 300 times, respectively, in 24 days. Veith et al.
(1979) exposed fathead minnows to 5.9 µg chlordane/litre for 32
days and obtained a CF of 37 800 for the whole body. Parrish et
al. (1978) similarly reported 16 000 for whole body in the
sheepshead minnow after exposure for 189 days. They also
determined the CF after only 28 days exposure and found a
comparable whole-body value of 15 300. A range of CFs between 9000
and 16 786 was reported by Schimmel et al. (1976a) when sheepshead
minnows were exposed to gamma-chlordane (in technical heptachlor)
at 1.1 - 2.8 µg/litre for 96 h. In a field study, long-term
exposure (209 days or less) of large-mouth bass to between 0.01 and
0.1 µg/litre chlordane gave concentration factors of between 157
and 3308 (Godsil & Johnson, 1968).
Food chain magnification is unlikely in terrestrial organisms.
Species of birds and mammals studied show little bioaccumulation,
probably because chlordane is rapidly broken down in homoiotherms.
There are very few data on birds. One report (Foster et al., 1972)
refers to a study on the accumulation of chlordane in laying hens
fed 0.1 mg/kg diet. CF values were maximal after 7 - 9 weeks, diet
to fat was between 0.01 and 3.3, and diet to eggs was between 0.01
and 2. After 3 weeks on an untreated diet, chlordane was not
detectable. McCaskey et al. (1968) dosed hens with the equivalent
of a diet containing 10 - 15 mg 60% technical chlordane/kg for 5
days and obtained a maximum CF value in eggs of 0.38 on day 6.
8.5. Population and Community Effects
The fate of 14C-chlordane was investigated in a terrestrial-
aquatic model ecosystem composed of an alga (Oedogonium), snails
(Physa), mosquito larvae (Culex), and fish (Gambusia)
(Sanborn et al., 1976). Accumulation of chlordane residues was
evident in all organisms in the ecosystem, but no toxic effects
were reported.
Exposure of a natural phytoplankton population to 5 and 10 µg
chlordane/litre for 5 days had virtually no effect on its species
composition (Biggs et al., 1978).
8.6. Effects on the Abiotic Environment
No data are available on abiotic effects.
8.7. Appraisal
Data on aquatic toxicity require interpretation. Although the
solubility of chlordane in water has been measured at between 6
and 9 µg/litre, in many studies on its aquatic toxicity, chlordane
has been applied at much higher nominal concentrations. Either the
chlordane has not been in solution or was added with a solvent.
Actual levels of chlordane in natural waters rarely exceed 250
ng/litre with most levels below 20 ng/litre. Test levels are
therefore unrealistically high. The data should thus be critically
examined unless the actual concentrations experienced by the test
organisms were measured.
Few long-term studies of the chronic effects of chlordane are
available. Data do not define threshold levels, although they
suggest levels at which effects might be expected. Data seldom
provide quantitative dose-response relationships.
Effects of chlordane on primary producers in the aquatic food
chain are largely unknown because studies have used unrealistically
high concentrations. Some data on lethal doses for aquatic
organisms are available, but data on sub-lethal effects on
reproduction or behaviour are not. There are data on the acute
toxicity of chlordane for fish at concentrations approaching the
water solubility, but few on long-term exposure at lower doses.
Most terrestrial studies have been on soil organisms. Effects
here might be due to the heptachlor in technical chlordane or to a
combination of heptachlor and chlordane. Only very high
application rates of chlordane affected arthropods. Field studies
do not indicate direct toxicity but a combination of toxicity and
avoidance of the compound.
Of major importance is the clear toxicity of chlordane for
earthworms with implications for soil fertility. Molluscs also
appear to be particularly sensitive to chlordane.
9. PREVIOUS EVALUATIONS OF CHLORDANE BY INTERNATIONAL BODIES
An IARC Working Group (IARC, 1982) concluded that the available
epidemiological studies on chlordane were inadequate for the
evaluation of the cancer risk for man and that there was limited
evidence of the carcinogenicity of chlordane for experimental
animals.
WHO has recommended a guideline value of 0.3 µg/litre for
chlordane (total isomers) in drinking-water (WHO, 1982).
The Joint Meeting on Pesticide Residues (JMPR) reviewed
residues and toxicity data on chlordane on several occasions in the
past (1965, 1967, 1970). In November 1972, it re-established
residue tolerences ranging from 0.02 - 0.5 mg/kg for the sum of
alpha- and gamma-isomers of chlordane and oxychlordane (FAO/WHO,
1973). The acceptable daily intake (ADI) for human beings of
0 - 0.001 mg/kg body weight was confirmed in December 1977
(FAO/WHO, 1978). This was based on no-observed-adverse-effect-
levels of:
- 5 mg/kg in the diet, equivalent to 0.25 mg/kg body
weight in the rat; and
- 3 mg/kg in the diet, equivalent to 0.075 mg/kg body
weight in the dog.
Both "no-observed-adverse-effect-levels" and the ADI were
reviewed by the 1982 JMPR (FAO/WHO, 1983). The ADI was given
"temporary" status pending the results of toxicology studies still
in progress.
WHO (1984), in its "Guidelines to the Use of the WHO
Recommended Classification of Pesticides by Hazard", classified
technical chlordane as moderately hazardous.
The WHO/FAO (1978) has issued practical advice in its "Data
Sheet on Pesticides" including one on Chlordane (No. 36) dealing
with labelling, safe-handling, transport, storage, disposal,
decontamination, training and medical supervision of workers, first
aid and medical treatment.
Regulatory standards established by national bodies in 12
different countries (Argentina, Brazil, Czechoslovakia, the Federal
Republic of Germany, India, Japan, Kenya, Mexico, Sweden, the
United Kingdom, the USA, and the USSR) and the EEC is available
from the IRPTC (International Register of Potentially Toxic
Chemicals) Legal file (IRPTC, 1983).
The CEC reviewed the data available on chlordane in 1981.
10. EVALUATION OF HEALTH RISKS FOR MAN AND EFFECTS ON THE
ENVIRONMENT
10.1. Chlordane Toxicity
Chlordane is readily absorbed in both animals and man via the
skin, via ingestion, and probably also by inhalation. Some
accumulation occurs in the body on repeated exposure - mainly in
adipose tissue. Elimination from the body is fairly slow. The
half-life in various species, including man, is of the order of a
few weeks.
The oral LD50 values of chlordane in the rat range from
200 - 590 mg/kg body weight. Thus, chlordane is moderately toxic
in acute exposures.
Acute poisoning in man and animals is characterized by
manifestations of central nervous system stimulation such as
disorientation, tremors, and convulsions. Death may follow
respiratory failure.
In experimental animals (rats and dogs), prolonged exposure
to levels in the diet exceeding 3 - 5 mg/kg resulted in the
induction of hepatic microsomal enzymes and, at a later stage,
liver hypertrophy with histological changes. At higher levels
(i.e., > 15 mg/kg body weight per day), chlordane is hepatotoxic.
For no-observed-adverse-effect levels see section 6.1.1.
At dosages above 30 mg/kg diet, chlordane interferes with
reproduction in rats and mice, but this was reversible after
exposure ceased. There are no indications for teratogenicity in
the rabbit at 15 mg/kg body weight per day.
Chlordane produces hepatocellular carcinomas in mice. It
is not generally active in short-term tests designed to measure
genetic activity. Chlordane can interfere with cell to cell
communication in vitro, a characteristic of many promoting agents.
10.2. Exposure to Chlordane
Food is the major source of exposure of the general population
to chlordane, but the use of chlordane on food crops has decreased
and residues in food from animal origin are low. Some chlordane
exposure can occur in buildings where chlordane has been used for
termite or other insect control.
No adverse health effects have been reported in workers engaged
in the manufacture of chlordane or in pest-control operations,
where exposure could be quite high. However, several cases of
accidental and suicidal poisoning in man have been reported
resulting in the symptoms described in section 7.1.
10.3. Evaluation of Overall Environmental Effects
Chlordane is used primarily to control soil pests. Technical
chlordane is a mixture of chlorinated hydrocarbons and contains
heptachlor, which might contribute significantly to the
insecticidal properties of the technical formulation.
About half of the chlordane applied to soil disappears in the
first season, presumably by volatalisation or by "run-off" into
surface waters. Remaining residues persist for several seasons.
If chlordane is applied annually for several successive seasons,
residues accumulate in the soil. Most chlordane persists in the
cultivated levels, since there is little leaching into subsoil.
The high rate of metabolism of chlordane in warm-blooded
animals means that there is little possibility of accumulation in
these animals or magnification in food chains at this level.
Concentration factors are generally modest in aquatic organisms;
this combined with its low solubility in water means that chlordane
presents a limited hazard for aquatic vertebrates. Long-term
effects are not sufficiently well-documented to say that there is
not a potential hazard for fish, but this seems unlikely from the
information available, as far as temperate areas are concerned.
The compound shows a higher toxicity at higher temperatures.
Significant mortality in tropical species of fish at concentrations
well within the solubility of the compound, suggest that chlordane
may be a greater aquatic hazard at lower latitudes.
The high toxicity of chlordane for earthworms may constitute
its greatest potential hazard. The long-term effects of reduced
numbers of earthworms in the soil cannot be readily assessed
because the ecology of the animal is still poorly understood.
10.4. Evaluation of Risks for Human Health and the Environment
Although there is no evidence that incriminates chlordane as a
human carcinogen, the suspicion principally arising from the mouse
carcinogenicity studies cannot be entirely put aside. Further
research is required to elucidate this problem. Nevertheless, in
the present state of knowledge, it is concluded that:
1. As long as occupational hygiene procedures are maintained
to keep exposure levels to a minimum, whether or not by the
imposition of maximum allowable concentrations, there is
little reason to believe that workers will be at risk from
their handling, or contacts, with chlordane.
2. For the general population, consumers should suffer no
adverse effects from chlordane as food residues, provided
that the intake is kept within the temporary ADI set by the
Joint FAO/WHO Meeting.
In certain regions of the world, the exposure of the
general population to chlordane may be augmented by its
use as a termiticide in buildings.
3. Apart from the possible long-term adverse effects on
aquatic organisms in tropical areas and the depleted soil
fertility that may arise, in time, from the suppression of
the earthworm population, chlordane seems to cause little
environmental concern in its normal use as a termiticide
and in other non-agricultural applications.
REFERENCES
AHMED, F.E., HART, R.W., & LEWIS, N.J. (1977a) Pesticide
induced DNA damage and its repair in cultured human cells.
Mutat. Res., 42: 161-174.
AHMED, F.E., LEWIS, N.J., & HART, R.W. (1977b) Pesticide
induced ouabain resistant mutants in Chinese hamster. Chem.
Biol. Interactions, 19: 369-374.
ALDRICH, F.D. & HOLMES, J.H. (1969) Acute chlordane
intoxication in a child. Case report with toxicological
data. Arch. environ. Health, 19: 129-132.
AL-HACHIM, G.M. & AL-BAKER, A. (1973) Effects of chlordane
on conditioned avoidance response, brain seizure threshold and
open-field performance of prenatally-treated mice. Br. J.
Pharmacol., 49: 311-315.
ALVAREZ, W.C. & HYMAN, S. (1953) Absence of toxic
manifestations in workers exposed to chlordane. Arch. ind.
Hyg. occup. Med., 7: 197-210.
AMBROSE, A.M., CHRISTENSEN, H.E., ROBBINS, D.J., & RATHER,
L.J. (1953) Toxicology and pharmacological studies on
chlordane. Arch. ind. Hyg. occup. Med., 1: 197-210.
ARNOLD, D.W., KENNEDY, G.L., KEPLINGER, M.L., CALANDRA, J.C.,
& CALO, C.J. (1977) Dominant lethal studies with technical
chlordane, HCS-3260, and heptachlor: Heptachlor epoxide.
J. Toxicol. environ. Health, 2: 547-555.
ATALLAH, Y.H., WHITACRE, D.M., & POLEN, P.B. (1977) Artifacts in
monitoring - related analysis of human adipose tissue for some
organochlorine pesticides. Chemosphere, 6: 17-20.
ATALLAH, Y.H., WHITACRE, D.M., & HOO, B.L. (1979)
Comparative volatility of liquid and granular formations of
chlordane and heptachlor from soil. Bull. environ. Contam.
Toxicol., 22: 570-574.
BACHMANN, K.A. & BURKMAN, A.M. (1974) Topical application of
a chlordane containing ectoparasiticide: Effect on plasma
half-life of warfarin in dogs. Pharmacologist, 16: 284.
BALBA, H.M. & SAHA, J.G. (1978) Studies on the distribution,
excretion, and metabolism of alpha- and gamma-isomers of 14C
chlordane in rabbits. J. environ. Sci. Health, B13: 211-233.
BARNETT, R.W. & DOROUGH, H.W. (1974) Metabolism of chlordane
in rats. J. agric. food Chem., 22: 612-619.
BARNETT, R.W., D'ERCOLE, A.J., CAIN, J.D., & ARTHUR, R.D.
(1979) Organochlorine pesticide residues in human milk
samples from women living in north-west and north-east
Mississippi, 1973-1975. Pestic. Monit. J., 13: 47-51.
BARTHEL, W.F., HAWTHORNE, J.C., FORD, J.H., BOLTON, G.C.,
McDOWELL, L.L., GRISSINGER, E.H., & PARSONS, D.A. (1969)
Pesticide residues in sediments of the Lower Mississippi River
and its tributaries. Pestic. Monit. J., 3: 8-34.
BATTE, E.G. & TURK, R.D. (1948) Toxicity of some synthetic
insecticides to dogs. J. econ. Entomol., 41: 102-103.
BEVENUE, A., OGATA, J.N., & HYLIN, J.W. (1972a) Organo-
chlorine pesticides in rain water, Oahu, Hawaii, 1971-1972.
Bull. environ. Contam. Toxicol., 8: 238-241.
BEVENUE, A., HYLIN, J.W., KAWANO, Y., & KELLEY, T.W. (1972b)
Organochlorine pesticide residues in water, sediment, algae,
and fish, Hawaii, 1970-71. Pestic. Monit. J., 6: 56-64.
BIGGS, D.C., ROWLAND, R.G., O'CONNORS, H.B., POWERS, C.D., &
WURSTER, C.F. (l978) A comparison of the effects of
chlordane and PCB on the growth, photosynthesis and cell size
of estuarine phytoplankton. Environ. Pollut., 15: 253-263.
BIROS, F.J. & ENOS, H.F. (1973) Oxychlordane residues in
human adipose tissue. Bull. environ. Contam. Toxicol., 10:
257-260.
BOYD, J.C. (1971) Field study of a chlordane residue
problem: Soil and plant relationships. Bull. environ. Contam.
Toxicol., 6: 177-182.
BOYD, E.M. (1972) Chlordane. In: Protein deficiency and
pesticide toxicity, Springfield, Ill., Charles C. Thomas,
468 pp.
BRIMFIELD, A.A. & STREET, J.C. (1979) Mammalian
biotransformation of chlordane: in vivo and primary hepatic
comparisons. Ann. New York Acad. Sci., 320: 247-256.
BRIMFIELD, A.A., STREET, J.C., FUTRELL, J., & CHATFIELD, D.A.
(1978) Identification of products arising from the metabolism
of cis- and trans-chlordane in rat liver microsomes in vitro:
Outline of a possible metabolic pathway. Pestic. Biochem.
Physiol., 9: 84-95.
BRODTMANN, N.V., Jr (1976) Continuous analysis of
chlorinated hydrocarbon pesticides in the lower Mississippi
river. Bull. environ. Contam. Toxicol., 15: 33-39.
BRUBAKER, P.E., FLAMM, W.G., & BERNHEIM, N.J. (1970) Effect
of gamma-chlordane on synchronized lymphoma cells and
inhibition of cell division. Nature (Lond.), 226: 548-549.
BUCK, W.B., OSWEILER, G.D., & VAN GELDER, G.A. (1973)
Clinical and diagnostic veterinary toxicology, Dubuque, Iowa,
Kendall Hunt.
BUGG, J.C., HIGGINS, J.E., & ROBERTSON, E.A. (l967) Residues
in fish, wildlife and estuaries: chlorinated pesticide levels
in the eastern oyster (Crassostrea virginica) from selected
areas of the South Atlantic and Gulf of Mexico. Pestic. Monit.
J., 1: 9-12.
BUTLER, P.A. (l963) Commercial fishery investigations,
Washington DC, US Department of the Interior, Fish and
Wildlife Service, pp. 5-28 (Circular 199).
BUTLER, P.A. & SCHUTZMANN, R.L. (1978) Residues in
pesticides and PCBs in estuarine fish, 1972-1976; National
Pesticide Monitoring Program. Pestic. Monit. J., 12: 51-59.
CALDWELL, R.S. (l977) Biological effects of pesticides on
the Dungeness crab, Washington DC, US Environment Protection
Agency (Report No. EPA 600/3-77-131).
CANADA, NATIONAL RESEARCH COUNCIL (1974) Chlordane: Its
effects on Canadian ecosystems and its chemistry, Canada, NRC
(Report Monograph Non Serials 189).
CARDWELL, R.D., FOREMAN, D.G., PAYNE, T.R., & WILBUR, D.J.
(l977) Acute and chronic toxicity of chlordane to fish and
invertebrates, Washington DC, US Environment Protection
Agency, 126 pp (Report No. EPA 600/3-77-019).
CAREY, A.E., WIERSMA, G.B., TAI, H., & MITCHELL, W.G. (1973)
Organochlorine pesticide residues in soils and crops of the
Corn Belt region, United States, 1970. Pestic. Monit. J., 6:
369-376.
CASPER, V.L. (l967) Galveston Bay pesticide study - water
and oyster samples analysed for pesticide residues following
mosquito control programme. Pestic. Monit. J., 1: 13-15.
CEC (1981) Criteria (dose-effects relationship) for organo-
chlorine pesticides, Oxford, CEC, Pergamon Press.
CHAMBERS, C. & DUTTA, S.K. (1976) Mutagenic tests of
chlordane on different microbial tester strains. Genetics,
83: S13.
CLARK, D.R., Jr & KRYNITSKY, A. (1978) Organochlorine
residues and reproduction in the little brown bat, Laurel,
Maryland, June 1976. Pestic. Monit. J., 12: 113-116.
CLARK, D.R., Jr & PROUTY, R.M. (1976) Organochlorine
residues in three bat species from four localities in Maryland
and West Virginia, 1973. Pestic. Monit. J., 10: 44-53.
CLEGG, T.J. & KOEVENIG, J.L. (l974) The effect of four
chlorinated hydrocarbon pesticides and one organophosphate
pesticide on ATP levels in three species of photosynthesizing
fresh-water algae. Bot. Gaz., 135: 368-372.
CLEMENS, H.P. & SNEED, K.E. (l959) Lethal doses of several
commercial chemicals for fingerling channel catfish,
Washington DC, US Department of the Interior, Fish and
Wildlife Service, 10 pp (Special Scientific Report on
Fisheries No. 316).
COCHRANE, W.P. & GREENHALGH, R. (1976) Chemical composition
of technical chlordane. J. Assoc. Off. Anal. Chem., 59:
696-702.
COCHRANE, W.P., PARLAR, H., GAEB, S., & KORTE, F. (1975)
Structural elucidation of the chlordene isomer constituents of
technical chlordane. J. agric. food Chem., 23: 882-886.
COPE, O.B. (l965) Sport fishery investigations, Washington
DC, US Department of the Interior, Fish and Wildlife Service
(Circular 226).
COPE, O.B., GJULLIN, C.M., & STORM, A. (l947) Effects of
some insecticides on trout and salmon in Alaska, with
reference to black-fly control. Trans. Am. Fish. Soc., 77:
160-177.
CORBETT, D.C.M. & WEBB, R.M. (l968) Effect of herbicides in
minimum tillage, Suffolk, England, Richard Clay (The Chaucer
Press) Ltd., pp. 158-159 (Report of the Rothamsted
Experimental Station for l967).
COUNCIL FOR AGRICULTURAL SCIENCE & TECHNOLOGY (1975)
Chlordane and heptachlor, Ames, Iowa, Iowa State University,
Department of Agronomy, pp. 1-71 (Report No. 47, Oct. 3).
CRANMER, J.S., AVERY, D.L., GRADY, R.R., & KITAY, J.I. (1978)
Postnatal endocrine dysfunction resulting from prenatal
exposure to carbofuran diazinon or chlordane. J. environ.
Pathol. Toxicol., 2: 357-370.
CROCKETT, A.B., WIERSMA, G.B., TAI, H., MITCHELL, W.G., SAND,
P.F., & CAREY, A.E. (1974) Pesticide residue levels in soils
and crops, FY-70: National soils monitoring program II.
Pestic. Monit. J., 8: 69-97.
CURLEY, A. & GARRETTSON, L.K. (1969) Acute chlordane
poisoning: clinical and chemical studies. Arch. environ.
Health, 18: 211-215.
DATTA, K.K., GUPTA, P.C., & DIKSHITH, T.S.S. (1975) Effect
of chlordane on the skin of male guinea pigs. In: Zaidi,
S.H., ed. Environmental pollution and human health, Lucknow,
India, Industrial Toxicology Research Centre, pp. 608-611.
DAVIS, R.W., FRIEDHOFF, J.M., & WEDENEYER, G.A. (l972)
Organochlorine insecticide, herbicide and polychlorinated
biphenyl (PCB) inhibition of NaK-ATPase in rainbow trout.
Bull. environ. Contam. Toxicol., 8: 69-72.
DEN TONKELAAR, E.M. & VAN ESCH, G.J. (1974) No-effect levels
of organochlorine pesticides based on induction of microsomal
liver enzymes in short-term toxicity experiments. Toxicology,
2: 371-380.
DERBES, J.V., DENT, H.J., FOREST, W.W., & JOHNSON, M.F.
(1955) Fatal chlordane poisoning. J. Am. Med. Assoc.,
158(15): 1367-1369.
DEWITT, J.B., STICKEL, W.H., & SPRINGER, P.F. (1963)
Wildlife studies, Patuxent Wildlife Research Centre l961-l962,
Washington DC, US Department of the Interior, Fish and
Wildlife Service, pp. 74-96 (Circular 167).
DITRAGLIA, D., BROWN, D.P., NAMEKATA, T., & IVERSON N.
(1981) Mortality study of workers employed at organochlorine
pesticide manufacturing plants. Scand. J. Work Environ.
Health, 7(Suppl. 4): 140-146.
DOANE, C.C. (l962) Effects of certain insecticides on
earthworms. J. econ. Entomol., 55: 416-418.
DRUMMOND, L., CHETTY, K.N., BAILEY, M., & DESAIAH, D. (1980)
in vivo effect of chlordane on rat brain ATP-ase system. Fed.
Proc., 39: 998.
DUFFY, J.R. & WONG, N. (1967) Residues of organochlorine
insecticides and their metabolites in soil in the Atlantic
Provinces of Canada. J. agric. food Chem., 15(3): 457-464.
EDWARDS, C.A. (l965a) Some side-effects resulting from the
use of persistent insecticides. Ann. appl. Biol., 55: 329-331.
EDWARDS, C.A. (l965b) Effects of pesticide residues on soil
invertebrates and plants. In: Goodman, G.T., Edwards, R.W., &
Lambert, J.M., ed. Ecology and the industrial society,
Reading, England, British Ecological Society, Symposium No. 5,
pp. 239-261.
EPSTEIN, S.S. (1976) Carcinogenicity of heptachlor and
chlordane. Sci. total Environ., 6: 103-154.
EPSTEIN, S.S., ARNOLD, E., ANDREA, J., BASS, W., & BISHOP, Y.
(1972) Detection of chemical mutagens by the dominant lethal
assay in the mouse. Toxicol. appl. Pharmacol., 23: 288-335.
ERCEGOVICH, C.D. & RASHID, K.A. (1977) Mutagenesis induced
in mutant strains of Salmonella typhimurium by pesticides.
Soc. Abstr. Pap., 174: 43.
FAO/WHO (1968) 1967 Evaluations of some pesticide residues
in food, Rome, Food and Agriculture Organization of the United
Nations (The Monographs).
FAO/WHO (1973) 1972 Evaluations of some pesticide residues
in food, Rome, Food and Agriculture Organization of the United
Nations.
FAO/WHO (1978) 1977 Evaluations of some pesticide residues
in food, Rome, Food and Agriculture Organization of the United
Nations.
FAO/WHO (1981) Joint FAO/WHO food and animal feed
contamination monitoring programme. Summary of data received
from collaborating centres - 1977-1980. Part B - Contaminants,
Geneva, World Health Organization.
FAO/WHO (1983) Pesticide residues in food - 1982, Rome, Food
and Agriculture Organization of the United Nations, pp. 16-17
(FAO Plant Production and Protection Paper 46).
FISHBEIN, W.I., WHITE, J.V., & ISAACS, H.J. (1964) Survey of
workers exposed to chlordane. Ind. Med. Surg., 33: 726-727.
FITZHUGH, O.G. & FAIRCHILD, H.E. (1976) Pesticidal aspects
of chlordane in relation to man and the environment,
Washington DC, US Environmental Protection Agency, US NTIS,
114 pp (PB Rep., ISS PB- 257107).
FOLMAR, L.C. (1978) In vitro inhibition of rat brain
ATPase, pNPPase, and ATP-32p exchange by chlorinated-diphenyl
ethanes and cyclodiene insecticides. Bull. environ. Contam.
Toxicol., 19: 481-488.
FOSTER, T.S., MORLEY, H.V., PURKAYASTHA, R., GREENHALGH, R., &
HUNT, J.R. (l972) Residues in eggs and tissues of hens fed a
ration containing low levels of pesticides with and without
charcoal. J. econ. Entomol., 65: 982-988.
FOUTS, J.R. (1963) Factors influencing the metabolism of
drugs in liver microsomes. Ann. New York Acad. Sci., 104:
875-880.
FOX, C.J.S. (l958) Some effects of insecticides on the
wireworms and vegetation of grassland in Nova Scotia. In:
Proceedings of the Tenth Congress on Entomology, Canada,
Vol. 3, pp. 297-300.
FRANK, R., BRAUN, H.E., HOLDRINET, M., DODGE, D.P., & NAPSZY,
S.J. (1978a) Residues of organochlorine insecticides and
polychlorinated biphenyls in fish from lakes Saint Clair and
Erie, Canada, 1968-1976. Pestic. Monit. J., 12: 69-80.
FRANK, R., HOLDRINET, M., BRAUN, H.E., DODGE, D.P., &
SPRANGLER, G.E. (1978b) Residues of organochlorine insecticides
and polychlorinated biphenyls in fish from lakes Huron and
Superior, Canada, 1968-1976. Pestic. Monit. J., 12: 60-68.
GAEB, S., BORN, L., PARLAR, H., & KORTE, F. (1977)
Structural elucidation of an octachloro component of technical
chlordane (Compound K) by spectroscopic and X-ray methods.
J. agric. food Chem., 25: 1365-1371.
GAINES, T.B. (1969) Acute toxicity of pesticides. Toxicol.
appl. Pharmacol., 14: 525-534.
GISH, C.D. (l970) Pesticides in soil. Organochlorine
insecticide residues in soils and soil invertebrates from
agricultural land. Pestic. Monit. J., 3: 241-252.
GLOOSCHENKO, V. & LOTT, J.N.A. (l977) The effects of
chlordane on the green algae, Scenedesmus quadricauda and
Chlamydomonas sp. Can. J. Bot., 55: 2866-2872.
GLOOSCHENKO, W.A., STRACHAN, W.M.J., & SAMPSON, R.C.J.
(1976) Residues in water: distribution of pesticides and
polychlorinated biphenyls in water, sediments, and seston of
the Upper Great Lakes - 1974. Pestic. Monit. J., 10: 61-67.
GODSIL, P.J. & JOHNSON, W.C. (l968) Residues in fish,
wildlife and estuaries. Pesticide monitoring of the aquatic
biota at the Tule Lake National Wildlife Refuge. Pestic.
Monit. J., 1: 21-26.
GOES, T.R., SAVAGE, E.P., & BOYD, W.L. (1978) In vitro
inhibition of oral viridans streptococci by chlordane. Arch.
environ. Contam. Toxicol., 7: 449-456.
GOULD, E. & HAMPSTEAD, E.O. (l95l) The toxicity of
cumulative spray residues in soils. J. econ. Entomol., 44:
713-717.
GOWEN, J.A., WIERSMA, G.B., TAI, H., & MITCHELL, W.G. (1976)
Pesticide levels in hay and soils from nine states, 1971.
Pestic. Monit. J., 10: 114-116.
GRIMES, D.J. & MORRISON, S.M. (1975) Bacterial
bioconcentration of chlorinated hydrocarbon insecticides from
aqueous systems. Microbiol. Ecol., 2: 43-59.
HANSON, W.R. (1952) Effects of some herbicides and
insecticides on the biota of North Dakota marshes. J. Wildl.
Manage., 16: 299-308.
HARRINGTON, J.M., BAKER, E.L., Jr, FOLLAND, D.S., SAUCIER,
J.W., & SANDIFER, S.H. (1978) Chlordane contamination of a
municipal water system. Environ. Res., 15: 155-159.
HARRIS, C.R. & SANS, W.W. (1976) Persistence of velsicol
HCS-3260 (AG-chlordane) in mineral and organic soil. Proc.
Entomol. Soc. Ontario, 106: 34-38.
HART, L.G. & FOUTS, J.R. (1965) Studies of the possible
mechanisms by which chlordane stimulates hepatic microsomal
drug metabolism in the rat. Biochem. Pharmacol., 14: 263-272.
HART, L.G., SHULTICE, R.W., & FOUTS, J.R. (1963) Stimulatory
effects of chlordane on hepatic microsomal drug metabolism in
the rat. Toxicol. appl. Pharmacol., 5: 371-386.
HENDERSON, C., PICKERING, Q.H., & TARZWELL, C.M. (1959) The
relative toxicity of ten chlorinated hydrocarbon insecticides
to four species of fish. Trans. Am. Fish. Soc., 88: 23-32.
HENDERSON, C., JOHNSON, W.L., & INGLIS, A. (1969)
Organochlorine insecticide residues in fish. Pestic. Monit.
J., 3: 145-171.
HENDERSON, C., INGLIS, A., & JOHNSON, W.L. (1971)
Organochlorine insecticide residues in fish - Fall 1969.
National pesticide monitoring program. Pestic. Monit. J., 5:
1-11.
HERRICK, G.M., FRY, J.I., FONG, W.G., & GOLDEN, D.C. (1969)
Insecticide residues in eggs resulting from the dusting and
short-term feeding of low levels of chlorinated hydrocarbon
insecticides to hens. J. agric. food Chem., 17: 291-295.
HILL, E.F., HEATH, R.G., SPANN, J.W., & WILLIAMS, J.D.
(1975) Lethal dietary toxicities of environmental pollutants
to birds, Washington DC, US Department of the Interior, Fish
and Wildlife Service, 61 pp (Special Scientific Report No.
191).
HODGE, H.C. & STERNER, J.H. (1956) Combine and tabulation of
toxicity classes. In: Spector, W.B., ed. Handbook of
toxicology, Philadelphia, Pennsylvania, W.B. Saunders Company,
Vol. 10.
HOPKINS, A.R. & KIRK, V.M. (1957) Effect of several
insecticides on the English Red Worm. J. econ. Entomol., 50:
699-700.
HRDINA, P.D., PETERS, D.A.V., & SINGHAL, R.L. (1973) Acute
neurotoxic effects of alpha-chlordane and alterations in brain
biogenic amines. Proc. Can. Fed. Biol. Soc., 16: 103.
HYDE, K.M. & FALKENBERG, R.L. (1976) Neuroelectrical
disturbance as indicator of chronic chlordane toxicity.
Toxicol. appl. Pharmacol., 37: 499-515.
HYDE, K.M., CRANDALL, J.C., KORTMAN, K.E., & MCCOY, W.K.
(1978) EEG, ECG, and respiratory response to acute
insecticide exposure. Bull. environ. Contam. Toxicol., 19:
47-55.
IARC (1979) Some halogenated hydrocarbons. Chlordane, Lyons,
International Agency for Research on Cancer, pp. 45-65
(Monographs on the Evaluation of the Carcinogenic Risk of
Chemicals to Humans, No. 20).
IARC (1982) Chemicals and industrial processes associated
with cancer in humans, Lyons, International Agency for
Research on Cancer, pp. 80-83 (Monographs on the Evaluation of
the Carcinogenic Risk of Chemicals to Humans, Suppl. 4).
ILO (1980) Occupational exposure limits for airborne toxic
substances, 2nd (revised) ed., Geneva, International Labour
Office (Occupational Safety and Health Series No. 37).
INFANTE, P.F., EPSTEIN, S.S., & NEWTON, W.A., Jr (1978)
Blood dyscrasias and childhood tumors and exposure to
chlordane and heptachlor. Scand. J. Work Environ. Health, 4:
137-150.
INGLE, L. (1952) Chronic oral toxicity of chlordane to
rats. Arch. ind. Hyg. occup. Med., 6: 357-367.
INGLE, L. (1965a) Effects of 1-hydroxychlordene when
incorporated into the diets of rats for 224 days, Urbana,
Illinois, University of Illinois, Department of Zoology
(Report for Velsicol Chemical Corporation).
INGLE, L. (1965b) Monograph on chlordane - toxicological &
pharmacological properties, Urbana, Illinois, University of
Illinois, Food & Drug Library (Library of Congress, Card
No. 65-28686A).
INGLE, L. (1969) Albino rats subjected to alpha and gamma
chlordane incorporated into the daily diet for 78 weeks
(Report from the University of Illinois).
INRS (1983) Valeurs limites pour les concentrations des
substances dangereuses dans l'air des locaux de travail,
Paris, France, Institut National de Recherche et de Sécurité
pour la Préventions des Accidents du Travail et des Maladies
Professionelles (Cahiers de notes documentaires, No. 110).
IRDC (1967) Chlordane, two-year chronic feeding study in the
beagle dog, Mattawan, Michigan, International Research and
Development Corporation (Report 163-001, sponsored by Velsicol
Chemical Corporation).
IRDC (1972) Chlordane, teratology study in rabbits,
Mattawan, Michigan, International Research and Development
Corporation (Report 163-106, sponsored by Velsicol Chemical
Corporation).
IRPTC (1983) IRPTC Legal file 1983, Geneva, International
Register of Potentially Toxic Chemicals, United Nations
Environment Programme (UNEP), Vols. I & II.
IVIE, G.W., KNOW, J.R., KHALIFA, S., YAMAMOTO, I., & CASIDA,
J.E. (1972) Novel photoproducts of heptachlor epoxide,
trans-chlordane, and trans-nonachlor. Bull. environ. Contam.
Toxicol., 7: 376-382.
JENSEN, A.A. (1983) Chemical contaminants in human milk.
Residue Rev., 89: 1-128.
KACEW, S. & SINGHAL, R.L. (1973a) Metabolic alterations after
chronic exposure to alpha-chlordane. Toxicol. appl.
Pharmacol., 24: 539-544.
KACEW, S. & SINGHAL, R.L. (1973b) The influence of
p,p'-DDT, alpha-chlordane, heptachlor and endrin on hepatic
and renal carbohydrate metabolism and cyclic AMP-adenyl
cyclase system. Life Sci., 13: 1363-1371.
KADAM, A.N., BENDRE, S.B., & GHATGE, B.B. (1978) Gas
chromatography of technical chlordane and identification of
some of the components. Pesticides, 12: 13-15.
KAREL, A.K. (1976) Acute chlordane toxicity on the serum
alkaline phosphatase activity of Meriones hurricanae, Jerdon
(gerbil). Arch. int. Physiol. Biochem., 84: 63-68.
KAREL, A.K. & SAXENA, S.C. (1976) Chronic chlordane
toxicity: Effect on blood biochemistry of Meriones hurricanae,
Jerdon, the Indian desert gerbil. Pestic. Biochem. Physiol.,
6: 111-114.
KEPLINGER, M.L., DEICHMANN, W.B., & SALA, F. (1968) Effects
of pesticides on reproduction in mice. Ind. Med. Surg., 37:
525.
KOCH, R.B., CUTCOMB, L.K., & YAP, H.H. (1971) Inhibition of
oligomycin sensitive and insensitive fish adenosine
triphosphatase activity by chlorinated hydrocarbon
insecticides. Biochem. Pharmacol., 20: 3243-3245.
KONAR, S.K. (1968) Experimental use of chlordane in fishery
management. Prog. Fish. Cult., 30: 96-99.
KRAYBILL, H.F.T. (1977a) The determination of carcinogenesis
induced by trace contaminants in potable water. In: Borchardt,
J.A., Cleland, J.K., Redman, W.J., & Olivier, J., ed. Viruses
and trace contaminants in water and wastewater, Ann Arbor,
Michigan, Ann Arbor Science Publishers, Inc., pp. 109-123
(Seminar, Ann Arbor, Michigan, January 26-28, 1977. XIV 249P).
KUTZ, F.W., YOBS, A.R., STRASSMAN, S.C. (1976) Organochlorine
pesticide residues in human adipose tissue. Bull. Soc.
Pharmacol. Environ. Pathol., 4(1): 17-19.
LANGLOIS, B.E. & SIDES, K.G. (1972) Effect of heptachlor and
related compounds on growth of Staphylococcus aureus. Bull
environ. Contam. Toxicol., 8: 158-164.
LAWRENCE, J.M. (1950) Toxicity of some new insecticides to
several species of pondfish. Prog. Fish. Cult., 12: 141-146.
LAWRENCE, J.H., BARRON, R.P., CHEN, J.Y.T., LOMBARDO, P., &
BENSON, W.R. (1970) Note on identification of a chlordane
metabolite found in milk and cheese. J. Assoc. Off. Agric.
Chem., 53: 261-262.
LEGG, D.C. (1968) Comparison of various worm killing
chemicals. J. Sports Turf Res. Inst., 44: 47-48.
LEHMAN, A.J. (1952a) Chemicals in foods: A report to the
Association of Food & Drug Officials on current developments.
Part II. Pesticides Section II: Dermal Toxicity. Assoc. Food
Drug Off. Q. Bull., 16: 3-9.
LEHMAN, A.J. (1952b) A report to the Association of Food and
Drug Officials on current developments. Section III: Subacute
and chronic toxicity. Assoc. Food Drug Off. Q. Bull., 16:
47-53.
LENSKY, P. & EVANS, H.L. (1952) Human poisoning by
chlordane. Report of a case. J. Am. Med. Assoc., 121: 826-827.
LICHTENSTEIN, E.P. & POLIUKA, J.B. (1959) Persistence of
some chlorinated hydrocarbon insecticides in turf soils.
J. econ. Entomol., 52(2): 280-293.
LICHTENSTEIN, E.P., SCHULTZ, K.K., FUHREMANN, T.W., & LIANG,
T.T. (l970) Degradation of aldrin and heptachlor in field
soils during a ten-year period translocation into crops.
J. agric. food Chem., 18: 100-106.
LIDGATE, H.J. (1966) Earthworm control with chlordane.
J. Sports Turf Res. Inst., 42: 5-8.
LINDUSKA, J.P. & SURBER, E.W. (1948) Effect of DDT and other
insecticides on fish and wildlife. Summary of investigations
during 1947, Washington DC, US Department of the Interior,
Fish and Wildlife Service, 19 pp (Circular 15).
