
FAO Meeting Report No. PL/1965/10/1
WHO/Food Add./27.65
EVALUATION OF THE TOXICITY OF PESTICIDE RESIDUES IN FOOD
The content of this document is the result of the deliberations of the
Joint Meeting of the FAO Committee on Pesticides in Agriculture and
the WHO Expert Committee on Pesticide Residues, which met in Rome,
15-22 March 19651
Food and Agriculture Organization of the United Nations
World Health Organization
1965
1 Report of the second joint meeting of the FAO Committee on
Pesticides in Agriculture and the WHO Expert Committee on Pesticide
Residues, FAO Meeting Report No. PL/1965/10; WHO/Food Add./26.65
CHLORDANE
Chemical name
1,2,4,5,6,7,8,8-octachloro-3a,4,7,7a-tetrahydro-4,7,
methanoindane or
1,2,4,5,6,7,10,10-octachloro-4-7-8-9-tetrahydro-4,7-methyleneindane or
1,2,4,5,6,7,8,8-octachloro-2,3,3a,4,7,7a-hexahydro-4,7-methanoindane.
Synonyms
Toxichlor; Octachlorodihydro-dicyclopentadiene; Octachlor.
Empirical formula
C10H6Cl8
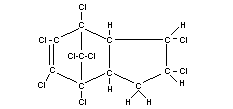
Structural formula
 BIOLOGICAL DATA
Biochemical aspects
Chlordane is absorbed from the alimentary tract. It is stored in
adipose tissue of sheep, goats, and cows and accumulates in the milk.
Cows' milk contained 0.1 to 0.2 ppm of chlordane after the animals had
been fed a diet containing a concentration equivalent to 0.36 to 0.42
mg/kg body-weight for 150 days (Carter et al., 1953). Some
water-soluble metabolites are excreted. Organically bound chlorine is
excreted in the urine of rabbits (Stohlman & Smith, 1950).
Both technical chlordane and one of its pure isomers
(gamma-chlordane) have been shown to have stimulating effects on rat
liver microsomes for the metabolism of certain drugs (Burns et al.,
1965; Hart & Fouts, 1963; Hart et al., 1963; Kutzman et al., 1964).
Acute toxicity
Animal Route LD50 mg/kg References
body-weight
Rat Oral 200-590* Ambrose et al., 1953
Ingle, 1954
Stohlman et al., 1950
Oral 335-430 Gaines, 1960
150-225 Ingle, 1954
Mouse Oral 430 US Food & Drug Admin., 1947
Rabbit Oral 100-300* Stohlman et al., 1950
20-40 Ingle, 1954
Goat Oral 180 Welch, 1948
Sheep Oral 500-1000 Welch, 1948
Chicken Oral 220-230 Turner & Eden, 1952
* The differences are explained by the use of different solvents, and
by the fact that the chlordane mentioned in the older literature
contained a considerable amount of the very toxic hexachlorocyclo
pentadiene (Ingle, 1954; Lehman, 1952).
Man. In adults a dose of 104 mg/kg proved fatal (Derbes et al.,
1955), An 18-year-old female showed convulsions but recovered after a
dose of approximately 30 mg/kg (amount retained after vomiting
estimated to be 10 mg/kg). In two infants respectively 15 months and 3
years of age, 10 and 40 mg/kg gave severe poisoning (Stormont &
Conley, 1955).
Short-term studies
Rat. When a diet containing 1000 ppm of chlordane was fed to 12
male rats, all of them died within 10 days. At 500 ppm 12/12 died
within 70 days, at 300 ppm 9/12 were alive after 100 days (Stohlman et
al., 1950).
Daily oral doses of 6.25-25 mg/kg given to 5 rats for 15 days
produced no tremors or convulsions, but daily doses of 50 mg/kg
produced toxic symptoms and 2 of the animals died. With 100 mg/kg all
the animals died (Ambrose et al., 1953). Intracytoplasmic bodies in
the liver-cells were found at all levels and their number was in
proportion to the dose used (Ambrose et al., 1953).
Rats in groups of 12 (6 females and 6 males) were fed for periods
up to 9 months, 2.5 ppm or 25 ppm of a sample of technical chlordane
containing 60-75% chlordane and 25-40% unrelated products.
Centrolobular cell hypertrophy, peripheral migration of cytoplasmic
granules and the presence of cytoplasmic bodies were observed in 1
male at 2.5 ppm and in 5 males at 12.5 ppm (Ortega et al., 1957).
Dog. Chlordane was given in varying oral doses to dogs for 7
days; convulsions were seen in 1 dog at 200 mg/kg (lowest dose) but
700 mg/kg (highest dose) did not produce any effect (Batte & Turk,
1948).
When 4 groups of 2 to 4 dogs were given chlordane orally in doses
between 5 and 80 mg/kg body-weight daily they all died within periods
of 25 days to 93 weeks (Lehman, 1952).
Sheep. Chlordane administered by stomach-tube to sheep in a
dose of 0.5 g/kg body-weight produced toxic symptom (incoordination,
partial blindness) in 5 to 6 days. A dose of 1 g/kg body-weight
produced severe respiratory and nervous symptoms at 16 hours and death
after 48 hours (Welch, 1948).
