
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
ENVIRONMENTAL HEALTH CRITERIA 94
PERMETHRIN
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization.
Published under the joint sponsorship of
the United Nations Environment Programme,
the International Labour Organisation,
and the World Health Organization
World Health Orgnization
Geneva, 1990
The International Programme on Chemical Safety (IPCS) is a
joint venture of the United Nations Environment Programme, the
International Labour Organisation, and the World Health
Organization. The main objective of the IPCS is to carry out and
disseminate evaluations of the effects of chemicals on human health
and the quality of the environment. Supporting activities include
the development of epidemiological, experimental laboratory, and
risk-assessment methods that could produce internationally
comparable results, and the development of manpower in the field of
toxicology. Other activities carried out by the IPCS include the
development of know-how for coping with chemical accidents,
coordination of laboratory testing and epidemiological studies, and
promotion of research on the mechanisms of the biological action of
chemicals.
WHO Library Cataloguing in Publication Data
Permethrin.
(Environmental health criteria ; 94)
1.Pyrethrins. I.Series
ISBN 92 4 154294 2 (NLM Classification: WA 240)
ISSN 0250-863X
The World Health Organization welcomes requests for permission
to reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made
to the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 1990
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved.
The designations employed and the presentation of the material
in this publication do not imply the expression of any opinion
whatsoever on the part of the Secretariat of the World Health
Organization concerning the legal status of any country, territory,
city or area or of its authorities, or concerning the delimitation
of its frontiers or boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar
nature that are not mentioned. Errors and omissions excepted, the
names of proprietary products are distinguished by initial capital
letters.
CONTENTS
ENVIRONMENTAL HEALTH CRITERIA FOR PERMETHRIN
INTRODUCTION
1. SUMMARY, EVALUATION, CONCLUSIONS, AND RECOMMENDATION
1.1. Summary and evaluation
1.1.1. Identity, physical and chemical properties, analytical
methods
1.1.2. Production and use
1.1.3. Human exposure
1.1.4. Environmental fate
1.1.5. Kinetics and metabolism
1.1.6. Effects on organisms in the environment
1.1.7. Effects on experimental animals and in vitro test systems
1.1.8. Effects on human beings
1.2. Conclusions
1.2.1. General population
1.2.2. Occupational exposure
1.2.3. Environment
1.3. Recommendations
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1. Chemical identity
2.2. Physical and chemical properties
2.3. Analytical methods
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE; ENVIRONMENTAL LEVELS
3.1. Industrial production
3.2. Use pattern
3.3. Residues in food and other products
3.4. Residues in the environment
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
4.1. Transport and distribution between media
4.2. Photodecomposition
4.3. Degradation in plants
4.4. Degradation in soils
5. KINETICS AND METABOLISM
5.1. Metabolism in mammals
5.1.1. Mouse
5.1.2. Rat
5.1.3. Goat
5.1.4. Cow
5.1.5. Man
5.2. Metabolism in hens
5.3. Enzymatic systems for biotransformation
6. EFFECTS ON THE ENVIRONMENT
6.1. Toxicity to aquatic organisms
6.1.1. Aquatic microorganisms
6.1.2. Aquatic invertebrates
6.1.3. Fish
6.1.4. Field studies and community effects
6.2. Toxicity to terrestrial organisms
6.2.1. Soil microorganisms
6.2.2. Terrestrial invertebrates
6.2.3. Birds
6.2.4. Mammals
6.3. Uptake, loss, bioaccumulation, and biomagnification
7. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
7.1. Acute toxicity
7.2. Subacute and subchronic toxicity
7.2.1. Oral exposure
7.2.1.1 Mouse
7.2.1.2 Rat
7.2.1.3 Dog
7.2.1.4 Rabbit
7.2.1.5 Cow
7.2.2. Dermal exposure
7.2.3. Inhalation exposure
7.3. Primary irritation
7.3.1. Skin irritation
7.3.2. Eye irritation
7.4. Sensitization
7.5. Long-term toxicity
7.5.1. Mouse
7.5.2. Rat
7.6. Carcinogenesis
7.6.1. Mouse
7.6.1.1 ICI study
7.6.1.2 FMC II study
7.6.1.3 BW study
7.6.1.4 Appraisal of mouse studies on carcinogenicity
7.6.2. Rat
7.6.2.1 ICI study
7.6.2.2 BW study
7.6.2.3 Appraisal of rat studies on carcinogenicity
7.7. Mutagenicity
7.7.1. Microorganism and insects
7.7.2. Mammals
7.8. Teratogenicity and reproduction studies
7.8.1. Teratogenicity studies
7.8.1.1 Mouse
7.8.1.2 Rat
7.8.1.3 Rabbit
7.8.2. Reproduction studies
7.8.2.1 Rat
7.9. Neurotoxicity
7.9.1. Rat
7.9.2. Hen
7.10. Behavioural effects
7.11. Miscellaneous studies
7.12. Mechanism of toxicity (mode of action)
8. EFFECTS ON HUMANS
8.1. Occupational exposure
8.2. Clinical studies
9. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
REFERENCES
APPENDIX I
FRENCH TRANSLATION OF SUMMARY, EVALUATION, CONCLUSIONS, AND RECOMMENDATIONS
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR PERMETHRIN
Members
Dr V. Benes, Department of Toxicology and Reference Laboratory, Insti-
tute of Hygiene and Epidemiology, Prague, Czechoslovakia
Dr S. Dobson, Institute of Terrestrial Ecology, Monks Wood Experimental
Station, Huntingdon, United Kingdom
Dr Y. Hayashi, Division of Pathology, National Institute of Hygienic
Sciences, Tokyo, Japan
Dr S. Johnson, Hazard Evaluation Division, Office of Pesticide Pro-
gramme, US Environmental Protection Agency, Washington DC, USA
(Chairman)
Dr S.K. Kashyap, National Institute of Occupational Health (ICMR)
Ahmedabad, India (Vice-Chairman)
Dr Yu. I. Kundiev, Research Institute of Labour, Hygiene, and Occu-
pational Diseases, Kiev, USSR
Dr J.P. Leahey, ICI Agrochemicals, Jealotts Hill Research Station,
Bracknell, United Kingdom (Rapporteur)
Dr J. Miyamoto, Takarazuka Research Centre, Sumitomo Chemical Company,
Takarazuka, Hyogo, Japan
Dr Y. Takenaka, Division of Information on Chemical Safety, National
Institute of Hygienic Sciences, Tokyo, Japan
Representatives of Other Organizations
Dr M. Ikeda, International Commission on Occupational Health. Depart-
ment of Environmental Health, Tohoku University, School of Medicine,
Sendai, Japan
Dr H. Naito, World Federation of Poison Control Centres and Clinical
Toxicology. Institute of Clinical Medicine, University of Tsukuba,
Tsukuba-Shi, Ibaraki, Japan
Observers
Dr M. Matsuo, Sumitomo Chemical Company, Biochemistry & Toxicology
Laboratory, Osaka, Japan
Dr Y. Okuno, Sumitomo Chemical Company, Biochemistry & Toxicology
Laboratory, Osaka, Japan
Dr N. Punja, International Group of National Association of Manufac-
turers of Agrochemical Products (GIFAP), ICI Plant Protection
Division, Fenhurst, Haslemere, United Kingdom
Secretariat
Dr K.W. Jager, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland (Secretary)
Dr R. Plestina, Division of Vector Control, Delivery and Management of
Vector Control, World Health Organization, Geneva, Switzerland
Dr J. Sekizawa, Section of Information and Investigation, Division of
Information on Chemical Safety, National Institute of Hygienic
Sciences, Tokyo, Japan (Rapporteur)
NOTE TO READERS OF THE CRITERIA DOCUMENTS
Every effort has been made to present information in the criteria
documents as accurately as possible without unduly delaying their pub-
lication. In the interest of all users of the environmental health
criteria documents, readers are kindly requested to communicate any
errors that may have occurred to the Manager of the International Pro-
gramme on Chemical Safety, World Health Organization, Geneva, Switzer-
land, in order that they may be included in corrigenda, which will
appear in subsequent volumes.
* * *
A detailed data profile and a legal file can be obtained from the
International Register of Potentially Toxic Chemicals, Palais des
Nations, 1211 Geneva 10, Switzerland (Telephone No. 7988400 or
7985850).
* * *
The proprietary information contained in this document cannot
replace documentation for registration purposes, because the latter has
to be closely linked to the source, the manufacturing route and the
purity/impurities of the substance to be registered. The data should
be used in accordance with paragraphs 82-84 and recommendations
paragraph 90 of the Second FAO Government Consultation (FAO, 1982).
ENVIRONMENTAL HEALTH CRITERIA FOR PERMETHRIN
A WHO Task Group on Environmental Health Criteria for Fenvalerate,
Permethrin, and d-Phenothrin met in Tokyo from 4 to 8 July 1988. This
meeting was convened with the financial assistance of the Ministry of
Health and Welfare, Tokyo, Japan, and was hosted by the National Insti-
tute of Hygienic Sciences (NIHS) in Tokyo.
Dr T. Furukawa and Dr K. Shirota opened the meeting on behalf of
the Ministry of Health and Welfare, and Dr A. Tanimura, Director-Gen-
eral of the NIHS welcomed the participants to the Institute. Dr M.
Mercier, Manager of the International Programme on Chemical Safety wel-
comed the participants on behalf of the three IPCS cooperating organ-
izations (UNEP/ILO/WHO). The group reviewed and revised the draft mono-
graph and made an evaluation of the risks for human health and the
environment from exposure to permethrin.
The first draft of this document was prepared by DR J. MIYAMOTO and
DR M. MATSUO of Sumitomo Chemical Company with the assistance of the
staff of the National Institute of Hygienic Sciences, Tokyo, Japan.
Dr I. Yamamoto of the Tokyo University of Agriculture and Dr M. Eto of
Kyushu University, Japan, assisted with the finalization of the draft.
The second draft was prepared by DR J. SEKIZAWA, NIHS, Tokyo, incor-
porating comments received following circulation of the first draft to
the IPCS contact points for Environmental Health Criteria documents.
Dr K.W. Jager and Dr P.G. Jenkins, both members of the IPCS Central
Unit, were responsible for the technical development and editing,
respectively, of this monograph.
The assistance of the Sumitomo Chemical Company, Japan, and ICI
Agrochemicals, United Kingdom, in making available to the IPCS and the
Task Group their toxicological proprietary information on permethrin is
gratefully acknowledged. This allowed the Task Group to make its
evaluation on the basis of more complete data.
* * *
The United Kingdom Department of Health and Social Security
generously supported the cost of printing.
ABBREVIATIONS
ai active ingredient
Cl2CA 3-(2,2-dichlorovinyl)-2,2-dimethylcyclopro-
panecarboxyclic acid
ECG electrocardiogram
EEG electroencephalogram
FID flame ionization detector
GC gas chromatography
GC-ECD gas chromatography with electron capture
detector
GC-SIM gas chromatography with selected ion
monitoring
GLC gas-liquid chromatography
HPLC high-performance liquid chromatography
JMPR Joint FAO/WHO Meeting on Pesticide Residues
NOEL no-observed-effect level
PBacid 3-phenoxybenzoic acid
PBalc 3-phenoxybenzyl alcohol
PBald 3-phenoxybenzaldehyde
TLC thin-layer chromatography
INTRODUCTION
SYNTHETIC PYRETHROIDS - A PROFILE
1. During investigations to modify the chemical structures of natural
pyrethrins, a certain number of synthetic pyrethroids were produced
with improved physical and chemical properties and greater biologi-
cal activity. Several of the earlier synthetic pyrethroids were
successfully commercialized, mainly for the control of household
insects. Other more recent pyrethroids have been introduced as
agricultural insecticides because of their excellent activity
against a wide range of insect pests and their non-persistence in
the environment.
2. The pyrethroids constitute another group of insecticides in ad-
dition to organochlorine, organophosphorus, carbamate, and other
compounds. Pyrethroids commercially available to date include
allethrin, resmethrin, d-phenothrin, and tetramethrin (for insects
of public health importance), and cypermethrin, deltamethrin,
fenvalerate, and permethrin (mainly for agricultural insects).
Other pyrethroids are also available including furamethrin, kadeth-
rin, and tellallethrin (usually for household insects), fenpropath-
rin, tralomethrin, cyhalothrin, lambda-cyhalothrin, tefluthrin,
cyfluthrin, flucythrinate, fluvalinate, and biphenate (for agricul-
tural insects).
3. Toxicological evaluations of several synthetic pyrethroids have
been performed by the FAO/WHO Joint Meeting on Pesticide Residues
(JMPR). The acceptable daily intake (ADI) has been estimated by
the JMPR for cypermethrin, deltamethrin, fenvalerate, permethrin,
d-phenothrin, cyfluthrin, cyhalothrin, and flucythrinate.
4. Chemically, synthetic pyrethroids are esters of specific acids
(e.g., chrysanthemic acid, halo-substituted chrysanthemic acid, 2-
(4-chlorophenyl)-3-methylbutyric acid) and alcohols (e.g.,
allethrolone, 3-phenoxybenzyl alcohol). For certain pyrethroids,
asymmetric centre(s) exist in the acid and/or alcohol moiety, and
the commercial products sometimes consist of a mixture of both
optical (1R/1S or d/1) and geometric (cis/trans) isomers. However,
most of the insecticidal activity of such products may reside in
only one or two isomers. Some of the products (e.g., d-phenothrin,
deltamethrin) consist only of such active isomer(s).
5. Synthetic pyrethroids are neuropoisons acting on the axons in the
peripheral and central nervous systems by interacting with sodium
channels in mammals and/or insects. A single dose produces toxic
signs in mammals, such as tremors, hyperexcitability, salivation,
choreoathetosis, and paralysis. The signs disappear fairly rap-
idly, and the animals recover, generally within a week. At near-
lethal dose levels, synthetic pyrethroids cause transient changes
in the nervous system, such as axonal swelling and/or breaks and
myelin degeneration in sciatic nerves. They are not considered to
cause delayed neurotoxicity of the kind induced by some organo-
phosphorus compounds. The mechanism of toxicity of synthetic
pyrethroids and their classification into two types are discussed
in the Appendix.
6. Some pyrethroids (e.g., deltamethrin, fenvalerate, cyhalothrin,
lambda-cyhalothrin, flucythrinate, and cypermethrin) may cause a
transient itching and/or burning sensation in exposed human skin.
7. Synthetic pyrethroids are generally metabolized in mammals through
ester hydrolysis, oxidation, and conjugation, and there is no tend-
ency to accumulate in tissues. In the environment, synthetic py-
rethroids are fairly rapidly degraded in soil and in plants. Ester
hydrolysis and oxidation at various sites on the molecule are the
major degradation processes. The pyrethroids are strongly adsorbed
on soil and sediments, and hardly eluted with water. There is
little tendency for bioaccumulation in organisms.
8. Because of low application rates and rapid degradation in the
environment, residues in food are generally low.
9. Synthetic pyrethroids have been shown to be toxic for fish, aquatic
arthropods, and honey bees in laboratory tests. But, in practical
usage, no serious adverse effects have been noticed because of the
low rates of application and lack of persistence in the environ-
ment. The toxicity of synthetic pyrethroids in birds and domestic
animals is low.
10. In addition to the evaluation documents of FAO/WHO, there are sev-
eral good reviews and books on the chemistry, metabolism, mammalian
toxicity, environmental effects, etc. of synthetic pyrethroids,
including those by Elliott (1977), Miyamoto (1981), Miyamoto &
Kearney (1983), and Leahey (1985).
1. SUMMARY AND EVALUATION, CONCLUSIONS, RECOMMENDATION
1.1 Summary and Evaluation
1.1.1 Identity, physical and chemical properties, analytical methods
Permethrin was first synthesized in 1973 and marketed in 1977 as a
photostable pyrethroid. It is an ester of the dichloro analogue of
chrysanthemic acid, 3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropane-carb-
oxylic acid (Cl2CA), and 3-phenoxybenzyl alcohol. Technical products
are a mixture of four stereoisomers with the configurations [1R,trans],
[1R,cis], [1S,trans], and [1S,cis] in the approximate ratio of 3:2:3:2.
The ratio of cis:trans is around 2:3 and 1R:1S is 1:1 (racemic). The
[1R,cis] isomer is the most insecticidally active of the isomers,
followed by the [1R,trans] isomer.
Technical grade permethrin is a brown or yellowish brown liquid
which may crystallize partly at room temperature. The melting point is
approximately 35°C and the boiling point is 220°C at 0.05 mmHg. The
specific gravity is 1.214 at 25°C and the vapour pressure is 1.3 µPa
at 20°C. Permethrin is almost insoluble in water (0.2 mg/litre at
30°C), but is soluble in organic solvents such as acetone, hexane, and
xylene. It is stable to light and heat, but unstable in alkaline
media.
Residue and environmental analyses are performed using a gas
chromatograph equipped with an electron capture detector (minimum
detectable concentration of 0.005 mg/kg). Technical products are ana-
lysed using a gas chromatograph with a flame ionization detector.
1.1.2 Production and use
Approximately 600 tonnes per year of permethrin is at present used
world-wide, mostly for agricultural purposes. It has a potential appli-
cation in the protection of stored grain and it has been used in aerial
application for forest protection and vector control, for the control
of noxious insects in the household and on cattle, for the control of
body lice, and in mosquito nets.
Permethrin is formulated as emulsifiable concentrate, ultra-low-
volume concentrate, wettable powder, and dustable powder.
1.1.3 Human exposure
The rate of decline of residue levels in various crops is fairly
slow, half-lives ranging from about 1 to 3 weeks depending on the crop.
However, when permethrin is used as recommended, there is no signifi-
cant increase in residues following repeated application.
Exposure of the general population to permethrin is mainly via
dietary residues. Residue levels in crops grown according to good agri-
cultural practice are generally low. The resulting exposure of the
general population is expected to be low, but precise data in the form
of total-diet studies is lacking.
Information on occupational exposure to permethrin is very lim-
ited.
1.1.4 Environmental fate
In laboratory studies, permethrin has been shown to degrade in soil
with a half-life of 28 days or less. The trans isomer degraded more
rapidly than the cis isomer, ester cleavage being the major initial
degradative reaction. The compounds generated by ester cleavage were
then further oxidised, eventually yielding carbon dioxide as the major
terminal product. Studies to investigate the leaching potential of
permethrin and its degradates showed that very little downward movement
occurs in soil.
Permethrin deposited on plants degrades with a half-life of
approximately 10 days. Ester cleavage and conjugation of the acid and
alcohol released is the major degradation pathway. Hydroxylation at
various positions of the molecule and photo-induced cis-trans inter-
conversion also occur.
In water and on soil surfaces permethrin is photodegraded by sun-
light. Ester cleavage and cis-trans interconversion are, as with
plants, the major reactions.
In general, the degradative processes which occur in the environ-
ment lead to less toxic products.
Permethrin disappears rapidly from the environment, in 6-24 h from
ponds and streams, 7 days from pond sediment, and 58 days from foliage
and soil in a forest. From cotton leaves in a field, 30% of the com-
pound was lost within 1 week.
Under aerobic conditions in soil, permethrin degrades with a half-
life of 28 days.
There is very little movement of permethrin in the environment, and
it is unlikely that it will attain significant levels in the environ-
ment.
1.1.5 Kinetics and metabolism
Permethrin administered to mammals was rapidly metabolized and
almost completely excreted in urine and faeces within 12 days. The
trans isomer, being much more susceptible to esterase attack than the
cis isomer, was eliminated faster than the cis isomer. The major meta-
bolic reactions were ester cleavage and oxidation, particularly at the
terminal aromatic ring of the phenoxybenzyl moiety and the geminal
dimethyl group of the cyclopropane ring, followed by conjugation. Less
than 0.7% of the dose was detected in the milk of goats or cows admin-
istered permethrin orally.
1.1.6 Effects on organisms in the environment
In laboratory tests, permethrin has been shown to be highly toxic
for aquatic arthropods, LC50 values ranging from 0.018 µg/litre for
larval stone crabs to 1.26 µg/litre for a cladoceran. It is also
highly toxic for fish, with 96-h LC50 values ranging from 0.62 µg/litre
for larval rainbow trout to 314 µg/litre for adult rainbow trout.
The no-observed-effect level for early life stages of the sheepshead
minnow over 28 days is 10 µg/litre and the chronic no-effect level
for fathead minnow is 0.66-1.4 µg/litre. Permethrin is less toxic to
aquatic molluscs and amphibia, 96-h LC50 values being >1000 µg/litre and
7000 µg/litre, respectively.
In field tests and in the use of the compound under practical con-
ditions, this high potential toxicity is not manifested. An extensive
literature exists on the effects of using permethrin in agriculture,
forestry, and in vector control in many parts of the world. Some
aquatic arthropods are killed, particularly when water is over-sprayed
but the effects on populations of organisms is temporary. There have
been no reports of fish killed in the field. This reduced toxicity in
the field is related to the strong adsorption of the compound to sedi-
ments and its rapid degradation. Sediment-bound permethrin is toxic to
burrowing organisms but this effect also is temporary.
Permethrin is highly toxic for honey bees. The topical LD50 is
0.11 µg/bee, but there is a strong repellent effect of permethrin to
bees which reduced the toxic effect in practice. There is no evidence
for significant kills of honey bees under normal use. Permethrin is
more toxic to predator mites than to the target pest species.
Permethrin has very low toxicity to birds when given orally or fed
in the diet. The LD50 is >3000 mg/kg body weight for acute single
oral dosage and for dietary exposure it is >5000 mg/kg diet. It has no
effect on reproduction in the hen at a dose of 40 mg/kg diet.
Permethrin is readily taken up by aquatic organisms, bioconcen-
tration factors ranging from 43 to 750 for various organisms. In all
the aquatic organisms studied, absorbed permethrin is rapidly lost on
transfer to clean water. There is no bioaccumulation in birds. The
compound can, therefore, be regarded as having no tendency to bio-
accumulate in practice.
1.1.7 Effects on experimental animals and in vitro test systems
Permethrin has a low acute toxicity to rats, mice, rabbits, and
guinea-pigs, though the LD50 value varies considerably according to
the vehicle used and the cis:trans isomeric ratio. Signs of acute
poisoning become apparent within 2 h of dosing and persist for up to
3 days. [1R, cis ]- and [1R, trans ]-permethrin belong to the type I
group of pyrethroids, which typically cause tremor (T-syndrome), inco-
ordination, hyperactivity, prostration, and paralysis. Core temperature
is markedly increased during poisoning.
None of the metabolites of permethrin shows a higher acute (oral or
intraperitoneal) toxicity than permethrin itself.
Permethrin caused a mild primary irritation of the intact and
abraded skin of rabbits but did not cause a photochemical irritation
reaction after exposure of treated areas of rabbit skin to ultra-violet
light. Permethrin did not cause a sensitization reaction in guinea-
pigs.
Oral subacute and subchronic toxicity studies of permethrin have
been performed in rats and mice at dose levels up to 10 000 mg/kg diet
and for 14 days to 26 weeks in duration. Changes detected at the
higher level were an increase in liver/body weight ratio, hypertrophy
in the liver, and clinical signs of poisoning such as tremor. The no-
observed-effects levels (NOEL) in rats ranged from 20 mg/kg diet (in
studies lasting 90 days or 6 months) to 1500 mg/kg diet (in a 6-month
study).
NOEL values in dogs ranged from 5 mg/kg body weight in 3-month
studies to 250 mg/kg body weight in 6-month studies.
In long-term studies in mice and rats, an increase in liver weight
was found which was considered to be associated with an induction of
the liver microsomal enzyme system.
The NOEL in a 2-year rat study was 100 mg/kg diet, corresponding to
5.0 mg/kg body weight.
There were indications, from three long-term mouse studies, of
oncogenicity in the lungs of one strain of mouse (females only) at the
highest dose level (5 g/kg diet). Studies in rats revealed no oncogenic
potential in either sex.
Permethrin was not mutagenic in in vivo or in vitro studies.
Toxicological evidence from mutagenicity studies and from long-term
mouse and rat studies suggests that permethrin's oncogenic potential is
very low, is limited to female mice, and is probably epigenetic.
Permethrin is not teratogenic to rats, mice, or rabbits at dose
levels up to 225, 150, and 1800 mg/kg body weight, respectively.
In a 3-generation reproduction study, permethrin did not induce
adverse effects at levels up to 2500 mg/kg diet.
Permethrin fed to rats at high dose levels (6600-7000 mg/kg diet)
for 14 days induced sciatic nerve damage in one study but did not
produce any ultrastructural changes in the sciatic nerve in another
study. Permethrin did not cause delayed neurotoxicity in hens.
1.1.8 Effects on human beings
Permethrin can induce skin sensations and paraesthesia in exposed
workers, which develop after a latent period of approximately 30 min,
peak by 8 h and disappear within 24 h. Numbness, itching, tingling, and
burning are symptoms frequently reported.
No poisoning cases have been reported.
The likelihood of oncogenic effects in human beings is extremely
low or non-existent.
There are no indications that permethrin has an adverse effect on
human beings when used as recommended.
1.2 Conclusions
1.2.1 General population
The exposure of the general population to permethrin is expected to
be low. It is not likely to present a hazard provided it is used as
recommended.
1.2.2 Occupational exposure
With reasonable work practices, hygiene measures, and safety pre-
cautions, permethrin is unlikely to present a hazard to those exposed
occupationally.
1.2.3 Environment
It is unlikely that permethrin or its degradation products will
attain levels of environmental significance provided that recommended
application rates are used. Under laboratory conditions permethrin is
highly toxic to fish, aquatic anthropods, and honey bees. However,
lasting adverse effects are not likely to occur under field conditions
provided it is used as recommended.
1.3 Recommendations
Although dietary levels arising from recommended usage are con-
sidered to be low, confirmation of this through inclusion of permethrin
in monitoring studies should be considered.
No adverse effects have been reported following human exposure to
permethrin during the many years of its use. Nevertheless, it would be
wise to maintain observations of human exposure.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1 Chemical Identity
Permethrin was synthesized as one of the new photostable
pyrethroids by Elliott et al. (1973). It is prepared by the esterifi-
cation of the dichloro analogue of chrysanthemic acid, i.e. (1R, cis;
1R, trans; 1S, cis; 1S, trans )-3-(2,2-dichlorovinyl)-2,2-dimethyl-cyclo-
propanecarboxyclic acid (Cl2CA), with 3-phenoxybenzyl alcohol (PBalc).
It contains four stereoisomers due to the chirality of the cyclopropane
ring (Fig. 1). The cis:trans isomer ratio is reported to be 2:3 and the
optical ratio of 1R:1S is 1:1 (racemic) (FAO/WHO, 1980b). Thus,
permethrin contains the [1R,trans], [1R,cis], [1S,trans], and [1S,cis]
isomers in the approximate ratio of 3:2:3:2. Table 1 gives further
details of the chemical identity of permethrin.
The [1R,cis] isomer is the most insecticidally active among the
isomers, followed by the [1R,trans] isomer.
Molecular formula: C21H20Cl2O3
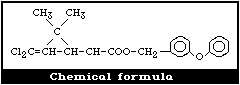
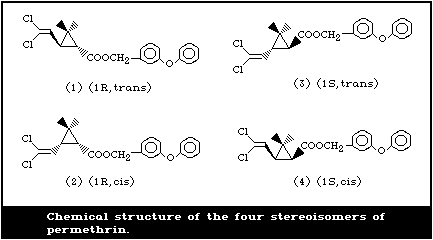 Table 1. Chemical identity of permethrin and its various stereoisomeric compositions
------------------------------------------------------------------------------------------------------
Common name/ CAS Index name (9Cl) Stereoisomeric Synonyms and
CAS Registry No./ compositionc trade names
NIOSH Accession No.a Stereospecific nameb
------------------------------------------------------------------------------------------------------
Permethrin Cyclopropanecarboxylic acid, (1):(2):(3):(4) Permethrina, Ambush,
52645-53-1 3-(2,2-dichloroethenyl)-2,2-dimethyl-, =3:2:3:2 Pounce, Outflank,
GZ1255000 (3-phenoxyphenyl)methyl ester Extin, Ectiban,
Stockade, NRDC143,
3-Phenoxybenzyl (1RS, cis,trans )-3- FMC33297, S-3151,
(2,2-dichlorovinyl)-2,2-dimethyl- SBP-1513, PP557,
cyclopropanecarboxylate A13-29158, BW-21-Z
(+)- cis -Permethrin same as permethrin - -
54774-45-7
GZ1257000 3-Phenoxybenzyl (1R, cis )-
3-(2,2-dichlorovinyl)-2,2-dimethyl-
cyclopropanecarboxylate
Permethrin same as permethrin cis:trans =2:3 -
(racemic mixture)
- 3-Phenoxybenzyl (1R, cis,trans)-3-
GZ1261000 (2,2-dichlorovinyl)-2,2-dimethyl-
cyclopropanecarboxylate
(+)- trans -Permethrin same as permethrin - -
51877-74-8
GZ1260000 3-Phenoxybenzyl (1R, trans )-3-
(2,2-dichlorovinyl)-2,2-dimethyl-
cyclopropanecarboxylate
cis-Permethrin same as permethrin
61949-76-6
GZ1251540 3-Phenoxybenzyl (1RS, cis )-3- - -
(2,2-dichlorovinyl)-2,2-dimethyl-
cyclopropanecarboxylate
------------------------------------------------------------------------------------------------------
a Registry of Toxic Effects of Chemical Substances (RTECS) (1981-1982 edition).
b (1R), (+) or (1S), (-) in the acid part of permethrin signifies the same stereospecific
conformation, respectively.
c Numbers in parentheses identify the structures shown in Fig. 1.
2.2 Physical and Chemical Properties
The physical and chemical properties of technical permethrin
(cis/trans isomeric ratio = 40:60, purity not less than 89%) are
summarized in Table 2. Permethrin is stable to heat and light. It is
more resistant in acidic media than alkaline, with an optimum stability
at pH 4.
Table 2. Physicochemical properties of technical permethrina
----------------------------------------------------------------------
Physical state crystal or viscous liquid
Colour yellow brown to brown
Relative molecular mass 391.31
Melting point 34 - 39 °C
63 - 65 °C (cis); 44 - 47 °C (trans)
Boiling point 220 °C (6.67 Pa), 200 °C (1.33 Pa)
Water solubility (30 °C) 0.2 mg/litre
Solubility in organic soluble or miscible with most organic
solvents (25 °C) solvents: acetone (450 g/litre), hexane
(> 1 kg/kg), methanol (258 g/kg), xylene
(> 1 kg/kg)
Density (25 °C) 1.214
Vapor pressure (20 °C) Technical grade : 1.3 µPa
Pure : 2.5 µPa (cis), 1.5 µPa (trans)
Octanol-water partition 6.5b
coefficient (log Pow)
----------------------------------------------------------------------
a From: Meister et al. (1983); Worthing & Walker (1987); FAO/WHO
(1980b); Wells et al. (1986)
b From: Schimmel et al. (1983)
2.3 Analytical Methods
Methods for the analysis of permethrin are summarized in Table 3.
The common procedure of residue and environmental analysis consists of
(a) extraction, (b) partition, (c) chromatographic separation (clean
up), and (d) quantitative and qualitative analysis of the insecticide
by analytical instruments. Table 3 also indicates minimum detectable
concentration (MDC) and percentage recovery.
To analyse technical grade permethrin, the product is dissolved in
chloroform, together with dioctyl phthalate (as an internal standard),
and the solution is injected into a GLC system equipped with flame
ionization detector (FID) (Horiba et al., 1977).
The Joint FAO/WHO Codex Alimentarius Committee has published rec-
ommendations for methods of analysis of permethrin residues (FAO/WHO,
1985c).
In the internationally accepted CIPAC (Collaborative International
Pesticide Analytical Council) method for permethrin analysis, the prod-
uct is dissolved in 4-methylpentan-2-one containing n-octacosane as
internal standard. Separation is carried out by GLC on a column of
chromosorb W-HP coated with silicone OV 210 (Henriet et al., 1985).
A gas chromatographic method for determining permethrin in techni-
cal and formulated products has been developed and subjected to a col-
laborative study involving 19 laboratories (Tyler, 1987). The column
used was a 1.0 m x 4 mm glass column packed with 3% OV-210 on chromo-
sorb W-HP. When five samples of technical material (90-95%), eight of
emulsifiable concentrates (10-50%), two of wettable powders (20-30%),
one of dustable powder (1-2%), and one of water-dispersible granules
(1-2%) were analysed, the coefficient of variation of the results
obtained ranged from 0.79 to 4.24%. The method was adopted as an
official first-action method by the Association of Official Analytical
Chemists.
Table 3. Analytical methods for permethrin
-------------------------------------------------------------------------------------------------------------------------------------
Sample Sample preparation Determination MDCc % Recovery Reference
GLC or HPLC; detector,b (fortification
Extraction Partition Clean up carrier, flow, column, level)
solvent Column Elution temperature, retention (mg/litre)d
time
-------------------------------------------------------------------------------------------------------------------------------------
Residue analysis
apple n-hexane/ ext.sol.a Silica gel CH2Cl2 ECD-GC,N2, 50 ml/min, 0.01 91 - 106 Baker &
acetone : /H2O 1 m, 3% OV-7, 235 °C (0.1 - 1.0) Bottomley
(1/1) (1982)
pear n-hexane/ ext.sol.a Silica gel CH2Cl2 HPLC UV-206 nm, 25 cm 0.05 81 - 95 Baker &
acetone : /H2O ODS, propan-2-ol, (0.1 - 1.0) Bottomley
(1/1) 1 ml/min (1982)
blueberry acetone n-hexane/ Florisil benzene/ ECD-GC, N2, 60 ml/min, 0.01 cis:79.6 - 87.1 MacPhee
sat.NaCl n-hexane 0.9 m, 3% OV-210, 200 °C, (0.05 - 0.25) et al.
(4/1) 7.0(cis), 8.3 (trans) min trans:73.3 - 84.2 (1982)
(0.05 - 0.25)
celery CH3CN n-hexane/ Florisil CH3CN/ ECD-GC, N2, 100 ml/min 0.005 94.2 - 97.0 Braun &
2% NaCl CH2Cl2/ 1.8 m, Ultra-Bond 20M, (0.01 - 1.0) Stanek
n-hexane 220 °C, 3.5, 4.1 min (1982)
(0.35/50/50)
corn pentane CH3CN/ Alumina pentane/ FID-GC, N2, 28 ml/min 0.2 87.5 - 105 Simonaitis
pentane ethyl acetate 1.22 m, 5% OV-225, 250 °C, (0.2 - 22) & Cail
(97/3) 9.5(cis), 10.0 (trans) min (1977)
beef CH3CN/ n-hexane Florisil CH3CN/ ECD-GC, N2, 100 ml/min 0.005 82.9 - 89.9 Braun &
muscle H2O 2% NaCl CH2Cl2/ 1.8 m, Ultra-Bond 20M, (0.01 - 1.0) Stanek
(85/15) n-hexane 220 °C, 3.5(cis), (1982)
(0.35/50/50) 4.1 (trans) min
-------------------------------------------------------------------------------------------------------------------------------------
Table 3 (contd.)
-------------------------------------------------------------------------------------------------------------------------------------
Sample Sample preparation Determination MDCc % Recovery Reference
GLC or HPLC; detector,b (fortification
Extraction Partition Clean up carrier, flow, column, level)
solvent Column Elution temperature, retention (mg/litre)d
time
-------------------------------------------------------------------------------------------------------------------------------------
Environmental analysis
waste XAD-2 Florisil n-hexane GC-SIM/MS, He, 25 ml/min, 50 95 (0.11) Siegel
water resin, /ether 1.8 m, SP-2250, 230 °C, ng/litre 95 (0.26) et al.
ether (9/1) 3.5(cis), 3.7 (trans) min (1980)
runoff n-hexane ECD-GC, N2, 150 ml/min, 100 97 Carroll
(sediment 0.91 m, 5% SP-2330, 215 °C, ng/litre et al.
+ water) 3(cis), 4 (trans) min (1981)
Product analysis
technical CHCl3 FID-GC, N2, 40 ml/min, Horiba
grade 2% LAC-2R-446, 200 °C et al.
(1977)
-------------------------------------------------------------------------------------------------------------------------------------
a ext. sol = extraction solvent.
b detector (ECD-GC = Coulson electrolytic conductivity detector-GC; GC-SIM/MS = GC-selected ion monitoring with mass spectroscopy).
c MDC = minimum detectable concentration (mg/kg, unless stated otherwise).
d fortification level indicates the concentration of permethrin added to control samples for the measurement of recovery.
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE; ENVIRONMENTAL LEVELS
3.1 Industrial Production
Permethrin was first marketed in 1977. Worldwide production fig-
ures (1979-1982) are shown in Table 4.
Table 4. World-wide production of permethrin
---------------------------------------------------------
Year Production Reference
(tonnes)
---------------------------------------------------------
1979 800 Wood Mackenzie (1980)
1980 860 Wood Mackenzie (1981)
1981 660 - 700 Wood Mackenzie (1982, 1983)
1982 650 Wood Mackenzie (1983)
1983 600 Wood Mackenzie (1984)
1984 335 Battelle (1986)
---------------------------------------------------------
3.2. Use Pattern
Permethrin is a photostable synthetic pyrethroid. It possesses a
high level of activity against Leptidoptera and is also effective
against Hemiptera, Diptera, and Coleoptera. It is a stomach and contact
insectide, but it has very little fumigant activity. Permethrin is not
plant systemic. It is fast acting and effective against all growth
stages, particularly larvae. Permethrin also has significant repellent
action. It is effective against insects at low rates of application
and is sufficiently photostable to be of wide-ranging practical use in
agriculture.
Permethrin is mostly used on cotton plants (61% of consumption).
The major consumer countries in 1980 were the USA (263 tonnes), Brazil
(38 tonnes), Mexico (36 tonnes), and Central America (27 tonnes)
(Battelle, 1982).
Other crops to which permethrin is applied are corn, soybean, cof-
fee, tobacco, oil seed rape, wheat, barley, alfalfa, vegetables, and
fruits. In addition to its pre-harvest usage, permethrin has a poten-
tial application in the protection of stored grain. For example, per-
methrin has been applied to sorghum or wheat in large scale trials in
Australia (FAO/WHO, 1981b, 1982b).
Permethrin is also used for the control of insects in household and
animal facilities (Battelle, 1982) or in forest pest control, as a fog
in mushroom houses, and as a wood preservative. Other applications are
in public health, particularly for insect control in aircraft, treat-
ment of mosquito nets, and human lice control.
It is formulated in emulsifiable concentrates (1.25-50%), ultra-low-
volume formulations (5%), wettable powders (25%), and fogging formu-
lations (2-5%) (FAO/WHO, 1980b). Permethrin is normally effective at
50 g ai/ha on leaf brassicae, whereas 100 g ai/ha is often needed under
more severe conditions in the Americas, Africa, and South-East Asia.
The concentration in most working dilutions is 0.04-0.08% (w/v).
3.3 Residues in Food and Other Products
As might be expected for a compound which is non-systemic and also
fairly stable on leaf surfaces, the amount of residue found on differ-
ent parts of crops depends largely on the direct exposure at the time
of application. This is particularly marked with leafy vegetables such
as lettuce and cabbage where residue levels on wrapper leaves are
usually many times (e.g., 10-100) higher than those on central heads
(as trimmed for commercial distribution). Similarly, residues on fruit
such as lemons, citrus, and kiwi fruits are almost entirely confined to
the peel or similar outer protective surfaces. This is illustrated by
the 1979 Joint FAO/WHO Meeting on Pesticide Residue (JMPR) evaluation,
which contains findings from the examinations of samples of cabbage,
lettuce, oranges, melons, and kiwi fruit (FAO/WHO, 1980b).
Residue levels in cotton seeds are influenced by the degree of boll
ripening/opening at the time of last spraying. Levels in root and tuber
vegetables are usually less than 0.05 mg/kg (FAO/WHO, 1980b).
Ground and aerial applications have been found to yield similar
residue levels in a wide range of vegetable and field crops. Simi-
larly, there were no major differences in residue levels in greenhouse
curcurbitae and solanaceae following spray and fogging applications at
effective rates under similar conditions (FAO/WHO, 1980b).
Supervised trials and residue analyses have been performed on a
variety of crops such as field crops, foliar and root vegetables,
trees, soft fruits, and fruiting vegetables. Comprehensive summaries of
reports (more than 5000 individual residue results on approximately 60
crops from 17 countries) were described in the evaluation reports of
the JMPR, (FAO/WHO 1980b, 1981b, 1982b, 1983b, 1984b, 1985b, 1986b). A
comprehensive list of maximum residue limits for a large number of
commodities resulted from these evaluations (FAO/WHO 1986c).
The rate of decline of residue levels in various crops is fairly
slow, half-life periods ranging from about 1 to 3 weeks depending on
the crop. However, there is no obvious build-up of residues following
repeated application within the rates and frequencies that are needed
to obtain good insect control (FAO/WHO, 1980b).
Residues were measured in cotton seeds in supervised trials during
1975-1977 in the USA. When emulsifiable concentrate formulations (25-
40%) of permethrin were applied to fields at rates of 110 or 450 g
ai/ha (3 to 16 times, until 0 to 76 days before harvest), the average
residue level in cotton seeds was 0.03-0.08 mg/kg, the highest values
ranging from 0.03 to 0.27 mg/kg in 27 samples (FAO/WHO, 1980b).
Similar results were obtained when sweet corn was treated 6-13
times with 25% emulsifiable concentrate at a rate of 280-450 g ai/ha.
The residue levels at 0-4 days after the last application were <0.01-
0.12 mg/kg (Ussary, 1978, 1979).
Wheat grains treated with permethrin at a rate of 0.5-5.0 mg/kg
revealed a residue level of 0.36-4.5 mg/kg after 9 months of storage
(Halls, 1981). When wheat containing a residue level of 1.09 mg/kg was
subjected to milling and baking processes, the level of the permethrin
residue declined to 0.12 mg/kg in white bread (Halls & Periam, 1980).
Groups of three cows were fed cis/trans (40/60)-permethrin at rates
of 0.2, 1.0, 10, 50, or 150 mg/kg diet for 28-31 days. Mean plateau
levels in whole milk were <0.01 µg/g and 0.3 µg/g at dietary levels
of 0.2 mg/kg and 150 mg/kg, respectively. These levels, however, de-
clined rapidly to <0.01 µg/g within 5 days after permethrin adminis-
tration ceased. Residue levels of <0.01-0.04 µg/g fat and 2.8-6.2 µg/g
fat were found in the perirenal fat of cows that were given permethrin
at dietary levels of 0.2 mg/kg and 150 mg/kg, respectively (Edwards &
Iswaran, 1977; Swaine & Sapiets, 1981a, 1981b).
In studies by Ussary & Braithwaite (1980), cows were given six
whole-body sprays of permethrin at a rate of 1.0 g ai/cow with an
interval of 14 days between each spray. They were allowed free access
to a self-oiler containing a solution of 0.03 g ai/litre (ensuring at
least two applications per day for a period of 10 weeks). The cows were
housed in premises that were sprayed at a rate of 0.06 g ai/m2, six
sprays taking place with a 14-day interval between sprays (the cows had
free access to the premises during spraying). This degree of exposure
is at the highest end of the range that is likely to occur in normal
husbandry practice. When cows were slaughtered five days after the
sixth application, the permethrin levels in muscle, liver, and kidney
were low (<0.01 mg/kg tissue). The highest residue levels detected were
0.10 mg/kg and 0.04 mg/kg in the intestinal and subcutaneous fat,
respectively.
Lactating cows (three/group) fed permethrin at dose levels of 0,
0.2, 1.0, 10, or 50 mg/kg diet for 28 days showed no mortality, and
growth and milk production were normal. Permethrin residues were ob-
served in the milk within 3 days at the two highest dietary levels;
levels appeared to reach a plateau rapidly and not to increase with
time. Analysis of individual cis and trans isomers showed that the
ratio of permethrin isomers in milk appeared to change during the
course of the study with the cis isomer predominating. Permethrin resi-
dues were not found in the tissues of animals that received doses of
1 mg/kg or less. At dose levels of 10 or 50 mg/kg, residues were de-
tected in the tissues, predominantly in the fat. Low levels were also
present in the muscle and kidney at the highest dose level. Permethrin
did not appear to accumulate in the fat but to reach a plateau rapidly
(Edwards & Iswaran, 1977).
3.4 Residues in the Environment
Data on precise levels of permethrin residues in the air, water, or
soil are not available. However, an assessment of the environmental
residues resulting from permethrin application has been made in some
studies.
Permethrin deposits and airborne concentrations have been measured
downwind from a single swath application using a back-pack mist blower.
Samples from Kromekote cards (to assess droplet density and size dis-
tribution), glass plates, water surface, bronze rods, and air samplers
were collected, cleaned up, and analysed by HPLC (Sundaram et al.,
1987). Permethrin deposits on all static collectors were greatest
within 30 m of the spray swath. Beyond 30 m downwind, the amounts of
the insecticide trapped by various collectors were extremely low and
were barely detectable.
Lindquist et al. (1987) measured permethrin concentrations in
greenhouse air and deposition on glass plates following application by
several different methods. Highest airborne residues were found after
thermal pulse-jet applications and lowest after hydraulic sprayings.
Most airborne residues were detected within 4 h of application. Surface
residues were highest after hydraulic and mechanical aerosol appli-
cations. Thermal pulse-jet applications resulted in low surface resi-
dues.
Agnihotri et al. (1986) evaluated the persistence of permethrin in
water and sediment contained in open trenches (3 m x 1 m x 30 cm) lined
with alkathene sheet. Insecticide emulsion was sprayed on the surface
of water at the normal recommended dosage and at twice this value. The
dissipation of the insecticide from the water was rapid, about 87-90%
of the pesticide being lost within 24 h at both rates of application.
However, residues were found to be absorbed by the sediment and these
persisted even beyond 30 days. In soil, persistence was moderate,
lasting for around 30 days.
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
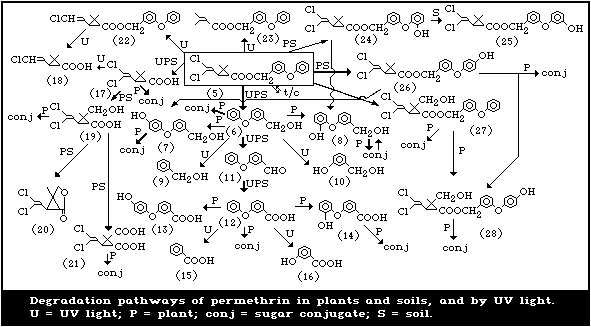
The degradation pathways of permethrin by ultraviolet light, in
soils, and in plants are summarized in Fig. 2.
Table 1. Chemical identity of permethrin and its various stereoisomeric compositions
------------------------------------------------------------------------------------------------------
Common name/ CAS Index name (9Cl) Stereoisomeric Synonyms and
CAS Registry No./ compositionc trade names
NIOSH Accession No.a Stereospecific nameb
------------------------------------------------------------------------------------------------------
Permethrin Cyclopropanecarboxylic acid, (1):(2):(3):(4) Permethrina, Ambush,
52645-53-1 3-(2,2-dichloroethenyl)-2,2-dimethyl-, =3:2:3:2 Pounce, Outflank,
GZ1255000 (3-phenoxyphenyl)methyl ester Extin, Ectiban,
Stockade, NRDC143,
3-Phenoxybenzyl (1RS, cis,trans )-3- FMC33297, S-3151,
(2,2-dichlorovinyl)-2,2-dimethyl- SBP-1513, PP557,
cyclopropanecarboxylate A13-29158, BW-21-Z
(+)- cis -Permethrin same as permethrin - -
54774-45-7
GZ1257000 3-Phenoxybenzyl (1R, cis )-
3-(2,2-dichlorovinyl)-2,2-dimethyl-
cyclopropanecarboxylate
Permethrin same as permethrin cis:trans =2:3 -
(racemic mixture)
- 3-Phenoxybenzyl (1R, cis,trans)-3-
GZ1261000 (2,2-dichlorovinyl)-2,2-dimethyl-
cyclopropanecarboxylate
(+)- trans -Permethrin same as permethrin - -
51877-74-8
GZ1260000 3-Phenoxybenzyl (1R, trans )-3-
(2,2-dichlorovinyl)-2,2-dimethyl-
cyclopropanecarboxylate
cis-Permethrin same as permethrin
61949-76-6
GZ1251540 3-Phenoxybenzyl (1RS, cis )-3- - -
(2,2-dichlorovinyl)-2,2-dimethyl-
cyclopropanecarboxylate
------------------------------------------------------------------------------------------------------
a Registry of Toxic Effects of Chemical Substances (RTECS) (1981-1982 edition).
b (1R), (+) or (1S), (-) in the acid part of permethrin signifies the same stereospecific
conformation, respectively.
c Numbers in parentheses identify the structures shown in Fig. 1.
2.2 Physical and Chemical Properties
The physical and chemical properties of technical permethrin
(cis/trans isomeric ratio = 40:60, purity not less than 89%) are
summarized in Table 2. Permethrin is stable to heat and light. It is
more resistant in acidic media than alkaline, with an optimum stability
at pH 4.
Table 2. Physicochemical properties of technical permethrina
----------------------------------------------------------------------
Physical state crystal or viscous liquid
Colour yellow brown to brown
Relative molecular mass 391.31
Melting point 34 - 39 °C
63 - 65 °C (cis); 44 - 47 °C (trans)
Boiling point 220 °C (6.67 Pa), 200 °C (1.33 Pa)
Water solubility (30 °C) 0.2 mg/litre
Solubility in organic soluble or miscible with most organic
solvents (25 °C) solvents: acetone (450 g/litre), hexane
(> 1 kg/kg), methanol (258 g/kg), xylene
(> 1 kg/kg)
Density (25 °C) 1.214
Vapor pressure (20 °C) Technical grade : 1.3 µPa
Pure : 2.5 µPa (cis), 1.5 µPa (trans)
Octanol-water partition 6.5b
coefficient (log Pow)
----------------------------------------------------------------------
a From: Meister et al. (1983); Worthing & Walker (1987); FAO/WHO
(1980b); Wells et al. (1986)
b From: Schimmel et al. (1983)
2.3 Analytical Methods
Methods for the analysis of permethrin are summarized in Table 3.
The common procedure of residue and environmental analysis consists of
(a) extraction, (b) partition, (c) chromatographic separation (clean
up), and (d) quantitative and qualitative analysis of the insecticide
by analytical instruments. Table 3 also indicates minimum detectable
concentration (MDC) and percentage recovery.
To analyse technical grade permethrin, the product is dissolved in
chloroform, together with dioctyl phthalate (as an internal standard),
and the solution is injected into a GLC system equipped with flame
ionization detector (FID) (Horiba et al., 1977).
The Joint FAO/WHO Codex Alimentarius Committee has published rec-
ommendations for methods of analysis of permethrin residues (FAO/WHO,
1985c).
In the internationally accepted CIPAC (Collaborative International
Pesticide Analytical Council) method for permethrin analysis, the prod-
uct is dissolved in 4-methylpentan-2-one containing n-octacosane as
internal standard. Separation is carried out by GLC on a column of
chromosorb W-HP coated with silicone OV 210 (Henriet et al., 1985).
A gas chromatographic method for determining permethrin in techni-
cal and formulated products has been developed and subjected to a col-
laborative study involving 19 laboratories (Tyler, 1987). The column
used was a 1.0 m x 4 mm glass column packed with 3% OV-210 on chromo-
sorb W-HP. When five samples of technical material (90-95%), eight of
emulsifiable concentrates (10-50%), two of wettable powders (20-30%),
one of dustable powder (1-2%), and one of water-dispersible granules
(1-2%) were analysed, the coefficient of variation of the results
obtained ranged from 0.79 to 4.24%. The method was adopted as an
official first-action method by the Association of Official Analytical
Chemists.
Table 3. Analytical methods for permethrin
-------------------------------------------------------------------------------------------------------------------------------------
Sample Sample preparation Determination MDCc % Recovery Reference
GLC or HPLC; detector,b (fortification
Extraction Partition Clean up carrier, flow, column, level)
solvent Column Elution temperature, retention (mg/litre)d
time
-------------------------------------------------------------------------------------------------------------------------------------
Residue analysis
apple n-hexane/ ext.sol.a Silica gel CH2Cl2 ECD-GC,N2, 50 ml/min, 0.01 91 - 106 Baker &
acetone : /H2O 1 m, 3% OV-7, 235 °C (0.1 - 1.0) Bottomley
(1/1) (1982)
pear n-hexane/ ext.sol.a Silica gel CH2Cl2 HPLC UV-206 nm, 25 cm 0.05 81 - 95 Baker &
acetone : /H2O ODS, propan-2-ol, (0.1 - 1.0) Bottomley
(1/1) 1 ml/min (1982)
blueberry acetone n-hexane/ Florisil benzene/ ECD-GC, N2, 60 ml/min, 0.01 cis:79.6 - 87.1 MacPhee
sat.NaCl n-hexane 0.9 m, 3% OV-210, 200 °C, (0.05 - 0.25) et al.
(4/1) 7.0(cis), 8.3 (trans) min trans:73.3 - 84.2 (1982)
(0.05 - 0.25)
celery CH3CN n-hexane/ Florisil CH3CN/ ECD-GC, N2, 100 ml/min 0.005 94.2 - 97.0 Braun &
2% NaCl CH2Cl2/ 1.8 m, Ultra-Bond 20M, (0.01 - 1.0) Stanek
n-hexane 220 °C, 3.5, 4.1 min (1982)
(0.35/50/50)
corn pentane CH3CN/ Alumina pentane/ FID-GC, N2, 28 ml/min 0.2 87.5 - 105 Simonaitis
pentane ethyl acetate 1.22 m, 5% OV-225, 250 °C, (0.2 - 22) & Cail
(97/3) 9.5(cis), 10.0 (trans) min (1977)
beef CH3CN/ n-hexane Florisil CH3CN/ ECD-GC, N2, 100 ml/min 0.005 82.9 - 89.9 Braun &
muscle H2O 2% NaCl CH2Cl2/ 1.8 m, Ultra-Bond 20M, (0.01 - 1.0) Stanek
(85/15) n-hexane 220 °C, 3.5(cis), (1982)
(0.35/50/50) 4.1 (trans) min
-------------------------------------------------------------------------------------------------------------------------------------
Table 3 (contd.)
-------------------------------------------------------------------------------------------------------------------------------------
Sample Sample preparation Determination MDCc % Recovery Reference
GLC or HPLC; detector,b (fortification
Extraction Partition Clean up carrier, flow, column, level)
solvent Column Elution temperature, retention (mg/litre)d
time
-------------------------------------------------------------------------------------------------------------------------------------
Environmental analysis
waste XAD-2 Florisil n-hexane GC-SIM/MS, He, 25 ml/min, 50 95 (0.11) Siegel
water resin, /ether 1.8 m, SP-2250, 230 °C, ng/litre 95 (0.26) et al.
ether (9/1) 3.5(cis), 3.7 (trans) min (1980)
runoff n-hexane ECD-GC, N2, 150 ml/min, 100 97 Carroll
(sediment 0.91 m, 5% SP-2330, 215 °C, ng/litre et al.
+ water) 3(cis), 4 (trans) min (1981)
Product analysis
technical CHCl3 FID-GC, N2, 40 ml/min, Horiba
grade 2% LAC-2R-446, 200 °C et al.
(1977)
-------------------------------------------------------------------------------------------------------------------------------------
a ext. sol = extraction solvent.
b detector (ECD-GC = Coulson electrolytic conductivity detector-GC; GC-SIM/MS = GC-selected ion monitoring with mass spectroscopy).
c MDC = minimum detectable concentration (mg/kg, unless stated otherwise).
d fortification level indicates the concentration of permethrin added to control samples for the measurement of recovery.
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE; ENVIRONMENTAL LEVELS
3.1 Industrial Production
Permethrin was first marketed in 1977. Worldwide production fig-
ures (1979-1982) are shown in Table 4.
Table 4. World-wide production of permethrin
---------------------------------------------------------
Year Production Reference
(tonnes)
---------------------------------------------------------
1979 800 Wood Mackenzie (1980)
1980 860 Wood Mackenzie (1981)
1981 660 - 700 Wood Mackenzie (1982, 1983)
1982 650 Wood Mackenzie (1983)
1983 600 Wood Mackenzie (1984)
1984 335 Battelle (1986)
---------------------------------------------------------
3.2. Use Pattern
Permethrin is a photostable synthetic pyrethroid. It possesses a
high level of activity against Leptidoptera and is also effective
against Hemiptera, Diptera, and Coleoptera. It is a stomach and contact
insectide, but it has very little fumigant activity. Permethrin is not
plant systemic. It is fast acting and effective against all growth
stages, particularly larvae. Permethrin also has significant repellent
action. It is effective against insects at low rates of application
and is sufficiently photostable to be of wide-ranging practical use in
agriculture.
Permethrin is mostly used on cotton plants (61% of consumption).
The major consumer countries in 1980 were the USA (263 tonnes), Brazil
(38 tonnes), Mexico (36 tonnes), and Central America (27 tonnes)
(Battelle, 1982).
Other crops to which permethrin is applied are corn, soybean, cof-
fee, tobacco, oil seed rape, wheat, barley, alfalfa, vegetables, and
fruits. In addition to its pre-harvest usage, permethrin has a poten-
tial application in the protection of stored grain. For example, per-
methrin has been applied to sorghum or wheat in large scale trials in
Australia (FAO/WHO, 1981b, 1982b).
Permethrin is also used for the control of insects in household and
animal facilities (Battelle, 1982) or in forest pest control, as a fog
in mushroom houses, and as a wood preservative. Other applications are
in public health, particularly for insect control in aircraft, treat-
ment of mosquito nets, and human lice control.
It is formulated in emulsifiable concentrates (1.25-50%), ultra-low-
volume formulations (5%), wettable powders (25%), and fogging formu-
lations (2-5%) (FAO/WHO, 1980b). Permethrin is normally effective at
50 g ai/ha on leaf brassicae, whereas 100 g ai/ha is often needed under
more severe conditions in the Americas, Africa, and South-East Asia.
The concentration in most working dilutions is 0.04-0.08% (w/v).
3.3 Residues in Food and Other Products
As might be expected for a compound which is non-systemic and also
fairly stable on leaf surfaces, the amount of residue found on differ-
ent parts of crops depends largely on the direct exposure at the time
of application. This is particularly marked with leafy vegetables such
as lettuce and cabbage where residue levels on wrapper leaves are
usually many times (e.g., 10-100) higher than those on central heads
(as trimmed for commercial distribution). Similarly, residues on fruit
such as lemons, citrus, and kiwi fruits are almost entirely confined to
the peel or similar outer protective surfaces. This is illustrated by
the 1979 Joint FAO/WHO Meeting on Pesticide Residue (JMPR) evaluation,
which contains findings from the examinations of samples of cabbage,
lettuce, oranges, melons, and kiwi fruit (FAO/WHO, 1980b).
Residue levels in cotton seeds are influenced by the degree of boll
ripening/opening at the time of last spraying. Levels in root and tuber
vegetables are usually less than 0.05 mg/kg (FAO/WHO, 1980b).
Ground and aerial applications have been found to yield similar
residue levels in a wide range of vegetable and field crops. Simi-
larly, there were no major differences in residue levels in greenhouse
curcurbitae and solanaceae following spray and fogging applications at
effective rates under similar conditions (FAO/WHO, 1980b).
Supervised trials and residue analyses have been performed on a
variety of crops such as field crops, foliar and root vegetables,
trees, soft fruits, and fruiting vegetables. Comprehensive summaries of
reports (more than 5000 individual residue results on approximately 60
crops from 17 countries) were described in the evaluation reports of
the JMPR, (FAO/WHO 1980b, 1981b, 1982b, 1983b, 1984b, 1985b, 1986b). A
comprehensive list of maximum residue limits for a large number of
commodities resulted from these evaluations (FAO/WHO 1986c).
The rate of decline of residue levels in various crops is fairly
slow, half-life periods ranging from about 1 to 3 weeks depending on
the crop. However, there is no obvious build-up of residues following
repeated application within the rates and frequencies that are needed
to obtain good insect control (FAO/WHO, 1980b).
Residues were measured in cotton seeds in supervised trials during
1975-1977 in the USA. When emulsifiable concentrate formulations (25-
40%) of permethrin were applied to fields at rates of 110 or 450 g
ai/ha (3 to 16 times, until 0 to 76 days before harvest), the average
residue level in cotton seeds was 0.03-0.08 mg/kg, the highest values
ranging from 0.03 to 0.27 mg/kg in 27 samples (FAO/WHO, 1980b).
Similar results were obtained when sweet corn was treated 6-13
times with 25% emulsifiable concentrate at a rate of 280-450 g ai/ha.
The residue levels at 0-4 days after the last application were <0.01-
0.12 mg/kg (Ussary, 1978, 1979).
Wheat grains treated with permethrin at a rate of 0.5-5.0 mg/kg
revealed a residue level of 0.36-4.5 mg/kg after 9 months of storage
(Halls, 1981). When wheat containing a residue level of 1.09 mg/kg was
subjected to milling and baking processes, the level of the permethrin
residue declined to 0.12 mg/kg in white bread (Halls & Periam, 1980).
Groups of three cows were fed cis/trans (40/60)-permethrin at rates
of 0.2, 1.0, 10, 50, or 150 mg/kg diet for 28-31 days. Mean plateau
levels in whole milk were <0.01 µg/g and 0.3 µg/g at dietary levels
of 0.2 mg/kg and 150 mg/kg, respectively. These levels, however, de-
clined rapidly to <0.01 µg/g within 5 days after permethrin adminis-
tration ceased. Residue levels of <0.01-0.04 µg/g fat and 2.8-6.2 µg/g
fat were found in the perirenal fat of cows that were given permethrin
at dietary levels of 0.2 mg/kg and 150 mg/kg, respectively (Edwards &
Iswaran, 1977; Swaine & Sapiets, 1981a, 1981b).
In studies by Ussary & Braithwaite (1980), cows were given six
whole-body sprays of permethrin at a rate of 1.0 g ai/cow with an
interval of 14 days between each spray. They were allowed free access
to a self-oiler containing a solution of 0.03 g ai/litre (ensuring at
least two applications per day for a period of 10 weeks). The cows were
housed in premises that were sprayed at a rate of 0.06 g ai/m2, six
sprays taking place with a 14-day interval between sprays (the cows had
free access to the premises during spraying). This degree of exposure
is at the highest end of the range that is likely to occur in normal
husbandry practice. When cows were slaughtered five days after the
sixth application, the permethrin levels in muscle, liver, and kidney
were low (<0.01 mg/kg tissue). The highest residue levels detected were
0.10 mg/kg and 0.04 mg/kg in the intestinal and subcutaneous fat,
respectively.
Lactating cows (three/group) fed permethrin at dose levels of 0,
0.2, 1.0, 10, or 50 mg/kg diet for 28 days showed no mortality, and
growth and milk production were normal. Permethrin residues were ob-
served in the milk within 3 days at the two highest dietary levels;
levels appeared to reach a plateau rapidly and not to increase with
time. Analysis of individual cis and trans isomers showed that the
ratio of permethrin isomers in milk appeared to change during the
course of the study with the cis isomer predominating. Permethrin resi-
dues were not found in the tissues of animals that received doses of
1 mg/kg or less. At dose levels of 10 or 50 mg/kg, residues were de-
tected in the tissues, predominantly in the fat. Low levels were also
present in the muscle and kidney at the highest dose level. Permethrin
did not appear to accumulate in the fat but to reach a plateau rapidly
(Edwards & Iswaran, 1977).
3.4 Residues in the Environment
Data on precise levels of permethrin residues in the air, water, or
soil are not available. However, an assessment of the environmental
residues resulting from permethrin application has been made in some
studies.
Permethrin deposits and airborne concentrations have been measured
downwind from a single swath application using a back-pack mist blower.
Samples from Kromekote cards (to assess droplet density and size dis-
tribution), glass plates, water surface, bronze rods, and air samplers
were collected, cleaned up, and analysed by HPLC (Sundaram et al.,
1987). Permethrin deposits on all static collectors were greatest
within 30 m of the spray swath. Beyond 30 m downwind, the amounts of
the insecticide trapped by various collectors were extremely low and
were barely detectable.
Lindquist et al. (1987) measured permethrin concentrations in
greenhouse air and deposition on glass plates following application by
several different methods. Highest airborne residues were found after
thermal pulse-jet applications and lowest after hydraulic sprayings.
Most airborne residues were detected within 4 h of application. Surface
residues were highest after hydraulic and mechanical aerosol appli-
cations. Thermal pulse-jet applications resulted in low surface resi-
dues.
Agnihotri et al. (1986) evaluated the persistence of permethrin in
water and sediment contained in open trenches (3 m x 1 m x 30 cm) lined
with alkathene sheet. Insecticide emulsion was sprayed on the surface
of water at the normal recommended dosage and at twice this value. The
dissipation of the insecticide from the water was rapid, about 87-90%
of the pesticide being lost within 24 h at both rates of application.
However, residues were found to be absorbed by the sediment and these
persisted even beyond 30 days. In soil, persistence was moderate,
lasting for around 30 days.
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
The degradation pathways of permethrin by ultraviolet light, in
soils, and in plants are summarized in Fig. 2.
 4.1 Transport and Distribution between Media
In laboratory studies, permethrin in water was rapidly adsorbed
onto lake sediments or soil columns and was not desorbed or eluted
easily from them. However in forest spray trials, permethrin residues
were not only dissipated from water streams very rapidly but also did
not accumulate much in the bottom sediment. This was explained by the
fact that the low density of permethrin and its insolubility in water
prevented it from reaching bottom sediments. Residues in forest litter
and exposed soils were more stable. Low levels of degradation products
can be translocated from soils to plants.
When a 640-ha forest block in northern Ontario, Canada, was sprayed
once with permethrin at 17.5 g ai/ha, residues in water persisted for
less than 96 h and attained peak concentrations of 147.0 µg/litre in
ponds and 2.5 µg/litre in streams after one hour. Accumulation and
persistence of the pesticide in bottom sediment were negligible. Resi-
due levels in the treated streams ranged from 0.05 to 0.89 µg/litre and
persisted for a maximum of 96 h, but in another case, residues fell to
a non-detectable level (less than 0.05 µg/l) after 6-24 h. Permethrin
residues appeared 2.1 km downstream from the treatment block 6 h after
spraying. The level reached a peak of 0.18 µg/litre at 12 h and did
not persist beyond 96 h. Accumulation of the insecticide in pond sedi-
ment was minimal (5-8 µg/kg) and persisted for less than 7 days. No
permethrin residue was found in stream sediments. The sprayed per-
methrin formulation had a density (0.88 g/ml) less than that of water
and was practically insoluble in water. It therefore formed a surface
film when brought into contact with stagnant or slowly moving water.
This significantly reduced the likelihood of the insecticide reaching
the bottom sediment or exposing fish in the treated ponds and streams.
Insecticide residues in foliage, soil, and litter were more stable than
in water and remained at detectable concentrations to the end of the
58-day sampling period. Deciduous and coniferous foliage contained
permethrin residues ranging from 0.02 to 0.78 mg/kg and retained con-
centrations of 0.02-0.05 mg/kg for at least 57 days. Forest litter
within the treatment block showed a residue level of 0.07 mg/kg 58 days
after the pesticide application. The permethrin residue levels in ex-
posed soil in the treatment block were fairly constant (0.04-0.07mg/kg)
for up to 58 days (Kingsbury & Kreutzweiser, 1980a).
In another field test where permethrin was sprayed (17.5 g ai/ha)
twice at intervals of 9 or 10 days in two forest blocks in Quebec,
Canada, the stagnant water in the sprayed region contained permethrin
levels of no more than 0.62 µg/litre and 0.84 µg/litre after the
initial and second applications, respectively. Samples from the streams
showed residue levels ranging from 0.05 to 1.84 µg/litre. Permethrin
concentrations in the water persisted at mean levels of 0.15 µg/litre
for 96 h and 0.03 µg/litre for 48 h after the first and second appli-
cations, respectively (detection limit: 0.01 µg/litre). Sediments
collected from a pond and streams, contained 30-95 µg permethrin/kg.
Accumulation of residual permethrin in stream sediment 4.5 km down-
stream from the treatment block was minimal. Permethrin residue levels
in forest litter increased substantially following the second appli-
cation. Mean concentrations ranged from 0.01 mg/kg to 0.053 mg/kg but
fell to non-detectable levels within 59 days (Kreutzweiser, 1982).
In a laboratory adsorption-desorption study, more than 95% of per-
methrin in aqueous solutions (6-42 µg/litre) was rapidly adsorbed
onto lake sediment, and the adsorbed insecticide was not easily de-
sorbed from the sediment by several water rinses. A high distribution
coefficient (i.e., g adsorbed per g sediment divided by g per ml of
solution) of 389 ml/g was obtained from the adsorption isotherm. Per-
methrin in aqueous solution applied to the surface of a sediment column
did not penetrate through more than 2 cm of the sediment (Sharom &
Solomon, 1981).
In a laboratory soil-leaching experiment, 14C-labelled (+)- cis or
(+)- trans- permethrin was incubated with two types of soils (light
clay soil of Kodaira and sandy clay loam soil of Azuchi) for 0 day or
21 days, then these permethrin-soil mixtures were applied to the top of
a soil column and eluted with water. When a mixture with no pre-
incubation was applied to the column, only 1.0 to 3.4% of the radio-
carbon was found in lower layer and no radiocarbon was eluted. However,
the degradation products from the pre-incubated samples were eluted
with water to a slight extent (see section 4.4) (Kaneko et al., 1978).
Similar results were obtained by Kaufman et al. (1981) in soil
mobility studies using soil TLC methods.
The uptake of permethrin and its degradation products by plants
from soil was studied by Leahey & Carpenter (1980). Sandy loam soil was
treated separately with [14C-cyclopropyl]- and [14C-phenyl]-permethrin
at a spray application rate of 2 kg/ha. The top 8 cm of the treated
soil was thoroughly mixed, and sugar beet, wheat, lettuce, and cotton
seeds were sown at intervals of 30, 60, and 120 days after treatment.
Low radioactive residues (up to 0.86 mg/kg) were detected in mature
plants, but the residues were higher in crops grown in soil treated
with [14C-cyclopropyl]-permethrin. It appeared that certain carboxylic
acid metabolites formed in the soil were subsequently taken up by the
plants. However, under field conditions, no residues of permethrin or
its metabolites were detected in crops sown 60 days or more after soil
treatment (Swaine et al., 1978).
4.2 Photodecomposition
Appraisal
Photochemical studies of permethrin in thin films and in solution
have shown it to be much more stable to light (10-100 times) than
synthetic pyrethroids developed earlier. In solution, photoisomeriz-
ation at the 1,3-bond of the cyclopropane ring and ester cleavage were
shown to be the major reactions.
In a thin film on plywood, permethrin remained insecticidally
active after 26 days, compared with 4-8 days and <2 days for phenothrin
and resmethrin, respectively. When exposed to daylight as a thin film
(0.2 mg/cm2) indoors near a window, phenothrin photodecomposed with a
half-life of about 6 days, whereas 60% of applied permethrin remained
undecomposed after 20 days. Thus, replacement of the isobutenyl group
with the dichlorovinyl substituent significantly enhanced the photo-
stability of permethrin. Permethrin was reported to be 10-100 times
more photostable than other pyrethroids synthesized earlier (Elliott et
al., 1973).
The photolysis of [1RS, trans ]- or [1RS, cis ]-permethrin (5)a has
been examined using materials labelled with 14C at the carboxy (acid)
or benzyl (alcohol) group (Fig. 2). On irradiation with ultraviolet
light (peak wavelength: 290-320 nm), both permethrin isomers decomposed
slightly faster in hexane than in methanol. In both solvents, the cis
isomer photodecomposed about 1.6 times faster (T´ = 43-58 min) than the
trans isomer. The photodecomposition reaction involved extensive
isomerization of the cyclopropane ring, i.e. interconversion of the
trans and cis isomers. This probably occurred via a triplet energy
state forming the diradical intermediate through Cl-C3 bond fission,
since the reaction was efficiently quenched by 1,3-cyclohexadiene. The
isomerization reaction reached a state of equilibrium after 1-4 h of
irradiation and the more thermodynamically stable trans isomer
constituted 65-70% of the isomer mixture. Apart from the isomerization
reaction, ester cleavage was the major photolytic reaction. As the
result of ester cleavage and other photolytic reactions, products
formed from permethrin also included smaller or trace amounts of
monochloro-permethrin (22) (from reductive dechlorination), 3-phenoxy-
benzaldehyde (PBald) (11), 3-phenoxybenzoic acid (PBacid) (12),
3-phenoxybenzyl-3,3-dimethylacrylate (23) (from diradical intermedi-
ate), and benzyl alcohols (9,10), as well as their corresponding acids
(15,16). In addition, large amounts of unidentified polar products were
-----------------------------------------------------------------------
a Numbers in parentheses refer to the corresponding numbers in Fig.2
detected, especially in water. Permethrin and monochloro-permethrin
(0.1-0.5 g) did not undergo photo-oxidation or other reactions within 7
days in oxygenated methanol solution using Rose Bengal as a sensitizer.
Thus the chlorine atoms at the vinyl position had a pronounced effect
in protecting this substituent from oxidation or epoxidation, as com-
pared with the isobutenyl in chrysanthemate (Holmstead et. al., 1978).
Holmstead et al. (1978) also investigated the photodegradation of
permethrin on a soil surface. The degradation on soil was similar to
the degradation pathways established in solution, but the rate of de-
gradation was slower and photo-isomerisation less important. Exposure
of the permethrin isomers on Dunkirk silt loam soil for 48 h resulted
in about a 55% loss of permethrin under sunlight and about a 35% loss
in the dark. The amount of unextractable material was about 6% in the
dark and about 18% in the light. On soil, permethrin did not undergo
extensive isomerization of the cyclopropane ring as it did in solution.
There was little difference in the amount of free acid detected in the
dark or in light, and 3-phenoxybenzyl alcohol (PBalc) (6) (approxi-
mately 5%) was the major cleavage product of the alcohol moiety. Other
products detected in trace amounts were essentially similar to those
present in solutions that had undergone photolysis.
4.3 Degradation in Plants
Appraisal
Thorough investigations of the fate of permethrin in plants have
been performed using bean plants and cotton plants. No significant
differences in the types of metabolic pathways were detected for the
two plant species. Very little translocation of permethrin or its
metabolites was observed following either topical application or stem
injection of permethrin to plants. Photochemical reactions played an
important role in the fate of permethrin applied to the surface of
plants. A major degradation pathway in plants was ester cleavage,
followed by rapid conjugation with sugars of the Cl2 CA and PBalc thus
formed.
The metabolism of the [1R,trans] and [1R,cis] isomers of 14C-per-
methrin, labelled separately in the dichlorovinyl and benzyl carbon
atoms, in snap bean seedlings has been studied in the greenhouse.
Whole-body autoradiography of the plants showed that little trans-
location of radiolabelled permethrin or its metabolites had occurred.
The amounts of radiocarbon remaining after 14 days were 13-17% of the
dose in the surface wash, 46-58% in the methanol extract, and 8-14% un-
extracted in the plant residues. Some interconversion of the trans and
cis isomers occurred and the cis isomer was slightly more persistent
than the trans isomer. The initial half-lives of the cis and trans
isomers of permethrin in the seedlings were 9 and 7 days, respectively.
A large number of metabolites were detected in the plant extracts, the
major ones from the alcohol moiety being PBalc (6) and its correspond-
ing 2'- (8) or 4'-hydroxy (7) derivatives, which occurred mainly as
glucoside conjugates (Fig. 1). There were seven or eight additional
minor unidentified products. The cis and trans isomers of 3-(2,2-
dichlorovinyl)-2,2-dimethylcyclopropanecarboxyclic acid (Cl2CA) (17)
were the major metabolites from the acid moiety and occurred mainly as
conjugated forms. In addition, trace amounts of the 2'- (24) or 4'-
hydroxy (26) derivatives of permethrin were also detected. From the
hydrolysis experiments using beta-glucosidase, it was inferred that the
sugar concerned was glucose, but no detailed evidence of the identity
was obtained (Ohkawa et al., 1977).
In a separate study, Gaughan & Casida (1978) examined the metab-
olism of the [1RS,trans] and [1RS,cis] isomers of permethrin in snap
beans in the glasshouse and in cotton both in the glasshouse and out-
doors. Individual leaves of snap beans and cotton plants were treated
with 1 µg of cis- or trans-14C-permethrin labelled either at the
carboxy or methylene carbon. Under field conditions, about 30% of the
radiolabel was lost from cotton plants within one week after appli-
cation and some trans/cis isomerization at the cyclopropane ring took
place by photodecomposition. trans-Permethrin was metabolized more
rapidly than the cis isomer. The major degradation pathway was again
hydrolysis, followed by rapid conjugation of Cl2CA (17) and PBalc (6)
with sugars. There were at least two types of conjugates; the minor one
was a glycoside readily cleaved by beta-glucosidase and the major one
was a conjugate which was resistant to beta-glucosidase but was readily
cleaved by cellulase. Other products identified included the hydroxy-
lated compounds reported by Ohkawa et al. (1977) in their study of
beans treated with permethrin. In addition, hydroxylation at either of
the two methyl groups in the acid moiety (27) with subsequent conju-
gation occurred to a greater extent with the more stable cis isomer.
Similar metabolites to those formed under field conditions were
detected in bean and cotton plants under glasshouse conditions.
Roberts & Wright (1981) studied the conjugation of 14C-PBalc in
cotton plants using abscised leaves to obtain more information on the
nature of the conjugates produced. The alcohol was rapidly converted
to glucosyl 3-phenoxybenzyl ether and subsequently to more polar sub-
stances such as disaccharide conjugates with glucose and pentose (prob-
ably xylose or arabinose) sugars. The alcohol and its monosaccharide
and disaccharide conjugates underwent interconversion in the cotton
leaves. The evidence was obtained from experiments with 14C-glucose,
which showed the ready exchange of the glucose units of the conjugates
with free glucose in the leaves. No larger sugar conjugates of PBalc
were detected in plants.
From the above studies, it can be concluded that the types of prod-
ucts formed from permethrin in plants are similar to those formed in
mammals, except for the nature of the conjugates (see section 5.1).
4.4 Degradation in Soils
Appraisal
Several studies on the degradation of permethrin in a wide variety of
soil types have been carried out. These studies used permethrin labelled
with 14 C at different positions, so that the fate of virtually all of the
significant sub-units of the molecule has been traced. In all soil types
degradation is fairly rapid under aerobic conditions, conversion to 14CO2
being the major ultimate fate of the 14 C. With all soils and all positions
of radiolabelling, the formation of unextractable residues is a major occur-
rence. Under anaerobic conditions, similar degradation processes seem to
occur, but the rate of ultimate conversion to 14 CO2 is slower than under
aerobic conditions.
Kaufman et al. (1977) studied the degradation of cis and trans iso-
mers of permethrin in five soils under aerobic, anaerobic, and steril-
ized conditions. Soils were treated with 14C-permethrin labelled sep-
arately in the carboxy and methylene groups at a dose rate of 224 g/ha
and stored under aerobic conditions at 25°C. Degradation of permethrin
was rapid in four of five soils, with the trans isomer decomposing more
rapidly than the cis isomer. The initial half-lives were less than 28
days in all but one soil. Rapid evolution of 14CO2 was observed. In
Hagerstown silty clay loam soil, 62% of methylene- and 52% of carboxy-
labelled permethrin were converted to 14CO2 in 27 days. Only 15%
(methylene-labelled) to 19% (carboxy-labelled) of the applied radio-
label was extractable with methanol, 25-27% remaining unextracted and
associated with soil organic matter. Microbial metabolism was involved
in permethrin degradation and the major route was hydrolysis of the
ester linkage to form PBalc and Cl2CA, the former product being
subsequently oxidized to PBacid. In contrast, less than 0.3% of
14CO2 was evolved from soils treated with sodium azide (an inhibitor
of microbial growth) or when the soil was incubated under waterlogged
anaerobic conditions.
Kaneko et al. (1978) reported the degradation in two Japanese soils
of 14C-permethrin labelled separately in the dichlorovinyl and methy-
lene groups. The initial half-lives of the trans and cis isomers were
6-9 days and 12 days, respectively, in soils treated at a rate of 1 mg/
kg and stored at 25°C under aerobic upland conditions. 14CO2 was
evolved at rates similar to those observed by Kaufman et al. (1981). As
one of the 14C-preparations was different in labelled position from
those used in the earlier work, the evolution of 14CO2 was the evi-
dence for extensive degradation of the cyclopropyl moiety after hy-
drolysis in the soils. In addition to the hydrolysis products, several
oxidation products were identified, including 3-(2,2-dichlorovinyl)-
2-methyl-2-hydroxymethyl-cyclopropanecarboxylic acid (19) and 3-(4-
hydroxyphenoxy)benzyl- 3-(2, 2-dichlorovinyl)-2,2-dimethylcyclopropane-
carboxylate (26).
The degradation of permethrin was studied in a flooded Memphis silt
loam soil incubated at 25°C, [14C-carbonyl]- cis-, [14C-carbonyl]- trans-,
and [methylene-14C]- cis-permethrin being added to the soil at rates
of 0.1 and 1.0 mg/kg. The soils were analyzed after 0, 4, 8, 16, 32,
and 64 days to determine the distribution of 14C in CO2, solvent-
extractable compounds, water-soluble polar compounds, and soil-bounds
residues. Thin-layer chromatographic analysis of the organic solvent
extracts showed that trans-permethrin was more rapidly degraded than
the cis isomer. After 64 days, the amounts of 14C-trans-permethrin remain-
ing were 34.2% (at 0.1 mg/kg) and 30.3% (at 1.0 mg/kg) of the applied
14C, and those of 14C- cis-permethrin were 73.4% (at 0.1 mg/kg) and
69.8% (at 1.0 mg/kg). Two metabolites, 3-(2,2-dichloro-vinyl)-2,2-
dimethylcyclopropanecarboxylic acid (17) and PBalc (6), resulting from
permethrin hydrolysis were identified. Other metabolites were PBacid
(12) and PBald (11). Fragmentation of (17) and (12) to CO2 was not
extensive, and cumulative 14CO2 recoveries were less than 3.5% for
all treatments during the 64-day incubation period. The metabolism
of trans-permethrin resulted in the accumulation of polar compounds in
the water. Soil-bound residues gradually increased with time and
accounted for 3.3-11.4% of the 14C activity after 64 days. The
largest percentage of soil-bound 14C residue was in the fulvic acid
fraction (Jordan & Kaufman, 1986).
When 14C-permethrin preincubated with soil for 21 days was applied
on top of a soil column and eluted with water, 7.9-17.2% of the applied
radiocarbon was recovered in the lower layers of the column and 0.3-
2.6% was found in effluents (Kaneko et al., 1978). Only degradation
products of permethrin, such as PBacid (12) and 3-(4-hydroxyphenoxy)-
benzyl-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropanecarboxylate (26)
were identified in the effluent. Permethrin was not present in the
effluent (see section 4.1).
The persistence of permethrin in soil was studied in aqueous sus-
pensions of soil spiked with permethrin at a rate of 17.8 mg/kg under a
range of redox potential (-150, +50, +250, and +450 mV) and at pH 5.5,
7.0, and 8.0. The results of this study indicated that both the pH and
redox potential significantly influence the degradation of permethrin.
After 25 days, permethrin disappeared almost completely under well
oxidized (+450 mV) conditions at all three pH levels. Under reduced
conditions (-150 mV), only about 40% of the applied permethrin was
degraded. The rate of degradation of permethrin was moderate at weakly
oxidized (+250 mV) and moderately reduced (+50 mV) conditions at pH 8.
Thus, permethrin was lost more rapidly under oxidizing conditions, and
increasing the pH enhanced this loss under moderately reduced and
weakly oxidized conditions (Gambrell et al., 1981).
Jordan et al. (1982) investigated the effect of temperature on the
degradation of permethrin in soil. Dubbs fine sandy loam soil was
treated with [14C-carbonyl]- cis, trans-permethrin at a rate of 1
mg/kg and incubated at 10, 25, and 40°C for up to 64 days. The half-
lives of disappearance for trans and cis isomers were 14 and 55 days at
10°C, 5 and 12 days at 25°C, and 4 and 27 days at 40°C, respectively.
The most rapid rate of degradation of permethrin occurred at 25°C, per-
methrin being converted to Cl2CA (17) and ultimately to 14CO2. At
40°C rapid degradation of permethrin to Cl2CA also occurred, but
further degradation of Cl2CA to 14CO2 was reduced. The amount of
14CO2 evolved after 64 days was 56% at 25°C, compared with 29% at
10°C and 24% at 40°C.
Lord et al. (1982) investigated the factors affecting the persist-
ence of permethrin in three loam soils under laboratory conditions.
The degradation of trans-permethrin (4 mg/kg) at 30°C was similar at
three moisture contents ranging from 40 to 80% of water-holding
capacity, but more rapid degradation occurred in an aqueous soil sus-
pension system probably due to better distribution of the insecticide.
Four repeated applications of permethrin (4 mg/kg) at 20-day intervals,
or addition of nutrients including sucrose (1 mg/kg), powdered cellu-
lose (100 mg/kg), and NH4Cl (80 mg/kg) plus K2HPO4 (260 mg/kg) to
soils caused no drastic changes in the rate of degradation of per-
methrin.
The influence of organic materials on the degradation of permethrin
in soil was also studied by Doyle et al. (1981). [14C-carbonyl]- cis-per-
methrin was added to silty loam soil which had been pretreated with
sewage sludge or dairy manure at rates of 0, 50, or 100 tonnes/ha, and
total CO2 and 14CO2 evolution were monitored regularly throughout a
60-day incubation period at 25°C. The incorporation of sewage or dairy
manure at the rate of 50 and 100 tonnes/ha increased permethrin break-
down by 87% and 149% (sewage), or 64% and 134% (dairy manure) based on
the values measured in unamended soil, respectively. In the waste-
amended soils, a lag period of 28-38 days during which time virtually
no 14CO2 was evolved, was followed by a rapid evolution of 14CO2 before
the rate became stabilized. The highest rates (0.21-0.22% per day) were
observed in soils amended with either dairy manure or sewage sludge at
100 tonnes/ha. The rate of 14CO2 formation correlated directly with
the total microbial activity, as measured by total CO2 production.
In studies by Williams & Brown (1979), the persistence of per-
methrin in six soils was compared with that of fenvalerate under lab-
oratory conditions. The soils were treated with one of the insecticides
at 1 mg/kg and incubated under aerobic conditions for 16 weeks at a
temperature alternating between 20°C for 15 h and 10°C for 9 h to simu-
late the actual field conditions. With the exception of organic soil
from Cloverdale, degradation of permethrin was rapid in all soils, with
half-lives of 3 weeks or less. Under identical conditions, the half
life for fenvalerate was about 7 weeks. Again, trans-permethrin was
lost more rapidly than the cis isomer, and there was very little loss
of either insecticide in the sterilized soils. With Cloverdale organic
soil, a greater degree of adsorption onto the soil organic fraction
might have contributed to the slower degradation rate.
When soil was treated with [14C-cyclopropyl]-permethrin, sugar
beet grown on the treated soil was found to contain radiolabelled
conjugates of Cl2CA and 3-(2,2-dichlorovinyl)-2-methylcyclopropane-
1,2-dicarboxylic acid (21) (Leahey & Carpenter, 1980). It was possible
that both carboxylic acids were formed in the soil and were sub-
sequently taken up by the plants (see section 4.1).
5. KINETICS AND METABOLISM
5.1 Metabolism in Mammals
Appraisal
The metabolic pathways of permethrin in mammals are summarized in
Fig. 3.
The metabolism of permethrin has been studied in great detail in
various species of mammals, using a variety of radiolabelled isomers.
Permethrin administered to mammals was rapidly metabolized and almost
completely eliminated from the body within a short period of time. The
trans isomer of permethrin was eliminated more rapidly that the cis
isomer. Radiocarbon from trans- permethrin was excreted mostly in
urine, whereas that from the cis isomer was eliminated both in urine
and faeces to a similar extent. Expiration as CO2 contributed little
to its elimination in mammals. Major routes of metabolism for both
trans and cis isomers were ester cleavage and oxidation of the 4'-
position of the terminal aromatic ring. A less important reaction in
mammals was hydroxylation of the geminal dimethyl group of the cyclo-
propane ring. Major metabolites thus formed were Cl2 CA in free and
glucuronide form, sulfate conjugate of 4'-hydroxy-3-phenoxybenzoic
acid, PBacid in free and conjugate form, and hydroxymethyl-Cl2 CA as a
glucuronide conjugate. This latter compound was also isolated as a
lactone where the hydroxymethyl group and the carboxy group had a cis
configuration.
4.1 Transport and Distribution between Media
In laboratory studies, permethrin in water was rapidly adsorbed
onto lake sediments or soil columns and was not desorbed or eluted
easily from them. However in forest spray trials, permethrin residues
were not only dissipated from water streams very rapidly but also did
not accumulate much in the bottom sediment. This was explained by the
fact that the low density of permethrin and its insolubility in water
prevented it from reaching bottom sediments. Residues in forest litter
and exposed soils were more stable. Low levels of degradation products
can be translocated from soils to plants.
When a 640-ha forest block in northern Ontario, Canada, was sprayed
once with permethrin at 17.5 g ai/ha, residues in water persisted for
less than 96 h and attained peak concentrations of 147.0 µg/litre in
ponds and 2.5 µg/litre in streams after one hour. Accumulation and
persistence of the pesticide in bottom sediment were negligible. Resi-
due levels in the treated streams ranged from 0.05 to 0.89 µg/litre and
persisted for a maximum of 96 h, but in another case, residues fell to
a non-detectable level (less than 0.05 µg/l) after 6-24 h. Permethrin
residues appeared 2.1 km downstream from the treatment block 6 h after
spraying. The level reached a peak of 0.18 µg/litre at 12 h and did
not persist beyond 96 h. Accumulation of the insecticide in pond sedi-
ment was minimal (5-8 µg/kg) and persisted for less than 7 days. No
permethrin residue was found in stream sediments. The sprayed per-
methrin formulation had a density (0.88 g/ml) less than that of water
and was practically insoluble in water. It therefore formed a surface
film when brought into contact with stagnant or slowly moving water.
This significantly reduced the likelihood of the insecticide reaching
the bottom sediment or exposing fish in the treated ponds and streams.
Insecticide residues in foliage, soil, and litter were more stable than
in water and remained at detectable concentrations to the end of the
58-day sampling period. Deciduous and coniferous foliage contained
permethrin residues ranging from 0.02 to 0.78 mg/kg and retained con-
centrations of 0.02-0.05 mg/kg for at least 57 days. Forest litter
within the treatment block showed a residue level of 0.07 mg/kg 58 days
after the pesticide application. The permethrin residue levels in ex-
posed soil in the treatment block were fairly constant (0.04-0.07mg/kg)
for up to 58 days (Kingsbury & Kreutzweiser, 1980a).
In another field test where permethrin was sprayed (17.5 g ai/ha)
twice at intervals of 9 or 10 days in two forest blocks in Quebec,
Canada, the stagnant water in the sprayed region contained permethrin
levels of no more than 0.62 µg/litre and 0.84 µg/litre after the
initial and second applications, respectively. Samples from the streams
showed residue levels ranging from 0.05 to 1.84 µg/litre. Permethrin
concentrations in the water persisted at mean levels of 0.15 µg/litre
for 96 h and 0.03 µg/litre for 48 h after the first and second appli-
cations, respectively (detection limit: 0.01 µg/litre). Sediments
collected from a pond and streams, contained 30-95 µg permethrin/kg.
Accumulation of residual permethrin in stream sediment 4.5 km down-
stream from the treatment block was minimal. Permethrin residue levels
in forest litter increased substantially following the second appli-
cation. Mean concentrations ranged from 0.01 mg/kg to 0.053 mg/kg but
fell to non-detectable levels within 59 days (Kreutzweiser, 1982).
In a laboratory adsorption-desorption study, more than 95% of per-
methrin in aqueous solutions (6-42 µg/litre) was rapidly adsorbed
onto lake sediment, and the adsorbed insecticide was not easily de-
sorbed from the sediment by several water rinses. A high distribution
coefficient (i.e., g adsorbed per g sediment divided by g per ml of
solution) of 389 ml/g was obtained from the adsorption isotherm. Per-
methrin in aqueous solution applied to the surface of a sediment column
did not penetrate through more than 2 cm of the sediment (Sharom &
Solomon, 1981).
In a laboratory soil-leaching experiment, 14C-labelled (+)- cis or
(+)- trans- permethrin was incubated with two types of soils (light
clay soil of Kodaira and sandy clay loam soil of Azuchi) for 0 day or
21 days, then these permethrin-soil mixtures were applied to the top of
a soil column and eluted with water. When a mixture with no pre-
incubation was applied to the column, only 1.0 to 3.4% of the radio-
carbon was found in lower layer and no radiocarbon was eluted. However,
the degradation products from the pre-incubated samples were eluted
with water to a slight extent (see section 4.4) (Kaneko et al., 1978).
Similar results were obtained by Kaufman et al. (1981) in soil
mobility studies using soil TLC methods.
The uptake of permethrin and its degradation products by plants
from soil was studied by Leahey & Carpenter (1980). Sandy loam soil was
treated separately with [14C-cyclopropyl]- and [14C-phenyl]-permethrin
at a spray application rate of 2 kg/ha. The top 8 cm of the treated
soil was thoroughly mixed, and sugar beet, wheat, lettuce, and cotton
seeds were sown at intervals of 30, 60, and 120 days after treatment.
Low radioactive residues (up to 0.86 mg/kg) were detected in mature
plants, but the residues were higher in crops grown in soil treated
with [14C-cyclopropyl]-permethrin. It appeared that certain carboxylic
acid metabolites formed in the soil were subsequently taken up by the
plants. However, under field conditions, no residues of permethrin or
its metabolites were detected in crops sown 60 days or more after soil
treatment (Swaine et al., 1978).
4.2 Photodecomposition
Appraisal
Photochemical studies of permethrin in thin films and in solution
have shown it to be much more stable to light (10-100 times) than
synthetic pyrethroids developed earlier. In solution, photoisomeriz-
ation at the 1,3-bond of the cyclopropane ring and ester cleavage were
shown to be the major reactions.
In a thin film on plywood, permethrin remained insecticidally
active after 26 days, compared with 4-8 days and <2 days for phenothrin
and resmethrin, respectively. When exposed to daylight as a thin film
(0.2 mg/cm2) indoors near a window, phenothrin photodecomposed with a
half-life of about 6 days, whereas 60% of applied permethrin remained
undecomposed after 20 days. Thus, replacement of the isobutenyl group
with the dichlorovinyl substituent significantly enhanced the photo-
stability of permethrin. Permethrin was reported to be 10-100 times
more photostable than other pyrethroids synthesized earlier (Elliott et
al., 1973).
The photolysis of [1RS, trans ]- or [1RS, cis ]-permethrin (5)a has
been examined using materials labelled with 14C at the carboxy (acid)
or benzyl (alcohol) group (Fig. 2). On irradiation with ultraviolet
light (peak wavelength: 290-320 nm), both permethrin isomers decomposed
slightly faster in hexane than in methanol. In both solvents, the cis
isomer photodecomposed about 1.6 times faster (T´ = 43-58 min) than the
trans isomer. The photodecomposition reaction involved extensive
isomerization of the cyclopropane ring, i.e. interconversion of the
trans and cis isomers. This probably occurred via a triplet energy
state forming the diradical intermediate through Cl-C3 bond fission,
since the reaction was efficiently quenched by 1,3-cyclohexadiene. The
isomerization reaction reached a state of equilibrium after 1-4 h of
irradiation and the more thermodynamically stable trans isomer
constituted 65-70% of the isomer mixture. Apart from the isomerization
reaction, ester cleavage was the major photolytic reaction. As the
result of ester cleavage and other photolytic reactions, products
formed from permethrin also included smaller or trace amounts of
monochloro-permethrin (22) (from reductive dechlorination), 3-phenoxy-
benzaldehyde (PBald) (11), 3-phenoxybenzoic acid (PBacid) (12),
3-phenoxybenzyl-3,3-dimethylacrylate (23) (from diradical intermedi-
ate), and benzyl alcohols (9,10), as well as their corresponding acids
(15,16). In addition, large amounts of unidentified polar products were
-----------------------------------------------------------------------
a Numbers in parentheses refer to the corresponding numbers in Fig.2
detected, especially in water. Permethrin and monochloro-permethrin
(0.1-0.5 g) did not undergo photo-oxidation or other reactions within 7
days in oxygenated methanol solution using Rose Bengal as a sensitizer.
Thus the chlorine atoms at the vinyl position had a pronounced effect
in protecting this substituent from oxidation or epoxidation, as com-
pared with the isobutenyl in chrysanthemate (Holmstead et. al., 1978).
Holmstead et al. (1978) also investigated the photodegradation of
permethrin on a soil surface. The degradation on soil was similar to
the degradation pathways established in solution, but the rate of de-
gradation was slower and photo-isomerisation less important. Exposure
of the permethrin isomers on Dunkirk silt loam soil for 48 h resulted
in about a 55% loss of permethrin under sunlight and about a 35% loss
in the dark. The amount of unextractable material was about 6% in the
dark and about 18% in the light. On soil, permethrin did not undergo
extensive isomerization of the cyclopropane ring as it did in solution.
There was little difference in the amount of free acid detected in the
dark or in light, and 3-phenoxybenzyl alcohol (PBalc) (6) (approxi-
mately 5%) was the major cleavage product of the alcohol moiety. Other
products detected in trace amounts were essentially similar to those
present in solutions that had undergone photolysis.
4.3 Degradation in Plants
Appraisal
Thorough investigations of the fate of permethrin in plants have
been performed using bean plants and cotton plants. No significant
differences in the types of metabolic pathways were detected for the
two plant species. Very little translocation of permethrin or its
metabolites was observed following either topical application or stem
injection of permethrin to plants. Photochemical reactions played an
important role in the fate of permethrin applied to the surface of
plants. A major degradation pathway in plants was ester cleavage,
followed by rapid conjugation with sugars of the Cl2 CA and PBalc thus
formed.
The metabolism of the [1R,trans] and [1R,cis] isomers of 14C-per-
methrin, labelled separately in the dichlorovinyl and benzyl carbon
atoms, in snap bean seedlings has been studied in the greenhouse.
Whole-body autoradiography of the plants showed that little trans-
location of radiolabelled permethrin or its metabolites had occurred.
The amounts of radiocarbon remaining after 14 days were 13-17% of the
dose in the surface wash, 46-58% in the methanol extract, and 8-14% un-
extracted in the plant residues. Some interconversion of the trans and
cis isomers occurred and the cis isomer was slightly more persistent
than the trans isomer. The initial half-lives of the cis and trans
isomers of permethrin in the seedlings were 9 and 7 days, respectively.
A large number of metabolites were detected in the plant extracts, the
major ones from the alcohol moiety being PBalc (6) and its correspond-
ing 2'- (8) or 4'-hydroxy (7) derivatives, which occurred mainly as
glucoside conjugates (Fig. 1). There were seven or eight additional
minor unidentified products. The cis and trans isomers of 3-(2,2-
dichlorovinyl)-2,2-dimethylcyclopropanecarboxyclic acid (Cl2CA) (17)
were the major metabolites from the acid moiety and occurred mainly as
conjugated forms. In addition, trace amounts of the 2'- (24) or 4'-
hydroxy (26) derivatives of permethrin were also detected. From the
hydrolysis experiments using beta-glucosidase, it was inferred that the
sugar concerned was glucose, but no detailed evidence of the identity
was obtained (Ohkawa et al., 1977).
In a separate study, Gaughan & Casida (1978) examined the metab-
olism of the [1RS,trans] and [1RS,cis] isomers of permethrin in snap
beans in the glasshouse and in cotton both in the glasshouse and out-
doors. Individual leaves of snap beans and cotton plants were treated
with 1 µg of cis- or trans-14C-permethrin labelled either at the
carboxy or methylene carbon. Under field conditions, about 30% of the
radiolabel was lost from cotton plants within one week after appli-
cation and some trans/cis isomerization at the cyclopropane ring took
place by photodecomposition. trans-Permethrin was metabolized more
rapidly than the cis isomer. The major degradation pathway was again
hydrolysis, followed by rapid conjugation of Cl2CA (17) and PBalc (6)
with sugars. There were at least two types of conjugates; the minor one
was a glycoside readily cleaved by beta-glucosidase and the major one
was a conjugate which was resistant to beta-glucosidase but was readily
cleaved by cellulase. Other products identified included the hydroxy-
lated compounds reported by Ohkawa et al. (1977) in their study of
beans treated with permethrin. In addition, hydroxylation at either of
the two methyl groups in the acid moiety (27) with subsequent conju-
gation occurred to a greater extent with the more stable cis isomer.
Similar metabolites to those formed under field conditions were
detected in bean and cotton plants under glasshouse conditions.
Roberts & Wright (1981) studied the conjugation of 14C-PBalc in
cotton plants using abscised leaves to obtain more information on the
nature of the conjugates produced. The alcohol was rapidly converted
to glucosyl 3-phenoxybenzyl ether and subsequently to more polar sub-
stances such as disaccharide conjugates with glucose and pentose (prob-
ably xylose or arabinose) sugars. The alcohol and its monosaccharide
and disaccharide conjugates underwent interconversion in the cotton
leaves. The evidence was obtained from experiments with 14C-glucose,
which showed the ready exchange of the glucose units of the conjugates
with free glucose in the leaves. No larger sugar conjugates of PBalc
were detected in plants.
From the above studies, it can be concluded that the types of prod-
ucts formed from permethrin in plants are similar to those formed in
mammals, except for the nature of the conjugates (see section 5.1).
4.4 Degradation in Soils
Appraisal
Several studies on the degradation of permethrin in a wide variety of
soil types have been carried out. These studies used permethrin labelled
with 14 C at different positions, so that the fate of virtually all of the
significant sub-units of the molecule has been traced. In all soil types
degradation is fairly rapid under aerobic conditions, conversion to 14CO2
being the major ultimate fate of the 14 C. With all soils and all positions
of radiolabelling, the formation of unextractable residues is a major occur-
rence. Under anaerobic conditions, similar degradation processes seem to
occur, but the rate of ultimate conversion to 14 CO2 is slower than under
aerobic conditions.
Kaufman et al. (1977) studied the degradation of cis and trans iso-
mers of permethrin in five soils under aerobic, anaerobic, and steril-
ized conditions. Soils were treated with 14C-permethrin labelled sep-
arately in the carboxy and methylene groups at a dose rate of 224 g/ha
and stored under aerobic conditions at 25°C. Degradation of permethrin
was rapid in four of five soils, with the trans isomer decomposing more
rapidly than the cis isomer. The initial half-lives were less than 28
days in all but one soil. Rapid evolution of 14CO2 was observed. In
Hagerstown silty clay loam soil, 62% of methylene- and 52% of carboxy-
labelled permethrin were converted to 14CO2 in 27 days. Only 15%
(methylene-labelled) to 19% (carboxy-labelled) of the applied radio-
label was extractable with methanol, 25-27% remaining unextracted and
associated with soil organic matter. Microbial metabolism was involved
in permethrin degradation and the major route was hydrolysis of the
ester linkage to form PBalc and Cl2CA, the former product being
subsequently oxidized to PBacid. In contrast, less than 0.3% of
14CO2 was evolved from soils treated with sodium azide (an inhibitor
of microbial growth) or when the soil was incubated under waterlogged
anaerobic conditions.
Kaneko et al. (1978) reported the degradation in two Japanese soils
of 14C-permethrin labelled separately in the dichlorovinyl and methy-
lene groups. The initial half-lives of the trans and cis isomers were
6-9 days and 12 days, respectively, in soils treated at a rate of 1 mg/
kg and stored at 25°C under aerobic upland conditions. 14CO2 was
evolved at rates similar to those observed by Kaufman et al. (1981). As
one of the 14C-preparations was different in labelled position from
those used in the earlier work, the evolution of 14CO2 was the evi-
dence for extensive degradation of the cyclopropyl moiety after hy-
drolysis in the soils. In addition to the hydrolysis products, several
oxidation products were identified, including 3-(2,2-dichlorovinyl)-
2-methyl-2-hydroxymethyl-cyclopropanecarboxylic acid (19) and 3-(4-
hydroxyphenoxy)benzyl- 3-(2, 2-dichlorovinyl)-2,2-dimethylcyclopropane-
carboxylate (26).
The degradation of permethrin was studied in a flooded Memphis silt
loam soil incubated at 25°C, [14C-carbonyl]- cis-, [14C-carbonyl]- trans-,
and [methylene-14C]- cis-permethrin being added to the soil at rates
of 0.1 and 1.0 mg/kg. The soils were analyzed after 0, 4, 8, 16, 32,
and 64 days to determine the distribution of 14C in CO2, solvent-
extractable compounds, water-soluble polar compounds, and soil-bounds
residues. Thin-layer chromatographic analysis of the organic solvent
extracts showed that trans-permethrin was more rapidly degraded than
the cis isomer. After 64 days, the amounts of 14C-trans-permethrin remain-
ing were 34.2% (at 0.1 mg/kg) and 30.3% (at 1.0 mg/kg) of the applied
14C, and those of 14C- cis-permethrin were 73.4% (at 0.1 mg/kg) and
69.8% (at 1.0 mg/kg). Two metabolites, 3-(2,2-dichloro-vinyl)-2,2-
dimethylcyclopropanecarboxylic acid (17) and PBalc (6), resulting from
permethrin hydrolysis were identified. Other metabolites were PBacid
(12) and PBald (11). Fragmentation of (17) and (12) to CO2 was not
extensive, and cumulative 14CO2 recoveries were less than 3.5% for
all treatments during the 64-day incubation period. The metabolism
of trans-permethrin resulted in the accumulation of polar compounds in
the water. Soil-bound residues gradually increased with time and
accounted for 3.3-11.4% of the 14C activity after 64 days. The
largest percentage of soil-bound 14C residue was in the fulvic acid
fraction (Jordan & Kaufman, 1986).
When 14C-permethrin preincubated with soil for 21 days was applied
on top of a soil column and eluted with water, 7.9-17.2% of the applied
radiocarbon was recovered in the lower layers of the column and 0.3-
2.6% was found in effluents (Kaneko et al., 1978). Only degradation
products of permethrin, such as PBacid (12) and 3-(4-hydroxyphenoxy)-
benzyl-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropanecarboxylate (26)
were identified in the effluent. Permethrin was not present in the
effluent (see section 4.1).
The persistence of permethrin in soil was studied in aqueous sus-
pensions of soil spiked with permethrin at a rate of 17.8 mg/kg under a
range of redox potential (-150, +50, +250, and +450 mV) and at pH 5.5,
7.0, and 8.0. The results of this study indicated that both the pH and
redox potential significantly influence the degradation of permethrin.
After 25 days, permethrin disappeared almost completely under well
oxidized (+450 mV) conditions at all three pH levels. Under reduced
conditions (-150 mV), only about 40% of the applied permethrin was
degraded. The rate of degradation of permethrin was moderate at weakly
oxidized (+250 mV) and moderately reduced (+50 mV) conditions at pH 8.
Thus, permethrin was lost more rapidly under oxidizing conditions, and
increasing the pH enhanced this loss under moderately reduced and
weakly oxidized conditions (Gambrell et al., 1981).
Jordan et al. (1982) investigated the effect of temperature on the
degradation of permethrin in soil. Dubbs fine sandy loam soil was
treated with [14C-carbonyl]- cis, trans-permethrin at a rate of 1
mg/kg and incubated at 10, 25, and 40°C for up to 64 days. The half-
lives of disappearance for trans and cis isomers were 14 and 55 days at
10°C, 5 and 12 days at 25°C, and 4 and 27 days at 40°C, respectively.
The most rapid rate of degradation of permethrin occurred at 25°C, per-
methrin being converted to Cl2CA (17) and ultimately to 14CO2. At
40°C rapid degradation of permethrin to Cl2CA also occurred, but
further degradation of Cl2CA to 14CO2 was reduced. The amount of
14CO2 evolved after 64 days was 56% at 25°C, compared with 29% at
10°C and 24% at 40°C.
Lord et al. (1982) investigated the factors affecting the persist-
ence of permethrin in three loam soils under laboratory conditions.
The degradation of trans-permethrin (4 mg/kg) at 30°C was similar at
three moisture contents ranging from 40 to 80% of water-holding
capacity, but more rapid degradation occurred in an aqueous soil sus-
pension system probably due to better distribution of the insecticide.
Four repeated applications of permethrin (4 mg/kg) at 20-day intervals,
or addition of nutrients including sucrose (1 mg/kg), powdered cellu-
lose (100 mg/kg), and NH4Cl (80 mg/kg) plus K2HPO4 (260 mg/kg) to
soils caused no drastic changes in the rate of degradation of per-
methrin.
The influence of organic materials on the degradation of permethrin
in soil was also studied by Doyle et al. (1981). [14C-carbonyl]- cis-per-
methrin was added to silty loam soil which had been pretreated with
sewage sludge or dairy manure at rates of 0, 50, or 100 tonnes/ha, and
total CO2 and 14CO2 evolution were monitored regularly throughout a
60-day incubation period at 25°C. The incorporation of sewage or dairy
manure at the rate of 50 and 100 tonnes/ha increased permethrin break-
down by 87% and 149% (sewage), or 64% and 134% (dairy manure) based on
the values measured in unamended soil, respectively. In the waste-
amended soils, a lag period of 28-38 days during which time virtually
no 14CO2 was evolved, was followed by a rapid evolution of 14CO2 before
the rate became stabilized. The highest rates (0.21-0.22% per day) were
observed in soils amended with either dairy manure or sewage sludge at
100 tonnes/ha. The rate of 14CO2 formation correlated directly with
the total microbial activity, as measured by total CO2 production.
In studies by Williams & Brown (1979), the persistence of per-
methrin in six soils was compared with that of fenvalerate under lab-
oratory conditions. The soils were treated with one of the insecticides
at 1 mg/kg and incubated under aerobic conditions for 16 weeks at a
temperature alternating between 20°C for 15 h and 10°C for 9 h to simu-
late the actual field conditions. With the exception of organic soil
from Cloverdale, degradation of permethrin was rapid in all soils, with
half-lives of 3 weeks or less. Under identical conditions, the half
life for fenvalerate was about 7 weeks. Again, trans-permethrin was
lost more rapidly than the cis isomer, and there was very little loss
of either insecticide in the sterilized soils. With Cloverdale organic
soil, a greater degree of adsorption onto the soil organic fraction
might have contributed to the slower degradation rate.
When soil was treated with [14C-cyclopropyl]-permethrin, sugar
beet grown on the treated soil was found to contain radiolabelled
conjugates of Cl2CA and 3-(2,2-dichlorovinyl)-2-methylcyclopropane-
1,2-dicarboxylic acid (21) (Leahey & Carpenter, 1980). It was possible
that both carboxylic acids were formed in the soil and were sub-
sequently taken up by the plants (see section 4.1).
5. KINETICS AND METABOLISM
5.1 Metabolism in Mammals
Appraisal
The metabolic pathways of permethrin in mammals are summarized in
Fig. 3.
The metabolism of permethrin has been studied in great detail in
various species of mammals, using a variety of radiolabelled isomers.
Permethrin administered to mammals was rapidly metabolized and almost
completely eliminated from the body within a short period of time. The
trans isomer of permethrin was eliminated more rapidly that the cis
isomer. Radiocarbon from trans- permethrin was excreted mostly in
urine, whereas that from the cis isomer was eliminated both in urine
and faeces to a similar extent. Expiration as CO2 contributed little
to its elimination in mammals. Major routes of metabolism for both
trans and cis isomers were ester cleavage and oxidation of the 4'-
position of the terminal aromatic ring. A less important reaction in
mammals was hydroxylation of the geminal dimethyl group of the cyclo-
propane ring. Major metabolites thus formed were Cl2 CA in free and
glucuronide form, sulfate conjugate of 4'-hydroxy-3-phenoxybenzoic
acid, PBacid in free and conjugate form, and hydroxymethyl-Cl2 CA as a
glucuronide conjugate. This latter compound was also isolated as a
lactone where the hydroxymethyl group and the carboxy group had a cis
configuration.
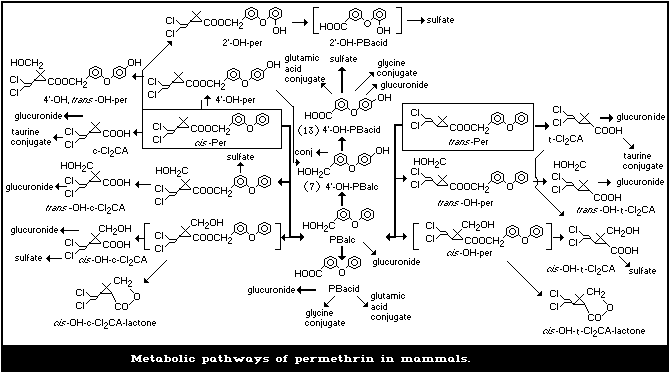 5.1.1 Mouse
In studies by Shah et al. (1981), 14C- cis-permethrin was applied
to the clipped skin of mice at a level of 1 mg/kg body weight in 0.1 ml
of acetone. The mice were restrained until the solvent had evaporated
and then placed in mouse metabolism cages. They were sacrificed at 1,
5, 15, 50, 480, and 2880 min after treatment and examined for absorp-
tion, distribution, and excretion of the insecticide. About 40% of the
applied permethrin had moved from the site of application within 5 min
and appeared to move rapidly to other parts of the body.
5.1.2 Rat
When a preparation of [1RS, trans ]- or [1RS, cis]-permethrin (14C-
labelled in the alcohol or acid moiety) was administered orally to male
rats at levels of 1.6-4.8 mg/kg, the compounds were rapidly metabolized
and labels in the acid and alcohol fragments were almost completely
eliminated from the body within a few days. The radiocarbon (alcohol
or acid label) from the cis isomer was eliminated in the urine (52-54%
of the dose) and the faeces (45-47%), whereas 79-82% of the radiocarbon
from the trans isomer appeared in the urine and 16-18% in the faeces
within 12 days after administration. The 14CO2 contained in the ex-
pired air corresponded to less than 0.5% of the dose. The tissue
residues were very low, although the cis isomer showed relatively
higher residue levels (0.46-0.62 mg/kg tissue) in the fat (Gaughan et
al., 1977). The major metabolite from the acid moiety was Cl2CA (17),
which was mostly excreted in the urine, conjugated with glucuronic
acid. This accounted for 50-63% of the dose from trans-permethrin and
15-22% from cis-permethrin. Oxidation at either of the geminal di-
methyl groups occurred to the extent of 4.3-10.4% (trans) or 12.2-14.9%
(cis), and these oxidised products were eliminated in the urine and
faeces as such or as the lactone or glucuronide. The major metabolite
from the alcohol moiety was 3-(4'-hydroxyphenoxy)benzoic acid (4'-OH-
PBacid) sulfate, accounting for 30.7-42.8% of the dose (trans) and
19.5-29.3% (cis). From cis-permethrin, 2'-OH-PBacid sulfate (about 3%)
was identified. Another significant metabolite was PBacid, which oc-
curred free and as glucuronide or glycine conjugates, and accounted for
25-31% (trans) and 5.7-10.1% (cis) of the dosed radiocarbon. Except for
a trace of PBacid, all the above metabolites from the alcohol moiety
were excreted entirely in the urine. However, the faeces of rats dosed
with trans-permethrin contained 1-2% of the radioactive dose as PBalc.
Thus substantial portions of the radioactive metabolites in the
recovered excreta were identified. The proposed metabolic pathways for
cis- and trans-permethrin are shown in Fig. 2. The five principle
sites of metabolic attack in both permethrin isomers were ester
cleavage, oxidation at the trans- and cis-methyl of the geminal
dimethyl group of the acid moiety, and oxidation at the 2'- and 4'-
positions of the phenoxy group. Conjugation of the resultant car-
boxylic acids, alcohols, and phenols with glucuronic acid, glycine, and
sulfuric acid occurred to varying extent. cis-Permethrin (29) was more
stable than trans-permethrin (30), and the cis isomer yielded four
faecally excreted ester metabolites that resulted from hydroxylation at
the 2'- or 4'-position of the phenoxy group or at the trans- or cis-
methyl group on the cyclopropane ring (e.g., (35'), (36')). The ester-
cleaved metabolites were extensively excreted into the urine whereas
the metabolites retaining an ester bond were found only in the faeces.
The major metabolite from the acid moiety of both isomers was Cl2CA
(31,31') in free (1-8%) and glucuronide (14-42%) forms. Other signifi-
cant metabolites were trans-OH-Cl2CA (32,32') (1-5%) and cis-OH-
Cl2CA (33,33') in the free (3-5%), lactone (34,34') (0-4%) and
glucuronide (1-2%) forms. On the other hand, the alcohol moiety
released after cleavage of the ester bond of both isomers was converted
mainly to the sulfate of 3-(4'-hydroxyphenoxy)benzoic acid (4'-OH-
PBacid) (13) (29-43% of the dose) and PBacid (12) in the free (1-10%)
and glucuronide (7-15%) forms. Other significant metabolites of the
alcohol moiety were PBalc (6), PBacid-glycine and the sulfate of 3-(2'-
hydroxyphenoxy) benzoic acid (2'-OH-PBacid) (14). [1RS, trans ]- and
[1RS, cis ]-permethrin showed no significant differences in metabolic
fate in the rat from [1R, trans ]- and [1R, cis ]-permethrin, respect-
ively (Elliott et al., 1976; Gaughan et al., 1977).
5.1.3 Goat
When ten consecutive oral doses of 14C- trans- or 14C- cis-permethrin
(labelled in the acid or alcohol moieties) at 0.2-0.3 mg/kg body
weight/day were given to lactating goats, they excreted 72-79% and 25-
36% of the trans and cis isomer doses, respectively, in urine and 12-
15% and 52-68%, respectively, in the faeces. The amounts of the
radiocarbon appearing in the milk were less than 0.7% with any one of
the four 14C-labelled preparations. Concerning the tissue residues 24h
after the last dose, detectable levels of radiocarbon were found in
most tissues, but none was higher than 0.04 mg/kg for the trans isomer
or 0.25 mg/kg for the cis isomer (Hunt & Gilbert, 1977).
The permethrin metabolites in goats were formed through cleavage of
the ester linkage, hydroxylation at the cis- or trans-methyl of the
geminal dimethyl group, and hydroxylation at the 4'-position of the
phenoxybenzyl moiety. Some of these metabolic products were further
oxidized and/or conjugated with glycine, glutamic acid and glucuronic
acid. The major compounds found in faeces after dosing with cis-per-
methrin were unmetabolized parent compound, 4'-OH-permethrin (35'),
trans-OH-permethrin (36'), PBalc, cis-OH- cis-Cl2CA-lactone (34') and
eight unidentified ester metabolites (Fig. 2). The faeces of goats
treated with the trans isomer contained large amounts of the parent
compound (41-79% of the faecal 14C) and of PBalc (8-25%) and cis-OH-
trans-Cl2CA-lactone (34). Also, three unidentified ester metab-
olites were found (8-23%). On the other hand, major urinary metab-
olites from the alcohol moiety of both isomers were PBacid-glycine (7-
9% of the urinary 14C) and 4'-OH-PBacid-glycine (4-12%). PBalc,
PBacid, 4'-OH-PBalc (7), 4'-OH-PBacid, PBacid-glutamic acid and 4'-OH-
PBacid-glutamic acid were also identified as minor metabolites. The
urine of goats treated with both isomers contained, as major compo-
nents, Cl2CA in the free form (2-47% of the urinary 14C) and as a
glucuronide (27-71%). Cl2CA-glucuronide was obtained to a larger
extent with the trans isomer than with the cis isomer. Other major
metabolites of the cis isomer were cis-OH-Cl2CA (33') (9-11%) and
cis-OH- cis-Cl2CA-lactone (34') (11-16%). trans-OH-Cl2CA (32,32') was
detected as a minor metabolite of both isomers. The milk of goats con-
tained the parent compounds, PBacid-glycine, and 4 -OH-PBacid-glycine.
On administration of the cis isomer, a larger amount of the parent
compound was excreted in the milk than in the case of the trans isomer.
Comparatively, when the trans isomer was administered, PBacid-glycine
was detected in the milk to a larger extent than with the cis isomer.
Most of the radioactivity in the fat was attributable to the parent
compound, or ester metabolites such as trans-OH-permethrin (36,36')
and trans-OH-permethrin conjugate (Ivie & Hunt, 1980).
5.1.4 Cow
When four lactating Jersey cows were orally administered 14C- trans- or
cis-permethrin preparations (labelled either in the alcohol or acid
moiety; three doses of ca. 1 mg/kg body weight at 24-h intervals), the
radiocarbon was almost completely eliminated in the faeces and urine 12
or 13 days after the initial dose. There was more faecal elimination of
the radiocarbon and higher tissue residue levels in the fat with the
cis isomer than the trans isomer. The 14C blood level reached a tran-
sient peak shortly after each dose and decreased to an insignificant
level within 2 to 4 days after the last dose. Higher blood levels were
attained with 14C- trans-permethrin labelled in the acid moiety than
when labelled in the alcohol moiety. This difference arising from
labelling positions was not evident with cis-permethrin. The radio-
carbon excreted in the milk was less than 0.5% of the dose. The lowest
14C level in milk was obtained from 14C- trans-permethrin (acid
moiety labelling) and the highest with 14C-trans-permethrin (alcohol
moiety labelling). With all labelled preparations, however, the radio-
carbon levels in milk decreased to <100 µg/litre within 2 to 4 days
after treatment ceased. The only radiolabelled compound recovered from
milk, in the case of the trans isomer, was unmetabolized permethrin,
whereas with the cis isomer 85% of the radiocarbon was as parent com-
pound and 15% as trans-OH- cis-permethrin (36 ). The metabolic reac-
tions of permethrin in cows were similar to those in rats and hens. In
cows, the permethrin isomers, their mono- and dihydroxy derivatives,
and PBalc, appeared only in the faeces, while the cis-OH-Cl2CA-
lactones (34,34') appeared in both faeces and urine. The remaining
metabolites appeared only in the urine. Although a slightly larger
portion of cis-permethrin than trans-permethrin was excreted un-
changed, there were similar amounts of ester metabolites with both iso-
mers. These ester metabolites were hydroxylated at the trans- or cis-
methyl positions of the geminal dimethyl group, at the 4'-position of
the phenoxybenzyl group, or at both the geminal dimethyl and phenoxy
groups. The preferred hydroxylation site with both isomers was the
trans-methyl group. The major metabolites from the acid moieties of
both isomers was the corresponding cis-OH-Cl2CA (33,33') and its
lactone and Cl2CA-glucuronide, while trans-OH- cis-Cl2CA (32') was also
a major metabolite from cis-permethrin. On the other hand, the major
metabolites from the alcohol moiety of both isomers were PBacid-glycine
(3-11% of the dose), PBalc (8-10%), and PBacid-glutamic acid (12-28%)
(Gaughan et al., 1978a).
5.1.5 Man
Two human volunteers, who consumed about 2 and 4 mg of permethrin
(25:75), respectively, excreted 18-37% and 32-39% of the administered
dose, detected as the metabolite Cl2CA, after acid hydrolysis of
their urine collected over 24 h (Cridland & Weatherley, 1977a,b).
5.2 Metabolism in Hens
A mixture (cis:trans = 25:75) of permethrin labelled with 14C in
the alcohol moiety was sprayed on 28 hens at doses of 3.77 or 11.94
mg/hen. The hens treated with the low dose showed no detectable levels
of radiocarbon in the gizzard, heart, lung, muscle, or egg white 24 h
after spraying, but the radiocarbon in the egg yolk reached a maximum
level of 0.049 mg/kg 5 days after treatment. The concentration of per-
methrin residues in the fat reached a peak 7 days after treatment and
no significant radioactivity was detectable after 4 weeks. With the
high dose, the radiocarbon in the skin had reached 6.69 mg/kg after 3
days. Small quantities of the radiocarbon were found in the egg yolks
(0.121 mg/kg) after 5 days and fat (0.110 mg/kg) after 1 day (Hunt et
al., 1979).
When White Leghorn hens were treated orally three consecutive days
with one of four 14C- trans- and cis-permethrin isomers labelled in
the alcohol or acid moieties at 10 mg/kg body weight, they showed no
signs of poisoning. More than 87% of the radiocarbon from the four
labelled preparations was found in the excreta 9 days after the initial
dose, 0.7-4.7% of the dose was exhaled as 14CO2, and 0.12-0.47% and
0.06-0.66% of the radiocarbon was recovered in egg yolk and fat (sub-
cutaneous and visceral fat), respectively. Both the cis isomers
labelled in the alcohol and acid moieties showed recoveries 3 to >10
times higher in the fat and egg yolk than those shown by the corre-
sponding trans isomers. The excreta (0-72 h) contained 1.7 times more
cis-permethrin than trans-permethrin. Hydroxylated ester metab-
olites of trans-permethrin were not excreted, but four monohydroxy and
dihydroxy esters (i.e., trans-OH-permethrin, 4'-OH-permethrin, 4'-OH,
trans-OH-permethrin (37) and trans-OH-permethrin sulfate) of cis-per-
methrin were found. Metabolites from the acid moieties of both isomers
were the Cl2CA isomers in free, glucuronide, and taurine conjugate
forms, trans-OH-Cl2CA (32,32'), cis-OH-Cl2CA (33,33'), cis-OH-Cl2CA
lactone (34,34'), and cis-OH-Cl2CA sulfate. trans-OH-Cl2CA (32,32')
was obtained from the cis isomer to larger extents than from the trans
isomer, whereas the amounts of cis-OH-Cl2CA (33,33') were larger with
the trans isomer than with the cis isomer. The metabolites from the
alcohol moiety included PBalc, PBacid, their 4'-hydroxy-derivatives and
the corresponding sulfate, the glucuronide of PBalc, and a variety of
unidentified conjugates of 4'-OH-PBalc (7) and 4'-OH-PBacid (13). The
taurine conjugate of PBacid was not detected. The metabolites produced
in largest amounts were the unidentified conjugates of 4'-OH-PBalc (6-
13% of the dose) and 4'-OH-PBacid (2-11%). The yolk of eggs 5 and 6
days after initial dosing contained 4.4 times more cis-permethrin than
trans-permethrin in unchanged form and the same ester metabolites of
cis-permethrin as those found in the excreta. Other metabolites in
the yolk were generally the same as those in the excreta. Overall,
cis-permethrin appeared at higher levels than trans-permethrin in
the egg yolk, fatty tissues, and excreta. Radiocarbon from cis-permethrin
preparations also persisted longer in the blood than that from trans-per-
permethrin preparations. It probably resulted from more rapid ester
cleavage of the trans isomer than the cis isomer, based on the relative
amounts of hydrolysis products from the two isomers in hen excreta
(Gaughan et al., 1978b).