LONG, W.H., ANDERSON, H.L., ISA, A.L., & KYLE, M.L. (1967)
Sugarcane growth responses to chlordane and microarthropods
and effects of chlordane on soil fauna. J. econ. Entomol., 60:
623-629.
LORA, R.P., MARTEACHE, A.H., VILLAR, L.M.P., GIMENEZ, R.L.,
VILLAREJO, M.J., & PEREZ, J.I. (l979) [Organochlorine
pesticides in the milk of Spanish humans.] Rev. Esp. pediatr.,
35: 93 (in Spanish).
LUDEMANN, D. & NEUMANN, H. (1962) [On the effects of the
latest contact insecticide on fresh water animals.] Anz.
Schaelingskd., 35: 5-9 (in German).
LUDKE, J.L. (1976) Organochlorine pesticide residues
associated with mortality: additivity of chlordane and endrin.
Bull. environ. Contam. Toxicol., 16: 253-260.
MACEK, K.J., HUTCHINSON, C., & COPE, O.B. (1969) The effects
of temperature on the susceptibility of bluegills and rainbow
trout to selected pesticides. Bull. environ. Contam. Toxicol.,
4: 174-183.
MACMAHON, B. & WANG, H.H. (1982) A second follow-up of
mortality in a cohort of pesticide applicators, Cambridge,
Massachusetts, Harvard School of Public Health, Department of
Epidemiology.
MADHUKAR, B.V. & MATSUMURA, F. (1979) Comparison of
induction patterns of rat hepatic microsomal mixed-function
oxidases by pesticides and related chemicals. Pestic.
Biochem. Physiol., 11: 301-308.
MAGNANI, B., POWERS, C.D., WURSTER, C.F., & O'CONNORS, H.B.
(1978) Effects of chlordane and heptachlor on the marine dino
flagellate Exuviella baltica Lohmann. Bull. environ. Contam.
Toxicol., 20: 1-8.
MAGUIRE, J. & WATKIN, N. (1975) Carbonic anhydrase
inhibition. Bull. environ. Contam. Toxicol., 13: 625-629.
MAHON, D.C. (1977) Interactions, in rats, between carbon
tetrachloride-induced liver cirrhosis and chronic treatment
with the insecticide chlordane. Diss. Abstr. Int., 38: 4012B.
MAHON, D.C. & OLOFFS, P.C. (1979) Effects of sub-chronic
low-level dietary intake of chlordane on rats with cirrhosis
of the liver. J. environ. Sci. Health, B14: 227-246.
MAHON, D.C., NAIR, K.K., & OLOFFS, P.C. (1979) DNA in rat
hepatocyte nuclei: Effects of treatment with low levels of
carbon tetrachloride and/or chlordane. Can. J. Zool., 57:
1003-1009.
MASLANSKY, C.J. & WILLIAMS, G.M. (1981) Evidence for an
epigenetic mode of action in organochlorine pesticide
hepatocarcinogenicity: A lack of genotoxicity in rat, mouse
and hamster hepatocytes. J. Toxicol. environ. Health, 8:
121-130.
MASTRI, C., KEPLINGER, M.L., & FANCHER, O.E. (1969a) Acute
oral toxicity study on synthetic (X) in male and female
albino rats, Illinois, Industrial Bio-Test Laboratories
(Report for Velsicol Chemical Corporation).
MASTRI, C., KEPLINGER, M.L., & FANCHER, O.E. (1969b) Acute
oral toxicity study on 4 chlordenes in albino rats, Illinois,
Industrial Bio-Test Laboratories (Report for Velsicol Chemical
Corporation).
MASTRI, C., KEPLINGER, M.L., & FANCHER, O.E. (1969c) Acute
oral toxicity study on 2 chlordenes in female albino rats,
Illinois, Industrial Bio-Test Laboratories (Report for
Velsicol Chemical Corporation).
MATTRAW, H.C., Jr (1975) Occurrence of chlorinated hydro-
carbon insecticides, Southern Florida, 1968-1972. Pestic.
Monit. J., 9: 106-114.
MCCASKEY, T.A., STENP, A.R., LISKA, B.J., & STADELMAN, W.J.
(1968) Residues in egg yolks and raw and cooked tissues from
laying hens administered selected chlorinated hydrocarbon
insecticides. Poult. Sci., 47: 564-569.
MCCLEESE, D.W., BURRIDGE, L.E., & VAN DINTER, J. (1982)
Toxicities of five organochlorine compounds in water and
sediment to Nereis virens. Bull. environ. Contam. Toxicol.,
28: 216-220.
MCGILL, H.C. (1979) Pilot study of the effects of pesticides
on blood lipoproteins, arteries, and cardiac muscle of
baboons, Washington DC, US Environmental Protection Agency.
MEHRLE, P.M., JOHNSON, W.W., & MEYER, F.L. (1974)
Nutritional effects of chlordane toxicity to rainbow trout.
Bull. environ. Contam. Toxicol., 12: 513-517.
MEITH-AVCIN, N., WARLEN, S.M., & BARBER, R.T. (1973)
Organochlorine insecticide residues in a Bathyl-Demersal Fish
from 2500 metres. Environ. Lett., 5: 215-221.
MES, J. & DAVIES, D.J. (1978) Variation in the
polychlorinated biphenyl and organochlor pesticide residues
during human breastfeeding and its diurnal pattern.
Chemosphere, 7: 699-706.
MES, J., COFFIN, D.E., & CAMPBELL, D.T. (1974)
Polychlorinated biphenyl and organochlorine pesticide residues
in Canadian chicken eggs. Pestic. Monit. J., 8: 8-11.
MIYAZAKI, T., AKIYAMA, K., KANEKO, S., HORII, S., & YAMAGISHI,
T. (1980) Chlordane residues in human milk. Bull. environ.
Contam. Toxicol., 25: 518.
MOORE, R., TORO, E., STANTON, M., & KHAN, M.A.Q. (1977)
Absorption and elimination of 14C-alpha and gamma-chlordane by
a fresh-water alga, daphnid, and goldfish. Arch. environ.
Contam. Toxicol., 6: 411-420.
MORGAN, D.P. & ROAN, C.C. (1969) Renal function in persons
occupationally exposed to pesticides. Arch. environ. Health
19: 633-636.
NAKAS, J.P. (1977) Chlordane. Inhibition of endopeptidase
activity in the marine bacterium, Aeromonas proteolytica, New
Jersey, Rutgers State University, 123 pp (Dissertation).
NAQVI, S.M. (1973) Toxicity of twenty-three insecticides to
a tubificid worm Branchiura sowerbyi from the Mississippi
Delta. J. econ. Entomol., 66: 70-74.
NCI (1977) Bioassay of chlordane for possible
carcinogenicity, Bethesda, Maryland, National Cancer Institute
(CAS NO. 57-74-9).
OLOFFS, P.C., ALBRIGHT, L.J., & SZETO, S.Y. (1978)
Persistence of residues in water and sediment of a fresh-water
lake after surface application of technical chlordane.
J. environ. Sci. Health, B13: 47-58.
ONSAGER, J.A., RUSK, H.W., & BUTLER, L.I. (1970) Residues of
aldrin, dieldrin, chlordane in soil and sugar beets. J. econ.
Entomol., 63: 1143-1146.
ORTEGA, P., HAYES, W.J., & DURHAM, W.F. (1957) Pathologic
changes in the liver of rats after feeding low levels of
various insecticides. Am. Med. Assoc. Arch. Pathol., 64:
614-622.
PARLAR, H., HUSTERT, K., GAEB, S., & KORTE, F. (1979)
Isolation, identification, and chromatographic characterization
of some chlorinated C10 hydrocarbons in technical chlordane.
J. agric. food Chem., 27: 278-283.
PARRISH, P.R., SCHIMMEL, S.C., HANSEN, D.J., PATRICK, J.M., &
FORESTER, J. (1976) Chlordane: effects on several estuarine
organisms. J. Toxicol. environ. Health, 1: 485-494.
PARRISH, P.R., DYAR, E.A., ENOS, J.M., & WILSON, W.G. (1978)
Chronic toxicity of chlordane, trifluralin, and pentachlorophenol
to sheepshead minnows (Cyprinodon variegatus), Washington DC,
US Environmental Protection Agency (Report No. EPA-600/3-78-010).
PLANK, J., KEPLINGER, M.L., FANCHER, O.E., & RICHTER, W.R.
(1970) 90-day subacute oral toxicity of synthetic "X"
(oxychlordane) in albino rats, Illinois, Industrial Bio-Test
Laboratories (Report for Velsicol Chemical Corporation).
POHL, R.J. & FOUTS, J.R. (1977) Xenobiotic metabolism in
skin of hairless mice exposed to ultraviolet radiation,
aroclor 1260, or chlordane. Environ. Health Perspect., 20:
230.
POLEN, P.B., HESTER, M., & BENZIGER, J. (1971)
Characterization of oxychlordane, animal metabolite of
chlordane. Bull. environ. Contam. Toxicol., 5: 521-528.
POONAWALLA, N.H. & KORTE, F. (1971) Metabolism of trans-
chlordane-14C and isolation and identification of its
metabolites from the urine of rabbits. J. agric. food Chem.,
19: 467.
PRINCI, F. & SPURBECK, G.H. (1951) A study of workers
exposed to the insecticides chlordane, aldrin and dieldrin.
Arch. ind. Hyg. occup. Med., 3: 64-72.
REINKE, J., UTHE, J.F., & JAMIESON, D. (1972) Organochlorine
pesticide residues in commercially caught fish in Canada,
1970. Pestic. Monit. J., 6: 43.
ROBERTS, J.R., DEFREITAS, A.S.W., & GIDNEY, M.A.J. (1977)
Influence of lipid pool size on bioaccumulation of the
insecticide chlordane by northern redhorse suckers ( Moxostoma
macrolepidotum). J. Fish. Res. Board Can., 34: 89-97.
SAHA, J.A. & SUMNER, A.K. (1971) Organochlorine insecticide
residues in soil from vegetable farms in Saskatchewan.
Pestic. Monit. J., 5(1): 28-31.
SANBORN, J.R., METCALF, R.L., BRUCE, W.N., & LU, P.-Y.
(1976) The fate of chlordane and toxaphene in a
terrestrial-aquatic model ecosystem. Environ. Entomol., 5:
533-538.
SANDERS, H.O. & COPE, O.B. (1966) Toxicities of several
pesticides to two species of cladocerans. Trans. Am. Fish.
Soc., 95: 165-169.
SASCHENBRECKER, P.W. (1976) Levels of terminal pesticide
residues in Canadian meat. Can. vet. J., 17: 158-163.
SAVAGE, E.P. (1976) National study to determine levels of
chlorinated hydrocarbon insecticides in human milk: 1975-76,
Washington DC, US Environmental Protection Agency (Report,
Iss. EPA/540/9-78/005, Order no. PB284393).
SAVAGE, E.P., KEEFE, T.J., TESSARI, J.D., WHEELER, H.W.,
APPLEHANS, F.M., GOES, E.A., & FORD, S.A. (1981) National
study of chlorinated hydrocarbon insecticide residues in human
milk. Am. J. Epidemiol., 113: 413.
SCHIMMEL, S.C., PATRICK, J.M., & FORESTER, J. (1976a)
Heptachlor: toxicity to and uptake by several estuarine
organisms. J. Toxicol. environ. Health, 1: 955-965.
SCHIMMEL, S.C., PATRICK, J.M., & FORESTER, J. (1976b)
Heptachalor: uptake, depuration, retention, and metabolism by
spot Leiostomus xanthurus. J. Toxicol. environ. Health, 2:
169-178.
SCHWEMMER, B., COCHRANE, W.P., & POLAN, P.B. (1970)
Oxychlordane, animal metabolite of chlordane, isolation and
synthesis. Science, 169: 1087.
SHINDELL & ASSOCIATES (1981) Report of epidemiologic study
of the employees of Velsicol Chemical Corporation Plant,
Memphis, Tennessee, January 1952 - December 1979, Milwaukee,
Wisconsin (Report of a study sponsored by Velsicol Chemical
Coporation).
SIMMON, V.F., KAUHANEN, K., & TARDIFF, R.G. (1977) Mutagenic
activity of chemicals identified in drinking water. Dev.
Toxicol. environ. Sci., 2: 249-258.
SIMS, G.G., CAMPBELL, J.R., ZEMLYAK, F., & GRAHAM, J.M.
(1977) Organochlorine residues in fish and fishery products
from the Northwest Atlantic. Bull. environ. Contam. Toxicol.,
18: 697-705.
SINGHAL, R.L. & KACEW, S. (1973) Evidence for the role of
cyclic AMP in the mechanism of action of organochlorine
pesticides. J. Cell Biol., 59: 321.
SINGHAL, R.L. & KACEW, S. (1976) The role of cyclic AMP in
chlorinated hydrocarbon-induced toxicity. Fed. Proc., 35:
2618-2663.
SOVOCOOL, G.W. & LEWIS, R.G. (1975) The identification of
trace levels of organic pollutants in human tissue compounds
related to chlordane/heptachlor exposure. Environ. Health, 9:
265-279.
SOVOCOOL, G.W., LEWIS, R.G., HARLESS, R.L., WILSON, N.K., &
ZEHR, R.D. (1977) Analysis of technical chlordane by gas
chromatography mass spectrometry. Anal. Chem., 49: 734-740.
SPENCER, E.Y. (1973) Guide to the chemicals used in crop
protection, 6th ed., Ontario, Research Branch Agriculture
Canada, pp. 94-95 (Publication No. 1093).
SPYKER-CRANMER, J.M., BARRETT, J.B., AVERY, D.L., & CRANMER,
M.F. (1982) Immunoteratology of chlordane: Cell-mediated and
humoral immune responses in adult mice expressed in utero.
Toxicol. appl. Pharmacol., 62: 402-408.
STARR, H.G., Jr, ALDRICH, F.D., MCDOUGAL, W.D. III, & MOUNCE,
L.M. (1974) Contribution of household dust to the human
exposure to pesticides. Pestic. Monit. J., 8: 209-212.
STAUFFER, T.B. (1977) Chlordane volatility, Florida, Civil
and Environmental Engineering Development Office, pp. 1-29
(CEEDO-TR-77-9).
STENGER, R.J., PORWAY, M., JOHNSON, J.E.A., & DATTA, R.K.
(1975) Effects of chlordane pretreatment on the
hepatotoxicity of carbon tetrachloride. Exp. mol. Pathol.,
23: 144-153.
STEWART, D.K.R. (1975) Chlordane uptake from soil by root
crops. Environ. Entomol., 4: 254-256.
STOHLMAN, E.F., THORP, W.T.S, & SMITH, M.I. (1950) Toxic
action of chlordane. J. ind. Hyg. occup. Med., 1: 13-19.
STRASSMAN, S.C. & KUTZ, F.W. (1977) Insecticide residues in
human milk from Arkansas and Mississippi, 1973-74. Pestic.
Monit. J., 10: 130-133.
STREET, J.C. & BLAU, S.E. (1972) Oxychlordane - accumulation
in rat adipose tissue on feeding chlordane isomers or
technical chlordane. J. agric. food Chem., 20: 395-397.
STREET, J.C., MAYER, F.L., & WAGSTAFF, J. (1969) Ecological
significance of pesticide interactions. Ind. Med. Surg., 38:
409-414.
SURBER, E.W. (1948) Chemical control agents and their
effects on fish. Prog. Fish. Cult., 10: 125-131.
TALAMANTES, F. & JANG, H. (1977) Effects of chlordane
isomers administered to female mice during the neonatal
period. J. Toxicol. environ. Health, 3: 713-720.
TASHIRO, S. & MATSUMURA, F. (1977) Metabolic routes of cis-
and trans-chlordane in rats. J. agric. food Chem., 25:
872-880.
TAYLOR, J.R., CALABRESE, V.P., & BLANKE, R.V. (1979)
Organochlorine and other insecticides. In: Vinken, P.J. &
Bruyn, G.W., ed. Handbook of clinical neurology, Vol. 36.
Intoxications of the nervous system, Amsterdam, New York,
Elsevier/North-Holland Biomedical Press, Part 1.
TELANG, S., TONG, C., & WILLIAMS, G.M. (1982) Epigenetic
membrane effects of a possible tumour promoting type on
cultured liver cells by the non-genotoxic organochlorine
pesticides chlordane and heptachlor. Carcinogenesis, 3:
1175-1178.
TONG, C., FAZIO, M., & WILLIAMS, G.M. (1981) Rat
hepatocyte-mediated mutagenesis of human cells by carcinogenic
polycyclic aromatic hydrocarbons but not organochlorine
pesticides. Proc. Soc. Exp. Biol. Med., 167: 572-575.
TRUDGILL, P.W., WIDDUS, R., & REES, J.S. (1971) Effects of
organochlorine insecticides on bacterial growth, respiration,
and viability. J. gen. Microbiol., 69: 1-13.
TRUHAUT, R., GAK, J.C., & GRAILLOT, C. (1974) Study of the
modalities and action mechanisms of organochlorine
insecticides. I. Comparative study of the acute toxicity in
hamster and rat. J. Eur. Toxicol., 7: 159-166.
TUCKER, R.K. & CRABTREE, D.G. (1970) Handbook of toxicity to
wildlife, Washington DC, US Department of the Interior, Bureau
of Sport Fishing and Wildlife Research (Publication No. 84).
US EPA (1976a) Pesticidal aspects of chlordane in relation
to man and the environment, Washington DC, US Environmental
Protection Agency (US Department of Commerce, National
Technical Information Service, publication No. PB-2577107).
US EPA (1976b) Pesticidal aspects of chlordane and
heptachlor in relation to man and the environment. A further
review 1972-1976, Washington DC, US Environmental Protection
Agency, p. 33 (US Department of Commerce, National Technical
Information Service, publication No. PB-258339).
US NAS (1977) An evaluation of the carcinogenicity of
chlordane and heptachlor. Natl Acad. Sci. J., October,
Washington, DC.
VEITH, G.D., DEFOE, D.L., & BERGSTEDT, B.V. (1979) Measuring
and estimating the bioconcentration factor of chemicals in
fish. J. Fish. Res. Board Can., 36: 1040-1048.
VILLENEUVE, D.C., GRANT, D.L., & PHILLIPS, W.E. (1972)
Modification of pentobarbital sleeping times in rats following
chronic polychlorinated biphenyl ingestion. Bull. environ.
Contam. Toxicol., 7: 264-269.
VON RUMKER, R., LAWLESS, E.W., MEINERS, A.F., LAWRENCE, K.A.,
KELSO, G.L., & HORAY, F. (1974) Production, distribution,
use and environmental impact potential of selected pesticides.
Natl Tech. Inf. Serv. (PB-236), 795: 439.
WANG, H.H. & GRUFFENMAN, S. (1981) Aplastic anemia and
occupational pesticide exposure: A case-control study.
J. occup. Med., 23: 364-366.
WANG, H.H. & MACMAHON, B. (1979a) Mortality of workers
employed in the manufacture of chlordane and heptachlor.
J. occup. Med., 26: 745-748.
WANG, H.H. & MACMAHON, B. (1979b) Mortality of pesticide
applicators. J. occup. Med., 26: 741-744.
WAZETER, F.X. (1967) Two-year chronic feeding study in the
beagle dog, Mattawan, Michigan, International Research and
Development Corporation (Report for Velsicol Chemical
Corporation).
WAZETER, F.X., BUTLER, R.H., GEIL, R.G., & REHKAMPER, J.A.
(1968) Alpha-chlordane, gamma-chlordane, alpha gamma-
chlordane. Comparative acute oral toxicity (LD50) in male
albino rats, Mattawan, Michigan, International Research and
Development Corporation (Report for Velsicol Chemical
Corporation.
WELCH, R.M. (1948) Tests of the toxicity to sheep and cattle
of certain of the newer insecticides. J. econ. Entomol., 41:
36-39.
WELCH, R.M., LEVIN, W., KUNTZMAN, R., JACOBSON, M., & CONNEY,
A.H. (1971) Effect of halogenated hydrocarbon insecticides
on the metabolism and uterotropic action of estrogens in rats
and mice. Toxicol. appl. Pharmacol., 19: 234-246.
WHO (1982) Guidelines for drinking water quality, Vol. 1,
Recommendations, Geneva, World Health Organization, 82 pp
(EFP/82.39).
WHO (1984) The use of WHO recommended classification of
pesticides by hazard, Geneva, World Health Organization
(Unpublished Report VBC/84.2).
WHO/FAO (1978) Chlordane, Geneva, World Health Organization
(Data Sheets on pesticides No. 36).
WILLIAMS, G.M. (1979) Liver cell culture systems for the
study of hepatocarcinogenesis. In: Margison, G.P., ed.
Advances in medical oncology research and education, Oxford,
Pergamon Press, Vol. 1, pp. 273-280.
WILLIAMS, C.H. & CASTERLINE, J.L., Jr (1970) Effects on
toxicity and on enzyme activity of the interactions between
aldrin, chlordane, piperonyl butoxide and banol in rats.
Proc. Soc. Exp. Biol. Med., 135: 46-50.
WILLIAMS, C.H., CASTERLINE, J.L., & JACOBSON, K.H. (1967)
Studies of toxicity and enzyme activity from interaction
between chlorinated hydrocarbon and carbamate insecticides.
Toxicol. appl. Pharmacol., 11: 302-307.
WILSON, A.J. (1965) Chemical assays. In: Annual report for
the fiscal year ending June 30, 1965, Gulfbreeze, Florida,
Bureau of Community Fisheries, Biology Laboratory, 247 pp
(Circular 6-7).
WILSON, D.M. & OLOFFS, P.C. (1973a) Residues in alfalfa
following soil treatment with high purity chlordane (Velsicol
HCS-3260). Bull. environ. Contam. Toxicol., 9: 337-344.
WILSON, D.M. & OLOFFS, P.C. (1973b) Persistence and movement
of a and q chlordane in soils following treatment with
high-purity chlordane (Velsicol HCS-3260). Can. J. Soil Sci.,
53: 465-472.
WRIGHT, L.H., LEWIS, R.G., CRIST, H.L., SOVOCOOL, G.W., &
SIMPSON, J.M. (1978) The identification of polychlorinated
terphenyls at trace levels in human adipose tissue by gas
chromatography/mass spectrometry. J. anal. Toxicol., 2: 76-79.
 Molecular formula: C10H6C18
CAS chemical name: 1,2,4,5,6,7,8,8-octachloro-2,3,3a,4,
7,7a-hexahydro-4,7-methano-1H-indene
Common trade names: Aspon, Belt, CD 68, Chlorindan,
Chlorkil, Chlordane, Corodan,
Cortilan-neu, Dowchlor, HCS 3260,
Kypchlor, M140, Niran, Octachlor,
Octaterr, Ortho-Klor, Synklor, Tat
Chlor 4, Topichlor, Toxichlor,
Velsicol-1068
CAS registry number: 57-47-9
Relative molecular mass: 409.8
2.2. Properties and Analytical Methods
2.2.1. Physical and chemical properties
Chlordane is a viscous, light yellow to amber-coloured liquid
(IARC, 1979) with a melting point of 106 - 107 °C for the alpha-
isomer and 104 - 105 °C for the gamma-isomer. It has a density of
1.59 - 1.63 g/ml and a vapour pressure of 1 x 10-5 mm Hg at 25 °C.
It is insoluble in water but soluble in most organic solvents.
The major isomers of chlordane have the endo-endo-configuration
on the carbon skeleton (US EPA, 1976a,b). However, the term
chlordane actually refers to a complex mixture of chlordane
isomers, other chlorinated hydrocarbons and by-products. According
to the Canada National Research Council (1974), the technical
product is described as follows:
"Technical chlordane is a mixture of insecticidal
components, including chlorinated addition and substitution
derivatives of 4,7-methano-3a,4,7,7a-tetrahydro-indane.
The chlorine content is 64-67%. The principal components
are alpha- and gamma-chlordane (C10H6 x Cl8), heptachlor
(C10H5Cl7) and non-achlor (C10H5Cl9). Technical chlordane
conforms to the biological, chemical and physical properties
of reference technical chlordane".
The production of technical chlordane is strictly controlled
and its composition varies within narrow limits (Canada, National
Research Council, 1974). Technical chlordane is a mixture of at
least 26 different components, mainly however alpha- and gamma-
chlordane. Up to 14 distinct chromatographic components have been
described (Cochrane et al., 1975; Cochrane & Greenhalgh, 1976; US
EPA, 1976a,b; Gaeb et al., 1977; Sovocool et al., 1977; Kadam et
al., 1978; Parlar et al., 1979). The composition has been
essentially, but not completely, worked out. Chlordane is
available in the USA in five basic formulations (von Rumker et al.,
1974, IARC, 1979), including 5% granules, oil solutions containing
chlordane at 2 - 200 g/litre, and emulsifiable concentrates
containing chlordane at 400 - 800 g/litre.
2.2.2. Analytical methods
Determination of chlordane residues is difficult because
of the complex nature of the components and the fact that each
component degrades independently. Resulting residues may bear
little relation to the proportions in the technical product
(Council for Agriculture Science and Technology, 1975).
Separation from interfering materials can be carried out by thin-
layer chromatography or other partition and clean-up methods (US
EPA, 1976a,b). Extraction from crops, other plant products, dairy
products, plants, and oils was achieved with an 80 - 110%
efficiency using acetonitrile for extraction, petroleum ether for
partitioning, and clean-up on a Florisil column (Canada, National
Research Council, 1974). Gel-permeation chromatography can also be
used for clean-up, particularly with human adipose tissue (Wright
et al., 1978).
The principal method for the qualitative and quantitative
estimation of chlordane isomers is gas-liquid chromatography with
electron capture detection (US EPA, 1976a,b). This method has a
high sensitivity and specificity (Canada, National Research
Council, 1974; Cochrane et al., 1975). According to Atallah et al.
(1977), the highly sensitive electron capture detector can,
however, lead to the incorrect identification of residues of
chlordane and its metabolites. Confirmation of gas-chromatographic
analysis can be carried out with GLC-Mass spectrometry, a method
that can also give a better determination of some of the components
such as heptachlorepoxide (US EPA, 1976a,b). Other methods of
detection include bioassay, carbon-skeleton chromatography,
colorimetric, and total chlorine methods (US EPA, 1976a,b).
Analysis for total organically-bound chlorine (Canada, National
Research Council, 1974) remains the preferred method for the
determination of technical chlordane and the active ingredient
(chlordane) in formulations.
3. SOURCES OF ENVIRONMENTAL POLLUTION, ENVIRONMENTAL
TRANSPORT AND DISTRIBUTION
3.1. Sources of Environmental Pollution
3.1.1. Industrial production and uses
Chlordane was first prepared in the 1940s by exhaustive
chlorination of the cyclopentadiene-hexachlorocyclopentadiene
adduct (IARC, 1979). It was first described as an insecticide in
1945 by Kearns (Spencer, 1973).
Chlordane is produced commercially by reacting hexachloro-
cyclopentadiene with cyclopentadiene to form chlordene, which is
then chlorinated to produce chlordane (IARC, 1979). Chlordane was
first produced commercially in the USA in 1947 (IARC, 1979).
Production in the USA, in 1974, amounted to 9.5 million kg (IARC,
1979). Chlordane is not produced in Europe nor has it ever been
manufactured in Japan (IARC, 1979). In Japan, the only permitted
use of the compound is for the control of termites. It is also
used against wood-boring beetles and in ant baits. Both the
amounts of chlordane produced and used have decreased considerably
in recent years (WHO, 1982).
Chlordane has been used as an insecticide for more than 35
years. It is a versatile, broad spectrum, contact insecticide and
is used mainly for non-agricultural purposes (primarily for the
protection of structures, but also on lawn and turf, ornamental
trees, and drainage ditches) (von Rumker et al., 1974). Further-
more, it is used on corn, potatoes, and livestock. In 1978, a US
EPA cancellation proceeding led to a settlement on contested uses.
This settlement allowed for limited usage by crop, location, amount
allowed, and maximum time interval for use.
Since 1 July 1983, the only use of chlordane approved in the
USA is for the control of underground termites (IARC, 1979). In
Canada, the use of chlordane is controlled under the Pest Control
Products Act and it is used for the protection of structures,
ornamental plants, lawns, and various crops. Accepted uses vary
from province to provide (Canada, National Research Council, 1974).
3.2. Environmental Transport and Distribution
3.2.1. Air
Entry into the atmosphere occurs mainly through aerial
applications of dusts and sprays, soil erosion by the wind, and
volatilization from soil and water (Canada, National Research
Council, 1974).
3.2.2. Water
Few data are available on the routes of entry or the behaviour
and fate of chlordane in aquatic systems. It can be assumed that
not much originates from ground water since there is little
leaching of chlordane. One possible source is surface run-off, but
no studies have tested the extent of this assumption. Another
source is rain; however, in two studies, chlordane levels did not
exceed 2 - 3 ng/litre rain water (Bevenue et al., 1972a; US EPA,
1976a,b).
One important aspect of chlordane residues is that they
accumulate in sediment. The fate and behaviour of chlordane was
investigated in an isolated fresh water lake, previously free from
pesticide residues (Oloffs et al., 1978). The lake was treated
with technical chlordane at 10 µg/litre, and sediment samples were
analysed for chlordane residues 7, 24, 52, 279, and 421 days after
treatment. It was found that water residue concentrations declined
rapidly. After 7 days, only 46.1% of the chlordane residue
remained. After 421 days, residues were still detectable, but all
levels were below 0.01% of the initial concentration. It was
observed that chlordane residues moved quickly to the bottom
sediment and persisted there. Mean residue levels in sediment were
35.29 µg/kg wet weight after 7 days and 10.31 µg/kg after 421 days.
3.2.3. Soil
Chlordane is used almost exclusively as a soil insecticide to
control soil pests such as termites (Canada, National Research
Council, 1974). Thus, residues of chlordane are mainly present in
this environmental compartment. In most temperate climates, only
the two chlordane isomers generally persist (Canada, National
Research Council, 1974). For example, in Nova Scotia, chlordane
was applied at 5 kg/ha per year to sandy loam soil for 3 years.
Fifteen years later, approximately 15% of the residues remained,
the alpha and gammma isomers being the major components (US EPA,
1976a,b).
The components of technical chlordane are relatively insoluble
in water and are readily adsorbed onto soil particles. As a
result, one of the characteristics of soil residues is that they do
not migrate readily through the soil profile (Canada, National
Research Council, 1974; von Rumker et al., 1974). In general, not
more than 15% of the residues migrate below the cultivated layer
(Canada, National Research Council, 1974). As a result, residues
are not likely to become a serious contaminant of the lower soil
strata or deep water sources (Canada, National Research Council,
1974). The organic matter and moisture contents of the soil can
affect the volatilization of chlordane components (Stauffer, 1977).
The organic matter causes greater adsorption and thus reduces
volatilization while soil moisture increases volatilization
(Stauffer, 1977). Also, liquid formulations are more volatile than
granular (Atallah et al., 1979).
3.2.4. Abiotic degradation
Chlordane is stable to light under normal conditions. When it
is exposed to photosensitizers such as rotenone or benzophenone and
short irradiation exposure at wavelengths above 300 nm, some
components will isomerize (Canada, National Research Council,
1974). No detectable degradation products were formed on plant
foliage in the absence of a photosensitizer (Ivie et al., 1972).
3.2.5. Biodegradation
Three conversion products of gamma-chlordane were found in
white cabbage and carrots, 4 weeks after application. One of the
two metabolites isolated from white cabbage (35% of the total), was
given the chlordene chlorohydrin structure. The other isolated
metabolite (15% of the total) was assigned the dihydroxy-beta-
dihydroheptachlor structure. The third metabolite was not
identified. 1,2-Dichlorochlordene, oxychlordane, and photo-alpha-
chlordane, as well as the parent chlordane compounds, were found in
alfalfa after treatment of the soil with chlordane (Canada,
National Research Council, 1974).
Oxychlordane (or 1,2-dichlorochlordene epoxide) is the common
metabolite derived from both alpha- and gamma-chlordane. It has
been found in the fat of pigs fed either of the isomers and in the
milk and cheese from cows fed alfalfa treated with technical
chlordane. According to some authors, alpha- and gamma-chlordane
give rise to oxychlordane via the intermediate 1,2-dichloro-
chlordene (Canada, National Research Council, 1974).
4. ENVIRONMENTAL LEVELS AND EXPOSURES
4.1. Environmental Levels
4.1.1. Air
Generally, atmospheric concentrations of chlordane appear to be
insignificant. However, chlordane has been detected in the air of
buildings where the compound has been used for termite or other
insect control (US EPA, 1976a,b). Insufficient information is
available on this.
4.1.2. Water
Data from several studies indicate that contamination of water
with chlordane is not a widespread problem (US EPA, 1976a,b), and
that, generally, water residue levels are non-measureable or very
low (Canada, National Research Council, 1974).
In a study of the bottom material of 26 tributary streams in
San Fransisco Bay, chlordane was found to be ubiquitous at
concentrations ranging from a trace to 800 µg/kg (Oloffs et al.,
1978).
No chlordane was detected in 188 samples of surface water from
southern Florida but it was detected in 30% of 214 sediment samples
(Mattraw, 1975). In a study in Hawaii in 1970-71 (Bevenue et
al., 1972b), chlordane was found in drinking-water in 9% of samples
at a mean level of 1.0 ng/litre. Chlordane was detected in non-
potable waters from canals at levels ranging from 3.7 - 9.1
ng/litre. Again, sediments showed much higher levels of 190 - 378
µg/kg. Chlordane was found to occur in the lower Mississippi river
almost continuously throughout 1974 at values ranging from 1.3 to
2.9 ng/litre (Brodtmann, 1976). In a study in the lower
Mississippi (Barthel et al., 1969), during 1964-67, chlordane
residues ranged from 0.80 to 2.80 mg/kg in river bed material
samples. In tributaries of the river, values ranged from 0.56 -
6.44 mg/kg, in 13 out of 348 samples. No chlordane was detected in
any water or sediment samples taken from the upper Great Lakes in
1974 (Glooschenko et al., 1976). In one study in 1976 (Harrington
et al., 1978), chlordane contamination of a municipal water system
was reported, concentrations of chlordane accidently rising to 1.2
g/litre.
4.1.3. Soil
One study has shown that the alpha- and gamma-isomers of
chlordane are less persistent in mineral sand soil than in organic
mucky soil (Harris & Sans, 1976). Data from the National Soils
Monitoring Program in 1970 showed that chlordane occurred in 0.07%
of 1346 sites in 35 States. The range of residues was 0.01 - 13.34
mg/kg dry weight with a mean of 0.08 mg/kg (Crockett et al., 1974).
Monitoring of the corn belt region in the USA (12 States) in 1970
showed chlordane to be one of the most commonly detected
insecticides with values of ND - 0.20 mg/kg (Carey et al., 1973).
Data from 9 States in 1971 detected chlordane in one soil sample at
0.04 mg/kg dry weight (Gowen et al., 1976). Monitoring in 1973
showed residues ranging from 0.001 - 0.020 mg/kg with generally
higher values in urban areas. When urban soils were tested in 14
cities in the USA in 1970, values ranged from 0.01 - 1.27 mg/kg.
In the Atlantic provinces of Canada, chlordane was detected in less
than 10% of agricultural lands at concentrations below 1 mg/kg dry
weight (Duffy & Wong, 1967). In another study on chlordane in
Saskatchewan soils (Saha & Sumner, 1971), 7 out of 41 samples
contained chlordane with residue values ranging from 0.01 to 3.91
mg/kg dry weight. The duration of soil contamination has been
studied by several investigators. Using bioassay techniques, it
was found in one study that 15% of active ingredients remained in
turf soils in Wisconsin after 12 years (Lichtenstein & Poliuka,
1959). In a study in 1970 (Lichtenstein, 1970), it was found that
10 years after application of chlordane at 8.5 kg /ha,
approximately 18 - 20% remained.
4.1.4. Crops and wildlife
Since chlordane residues are present predominantly in the soil,
translocation into plants is an important factor. In most
temperate climates, alpha- and gamma-chlordane are the principal
plant residues. In the Canadian climate, the composition of plant
residues resembles that of technical chlordane (Canada, National
Research Council, 1974). In a field study on soil/plant
relationships, it was found that the relationship between residues
in soil and those in crops was not consistent and consequently not
predictable (Boyd, 1971).
In a three-year study, Onsager et al. (1970) monitored
chlordane residues in sugar beets cultivated on loam soil treated
once at 6 different application rates ranging from 1.4 - 22.4
kg/ha. In the first growing season, only sugar beets treated at
the two lowest rates (1.4 and 2.8 mg/kg) showed residues below 0.3
mg/kg dry weight. In the last two seasons, beets from soil treated
at all rates contained residues below this value. In another
study, uptake by root crops was shown to be related to the soil
type (Stewart, 1975). When chlordane was applied to sandy loam
soil with 12% clay at 15 kg/ha, residues in beets, carrots,
parsnips, potatoes, and rutabagas were 0.03, 0.26, 0.24, 0.04, and
0.01 mg/kg, respectively. In sandy loam containing 28% clay,
values were 0.01, 0.07, 0.12, 0.15, and 0.01 mg/kg, respectively.
A study of residues in alfalfa following applications of high
purity chlordane showed that during the first 4 months following
treatment, oxychlordane and photo-alpha-chlordane accounted for 16%
and 17% of the residues, respectively (Wilson & Oloffs, 1973a).
Generally, no detectable residues of chlordane were found in
wildlife such as birds (Canada, National Research Council, 1974;
Fitzhugh & Fairchild, 1976; Clark & Krynitsky, 1978). However,
in one study (Clark & Prouty, 1976), mean total residues of
oxychlordane ranging from 0.11 to 6.63 µg found in the carcasses
of bats from Maryland and Virginia were attributed to chlordane
use.
In extensive surveys, residues in fish have generally been low.
In 1976, residues in several species from Lake Erie and Lake Saint
Claire in Canada were found to range from non-detectable to 0.046
mg/kg fresh weight (Frank et al., 1978a,b). Residues in Canadian
commercially caught fish in 1970 were not detectable (Reinke et
al., 1972). The National Pesticide Monitoring Program from 1967-68
found chlordane residues in 128 out of 590 fish samples at levels
generally less than 0.5 mg/kg (Wilson & Oloffs, 1973b). From
1972-76, chlordane residues were found in only 3% of the samples of
estuarine fish in the USA (Butler & Schutzmann, 1978). Chlordane
residues were also not detectable in fish and fishery products from
the Northwestern Atlantic (Meith-Avcin et al., 1973; Sims et al.,
1977).
4.1.5. Food
There have been many studies in Canada, Great Britain, the USA,
and other countries on the occurrence of pesticide residues in
food. Generally, the results of these studies showed that residues
of chlordane seldom occur and uptake by man is negligible (Canada,
National Research Council, 1974). Residue tolerances for chlordane
have been established at the following levels: Belgium, Luxembourg,
and the Netherlands, 0.1 mg/kg, Canada, 0.3 mg/kg, European
Economic Community, 0.2 mg/kg and the USA, 0.3 mg/kg. These levels
are for a wide variety of foods (US EPA, 1976a,b). A temporary
acceptable daily intake (ADI) for human beings for the sum of the
alpha- and gamma-isomers of chlordane and oxychlordane of 0 - 0.001
mg/kg body weight was advised by the Joint Meeting on Pesticide
Residues (FAO/WHO, 1983). Chlordane is rarely present in market
basket surveys, and then only at low levels. It is not among the
top 10 chlorinated pesticides usually found as residues in food (US
EPA, 1976a,b). For example, in a survey in the USA from 1963 to
1969, chlordane residues were found in less than 1% of the samples
and ranged from 1 - 5 µg/kg.