Long-term studies
Rat. In one experiment published in 1952, 24 rats (12 of each
sex) were given 2.5, 25 and 75 ppm of chlordane in the diet for 2
years. The sample of chlordane used had an LD50 of 450 mg/kg (Lehman,
1951). It was found that 25 and 75 ppm gave moderate to severe signs
of intoxication; 2.5 ppm still caused liver histological damage, the
nature of which has not been reported (Lehman, 1952).
In another experiment, groups of 40 rats (20 males and 20
females) were fed concentrations of 5, 10, 30, 150 and 300 ppm of
"technical chlordane" in the diet over a 2-year period. Throughout the
experiment tremors and convulsions appeared or could be induced at 30
or more ppm. Following fasting, no neurological symptoms appeared at 5
or 10 ppm. Growth rate was affected at 150 or 300 ppm. Liver
histological damage was observed in the form of hypertrophy of
centrolobular cells, cytoplasmic oxyphilia and hyalinization, nuclear
karyorhexis or cellular pyknosis, presence of fat in the cytoplasm and
some bile-duct proliferation. These changes were obvious at 150-300
ppm slight at 30 ppm, minimal at 10 ppm and absent at 5 ppm (Ingle,
1952).
In a subsequent experiment from the same laboratory, which was
carried on between late 1953 and late 1955, "technical chlordane of
recent manufacture" was used. Groups of 40 rats were given chlordane
at 2.5, 5, 10, 25, 50, 75, 150 or 300 ppm. A control group was given
no chlordane. Changes concerning food consumption, growth and
mortality were seen only in the 300 ppm group. Liver cell changes were
not present in the animals given 2.5-25 ppm. At 50 ppm only
"cytoplasmic peripheralization" was present. At higher doses the
changes were as those previously described (Ingle, 1955).
In a study published in 1953 a sample of chlordane exhibiting an
oral LD50 for the rat of 590 mg/kg was used. Groups of 5 rats of each
sex were given 0, 10, 20, 40, 80, 160, 320, 640 or 1280 ppm of
chlordane in their diets for approximately 407 days. The animals at
640 and 1280 ppm died early. At lower dosages, survival was
unaffected. Increased liver-weight (in comparison with the control
group) was observed over 320 ppm. In a sample of liver of a male at
320 ppm the average nuclear volume was 377µ3 compared to 268µ3 in a
control rat. Cytoplasmic vacuoles containing fat and clusters of
granules at the periphery of the cytoplasm were often seen. In the
males they were equivocal at 10 ppm, absent at 20 ppm and infrequent
at 40 ppm. In the females these lesions were common and were seen only
at 80 ppm and over (Ambrose et al., 1953).
Comments on experimental studies reported
It appears that at least in two reports concerning the rat a dose
level was found at which no histopathological changes occurred
(Ambrose et al., 1953; Ingle, 1955). Such dose levels appear to be in
the order of 20-25 ppm in the diet (1-1.25 mg/kg/day). This figure
applies to samples of "late" chlordane and would require clear
specifications concerning the formulations of chlordane falling into
this category. This cannot be done at the present time because of
incomplete information about chemical structure and toxicity of
impurities occurring in technical chlordane.
EVALUATION
Estimate of acceptable daily intake for man
Because:
(i) there is still some doubt about the composition of the chlordane
entering into commerce;
(ii) some metabolic problems, e.g. the effects of chlordane on the
hydroxylation of steroids, are not resolved (Burns et al., 1965;
Kutzman et al., 1964);
(iii) of the fact that, with one or two small exceptions, the animal
experiments have been limited to only one species, viz. the rat;
(iv) of the possible persistence of this compound in the environment,
the Committee considers that caution should still be exercised, and
that every effort should be made to see that the intake of chlordane
for man should be kept at the lowest possible level.
Further work required
Standardization of the technical product. Investigation on the
nature and toxicity of the residue occurring in the plant.
Determination of a maximum no-effect level in other species than the
rat. Long-term toxicity in other species than the rat. Reproduction
studies.
REFERENCES
Ambrose, A. M. Christensen, H., Robbins D. & Rather. L. (1953) Arch.
Industr. Hyg., 7, 197
Batte, E. G. & Turk, R. D. (1948) J. econ. Ent., 41, 102-103
Burns, J. J., Cucinell. S. A.. Koster, R. & Conney, A. H. (1965)
Ann. N.Y. Acad. Sci., 123, 273
Carter, R. H. et al. (1953) J. Dairy Science, 36, 1172
Dadey, J. L. & Kammer, A. G. (1953) J. Amer. med. Ass., 153, 723
Derbes, J. V. et al. (1955) J. Amer. med. Ass., 158, 1367
Gaines. T. G. (1960) Toxicol. Appl. Pharmacol., 2, 88
Hart, L. G. & Fouts, J. R. (1963) Proc. Soc. exp. Biol. (N.Y.),
114, 388
Hart, L. G., Shultice, R. W. & Fouts, J. R. (1963) Toxicol. Appl.
