
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
ENVIRONMENTAL HEALTH CRITERIA 68
HYDRAZINE
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization.
Published under the joint sponsorship of
the United Nations Environment Programme,
the International Labour Organisation,
and the World Health Organization
World Health Orgnization
Geneva, 1987
The International Programme on Chemical Safety (IPCS) is a
joint venture of the United Nations Environment Programme, the
International Labour Organisation, and the World Health
Organization. The main objective of the IPCS is to carry out and
disseminate evaluations of the effects of chemicals on human health
and the quality of the environment. Supporting activities include
the development of epidemiological, experimental laboratory, and
risk-assessment methods that could produce internationally
comparable results, and the development of manpower in the field of
toxicology. Other activities carried out by the IPCS include the
development of know-how for coping with chemical accidents,
coordination of laboratory testing and epidemiological studies, and
promotion of research on the mechanisms of the biological action of
chemicals.
ISBN 92 4 154268 3
The World Health Organization welcomes requests for permission
to reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made
to the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 1987
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved.
The designations employed and the presentation of the material
in this publication do not imply the expression of any opinion
whatsoever on the part of the Secretariat of the World Health
Organization concerning the legal status of any country, territory,
city or area or of its authorities, or concerning the delimitation
of its frontiers or boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar
nature that are not mentioned. Errors and omissions excepted, the
names of proprietary products are distinguished by initial capital
letters.
CONTENTS
ENVIRONMENTAL HEALTH CRITERIA FOR HYDRAZINE
1. SUMMARY
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1. Identity
2.2. Physical and chemical properties
2.3. Analytical methods
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1. Natural occurrence
3.2. Man-made sources
3.2.1. Industrial production
3.2.2. Methods of transport
3.2.3. Disposal of waste
3.3. Use pattern
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION AND TRANSFORMATION
4.1. Transport and distribution between media
4.2. Abiotic degradation
4.3. Biodegradation
4.4. Interactions with soil
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1. Environmental levels
5.2. General population exposure
5.3. Occupational exposure
5.4. Populations at special risk
6. KINETICS AND METABOLISM
6.1. Absorption and distribution
6.2. Metabolism and excretion
6.3. Reaction with body components
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
7.1. Aquatic organisms
7.2. Microorganisms
7.3. Plants
8. EFFECTS ON EXPERIMENTAL ANIMALS
8.1. Single exposures
8.2. Short-term exposures
8.2.1. Inhalation exposure
8.2.2. Other routes of exposure
8.3. Biochemical effects and mechanisms of toxicity
8.3.1. Effects on lipid metabolism
8.3.2. Effects on carbohydrate and protein metabolism
8.3.3. Effects on mitochondrial oxidation
8.3.4. Effects on microsomal oxidation
8.3.5. Effects on the central nervous system
8.4. Reproduction, embryotoxIcity, and teratogenicity
8.5. Mutagenicity and related end-points
8.5.1. DNA damage
8.5.2. Mutation and chromosomal effects
8.5.3. Cell transformation
8.6. Carcinogenicity
8.6.1. Inhalation exposure
8.6.2. Oral exposure
9. EFFECTS ON MAN
9.1. Poisoning incidents
9.2. Occupational exposure
9.2.1. Inhalation exposure
9.2.2. Skin and eye irritation; sensitization
9.2.3. Mortality studies
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1. Evaluation of human health risks
10.2. Evaluation of effects on the environment
11. RECOMMENDATIONS FOR FURTHER STUDIES
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
REFERENCES
WHO TASK GROUP ON HYDRAZINE
Members
Dr B. Gilbert, CODETEC, University City, Campinas, Brazil
Professor P. Grasso, Robens Institute, University of Surrey,
Guildford, Surrey, United Kingdom
Mr M. Greenberg, Environmental and Criteria Assessment Office,
US Environmental Protection Agency MD-52, Research Triangle
Park, North Carolina, USA
Professor M. Ikeda, Department of Environmental Health, Tohoku
University School of Medicine, Sendai, Japan (Chairman)
Dr N.N. Litvinov, A.N. Sysin Institute of General and Community
Hygiene, USSR Academy of Medical Science, Moscow, USSR
(Vice-Chairman)
Dr G.B. Maru, Carcinogenesis Division, Cancer Research Insti-
tute, Tata Memorial Center, Parel, Bombay, India
Professor M. Noweir, Occupational Health Research Centre, High
Institute of Public Health, University of Alexandria,
Alexandria, Egypt
Dr E. Rauckman, Carcinogenesis and Toxicological Evaluation
Branch, National Institute of Environmental Health Sciences,
National Toxicology Program, Research Triangle Park, North
Carolina, USA
Professor D.J. Reed, Environmental Health Sciences Center,
Oregon State University, Corvallis, Oregon, USA
Dr E. Rosskamp, Institute for Water, Soil and Air Hygiene of
the Federal Ministry of Health, Berlin (West)
Dr S. Susten, Document Development Branch, Division of Stan-
dards Development and Technology Transfer, National
Institute for Occupational Safety and Health, Cincinnati,
Ohio, USA (Rapporteur)
Professor J.A. Timbrell, University of London, School of
Pharmacy, Toxicology Unit, London, United Kingdom
Observers
Dr P. Schmidt (European Chemical Industry Ecology and Toxico-
logy Centre), Bayer AG, Leverkusen-Bayerwerk, Federal
Republic of Germany
Dr D. Steinhoff (European Chemical Industry Ecology and Toxico-
logy Centre), Bayer AG, Institute for Toxicology, Wuppertal,
Federal Republic of Germany
Secretariat
Professor F. Valic, Andrija Stampar School of Public Health,
University of Zagreb, Zagreb, Yugoslavia (Secretary) a
Dr T. Ng, Office of Occupational Health, World Health Organ-
ization, Geneva, Switzerland
Ms F. Ouane, International Register of Potentially Toxic
Chemicals, United Nations Environment Programme, Geneva,
Switzerland
Dr T. Vermeire, National Institute of Public Health and
Environmental Hygiene, Bilthoven, Netherlands (Temporary
Adviser)
Mr J. Wilbourn, Unit of Carcinogen Identification and
Evaluation, International Agency for Research on Cancer,
Lyons, France
------------------------
a IPCS Consultant.