It has already been shown that crops will translocate chlordane
residues from the soil. Generally, the amounts in crops are low.
Residues tend to accumulate in the crude oils of oil-seed crops at
levels higher than those in the original seed and in the oil-seed
meal. However, these levels are reduced by refining processes.
Chlordane residues were found in meat, milk, and eggs. Residues in
feed crops or from direct applications to cattle and poultry were
shown to result in significant residues in milk, meat, and eggs (US
EPA, 1976a,b). In a study on eggs in Canada, gamma-chlordane was
found in 78% of the samples with a mean value of 2 µg/kg fresh
weight and alpha-chlordane in 81% of the eggs with a mean value of
1 µg/kg (Mes et al., 1974). In another study (Herrick et al.,
1969), no residues were found in the eggs of chickens fed chlordane
in their diet at 0.08 mg/kg for a week.
In a study on samples of cow's milk analysed in the USA, 87%
were positive for chlordane with levels ranging from 0.02 to 0.06
mg/litre (IARC, 1979). In another study (US EPA, 1976a,b), the
milk of cows grazing on pastures with chlordane applied at 0.55
kg/ha contained an average chlordane concentration of 0.03
mg/litre. No residues were found at lower treatment levels.
Chlordane was also found in Canadian meat samples at levels ranging
from 0 to 106 µg/kg in beef, 0 to 32 µg/kg in pork, and 0 to 70
µg/kg in fowl (Saschenbrecker, 1976).
4.1.6. Human milk
Several studies on pesticide residues in human breast milk did
not reveal any residues of chlordane, but oxychlordane, trans-
nonachlor, and heptachlor epoxide were found, which may be related
to chlordane exposure. In a study on 54 women in Arkansas and
Mississippi from 1973-74, breast milk contained oxychlordane at
0.005 mg/litre, heptachlor epoxide at 0.004 mg/litre, and trans-
nonachlor at 0.001 mg/litre (Strassman & Kutz, 1977). In another
study on 34 samples of breast milk in Northern Mississippi from
1973-75, oxychlordane levels were found of 0.005 mg/litre in high-
pesticide-usage areas and 0.002 mg/litre in low-usage areas
(Barnett et al., 1979). In a survey involving 1436 lactating
mothers in the USA, the mean levels of oxychlordane in the milk
ranged from 75.4 - 116 µg/litre on an adjusted fat basis (Savage,
1976). In a study of Canadian human milk samples in 1974,
oxychlordane was found in 77% of the samples, trans-nonachlor in
68%, and heptachlor epoxide in 69%, each at a mean level of 1
mg/litre whole milk (Mes & Davies, 1978).
Jensen (1983) recently reviewed the levels of chlordane
and oxychlordane in human milk, and his data, including the
above-mentioned studies as well as more recent data, are
reproduced in Table 1.
4.2. General Population Exposure
Oxychlordane was found together with other organochlorine
pesticides in human fat samples at levels ranging from 0.03 to
0.4 mg/kg wet weight (mean 0.14 mg/kg) in residents of the USA
(Biros & Enos, 1973). Sovocool & Lewis (1975) also reported
the identification of oxychlordane in human fat. As indicated
by Biros & Enos (1973), the occurrence of oxychlordane
residues in human adipose tissue in the general population may
reflect previous exposure to chlordane and/or oxychlordane.
This organochlorine compound is included in the human tissue
residue monitoring program (Kutz et al., 1976).
4.3. Occupational Exposure
Permissible levels of exposure to chlordane in the workplace
air have been adopted in different countries (ILO, 1980). Examples
include: 0.5 mg/m3 as a time-weighted average concentration in
Belgium, Finland, Japan, the Netherlands, and the USA (both OSHA
and ACGIH), 0.3 mg/m3 as a time-weighted average and 0.6 mg/m3
as a ceiling concentration in Romania, and 0.01 mg/m3 as maximum
allowable concentration in the USSR.
People primarily exposed are those employed in the application
of chlordane for the control of insects and pests (IARC, 1979).
Chlordane has been found in household dust in the homes of farmers
(mean level 5.79 mg/kg air-dried dust) and pesticide formulators
(mean level 23.11 mg/kg) (Starr et al., 1974).
Table 1. Chlordane and oxychlordane in human milka
------------------------------------------------------------------------------------------------------
Oxychlordane and
chlordane content inb
---------------------
No. of samples Fat Whole milk Milk fat
Area, year (% positive) % (mean) (mg/litre) (mg/kg) References
------------------------------------------------------------------------------------------------------
Canada (1975) 100 2.2 1 - Mes & Davies (1978)
(< 2)
Japan
Tokyo (1978) 11 - 0.5 - Miyazaki et al. (1980)
(0.1 - 1.0)
Tokyo (1979) 12 - 0.5 - Miyazaki et al. (1980)
(0.3 - 1.1)
Mexico (1976) 620 - - 0.40 (median) FAO/WHO (1981)
Spain (1979) 45 (17.8%) - 0.3c 0.026c Lora et al. (1979)
(0 - 0.72)
USA
Arkansas/Mississippi 57 (46%) 3.0 12/10 - Strassman & Kutz (1977);
(1973-4) (0 - 20) FAO/WHO (1981)
------------------------------------------------------------------------------------------------------
Table 1. (contd.)
------------------------------------------------------------------------------------------------------
Oxychlordane and
chlordane content inb
---------------------
No. of samples Fat Whole milk Milk fat
Area, year (% positive) % (mean) (mg/litre) (mg/kg) References
------------------------------------------------------------------------------------------------------
Hawaii (1979-80) 50 (100%) 3.2 - 0.059 Takahashi et al. (1981)
(0.01 - 0.16)
Mississippi (1973-5) 34 (100%) - 5 0.13 Barnett et al. (1979)
(pesticide area) (1 - 22) (0.03 - 0.70)
Mississippi (1973-5) 6 (68%) - 2 0.05 Barnett et al. (1979)
(non-pesticide area) (0 - 4) (0 - 0.12)
USA-NE (1975) 233 - - 0.08 ± 0.05 Savage (1976);
Savage et al. (1981)
USA-SE (1975) 288 - - 0.12 ± 0.15 Savage (1976);
Savage et al. (1981)
USA-MW (1975) 378 - - 0.08 ± 0.05 Savage (1976);
Savage et al. (1981)
USA-SW (1975) 388 - - 0.11 ± 0.35 Savage (1976);
Savage et al. (1981)
USA-NW (1975) 149 - - 0.08 ± 0.05 Savage (1976);
Savage et al. (1981)
USA (total) (1975) 1 436 (74%) - 2 (median) 0.096 ± 0.195 Savage (1976);
(0.013 - 0.57) Savage et al. (1981)
FAO/WHO (1981)
------------------------------------------------------------------------------------------------------
a From: Jensen (1983).
b Results are expressed as means ± S.D. Ranges are listed in parentheses below.
c Chlordane.
5. KINETICS AND METABOLISM
5.1. Absorption
In studies on 4 male rabbits, a combination of 14C-alpha and
gamma-chlordane (approximately 1700 mg of each, administered orally
in 4 doses at 4-day intervals), was well absorbed (Balba & Saha,
1978). Brief exposure of dogs to topically applied chlordane
solutions (3.2 g/litre) resulted in a significant and long-lasting
decrease in the biological half-life of orally-administered
warfarin (Bachmann & Burkman, 1974).
5.2. Distribution and Storage
Studies using radio-labelled chlordane showed that after oral
administration, the radioactivity was well distributed in tissues
of rats (Barnett & Dorough, 1974) and rabbits (Balba & Saha, 1978).
Rats, whether being treated with single oral doses of chlordane or
fed diets containing this compound, retained the highest levels of
residues in adipose tissue, followed by the liver, kidney, brain,
and muscle. More of the gamma-isomers was retained than of the
alpha-isomer. Residues in the fat of rats fed radiolabelled
chlordane (3:1 alpha- and gamma-chlordane) at 1, 5, and 25 mg/kg
diet for 56 days were approximately 3 times higher than those in
the diets. Oxychlordane was the most persistent residue in the
tissues of these rats after chlordane was removed from the diet
(Barnett & Dorough, 1974). The tissue distribution of chlordane in
rabbits was found to be similar to that in rats (Poonawalla &
Korte, 1971; Balba & Saha, 1978).
5.3. Biotransformation
Poonawalla & Korte (1971) showed that 70% of gamma-chlordane
fed to rabbits was excreted in the urine in the form of
metabolities, a.o., gamma-1-hydroxy-2-chlorodihydrochlordene and
1,2-dihydroxydichlordene.
Oxychlordane has been isolated from the fat of dogs, rats
(Polen et al., 1971), pigs (Schwemmer et al., 1970), and cattle
(Lawrence et al., 1970). It has also been isolated from human fat
(Biros & Enos, 1973). Barnett & Dorough (1974) indicated that the
faecal extracts of rats fed 14C-chlordane showed the presence of
eight radioactive areas on the TLC plate. Although the structures
of the metabolites were not fully elucidated, they were tentatively
identified as mono-, di-, and tri-hydroxylated products of
chlordane.
The major route of metabolism for both alpha- and gamma-
chlordane was via dichlorochlordene and oxychlordane (Tashiro &
Matsumura, 1977). The results of these studies were in general
agreement with the proposal of Street & Blau (1972) and the results
of in vitro metabolism studies of Brimfield et al. (1978).
Tashiro & Matsumura (1977) were able to isolate 1-exo-hydroxy-2-
endo-chloro-2,3-exoepoxychlordene, and found another major
metabolic route for alpha-chlordane that involved a more direct
hydroxylation reaction to form 1-exo-hydroxy-dihydrochlordenes and
1,2-gamma-dihydroxydihydrochlordene. Both Brimfield et al. (1978)
and Tashiro & Matsumura (1977) indicated that oxychlordane and, to
a lesser extent, heptachlor were metabolites of chlordane.
However, they did not agree as to whether these two metabolites
would be terminal residues or intermediates in the metabolic
pathways of chlordane. Recent investigations have indicated that
other metabolites were present in the urine of rabbits fed
chlordane. Thus, alpha-chlordane gave rise to 1-hydroxy-2-
chlorochlordene, 1-hydroxychlordene, and gamma-chlordene
chlorhydrin. Administration of the gamma-isomer resulted in
excretion in the urine of the rabbits of 1,2-dichlorochlordene,
1-hydroxy-2-chlorochlordene, gamma-chlordene chlorhydrin, and
3-hydroxychlordane (Balba & Saha, 1978).
In vitro metabolism studies have been summarized by Brimfield
& Street (1979). By incubation of alpha- and gamma-chlordane with
rat liver postmitochondrial supernatant, dichlorchlordene and
oxychlordane were isolated, a result that was similar to those from
in vivo studies. Hart et al. (1963) and Hart & Fouts (1965)
reported that chlordane induced non-specific microsomal enzyme
activity in the rat, resembling, from this point of view,
phenobarbital.
5.4. Elimination and Excretion
Elimination of radiolabelled chlordane (3:1 alpha- and gamma-
chlordane) and the individual isomers was studied in rats. Single
oral doses of 0.05, 0.2, and 1 mg/kg body weight in corn oil were
almost completely eliminated after 7 days; 24 h after administra-
tion, 70% of alpha-chlordane and 60% of the gamma-isomer were
excreted. Female rats excreted more of the dose in the urine than
the males (Barnett & Dorough, 1974).
6. STUDIES ON EXPERIMENTAL ANIMALS
The toxicity and the residue data on chlordane including some
unpublished studies have been reviewed several times by
international bodies such as FAO/WHO (1968, 1973, 1978, 1981,
1983), CEC (1981), and IARC (1979). For their conclusions, refer
to section 9.
6.1. Short-term Exposures
6.1.1. Oral exposure
The acute toxicity of chlordane in several animal species is
shown in Table 2.
The signs associated with acute chlordane poisoning include
ataxia, convulsions, respiratory failure, and cyanosis followed by
death (US EPA, 1976a,b). Correlation between respiratory
difficulty and EEG patterns suggest that respiratory failure is a
contributing factor in chlordane-induced mortality (Hyde &
Falkenberg, 1976). Pathological manifestations include haemorrhage
in the gastrointestinal tract, kidneys, lung, and heart as well as
pulmonary congestion and oedema, and degenerative changes in the
central nervous system (US EPA, 1976a,b).
Seven dogs were given chlordane in single oral doses ranging
from 200 - 700 mg/kg body weight. Convulsions were seen in one dog
at 200 mg/kg (lowest dose) but 700 mg/kg (highest dose) did not
induce any effects (Batte & Turk, 1948). Four groups of 2 - 4 dogs
were given chlordane orally in doses of 5 - 200 mg/kg body weight.
All of the dogs died within 25 days to 93 weeks (Lehman, 1952b).
Chlordane administered by stomach-tube to sheep at 500 mg/kg
body weight induced toxic symptoms (incoordination, partial
blindness). Full recovery occurred in 5 - 6 days. A dose of 1000
mg/kg body weight induced severe respiratory and nervous symptoms
16 h after treatment and death after 48 h (Welch, 1948).
When a diet containing 1000 mg chlordane/kg was fed to 12 male
rats, all of them died within 10 days (Stohlman et al., 1950). At
500 mg/kg all 12 died within 70 days and, at 300 mg/kg, 9 animals
out of 12 were alive after 100 days. Daily oral doses of 6.25 - 25
mg/kg body weight administered to 5 rats for 15 days did not induce
tremors or convulsions, but daily doses of 50 mg/kg body weight
induced toxic symptoms, and 2 of the animals died (Ambrose et al.,
1953). Cytoplasmic bodies in the liver cells were observed in all
groups and were dose-related.
Table 2. Acute toxicity of chlordane in experimental animals
---------------------------------------------------------------------------
Species Sex Route Vehicle LD50 Reference
(mg/kg)
---------------------------------------------------------------------------
Rat F dermal xylene 530 Gaines (1969)
Rat M dermal xylene 205 Gaines (1969a)
Rabbit NS dermal "early" < 780 Ingle (1965b)
chlordane
Rabbit NS dermal "later" 1100 - 1200 Ingle (1965b)
chlordane
(more purified)
Rat M oral peanut oil 335 Gaines (1969)
Rat F oral peanut oil 430 Gaines (1969)
Rat NS oral variety 200 - 590a Ambrose et al. (1953);
Ingle (1965a)
Rat NS oral NS 283 Buck et al. (1973)
Rat NS oral NS 350 Truhaut et al. (1974)
Rabbit NS oral NS 100 - 300a Stohlman et al. (1950)
Rabbit NS oral NS 20 - 40a Ingle (unpublished
data, 1955)
Hamster NS oral NS 1720 Truhaut et al. (1974)
Goat NS oral NS 180 Welch (1948)
Sheep NS oral NS 500 - 1000 Welch (1948)
Chicken NS oral NS 220 - 230 FAO/WHO (1968)
Mallard NS oral NS 1200 Buck et al. (1973)
Cow NS oral NS 25 - 90 Buck et al. (1973)
---------------------------------------------------------------------------
a The wide range is explained by the use of different solvents and the
fact that chlordane produced before 1950 contained a considerable
amount of hexachlorocyclopentadiene.
NS - Not specified.
6.1.2. Dermal exposure
The single-dose dermal LD50 of "early" chlordane in rabbits
was reported to be less than 780 mg/kg body weight (Ingle, 1965b),
and it was noted that this concentration caused severe skin
irritation, tremors, and convulsions (Lehman, 1952a). The dermal
LD50 of the later, more purified chlordane was 1100 - 1200 mg/kg
(Ingle, 1965b).
6.1.3. Parenteral exposure
Male gerbils were dosed intramuscularly with chlordane at 2.5
mg/kg body weight every 3 days for 45 days. Treatment induced
hyperproteinemia, hyperglycemia, and enhanced serum alkaline and
acid phosphatase activities (Karel & Saxena, 1976).
6.2. Long-term Exposures
6.2.1. Oral exposure
Groups of 4 - 7 male and 4 - 7 female dogs were fed dietary
levels of 0, 0.3, 3, 15, or 30 mg chlordane/kg for 2 years.
Abnormalities in the results of clinical liver function tests were
seen in the 15 and 30 mg/kg groups. In animals selected for
necropsy at the end of the first year, increased relative liver
weights and associated hepatocellular changes were found at 30
mg/kg; at the end of two years, dose-related increases in relative
liver weights were found at 15 and 30 mg/kg, with non-dose-related
hepatocellular changes. There was no difference between the
severity of the liver lesions of the 30 mg/kg animals and those of
four animals withdrawn from 30 mg/kg treatment for eight months
prior to sacrifice. Liver biopsies on two animals of the 30 mg/kg
group at 1, 3, and 6 months showed hepatocellular changes at 6
months but not at 1 or 3 months. No adverse effects were seen on
behaviour, appearance, survival, weight gain, blood picture, or the
results of periodic physical examinations, at any level (Wazeter,
1967).
Twenty-four rats, 12 of each sex, were fed dietary levels of
2.5, 25, or 75 mg chlordane/kg for 2 years (Lehman, 1952b). The
two higher levels caused moderate to severe signs of toxicity. The
lowest level caused histological liver changes. Rats were fed
technical chlordane (early production) for two years at levels in
the diet of 0, 5, 10, 30, 150 or 300 mg/kg (Ingle, 1952; 1965a).
Convulsions and tremors were observed in animals receiving 150
mg/kg or more. Hepatocellular alterations consisting of
hypertrophy, cytoplasmic oxyphilia and hyalinization, karyorrhexis,
karyolysis, and cell necrosis were obvious at 150 and 300 mg/kg,
slight at 30 mg/kg, minimal at 10 mg/kg and absent at 5 mg/kg.
Growth was retarded and liver weight and mortality rate increased
at 150 and 300 mg/kg. In a subsequent study on rats (Ingle,
1965a), technical chlordane of later production containing fewer
by-products was administered at levels of 2.5 - 300 mg/kg diet for
2 years. Changes in food consumption, growth, and mortality rate
were seen only at the highest dose. Cellular alterations were seen
at 50 mg/kg and higher.
Rats were fed chlordane at levels ranging from 10 to 1280 mg/kg
diet for 407 days (Ambrose et al., 1953). The animals in the two
highest dose groups died early; liver weight was increased at 320
mg/kg; fatty infiltration and cytoplasmic margination were seen in
the liver parenchymatous cells in males at 40 mg/kg and above, and
in females at 80 mg/kg and above.
Groups, each comprising 20 male and 20 female rats, were fed
dietary levels of 0, 5, 15, 25, or 35 mg/kg of alpha-chlordane, 15,
25, 35, or 75 mg/kg of gamma-chlordane or 5, 15, 25, 35 or 50 mg/kg
of a 1:1 mixture of alpha- and gamma-chlordane (Ingle, 1969). In
the group fed alpha-chlordane, growth retardation became apparent
in the rats fed 35 mg/kg after 4 months in males and after 5 months
in females; with gamma-chlordane, the 75 mg/kg group of males only
displayed growth retardation after 8 months. With the mixture,
growth retardation was evident in both sexes fed 50 mg/kg,
beginning earlier in males than in females. Growth retardation was
not evident in any group fed lower doses of either isomer. Food
consumption bore a relationship to growth. Increased mortality
rates for both sexes became significant in the groups fed alpha-
chlordane at 35 mg/kg, gamma-chlordane at 75 mg/kg, or the alpha-
gamma mixture at 50 mg/kg. Haematocrit was normal for all test
groups. Autopsy did not reveal any gross pathological lesions.
There was no evidence of tumours. Histological examination did not
show any changes from feeding chlordane in any organ except the
liver. Compression of sinusoids due to slight hepatic cell
hypertrophy in the centrolobular region and minimal bile duct
proliferation were evident with administration of alpha-chlordane
at 35 mg/kg. The same changes were noted, but were minimal with
the same isomer at 25 mg/kg. Slight to moderate cytoplasmic
homogeneity of the hepatic cells in the centrolobular region,
minimal cytoplasmic margination, and minimal cell hypertrophy with
compressed sinusoids were noted with administration of gamma-
chlordane at 75 mg/kg. Slight cytoplasmic homogeneity of hepatic
cells in the centrolobular region and occasional cytoplasmic
margination were observed with the alpha-gamma mixture at 50 mg/kg.
The above alterations were minimal with the same mixture at 35
mg/kg. No liver changes were evident after feeding lower levels of
the chlordane isomers.
Groups of 6 female and 6 male rats were fed 2.5 mg or 25 mg of
a sample of technical chlordane containing 60 - 75% chlordane and
25 - 40% unrelated products per kg diet for up to 9 months (Ortega
et al., 1957). Centrolobular cell hypertrophy, cytoplasmic
margination, and cytoplasmic bodies were observed in the liver in 1
male fed 2.5 mg/kg and in 5 males fed 25 mg/kg. No changes were
seen in females.
In a two-year feeding study, pure-bred male and female beagle
dogs were fed chlordane at levels of 0, 0.3, 3.0, 15, or 30 mg/kg
diet. No adverse treatment-related alterations were observed in
behaviour, appearance, eye examination, body weight, food
consumption, haematology, or plasma biochemistry. Some liver
enzyme activities were altered throughout the study at the 15 and
30 mg/kg levels. Relative liver weights were slightly increased
after two years in the two highest groups. Treatment-related
microscopic changes, observed in dogs fed the two highest levels,
consisted of enlargement of centrolobular hepatocytes with
margination of coarse cytoplasmic granules (IRDC, 1967).
The Joint Meeting on Pesticide Residues (JMPR) reviewed the
toxicity data on chlordane at its 1977 meeting (FAO/WHO 1978) and
decided on the following "no-observed-adverse-effect levels":
- rat: 5 mg/kg in the diet, equivalent to 0.25 mg/kg
body weight; and
- dog: 3 mg/kg in the diet, equivalent to 0.075 mg/kg
body weight.
These "no-observed-adverse-effect levels" were confirmed by the
1982 JMPR (FAO/WHO, 1983).
6.2.2. Dermal exposure
When male guinea-pigs were exposed to chlordane at 67 mg/kg
body weight/day, through dermal painting for 90 days, mild
degenerative changes in the skin and testis were evident (Datta et
al., 1975).
The ninety-day repeated daily dose LD50 for rabbits was
reported in the paper by Lehman (1952a) (section 6.1.2) to be from
20 - 40 mg/kg body weight. Ingle (1965b) reviewed the dermal
toxicity of chlordane and attributed the toxicity of early
technical chlordane to the significant content of hexachloro-
cyclopentadiene (HCPD). A more pure product, which did not contain
significant quantities of HCPD, was only half as toxic to rabbits
as the earlier chlordane and did not cause any skin irritation or
damage to mucous membranes.
6.3. Reproduction Studies and Teratogenicity
Rats, maintained from weaning on a diet containing a chlordane
level of 320 mg/kg, showed reduced rates of mating, of viable
litters, and an increased rate of death of progeny prior to
weaning. It was concluded that, at this dosage, chlordane
interfered with both fertility and litter survival (Ambrose et al.,
1953). Groups of 10 male and 20 female rats were used in a 3-
generation study at dietary levels of technical chlordane of 0,
0.3, 3, 15, 30, and 60 mg/kg (Ingle, unpublished data, 1967). Two
litters in each filial generation were studied. Levels up to and
including 30 mg/kg did not have any effect on fertility, number of
offspring, or weight, growth, or mortality rate of the young
animals to weaning age. Autopsy of animals after weaning did not
reveal any gross or microscopic differences between the groups. At
60 mg/kg, there was a high (10.6%) mortality rate in the second F3
generation litters during the latter part of the nursing period;
these animals showed gross and microscopic pathological changes,
comparable with those characteristic for chlordane intoxication.
However, survivors of this generation did not show any tissue
changes at all. A third set of F3 litters at 60 mg/kg suffered 17%
mortality during the nursing period, with symptomatology and gross
and microscopic tissue changes characteristic of chlordane
intoxication. Third F3 generation litters from dams removed from
the 60 mg/kg group and placed on chlordane-free diets for 30 days
prior to remating showed no differences in any respect from control
litters. No evidence of teratogenicity was found in this study.
Hens and cocks fed up to 0.3 mg chlordane/kg diet did not show
any toxic symptoms or any adverse effects on egg weight,
hatchability, or growth of chicks (Biotox, unpublished data, 1969).
Mice fed diets containing chlordane at 25 - 100 mg/kg for 6
generations showed decreased viability in the first and second
generations at 100 mg/kg; in the third generation at this level, no
offspring were produced (Keplinger et al., 1968). At 50 mg/kg,
viability was reduced in the fourth and fifth generations, and at
25 mg/kg no statistically significant effects were observed, even
after 6 generations.
Chlordane was administered to rabbits orally at levels of 1.0,
5.0, and 15 mg/kg body weight per day on the 6th - 18th days of
gestation. A control group and a positive control group were used.
No changes were seen in behaviour, appearance, or body weight.
Miscarriages were seen in 3 rabbits at the 1.0 mg/kg level and one
rabbit at 15 mg/kg dose level. No effects on any of the maternal
or fetal parameters were noted. No teratogenic effects were noted
(IRDC, 1972).
6.4. Mutagenicity
Alpha-Chlordane, gamma-chlordane, and chlordene were tested in
the Ames Salmonella microsome assay and were not mutagenic (Simmon
et al., 1977). Chlordane was not mutagenic when tested using 5
different strains of Salmonella typhimurium in the Ames assay
(Ercegovich & Rashid, 1977). Chlordane was shown to enhance the
number of ouabain-resistant mutants in Chinese hamster V79 cells
and was considered weakly mutagenic (Ahmed et al., 1977b).
Chlordane induced unscheduled DNA synthesis in SV-40 human
cells in culture without activation (Ahmed et al., 1977a). It was
established that chlordane-treated cells did not (for the most
part) re-enter mitosis. They were, instead, arrested somewhere
between the G1 and G2 phases of the cell cycle. Studies involving
DNA synthesis were undertaken to determine more precisely at which
phase (G1, S, G2) the cells are blocked. The data showed that the
treated cells were as competant in DNA replication as the control
cells. In both control and treated cultures, 25 - 30% of total DNA
persisted as light-density material indicating that some of the
pre-existing DNA never engaged in DNA synthesis. Either a large
fraction of cells failed to complete DNA synthesis or 25 - 30% of
the cells did not enter phase S. In any case, treated and control
cells behaved the same in terms of DNA synthesis, indicating that
treatment of the cells with chlordane blocked the cells at the G2
stage of the cell cycle (Brubaker et al., 1970).
Chlordane induced gene conversions in Saccharomyces cerevisiae
strain D4 (Chambers & Dutta, 1976).
Neither alpha-chlordane (42, 58, and 290 mg/kg body weight
single ip doses or 5 daily oral doses of 75 mg/kg body weight) nor
the gamma-isomer (5 daily oral doses of 50 mg/kg body weight) had a
significant effect in a dominant lethal assay on mice (Epstein et
al., 1972). Technical chlordane at dose levels of 50 or 100 mg/kg
body weight in a dominant lethal study using mice failed to induce
any dominant lethal changes (Arnold et al., 1977).
More recent studies on animal and human cells in culture have
shown that chlordane is not mutagenic or is only weakly mutagenic
(Williams, 1979; Maslansky & Williams, 1981; Tong et al., 1981).
Further work by Telang et al. (1982) showed that chlordane was not
mutagenic to an adult rat liver cell line but inhibited cell to
cell communication in a rat liver 6-thioguanine resistant/sensitive
cell line. Telang et al. proposed that chlordane was exhibiting
properties exerted by many promoting agents.
6.5. Carcinogenicity
Epstein (1976) reported a previously unpublished study by the
International Research and Development Corporation, carried out in
1973, in which groups of 100 male and 100 female Charles River CD-1
mice, 6 weeks of age, were fed technical-grade chlordane (purity
not given) at 5, 25, and 50 mg/kg diet, for 18 months. Excluding
10 animals sacrificed from each group for interim study at 6
months, mortality rates at 18 months ranged from 27 - 49%, except
in males and females receiving the 50 mg/kg diet, in which the
mortality rates were 86 and 75%, respectively. A relatively large
number of the deceased animals was lost due to autolysis. A dose-
related increased incidence of liver hyperplastic nodules was
reported in the 25 and 50 mg/kg diet test groups and a dose-related
increased incidence of liver cell hypertrophy was found in all test
groups. A significant incidence of hepatocellular carcinomas
compared with controls was also reported. In the males receiving
chlordane at 0, 5, 25, or 50 mg/kg diet, hepatocellular carcinomas
were found in 3/33, 5/55, 41/52, and 32/39 animals, respectively;
in females, the respective incidences were 0/45, 0/61, 32/50, and
26/37.
Groups of 50 male and 50 female B6C3F1 hybrid mice, 5 weeks of
age, were fed analytical-grade chlordane, consisting of 94.8%
chlordane (71.7% alpha-chlordane and 23.1% gamma-chlordane), 0.3%
heptachlor, 0.6% nonachlor, 1.1% hexachlorocyclopentadiene, 0.25%
chlordene isomers, and other chlorinated compounds for 80 weeks
(NCI, 1977). Males received initial levels of 20 or 40 mg/kg diet,
and females 40 and 80 mg/kg diet; time-weighted average dietary
concentrations were 30 and 56 mg/kg for males and 30 and 64 mg/kg
diet for females. There were 20 male and 10 female matched
controls and 100 male and 80 female pooled controls. Survival in
all groups was relatively high, i.e., over 60% in treated males,
over 80% in treated females, and over 90% in male and female
controls. A dose-related increase in the incidence of
hepatocellular carcinomas was found in males and females. The
incidences were 43/49 and 34/49 in high-dose males and females,
respectively, and 16/48 and 3/47 in low-dose males and females,
respectively, compared with 2/18 and 0/19 in male and female
matched controls, respectively.
Groups of 50 male and 50 female, 5-week-old Osborne-Mendel rats
were given analytical-grade chlordane in the diet for 80 weeks, at
initial levels of 400 and 800 mg/kg for males and 200 and 400 mg/kg
for females (NCI, 1977). The levels had to be reduced during the
study because of adverse toxic effects; the time-weighted average
dietary concentrations were 407 and 203 mg/kg for males and 241 and
121 mg/kg for females. There were 10 male and 10 female matched
controls and 60 male and 60 female pooled controls. Survivors were
killed at 80 weeks, at which time approximately 50% of treated and
control males and 60% of treated females and 90% of control females
were still alive. In all treated animals combined, there was an
excess incidence of follicular-cell thyroid neoplasms (10/75 in
treated females and 7/65 in treated males versus 0/10, 3/58, 0/6,
and 4/51 in matched and pooled female and male controls); there was
an excess of malignant fibrous histiocytomas in the treated male
groups (8/88 versus 0/8 and 2/58 in matched and pooled male
controls).
A committee of the National Academy of Sciences (NAS) in the
USA was asked to review all available carcinogenicity data on
chlordane as part of the cancellation hearings. Chlordane was not
found to be carcinogenic in rats and the only target organ site for
carcinogenic response in certain strains of mice was the liver.
The committee concluded that "there are no adequate data to show
that these compounds are carcinogenic in humans, but because of
their carcinogenicity in certain mouse strains and the extensive
similarity of the carcinogenic action of chemicals in animals and
in humans, the committee concluded that chlordane, heptachlor
and/or their metabolites may be carcinogenic in humans. Although
the magnitude of risk is greater than if no carcinogenicity had
been found in certain mouse strains, in the opinion of the
committee the magnitude of risk cannot be reliably estimated
because of the uncertainties in the available data and in the
extrapolation of carcinogenicity data from laboratory animals to
humans" (US NAS, 1977).
IARC (1979), in its evaluation of the carcinogenic risk of
chlordane, concluded: "There is sufficient evidence that chlordane
is carcinogenic in mice." In 1982, another IARC Working Group
reviewed existing data on chlordane and concluded that there was
limited evidence for the carcinogenicity of chlordane for
experimental animals (IARC, 1982). The group of Williams (Telang
et al., 1982) suggested that chlordane had the properties of many
promoting agents.
6.6. Behavioural Studies
Offspring of chlordane-treated mice (1 or 2.5 mg/kg body weight
for 7 consecutive days) made fewer conditioned avoidance responses
than controls (Al-Hachim & Al-Baker, 1973). In addition, progeny
of mothers receiving the higher dose were more active than the
controls.
6.7. Other Studies
Chlordane induces hepatic mixed-function oxidase enzymes in
rats (Fouts, 1963; Hart et al., 1963; Hart & Fouts, 1965;
Villeneuve et al., 1972; den Tonkelaar et al., 1974; Madhukar &
Matsumura, 1979) and enhances estrone metabolism in rats and mice
(Welch et al., 1971). Chlordane has been shown to inhibit skin 7-
ethoxycoumarin de-ethylase activity (7-EC) (EC 1.14.13) in mice at
doses which induced hepatic 7-EC activity (Pohl & Fouts, 1977).
Several studies were carried out in which rats were fed chlordane
at levels of 2, 5, 10, 20, or 50 mg/kg diet during two weeks (den
Tonkelaar et al., 1974). At the end of this period, the liver
microsomal enzymes hexobarbital oxidase (EC 1.1), aminopyrine
demethylase (EC 1.5.3), and aniline hydroxylase (EC 1.14.14) were
determined. A no-observed-adverse-effect level of 5 mg/kg was
found for chlordane. Chlordane inhibition of rat brain ATPase
activity (Folmar, 1978) and bovine carbonic anhydrase (EC 4.2.1.1)
(Maguire & Watkin, 1975) has been demonstrated in in vitro
systems. The in vivo inhibition of rat brain ATPase has also
been reported (Drummond et al., 1980).
Three female and 3 male baboons were fed atherogenic diets to
which chlordane was added at 0.1 or 1 mg/kg body weight per day for
two years. At the higher dose level, chlordane increased
cytochrome P-450 activity significantly, otherwise no adverse
effects on general health or on any major organ systems were found
(McGill, 1979).
Acute intramuscular doses (50 mg/kg body weight) of chlordane
have been shown to increase the alkaline phosphatase (EC 3.1.3.1)
activity in gerbils temporarily (Karel, 1976) and stimulate
gluconeogenetic enzymes in the liver and kidney cortex of rats
(Kacew & Singhal, 1973a). Oral administration of 200 mg alpha-
chlordane/kg body weight to mature Wistar rats also induced
elevated serum levels of glucose and urea with a concomitant
decrease in liver glycogen at sacrifice, 1 h later (Kacew &
Singhal, 1973b). The chlordane-induced alterations have been
attributed to the enhanced ability of these organs to synthesize
cyclic AMP (Kacew & Singhal, 1973b, 1974; Singhal & Kacew, 1973,
1976; Kraybill, 1977).
Rats were administered chlordane ip daily for 42 days at levels
of 0.15, 1.75, or 25.0 mg/kg body weight. Results revealed "dose-
dependent alterations of brain potentials without behavioural signs
of chronic toxicity" (Hyde & Falkenberg, 1976, Hyde et al., 1978).
Chlordane has also been shown to influence brain biogenic amines
including acetylcholine (Hrdina et al., 1973).
Prenatal exposure to 0.16 mg chlordane/kg body weight, in
peanut butter, on each day of gestation, resulted in increased
plasma corticosterone levels in adult male mice (Cranmer et al.,
1978). When neonatal mice were treated with 0.075 mg alpha- or
gamma-chlordane on days 2 - 4 after birth, growth rates were
depressed and eye and vaginal opening were delayed (Talamantes &
Jang, 1977).
Female Balb C mice were mated and treated with chlordane at
0.16 or 8 mg/kg body weight throughout gestation. Decreased cell-
mediated immune competence was found in offspring of high-dose-
treated females, at 101 days of age, challenged with oxazolone
(Spyker-Cranmer et al., 1982).
Chlordane added to the medium at a concentration of
1 mg/kg inhibited the growth of Streptococcus viridans in
vitro by more than 50%. Total growth inhibition occurred at a
chlordane concentration of 3 mg/kg (Goes et al., 1978).
6.8. Factors Influencing Toxicity
Metabolites
The acute oral toxicity of chlordane isomers and their
metabolites is summarized in Table 3.
Table 3. Toxicity of chlordane isomers and metabolites
-----------------------------------------------------------------------
Compound Species LD50 (mg/kg Reference
(sex) body weight)
-----------------------------------------------------------------------
alpha-chlordane rat (M) 392 Wazeter et al. (1968)
gamma-chlordane rat (M) 327 Wazeter et al. (1968)
alpha + gamma- rat (M) 371 Wazeter et al. (1968)
chlordane (1:1 ratio)
oxychlordane rat (M,F) 19.1 Mastri et al. (1969a)
chlordene rat (M,F) over 4600 Mastri et al. (1969b)
3-chlorchlordene rat (M,F) over 4600 Mastri et al. (1969b)
1-hydroxychlordene rat (M,F) over 4600 Mastri et al. (1969b)
chlordene epoxide rat (M,F) over 4600 Mastri et al. (1969c)
1-hydroxy, 2,3-epoxy rat (F) over 4600 Mastri et al. (1969c)
chlordene
2-chlorchlordene rat (F) over 10 200 Mastri et al. (1969c)
-----------------------------------------------------------------------
Only oxychlordane was more toxic than the parent compounds.
Two male and 2 female rats were given the chlordane metabolite
oxychlordane in their diet, at a level of 2.0 mg/kg body weight
(Plank et al., 1970). Following 90 days feeding, the surviving
animals were sacrificed. Body-weight gain, food consumption,
behaviour, mortality rate, organ weights and organ to body weight
ratios, and the results of haematological and blood chemistry tests
and urological studies were considered to be within the normal
range for the strain of rat used. No gross pathological
abnormalities were evident, and no histopathological lesions could
be attributed to oxychlordane.
Groups of 25 male and 25 female rats were fed dietary levels of
1-hydroxychlordene of 0, 100, 250, 500, 1000, and 2000 mg/kg for up
to 224 days (Ingle, 1965a). After 110 days, 3 females from each
feeding level were mated with males at all levels. The mortality
rate in all groups was low, and no statistically significant
differences existed. No gross abnormalities were revealed at
autopsy after 224 days. The histopathological study of the
visceral organs did not show any pathological effects. Slight to
moderate hyperplasia of the smooth endoplasmatic reticulum and
cytoplasmic margination of a few liver cells were noted at the 1000
and 2000 mg/kg levels.
Interactions
Chlordane has been shown to exert a protective effect against
several organophosphorous and carbamate insecticides (Williams et
al., 1967; Street et al., 1969; Williams & Casterline, 1970; US
EPA, 1976a,b).
Protein deficiency has been shown to double the acute toxicity
of chlordane in rats (Boyd, 1972).
Chlordane has also been shown to increase the hepatotoxic
effects of carbon tetrachloride in the rat (Stenger et al., 1975;
Mahon, 1977; Mahon & Oloffs, 1979; Mahon et al., 1979).