5.3 Enzymatic Systems for Biotransformation
In studies by Shono et al. (1979), 1 µg each of [1RS, trans ]-permethrin
or [1RS, cis ]-permethrin was incubated at 37°C for 30 min with 2.2 ml
of ca. 10% rat and mouse liver microsomes under the following
conditions:
* microsomes treated with tetraethyl pyrophosphate (TEPP) (no ester-
ase and oxidase activity),
* normal microsomes (esterase activity),
* TEPP-treated microsomes plus NADPH (oxidase activity),
* normal microsomes plus NADPH (esterase plus oxidase activity).
Each esterase preparation hydrolyzed trans-permethrin to a much
greater extent than the corresponding cis isomer. In contrast, oxidat-
ive metabolism was greater for cis-permethrin than for trans-permethrin
except with the mouse microsomes, where the reactions of both isomers
proceeded to a similar extent. Aryl hydroxylation occurred at the 4'-
and 6-positions with the mouse enzymes but only at the 4'-position with
the rat enzymes. Hydroxylation at the 2'-position was observed only
with the cis-permethrin and mouse oxidase system. The amount of trans-
hydroxymethyl ester metabolites exceeded that of the corresponding
cis-hydroxymethyl compounds except with rat enzymes acting on trans-
permethrin. In general, oxidative activity with rat microsomes was
weaker than that with mouse microsomes. The dihydroxy ester metabolite
was evident only with cis-permethrin. The cis-hydroxymethyl ester
derivatives of trans-permethrin were further oxidized to the corre-
sponding aldehyde and carboxylic acid by the mouse enzymes. The pre-
ferred sites of hydroxylation, based on all identified metabolites in
the oxidase and esterase-plus-oxidase systems, were as follows (Shono
et al., 1979):
trans-permethrin
mouse: cis-methyl > trans-methyl > 4'-carbon = 6-carbon
rat: 4'-carbon = cis-methyl > trans-methyl
cis-permethrin
mouse: trans-methyl > cis-methyl = 4'-carbon > 6-carbon
> 2'-carbon
rat: 4'-carbon = trans-methyl > cis-methyl
When 100 nmol each of [1R, trans]-, [1S, trans]-, [1RS, trans]-, [1R, cis]-,
[1S, cis ]-, or [1RS, cis ]-permethrin were incubated individually with
2.5 ml of mouse liver microsome (1.5-2.0 mg of protein), the trans iso-
mers were much more rapidly hydrolyzed than the corresponding cis iso-
mers. Of the trans isomers, [1S,trans] isomer was hydrolyzed to a
greater extent than the other trans isomers. On the other hand, when
esterase activity was suppressed, there were no distinct differences in
the oxidative metabolic rates between trans and cis isomers (Soderlund
& Casida, 1977).
The persistence of isomers of permethrin, cypermethrin, delta-
methrin, and fenvalerate in the fat and brain after oral or intraper-
itoneal administration of these pesticides to rats was compared by
Marei et al. (1982). Residues in fat and brain were much higher and more
persistent with cis-permethrin than with trans-permethrin or the
alpha-cyano phenoxybenzyl pyrethroids (cypermethrin, fenvalerate, delta-
methrin). Brain levels of trans-permethrin (but not of cis-permethrin)
were greatly elevated after pretreatment with pyrethroid esterase and
oxidase inhibitors (i.e. tri- o-cresyl phosphate, S,S,S-tributyl phosph-
orotrithioate, phenyl saligenin cyclic phosphanate as esterase inhibit-
ors and piperonyl butoxide as oxidase inhibitor).
Pyrethroid carboxyesterase(s) that hydrolyze esters of chrysan-
themic acid were purified by Suzuki & Miyamoto (1978) from rat liver
microsomes by cholic acid solubilization, ammonium sulfate fraction-
ation, heat treatment, and DEAE-Sephadex A-50 column chromatography.
The 45-fold purified enzyme (38% yield) was thought to consist of a
single protein with a relative molecular mass of approximate 74 000, a
Michaelis constant (Km) of 0.21 mmol/litre for [1R, trans ]-phenothrin,
and an optimum pH of 7-9. It was susceptible to inhibition by organo-
phosphate and carbamate insecticides and insensitive to p-chloromerc-
urybenzoic acid and to mercuric and cupric ions. The enzyme seemed
to require neither coenzymes nor cofactors and hydrolysed trans isomers
of several synthetic pyrethroids (tetramethrin, resmethrin, trans- or
cis-phenothrin and permethrin) well, at more or less similar rates. On
the other hand, the cis isomers were hydrolysed at rates one-fifth to
one-tenth of those of the trans counterparts. The purified pyrethroid
carboxyesterase was apparently identical in nature to malathion carbox-
yesterase and p-nitro phenyl acetate carboxyesterase (Suzuki &
Miyamoto, 1978).
6. EFFECTS ON THE ENVIRONMENT
Acute toxicity data of permethrin on aquatic and terrestrial non-
target organisms are summarized in Tables 5 and 6, respectively.
6.1 Toxicity to Aquatic Organisms
6.1.1 Aquatic microorganisms
Stratton & Corke (1982) investigated the toxicity of permethrin and
ten of its degradation products on the growth, photosynthesis, and
acetylene-reducing activity of two species of green algae ( Chlorella
pyrenoidosa and Scenedesmus quadricaudata ) and three species of cyano-
bacteria ( Anabaena spp.). Permethrin itself was relatively non-toxic
to photosynthesis (EC50 values >100 mg/litre) and to acetylene re-
duction (EC50 values >100 mg/litre). Its degradation products were
similarly non-toxic to green algae. However, the cyanobacteria were
susceptible to some of the breakdown products of permethrin. Growth
was the most sensitive parameter with growth yield showing EC50 values
of 2.5, 2.2, and 1.4 mg/litre for the cyanobacteria and 2.8 and 4.3 mg/
litre for the green algae with PBalc and similar values for three of
the five test species with PBald. A complex test system found interac-
tions between the various metabolites and the parent compound which
were sometimes additive and sometimes synergistic. The authors con-
cluded that it is difficult to assess the true toxicity of compounds to
soil and water microorganisms without considering the breakdown prod-
ucts. The cyanobacteria are significant nitrogen-fixing organisms in
wet tropical soils.
6.1.2 Aquatic invertebrates
Non-target invertebrates, except molluscs, are more sensitive to
permethrin than fish, as shown in Table 5.
During exposure of permethrin for up to 28 days, the caddisfly
(Brachycentrus americanus) and the stonefly (Pteronarcys dorsata)
showed behavioural changes or death at concentrations as low as 0.022
µg/litre (Anderson, 1982).
A 3-h exposure to permethrin, at 50 mg/litre, was not lethal to
Daphnia pulex. The no-effect levels were 1 µg/litre for racemic, 1R
or (+)-trans, and 1R or (+)-cis, and 50 µg/litre for 1S or (-)-trans
and 1S or (-)-cis isomers (Miyamoto, 1976).
Zitko et al. (1979) established lethal threshold values for the
lobster Homarus americanus of 7.00 µg/litre for technical permethrin
and 0.40 µg/litre for [1R, cis ]-permethrin.
Larval oyster and bullfrog (tadpole) are highly tolerant to the
insecticide, with LC50 values of >1000 and 7000 µg/litre, respect-
ively.
Stratton & Corke (1981) reported that the 48-h LC50 of permethrin
to juvenile and adult waterfleas Daphnia magna was 0.2-0.6 µg/litre. A
further series of experiments involved the addition of algae or
bacteria to the cultures of daphnids, since feeding of daphnids during
these tests had been reported to reduce the toxicity of several
chemicals to the animals. With permethrin, however, algae in the test
vessel increased the lethal effect of the compound. Algae, bacteria,
and also inert silica powder adhered to the swimming antennae of the
daphnids, causing the daphnids to sink and die on the bottom of the
flasks. The effect was greatest with adults; the shed carapaces of
juvenile showed the same adhesion of particulates but moulting pro-
tected the juveniles to some degree. This raised toxicity was due to a
direct effect of the permethrin on the daphnids and not to a tendency
for the compound to cause flocculation of the suspended material.
Friesen et al. (1983) tested the toxicity of permethrin to
sediment-living nymphs of the mayfly Hexagenia rigida. In test vessels
containing water without sediment, the 6-h LC50 was estimated to lie
between 0.58 and 2.06 µg/litre; no nymphs survived exposure to water
concentrations of 7.63 µg/litre. In the presence of sediment,
lethality was reduced; there was 88% mortality of nymphs exposed to
permethrin in water at 7.63 µg/litre after 24-h exposure. Mortality
reached 100% only after 7 days exposure with sediment. Maximum concen-
trations of permethrin in the sediment over the 7 days were estimated
to be 50 µg/kg dry weight. The authors also exposed nymphs to sedi-
ment previously exposed to permethrin. The initial water concentration
was again 7.63 µg/litre, and the sediment was left for 8 days to take
up the insecticide before the water was decanted off. Nymphs were
introduced along with clean water over the contaminated sediment. There
was 100% mortality in the exposed nymphs. Long-term exposure to both
water and sediment contaminated with permethrin led to increasing
mortality up to 4 weeks; there was little further mortality between 4
and 10 weeks. Lethality reached 100% after exposure to either water or
sediment at a simulated application rate of 7.3 g/ha over 10 weeks (95%
at 4 weeks), whereas a simulated exposure equivalent to 0.6 g/ha led to
74% mortality after a 10-week exposure of the nymphs in water and 45%
after exposure of the nymphs to sediment. The authors comment that it
is not yet possible to state a concentration of permethrin in sediment
which is sufficiently low to permit successful recolonization of con-
taminated sediment.
6.1.3 Fish
Permethrin is highly toxic to fish, as shown in Table 5. Prep-
arations using an emulsifiable concentrate of permethrin enhanced its
toxicity twofold (Coats & O'Donnell-Jeffery, 1979).
The lethal toxicity of permethrin varied inversely with water
temperature, particularly between 10 and 20°C, and with body weight
between 1 and 50 g. There was a 10-fold difference between the 96-h
LC50 values at 10 and 20°C. At 15°C, a large trout (200 g) was con-
siderably more (about 100 times) tolerant than a small fish (1 g)
(Kumaraguru & Beamish, 1981).
Toxicity to fish is linked more with the nature of the optical iso-
mers than with that of the stereoisomers; i.e. 1R isomers are more
toxic than 1S isomers. Trans and cis isomers are of similar toxicity
(Miyamoto, 1976).
Zitko et al. (1979) established lethal threshold values for the
Atlantic salmon Salmo salar of 8.8 µg/litre for technical permethrin
and 1.34 µg/litre for [1R, cis ]-permethrin.
Hansen et al. (1983) exposed embryos and the hatched fry of
sheepshead minnow (Cyprinodon variegatus), continuously over 28 days,
to concentrations of permethrin of 1.25, 2.5, 5.0, 10, 20, or 40 µg/
litre. The survival of embryos was unaffected by any of the test
concentrations. Fry were affected by exposure to 20 µg/litre or more
but unaffected by 10 µg/litre. The toxicity curve was steep; 99% of fry
survived at 10 µg/litre but only 1% at 20 µg/litre. The authors
estimated the ratio between the 96-h LC50 and the NOEL to be 0.8.
Holdway & Dixon (1988) exposed larval fish (white sucker,
Catastomus commersoni, and flagfish, Jordanella floridae ) to per-
methrin in a single 2-h pulse and examined lethality over the following
96-h. They examined the effect of age, and whether or not the fish were
fed, upon the toxic effect of the insecticide. Feeding decreased the
toxicity of permethrin to flagfish at 2 and 4 days of age but not at 8
days. Age was the most important factor affecting toxicity. The 96-h
LC50 (from exposure for 2 h) was 5.55 mg/litre, 7.91 mg/litre, and
0.57 mg/litre for flagfish of age 2, 4, and 8 days, respectively.
White suckers were most susceptible to permethrin at 20 days of age,
with a 2-h LC50 of 10.0 µg/litre. Unfed white suckers at 13 and 20
days of age were highly susceptible to permethrin, with LC50 values of
2.0 and 1.0 µg/litre, respectively. The authors pointed out that
permethrin is toxic to cladocerans (waterfleas) at levels of 0.5 µg/
litre and that fish could suffer both from the direct toxic effect of
the insecticide and the added effect experienced during food deprivation.
When used for mosquito control, the safety margins (LC50 fish/LC50
mosquito larvae) for permethrin and cis-permethrin are 2-40 and 25-65,
respectively (Mulla et al., 1978a). When intraperitoneally injected
into rainbow trout, the trans- and cis-permethrin isomers were about
110 and 5 times, respectively, more toxic to trout than to mice,
(Glickman et al., 1981).
Rainbow trout exposed to sublethal concentrations of permethrin in
water (0.09-0.35 µg/litre) or in food (85-350 µg/kg) in 20-40-day
experiments showed similar branchial changes, i.e. epithelial separa-
tion or necrosis, mucus cell hyperplasia, clubbing of epithelial cells,
or hyperplasia and fusion of adjacent secondary lamellae (Kumaraguru et
al., 1982).
6.1.4 Field studies and community effects
In studies by Mulla et al. (1975), permethrin was applied to ponds
at rates of 56 g/ha and 112 g/ha in field trials. The numbers of
Tanypodinae (mostly Pentaneura and Tanypus ) and Chironominae (mostly
Tanytarsus and Chironomus ) midge larvae were slightly depressed by
the 56 g/ha treatment. Mayfly (mostly Baetis sp.) naiads and diving
beetle ( Hydrophilidae and Dytiscidae ) larvae and adults were also
affected. However, Copepoda (mostly Cyclops and Diaptomus ) and
Ostracoda (mostly Cypricercus and Cyprinotus ) were not greatly affec-
ted. The effect on these non-target organisms was much greater at the
higher dose level of permethrin, except for the ostracods. It was con-
cluded that permethrin affected mayfly naiads severely during the
exposure period. Most populations recovered within 2 weeks following
exposure.
Mayfly naiads (mostly baetids) were also adversely affected by per-
methrin at 5.6-28 g/ha and by its cis isomer at 2.8-28 g/ha. There was
a slight recovery within 1-3 weeks after treatment (Mulla et al.,
1978b).
Permethrin was applied weekly for 6 or 8 successive weeks at the
mosquito larvicidal rate of 28 g/ha (and at a rate 5 times higher) to
ponds where 20 individuals of mosquito fish or desert pupfish were
maintained. The insecticide produced no adverse effects on the two
species of fish, and the number of fish in the treated ponds increased
markedly during the experiment. At the higher rate, mats of algae were
formed, probably as the result of elimination by permethrin of her-
bivorous arthropods that feed on the algae (Mulla et al., 1981).
Kaushik et al. (1985) investigated the effect of permethrin on the
pelagic zooplankton of a 10-ha lake in southern Ontario, Canada. The
insecticide was applied to give nominal water concentrations of 0.5,
5.0, or 50 µg/litre in in situ aquatic enclosures of 5 x 5 x 5 m.
Macrozooplankton (daphnids and copepods) were most susceptible to the
insecticide. The numbers, which in untreated enclosures were 100-1000
organisms per litre of water, fell in the days immediately following
treatment to 1-10 at 0.5 µg/litre, 0.1-1.0 at 5.0 µg/litre, and
0.01-0.1 organisms per litre at 50 µg/litre of permethrin (nominal).
Microzooplankton (mainly rotifers) were unaffected by all doses except
the highest. At this dose, numbers fell transitorily to about one tenth
of their control levels (about 1000 organisms per litre). In all cases
of treatment, rotifer numbers increased between 5- and 10-fold 20 to
100 days after treatment. The authors attributed this rise in numbers
to the resistance of the organisms to the insecticide coupled with a re-
duction in the predator organisms that normally feed on the rotifers.
Populations of macrozooplankton had returned to normal within 250 days
of treatment (after the winter freeze) even with the highest dose of
50 µg/litre. Recovery was quicker with the lower doses (about 60 days
for treatment at 5 µg/litre and 30 days for most species at 0.5 µg/
litre). Despite this recovery in overall numbers of zooplankton, there
was a decrease in the species diversity of the larger, predator organ-
isms at all treatment levels. The enclosures, of course, prevented
immigration from the surrounding areas of water.
Helson et al. (1986) placed two species of aquatic arthropods (the
amphipod Gammarus pseudolimnaeus and the mosquito Aedes aegypti ) in
open containers of different sizes downwind from the application of
permethrin to young spruce trees for control of defoliators. The insec-
ticide was applied to trees 0.75-0.8 m tall in a single swath from a
mistblower backpack. Nominal application rates of 36 g ai/ha were used
with a swath width of 10 m, and standard and ultra-low volume appli-
cations were made. The mortality of Gammarus after 48 h averaged 95%
(range 76-100%) in the first trial and 85% (range 37-100%) in a dupli-
cate trial in containers 10 m downwind from the spraying. The effect
was reduced to 12% and 18% at a distance of 30 m from the spraying and
further reduced to an average of 5% 50 m from the spray. Mosquito
larvae were examined only in the second trial and showed 76% (37-100%)
at 10 m falling to 6% and 2% at 30 and 50 m, respectively, from the
spray. Mortality increased over the following 9 days. The authors also
determined 48-h LC50 values for the two organisms in containers simi-
larly placed in the field. These were 0.37 µg/litre and 0.69 µg/litre for
Gammarus and mosquito larvae, respectively, while LC95 values were
0.61 µg/litre for Gammarus and 1.14 µg/litre for mosquito larvae.
The authors regarded these data as a "worst case" , since sediment in
natural water and flowing water in streams could be expected to reduce
the toxic effect of the permethrin. They concluded that a 30-m safety
zone needs to be left using this application method between a spraying
area and natural waters to avoid killing aquatic arthropods.
When permethrin was applied by airplane to the surface of a creek
at a nominal rate of 70 g ai/ha, the actual concentration that reached
the ground was 13.4 g ai/ha. Dramatic, but short-lived, increases in
the drift of aquatic insects (particularly large catches of springtail
(Collembola), mayfly nymphs (Ephemeroptera heptageniidea), water
scavenger beetle larvae (Coleoptera hydrophilidae), midge larvae and
pupae, water boatmen (Hemiptera corixidae), predaceous diving beetles
(Coleoptera dytiscidae), and caddisfly larvae (Trichoptera)) occurred
after treatment. No effects on populations of organisms that inhabited
the bottom layer of the creek were noticeable. The permethrin sprayed
had little effect on caged or native fish and no fish mortality was re-
corded due to the treatment. From these data, it could be inferred that
permethrin had no significant impact on the aquatic system (Kingsbury,
1976).
After an aerial application of permethrin at 17.5 g ai/ha, residues
attained peak concentrations of 147.0 µg/litre in ponds and 2.5 µg/litre
in streams, but accumulations and persistence of the pesticide in
bottom sediment were negligible. Noticeable increases in the number of
drifting organisms occurred in the treatment block ( Ephemeroptera hepta-
geniidae, Baetidae, and Plecoptera nymphs ) and 2.1 km downstream
(mayfly and stonefly nymphs) over a 24-h period immediately after the
spray. A slight reduction in the bottom fauna also occurred
downstream. When exposed in cages in the ponds, yellow perch (Perca flu-
vescens) did not exhibit any adverse effects; little or no accumulated
permethrin residues were detectable in the fish following exposure. Ob-
servations of the headwater ponds indicated that permethrin application
resulted in noticeable levels of distress and mortality to surface and
littoral invertebrates and produced a similar impact on benthic organ-
isms (Kingsbury & Kreutzweiser, 1980a).
Table 5. Acute toxicity of permethrin to non-target aquatic organisms
--------------------------------------------------------------------------------------------------------------------------------------------
Species Size Duration Toxicitya Formu- Sys- Tempera- pH Hardness Reference
of test (µg/litre) lationd teme ture (°C)
--------------------------------------------------------------------------------------------------------------------------------------------
A. Freshwater Organisms
Arthropods
Crayfish 0.8 - 1.2 cm, 96 h 0.39 EC S 24 100 Jolly et al. (1978)
(Procambarus clarkii) (0.05 g)
2 - 3 cm, 96 h 0.62 EC S 24 100 Jolly et al. (1978)
(0.5 g)
Water flea (Daphnia pulex) 3 h > 50 000 T S 25 Miyamoto (1976)
3 h > 50 000 (+)-trans S 25 Miyamoto (1976)
3 h > 50 000 (+)-cis S 25 Miyamoto (1976)
3 h > 50 000 (-)-trans S 25 Miyamoto (1976)
3 h > 50 000 (-)-cis S 25 Miyamoto (1976)
Water flea (Daphnia magna) 1st instar 48 h 1.26 T S 18 7.4 42 Mayer &
Amphipod immature 96 h 0.17 T S 17 7.4 42 Ellersieck (1986)
(Gammarus pseudolimnaeus) Mayer &
Midge (Chironomus plumosus) 3rd instar 48 h 0.56 T S 22 7.4 42 Ellersieck (1986)
Caddisfly 21 days 0.17 T F 15 7.6 - 7.8 46 - 48 Anderson (1982)
(Brachycentrus americanus)
Fish
Salmon (Salmo salar) 6.2 cm, 5.3 g 96 h 12 T R 10 McLeese et al.
(1980)
Rainbow trout 1 g 96 h 0.62 T F 5 7.9 - 8.2 358 - 363 Kumaraguru &
(Salmo gairdneri) 1 g 96 h 0.69 T F 10 7.9 - 8.2 358 - 363 Beamish (1981)
1 g 96 h 3.17 T F 15 7.9 - 8.2 358 - 363 Kumaraguru &
1 g 96 h 6.43 T F 20 7.9 - 8.2 358 - 363 Beamish (1981)
5 g 96 h 6.43 T F 15 7.9 - 8.2 358 - 363 Kumaraguru &
20 g 96 h ca. 50 T F 15 7.9 - 8.2 358 - 363 Beamish (1981)
50 g 96 h 287 T F 15 7.9 - 8.2 358 - 363 Kumaraguru &
200 g 96 h 314 T F 15 7.9 - 8.2 358 - 363 Beamish (1981)
6 cm, 3 g 24 h 135 T 10 7.5 110 Coats & O'Donnell-
6 cm, 3 g 24 h 61 EC 10 7.5 110 Jeffery (1979)
--------------------------------------------------------------------------------------------------------------------------------------------
Table 5. (contd.)
--------------------------------------------------------------------------------------------------------------------------------------------
Species Size Duration Toxicitya Formu- Sys- Tempera- pH Hardness Reference
of test (µg/litre) lationd teme ture (°C)
--------------------------------------------------------------------------------------------------------------------------------------------
Rainbow trout (contd.)
(Salmo gairdneri) 5 - 6 cm 48 h 6.0 EC 12- Mulla et al.
5 - 6 cm 48 h 7.0 cis, EC 25.5 (1978a)
2 - 4 g 24 h 18 T S 12 Glickman
2 - 4 g 24 h 25 cis S 12 et al.
2 - 4 g 24 h 14 trans S 12 (1981)
Killifish adult 48 h 41 T S 25 Miyamoto (1976)
(Oryzias latipes) adult 48 h 17 (+)-trans S 25 Miyamoto (1976)
adult 48 h 13 (+)-cis S 25 Miyamoto (1976)
adult 48 h > 10 000 (-)-trans S 25 Miyamoto (1976)
adult 48 h > 10 000 (-)-cis S 25 Miyamoto (1976)
Channel catfish 1.4 - 1.7 cm, 96 h 1.1 EC S 24 100 Jolly et al. (1978)
(Ictalurus punctatus) (0.02 g)
Largemouth Bass 4.5 - 5.5 cm, 96 h 8.5 EC S 24 100 Jolly et al. (1978)
(Micropterus salmoides) (1.14 g)
Mosquitofish 1.5 - 2.5 cm, 96 h 15 EC S 24 100 Jolly et al. (1978)
(Gambusia affinis) (0.25 g)
4 - 5 cm 48 h 97.0 EC 8.8 - 16 Mulla et al.
4 - 5 cm 48 h 13.0 cis, EC 8.8 - 16 (1978a)
Brook trout 1.2 g 96 h 3.2 T S 12 7.5 40 Mayer & Ellersieck
(Salvelinus foutinalis) (1986)
Fathead minnow 0.6 g 96 h 5.7 T S 22 7.3 38 Mayer & Ellersieck
(Pimephales promelas) (1986)
Bluegill sunfish 0.7 g 96 h 5.0 T S 22 7.3 38 Mayer & Ellersieck
(Lepomis macrochirus) (1986)
Desert pupfish 4 - 5 cm 48 h 5.0 EC S 11 - 16.5 Mulla et al.
(Cyprinodon macularis) 4 - 5 cm 48 h 5.0 cis, EC S 11 - 16.5 (1978a)
Tilapia mossambica 5 - 6 cm 48 h 44.0 EC S 15 - 21.4 Mulla et al.
5 - 6 cm 48 h 5.6 cis, EC S 15 - 21.4 (1978a)
Amphibian
Bullfrog, tadpole 0.6 - 0.8 cm 96 h 7033 EC S 24 100 Jolly et al. (1978)
(Rana catesbeiana)
--------------------------------------------------------------------------------------------------------------------------------------------
Table 5. (contd.)
--------------------------------------------------------------------------------------------------------------------------------------------
Species Size Duration Toxicitya Formu- Sys- Tempera- pH Salinity Reference
of test (µg/litre) lationd teme ture (°C) (°/oo)
--------------------------------------------------------------------------------------------------------------------------------------------
B. Estuarine and Marine Organisms
Algae
Skeletonema costatum 96 h 92b T 20 Borthwich & Walsh
(1981)
Molluscs
Oyster (Crassostrea virginica) 2-h larva 48 h > 1000c T S 25 20 Borthwich & Walsh
(1981)
Arthropods
Lobster (Homarus americanus) 450 g 96 h 0.73 T R 10 30 McLeese et al.
Shrimp (Crangon Septemspinosa) 1.3 g 96 h 0.13 T R 10 (1980)
Shrimp (Mysidopsis bahia) 1-day, 96 h 0.046 T S 25 20 Borthwich
juvenile & Walsh
Stone crab (Menippe mercenaria) Zoea larva 96 h 0.018 T S 25 20 (1981)
Pink shrimp (Penaeus duorarum) adult 96 h 0.22 T F 25 25 Mayer (1987)
Fish
Harpacticoid (Nitocra spinipes) 3-6 weeks old 96 h 0.6 EC S 20 - 22 7.8 7 Linden et al.
Bleak (Alburnus alburnus) 8 cm 96 h 4 - 8 EC S 10 7.8 7 (1979)
Sheepshead minnow 28-day fry 96 h 88 T S 25 20 Borthwich & Walsh
(Cyprinodon variegatus) (1981)
adult 96 h 7.8 T F 30 22 Mayer (1987)
Atlantic silverside adult 96 h 2.2 T F 26 25 Mayer (1987)
(Menidia menidia)
Striped mullet (Mugil cephalus) juvenile 96 h 5.5 T F 24 19 Mayer (1987)
--------------------------------------------------------------------------------------------------------------------------------------------
a Values are LC50 unless stated otherwise.
b EC50 (growth inhibition).
c EC50 (abnormal development).
d T = Technical, EC = Emulsifiable concentrate.
e R = Renewal, S = Static, F = Flow-through.
f expressed as mg CaCO3/litre.
When permethrin was sprayed at 8.8, 17.5, 35.0, or 70.0 g ai/ha by
aeroplane over small trout streams, the impact on aquatic invertebrates
and effects on the general fish population correlated with the dose.
There was an increase in the number of organisms drifting downstream,
the major ones being mayflies, followed by caddisflies, stoneflies, and
chironomids. The total number of drifting organisms was greater than
the pre-spray average by factors of 303, 699, 4960, and 6450 with spray
concentrations of 8.8, 17.5, 35.0, and 70.0 g ai/ha, respectively. A
return to pre-spray drift levels was evident within 36 h after appli-
cation at 8.8 and 17.5 g ai/ha whereas drifting of organism persisted
for up to 72 h at the higher application rates of 35 and 70 g ai/ha.
Following the spraying of permethrin at these higher doses, there was
no evidence of fish mortality, but there appeared a dramatic change in
the diets of fish (such as the native brook trout and sculpins). These
fish became virtually completely dependent on terrestrial invertebrates
rather than on the aquatic insects for food. When the rate of appli-
cation was low (8.8 g ai/ha) permethrin did not appear to affect these
fish, either in mortality rate or in their diet composition (Kingsbury
& Kreutzweiser, 1980b).
Serial applications of permethrin, once or twice at 17.5 g ai/ha,
resulted in catastrophic drift of aquatic invertebrates and substantial
depletion of benthos in streams within the application blocks and up to
2 km downstream. Despite massive disturbances of benthos, repopulation
of bottom fauna was evident within 2.5 months and had virtually re-
turned to normal within 3.5 months. Permethrin residues attained peak
levels of 1.35 µg/litre in standing water and 1.94 µg/litre in
flowing water in the sprayed regions. The residue persisted at low
concentrations for up to 96 h after spraying (Kreutzweiser, 1982).
In a study by Kingsbury & Kreutzweiser (1987), aerial applications
of permethrin to forests over several seasons at 8.8, 17.5, 35, and 70
g ai/ha did not cause mortality to native and caged fish (minnows,
mudminnows, perch, and under-yearling and yearling Atlantic salmon).
The composition of the salmonid diet was subsequently altered from
aquatic insects (mayfly nymphs, stonefly nymphs, and various aquatic
fly larvae) to terrestrial arthropods. The duration of changes ranged
from a few months after applications of 8.8 and 17.5 g ai/ha to a year
or longer after treatment with 35 and 70 g ai/ha. There were temporary
reductions in fish growth rate and fish densities in the treated area,
which returned to normal within four months after treatment. In the
same study, the effect on stream invertebrates was also evaluated.
Large drifts of invertebrates were observed immediately after appli-
cations and continued for 24 to 72 h. Although the peak of permethrin
residues in stream water was higher after the second application (0.36-
1.80 µg/litre) than the first (0.25-0.62 µg/litre), drift response
to the second application ranged from 6 to 62% of the first drift,
indicating that first application deleted susceptible invertebrates
(e.g., Ephemeroptera nymphs, Plecoptera, Trichoptera, and the
Diptera families) and that a much smaller residual population re-
sponded to the second treatment. Recovery of benthic fauna was appar-
ent between 1 and 18 months after spraying. The double treatment
reduced benthos density to a point at which recovery of numbers was
slower than after the single application (Kreutzweiser & Kingsbury,
1987).
6.2 Toxicity to Terrestrial Organisms
6.2.1 Soil microorganisms
Mathur et al. (1980) applied permethrin (Ambush 5 G) to Canadian
soils with a high content of organic material at a rate of 2.24 kg/ha.
Lettuce or carrots were grown on the plots. Soil cores were taken at
regular intervals and bacterial and fungal numbers were estimated, soil
nutrients were measured, and acid phosphatase activity in the soil was
monitored. Residues of permethrin persisted throughout the growing
season up to crop harvest, when 65% of the original concentration in
soil was found (113 days after application). This reflected the poor
breakdown of permethrin in organic soils compared to mineral soils.
Permethrin suppressed bacterial and actinomycete populations in samples
taken 1, 9, and 27 days after application, but control levels were re-
gained after 41 days (the next sampling time). The available nitrogen
and phosphorus was lower in treated soil at some points during the
study, but these changes were not consistent. Soil respiration and acid
phosphatase activity were higher in permethrin-treated plots, though
not consistently so. Permethrin had a greater effect when carrots were
grown than when lettuce was grown. The yield of neither crop was
affected. Most of the effects reported were transitory and none were
of overall significance for either the soil or the crop.
6.2.2 Terrestrial invertebrates
Under laboratory conditions, permethrin is highly toxic to certain
beneficial insects or natural enemies of pests, as shown in Table 6.
Cox & Wilson (1984) treated honey bee workers topically with a sub-
lethal dose of permethrin (0.09 µg/bee in 1.0 µl of acetone applied
to the thorax). This dose gave no higher mortality than treatment with
acetone alone. The bees, which were individually tagged, were housed in
an observation hive and trained before the experiment to feed at an
artificial feeding station 5 m from the hive. The experiment was con-
ducted at 35°C because at lower temperatures this dose resulted in
mortality. Treated bees made less foraging trips than controls and gave
food less frequently to other bees in the hive. Other behaviours were
increased in treated bees, i.e., self-cleaning, trembling dance, ab-
domen tucking, and rotating and cleaning of abdomen while rubbing the
hind legs together. Gerig (1985) also reported minimal lethal effects
on honey bees of permethrin used at recommended rates and that the
insecticide had a strong repellent effect.
Table 6. Acute toxicity of permethrin to non-target terrestrial organisms
-----------------------------------------------------------------------------------------------------------------------------
Species Size Application Duration Toxicitya Temperature Reference
(°C)
-----------------------------------------------------------------------------------------------------------------------------
Bird
Hen oral > 1.5 g/kg b.w.b Millner & Butterworth
(1977)
Chicken oral > 3 g/kg b.w.b Worthing & Walker
Japanese quail oral 5 days > 13.5 g/kg b.w.b (1983)
diet 5 days > 5 g/kg dietc Hill & Camardese
diet 5 days > 5 g/kg dietd (1986)
Mallard duck diet 5 days > 27.5 g/kg diet Ross et al. (1976b)
Mallard duck oral > 13.5 g/kg b.w.b Ross et al. (1976a)
Starling diet 5 days > 27.5 g/kg diet Ross et al. (1976d)
Starling oral > 38 g/kg b.w.b Ross et al. (1976c)
Ring-necked pheasant diet 5 days > 27.5 g/kg diet Ross et al. (1976e)
Ring-necked pheasant oral > 13.5 g/kg b.w.b Ross et al. (1977a)
Arthropods
Honeybee (Apis mellifera) contact 0.11 µg/beeb 26 - 27 Stevenson et al. (1978)
oral 0.28 µg/beeb 26 - 27 Stevenson et al. (1978)
Insect parasite
Ichneumoid (Campoletis adult male film 24 h 0.31 µg/vial Plapp & Vinson (1977)
sonorensis)
Insect predator
Carabid (Pterostichus adult 0.16 g topical > 2000 µg/insectb 21 Hagley et al. (1980)
melanarius)
Carabid (Harpalus affinis) adult 0.05 g topical 116 µg/insectb 21 Hagley et al. (1980)
Carabid (Amara sp.) adult 0.03 g topical 25 µg/insectb 21 Hagley et al. (1980)
Earwig (Labidura raparia) mature soil 0.1 kg 6% mortality Workman (1977)
ai/ha
mature soil 0.2 kg 50% mortality Workman (1977)
ai/ha
Green lacewing larvae
(Chrysopa carnea) 5-6 days old film 9.87 µg/vial 25 Plapp & Bull (1978)
Predaceous mite species
Mataseiulus occidentalis adult female slide-dip 0.72, 1.32, 27.5 Roush & Hoy (1978)
(3 strains) method 14.8 mg ai/litre
young gravid leaf-disc 2.8 mg ai/litre 27 Hoy et al. (1979)
female method
(Amblyseius fallacis) adult female slide-dip 14 mg ai/litre 27 Rock (1979)
method
-----------------------------------------------------------------------------------------------------------------------------
a LC50 values, unless stated otherwise.
b LD50 values; b.w. = body weight.
c Technical product.
d Emulsifiable concentrate.
Pike et al. (1982) applied permethrin by helicopter at a rate of
0.22 kg ai/ha to fields of maize (Zea mays). The applications were
made early in the morning before bees were actively foraging for pollen
and were repeated every 3 to 6 days (to a maximum of six applications
per season). The trial was repeated for 3 years. There was no differ-
ence in the number of dead bees per hive between treated and control
areas, but a marked reduction in the number of bees foraging in treated
fields, indicating avoidance of permethrin. The authors concluded that
treatment of corn fields with permethrin at the normal application rate
is safe for bees as long as the application does not coincide with bee
activity in the area.
The acute toxicity values of permethrin to tobacco budworm
(Heliothis virescens), to the green lacewing (Chrysopa carnea), a
predator of tobacco budworm, and to Campoletis sonorensis, an
ichneumoid parasite of tobacco budworm, showed that permethrin was
approximately 18 times less toxic to the predators than to the pest
(Plapp & Bull, 1978; Plapp & Vinson, 1977).
Larvae of the green lacewing exhibited marked tolerance to per-
methrin, and to its cis or trans isomers, when dosed topically with
250 µg per insect (about 25 000 µg/g). This value is ca. 10 000 times
greater than the LD50 value for the tobacco budworm (Shour & Crowder,
1980).
Workman (1977) added permethrin to loamy sand soil into which the
striped earwig (Labidura riparia), an effective insect predator of
the cabbage looper, was introduced. The insecticide was of low toxicity
to the earwig at dosage rates which gave good looper control.
The susceptibility of carabids to permethrin appears to be in-
versely related to beetle size, as shown in Table 6. When permethrin
was applied at a concentration of 0.21-0.85 kg ai/ha to an apple
orchard, it did not significantly affect the numbers of Pterostichus
melanarius at any time during the season, but the numbers of Harpalus
affinis and Amara sp. were significantly reduced 3-5 days after appli-
cation. This result reflected the toxicity ratings by means of LD50
values obtained in laboratory studies. The total seasonal numbers of
these carabids were not significantly affected by permethrin, owing to
short residual effects (Hagley et al., 1980).
In laboratory tests, LC50 values of permethrin for two strains of
spider mites (Tetranychus urticae) were ca. 20-40 times greater than
those for three strains of predator mites (Mataseiulus occidentalis)
(Roush & Hoy, 1978), and ca.15 times greater than those for Amblyseius
fallacis (Rock, 1979). These studies indicate that the use of per-
methrin at the recommended rates of 60-120 mg ai/litre would be detri-
mental to orchard integrated mite control programs.
In laboratory tests, the Pacific spider mite (T. pacificus) has
been found to be 40 times more tolerant to permethrin than the predator
mite (M. occidentalis), (Hoy et al., 1979). The spraying of vineyards
with 15 or 30 mg ai/litre resulted in substantially higher populations
of T. pacificus and E. willamettei for about one month due to re-
duction in predator species numbers. Similarly, spraying with 60 or
120 mg ai/litre produced a subsequent increase of Eotetranychus
willamettei late in August and September for the above reason (Hoy et
al., 1979).
When permethrin was applied to apple trees at a concentration of
40 mg/litre, no predator mites (Typhlodromus pyri) were found for 4-6
weeks, and only small numbers were found 10 weeks after the spray. On
the other hand, permethrin had no appreciable toxicity to the spider
mite (Panonychus ulmi). The virtual elimination of the predatory mite
by permethrin spraying led to a marked population increase of P. ulmi
later in the same season (Aliniazee & Cranham, 1980).
In apple and pear orchards, applications of permethrin at 30 mg
ai/litre reduced the numbers of a predatory mite (M. occidentalis) to
almost zero and dramatically increased the populations of spider mites
(T. urticae, Tetranychus mcdanieli, or P. ulmi) (Hoyt et al., 1978).
From the above findings it appeared that permethrin, when applied
according to recommendations, is relatively harmless to insect pred-
ators, with the exception of predaceous mite species. Everts et al.
(1985) investigated the effects of permethrin on beneficial terrestrial
arthropods in the soil and vegetation in areas surrounding applications
of the insecticide to control tsetse flies in the Ivory Coast, West
Africa. Permethrin was used as a 2.5% wettable powder at a rate of 121
g ai/ha during January (a minimum temperature of 20.0°C and a maximum
of 36.0°C). The permethrin application significantly reduced popu-
lations of Coleoptera, Lepidoptera, Ephydridae, Chloropidae, Muscidae,
Ichneumonidea, Chalcidoidea and Proctotrupoidea. The populations of
almost all these groups were found to recover to normal levels within 2
months, i.e., before the likely time of respraying for the control of
the tsetse flies. However, one Proctotrupoid genus, Cremastobaeus, was
eliminated by permethrin treatment.
6.2.3 Birds
Neither an acute oral nor a dietary LD50 or LC50 has been estab-
lished accurately because of the very low toxicity of permethrin to
birds (Table 6). The acute LD50 is >3000 mg/kg body weight and the
dietary toxicity >5000 mg/kg diet. (Worthing & Walker, 1983; Hill &
Camardese, 1986).
The inclusion of permethrin at up to 40 mg/kg in the diet of laying
hens for 28 days had no adverse effects on the health of parent birds
or on egg production quality, hatchability, or the viability of the
chicks produced (Ross et al. 1977b).
6.2.4 Mammals
Racey & Swift (1986) treated roosting boxes for pipistrelle bats
with various wood preservatives and allowed the bats to roost in the
boxes for up to 154 days. There were no toxic effects of mixtures of
synthetic pyrethroids including permethrin. The authors concluded that
the insecticide component of wood preservatives should be pyrethroids
when bats are present in the area.
6.3 Uptake, Loss, Bioaccumulation and Biomagnification
Proposed metabolic pathways of permethrin in fish are summarized in
Fig. 4.
5.1.1 Mouse
In studies by Shah et al. (1981), 14C- cis-permethrin was applied
to the clipped skin of mice at a level of 1 mg/kg body weight in 0.1 ml
of acetone. The mice were restrained until the solvent had evaporated
and then placed in mouse metabolism cages. They were sacrificed at 1,
5, 15, 50, 480, and 2880 min after treatment and examined for absorp-
tion, distribution, and excretion of the insecticide. About 40% of the
applied permethrin had moved from the site of application within 5 min
and appeared to move rapidly to other parts of the body.
5.1.2 Rat
When a preparation of [1RS, trans ]- or [1RS, cis]-permethrin (14C-
labelled in the alcohol or acid moiety) was administered orally to male
rats at levels of 1.6-4.8 mg/kg, the compounds were rapidly metabolized
and labels in the acid and alcohol fragments were almost completely
eliminated from the body within a few days. The radiocarbon (alcohol
or acid label) from the cis isomer was eliminated in the urine (52-54%
of the dose) and the faeces (45-47%), whereas 79-82% of the radiocarbon
from the trans isomer appeared in the urine and 16-18% in the faeces
within 12 days after administration. The 14CO2 contained in the ex-
pired air corresponded to less than 0.5% of the dose. The tissue
residues were very low, although the cis isomer showed relatively
higher residue levels (0.46-0.62 mg/kg tissue) in the fat (Gaughan et
al., 1977). The major metabolite from the acid moiety was Cl2CA (17),
which was mostly excreted in the urine, conjugated with glucuronic
acid. This accounted for 50-63% of the dose from trans-permethrin and
15-22% from cis-permethrin. Oxidation at either of the geminal di-
methyl groups occurred to the extent of 4.3-10.4% (trans) or 12.2-14.9%
(cis), and these oxidised products were eliminated in the urine and
faeces as such or as the lactone or glucuronide. The major metabolite
from the alcohol moiety was 3-(4'-hydroxyphenoxy)benzoic acid (4'-OH-
PBacid) sulfate, accounting for 30.7-42.8% of the dose (trans) and
19.5-29.3% (cis). From cis-permethrin, 2'-OH-PBacid sulfate (about 3%)
was identified. Another significant metabolite was PBacid, which oc-
curred free and as glucuronide or glycine conjugates, and accounted for
25-31% (trans) and 5.7-10.1% (cis) of the dosed radiocarbon. Except for
a trace of PBacid, all the above metabolites from the alcohol moiety
were excreted entirely in the urine. However, the faeces of rats dosed
with trans-permethrin contained 1-2% of the radioactive dose as PBalc.
Thus substantial portions of the radioactive metabolites in the
recovered excreta were identified. The proposed metabolic pathways for
cis- and trans-permethrin are shown in Fig. 2. The five principle
sites of metabolic attack in both permethrin isomers were ester
cleavage, oxidation at the trans- and cis-methyl of the geminal
dimethyl group of the acid moiety, and oxidation at the 2'- and 4'-
positions of the phenoxy group. Conjugation of the resultant car-
boxylic acids, alcohols, and phenols with glucuronic acid, glycine, and
sulfuric acid occurred to varying extent. cis-Permethrin (29) was more
stable than trans-permethrin (30), and the cis isomer yielded four
faecally excreted ester metabolites that resulted from hydroxylation at
the 2'- or 4'-position of the phenoxy group or at the trans- or cis-
methyl group on the cyclopropane ring (e.g., (35'), (36')). The ester-
cleaved metabolites were extensively excreted into the urine whereas
the metabolites retaining an ester bond were found only in the faeces.
The major metabolite from the acid moiety of both isomers was Cl2CA
(31,31') in free (1-8%) and glucuronide (14-42%) forms. Other signifi-
cant metabolites were trans-OH-Cl2CA (32,32') (1-5%) and cis-OH-
Cl2CA (33,33') in the free (3-5%), lactone (34,34') (0-4%) and
glucuronide (1-2%) forms. On the other hand, the alcohol moiety
released after cleavage of the ester bond of both isomers was converted
mainly to the sulfate of 3-(4'-hydroxyphenoxy)benzoic acid (4'-OH-
PBacid) (13) (29-43% of the dose) and PBacid (12) in the free (1-10%)
and glucuronide (7-15%) forms. Other significant metabolites of the
alcohol moiety were PBalc (6), PBacid-glycine and the sulfate of 3-(2'-
hydroxyphenoxy) benzoic acid (2'-OH-PBacid) (14). [1RS, trans ]- and
[1RS, cis ]-permethrin showed no significant differences in metabolic
fate in the rat from [1R, trans ]- and [1R, cis ]-permethrin, respect-
ively (Elliott et al., 1976; Gaughan et al., 1977).
5.1.3 Goat
When ten consecutive oral doses of 14C- trans- or 14C- cis-permethrin
(labelled in the acid or alcohol moieties) at 0.2-0.3 mg/kg body
weight/day were given to lactating goats, they excreted 72-79% and 25-
36% of the trans and cis isomer doses, respectively, in urine and 12-
15% and 52-68%, respectively, in the faeces. The amounts of the
radiocarbon appearing in the milk were less than 0.7% with any one of
the four 14C-labelled preparations. Concerning the tissue residues 24h
after the last dose, detectable levels of radiocarbon were found in
most tissues, but none was higher than 0.04 mg/kg for the trans isomer
or 0.25 mg/kg for the cis isomer (Hunt & Gilbert, 1977).
The permethrin metabolites in goats were formed through cleavage of
the ester linkage, hydroxylation at the cis- or trans-methyl of the
geminal dimethyl group, and hydroxylation at the 4'-position of the
phenoxybenzyl moiety. Some of these metabolic products were further
oxidized and/or conjugated with glycine, glutamic acid and glucuronic
acid. The major compounds found in faeces after dosing with cis-per-
methrin were unmetabolized parent compound, 4'-OH-permethrin (35'),
trans-OH-permethrin (36'), PBalc, cis-OH- cis-Cl2CA-lactone (34') and
eight unidentified ester metabolites (Fig. 2). The faeces of goats
treated with the trans isomer contained large amounts of the parent
compound (41-79% of the faecal 14C) and of PBalc (8-25%) and cis-OH-
trans-Cl2CA-lactone (34). Also, three unidentified ester metab-
olites were found (8-23%). On the other hand, major urinary metab-
olites from the alcohol moiety of both isomers were PBacid-glycine (7-
9% of the urinary 14C) and 4'-OH-PBacid-glycine (4-12%). PBalc,
PBacid, 4'-OH-PBalc (7), 4'-OH-PBacid, PBacid-glutamic acid and 4'-OH-
PBacid-glutamic acid were also identified as minor metabolites. The
urine of goats treated with both isomers contained, as major compo-
nents, Cl2CA in the free form (2-47% of the urinary 14C) and as a
glucuronide (27-71%). Cl2CA-glucuronide was obtained to a larger
extent with the trans isomer than with the cis isomer. Other major
metabolites of the cis isomer were cis-OH-Cl2CA (33') (9-11%) and
cis-OH- cis-Cl2CA-lactone (34') (11-16%). trans-OH-Cl2CA (32,32') was
detected as a minor metabolite of both isomers. The milk of goats con-
tained the parent compounds, PBacid-glycine, and 4 -OH-PBacid-glycine.
On administration of the cis isomer, a larger amount of the parent
compound was excreted in the milk than in the case of the trans isomer.
Comparatively, when the trans isomer was administered, PBacid-glycine
was detected in the milk to a larger extent than with the cis isomer.
Most of the radioactivity in the fat was attributable to the parent
compound, or ester metabolites such as trans-OH-permethrin (36,36')
and trans-OH-permethrin conjugate (Ivie & Hunt, 1980).
5.1.4 Cow
When four lactating Jersey cows were orally administered 14C- trans- or
cis-permethrin preparations (labelled either in the alcohol or acid
moiety; three doses of ca. 1 mg/kg body weight at 24-h intervals), the
radiocarbon was almost completely eliminated in the faeces and urine 12
or 13 days after the initial dose. There was more faecal elimination of
the radiocarbon and higher tissue residue levels in the fat with the
cis isomer than the trans isomer. The 14C blood level reached a tran-
sient peak shortly after each dose and decreased to an insignificant
level within 2 to 4 days after the last dose. Higher blood levels were
attained with 14C- trans-permethrin labelled in the acid moiety than
when labelled in the alcohol moiety. This difference arising from
labelling positions was not evident with cis-permethrin. The radio-
carbon excreted in the milk was less than 0.5% of the dose. The lowest
14C level in milk was obtained from 14C- trans-permethrin (acid
moiety labelling) and the highest with 14C-trans-permethrin (alcohol
moiety labelling). With all labelled preparations, however, the radio-
carbon levels in milk decreased to <100 µg/litre within 2 to 4 days
after treatment ceased. The only radiolabelled compound recovered from
milk, in the case of the trans isomer, was unmetabolized permethrin,
whereas with the cis isomer 85% of the radiocarbon was as parent com-
pound and 15% as trans-OH- cis-permethrin (36 ). The metabolic reac-
tions of permethrin in cows were similar to those in rats and hens. In
cows, the permethrin isomers, their mono- and dihydroxy derivatives,
and PBalc, appeared only in the faeces, while the cis-OH-Cl2CA-
lactones (34,34') appeared in both faeces and urine. The remaining
metabolites appeared only in the urine. Although a slightly larger
portion of cis-permethrin than trans-permethrin was excreted un-
changed, there were similar amounts of ester metabolites with both iso-
mers. These ester metabolites were hydroxylated at the trans- or cis-
methyl positions of the geminal dimethyl group, at the 4'-position of
the phenoxybenzyl group, or at both the geminal dimethyl and phenoxy
groups. The preferred hydroxylation site with both isomers was the
trans-methyl group. The major metabolites from the acid moieties of
both isomers was the corresponding cis-OH-Cl2CA (33,33') and its
lactone and Cl2CA-glucuronide, while trans-OH- cis-Cl2CA (32') was also
a major metabolite from cis-permethrin. On the other hand, the major
metabolites from the alcohol moiety of both isomers were PBacid-glycine
(3-11% of the dose), PBalc (8-10%), and PBacid-glutamic acid (12-28%)
(Gaughan et al., 1978a).
5.1.5 Man
Two human volunteers, who consumed about 2 and 4 mg of permethrin
(25:75), respectively, excreted 18-37% and 32-39% of the administered
dose, detected as the metabolite Cl2CA, after acid hydrolysis of
their urine collected over 24 h (Cridland & Weatherley, 1977a,b).
5.2 Metabolism in Hens
A mixture (cis:trans = 25:75) of permethrin labelled with 14C in
the alcohol moiety was sprayed on 28 hens at doses of 3.77 or 11.94
mg/hen. The hens treated with the low dose showed no detectable levels
of radiocarbon in the gizzard, heart, lung, muscle, or egg white 24 h
after spraying, but the radiocarbon in the egg yolk reached a maximum
level of 0.049 mg/kg 5 days after treatment. The concentration of per-
methrin residues in the fat reached a peak 7 days after treatment and
no significant radioactivity was detectable after 4 weeks. With the
high dose, the radiocarbon in the skin had reached 6.69 mg/kg after 3
days. Small quantities of the radiocarbon were found in the egg yolks
(0.121 mg/kg) after 5 days and fat (0.110 mg/kg) after 1 day (Hunt et
al., 1979).
When White Leghorn hens were treated orally three consecutive days
with one of four 14C- trans- and cis-permethrin isomers labelled in
the alcohol or acid moieties at 10 mg/kg body weight, they showed no
signs of poisoning. More than 87% of the radiocarbon from the four
labelled preparations was found in the excreta 9 days after the initial
dose, 0.7-4.7% of the dose was exhaled as 14CO2, and 0.12-0.47% and
0.06-0.66% of the radiocarbon was recovered in egg yolk and fat (sub-
cutaneous and visceral fat), respectively. Both the cis isomers
labelled in the alcohol and acid moieties showed recoveries 3 to >10
times higher in the fat and egg yolk than those shown by the corre-
sponding trans isomers. The excreta (0-72 h) contained 1.7 times more
cis-permethrin than trans-permethrin. Hydroxylated ester metab-
olites of trans-permethrin were not excreted, but four monohydroxy and
dihydroxy esters (i.e., trans-OH-permethrin, 4'-OH-permethrin, 4'-OH,
trans-OH-permethrin (37) and trans-OH-permethrin sulfate) of cis-per-
methrin were found. Metabolites from the acid moieties of both isomers
were the Cl2CA isomers in free, glucuronide, and taurine conjugate
forms, trans-OH-Cl2CA (32,32'), cis-OH-Cl2CA (33,33'), cis-OH-Cl2CA
lactone (34,34'), and cis-OH-Cl2CA sulfate. trans-OH-Cl2CA (32,32')
was obtained from the cis isomer to larger extents than from the trans
isomer, whereas the amounts of cis-OH-Cl2CA (33,33') were larger with
the trans isomer than with the cis isomer. The metabolites from the
alcohol moiety included PBalc, PBacid, their 4'-hydroxy-derivatives and
the corresponding sulfate, the glucuronide of PBalc, and a variety of
unidentified conjugates of 4'-OH-PBalc (7) and 4'-OH-PBacid (13). The
taurine conjugate of PBacid was not detected. The metabolites produced
in largest amounts were the unidentified conjugates of 4'-OH-PBalc (6-
13% of the dose) and 4'-OH-PBacid (2-11%). The yolk of eggs 5 and 6
days after initial dosing contained 4.4 times more cis-permethrin than
trans-permethrin in unchanged form and the same ester metabolites of
cis-permethrin as those found in the excreta. Other metabolites in
the yolk were generally the same as those in the excreta. Overall,
cis-permethrin appeared at higher levels than trans-permethrin in
the egg yolk, fatty tissues, and excreta. Radiocarbon from cis-permethrin
preparations also persisted longer in the blood than that from trans-per-
permethrin preparations. It probably resulted from more rapid ester
cleavage of the trans isomer than the cis isomer, based on the relative
amounts of hydrolysis products from the two isomers in hen excreta
(Gaughan et al., 1978b).
5.3 Enzymatic Systems for Biotransformation
In studies by Shono et al. (1979), 1 µg each of [1RS, trans ]-permethrin
or [1RS, cis ]-permethrin was incubated at 37°C for 30 min with 2.2 ml
of ca. 10% rat and mouse liver microsomes under the following
conditions:
* microsomes treated with tetraethyl pyrophosphate (TEPP) (no ester-
ase and oxidase activity),
* normal microsomes (esterase activity),
* TEPP-treated microsomes plus NADPH (oxidase activity),
* normal microsomes plus NADPH (esterase plus oxidase activity).
Each esterase preparation hydrolyzed trans-permethrin to a much
greater extent than the corresponding cis isomer. In contrast, oxidat-
ive metabolism was greater for cis-permethrin than for trans-permethrin
except with the mouse microsomes, where the reactions of both isomers
proceeded to a similar extent. Aryl hydroxylation occurred at the 4'-
and 6-positions with the mouse enzymes but only at the 4'-position with
the rat enzymes. Hydroxylation at the 2'-position was observed only
with the cis-permethrin and mouse oxidase system. The amount of trans-
hydroxymethyl ester metabolites exceeded that of the corresponding
cis-hydroxymethyl compounds except with rat enzymes acting on trans-
permethrin. In general, oxidative activity with rat microsomes was
weaker than that with mouse microsomes. The dihydroxy ester metabolite
was evident only with cis-permethrin. The cis-hydroxymethyl ester
derivatives of trans-permethrin were further oxidized to the corre-
sponding aldehyde and carboxylic acid by the mouse enzymes. The pre-
ferred sites of hydroxylation, based on all identified metabolites in
the oxidase and esterase-plus-oxidase systems, were as follows (Shono
et al., 1979):
trans-permethrin
mouse: cis-methyl > trans-methyl > 4'-carbon = 6-carbon
rat: 4'-carbon = cis-methyl > trans-methyl
cis-permethrin
mouse: trans-methyl > cis-methyl = 4'-carbon > 6-carbon
> 2'-carbon
rat: 4'-carbon = trans-methyl > cis-methyl
When 100 nmol each of [1R, trans]-, [1S, trans]-, [1RS, trans]-, [1R, cis]-,
[1S, cis ]-, or [1RS, cis ]-permethrin were incubated individually with
2.5 ml of mouse liver microsome (1.5-2.0 mg of protein), the trans iso-
mers were much more rapidly hydrolyzed than the corresponding cis iso-
mers. Of the trans isomers, [1S,trans] isomer was hydrolyzed to a
greater extent than the other trans isomers. On the other hand, when
esterase activity was suppressed, there were no distinct differences in
the oxidative metabolic rates between trans and cis isomers (Soderlund
& Casida, 1977).
The persistence of isomers of permethrin, cypermethrin, delta-
methrin, and fenvalerate in the fat and brain after oral or intraper-
itoneal administration of these pesticides to rats was compared by
Marei et al. (1982). Residues in fat and brain were much higher and more
persistent with cis-permethrin than with trans-permethrin or the
alpha-cyano phenoxybenzyl pyrethroids (cypermethrin, fenvalerate, delta-
methrin). Brain levels of trans-permethrin (but not of cis-permethrin)
were greatly elevated after pretreatment with pyrethroid esterase and
oxidase inhibitors (i.e. tri- o-cresyl phosphate, S,S,S-tributyl phosph-
orotrithioate, phenyl saligenin cyclic phosphanate as esterase inhibit-
ors and piperonyl butoxide as oxidase inhibitor).
Pyrethroid carboxyesterase(s) that hydrolyze esters of chrysan-
themic acid were purified by Suzuki & Miyamoto (1978) from rat liver
microsomes by cholic acid solubilization, ammonium sulfate fraction-
ation, heat treatment, and DEAE-Sephadex A-50 column chromatography.
The 45-fold purified enzyme (38% yield) was thought to consist of a
single protein with a relative molecular mass of approximate 74 000, a
Michaelis constant (Km) of 0.21 mmol/litre for [1R, trans ]-phenothrin,
and an optimum pH of 7-9. It was susceptible to inhibition by organo-
phosphate and carbamate insecticides and insensitive to p-chloromerc-
urybenzoic acid and to mercuric and cupric ions. The enzyme seemed
to require neither coenzymes nor cofactors and hydrolysed trans isomers
of several synthetic pyrethroids (tetramethrin, resmethrin, trans- or
cis-phenothrin and permethrin) well, at more or less similar rates. On
the other hand, the cis isomers were hydrolysed at rates one-fifth to
one-tenth of those of the trans counterparts. The purified pyrethroid
carboxyesterase was apparently identical in nature to malathion carbox-
yesterase and p-nitro phenyl acetate carboxyesterase (Suzuki &
Miyamoto, 1978).
6. EFFECTS ON THE ENVIRONMENT
Acute toxicity data of permethrin on aquatic and terrestrial non-
target organisms are summarized in Tables 5 and 6, respectively.
6.1 Toxicity to Aquatic Organisms
6.1.1 Aquatic microorganisms
Stratton & Corke (1982) investigated the toxicity of permethrin and
ten of its degradation products on the growth, photosynthesis, and
acetylene-reducing activity of two species of green algae ( Chlorella
pyrenoidosa and Scenedesmus quadricaudata ) and three species of cyano-
bacteria ( Anabaena spp.). Permethrin itself was relatively non-toxic
to photosynthesis (EC50 values >100 mg/litre) and to acetylene re-
duction (EC50 values >100 mg/litre). Its degradation products were
similarly non-toxic to green algae. However, the cyanobacteria were
susceptible to some of the breakdown products of permethrin. Growth
was the most sensitive parameter with growth yield showing EC50 values
of 2.5, 2.2, and 1.4 mg/litre for the cyanobacteria and 2.8 and 4.3 mg/
litre for the green algae with PBalc and similar values for three of
the five test species with PBald. A complex test system found interac-
tions between the various metabolites and the parent compound which
were sometimes additive and sometimes synergistic. The authors con-
cluded that it is difficult to assess the true toxicity of compounds to
soil and water microorganisms without considering the breakdown prod-
ucts. The cyanobacteria are significant nitrogen-fixing organisms in
wet tropical soils.
6.1.2 Aquatic invertebrates
Non-target invertebrates, except molluscs, are more sensitive to
permethrin than fish, as shown in Table 5.
During exposure of permethrin for up to 28 days, the caddisfly
(Brachycentrus americanus) and the stonefly (Pteronarcys dorsata)
showed behavioural changes or death at concentrations as low as 0.022
µg/litre (Anderson, 1982).
A 3-h exposure to permethrin, at 50 mg/litre, was not lethal to
Daphnia pulex. The no-effect levels were 1 µg/litre for racemic, 1R
or (+)-trans, and 1R or (+)-cis, and 50 µg/litre for 1S or (-)-trans
and 1S or (-)-cis isomers (Miyamoto, 1976).
Zitko et al. (1979) established lethal threshold values for the
lobster Homarus americanus of 7.00 µg/litre for technical permethrin
and 0.40 µg/litre for [1R, cis ]-permethrin.
Larval oyster and bullfrog (tadpole) are highly tolerant to the
insecticide, with LC50 values of >1000 and 7000 µg/litre, respect-
ively.
Stratton & Corke (1981) reported that the 48-h LC50 of permethrin
to juvenile and adult waterfleas Daphnia magna was 0.2-0.6 µg/litre. A
further series of experiments involved the addition of algae or
bacteria to the cultures of daphnids, since feeding of daphnids during
these tests had been reported to reduce the toxicity of several
chemicals to the animals. With permethrin, however, algae in the test
vessel increased the lethal effect of the compound. Algae, bacteria,
and also inert silica powder adhered to the swimming antennae of the
daphnids, causing the daphnids to sink and die on the bottom of the
flasks. The effect was greatest with adults; the shed carapaces of
juvenile showed the same adhesion of particulates but moulting pro-
tected the juveniles to some degree. This raised toxicity was due to a
direct effect of the permethrin on the daphnids and not to a tendency
for the compound to cause flocculation of the suspended material.
Friesen et al. (1983) tested the toxicity of permethrin to
sediment-living nymphs of the mayfly Hexagenia rigida. In test vessels
containing water without sediment, the 6-h LC50 was estimated to lie
between 0.58 and 2.06 µg/litre; no nymphs survived exposure to water
concentrations of 7.63 µg/litre. In the presence of sediment,
lethality was reduced; there was 88% mortality of nymphs exposed to
permethrin in water at 7.63 µg/litre after 24-h exposure. Mortality
reached 100% only after 7 days exposure with sediment. Maximum concen-
trations of permethrin in the sediment over the 7 days were estimated
to be 50 µg/kg dry weight. The authors also exposed nymphs to sedi-
ment previously exposed to permethrin. The initial water concentration
was again 7.63 µg/litre, and the sediment was left for 8 days to take
up the insecticide before the water was decanted off. Nymphs were
introduced along with clean water over the contaminated sediment. There
was 100% mortality in the exposed nymphs. Long-term exposure to both
water and sediment contaminated with permethrin led to increasing
mortality up to 4 weeks; there was little further mortality between 4
and 10 weeks. Lethality reached 100% after exposure to either water or
sediment at a simulated application rate of 7.3 g/ha over 10 weeks (95%
at 4 weeks), whereas a simulated exposure equivalent to 0.6 g/ha led to
74% mortality after a 10-week exposure of the nymphs in water and 45%
after exposure of the nymphs to sediment. The authors comment that it
is not yet possible to state a concentration of permethrin in sediment
which is sufficiently low to permit successful recolonization of con-
taminated sediment.
6.1.3 Fish
Permethrin is highly toxic to fish, as shown in Table 5. Prep-
arations using an emulsifiable concentrate of permethrin enhanced its
toxicity twofold (Coats & O'Donnell-Jeffery, 1979).
The lethal toxicity of permethrin varied inversely with water
temperature, particularly between 10 and 20°C, and with body weight
between 1 and 50 g. There was a 10-fold difference between the 96-h
LC50 values at 10 and 20°C. At 15°C, a large trout (200 g) was con-
siderably more (about 100 times) tolerant than a small fish (1 g)
(Kumaraguru & Beamish, 1981).
Toxicity to fish is linked more with the nature of the optical iso-
mers than with that of the stereoisomers; i.e. 1R isomers are more
toxic than 1S isomers. Trans and cis isomers are of similar toxicity
(Miyamoto, 1976).
Zitko et al. (1979) established lethal threshold values for the
Atlantic salmon Salmo salar of 8.8 µg/litre for technical permethrin
and 1.34 µg/litre for [1R, cis ]-permethrin.
Hansen et al. (1983) exposed embryos and the hatched fry of
sheepshead minnow (Cyprinodon variegatus), continuously over 28 days,
to concentrations of permethrin of 1.25, 2.5, 5.0, 10, 20, or 40 µg/
litre. The survival of embryos was unaffected by any of the test
concentrations. Fry were affected by exposure to 20 µg/litre or more
but unaffected by 10 µg/litre. The toxicity curve was steep; 99% of fry
survived at 10 µg/litre but only 1% at 20 µg/litre. The authors
estimated the ratio between the 96-h LC50 and the NOEL to be 0.8.
Holdway & Dixon (1988) exposed larval fish (white sucker,
Catastomus commersoni, and flagfish, Jordanella floridae ) to per-
methrin in a single 2-h pulse and examined lethality over the following
96-h. They examined the effect of age, and whether or not the fish were
fed, upon the toxic effect of the insecticide. Feeding decreased the
toxicity of permethrin to flagfish at 2 and 4 days of age but not at 8
days. Age was the most important factor affecting toxicity. The 96-h
LC50 (from exposure for 2 h) was 5.55 mg/litre, 7.91 mg/litre, and
0.57 mg/litre for flagfish of age 2, 4, and 8 days, respectively.
White suckers were most susceptible to permethrin at 20 days of age,
with a 2-h LC50 of 10.0 µg/litre. Unfed white suckers at 13 and 20
days of age were highly susceptible to permethrin, with LC50 values of
2.0 and 1.0 µg/litre, respectively. The authors pointed out that
permethrin is toxic to cladocerans (waterfleas) at levels of 0.5 µg/
litre and that fish could suffer both from the direct toxic effect of
the insecticide and the added effect experienced during food deprivation.
When used for mosquito control, the safety margins (LC50 fish/LC50
mosquito larvae) for permethrin and cis-permethrin are 2-40 and 25-65,
respectively (Mulla et al., 1978a). When intraperitoneally injected
into rainbow trout, the trans- and cis-permethrin isomers were about
110 and 5 times, respectively, more toxic to trout than to mice,
(Glickman et al., 1981).
Rainbow trout exposed to sublethal concentrations of permethrin in
water (0.09-0.35 µg/litre) or in food (85-350 µg/kg) in 20-40-day
experiments showed similar branchial changes, i.e. epithelial separa-
tion or necrosis, mucus cell hyperplasia, clubbing of epithelial cells,
or hyperplasia and fusion of adjacent secondary lamellae (Kumaraguru et
al., 1982).
6.1.4 Field studies and community effects
In studies by Mulla et al. (1975), permethrin was applied to ponds
at rates of 56 g/ha and 112 g/ha in field trials. The numbers of
Tanypodinae (mostly Pentaneura and Tanypus ) and Chironominae (mostly
Tanytarsus and Chironomus ) midge larvae were slightly depressed by
the 56 g/ha treatment. Mayfly (mostly Baetis sp.) naiads and diving
beetle ( Hydrophilidae and Dytiscidae ) larvae and adults were also
affected. However, Copepoda (mostly Cyclops and Diaptomus ) and
Ostracoda (mostly Cypricercus and Cyprinotus ) were not greatly affec-
ted. The effect on these non-target organisms was much greater at the
higher dose level of permethrin, except for the ostracods. It was con-
cluded that permethrin affected mayfly naiads severely during the
exposure period. Most populations recovered within 2 weeks following
exposure.
Mayfly naiads (mostly baetids) were also adversely affected by per-
methrin at 5.6-28 g/ha and by its cis isomer at 2.8-28 g/ha. There was
a slight recovery within 1-3 weeks after treatment (Mulla et al.,
1978b).
Permethrin was applied weekly for 6 or 8 successive weeks at the
mosquito larvicidal rate of 28 g/ha (and at a rate 5 times higher) to
ponds where 20 individuals of mosquito fish or desert pupfish were
maintained. The insecticide produced no adverse effects on the two
species of fish, and the number of fish in the treated ponds increased
markedly during the experiment. At the higher rate, mats of algae were
formed, probably as the result of elimination by permethrin of her-
bivorous arthropods that feed on the algae (Mulla et al., 1981).
Kaushik et al. (1985) investigated the effect of permethrin on the
pelagic zooplankton of a 10-ha lake in southern Ontario, Canada. The
insecticide was applied to give nominal water concentrations of 0.5,
5.0, or 50 µg/litre in in situ aquatic enclosures of 5 x 5 x 5 m.
Macrozooplankton (daphnids and copepods) were most susceptible to the
insecticide. The numbers, which in untreated enclosures were 100-1000
organisms per litre of water, fell in the days immediately following
treatment to 1-10 at 0.5 µg/litre, 0.1-1.0 at 5.0 µg/litre, and
0.01-0.1 organisms per litre at 50 µg/litre of permethrin (nominal).
Microzooplankton (mainly rotifers) were unaffected by all doses except
the highest. At this dose, numbers fell transitorily to about one tenth
of their control levels (about 1000 organisms per litre). In all cases
of treatment, rotifer numbers increased between 5- and 10-fold 20 to
100 days after treatment. The authors attributed this rise in numbers
to the resistance of the organisms to the insecticide coupled with a re-
duction in the predator organisms that normally feed on the rotifers.
Populations of macrozooplankton had returned to normal within 250 days
of treatment (after the winter freeze) even with the highest dose of
50 µg/litre. Recovery was quicker with the lower doses (about 60 days
for treatment at 5 µg/litre and 30 days for most species at 0.5 µg/
litre). Despite this recovery in overall numbers of zooplankton, there
was a decrease in the species diversity of the larger, predator organ-
isms at all treatment levels. The enclosures, of course, prevented
immigration from the surrounding areas of water.
Helson et al. (1986) placed two species of aquatic arthropods (the
amphipod Gammarus pseudolimnaeus and the mosquito Aedes aegypti ) in
open containers of different sizes downwind from the application of
permethrin to young spruce trees for control of defoliators. The insec-
ticide was applied to trees 0.75-0.8 m tall in a single swath from a
mistblower backpack. Nominal application rates of 36 g ai/ha were used
with a swath width of 10 m, and standard and ultra-low volume appli-
cations were made. The mortality of Gammarus after 48 h averaged 95%
(range 76-100%) in the first trial and 85% (range 37-100%) in a dupli-
cate trial in containers 10 m downwind from the spraying. The effect
was reduced to 12% and 18% at a distance of 30 m from the spraying and
further reduced to an average of 5% 50 m from the spray. Mosquito
larvae were examined only in the second trial and showed 76% (37-100%)
at 10 m falling to 6% and 2% at 30 and 50 m, respectively, from the
spray. Mortality increased over the following 9 days. The authors also
determined 48-h LC50 values for the two organisms in containers simi-
larly placed in the field. These were 0.37 µg/litre and 0.69 µg/litre for
Gammarus and mosquito larvae, respectively, while LC95 values were
0.61 µg/litre for Gammarus and 1.14 µg/litre for mosquito larvae.
The authors regarded these data as a "worst case" , since sediment in
natural water and flowing water in streams could be expected to reduce
the toxic effect of the permethrin. They concluded that a 30-m safety
zone needs to be left using this application method between a spraying
area and natural waters to avoid killing aquatic arthropods.
When permethrin was applied by airplane to the surface of a creek
at a nominal rate of 70 g ai/ha, the actual concentration that reached
the ground was 13.4 g ai/ha. Dramatic, but short-lived, increases in
the drift of aquatic insects (particularly large catches of springtail
(Collembola), mayfly nymphs (Ephemeroptera heptageniidea), water
scavenger beetle larvae (Coleoptera hydrophilidae), midge larvae and
pupae, water boatmen (Hemiptera corixidae), predaceous diving beetles
(Coleoptera dytiscidae), and caddisfly larvae (Trichoptera)) occurred
after treatment. No effects on populations of organisms that inhabited
the bottom layer of the creek were noticeable. The permethrin sprayed
had little effect on caged or native fish and no fish mortality was re-
corded due to the treatment. From these data, it could be inferred that
permethrin had no significant impact on the aquatic system (Kingsbury,
1976).
After an aerial application of permethrin at 17.5 g ai/ha, residues
attained peak concentrations of 147.0 µg/litre in ponds and 2.5 µg/litre
in streams, but accumulations and persistence of the pesticide in
bottom sediment were negligible. Noticeable increases in the number of
drifting organisms occurred in the treatment block ( Ephemeroptera hepta-
geniidae, Baetidae, and Plecoptera nymphs ) and 2.1 km downstream
(mayfly and stonefly nymphs) over a 24-h period immediately after the
spray. A slight reduction in the bottom fauna also occurred
downstream. When exposed in cages in the ponds, yellow perch (Perca flu-
vescens) did not exhibit any adverse effects; little or no accumulated
permethrin residues were detectable in the fish following exposure. Ob-
servations of the headwater ponds indicated that permethrin application
resulted in noticeable levels of distress and mortality to surface and
littoral invertebrates and produced a similar impact on benthic organ-
isms (Kingsbury & Kreutzweiser, 1980a).
Table 5. Acute toxicity of permethrin to non-target aquatic organisms
--------------------------------------------------------------------------------------------------------------------------------------------
Species Size Duration Toxicitya Formu- Sys- Tempera- pH Hardness Reference
of test (µg/litre) lationd teme ture (°C)
--------------------------------------------------------------------------------------------------------------------------------------------
A. Freshwater Organisms
Arthropods
Crayfish 0.8 - 1.2 cm, 96 h 0.39 EC S 24 100 Jolly et al. (1978)
(Procambarus clarkii) (0.05 g)
2 - 3 cm, 96 h 0.62 EC S 24 100 Jolly et al. (1978)
(0.5 g)
Water flea (Daphnia pulex) 3 h > 50 000 T S 25 Miyamoto (1976)
3 h > 50 000 (+)-trans S 25 Miyamoto (1976)
3 h > 50 000 (+)-cis S 25 Miyamoto (1976)
3 h > 50 000 (-)-trans S 25 Miyamoto (1976)
3 h > 50 000 (-)-cis S 25 Miyamoto (1976)
Water flea (Daphnia magna) 1st instar 48 h 1.26 T S 18 7.4 42 Mayer &
Amphipod immature 96 h 0.17 T S 17 7.4 42 Ellersieck (1986)
(Gammarus pseudolimnaeus) Mayer &
Midge (Chironomus plumosus) 3rd instar 48 h 0.56 T S 22 7.4 42 Ellersieck (1986)
Caddisfly 21 days 0.17 T F 15 7.6 - 7.8 46 - 48 Anderson (1982)
(Brachycentrus americanus)
Fish
Salmon (Salmo salar) 6.2 cm, 5.3 g 96 h 12 T R 10 McLeese et al.
(1980)
Rainbow trout 1 g 96 h 0.62 T F 5 7.9 - 8.2 358 - 363 Kumaraguru &
(Salmo gairdneri) 1 g 96 h 0.69 T F 10 7.9 - 8.2 358 - 363 Beamish (1981)
1 g 96 h 3.17 T F 15 7.9 - 8.2 358 - 363 Kumaraguru &
1 g 96 h 6.43 T F 20 7.9 - 8.2 358 - 363 Beamish (1981)
5 g 96 h 6.43 T F 15 7.9 - 8.2 358 - 363 Kumaraguru &
20 g 96 h ca. 50 T F 15 7.9 - 8.2 358 - 363 Beamish (1981)
50 g 96 h 287 T F 15 7.9 - 8.2 358 - 363 Kumaraguru &
200 g 96 h 314 T F 15 7.9 - 8.2 358 - 363 Beamish (1981)
6 cm, 3 g 24 h 135 T 10 7.5 110 Coats & O'Donnell-
6 cm, 3 g 24 h 61 EC 10 7.5 110 Jeffery (1979)
--------------------------------------------------------------------------------------------------------------------------------------------
Table 5. (contd.)
--------------------------------------------------------------------------------------------------------------------------------------------
Species Size Duration Toxicitya Formu- Sys- Tempera- pH Hardness Reference
of test (µg/litre) lationd teme ture (°C)
--------------------------------------------------------------------------------------------------------------------------------------------
Rainbow trout (contd.)
(Salmo gairdneri) 5 - 6 cm 48 h 6.0 EC 12- Mulla et al.
5 - 6 cm 48 h 7.0 cis, EC 25.5 (1978a)
2 - 4 g 24 h 18 T S 12 Glickman
2 - 4 g 24 h 25 cis S 12 et al.
2 - 4 g 24 h 14 trans S 12 (1981)
Killifish adult 48 h 41 T S 25 Miyamoto (1976)
(Oryzias latipes) adult 48 h 17 (+)-trans S 25 Miyamoto (1976)
adult 48 h 13 (+)-cis S 25 Miyamoto (1976)
adult 48 h > 10 000 (-)-trans S 25 Miyamoto (1976)
adult 48 h > 10 000 (-)-cis S 25 Miyamoto (1976)
Channel catfish 1.4 - 1.7 cm, 96 h 1.1 EC S 24 100 Jolly et al. (1978)
(Ictalurus punctatus) (0.02 g)
Largemouth Bass 4.5 - 5.5 cm, 96 h 8.5 EC S 24 100 Jolly et al. (1978)
(Micropterus salmoides) (1.14 g)
Mosquitofish 1.5 - 2.5 cm, 96 h 15 EC S 24 100 Jolly et al. (1978)
(Gambusia affinis) (0.25 g)
4 - 5 cm 48 h 97.0 EC 8.8 - 16 Mulla et al.
4 - 5 cm 48 h 13.0 cis, EC 8.8 - 16 (1978a)
Brook trout 1.2 g 96 h 3.2 T S 12 7.5 40 Mayer & Ellersieck
(Salvelinus foutinalis) (1986)
Fathead minnow 0.6 g 96 h 5.7 T S 22 7.3 38 Mayer & Ellersieck
(Pimephales promelas) (1986)
Bluegill sunfish 0.7 g 96 h 5.0 T S 22 7.3 38 Mayer & Ellersieck
(Lepomis macrochirus) (1986)
Desert pupfish 4 - 5 cm 48 h 5.0 EC S 11 - 16.5 Mulla et al.
(Cyprinodon macularis) 4 - 5 cm 48 h 5.0 cis, EC S 11 - 16.5 (1978a)
Tilapia mossambica 5 - 6 cm 48 h 44.0 EC S 15 - 21.4 Mulla et al.
5 - 6 cm 48 h 5.6 cis, EC S 15 - 21.4 (1978a)
Amphibian
Bullfrog, tadpole 0.6 - 0.8 cm 96 h 7033 EC S 24 100 Jolly et al. (1978)
(Rana catesbeiana)
--------------------------------------------------------------------------------------------------------------------------------------------
Table 5. (contd.)
--------------------------------------------------------------------------------------------------------------------------------------------
Species Size Duration Toxicitya Formu- Sys- Tempera- pH Salinity Reference
of test (µg/litre) lationd teme ture (°C) (°/oo)
--------------------------------------------------------------------------------------------------------------------------------------------
B. Estuarine and Marine Organisms
Algae
Skeletonema costatum 96 h 92b T 20 Borthwich & Walsh
(1981)
Molluscs
Oyster (Crassostrea virginica) 2-h larva 48 h > 1000c T S 25 20 Borthwich & Walsh
(1981)
Arthropods
Lobster (Homarus americanus) 450 g 96 h 0.73 T R 10 30 McLeese et al.
Shrimp (Crangon Septemspinosa) 1.3 g 96 h 0.13 T R 10 (1980)
Shrimp (Mysidopsis bahia) 1-day, 96 h 0.046 T S 25 20 Borthwich
juvenile & Walsh
Stone crab (Menippe mercenaria) Zoea larva 96 h 0.018 T S 25 20 (1981)
Pink shrimp (Penaeus duorarum) adult 96 h 0.22 T F 25 25 Mayer (1987)
Fish
Harpacticoid (Nitocra spinipes) 3-6 weeks old 96 h 0.6 EC S 20 - 22 7.8 7 Linden et al.
Bleak (Alburnus alburnus) 8 cm 96 h 4 - 8 EC S 10 7.8 7 (1979)
Sheepshead minnow 28-day fry 96 h 88 T S 25 20 Borthwich & Walsh
(Cyprinodon variegatus) (1981)
adult 96 h 7.8 T F 30 22 Mayer (1987)
Atlantic silverside adult 96 h 2.2 T F 26 25 Mayer (1987)
(Menidia menidia)
Striped mullet (Mugil cephalus) juvenile 96 h 5.5 T F 24 19 Mayer (1987)
--------------------------------------------------------------------------------------------------------------------------------------------
a Values are LC50 unless stated otherwise.
b EC50 (growth inhibition).
c EC50 (abnormal development).
d T = Technical, EC = Emulsifiable concentrate.
e R = Renewal, S = Static, F = Flow-through.
f expressed as mg CaCO3/litre.
When permethrin was sprayed at 8.8, 17.5, 35.0, or 70.0 g ai/ha by
aeroplane over small trout streams, the impact on aquatic invertebrates
and effects on the general fish population correlated with the dose.
There was an increase in the number of organisms drifting downstream,
the major ones being mayflies, followed by caddisflies, stoneflies, and
chironomids. The total number of drifting organisms was greater than
the pre-spray average by factors of 303, 699, 4960, and 6450 with spray
concentrations of 8.8, 17.5, 35.0, and 70.0 g ai/ha, respectively. A
return to pre-spray drift levels was evident within 36 h after appli-
cation at 8.8 and 17.5 g ai/ha whereas drifting of organism persisted
for up to 72 h at the higher application rates of 35 and 70 g ai/ha.
Following the spraying of permethrin at these higher doses, there was
no evidence of fish mortality, but there appeared a dramatic change in
the diets of fish (such as the native brook trout and sculpins). These
fish became virtually completely dependent on terrestrial invertebrates
rather than on the aquatic insects for food. When the rate of appli-
cation was low (8.8 g ai/ha) permethrin did not appear to affect these
fish, either in mortality rate or in their diet composition (Kingsbury
& Kreutzweiser, 1980b).
Serial applications of permethrin, once or twice at 17.5 g ai/ha,
resulted in catastrophic drift of aquatic invertebrates and substantial
depletion of benthos in streams within the application blocks and up to
2 km downstream. Despite massive disturbances of benthos, repopulation
of bottom fauna was evident within 2.5 months and had virtually re-
turned to normal within 3.5 months. Permethrin residues attained peak
levels of 1.35 µg/litre in standing water and 1.94 µg/litre in
flowing water in the sprayed regions. The residue persisted at low
concentrations for up to 96 h after spraying (Kreutzweiser, 1982).
In a study by Kingsbury & Kreutzweiser (1987), aerial applications
of permethrin to forests over several seasons at 8.8, 17.5, 35, and 70
g ai/ha did not cause mortality to native and caged fish (minnows,
mudminnows, perch, and under-yearling and yearling Atlantic salmon).
The composition of the salmonid diet was subsequently altered from
aquatic insects (mayfly nymphs, stonefly nymphs, and various aquatic
fly larvae) to terrestrial arthropods. The duration of changes ranged
from a few months after applications of 8.8 and 17.5 g ai/ha to a year
or longer after treatment with 35 and 70 g ai/ha. There were temporary
reductions in fish growth rate and fish densities in the treated area,
which returned to normal within four months after treatment. In the
same study, the effect on stream invertebrates was also evaluated.
Large drifts of invertebrates were observed immediately after appli-
cations and continued for 24 to 72 h. Although the peak of permethrin
residues in stream water was higher after the second application (0.36-
1.80 µg/litre) than the first (0.25-0.62 µg/litre), drift response
to the second application ranged from 6 to 62% of the first drift,
indicating that first application deleted susceptible invertebrates
(e.g., Ephemeroptera nymphs, Plecoptera, Trichoptera, and the
Diptera families) and that a much smaller residual population re-
sponded to the second treatment. Recovery of benthic fauna was appar-
ent between 1 and 18 months after spraying. The double treatment
reduced benthos density to a point at which recovery of numbers was
slower than after the single application (Kreutzweiser & Kingsbury,
1987).
6.2 Toxicity to Terrestrial Organisms
6.2.1 Soil microorganisms
Mathur et al. (1980) applied permethrin (Ambush 5 G) to Canadian
soils with a high content of organic material at a rate of 2.24 kg/ha.
Lettuce or carrots were grown on the plots. Soil cores were taken at
regular intervals and bacterial and fungal numbers were estimated, soil
nutrients were measured, and acid phosphatase activity in the soil was
monitored. Residues of permethrin persisted throughout the growing
season up to crop harvest, when 65% of the original concentration in
soil was found (113 days after application). This reflected the poor
breakdown of permethrin in organic soils compared to mineral soils.
Permethrin suppressed bacterial and actinomycete populations in samples
taken 1, 9, and 27 days after application, but control levels were re-
gained after 41 days (the next sampling time). The available nitrogen
and phosphorus was lower in treated soil at some points during the
study, but these changes were not consistent. Soil respiration and acid
phosphatase activity were higher in permethrin-treated plots, though
not consistently so. Permethrin had a greater effect when carrots were
grown than when lettuce was grown. The yield of neither crop was
affected. Most of the effects reported were transitory and none were
of overall significance for either the soil or the crop.
6.2.2 Terrestrial invertebrates
Under laboratory conditions, permethrin is highly toxic to certain
beneficial insects or natural enemies of pests, as shown in Table 6.
Cox & Wilson (1984) treated honey bee workers topically with a sub-
lethal dose of permethrin (0.09 µg/bee in 1.0 µl of acetone applied
to the thorax). This dose gave no higher mortality than treatment with
acetone alone. The bees, which were individually tagged, were housed in
an observation hive and trained before the experiment to feed at an
artificial feeding station 5 m from the hive. The experiment was con-
ducted at 35°C because at lower temperatures this dose resulted in
mortality. Treated bees made less foraging trips than controls and gave
food less frequently to other bees in the hive. Other behaviours were
increased in treated bees, i.e., self-cleaning, trembling dance, ab-
domen tucking, and rotating and cleaning of abdomen while rubbing the
hind legs together. Gerig (1985) also reported minimal lethal effects
on honey bees of permethrin used at recommended rates and that the
insecticide had a strong repellent effect.
Table 6. Acute toxicity of permethrin to non-target terrestrial organisms
-----------------------------------------------------------------------------------------------------------------------------
Species Size Application Duration Toxicitya Temperature Reference
(°C)
-----------------------------------------------------------------------------------------------------------------------------
Bird
Hen oral > 1.5 g/kg b.w.b Millner & Butterworth
(1977)
Chicken oral > 3 g/kg b.w.b Worthing & Walker
Japanese quail oral 5 days > 13.5 g/kg b.w.b (1983)
diet 5 days > 5 g/kg dietc Hill & Camardese
diet 5 days > 5 g/kg dietd (1986)
Mallard duck diet 5 days > 27.5 g/kg diet Ross et al. (1976b)
Mallard duck oral > 13.5 g/kg b.w.b Ross et al. (1976a)
Starling diet 5 days > 27.5 g/kg diet Ross et al. (1976d)
Starling oral > 38 g/kg b.w.b Ross et al. (1976c)
Ring-necked pheasant diet 5 days > 27.5 g/kg diet Ross et al. (1976e)
Ring-necked pheasant oral > 13.5 g/kg b.w.b Ross et al. (1977a)
Arthropods
Honeybee (Apis mellifera) contact 0.11 µg/beeb 26 - 27 Stevenson et al. (1978)
oral 0.28 µg/beeb 26 - 27 Stevenson et al. (1978)
Insect parasite
Ichneumoid (Campoletis adult male film 24 h 0.31 µg/vial Plapp & Vinson (1977)
sonorensis)
Insect predator
Carabid (Pterostichus adult 0.16 g topical > 2000 µg/insectb 21 Hagley et al. (1980)
melanarius)
Carabid (Harpalus affinis) adult 0.05 g topical 116 µg/insectb 21 Hagley et al. (1980)
Carabid (Amara sp.) adult 0.03 g topical 25 µg/insectb 21 Hagley et al. (1980)
Earwig (Labidura raparia) mature soil 0.1 kg 6% mortality Workman (1977)
ai/ha
mature soil 0.2 kg 50% mortality Workman (1977)
ai/ha
Green lacewing larvae
(Chrysopa carnea) 5-6 days old film 9.87 µg/vial 25 Plapp & Bull (1978)
Predaceous mite species
Mataseiulus occidentalis adult female slide-dip 0.72, 1.32, 27.5 Roush & Hoy (1978)
(3 strains) method 14.8 mg ai/litre
young gravid leaf-disc 2.8 mg ai/litre 27 Hoy et al. (1979)
female method
(Amblyseius fallacis) adult female slide-dip 14 mg ai/litre 27 Rock (1979)
method
-----------------------------------------------------------------------------------------------------------------------------
a LC50 values, unless stated otherwise.
b LD50 values; b.w. = body weight.
c Technical product.
d Emulsifiable concentrate.
Pike et al. (1982) applied permethrin by helicopter at a rate of
0.22 kg ai/ha to fields of maize (Zea mays). The applications were
made early in the morning before bees were actively foraging for pollen
and were repeated every 3 to 6 days (to a maximum of six applications
per season). The trial was repeated for 3 years. There was no differ-
ence in the number of dead bees per hive between treated and control
areas, but a marked reduction in the number of bees foraging in treated
fields, indicating avoidance of permethrin. The authors concluded that
treatment of corn fields with permethrin at the normal application rate
is safe for bees as long as the application does not coincide with bee
activity in the area.
The acute toxicity values of permethrin to tobacco budworm
(Heliothis virescens), to the green lacewing (Chrysopa carnea), a
predator of tobacco budworm, and to Campoletis sonorensis, an
ichneumoid parasite of tobacco budworm, showed that permethrin was
approximately 18 times less toxic to the predators than to the pest
(Plapp & Bull, 1978; Plapp & Vinson, 1977).
Larvae of the green lacewing exhibited marked tolerance to per-
methrin, and to its cis or trans isomers, when dosed topically with
250 µg per insect (about 25 000 µg/g). This value is ca. 10 000 times
greater than the LD50 value for the tobacco budworm (Shour & Crowder,
1980).
Workman (1977) added permethrin to loamy sand soil into which the
striped earwig (Labidura riparia), an effective insect predator of
the cabbage looper, was introduced. The insecticide was of low toxicity
to the earwig at dosage rates which gave good looper control.
The susceptibility of carabids to permethrin appears to be in-
versely related to beetle size, as shown in Table 6. When permethrin
was applied at a concentration of 0.21-0.85 kg ai/ha to an apple
orchard, it did not significantly affect the numbers of Pterostichus
melanarius at any time during the season, but the numbers of Harpalus
affinis and Amara sp. were significantly reduced 3-5 days after appli-
cation. This result reflected the toxicity ratings by means of LD50
values obtained in laboratory studies. The total seasonal numbers of
these carabids were not significantly affected by permethrin, owing to
short residual effects (Hagley et al., 1980).
In laboratory tests, LC50 values of permethrin for two strains of
spider mites (Tetranychus urticae) were ca. 20-40 times greater than
those for three strains of predator mites (Mataseiulus occidentalis)
(Roush & Hoy, 1978), and ca.15 times greater than those for Amblyseius
fallacis (Rock, 1979). These studies indicate that the use of per-
methrin at the recommended rates of 60-120 mg ai/litre would be detri-
mental to orchard integrated mite control programs.
In laboratory tests, the Pacific spider mite (T. pacificus) has
been found to be 40 times more tolerant to permethrin than the predator
mite (M. occidentalis), (Hoy et al., 1979). The spraying of vineyards
with 15 or 30 mg ai/litre resulted in substantially higher populations
of T. pacificus and E. willamettei for about one month due to re-
duction in predator species numbers. Similarly, spraying with 60 or
120 mg ai/litre produced a subsequent increase of Eotetranychus
willamettei late in August and September for the above reason (Hoy et
al., 1979).
When permethrin was applied to apple trees at a concentration of
40 mg/litre, no predator mites (Typhlodromus pyri) were found for 4-6
weeks, and only small numbers were found 10 weeks after the spray. On
the other hand, permethrin had no appreciable toxicity to the spider
mite (Panonychus ulmi). The virtual elimination of the predatory mite
by permethrin spraying led to a marked population increase of P. ulmi
later in the same season (Aliniazee & Cranham, 1980).
In apple and pear orchards, applications of permethrin at 30 mg
ai/litre reduced the numbers of a predatory mite (M. occidentalis) to
almost zero and dramatically increased the populations of spider mites
(T. urticae, Tetranychus mcdanieli, or P. ulmi) (Hoyt et al., 1978).
From the above findings it appeared that permethrin, when applied
according to recommendations, is relatively harmless to insect pred-
ators, with the exception of predaceous mite species. Everts et al.
(1985) investigated the effects of permethrin on beneficial terrestrial
arthropods in the soil and vegetation in areas surrounding applications
of the insecticide to control tsetse flies in the Ivory Coast, West
Africa. Permethrin was used as a 2.5% wettable powder at a rate of 121
g ai/ha during January (a minimum temperature of 20.0°C and a maximum
of 36.0°C). The permethrin application significantly reduced popu-
lations of Coleoptera, Lepidoptera, Ephydridae, Chloropidae, Muscidae,
Ichneumonidea, Chalcidoidea and Proctotrupoidea. The populations of
almost all these groups were found to recover to normal levels within 2
months, i.e., before the likely time of respraying for the control of
the tsetse flies. However, one Proctotrupoid genus, Cremastobaeus, was
eliminated by permethrin treatment.
6.2.3 Birds
Neither an acute oral nor a dietary LD50 or LC50 has been estab-
lished accurately because of the very low toxicity of permethrin to
birds (Table 6). The acute LD50 is >3000 mg/kg body weight and the
dietary toxicity >5000 mg/kg diet. (Worthing & Walker, 1983; Hill &
Camardese, 1986).
The inclusion of permethrin at up to 40 mg/kg in the diet of laying
hens for 28 days had no adverse effects on the health of parent birds
or on egg production quality, hatchability, or the viability of the
chicks produced (Ross et al. 1977b).
6.2.4 Mammals
Racey & Swift (1986) treated roosting boxes for pipistrelle bats
with various wood preservatives and allowed the bats to roost in the
boxes for up to 154 days. There were no toxic effects of mixtures of
synthetic pyrethroids including permethrin. The authors concluded that
the insecticide component of wood preservatives should be pyrethroids
when bats are present in the area.
6.3 Uptake, Loss, Bioaccumulation and Biomagnification
Proposed metabolic pathways of permethrin in fish are summarized in
Fig. 4.
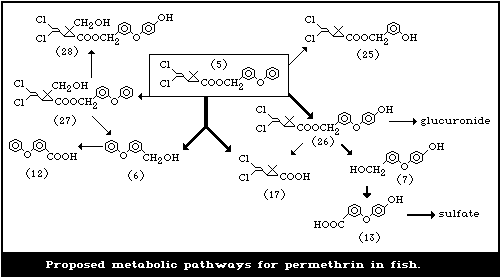 When rainbow trout (Salmo gairdneri) were held in static water
containing 5 µg/litre of 14C-permethrin for 24 h, both cis and trans
isomers were similarly taken up into the fish. The bioaccumulation
ratios for total radiocarbon in the blood, muscle, liver, and fat of
the fish were 30, 30, 300, and 400, respectively. When the fish were
transferred to fresh running water, radioactivity was eliminated, with
initial half-lives of 9-35 h, from all the tissues except fat, where
little decay in 14C-permethrin concentrations occurred. When rainbow
trout were injected intraperitoneally with 14C-permethrin at a rate of
0.5 mg/kg, 32-43% of the dose was recovered after 48 h in the bile, 3-
7% in the urine, and 31-42% in the carcass. However, the urine and bile
of the trout injected with the trans isomer contained higher levels of
radioactivity. In the bile, the major metabolite was the glucuronide
conjugate of 3-(4-hydroxyphenoxy)-benzyl-3-(2,2-dichlorovinyl)-2,2-
dimethyl-cyclopropanecarboxylate (26) and there were few metabolites
formed by hydrolysis (Fig. 4). The urine contained principally the
sulfate conjugates of polar products. The ability of rainbow trout to
hydrolyze permethrin in vivo appeared minimal (Glickman et al., 1981).
The rates of trans-permethrin hydrolysis in trout liver, kidney,
and plasma incubated at 12°C were approximately 166, 38, and 59 times,
respectively, lower than those in the corresponding mouse tissues
incubated at 37°C. Although an increase in the incubation temperature
from 12°C to 37°C caused an increase in the rate of trans-permethrin
hydrolysis by trout liver microsomes, trans-permethrin was hydrolyzed
about 45 times slower than by mouse liver microsomes at 37°C. The
hydrolysis of permethrin in trout plasma, however, was higher than that
in trout liver microsomes (Glickman & Lech, 1981).
When the microsomal preparations of both carp and rainbow trout
were fortified with NADPH, the carp microsomes oxidized permethrin iso-
mers more actively than the trout microsomes. Also, larger amounts of
hydroxy ester metabolites were recovered with the cis isomer than with
the trans isomer. The preferred site of oxidation of both isomers by
the carp and trout microsomes was the 4'-position (26) of the phenoxy-
benzyl moiety. The geminal dimethyl group was attacked in preference to
the methyl group situated trans to the carboxy group. trans-Permethrin
primarily underwent hydrolysis by both carp and trout liver microsomes
in the presence or absence of NADPH to yield PBalc (6) and Cl2CA (17)
(Glickman et al., 1979). In this respect, the results of in vitro
studies were different from those obtained in vivo.
Juvenile Atlantic salmon (lipid content 4.2%), exposed for 96 h to
static water containing 22 µg/litre permethrin, took up the insecti-
cide with a bioaccumulation ratio of 55. Dead juvenile salmon exposed
for 12.5 h to 0.098-0.994 mg/litre contained 2.21-3.69 µg/g (Zitko et
al., 1977).
Residues of approximately 0.5-1.2 mg/kg were detected in dead
juvenile Atlantic salmon exposed for 10-89 h to static water containing
permethrin at 6.9-85 µg/litre, the bioaccumulation ratio ranging from
14 to 73. The insecticide was not detected (detection limit 5 ng/g) in
dead lobster hepatopancreas or in dead shrimp (McLeese et al., 1980).
When stoneflies (Pteronarcys dorsata) were exposed for 28 days to
running water containing permethrin at 0.029-0.21 mg/litre, the bioac-
cumulation ratios of the survivors ranged from 43 to 570 (average, 183;
standard deviation, 171) (Anderson, 1982).
When carp were exposed to a 14C-permethrin isomer (phenoxyphenyl-
labelled [1R,trans], [1R,cis], [1S,trans], or [1S,cis] isomers) in a
flow-through system at 25°C, the concentrations of 14C and permethrin
isomers in the fish body reached an equilibrium on days 7-9 of ex-
posure. The bioaccumulation ratios of the permethrin isomers at equi-
librium were 330-750. When the fish were transferred to fresh water,
the permethrin isomers, as well as their metabolites, were rapidly
excreted. The biological half-lives for the permethrin isomers were
2.0-2.8 days. The major metabolic reactions involved were oxidation at
the 4 -position of the alcohol moiety or the methyl group of the acid
moiety, cleavage of the ester linkage, and conjugation of the resultant
alcohols and phenols with glucuronic acid or sulfuric acid (Ohshima et
al., 1988).
Bioconcentration factors for sheepshead minnows ( Cyprinodon vari-
egatus exposed to permethrin at concentrations between 1.25 and
10 µg/litre for 28 days from hatching varied between 290 and 620.
Maximum bioconcentration occurred after exposure at 2.5 µg/litre, and
a maximum residue of 5.7 mg/kg occurred after exposure at 10 µg/litre
(the concentrations were for whole fish) (Hansen et al., 1983).
Permethrin and its metabolites are not accumulated in birds. During
repeated dosing to quails and to mallard ducks, very similar patterns
and levels of both the appearance and depletion of radioactive residue
in tissues were found. The level in fat, which was small, reached a
plateau during a 28-day period. In all tissues, residues declined
extensively during a 14-day period after the final dose (Leahey et al.,
1977).
7. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
Toxicological profiles of permethrins with different isomeric com-
positions (cis:trans ratios of 40:60 or 25:75) were compared in a range
of toxicological studies. The toxicological profile of permethrin
(25:75) resembles that of permethrin (40:60) except that it is less
acutely toxic than permethrin (40:60).
7.1 Acute Toxicity
Table 7 shows the results of acute toxicity tests of permethrin
with various animal species. Aqueous suspensions usually produced the
least toxic results, LD50 values ranging from 3000 to >4000 mg/kg body
weight. However, corn oil is the more standard vehicle for pyrethroids
and yielded LD50 values of about 500 mg/kg (in all studies except one)
for oral administration in rats and mice.
Following oral administration of permethrin to rats, signs of
poisoning became apparent within 2 h after dosing and persisted for up
to 3 days. At lethal levels, these signs included whole body tremors of
varying degree from slight to convulsive, which in some cases were ac-
companied by salivation. Associated signs were hyperactivity and hyper-
excitability to external stimuli, urination and defecation, ataxia, and
lacrimation (Parkinson, 1978; Litchfield, 1983).
Table 8 gives the acute oral toxicity of three lots of permethrin
to rats. The observed ten-fold decrease in LD50 when corn oil or olive
oil were used could be due to enhanced absorption of the insecticide
(Metker et al, 1977).
The acute oral toxicity of permethrin (25:75) to groups of six
female C.S.E. Wistar rats was determined in five different vehicles
(Table 9). Most symptoms of acute poisoning developed within 12 h of
dosing and consisted of muscular tremors, hypersensitivity to stimuli,
and staining of abdominal fur. The majority of deaths occurred between
1 and 3 days (Wallwork & Malone, 1974).
Groups of female Sprague-Dawley rats, either fed ad libitum or
starved for 24 h beforehand, were given a single oral dose of
permethrin (25:75) (94.1% purity) in corn oil solution (40% w/v) at
750, 1500, 3000, or 6000 mg/kg. Permethrin was more toxic in starved
animals (LD50 = 3000 mg/kg) than in animals that had been fed (LD50 =
4251 mg/kg) (Piercy et al., 1976).
The acutetoxicity of permethrin with various cis- and trans-per-
methrin ratios is indicated in Table 10. These data clearly demonstrate
that cis-permethrin is more toxic than trans-permethrin to rats and
mice.
Table 7. Acute toxicity of permethrin administered to various
animal species
-------------------------------------------------------------------------
Species Sex Routea Vehicleb LD50 Reference
(mg/kg body
weight)
-------------------------------------------------------------------------
Rat M oral waterc 2949 Parkinson 1978
F oral water > 4000 Parkinson et al. 1976
M oral DMSO 1500 Clark 1978
F oral DMSO 1000 Clark 1978
M oral corn oil 500 Jaggers & Parkinson 1979
M oral corn oil 430 Kohda et al. 1979a
F oral corn oil 470 Kohda et al. 1979a
M&F oral corn oil 1200 Braun & Killeen 1975
M&F oral water 1725 Sasinovich & Panshina 1987
M dermal water > 5176 Parkinson 1978
F dermal noned > 4000 Parkinson et al. 1976
M dermal noned > 2500 Kohda et al. 1979a
F dermal noned > 2500 Kohda et al. 1979a
M&F dermal xylene > 750 Clark 1978
M&F dermal none 2000 Sasinovich & Panshina 1987
M sc corn oil 7800 Kohda et al. 1979a
F sc corn oil 6600 Kohda et al. 1979a
M ip water > 3200 Parkinson et al. 1976
F ip water > 3200 Parkinson et al. 1976
ip 463 - 1725 Sasinovich & Panshina 1987
Mouse F oral water > 4000 Parkinson et al. 1976
M&F oral DMSO 250 - 500 Clark 1978
M oral corn oil 650 Kohda et al. 1979a
F oral corn oil 540 Kohda et al. 1979a
M dermal noned > 2500 Kohda et al. 1979a
F dermal noned > 2500 Kohda et al. 1979a
M sc corn oil > 10 000 Kohda et al. 1979a
F sc corn oil 10 000 Kohda et al. 1979a
Rabbit F oral waterc > 4000 Parkinson et al. 1976
F dermal noned > 2000 Parkinson et al. 1976
Guinea- M oral water > 4000 Parkinson et al. 1976
pig
Hen oral > 1500 Milner & Butterworth 1977
-------------------------------------------------------------------------
a sc = subcutaneous; ip = intraperitoneal.
b DMSO = dimethyl sulfoxide.
c as an aqueous suspension.
d technical material applied without vehicle.
Table 8. Acute oral toxicity of three lots of permethrin to rats
------------------------------------------------------------------
Lot No.c Strain Sex Solvent LD50
(mg/kg)
------------------------------------------------------------------
827-RSP-1422 Sprague-Dawley Male None 5010
Female None 3801
827-RTP-1450 Sprague-Dawley-1a Male Corn oil 563
Sprague-Dawley-2b Male Corn oil 383
827-RTP-1450 Long-Evans Male None 4892
Female None 2712
8719-RTP-1450 Sprague-Dawley Male Olive oil 584
Female Corn oil 413
------------------------------------------------------------------
a Average body weight was 220 g.
b Average body weight was 321 g.
c Isomeric composition and the purity of the compound in each lot
were as follows:
--------------------------------------------
Lot No. Isomeric Ratio Stated
cis trans Purity
--------------------------------------------
827-RSP-1422 44% 56% 93.6%
827-RTP-1450 45% 55% 95.0%
8719-RTP-1450 46.5% 53.5% 92.4%
--------------------------------------------
Table 9. Acute toxicity of permethrin (25:75) to rats
------------------------------------------------------
Vehicle LD50 (mg/kg)
------------------------------------------------------
Neat undiluted permethrin (control) > 20 000
40% w/v in corn oil 4672
40% w/v in petroleum distillate > 8000
40% w/v in dimethylsulfoxide > 8000
20% w/v in glycerol > 5048
------------------------------------------------------
Table 10. Acute toxicity of permethrins with various cis:trans
isomeric ratios
---------------------------------------------------------------------------
Permethrin LD50
(cis:trans) Animal Sex Routea (mg/kg body Reference
weight)
---------------------------------------------------------------------------
80:20 Rat F oral 396 Jaggers & Parkinson (1979)
57:43 F oral 333
50:50 F oral 748
40:60 F oral 630
20:80 F oral 2800
99:1 Mouse ip 108 Glickman et al. (1982)
40:60 ip 514
1:99 ip > 800
99:1 Mouse iv 17 Glickman et al. (1982)
40:60 iv 31
1:99 iv > 135
---------------------------------------------------------------------------
a ip = intraperitoneal; iv = intravenous.
Table 11 shows the results of acute oral toxicity tests of the
metabolites of permethrin on rats (FAO/WHO, 1980b).
Table 11. Acute oral toxicity to rats of several metabolites
of permethrin
---------------------------------------------------------------
Chemical No.a LD50 (mg/kg
body weight)
---------------------------------------------------------------
3-phenoxybenzyl alcohol 6 1330
3-(2,2-dichlorovinyl)-2,2-dimethylcyclo- 17 980
propanecarboxylic acid
3-phenoxybenzaldehyde 11 600
---------------------------------------------------------------
a Chemical identification no. used in Fig. 3.
Table 12 shows the results of the acute intraperitoneal toxicity to
mice of permethrin metabolites (Kohda et al., 1979b).
Table 12. Acute intraperitoneal toxicity to mice of several
permethrin metabolites
---------------------------------------------------------------------
Chemicala No.b LD50 (mg/kg body weight)
Male Female
---------------------------------------------------------------------
3-phenoxybenzyl alcohol 6 71 424
3-(4 -hydroxyphenoxy)benzyl alcohol 7 750 - 1000 750 - 1000
3-(2 -hydroxyphenoxy)benzyl alcohol 8 876 778
3-phenoxybenzoic acid 12 154 169
3-(4 -hydroxyphenoxy)benzoic acid 13 783 745
3-(2 -hydroxyphenoxy)benzoic acid 14 859 912
3-phenoxybenzaldehyde 11 415 416
---------------------------------------------------------------------
a All compounds were dissolved in corn oil, except 3-phenoxybenzoic
acid, which was dissolved in DMSO.
b Chemical identification no. used in Fig. 3.
7.2 Subacute and Subchronic Toxicity
7.2.1 Oral exposure
7.2.1.1 Mouse
When male and female Alderly Park mice (20 of each sex per group)
were fed permethrin in the diet at levels of 0, 200, 400, 2000, or
4000 mg/kg diet for 28 days, mortality, growth, and food utilization
were normal for all animals. One additional group (permethrin level of
80 mg/kg for 2 weeks and 10 000 mg/kg for the final 2 weeks) showed
weight loss and poor food utilization when feeding with 10 000 mg/kg
began. Animals fed permethrin at 2000 mg/kg or more showed increased
liver weight and liver-to-body weight ratio. Higher weight and organ-
to-body weight ratios were also observed in the kidney, heart, and
spleen of males receiving a dose of 10 000 mg/kg. Gross tissue changes
were observed in females at 2000 and 10 000 mg/kg. On histopathological
examination, regenerating tubules in the renal cortex and hypertrophy
of centrilobular hepatocytes with cytoplasmic eosinophilia, which were
not dose related, were observed in all the treated animals (Clapp et
al., 1977b).
In a study by Wallwork et al. (1974a), groups of six mature female
mice received daily oral doses of permethrin (25:75) in corn oil at 0,
200, 400, 800, or 1600 mg/kg body weight for 10 consecutive days.
Signs of acute toxicity, such as spasm and convulsion, were seen only
in the highest dose group, half of which died after the initial dose.
No significant changes in haematology, clinical chemistry, or body
weights on the 11th day of dosing were recorded. The mice treated at
800 and 1600 mg/kg body weight exhibited increased liver weights.
7.2.1.2 Rat
Sprague-Dawley rats (six of each sex per group) were fed permethrin
in the diet for 14 days at dose levels of 54, 108, 216, 432, 864, or
1728 mg/kg body weight per day. All rats surviving to term were sacri-
ficed and various organs and tissues were examined histopathologically.
At the two highest dose levels, all animals died except one female fed
864 mg/kg. Muscle tremors were noted in all animals at 432 mg/kg, but
doses of 216 mg/kg or less caused no toxic signs in either males or
females. There was a statistically significant increase in average
liver-to-body weight ratios at 432 mg/kg, but compound-related histo-
logical changes were not observed in any of the tissues or organs. The
maximum NOEL in this study was 216 mg/kg (Metker et al., 1977).
In studies by Metker et al. (1977), Long-Evans rats (six of each
sex per group) were fed permethrin in the diet for 14 days at dose
levels of 0, 27, 54, 108, 216, or 432 mg/kg body weight per day. All
rats surviving to term were sacrificed and various organs and tissues
were examined histopathologically. At a dose of 432 mg/kg, three out of
six females died within the first five days. Muscle tremors were noted
in all surviving animals at 216 and 432 mg/kg. There was a statisti-
cally significant increase among female animals in the average liver-
to-body weight ratio. Compound-related histological changes were not
observed in any of the tissues or organs examined. The maximum dietary
NOEL was 108 mg/kg body weight per day.
When young male and female Wistar rats (8 of each sex per group)
were fed permethrin in the diet at dose levels of 0, 200, 500, 1000,
2500, 5000, or 10 000 mg/kg diet for 4 weeks, all rats that received
the highest dose died within 3 days. Mortality was evident at 5000
mg/kg, and hyperexcitability was observed in animals that received
2500 mg/kg. Other non-specific signs of poisoning were observed at
1000 mg/kg on the first day of the study only. Food consumption and
growth were reduced in the animals dosed at 5000 mg/kg. There was no
effect on haematological parameters, clinical chemistry, or urinalysis
except for a reduction in urinary protein excretion in males at 5000
mg/kg. Liver weight and liver-to-body weight ratios were increased in
males at 2500 mg/kg or more and in females at 1000 mg/kg or more. This
study had been designed as a preliminary range-finding test for long-
term dietary administration (Clapp et al., 1977a).
In a study of the reversibility of hepatic changes in rats follow-
ing short-term dietary administration of permethrin, female Wistar rats
(48 rats per group) were fed permethrin at levels of 0 or 2500 mg/kg
diet for 28 days. At the end of the feeding trial, rats were either
sacrificed or maintained on control diets and sacrificed at 1, 4, or
8 weeks after the termination of dosing. There was no mortality, but
food consumption, food utilization, and body weight were reduced in the
permethrin-treated rats during the administration period. However, the
animals gained weight rapidly after the dosing period and there was no
difference in body weight between control and test animals at the end
of the study period. After the 4 weeks of permethrin dosing, signifi-
cantly higher absolute and relative liver weights were observed. During
the 8-week recovery period, the relative liver weight of permethrin-
treated animals was significantly higher than the control values, but
the absolute weights of the liver of control and test animals were
similar. There were no effects of permethrin on plasma alanine transam-
inase over the course of the study. Oxidative enzyme activity in liver
microsomes was significantly higher in test animals than in controls at
the end of dosing and 1 week later. The activity of liver microsomal
enzymes in the permethrin-treated animals was normal 4 weeks after
dosing but was elevated 8 weeks after dosing. The amount of smooth
endoplasmic reticulum in rat liver cells was significantly increased as
a result of permethrin dosing, but within 4 weeks after dosing, there
were no significant histological differences in the liver between
treated and control animals (Bradbrook et al., 1977).
When male and female Charles River (CD) rats (six of each sex per
group) were fed permethrin at levels of 0, 30, 100, 300, 1000, or 3000
mg/kg diet for five weeks, persistent tremors were evident in animals
fed at 3000 mg/kg although no mortality was observed. Growth was
inhibited in both males and females at this dose level. Relative liver
weight was increased in both the males (1000 mg/kg or more) and females
(3000 mg/kg). Slight effects on certain clinical chemistry parameters,
such as increased prothrombin times in males, were noted at the 3000
mg/kg level. Examination of tissues and organs of the animals receiving
the two highest doses did not show any unusual effects as a result of
permethrin in the diet (Butterworth & Hend, 1976).
Male and female Long-Evans rats (10 of each sex per group) fed
permethrin in the diet at dose levels of 0, 20, 100, or 500 mg/kg diet
for 90 days showed no mortality, and the growth and food consumption of
all animals were normal. The results of haematology, clinical chemis-
try, urinalysis, and ophthalmological examinations were also normal.
Tremors were noted in some animals at the highest dose level, mainly
during the first week of treatment. There were significant increases
in absolute and relative liver weights at the two highest dose levels.
These increases were consistent with data from microscopic examination
of the liver showing compound-related centrilobular hepatocyte hyper-
trophy in both males and females. There were no significant effects at
the 20-mg/kg level, although slight hepatic effects were reported in a
few of the male rats (Killeen & Rapp., 1976b).
In studies by Metker et al. (1977), Sprague-Dawley rats (10 of each
sex per group) were fed permethrin in the diet for 90 days at dose
levels of 0, 9, 27, 85, 270, or 850 mg/kg body weight per day. All
rats surviving to term were killed and various tissues and organs from
each animal were examined histopathologically. At 850 mg/kg, all male
and female rats died. An increase in the average liver-to-body weight
ratio was noted in both male and female rats fed 270 mg/kg. Compound-
related histological changes were not observed in any of the tissues
and organs examined. The minimum effect level was 270 mg/kg per day.
At 85 mg/kg no effects were observed.
When male and female Sprague-Dawley rats (16 of each sex per group)
were fed permethrin in the diet at dose levels of 0, 375, 750, 1500, or
3000 mg/kg diet for 6 months, there was no mortality and all animals
exhibited normal growth and normal food and water consumption. Urinal-
ysis, haematological values, and clinical biochemistry parameters
showed no changes related to permethrin dosing. Signs of hyperexcit-
ability and tremors were observed during the study in animals dosed at
3000 mg/kg and the liver weight and liver-to-body weight ratio of these
animals were slightly increased. There were no significant histopatho-
logical findings attributable to the presence of the permethrin in the
diet. The NOEL was 1500 mg/kg (Kadota et al., 1975).
In a study designed to evaluate liver hypertrophy, male and female
Wistar rats were fed permethrin at levels of 0, 20, 100, or 1000 mg/kg
diet for 26 weeks. There was no mortality, and the growth and food con-
sumption of the animals were normal. Although the mean liver weight was
increased at all dose levels, a significant increase was noted only at
the highest dose level. The increase in liver weight at this dose level
was accompanied by an increase in the smooth endoplasmic reticulum and
in biochemical parameters associated with microsomal oxidative mechan-
isms. At a dose level of 100 mg/kg, there were slight, non-significant
increases in biochemical activities. No effects on any of the par-
ameters measured were observed in animals dosed at 20 mg/kg (Hart et
al., 1977c).
In a study by Wallwork et al. (1974b), groups of five to six female
Charles River CDI rats received permethrin (25:75) in corn oil at 0,
200, 400, or 800 mg/kg body weight by daily gavage for 10 days. The
animals were sacrificed on the eleventh day so that haematological and
clinical chemical parameters and organ weights could be investigated.
Permethrin (25:75) gave a toxicity profile similar to that of
permethrin (40:60).
7.2.1.3 Dog
Beagle dogs (four of each sex per group) fed permethrin in gelatin
capsules daily for 3 months at dose levels of 0, 5, 50, or 500 mg/kg
body weight showed no mortality, but clinical signs of poisoning were
noted at various times in both males and females at the highest dose
level. Growth and food consumption, as well as clinical chemical,
haematological, and urinalysis parameters, were normal. The liver
weights and liver-to-body weight ratios of animals that received per-
methrin at 50 mg/kg or more were significantly increased. Histopatho-
logical examination did not reveal any changes attributable to per-
methrin (Killeen & Rapp, 1976a).
Beagle dogs (four of each sex per group) administered permethrin in
gelatin capsules daily for 13 weeks at dose levels of 0, 10, 100, and
2000 mg/kg body weight likewise showed no mortality, but clinical signs
of poisoning were evident at 2000 mg/kg. Haematological, clinical
chemical, and urinalysis values were normal in all animals. There was a
slight increase in the liver weight of animals dosed at 2000 mg/kg/day,
but no accompanying histopathological changes in the liver (Edwards et
al., 1976).
When two beagle dogs were given daily oral doses of permethrin
(25:75) at 500 mg/kg body weight for 14 days, there were no clinical
signs of toxicity or significant effects of the treatment on body
weight or on clinical chemistry or haematological parameters (Chesher
et al., 1975a).
Groups of four male and four female beagle dogs, given encapsulated
permethrin [(25:75) 4.5% w/v] at 0, 10, 50, or 250 mg/kg body weight
for 6 months, revealed no signs of toxicity and no effect on body
weight. Ophthalmoscopy and electrocardiography showed no abnormalities.
At necropsy, there were no gross pathological or significant histo-
pathological findings. Haematological and clinical chemistry para-
meters, including plasma antipyrine elimination rate, were unaffected
by treatment. The results of this study indicate that daily oral doses
up to 250 mg/kg body weight do not adversely affect beagle dogs
(Reynolds et al., 1978).
7.2.1.4 Rabbits
In a study by Chesher & Malone (1974a), groups of five female Dutch
rabbits received permethrin (25:75) in 10 daily doses by gavage in corn
oil at 0, 200, 400, or 800 mg/kg body weight. The animals were killed
on the eleventh day so that clinical chemical, haematological para-
meters, and organ weights could be investigated. One rabbit, dosed at
400 mg/kg, exhibited mild hyperactivity and muscular fasciculation, but
only at days 6 and 7. Although all animals, including the controls,
exhibited some degree of weight loss, it was most marked in the high-
dose group. There were no significant haematological or clinical chem-
istry findings, but there was some decrease in liver and kidney weight
and also some enlargement of adrenal gland weights in all treated
groups.
7.2.1.5 Cow
Lactating cows (three per group) fed permethrin in the diet at dose
levels of 0, 0.2, 1.0, 10, or 50 mg/kg diet for 28 days showed no mor-
tality. Growth and milk production were normal, and no histopathologi-
cal changes in the tissues were observed (Edwards & Iswaran, 1977).
7.2.2 Dermal Exposure
Technical grade permethrin was applied daily to the clipped skin of
New Zealand White rabbits (eight males per group) at dose levels of 0,
0.10, 0.32, or 1.0 g/kg body weight, each day for 21 consecutive days.
The application site was abraded on the first test day in half of the
animals in each group. Blood samples were drawn weekly from the animals
for clinical chemistry studies. All animals were killed on the tenth
day after exposure ceased. Various tissues and organs were taken from
each animal and examined for microscopic lesions. A moderate primary
irritation of the skin was produced by permethrin. No significant
changes in body weight, organ weight, or clinical chemistry values were
evident, neither were there any compound-related lesions in the skin or
other tissues (Metker et al., 1977).
In further studies by Metker et al. (1977), permethrin (dissolved
in acetone) or acetone (as a control) was placed on the skin twice a
week for 3 weeks to 6 groups of 10 shaved male New Zealand White rab-
bits. Cotton cloth treated with permethrin (1.25 or 0.125 mg/cm2) was
applied to the skin over 1 ml of artificial sweat (salt solution imi-
tating sweat). In the case of other rabbits, similarly treated, the
sweat was omitted. In the control groups, acetone-treated cotton cloth
with or without 1 ml of sweat was used. Blood samples were collected
once a week for clinical chemistry determinations. All animals sur-
viving to term were killed and various tissues and organs from each
animal were examined for microscopic lesions. No significant changes
were noted in rabbit body weight or organ-to-body weight at the end of
the 21-day test, and no skin irritation was observed. There were no
significant changes in clinical chemistry values in the treated groups
and no compound-related lesions in the skin or other tissues and organs
examined (Metker et al., 1977).
7.2.3 Inhalation exposure
The inhalation toxicity of technical grade permethrin was deter-
mined in three species of laboratory animals. Male Hartley guinea-pigs,
male and female Sprague-Dawley rats, and male and female beagle dogs
were exposed to an aerosol of permethrin at concentrations of 125, 250,
or 500 mg/m3, 6 h per day, 5 days per week for 13 weeks. The mass
median diameter of the aerosol droplets was 5.1 µm, and 85% of the
total droplets had a diameter of 1.0 µm or less. At 500 mg/m3, tremors
and convulsions occurred in the rats during the first week of exposure
but disappeared in the second week. There was no difference in oxygen
consumption between control and treated rats. Urine metabolite studies
indicated that permethrin was rapidly metabolized and excreted. Post-
exposure experiments in male rats showed that the hexobarbital-induced
sleeping time was significantly shortened after exposures at 500
mg/m3 but not at lower doses. No clinical signs of poisoning were
observed in the guinea-pigs and dogs when exposed to aerosols of per-
methrin under similar conditions. Pulmonary function, clinical chemis-
try parameters, and blood cell counts were unaffected in the beagle
dogs following exposure. No compound-related gross or microscopic
pathological changes or other permanent changes were observed in the
dogs, rats, or guinea-pigs as a result of permethrin inhalation
(Metker, 1978).
7.3 Primary Irritation
7.3.1 Skin irritation
When undiluted technical permethrin (91.3% purity, 0.5 ml) was
applied to the clipped dorsal surface of Japanese White rabbits, there
was no irritation (Okuno et al., 1976).
Single applications of 0.05 ml of 25% (w/v) permethrin (in 95%
ethanol) or 10% (w/v) oil of bergamot solution (in 95% ethanol) (posi-
tive control) were applied to the intact skin of six rabbits. Five
minutes later, some of the rabbits were exposed to UV light (365 nm) at
a distance of 10-15 cm for 30 min (the intensity of UV light was not
mentioned). Skin treated with the positive control solution and
irradiated exhibited a greater irritation reaction than did non-
irradiated skin. Permethrin did not cause any irritation reaction
under the test conditions with or without irradiation (Metker et al.,
1977).
When a permethrin formulation was applied to the clipped dorsal
surface (0.13 mg/cm2) of six New Zealand White rabbits (three of each
sex) once a day for 16 days, a slight erythema appeared, which corre-
lated with increased cutaneous blood flow measured by laser Doppler
velocimetry. No significant histopathological changes were detected
(Flannigan et al., 1985a).
7.3.2 Eye irritation
In a study by Okuno et al. (1976), 0.1 ml of undiluted technical
permethrin (91.3% purity) was applied to the eyes of Japanese White
rabbits. The eyes were washed with distilled water 5 min or 24 h after
the application of permethrin. No eye irritation was observed.
Undiluted permethrin applied to the eyes of female rabbits caused
minimal pain, redness, chemosis of the conjunctiva, and a slight dis-
charge (Parkinson et al., 1976).
Permethrin (25:75) (40% in corn oil) did not produce any ocular
effects when 0.1 ml was instilled into the ocular sac of New Zealand
rabbits (Chesher & Malone, 1974c).
7.4 Sensitization
In a study by Parkinson et al. (1976), guinea-pigs were dermally
administered permethrin as a 10% solution in dimethylformamide for
3 consecutive days. This was followed 4 days later by challenge doses
of 0.1%, 1%, and 10% solutions of permethrin in dimethylformamide. Only
very slight erythema was observed. Permethrin was therefore considered
to be either non-sensitizing or only mildly so.
Guinea-pigs (10 per group) were initially injected intradermally
with 0.1 ml permethrin solution and 14 days later were challenged with
an intradermal injection (0.1 ml) of either a 0.1% solution of permeth-
rin or dinitrochlorobenzene (DNCB). Five other animals per group
received intradermally a challenge dose of 0.1% permethrin or DNCB
without a prior sensitizing dose. The positive control substance (DNCB)
elicited sensitization reactions in all guinea-pigs when examined 24
and 48 h after the challenge dose, whereas permethrin did not cause any
sensitization reactions (Metker et al., 1977; Metker, 1978).
Permethrin (25:75) in corn oil (1% w/v) or Freund's complete
adjuvant (1% w/v) did not produce dermal irritation or sensitization in
groups of 10 male guinea-pigs when applied as a 25% dispersion in
petrolatum. The positive control, DNCB, (5% w/v) in petrolatum produced
marked sensitization (Chesher & Malone, 1974b).