Pharmacol., 5, 371
Ingle, L. (1952) Arch. industr. Hyg., 6, 357
Ingle, L. (1954) Velsicol Special Bull., 309-7
Ingle, L. (1955) Data submitted by Velsicol Corporation to WHO in 1964
Kutzman, R., Jackobson, M., Schneidman, K. & Conney, A. H. (1964)
J. Pharmacol. exp. Ther., 146, 280
Lehman, A. J. (1951) Assoc. Food and Drug Officials Bull., 15, 122
Lehman, A. J. (1952) Quart. Bull. Assoc. Food and Drug Officials
U.S., 16, 47
Ortega, P., Hayes, W. J. & Durham, W. F. (1957) A.M.A. Arch. Path.,
64, 614
Stohlman, E. F. & Smith, M. I. (1950) Advances in Chem. Ser., 1,
228
Stohlman, E. F., Thorp, W. T. S. & Smith, M. I. (1950) Arch.
industr. Hyg., 1, 13-19
Stormont, R. T. & Conley, B. E. (1955) J. Amer. med. Ass., 158,
(15), 1364
Turner, H. F. & Eden, W. G. (1952) J. econ. Ent., 45, 130
US Food and Drug Administration (1947) Quarterly Report No. 3
Welch, H. (1948) J. econ. Entomol., 41, 36-39
BIOLOGICAL DATA
Biochemical aspects
Chlordane is absorbed from the alimentary tract. It is stored in
adipose tissue of sheep, goats, and cows and accumulates in the milk.
Cows' milk contained 0.1 to 0.2 ppm of chlordane after the animals had
been fed a diet containing a concentration equivalent to 0.36 to 0.42
mg/kg body-weight for 150 days (Carter et al., 1953). Some
water-soluble metabolites are excreted. Organically bound chlorine is
excreted in the urine of rabbits (Stohlman & Smith, 1950).
Both technical chlordane and one of its pure isomers
(gamma-chlordane) have been shown to have stimulating effects on rat
liver microsomes for the metabolism of certain drugs (Burns et al.,
1965; Hart & Fouts, 1963; Hart et al., 1963; Kutzman et al., 1964).
Acute toxicity
Animal Route LD50 mg/kg References
body-weight
Rat Oral 200-590* Ambrose et al., 1953
Ingle, 1954
Stohlman et al., 1950
Oral 335-430 Gaines, 1960
150-225 Ingle, 1954
Mouse Oral 430 US Food & Drug Admin., 1947
Rabbit Oral 100-300* Stohlman et al., 1950
20-40 Ingle, 1954
Goat Oral 180 Welch, 1948
Sheep Oral 500-1000 Welch, 1948
Chicken Oral 220-230 Turner & Eden, 1952
* The differences are explained by the use of different solvents, and
by the fact that the chlordane mentioned in the older literature
contained a considerable amount of the very toxic hexachlorocyclo
pentadiene (Ingle, 1954; Lehman, 1952).
Man. In adults a dose of 104 mg/kg proved fatal (Derbes et al.,
1955), An 18-year-old female showed convulsions but recovered after a
dose of approximately 30 mg/kg (amount retained after vomiting
estimated to be 10 mg/kg). In two infants respectively 15 months and 3
years of age, 10 and 40 mg/kg gave severe poisoning (Stormont &
Conley, 1955).
Short-term studies
Rat. When a diet containing 1000 ppm of chlordane was fed to 12
male rats, all of them died within 10 days. At 500 ppm 12/12 died
within 70 days, at 300 ppm 9/12 were alive after 100 days (Stohlman et
al., 1950).
Daily oral doses of 6.25-25 mg/kg given to 5 rats for 15 days
produced no tremors or convulsions, but daily doses of 50 mg/kg
produced toxic symptoms and 2 of the animals died. With 100 mg/kg all
the animals died (Ambrose et al., 1953). Intracytoplasmic bodies in
the liver-cells were found at all levels and their number was in
proportion to the dose used (Ambrose et al., 1953).
Rats in groups of 12 (6 females and 6 males) were fed for periods
up to 9 months, 2.5 ppm or 25 ppm of a sample of technical chlordane
containing 60-75% chlordane and 25-40% unrelated products.
Centrolobular cell hypertrophy, peripheral migration of cytoplasmic
granules and the presence of cytoplasmic bodies were observed in 1
male at 2.5 ppm and in 5 males at 12.5 ppm (Ortega et al., 1957).
Dog. Chlordane was given in varying oral doses to dogs for 7
days; convulsions were seen in 1 dog at 200 mg/kg (lowest dose) but
700 mg/kg (highest dose) did not produce any effect (Batte & Turk,
1948).
When 4 groups of 2 to 4 dogs were given chlordane orally in doses
between 5 and 80 mg/kg body-weight daily they all died within periods
of 25 days to 93 weeks (Lehman, 1952).
Sheep. Chlordane administered by stomach-tube to sheep in a
dose of 0.5 g/kg body-weight produced toxic symptom (incoordination,
partial blindness) in 5 to 6 days. A dose of 1 g/kg body-weight
produced severe respiratory and nervous symptoms at 16 hours and death
after 48 hours (Welch, 1948).
Long-term studies
Rat. In one experiment published in 1952, 24 rats (12 of each
sex) were given 2.5, 25 and 75 ppm of chlordane in the diet for 2
years. The sample of chlordane used had an LD50 of 450 mg/kg (Lehman,
1951). It was found that 25 and 75 ppm gave moderate to severe signs
of intoxication; 2.5 ppm still caused liver histological damage, the
nature of which has not been reported (Lehman, 1952).