NOTE TO READERS OF THE CRITERIA DOCUMENTS
Every effort has been made to present information in the
criteria documents as accurately as possible without unduly
delaying their publication. In the interest of all users of the
environmental health criteria documents, readers are kindly
requested to communicate any errors that may have occurred to
the Manager of the International Programme on Chemical Safety,
World Health Organization, Geneva, Switzerland, in order that
they may be included in corrigenda, which will appear in
subsequent volumes.
* * *
A detailed data profile and a legal file can be obtained
from the International Register of Potentially Toxic Chemicals,
Palais des Nations, 1211 Geneva 10, Switzerland (Telephone no.
988400 - 985850).
ENVIRONMENTAL HEALTH CRITERIA FOR HYDRAZINE
A WHO Task Group on Environmental Health Criteria for
Hydrazine met in Geneva from 25 to 30 August, 1986.
Professor F. Valic opened the meeting on behalf of the Director-
General. The Task Group reviewed and revised the draft criteria
document and made an evaluation of the health risks of exposure
to hydrazine.
The draft of this document were prepared by DR T. VERMEIRE
of the National Institute of Public Health and Environmental
Hygiene, Bilthoven, the Netherlands.
The efforts of all who helped in the preparation and
finalization of the document are gratefully acknowledged.
* * *
Partial financial support for the publication of this
criteria document was kindly provided by the United States
Department of Health and Human Services, through a contract from
the National Institute of Environmental Health Sciences,
Research Triangle Park, North Carolina, USA - a WHO
Collaborating Centre for Environmental Health Effects. The
United Kingdom Department of Health and Social Security
generously supported the costs of printing.
1. SUMMARY
Anhydrous hydrazine is a caustic, fuming, hygroscopic liquid
at ordinary temperature and pressure. The odour perception
threshold is 3 - 9 mg/m3. It decomposes on heating or when
exposed to ultraviolet radiation to form ammonia, hydrogen, and
nitrogen. This reaction may be explosive, especially when
catalysed by certain metals and metal oxides. Spontaneous
ignition can occur in contact with porous materials. Hydrazine
hydrate, the principal compound produced, contains 64% by weight
hydrazine. Hydrazine is basic and is a strong reducing agent.
In 1981, the world production capacity of hydrazine was
estimated to be in excess of 35 000 tonnes.
Sensitive analytical methods have been developed for the
determination of hydrazine in air, water, biota, food, drugs,
and cigarette smoke. Minimum detection limits reported are
2 µg/m3 air (gas chromatography), 5 µg/litre water (colori-
metry), 1 µg/litre blood, plasma, or urine (gas chromato-
graphy/mass spectrometry), and 3000 µg/kg drug (gas chromato-
graphy).
Hydrazine is not known to occur naturally, except perhaps in
the tobacco plant. Currently, the primary uses of hydrazine
hydrate are as a raw material in the manufacture of agricultural
chemicals, blowing agents, polymerization catalysts, and pharm-
aceutical products, and as a corrosion inhibitor in boiler
water. Both the hydrate and anhydrous hydrazine are used as
propellant fuels.
Emission factors for loss of hydrazine into the atmosphere,
estimated for the Federal Republic of Germany, amounted to
0.06 - 0.08 kg/tonne of hydrazine produced and 0.02 - 0.03
kg/tonne of hydrazine during handling and further processing.
Accidental discharges into air, water, and soil can result from
bulk storage, handling, transport, and improper waste disposal.
At production facilities, data show that concentrations of
up to 0.35 mg/m3 and, occasionally, up to 1.18 mg/m3, can occur
during production under normal conditions. During handling of
the fuel, concentrations of up to 0.25 mg/m3 have been measured
under normal conditions, exceptionally rising to 2.59 mg/m3. A
level of 800 mg/m3 was measured at the site of a leak in an
industrial plant. The general population may be exposed to
hydrazine vapour via accidental discharge. Evaporation of
hydrazine from a liquid spill can be sufficient to generate an
atmospheric concentration as high as 4 mg/m3, 2 km downwind of
the spill.
Hydrazine is degraded rapidly in air through reactions with
ozone, hydroxyl radicals, and nitrogen dioxide. In polluted
air, the life-time of hydrazine is estimated to be of the order
of minutes. In a clean atmosphere, the life-time will be
approximately 1 h. In soil, aqueous hydrazine is adsorbed and
decomposed on clay surfaces under aerobic conditions. The rate
of degradation of hydrazine in water is highly dependent on
factors such as pH, temperature, oxygen content, alkalinity,
hardness, and the presence of organic material and metal ions.
The compound is biodegradable by microorganisms in activated
sludge. However, at concentrations above 1 mg/litre, it is also
toxic for these microorganisms.
The blue algae Microcystis aeruginosa was the most sensitive
aquatic species tested with respect to hydrazine; the 10-day
toxicity threshold was reported to be 0.00008 mg/litre. Fish
species showed LC50 values of between 0.54 and 5.98 mg/litre. A
test to assess damage to the embryo showed a lowest-observed-
adverse-effect level of 0.1 mg/litre for the fathead minnow. In
view of the low octanol/water partition coefficient of hydrazine
and its ready degradation, it will not bioaccumulate. Hydrazine
is toxic for plants and can inhibit germination.
Hydrazine is absorbed rapidly through the skin or via other
routes of exposure. It is rapidly distributed to, and eliminated
from, most tissues. In mice and rats, part of the absorbed
hydrazine is excreted unchanged, and part as labile conjugates
or as acid-hydrolysable derivatives via the urine. When
hydrazine is metabolized, a significant amount of nitrogen is
produced, which is excreted via the lungs.
In human beings, hydrazine is irritating to the skin, eyes,
and respiratory tract. It is a strong skin sensitizer. In a
number of cases of accidental exposure, severe adverse effects
were observed, principally in the central nervous system, liver,
and kidneys.
In experimental animals, in addition to the above effects,
common observations following single exposure include loss of
body weight, anaemia, hypoglycaemia, fatty degeneration of the
liver, and convulsions. Continuous exposure of mice, rats,
monkeys, and dogs to levels of 0.26 and 1.3 mg/m3 for 6 months
resulted in adverse effects in all species at 1.3 mg/m3 and in
mice (fatty liver) and rats (decrease in growth), also at 0.26
mg/m3. When rats were treated with hydrazine in the drinking-
water at 0.0003 - 0.3 mg/kg body weight per day, no adverse
effects were observed at levels of 0.003 mg/kg or less. This is
the only study in which a no-observed-adverse-effect level by
the oral route in rats has been reported. No data are available
for establishing a no-observed-adverse-effect level by the
inhalation route.