7. EFFECTS ON MAN: EPIDEMIOLOGICAL AND CLINICAL STUDIES
7.1. Poisoning Incidents
A 15-month-old girl ingested a mouthful of chlordane suspension
and, after 3 h, displayed tremors and incoordination (Lensky &
Evans, 1952). Repeated seizures developed and she was treated with
ethyl chloride, amobarbital, and gastric lavage with magnesium
sulfate. The child recovered completely and ataxia and
excitability disappeared after 2 - 3 weeks. At 26 years of age,
she was in excellent health and appeared not to suffer any
consequences from the childhood episode (Taylor et al., 1979).
A 2-year-old child had drunk an unknown amount of a 74%
formulation of chlordane (Curley & Garrettson, 1969). Vomiting
preceeded convulsions, which were controlled by phenobarbital; the
EEG pattern was normal within 40 h and the child recovered.
A similar poisoning incident was observed with a 4-year-old
child (Aldrich & Holmes, 1969). Convulsions were treated with
phenobarbital. As with the previous case, the individual
recovered.
Two other cases of chlordane poisoning were reported in 1955
(Derbes et al., 1955). One was caused by the absorption of
accidentally-spilled chlordane and the other was a suicide attempt
where the individual (female) swallowed 6 g of chlordane (104 mg/kg
body weight) and died 9 1/2 days after the incident (Derbes et al.,
1955).
When a section of a municipal water system in Chattanooga,
Tennessee, USA was contaminated with chlordane in concentrations of
up to 1.2 g/litre in 1976, 13 persons showed gastrointestinal
and/or neurological symptoms (Harrington et al., 1978).
7.2. Occupational and Epidemiological Studies
No deleterious effects associated with occupational exposure to
chlordane have been reported. Twenty-two men, who had been
occupationally exposed to chlordane during its manufacture for
periods of 1 - 3 years, did not show any evidence of intoxication
(Princi & Spurbeck, 1951). Other clinical studies have been
reported on men engaged in the manufacture of chlordane (Alvarez &
Hyman, 1953; Fishbein et al., 1964; Morgan & Roan, 1969).
Infante et al. (1978) reviewed 25 previously reported cases of
blood dyscrasia together with a small number of newly identified
cases of aplastic anaemia, leukaemia, or neuroblastoma in children
in relation to their possible association with pre- and post-natal
chlordane or heptachlor exposure and reported an anecdotal
relationship. However, in a case-control study, no association was
found between blood dyscrasias and occupational exposure to a
number of pesticides including chlordane (Wang & Gruffenman, 1981).
Wang & MacMahon (1979 a,b) studied a cohort of workers engaged
in the manufacture of chlordane, heptachlor, and endrin and another
cohort of 16 000 pesticide-spraying personnel, including termite-
control workers. Both studies showed a deficit of deaths from all
cancers and slight excesses of lung, skin, or bladder cancer that
were not statistically significant.
In 1982, an IARC Working Group concluded that the above studies
were inadequate to evaluate the carcinogenicity of chlordane for
human beings (IARC, 1982).
Shindell & Associates (1981) studied the mortality experience
of 783 workers engaged in the manufacture of chlordane and
heptachlor. Workers had been employed for a minimum of 3 months 5,
10, 15, or 20 years ago. SMRs for cancer were not increased among
124 deaths.
In a retrospective cohort study of workers involved in the
production of chlorinated hydrocarbon pesticides, Ditraglia et al.
(1981) studied the workers in a chlordane-manufacturing plant; the
same workers were also studied by Wang & MacMahon (1979a). SMRs
for all cancer deaths were lower than expected; a slight excess of
stomach cancer (3 vs 0.99 expected), which was observed, was not
statistically significant. The number of workers studied was small
and further follow-up of the cohort was recommended by the authors.
MacMahon & Wang (1982) carried out a second follow-up study of
mortality rates in a cohort of workers employed in spraying
pesticides, including termite-control workers. Among 540 deaths
for which the cause was ascertainable, small excesses of bladder
cancer in termite-control operators and of skin and lung cancer in
other operators were observed, but these were not statistically
significant.
7.3. Treatment of Poisoning
In case of overexposure, medical advice should be sought
immediately.
Treatment before person is seen by a physician
The person should stop work immediately, remove contaminated
clothing and wash the affected skin with soap and water, if
available, flushing the area with large quantities of water. If
swallowed, vomiting should be induced, if the person is conscious
(FAO/WHO, 1978).
Medical Treatment
If the pesticide has been ingested, gastric lavage should be
Performed with 2 - 4 litres of water, followed by saline
purgatives. Barbiturates (preferably phenobarbitone or
pentobarbitone) or diazepam should be given intramuscularly or
intravenously in sufficient dosage to control restlessness or
convulsions. Mechanical respiratory assistance with oxygen may be
required. Calcium gluconate, 10% in 10 ml, injected
intramuscularly at 4-h intervals, may be helpful. Contraindicated
are oily purgatives, epinephrine, and other adrenergic drugs and
central stimulants of all types (FAO/WHO, 1978).
8. EFFECTS ON THE ENVIRONMENT
8.1. Toxicity for Aquatic Organisms
Data on the toxicity of chlordane for aquatic organisms are
given in Table 4. A more comprehensive table, listing different
conditions and exposure times is available on request from IRPTC,
Geneva, Switzerland. It is of importance for the interpretation of
these data to note that a change in the purity of technical of
chlordane occurred in the early 1950s.
Studies of the effects of chlordane on fish began with the
application of the original material to rainbow trout by Cope et
al. (1947). They determined minimum disabling 24-h doses of
chlordane in a xylene emulsion, an acetone solution, a fuel oil
solution, and a Velsicol AR-50 solution, and found this to be
higher than 6 mg chlordane/litre. An application of 1.12 kg/ha of
a field formulation of chlordane to a small pond killed 87% of
bluegills present (Surber, 1948), application of 0.56 kg/ha killed
some bluegills whilst at 0.28 kg/ha all fish survived (Linduska &
Surber, 1948). In a study by Lawrence (1950) on bluegill
fingerlings, large-mouth black bass fingerlings, and juvenile
goldfish in aquaria, no deaths occurred at 100 µg chlordane/litre
(original formulation), whilst at 200 µg/litre, a 30-h exposure
killed bass, and an 87-h exposure killed bluegills; goldfish were
not affected. In earthen ponds, large-mouth black bass fingerlings
were killed by a concentration of 200 µg/litre, but bluegills and
fingerlings and juvenile goldfish survived.
Studies using the current formulation of chlordane are
summarised in Table 4. A definite temperature effect demonstrated
by Macek et al. (1969) during acute exposures, i.e., fish showed a
greater susceptibility at higher temperatures, was not present in
96-h exposure studies. Temperature effects were also noted in
toxicity tests on tubificid worms, Branchuria sowerbyi (Naqvi,
1973). In static tests, 500 µg chlordane/litre caused 100%
mortality at 4.4° and 32 °C, but no mortality at 21 °C. Nutrition
has been shown to affect chlordane toxicity in rainbow trout, with
96-h LC50s ranging from 8.2 to 47 µg/litre, depending on the
composition of the diet given to the fish (Merhle et al., 1974).
Recent in vitro studies on bluegills (Koch et al., 1971)
and on rainbow trout (Davis et al., 1972) have shown that chlordane
acts as an inhibitor of ATPase systems.
8.2. Toxicity for Terrestrial Organisms
Studies of the effects of chlordane on soil microfauna have
been limited to work on nematodes. Populations of plant-feeding
nematodes were reduced by an insecticide mixture containing
chlordane (as well as DDT, diazinon, and zinophos) in July,
following application in March, but at no other time during the
study period (Corbett & Webb, 1968). Nematodes are generally
unaffected by most soil insecticides (Edwards, 1965a).
Table 4. Toxicity of chlordane for aquatic organisms
---------------------------------------------------------------------------------------------------------
Organism Age/ Temp pH Flow/ Grade Hard- alk sal End Para- Concen- Reference
size (° C) stat ness (mg/ o/oo point meter tration
(mg/ litre) (µg/
litre) litre)
---------------------------------------------------------------------------------------------------------
Eastern oyster 29-53 31.6 flow tech 27.3 % reduc- 96-h 6.2 Parrish et
(Crassostrea mm tion shell EC50 al. (1976)
virginica) deposition
Annelid stat sea 288-h 220 McLeese et
(Nereis virens) water LC50 al. (1982)
Scud 5 mm 16.7 7.9 flow tech 148 152 immobili- 168-h 97.1 Cardwell et
(Hyalella juv. sation EC50 al. (1977)
azteca)
Cladoceran 1st 15.5 7.4- stat immobili- 48-h 29 Sanders &
( Daphnia pulex) instar 7.8 sation EC50 Cope (1966)
Pink shrimp 50-65 28.4 flow tech 21.8 96-h 0.4 Parrish et
(Penaeus mm LC50 al. (1976)
duorarum)
Dungeness crab adult 13 stat tech 25 96-h 220 Caldwell
(Cancer LC50 (1977)
magister) zoeal 13 stat tech 25 immobili- 96-h 1.3
sation EC50
Backswimmer 5-6 mm 18-24 stat 25% EM 168-h 0.79 Konar (1968)
(Notonecta) sp LC50
Water scorpion 24-28 18-24 stat 25% EM 168-h 182 Konar (1968)
(Nepa) sp mm LC50
Bluegill 38-84 25 7.1 flow 100% 20 18 96-h 22 Henderson et
(Lepomis mm LC50 al. (1959)
macrochirus)
---------------------------------------------------------------------------------------------------------
Table 4. (contd.)
---------------------------------------------------------------------------------------------------------
Organism Age/ Temp pH Flow/ Grade Hard- alk sal End Para- Concen- Reference
size (° C) stat ness (mg/ o/oo point meter tration
(mg/ litre) (µg/
litre) litre)
---------------------------------------------------------------------------------------------------------
Fathead minnow 1 day 21/25 7.7 flow tech 156 166 major 11-mo Cardwell et.
(Pimephales chronic lowest al. (1977)
promelas) effects dose
life
cycle
tests
38-84 25 7.1 flow 100% 20 18 96-h 52 Henderson et
mm LC50 al. (1959)
38-84 25 7.1 flow 75% 20 18 96-h 170 Henderson et
mm EM LC50 al. (1959)
Rainbow trout 0.9 g 13 tech 96-h 7.8 Cope (1965)
(Salmo gairdneri) LC50
Murrel 24-26 18-24 stat 25% EM 168-h 0.51 Konar (1968)
(Channa mm LC50
punctatus)
48-50 18-24 stat 25% EM 168-h 3 Konar (1968)
mm LC50
100- 18-24 stat 25% EM 168-h 25.5 Konar (1968)
105 mm LC50
Tropical fish juv. 18-24 stat 25% EM 168-h 0.7- Konar (1968)
LC50 3.7
Channel catfish finger 25 stat tech 96-h 500 Clemens &
(Ictalurus ling LC50 Sneed (1959)
punctatus)
Common toad tad- 18-20 stat 48-h 2000 Ludemann &
(Bufo bufo) pole LC50 Neumann
(1962)
---------------------------------------------------------------------------------------------------------
Soil fauna populations (mainly arthropods with a small
percentage of earthworms and nematodes) were reduced to very low
levels by the normal application rate (112 kg/ha per year) of a
commercial formulation of chlordane (Gould & Hampstead, 1951).
However, Long et al. (1967) did not find any significant reductions
in most soil arthropod populations following application of
technical chlordane to sugar cane at 2.24 kg/ha, although numbers
of Diplura and Pauropoda were reduced. In a summary of the effects
of pesticides on soil invertebrates, Edwards (1965b) stated that
chlordane caused a large reduction in the numbers of Coleoptera,
Diptera, hemiedaphic Collembola, and non-predatory mites, but
little reduction in the numbers of edaphic Collembola or predatory
mites at application rates of 1.12 - 2.24 kg/ha. He also stated
that chlordane was lethal for fly and beetle larvae. Fox (1958)
produced a few data indicating that carabid and staphylinid beetle
populations returned to normal 3 years after a field application
of chlordane (as a 40% wettable powder) at 8.96 or 11.2 kg/ha.
Chlordane is toxic both to earthworms and to enchytraeid worms
at application rates well within the range of normal usage (Hopkins
& Kirk, 1957; Doane, 1962). The toxicity of chlordane for earth-
worms is given in Table 5. Legg (1968) made a single application
of 25% EC chlordane at 9.0, 13.4, or 20.2 kg/ha on closely-mown
turf and counted worm casts for up to 13 months. After 19 days,
there were reductions of 52, 72, and 98%, respectively, for the 3
doses compared with control plots; after 13 months, the reductions
were 89, 95, and 97%, respectively. Long et al. (1967) reported a
significant reduction in earthworm populations, 6 - 11 months after
an application of chlordane at 2.2 kg/ha. A reduction in worm
casts to zero, 1 year after an application of chlordane at 11.2
kg/ha, either as granules or in spray formulation was shown by
Doane (1962). Lidgate (1966) applied chlordane, either as 20%
granules or 75% EC diluted with water, at rates between 13.4 and
35.2 kg/ha, to a putting green and measured worm activity by the
number of worm casts. The first count of casts, 18 days after
treatment, showed that worm activity was significantly depressed by
granular applications of 17.6 and 35.2 kg/ha but not by spray
formulations of 13.4 or 26.4 kg/ha. Activity became depressed on
sprayed plots about 8 weeks after treatment. There were still
significantly fewer worm casts on treated plots, 5 years later.
Table 5. Toxicity of chlordane for earthworms
-----------------------------------------------------------------------------------------
Organism Grade Application Concen- Effect Reference
method tration
(kg/ha)
-----------------------------------------------------------------------------------------
Earthworm 25% EC spray 9 52% reduction in worm Legg (1968)
(Lumbricus casts at 19 days
terrestris)
25% EC spray 13.4 72% reduction in worm Legg (1968)
casts at 19 days
25% EC spray 20.2 98% reduction in worm Legg (1968)
casts at 19 days
25% EC spray 9 89% reduction in worm Legg (1968)
casts at 1 year
25% EC spray 13.4 95% reduction in worm Legg (1968)
casts at 1 year
25% EC spray 20.2 97% reduction in worm Legg (1968)
casts at 1 year
Red earth- 5% dust soil 35 0% mortality in Hopkins & Kirk (1957)
worm incorpor- 4 days
(Eisenia ation
sp)
5% dust soil 70 46% mortality in Hopkins & Kirk (1957)
incorpor- 4 days
ation
5% dust soil 141 40% mortality in Hopkins & Kirk (1957)
incorpor- 4 days
ation
5% dust soil 282 79% mortality in Hopkins & Kirk (1957)
incorpor- 4 days
ation
5% dust soil 100 96-h LC50 Hopkins & Kirk (1957)
incorpor-
ation
-----------------------------------------------------------------------------------------
The toxicity of chlordane for birds, when given in the diet, is
summarised in Table 6. LC50 values as mg/kg diet ranged from 170
to 858 in studies where chlordane was given for between 5 days and
100 weeks. When chlordane was applied to marshland at 1.12 kg/ha,
the fecundity of marsh birds was affected (Hanson, 1952); blue-
winged teal and shovelers did not produce any young, and coot and
redwinged-blackbirds produced 60% fewer young. It was postulated
that chlordane had caused disruption in food cycles in the marsh
and that this was the probable cause of reproductive failure in the
birds.
8.3. Toxicity for Microorganisms
Some effects of chlordane on microorganisms are summarised in
Table 7. Its effects may be due, at least in part, to inhibition
of enzyme activity (Nakas, 1977). Some work has been reported on
the effects of chlordane on soil microorganisms. Gram-positive
bacteria appear to be more sensitive to chlordane than gram-
negative bacteria, since the growth of gram-positives was inhibited
whilst that of gram-negatives was unaffected (Trudgill et al.,
1971). When Bacillus subtilis cultures were treated with technical
chlordane at 20 mg/litre, they ceased to grow. Viable count and
respiration rate fell to zero after about 3 h of exposure. The
actual concentration experienced by the bacteria is not known, but
it is likely to be less than the 20 mg/litre added because of the
poor solubility of chlordane in aqueous solution. Langlois & Sides
(1972) investigated the effects of constituents of technical
chlordane on the growth of Staphylococcus aureus. The viability
of the culture, the length of the lag-phase, and the generation
time were affected by the amount of chlordane and gamma-chlordane
applied.
8.4. Bioaccumulation and Biomagnification
Grimes & Morrison (1975) examined the uptake of chlordane by 13
types of bacteria and found that although the uptake of alpha- and
beta-isomers of chlordane was the same for any one species, the
concentration factors (CF) differed greatly between species. The
CFs ranged from a few hundred to several thousand, with 3 species
giving much higher values. The highest CF was 53 000 for
Caulobacter vibrioides. Caulobacter cells were found to contain 4
distinct lipid-containing materials, and this was offered as an
explanation of the high CF. Sanborn et al. (1976) used unlabelled
chlordane and labelled 14C-chlordane on filamentous Oedogonium
alga and obtained CFs of 49 500 and 98 386. The lower figure may
be due to uncertainties in determining chlordane in solution and in
the alga. Moore et al. (1977), using the planktonic alga
Ankistrodesmus amalloides, obtained a very much lower CF of 5560,
but even this species shows accumulation potential.
Table 6. Toxicity of chlordane for birds
--------------------------------------------------------------------------------
Species Age Route Parameter Concen- Reference
trationa
(mg/kg)
--------------------------------------------------------------------------------
Mallard 4 - 5 mo oral acute LD50 1200 Tucker & Crabtree (1970)
10 days diet 5-day LC50b 858 Hill et al. (1975)
Bobwhite 14 weeks diet 10-week LC0 10 Ludke (1976)
quail 17 days diet 5-day LC50b 331 Hill et al. (1975)
young diet 100-day LC50 100 DeWitt et al. (1963)
young diet 10-day LC50 250 DeWitt et al. (1963)
adult diet 100-day LC50 250 DeWitt et al. (1963)
Japanese 7 days diet 5-day LC50b 350 Hill et al. (1975)
quail
Ring-necked 15 days diet 5-day LC50b 430 Hill et al. (1975)
pheasant young diet 10-day LC50 500 DeWitt et al. (1963)
young diet 100-day LC50 50 DeWitt et al. (1963)
adult diet 100-day LC50 200 DeWitt et al. (1963)
Cowbird adult diet 30-week LC50 500 DeWitt et al. (1963)
--------------------------------------------------------------------------------
a Concentration in mg/kg body weight for oral dosing; concentration in mg/kg
diet for dietary dosing.
b 5 days of treated diet followed by 3 days of clean diet. Mortality rate
determined on day 8.
Table 7. Toxicity of chlordane for microorganisms
----------------------------------------------------------------------------------------
Organism F/M/ Temp Grade Solvent Endpoint Time Concen- Reference
S (° C) tration
(µg/
litre)a
----------------------------------------------------------------------------------------
Scenedesmus F 23 tech acetone stimulation of 5 - 7 0.1- Glooschenko &
quadricauda cell division days 100 Lott (1977)
F 23 tech acetone inhibition of 1 - 5 50 & Glooschenko &
photosynthesis days 100 Lott (1977)
Chlamydomonas S 23 tech acetone stimulation of 7 - 11 0.1- Glooschenko &
sp cell division days 50 Lott (1977)
S 23 tech acetone stimulation of 3 - 4 0.1- Glooschenko &
respiration h 100 Lott (1977)
S 23 tech acetone inhibition of 7 - 11 100 Glooschenko &
cell division days Lott (1977)
F 23- 60% acetone 50% reduction 3 days 100 000 Clegg &
25 ATPase Koevenig
activity; (1974)
no effect on
cell density
Volvox sp F 18- 20% none 100% mortality 7 days 1 Konar (1968)
Pandorina sp 24 EM
Closterium sp
Chlorella F 23- 60% acetone reduced ATPase 3 days 100 000 Clegg &
ellipsoidea 25 levels; Koevenig
Euglena no effect on (1974)
elastica cell density
Exuviella M 60% methanol virtual cessa- 7 days 50 Magnani et
baltica tion of growth al. (1978)
Natural M 60% acetone 94% decrease 4 h 1000 Butler (1963)
estuarine in product-
phytoplankton ivity
Estuarine M 7-14 60% methanol no effect 5 days 5 Biggs et al.
phytoplankton M 7-14 60% methanol growth and 14C 5 days 10 (1978)
uptake reduced
in laboratory
Bacillus S tech acetone growth ceased; 3 h 20 000 Trudgill et
subtilis decline in al. (1971)
viable count;
respiration
to zero
----------------------------------------------------------------------------------------
a Solubility of chlordane: 6 - 9 µg/litre. F = freshwater; M = marine; S = soil.
Accumulation of alpha- and and gamma-isomers of chlordane and
nonachlor, and chlordenes was studied by Cardwell et al. (1977) in
3 species of freshwater invertebrates. Chironomus larvae did not
show any detectable accumulation. After one week's exposure to 1.7 -
21.6 µg/litre, Daphnia magna gave CFs ranging from 15 000 to
175 000. After 65 days of exposure to 1.4 - 11.5 µ/litre, Hyallela
azteca showed CFs ranging from 41 000 to 144 000. After a 24-h
exposure to 0.5 µg alpha- and gamma-chlordane/litre, Daphnia pulex
gave a CF of 24 000 (Moore et al., 1977). Sanborn et al. (1976)
found a CF of 6132 for insect larvae. A fresh-water gastropod
Physa sp., concentrated chlordane 132 613 times (Sanborn et al.,
1976). Little bioaccumulation has been observed in the marine
invertebrate species studied. Wilson (1965) exposed oysters to
0.01 mg chlordane/litre and determined a CF of 7300, which is
considerably lower than that for fresh-water gastropods. Water and
oyster samples from Galveston Bay, Texas were analysed following an
extensive mosquito-control programme (Casper, 1967). Oysters
sampled did not contain any detectable chlordane and only 2 out of
9 water samples gave a positive result of less than 0.001 µg/litre.
Chlordane was detected by Bugg et al. (1967) in 20 out of 133
oyster samples taken from the South Atlantic and the Gulf of
Mexico, but 19 of these gave values of less than 0.01 mg/kg drained
weight. Clams, which were living in water containing chlordane at
0.01 µg/litre for 106 days, showed CFs of 1000 or less (Godsil &
Johnson, 1968). Parrish et al. (1976) reported that chlordane was
concentrated in the tissues of the estuarine pink and grass shrimps
at 1000 - 2300 times levels measured in water.
Few data are available on soil invertebrates. One report on
earthworms has been published by Gish (1970), who measured the
levels of gamma-chlordane in the soil and earthworms. CFs of 0.37,
7.1, 10.6, and 152 were obtained for 4 worms in agricultural soils.
Several studies are available on fresh-water fish. Henderson et
al. (1969) found that fish from Atlantic-coast streams, which gave
positive samples, contained between 0.1 and 7.29 mg chlordane/kg
(whole fish wet weight), fish from the Great Lake drainage areas
contained between 0.01 and 0.39 mg/kg, and fish from the
Mississippi River system contained between 0.01 and 0.72 mg/kg.
Fish from other systems (Hudson Bay, Colorado River, Interior
basins, Californian streams, Columbia River, Pacific coast and
Alaskan streams) contained less than 0.01 mg/kg. A further study
by Henderson et al. (1971) yielded 16 positive samples out of 666
fish taken from 50 sites, giving chlordane levels of between 0.09
and 13.5 mg/kg (whole fish wet weight). Working on chlordane
accumulation in sucker-fish, Roberts et al. (1977) showed that
accumulation from food was directly proportional to the lipid
levels of the fish. Chlordane was given to the northern redhorse
sucker, Moxostoma macrolepidotum, in the feed at 45 g/kg dry
feed for 5 consecutive days and to the white sucker, Catostomus
commersoni, directly to the stomach in a single dose of 340 mg
in corn oil. Both tests gave CF values of less than, or equal, to
0.52. CF values obtained in fish by uptake from the food were
lower than those obtained by uptake from water (goldfish at a CF of
162 with chlordane in diet (Moore et al., 1977); mosquito fish at a
CF of 8258 with chlordane in diet and in water (Sanborn et al.,
1976)). This suggests that chlordane is taken up directly from
water (bioaccumulated) more than it is from ingested food
(biomagnified).
Schimmel et al. (1976a,b) reported that CFs for two species of
marine fish were similar to those found in fresh-water species.
Spot and sheepshead minnow concentrated gamma-chlordane 3700 - 14
800 and 9000 - 16 800 times, respectively, in 4 days, and 3300 -
5100 and 10 300 times, respectively, in 24 days. Veith et al.
(1979) exposed fathead minnows to 5.9 µg chlordane/litre for 32
days and obtained a CF of 37 800 for the whole body. Parrish et
al. (1978) similarly reported 16 000 for whole body in the
sheepshead minnow after exposure for 189 days. They also
determined the CF after only 28 days exposure and found a
comparable whole-body value of 15 300. A range of CFs between 9000
and 16 786 was reported by Schimmel et al. (1976a) when sheepshead
minnows were exposed to gamma-chlordane (in technical heptachlor)
at 1.1 - 2.8 µg/litre for 96 h. In a field study, long-term
exposure (209 days or less) of large-mouth bass to between 0.01 and
0.1 µg/litre chlordane gave concentration factors of between 157
and 3308 (Godsil & Johnson, 1968).
Food chain magnification is unlikely in terrestrial organisms.
Species of birds and mammals studied show little bioaccumulation,
probably because chlordane is rapidly broken down in homoiotherms.
There are very few data on birds. One report (Foster et al., 1972)
refers to a study on the accumulation of chlordane in laying hens
fed 0.1 mg/kg diet. CF values were maximal after 7 - 9 weeks, diet
to fat was between 0.01 and 3.3, and diet to eggs was between 0.01
and 2. After 3 weeks on an untreated diet, chlordane was not
detectable. McCaskey et al. (1968) dosed hens with the equivalent
of a diet containing 10 - 15 mg 60% technical chlordane/kg for 5
days and obtained a maximum CF value in eggs of 0.38 on day 6.
8.5. Population and Community Effects
The fate of 14C-chlordane was investigated in a terrestrial-
aquatic model ecosystem composed of an alga (Oedogonium), snails
(Physa), mosquito larvae (Culex), and fish (Gambusia)
(Sanborn et al., 1976). Accumulation of chlordane residues was
evident in all organisms in the ecosystem, but no toxic effects
were reported.
Exposure of a natural phytoplankton population to 5 and 10 µg
chlordane/litre for 5 days had virtually no effect on its species
composition (Biggs et al., 1978).
8.6. Effects on the Abiotic Environment
No data are available on abiotic effects.
8.7. Appraisal
Data on aquatic toxicity require interpretation. Although the
solubility of chlordane in water has been measured at between 6
and 9 µg/litre, in many studies on its aquatic toxicity, chlordane
has been applied at much higher nominal concentrations. Either the
chlordane has not been in solution or was added with a solvent.
Actual levels of chlordane in natural waters rarely exceed 250
ng/litre with most levels below 20 ng/litre. Test levels are
therefore unrealistically high. The data should thus be critically
examined unless the actual concentrations experienced by the test
organisms were measured.
Few long-term studies of the chronic effects of chlordane are
available. Data do not define threshold levels, although they
suggest levels at which effects might be expected. Data seldom
provide quantitative dose-response relationships.
Effects of chlordane on primary producers in the aquatic food
chain are largely unknown because studies have used unrealistically
high concentrations. Some data on lethal doses for aquatic
organisms are available, but data on sub-lethal effects on
reproduction or behaviour are not. There are data on the acute
toxicity of chlordane for fish at concentrations approaching the
water solubility, but few on long-term exposure at lower doses.
Most terrestrial studies have been on soil organisms. Effects
here might be due to the heptachlor in technical chlordane or to a
combination of heptachlor and chlordane. Only very high
application rates of chlordane affected arthropods. Field studies
do not indicate direct toxicity but a combination of toxicity and
avoidance of the compound.
Of major importance is the clear toxicity of chlordane for
earthworms with implications for soil fertility. Molluscs also
appear to be particularly sensitive to chlordane.
9. PREVIOUS EVALUATIONS OF CHLORDANE BY INTERNATIONAL BODIES
An IARC Working Group (IARC, 1982) concluded that the available
epidemiological studies on chlordane were inadequate for the
evaluation of the cancer risk for man and that there was limited
evidence of the carcinogenicity of chlordane for experimental
animals.
WHO has recommended a guideline value of 0.3 µg/litre for
chlordane (total isomers) in drinking-water (WHO, 1982).
The Joint Meeting on Pesticide Residues (JMPR) reviewed
residues and toxicity data on chlordane on several occasions in the
past (1965, 1967, 1970). In November 1972, it re-established
residue tolerences ranging from 0.02 - 0.5 mg/kg for the sum of
alpha- and gamma-isomers of chlordane and oxychlordane (FAO/WHO,
1973). The acceptable daily intake (ADI) for human beings of
0 - 0.001 mg/kg body weight was confirmed in December 1977
(FAO/WHO, 1978). This was based on no-observed-adverse-effect-
levels of:
- 5 mg/kg in the diet, equivalent to 0.25 mg/kg body
weight in the rat; and
- 3 mg/kg in the diet, equivalent to 0.075 mg/kg body
weight in the dog.
Both "no-observed-adverse-effect-levels" and the ADI were
reviewed by the 1982 JMPR (FAO/WHO, 1983). The ADI was given
"temporary" status pending the results of toxicology studies still
in progress.
WHO (1984), in its "Guidelines to the Use of the WHO
Recommended Classification of Pesticides by Hazard", classified
technical chlordane as moderately hazardous.
The WHO/FAO (1978) has issued practical advice in its "Data
Sheet on Pesticides" including one on Chlordane (No. 36) dealing
with labelling, safe-handling, transport, storage, disposal,
decontamination, training and medical supervision of workers, first
aid and medical treatment.
Regulatory standards established by national bodies in 12
different countries (Argentina, Brazil, Czechoslovakia, the Federal
Republic of Germany, India, Japan, Kenya, Mexico, Sweden, the
United Kingdom, the USA, and the USSR) and the EEC is available
from the IRPTC (International Register of Potentially Toxic
Chemicals) Legal file (IRPTC, 1983).
The CEC reviewed the data available on chlordane in 1981.
10. EVALUATION OF HEALTH RISKS FOR MAN AND EFFECTS ON THE
ENVIRONMENT
10.1. Chlordane Toxicity
Chlordane is readily absorbed in both animals and man via the
skin, via ingestion, and probably also by inhalation. Some
accumulation occurs in the body on repeated exposure - mainly in
adipose tissue. Elimination from the body is fairly slow. The
half-life in various species, including man, is of the order of a
few weeks.
The oral LD50 values of chlordane in the rat range from
200 - 590 mg/kg body weight. Thus, chlordane is moderately toxic
in acute exposures.
Acute poisoning in man and animals is characterized by
manifestations of central nervous system stimulation such as
disorientation, tremors, and convulsions. Death may follow
respiratory failure.
In experimental animals (rats and dogs), prolonged exposure
to levels in the diet exceeding 3 - 5 mg/kg resulted in the
induction of hepatic microsomal enzymes and, at a later stage,
liver hypertrophy with histological changes. At higher levels
(i.e., > 15 mg/kg body weight per day), chlordane is hepatotoxic.
For no-observed-adverse-effect levels see section 6.1.1.
At dosages above 30 mg/kg diet, chlordane interferes with
reproduction in rats and mice, but this was reversible after
exposure ceased. There are no indications for teratogenicity in
the rabbit at 15 mg/kg body weight per day.
Chlordane produces hepatocellular carcinomas in mice. It
is not generally active in short-term tests designed to measure
genetic activity. Chlordane can interfere with cell to cell
communication in vitro, a characteristic of many promoting agents.
10.2. Exposure to Chlordane
Food is the major source of exposure of the general population
to chlordane, but the use of chlordane on food crops has decreased
and residues in food from animal origin are low. Some chlordane
exposure can occur in buildings where chlordane has been used for
termite or other insect control.
No adverse health effects have been reported in workers engaged
in the manufacture of chlordane or in pest-control operations,
where exposure could be quite high. However, several cases of
accidental and suicidal poisoning in man have been reported
resulting in the symptoms described in section 7.1.
10.3. Evaluation of Overall Environmental Effects
Chlordane is used primarily to control soil pests. Technical
chlordane is a mixture of chlorinated hydrocarbons and contains
heptachlor, which might contribute significantly to the
insecticidal properties of the technical formulation.
About half of the chlordane applied to soil disappears in the
first season, presumably by volatalisation or by "run-off" into
surface waters. Remaining residues persist for several seasons.
If chlordane is applied annually for several successive seasons,
residues accumulate in the soil. Most chlordane persists in the
cultivated levels, since there is little leaching into subsoil.
The high rate of metabolism of chlordane in warm-blooded
animals means that there is little possibility of accumulation in
these animals or magnification in food chains at this level.
Concentration factors are generally modest in aquatic organisms;
this combined with its low solubility in water means that chlordane
presents a limited hazard for aquatic vertebrates. Long-term
effects are not sufficiently well-documented to say that there is
not a potential hazard for fish, but this seems unlikely from the
information available, as far as temperate areas are concerned.
The compound shows a higher toxicity at higher temperatures.
Significant mortality in tropical species of fish at concentrations
well within the solubility of the compound, suggest that chlordane
may be a greater aquatic hazard at lower latitudes.
The high toxicity of chlordane for earthworms may constitute
its greatest potential hazard. The long-term effects of reduced
numbers of earthworms in the soil cannot be readily assessed
because the ecology of the animal is still poorly understood.
10.4. Evaluation of Risks for Human Health and the Environment
Although there is no evidence that incriminates chlordane as a
human carcinogen, the suspicion principally arising from the mouse
carcinogenicity studies cannot be entirely put aside. Further
research is required to elucidate this problem. Nevertheless, in
the present state of knowledge, it is concluded that:
1. As long as occupational hygiene procedures are maintained
to keep exposure levels to a minimum, whether or not by the
imposition of maximum allowable concentrations, there is
little reason to believe that workers will be at risk from
their handling, or contacts, with chlordane.
2. For the general population, consumers should suffer no
adverse effects from chlordane as food residues, provided
that the intake is kept within the temporary ADI set by the
Joint FAO/WHO Meeting.
In certain regions of the world, the exposure of the
general population to chlordane may be augmented by its
use as a termiticide in buildings.
3. Apart from the possible long-term adverse effects on
aquatic organisms in tropical areas and the depleted soil
fertility that may arise, in time, from the suppression of
the earthworm population, chlordane seems to cause little
environmental concern in its normal use as a termiticide
and in other non-agricultural applications.
REFERENCES
AHMED, F.E., HART, R.W., & LEWIS, N.J. (1977a) Pesticide
induced DNA damage and its repair in cultured human cells.
Mutat. Res., 42: 161-174.
AHMED, F.E., LEWIS, N.J., & HART, R.W. (1977b) Pesticide
induced ouabain resistant mutants in Chinese hamster. Chem.
Biol. Interactions, 19: 369-374.
ALDRICH, F.D. & HOLMES, J.H. (1969) Acute chlordane
intoxication in a child. Case report with toxicological
data. Arch. environ. Health, 19: 129-132.
AL-HACHIM, G.M. & AL-BAKER, A. (1973) Effects of chlordane
on conditioned avoidance response, brain seizure threshold and
open-field performance of prenatally-treated mice. Br. J.
Pharmacol., 49: 311-315.
ALVAREZ, W.C. & HYMAN, S. (1953) Absence of toxic
manifestations in workers exposed to chlordane. Arch. ind.
Hyg. occup. Med., 7: 197-210.
AMBROSE, A.M., CHRISTENSEN, H.E., ROBBINS, D.J., & RATHER,
L.J. (1953) Toxicology and pharmacological studies on
chlordane. Arch. ind. Hyg. occup. Med., 1: 197-210.
ARNOLD, D.W., KENNEDY, G.L., KEPLINGER, M.L., CALANDRA, J.C.,
& CALO, C.J. (1977) Dominant lethal studies with technical
chlordane, HCS-3260, and heptachlor: Heptachlor epoxide.
J. Toxicol. environ. Health, 2: 547-555.
ATALLAH, Y.H., WHITACRE, D.M., & POLEN, P.B. (1977) Artifacts in
monitoring - related analysis of human adipose tissue for some
organochlorine pesticides. Chemosphere, 6: 17-20.
ATALLAH, Y.H., WHITACRE, D.M., & HOO, B.L. (1979)
Comparative volatility of liquid and granular formations of
chlordane and heptachlor from soil. Bull. environ. Contam.
Toxicol., 22: 570-574.
BACHMANN, K.A. & BURKMAN, A.M. (1974) Topical application of
a chlordane containing ectoparasiticide: Effect on plasma
half-life of warfarin in dogs. Pharmacologist, 16: 284.
BALBA, H.M. & SAHA, J.G. (1978) Studies on the distribution,
excretion, and metabolism of alpha- and gamma-isomers of 14C
chlordane in rabbits. J. environ. Sci. Health, B13: 211-233.
BARNETT, R.W. & DOROUGH, H.W. (1974) Metabolism of chlordane
in rats. J. agric. food Chem., 22: 612-619.
BARNETT, R.W., D'ERCOLE, A.J., CAIN, J.D., & ARTHUR, R.D.
(1979) Organochlorine pesticide residues in human milk
samples from women living in north-west and north-east
Mississippi, 1973-1975. Pestic. Monit. J., 13: 47-51.
BARTHEL, W.F., HAWTHORNE, J.C., FORD, J.H., BOLTON, G.C.,
McDOWELL, L.L., GRISSINGER, E.H., & PARSONS, D.A. (1969)
Pesticide residues in sediments of the Lower Mississippi River
and its tributaries. Pestic. Monit. J., 3: 8-34.
BATTE, E.G. & TURK, R.D. (1948) Toxicity of some synthetic
insecticides to dogs. J. econ. Entomol., 41: 102-103.
BEVENUE, A., OGATA, J.N., & HYLIN, J.W. (1972a) Organo-
chlorine pesticides in rain water, Oahu, Hawaii, 1971-1972.
Bull. environ. Contam. Toxicol., 8: 238-241.
BEVENUE, A., HYLIN, J.W., KAWANO, Y., & KELLEY, T.W. (1972b)
Organochlorine pesticide residues in water, sediment, algae,
and fish, Hawaii, 1970-71. Pestic. Monit. J., 6: 56-64.
BIGGS, D.C., ROWLAND, R.G., O'CONNORS, H.B., POWERS, C.D., &
WURSTER, C.F. (l978) A comparison of the effects of
chlordane and PCB on the growth, photosynthesis and cell size
of estuarine phytoplankton. Environ. Pollut., 15: 253-263.
BIROS, F.J. & ENOS, H.F. (1973) Oxychlordane residues in
human adipose tissue. Bull. environ. Contam. Toxicol., 10:
257-260.
BOYD, J.C. (1971) Field study of a chlordane residue
problem: Soil and plant relationships. Bull. environ. Contam.
Toxicol., 6: 177-182.
BOYD, E.M. (1972) Chlordane. In: Protein deficiency and
pesticide toxicity, Springfield, Ill., Charles C. Thomas,
468 pp.
BRIMFIELD, A.A. & STREET, J.C. (1979) Mammalian
biotransformation of chlordane: in vivo and primary hepatic
comparisons. Ann. New York Acad. Sci., 320: 247-256.
BRIMFIELD, A.A., STREET, J.C., FUTRELL, J., & CHATFIELD, D.A.
(1978) Identification of products arising from the metabolism
of cis- and trans-chlordane in rat liver microsomes in vitro:
Outline of a possible metabolic pathway. Pestic. Biochem.