7.5 Long-term Toxicity
7.5.1 Mouse
SPF Alderly Park strain mice (70 males and 70 females per group)
were fed permethrin (cis 35-45%; trans 65-55%) at dose levels of 0,
250, 1000, or 2500 mg/kg diet for 2 years. Ten males and ten females
were designated for interim kills at 26 and 52 weeks. The mortality
rate was unaffected by the administration of permethrin. Growth was
slightly decreased at the two highest dose levels at various periods
during the study. At the interim sacrifice of 52 weeks and at the end
of study, a significant dose-dependent increase in liver-to-body weight
ratio was observed at the two highest dose levels in females (with 2500
mg/kg only at the end of the study) and at the highest dose level in
males. Hepatic aminopyrine N-demethylase activity was also substan-
tially increased, although not consistently, in both male and female
mice given the highest dose. Gross and microscopic examination of
tissues and organs (and specific examination for hepatic neoplasia) did
not reveal any significant carcinogenic effects as a result of per-
methrin administration. Many of the non-tumour abnormalities observed
were considered to be those associated with aging of the mice, charac-
terized by increased eosinophilia of the centrilobular hepatocytes.
Also, a decrease in vacuolation of the proximal tubular epithelium of
the kidney was noted at all dietary levels in males. A high incidence
of lung adenomas was observed with all animals in the study, but stat-
istical analysis suggested that this was not related to permethrin
feeding. Electron-microscopic examination of subcellular liver com-
ponents showed a proliferation of the smooth endoplasmic reticulum at
dose levels of 1000 and 2500 mg/kg. No notable effects on the sciatic
nerve were found as the result of permethrin administration (Ishmael &
Litchfield, 1988).
In studies by Hogan & Rinehart (1977) and Rapp (1978), CD-1 strain
mice (75 of each sex per group) were fed permethrin in the diet for
104 weeks. Alterations were made in the dietary dose levels during the
course of the study. From weeks 1 to 19, the animals were given dose
levels of 0, 20, 100, and 500 mg/kg diet. At week 19, the dose level
of 500 mg/kg was increased to 5000 mg/kg and maintained for 2 weeks
before returning to 500 mg/kg. At week 21, the 100 mg/kg dose was
increased to 4000 mg/kg and maintained for the remainder of the dosing
period. Growth was inhibited in males at 4000 mg/kg. With the exception
of a reduced blood glucose level in the animals receiving 4000 mg/kg,
dietary administration of permethrin had no other effects on haema-
tology or clinical chemistry parameters in the mouse. The liver weight
was higher than it was in control animals in both male and female
animals at a dose level of 500 mg/kg or more. In addition, the heart
weight was higher at 4000 mg/kg. Neoplastic changes, not associated
with dietary levels of permethrin, were observed in some animals in all
groups. While there was no direct effect with respect to hepatic neo-
plasms, it was noted that hepatocellular hypertrophy, pleomorphism, and
degeneration occurred in treated mice with increased frequency and
appeared to show a dose-response relationship. No oncogenic effects
were observed in the test animals.
7.5.2 Rat
Wistar rats (60 of each sex per group) were fed permethrin in the
diet at dose levels of 0, 500, 1000, or 2500 mg/kg diet for 2 years,
and twelve rats of each sex per group were sacrificed at 1 year. Signs
of poisoning such as tremors and hyperexcitability were noted during
the first 2 weeks of dosing in the animals that received the highest
dose. There was no mortality attributable to permethrin, and growth and
food consumption were unaffected. There were no effects on haema-
tological, ophthalmological, urological, or other clinical chemistry
parameters. Liver aminopyrine N-demethylase activity was increased in
all permethrin-treated animals. Bone marrow smears of the animals
showed no unusual findings. Gross and microscopic examination of
tissues and organs was performed after 1 and 2 years, and all animals
that died with neoplastic changes were examined. Liver weights were
higher after 1 year of dosing in male and female rats that received
permethrin at 2500 mg/kg than in the control animals. After 2 years,
the liver weight and liver-to-body weight ratios were higher in all
permethrin-treated males than in the corresponding controls. In the
females, higher values of absolute and relative liver weights, compared
to the controls, were recorded only in the group of animals dosed at
1000 mg/kg. Kidney weight was also increased, predominantly in males,
at all dose levels. Hepatocyte vacuolation was seen at 1 year in males
fed at the highest dose level only and in females at all dose levels.
The smooth endoplasmic reticulum showed significant increases at 52
weeks in both males and females at all dietary levels. At the end of
the study, notable endoplasmic reticulum increases were detected only
at the highest dose levels, although non-significant increases were
noted at all dose levels in both males and females. Examination of the
sciatic nerve showed no effects attributable to permethrin. No
oncogenic effects were noted at any dose level (Ishmael & Litchfield,
1988).
Long-Evans rats (60 males and 60 females per group) fed permethrin
in the diet at dose levels of 0, 20, 100, or 500 mg/kg for 2 years did
not reveal any mortality or adverse effects on growth, food consump-
tion, or behaviour attributable to the administration. Haematology,
clinical chemistry, and urinalysis measurements were performed at
either 6 months or 1 year and at the end of the study. There were no
compound-related effects on a wide variety of parameters examined, and
ophthalmological examination indicated no abnormalities. Blood glucose
levels were higher in the highest-dose males at 24 months and in the
highest-dose females at 18 months, compared to the values of the
control animals. Two independent evaluations of the histopathological
data concluded that there was no oncogenic potential for permethrin.
While there was a dose-dependent increase in gross liver weight in both
males and females, these values are small and not statistically
significant. The NOEL for general toxicity in this study was estimated
to be 100 mg/kg (Braun & Rinehart, 1977; Billups 1978a, b).
7.6 Carcinogenesis
Summaries by Paynter et al. (1982) of toxicological data from seven
long-term chronic toxicity/oncogenicity studies (four in mouse and
three in rat) carried out by ICI, FMC, and Burroughs-Welcome (BW) have
been made available by the US EPA. One rat study and one mouse study
performed by ICI were recently published (Ishmael & Litchfield, 1988),
and one rat study and one mouse study carried out by BW were cited in
FAO/WHO (1988). A report of the FIFRA Scientific Advisory Panel which
reviewed this data was also made available (US EPA, 1981). Table 13
summarizes the basic design of each of these studies, some of which are
also discussed in section 7.5.
7.6.1 Mouse
One of the four mouse studies referenced in Table 13 (FMC I) was
not considered for evaluation because of dose level changes in the mid-
and high-level groups and problems in histopathological methodology.
7.6.1.1 ICI study
Relevant non-oncogenic effects observed during the study consisted
of increased mortality, increased liver aminopyrine- N-demethylase ac-
tivity, increased liver weight, and eosinophilia of hepatocytes in both
males and females at 2500 mg/kg diet. The liver changes observed in
this study were considered to be related in large measure to the
induction of liver microsomal enzyme activity. Minimal liver changes
were also observed at 1000 mg/kg, but not at 250 mg/kg. A slight
increase in lung adenomas was observed in male mice at the highest dose
level. However, there was some uncertainty as to whether this increase
was related to permethrin ingestion.
7.6.1.2 FMC II study
Relevant non-oncogenic effects observed during the study consisted
of increased mortality in males at 2000 mg/kg diet, increased liver
weight in females at 2500 mg/kg and 5000 mg/kg, and increased lung
weight in females at 5000 mg/kg. Histopathologically, dose-related
"focal alveolar cell proliferation" (increased numbers of lung cells)
was observed in permethrin-treated females. As regards oncogenic
effects, there was an increased incidence of bronchio-alveolar adenomas
in female mice only. The number of female mice with adenomas and/or
carcinomas (15/74, 24/72, 35/74, and 44/75 at the four dose levels)
revealed a statistically significant dose-response relationship. Male
mice did not show this effect. However, some doubt was expressed by
the FIFRA Scientific Advisory Panel concerning the conduct of this
study.
7.6.1.3 BW study
Non-oncogenic effects observed during the study consisted of
slightly decreased mortality in females at 50 and 250 mg/kg per day,
increased liver weights in males, and increased kidney weights in
females at 250 mg/kg per day. Histopathologically, an increased inci-
dence in cuboidal/columnar metaplasia of the alveolar epithelium was
observed in the lungs of male and female mice at the highest dose. The
oncogenicity data indicated a dose-related trend in females, but not in
males, for adenomatous tumours in the lungs. No notable pattern was
observed for other neoplasms at any dose level.
7.6.1.4 Appraisal of mouse studies on carginogenicity
Consistent findings in the above three mouse studies at high dose
levels were liver changes known to be associated with the induction of
the liver microsomal enzyme system. Other histopathological effects
observed in liver, not usually associated with microsomal induction,
included multifocal hepatocytomegaly and hepatocytic pigmentation. The
incidence of lung adenomas for each of the three mouse studies is given
in Table 14.
Among the three long-term mouse studies, there was evidence of
permethrin oncogenicity in the lungs in one strain (CD-1 female only)
at the highest dose level only.
Although there was a difference between the control and treated
groups in terms of lung adenomas in these studies, these differences
were not significant when compared with historical control values. The
oncogenicity potential, as evaluated by the FIFRA Scientific Advisory
Panel, was considered to be very weak.
Table 13. Chronic toxicity and carcinogenicity studies in mice and rats
------------------------------------------------------------------------------------------------------
Study
performed Species Strain No. of Duration Dose Reference
by animals (weeks) (mg/kg diet)
------------------------------------------------------------------------------------------------------
ICI mouse Alderly Park 70 98 0, 250, 1000, 2500 Ishmael & Litchfield (1988)
(SPF)
FMC I mouse CD-1 75 104 0, 20, 500, 4000 Hogan & Rinehart (1977);
Rapp (1978)
FMC II mouse CD-1 75 104 M:0, 20, 500, 4000 Bio Dynamics (1979)
F:0, 20, 2500, 5000
BW mouse CFLP 75 92 0a, 10a, 50b, 250b James et al. (1980)
ICI rat Wistar 60 104 0, 500, 1000, 2500 Ishmael & Litchfield (1988)
FMC rat Long-Evans 60 104 0, 20, 100, 500 Braun & Rinehart (1977);
Billups (1978a,b)
BW rat Wistar 60 104 0, 10b, 50b, 250b McSheehy & Finn (1980)
------------------------------------------------------------------------------------------------------
a 100 animals as control.
b mg/kg body weight; permethrin 25% cis, 75% trans.
Table 14. Comparison of lung adenomas (%) in three mouse studies
------------------------------------------------------------------------
Male Female
control low mid high control low mid high
dose dose dose dose dose dose
------------------------------------------------------------------------
ICI study 20 12 26 32 22 16 20 30
FMC II study 22 23 28 25 16(20a) 17 35 35
BW study 26 19 23 22 3(20b) 7 10 20
------------------------------------------------------------------------
a Historical control values ranged from 23 to 60%, with a mean
of 20.4%.
b Historical control values ranged from 7.5 to 30.0%, with a mean
of 20.4%.
7.6.2 Rat
One of the three long-term rat studies referred to in Table 13,
i.e. the FMC rat study, was excluded from examination because of
serious flaws in histopathological methodology.
7.6.2.1 ICI study
Relevant non-oncogenic effects observed consisted of increased
mortality in males and decreased mortality in females at 2500 mg/kg
diet, increased liver weights in both males and females at 1000 and
2500 mg/kg and in males only at 500 mg/kg, increased liver aminopyrine-
N-demethylase activity in both males and females at 1000 and 2500
mg/kg, and hepatocyte vacuolization or hypertrophy in males and females
at 1000 and 2500 mg/kg. Additional effects observed were increased
kidney weight in males at all treatment levels and increased pituitary
weight in males at 1000 and 2500 mg/kg.
No tumours related to the ingestion of permethrin were observed in
this study.
7.6.2.2 BW study
Non-oncogenic effects observed at 250 mg/kg per day were increased
mortality in males, occasional body tremors in males and females, in-
creased liver weight in males, hepatocyte hypertrophy in males and fe-
males, and focal changes of the thyroid follicles in males and females.
The microscopic liver and thyroid changes were also observed at 50
mg/kg per day in both sexes.
With respect to tumours (including rare, unusual, or malignant neo-
plasms), none of the tumour types observed in this study were con-
sidered to be related to the ingestion of permethrin.
7.6.2.3 Appraisal of rat studies on carcinogenicity
No evidence of oncogenicity was observed in the rat studies.
7.7 Mutagenicity
7.7.1 Microorganism and insects
The mutagenic activity of permethrin was evaluated using the Ames
test. There was no increase in the number of revertant colonies at
doses up to 2500 µg permethrin/plate in five strains of Salmonella
typhimurium (TA1535, TA1537, TA1538, TA98, and TA100) with or without
S9-mix prepared from rat liver or S9 prepared from PCB-treated mice
(Longstaff, 1976; Newell & Skinner, 1976; Simmon, 1976; Suzuki, 1977).
Permethrin and six other synthetic pyrethroids were tested for
mutagenicity in S. typhimurium TA98 and TA100 strains in the presence
and absence of a metabolic-activation system. All pyrethroids tested
gave negative results (Pluijmen et al., 1984).
Two reverse-mutation tests in Escherichia coli WP2 also gave nega-
tive results (Newell & Skinner, 1976; Simmon, 1976).
When tested for mutagenicity in V79 Chinese hamster cells, permeth-
rin and five other synthetic pyrethroids were shown to be non-mutagenic
(Pluijmen et al., 1984).
In a host-mediated assay, permethrin (200 mg/kg body weight) was
orally administered to ICR mice. The indicator organism, S. typhimurium
G46, harvested from the abdominal cavity of mice 3 h after treatment,
did not reveal any mutagenic effect (Shirasu et al., 1979). In another
host-mediated assay employing a similar test system, (+)- trans-permethrin
at dose levels of 600 and 3000 mg/kg body weight and (+)- cis-permethrin
at 21 and 54 mg/kg body weight gave negative results (Miyamoto, 1976).
Permethrin was tested for its ability to induce complete and
partial chromosome loss in Drosophila melanogaster males by adding
5 mg/litre (soaked onto a filter paper) to the feeding solution.
Treated males were mated with mus-302 repair-defective females to
detect chromosome loss in the zygotes. Permethrin did not induce a
significant increase in chromosome loss, compared to negative controls
(Woodruff et al., 1983).
7.7.2 Mammals
An in vivo cytogenetic test was performed in Alderly Park rats to
assess the ability of permethrin to induce chromosomal aberration.
Permethrin was administered to groups of eight males by a single intra-
peritoneal injection or by five daily injections at doses of 600, 3000,
or 6000 mg/kg. The cytogenetic effect on bone marrow cells was evalu-
ated 24 h after the single injection and 6 h after the last multiple
dosing. No differences were observed in the rate of chromosomal aber-
rations between any permethrin-treated groups and the vehicle controls.
Two positive controls (trimethyl phosphate and mitomycin C) produced a
significantly higher incidence of chromosomal aberrations (Anderson &
Richardson, 1976).
Permethrin (25:75) gave a negative response when mouse lymphoma
L5178Y cells were treated with permethrin (up to 125 µg/ml) with or
without activation (Clive, 1977).
In dominant lethal studies, permethrin dissolved in corn oil was
administered orally for five successive days to groups of male CD mice
(15 per group) at doses of 15, 48, or 150 mg/kg. Each male was mated
with 16 virgin females, and on the 12th day of gestation the females
were killed. There was no dose-related effect on pregnancy or early or
late fetal deaths. Administration of permethrin thus had no dominant
lethal effect on male mice. On the other hand, the positive control
(ethylmethanesulfonate) induced pre-implantation losses and the early
death of embryos (McGregor & Wickramaratne, 1976a; Chesher et al.,
1975b).
7.8 Teratogenicity and Reproduction Studies
7.8.1 Teratogenicity studies
7.8.1.1 Mouse
In studies by Kohda et al. (1976b), groups of pregnant ICR mice (27
to 32 mice per group) were orally administered permethrin at dose
levels of 0, 15, 50, or 150 mg/kg body weight from day 7 to day 12 of
pregnancy. On day 18, two-thirds of the animals were sacrificed and
examined for implantation and resorption sites. Viable offspring were
examined for somatic and skeletal abnormalities, and, after 3 weeks of
lactation, pups were examined for behavioral abnormalities and for
differentiation and growth. At 6 weeks of age, all animals were sacri-
ficed and subjected to internal and external examination. There were no
effects on maternal toxicity over the course of the study. Growth and
differentiation of pregnant females were not affected by permethrin,
nor were the number of implantation sites or litter size adversely
affected. The size of individual pups and the incidence of gross
external, internal, and skeletal abnormalities were not significantly
different from those in the control mice. Permethrin, at dose levels
up to and including 150 mg/kg, did not appear to affect those animals
allowed to bear and wean young. The growth of young animals did not
appear to differ from control values, and, 3 weeks after weaning, the
surviving animals did not differ from controls with respect to growth
or major organ changes. There was no teratogenicity associated with
permethrin in this mouse bioassay, although the duration of dosing was
a little too short to cover both the early and late stage of organ
development (Kohda et al., 1976b).
7.8.1.2 Rat
In studies by McGregor & Wickramaratne (1976b), pregnant CD rats
(20 rats per group) were orally administered permethrin at dose levels
of 0, 22.5, 71.0, or 225 mg/kg from day 6 to day 16 of gestation. On
day 20, the animals were sacrificed and the corpora lutea were exam-
ined. Somatic and skeletal investigations were performed on the
fetuses. No adverse toxicological response was seen at the highest dose
used. There were no abortions or maternal deaths and no significant
differences in pregnancy frequency, corpora lutea, or total number of
implantations between treated and control rats. Placental and fetal
weights were similar to those of the controls and no skeletal or struc-
tural abnormalities were observed. Based upon the standard teratologi-
cal rat bioassay, permethrin did not show any teratological potential.
Pregnant Sprague-Dawley rats (29-34 rats per group) were orally
administered permethrin at dose levels of 0, 10, 20, or 50 mg/kg body
weight from day 9 to day 14 of pregnancy. On day 20, approximately two-
thirds of the pregnant females were sacrificed and the remaining rats
were allowed to deliver and wean pups. After lactation, the pups were
examined for behaviour and for growth and differentiation before being
sacrificed at 6 weeks of age and examined for internal and external
gross malformations. Pregnant females fed at the highest dose showed
toxic signs of poisoning, including ataxia, tremor, and a slight
reduction in body weight. There was no mortality, although fetal loss
at the highest dose level was slightly higher than that in the control
animals. A slightly higher incidence of non-ossified sternebra was
noted at 50 mg/kg. The number of implantation sites and the litter size
were not affected, and growth and differentiation were similarly unaf-
fected. Internal and external examination showed that, with the excep-
tion of the slight skeletal variation noted at 50 mg/kg, there were no
permethrin-associated changes. In those animals allowed to bear and
wean pups, there were no notable differences from control values with
respect to gestation, implantation sites, delivery, and numbers of live
young. Growth and differentiation of the offspring did not appear to
be affected by permethrin, and there were no abnormalities with respect
to gross pathology or in the weights of major tissues and organs at the
conclusion of the study. Permethrin did not elicit a teratogenic effect
in this bioassay (Kohda et al., 1976a).
In a study by Metker et al. (1977), permethrin (4, 41, and 83
mg/kg/diet), aspirin (200 mg/kg diet), and corn oil (2 ml/kg diet) were
each administered to groups of 20 pre-impregnated Sprague-Dawley rats
from day 6 to day 16 of gestation. The animals were sacrificed on day
20 of gestation, and the fetuses were removed and examined for gross
abnormalities, sex, weight, and body length. The administration of
aspirin (the positive control) resulted in significantly lower body
weight and length and a variety of abnormalities including craniorachi-
oschisis in the foetuses. Permethrin, administered to pregnant rats
during gestation by intragastric intubation, did not appear to present
a teratogenic or lethal hazard to the developing fetus.
When groups of 22 female Wistar rats received permethrin (25:75) at
0 or 200 mg/kg body weight in corn oil by daily oral gavage on days 6-
16 (inclusive) of pregnancy, treatment was without apparent effect on
maternal body weight gain or general conditions. The animals were
sacrificed on day 20 so that their uterine contents could be examined.
Treatment had no effect on the number of corpora lutea, implantations,
live fetuses, early and late fetal deaths, or fetal abnormalities.
Examination of the fetuses, which included dissection and skeletal
staining, showed no morphological effects of treatment. These results
indicate that permethrin (25:75) at 200 mg/kg body weight per day is
not fetotoxic to rats (James, 1974).
7.8.1.3 Rabbit
Mated female Dutch rabbits (18 per group) were orally administered
permethrin (at dose levels of 0, 600, 1200, or 1800 mg/kg body weight
per day) in 0.5% v/v aqueous Tween 80 from days 6-18 inclusive of
pregnancy. On day 29 of pregnancy the animals were killed and their
uteri were examined for resorptions and live implantations. The fetuses
were examined for gross abnormalities of skeleton and soft tissue. At
all dose levels, permethrin depressed body weight gain during dosing
and was embryotoxic at the two highest dose levels. It was toxic to
the dams at 1800 mg/kg body weight per day, but no teratogenic activity
was detectable at any dose level (Richards et al., 1980).
7.8.2 Reproduction Studies
7.8.2.1 Rat
Groups of Long-Evans rats (10 males and 20 females per group) were
fed permethrin at dose levels of 0, 20, and 100 mg/kg diet in a 3-
generation (two litters per generation) reproduction study. There was
no effect on mortality, mating, pregnancy, or fertility, with the
exception of the F2 mating index, which was reduced in both controls
and treatment groups. Pup survival and growth were unaffected. Haema-
tological evaluations of F2 adults between the second and third mating
showed no unusual effects. This study indicated that dietary permethrin
does not adversely affect reproduction in the rat (Schroeder &
Rinehart, 1977).
In studies by Hodge et al. (1977), groups of Wistar rats (12 males
and 24 females per group) were fed permethrin at dose levels of 0, 500,
1000, and 2500 mg/kg diet for 12 weeks. At 12 weeks the animals were
mated to initiate a standard 3-generation (two litters per generation)
reproduction study. Clinical signs of acute poisoning (tremors, etc.)
were noted, predominantly in females given the highest dose. There
were no effects attributable to permethrin on fertility, gestation,
viability of pups, sex ratio, litter size, or lactation. Ten male and
female weanlings from the second litter of the F3 generation were
examined for histopathological changes. Centrilobular hypertrophy and
cytoplasmic eosinophilia were observed at all dose levels, the inci-
dence and severity of which appeared to be dose dependent. Rats in the
third litter of the F3 generation were sacrificed on day 12 of ges-
tation for teratogenic examination, but no abnormalities were observed.
Based on the results of this study, permethrin does not appear to
induce reproductive toxicity in rats.
Spencer and Berhance (1982) fed female Sprague-Dawley rats (5-8
rats per group) permethrin in the diet at levels of 0, 500, 1000, 1500,
2000, 2500, 3000, 3500, and 4000 mg/kg diet from day 6 to day 15 of
pregnancy. Laparotomy was performed on day 20 of gestation, and the
number of live fetuses was determined. Placentae were excised and
cleaned of extraneous connective tissue. Analysis of the protein and
glycogen contents of the placentae on day 16 of pregnancy indicated
that they were only influenced by very high doses (2500-4000 mg/kg
diet) of permethrin. Analysis of variance indicated no significant
effect on protein level, but the treatment did decrease the glycogen
concentration. No significant dose-related effects on implantational
sites/intrauterine fetuses were observed. These results appeared to
confirm that permethrin possesses low mammalian toxicity.
In a 3-generation reproduction study, groups of 20 male and 20 fe-
male Wistar COBS rats received permethrin (25:75) in the diet at 0, 5,
30, and 180 mg/kg body weight per day during growth, mating, gestation,
parturition, and lactation for three generations, each with two
litters. Fetal toxicity and teratogenicity was assessed in the second
pregnancy of the F2 generation. Treatment with permethrin had no ef-
fect on general behaviour or condition, food intake, body weight gain,
or pregnancy rate of the dams, or on parturition, sex ratio, or pup
weight. A small number of rats of each group developed eye abnor-
malities, including ocular haemorrhage and chronic glaucoma, but this
was not related to the treatment. Examination of F3b fetuses showed
no treatment-related effect on sex ratio, body weight, or the occur-
rence of visceral or skeletal abnormalities. This study indicated that
permethrin (25:75) has no effect on the reproduction of rats at doses
up to 180 mg/kg body weight per day (James, 1979).
7.9 Neurotoxicity
7.9.1 Rat
When male and female Charles River rats (six of each sex per group)
were fed permethrin at dose levels of 0 or 6000 mg/kg diet for up to
14 days, severe clinical signs of poisoning were evident in all the
permethrin-treated rats. Only one permethrin-treated male survived the
14-day trial. Fragmented and swollen sciatic nerve axons and myelin
degeneration were observed in four out of five permethrin-treated ani-
mals (Hend & Butterworth, 1977).
In a short-term study designed to assess the effects of high con-
centrations of permethrin on the sciatic nerve, male Wistar rats (10
per group) were fed permethrin at dose levels of 0, 2500, 3000, 3750,
4500, 5000, and 7000 mg/kg diet for 14 days. Clinical signs of acute
poisoning and death occurred in the animals that were dosed at 5000 or
7000 mg/kg. Some rats that received the lower dose levels showed slight
to moderate tremors, and food consumption and growth were reduced in
these animals. At the two lowest dose levels, clinical signs of poison-
ing disappeared within the first week whereas, at the higher dose
levels, signs of poisoning persisted throughout the study. Rats
receiving permethrin at doses of up to 4500 mg/kg showed no ultra-
structural changes in their sciatic nerve. A variety of mild ultra-
structural changes, such as vacuolation and swelling of unmyelinated
fibres and hypertrophy of Schwann cells, were observed in the nerves of
rats receiving 5000 mg/kg (Glaister et al., 1977).
A detailed morphological evaluation of the nervous system was per-
formed on rats in two long-term feeding studies. In the first, Long-
Evans rats were fed diets containing permethrin at concentrations of 0,
20, 100, or 500 mg/kg diet for 2 years, and five male and five female
randomly selected survivors from each group were examined. In the
second study, Long-Evans rats were fed diets containing permethrin at
concentrations of 0, 20, or 100 mg/kg diet for three successive gener-
ations, and five male and five female rats from each group were ran-
domly selected from the third generation parental animals. Examination
of central and peripheral nerves and of extensive morphometric data and
teased myelinated fibers of distal sural and tibial nerves and of the
maxillary division of the fifth cranial nerve did not reveal any
changes attributable to the feeding of the pesticide (Dyck et al.,
1984).
When groups of 10 male and 10 female Sprague-Dawley rats were given
permethrin (25:75) (94.5% pure) at 4000, 6000, or 9000 mk/kg diet for
21 days, all animals developed severe trembling and lost weight. Some
of the highest-dose rats of each sex died. Subsequent examination of
brain, spinal cord, trigeminal and dorsal root ganglia, proximal and
distal root trunks, and terminal motor and sensory nerves revealed no
consistent histopathological abnormalities (Dayan, 1980).
7.9.2 Hen
Hens were administered permethrin orally (cis:trans=1:1) (as a 40%
w/v solution in dimethylsulfoxide) at a daily dose level of 1 g/kg body
weight for 5 days. After 3 weeks, the dosing regimen was repeated, and
the animals were maintained for an additional period of 3 weeks before
being sacrificed. There were no signs of neurological disturbance or
mortality in any of the animals. All hens treated with tri- ortho-cresyl phos-
phate (TOCP) (positive control) displayed clinical and histopathologi-
cal evidence of neurotoxicity, whereas none of the birds dosed with
permethrin showed any signs of intoxication. Histological examination
of nerve tissues revealed no lesions. Hence, permethrin was considered
to have no delayed neurotoxic potential such as that associated with
certain organophosphates (Millner & Butterworth, 1977).
In studies by Ross & Prentice (1977), 15 adult hens were orally
administered permethrin at 9 g/kg body weight and again 21 days later.
After a further 21 days, they were sacrificed. All positive control
animals (given TOCP at 500 mg/kg) showed signs of delayed neurotoxicity
ranging from slight muscular incoordination to paralysis. No signs of
ataxia were recorded in any of the hens in the permethrin-treated or
negative control groups. Histopathological examination of the nervous
tissues of permethrin-treated animals revealed none of the degenerative
changes noted in the tissues of the positive controls.
7.10 Behavioural Effects
Behavioural observations were carried out on immature male Sprague-
Dawley rats habituated to inhalation of permethrin aerosols. Habitu-
ation was carried out by exposing three groups of rats (five per group)
to aerosols of permethrin firstly at 500 mg/m3 for 21 days, then at
1000 mg/m3 for an additional 21 days. Three other groups of rats (five
per group) served as controls; they were similarly treated but were not
exposed to permethrin. At the end of this conditioning period, all
rats, including the controls, were exposed to a permethrin aerosol at
5000 mg/m3 for 4 h. At the end of the habituation period, there were
no differences in retention of avoidance training or the ability to
learn the same task between controls and aerosol-exposed groups. How-
ever, after exposure to permethrin at 5000 mg/m3, the non-habituated
control group of rats showed significantly lower retention capacity
compared with the habituated rats or with their own pre-exposure per-
formances. The non-habituated control rats also showed decreases in
coordination and balance and a higher incidence of conflict behaviour
and tremors. The performance of the rats in the habituated groups was
not changed (Sherman, 1979).
7.11 Miscellaneous Studies
The pharmacological action of permethrin on nictitating membrane,
blood pressure, respiration, heart rate, and isolated ileum was inves-
tigated in the rabbit, guinea-pig, and cat. Permethrin reduced the
incidence and amplitude of contraction of isolated rabbit ileum but
induced no changes in a similar preparation from the guinea-pig. Intra-
venous administration of permethrin at doses of 4 mg/kg or more affec-
ted blood pressure and respiration in all animals. The hypotensive
effect was not affected by pretreatment with atropine or propanolol.
Permethrin was shown to produce slight contraction of the nictitating
membrane. An increase in pulse rate was observed in the electrocardio-
gram (ECG) of the rabbit at dose levels above 4 mg/kg, but was not
accompanied by changes in the wave pattern (Nomura & Segawa, 1979).
In the Japanese White rabbit, the intravenous administration of
lethal doses of permethrin caused changes in the electroencephalogram
(EEG) tracings. Spike waves and an increased amplitude of slow waves
were induced at 100 mg/kg body weight. At a sub-lethal dose of 30
mg/kg, permethrin did not induce changes in the EEG (Takahashi et al.,
1979).
There was no change in hexobarbital-induced sleeping time in ICR
mice intraperitoneously administered a single dose of permethrin at
dose levels of up to 2000 mg/kg body weight (Takahashi et al., 1979).
Sprague-Dawley rats (three groups of 10 rats each) were pretreated
for four consecutive days with an intraperitoneal injection of either
sodium phenobarbital at 100 mg/kg (positive control group), permethrin
at 575 mg/kg (test group), or corn oil at 2.0 ml/kg (solvent control
group). On the fifth test day the rats received an intraperitoneal
injection of hexobarbital (220 mg/kg). The hexobarbital-induced sleep-
ing times of the permethrin-treated rats were significantly shorter
than those of the solvent control animals but were similar to those of
the phenobarbital positive control (Metker et al., 1977).
Permethrin and cypermethrin were evaluated for their ability to
alter microsomal cytochrome P-450 and NADPH cytochrome c reductase in
Long-Evans rats. When permethrin (cis:trans = 80:20) was orally admin-
istered to rats at 50 mg/kg body weight per day, it increased cyto-
chrome P-450 after 4, 8, or 12 days of administration and NADPH cyto-
chrome c reductase after 8 or 12 days, whereas cypermethrin (alpha-cyano
analogue of permethrin) did not induce either cytochrome P-450 or the
reductase. Neither of the two pyrethroids altered body weight gain
(Carlson & Schoening, 1980).
7.12 Mechanism of Toxicity (Mode of Action)
Based on the signs of toxicity to mammals (Verschoyle & Aldridge,
1980; Lawrence & Casida, 1982) and to cockroaches (Gammon et al.,
1981), pyrethroids may be classified into two types: Type I and Type II
compounds (see Appendix I). 1R- cis- and 1R- trans-permethrin belong
to Type I. Electrophysiological recordings from dosed cockroaches
reveal trains of cercal sensory spikes and, sometimes, spike trains
from the cercal motor nerves and the central nervous system. The signs
of poisoning caused by Type I pyrethroid compounds are restlessness,
incoordination, hyperactivity, prostration, and paralysis (Gammon et
al., 1981).
1RS- cis-permethrin and 1RS- trans-permethrin cause tremor (known
as T-syndrome) when injected intravenously into rats at a dose level of
more than 270 mg/kg body weight. The onset of the T-syndrome is usually
rapid. Rats suffering from T-syndrome exhibit aggressive sparring be-
haviour and increased sensitivity to external stimuli. This is followed
by the appearance of a slight tremor, which gradually becomes more
severe and finally reaches a state of prostration and vigorous whole
body tremor. The core temperature is markedly increased during poison-
ing; this may result from the excessive muscular activity associated
with tremor (Verschoyle & Aldridge, 1980).
Exposure of sensory nerve fibres from clawed frogs (Xenopus laevis)
to permethrin (10-7 to 10-5 mol/litre) resulted in marked repetitive
activity. This heightened activity was not observed in the motor
fibres of frogs that were similarly tested. Treatment of isolated
lateral-line preparations of frogs with permethrin (5 x 10-6 mol/litre)
also resulted in pronounced repetitive activity (Van den Bercken &
Vijverberg, 1980b).
Permethrin (cis, trans, and technical grade) and deltamethrin, as
representatives of the non-cyano- and cyano-containing classes,
respectively, of synthetic pyrethroids, were examined regarding their
major site of action on the mammalian nervous system in mice. ED50
values for the ability of both types of pyrethroids to cause pros-
tration and loss of righting reflex were estimated following either
intravenous or intracerebroventricular injections. The comparative
potencies of the four pyrethroids (deltamethrin > cis-permethrin >
technical grade permethrin > trans-permethrin) were the same following
either route of administration. All four compounds tested showed a much
greater potency (> 200-fold for deltamethrin, cis-permethrin, and
technical grade permethrin, and 85-fold for trans-permethrin) after
intracerebroventricular administration than after intravenous admin-
istration. In addition, the poisoning symptoms seen following direct
central injection were almost identical to those obtained with periph-
eral administration. These results suggest that poisoning from both
classes of pyrethroids in mammals is due predominantly to central
mechanisms (Staatz et al., 1982).
8. EFFECTS ON HUMANS
Although permethrin has been used for many years, no human poison-
ing cases have been reported.
8.1 Occupational Exposure
Data on permethrin human toxicity are scanty. Studies of occu-
pational exposure to permethrin were reported in Sweden (Kolmodin-
Hedman et al., 1982). In the first study, six forestry workers using
an aqueous emulsion of permethrin (trans:cis=75:25) were studied. The
duties of these workers involved dipping conifer seedlings in a 2%
aqueous emulsion of permethrin (one worker) and packaging the
permethrin-treated seedlings (five workers). The permethrin concen-
trations in the breathing zones of these workers varied between 0.011
and 0.085 mg/m3. One person excreted permethrin metabolites at
0.26 µg/ml urine the following morning, but the same afternoon the
urinary excretion of permethrin residues was below the detection limit
of 0.1 µg/ml. The urine from other workers did not contain detectable
amounts of permethrin residues. No symptoms of permethrin poisoning
were reported in this field study. The second study, performed on the
basis of a questionnaire and interviews, was conducted 1-2 months after
the planting season. It involved 87 persons at various plant nurseries
that used the insecticide (trans:cis=60:40 or 75/25). This study showed
that the major work-related symptoms amongst workers were irritative,
such as itching and burning of the skin, and itching and irritation of
the eyes. Irritative symptoms in the skin and upper respiratory tract
were reported in 63% of workers who were exposed to permethrin
(trans:cis=75:25) and 33% who were exposed to permethrin with a
different isomeric composition (trans:cis=60:40). The frequency of each
symptom was about 10% in each case. Increased nasal secretion was
reported by 13% of the workers handling permethrin (trans:cis= 75:25).
Le Quesne et al. (1980) examined 23 laboratory workers involved in
field trials, formulation, or general laboratory work with permethrin,
cypermethrin, fenvalerate, and fenpropathrin. The study involved
electrophysiological monitoring and interviews to ascertain subjective
symptoms. The most frequently reported symptom was a facial sensation
described as tingling, burning, or nettle rash by workers who had ex-
perienced it on one or more occasions. This sensation usually occurred
about 30 min to 3 h after exposure and lasted for about 30 min to 8 h.
Apparently this did not occur when permethrin alone was involved. All
the workers were examined neurologically and no abnormal findings were
recorded. Electro-physiological measurements from these workers were
compared with those of an age-matched control group. No difference in
response was found between the two groups.
Studies of pesticide contamination of clothing worn by crop con-
sultants during permethrin application to soybean fields were performed
to assess the extent of dermal exposure to the pesticide. The suits
and T-shirts were removed and wrapped in aluminum foil, placed on ice
and transported to the laboratory where they were held in a freezer
until residue analysis was performed. Specimens from the thigh, arm,
and chest of each suit and from the arm and chest of each T-shirt were
collected, extracted with hexane and analyzed by GC-ECD. Measurable
residues of permethrin were detected only in leg specimens (Cloud et
al., 1987).
As part of an evaluation of permethrin (25:75) (5% wettable powder)
in Nigeria, medical surveillance, including urinalysis of staff engaged
in bagging, mixing, or spraying, was undertaken. Medical history,
pulse, and blood pressure were recorded and urine was collected twice
daily. This surveillance revealed no effects attributable to permeth-
rin. Despite the use of protective clothing, up to 2 mg of permethrin
was excreted within 24 h of exposure (Rishikesh et al., 1978).
8.2 Clinical Studies
Flannigan & Tucker (1985) and Flannigan et al. (1985a,b) studied
the difference in the degree of paraesthesia induced by a number of
pyrethroids. On five occasions, 0.05 ml of field-strength-formulated
permethrin (0.13 mg/cm2) was applied to a 4 cm2 area of earlobe.
The opposite earlobe received distilled water. Participant evaluation
after each application continued for 48 h and involved description of
the cutaneous sensations. Each participant was treated after each
application with one of the remaining compounds. Permethrin, like the
other pyrethroids, induced skin sensations. Paraesthesia developed with
a latency period of approximately 30 min, peaked by 8 h, and deterio-
rated within 24 h. In the case of permethrin these sensations were
approximately four times less marked than those induced by cypermethrin
and fenvalerate, which both contain an alpha-cyano-group. It was also
found that local application of dl-alpha-tocopheryl acetate markedly
inhibited the occurrence of skin sensations.
To assess the safety of permethrin dusts for the control of human
body lice, approximately 350 people were individually dusted with 50 g
of powder containing either 2.5 or 5.0 g permethrin/kg. Urine samples,
taken at the time of treatment and subsequently, indicated that maximal
absorption of permethrin was 39 µg/kg, 24 h after treatment (Nassif
et al., 1980).
In a study to assess the degree of dermal absorption of permethrin
from impregnated clothing, a group of 10 male volunteer soldiers for
48 h wore military clothing that had previously been treated with an
aqueous suspension of permethrin (0.2% w/v). Subsequent analysis
showed that the mean permethrin (25:75) concentration of the shirts
and trousers was 0.32 g/100 g. However, the average individual
exposure to permethrin was 3.8 mg/day. No volunteers complained of
irritation and there were no abnormal findings on physical examination
(Farquhar et al., 1981a).
When dermally exposed to permethrin [(25:75) 1% w/w in soft
paraffin] for up to 9 days using a patch test, 2 out of 17 volunteers
developed mild erythema (Pegum & Doughty, 1978).
To assess the human tolerance, absorption, and persistence of
permethrin when used against human lice, 10 adult volunteers (four men,
six women) were treated with 15-40 ml of permethrin (25:75) (1%) head
louse solution. Their hair was allowed to dry naturally and then
washed with baby shampoo. Urine samples were collected at 0-24, 24-48,
120-144, and 336-360 h to measure dermal absorption. On assessment, 3
out of 10 volunteers developed mild, patchy erythema, which faded
between days 4-7. Permethrin excretion during the first 24 h was only
about 1% of the applied dose, while the cumulative maximum over 14 days
was only about 5.5 mg (Farquhar et al., 1981b).
9. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
The Joint FAO/WHO Meeting on Pesticide Residues (JMPR) discussed
and evaluated permethrin in 1979, 1980, 1981, 1982, 1983, 1984, 1985,
and 1987 (FAO/WHO, 1980a,b; 1981a,b; 1982a,b; 1983a,b; 1984a,b;
1985a,b; 1986a,b; 1987).
An acceptable daily intake (ADI) of 0-0.05 mg/kg body weight
(cis:trans ratios of 40:60 and 27:75) was established in 1985.
Maximum residue limits of 0.01-50 mg/kg for specified foods and 20-
100 mg/kg dry weight for specified feed have been proposed (FAO/WHO,
1986).
In the WHO recommended classification of pesticides by hazard,
permethrin as a technical product is classified in class II (i.e., as
moderately hazardous) (WHO, 1988). A WHO/FAO data sheet on permethrin
exists (WHO/FAO, 1984).
REFERENCES
AGNIHOTRI, N.P., JAIN, H.K., & GAJBHIYE, V.T. (1986) Persistence of
some synthetic pyrethroid insecticides in soil, water and sediment -
Part I. J. entomol. Res., 10: 147-151.
ALINIAZEE, M.T. & CRANHAM, J.E. (1980) Effect of four synthetic
pyrethroids on a predatory mite, Typhlodromus pyri, and its prey,
Panonychus ulmi, on apples in southeast England. Environ. Entomol., 9:
436-439.
ANDERSON, D. & RICHARDSON, C.R. (1976) Permethrin (PP557):
cytogenetic study in the rat (Report No. CTL/P/296) (Unpublished
report submitted to WHO by ICI Central Toxicology Laboratory).
ANDERSON, R.L. (1982) Toxicity of fenvalerate and permethrin to
several nontarget aquatic invertebrates. Environ. Entomol., 11: 1251-
1257.
BAKER, P.G. & BOTTOMLEY, P. (1982) Determination of residues of
synthetic pyrethroids in fruit and vegetables by gas-liquid and high-
performance liquid chromatography. Analyst, 107: 206-212.
BATTELLE (1982) Pesticide programme of research and market planning.
Part I: Insecticides, Geneva, Institut Battelle (October 1982).
BATTELLE (1986) World Pesticide Programme, Insecticide II, 1984,
Geneva, Institut Battelle.
BILLUPS, L.H. (1978a) Histopathologic examination of a twenty-four
month toxicity/carcinogenicity study of compound FMC33297 in rats, (Un-
published report submitted to WHO by FMC Corporation, Environmental
Pathology Services).
BILLUPS, L.H. (1978b) Twenty-four month toxicity/carcinogenicity
study of compound FMC33297 in rats (Unpublished report submitted to
WHO by FMC Corporation, Environmental Pathology Services).
BIO-DYNAMICS (1979) Twenty-four month toxicity/carcinogenicity study
of permethrin in mice (Bio-Dynamics Inc. Project No. 76-1695/9)
(Unpublished report submitted to WHO by FMC Corporation).
BORTHWICH, P.W. & WALSH, G.E. (1981) Initial toxicological assessment
of Ambush, Bolero, Bux, Dursban, Fentrifanil, Larvin, and Pydrin:
Static acute toxicity tests with selected estuarine algae,
invertebrates, and fish, Springfield, Virginia, National Technical
Information Service, pp. 1-9 (EPA-600/4-81-076).
BRADBROOK, C., BANHAM, P.B., GORE, C.W., PRATT, I., & WEIGHT,
T.M. (1977) PP557: A study of the reversibility of hepatic
biochemical and ultrastructural changes in the rat (Report No.
CTL/P/354) (Unpublished Data submitted to WHO by ICI Central Toxicology
Laboratory).
BRAUN, W.G. & KILLEEN, J.C. (1975) Acute oral toxicity in rat:
compound no. FMC33297 (Bio-Dynamics Inc. Project) (Unpublished report
submitted to WHO by FMC Corporation).
BRAUN, W.G. & RINEHART, W.E. (1977) A twenty-four month oral
toxicity/carcinogenicity study of FMC33297 in rats (Bio-Dynamics Inc.
Project) (Unpublished report submitted to WHO by FMC Corporation).
BRAUN, H.E. & STANEK, J. (1982) Application of the AOAC multi-residue
method to determination of synthetic pyrethroid residues in celery and
animal products. J. Assoc. Off. Anal. Chem., 65: 685-689.
BUTTERWORTH, S.T.G. & HEND, R.W. (1976) Toxicity studies on the
insecticide WL43470: a five week feeding study in rats (Report No.
TLGR.0056.76) (Unpublished data submitted to WHO by Shell Research
Ltd).
CARLSON, G.P. & SCHOENIG, G.P. (1980) Induction of liver microsomal
NADPH cytochrome c reductase and cytochrome P-450 by some new synthetic
pyrethroids. Toxicol. appl. Pharmacol., 52: 507-512.
CARROLL, B.R., WILLIS, G.H., & GRAVES, J.B. (1981) Permethrin
concentration of cotton plants, persistence in soil and loss in runoff.
J. environ. Qual., 10: 497-500.
CHESHER, B.C. & MALONE, J.C. (1974a) 10-day cumulative oral toxicity
study with 21Z73 in rabbits, Berkhamsted, Wellcome Research
Laboratories (Report No. HEFG 74-7) (Unpublished data submitted to
WHO).
CHESHER, B.C. & MALONE, J.C. (1974b) Guinea pig sensitization study
with 21Z73 using the maximisation test method, Berkhamsted, Wellcome
Research Laboratories (Report No. HEFG 74-3) (Unpublished data
submitted to WHO).
CHESHER, B.C. & MALONE, J.C. (1974c) Occular irritancy of 21Z73 in
rabbits, Berkhamsted, Wellcome Research Laboratories (Report no. HEFG
74-6) (Unpublished data submitted to WHO).
CHESHER, B.C., CLAMPITT, R.C., & MALONE, J.C. (1975) 21Z73 -
Preliminary investigations into the cumulative oral toxicity on dogs,
Berkhamsted, Wellcome Research Laboratories (Report no. HEFG 75-9)
(Unpublished data submitted to WHO).
CLAPP, M.J.L., BANHAM, P.B., CHART, I.S., GLAISTER, J., GORE, C., &
MOYES, A. (1977a) PP557: 28 day feeding study in rats (Report No.
CTL/P/355) (Unpublished data submitted to WHO by ICI Central Toxicology
Laboratory).
CLAPP, M.J.L., BANHAM, P.B., GLAISTER, J.R., & MOYES, A. (1977b)
PP557: 28 day feeding study in mice (Report No. CTL/P/354)
(Unpublished data submitted to WHO by ICI Central Toxicology
Laboratory).
CLARK, D.G. (1978) Toxicology of WL 43479: acute toxicity of WL43479,
Sittingbourne, Shell Research Ltd (Report No. TLGR. 0043.78)
(Unpublished data submitted to WHO).
CLIVE, C.D. (1977) Mutagenicity of BW21Z73 in L5178Y/TK mouse
lymphoma cells with and without exogenous metabolic activation, Re-
search Triangle Park, NC, Burroughs Wellcome Co. (Report No.
TTEP/77/0011) (Unpublished data submitted to WHO).
CLOUD, R.M., ZIMPFER, M.L., YANES, J.JR., BOETHEL, D.J., BUCO, P.,
& HARMON, C.W. (1987) Insecticide residues on clothing worn by crop
consultants in soybean fields treated with non-conventional application
technology. Bull. environ. Contam. Toxicol., 38: 277-282.
COATS, J.R. & O'DONNELL-JEFFERY, N.L. (1979) Toxicity of four
synthetic pyrethroid insecticides to rainbow trout. Bull. environ.
Contam. Toxicol., 23: 250-255.
COX, R.L. & WILSON, W.T. (1984) Effects of permethrin on the behavior
of individually tagged honey bees, Apis mellifera L. (Hymenoptera:
Apidae). Environ. Entomol., 13: 375-378.
CRIDLAND, J.S. & WEATHERLEY, B.C. (1977a) Urinary excretion in man of
3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropane carboxylic acid ("CVA")
after oral ingestion of permethrin (NRDC 143) - A first report, Bech-
enham, Wellcome Research Laboratories (Report No. BDPE-77-1)
(Unpublished data submitted to WHO).
CRIDLAND, J.S. & WEATHERLEY, B.C. (1977b) An estimate of permethrin
(NRDC 143; OMS1821) absorbed by people employed in a field trial of the
insecticide, Bechenham, Wellcome Research Laboratories (Report No.
BDPE-77-3) (Unpublished data submitted to WHO).
DAYAN, A.D. (1980) 21-day neuropathological study in the Sprague-
Dawley rat of permethrin (212732J) administered in the diet,
Berkhamsted, Wellcome Research Laboratories (Report No. BP 80-48)
(Unpublished report submitted to WHO by Wellcome Foundation Ltd).
DOYLE, R.D., KAUFMAN, D.D., BURT, G.W., & DOUGLASS, L. (1981)
Degradation of cis-permethrin in soil amended with sewage sludge or
dairy manure. J. agric. food Chem., 29: 412-414.
DYCK, P.J., SHIMONO, M., SCHOENING, G.P., LAIS, A.C., OVIATT, K.F.,
& SPARKS, M.F. (1984) The evaluation of a new synthetic pyrethroid
pesticide (permethrin) for neurotoxicity. J. environ. Pathol. Toxicol.
Oncol., 5: 109-117.
EDWARDS, D.B., OSBORN, B.E., DENT, N.J., & KINCH, D.A. (1976)
Toxicity study in beagle dogs (oral administration for three months),
Inveresk, United Kingdom, Inveresk Research International Ltd
(Submitted to WHO by ICI Ltd).
EDWARDS, M.J. & ISWARAN, T.J. (1977) Permethrin: residue transfer and
toxicology study with cows fed treated grass nuts (Report No.
TMJ1519/B) (Unpublished report submitted to WHO by ICI Plant Protection
Division).
ELLIOTT, M. (1977) Synthetic pyrethroids, Washington, DC, American
Chemical Society, p. 229 (ACS Symposium Series 42).
ELLIOTT, M., FARNHAM, A.W., JANES, N.F., NEEDHAM, P.H.,
PULMAN, D.A., & STEVENSON, J.H. (1973) A photostable pyrethroid.
Nature (Lond.), 246: 169-170.
ELLIOTT, M., JANES, N.F., PULMAN, D.A., GAUGHAN, L.C., UNAI, T.,
& CASIDA, J.E. (1976) Radiosynthesis and metabolism in rats of the 1R-
isomers of the insecticide permethrin. J. agric. food Chem., 24: 270-
276.
EVANS, M.H. (1976) End-plate potentials in frog muscle exposed to a
synthetic pyrethroid Pestic. Biochem. Physiol., 6: 547-550.
EVERTS, J.W., KORTENHOFF, B.A., HOOGLAND, H., VLUG, H.J.,
JOCQUE, R., & KOEMAN, J.H. (1985) Effects on non-target terrestrial
arthropods of synthetic pyrethroids used for the control of the tsetse
fly (Glossina spp.) in settlement areas of the southern Ivory Coast,
Africa. Arch. environ. Contam. Toxicol., 14: 641-650.
FAO (1982) Second Government Consultation on International
Harmonization of Pesticide Registration Requirements, Rome, 11-15
October 1982, Rome, Food and Agriculture Organization of the United
Nations.
FAO/WHO (1980a) Pesticide residues in food. Report of the 1979 Joint
Meeting of the FAO Panel of Experts on Pesticide Residues in Food and
the Environment and the WHO Expert Group on Pesticide Residues, Rome,
Food and Agriculture Organization of the United Nations, pp. 52-55 (FAO
Plant Production and Protection Paper 20).
FAO/WHO (1980b) 1979 Evaluations of some pesticide residues in food,
Rome, Food and Agriculture Organization of the United Nations,
pp. 369- 425 (FAO Plant Production and Protection Paper 20 Sup).
FAO/WHO (1981a) Pesticide residues in food. Report of the 1980 Joint
Meeting of the FAO Panel of Experts on Pesticide Residues in Food and
the Environment and the WHO Expert Group on Pesticide Residues, Rome,
Food and Agriculture Organization of the United Nations, pp. 50-51 (FAO
Plant Production and Protection Paper 26).
FAO/WHO (1981b) 1980 Evaluations of some pesticide residues in
food, Rome, Food and Agriculture Organization of the United Nations,
pp. 360-393 (FAO Plant Production and Protection Paper 26 Sup).
FAO/WHO (1982a) Pesticide residues in food. Report of the 1981
Joint Meeting of the FAO Panel of Experts on Pesticide Residues in Food
and the Environment and the WHO Expert Group on Pesticide Residues,
Rome, Food and Agriculture Organization of the United Nations, pp.
37-39 (FAO Plant Production and Protection Paper 37).
FAO/WHO (1982b) 1981 Evaluations of some pesticide residues in food,
Rome, Food and Agriculture Organization of the United Nations,
pp. 385-425 (FAO Plant Production and Protection Paper 42).
FAO/WHO (1983a) Pesticide residues in food. Report of the 1982 Joint
Meeting of the FAO Panel of Experts on Pesticide Residues in Food
and the Environment and the WHO Expert Group on Pesticide Residues,
Rome, Food and Agriculture Organization of the United Nations, pp. 36-
38 (FAO Plant Production and Protection Paper 46).
FAO/WHO (1983b) 1982 Evaluations of some pesticide residues in food,
Rome, Food and Agriculture Organization of the United Nations,
pp. 329-350 (FAO Plant Production and Protection Paper 49).
FAO/WHO (1984a) Pesticide residues in food. Report of the 1983
Joint Meeting of the FAO Panel of Experts on Pesticide Residues in Food
and the Environment and the WHO Expert Group on Pesticide Residues,
Rome, Food and Agriculture Organization of the United Nations, p. 38,
57, 62, 65 (FAO Plant Production and Protection Paper 56).
FAO/WHO (1984b) 1983 Evaluations of some pesticide residues in food,
Rome, Food and Agriculture Organization of the United Nations,
pp. 307-313, 501 (FAO Plant Production and Protection Paper 61).
FAO/WHO (1985a) Pesticide residues in food. Report of the 1984 Joint
Meeting of the FAO Panel of Experts on Pesticide Residues in Food and
the Environment and the WHO Expert Group on Pesticide Residues, Rome,
Food and Agriculture Organization of the United Nations, pp. 63, 85, 93
(FAO Plant Production and Protection Paper 62).
FAO/WHO (1985b) 1984 Evaluations of some pesticide residues in food,
Rome, Food and Agriculture Organization of the United Nations,
pp. 441-444, 723 (FAO Plant Production and Protection Paper 67).
FAO/WHO (1985c) Guide to Codex recommendations concerning pesticide
residues. Part 8. Recommendations for methods of analysis of pesticide
residues, 3rd ed., Rome, Codex Committee on Pesticide Residues.
FAO/WHO (1986a) Pesticide residues in food. Report of the 1985 Joint
Meeting of the FAO Panel of Experts on Pesticide Residues in Food and
the Environment and the WHO Expert Group on Pesticide Residues, Rome,
Food and Agriculture Organization of the United Nations, p. 37 (FAO
Plant Production and Protection Paper 68).
FAO/WHO (1986b) 1985 Evaluations of some pesticide residues in food,
Part I - Residues, Rome, Food and Agriculture Organization of the
United Nations, pp. 201-203 (FAO Plant Production and Protection Paper
No 72/1).
FAO/WHO (1986c) Codex Maximum Limits for pesticide residues, 2nd ed.,
Rome, Food and Agriculture Organization of the United Nations, Joint
FAO/WHO Food Standards Programme, Codex Alimentarius Commission,
(CAC XIII).
FAO/WHO (1987) Pesticide residues in food. Report of the 1987 Joint
Meeting of the FAO Panel of Experts on Pesticide residues in Food and
the Environment and the WHO Expert Group on Pesticide Residues, Rome,
Food and Agriculture Organization of the United Nations, p. 33 (FAO
Plant Production and Protection Paper 84).
FAO/WHO (1988) Permethrin. In: Pesticide residues in food. Part II -
Toxicology, Rome, Food and Agriculture Organization of the United
Nations, pp. 101-110 (FAO Plant Production and Protection Paper 86/2).
FARQUHAR, J.A., HUTCHINSON, D.B.A., PERIAM, A.W., & SPARKES,
R.G. (1981a) An investigation into the absorption of permethrin from
impregnated clothing, Bechenham, Wellcome Research Laboratories (Report
No. BKDL/81/1) (Unpublished data submitted to WHO).
FARQUHAR, J.A., HUTCHINSON, D.B.A., PERIAM, A.W., & SPARKES,
R.G. (1981b) An investigation of tolerance to absorption of and
persistence of permethrin applied as a 1% lotion to human hair,
Bechenham, Wellcome Research Laboratories (Report No. BKDL/81/2)
(Unpublished data submitted to WHO).
FLANNIGAN, S.A. & TUCKER, S.B. (1985) Variation in cutaneous
sensation between synthetic pyrethroid insecticides. Contact Derma-
titis, 13: 140-147.
FLANNIGAN, S.A., TUCKER, S.B., MARCUS, M.K., ROSS, C.E.,
FAIRCHILD, E.J., GRIMES, B.A., & HARRIST, R.B. (1985a) Primary
irritant contact dermatitis from synthetic pyrethroid insecticide
exposure. Arch. Toxicol., 56, 288-294.
FLANNIGAN, S.A., TUCKER, S.B., KEY, M.M., ROSS, C.E., FAIRCHILD,
E.J., GRIMES, B.A., & HARRIST, R.B. (1985b) Synthetic pyrethroid
insecticides: a dermatological evaluation. Br. J. ind. Med., 42: 363-
372.
FRIESEN, M.K., GALLOWAY, T.D., & FLANNAGAN, J.F. (1983) Toxicity
of the insecticide permethrin in water and sediment to nymphs of the
burrowing mayfly Hexagenia rigida (Ephemeroptera: Ephemeridae). Can.
Entomol., 115: 1007-1014.
GAMBRELL, R.P., REDDY, C.N., COLLARD, V., GREEN, G., & PATRICK,
W.H., Jr. (1981) Behavior of DDT, kepone and permethrin in sediment-
water systems under different oxidation-reduction and pH conditions,
Washington, DC, US Environmental Protection Agency (EPA 600/3-81-
038).
GAMMON, D.W. & CASIDA, J.E. (1983) Pyrethroids of the most potent
class antagonize GABA action at the crayfish neuromuscular junction.
Neurosci. Lett., 40: 163-168.
GAMMON, D.W., BROWN, M.A., & CASIDA, J.E. (1981) Two classes of
pyrethroid action in the cockroach. Pestic. Biochem. Physiol., 15:
181-191.
GAMMON, D.W., LAWRENCE, L.J., & CASIDA, J.E. (1982) Pyrethroid
toxicology: Protective effects of Diazepam and phenobarbital in the
mouse and the cockroach. Toxicol. appl. Pharmacol., 66: 290-296.
GAUGHAN, L.C. & CASIDA, J.E. (1978) Degradation of trans- and cis-
permethrin on cotton and bean plants. J. agric. food Chem., 26: 525-
528.
GAUGHAN, L.C., UNAI, T., & CASIDA, J.E. (1977) Permethrin metabolism
in rats. J. agric. food Chem., 25: 9-17.
GAUGHAN, L.C., ROBINSON, R.A., & CASIDA, J.E. (1978b) Distribution
and metabolic fate of trans- and cis-permethrin in laying hens. J.
agric. food Chem., 26: 1374-1380.
GAUGHAN, L.C., ACKERMAN, M.E., UNAI, T., & CASIDA, J.E. (1978a)
Distribution and metabolism of trans- and cis-permethrin in lactating
Jersey cows. J. agric. food Chem., 26: 613-618.
GERIG, L. (1985) Testing the toxicity of synthetic pyrethroid insecti-
cides to bees. Pestic. Sci., 16: 206-207.
GLAISTER, J.R., PRATT, I., & RICHARDS, D. (1977) Effects of high
dietary levels of PP557 on clinical behaviour and structure of sciatic
nerves in rats (Report No. CTL/P/317) (Unpublished data submitted to
WHO by ICI Central Toxicology Laboratory).
GLICKMAN, A.H. & CASIDA, J.E. (1982) Species and structural variations
affecting pyrethroid neurotoxicity. Neurobehav. Toxicol. Teratol., 4:
(6) 793-799.
GLICKMAN, A.H. & LECH, J.J. (1981) Hydrolysis of permethrin, a
pyrethroid insecticide, by rainbow trout and mouse tissues in vitro : A
comparative study. Toxicol. appl. Pharmacol., 60: 186-192.
GLICKMAN, A.H., SHONO, T., CASIDA, J.E., & LECH, J.J. (1979) In vitro
metabolism of permethrin isomers by carp and rainbow trout liver
microsomes. J. agric. food Chem., 27: 1038-1041.
GLICKMAN, A.H., HAMID, A.AR., RICKERT, D.E., & LECH, J.J. (1981)
Elimination and metabolism of permethrin isomers in rainbow trout.
Toxicol. appl. Pharmacol., 57: 88-98.
GLICKMAN, A.H., WEITMAN, S.D., & LECH, J.J. (1982) Different toxicity
of trans-permethrin in rainbow trout and mice. I. Role of biotransfor-
mation. Toxicol. appl. Pharmacol., 66: 153-161.
HAGLEY, E.A.C., PREE, D.J., & HOLLIDAY, N.J. (1980) Toxicity of
insecticides to some orchard carabids (Coleoptera carabidae). Can. Entomol.,
112: 457-462.
HALLS, G.R.H. (1981) The fate of permethrin residues on wheat during 9
months storage in Australia (Report No. HEFH 81-1) (Unpublished data
submitted to WHO by Wellcome Research Laboratories).
HALLS, G.R.H. & PERIAM, A.W. (1980) The fate of permethrin residues on
wheat during storage and after milling and baking - results after 9, 12
and 15 months storage (Report HEFH 80-3) (Unpublished data submitted to
WHO by Wellcome Research Laboratories).
HANSEN, D.J., GOODMAN, L.R., MOORE, J.C., & HIGDON, P.K. (1983)
Effects of the synthetic pyrethroids AC 222, 705, permethrin and
fenvalerate on sheepshead minnows in early life stage of toxicity
tests. Environ. Toxicol. Chem., 2: 251-258.
HART, D., BANHAM, P.B., GORE, C.W., PRATT, I., & WEIGHT, T.M.
(1977c) PP557: Liver hypertrophy study in rats-dietary administration
over 26 weeks (Report No. CTL/P/360) (Unpublished data submitted to WHO
by ICI Central Toxicology Laboratory).
HELSON, B.V., KINGSBURY, P.D., & DE GROOT, P. (1986) The use of
bioassays to assess aquatic arthropod mortality from permethrin drift
deposits. Aquat. Toxicol., 9: 253-262.
HEND, R.W. & BUTTERWORTH, S.T.G. (1977) Toxicity of insecticides: a
short-term feeding study of WL 43379 in rats (Report No. TLGR.0108.77)
(Unpublished data submitted to WHO by Shell Research Ltd).
HENRIET, J., MARTIJN, A., & POLVISEN, H.H. (1985) CIPAC Handbook.
Volume 1C: Analysis of technical and formulated pesticides, Hertford-
shire, Collaborative International Pesticides Analytical Council Ltd,
pp. 2172-2173.
HILL, E.F. & CAMARDESE, M.B. (1986) Lethal dietary toxicities of
environmental contaminants and pesticides to Coturnix, Washington, DC,
United States Department of the Interior, Fish and Wildlife Service,
p.111 (Fish and Wildlife Technical Report No. 2).
HODGE, M.C.E., BANHAM, P.B., GLAISTER, J.R., RICHARDS, D.,
TAYLOR, K., & WEIGHT, T.M. (1977) PP557: Three generation reproduction
study in rats (Unpublished report submitted to WHO by ICI Central
Toxicology Laboratory).
HOGAN, G.K. & RINEHART, W.E. (1977) A twenty-four month oral
carcinogenicity study of FMC 33297 in mice (Bio-Dynamics Inc. Project)
(Unpublished report submitted to WHO by FMC Corporation).
HOLDWAY, D.A. & DIXON, D.G. (1988) Acute toxicity of permethrin or
glyphosate pulse exposure to larval white sucker (Catostomus commer-
soni) and juvenile flagfish (Jordanella floridae) as modified by age
and ration level. Environ. Toxicol. Chem., 7: 63-68.
HOLMSTEAD, R.L., CASIDA, J.E., RUZO, L.O., & FULLER, D.G. (1978)
Pyrethroid photodecomposition: Permethrin. J. agric. food Chem., 26:
590-595.
HORIBA, M., KOBAYASHI, A., & MURANO, A. (1977) Gas-liquid
chromatographic determination of a new pyrethroid, permethrin (S-3151)
and its optical isomers. Agric. biol. Chem., 41: 581-586.
HOY, M.A., FLAHERTY, D., PEACOCK, W., & CULVER, D. (1979)
Vineyard and laboratory evaluations of methomyl, dimethoate, and
permethrin for a grape pest management program in the San Joaquin
Valley of California. J. econ. Entomol., 72: 250-255.
HOYT, S.C., WESTIGARD, P.H., & BURTS, E.C. (1978) Effects of two
synthetic pyrethroids on the codling moth, pear psylla, and various
mite species in northwest apple and pear orchards. J. econ. Entomol.,
71: 431-434.
HUNT, L.M. & GILBERT, B.N. (1977) Distribution and excretion rates of
14C-labelled permethrin isomers administered orally to four lactating
goats for 10 days. J. agric. food Chem., 25: 673-676.
HUNT, L.M., GILBERT, B.N., & LEMEILLEUR, C.A. (1979) Distri-
bution and depletion of radioactivity in hens treated dermally with
14C-labelled permethrin. Poult. Sci., 58: 1197-1201.
ISHMAEL, J. & LITCHFIELD, M.H. (1988) Chronic toxicity and
carcinogenic evaluation of permethrin in rats and mice. Fundam. appl.
Toxicol., 11: 308-311.
IVIE, G.W. & HUNT, L. (1980) Metabolites of cis- and trans-permethrin
in lactating goats. J. agric. food Chem., 28: 1131-1138.
JAGGERS, S.E. & PARKINSON, G.R. (1979) Permethrin: summary and review
of acute toxicities in laboratory species (Report No. CTL/P/461)
(Unpublished data submitted to WHO by ICI Central Toxicology
Laboratory).
JAMES, J.A. (1974) Fetal toxicity study of 21Z73 (NRDC 143) in the
rat (Report No. BPAT 74-10) Bechenham, Wellcome Research Laboratories
(Unpublished data submitted to WHO).
JAMES, J.A., (1979) A multigeneration reproduction study of 21Z73
(permethrin) in the rat (Report No. BPAT 79-3) Bechenham, Wellcome
Research Laboratories (Unpublished data submitted to WHO).
JAMES, J.A., TAYLOR, P.E., & ROE, F.J.C. (1980) Carcinogenicity study
in mice with permethrin, Bechenham, Wellcome Research Laboratories,
(Report No. HEFG 80-29) (Unpublished data submitted to WHO).
JOLLY, A.L., Jr., AVAULT, J.W., Jr., KOONCE, K.L., & GRAVES, J.B.
(1978) Acute toxicity of permethrin to several aquatic animals. Trans.
Am. Fish. Soc., 107: 825-827.
JORDAN, E.G. & KAUFMAN, D.D. (1986) Degradation of cis- and trans-
permethrin in flooded soil. J. agric. food Chem., 34: 880-884.
JORDAN, E.G., KAUFMAN, D.D., & KAYSER, A.J. (1982) The effect of soil
temperature on the degradation of cis-, trans-permethrin in soil. J.
environ. Sci. Health, B 17: 1-17.
KADOTA, T., MIYAMOTO, J., & ITO, N. (1975) Six-month subacute oral
toxicity of NRDC in Sprague-Dawley rats (Unpublished data submitted to
WHO by Sumitomo Chemical Co.).
KANEKO, H., OHKAWA, H., & MIYAMOTO, J. (1978) Degradation and
movement of permethrin isomers in soil. J. Pestic. Sci., 3: 43-51.
KAUFMAN, D.D., HAYES, S.C., JORDAN, E.G., & KAYSER, A.J. (1977)
Permethrin degradation in soil and microbial cultures. In: Elliott, M.,
ed. Synthetic pyrethroids, Washington, DC, American Chemical Society,
pp. 147-161 (ACS symposium series 42).
KAUFMAN, D.D., RUSSEL, B.A., HELLING, C.S., & KAYSER, A.J. (1981)
Movement of cypermethrin, deltamethrin, permethrin and their
degradation products in soil. J. agric. food Chem., 29: 239-245.
KAUSHIK, N.K., STEPHENSON, G.L., SOLOMON, K.R., & DAY, K.E.
(1985) Impact of permethrin on zooplankton communities in
limnocorrals. Can. J. Fish. aquat. Sci., 42: 77-85.
KILLEEN, J.C. & RAPP, W.R. (1976a) A three month oral toxicity study
of FMC 33297 in Beagle dogs (Bio-Dynamics Inc. Project) (Unpublished
report submitted to WHO by FMC Corporation).
KILLEEN, J.C. & RAPP, W.R. (1976b) A three month oral toxicity study
of FMC 33297 in rats (Bio-Dynamics Inc. Project) (Unpublished report
submitted to WHO by FMC Corporation).
KINGSBURY, P.D. (1976) Effects of an aerial application of the syn-
thetic pyrethroid permethrin on a forest stream. Manitoba Entomol., 10:
9-17.
KINGSBURY, P.D. & KREUTZWEISER, D.P. (1980a) Environmental impact
assessment of a semi-operational permethrin application. Sault Ste.
Marie, Ontario, Canada, Forest Pest Management Institute. Report No.
FPM-X 30: pp.47.
KINGSBURY, P.D. & KREUTZWEISER, D.P. (1980b) Dosage-effect studies
on the impact of permethrin on trout streams. Sault Ste. Marie,
Ontario, Canada, Forest Pest Management Institute Report No. FPM-X 31.
KINGSBURY, P.D. & KREUTZWEISER, D.P. (1987) Permethrin treatments in
Canadian forests. Part 1: Impact on fish. Pestic. Sci., 19: 35-48.
KOHDA, H., KADOTA, T., & MIYAMOTO, J. (1976a) Teratogenic evaluation
with permethrin in rats (Unpublished report submitted to WHO by
Sumitomo Chemical Co.).
KOHDA, H., KADOTA, T., & MIYAMOTO, J. (1976b) Teratogenic evaluation
with permethrin in mice (Unpublished report submitted to WHO by
Sumitomo Chemical Co.).
KOHDA, H., KADOTA, T., & MIYAMOTO, J. (1979a) Acute oral, dermal and
subcutaneous toxicities of permethrin in rats and mice (Unpblished
report submitted to WHO by Sumitomo Chemical Co.).
KOHDA, H., KANEDO, H., OHKAWA, H., KADOTA, T., & MIYAMOTO, J.
(1979b) Acute intraperitoneal toxicity of permethrin metabolites in
mice (Unpublished report submitted to WHO by Sumitomo Chemical Co.).
KOLMODIN-HEDMAN, B., SWENSSON, A., & AKERBLOM, M. (1982)
Occupational exposure to some synthetic pyrethroids (permethrin and
fenvalerate). Arch. Toxicol., 50: 27-33.
KREUTZWEISER, D.P. (1982) The effects of permethrin on the invertebrate
fauna of a Quebec forest. Sault Ste Marie, Ontario, Canada, Forest Pest
Management Institute, Report No. FPM-X 50: pp.44.
KREUTZWEISER, D.P. & KINGSBURY, P.D. (1987) Permethrin treatments
in Canadian forests. Part 2: Impact on stream invertebrates. Pestic.
Sci., 19: 49-60.
KUMARAGURU, A.K. & BEAMISH, F.W.H. (1981) Lethal toxicity of
permethrin (NRDC-143) to rainbow trout, Salmo gairdneri, in relation to
body weight and water temperature. Water Res., 15: 503-505.
KUMARAGURU, A.K., BEAMISH, F.W.H., & FERGUSON, H.W. (1982)
Direct and circulatory paths of permethrin (NRDC-143) causing
histopathological changes in the gills of rainbow trout, Salmo
gairdneri Richardson. J. Fish Biol., 20: 87-91.
LAWRENCE, L.J. & CASIDA, J.E. (1982) Pyrethroid toxicology: Mouse
intracerebral structure-toxicity relationship. Pestic. Biochem. Physiol.,
18: 9-14.
LAWRENCE, L.J. & CASIDA, J.E. (1983) Stereospecific action of
pyrethroid insecticides on the gamma-aminobutyric acid receptor-
ionophore complex. Science, 221: 1399-1401.
LAWRENCE, L.J., GEE, K.W., & YAMAMURA, H.I. (1985) Interactions of
pyrethroid insecticides with chloride ionophore-associated binding
sites. Neurotoxicology, 6: 87-98.
LEAHEY, J.P. (1985) The pyrethroid insecticides, London, Taylor &
Francis Ltd, p. 440.
LEAHEY, J.P. & CARPENTER, P.K. (1980) The uptake of metabolites
of permethrin by plants grown in soil treated with [14C]permethrin.
Pestic. Sci., 11: 279-289.
LEAHEY, J.P., BEWICK, D.W., & SAUNDERS, R. (1977) Accumulation and
depletion of radioactive residues in the tissues of Mallard duck and
Japanese quail following daily dosing with 14 C-permethrin (Unpublished
report submitted to WHO by ICI Plant Protection Division).
LE QUESNE, P.N., MAXWELL, I.C., & BUTTERWORTH, T.G. (1980)
Transient facial sensory symptoms following exposure to synthetic
pyrethroids: a clinical and electorophysiological assessment. Neurotoxi-
cology, 2: 1-11.
LINDEN, E., BENGTSSON, B.-E., SVANBERG, O., & SUNDSTROM, G.
(1979) The acute toxicity of 78 chemicals and pesticide formulations
against two brackish water organisms, the Bleak (Alburnus alburnus) and
the Nitocra spinipes. Chemosphere, 11/12: 843-851.
LINDQUIST, R.K., KRUEGER, H.R., & POWELL, C.C., Jr. (1987) Airborne
and surface residues of permethrin after high- and low-volume
applications in greenhouses. J. environ. Sci. Health, B22: 15-27.
LITCHFIELD, M.H. (1983) Characterisation of the principal mammalian
toxicological and biological actions of synthetic pyrethroids. In:
Miyamoto, J. & Kearney, P.C., ed. Pesticide chemistry: human welfare
and the environment. II. Natural products, Oxford, Pergamon Press, pp.
207-211.
LONGSTAFF, E. (1976) Permethrin: short-term predictive tests for
carcinogenicity: results from the Ames test (Report No. CTL/P/301)
(Unpublished data submitted to WHO by ICI Central Toxicology
Laboratory).
LORD, K.A., MCKINLEY, M., & WALKER, N. (1982) Degradation of
permethrin in soils. Environ. Pollut. A29: 81-90.
LUND, A.E. & NARAHASHI, T. (1983) Kinetics of sodium channel
modification as the basis for the variation in the nerve membrane
effects of pyrethroids and DDT analogs. Pestic. Biochem. Physiol., 20:
203-216.
MCGREGOR, D.B. & WICKRAMARATNE, G.A., de S. (1976a) Dominant
lethal study in mice of ICI-PP557 (Report No. 623, Inveresk Research
International Project No. 406722) (Unpublished data submitted to WHO by
ICI Ltd).
MCGREGOR, D.B. & WICKRAMARATNE, G.A., de S. (1976b) Terato-
genicity study in rats of ICI-PP557 (Inveresk Research International
Project No. 404898) (Unpublished data submitted to WHO by ICI Ltd.).
MCLEESE, D.W., METCALFE, C.D., & ZITKO, V. (1980) Lethality of
permethrin, cypermethrin and fenvalerate to salmon, lobster and shrimp.
Bull. environ. Contam. Toxicol., 25: 950-955.
MACPHEE, A., GAUL, S., & RAGAB, M.T.H. (1982) Control of blueberry
thrips, Frankliniella vaccinii Morga, with permethrin and effect on
yield and residue in fruit. J. environ. Sci. Health, B17: 183-193.
MCSHEEHY, T.W. & FINN, J.P. (1980) 21Z: Potential toxicity and
oncogenicity in dietary administration to rats for a period of 104
weeks (Report No. HEFG 80-33) (Unpublished data of Wellcome Research
Laboratories).
MAREI, A.E.M., RUZO, L.O., & CASIDA, J.E. (1982) Analysis and
persistence of permethrin, cypermethrin, deltamethrin and fenvalerate
in the fat and brains of treated rats. J. agric. food Chem., 30: 558-
562.
MATHUR, S.P., BELANGER, A., HAMILTON, H.A., & KHAN, S.U. (1980)
Influence on microflora and persistence of field-applied disulfoton,
permethrin and prometryne in an organic soil. Pedobiologia, 20: 237-
242.
MAYER, F.L., Jr. (1987) Acute toxicity handbook of chemicals to
estuarine organisms, Springfield, Virginia, National Technical
Information Service, p. 158 EPA/600/8-87/017.
MAYER, F.L., Jr. & ELLERSIECK, M.R. (1986) Manual of acute toxicity:
Interpretation and data base for 410 chemicals and 66 species of
freshwater animals, Washington, DC, United States Department of the
Interior, Fish and Wildlife Service, pp.377-378.
MEISTER, R.T., BERG, G.L., SINE, C., MEISTER, S., & POPLYK, J. (1983)
Farm chemicals handbook. Section C. Pesticide dictionary, Wilboughby,
Ohio, Meister Publishing Co., pp. C180-C181.
METKER, L.W. (1978) Subchronic inhalation toxicity of 3-(phenoxy-
phenyl)methyl(+)-cis,trans-3-(2,2-dichloroethenyl)-2,2-dimethylcyclopro-
panecarboxylate (permethrin), Aberdeen Proving Ground, Maryland, US
Army Environmental Hygiene Agency (Report No. 75-51-0026-80).
METKER, L.W., ANGERHOFER, R.A., POPE, C.R., & SWENTZEL, K.C.
(1977) Toxicology evaluation of 3-(phenoxyphenyl)methyl(+)-cis,trans-3-
(2,2-dichloroethenyl)-2,2-dimethylcyclopropanecarboxylate (permethrin),
Environmental Hygiene Agency (Report No. 75-51-0837-78).
MILLNER, C.K. & BUTTERWORTH, S.T.G. (1977) Toxicity of pyrethroid
insecticides: investigation of the neurotoxic potential of WL 43479 to
adult hens, (Report No. TLGR.0069.77) (Unpublished data submitted to
WHO by Shell Research Ltd).
MIYAMOTO, J. (1976) Degradation, metabolism and toxicity of synthetic
pyrethroids. Environ. Health Perspect., 14: 15-28.
MIYAMOTO, J. (1981) The chemistry, metabolism and residue analysis of
synthetic pyrethroids, Pure appl. Chem., 53: 1967-2022.
MIYAMOTO, J. & KEARNEY, P.C. (1983) Pesticide chemistry-human welfare
and the environment. Proceedings of the Fifth International Congress of
Pesticide Chemistry, Kyoto, Japan, 29 August-4 September 1982, Oxford,
Pergamon Press, Vol. 1-4.
MULLA, M.S., DARWAZEH, H.A., & MAJORI, G. (1975) Field efficacy of
some promising mosquito larvicides and their effect on nontarget
organisms. Mosq. News, 35: 179-185.
MULLA, M.S., NAVVAB-GOJRATI, H.A., & DARWAZEH, H.A. (1978a)
Toxicity of mosquito larvicidal pyrethroids to four species of
freshwater fishes. Environ. Entomol., 7: 428-430.
MULLA, M.S., NAVVAB-GOJRATI, H.A., & DARWAZEH, H.A. (l978b)
Biological activity and longevity of new synthetic pyrethroids against
mosquitoes and some nontarget insects. Mosq. News, 38: 90-96.
MULLA, M.S., DARWAZEH, H.A., & DHILLON, K.S. (1981) Impact and
joint action of decamethrin and permethrin and freshwater fishes on
mosquitoes. Bull. environ. Contam. Toxicol., 26: 689-695.
NASSIF, M., BROOKE, J.P., HUTCHINSON, D.B.A., & KAMEL, O.M. (1980)
Studies with permethrin against body lice in Egypt. Pestic. Sci., 11:
679-684.
NEWELL, G.W. & SKINNER W.A. (1976) In vitro microbiological
mutagenicity study of an FMC corporation compound (Unpublished report
submitted to WHO by FMC Corporation).
NOMURA, Y. & SEGAWA, T. (1979) Pharmacological study of permethrin:
effects of isolated ileum, nictitating membrane, respiration, blood
pressure and electrocardiography (Unpublished report submitted to WHO
by Sumitomo Chemical Co.).
OHKAWA, H., KANEKO, H., & MIYAMOTO, J. (1977) Metabolism of
permethrin in bean plants. J. Pestic. Sci., 2: 67-76.
OHSHIMA, M., TAKIMOTO, Y., & MATSUDA, T. (1988) Accumulation and
metabolism of 14 C-permethrin in carp (Cyprinus carpio) (Unpublished
data submitted to WHO by Sumitomo Chemical Co.).
OKUNO, Y., MIYAGAWA, H., HOSOI, R., & KADOTA, T. (1976) Skin and
eye irritation study of permethrin (S-3151) in rabbits (Unpublished
report submitted to WHO by Sumitomo Chemical Co.).
PARKINSON, G.R. (1978) Permethrin: acute toxicity to male rats (Report
No. CTL/P/388) (Unpublished data submitted to WHO by ICI Central
Toxicology Laboratory).
PARKINSON, G.R., BERRY, P.N., GLAISTER, J., GORE, C.W., LEFEVRE,
V.K., & MURPHY, J.A. (1976) PP557 (Permethrin): acute and sub-acute
toxicity, (Report No. CTL/P/225) (Unpublished data submitted to WHO by
ICI Central Toxicology Laboratory).
PAYNTER, O.C., BUDD, E.R., & LITT, B.D. (1982) Permethrin. Assessment
of chronic and oncogenic effects: A summary, Washington, DC, Toxicology
Branch, Hazard Evaluation Division, Office of Pesticide Programs, US
Environmental Protection Agency (Unpublished data submitted to WHO).
PEGUM, J.S. & DOUGHTY, B.J. (1978) Human skin patch test with
pyrethrins, kadethrin, permethrin and decamethrin, Berkhamsted Hill,
Wellcome Group Research and Development (Report No. NEFJ 78-2)
(Unpublished data submitted to WHO).
PIERCY, D.W.T., REYNOLDS, J., & JAMES, J.A. (1976) Effects of diet on
the acute toxicity of permethrin in the female rat, Berkhamsted Hill,
Wellcome Research and Development (Report no. 77-11) (Unpublished data
submitted to WHO).
PIKE, K.S., MAYER, D.F., GLAZER, M., & KIOUS, C. (1982) Effects of
permethrin on mortality and foraging behavior of honey bees in sweet
corn. Environ. Entomol., 11: 951-953.
PLAPP, F.W., Jr & BULL, D.L. (1978) Toxicity and selectivity of some
insecticides to Chrysopa carnea, a predator of the tobacco budworm.
Environ. Entomol., 7: 431-434.
PLAPP, F.W., Jr & VINSON, S.B. (1977) Comparative toxicities of some
insecticides to the tobacco budworm and its ichneumonid parasite,
Campoletis sonorensis. Environ. Entomol., 6: 381-384.
PLUIJMEN, M., DREVON, C., MONTESANO, R., MALAVEILLE, C.,
HAUTEFEUILLE, A., & BARTSCH, H. (1984) Lack of mutagenicity of
synthetic pyrethroids in Salmonella typhimurium strains in V79 Chinese
hamster cells. Mutat. Res., 137: 7-15.
RACEY, P.A. & SWIFT, S.M. (1986) The residual effect of remedial
timber treatments on bats. Biol. Conserv., 35: 205-214.
RAPP, W.R. (1978) Twenty-four month oral toxicity/oncogenicity study
of FMC33297 in mice. Histopathology report (Unpublished data of FMC
Corporation).
REYNOLDS, J., PIERCY, D.W.T., CLAMPITT, R.B., JAMES, J.A.,
THOMPSON, P.M., FAREBROTHER, D.A., & DAYAN, A.D. (1978)
Permethrin oral administration to dogs for 6 months, Berkhamsted Hill,
Wellcome Research Laboratories (Report No. HEFG 78-14) (Unpublished
data submitted to WHO).
RICHARDS, D., BANHAM, P.B., KILMARTIN, M., & WEIGHT, T.M. (1980)
Permethrin: Teratogenicity study in the rabbit (Report No. CTL/P/523)
(Unpublished report submitted to WHO by ICI Ltd).
RISHIKESH, N., CLARKE, J.L., MATHIN, H.L., KING, J.S., & PEARSON, J.
(1978) Evaluation of decamethrins and permethrin against Anopheles
gambiae and Anopheles funestus in a village trial in Nigeria
(WHO/VBC/78.679) (Unpublished report of Division of Vector Biology
Control, World Health Organization).
ROBERTS, T.R. & WRIGHT, A.N. (1981) The metabolism of 3-phenoxybenzyl
alcohol, a pyrethroid metabolite, in plants. Pestic. Sci., 12: 161-
169.
ROCK, G.C. (1979) Relative toxicity of two synthetic pyrethroids to a
predator Amblyseius fallacis and its prey Tetranychus urticae. J. econ.
Entomol., 72: 293-294.
ROSS, D.B. & PRENTICE, D.E. (1977) Examination of permethrin (PP557)
for neurotoxicity in the domestic hen, Huntingdon, Huntingdon Research
Centre (Report No. ICI/157-NT/77468) (Unpublished data submitted to WHO
by FMC Corporation and ICI Ltd).
ROSS, D.B., CAMERON, D.M., & ROBERTS, N.L. (1976a) The acute oral
toxicity (LD50 ) of PP557 (permethrin) to Mallard ducks, Huntingdon,
Huntingdon Research Centre (Report No. ICI 68/WL/7639) (Unpublished
data submitted to WHO by ICI Ltd).
ROSS, D.B., CAMERON, D.M., & ROBERTS, N.L. (1976b) The sub-acute
toxicity (LC50 ) of PP557 (permethrin) to Mallard ducks, Huntingdon,
Huntingdon Research Centre (Report No. ICI 68/WL/75837) (Unpublished
data submitted to WHO by ICI Ltd).
ROSS, D.B., CAMERON, D.M., & ROBERTS, N.L. (1976c) The acute oral
toxicity (LD50 ) of PP557 (permethrin) to Starling, Huntingdon,
Huntingdon Research Centre (Report No. ICI 68/WL/7637) (Unpublished
data submitted to WHO by ICI Ltd).
ROSS, D.B., CAMERON, D.M., & ROBERTS, N.L. (1976d) The sub-acute
toxicity (LC50 ) of PP557 (permethrin) to Starling, Huntingdon,
Huntingdon Research Centre (Report No. ICI 68/WL/7636) (Unpublished
data submitted to WHO by ICI Ltd).
ROSS, D.B., CAMERON, D.M., & ROBERTS, N.L. (1976e) The sub-acute
toxicity (LC50 ) of PP557 (permethrin) to Ring-necked pheasant,
Huntingdon, Huntingdon Research Centre (Report No. ICI 68/WL/75839)
(Unpublished data submitted to WHO by ICI Ltd).
ROSS, D.B., CAMERON, D.M., & ROBERTS, N.L. (1977a) The acute oral
toxicity (LD50 ) of PP557 (permethrin) to Ring-necked pheasant,
Huntingdon, Huntingdon Research Centre (Report No. ICI 68/WL/77154)
(Unpublished data submitted to WHO by ICI Ltd).
ROSS, D.B., PRENTICE, D.E., MAJEED, S.K., GIBSON, W.A., CAMERON,
D.M., CAMERON, M. McD., & ROBERTS, N.L. (1977b) The incorporation of
permethrin in the diet of laying hens (part I), Huntingdon, Huntingdon
Research Centre (Report No. ICI 152/77387) (Unpublished data submitted
to WHO by ICI Ltd).
ROUSH, R.T. & HOY, M.A. (1978) Relative toxicity of permethrin to a
predator, Metaseiulus occidentalis, and its prey, Tetranychus urticae.
Environ. Entomol., 7: 287-288.
RUIGT, G.S.F. & VAN DEN BERCKEN, J. (1986) Action of pyrethroids on a
nerve-muscle preparation of the clawed frog, Xenopus laevis. Pestic.
Biochem. Physiol., 25: 176-187.
SASINOVICH, L.M. & PANSHINA, T.N. (1987) [Substantiation of hygienic
rules for synthetic pyrethroid content in the work zone air]. Gig. Tr.
prof. Zabol., 8: 48-50 (in Russian).
SCHROEDER, R.E. & RINEHART, W.E. (1977) A three generation
reproduction study of FMC33297 in rats (Bio-Dynamics Inc Project)
(Unpublished report submitted to WHO by FMC Corporation).
SHAH, P.V., MONROE, R.J., & GUTHRIE, F.E. (1981) Comparative rates of
dermal penetration of insecticides in mice. Toxicol. appl. Pharmacol.,
59: 414-423.
SHAROM, M.S. & SOLOMON, K.R. (1981) Adsorption-desorption,
degradation, and distribution of permethrin in aqueous systems. J.
agric. food Chem., 29: 1122-1125.
SHERMAN, R.A. (1979) Preliminary behavioural assessment of habituation
to the insecticide permethrin, Aberdeen Proving Ground, Maryland, US
Army Environmental Hygiene Agency (Report No. 75-51-002679).
SHIRASU, Y., MORIYA, M., & OTA, T. (1979) Mutagenicity of S-3151 in
bacterial test systems (Unpublished report submitted to WHO by Sumitomo
Chemical Co.).
SHONO, T., OHSAWA, K., & CASIDA, J.E. (1979) Metabolism of trans- and
cis-permethrin, trans- and cis-cypermethrin, and decamethrin by
microsomal enzymes. J. agric. food Chem., 27: 316-325.
SHOUR, M.H. & CROWDER, L.A. (1980) Effects of pyrethroid insecticides
on the common green lacewing. J. econ. Entomol., 73: 306-309.
SIEGEL, M.M., HILDEBRAND, B.E., & HALL, D.R. (1980) Determination of
permethrin in environmental waters by gas chromatography-selected ion
monitoring-mass spectroscopy using ion counting detection. Int. J.
environ. anal. Chem., 8: 107-126.
SIMMON, V.F. (1976) In vitro microbiological mutagenicity study of an
FMC corporation compound, Menlo Park, Stanford Research Institute,
(Report No. LSC-4768) (Unpublished report submitted to WHO).
SIMONAITIS, R.A. & CAIL, R.S. (1977) Gas chromatographic determination
of residues of the synthetic pyrethroid FMC33297. In: Elliott, M., ed.
Synthetic pyrethroids, Washington, DC, American Chemical Society,
pp. 211-223 (ACS Symposium Series U2).
SODERLUND, D.M. & CASIDA, J.E. (1977) Effects of pyrethroid structure
on rate of hydrolysis and oxidation by mouse liver microsomal enzymes.
Pestic. Biochem. Physiol., 7: 391-401.
SPENCER, F. & BERHANCE, Z. (1982) Uterine and fetal characteristics in
rats following a post-implantational exposure to permethrin. Bull.
environ. Contam. Toxicol., 29: 84-88.
STAATZ, C.G., BLOOM, A.S., & LECH, J.J. (1982) A pharmacological
study of pyrethroid neurotoxicity in mice. Pestic. Biochem. Physiol.,
17: 287-292.
STEVENSON, J.H., NEEDHAM, P.H., & WALKER, J. (1978) Poisoning of
honeybee by pesticides: Investigation of the changing pattern in
Britain over 20 years. Rothamsted Experimental Station Report (1977),
Part 2: 55-72.
STRATTON, G.W. & CORKE, C.T. (1981) Interaction of permethrin with
Daphnia magna in the presence and absence of particulate material.
Environ. Pollut., A24: 135-144.
STRATTON, G.W. & CORKE, C.T. (1982) Toxicity of the insecticide
permethrin and some degradation products towards algae and
cyanobacteria. Environ. Pollut. A29: 71-80.
SUNDARAM, K.M.S., DE GROOT, P., & SUNDARAM, A. (1987)
Permethrin deposits and airborne concentrations downwind from a single
swath application using a back pack mist blower. J. environ. Sci.
Health, B22: 171-193.
SUZUKI, H. (1977) Studies on the mutagenicity of some pyrethroids on
salmonella strains in the presence of mouse hepatic S9 fractions
(Unpublished report submitted to WHO by Sumitomo Chemical Co.).
SWAINE, H. & SAPIETS, A. (1981a) Cypermethrin: residue transfer study
with dairy cows fed on a diet containing the insecticide (Report No.
RJ0186B) (Unpublished data submitted to WHO by ICI Ltd).
SWAINE, H. & SAPIETS, A. (1981b) Cypermethrin: residue levels of the
major metabolites of the insecticide in the milk and tissues of dairy
cows fed on a diet containing cypermethrin at 50 mg/kg (Report No.
RJ0198B) (Unpublished data submitted to WHO by ICI Ltd).
SWAINE, H., EDWARD, M.J., & USSARY, J.P. (1978) Permethrin crop
protection study (Report No. TMV 0378B) (Unpublished data submitted to
WHO by ICI Ltd).
TAKAHASHI, K., OKUDA, No., & SHIRASU, Y. (1979) Effects of
permethrin on hexobarbital induced sleeping time in mice and electro-
encephalography in rabbits (Unpublished report submitted to WHO by
Sumitomo Chemical Co.).
TYLER, J.F.C. (1987) Gas chromatographic method for determination of
permethrin in pesticide formulations: Collaborative study. J. Assoc.
Off. Anal. Chem., 70(1): 53-55.
US EPA (1981) Advisary opinion on the oncogenic potential of
permethrin, Washington, DC, US Environmental Protection Agency
(Unpublished report submitted to WHO).
USSARY, J.P. (1979) Permethrin residues on sweet corn (Report No.
TMU0438B) (Unpublished data submitted to WHO by ICI Americas Inc.).
USSARY, J.P. (1978) Permethrin residues on sweet corn (Report No.
TMU0413B) (Unpublished data submitted to WHO by ICI Americas Inc.).
USSARY, J.P. & BRAITHWAITE, G.B. (1980) Residues of permethrin and 3-
phenoxy-benzyl alcohol in cow tissues (Trial No. 35NC79-001. Report
No. TMU0493B) (Unpublished data submitted to WHO by ICI Americas
Inc.).
VAN DEN BERCKEN, J. (1977) The action of allethrin on the peripheral
nervous system of the frog. Pestic. Sci., 8: 692-699.
VAN DEN BERCKEN, J. & VIJVERBERG, H.P.M. (1980a) Voltage clamp
studies on the effects of allethrin and DDT on the sodium channels in
frog myelinated nerve membrane. In: Insect neurobiology and pesticide
action, London, Society of Chemical Industry, pp. 79-85.
VAN DEN BERCKEN, J. & VIJVERBERG, H.P.M. (1980b) Effects of
insecticides on the sensory system of Xenopus. In: Insect neurobiology
and pesticide action, London, Society of Chemical Industry, pp.391-
397.
VAN DEN BERCKEN, J., AKKERMANS, L.M.A., & VAN DER ZALM,
J.M., (1973) DDT-like action of allethrin in the sensory nervous
system of Xenopus laevis. Eur. J. Pharmacol., 21: 95-106.
VAN DEN BERCKEN, J., KROESE, A.B.A., & AKKERMANS, L.M.A.
(1979) Effect of insecticides on the sensory nervous system. In:
Narahashi, T., ed. Neurotoxicology of insecticides and pheromones,
New York, London, Plenum Press, pp. 183-210.
VERSCHOYLE, R.D. & ALDRIDGE, W.N. (1980) Structure-activity
relationship of some pyrethrins to rats. Arch. Toxicol., 45: 325-329.
VIJVERBERG, H.P.M. & VAN DEN BERCKEN, J. (1979) Frequency-
dependent effects of the pyrethroid insecticide decamethrin in frog
myelinated nerve fibres. Eur. J. Phamacol., 58: 501-504.
VIJVERBERG, H.P.M. & VAN DEN BERCKEN, J. (1982) Action of
pyrethroid insecticides on the vertebrate nervous system. Neuropathol.
appl. Neurobiol., 8: 421-440.
VIJVERBERG, H.P.M., RUIGT, G.S.F., & VAN DEN BERCKEN, J. (1982a)
Structure-related effects of pyrethroid insecticides on the lateral-
line sense organ and on peripheral nerves of the clawed frog, Xenopus
laevis. Pestic. Biochem. Physiol., 18: 315-324.
VIJVERBERG, H.P.M., VAN DER ZALM, J.M., & VAN DEN BERCKEN, J.
(1982b) Similar mode of action of pyrethroids and DDT on sodium
channel gating in mylinated nerves. Nature (Lond.), 295: 601-603.
VIJVERBERG, H.P.M., VAN DER ZALM, J.M., VAN KLEEF, R.G.D.M., &
VAN DEN BERCKEN, J. (1983) Temperature and structure-dependent
interaction of pyrethroids with the sodium channels in frog node of
Ranvier. Biochim. Biophys. Acta, 728: 73-82.
WALLWORK, L.M. & MALONE, J.C. (1974) 21Z73 (25:75) effect of
different solvents on the rat oral toxicity (Report No. HEFG 75-4)
Berkhamsted, Wellcome Research Laboratories (Unpublished data submitted
to WHO).
WALLWORK, L.M., CLAMPITT, R.B., & MALONE, J.C. (1974a) 10-day
cumulative oral toxicity study with 21Z73 in mice, Berkhamsted,
Wellcome Research Laboratories (Report No. HEFG 74-9) (Unpublished
data submitted to WHO).
WALLWORK, L.M., CLAMPITT, R.B., & MALONE, J.C. (1974b) 10-day
cumulative oral toxicity study with 21Z73 in rats, Berkhamsted,
Wellcome Research Laboratories (Unpublished report submitted to WHO).
WELLS, D., GRAYSON, B.T., & LANGNER, E. (1986) Vapour pressure of
permethrin. Pestic. Sci., 17: 473-476.
WHO (1979) WHO Technical Report Series, No. 634 (Safe use of
pesticides. Third Report of the WHO Expert Committee on Vector Biology
and Control), pp. 18-23.
WHO (1988) The WHO recommended classification of pesticides by
hazard: Guidelines to classification 1988-89, Geneva, World Health
Organization (Unpublished report VBC/88.953).
WHO/FAO (1984) Data sheet on pesticides No. 51: Permethrin, Geneva,
World Health Organization (VBC/DS/84.51).
WILLIAMS, I.H. & BROWN, M.J. (1979) Persistence of permethrin and WL
43775 in soil. J. agric. food Chem., 27: 130-132.
WOODRUFF, R.C., PHILLIPS, J.P., & IRWIN, D. (1983) Pesticide-induced
complete and partial chromosome loss in screens with repair-defective
females of Drosophila melanogaster. Environ. Mutagen., 5: 835-846.
WOOD, MACKENZIE & Co. (1980) Pyrethroids. Agrochem. Monit., 9: 3-14.
WOOD, MACKENZIE & Co. (1981) Pyrethroids. Agrochem. Monit., 15: 3-27.
WOOD, MACKENZIE & Co. (1982) Pyrethroids. Agrochem. Monit., 21: 3-17.
WOOD, MACKENZIE & Co. (1983) Pyrethroids. Agrochem. Monit., 27: 3-12.
WOOD, MACKENZIE & Co. (1984) Pyrethroids. Agrochem. Monit., 33: 2-12.
WORKMAN, R.B. (1977) Pesticides toxic to striped earwig, an important
insect predator. Proc. Florida State Hortic. Soc., 90: 401-402.
WORTHING, C.R. & WALKER, S.B. (1983) The pesticide manual, 7th ed.,
Croydon, British Crop Protection Council, p. 427.
WORTHING, C.R. & WALKER, S.B. (1987) Permethrin. In: The pesticide
manual, 8th ed., Croydon, British Crop Protection Council, pp. 647-
648.
WOUTERS, W. & VAN DEN BERCKEN, J. (1978) Action of pyrethroids. Gen.
Pharmacol., 9: 387-398.
ZITKO, V., CARSON, W.G., & METCALFE, C.D. (1977) Toxicity of
pyrethroids to juvenile atlantic salmon. Bull. environ. Contam.
Toxicol., 18: 35-41.
ZITKO, V., MCLEESE, D.W., METCALFE, C.D., & CARSON, W.G. (1979)
Toxicity of permethrin, decamethrin, and related pyrethroids to salmon
and lobster. Bull. environ. Contam. Toxicol., 21: 338-343.
APPENDIX I
On the basis of electrophysiological studies with peripheral nerve
preparations of frogs ( Xenopus laevis; Rana temporaria, and Rana
esculenta ), it is possible to distinguish between 2 classes of
pyrethroid insecticides: (Type I and Type II). A similar distinction
between these 2 classes of pyrethroids has been made on the basis of
the symptoms of toxicity in mammals and insects (Van den Bercken et
al., 1979; WHO, 1979; Verschoyle & Aldridge, 1980; Glickman & Casida,
1982; Lawrence & Casida, 1982). The same distinction was found in
studies on cockroaches (Gammon et al., 1981).
Based on the binding assay on the gamma-aminobutyric acid (GABA)
receptor-ionophore complex, synthetic pyrethroids can also be
classified into two types: the alpha-cyano-3-phenoxybenzyl pyrethroids
and the non-cyano pyrethroids (Gammon et al., 1982; Gammon & Casida,
1983; Lawrence & Casida, 1983; Lawrence et al., 1985).
Pyrethroids that do not contain an alpha-cyano group (allethrin,
d-phenothrin, permethrin, tetramethrin, cismethrin, and bioresmethrin)
(Type I: T-syndrome)
The pyrethroids that do not contain an alpha-cyano group give rise
to pronounced repetitive activity in sense organs and in sensory nerve
fibres (Van den Bercken et al., 1973). At room temperature, this
repetitive activity usually consists of trains of 3-10 impulses and
occasionally up to 25 impulses. Train duration is between 10 and 5
milliseconds.
These compounds also induce pronounced repetitive firing of the
presynaptic motor nerve terminal in the neuromuscular junction (Van den
Bercken, 1977). There was no significant effect of the insecticide on
neurotransmitter release or on the sensitivity of the subsynaptic
membrane, nor on the muscle fibre membrane. Presynaptic repetitive
firing was also observed in the sympathetic ganglion treated with these
pyrethroids.
In the lateral-line sense organ and in the motor nerve terminal,
but not in the cutaneous touch receptor or in sensory nerve fibres, the
pyrethroid-induced repetitive activity increases dramatically as the
temperature is lowered, and a decrease of 5°C in temperature may cause
a more than 3-fold increase in the number of repetitive impulses per
train. This effect is easily reversed by raising the temperature. The
origin of this "negative temperature coefficient" is not clear
(Vijverberg et al., 1983).
Synthetic pyrethroids act directly on the axon through interference
with the sodium channel gating mechanism that underlies the generation
and conduction of each nerve impulse. The transitional state of the
sodium channel is controlled by 2 separately acting gating mechanisms,
referred to as the activation gate and the inactivation gate. Since
pyrethroids only appear to affect the sodium current during
depolarization, the rapid opening of the activation gate and the slow
closing of the inactivation gate proceed normally. However, once the
sodium channel is open, the activation gate is restrained in the open
position by the pyrethroid molecule. While all pyrethroids have
essentially the same basic mechanism of action, however, the rate of
relaxation differs substantially for the various pyrethroids (Flannigan
& Tucker, 1985).
In the isolated node of Ranvier, allethrin causes prolongation of
the transient increase in sodium permeability of the nerve membrane
during excitation (Van den Bercken & Vijverberg, 1980a,b). Evidence so
far available indicates that allethrin selectively slows down the
closing of the activation gate of a fraction of the sodium channels
that open during depolarization of the membrane. The time constant of
closing of the activation gate in the allethrin-affected channels is
about 100 milliseconds compared with less than 100 microseconds in the
normal sodium channel, i.e., it is slowed down by a factor of more than
100. This results in a marked prolongation of the sodium current
across the nerve membrane during excitation, and this prolonged sodium
current is directly responsible for the repetitive activity induced by
allethrin (Vijverberg et al., 1983).
The effects of cismethrin on synaptic transmission in the frog
neuromuscular junction, as reported by Evans (1976), are almost
identical to those of allethrin, i.e., presynaptic repetitive firing,
and no significant effects on transmitter release or on the subsynaptic
membrane.
Interestingly, the action of these pyrethroids closely resembles
that of the insecticide DDT in the peripheral nervous system of the
frog. DDT also causes pronounced repetitive activity in sense organs,
in sensory nerve fibres, and in motor nerve terminals, due to a
prolongation of the transient increase in sodium permeability of the
nerve membrane during excitation. Recently, it was demonstrated that
allethrin and DDT have essentially the same effect on sodium channels
in frog myelinated nerve membrane. Both compounds slow down the rate
of closing of a fraction of the sodium channels that open on
depolarization of the membrane (Van den Bercken et al., 1973, 1979;
Vijverberg et al., 1982b).
In the electrophysiological experiments using giant axons of
crayfish, the type I pyrethroids and DDT analogues retain sodium
channels in a modified open state only intermittently, cause large
depolarizing after-potentials, and evoke repetitive firing with minimal
effect on the resting potential (Lund & Narahashi, 1983).
These results strongly suggest that permethrin and cismethrin, like
allethrin, primarily affect the sodium channels in the nerve membrane
and cause a prolongation of the transient increase in sodium
permeability of the membrane during excitation.
The effects of pyrethroids on end-plate and muscle action
potentials were studied in the pectoralis nerve-muscle preparation of
the clawed frog ( Xenopus laevis ). Type I pyrethroids (allethrin,
cismethrin, bioresmethrin, and 1R, cis-phenothrin) caused moderate
presynaptic repetitive activity, resulting in the occurrence of
multiple end-plate potentials (Ruigt & Van den Bercken, 1986).
Pyrethroids with an alpha-cyano group on the 3-phenoxybenzyl alcohol
(deltamethrin, cypermethrin, cyhalothrin, lambda-cyhalothrin,
fenvalerate, and fenpropanate) (Type II: CS-syndrome)
The pyrethroids with an alpha-cyano group cause an intense repetitive
activity in the lateral line organ in the form of long-lasting trains
of impulses (Vijverberg et al., 1982a). Such a train may last for up
to 1 min and contains thousands of impulses. The duration of the
trains and the number of impulses per train increase markedly on
lowering the temperature. Cypermethrin does not cause repetitive
activity in myelinated nerve fibres. Instead, this pyrethroid causes a
frequency-dependent depression of the nervous impulse, brought about by
a progressive depolarization of the nerve membrane as a result of the
summation of depolarizing after-potentials during train stimulation
(Vijverberg & Van den Bercken, 1979; Vijverberg et al., 1983).
In the isolated node of Ranvier, cypermethrin, like allethrin,
specifically affects the sodium channels of the nerve membrane and
causes a long-lasting prolongation of the transient increase in sodium
permeability during excitation, presumably by slowing down the closing
of the activation gate of the sodium channel (Vijverberg & Van den
Bercken, 1979; Vijverberg et al., 1983). The time constant of closing
of the activation gate in the cypermethrin-affected channels is
prolonged to more than 100 milliseconds. Apparently, the amplitude of
the prolonged sodium current after cypermethrin is too small to induce
repetitive activity in nerve fibres, but is sufficient to cause the
long-lasting repetitive firing in the lateral-line sense organ.
These results suggest that alpha-cyano pyrethroids primarily affect
the sodium channels in the nerve membrane and cause a long-lasting
prolongation of the transient increase in sodium permeability of the
membrane during excitation.
In the electrophysiological experiments using giant axons of
crayfish, the Type II pyrethroids retain sodium channels in a modified
continuous open state persistently, depolarize the membrane, and block
the action potential without causing repetitive firing (Lund &
Narahashi, 1983).
Diazepam, which facilitates GABA reaction, delayed the onset of
action of deltamethrin and fenvalerate, but not permethrin and
allethrin, in both the mouse and cockroach. Possible mechanisms of the
Type II pyrethroid syndrome include action at the GABA receptor complex
or a closely linked class of neuroreceptor (Gammon et al., 1982).
The Type II syndrome of intracerebrally administered pyrethroids
closely approximates that of the convulsant picrotoxin (PTX).
Deltamethrin inhibits the binding of [3H]-dihydropicrotoxin to rat
brain synaptic membranes, whereas the non-toxic R epimer of
deltamethrin is inactive. These findings suggest a possible relation
between the Type II pyrethroid action and the GABA receptor complex.
The stereospecific correlation between the toxicity of Type II
pyrethroids and their potency to inhibit the [35S]-TBPS binding was
established using a radioligand, [35S]- t-butyl-bicyclophosphoro-
thionate [35S]-TBPS. Studies with 37 pyrethroids revealed an
absolute correlation, without any false positive or negative, between
mouse intracerebral toxicity and in vitro inhibition: all toxic cyano
compounds including deltamethrin, 1R, cis-cypermethrin, 1R, trans-
cypermethrin, and [2S,alphaS]-fenvalerate were inhibitors, but their
non-toxic stereoisomers were not; non-cyano pyrethroids were much less
potent or were inactive (Lawrence & Casida, 1983).
In the [35S]-TBPS and [3H]-Ro 5-4864 (a convulsant
benzodiazepine radioligand) binding assay, the inhibitory potencies of
pyrethroids were closely related to their mammalian toxicities. The
most toxic pyrethroids of Type II were the most potent inhibitors of
[3H]-Ro 5-4864 specific binding to rat brain membranes. The [3H]-
dihydropicrotoxin and [ 35S]-TBPS binding studies with pyrethroids
strongly indicated that Type II effects of pyrethroids are mediated, at
least in part, through an interaction with a GABA-regulated chloride
ionophore-associated binding site. Moreover, studies with [3H]-Ro 5-
4864 support this hypothesis and, in addition, indicate that the
pyrethroid-binding site may be very closely related to the convulsant
benzodiazepine site of action (Lawrence et al., 1985).
The Type II pyrethroids (deltamethrin, 1R, cis-cypermethrin and
[2S,alphaS]-fenvalerate) increased the input resistance of crayfish
claw opener muscle fibres bathed in GABA. In contrast, two non-insect-
icidal stereoisomers and Type I pyrethroids (permethrin, resmethrin,
allethrin) were inactive. Therefore, cyanophenoxybenzyl pyrethroids
appear to act on the GABA receptor-ionophore complex (Gammon & Casida,
1983).
The effects of pyrethroids on end-plate and muscle action
potentials were studied in the pectoralis nerve-muscle preparation of
the clawed frog ( Xenopus laevis). Type II pyrethroids (cypermethrin
and deltamethrin) induced trains of repetitive muscle action potentials
without presynaptic repetitive activity. However, an intermediate
group of pyrethroids (1R-permethrin, cyphenothrin, and fenvalerate)
caused both types of effect. Thus, in muscle or nerve membrane the
pyrethroid induced repetitive activities due to a prolongation of the
sodium current. But no clear distinction was observed between non-
cyano and alpha-cyano pyrethroids (Ruigt & Van den Bercken, 1986).
Appraisal
In summary, the results strongly suggest that the primary target
site of pyrethroid insecticides in the vertebrate nervous system is the
sodium channel in the nerve membrane. Pyrethroids without an alpha-cyano
group (allethrin, d-phenothrin, permethrin, and cismethrin) cause a
moderate prolongation of the transient increase in sodium permeability
of the nerve membrane during excitation. This results in relatively
short trains of repetitive nerve impulses in sense organs, sensory
(afferent) nerve fibres, and, in effect, nerve terminals. On the other
hand, the alpha-cyano pyrethroids cause a long-lasting prolongation of
the transient increase in sodium permeability of the nerve membrane
during excitation. This results in long-lasting trains of repetitive
impulses in sense organs and a frequency-dependent depression of the
nerve impulse in nerve fibres. The difference in effects between
permethrin and cypermethrin, which have identical molecular structures
except for the presence of an alpha-cyano group on the phenoxybenzyl
alcohol, indicates that it is this alpha-cyano group that is responsible
for the long-lasting prolongation of the sodium permeability.
Since the mechanisms responsible for nerve impulse generation and
conduction are basically the same throughout the entire nervous system,
pyrethroids may also induce repetitive activity in various parts of the
brain. The difference in symptoms of poisoning by alpha-cyano
pyrethroids, compared with the classical pyrethroids, is not
necessarily due to an exclusive central site of action. It may be
related to the long-lasting repetitive activity in sense organs and
possibly in other parts of the nervous system, which, in a more advance
state of poisoning, may be accompanied by a frequency-dependent
depression of the nervous impulse.
Pyrethroids also cause pronounced repetitive activity and a
prolongation of the transient increase in sodium permeability of the
nerve membrane in insects and other invertebrates. Available
information indicates that the sodium channel in the nerve membrane is
also the most important target site of pyrethroids in the invertebrate
nervous system (Wouters & Van den Bercken, 1978; WHO, 1979).
Because of the universal character of the processes underlying
nerve excitability, the action of pyrethroids should not be considered
restricted to particular animal species, or to a certain region of the
nervous system. Although it has been established that sense organs and
nerve endings are the most vulnerable to the action of pyrethroids, the
ultimate lesion that causes death will depend on the animal species,
environmental conditions, and on the chemical structure and physical
characteristics of the pyrethroid molecule (Vijverberg & Van den
Bercken, 1982).
RESUME, EVALUATION, CONLUSIONS ET RECOMMANDATIONS
1. Résumé et Evaluation
1.1 Identité, propriétés physicochimiques et méthodes d'analyse
La perméthrine, un pyréthroide photostable, a été synthétisée pour
la première fois en 1973 et commercialisée en 1977. C'est un ester de
l'analogue dichloré de l'acide chrysanthémique, à savoir l'acide
(dichloro-2,2 vinyl)-3 diméthyl-2,2 cyclopropanecarboxylique (Cl2CA) et
de l'alcool phénoxy-3 benzylique. Les produits techniques se présentent
sous la forme d'un mélange de quatre stéréoisomères ayant les
configurations [1R,trans], [1R,cis], [1S,trans] et [1S,cis] dans les
proportions approximatives de 3:2:3:2. Le rapport cis/trans est
d'environ deux tiers, les isomères 1R et 1S étant présents en quantités
égales (racémique). C'est l'isomère [1R,cis] qui est l'insecticide le
plus actif; vient ensuite l'isomère [1R,trans].
La perméthrine de qualité technique se présente sous la forme d'un
liquide brun ou brun jaunâtre qui peut partiellement cristalliser à la
température ambiante. Son point de fusion est d'environ 35°C et son
point d'ébullition de 220°C sous 0,05 mmHg. La densité est de 1,214 à
25°C et la tension de vapeur de 1,3 µPa à 20°C. La perméthrine est
pratiquement insoluble dans l'eau (moins de 0,2 mg par litre à 25°C),
mais elle est soluble dans certains solvants organiques tels que
l'acétone, l'hexane et le xylène. Elle est stable à la lumière et à la
chaleur, mais instable en milieu alcalin.
Le dosage des résidus et les analyses écotoxicologiques s'effect-
uent par chromatographie en phase gazeuse avec détection par capture
d'électrons (concentration minimale décelable de 0,005 mg/kg). Pour
l'analyse des produits techniques, on a recours à la chromatographie en
phase gazeuse avec détection par ionisation de flamme.
1.2 Production et usage
On utilise actuellement dans le monde environ 600 tonnes de
perméthrine par an, essentiellement en agriculture. Ce produit pourrait
être utilisé pour la protection des céréales ensilées et on l'emploie
en épandage aérien pour la protection des forêts, la lutte anti-
vectorielle, la destruction des insectes incommodants dans les
habitations, le déparasitage des bestiaux, la destruction des poux du
corps et l'imprégnation des moustiquaires.
La perméthrine est présentée en concentré émulsionnable, en
concentré pour application à très bas volume, en poudre mouillable, en
poudre pour poudrage et en aérosols.
1.3 Exposition humaine
Les teneurs en résidus des diverses récoltes diminuent assez
lentement, le temps de demi-élimination allant de une à trois semaines
selon les plantes. Toutefois, lorsqu'elle est utilisée conformément
aux recommandations, la perméthrine ne donne pas lieu à une accumu-
lation de résidus, même après plusieurs traitements.
Pour ce qui est de la population dans son ensemble, la voie
d'exposition à la perméthrine est essentiellement alimentaire. Les taux
de résidus dans les plantes correctement cultivées sont généralement
faibles. L'exposition qui pourrait en résulter pour la population
générale est vraisemblablement faible, mais on manque de données
précises provenant d'études sur la ration totale.
On est très peu documenté sur l'exposition professionnelle à la
perméthrine.
1.4 Destinée dans l'environnement
On a montré en laboratoire que la perméthrine se dégrade dans le
sol avec une demi-vie d'environ 28 jours. L'isomère trans se décompose
plus rapidement que l'isomère cis, la principale réaction de décompo-
sition initiale étant le clivage du groupement ester. Cette réaction
donne naissance à des composés qui subissent une oxydation plus poussée
aboutissant à l'anhydride carbonique comme produit final. On a montré,
en étudiant le potentiel de lessivage de la perméthrine et de ses
produits de dégradation, que toutes ces substances pénétraient peu
profondément dans le sol.
La perméthrine déposée sur les végétaux se dégrade avec une demi-
vie d'environ 10 jours. La principale voie de dégradation comporte le
clivage du groupement ester et la conjugaison de l'acide et de l'alcool
qui en résultent. Il se produit également une hydroxylation en divers
points de la molécule ainsi qu'une interconversion cis-trans photo-
induite.
Dans l'eau et à la surface du sol, la perméthrine est décomposée
par le rayonnement solaire. Là encore, le clivage du groupement ester
et l'interconversion cis-trans sont les principales réactions.
En général, ce processus de décomposition environnementale conduit
à des produits de moindre toxicité.
La perméthrine disparaît rapidement de l'environnement, en six
à 24 heures dans les étangs et les cours d'eau, en sept jours dans les
sédiments des étangs, et en 58 jours dans les feuilles et le sol des
forêts. On a constaté que 30% du composé disparaissaient en une semaine
des feuilles d'une plantation de cotonniers.
En conditions d'aérobiose dans le sol, la perméthrine se décompose
avec une demi-vie de 28 jours.
La perméthrine se déplace peu dans l'environnement et il est
improbable qu'elle s'y accumule en quantité notable.
1.5 Cinétique et métabolisme
Administrée à des mammifères, la perméthrine est rapidement
métabolisée et presqu'entièrement excrétée dans les urines et les
matières fécales en l'espace de 12 jours. L'isomère trans, beaucoup
plus sensible à l'attaque par l'estérase que l'isomère cis, s'élimine
plus rapidement que ce dernier. Les principales réactions métaboliques
sont le clivage du groupement ester et l'oxydation, particulièrement au
niveau du cycle aromatique terminal, du reste phénoxybenzylique et du
groupe diméthyle géminé du cycle cyclopropane, réactions qui sont
suivies d'une conjugaison. On a retrouvé moins de 0,7% de la dose
initiale dans le lait de chèvres et de vaches à qui l'on avait
administré de la perméthrine par voie orale.
1.6 Effets sur les êtres vivants dans leur milieu naturel
Des épreuves de laboratoire ont montré que la perméthrine était
extrêmement toxique pour les arthropodes aquatiques, la CL50 allant de
0,018 µg/litre pour la larve d'un crabe comestible à 1,26 µg/litre pour
un cladocère. Elle est également très toxique pour les poissons, la
CL50 à 96 heures allant de 0,62 µg/litre pour la larve de truite
arc-en-ciel à 314 µg/litre pour la truite adulte. La dose sans effet
observable au début du cycle évolutif du vairon est 10 µg/litre sur 28
jours, la dose chronique étant de 0,66 à 1,4 µg/litre pour un autre
cyprinidé, Pimephas promelas. La perméthrine est moins toxique pour
les mollusques aquatiques et les amphibiens, les valeurs de la CL50 à
96 heures se situant respectivement à plus de 1000 µg/litre et
7000 µg/litre.
Lors d'essais sur le terrain et en utilisation normale, cette forte
toxicité potentielle ne se manifeste pas. Il existe une abondante
littérature sur les effets de la perméthrine utilisée en agriculture,
dans les exploitations forestières ainsi que pour la lutte antivectori-
elle dans de nombreuses régions du monde. Il y a une certaine mortalité
pour les arthropodes aquatiques, notamment lorsque l'on traite les eaux
en surface, mais les effets sur les populations ne sont que tempor-
aires. Il n'y a pas eu de cas de mortalité chez les poissons. Cette
moindre toxicité qui se manifeste sur le terrain provient de la forte
adsorption du composé par les sédiments et de sa décomposition rapide.
La perméthrine fixée sur les sédiments est toxique pour les
organismes fouisseurs, mais là encore, l'effet est temporaire. La
perméthrine est extrêmement toxique pour les abeilles. La DL50 topique est
de 0,11 µg par abeille mais le fort effet répulsif qu'elle exerce sur
l'insecte en réduit les effets toxiques dans la pratique. Rien
n'indique qu'il puisse y avoir une forte mortalité des abeilles en
utilisation normale. La perméthrine est plus toxique pour les acariens
prédateurs que pour les espèces nuisibles visées.
La perméthrine est très peu toxique pour les oiseaux, en
administration orale ou par voie alimentaire. La DL50 aiguë est
supérieure à 3000 mg par kg de poids corporel en une seule
administration et supérieure à 5000 mg par kg de nourriture quand le
produit est mêlé à la ration. Elle n'a aucun effet sur la reproduction
de la poule à la dose de 40 mg/kg de nourriture.
Les organismes aquatiques accumulent facilement la perméthrine, le
facteur de concentration allant de 43 à 750 selon les espèces. Chez
tous les organismes aquatiques étudiés, la perméthrine disparaît
rapidement lorsque les animaux sont remis en eau propre. Il n'y a
aucune accumulation chez les oiseaux. On peut donc considérer que ce
composé ne présente en pratique aucune tendence à la bioaccumulation.
1.7 Effets sur les animaux d'expérience et sur les systèmes
d'épreuve in vitro
La perméthrine n'a qu'une faible toxicité aiguë pour les rats, les
souris, les lapins et les cobayes, encore que la DL50 varie
considérablement selon le véhicule utilisé et le rapport des isomères
cis/trans. Les signes d'intoxication aiguë apparaissent en deux heures
et persistent jusqu'à trois jours. Les isomères [1R,cis] et [1R,trans]
appartiennent aux pyréthroïdes du type-I dont les effets caractérist-
iques sont les tremblements (syndrome T) la perte de coordination,
l'hyperactivité, la prostration et la paralysie. L'intoxication pro-
voque une augmentation notable de la température centrale.
Aucun des métabolites de la perméthrine ne présente une toxicité
aiguë (par voie orale ou intrapéritonéale) supérieure à celle de la
perméthrine elle-même.
La perméthrine a provoqué une légère irritation de la peau intacte
ou abrasée chez le lapin, mais il n'y a pas eu d'irritation d'origine
photochimique après exposition aux rayons ultra-violets de zones de la
peau traitées par cet insecticide. Elle ne suscite en revanche aucune
réaction de sensibilisation chez le cobaye.
Des études de toxicité orale subaiguë et subchronique ont été
effectuées chez le rat et la souris à des doses allant jusqu'à
10 000 mg/kg de nourriture et pendant des périodes s'étendant de 14 à
26 semaines. A la dose la plus forte, on a observé une augmentation du
rapport poids du foie/poids du corps, une hypertrophie du foie et des
signes cliniques d'intoxication tels que des tremblements. Chez le rat,
la dose sans effet observable varie de 20 mg/kg de nourriture (pour les
études de 90 jours ou de six mois) à 150 mg/kg de nourriture (lors
d'une étude de six mois).
Chez le chien, ces valeurs ont été trouvées égales à 50 mg/kg de
poids corporel et à 100 mg/kg de poids corporel, respectivement, lors
de deux études de trois mois.
Des études à long terme sur des souris et des rats ont révélé une
augmentation du poids du foie, vraisemblablement liée à l'induction du
système enzymatique des microsomes hépatiques.
Une étude de deux ans sur des rats a fait ressortir une dose sans
effet observable de 100 mg/kg de nourriture, soit 5 mg/kg de poids
corporel.
D'après trois études à long terme sur la souris, il semblerait
qu'il existe un certain pouvoir cancérogène au niveau des poumons pour
au moins une souche de souris (uniquement des femelles) à la dose la
plus élevée étudiée, soit 5 g/kg de nourriture. Aucun effet oncogène
n'a été observé chez des rats des deux sexes.
La perméthrine n'est mutagène ni in vivo ni in vitro .
Les études de mutagénicité ainsi que des études à long terme sur la
souris et le rat indiquent que le pouvoir oncogène de la perméthrine
est très faible, qu'il se limite aux souris femelles et qu'il s'agit
probablement d'un phénomène épigénétique.
La perméthrine n'est pas tératogène pour le rat, la souris ou le
lapin, à des doses allant respectivement jusqu'à 225, 150 et 1800 mg/kg
de poids corporel.
La perméthrine n'a pas produit d'effet indésirable à des doses
allant jusqu'à 2500 mg/kg de nourriture lors d'une étude de repro-
duction portant sur trois générations.
Administrée à des rats à forte dose (6600 à 7000 mg/kg de
nourriture) pendant 14 jours, l'insecticide a provoqué des lésions au
niveau du nerf sciatique; cependant une autre étude n'a pas confirmé la
présence d'altérations ultrastructurales à ce niveau. Chez la poule on
n'a pas observé de neurotoxicité retardée.
1.8 Effets sur l'homme
La perméthrine peut provoquer un certain nombre de sensations au
niveau de la peau ainsi que des parésthésies chez les travailleurs
exposés, symptômes qui apparaissent après une période de latence
d'environ 30 minutes, passent par un maximum au bout de huit heures et
disparaissent en 24 heures. Parmi les symptômes les plus fréquemment
signalés, figurent un engourdissement, des démangeaisons, des four-
millements et des sensations de brûlure.
Aucun cas d'intoxication n'a été signalé.
La probabilité d'effets oncogènes chez l'homme est extrêmement
faible, voire nulle.
Rien n'indique que la perméthrine puisse exercer des effets indési-
rables sur l'homme si elle est utilisée conformément aux recommand-
ations.
2. Conclusions
2.1 Population générale
La population dans son ensemble est vraisemblablement très peu
exposée à la perméthrine. Cet insecticide ne devrait présenter aucun
danger s'il est utilisé conformément aux recommandations.
2.2 Exposition professionnelle
Utilisée de manière raisonnable et moyennant certaines mesures
d'hygiène et de sécurité, la perméthrine ne devrait présenter aucun
danger pour les personnes qui lui sont exposées de par leur
profession.
2.3 Environnement
La perméthrine ou ses produits de décomposition ne devraient pas
atteindre dans le milieu des concentrations critiques dans la mesure où
l'insecticide est utilisé aux doses recommandées. Au laboratoire, la
perméthrine est extrêmement toxique pour les poissons, les arthropodes
aquatiques et les abeilles. Toutefois il est improbable que des effets
indésirables durables se produisent en situation réelle si l'insecti-
cide est utilisé conformément aux recommandations.