Data are lacking concerning the effects of hydrazine on the
human embryo or fetus. In studies on rats and mice, hydrazine
administered by injection, orally, or through inhalation pro-
duced adverse effects on embryos and fetuses, when administered
at doses that were toxic for the mother. The adverse effects
observed in these studies included increased resorptions,
reduced fetal weight, increased perinatal mortality, and
increased incidences of litters and fetuses with abnormalities.
The abnormalities observed were primarily supernumerary and
fused ribs, delayed ossification, moderate hydronephrosis, and
moderate dilation of brain ventricle. These abnormalities were
considered to be minor by the authors. On the basis of these
studies, it was concluded that, in the absence of human data, it
is prudent to assume that hydrazine would have an adverse effect
on the human embryo or fetus at levels near those producing
toxic effects in the mothers. Such exposures may occur from
accidental spillages.
Hydrazine caused increased DNA damage and repair in vitro.
No increased unscheduled DNA synthesis was observed in the germ
cells of mice after exposure in vivo. Hydrazine induced indir-
ect methylation of O6 and N7 of guanine in the liver DNA of
rodents after in vivo exposure to toxic doses. It also induced
gene mutations and chromosome aberrations in a variety of test
systems including plants, phages, bacteria, fungi, Drosophila,
and mammalian cells in vitro . However, in gene mutation assays
using bacteria, there were variable responses following the
addition of rat liver metabolic activation systems. Hydrazine
was found to transform hamster and human cells in vitro . It did
not induce chromosome aberrations, micronuclei, or dominant
lethals in mice in vivo, but chromosomal aberrations were
reported in rats in vivo .
Hydrazine vapour induced nasal tumours, most of which were
benign, in Fischer 344 rats, following inhalation exposure for
12 months to concentrations of 1.3 or 6.5 mg/m3 with subsequent
observation for 18 months, and in Syrian golden hamsters exposed
to 6.5 mg/m3 with subsequent observation for 12 months. Such
effects were not seen in C57BL/6 mice exposed to concentrations
of 0.06, 0.33, or 1.3 mg/m3 for 12 months followed by 15 months
of observation, except for an increased incidence of lung
adenomas of borderline significance at 1.3 mg/m3. In several
limited gavage and drinking-water studies, hydrazine induced an
increased incidence, in some cases dose-related, of multiple
pulmonary tumours in various mouse strains and in Cb/Se rats.
In CBA/Cb/Se and BALB/c/CB/Se mice, an increased incidence of
hepatocarcinomas was also induced. A very low, but increased,
incidence of hepatocarcinomas was observed in male Cb/Se rats.
No tumours were observed in hamsters.
On the basis of the carcinogenicity studies on experimental
animals, there is evidence that hydrazine is an animal carcino-
gen. Human data are inadequate. Hydrazine induced DNA
damage in vitro, methylation of DNA guanine in vivo, and
positive results in in vitro mutagenesis assays.
Making an overall evaluation, hydrazine can be regarded as
posing little hazard for the general population at normal
ambient levels. However, in the work-place and under conditions
of accidental exposure, hydrazine can present a significant
health hazard. Human data are limited but show that hydrazine
may affect the central nervous system, liver, and kidneys. In
addition, it may produce skin and eye irritation and skin sensi-
tization. The results of animal studies suggest that effects on
human beings may also include embryotoxicity at levels near
those producing toxic effects in the mothers and adverse effects
on the respiratory system. On the basis of the evidence of
carcinogenicity in animals and positive results in short-term
tests, it would be prudent to consider hydrazine to be a
possible human carcinogen and, therefore, the levels in the
environment should be kept as low as feasible. It can also be
concluded that hydrazine may present a hazard for the aquatic
environment and plant life.
Further studies are needed on: (a) dose-response in relation
to DNA alkylation, damage to the nasal epithelium, and pulmonary
effects; (b) skin sensitization, focusing on cross-reactivity
with hydrazine derivatives; (c) dermal irritation-promotion;
(d) dose-response in sensitive fish species in relation to diet;
(e) metabolism with regard to effects on DNA; and (f) effects of
continuous low-level exposure on reproduction in sensitive
rodent species.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1. Identity
Chemical formula: N2H4
Chemical structure: H H
| |
N - N
| |
H H
Relative molecular mass: 32.05
Common name: hydrazine
Common synonyms: diamide, diamine, anhydrous hydra-
zine, hydrazine base
Common trade names: Aerozine-50 (a 1:1 w/w fuel mixture
(of mixtures) of anhydrous hydrazine and 1,1-
dimethylhydrazine); Hydrazine
hydrate (N2H4 H2O) (a 1:1 molar
mixture of anhydrous hydrazine and
water; Levoxin (a 15-64% aqueous
solution); SCAV-OX (a 35-64% aqueous
solution); Zerox (a 15-64% aqueous
solution)
CAS chemical name: hydrazine
CAS registry number: 302-01-2
Conversion factors: 1 ppm = 1.31 mg/m3 at 25 °C and
101.3 kPa (760 mmHg)
1 mg/m3 = 0.76 ppm
Hydrazine exposures are always expressed as exposure to free
base N2H4. The actual compound used is given in parentheses
(sections, 6, 8, 9).
2.2. Physical and Chemical Properties
Anhydrous hydrazine is a caustic, fuming, highly polar,
weakly basic, hygroscopic liquid at ordinary temperature and
pressure. It is a combustible substance, burning with a blue
flame. The pure compound decomposes on heating or when exposed
to ultraviolet radiation to form ammonia, hydrogen, and nitro-
gen. This reaction may be explosive, especially when catalysed
by certain metals and metal oxides. Hydrazine can ignite spon-
taneously in air, when in contact with porous materials. In
aqueous solution, considerable hydrogen bonding takes place.
Hydrazine and water form a constant boiling mixture that con-
tains 68% by weight of hydrazine and boils at 120.5 °C. Hydra-
zine and water also form the compound hydrazine monohydrate,
which contains 64% hydrazine by weight. Hydrazine solutions in
water have basic properties. Hydrazine is a powerful reducing
agent. Autooxidation occurs in alkaline solutions and is
strongly catalysed by metal ions, notably copper, yielding
hydrogen peroxide as a by-product. Decomposition of aqueous
hydrazine occurs in the presence of metal catalysts such as
platinum or Raney nickel.