Physiol., 9: 84-95.
BRODTMANN, N.V., Jr (1976) Continuous analysis of
chlorinated hydrocarbon pesticides in the lower Mississippi
river. Bull. environ. Contam. Toxicol., 15: 33-39.
BRUBAKER, P.E., FLAMM, W.G., & BERNHEIM, N.J. (1970) Effect
of gamma-chlordane on synchronized lymphoma cells and
inhibition of cell division. Nature (Lond.), 226: 548-549.
BUCK, W.B., OSWEILER, G.D., & VAN GELDER, G.A. (1973)
Clinical and diagnostic veterinary toxicology, Dubuque, Iowa,
Kendall Hunt.
BUGG, J.C., HIGGINS, J.E., & ROBERTSON, E.A. (l967) Residues
in fish, wildlife and estuaries: chlorinated pesticide levels
in the eastern oyster (Crassostrea virginica) from selected
areas of the South Atlantic and Gulf of Mexico. Pestic. Monit.
J., 1: 9-12.
BUTLER, P.A. (l963) Commercial fishery investigations,
Washington DC, US Department of the Interior, Fish and
Wildlife Service, pp. 5-28 (Circular 199).
BUTLER, P.A. & SCHUTZMANN, R.L. (1978) Residues in
pesticides and PCBs in estuarine fish, 1972-1976; National
Pesticide Monitoring Program. Pestic. Monit. J., 12: 51-59.
CALDWELL, R.S. (l977) Biological effects of pesticides on
the Dungeness crab, Washington DC, US Environment Protection
Agency (Report No. EPA 600/3-77-131).
CANADA, NATIONAL RESEARCH COUNCIL (1974) Chlordane: Its
effects on Canadian ecosystems and its chemistry, Canada, NRC
(Report Monograph Non Serials 189).
CARDWELL, R.D., FOREMAN, D.G., PAYNE, T.R., & WILBUR, D.J.
(l977) Acute and chronic toxicity of chlordane to fish and
invertebrates, Washington DC, US Environment Protection
Agency, 126 pp (Report No. EPA 600/3-77-019).
CAREY, A.E., WIERSMA, G.B., TAI, H., & MITCHELL, W.G. (1973)
Organochlorine pesticide residues in soils and crops of the
Corn Belt region, United States, 1970. Pestic. Monit. J., 6:
369-376.
CASPER, V.L. (l967) Galveston Bay pesticide study - water
and oyster samples analysed for pesticide residues following
mosquito control programme. Pestic. Monit. J., 1: 13-15.
CEC (1981) Criteria (dose-effects relationship) for organo-
chlorine pesticides, Oxford, CEC, Pergamon Press.
CHAMBERS, C. & DUTTA, S.K. (1976) Mutagenic tests of
chlordane on different microbial tester strains. Genetics,
83: S13.
CLARK, D.R., Jr & KRYNITSKY, A. (1978) Organochlorine
residues and reproduction in the little brown bat, Laurel,
Maryland, June 1976. Pestic. Monit. J., 12: 113-116.
CLARK, D.R., Jr & PROUTY, R.M. (1976) Organochlorine
residues in three bat species from four localities in Maryland
and West Virginia, 1973. Pestic. Monit. J., 10: 44-53.
CLEGG, T.J. & KOEVENIG, J.L. (l974) The effect of four
chlorinated hydrocarbon pesticides and one organophosphate
pesticide on ATP levels in three species of photosynthesizing
fresh-water algae. Bot. Gaz., 135: 368-372.
CLEMENS, H.P. & SNEED, K.E. (l959) Lethal doses of several
commercial chemicals for fingerling channel catfish,
Washington DC, US Department of the Interior, Fish and
Wildlife Service, 10 pp (Special Scientific Report on
Fisheries No. 316).
COCHRANE, W.P. & GREENHALGH, R. (1976) Chemical composition
of technical chlordane. J. Assoc. Off. Anal. Chem., 59:
696-702.
COCHRANE, W.P., PARLAR, H., GAEB, S., & KORTE, F. (1975)
Structural elucidation of the chlordene isomer constituents of
technical chlordane. J. agric. food Chem., 23: 882-886.
COPE, O.B. (l965) Sport fishery investigations, Washington
DC, US Department of the Interior, Fish and Wildlife Service
(Circular 226).
COPE, O.B., GJULLIN, C.M., & STORM, A. (l947) Effects of
some insecticides on trout and salmon in Alaska, with
reference to black-fly control. Trans. Am. Fish. Soc., 77:
160-177.
CORBETT, D.C.M. & WEBB, R.M. (l968) Effect of herbicides in
minimum tillage, Suffolk, England, Richard Clay (The Chaucer
Press) Ltd., pp. 158-159 (Report of the Rothamsted
Experimental Station for l967).
COUNCIL FOR AGRICULTURAL SCIENCE & TECHNOLOGY (1975)
Chlordane and heptachlor, Ames, Iowa, Iowa State University,
Department of Agronomy, pp. 1-71 (Report No. 47, Oct. 3).
CRANMER, J.S., AVERY, D.L., GRADY, R.R., & KITAY, J.I. (1978)
Postnatal endocrine dysfunction resulting from prenatal
exposure to carbofuran diazinon or chlordane. J. environ.
Pathol. Toxicol., 2: 357-370.
CROCKETT, A.B., WIERSMA, G.B., TAI, H., MITCHELL, W.G., SAND,
P.F., & CAREY, A.E. (1974) Pesticide residue levels in soils
and crops, FY-70: National soils monitoring program II.
Pestic. Monit. J., 8: 69-97.
CURLEY, A. & GARRETTSON, L.K. (1969) Acute chlordane
poisoning: clinical and chemical studies. Arch. environ.
Health, 18: 211-215.
DATTA, K.K., GUPTA, P.C., & DIKSHITH, T.S.S. (1975) Effect
of chlordane on the skin of male guinea pigs. In: Zaidi,
S.H., ed. Environmental pollution and human health, Lucknow,
India, Industrial Toxicology Research Centre, pp. 608-611.
DAVIS, R.W., FRIEDHOFF, J.M., & WEDENEYER, G.A. (l972)
Organochlorine insecticide, herbicide and polychlorinated
biphenyl (PCB) inhibition of NaK-ATPase in rainbow trout.
Bull. environ. Contam. Toxicol., 8: 69-72.
DEN TONKELAAR, E.M. & VAN ESCH, G.J. (1974) No-effect levels
of organochlorine pesticides based on induction of microsomal
liver enzymes in short-term toxicity experiments. Toxicology,
2: 371-380.
DERBES, J.V., DENT, H.J., FOREST, W.W., & JOHNSON, M.F.
(1955) Fatal chlordane poisoning. J. Am. Med. Assoc.,
158(15): 1367-1369.
DEWITT, J.B., STICKEL, W.H., & SPRINGER, P.F. (1963)
Wildlife studies, Patuxent Wildlife Research Centre l961-l962,
Washington DC, US Department of the Interior, Fish and
Wildlife Service, pp. 74-96 (Circular 167).
DITRAGLIA, D., BROWN, D.P., NAMEKATA, T., & IVERSON N.
(1981) Mortality study of workers employed at organochlorine
pesticide manufacturing plants. Scand. J. Work Environ.
Health, 7(Suppl. 4): 140-146.
DOANE, C.C. (l962) Effects of certain insecticides on
earthworms. J. econ. Entomol., 55: 416-418.
DRUMMOND, L., CHETTY, K.N., BAILEY, M., & DESAIAH, D. (1980)
in vivo effect of chlordane on rat brain ATP-ase system. Fed.
Proc., 39: 998.
DUFFY, J.R. & WONG, N. (1967) Residues of organochlorine
insecticides and their metabolites in soil in the Atlantic
Provinces of Canada. J. agric. food Chem., 15(3): 457-464.
EDWARDS, C.A. (l965a) Some side-effects resulting from the
use of persistent insecticides. Ann. appl. Biol., 55: 329-331.
EDWARDS, C.A. (l965b) Effects of pesticide residues on soil
invertebrates and plants. In: Goodman, G.T., Edwards, R.W., &
Lambert, J.M., ed. Ecology and the industrial society,
Reading, England, British Ecological Society, Symposium No. 5,
pp. 239-261.
EPSTEIN, S.S. (1976) Carcinogenicity of heptachlor and
chlordane. Sci. total Environ., 6: 103-154.
EPSTEIN, S.S., ARNOLD, E., ANDREA, J., BASS, W., & BISHOP, Y.
(1972) Detection of chemical mutagens by the dominant lethal
assay in the mouse. Toxicol. appl. Pharmacol., 23: 288-335.
ERCEGOVICH, C.D. & RASHID, K.A. (1977) Mutagenesis induced
in mutant strains of Salmonella typhimurium by pesticides.
Soc. Abstr. Pap., 174: 43.
FAO/WHO (1968) 1967 Evaluations of some pesticide residues
in food, Rome, Food and Agriculture Organization of the United
Nations (The Monographs).
FAO/WHO (1973) 1972 Evaluations of some pesticide residues
in food, Rome, Food and Agriculture Organization of the United
Nations.
FAO/WHO (1978) 1977 Evaluations of some pesticide residues
in food, Rome, Food and Agriculture Organization of the United
Nations.
FAO/WHO (1981) Joint FAO/WHO food and animal feed
contamination monitoring programme. Summary of data received
from collaborating centres - 1977-1980. Part B - Contaminants,
Geneva, World Health Organization.
FAO/WHO (1983) Pesticide residues in food - 1982, Rome, Food
and Agriculture Organization of the United Nations, pp. 16-17
(FAO Plant Production and Protection Paper 46).
FISHBEIN, W.I., WHITE, J.V., & ISAACS, H.J. (1964) Survey of
workers exposed to chlordane. Ind. Med. Surg., 33: 726-727.
FITZHUGH, O.G. & FAIRCHILD, H.E. (1976) Pesticidal aspects
of chlordane in relation to man and the environment,
Washington DC, US Environmental Protection Agency, US NTIS,
114 pp (PB Rep., ISS PB- 257107).
FOLMAR, L.C. (1978) In vitro inhibition of rat brain
ATPase, pNPPase, and ATP-32p exchange by chlorinated-diphenyl
ethanes and cyclodiene insecticides. Bull. environ. Contam.
Toxicol., 19: 481-488.
FOSTER, T.S., MORLEY, H.V., PURKAYASTHA, R., GREENHALGH, R., &
HUNT, J.R. (l972) Residues in eggs and tissues of hens fed a
ration containing low levels of pesticides with and without
charcoal. J. econ. Entomol., 65: 982-988.
FOUTS, J.R. (1963) Factors influencing the metabolism of
drugs in liver microsomes. Ann. New York Acad. Sci., 104:
875-880.
FOX, C.J.S. (l958) Some effects of insecticides on the
wireworms and vegetation of grassland in Nova Scotia. In:
Proceedings of the Tenth Congress on Entomology, Canada,
Vol. 3, pp. 297-300.
FRANK, R., BRAUN, H.E., HOLDRINET, M., DODGE, D.P., & NAPSZY,
S.J. (1978a) Residues of organochlorine insecticides and
polychlorinated biphenyls in fish from lakes Saint Clair and
Erie, Canada, 1968-1976. Pestic. Monit. J., 12: 69-80.
FRANK, R., HOLDRINET, M., BRAUN, H.E., DODGE, D.P., &
SPRANGLER, G.E. (1978b) Residues of organochlorine insecticides
and polychlorinated biphenyls in fish from lakes Huron and
Superior, Canada, 1968-1976. Pestic. Monit. J., 12: 60-68.
GAEB, S., BORN, L., PARLAR, H., & KORTE, F. (1977)
Structural elucidation of an octachloro component of technical
chlordane (Compound K) by spectroscopic and X-ray methods.
J. agric. food Chem., 25: 1365-1371.
GAINES, T.B. (1969) Acute toxicity of pesticides. Toxicol.
appl. Pharmacol., 14: 525-534.
GISH, C.D. (l970) Pesticides in soil. Organochlorine
insecticide residues in soils and soil invertebrates from
agricultural land. Pestic. Monit. J., 3: 241-252.
GLOOSCHENKO, V. & LOTT, J.N.A. (l977) The effects of
chlordane on the green algae, Scenedesmus quadricauda and
Chlamydomonas sp. Can. J. Bot., 55: 2866-2872.
GLOOSCHENKO, W.A., STRACHAN, W.M.J., & SAMPSON, R.C.J.
(1976) Residues in water: distribution of pesticides and
polychlorinated biphenyls in water, sediments, and seston of
the Upper Great Lakes - 1974. Pestic. Monit. J., 10: 61-67.
GODSIL, P.J. & JOHNSON, W.C. (l968) Residues in fish,
wildlife and estuaries. Pesticide monitoring of the aquatic
biota at the Tule Lake National Wildlife Refuge. Pestic.
Monit. J., 1: 21-26.
GOES, T.R., SAVAGE, E.P., & BOYD, W.L. (1978) In vitro
inhibition of oral viridans streptococci by chlordane. Arch.
environ. Contam. Toxicol., 7: 449-456.
GOULD, E. & HAMPSTEAD, E.O. (l95l) The toxicity of
cumulative spray residues in soils. J. econ. Entomol., 44:
713-717.
GOWEN, J.A., WIERSMA, G.B., TAI, H., & MITCHELL, W.G. (1976)
Pesticide levels in hay and soils from nine states, 1971.
Pestic. Monit. J., 10: 114-116.
GRIMES, D.J. & MORRISON, S.M. (1975) Bacterial
bioconcentration of chlorinated hydrocarbon insecticides from
aqueous systems. Microbiol. Ecol., 2: 43-59.
HANSON, W.R. (1952) Effects of some herbicides and
insecticides on the biota of North Dakota marshes. J. Wildl.
Manage., 16: 299-308.
HARRINGTON, J.M., BAKER, E.L., Jr, FOLLAND, D.S., SAUCIER,
J.W., & SANDIFER, S.H. (1978) Chlordane contamination of a
municipal water system. Environ. Res., 15: 155-159.
HARRIS, C.R. & SANS, W.W. (1976) Persistence of velsicol
HCS-3260 (AG-chlordane) in mineral and organic soil. Proc.
Entomol. Soc. Ontario, 106: 34-38.
HART, L.G. & FOUTS, J.R. (1965) Studies of the possible
mechanisms by which chlordane stimulates hepatic microsomal
drug metabolism in the rat. Biochem. Pharmacol., 14: 263-272.
HART, L.G., SHULTICE, R.W., & FOUTS, J.R. (1963) Stimulatory
effects of chlordane on hepatic microsomal drug metabolism in
the rat. Toxicol. appl. Pharmacol., 5: 371-386.
HENDERSON, C., PICKERING, Q.H., & TARZWELL, C.M. (1959) The
relative toxicity of ten chlorinated hydrocarbon insecticides
to four species of fish. Trans. Am. Fish. Soc., 88: 23-32.
HENDERSON, C., JOHNSON, W.L., & INGLIS, A. (1969)
Organochlorine insecticide residues in fish. Pestic. Monit.
J., 3: 145-171.
HENDERSON, C., INGLIS, A., & JOHNSON, W.L. (1971)
Organochlorine insecticide residues in fish - Fall 1969.
National pesticide monitoring program. Pestic. Monit. J., 5:
1-11.
HERRICK, G.M., FRY, J.I., FONG, W.G., & GOLDEN, D.C. (1969)
Insecticide residues in eggs resulting from the dusting and
short-term feeding of low levels of chlorinated hydrocarbon
insecticides to hens. J. agric. food Chem., 17: 291-295.
HILL, E.F., HEATH, R.G., SPANN, J.W., & WILLIAMS, J.D.
(1975) Lethal dietary toxicities of environmental pollutants
to birds, Washington DC, US Department of the Interior, Fish
and Wildlife Service, 61 pp (Special Scientific Report No.
191).
HODGE, H.C. & STERNER, J.H. (1956) Combine and tabulation of
toxicity classes. In: Spector, W.B., ed. Handbook of
toxicology, Philadelphia, Pennsylvania, W.B. Saunders Company,
Vol. 10.
HOPKINS, A.R. & KIRK, V.M. (1957) Effect of several
insecticides on the English Red Worm. J. econ. Entomol., 50:
699-700.
HRDINA, P.D., PETERS, D.A.V., & SINGHAL, R.L. (1973) Acute
neurotoxic effects of alpha-chlordane and alterations in brain
biogenic amines. Proc. Can. Fed. Biol. Soc., 16: 103.
HYDE, K.M. & FALKENBERG, R.L. (1976) Neuroelectrical
disturbance as indicator of chronic chlordane toxicity.
Toxicol. appl. Pharmacol., 37: 499-515.
HYDE, K.M., CRANDALL, J.C., KORTMAN, K.E., & MCCOY, W.K.
(1978) EEG, ECG, and respiratory response to acute
insecticide exposure. Bull. environ. Contam. Toxicol., 19:
47-55.
IARC (1979) Some halogenated hydrocarbons. Chlordane, Lyons,
International Agency for Research on Cancer, pp. 45-65
(Monographs on the Evaluation of the Carcinogenic Risk of
Chemicals to Humans, No. 20).
IARC (1982) Chemicals and industrial processes associated
with cancer in humans, Lyons, International Agency for
Research on Cancer, pp. 80-83 (Monographs on the Evaluation of
the Carcinogenic Risk of Chemicals to Humans, Suppl. 4).
ILO (1980) Occupational exposure limits for airborne toxic
substances, 2nd (revised) ed., Geneva, International Labour
Office (Occupational Safety and Health Series No. 37).
INFANTE, P.F., EPSTEIN, S.S., & NEWTON, W.A., Jr (1978)
Blood dyscrasias and childhood tumors and exposure to
chlordane and heptachlor. Scand. J. Work Environ. Health, 4:
137-150.
INGLE, L. (1952) Chronic oral toxicity of chlordane to
rats. Arch. ind. Hyg. occup. Med., 6: 357-367.
INGLE, L. (1965a) Effects of 1-hydroxychlordene when
incorporated into the diets of rats for 224 days, Urbana,
Illinois, University of Illinois, Department of Zoology
(Report for Velsicol Chemical Corporation).
INGLE, L. (1965b) Monograph on chlordane - toxicological &
pharmacological properties, Urbana, Illinois, University of
Illinois, Food & Drug Library (Library of Congress, Card
No. 65-28686A).
INGLE, L. (1969) Albino rats subjected to alpha and gamma
chlordane incorporated into the daily diet for 78 weeks
(Report from the University of Illinois).
INRS (1983) Valeurs limites pour les concentrations des
substances dangereuses dans l'air des locaux de travail,
Paris, France, Institut National de Recherche et de Sécurité
pour la Préventions des Accidents du Travail et des Maladies
Professionelles (Cahiers de notes documentaires, No. 110).
IRDC (1967) Chlordane, two-year chronic feeding study in the
beagle dog, Mattawan, Michigan, International Research and
Development Corporation (Report 163-001, sponsored by Velsicol
Chemical Corporation).
IRDC (1972) Chlordane, teratology study in rabbits,
Mattawan, Michigan, International Research and Development
Corporation (Report 163-106, sponsored by Velsicol Chemical
Corporation).
IRPTC (1983) IRPTC Legal file 1983, Geneva, International
Register of Potentially Toxic Chemicals, United Nations
Environment Programme (UNEP), Vols. I & II.
IVIE, G.W., KNOW, J.R., KHALIFA, S., YAMAMOTO, I., & CASIDA,
J.E. (1972) Novel photoproducts of heptachlor epoxide,
trans-chlordane, and trans-nonachlor. Bull. environ. Contam.
Toxicol., 7: 376-382.
JENSEN, A.A. (1983) Chemical contaminants in human milk.
Residue Rev., 89: 1-128.
KACEW, S. & SINGHAL, R.L. (1973a) Metabolic alterations after
chronic exposure to alpha-chlordane. Toxicol. appl.
Pharmacol., 24: 539-544.
KACEW, S. & SINGHAL, R.L. (1973b) The influence of
p,p'-DDT, alpha-chlordane, heptachlor and endrin on hepatic
and renal carbohydrate metabolism and cyclic AMP-adenyl
cyclase system. Life Sci., 13: 1363-1371.
KADAM, A.N., BENDRE, S.B., & GHATGE, B.B. (1978) Gas
chromatography of technical chlordane and identification of
some of the components. Pesticides, 12: 13-15.
KAREL, A.K. (1976) Acute chlordane toxicity on the serum
alkaline phosphatase activity of Meriones hurricanae, Jerdon
(gerbil). Arch. int. Physiol. Biochem., 84: 63-68.
KAREL, A.K. & SAXENA, S.C. (1976) Chronic chlordane
toxicity: Effect on blood biochemistry of Meriones hurricanae,
Jerdon, the Indian desert gerbil. Pestic. Biochem. Physiol.,
6: 111-114.
KEPLINGER, M.L., DEICHMANN, W.B., & SALA, F. (1968) Effects
of pesticides on reproduction in mice. Ind. Med. Surg., 37:
525.
KOCH, R.B., CUTCOMB, L.K., & YAP, H.H. (1971) Inhibition of
oligomycin sensitive and insensitive fish adenosine
triphosphatase activity by chlorinated hydrocarbon
insecticides. Biochem. Pharmacol., 20: 3243-3245.
KONAR, S.K. (1968) Experimental use of chlordane in fishery
management. Prog. Fish. Cult., 30: 96-99.
KRAYBILL, H.F.T. (1977a) The determination of carcinogenesis
induced by trace contaminants in potable water. In: Borchardt,
J.A., Cleland, J.K., Redman, W.J., & Olivier, J., ed. Viruses
and trace contaminants in water and wastewater, Ann Arbor,
Michigan, Ann Arbor Science Publishers, Inc., pp. 109-123
(Seminar, Ann Arbor, Michigan, January 26-28, 1977. XIV 249P).
KUTZ, F.W., YOBS, A.R., STRASSMAN, S.C. (1976) Organochlorine
pesticide residues in human adipose tissue. Bull. Soc.
Pharmacol. Environ. Pathol., 4(1): 17-19.
LANGLOIS, B.E. & SIDES, K.G. (1972) Effect of heptachlor and
related compounds on growth of Staphylococcus aureus. Bull
environ. Contam. Toxicol., 8: 158-164.
LAWRENCE, J.M. (1950) Toxicity of some new insecticides to
several species of pondfish. Prog. Fish. Cult., 12: 141-146.
LAWRENCE, J.H., BARRON, R.P., CHEN, J.Y.T., LOMBARDO, P., &
BENSON, W.R. (1970) Note on identification of a chlordane
metabolite found in milk and cheese. J. Assoc. Off. Agric.
Chem., 53: 261-262.
LEGG, D.C. (1968) Comparison of various worm killing
chemicals. J. Sports Turf Res. Inst., 44: 47-48.
LEHMAN, A.J. (1952a) Chemicals in foods: A report to the
Association of Food & Drug Officials on current developments.
Part II. Pesticides Section II: Dermal Toxicity. Assoc. Food
Drug Off. Q. Bull., 16: 3-9.
LEHMAN, A.J. (1952b) A report to the Association of Food and
Drug Officials on current developments. Section III: Subacute
and chronic toxicity. Assoc. Food Drug Off. Q. Bull., 16:
47-53.
LENSKY, P. & EVANS, H.L. (1952) Human poisoning by
chlordane. Report of a case. J. Am. Med. Assoc., 121: 826-827.
LICHTENSTEIN, E.P. & POLIUKA, J.B. (1959) Persistence of
some chlorinated hydrocarbon insecticides in turf soils.
J. econ. Entomol., 52(2): 280-293.
LICHTENSTEIN, E.P., SCHULTZ, K.K., FUHREMANN, T.W., & LIANG,
T.T. (l970) Degradation of aldrin and heptachlor in field
soils during a ten-year period translocation into crops.
J. agric. food Chem., 18: 100-106.
LIDGATE, H.J. (1966) Earthworm control with chlordane.
J. Sports Turf Res. Inst., 42: 5-8.
LINDUSKA, J.P. & SURBER, E.W. (1948) Effect of DDT and other
insecticides on fish and wildlife. Summary of investigations
during 1947, Washington DC, US Department of the Interior,
Fish and Wildlife Service, 19 pp (Circular 15).
LONG, W.H., ANDERSON, H.L., ISA, A.L., & KYLE, M.L. (1967)
Sugarcane growth responses to chlordane and microarthropods
and effects of chlordane on soil fauna. J. econ. Entomol., 60:
623-629.
LORA, R.P., MARTEACHE, A.H., VILLAR, L.M.P., GIMENEZ, R.L.,
VILLAREJO, M.J., & PEREZ, J.I. (l979) [Organochlorine
pesticides in the milk of Spanish humans.] Rev. Esp. pediatr.,
35: 93 (in Spanish).
LUDEMANN, D. & NEUMANN, H. (1962) [On the effects of the
latest contact insecticide on fresh water animals.] Anz.
Schaelingskd., 35: 5-9 (in German).
LUDKE, J.L. (1976) Organochlorine pesticide residues
associated with mortality: additivity of chlordane and endrin.
Bull. environ. Contam. Toxicol., 16: 253-260.
MACEK, K.J., HUTCHINSON, C., & COPE, O.B. (1969) The effects
of temperature on the susceptibility of bluegills and rainbow
trout to selected pesticides. Bull. environ. Contam. Toxicol.,
4: 174-183.
MACMAHON, B. & WANG, H.H. (1982) A second follow-up of
mortality in a cohort of pesticide applicators, Cambridge,
Massachusetts, Harvard School of Public Health, Department of
Epidemiology.
MADHUKAR, B.V. & MATSUMURA, F. (1979) Comparison of
induction patterns of rat hepatic microsomal mixed-function
oxidases by pesticides and related chemicals. Pestic.
Biochem. Physiol., 11: 301-308.
MAGNANI, B., POWERS, C.D., WURSTER, C.F., & O'CONNORS, H.B.
(1978) Effects of chlordane and heptachlor on the marine dino
flagellate Exuviella baltica Lohmann. Bull. environ. Contam.
Toxicol., 20: 1-8.
MAGUIRE, J. & WATKIN, N. (1975) Carbonic anhydrase
inhibition. Bull. environ. Contam. Toxicol., 13: 625-629.
MAHON, D.C. (1977) Interactions, in rats, between carbon
tetrachloride-induced liver cirrhosis and chronic treatment
with the insecticide chlordane. Diss. Abstr. Int., 38: 4012B.
MAHON, D.C. & OLOFFS, P.C. (1979) Effects of sub-chronic
low-level dietary intake of chlordane on rats with cirrhosis
of the liver. J. environ. Sci. Health, B14: 227-246.
MAHON, D.C., NAIR, K.K., & OLOFFS, P.C. (1979) DNA in rat
hepatocyte nuclei: Effects of treatment with low levels of
carbon tetrachloride and/or chlordane. Can. J. Zool., 57:
1003-1009.
MASLANSKY, C.J. & WILLIAMS, G.M. (1981) Evidence for an
epigenetic mode of action in organochlorine pesticide
hepatocarcinogenicity: A lack of genotoxicity in rat, mouse
and hamster hepatocytes. J. Toxicol. environ. Health, 8:
121-130.
MASTRI, C., KEPLINGER, M.L., & FANCHER, O.E. (1969a) Acute
oral toxicity study on synthetic (X) in male and female
albino rats, Illinois, Industrial Bio-Test Laboratories
(Report for Velsicol Chemical Corporation).
MASTRI, C., KEPLINGER, M.L., & FANCHER, O.E. (1969b) Acute
oral toxicity study on 4 chlordenes in albino rats, Illinois,
Industrial Bio-Test Laboratories (Report for Velsicol Chemical
Corporation).
MASTRI, C., KEPLINGER, M.L., & FANCHER, O.E. (1969c) Acute
oral toxicity study on 2 chlordenes in female albino rats,
Illinois, Industrial Bio-Test Laboratories (Report for
Velsicol Chemical Corporation).
MATTRAW, H.C., Jr (1975) Occurrence of chlorinated hydro-
carbon insecticides, Southern Florida, 1968-1972. Pestic.
Monit. J., 9: 106-114.
MCCASKEY, T.A., STENP, A.R., LISKA, B.J., & STADELMAN, W.J.
(1968) Residues in egg yolks and raw and cooked tissues from
laying hens administered selected chlorinated hydrocarbon
insecticides. Poult. Sci., 47: 564-569.
MCCLEESE, D.W., BURRIDGE, L.E., & VAN DINTER, J. (1982)
Toxicities of five organochlorine compounds in water and
sediment to Nereis virens. Bull. environ. Contam. Toxicol.,
28: 216-220.
MCGILL, H.C. (1979) Pilot study of the effects of pesticides
on blood lipoproteins, arteries, and cardiac muscle of
baboons, Washington DC, US Environmental Protection Agency.
MEHRLE, P.M., JOHNSON, W.W., & MEYER, F.L. (1974)
Nutritional effects of chlordane toxicity to rainbow trout.
Bull. environ. Contam. Toxicol., 12: 513-517.
MEITH-AVCIN, N., WARLEN, S.M., & BARBER, R.T. (1973)
Organochlorine insecticide residues in a Bathyl-Demersal Fish
from 2500 metres. Environ. Lett., 5: 215-221.
MES, J. & DAVIES, D.J. (1978) Variation in the
polychlorinated biphenyl and organochlor pesticide residues
during human breastfeeding and its diurnal pattern.
Chemosphere, 7: 699-706.
MES, J., COFFIN, D.E., & CAMPBELL, D.T. (1974)
Polychlorinated biphenyl and organochlorine pesticide residues
in Canadian chicken eggs. Pestic. Monit. J., 8: 8-11.
MIYAZAKI, T., AKIYAMA, K., KANEKO, S., HORII, S., & YAMAGISHI,
T. (1980) Chlordane residues in human milk. Bull. environ.
Contam. Toxicol., 25: 518.
MOORE, R., TORO, E., STANTON, M., & KHAN, M.A.Q. (1977)
Absorption and elimination of 14C-alpha and gamma-chlordane by
a fresh-water alga, daphnid, and goldfish. Arch. environ.
Contam. Toxicol., 6: 411-420.
MORGAN, D.P. & ROAN, C.C. (1969) Renal function in persons
occupationally exposed to pesticides. Arch. environ. Health
19: 633-636.
NAKAS, J.P. (1977) Chlordane. Inhibition of endopeptidase
activity in the marine bacterium, Aeromonas proteolytica, New
Jersey, Rutgers State University, 123 pp (Dissertation).
NAQVI, S.M. (1973) Toxicity of twenty-three insecticides to
a tubificid worm Branchiura sowerbyi from the Mississippi
Delta. J. econ. Entomol., 66: 70-74.
NCI (1977) Bioassay of chlordane for possible
carcinogenicity, Bethesda, Maryland, National Cancer Institute
(CAS NO.
Molecular formula: C10H6C18
CAS chemical name: 1,2,4,5,6,7,8,8-octachloro-2,3,3a,4,
7,7a-hexahydro-4,7-methano-1H-indene
Common trade names: Aspon, Belt, CD 68, Chlorindan,
Chlorkil, Chlordane, Corodan,
Cortilan-neu, Dowchlor, HCS 3260,
Kypchlor, M140, Niran, Octachlor,
Octaterr, Ortho-Klor, Synklor, Tat
Chlor 4, Topichlor, Toxichlor,
Velsicol-1068
CAS registry number: 57-47-9
Relative molecular mass: 409.8
2.2. Properties and Analytical Methods
2.2.1. Physical and chemical properties
Chlordane is a viscous, light yellow to amber-coloured liquid
(IARC, 1979) with a melting point of 106 - 107 °C for the alpha-
isomer and 104 - 105 °C for the gamma-isomer. It has a density of
1.59 - 1.63 g/ml and a vapour pressure of 1 x 10-5 mm Hg at 25 °C.
It is insoluble in water but soluble in most organic solvents.
The major isomers of chlordane have the endo-endo-configuration
on the carbon skeleton (US EPA, 1976a,b). However, the term
chlordane actually refers to a complex mixture of chlordane
isomers, other chlorinated hydrocarbons and by-products. According
to the Canada National Research Council (1974), the technical
product is described as follows:
"Technical chlordane is a mixture of insecticidal
components, including chlorinated addition and substitution
derivatives of 4,7-methano-3a,4,7,7a-tetrahydro-indane.
The chlorine content is 64-67%. The principal components
are alpha- and gamma-chlordane (C10H6 x Cl8), heptachlor
(C10H5Cl7) and non-achlor (C10H5Cl9). Technical chlordane
conforms to the biological, chemical and physical properties
of reference technical chlordane".
The production of technical chlordane is strictly controlled
and its composition varies within narrow limits (Canada, National
Research Council, 1974). Technical chlordane is a mixture of at
least 26 different components, mainly however alpha- and gamma-
chlordane. Up to 14 distinct chromatographic components have been
described (Cochrane et al., 1975; Cochrane & Greenhalgh, 1976; US
EPA, 1976a,b; Gaeb et al., 1977; Sovocool et al., 1977; Kadam et
al., 1978; Parlar et al., 1979). The composition has been
essentially, but not completely, worked out. Chlordane is
available in the USA in five basic formulations (von Rumker et al.,
1974, IARC, 1979), including 5% granules, oil solutions containing
chlordane at 2 - 200 g/litre, and emulsifiable concentrates
containing chlordane at 400 - 800 g/litre.
2.2.2. Analytical methods
Determination of chlordane residues is difficult because
of the complex nature of the components and the fact that each
component degrades independently. Resulting residues may bear
little relation to the proportions in the technical product
(Council for Agriculture Science and Technology, 1975).
Separation from interfering materials can be carried out by thin-
layer chromatography or other partition and clean-up methods (US
EPA, 1976a,b). Extraction from crops, other plant products, dairy
products, plants, and oils was achieved with an 80 - 110%
efficiency using acetonitrile for extraction, petroleum ether for
partitioning, and clean-up on a Florisil column (Canada, National
Research Council, 1974). Gel-permeation chromatography can also be
used for clean-up, particularly with human adipose tissue (Wright
et al., 1978).
The principal method for the qualitative and quantitative
estimation of chlordane isomers is gas-liquid chromatography with
electron capture detection (US EPA, 1976a,b). This method has a
high sensitivity and specificity (Canada, National Research
Council, 1974; Cochrane et al., 1975). According to Atallah et al.
(1977), the highly sensitive electron capture detector can,
however, lead to the incorrect identification of residues of
chlordane and its metabolites. Confirmation of gas-chromatographic
analysis can be carried out with GLC-Mass spectrometry, a method
that can also give a better determination of some of the components
such as heptachlorepoxide (US EPA, 1976a,b). Other methods of
detection include bioassay, carbon-skeleton chromatography,
colorimetric, and total chlorine methods (US EPA, 1976a,b).
Analysis for total organically-bound chlorine (Canada, National
Research Council, 1974) remains the preferred method for the
determination of technical chlordane and the active ingredient
(chlordane) in formulations.
3. SOURCES OF ENVIRONMENTAL POLLUTION, ENVIRONMENTAL
TRANSPORT AND DISTRIBUTION
3.1. Sources of Environmental Pollution
3.1.1. Industrial production and uses
Chlordane was first prepared in the 1940s by exhaustive
chlorination of the cyclopentadiene-hexachlorocyclopentadiene
adduct (IARC, 1979). It was first described as an insecticide in
1945 by Kearns (Spencer, 1973).
Chlordane is produced commercially by reacting hexachloro-
cyclopentadiene with cyclopentadiene to form chlordene, which is
then chlorinated to produce chlordane (IARC, 1979). Chlordane was
first produced commercially in the USA in 1947 (IARC, 1979).
Production in the USA, in 1974, amounted to 9.5 million kg (IARC,
1979). Chlordane is not produced in Europe nor has it ever been
manufactured in Japan (IARC, 1979). In Japan, the only permitted
use of the compound is for the control of termites. It is also
used against wood-boring beetles and in ant baits. Both the
amounts of chlordane produced and used have decreased considerably
in recent years (WHO, 1982).
Chlordane has been used as an insecticide for more than 35
years. It is a versatile, broad spectrum, contact insecticide and
is used mainly for non-agricultural purposes (primarily for the
protection of structures, but also on lawn and turf, ornamental
trees, and drainage ditches) (von Rumker et al., 1974). Further-
more, it is used on corn, potatoes, and livestock. In 1978, a US
EPA cancellation proceeding led to a settlement on contested uses.
This settlement allowed for limited usage by crop, location, amount
allowed, and maximum time interval for use.
Since 1 July 1983, the only use of chlordane approved in the
USA is for the control of underground termites (IARC, 1979). In
Canada, the use of chlordane is controlled under the Pest Control
Products Act and it is used for the protection of structures,
ornamental plants, lawns, and various crops. Accepted uses vary
from province to provide (Canada, National Research Council, 1974).
3.2. Environmental Transport and Distribution
3.2.1. Air
Entry into the atmosphere occurs mainly through aerial
applications of dusts and sprays, soil erosion by the wind, and
volatilization from soil and water (Canada, National Research
Council, 1974).
3.2.2. Water
Few data are available on the routes of entry or the behaviour
and fate of chlordane in aquatic systems. It can be assumed that
not much originates from ground water since there is little
leaching of chlordane. One possible source is surface run-off, but
no studies have tested the extent of this assumption. Another
source is rain; however, in two studies, chlordane levels did not
exceed 2 - 3 ng/litre rain water (Bevenue et al., 1972a; US EPA,
1976a,b).
One important aspect of chlordane residues is that they
accumulate in sediment. The fate and behaviour of chlordane was
investigated in an isolated fresh water lake, previously free from
pesticide residues (Oloffs et al., 1978). The lake was treated
with technical chlordane at 10 µg/litre, and sediment samples were
analysed for chlordane residues 7, 24, 52, 279, and 421 days after
treatment. It was found that water residue concentrations declined
rapidly. After 7 days, only 46.1% of the chlordane residue
remained. After 421 days, residues were still detectable, but all
levels were below 0.01% of the initial concentration. It was
observed that chlordane residues moved quickly to the bottom
sediment and persisted there. Mean residue levels in sediment were
35.29 µg/kg wet weight after 7 days and 10.31 µg/kg after 421 days.
3.2.3. Soil
Chlordane is used almost exclusively as a soil insecticide to
control soil pests such as termites (Canada, National Research
Council, 1974). Thus, residues of chlordane are mainly present in
this environmental compartment. In most temperate climates, only
the two chlordane isomers generally persist (Canada, National
Research Council, 1974). For example, in Nova Scotia, chlordane
was applied at 5 kg/ha per year to sandy loam soil for 3 years.
Fifteen years later, approximately 15% of the residues remained,
the alpha and gammma isomers being the major components (US EPA,
1976a,b).
The components of technical chlordane are relatively insoluble
in water and are readily adsorbed onto soil particles. As a
result, one of the characteristics of soil residues is that they do
not migrate readily through the soil profile (Canada, National
Research Council, 1974; von Rumker et al., 1974). In general, not
more than 15% of the residues migrate below the cultivated layer
(Canada, National Research Council, 1974). As a result, residues
are not likely to become a serious contaminant of the lower soil
strata or deep water sources (Canada, National Research Council,
1974). The organic matter and moisture contents of the soil can
affect the volatilization of chlordane components (Stauffer, 1977).