In another experiment, groups of 40 rats (20 males and 20
females) were fed concentrations of 5, 10, 30, 150 and 300 ppm of
"technical chlordane" in the diet over a 2-year period. Throughout the
experiment tremors and convulsions appeared or could be induced at 30
or more ppm. Following fasting, no neurological symptoms appeared at 5
or 10 ppm. Growth rate was affected at 150 or 300 ppm. Liver
histological damage was observed in the form of hypertrophy of
centrolobular cells, cytoplasmic oxyphilia and hyalinization, nuclear
karyorhexis or cellular pyknosis, presence of fat in the cytoplasm and
some bile-duct proliferation. These changes were obvious at 150-300
ppm slight at 30 ppm, minimal at 10 ppm and absent at 5 ppm (Ingle,
1952).
In a subsequent experiment from the same laboratory, which was
carried on between late 1953 and late 1955, "technical chlordane of
recent manufacture" was used. Groups of 40 rats were given chlordane
at 2.5, 5, 10, 25, 50, 75, 150 or 300 ppm. A control group was given
no chlordane. Changes concerning food consumption, growth and
mortality were seen only in the 300 ppm group. Liver cell changes were
not present in the animals given 2.5-25 ppm. At 50 ppm only
"cytoplasmic peripheralization" was present. At higher doses the
changes were as those previously described (Ingle, 1955).
In a study published in 1953 a sample of chlordane exhibiting an
oral LD50 for the rat of 590 mg/kg was used. Groups of 5 rats of each
sex were given 0, 10, 20, 40, 80, 160, 320, 640 or 1280 ppm of
chlordane in their diets for approximately 407 days. The animals at
640 and 1280 ppm died early. At lower dosages, survival was
unaffected. Increased liver-weight (in comparison with the control
group) was observed over 320 ppm. In a sample of liver of a male at
320 ppm the average nuclear volume was 377µ3 compared to 268µ3 in a
control rat. Cytoplasmic vacuoles containing fat and clusters of
granules at the periphery of the cytoplasm were often seen. In the
males they were equivocal at 10 ppm, absent at 20 ppm and infrequent
at 40 ppm. In the females these lesions were common and were seen only
at 80 ppm and over (Ambrose et al., 1953).
Comments on experimental studies reported
It appears that at least in two reports concerning the rat a dose
level was found at which no histopathological changes occurred
(Ambrose et al., 1953; Ingle, 1955). Such dose levels appear to be in
the order of 20-25 ppm in the diet (1-1.25 mg/kg/day). This figure
applies to samples of "late" chlordane and would require clear
specifications concerning the formulations of chlordane falling into
this category. This cannot be done at the present time because of
incomplete information about chemical structure and toxicity of
impurities occurring in technical chlordane.
EVALUATION
Estimate of acceptable daily intake for man
Because:
(i) there is still some doubt about the composition of the chlordane
entering into commerce;
(ii) some metabolic problems, e.g. the effects of chlordane on the
hydroxylation of steroids, are not resolved (Burns et al., 1965;
Kutzman et al., 1964);
(iii) of the fact that, with one or two small exceptions, the animal
experiments have been limited to only one species, viz. the rat;
(iv) of the possible persistence of this compound in the environment,
the Committee considers that caution should still be exercised, and
that every effort should be made to see that the intake of chlordane
for man should be kept at the lowest possible level.
Further work required
Standardization of the technical product. Investigation on the
nature and toxicity of the residue occurring in the plant.
Determination of a maximum no-effect level in other species than the
rat. Long-term toxicity in other species than the rat. Reproduction
studies.
REFERENCES
Ambrose, A. M. Christensen, H., Robbins D. & Rather. L. (1953) Arch.
Industr. Hyg., 7, 197
Batte, E. G. & Turk, R. D. (1948) J. econ. Ent., 41, 102-103
Burns, J. J., Cucinell. S. A.. Koster, R. & Conney, A. H. (1965)
Ann. N.Y. Acad. Sci., 123, 273
Carter, R. H. et al. (1953) J. Dairy Science, 36, 1172
Dadey, J. L. & Kammer, A. G. (1953) J. Amer. med. Ass., 153, 723
Derbes, J. V. et al. (1955) J. Amer. med. Ass., 158, 1367
Gaines. T. G. (1960) Toxicol. Appl. Pharmacol., 2, 88
Hart, L. G. & Fouts, J. R. (1963) Proc. Soc. exp. Biol. (N.Y.),
114, 388
Hart, L. G., Shultice, R. W. & Fouts, J. R. (1963) Toxicol. Appl.