Some physical and chemical properties of hydrazine and its
hydrate are given in Table 1.
2.3. Analytical Methods
A selection of analytical methods for the determination of
hydrazine in air, water, biota, drugs, and smoke is presented in
Table 2. A review of methods can be found in Schmidt (1984).
Gas chromatographic methods are the most specific assays
available providing that the chromatographic behaviour of
hydrazine is improved by derivatization, for example, by
reaction with p-dimethylaminobenzaldehyde or 2,4-pentanedione.
Hydrazine can be determined simultaneously with its derivatives
using these methods. Colorimetric and titrimetric methods are
subject to interference, especially by hydrazine derivatives.
Direct-reading papers or indicating tubes, based on these
colorimetric methods, are available commercially with reported
detection limits of 65 µg/m3 for tapes and 330 µg/m3 for tubes
(US NIOSH, 1978; Schmidt, 1984).
The instability of hydrazine can present a problem in
aqueous samples. Usually, acidification of the samples with
sulfuric acid will prevent degradation of hydrazine.
Table 1. Some physical and chemical properties of hydrazine
and its hydrate
---------------------------------------------------------------------------
Property Anhydrous hydrazine Hydrazine hydrate
(100% N2H4) (64% N2H4)
---------------------------------------------------------------------------
Physical state liquid liquid
Colour colourless colourless
Odour ammoniacal and pungent ammoniacal and pungent
Odour perception 3 - 9 mg/m3a 3 - 9 mg/m3
Melting point 2 °C -51.5 °C
Boiling point 113.5 °C 120.1 °C (azeotrope)
Flash point 38 °C (open cup) 75 °C (open cup)
Flammable limits 1.8 - 100% 3.4 - 100%
Vapour pressure 1.39 kPa (10.4 mmHg) 1 kPa (7.5 mmHg) at 20 °C
at 20 °C
Density 1008 g/litre 1032 g/litre at 20 °C
at 20 °C
Relative vapour density 1.1
log n-octanol/water -3.08
partition coefficient
Solubility in water infinite infinite
Surface tension 66.7 dyne/cm at 25 °C 74.2 dyne/cm at 25 °C
-----------------------------------------------------------------------------
a From: Jacobson et al. (1955, 1958).
Table 2. Sampling, preparation, analysis
---------------------------------------------------------------------------------------------------------
Medium Sampling method/ Analytical method Detection Comments Reference
pretreatment limit
---------------------------------------------------------------------------------------------------------
Air trapping in dilute colorimetry after reac- 20 µg/m3 sample size 100 litre; US NIOSH
hydrochloric acid tion with p-dimethyl- suitable for personal and (1977a)
aminobenzaldehyde area monitoring; rec-
ommended range is
589 - 3440 µg/m3
Air trapping on sulfuric gas chromatography with 2 µg/m3 sample size 96 litre; US NIOSH
acid-coated silica- flame-ionization detec- suitable for personal and (1977b)
gel; desorption tion after derivatiza- area monitoring; rec-
with water tion with 2-furaldehyde ommended range is
and extraction into 2 - 60 000 µg/m3
ethyl acetate
Air trapping in chilled gas chromatography of 5 µg/m3 sample size 2 litre; Holtzclaw
acetone acetone derivative with suitable for area et al.
a nitrogen detector monitoring (1984)
Water sample must be acidi- colorimetry after re- 5 µg/ recommended range is ASTM (1981);
fied when not anal- action with p-dimethyl- litre 5 - 150 µg/litre Velte
ysed immediately aminobenzaldehyde (1984)
Water polarography after re- 50 µg/ Slonim &
action with 5-nitro- litre Gisclard
salicylaldehyde (1976)
Water sample adjusted to gas chromatography with 100 µg/ recommended range is Dee (1971)
pH 6 - 9 flame-ionization detec- litre 100 - 50 000 µg/litre
tion after derivatiza-
tion with 2,4-pentane-
dione
Urine, sample adjusted to gas chromatography with 400 µg/ p-bromobenzaldehyde Timbrell
water pH 3 nitrogen detection after litre used as internal standard et al.
derivatization with p- (1977)
chlorobenzaldehyde and
extraction with methylene
chloride
---------------------------------------------------------------------------------------------------------
Table 2. (contd.)
---------------------------------------------------------------------------------------------------------
Medium Sampling method/ Analytical method Detection Comments Reference
pretreatment limit
---------------------------------------------------------------------------------------------------------
Blood blood pretreated colorimetry after re- 200 µg/ detectable range 200 - Reynolds &
with trichloroacetic action with p-dimethyl- litre 2900 µg/litre; a serum Thomas
acid to precipitate aminobenzaldehyde blank should be included (1965);
protein Springer
et al.
Urine urine adjusted to pH 3 (1981)
Blood fluorimetry after reac- 5 µg/ Lewalter
tion with p-dimethyl- litre et al.
aminobenzaldehyde (1984)
Drugs sample dissolved gas chromatography with 3000 µg/kg the method was used in Matsui et
in water and nitrogen detection after drug or the analysis of isoniazid al. (1983)
centrifuged derivatization with ben- formul- and hydralazine
zaldehyde and extraction ation
into n-heptane
Cigar- trapping in penta- gas chromatography with 0.002 µg/ the method was also used Liu et al.
ette fluorobenzaldehyde electron-capture detec- 20 cigar- for the analysis of (1974)
smoke in methanol tion after extraction ettes tobacco
with ether and enrich-
ment by thin-layer chroma-
tography with elution by
ether
Plasma, gas chromatography/mass 1 µg/ 15 N -hydrazine used as Timbrell
biolo- spectrometry after deriv- litre the internal standard et al.
gical atization with pentafluoro- (1982);
media benzaldehyde, adsorption Blair
on silica, elution with et al.
hexane (1985)
---------------------------------------------------------------------------------------------------------
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1. Natural Occurrence
The only natural occurrence of hydrazine reported was in the
tobacco plant (Liu et al., 1974). Model system studies have
indicated that nitrogenase-bound hydrazine may be an inter-
mediate in biological nitrogen fixation (Jackson et al., 1968;
Mitchell & Scarle, 1972; Thorneley et al., 1978).