The organic matter causes greater adsorption and thus reduces
volatilization while soil moisture increases volatilization
(Stauffer, 1977). Also, liquid formulations are more volatile than
granular (Atallah et al., 1979).
3.2.4. Abiotic degradation
Chlordane is stable to light under normal conditions. When it
is exposed to photosensitizers such as rotenone or benzophenone and
short irradiation exposure at wavelengths above 300 nm, some
components will isomerize (Canada, National Research Council,
1974). No detectable degradation products were formed on plant
foliage in the absence of a photosensitizer (Ivie et al., 1972).
3.2.5. Biodegradation
Three conversion products of gamma-chlordane were found in
white cabbage and carrots, 4 weeks after application. One of the
two metabolites isolated from white cabbage (35% of the total), was
given the chlordene chlorohydrin structure. The other isolated
metabolite (15% of the total) was assigned the dihydroxy-beta-
dihydroheptachlor structure. The third metabolite was not
identified. 1,2-Dichlorochlordene, oxychlordane, and photo-alpha-
chlordane, as well as the parent chlordane compounds, were found in
alfalfa after treatment of the soil with chlordane (Canada,
National Research Council, 1974).
Oxychlordane (or 1,2-dichlorochlordene epoxide) is the common
metabolite derived from both alpha- and gamma-chlordane. It has
been found in the fat of pigs fed either of the isomers and in the
milk and cheese from cows fed alfalfa treated with technical
chlordane. According to some authors, alpha- and gamma-chlordane
give rise to oxychlordane via the intermediate 1,2-dichloro-
chlordene (Canada, National Research Council, 1974).
4. ENVIRONMENTAL LEVELS AND EXPOSURES
4.1. Environmental Levels
4.1.1. Air
Generally, atmospheric concentrations of chlordane appear to be
insignificant. However, chlordane has been detected in the air of
buildings where the compound has been used for termite or other
insect control (US EPA, 1976a,b). Insufficient information is
available on this.
4.1.2. Water
Data from several studies indicate that contamination of water
with chlordane is not a widespread problem (US EPA, 1976a,b), and
that, generally, water residue levels are non-measureable or very
low (Canada, National Research Council, 1974).
In a study of the bottom material of 26 tributary streams in
San Fransisco Bay, chlordane was found to be ubiquitous at
concentrations ranging from a trace to 800 µg/kg (Oloffs et al.,
1978).
No chlordane was detected in 188 samples of surface water from
southern Florida but it was detected in 30% of 214 sediment samples
(Mattraw, 1975). In a study in Hawaii in 1970-71 (Bevenue et
al., 1972b), chlordane was found in drinking-water in 9% of samples
at a mean level of 1.0 ng/litre. Chlordane was detected in non-
potable waters from canals at levels ranging from 3.7 - 9.1
ng/litre. Again, sediments showed much higher levels of 190 - 378
µg/kg. Chlordane was found to occur in the lower Mississippi river
almost continuously throughout 1974 at values ranging from 1.3 to
2.9 ng/litre (Brodtmann, 1976). In a study in the lower
Mississippi (Barthel et al., 1969), during 1964-67, chlordane
residues ranged from 0.80 to 2.80 mg/kg in river bed material
samples. In tributaries of the river, values ranged from 0.56 -
6.44 mg/kg, in 13 out of 348 samples. No chlordane was detected in
any water or sediment samples taken from the upper Great Lakes in
1974 (Glooschenko et al., 1976). In one study in 1976 (Harrington
et al., 1978), chlordane contamination of a municipal water system
was reported, concentrations of chlordane accidently rising to 1.2
g/litre.
4.1.3. Soil
One study has shown that the alpha- and gamma-isomers of
chlordane are less persistent in mineral sand soil than in organic
mucky soil (Harris & Sans, 1976). Data from the National Soils
Monitoring Program in 1970 showed that chlordane occurred in 0.07%
of 1346 sites in 35 States. The range of residues was 0.01 - 13.34
mg/kg dry weight with a mean of 0.08 mg/kg (Crockett et al., 1974).
Monitoring of the corn belt region in the USA (12 States) in 1970
showed chlordane to be one of the most commonly detected
insecticides with values of ND - 0.20 mg/kg (Carey et al., 1973).
Data from 9 States in 1971 detected chlordane in one soil sample at
0.04 mg/kg dry weight (Gowen et al., 1976). Monitoring in 1973
showed residues ranging from 0.001 - 0.020 mg/kg with generally
higher values in urban areas. When urban soils were tested in 14
cities in the USA in 1970, values ranged from 0.01 - 1.27 mg/kg.
In the Atlantic provinces of Canada, chlordane was detected in less
than 10% of agricultural lands at concentrations below 1 mg/kg dry
weight (Duffy & Wong, 1967). In another study on chlordane in
Saskatchewan soils (Saha & Sumner, 1971), 7 out of 41 samples
contained chlordane with residue values ranging from 0.01 to 3.91
mg/kg dry weight. The duration of soil contamination has been
studied by several investigators. Using bioassay techniques, it
was found in one study that 15% of active ingredients remained in
turf soils in Wisconsin after 12 years (Lichtenstein & Poliuka,
1959). In a study in 1970 (Lichtenstein, 1970), it was found that
10 years after application of chlordane at 8.5 kg /ha,
approximately 18 - 20% remained.
4.1.4. Crops and wildlife
Since chlordane residues are present predominantly in the soil,
translocation into plants is an important factor. In most
temperate climates, alpha- and gamma-chlordane are the principal
plant residues. In the Canadian climate, the composition of plant
residues resembles that of technical chlordane (Canada, National
Research Council, 1974). In a field study on soil/plant
relationships, it was found that the relationship between residues
in soil and those in crops was not consistent and consequently not
predictable (Boyd, 1971).
In a three-year study, Onsager et al. (1970) monitored
chlordane residues in sugar beets cultivated on loam soil treated
once at 6 different application rates ranging from 1.4 - 22.4
kg/ha. In the first growing season, only sugar beets treated at
the two lowest rates (1.4 and 2.8 mg/kg) showed residues below 0.3
mg/kg dry weight. In the last two seasons, beets from soil treated
at all rates contained residues below this value. In another
study, uptake by root crops was shown to be related to the soil
type (Stewart, 1975). When chlordane was applied to sandy loam
soil with 12% clay at 15 kg/ha, residues in beets, carrots,
parsnips, potatoes, and rutabagas were 0.03, 0.26, 0.24, 0.04, and
0.01 mg/kg, respectively. In sandy loam containing 28% clay,
values were 0.01, 0.07, 0.12, 0.15, and 0.01 mg/kg, respectively.
A study of residues in alfalfa following applications of high
purity chlordane showed that during the first 4 months following
treatment, oxychlordane and photo-alpha-chlordane accounted for 16%
and 17% of the residues, respectively (Wilson & Oloffs, 1973a).
Generally, no detectable residues of chlordane were found in
wildlife such as birds (Canada, National Research Council, 1974;
Fitzhugh & Fairchild, 1976; Clark & Krynitsky, 1978). However,
in one study (Clark & Prouty, 1976), mean total residues of
oxychlordane ranging from 0.11 to 6.63 µg found in the carcasses
of bats from Maryland and Virginia were attributed to chlordane
use.
In extensive surveys, residues in fish have generally been low.
In 1976, residues in several species from Lake Erie and Lake Saint
Claire in Canada were found to range from non-detectable to 0.046
mg/kg fresh weight (Frank et al., 1978a,b). Residues in Canadian
commercially caught fish in 1970 were not detectable (Reinke et
al., 1972). The National Pesticide Monitoring Program from 1967-68
found chlordane residues in 128 out of 590 fish samples at levels
generally less than 0.5 mg/kg (Wilson & Oloffs, 1973b). From
1972-76, chlordane residues were found in only 3% of the samples of
estuarine fish in the USA (Butler & Schutzmann, 1978). Chlordane
residues were also not detectable in fish and fishery products from
the Northwestern Atlantic (Meith-Avcin et al., 1973; Sims et al.,
1977).
4.1.5. Food
There have been many studies in Canada, Great Britain, the USA,
and other countries on the occurrence of pesticide residues in
food. Generally, the results of these studies showed that residues
of chlordane seldom occur and uptake by man is negligible (Canada,
National Research Council, 1974). Residue tolerances for chlordane
have been established at the following levels: Belgium, Luxembourg,
and the Netherlands, 0.1 mg/kg, Canada, 0.3 mg/kg, European
Economic Community, 0.2 mg/kg and the USA, 0.3 mg/kg. These levels
are for a wide variety of foods (US EPA, 1976a,b). A temporary
acceptable daily intake (ADI) for human beings for the sum of the
alpha- and gamma-isomers of chlordane and oxychlordane of 0 - 0.001
mg/kg body weight was advised by the Joint Meeting on Pesticide
Residues (FAO/WHO, 1983). Chlordane is rarely present in market
basket surveys, and then only at low levels. It is not among the
top 10 chlorinated pesticides usually found as residues in food (US
EPA, 1976a,b). For example, in a survey in the USA from 1963 to
1969, chlordane residues were found in less than 1% of the samples
and ranged from 1 - 5 µg/kg.
It has already been shown that crops will translocate chlordane
residues from the soil. Generally, the amounts in crops are low.
Residues tend to accumulate in the crude oils of oil-seed crops at
levels higher than those in the original seed and in the oil-seed
meal. However, these levels are reduced by refining processes.
Chlordane residues were found in meat, milk, and eggs. Residues in
feed crops or from direct applications to cattle and poultry were
shown to result in significant residues in milk, meat, and eggs (US
EPA, 1976a,b). In a study on eggs in Canada, gamma-chlordane was
found in 78% of the samples with a mean value of 2 µg/kg fresh
weight and alpha-chlordane in 81% of the eggs with a mean value of
1 µg/kg (Mes et al., 1974). In another study (Herrick et al.,
1969), no residues were found in the eggs of chickens fed chlordane
in their diet at 0.08 mg/kg for a week.
In a study on samples of cow's milk analysed in the USA, 87%
were positive for chlordane with levels ranging from 0.02 to 0.06
mg/litre (IARC, 1979). In another study (US EPA, 1976a,b), the
milk of cows grazing on pastures with chlordane applied at 0.55
kg/ha contained an average chlordane concentration of 0.03
mg/litre. No residues were found at lower treatment levels.
Chlordane was also found in Canadian meat samples at levels ranging
from 0 to 106 µg/kg in beef, 0 to 32 µg/kg in pork, and 0 to 70
µg/kg in fowl (Saschenbrecker, 1976).
4.1.6. Human milk
Several studies on pesticide residues in human breast milk did
not reveal any residues of chlordane, but oxychlordane, trans-
nonachlor, and heptachlor epoxide were found, which may be related
to chlordane exposure. In a study on 54 women in Arkansas and
Mississippi from 1973-74, breast milk contained oxychlordane at
0.005 mg/litre, heptachlor epoxide at 0.004 mg/litre, and trans-
nonachlor at 0.001 mg/litre (Strassman & Kutz, 1977). In another
study on 34 samples of breast milk in Northern Mississippi from
1973-75, oxychlordane levels were found of 0.005 mg/litre in high-
pesticide-usage areas and 0.002 mg/litre in low-usage areas
(Barnett et al., 1979). In a survey involving 1436 lactating
mothers in the USA, the mean levels of oxychlordane in the milk
ranged from 75.4 - 116 µg/litre on an adjusted fat basis (Savage,
1976). In a study of Canadian human milk samples in 1974,
oxychlordane was found in 77% of the samples, trans-nonachlor in
68%, and heptachlor epoxide in 69%, each at a mean level of 1
mg/litre whole milk (Mes & Davies, 1978).
Jensen (1983) recently reviewed the levels of chlordane
and oxychlordane in human milk, and his data, including the
above-mentioned studies as well as more recent data, are
reproduced in Table 1.
4.2. General Population Exposure
Oxychlordane was found together with other organochlorine
pesticides in human fat samples at levels ranging from 0.03 to
0.4 mg/kg wet weight (mean 0.14 mg/kg) in residents of the USA
(Biros & Enos, 1973). Sovocool & Lewis (1975) also reported
the identification of oxychlordane in human fat. As indicated
by Biros & Enos (1973), the occurrence of oxychlordane
residues in human adipose tissue in the general population may
reflect previous exposure to chlordane and/or oxychlordane.
This organochlorine compound is included in the human tissue
residue monitoring program (Kutz et al., 1976).
4.3. Occupational Exposure
Permissible levels of exposure to chlordane in the workplace
air have been adopted in different countries (ILO, 1980). Examples
include: 0.5 mg/m3 as a time-weighted average concentration in
Belgium, Finland, Japan, the Netherlands, and the USA (both OSHA
and ACGIH), 0.3 mg/m3 as a time-weighted average and 0.6 mg/m3
as a ceiling concentration in Romania, and 0.01 mg/m3 as maximum
allowable concentration in the USSR.
People primarily exposed are those employed in the application
of chlordane for the control of insects and pests (IARC, 1979).
Chlordane has been found in household dust in the homes of farmers
(mean level 5.79 mg/kg air-dried dust) and pesticide formulators
(mean level 23.11 mg/kg) (Starr et al., 1974).
Table 1. Chlordane and oxychlordane in human milka
------------------------------------------------------------------------------------------------------
Oxychlordane and
chlordane content inb
---------------------
No. of samples Fat Whole milk Milk fat
Area, year (% positive) % (mean) (mg/litre) (mg/kg) References
------------------------------------------------------------------------------------------------------
Canada (1975) 100 2.2 1 - Mes & Davies (1978)
(< 2)
Japan
Tokyo (1978) 11 - 0.5 - Miyazaki et al. (1980)
(0.1 - 1.0)
Tokyo (1979) 12 - 0.5 - Miyazaki et al. (1980)
(0.3 - 1.1)
Mexico (1976) 620 - - 0.40 (median) FAO/WHO (1981)
Spain (1979) 45 (17.8%) - 0.3c 0.026c Lora et al. (1979)
(0 - 0.72)
USA
Arkansas/Mississippi 57 (46%) 3.0 12/10 - Strassman & Kutz (1977);
(1973-4) (0 - 20) FAO/WHO (1981)
------------------------------------------------------------------------------------------------------
Table 1. (contd.)
------------------------------------------------------------------------------------------------------
Oxychlordane and
chlordane content inb
---------------------
No. of samples Fat Whole milk Milk fat
Area, year (% positive) % (mean) (mg/litre) (mg/kg) References
------------------------------------------------------------------------------------------------------
Hawaii (1979-80) 50 (100%) 3.2 - 0.059 Takahashi et al. (1981)
(0.01 - 0.16)
Mississippi (1973-5) 34 (100%) - 5 0.13 Barnett et al. (1979)
(pesticide area) (1 - 22) (0.03 - 0.70)
Mississippi (1973-5) 6 (68%) - 2 0.05 Barnett et al. (1979)
(non-pesticide area) (0 - 4) (0 - 0.12)
USA-NE (1975) 233 - - 0.08 ± 0.05 Savage (1976);
Savage et al. (1981)
USA-SE (1975) 288 - - 0.12 ± 0.15 Savage (1976);
Savage et al. (1981)
USA-MW (1975) 378 - - 0.08 ± 0.05 Savage (1976);
Savage et al. (1981)
USA-SW (1975) 388 - - 0.11 ± 0.35 Savage (1976);
Savage et al. (1981)
USA-NW (1975) 149 - - 0.08 ± 0.05 Savage (1976);
Savage et al. (1981)
USA (total) (1975) 1 436 (74%) - 2 (median) 0.096 ± 0.195 Savage (1976);
(0.013 - 0.57) Savage et al. (1981)
FAO/WHO (1981)
------------------------------------------------------------------------------------------------------
a From: Jensen (1983).
b Results are expressed as means ± S.D. Ranges are listed in parentheses below.
c Chlordane.
5. KINETICS AND METABOLISM
5.1. Absorption
In studies on 4 male rabbits, a combination of 14C-alpha and
gamma-chlordane (approximately 1700 mg of each, administered orally
in 4 doses at 4-day intervals), was well absorbed (Balba & Saha,
1978). Brief exposure of dogs to topically applied chlordane
solutions (3.2 g/litre) resulted in a significant and long-lasting
decrease in the biological half-life of orally-administered
warfarin (Bachmann & Burkman, 1974).
5.2. Distribution and Storage
Studies using radio-labelled chlordane showed that after oral
administration, the radioactivity was well distributed in tissues
of rats (Barnett & Dorough, 1974) and rabbits (Balba & Saha, 1978).
Rats, whether being treated with single oral doses of chlordane or
fed diets containing this compound, retained the highest levels of
residues in adipose tissue, followed by the liver, kidney, brain,
and muscle. More of the gamma-isomers was retained than of the
alpha-isomer. Residues in the fat of rats fed radiolabelled
chlordane (3:1 alpha- and gamma-chlordane) at 1, 5, and 25 mg/kg
diet for 56 days were approximately 3 times higher than those in
the diets. Oxychlordane was the most persistent residue in the
tissues of these rats after chlordane was removed from the diet
(Barnett & Dorough, 1974). The tissue distribution of chlordane in
rabbits was found to be similar to that in rats (Poonawalla &
Korte, 1971; Balba & Saha, 1978).
5.3. Biotransformation
Poonawalla & Korte (1971) showed that 70% of gamma-chlordane
fed to rabbits was excreted in the urine in the form of
metabolities, a.o., gamma-1-hydroxy-2-chlorodihydrochlordene and
1,2-dihydroxydichlordene.
Oxychlordane has been isolated from the fat of dogs, rats
(Polen et al., 1971), pigs (Schwemmer et al., 1970), and cattle
(Lawrence et al., 1970). It has also been isolated from human fat
(Biros & Enos, 1973). Barnett & Dorough (1974) indicated that the
faecal extracts of rats fed 14C-chlordane showed the presence of
eight radioactive areas on the TLC plate. Although the structures
of the metabolites were not fully elucidated, they were tentatively
identified as mono-, di-, and tri-hydroxylated products of
chlordane.
The major route of metabolism for both alpha- and gamma-
chlordane was via dichlorochlordene and oxychlordane (Tashiro &
Matsumura, 1977). The results of these studies were in general
agreement with the proposal of Street & Blau (1972) and the results
of in vitro metabolism studies of Brimfield et al. (1978).
Tashiro & Matsumura (1977) were able to isolate 1-exo-hydroxy-2-
endo-chloro-2,3-exoepoxychlordene, and found another major
metabolic route for alpha-chlordane that involved a more direct
hydroxylation reaction to form 1-exo-hydroxy-dihydrochlordenes and
1,2-gamma-dihydroxydihydrochlordene. Both Brimfield et al. (1978)
and Tashiro & Matsumura (1977) indicated that oxychlordane and, to
a lesser extent, heptachlor were metabolites of chlordane.
However, they did not agree as to whether these two metabolites
would be terminal residues or intermediates in the metabolic
pathways of chlordane. Recent investigations have indicated that
other metabolites were present in the urine of rabbits fed
chlordane. Thus, alpha-chlordane gave rise to 1-hydroxy-2-
chlorochlordene, 1-hydroxychlordene, and gamma-chlordene
chlorhydrin. Administration of the gamma-isomer resulted in
excretion in the urine of the rabbits of 1,2-dichlorochlordene,
1-hydroxy-2-chlorochlordene, gamma-chlordene chlorhydrin, and
3-hydroxychlordane (Balba & Saha, 1978).
In vitro metabolism studies have been summarized by Brimfield
& Street (1979). By incubation of alpha- and gamma-chlordane with
rat liver postmitochondrial supernatant, dichlorchlordene and
oxychlordane were isolated, a result that was similar to those from
in vivo studies. Hart et al. (1963) and Hart & Fouts (1965)
reported that chlordane induced non-specific microsomal enzyme
activity in the rat, resembling, from this point of view,
phenobarbital.
5.4. Elimination and Excretion
Elimination of radiolabelled chlordane (3:1 alpha- and gamma-
chlordane) and the individual isomers was studied in rats. Single
oral doses of 0.05, 0.2, and 1 mg/kg body weight in corn oil were
almost completely eliminated after 7 days; 24 h after administra-
tion, 70% of alpha-chlordane and 60% of the gamma-isomer were
excreted. Female rats excreted more of the dose in the urine than
the males (Barnett & Dorough, 1974).
6. STUDIES ON EXPERIMENTAL ANIMALS
The toxicity and the residue data on chlordane including some
unpublished studies have been reviewed several times by
international bodies such as FAO/WHO (1968, 1973, 1978, 1981,
1983), CEC (1981), and IARC (1979). For their conclusions, refer
to section 9.
6.1. Short-term Exposures
6.1.1. Oral exposure
The acute toxicity of chlordane in several animal species is
shown in Table 2.
The signs associated with acute chlordane poisoning include
ataxia, convulsions, respiratory failure, and cyanosis followed by
death (US EPA, 1976a,b). Correlation between respiratory
difficulty and EEG patterns suggest that respiratory failure is a
contributing factor in chlordane-induced mortality (Hyde &
Falkenberg, 1976). Pathological manifestations include haemorrhage
in the gastrointestinal tract, kidneys, lung, and heart as well as
pulmonary congestion and oedema, and degenerative changes in the
central nervous system (US EPA, 1976a,b).
Seven dogs were given chlordane in single oral doses ranging
from 200 - 700 mg/kg body weight. Convulsions were seen in one dog
at 200 mg/kg (lowest dose) but 700 mg/kg (highest dose) did not
induce any effects (Batte & Turk, 1948). Four groups of 2 - 4 dogs
were given chlordane orally in doses of 5 - 200 mg/kg body weight.
All of the dogs died within 25 days to 93 weeks (Lehman, 1952b).
Chlordane administered by stomach-tube to sheep at 500 mg/kg
body weight induced toxic symptoms (incoordination, partial
blindness). Full recovery occurred in 5 - 6 days. A dose of 1000
mg/kg body weight induced severe respiratory and nervous symptoms
16 h after treatment and death after 48 h (Welch, 1948).
When a diet containing 1000 mg chlordane/kg was fed to 12 male
rats, all of them died within 10 days (Stohlman et al., 1950). At
500 mg/kg all 12 died within 70 days and, at 300 mg/kg, 9 animals
out of 12 were alive after 100 days. Daily oral doses of 6.25 - 25
mg/kg body weight administered to 5 rats for 15 days did not induce
tremors or convulsions, but daily doses of 50 mg/kg body weight
induced toxic symptoms, and 2 of the animals died (Ambrose et al.,
1953). Cytoplasmic bodies in the liver cells were observed in all
groups and were dose-related.
Table 2. Acute toxicity of chlordane in experimental animals
---------------------------------------------------------------------------
Species Sex Route Vehicle LD50 Reference
(mg/kg)
---------------------------------------------------------------------------
Rat F dermal xylene 530 Gaines (1969)
Rat M dermal xylene 205 Gaines (1969a)
Rabbit NS dermal "early" < 780 Ingle (1965b)
chlordane
Rabbit NS dermal "later" 1100 - 1200 Ingle (1965b)
chlordane
(more purified)
Rat M oral peanut oil 335 Gaines (1969)
Rat F oral peanut oil 430 Gaines (1969)
Rat NS oral variety 200 - 590a Ambrose et al. (1953);
Ingle (1965a)
Rat NS oral NS 283 Buck et al. (1973)
Rat NS oral NS 350 Truhaut et al. (1974)
Rabbit NS oral NS 100 - 300a Stohlman et al. (1950)
Rabbit NS oral NS 20 - 40a Ingle (unpublished
data, 1955)
Hamster NS oral NS 1720 Truhaut et al. (1974)
Goat NS oral NS 180 Welch (1948)
Sheep NS oral NS 500 - 1000 Welch (1948)
Chicken NS oral NS 220 - 230 FAO/WHO (1968)
Mallard NS oral NS 1200 Buck et al. (1973)
Cow NS oral NS 25 - 90 Buck et al. (1973)
---------------------------------------------------------------------------
a The wide range is explained by the use of different solvents and the
fact that chlordane produced before 1950 contained a considerable
amount of hexachlorocyclopentadiene.
NS - Not specified.
6.1.2. Dermal exposure
The single-dose dermal LD50 of "early" chlordane in rabbits
was reported to be less than 780 mg/kg body weight (Ingle, 1965b),
and it was noted that this concentration caused severe skin
irritation, tremors, and convulsions (Lehman, 1952a). The dermal
LD50 of the later, more purified chlordane was 1100 - 1200 mg/kg
(Ingle, 1965b).
6.1.3. Parenteral exposure
Male gerbils were dosed intramuscularly with chlordane at 2.5
mg/kg body weight every 3 days for 45 days. Treatment induced
hyperproteinemia, hyperglycemia, and enhanced serum alkaline and
acid phosphatase activities (Karel & Saxena, 1976).
6.2. Long-term Exposures
6.2.1. Oral exposure
Groups of 4 - 7 male and 4 - 7 female dogs were fed dietary
levels of 0, 0.3, 3, 15, or 30 mg chlordane/kg for 2 years.
Abnormalities in the results of clinical liver function tests were
seen in the 15 and 30 mg/kg groups. In animals selected for
necropsy at the end of the first year, increased relative liver
weights and associated hepatocellular changes were found at 30
mg/kg; at the end of two years, dose-related increases in relative
liver weights were found at 15 and 30 mg/kg, with non-dose-related
hepatocellular changes. There was no difference between the
severity of the liver lesions of the 30 mg/kg animals and those of
four animals withdrawn from 30 mg/kg treatment for eight months
prior to sacrifice. Liver biopsies on two animals of the 30 mg/kg
group at 1, 3, and 6 months showed hepatocellular changes at 6
months but not at 1 or 3 months. No adverse effects were seen on
behaviour, appearance, survival, weight gain, blood picture, or the
results of periodic physical examinations, at any level (Wazeter,
1967).
Twenty-four rats, 12 of each sex, were fed dietary levels of
2.5, 25, or 75 mg chlordane/kg for 2 years (Lehman, 1952b). The
two higher levels caused moderate to severe signs of toxicity. The
lowest level caused histological liver changes. Rats were fed
technical chlordane (early production) for two years at levels in
the diet of 0, 5, 10, 30, 150 or 300 mg/kg (Ingle, 1952; 1965a).
Convulsions and tremors were observed in animals receiving 150
mg/kg or more. Hepatocellular alterations consisting of
hypertrophy, cytoplasmic oxyphilia and hyalinization, karyorrhexis,
karyolysis, and cell necrosis were obvious at 150 and 300 mg/kg,
slight at 30 mg/kg, minimal at 10 mg/kg and absent at 5 mg/kg.
Growth was retarded and liver weight and mortality rate increased
at 150 and 300 mg/kg. In a subsequent study on rats (Ingle,
1965a), technical chlordane of later production containing fewer
by-products was administered at levels of 2.5 - 300 mg/kg diet for
2 years. Changes in food consumption, growth, and mortality rate
were seen only at the highest dose. Cellular alterations were seen
at 50 mg/kg and higher.
Rats were fed chlordane at levels ranging from 10 to 1280 mg/kg
diet for 407 days (Ambrose et al., 1953). The animals in the two
highest dose groups died early; liver weight was increased at 320
mg/kg; fatty infiltration and cytoplasmic margination were seen in
the liver parenchymatous cells in males at 40 mg/kg and above, and
in females at 80 mg/kg and above.
Groups, each comprising 20 male and 20 female rats, were fed
dietary levels of 0, 5, 15, 25, or 35 mg/kg of alpha-chlordane, 15,
25, 35, or 75 mg/kg of gamma-chlordane or 5, 15, 25, 35 or 50 mg/kg
of a 1:1 mixture of alpha- and gamma-chlordane (Ingle, 1969). In
the group fed alpha-chlordane, growth retardation became apparent
in the rats fed 35 mg/kg after 4 months in males and after 5 months
in females; with gamma-chlordane, the 75 mg/kg group of males only
displayed growth retardation after 8 months. With the mixture,
growth retardation was evident in both sexes fed 50 mg/kg,
beginning earlier in males than in females. Growth retardation was
not evident in any group fed lower doses of either isomer. Food
consumption bore a relationship to growth. Increased mortality
rates for both sexes became significant in the groups fed alpha-
chlordane at 35 mg/kg, gamma-chlordane at 75 mg/kg, or the alpha-
gamma mixture at 50 mg/kg. Haematocrit was normal for all test
groups. Autopsy did not reveal any gross pathological lesions.
There was no evidence of tumours. Histological examination did not
show any changes from feeding chlordane in any organ except the
liver. Compression of sinusoids due to slight hepatic cell
hypertrophy in the centrolobular region and minimal bile duct
proliferation were evident with administration of alpha-chlordane
at 35 mg/kg. The same changes were noted, but were minimal with
the same isomer at 25 mg/kg. Slight to moderate cytoplasmic
homogeneity of the hepatic cells in the centrolobular region,
minimal cytoplasmic margination, and minimal cell hypertrophy with
compressed sinusoids were noted with administration of gamma-
chlordane at 75 mg/kg. Slight cytoplasmic homogeneity of hepatic
cells in the centrolobular region and occasional cytoplasmic
margination were observed with the alpha-gamma mixture at 50 mg/kg.
The above alterations were minimal with the same mixture at 35
mg/kg. No liver changes were evident after feeding lower levels of
the chlordane isomers.
Groups of 6 female and 6 male rats were fed 2.5 mg or 25 mg of
a sample of technical chlordane containing 60 - 75% chlordane and
25 - 40% unrelated products per kg diet for up to 9 months (Ortega
et al., 1957). Centrolobular cell hypertrophy, cytoplasmic
margination, and cytoplasmic bodies were observed in the liver in 1
male fed 2.5 mg/kg and in 5 males fed 25 mg/kg. No changes were
seen in females.
In a two-year feeding study, pure-bred male and female beagle
dogs were fed chlordane at levels of 0, 0.3, 3.0, 15, or 30 mg/kg
diet. No adverse treatment-related alterations were observed in
behaviour, appearance, eye examination, body weight, food
consumption, haematology, or plasma biochemistry. Some liver
enzyme activities were altered throughout the study at the 15 and
30 mg/kg levels. Relative liver weights were slightly increased
after two years in the two highest groups. Treatment-related
microscopic changes, observed in dogs fed the two highest levels,
consisted of enlargement of centrolobular hepatocytes with
margination of coarse cytoplasmic granules (IRDC, 1967).
The Joint Meeting on Pesticide Residues (JMPR) reviewed the
toxicity data on chlordane at its 1977 meeting (FAO/WHO 1978) and
decided on the following "no-observed-adverse-effect levels":
- rat: 5 mg/kg in the diet, equivalent to 0.25 mg/kg
body weight; and
- dog: 3 mg/kg in the diet, equivalent to 0.075 mg/kg
body weight.
These "no-observed-adverse-effect levels" were confirmed by the
1982 JMPR (FAO/WHO, 1983).
6.2.2. Dermal exposure
When male guinea-pigs were exposed to chlordane at 67 mg/kg
body weight/day, through dermal painting for 90 days, mild
degenerative changes in the skin and testis were evident (Datta et
al., 1975).
The ninety-day repeated daily dose LD50 for rabbits was
reported in the paper by Lehman (1952a) (section 6.1.2) to be from
20 - 40 mg/kg body weight. Ingle (1965b) reviewed the dermal
toxicity of chlordane and attributed the toxicity of early
technical chlordane to the significant content of hexachloro-
cyclopentadiene (HCPD). A more pure product, which did not contain
significant quantities of HCPD, was only half as toxic to rabbits
as the earlier chlordane and did not cause any skin irritation or
damage to mucous membranes.
6.3. Reproduction Studies and Teratogenicity
Rats, maintained from weaning on a diet containing a chlordane
level of 320 mg/kg, showed reduced rates of mating, of viable
litters, and an increased rate of death of progeny prior to
weaning. It was concluded that, at this dosage, chlordane
interfered with both fertility and litter survival (Ambrose et al.,
1953). Groups of 10 male and 20 female rats were used in a 3-
generation study at dietary levels of technical chlordane of 0,
0.3, 3, 15, 30, and 60 mg/kg (Ingle, unpublished data, 1967). Two
litters in each filial generation were studied. Levels up to and
including 30 mg/kg did not have any effect on fertility, number of
offspring, or weight, growth, or mortality rate of the young
animals to weaning age. Autopsy of animals after weaning did not
reveal any gross or microscopic differences between the groups. At
60 mg/kg, there was a high (10.6%) mortality rate in the second F3
generation litters during the latter part of the nursing period;
these animals showed gross and microscopic pathological changes,
comparable with those characteristic for chlordane intoxication.
However, survivors of this generation did not show any tissue
changes at all. A third set of F3 litters at 60 mg/kg suffered 17%
mortality during the nursing period, with symptomatology and gross
and microscopic tissue changes characteristic of chlordane
intoxication. Third F3 generation litters from dams removed from
the 60 mg/kg group and placed on chlordane-free diets for 30 days
prior to remating showed no differences in any respect from control
litters. No evidence of teratogenicity was found in this study.
Hens and cocks fed up to 0.3 mg chlordane/kg diet did not show
any toxic symptoms or any adverse effects on egg weight,
hatchability, or growth of chicks (Biotox, unpublished data, 1969).
Mice fed diets containing chlordane at 25 - 100 mg/kg for 6
generations showed decreased viability in the first and second
generations at 100 mg/kg; in the third generation at this level, no
offspring were produced (Keplinger et al., 1968). At 50 mg/kg,
viability was reduced in the fourth and fifth generations, and at
25 mg/kg no statistically significant effects were observed, even
after 6 generations.
Chlordane was administered to rabbits orally at levels of 1.0,
5.0, and 15 mg/kg body weight per day on the 6th - 18th days of
gestation. A control group and a positive control group were used.
No changes were seen in behaviour, appearance, or body weight.
Miscarriages were seen in 3 rabbits at the 1.0 mg/kg level and one
rabbit at 15 mg/kg dose level. No effects on any of the maternal
or fetal parameters were noted. No teratogenic effects were noted
(IRDC, 1972).
6.4. Mutagenicity
Alpha-Chlordane, gamma-chlordane, and chlordene were tested in
the Ames Salmonella microsome assay and were not mutagenic (Simmon
et al., 1977). Chlordane was not mutagenic when tested using 5
different strains of Salmonella typhimurium in the Ames assay
(Ercegovich & Rashid, 1977). Chlordane was shown to enhance the
number of ouabain-resistant mutants in Chinese hamster V79 cells
and was considered weakly mutagenic (Ahmed et al., 1977b).
Chlordane induced unscheduled DNA synthesis in SV-40 human
cells in culture without activation (Ahmed et al., 1977a). It was
established that chlordane-treated cells did not (for the most
part) re-enter mitosis. They were, instead, arrested somewhere
between the G1 and G2 phases of the cell cycle. Studies involving
DNA synthesis were undertaken to determine more precisely at which
phase (G1, S, G2) the cells are blocked. The data showed that the
treated cells were as competant in DNA replication as the control
cells. In both control and treated cultures, 25 - 30% of total DNA
persisted as light-density material indicating that some of the
pre-existing DNA never engaged in DNA synthesis. Either a large
fraction of cells failed to complete DNA synthesis or 25 - 30% of
the cells did not enter phase S. In any case, treated and control
cells behaved the same in terms of DNA synthesis, indicating that
treatment of the cells with chlordane blocked the cells at the G2
stage of the cell cycle (Brubaker et al., 1970).
Chlordane induced gene conversions in Saccharomyces cerevisiae
strain D4 (Chambers & Dutta, 1976).
Neither alpha-chlordane (42, 58, and 290 mg/kg body weight
single ip doses or 5 daily oral doses of 75 mg/kg body weight) nor
the gamma-isomer (5 daily oral doses of 50 mg/kg body weight) had a
significant effect in a dominant lethal assay on mice (Epstein et
al., 1972). Technical chlordane at dose levels of 50 or 100 mg/kg
body weight in a dominant lethal study using mice failed to induce
any dominant lethal changes (Arnold et al., 1977).
More recent studies on animal and human cells in culture have
shown that chlordane is not mutagenic or is only weakly mutagenic
(Williams, 1979; Maslansky & Williams, 1981; Tong et al., 1981).
Further work by Telang et al. (1982) showed that chlordane was not
mutagenic to an adult rat liver cell line but inhibited cell to
cell communication in a rat liver 6-thioguanine resistant/sensitive
cell line. Telang et al. proposed that chlordane was exhibiting
properties exerted by many promoting agents.
6.5. Carcinogenicity
Epstein (1976) reported a previously unpublished study by the
International Research and Development Corporation, carried out in
1973, in which groups of 100 male and 100 female Charles River CD-1
mice, 6 weeks of age, were fed technical-grade chlordane (purity
not given) at 5, 25, and 50 mg/kg diet, for 18 months. Excluding
10 animals sacrificed from each group for interim study at 6
months, mortality rates at 18 months ranged from 27 - 49%, except
in males and females receiving the 50 mg/kg diet, in which the
mortality rates were 86 and 75%, respectively. A relatively large
number of the deceased animals was lost due to autolysis. A dose-
related increased incidence of liver hyperplastic nodules was
reported in the 25 and 50 mg/kg diet test groups and a dose-related
increased incidence of liver cell hypertrophy was found in all test
groups. A significant incidence of hepatocellular carcinomas
compared with controls was also reported. In the males receiving
chlordane at 0, 5, 25, or 50 mg/kg diet, hepatocellular carcinomas
were found in 3/33, 5/55, 41/52, and 32/39 animals, respectively;
in females, the respective incidences were 0/45, 0/61, 32/50, and
26/37.
Groups of 50 male and 50 female B6C3F1 hybrid mice, 5 weeks of
age, were fed analytical-grade chlordane, consisting of 94.8%
chlordane (71.7% alpha-chlordane and 23.1% gamma-chlordane), 0.3%
heptachlor, 0.6% nonachlor, 1.1% hexachlorocyclopentadiene, 0.25%
chlordene isomers, and other chlorinated compounds for 80 weeks
(NCI, 1977). Males received initial levels of 20 or 40 mg/kg diet,
and females 40 and 80 mg/kg diet; time-weighted average dietary
concentrations were 30 and 56 mg/kg for males and 30 and 64 mg/kg
diet for females. There were 20 male and 10 female matched
controls and 100 male and 80 female pooled controls. Survival in
all groups was relatively high, i.e., over 60% in treated males,
over 80% in treated females, and over 90% in male and female
controls. A dose-related increase in the incidence of
hepatocellular carcinomas was found in males and females. The
incidences were 43/49 and 34/49 in high-dose males and females,
respectively, and 16/48 and 3/47 in low-dose males and females,
respectively, compared with 2/18 and 0/19 in male and female
matched controls, respectively.
Groups of 50 male and 50 female, 5-week-old Osborne-Mendel rats
were given analytical-grade chlordane in the diet for 80 weeks, at
initial levels of 400 and 800 mg/kg for males and 200 and 400 mg/kg
for females (NCI, 1977). The levels had to be reduced during the
study because of adverse toxic effects; the time-weighted average
dietary concentrations were 407 and 203 mg/kg for males and 241 and
121 mg/kg for females. There were 10 male and 10 female matched
controls and 60 male and 60 female pooled controls. Survivors were
killed at 80 weeks, at which time approximately 50% of treated and
control males and 60% of treated females and 90% of control females
were still alive. In all treated animals combined, there was an
excess incidence of follicular-cell thyroid neoplasms (10/75 in
treated females and 7/65 in treated males versus 0/10, 3/58, 0/6,
and 4/51 in matched and pooled female and male controls); there was
an excess of malignant fibrous histiocytomas in the treated male
groups (8/88 versus 0/8 and 2/58 in matched and pooled male
controls).