3. Recommandations
Les concentrations alimentaires résultant d'une utilisation
conforme aux recommandations sont en principe faibles, toutefois il
serait bon de confirmer cette hypothèse en étendant la surveillance à
la perméthrine.
La perméthrine est utilisée depuis de nombreuses années sans
qu'aucun effet indésirable n'ait été signalé à la suite d'une
exposition humaine. Néanmoins il serait bon de poursuivre les
observations sur ce type d'exposition.
When rainbow trout (Salmo gairdneri) were held in static water
containing 5 µg/litre of 14C-permethrin for 24 h, both cis and trans
isomers were similarly taken up into the fish. The bioaccumulation
ratios for total radiocarbon in the blood, muscle, liver, and fat of
the fish were 30, 30, 300, and 400, respectively. When the fish were
transferred to fresh running water, radioactivity was eliminated, with
initial half-lives of 9-35 h, from all the tissues except fat, where
little decay in 14C-permethrin concentrations occurred. When rainbow
trout were injected intraperitoneally with 14C-permethrin at a rate of
0.5 mg/kg, 32-43% of the dose was recovered after 48 h in the bile, 3-
7% in the urine, and 31-42% in the carcass. However, the urine and bile
of the trout injected with the trans isomer contained higher levels of
radioactivity. In the bile, the major metabolite was the glucuronide
conjugate of 3-(4-hydroxyphenoxy)-benzyl-3-(2,2-dichlorovinyl)-2,2-
dimethyl-cyclopropanecarboxylate (26) and there were few metabolites
formed by hydrolysis (Fig. 4). The urine contained principally the
sulfate conjugates of polar products. The ability of rainbow trout to
hydrolyze permethrin in vivo appeared minimal (Glickman et al., 1981).
The rates of trans-permethrin hydrolysis in trout liver, kidney,
and plasma incubated at 12°C were approximately 166, 38, and 59 times,
respectively, lower than those in the corresponding mouse tissues
incubated at 37°C. Although an increase in the incubation temperature
from 12°C to 37°C caused an increase in the rate of trans-permethrin
hydrolysis by trout liver microsomes, trans-permethrin was hydrolyzed
about 45 times slower than by mouse liver microsomes at 37°C. The
hydrolysis of permethrin in trout plasma, however, was higher than that
in trout liver microsomes (Glickman & Lech, 1981).
When the microsomal preparations of both carp and rainbow trout
were fortified with NADPH, the carp microsomes oxidized permethrin iso-
mers more actively than the trout microsomes. Also, larger amounts of
hydroxy ester metabolites were recovered with the cis isomer than with
the trans isomer. The preferred site of oxidation of both isomers by
the carp and trout microsomes was the 4'-position (26) of the phenoxy-
benzyl moiety. The geminal dimethyl group was attacked in preference to
the methyl group situated trans to the carboxy group. trans-Permethrin
primarily underwent hydrolysis by both carp and trout liver microsomes
in the presence or absence of NADPH to yield PBalc (6) and Cl2CA (17)
(Glickman et al., 1979). In this respect, the results of in vitro
studies were different from those obtained in vivo.
Juvenile Atlantic salmon (lipid content 4.2%), exposed for 96 h to
static water containing 22 µg/litre permethrin, took up the insecti-
cide with a bioaccumulation ratio of 55. Dead juvenile salmon exposed
for 12.5 h to 0.098-0.994 mg/litre contained 2.21-3.69 µg/g (Zitko et
al., 1977).
Residues of approximately 0.5-1.2 mg/kg were detected in dead
juvenile Atlantic salmon exposed for 10-89 h to static water containing
permethrin at 6.9-85 µg/litre, the bioaccumulation ratio ranging from
14 to 73. The insecticide was not detected (detection limit 5 ng/g) in
dead lobster hepatopancreas or in dead shrimp (McLeese et al., 1980).
When stoneflies (Pteronarcys dorsata) were exposed for 28 days to
running water containing permethrin at 0.029-0.21 mg/litre, the bioac-
cumulation ratios of the survivors ranged from 43 to 570 (average, 183;
standard deviation, 171) (Anderson, 1982).
When carp were exposed to a 14C-permethrin isomer (phenoxyphenyl-
labelled [1R,trans], [1R,cis], [1S,trans], or [1S,cis] isomers) in a
flow-through system at 25°C, the concentrations of 14C and permethrin
isomers in the fish body reached an equilibrium on days 7-9 of ex-
posure. The bioaccumulation ratios of the permethrin isomers at equi-
librium were 330-750. When the fish were transferred to fresh water,
the permethrin isomers, as well as their metabolites, were rapidly
excreted. The biological half-lives for the permethrin isomers were
2.0-2.8 days. The major metabolic reactions involved were oxidation at
the 4 -position of the alcohol moiety or the methyl group of the acid
moiety, cleavage of the ester linkage, and conjugation of the resultant
alcohols and phenols with glucuronic acid or sulfuric acid (Ohshima et
al., 1988).
Bioconcentration factors for sheepshead minnows ( Cyprinodon vari-
egatus exposed to permethrin at concentrations between 1.25 and
10 µg/litre for 28 days from hatching varied between 290 and 620.
Maximum bioconcentration occurred after exposure at 2.5 µg/litre, and
a maximum residue of 5.7 mg/kg occurred after exposure at 10 µg/litre
(the concentrations were for whole fish) (Hansen et al., 1983).
Permethrin and its metabolites are not accumulated in birds. During
repeated dosing to quails and to mallard ducks, very similar patterns
and levels of both the appearance and depletion of radioactive residue
in tissues were found. The level in fat, which was small, reached a
plateau during a 28-day period. In all tissues, residues declined
extensively during a 14-day period after the final dose (Leahey et al.,
1977).
7. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
Toxicological profiles of permethrins with different isomeric com-
positions (cis:trans ratios of 40:60 or 25:75) were compared in a range
of toxicological studies. The toxicological profile of permethrin
(25:75) resembles that of permethrin (40:60) except that it is less
acutely toxic than permethrin (40:60).
7.1 Acute Toxicity
Table 7 shows the results of acute toxicity tests of permethrin
with various animal species. Aqueous suspensions usually produced the
least toxic results, LD50 values ranging from 3000 to >4000 mg/kg body
weight. However, corn oil is the more standard vehicle for pyrethroids
and yielded LD50 values of about 500 mg/kg (in all studies except one)
for oral administration in rats and mice.
Following oral administration of permethrin to rats, signs of
poisoning became apparent within 2 h after dosing and persisted for up
to 3 days. At lethal levels, these signs included whole body tremors of
varying degree from slight to convulsive, which in some cases were ac-
companied by salivation. Associated signs were hyperactivity and hyper-
excitability to external stimuli, urination and defecation, ataxia, and
lacrimation (Parkinson, 1978; Litchfield, 1983).
Table 8 gives the acute oral toxicity of three lots of permethrin
to rats. The observed ten-fold decrease in LD50 when corn oil or olive
oil were used could be due to enhanced absorption of the insecticide
(Metker et al, 1977).
The acute oral toxicity of permethrin (25:75) to groups of six
female C.S.E. Wistar rats was determined in five different vehicles
(Table 9). Most symptoms of acute poisoning developed within 12 h of
dosing and consisted of muscular tremors, hypersensitivity to stimuli,
and staining of abdominal fur. The majority of deaths occurred between
1 and 3 days (Wallwork & Malone, 1974).
Groups of female Sprague-Dawley rats, either fed ad libitum or
starved for 24 h beforehand, were given a single oral dose of
permethrin (25:75) (94.1% purity) in corn oil solution (40% w/v) at
750, 1500, 3000, or 6000 mg/kg. Permethrin was more toxic in starved
animals (LD50 = 3000 mg/kg) than in animals that had been fed (LD50 =
4251 mg/kg) (Piercy et al., 1976).
The acutetoxicity of permethrin with various cis- and trans-per-
methrin ratios is indicated in Table 10. These data clearly demonstrate
that cis-permethrin is more toxic than trans-permethrin to rats and
mice.
Table 7. Acute toxicity of permethrin administered to various
animal species
-------------------------------------------------------------------------
Species Sex Routea Vehicleb LD50 Reference
(mg/kg body
weight)
-------------------------------------------------------------------------
Rat M oral waterc 2949 Parkinson 1978
F oral water > 4000 Parkinson et al. 1976
M oral DMSO 1500 Clark 1978
F oral DMSO 1000 Clark 1978
M oral corn oil 500 Jaggers & Parkinson 1979
M oral corn oil 430 Kohda et al. 1979a
F oral corn oil 470 Kohda et al. 1979a
M&F oral corn oil 1200 Braun & Killeen 1975
M&F oral water 1725 Sasinovich & Panshina 1987
M dermal water > 5176 Parkinson 1978
F dermal noned > 4000 Parkinson et al. 1976
M dermal noned > 2500 Kohda et al. 1979a
F dermal noned > 2500 Kohda et al. 1979a
M&F dermal xylene > 750 Clark 1978
M&F dermal none 2000 Sasinovich & Panshina 1987
M sc corn oil 7800 Kohda et al. 1979a
F sc corn oil 6600 Kohda et al. 1979a
M ip water > 3200 Parkinson et al. 1976
F ip water > 3200 Parkinson et al. 1976
ip 463 - 1725 Sasinovich & Panshina 1987
Mouse F oral water > 4000 Parkinson et al. 1976
M&F oral DMSO 250 - 500 Clark 1978
M oral corn oil 650 Kohda et al. 1979a
F oral corn oil 540 Kohda et al. 1979a
M dermal noned > 2500 Kohda et al. 1979a
F dermal noned > 2500 Kohda et al. 1979a
M sc corn oil > 10 000 Kohda et al. 1979a
F sc corn oil 10 000 Kohda et al. 1979a
Rabbit F oral waterc > 4000 Parkinson et al. 1976
F dermal noned > 2000 Parkinson et al. 1976
Guinea- M oral water > 4000 Parkinson et al. 1976
pig
Hen oral > 1500 Milner & Butterworth 1977
-------------------------------------------------------------------------
a sc = subcutaneous; ip = intraperitoneal.
b DMSO = dimethyl sulfoxide.
c as an aqueous suspension.
d technical material applied without vehicle.
Table 8. Acute oral toxicity of three lots of permethrin to rats
------------------------------------------------------------------
Lot No.c Strain Sex Solvent LD50
(mg/kg)
------------------------------------------------------------------
827-RSP-1422 Sprague-Dawley Male None 5010
Female None 3801
827-RTP-1450 Sprague-Dawley-1a Male Corn oil 563
Sprague-Dawley-2b Male Corn oil 383
827-RTP-1450 Long-Evans Male None 4892
Female None 2712
8719-RTP-1450 Sprague-Dawley Male Olive oil 584
Female Corn oil 413
------------------------------------------------------------------
a Average body weight was 220 g.
b Average body weight was 321 g.
c Isomeric composition and the purity of the compound in each lot
were as follows:
--------------------------------------------
Lot No. Isomeric Ratio Stated
cis trans Purity
--------------------------------------------
827-RSP-1422 44% 56% 93.6%
827-RTP-1450 45% 55% 95.0%
8719-RTP-1450 46.5% 53.5% 92.4%
--------------------------------------------
Table 9. Acute toxicity of permethrin (25:75) to rats
------------------------------------------------------
Vehicle LD50 (mg/kg)
------------------------------------------------------
Neat undiluted permethrin (control) > 20 000
40% w/v in corn oil 4672
40% w/v in petroleum distillate > 8000
40% w/v in dimethylsulfoxide > 8000
20% w/v in glycerol > 5048
------------------------------------------------------
Table 10. Acute toxicity of permethrins with various cis:trans
isomeric ratios
---------------------------------------------------------------------------
Permethrin LD50
(cis:trans) Animal Sex Routea (mg/kg body Reference
weight)
---------------------------------------------------------------------------
80:20 Rat F oral 396 Jaggers & Parkinson (1979)
57:43 F oral 333
50:50 F oral 748
40:60 F oral 630
20:80 F oral 2800
99:1 Mouse ip 108 Glickman et al. (1982)
40:60 ip 514
1:99 ip > 800
99:1 Mouse iv 17 Glickman et al. (1982)
40:60 iv 31
1:99 iv > 135
---------------------------------------------------------------------------
a ip = intraperitoneal; iv = intravenous.
Table 11 shows the results of acute oral toxicity tests of the
metabolites of permethrin on rats (FAO/WHO, 1980b).
Table 11. Acute oral toxicity to rats of several metabolites
of permethrin
---------------------------------------------------------------
Chemical No.a LD50 (mg/kg
body weight)
---------------------------------------------------------------
3-phenoxybenzyl alcohol 6 1330
3-(2,2-dichlorovinyl)-2,2-dimethylcyclo- 17 980
propanecarboxylic acid
3-phenoxybenzaldehyde 11 600
---------------------------------------------------------------
a Chemical identification no. used in Fig. 3.
Table 12 shows the results of the acute intraperitoneal toxicity to
mice of permethrin metabolites (Kohda et al., 1979b).
Table 12. Acute intraperitoneal toxicity to mice of several
permethrin metabolites
---------------------------------------------------------------------
Chemicala No.b LD50 (mg/kg body weight)
Male Female
---------------------------------------------------------------------
3-phenoxybenzyl alcohol 6 71 424
3-(4 -hydroxyphenoxy)benzyl alcohol 7 750 - 1000 750 - 1000
3-(2 -hydroxyphenoxy)benzyl alcohol 8 876 778
3-phenoxybenzoic acid 12 154 169
3-(4 -hydroxyphenoxy)benzoic acid 13 783 745
3-(2 -hydroxyphenoxy)benzoic acid 14 859 912
3-phenoxybenzaldehyde 11 415 416
---------------------------------------------------------------------
a All compounds were dissolved in corn oil, except 3-phenoxybenzoic
acid, which was dissolved in DMSO.
b Chemical identification no. used in Fig. 3.
7.2 Subacute and Subchronic Toxicity
7.2.1 Oral exposure
7.2.1.1 Mouse
When male and female Alderly Park mice (20 of each sex per group)
were fed permethrin in the diet at levels of 0, 200, 400, 2000, or
4000 mg/kg diet for 28 days, mortality, growth, and food utilization
were normal for all animals. One additional group (permethrin level of
80 mg/kg for 2 weeks and 10 000 mg/kg for the final 2 weeks) showed
weight loss and poor food utilization when feeding with 10 000 mg/kg
began. Animals fed permethrin at 2000 mg/kg or more showed increased
liver weight and liver-to-body weight ratio. Higher weight and organ-
to-body weight ratios were also observed in the kidney, heart, and
spleen of males receiving a dose of 10 000 mg/kg. Gross tissue changes
were observed in females at 2000 and 10 000 mg/kg. On histopathological
examination, regenerating tubules in the renal cortex and hypertrophy
of centrilobular hepatocytes with cytoplasmic eosinophilia, which were
not dose related, were observed in all the treated animals (Clapp et
al., 1977b).
In a study by Wallwork et al. (1974a), groups of six mature female
mice received daily oral doses of permethrin (25:75) in corn oil at 0,
200, 400, 800, or 1600 mg/kg body weight for 10 consecutive days.
Signs of acute toxicity, such as spasm and convulsion, were seen only
in the highest dose group, half of which died after the initial dose.
No significant changes in haematology, clinical chemistry, or body
weights on the 11th day of dosing were recorded. The mice treated at
800 and 1600 mg/kg body weight exhibited increased liver weights.
7.2.1.2 Rat
Sprague-Dawley rats (six of each sex per group) were fed permethrin
in the diet for 14 days at dose levels of 54, 108, 216, 432, 864, or
1728 mg/kg body weight per day. All rats surviving to term were sacri-
ficed and various organs and tissues were examined histopathologically.
At the two highest dose levels, all animals died except one female fed
864 mg/kg. Muscle tremors were noted in all animals at 432 mg/kg, but
doses of 216 mg/kg or less caused no toxic signs in either males or
females. There was a statistically significant increase in average
liver-to-body weight ratios at 432 mg/kg, but compound-related histo-
logical changes were not observed in any of the tissues or organs. The
maximum NOEL in this study was 216 mg/kg (Metker et al., 1977).
In studies by Metker et al. (1977), Long-Evans rats (six of each
sex per group) were fed permethrin in the diet for 14 days at dose
levels of 0, 27, 54, 108, 216, or 432 mg/kg body weight per day. All
rats surviving to term were sacrificed and various organs and tissues
were examined histopathologically. At a dose of 432 mg/kg, three out of
six females died within the first five days. Muscle tremors were noted
in all surviving animals at 216 and 432 mg/kg. There was a statisti-
cally significant increase among female animals in the average liver-
to-body weight ratio. Compound-related histological changes were not
observed in any of the tissues or organs examined. The maximum dietary
NOEL was 108 mg/kg body weight per day.
When young male and female Wistar rats (8 of each sex per group)
were fed permethrin in the diet at dose levels of 0, 200, 500, 1000,
2500, 5000, or 10 000 mg/kg diet for 4 weeks, all rats that received
the highest dose died within 3 days. Mortality was evident at 5000
mg/kg, and hyperexcitability was observed in animals that received
2500 mg/kg. Other non-specific signs of poisoning were observed at
1000 mg/kg on the first day of the study only. Food consumption and
growth were reduced in the animals dosed at 5000 mg/kg. There was no
effect on haematological parameters, clinical chemistry, or urinalysis
except for a reduction in urinary protein excretion in males at 5000
mg/kg. Liver weight and liver-to-body weight ratios were increased in
males at 2500 mg/kg or more and in females at 1000 mg/kg or more. This
study had been designed as a preliminary range-finding test for long-
term dietary administration (Clapp et al., 1977a).
In a study of the reversibility of hepatic changes in rats follow-
ing short-term dietary administration of permethrin, female Wistar rats
(48 rats per group) were fed permethrin at levels of 0 or 2500 mg/kg
diet for 28 days. At the end of the feeding trial, rats were either
sacrificed or maintained on control diets and sacrificed at 1, 4, or
8 weeks after the termination of dosing. There was no mortality, but
food consumption, food utilization, and body weight were reduced in the
permethrin-treated rats during the administration period. However, the
animals gained weight rapidly after the dosing period and there was no
difference in body weight between control and test animals at the end
of the study period. After the 4 weeks of permethrin dosing, signifi-
cantly higher absolute and relative liver weights were observed. During
the 8-week recovery period, the relative liver weight of permethrin-
treated animals was significantly higher than the control values, but
the absolute weights of the liver of control and test animals were
similar. There were no effects of permethrin on plasma alanine transam-
inase over the course of the study. Oxidative enzyme activity in liver
microsomes was significantly higher in test animals than in controls at
the end of dosing and 1 week later. The activity of liver microsomal
enzymes in the permethrin-treated animals was normal 4 weeks after
dosing but was elevated 8 weeks after dosing. The amount of smooth
endoplasmic reticulum in rat liver cells was significantly increased as
a result of permethrin dosing, but within 4 weeks after dosing, there
were no significant histological differences in the liver between
treated and control animals (Bradbrook et al., 1977).
When male and female Charles River (CD) rats (six of each sex per
group) were fed permethrin at levels of 0, 30, 100, 300, 1000, or 3000
mg/kg diet for five weeks, persistent tremors were evident in animals
fed at 3000 mg/kg although no mortality was observed. Growth was
inhibited in both males and females at this dose level. Relative liver
weight was increased in both the males (1000 mg/kg or more) and females
(3000 mg/kg). Slight effects on certain clinical chemistry parameters,
such as increased prothrombin times in males, were noted at the 3000
mg/kg level. Examination of tissues and organs of the animals receiving
the two highest doses did not show any unusual effects as a result of
permethrin in the diet (Butterworth & Hend, 1976).
Male and female Long-Evans rats (10 of each sex per group) fed
permethrin in the diet at dose levels of 0, 20, 100, or 500 mg/kg diet
for 90 days showed no mortality, and the growth and food consumption of
all animals were normal. The results of haematology, clinical chemis-
try, urinalysis, and ophthalmological examinations were also normal.
Tremors were noted in some animals at the highest dose level, mainly
during the first week of treatment. There were significant increases
in absolute and relative liver weights at the two highest dose levels.
These increases were consistent with data from microscopic examination
of the liver showing compound-related centrilobular hepatocyte hyper-
trophy in both males and females. There were no significant effects at
the 20-mg/kg level, although slight hepatic effects were reported in a
few of the male rats (Killeen & Rapp., 1976b).
In studies by Metker et al. (1977), Sprague-Dawley rats (10 of each
sex per group) were fed permethrin in the diet for 90 days at dose
levels of 0, 9, 27, 85, 270, or 850 mg/kg body weight per day. All
rats surviving to term were killed and various tissues and organs from
each animal were examined histopathologically. At 850 mg/kg, all male
and female rats died. An increase in the average liver-to-body weight
ratio was noted in both male and female rats fed 270 mg/kg. Compound-
related histological changes were not observed in any of the tissues
and organs examined. The minimum effect level was 270 mg/kg per day.
At 85 mg/kg no effects were observed.
When male and female Sprague-Dawley rats (16 of each sex per group)
were fed permethrin in the diet at dose levels of 0, 375, 750, 1500, or
3000 mg/kg diet for 6 months, there was no mortality and all animals
exhibited normal growth and normal food and water consumption. Urinal-
ysis, haematological values, and clinical biochemistry parameters
showed no changes related to permethrin dosing. Signs of hyperexcit-
ability and tremors were observed during the study in animals dosed at
3000 mg/kg and the liver weight and liver-to-body weight ratio of these
animals were slightly increased. There were no significant histopatho-
logical findings attributable to the presence of the permethrin in the
diet. The NOEL was 1500 mg/kg (Kadota et al., 1975).
In a study designed to evaluate liver hypertrophy, male and female
Wistar rats were fed permethrin at levels of 0, 20, 100, or 1000 mg/kg
diet for 26 weeks. There was no mortality, and the growth and food con-
sumption of the animals were normal. Although the mean liver weight was
increased at all dose levels, a significant increase was noted only at
the highest dose level. The increase in liver weight at this dose level
was accompanied by an increase in the smooth endoplasmic reticulum and
in biochemical parameters associated with microsomal oxidative mechan-
isms. At a dose level of 100 mg/kg, there were slight, non-significant
increases in biochemical activities. No effects on any of the par-
ameters measured were observed in animals dosed at 20 mg/kg (Hart et
al., 1977c).
In a study by Wallwork et al. (1974b), groups of five to six female
Charles River CDI rats received permethrin (25:75) in corn oil at 0,
200, 400, or 800 mg/kg body weight by daily gavage for 10 days. The
animals were sacrificed on the eleventh day so that haematological and
clinical chemical parameters and organ weights could be investigated.
Permethrin (25:75) gave a toxicity profile similar to that of
permethrin (40:60).
7.2.1.3 Dog
Beagle dogs (four of each sex per group) fed permethrin in gelatin
capsules daily for 3 months at dose levels of 0, 5, 50, or 500 mg/kg
body weight showed no mortality, but clinical signs of poisoning were
noted at various times in both males and females at the highest dose
level. Growth and food consumption, as well as clinical chemical,
haematological, and urinalysis parameters, were normal. The liver
weights and liver-to-body weight ratios of animals that received per-
methrin at 50 mg/kg or more were significantly increased. Histopatho-
logical examination did not reveal any changes attributable to per-
methrin (Killeen & Rapp, 1976a).
Beagle dogs (four of each sex per group) administered permethrin in
gelatin capsules daily for 13 weeks at dose levels of 0, 10, 100, and
2000 mg/kg body weight likewise showed no mortality, but clinical signs
of poisoning were evident at 2000 mg/kg. Haematological, clinical
chemical, and urinalysis values were normal in all animals. There was a
slight increase in the liver weight of animals dosed at 2000 mg/kg/day,
but no accompanying histopathological changes in the liver (Edwards et
al., 1976).
When two beagle dogs were given daily oral doses of permethrin
(25:75) at 500 mg/kg body weight for 14 days, there were no clinical
signs of toxicity or significant effects of the treatment on body
weight or on clinical chemistry or haematological parameters (Chesher
et al., 1975a).
Groups of four male and four female beagle dogs, given encapsulated
permethrin [(25:75) 4.5% w/v] at 0, 10, 50, or 250 mg/kg body weight
for 6 months, revealed no signs of toxicity and no effect on body
weight. Ophthalmoscopy and electrocardiography showed no abnormalities.
At necropsy, there were no gross pathological or significant histo-
pathological findings. Haematological and clinical chemistry para-
meters, including plasma antipyrine elimination rate, were unaffected
by treatment. The results of this study indicate that daily oral doses
up to 250 mg/kg body weight do not adversely affect beagle dogs
(Reynolds et al., 1978).
7.2.1.4 Rabbits
In a study by Chesher & Malone (1974a), groups of five female Dutch
rabbits received permethrin (25:75) in 10 daily doses by gavage in corn
oil at 0, 200, 400, or 800 mg/kg body weight. The animals were killed
on the eleventh day so that clinical chemical, haematological para-
meters, and organ weights could be investigated. One rabbit, dosed at
400 mg/kg, exhibited mild hyperactivity and muscular fasciculation, but
only at days 6 and 7. Although all animals, including the controls,
exhibited some degree of weight loss, it was most marked in the high-
dose group. There were no significant haematological or clinical chem-
istry findings, but there was some decrease in liver and kidney weight
and also some enlargement of adrenal gland weights in all treated
groups.
7.2.1.5 Cow
Lactating cows (three per group) fed permethrin in the diet at dose
levels of 0, 0.2, 1.0, 10, or 50 mg/kg diet for 28 days showed no mor-
tality. Growth and milk production were normal, and no histopathologi-
cal changes in the tissues were observed (Edwards & Iswaran, 1977).
7.2.2 Dermal Exposure
Technical grade permethrin was applied daily to the clipped skin of
New Zealand White rabbits (eight males per group) at dose levels of 0,
0.10, 0.32, or 1.0 g/kg body weight, each day for 21 consecutive days.
The application site was abraded on the first test day in half of the
animals in each group. Blood samples were drawn weekly from the animals
for clinical chemistry studies. All animals were killed on the tenth
day after exposure ceased. Various tissues and organs were taken from
each animal and examined for microscopic lesions. A moderate primary
irritation of the skin was produced by permethrin. No significant
changes in body weight, organ weight, or clinical chemistry values were
evident, neither were there any compound-related lesions in the skin or
other tissues (Metker et al., 1977).
In further studies by Metker et al. (1977), permethrin (dissolved
in acetone) or acetone (as a control) was placed on the skin twice a
week for 3 weeks to 6 groups of 10 shaved male New Zealand White rab-
bits. Cotton cloth treated with permethrin (1.25 or 0.125 mg/cm2) was
applied to the skin over 1 ml of artificial sweat (salt solution imi-
tating sweat). In the case of other rabbits, similarly treated, the
sweat was omitted. In the control groups, acetone-treated cotton cloth
with or without 1 ml of sweat was used. Blood samples were collected
once a week for clinical chemistry determinations. All animals sur-
viving to term were killed and various tissues and organs from each
animal were examined for microscopic lesions. No significant changes
were noted in rabbit body weight or organ-to-body weight at the end of
the 21-day test, and no skin irritation was observed. There were no
significant changes in clinical chemistry values in the treated groups
and no compound-related lesions in the skin or other tissues and organs
examined (Metker et al., 1977).
7.2.3 Inhalation exposure
The inhalation toxicity of technical grade permethrin was deter-
mined in three species of laboratory animals. Male Hartley guinea-pigs,
male and female Sprague-Dawley rats, and male and female beagle dogs
were exposed to an aerosol of permethrin at concentrations of 125, 250,
or 500 mg/m3, 6 h per day, 5 days per week for 13 weeks. The mass
median diameter of the aerosol droplets was 5.1 µm, and 85% of the
total droplets had a diameter of 1.0 µm or less. At 500 mg/m3, tremors
and convulsions occurred in the rats during the first week of exposure
but disappeared in the second week. There was no difference in oxygen
consumption between control and treated rats. Urine metabolite studies
indicated that permethrin was rapidly metabolized and excreted. Post-
exposure experiments in male rats showed that the hexobarbital-induced
sleeping time was significantly shortened after exposures at 500
mg/m3 but not at lower doses. No clinical signs of poisoning were
observed in the guinea-pigs and dogs when exposed to aerosols of per-
methrin under similar conditions. Pulmonary function, clinical chemis-
try parameters, and blood cell counts were unaffected in the beagle
dogs following exposure. No compound-related gross or microscopic
pathological changes or other permanent changes were observed in the
dogs, rats, or guinea-pigs as a result of permethrin inhalation
(Metker, 1978).
7.3 Primary Irritation
7.3.1 Skin irritation
When undiluted technical permethrin (91.3% purity, 0.5 ml) was
applied to the clipped dorsal surface of Japanese White rabbits, there
was no irritation (Okuno et al., 1976).
Single applications of 0.05 ml of 25% (w/v) permethrin (in 95%
ethanol) or 10% (w/v) oil of bergamot solution (in 95% ethanol) (posi-
tive control) were applied to the intact skin of six rabbits. Five
minutes later, some of the rabbits were exposed to UV light (365 nm) at
a distance of 10-15 cm for 30 min (the intensity of UV light was not
mentioned). Skin treated with the positive control solution and
irradiated exhibited a greater irritation reaction than did non-
irradiated skin. Permethrin did not cause any irritation reaction
under the test conditions with or without irradiation (Metker et al.,
1977).
When a permethrin formulation was applied to the clipped dorsal
surface (0.13 mg/cm2) of six New Zealand White rabbits (three of each
sex) once a day for 16 days, a slight erythema appeared, which corre-
lated with increased cutaneous blood flow measured by laser Doppler
velocimetry. No significant histopathological changes were detected
(Flannigan et al., 1985a).
7.3.2 Eye irritation
In a study by Okuno et al. (1976), 0.1 ml of undiluted technical
permethrin (91.3% purity) was applied to the eyes of Japanese White
rabbits. The eyes were washed with distilled water 5 min or 24 h after
the application of permethrin. No eye irritation was observed.
Undiluted permethrin applied to the eyes of female rabbits caused
minimal pain, redness, chemosis of the conjunctiva, and a slight dis-
charge (Parkinson et al., 1976).
Permethrin (25:75) (40% in corn oil) did not produce any ocular
effects when 0.1 ml was instilled into the ocular sac of New Zealand
rabbits (Chesher & Malone, 1974c).
7.4 Sensitization
In a study by Parkinson et al. (1976), guinea-pigs were dermally
administered permethrin as a 10% solution in dimethylformamide for
3 consecutive days. This was followed 4 days later by challenge doses
of 0.1%, 1%, and 10% solutions of permethrin in dimethylformamide. Only
very slight erythema was observed. Permethrin was therefore considered
to be either non-sensitizing or only mildly so.
Guinea-pigs (10 per group) were initially injected intradermally
with 0.1 ml permethrin solution and 14 days later were challenged with
an intradermal injection (0.1 ml) of either a 0.1% solution of permeth-
rin or dinitrochlorobenzene (DNCB). Five other animals per group
received intradermally a challenge dose of 0.1% permethrin or DNCB
without a prior sensitizing dose. The positive control substance (DNCB)
elicited sensitization reactions in all guinea-pigs when examined 24
and 48 h after the challenge dose, whereas permethrin did not cause any
sensitization reactions (Metker et al., 1977; Metker, 1978).
Permethrin (25:75) in corn oil (1% w/v) or Freund's complete
adjuvant (1% w/v) did not produce dermal irritation or sensitization in
groups of 10 male guinea-pigs when applied as a 25% dispersion in
petrolatum. The positive control, DNCB, (5% w/v) in petrolatum produced
marked sensitization (Chesher & Malone, 1974b).
7.5 Long-term Toxicity
7.5.1 Mouse
SPF Alderly Park strain mice (70 males and 70 females per group)
were fed permethrin (cis 35-45%; trans 65-55%) at dose levels of 0,
250, 1000, or 2500 mg/kg diet for 2 years. Ten males and ten females
were designated for interim kills at 26 and 52 weeks. The mortality
rate was unaffected by the administration of permethrin. Growth was
slightly decreased at the two highest dose levels at various periods
during the study. At the interim sacrifice of 52 weeks and at the end
of study, a significant dose-dependent increase in liver-to-body weight
ratio was observed at the two highest dose levels in females (with 2500
mg/kg only at the end of the study) and at the highest dose level in
males. Hepatic aminopyrine N-demethylase activity was also substan-
tially increased, although not consistently, in both male and female
mice given the highest dose. Gross and microscopic examination of
tissues and organs (and specific examination for hepatic neoplasia) did
not reveal any significant carcinogenic effects as a result of per-
methrin administration. Many of the non-tumour abnormalities observed
were considered to be those associated with aging of the mice, charac-
terized by increased eosinophilia of the centrilobular hepatocytes.
Also, a decrease in vacuolation of the proximal tubular epithelium of
the kidney was noted at all dietary levels in males. A high incidence
of lung adenomas was observed with all animals in the study, but stat-
istical analysis suggested that this was not related to permethrin
feeding. Electron-microscopic examination of subcellular liver com-
ponents showed a proliferation of the smooth endoplasmic reticulum at
dose levels of 1000 and 2500 mg/kg. No notable effects on the sciatic
nerve were found as the result of permethrin administration (Ishmael &
Litchfield, 1988).
In studies by Hogan & Rinehart (1977) and Rapp (1978), CD-1 strain
mice (75 of each sex per group) were fed permethrin in the diet for
104 weeks. Alterations were made in the dietary dose levels during the
course of the study. From weeks 1 to 19, the animals were given dose
levels of 0, 20, 100, and 500 mg/kg diet. At week 19, the dose level
of 500 mg/kg was increased to 5000 mg/kg and maintained for 2 weeks
before returning to 500 mg/kg. At week 21, the 100 mg/kg dose was
increased to 4000 mg/kg and maintained for the remainder of the dosing
period. Growth was inhibited in males at 4000 mg/kg. With the exception
of a reduced blood glucose level in the animals receiving 4000 mg/kg,
dietary administration of permethrin had no other effects on haema-
tology or clinical chemistry parameters in the mouse. The liver weight
was higher than it was in control animals in both male and female
animals at a dose level of 500 mg/kg or more. In addition, the heart
weight was higher at 4000 mg/kg. Neoplastic changes, not associated
with dietary levels of permethrin, were observed in some animals in all
groups. While there was no direct effect with respect to hepatic neo-
plasms, it was noted that hepatocellular hypertrophy, pleomorphism, and
degeneration occurred in treated mice with increased frequency and
appeared to show a dose-response relationship. No oncogenic effects
were observed in the test animals.
7.5.2 Rat
Wistar rats (60 of each sex per group) were fed permethrin in the
diet at dose levels of 0, 500, 1000, or 2500 mg/kg diet for 2 years,
and twelve rats of each sex per group were sacrificed at 1 year. Signs
of poisoning such as tremors and hyperexcitability were noted during
the first 2 weeks of dosing in the animals that received the highest
dose. There was no mortality attributable to permethrin, and growth and
food consumption were unaffected. There were no effects on haema-
tological, ophthalmological, urological, or other clinical chemistry
parameters. Liver aminopyrine N-demethylase activity was increased in
all permethrin-treated animals. Bone marrow smears of the animals
showed no unusual findings. Gross and microscopic examination of
tissues and organs was performed after 1 and 2 years, and all animals
that died with neoplastic changes were examined. Liver weights were
higher after 1 year of dosing in male and female rats that received
permethrin at 2500 mg/kg than in the control animals. After 2 years,
the liver weight and liver-to-body weight ratios were higher in all
permethrin-treated males than in the corresponding controls. In the
females, higher values of absolute and relative liver weights, compared
to the controls, were recorded only in the group of animals dosed at
1000 mg/kg. Kidney weight was also increased, predominantly in males,
at all dose levels. Hepatocyte vacuolation was seen at 1 year in males
fed at the highest dose level only and in females at all dose levels.
The smooth endoplasmic reticulum showed significant increases at 52
weeks in both males and females at all dietary levels. At the end of
the study, notable endoplasmic reticulum increases were detected only
at the highest dose levels, although non-significant increases were
noted at all dose levels in both males and females. Examination of the
sciatic nerve showed no effects attributable to permethrin. No
oncogenic effects were noted at any dose level (Ishmael & Litchfield,
1988).
Long-Evans rats (60 males and 60 females per group) fed permethrin
in the diet at dose levels of 0, 20, 100, or 500 mg/kg for 2 years did
not reveal any mortality or adverse effects on growth, food consump-
tion, or behaviour attributable to the administration. Haematology,
clinical chemistry, and urinalysis measurements were performed at
either 6 months or 1 year and at the end of the study. There were no
compound-related effects on a wide variety of parameters examined, and
ophthalmological examination indicated no abnormalities. Blood glucose
levels were higher in the highest-dose males at 24 months and in the
highest-dose females at 18 months, compared to the values of the
control animals. Two independent evaluations of the histopathological
data concluded that there was no oncogenic potential for permethrin.
While there was a dose-dependent increase in gross liver weight in both
males and females, these values are small and not statistically
significant. The NOEL for general toxicity in this study was estimated
to be 100 mg/kg (Braun & Rinehart, 1977; Billups 1978a, b).
7.6 Carcinogenesis
Summaries by Paynter et al. (1982) of toxicological data from seven
long-term chronic toxicity/oncogenicity studies (four in mouse and
three in rat) carried out by ICI, FMC, and Burroughs-Welcome (BW) have
been made available by the US EPA. One rat study and one mouse study
performed by ICI were recently published (Ishmael & Litchfield, 1988),
and one rat study and one mouse study carried out by BW were cited in
FAO/WHO (1988). A report of the FIFRA Scientific Advisory Panel which
reviewed this data was also made available (US EPA, 1981). Table 13
summarizes the basic design of each of these studies, some of which are
also discussed in section 7.5.
7.6.1 Mouse
One of the four mouse studies referenced in Table 13 (FMC I) was
not considered for evaluation because of dose level changes in the mid-
and high-level groups and problems in histopathological methodology.
7.6.1.1 ICI study
Relevant non-oncogenic effects observed during the study consisted
of increased mortality, increased liver aminopyrine- N-demethylase ac-
tivity, increased liver weight, and eosinophilia of hepatocytes in both
males and females at 2500 mg/kg diet. The liver changes observed in
this study were considered to be related in large measure to the
induction of liver microsomal enzyme activity. Minimal liver changes
were also observed at 1000 mg/kg, but not at 250 mg/kg. A slight
increase in lung adenomas was observed in male mice at the highest dose
level. However, there was some uncertainty as to whether this increase
was related to permethrin ingestion.
7.6.1.2 FMC II study
Relevant non-oncogenic effects observed during the study consisted
of increased mortality in males at 2000 mg/kg diet, increased liver
weight in females at 2500 mg/kg and 5000 mg/kg, and increased lung
weight in females at 5000 mg/kg. Histopathologically, dose-related
"focal alveolar cell proliferation" (increased numbers of lung cells)
was observed in permethrin-treated females. As regards oncogenic
effects, there was an increased incidence of bronchio-alveolar adenomas
in female mice only. The number of female mice with adenomas and/or
carcinomas (15/74, 24/72, 35/74, and 44/75 at the four dose levels)
revealed a statistically significant dose-response relationship. Male
mice did not show this effect. However, some doubt was expressed by
the FIFRA Scientific Advisory Panel concerning the conduct of this
study.
7.6.1.3 BW study
Non-oncogenic effects observed during the study consisted of
slightly decreased mortality in females at 50 and 250 mg/kg per day,
increased liver weights in males, and increased kidney weights in
females at 250 mg/kg per day. Histopathologically, an increased inci-
dence in cuboidal/columnar metaplasia of the alveolar epithelium was
observed in the lungs of male and female mice at the highest dose. The
oncogenicity data indicated a dose-related trend in females, but not in
males, for adenomatous tumours in the lungs. No notable pattern was
observed for other neoplasms at any dose level.
7.6.1.4 Appraisal of mouse studies on carginogenicity
Consistent findings in the above three mouse studies at high dose
levels were liver changes known to be associated with the induction of
the liver microsomal enzyme system. Other histopathological effects
observed in liver, not usually associated with microsomal induction,
included multifocal hepatocytomegaly and hepatocytic pigmentation. The
incidence of lung adenomas for each of the three mouse studies is given
in Table 14.
Among the three long-term mouse studies, there was evidence of
permethrin oncogenicity in the lungs in one strain (CD-1 female only)
at the highest dose level only.
Although there was a difference between the control and treated
groups in terms of lung adenomas in these studies, these differences
were not significant when compared with historical control values. The
oncogenicity potential, as evaluated by the FIFRA Scientific Advisory
Panel, was considered to be very weak.
Table 13. Chronic toxicity and carcinogenicity studies in mice and rats
------------------------------------------------------------------------------------------------------
Study
performed Species Strain No. of Duration Dose Reference
by animals (weeks) (mg/kg diet)
------------------------------------------------------------------------------------------------------
ICI mouse Alderly Park 70 98 0, 250, 1000, 2500 Ishmael & Litchfield (1988)
(SPF)
FMC I mouse CD-1 75 104 0, 20, 500, 4000 Hogan & Rinehart (1977);
Rapp (1978)
FMC II mouse CD-1 75 104 M:0, 20, 500, 4000 Bio Dynamics (1979)
F:0, 20, 2500, 5000
BW mouse CFLP 75 92 0a, 10a, 50b, 250b James et al. (1980)
ICI rat Wistar 60 104 0, 500, 1000, 2500 Ishmael & Litchfield (1988)
FMC rat Long-Evans 60 104 0, 20, 100, 500 Braun & Rinehart (1977);
Billups (1978a,b)
BW rat Wistar 60 104 0, 10b, 50b, 250b McSheehy & Finn (1980)
------------------------------------------------------------------------------------------------------
a 100 animals as control.
b mg/kg body weight; permethrin 25% cis, 75% trans.
Table 14. Comparison of lung adenomas (%) in three mouse studies
------------------------------------------------------------------------
Male Female
control low mid high control low mid high
dose dose dose dose dose dose
------------------------------------------------------------------------
ICI study 20 12 26 32 22 16 20 30
FMC II study 22 23 28 25 16(20a) 17 35 35
BW study 26 19 23 22 3(20b) 7 10 20
------------------------------------------------------------------------
a Historical control values ranged from 23 to 60%, with a mean
of 20.4%.
b Historical control values ranged from 7.5 to 30.0%, with a mean
of 20.4%.
7.6.2 Rat
One of the three long-term rat studies referred to in Table 13,
i.e. the FMC rat study, was excluded from examination because of
serious flaws in histopathological methodology.
7.6.2.1 ICI study
Relevant non-oncogenic effects observed consisted of increased
mortality in males and decreased mortality in females at 2500 mg/kg
diet, increased liver weights in both males and females at 1000 and
2500 mg/kg and in males only at 500 mg/kg, increased liver aminopyrine-
N-demethylase activity in both males and females at 1000 and 2500
mg/kg, and hepatocyte vacuolization or hypertrophy in males and females
at 1000 and 2500 mg/kg. Additional effects observed were increased
kidney weight in males at all treatment levels and increased pituitary
weight in males at 1000 and 2500 mg/kg.
No tumours related to the ingestion of permethrin were observed in
this study.
7.6.2.2 BW study
Non-oncogenic effects observed at 250 mg/kg per day were increased
mortality in males, occasional body tremors in males and females, in-
creased liver weight in males, hepatocyte hypertrophy in males and fe-
males, and focal changes of the thyroid follicles in males and females.
The microscopic liver and thyroid changes were also observed at 50
mg/kg per day in both sexes.
With respect to tumours (including rare, unusual, or malignant neo-
plasms), none of the tumour types observed in this study were con-
sidered to be related to the ingestion of permethrin.
7.6.2.3 Appraisal of rat studies on carcinogenicity
No evidence of oncogenicity was observed in the rat studies.
7.7 Mutagenicity
7.7.1 Microorganism and insects
The mutagenic activity of permethrin was evaluated using the Ames
test. There was no increase in the number of revertant colonies at
doses up to 2500 µg permethrin/plate in five strains of Salmonella
typhimurium (TA1535, TA1537, TA1538, TA98, and TA100) with or without
S9-mix prepared from rat liver or S9 prepared from PCB-treated mice
(Longstaff, 1976; Newell & Skinner, 1976; Simmon, 1976; Suzuki, 1977).
Permethrin and six other synthetic pyrethroids were tested for
mutagenicity in S. typhimurium TA98 and TA100 strains in the presence
and absence of a metabolic-activation system. All pyrethroids tested
gave negative results (Pluijmen et al., 1984).
Two reverse-mutation tests in Escherichia coli WP2 also gave nega-
tive results (Newell & Skinner, 1976; Simmon, 1976).
When tested for mutagenicity in V79 Chinese hamster cells, permeth-
rin and five other synthetic pyrethroids were shown to be non-mutagenic
(Pluijmen et al., 1984).
In a host-mediated assay, permethrin (200 mg/kg body weight) was
orally administered to ICR mice. The indicator organism, S. typhimurium
G46, harvested from the abdominal cavity of mice 3 h after treatment,
did not reveal any mutagenic effect (Shirasu et al., 1979). In another
host-mediated assay employing a similar test system, (+)- trans-permethrin
at dose levels of 600 and 3000 mg/kg body weight and (+)- cis-permethrin
at 21 and 54 mg/kg body weight gave negative results (Miyamoto, 1976).
Permethrin was tested for its ability to induce complete and
partial chromosome loss in Drosophila melanogaster males by adding
5 mg/litre (soaked onto a filter paper) to the feeding solution.
Treated males were mated with mus-302 repair-defective females to
detect chromosome loss in the zygotes. Permethrin did not induce a
significant increase in chromosome loss, compared to negative controls
(Woodruff et al., 1983).
7.7.2 Mammals
An in vivo cytogenetic test was performed in Alderly Park rats to
assess the ability of permethrin to induce chromosomal aberration.
Permethrin was administered to groups of eight males by a single intra-
peritoneal injection or by five daily injections at doses of 600, 3000,
or 6000 mg/kg. The cytogenetic effect on bone marrow cells was evalu-
ated 24 h after the single injection and 6 h after the last multiple
dosing. No differences were observed in the rate of chromosomal aber-
rations between any permethrin-treated groups and the vehicle controls.
Two positive controls (trimethyl phosphate and mitomycin C) produced a
significantly higher incidence of chromosomal aberrations (Anderson &
Richardson, 1976).
Permethrin (25:75) gave a negative response when mouse lymphoma
L5178Y cells were treated with permethrin (up to 125 µg/ml) with or
without activation (Clive, 1977).
In dominant lethal studies, permethrin dissolved in corn oil was
administered orally for five successive days to groups of male CD mice
(15 per group) at doses of 15, 48, or 150 mg/kg. Each male was mated
with 16 virgin females, and on the 12th day of gestation the females
were killed. There was no dose-related effect on pregnancy or early or
late fetal deaths. Administration of permethrin thus had no dominant
lethal effect on male mice. On the other hand, the positive control
(ethylmethanesulfonate) induced pre-implantation losses and the early
death of embryos (McGregor & Wickramaratne, 1976a; Chesher et al.,
1975b).
7.8 Teratogenicity and Reproduction Studies
7.8.1 Teratogenicity studies
7.8.1.1 Mouse
In studies by Kohda et al. (1976b), groups of pregnant ICR mice (27
to 32 mice per group) were orally administered permethrin at dose
levels of 0, 15, 50, or 150 mg/kg body weight from day 7 to day 12 of
pregnancy. On day 18, two-thirds of the animals were sacrificed and
examined for implantation and resorption sites. Viable offspring were
examined for somatic and skeletal abnormalities, and, after 3 weeks of
lactation, pups were examined for behavioral abnormalities and for
differentiation and growth. At 6 weeks of age, all animals were sacri-
ficed and subjected to internal and external examination. There were no
effects on maternal toxicity over the course of the study. Growth and
differentiation of pregnant females were not affected by permethrin,
nor were the number of implantation sites or litter size adversely
affected. The size of individual pups and the incidence of gross
external, internal, and skeletal abnormalities were not significantly
different from those in the control mice. Permethrin, at dose levels
up to and including 150 mg/kg, did not appear to affect those animals
allowed to bear and wean young. The growth of young animals did not
appear to differ from control values, and, 3 weeks after weaning, the
surviving animals did not differ from controls with respect to growth
or major organ changes. There was no teratogenicity associated with
permethrin in this mouse bioassay, although the duration of dosing was
a little too short to cover both the early and late stage of organ
development (Kohda et al., 1976b).
7.8.1.2 Rat
In studies by McGregor & Wickramaratne (1976b), pregnant CD rats
(20 rats per group) were orally administered permethrin at dose levels
of 0, 22.5, 71.0, or 225 mg/kg from day 6 to day 16 of gestation. On
day 20, the animals were sacrificed and the corpora lutea were exam-
ined. Somatic and skeletal investigations were performed on the
fetuses. No adverse toxicological response was seen at the highest dose
used. There were no abortions or maternal deaths and no significant
differences in pregnancy frequency, corpora lutea, or total number of
implantations between treated and control rats. Placental and fetal
weights were similar to those of the controls and no skeletal or struc-
tural abnormalities were observed. Based upon the standard teratologi-
cal rat bioassay, permethrin did not show any teratological potential.
Pregnant Sprague-Dawley rats (29-34 rats per group) were orally
administered permethrin at dose levels of 0, 10, 20, or 50 mg/kg body
weight from day 9 to day 14 of pregnancy. On day 20, approximately two-
thirds of the pregnant females were sacrificed and the remaining rats
were allowed to deliver and wean pups. After lactation, the pups were
examined for behaviour and for growth and differentiation before being
sacrificed at 6 weeks of age and examined for internal and external
gross malformations. Pregnant females fed at the highest dose showed
toxic signs of poisoning, including ataxia, tremor, and a slight
reduction in body weight. There was no mortality, although fetal loss
at the highest dose level was slightly higher than that in the control
animals. A slightly higher incidence of non-ossified sternebra was
noted at 50 mg/kg. The number of implantation sites and the litter size
were not affected, and growth and differentiation were similarly unaf-
fected. Internal and external examination showed that, with the excep-
tion of the slight skeletal variation noted at 50 mg/kg, there were no
permethrin-associated changes. In those animals allowed to bear and
wean pups, there were no notable differences from control values with
respect to gestation, implantation sites, delivery, and numbers of live
young. Growth and differentiation of the offspring did not appear to
be affected by permethrin, and there were no abnormalities with respect
to gross pathology or in the weights of major tissues and organs at the
conclusion of the study. Permethrin did not elicit a teratogenic effect
in this bioassay (Kohda et al., 1976a).
In a study by Metker et al. (1977), permethrin (4, 41, and 83
mg/kg/diet), aspirin (200 mg/kg diet), and corn oil (2 ml/kg diet) were
each administered to groups of 20 pre-impregnated Sprague-Dawley rats
from day 6 to day 16 of gestation. The animals were sacrificed on day
20 of gestation, and the fetuses were removed and examined for gross
abnormalities, sex, weight, and body length. The administration of
aspirin (the positive control) resulted in significantly lower body
weight and length and a variety of abnormalities including craniorachi-
oschisis in the foetuses. Permethrin, administered to pregnant rats
during gestation by intragastric intubation, did not appear to present
a teratogenic or lethal hazard to the developing fetus.
When groups of 22 female Wistar rats received permethrin (25:75) at
0 or 200 mg/kg body weight in corn oil by daily oral gavage on days 6-
16 (inclusive) of pregnancy, treatment was without apparent effect on
maternal body weight gain or general conditions. The animals were
sacrificed on day 20 so that their uterine contents could be examined.
Treatment had no effect on the number of corpora lutea, implantations,
live fetuses, early and late fetal deaths, or fetal abnormalities.
Examination of the fetuses, which included dissection and skeletal
staining, showed no morphological effects of treatment. These results
indicate that permethrin (25:75) at 200 mg/kg body weight per day is
not fetotoxic to rats (James, 1974).
7.8.1.3 Rabbit
Mated female Dutch rabbits (18 per group) were orally administered
permethrin (at dose levels of 0, 600, 1200, or 1800 mg/kg body weight
per day) in 0.5% v/v aqueous Tween 80 from days 6-18 inclusive of
pregnancy. On day 29 of pregnancy the animals were killed and their
uteri were examined for resorptions and live implantations. The fetuses
were examined for gross abnormalities of skeleton and soft tissue. At
all dose levels, permethrin depressed body weight gain during dosing
and was embryotoxic at the two highest dose levels. It was toxic to
the dams at 1800 mg/kg body weight per day, but no teratogenic activity
was detectable at any dose level (Richards et al., 1980).
7.8.2 Reproduction Studies
7.8.2.1 Rat
Groups of Long-Evans rats (10 males and 20 females per group) were
fed permethrin at dose levels of 0, 20, and 100 mg/kg diet in a 3-
generation (two litters per generation) reproduction study. There was
no effect on mortality, mating, pregnancy, or fertility, with the
exception of the F2 mating index, which was reduced in both controls
and treatment groups. Pup survival and growth were unaffected. Haema-
tological evaluations of F2 adults between the second and third mating
showed no unusual effects. This study indicated that dietary permethrin
does not adversely affect reproduction in the rat (Schroeder &
Rinehart, 1977).
In studies by Hodge et al. (1977), groups of Wistar rats (12 males
and 24 females per group) were fed permethrin at dose levels of 0, 500,
1000, and 2500 mg/kg diet for 12 weeks. At 12 weeks the animals were
mated to initiate a standard 3-generation (two litters per generation)
reproduction study. Clinical signs of acute poisoning (tremors, etc.)
were noted, predominantly in females given the highest dose. There
were no effects attributable to permethrin on fertility, gestation,
viability of pups, sex ratio, litter size, or lactation. Ten male and
female weanlings from the second litter of the F3 generation were
examined for histopathological changes. Centrilobular hypertrophy and
cytoplasmic eosinophilia were observed at all dose levels, the inci-
dence and severity of which appeared to be dose dependent. Rats in the
third litter of the F3 generation were sacrificed on day 12 of ges-
tation for teratogenic examination, but no abnormalities were observed.
Based on the results of this study, permethrin does not appear to
induce reproductive toxicity in rats.
Spencer and Berhance (1982) fed female Sprague-Dawley rats (5-8
rats per group) permethrin in the diet at levels of 0, 500, 1000, 1500,
2000, 2500, 3000, 3500, and 4000 mg/kg diet from day 6 to day 15 of
pregnancy. Laparotomy was performed on day 20 of gestation, and the
number of live fetuses was determined. Placentae were excised and
cleaned of extraneous connective tissue. Analysis of the protein and
glycogen contents of the placentae on day 16 of pregnancy indicated
that they were only influenced by very high doses (2500-4000 mg/kg
diet) of permethrin. Analysis of variance indicated no significant
effect on protein level, but the treatment did decrease the glycogen
concentration. No significant dose-related effects on implantational
sites/intrauterine fetuses were observed. These results appeared to
confirm that permethrin possesses low mammalian toxicity.
In a 3-generation reproduction study, groups of 20 male and 20 fe-
male Wistar COBS rats received permethrin (25:75) in the diet at 0, 5,
30, and 180 mg/kg body weight per day during growth, mating, gestation,
parturition, and lactation for three generations, each with two
litters. Fetal toxicity and teratogenicity was assessed in the second
pregnancy of the F2 generation. Treatment with permethrin had no ef-
fect on general behaviour or condition, food intake, body weight gain,
or pregnancy rate of the dams, or on parturition, sex ratio, or pup
weight. A small number of rats of each group developed eye abnor-
malities, including ocular haemorrhage and chronic glaucoma, but this
was not related to the treatment. Examination of F3b fetuses showed
no treatment-related effect on sex ratio, body weight, or the occur-
rence of visceral or skeletal abnormalities. This study indicated that
permethrin (25:75) has no effect on the reproduction of rats at doses
up to 180 mg/kg body weight per day (James, 1979).
7.9 Neurotoxicity
7.9.1 Rat
When male and female Charles River rats (six of each sex per group)
were fed permethrin at dose levels of 0 or 6000 mg/kg diet for up to
14 days, severe clinical signs of poisoning were evident in all the
permethrin-treated rats. Only one permethrin-treated male survived the
14-day trial. Fragmented and swollen sciatic nerve axons and myelin
degeneration were observed in four out of five permethrin-treated ani-
mals (Hend & Butterworth, 1977).
In a short-term study designed to assess the effects of high con-
centrations of permethrin on the sciatic nerve, male Wistar rats (10
per group) were fed permethrin at dose levels of 0, 2500, 3000, 3750,
4500, 5000, and 7000 mg/kg diet for 14 days. Clinical signs of acute
poisoning and death occurred in the animals that were dosed at 5000 or
7000 mg/kg. Some rats that received the lower dose levels showed slight
to moderate tremors, and food consumption and growth were reduced in
these animals. At the two lowest dose levels, clinical signs of poison-
ing disappeared within the first week whereas, at the higher dose
levels, signs of poisoning persisted throughout the study. Rats
receiving permethrin at doses of up to 4500 mg/kg showed no ultra-
structural changes in their sciatic nerve. A variety of mild ultra-
structural changes, such as vacuolation and swelling of unmyelinated
fibres and hypertrophy of Schwann cells, were observed in the nerves of
rats receiving 5000 mg/kg (Glaister et al., 1977).
A detailed morphological evaluation of the nervous system was per-
formed on rats in two long-term feeding studies. In the first, Long-
Evans rats were fed diets containing permethrin at concentrations of 0,
20, 100, or 500 mg/kg diet for 2 years, and five male and five female
randomly selected survivors from each group were examined. In the
second study, Long-Evans rats were fed diets containing permethrin at
concentrations of 0, 20, or 100 mg/kg diet for three successive gener-
ations, and five male and five female rats from each group were ran-
domly selected from the third generation parental animals. Examination
of central and peripheral nerves and of extensive morphometric data and
teased myelinated fibers of distal sural and tibial nerves and of the
maxillary division of the fifth cranial nerve did not reveal any
changes attributable to the feeding of the pesticide (Dyck et al.,
1984).
When groups of 10 male and 10 female Sprague-Dawley rats were given
permethrin (25:75) (94.5% pure) at 4000, 6000, or 9000 mk/kg diet for
21 days, all animals developed severe trembling and lost weight. Some
of the highest-dose rats of each sex died. Subsequent examination of
brain, spinal cord, trigeminal and dorsal root ganglia, proximal and
distal root trunks, and terminal motor and sensory nerves revealed no
consistent histopathological abnormalities (Dayan, 1980).
7.9.2 Hen
Hens were administered permethrin orally (cis:trans=1:1) (as a 40%
w/v solution in dimethylsulfoxide) at a daily dose level of 1 g/kg body
weight for 5 days. After 3 weeks, the dosing regimen was repeated, and
the animals were maintained for an additional period of 3 weeks before
being sacrificed. There were no signs of neurological disturbance or
mortality in any of the animals. All hens treated with tri- ortho-cresyl phos-
phate (TOCP) (positive control) displayed clinical and histopathologi-
cal evidence of neurotoxicity, whereas none of the birds dosed with
permethrin showed any signs of intoxication. Histological examination
of nerve tissues revealed no lesions. Hence, permethrin was considered
to have no delayed neurotoxic potential such as that associated with
certain organophosphates (Millner & Butterworth, 1977).
In studies by Ross & Prentice (1977), 15 adult hens were orally
administered permethrin at 9 g/kg body weight and again 21 days later.
After a further 21 days, they were sacrificed. All positive control
animals (given TOCP at 500 mg/kg) showed signs of delayed neurotoxicity
ranging from slight muscular incoordination to paralysis. No signs of
ataxia were recorded in any of the hens in the permethrin-treated or
negative control groups. Histopathological examination of the nervous
tissues of permethrin-treated animals revealed none of the degenerative
changes noted in the tissues of the positive controls.
7.10 Behavioural Effects
Behavioural observations were carried out on immature male Sprague-
Dawley rats habituated to inhalation of permethrin aerosols. Habitu-
ation was carried out by exposing three groups of rats (five per group)
to aerosols of permethrin firstly at 500 mg/m3 for 21 days, then at
1000 mg/m3 for an additional 21 days. Three other groups of rats (five
per group) served as controls; they were similarly treated but were not
exposed to permethrin. At the end of this conditioning period, all
rats, including the controls, were exposed to a permethrin aerosol at
5000 mg/m3 for 4 h. At the end of the habituation period, there were
no differences in retention of avoidance training or the ability to
learn the same task between controls and aerosol-exposed groups. How-
ever, after exposure to permethrin at 5000 mg/m3, the non-habituated
control group of rats showed significantly lower retention capacity
compared with the habituated rats or with their own pre-exposure per-
formances. The non-habituated control rats also showed decreases in
coordination and balance and a higher incidence of conflict behaviour
and tremors. The performance of the rats in the habituated groups was
not changed (Sherman, 1979).
7.11 Miscellaneous Studies
The pharmacological action of permethrin on nictitating membrane,
blood pressure, respiration, heart rate, and isolated ileum was inves-
tigated in the rabbit, guinea-pig, and cat. Permethrin reduced the
incidence and amplitude of contraction of isolated rabbit ileum but
induced no changes in a similar preparation from the guinea-pig. Intra-
venous administration of permethrin at doses of 4 mg/kg or more affec-
ted blood pressure and respiration in all animals. The hypotensive
effect was not affected by pretreatment with atropine or propanolol.
Permethrin was shown to produce slight contraction of the nictitating
membrane. An increase in pulse rate was observed in the electrocardio-
gram (ECG) of the rabbit at dose levels above 4 mg/kg, but was not
accompanied by changes in the wave pattern (Nomura & Segawa, 1979).
In the Japanese White rabbit, the intravenous administration of
lethal doses of permethrin caused changes in the electroencephalogram
(EEG) tracings. Spike waves and an increased amplitude of slow waves
were induced at 100 mg/kg body weight. At a sub-lethal dose of 30
mg/kg, permethrin did not induce changes in the EEG (Takahashi et al.,
1979).
There was no change in hexobarbital-induced sleeping time in ICR
mice intraperitoneously administered a single dose of permethrin at
dose levels of up to 2000 mg/kg body weight (Takahashi et al., 1979).
Sprague-Dawley rats (three groups of 10 rats each) were pretreated
for four consecutive days with an intraperitoneal injection of either
sodium phenobarbital at 100 mg/kg (positive control group), permethrin
at 575 mg/kg (test group), or corn oil at 2.0 ml/kg (solvent control
group). On the fifth test day the rats received an intraperitoneal
injection of hexobarbital (220 mg/kg). The hexobarbital-induced sleep-
ing times of the permethrin-treated rats were significantly shorter
than those of the solvent control animals but were similar to those of
the phenobarbital positive control (Metker et al., 1977).
Permethrin and cypermethrin were evaluated for their ability to
alter microsomal cytochrome P-450 and NADPH cytochrome c reductase in
Long-Evans rats. When permethrin (cis:trans = 80:20) was orally admin-
istered to rats at 50 mg/kg body weight per day, it increased cyto-
chrome P-450 after 4, 8, or 12 days of administration and NADPH cyto-
chrome c reductase after 8 or 12 days, whereas cypermethrin (alpha-cyano
analogue of permethrin) did not induce either cytochrome P-450 or the
reductase. Neither of the two pyrethroids altered body weight gain
(Carlson & Schoening, 1980).
7.12 Mechanism of Toxicity (Mode of Action)
Based on the signs of toxicity to mammals (Verschoyle & Aldridge,
1980; Lawrence & Casida, 1982) and to cockroaches (Gammon et al.,
1981), pyrethroids may be classified into two types: Type I and Type II
compounds (see Appendix I). 1R- cis- and 1R- trans-permethrin belong
to Type I. Electrophysiological recordings from dosed cockroaches
reveal trains of cercal sensory spikes and, sometimes, spike trains
from the cercal motor nerves and the central nervous system. The signs
of poisoning caused by Type I pyrethroid compounds are restlessness,
incoordination, hyperactivity, prostration, and paralysis (Gammon et
al., 1981).
1RS- cis-permethrin and 1RS- trans-permethrin cause tremor (known
as T-syndrome) when injected intravenously into rats at a dose level of
more than 270 mg/kg body weight. The onset of the T-syndrome is usually
rapid. Rats suffering from T-syndrome exhibit aggressive sparring be-
haviour and increased sensitivity to external stimuli. This is followed
by the appearance of a slight tremor, which gradually becomes more
severe and finally reaches a state of prostration and vigorous whole
body tremor. The core temperature is markedly increased during poison-
ing; this may result from the excessive muscular activity associated
with tremor (Verschoyle & Aldridge, 1980).
Exposure of sensory nerve fibres from clawed frogs (Xenopus laevis)
to permethrin (10-7 to 10-5 mol/litre) resulted in marked repetitive
activity. This heightened activity was not observed in the motor
fibres of frogs that were similarly tested. Treatment of isolated
lateral-line preparations of frogs with permethrin (5 x 10-6 mol/litre)
also resulted in pronounced repetitive activity (Van den Bercken &
Vijverberg, 1980b).
Permethrin (cis, trans, and technical grade) and deltamethrin, as
representatives of the non-cyano- and cyano-containing classes,
respectively, of synthetic pyrethroids, were examined regarding their
major site of action on the mammalian nervous system in mice. ED50
values for the ability of both types of pyrethroids to cause pros-
tration and loss of righting reflex were estimated following either
intravenous or intracerebroventricular injections. The comparative
potencies of the four pyrethroids (deltamethrin > cis-permethrin >
technical grade permethrin > trans-permethrin) were the same following
either route of administration. All four compounds tested showed a much
greater potency (> 200-fold for deltamethrin, cis-permethrin, and
technical grade permethrin, and 85-fold for trans-permethrin) after
intracerebroventricular administration than after intravenous admin-
istration. In addition, the poisoning symptoms seen following direct
central injection were almost identical to those obtained with periph-
eral administration. These results suggest that poisoning from both
classes of pyrethroids in mammals is due predominantly to central
mechanisms (Staatz et al., 1982).
8. EFFECTS ON HUMANS
Although permethrin has been used for many years, no human poison-
ing cases have been reported.
8.1 Occupational Exposure
Data on permethrin human toxicity are scanty. Studies of occu-
pational exposure to permethrin were reported in Sweden (Kolmodin-
Hedman et al., 1982). In the first study, six forestry workers using
an aqueous emulsion of permethrin (trans:cis=75:25) were studied. The
duties of these workers involved dipping conifer seedlings in a 2%
aqueous emulsion of permethrin (one worker) and packaging the
permethrin-treated seedlings (five workers). The permethrin concen-
trations in the breathing zones of these workers varied between 0.011
and 0.085 mg/m3. One person excreted permethrin metabolites at
0.26 µg/ml urine the following morning, but the same afternoon the
urinary excretion of permethrin residues was below the detection limit
of 0.1 µg/ml. The urine from other workers did not contain detectable
amounts of permethrin residues. No symptoms of permethrin poisoning
were reported in this field study. The second study, performed on the
basis of a questionnaire and interviews, was conducted 1-2 months after
the planting season. It involved 87 persons at various plant nurseries
that used the insecticide (trans:cis=60:40 or 75/25). This study showed
that the major work-related symptoms amongst workers were irritative,
such as itching and burning of the skin, and itching and irritation of
the eyes. Irritative symptoms in the skin and upper respiratory tract
were reported in 63% of workers who were exposed to permethrin
(trans:cis=75:25) and 33% who were exposed to permethrin with a
different isomeric composition (trans:cis=60:40). The frequency of each
symptom was about 10% in each case. Increased nasal secretion was
reported by 13% of the workers handling permethrin (trans:cis= 75:25).
Le Quesne et al. (1980) examined 23 laboratory workers involved in
field trials, formulation, or general laboratory work with permethrin,
cypermethrin, fenvalerate, and fenpropathrin. The study involved
electrophysiological monitoring and interviews to ascertain subjective
symptoms. The most frequently reported symptom was a facial sensation
described as tingling, burning, or nettle rash by workers who had ex-
perienced it on one or more occasions. This sensation usually occurred
about 30 min to 3 h after exposure and lasted for about 30 min to 8 h.
Apparently this did not occur when permethrin alone was involved. All
the workers were examined neurologically and no abnormal findings were
recorded. Electro-physiological measurements from these workers were
compared with those of an age-matched control group. No difference in
response was found between the two groups.
Studies of pesticide contamination of clothing worn by crop con-
sultants during permethrin application to soybean fields were performed
to assess the extent of dermal exposure to the pesticide. The suits
and T-shirts were removed and wrapped in aluminum foil, placed on ice
and transported to the laboratory where they were held in a freezer
until residue analysis was performed. Specimens from the thigh, arm,
and chest of each suit and from the arm and chest of each T-shirt were
collected, extracted with hexane and analyzed by GC-ECD. Measurable
residues of permethrin were detected only in leg specimens (Cloud et
al., 1987).
As part of an evaluation of permethrin (25:75) (5% wettable powder)
in Nigeria, medical surveillance, including urinalysis of staff engaged
in bagging, mixing, or spraying, was undertaken. Medical history,
pulse, and blood pressure were recorded and urine was collected twice
daily. This surveillance revealed no effects attributable to permeth-
rin. Despite the use of protective clothing, up to 2 mg of permethrin
was excreted within 24 h of exposure (Rishikesh et al., 1978).
8.2 Clinical Studies
Flannigan & Tucker (1985) and Flannigan et al. (1985a,b) studied
the difference in the degree of paraesthesia induced by a number of
pyrethroids. On five occasions, 0.05 ml of field-strength-formulated
permethrin (0.13 mg/cm2) was applied to a 4 cm2 area of earlobe.
The opposite earlobe received distilled water. Participant evaluation
after each application continued for 48 h and involved description of
the cutaneous sensations. Each participant was treated after each
application with one of the remaining compounds. Permethrin, like the
other pyrethroids, induced skin sensations. Paraesthesia developed with
a latency period of approximately 30 min, peaked by 8 h, and deterio-
rated within 24 h. In the case of permethrin these sensations were
approximately four times less marked than those induced by cypermethrin
and fenvalerate, which both contain an alpha-cyano-group. It was also
found that local application of dl-alpha-tocopheryl acetate markedly
inhibited the occurrence of skin sensations.
To assess the safety of permethrin dusts for the control of human
body lice, approximately 350 people were individually dusted with 50 g
of powder containing either 2.5 or 5.0 g permethrin/kg. Urine samples,
taken at the time of treatment and subsequently, indicated that maximal
absorption of permethrin was 39 µg/kg, 24 h after treatment (Nassif
et al., 1980).
In a study to assess the degree of dermal absorption of permethrin
from impregnated clothing, a group of 10 male volunteer soldiers for
48 h wore military clothing that had previously been treated with an
aqueous suspension of permethrin (0.2% w/v). Subsequent analysis
showed that the mean permethrin (25:75) concentration of the shirts
and trousers was 0.32 g/100 g. However, the average individual
exposure to permethrin was 3.8 mg/day. No volunteers complained of
irritation and there were no abnormal findings on physical examination
(Farquhar et al., 1981a).
When dermally exposed to permethrin [(25:75) 1% w/w in soft
paraffin] for up to 9 days using a patch test, 2 out of 17 volunteers
developed mild erythema (Pegum & Doughty, 1978).
To assess the human tolerance, absorption, and persistence of
permethrin when used against human lice, 10 adult volunteers (four men,
six women) were treated with 15-40 ml of permethrin (25:75) (1%) head
louse solution. Their hair was allowed to dry naturally and then
washed with baby shampoo. Urine samples were collected at 0-24, 24-48,
120-144, and 336-360 h to measure dermal absorption. On assessment, 3
out of 10 volunteers developed mild, patchy erythema, which faded
between days 4-7. Permethrin excretion during the first 24 h was only
about 1% of the applied dose, while the cumulative maximum over 14 days
was only about 5.5 mg (Farquhar et al., 1981b).
9. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
The Joint FAO/WHO Meeting on Pesticide Residues (JMPR) discussed
and evaluated permethrin in 1979, 1980, 1981, 1982, 1983, 1984, 1985,
and 1987 (FAO/WHO, 1980a,b; 1981a,b; 1982a,b; 1983a,b; 1984a,b;
1985a,b; 1986a,b; 1987).
An acceptable daily intake (ADI) of 0-0.05 mg/kg body weight
(cis:trans ratios of 40:60 and 27:75) was established in 1985.
Maximum residue limits of 0.01-50 mg/kg for specified foods and 20-
100 mg/kg dry weight for specified feed have been proposed (FAO/WHO,
1986).
In the WHO recommended classification of pesticides by hazard,
permethrin as a technical product is classified in class II (i.e., as
moderately hazardous) (WHO, 1988). A WHO/FAO data sheet on permethrin
exists (WHO/FAO, 1984).
REFERENCES
AGNIHOTRI, N.P., JAIN, H.K., & GAJBHIYE, V.T. (1986) Persistence of
some synthetic pyrethroid insecticides in soil, water and sediment -
Part I. J. entomol. Res., 10: 147-151.
ALINIAZEE, M.T. & CRANHAM, J.E. (1980) Effect of four synthetic
pyrethroids on a predatory mite, Typhlodromus pyri, and its prey,
Panonychus ulmi, on apples in southeast England. Environ. Entomol., 9:
436-439.
ANDERSON, D. & RICHARDSON, C.R. (1976) Permethrin (PP557):
cytogenetic study in the rat (Report No. CTL/P/296) (Unpublished
report submitted to WHO by ICI Central Toxicology Laboratory).
ANDERSON, R.L. (1982) Toxicity of fenvalerate and permethrin to
several nontarget aquatic invertebrates. Environ. Entomol., 11: 1251-
1257.
BAKER, P.G. & BOTTOMLEY, P. (1982) Determination of residues of
synthetic pyrethroids in fruit and vegetables by gas-liquid and high-
performance liquid chromatography. Analyst, 107: 206-212.
BATTELLE (1982) Pesticide programme of research and market planning.
Part I: Insecticides, Geneva, Institut Battelle (October 1982).
BATTELLE (1986) World Pesticide Programme, Insecticide II, 1984,
Geneva, Institut Battelle.
BILLUPS, L.H. (1978a) Histopathologic examination of a twenty-four
month toxicity/carcinogenicity study of compound FMC33297 in rats, (Un-
published report submitted to WHO by FMC Corporation, Environmental
Pathology Services).
BILLUPS, L.H. (1978b) Twenty-four month toxicity/carcinogenicity
study of compound FMC33297 in rats (Unpublished report submitted to
WHO by FMC Corporation, Environmental Pathology Services).
BIO-DYNAMICS (1979) Twenty-four month toxicity/carcinogenicity study
of permethrin in mice (Bio-Dynamics Inc. Project No. 76-1695/9)
(Unpublished report submitted to WHO by FMC Corporation).
BORTHWICH, P.W. & WALSH, G.E. (1981) Initial toxicological assessment
of Ambush, Bolero, Bux, Dursban, Fentrifanil, Larvin, and Pydrin:
Static acute toxicity tests with selected estuarine algae,
invertebrates, and fish, Springfield, Virginia, National Technical
Information Service, pp. 1-9 (EPA-600/4-81-076).
BRADBROOK, C., BANHAM, P.B., GORE, C.W., PRATT, I., & WEIGHT,
T.M. (1977) PP557: A study of the reversibility of hepatic
biochemical and ultrastructural changes in the rat (Report No.
CTL/P/354) (Unpublished Data submitted to WHO by ICI Central Toxicology
Laboratory).
BRAUN, W.G. & KILLEEN, J.C. (1975) Acute oral toxicity in rat:
compound no. FMC33297 (Bio-Dynamics Inc. Project) (Unpublished report
submitted to WHO by FMC Corporation).
BRAUN, W.G. & RINEHART, W.E. (1977) A twenty-four month oral
toxicity/carcinogenicity study of FMC33297 in rats (Bio-Dynamics Inc.
Project) (Unpublished report submitted to WHO by FMC Corporation).
BRAUN, H.E. & STANEK, J. (1982) Application of the AOAC multi-residue
method to determination of synthetic pyrethroid residues in celery and
animal products. J. Assoc. Off. Anal. Chem., 65: 685-689.
BUTTERWORTH, S.T.G. & HEND, R.W. (1976) Toxicity studies on the
insecticide WL43470: a five week feeding study in rats (Report No.
TLGR.0056.76) (Unpublished data submitted to WHO by Shell Research
Ltd).
CARLSON, G.P. & SCHOENIG, G.P. (1980) Induction of liver microsomal
NADPH cytochrome c reductase and cytochrome P-450 by some new synthetic
pyrethroids. Toxicol. appl. Pharmacol., 52: 507-512.
CARROLL, B.R., WILLIS, G.H., & GRAVES, J.B. (1981) Permethrin
concentration of cotton plants, persistence in soil and loss in runoff.
J. environ. Qual., 10: 497-500.
CHESHER, B.C. & MALONE, J.C. (1974a) 10-day cumulative oral toxicity
study with 21Z73 in rabbits, Berkhamsted, Wellcome Research
Laboratories (Report No. HEFG 74-7) (Unpublished data submitted to
WHO).
CHESHER, B.C. & MALONE, J.C. (1974b) Guinea pig sensitization study
with 21Z73 using the maximisation test method, Berkhamsted, Wellcome
Research Laboratories (Report No. HEFG 74-3) (Unpublished data
submitted to WHO).
CHESHER, B.C. & MALONE, J.C. (1974c) Occular irritancy of 21Z73 in
rabbits, Berkhamsted, Wellcome Research Laboratories (Report no. HEFG
74-6) (Unpublished data submitted to WHO).
CHESHER, B.C., CLAMPITT, R.C., & MALONE, J.C. (1975) 21Z73 -
Preliminary investigations into the cumulative oral toxicity on dogs,
Berkhamsted, Wellcome Research Laboratories (Report no. HEFG 75-9)
(Unpublished data submitted to WHO).
CLAPP, M.J.L., BANHAM, P.B., CHART, I.S., GLAISTER, J., GORE, C., &
MOYES, A. (1977a) PP557: 28 day feeding study in rats (Report No.
CTL/P/355) (Unpublished data submitted to WHO by ICI Central Toxicology
Laboratory).
CLAPP, M.J.L., BANHAM, P.B., GLAISTER, J.R., & MOYES, A. (1977b)
PP557: 28 day feeding study in mice (Report No. CTL/P/354)
(Unpublished data submitted to WHO by ICI Central Toxicology
Laboratory).
CLARK, D.G. (1978) Toxicology of WL 43479: acute toxicity of WL43479,
Sittingbourne, Shell Research Ltd (Report No. TLGR. 0043.78)
(Unpublished data submitted to WHO).
CLIVE, C.D. (1977) Mutagenicity of BW21Z73 in L5178Y/TK mouse
lymphoma cells with and without exogenous metabolic activation, Re-
search Triangle Park, NC, Burroughs Wellcome Co. (Report No.
TTEP/77/0011) (Unpublished data submitted to WHO).
CLOUD, R.M., ZIMPFER, M.L., YANES, J.JR., BOETHEL, D.J., BUCO, P.,
& HARMON, C.W. (1987) Insecticide residues on clothing worn by crop
consultants in soybean fields treated with non-conventional application
technology. Bull. environ. Contam. Toxicol., 38: 277-282.
COATS, J.R. & O'DONNELL-JEFFERY, N.L. (1979) Toxicity of four
synthetic pyrethroid insecticides to rainbow trout. Bull. environ.
Contam. Toxicol., 23: 250-255.
COX, R.L. & WILSON, W.T. (1984) Effects of permethrin on the behavior
of individually tagged honey bees, Apis mellifera L. (Hymenoptera:
Apidae). Environ. Entomol., 13: 375-378.
CRIDLAND, J.S. & WEATHERLEY, B.C. (1977a) Urinary excretion in man of
3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropane carboxylic acid ("CVA")
after oral ingestion of permethrin (NRDC 143) - A first report, Bech-
enham, Wellcome Research Laboratories (Report No. BDPE-77-1)
(Unpublished data submitted to WHO).
CRIDLAND, J.S. & WEATHERLEY, B.C. (1977b) An estimate of permethrin
(NRDC 143; OMS1821) absorbed by people employed in a field trial of the
insecticide, Bechenham, Wellcome Research Laboratories (Report No.
BDPE-77-3) (Unpublished data submitted to WHO).
DAYAN, A.D. (1980) 21-day neuropathological study in the Sprague-
Dawley rat of permethrin (212732J) administered in the diet,
Berkhamsted, Wellcome Research Laboratories (Report No. BP 80-48)
(Unpublished report submitted to WHO by Wellcome Foundation Ltd).
DOYLE, R.D., KAUFMAN, D.D., BURT, G.W., & DOUGLASS, L. (1981)
Degradation of cis-permethrin in soil amended with sewage sludge or
dairy manure. J. agric. food Chem., 29: 412-414.
DYCK, P.J., SHIMONO, M., SCHOENING, G.P., LAIS, A.C., OVIATT, K.F.,
& SPARKS, M.F. (1984) The evaluation of a new synthetic pyrethroid
pesticide (permethrin) for neurotoxicity. J. environ. Pathol. Toxicol.
Oncol., 5: 109-117.
EDWARDS, D.B., OSBORN, B.E., DENT, N.J., & KINCH, D.A. (1976)
Toxicity study in beagle dogs (oral administration for three months),
Inveresk, United Kingdom, Inveresk Research International Ltd
(Submitted to WHO by ICI Ltd).
EDWARDS, M.J. & ISWARAN, T.J. (1977) Permethrin: residue transfer and
toxicology study with cows fed treated grass nuts (Report No.
TMJ1519/B) (Unpublished report submitted to WHO by ICI Plant Protection
Division).
ELLIOTT, M. (1977) Synthetic pyrethroids, Washington, DC, American
Chemical Society, p. 229 (ACS Symposium Series 42).
ELLIOTT, M., FARNHAM, A.W., JANES, N.F., NEEDHAM, P.H.,
PULMAN, D.A., & STEVENSON, J.H. (1973) A photostable pyrethroid.
Nature (Lond.), 246: 169-170.
ELLIOTT, M., JANES, N.F., PULMAN, D.A., GAUGHAN, L.C., UNAI, T.,
& CASIDA, J.E. (1976) Radiosynthesis and metabolism in rats of the 1R-
isomers of the insecticide permethrin. J. agric. food Chem., 24: 270-
276.
EVANS, M.H. (1976) End-plate potentials in frog muscle exposed to a
synthetic pyrethroid Pestic. Biochem. Physiol., 6: 547-550.
EVERTS, J.W., KORTENHOFF, B.A., HOOGLAND, H., VLUG, H.J.,
JOCQUE, R., & KOEMAN, J.H. (1985) Effects on non-target terrestrial
arthropods of synthetic pyrethroids used for the control of the tsetse
fly (Glossina spp.) in settlement areas of the southern Ivory Coast,
Africa. Arch. environ. Contam. Toxicol., 14: 641-650.
FAO (1982) Second Government Consultation on International
Harmonization of Pesticide Registration Requirements, Rome, 11-15
October 1982, Rome, Food and Agriculture Organization of the United
Nations.
FAO/WHO (1980a) Pesticide residues in food. Report of the 1979 Joint
Meeting of the FAO Panel of Experts on Pesticide Residues in Food and
the Environment and the WHO Expert Group on Pesticide Residues, Rome,
Food and Agriculture Organization of the United Nations, pp. 52-55 (FAO
Plant Production and Protection Paper 20).
FAO/WHO (1980b) 1979 Evaluations of some pesticide residues in food,
Rome, Food and Agriculture Organization of the United Nations,
pp. 369- 425 (FAO Plant Production and Protection Paper 20 Sup).
FAO/WHO (1981a) Pesticide residues in food. Report of the 1980 Joint
Meeting of the FAO Panel of Experts on Pesticide Residues in Food and
the Environment and the WHO Expert Group on Pesticide Residues, Rome,
Food and Agriculture Organization of the United Nations, pp. 50-51 (FAO
Plant Production and Protection Paper 26).
FAO/WHO (1981b) 1980 Evaluations of some pesticide residues in
food, Rome, Food and Agriculture Organization of the United Nations,
pp. 360-393 (FAO Plant Production and Protection Paper 26 Sup).
FAO/WHO (1982a) Pesticide residues in food. Report of the 1981
Joint Meeting of the FAO Panel of Experts on Pesticide Residues in Food
and the Environment and the WHO Expert Group on Pesticide Residues,
Rome, Food and Agriculture Organization of the United Nations, pp.
37-39 (FAO Plant Production and Protection Paper 37).
FAO/WHO (1982b) 1981 Evaluations of some pesticide residues in food,
Rome, Food and Agriculture Organization of the United Nations,
pp. 385-425 (FAO Plant Production and Protection Paper 42).
FAO/WHO (1983a) Pesticide residues in food. Report of the 1982 Joint
Meeting of the FAO Panel of Experts on Pesticide Residues in Food
and the Environment and the WHO Expert Group on Pesticide Residues,
Rome, Food and Agriculture Organization of the United Nations, pp. 36-
38 (FAO Plant Production and Protection Paper 46).
FAO/WHO (1983b) 1982 Evaluations of some pesticide residues in food,
Rome, Food and Agriculture Organization of the United Nations,
pp. 329-350 (FAO Plant Production and Protection Paper 49).
FAO/WHO (1984a) Pesticide residues in food. Report of the 1983
Joint Meeting of the FAO Panel of Experts on Pesticide Residues in Food
and the Environment and the WHO Expert Group on Pesticide Residues,
Rome, Food and Agriculture Organization of the United Nations, p. 38,
57, 62, 65 (FAO Plant Production and Protection Paper 56).
FAO/WHO (1984b) 1983 Evaluations of some pesticide residues in food,
Rome, Food and Agriculture Organization of the United Nations,
pp. 307-313, 501 (FAO Plant Production and Protection Paper 61).
FAO/WHO (1985a) Pesticide residues in food. Report of the 1984 Joint
Meeting of the FAO Panel of Experts on Pesticide Residues in Food and
the Environment and the WHO Expert Group on Pesticide Residues, Rome,
Food and Agriculture Organization of the United Nations, pp. 63, 85, 93
(FAO Plant Production and Protection Paper 62).
FAO/WHO (1985b) 1984 Evaluations of some pesticide residues in food,
Rome, Food and Agriculture Organization of the United Nations,
pp. 441-444, 723 (FAO Plant Production and Protection Paper 67).
FAO/WHO (1985c) Guide to Codex recommendations concerning pesticide
residues. Part 8. Recommendations for methods of analysis of pesticide
residues, 3rd ed., Rome, Codex Committee on Pesticide Residues.
FAO/WHO (1986a) Pesticide residues in food. Report of the 1985 Joint
Meeting of the FAO Panel of Experts on Pesticide Residues in Food and
the Environment and the WHO Expert Group on Pesticide Residues, Rome,
Food and Agriculture Organization of the United Nations, p. 37 (FAO
Plant Production and Protection Paper 68).
FAO/WHO (1986b) 1985 Evaluations of some pesticide residues in food,
Part I - Residues, Rome, Food and Agriculture Organization of the
United Nations, pp. 201-203 (FAO Plant Production and Protection Paper
No 72/1).
FAO/WHO (1986c) Codex Maximum Limits for pesticide residues, 2nd ed.,
Rome, Food and Agriculture Organization of the United Nations, Joint
FAO/WHO Food Standards Programme, Codex Alimentarius Commission,
(CAC XIII).
FAO/WHO (1987) Pesticide residues in food. Report of the 1987 Joint
Meeting of the FAO Panel of Experts on Pesticide residues in Food and
the Environment and the WHO Expert Group on Pesticide Residues, Rome,
Food and Agriculture Organization of the United Nations, p. 33 (FAO
Plant Production and Protection Paper 84).
FAO/WHO (1988) Permethrin. In: Pesticide residues in food. Part II -
Toxicology, Rome, Food and Agriculture Organization of the United
Nations, pp. 101-110 (FAO Plant Production and Protection Paper 86/2).
FARQUHAR, J.A., HUTCHINSON, D.B.A., PERIAM, A.W., & SPARKES,
R.G. (1981a) An investigation into the absorption of permethrin from
impregnated clothing, Bechenham, Wellcome Research Laboratories (Report
No. BKDL/81/1) (Unpublished data submitted to WHO).
FARQUHAR, J.A., HUTCHINSON, D.B.A., PERIAM, A.W., & SPARKES,
R.G. (1981b) An investigation of tolerance to absorption of and
persistence of permethrin applied as a 1% lotion to human hair,
Bechenham, Wellcome Research Laboratories (Report No. BKDL/81/2)
(Unpublished data submitted to WHO).
FLANNIGAN, S.A. & TUCKER, S.B. (1985) Variation in cutaneous
sensation between synthetic pyrethroid insecticides. Contact Derma-
titis, 13: 140-147.
FLANNIGAN, S.A., TUCKER, S.B., MARCUS, M.K., ROSS, C.E.,
FAIRCHILD, E.J., GRIMES, B.A., & HARRIST, R.B. (1985a) Primary
irritant contact dermatitis from synthetic pyrethroid insecticide
exposure. Arch. Toxicol., 56, 288-294.
FLANNIGAN, S.A., TUCKER, S.B., KEY, M.M., ROSS, C.E., FAIRCHILD,
E.J., GRIMES, B.A., & HARRIST, R.B. (1985b) Synthetic pyrethroid
insecticides: a dermatological evaluation. Br. J. ind. Med., 42: 363-
372.
FRIESEN, M.K., GALLOWAY, T.D., & FLANNAGAN, J.F. (1983) Toxicity
of the insecticide permethrin in water and sediment to nymphs of the
burrowing mayfly Hexagenia rigida (Ephemeroptera: Ephemeridae). Can.
Entomol., 115: 1007-1014.
GAMBRELL, R.P., REDDY, C.N., COLLARD, V., GREEN, G., & PATRICK,
W.H., Jr. (1981) Behavior of DDT, kepone and permethrin in sediment-
water systems under different oxidation-reduction and pH conditions,
Washington, DC, US Environmental Protection Agency (EPA 600/3-81-
038).
GAMMON, D.W. & CASIDA, J.E. (1983) Pyrethroids of the most potent
class antagonize GABA action at the crayfish neuromuscular junction.
Neurosci. Lett., 40: 163-168.
GAMMON, D.W., BROWN, M.A., & CASIDA, J.E. (1981) Two classes of
pyrethroid action in the cockroach. Pestic. Biochem. Physiol., 15:
181-191.
GAMMON, D.W., LAWRENCE, L.J., & CASIDA, J.E. (1982) Pyrethroid
toxicology: Protective effects of Diazepam and phenobarbital in the
mouse and the cockroach. Toxicol. appl. Pharmacol., 66: 290-296.
GAUGHAN, L.C. & CASIDA, J.E. (1978) Degradation of trans- and cis-
permethrin on cotton and bean plants. J. agric. food Chem., 26: 525-
528.
GAUGHAN, L.C., UNAI, T., & CASIDA, J.E. (1977) Permethrin metabolism
in rats. J. agric. food Chem., 25: 9-17.
GAUGHAN, L.C., ROBINSON, R.A., & CASIDA, J.E. (1978b) Distribution
and metabolic fate of trans- and cis-permethrin in laying hens. J.
agric. food Chem., 26: 1374-1380.
GAUGHAN, L.C., ACKERMAN, M.E., UNAI, T., & CASIDA, J.E. (1978a)
Distribution and metabolism of trans- and cis-permethrin in lactating
Jersey cows. J. agric. food Chem., 26: 613-618.
GERIG, L. (1985) Testing the toxicity of synthetic pyrethroid insecti-
cides to bees. Pestic. Sci., 16: 206-207.
GLAISTER, J.R., PRATT, I., & RICHARDS, D. (1977) Effects of high
dietary levels of PP557 on clinical behaviour and structure of sciatic
nerves in rats (Report No. CTL/P/317) (Unpublished data submitted to
WHO by ICI Central Toxicology Laboratory).
GLICKMAN, A.H. & CASIDA, J.E. (1982) Species and structural variations
affecting pyrethroid neurotoxicity. Neurobehav. Toxicol. Teratol., 4:
(6) 793-799.
GLICKMAN, A.H. & LECH, J.J. (1981) Hydrolysis of permethrin, a
pyrethroid insecticide, by rainbow trout and mouse tissues in vitro : A
comparative study. Toxicol. appl. Pharmacol., 60: 186-192.
GLICKMAN, A.H., SHONO, T., CASIDA, J.E., & LECH, J.J. (1979) In vitro
metabolism of permethrin isomers by carp and rainbow trout liver
microsomes. J. agric. food Chem., 27: 1038-1041.
GLICKMAN, A.H., HAMID, A.AR., RICKERT, D.E., & LECH, J.J. (1981)
Elimination and metabolism of permethrin isomers in rainbow trout.
Toxicol. appl. Pharmacol., 57: 88-98.
GLICKMAN, A.H., WEITMAN, S.D., & LECH, J.J. (1982) Different toxicity
of trans-permethrin in rainbow trout and mice. I. Role of biotransfor-
mation. Toxicol. appl. Pharmacol., 66: 153-161.
HAGLEY, E.A.C., PREE, D.J., & HOLLIDAY, N.J. (1980) Toxicity of
insecticides to some orchard carabids (Coleoptera carabidae). Can. Entomol.,
112: 457-462.
HALLS, G.R.H. (1981) The fate of permethrin residues on wheat during 9
months storage in Australia (Report No. HEFH 81-1) (Unpublished data
submitted to WHO by Wellcome Research Laboratories).
HALLS, G.R.H. & PERIAM, A.W. (1980) The fate of permethrin residues on
wheat during storage and after milling and baking - results after 9, 12
and 15 months storage (Report HEFH 80-3) (Unpublished data submitted to
WHO by Wellcome Research Laboratories).
HANSEN, D.J., GOODMAN, L.R., MOORE, J.C., & HIGDON, P.K. (1983)
Effects of the synthetic pyrethroids AC 222, 705, permethrin and
fenvalerate on sheepshead minnows in early life stage of toxicity
tests. Environ. Toxicol. Chem., 2: 251-258.
HART, D., BANHAM, P.B., GORE, C.W., PRATT, I., & WEIGHT, T.M.
(1977c) PP557: Liver hypertrophy study in rats-dietary administration
over 26 weeks (Report No. CTL/P/360) (Unpublished data submitted to WHO
by ICI Central Toxicology Laboratory).
HELSON, B.V., KINGSBURY, P.D., & DE GROOT, P. (1986) The use of
bioassays to assess aquatic arthropod mortality from permethrin drift
deposits. Aquat. Toxicol., 9: 253-262.
HEND, R.W. & BUTTERWORTH, S.T.G. (1977) Toxicity of insecticides: a
short-term feeding study of WL 43379 in rats (Report No. TLGR.0108.77)
(Unpublished data submitted to WHO by Shell Research Ltd).
HENRIET, J., MARTIJN, A., & POLVISEN, H.H. (1985) CIPAC Handbook.
Volume 1C: Analysis of technical and formulated pesticides, Hertford-
shire, Collaborative International Pesticides Analytical Council Ltd,
pp. 2172-2173.
HILL, E.F. & CAMARDESE, M.B. (1986) Lethal dietary toxicities of
environmental contaminants and pesticides to Coturnix, Washington, DC,
United States Department of the Interior, Fish and Wildlife Service,
p.111 (Fish and Wildlife Technical Report No. 2).
HODGE, M.C.E., BANHAM, P.B., GLAISTER, J.R., RICHARDS, D.,
TAYLOR, K., & WEIGHT, T.M. (1977) PP557: Three generation reproduction
study in rats (Unpublished report submitted to WHO by ICI Central
Toxicology Laboratory).
HOGAN, G.K. & RINEHART, W.E. (1977) A twenty-four month oral
carcinogenicity study of FMC 33297 in mice (Bio-Dynamics Inc. Project)
(Unpublished report submitted to WHO by FMC Corporation).
HOLDWAY, D.A. & DIXON, D.G. (1988) Acute toxicity of permethrin or
glyphosate pulse exposure to larval white sucker (Catostomus commer-
soni) and juvenile flagfish (Jordanella floridae) as modified by age
and ration level. Environ. Toxicol. Chem., 7: 63-68.
HOLMSTEAD, R.L., CASIDA, J.E., RUZO, L.O., & FULLER, D.G. (1978)
Pyrethroid photodecomposition: Permethrin. J. agric. food Chem., 26:
590-595.
HORIBA, M., KOBAYASHI, A., & MURANO, A. (1977) Gas-liquid
chromatographic determination of a new pyrethroid, permethrin (S-3151)
and its optical isomers. Agric. biol. Chem., 41: 581-586.
HOY, M.A., FLAHERTY, D., PEACOCK, W., & CULVER, D. (1979)
Vineyard and laboratory evaluations of methomyl, dimethoate, and
permethrin for a grape pest management program in the San Joaquin
Valley of California. J. econ. Entomol., 72: 250-255.
HOYT, S.C., WESTIGARD, P.H., & BURTS, E.C. (1978) Effects of two
synthetic pyrethroids on the codling moth, pear psylla, and various
mite species in northwest apple and pear orchards. J. econ. Entomol.,
71: 431-434.
HUNT, L.M. & GILBERT, B.N. (1977) Distribution and excretion rates of
14C-labelled permethrin isomers administered orally to four lactating
goats for 10 days. J. agric. food Chem., 25: 673-676.
HUNT, L.M., GILBERT, B.N., & LEMEILLEUR, C.A. (1979) Distri-
bution and depletion of radioactivity in hens treated dermally with
14C-labelled permethrin. Poult. Sci., 58: 1197-1201.
ISHMAEL, J. & LITCHFIELD, M.H. (1988) Chronic toxicity and
carcinogenic evaluation of permethrin in rats and mice. Fundam. appl.
Toxicol., 11: 308-311.
IVIE, G.W. & HUNT, L. (1980) Metabolites of cis- and trans-permethrin
in lactating goats. J. agric. food Chem., 28: 1131-1138.
JAGGERS, S.E. & PARKINSON, G.R. (1979) Permethrin: summary and review
of acute toxicities in laboratory species (Report No. CTL/P/461)
(Unpublished data submitted to WHO by ICI Central Toxicology
Laboratory).
JAMES, J.A. (1974) Fetal toxicity study of 21Z73 (NRDC 143) in the
rat (Report No. BPAT 74-10) Bechenham, Wellcome Research Laboratories
(Unpublished data submitted to WHO).
JAMES, J.A., (1979) A multigeneration reproduction study of 21Z73
(permethrin) in the rat (Report No. BPAT 79-3) Bechenham, Wellcome
Research Laboratories (Unpublished data submitted to WHO).
JAMES, J.A., TAYLOR, P.E., & ROE, F.J.C. (1980) Carcinogenicity study
in mice with permethrin, Bechenham, Wellcome Research Laboratories,
(Report No. HEFG 80-29) (Unpublished data submitted to WHO).
JOLLY, A.L., Jr., AVAULT, J.W., Jr., KOONCE, K.L., & GRAVES, J.B.
(1978) Acute toxicity of permethrin to several aquatic animals. Trans.
Am. Fish. Soc., 107: 825-827.
JORDAN, E.G. & KAUFMAN, D.D. (1986) Degradation of cis- and trans-
permethrin in flooded soil. J. agric. food Chem., 34: 880-884.
JORDAN, E.G., KAUFMAN, D.D., & KAYSER, A.J. (1982) The effect of soil
temperature on the degradation of cis-, trans-permethrin in soil. J.
environ. Sci. Health, B 17: 1-17.
KADOTA, T., MIYAMOTO, J., & ITO, N. (1975) Six-month subacute oral
toxicity of NRDC in Sprague-Dawley rats (Unpublished data submitted to
WHO by Sumitomo Chemical Co.).
KANEKO, H., OHKAWA, H., & MIYAMOTO, J. (1978) Degradation and
movement of permethrin isomers in soil. J. Pestic. Sci., 3: 43-51.
KAUFMAN, D.D., HAYES, S.C., JORDAN, E.G., & KAYSER, A.J. (1977)
Permethrin degradation in soil and microbial cultures. In: Elliott, M.,
ed. Synthetic pyrethroids, Washington, DC, American Chemical Society,
pp. 147-161 (ACS symposium series 42).
KAUFMAN, D.D., RUSSEL, B.A., HELLING, C.S., & KAYSER, A.J. (1981)
Movement of cypermethrin, deltamethrin, permethrin and their
degradation products in soil. J. agric. food Chem., 29: 239-245.
KAUSHIK, N.K., STEPHENSON, G.L., SOLOMON, K.R., & DAY, K.E.
(1985) Impact of permethrin on zooplankton communities in
limnocorrals. Can. J. Fish. aquat. Sci., 42: 77-85.
KILLEEN, J.C. & RAPP, W.R. (1976a) A three month oral toxicity study
of FMC 33297 in Beagle dogs (Bio-Dynamics Inc. Project) (Unpublished
report submitted to WHO by FMC Corporation).
KILLEEN, J.C. & RAPP, W.R. (1976b) A three month oral toxicity study
of FMC 33297 in rats (Bio-Dynamics Inc. Project) (Unpublished report
submitted to WHO by FMC Corporation).
KINGSBURY, P.D. (1976) Effects of an aerial application of the syn-
thetic pyrethroid permethrin on a forest stream. Manitoba Entomol., 10:
9-17.
KINGSBURY, P.D. & KREUTZWEISER, D.P. (1980a) Environmental impact
assessment of a semi-operational permethrin application. Sault Ste.
Marie, Ontario, Canada, Forest Pest Management Institute. Report No.
FPM-X 30: pp.47.
KINGSBURY, P.D. & KREUTZWEISER, D.P. (1980b) Dosage-effect studies
on the impact of permethrin on trout streams. Sault Ste. Marie,
Ontario, Canada, Forest Pest Management Institute Report No. FPM-X 31.
KINGSBURY, P.D. & KREUTZWEISER, D.P. (1987) Permethrin treatments in
Canadian forests. Part 1: Impact on fish. Pestic. Sci., 19: 35-48.
KOHDA, H., KADOTA, T., & MIYAMOTO, J. (1976a) Teratogenic evaluation
with permethrin in rats (Unpublished report submitted to WHO by
Sumitomo Chemical Co.).
KOHDA, H., KADOTA, T., & MIYAMOTO, J. (1976b) Teratogenic evaluation
with permethrin in mice (Unpublished report submitted to WHO by
Sumitomo Chemical Co.).
KOHDA, H., KADOTA, T., & MIYAMOTO, J. (1979a) Acute oral, dermal and
subcutaneous toxicities of permethrin in rats and mice (Unpblished
report submitted to WHO by Sumitomo Chemical Co.).
KOHDA, H., KANEDO, H., OHKAWA, H., KADOTA, T., & MIYAMOTO, J.
(1979b) Acute intraperitoneal toxicity of permethrin metabolites in
mice (Unpublished report submitted to WHO by Sumitomo Chemical Co.).
KOLMODIN-HEDMAN, B., SWENSSON, A., & AKERBLOM, M. (1982)
Occupational exposure to some synthetic pyrethroids (permethrin and
fenvalerate). Arch. Toxicol., 50: 27-33.
KREUTZWEISER, D.P. (1982) The effects of permethrin on the invertebrate
fauna of a Quebec forest. Sault Ste Marie, Ontario, Canada, Forest Pest
Management Institute, Report No. FPM-X 50: pp.44.
KREUTZWEISER, D.P. & KINGSBURY, P.D. (1987) Permethrin treatments
in Canadian forests. Part 2: Impact on stream invertebrates. Pestic.
Sci., 19: 49-60.
KUMARAGURU, A.K. & BEAMISH, F.W.H. (1981) Lethal toxicity of
permethrin (NRDC-143) to rainbow trout, Salmo gairdneri, in relation to
body weight and water temperature. Water Res., 15: 503-505.
KUMARAGURU, A.K., BEAMISH, F.W.H., & FERGUSON, H.W. (1982)
Direct and circulatory paths of permethrin (NRDC-143) causing
histopathological changes in the gills of rainbow trout, Salmo
gairdneri Richardson. J. Fish Biol., 20: 87-91.
LAWRENCE, L.J. & CASIDA, J.E. (1982) Pyrethroid toxicology: Mouse
intracerebral structure-toxicity relationship. Pestic. Biochem. Physiol.,
18: 9-14.
LAWRENCE, L.J. & CASIDA, J.E. (1983) Stereospecific action of
pyrethroid insecticides on the gamma-aminobutyric acid receptor-
ionophore complex. Science, 221: 1399-1401.
LAWRENCE, L.J., GEE, K.W., & YAMAMURA, H.I. (1985) Interactions of
pyrethroid insecticides with chloride ionophore-associated binding
sites. Neurotoxicology, 6: 87-98.
LEAHEY, J.P. (1985) The pyrethroid insecticides, London, Taylor &
Francis Ltd, p. 440.
LEAHEY, J.P. & CARPENTER, P.K. (1980) The uptake of metabolites
of permethrin by plants grown in soil treated with [14C]permethrin.
Pestic. Sci., 11: 279-289.
LEAHEY, J.P., BEWICK, D.W., & SAUNDERS, R. (1977) Accumulation and
depletion of radioactive residues in the tissues of Mallard duck and
Japanese quail following daily dosing with 14 C-permethrin (Unpublished
report submitted to WHO by ICI Plant Protection Division).
LE QUESNE, P.N., MAXWELL, I.C., & BUTTERWORTH, T.G. (1980)
Transient facial sensory symptoms following exposure to synthetic
pyrethroids: a clinical and electorophysiological assessment. Neurotoxi-
cology, 2: 1-11.
LINDEN, E., BENGTSSON, B.-E., SVANBERG, O., & SUNDSTROM, G.
(1979) The acute toxicity of 78 chemicals and pesticide formulations
against two brackish water organisms, the Bleak (Alburnus alburnus) and
the Nitocra spinipes. Chemosphere, 11/12: 843-851.
LINDQUIST, R.K., KRUEGER, H.R., & POWELL, C.C., Jr. (1987) Airborne
and surface residues of permethrin after high- and low-volume
applications in greenhouses. J. environ. Sci. Health, B22: 15-27.
LITCHFIELD, M.H. (1983) Characterisation of the principal mammalian
toxicological and biological actions of synthetic pyrethroids. In:
Miyamoto, J. & Kearney, P.C., ed. Pesticide chemistry: human welfare
and the environment. II. Natural products, Oxford, Pergamon Press, pp.
207-211.
LONGSTAFF, E. (1976) Permethrin: short-term predictive tests for
carcinogenicity: results from the Ames test (Report No. CTL/P/301)
(Unpublished data submitted to WHO by ICI Central Toxicology
Laboratory).
LORD, K.A., MCKINLEY, M., & WALKER, N. (1982) Degradation of
permethrin in soils. Environ. Pollut. A29: 81-90.
LUND, A.E. & NARAHASHI, T. (1983) Kinetics of sodium channel
modification as the basis for the variation in the nerve membrane
effects of pyrethroids and DDT analogs. Pestic. Biochem. Physiol., 20:
203-216.
MCGREGOR, D.B. & WICKRAMARATNE, G.A., de S. (1976a) Dominant
lethal study in mice of ICI-PP557 (Report No. 623, Inveresk Research
International Project No. 406722) (Unpublished data submitted to WHO by
ICI Ltd).
MCGREGOR, D.B. & WICKRAMARATNE, G.A., de S. (1976b) Terato-
genicity study in rats of ICI-PP557 (Inveresk Research International
Project No. 404898) (Unpublished data submitted to WHO by ICI Ltd.).
MCLEESE, D.W., METCALFE, C.D., & ZITKO, V. (1980) Lethality of
permethrin, cypermethrin and fenvalerate to salmon, lobster and shrimp.
Bull. environ. Contam. Toxicol., 25: 950-955.
MACPHEE, A., GAUL, S., & RAGAB, M.T.H. (1982) Control of blueberry
thrips, Frankliniella vaccinii Morga, with permethrin and effect on
yield and residue in fruit. J. environ. Sci. Health, B17: 183-193.
MCSHEEHY, T.W. & FINN, J.P. (1980) 21Z: Potential toxicity and
oncogenicity in dietary administration to rats for a period of 104
weeks (Report No. HEFG 80-33) (Unpublished data of Wellcome Research
Laboratories).
MAREI, A.E.M., RUZO, L.O., & CASIDA, J.E. (1982) Analysis and
persistence of permethrin, cypermethrin, deltamethrin and fenvalerate
in the fat and brains of treated rats. J. agric. food Chem., 30: 558-
562.
MATHUR, S.P., BELANGER, A., HAMILTON, H.A., & KHAN, S.U. (1980)
Influence on microflora and persistence of field-applied disulfoton,
permethrin and prometryne in an organic soil. Pedobiologia, 20: 237-
242.
MAYER, F.L., Jr. (1987) Acute toxicity handbook of chemicals to
estuarine organisms, Springfield, Virginia, National Technical
Information Service, p. 158 EPA/600/8-87/017.
MAYER, F.L., Jr. & ELLERSIECK, M.R. (1986) Manual of acute toxicity:
Interpretation and data base for 410 chemicals and 66 species of
freshwater animals, Washington, DC, United States Department of the
Interior, Fish and Wildlife Service, pp.377-378.
MEISTER, R.T., BERG, G.L., SINE, C., MEISTER, S., & POPLYK, J. (1983)
Farm chemicals handbook. Section C. Pesticide dictionary, Wilboughby,
Ohio, Meister Publishing Co., pp. C180-C181.
METKER, L.W. (1978) Subchronic inhalation toxicity of 3-(phenoxy-
phenyl)methyl(+)-cis,trans-3-(2,2-dichloroethenyl)-2,2-dimethylcyclopro-
panecarboxylate (permethrin), Aberdeen Proving Ground, Maryland, US
Army Environmental Hygiene Agency (Report No. 75-51-0026-80).
METKER, L.W., ANGERHOFER, R.A., POPE, C.R., & SWENTZEL, K.C.
(1977) Toxicology evaluation of 3-(phenoxyphenyl)methyl(+)-cis,trans-3-
(2,2-dichloroethenyl)-2,2-dimethylcyclopropanecarboxylate (permethrin),
Environmental Hygiene Agency (Report No. 75-51-0837-78).
MILLNER, C.K. & BUTTERWORTH, S.T.G. (1977) Toxicity of pyrethroid
insecticides: investigation of the neurotoxic potential of WL 43479 to
adult hens, (Report No. TLGR.0069.77) (Unpublished data submitted to
WHO by Shell Research Ltd).
MIYAMOTO, J. (1976) Degradation, metabolism and toxicity of synthetic
pyrethroids. Environ. Health Perspect., 14: 15-28.
MIYAMOTO, J. (1981) The chemistry, metabolism and residue analysis of
synthetic pyrethroids, Pure appl. Chem., 53: 1967-2022.
MIYAMOTO, J. & KEARNEY, P.C. (1983) Pesticide chemistry-human welfare
and the environment. Proceedings of the Fifth International Congress of
Pesticide Chemistry, Kyoto, Japan, 29 August-4 September 1982, Oxford,
Pergamon Press, Vol. 1-4.
MULLA, M.S., DARWAZEH, H.A., & MAJORI, G. (1975) Field efficacy of
some promising mosquito larvicides and their effect on nontarget
organisms. Mosq. News, 35: 179-185.
MULLA, M.S., NAVVAB-GOJRATI, H.A., & DARWAZEH, H.A. (1978a)
Toxicity of mosquito larvicidal pyrethroids to four species of
freshwater fishes. Environ. Entomol., 7: 428-430.
MULLA, M.S., NAVVAB-GOJRATI, H.A., & DARWAZEH, H.A. (l978b)
Biological activity and longevity of new synthetic pyrethroids against
mosquitoes and some nontarget insects. Mosq. News, 38: 90-96.
MULLA, M.S., DARWAZEH, H.A., & DHILLON, K.S. (1981) Impact and
joint action of decamethrin and permethrin and freshwater fishes on
mosquitoes. Bull. environ. Contam. Toxicol., 26: 689-695.
NASSIF, M., BROOKE, J.P., HUTCHINSON, D.B.A., & KAMEL, O.M. (1980)
Studies with permethrin against body lice in Egypt. Pestic. Sci., 11:
679-684.
NEWELL, G.W. & SKINNER W.A. (1976) In vitro microbiological
mutagenicity study of an FMC corporation compound (Unpublished report
submitted to WHO by FMC Corporation).
NOMURA, Y. & SEGAWA, T. (1979) Pharmacological study of permethrin:
effects of isolated ileum, nictitating membrane, respiration, blood
pressure and electrocardiography (Unpublished report submitted to WHO
by Sumitomo Chemical Co.).
OHKAWA, H., KANEKO, H., & MIYAMOTO, J. (1977) Metabolism of
permethrin in bean plants. J. Pestic. Sci., 2: 67-76.
OHSHIMA, M., TAKIMOTO, Y., & MATSUDA, T. (1988) Accumulation and
metabolism of 14 C-permethrin in carp (Cyprinus carpio) (Unpublished
data submitted to WHO by Sumitomo Chemical Co.).
OKUNO, Y., MIYAGAWA, H., HOSOI, R., & KADOTA, T. (1976) Skin and
eye irritation study of permethrin (S-3151) in rabbits (Unpublished
report submitted to WHO by Sumitomo Chemical Co.).
PARKINSON, G.R. (1978) Permethrin: acute toxicity to male rats (Report
No. CTL/P/388) (Unpublished data submitted to WHO by ICI Central
Toxicology Laboratory).
PARKINSON, G.R., BERRY, P.N., GLAISTER, J., GORE, C.W., LEFEVRE,
V.K., & MURPHY, J.A. (1976) PP557 (Permethrin): acute and sub-acute
toxicity, (Report No. CTL/P/225) (Unpublished data submitted to WHO by
ICI Central Toxicology Laboratory).
PAYNTER, O.C., BUDD, E.R., & LITT, B.D. (1982) Permethrin. Assessment
of chronic and oncogenic effects: A summary, Washington, DC, Toxicology
Branch, Hazard Evaluation Division, Office of Pesticide Programs, US
Environmental Protection Agency (Unpublished data submitted to WHO).
PEGUM, J.S. & DOUGHTY, B.J. (1978) Human skin patch test with
pyrethrins, kadethrin, permethrin and decamethrin, Berkhamsted Hill,
Wellcome Group Research and Development (Report No. NEFJ 78-2)
(Unpublished data submitted to WHO).
PIERCY, D.W.T., REYNOLDS, J., & JAMES, J.A. (1976) Effects of diet on
the acute toxicity of permethrin in the female rat, Berkhamsted Hill,
Wellcome Research and Development (Report no. 77-11) (Unpublished data
submitted to WHO).
PIKE, K.S., MAYER, D.F., GLAZER, M., & KIOUS, C. (1982) Effects of
permethrin on mortality and foraging behavior of honey bees in sweet
corn. Environ. Entomol., 11: 951-953.
PLAPP, F.W., Jr & BULL, D.L. (1978) Toxicity and selectivity of some
insecticides to Chrysopa carnea, a predator of the tobacco budworm.
Environ. Entomol., 7: 431-434.
PLAPP, F.W., Jr & VINSON, S.B. (1977) Comparative toxicities of some
insecticides to the tobacco budworm and its ichneumonid parasite,
Campoletis sonorensis. Environ. Entomol., 6: 381-384.
PLUIJMEN, M., DREVON, C., MONTESANO, R., MALAVEILLE, C.,
HAUTEFEUILLE, A., & BARTSCH, H. (1984) Lack of mutagenicity of
synthetic pyrethroids in Salmonella typhimurium strains in V79 Chinese
hamster cells. Mutat. Res., 137: 7-15.
RACEY, P.A. & SWIFT, S.M. (1986) The residual effect of remedial
timber treatments on bats. Biol. Conserv., 35: 205-214.
RAPP, W.R. (1978) Twenty-four month oral toxicity/oncogenicity study
of FMC33297 in mice. Histopathology report (Unpublished data of FMC
Corporation).
REYNOLDS, J., PIERCY, D.W.T., CLAMPITT, R.B., JAMES, J.A.,
THOMPSON, P.M., FAREBROTHER, D.A., & DAYAN, A.D. (1978)
Permethrin oral administration to dogs for 6 months, Berkhamsted Hill,
Wellcome Research Laboratories (Report No. HEFG 78-14) (Unpublished
data submitted to WHO).
RICHARDS, D., BANHAM, P.B., KILMARTIN, M., & WEIGHT, T.M. (1980)
Permethrin: Teratogenicity study in the rabbit (Report No. CTL/P/523)
(Unpublished report submitted to WHO by ICI Ltd).
RISHIKESH, N., CLARKE, J.L., MATHIN, H.L., KING, J.S., & PEARSON, J.
(1978) Evaluation of decamethrins and permethrin against Anopheles
gambiae and Anopheles funestus in a village trial in Nigeria
(WHO/VBC/78.679) (Unpublished report of Division of Vector Biology
Control, World Health Organization).
ROBERTS, T.R. & WRIGHT, A.N. (1981) The metabolism of 3-phenoxybenzyl
alcohol, a pyrethroid metabolite, in plants. Pestic. Sci., 12: 161-
169.
ROCK, G.C. (1979) Relative toxicity of two synthetic pyrethroids to a
predator Amblyseius fallacis and its prey Tetranychus urticae. J. econ.
Entomol., 72: 293-294.
ROSS, D.B. & PRENTICE, D.E. (1977) Examination of permethrin (PP557)
for neurotoxicity in the domestic hen, Huntingdon, Huntingdon Research
Centre (Report No. ICI/157-NT/77468) (Unpublished data submitted to WHO
by FMC Corporation and ICI Ltd).
ROSS, D.B., CAMERON, D.M., & ROBERTS, N.L. (1976a) The acute oral
toxicity (LD50 ) of PP557 (permethrin) to Mallard ducks, Huntingdon,
Huntingdon Research Centre (Report No. ICI 68/WL/7639) (Unpublished
data submitted to WHO by ICI Ltd).
ROSS, D.B., CAMERON, D.M., & ROBERTS, N.L. (1976b) The sub-acute
toxicity (LC50 ) of PP557 (permethrin) to Mallard ducks, Huntingdon,
Huntingdon Research Centre (Report No. ICI 68/WL/75837) (Unpublished
data submitted to WHO by ICI Ltd).
ROSS, D.B., CAMERON, D.M., & ROBERTS, N.L. (1976c) The acute oral
toxicity (LD50 ) of PP557 (permethrin) to Starling, Huntingdon,
Huntingdon Research Centre (Report No. ICI 68/WL/7637) (Unpublished
data submitted to WHO by ICI Ltd).
ROSS, D.B., CAMERON, D.M., & ROBERTS, N.L. (1976d) The sub-acute
toxicity (LC50 ) of PP557 (permethrin) to Starling, Huntingdon,
Huntingdon Research Centre (Report No. ICI 68/WL/7636) (Unpublished
data submitted to WHO by ICI Ltd).
ROSS, D.B., CAMERON, D.M., & ROBERTS, N.L. (1976e) The sub-acute
toxicity (LC50 ) of PP557 (permethrin) to Ring-necked pheasant,
Huntingdon, Huntingdon Research Centre (Report No. ICI 68/WL/75839)
(Unpublished data submitted to WHO by ICI Ltd).
ROSS, D.B., CAMERON, D.M., & ROBERTS, N.L. (1977a) The acute oral
toxicity (LD50 ) of PP557 (permethrin) to Ring-necked pheasant,
Huntingdon, Huntingdon Research Centre (Report No. ICI 68/WL/77154)
(Unpublished data submitted to WHO by ICI Ltd).
ROSS, D.B., PRENTICE, D.E., MAJEED, S.K., GIBSON, W.A., CAMERON,
D.M., CAMERON, M. McD., & ROBERTS, N.L. (1977b) The incorporation of
permethrin in the diet of laying hens (part I), Huntingdon, Huntingdon
Research Centre (Report No. ICI 152/77387) (Unpublished data submitted
to WHO by ICI Ltd).
ROUSH, R.T. & HOY, M.A. (1978) Relative toxicity of permethrin to a
predator, Metaseiulus occidentalis, and its prey, Tetranychus urticae.
Environ. Entomol., 7: 287-288.
RUIGT, G.S.F. & VAN DEN BERCKEN, J. (1986) Action of pyrethroids on a
nerve-muscle preparation of the clawed frog, Xenopus laevis. Pestic.
Biochem. Physiol., 25: 176-187.
SASINOVICH, L.M. & PANSHINA, T.N. (1987) [Substantiation of hygienic
rules for synthetic pyrethroid content in the work zone air]. Gig. Tr.
prof. Zabol., 8: 48-50 (in Russian).
SCHROEDER, R.E. & RINEHART, W.E. (1977) A three generation
reproduction study of FMC33297 in rats (Bio-Dynamics Inc Project)
(Unpublished report submitted to WHO by FMC Corporation).
SHAH, P.V., MONROE, R.J., & GUTHRIE, F.E. (1981) Comparative rates of
dermal penetration of insecticides in mice. Toxicol. appl. Pharmacol.,
59: 414-423.
SHAROM, M.S. & SOLOMON, K.R. (1981) Adsorption-desorption,
degradation, and distribution of permethrin in aqueous systems. J.
agric. food Chem., 29: 1122-1125.
SHERMAN, R.A. (1979) Preliminary behavioural assessment of habituation
to the insecticide permethrin, Aberdeen Proving Ground, Maryland, US
Army Environmental Hygiene Agency (Report No. 75-51-002679).
SHIRASU, Y., MORIYA, M., & OTA, T. (1979) Mutagenicity of S-3151 in
bacterial test systems (Unpublished report submitted to WHO by Sumitomo
Chemical Co.).
SHONO, T., OHSAWA, K., & CASIDA, J.E. (1979) Metabolism of trans- and
cis-permethrin, trans- and cis-cypermethrin, and decamethrin by
microsomal enzymes. J. agric. food Chem., 27: 316-325.
SHOUR, M.H. & CROWDER, L.A. (1980) Effects of pyrethroid insecticides
on the common green lacewing. J. econ. Entomol., 73: 306-309.
SIEGEL, M.M., HILDEBRAND, B.E., & HALL, D.R. (1980) Determination of
permethrin in environmental waters by gas chromatography-selected ion
monitoring-mass spectroscopy using ion counting detection. Int. J.
environ. anal. Chem., 8: 107-126.
SIMMON, V.F. (1976) In vitro microbiological mutagenicity study of an
FMC corporation compound, Menlo Park, Stanford Research Institute,
(Report No. LSC-4768) (Unpublished report submitted to WHO).
SIMONAITIS, R.A. & CAIL, R.S. (1977) Gas chromatographic determination
of residues of the synthetic pyrethroid FMC33297. In: Elliott, M., ed.
Synthetic pyrethroids, Washington, DC, American Chemical Society,
pp. 211-223 (ACS Symposium Series U2).
SODERLUND, D.M. & CASIDA, J.E. (1977) Effects of pyrethroid structure
on rate of hydrolysis and oxidation by mouse liver microsomal enzymes.
Pestic. Biochem. Physiol., 7: 391-401.
SPENCER, F. & BERHANCE, Z. (1982) Uterine and fetal characteristics in
rats following a post-implantational exposure to permethrin. Bull.
environ. Contam. Toxicol., 29: 84-88.
STAATZ, C.G., BLOOM, A.S., & LECH, J.J. (1982) A pharmacological
study of pyrethroid neurotoxicity in mice. Pestic. Biochem. Physiol.,
17: 287-292.
STEVENSON, J.H., NEEDHAM, P.H., & WALKER, J. (1978) Poisoning of
honeybee by pesticides: Investigation of the changing pattern in
Britain over 20 years. Rothamsted Experimental Station Report (1977),
Part 2: 55-72.
STRATTON, G.W. & CORKE, C.T. (1981) Interaction of permethrin with
Daphnia magna in the presence and absence of particulate material.
Environ. Pollut., A24: 135-144.
STRATTON, G.W. & CORKE, C.T. (1982) Toxicity of the insecticide
permethrin and some degradation products towards algae and
cyanobacteria. Environ. Pollut. A29: 71-80.
SUNDARAM, K.M.S., DE GROOT, P., & SUNDARAM, A. (1987)
Permethrin deposits and airborne concentrations downwind from a single
swath application using a back pack mist blower. J. environ. Sci.
Health, B22: 171-193.
SUZUKI, H. (1977) Studies on the mutagenicity of some pyrethroids on
salmonella strains in the presence of mouse hepatic S9 fractions
(Unpublished report submitted to WHO by Sumitomo Chemical Co.).
SWAINE, H. & SAPIETS, A. (1981a) Cypermethrin: residue transfer study
with dairy cows fed on a diet containing the insecticide (Report No.
RJ0186B) (Unpublished data submitted to WHO by ICI Ltd).
SWAINE, H. & SAPIETS, A. (1981b) Cypermethrin: residue levels of the
major metabolites of the insecticide in the milk and tissues of dairy
cows fed on a diet containing cypermethrin at 50 mg/kg (Report No.
RJ0198B) (Unpublished data submitted to WHO by ICI Ltd).
SWAINE, H., EDWARD, M.J., & USSARY, J.P. (1978) Permethrin crop
protection study (Report No. TMV 0378B) (Unpublished data submitted to
WHO by ICI Ltd).
TAKAHASHI, K., OKUDA, No., & SHIRASU, Y. (1979) Effects of
permethrin on hexobarbital induced sleeping time in mice and electro-
encephalography in rabbits (Unpublished report submitted to WHO by
Sumitomo Chemical Co.).
TYLER, J.F.C. (1987) Gas chromatographic method for determination of
permethrin in pesticide formulations: Collaborative study. J. Assoc.
Off. Anal. Chem., 70(1): 53-55.
US EPA (1981) Advisary opinion on the oncogenic potential of
permethrin, Washington, DC, US Environmental Protection Agency
(Unpublished report submitted to WHO).
USSARY, J.P. (1979) Permethrin residues on sweet corn (Report No.
TMU0438B) (Unpublished data submitted to WHO by ICI Americas Inc.).
USSARY, J.P. (1978) Permethrin residues on sweet corn (Report No.
TMU0413B) (Unpublished data submitted to WHO by ICI Americas Inc.).
USSARY, J.P. & BRAITHWAITE, G.B. (1980) Residues of permethrin and 3-
phenoxy-benzyl alcohol in cow tissues (Trial No. 35NC79-001. Report
No. TMU0493B) (Unpublished data submitted to WHO by ICI Americas
Inc.).
VAN DEN BERCKEN, J. (1977) The action of allethrin on the peripheral
nervous system of the frog. Pestic. Sci., 8: 692-699.
VAN DEN BERCKEN, J. & VIJVERBERG, H.P.M. (1980a) Voltage clamp
studies on the effects of allethrin and DDT on the sodium channels in
frog myelinated nerve membrane. In: Insect neurobiology and pesticide
action, London, Society of Chemical Industry, pp. 79-85.
VAN DEN BERCKEN, J. & VIJVERBERG, H.P.M. (1980b) Effects of
insecticides on the sensory system of Xenopus. In: Insect neurobiology
and pesticide action, London, Society of Chemical Industry, pp.391-
397.
VAN DEN BERCKEN, J., AKKERMANS, L.M.A., & VAN DER ZALM,
J.M., (1973) DDT-like action of allethrin in the sensory nervous
system of Xenopus laevis. Eur. J. Pharmacol., 21: 95-106.