3.2. Man-Made Sources
Hydrazine can be released into the atmosphere during venting
operations, storage, and transfer. In the Federal Republic of
Germany, the emission factor for the production of hydrazine is
estimated to be 0.06 - 0.08 kg/tonne. It is estimated that
0.02 - 0.03 kg of hydrazine is lost to the environment for each
tonne of hydrazine subjected to handling and further processing
(Brugger, 1983). Accidental discharge into water, air, and soil
can result from bulk storage, handling, transport, and improper
waste disposal.
3.2.1. Industrial productiona
Most production methods are based on the ketazine process, a
variation of the Raschig process, in which ammonia is oxidized
by chlorine or chloramine in the presence of aliphatic ketones,
usually acetone. The resulting ketazine is then hydrolysed to
hydrazine. In a recent method, hydrogen peroxide is used to
oxidize ammonia in the presence of a ketone. A production
process of minor importance involves the reaction between urea
and sodium hypochlorite (Schiessl, 1980; Schmidt, 1984).
The world production capacity was estimated to be about
36 000 tonnes in 1981, not including countries with planned
economies (Schmidt, 1984). In addition to hydrazine hydrate, a
small amount of anhydrous hydrazine is produced. In 1964,
domestic consumption in the USA was approximately 7000 tonnes
(Raphaelian, 1966). In 1974, the total production in the USA
was reported to be 17 000 tonnes (US NIOSH, 1978; Schmidt,
1984). A more recent estimate for the USA is a production
capacity of 17 240 tonnes in 1979. In the same year, the
production capacity was 6400 tonnes in the Federal Republic of
Germany, 3200 tonnes in France, 6500 tonnes in Japan, and 1900
tonnes in the United Kingdom (Schiessl, 1980; Schmidt, 1984).
3.2.2. Methods of transport
In 1978, it was reported that the US Department of Energy
Management annually transported an average of 600 tonnes of
hydrazine fuel and 900 tonnes of hydrazine-1,1-dimethylhydrazine
fuel (Aerozine-50) by rail, road, and ship. These fuels were
transported in aluminium tank cars, stainless steel tank
trailers, or in stainless steel drums (Watje, 1978).
Current international regulations require the transport of
hydrazine hydrate and its aqueous solutions in metal containers
with polyethylene liners, in plastic canisters, or in stainless
steel containers.
3.2.3. Disposal of waste
Hydrazine has been disposed of by dilution with water to
form at least a 400 g/litre solution, followed by neutralization
with dilute sulfuric acid and drainage into a sewer with
abundant water (IRPTC, 1985). However, it should be noted that
even very dilute solutions of 0.1 mg/litre can be toxic for
aquatic life (section 7.1). Alternatively, hydrazine has been
burnt in an open pit after the addition of a hydrocarbon solvent
(IRPTC, 1985). A better procedure is to dilute with abundant
water and then oxidize the diluted solution (to below
20 g/litre) with hydrogen peroxide, calcium hypochlorite, or
sodium hypochlorite before draining into a sewer (NEPSS, 1975).
Hydrazine vapour emissions can be controlled by scrubbing,
using water as the scrubbing liquid, or by the direct flame of
catalytic incineraton (Gordon & Lewandowski, 1980).
Hydrazine sulfate, a commonly-used derivative, may be
disposed of by incineration (IRPTC, 1985).
3.3. Use Pattern
The first important use of hydrazine was as a rocket
propellant. In 1964, 73% of the hydrazine consumed was used for
this purpose in the USA. The remainder was mainly used as an
intermediate in the synthesis of agricultural chemicals such as
maleic hydrazine, blowing agents for plastics, drugs such as the
antitubercular isoniazid and the antihypertensive hydralazine,
and the solder fluxes, hydrazine bromide and hydrazine chloride.
Aqeous hydrazine was in use at that time as a corrosion
inhibitor in boiler water (Raphaelian, 1966). This use pattern
has shifted towards a relatively greater use of hydrazine as a
chemical intermediate. For the year 1977, it was estimated that
only 5% of the world production of hydrazine was used as fuel,
while 19% was used for boiler water treatment, 32% for the
synthesis of agricultural chemicals, and 34% for the synthesis
of blowing agents (Schiessl, 1980). Currently, the main use of
hydrazine hydrate as a raw material is in the manufacture of
agricultural chemicals (40%), blowing agents (33%), polymeriza-
tion catalysts, and pharmaceutical products. Use as a corrosion
inhibitor in boiler water (15%) continues, and there are appli-
cations as a chemical reducing agent in the metal-plating, metal
---------------------------------------
a All production capacities and consumption figures were
calculated for anhydrous hydrazine, although actual
production and consumption was of hydrazine hydrate or of
more dilute aqueous mixtures.
recovery, and photographic industries, and as an ignitor in
explosives (Schiessl, 1980; Schmidt, 1984). Smaller amounts are
used as a rocket propellant and emergency fuel (Pitts et al.,
1980; Wald et al., 1984). A very small amount of anhydrous
hydrazine is used as a monopropellant in space vehicles and
satellites (Schmidt, 1984).
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
4.1. Transport and Distribution Between Media
Pure hydrazine has a low vapour pressure and is highly
soluble in water. Nevertheless, the evaporation rate from a
liquid spill can be sufficient to generate an atmospheric
concentration of 4 mg/m3, 2 km downwind under unfavourable
meterological conditions. Dilution with large amounts of water
reduces the evaporation rate significantly (MacNaughton et al.,
1981).
4.2. Abiotic Degradation
Alkaline solutions of hydrazine in water can be subject to
autooxidation by dissolved oxygen. Hydrogen peroxide is an
important intermediate (Audrieth & Ogg, 1951). In acidic
solutions or in the absence of metal ions, notably copper, no
appreciable degradation was observed in aerated distilled water
(Gormley & Ford, 1973; MacNaughton et al., 1981). Gormely &
Ford (1973) measured a rapid oxygen depletion from 0.02 to 0.1%
alkaline solutions of hydrazine in the presence of copper ions
and developed a mathematical expression relating the aqueous
degradation rate of hydrazine to the concentrations of hydra-
zine, copper ions, and oxygen at constant pH and temperature.
Degradation rates for dilute hydrazine solutions were highly
variable (Slonim & Gisclard, 1976; MacNaughton et al., 1981).
Hydrazine was almost completely degraded within one day in muddy
river water, sampled directly after a rain storm. However, in
softened, filtered water at the same temperature and with the
same dissolved oxygen content, but a lower initial pH, little
degradation occurred within 4 days. The main factors that
favour abiotic hydrazine degradation are the presence of certain
metal ions, organic material, in general, and organic oxidizers,
in particular, increased hardness, and high pH (Slonim &
Gisclard, 1976).