A committee of the National Academy of Sciences (NAS) in the
USA was asked to review all available carcinogenicity data on
chlordane as part of the cancellation hearings. Chlordane was not
found to be carcinogenic in rats and the only target organ site for
carcinogenic response in certain strains of mice was the liver.
The committee concluded that "there are no adequate data to show
that these compounds are carcinogenic in humans, but because of
their carcinogenicity in certain mouse strains and the extensive
similarity of the carcinogenic action of chemicals in animals and
in humans, the committee concluded that chlordane, heptachlor
and/or their metabolites may be carcinogenic in humans. Although
the magnitude of risk is greater than if no carcinogenicity had
been found in certain mouse strains, in the opinion of the
committee the magnitude of risk cannot be reliably estimated
because of the uncertainties in the available data and in the
extrapolation of carcinogenicity data from laboratory animals to
humans" (US NAS, 1977).
IARC (1979), in its evaluation of the carcinogenic risk of
chlordane, concluded: "There is sufficient evidence that chlordane
is carcinogenic in mice." In 1982, another IARC Working Group
reviewed existing data on chlordane and concluded that there was
limited evidence for the carcinogenicity of chlordane for
experimental animals (IARC, 1982). The group of Williams (Telang
et al., 1982) suggested that chlordane had the properties of many
promoting agents.
6.6. Behavioural Studies
Offspring of chlordane-treated mice (1 or 2.5 mg/kg body weight
for 7 consecutive days) made fewer conditioned avoidance responses
than controls (Al-Hachim & Al-Baker, 1973). In addition, progeny
of mothers receiving the higher dose were more active than the
controls.
6.7. Other Studies
Chlordane induces hepatic mixed-function oxidase enzymes in
rats (Fouts, 1963; Hart et al., 1963; Hart & Fouts, 1965;
Villeneuve et al., 1972; den Tonkelaar et al., 1974; Madhukar &
Matsumura, 1979) and enhances estrone metabolism in rats and mice
(Welch et al., 1971). Chlordane has been shown to inhibit skin 7-
ethoxycoumarin de-ethylase activity (7-EC) (EC 1.14.13) in mice at
doses which induced hepatic 7-EC activity (Pohl & Fouts, 1977).
Several studies were carried out in which rats were fed chlordane
at levels of 2, 5, 10, 20, or 50 mg/kg diet during two weeks (den
Tonkelaar et al., 1974). At the end of this period, the liver
microsomal enzymes hexobarbital oxidase (EC 1.1), aminopyrine
demethylase (EC 1.5.3), and aniline hydroxylase (EC 1.14.14) were
determined. A no-observed-adverse-effect level of 5 mg/kg was
found for chlordane. Chlordane inhibition of rat brain ATPase
activity (Folmar, 1978) and bovine carbonic anhydrase (EC 4.2.1.1)
(Maguire & Watkin, 1975) has been demonstrated in in vitro
systems. The in vivo inhibition of rat brain ATPase has also
been reported (Drummond et al., 1980).
Three female and 3 male baboons were fed atherogenic diets to
which chlordane was added at 0.1 or 1 mg/kg body weight per day for
two years. At the higher dose level, chlordane increased
cytochrome P-450 activity significantly, otherwise no adverse
effects on general health or on any major organ systems were found
(McGill, 1979).
Acute intramuscular doses (50 mg/kg body weight) of chlordane
have been shown to increase the alkaline phosphatase (EC 3.1.3.1)
activity in gerbils temporarily (Karel, 1976) and stimulate
gluconeogenetic enzymes in the liver and kidney cortex of rats
(Kacew & Singhal, 1973a). Oral administration of 200 mg alpha-
chlordane/kg body weight to mature Wistar rats also induced
elevated serum levels of glucose and urea with a concomitant
decrease in liver glycogen at sacrifice, 1 h later (Kacew &
Singhal, 1973b). The chlordane-induced alterations have been
attributed to the enhanced ability of these organs to synthesize
cyclic AMP (Kacew & Singhal, 1973b, 1974; Singhal & Kacew, 1973,
1976; Kraybill, 1977).
Rats were administered chlordane ip daily for 42 days at levels
of 0.15, 1.75, or 25.0 mg/kg body weight. Results revealed "dose-
dependent alterations of brain potentials without behavioural signs
of chronic toxicity" (Hyde & Falkenberg, 1976, Hyde et al., 1978).
Chlordane has also been shown to influence brain biogenic amines
including acetylcholine (Hrdina et al., 1973).
Prenatal exposure to 0.16 mg chlordane/kg body weight, in
peanut butter, on each day of gestation, resulted in increased
plasma corticosterone levels in adult male mice (Cranmer et al.,
1978). When neonatal mice were treated with 0.075 mg alpha- or
gamma-chlordane on days 2 - 4 after birth, growth rates were
depressed and eye and vaginal opening were delayed (Talamantes &
Jang, 1977).
Female Balb C mice were mated and treated with chlordane at
0.16 or 8 mg/kg body weight throughout gestation. Decreased cell-
mediated immune competence was found in offspring of high-dose-
treated females, at 101 days of age, challenged with oxazolone
(Spyker-Cranmer et al., 1982).
Chlordane added to the medium at a concentration of
1 mg/kg inhibited the growth of Streptococcus viridans in
vitro by more than 50%. Total growth inhibition occurred at a
chlordane concentration of 3 mg/kg (Goes et al., 1978).
6.8. Factors Influencing Toxicity
Metabolites
The acute oral toxicity of chlordane isomers and their
metabolites is summarized in Table 3.
Table 3. Toxicity of chlordane isomers and metabolites
-----------------------------------------------------------------------
Compound Species LD50 (mg/kg Reference
(sex) body weight)
-----------------------------------------------------------------------
alpha-chlordane rat (M) 392 Wazeter et al. (1968)
gamma-chlordane rat (M) 327 Wazeter et al. (1968)
alpha + gamma- rat (M) 371 Wazeter et al. (1968)
chlordane (1:1 ratio)
oxychlordane rat (M,F) 19.1 Mastri et al. (1969a)
chlordene rat (M,F) over 4600 Mastri et al. (1969b)
3-chlorchlordene rat (M,F) over 4600 Mastri et al. (1969b)
1-hydroxychlordene rat (M,F) over 4600 Mastri et al. (1969b)
chlordene epoxide rat (M,F) over 4600 Mastri et al. (1969c)
1-hydroxy, 2,3-epoxy rat (F) over 4600 Mastri et al. (1969c)
chlordene
2-chlorchlordene rat (F) over 10 200 Mastri et al. (1969c)
-----------------------------------------------------------------------
Only oxychlordane was more toxic than the parent compounds.
Two male and 2 female rats were given the chlordane metabolite
oxychlordane in their diet, at a level of 2.0 mg/kg body weight
(Plank et al., 1970). Following 90 days feeding, the surviving
animals were sacrificed. Body-weight gain, food consumption,
behaviour, mortality rate, organ weights and organ to body weight
ratios, and the results of haematological and blood chemistry tests
and urological studies were considered to be within the normal
range for the strain of rat used. No gross pathological
abnormalities were evident, and no histopathological lesions could
be attributed to oxychlordane.
Groups of 25 male and 25 female rats were fed dietary levels of
1-hydroxychlordene of 0, 100, 250, 500, 1000, and 2000 mg/kg for up
to 224 days (Ingle, 1965a). After 110 days, 3 females from each
feeding level were mated with males at all levels. The mortality
rate in all groups was low, and no statistically significant
differences existed. No gross abnormalities were revealed at
autopsy after 224 days. The histopathological study of the
visceral organs did not show any pathological effects. Slight to
moderate hyperplasia of the smooth endoplasmatic reticulum and
cytoplasmic margination of a few liver cells were noted at the 1000
and 2000 mg/kg levels.
Interactions
Chlordane has been shown to exert a protective effect against
several organophosphorous and carbamate insecticides (Williams et
al., 1967; Street et al., 1969; Williams & Casterline, 1970; US
EPA, 1976a,b).
Protein deficiency has been shown to double the acute toxicity
of chlordane in rats (Boyd, 1972).
Chlordane has also been shown to increase the hepatotoxic
effects of carbon tetrachloride in the rat (Stenger et al., 1975;
Mahon, 1977; Mahon & Oloffs, 1979; Mahon et al., 1979).
7. EFFECTS ON MAN: EPIDEMIOLOGICAL AND CLINICAL STUDIES
7.1. Poisoning Incidents
A 15-month-old girl ingested a mouthful of chlordane suspension
and, after 3 h, displayed tremors and incoordination (Lensky &
Evans, 1952). Repeated seizures developed and she was treated with
ethyl chloride, amobarbital, and gastric lavage with magnesium
sulfate. The child recovered completely and ataxia and
excitability disappeared after 2 - 3 weeks. At 26 years of age,
she was in excellent health and appeared not to suffer any
consequences from the childhood episode (Taylor et al., 1979).
A 2-year-old child had drunk an unknown amount of a 74%
formulation of chlordane (Curley & Garrettson, 1969). Vomiting
preceeded convulsions, which were controlled by phenobarbital; the
EEG pattern was normal within 40 h and the child recovered.
A similar poisoning incident was observed with a 4-year-old
child (Aldrich & Holmes, 1969). Convulsions were treated with
phenobarbital. As with the previous case, the individual
recovered.
Two other cases of chlordane poisoning were reported in 1955
(Derbes et al., 1955). One was caused by the absorption of
accidentally-spilled chlordane and the other was a suicide attempt
where the individual (female) swallowed 6 g of chlordane (104 mg/kg
body weight) and died 9 1/2 days after the incident (Derbes et al.,
1955).
When a section of a municipal water system in Chattanooga,
Tennessee, USA was contaminated with chlordane in concentrations of
up to 1.2 g/litre in 1976, 13 persons showed gastrointestinal
and/or neurological symptoms (Harrington et al., 1978).
7.2. Occupational and Epidemiological Studies
No deleterious effects associated with occupational exposure to
chlordane have been reported. Twenty-two men, who had been
occupationally exposed to chlordane during its manufacture for
periods of 1 - 3 years, did not show any evidence of intoxication
(Princi & Spurbeck, 1951). Other clinical studies have been
reported on men engaged in the manufacture of chlordane (Alvarez &
Hyman, 1953; Fishbein et al., 1964; Morgan & Roan, 1969).
Infante et al. (1978) reviewed 25 previously reported cases of
blood dyscrasia together with a small number of newly identified
cases of aplastic anaemia, leukaemia, or neuroblastoma in children
in relation to their possible association with pre- and post-natal
chlordane or heptachlor exposure and reported an anecdotal
relationship. However, in a case-control study, no association was
found between blood dyscrasias and occupational exposure to a
number of pesticides including chlordane (Wang & Gruffenman, 1981).
Wang & MacMahon (1979 a,b) studied a cohort of workers engaged
in the manufacture of chlordane, heptachlor, and endrin and another
cohort of 16 000 pesticide-spraying personnel, including termite-
control workers. Both studies showed a deficit of deaths from all
cancers and slight excesses of lung, skin, or bladder cancer that
were not statistically significant.
In 1982, an IARC Working Group concluded that the above studies
were inadequate to evaluate the carcinogenicity of chlordane for
human beings (IARC, 1982).
Shindell & Associates (1981) studied the mortality experience
of 783 workers engaged in the manufacture of chlordane and
heptachlor. Workers had been employed for a minimum of 3 months 5,
10, 15, or 20 years ago. SMRs for cancer were not increased among
124 deaths.
In a retrospective cohort study of workers involved in the
production of chlorinated hydrocarbon pesticides, Ditraglia et al.
(1981) studied the workers in a chlordane-manufacturing plant; the
same workers were also studied by Wang & MacMahon (1979a). SMRs
for all cancer deaths were lower than expected; a slight excess of
stomach cancer (3 vs 0.99 expected), which was observed, was not
statistically significant. The number of workers studied was small
and further follow-up of the cohort was recommended by the authors.
MacMahon & Wang (1982) carried out a second follow-up study of
mortality rates in a cohort of workers employed in spraying
pesticides, including termite-control workers. Among 540 deaths
for which the cause was ascertainable, small excesses of bladder
cancer in termite-control operators and of skin and lung cancer in
other operators were observed, but these were not statistically
significant.
7.3. Treatment of Poisoning
In case of overexposure, medical advice should be sought
immediately.
Treatment before person is seen by a physician
The person should stop work immediately, remove contaminated
clothing and wash the affected skin with soap and water, if
available, flushing the area with large quantities of water. If
swallowed, vomiting should be induced, if the person is conscious
(FAO/WHO, 1978).
Medical Treatment
If the pesticide has been ingested, gastric lavage should be
Performed with 2 - 4 litres of water, followed by saline
purgatives. Barbiturates (preferably phenobarbitone or
pentobarbitone) or diazepam should be given intramuscularly or
intravenously in sufficient dosage to control restlessness or
convulsions. Mechanical respiratory assistance with oxygen may be
required. Calcium gluconate, 10% in 10 ml, injected
intramuscularly at 4-h intervals, may be helpful. Contraindicated
are oily purgatives, epinephrine, and other adrenergic drugs and
central stimulants of all types (FAO/WHO, 1978).
8. EFFECTS ON THE ENVIRONMENT
8.1. Toxicity for Aquatic Organisms
Data on the toxicity of chlordane for aquatic organisms are
given in Table 4. A more comprehensive table, listing different
conditions and exposure times is available on request from IRPTC,
Geneva, Switzerland. It is of importance for the interpretation of
these data to note that a change in the purity of technical of
chlordane occurred in the early 1950s.
Studies of the effects of chlordane on fish began with the
application of the original material to rainbow trout by Cope et
al. (1947). They determined minimum disabling 24-h doses of
chlordane in a xylene emulsion, an acetone solution, a fuel oil
solution, and a Velsicol AR-50 solution, and found this to be
higher than 6 mg chlordane/litre. An application of 1.12 kg/ha of
a field formulation of chlordane to a small pond killed 87% of
bluegills present (Surber, 1948), application of 0.56 kg/ha killed
some bluegills whilst at 0.28 kg/ha all fish survived (Linduska &
Surber, 1948). In a study by Lawrence (1950) on bluegill
fingerlings, large-mouth black bass fingerlings, and juvenile
goldfish in aquaria, no deaths occurred at 100 µg chlordane/litre
(original formulation), whilst at 200 µg/litre, a 30-h exposure
killed bass, and an 87-h exposure killed bluegills; goldfish were
not affected. In earthen ponds, large-mouth black bass fingerlings
were killed by a concentration of 200 µg/litre, but bluegills and
fingerlings and juvenile goldfish survived.
Studies using the current formulation of chlordane are
summarised in Table 4. A definite temperature effect demonstrated
by Macek et al. (1969) during acute exposures, i.e., fish showed a
greater susceptibility at higher temperatures, was not present in
96-h exposure studies. Temperature effects were also noted in
toxicity tests on tubificid worms, Branchuria sowerbyi (Naqvi,
1973). In static tests, 500 µg chlordane/litre caused 100%
mortality at 4.4° and 32 °C, but no mortality at 21 °C. Nutrition
has been shown to affect chlordane toxicity in rainbow trout, with
96-h LC50s ranging from 8.2 to 47 µg/litre, depending on the
composition of the diet given to the fish (Merhle et al., 1974).
Recent in vitro studies on bluegills (Koch et al., 1971)
and on rainbow trout (Davis et al., 1972) have shown that chlordane
acts as an inhibitor of ATPase systems.
8.2. Toxicity for Terrestrial Organisms
Studies of the effects of chlordane on soil microfauna have
been limited to work on nematodes. Populations of plant-feeding
nematodes were reduced by an insecticide mixture containing
chlordane (as well as DDT, diazinon, and zinophos) in July,
following application in March, but at no other time during the
study period (Corbett & Webb, 1968). Nematodes are generally
unaffected by most soil insecticides (Edwards, 1965a).
Table 4. Toxicity of chlordane for aquatic organisms
---------------------------------------------------------------------------------------------------------
Organism Age/ Temp pH Flow/ Grade Hard- alk sal End Para- Concen- Reference
size (° C) stat ness (mg/ o/oo point meter tration
(mg/ litre) (µg/
litre) litre)
---------------------------------------------------------------------------------------------------------
Eastern oyster 29-53 31.6 flow tech 27.3 % reduc- 96-h 6.2 Parrish et
(Crassostrea mm tion shell EC50 al. (1976)
virginica) deposition
Annelid stat sea 288-h 220 McLeese et
(Nereis virens) water LC50 al. (1982)
Scud 5 mm 16.7 7.9 flow tech 148 152 immobili- 168-h 97.1 Cardwell et
(Hyalella juv. sation EC50 al. (1977)
azteca)
Cladoceran 1st 15.5 7.4- stat immobili- 48-h 29 Sanders &
( Daphnia pulex) instar 7.8 sation EC50 Cope (1966)
Pink shrimp 50-65 28.4 flow tech 21.8 96-h 0.4 Parrish et
(Penaeus mm LC50 al. (1976)
duorarum)
Dungeness crab adult 13 stat tech 25 96-h 220 Caldwell
(Cancer LC50 (1977)
magister) zoeal 13 stat tech 25 immobili- 96-h 1.3
sation EC50
Backswimmer 5-6 mm 18-24 stat 25% EM 168-h 0.79 Konar (1968)
(Notonecta) sp LC50
Water scorpion 24-28 18-24 stat 25% EM 168-h 182 Konar (1968)
(Nepa) sp mm LC50
Bluegill 38-84 25 7.1 flow 100% 20 18 96-h 22 Henderson et
(Lepomis mm LC50 al. (1959)
macrochirus)
---------------------------------------------------------------------------------------------------------
Table 4. (contd.)
---------------------------------------------------------------------------------------------------------
Organism Age/ Temp pH Flow/ Grade Hard- alk sal End Para- Concen- Reference
size (° C) stat ness (mg/ o/oo point meter tration
(mg/ litre) (µg/
litre) litre)
---------------------------------------------------------------------------------------------------------
Fathead minnow 1 day 21/25 7.7 flow tech 156 166 major 11-mo Cardwell et.
(Pimephales chronic lowest al. (1977)
promelas) effects dose
life
cycle
tests
38-84 25 7.1 flow 100% 20 18 96-h 52 Henderson et
mm LC50 al. (1959)
38-84 25 7.1 flow 75% 20 18 96-h 170 Henderson et
mm EM LC50 al. (1959)
Rainbow trout 0.9 g 13 tech 96-h 7.8 Cope (1965)
(Salmo gairdneri) LC50
Murrel 24-26 18-24 stat 25% EM 168-h 0.51 Konar (1968)
(Channa mm LC50
punctatus)
48-50 18-24 stat 25% EM 168-h 3 Konar (1968)
mm LC50
100- 18-24 stat 25% EM 168-h 25.5 Konar (1968)
105 mm LC50
Tropical fish juv. 18-24 stat 25% EM 168-h 0.7- Konar (1968)
LC50 3.7
Channel catfish finger 25 stat tech 96-h 500 Clemens &
(Ictalurus ling LC50 Sneed (1959)
punctatus)
Common toad tad- 18-20 stat 48-h 2000 Ludemann &
(Bufo bufo) pole LC50 Neumann
(1962)
---------------------------------------------------------------------------------------------------------
Soil fauna populations (mainly arthropods with a small
percentage of earthworms and nematodes) were reduced to very low
levels by the normal application rate (112 kg/ha per year) of a
commercial formulation of chlordane (Gould & Hampstead, 1951).
However, Long et al. (1967) did not find any significant reductions
in most soil arthropod populations following application of
technical chlordane to sugar cane at 2.24 kg/ha, although numbers
of Diplura and Pauropoda were reduced. In a summary of the effects
of pesticides on soil invertebrates, Edwards (1965b) stated that
chlordane caused a large reduction in the numbers of Coleoptera,
Diptera, hemiedaphic Collembola, and non-predatory mites, but
little reduction in the numbers of edaphic Collembola or predatory
mites at application rates of 1.12 - 2.24 kg/ha. He also stated
that chlordane was lethal for fly and beetle larvae. Fox (1958)
produced a few data indicating that carabid and staphylinid beetle
populations returned to normal 3 years after a field application
of chlordane (as a 40% wettable powder) at 8.96 or 11.2 kg/ha.
Chlordane is toxic both to earthworms and to enchytraeid worms
at application rates well within the range of normal usage (Hopkins
& Kirk, 1957; Doane, 1962). The toxicity of chlordane for earth-
worms is given in Table 5. Legg (1968) made a single application
of 25% EC chlordane at 9.0, 13.4, or 20.2 kg/ha on closely-mown
turf and counted worm casts for up to 13 months. After 19 days,
there were reductions of 52, 72, and 98%, respectively, for the 3
doses compared with control plots; after 13 months, the reductions
were 89, 95, and 97%, respectively. Long et al. (1967) reported a
significant reduction in earthworm populations, 6 - 11 months after
an application of chlordane at 2.2 kg/ha. A reduction in worm
casts to zero, 1 year after an application of chlordane at 11.2
kg/ha, either as granules or in spray formulation was shown by
Doane (1962). Lidgate (1966) applied chlordane, either as 20%
granules or 75% EC diluted with water, at rates between 13.4 and
35.2 kg/ha, to a putting green and measured worm activity by the
number of worm casts. The first count of casts, 18 days after
treatment, showed that worm activity was significantly depressed by
granular applications of 17.6 and 35.2 kg/ha but not by spray
formulations of 13.4 or 26.4 kg/ha. Activity became depressed on
sprayed plots about 8 weeks after treatment. There were still
significantly fewer worm casts on treated plots, 5 years later.
Table 5. Toxicity of chlordane for earthworms
-----------------------------------------------------------------------------------------
Organism Grade Application Concen- Effect Reference
method tration
(kg/ha)
-----------------------------------------------------------------------------------------
Earthworm 25% EC spray 9 52% reduction in worm Legg (1968)
(Lumbricus casts at 19 days
terrestris)
25% EC spray 13.4 72% reduction in worm Legg (1968)
casts at 19 days
25% EC spray 20.2 98% reduction in worm Legg (1968)
casts at 19 days
25% EC spray 9 89% reduction in worm Legg (1968)
casts at 1 year
25% EC spray 13.4 95% reduction in worm Legg (1968)
casts at 1 year
25% EC spray 20.2 97% reduction in worm Legg (1968)
casts at 1 year
Red earth- 5% dust soil 35 0% mortality in Hopkins & Kirk (1957)
worm incorpor- 4 days
(Eisenia ation
sp)
5% dust soil 70 46% mortality in Hopkins & Kirk (1957)
incorpor- 4 days
ation
5% dust soil 141 40% mortality in Hopkins & Kirk (1957)
incorpor- 4 days
ation
5% dust soil 282 79% mortality in Hopkins & Kirk (1957)
incorpor- 4 days
ation
5% dust soil 100 96-h LC50 Hopkins & Kirk (1957)
incorpor-
ation
-----------------------------------------------------------------------------------------
The toxicity of chlordane for birds, when given in the diet, is
summarised in Table 6. LC50 values as mg/kg diet ranged from 170
to 858 in studies where chlordane was given for between 5 days and
100 weeks. When chlordane was applied to marshland at 1.12 kg/ha,
the fecundity of marsh birds was affected (Hanson, 1952); blue-
winged teal and shovelers did not produce any young, and coot and
redwinged-blackbirds produced 60% fewer young. It was postulated
that chlordane had caused disruption in food cycles in the marsh
and that this was the probable cause of reproductive failure in the
birds.
8.3. Toxicity for Microorganisms
Some effects of chlordane on microorganisms are summarised in
Table 7. Its effects may be due, at least in part, to inhibition
of enzyme activity (Nakas, 1977). Some work has been reported on
the effects of chlordane on soil microorganisms. Gram-positive
bacteria appear to be more sensitive to chlordane than gram-
negative bacteria, since the growth of gram-positives was inhibited
whilst that of gram-negatives was unaffected (Trudgill et al.,
1971). When Bacillus subtilis cultures were treated with technical
chlordane at 20 mg/litre, they ceased to grow. Viable count and
respiration rate fell to zero after about 3 h of exposure. The
actual concentration experienced by the bacteria is not known, but
it is likely to be less than the 20 mg/litre added because of the
poor solubility of chlordane in aqueous solution. Langlois & Sides
(1972) investigated the effects of constituents of technical
chlordane on the growth of Staphylococcus aureus. The viability
of the culture, the length of the lag-phase, and the generation
time were affected by the amount of chlordane and gamma-chlordane
applied.
8.4. Bioaccumulation and Biomagnification
Grimes & Morrison (1975) examined the uptake of chlordane by 13
types of bacteria and found that although the uptake of alpha- and
beta-isomers of chlordane was the same for any one species, the
concentration factors (CF) differed greatly between species. The
CFs ranged from a few hundred to several thousand, with 3 species
giving much higher values. The highest CF was 53 000 for
Caulobacter vibrioides. Caulobacter cells were found to contain 4
distinct lipid-containing materials, and this was offered as an
explanation of the high CF. Sanborn et al. (1976) used unlabelled
chlordane and labelled 14C-chlordane on filamentous Oedogonium
alga and obtained CFs of 49 500 and 98 386. The lower figure may
be due to uncertainties in determining chlordane in solution and in
the alga. Moore et al. (1977), using the planktonic alga
Ankistrodesmus amalloides, obtained a very much lower CF of 5560,
but even this species shows accumulation potential.
Table 6. Toxicity of chlordane for birds
--------------------------------------------------------------------------------
Species Age Route Parameter Concen- Reference
trationa
(mg/kg)
--------------------------------------------------------------------------------
Mallard 4 - 5 mo oral acute LD50 1200 Tucker & Crabtree (1970)
10 days diet 5-day LC50b 858 Hill et al. (1975)
Bobwhite 14 weeks diet 10-week LC0 10 Ludke (1976)
quail 17 days diet 5-day LC50b 331 Hill et al. (1975)
young diet 100-day LC50 100 DeWitt et al. (1963)
young diet 10-day LC50 250 DeWitt et al. (1963)
adult diet 100-day LC50 250 DeWitt et al. (1963)
Japanese 7 days diet 5-day LC50b 350 Hill et al. (1975)
quail
Ring-necked 15 days diet 5-day LC50b 430 Hill et al. (1975)
pheasant young diet 10-day LC50 500 DeWitt et al. (1963)
young diet 100-day LC50 50 DeWitt et al. (1963)
adult diet 100-day LC50 200 DeWitt et al. (1963)
Cowbird adult diet 30-week LC50 500 DeWitt et al. (1963)
--------------------------------------------------------------------------------
a Concentration in mg/kg body weight for oral dosing; concentration in mg/kg
diet for dietary dosing.
b 5 days of treated diet followed by 3 days of clean diet. Mortality rate
determined on day 8.
Table 7. Toxicity of chlordane for microorganisms
----------------------------------------------------------------------------------------
Organism F/M/ Temp Grade Solvent Endpoint Time Concen- Reference
S (° C) tration
(µg/
litre)a
----------------------------------------------------------------------------------------
Scenedesmus F 23 tech acetone stimulation of 5 - 7 0.1- Glooschenko &
quadricauda cell division days 100 Lott (1977)
F 23 tech acetone inhibition of 1 - 5 50 & Glooschenko &
photosynthesis days 100 Lott (1977)
Chlamydomonas S 23 tech acetone stimulation of 7 - 11 0.1- Glooschenko &
sp cell division days 50 Lott (1977)
S 23 tech acetone stimulation of 3 - 4 0.1- Glooschenko &
respiration h 100 Lott (1977)
S 23 tech acetone inhibition of 7 - 11 100 Glooschenko &
cell division days Lott (1977)
F 23- 60% acetone 50% reduction 3 days 100 000 Clegg &
25 ATPase Koevenig
activity; (1974)
no effect on
cell density
Volvox sp F 18- 20% none 100% mortality 7 days 1 Konar (1968)
Pandorina sp 24 EM
Closterium sp
Chlorella F 23- 60% acetone reduced ATPase 3 days 100 000 Clegg &
ellipsoidea 25 levels; Koevenig
Euglena no effect on (1974)
elastica cell density
Exuviella M 60% methanol virtual cessa- 7 days 50 Magnani et
baltica tion of growth al. (1978)
Natural M 60% acetone 94% decrease 4 h 1000 Butler (1963)
estuarine in product-
phytoplankton ivity
Estuarine M 7-14 60% methanol no effect 5 days 5 Biggs et al.
phytoplankton M 7-14 60% methanol growth and 14C 5 days 10 (1978)
uptake reduced
in laboratory
Bacillus S tech acetone growth ceased; 3 h 20 000 Trudgill et
subtilis decline in al. (1971)
viable count;
respiration
to zero
----------------------------------------------------------------------------------------
a Solubility of chlordane: 6 - 9 µg/litre. F = freshwater; M = marine; S = soil.
Accumulation of alpha- and and gamma-isomers of chlordane and
nonachlor, and chlordenes was studied by Cardwell et al. (1977) in
3 species of freshwater invertebrates. Chironomus larvae did not
show any detectable accumulation. After one week's exposure to 1.7 -
21.6 µg/litre, Daphnia magna gave CFs ranging from 15 000 to
175 000. After 65 days of exposure to 1.4 - 11.5 µ/litre, Hyallela
azteca showed CFs ranging from 41 000 to 144 000. After a 24-h
exposure to 0.5 µg alpha- and gamma-chlordane/litre, Daphnia pulex
gave a CF of 24 000 (Moore et al., 1977). Sanborn et al. (1976)
found a CF of 6132 for insect larvae. A fresh-water gastropod
Physa sp., concentrated chlordane 132 613 times (Sanborn et al.,
1976). Little bioaccumulation has been observed in the marine
invertebrate species studied. Wilson (1965) exposed oysters to
0.01 mg chlordane/litre and determined a CF of 7300, which is
considerably lower than that for fresh-water gastropods. Water and
oyster samples from Galveston Bay, Texas were analysed following an
extensive mosquito-control programme (Casper, 1967). Oysters
sampled did not contain any detectable chlordane and only 2 out of
9 water samples gave a positive result of less than 0.001 µg/litre.
Chlordane was detected by Bugg et al. (1967) in 20 out of 133
oyster samples taken from the South Atlantic and the Gulf of
Mexico, but 19 of these gave values of less than 0.01 mg/kg drained
weight. Clams, which were living in water containing chlordane at
0.01 µg/litre for 106 days, showed CFs of 1000 or less (Godsil &
Johnson, 1968). Parrish et al. (1976) reported that chlordane was
concentrated in the tissues of the estuarine pink and grass shrimps
at 1000 - 2300 times levels measured in water.
Few data are available on soil invertebrates. One report on
earthworms has been published by Gish (1970), who measured the
levels of gamma-chlordane in the soil and earthworms. CFs of 0.37,
7.1, 10.6, and 152 were obtained for 4 worms in agricultural soils.
Several studies are available on fresh-water fish. Henderson et
al. (1969) found that fish from Atlantic-coast streams, which gave
positive samples, contained between 0.1 and 7.29 mg chlordane/kg
(whole fish wet weight), fish from the Great Lake drainage areas
contained between 0.01 and 0.39 mg/kg, and fish from the
Mississippi River system contained between 0.01 and 0.72 mg/kg.
Fish from other systems (Hudson Bay, Colorado River, Interior
basins, Californian streams, Columbia River, Pacific coast and
Alaskan streams) contained less than 0.01 mg/kg. A further study
by Henderson et al. (1971) yielded 16 positive samples out of 666
fish taken from 50 sites, giving chlordane levels of between 0.09
and 13.5 mg/kg (whole fish wet weight). Working on chlordane
accumulation in sucker-fish, Roberts et al. (1977) showed that
accumulation from food was directly proportional to the lipid
levels of the fish. Chlordane was given to the northern redhorse
sucker, Moxostoma macrolepidotum, in the feed at 45 g/kg dry
feed for 5 consecutive days and to the white sucker, Catostomus
commersoni, directly to the stomach in a single dose of 340 mg
in corn oil. Both tests gave CF values of less than, or equal, to
0.52. CF values obtained in fish by uptake from the food were
lower than those obtained by uptake from water (goldfish at a CF of
162 with chlordane in diet (Moore et al., 1977); mosquito fish at a
CF of 8258 with chlordane in diet and in water (Sanborn et al.,
1976)). This suggests that chlordane is taken up directly from
water (bioaccumulated) more than it is from ingested food
(biomagnified).
Schimmel et al. (1976a,b) reported that CFs for two species of
marine fish were similar to those found in fresh-water species.
Spot and sheepshead minnow concentrated gamma-chlordane 3700 - 14
800 and 9000 - 16 800 times, respectively, in 4 days, and 3300 -
5100 and 10 300 times, respectively, in 24 days. Veith et al.
(1979) exposed fathead minnows to 5.9 µg chlordane/litre for 32
days and obtained a CF of 37 800 for the whole body. Parrish et
al. (1978) similarly reported 16 000 for whole body in the
sheepshead minnow after exposure for 189 days. They also
determined the CF after only 28 days exposure and found a
comparable whole-body value of 15 300. A range of CFs between 9000
and 16 786 was reported by Schimmel et al. (1976a) when sheepshead
minnows were exposed to gamma-chlordane (in technical heptachlor)
at 1.1 - 2.8 µg/litre for 96 h. In a field study, long-term
exposure (209 days or less) of large-mouth bass to between 0.01 and
0.1 µg/litre chlordane gave concentration factors of between 157
and 3308 (Godsil & Johnson, 1968).
Food chain magnification is unlikely in terrestrial organisms.
Species of birds and mammals studied show little bioaccumulation,
probably because chlordane is rapidly broken down in homoiotherms.
There are very few data on birds. One report (Foster et al., 1972)
refers to a study on the accumulation of chlordane in laying hens
fed 0.1 mg/kg diet. CF values were maximal after 7 - 9 weeks, diet
to fat was between 0.01 and 3.3, and diet to eggs was between 0.01
and 2. After 3 weeks on an untreated diet, chlordane was not
detectable. McCaskey et al. (1968) dosed hens with the equivalent
of a diet containing 10 - 15 mg 60% technical chlordane/kg for 5
days and obtained a maximum CF value in eggs of 0.38 on day 6.
8.5. Population and Community Effects
The fate of 14C-chlordane was investigated in a terrestrial-
aquatic model ecosystem composed of an alga (Oedogonium), snails
(Physa), mosquito larvae (Culex), and fish (Gambusia)
(Sanborn et al., 1976). Accumulation of chlordane residues was
evident in all organisms in the ecosystem, but no toxic effects
were reported.
Exposure of a natural phytoplankton population to 5 and 10 µg
chlordane/litre for 5 days had virtually no effect on its species
composition (Biggs et al., 1978).
8.6. Effects on the Abiotic Environment
No data are available on abiotic effects.
8.7. Appraisal
Data on aquatic toxicity require interpretation. Although the
solubility of chlordane in water has been measured at between 6
and 9 µg/litre, in many studies on its aquatic toxicity, chlordane
has been applied at much higher nominal concentrations. Either the
chlordane has not been in solution or was added with a solvent.
Actual levels of chlordane in natural waters rarely exceed 250
ng/litre with most levels below 20 ng/litre. Test levels are
therefore unrealistically high. The data should thus be critically
examined unless the actual concentrations experienced by the test
organisms were measured.
Few long-term studies of the chronic effects of chlordane are
available. Data do not define threshold levels, although they
suggest levels at which effects might be expected. Data seldom
provide quantitative dose-response relationships.
Effects of chlordane on primary producers in the aquatic food
chain are largely unknown because studies have used unrealistically
high concentrations. Some data on lethal doses for aquatic
organisms are available, but data on sub-lethal effects on
reproduction or behaviour are not. There are data on the acute
toxicity of chlordane for fish at concentrations approaching the
water solubility, but few on long-term exposure at lower doses.
Most terrestrial studies have been on soil organisms. Effects
here might be due to the heptachlor in technical chlordane or to a
combination of heptachlor and chlordane. Only very high
application rates of chlordane affected arthropods. Field studies
do not indicate direct toxicity but a combination of toxicity and
avoidance of the compound.
Of major importance is the clear toxicity of chlordane for
earthworms with implications for soil fertility. Molluscs also
appear to be particularly sensitive to chlordane.
9. PREVIOUS EVALUATIONS OF CHLORDANE BY INTERNATIONAL BODIES
An IARC Working Group (IARC, 1982) concluded that the available
epidemiological studies on chlordane were inadequate for the
evaluation of the cancer risk for man and that there was limited
evidence of the carcinogenicity of chlordane for experimental
animals.
WHO has recommended a guideline value of 0.3 µg/litre for
chlordane (total isomers) in drinking-water (WHO, 1982).
The Joint Meeting on Pesticide Residues (JMPR) reviewed
residues and toxicity data on chlordane on several occasions in the
past (1965, 1967, 1970). In November 1972, it re-established
residue tolerences ranging from 0.02 - 0.5 mg/kg for the sum of
alpha- and gamma-isomers of chlordane and oxychlordane (FAO/WHO,
1973). The acceptable daily intake (ADI) for human beings of
0 - 0.001 mg/kg body weight was confirmed in December 1977
(FAO/WHO, 1978). This was based on no-observed-adverse-effect-
levels of:
- 5 mg/kg in the diet, equivalent to 0.25 mg/kg body
weight in the rat; and
- 3 mg/kg in the diet, equivalent to 0.075 mg/kg body
weight in the dog.
Both "no-observed-adverse-effect-levels" and the ADI were
reviewed by the 1982 JMPR (FAO/WHO, 1983). The ADI was given
"temporary" status pending the results of toxicology studies still
in progress.
WHO (1984), in its "Guidelines to the Use of the WHO
Recommended Classification of Pesticides by Hazard", classified
technical chlordane as moderately hazardous.
The WHO/FAO (1978) has issued practical advice in its "Data
Sheet on Pesticides" including one on Chlordane (No. 36) dealing
with labelling, safe-handling, transport, storage, disposal,
decontamination, training and medical supervision of workers, first
aid and medical treatment.
Regulatory standards established by national bodies in 12
different countries (Argentina, Brazil, Czechoslovakia, the Federal
Republic of Germany, India, Japan, Kenya, Mexico, Sweden, the
United Kingdom, the USA, and the USSR) and the EEC is available
from the IRPTC (International Register of Potentially Toxic
Chemicals) Legal file (IRPTC, 1983).
The CEC reviewed the data available on chlordane in 1981.
10. EVALUATION OF HEALTH RISKS FOR MAN AND EFFECTS ON THE
ENVIRONMENT
10.1. Chlordane Toxicity
Chlordane is readily absorbed in both animals and man via the
skin, via ingestion, and probably also by inhalation. Some
accumulation occurs in the body on repeated exposure - mainly in
adipose tissue. Elimination from the body is fairly slow. The
half-life in various species, including man, is of the order of a
few weeks.
The oral LD50 values of chlordane in the rat range from
200 - 590 mg/kg body weight. Thus, chlordane is moderately toxic
in acute exposures.
Acute poisoning in man and animals is characterized by
manifestations of central nervous system stimulation such as
disorientation, tremors, and convulsions. Death may follow
respiratory failure.