Pharmacol., 5, 371
Ingle, L. (1952) Arch. industr. Hyg., 6, 357
Ingle, L. (1954) Velsicol Special Bull., 309-7
Ingle, L. (1955) Data submitted by Velsicol Corporation to WHO in 1964
Kutzman, R., Jackobson, M., Schneidman, K. & Conney, A. H. (1964)
J. Pharmacol. exp. Ther., 146, 280
Lehman, A. J. (1951) Assoc. Food and Drug Officials Bull., 15, 122
Lehman, A. J. (1952) Quart. Bull. Assoc. Food and Drug Officials
U.S., 16, 47
Ortega, P., Hayes, W. J. & Durham, W. F. (1957) A.M.A. Arch. Path.,
64, 614
Stohlman, E. F. & Smith, M. I. (1950) Advances in Chem. Ser., 1,
228
Stohlman, E. F., Thorp, W. T. S. & Smith, M. I. (1950) Arch.
industr. Hyg., 1, 13-19
Stormont, R. T. & Conley, B. E. (1955) J. Amer. med. Ass., 158,
(15), 1364
Turner, H. F. & Eden, W. G. (1952) J. econ. Ent., 45, 130
US Food and Drug Administration (1947) Quarterly Report No. 3
Welch, H. (1948) J. econ. Entomol., 41, 36-39
 BIOLOGICAL DATA
Biochemical aspects
Chlordane is absorbed from the alimentary tract. It is stored in
adipose tissue of sheep, goats, and cows and accumulates in the milk.
Cows' milk contained 0.1 to 0.2 ppm of chlordane after the animals had
been fed a diet containing a concentration equivalent to 0.36 to 0.42
mg/kg body-weight for 150 days (Carter et al., 1953). Some
water-soluble metabolites are excreted. Organically bound chlorine is
excreted in the urine of rabbits (Stohlman & Smith, 1950).
Both technical chlordane and one of its pure isomers
(gamma-chlordane) have been shown to have stimulating effects on rat
liver microsomes for the metabolism of certain drugs (Burns et al.,
1965; Hart & Fouts, 1963; Hart et al., 1963; Kutzman et al., 1964).
Acute toxicity
Animal Route LD50 mg/kg References
body-weight
Rat Oral 200-590* Ambrose et al., 1953
Ingle, 1954
Stohlman et al., 1950
Oral 335-430 Gaines, 1960
150-225 Ingle, 1954
Mouse Oral 430 US Food & Drug Admin., 1947
Rabbit Oral 100-300* Stohlman et al., 1950
20-40 Ingle, 1954
Goat Oral 180 Welch, 1948
Sheep Oral 500-1000 Welch, 1948
Chicken Oral 220-230 Turner & Eden, 1952
* The differences are explained by the use of different solvents, and
by the fact that the chlordane mentioned in the older literature
contained a considerable amount of the very toxic hexachlorocyclo
pentadiene (Ingle, 1954; Lehman, 1952).
Man. In adults a dose of 104 mg/kg proved fatal (Derbes et al.,
1955), An 18-year-old female showed convulsions but recovered after a
dose of approximately 30 mg/kg (amount retained after vomiting
estimated to be 10 mg/kg). In two infants respectively 15 months and 3
years of age, 10 and 40 mg/kg gave severe poisoning (Stormont &
Conley, 1955).
Short-term studies
Rat. When a diet containing 1000 ppm of chlordane was fed to 12
male rats, all of them died within 10 days. At 500 ppm 12/12 died
within 70 days, at 300 ppm 9/12 were alive after 100 days (Stohlman et
al., 1950).
Daily oral doses of 6.25-25 mg/kg given to 5 rats for 15 days
produced no tremors or convulsions, but daily doses of 50 mg/kg
produced toxic symptoms and 2 of the animals died. With 100 mg/kg all
the animals died (Ambrose et al., 1953). Intracytoplasmic bodies in
the liver-cells were found at all levels and their number was in
proportion to the dose used (Ambrose et al., 1953).
Rats in groups of 12 (6 females and 6 males) were fed for periods
up to 9 months, 2.5 ppm or 25 ppm of a sample of technical chlordane
containing 60-75% chlordane and 25-40% unrelated products.
Centrolobular cell hypertrophy, peripheral migration of cytoplasmic
granules and the presence of cytoplasmic bodies were observed in 1
male at 2.5 ppm and in 5 males at 12.5 ppm (Ortega et al., 1957).
Dog. Chlordane was given in varying oral doses to dogs for 7
days; convulsions were seen in 1 dog at 200 mg/kg (lowest dose) but
700 mg/kg (highest dose) did not produce any effect (Batte & Turk,
1948).
When 4 groups of 2 to 4 dogs were given chlordane orally in doses
between 5 and 80 mg/kg body-weight daily they all died within periods
of 25 days to 93 weeks (Lehman, 1952).
Sheep. Chlordane administered by stomach-tube to sheep in a
dose of 0.5 g/kg body-weight produced toxic symptom (incoordination,
partial blindness) in 5 to 6 days. A dose of 1 g/kg body-weight
produced severe respiratory and nervous symptoms at 16 hours and death
after 48 hours (Welch, 1948).
Long-term studies
Rat. In one experiment published in 1952, 24 rats (12 of each
sex) were given 2.5, 25 and 75 ppm of chlordane in the diet for 2
years. The sample of chlordane used had an LD50 of 450 mg/kg (Lehman,
1951). It was found that 25 and 75 ppm gave moderate to severe signs
of intoxication; 2.5 ppm still caused liver histological damage, the
nature of which has not been reported (Lehman, 1952).