In air, hydrazine can be oxidized in a number of different
ways. There is no information on how hydrazine in the atmo-
sphere is degraded. The destruction of hydrazine by ozone and
by hydroxyl radicals has been experimentally investigated (Hack
et al., 1974; Harris et al., 1979, Pitts et al., 1980). The
rate of hydroxyl radical reaction with hydrazine was found to be
a linear function of the hydrazine concentration, independent of
temperature and pressure. Assuming an average hydroxyl radical
concentration of 106 radicals/cm3 for the lower troposphere, the
half-life of hydrazine with respect to this radical is estimated
to be about 3 h (Harris et al., 1979; Pitts et al., 1980).
Assuming an average level of 80 µg ozone/m3 air (Singh et al.,
1978), the lifetime of hydrazine with respect to ozone would be
approximately 1 h. Nitrogen dioxide also reacts with hydrazine
(Pitts et al., 1980; Tuazon et al., 1982). In a polluted atmo-
sphere, the lifetime would be of the order of minutes (Pitts et
al., 1980; Tuazon et al., 1982; Schmidt, 1984). Diazene, hydro-
gen peroxide, and small amounts of nitrous oxide and ammonia
have been identified as products of these reactions (Tuazon et
al., 1982).
4.3. Biodegradation
Hydrazine has been shown to be co-metabolized mainly to
nitrogen gas by the nitrifying bacterium Nitrosomonas (Kane &
Williamson, 1983). Preliminary studies have also indicated that
hydrazine can be reduced to ammonia by nitrogenase isolated from
the nitrogen-fixing bacterium Azobacter vinelandii (Davis,
1980). When hydrazine was added continously to a waste-water
treatment plant, only concentrations below 1 mg/litre ensured
complete absence of the compound from the effluent, without
inhibiting treatment efficiency (Farmwald & MacNaughton, 1981).
4.4. Interactions with Soil
Heck et al. (1963) found that dilute hydrazine was adsorbed
in a column of soil or decomposed, if the soil contained a
moderate amount of clay. Probably, decomposition on clay
particles was more important than adsorption. Another factor
influencing adsorption is the organic content of the soil
(Isaacson et al., 1984). Dilute hydrazine leached completely
through a column of sand (Heck et al., 1963).
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1. Environmental Levels
No data on environmental levels of hydrazine are available.
This is because degradation is so rapid that measureable levels
are not normally encountered (Schmidt, 1984).
5.2. General Population Exposure
Hydrazine levels of 23 - 43 ng/cigarette were found in
cigarette smoke by Liu et al. (1974).
Traces of hydrazine have been found in samples of commercial
maleic hydrazide, one of the uses of which is to inhibit sucker
growth on tobacco. However, the amount of hydrazine measured in
tobacco that had been treated with maleic hydrazide (12 - 51
ng/cigarette) was not very much different from that measured in
untreated tobacco (14 - 22 ng/cigarette), indicating another
source of hydrazine in tobacco (Liu et al., 1974).
It has been reported that analyses of hydrazine-treated
boiler water and the condensate of steam, which could have been
in contact with food, confirmed the presence of hydrazine (US
FDA, 1979).
District heating water has been mentioned as an additional
potential route of accidental human exposure. This water may
contain a low concentration of hydrazine as a corrosion inhi-
bitor. If this water is used to heat tap water and there is a
leak inside the heat-exchanger at the user end, the tap water
may be contaminated. Cases have been reported in which hot
water became contaminated with levels of up to 10.72 mg/litre
and drinking-water, up to 0.47 mg/litre (Bodenschatz, 1986).
5.3. Occupational Exposure
Workers may be exposed to hydrazine at facilities producing
hydrazine itself and those producing its salts and derivatives,
at propulsion testing and rocket launching sites, and at
locations where aircraft using hydrazine as an emergency fuel
are assembled or refueled. Workers at plants using high-
pressure boilers are potentially exposed to relatively dilute
solutions of hydrazine. The number of workers and levels of
exposure for sites in the USA are given in Table 3 (US NIOSH,
1986). Earlier and essentially similar data are reported by US
NIOSH (1978) and Suggs et al. (1980).
Workers normally exposed to anhydrous or concentrated
hydrazine are provided with respiratory and skin protection.
The difference between air levels outside and inside protective
masks was illustrated by Cook et al. (1979) who found levels of
0.29 - 2.59 mg/m3 outside the masks at a rocket propellant-
handling facility, and levels below the detection limit of 0.013
mg/m3 inside the masks.
Much higher levels (800 mg/m3) were observed at the site of
a leak (Suggs et al., 1980).
Table 3. Occupational exposure to hydrazine in the USA
---------------------------------------------------------------------
Site Approximate numbers Measured levels (mg/m3)
exposed Normal Exceptional
Normal Potential
---------------------------------------------------------------------
A Rocket testing 10 100 0.01-0.02 0.14a
B Production 100 800 < 0.13 0.13-0.26b
C F-16 fighter 32 - 0.04-0.05
station
D Rocket testing 10 300 no data no data
E F-16 assembly 51 16 500 0.04-0.25 no data
F Space-craft no data 14 000 no data no data
launching
G Derivative manu- < 25 no data < 0.13 ca. 0.13
facturer
H Production no data 1100 < 0.35 < 1.18
---------------------------------------------------------------------
a Level measured during aeration of the waste-water holding pond.
b Short-term samples during specific operations.
5.4. Populations at Special Risk
Although not strictly an environmental occurrence of
hydrazine, the presence of this compound in inadequately
purified or aged medicinal drugs can expose a section of the
human population to hydrazine. Two drugs that exemplify this
exposure risk are isoniazid (Spinkova, 1971; Matsui et al.,
1983; Blair et al., 1985) and hydralazine (Matsui et al., 1983;
Blair et al., 1985). Hydrazine can also be formed during the
metabolism of these drugs (Noda et al., 1978; Timbrell &
Harland, 1979).
Recently, hydrazine was detected in the plasma of 8 healthy
male volunteers taking isoniazid for 2 weeks and in the plasma
of 8 out of 14 hypertensive patients treated with, among others,
hydralazine. After 2 weeks of dosing with isoniazid, the
average level of acid-labile hydrazine in men of a slow
acetylator phenotype was 2.7 times higher than in men of a rapid
acetylator phenotype (Blair et al., 1985).