In experimental animals (rats and dogs), prolonged exposure
to levels in the diet exceeding 3 - 5 mg/kg resulted in the
induction of hepatic microsomal enzymes and, at a later stage,
liver hypertrophy with histological changes. At higher levels
(i.e., > 15 mg/kg body weight per day), chlordane is hepatotoxic.
For no-observed-adverse-effect levels see section 6.1.1.
At dosages above 30 mg/kg diet, chlordane interferes with
reproduction in rats and mice, but this was reversible after
exposure ceased. There are no indications for teratogenicity in
the rabbit at 15 mg/kg body weight per day.
Chlordane produces hepatocellular carcinomas in mice. It
is not generally active in short-term tests designed to measure
genetic activity. Chlordane can interfere with cell to cell
communication in vitro, a characteristic of many promoting agents.
10.2. Exposure to Chlordane
Food is the major source of exposure of the general population
to chlordane, but the use of chlordane on food crops has decreased
and residues in food from animal origin are low. Some chlordane
exposure can occur in buildings where chlordane has been used for
termite or other insect control.
No adverse health effects have been reported in workers engaged
in the manufacture of chlordane or in pest-control operations,
where exposure could be quite high. However, several cases of
accidental and suicidal poisoning in man have been reported
resulting in the symptoms described in section 7.1.
10.3. Evaluation of Overall Environmental Effects
Chlordane is used primarily to control soil pests. Technical
chlordane is a mixture of chlorinated hydrocarbons and contains
heptachlor, which might contribute significantly to the
insecticidal properties of the technical formulation.
About half of the chlordane applied to soil disappears in the
first season, presumably by volatalisation or by "run-off" into
surface waters. Remaining residues persist for several seasons.
If chlordane is applied annually for several successive seasons,
residues accumulate in the soil. Most chlordane persists in the
cultivated levels, since there is little leaching into subsoil.
The high rate of metabolism of chlordane in warm-blooded
animals means that there is little possibility of accumulation in
these animals or magnification in food chains at this level.
Concentration factors are generally modest in aquatic organisms;
this combined with its low solubility in water means that chlordane
presents a limited hazard for aquatic vertebrates. Long-term
effects are not sufficiently well-documented to say that there is
not a potential hazard for fish, but this seems unlikely from the
information available, as far as temperate areas are concerned.
The compound shows a higher toxicity at higher temperatures.
Significant mortality in tropical species of fish at concentrations
well within the solubility of the compound, suggest that chlordane
may be a greater aquatic hazard at lower latitudes.
The high toxicity of chlordane for earthworms may constitute
its greatest potential hazard. The long-term effects of reduced
numbers of earthworms in the soil cannot be readily assessed
because the ecology of the animal is still poorly understood.
10.4. Evaluation of Risks for Human Health and the Environment
Although there is no evidence that incriminates chlordane as a
human carcinogen, the suspicion principally arising from the mouse
carcinogenicity studies cannot be entirely put aside. Further
research is required to elucidate this problem. Nevertheless, in
the present state of knowledge, it is concluded that:
1. As long as occupational hygiene procedures are maintained
to keep exposure levels to a minimum, whether or not by the
imposition of maximum allowable concentrations, there is
little reason to believe that workers will be at risk from
their handling, or contacts, with chlordane.
2. For the general population, consumers should suffer no
adverse effects from chlordane as food residues, provided
that the intake is kept within the temporary ADI set by the
Joint FAO/WHO Meeting.
In certain regions of the world, the exposure of the
general population to chlordane may be augmented by its
use as a termiticide in buildings.
3. Apart from the possible long-term adverse effects on
aquatic organisms in tropical areas and the depleted soil
fertility that may arise, in time, from the suppression of
the earthworm population, chlordane seems to cause little
environmental concern in its normal use as a termiticide
and in other non-agricultural applications.
REFERENCES
AHMED, F.E., HART, R.W., & LEWIS, N.J. (1977a) Pesticide
induced DNA damage and its repair in cultured human cells.
Mutat. Res., 42: 161-174.
AHMED, F.E., LEWIS, N.J., & HART, R.W. (1977b) Pesticide
induced ouabain resistant mutants in Chinese hamster. Chem.
Biol. Interactions, 19: 369-374.
ALDRICH, F.D. & HOLMES, J.H. (1969) Acute chlordane
intoxication in a child. Case report with toxicological
data. Arch. environ. Health, 19: 129-132.
AL-HACHIM, G.M. & AL-BAKER, A. (1973) Effects of chlordane
on conditioned avoidance response, brain seizure threshold and
open-field performance of prenatally-treated mice. Br. J.
Pharmacol., 49: 311-315.
ALVAREZ, W.C. & HYMAN, S. (1953) Absence of toxic
manifestations in workers exposed to chlordane. Arch. ind.
Hyg. occup. Med., 7: 197-210.
AMBROSE, A.M., CHRISTENSEN, H.E., ROBBINS, D.J., & RATHER,
L.J. (1953) Toxicology and pharmacological studies on
chlordane. Arch. ind. Hyg. occup. Med., 1: 197-210.
ARNOLD, D.W., KENNEDY, G.L., KEPLINGER, M.L., CALANDRA, J.C.,
& CALO, C.J. (1977) Dominant lethal studies with technical
chlordane, HCS-3260, and heptachlor: Heptachlor epoxide.
J. Toxicol. environ. Health, 2: 547-555.
ATALLAH, Y.H., WHITACRE, D.M., & POLEN, P.B. (1977) Artifacts in
monitoring - related analysis of human adipose tissue for some
organochlorine pesticides. Chemosphere, 6: 17-20.
ATALLAH, Y.H., WHITACRE, D.M., & HOO, B.L. (1979)
Comparative volatility of liquid and granular formations of
chlordane and heptachlor from soil. Bull. environ. Contam.
Toxicol., 22: 570-574.
BACHMANN, K.A. & BURKMAN, A.M. (1974) Topical application of
a chlordane containing ectoparasiticide: Effect on plasma
half-life of warfarin in dogs. Pharmacologist, 16: 284.
BALBA, H.M. & SAHA, J.G. (1978) Studies on the distribution,
excretion, and metabolism of alpha- and gamma-isomers of 14C
chlordane in rabbits. J. environ. Sci. Health, B13: 211-233.
BARNETT, R.W. & DOROUGH, H.W. (1974) Metabolism of chlordane
in rats. J. agric. food Chem., 22: 612-619.
BARNETT, R.W., D'ERCOLE, A.J., CAIN, J.D., & ARTHUR, R.D.
(1979) Organochlorine pesticide residues in human milk
samples from women living in north-west and north-east
Mississippi, 1973-1975. Pestic. Monit. J., 13: 47-51.
BARTHEL, W.F., HAWTHORNE, J.C., FORD, J.H., BOLTON, G.C.,
McDOWELL, L.L., GRISSINGER, E.H., & PARSONS, D.A. (1969)
Pesticide residues in sediments of the Lower Mississippi River
and its tributaries. Pestic. Monit. J., 3: 8-34.
BATTE, E.G. & TURK, R.D. (1948) Toxicity of some synthetic
insecticides to dogs. J. econ. Entomol., 41: 102-103.
BEVENUE, A., OGATA, J.N., & HYLIN, J.W. (1972a) Organo-
chlorine pesticides in rain water, Oahu, Hawaii, 1971-1972.
Bull. environ. Contam. Toxicol., 8: 238-241.
BEVENUE, A., HYLIN, J.W., KAWANO, Y., & KELLEY, T.W. (1972b)
Organochlorine pesticide residues in water, sediment, algae,
and fish, Hawaii, 1970-71. Pestic. Monit. J., 6: 56-64.
BIGGS, D.C., ROWLAND, R.G., O'CONNORS, H.B., POWERS, C.D., &
WURSTER, C.F. (l978) A comparison of the effects of
chlordane and PCB on the growth, photosynthesis and cell size
of estuarine phytoplankton. Environ. Pollut., 15: 253-263.
BIROS, F.J. & ENOS, H.F. (1973) Oxychlordane residues in
human adipose tissue. Bull. environ. Contam. Toxicol., 10:
257-260.
BOYD, J.C. (1971) Field study of a chlordane residue
problem: Soil and plant relationships. Bull. environ. Contam.
Toxicol., 6: 177-182.
BOYD, E.M. (1972) Chlordane. In: Protein deficiency and
pesticide toxicity, Springfield, Ill., Charles C. Thomas,
468 pp.
BRIMFIELD, A.A. & STREET, J.C. (1979) Mammalian
biotransformation of chlordane: in vivo and primary hepatic
comparisons. Ann. New York Acad. Sci., 320: 247-256.
BRIMFIELD, A.A., STREET, J.C., FUTRELL, J., & CHATFIELD, D.A.
(1978) Identification of products arising from the metabolism
of cis- and trans-chlordane in rat liver microsomes in vitro:
Outline of a possible metabolic pathway. Pestic. Biochem.
Physiol., 9: 84-95.
BRODTMANN, N.V., Jr (1976) Continuous analysis of
chlorinated hydrocarbon pesticides in the lower Mississippi
river. Bull. environ. Contam. Toxicol., 15: 33-39.
BRUBAKER, P.E., FLAMM, W.G., & BERNHEIM, N.J. (1970) Effect
of gamma-chlordane on synchronized lymphoma cells and
inhibition of cell division. Nature (Lond.), 226: 548-549.
BUCK, W.B., OSWEILER, G.D., & VAN GELDER, G.A. (1973)
Clinical and diagnostic veterinary toxicology, Dubuque, Iowa,
Kendall Hunt.
BUGG, J.C., HIGGINS, J.E., & ROBERTSON, E.A. (l967) Residues
in fish, wildlife and estuaries: chlorinated pesticide levels
in the eastern oyster (Crassostrea virginica) from selected
areas of the South Atlantic and Gulf of Mexico. Pestic. Monit.
J., 1: 9-12.
BUTLER, P.A. (l963) Commercial fishery investigations,
Washington DC, US Department of the Interior, Fish and
Wildlife Service, pp. 5-28 (Circular 199).
BUTLER, P.A. & SCHUTZMANN, R.L. (1978) Residues in
pesticides and PCBs in estuarine fish, 1972-1976; National
Pesticide Monitoring Program. Pestic. Monit. J., 12: 51-59.
CALDWELL, R.S. (l977) Biological effects of pesticides on
the Dungeness crab, Washington DC, US Environment Protection
Agency (Report No. EPA 600/3-77-131).
CANADA, NATIONAL RESEARCH COUNCIL (1974) Chlordane: Its
effects on Canadian ecosystems and its chemistry, Canada, NRC
(Report Monograph Non Serials 189).
CARDWELL, R.D., FOREMAN, D.G., PAYNE, T.R., & WILBUR, D.J.
(l977) Acute and chronic toxicity of chlordane to fish and
invertebrates, Washington DC, US Environment Protection
Agency, 126 pp (Report No. EPA 600/3-77-019).
CAREY, A.E., WIERSMA, G.B., TAI, H., & MITCHELL, W.G. (1973)
Organochlorine pesticide residues in soils and crops of the
Corn Belt region, United States, 1970. Pestic. Monit. J., 6:
369-376.
CASPER, V.L. (l967) Galveston Bay pesticide study - water
and oyster samples analysed for pesticide residues following
mosquito control programme. Pestic. Monit. J., 1: 13-15.
CEC (1981) Criteria (dose-effects relationship) for organo-
chlorine pesticides, Oxford, CEC, Pergamon Press.
CHAMBERS, C. & DUTTA, S.K. (1976) Mutagenic tests of
chlordane on different microbial tester strains. Genetics,
83: S13.
CLARK, D.R., Jr & KRYNITSKY, A. (1978) Organochlorine
residues and reproduction in the little brown bat, Laurel,
Maryland, June 1976. Pestic. Monit. J., 12: 113-116.
CLARK, D.R., Jr & PROUTY, R.M. (1976) Organochlorine
residues in three bat species from four localities in Maryland
and West Virginia, 1973. Pestic. Monit. J., 10: 44-53.
CLEGG, T.J. & KOEVENIG, J.L. (l974) The effect of four
chlorinated hydrocarbon pesticides and one organophosphate
pesticide on ATP levels in three species of photosynthesizing
fresh-water algae. Bot. Gaz., 135: 368-372.
CLEMENS, H.P. & SNEED, K.E. (l959) Lethal doses of several
commercial chemicals for fingerling channel catfish,
Washington DC, US Department of the Interior, Fish and
Wildlife Service, 10 pp (Special Scientific Report on
Fisheries No. 316).
COCHRANE, W.P. & GREENHALGH, R. (1976) Chemical composition
of technical chlordane. J. Assoc. Off. Anal. Chem., 59:
696-702.
COCHRANE, W.P., PARLAR, H., GAEB, S., & KORTE, F. (1975)
Structural elucidation of the chlordene isomer constituents of
technical chlordane. J. agric. food Chem., 23: 882-886.
COPE, O.B. (l965) Sport fishery investigations, Washington
DC, US Department of the Interior, Fish and Wildlife Service
(Circular 226).
COPE, O.B., GJULLIN, C.M., & STORM, A. (l947) Effects of
some insecticides on trout and salmon in Alaska, with
reference to black-fly control. Trans. Am. Fish. Soc., 77:
160-177.
CORBETT, D.C.M. & WEBB, R.M. (l968) Effect of herbicides in
minimum tillage, Suffolk, England, Richard Clay (The Chaucer
Press) Ltd., pp. 158-159 (Report of the Rothamsted
Experimental Station for l967).
COUNCIL FOR AGRICULTURAL SCIENCE & TECHNOLOGY (1975)
Chlordane and heptachlor, Ames, Iowa, Iowa State University,
Department of Agronomy, pp. 1-71 (Report No. 47, Oct. 3).
CRANMER, J.S., AVERY, D.L., GRADY, R.R., & KITAY, J.I. (1978)
Postnatal endocrine dysfunction resulting from prenatal
exposure to carbofuran diazinon or chlordane. J. environ.
Pathol. Toxicol., 2: 357-370.
CROCKETT, A.B., WIERSMA, G.B., TAI, H., MITCHELL, W.G., SAND,
P.F., & CAREY, A.E. (1974) Pesticide residue levels in soils
and crops, FY-70: National soils monitoring program II.
Pestic. Monit. J., 8: 69-97.
CURLEY, A. & GARRETTSON, L.K. (1969) Acute chlordane
poisoning: clinical and chemical studies. Arch. environ.
Health, 18: 211-215.
DATTA, K.K., GUPTA, P.C., & DIKSHITH, T.S.S. (1975) Effect
of chlordane on the skin of male guinea pigs. In: Zaidi,
S.H., ed. Environmental pollution and human health, Lucknow,
India, Industrial Toxicology Research Centre, pp. 608-611.
DAVIS, R.W., FRIEDHOFF, J.M., & WEDENEYER, G.A. (l972)
Organochlorine insecticide, herbicide and polychlorinated
biphenyl (PCB) inhibition of NaK-ATPase in rainbow trout.
Bull. environ. Contam. Toxicol., 8: 69-72.
DEN TONKELAAR, E.M. & VAN ESCH, G.J. (1974) No-effect levels
of organochlorine pesticides based on induction of microsomal
liver enzymes in short-term toxicity experiments. Toxicology,
2: 371-380.
DERBES, J.V., DENT, H.J., FOREST, W.W., & JOHNSON, M.F.
(1955) Fatal chlordane poisoning. J. Am. Med. Assoc.,
158(15): 1367-1369.
DEWITT, J.B., STICKEL, W.H., & SPRINGER, P.F. (1963)
Wildlife studies, Patuxent Wildlife Research Centre l961-l962,
Washington DC, US Department of the Interior, Fish and
Wildlife Service, pp. 74-96 (Circular 167).
DITRAGLIA, D., BROWN, D.P., NAMEKATA, T., & IVERSON N.
(1981) Mortality study of workers employed at organochlorine
pesticide manufacturing plants. Scand. J. Work Environ.
Health, 7(Suppl. 4): 140-146.
DOANE, C.C. (l962) Effects of certain insecticides on
earthworms. J. econ. Entomol., 55: 416-418.
DRUMMOND, L., CHETTY, K.N., BAILEY, M., & DESAIAH, D. (1980)
in vivo effect of chlordane on rat brain ATP-ase system. Fed.
Proc., 39: 998.
DUFFY, J.R. & WONG, N. (1967) Residues of organochlorine
insecticides and their metabolites in soil in the Atlantic
Provinces of Canada. J. agric. food Chem., 15(3): 457-464.
EDWARDS, C.A. (l965a) Some side-effects resulting from the
use of persistent insecticides. Ann. appl. Biol., 55: 329-331.
EDWARDS, C.A. (l965b) Effects of pesticide residues on soil
invertebrates and plants. In: Goodman, G.T., Edwards, R.W., &
Lambert, J.M., ed. Ecology and the industrial society,
Reading, England, British Ecological Society, Symposium No. 5,
pp. 239-261.
EPSTEIN, S.S. (1976) Carcinogenicity of heptachlor and
chlordane. Sci. total Environ., 6: 103-154.
EPSTEIN, S.S., ARNOLD, E., ANDREA, J., BASS, W., & BISHOP, Y.
(1972) Detection of chemical mutagens by the dominant lethal
assay in the mouse. Toxicol. appl. Pharmacol., 23: 288-335.
ERCEGOVICH, C.D. & RASHID, K.A. (1977) Mutagenesis induced
in mutant strains of Salmonella typhimurium by pesticides.
Soc. Abstr. Pap., 174: 43.
FAO/WHO (1968) 1967 Evaluations of some pesticide residues
in food, Rome, Food and Agriculture Organization of the United
Nations (The Monographs).
FAO/WHO (1973) 1972 Evaluations of some pesticide residues
in food, Rome, Food and Agriculture Organization of the United
Nations.
FAO/WHO (1978) 1977 Evaluations of some pesticide residues
in food, Rome, Food and Agriculture Organization of the United
Nations.
FAO/WHO (1981) Joint FAO/WHO food and animal feed
contamination monitoring programme. Summary of data received
from collaborating centres - 1977-1980. Part B - Contaminants,
Geneva, World Health Organization.
FAO/WHO (1983) Pesticide residues in food - 1982, Rome, Food
and Agriculture Organization of the United Nations, pp. 16-17
(FAO Plant Production and Protection Paper 46).
FISHBEIN, W.I., WHITE, J.V., & ISAACS, H.J. (1964) Survey of
workers exposed to chlordane. Ind. Med. Surg., 33: 726-727.
FITZHUGH, O.G. & FAIRCHILD, H.E. (1976) Pesticidal aspects
of chlordane in relation to man and the environment,
Washington DC, US Environmental Protection Agency, US NTIS,
114 pp (PB Rep., ISS PB- 257107).
FOLMAR, L.C. (1978) In vitro inhibition of rat brain
ATPase, pNPPase, and ATP-32p exchange by chlorinated-diphenyl
ethanes and cyclodiene insecticides. Bull. environ. Contam.
Toxicol., 19: 481-488.
FOSTER, T.S., MORLEY, H.V., PURKAYASTHA, R., GREENHALGH, R., &
HUNT, J.R. (l972) Residues in eggs and tissues of hens fed a
ration containing low levels of pesticides with and without
charcoal. J. econ. Entomol., 65: 982-988.
FOUTS, J.R. (1963) Factors influencing the metabolism of
drugs in liver microsomes. Ann. New York Acad. Sci., 104:
875-880.
FOX, C.J.S. (l958) Some effects of insecticides on the
wireworms and vegetation of grassland in Nova Scotia. In:
Proceedings of the Tenth Congress on Entomology, Canada,
Vol. 3, pp. 297-300.
FRANK, R., BRAUN, H.E., HOLDRINET, M., DODGE, D.P., & NAPSZY,
S.J. (1978a) Residues of organochlorine insecticides and
polychlorinated biphenyls in fish from lakes Saint Clair and
Erie, Canada, 1968-1976. Pestic. Monit. J., 12: 69-80.
FRANK, R., HOLDRINET, M., BRAUN, H.E., DODGE, D.P., &
SPRANGLER, G.E. (1978b) Residues of organochlorine insecticides
and polychlorinated biphenyls in fish from lakes Huron and
Superior, Canada, 1968-1976. Pestic. Monit. J., 12: 60-68.
GAEB, S., BORN, L., PARLAR, H., & KORTE, F. (1977)
Structural elucidation of an octachloro component of technical
chlordane (Compound K) by spectroscopic and X-ray methods.
J. agric. food Chem., 25: 1365-1371.
GAINES, T.B. (1969) Acute toxicity of pesticides. Toxicol.
appl. Pharmacol., 14: 525-534.
GISH, C.D. (l970) Pesticides in soil. Organochlorine
insecticide residues in soils and soil invertebrates from
agricultural land. Pestic. Monit. J., 3: 241-252.
GLOOSCHENKO, V. & LOTT, J.N.A. (l977) The effects of
chlordane on the green algae, Scenedesmus quadricauda and
Chlamydomonas sp. Can. J. Bot., 55: 2866-2872.
GLOOSCHENKO, W.A., STRACHAN, W.M.J., & SAMPSON, R.C.J.
(1976) Residues in water: distribution of pesticides and
polychlorinated biphenyls in water, sediments, and seston of
the Upper Great Lakes - 1974. Pestic. Monit. J., 10: 61-67.
GODSIL, P.J. & JOHNSON, W.C. (l968) Residues in fish,
wildlife and estuaries. Pesticide monitoring of the aquatic
biota at the Tule Lake National Wildlife Refuge. Pestic.
Monit. J., 1: 21-26.
GOES, T.R., SAVAGE, E.P., & BOYD, W.L. (1978) In vitro
inhibition of oral viridans streptococci by chlordane. Arch.
environ. Contam. Toxicol., 7: 449-456.
GOULD, E. & HAMPSTEAD, E.O. (l95l) The toxicity of
cumulative spray residues in soils. J. econ. Entomol., 44:
713-717.
GOWEN, J.A., WIERSMA, G.B., TAI, H., & MITCHELL, W.G. (1976)
Pesticide levels in hay and soils from nine states, 1971.
Pestic. Monit. J., 10: 114-116.
GRIMES, D.J. & MORRISON, S.M. (1975) Bacterial
bioconcentration of chlorinated hydrocarbon insecticides from
aqueous systems. Microbiol. Ecol., 2: 43-59.
HANSON, W.R. (1952) Effects of some herbicides and
insecticides on the biota of North Dakota marshes. J. Wildl.
Manage., 16: 299-308.
HARRINGTON, J.M., BAKER, E.L., Jr, FOLLAND, D.S., SAUCIER,
J.W., & SANDIFER, S.H. (1978) Chlordane contamination of a
municipal water system. Environ. Res., 15: 155-159.
HARRIS, C.R. & SANS, W.W. (1976) Persistence of velsicol
HCS-3260 (AG-chlordane) in mineral and organic soil. Proc.
Entomol. Soc. Ontario, 106: 34-38.
HART, L.G. & FOUTS, J.R. (1965) Studies of the possible
mechanisms by which chlordane stimulates hepatic microsomal
drug metabolism in the rat. Biochem. Pharmacol., 14: 263-272.
HART, L.G., SHULTICE, R.W., & FOUTS, J.R. (1963) Stimulatory
effects of chlordane on hepatic microsomal drug metabolism in
the rat. Toxicol. appl. Pharmacol., 5: 371-386.
HENDERSON, C., PICKERING, Q.H., & TARZWELL, C.M. (1959) The
relative toxicity of ten chlorinated hydrocarbon insecticides
to four species of fish. Trans. Am. Fish. Soc., 88: 23-32.
HENDERSON, C., JOHNSON, W.L., & INGLIS, A. (1969)
Organochlorine insecticide residues in fish. Pestic. Monit.
J., 3: 145-171.
HENDERSON, C., INGLIS, A., & JOHNSON, W.L. (1971)
Organochlorine insecticide residues in fish - Fall 1969.
National pesticide monitoring program. Pestic. Monit. J., 5:
1-11.
HERRICK, G.M., FRY, J.I., FONG, W.G., & GOLDEN, D.C. (1969)
Insecticide residues in eggs resulting from the dusting and
short-term feeding of low levels of chlorinated hydrocarbon
insecticides to hens. J. agric. food Chem., 17: 291-295.
HILL, E.F., HEATH, R.G., SPANN, J.W., & WILLIAMS, J.D.
(1975) Lethal dietary toxicities of environmental pollutants
to birds, Washington DC, US Department of the Interior, Fish
and Wildlife Service, 61 pp (Special Scientific Report No.
191).
HODGE, H.C. & STERNER, J.H. (1956) Combine and tabulation of
toxicity classes. In: Spector, W.B., ed. Handbook of
toxicology, Philadelphia, Pennsylvania, W.B. Saunders Company,
Vol. 10.
HOPKINS, A.R. & KIRK, V.M. (1957) Effect of several
insecticides on the English Red Worm. J. econ. Entomol., 50:
699-700.
HRDINA, P.D., PETERS, D.A.V., & SINGHAL, R.L. (1973) Acute
neurotoxic effects of alpha-chlordane and alterations in brain
biogenic amines. Proc. Can. Fed. Biol. Soc., 16: 103.
HYDE, K.M. & FALKENBERG, R.L. (1976) Neuroelectrical
disturbance as indicator of chronic chlordane toxicity.
Toxicol. appl. Pharmacol., 37: 499-515.
HYDE, K.M., CRANDALL, J.C., KORTMAN, K.E., & MCCOY, W.K.
(1978) EEG, ECG, and respiratory response to acute
insecticide exposure. Bull. environ. Contam. Toxicol., 19:
47-55.
IARC (1979) Some halogenated hydrocarbons. Chlordane, Lyons,
International Agency for Research on Cancer, pp. 45-65
(Monographs on the Evaluation of the Carcinogenic Risk of
Chemicals to Humans, No. 20).
IARC (1982) Chemicals and industrial processes associated
with cancer in humans, Lyons, International Agency for
Research on Cancer, pp. 80-83 (Monographs on the Evaluation of
the Carcinogenic Risk of Chemicals to Humans, Suppl. 4).
ILO (1980) Occupational exposure limits for airborne toxic
substances, 2nd (revised) ed., Geneva, International Labour
Office (Occupational Safety and Health Series No. 37).
INFANTE, P.F., EPSTEIN, S.S., & NEWTON, W.A., Jr (1978)
Blood dyscrasias and childhood tumors and exposure to
chlordane and heptachlor. Scand. J. Work Environ. Health, 4:
137-150.
INGLE, L. (1952) Chronic oral toxicity of chlordane to
rats. Arch. ind. Hyg. occup. Med., 6: 357-367.
INGLE, L. (1965a) Effects of 1-hydroxychlordene when
incorporated into the diets of rats for 224 days, Urbana,
Illinois, University of Illinois, Department of Zoology
(Report for Velsicol Chemical Corporation).
INGLE, L. (1965b) Monograph on chlordane - toxicological &
pharmacological properties, Urbana, Illinois, University of
Illinois, Food & Drug Library (Library of Congress, Card
No. 65-28686A).
INGLE, L. (1969) Albino rats subjected to alpha and gamma
chlordane incorporated into the daily diet for 78 weeks
(Report from the University of Illinois).
INRS (1983) Valeurs limites pour les concentrations des
substances dangereuses dans l'air des locaux de travail,
Paris, France, Institut National de Recherche et de Sécurité
pour la Préventions des Accidents du Travail et des Maladies
Professionelles (Cahiers de notes documentaires, No. 110).
IRDC (1967) Chlordane, two-year chronic feeding study in the
beagle dog, Mattawan, Michigan, International Research and
Development Corporation (Report 163-001, sponsored by Velsicol
Chemical Corporation).
IRDC (1972) Chlordane, teratology study in rabbits,
Mattawan, Michigan, International Research and Development
Corporation (Report 163-106, sponsored by Velsicol Chemical
Corporation).
IRPTC (1983) IRPTC Legal file 1983, Geneva, International
Register of Potentially Toxic Chemicals, United Nations
Environment Programme (UNEP), Vols. I & II.
IVIE, G.W., KNOW, J.R., KHALIFA, S., YAMAMOTO, I., & CASIDA,
J.E. (1972) Novel photoproducts of heptachlor epoxide,
trans-chlordane, and trans-nonachlor. Bull. environ. Contam.
Toxicol., 7: 376-382.
JENSEN, A.A. (1983) Chemical contaminants in human milk.
Residue Rev., 89: 1-128.
KACEW, S. & SINGHAL, R.L. (1973a) Metabolic alterations after
chronic exposure to alpha-chlordane. Toxicol. appl.
Pharmacol., 24: 539-544.
KACEW, S. & SINGHAL, R.L. (1973b) The influence of
p,p'-DDT, alpha-chlordane, heptachlor and endrin on hepatic
and renal carbohydrate metabolism and cyclic AMP-adenyl
cyclase system. Life Sci., 13: 1363-1371.
KADAM, A.N., BENDRE, S.B., & GHATGE, B.B. (1978) Gas
chromatography of technical chlordane and identification of
some of the components. Pesticides, 12: 13-15.
KAREL, A.K. (1976) Acute chlordane toxicity on the serum
alkaline phosphatase activity of Meriones hurricanae, Jerdon
(gerbil). Arch. int. Physiol. Biochem., 84: 63-68.
KAREL, A.K. & SAXENA, S.C. (1976) Chronic chlordane
toxicity: Effect on blood biochemistry of Meriones hurricanae,
Jerdon, the Indian desert gerbil. Pestic. Biochem. Physiol.,
6: 111-114.
KEPLINGER, M.L., DEICHMANN, W.B., & SALA, F. (1968) Effects
of pesticides on reproduction in mice. Ind. Med. Surg., 37:
525.
KOCH, R.B., CUTCOMB, L.K., & YAP, H.H. (1971) Inhibition of
oligomycin sensitive and insensitive fish adenosine
triphosphatase activity by chlorinated hydrocarbon
insecticides. Biochem. Pharmacol., 20: 3243-3245.
KONAR, S.K. (1968) Experimental use of chlordane in fishery
management. Prog. Fish. Cult., 30: 96-99.
KRAYBILL, H.F.T. (1977a) The determination of carcinogenesis
induced by trace contaminants in potable water. In: Borchardt,
J.A., Cleland, J.K., Redman, W.J., & Olivier, J., ed. Viruses
and trace contaminants in water and wastewater, Ann Arbor,
Michigan, Ann Arbor Science Publishers, Inc., pp. 109-123
(Seminar, Ann Arbor, Michigan, January 26-28, 1977. XIV 249P).
KUTZ, F.W., YOBS, A.R., STRASSMAN, S.C. (1976) Organochlorine
pesticide residues in human adipose tissue. Bull. Soc.
Pharmacol. Environ. Pathol., 4(1): 17-19.
LANGLOIS, B.E. & SIDES, K.G. (1972) Effect of heptachlor and
related compounds on growth of Staphylococcus aureus. Bull
environ. Contam. Toxicol., 8: 158-164.
LAWRENCE, J.M. (1950) Toxicity of some new insecticides to
several species of pondfish. Prog. Fish. Cult., 12: 141-146.
LAWRENCE, J.H., BARRON, R.P., CHEN, J.Y.T., LOMBARDO, P., &
BENSON, W.R. (1970) Note on identification of a chlordane
metabolite found in milk and cheese. J. Assoc. Off. Agric.
Chem., 53: 261-262.
LEGG, D.C. (1968) Comparison of various worm killing
chemicals. J. Sports Turf Res. Inst., 44: 47-48.
LEHMAN, A.J. (1952a) Chemicals in foods: A report to the
Association of Food & Drug Officials on current developments.
Part II. Pesticides Section II: Dermal Toxicity. Assoc. Food
Drug Off. Q. Bull., 16: 3-9.
LEHMAN, A.J. (1952b) A report to the Association of Food and
Drug Officials on current developments. Section III: Subacute
and chronic toxicity. Assoc. Food Drug Off. Q. Bull., 16:
47-53.
LENSKY, P. & EVANS, H.L. (1952) Human poisoning by
chlordane. Report of a case. J. Am. Med. Assoc., 121: 826-827.
LICHTENSTEIN, E.P. & POLIUKA, J.B. (1959) Persistence of
some chlorinated hydrocarbon insecticides in turf soils.
J. econ. Entomol., 52(2): 280-293.
LICHTENSTEIN, E.P., SCHULTZ, K.K., FUHREMANN, T.W., & LIANG,
T.T. (l970) Degradation of aldrin and heptachlor in field
soils during a ten-year period translocation into crops.
J. agric. food Chem., 18: 100-106.
LIDGATE, H.J. (1966) Earthworm control with chlordane.
J. Sports Turf Res. Inst., 42: 5-8.
LINDUSKA, J.P. & SURBER, E.W. (1948) Effect of DDT and other
insecticides on fish and wildlife. Summary of investigations
during 1947, Washington DC, US Department of the Interior,
Fish and Wildlife Service, 19 pp (Circular 15).
LONG, W.H., ANDERSON, H.L., ISA, A.L., & KYLE, M.L. (1967)
Sugarcane growth responses to chlordane and microarthropods
and effects of chlordane on soil fauna. J. econ. Entomol., 60:
623-629.
LORA, R.P., MARTEACHE, A.H., VILLAR, L.M.P., GIMENEZ, R.L.,
VILLAREJO, M.J., & PEREZ, J.I. (l979) [Organochlorine
pesticides in the milk of Spanish humans.] Rev. Esp. pediatr.,
35: 93 (in Spanish).
LUDEMANN, D. & NEUMANN, H. (1962) [On the effects of the
latest contact insecticide on fresh water animals.] Anz.
Schaelingskd., 35: 5-9 (in German).
LUDKE, J.L. (1976) Organochlorine pesticide residues
associated with mortality: additivity of chlordane and endrin.
Bull. environ. Contam. Toxicol., 16: 253-260.
MACEK, K.J., HUTCHINSON, C., & COPE, O.B. (1969) The effects
of temperature on the susceptibility of bluegills and rainbow
trout to selected pesticides. Bull. environ. Contam. Toxicol.,
4: 174-183.
MACMAHON, B. & WANG, H.H. (1982) A second follow-up of
mortality in a cohort of pesticide applicators, Cambridge,
Massachusetts, Harvard School of Public Health, Department of
Epidemiology.
MADHUKAR, B.V. & MATSUMURA, F. (1979) Comparison of
induction patterns of rat hepatic microsomal mixed-function
oxidases by pesticides and related chemicals. Pestic.
Biochem. Physiol., 11: 301-308.
MAGNANI, B., POWERS, C.D., WURSTER, C.F., & O'CONNORS, H.B.
(1978) Effects of chlordane and heptachlor on the marine dino
flagellate Exuviella baltica Lohmann. Bull. environ. Contam.
Toxicol., 20: 1-8.
MAGUIRE, J. & WATKIN, N. (1975) Carbonic anhydrase
inhibition. Bull. environ. Contam. Toxicol., 13: 625-629.
MAHON, D.C. (1977) Interactions, in rats, between carbon
tetrachloride-induced liver cirrhosis and chronic treatment
with the insecticide chlordane. Diss. Abstr. Int., 38: 4012B.
MAHON, D.C. & OLOFFS, P.C. (1979) Effects of sub-chronic
low-level dietary intake of chlordane on rats with cirrhosis
of the liver. J. environ. Sci. Health, B14: 227-246.
MAHON, D.C., NAIR, K.K., & OLOFFS, P.C. (1979) DNA in rat
hepatocyte nuclei: Effects of treatment with low levels of
carbon tetrachloride and/or chlordane. Can. J. Zool., 57:
1003-1009.
MASLANSKY, C.J. & WILLIAMS, G.M. (1981) Evidence for an
epigenetic mode of action in organochlorine pesticide
hepatocarcinogenicity: A lack of genotoxicity in rat, mouse
and hamster hepatocytes. J. Toxicol. environ. Health, 8:
121-130.
MASTRI, C., KEPLINGER, M.L., & FANCHER, O.E. (1969a) Acute
oral toxicity study on synthetic (X) in male and female
albino rats, Illinois, Industrial Bio-Test Laboratories
(Report for Velsicol Chemical Corporation).
MASTRI, C., KEPLINGER, M.L., & FANCHER, O.E. (1969b) Acute
oral toxicity study on 4 chlordenes in albino rats, Illinois,
Industrial Bio-Test Laboratories (Report for Velsicol Chemical
Corporation).
MASTRI, C., KEPLINGER, M.L., & FANCHER, O.E. (1969c) Acute
oral toxicity study on 2 chlordenes in female albino rats,
Illinois, Industrial Bio-Test Laboratories (Report for
Velsicol Chemical Corporation).
MATTRAW, H.C., Jr (1975) Occurrence of chlorinated hydro-
carbon insecticides, Southern Florida, 1968-1972. Pestic.
Monit. J., 9: 106-114.
MCCASKEY, T.A., STENP, A.R., LISKA, B.J., & STADELMAN, W.J.
(1968) Residues in egg yolks and raw and cooked tissues from
laying hens administered selected chlorinated hydrocarbon
insecticides. Poult. Sci., 47: 564-569.
MCCLEESE, D.W., BURRIDGE, L.E., & VAN DINTER, J. (1982)
Toxicities of five organochlorine compounds in water and
sediment to Nereis virens. Bull. environ. Contam. Toxicol.,
28: 216-220.
MCGILL, H.C. (1979) Pilot study of the effects of pesticides
on blood lipoproteins, arteries, and cardiac muscle of
baboons, Washington DC, US Environmental Protection Agency.
MEHRLE, P.M., JOHNSON, W.W., & MEYER, F.L. (1974)
Nutritional effects of chlordane toxicity to rainbow trout.
Bull. environ. Contam. Toxicol., 12: 513-517.
MEITH-AVCIN, N., WARLEN, S.M., & BARBER, R.T. (1973)
Organochlorine insecticide residues in a Bathyl-Demersal Fish
from 2500 metres. Environ. Lett., 5: 215-221.
MES, J. & DAVIES, D.J. (1978) Variation in the
polychlorinated biphenyl and organochlor pesticide residues
during human breastfeeding and its diurnal pattern.
Chemosphere, 7: 699-706.
MES, J., COFFIN, D.E., & CAMPBELL, D.T. (1974)
Polychlorinated biphenyl and organochlorine pesticide residues
in Canadian chicken eggs. Pestic. Monit. J., 8: 8-11.
MIYAZAKI, T., AKIYAMA, K., KANEKO, S., HORII, S., & YAMAGISHI,
T. (1980) Chlordane residues in human milk. Bull. environ.
Contam. Toxicol., 25: 518.
MOORE, R., TORO, E., STANTON, M., & KHAN, M.A.Q. (1977)
Absorption and elimination of 14C-alpha and gamma-chlordane by
a fresh-water alga, daphnid, and goldfish. Arch. environ.
Contam. Toxicol., 6: 411-420.
MORGAN, D.P. & ROAN, C.C. (1969) Renal function in persons
occupationally exposed to pesticides. Arch. environ. Health
19: 633-636.
NAKAS, J.P. (1977) Chlordane. Inhibition of endopeptidase
activity in the marine bacterium, Aeromonas proteolytica, New
Jersey, Rutgers State University, 123 pp (Dissertation).
NAQVI, S.M. (1973) Toxicity of twenty-three insecticides to
a tubificid worm Branchiura sowerbyi from the Mississippi
Delta. J. econ. Entomol., 66: 70-74.
NCI (1977) Bioassay of chlordane for possible
carcinogenicity, Bethesda, Maryland, National Cancer Institute
(CAS NO. 