VAN DEN BERCKEN, J., KROESE, A.B.A., & AKKERMANS, L.M.A.
(1979) Effect of insecticides on the sensory nervous system. In:
Narahashi, T., ed. Neurotoxicology of insecticides and pheromones,
New York, London, Plenum Press, pp. 183-210.
VERSCHOYLE, R.D. & ALDRIDGE, W.N. (1980) Structure-activity
relationship of some pyrethrins to rats. Arch. Toxicol., 45: 325-329.
VIJVERBERG, H.P.M. & VAN DEN BERCKEN, J. (1979) Frequency-
dependent effects of the pyrethroid insecticide decamethrin in frog
myelinated nerve fibres. Eur. J. Phamacol., 58: 501-504.
VIJVERBERG, H.P.M. & VAN DEN BERCKEN, J. (1982) Action of
pyrethroid insecticides on the vertebrate nervous system. Neuropathol.
appl. Neurobiol., 8: 421-440.
VIJVERBERG, H.P.M., RUIGT, G.S.F., & VAN DEN BERCKEN, J. (1982a)
Structure-related effects of pyrethroid insecticides on the lateral-
line sense organ and on peripheral nerves of the clawed frog, Xenopus
laevis. Pestic. Biochem. Physiol., 18: 315-324.
VIJVERBERG, H.P.M., VAN DER ZALM, J.M., & VAN DEN BERCKEN, J.
(1982b) Similar mode of action of pyrethroids and DDT on sodium
channel gating in mylinated nerves. Nature (Lond.), 295: 601-603.
VIJVERBERG, H.P.M., VAN DER ZALM, J.M., VAN KLEEF, R.G.D.M., &
VAN DEN BERCKEN, J. (1983) Temperature and structure-dependent
interaction of pyrethroids with the sodium channels in frog node of
Ranvier. Biochim. Biophys. Acta, 728: 73-82.
WALLWORK, L.M. & MALONE, J.C. (1974) 21Z73 (25:75) effect of
different solvents on the rat oral toxicity (Report No. HEFG 75-4)
Berkhamsted, Wellcome Research Laboratories (Unpublished data submitted
to WHO).
WALLWORK, L.M., CLAMPITT, R.B., & MALONE, J.C. (1974a) 10-day
cumulative oral toxicity study with 21Z73 in mice, Berkhamsted,
Wellcome Research Laboratories (Report No. HEFG 74-9) (Unpublished
data submitted to WHO).
WALLWORK, L.M., CLAMPITT, R.B., & MALONE, J.C. (1974b) 10-day
cumulative oral toxicity study with 21Z73 in rats, Berkhamsted,
Wellcome Research Laboratories (Unpublished report submitted to WHO).
WELLS, D., GRAYSON, B.T., & LANGNER, E. (1986) Vapour pressure of
permethrin. Pestic. Sci., 17: 473-476.
WHO (1979) WHO Technical Report Series, No. 634 (Safe use of
pesticides. Third Report of the WHO Expert Committee on Vector Biology
and Control), pp. 18-23.
WHO (1988) The WHO recommended classification of pesticides by
hazard: Guidelines to classification 1988-89, Geneva, World Health
Organization (Unpublished report VBC/88.953).
WHO/FAO (1984) Data sheet on pesticides No. 51: Permethrin, Geneva,
World Health Organization (VBC/DS/84.51).
WILLIAMS, I.H. & BROWN, M.J. (1979) Persistence of permethrin and WL
43775 in soil. J. agric. food Chem., 27: 130-132.
WOODRUFF, R.C., PHILLIPS, J.P., & IRWIN, D. (1983) Pesticide-induced
complete and partial chromosome loss in screens with repair-defective
females of Drosophila melanogaster. Environ. Mutagen., 5: 835-846.
WOOD, MACKENZIE & Co. (1980) Pyrethroids. Agrochem. Monit., 9: 3-14.
WOOD, MACKENZIE & Co. (1981) Pyrethroids. Agrochem. Monit., 15: 3-27.
WOOD, MACKENZIE & Co. (1982) Pyrethroids. Agrochem. Monit., 21: 3-17.
WOOD, MACKENZIE & Co. (1983) Pyrethroids. Agrochem. Monit., 27: 3-12.
WOOD, MACKENZIE & Co. (1984) Pyrethroids. Agrochem. Monit., 33: 2-12.
WORKMAN, R.B. (1977) Pesticides toxic to striped earwig, an important
insect predator. Proc. Florida State Hortic. Soc., 90: 401-402.
WORTHING, C.R. & WALKER, S.B. (1983) The pesticide manual, 7th ed.,
Croydon, British Crop Protection Council, p. 427.
WORTHING, C.R. & WALKER, S.B. (1987) Permethrin. In: The pesticide
manual, 8th ed., Croydon, British Crop Protection Council, pp. 647-
648.
WOUTERS, W. & VAN DEN BERCKEN, J. (1978) Action of pyrethroids. Gen.
Pharmacol., 9: 387-398.
ZITKO, V., CARSON, W.G., & METCALFE, C.D. (1977) Toxicity of
pyrethroids to juvenile atlantic salmon. Bull. environ. Contam.
Toxicol., 18: 35-41.
ZITKO, V., MCLEESE, D.W., METCALFE, C.D., & CARSON, W.G. (1979)
Toxicity of permethrin, decamethrin, and related pyrethroids to salmon
and lobster. Bull. environ. Contam. Toxicol., 21: 338-343.
APPENDIX I
On the basis of electrophysiological studies with peripheral nerve
preparations of frogs ( Xenopus laevis; Rana temporaria, and Rana
esculenta ), it is possible to distinguish between 2 classes of
pyrethroid insecticides: (Type I and Type II). A similar distinction
between these 2 classes of pyrethroids has been made on the basis of
the symptoms of toxicity in mammals and insects (Van den Bercken et
al., 1979; WHO, 1979; Verschoyle & Aldridge, 1980; Glickman & Casida,
1982; Lawrence & Casida, 1982). The same distinction was found in
studies on cockroaches (Gammon et al., 1981).
Based on the binding assay on the gamma-aminobutyric acid (GABA)
receptor-ionophore complex, synthetic pyrethroids can also be
classified into two types: the alpha-cyano-3-phenoxybenzyl pyrethroids
and the non-cyano pyrethroids (Gammon et al., 1982; Gammon & Casida,
1983; Lawrence & Casida, 1983; Lawrence et al., 1985).
Pyrethroids that do not contain an alpha-cyano group (allethrin,
d-phenothrin, permethrin, tetramethrin, cismethrin, and bioresmethrin)
(Type I: T-syndrome)
The pyrethroids that do not contain an alpha-cyano group give rise
to pronounced repetitive activity in sense organs and in sensory nerve
fibres (Van den Bercken et al., 1973). At room temperature, this
repetitive activity usually consists of trains of 3-10 impulses and
occasionally up to 25 impulses. Train duration is between 10 and 5
milliseconds.
These compounds also induce pronounced repetitive firing of the
presynaptic motor nerve terminal in the neuromuscular junction (Van den
Bercken, 1977). There was no significant effect of the insecticide on
neurotransmitter release or on the sensitivity of the subsynaptic
membrane, nor on the muscle fibre membrane. Presynaptic repetitive
firing was also observed in the sympathetic ganglion treated with these
pyrethroids.
In the lateral-line sense organ and in the motor nerve terminal,
but not in the cutaneous touch receptor or in sensory nerve fibres, the
pyrethroid-induced repetitive activity increases dramatically as the
temperature is lowered, and a decrease of 5°C in temperature may cause
a more than 3-fold increase in the number of repetitive impulses per
train. This effect is easily reversed by raising the temperature. The
origin of this "negative temperature coefficient" is not clear
(Vijverberg et al., 1983).
Synthetic pyrethroids act directly on the axon through interference
with the sodium channel gating mechanism that underlies the generation
and conduction of each nerve impulse. The transitional state of the
sodium channel is controlled by 2 separately acting gating mechanisms,
referred to as the activation gate and the inactivation gate. Since
pyrethroids only appear to affect the sodium current during
depolarization, the rapid opening of the activation gate and the slow
closing of the inactivation gate proceed normally. However, once the
sodium channel is open, the activation gate is restrained in the open
position by the pyrethroid molecule. While all pyrethroids have
essentially the same basic mechanism of action, however, the rate of
relaxation differs substantially for the various pyrethroids (Flannigan
& Tucker, 1985).
In the isolated node of Ranvier, allethrin causes prolongation of
the transient increase in sodium permeability of the nerve membrane
during excitation (Van den Bercken & Vijverberg, 1980a,b). Evidence so
far available indicates that allethrin selectively slows down the
closing of the activation gate of a fraction of the sodium channels
that open during depolarization of the membrane. The time constant of
closing of the activation gate in the allethrin-affected channels is
about 100 milliseconds compared with less than 100 microseconds in the
normal sodium channel, i.e., it is slowed down by a factor of more than
100. This results in a marked prolongation of the sodium current
across the nerve membrane during excitation, and this prolonged sodium
current is directly responsible for the repetitive activity induced by
allethrin (Vijverberg et al., 1983).
The effects of cismethrin on synaptic transmission in the frog
neuromuscular junction, as reported by Evans (1976), are almost
identical to those of allethrin, i.e., presynaptic repetitive firing,
and no significant effects on transmitter release or on the subsynaptic
membrane.
Interestingly, the action of these pyrethroids closely resembles
that of the insecticide DDT in the peripheral nervous system of the
frog. DDT also causes pronounced repetitive activity in sense organs,
in sensory nerve fibres, and in motor nerve terminals, due to a
prolongation of the transient increase in sodium permeability of the
nerve membrane during excitation. Recently, it was demonstrated that
allethrin and DDT have essentially the same effect on sodium channels
in frog myelinated nerve membrane. Both compounds slow down the rate
of closing of a fraction of the sodium channels that open on
depolarization of the membrane (Van den Bercken et al., 1973, 1979;
Vijverberg et al., 1982b).
In the electrophysiological experiments using giant axons of
crayfish, the type I pyrethroids and DDT analogues retain sodium
channels in a modified open state only intermittently, cause large
depolarizing after-potentials, and evoke repetitive firing with minimal
effect on the resting potential (Lund & Narahashi, 1983).
These results strongly suggest that permethrin and cismethrin, like
allethrin, primarily affect the sodium channels in the nerve membrane
and cause a prolongation of the transient increase in sodium
permeability of the membrane during excitation.
The effects of pyrethroids on end-plate and muscle action
potentials were studied in the pectoralis nerve-muscle preparation of
the clawed frog ( Xenopus laevis ). Type I pyrethroids (allethrin,
cismethrin, bioresmethrin, and 1R, cis-phenothrin) caused moderate
presynaptic repetitive activity, resulting in the occurrence of
multiple end-plate potentials (Ruigt & Van den Bercken, 1986).
Pyrethroids with an alpha-cyano group on the 3-phenoxybenzyl alcohol
(deltamethrin, cypermethrin, cyhalothrin, lambda-cyhalothrin,
fenvalerate, and fenpropanate) (Type II: CS-syndrome)
The pyrethroids with an alpha-cyano group cause an intense repetitive
activity in the lateral line organ in the form of long-lasting trains
of impulses (Vijverberg et al., 1982a). Such a train may last for up
to 1 min and contains thousands of impulses. The duration of the
trains and the number of impulses per train increase markedly on
lowering the temperature. Cypermethrin does not cause repetitive
activity in myelinated nerve fibres. Instead, this pyrethroid causes a
frequency-dependent depression of the nervous impulse, brought about by
a progressive depolarization of the nerve membrane as a result of the
summation of depolarizing after-potentials during train stimulation
(Vijverberg & Van den Bercken, 1979; Vijverberg et al., 1983).
In the isolated node of Ranvier, cypermethrin, like allethrin,
specifically affects the sodium channels of the nerve membrane and
causes a long-lasting prolongation of the transient increase in sodium
permeability during excitation, presumably by slowing down the closing
of the activation gate of the sodium channel (Vijverberg & Van den
Bercken, 1979; Vijverberg et al., 1983). The time constant of closing
of the activation gate in the cypermethrin-affected channels is
prolonged to more than 100 milliseconds. Apparently, the amplitude of
the prolonged sodium current after cypermethrin is too small to induce
repetitive activity in nerve fibres, but is sufficient to cause the
long-lasting repetitive firing in the lateral-line sense organ.
These results suggest that alpha-cyano pyrethroids primarily affect
the sodium channels in the nerve membrane and cause a long-lasting
prolongation of the transient increase in sodium permeability of the
membrane during excitation.
In the electrophysiological experiments using giant axons of
crayfish, the Type II pyrethroids retain sodium channels in a modified
continuous open state persistently, depolarize the membrane, and block
the action potential without causing repetitive firing (Lund &
Narahashi, 1983).
Diazepam, which facilitates GABA reaction, delayed the onset of
action of deltamethrin and fenvalerate, but not permethrin and
allethrin, in both the mouse and cockroach. Possible mechanisms of the
Type II pyrethroid syndrome include action at the GABA receptor complex
or a closely linked class of neuroreceptor (Gammon et al., 1982).
The Type II syndrome of intracerebrally administered pyrethroids
closely approximates that of the convulsant picrotoxin (PTX).
Deltamethrin inhibits the binding of [3H]-dihydropicrotoxin to rat
brain synaptic membranes, whereas the non-toxic R epimer of
deltamethrin is inactive. These findings suggest a possible relation
between the Type II pyrethroid action and the GABA receptor complex.
The stereospecific correlation between the toxicity of Type II
pyrethroids and their potency to inhibit the [35S]-TBPS binding was
established using a radioligand, [35S]- t-butyl-bicyclophosphoro-
thionate [35S]-TBPS. Studies with 37 pyrethroids revealed an
absolute correlation, without any false positive or negative, between
mouse intracerebral toxicity and in vitro inhibition: all toxic cyano
compounds including deltamethrin, 1R, cis-cypermethrin, 1R, trans-
cypermethrin, and [2S,alphaS]-fenvalerate were inhibitors, but their
non-toxic stereoisomers were not; non-cyano pyrethroids were much less
potent or were inactive (Lawrence & Casida, 1983).
In the [35S]-TBPS and [3H]-Ro 5-4864 (a convulsant
benzodiazepine radioligand) binding assay, the inhibitory potencies of
pyrethroids were closely related to their mammalian toxicities. The
most toxic pyrethroids of Type II were the most potent inhibitors of
[3H]-Ro 5-4864 specific binding to rat brain membranes. The [3H]-
dihydropicrotoxin and [ 35S]-TBPS binding studies with pyrethroids
strongly indicated that Type II effects of pyrethroids are mediated, at
least in part, through an interaction with a GABA-regulated chloride
ionophore-associated binding site. Moreover, studies with [3H]-Ro 5-
4864 support this hypothesis and, in addition, indicate that the
pyrethroid-binding site may be very closely related to the convulsant
benzodiazepine site of action (Lawrence et al., 1985).
The Type II pyrethroids (deltamethrin, 1R, cis-cypermethrin and
[2S,alphaS]-fenvalerate) increased the input resistance of crayfish
claw opener muscle fibres bathed in GABA. In contrast, two non-insect-
icidal stereoisomers and Type I pyrethroids (permethrin, resmethrin,
allethrin) were inactive. Therefore, cyanophenoxybenzyl pyrethroids
appear to act on the GABA receptor-ionophore complex (Gammon & Casida,
1983).
The effects of pyrethroids on end-plate and muscle action
potentials were studied in the pectoralis nerve-muscle preparation of
the clawed frog ( Xenopus laevis). Type II pyrethroids (cypermethrin
and deltamethrin) induced trains of repetitive muscle action potentials
without presynaptic repetitive activity. However, an intermediate
group of pyrethroids (1R-permethrin, cyphenothrin, and fenvalerate)
caused both types of effect. Thus, in muscle or nerve membrane the
pyrethroid induced repetitive activities due to a prolongation of the
sodium current. But no clear distinction was observed between non-
cyano and alpha-cyano pyrethroids (Ruigt & Van den Bercken, 1986).
Appraisal
In summary, the results strongly suggest that the primary target
site of pyrethroid insecticides in the vertebrate nervous system is the
sodium channel in the nerve membrane. Pyrethroids without an alpha-cyano
group (allethrin, d-phenothrin, permethrin, and cismethrin) cause a
moderate prolongation of the transient increase in sodium permeability
of the nerve membrane during excitation. This results in relatively
short trains of repetitive nerve impulses in sense organs, sensory
(afferent) nerve fibres, and, in effect, nerve terminals. On the other
hand, the alpha-cyano pyrethroids cause a long-lasting prolongation of
the transient increase in sodium permeability of the nerve membrane
during excitation. This results in long-lasting trains of repetitive
impulses in sense organs and a frequency-dependent depression of the
nerve impulse in nerve fibres. The difference in effects between
permethrin and cypermethrin, which have identical molecular structures
except for the presence of an alpha-cyano group on the phenoxybenzyl
alcohol, indicates that it is this alpha-cyano group that is responsible
for the long-lasting prolongation of the sodium permeability.
Since the mechanisms responsible for nerve impulse generation and
conduction are basically the same throughout the entire nervous system,
pyrethroids may also induce repetitive activity in various parts of the
brain. The difference in symptoms of poisoning by alpha-cyano
pyrethroids, compared with the classical pyrethroids, is not
necessarily due to an exclusive central site of action. It may be
related to the long-lasting repetitive activity in sense organs and
possibly in other parts of the nervous system, which, in a more advance
state of poisoning, may be accompanied by a frequency-dependent
depression of the nervous impulse.
Pyrethroids also cause pronounced repetitive activity and a
prolongation of the transient increase in sodium permeability of the
nerve membrane in insects and other invertebrates. Available
information indicates that the sodium channel in the nerve membrane is
also the most important target site of pyrethroids in the invertebrate
nervous system (Wouters & Van den Bercken, 1978; WHO, 1979).
Because of the universal character of the processes underlying
nerve excitability, the action of pyrethroids should not be considered
restricted to particular animal species, or to a certain region of the
nervous system. Although it has been established that sense organs and
nerve endings are the most vulnerable to the action of pyrethroids, the
ultimate lesion that causes death will depend on the animal species,
environmental conditions, and on the chemical structure and physical
characteristics of the pyrethroid molecule (Vijverberg & Van den
Bercken, 1982).
RESUME, EVALUATION, CONLUSIONS ET RECOMMANDATIONS
1. Résumé et Evaluation
1.1 Identité, propriétés physicochimiques et méthodes d'analyse
La perméthrine, un pyréthroide photostable, a été synthétisée pour
la première fois en 1973 et commercialisée en 1977. C'est un ester de
l'analogue dichloré de l'acide chrysanthémique, à savoir l'acide
(dichloro-2,2 vinyl)-3 diméthyl-2,2 cyclopropanecarboxylique (Cl2CA) et
de l'alcool phénoxy-3 benzylique. Les produits techniques se présentent
sous la forme d'un mélange de quatre stéréoisomères ayant les
configurations [1R,trans], [1R,cis], [1S,trans] et [1S,cis] dans les
proportions approximatives de 3:2:3:2. Le rapport cis/trans est
d'environ deux tiers, les isomères 1R et 1S étant présents en quantités
égales (racémique). C'est l'isomère [1R,cis] qui est l'insecticide le
plus actif; vient ensuite l'isomère [1R,trans].
La perméthrine de qualité technique se présente sous la forme d'un
liquide brun ou brun jaunâtre qui peut partiellement cristalliser à la
température ambiante. Son point de fusion est d'environ 35°C et son
point d'ébullition de 220°C sous 0,05 mmHg. La densité est de 1,214 à
25°C et la tension de vapeur de 1,3 µPa à 20°C. La perméthrine est
pratiquement insoluble dans l'eau (moins de 0,2 mg par litre à 25°C),
mais elle est soluble dans certains solvants organiques tels que
l'acétone, l'hexane et le xylène. Elle est stable à la lumière et à la
chaleur, mais instable en milieu alcalin.
Le dosage des résidus et les analyses écotoxicologiques s'effect-
uent par chromatographie en phase gazeuse avec détection par capture
d'électrons (concentration minimale décelable de 0,005 mg/kg). Pour
l'analyse des produits techniques, on a recours à la chromatographie en
phase gazeuse avec détection par ionisation de flamme.
1.2 Production et usage
On utilise actuellement dans le monde environ 600 tonnes de
perméthrine par an, essentiellement en agriculture. Ce produit pourrait
être utilisé pour la protection des céréales ensilées et on l'emploie
en épandage aérien pour la protection des forêts, la lutte anti-
vectorielle, la destruction des insectes incommodants dans les
habitations, le déparasitage des bestiaux, la destruction des poux du
corps et l'imprégnation des moustiquaires.
La perméthrine est présentée en concentré émulsionnable, en
concentré pour application à très bas volume, en poudre mouillable, en
poudre pour poudrage et en aérosols.
1.3 Exposition humaine
Les teneurs en résidus des diverses récoltes diminuent assez
lentement, le temps de demi-élimination allant de une à trois semaines
selon les plantes. Toutefois, lorsqu'elle est utilisée conformément
aux recommandations, la perméthrine ne donne pas lieu à une accumu-
lation de résidus, même après plusieurs traitements.
Pour ce qui est de la population dans son ensemble, la voie
d'exposition à la perméthrine est essentiellement alimentaire. Les taux
de résidus dans les plantes correctement cultivées sont généralement
faibles. L'exposition qui pourrait en résulter pour la population
générale est vraisemblablement faible, mais on manque de données
précises provenant d'études sur la ration totale.
On est très peu documenté sur l'exposition professionnelle à la
perméthrine.
1.4 Destinée dans l'environnement
On a montré en laboratoire que la perméthrine se dégrade dans le
sol avec une demi-vie d'environ 28 jours. L'isomère trans se décompose
plus rapidement que l'isomère cis, la principale réaction de décompo-
sition initiale étant le clivage du groupement ester. Cette réaction
donne naissance à des composés qui subissent une oxydation plus poussée
aboutissant à l'anhydride carbonique comme produit final. On a montré,
en étudiant le potentiel de lessivage de la perméthrine et de ses
produits de dégradation, que toutes ces substances pénétraient peu
profondément dans le sol.
La perméthrine déposée sur les végétaux se dégrade avec une demi-
vie d'environ 10 jours. La principale voie de dégradation comporte le
clivage du groupement ester et la conjugaison de l'acide et de l'alcool
qui en résultent. Il se produit également une hydroxylation en divers
points de la molécule ainsi qu'une interconversion cis-trans photo-
induite.
Dans l'eau et à la surface du sol, la perméthrine est décomposée
par le rayonnement solaire. Là encore, le clivage du groupement ester
et l'interconversion cis-trans sont les principales réactions.
En général, ce processus de décomposition environnementale conduit
à des produits de moindre toxicité.
La perméthrine disparaît rapidement de l'environnement, en six
à 24 heures dans les étangs et les cours d'eau, en sept jours dans les
sédiments des étangs, et en 58 jours dans les feuilles et le sol des
forêts. On a constaté que 30% du composé disparaissaient en une semaine
des feuilles d'une plantation de cotonniers.
En conditions d'aérobiose dans le sol, la perméthrine se décompose
avec une demi-vie de 28 jours.
La perméthrine se déplace peu dans l'environnement et il est
improbable qu'elle s'y accumule en quantité notable.
1.5 Cinétique et métabolisme
Administrée à des mammifères, la perméthrine est rapidement
métabolisée et presqu'entièrement excrétée dans les urines et les
matières fécales en l'espace de 12 jours. L'isomère trans, beaucoup
plus sensible à l'attaque par l'estérase que l'isomère cis, s'élimine
plus rapidement que ce dernier. Les principales réactions métaboliques
sont le clivage du groupement ester et l'oxydation, particulièrement au
niveau du cycle aromatique terminal, du reste phénoxybenzylique et du
groupe diméthyle géminé du cycle cyclopropane, réactions qui sont
suivies d'une conjugaison. On a retrouvé moins de 0,7% de la dose
initiale dans le lait de chèvres et de vaches à qui l'on avait
administré de la perméthrine par voie orale.
1.6 Effets sur les êtres vivants dans leur milieu naturel
Des épreuves de laboratoire ont montré que la perméthrine était
extrêmement toxique pour les arthropodes aquatiques, la CL50 allant de
0,018 µg/litre pour la larve d'un crabe comestible à 1,26 µg/litre pour
un cladocère. Elle est également très toxique pour les poissons, la
CL50 à 96 heures allant de 0,62 µg/litre pour la larve de truite
arc-en-ciel à 314 µg/litre pour la truite adulte. La dose sans effet
observable au début du cycle évolutif du vairon est 10 µg/litre sur 28
jours, la dose chronique étant de 0,66 à 1,4 µg/litre pour un autre
cyprinidé, Pimephas promelas. La perméthrine est moins toxique pour
les mollusques aquatiques et les amphibiens, les valeurs de la CL50 à
96 heures se situant respectivement à plus de 1000 µg/litre et
7000 µg/litre.
Lors d'essais sur le terrain et en utilisation normale, cette forte
toxicité potentielle ne se manifeste pas. Il existe une abondante
littérature sur les effets de la perméthrine utilisée en agriculture,
dans les exploitations forestières ainsi que pour la lutte antivectori-
elle dans de nombreuses régions du monde. Il y a une certaine mortalité
pour les arthropodes aquatiques, notamment lorsque l'on traite les eaux
en surface, mais les effets sur les populations ne sont que tempor-
aires. Il n'y a pas eu de cas de mortalité chez les poissons. Cette
moindre toxicité qui se manifeste sur le terrain provient de la forte
adsorption du composé par les sédiments et de sa décomposition rapide.
La perméthrine fixée sur les sédiments est toxique pour les
organismes fouisseurs, mais là encore, l'effet est temporaire. La
perméthrine est extrêmement toxique pour les abeilles. La DL50 topique est
de 0,11 µg par abeille mais le fort effet répulsif qu'elle exerce sur
l'insecte en réduit les effets toxiques dans la pratique. Rien
n'indique qu'il puisse y avoir une forte mortalité des abeilles en
utilisation normale. La perméthrine est plus toxique pour les acariens
prédateurs que pour les espèces nuisibles visées.
La perméthrine est très peu toxique pour les oiseaux, en
administration orale ou par voie alimentaire. La DL50 aiguë est
supérieure à 3000 mg par kg de poids corporel en une seule
administration et supérieure à 5000 mg par kg de nourriture quand le
produit est mêlé à la ration. Elle n'a aucun effet sur la reproduction
de la poule à la dose de 40 mg/kg de nourriture.
Les organismes aquatiques accumulent facilement la perméthrine, le
facteur de concentration allant de 43 à 750 selon les espèces. Chez
tous les organismes aquatiques étudiés, la perméthrine disparaît
rapidement lorsque les animaux sont remis en eau propre. Il n'y a
aucune accumulation chez les oiseaux. On peut donc considérer que ce
composé ne présente en pratique aucune tendence à la bioaccumulation.
1.7 Effets sur les animaux d'expérience et sur les systèmes
d'épreuve in vitro
La perméthrine n'a qu'une faible toxicité aiguë pour les rats, les
souris, les lapins et les cobayes, encore que la DL50 varie
considérablement selon le véhicule utilisé et le rapport des isomères
cis/trans. Les signes d'intoxication aiguë apparaissent en deux heures
et persistent jusqu'à trois jours. Les isomères [1R,cis] et [1R,trans]
appartiennent aux pyréthroïdes du type-I dont les effets caractérist-
iques sont les tremblements (syndrome T) la perte de coordination,
l'hyperactivité, la prostration et la paralysie. L'intoxication pro-
voque une augmentation notable de la température centrale.
Aucun des métabolites de la perméthrine ne présente une toxicité
aiguë (par voie orale ou intrapéritonéale) supérieure à celle de la
perméthrine elle-même.
La perméthrine a provoqué une légère irritation de la peau intacte
ou abrasée chez le lapin, mais il n'y a pas eu d'irritation d'origine
photochimique après exposition aux rayons ultra-violets de zones de la
peau traitées par cet insecticide. Elle ne suscite en revanche aucune
réaction de sensibilisation chez le cobaye.
Des études de toxicité orale subaiguë et subchronique ont été
effectuées chez le rat et la souris à des doses allant jusqu'à
10 000 mg/kg de nourriture et pendant des périodes s'étendant de 14 à
26 semaines. A la dose la plus forte, on a observé une augmentation du
rapport poids du foie/poids du corps, une hypertrophie du foie et des
signes cliniques d'intoxication tels que des tremblements. Chez le rat,
la dose sans effet observable varie de 20 mg/kg de nourriture (pour les
études de 90 jours ou de six mois) à 150 mg/kg de nourriture (lors
d'une étude de six mois).
Chez le chien, ces valeurs ont été trouvées égales à 50 mg/kg de
poids corporel et à 100 mg/kg de poids corporel, respectivement, lors
de deux études de trois mois.
Des études à long terme sur des souris et des rats ont révélé une
augmentation du poids du foie, vraisemblablement liée à l'induction du
système enzymatique des microsomes hépatiques.
Une étude de deux ans sur des rats a fait ressortir une dose sans
effet observable de 100 mg/kg de nourriture, soit 5 mg/kg de poids
corporel.
D'après trois études à long terme sur la souris, il semblerait
qu'il existe un certain pouvoir cancérogène au niveau des poumons pour
au moins une souche de souris (uniquement des femelles) à la dose la
plus élevée étudiée, soit 5 g/kg de nourriture. Aucun effet oncogène
n'a été observé chez des rats des deux sexes.
La perméthrine n'est mutagène ni in vivo ni in vitro .
Les études de mutagénicité ainsi que des études à long terme sur la
souris et le rat indiquent que le pouvoir oncogène de la perméthrine
est très faible, qu'il se limite aux souris femelles et qu'il s'agit
probablement d'un phénomène épigénétique.
La perméthrine n'est pas tératogène pour le rat, la souris ou le
lapin, à des doses allant respectivement jusqu'à 225, 150 et 1800 mg/kg
de poids corporel.
La perméthrine n'a pas produit d'effet indésirable à des doses
allant jusqu'à 2500 mg/kg de nourriture lors d'une étude de repro-
duction portant sur trois générations.
Administrée à des rats à forte dose (6600 à 7000 mg/kg de
nourriture) pendant 14 jours, l'insecticide a provoqué des lésions au
niveau du nerf sciatique; cependant une autre étude n'a pas confirmé la
présence d'altérations ultrastructurales à ce niveau. Chez la poule on
n'a pas observé de neurotoxicité retardée.
1.8 Effets sur l'homme
La perméthrine peut provoquer un certain nombre de sensations au
niveau de la peau ainsi que des parésthésies chez les travailleurs
exposés, symptômes qui apparaissent après une période de latence
d'environ 30 minutes, passent par un maximum au bout de huit heures et
disparaissent en 24 heures. Parmi les symptômes les plus fréquemment
signalés, figurent un engourdissement, des démangeaisons, des four-
millements et des sensations de brûlure.
Aucun cas d'intoxication n'a été signalé.
La probabilité d'effets oncogènes chez l'homme est extrêmement
faible, voire nulle.
Rien n'indique que la perméthrine puisse exercer des effets indési-
rables sur l'homme si elle est utilisée conformément aux recommand-
ations.
2. Conclusions
2.1 Population générale
La population dans son ensemble est vraisemblablement très peu
exposée à la perméthrine. Cet insecticide ne devrait présenter aucun
danger s'il est utilisé conformément aux recommandations.
2.2 Exposition professionnelle
Utilisée de manière raisonnable et moyennant certaines mesures
d'hygiène et de sécurité, la perméthrine ne devrait présenter aucun
danger pour les personnes qui lui sont exposées de par leur
profession.
2.3 Environnement
La perméthrine ou ses produits de décomposition ne devraient pas
atteindre dans le milieu des concentrations critiques dans la mesure où
l'insecticide est utilisé aux doses recommandées. Au laboratoire, la
perméthrine est extrêmement toxique pour les poissons, les arthropodes
aquatiques et les abeilles. Toutefois il est improbable que des effets
indésirables durables se produisent en situation réelle si l'insecti-
cide est utilisé conformément aux recommandations.
3. Recommandations
Les concentrations alimentaires résultant d'une utilisation
conforme aux recommandations sont en principe faibles, toutefois il
serait bon de confirmer cette hypothèse en étendant la surveillance à
la perméthrine.
La perméthrine est utilisée depuis de nombreuses années sans
qu'aucun effet indésirable n'ait été signalé à la suite d'une
exposition humaine. Néanmoins il serait bon de poursuivre les
observations sur ce type d'exposition.
See Also:
Toxicological Abbreviations
Permethrin (HSG 33, 1989)
Permethrin (ICSC)
PERMETHRIN (JECFA Evaluation)
Permethrin (Pesticide residues in food: 1979 evaluations)
Permethrin (Pesticide residues in food: 1980 evaluations)
Permethrin (Pesticide residues in food: 1981 evaluations)
Permethrin (Pesticide residues in food: 1982 evaluations)
Permethrin (Pesticide residues in food: 1983 evaluations)
Permethrin (Pesticide residues in food: 1984 evaluations)
Permethrin (Pesticide residues in food: 1987 evaluations Part II Toxicology)
Permethrin (JMPR Evaluations 1999 Part II Toxicological)
Permethrin (UKPID)
Permethrin (IARC Summary & Evaluation, Volume 53, 1991)

 Table 1. Chemical identity of permethrin and its various stereoisomeric compositions
------------------------------------------------------------------------------------------------------
Common name/ CAS Index name (9Cl) Stereoisomeric Synonyms and
CAS Registry No./ compositionc trade names
NIOSH Accession No.a Stereospecific nameb
------------------------------------------------------------------------------------------------------
Permethrin Cyclopropanecarboxylic acid, (1):(2):(3):(4) Permethrina, Ambush,
Table 1. Chemical identity of permethrin and its various stereoisomeric compositions
------------------------------------------------------------------------------------------------------
Common name/ CAS Index name (9Cl) Stereoisomeric Synonyms and
CAS Registry No./ compositionc trade names
NIOSH Accession No.a Stereospecific nameb
------------------------------------------------------------------------------------------------------
Permethrin Cyclopropanecarboxylic acid, (1):(2):(3):(4) Permethrina, Ambush,
 4.1 Transport and Distribution between Media
In laboratory studies, permethrin in water was rapidly adsorbed
onto lake sediments or soil columns and was not desorbed or eluted
easily from them. However in forest spray trials, permethrin residues
were not only dissipated from water streams very rapidly but also did
not accumulate much in the bottom sediment. This was explained by the
fact that the low density of permethrin and its insolubility in water
prevented it from reaching bottom sediments. Residues in forest litter
and exposed soils were more stable. Low levels of degradation products
can be translocated from soils to plants.
When a 640-ha forest block in northern Ontario, Canada, was sprayed
once with permethrin at 17.5 g ai/ha, residues in water persisted for
less than 96 h and attained peak concentrations of 147.0 µg/litre in
ponds and 2.5 µg/litre in streams after one hour. Accumulation and
persistence of the pesticide in bottom sediment were negligible. Resi-
due levels in the treated streams ranged from 0.05 to 0.89 µg/litre and
persisted for a maximum of 96 h, but in another case, residues fell to
a non-detectable level (less than 0.05 µg/l) after 6-24 h. Permethrin
residues appeared 2.1 km downstream from the treatment block 6 h after
spraying. The level reached a peak of 0.18 µg/litre at 12 h and did
not persist beyond 96 h. Accumulation of the insecticide in pond sedi-
ment was minimal (5-8 µg/kg) and persisted for less than 7 days. No
permethrin residue was found in stream sediments. The sprayed per-
methrin formulation had a density (0.88 g/ml) less than that of water
and was practically insoluble in water. It therefore formed a surface
film when brought into contact with stagnant or slowly moving water.
This significantly reduced the likelihood of the insecticide reaching
the bottom sediment or exposing fish in the treated ponds and streams.
Insecticide residues in foliage, soil, and litter were more stable than
in water and remained at detectable concentrations to the end of the
58-day sampling period. Deciduous and coniferous foliage contained
permethrin residues ranging from 0.02 to 0.78 mg/kg and retained con-
centrations of 0.02-0.05 mg/kg for at least 57 days. Forest litter
within the treatment block showed a residue level of 0.07 mg/kg 58 days
after the pesticide application. The permethrin residue levels in ex-
posed soil in the treatment block were fairly constant (0.04-0.07mg/kg)
for up to 58 days (Kingsbury & Kreutzweiser, 1980a).
In another field test where permethrin was sprayed (17.5 g ai/ha)
twice at intervals of 9 or 10 days in two forest blocks in Quebec,
Canada, the stagnant water in the sprayed region contained permethrin
levels of no more than 0.62 µg/litre and 0.84 µg/litre after the
initial and second applications, respectively. Samples from the streams
showed residue levels ranging from 0.05 to 1.84 µg/litre. Permethrin
concentrations in the water persisted at mean levels of 0.15 µg/litre
for 96 h and 0.03 µg/litre for 48 h after the first and second appli-
cations, respectively (detection limit: 0.01 µg/litre). Sediments
collected from a pond and streams, contained 30-95 µg permethrin/kg.
Accumulation of residual permethrin in stream sediment 4.5 km down-
stream from the treatment block was minimal. Permethrin residue levels
in forest litter increased substantially following the second appli-
cation. Mean concentrations ranged from 0.01 mg/kg to 0.053 mg/kg but
fell to non-detectable levels within 59 days (Kreutzweiser, 1982).
In a laboratory adsorption-desorption study, more than 95% of per-
methrin in aqueous solutions (6-42 µg/litre) was rapidly adsorbed
onto lake sediment, and the adsorbed insecticide was not easily de-
sorbed from the sediment by several water rinses. A high distribution
coefficient (i.e., g adsorbed per g sediment divided by g per ml of
solution) of 389 ml/g was obtained from the adsorption isotherm. Per-
methrin in aqueous solution applied to the surface of a sediment column
did not penetrate through more than 2 cm of the sediment (Sharom &
Solomon, 1981).
In a laboratory soil-leaching experiment, 14C-labelled (+)- cis or
(+)- trans- permethrin was incubated with two types of soils (light
clay soil of Kodaira and sandy clay loam soil of Azuchi) for 0 day or
21 days, then these permethrin-soil mixtures were applied to the top of
a soil column and eluted with water. When a mixture with no pre-
incubation was applied to the column, only 1.0 to 3.4% of the radio-
carbon was found in lower layer and no radiocarbon was eluted. However,
the degradation products from the pre-incubated samples were eluted
with water to a slight extent (see section 4.4) (Kaneko et al., 1978).
Similar results were obtained by Kaufman et al. (1981) in soil
mobility studies using soil TLC methods.
The uptake of permethrin and its degradation products by plants
from soil was studied by Leahey & Carpenter (1980). Sandy loam soil was
treated separately with [14C-cyclopropyl]- and [14C-phenyl]-permethrin
at a spray application rate of 2 kg/ha. The top 8 cm of the treated
soil was thoroughly mixed, and sugar beet, wheat, lettuce, and cotton
seeds were sown at intervals of 30, 60, and 120 days after treatment.
Low radioactive residues (up to 0.86 mg/kg) were detected in mature
plants, but the residues were higher in crops grown in soil treated
with [14C-cyclopropyl]-permethrin. It appeared that certain carboxylic
acid metabolites formed in the soil were subsequently taken up by the
plants. However, under field conditions, no residues of permethrin or
its metabolites were detected in crops sown 60 days or more after soil
treatment (Swaine et al., 1978).
4.2 Photodecomposition
Appraisal
Photochemical studies of permethrin in thin films and in solution
have shown it to be much more stable to light (10-100 times) than
synthetic pyrethroids developed earlier. In solution, photoisomeriz-
ation at the 1,3-bond of the cyclopropane ring and ester cleavage were
shown to be the major reactions.
In a thin film on plywood, permethrin remained insecticidally
active after 26 days, compared with 4-8 days and <2 days for phenothrin
and resmethrin, respectively. When exposed to daylight as a thin film
(0.2 mg/cm2) indoors near a window, phenothrin photodecomposed with a
half-life of about 6 days, whereas 60% of applied permethrin remained
undecomposed after 20 days. Thus, replacement of the isobutenyl group
with the dichlorovinyl substituent significantly enhanced the photo-
stability of permethrin. Permethrin was reported to be 10-100 times
more photostable than other pyrethroids synthesized earlier (Elliott et
al., 1973).
The photolysis of [1RS, trans ]- or [1RS, cis ]-permethrin (5)a has
been examined using materials labelled with 14C at the carboxy (acid)
or benzyl (alcohol) group (Fig. 2). On irradiation with ultraviolet
light (peak wavelength: 290-320 nm), both permethrin isomers decomposed
slightly faster in hexane than in methanol. In both solvents, the cis
isomer photodecomposed about 1.6 times faster (T´ = 43-58 min) than the
trans isomer. The photodecomposition reaction involved extensive
isomerization of the cyclopropane ring, i.e. interconversion of the
trans and cis isomers. This probably occurred via a triplet energy
state forming the diradical intermediate through Cl-C3 bond fission,
since the reaction was efficiently quenched by 1,3-cyclohexadiene. The
isomerization reaction reached a state of equilibrium after 1-4 h of
irradiation and the more thermodynamically stable trans isomer
constituted 65-70% of the isomer mixture. Apart from the isomerization
reaction, ester cleavage was the major photolytic reaction. As the
result of ester cleavage and other photolytic reactions, products
formed from permethrin also included smaller or trace amounts of
monochloro-permethrin (22) (from reductive dechlorination), 3-phenoxy-
benzaldehyde (PBald) (11), 3-phenoxybenzoic acid (PBacid) (12),
3-phenoxybenzyl-3,3-dimethylacrylate (23) (from diradical intermedi-
ate), and benzyl alcohols (9,10), as well as their corresponding acids
(15,16). In addition, large amounts of unidentified polar products were
-----------------------------------------------------------------------
a Numbers in parentheses refer to the corresponding numbers in Fig.2
detected, especially in water. Permethrin and monochloro-permethrin
(0.1-0.5 g) did not undergo photo-oxidation or other reactions within 7
days in oxygenated methanol solution using Rose Bengal as a sensitizer.
Thus the chlorine atoms at the vinyl position had a pronounced effect
in protecting this substituent from oxidation or epoxidation, as com-
pared with the isobutenyl in chrysanthemate (Holmstead et. al., 1978).
Holmstead et al. (1978) also investigated the photodegradation of
permethrin on a soil surface. The degradation on soil was similar to
the degradation pathways established in solution, but the rate of de-
gradation was slower and photo-isomerisation less important. Exposure
of the permethrin isomers on Dunkirk silt loam soil for 48 h resulted
in about a 55% loss of permethrin under sunlight and about a 35% loss
in the dark. The amount of unextractable material was about 6% in the
dark and about 18% in the light. On soil, permethrin did not undergo
extensive isomerization of the cyclopropane ring as it did in solution.
There was little difference in the amount of free acid detected in the
dark or in light, and 3-phenoxybenzyl alcohol (PBalc) (6) (approxi-
mately 5%) was the major cleavage product of the alcohol moiety. Other
products detected in trace amounts were essentially similar to those
present in solutions that had undergone photolysis.
4.3 Degradation in Plants
Appraisal
Thorough investigations of the fate of permethrin in plants have
been performed using bean plants and cotton plants. No significant
differences in the types of metabolic pathways were detected for the
two plant species. Very little translocation of permethrin or its
metabolites was observed following either topical application or stem
injection of permethrin to plants. Photochemical reactions played an
important role in the fate of permethrin applied to the surface of
plants. A major degradation pathway in plants was ester cleavage,
followed by rapid conjugation with sugars of the Cl2 CA and PBalc thus
formed.
The metabolism of the [1R,trans] and [1R,cis] isomers of 14C-per-
methrin, labelled separately in the dichlorovinyl and benzyl carbon
atoms, in snap bean seedlings has been studied in the greenhouse.
Whole-body autoradiography of the plants showed that little trans-
location of radiolabelled permethrin or its metabolites had occurred.
The amounts of radiocarbon remaining after 14 days were 13-17% of the
dose in the surface wash, 46-58% in the methanol extract, and 8-14% un-
extracted in the plant residues. Some interconversion of the trans and
cis isomers occurred and the cis isomer was slightly more persistent
than the trans isomer. The initial half-lives of the cis and trans
isomers of permethrin in the seedlings were 9 and 7 days, respectively.
A large number of metabolites were detected in the plant extracts, the
major ones from the alcohol moiety being PBalc (6) and its correspond-
ing 2'- (8) or 4'-hydroxy (7) derivatives, which occurred mainly as
glucoside conjugates (Fig. 1). There were seven or eight additional
minor unidentified products. The cis and trans isomers of 3-(2,2-
dichlorovinyl)-2,2-dimethylcyclopropanecarboxyclic acid (Cl2CA) (17)
were the major metabolites from the acid moiety and occurred mainly as
conjugated forms. In addition, trace amounts of the 2'- (24) or 4'-
hydroxy (26) derivatives of permethrin were also detected. From the
hydrolysis experiments using beta-glucosidase, it was inferred that the
sugar concerned was glucose, but no detailed evidence of the identity
was obtained (Ohkawa et al., 1977).
In a separate study, Gaughan & Casida (1978) examined the metab-
olism of the [1RS,trans] and [1RS,cis] isomers of permethrin in snap
beans in the glasshouse and in cotton both in the glasshouse and out-
doors. Individual leaves of snap beans and cotton plants were treated
with 1 µg of cis- or trans-14C-permethrin labelled either at the
carboxy or methylene carbon. Under field conditions, about 30% of the
radiolabel was lost from cotton plants within one week after appli-
cation and some trans/cis isomerization at the cyclopropane ring took
place by photodecomposition. trans-Permethrin was metabolized more
rapidly than the cis isomer. The major degradation pathway was again
hydrolysis, followed by rapid conjugation of Cl2CA (17) and PBalc (6)
with sugars. There were at least two types of conjugates; the minor one
was a glycoside readily cleaved by beta-glucosidase and the major one
was a conjugate which was resistant to beta-glucosidase but was readily
cleaved by cellulase. Other products identified included the hydroxy-
lated compounds reported by Ohkawa et al. (1977) in their study of
beans treated with permethrin. In addition, hydroxylation at either of
the two methyl groups in the acid moiety (27) with subsequent conju-
gation occurred to a greater extent with the more stable cis isomer.
Similar metabolites to those formed under field conditions were
detected in bean and cotton plants under glasshouse conditions.
Roberts & Wright (1981) studied the conjugation of 14C-PBalc in
cotton plants using abscised leaves to obtain more information on the
nature of the conjugates produced. The alcohol was rapidly converted
to glucosyl 3-phenoxybenzyl ether and subsequently to more polar sub-
stances such as disaccharide conjugates with glucose and pentose (prob-
ably xylose or arabinose) sugars. The alcohol and its monosaccharide
and disaccharide conjugates underwent interconversion in the cotton
leaves. The evidence was obtained from experiments with 14C-glucose,
which showed the ready exchange of the glucose units of the conjugates
with free glucose in the leaves. No larger sugar conjugates of PBalc
were detected in plants.
From the above studies, it can be concluded that the types of prod-
ucts formed from permethrin in plants are similar to those formed in
mammals, except for the nature of the conjugates (see section 5.1).
4.4 Degradation in Soils
Appraisal
Several studies on the degradation of permethrin in a wide variety of
soil types have been carried out. These studies used permethrin labelled
with 14 C at different positions, so that the fate of virtually all of the
significant sub-units of the molecule has been traced. In all soil types
degradation is fairly rapid under aerobic conditions, conversion to 14CO2
being the major ultimate fate of the 14 C. With all soils and all positions
of radiolabelling, the formation of unextractable residues is a major occur-
rence. Under anaerobic conditions, similar degradation processes seem to
occur, but the rate of ultimate conversion to 14 CO2 is slower than under
aerobic conditions.
Kaufman et al. (1977) studied the degradation of cis and trans iso-
mers of permethrin in five soils under aerobic, anaerobic, and steril-
ized conditions. Soils were treated with 14C-permethrin labelled sep-
arately in the carboxy and methylene groups at a dose rate of 224 g/ha
and stored under aerobic conditions at 25°C. Degradation of permethrin
was rapid in four of five soils, with the trans isomer decomposing more
rapidly than the cis isomer. The initial half-lives were less than 28
days in all but one soil. Rapid evolution of 14CO2 was observed. In
Hagerstown silty clay loam soil, 62% of methylene- and 52% of carboxy-
labelled permethrin were converted to 14CO2 in 27 days. Only 15%
(methylene-labelled) to 19% (carboxy-labelled) of the applied radio-
label was extractable with methanol, 25-27% remaining unextracted and
associated with soil organic matter. Microbial metabolism was involved
in permethrin degradation and the major route was hydrolysis of the
ester linkage to form PBalc and Cl2CA, the former product being
subsequently oxidized to PBacid. In contrast, less than 0.3% of
14CO2 was evolved from soils treated with sodium azide (an inhibitor
of microbial growth) or when the soil was incubated under waterlogged
anaerobic conditions.
Kaneko et al. (1978) reported the degradation in two Japanese soils
of 14C-permethrin labelled separately in the dichlorovinyl and methy-
lene groups. The initial half-lives of the trans and cis isomers were
6-9 days and 12 days, respectively, in soils treated at a rate of 1 mg/
kg and stored at 25°C under aerobic upland conditions. 14CO2 was
evolved at rates similar to those observed by Kaufman et al. (1981). As
one of the 14C-preparations was different in labelled position from
those used in the earlier work, the evolution of 14CO2 was the evi-
dence for extensive degradation of the cyclopropyl moiety after hy-
drolysis in the soils. In addition to the hydrolysis products, several
oxidation products were identified, including 3-(2,2-dichlorovinyl)-
2-methyl-2-hydroxymethyl-cyclopropanecarboxylic acid (19) and 3-(4-
hydroxyphenoxy)benzyl- 3-(2, 2-dichlorovinyl)-2,2-dimethylcyclopropane-
carboxylate (26).
The degradation of permethrin was studied in a flooded Memphis silt
loam soil incubated at 25°C, [14C-carbonyl]- cis-, [14C-carbonyl]- trans-,
and [methylene-14C]- cis-permethrin being added to the soil at rates
of 0.1 and 1.0 mg/kg. The soils were analyzed after 0, 4, 8, 16, 32,
and 64 days to determine the distribution of 14C in CO2, solvent-
extractable compounds, water-soluble polar compounds, and soil-bounds
residues. Thin-layer chromatographic analysis of the organic solvent
extracts showed that trans-permethrin was more rapidly degraded than
the cis isomer. After 64 days, the amounts of 14C-trans-permethrin remain-
ing were 34.2% (at 0.1 mg/kg) and 30.3% (at 1.0 mg/kg) of the applied
14C, and those of 14C- cis-permethrin were 73.4% (at 0.1 mg/kg) and
69.8% (at 1.0 mg/kg). Two metabolites, 3-(2,2-dichloro-vinyl)-2,2-
dimethylcyclopropanecarboxylic acid (17) and PBalc (6), resulting from
permethrin hydrolysis were identified. Other metabolites were PBacid
(12) and PBald (11). Fragmentation of (17) and (12) to CO2 was not
extensive, and cumulative 14CO2 recoveries were less than 3.5% for
all treatments during the 64-day incubation period. The metabolism
of trans-permethrin resulted in the accumulation of polar compounds in
the water. Soil-bound residues gradually increased with time and
accounted for 3.3-11.4% of the 14C activity after 64 days. The
largest percentage of soil-bound 14C residue was in the fulvic acid
fraction (Jordan & Kaufman, 1986).
When 14C-permethrin preincubated with soil for 21 days was applied
on top of a soil column and eluted with water, 7.9-17.2% of the applied
radiocarbon was recovered in the lower layers of the column and 0.3-
2.6% was found in effluents (Kaneko et al., 1978). Only degradation
products of permethrin, such as PBacid (12) and 3-(4-hydroxyphenoxy)-
benzyl-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropanecarboxylate (26)
were identified in the effluent. Permethrin was not present in the
effluent (see section 4.1).
The persistence of permethrin in soil was studied in aqueous sus-
pensions of soil spiked with permethrin at a rate of 17.8 mg/kg under a
range of redox potential (-150, +50, +250, and +450 mV) and at pH 5.5,
7.0, and 8.0. The results of this study indicated that both the pH and
redox potential significantly influence the degradation of permethrin.
After 25 days, permethrin disappeared almost completely under well
oxidized (+450 mV) conditions at all three pH levels. Under reduced
conditions (-150 mV), only about 40% of the applied permethrin was
degraded. The rate of degradation of permethrin was moderate at weakly
oxidized (+250 mV) and moderately reduced (+50 mV) conditions at pH 8.
Thus, permethrin was lost more rapidly under oxidizing conditions, and
increasing the pH enhanced this loss under moderately reduced and
weakly oxidized conditions (Gambrell et al., 1981).
Jordan et al. (1982) investigated the effect of temperature on the
degradation of permethrin in soil. Dubbs fine sandy loam soil was
treated with [14C-carbonyl]- cis, trans-permethrin at a rate of 1
mg/kg and incubated at 10, 25, and 40°C for up to 64 days. The half-
lives of disappearance for trans and cis isomers were 14 and 55 days at
10°C, 5 and 12 days at 25°C, and 4 and 27 days at 40°C, respectively.
The most rapid rate of degradation of permethrin occurred at 25°C, per-
methrin being converted to Cl2CA (17) and ultimately to 14CO2. At
40°C rapid degradation of permethrin to Cl2CA also occurred, but
further degradation of Cl2CA to 14CO2 was reduced. The amount of
14CO2 evolved after 64 days was 56% at 25°C, compared with 29% at
10°C and 24% at 40°C.
Lord et al. (1982) investigated the factors affecting the persist-
ence of permethrin in three loam soils under laboratory conditions.
The degradation of trans-permethrin (4 mg/kg) at 30°C was similar at
three moisture contents ranging from 40 to 80% of water-holding
capacity, but more rapid degradation occurred in an aqueous soil sus-
pension system probably due to better distribution of the insecticide.
Four repeated applications of permethrin (4 mg/kg) at 20-day intervals,
or addition of nutrients including sucrose (1 mg/kg), powdered cellu-
lose (100 mg/kg), and NH4Cl (80 mg/kg) plus K2HPO4 (260 mg/kg) to
soils caused no drastic changes in the rate of degradation of per-
methrin.
The influence of organic materials on the degradation of permethrin
in soil was also studied by Doyle et al. (1981). [14C-carbonyl]- cis-per-
methrin was added to silty loam soil which had been pretreated with
sewage sludge or dairy manure at rates of 0, 50, or 100 tonnes/ha, and
total CO2 and 14CO2 evolution were monitored regularly throughout a
60-day incubation period at 25°C. The incorporation of sewage or dairy
manure at the rate of 50 and 100 tonnes/ha increased permethrin break-
down by 87% and 149% (sewage), or 64% and 134% (dairy manure) based on
the values measured in unamended soil, respectively. In the waste-
amended soils, a lag period of 28-38 days during which time virtually
no 14CO2 was evolved, was followed by a rapid evolution of 14CO2 before
the rate became stabilized. The highest rates (0.21-0.22% per day) were
observed in soils amended with either dairy manure or sewage sludge at
100 tonnes/ha. The rate of 14CO2 formation correlated directly with
the total microbial activity, as measured by total CO2 production.
In studies by Williams & Brown (1979), the persistence of per-
methrin in six soils was compared with that of fenvalerate under lab-
oratory conditions. The soils were treated with one of the insecticides
at 1 mg/kg and incubated under aerobic conditions for 16 weeks at a
temperature alternating between 20°C for 15 h and 10°C for 9 h to simu-
late the actual field conditions. With the exception of organic soil
from Cloverdale, degradation of permethrin was rapid in all soils, with
half-lives of 3 weeks or less. Under identical conditions, the half
life for fenvalerate was about 7 weeks. Again, trans-permethrin was
lost more rapidly than the cis isomer, and there was very little loss
of either insecticide in the sterilized soils. With Cloverdale organic
soil, a greater degree of adsorption onto the soil organic fraction
might have contributed to the slower degradation rate.
When soil was treated with [14C-cyclopropyl]-permethrin, sugar
beet grown on the treated soil was found to contain radiolabelled
conjugates of Cl2CA and 3-(2,2-dichlorovinyl)-2-methylcyclopropane-
1,2-dicarboxylic acid (21) (Leahey & Carpenter, 1980). It was possible
that both carboxylic acids were formed in the soil and were sub-
sequently taken up by the plants (see section 4.1).
5. KINETICS AND METABOLISM
5.1 Metabolism in Mammals
Appraisal
The metabolic pathways of permethrin in mammals are summarized in
Fig. 3.
The metabolism of permethrin has been studied in great detail in
various species of mammals, using a variety of radiolabelled isomers.
Permethrin administered to mammals was rapidly metabolized and almost
completely eliminated from the body within a short period of time. The
trans isomer of permethrin was eliminated more rapidly that the cis
isomer. Radiocarbon from trans- permethrin was excreted mostly in
urine, whereas that from the cis isomer was eliminated both in urine
and faeces to a similar extent. Expiration as CO2 contributed little
to its elimination in mammals. Major routes of metabolism for both
trans and cis isomers were ester cleavage and oxidation of the 4'-
position of the terminal aromatic ring. A less important reaction in
mammals was hydroxylation of the geminal dimethyl group of the cyclo-
propane ring. Major metabolites thus formed were Cl2 CA in free and
glucuronide form, sulfate conjugate of 4'-hydroxy-3-phenoxybenzoic
acid, PBacid in free and conjugate form, and hydroxymethyl-Cl2 CA as a
glucuronide conjugate. This latter compound was also isolated as a
lactone where the hydroxymethyl group and the carboxy group had a cis
configuration.
4.1 Transport and Distribution between Media
In laboratory studies, permethrin in water was rapidly adsorbed
onto lake sediments or soil columns and was not desorbed or eluted
easily from them. However in forest spray trials, permethrin residues
were not only dissipated from water streams very rapidly but also did
not accumulate much in the bottom sediment. This was explained by the
fact that the low density of permethrin and its insolubility in water
prevented it from reaching bottom sediments. Residues in forest litter
and exposed soils were more stable. Low levels of degradation products
can be translocated from soils to plants.
When a 640-ha forest block in northern Ontario, Canada, was sprayed
once with permethrin at 17.5 g ai/ha, residues in water persisted for
less than 96 h and attained peak concentrations of 147.0 µg/litre in
ponds and 2.5 µg/litre in streams after one hour. Accumulation and
persistence of the pesticide in bottom sediment were negligible. Resi-
due levels in the treated streams ranged from 0.05 to 0.89 µg/litre and
persisted for a maximum of 96 h, but in another case, residues fell to
a non-detectable level (less than 0.05 µg/l) after 6-24 h. Permethrin
residues appeared 2.1 km downstream from the treatment block 6 h after
spraying. The level reached a peak of 0.18 µg/litre at 12 h and did
not persist beyond 96 h. Accumulation of the insecticide in pond sedi-
ment was minimal (5-8 µg/kg) and persisted for less than 7 days. No
permethrin residue was found in stream sediments. The sprayed per-
methrin formulation had a density (0.88 g/ml) less than that of water
and was practically insoluble in water. It therefore formed a surface
film when brought into contact with stagnant or slowly moving water.
This significantly reduced the likelihood of the insecticide reaching
the bottom sediment or exposing fish in the treated ponds and streams.
Insecticide residues in foliage, soil, and litter were more stable than
in water and remained at detectable concentrations to the end of the
58-day sampling period. Deciduous and coniferous foliage contained
permethrin residues ranging from 0.02 to 0.78 mg/kg and retained con-
centrations of 0.02-0.05 mg/kg for at least 57 days. Forest litter
within the treatment block showed a residue level of 0.07 mg/kg 58 days
after the pesticide application. The permethrin residue levels in ex-
posed soil in the treatment block were fairly constant (0.04-0.07mg/kg)
for up to 58 days (Kingsbury & Kreutzweiser, 1980a).
In another field test where permethrin was sprayed (17.5 g ai/ha)
twice at intervals of 9 or 10 days in two forest blocks in Quebec,
Canada, the stagnant water in the sprayed region contained permethrin
levels of no more than 0.62 µg/litre and 0.84 µg/litre after the
initial and second applications, respectively. Samples from the streams
showed residue levels ranging from 0.05 to 1.84 µg/litre. Permethrin
concentrations in the water persisted at mean levels of 0.15 µg/litre
for 96 h and 0.03 µg/litre for 48 h after the first and second appli-
cations, respectively (detection limit: 0.01 µg/litre). Sediments
collected from a pond and streams, contained 30-95 µg permethrin/kg.
Accumulation of residual permethrin in stream sediment 4.5 km down-
stream from the treatment block was minimal. Permethrin residue levels
in forest litter increased substantially following the second appli-
cation. Mean concentrations ranged from 0.01 mg/kg to 0.053 mg/kg but
fell to non-detectable levels within 59 days (Kreutzweiser, 1982).
In a laboratory adsorption-desorption study, more than 95% of per-
methrin in aqueous solutions (6-42 µg/litre) was rapidly adsorbed
onto lake sediment, and the adsorbed insecticide was not easily de-
sorbed from the sediment by several water rinses. A high distribution
coefficient (i.e., g adsorbed per g sediment divided by g per ml of
solution) of 389 ml/g was obtained from the adsorption isotherm. Per-
methrin in aqueous solution applied to the surface of a sediment column
did not penetrate through more than 2 cm of the sediment (Sharom &
Solomon, 1981).
In a laboratory soil-leaching experiment, 14C-labelled (+)- cis or
(+)- trans- permethrin was incubated with two types of soils (light
clay soil of Kodaira and sandy clay loam soil of Azuchi) for 0 day or
21 days, then these permethrin-soil mixtures were applied to the top of
a soil column and eluted with water. When a mixture with no pre-
incubation was applied to the column, only 1.0 to 3.4% of the radio-
carbon was found in lower layer and no radiocarbon was eluted. However,
the degradation products from the pre-incubated samples were eluted
with water to a slight extent (see section 4.4) (Kaneko et al., 1978).
Similar results were obtained by Kaufman et al. (1981) in soil
mobility studies using soil TLC methods.
The uptake of permethrin and its degradation products by plants
from soil was studied by Leahey & Carpenter (1980). Sandy loam soil was
treated separately with [14C-cyclopropyl]- and [14C-phenyl]-permethrin
at a spray application rate of 2 kg/ha. The top 8 cm of the treated
soil was thoroughly mixed, and sugar beet, wheat, lettuce, and cotton
seeds were sown at intervals of 30, 60, and 120 days after treatment.
Low radioactive residues (up to 0.86 mg/kg) were detected in mature
plants, but the residues were higher in crops grown in soil treated
with [14C-cyclopropyl]-permethrin. It appeared that certain carboxylic
acid metabolites formed in the soil were subsequently taken up by the
plants. However, under field conditions, no residues of permethrin or
its metabolites were detected in crops sown 60 days or more after soil
treatment (Swaine et al., 1978).
4.2 Photodecomposition
Appraisal
Photochemical studies of permethrin in thin films and in solution
have shown it to be much more stable to light (10-100 times) than
synthetic pyrethroids developed earlier. In solution, photoisomeriz-
ation at the 1,3-bond of the cyclopropane ring and ester cleavage were
shown to be the major reactions.
In a thin film on plywood, permethrin remained insecticidally
active after 26 days, compared with 4-8 days and <2 days for phenothrin
and resmethrin, respectively. When exposed to daylight as a thin film
(0.2 mg/cm2) indoors near a window, phenothrin photodecomposed with a
half-life of about 6 days, whereas 60% of applied permethrin remained
undecomposed after 20 days. Thus, replacement of the isobutenyl group
with the dichlorovinyl substituent significantly enhanced the photo-
stability of permethrin. Permethrin was reported to be 10-100 times
more photostable than other pyrethroids synthesized earlier (Elliott et
al., 1973).
The photolysis of [1RS, trans ]- or [1RS, cis ]-permethrin (5)a has
been examined using materials labelled with 14C at the carboxy (acid)
or benzyl (alcohol) group (Fig. 2). On irradiation with ultraviolet
light (peak wavelength: 290-320 nm), both permethrin isomers decomposed
slightly faster in hexane than in methanol. In both solvents, the cis
isomer photodecomposed about 1.6 times faster (T´ = 43-58 min) than the
trans isomer. The photodecomposition reaction involved extensive
isomerization of the cyclopropane ring, i.e. interconversion of the
trans and cis isomers. This probably occurred via a triplet energy
state forming the diradical intermediate through Cl-C3 bond fission,
since the reaction was efficiently quenched by 1,3-cyclohexadiene. The
isomerization reaction reached a state of equilibrium after 1-4 h of
irradiation and the more thermodynamically stable trans isomer
constituted 65-70% of the isomer mixture. Apart from the isomerization
reaction, ester cleavage was the major photolytic reaction. As the
result of ester cleavage and other photolytic reactions, products
formed from permethrin also included smaller or trace amounts of
monochloro-permethrin (22) (from reductive dechlorination), 3-phenoxy-
benzaldehyde (PBald) (11), 3-phenoxybenzoic acid (PBacid) (12),
3-phenoxybenzyl-3,3-dimethylacrylate (23) (from diradical intermedi-
ate), and benzyl alcohols (9,10), as well as their corresponding acids
(15,16). In addition, large amounts of unidentified polar products were
-----------------------------------------------------------------------
a Numbers in parentheses refer to the corresponding numbers in Fig.2
detected, especially in water. Permethrin and monochloro-permethrin
(0.1-0.5 g) did not undergo photo-oxidation or other reactions within 7
days in oxygenated methanol solution using Rose Bengal as a sensitizer.
Thus the chlorine atoms at the vinyl position had a pronounced effect
in protecting this substituent from oxidation or epoxidation, as com-
pared with the isobutenyl in chrysanthemate (Holmstead et. al., 1978).
Holmstead et al. (1978) also investigated the photodegradation of
permethrin on a soil surface. The degradation on soil was similar to
the degradation pathways established in solution, but the rate of de-
gradation was slower and photo-isomerisation less important. Exposure
of the permethrin isomers on Dunkirk silt loam soil for 48 h resulted
in about a 55% loss of permethrin under sunlight and about a 35% loss
in the dark. The amount of unextractable material was about 6% in the
dark and about 18% in the light. On soil, permethrin did not undergo
extensive isomerization of the cyclopropane ring as it did in solution.
There was little difference in the amount of free acid detected in the
dark or in light, and 3-phenoxybenzyl alcohol (PBalc) (6) (approxi-
mately 5%) was the major cleavage product of the alcohol moiety. Other
products detected in trace amounts were essentially similar to those
present in solutions that had undergone photolysis.
4.3 Degradation in Plants
Appraisal
Thorough investigations of the fate of permethrin in plants have
been performed using bean plants and cotton plants. No significant
differences in the types of metabolic pathways were detected for the
two plant species. Very little translocation of permethrin or its
metabolites was observed following either topical application or stem
injection of permethrin to plants. Photochemical reactions played an
important role in the fate of permethrin applied to the surface of
plants. A major degradation pathway in plants was ester cleavage,
followed by rapid conjugation with sugars of the Cl2 CA and PBalc thus
formed.
The metabolism of the [1R,trans] and [1R,cis] isomers of 14C-per-
methrin, labelled separately in the dichlorovinyl and benzyl carbon
atoms, in snap bean seedlings has been studied in the greenhouse.
Whole-body autoradiography of the plants showed that little trans-
location of radiolabelled permethrin or its metabolites had occurred.
The amounts of radiocarbon remaining after 14 days were 13-17% of the
dose in the surface wash, 46-58% in the methanol extract, and 8-14% un-
extracted in the plant residues. Some interconversion of the trans and
cis isomers occurred and the cis isomer was slightly more persistent
than the trans isomer. The initial half-lives of the cis and trans
isomers of permethrin in the seedlings were 9 and 7 days, respectively.
A large number of metabolites were detected in the plant extracts, the
major ones from the alcohol moiety being PBalc (6) and its correspond-
ing 2'- (8) or 4'-hydroxy (7) derivatives, which occurred mainly as
glucoside conjugates (Fig. 1). There were seven or eight additional
minor unidentified products. The cis and trans isomers of 3-(2,2-
dichlorovinyl)-2,2-dimethylcyclopropanecarboxyclic acid (Cl2CA) (17)
were the major metabolites from the acid moiety and occurred mainly as
conjugated forms. In addition, trace amounts of the 2'- (24) or 4'-
hydroxy (26) derivatives of permethrin were also detected. From the
hydrolysis experiments using beta-glucosidase, it was inferred that the
sugar concerned was glucose, but no detailed evidence of the identity
was obtained (Ohkawa et al., 1977).
In a separate study, Gaughan & Casida (1978) examined the metab-
olism of the [1RS,trans] and [1RS,cis] isomers of permethrin in snap
beans in the glasshouse and in cotton both in the glasshouse and out-
doors. Individual leaves of snap beans and cotton plants were treated
with 1 µg of cis- or trans-14C-permethrin labelled either at the
carboxy or methylene carbon. Under field conditions, about 30% of the
radiolabel was lost from cotton plants within one week after appli-
cation and some trans/cis isomerization at the cyclopropane ring took
place by photodecomposition. trans-Permethrin was metabolized more
rapidly than the cis isomer. The major degradation pathway was again
hydrolysis, followed by rapid conjugation of Cl2CA (17) and PBalc (6)
with sugars. There were at least two types of conjugates; the minor one
was a glycoside readily cleaved by beta-glucosidase and the major one
was a conjugate which was resistant to beta-glucosidase but was readily
cleaved by cellulase. Other products identified included the hydroxy-
lated compounds reported by Ohkawa et al. (1977) in their study of
beans treated with permethrin. In addition, hydroxylation at either of
the two methyl groups in the acid moiety (27) with subsequent conju-
gation occurred to a greater extent with the more stable cis isomer.
Similar metabolites to those formed under field conditions were
detected in bean and cotton plants under glasshouse conditions.
Roberts & Wright (1981) studied the conjugation of 14C-PBalc in
cotton plants using abscised leaves to obtain more information on the
nature of the conjugates produced. The alcohol was rapidly converted
to glucosyl 3-phenoxybenzyl ether and subsequently to more polar sub-
stances such as disaccharide conjugates with glucose and pentose (prob-
ably xylose or arabinose) sugars. The alcohol and its monosaccharide
and disaccharide conjugates underwent interconversion in the cotton
leaves. The evidence was obtained from experiments with 14C-glucose,
which showed the ready exchange of the glucose units of the conjugates
with free glucose in the leaves. No larger sugar conjugates of PBalc
were detected in plants.
From the above studies, it can be concluded that the types of prod-
ucts formed from permethrin in plants are similar to those formed in
mammals, except for the nature of the conjugates (see section 5.1).
4.4 Degradation in Soils
Appraisal
Several studies on the degradation of permethrin in a wide variety of
soil types have been carried out. These studies used permethrin labelled
with 14 C at different positions, so that the fate of virtually all of the
significant sub-units of the molecule has been traced. In all soil types
degradation is fairly rapid under aerobic conditions, conversion to 14CO2
being the major ultimate fate of the 14 C. With all soils and all positions
of radiolabelling, the formation of unextractable residues is a major occur-
rence. Under anaerobic conditions, similar degradation processes seem to
occur, but the rate of ultimate conversion to 14 CO2 is slower than under
aerobic conditions.
Kaufman et al. (1977) studied the degradation of cis and trans iso-
mers of permethrin in five soils under aerobic, anaerobic, and steril-
ized conditions. Soils were treated with 14C-permethrin labelled sep-
arately in the carboxy and methylene groups at a dose rate of 224 g/ha
and stored under aerobic conditions at 25°C. Degradation of permethrin
was rapid in four of five soils, with the trans isomer decomposing more
rapidly than the cis isomer. The initial half-lives were less than 28
days in all but one soil. Rapid evolution of 14CO2 was observed. In
Hagerstown silty clay loam soil, 62% of methylene- and 52% of carboxy-
labelled permethrin were converted to 14CO2 in 27 days. Only 15%
(methylene-labelled) to 19% (carboxy-labelled) of the applied radio-
label was extractable with methanol, 25-27% remaining unextracted and
associated with soil organic matter. Microbial metabolism was involved
in permethrin degradation and the major route was hydrolysis of the
ester linkage to form PBalc and Cl2CA, the former product being
subsequently oxidized to PBacid. In contrast, less than 0.3% of
14CO2 was evolved from soils treated with sodium azide (an inhibitor
of microbial growth) or when the soil was incubated under waterlogged
anaerobic conditions.
Kaneko et al. (1978) reported the degradation in two Japanese soils
of 14C-permethrin labelled separately in the dichlorovinyl and methy-
lene groups. The initial half-lives of the trans and cis isomers were
6-9 days and 12 days, respectively, in soils treated at a rate of 1 mg/
kg and stored at 25°C under aerobic upland conditions. 14CO2 was
evolved at rates similar to those observed by Kaufman et al. (1981). As
one of the 14C-preparations was different in labelled position from
those used in the earlier work, the evolution of 14CO2 was the evi-
dence for extensive degradation of the cyclopropyl moiety after hy-
drolysis in the soils. In addition to the hydrolysis products, several
oxidation products were identified, including 3-(2,2-dichlorovinyl)-
2-methyl-2-hydroxymethyl-cyclopropanecarboxylic acid (19) and 3-(4-
hydroxyphenoxy)benzyl- 3-(2, 2-dichlorovinyl)-2,2-dimethylcyclopropane-
carboxylate (26).
The degradation of permethrin was studied in a flooded Memphis silt
loam soil incubated at 25°C, [14C-carbonyl]- cis-, [14C-carbonyl]- trans-,
and [methylene-14C]- cis-permethrin being added to the soil at rates
of 0.1 and 1.0 mg/kg. The soils were analyzed after 0, 4, 8, 16, 32,
and 64 days to determine the distribution of 14C in CO2, solvent-
extractable compounds, water-soluble polar compounds, and soil-bounds
residues. Thin-layer chromatographic analysis of the organic solvent
extracts showed that trans-permethrin was more rapidly degraded than
the cis isomer. After 64 days, the amounts of 14C-trans-permethrin remain-
ing were 34.2% (at 0.1 mg/kg) and 30.3% (at 1.0 mg/kg) of the applied
14C, and those of 14C- cis-permethrin were 73.4% (at 0.1 mg/kg) and
69.8% (at 1.0 mg/kg). Two metabolites, 3-(2,2-dichloro-vinyl)-2,2-
dimethylcyclopropanecarboxylic acid (17) and PBalc (6), resulting from
permethrin hydrolysis were identified. Other metabolites were PBacid
(12) and PBald (11). Fragmentation of (17) and (12) to CO2 was not
extensive, and cumulative 14CO2 recoveries were less than 3.5% for
all treatments during the 64-day incubation period. The metabolism
of trans-permethrin resulted in the accumulation of polar compounds in
the water. Soil-bound residues gradually increased with time and
accounted for 3.3-11.4% of the 14C activity after 64 days. The
largest percentage of soil-bound 14C residue was in the fulvic acid
fraction (Jordan & Kaufman, 1986).
When 14C-permethrin preincubated with soil for 21 days was applied
on top of a soil column and eluted with water, 7.9-17.2% of the applied
radiocarbon was recovered in the lower layers of the column and 0.3-
2.6% was found in effluents (Kaneko et al., 1978). Only degradation
products of permethrin, such as PBacid (12) and 3-(4-hydroxyphenoxy)-
benzyl-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropanecarboxylate (26)
were identified in the effluent. Permethrin was not present in the
effluent (see section 4.1).
The persistence of permethrin in soil was studied in aqueous sus-
pensions of soil spiked with permethrin at a rate of 17.8 mg/kg under a
range of redox potential (-150, +50, +250, and +450 mV) and at pH 5.5,
7.0, and 8.0. The results of this study indicated that both the pH and
redox potential significantly influence the degradation of permethrin.
After 25 days, permethrin disappeared almost completely under well
oxidized (+450 mV) conditions at all three pH levels. Under reduced
conditions (-150 mV), only about 40% of the applied permethrin was
degraded. The rate of degradation of permethrin was moderate at weakly
oxidized (+250 mV) and moderately reduced (+50 mV) conditions at pH 8.
Thus, permethrin was lost more rapidly under oxidizing conditions, and
increasing the pH enhanced this loss under moderately reduced and
weakly oxidized conditions (Gambrell et al., 1981).
Jordan et al. (1982) investigated the effect of temperature on the
degradation of permethrin in soil. Dubbs fine sandy loam soil was
treated with [14C-carbonyl]- cis, trans-permethrin at a rate of 1
mg/kg and incubated at 10, 25, and 40°C for up to 64 days. The half-
lives of disappearance for trans and cis isomers were 14 and 55 days at
10°C, 5 and 12 days at 25°C, and 4 and 27 days at 40°C, respectively.
The most rapid rate of degradation of permethrin occurred at 25°C, per-
methrin being converted to Cl2CA (17) and ultimately to 14CO2. At
40°C rapid degradation of permethrin to Cl2CA also occurred, but
further degradation of Cl2CA to 14CO2 was reduced. The amount of
14CO2 evolved after 64 days was 56% at 25°C, compared with 29% at
10°C and 24% at 40°C.
Lord et al. (1982) investigated the factors affecting the persist-
ence of permethrin in three loam soils under laboratory conditions.
The degradation of trans-permethrin (4 mg/kg) at 30°C was similar at
three moisture contents ranging from 40 to 80% of water-holding
capacity, but more rapid degradation occurred in an aqueous soil sus-
pension system probably due to better distribution of the insecticide.
Four repeated applications of permethrin (4 mg/kg) at 20-day intervals,
or addition of nutrients including sucrose (1 mg/kg), powdered cellu-
lose (100 mg/kg), and NH4Cl (80 mg/kg) plus K2HPO4 (260 mg/kg) to
soils caused no drastic changes in the rate of degradation of per-
methrin.
The influence of organic materials on the degradation of permethrin
in soil was also studied by Doyle et al. (1981). [14C-carbonyl]- cis-per-
methrin was added to silty loam soil which had been pretreated with
sewage sludge or dairy manure at rates of 0, 50, or 100 tonnes/ha, and
total CO2 and 14CO2 evolution were monitored regularly throughout a
60-day incubation period at 25°C. The incorporation of sewage or dairy
manure at the rate of 50 and 100 tonnes/ha increased permethrin break-
down by 87% and 149% (sewage), or 64% and 134% (dairy manure) based on
the values measured in unamended soil, respectively. In the waste-
amended soils, a lag period of 28-38 days during which time virtually
no 14CO2 was evolved, was followed by a rapid evolution of 14CO2 before
the rate became stabilized. The highest rates (0.21-0.22% per day) were
observed in soils amended with either dairy manure or sewage sludge at
100 tonnes/ha. The rate of 14CO2 formation correlated directly with
the total microbial activity, as measured by total CO2 production.
In studies by Williams & Brown (1979), the persistence of per-
methrin in six soils was compared with that of fenvalerate under lab-
oratory conditions. The soils were treated with one of the insecticides
at 1 mg/kg and incubated under aerobic conditions for 16 weeks at a
temperature alternating between 20°C for 15 h and 10°C for 9 h to simu-
late the actual field conditions. With the exception of organic soil
from Cloverdale, degradation of permethrin was rapid in all soils, with
half-lives of 3 weeks or less. Under identical conditions, the half
life for fenvalerate was about 7 weeks. Again, trans-permethrin was
lost more rapidly than the cis isomer, and there was very little loss
of either insecticide in the sterilized soils. With Cloverdale organic
soil, a greater degree of adsorption onto the soil organic fraction
might have contributed to the slower degradation rate.
When soil was treated with [14C-cyclopropyl]-permethrin, sugar
beet grown on the treated soil was found to contain radiolabelled
conjugates of Cl2CA and 3-(2,2-dichlorovinyl)-2-methylcyclopropane-
1,2-dicarboxylic acid (21) (Leahey & Carpenter, 1980). It was possible
that both carboxylic acids were formed in the soil and were sub-
sequently taken up by the plants (see section 4.1).
5. KINETICS AND METABOLISM
5.1 Metabolism in Mammals
Appraisal
The metabolic pathways of permethrin in mammals are summarized in
Fig. 3.
The metabolism of permethrin has been studied in great detail in
various species of mammals, using a variety of radiolabelled isomers.
Permethrin administered to mammals was rapidly metabolized and almost
completely eliminated from the body within a short period of time. The
trans isomer of permethrin was eliminated more rapidly that the cis
isomer. Radiocarbon from trans- permethrin was excreted mostly in
urine, whereas that from the cis isomer was eliminated both in urine
and faeces to a similar extent. Expiration as CO2 contributed little
to its elimination in mammals. Major routes of metabolism for both
trans and cis isomers were ester cleavage and oxidation of the 4'-
position of the terminal aromatic ring. A less important reaction in
mammals was hydroxylation of the geminal dimethyl group of the cyclo-
propane ring. Major metabolites thus formed were Cl2 CA in free and
glucuronide form, sulfate conjugate of 4'-hydroxy-3-phenoxybenzoic
acid, PBacid in free and conjugate form, and hydroxymethyl-Cl2 CA as a
glucuronide conjugate. This latter compound was also isolated as a
lactone where the hydroxymethyl group and the carboxy group had a cis
configuration.
 5.1.1 Mouse
In studies by Shah et al. (1981), 14C- cis-permethrin was applied
to the clipped skin of mice at a level of 1 mg/kg body weight in 0.1 ml
of acetone. The mice were restrained until the solvent had evaporated
and then placed in mouse metabolism cages. They were sacrificed at 1,
5, 15, 50, 480, and 2880 min after treatment and examined for absorp-
tion, distribution, and excretion of the insecticide. About 40% of the
applied permethrin had moved from the site of application within 5 min
and appeared to move rapidly to other parts of the body.
5.1.2 Rat
When a preparation of [1RS, trans ]- or [1RS, cis]-permethrin (14C-
labelled in the alcohol or acid moiety) was administered orally to male
rats at levels of 1.6-4.8 mg/kg, the compounds were rapidly metabolized
and labels in the acid and alcohol fragments were almost completely
eliminated from the body within a few days. The radiocarbon (alcohol
or acid label) from the cis isomer was eliminated in the urine (52-54%
of the dose) and the faeces (45-47%), whereas 79-82% of the radiocarbon
from the trans isomer appeared in the urine and 16-18% in the faeces
within 12 days after administration. The 14CO2 contained in the ex-
pired air corresponded to less than 0.5% of the dose. The tissue
residues were very low, although the cis isomer showed relatively
higher residue levels (0.46-0.62 mg/kg tissue) in the fat (Gaughan et
al., 1977). The major metabolite from the acid moiety was Cl2CA (17),
which was mostly excreted in the urine, conjugated with glucuronic
acid. This accounted for 50-63% of the dose from trans-permethrin and
15-22% from cis-permethrin. Oxidation at either of the geminal di-
methyl groups occurred to the extent of 4.3-10.4% (trans) or 12.2-14.9%
(cis), and these oxidised products were eliminated in the urine and
faeces as such or as the lactone or glucuronide. The major metabolite
from the alcohol moiety was 3-(4'-hydroxyphenoxy)benzoic acid (4'-OH-
PBacid) sulfate, accounting for 30.7-42.8% of the dose (trans) and
19.5-29.3% (cis). From cis-permethrin, 2'-OH-PBacid sulfate (about 3%)
was identified. Another significant metabolite was PBacid, which oc-
curred free and as glucuronide or glycine conjugates, and accounted for
25-31% (trans) and 5.7-10.1% (cis) of the dosed radiocarbon. Except for
a trace of PBacid, all the above metabolites from the alcohol moiety
were excreted entirely in the urine. However, the faeces of rats dosed
with trans-permethrin contained 1-2% of the radioactive dose as PBalc.
Thus substantial portions of the radioactive metabolites in the
recovered excreta were identified. The proposed metabolic pathways for
cis- and trans-permethrin are shown in Fig. 2. The five principle
sites of metabolic attack in both permethrin isomers were ester
cleavage, oxidation at the trans- and cis-methyl of the geminal
dimethyl group of the acid moiety, and oxidation at the 2'- and 4'-
positions of the phenoxy group. Conjugation of the resultant car-
boxylic acids, alcohols, and phenols with glucuronic acid, glycine, and
sulfuric acid occurred to varying extent. cis-Permethrin (29) was more
stable than trans-permethrin (30), and the cis isomer yielded four
faecally excreted ester metabolites that resulted from hydroxylation at
the 2'- or 4'-position of the phenoxy group or at the trans- or cis-
methyl group on the cyclopropane ring (e.g., (35'), (36')). The ester-
cleaved metabolites were extensively excreted into the urine whereas
the metabolites retaining an ester bond were found only in the faeces.
The major metabolite from the acid moiety of both isomers was Cl2CA
(31,31') in free (1-8%) and glucuronide (14-42%) forms. Other signifi-
cant metabolites were trans-OH-Cl2CA (32,32') (1-5%) and cis-OH-
Cl2CA (33,33') in the free (3-5%), lactone (34,34') (0-4%) and
glucuronide (1-2%) forms. On the other hand, the alcohol moiety
released after cleavage of the ester bond of both isomers was converted
mainly to the sulfate of 3-(4'-hydroxyphenoxy)benzoic acid (4'-OH-
PBacid) (13) (29-43% of the dose) and PBacid (12) in the free (1-10%)
and glucuronide (7-15%) forms. Other significant metabolites of the
alcohol moiety were PBalc (6), PBacid-glycine and the sulfate of 3-(2'-
hydroxyphenoxy) benzoic acid (2'-OH-PBacid) (14). [1RS, trans ]- and
[1RS, cis ]-permethrin showed no significant differences in metabolic
fate in the rat from [1R, trans ]- and [1R, cis ]-permethrin, respect-
ively (Elliott et al., 1976; Gaughan et al., 1977).
5.1.3 Goat
When ten consecutive oral doses of 14C- trans- or 14C- cis-permethrin
(labelled in the acid or alcohol moieties) at 0.2-0.3 mg/kg body
weight/day were given to lactating goats, they excreted 72-79% and 25-
36% of the trans and cis isomer doses, respectively, in urine and 12-
15% and 52-68%, respectively, in the faeces. The amounts of the
radiocarbon appearing in the milk were less than 0.7% with any one of
the four 14C-labelled preparations. Concerning the tissue residues 24h
after the last dose, detectable levels of radiocarbon were found in
most tissues, but none was higher than 0.04 mg/kg for the trans isomer
or 0.25 mg/kg for the cis isomer (Hunt & Gilbert, 1977).
The permethrin metabolites in goats were formed through cleavage of
the ester linkage, hydroxylation at the cis- or trans-methyl of the
geminal dimethyl group, and hydroxylation at the 4'-position of the
phenoxybenzyl moiety. Some of these metabolic products were further
oxidized and/or conjugated with glycine, glutamic acid and glucuronic
acid. The major compounds found in faeces after dosing with cis-per-
methrin were unmetabolized parent compound, 4'-OH-permethrin (35'),
trans-OH-permethrin (36'), PBalc, cis-OH- cis-Cl2CA-lactone (34') and
eight unidentified ester metabolites (Fig. 2). The faeces of goats
treated with the trans isomer contained large amounts of the parent
compound (41-79% of the faecal 14C) and of PBalc (8-25%) and cis-OH-
trans-Cl2CA-lactone (34). Also, three unidentified ester metab-
olites were found (8-23%). On the other hand, major urinary metab-
olites from the alcohol moiety of both isomers were PBacid-glycine (7-
9% of the urinary 14C) and 4'-OH-PBacid-glycine (4-12%). PBalc,
PBacid, 4'-OH-PBalc (7), 4'-OH-PBacid, PBacid-glutamic acid and 4'-OH-
PBacid-glutamic acid were also identified as minor metabolites. The
urine of goats treated with both isomers contained, as major compo-
nents, Cl2CA in the free form (2-47% of the urinary 14C) and as a
glucuronide (27-71%). Cl2CA-glucuronide was obtained to a larger
extent with the trans isomer than with the cis isomer. Other major
metabolites of the cis isomer were cis-OH-Cl2CA (33') (9-11%) and
cis-OH- cis-Cl2CA-lactone (34') (11-16%). trans-OH-Cl2CA (32,32') was
detected as a minor metabolite of both isomers. The milk of goats con-
tained the parent compounds, PBacid-glycine, and 4 -OH-PBacid-glycine.
On administration of the cis isomer, a larger amount of the parent
compound was excreted in the milk than in the case of the trans isomer.
Comparatively, when the trans isomer was administered, PBacid-glycine
was detected in the milk to a larger extent than with the cis isomer.
Most of the radioactivity in the fat was attributable to the parent
compound, or ester metabolites such as trans-OH-permethrin (36,36')
and trans-OH-permethrin conjugate (Ivie & Hunt, 1980).
5.1.4 Cow
When four lactating Jersey cows were orally administered 14C- trans- or
cis-permethrin preparations (labelled either in the alcohol or acid
moiety; three doses of ca. 1 mg/kg body weight at 24-h intervals), the
radiocarbon was almost completely eliminated in the faeces and urine 12
or 13 days after the initial dose. There was more faecal elimination of
the radiocarbon and higher tissue residue levels in the fat with the
cis isomer than the trans isomer. The 14C blood level reached a tran-
sient peak shortly after each dose and decreased to an insignificant
level within 2 to 4 days after the last dose. Higher blood levels were
attained with 14C- trans-permethrin labelled in the acid moiety than
when labelled in the alcohol moiety. This difference arising from
labelling positions was not evident with cis-permethrin. The radio-
carbon excreted in the milk was less than 0.5% of the dose. The lowest
14C level in milk was obtained from 14C- trans-permethrin (acid
moiety labelling) and the highest with 14C-trans-permethrin (alcohol
moiety labelling). With all labelled preparations, however, the radio-
carbon levels in milk decreased to <100 µg/litre within 2 to 4 days
after treatment ceased. The only radiolabelled compound recovered from
milk, in the case of the trans isomer, was unmetabolized permethrin,
whereas with the cis isomer 85% of the radiocarbon was as parent com-
pound and 15% as trans-OH- cis-permethrin (36 ). The metabolic reac-
tions of permethrin in cows were similar to those in rats and hens. In
cows, the permethrin isomers, their mono- and dihydroxy derivatives,
and PBalc, appeared only in the faeces, while the cis-OH-Cl2CA-
lactones (34,34') appeared in both faeces and urine. The remaining
metabolites appeared only in the urine. Although a slightly larger
portion of cis-permethrin than trans-permethrin was excreted un-
changed, there were similar amounts of ester metabolites with both iso-
mers. These ester metabolites were hydroxylated at the trans- or cis-
methyl positions of the geminal dimethyl group, at the 4'-position of
the phenoxybenzyl group, or at both the geminal dimethyl and phenoxy
groups. The preferred hydroxylation site with both isomers was the
trans-methyl group. The major metabolites from the acid moieties of
both isomers was the corresponding cis-OH-Cl2CA (33,33') and its
lactone and Cl2CA-glucuronide, while trans-OH- cis-Cl2CA (32') was also
a major metabolite from cis-permethrin. On the other hand, the major
metabolites from the alcohol moiety of both isomers were PBacid-glycine
(3-11% of the dose), PBalc (8-10%), and PBacid-glutamic acid (12-28%)
(Gaughan et al., 1978a).
5.1.5 Man
Two human volunteers, who consumed about 2 and 4 mg of permethrin
(25:75), respectively, excreted 18-37% and 32-39% of the administered
dose, detected as the metabolite Cl2CA, after acid hydrolysis of
their urine collected over 24 h (Cridland & Weatherley, 1977a,b).
5.2 Metabolism in Hens
A mixture (cis:trans = 25:75) of permethrin labelled with 14C in
the alcohol moiety was sprayed on 28 hens at doses of 3.77 or 11.94
mg/hen. The hens treated with the low dose showed no detectable levels
of radiocarbon in the gizzard, heart, lung, muscle, or egg white 24 h
after spraying, but the radiocarbon in the egg yolk reached a maximum
level of 0.049 mg/kg 5 days after treatment. The concentration of per-
methrin residues in the fat reached a peak 7 days after treatment and
no significant radioactivity was detectable after 4 weeks. With the
high dose, the radiocarbon in the skin had reached 6.69 mg/kg after 3
days. Small quantities of the radiocarbon were found in the egg yolks
(0.121 mg/kg) after 5 days and fat (0.110 mg/kg) after 1 day (Hunt et
al., 1979).
When White Leghorn hens were treated orally three consecutive days
with one of four 14C- trans- and cis-permethrin isomers labelled in
the alcohol or acid moieties at 10 mg/kg body weight, they showed no
signs of poisoning. More than 87% of the radiocarbon from the four
labelled preparations was found in the excreta 9 days after the initial
dose, 0.7-4.7% of the dose was exhaled as 14CO2, and 0.12-0.47% and
0.06-0.66% of the radiocarbon was recovered in egg yolk and fat (sub-
cutaneous and visceral fat), respectively. Both the cis isomers
labelled in the alcohol and acid moieties showed recoveries 3 to >10
times higher in the fat and egg yolk than those shown by the corre-
sponding trans isomers. The excreta (0-72 h) contained 1.7 times more
cis-permethrin than trans-permethrin. Hydroxylated ester metab-
olites of trans-permethrin were not excreted, but four monohydroxy and
dihydroxy esters (i.e., trans-OH-permethrin, 4'-OH-permethrin, 4'-OH,
trans-OH-permethrin (37) and trans-OH-permethrin sulfate) of cis-per-
methrin were found. Metabolites from the acid moieties of both isomers
were the Cl2CA isomers in free, glucuronide, and taurine conjugate
forms, trans-OH-Cl2CA (32,32'), cis-OH-Cl2CA (33,33'), cis-OH-Cl2CA
lactone (34,34'), and cis-OH-Cl2CA sulfate. trans-OH-Cl2CA (32,32')
was obtained from the cis isomer to larger extents than from the trans
isomer, whereas the amounts of cis-OH-Cl2CA (33,33') were larger with
the trans isomer than with the cis isomer. The metabolites from the
alcohol moiety included PBalc, PBacid, their 4'-hydroxy-derivatives and
the corresponding sulfate, the glucuronide of PBalc, and a variety of
unidentified conjugates of 4'-OH-PBalc (7) and 4'-OH-PBacid (13). The
taurine conjugate of PBacid was not detected. The metabolites produced
in largest amounts were the unidentified conjugates of 4'-OH-PBalc (6-
13% of the dose) and 4'-OH-PBacid (2-11%). The yolk of eggs 5 and 6
days after initial dosing contained 4.4 times more cis-permethrin than
trans-permethrin in unchanged form and the same ester metabolites of
cis-permethrin as those found in the excreta. Other metabolites in
the yolk were generally the same as those in the excreta. Overall,
cis-permethrin appeared at higher levels than trans-permethrin in
the egg yolk, fatty tissues, and excreta. Radiocarbon from cis-permethrin
preparations also persisted longer in the blood than that from trans-per-
permethrin preparations. It probably resulted from more rapid ester
cleavage of the trans isomer than the cis isomer, based on the relative
amounts of hydrolysis products from the two isomers in hen excreta
(Gaughan et al., 1978b).
5.3 Enzymatic Systems for Biotransformation
In studies by Shono et al. (1979), 1 µg each of [1RS, trans ]-permethrin
or [1RS, cis ]-permethrin was incubated at 37°C for 30 min with 2.2 ml
of ca. 10% rat and mouse liver microsomes under the following
conditions:
* microsomes treated with tetraethyl pyrophosphate (TEPP) (no ester-
ase and oxidase activity),
* normal microsomes (esterase activity),
* TEPP-treated microsomes plus NADPH (oxidase activity),
* normal microsomes plus NADPH (esterase plus oxidase activity).
Each esterase preparation hydrolyzed trans-permethrin to a much
greater extent than the corresponding cis isomer. In contrast, oxidat-
ive metabolism was greater for cis-permethrin than for trans-permethrin
except with the mouse microsomes, where the reactions of both isomers
proceeded to a similar extent. Aryl hydroxylation occurred at the 4'-
and 6-positions with the mouse enzymes but only at the 4'-position with
the rat enzymes. Hydroxylation at the 2'-position was observed only
with the cis-permethrin and mouse oxidase system. The amount of trans-
hydroxymethyl ester metabolites exceeded that of the corresponding
cis-hydroxymethyl compounds except with rat enzymes acting on trans-
permethrin. In general, oxidative activity with rat microsomes was
weaker than that with mouse microsomes. The dihydroxy ester metabolite
was evident only with cis-permethrin. The cis-hydroxymethyl ester
derivatives of trans-permethrin were further oxidized to the corre-
sponding aldehyde and carboxylic acid by the mouse enzymes. The pre-
ferred sites of hydroxylation, based on all identified metabolites in
the oxidase and esterase-plus-oxidase systems, were as follows (Shono
et al., 1979):
trans-permethrin
mouse: cis-methyl > trans-methyl > 4'-carbon = 6-carbon
rat: 4'-carbon = cis-methyl > trans-methyl
cis-permethrin
mouse: trans-methyl > cis-methyl = 4'-carbon > 6-carbon
> 2'-carbon
rat: 4'-carbon = trans-methyl > cis-methyl
When 100 nmol each of [1R, trans]-, [1S, trans]-, [1RS, trans]-, [1R, cis]-,
[1S, cis ]-, or [1RS, cis ]-permethrin were incubated individually with
2.5 ml of mouse liver microsome (1.5-2.0 mg of protein), the trans iso-
mers were much more rapidly hydrolyzed than the corresponding cis iso-
mers. Of the trans isomers, [1S,trans] isomer was hydrolyzed to a
greater extent than the other trans isomers. On the other hand, when
esterase activity was suppressed, there were no distinct differences in
the oxidative metabolic rates between trans and cis isomers (Soderlund
& Casida, 1977).
The persistence of isomers of permethrin, cypermethrin, delta-
methrin, and fenvalerate in the fat and brain after oral or intraper-
itoneal administration of these pesticides to rats was compared by
Marei et al. (1982). Residues in fat and brain were much higher and more
persistent with cis-permethrin than with trans-permethrin or the
alpha-cyano phenoxybenzyl pyrethroids (cypermethrin, fenvalerate, delta-
methrin). Brain levels of trans-permethrin (but not of cis-permethrin)
were greatly elevated after pretreatment with pyrethroid esterase and
oxidase inhibitors (i.e. tri- o-cresyl phosphate, S,S,S-tributyl phosph-
orotrithioate, phenyl saligenin cyclic phosphanate as esterase inhibit-
ors and piperonyl butoxide as oxidase inhibitor).
Pyrethroid carboxyesterase(s) that hydrolyze esters of chrysan-
themic acid were purified by Suzuki & Miyamoto (1978) from rat liver
microsomes by cholic acid solubilization, ammonium sulfate fraction-
ation, heat treatment, and DEAE-Sephadex A-50 column chromatography.
The 45-fold purified enzyme (38% yield) was thought to consist of a
single protein with a relative molecular mass of approximate 74 000, a
Michaelis constant (Km) of 0.21 mmol/litre for [1R, trans ]-phenothrin,
and an optimum pH of 7-9. It was susceptible to inhibition by organo-
phosphate and carbamate insecticides and insensitive to p-chloromerc-
urybenzoic acid and to mercuric and cupric ions. The enzyme seemed
to require neither coenzymes nor cofactors and hydrolysed trans isomers
of several synthetic pyrethroids (tetramethrin, resmethrin, trans- or
cis-phenothrin and permethrin) well, at more or less similar rates. On
the other hand, the cis isomers were hydrolysed at rates one-fifth to
one-tenth of those of the trans counterparts. The purified pyrethroid
carboxyesterase was apparently identical in nature to malathion carbox-
yesterase and p-nitro phenyl acetate carboxyesterase (Suzuki &
Miyamoto, 1978).
6. EFFECTS ON THE ENVIRONMENT
Acute toxicity data of permethrin on aquatic and terrestrial non-
target organisms are summarized in Tables 5 and 6, respectively.
6.1 Toxicity to Aquatic Organisms
6.1.1 Aquatic microorganisms
Stratton & Corke (1982) investigated the toxicity of permethrin and
ten of its degradation products on the growth, photosynthesis, and
acetylene-reducing activity of two species of green algae ( Chlorella
pyrenoidosa and Scenedesmus quadricaudata ) and three species of cyano-
bacteria ( Anabaena spp.). Permethrin itself was relatively non-toxic
to photosynthesis (EC50 values >100 mg/litre) and to acetylene re-
duction (EC50 values >100 mg/litre). Its degradation products were
similarly non-toxic to green algae. However, the cyanobacteria were
susceptible to some of the breakdown products of permethrin. Growth
was the most sensitive parameter with growth yield showing EC50 values
of 2.5, 2.2, and 1.4 mg/litre for the cyanobacteria and 2.8 and 4.3 mg/
litre for the green algae with PBalc and similar values for three of
the five test species with PBald. A complex test system found interac-
tions between the various metabolites and the parent compound which
were sometimes additive and sometimes synergistic. The authors con-
cluded that it is difficult to assess the true toxicity of compounds to
soil and water microorganisms without considering the breakdown prod-
ucts. The cyanobacteria are significant nitrogen-fixing organisms in
wet tropical soils.
6.1.2 Aquatic invertebrates
Non-target invertebrates, except molluscs, are more sensitive to
permethrin than fish, as shown in Table 5.
During exposure of permethrin for up to 28 days, the caddisfly
(Brachycentrus americanus) and the stonefly (Pteronarcys dorsata)
showed behavioural changes or death at concentrations as low as 0.022
µg/litre (Anderson, 1982).
A 3-h exposure to permethrin, at 50 mg/litre, was not lethal to
Daphnia pulex. The no-effect levels were 1 µg/litre for racemic, 1R
or (+)-trans, and 1R or (+)-cis, and 50 µg/litre for 1S or (-)-trans
and 1S or (-)-cis isomers (Miyamoto, 1976).
Zitko et al. (1979) established lethal threshold values for the
lobster Homarus americanus of 7.00 µg/litre for technical permethrin
and 0.40 µg/litre for [1R, cis ]-permethrin.
Larval oyster and bullfrog (tadpole) are highly tolerant to the
insecticide, with LC50 values of >1000 and 7000 µg/litre, respect-
ively.
Stratton & Corke (1981) reported that the 48-h LC50 of permethrin
to juvenile and adult waterfleas Daphnia magna was 0.2-0.6 µg/litre. A
further series of experiments involved the addition of algae or
bacteria to the cultures of daphnids, since feeding of daphnids during
these tests had been reported to reduce the toxicity of several
chemicals to the animals. With permethrin, however, algae in the test
vessel increased the lethal effect of the compound. Algae, bacteria,
and also inert silica powder adhered to the swimming antennae of the
daphnids, causing the daphnids to sink and die on the bottom of the
flasks. The effect was greatest with adults; the shed carapaces of
juvenile showed the same adhesion of particulates but moulting pro-
tected the juveniles to some degree. This raised toxicity was due to a
direct effect of the permethrin on the daphnids and not to a tendency
for the compound to cause flocculation of the suspended material.
Friesen et al. (1983) tested the toxicity of permethrin to
sediment-living nymphs of the mayfly Hexagenia rigida. In test vessels
containing water without sediment, the 6-h LC50 was estimated to lie
between 0.58 and 2.06 µg/litre; no nymphs survived exposure to water
concentrations of 7.63 µg/litre. In the presence of sediment,
lethality was reduced; there was 88% mortality of nymphs exposed to
permethrin in water at 7.63 µg/litre after 24-h exposure. Mortality
reached 100% only after 7 days exposure with sediment. Maximum concen-
trations of permethrin in the sediment over the 7 days were estimated
to be 50 µg/kg dry weight. The authors also exposed nymphs to sedi-
ment previously exposed to permethrin. The initial water concentration
was again 7.63 µg/litre, and the sediment was left for 8 days to take
up the insecticide before the water was decanted off. Nymphs were
introduced along with clean water over the contaminated sediment. There
was 100% mortality in the exposed nymphs. Long-term exposure to both
water and sediment contaminated with permethrin led to increasing
mortality up to 4 weeks; there was little further mortality between 4
and 10 weeks. Lethality reached 100% after exposure to either water or
sediment at a simulated application rate of 7.3 g/ha over 10 weeks (95%
at 4 weeks), whereas a simulated exposure equivalent to 0.6 g/ha led to
74% mortality after a 10-week exposure of the nymphs in water and 45%
after exposure of the nymphs to sediment. The authors comment that it
is not yet possible to state a concentration of permethrin in sediment
which is sufficiently low to permit successful recolonization of con-
taminated sediment.
6.1.3 Fish
Permethrin is highly toxic to fish, as shown in Table 5. Prep-
arations using an emulsifiable concentrate of permethrin enhanced its
toxicity twofold (Coats & O'Donnell-Jeffery, 1979).
The lethal toxicity of permethrin varied inversely with water
temperature, particularly between 10 and 20°C, and with body weight
between 1 and 50 g. There was a 10-fold difference between the 96-h
LC50 values at 10 and 20°C. At 15°C, a large trout (200 g) was con-
siderably more (about 100 times) tolerant than a small fish (1 g)
(Kumaraguru & Beamish, 1981).
Toxicity to fish is linked more with the nature of the optical iso-
mers than with that of the stereoisomers; i.e. 1R isomers are more
toxic than 1S isomers. Trans and cis isomers are of similar toxicity
(Miyamoto, 1976).
Zitko et al. (1979) established lethal threshold values for the
Atlantic salmon Salmo salar of 8.8 µg/litre for technical permethrin
and 1.34 µg/litre for [1R, cis ]-permethrin.
Hansen et al. (1983) exposed embryos and the hatched fry of
sheepshead minnow (Cyprinodon variegatus), continuously over 28 days,
to concentrations of permethrin of 1.25, 2.5, 5.0, 10, 20, or 40 µg/
litre. The survival of embryos was unaffected by any of the test
concentrations. Fry were affected by exposure to 20 µg/litre or more
but unaffected by 10 µg/litre. The toxicity curve was steep; 99% of fry
survived at 10 µg/litre but only 1% at 20 µg/litre. The authors
estimated the ratio between the 96-h LC50 and the NOEL to be 0.8.
Holdway & Dixon (1988) exposed larval fish (white sucker,
Catastomus commersoni, and flagfish, Jordanella floridae ) to per-
methrin in a single 2-h pulse and examined lethality over the following
96-h. They examined the effect of age, and whether or not the fish were
fed, upon the toxic effect of the insecticide. Feeding decreased the
toxicity of permethrin to flagfish at 2 and 4 days of age but not at 8
days. Age was the most important factor affecting toxicity. The 96-h
LC50 (from exposure for 2 h) was 5.55 mg/litre, 7.91 mg/litre, and
0.57 mg/litre for flagfish of age 2, 4, and 8 days, respectively.
White suckers were most susceptible to permethrin at 20 days of age,
with a 2-h LC50 of 10.0 µg/litre. Unfed white suckers at 13 and 20
days of age were highly susceptible to permethrin, with LC50 values of
2.0 and 1.0 µg/litre, respectively. The authors pointed out that
permethrin is toxic to cladocerans (waterfleas) at levels of 0.5 µg/
litre and that fish could suffer both from the direct toxic effect of
the insecticide and the added effect experienced during food deprivation.
When used for mosquito control, the safety margins (LC50 fish/LC50
mosquito larvae) for permethrin and cis-permethrin are 2-40 and 25-65,
respectively (Mulla et al., 1978a). When intraperitoneally injected
into rainbow trout, the trans- and cis-permethrin isomers were about
110 and 5 times, respectively, more toxic to trout than to mice,
(Glickman et al., 1981).
Rainbow trout exposed to sublethal concentrations of permethrin in
water (0.09-0.35 µg/litre) or in food (85-350 µg/kg) in 20-40-day
experiments showed similar branchial changes, i.e. epithelial separa-
tion or necrosis, mucus cell hyperplasia, clubbing of epithelial cells,
or hyperplasia and fusion of adjacent secondary lamellae (Kumaraguru et
al., 1982).
6.1.4 Field studies and community effects
In studies by Mulla et al. (1975), permethrin was applied to ponds
at rates of 56 g/ha and 112 g/ha in field trials. The numbers of
Tanypodinae (mostly Pentaneura and Tanypus ) and Chironominae (mostly
Tanytarsus and Chironomus ) midge larvae were slightly depressed by
the 56 g/ha treatment. Mayfly (mostly Baetis sp.) naiads and diving
beetle ( Hydrophilidae and Dytiscidae ) larvae and adults were also
affected. However, Copepoda (mostly Cyclops and Diaptomus ) and
Ostracoda (mostly Cypricercus and Cyprinotus ) were not greatly affec-
ted. The effect on these non-target organisms was much greater at the
higher dose level of permethrin, except for the ostracods. It was con-
cluded that permethrin affected mayfly naiads severely during the
exposure period. Most populations recovered within 2 weeks following
exposure.
Mayfly naiads (mostly baetids) were also adversely affected by per-
methrin at 5.6-28 g/ha and by its cis isomer at 2.8-28 g/ha. There was
a slight recovery within 1-3 weeks after treatment (Mulla et al.,
1978b).
Permethrin was applied weekly for 6 or 8 successive weeks at the
mosquito larvicidal rate of 28 g/ha (and at a rate 5 times higher) to
ponds where 20 individuals of mosquito fish or desert pupfish were
maintained. The insecticide produced no adverse effects on the two
species of fish, and the number of fish in the treated ponds increased
markedly during the experiment. At the higher rate, mats of algae were
formed, probably as the result of elimination by permethrin of her-
bivorous arthropods that feed on the algae (Mulla et al., 1981).
Kaushik et al. (1985) investigated the effect of permethrin on the
pelagic zooplankton of a 10-ha lake in southern Ontario, Canada. The
insecticide was applied to give nominal water concentrations of 0.5,
5.0, or 50 µg/litre in in situ aquatic enclosures of 5 x 5 x 5 m.
Macrozooplankton (daphnids and copepods) were most susceptible to the
insecticide. The numbers, which in untreated enclosures were 100-1000
organisms per litre of water, fell in the days immediately following
treatment to 1-10 at 0.5 µg/litre, 0.1-1.0 at 5.0 µg/litre, and
0.01-0.1 organisms per litre at 50 µg/litre of permethrin (nominal).
Microzooplankton (mainly rotifers) were unaffected by all doses except
the highest. At this dose, numbers fell transitorily to about one tenth
of their control levels (about 1000 organisms per litre). In all cases
of treatment, rotifer numbers increased between 5- and 10-fold 20 to
100 days after treatment. The authors attributed this rise in numbers
to the resistance of the organisms to the insecticide coupled with a re-
duction in the predator organisms that normally feed on the rotifers.
Populations of macrozooplankton had returned to normal within 250 days
of treatment (after the winter freeze) even with the highest dose of
50 µg/litre. Recovery was quicker with the lower doses (about 60 days
for treatment at 5 µg/litre and 30 days for most species at 0.5 µg/
litre). Despite this recovery in overall numbers of zooplankton, there
was a decrease in the species diversity of the larger, predator organ-
isms at all treatment levels. The enclosures, of course, prevented
immigration from the surrounding areas of water.
Helson et al. (1986) placed two species of aquatic arthropods (the
amphipod Gammarus pseudolimnaeus and the mosquito Aedes aegypti ) in
open containers of different sizes downwind from the application of
permethrin to young spruce trees for control of defoliators. The insec-
ticide was applied to trees 0.75-0.8 m tall in a single swath from a
mistblower backpack. Nominal application rates of 36 g ai/ha were used
with a swath width of 10 m, and standard and ultra-low volume appli-
cations were made. The mortality of Gammarus after 48 h averaged 95%
(range 76-100%) in the first trial and 85% (range 37-100%) in a dupli-
cate trial in containers 10 m downwind from the spraying. The effect
was reduced to 12% and 18% at a distance of 30 m from the spraying and
further reduced to an average of 5% 50 m from the spray. Mosquito
larvae were examined only in the second trial and showed 76% (37-100%)
at 10 m falling to 6% and 2% at 30 and 50 m, respectively, from the
spray. Mortality increased over the following 9 days. The authors also
determined 48-h LC50 values for the two organisms in containers simi-
larly placed in the field. These were 0.37 µg/litre and 0.69 µg/litre for
Gammarus and mosquito larvae, respectively, while LC95 values were
0.61 µg/litre for Gammarus and 1.14 µg/litre for mosquito larvae.
The authors regarded these data as a "worst case" , since sediment in
natural water and flowing water in streams could be expected to reduce
the toxic effect of the permethrin. They concluded that a 30-m safety
zone needs to be left using this application method between a spraying
area and natural waters to avoid killing aquatic arthropods.
When permethrin was applied by airplane to the surface of a creek
at a nominal rate of 70 g ai/ha, the actual concentration that reached
the ground was 13.4 g ai/ha. Dramatic, but short-lived, increases in
the drift of aquatic insects (particularly large catches of springtail
(Collembola), mayfly nymphs (Ephemeroptera heptageniidea), water
scavenger beetle larvae (Coleoptera hydrophilidae), midge larvae and
pupae, water boatmen (Hemiptera corixidae), predaceous diving beetles
(Coleoptera dytiscidae), and caddisfly larvae (Trichoptera)) occurred
after treatment. No effects on populations of organisms that inhabited
the bottom layer of the creek were noticeable. The permethrin sprayed
had little effect on caged or native fish and no fish mortality was re-
corded due to the treatment. From these data, it could be inferred that
permethrin had no significant impact on the aquatic system (Kingsbury,
1976).
After an aerial application of permethrin at 17.5 g ai/ha, residues
attained peak concentrations of 147.0 µg/litre in ponds and 2.5 µg/litre
in streams, but accumulations and persistence of the pesticide in
bottom sediment were negligible. Noticeable increases in the number of
drifting organisms occurred in the treatment block ( Ephemeroptera hepta-
geniidae, Baetidae, and Plecoptera nymphs ) and 2.1 km downstream
(mayfly and stonefly nymphs) over a 24-h period immediately after the
spray. A slight reduction in the bottom fauna also occurred
downstream. When exposed in cages in the ponds, yellow perch (Perca flu-
vescens) did not exhibit any adverse effects; little or no accumulated
permethrin residues were detectable in the fish following exposure. Ob-
servations of the headwater ponds indicated that permethrin application
resulted in noticeable levels of distress and mortality to surface and
littoral invertebrates and produced a similar impact on benthic organ-
isms (Kingsbury & Kreutzweiser, 1980a).
Table 5. Acute toxicity of permethrin to non-target aquatic organisms
--------------------------------------------------------------------------------------------------------------------------------------------
Species Size Duration Toxicitya Formu- Sys- Tempera- pH Hardness Reference
of test (µg/litre) lationd teme ture (°C)
--------------------------------------------------------------------------------------------------------------------------------------------
A. Freshwater Organisms
Arthropods
Crayfish 0.8 - 1.2 cm, 96 h 0.39 EC S 24 100 Jolly et al. (1978)
(Procambarus clarkii) (0.05 g)
2 - 3 cm, 96 h 0.62 EC S 24 100 Jolly et al. (1978)
(0.5 g)
Water flea (Daphnia pulex) 3 h > 50 000 T S 25 Miyamoto (1976)
3 h > 50 000 (+)-trans S 25 Miyamoto (1976)
3 h > 50 000 (+)-cis S 25 Miyamoto (1976)
3 h > 50 000 (-)-trans S 25 Miyamoto (1976)
3 h > 50 000 (-)-cis S 25 Miyamoto (1976)
Water flea (Daphnia magna) 1st instar 48 h 1.26 T S 18 7.4 42 Mayer &
Amphipod immature 96 h 0.17 T S 17 7.4 42 Ellersieck (1986)
(Gammarus pseudolimnaeus) Mayer &
Midge (Chironomus plumosus) 3rd instar 48 h 0.56 T S 22 7.4 42 Ellersieck (1986)
Caddisfly 21 days 0.17 T F 15 7.6 - 7.8 46 - 48 Anderson (1982)
(Brachycentrus americanus)
Fish
Salmon (Salmo salar) 6.2 cm, 5.3 g 96 h 12 T R 10 McLeese et al.
(1980)
Rainbow trout 1 g 96 h 0.62 T F 5 7.9 - 8.2 358 - 363 Kumaraguru &
(Salmo gairdneri) 1 g 96 h 0.69 T F 10 7.9 - 8.2 358 - 363 Beamish (1981)
1 g 96 h 3.17 T F 15 7.9 - 8.2 358 - 363 Kumaraguru &
1 g 96 h 6.43 T F 20 7.9 - 8.2 358 - 363 Beamish (1981)
5 g 96 h 6.43 T F 15 7.9 - 8.2 358 - 363 Kumaraguru &
20 g 96 h ca. 50 T F 15 7.9 - 8.2 358 - 363 Beamish (1981)
50 g 96 h 287 T F 15 7.9 - 8.2 358 - 363 Kumaraguru &
200 g 96 h 314 T F 15 7.9 - 8.2 358 - 363 Beamish (1981)
6 cm, 3 g 24 h 135 T 10 7.5 110 Coats & O'Donnell-
6 cm, 3 g 24 h 61 EC 10 7.5 110 Jeffery (1979)
--------------------------------------------------------------------------------------------------------------------------------------------
Table 5. (contd.)
--------------------------------------------------------------------------------------------------------------------------------------------
Species Size Duration Toxicitya Formu- Sys- Tempera- pH Hardness Reference
of test (µg/litre) lationd teme ture (°C)
--------------------------------------------------------------------------------------------------------------------------------------------
Rainbow trout (contd.)
(Salmo gairdneri) 5 - 6 cm 48 h 6.0 EC 12- Mulla et al.
5 - 6 cm 48 h 7.0 cis, EC 25.5 (1978a)
2 - 4 g 24 h 18 T S 12 Glickman
2 - 4 g 24 h 25 cis S 12 et al.
2 - 4 g 24 h 14 trans S 12 (1981)
Killifish adult 48 h 41 T S 25 Miyamoto (1976)
(Oryzias latipes) adult 48 h 17 (+)-trans S 25 Miyamoto (1976)
adult 48 h 13 (+)-cis S 25 Miyamoto (1976)
adult 48 h > 10 000 (-)-trans S 25 Miyamoto (1976)
adult 48 h > 10 000 (-)-cis S 25 Miyamoto (1976)
Channel catfish 1.4 - 1.7 cm, 96 h 1.1 EC S 24 100 Jolly et al. (1978)
(Ictalurus punctatus) (0.02 g)
Largemouth Bass 4.5 - 5.5 cm, 96 h 8.5 EC S 24 100 Jolly et al. (1978)
(Micropterus salmoides) (1.14 g)
Mosquitofish 1.5 - 2.5 cm, 96 h 15 EC S 24 100 Jolly et al. (1978)
(Gambusia affinis) (0.25 g)
4 - 5 cm 48 h 97.0 EC 8.8 - 16 Mulla et al.
4 - 5 cm 48 h 13.0 cis, EC 8.8 - 16 (1978a)
Brook trout 1.2 g 96 h 3.2 T S 12 7.5 40 Mayer & Ellersieck
(Salvelinus foutinalis) (1986)
Fathead minnow 0.6 g 96 h 5.7 T S 22 7.3 38 Mayer & Ellersieck
(Pimephales promelas) (1986)
Bluegill sunfish 0.7 g 96 h 5.0 T S 22 7.3 38 Mayer & Ellersieck
(Lepomis macrochirus) (1986)
Desert pupfish 4 - 5 cm 48 h 5.0 EC S 11 - 16.5 Mulla et al.
(Cyprinodon macularis) 4 - 5 cm 48 h 5.0 cis, EC S 11 - 16.5 (1978a)
Tilapia mossambica 5 - 6 cm 48 h 44.0 EC S 15 - 21.4 Mulla et al.
5 - 6 cm 48 h 5.6 cis, EC S 15 - 21.4 (1978a)
Amphibian
Bullfrog, tadpole 0.6 - 0.8 cm 96 h 7033 EC S 24 100 Jolly et al. (1978)
(Rana catesbeiana)
--------------------------------------------------------------------------------------------------------------------------------------------
Table 5. (contd.)
--------------------------------------------------------------------------------------------------------------------------------------------
Species Size Duration Toxicitya Formu- Sys- Tempera- pH Salinity Reference
of test (µg/litre) lationd teme ture (°C) (°/oo)
--------------------------------------------------------------------------------------------------------------------------------------------
B. Estuarine and Marine Organisms
Algae
Skeletonema costatum 96 h 92b T 20 Borthwich & Walsh
(1981)
Molluscs
Oyster (Crassostrea virginica) 2-h larva 48 h > 1000c T S 25 20 Borthwich & Walsh
(1981)
Arthropods
Lobster (Homarus americanus) 450 g 96 h 0.73 T R 10 30 McLeese et al.
Shrimp (Crangon Septemspinosa) 1.3 g 96 h 0.13 T R 10 (1980)
Shrimp (Mysidopsis bahia) 1-day, 96 h 0.046 T S 25 20 Borthwich
juvenile & Walsh
Stone crab (Menippe mercenaria) Zoea larva 96 h 0.018 T S 25 20 (1981)
Pink shrimp (Penaeus duorarum) adult 96 h 0.22 T F 25 25 Mayer (1987)
Fish
Harpacticoid (Nitocra spinipes) 3-6 weeks old 96 h 0.6 EC S 20 - 22 7.8 7 Linden et al.
Bleak (Alburnus alburnus) 8 cm 96 h 4 - 8 EC S 10 7.8 7 (1979)
Sheepshead minnow 28-day fry 96 h 88 T S 25 20 Borthwich & Walsh
(Cyprinodon variegatus) (1981)
adult 96 h 7.8 T F 30 22 Mayer (1987)
Atlantic silverside adult 96 h 2.2 T F 26 25 Mayer (1987)
(Menidia menidia)
Striped mullet (Mugil cephalus) juvenile 96 h 5.5 T F 24 19 Mayer (1987)
--------------------------------------------------------------------------------------------------------------------------------------------
a Values are LC50 unless stated otherwise.
b EC50 (growth inhibition).
c EC50 (abnormal development).
d T = Technical, EC = Emulsifiable concentrate.
e R = Renewal, S = Static, F = Flow-through.
f expressed as mg CaCO3/litre.
When permethrin was sprayed at 8.8, 17.5, 35.0, or 70.0 g ai/ha by
aeroplane over small trout streams, the impact on aquatic invertebrates
and effects on the general fish population correlated with the dose.
There was an increase in the number of organisms drifting downstream,
the major ones being mayflies, followed by caddisflies, stoneflies, and
chironomids. The total number of drifting organisms was greater than
the pre-spray average by factors of 303, 699, 4960, and 6450 with spray
concentrations of 8.8, 17.5, 35.0, and 70.0 g ai/ha, respectively. A
return to pre-spray drift levels was evident within 36 h after appli-
cation at 8.8 and 17.5 g ai/ha whereas drifting of organism persisted
for up to 72 h at the higher application rates of 35 and 70 g ai/ha.
Following the spraying of permethrin at these higher doses, there was
no evidence of fish mortality, but there appeared a dramatic change in
the diets of fish (such as the native brook trout and sculpins). These
fish became virtually completely dependent on terrestrial invertebrates
rather than on the aquatic insects for food. When the rate of appli-
cation was low (8.8 g ai/ha) permethrin did not appear to affect these
fish, either in mortality rate or in their diet composition (Kingsbury
& Kreutzweiser, 1980b).
Serial applications of permethrin, once or twice at 17.5 g ai/ha,
resulted in catastrophic drift of aquatic invertebrates and substantial
depletion of benthos in streams within the application blocks and up to
2 km downstream. Despite massive disturbances of benthos, repopulation
of bottom fauna was evident within 2.5 months and had virtually re-
turned to normal within 3.5 months. Permethrin residues attained peak
levels of 1.35 µg/litre in standing water and 1.94 µg/litre in
flowing water in the sprayed regions. The residue persisted at low
concentrations for up to 96 h after spraying (Kreutzweiser, 1982).
In a study by Kingsbury & Kreutzweiser (1987), aerial applications
of permethrin to forests over several seasons at 8.8, 17.5, 35, and 70
g ai/ha did not cause mortality to native and caged fish (minnows,
mudminnows, perch, and under-yearling and yearling Atlantic salmon).
The composition of the salmonid diet was subsequently altered from
aquatic insects (mayfly nymphs, stonefly nymphs, and various aquatic
fly larvae) to terrestrial arthropods. The duration of changes ranged
from a few months after applications of 8.8 and 17.5 g ai/ha to a year
or longer after treatment with 35 and 70 g ai/ha. There were temporary
reductions in fish growth rate and fish densities in the treated area,
which returned to normal within four months after treatment. In the
same study, the effect on stream invertebrates was also evaluated.
Large drifts of invertebrates were observed immediately after appli-
cations and continued for 24 to 72 h. Although the peak of permethrin
residues in stream water was higher after the second application (0.36-
1.80 µg/litre) than the first (0.25-0.62 µg/litre), drift response
to the second application ranged from 6 to 62% of the first drift,
indicating that first application deleted susceptible invertebrates
(e.g., Ephemeroptera nymphs, Plecoptera, Trichoptera, and the
Diptera families) and that a much smaller residual population re-
sponded to the second treatment. Recovery of benthic fauna was appar-
ent between 1 and 18 months after spraying. The double treatment
reduced benthos density to a point at which recovery of numbers was
slower than after the single application (Kreutzweiser & Kingsbury,
1987).
6.2 Toxicity to Terrestrial Organisms
6.2.1 Soil microorganisms
Mathur et al. (1980) applied permethrin (Ambush 5 G) to Canadian
soils with a high content of organic material at a rate of 2.24 kg/ha.
Lettuce or carrots were grown on the plots. Soil cores were taken at
regular intervals and bacterial and fungal numbers were estimated, soil
nutrients were measured, and acid phosphatase activity in the soil was
monitored. Residues of permethrin persisted throughout the growing
season up to crop harvest, when 65% of the original concentration in
soil was found (113 days after application). This reflected the poor
breakdown of permethrin in organic soils compared to mineral soils.
Permethrin suppressed bacterial and actinomycete populations in samples
taken 1, 9, and 27 days after application, but control levels were re-
gained after 41 days (the next sampling time). The available nitrogen
and phosphorus was lower in treated soil at some points during the
study, but these changes were not consistent. Soil respiration and acid
phosphatase activity were higher in permethrin-treated plots, though
not consistently so. Permethrin had a greater effect when carrots were
grown than when lettuce was grown. The yield of neither crop was
affected. Most of the effects reported were transitory and none were
of overall significance for either the soil or the crop.
6.2.2 Terrestrial invertebrates
Under laboratory conditions, permethrin is highly toxic to certain
beneficial insects or natural enemies of pests, as shown in Table 6.
Cox & Wilson (1984) treated honey bee workers topically with a sub-
lethal dose of permethrin (0.09 µg/bee in 1.0 µl of acetone applied
to the thorax). This dose gave no higher mortality than treatment with
acetone alone. The bees, which were individually tagged, were housed in
an observation hive and trained before the experiment to feed at an
artificial feeding station 5 m from the hive. The experiment was con-
ducted at 35°C because at lower temperatures this dose resulted in
mortality. Treated bees made less foraging trips than controls and gave
food less frequently to other bees in the hive. Other behaviours were
increased in treated bees, i.e., self-cleaning, trembling dance, ab-
domen tucking, and rotating and cleaning of abdomen while rubbing the
hind legs together. Gerig (1985) also reported minimal lethal effects
on honey bees of permethrin used at recommended rates and that the
insecticide had a strong repellent effect.