6. KINETICS AND METABOLISM
6.1. Absorption and Distribution
When undiluted hydrazine (free base) was applied to the
uncovered skin of dogs, the compound was detectable in plasma
within 30 seconds. Maximum concentrations were reached 1 - 3 h
after application. The concentration of hydrazine in blood
increased with dose (Smith & Clark, 1972).
An aqueous solution (700 g/litre) was administered dermally
at a dose of 12 mg hydrazine (free base)/kg body weight to
groups of 4 rabbits by fixing a piece of fibre glass screen to
an area of shaved skin. The area was not covered, but
corrections were made for evaporation loss. Hydrazine was
rapidly detectable in serum and reached a maximum concentration
of 10 mg/litre approximately 1 h after application. The half-
life of disappearance from serum was 2.3 h. The apparent volume
of distribution was determined to be 630 ml/kg body weight. It
was calculated that 55% of the applied hydrazine was absorbed
percutaneously (Andersen & Keller, 1984).
Following intraperitoneal (ip) injection of 32 mg hydra-
zine/kg body weight in rats or mice (free base and hydrazine
sulfate, respectively), peak concentrations of hydrazine in
blood of approximately 10 mg/litre occurred almost immediately,
and then the hydrazine disappeared rapidly from the blood
(Springer et al., 1981; Nelson & Gordon, 1982). A half-life of
44 min was observed in the blood of the rats during the first 3
h following exposure, followed by a slower phase with a half-
life of 27 h (Springer et al., 1981). When rats were exposed to
hydrazine vapour at concentrations of between 0 and 40 mg/m3
(free base), the blood concentration of hydrazine increased with
exposure. After 6 h of exposure to a hydrazine concentration of
20 - 25 mg/m3, a blood concentration of 0.64 mg/litre was
measured (Dost et al., 1981).
Hydrazine was distributed rapidly in most tissues of mice
and rats after ip or subcutaneous (sc) exposure. Elimination
from these tissues also occurred rapidly (Dambrauskas & Cornish,
1964; Nelson & Gordon, 1982; Kaneo et al., 1984). For example,
24 h after the injection of 30 mg (ip to mice, hydrazine
sulfate) or 60 mg (sc to rats, free base) hydrazine/kg body
weight, less than 15% of the hydrazine present in the various
tissues at 2 h was retained in these tissues at 18 h
(Dambrauskas & Cornish, 1964; Nelson & Gordon, 1982). The
highest levels of hydrazine were measured in the kidneys of both
rats (Dambrauskas & Cornish, 1964; Kaneo et al., 1984) and mice
(Nelson & Gordon, 1982), levels in other tissues being much
lower. In rats, the greater part of sc-administered hydrazine,
recoverable from tissues and blood (approximately 75%), was
recovered from skin and muscles (Dambrauskas & Cornish, 1964).
6.2. Metabolism and Excretion
A significant part of hydrazine (free base), administered
sc, ip, or intravenously (iv), was excreted unchanged or as
acetylhydrazine in the urine of dogs (McKennis et al., 1955) and
rabbits (McKennis et al., 1959). After acid hydrolysis, small
quantities of 1,2-diacetylhydrazine were identified in the urine
of rabbits, but not in that of dogs (McKennis et al., 1959).
Dambrauskas & Cornish (1964) administered 60 mg hydrazine (free
base)/kg body weight, sc, to mice and rats and recovered 48.3
and 27.3% of the dose, respectively, in the urine, as hydrazine
or acetylhydrazine, while almost none was left in the body.
Approximately 14% of an ip dose of 5 mg/kg body weight (hydra-
zine hydrate) was recovered in the urine of rats as hydrazine
(10.3%), acetylhydrazine (2.2%), and diacetylhydrazine (1.2%)
(Wright & Timbrell, 1978). In a similar study on rats, 30% of
sc doses of 2.6 and 5.1 mg/kg body weight (hydrazine monohydro-
chloride) was recovered in the urine as hydrazine (19%),
acetylhydrazine (10%), and small quantities of diacetylhydrazine
(Perry et al., 1981). When rats were injected sc with 9.9 mg
hydrazine (hydrazine sulfate), the percentages of the dose
recovered as acetylhydrazine and diacetylhydrazine were 2.9 and
2.5%, respectively, in 48-h urine samples (Kaneo et al., 1984).
In spite of the variation in these results, it is clear that the
major part of the hydrazine administered is not accounted for.
Noda et al. (1985b) studied the effects of microsomal enzyme
inducers on hydrazine disposition in rats. After iv administra-
tion of 1.2 mg hydrazine (hydrazine sulfate)/kg body weight to
rats, the plasma half-life was decreased from 1.69 to 1.2 h and
1.03 h by pretreatment of the animals with rifampicin and pheno-
barbital, respectively. Urinary excretion of hydrazine was
significantly decreased by phenobarbital pretreatment from 21.3%
to 14.6% of the dose.
In vitro studies revealed that both oxyhaemoglobin in
erythrocytes and liver microsomal oxygenases can catalyse the
oxidation of hydrazine to nitrogen (Clark et al., 1968; Springer
et al., 1981; Nelson & Gordon, 1982). Diazene (C4H4N2) has been
proposed as a probable intermediate (Nelson & Gordon, 1982).
Rat liver cytochrome P-450 has been implicated in the formation
of a free radical intermediate that must be a precursor of
diazene during microsomal oxidation of hydrazine (Noda et al.,
1985a). Tracer balance studies with 15N-labelled hydrazine have
shown that rats and mice can convert hydrazine to nitrogen gas,
which is excreted via the lungs. The results of these studies
are summarized in Table 4.
Table 4. Tracer-balance studies with 15N-labelled hydrazine
----------------------------------------------------------------------------------------
Species Route Dose Medium Metabolites % of Reference
(mg/kg dose
body weight)
----------------------------------------------------------------------------------------
Rat ip 32 expired air 15N-nitrogen 25 Springer
(free et al.
base) urine hydrazine, 30 (1981)
acetylhydrazine;
acid-hydrolysable 20
derivativesa;
15N-ammonia NDb
bile hydrazine and acid- < 1
hydrolysable
derivatives
Mouse ipc 32 expired air, 15N-nitrogen 30 - 35 Nelson &
(hydrazine Gordon
sulfate) urine hydrazine or labile 15 (1982)
conjugates;
acid-hydrolysable 25
derivatives
----------------------------------------------------------------------------------------
a Excluding acetylhydrazine.
b ND = not detected.
c sc and iv injection resulted in minor differences in conversion.