In another experiment, groups of 40 rats (20 males and 20
females) were fed concentrations of 5, 10, 30, 150 and 300 ppm of
"technical chlordane" in the diet over a 2-year period. Throughout the
experiment tremors and convulsions appeared or could be induced at 30
or more ppm. Following fasting, no neurological symptoms appeared at 5
or 10 ppm. Growth rate was affected at 150 or 300 ppm. Liver
histological damage was observed in the form of hypertrophy of
centrolobular cells, cytoplasmic oxyphilia and hyalinization, nuclear
karyorhexis or cellular pyknosis, presence of fat in the cytoplasm and
some bile-duct proliferation. These changes were obvious at 150-300
ppm slight at 30 ppm, minimal at 10 ppm and absent at 5 ppm (Ingle,
1952).
In a subsequent experiment from the same laboratory, which was
carried on between late 1953 and late 1955, "technical chlordane of
recent manufacture" was used. Groups of 40 rats were given chlordane
at 2.5, 5, 10, 25, 50, 75, 150 or 300 ppm. A control group was given
no chlordane. Changes concerning food consumption, growth and
mortality were seen only in the 300 ppm group. Liver cell changes were
not present in the animals given 2.5-25 ppm. At 50 ppm only
"cytoplasmic peripheralization" was present. At higher doses the
changes were as those previously described (Ingle, 1955).
In a study published in 1953 a sample of chlordane exhibiting an
oral LD50 for the rat of 590 mg/kg was used. Groups of 5 rats of each
sex were given 0, 10, 20, 40, 80, 160, 320, 640 or 1280 ppm of
chlordane in their diets for approximately 407 days. The animals at
640 and 1280 ppm died early. At lower dosages, survival was
unaffected. Increased liver-weight (in comparison with the control
group) was observed over 320 ppm. In a sample of liver of a male at
320 ppm the average nuclear volume was 377µ3 compared to 268µ3 in a
control rat. Cytoplasmic vacuoles containing fat and clusters of
granules at the periphery of the cytoplasm were often seen. In the
males they were equivocal at 10 ppm, absent at 20 ppm and infrequent
at 40 ppm. In the females these lesions were common and were seen only
at 80 ppm and over (Ambrose et al., 1953).
Comments on experimental studies reported
It appears that at least in two reports concerning the rat a dose
level was found at which no histopathological changes occurred
(Ambrose et al., 1953; Ingle, 1955). Such dose levels appear to be in
the order of 20-25 ppm in the diet (1-1.25 mg/kg/day). This figure
applies to samples of "late" chlordane and would require clear
specifications concerning the formulations of chlordane falling into
this category. This cannot be done at the present time because of
incomplete information about chemical structure and toxicity of
impurities occurring in technical chlordane.
EVALUATION
Estimate of acceptable daily intake for man
Because:
(i) there is still some doubt about the composition of the chlordane
entering into commerce;
(ii) some metabolic problems, e.g. the effects of chlordane on the
hydroxylation of steroids, are not resolved (Burns et al., 1965;
Kutzman et al., 1964);
(iii) of the fact that, with one or two small exceptions, the animal
experiments have been limited to only one species, viz. the rat;
(iv) of the possible persistence of this compound in the environment,
the Committee considers that caution should still be exercised, and
that every effort should be made to see that the intake of chlordane
for man should be kept at the lowest possible level.
Further work required
Standardization of the technical product. Investigation on the
nature and toxicity of the residue occurring in the plant.
Determination of a maximum no-effect level in other species than the
rat. Long-term toxicity in other species than the rat. Reproduction
studies.
REFERENCES
Ambrose, A. M. Christensen, H., Robbins D. & Rather. L. (1953) Arch.
Industr. Hyg., 7, 197
Batte, E. G. & Turk, R. D. (1948) J. econ. Ent., 41, 102-103
Burns, J. J., Cucinell. S. A.. Koster, R. & Conney, A. H. (1965)
Ann. N.Y. Acad. Sci., 123, 273
Carter, R. H. et al. (1953) J. Dairy Science, 36, 1172
Dadey, J. L. & Kammer, A. G. (1953) J. Amer. med. Ass., 153, 723
Derbes, J. V. et al. (1955) J. Amer. med. Ass., 158, 1367
Gaines. T. G. (1960) Toxicol. Appl. Pharmacol., 2, 88
Hart, L. G. & Fouts, J. R. (1963) Proc. Soc. exp. Biol. (N.Y.),
114, 388
Hart, L. G., Shultice, R. W. & Fouts, J. R. (1963) Toxicol. Appl.
Pharmacol., 5, 371
Ingle, L. (1952) Arch. industr. Hyg., 6, 357
Ingle, L. (1954) Velsicol Special Bull., 309-7
Ingle, L. (1955) Data submitted by Velsicol Corporation to WHO in 1964
Kutzman, R., Jackobson, M., Schneidman, K. & Conney, A. H. (1964)
J. Pharmacol. exp. Ther., 146, 280
Lehman, A. J. (1951) Assoc. Food and Drug Officials Bull., 15, 122
Lehman, A. J. (1952) Quart. Bull. Assoc. Food and Drug Officials
U.S., 16, 47
Ortega, P., Hayes, W. J. & Durham, W. F. (1957) A.M.A. Arch. Path.,
64, 614
Stohlman, E. F. & Smith, M. I. (1950) Advances in Chem. Ser., 1,
228
Stohlman, E. F., Thorp, W. T. S. & Smith, M. I. (1950) Arch.
industr. Hyg., 1, 13-19
Stormont, R. T. & Conley, B. E. (1955) J. Amer. med. Ass., 158,
(15), 1364
Turner, H. F. & Eden, W. G. (1952) J. econ. Ent., 45, 130
US Food and Drug Administration (1947) Quarterly Report No. 3
Welch, H. (1948) J. econ. Entomol., 41, 36-39
BIOLOGICAL DATA
Biochemical aspects
Chlordane is absorbed from the alimentary tract. It is stored in
adipose tissue of sheep, goats, and cows and accumulates in the milk.