Table 6. Acute toxicity of permethrin to non-target terrestrial organisms
-----------------------------------------------------------------------------------------------------------------------------
Species Size Application Duration Toxicitya Temperature Reference
(°C)
-----------------------------------------------------------------------------------------------------------------------------
Bird
Hen oral > 1.5 g/kg b.w.b Millner & Butterworth
(1977)
Chicken oral > 3 g/kg b.w.b Worthing & Walker
Japanese quail oral 5 days > 13.5 g/kg b.w.b (1983)
diet 5 days > 5 g/kg dietc Hill & Camardese
diet 5 days > 5 g/kg dietd (1986)
Mallard duck diet 5 days > 27.5 g/kg diet Ross et al. (1976b)
Mallard duck oral > 13.5 g/kg b.w.b Ross et al. (1976a)
Starling diet 5 days > 27.5 g/kg diet Ross et al. (1976d)
Starling oral > 38 g/kg b.w.b Ross et al. (1976c)
Ring-necked pheasant diet 5 days > 27.5 g/kg diet Ross et al. (1976e)
Ring-necked pheasant oral > 13.5 g/kg b.w.b Ross et al. (1977a)
Arthropods
Honeybee (Apis mellifera) contact 0.11 µg/beeb 26 - 27 Stevenson et al. (1978)
oral 0.28 µg/beeb 26 - 27 Stevenson et al. (1978)
Insect parasite
Ichneumoid (Campoletis adult male film 24 h 0.31 µg/vial Plapp & Vinson (1977)
sonorensis)
Insect predator
Carabid (Pterostichus adult 0.16 g topical > 2000 µg/insectb 21 Hagley et al. (1980)
melanarius)
Carabid (Harpalus affinis) adult 0.05 g topical 116 µg/insectb 21 Hagley et al. (1980)
Carabid (Amara sp.) adult 0.03 g topical 25 µg/insectb 21 Hagley et al. (1980)
Earwig (Labidura raparia) mature soil 0.1 kg 6% mortality Workman (1977)
ai/ha
mature soil 0.2 kg 50% mortality Workman (1977)
ai/ha
Green lacewing larvae
(Chrysopa carnea) 5-6 days old film 9.87 µg/vial 25 Plapp & Bull (1978)
Predaceous mite species
Mataseiulus occidentalis adult female slide-dip 0.72, 1.32, 27.5 Roush & Hoy (1978)
(3 strains) method 14.8 mg ai/litre
young gravid leaf-disc 2.8 mg ai/litre 27 Hoy et al. (1979)
female method
(Amblyseius fallacis) adult female slide-dip 14 mg ai/litre 27 Rock (1979)
method
-----------------------------------------------------------------------------------------------------------------------------
a LC50 values, unless stated otherwise.
b LD50 values; b.w. = body weight.
c Technical product.
d Emulsifiable concentrate.
Pike et al. (1982) applied permethrin by helicopter at a rate of
0.22 kg ai/ha to fields of maize (Zea mays). The applications were
made early in the morning before bees were actively foraging for pollen
and were repeated every 3 to 6 days (to a maximum of six applications
per season). The trial was repeated for 3 years. There was no differ-
ence in the number of dead bees per hive between treated and control
areas, but a marked reduction in the number of bees foraging in treated
fields, indicating avoidance of permethrin. The authors concluded that
treatment of corn fields with permethrin at the normal application rate
is safe for bees as long as the application does not coincide with bee
activity in the area.
The acute toxicity values of permethrin to tobacco budworm
(Heliothis virescens), to the green lacewing (Chrysopa carnea), a
predator of tobacco budworm, and to Campoletis sonorensis, an
ichneumoid parasite of tobacco budworm, showed that permethrin was
approximately 18 times less toxic to the predators than to the pest
(Plapp & Bull, 1978; Plapp & Vinson, 1977).
Larvae of the green lacewing exhibited marked tolerance to per-
methrin, and to its cis or trans isomers, when dosed topically with
250 µg per insect (about 25 000 µg/g). This value is ca. 10 000 times
greater than the LD50 value for the tobacco budworm (Shour & Crowder,
1980).
Workman (1977) added permethrin to loamy sand soil into which the
striped earwig (Labidura riparia), an effective insect predator of
the cabbage looper, was introduced. The insecticide was of low toxicity
to the earwig at dosage rates which gave good looper control.
The susceptibility of carabids to permethrin appears to be in-
versely related to beetle size, as shown in Table 6. When permethrin
was applied at a concentration of 0.21-0.85 kg ai/ha to an apple
orchard, it did not significantly affect the numbers of Pterostichus
melanarius at any time during the season, but the numbers of Harpalus
affinis and Amara sp. were significantly reduced 3-5 days after appli-
cation. This result reflected the toxicity ratings by means of LD50
values obtained in laboratory studies. The total seasonal numbers of
these carabids were not significantly affected by permethrin, owing to
short residual effects (Hagley et al., 1980).
In laboratory tests, LC50 values of permethrin for two strains of
spider mites (Tetranychus urticae) were ca. 20-40 times greater than
those for three strains of predator mites (Mataseiulus occidentalis)
(Roush & Hoy, 1978), and ca.15 times greater than those for Amblyseius
fallacis (Rock, 1979). These studies indicate that the use of per-
methrin at the recommended rates of 60-120 mg ai/litre would be detri-
mental to orchard integrated mite control programs.
In laboratory tests, the Pacific spider mite (T. pacificus) has
been found to be 40 times more tolerant to permethrin than the predator
mite (M. occidentalis), (Hoy et al., 1979). The spraying of vineyards
with 15 or 30 mg ai/litre resulted in substantially higher populations
of T. pacificus and E. willamettei for about one month due to re-
duction in predator species numbers. Similarly, spraying with 60 or
120 mg ai/litre produced a subsequent increase of Eotetranychus
willamettei late in August and September for the above reason (Hoy et
al., 1979).
When permethrin was applied to apple trees at a concentration of
40 mg/litre, no predator mites (Typhlodromus pyri) were found for 4-6
weeks, and only small numbers were found 10 weeks after the spray. On
the other hand, permethrin had no appreciable toxicity to the spider
mite (Panonychus ulmi). The virtual elimination of the predatory mite
by permethrin spraying led to a marked population increase of P. ulmi
later in the same season (Aliniazee & Cranham, 1980).
In apple and pear orchards, applications of permethrin at 30 mg
ai/litre reduced the numbers of a predatory mite (M. occidentalis) to
almost zero and dramatically increased the populations of spider mites
(T. urticae, Tetranychus mcdanieli, or P. ulmi) (Hoyt et al., 1978).
From the above findings it appeared that permethrin, when applied
according to recommendations, is relatively harmless to insect pred-
ators, with the exception of predaceous mite species. Everts et al.
(1985) investigated the effects of permethrin on beneficial terrestrial
arthropods in the soil and vegetation in areas surrounding applications
of the insecticide to control tsetse flies in the Ivory Coast, West
Africa. Permethrin was used as a 2.5% wettable powder at a rate of 121
g ai/ha during January (a minimum temperature of 20.0°C and a maximum
of 36.0°C). The permethrin application significantly reduced popu-
lations of Coleoptera, Lepidoptera, Ephydridae, Chloropidae, Muscidae,
Ichneumonidea, Chalcidoidea and Proctotrupoidea. The populations of
almost all these groups were found to recover to normal levels within 2
months, i.e., before the likely time of respraying for the control of
the tsetse flies. However, one Proctotrupoid genus, Cremastobaeus, was
eliminated by permethrin treatment.
6.2.3 Birds
Neither an acute oral nor a dietary LD50 or LC50 has been estab-
lished accurately because of the very low toxicity of permethrin to
birds (Table 6). The acute LD50 is >3000 mg/kg body weight and the
dietary toxicity >5000 mg/kg diet. (Worthing & Walker, 1983; Hill &
Camardese, 1986).
The inclusion of permethrin at up to 40 mg/kg in the diet of laying
hens for 28 days had no adverse effects on the health of parent birds
or on egg production quality, hatchability, or the viability of the
chicks produced (Ross et al. 1977b).
6.2.4 Mammals
Racey & Swift (1986) treated roosting boxes for pipistrelle bats
with various wood preservatives and allowed the bats to roost in the
boxes for up to 154 days. There were no toxic effects of mixtures of
synthetic pyrethroids including permethrin. The authors concluded that
the insecticide component of wood preservatives should be pyrethroids
when bats are present in the area.
6.3 Uptake, Loss, Bioaccumulation and Biomagnification
Proposed metabolic pathways of permethrin in fish are summarized in
Fig. 4.
5.1.1 Mouse
In studies by Shah et al. (1981), 14C- cis-permethrin was applied
to the clipped skin of mice at a level of 1 mg/kg body weight in 0.1 ml
of acetone. The mice were restrained until the solvent had evaporated
and then placed in mouse metabolism cages. They were sacrificed at 1,
5, 15, 50, 480, and 2880 min after treatment and examined for absorp-
tion, distribution, and excretion of the insecticide. About 40% of the
applied permethrin had moved from the site of application within 5 min
and appeared to move rapidly to other parts of the body.
5.1.2 Rat
When a preparation of [1RS, trans ]- or [1RS, cis]-permethrin (14C-
labelled in the alcohol or acid moiety) was administered orally to male
rats at levels of 1.6-4.8 mg/kg, the compounds were rapidly metabolized
and labels in the acid and alcohol fragments were almost completely
eliminated from the body within a few days. The radiocarbon (alcohol
or acid label) from the cis isomer was eliminated in the urine (52-54%
of the dose) and the faeces (45-47%), whereas 79-82% of the radiocarbon
from the trans isomer appeared in the urine and 16-18% in the faeces
within 12 days after administration. The 14CO2 contained in the ex-
pired air corresponded to less than 0.5% of the dose. The tissue
residues were very low, although the cis isomer showed relatively
higher residue levels (0.46-0.62 mg/kg tissue) in the fat (Gaughan et
al., 1977). The major metabolite from the acid moiety was Cl2CA (17),
which was mostly excreted in the urine, conjugated with glucuronic
acid. This accounted for 50-63% of the dose from trans-permethrin and
15-22% from cis-permethrin. Oxidation at either of the geminal di-
methyl groups occurred to the extent of 4.3-10.4% (trans) or 12.2-14.9%
(cis), and these oxidised products were eliminated in the urine and
faeces as such or as the lactone or glucuronide. The major metabolite
from the alcohol moiety was 3-(4'-hydroxyphenoxy)benzoic acid (4'-OH-
PBacid) sulfate, accounting for 30.7-42.8% of the dose (trans) and
19.5-29.3% (cis). From cis-permethrin, 2'-OH-PBacid sulfate (about 3%)
was identified. Another significant metabolite was PBacid, which oc-
curred free and as glucuronide or glycine conjugates, and accounted for
25-31% (trans) and 5.7-10.1% (cis) of the dosed radiocarbon. Except for
a trace of PBacid, all the above metabolites from the alcohol moiety
were excreted entirely in the urine. However, the faeces of rats dosed
with trans-permethrin contained 1-2% of the radioactive dose as PBalc.
Thus substantial portions of the radioactive metabolites in the
recovered excreta were identified. The proposed metabolic pathways for
cis- and trans-permethrin are shown in Fig. 2. The five principle
sites of metabolic attack in both permethrin isomers were ester
cleavage, oxidation at the trans- and cis-methyl of the geminal
dimethyl group of the acid moiety, and oxidation at the 2'- and 4'-
positions of the phenoxy group. Conjugation of the resultant car-
boxylic acids, alcohols, and phenols with glucuronic acid, glycine, and
sulfuric acid occurred to varying extent. cis-Permethrin (29) was more
stable than trans-permethrin (30), and the cis isomer yielded four
faecally excreted ester metabolites that resulted from hydroxylation at
the 2'- or 4'-position of the phenoxy group or at the trans- or cis-
methyl group on the cyclopropane ring (e.g., (35'), (36')). The ester-
cleaved metabolites were extensively excreted into the urine whereas
the metabolites retaining an ester bond were found only in the faeces.
The major metabolite from the acid moiety of both isomers was Cl2CA
(31,31') in free (1-8%) and glucuronide (14-42%) forms. Other signifi-
cant metabolites were trans-OH-Cl2CA (32,32') (1-5%) and cis-OH-
Cl2CA (33,33') in the free (3-5%), lactone (34,34') (0-4%) and
glucuronide (1-2%) forms. On the other hand, the alcohol moiety
released after cleavage of the ester bond of both isomers was converted
mainly to the sulfate of 3-(4'-hydroxyphenoxy)benzoic acid (4'-OH-
PBacid) (13) (29-43% of the dose) and PBacid (12) in the free (1-10%)
and glucuronide (7-15%) forms. Other significant metabolites of the
alcohol moiety were PBalc (6), PBacid-glycine and the sulfate of 3-(2'-
hydroxyphenoxy) benzoic acid (2'-OH-PBacid) (14). [1RS, trans ]- and
[1RS, cis ]-permethrin showed no significant differences in metabolic
fate in the rat from [1R, trans ]- and [1R, cis ]-permethrin, respect-
ively (Elliott et al., 1976; Gaughan et al., 1977).
5.1.3 Goat
When ten consecutive oral doses of 14C- trans- or 14C- cis-permethrin
(labelled in the acid or alcohol moieties) at 0.2-0.3 mg/kg body
weight/day were given to lactating goats, they excreted 72-79% and 25-
36% of the trans and cis isomer doses, respectively, in urine and 12-
15% and 52-68%, respectively, in the faeces. The amounts of the
radiocarbon appearing in the milk were less than 0.7% with any one of
the four 14C-labelled preparations. Concerning the tissue residues 24h
after the last dose, detectable levels of radiocarbon were found in
most tissues, but none was higher than 0.04 mg/kg for the trans isomer
or 0.25 mg/kg for the cis isomer (Hunt & Gilbert, 1977).
The permethrin metabolites in goats were formed through cleavage of
the ester linkage, hydroxylation at the cis- or trans-methyl of the
geminal dimethyl group, and hydroxylation at the 4'-position of the
phenoxybenzyl moiety. Some of these metabolic products were further
oxidized and/or conjugated with glycine, glutamic acid and glucuronic
acid. The major compounds found in faeces after dosing with cis-per-
methrin were unmetabolized parent compound, 4'-OH-permethrin (35'),
trans-OH-permethrin (36'), PBalc, cis-OH- cis-Cl2CA-lactone (34') and
eight unidentified ester metabolites (Fig. 2). The faeces of goats
treated with the trans isomer contained large amounts of the parent
compound (41-79% of the faecal 14C) and of PBalc (8-25%) and cis-OH-
trans-Cl2CA-lactone (34). Also, three unidentified ester metab-
olites were found (8-23%). On the other hand, major urinary metab-
olites from the alcohol moiety of both isomers were PBacid-glycine (7-
9% of the urinary 14C) and 4'-OH-PBacid-glycine (4-12%). PBalc,
PBacid, 4'-OH-PBalc (7), 4'-OH-PBacid, PBacid-glutamic acid and 4'-OH-
PBacid-glutamic acid were also identified as minor metabolites. The
urine of goats treated with both isomers contained, as major compo-
nents, Cl2CA in the free form (2-47% of the urinary 14C) and as a
glucuronide (27-71%). Cl2CA-glucuronide was obtained to a larger
extent with the trans isomer than with the cis isomer. Other major
metabolites of the cis isomer were cis-OH-Cl2CA (33') (9-11%) and
cis-OH- cis-Cl2CA-lactone (34') (11-16%). trans-OH-Cl2CA (32,32') was
detected as a minor metabolite of both isomers. The milk of goats con-
tained the parent compounds, PBacid-glycine, and 4 -OH-PBacid-glycine.
On administration of the cis isomer, a larger amount of the parent
compound was excreted in the milk than in the case of the trans isomer.
Comparatively, when the trans isomer was administered, PBacid-glycine
was detected in the milk to a larger extent than with the cis isomer.
Most of the radioactivity in the fat was attributable to the parent
compound, or ester metabolites such as trans-OH-permethrin (36,36')
and trans-OH-permethrin conjugate (Ivie & Hunt, 1980).
5.1.4 Cow
When four lactating Jersey cows were orally administered 14C- trans- or
cis-permethrin preparations (labelled either in the alcohol or acid
moiety; three doses of ca. 1 mg/kg body weight at 24-h intervals), the
radiocarbon was almost completely eliminated in the faeces and urine 12
or 13 days after the initial dose. There was more faecal elimination of
the radiocarbon and higher tissue residue levels in the fat with the
cis isomer than the trans isomer. The 14C blood level reached a tran-
sient peak shortly after each dose and decreased to an insignificant
level within 2 to 4 days after the last dose. Higher blood levels were
attained with 14C- trans-permethrin labelled in the acid moiety than
when labelled in the alcohol moiety. This difference arising from
labelling positions was not evident with cis-permethrin. The radio-
carbon excreted in the milk was less than 0.5% of the dose. The lowest
14C level in milk was obtained from 14C- trans-permethrin (acid
moiety labelling) and the highest with 14C-trans-permethrin (alcohol
moiety labelling). With all labelled preparations, however, the radio-
carbon levels in milk decreased to <100 µg/litre within 2 to 4 days
after treatment ceased. The only radiolabelled compound recovered from
milk, in the case of the trans isomer, was unmetabolized permethrin,
whereas with the cis isomer 85% of the radiocarbon was as parent com-
pound and 15% as trans-OH- cis-permethrin (36 ). The metabolic reac-
tions of permethrin in cows were similar to those in rats and hens. In
cows, the permethrin isomers, their mono- and dihydroxy derivatives,
and PBalc, appeared only in the faeces, while the cis-OH-Cl2CA-
lactones (34,34') appeared in both faeces and urine. The remaining
metabolites appeared only in the urine. Although a slightly larger
portion of cis-permethrin than trans-permethrin was excreted un-
changed, there were similar amounts of ester metabolites with both iso-
mers. These ester metabolites were hydroxylated at the trans- or cis-
methyl positions of the geminal dimethyl group, at the 4'-position of
the phenoxybenzyl group, or at both the geminal dimethyl and phenoxy
groups. The preferred hydroxylation site with both isomers was the
trans-methyl group. The major metabolites from the acid moieties of
both isomers was the corresponding cis-OH-Cl2CA (33,33') and its
lactone and Cl2CA-glucuronide, while trans-OH- cis-Cl2CA (32') was also
a major metabolite from cis-permethrin. On the other hand, the major
metabolites from the alcohol moiety of both isomers were PBacid-glycine
(3-11% of the dose), PBalc (8-10%), and PBacid-glutamic acid (12-28%)
(Gaughan et al., 1978a).
5.1.5 Man
Two human volunteers, who consumed about 2 and 4 mg of permethrin
(25:75), respectively, excreted 18-37% and 32-39% of the administered
dose, detected as the metabolite Cl2CA, after acid hydrolysis of
their urine collected over 24 h (Cridland & Weatherley, 1977a,b).
5.2 Metabolism in Hens
A mixture (cis:trans = 25:75) of permethrin labelled with 14C in
the alcohol moiety was sprayed on 28 hens at doses of 3.77 or 11.94
mg/hen. The hens treated with the low dose showed no detectable levels
of radiocarbon in the gizzard, heart, lung, muscle, or egg white 24 h
after spraying, but the radiocarbon in the egg yolk reached a maximum
level of 0.049 mg/kg 5 days after treatment. The concentration of per-
methrin residues in the fat reached a peak 7 days after treatment and
no significant radioactivity was detectable after 4 weeks. With the
high dose, the radiocarbon in the skin had reached 6.69 mg/kg after 3
days. Small quantities of the radiocarbon were found in the egg yolks
(0.121 mg/kg) after 5 days and fat (0.110 mg/kg) after 1 day (Hunt et
al., 1979).
When White Leghorn hens were treated orally three consecutive days
with one of four 14C- trans- and cis-permethrin isomers labelled in
the alcohol or acid moieties at 10 mg/kg body weight, they showed no
signs of poisoning. More than 87% of the radiocarbon from the four
labelled preparations was found in the excreta 9 days after the initial
dose, 0.7-4.7% of the dose was exhaled as 14CO2, and 0.12-0.47% and
0.06-0.66% of the radiocarbon was recovered in egg yolk and fat (sub-
cutaneous and visceral fat), respectively. Both the cis isomers
labelled in the alcohol and acid moieties showed recoveries 3 to >10
times higher in the fat and egg yolk than those shown by the corre-
sponding trans isomers. The excreta (0-72 h) contained 1.7 times more
cis-permethrin than trans-permethrin. Hydroxylated ester metab-
olites of trans-permethrin were not excreted, but four monohydroxy and
dihydroxy esters (i.e., trans-OH-permethrin, 4'-OH-permethrin, 4'-OH,
trans-OH-permethrin (37) and trans-OH-permethrin sulfate) of cis-per-
methrin were found. Metabolites from the acid moieties of both isomers
were the Cl2CA isomers in free, glucuronide, and taurine conjugate
forms, trans-OH-Cl2CA (32,32'), cis-OH-Cl2CA (33,33'), cis-OH-Cl2CA
lactone (34,34'), and cis-OH-Cl2CA sulfate. trans-OH-Cl2CA (32,32')
was obtained from the cis isomer to larger extents than from the trans
isomer, whereas the amounts of cis-OH-Cl2CA (33,33') were larger with
the trans isomer than with the cis isomer. The metabolites from the
alcohol moiety included PBalc, PBacid, their 4'-hydroxy-derivatives and
the corresponding sulfate, the glucuronide of PBalc, and a variety of
unidentified conjugates of 4'-OH-PBalc (7) and 4'-OH-PBacid (13). The
taurine conjugate of PBacid was not detected. The metabolites produced
in largest amounts were the unidentified conjugates of 4'-OH-PBalc (6-
13% of the dose) and 4'-OH-PBacid (2-11%). The yolk of eggs 5 and 6
days after initial dosing contained 4.4 times more cis-permethrin than
trans-permethrin in unchanged form and the same ester metabolites of
cis-permethrin as those found in the excreta. Other metabolites in
the yolk were generally the same as those in the excreta. Overall,
cis-permethrin appeared at higher levels than trans-permethrin in
the egg yolk, fatty tissues, and excreta. Radiocarbon from cis-permethrin
preparations also persisted longer in the blood than that from trans-per-
permethrin preparations. It probably resulted from more rapid ester
cleavage of the trans isomer than the cis isomer, based on the relative
amounts of hydrolysis products from the two isomers in hen excreta
(Gaughan et al., 1978b).
5.3 Enzymatic Systems for Biotransformation
In studies by Shono et al. (1979), 1 µg each of [1RS, trans ]-permethrin
or [1RS, cis ]-permethrin was incubated at 37°C for 30 min with 2.2 ml
of ca. 10% rat and mouse liver microsomes under the following
conditions:
* microsomes treated with tetraethyl pyrophosphate (TEPP) (no ester-
ase and oxidase activity),
* normal microsomes (esterase activity),
* TEPP-treated microsomes plus NADPH (oxidase activity),
* normal microsomes plus NADPH (esterase plus oxidase activity).
Each esterase preparation hydrolyzed trans-permethrin to a much
greater extent than the corresponding cis isomer. In contrast, oxidat-
ive metabolism was greater for cis-permethrin than for trans-permethrin
except with the mouse microsomes, where the reactions of both isomers
proceeded to a similar extent. Aryl hydroxylation occurred at the 4'-
and 6-positions with the mouse enzymes but only at the 4'-position with
the rat enzymes. Hydroxylation at the 2'-position was observed only
with the cis-permethrin and mouse oxidase system. The amount of trans-
hydroxymethyl ester metabolites exceeded that of the corresponding
cis-hydroxymethyl compounds except with rat enzymes acting on trans-
permethrin. In general, oxidative activity with rat microsomes was
weaker than that with mouse microsomes. The dihydroxy ester metabolite
was evident only with cis-permethrin. The cis-hydroxymethyl ester
derivatives of trans-permethrin were further oxidized to the corre-
sponding aldehyde and carboxylic acid by the mouse enzymes. The pre-
ferred sites of hydroxylation, based on all identified metabolites in
the oxidase and esterase-plus-oxidase systems, were as follows (Shono
et al., 1979):
trans-permethrin
mouse: cis-methyl > trans-methyl > 4'-carbon = 6-carbon
rat: 4'-carbon = cis-methyl > trans-methyl
cis-permethrin
mouse: trans-methyl > cis-methyl = 4'-carbon > 6-carbon
> 2'-carbon
rat: 4'-carbon = trans-methyl > cis-methyl
When 100 nmol each of [1R, trans]-, [1S, trans]-, [1RS, trans]-, [1R, cis]-,
[1S, cis ]-, or [1RS, cis ]-permethrin were incubated individually with
2.5 ml of mouse liver microsome (1.5-2.0 mg of protein), the trans iso-
mers were much more rapidly hydrolyzed than the corresponding cis iso-
mers. Of the trans isomers, [1S,trans] isomer was hydrolyzed to a
greater extent than the other trans isomers. On the other hand, when
esterase activity was suppressed, there were no distinct differences in
the oxidative metabolic rates between trans and cis isomers (Soderlund
& Casida, 1977).
The persistence of isomers of permethrin, cypermethrin, delta-
methrin, and fenvalerate in the fat and brain after oral or intraper-
itoneal administration of these pesticides to rats was compared by
Marei et al. (1982). Residues in fat and brain were much higher and more
persistent with cis-permethrin than with trans-permethrin or the
alpha-cyano phenoxybenzyl pyrethroids (cypermethrin, fenvalerate, delta-
methrin). Brain levels of trans-permethrin (but not of cis-permethrin)
were greatly elevated after pretreatment with pyrethroid esterase and
oxidase inhibitors (i.e. tri- o-cresyl phosphate, S,S,S-tributyl phosph-
orotrithioate, phenyl saligenin cyclic phosphanate as esterase inhibit-
ors and piperonyl butoxide as oxidase inhibitor).
Pyrethroid carboxyesterase(s) that hydrolyze esters of chrysan-
themic acid were purified by Suzuki & Miyamoto (1978) from rat liver
microsomes by cholic acid solubilization, ammonium sulfate fraction-
ation, heat treatment, and DEAE-Sephadex A-50 column chromatography.
The 45-fold purified enzyme (38% yield) was thought to consist of a
single protein with a relative molecular mass of approximate 74 000, a
Michaelis constant (Km) of 0.21 mmol/litre for [1R, trans ]-phenothrin,
and an optimum pH of 7-9. It was susceptible to inhibition by organo-
phosphate and carbamate insecticides and insensitive to p-chloromerc-
urybenzoic acid and to mercuric and cupric ions. The enzyme seemed
to require neither coenzymes nor cofactors and hydrolysed trans isomers
of several synthetic pyrethroids (tetramethrin, resmethrin, trans- or
cis-phenothrin and permethrin) well, at more or less similar rates. On
the other hand, the cis isomers were hydrolysed at rates one-fifth to
one-tenth of those of the trans counterparts. The purified pyrethroid
carboxyesterase was apparently identical in nature to malathion carbox-
yesterase and p-nitro phenyl acetate carboxyesterase (Suzuki &
Miyamoto, 1978).
6. EFFECTS ON THE ENVIRONMENT
Acute toxicity data of permethrin on aquatic and terrestrial non-
target organisms are summarized in Tables 5 and 6, respectively.
6.1 Toxicity to Aquatic Organisms
6.1.1 Aquatic microorganisms
Stratton & Corke (1982) investigated the toxicity of permethrin and
ten of its degradation products on the growth, photosynthesis, and
acetylene-reducing activity of two species of green algae ( Chlorella
pyrenoidosa and Scenedesmus quadricaudata ) and three species of cyano-
bacteria ( Anabaena spp.). Permethrin itself was relatively non-toxic
to photosynthesis (EC50 values >100 mg/litre) and to acetylene re-
duction (EC50 values >100 mg/litre). Its degradation products were
similarly non-toxic to green algae. However, the cyanobacteria were
susceptible to some of the breakdown products of permethrin. Growth
was the most sensitive parameter with growth yield showing EC50 values
of 2.5, 2.2, and 1.4 mg/litre for the cyanobacteria and 2.8 and 4.3 mg/
litre for the green algae with PBalc and similar values for three of
the five test species with PBald. A complex test system found interac-
tions between the various metabolites and the parent compound which
were sometimes additive and sometimes synergistic. The authors con-
cluded that it is difficult to assess the true toxicity of compounds to
soil and water microorganisms without considering the breakdown prod-
ucts. The cyanobacteria are significant nitrogen-fixing organisms in
wet tropical soils.
6.1.2 Aquatic invertebrates
Non-target invertebrates, except molluscs, are more sensitive to
permethrin than fish, as shown in Table 5.
During exposure of permethrin for up to 28 days, the caddisfly
(Brachycentrus americanus) and the stonefly (Pteronarcys dorsata)
showed behavioural changes or death at concentrations as low as 0.022
µg/litre (Anderson, 1982).
A 3-h exposure to permethrin, at 50 mg/litre, was not lethal to
Daphnia pulex. The no-effect levels were 1 µg/litre for racemic, 1R
or (+)-trans, and 1R or (+)-cis, and 50 µg/litre for 1S or (-)-trans
and 1S or (-)-cis isomers (Miyamoto, 1976).
Zitko et al. (1979) established lethal threshold values for the
lobster Homarus americanus of 7.00 µg/litre for technical permethrin
and 0.40 µg/litre for [1R, cis ]-permethrin.
Larval oyster and bullfrog (tadpole) are highly tolerant to the
insecticide, with LC50 values of >1000 and 7000 µg/litre, respect-
ively.
Stratton & Corke (1981) reported that the 48-h LC50 of permethrin
to juvenile and adult waterfleas Daphnia magna was 0.2-0.6 µg/litre. A
further series of experiments involved the addition of algae or
bacteria to the cultures of daphnids, since feeding of daphnids during
these tests had been reported to reduce the toxicity of several
chemicals to the animals. With permethrin, however, algae in the test
vessel increased the lethal effect of the compound. Algae, bacteria,
and also inert silica powder adhered to the swimming antennae of the
daphnids, causing the daphnids to sink and die on the bottom of the
flasks. The effect was greatest with adults; the shed carapaces of
juvenile showed the same adhesion of particulates but moulting pro-
tected the juveniles to some degree. This raised toxicity was due to a
direct effect of the permethrin on the daphnids and not to a tendency
for the compound to cause flocculation of the suspended material.
Friesen et al. (1983) tested the toxicity of permethrin to
sediment-living nymphs of the mayfly Hexagenia rigida. In test vessels
containing water without sediment, the 6-h LC50 was estimated to lie
between 0.58 and 2.06 µg/litre; no nymphs survived exposure to water
concentrations of 7.63 µg/litre. In the presence of sediment,
lethality was reduced; there was 88% mortality of nymphs exposed to
permethrin in water at 7.63 µg/litre after 24-h exposure. Mortality
reached 100% only after 7 days exposure with sediment. Maximum concen-
trations of permethrin in the sediment over the 7 days were estimated
to be 50 µg/kg dry weight. The authors also exposed nymphs to sedi-
ment previously exposed to permethrin. The initial water concentration
was again 7.63 µg/litre, and the sediment was left for 8 days to take
up the insecticide before the water was decanted off. Nymphs were
introduced along with clean water over the contaminated sediment. There
was 100% mortality in the exposed nymphs. Long-term exposure to both
water and sediment contaminated with permethrin led to increasing
mortality up to 4 weeks; there was little further mortality between 4
and 10 weeks. Lethality reached 100% after exposure to either water or
sediment at a simulated application rate of 7.3 g/ha over 10 weeks (95%
at 4 weeks), whereas a simulated exposure equivalent to 0.6 g/ha led to
74% mortality after a 10-week exposure of the nymphs in water and 45%
after exposure of the nymphs to sediment. The authors comment that it
is not yet possible to state a concentration of permethrin in sediment
which is sufficiently low to permit successful recolonization of con-
taminated sediment.
6.1.3 Fish
Permethrin is highly toxic to fish, as shown in Table 5. Prep-
arations using an emulsifiable concentrate of permethrin enhanced its
toxicity twofold (Coats & O'Donnell-Jeffery, 1979).
The lethal toxicity of permethrin varied inversely with water
temperature, particularly between 10 and 20°C, and with body weight
between 1 and 50 g. There was a 10-fold difference between the 96-h
LC50 values at 10 and 20°C. At 15°C, a large trout (200 g) was con-
siderably more (about 100 times) tolerant than a small fish (1 g)
(Kumaraguru & Beamish, 1981).
Toxicity to fish is linked more with the nature of the optical iso-
mers than with that of the stereoisomers; i.e. 1R isomers are more
toxic than 1S isomers. Trans and cis isomers are of similar toxicity
(Miyamoto, 1976).
Zitko et al. (1979) established lethal threshold values for the
Atlantic salmon Salmo salar of 8.8 µg/litre for technical permethrin
and 1.34 µg/litre for [1R, cis ]-permethrin.
Hansen et al. (1983) exposed embryos and the hatched fry of
sheepshead minnow (Cyprinodon variegatus), continuously over 28 days,
to concentrations of permethrin of 1.25, 2.5, 5.0, 10, 20, or 40 µg/
litre. The survival of embryos was unaffected by any of the test
concentrations. Fry were affected by exposure to 20 µg/litre or more
but unaffected by 10 µg/litre. The toxicity curve was steep; 99% of fry
survived at 10 µg/litre but only 1% at 20 µg/litre. The authors
estimated the ratio between the 96-h LC50 and the NOEL to be 0.8.
Holdway & Dixon (1988) exposed larval fish (white sucker,
Catastomus commersoni, and flagfish, Jordanella floridae ) to per-
methrin in a single 2-h pulse and examined lethality over the following
96-h. They examined the effect of age, and whether or not the fish were
fed, upon the toxic effect of the insecticide. Feeding decreased the
toxicity of permethrin to flagfish at 2 and 4 days of age but not at 8
days. Age was the most important factor affecting toxicity. The 96-h
LC50 (from exposure for 2 h) was 5.55 mg/litre, 7.91 mg/litre, and
0.57 mg/litre for flagfish of age 2, 4, and 8 days, respectively.
White suckers were most susceptible to permethrin at 20 days of age,
with a 2-h LC50 of 10.0 µg/litre. Unfed white suckers at 13 and 20
days of age were highly susceptible to permethrin, with LC50 values of
2.0 and 1.0 µg/litre, respectively. The authors pointed out that
permethrin is toxic to cladocerans (waterfleas) at levels of 0.5 µg/
litre and that fish could suffer both from the direct toxic effect of
the insecticide and the added effect experienced during food deprivation.
When used for mosquito control, the safety margins (LC50 fish/LC50
mosquito larvae) for permethrin and cis-permethrin are 2-40 and 25-65,
respectively (Mulla et al., 1978a). When intraperitoneally injected
into rainbow trout, the trans- and cis-permethrin isomers were about
110 and 5 times, respectively, more toxic to trout than to mice,
(Glickman et al., 1981).
Rainbow trout exposed to sublethal concentrations of permethrin in
water (0.09-0.35 µg/litre) or in food (85-350 µg/kg) in 20-40-day
experiments showed similar branchial changes, i.e. epithelial separa-
tion or necrosis, mucus cell hyperplasia, clubbing of epithelial cells,
or hyperplasia and fusion of adjacent secondary lamellae (Kumaraguru et
al., 1982).
6.1.4 Field studies and community effects
In studies by Mulla et al. (1975), permethrin was applied to ponds
at rates of 56 g/ha and 112 g/ha in field trials. The numbers of
Tanypodinae (mostly Pentaneura and Tanypus ) and Chironominae (mostly
Tanytarsus and Chironomus ) midge larvae were slightly depressed by
the 56 g/ha treatment. Mayfly (mostly Baetis sp.) naiads and diving
beetle ( Hydrophilidae and Dytiscidae ) larvae and adults were also
affected. However, Copepoda (mostly Cyclops and Diaptomus ) and
Ostracoda (mostly Cypricercus and Cyprinotus ) were not greatly affec-
ted. The effect on these non-target organisms was much greater at the
higher dose level of permethrin, except for the ostracods. It was con-
cluded that permethrin affected mayfly naiads severely during the
exposure period. Most populations recovered within 2 weeks following
exposure.
Mayfly naiads (mostly baetids) were also adversely affected by per-
methrin at 5.6-28 g/ha and by its cis isomer at 2.8-28 g/ha. There was
a slight recovery within 1-3 weeks after treatment (Mulla et al.,
1978b).
Permethrin was applied weekly for 6 or 8 successive weeks at the
mosquito larvicidal rate of 28 g/ha (and at a rate 5 times higher) to
ponds where 20 individuals of mosquito fish or desert pupfish were
maintained. The insecticide produced no adverse effects on the two
species of fish, and the number of fish in the treated ponds increased
markedly during the experiment. At the higher rate, mats of algae were
formed, probably as the result of elimination by permethrin of her-
bivorous arthropods that feed on the algae (Mulla et al., 1981).
Kaushik et al. (1985) investigated the effect of permethrin on the
pelagic zooplankton of a 10-ha lake in southern Ontario, Canada. The
insecticide was applied to give nominal water concentrations of 0.5,
5.0, or 50 µg/litre in in situ aquatic enclosures of 5 x 5 x 5 m.
Macrozooplankton (daphnids and copepods) were most susceptible to the
insecticide. The numbers, which in untreated enclosures were 100-1000
organisms per litre of water, fell in the days immediately following
treatment to 1-10 at 0.5 µg/litre, 0.1-1.0 at 5.0 µg/litre, and
0.01-0.1 organisms per litre at 50 µg/litre of permethrin (nominal).
Microzooplankton (mainly rotifers) were unaffected by all doses except
the highest. At this dose, numbers fell transitorily to about one tenth
of their control levels (about 1000 organisms per litre). In all cases
of treatment, rotifer numbers increased between 5- and 10-fold 20 to
100 days after treatment. The authors attributed this rise in numbers
to the resistance of the organisms to the insecticide coupled with a re-
duction in the predator organisms that normally feed on the rotifers.
Populations of macrozooplankton had returned to normal within 250 days
of treatment (after the winter freeze) even with the highest dose of
50 µg/litre. Recovery was quicker with the lower doses (about 60 days
for treatment at 5 µg/litre and 30 days for most species at 0.5 µg/
litre). Despite this recovery in overall numbers of zooplankton, there
was a decrease in the species diversity of the larger, predator organ-
isms at all treatment levels. The enclosures, of course, prevented
immigration from the surrounding areas of water.
Helson et al. (1986) placed two species of aquatic arthropods (the
amphipod Gammarus pseudolimnaeus and the mosquito Aedes aegypti ) in
open containers of different sizes downwind from the application of
permethrin to young spruce trees for control of defoliators. The insec-
ticide was applied to trees 0.75-0.8 m tall in a single swath from a
mistblower backpack. Nominal application rates of 36 g ai/ha were used
with a swath width of 10 m, and standard and ultra-low volume appli-
cations were made. The mortality of Gammarus after 48 h averaged 95%
(range 76-100%) in the first trial and 85% (range 37-100%) in a dupli-
cate trial in containers 10 m downwind from the spraying. The effect
was reduced to 12% and 18% at a distance of 30 m from the spraying and
further reduced to an average of 5% 50 m from the spray. Mosquito
larvae were examined only in the second trial and showed 76% (37-100%)
at 10 m falling to 6% and 2% at 30 and 50 m, respectively, from the
spray. Mortality increased over the following 9 days. The authors also
determined 48-h LC50 values for the two organisms in containers simi-
larly placed in the field. These were 0.37 µg/litre and 0.69 µg/litre for
Gammarus and mosquito larvae, respectively, while LC95 values were
0.61 µg/litre for Gammarus and 1.14 µg/litre for mosquito larvae.
The authors regarded these data as a "worst case" , since sediment in
natural water and flowing water in streams could be expected to reduce
the toxic effect of the permethrin. They concluded that a 30-m safety
zone needs to be left using this application method between a spraying
area and natural waters to avoid killing aquatic arthropods.
When permethrin was applied by airplane to the surface of a creek
at a nominal rate of 70 g ai/ha, the actual concentration that reached
the ground was 13.4 g ai/ha. Dramatic, but short-lived, increases in
the drift of aquatic insects (particularly large catches of springtail
(Collembola), mayfly nymphs (Ephemeroptera heptageniidea), water
scavenger beetle larvae (Coleoptera hydrophilidae), midge larvae and
pupae, water boatmen (Hemiptera corixidae), predaceous diving beetles
(Coleoptera dytiscidae), and caddisfly larvae (Trichoptera)) occurred
after treatment. No effects on populations of organisms that inhabited
the bottom layer of the creek were noticeable. The permethrin sprayed
had little effect on caged or native fish and no fish mortality was re-
corded due to the treatment. From these data, it could be inferred that
permethrin had no significant impact on the aquatic system (Kingsbury,
1976).
After an aerial application of permethrin at 17.5 g ai/ha, residues
attained peak concentrations of 147.0 µg/litre in ponds and 2.5 µg/litre
in streams, but accumulations and persistence of the pesticide in
bottom sediment were negligible. Noticeable increases in the number of
drifting organisms occurred in the treatment block ( Ephemeroptera hepta-
geniidae, Baetidae, and Plecoptera nymphs ) and 2.1 km downstream
(mayfly and stonefly nymphs) over a 24-h period immediately after the
spray. A slight reduction in the bottom fauna also occurred
downstream. When exposed in cages in the ponds, yellow perch (Perca flu-
vescens) did not exhibit any adverse effects; little or no accumulated
permethrin residues were detectable in the fish following exposure. Ob-
servations of the headwater ponds indicated that permethrin application
resulted in noticeable levels of distress and mortality to surface and
littoral invertebrates and produced a similar impact on benthic organ-
isms (Kingsbury & Kreutzweiser, 1980a).
Table 5. Acute toxicity of permethrin to non-target aquatic organisms
--------------------------------------------------------------------------------------------------------------------------------------------
Species Size Duration Toxicitya Formu- Sys- Tempera- pH Hardness Reference
of test (µg/litre) lationd teme ture (°C)
--------------------------------------------------------------------------------------------------------------------------------------------
A. Freshwater Organisms
Arthropods
Crayfish 0.8 - 1.2 cm, 96 h 0.39 EC S 24 100 Jolly et al. (1978)
(Procambarus clarkii) (0.05 g)
2 - 3 cm, 96 h 0.62 EC S 24 100 Jolly et al. (1978)
(0.5 g)
Water flea (Daphnia pulex) 3 h > 50 000 T S 25 Miyamoto (1976)
3 h > 50 000 (+)-trans S 25 Miyamoto (1976)
3 h > 50 000 (+)-cis S 25 Miyamoto (1976)
3 h > 50 000 (-)-trans S 25 Miyamoto (1976)
3 h > 50 000 (-)-cis S 25 Miyamoto (1976)
Water flea (Daphnia magna) 1st instar 48 h 1.26 T S 18 7.4 42 Mayer &
Amphipod immature 96 h 0.17 T S 17 7.4 42 Ellersieck (1986)
(Gammarus pseudolimnaeus) Mayer &
Midge (Chironomus plumosus) 3rd instar 48 h 0.56 T S 22 7.4 42 Ellersieck (1986)
Caddisfly 21 days 0.17 T F 15 7.6 - 7.8 46 - 48 Anderson (1982)
(Brachycentrus americanus)
Fish
Salmon (Salmo salar) 6.2 cm, 5.3 g 96 h 12 T R 10 McLeese et al.
(1980)
Rainbow trout 1 g 96 h 0.62 T F 5 7.9 - 8.2 358 - 363 Kumaraguru &
(Salmo gairdneri) 1 g 96 h 0.69 T F 10 7.9 - 8.2 358 - 363 Beamish (1981)
1 g 96 h 3.17 T F 15 7.9 - 8.2 358 - 363 Kumaraguru &
1 g 96 h 6.43 T F 20 7.9 - 8.2 358 - 363 Beamish (1981)
5 g 96 h 6.43 T F 15 7.9 - 8.2 358 - 363 Kumaraguru &
20 g 96 h ca. 50 T F 15 7.9 - 8.2 358 - 363 Beamish (1981)
50 g 96 h 287 T F 15 7.9 - 8.2 358 - 363 Kumaraguru &
200 g 96 h 314 T F 15 7.9 - 8.2 358 - 363 Beamish (1981)
6 cm, 3 g 24 h 135 T 10 7.5 110 Coats & O'Donnell-
6 cm, 3 g 24 h 61 EC 10 7.5 110 Jeffery (1979)
--------------------------------------------------------------------------------------------------------------------------------------------
Table 5. (contd.)
--------------------------------------------------------------------------------------------------------------------------------------------
Species Size Duration Toxicitya Formu- Sys- Tempera- pH Hardness Reference
of test (µg/litre) lationd teme ture (°C)
--------------------------------------------------------------------------------------------------------------------------------------------
Rainbow trout (contd.)
(Salmo gairdneri) 5 - 6 cm 48 h 6.0 EC 12- Mulla et al.
5 - 6 cm 48 h 7.0 cis, EC 25.5 (1978a)
2 - 4 g 24 h 18 T S 12 Glickman
2 - 4 g 24 h 25 cis S 12 et al.
2 - 4 g 24 h 14 trans S 12 (1981)
Killifish adult 48 h 41 T S 25 Miyamoto (1976)
(Oryzias latipes) adult 48 h 17 (+)-trans S 25 Miyamoto (1976)
adult 48 h 13 (+)-cis S 25 Miyamoto (1976)
adult 48 h > 10 000 (-)-trans S 25 Miyamoto (1976)
adult 48 h > 10 000 (-)-cis S 25 Miyamoto (1976)
Channel catfish 1.4 - 1.7 cm, 96 h 1.1 EC S 24 100 Jolly et al. (1978)
(Ictalurus punctatus) (0.02 g)
Largemouth Bass 4.5 - 5.5 cm, 96 h 8.5 EC S 24 100 Jolly et al. (1978)
(Micropterus salmoides) (1.14 g)
Mosquitofish 1.5 - 2.5 cm, 96 h 15 EC S 24 100 Jolly et al. (1978)
(Gambusia affinis) (0.25 g)
4 - 5 cm 48 h 97.0 EC 8.8 - 16 Mulla et al.
4 - 5 cm 48 h 13.0 cis, EC 8.8 - 16 (1978a)
Brook trout 1.2 g 96 h 3.2 T S 12 7.5 40 Mayer & Ellersieck
(Salvelinus foutinalis) (1986)
Fathead minnow 0.6 g 96 h 5.7 T S 22 7.3 38 Mayer & Ellersieck
(Pimephales promelas) (1986)
Bluegill sunfish 0.7 g 96 h 5.0 T S 22 7.3 38 Mayer & Ellersieck
(Lepomis macrochirus) (1986)
Desert pupfish 4 - 5 cm 48 h 5.0 EC S 11 - 16.5 Mulla et al.
(Cyprinodon macularis) 4 - 5 cm 48 h 5.0 cis, EC S 11 - 16.5 (1978a)
Tilapia mossambica 5 - 6 cm 48 h 44.0 EC S 15 - 21.4 Mulla et al.
5 - 6 cm 48 h 5.6 cis, EC S 15 - 21.4 (1978a)
Amphibian
Bullfrog, tadpole 0.6 - 0.8 cm 96 h 7033 EC S 24 100 Jolly et al. (1978)
(Rana catesbeiana)
--------------------------------------------------------------------------------------------------------------------------------------------
Table 5. (contd.)
--------------------------------------------------------------------------------------------------------------------------------------------
Species Size Duration Toxicitya Formu- Sys- Tempera- pH Salinity Reference
of test (µg/litre) lationd teme ture (°C) (°/oo)
--------------------------------------------------------------------------------------------------------------------------------------------
B. Estuarine and Marine Organisms
Algae
Skeletonema costatum 96 h 92b T 20 Borthwich & Walsh
(1981)
Molluscs
Oyster (Crassostrea virginica) 2-h larva 48 h > 1000c T S 25 20 Borthwich & Walsh
(1981)
Arthropods
Lobster (Homarus americanus) 450 g 96 h 0.73 T R 10 30 McLeese et al.
Shrimp (Crangon Septemspinosa) 1.3 g 96 h 0.13 T R 10 (1980)
Shrimp (Mysidopsis bahia) 1-day, 96 h 0.046 T S 25 20 Borthwich
juvenile & Walsh
Stone crab (Menippe mercenaria) Zoea larva 96 h 0.018 T S 25 20 (1981)
Pink shrimp (Penaeus duorarum) adult 96 h 0.22 T F 25 25 Mayer (1987)
Fish
Harpacticoid (Nitocra spinipes) 3-6 weeks old 96 h 0.6 EC S 20 - 22 7.8 7 Linden et al.
Bleak (Alburnus alburnus) 8 cm 96 h 4 - 8 EC S 10 7.8 7 (1979)
Sheepshead minnow 28-day fry 96 h 88 T S 25 20 Borthwich & Walsh
(Cyprinodon variegatus) (1981)
adult 96 h 7.8 T F 30 22 Mayer (1987)
Atlantic silverside adult 96 h 2.2 T F 26 25 Mayer (1987)
(Menidia menidia)
Striped mullet (Mugil cephalus) juvenile 96 h 5.5 T F 24 19 Mayer (1987)
--------------------------------------------------------------------------------------------------------------------------------------------
a Values are LC50 unless stated otherwise.
b EC50 (growth inhibition).
c EC50 (abnormal development).
d T = Technical, EC = Emulsifiable concentrate.
e R = Renewal, S = Static, F = Flow-through.
f expressed as mg CaCO3/litre.
When permethrin was sprayed at 8.8, 17.5, 35.0, or 70.0 g ai/ha by
aeroplane over small trout streams, the impact on aquatic invertebrates
and effects on the general fish population correlated with the dose.
There was an increase in the number of organisms drifting downstream,
the major ones being mayflies, followed by caddisflies, stoneflies, and
chironomids. The total number of drifting organisms was greater than
the pre-spray average by factors of 303, 699, 4960, and 6450 with spray
concentrations of 8.8, 17.5, 35.0, and 70.0 g ai/ha, respectively. A
return to pre-spray drift levels was evident within 36 h after appli-
cation at 8.8 and 17.5 g ai/ha whereas drifting of organism persisted
for up to 72 h at the higher application rates of 35 and 70 g ai/ha.
Following the spraying of permethrin at these higher doses, there was
no evidence of fish mortality, but there appeared a dramatic change in
the diets of fish (such as the native brook trout and sculpins). These
fish became virtually completely dependent on terrestrial invertebrates
rather than on the aquatic insects for food. When the rate of appli-
cation was low (8.8 g ai/ha) permethrin did not appear to affect these
fish, either in mortality rate or in their diet composition (Kingsbury
& Kreutzweiser, 1980b).
Serial applications of permethrin, once or twice at 17.5 g ai/ha,
resulted in catastrophic drift of aquatic invertebrates and substantial
depletion of benthos in streams within the application blocks and up to
2 km downstream. Despite massive disturbances of benthos, repopulation
of bottom fauna was evident within 2.5 months and had virtually re-
turned to normal within 3.5 months. Permethrin residues attained peak
levels of 1.35 µg/litre in standing water and 1.94 µg/litre in
flowing water in the sprayed regions. The residue persisted at low
concentrations for up to 96 h after spraying (Kreutzweiser, 1982).
In a study by Kingsbury & Kreutzweiser (1987), aerial applications
of permethrin to forests over several seasons at 8.8, 17.5, 35, and 70
g ai/ha did not cause mortality to native and caged fish (minnows,
mudminnows, perch, and under-yearling and yearling Atlantic salmon).
The composition of the salmonid diet was subsequently altered from
aquatic insects (mayfly nymphs, stonefly nymphs, and various aquatic
fly larvae) to terrestrial arthropods. The duration of changes ranged
from a few months after applications of 8.8 and 17.5 g ai/ha to a year
or longer after treatment with 35 and 70 g ai/ha. There were temporary
reductions in fish growth rate and fish densities in the treated area,
which returned to normal within four months after treatment. In the
same study, the effect on stream invertebrates was also evaluated.
Large drifts of invertebrates were observed immediately after appli-
cations and continued for 24 to 72 h. Although the peak of permethrin
residues in stream water was higher after the second application (0.36-
1.80 µg/litre) than the first (0.25-0.62 µg/litre), drift response
to the second application ranged from 6 to 62% of the first drift,
indicating that first application deleted susceptible invertebrates
(e.g., Ephemeroptera nymphs, Plecoptera, Trichoptera, and the
Diptera families) and that a much smaller residual population re-
sponded to the second treatment. Recovery of benthic fauna was appar-
ent between 1 and 18 months after spraying. The double treatment
reduced benthos density to a point at which recovery of numbers was
slower than after the single application (Kreutzweiser & Kingsbury,
1987).
6.2 Toxicity to Terrestrial Organisms
6.2.1 Soil microorganisms
Mathur et al. (1980) applied permethrin (Ambush 5 G) to Canadian
soils with a high content of organic material at a rate of 2.24 kg/ha.
Lettuce or carrots were grown on the plots. Soil cores were taken at
regular intervals and bacterial and fungal numbers were estimated, soil
nutrients were measured, and acid phosphatase activity in the soil was
monitored. Residues of permethrin persisted throughout the growing
season up to crop harvest, when 65% of the original concentration in
soil was found (113 days after application). This reflected the poor
breakdown of permethrin in organic soils compared to mineral soils.
Permethrin suppressed bacterial and actinomycete populations in samples
taken 1, 9, and 27 days after application, but control levels were re-
gained after 41 days (the next sampling time). The available nitrogen
and phosphorus was lower in treated soil at some points during the
study, but these changes were not consistent. Soil respiration and acid
phosphatase activity were higher in permethrin-treated plots, though
not consistently so. Permethrin had a greater effect when carrots were
grown than when lettuce was grown. The yield of neither crop was
affected. Most of the effects reported were transitory and none were
of overall significance for either the soil or the crop.
6.2.2 Terrestrial invertebrates
Under laboratory conditions, permethrin is highly toxic to certain
beneficial insects or natural enemies of pests, as shown in Table 6.
Cox & Wilson (1984) treated honey bee workers topically with a sub-
lethal dose of permethrin (0.09 µg/bee in 1.0 µl of acetone applied
to the thorax). This dose gave no higher mortality than treatment with
acetone alone. The bees, which were individually tagged, were housed in
an observation hive and trained before the experiment to feed at an
artificial feeding station 5 m from the hive. The experiment was con-
ducted at 35°C because at lower temperatures this dose resulted in
mortality. Treated bees made less foraging trips than controls and gave
food less frequently to other bees in the hive. Other behaviours were
increased in treated bees, i.e., self-cleaning, trembling dance, ab-
domen tucking, and rotating and cleaning of abdomen while rubbing the
hind legs together. Gerig (1985) also reported minimal lethal effects
on honey bees of permethrin used at recommended rates and that the
insecticide had a strong repellent effect.
Table 6. Acute toxicity of permethrin to non-target terrestrial organisms
-----------------------------------------------------------------------------------------------------------------------------
Species Size Application Duration Toxicitya Temperature Reference
(°C)
-----------------------------------------------------------------------------------------------------------------------------
Bird
Hen oral > 1.5 g/kg b.w.b Millner & Butterworth
(1977)
Chicken oral > 3 g/kg b.w.b Worthing & Walker
Japanese quail oral 5 days > 13.5 g/kg b.w.b (1983)
diet 5 days > 5 g/kg dietc Hill & Camardese
diet 5 days > 5 g/kg dietd (1986)
Mallard duck diet 5 days > 27.5 g/kg diet Ross et al. (1976b)
Mallard duck oral > 13.5 g/kg b.w.b Ross et al. (1976a)
Starling diet 5 days > 27.5 g/kg diet Ross et al. (1976d)
Starling oral > 38 g/kg b.w.b Ross et al. (1976c)
Ring-necked pheasant diet 5 days > 27.5 g/kg diet Ross et al. (1976e)
Ring-necked pheasant oral > 13.5 g/kg b.w.b Ross et al. (1977a)
Arthropods
Honeybee (Apis mellifera) contact 0.11 µg/beeb 26 - 27 Stevenson et al. (1978)
oral 0.28 µg/beeb 26 - 27 Stevenson et al. (1978)
Insect parasite
Ichneumoid (Campoletis adult male film 24 h 0.31 µg/vial Plapp & Vinson (1977)
sonorensis)
Insect predator
Carabid (Pterostichus adult 0.16 g topical > 2000 µg/insectb 21 Hagley et al. (1980)
melanarius)
Carabid (Harpalus affinis) adult 0.05 g topical 116 µg/insectb 21 Hagley et al. (1980)
Carabid (Amara sp.) adult 0.03 g topical 25 µg/insectb 21 Hagley et al. (1980)
Earwig (Labidura raparia) mature soil 0.1 kg 6% mortality Workman (1977)
ai/ha
mature soil 0.2 kg 50% mortality Workman (1977)
ai/ha
Green lacewing larvae
(Chrysopa carnea) 5-6 days old film 9.87 µg/vial 25 Plapp & Bull (1978)
Predaceous mite species
Mataseiulus occidentalis adult female slide-dip 0.72, 1.32, 27.5 Roush & Hoy (1978)
(3 strains) method 14.8 mg ai/litre
young gravid leaf-disc 2.8 mg ai/litre 27 Hoy et al. (1979)
female method
(Amblyseius fallacis) adult female slide-dip 14 mg ai/litre 27 Rock (1979)
method
-----------------------------------------------------------------------------------------------------------------------------
a LC50 values, unless stated otherwise.
b LD50 values; b.w. = body weight.
c Technical product.
d Emulsifiable concentrate.
Pike et al. (1982) applied permethrin by helicopter at a rate of
0.22 kg ai/ha to fields of maize (Zea mays). The applications were
made early in the morning before bees were actively foraging for pollen
and were repeated every 3 to 6 days (to a maximum of six applications
per season). The trial was repeated for 3 years. There was no differ-
ence in the number of dead bees per hive between treated and control
areas, but a marked reduction in the number of bees foraging in treated
fields, indicating avoidance of permethrin. The authors concluded that
treatment of corn fields with permethrin at the normal application rate
is safe for bees as long as the application does not coincide with bee
activity in the area.
The acute toxicity values of permethrin to tobacco budworm
(Heliothis virescens), to the green lacewing (Chrysopa carnea), a
predator of tobacco budworm, and to Campoletis sonorensis, an
ichneumoid parasite of tobacco budworm, showed that permethrin was
approximately 18 times less toxic to the predators than to the pest
(Plapp & Bull, 1978; Plapp & Vinson, 1977).
Larvae of the green lacewing exhibited marked tolerance to per-
methrin, and to its cis or trans isomers, when dosed topically with
250 µg per insect (about 25 000 µg/g). This value is ca. 10 000 times
greater than the LD50 value for the tobacco budworm (Shour & Crowder,
1980).
Workman (1977) added permethrin to loamy sand soil into which the
striped earwig (Labidura riparia), an effective insect predator of
the cabbage looper, was introduced. The insecticide was of low toxicity
to the earwig at dosage rates which gave good looper control.
The susceptibility of carabids to permethrin appears to be in-
versely related to beetle size, as shown in Table 6. When permethrin
was applied at a concentration of 0.21-0.85 kg ai/ha to an apple
orchard, it did not significantly affect the numbers of Pterostichus
melanarius at any time during the season, but the numbers of Harpalus
affinis and Amara sp. were significantly reduced 3-5 days after appli-
cation. This result reflected the toxicity ratings by means of LD50
values obtained in laboratory studies. The total seasonal numbers of
these carabids were not significantly affected by permethrin, owing to
short residual effects (Hagley et al., 1980).
In laboratory tests, LC50 values of permethrin for two strains of
spider mites (Tetranychus urticae) were ca. 20-40 times greater than
those for three strains of predator mites (Mataseiulus occidentalis)
(Roush & Hoy, 1978), and ca.15 times greater than those for Amblyseius
fallacis (Rock, 1979). These studies indicate that the use of per-
methrin at the recommended rates of 60-120 mg ai/litre would be detri-
mental to orchard integrated mite control programs.
In laboratory tests, the Pacific spider mite (T. pacificus) has
been found to be 40 times more tolerant to permethrin than the predator
mite (M. occidentalis), (Hoy et al., 1979). The spraying of vineyards
with 15 or 30 mg ai/litre resulted in substantially higher populations
of T. pacificus and E. willamettei for about one month due to re-
duction in predator species numbers. Similarly, spraying with 60 or
120 mg ai/litre produced a subsequent increase of Eotetranychus
willamettei late in August and September for the above reason (Hoy et
al., 1979).
When permethrin was applied to apple trees at a concentration of
40 mg/litre, no predator mites (Typhlodromus pyri) were found for 4-6
weeks, and only small numbers were found 10 weeks after the spray. On
the other hand, permethrin had no appreciable toxicity to the spider
mite (Panonychus ulmi). The virtual elimination of the predatory mite
by permethrin spraying led to a marked population increase of P. ulmi
later in the same season (Aliniazee & Cranham, 1980).
In apple and pear orchards, applications of permethrin at 30 mg
ai/litre reduced the numbers of a predatory mite (M. occidentalis) to
almost zero and dramatically increased the populations of spider mites
(T. urticae, Tetranychus mcdanieli, or P. ulmi) (Hoyt et al., 1978).
From the above findings it appeared that permethrin, when applied
according to recommendations, is relatively harmless to insect pred-
ators, with the exception of predaceous mite species. Everts et al.
(1985) investigated the effects of permethrin on beneficial terrestrial
arthropods in the soil and vegetation in areas surrounding applications
of the insecticide to control tsetse flies in the Ivory Coast, West
Africa. Permethrin was used as a 2.5% wettable powder at a rate of 121
g ai/ha during January (a minimum temperature of 20.0°C and a maximum
of 36.0°C). The permethrin application significantly reduced popu-
lations of Coleoptera, Lepidoptera, Ephydridae, Chloropidae, Muscidae,
Ichneumonidea, Chalcidoidea and Proctotrupoidea. The populations of
almost all these groups were found to recover to normal levels within 2
months, i.e., before the likely time of respraying for the control of
the tsetse flies. However, one Proctotrupoid genus, Cremastobaeus, was
eliminated by permethrin treatment.
6.2.3 Birds
Neither an acute oral nor a dietary LD50 or LC50 has been estab-
lished accurately because of the very low toxicity of permethrin to
birds (Table 6). The acute LD50 is >3000 mg/kg body weight and the
dietary toxicity >5000 mg/kg diet. (Worthing & Walker, 1983; Hill &
Camardese, 1986).
The inclusion of permethrin at up to 40 mg/kg in the diet of laying
hens for 28 days had no adverse effects on the health of parent birds
or on egg production quality, hatchability, or the viability of the
chicks produced (Ross et al. 1977b).
6.2.4 Mammals
Racey & Swift (1986) treated roosting boxes for pipistrelle bats
with various wood preservatives and allowed the bats to roost in the
boxes for up to 154 days. There were no toxic effects of mixtures of
synthetic pyrethroids including permethrin. The authors concluded that
the insecticide component of wood preservatives should be pyrethroids
when bats are present in the area.
6.3 Uptake, Loss, Bioaccumulation and Biomagnification
Proposed metabolic pathways of permethrin in fish are summarized in
Fig. 4.
 When rainbow trout (Salmo gairdneri) were held in static water
containing 5 µg/litre of 14C-permethrin for 24 h, both cis and trans
isomers were similarly taken up into the fish. The bioaccumulation
ratios for total radiocarbon in the blood, muscle, liver, and fat of
the fish were 30, 30, 300, and 400, respectively. When the fish were
transferred to fresh running water, radioactivity was eliminated, with
initial half-lives of 9-35 h, from all the tissues except fat, where
little decay in 14C-permethrin concentrations occurred. When rainbow
trout were injected intraperitoneally with 14C-permethrin at a rate of
0.5 mg/kg, 32-43% of the dose was recovered after 48 h in the bile, 3-
7% in the urine, and 31-42% in the carcass. However, the urine and bile
of the trout injected with the trans isomer contained higher levels of
radioactivity. In the bile, the major metabolite was the glucuronide
conjugate of 3-(4-hydroxyphenoxy)-benzyl-3-(2,2-dichlorovinyl)-2,2-
dimethyl-cyclopropanecarboxylate (26) and there were few metabolites
formed by hydrolysis (Fig. 4). The urine contained principally the
sulfate conjugates of polar products. The ability of rainbow trout to
hydrolyze permethrin in vivo appeared minimal (Glickman et al., 1981).
The rates of trans-permethrin hydrolysis in trout liver, kidney,
and plasma incubated at 12°C were approximately 166, 38, and 59 times,
respectively, lower than those in the corresponding mouse tissues
incubated at 37°C. Although an increase in the incubation temperature
from 12°C to 37°C caused an increase in the rate of trans-permethrin
hydrolysis by trout liver microsomes, trans-permethrin was hydrolyzed
about 45 times slower than by mouse liver microsomes at 37°C. The
hydrolysis of permethrin in trout plasma, however, was higher than that
in trout liver microsomes (Glickman & Lech, 1981).
When the microsomal preparations of both carp and rainbow trout
were fortified with NADPH, the carp microsomes oxidized permethrin iso-
mers more actively than the trout microsomes. Also, larger amounts of
hydroxy ester metabolites were recovered with the cis isomer than with
the trans isomer. The preferred site of oxidation of both isomers by
the carp and trout microsomes was the 4'-position (26) of the phenoxy-
benzyl moiety. The geminal dimethyl group was attacked in preference to
the methyl group situated trans to the carboxy group. trans-Permethrin
primarily underwent hydrolysis by both carp and trout liver microsomes
in the presence or absence of NADPH to yield PBalc (6) and Cl2CA (17)
(Glickman et al., 1979). In this respect, the results of in vitro
studies were different from those obtained in vivo.
Juvenile Atlantic salmon (lipid content 4.2%), exposed for 96 h to
static water containing 22 µg/litre permethrin, took up the insecti-
cide with a bioaccumulation ratio of 55. Dead juvenile salmon exposed
for 12.5 h to 0.098-0.994 mg/litre contained 2.21-3.69 µg/g (Zitko et
al., 1977).
Residues of approximately 0.5-1.2 mg/kg were detected in dead
juvenile Atlantic salmon exposed for 10-89 h to static water containing
permethrin at 6.9-85 µg/litre, the bioaccumulation ratio ranging from
14 to 73. The insecticide was not detected (detection limit 5 ng/g) in
dead lobster hepatopancreas or in dead shrimp (McLeese et al., 1980).
When stoneflies (Pteronarcys dorsata) were exposed for 28 days to
running water containing permethrin at 0.029-0.21 mg/litre, the bioac-
cumulation ratios of the survivors ranged from 43 to 570 (average, 183;
standard deviation, 171) (Anderson, 1982).
When carp were exposed to a 14C-permethrin isomer (phenoxyphenyl-
labelled [1R,trans], [1R,cis], [1S,trans], or [1S,cis] isomers) in a
flow-through system at 25°C, the concentrations of 14C and permethrin
isomers in the fish body reached an equilibrium on days 7-9 of ex-
posure. The bioaccumulation ratios of the permethrin isomers at equi-
librium were 330-750. When the fish were transferred to fresh water,
the permethrin isomers, as well as their metabolites, were rapidly
excreted. The biological half-lives for the permethrin isomers were
2.0-2.8 days. The major metabolic reactions involved were oxidation at
the 4 -position of the alcohol moiety or the methyl group of the acid
moiety, cleavage of the ester linkage, and conjugation of the resultant
alcohols and phenols with glucuronic acid or sulfuric acid (Ohshima et
al., 1988).
Bioconcentration factors for sheepshead minnows ( Cyprinodon vari-
egatus exposed to permethrin at concentrations between 1.25 and
10 µg/litre for 28 days from hatching varied between 290 and 620.
Maximum bioconcentration occurred after exposure at 2.5 µg/litre, and
a maximum residue of 5.7 mg/kg occurred after exposure at 10 µg/litre
(the concentrations were for whole fish) (Hansen et al., 1983).
Permethrin and its metabolites are not accumulated in birds. During
repeated dosing to quails and to mallard ducks, very similar patterns
and levels of both the appearance and depletion of radioactive residue
in tissues were found. The level in fat, which was small, reached a
plateau during a 28-day period. In all tissues, residues declined
extensively during a 14-day period after the final dose (Leahey et al.,
1977).
7. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
Toxicological profiles of permethrins with different isomeric com-
positions (cis:trans ratios of 40:60 or 25:75) were compared in a range
of toxicological studies. The toxicological profile of permethrin
(25:75) resembles that of permethrin (40:60) except that it is less
acutely toxic than permethrin (40:60).
7.1 Acute Toxicity
Table 7 shows the results of acute toxicity tests of permethrin
with various animal species. Aqueous suspensions usually produced the
least toxic results, LD50 values ranging from 3000 to >4000 mg/kg body
weight. However, corn oil is the more standard vehicle for pyrethroids
and yielded LD50 values of about 500 mg/kg (in all studies except one)
for oral administration in rats and mice.
Following oral administration of permethrin to rats, signs of
poisoning became apparent within 2 h after dosing and persisted for up
to 3 days. At lethal levels, these signs included whole body tremors of
varying degree from slight to convulsive, which in some cases were ac-
companied by salivation. Associated signs were hyperactivity and hyper-
excitability to external stimuli, urination and defecation, ataxia, and
lacrimation (Parkinson, 1978; Litchfield, 1983).
Table 8 gives the acute oral toxicity of three lots of permethrin
to rats. The observed ten-fold decrease in LD50 when corn oil or olive
oil were used could be due to enhanced absorption of the insecticide
(Metker et al, 1977).
The acute oral toxicity of permethrin (25:75) to groups of six
female C.S.E. Wistar rats was determined in five different vehicles
(Table 9). Most symptoms of acute poisoning developed within 12 h of
dosing and consisted of muscular tremors, hypersensitivity to stimuli,
and staining of abdominal fur. The majority of deaths occurred between
1 and 3 days (Wallwork & Malone, 1974).
Groups of female Sprague-Dawley rats, either fed ad libitum or
starved for 24 h beforehand, were given a single oral dose of
permethrin (25:75) (94.1% purity) in corn oil solution (40% w/v) at
750, 1500, 3000, or 6000 mg/kg. Permethrin was more toxic in starved
animals (LD50 = 3000 mg/kg) than in animals that had been fed (LD50 =
4251 mg/kg) (Piercy et al., 1976).
The acutetoxicity of permethrin with various cis- and trans-per-
methrin ratios is indicated in Table 10. These data clearly demonstrate
that cis-permethrin is more toxic than trans-permethrin to rats and
mice.
Table 7. Acute toxicity of permethrin administered to various
animal species
-------------------------------------------------------------------------
Species Sex Routea Vehicleb LD50 Reference
(mg/kg body
weight)
-------------------------------------------------------------------------
Rat M oral waterc 2949 Parkinson 1978
F oral water > 4000 Parkinson et al. 1976
M oral DMSO 1500 Clark 1978
F oral DMSO 1000 Clark 1978
M oral corn oil 500 Jaggers & Parkinson 1979
M oral corn oil 430 Kohda et al. 1979a
F oral corn oil 470 Kohda et al. 1979a
M&F oral corn oil 1200 Braun & Killeen 1975
M&F oral water 1725 Sasinovich & Panshina 1987
M dermal water > 5176 Parkinson 1978
F dermal noned > 4000 Parkinson et al. 1976
M dermal noned > 2500 Kohda et al. 1979a
F dermal noned > 2500 Kohda et al. 1979a
M&F dermal xylene > 750 Clark 1978
M&F dermal none 2000 Sasinovich & Panshina 1987
M sc corn oil 7800 Kohda et al. 1979a
F sc corn oil 6600 Kohda et al. 1979a
M ip water > 3200 Parkinson et al. 1976
F ip water > 3200 Parkinson et al. 1976
ip 463 - 1725 Sasinovich & Panshina 1987
Mouse F oral water > 4000 Parkinson et al. 1976
M&F oral DMSO 250 - 500 Clark 1978
M oral corn oil 650 Kohda et al. 1979a
F oral corn oil 540 Kohda et al. 1979a
M dermal noned > 2500 Kohda et al. 1979a
F dermal noned > 2500 Kohda et al. 1979a
M sc corn oil > 10 000 Kohda et al. 1979a
F sc corn oil 10 000 Kohda et al. 1979a
Rabbit F oral waterc > 4000 Parkinson et al. 1976
F dermal noned > 2000 Parkinson et al. 1976
Guinea- M oral water > 4000 Parkinson et al. 1976
pig
Hen oral > 1500 Milner & Butterworth 1977
-------------------------------------------------------------------------
a sc = subcutaneous; ip = intraperitoneal.
b DMSO = dimethyl sulfoxide.
c as an aqueous suspension.
d technical material applied without vehicle.
Table 8. Acute oral toxicity of three lots of permethrin to rats
------------------------------------------------------------------
Lot No.c Strain Sex Solvent LD50
(mg/kg)
------------------------------------------------------------------
827-RSP-1422 Sprague-Dawley Male None 5010
Female None 3801
827-RTP-1450 Sprague-Dawley-1a Male Corn oil 563
Sprague-Dawley-2b Male Corn oil 383
827-RTP-1450 Long-Evans Male None 4892
Female None 2712
8719-RTP-1450 Sprague-Dawley Male Olive oil 584
Female Corn oil 413
------------------------------------------------------------------
a Average body weight was 220 g.
b Average body weight was 321 g.
c Isomeric composition and the purity of the compound in each lot
were as follows:
--------------------------------------------
Lot No. Isomeric Ratio Stated
cis trans Purity
--------------------------------------------
827-RSP-1422 44% 56% 93.6%
827-RTP-1450 45% 55% 95.0%
8719-RTP-1450 46.5% 53.5% 92.4%
--------------------------------------------
Table 9. Acute toxicity of permethrin (25:75) to rats
------------------------------------------------------
Vehicle LD50 (mg/kg)
------------------------------------------------------
Neat undiluted permethrin (control) > 20 000
40% w/v in corn oil 4672
40% w/v in petroleum distillate > 8000
40% w/v in dimethylsulfoxide > 8000
20% w/v in glycerol > 5048
------------------------------------------------------
Table 10. Acute toxicity of permethrins with various cis:trans
isomeric ratios
---------------------------------------------------------------------------
Permethrin LD50
(cis:trans) Animal Sex Routea (mg/kg body Reference
weight)
---------------------------------------------------------------------------
80:20 Rat F oral 396 Jaggers & Parkinson (1979)
57:43 F oral 333
50:50 F oral 748
40:60 F oral 630
20:80 F oral 2800
99:1 Mouse ip 108 Glickman et al. (1982)
40:60 ip 514
1:99 ip > 800
99:1 Mouse iv 17 Glickman et al. (1982)
40:60 iv 31
1:99 iv > 135
---------------------------------------------------------------------------
a ip = intraperitoneal; iv = intravenous.
Table 11 shows the results of acute oral toxicity tests of the
metabolites of permethrin on rats (FAO/WHO, 1980b).
Table 11. Acute oral toxicity to rats of several metabolites
of permethrin
---------------------------------------------------------------
Chemical No.a LD50 (mg/kg
body weight)
---------------------------------------------------------------
3-phenoxybenzyl alcohol 6 1330
3-(2,2-dichlorovinyl)-2,2-dimethylcyclo- 17 980
propanecarboxylic acid
3-phenoxybenzaldehyde 11 600
---------------------------------------------------------------
a Chemical identification no. used in Fig. 3.
Table 12 shows the results of the acute intraperitoneal toxicity to
mice of permethrin metabolites (Kohda et al., 1979b).
Table 12. Acute intraperitoneal toxicity to mice of several
permethrin metabolites
---------------------------------------------------------------------
Chemicala No.b LD50 (mg/kg body weight)
Male Female
---------------------------------------------------------------------
3-phenoxybenzyl alcohol 6 71 424
3-(4 -hydroxyphenoxy)benzyl alcohol 7 750 - 1000 750 - 1000
3-(2 -hydroxyphenoxy)benzyl alcohol 8 876 778
3-phenoxybenzoic acid 12 154 169
3-(4 -hydroxyphenoxy)benzoic acid 13 783 745
3-(2 -hydroxyphenoxy)benzoic acid 14 859 912
3-phenoxybenzaldehyde 11 415 416
---------------------------------------------------------------------
a All compounds were dissolved in corn oil, except 3-phenoxybenzoic
acid, which was dissolved in DMSO.
b Chemical identification no. used in Fig. 3.
7.2 Subacute and Subchronic Toxicity
7.2.1 Oral exposure
7.2.1.1 Mouse
When male and female Alderly Park mice (20 of each sex per group)
were fed permethrin in the diet at levels of 0, 200, 400, 2000, or
4000 mg/kg diet for 28 days, mortality, growth, and food utilization
were normal for all animals. One additional group (permethrin level of
80 mg/kg for 2 weeks and 10 000 mg/kg for the final 2 weeks) showed
weight loss and poor food utilization when feeding with 10 000 mg/kg
began. Animals fed permethrin at 2000 mg/kg or more showed increased
liver weight and liver-to-body weight ratio. Higher weight and organ-
to-body weight ratios were also observed in the kidney, heart, and
spleen of males receiving a dose of 10 000 mg/kg. Gross tissue changes
were observed in females at 2000 and 10 000 mg/kg. On histopathological
examination, regenerating tubules in the renal cortex and hypertrophy
of centrilobular hepatocytes with cytoplasmic eosinophilia, which were
not dose related, were observed in all the treated animals (Clapp et
al., 1977b).
In a study by Wallwork et al. (1974a), groups of six mature female
mice received daily oral doses of permethrin (25:75) in corn oil at 0,
200, 400, 800, or 1600 mg/kg body weight for 10 consecutive days.
Signs of acute toxicity, such as spasm and convulsion, were seen only
in the highest dose group, half of which died after the initial dose.
No significant changes in haematology, clinical chemistry, or body
weights on the 11th day of dosing were recorded. The mice treated at
800 and 1600 mg/kg body weight exhibited increased liver weights.
7.2.1.2 Rat
Sprague-Dawley rats (six of each sex per group) were fed permethrin
in the diet for 14 days at dose levels of 54, 108, 216, 432, 864, or
1728 mg/kg body weight per day. All rats surviving to term were sacri-
ficed and various organs and tissues were examined histopathologically.
At the two highest dose levels, all animals died except one female fed
864 mg/kg. Muscle tremors were noted in all animals at 432 mg/kg, but
doses of 216 mg/kg or less caused no toxic signs in either males or
females. There was a statistically significant increase in average
liver-to-body weight ratios at 432 mg/kg, but compound-related histo-
logical changes were not observed in any of the tissues or organs. The
maximum NOEL in this study was 216 mg/kg (Metker et al., 1977).
In studies by Metker et al. (1977), Long-Evans rats (six of each
sex per group) were fed permethrin in the diet for 14 days at dose
levels of 0, 27, 54, 108, 216, or 432 mg/kg body weight per day. All
rats surviving to term were sacrificed and various organs and tissues
were examined histopathologically. At a dose of 432 mg/kg, three out of
six females died within the first five days. Muscle tremors were noted
in all surviving animals at 216 and 432 mg/kg. There was a statisti-
cally significant increase among female animals in the average liver-
to-body weight ratio. Compound-related histological changes were not
observed in any of the tissues or organs examined. The maximum dietary
NOEL was 108 mg/kg body weight per day.
When young male and female Wistar rats (8 of each sex per group)
were fed permethrin in the diet at dose levels of 0, 200, 500, 1000,
2500, 5000, or 10 000 mg/kg diet for 4 weeks, all rats that received
the highest dose died within 3 days. Mortality was evident at 5000
mg/kg, and hyperexcitability was observed in animals that received
2500 mg/kg. Other non-specific signs of poisoning were observed at
1000 mg/kg on the first day of the study only. Food consumption and
growth were reduced in the animals dosed at 5000 mg/kg. There was no
effect on haematological parameters, clinical chemistry, or urinalysis
except for a reduction in urinary protein excretion in males at 5000
mg/kg. Liver weight and liver-to-body weight ratios were increased in
males at 2500 mg/kg or more and in females at 1000 mg/kg or more. This
study had been designed as a preliminary range-finding test for long-
term dietary administration (Clapp et al., 1977a).
In a study of the reversibility of hepatic changes in rats follow-
ing short-term dietary administration of permethrin, female Wistar rats
(48 rats per group) were fed permethrin at levels of 0 or 2500 mg/kg
diet for 28 days. At the end of the feeding trial, rats were either
sacrificed or maintained on control diets and sacrificed at 1, 4, or
8 weeks after the termination of dosing. There was no mortality, but
food consumption, food utilization, and body weight were reduced in the
permethrin-treated rats during the administration period. However, the
animals gained weight rapidly after the dosing period and there was no
difference in body weight between control and test animals at the end
of the study period. After the 4 weeks of permethrin dosing, signifi-
cantly higher absolute and relative liver weights were observed. During
the 8-week recovery period, the relative liver weight of permethrin-
treated animals was significantly higher than the control values, but
the absolute weights of the liver of control and test animals were
similar. There were no effects of permethrin on plasma alanine transam-
inase over the course of the study. Oxidative enzyme activity in liver
microsomes was significantly higher in test animals than in controls at
the end of dosing and 1 week later. The activity of liver microsomal
enzymes in the permethrin-treated animals was normal 4 weeks after
dosing but was elevated 8 weeks after dosing. The amount of smooth
endoplasmic reticulum in rat liver cells was significantly increased as
a result of permethrin dosing, but within 4 weeks after dosing, there
were no significant histological differences in the liver between
treated and control animals (Bradbrook et al., 1977).
When male and female Charles River (CD) rats (six of each sex per
group) were fed permethrin at levels of 0, 30, 100, 300, 1000, or 3000
mg/kg diet for five weeks, persistent tremors were evident in animals
fed at 3000 mg/kg although no mortality was observed. Growth was
inhibited in both males and females at this dose level. Relative liver
weight was increased in both the males (1000 mg/kg or more) and females
(3000 mg/kg). Slight effects on certain clinical chemistry parameters,
such as increased prothrombin times in males, were noted at the 3000
mg/kg level. Examination of tissues and organs of the animals receiving
the two highest doses did not show any unusual effects as a result of
permethrin in the diet (Butterworth & Hend, 1976).
Male and female Long-Evans rats (10 of each sex per group) fed
permethrin in the diet at dose levels of 0, 20, 100, or 500 mg/kg diet
for 90 days showed no mortality, and the growth and food consumption of
all animals were normal. The results of haematology, clinical chemis-
try, urinalysis, and ophthalmological examinations were also normal.
Tremors were noted in some animals at the highest dose level, mainly
during the first week of treatment. There were significant increases
in absolute and relative liver weights at the two highest dose levels.
These increases were consistent with data from microscopic examination
of the liver showing compound-related centrilobular hepatocyte hyper-
trophy in both males and females. There were no significant effects at
the 20-mg/kg level, although slight hepatic effects were reported in a
few of the male rats (Killeen & Rapp., 1976b).
In studies by Metker et al. (1977), Sprague-Dawley rats (10 of each
sex per group) were fed permethrin in the diet for 90 days at dose
levels of 0, 9, 27, 85, 270, or 850 mg/kg body weight per day. All
rats surviving to term were killed and various tissues and organs from
each animal were examined histopathologically. At 850 mg/kg, all male
and female rats died. An increase in the average liver-to-body weight
ratio was noted in both male and female rats fed 270 mg/kg. Compound-
related histological changes were not observed in any of the tissues
and organs examined. The minimum effect level was 270 mg/kg per day.
At 85 mg/kg no effects were observed.
When male and female Sprague-Dawley rats (16 of each sex per group)
were fed permethrin in the diet at dose levels of 0, 375, 750, 1500, or
3000 mg/kg diet for 6 months, there was no mortality and all animals
exhibited normal growth and normal food and water consumption. Urinal-
ysis, haematological values, and clinical biochemistry parameters
showed no changes related to permethrin dosing. Signs of hyperexcit-
ability and tremors were observed during the study in animals dosed at
3000 mg/kg and the liver weight and liver-to-body weight ratio of these
animals were slightly increased. There were no significant histopatho-
logical findings attributable to the presence of the permethrin in the
diet. The NOEL was 1500 mg/kg (Kadota et al., 1975).
In a study designed to evaluate liver hypertrophy, male and female
Wistar rats were fed permethrin at levels of 0, 20, 100, or 1000 mg/kg
diet for 26 weeks. There was no mortality, and the growth and food con-
sumption of the animals were normal. Although the mean liver weight was
increased at all dose levels, a significant increase was noted only at
the highest dose level. The increase in liver weight at this dose level
was accompanied by an increase in the smooth endoplasmic reticulum and
in biochemical parameters associated with microsomal oxidative mechan-
isms. At a dose level of 100 mg/kg, there were slight, non-significant
increases in biochemical activities. No effects on any of the par-
ameters measured were observed in animals dosed at 20 mg/kg (Hart et
al., 1977c).
In a study by Wallwork et al. (1974b), groups of five to six female
Charles River CDI rats received permethrin (25:75) in corn oil at 0,
200, 400, or 800 mg/kg body weight by daily gavage for 10 days. The
animals were sacrificed on the eleventh day so that haematological and
clinical chemical parameters and organ weights could be investigated.
Permethrin (25:75) gave a toxicity profile similar to that of
permethrin (40:60).
7.2.1.3 Dog
Beagle dogs (four of each sex per group) fed permethrin in gelatin
capsules daily for 3 months at dose levels of 0, 5, 50, or 500 mg/kg
body weight showed no mortality, but clinical signs of poisoning were
noted at various times in both males and females at the highest dose
level. Growth and food consumption, as well as clinical chemical,
haematological, and urinalysis parameters, were normal. The liver
weights and liver-to-body weight ratios of animals that received per-
methrin at 50 mg/kg or more were significantly increased. Histopatho-
logical examination did not reveal any changes attributable to per-
methrin (Killeen & Rapp, 1976a).
Beagle dogs (four of each sex per group) administered permethrin in
gelatin capsules daily for 13 weeks at dose levels of 0, 10, 100, and
2000 mg/kg body weight likewise showed no mortality, but clinical signs
of poisoning were evident at 2000 mg/kg. Haematological, clinical
chemical, and urinalysis values were normal in all animals. There was a
slight increase in the liver weight of animals dosed at 2000 mg/kg/day,
but no accompanying histopathological changes in the liver (Edwards et
al., 1976).
When two beagle dogs were given daily oral doses of permethrin
(25:75) at 500 mg/kg body weight for 14 days, there were no clinical
signs of toxicity or significant effects of the treatment on body
weight or on clinical chemistry or haematological parameters (Chesher
et al., 1975a).
Groups of four male and four female beagle dogs, given encapsulated
permethrin [(25:75) 4.5% w/v] at 0, 10, 50, or 250 mg/kg body weight
for 6 months, revealed no signs of toxicity and no effect on body
weight. Ophthalmoscopy and electrocardiography showed no abnormalities.
At necropsy, there were no gross pathological or significant histo-
pathological findings. Haematological and clinical chemistry para-
meters, including plasma antipyrine elimination rate, were unaffected
by treatment. The results of this study indicate that daily oral doses
up to 250 mg/kg body weight do not adversely affect beagle dogs
(Reynolds et al., 1978).
7.2.1.4 Rabbits
In a study by Chesher & Malone (1974a), groups of five female Dutch
rabbits received permethrin (25:75) in 10 daily doses by gavage in corn
oil at 0, 200, 400, or 800 mg/kg body weight. The animals were killed
on the eleventh day so that clinical chemical, haematological para-
meters, and organ weights could be investigated. One rabbit, dosed at
400 mg/kg, exhibited mild hyperactivity and muscular fasciculation, but
only at days 6 and 7. Although all animals, including the controls,
exhibited some degree of weight loss, it was most marked in the high-
dose group. There were no significant haematological or clinical chem-
istry findings, but there was some decrease in liver and kidney weight
and also some enlargement of adrenal gland weights in all treated
groups.
7.2.1.5 Cow
Lactating cows (three per group) fed permethrin in the diet at dose
levels of 0, 0.2, 1.0, 10, or 50 mg/kg diet for 28 days showed no mor-
tality. Growth and milk production were normal, and no histopathologi-
cal changes in the tissues were observed (Edwards & Iswaran, 1977).
7.2.2 Dermal Exposure
Technical grade permethrin was applied daily to the clipped skin of
New Zealand White rabbits (eight males per group) at dose levels of 0,
0.10, 0.32, or 1.0 g/kg body weight, each day for 21 consecutive days.
The application site was abraded on the first test day in half of the
animals in each group. Blood samples were drawn weekly from the animals
for clinical chemistry studies. All animals were killed on the tenth
day after exposure ceased. Various tissues and organs were taken from
each animal and examined for microscopic lesions. A moderate primary
irritation of the skin was produced by permethrin. No significant
changes in body weight, organ weight, or clinical chemistry values were
evident, neither were there any compound-related lesions in the skin or
other tissues (Metker et al., 1977).
In further studies by Metker et al. (1977), permethrin (dissolved
in acetone) or acetone (as a control) was placed on the skin twice a
week for 3 weeks to 6 groups of 10 shaved male New Zealand White rab-
bits. Cotton cloth treated with permethrin (1.25 or 0.125 mg/cm2) was
applied to the skin over 1 ml of artificial sweat (salt solution imi-
tating sweat). In the case of other rabbits, similarly treated, the
sweat was omitted. In the control groups, acetone-treated cotton cloth
with or without 1 ml of sweat was used. Blood samples were collected
once a week for clinical chemistry determinations. All animals sur-
viving to term were killed and various tissues and organs from each
animal were examined for microscopic lesions. No significant changes
were noted in rabbit body weight or organ-to-body weight at the end of
the 21-day test, and no skin irritation was observed. There were no
significant changes in clinical chemistry values in the treated groups
and no compound-related lesions in the skin or other tissues and organs
examined (Metker et al., 1977).
7.2.3 Inhalation exposure
The inhalation toxicity of technical grade permethrin was deter-
mined in three species of laboratory animals. Male Hartley guinea-pigs,
male and female Sprague-Dawley rats, and male and female beagle dogs
were exposed to an aerosol of permethrin at concentrations of 125, 250,
or 500 mg/m3, 6 h per day, 5 days per week for 13 weeks. The mass
median diameter of the aerosol droplets was 5.1 µm, and 85% of the
total droplets had a diameter of 1.0 µm or less. At 500 mg/m3, tremors
and convulsions occurred in the rats during the first week of exposure
but disappeared in the second week. There was no difference in oxygen
consumption between control and treated rats. Urine metabolite studies
indicated that permethrin was rapidly metabolized and excreted. Post-
exposure experiments in male rats showed that the hexobarbital-induced
sleeping time was significantly shortened after exposures at 500
mg/m3 but not at lower doses. No clinical signs of poisoning were
observed in the guinea-pigs and dogs when exposed to aerosols of per-
methrin under similar conditions. Pulmonary function, clinical chemis-
try parameters, and blood cell counts were unaffected in the beagle
dogs following exposure. No compound-related gross or microscopic
pathological changes or other permanent changes were observed in the
dogs, rats, or guinea-pigs as a result of permethrin inhalation
(Metker, 1978).
7.3 Primary Irritation
7.3.1 Skin irritation
When undiluted technical permethrin (91.3% purity, 0.5 ml) was
applied to the clipped dorsal surface of Japanese White rabbits, there
was no irritation (Okuno et al., 1976).
Single applications of 0.05 ml of 25% (w/v) permethrin (in 95%
ethanol) or 10% (w/v) oil of bergamot solution (in 95% ethanol) (posi-
tive control) were applied to the intact skin of six rabbits. Five
minutes later, some of the rabbits were exposed to UV light (365 nm) at
a distance of 10-15 cm for 30 min (the intensity of UV light was not
mentioned). Skin treated with the positive control solution and
irradiated exhibited a greater irritation reaction than did non-
irradiated skin. Permethrin did not cause any irritation reaction
under the test conditions with or without irradiation (Metker et al.,
1977).
When a permethrin formulation was applied to the clipped dorsal
surface (0.13 mg/cm2) of six New Zealand White rabbits (three of each
sex) once a day for 16 days, a slight erythema appeared, which corre-
lated with increased cutaneous blood flow measured by laser Doppler
velocimetry. No significant histopathological changes were detected
(Flannigan et al., 1985a).
7.3.2 Eye irritation
In a study by Okuno et al. (1976), 0.1 ml of undiluted technical
permethrin (91.3% purity) was applied to the eyes of Japanese White
rabbits. The eyes were washed with distilled water 5 min or 24 h after
the application of permethrin. No eye irritation was observed.
Undiluted permethrin applied to the eyes of female rabbits caused
minimal pain, redness, chemosis of the conjunctiva, and a slight dis-
charge (Parkinson et al., 1976).
Permethrin (25:75) (40% in corn oil) did not produce any ocular
effects when 0.1 ml was instilled into the ocular sac of New Zealand
rabbits (Chesher & Malone, 1974c).
7.4 Sensitization
In a study by Parkinson et al. (1976), guinea-pigs were dermally
administered permethrin as a 10% solution in dimethylformamide for
3 consecutive days. This was followed 4 days later by challenge doses
of 0.1%, 1%, and 10% solutions of permethrin in dimethylformamide. Only
very slight erythema was observed. Permethrin was therefore considered
to be either non-sensitizing or only mildly so.
Guinea-pigs (10 per group) were initially injected intradermally
with 0.1 ml permethrin solution and 14 days later were challenged with
an intradermal injection (0.1 ml) of either a 0.1% solution of permeth-
rin or dinitrochlorobenzene (DNCB). Five other animals per group
received intradermally a challenge dose of 0.1% permethrin or DNCB
without a prior sensitizing dose. The positive control substance (DNCB)
elicited sensitization reactions in all guinea-pigs when examined 24
and 48 h after the challenge dose, whereas permethrin did not cause any
sensitization reactions (Metker et al., 1977; Metker, 1978).
Permethrin (25:75) in corn oil (1% w/v) or Freund's complete
adjuvant (1% w/v) did not produce dermal irritation or sensitization in
groups of 10 male guinea-pigs when applied as a 25% dispersion in
petrolatum. The positive control, DNCB, (5% w/v) in petrolatum produced
marked sensitization (Chesher & Malone, 1974b).
7.5 Long-term Toxicity
7.5.1 Mouse
SPF Alderly Park strain mice (70 males and 70 females per group)
were fed permethrin (cis 35-45%; trans 65-55%) at dose levels of 0,
250, 1000, or 2500 mg/kg diet for 2 years. Ten males and ten females
were designated for interim kills at 26 and 52 weeks. The mortality
rate was unaffected by the administration of permethrin. Growth was
slightly decreased at the two highest dose levels at various periods
during the study. At the interim sacrifice of 52 weeks and at the end
of study, a significant dose-dependent increase in liver-to-body weight
ratio was observed at the two highest dose levels in females (with 2500
mg/kg only at the end of the study) and at the highest dose level in
males. Hepatic aminopyrine N-demethylase activity was also substan-
tially increased, although not consistently, in both male and female
mice given the highest dose. Gross and microscopic examination of
tissues and organs (and specific examination for hepatic neoplasia) did
not reveal any significant carcinogenic effects as a result of per-
methrin administration. Many of the non-tumour abnormalities observed
were considered to be those associated with aging of the mice, charac-
terized by increased eosinophilia of the centrilobular hepatocytes.
Also, a decrease in vacuolation of the proximal tubular epithelium of
the kidney was noted at all dietary levels in males. A high incidence
of lung adenomas was observed with all animals in the study, but stat-
istical analysis suggested that this was not related to permethrin
feeding. Electron-microscopic examination of subcellular liver com-
ponents showed a proliferation of the smooth endoplasmic reticulum at
dose levels of 1000 and 2500 mg/kg. No notable effects on the sciatic
nerve were found as the result of permethrin administration (Ishmael &
Litchfield, 1988).
In studies by Hogan & Rinehart (1977) and Rapp (1978), CD-1 strain
mice (75 of each sex per group) were fed permethrin in the diet for
104 weeks. Alterations were made in the dietary dose levels during the
course of the study. From weeks 1 to 19, the animals were given dose
levels of 0, 20, 100, and 500 mg/kg diet. At week 19, the dose level
of 500 mg/kg was increased to 5000 mg/kg and maintained for 2 weeks
before returning to 500 mg/kg. At week 21, the 100 mg/kg dose was
increased to 4000 mg/kg and maintained for the remainder of the dosing
period. Growth was inhibited in males at 4000 mg/kg. With the exception
of a reduced blood glucose level in the animals receiving 4000 mg/kg,
dietary administration of permethrin had no other effects on haema-
tology or clinical chemistry parameters in the mouse. The liver weight
was higher than it was in control animals in both male and female
animals at a dose level of 500 mg/kg or more. In addition, the heart
weight was higher at 4000 mg/kg. Neoplastic changes, not associated
with dietary levels of permethrin, were observed in some animals in all
groups. While there was no direct effect with respect to hepatic neo-
plasms, it was noted that hepatocellular hypertrophy, pleomorphism, and
degeneration occurred in treated mice with increased frequency and
appeared to show a dose-response relationship. No oncogenic effects
were observed in the test animals.
7.5.2 Rat
Wistar rats (60 of each sex per group) were fed permethrin in the
diet at dose levels of 0, 500, 1000, or 2500 mg/kg diet for 2 years,
and twelve rats of each sex per group were sacrificed at 1 year. Signs
of poisoning such as tremors and hyperexcitability were noted during
the first 2 weeks of dosing in the animals that received the highest
dose. There was no mortality attributable to permethrin, and growth and
food consumption were unaffected. There were no effects on haema-
tological, ophthalmological, urological, or other clinical chemistry
parameters. Liver aminopyrine N-demethylase activity was increased in
all permethrin-treated animals. Bone marrow smears of the animals
showed no unusual findings. Gross and microscopic examination of
tissues and organs was performed after 1 and 2 years, and all animals
that died with neoplastic changes were examined. Liver weights were
higher after 1 year of dosing in male and female rats that received
permethrin at 2500 mg/kg than in the control animals. After 2 years,
the liver weight and liver-to-body weight ratios were higher in all
permethrin-treated males than in the corresponding controls. In the
females, higher values of absolute and relative liver weights, compared
to the controls, were recorded only in the group of animals dosed at
1000 mg/kg. Kidney weight was also increased, predominantly in males,
at all dose levels. Hepatocyte vacuolation was seen at 1 year in males
fed at the highest dose level only and in females at all dose levels.
The smooth endoplasmic reticulum showed significant increases at 52
weeks in both males and females at all dietary levels. At the end of
the study, notable endoplasmic reticulum increases were detected only
at the highest dose levels, although non-significant increases were
noted at all dose levels in both males and females. Examination of the
sciatic nerve showed no effects attributable to permethrin. No
oncogenic effects were noted at any dose level (Ishmael & Litchfield,
1988).
Long-Evans rats (60 males and 60 females per group) fed permethrin
in the diet at dose levels of 0, 20, 100, or 500 mg/kg for 2 years did
not reveal any mortality or adverse effects on growth, food consump-
tion, or behaviour attributable to the administration. Haematology,
clinical chemistry, and urinalysis measurements were performed at
either 6 months or 1 year and at the end of the study. There were no
compound-related effects on a wide variety of parameters examined, and
ophthalmological examination indicated no abnormalities. Blood glucose
levels were higher in the highest-dose males at 24 months and in the
highest-dose females at 18 months, compared to the values of the
control animals. Two independent evaluations of the histopathological
data concluded that there was no oncogenic potential for permethrin.
While there was a dose-dependent increase in gross liver weight in both
males and females, these values are small and not statistically
significant. The NOEL for general toxicity in this study was estimated
to be 100 mg/kg (Braun & Rinehart, 1977; Billups 1978a, b).
7.6 Carcinogenesis
Summaries by Paynter et al. (1982) of toxicological data from seven
long-term chronic toxicity/oncogenicity studies (four in mouse and
three in rat) carried out by ICI, FMC, and Burroughs-Welcome (BW) have
been made available by the US EPA. One rat study and one mouse study
performed by ICI were recently published (Ishmael & Litchfield, 1988),
and one rat study and one mouse study carried out by BW were cited in
FAO/WHO (1988). A report of the FIFRA Scientific Advisory Panel which
reviewed this data was also made available (US EPA, 1981). Table 13
summarizes the basic design of each of these studies, some of which are
also discussed in section 7.5.
7.6.1 Mouse
One of the four mouse studies referenced in Table 13 (FMC I) was
not considered for evaluation because of dose level changes in the mid-
and high-level groups and problems in histopathological methodology.
7.6.1.1 ICI study
Relevant non-oncogenic effects observed during the study consisted
of increased mortality, increased liver aminopyrine- N-demethylase ac-
tivity, increased liver weight, and eosinophilia of hepatocytes in both
males and females at 2500 mg/kg diet. The liver changes observed in
this study were considered to be related in large measure to the
induction of liver microsomal enzyme activity. Minimal liver changes
were also observed at 1000 mg/kg, but not at 250 mg/kg. A slight
increase in lung adenomas was observed in male mice at the highest dose
level. However, there was some uncertainty as to whether this increase
was related to permethrin ingestion.
7.6.1.2 FMC II study
Relevant non-oncogenic effects observed during the study consisted
of increased mortality in males at 2000 mg/kg diet, increased liver
weight in females at 2500 mg/kg and 5000 mg/kg, and increased lung
weight in females at 5000 mg/kg. Histopathologically, dose-related
"focal alveolar cell proliferation" (increased numbers of lung cells)
was observed in permethrin-treated females. As regards oncogenic
effects, there was an increased incidence of bronchio-alveolar adenomas
in female mice only. The number of female mice with adenomas and/or
carcinomas (15/74, 24/72, 35/74, and 44/75 at the four dose levels)
revealed a statistically significant dose-response relationship. Male
mice did not show this effect. However, some doubt was expressed by
the FIFRA Scientific Advisory Panel concerning the conduct of this
study.
7.6.1.3 BW study
Non-oncogenic effects observed during the study consisted of
slightly decreased mortality in females at 50 and 250 mg/kg per day,
increased liver weights in males, and increased kidney weights in
females at 250 mg/kg per day. Histopathologically, an increased inci-
dence in cuboidal/columnar metaplasia of the alveolar epithelium was
observed in the lungs of male and female mice at the highest dose. The
oncogenicity data indicated a dose-related trend in females, but not in
males, for adenomatous tumours in the lungs. No notable pattern was
observed for other neoplasms at any dose level.
7.6.1.4 Appraisal of mouse studies on carginogenicity
Consistent findings in the above three mouse studies at high dose
levels were liver changes known to be associated with the induction of
the liver microsomal enzyme system. Other histopathological effects
observed in liver, not usually associated with microsomal induction,
included multifocal hepatocytomegaly and hepatocytic pigmentation. The
incidence of lung adenomas for each of the three mouse studies is given
in Table 14.
Among the three long-term mouse studies, there was evidence of
permethrin oncogenicity in the lungs in one strain (CD-1 female only)
at the highest dose level only.
Although there was a difference between the control and treated
groups in terms of lung adenomas in these studies, these differences
were not significant when compared with historical control values. The
oncogenicity potential, as evaluated by the FIFRA Scientific Advisory
Panel, was considered to be very weak.
Table 13. Chronic toxicity and carcinogenicity studies in mice and rats
------------------------------------------------------------------------------------------------------
Study
performed Species Strain No. of Duration Dose Reference
by animals (weeks) (mg/kg diet)
------------------------------------------------------------------------------------------------------
ICI mouse Alderly Park 70 98 0, 250, 1000, 2500 Ishmael & Litchfield (1988)
(SPF)
FMC I mouse CD-1 75 104 0, 20, 500, 4000 Hogan & Rinehart (1977);
Rapp (1978)
FMC II mouse CD-1 75 104 M:0, 20, 500, 4000 Bio Dynamics (1979)
F:0, 20, 2500, 5000
BW mouse CFLP 75 92 0a, 10a, 50b, 250b James et al. (1980)
ICI rat Wistar 60 104 0, 500, 1000, 2500 Ishmael & Litchfield (1988)
FMC rat Long-Evans 60 104 0, 20, 100, 500 Braun & Rinehart (1977);
Billups (1978a,b)
BW rat Wistar 60 104 0, 10b, 50b, 250b McSheehy & Finn (1980)
------------------------------------------------------------------------------------------------------
a 100 animals as control.
b mg/kg body weight; permethrin 25% cis, 75% trans.
Table 14. Comparison of lung adenomas (%) in three mouse studies
------------------------------------------------------------------------
Male Female
control low mid high control low mid high
dose dose dose dose dose dose
------------------------------------------------------------------------
ICI study 20 12 26 32 22 16 20 30
FMC II study 22 23 28 25 16(20a) 17 35 35
BW study 26 19 23 22 3(20b) 7 10 20
------------------------------------------------------------------------
a Historical control values ranged from 23 to 60%, with a mean
of 20.4%.
b Historical control values ranged from 7.5 to 30.0%, with a mean
of 20.4%.
7.6.2 Rat
One of the three long-term rat studies referred to in Table 13,
i.e. the FMC rat study, was excluded from examination because of
serious flaws in histopathological methodology.
7.6.2.1 ICI study
Relevant non-oncogenic effects observed consisted of increased
mortality in males and decreased mortality in females at 2500 mg/kg
diet, increased liver weights in both males and females at 1000 and
2500 mg/kg and in males only at 500 mg/kg, increased liver aminopyrine-
N-demethylase activity in both males and females at 1000 and 2500
mg/kg, and hepatocyte vacuolization or hypertrophy in males and females
at 1000 and 2500 mg/kg. Additional effects observed were increased
kidney weight in males at all treatment levels and increased pituitary
weight in males at 1000 and 2500 mg/kg.
No tumours related to the ingestion of permethrin were observed in
this study.
7.6.2.2 BW study
Non-oncogenic effects observed at 250 mg/kg per day were increased
mortality in males, occasional body tremors in males and females, in-
creased liver weight in males, hepatocyte hypertrophy in males and fe-
males, and focal changes of the thyroid follicles in males and females.
The microscopic liver and thyroid changes were also observed at 50
mg/kg per day in both sexes.
With respect to tumours (including rare, unusual, or malignant neo-
plasms), none of the tumour types observed in this study were con-
sidered to be related to the ingestion of permethrin.
7.6.2.3 Appraisal of rat studies on carcinogenicity
No evidence of oncogenicity was observed in the rat studies.
7.7 Mutagenicity
7.7.1 Microorganism and insects
The mutagenic activity of permethrin was evaluated using the Ames
test. There was no increase in the number of revertant colonies at
doses up to 2500 µg permethrin/plate in five strains of Salmonella
typhimurium (TA1535, TA1537, TA1538, TA98, and TA100) with or without
S9-mix prepared from rat liver or S9 prepared from PCB-treated mice
(Longstaff, 1976; Newell & Skinner, 1976; Simmon, 1976; Suzuki, 1977).
Permethrin and six other synthetic pyrethroids were tested for
mutagenicity in S. typhimurium TA98 and TA100 strains in the presence
and absence of a metabolic-activation system. All pyrethroids tested
gave negative results (Pluijmen et al., 1984).
Two reverse-mutation tests in Escherichia coli WP2 also gave nega-
tive results (Newell & Skinner, 1976; Simmon, 1976).
When tested for mutagenicity in V79 Chinese hamster cells, permeth-
rin and five other synthetic pyrethroids were shown to be non-mutagenic
(Pluijmen et al., 1984).
In a host-mediated assay, permethrin (200 mg/kg body weight) was
orally administered to ICR mice. The indicator organism, S. typhimurium
G46, harvested from the abdominal cavity of mice 3 h after treatment,
did not reveal any mutagenic effect (Shirasu et al., 1979). In another
host-mediated assay employing a similar test system, (+)- trans-permethrin
at dose levels of 600 and 3000 mg/kg body weight and (+)- cis-permethrin
at 21 and 54 mg/kg body weight gave negative results (Miyamoto, 1976).
Permethrin was tested for its ability to induce complete and
partial chromosome loss in Drosophila melanogaster males by adding
5 mg/litre (soaked onto a filter paper) to the feeding solution.
Treated males were mated with mus-302 repair-defective females to
detect chromosome loss in the zygotes. Permethrin did not induce a
significant increase in chromosome loss, compared to negative controls
(Woodruff et al., 1983).
7.7.2 Mammals
An in vivo cytogenetic test was performed in Alderly Park rats to
assess the ability of permethrin to induce chromosomal aberration.
Permethrin was administered to groups of eight males by a single intra-
peritoneal injection or by five daily injections at doses of 600, 3000,
or 6000 mg/kg. The cytogenetic effect on bone marrow cells was evalu-
ated 24 h after the single injection and 6 h after the last multiple
dosing. No differences were observed in the rate of chromosomal aber-
rations between any permethrin-treated groups and the vehicle controls.
Two positive controls (trimethyl phosphate and mitomycin C) produced a
significantly higher incidence of chromosomal aberrations (Anderson &
Richardson, 1976).
Permethrin (25:75) gave a negative response when mouse lymphoma
L5178Y cells were treated with permethrin (up to 125 µg/ml) with or
without activation (Clive, 1977).
In dominant lethal studies, permethrin dissolved in corn oil was
administered orally for five successive days to groups of male CD mice
(15 per group) at doses of 15, 48, or 150 mg/kg. Each male was mated
with 16 virgin females, and on the 12th day of gestation the females
were killed. There was no dose-related effect on pregnancy or early or
late fetal deaths. Administration of permethrin thus had no dominant
lethal effect on male mice. On the other hand, the positive control
(ethylmethanesulfonate) induced pre-implantation losses and the early
death of embryos (McGregor & Wickramaratne, 1976a; Chesher et al.,
1975b).
7.8 Teratogenicity and Reproduction Studies
7.8.1 Teratogenicity studies
7.8.1.1 Mouse
In studies by Kohda et al. (1976b), groups of pregnant ICR mice (27
to 32 mice per group) were orally administered permethrin at dose
levels of 0, 15, 50, or 150 mg/kg body weight from day 7 to day 12 of
pregnancy. On day 18, two-thirds of the animals were sacrificed and
examined for implantation and resorption sites. Viable offspring were
examined for somatic and skeletal abnormalities, and, after 3 weeks of
lactation, pups were examined for behavioral abnormalities and for
differentiation and growth. At 6 weeks of age, all animals were sacri-
ficed and subjected to internal and external examination. There were no
effects on maternal toxicity over the course of the study. Growth and
differentiation of pregnant females were not affected by permethrin,
nor were the number of implantation sites or litter size adversely
affected. The size of individual pups and the incidence of gross
external, internal, and skeletal abnormalities were not significantly
different from those in the control mice. Permethrin, at dose levels
up to and including 150 mg/kg, did not appear to affect those animals
allowed to bear and wean young. The growth of young animals did not
appear to differ from control values, and, 3 weeks after weaning, the
surviving animals did not differ from controls with respect to growth
or major organ changes. There was no teratogenicity associated with
permethrin in this mouse bioassay, although the duration of dosing was
a little too short to cover both the early and late stage of organ
development (Kohda et al., 1976b).
7.8.1.2 Rat
In studies by McGregor & Wickramaratne (1976b), pregnant CD rats
(20 rats per group) were orally administered permethrin at dose levels
of 0, 22.5, 71.0, or 225 mg/kg from day 6 to day 16 of gestation. On
day 20, the animals were sacrificed and the corpora lutea were exam-
ined. Somatic and skeletal investigations were performed on the
fetuses. No adverse toxicological response was seen at the highest dose
used. There were no abortions or maternal deaths and no significant
differences in pregnancy frequency, corpora lutea, or total number of
implantations between treated and control rats. Placental and fetal
weights were similar to those of the controls and no skeletal or struc-
tural abnormalities were observed. Based upon the standard teratologi-
cal rat bioassay, permethrin did not show any teratological potential.
Pregnant Sprague-Dawley rats (29-34 rats per group) were orally
administered permethrin at dose levels of 0, 10, 20, or 50 mg/kg body
weight from day 9 to day 14 of pregnancy. On day 20, approximately two-
thirds of the pregnant females were sacrificed and the remaining rats
were allowed to deliver and wean pups. After lactation, the pups were
examined for behaviour and for growth and differentiation before being
sacrificed at 6 weeks of age and examined for internal and external
gross malformations. Pregnant females fed at the highest dose showed
toxic signs of poisoning, including ataxia, tremor, and a slight
reduction in body weight. There was no mortality, although fetal loss
at the highest dose level was slightly higher than that in the control
animals. A slightly higher incidence of non-ossified sternebra was
noted at 50 mg/kg. The number of implantation sites and the litter size
were not affected, and growth and differentiation were similarly unaf-
fected. Internal and external examination showed that, with the excep-
tion of the slight skeletal variation noted at 50 mg/kg, there were no
permethrin-associated changes. In those animals allowed to bear and
wean pups, there were no notable differences from control values with
respect to gestation, implantation sites, delivery, and numbers of live
young. Growth and differentiation of the offspring did not appear to
be affected by permethrin, and there were no abnormalities with respect
to gross pathology or in the weights of major tissues and organs at the
conclusion of the study. Permethrin did not elicit a teratogenic effect
in this bioassay (Kohda et al., 1976a).
In a study by Metker et al. (1977), permethrin (4, 41, and 83
mg/kg/diet), aspirin (200 mg/kg diet), and corn oil (2 ml/kg diet) were
each administered to groups of 20 pre-impregnated Sprague-Dawley rats
from day 6 to day 16 of gestation. The animals were sacrificed on day
20 of gestation, and the fetuses were removed and examined for gross
abnormalities, sex, weight, and body length. The administration of
aspirin (the positive control) resulted in significantly lower body
weight and length and a variety of abnormalities including craniorachi-
oschisis in the foetuses. Permethrin, administered to pregnant rats
during gestation by intragastric intubation, did not appear to present
a teratogenic or lethal hazard to the developing fetus.
When groups of 22 female Wistar rats received permethrin (25:75) at
0 or 200 mg/kg body weight in corn oil by daily oral gavage on days 6-
16 (inclusive) of pregnancy, treatment was without apparent effect on
maternal body weight gain or general conditions. The animals were
sacrificed on day 20 so that their uterine contents could be examined.
Treatment had no effect on the number of corpora lutea, implantations,
live fetuses, early and late fetal deaths, or fetal abnormalities.
Examination of the fetuses, which included dissection and skeletal
staining, showed no morphological effects of treatment. These results
indicate that permethrin (25:75) at 200 mg/kg body weight per day is
not fetotoxic to rats (James, 1974).
7.8.1.3 Rabbit
Mated female Dutch rabbits (18 per group) were orally administered
permethrin (at dose levels of 0, 600, 1200, or 1800 mg/kg body weight
per day) in 0.5% v/v aqueous Tween 80 from days 6-18 inclusive of
pregnancy. On day 29 of pregnancy the animals were killed and their
uteri were examined for resorptions and live implantations. The fetuses
were examined for gross abnormalities of skeleton and soft tissue. At
all dose levels, permethrin depressed body weight gain during dosing
and was embryotoxic at the two highest dose levels. It was toxic to
the dams at 1800 mg/kg body weight per day, but no teratogenic activity
was detectable at any dose level (Richards et al., 1980).
7.8.2 Reproduction Studies
7.8.2.1 Rat
Groups of Long-Evans rats (10 males and 20 females per group) were
fed permethrin at dose levels of 0, 20, and 100 mg/kg diet in a 3-
generation (two litters per generation) reproduction study. There was
no effect on mortality, mating, pregnancy, or fertility, with the
exception of the F2 mating index, which was reduced in both controls
and treatment groups. Pup survival and growth were unaffected. Haema-
tological evaluations of F2 adults between the second and third mating
showed no unusual effects. This study indicated that dietary permethrin
does not adversely affect reproduction in the rat (Schroeder &
Rinehart, 1977).
In studies by Hodge et al. (1977), groups of Wistar rats (12 males
and 24 females per group) were fed permethrin at dose levels of 0, 500,
1000, and 2500 mg/kg diet for 12 weeks. At 12 weeks the animals were
mated to initiate a standard 3-generation (two litters per generation)
reproduction study. Clinical signs of acute poisoning (tremors, etc.)
were noted, predominantly in females given the highest dose. There
were no effects attributable to permethrin on fertility, gestation,
viability of pups, sex ratio, litter size, or lactation. Ten male and
female weanlings from the second litter of the F3 generation were
examined for histopathological changes. Centrilobular hypertrophy and
cytoplasmic eosinophilia were observed at all dose levels, the inci-
dence and severity of which appeared to be dose dependent. Rats in the
third litter of the F3 generation were sacrificed on day 12 of ges-
tation for teratogenic examination, but no abnormalities were observed.
Based on the results of this study, permethrin does not appear to
induce reproductive toxicity in rats.
Spencer and Berhance (1982) fed female Sprague-Dawley rats (5-8
rats per group) permethrin in the diet at levels of 0, 500, 1000, 1500,
2000, 2500, 3000, 3500, and 4000 mg/kg diet from day 6 to day 15 of
pregnancy. Laparotomy was performed on day 20 of gestation, and the
number of live fetuses was determined. Placentae were excised and
cleaned of extraneous connective tissue. Analysis of the protein and
glycogen contents of the placentae on day 16 of pregnancy indicated
that they were only influenced by very high doses (2500-4000 mg/kg
diet) of permethrin. Analysis of variance indicated no significant
effect on protein level, but the treatment did decrease the glycogen
concentration. No significant dose-related effects on implantational
sites/intrauterine fetuses were observed. These results appeared to
confirm that permethrin possesses low mammalian toxicity.
In a 3-generation reproduction study, groups of 20 male and 20 fe-
male Wistar COBS rats received permethrin (25:75) in the diet at 0, 5,
30, and 180 mg/kg body weight per day during growth, mating, gestation,
parturition, and lactation for three generations, each with two
litters. Fetal toxicity and teratogenicity was assessed in the second
pregnancy of the F2 generation. Treatment with permethrin had no ef-
fect on general behaviour or condition, food intake, body weight gain,
or pregnancy rate of the dams, or on parturition, sex ratio, or pup
weight. A small number of rats of each group developed eye abnor-
malities, including ocular haemorrhage and chronic glaucoma, but this
was not related to the treatment. Examination of F3b fetuses showed
no treatment-related effect on sex ratio, body weight, or the occur-
rence of visceral or skeletal abnormalities. This study indicated that
permethrin (25:75) has no effect on the reproduction of rats at doses
up to 180 mg/kg body weight per day (James, 1979).
7.9 Neurotoxicity
7.9.1 Rat
When male and female Charles River rats (six of each sex per group)
were fed permethrin at dose levels of 0 or 6000 mg/kg diet for up to
14 days, severe clinical signs of poisoning were evident in all the
permethrin-treated rats. Only one permethrin-treated male survived the
14-day trial. Fragmented and swollen sciatic nerve axons and myelin
degeneration were observed in four out of five permethrin-treated ani-
mals (Hend & Butterworth, 1977).
In a short-term study designed to assess the effects of high con-
centrations of permethrin on the sciatic nerve, male Wistar rats (10
per group) were fed permethrin at dose levels of 0, 2500, 3000, 3750,
4500, 5000, and 7000 mg/kg diet for 14 days. Clinical signs of acute
poisoning and death occurred in the animals that were dosed at 5000 or
7000 mg/kg. Some rats that received the lower dose levels showed slight
to moderate tremors, and food consumption and growth were reduced in
these animals. At the two lowest dose levels, clinical signs of poison-
ing disappeared within the first week whereas, at the higher dose
levels, signs of poisoning persisted throughout the study. Rats
receiving permethrin at doses of up to 4500 mg/kg showed no ultra-
structural changes in their sciatic nerve. A variety of mild ultra-
structural changes, such as vacuolation and swelling of unmyelinated
fibres and hypertrophy of Schwann cells, were observed in the nerves of
rats receiving 5000 mg/kg (Glaister et al., 1977).
A detailed morphological evaluation of the nervous system was per-
formed on rats in two long-term feeding studies. In the first, Long-
Evans rats were fed diets containing permethrin at concentrations of 0,
20, 100, or 500 mg/kg diet for 2 years, and five male and five female
randomly selected survivors from each group were examined. In the
second study, Long-Evans rats were fed diets containing permethrin at
concentrations of 0, 20, or 100 mg/kg diet for three successive gener-
ations, and five male and five female rats from each group were ran-
domly selected from the third generation parental animals. Examination
of central and peripheral nerves and of extensive morphometric data and
teased myelinated fibers of distal sural and tibial nerves and of the
maxillary division of the fifth cranial nerve did not reveal any
changes attributable to the feeding of the pesticide (Dyck et al.,
1984).
When groups of 10 male and 10 female Sprague-Dawley rats were given
permethrin (25:75) (94.5% pure) at 4000, 6000, or 9000 mk/kg diet for
21 days, all animals developed severe trembling and lost weight. Some
of the highest-dose rats of each sex died. Subsequent examination of
brain, spinal cord, trigeminal and dorsal root ganglia, proximal and
distal root trunks, and terminal motor and sensory nerves revealed no
consistent histopathological abnormalities (Dayan, 1980).
7.9.2 Hen
Hens were administered permethrin orally (cis:trans=1:1) (as a 40%
w/v solution in dimethylsulfoxide) at a daily dose level of 1 g/kg body
weight for 5 days. After 3 weeks, the dosing regimen was repeated, and
the animals were maintained for an additional period of 3 weeks before
being sacrificed. There were no signs of neurological disturbance or
mortality in any of the animals. All hens treated with tri- ortho-cresyl phos-
phate (TOCP) (positive control) displayed clinical and histopathologi-
cal evidence of neurotoxicity, whereas none of the birds dosed with
permethrin showed any signs of intoxication. Histological examination
of nerve tissues revealed no lesions. Hence, permethrin was considered
to have no delayed neurotoxic potential such as that associated with
certain organophosphates (Millner & Butterworth, 1977).
In studies by Ross & Prentice (1977), 15 adult hens were orally
administered permethrin at 9 g/kg body weight and again 21 days later.
After a further 21 days, they were sacrificed. All positive control
animals (given TOCP at 500 mg/kg) showed signs of delayed neurotoxicity
ranging from slight muscular incoordination to paralysis. No signs of
ataxia were recorded in any of the hens in the permethrin-treated or
negative control groups. Histopathological examination of the nervous
tissues of permethrin-treated animals revealed none of the degenerative
changes noted in the tissues of the positive controls.
7.10 Behavioural Effects
Behavioural observations were carried out on immature male Sprague-
Dawley rats habituated to inhalation of permethrin aerosols. Habitu-
ation was carried out by exposing three groups of rats (five per group)
to aerosols of permethrin firstly at 500 mg/m3 for 21 days, then at
1000 mg/m3 for an additional 21 days. Three other groups of rats (five
per group) served as controls; they were similarly treated but were not
exposed to permethrin. At the end of this conditioning period, all
rats, including the controls, were exposed to a permethrin aerosol at
5000 mg/m3 for 4 h. At the end of the habituation period, there were
no differences in retention of avoidance training or the ability to
learn the same task between controls and aerosol-exposed groups. How-
ever, after exposure to permethrin at 5000 mg/m3, the non-habituated
control group of rats showed significantly lower retention capacity
compared with the habituated rats or with their own pre-exposure per-
formances. The non-habituated control rats also showed decreases in
coordination and balance and a higher incidence of conflict behaviour
and tremors. The performance of the rats in the habituated groups was
not changed (Sherman, 1979).
7.11 Miscellaneous Studies
The pharmacological action of permethrin on nictitating membrane,
blood pressure, respiration, heart rate, and isolated ileum was inves-
tigated in the rabbit, guinea-pig, and cat. Permethrin reduced the
incidence and amplitude of contraction of isolated rabbit ileum but
induced no changes in a similar preparation from the guinea-pig. Intra-
venous administration of permethrin at doses of 4 mg/kg or more affec-
ted blood pressure and respiration in all animals. The hypotensive
effect was not affected by pretreatment with atropine or propanolol.
Permethrin was shown to produce slight contraction of the nictitating
membrane. An increase in pulse rate was observed in the electrocardio-
gram (ECG) of the rabbit at dose levels above 4 mg/kg, but was not
accompanied by changes in the wave pattern (Nomura & Segawa, 1979).
In the Japanese White rabbit, the intravenous administration of
lethal doses of permethrin caused changes in the electroencephalogram
(EEG) tracings. Spike waves and an increased amplitude of slow waves
were induced at 100 mg/kg body weight. At a sub-lethal dose of 30
mg/kg, permethrin did not induce changes in the EEG (Takahashi et al.,
1979).
There was no change in hexobarbital-induced sleeping time in ICR
mice intraperitoneously administered a single dose of permethrin at
dose levels of up to 2000 mg/kg body weight (Takahashi et al., 1979).
Sprague-Dawley rats (three groups of 10 rats each) were pretreated
for four consecutive days with an intraperitoneal injection of either
sodium phenobarbital at 100 mg/kg (positive control group), permethrin
at 575 mg/kg (test group), or corn oil at 2.0 ml/kg (solvent control
group). On the fifth test day the rats received an intraperitoneal
injection of hexobarbital (220 mg/kg). The hexobarbital-induced sleep-
ing times of the permethrin-treated rats were significantly shorter
than those of the solvent control animals but were similar to those of
the phenobarbital positive control (Metker et al., 1977).
Permethrin and cypermethrin were evaluated for their ability to
alter microsomal cytochrome P-450 and NADPH cytochrome c reductase in
Long-Evans rats. When permethrin (cis:trans = 80:20) was orally admin-
istered to rats at 50 mg/kg body weight per day, it increased cyto-
chrome P-450 after 4, 8, or 12 days of administration and NADPH cyto-
chrome c reductase after 8 or 12 days, whereas cypermethrin (alpha-cyano
analogue of permethrin) did not induce either cytochrome P-450 or the
reductase. Neither of the two pyrethroids altered body weight gain
(Carlson & Schoening, 1980).
7.12 Mechanism of Toxicity (Mode of Action)
Based on the signs of toxicity to mammals (Verschoyle & Aldridge,
1980; Lawrence & Casida, 1982) and to cockroaches (Gammon et al.,
1981), pyrethroids may be classified into two types: Type I and Type II
compounds (see Appendix I). 1R- cis- and 1R- trans-permethrin belong
to Type I. Electrophysiological recordings from dosed cockroaches
reveal trains of cercal sensory spikes and, sometimes, spike trains
from the cercal motor nerves and the central nervous system. The signs
of poisoning caused by Type I pyrethroid compounds are restlessness,
incoordination, hyperactivity, prostration, and paralysis (Gammon et
al., 1981).
1RS- cis-permethrin and 1RS- trans-permethrin cause tremor (known
as T-syndrome) when injected intravenously into rats at a dose level of
more than 270 mg/kg body weight. The onset of the T-syndrome is usually
rapid. Rats suffering from T-syndrome exhibit aggressive sparring be-
haviour and increased sensitivity to external stimuli. This is followed
by the appearance of a slight tremor, which gradually becomes more
severe and finally reaches a state of prostration and vigorous whole
body tremor. The core temperature is markedly increased during poison-
ing; this may result from the excessive muscular activity associated
with tremor (Verschoyle & Aldridge, 1980).