Cows' milk contained 0.1 to 0.2 ppm of chlordane after the animals had
been fed a diet containing a concentration equivalent to 0.36 to 0.42
mg/kg body-weight for 150 days (Carter et al., 1953). Some
water-soluble metabolites are excreted. Organically bound chlorine is
excreted in the urine of rabbits (Stohlman & Smith, 1950).
Both technical chlordane and one of its pure isomers
(gamma-chlordane) have been shown to have stimulating effects on rat
liver microsomes for the metabolism of certain drugs (Burns et al.,
1965; Hart & Fouts, 1963; Hart et al., 1963; Kutzman et al., 1964).
Acute toxicity
Animal Route LD50 mg/kg References
body-weight
Rat Oral 200-590* Ambrose et al., 1953
Ingle, 1954
Stohlman et al., 1950
Oral 335-430 Gaines, 1960
150-225 Ingle, 1954
Mouse Oral 430 US Food & Drug Admin., 1947
Rabbit Oral 100-300* Stohlman et al., 1950
20-40 Ingle, 1954
Goat Oral 180 Welch, 1948
Sheep Oral 500-1000 Welch, 1948
Chicken Oral 220-230 Turner & Eden, 1952
* The differences are explained by the use of different solvents, and
by the fact that the chlordane mentioned in the older literature
contained a considerable amount of the very toxic hexachlorocyclo
pentadiene (Ingle, 1954; Lehman, 1952).
Man. In adults a dose of 104 mg/kg proved fatal (Derbes et al.,
1955), An 18-year-old female showed convulsions but recovered after a
dose of approximately 30 mg/kg (amount retained after vomiting
estimated to be 10 mg/kg). In two infants respectively 15 months and 3
years of age, 10 and 40 mg/kg gave severe poisoning (Stormont &
Conley, 1955).
Short-term studies
Rat. When a diet containing 1000 ppm of chlordane was fed to 12
male rats, all of them died within 10 days. At 500 ppm 12/12 died
within 70 days, at 300 ppm 9/12 were alive after 100 days (Stohlman et
al., 1950).
Daily oral doses of 6.25-25 mg/kg given to 5 rats for 15 days
produced no tremors or convulsions, but daily doses of 50 mg/kg
produced toxic symptoms and 2 of the animals died. With 100 mg/kg all
the animals died (Ambrose et al., 1953). Intracytoplasmic bodies in
the liver-cells were found at all levels and their number was in
proportion to the dose used (Ambrose et al., 1953).
Rats in groups of 12 (6 females and 6 males) were fed for periods
up to 9 months, 2.5 ppm or 25 ppm of a sample of technical chlordane
containing 60-75% chlordane and 25-40% unrelated products.
Centrolobular cell hypertrophy, peripheral migration of cytoplasmic
granules and the presence of cytoplasmic bodies were observed in 1
male at 2.5 ppm and in 5 males at 12.5 ppm (Ortega et al., 1957).
Dog. Chlordane was given in varying oral doses to dogs for 7
days; convulsions were seen in 1 dog at 200 mg/kg (lowest dose) but
700 mg/kg (highest dose) did not produce any effect (Batte & Turk,
1948).
When 4 groups of 2 to 4 dogs were given chlordane orally in doses
between 5 and 80 mg/kg body-weight daily they all died within periods
of 25 days to 93 weeks (Lehman, 1952).
Sheep. Chlordane administered by stomach-tube to sheep in a
dose of 0.5 g/kg body-weight produced toxic symptom (incoordination,
partial blindness) in 5 to 6 days. A dose of 1 g/kg body-weight
produced severe respiratory and nervous symptoms at 16 hours and death
after 48 hours (Welch, 1948).
Long-term studies
Rat. In one experiment published in 1952, 24 rats (12 of each
sex) were given 2.5, 25 and 75 ppm of chlordane in the diet for 2
years. The sample of chlordane used had an LD50 of 450 mg/kg (Lehman,
1951). It was found that 25 and 75 ppm gave moderate to severe signs
of intoxication; 2.5 ppm still caused liver histological damage, the
nature of which has not been reported (Lehman, 1952).
In another experiment, groups of 40 rats (20 males and 20
females) were fed concentrations of 5, 10, 30, 150 and 300 ppm of
"technical chlordane" in the diet over a 2-year period. Throughout the
experiment tremors and convulsions appeared or could be induced at 30
or more ppm. Following fasting, no neurological symptoms appeared at 5
or 10 ppm. Growth rate was affected at 150 or 300 ppm. Liver
histological damage was observed in the form of hypertrophy of
centrolobular cells, cytoplasmic oxyphilia and hyalinization, nuclear
karyorhexis or cellular pyknosis, presence of fat in the cytoplasm and
some bile-duct proliferation. These changes were obvious at 150-300
ppm slight at 30 ppm, minimal at 10 ppm and absent at 5 ppm (Ingle,
1952).