Exposure of sensory nerve fibres from clawed frogs (Xenopus laevis)
to permethrin (10-7 to 10-5 mol/litre) resulted in marked repetitive
activity. This heightened activity was not observed in the motor
fibres of frogs that were similarly tested. Treatment of isolated
lateral-line preparations of frogs with permethrin (5 x 10-6 mol/litre)
also resulted in pronounced repetitive activity (Van den Bercken &
Vijverberg, 1980b).
Permethrin (cis, trans, and technical grade) and deltamethrin, as
representatives of the non-cyano- and cyano-containing classes,
respectively, of synthetic pyrethroids, were examined regarding their
major site of action on the mammalian nervous system in mice. ED50
values for the ability of both types of pyrethroids to cause pros-
tration and loss of righting reflex were estimated following either
intravenous or intracerebroventricular injections. The comparative
potencies of the four pyrethroids (deltamethrin > cis-permethrin >
technical grade permethrin > trans-permethrin) were the same following
either route of administration. All four compounds tested showed a much
greater potency (> 200-fold for deltamethrin, cis-permethrin, and
technical grade permethrin, and 85-fold for trans-permethrin) after
intracerebroventricular administration than after intravenous admin-
istration. In addition, the poisoning symptoms seen following direct
central injection were almost identical to those obtained with periph-
eral administration. These results suggest that poisoning from both
classes of pyrethroids in mammals is due predominantly to central
mechanisms (Staatz et al., 1982).
8. EFFECTS ON HUMANS
Although permethrin has been used for many years, no human poison-
ing cases have been reported.
8.1 Occupational Exposure
Data on permethrin human toxicity are scanty. Studies of occu-
pational exposure to permethrin were reported in Sweden (Kolmodin-
Hedman et al., 1982). In the first study, six forestry workers using
an aqueous emulsion of permethrin (trans:cis=75:25) were studied. The
duties of these workers involved dipping conifer seedlings in a 2%
aqueous emulsion of permethrin (one worker) and packaging the
permethrin-treated seedlings (five workers). The permethrin concen-
trations in the breathing zones of these workers varied between 0.011
and 0.085 mg/m3. One person excreted permethrin metabolites at
0.26 µg/ml urine the following morning, but the same afternoon the
urinary excretion of permethrin residues was below the detection limit
of 0.1 µg/ml. The urine from other workers did not contain detectable
amounts of permethrin residues. No symptoms of permethrin poisoning
were reported in this field study. The second study, performed on the
basis of a questionnaire and interviews, was conducted 1-2 months after
the planting season. It involved 87 persons at various plant nurseries
that used the insecticide (trans:cis=60:40 or 75/25). This study showed
that the major work-related symptoms amongst workers were irritative,
such as itching and burning of the skin, and itching and irritation of
the eyes. Irritative symptoms in the skin and upper respiratory tract
were reported in 63% of workers who were exposed to permethrin
(trans:cis=75:25) and 33% who were exposed to permethrin with a
different isomeric composition (trans:cis=60:40). The frequency of each
symptom was about 10% in each case. Increased nasal secretion was
reported by 13% of the workers handling permethrin (trans:cis= 75:25).
Le Quesne et al. (1980) examined 23 laboratory workers involved in
field trials, formulation, or general laboratory work with permethrin,
cypermethrin, fenvalerate, and fenpropathrin. The study involved
electrophysiological monitoring and interviews to ascertain subjective
symptoms. The most frequently reported symptom was a facial sensation
described as tingling, burning, or nettle rash by workers who had ex-
perienced it on one or more occasions. This sensation usually occurred
about 30 min to 3 h after exposure and lasted for about 30 min to 8 h.
Apparently this did not occur when permethrin alone was involved. All
the workers were examined neurologically and no abnormal findings were
recorded. Electro-physiological measurements from these workers were
compared with those of an age-matched control group. No difference in
response was found between the two groups.
Studies of pesticide contamination of clothing worn by crop con-
sultants during permethrin application to soybean fields were performed
to assess the extent of dermal exposure to the pesticide. The suits
and T-shirts were removed and wrapped in aluminum foil, placed on ice
and transported to the laboratory where they were held in a freezer
until residue analysis was performed. Specimens from the thigh, arm,
and chest of each suit and from the arm and chest of each T-shirt were
collected, extracted with hexane and analyzed by GC-ECD. Measurable
residues of permethrin were detected only in leg specimens (Cloud et
al., 1987).
As part of an evaluation of permethrin (25:75) (5% wettable powder)
in Nigeria, medical surveillance, including urinalysis of staff engaged
in bagging, mixing, or spraying, was undertaken. Medical history,
pulse, and blood pressure were recorded and urine was collected twice
daily. This surveillance revealed no effects attributable to permeth-
rin. Despite the use of protective clothing, up to 2 mg of permethrin
was excreted within 24 h of exposure (Rishikesh et al., 1978).
8.2 Clinical Studies
Flannigan & Tucker (1985) and Flannigan et al. (1985a,b) studied
the difference in the degree of paraesthesia induced by a number of
pyrethroids. On five occasions, 0.05 ml of field-strength-formulated
permethrin (0.13 mg/cm2) was applied to a 4 cm2 area of earlobe.
The opposite earlobe received distilled water. Participant evaluation
after each application continued for 48 h and involved description of
the cutaneous sensations. Each participant was treated after each
application with one of the remaining compounds. Permethrin, like the
other pyrethroids, induced skin sensations. Paraesthesia developed with
a latency period of approximately 30 min, peaked by 8 h, and deterio-
rated within 24 h. In the case of permethrin these sensations were
approximately four times less marked than those induced by cypermethrin
and fenvalerate, which both contain an alpha-cyano-group. It was also
found that local application of dl-alpha-tocopheryl acetate markedly
inhibited the occurrence of skin sensations.
To assess the safety of permethrin dusts for the control of human
body lice, approximately 350 people were individually dusted with 50 g
of powder containing either 2.5 or 5.0 g permethrin/kg. Urine samples,
taken at the time of treatment and subsequently, indicated that maximal
absorption of permethrin was 39 µg/kg, 24 h after treatment (Nassif
et al., 1980).
In a study to assess the degree of dermal absorption of permethrin
from impregnated clothing, a group of 10 male volunteer soldiers for
48 h wore military clothing that had previously been treated with an
aqueous suspension of permethrin (0.2% w/v). Subsequent analysis
showed that the mean permethrin (25:75) concentration of the shirts
and trousers was 0.32 g/100 g. However, the average individual
exposure to permethrin was 3.8 mg/day. No volunteers complained of
irritation and there were no abnormal findings on physical examination
(Farquhar et al., 1981a).
When dermally exposed to permethrin [(25:75) 1% w/w in soft
paraffin] for up to 9 days using a patch test, 2 out of 17 volunteers
developed mild erythema (Pegum & Doughty, 1978).
To assess the human tolerance, absorption, and persistence of
permethrin when used against human lice, 10 adult volunteers (four men,
six women) were treated with 15-40 ml of permethrin (25:75) (1%) head
louse solution. Their hair was allowed to dry naturally and then
washed with baby shampoo. Urine samples were collected at 0-24, 24-48,
120-144, and 336-360 h to measure dermal absorption. On assessment, 3
out of 10 volunteers developed mild, patchy erythema, which faded
between days 4-7. Permethrin excretion during the first 24 h was only
about 1% of the applied dose, while the cumulative maximum over 14 days
was only about 5.5 mg (Farquhar et al., 1981b).
9. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
The Joint FAO/WHO Meeting on Pesticide Residues (JMPR) discussed
and evaluated permethrin in 1979, 1980, 1981, 1982, 1983, 1984, 1985,
and 1987 (FAO/WHO, 1980a,b; 1981a,b; 1982a,b; 1983a,b; 1984a,b;
1985a,b; 1986a,b; 1987).
An acceptable daily intake (ADI) of 0-0.05 mg/kg body weight
(cis:trans ratios of 40:60 and 27:75) was established in 1985.
Maximum residue limits of 0.01-50 mg/kg for specified foods and 20-
100 mg/kg dry weight for specified feed have been proposed (FAO/WHO,
1986).
In the WHO recommended classification of pesticides by hazard,
permethrin as a technical product is classified in class II (i.e., as
moderately hazardous) (WHO, 1988). A WHO/FAO data sheet on permethrin
exists (WHO/FAO, 1984).
REFERENCES
AGNIHOTRI, N.P., JAIN, H.K., & GAJBHIYE, V.T. (1986) Persistence of
some synthetic pyrethroid insecticides in soil, water and sediment -
Part I. J. entomol. Res., 10: 147-151.
ALINIAZEE, M.T. & CRANHAM, J.E. (1980) Effect of four synthetic
pyrethroids on a predatory mite, Typhlodromus pyri, and its prey,
Panonychus ulmi, on apples in southeast England. Environ. Entomol., 9:
436-439.
ANDERSON, D. & RICHARDSON, C.R. (1976) Permethrin (PP557):
cytogenetic study in the rat (Report No. CTL/P/296) (Unpublished
report submitted to WHO by ICI Central Toxicology Laboratory).
ANDERSON, R.L. (1982) Toxicity of fenvalerate and permethrin to
several nontarget aquatic invertebrates. Environ. Entomol., 11: 1251-
1257.
BAKER, P.G. & BOTTOMLEY, P. (1982) Determination of residues of
synthetic pyrethroids in fruit and vegetables by gas-liquid and high-
performance liquid chromatography. Analyst, 107: 206-212.
BATTELLE (1982) Pesticide programme of research and market planning.
Part I: Insecticides, Geneva, Institut Battelle (October 1982).
BATTELLE (1986) World Pesticide Programme, Insecticide II, 1984,
Geneva, Institut Battelle.
BILLUPS, L.H. (1978a) Histopathologic examination of a twenty-four
month toxicity/carcinogenicity study of compound FMC33297 in rats, (Un-
published report submitted to WHO by FMC Corporation, Environmental
Pathology Services).
BILLUPS, L.H. (1978b) Twenty-four month toxicity/carcinogenicity
study of compound FMC33297 in rats (Unpublished report submitted to
WHO by FMC Corporation, Environmental Pathology Services).
BIO-DYNAMICS (1979) Twenty-four month toxicity/carcinogenicity study
of permethrin in mice (Bio-Dynamics Inc. Project No. 76-1695/9)
(Unpublished report submitted to WHO by FMC Corporation).
BORTHWICH, P.W. & WALSH, G.E. (1981) Initial toxicological assessment
of Ambush, Bolero, Bux, Dursban, Fentrifanil, Larvin, and Pydrin:
Static acute toxicity tests with selected estuarine algae,
invertebrates, and fish, Springfield, Virginia, National Technical
Information Service, pp. 1-9 (EPA-600/4-81-076).
BRADBROOK, C., BANHAM, P.B., GORE, C.W., PRATT, I., & WEIGHT,
T.M. (1977) PP557: A study of the reversibility of hepatic
biochemical and ultrastructural changes in the rat (Report No.
CTL/P/354) (Unpublished Data submitted to WHO by ICI Central Toxicology
Laboratory).
BRAUN, W.G. & KILLEEN, J.C. (1975) Acute oral toxicity in rat:
compound no. FMC33297 (Bio-Dynamics Inc. Project) (Unpublished report
submitted to WHO by FMC Corporation).
BRAUN, W.G. & RINEHART, W.E. (1977) A twenty-four month oral
toxicity/carcinogenicity study of FMC33297 in rats (Bio-Dynamics Inc.
Project) (Unpublished report submitted to WHO by FMC Corporation).
BRAUN, H.E. & STANEK, J. (1982) Application of the AOAC multi-residue
method to determination of synthetic pyrethroid residues in celery and
animal products. J. Assoc. Off. Anal. Chem., 65: 685-689.
BUTTERWORTH, S.T.G. & HEND, R.W. (1976) Toxicity studies on the
insecticide WL43470: a five week feeding study in rats (Report No.
TLGR.0056.76) (Unpublished data submitted to WHO by Shell Research
Ltd).
CARLSON, G.P. & SCHOENIG, G.P. (1980) Induction of liver microsomal
NADPH cytochrome c reductase and cytochrome P-450 by some new synthetic
pyrethroids. Toxicol. appl. Pharmacol., 52: 507-512.
CARROLL, B.R., WILLIS, G.H., & GRAVES, J.B. (1981) Permethrin
concentration of cotton plants, persistence in soil and loss in runoff.
J. environ. Qual., 10: 497-500.
CHESHER, B.C. & MALONE, J.C. (1974a) 10-day cumulative oral toxicity
study with 21Z73 in rabbits, Berkhamsted, Wellcome Research
Laboratories (Report No. HEFG 74-7) (Unpublished data submitted to
WHO).
CHESHER, B.C. & MALONE, J.C. (1974b) Guinea pig sensitization study
with 21Z73 using the maximisation test method, Berkhamsted, Wellcome
Research Laboratories (Report No. HEFG 74-3) (Unpublished data
submitted to WHO).
CHESHER, B.C. & MALONE, J.C. (1974c) Occular irritancy of 21Z73 in
rabbits, Berkhamsted, Wellcome Research Laboratories (Report no. HEFG
74-6) (Unpublished data submitted to WHO).
CHESHER, B.C., CLAMPITT, R.C., & MALONE, J.C. (1975) 21Z73 -
Preliminary investigations into the cumulative oral toxicity on dogs,
Berkhamsted, Wellcome Research Laboratories (Report no. HEFG 75-9)
(Unpublished data submitted to WHO).
CLAPP, M.J.L., BANHAM, P.B., CHART, I.S., GLAISTER, J., GORE, C., &
MOYES, A. (1977a) PP557: 28 day feeding study in rats (Report No.
CTL/P/355) (Unpublished data submitted to WHO by ICI Central Toxicology
Laboratory).
CLAPP, M.J.L., BANHAM, P.B., GLAISTER, J.R., & MOYES, A. (1977b)
PP557: 28 day feeding study in mice (Report No. CTL/P/354)
(Unpublished data submitted to WHO by ICI Central Toxicology
Laboratory).
CLARK, D.G. (1978) Toxicology of WL 43479: acute toxicity of WL43479,
Sittingbourne, Shell Research Ltd (Report No. TLGR. 0043.78)
(Unpublished data submitted to WHO).
CLIVE, C.D. (1977) Mutagenicity of BW21Z73 in L5178Y/TK mouse
lymphoma cells with and without exogenous metabolic activation, Re-
search Triangle Park, NC, Burroughs Wellcome Co. (Report No.
TTEP/77/0011) (Unpublished data submitted to WHO).
CLOUD, R.M., ZIMPFER, M.L., YANES, J.JR., BOETHEL, D.J., BUCO, P.,
& HARMON, C.W. (1987) Insecticide residues on clothing worn by crop
consultants in soybean fields treated with non-conventional application
technology. Bull. environ. Contam. Toxicol., 38: 277-282.
COATS, J.R. & O'DONNELL-JEFFERY, N.L. (1979) Toxicity of four
synthetic pyrethroid insecticides to rainbow trout. Bull. environ.
Contam. Toxicol., 23: 250-255.
COX, R.L. & WILSON, W.T. (1984) Effects of permethrin on the behavior
of individually tagged honey bees, Apis mellifera L. (Hymenoptera:
Apidae). Environ. Entomol., 13: 375-378.
CRIDLAND, J.S. & WEATHERLEY, B.C. (1977a) Urinary excretion in man of
3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropane carboxylic acid ("CVA")
after oral ingestion of permethrin (NRDC 143) - A first report, Bech-
enham, Wellcome Research Laboratories (Report No. BDPE-77-1)
(Unpublished data submitted to WHO).
CRIDLAND, J.S. & WEATHERLEY, B.C. (1977b) An estimate of permethrin
(NRDC 143; OMS1821) absorbed by people employed in a field trial of the
insecticide, Bechenham, Wellcome Research Laboratories (Report No.
BDPE-77-3) (Unpublished data submitted to WHO).
DAYAN, A.D. (1980) 21-day neuropathological study in the Sprague-
Dawley rat of permethrin (212732J) administered in the diet,
Berkhamsted, Wellcome Research Laboratories (Report No. BP 80-48)
(Unpublished report submitted to WHO by Wellcome Foundation Ltd).
DOYLE, R.D., KAUFMAN, D.D., BURT, G.W., & DOUGLASS, L. (1981)
Degradation of cis-permethrin in soil amended with sewage sludge or
dairy manure. J. agric. food Chem., 29: 412-414.
DYCK, P.J., SHIMONO, M., SCHOENING, G.P., LAIS, A.C., OVIATT, K.F.,
& SPARKS, M.F. (1984) The evaluation of a new synthetic pyrethroid
pesticide (permethrin) for neurotoxicity. J. environ. Pathol. Toxicol.
Oncol., 5: 109-117.
EDWARDS, D.B., OSBORN, B.E., DENT, N.J., & KINCH, D.A. (1976)
Toxicity study in beagle dogs (oral administration for three months),
Inveresk, United Kingdom, Inveresk Research International Ltd
(Submitted to WHO by ICI Ltd).
EDWARDS, M.J. & ISWARAN, T.J. (1977) Permethrin: residue transfer and
toxicology study with cows fed treated grass nuts (Report No.
TMJ1519/B) (Unpublished report submitted to WHO by ICI Plant Protection
Division).
ELLIOTT, M. (1977) Synthetic pyrethroids, Washington, DC, American
Chemical Society, p. 229 (ACS Symposium Series 42).
ELLIOTT, M., FARNHAM, A.W., JANES, N.F., NEEDHAM, P.H.,
PULMAN, D.A., & STEVENSON, J.H. (1973) A photostable pyrethroid.
Nature (Lond.), 246: 169-170.
ELLIOTT, M., JANES, N.F., PULMAN, D.A., GAUGHAN, L.C., UNAI, T.,
& CASIDA, J.E. (1976) Radiosynthesis and metabolism in rats of the 1R-
isomers of the insecticide permethrin. J. agric. food Chem., 24: 270-
276.
EVANS, M.H. (1976) End-plate potentials in frog muscle exposed to a
synthetic pyrethroid Pestic. Biochem. Physiol., 6: 547-550.
EVERTS, J.W., KORTENHOFF, B.A., HOOGLAND, H., VLUG, H.J.,
JOCQUE, R., & KOEMAN, J.H. (1985) Effects on non-target terrestrial
arthropods of synthetic pyrethroids used for the control of the tsetse
fly (Glossina spp.) in settlement areas of the southern Ivory Coast,
Africa. Arch. environ. Contam. Toxicol., 14: 641-650.
FAO (1982) Second Government Consultation on International
Harmonization of Pesticide Registration Requirements, Rome, 11-15
October 1982, Rome, Food and Agriculture Organization of the United
Nations.
FAO/WHO (1980a) Pesticide residues in food. Report of the 1979 Joint
Meeting of the FAO Panel of Experts on Pesticide Residues in Food and
the Environment and the WHO Expert Group on Pesticide Residues, Rome,
Food and Agriculture Organization of the United Nations, pp. 52-55 (FAO
Plant Production and Protection Paper 20).
FAO/WHO (1980b) 1979 Evaluations of some pesticide residues in food,
Rome, Food and Agriculture Organization of the United Nations,
pp. 369- 425 (FAO Plant Production and Protection Paper 20 Sup).
FAO/WHO (1981a) Pesticide residues in food. Report of the 1980 Joint
Meeting of the FAO Panel of Experts on Pesticide Residues in Food and
the Environment and the WHO Expert Group on Pesticide Residues, Rome,
Food and Agriculture Organization of the United Nations, pp. 50-51 (FAO
Plant Production and Protection Paper 26).
FAO/WHO (1981b) 1980 Evaluations of some pesticide residues in
food, Rome, Food and Agriculture Organization of the United Nations,
pp. 360-393 (FAO Plant Production and Protection Paper 26 Sup).
FAO/WHO (1982a) Pesticide residues in food. Report of the 1981
Joint Meeting of the FAO Panel of Experts on Pesticide Residues in Food
and the Environment and the WHO Expert Group on Pesticide Residues,
Rome, Food and Agriculture Organization of the United Nations, pp.
37-39 (FAO Plant Production and Protection Paper 37).
FAO/WHO (1982b) 1981 Evaluations of some pesticide residues in food,
Rome, Food and Agriculture Organization of the United Nations,
pp. 385-425 (FAO Plant Production and Protection Paper 42).
FAO/WHO (1983a) Pesticide residues in food. Report of the 1982 Joint
Meeting of the FAO Panel of Experts on Pesticide Residues in Food
and the Environment and the WHO Expert Group on Pesticide Residues,
Rome, Food and Agriculture Organization of the United Nations, pp. 36-
38 (FAO Plant Production and Protection Paper 46).
FAO/WHO (1983b) 1982 Evaluations of some pesticide residues in food,
Rome, Food and Agriculture Organization of the United Nations,
pp. 329-350 (FAO Plant Production and Protection Paper 49).
FAO/WHO (1984a) Pesticide residues in food. Report of the 1983
Joint Meeting of the FAO Panel of Experts on Pesticide Residues in Food
and the Environment and the WHO Expert Group on Pesticide Residues,
Rome, Food and Agriculture Organization of the United Nations, p. 38,
57, 62, 65 (FAO Plant Production and Protection Paper 56).
FAO/WHO (1984b) 1983 Evaluations of some pesticide residues in food,
Rome, Food and Agriculture Organization of the United Nations,
pp. 307-313, 501 (FAO Plant Production and Protection Paper 61).
FAO/WHO (1985a) Pesticide residues in food. Report of the 1984 Joint
Meeting of the FAO Panel of Experts on Pesticide Residues in Food and
the Environment and the WHO Expert Group on Pesticide Residues, Rome,
Food and Agriculture Organization of the United Nations, pp. 63, 85, 93
(FAO Plant Production and Protection Paper 62).
FAO/WHO (1985b) 1984 Evaluations of some pesticide residues in food,
Rome, Food and Agriculture Organization of the United Nations,
pp. 441-444, 723 (FAO Plant Production and Protection Paper 67).
FAO/WHO (1985c) Guide to Codex recommendations concerning pesticide
residues. Part 8. Recommendations for methods of analysis of pesticide
residues, 3rd ed., Rome, Codex Committee on Pesticide Residues.
FAO/WHO (1986a) Pesticide residues in food. Report of the 1985 Joint
Meeting of the FAO Panel of Experts on Pesticide Residues in Food and
the Environment and the WHO Expert Group on Pesticide Residues, Rome,
Food and Agriculture Organization of the United Nations, p. 37 (FAO
Plant Production and Protection Paper 68).
FAO/WHO (1986b) 1985 Evaluations of some pesticide residues in food,
Part I - Residues, Rome, Food and Agriculture Organization of the
United Nations, pp. 201-203 (FAO Plant Production and Protection Paper
No 72/1).
FAO/WHO (1986c) Codex Maximum Limits for pesticide residues, 2nd ed.,
Rome, Food and Agriculture Organization of the United Nations, Joint
FAO/WHO Food Standards Programme, Codex Alimentarius Commission,
(CAC XIII).
FAO/WHO (1987) Pesticide residues in food. Report of the 1987 Joint
Meeting of the FAO Panel of Experts on Pesticide residues in Food and
the Environment and the WHO Expert Group on Pesticide Residues, Rome,
Food and Agriculture Organization of the United Nations, p. 33 (FAO
Plant Production and Protection Paper 84).
FAO/WHO (1988) Permethrin. In: Pesticide residues in food. Part II -
Toxicology, Rome, Food and Agriculture Organization of the United
Nations, pp. 101-110 (FAO Plant Production and Protection Paper 86/2).
FARQUHAR, J.A., HUTCHINSON, D.B.A., PERIAM, A.W., & SPARKES,
R.G. (1981a) An investigation into the absorption of permethrin from
impregnated clothing, Bechenham, Wellcome Research Laboratories (Report
No. BKDL/81/1) (Unpublished data submitted to WHO).
FARQUHAR, J.A., HUTCHINSON, D.B.A., PERIAM, A.W., & SPARKES,
R.G. (1981b) An investigation of tolerance to absorption of and
persistence of permethrin applied as a 1% lotion to human hair,
Bechenham, Wellcome Research Laboratories (Report No. BKDL/81/2)
(Unpublished data submitted to WHO).
FLANNIGAN, S.A. & TUCKER, S.B. (1985) Variation in cutaneous
sensation between synthetic pyrethroid insecticides. Contact Derma-
titis, 13: 140-147.
FLANNIGAN, S.A., TUCKER, S.B., MARCUS, M.K., ROSS, C.E.,
FAIRCHILD, E.J., GRIMES, B.A., & HARRIST, R.B. (1985a) Primary
irritant contact dermatitis from synthetic pyrethroid insecticide
exposure. Arch. Toxicol., 56, 288-294.
FLANNIGAN, S.A., TUCKER, S.B., KEY, M.M., ROSS, C.E., FAIRCHILD,
E.J., GRIMES, B.A., & HARRIST, R.B. (1985b) Synthetic pyrethroid
insecticides: a dermatological evaluation. Br. J. ind. Med., 42: 363-
372.
FRIESEN, M.K., GALLOWAY, T.D., & FLANNAGAN, J.F. (1983) Toxicity
of the insecticide permethrin in water and sediment to nymphs of the
burrowing mayfly Hexagenia rigida (Ephemeroptera: Ephemeridae). Can.
Entomol., 115: 1007-1014.
GAMBRELL, R.P., REDDY, C.N., COLLARD, V., GREEN, G., & PATRICK,
W.H., Jr. (1981) Behavior of DDT, kepone and permethrin in sediment-
water systems under different oxidation-reduction and pH conditions,
Washington, DC, US Environmental Protection Agency (EPA 600/3-81-
038).
GAMMON, D.W. & CASIDA, J.E. (1983) Pyrethroids of the most potent
class antagonize GABA action at the crayfish neuromuscular junction.
Neurosci. Lett., 40: 163-168.
GAMMON, D.W., BROWN, M.A., & CASIDA, J.E. (1981) Two classes of
pyrethroid action in the cockroach. Pestic. Biochem. Physiol., 15:
181-191.
GAMMON, D.W., LAWRENCE, L.J., & CASIDA, J.E. (1982) Pyrethroid
toxicology: Protective effects of Diazepam and phenobarbital in the
mouse and the cockroach. Toxicol. appl. Pharmacol., 66: 290-296.
GAUGHAN, L.C. & CASIDA, J.E. (1978) Degradation of trans- and cis-
permethrin on cotton and bean plants. J. agric. food Chem., 26: 525-
528.
GAUGHAN, L.C., UNAI, T., & CASIDA, J.E. (1977) Permethrin metabolism
in rats. J. agric. food Chem., 25: 9-17.
GAUGHAN, L.C., ROBINSON, R.A., & CASIDA, J.E. (1978b) Distribution
and metabolic fate of trans- and cis-permethrin in laying hens. J.
agric. food Chem., 26: 1374-1380.
GAUGHAN, L.C., ACKERMAN, M.E., UNAI, T., & CASIDA, J.E. (1978a)
Distribution and metabolism of trans- and cis-permethrin in lactating
Jersey cows. J. agric. food Chem., 26: 613-618.
GERIG, L. (1985) Testing the toxicity of synthetic pyrethroid insecti-
cides to bees. Pestic. Sci., 16: 206-207.
GLAISTER, J.R., PRATT, I., & RICHARDS, D. (1977) Effects of high
dietary levels of PP557 on clinical behaviour and structure of sciatic
nerves in rats (Report No. CTL/P/317) (Unpublished data submitted to
WHO by ICI Central Toxicology Laboratory).
GLICKMAN, A.H. & CASIDA, J.E. (1982) Species and structural variations
affecting pyrethroid neurotoxicity. Neurobehav. Toxicol. Teratol., 4:
(6) 793-799.
GLICKMAN, A.H. & LECH, J.J. (1981) Hydrolysis of permethrin, a
pyrethroid insecticide, by rainbow trout and mouse tissues in vitro : A
comparative study. Toxicol. appl. Pharmacol., 60: 186-192.
GLICKMAN, A.H., SHONO, T., CASIDA, J.E., & LECH, J.J. (1979) In vitro
metabolism of permethrin isomers by carp and rainbow trout liver
microsomes. J. agric. food Chem., 27: 1038-1041.
GLICKMAN, A.H., HAMID, A.AR., RICKERT, D.E., & LECH, J.J. (1981)
Elimination and metabolism of permethrin isomers in rainbow trout.
Toxicol. appl. Pharmacol., 57: 88-98.
GLICKMAN, A.H., WEITMAN, S.D., & LECH, J.J. (1982) Different toxicity
of trans-permethrin in rainbow trout and mice. I. Role of biotransfor-
mation. Toxicol. appl. Pharmacol., 66: 153-161.
HAGLEY, E.A.C., PREE, D.J., & HOLLIDAY, N.J. (1980) Toxicity of
insecticides to some orchard carabids (Coleoptera carabidae). Can. Entomol.,
112: 457-462.
HALLS, G.R.H. (1981) The fate of permethrin residues on wheat during 9
months storage in Australia (Report No. HEFH 81-1) (Unpublished data
submitted to WHO by Wellcome Research Laboratories).
HALLS, G.R.H. & PERIAM, A.W. (1980) The fate of permethrin residues on
wheat during storage and after milling and baking - results after 9, 12
and 15 months storage (Report HEFH 80-3) (Unpublished data submitted to
WHO by Wellcome Research Laboratories).
HANSEN, D.J., GOODMAN, L.R., MOORE, J.C., & HIGDON, P.K. (1983)
Effects of the synthetic pyrethroids AC 222, 705, permethrin and
fenvalerate on sheepshead minnows in early life stage of toxicity
tests. Environ. Toxicol. Chem., 2: 251-258.
HART, D., BANHAM, P.B., GORE, C.W., PRATT, I., & WEIGHT, T.M.
(1977c) PP557: Liver hypertrophy study in rats-dietary administration
over 26 weeks (Report No. CTL/P/360) (Unpublished data submitted to WHO
by ICI Central Toxicology Laboratory).
HELSON, B.V., KINGSBURY, P.D., & DE GROOT, P. (1986) The use of
bioassays to assess aquatic arthropod mortality from permethrin drift
deposits. Aquat. Toxicol., 9: 253-262.
HEND, R.W. & BUTTERWORTH, S.T.G. (1977) Toxicity of insecticides: a
short-term feeding study of WL 43379 in rats (Report No. TLGR.0108.77)
(Unpublished data submitted to WHO by Shell Research Ltd).
HENRIET, J., MARTIJN, A., & POLVISEN, H.H. (1985) CIPAC Handbook.
Volume 1C: Analysis of technical and formulated pesticides, Hertford-
shire, Collaborative International Pesticides Analytical Council Ltd,
pp. 2172-2173.
HILL, E.F. & CAMARDESE, M.B. (1986) Lethal dietary toxicities of
environmental contaminants and pesticides to Coturnix, Washington, DC,
United States Department of the Interior, Fish and Wildlife Service,
p.111 (Fish and Wildlife Technical Report No. 2).
HODGE, M.C.E., BANHAM, P.B., GLAISTER, J.R., RICHARDS, D.,
TAYLOR, K., & WEIGHT, T.M. (1977) PP557: Three generation reproduction
study in rats (Unpublished report submitted to WHO by ICI Central
Toxicology Laboratory).
HOGAN, G.K. & RINEHART, W.E. (1977) A twenty-four month oral
carcinogenicity study of FMC 33297 in mice (Bio-Dynamics Inc. Project)
(Unpublished report submitted to WHO by FMC Corporation).
HOLDWAY, D.A. & DIXON, D.G. (1988) Acute toxicity of permethrin or
glyphosate pulse exposure to larval white sucker (Catostomus commer-
soni) and juvenile flagfish (Jordanella floridae) as modified by age
and ration level. Environ. Toxicol. Chem., 7: 63-68.
HOLMSTEAD, R.L., CASIDA, J.E., RUZO, L.O., & FULLER, D.G. (1978)
Pyrethroid photodecomposition: Permethrin. J. agric. food Chem., 26:
590-595.
HORIBA, M., KOBAYASHI, A., & MURANO, A. (1977) Gas-liquid
chromatographic determination of a new pyrethroid, permethrin (S-3151)
and its optical isomers. Agric. biol. Chem., 41: 581-586.
HOY, M.A., FLAHERTY, D., PEACOCK, W., & CULVER, D. (1979)
Vineyard and laboratory evaluations of methomyl, dimethoate, and
permethrin for a grape pest management program in the San Joaquin
Valley of California. J. econ. Entomol., 72: 250-255.
HOYT, S.C., WESTIGARD, P.H., & BURTS, E.C. (1978) Effects of two
synthetic pyrethroids on the codling moth, pear psylla, and various
mite species in northwest apple and pear orchards. J. econ. Entomol.,
71: 431-434.
HUNT, L.M. & GILBERT, B.N. (1977) Distribution and excretion rates of
14C-labelled permethrin isomers administered orally to four lactating
goats for 10 days. J. agric. food Chem., 25: 673-676.
HUNT, L.M., GILBERT, B.N., & LEMEILLEUR, C.A. (1979) Distri-
bution and depletion of radioactivity in hens treated dermally with
14C-labelled permethrin. Poult. Sci., 58: 1197-1201.
ISHMAEL, J. & LITCHFIELD, M.H. (1988) Chronic toxicity and
carcinogenic evaluation of permethrin in rats and mice. Fundam. appl.
Toxicol., 11: 308-311.
IVIE, G.W. & HUNT, L. (1980) Metabolites of cis- and trans-permethrin
in lactating goats. J. agric. food Chem., 28: 1131-1138.
JAGGERS, S.E. & PARKINSON, G.R. (1979) Permethrin: summary and review
of acute toxicities in laboratory species (Report No. CTL/P/461)
(Unpublished data submitted to WHO by ICI Central Toxicology
Laboratory).
JAMES, J.A. (1974) Fetal toxicity study of 21Z73 (NRDC 143) in the
rat (Report No. BPAT 74-10) Bechenham, Wellcome Research Laboratories
(Unpublished data submitted to WHO).
JAMES, J.A., (1979) A multigeneration reproduction study of 21Z73
(permethrin) in the rat (Report No. BPAT 79-3) Bechenham, Wellcome
Research Laboratories (Unpublished data submitted to WHO).
JAMES, J.A., TAYLOR, P.E., & ROE, F.J.C. (1980) Carcinogenicity study
in mice with permethrin, Bechenham, Wellcome Research Laboratories,
(Report No. HEFG 80-29) (Unpublished data submitted to WHO).
JOLLY, A.L., Jr., AVAULT, J.W., Jr., KOONCE, K.L., & GRAVES, J.B.
(1978) Acute toxicity of permethrin to several aquatic animals. Trans.
Am. Fish. Soc., 107: 825-827.
JORDAN, E.G. & KAUFMAN, D.D. (1986) Degradation of cis- and trans-
permethrin in flooded soil. J. agric. food Chem., 34: 880-884.
JORDAN, E.G., KAUFMAN, D.D., & KAYSER, A.J. (1982) The effect of soil
temperature on the degradation of cis-, trans-permethrin in soil. J.
environ. Sci. Health, B 17: 1-17.
KADOTA, T., MIYAMOTO, J., & ITO, N. (1975) Six-month subacute oral
toxicity of NRDC in Sprague-Dawley rats (Unpublished data submitted to
WHO by Sumitomo Chemical Co.).
KANEKO, H., OHKAWA, H., & MIYAMOTO, J. (1978) Degradation and
movement of permethrin isomers in soil. J. Pestic. Sci., 3: 43-51.
KAUFMAN, D.D., HAYES, S.C., JORDAN, E.G., & KAYSER, A.J. (1977)
Permethrin degradation in soil and microbial cultures. In: Elliott, M.,
ed. Synthetic pyrethroids, Washington, DC, American Chemical Society,
pp. 147-161 (ACS symposium series 42).
KAUFMAN, D.D., RUSSEL, B.A., HELLING, C.S., & KAYSER, A.J. (1981)
Movement of cypermethrin, deltamethrin, permethrin and their
degradation products in soil. J. agric. food Chem., 29: 239-245.
KAUSHIK, N.K., STEPHENSON, G.L., SOLOMON, K.R., & DAY, K.E.
(1985) Impact of permethrin on zooplankton communities in
limnocorrals. Can. J. Fish. aquat. Sci., 42: 77-85.
KILLEEN, J.C. & RAPP, W.R. (1976a) A three month oral toxicity study
of FMC 33297 in Beagle dogs (Bio-Dynamics Inc. Project) (Unpublished
report submitted to WHO by FMC Corporation).
KILLEEN, J.C. & RAPP, W.R. (1976b) A three month oral toxicity study
of FMC 33297 in rats (Bio-Dynamics Inc. Project) (Unpublished report
submitted to WHO by FMC Corporation).
KINGSBURY, P.D. (1976) Effects of an aerial application of the syn-
thetic pyrethroid permethrin on a forest stream. Manitoba Entomol., 10:
9-17.
KINGSBURY, P.D. & KREUTZWEISER, D.P. (1980a) Environmental impact
assessment of a semi-operational permethrin application. Sault Ste.
Marie, Ontario, Canada, Forest Pest Management Institute. Report No.
FPM-X 30: pp.47.
KINGSBURY, P.D. & KREUTZWEISER, D.P. (1980b) Dosage-effect studies
on the impact of permethrin on trout streams. Sault Ste. Marie,
Ontario, Canada, Forest Pest Management Institute Report No. FPM-X 31.
KINGSBURY, P.D. & KREUTZWEISER, D.P. (1987) Permethrin treatments in
Canadian forests. Part 1: Impact on fish. Pestic. Sci., 19: 35-48.
KOHDA, H., KADOTA, T., & MIYAMOTO, J. (1976a) Teratogenic evaluation
with permethrin in rats (Unpublished report submitted to WHO by
Sumitomo Chemical Co.).
KOHDA, H., KADOTA, T., & MIYAMOTO, J. (1976b) Teratogenic evaluation
with permethrin in mice (Unpublished report submitted to WHO by
Sumitomo Chemical Co.).
KOHDA, H., KADOTA, T., & MIYAMOTO, J. (1979a) Acute oral, dermal and
subcutaneous toxicities of permethrin in rats and mice (Unpblished
report submitted to WHO by Sumitomo Chemical Co.).
KOHDA, H., KANEDO, H., OHKAWA, H., KADOTA, T., & MIYAMOTO, J.
(1979b) Acute intraperitoneal toxicity of permethrin metabolites in
mice (Unpublished report submitted to WHO by Sumitomo Chemical Co.).
KOLMODIN-HEDMAN, B., SWENSSON, A., & AKERBLOM, M. (1982)
Occupational exposure to some synthetic pyrethroids (permethrin and
fenvalerate). Arch. Toxicol., 50: 27-33.
KREUTZWEISER, D.P. (1982) The effects of permethrin on the invertebrate
fauna of a Quebec forest. Sault Ste Marie, Ontario, Canada, Forest Pest
Management Institute, Report No. FPM-X 50: pp.44.
KREUTZWEISER, D.P. & KINGSBURY, P.D. (1987) Permethrin treatments
in Canadian forests. Part 2: Impact on stream invertebrates. Pestic.
Sci., 19: 49-60.
KUMARAGURU, A.K. & BEAMISH, F.W.H. (1981) Lethal toxicity of
permethrin (NRDC-143) to rainbow trout, Salmo gairdneri, in relation to
body weight and water temperature. Water Res., 15: 503-505.
KUMARAGURU, A.K., BEAMISH, F.W.H., & FERGUSON, H.W. (1982)
Direct and circulatory paths of permethrin (NRDC-143) causing
histopathological changes in the gills of rainbow trout, Salmo
gairdneri Richardson. J. Fish Biol., 20: 87-91.
LAWRENCE, L.J. & CASIDA, J.E. (1982) Pyrethroid toxicology: Mouse
intracerebral structure-toxicity relationship. Pestic. Biochem. Physiol.,
18: 9-14.
LAWRENCE, L.J. & CASIDA, J.E. (1983) Stereospecific action of
pyrethroid insecticides on the gamma-aminobutyric acid receptor-
ionophore complex. Science, 221: 1399-1401.
LAWRENCE, L.J., GEE, K.W., & YAMAMURA, H.I. (1985) Interactions of
pyrethroid insecticides with chloride ionophore-associated binding
sites. Neurotoxicology, 6: 87-98.
LEAHEY, J.P. (1985) The pyrethroid insecticides, London, Taylor &
Francis Ltd, p. 440.
LEAHEY, J.P. & CARPENTER, P.K. (1980) The uptake of metabolites
of permethrin by plants grown in soil treated with [14C]permethrin.
Pestic. Sci., 11: 279-289.
LEAHEY, J.P., BEWICK, D.W., & SAUNDERS, R. (1977) Accumulation and
depletion of radioactive residues in the tissues of Mallard duck and
Japanese quail following daily dosing with 14 C-permethrin (Unpublished
report submitted to WHO by ICI Plant Protection Division).
LE QUESNE, P.N., MAXWELL, I.C., & BUTTERWORTH, T.G. (1980)
Transient facial sensory symptoms following exposure to synthetic
pyrethroids: a clinical and electorophysiological assessment. Neurotoxi-
cology, 2: 1-11.
LINDEN, E., BENGTSSON, B.-E., SVANBERG, O., & SUNDSTROM, G.
(1979) The acute toxicity of 78 chemicals and pesticide formulations
against two brackish water organisms, the Bleak (Alburnus alburnus) and
the Nitocra spinipes. Chemosphere, 11/12: 843-851.
LINDQUIST, R.K., KRUEGER, H.R., & POWELL, C.C., Jr. (1987) Airborne
and surface residues of permethrin after high- and low-volume
applications in greenhouses. J. environ. Sci. Health, B22: 15-27.
LITCHFIELD, M.H. (1983) Characterisation of the principal mammalian
toxicological and biological actions of synthetic pyrethroids. In:
Miyamoto, J. & Kearney, P.C., ed. Pesticide chemistry: human welfare
and the environment. II. Natural products, Oxford, Pergamon Press, pp.
207-211.
LONGSTAFF, E. (1976) Permethrin: short-term predictive tests for
carcinogenicity: results from the Ames test (Report No. CTL/P/301)
(Unpublished data submitted to WHO by ICI Central Toxicology
Laboratory).
LORD, K.A., MCKINLEY, M., & WALKER, N. (1982) Degradation of
permethrin in soils. Environ. Pollut. A29: 81-90.
LUND, A.E. & NARAHASHI, T. (1983) Kinetics of sodium channel
modification as the basis for the variation in the nerve membrane
effects of pyrethroids and DDT analogs. Pestic. Biochem. Physiol., 20:
203-216.
MCGREGOR, D.B. & WICKRAMARATNE, G.A., de S. (1976a) Dominant
lethal study in mice of ICI-PP557 (Report No. 623, Inveresk Research
International Project No. 406722) (Unpublished data submitted to WHO by
ICI Ltd).
MCGREGOR, D.B. & WICKRAMARATNE, G.A., de S. (1976b) Terato-
genicity study in rats of ICI-PP557 (Inveresk Research International
Project No. 404898) (Unpublished data submitted to WHO by ICI Ltd.).
MCLEESE, D.W., METCALFE, C.D., & ZITKO, V. (1980) Lethality of
permethrin, cypermethrin and fenvalerate to salmon, lobster and shrimp.
Bull. environ. Contam. Toxicol., 25: 950-955.
MACPHEE, A., GAUL, S., & RAGAB, M.T.H. (1982) Control of blueberry
thrips, Frankliniella vaccinii Morga, with permethrin and effect on
yield and residue in fruit. J. environ. Sci. Health, B17: 183-193.
MCSHEEHY, T.W. & FINN, J.P. (1980) 21Z: Potential toxicity and
oncogenicity in dietary administration to rats for a period of 104
weeks (Report No. HEFG 80-33) (Unpublished data of Wellcome Research
Laboratories).
MAREI, A.E.M., RUZO, L.O., & CASIDA, J.E. (1982) Analysis and
persistence of permethrin, cypermethrin, deltamethrin and fenvalerate
in the fat and brains of treated rats. J. agric. food Chem., 30: 558-
562.
MATHUR, S.P., BELANGER, A., HAMILTON, H.A., & KHAN, S.U. (1980)
Influence on microflora and persistence of field-applied disulfoton,
permethrin and prometryne in an organic soil. Pedobiologia, 20: 237-
242.
MAYER, F.L., Jr. (1987) Acute toxicity handbook of chemicals to
estuarine organisms, Springfield, Virginia, National Technical
Information Service, p. 158 EPA/600/8-87/017.
MAYER, F.L., Jr. & ELLERSIECK, M.R. (1986) Manual of acute toxicity:
Interpretation and data base for 410 chemicals and 66 species of
freshwater animals, Washington, DC, United States Department of the
Interior, Fish and Wildlife Service, pp.377-378.
MEISTER, R.T., BERG, G.L., SINE, C., MEISTER, S., & POPLYK, J. (1983)
Farm chemicals handbook. Section C. Pesticide dictionary, Wilboughby,
Ohio, Meister Publishing Co., pp. C180-C181.
METKER, L.W. (1978) Subchronic inhalation toxicity of 3-(phenoxy-
phenyl)methyl(+)-cis,trans-3-(2,2-dichloroethenyl)-2,2-dimethylcyclopro-
panecarboxylate (permethrin), Aberdeen Proving Ground, Maryland, US
Army Environmental Hygiene Agency (Report No. 75-51-0026-80).
METKER, L.W., ANGERHOFER, R.A., POPE, C.R., & SWENTZEL, K.C.
(1977) Toxicology evaluation of 3-(phenoxyphenyl)methyl(+)-cis,trans-3-
(2,2-dichloroethenyl)-2,2-dimethylcyclopropanecarboxylate (permethrin),
Environmental Hygiene Agency (Report No. 75-51-0837-78).
MILLNER, C.K. & BUTTERWORTH, S.T.G. (1977) Toxicity of pyrethroid
insecticides: investigation of the neurotoxic potential of WL 43479 to
adult hens, (Report No. TLGR.0069.77) (Unpublished data submitted to
WHO by Shell Research Ltd).
MIYAMOTO, J. (1976) Degradation, metabolism and toxicity of synthetic
pyrethroids. Environ. Health Perspect., 14: 15-28.
MIYAMOTO, J. (1981) The chemistry, metabolism and residue analysis of
synthetic pyrethroids, Pure appl. Chem., 53: 1967-2022.
MIYAMOTO, J. & KEARNEY, P.C. (1983) Pesticide chemistry-human welfare
and the environment. Proceedings of the Fifth International Congress of
Pesticide Chemistry, Kyoto, Japan, 29 August-4 September 1982, Oxford,
Pergamon Press, Vol. 1-4.
MULLA, M.S., DARWAZEH, H.A., & MAJORI, G. (1975) Field efficacy of
some promising mosquito larvicides and their effect on nontarget
organisms. Mosq. News, 35: 179-185.
MULLA, M.S., NAVVAB-GOJRATI, H.A., & DARWAZEH, H.A. (1978a)
Toxicity of mosquito larvicidal pyrethroids to four species of
freshwater fishes. Environ. Entomol., 7: 428-430.
MULLA, M.S., NAVVAB-GOJRATI, H.A., & DARWAZEH, H.A. (l978b)
Biological activity and longevity of new synthetic pyrethroids against
mosquitoes and some nontarget insects. Mosq. News, 38: 90-96.
MULLA, M.S., DARWAZEH, H.A., & DHILLON, K.S. (1981) Impact and
joint action of decamethrin and permethrin and freshwater fishes on
mosquitoes. Bull. environ. Contam. Toxicol., 26: 689-695.
NASSIF, M., BROOKE, J.P., HUTCHINSON, D.B.A., & KAMEL, O.M. (1980)
Studies with permethrin against body lice in Egypt. Pestic. Sci., 11:
679-684.
NEWELL, G.W. & SKINNER W.A. (1976) In vitro microbiological
mutagenicity study of an FMC corporation compound (Unpublished report
submitted to WHO by FMC Corporation).
NOMURA, Y. & SEGAWA, T. (1979) Pharmacological study of permethrin:
effects of isolated ileum, nictitating membrane, respiration, blood
pressure and electrocardiography (Unpublished report submitted to WHO
by Sumitomo Chemical Co.).
OHKAWA, H., KANEKO, H., & MIYAMOTO, J. (1977) Metabolism of
permethrin in bean plants. J. Pestic. Sci., 2: 67-76.
OHSHIMA, M., TAKIMOTO, Y., & MATSUDA, T. (1988) Accumulation and
metabolism of 14 C-permethrin in carp (Cyprinus carpio) (Unpublished
data submitted to WHO by Sumitomo Chemical Co.).
OKUNO, Y., MIYAGAWA, H., HOSOI, R., & KADOTA, T. (1976) Skin and
eye irritation study of permethrin (S-3151) in rabbits (Unpublished
report submitted to WHO by Sumitomo Chemical Co.).
PARKINSON, G.R. (1978) Permethrin: acute toxicity to male rats (Report
No. CTL/P/388) (Unpublished data submitted to WHO by ICI Central
Toxicology Laboratory).
PARKINSON, G.R., BERRY, P.N., GLAISTER, J., GORE, C.W., LEFEVRE,
V.K., & MURPHY, J.A. (1976) PP557 (Permethrin): acute and sub-acute
toxicity, (Report No. CTL/P/225) (Unpublished data submitted to WHO by
ICI Central Toxicology Laboratory).
PAYNTER, O.C., BUDD, E.R., & LITT, B.D. (1982) Permethrin. Assessment
of chronic and oncogenic effects: A summary, Washington, DC, Toxicology
Branch, Hazard Evaluation Division, Office of Pesticide Programs, US
Environmental Protection Agency (Unpublished data submitted to WHO).
PEGUM, J.S. & DOUGHTY, B.J. (1978) Human skin patch test with
pyrethrins, kadethrin, permethrin and decamethrin, Berkhamsted Hill,
Wellcome Group Research and Development (Report No. NEFJ 78-2)
(Unpublished data submitted to WHO).
PIERCY, D.W.T., REYNOLDS, J., & JAMES, J.A. (1976) Effects of diet on
the acute toxicity of permethrin in the female rat, Berkhamsted Hill,
Wellcome Research and Development (Report no. 77-11) (Unpublished data
submitted to WHO).
PIKE, K.S., MAYER, D.F., GLAZER, M., & KIOUS, C. (1982) Effects of
permethrin on mortality and foraging behavior of honey bees in sweet
corn. Environ. Entomol., 11: 951-953.
PLAPP, F.W., Jr & BULL, D.L. (1978) Toxicity and selectivity of some
insecticides to Chrysopa carnea, a predator of the tobacco budworm.
Environ. Entomol., 7: 431-434.
PLAPP, F.W., Jr & VINSON, S.B. (1977) Comparative toxicities of some
insecticides to the tobacco budworm and its ichneumonid parasite,
Campoletis sonorensis. Environ. Entomol., 6: 381-384.
PLUIJMEN, M., DREVON, C., MONTESANO, R., MALAVEILLE, C.,
HAUTEFEUILLE, A., & BARTSCH, H. (1984) Lack of mutagenicity of
synthetic pyrethroids in Salmonella typhimurium strains in V79 Chinese
hamster cells. Mutat. Res., 137: 7-15.
RACEY, P.A. & SWIFT, S.M. (1986) The residual effect of remedial
timber treatments on bats. Biol. Conserv., 35: 205-214.
RAPP, W.R. (1978) Twenty-four month oral toxicity/oncogenicity study
of FMC33297 in mice. Histopathology report (Unpublished data of FMC
Corporation).
REYNOLDS, J., PIERCY, D.W.T., CLAMPITT, R.B., JAMES, J.A.,
THOMPSON, P.M., FAREBROTHER, D.A., & DAYAN, A.D. (1978)
Permethrin oral administration to dogs for 6 months, Berkhamsted Hill,
Wellcome Research Laboratories (Report No. HEFG 78-14) (Unpublished
data submitted to WHO).
RICHARDS, D., BANHAM, P.B., KILMARTIN, M., & WEIGHT, T.M. (1980)
Permethrin: Teratogenicity study in the rabbit (Report No. CTL/P/523)
(Unpublished report submitted to WHO by ICI Ltd).
RISHIKESH, N., CLARKE, J.L., MATHIN, H.L., KING, J.S., & PEARSON, J.
(1978) Evaluation of decamethrins and permethrin against Anopheles
gambiae and Anopheles funestus in a village trial in Nigeria
(WHO/VBC/78.679) (Unpublished report of Division of Vector Biology
Control, World Health Organization).
ROBERTS, T.R. & WRIGHT, A.N. (1981) The metabolism of 3-phenoxybenzyl
alcohol, a pyrethroid metabolite, in plants. Pestic. Sci., 12: 161-
169.
ROCK, G.C. (1979) Relative toxicity of two synthetic pyrethroids to a
predator Amblyseius fallacis and its prey Tetranychus urticae. J. econ.
Entomol., 72: 293-294.
ROSS, D.B. & PRENTICE, D.E. (1977) Examination of permethrin (PP557)
for neurotoxicity in the domestic hen, Huntingdon, Huntingdon Research
Centre (Report No. ICI/157-NT/77468) (Unpublished data submitted to WHO
by FMC Corporation and ICI Ltd).
ROSS, D.B., CAMERON, D.M., & ROBERTS, N.L. (1976a) The acute oral
toxicity (LD50 ) of PP557 (permethrin) to Mallard ducks, Huntingdon,
Huntingdon Research Centre (Report No. ICI 68/WL/7639) (Unpublished
data submitted to WHO by ICI Ltd).
ROSS, D.B., CAMERON, D.M., & ROBERTS, N.L. (1976b) The sub-acute
toxicity (LC50 ) of PP557 (permethrin) to Mallard ducks, Huntingdon,
Huntingdon Research Centre (Report No. ICI 68/WL/75837) (Unpublished
data submitted to WHO by ICI Ltd).
ROSS, D.B., CAMERON, D.M., & ROBERTS, N.L. (1976c) The acute oral
toxicity (LD50 ) of PP557 (permethrin) to Starling, Huntingdon,
Huntingdon Research Centre (Report No. ICI 68/WL/7637) (Unpublished
data submitted to WHO by ICI Ltd).
ROSS, D.B., CAMERON, D.M., & ROBERTS, N.L. (1976d) The sub-acute
toxicity (LC50 ) of PP557 (permethrin) to Starling, Huntingdon,
Huntingdon Research Centre (Report No. ICI 68/WL/7636) (Unpublished
data submitted to WHO by ICI Ltd).
ROSS, D.B., CAMERON, D.M., & ROBERTS, N.L. (1976e) The sub-acute
toxicity (LC50 ) of PP557 (permethrin) to Ring-necked pheasant,
Huntingdon, Huntingdon Research Centre (Report No. ICI 68/WL/75839)
(Unpublished data submitted to WHO by ICI Ltd).
ROSS, D.B., CAMERON, D.M., & ROBERTS, N.L. (1977a) The acute oral
toxicity (LD50 ) of PP557 (permethrin) to Ring-necked pheasant,
Huntingdon, Huntingdon Research Centre (Report No. ICI 68/WL/77154)
(Unpublished data submitted to WHO by ICI Ltd).
ROSS, D.B., PRENTICE, D.E., MAJEED, S.K., GIBSON, W.A., CAMERON,
D.M., CAMERON, M. McD., & ROBERTS, N.L. (1977b) The incorporation of
permethrin in the diet of laying hens (part I), Huntingdon, Huntingdon
Research Centre (Report No. ICI 152/77387) (Unpublished data submitted
to WHO by ICI Ltd).
ROUSH, R.T. & HOY, M.A. (1978) Relative toxicity of permethrin to a
predator, Metaseiulus occidentalis, and its prey, Tetranychus urticae.
Environ. Entomol., 7: 287-288.
RUIGT, G.S.F. & VAN DEN BERCKEN, J. (1986) Action of pyrethroids on a
nerve-muscle preparation of the clawed frog, Xenopus laevis. Pestic.
Biochem. Physiol., 25: 176-187.
SASINOVICH, L.M. & PANSHINA, T.N. (1987) [Substantiation of hygienic
rules for synthetic pyrethroid content in the work zone air]. Gig. Tr.
prof. Zabol., 8: 48-50 (in Russian).
SCHROEDER, R.E. & RINEHART, W.E. (1977) A three generation
reproduction study of FMC33297 in rats (Bio-Dynamics Inc Project)
(Unpublished report submitted to WHO by FMC Corporation).
SHAH, P.V., MONROE, R.J., & GUTHRIE, F.E. (1981) Comparative rates of
dermal penetration of insecticides in mice. Toxicol. appl. Pharmacol.,
59: 414-423.
SHAROM, M.S. & SOLOMON, K.R. (1981) Adsorption-desorption,
degradation, and distribution of permethrin in aqueous systems. J.
agric. food Chem., 29: 1122-1125.
SHERMAN, R.A. (1979) Preliminary behavioural assessment of habituation
to the insecticide permethrin, Aberdeen Proving Ground, Maryland, US
Army Environmental Hygiene Agency (Report No. 75-51-002679).
SHIRASU, Y., MORIYA, M., & OTA, T. (1979) Mutagenicity of S-3151 in
bacterial test systems (Unpublished report submitted to WHO by Sumitomo
Chemical Co.).
SHONO, T., OHSAWA, K., & CASIDA, J.E. (1979) Metabolism of trans- and
cis-permethrin, trans- and cis-cypermethrin, and decamethrin by
microsomal enzymes. J. agric. food Chem., 27: 316-325.
SHOUR, M.H. & CROWDER, L.A. (1980) Effects of pyrethroid insecticides
on the common green lacewing. J. econ. Entomol., 73: 306-309.
SIEGEL, M.M., HILDEBRAND, B.E., & HALL, D.R. (1980) Determination of
permethrin in environmental waters by gas chromatography-selected ion
monitoring-mass spectroscopy using ion counting detection. Int. J.
environ. anal. Chem., 8: 107-126.
SIMMON, V.F. (1976) In vitro microbiological mutagenicity study of an
FMC corporation compound, Menlo Park, Stanford Research Institute,
(Report No. LSC-4768) (Unpublished report submitted to WHO).
SIMONAITIS, R.A. & CAIL, R.S. (1977) Gas chromatographic determination
of residues of the synthetic pyrethroid FMC33297. In: Elliott, M., ed.
Synthetic pyrethroids, Washington, DC, American Chemical Society,
pp. 211-223 (ACS Symposium Series U2).
SODERLUND, D.M. & CASIDA, J.E. (1977) Effects of pyrethroid structure
on rate of hydrolysis and oxidation by mouse liver microsomal enzymes.
Pestic. Biochem. Physiol., 7: 391-401.
SPENCER, F. & BERHANCE, Z. (1982) Uterine and fetal characteristics in
rats following a post-implantational exposure to permethrin. Bull.
environ. Contam. Toxicol., 29: 84-88.
STAATZ, C.G., BLOOM, A.S., & LECH, J.J. (1982) A pharmacological
study of pyrethroid neurotoxicity in mice. Pestic. Biochem. Physiol.,
17: 287-292.
STEVENSON, J.H., NEEDHAM, P.H., & WALKER, J. (1978) Poisoning of
honeybee by pesticides: Investigation of the changing pattern in
Britain over 20 years. Rothamsted Experimental Station Report (1977),
Part 2: 55-72.
STRATTON, G.W. & CORKE, C.T. (1981) Interaction of permethrin with
Daphnia magna in the presence and absence of particulate material.
Environ. Pollut., A24: 135-144.
STRATTON, G.W. & CORKE, C.T. (1982) Toxicity of the insecticide
permethrin and some degradation products towards algae and
cyanobacteria. Environ. Pollut. A29: 71-80.
SUNDARAM, K.M.S., DE GROOT, P., & SUNDARAM, A. (1987)
Permethrin deposits and airborne concentrations downwind from a single
swath application using a back pack mist blower. J. environ. Sci.
Health, B22: 171-193.
SUZUKI, H. (1977) Studies on the mutagenicity of some pyrethroids on
salmonella strains in the presence of mouse hepatic S9 fractions
(Unpublished report submitted to WHO by Sumitomo Chemical Co.).
SWAINE, H. & SAPIETS, A. (1981a) Cypermethrin: residue transfer study
with dairy cows fed on a diet containing the insecticide (Report No.
RJ0186B) (Unpublished data submitted to WHO by ICI Ltd).
SWAINE, H. & SAPIETS, A. (1981b) Cypermethrin: residue levels of the
major metabolites of the insecticide in the milk and tissues of dairy
cows fed on a diet containing cypermethrin at 50 mg/kg (Report No.
RJ0198B) (Unpublished data submitted to WHO by ICI Ltd).
SWAINE, H., EDWARD, M.J., & USSARY, J.P. (1978) Permethrin crop
protection study (Report No. TMV 0378B) (Unpublished data submitted to
WHO by ICI Ltd).
TAKAHASHI, K., OKUDA, No., & SHIRASU, Y. (1979) Effects of
permethrin on hexobarbital induced sleeping time in mice and electro-
encephalography in rabbits (Unpublished report submitted to WHO by
Sumitomo Chemical Co.).
TYLER, J.F.C. (1987) Gas chromatographic method for determination of
permethrin in pesticide formulations: Collaborative study. J. Assoc.
Off. Anal. Chem., 70(1): 53-55.
US EPA (1981) Advisary opinion on the oncogenic potential of
permethrin, Washington, DC, US Environmental Protection Agency
(Unpublished report submitted to WHO).
USSARY, J.P. (1979) Permethrin residues on sweet corn (Report No.
TMU0438B) (Unpublished data submitted to WHO by ICI Americas Inc.).
USSARY, J.P. (1978) Permethrin residues on sweet corn (Report No.
TMU0413B) (Unpublished data submitted to WHO by ICI Americas Inc.).
USSARY, J.P. & BRAITHWAITE, G.B. (1980) Residues of permethrin and 3-
phenoxy-benzyl alcohol in cow tissues (Trial No. 35NC79-001. Report
No. TMU0493B) (Unpublished data submitted to WHO by ICI Americas
Inc.).
VAN DEN BERCKEN, J. (1977) The action of allethrin on the peripheral
nervous system of the frog. Pestic. Sci., 8: 692-699.
VAN DEN BERCKEN, J. & VIJVERBERG, H.P.M. (1980a) Voltage clamp
studies on the effects of allethrin and DDT on the sodium channels in
frog myelinated nerve membrane. In: Insect neurobiology and pesticide
action, London, Society of Chemical Industry, pp. 79-85.
VAN DEN BERCKEN, J. & VIJVERBERG, H.P.M. (1980b) Effects of
insecticides on the sensory system of Xenopus. In: Insect neurobiology
and pesticide action, London, Society of Chemical Industry, pp.391-
397.
VAN DEN BERCKEN, J., AKKERMANS, L.M.A., & VAN DER ZALM,
J.M., (1973) DDT-like action of allethrin in the sensory nervous
system of Xenopus laevis. Eur. J. Pharmacol., 21: 95-106.
VAN DEN BERCKEN, J., KROESE, A.B.A., & AKKERMANS, L.M.A.
(1979) Effect of insecticides on the sensory nervous system. In:
Narahashi, T., ed. Neurotoxicology of insecticides and pheromones,
New York, London, Plenum Press, pp. 183-210.
VERSCHOYLE, R.D. & ALDRIDGE, W.N. (1980) Structure-activity
relationship of some pyrethrins to rats. Arch. Toxicol., 45: 325-329.
VIJVERBERG, H.P.M. & VAN DEN BERCKEN, J. (1979) Frequency-
dependent effects of the pyrethroid insecticide decamethrin in frog
myelinated nerve fibres. Eur. J. Phamacol., 58: 501-504.
VIJVERBERG, H.P.M. & VAN DEN BERCKEN, J. (1982) Action of
pyrethroid insecticides on the vertebrate nervous system. Neuropathol.
appl. Neurobiol., 8: 421-440.
VIJVERBERG, H.P.M., RUIGT, G.S.F., & VAN DEN BERCKEN, J. (1982a)
Structure-related effects of pyrethroid insecticides on the lateral-
line sense organ and on peripheral nerves of the clawed frog, Xenopus
laevis. Pestic. Biochem. Physiol., 18: 315-324.
VIJVERBERG, H.P.M., VAN DER ZALM, J.M., & VAN DEN BERCKEN, J.
(1982b) Similar mode of action of pyrethroids and DDT on sodium
channel gating in mylinated nerves. Nature (Lond.), 295: 601-603.
VIJVERBERG, H.P.M., VAN DER ZALM, J.M., VAN KLEEF, R.G.D.M., &
VAN DEN BERCKEN, J. (1983) Temperature and structure-dependent
interaction of pyrethroids with the sodium channels in frog node of
Ranvier. Biochim. Biophys. Acta, 728: 73-82.
WALLWORK, L.M. & MALONE, J.C. (1974) 21Z73 (25:75) effect of
different solvents on the rat oral toxicity (Report No. HEFG 75-4)
Berkhamsted, Wellcome Research Laboratories (Unpublished data submitted
to WHO).
WALLWORK, L.M., CLAMPITT, R.B., & MALONE, J.C. (1974a) 10-day
cumulative oral toxicity study with 21Z73 in mice, Berkhamsted,
Wellcome Research Laboratories (Report No. HEFG 74-9) (Unpublished
data submitted to WHO).
WALLWORK, L.M., CLAMPITT, R.B., & MALONE, J.C. (1974b) 10-day
cumulative oral toxicity study with 21Z73 in rats, Berkhamsted,
Wellcome Research Laboratories (Unpublished report submitted to WHO).
WELLS, D., GRAYSON, B.T., & LANGNER, E. (1986) Vapour pressure of
permethrin. Pestic. Sci., 17: 473-476.
WHO (1979) WHO Technical Report Series, No. 634 (Safe use of
pesticides. Third Report of the WHO Expert Committee on Vector Biology
and Control), pp. 18-23.
WHO (1988) The WHO recommended classification of pesticides by
hazard: Guidelines to classification 1988-89, Geneva, World Health
Organization (Unpublished report VBC/88.953).
WHO/FAO (1984) Data sheet on pesticides No. 51: Permethrin, Geneva,
World Health Organization (VBC/DS/84.51).
WILLIAMS, I.H. & BROWN, M.J. (1979) Persistence of permethrin and WL
43775 in soil. J. agric. food Chem., 27: 130-132.
WOODRUFF, R.C., PHILLIPS, J.P., & IRWIN, D. (1983) Pesticide-induced
complete and partial chromosome loss in screens with repair-defective
females of Drosophila melanogaster. Environ. Mutagen., 5: 835-846.
WOOD, MACKENZIE & Co. (1980) Pyrethroids. Agrochem. Monit., 9: 3-14.
WOOD, MACKENZIE & Co. (1981) Pyrethroids. Agrochem. Monit., 15: 3-27.
WOOD, MACKENZIE & Co. (1982) Pyrethroids. Agrochem. Monit., 21: 3-17.
WOOD, MACKENZIE & Co. (1983) Pyrethroids. Agrochem. Monit., 27: 3-12.
WOOD, MACKENZIE & Co. (1984) Pyrethroids. Agrochem. Monit., 33: 2-12.
WORKMAN, R.B. (1977) Pesticides toxic to striped earwig, an important
insect predator. Proc. Florida State Hortic. Soc., 90: 401-402.
WORTHING, C.R. & WALKER, S.B. (1983) The pesticide manual, 7th ed.,
Croydon, British Crop Protection Council, p. 427.
WORTHING, C.R. & WALKER, S.B. (1987) Permethrin. In: The pesticide
manual, 8th ed., Croydon, British Crop Protection Council, pp. 647-
648.
WOUTERS, W. & VAN DEN BERCKEN, J. (1978) Action of pyrethroids. Gen.
Pharmacol., 9: 387-398.
ZITKO, V., CARSON, W.G., & METCALFE, C.D. (1977) Toxicity of
pyrethroids to juvenile atlantic salmon. Bull. environ. Contam.
Toxicol., 18: 35-41.
ZITKO, V., MCLEESE, D.W., METCALFE, C.D., & CARSON, W.G. (1979)
Toxicity of permethrin, decamethrin, and related pyrethroids to salmon
and lobster. Bull. environ. Contam. Toxicol., 21: 338-343.
APPENDIX I
On the basis of electrophysiological studies with peripheral nerve
preparations of frogs ( Xenopus laevis; Rana temporaria, and Rana
esculenta ), it is possible to distinguish between 2 classes of
pyrethroid insecticides: (Type I and Type II). A similar distinction
between these 2 classes of pyrethroids has been made on the basis of
the symptoms of toxicity in mammals and insects (Van den Bercken et
al., 1979; WHO, 1979; Verschoyle & Aldridge, 1980; Glickman & Casida,
1982; Lawrence & Casida, 1982). The same distinction was found in
studies on cockroaches (Gammon et al., 1981).
Based on the binding assay on the gamma-aminobutyric acid (GABA)
receptor-ionophore complex, synthetic pyrethroids can also be
classified into two types: the alpha-cyano-3-phenoxybenzyl pyrethroids
and the non-cyano pyrethroids (Gammon et al., 1982; Gammon & Casida,
1983; Lawrence & Casida, 1983; Lawrence et al., 1985).
Pyrethroids that do not contain an alpha-cyano group (allethrin,
d-phenothrin, permethrin, tetramethrin, cismethrin, and bioresmethrin)
(Type I: T-syndrome)
The pyrethroids that do not contain an alpha-cyano group give rise
to pronounced repetitive activity in sense organs and in sensory nerve
fibres (Van den Bercken et al., 1973). At room temperature, this
repetitive activity usually consists of trains of 3-10 impulses and
occasionally up to 25 impulses. Train duration is between 10 and 5
milliseconds.
These compounds also induce pronounced repetitive firing of the
presynaptic motor nerve terminal in the neuromuscular junction (Van den
Bercken, 1977). There was no significant effect of the insecticide on
neurotransmitter release or on the sensitivity of the subsynaptic
membrane, nor on the muscle fibre membrane. Presynaptic repetitive
firing was also observed in the sympathetic ganglion treated with these
pyrethroids.
In the lateral-line sense organ and in the motor nerve terminal,
but not in the cutaneous touch receptor or in sensory nerve fibres, the
pyrethroid-induced repetitive activity increases dramatically as the
temperature is lowered, and a decrease of 5°C in temperature may cause
a more than 3-fold increase in the number of repetitive impulses per
train. This effect is easily reversed by raising the temperature. The
origin of this "negative temperature coefficient" is not clear
(Vijverberg et al., 1983).
Synthetic pyrethroids act directly on the axon through interference
with the sodium channel gating mechanism that underlies the generation
and conduction of each nerve impulse. The transitional state of the
sodium channel is controlled by 2 separately acting gating mechanisms,
referred to as the activation gate and the inactivation gate. Since
pyrethroids only appear to affect the sodium current during
depolarization, the rapid opening of the activation gate and the slow
closing of the inactivation gate proceed normally. However, once the
sodium channel is open, the activation gate is restrained in the open
position by the pyrethroid molecule. While all pyrethroids have
essentially the same basic mechanism of action, however, the rate of
relaxation differs substantially for the various pyrethroids (Flannigan
& Tucker, 1985).
In the isolated node of Ranvier, allethrin causes prolongation of
the transient increase in sodium permeability of the nerve membrane
during excitation (Van den Bercken & Vijverberg, 1980a,b). Evidence so
far available indicates that allethrin selectively slows down the
closing of the activation gate of a fraction of the sodium channels
that open during depolarization of the membrane. The time constant of
closing of the activation gate in the allethrin-affected channels is
about 100 milliseconds compared with less than 100 microseconds in the
normal sodium channel, i.e., it is slowed down by a factor of more than
100. This results in a marked prolongation of the sodium current
across the nerve membrane during excitation, and this prolonged sodium
current is directly responsible for the repetitive activity induced by
allethrin (Vijverberg et al., 1983).
The effects of cismethrin on synaptic transmission in the frog
neuromuscular junction, as reported by Evans (1976), are almost
identical to those of allethrin, i.e., presynaptic repetitive firing,
and no significant effects on transmitter release or on the subsynaptic
membrane.
Interestingly, the action of these pyrethroids closely resembles
that of the insecticide DDT in the peripheral nervous system of the
frog. DDT also causes pronounced repetitive activity in sense organs,
in sensory nerve fibres, and in motor nerve terminals, due to a
prolongation of the transient increase in sodium permeability of the
nerve membrane during excitation. Recently, it was demonstrated that
allethrin and DDT have essentially the same effect on sodium channels
in frog myelinated nerve membrane. Both compounds slow down the rate
of closing of a fraction of the sodium channels that open on
depolarization of the membrane (Van den Bercken et al., 1973, 1979;
Vijverberg et al., 1982b).
In the electrophysiological experiments using giant axons of
crayfish, the type I pyrethroids and DDT analogues retain sodium
channels in a modified open state only intermittently, cause large
depolarizing after-potentials, and evoke repetitive firing with minimal
effect on the resting potential (Lund & Narahashi, 1983).
These results strongly suggest that permethrin and cismethrin, like
allethrin, primarily affect the sodium channels in the nerve membrane
and cause a prolongation of the transient increase in sodium
permeability of the membrane during excitation.
The effects of pyrethroids on end-plate and muscle action
potentials were studied in the pectoralis nerve-muscle preparation of
the clawed frog ( Xenopus laevis ). Type I pyrethroids (allethrin,
cismethrin, bioresmethrin, and 1R, cis-phenothrin) caused moderate
presynaptic repetitive activity, resulting in the occurrence of
multiple end-plate potentials (Ruigt & Van den Bercken, 1986).
Pyrethroids with an alpha-cyano group on the 3-phenoxybenzyl alcohol
(deltamethrin, cypermethrin, cyhalothrin, lambda-cyhalothrin,
fenvalerate, and fenpropanate) (Type II: CS-syndrome)
The pyrethroids with an alpha-cyano group cause an intense repetitive
activity in the lateral line organ in the form of long-lasting trains
of impulses (Vijverberg et al., 1982a). Such a train may last for up
to 1 min and contains thousands of impulses. The duration of the
trains and the number of impulses per train increase markedly on
lowering the temperature. Cypermethrin does not cause repetitive
activity in myelinated nerve fibres. Instead, this pyrethroid causes a
frequency-dependent depression of the nervous impulse, brought about by
a progressive depolarization of the nerve membrane as a result of the
summation of depolarizing after-potentials during train stimulation
(Vijverberg & Van den Bercken, 1979; Vijverberg et al., 1983).
In the isolated node of Ranvier, cypermethrin, like allethrin,
specifically affects the sodium channels of the nerve membrane and
causes a long-lasting prolongation of the transient increase in sodium
permeability during excitation, presumably by slowing down the closing
of the activation gate of the sodium channel (Vijverberg & Van den
Bercken, 1979; Vijverberg et al., 1983). The time constant of closing
of the activation gate in the cypermethrin-affected channels is
prolonged to more than 100 milliseconds. Apparently, the amplitude of
the prolonged sodium current after cypermethrin is too small to induce
repetitive activity in nerve fibres, but is sufficient to cause the
long-lasting repetitive firing in the lateral-line sense organ.
These results suggest that alpha-cyano pyrethroids primarily affect
the sodium channels in the nerve membrane and cause a long-lasting
prolongation of the transient increase in sodium permeability of the
membrane during excitation.
In the electrophysiological experiments using giant axons of
crayfish, the Type II pyrethroids retain sodium channels in a modified
continuous open state persistently, depolarize the membrane, and block
the action potential without causing repetitive firing (Lund &
Narahashi, 1983).
Diazepam, which facilitates GABA reaction, delayed the onset of
action of deltamethrin and fenvalerate, but not permethrin and
allethrin, in both the mouse and cockroach. Possible mechanisms of the
Type II pyrethroid syndrome include action at the GABA receptor complex
or a closely linked class of neuroreceptor (Gammon et al., 1982).
The Type II syndrome of intracerebrally administered pyrethroids
closely approximates that of the convulsant picrotoxin (PTX).
Deltamethrin inhibits the binding of [3H]-dihydropicrotoxin to rat
brain synaptic membranes, whereas the non-toxic R epimer of
deltamethrin is inactive. These findings suggest a possible relation
between the Type II pyrethroid action and the GABA receptor complex.
The stereospecific correlation between the toxicity of Type II
pyrethroids and their potency to inhibit the [35S]-TBPS binding was
established using a radioligand, [35S]- t-butyl-bicyclophosphoro-
thionate [35S]-TBPS. Studies with 37 pyrethroids revealed an
absolute correlation, without any false positive or negative, between
mouse intracerebral toxicity and in vitro inhibition: all toxic cyano
compounds including deltamethrin, 1R, cis-cypermethrin, 1R, trans-
cypermethrin, and [2S,alphaS]-fenvalerate were inhibitors, but their
non-toxic stereoisomers were not; non-cyano pyrethroids were much less
potent or were inactive (Lawrence & Casida, 1983).
In the [35S]-TBPS and [3H]-Ro 5-4864 (a convulsant
benzodiazepine radioligand) binding assay, the inhibitory potencies of
pyrethroids were closely related to their mammalian toxicities. The
most toxic pyrethroids of Type II were the most potent inhibitors of
[3H]-Ro 5-4864 specific binding to rat brain membranes. The [3H]-
dihydropicrotoxin and [ 35S]-TBPS binding studies with pyrethroids
strongly indicated that Type II effects of pyrethroids are mediated, at
least in part, through an interaction with a GABA-regulated chloride
ionophore-associated binding site. Moreover, studies with [3H]-Ro 5-
4864 support this hypothesis and, in addition, indicate that the
pyrethroid-binding site may be very closely related to the convulsant
benzodiazepine site of action (Lawrence et al., 1985).
The Type II pyrethroids (deltamethrin, 1R, cis-cypermethrin and
[2S,alphaS]-fenvalerate) increased the input resistance of crayfish
claw opener muscle fibres bathed in GABA. In contrast, two non-insect-
icidal stereoisomers and Type I pyrethroids (permethrin, resmethrin,
allethrin) were inactive. Therefore, cyanophenoxybenzyl pyrethroids
appear to act on the GABA receptor-ionophore complex (Gammon & Casida,
1983).
The effects of pyrethroids on end-plate and muscle action
potentials were studied in the pectoralis nerve-muscle preparation of
the clawed frog ( Xenopus laevis). Type II pyrethroids (cypermethrin
and deltamethrin) induced trains of repetitive muscle action potentials
without presynaptic repetitive activity. However, an intermediate
group of pyrethroids (1R-permethrin, cyphenothrin, and fenvalerate)
caused both types of effect. Thus, in muscle or nerve membrane the
pyrethroid induced repetitive activities due to a prolongation of the
sodium current. But no clear distinction was observed between non-
cyano and alpha-cyano pyrethroids (Ruigt & Van den Bercken, 1986).
Appraisal
In summary, the results strongly suggest that the primary target
site of pyrethroid insecticides in the vertebrate nervous system is the
sodium channel in the nerve membrane. Pyrethroids without an alpha-cyano
group (allethrin, d-phenothrin, permethrin, and cismethrin) cause a
moderate prolongation of the transient increase in sodium permeability
of the nerve membrane during excitation. This results in relatively
short trains of repetitive nerve impulses in sense organs, sensory
(afferent) nerve fibres, and, in effect, nerve terminals. On the other
hand, the alpha-cyano pyrethroids cause a long-lasting prolongation of
the transient increase in sodium permeability of the nerve membrane
during excitation. This results in long-lasting trains of repetitive
impulses in sense organs and a frequency-dependent depression of the
nerve impulse in nerve fibres. The difference in effects between
permethrin and cypermethrin, which have identical molecular structures
except for the presence of an alpha-cyano group on the phenoxybenzyl
alcohol, indicates that it is this alpha-cyano group that is responsible
for the long-lasting prolongation of the sodium permeability.
Since the mechanisms responsible for nerve impulse generation and
conduction are basically the same throughout the entire nervous system,
pyrethroids may also induce repetitive activity in various parts of the
brain. The difference in symptoms of poisoning by alpha-cyano
pyrethroids, compared with the classical pyrethroids, is not
necessarily due to an exclusive central site of action. It may be
related to the long-lasting repetitive activity in sense organs and
possibly in other parts of the nervous system, which, in a more advance
state of poisoning, may be accompanied by a frequency-dependent
depression of the nervous impulse.
Pyrethroids also cause pronounced repetitive activity and a
prolongation of the transient increase in sodium permeability of the
nerve membrane in insects and other invertebrates. Available
information indicates that the sodium channel in the nerve membrane is
also the most important target site of pyrethroids in the invertebrate
nervous system (Wouters & Van den Bercken, 1978; WHO, 1979).
Because of the universal character of the processes underlying
nerve excitability, the action of pyrethroids should not be considered
restricted to particular animal species, or to a certain region of the
nervous system. Although it has been established that sense organs and
nerve endings are the most vulnerable to the action of pyrethroids, the
ultimate lesion that causes death will depend on the animal species,
environmental conditions, and on the chemical structure and physical
characteristics of the pyrethroid molecule (Vijverberg & Van den
Bercken, 1982).
RESUME, EVALUATION, CONLUSIONS ET RECOMMANDATIONS
1. Résumé et Evaluation
1.1 Identité, propriétés physicochimiques et méthodes d'analyse
La perméthrine, un pyréthroide photostable, a été synthétisée pour
la première fois en 1973 et commercialisée en 1977. C'est un ester de
l'analogue dichloré de l'acide chrysanthémique, à savoir l'acide
(dichloro-2,2 vinyl)-3 diméthyl-2,2 cyclopropanecarboxylique (Cl2CA) et
de l'alcool phénoxy-3 benzylique. Les produits techniques se présentent
sous la forme d'un mélange de quatre stéréoisomères ayant les
configurations [1R,trans], [1R,cis], [1S,trans] et [1S,cis] dans les
proportions approximatives de 3:2:3:2. Le rapport cis/trans est
d'environ deux tiers, les isomères 1R et 1S étant présents en quantités
égales (racémique). C'est l'isomère [1R,cis] qui est l'insecticide le
plus actif; vient ensuite l'isomère [1R,trans].
La perméthrine de qualité technique se présente sous la forme d'un
liquide brun ou brun jaunâtre qui peut partiellement cristalliser à la
température ambiante. Son point de fusion est d'environ 35°C et son
point d'ébullition de 220°C sous 0,05 mmHg. La densité est de 1,214 à
25°C et la tension de vapeur de 1,3 µPa à 20°C. La perméthrine est
pratiquement insoluble dans l'eau (moins de 0,2 mg par litre à 25°C),
mais elle est soluble dans certains solvants organiques tels que
l'acétone, l'hexane et le xylène. Elle est stable à la lumière et à la
chaleur, mais instable en milieu alcalin.
Le dosage des résidus et les analyses écotoxicologiques s'effect-
uent par chromatographie en phase gazeuse avec détection par capture
d'électrons (concentration minimale décelable de 0,005 mg/kg). Pour
l'analyse des produits techniques, on a recours à la chromatographie en
phase gazeuse avec détection par ionisation de flamme.
1.2 Production et usage
On utilise actuellement dans le monde environ 600 tonnes de
perméthrine par an, essentiellement en agriculture. Ce produit pourrait
être utilisé pour la protection des céréales ensilées et on l'emploie
en épandage aérien pour la protection des forêts, la lutte anti-
vectorielle, la destruction des insectes incommodants dans les
habitations, le déparasitage des bestiaux, la destruction des poux du
corps et l'imprégnation des moustiquaires.
La perméthrine est présentée en concentré émulsionnable, en
concentré pour application à très bas volume, en poudre mouillable, en
poudre pour poudrage et en aérosols.
1.3 Exposition humaine
Les teneurs en résidus des diverses récoltes diminuent assez
lentement, le temps de demi-élimination allant de une à trois semaines
selon les plantes. Toutefois, lorsqu'elle est utilisée conformément
aux recommandations, la perméthrine ne donne pas lieu à une accumu-
lation de résidus, même après plusieurs traitements.
Pour ce qui est de la population dans son ensemble, la voie
d'exposition à la perméthrine est essentiellement alimentaire. Les taux
de résidus dans les plantes correctement cultivées sont généralement
faibles. L'exposition qui pourrait en résulter pour la population
générale est vraisemblablement faible, mais on manque de données
précises provenant d'études sur la ration totale.
On est très peu documenté sur l'exposition professionnelle à la
perméthrine.
1.4 Destinée dans l'environnement
On a montré en laboratoire que la perméthrine se dégrade dans le
sol avec une demi-vie d'environ 28 jours. L'isomère trans se décompose
plus rapidement que l'isomère cis, la principale réaction de décompo-
sition initiale étant le clivage du groupement ester. Cette réaction
donne naissance à des composés qui subissent une oxydation plus poussée
aboutissant à l'anhydride carbonique comme produit final. On a montré,
en étudiant le potentiel de lessivage de la perméthrine et de ses
produits de dégradation, que toutes ces substances pénétraient peu
profondément dans le sol.
La perméthrine déposée sur les végétaux se dégrade avec une demi-
vie d'environ 10 jours. La principale voie de dégradation comporte le
clivage du groupement ester et la conjugaison de l'acide et de l'alcool
qui en résultent. Il se produit également une hydroxylation en divers
points de la molécule ainsi qu'une interconversion cis-trans photo-
induite.
Dans l'eau et à la surface du sol, la perméthrine est décomposée
par le rayonnement solaire. Là encore, le clivage du groupement ester
et l'interconversion cis-trans sont les principales réactions.
En général, ce processus de décomposition environnementale conduit
à des produits de moindre toxicité.
La perméthrine disparaît rapidement de l'environnement, en six
à 24 heures dans les étangs et les cours d'eau, en sept jours dans les
sédiments des étangs, et en 58 jours dans les feuilles et le sol des
forêts. On a constaté que 30% du composé disparaissaient en une semaine
des feuilles d'une plantation de cotonniers.
En conditions d'aérobiose dans le sol, la perméthrine se décompose
avec une demi-vie de 28 jours.
La perméthrine se déplace peu dans l'environnement et il est
improbable qu'elle s'y accumule en quantité notable.
1.5 Cinétique et métabolisme
Administrée à des mammifères, la perméthrine est rapidement
métabolisée et presqu'entièrement excrétée dans les urines et les
matières fécales en l'espace de 12 jours. L'isomère trans, beaucoup
plus sensible à l'attaque par l'estérase que l'isomère cis, s'élimine
plus rapidement que ce dernier. Les principales réactions métaboliques
sont le clivage du groupement ester et l'oxydation, particulièrement au
niveau du cycle aromatique terminal, du reste phénoxybenzylique et du
groupe diméthyle géminé du cycle cyclopropane, réactions qui sont
suivies d'une conjugaison. On a retrouvé moins de 0,7% de la dose
initiale dans le lait de chèvres et de vaches à qui l'on avait
administré de la perméthrine par voie orale.
1.6 Effets sur les êtres vivants dans leur milieu naturel
Des épreuves de laboratoire ont montré que la perméthrine était
extrêmement toxique pour les arthropodes aquatiques, la CL50 allant de
0,018 µg/litre pour la larve d'un crabe comestible à 1,26 µg/litre pour
un cladocère. Elle est également très toxique pour les poissons, la
CL50 à 96 heures allant de 0,62 µg/litre pour la larve de truite
arc-en-ciel à 314 µg/litre pour la truite adulte. La dose sans effet
observable au début du cycle évolutif du vairon est 10 µg/litre sur 28
jours, la dose chronique étant de 0,66 à 1,4 µg/litre pour un autre
cyprinidé, Pimephas promelas. La perméthrine est moins toxique pour
les mollusques aquatiques et les amphibiens, les valeurs de la CL50 à
96 heures se situant respectivement à plus de 1000 µg/litre et
7000 µg/litre.
Lors d'essais sur le terrain et en utilisation normale, cette forte
toxicité potentielle ne se manifeste pas. Il existe une abondante
littérature sur les effets de la perméthrine utilisée en agriculture,
dans les exploitations forestières ainsi que pour la lutte antivectori-
elle dans de nombreuses régions du monde. Il y a une certaine mortalité
pour les arthropodes aquatiques, notamment lorsque l'on traite les eaux
en surface, mais les effets sur les populations ne sont que tempor-
aires. Il n'y a pas eu de cas de mortalité chez les poissons. Cette
moindre toxicité qui se manifeste sur le terrain provient de la forte
adsorption du composé par les sédiments et de sa décomposition rapide.
La perméthrine fixée sur les sédiments est toxique pour les
organismes fouisseurs, mais là encore, l'effet est temporaire. La
perméthrine est extrêmement toxique pour les abeilles. La DL50 topique est
de 0,11 µg par abeille mais le fort effet répulsif qu'elle exerce sur
l'insecte en réduit les effets toxiques dans la pratique. Rien
n'indique qu'il puisse y avoir une forte mortalité des abeilles en
utilisation normale. La perméthrine est plus toxique pour les acariens
prédateurs que pour les espèces nuisibles visées.
La perméthrine est très peu toxique pour les oiseaux, en
administration orale ou par voie alimentaire. La DL50 aiguë est
supérieure à 3000 mg par kg de poids corporel en une seule
administration et supérieure à 5000 mg par kg de nourriture quand le
produit est mêlé à la ration. Elle n'a aucun effet sur la reproduction
de la poule à la dose de 40 mg/kg de nourriture.
Les organismes aquatiques accumulent facilement la perméthrine, le
facteur de concentration allant de 43 à 750 selon les espèces. Chez
tous les organismes aquatiques étudiés, la perméthrine disparaît
rapidement lorsque les animaux sont remis en eau propre. Il n'y a
aucune accumulation chez les oiseaux. On peut donc considérer que ce
composé ne présente en pratique aucune tendence à la bioaccumulation.
1.7 Effets sur les animaux d'expérience et sur les systèmes
d'épreuve in vitro
La perméthrine n'a qu'une faible toxicité aiguë pour les rats, les
souris, les lapins et les cobayes, encore que la DL50 varie
considérablement selon le véhicule utilisé et le rapport des isomères
cis/trans. Les signes d'intoxication aiguë apparaissent en deux heures
et persistent jusqu'à trois jours. Les isomères [1R,cis] et [1R,trans]
appartiennent aux pyréthroïdes du type-I dont les effets caractérist-
iques sont les tremblements (syndrome T) la perte de coordination,
l'hyperactivité, la prostration et la paralysie. L'intoxication pro-
voque une augmentation notable de la température centrale.
Aucun des métabolites de la perméthrine ne présente une toxicité
aiguë (par voie orale ou intrapéritonéale) supérieure à celle de la
perméthrine elle-même.
La perméthrine a provoqué une légère irritation de la peau intacte
ou abrasée chez le lapin, mais il n'y a pas eu d'irritation d'origine
photochimique après exposition aux rayons ultra-violets de zones de la
peau traitées par cet insecticide. Elle ne suscite en revanche aucune
réaction de sensibilisation chez le cobaye.
Des études de toxicité orale subaiguë et subchronique ont été
effectuées chez le rat et la souris à des doses allant jusqu'à
10 000 mg/kg de nourriture et pendant des périodes s'étendant de 14 à
26 semaines. A la dose la plus forte, on a observé une augmentation du
rapport poids du foie/poids du corps, une hypertrophie du foie et des
signes cliniques d'intoxication tels que des tremblements. Chez le rat,
la dose sans effet observable varie de 20 mg/kg de nourriture (pour les
études de 90 jours ou de six mois) à 150 mg/kg de nourriture (lors
d'une étude de six mois).
Chez le chien, ces valeurs ont été trouvées égales à 50 mg/kg de
poids corporel et à 100 mg/kg de poids corporel, respectivement, lors
de deux études de trois mois.
Des études à long terme sur des souris et des rats ont révélé une
augmentation du poids du foie, vraisemblablement liée à l'induction du
système enzymatique des microsomes hépatiques.
Une étude de deux ans sur des rats a fait ressortir une dose sans
effet observable de 100 mg/kg de nourriture, soit 5 mg/kg de poids
corporel.
D'après trois études à long terme sur la souris, il semblerait
qu'il existe un certain pouvoir cancérogène au niveau des poumons pour
au moins une souche de souris (uniquement des femelles) à la dose la
plus élevée étudiée, soit 5 g/kg de nourriture. Aucun effet oncogène
n'a été observé chez des rats des deux sexes.
La perméthrine n'est mutagène ni in vivo ni in vitro .
Les études de mutagénicité ainsi que des études à long terme sur la
souris et le rat indiquent que le pouvoir oncogène de la perméthrine
est très faible, qu'il se limite aux souris femelles et qu'il s'agit
probablement d'un phénomène épigénétique.
La perméthrine n'est pas tératogène pour le rat, la souris ou le
lapin, à des doses allant respectivement jusqu'à 225, 150 et 1800 mg/kg
de poids corporel.
La perméthrine n'a pas produit d'effet indésirable à des doses
allant jusqu'à 2500 mg/kg de nourriture lors d'une étude de repro-
duction portant sur trois générations.
Administrée à des rats à forte dose (6600 à 7000 mg/kg de
nourriture) pendant 14 jours, l'insecticide a provoqué des lésions au
niveau du nerf sciatique; cependant une autre étude n'a pas confirmé la
présence d'altérations ultrastructurales à ce niveau. Chez la poule on
n'a pas observé de neurotoxicité retardée.
1.8 Effets sur l'homme
La perméthrine peut provoquer un certain nombre de sensations au
niveau de la peau ainsi que des parésthésies chez les travailleurs
exposés, symptômes qui apparaissent après une période de latence
d'environ 30 minutes, passent par un maximum au bout de huit heures et
disparaissent en 24 heures. Parmi les symptômes les plus fréquemment
signalés, figurent un engourdissement, des démangeaisons, des four-
millements et des sensations de brûlure.
Aucun cas d'intoxication n'a été signalé.
La probabilité d'effets oncogènes chez l'homme est extrêmement
faible, voire nulle.
Rien n'indique que la perméthrine puisse exercer des effets indési-
rables sur l'homme si elle est utilisée conformément aux recommand-
ations.
2. Conclusions
2.1 Population générale
La population dans son ensemble est vraisemblablement très peu
exposée à la perméthrine. Cet insecticide ne devrait présenter aucun
danger s'il est utilisé conformément aux recommandations.
2.2 Exposition professionnelle
Utilisée de manière raisonnable et moyennant certaines mesures
d'hygiène et de sécurité, la perméthrine ne devrait présenter aucun
danger pour les personnes qui lui sont exposées de par leur
profession.
2.3 Environnement
La perméthrine ou ses produits de décomposition ne devraient pas
atteindre dans le milieu des concentrations critiques dans la mesure où
l'insecticide est utilisé aux doses recommandées. Au laboratoire, la
perméthrine est extrêmement toxique pour les poissons, les arthropodes
aquatiques et les abeilles. Toutefois il est improbable que des effets
indésirables durables se produisent en situation réelle si l'insecti-
cide est utilisé conformément aux recommandations.
3. Recommandations
Les concentrations alimentaires résultant d'une utilisation
conforme aux recommandations sont en principe faibles, toutefois il
serait bon de confirmer cette hypothèse en étendant la surveillance à
la perméthrine.
La perméthrine est utilisée depuis de nombreuses années sans
qu'aucun effet indésirable n'ait été signalé à la suite d'une
exposition humaine. Néanmoins il serait bon de poursuivre les
observations sur ce type d'exposition.
When rainbow trout (Salmo gairdneri) were held in static water
containing 5 µg/litre of 14C-permethrin for 24 h, both cis and trans
isomers were similarly taken up into the fish. The bioaccumulation
ratios for total radiocarbon in the blood, muscle, liver, and fat of
the fish were 30, 30, 300, and 400, respectively. When the fish were
transferred to fresh running water, radioactivity was eliminated, with
initial half-lives of 9-35 h, from all the tissues except fat, where
little decay in 14C-permethrin concentrations occurred. When rainbow
trout were injected intraperitoneally with 14C-permethrin at a rate of
0.5 mg/kg, 32-43% of the dose was recovered after 48 h in the bile, 3-
7% in the urine, and 31-42% in the carcass. However, the urine and bile
of the trout injected with the trans isomer contained higher levels of
radioactivity. In the bile, the major metabolite was the glucuronide
conjugate of 3-(4-hydroxyphenoxy)-benzyl-3-(2,2-dichlorovinyl)-2,2-
dimethyl-cyclopropanecarboxylate (26) and there were few metabolites
formed by hydrolysis (Fig. 4). The urine contained principally the
sulfate conjugates of polar products. The ability of rainbow trout to
hydrolyze permethrin in vivo appeared minimal (Glickman et al., 1981).
The rates of trans-permethrin hydrolysis in trout liver, kidney,
and plasma incubated at 12°C were approximately 166, 38, and 59 times,
respectively, lower than those in the corresponding mouse tissues
incubated at 37°C. Although an increase in the incubation temperature
from 12°C to 37°C caused an increase in the rate of trans-permethrin
hydrolysis by trout liver microsomes, trans-permethrin was hydrolyzed
about 45 times slower than by mouse liver microsomes at 37°C. The
hydrolysis of permethrin in trout plasma, however, was higher than that
in trout liver microsomes (Glickman & Lech, 1981).
When the microsomal preparations of both carp and rainbow trout
were fortified with NADPH, the carp microsomes oxidized permethrin iso-
mers more actively than the trout microsomes. Also, larger amounts of
hydroxy ester metabolites were recovered with the cis isomer than with
the trans isomer. The preferred site of oxidation of both isomers by
the carp and trout microsomes was the 4'-position (26) of the phenoxy-
benzyl moiety. The geminal dimethyl group was attacked in preference to
the methyl group situated trans to the carboxy group. trans-Permethrin
primarily underwent hydrolysis by both carp and trout liver microsomes
in the presence or absence of NADPH to yield PBalc (6) and Cl2CA (17)
(Glickman et al., 1979). In this respect, the results of in vitro
studies were different from those obtained in vivo.
Juvenile Atlantic salmon (lipid content 4.2%), exposed for 96 h to
static water containing 22 µg/litre permethrin, took up the insecti-
cide with a bioaccumulation ratio of 55. Dead juvenile salmon exposed
for 12.5 h to 0.098-0.994 mg/litre contained 2.21-3.69 µg/g (Zitko et
al., 1977).
Residues of approximately 0.5-1.2 mg/kg were detected in dead
juvenile Atlantic salmon exposed for 10-89 h to static water containing
permethrin at 6.9-85 µg/litre, the bioaccumulation ratio ranging from
14 to 73. The insecticide was not detected (detection limit 5 ng/g) in
dead lobster hepatopancreas or in dead shrimp (McLeese et al., 1980).
When stoneflies (Pteronarcys dorsata) were exposed for 28 days to
running water containing permethrin at 0.029-0.21 mg/litre, the bioac-
cumulation ratios of the survivors ranged from 43 to 570 (average, 183;
standard deviation, 171) (Anderson, 1982).
When carp were exposed to a 14C-permethrin isomer (phenoxyphenyl-
labelled [1R,trans], [1R,cis], [1S,trans], or [1S,cis] isomers) in a
flow-through system at 25°C, the concentrations of 14C and permethrin
isomers in the fish body reached an equilibrium on days 7-9 of ex-
posure. The bioaccumulation ratios of the permethrin isomers at equi-
librium were 330-750. When the fish were transferred to fresh water,
the permethrin isomers, as well as their metabolites, were rapidly
excreted. The biological half-lives for the permethrin isomers were
2.0-2.8 days. The major metabolic reactions involved were oxidation at
the 4 -position of the alcohol moiety or the methyl group of the acid
moiety, cleavage of the ester linkage, and conjugation of the resultant
alcohols and phenols with glucuronic acid or sulfuric acid (Ohshima et
al., 1988).
Bioconcentration factors for sheepshead minnows ( Cyprinodon vari-
egatus exposed to permethrin at concentrations between 1.25 and
10 µg/litre for 28 days from hatching varied between 290 and 620.
Maximum bioconcentration occurred after exposure at 2.5 µg/litre, and
a maximum residue of 5.7 mg/kg occurred after exposure at 10 µg/litre
(the concentrations were for whole fish) (Hansen et al., 1983).
Permethrin and its metabolites are not accumulated in birds. During
repeated dosing to quails and to mallard ducks, very similar patterns
and levels of both the appearance and depletion of radioactive residue
in tissues were found. The level in fat, which was small, reached a
plateau during a 28-day period. In all tissues, residues declined
extensively during a 14-day period after the final dose (Leahey et al.,
1977).
7. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
Toxicological profiles of permethrins with different isomeric com-
positions (cis:trans ratios of 40:60 or 25:75) were compared in a range
of toxicological studies. The toxicological profile of permethrin
(25:75) resembles that of permethrin (40:60) except that it is less
acutely toxic than permethrin (40:60).
7.1 Acute Toxicity
Table 7 shows the results of acute toxicity tests of permethrin
with various animal species. Aqueous suspensions usually produced the
least toxic results, LD50 values ranging from 3000 to >4000 mg/kg body
weight. However, corn oil is the more standard vehicle for pyrethroids
and yielded LD50 values of about 500 mg/kg (in all studies except one)
for oral administration in rats and mice.
Following oral administration of permethrin to rats, signs of
poisoning became apparent within 2 h after dosing and persisted for up
to 3 days. At lethal levels, these signs included whole body tremors of
varying degree from slight to convulsive, which in some cases were ac-
companied by salivation. Associated signs were hyperactivity and hyper-
excitability to external stimuli, urination and defecation, ataxia, and
lacrimation (Parkinson, 1978; Litchfield, 1983).
Table 8 gives the acute oral toxicity of three lots of permethrin
to rats. The observed ten-fold decrease in LD50 when corn oil or olive
oil were used could be due to enhanced absorption of the insecticide
(Metker et al, 1977).
The acute oral toxicity of permethrin (25:75) to groups of six
female C.S.E. Wistar rats was determined in five different vehicles
(Table 9). Most symptoms of acute poisoning developed within 12 h of
dosing and consisted of muscular tremors, hypersensitivity to stimuli,
and staining of abdominal fur. The majority of deaths occurred between
1 and 3 days (Wallwork & Malone, 1974).
Groups of female Sprague-Dawley rats, either fed ad libitum or
starved for 24 h beforehand, were given a single oral dose of
permethrin (25:75) (94.1% purity) in corn oil solution (40% w/v) at
750, 1500, 3000, or 6000 mg/kg. Permethrin was more toxic in starved
animals (LD50 = 3000 mg/kg) than in animals that had been fed (LD50 =
4251 mg/kg) (Piercy et al., 1976).
The acutetoxicity of permethrin with various cis- and trans-per-
methrin ratios is indicated in Table 10. These data clearly demonstrate
that cis-permethrin is more toxic than trans-permethrin to rats and
mice.
Table 7. Acute toxicity of permethrin administered to various
animal species
-------------------------------------------------------------------------
Species Sex Routea Vehicleb LD50 Reference
(mg/kg body
weight)
-------------------------------------------------------------------------
Rat M oral waterc 2949 Parkinson 1978
F oral water > 4000 Parkinson et al. 1976
M oral DMSO 1500 Clark 1978
F oral DMSO 1000 Clark 1978
M oral corn oil 500 Jaggers & Parkinson 1979
M oral corn oil 430 Kohda et al. 1979a
F oral corn oil 470 Kohda et al. 1979a
M&F oral corn oil 1200 Braun & Killeen 1975
M&F oral water 1725 Sasinovich & Panshina 1987
M dermal water > 5176 Parkinson 1978
F dermal noned > 4000 Parkinson et al. 1976
M dermal noned > 2500 Kohda et al. 1979a
F dermal noned > 2500 Kohda et al. 1979a
M&F dermal xylene > 750 Clark 1978
M&F dermal none 2000 Sasinovich & Panshina 1987
M sc corn oil 7800 Kohda et al. 1979a
F sc corn oil 6600 Kohda et al. 1979a
M ip water > 3200 Parkinson et al. 1976
F ip water > 3200 Parkinson et al. 1976
ip 463 - 1725 Sasinovich & Panshina 1987
Mouse F oral water > 4000 Parkinson et al. 1976
M&F oral DMSO 250 - 500 Clark 1978
M oral corn oil 650 Kohda et al. 1979a
F oral corn oil 540 Kohda et al. 1979a
M dermal noned > 2500 Kohda et al. 1979a
F dermal noned > 2500 Kohda et al. 1979a
M sc corn oil > 10 000 Kohda et al. 1979a
F sc corn oil 10 000 Kohda et al. 1979a
Rabbit F oral waterc > 4000 Parkinson et al. 1976
F dermal noned > 2000 Parkinson et al. 1976
Guinea- M oral water > 4000 Parkinson et al. 1976
pig
Hen oral > 1500 Milner & Butterworth 1977
-------------------------------------------------------------------------
a sc = subcutaneous; ip = intraperitoneal.
b DMSO = dimethyl sulfoxide.
c as an aqueous suspension.
d technical material applied without vehicle.
Table 8. Acute oral toxicity of three lots of permethrin to rats
------------------------------------------------------------------
Lot No.c Strain Sex Solvent LD50
(mg/kg)
------------------------------------------------------------------
827-RSP-1422 Sprague-Dawley Male None 5010
Female None 3801
827-RTP-1450 Sprague-Dawley-1a Male Corn oil 563
Sprague-Dawley-2b Male Corn oil 383
827-RTP-1450 Long-Evans Male None 4892
Female None 2712
8719-RTP-1450 Sprague-Dawley Male Olive oil 584
Female Corn oil 413
------------------------------------------------------------------
a Average body weight was 220 g.
b Average body weight was 321 g.
c Isomeric composition and the purity of the compound in each lot
were as follows:
--------------------------------------------
Lot No. Isomeric Ratio Stated
cis trans Purity
--------------------------------------------
827-RSP-1422 44% 56% 93.6%
827-RTP-1450 45% 55% 95.0%
8719-RTP-1450 46.5% 53.5% 92.4%
--------------------------------------------
Table 9. Acute toxicity of permethrin (25:75) to rats
------------------------------------------------------
Vehicle LD50 (mg/kg)
------------------------------------------------------
Neat undiluted permethrin (control) > 20 000
40% w/v in corn oil 4672
40% w/v in petroleum distillate > 8000
40% w/v in dimethylsulfoxide > 8000
20% w/v in glycerol > 5048
------------------------------------------------------
Table 10. Acute toxicity of permethrins with various cis:trans
isomeric ratios
---------------------------------------------------------------------------
Permethrin LD50
(cis:trans) Animal Sex Routea (mg/kg body Reference
weight)
---------------------------------------------------------------------------
80:20 Rat F oral 396 Jaggers & Parkinson (1979)
57:43 F oral 333
50:50 F oral 748
40:60 F oral 630
20:80 F oral 2800
99:1 Mouse ip 108 Glickman et al. (1982)
40:60 ip 514
1:99 ip > 800
99:1 Mouse iv 17 Glickman et al. (1982)
40:60 iv 31
1:99 iv > 135
---------------------------------------------------------------------------
a ip = intraperitoneal; iv = intravenous.
Table 11 shows the results of acute oral toxicity tests of the
metabolites of permethrin on rats (FAO/WHO, 1980b).
Table 11. Acute oral toxicity to rats of several metabolites
of permethrin
---------------------------------------------------------------
Chemical No.a LD50 (mg/kg
body weight)
---------------------------------------------------------------
3-phenoxybenzyl alcohol 6 1330
3-(2,2-dichlorovinyl)-2,2-dimethylcyclo- 17 980
propanecarboxylic acid
3-phenoxybenzaldehyde 11 600
---------------------------------------------------------------
a Chemical identification no. used in Fig. 3.
Table 12 shows the results of the acute intraperitoneal toxicity to
mice of permethrin metabolites (Kohda et al., 1979b).
Table 12. Acute intraperitoneal toxicity to mice of several
permethrin metabolites
---------------------------------------------------------------------
Chemicala No.b LD50 (mg/kg body weight)
Male Female
---------------------------------------------------------------------
3-phenoxybenzyl alcohol 6 71 424
3-(4 -hydroxyphenoxy)benzyl alcohol 7 750 - 1000 750 - 1000
3-(2 -hydroxyphenoxy)benzyl alcohol 8 876 778
3-phenoxybenzoic acid 12 154 169
3-(4 -hydroxyphenoxy)benzoic acid 13 783 745
3-(2 -hydroxyphenoxy)benzoic acid 14 859 912
3-phenoxybenzaldehyde 11 415 416
---------------------------------------------------------------------
a All compounds were dissolved in corn oil, except 3-phenoxybenzoic
acid, which was dissolved in DMSO.
b Chemical identification no. used in Fig. 3.
7.2 Subacute and Subchronic Toxicity
7.2.1 Oral exposure
7.2.1.1 Mouse
When male and female Alderly Park mice (20 of each sex per group)
were fed permethrin in the diet at levels of 0, 200, 400, 2000, or
4000 mg/kg diet for 28 days, mortality, growth, and food utilization
were normal for all animals. One additional group (permethrin level of
80 mg/kg for 2 weeks and 10 000 mg/kg for the final 2 weeks) showed
weight loss and poor food utilization when feeding with 10 000 mg/kg
began. Animals fed permethrin at 2000 mg/kg or more showed increased
liver weight and liver-to-body weight ratio. Higher weight and organ-
to-body weight ratios were also observed in the kidney, heart, and
spleen of males receiving a dose of 10 000 mg/kg. Gross tissue changes
were observed in females at 2000 and 10 000 mg/kg. On histopathological
examination, regenerating tubules in the renal cortex and hypertrophy
of centrilobular hepatocytes with cytoplasmic eosinophilia, which were
not dose related, were observed in all the treated animals (Clapp et
al., 1977b).
In a study by Wallwork et al. (1974a), groups of six mature female
mice received daily oral doses of permethrin (25:75) in corn oil at 0,
200, 400, 800, or 1600 mg/kg body weight for 10 consecutive days.
Signs of acute toxicity, such as spasm and convulsion, were seen only
in the highest dose group, half of which died after the initial dose.
No significant changes in haematology, clinical chemistry, or body
weights on the 11th day of dosing were recorded. The mice treated at
800 and 1600 mg/kg body weight exhibited increased liver weights.
7.2.1.2 Rat
Sprague-Dawley rats (six of each sex per group) were fed permethrin
in the diet for 14 days at dose levels of 54, 108, 216, 432, 864, or
1728 mg/kg body weight per day. All rats surviving to term were sacri-
ficed and various organs and tissues were examined histopathologically.
At the two highest dose levels, all animals died except one female fed
864 mg/kg. Muscle tremors were noted in all animals at 432 mg/kg, but
doses of 216 mg/kg or less caused no toxic signs in either males or
females. There was a statistically significant increase in average
liver-to-body weight ratios at 432 mg/kg, but compound-related histo-
logical changes were not observed in any of the tissues or organs. The
maximum NOEL in this study was 216 mg/kg (Metker et al., 1977).
In studies by Metker et al. (1977), Long-Evans rats (six of each
sex per group) were fed permethrin in the diet for 14 days at dose
levels of 0, 27, 54, 108, 216, or 432 mg/kg body weight per day. All
rats surviving to term were sacrificed and various organs and tissues
were examined histopathologically. At a dose of 432 mg/kg, three out of
six females died within the first five days. Muscle tremors were noted
in all surviving animals at 216 and 432 mg/kg. There was a statisti-
cally significant increase among female animals in the average liver-
to-body weight ratio. Compound-related histological changes were not
observed in any of the tissues or organs examined. The maximum dietary
NOEL was 108 mg/kg body weight per day.
When young male and female Wistar rats (8 of each sex per group)
were fed permethrin in the diet at dose levels of 0, 200, 500, 1000,
2500, 5000, or 10 000 mg/kg diet for 4 weeks, all rats that received
the highest dose died within 3 days. Mortality was evident at 5000
mg/kg, and hyperexcitability was observed in animals that received
2500 mg/kg. Other non-specific signs of poisoning were observed at
1000 mg/kg on the first day of the study only. Food consumption and
growth were reduced in the animals dosed at 5000 mg/kg. There was no
effect on haematological parameters, clinical chemistry, or urinalysis
except for a reduction in urinary protein excretion in males at 5000
mg/kg. Liver weight and liver-to-body weight ratios were increased in
males at 2500 mg/kg or more and in females at 1000 mg/kg or more. This
study had been designed as a preliminary range-finding test for long-
term dietary administration (Clapp et al., 1977a).
In a study of the reversibility of hepatic changes in rats follow-
ing short-term dietary administration of permethrin, female Wistar rats
(48 rats per group) were fed permethrin at levels of 0 or 2500 mg/kg
diet for 28 days. At the end of the feeding trial, rats were either
sacrificed or maintained on control diets and sacrificed at 1, 4, or
8 weeks after the termination of dosing. There was no mortality, but
food consumption, food utilization, and body weight were reduced in the
permethrin-treated rats during the administration period. However, the
animals gained weight rapidly after the dosing period and there was no
difference in body weight between control and test animals at the end
of the study period. After the 4 weeks of permethrin dosing, signifi-
cantly higher absolute and relative liver weights were observed. During
the 8-week recovery period, the relative liver weight of permethrin-
treated animals was significantly higher than the control values, but
the absolute weights of the liver of control and test animals were
similar. There were no effects of permethrin on plasma alanine transam-
inase over the course of the study. Oxidative enzyme activity in liver
microsomes was significantly higher in test animals than in controls at
the end of dosing and 1 week later. The activity of liver microsomal
enzymes in the permethrin-treated animals was normal 4 weeks after
dosing but was elevated 8 weeks after dosing. The amount of smooth
endoplasmic reticulum in rat liver cells was significantly increased as
a result of permethrin dosing, but within 4 weeks after dosing, there
were no significant histological differences in the liver between
treated and control animals (Bradbrook et al., 1977).
When male and female Charles River (CD) rats (six of each sex per
group) were fed permethrin at levels of 0, 30, 100, 300, 1000, or 3000
mg/kg diet for five weeks, persistent tremors were evident in animals
fed at 3000 mg/kg although no mortality was observed. Growth was
inhibited in both males and females at this dose level. Relative liver
weight was increased in both the males (1000 mg/kg or more) and females
(3000 mg/kg). Slight effects on certain clinical chemistry parameters,
such as increased prothrombin times in males, were noted at the 3000
mg/kg level. Examination of tissues and organs of the animals receiving
the two highest doses did not show any unusual effects as a result of
permethrin in the diet (Butterworth & Hend, 1976).
Male and female Long-Evans rats (10 of each sex per group) fed
permethrin in the diet at dose levels of 0, 20, 100, or 500 mg/kg diet
for 90 days showed no mortality, and the growth and food consumption of
all animals were normal. The results of haematology, clinical chemis-
try, urinalysis, and ophthalmological examinations were also normal.
Tremors were noted in some animals at the highest dose level, mainly
during the first week of treatment. There were significant increases
in absolute and relative liver weights at the two highest dose levels.
These increases were consistent with data from microscopic examination
of the liver showing compound-related centrilobular hepatocyte hyper-
trophy in both males and females. There were no significant effects at
the 20-mg/kg level, although slight hepatic effects were reported in a
few of the male rats (Killeen & Rapp., 1976b).
In studies by Metker et al. (1977), Sprague-Dawley rats (10 of each
sex per group) were fed permethrin in the diet for 90 days at dose
levels of 0, 9, 27, 85, 270, or 850 mg/kg body weight per day. All
rats surviving to term were killed and various tissues and organs from
each animal were examined histopathologically. At 850 mg/kg, all male
and female rats died. An increase in the average liver-to-body weight
ratio was noted in both male and female rats fed 270 mg/kg. Compound-
related histological changes were not observed in any of the tissues
and organs examined. The minimum effect level was 270 mg/kg per day.
At 85 mg/kg no effects were observed.
When male and female Sprague-Dawley rats (16 of each sex per group)
were fed permethrin in the diet at dose levels of 0, 375, 750, 1500, or
3000 mg/kg diet for 6 months, there was no mortality and all animals
exhibited normal growth and normal food and water consumption. Urinal-
ysis, haematological values, and clinical biochemistry parameters
showed no changes related to permethrin dosing. Signs of hyperexcit-
ability and tremors were observed during the study in animals dosed at
3000 mg/kg and the liver weight and liver-to-body weight ratio of these
animals were slightly increased. There were no significant histopatho-
logical findings attributable to the presence of the permethrin in the
diet. The NOEL was 1500 mg/kg (Kadota et al., 1975).
In a study designed to evaluate liver hypertrophy, male and female
Wistar rats were fed permethrin at levels of 0, 20, 100, or 1000 mg/kg
diet for 26 weeks. There was no mortality, and the growth and food con-
sumption of the animals were normal. Although the mean liver weight was
increased at all dose levels, a significant increase was noted only at
the highest dose level. The increase in liver weight at this dose level
was accompanied by an increase in the smooth endoplasmic reticulum and
in biochemical parameters associated with microsomal oxidative mechan-
isms. At a dose level of 100 mg/kg, there were slight, non-significant
increases in biochemical activities. No effects on any of the par-
ameters measured were observed in animals dosed at 20 mg/kg (Hart et
al., 1977c).
In a study by Wallwork et al. (1974b), groups of five to six female
Charles River CDI rats received permethrin (25:75) in corn oil at 0,
200, 400, or 800 mg/kg body weight by daily gavage for 10 days. The
animals were sacrificed on the eleventh day so that haematological and
clinical chemical parameters and organ weights could be investigated.
Permethrin (25:75) gave a toxicity profile similar to that of
permethrin (40:60).
7.2.1.3 Dog
Beagle dogs (four of each sex per group) fed permethrin in gelatin
capsules daily for 3 months at dose levels of 0, 5, 50, or 500 mg/kg
body weight showed no mortality, but clinical signs of poisoning were
noted at various times in both males and females at the highest dose
level. Growth and food consumption, as well as clinical chemical,
haematological, and urinalysis parameters, were normal. The liver
weights and liver-to-body weight ratios of animals that received per-
methrin at 50 mg/kg or more were significantly increased. Histopatho-
logical examination did not reveal any changes attributable to per-
methrin (Killeen & Rapp, 1976a).
Beagle dogs (four of each sex per group) administered permethrin in
gelatin capsules daily for 13 weeks at dose levels of 0, 10, 100, and
2000 mg/kg body weight likewise showed no mortality, but clinical signs
of poisoning were evident at 2000 mg/kg. Haematological, clinical
chemical, and urinalysis values were normal in all animals. There was a
slight increase in the liver weight of animals dosed at 2000 mg/kg/day,
but no accompanying histopathological changes in the liver (Edwards et
al., 1976).
When two beagle dogs were given daily oral doses of permethrin
(25:75) at 500 mg/kg body weight for 14 days, there were no clinical
signs of toxicity or significant effects of the treatment on body
weight or on clinical chemistry or haematological parameters (Chesher
et al., 1975a).
Groups of four male and four female beagle dogs, given encapsulated
permethrin [(25:75) 4.5% w/v] at 0, 10, 50, or 250 mg/kg body weight
for 6 months, revealed no signs of toxicity and no effect on body
weight. Ophthalmoscopy and electrocardiography showed no abnormalities.
At necropsy, there were no gross pathological or significant histo-
pathological findings. Haematological and clinical chemistry para-
meters, including plasma antipyrine elimination rate, were unaffected
by treatment. The results of this study indicate that daily oral doses
up to 250 mg/kg body weight do not adversely affect beagle dogs
(Reynolds et al., 1978).
7.2.1.4 Rabbits
In a study by Chesher & Malone (1974a), groups of five female Dutch
rabbits received permethrin (25:75) in 10 daily doses by gavage in corn
oil at 0, 200, 400, or 800 mg/kg body weight. The animals were killed
on the eleventh day so that clinical chemical, haematological para-
meters, and organ weights could be investigated. One rabbit, dosed at
400 mg/kg, exhibited mild hyperactivity and muscular fasciculation, but
only at days 6 and 7. Although all animals, including the controls,
exhibited some degree of weight loss, it was most marked in the high-
dose group. There were no significant haematological or clinical chem-
istry findings, but there was some decrease in liver and kidney weight
and also some enlargement of adrenal gland weights in all treated
groups.
7.2.1.5 Cow
Lactating cows (three per group) fed permethrin in the diet at dose
levels of 0, 0.2, 1.0, 10, or 50 mg/kg diet for 28 days showed no mor-
tality. Growth and milk production were normal, and no histopathologi-
cal changes in the tissues were observed (Edwards & Iswaran, 1977).
7.2.2 Dermal Exposure
Technical grade permethrin was applied daily to the clipped skin of
New Zealand White rabbits (eight males per group) at dose levels of 0,
0.10, 0.32, or 1.0 g/kg body weight, each day for 21 consecutive days.
The application site was abraded on the first test day in half of the
animals in each group. Blood samples were drawn weekly from the animals
for clinical chemistry studies. All animals were killed on the tenth
day after exposure ceased. Various tissues and organs were taken from
each animal and examined for microscopic lesions. A moderate primary
irritation of the skin was produced by permethrin. No significant
changes in body weight, organ weight, or clinical chemistry values were
evident, neither were there any compound-related lesions in the skin or
other tissues (Metker et al., 1977).
In further studies by Metker et al. (1977), permethrin (dissolved
in acetone) or acetone (as a control) was placed on the skin twice a
week for 3 weeks to 6 groups of 10 shaved male New Zealand White rab-
bits. Cotton cloth treated with permethrin (1.25 or 0.125 mg/cm2) was
applied to the skin over 1 ml of artificial sweat (salt solution imi-
tating sweat). In the case of other rabbits, similarly treated, the
sweat was omitted. In the control groups, acetone-treated cotton cloth
with or without 1 ml of sweat was used. Blood samples were collected
once a week for clinical chemistry determinations. All animals sur-
viving to term were killed and various tissues and organs from each
animal were examined for microscopic lesions. No significant changes
were noted in rabbit body weight or organ-to-body weight at the end of
the 21-day test, and no skin irritation was observed. There were no
significant changes in clinical chemistry values in the treated groups
and no compound-related lesions in the skin or other tissues and organs
examined (Metker et al., 1977).
7.2.3 Inhalation exposure
The inhalation toxicity of technical grade permethrin was deter-
mined in three species of laboratory animals. Male Hartley guinea-pigs,
male and female Sprague-Dawley rats, and male and female beagle dogs
were exposed to an aerosol of permethrin at concentrations of 125, 250,
or 500 mg/m3, 6 h per day, 5 days per week for 13 weeks. The mass
median diameter of the aerosol droplets was 5.1 µm, and 85% of the
total droplets had a diameter of 1.0 µm or less. At 500 mg/m3, tremors
and convulsions occurred in the rats during the first week of exposure
but disappeared in the second week. There was no difference in oxygen
consumption between control and treated rats. Urine metabolite studies
indicated that permethrin was rapidly metabolized and excreted. Post-
exposure experiments in male rats showed that the hexobarbital-induced
sleeping time was significantly shortened after exposures at 500
mg/m3 but not at lower doses. No clinical signs of poisoning were
observed in the guinea-pigs and dogs when exposed to aerosols of per-
methrin under similar conditions. Pulmonary function, clinical chemis-
try parameters, and blood cell counts were unaffected in the beagle
dogs following exposure. No compound-related gross or microscopic
pathological changes or other permanent changes were observed in the
dogs, rats, or guinea-pigs as a result of permethrin inhalation
(Metker, 1978).
7.3 Primary Irritation
7.3.1 Skin irritation
When undiluted technical permethrin (91.3% purity, 0.5 ml) was
applied to the clipped dorsal surface of Japanese White rabbits, there
was no irritation (Okuno et al., 1976).
Single applications of 0.05 ml of 25% (w/v) permethrin (in 95%
ethanol) or 10% (w/v) oil of bergamot solution (in 95% ethanol) (posi-
tive control) were applied to the intact skin of six rabbits. Five
minutes later, some of the rabbits were exposed to UV light (365 nm) at
a distance of 10-15 cm for 30 min (the intensity of UV light was not
mentioned). Skin treated with the positive control solution and
irradiated exhibited a greater irritation reaction than did non-
irradiated skin. Permethrin did not cause any irritation reaction
under the test conditions with or without irradiation (Metker et al.,
1977).
When a permethrin formulation was applied to the clipped dorsal
surface (0.13 mg/cm2) of six New Zealand White rabbits (three of each
sex) once a day for 16 days, a slight erythema appeared, which corre-
lated with increased cutaneous blood flow measured by laser Doppler
velocimetry. No significant histopathological changes were detected
(Flannigan et al., 1985a).
7.3.2 Eye irritation
In a study by Okuno et al. (1976), 0.1 ml of undiluted technical
permethrin (91.3% purity) was applied to the eyes of Japanese White
rabbits. The eyes were washed with distilled water 5 min or 24 h after
the application of permethrin. No eye irritation was observed.
Undiluted permethrin applied to the eyes of female rabbits caused
minimal pain, redness, chemosis of the conjunctiva, and a slight dis-
charge (Parkinson et al., 1976).
Permethrin (25:75) (40% in corn oil) did not produce any ocular
effects when 0.1 ml was instilled into the ocular sac of New Zealand
rabbits (Chesher & Malone, 1974c).
7.4 Sensitization
In a study by Parkinson et al. (1976), guinea-pigs were dermally
administered permethrin as a 10% solution in dimethylformamide for
3 consecutive days. This was followed 4 days later by challenge doses
of 0.1%, 1%, and 10% solutions of permethrin in dimethylformamide. Only
very slight erythema was observed. Permethrin was therefore considered
to be either non-sensitizing or only mildly so.
Guinea-pigs (10 per group) were initially injected intradermally
with 0.1 ml permethrin solution and 14 days later were challenged with
an intradermal injection (0.1 ml) of either a 0.1% solution of permeth-
rin or dinitrochlorobenzene (DNCB). Five other animals per group
received intradermally a challenge dose of 0.1% permethrin or DNCB
without a prior sensitizing dose. The positive control substance (DNCB)
elicited sensitization reactions in all guinea-pigs when examined 24
and 48 h after the challenge dose, whereas permethrin did not cause any
sensitization reactions (Metker et al., 1977; Metker, 1978).
Permethrin (25:75) in corn oil (1% w/v) or Freund's complete
adjuvant (1% w/v) did not produce dermal irritation or sensitization in
groups of 10 male guinea-pigs when applied as a 25% dispersion in
petrolatum. The positive control, DNCB, (5% w/v) in petrolatum produced
marked sensitization (Chesher & Malone, 1974b).
7.5 Long-term Toxicity
7.5.1 Mouse
SPF Alderly Park strain mice (70 males and 70 females per group)
were fed permethrin (cis 35-45%; trans 65-55%) at dose levels of 0,
250, 1000, or 2500 mg/kg diet for 2 years. Ten males and ten females
were designated for interim kills at 26 and 52 weeks. The mortality
rate was unaffected by the administration of permethrin. Growth was
slightly decreased at the two highest dose levels at various periods
during the study. At the interim sacrifice of 52 weeks and at the end
of study, a significant dose-dependent increase in liver-to-body weight
ratio was observed at the two highest dose levels in females (with 2500
mg/kg only at the end of the study) and at the highest dose level in
males. Hepatic aminopyrine N-demethylase activity was also substan-
tially increased, although not consistently, in both male and female
mice given the highest dose. Gross and microscopic examination of
tissues and organs (and specific examination for hepatic neoplasia) did
not reveal any significant carcinogenic effects as a result of per-
methrin administration. Many of the non-tumour abnormalities observed
were considered to be those associated with aging of the mice, charac-
terized by increased eosinophilia of the centrilobular hepatocytes.