In a subsequent experiment from the same laboratory, which was
carried on between late 1953 and late 1955, "technical chlordane of
recent manufacture" was used. Groups of 40 rats were given chlordane
at 2.5, 5, 10, 25, 50, 75, 150 or 300 ppm. A control group was given
no chlordane. Changes concerning food consumption, growth and
mortality were seen only in the 300 ppm group. Liver cell changes were
not present in the animals given 2.5-25 ppm. At 50 ppm only
"cytoplasmic peripheralization" was present. At higher doses the
changes were as those previously described (Ingle, 1955).
In a study published in 1953 a sample of chlordane exhibiting an
oral LD50 for the rat of 590 mg/kg was used. Groups of 5 rats of each
sex were given 0, 10, 20, 40, 80, 160, 320, 640 or 1280 ppm of
chlordane in their diets for approximately 407 days. The animals at
640 and 1280 ppm died early. At lower dosages, survival was
unaffected. Increased liver-weight (in comparison with the control
group) was observed over 320 ppm. In a sample of liver of a male at
320 ppm the average nuclear volume was 377µ3 compared to 268µ3 in a
control rat. Cytoplasmic vacuoles containing fat and clusters of
granules at the periphery of the cytoplasm were often seen. In the
males they were equivocal at 10 ppm, absent at 20 ppm and infrequent
at 40 ppm. In the females these lesions were common and were seen only
at 80 ppm and over (Ambrose et al., 1953).
Comments on experimental studies reported
It appears that at least in two reports concerning the rat a dose
level was found at which no histopathological changes occurred
(Ambrose et al., 1953; Ingle, 1955). Such dose levels appear to be in
the order of 20-25 ppm in the diet (1-1.25 mg/kg/day). This figure
applies to samples of "late" chlordane and would require clear
specifications concerning the formulations of chlordane falling into
this category. This cannot be done at the present time because of
incomplete information about chemical structure and toxicity of
impurities occurring in technical chlordane.
EVALUATION
Estimate of acceptable daily intake for man
Because:
(i) there is still some doubt about the composition of the chlordane
entering into commerce;
(ii) some metabolic problems, e.g. the effects of chlordane on the
hydroxylation of steroids, are not resolved (Burns et al., 1965;
Kutzman et al., 1964);
(iii) of the fact that, with one or two small exceptions, the animal
experiments have been limited to only one species, viz. the rat;
(iv) of the possible persistence of this compound in the environment,
the Committee considers that caution should still be exercised, and
that every effort should be made to see that the intake of chlordane
for man should be kept at the lowest possible level.
Further work required
Standardization of the technical product. Investigation on the
nature and toxicity of the residue occurring in the plant.
Determination of a maximum no-effect level in other species than the
rat. Long-term toxicity in other species than the rat. Reproduction
studies.
REFERENCES
Ambrose, A. M. Christensen, H., Robbins D. & Rather. L. (1953) Arch.
Industr. Hyg., 7, 197
Batte, E. G. & Turk, R. D. (1948) J. econ. Ent., 41, 102-103
Burns, J. J., Cucinell. S. A.. Koster, R. & Conney, A. H. (1965)
Ann. N.Y. Acad. Sci., 123, 273
Carter, R. H. et al. (1953) J. Dairy Science, 36, 1172
Dadey, J. L. & Kammer, A. G. (1953) J. Amer. med. Ass., 153, 723
Derbes, J. V. et al. (1955) J. Amer. med. Ass., 158, 1367
Gaines. T. G. (1960) Toxicol. Appl. Pharmacol., 2, 88
Hart, L. G. & Fouts, J. R. (1963) Proc. Soc. exp. Biol. (N.Y.),
114, 388
Hart, L. G., Shultice, R. W. & Fouts, J. R. (1963) Toxicol. Appl.
Pharmacol., 5, 371
Ingle, L. (1952) Arch. industr. Hyg., 6, 357
Ingle, L. (1954) Velsicol Special Bull., 309-7
Ingle, L. (1955) Data submitted by Velsicol Corporation to WHO in 1964
Kutzman, R., Jackobson, M., Schneidman, K. & Conney, A. H. (1964)
J. Pharmacol. exp. Ther., 146, 280
Lehman, A. J. (1951) Assoc. Food and Drug Officials Bull., 15, 122
Lehman, A. J. (1952) Quart. Bull. Assoc. Food and Drug Officials
U.S., 16, 47
Ortega, P., Hayes, W. J. & Durham, W. F. (1957) A.M.A. Arch. Path.,
64, 614
Stohlman, E. F. & Smith, M. I. (1950) Advances in Chem. Ser., 1,
228
Stohlman, E. F., Thorp, W. T. S. & Smith, M. I. (1950) Arch.
industr. Hyg., 1, 13-19
Stormont, R. T. & Conley, B. E. (1955) J. Amer. med. Ass., 158,
(15), 1364
Turner, H. F. & Eden, W. G. (1952) J. econ. Ent., 45, 130
US Food and Drug Administration (1947) Quarterly Report No. 3
Welch, H. (1948) J. econ. Entomol., 41, 36-39