Also, a decrease in vacuolation of the proximal tubular epithelium of
the kidney was noted at all dietary levels in males. A high incidence
of lung adenomas was observed with all animals in the study, but stat-
istical analysis suggested that this was not related to permethrin
feeding. Electron-microscopic examination of subcellular liver com-
ponents showed a proliferation of the smooth endoplasmic reticulum at
dose levels of 1000 and 2500 mg/kg. No notable effects on the sciatic
nerve were found as the result of permethrin administration (Ishmael &
Litchfield, 1988).
In studies by Hogan & Rinehart (1977) and Rapp (1978), CD-1 strain
mice (75 of each sex per group) were fed permethrin in the diet for
104 weeks. Alterations were made in the dietary dose levels during the
course of the study. From weeks 1 to 19, the animals were given dose
levels of 0, 20, 100, and 500 mg/kg diet. At week 19, the dose level
of 500 mg/kg was increased to 5000 mg/kg and maintained for 2 weeks
before returning to 500 mg/kg. At week 21, the 100 mg/kg dose was
increased to 4000 mg/kg and maintained for the remainder of the dosing
period. Growth was inhibited in males at 4000 mg/kg. With the exception
of a reduced blood glucose level in the animals receiving 4000 mg/kg,
dietary administration of permethrin had no other effects on haema-
tology or clinical chemistry parameters in the mouse. The liver weight
was higher than it was in control animals in both male and female
animals at a dose level of 500 mg/kg or more. In addition, the heart
weight was higher at 4000 mg/kg. Neoplastic changes, not associated
with dietary levels of permethrin, were observed in some animals in all
groups. While there was no direct effect with respect to hepatic neo-
plasms, it was noted that hepatocellular hypertrophy, pleomorphism, and
degeneration occurred in treated mice with increased frequency and
appeared to show a dose-response relationship. No oncogenic effects
were observed in the test animals.
7.5.2 Rat
Wistar rats (60 of each sex per group) were fed permethrin in the
diet at dose levels of 0, 500, 1000, or 2500 mg/kg diet for 2 years,
and twelve rats of each sex per group were sacrificed at 1 year. Signs
of poisoning such as tremors and hyperexcitability were noted during
the first 2 weeks of dosing in the animals that received the highest
dose. There was no mortality attributable to permethrin, and growth and
food consumption were unaffected. There were no effects on haema-
tological, ophthalmological, urological, or other clinical chemistry
parameters. Liver aminopyrine N-demethylase activity was increased in
all permethrin-treated animals. Bone marrow smears of the animals
showed no unusual findings. Gross and microscopic examination of
tissues and organs was performed after 1 and 2 years, and all animals
that died with neoplastic changes were examined. Liver weights were
higher after 1 year of dosing in male and female rats that received
permethrin at 2500 mg/kg than in the control animals. After 2 years,
the liver weight and liver-to-body weight ratios were higher in all
permethrin-treated males than in the corresponding controls. In the
females, higher values of absolute and relative liver weights, compared
to the controls, were recorded only in the group of animals dosed at
1000 mg/kg. Kidney weight was also increased, predominantly in males,
at all dose levels. Hepatocyte vacuolation was seen at 1 year in males
fed at the highest dose level only and in females at all dose levels.
The smooth endoplasmic reticulum showed significant increases at 52
weeks in both males and females at all dietary levels. At the end of
the study, notable endoplasmic reticulum increases were detected only
at the highest dose levels, although non-significant increases were
noted at all dose levels in both males and females. Examination of the
sciatic nerve showed no effects attributable to permethrin. No
oncogenic effects were noted at any dose level (Ishmael & Litchfield,
1988).
Long-Evans rats (60 males and 60 females per group) fed permethrin
in the diet at dose levels of 0, 20, 100, or 500 mg/kg for 2 years did
not reveal any mortality or adverse effects on growth, food consump-
tion, or behaviour attributable to the administration. Haematology,
clinical chemistry, and urinalysis measurements were performed at
either 6 months or 1 year and at the end of the study. There were no
compound-related effects on a wide variety of parameters examined, and
ophthalmological examination indicated no abnormalities. Blood glucose
levels were higher in the highest-dose males at 24 months and in the
highest-dose females at 18 months, compared to the values of the
control animals. Two independent evaluations of the histopathological
data concluded that there was no oncogenic potential for permethrin.
While there was a dose-dependent increase in gross liver weight in both
males and females, these values are small and not statistically
significant. The NOEL for general toxicity in this study was estimated
to be 100 mg/kg (Braun & Rinehart, 1977; Billups 1978a, b).
7.6 Carcinogenesis
Summaries by Paynter et al. (1982) of toxicological data from seven
long-term chronic toxicity/oncogenicity studies (four in mouse and
three in rat) carried out by ICI, FMC, and Burroughs-Welcome (BW) have
been made available by the US EPA. One rat study and one mouse study
performed by ICI were recently published (Ishmael & Litchfield, 1988),
and one rat study and one mouse study carried out by BW were cited in
FAO/WHO (1988). A report of the FIFRA Scientific Advisory Panel which
reviewed this data was also made available (US EPA, 1981). Table 13
summarizes the basic design of each of these studies, some of which are
also discussed in section 7.5.
7.6.1 Mouse
One of the four mouse studies referenced in Table 13 (FMC I) was
not considered for evaluation because of dose level changes in the mid-
and high-level groups and problems in histopathological methodology.
7.6.1.1 ICI study
Relevant non-oncogenic effects observed during the study consisted
of increased mortality, increased liver aminopyrine- N-demethylase ac-
tivity, increased liver weight, and eosinophilia of hepatocytes in both
males and females at 2500 mg/kg diet. The liver changes observed in
this study were considered to be related in large measure to the
induction of liver microsomal enzyme activity. Minimal liver changes
were also observed at 1000 mg/kg, but not at 250 mg/kg. A slight
increase in lung adenomas was observed in male mice at the highest dose
level. However, there was some uncertainty as to whether this increase
was related to permethrin ingestion.
7.6.1.2 FMC II study
Relevant non-oncogenic effects observed during the study consisted
of increased mortality in males at 2000 mg/kg diet, increased liver
weight in females at 2500 mg/kg and 5000 mg/kg, and increased lung
weight in females at 5000 mg/kg. Histopathologically, dose-related
"focal alveolar cell proliferation" (increased numbers of lung cells)
was observed in permethrin-treated females. As regards oncogenic
effects, there was an increased incidence of bronchio-alveolar adenomas
in female mice only. The number of female mice with adenomas and/or
carcinomas (15/74, 24/72, 35/74, and 44/75 at the four dose levels)
revealed a statistically significant dose-response relationship. Male
mice did not show this effect. However, some doubt was expressed by
the FIFRA Scientific Advisory Panel concerning the conduct of this
study.
7.6.1.3 BW study
Non-oncogenic effects observed during the study consisted of
slightly decreased mortality in females at 50 and 250 mg/kg per day,
increased liver weights in males, and increased kidney weights in
females at 250 mg/kg per day. Histopathologically, an increased inci-
dence in cuboidal/columnar metaplasia of the alveolar epithelium was
observed in the lungs of male and female mice at the highest dose. The
oncogenicity data indicated a dose-related trend in females, but not in
males, for adenomatous tumours in the lungs. No notable pattern was
observed for other neoplasms at any dose level.
7.6.1.4 Appraisal of mouse studies on carginogenicity
Consistent findings in the above three mouse studies at high dose
levels were liver changes known to be associated with the induction of
the liver microsomal enzyme system. Other histopathological effects
observed in liver, not usually associated with microsomal induction,
included multifocal hepatocytomegaly and hepatocytic pigmentation. The
incidence of lung adenomas for each of the three mouse studies is given
in Table 14.
Among the three long-term mouse studies, there was evidence of
permethrin oncogenicity in the lungs in one strain (CD-1 female only)
at the highest dose level only.
Although there was a difference between the control and treated
groups in terms of lung adenomas in these studies, these differences
were not significant when compared with historical control values. The
oncogenicity potential, as evaluated by the FIFRA Scientific Advisory
Panel, was considered to be very weak.
Table 13. Chronic toxicity and carcinogenicity studies in mice and rats
------------------------------------------------------------------------------------------------------
Study
performed Species Strain No. of Duration Dose Reference
by animals (weeks) (mg/kg diet)
------------------------------------------------------------------------------------------------------
ICI mouse Alderly Park 70 98 0, 250, 1000, 2500 Ishmael & Litchfield (1988)
(SPF)
FMC I mouse CD-1 75 104 0, 20, 500, 4000 Hogan & Rinehart (1977);
Rapp (1978)
FMC II mouse CD-1 75 104 M:0, 20, 500, 4000 Bio Dynamics (1979)
F:0, 20, 2500, 5000
BW mouse CFLP 75 92 0a, 10a, 50b, 250b James et al. (1980)
ICI rat Wistar 60 104 0, 500, 1000, 2500 Ishmael & Litchfield (1988)
FMC rat Long-Evans 60 104 0, 20, 100, 500 Braun & Rinehart (1977);
Billups (1978a,b)
BW rat Wistar 60 104 0, 10b, 50b, 250b McSheehy & Finn (1980)
------------------------------------------------------------------------------------------------------
a 100 animals as control.
b mg/kg body weight; permethrin 25% cis, 75% trans.
Table 14. Comparison of lung adenomas (%) in three mouse studies
------------------------------------------------------------------------
Male Female
control low mid high control low mid high
dose dose dose dose dose dose
------------------------------------------------------------------------
ICI study 20 12 26 32 22 16 20 30
FMC II study 22 23 28 25 16(20a) 17 35 35
BW study 26 19 23 22 3(20b) 7 10 20
------------------------------------------------------------------------
a Historical control values ranged from 23 to 60%, with a mean
of 20.4%.
b Historical control values ranged from 7.5 to 30.0%, with a mean
of 20.4%.
7.6.2 Rat
One of the three long-term rat studies referred to in Table 13,
i.e. the FMC rat study, was excluded from examination because of
serious flaws in histopathological methodology.
7.6.2.1 ICI study
Relevant non-oncogenic effects observed consisted of increased
mortality in males and decreased mortality in females at 2500 mg/kg
diet, increased liver weights in both males and females at 1000 and
2500 mg/kg and in males only at 500 mg/kg, increased liver aminopyrine-
N-demethylase activity in both males and females at 1000 and 2500
mg/kg, and hepatocyte vacuolization or hypertrophy in males and females
at 1000 and 2500 mg/kg. Additional effects observed were increased
kidney weight in males at all treatment levels and increased pituitary
weight in males at 1000 and 2500 mg/kg.
No tumours related to the ingestion of permethrin were observed in
this study.
7.6.2.2 BW study
Non-oncogenic effects observed at 250 mg/kg per day were increased
mortality in males, occasional body tremors in males and females, in-
creased liver weight in males, hepatocyte hypertrophy in males and fe-
males, and focal changes of the thyroid follicles in males and females.
The microscopic liver and thyroid changes were also observed at 50
mg/kg per day in both sexes.
With respect to tumours (including rare, unusual, or malignant neo-
plasms), none of the tumour types observed in this study were con-
sidered to be related to the ingestion of permethrin.
7.6.2.3 Appraisal of rat studies on carcinogenicity
No evidence of oncogenicity was observed in the rat studies.
7.7 Mutagenicity
7.7.1 Microorganism and insects
The mutagenic activity of permethrin was evaluated using the Ames
test. There was no increase in the number of revertant colonies at
doses up to 2500 µg permethrin/plate in five strains of Salmonella
typhimurium (TA1535, TA1537, TA1538, TA98, and TA100) with or without
S9-mix prepared from rat liver or S9 prepared from PCB-treated mice
(Longstaff, 1976; Newell & Skinner, 1976; Simmon, 1976; Suzuki, 1977).
Permethrin and six other synthetic pyrethroids were tested for
mutagenicity in S. typhimurium TA98 and TA100 strains in the presence
and absence of a metabolic-activation system. All pyrethroids tested
gave negative results (Pluijmen et al., 1984).
Two reverse-mutation tests in Escherichia coli WP2 also gave nega-
tive results (Newell & Skinner, 1976; Simmon, 1976).
When tested for mutagenicity in V79 Chinese hamster cells, permeth-
rin and five other synthetic pyrethroids were shown to be non-mutagenic
(Pluijmen et al., 1984).
In a host-mediated assay, permethrin (200 mg/kg body weight) was
orally administered to ICR mice. The indicator organism, S. typhimurium
G46, harvested from the abdominal cavity of mice 3 h after treatment,
did not reveal any mutagenic effect (Shirasu et al., 1979). In another
host-mediated assay employing a similar test system, (+)- trans-permethrin
at dose levels of 600 and 3000 mg/kg body weight and (+)- cis-permethrin
at 21 and 54 mg/kg body weight gave negative results (Miyamoto, 1976).
Permethrin was tested for its ability to induce complete and
partial chromosome loss in Drosophila melanogaster males by adding
5 mg/litre (soaked onto a filter paper) to the feeding solution.
Treated males were mated with mus-302 repair-defective females to
detect chromosome loss in the zygotes. Permethrin did not induce a
significant increase in chromosome loss, compared to negative controls
(Woodruff et al., 1983).
7.7.2 Mammals
An in vivo cytogenetic test was performed in Alderly Park rats to
assess the ability of permethrin to induce chromosomal aberration.
Permethrin was administered to groups of eight males by a single intra-
peritoneal injection or by five daily injections at doses of 600, 3000,
or 6000 mg/kg. The cytogenetic effect on bone marrow cells was evalu-
ated 24 h after the single injection and 6 h after the last multiple
dosing. No differences were observed in the rate of chromosomal aber-
rations between any permethrin-treated groups and the vehicle controls.
Two positive controls (trimethyl phosphate and mitomycin C) produced a
significantly higher incidence of chromosomal aberrations (Anderson &
Richardson, 1976).
Permethrin (25:75) gave a negative response when mouse lymphoma
L5178Y cells were treated with permethrin (up to 125 µg/ml) with or
without activation (Clive, 1977).
In dominant lethal studies, permethrin dissolved in corn oil was
administered orally for five successive days to groups of male CD mice
(15 per group) at doses of 15, 48, or 150 mg/kg. Each male was mated
with 16 virgin females, and on the 12th day of gestation the females
were killed. There was no dose-related effect on pregnancy or early or
late fetal deaths. Administration of permethrin thus had no dominant
lethal effect on male mice. On the other hand, the positive control
(ethylmethanesulfonate) induced pre-implantation losses and the early
death of embryos (McGregor & Wickramaratne, 1976a; Chesher et al.,
1975b).
7.8 Teratogenicity and Reproduction Studies
7.8.1 Teratogenicity studies
7.8.1.1 Mouse
In studies by Kohda et al. (1976b), groups of pregnant ICR mice (27
to 32 mice per group) were orally administered permethrin at dose
levels of 0, 15, 50, or 150 mg/kg body weight from day 7 to day 12 of
pregnancy. On day 18, two-thirds of the animals were sacrificed and
examined for implantation and resorption sites. Viable offspring were
examined for somatic and skeletal abnormalities, and, after 3 weeks of
lactation, pups were examined for behavioral abnormalities and for
differentiation and growth. At 6 weeks of age, all animals were sacri-
ficed and subjected to internal and external examination. There were no
effects on maternal toxicity over the course of the study. Growth and
differentiation of pregnant females were not affected by permethrin,
nor were the number of implantation sites or litter size adversely
affected. The size of individual pups and the incidence of gross
external, internal, and skeletal abnormalities were not significantly
different from those in the control mice. Permethrin, at dose levels
up to and including 150 mg/kg, did not appear to affect those animals
allowed to bear and wean young. The growth of young animals did not
appear to differ from control values, and, 3 weeks after weaning, the
surviving animals did not differ from controls with respect to growth
or major organ changes. There was no teratogenicity associated with
permethrin in this mouse bioassay, although the duration of dosing was
a little too short to cover both the early and late stage of organ
development (Kohda et al., 1976b).
7.8.1.2 Rat
In studies by McGregor & Wickramaratne (1976b), pregnant CD rats
(20 rats per group) were orally administered permethrin at dose levels
of 0, 22.5, 71.0, or 225 mg/kg from day 6 to day 16 of gestation. On
day 20, the animals were sacrificed and the corpora lutea were exam-
ined. Somatic and skeletal investigations were performed on the
fetuses. No adverse toxicological response was seen at the highest dose
used. There were no abortions or maternal deaths and no significant
differences in pregnancy frequency, corpora lutea, or total number of
implantations between treated and control rats. Placental and fetal
weights were similar to those of the controls and no skeletal or struc-
tural abnormalities were observed. Based upon the standard teratologi-
cal rat bioassay, permethrin did not show any teratological potential.
Pregnant Sprague-Dawley rats (29-34 rats per group) were orally
administered permethrin at dose levels of 0, 10, 20, or 50 mg/kg body
weight from day 9 to day 14 of pregnancy. On day 20, approximately two-
thirds of the pregnant females were sacrificed and the remaining rats
were allowed to deliver and wean pups. After lactation, the pups were
examined for behaviour and for growth and differentiation before being
sacrificed at 6 weeks of age and examined for internal and external
gross malformations. Pregnant females fed at the highest dose showed
toxic signs of poisoning, including ataxia, tremor, and a slight
reduction in body weight. There was no mortality, although fetal loss
at the highest dose level was slightly higher than that in the control
animals. A slightly higher incidence of non-ossified sternebra was
noted at 50 mg/kg. The number of implantation sites and the litter size
were not affected, and growth and differentiation were similarly unaf-
fected. Internal and external examination showed that, with the excep-
tion of the slight skeletal variation noted at 50 mg/kg, there were no
permethrin-associated changes. In those animals allowed to bear and
wean pups, there were no notable differences from control values with
respect to gestation, implantation sites, delivery, and numbers of live
young. Growth and differentiation of the offspring did not appear to
be affected by permethrin, and there were no abnormalities with respect
to gross pathology or in the weights of major tissues and organs at the
conclusion of the study. Permethrin did not elicit a teratogenic effect
in this bioassay (Kohda et al., 1976a).
In a study by Metker et al. (1977), permethrin (4, 41, and 83
mg/kg/diet), aspirin (200 mg/kg diet), and corn oil (2 ml/kg diet) were
each administered to groups of 20 pre-impregnated Sprague-Dawley rats
from day 6 to day 16 of gestation. The animals were sacrificed on day
20 of gestation, and the fetuses were removed and examined for gross
abnormalities, sex, weight, and body length. The administration of
aspirin (the positive control) resulted in significantly lower body
weight and length and a variety of abnormalities including craniorachi-
oschisis in the foetuses. Permethrin, administered to pregnant rats
during gestation by intragastric intubation, did not appear to present
a teratogenic or lethal hazard to the developing fetus.
When groups of 22 female Wistar rats received permethrin (25:75) at
0 or 200 mg/kg body weight in corn oil by daily oral gavage on days 6-
16 (inclusive) of pregnancy, treatment was without apparent effect on
maternal body weight gain or general conditions. The animals were
sacrificed on day 20 so that their uterine contents could be examined.
Treatment had no effect on the number of corpora lutea, implantations,
live fetuses, early and late fetal deaths, or fetal abnormalities.
Examination of the fetuses, which included dissection and skeletal
staining, showed no morphological effects of treatment. These results
indicate that permethrin (25:75) at 200 mg/kg body weight per day is
not fetotoxic to rats (James, 1974).
7.8.1.3 Rabbit
Mated female Dutch rabbits (18 per group) were orally administered
permethrin (at dose levels of 0, 600, 1200, or 1800 mg/kg body weight
per day) in 0.5% v/v aqueous Tween 80 from days 6-18 inclusive of
pregnancy. On day 29 of pregnancy the animals were killed and their
uteri were examined for resorptions and live implantations. The fetuses
were examined for gross abnormalities of skeleton and soft tissue. At
all dose levels, permethrin depressed body weight gain during dosing
and was embryotoxic at the two highest dose levels. It was toxic to
the dams at 1800 mg/kg body weight per day, but no teratogenic activity
was detectable at any dose level (Richards et al., 1980).
7.8.2 Reproduction Studies
7.8.2.1 Rat
Groups of Long-Evans rats (10 males and 20 females per group) were
fed permethrin at dose levels of 0, 20, and 100 mg/kg diet in a 3-
generation (two litters per generation) reproduction study. There was
no effect on mortality, mating, pregnancy, or fertility, with the
exception of the F2 mating index, which was reduced in both controls
and treatment groups. Pup survival and growth were unaffected. Haema-
tological evaluations of F2 adults between the second and third mating
showed no unusual effects. This study indicated that dietary permethrin
does not adversely affect reproduction in the rat (Schroeder &
Rinehart, 1977).
In studies by Hodge et al. (1977), groups of Wistar rats (12 males
and 24 females per group) were fed permethrin at dose levels of 0, 500,
1000, and 2500 mg/kg diet for 12 weeks. At 12 weeks the animals were
mated to initiate a standard 3-generation (two litters per generation)
reproduction study. Clinical signs of acute poisoning (tremors, etc.)
were noted, predominantly in females given the highest dose. There
were no effects attributable to permethrin on fertility, gestation,
viability of pups, sex ratio, litter size, or lactation. Ten male and
female weanlings from the second litter of the F3 generation were
examined for histopathological changes. Centrilobular hypertrophy and
cytoplasmic eosinophilia were observed at all dose levels, the inci-
dence and severity of which appeared to be dose dependent. Rats in the
third litter of the F3 generation were sacrificed on day 12 of ges-
tation for teratogenic examination, but no abnormalities were observed.
Based on the results of this study, permethrin does not appear to
induce reproductive toxicity in rats.
Spencer and Berhance (1982) fed female Sprague-Dawley rats (5-8
rats per group) permethrin in the diet at levels of 0, 500, 1000, 1500,
2000, 2500, 3000, 3500, and 4000 mg/kg diet from day 6 to day 15 of
pregnancy. Laparotomy was performed on day 20 of gestation, and the
number of live fetuses was determined. Placentae were excised and
cleaned of extraneous connective tissue. Analysis of the protein and
glycogen contents of the placentae on day 16 of pregnancy indicated
that they were only influenced by very high doses (2500-4000 mg/kg
diet) of permethrin. Analysis of variance indicated no significant
effect on protein level, but the treatment did decrease the glycogen
concentration. No significant dose-related effects on implantational
sites/intrauterine fetuses were observed. These results appeared to
confirm that permethrin possesses low mammalian toxicity.
In a 3-generation reproduction study, groups of 20 male and 20 fe-
male Wistar COBS rats received permethrin (25:75) in the diet at 0, 5,
30, and 180 mg/kg body weight per day during growth, mating, gestation,
parturition, and lactation for three generations, each with two
litters. Fetal toxicity and teratogenicity was assessed in the second
pregnancy of the F2 generation. Treatment with permethrin had no ef-
fect on general behaviour or condition, food intake, body weight gain,
or pregnancy rate of the dams, or on parturition, sex ratio, or pup
weight. A small number of rats of each group developed eye abnor-
malities, including ocular haemorrhage and chronic glaucoma, but this
was not related to the treatment. Examination of F3b fetuses showed
no treatment-related effect on sex ratio, body weight, or the occur-
rence of visceral or skeletal abnormalities. This study indicated that
permethrin (25:75) has no effect on the reproduction of rats at doses
up to 180 mg/kg body weight per day (James, 1979).
7.9 Neurotoxicity
7.9.1 Rat
When male and female Charles River rats (six of each sex per group)
were fed permethrin at dose levels of 0 or 6000 mg/kg diet for up to
14 days, severe clinical signs of poisoning were evident in all the
permethrin-treated rats. Only one permethrin-treated male survived the
14-day trial. Fragmented and swollen sciatic nerve axons and myelin
degeneration were observed in four out of five permethrin-treated ani-
mals (Hend & Butterworth, 1977).
In a short-term study designed to assess the effects of high con-
centrations of permethrin on the sciatic nerve, male Wistar rats (10
per group) were fed permethrin at dose levels of 0, 2500, 3000, 3750,
4500, 5000, and 7000 mg/kg diet for 14 days. Clinical signs of acute
poisoning and death occurred in the animals that were dosed at 5000 or
7000 mg/kg. Some rats that received the lower dose levels showed slight
to moderate tremors, and food consumption and growth were reduced in
these animals. At the two lowest dose levels, clinical signs of poison-
ing disappeared within the first week whereas, at the higher dose
levels, signs of poisoning persisted throughout the study. Rats
receiving permethrin at doses of up to 4500 mg/kg showed no ultra-
structural changes in their sciatic nerve. A variety of mild ultra-
structural changes, such as vacuolation and swelling of unmyelinated
fibres and hypertrophy of Schwann cells, were observed in the nerves of
rats receiving 5000 mg/kg (Glaister et al., 1977).
A detailed morphological evaluation of the nervous system was per-
formed on rats in two long-term feeding studies. In the first, Long-
Evans rats were fed diets containing permethrin at concentrations of 0,
20, 100, or 500 mg/kg diet for 2 years, and five male and five female
randomly selected survivors from each group were examined. In the
second study, Long-Evans rats were fed diets containing permethrin at
concentrations of 0, 20, or 100 mg/kg diet for three successive gener-
ations, and five male and five female rats from each group were ran-
domly selected from the third generation parental animals. Examination
of central and peripheral nerves and of extensive morphometric data and
teased myelinated fibers of distal sural and tibial nerves and of the
maxillary division of the fifth cranial nerve did not reveal any
changes attributable to the feeding of the pesticide (Dyck et al.,
1984).
When groups of 10 male and 10 female Sprague-Dawley rats were given
permethrin (25:75) (94.5% pure) at 4000, 6000, or 9000 mk/kg diet for
21 days, all animals developed severe trembling and lost weight. Some
of the highest-dose rats of each sex died. Subsequent examination of
brain, spinal cord, trigeminal and dorsal root ganglia, proximal and
distal root trunks, and terminal motor and sensory nerves revealed no
consistent histopathological abnormalities (Dayan, 1980).
7.9.2 Hen
Hens were administered permethrin orally (cis:trans=1:1) (as a 40%
w/v solution in dimethylsulfoxide) at a daily dose level of 1 g/kg body
weight for 5 days. After 3 weeks, the dosing regimen was repeated, and
the animals were maintained for an additional period of 3 weeks before
being sacrificed. There were no signs of neurological disturbance or
mortality in any of the animals. All hens treated with tri- ortho-cresyl phos-
phate (TOCP) (positive control) displayed clinical and histopathologi-
cal evidence of neurotoxicity, whereas none of the birds dosed with
permethrin showed any signs of intoxication. Histological examination
of nerve tissues revealed no lesions. Hence, permethrin was considered
to have no delayed neurotoxic potential such as that associated with
certain organophosphates (Millner & Butterworth, 1977).
In studies by Ross & Prentice (1977), 15 adult hens were orally
administered permethrin at 9 g/kg body weight and again 21 days later.
After a further 21 days, they were sacrificed. All positive control
animals (given TOCP at 500 mg/kg) showed signs of delayed neurotoxicity
ranging from slight muscular incoordination to paralysis. No signs of
ataxia were recorded in any of the hens in the permethrin-treated or
negative control groups. Histopathological examination of the nervous
tissues of permethrin-treated animals revealed none of the degenerative
changes noted in the tissues of the positive controls.
7.10 Behavioural Effects
Behavioural observations were carried out on immature male Sprague-
Dawley rats habituated to inhalation of permethrin aerosols. Habitu-
ation was carried out by exposing three groups of rats (five per group)
to aerosols of permethrin firstly at 500 mg/m3 for 21 days, then at
1000 mg/m3 for an additional 21 days. Three other groups of rats (five
per group) served as controls; they were similarly treated but were not
exposed to permethrin. At the end of this conditioning period, all
rats, including the controls, were exposed to a permethrin aerosol at
5000 mg/m3 for 4 h. At the end of the habituation period, there were
no differences in retention of avoidance training or the ability to
learn the same task between controls and aerosol-exposed groups. How-
ever, after exposure to permethrin at 5000 mg/m3, the non-habituated
control group of rats showed significantly lower retention capacity
compared with the habituated rats or with their own pre-exposure per-
formances. The non-habituated control rats also showed decreases in
coordination and balance and a higher incidence of conflict behaviour
and tremors. The performance of the rats in the habituated groups was
not changed (Sherman, 1979).
7.11 Miscellaneous Studies
The pharmacological action of permethrin on nictitating membrane,
blood pressure, respiration, heart rate, and isolated ileum was inves-
tigated in the rabbit, guinea-pig, and cat. Permethrin reduced the
incidence and amplitude of contraction of isolated rabbit ileum but
induced no changes in a similar preparation from the guinea-pig. Intra-
venous administration of permethrin at doses of 4 mg/kg or more affec-
ted blood pressure and respiration in all animals. The hypotensive
effect was not affected by pretreatment with atropine or propanolol.
Permethrin was shown to produce slight contraction of the nictitating
membrane. An increase in pulse rate was observed in the electrocardio-
gram (ECG) of the rabbit at dose levels above 4 mg/kg, but was not
accompanied by changes in the wave pattern (Nomura & Segawa, 1979).
In the Japanese White rabbit, the intravenous administration of
lethal doses of permethrin caused changes in the electroencephalogram
(EEG) tracings. Spike waves and an increased amplitude of slow waves
were induced at 100 mg/kg body weight. At a sub-lethal dose of 30
mg/kg, permethrin did not induce changes in the EEG (Takahashi et al.,
1979).
There was no change in hexobarbital-induced sleeping time in ICR
mice intraperitoneously administered a single dose of permethrin at
dose levels of up to 2000 mg/kg body weight (Takahashi et al., 1979).
Sprague-Dawley rats (three groups of 10 rats each) were pretreated
for four consecutive days with an intraperitoneal injection of either
sodium phenobarbital at 100 mg/kg (positive control group), permethrin
at 575 mg/kg (test group), or corn oil at 2.0 ml/kg (solvent control
group). On the fifth test day the rats received an intraperitoneal
injection of hexobarbital (220 mg/kg). The hexobarbital-induced sleep-
ing times of the permethrin-treated rats were significantly shorter
than those of the solvent control animals but were similar to those of
the phenobarbital positive control (Metker et al., 1977).
Permethrin and cypermethrin were evaluated for their ability to
alter microsomal cytochrome P-450 and NADPH cytochrome c reductase in
Long-Evans rats. When permethrin (cis:trans = 80:20) was orally admin-
istered to rats at 50 mg/kg body weight per day, it increased cyto-
chrome P-450 after 4, 8, or 12 days of administration and NADPH cyto-
chrome c reductase after 8 or 12 days, whereas cypermethrin (alpha-cyano
analogue of permethrin) did not induce either cytochrome P-450 or the
reductase. Neither of the two pyrethroids altered body weight gain
(Carlson & Schoening, 1980).
7.12 Mechanism of Toxicity (Mode of Action)
Based on the signs of toxicity to mammals (Verschoyle & Aldridge,
1980; Lawrence & Casida, 1982) and to cockroaches (Gammon et al.,
1981), pyrethroids may be classified into two types: Type I and Type II
compounds (see Appendix I). 1R- cis- and 1R- trans-permethrin belong
to Type I. Electrophysiological recordings from dosed cockroaches
reveal trains of cercal sensory spikes and, sometimes, spike trains
from the cercal motor nerves and the central nervous system. The signs
of poisoning caused by Type I pyrethroid compounds are restlessness,
incoordination, hyperactivity, prostration, and paralysis (Gammon et
al., 1981).
1RS- cis-permethrin and 1RS- trans-permethrin cause tremor (known
as T-syndrome) when injected intravenously into rats at a dose level of
more than 270 mg/kg body weight. The onset of the T-syndrome is usually
rapid. Rats suffering from T-syndrome exhibit aggressive sparring be-
haviour and increased sensitivity to external stimuli. This is followed
by the appearance of a slight tremor, which gradually becomes more
severe and finally reaches a state of prostration and vigorous whole
body tremor. The core temperature is markedly increased during poison-
ing; this may result from the excessive muscular activity associated
with tremor (Verschoyle & Aldridge, 1980).
Exposure of sensory nerve fibres from clawed frogs (Xenopus laevis)
to permethrin (10-7 to 10-5 mol/litre) resulted in marked repetitive
activity. This heightened activity was not observed in the motor
fibres of frogs that were similarly tested. Treatment of isolated
lateral-line preparations of frogs with permethrin (5 x 10-6 mol/litre)
also resulted in pronounced repetitive activity (Van den Bercken &
Vijverberg, 1980b).
Permethrin (cis, trans, and technical grade) and deltamethrin, as
representatives of the non-cyano- and cyano-containing classes,
respectively, of synthetic pyrethroids, were examined regarding their
major site of action on the mammalian nervous system in mice. ED50
values for the ability of both types of pyrethroids to cause pros-
tration and loss of righting reflex were estimated following either
intravenous or intracerebroventricular injections. The comparative
potencies of the four pyrethroids (deltamethrin > cis-permethrin >
technical grade permethrin > trans-permethrin) were the same following
either route of administration. All four compounds tested showed a much
greater potency (> 200-fold for deltamethrin, cis-permethrin, and
technical grade permethrin, and 85-fold for trans-permethrin) after
intracerebroventricular administration than after intravenous admin-
istration. In addition, the poisoning symptoms seen following direct
central injection were almost identical to those obtained with periph-
eral administration. These results suggest that poisoning from both
classes of pyrethroids in mammals is due predominantly to central
mechanisms (Staatz et al., 1982).
8. EFFECTS ON HUMANS
Although permethrin has been used for many years, no human poison-
ing cases have been reported.
8.1 Occupational Exposure
Data on permethrin human toxicity are scanty. Studies of occu-
pational exposure to permethrin were reported in Sweden (Kolmodin-
Hedman et al., 1982). In the first study, six forestry workers using
an aqueous emulsion of permethrin (trans:cis=75:25) were studied. The
duties of these workers involved dipping conifer seedlings in a 2%
aqueous emulsion of permethrin (one worker) and packaging the
permethrin-treated seedlings (five workers). The permethrin concen-
trations in the breathing zones of these workers varied between 0.011
and 0.085 mg/m3. One person excreted permethrin metabolites at
0.26 µg/ml urine the following morning, but the same afternoon the
urinary excretion of permethrin residues was below the detection limit
of 0.1 µg/ml. The urine from other workers did not contain detectable
amounts of permethrin residues. No symptoms of permethrin poisoning
were reported in this field study. The second study, performed on the
basis of a questionnaire and interviews, was conducted 1-2 months after
the planting season. It involved 87 persons at various plant nurseries
that used the insecticide (trans:cis=60:40 or 75/25). This study showed
that the major work-related symptoms amongst workers were irritative,
such as itching and burning of the skin, and itching and irritation of
the eyes. Irritative symptoms in the skin and upper respiratory tract
were reported in 63% of workers who were exposed to permethrin
(trans:cis=75:25) and 33% who were exposed to permethrin with a
different isomeric composition (trans:cis=60:40). The frequency of each
symptom was about 10% in each case. Increased nasal secretion was
reported by 13% of the workers handling permethrin (trans:cis= 75:25).
Le Quesne et al. (1980) examined 23 laboratory workers involved in
field trials, formulation, or general laboratory work with permethrin,
cypermethrin, fenvalerate, and fenpropathrin. The study involved
electrophysiological monitoring and interviews to ascertain subjective
symptoms. The most frequently reported symptom was a facial sensation
described as tingling, burning, or nettle rash by workers who had ex-
perienced it on one or more occasions. This sensation usually occurred
about 30 min to 3 h after exposure and lasted for about 30 min to 8 h.
Apparently this did not occur when permethrin alone was involved. All
the workers were examined neurologically and no abnormal findings were
recorded. Electro-physiological measurements from these workers were
compared with those of an age-matched control group. No difference in
response was found between the two groups.
Studies of pesticide contamination of clothing worn by crop con-
sultants during permethrin application to soybean fields were performed
to assess the extent of dermal exposure to the pesticide. The suits
and T-shirts were removed and wrapped in aluminum foil, placed on ice
and transported to the laboratory where they were held in a freezer
until residue analysis was performed. Specimens from the thigh, arm,
and chest of each suit and from the arm and chest of each T-shirt were
collected, extracted with hexane and analyzed by GC-ECD. Measurable
residues of permethrin were detected only in leg specimens (Cloud et
al., 1987).
As part of an evaluation of permethrin (25:75) (5% wettable powder)
in Nigeria, medical surveillance, including urinalysis of staff engaged
in bagging, mixing, or spraying, was undertaken. Medical history,
pulse, and blood pressure were recorded and urine was collected twice
daily. This surveillance revealed no effects attributable to permeth-
rin. Despite the use of protective clothing, up to 2 mg of permethrin
was excreted within 24 h of exposure (Rishikesh et al., 1978).
8.2 Clinical Studies
Flannigan & Tucker (1985) and Flannigan et al. (1985a,b) studied
the difference in the degree of paraesthesia induced by a number of
pyrethroids. On five occasions, 0.05 ml of field-strength-formulated
permethrin (0.13 mg/cm2) was applied to a 4 cm2 area of earlobe.
The opposite earlobe received distilled water. Participant evaluation
after each application continued for 48 h and involved description of
the cutaneous sensations. Each participant was treated after each
application with one of the remaining compounds. Permethrin, like the
other pyrethroids, induced skin sensations. Paraesthesia developed with
a latency period of approximately 30 min, peaked by 8 h, and deterio-
rated within 24 h. In the case of permethrin these sensations were
approximately four times less marked than those induced by cypermethrin
and fenvalerate, which both contain an alpha-cyano-group. It was also
found that local application of dl-alpha-tocopheryl acetate markedly
inhibited the occurrence of skin sensations.
To assess the safety of permethrin dusts for the control of human
body lice, approximately 350 people were individually dusted with 50 g
of powder containing either 2.5 or 5.0 g permethrin/kg. Urine samples,
taken at the time of treatment and subsequently, indicated that maximal
absorption of permethrin was 39 µg/kg, 24 h after treatment (Nassif
et al., 1980).
In a study to assess the degree of dermal absorption of permethrin
from impregnated clothing, a group of 10 male volunteer soldiers for
48 h wore military clothing that had previously been treated with an
aqueous suspension of permethrin (0.2% w/v). Subsequent analysis
showed that the mean permethrin (25:75) concentration of the shirts
and trousers was 0.32 g/100 g. However, the average individual
exposure to permethrin was 3.8 mg/day. No volunteers complained of
irritation and there were no abnormal findings on physical examination
(Farquhar et al., 1981a).
When dermally exposed to permethrin [(25:75) 1% w/w in soft
paraffin] for up to 9 days using a patch test, 2 out of 17 volunteers
developed mild erythema (Pegum & Doughty, 1978).
To assess the human tolerance, absorption, and persistence of
permethrin when used against human lice, 10 adult volunteers (four men,
six women) were treated with 15-40 ml of permethrin (25:75) (1%) head
louse solution. Their hair was allowed to dry naturally and then
washed with baby shampoo. Urine samples were collected at 0-24, 24-48,
120-144, and 336-360 h to measure dermal absorption. On assessment, 3
out of 10 volunteers developed mild, patchy erythema, which faded
between days 4-7. Permethrin excretion during the first 24 h was only
about 1% of the applied dose, while the cumulative maximum over 14 days
was only about 5.5 mg (Farquhar et al., 1981b).
9. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
The Joint FAO/WHO Meeting on Pesticide Residues (JMPR) discussed
and evaluated permethrin in 1979, 1980, 1981, 1982, 1983, 1984, 1985,
and 1987 (FAO/WHO, 1980a,b; 1981a,b; 1982a,b; 1983a,b; 1984a,b;
1985a,b; 1986a,b; 1987).
An acceptable daily intake (ADI) of 0-0.05 mg/kg body weight
(cis:trans ratios of 40:60 and 27:75) was established in 1985.
Maximum residue limits of 0.01-50 mg/kg for specified foods and 20-
100 mg/kg dry weight for specified feed have been proposed (FAO/WHO,
1986).
In the WHO recommended classification of pesticides by hazard,
permethrin as a technical product is classified in class II (i.e., as
moderately hazardous) (WHO, 1988). A WHO/FAO data sheet on permethrin
exists (WHO/FAO, 1984).
REFERENCES
AGNIHOTRI, N.P., JAIN, H.K., & GAJBHIYE, V.T. (1986) Persistence of
some synthetic pyrethroid insecticides in soil, water and sediment -
Part I. J. entomol. Res., 10: 147-151.
ALINIAZEE, M.T. & CRANHAM, J.E. (1980) Effect of four synthetic
pyrethroids on a predatory mite, Typhlodromus pyri, and its prey,
Panonychus ulmi, on apples in southeast England. Environ. Entomol., 9:
436-439.
ANDERSON, D. & RICHARDSON, C.R. (1976) Permethrin (PP557):
cytogenetic study in the rat (Report No. CTL/P/296) (Unpublished
report submitted to WHO by ICI Central Toxicology Laboratory).
ANDERSON, R.L. (1982) Toxicity of fenvalerate and permethrin to
several nontarget aquatic invertebrates. Environ. Entomol., 11: 1251-
1257.
BAKER, P.G. & BOTTOMLEY, P. (1982) Determination of residues of
synthetic pyrethroids in fruit and vegetables by gas-liquid and high-
performance liquid chromatography. Analyst, 107: 206-212.
BATTELLE (1982) Pesticide programme of research and market planning.
Part I: Insecticides, Geneva, Institut Battelle (October 1982).
BATTELLE (1986) World Pesticide Programme, Insecticide II, 1984,
Geneva, Institut Battelle.
BILLUPS, L.H. (1978a) Histopathologic examination of a twenty-four
month toxicity/carcinogenicity study of compound FMC33297 in rats, (Un-
published report submitted to WHO by FMC Corporation, Environmental
Pathology Services).
BILLUPS, L.H. (1978b) Twenty-four month toxicity/carcinogenicity
study of compound FMC33297 in rats (Unpublished report submitted to
WHO by FMC Corporation, Environmental Pathology Services).
BIO-DYNAMICS (1979) Twenty-four month toxicity/carcinogenicity study
of permethrin in mice (Bio-Dynamics Inc. Project No. 76-1695/9)
(Unpublished report submitted to WHO by FMC Corporation).
BORTHWICH, P.W. & WALSH, G.E. (1981) Initial toxicological assessment
of Ambush, Bolero, Bux, Dursban, Fentrifanil, Larvin, and Pydrin:
Static acute toxicity tests with selected estuarine algae,
invertebrates, and fish, Springfield, Virginia, National Technical
Information Service, pp. 1-9 (EPA-600/4-81-076).
BRADBROOK, C., BANHAM, P.B., GORE, C.W., PRATT, I., & WEIGHT,
T.M. (1977) PP557: A study of the reversibility of hepatic
biochemical and ultrastructural changes in the rat (Report No.
CTL/P/354) (Unpublished Data submitted to WHO by ICI Central Toxicology
Laboratory).
BRAUN, W.G. & KILLEEN, J.C. (1975) Acute oral toxicity in rat:
compound no. FMC33297 (Bio-Dynamics Inc. Project) (Unpublished report
submitted to WHO by FMC Corporation).
BRAUN, W.G. & RINEHART, W.E. (1977) A twenty-four month oral
toxicity/carcinogenicity study of FMC33297 in rats (Bio-Dynamics Inc.
Project) (Unpublished report submitted to WHO by FMC Corporation).
BRAUN, H.E. & STANEK, J. (1982) Application of the AOAC multi-residue
method to determination of synthetic pyrethroid residues in celery and
animal products. J. Assoc. Off. Anal. Chem., 65: 685-689.
BUTTERWORTH, S.T.G. & HEND, R.W. (1976) Toxicity studies on the
insecticide WL43470: a five week feeding study in rats (Report No.
TLGR.0056.76) (Unpublished data submitted to WHO by Shell Research
Ltd).
CARLSON, G.P. & SCHOENIG, G.P. (1980) Induction of liver microsomal
NADPH cytochrome c reductase and cytochrome P-450 by some new synthetic
pyrethroids. Toxicol. appl. Pharmacol., 52: 507-512.
CARROLL, B.R., WILLIS, G.H., & GRAVES, J.B. (1981) Permethrin
concentration of cotton plants, persistence in soil and loss in runoff.
J. environ. Qual., 10: 497-500.
CHESHER, B.C. & MALONE, J.C. (1974a) 10-day cumulative oral toxicity
study with 21Z73 in rabbits, Berkhamsted, Wellcome Research
Laboratories (Report No. HEFG 74-7) (Unpublished data submitted to
WHO).
CHESHER, B.C. & MALONE, J.C. (1974b) Guinea pig sensitization study
with 21Z73 using the maximisation test method, Berkhamsted, Wellcome
Research Laboratories (Report No. HEFG 74-3) (Unpublished data
submitted to WHO).
CHESHER, B.C. & MALONE, J.C. (1974c) Occular irritancy of 21Z73 in
rabbits, Berkhamsted, Wellcome Research Laboratories (Report no. HEFG
74-6) (Unpublished data submitted to WHO).
CHESHER, B.C., CLAMPITT, R.C., & MALONE, J.C. (1975) 21Z73 -
Preliminary investigations into the cumulative oral toxicity on dogs,
Berkhamsted, Wellcome Research Laboratories (Report no. HEFG 75-9)
(Unpublished data submitted to WHO).
CLAPP, M.J.L., BANHAM, P.B., CHART, I.S., GLAISTER, J., GORE, C., &
MOYES, A. (1977a) PP557: 28 day feeding study in rats (Report No.
CTL/P/355) (Unpublished data submitted to WHO by ICI Central Toxicology
Laboratory).
CLAPP, M.J.L., BANHAM, P.B., GLAISTER, J.R., & MOYES, A. (1977b)
PP557: 28 day feeding study in mice (Report No. CTL/P/354)
(Unpublished data submitted to WHO by ICI Central Toxicology
Laboratory).
CLARK, D.G. (1978) Toxicology of WL 43479: acute toxicity of WL43479,
Sittingbourne, Shell Research Ltd (Report No. TLGR. 0043.78)
(Unpublished data submitted to WHO).
CLIVE, C.D. (1977) Mutagenicity of BW21Z73 in L5178Y/TK mouse
lymphoma cells with and without exogenous metabolic activation, Re-
search Triangle Park, NC, Burroughs Wellcome Co. (Report No.
TTEP/77/0011) (Unpublished data submitted to WHO).
CLOUD, R.M., ZIMPFER, M.L., YANES, J.JR., BOETHEL, D.J., BUCO, P.,
& HARMON, C.W. (1987) Insecticide residues on clothing worn by crop
consultants in soybean fields treated with non-conventional application
technology. Bull. environ. Contam. Toxicol., 38: 277-282.
COATS, J.R. & O'DONNELL-JEFFERY, N.L. (1979) Toxicity of four
synthetic pyrethroid insecticides to rainbow trout. Bull. environ.
Contam. Toxicol., 23: 250-255.
COX, R.L. & WILSON, W.T. (1984) Effects of permethrin on the behavior
of individually tagged honey bees, Apis mellifera L. (Hymenoptera:
Apidae). Environ. Entomol., 13: 375-378.
CRIDLAND, J.S. & WEATHERLEY, B.C. (1977a) Urinary excretion in man of
3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropane carboxylic acid ("CVA")
after oral ingestion of permethrin (NRDC 143) - A first report, Bech-
enham, Wellcome Research Laboratories (Report No. BDPE-77-1)
(Unpublished data submitted to WHO).
CRIDLAND, J.S. & WEATHERLEY, B.C. (1977b) An estimate of permethrin
(NRDC 143; OMS1821) absorbed by people employed in a field trial of the
insecticide, Bechenham, Wellcome Research Laboratories (Report No.
BDPE-77-3) (Unpublished data submitted to WHO).
DAYAN, A.D. (1980) 21-day neuropathological study in the Sprague-
Dawley rat of permethrin (212732J) administered in the diet,
Berkhamsted, Wellcome Research Laboratories (Report No. BP 80-48)
(Unpublished report submitted to WHO by Wellcome Foundation Ltd).
DOYLE, R.D., KAUFMAN, D.D., BURT, G.W., & DOUGLASS, L. (1981)
Degradation of cis-permethrin in soil amended with sewage sludge or
dairy manure. J. agric. food Chem., 29: 412-414.
DYCK, P.J., SHIMONO, M., SCHOENING, G.P., LAIS, A.C., OVIATT, K.F.,
& SPARKS, M.F. (1984) The evaluation of a new synthetic pyrethroid
pesticide (permethrin) for neurotoxicity. J. environ. Pathol. Toxicol.
Oncol., 5: 109-117.
EDWARDS, D.B., OSBORN, B.E., DENT, N.J., & KINCH, D.A. (1976)
Toxicity study in beagle dogs (oral administration for three months),
Inveresk, United Kingdom, Inveresk Research International Ltd
(Submitted to WHO by ICI Ltd).
EDWARDS, M.J. & ISWARAN, T.J. (1977) Permethrin: residue transfer and
toxicology study with cows fed treated grass nuts (Report No.
TMJ1519/B) (Unpublished report submitted to WHO by ICI Plant Protection
Division).
ELLIOTT, M. (1977) Synthetic pyrethroids, Washington, DC, American
Chemical Society, p. 229 (ACS Symposium Series 42).
ELLIOTT, M., FARNHAM, A.W., JANES, N.F., NEEDHAM, P.H.,
PULMAN, D.A., & STEVENSON, J.H. (1973) A photostable pyrethroid.
Nature (Lond.), 246: 169-170.
ELLIOTT, M., JANES, N.F., PULMAN, D.A., GAUGHAN, L.C., UNAI, T.,
& CASIDA, J.E. (1976) Radiosynthesis and metabolism in rats of the 1R-
isomers of the insecticide permethrin. J. agric. food Chem., 24: 270-
276.
EVANS, M.H. (1976) End-plate potentials in frog muscle exposed to a
synthetic pyrethroid Pestic. Biochem. Physiol., 6: 547-550.
EVERTS, J.W., KORTENHOFF, B.A., HOOGLAND, H., VLUG, H.J.,
JOCQUE, R., & KOEMAN, J.H. (1985) Effects on non-target terrestrial
arthropods of synthetic pyrethroids used for the control of the tsetse
fly (Glossina spp.) in settlement areas of the southern Ivory Coast,
Africa. Arch. environ. Contam. Toxicol., 14: 641-650.
FAO (1982) Second Government Consultation on International
Harmonization of Pesticide Registration Requirements, Rome, 11-15
October 1982, Rome, Food and Agriculture Organization of the United
Nations.
FAO/WHO (1980a) Pesticide residues in food. Report of the 1979 Joint
Meeting of the FAO Panel of Experts on Pesticide Residues in Food and
the Environment and the WHO Expert Group on Pesticide Residues, Rome,
Food and Agriculture Organization of the United Nations, pp. 52-55 (FAO
Plant Production and Protection Paper 20).
FAO/WHO (1980b) 1979 Evaluations of some pesticide residues in food,
Rome, Food and Agriculture Organization of the United Nations,
pp. 369- 425 (FAO Plant Production and Protection Paper 20 Sup).
FAO/WHO (1981a) Pesticide residues in food. Report of the 1980 Joint
Meeting of the FAO Panel of Experts on Pesticide Residues in Food and
the Environment and the WHO Expert Group on Pesticide Residues, Rome,
Food and Agriculture Organization of the United Nations, pp. 50-51 (FAO
Plant Production and Protection Paper 26).
FAO/WHO (1981b) 1980 Evaluations of some pesticide residues in
food, Rome, Food and Agriculture Organization of the United Nations,
pp. 360-393 (FAO Plant Production and Protection Paper 26 Sup).
FAO/WHO (1982a) Pesticide residues in food. Report of the 1981
Joint Meeting of the FAO Panel of Experts on Pesticide Residues in Food
and the Environment and the WHO Expert Group on Pesticide Residues,
Rome, Food and Agriculture Organization of the United Nations, pp.
37-39 (FAO Plant Production and Protection Paper 37).
FAO/WHO (1982b) 1981 Evaluations of some pesticide residues in food,
Rome, Food and Agriculture Organization of the United Nations,
pp. 385-425 (FAO Plant Production and Protection Paper 42).
FAO/WHO (1983a) Pesticide residues in food. Report of the 1982 Joint
Meeting of the FAO Panel of Experts on Pesticide Residues in Food
and the Environment and the WHO Expert Group on Pesticide Residues,
Rome, Food and Agriculture Organization of the United Nations, pp. 36-
38 (FAO Plant Production and Protection Paper 46).
FAO/WHO (1983b) 1982 Evaluations of some pesticide residues in food,
Rome, Food and Agriculture Organization of the United Nations,
pp. 329-350 (FAO Plant Production and Protection Paper 49).
FAO/WHO (1984a) Pesticide residues in food. Report of the 1983
Joint Meeting of the FAO Panel of Experts on Pesticide Residues in Food
and the Environment and the WHO Expert Group on Pesticide Residues,
Rome, Food and Agriculture Organization of the United Nations, p. 38,
57, 62, 65 (FAO Plant Production and Protection Paper 56).
FAO/WHO (1984b) 1983 Evaluations of some pesticide residues in food,
Rome, Food and Agriculture Organization of the United Nations,
pp. 307-313, 501 (FAO Plant Production and Protection Paper 61).
FAO/WHO (1985a) Pesticide residues in food. Report of the 1984 Joint
Meeting of the FAO Panel of Experts on Pesticide Residues in Food and
the Environment and the WHO Expert Group on Pesticide Residues, Rome,
Food and Agriculture Organization of the United Nations, pp. 63, 85, 93
(FAO Plant Production and Protection Paper 62).
FAO/WHO (1985b) 1984 Evaluations of some pesticide residues in food,
Rome, Food and Agriculture Organization of the United Nations,
pp. 441-444, 723 (FAO Plant Production and Protection Paper 67).
FAO/WHO (1985c) Guide to Codex recommendations concerning pesticide
residues. Part 8. Recommendations for methods of analysis of pesticide
residues, 3rd ed., Rome, Codex Committee on Pesticide Residues.
FAO/WHO (1986a) Pesticide residues in food. Report of the 1985 Joint
Meeting of the FAO Panel of Experts on Pesticide Residues in Food and
the Environment and the WHO Expert Group on Pesticide Residues, Rome,
Food and Agriculture Organization of the United Nations, p. 37 (FAO
Plant Production and Protection Paper 68).
FAO/WHO (1986b) 1985 Evaluations of some pesticide residues in food,
Part I - Residues, Rome, Food and Agriculture Organization of the
United Nations, pp. 201-203 (FAO Plant Production and Protection Paper
No 72/1).
FAO/WHO (1986c) Codex Maximum Limits for pesticide residues, 2nd ed.,
Rome, Food and Agriculture Organization of the United Nations, Joint
FAO/WHO Food Standards Programme, Codex Alimentarius Commission,
(CAC XIII).
FAO/WHO (1987) Pesticide residues in food. Report of the 1987 Joint
Meeting of the FAO Panel of Experts on Pesticide residues in Food and
the Environment and the WHO Expert Group on Pesticide Residues, Rome,
Food and Agriculture Organization of the United Nations, p. 33 (FAO
Plant Production and Protection Paper 84).
FAO/WHO (1988) Permethrin. In: Pesticide residues in food. Part II -
Toxicology, Rome, Food and Agriculture Organization of the United
Nations, pp. 101-110 (FAO Plant Production and Protection Paper 86/2).
FARQUHAR, J.A., HUTCHINSON, D.B.A., PERIAM, A.W., & SPARKES,
R.G. (1981a) An investigation into the absorption of permethrin from
impregnated clothing, Bechenham, Wellcome Research Laboratories (Report
No. BKDL/81/1) (Unpublished data submitted to WHO).
FARQUHAR, J.A., HUTCHINSON, D.B.A., PERIAM, A.W., & SPARKES,
R.G. (1981b) An investigation of tolerance to absorption of and
persistence of permethrin applied as a 1% lotion to human hair,
Bechenham, Wellcome Research Laboratories (Report No. BKDL/81/2)
(Unpublished data submitted to WHO).
FLANNIGAN, S.A. & TUCKER, S.B. (1985) Variation in cutaneous
sensation between synthetic pyrethroid insecticides. Contact Derma-
titis, 13: 140-147.
FLANNIGAN, S.A., TUCKER, S.B., MARCUS, M.K., ROSS, C.E.,
FAIRCHILD, E.J., GRIMES, B.A., & HARRIST, R.B. (1985a) Primary
irritant contact dermatitis from synthetic pyrethroid insecticide
exposure. Arch. Toxicol., 56, 288-294.
FLANNIGAN, S.A., TUCKER, S.B., KEY, M.M., ROSS, C.E., FAIRCHILD,
E.J., GRIMES, B.A., & HARRIST, R.B. (1985b) Synthetic pyrethroid
insecticides: a dermatological evaluation. Br. J. ind. Med., 42: 363-
372.
FRIESEN, M.K., GALLOWAY, T.D., & FLANNAGAN, J.F. (1983) Toxicity
of the insecticide permethrin in water and sediment to nymphs of the
burrowing mayfly Hexagenia rigida (Ephemeroptera: Ephemeridae). Can.
Entomol., 115: 1007-1014.
GAMBRELL, R.P., REDDY, C.N., COLLARD, V., GREEN, G., & PATRICK,
W.H., Jr. (1981) Behavior of DDT, kepone and permethrin in sediment-
water systems under different oxidation-reduction and pH conditions,
Washington, DC, US Environmental Protection Agency (EPA 600/3-81-
038).
GAMMON, D.W. & CASIDA, J.E. (1983) Pyrethroids of the most potent
class antagonize GABA action at the crayfish neuromuscular junction.
Neurosci. Lett., 40: 163-168.
GAMMON, D.W., BROWN, M.A., & CASIDA, J.E. (1981) Two classes of
pyrethroid action in the cockroach. Pestic. Biochem. Physiol., 15:
181-191.
GAMMON, D.W., LAWRENCE, L.J., & CASIDA, J.E. (1982) Pyrethroid
toxicology: Protective effects of Diazepam and phenobarbital in the
mouse and the cockroach. Toxicol. appl. Pharmacol., 66: 290-296.
GAUGHAN, L.C. & CASIDA, J.E. (1978) Degradation of trans- and cis-
permethrin on cotton and bean plants. J. agric. food Chem., 26: 525-
528.
GAUGHAN, L.C., UNAI, T., & CASIDA, J.E. (1977) Permethrin metabolism
in rats. J. agric. food Chem., 25: 9-17.
GAUGHAN, L.C., ROBINSON, R.A., & CASIDA, J.E. (1978b) Distribution
and metabolic fate of trans- and cis-permethrin in laying hens. J.
agric. food Chem., 26: 1374-1380.
GAUGHAN, L.C., ACKERMAN, M.E., UNAI, T., & CASIDA, J.E. (1978a)
Distribution and metabolism of trans- and cis-permethrin in lactating
Jersey cows. J. agric. food Chem., 26: 613-618.
GERIG, L. (1985) Testing the toxicity of synthetic pyrethroid insecti-
cides to bees. Pestic. Sci., 16: 206-207.
GLAISTER, J.R., PRATT, I., & RICHARDS, D. (1977) Effects of high
dietary levels of PP557 on clinical behaviour and structure of sciatic
nerves in rats (Report No. CTL/P/317) (Unpublished data submitted to
WHO by ICI Central Toxicology Laboratory).
GLICKMAN, A.H. & CASIDA, J.E. (1982) Species and structural variations
affecting pyrethroid neurotoxicity. Neurobehav. Toxicol. Teratol., 4:
(6) 793-799.
GLICKMAN, A.H. & LECH, J.J. (1981) Hydrolysis of permethrin, a
pyrethroid insecticide, by rainbow trout and mouse tissues in vitro : A
comparative study. Toxicol. appl. Pharmacol., 60: 186-192.
GLICKMAN, A.H., SHONO, T., CASIDA, J.E., & LECH, J.J. (1979) In vitro
metabolism of permethrin isomers by carp and rainbow trout liver
microsomes. J. agric. food Chem., 27: 1038-1041.
GLICKMAN, A.H., HAMID, A.AR., RICKERT, D.E., & LECH, J.J. (1981)
Elimination and metabolism of permethrin isomers in rainbow trout.
Toxicol. appl. Pharmacol., 57: 88-98.
GLICKMAN, A.H., WEITMAN, S.D., & LECH, J.J. (1982) Different toxicity
of trans-permethrin in rainbow trout and mice. I. Role of biotransfor-
mation. Toxicol. appl. Pharmacol., 66: 153-161.
HAGLEY, E.A.C., PREE, D.J., & HOLLIDAY, N.J. (1980) Toxicity of
insecticides to some orchard carabids (Coleoptera carabidae). Can. Entomol.,
112: 457-462.
HALLS, G.R.H. (1981) The fate of permethrin residues on wheat during 9
months storage in Australia (Report No. HEFH 81-1) (Unpublished data
submitted to WHO by Wellcome Research Laboratories).
HALLS, G.R.H. & PERIAM, A.W. (1980) The fate of permethrin residues on
wheat during storage and after milling and baking - results after 9, 12
and 15 months storage (Report HEFH 80-3) (Unpublished data submitted to
WHO by Wellcome Research Laboratories).
HANSEN, D.J., GOODMAN, L.R., MOORE, J.C., & HIGDON, P.K. (1983)
Effects of the synthetic pyrethroids AC 222, 705, permethrin and
fenvalerate on sheepshead minnows in early life stage of toxicity
tests. Environ. Toxicol. Chem., 2: 251-258.
HART, D., BANHAM, P.B., GORE, C.W., PRATT, I., & WEIGHT, T.M.
(1977c) PP557: Liver hypertrophy study in rats-dietary administration
over 26 weeks (Report No. CTL/P/360) (Unpublished data submitted to WHO
by ICI Central Toxicology Laboratory).
HELSON, B.V., KINGSBURY, P.D., & DE GROOT, P. (1986) The use of
bioassays to assess aquatic arthropod mortality from permethrin drift
deposits. Aquat. Toxicol., 9: 253-262.
HEND, R.W. & BUTTERWORTH, S.T.G. (1977) Toxicity of insecticides: a
short-term feeding study of WL 43379 in rats (Report No. TLGR.0108.77)
(Unpublished data submitted to WHO by Shell Research Ltd).
HENRIET, J., MARTIJN, A., & POLVISEN, H.H. (1985) CIPAC Handbook.
Volume 1C: Analysis of technical and formulated pesticides, Hertford-
shire, Collaborative International Pesticides Analytical Council Ltd,
pp. 2172-2173.
HILL, E.F. & CAMARDESE, M.B. (1986) Lethal dietary toxicities of
environmental contaminants and pesticides to Coturnix, Washington, DC,
United States Department of the Interior, Fish and Wildlife Service,
p.111 (Fish and Wildlife Technical Report No. 2).
HODGE, M.C.E., BANHAM, P.B., GLAISTER, J.R., RICHARDS, D.,
TAYLOR, K., & WEIGHT, T.M. (1977) PP557: Three generation reproduction
study in rats (Unpublished report submitted to WHO by ICI Central
Toxicology Laboratory).
HOGAN, G.K. & RINEHART, W.E. (1977) A twenty-four month oral
carcinogenicity study of FMC 33297 in mice (Bio-Dynamics Inc. Project)
(Unpublished report submitted to WHO by FMC Corporation).
HOLDWAY, D.A. & DIXON, D.G. (1988) Acute toxicity of permethrin or
glyphosate pulse exposure to larval white sucker (Catostomus commer-
soni) and juvenile flagfish (Jordanella floridae) as modified by age
and ration level. Environ. Toxicol. Chem., 7: 63-68.
HOLMSTEAD, R.L., CASIDA, J.E., RUZO, L.O., & FULLER, D.G. (1978)
Pyrethroid photodecomposition: Permethrin. J. agric. food Chem., 26:
590-595.
HORIBA, M., KOBAYASHI, A., & MURANO, A. (1977) Gas-liquid
chromatographic determination of a new pyrethroid, permethrin (S-3151)
and its optical isomers. Agric. biol. Chem., 41: 581-586.
HOY, M.A., FLAHERTY, D., PEACOCK, W., & CULVER, D. (1979)
Vineyard and laboratory evaluations of methomyl, dimethoate, and
permethrin for a grape pest management program in the San Joaquin
Valley of California. J. econ. Entomol., 72: 250-255.
HOYT, S.C., WESTIGARD, P.H., & BURTS, E.C. (1978) Effects of two
synthetic pyrethroids on the codling moth, pear psylla, and various
mite species in northwest apple and pear orchards. J. econ. Entomol.,
71: 431-434.
HUNT, L.M. & GILBERT, B.N. (1977) Distribution and excretion rates of
14C-labelled permethrin isomers administered orally to four lactating
goats for 10 days. J. agric. food Chem., 25: 673-676.
HUNT, L.M., GILBERT, B.N., & LEMEILLEUR, C.A. (1979) Distri-
bution and depletion of radioactivity in hens treated dermally with
14C-labelled permethrin. Poult. Sci., 58: 1197-1201.
ISHMAEL, J. & LITCHFIELD, M.H. (1988) Chronic toxicity and
carcinogenic evaluation of permethrin in rats and mice. Fundam. appl.
Toxicol., 11: 308-311.
IVIE, G.W. & HUNT, L. (1980) Metabolites of cis- and trans-permethrin
in lactating goats. J. agric. food Chem., 28: 1131-1138.
JAGGERS, S.E. & PARKINSON, G.R. (1979) Permethrin: summary and review
of acute toxicities in laboratory species (Report No. CTL/P/461)
(Unpublished data submitted to WHO by ICI Central Toxicology
Laboratory).
JAMES, J.A. (1974) Fetal toxicity study of 21Z73 (NRDC 143) in the
rat (Report No. BPAT 74-10) Bechenham, Wellcome Research Laboratories
(Unpublished data submitted to WHO).
JAMES, J.A., (1979) A multigeneration reproduction study of 21Z73
(permethrin) in the rat (Report No. BPAT 79-3) Bechenham, Wellcome
Research Laboratories (Unpublished data submitted to WHO).
JAMES, J.A., TAYLOR, P.E., & ROE, F.J.C. (1980) Carcinogenicity study
in mice with permethrin, Bechenham, Wellcome Research Laboratories,
(Report No. HEFG 80-29) (Unpublished data submitted to WHO).
JOLLY, A.L., Jr., AVAULT, J.W., Jr., KOONCE, K.L., & GRAVES, J.B.
(1978) Acute toxicity of permethrin to several aquatic animals. Trans.
Am. Fish. Soc., 107: 825-827.
JORDAN, E.G. & KAUFMAN, D.D. (1986) Degradation of cis- and trans-
permethrin in flooded soil. J. agric. food Chem., 34: 880-884.
JORDAN, E.G., KAUFMAN, D.D., & KAYSER, A.J. (1982) The effect of soil
temperature on the degradation of cis-, trans-permethrin in soil. J.
environ. Sci. Health, B 17: 1-17.
KADOTA, T., MIYAMOTO, J., & ITO, N. (1975) Six-month subacute oral
toxicity of NRDC in Sprague-Dawley rats (Unpublished data submitted to
WHO by Sumitomo Chemical Co.).
KANEKO, H., OHKAWA, H., & MIYAMOTO, J. (1978) Degradation and
movement of permethrin isomers in soil. J. Pestic. Sci., 3: 43-51.
KAUFMAN, D.D., HAYES, S.C., JORDAN, E.G., & KAYSER, A.J. (1977)
Permethrin degradation in soil and microbial cultures. In: Elliott, M.,
ed. Synthetic pyrethroids, Washington, DC, American Chemical Society,
pp. 147-161 (ACS symposium series 42).
KAUFMAN, D.D., RUSSEL, B.A., HELLING, C.S., & KAYSER, A.J. (1981)
Movement of cypermethrin, deltamethrin, permethrin and their
degradation products in soil. J. agric. food Chem., 29: 239-245.
KAUSHIK, N.K., STEPHENSON, G.L., SOLOMON, K.R., & DAY, K.E.
(1985) Impact of permethrin on zooplankton communities in
limnocorrals. Can. J. Fish. aquat. Sci., 42: 77-85.
KILLEEN, J.C. & RAPP, W.R. (1976a) A three month oral toxicity study
of FMC 33297 in Beagle dogs (Bio-Dynamics Inc. Project) (Unpublished
report submitted to WHO by FMC Corporation).
KILLEEN, J.C. & RAPP, W.R. (1976b) A three month oral toxicity study
of FMC 33297 in rats (Bio-Dynamics Inc. Project) (Unpublished report
submitted to WHO by FMC Corporation).
KINGSBURY, P.D. (1976) Effects of an aerial application of the syn-
thetic pyrethroid permethrin on a forest stream. Manitoba Entomol., 10:
9-17.
KINGSBURY, P.D. & KREUTZWEISER, D.P. (1980a) Environmental impact
assessment of a semi-operational permethrin application. Sault Ste.
Marie, Ontario, Canada, Forest Pest Management Institute. Report No.
FPM-X 30: pp.47.
KINGSBURY, P.D. & KREUTZWEISER, D.P. (1980b) Dosage-effect studies
on the impact of permethrin on trout streams. Sault Ste. Marie,
Ontario, Canada, Forest Pest Management Institute Report No. FPM-X 31.
KINGSBURY, P.D. & KREUTZWEISER, D.P. (1987) Permethrin treatments in
Canadian forests. Part 1: Impact on fish. Pestic. Sci., 19: 35-48.
KOHDA, H., KADOTA, T., & MIYAMOTO, J. (1976a) Teratogenic evaluation
with permethrin in rats (Unpublished report submitted to WHO by
Sumitomo Chemical Co.).
KOHDA, H., KADOTA, T., & MIYAMOTO, J. (1976b) Teratogenic evaluation
with permethrin in mice (Unpublished report submitted to WHO by
Sumitomo Chemical Co.).
KOHDA, H., KADOTA, T., & MIYAMOTO, J. (1979a) Acute oral, dermal and
subcutaneous toxicities of permethrin in rats and mice (Unpblished
report submitted to WHO by Sumitomo Chemical Co.).
KOHDA, H., KANEDO, H., OHKAWA, H., KADOTA, T., & MIYAMOTO, J.
(1979b) Acute intraperitoneal toxicity of permethrin metabolites in
mice (Unpublished report submitted to WHO by Sumitomo Chemical Co.).
KOLMODIN-HEDMAN, B., SWENSSON, A., & AKERBLOM, M. (1982)
Occupational exposure to some synthetic pyrethroids (permethrin and
fenvalerate). Arch. Toxicol., 50: 27-33.
KREUTZWEISER, D.P. (1982) The effects of permethrin on the invertebrate
fauna of a Quebec forest. Sault Ste Marie, Ontario, Canada, Forest Pest
Management Institute, Report No. FPM-X 50: pp.44.
KREUTZWEISER, D.P. & KINGSBURY, P.D. (1987) Permethrin treatments
in Canadian forests. Part 2: Impact on stream invertebrates. Pestic.
Sci., 19: 49-60.
KUMARAGURU, A.K. & BEAMISH, F.W.H. (1981) Lethal toxicity of
permethrin (NRDC-143) to rainbow trout, Salmo gairdneri, in relation to
body weight and water temperature. Water Res., 15: 503-505.
KUMARAGURU, A.K., BEAMISH, F.W.H., & FERGUSON, H.W. (1982)
Direct and circulatory paths of permethrin (NRDC-143) causing
histopathological changes in the gills of rainbow trout, Salmo
gairdneri Richardson. J. Fish Biol., 20: 87-91.
LAWRENCE, L.J. & CASIDA, J.E. (1982) Pyrethroid toxicology: Mouse
intracerebral structure-toxicity relationship. Pestic. Biochem. Physiol.,
18: 9-14.
LAWRENCE, L.J. & CASIDA, J.E. (1983) Stereospecific action of
pyrethroid insecticides on the gamma-aminobutyric acid receptor-
ionophore complex. Science, 221: 1399-1401.
LAWRENCE, L.J., GEE, K.W., & YAMAMURA, H.I. (1985) Interactions of
pyrethroid insecticides with chloride ionophore-associated binding
sites. Neurotoxicology, 6: 87-98.
LEAHEY, J.P. (1985) The pyrethroid insecticides, London, Taylor &
Francis Ltd, p. 440.
LEAHEY, J.P. & CARPENTER, P.K. (1980) The uptake of metabolites
of permethrin by plants grown in soil treated with [14C]permethrin.
Pestic. Sci., 11: 279-289.
LEAHEY, J.P., BEWICK, D.W., & SAUNDERS, R. (1977) Accumulation and
depletion of radioactive residues in the tissues of Mallard duck and
Japanese quail following daily dosing with 14 C-permethrin (Unpublished
report submitted to WHO by ICI Plant Protection Division).
LE QUESNE, P.N., MAXWELL, I.C., & BUTTERWORTH, T.G. (1980)
Transient facial sensory symptoms following exposure to synthetic
pyrethroids: a clinical and electorophysiological assessment. Neurotoxi-
cology, 2: 1-11.
LINDEN, E., BENGTSSON, B.-E., SVANBERG, O., & SUNDSTROM, G.
(1979) The acute toxicity of 78 chemicals and pesticide formulations
against two brackish water organisms, the Bleak (Alburnus alburnus) and
the Nitocra spinipes. Chemosphere, 11/12: 843-851.
LINDQUIST, R.K., KRUEGER, H.R., & POWELL, C.C., Jr. (1987) Airborne
and surface residues of permethrin after high- and low-volume
applications in greenhouses. J. environ. Sci. Health, B22: 15-27.
LITCHFIELD, M.H. (1983) Characterisation of the principal mammalian
toxicological and biological actions of synthetic pyrethroids. In:
Miyamoto, J. & Kearney, P.C., ed. Pesticide chemistry: human welfare
and the environment. II. Natural products, Oxford, Pergamon Press, pp.
207-211.
LONGSTAFF, E. (1976) Permethrin: short-term predictive tests for
carcinogenicity: results from the Ames test (Report No. CTL/P/301)
(Unpublished data submitted to WHO by ICI Central Toxicology
Laboratory).
LORD, K.A., MCKINLEY, M., & WALKER, N. (1982) Degradation of
permethrin in soils. Environ. Pollut. A29: 81-90.
LUND, A.E. & NARAHASHI, T. (1983) Kinetics of sodium channel
modification as the basis for the variation in the nerve membrane
effects of pyrethroids and DDT analogs. Pestic. Biochem. Physiol., 20:
203-216.
MCGREGOR, D.B. & WICKRAMARATNE, G.A., de S. (1976a) Dominant
lethal study in mice of ICI-PP557 (Report No. 623, Inveresk Research
International Project No. 406722) (Unpublished data submitted to WHO by
ICI Ltd).
MCGREGOR, D.B. & WICKRAMARATNE, G.A., de S. (1976b) Terato-
genicity study in rats of ICI-PP557 (Inveresk Research International
Project No. 404898) (Unpublished data submitted to WHO by ICI Ltd.).
MCLEESE, D.W., METCALFE, C.D., & ZITKO, V. (1980) Lethality of
permethrin, cypermethrin and fenvalerate to salmon, lobster and shrimp.
Bull. environ. Contam. Toxicol., 25: 950-955.
MACPHEE, A., GAUL, S., & RAGAB, M.T.H. (1982) Control of blueberry
thrips, Frankliniella vaccinii Morga, with permethrin and effect on
yield and residue in fruit. J. environ. Sci. Health, B17: 183-193.
MCSHEEHY, T.W. & FINN, J.P. (1980) 21Z: Potential toxicity and
oncogenicity in dietary administration to rats for a period of 104
weeks (Report No. HEFG 80-33) (Unpublished data of Wellcome Research
Laboratories).
MAREI, A.E.M., RUZO, L.O., & CASIDA, J.E. (1982) Analysis and
persistence of permethrin, cypermethrin, deltamethrin and fenvalerate
in the fat and brains of treated rats. J. agric. food Chem., 30: 558-
562.
MATHUR, S.P., BELANGER, A., HAMILTON, H.A., & KHAN, S.U. (1980)
Influence on microflora and persistence of field-applied disulfoton,
permethrin and prometryne in an organic soil. Pedobiologia, 20: 237-
242.
MAYER, F.L., Jr. (1987) Acute toxicity handbook of chemicals to
estuarine organisms, Springfield, Virginia, National Technical
Information Service, p. 158 EPA/600/8-87/017.
MAYER, F.L., Jr. & ELLERSIECK, M.R. (1986) Manual of acute toxicity:
Interpretation and data base for 410 chemicals and 66 species of
freshwater animals, Washington, DC, United States Department of the
Interior, Fish and Wildlife Service, pp.377-378.
MEISTER, R.T., BERG, G.L., SINE, C., MEISTER, S., & POPLYK, J. (1983)
Farm chemicals handbook. Section C. Pesticide dictionary, Wilboughby,
Ohio, Meister Publishing Co., pp. C180-C181.
METKER, L.W. (1978) Subchronic inhalation toxicity of 3-(phenoxy-
phenyl)methyl(+)-cis,trans-3-(2,2-dichloroethenyl)-2,2-dimethylcyclopro-
panecarboxylate (permethrin), Aberdeen Proving Ground, Maryland, US
Army Environmental Hygiene Agency (Report No. 75-51-0026-80).
METKER, L.W., ANGERHOFER, R.A., POPE, C.R., & SWENTZEL, K.C.
(1977) Toxicology evaluation of 3-(phenoxyphenyl)methyl(+)-cis,trans-3-
(2,2-dichloroethenyl)-2,2-dimethylcyclopropanecarboxylate (permethrin),
Environmental Hygiene Agency (Report No. 75-51-0837-78).
MILLNER, C.K. & BUTTERWORTH, S.T.G. (1977) Toxicity of pyrethroid
insecticides: investigation of the neurotoxic potential of WL 43479 to
adult hens, (Report No. TLGR.0069.77) (Unpublished data submitted to
WHO by Shell Research Ltd).
MIYAMOTO, J. (1976) Degradation, metabolism and toxicity of synthetic
pyrethroids. Environ. Health Perspect., 14: 15-28.
MIYAMOTO, J. (1981) The chemistry, metabolism and residue analysis of
synthetic pyrethroids, Pure appl. Chem., 53: 1967-2022.
MIYAMOTO, J. & KEARNEY, P.C. (1983) Pesticide chemistry-human welfare
and the environment. Proceedings of the Fifth International Congress of
Pesticide Chemistry, Kyoto, Japan, 29 August-4 September 1982, Oxford,
Pergamon Press, Vol. 1-4.
MULLA, M.S., DARWAZEH, H.A., & MAJORI, G. (1975) Field efficacy of
some promising mosquito larvicides and their effect on nontarget
organisms. Mosq. News, 35: 179-185.
MULLA, M.S., NAVVAB-GOJRATI, H.A., & DARWAZEH, H.A. (1978a)
Toxicity of mosquito larvicidal pyrethroids to four species of
freshwater fishes. Environ. Entomol., 7: 428-430.
MULLA, M.S., NAVVAB-GOJRATI, H.A., & DARWAZEH, H.A. (l978b)
Biological activity and longevity of new synthetic pyrethroids against
mosquitoes and some nontarget insects. Mosq. News, 38: 90-96.
MULLA, M.S., DARWAZEH, H.A., & DHILLON, K.S. (1981) Impact and
joint action of decamethrin and permethrin and freshwater fishes on
mosquitoes. Bull. environ. Contam. Toxicol., 26: 689-695.
NASSIF, M., BROOKE, J.P., HUTCHINSON, D.B.A., & KAMEL, O.M. (1980)
Studies with permethrin against body lice in Egypt. Pestic. Sci., 11:
679-684.
NEWELL, G.W. & SKINNER W.A. (1976) In vitro microbiological
mutagenicity study of an FMC corporation compound (Unpublished report
submitted to WHO by FMC Corporation).
NOMURA, Y. & SEGAWA, T. (1979) Pharmacological study of permethrin:
effects of isolated ileum, nictitating membrane, respiration, blood
pressure and electrocardiography (Unpublished report submitted to WHO
by Sumitomo Chemical Co.).
OHKAWA, H., KANEKO, H., & MIYAMOTO, J. (1977) Metabolism of
permethrin in bean plants. J. Pestic. Sci., 2: 67-76.
OHSHIMA, M., TAKIMOTO, Y., & MATSUDA, T. (1988) Accumulation and
metabolism of 14 C-permethrin in carp (Cyprinus carpio) (Unpublished
data submitted to WHO by Sumitomo Chemical Co.).
OKUNO, Y., MIYAGAWA, H., HOSOI, R., & KADOTA, T. (1976) Skin and
eye irritation study of permethrin (S-3151) in rabbits (Unpublished
report submitted to WHO by Sumitomo Chemical Co.).
PARKINSON, G.R. (1978) Permethrin: acute toxicity to male rats (Report
No. CTL/P/388) (Unpublished data submitted to WHO by ICI Central
Toxicology Laboratory).
PARKINSON, G.R., BERRY, P.N., GLAISTER, J., GORE, C.W., LEFEVRE,
V.K., & MURPHY, J.A. (1976) PP557 (Permethrin): acute and sub-acute
toxicity, (Report No. CTL/P/225) (Unpublished data submitted to WHO by
ICI Central Toxicology Laboratory).
PAYNTER, O.C., BUDD, E.R., & LITT, B.D. (1982) Permethrin. Assessment
of chronic and oncogenic effects: A summary, Washington, DC, Toxicology
Branch, Hazard Evaluation Division, Office of Pesticide Programs, US
Environmental Protection Agency (Unpublished data submitted to WHO).
PEGUM, J.S. & DOUGHTY, B.J. (1978) Human skin patch test with
pyrethrins, kadethrin, permethrin and decamethrin, Berkhamsted Hill,
Wellcome Group Research and Development (Report No. NEFJ 78-2)
(Unpublished data submitted to WHO).
PIERCY, D.W.T., REYNOLDS, J., & JAMES, J.A. (1976) Effects of diet on
the acute toxicity of permethrin in the female rat, Berkhamsted Hill,
Wellcome Research and Development (Report no. 77-11) (Unpublished data
submitted to WHO).
PIKE, K.S., MAYER, D.F., GLAZER, M., & KIOUS, C. (1982) Effects of
permethrin on mortality and foraging behavior of honey bees in sweet
corn. Environ. Entomol., 11: 951-953.
PLAPP, F.W., Jr & BULL, D.L. (1978) Toxicity and selectivity of some
insecticides to Chrysopa carnea, a predator of the tobacco budworm.
Environ. Entomol., 7: 431-434.
PLAPP, F.W., Jr & VINSON, S.B. (1977) Comparative toxicities of some
insecticides to the tobacco budworm and its ichneumonid parasite,
Campoletis sonorensis. Environ. Entomol., 6: 381-384.
PLUIJMEN, M., DREVON, C., MONTESANO, R., MALAVEILLE, C.,
HAUTEFEUILLE, A., & BARTSCH, H. (1984) Lack of mutagenicity of
synthetic pyrethroids in Salmonella typhimurium strains in V79 Chinese
hamster cells. Mutat. Res., 137: 7-15.
RACEY, P.A. & SWIFT, S.M. (1986) The residual effect of remedial
timber treatments on bats. Biol. Conserv., 35: 205-214.
RAPP, W.R. (1978) Twenty-four month oral toxicity/oncogenicity study
of FMC33297 in mice. Histopathology report (Unpublished data of FMC
Corporation).
REYNOLDS, J., PIERCY, D.W.T., CLAMPITT, R.B., JAMES, J.A.,
THOMPSON, P.M., FAREBROTHER, D.A., & DAYAN, A.D. (1978)
Permethrin oral administration to dogs for 6 months, Berkhamsted Hill,
Wellcome Research Laboratories (Report No. HEFG 78-14) (Unpublished
data submitted to WHO).
RICHARDS, D., BANHAM, P.B., KILMARTIN, M., & WEIGHT, T.M. (1980)
Permethrin: Teratogenicity study in the rabbit (Report No. CTL/P/523)
(Unpublished report submitted to WHO by ICI Ltd).
RISHIKESH, N., CLARKE, J.L., MATHIN, H.L., KING, J.S., & PEARSON, J.
(1978) Evaluation of decamethrins and permethrin against Anopheles
gambiae and Anopheles funestus in a village trial in Nigeria
(WHO/VBC/78.679) (Unpublished report of Division of Vector Biology
Control, World Health Organization).
ROBERTS, T.R. & WRIGHT, A.N. (1981) The metabolism of 3-phenoxybenzyl
alcohol, a pyrethroid metabolite, in plants. Pestic. Sci., 12: 161-
169.
ROCK, G.C. (1979) Relative toxicity of two synthetic pyrethroids to a
predator Amblyseius fallacis and its prey Tetranychus urticae. J. econ.
Entomol., 72: 293-294.
ROSS, D.B. & PRENTICE, D.E. (1977) Examination of permethrin (PP557)
for neurotoxicity in the domestic hen, Huntingdon, Huntingdon Research
Centre (Report No. ICI/157-NT/77468) (Unpublished data submitted to WHO
by FMC Corporation and ICI Ltd).
ROSS, D.B., CAMERON, D.M., & ROBERTS, N.L. (1976a) The acute oral
toxicity (LD50 ) of PP557 (permethrin) to Mallard ducks, Huntingdon,
Huntingdon Research Centre (Report No. ICI 68/WL/7639) (Unpublished
data submitted to WHO by ICI Ltd).
ROSS, D.B., CAMERON, D.M., & ROBERTS, N.L. (1976b) The sub-acute
toxicity (LC50 ) of PP557 (permethrin) to Mallard ducks, Huntingdon,
Huntingdon Research Centre (Report No. ICI 68/WL/75837) (Unpublished
data submitted to WHO by ICI Ltd).
ROSS, D.B., CAMERON, D.M., & ROBERTS, N.L. (1976c) The acute oral
toxicity (LD50 ) of PP557 (permethrin) to Starling, Huntingdon,
Huntingdon Research Centre (Report No. ICI 68/WL/7637) (Unpublished
data submitted to WHO by ICI Ltd).
ROSS, D.B., CAMERON, D.M., & ROBERTS, N.L. (1976d) The sub-acute
toxicity (LC50 ) of PP557 (permethrin) to Starling, Huntingdon,
Huntingdon Research Centre (Report No. ICI 68/WL/7636) (Unpublished
data submitted to WHO by ICI Ltd).
ROSS, D.B., CAMERON, D.M., & ROBERTS, N.L. (1976e) The sub-acute
toxicity (LC50 ) of PP557 (permethrin) to Ring-necked pheasant,
Huntingdon, Huntingdon Research Centre (Report No. ICI 68/WL/75839)
(Unpublished data submitted to WHO by ICI Ltd).
ROSS, D.B., CAMERON, D.M., & ROBERTS, N.L. (1977a) The acute oral
toxicity (LD50 ) of PP557 (permethrin) to Ring-necked pheasant,
Huntingdon, Huntingdon Research Centre (Report No. ICI 68/WL/77154)
(Unpublished data submitted to WHO by ICI Ltd).
ROSS, D.B., PRENTICE, D.E., MAJEED, S.K., GIBSON, W.A., CAMERON,
D.M., CAMERON, M. McD., & ROBERTS, N.L. (1977b) The incorporation of
permethrin in the diet of laying hens (part I), Huntingdon, Huntingdon
Research Centre (Report No. ICI 152/77387) (Unpublished data submitted
to WHO by ICI Ltd).
ROUSH, R.T. & HOY, M.A. (1978) Relative toxicity of permethrin to a
predator, Metaseiulus occidentalis, and its prey, Tetranychus urticae.
Environ. Entomol., 7: 287-288.
RUIGT, G.S.F. & VAN DEN BERCKEN, J. (1986) Action of pyrethroids on a
nerve-muscle preparation of the clawed frog, Xenopus laevis. Pestic.
Biochem. Physiol., 25: 176-187.
SASINOVICH, L.M. & PANSHINA, T.N. (1987) [Substantiation of hygienic
rules for synthetic pyrethroid content in the work zone air]. Gig. Tr.
prof. Zabol., 8: 48-50 (in Russian).
SCHROEDER, R.E. & RINEHART, W.E. (1977) A three generation
reproduction study of FMC33297 in rats (Bio-Dynamics Inc Project)
(Unpublished report submitted to WHO by FMC Corporation).
SHAH, P.V., MONROE, R.J., & GUTHRIE, F.E. (1981) Comparative rates of
dermal penetration of insecticides in mice. Toxicol. appl. Pharmacol.,
59: 414-423.
SHAROM, M.S. & SOLOMON, K.R. (1981) Adsorption-desorption,
degradation, and distribution of permethrin in aqueous systems. J.
agric. food Chem., 29: 1122-1125.
SHERMAN, R.A. (1979) Preliminary behavioural assessment of habituation
to the insecticide permethrin, Aberdeen Proving Ground, Maryland, US
Army Environmental Hygiene Agency (Report No. 75-51-002679).
SHIRASU, Y., MORIYA, M., & OTA, T. (1979) Mutagenicity of S-3151 in
bacterial test systems (Unpublished report submitted to WHO by Sumitomo
Chemical Co.).
SHONO, T., OHSAWA, K., & CASIDA, J.E. (1979) Metabolism of trans- and
cis-permethrin, trans- and cis-cypermethrin, and decamethrin by
microsomal enzymes. J. agric. food Chem., 27: 316-325.
SHOUR, M.H. & CROWDER, L.A. (1980) Effects of pyrethroid insecticides
on the common green lacewing. J. econ. Entomol., 73: 306-309.
SIEGEL, M.M., HILDEBRAND, B.E., & HALL, D.R. (1980) Determination of
permethrin in environmental waters by gas chromatography-selected ion
monitoring-mass spectroscopy using ion counting detection. Int. J.
environ. anal. Chem., 8: 107-126.
SIMMON, V.F. (1976) In vitro microbiological mutagenicity study of an
FMC corporation compound, Menlo Park, Stanford Research Institute,
(Report No. LSC-4768) (Unpublished report submitted to WHO).
SIMONAITIS, R.A. & CAIL, R.S. (1977) Gas chromatographic determination
of residues of the synthetic pyrethroid FMC33297. In: Elliott, M., ed.
Synthetic pyrethroids, Washington, DC, American Chemical Society,
pp. 211-223 (ACS Symposium Series U2).
SODERLUND, D.M. & CASIDA, J.E. (1977) Effects of pyrethroid structure
on rate of hydrolysis and oxidation by mouse liver microsomal enzymes.
Pestic. Biochem. Physiol., 7: 391-401.
SPENCER, F. & BERHANCE, Z. (1982) Uterine and fetal characteristics in
rats following a post-implantational exposure to permethrin. Bull.
environ. Contam. Toxicol., 29: 84-88.
STAATZ, C.G., BLOOM, A.S., & LECH, J.J. (1982) A pharmacological
study of pyrethroid neurotoxicity in mice. Pestic. Biochem. Physiol.,
17: 287-292.
STEVENSON, J.H., NEEDHAM, P.H., & WALKER, J. (1978) Poisoning of
honeybee by pesticides: Investigation of the changing pattern in
Britain over 20 years. Rothamsted Experimental Station Report (1977),
Part 2: 55-72.
STRATTON, G.W. & CORKE, C.T. (1981) Interaction of permethrin with
Daphnia magna in the presence and absence of particulate material.
Environ. Pollut., A24: 135-144.
STRATTON, G.W. & CORKE, C.T. (1982) Toxicity of the insecticide
permethrin and some degradation products towards algae and
cyanobacteria. Environ. Pollut. A29: 71-80.
SUNDARAM, K.M.S., DE GROOT, P., & SUNDARAM, A. (1987)
Permethrin deposits and airborne concentrations downwind from a single
swath application using a back pack mist blower. J. environ. Sci.
Health, B22: 171-193.
SUZUKI, H. (1977) Studies on the mutagenicity of some pyrethroids on
salmonella strains in the presence of mouse hepatic S9 fractions
(Unpublished report submitted to WHO by Sumitomo Chemical Co.).
SWAINE, H. & SAPIETS, A. (1981a) Cypermethrin: residue transfer study
with dairy cows fed on a diet containing the insecticide (Report No.
RJ0186B) (Unpublished data submitted to WHO by ICI Ltd).
SWAINE, H. & SAPIETS, A. (1981b) Cypermethrin: residue levels of the
major metabolites of the insecticide in the milk and tissues of dairy
cows fed on a diet containing cypermethrin at 50 mg/kg (Report No.
RJ0198B) (Unpublished data submitted to WHO by ICI Ltd).
SWAINE, H., EDWARD, M.J., & USSARY, J.P. (1978) Permethrin crop
protection study (Report No. TMV 0378B) (Unpublished data submitted to
WHO by ICI Ltd).
TAKAHASHI, K., OKUDA, No., & SHIRASU, Y. (1979) Effects of
permethrin on hexobarbital induced sleeping time in mice and electro-
encephalography in rabbits (Unpublished report submitted to WHO by
Sumitomo Chemical Co.).
TYLER, J.F.C. (1987) Gas chromatographic method for determination of
permethrin in pesticide formulations: Collaborative study. J. Assoc.
Off. Anal. Chem., 70(1): 53-55.
US EPA (1981) Advisary opinion on the oncogenic potential of
permethrin, Washington, DC, US Environmental Protection Agency
(Unpublished report submitted to WHO).
USSARY, J.P. (1979) Permethrin residues on sweet corn (Report No.
TMU0438B) (Unpublished data submitted to WHO by ICI Americas Inc.).
USSARY, J.P. (1978) Permethrin residues on sweet corn (Report No.
TMU0413B) (Unpublished data submitted to WHO by ICI Americas Inc.).
USSARY, J.P. & BRAITHWAITE, G.B. (1980) Residues of permethrin and 3-
phenoxy-benzyl alcohol in cow tissues (Trial No. 35NC79-001. Report
No. TMU0493B) (Unpublished data submitted to WHO by ICI Americas
Inc.).
VAN DEN BERCKEN, J. (1977) The action of allethrin on the peripheral
nervous system of the frog. Pestic. Sci., 8: 692-699.
VAN DEN BERCKEN, J. & VIJVERBERG, H.P.M. (1980a) Voltage clamp
studies on the effects of allethrin and DDT on the sodium channels in
frog myelinated nerve membrane. In: Insect neurobiology and pesticide
action, London, Society of Chemical Industry, pp. 79-85.
VAN DEN BERCKEN, J. & VIJVERBERG, H.P.M. (1980b) Effects of
insecticides on the sensory system of Xenopus. In: Insect neurobiology
and pesticide action, London, Society of Chemical Industry, pp.391-
397.
VAN DEN BERCKEN, J., AKKERMANS, L.M.A., & VAN DER ZALM,
J.M., (1973) DDT-like action of allethrin in the sensory nervous
system of Xenopus laevis. Eur. J. Pharmacol., 21: 95-106.
VAN DEN BERCKEN, J., KROESE, A.B.A., & AKKERMANS, L.M.A.
(1979) Effect of insecticides on the sensory nervous system. In:
Narahashi, T., ed. Neurotoxicology of insecticides and pheromones,
New York, London, Plenum Press, pp. 183-210.
VERSCHOYLE, R.D. & ALDRIDGE, W.N. (1980) Structure-activity
relationship of some pyrethrins to rats. Arch. Toxicol., 45: 325-329.
VIJVERBERG, H.P.M. & VAN DEN BERCKEN, J. (1979) Frequency-
dependent effects of the pyrethroid insecticide decamethrin in frog
myelinated nerve fibres. Eur. J. Phamacol., 58: 501-504.
VIJVERBERG, H.P.M. & VAN DEN BERCKEN, J. (1982) Action of
pyrethroid insecticides on the vertebrate nervous system. Neuropathol.
appl. Neurobiol., 8: 421-440.
VIJVERBERG, H.P.M., RUIGT, G.S.F., & VAN DEN BERCKEN, J. (1982a)
Structure-related effects of pyrethroid insecticides on the lateral-
line sense organ and on peripheral nerves of the clawed frog, Xenopus
laevis. Pestic. Biochem. Physiol., 18: 315-324.
VIJVERBERG, H.P.M., VAN DER ZALM, J.M., & VAN DEN BERCKEN, J.
(1982b) Similar mode of action of pyrethroids and DDT on sodium
channel gating in mylinated nerves. Nature (Lond.), 295: 601-603.
VIJVERBERG, H.P.M., VAN DER ZALM, J.M., VAN KLEEF, R.G.D.M., &
VAN DEN BERCKEN, J. (1983) Temperature and structure-dependent
interaction of pyrethroids with the sodium channels in frog node of
Ranvier. Biochim. Biophys. Acta, 728: 73-82.
WALLWORK, L.M. & MALONE, J.C. (1974) 21Z73 (25:75) effect of
different solvents on the rat oral toxicity (Report No. HEFG 75-4)
Berkhamsted, Wellcome Research Laboratories (Unpublished data submitted
to WHO).
WALLWORK, L.M., CLAMPITT, R.B., & MALONE, J.C. (1974a) 10-day
cumulative oral toxicity study with 21Z73 in mice, Berkhamsted,
Wellcome Research Laboratories (Report No. HEFG 74-9) (Unpublished
data submitted to WHO).
WALLWORK, L.M., CLAMPITT, R.B., & MALONE, J.C. (1974b) 10-day
cumulative oral toxicity study with 21Z73 in rats, Berkhamsted,
Wellcome Research Laboratories (Unpublished report submitted to WHO).
WELLS, D., GRAYSON, B.T., & LANGNER, E. (1986) Vapour pressure of
permethrin. Pestic. Sci., 17: 473-476.
WHO (1979) WHO Technical Report Series, No. 634 (Safe use of
pesticides. Third Report of the WHO Expert Committee on Vector Biology
and Control), pp. 18-23.
WHO (1988) The WHO recommended classification of pesticides by
hazard: Guidelines to classification 1988-89, Geneva, World Health
Organization (Unpublished report VBC/88.953).
WHO/FAO (1984) Data sheet on pesticides No. 51: Permethrin, Geneva,
World Health Organization (VBC/DS/84.51).
WILLIAMS, I.H. & BROWN, M.J. (1979) Persistence of permethrin and WL
43775 in soil. J. agric. food Chem., 27: 130-132.
WOODRUFF, R.C., PHILLIPS, J.P., & IRWIN, D. (1983) Pesticide-induced
complete and partial chromosome loss in screens with repair-defective
females of Drosophila melanogaster. Environ. Mutagen., 5: 835-846.
WOOD, MACKENZIE & Co. (1980) Pyrethroids. Agrochem. Monit., 9: 3-14.
WOOD, MACKENZIE & Co. (1981) Pyrethroids. Agrochem. Monit., 15: 3-27.
WOOD, MACKENZIE & Co. (1982) Pyrethroids. Agrochem. Monit., 21: 3-17.
WOOD, MACKENZIE & Co. (1983) Pyrethroids. Agrochem. Monit., 27: 3-12.
WOOD, MACKENZIE & Co. (1984) Pyrethroids. Agrochem. Monit., 33: 2-12.
WORKMAN, R.B. (1977) Pesticides toxic to striped earwig, an important
insect predator. Proc. Florida State Hortic. Soc., 90: 401-402.
WORTHING, C.R. & WALKER, S.B. (1983) The pesticide manual, 7th ed.,
Croydon, British Crop Protection Council, p. 427.
WORTHING, C.R. & WALKER, S.B. (1987) Permethrin. In: The pesticide
manual, 8th ed., Croydon, British Crop Protection Council, pp. 647-
648.
WOUTERS, W. & VAN DEN BERCKEN, J. (1978) Action of pyrethroids. Gen.
Pharmacol., 9: 387-398.
ZITKO, V., CARSON, W.G., & METCALFE, C.D. (1977) Toxicity of
pyrethroids to juvenile atlantic salmon. Bull. environ. Contam.
Toxicol., 18: 35-41.
ZITKO, V., MCLEESE, D.W., METCALFE, C.D., & CARSON, W.G. (1979)
Toxicity of permethrin, decamethrin, and related pyrethroids to salmon
and lobster. Bull. environ. Contam. Toxicol., 21: 338-343.
APPENDIX I
On the basis of electrophysiological studies with peripheral nerve
preparations of frogs ( Xenopus laevis; Rana temporaria, and Rana
esculenta ), it is possible to distinguish between 2 classes of
pyrethroid insecticides: (Type I and Type II). A similar distinction
between these 2 classes of pyrethroids has been made on the basis of
the symptoms of toxicity in mammals and insects (Van den Bercken et
al., 1979; WHO, 1979; Verschoyle & Aldridge, 1980; Glickman & Casida,
1982; Lawrence & Casida, 1982). The same distinction was found in
studies on cockroaches (Gammon et al., 1981).
Based on the binding assay on the gamma-aminobutyric acid (GABA)
receptor-ionophore complex, synthetic pyrethroids can also be
classified into two types: the alpha-cyano-3-phenoxybenzyl pyrethroids
and the non-cyano pyrethroids (Gammon et al., 1982; Gammon & Casida,
1983; Lawrence & Casida, 1983; Lawrence et al., 1985).
Pyrethroids that do not contain an alpha-cyano group (allethrin,
d-phenothrin, permethrin, tetramethrin, cismethrin, and bioresmethrin)
(Type I: T-syndrome)
The pyrethroids that do not contain an alpha-cyano group give rise
to pronounced repetitive activity in sense organs and in sensory nerve
fibres (Van den Bercken et al., 1973). At room temperature, this
repetitive activity usually consists of trains of 3-10 impulses and
occasionally up to 25 impulses. Train duration is between 10 and 5
milliseconds.
These compounds also induce pronounced repetitive firing of the
presynaptic motor nerve terminal in the neuromuscular junction (Van den
Bercken, 1977). There was no significant effect of the insecticide on
neurotransmitter release or on the sensitivity of the subsynaptic
membrane, nor on the muscle fibre membrane. Presynaptic repetitive
firing was also observed in the sympathetic ganglion treated with these
pyrethroids.
In the lateral-line sense organ and in the motor nerve terminal,
but not in the cutaneous touch receptor or in sensory nerve fibres, the
pyrethroid-induced repetitive activity increases dramatically as the
temperature is lowered, and a decrease of 5°C in temperature may cause
a more than 3-fold increase in the number of repetitive impulses per
train. This effect is easily reversed by raising the temperature. The
origin of this "negative temperature coefficient" is not clear
(Vijverberg et al., 1983).
Synthetic pyrethroids act directly on the axon through interference
with the sodium channel gating mechanism that underlies the generation
and conduction of each nerve impulse. The transitional state of the
sodium channel is controlled by 2 separately acting gating mechanisms,
referred to as the activation gate and the inactivation gate. Since
pyrethroids only appear to affect the sodium current during
depolarization, the rapid opening of the activation gate and the slow
closing of the inactivation gate proceed normally. However, once the
sodium channel is open, the activation gate is restrained in the open
position by the pyrethroid molecule. While all pyrethroids have
essentially the same basic mechanism of action, however, the rate of
relaxation differs substantially for the various pyrethroids (Flannigan
& Tucker, 1985).
In the isolated node of Ranvier, allethrin causes prolongation of
the transient increase in sodium permeability of the nerve membrane
during excitation (Van den Bercken & Vijverberg, 1980a,b). Evidence so
far available indicates that allethrin selectively slows down the
closing of the activation gate of a fraction of the sodium channels
that open during depolarization of the membrane. The time constant of
closing of the activation gate in the allethrin-affected channels is
about 100 milliseconds compared with less than 100 microseconds in the
normal sodium channel, i.e., it is slowed down by a factor of more than
100. This results in a marked prolongation of the sodium current
across the nerve membrane during excitation, and this prolonged sodium
current is directly responsible for the repetitive activity induced by
allethrin (Vijverberg et al., 1983).
The effects of cismethrin on synaptic transmission in the frog
neuromuscular junction, as reported by Evans (1976), are almost
identical to those of allethrin, i.e., presynaptic repetitive firing,
and no significant effects on transmitter release or on the subsynaptic
membrane.
Interestingly, the action of these pyrethroids closely resembles
that of the insecticide DDT in the peripheral nervous system of the
frog. DDT also causes pronounced repetitive activity in sense organs,
in sensory nerve fibres, and in motor nerve terminals, due to a
prolongation of the transient increase in sodium permeability of the
nerve membrane during excitation. Recently, it was demonstrated that
allethrin and DDT have essentially the same effect on sodium channels
in frog myelinated nerve membrane. Both compounds slow down the rate
of closing of a fraction of the sodium channels that open on
depolarization of the membrane (Van den Bercken et al., 1973, 1979;
Vijverberg et al., 1982b).
In the electrophysiological experiments using giant axons of
crayfish, the type I pyrethroids and DDT analogues retain sodium
channels in a modified open state only intermittently, cause large
depolarizing after-potentials, and evoke repetitive firing with minimal
effect on the resting potential (Lund & Narahashi, 1983).
These results strongly suggest that permethrin and cismethrin, like
allethrin, primarily affect the sodium channels in the nerve membrane
and cause a prolongation of the transient increase in sodium
permeability of the membrane during excitation.
The effects of pyrethroids on end-plate and muscle action
potentials were studied in the pectoralis nerve-muscle preparation of
the clawed frog ( Xenopus laevis ). Type I pyrethroids (allethrin,
cismethrin, bioresmethrin, and 1R, cis-phenothrin) caused moderate
presynaptic repetitive activity, resulting in the occurrence of
multiple end-plate potentials (Ruigt & Van den Bercken, 1986).
Pyrethroids with an alpha-cyano group on the 3-phenoxybenzyl alcohol
(deltamethrin, cypermethrin, cyhalothrin, lambda-cyhalothrin,
fenvalerate, and fenpropanate) (Type II: CS-syndrome)
The pyrethroids with an alpha-cyano group cause an intense repetitive
activity in the lateral line organ in the form of long-lasting trains
of impulses (Vijverberg et al., 1982a). Such a train may last for up
to 1 min and contains thousands of impulses. The duration of the
trains and the number of impulses per train increase markedly on
lowering the temperature. Cypermethrin does not cause repetitive
activity in myelinated nerve fibres. Instead, this pyrethroid causes a
frequency-dependent depression of the nervous impulse, brought about by
a progressive depolarization of the nerve membrane as a result of the
summation of depolarizing after-potentials during train stimulation
(Vijverberg & Van den Bercken, 1979; Vijverberg et al., 1983).
In the isolated node of Ranvier, cypermethrin, like allethrin,
specifically affects the sodium channels of the nerve membrane and
causes a long-lasting prolongation of the transient increase in sodium
permeability during excitation, presumably by slowing down the closing
of the activation gate of the sodium channel (Vijverberg & Van den
Bercken, 1979; Vijverberg et al., 1983). The time constant of closing
of the activation gate in the cypermethrin-affected channels is
prolonged to more than 100 milliseconds. Apparently, the amplitude of
the prolonged sodium current after cypermethrin is too small to induce
repetitive activity in nerve fibres, but is sufficient to cause the
long-lasting repetitive firing in the lateral-line sense organ.
These results suggest that alpha-cyano pyrethroids primarily affect
the sodium channels in the nerve membrane and cause a long-lasting
prolongation of the transient increase in sodium permeability of the
membrane during excitation.
In the electrophysiological experiments using giant axons of
crayfish, the Type II pyrethroids retain sodium channels in a modified
continuous open state persistently, depolarize the membrane, and block
the action potential without causing repetitive firing (Lund &
Narahashi, 1983).
Diazepam, which facilitates GABA reaction, delayed the onset of
action of deltamethrin and fenvalerate, but not permethrin and
allethrin, in both the mouse and cockroach. Possible mechanisms of the
Type II pyrethroid syndrome include action at the GABA receptor complex
or a closely linked class of neuroreceptor (Gammon et al., 1982).
The Type II syndrome of intracerebrally administered pyrethroids
closely approximates that of the convulsant picrotoxin (PTX).
Deltamethrin inhibits the binding of [3H]-dihydropicrotoxin to rat
brain synaptic membranes, whereas the non-toxic R epimer of
deltamethrin is inactive. These findings suggest a possible relation
between the Type II pyrethroid action and the GABA receptor complex.
The stereospecific correlation between the toxicity of Type II
pyrethroids and their potency to inhibit the [35S]-TBPS binding was
established using a radioligand, [35S]- t-butyl-bicyclophosphoro-
thionate [35S]-TBPS. Studies with 37 pyrethroids revealed an
absolute correlation, without any false positive or negative, between
mouse intracerebral toxicity and in vitro inhibition: all toxic cyano
compounds including deltamethrin, 1R, cis-cypermethrin, 1R, trans-
cypermethrin, and [2S,alphaS]-fenvalerate were inhibitors, but their
non-toxic stereoisomers were not; non-cyano pyrethroids were much less
potent or were inactive (Lawrence & Casida, 1983).
In the [35S]-TBPS and [3H]-Ro 5-4864 (a convulsant
benzodiazepine radioligand) binding assay, the inhibitory potencies of
pyrethroids were closely related to their mammalian toxicities. The
most toxic pyrethroids of Type II were the most potent inhibitors of
[3H]-Ro 5-4864 specific binding to rat brain membranes. The [3H]-
dihydropicrotoxin and [ 35S]-TBPS binding studies with pyrethroids
strongly indicated that Type II effects of pyrethroids are mediated, at
least in part, through an interaction with a GABA-regulated chloride
ionophore-associated binding site. Moreover, studies with [3H]-Ro 5-
4864 support this hypothesis and, in addition, indicate that the
pyrethroid-binding site may be very closely related to the convulsant
benzodiazepine site of action (Lawrence et al., 1985).
The Type II pyrethroids (deltamethrin, 1R, cis-cypermethrin and
[2S,alphaS]-fenvalerate) increased the input resistance of crayfish
claw opener muscle fibres bathed in GABA. In contrast, two non-insect-
icidal stereoisomers and Type I pyrethroids (permethrin, resmethrin,
allethrin) were inactive. Therefore, cyanophenoxybenzyl pyrethroids
appear to act on the GABA receptor-ionophore complex (Gammon & Casida,
1983).
The effects of pyrethroids on end-plate and muscle action
potentials were studied in the pectoralis nerve-muscle preparation of
the clawed frog ( Xenopus laevis). Type II pyrethroids (cypermethrin
and deltamethrin) induced trains of repetitive muscle action potentials
without presynaptic repetitive activity. However, an intermediate
group of pyrethroids (1R-permethrin, cyphenothrin, and fenvalerate)
caused both types of effect. Thus, in muscle or nerve membrane the
pyrethroid induced repetitive activities due to a prolongation of the
sodium current. But no clear distinction was observed between non-
cyano and alpha-cyano pyrethroids (Ruigt & Van den Bercken, 1986).
Appraisal
In summary, the results strongly suggest that the primary target
site of pyrethroid insecticides in the vertebrate nervous system is the
sodium channel in the nerve membrane. Pyrethroids without an alpha-cyano
group (allethrin, d-phenothrin, permethrin, and cismethrin) cause a
moderate prolongation of the transient increase in sodium permeability
of the nerve membrane during excitation. This results in relatively
short trains of repetitive nerve impulses in sense organs, sensory
(afferent) nerve fibres, and, in effect, nerve terminals. On the other
hand, the alpha-cyano pyrethroids cause a long-lasting prolongation of
the transient increase in sodium permeability of the nerve membrane
during excitation. This results in long-lasting trains of repetitive
impulses in sense organs and a frequency-dependent depression of the
nerve impulse in nerve fibres. The difference in effects between
permethrin and cypermethrin, which have identical molecular structures
except for the presence of an alpha-cyano group on the phenoxybenzyl
alcohol, indicates that it is this alpha-cyano group that is responsible
for the long-lasting prolongation of the sodium permeability.
Since the mechanisms responsible for nerve impulse generation and
conduction are basically the same throughout the entire nervous system,
pyrethroids may also induce repetitive activity in various parts of the
brain. The difference in symptoms of poisoning by alpha-cyano
pyrethroids, compared with the classical pyrethroids, is not
necessarily due to an exclusive central site of action. It may be
related to the long-lasting repetitive activity in sense organs and
possibly in other parts of the nervous system, which, in a more advance
state of poisoning, may be accompanied by a frequency-dependent
depression of the nervous impulse.
Pyrethroids also cause pronounced repetitive activity and a
prolongation of the transient increase in sodium permeability of the
nerve membrane in insects and other invertebrates. Available
information indicates that the sodium channel in the nerve membrane is
also the most important target site of pyrethroids in the invertebrate
nervous system (Wouters & Van den Bercken, 1978; WHO, 1979).
Because of the universal character of the processes underlying
nerve excitability, the action of pyrethroids should not be considered
restricted to particular animal species, or to a certain region of the
nervous system. Although it has been established that sense organs and
nerve endings are the most vulnerable to the action of pyrethroids, the
ultimate lesion that causes death will depend on the animal species,
environmental conditions, and on the chemical structure and physical
characteristics of the pyrethroid molecule (Vijverberg & Van den
Bercken, 1982).
RESUME, EVALUATION, CONLUSIONS ET RECOMMANDATIONS
1. Résumé et Evaluation
1.1 Identité, propriétés physicochimiques et méthodes d'analyse
La perméthrine, un pyréthroide photostable, a été synthétisée pour
la première fois en 1973 et commercialisée en 1977. C'est un ester de
l'analogue dichloré de l'acide chrysanthémique, à savoir l'acide
(dichloro-2,2 vinyl)-3 diméthyl-2,2 cyclopropanecarboxylique (Cl2CA) et
de l'alcool phénoxy-3 benzylique. Les produits techniques se présentent
sous la forme d'un mélange de quatre stéréoisomères ayant les
configurations [1R,trans], [1R,cis], [1S,trans] et [1S,cis] dans les
proportions approximatives de 3:2:3:2. Le rapport cis/trans est
d'environ deux tiers, les isomères 1R et 1S étant présents en quantités
égales (racémique). C'est l'isomère [1R,cis] qui est l'insecticide le
plus actif; vient ensuite l'isomère [1R,trans].
La perméthrine de qualité technique se présente sous la forme d'un
liquide brun ou brun jaunâtre qui peut partiellement cristalliser à la
température ambiante. Son point de fusion est d'environ 35°C et son
point d'ébullition de 220°C sous 0,05 mmHg. La densité est de 1,214 à
25°C et la tension de vapeur de 1,3 µPa à 20°C. La perméthrine est
pratiquement insoluble dans l'eau (moins de 0,2 mg par litre à 25°C),
mais elle est soluble dans certains solvants organiques tels que
l'acétone, l'hexane et le xylène. Elle est stable à la lumière et à la
chaleur, mais instable en milieu alcalin.
Le dosage des résidus et les analyses écotoxicologiques s'effect-
uent par chromatographie en phase gazeuse avec détection par capture
d'électrons (concentration minimale décelable de 0,005 mg/kg). Pour
l'analyse des produits techniques, on a recours à la chromatographie en
phase gazeuse avec détection par ionisation de flamme.
1.2 Production et usage
On utilise actuellement dans le monde environ 600 tonnes de
perméthrine par an, essentiellement en agriculture. Ce produit pourrait
être utilisé pour la protection des céréales ensilées et on l'emploie
en épandage aérien pour la protection des forêts, la lutte anti-
vectorielle, la destruction des insectes incommodants dans les
habitations, le déparasitage des bestiaux, la destruction des poux du
corps et l'imprégnation des moustiquaires.
La perméthrine est présentée en concentré émulsionnable, en
concentré pour application à très bas volume, en poudre mouillable, en
poudre pour poudrage et en aérosols.
1.3 Exposition humaine
Les teneurs en résidus des diverses récoltes diminuent assez
lentement, le temps de demi-élimination allant de une à trois semaines
selon les plantes. Toutefois, lorsqu'elle est utilisée conformément
aux recommandations, la perméthrine ne donne pas lieu à une accumu-
lation de résidus, même après plusieurs traitements.
Pour ce qui est de la population dans son ensemble, la voie
d'exposition à la perméthrine est essentiellement alimentaire. Les taux
de résidus dans les plantes correctement cultivées sont généralement
faibles. L'exposition qui pourrait en résulter pour la population
générale est vraisemblablement faible, mais on manque de données
précises provenant d'études sur la ration totale.
On est très peu documenté sur l'exposition professionnelle à la
perméthrine.
1.4 Destinée dans l'environnement
On a montré en laboratoire que la perméthrine se dégrade dans le
sol avec une demi-vie d'environ 28 jours. L'isomère trans se décompose
plus rapidement que l'isomère cis, la principale réaction de décompo-
sition initiale étant le clivage du groupement ester. Cette réaction
donne naissance à des composés qui subissent une oxydation plus poussée
aboutissant à l'anhydride carbonique comme produit final. On a montré,
en étudiant le potentiel de lessivage de la perméthrine et de ses
produits de dégradation, que toutes ces substances pénétraient peu
profondément dans le sol.
La perméthrine déposée sur les végétaux se dégrade avec une demi-
vie d'environ 10 jours. La principale voie de dégradation comporte le
clivage du groupement ester et la conjugaison de l'acide et de l'alcool
qui en résultent. Il se produit également une hydroxylation en divers
points de la molécule ainsi qu'une interconversion cis-trans photo-
induite.
Dans l'eau et à la surface du sol, la perméthrine est décomposée
par le rayonnement solaire. Là encore, le clivage du groupement ester
et l'interconversion cis-trans sont les principales réactions.
En général, ce processus de décomposition environnementale conduit
à des produits de moindre toxicité.
La perméthrine disparaît rapidement de l'environnement, en six
à 24 heures dans les étangs et les cours d'eau, en sept jours dans les
sédiments des étangs, et en 58 jours dans les feuilles et le sol des
forêts. On a constaté que 30% du composé disparaissaient en une semaine
des feuilles d'une plantation de cotonniers.
En conditions d'aérobiose dans le sol, la perméthrine se décompose
avec une demi-vie de 28 jours.
La perméthrine se déplace peu dans l'environnement et il est
improbable qu'elle s'y accumule en quantité notable.
1.5 Cinétique et métabolisme
Administrée à des mammifères, la perméthrine est rapidement
métabolisée et presqu'entièrement excrétée dans les urines et les
matières fécales en l'espace de 12 jours. L'isomère trans, beaucoup
plus sensible à l'attaque par l'estérase que l'isomère cis, s'élimine
plus rapidement que ce dernier. Les principales réactions métaboliques
sont le clivage du groupement ester et l'oxydation, particulièrement au
niveau du cycle aromatique terminal, du reste phénoxybenzylique et du
groupe diméthyle géminé du cycle cyclopropane, réactions qui sont
suivies d'une conjugaison. On a retrouvé moins de 0,7% de la dose
initiale dans le lait de chèvres et de vaches à qui l'on avait
administré de la perméthrine par voie orale.
1.6 Effets sur les êtres vivants dans leur milieu naturel
Des épreuves de laboratoire ont montré que la perméthrine était
extrêmement toxique pour les arthropodes aquatiques, la CL50 allant de
0,018 µg/litre pour la larve d'un crabe comestible à 1,26 µg/litre pour
un cladocère. Elle est également très toxique pour les poissons, la
CL50 à 96 heures allant de 0,62 µg/litre pour la larve de truite
arc-en-ciel à 314 µg/litre pour la truite adulte. La dose sans effet
observable au début du cycle évolutif du vairon est 10 µg/litre sur 28
jours, la dose chronique étant de 0,66 à 1,4 µg/litre pour un autre
cyprinidé, Pimephas promelas. La perméthrine est moins toxique pour
les mollusques aquatiques et les amphibiens, les valeurs de la CL50 à
96 heures se situant respectivement à plus de 1000 µg/litre et
7000 µg/litre.
Lors d'essais sur le terrain et en utilisation normale, cette forte
toxicité potentielle ne se manifeste pas. Il existe une abondante
littérature sur les effets de la perméthrine utilisée en agriculture,
dans les exploitations forestières ainsi que pour la lutte antivectori-
elle dans de nombreuses régions du monde. Il y a une certaine mortalité
pour les arthropodes aquatiques, notamment lorsque l'on traite les eaux
en surface, mais les effets sur les populations ne sont que tempor-
aires. Il n'y a pas eu de cas de mortalité chez les poissons. Cette
moindre toxicité qui se manifeste sur le terrain provient de la forte
adsorption du composé par les sédiments et de sa décomposition rapide.
La perméthrine fixée sur les sédiments est toxique pour les
organismes fouisseurs, mais là encore, l'effet est temporaire. La
perméthrine est extrêmement toxique pour les abeilles. La DL50 topique est
de 0,11 µg par abeille mais le fort effet répulsif qu'elle exerce sur
l'insecte en réduit les effets toxiques dans la pratique. Rien
n'indique qu'il puisse y avoir une forte mortalité des abeilles en
utilisation normale. La perméthrine est plus toxique pour les acariens
prédateurs que pour les espèces nuisibles visées.
La perméthrine est très peu toxique pour les oiseaux, en
administration orale ou par voie alimentaire. La DL50 aiguë est
supérieure à 3000 mg par kg de poids corporel en une seule
administration et supérieure à 5000 mg par kg de nourriture quand le
produit est mêlé à la ration. Elle n'a aucun effet sur la reproduction
de la poule à la dose de 40 mg/kg de nourriture.
Les organismes aquatiques accumulent facilement la perméthrine, le
facteur de concentration allant de 43 à 750 selon les espèces. Chez
tous les organismes aquatiques étudiés, la perméthrine disparaît
rapidement lorsque les animaux sont remis en eau propre. Il n'y a
aucune accumulation chez les oiseaux. On peut donc considérer que ce
composé ne présente en pratique aucune tendence à la bioaccumulation.
1.7 Effets sur les animaux d'expérience et sur les systèmes
d'épreuve in vitro
La perméthrine n'a qu'une faible toxicité aiguë pour les rats, les
souris, les lapins et les cobayes, encore que la DL50 varie
considérablement selon le véhicule utilisé et le rapport des isomères
cis/trans. Les signes d'intoxication aiguë apparaissent en deux heures
et persistent jusqu'à trois jours. Les isomères [1R,cis] et [1R,trans]
appartiennent aux pyréthroïdes du type-I dont les effets caractérist-
iques sont les tremblements (syndrome T) la perte de coordination,
l'hyperactivité, la prostration et la paralysie. L'intoxication pro-
voque une augmentation notable de la température centrale.
Aucun des métabolites de la perméthrine ne présente une toxicité
aiguë (par voie orale ou intrapéritonéale) supérieure à celle de la
perméthrine elle-même.
La perméthrine a provoqué une légère irritation de la peau intacte
ou abrasée chez le lapin, mais il n'y a pas eu d'irritation d'origine
photochimique après exposition aux rayons ultra-violets de zones de la
peau traitées par cet insecticide. Elle ne suscite en revanche aucune
réaction de sensibilisation chez le cobaye.
Des études de toxicité orale subaiguë et subchronique ont été
effectuées chez le rat et la souris à des doses allant jusqu'à
10 000 mg/kg de nourriture et pendant des périodes s'étendant de 14 à
26 semaines. A la dose la plus forte, on a observé une augmentation du
rapport poids du foie/poids du corps, une hypertrophie du foie et des
signes cliniques d'intoxication tels que des tremblements. Chez le rat,
la dose sans effet observable varie de 20 mg/kg de nourriture (pour les
études de 90 jours ou de six mois) à 150 mg/kg de nourriture (lors
d'une étude de six mois).
Chez le chien, ces valeurs ont été trouvées égales à 50 mg/kg de
poids corporel et à 100 mg/kg de poids corporel, respectivement, lors
de deux études de trois mois.
Des études à long terme sur des souris et des rats ont révélé une
augmentation du poids du foie, vraisemblablement liée à l'induction du
système enzymatique des microsomes hépatiques.
Une étude de deux ans sur des rats a fait ressortir une dose sans
effet observable de 100 mg/kg de nourriture, soit 5 mg/kg de poids
corporel.
D'après trois études à long terme sur la souris, il semblerait
qu'il existe un certain pouvoir cancérogène au niveau des poumons pour
au moins une souche de souris (uniquement des femelles) à la dose la
plus élevée étudiée, soit 5 g/kg de nourriture. Aucun effet oncogène
n'a été observé chez des rats des deux sexes.
La perméthrine n'est mutagène ni in vivo ni in vitro .
Les études de mutagénicité ainsi que des études à long terme sur la
souris et le rat indiquent que le pouvoir oncogène de la perméthrine
est très faible, qu'il se limite aux souris femelles et qu'il s'agit
probablement d'un phénomène épigénétique.
La perméthrine n'est pas tératogène pour le rat, la souris ou le
lapin, à des doses allant respectivement jusqu'à 225, 150 et 1800 mg/kg
de poids corporel.
La perméthrine n'a pas produit d'effet indésirable à des doses
allant jusqu'à 2500 mg/kg de nourriture lors d'une étude de repro-
duction portant sur trois générations.
Administrée à des rats à forte dose (6600 à 7000 mg/kg de
nourriture) pendant 14 jours, l'insecticide a provoqué des lésions au
niveau du nerf sciatique; cependant une autre étude n'a pas confirmé la
présence d'altérations ultrastructurales à ce niveau. Chez la poule on
n'a pas observé de neurotoxicité retardée.
1.8 Effets sur l'homme
La perméthrine peut provoquer un certain nombre de sensations au
niveau de la peau ainsi que des parésthésies chez les travailleurs
exposés, symptômes qui apparaissent après une période de latence
d'environ 30 minutes, passent par un maximum au bout de huit heures et
disparaissent en 24 heures. Parmi les symptômes les plus fréquemment
signalés, figurent un engourdissement, des démangeaisons, des four-
millements et des sensations de brûlure.
Aucun cas d'intoxication n'a été signalé.
La probabilité d'effets oncogènes chez l'homme est extrêmement
faible, voire nulle.
Rien n'indique que la perméthrine puisse exercer des effets indési-
rables sur l'homme si elle est utilisée conformément aux recommand-
ations.
2. Conclusions
2.1 Population générale
La population dans son ensemble est vraisemblablement très peu
exposée à la perméthrine. Cet insecticide ne devrait présenter aucun
danger s'il est utilisé conformément aux recommandations.
2.2 Exposition professionnelle
Utilisée de manière raisonnable et moyennant certaines mesures
d'hygiène et de sécurité, la perméthrine ne devrait présenter aucun
danger pour les personnes qui lui sont exposées de par leur
profession.
2.3 Environnement
La perméthrine ou ses produits de décomposition ne devraient pas
atteindre dans le milieu des concentrations critiques dans la mesure où
l'insecticide est utilisé aux doses recommandées. Au laboratoire, la
perméthrine est extrêmement toxique pour les poissons, les arthropodes
aquatiques et les abeilles. Toutefois il est improbable que des effets
indésirables durables se produisent en situation réelle si l'insecti-
cide est utilisé conformément aux recommandations.
3. Recommandations
Les concentrations alimentaires résultant d'une utilisation
conforme aux recommandations sont en principe faibles, toutefois il
serait bon de confirmer cette hypothèse en étendant la surveillance à
la perméthrine.
La perméthrine est utilisée depuis de nombreuses années sans
qu'aucun effet indésirable n'ait été signalé à la suite d'une
exposition humaine. Néanmoins il serait bon de poursuivre les
observations sur ce type d'exposition.