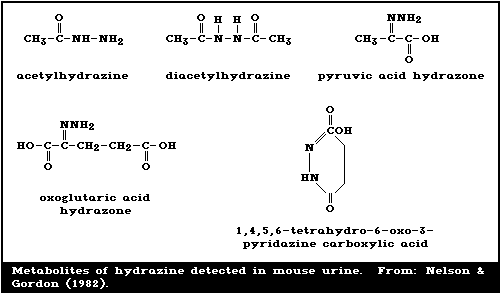
Nelson & Gordon (1982) reported the identification of some
of the acid-hydrolysable derivatives, as shown in Fig. 1. They
postulated that, when administered in vivo , hydrazine is rapidly
oxidized to nitrogen gas by haem constituents, including oxy-
haemoglobin and cytochrome P-450, and to a free radical of
hydrazine leading to diazene, which spontaneously decomposes to
nitrogen gas. After this initial release of nitrogen during the
first 15 - 30 min, nitrogen release is much slower, and acetyl-
ation and carbonyl group reactions are the dominant processes
leading to urinary products (Fig. 1). About 20 - 30% of the
hydrazine dose is expired as nitrogen gas in the first 2 h in
both rats and mice (Springer et al., 1981; Nelson & Gordon,
1982).
 Approximately 25% of the hydrazine dose remains unaccounted
for. Ammonia was found in the blood of dogs without
significantly elevated blood-urea nitrogen (Floyd, 1980).
Springer et al. (1981) did not find labelled ammonia in the
urine of rats exposed to 15N-hydrazine (Table 4). Therefore,
the ammonia in dogs was probably not derived from hydrazine
(section 8.3.2), but was the result of an effect on metabolic
pathways (Floyd, 1980; Springer et al., 1981).
6.3. Reaction With Body Components
No adduct formation between hydrazine and DNA in vivo has
been reported (Shank, 1983). Under non-physiological conditions,
hydrazine can react with pyrimidine bases. These reactions were
reviewed by Kimball (1977). Indirect methylation of guanines in
DNA following hydrazine exposure has been demonstrated and will
be discussed in section 8.5.1.
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
7.1. Aquatic Organisms
A summary of acute toxicity data is presented in table 5. It
should be realized that the rate of decay of hydrazine in the
aquatic environment depends on the conditions (section 4.2).
When the concentration of hydrazine is not monitored during
exposure, it should be noted that the toxic effects observed
must have occurred at concentrations lower than the nominal
ones, due to degradation of the compound. the increased toxicity
of hydrazine for guppies in soft water at a pH just below 7
compared with the toxicity in hard water at a pH of
approximately 8, as found by Slonim (1977), is at least partly
explained by the increased persistence of hydrazine in soft and
non-alkaline water. Taking into account the decay of hydrazine,
increases in water temperature were found to enhance the
toxicity of the compound for bluegills (hunt et al., 1981).
Teratogenicity and toxicity screening were reported using
the South African clawed toad (Greenhouse, 1976a,b), fathead
minnow (Henderson et al., 1981), and rainbow trout (Henderson et
al., 1983). Eggs of the South African clawed toad in the
cleavage were exposed to hydrazine until hatching. Survival and
development into normal larvae occurred at exposures below 10
mg/litre. At 10 mg/litre, 35% of the embryos were malformed at
hatching. The effect was dose-related. Additional studies
revealed that teratogenic effects appeared during neurulation
(Greenhouse, 1976a). When larvae of the South African clawed
toad were exposed to 1.0 mg hydrazine/litre water, for 120 h,
all died in 24 - 48 h following exposure. No significant effects
on survival and development were observed after exposure to 0.1
mg/litre, the next lower concentration tested (Greenhouse,
1976b).
Eggs of fathead minnows at the mid-cleavage stage were
exposed to hydrazine for 24 or 48 h. Embryos, exposed for 24 h,
to 0.1 mg/litre, showed several defects, such as slightly or
moderately subnormal heart beat, haemoglobin levels, body
movement amount of eye pigment. From 1 mg/litre upwards, the
responses were generally stronger; in addition, body pigment was
absent and developmental arrest was observed. Embryos exposed to
a hydrazine concentration of 1.0 mg/litre for 48 h appeared to
have little chance of survival. Surviving embryos showed severe
deformities and larvae exhibited reduced growth (Henderson et
al., 1981).
Table 5. Acute aquatic toxicity of hydrazine
---------------------------------------------------------------------------------------------------------
Organism Tempera- pH Hardness Flow/ Parameter Concentration Reference
ture (mg CaCO3 stata (mg/litre)
(°C) /litre)
---------------------------------------------------------------------------------------------------------
Bacteria
Pseudomonas putida 20 stat 16-h TT 0.019 Bringmann &
Kühn
(1980)b
Protozoa
Entosiphon sulcatum 25 6.9 stat 72-h TT 0.93 Bringmann &
Kühn (1981)b
Uronema paraduczi 25 6.9 stat 22-h TT 0.24 Bringmann &
Kühn (1981)b
Chilomenas paramecium 20 6.9 stat 48-h TT 0.002 Bringmann &
Kühn (1981)b
Algae
Green algae
(Chlorella pyrenoidosa) 23 6.8 75 stat 48-h EC50 ca.10c Heck et al.
48-h EC100 ca.100c (1963)d
Crustacea
Water flea 20 8.0 stat 24-h EC50 2.3c Bringmann &
(Daphnia pulex) Kühn (1982)
8.2 stat 24-h LC50 1.16c Heck et al.
(1963)
20 7.1-7.2 stat 24-h LC50 0.51 and 1.01 Velte (1984)e
48-h LC50 0.16 and 0.19
Amphibia
South African clawed
toad (Xenopus laevis)
eggs 8.2-8.7 stat LOEL 10c Greenhouse
(1976a)f
larvae stat 120-h LOEL 1.0c Greenhouse
120-h NOEL 0.1c (1976b)g
Table 5. (contd.)
---------------------------------------------------------------------------------------------------------
Organism Tempera- pH Hardness Flow/ Parameter Concentration Reference
ture (mg CaCO3 stata (mg/litre)
(°C) /litre)
---------------------------------------------------------------------------------------------------------
Fish (fresh-water)
Guppy (Lebistes reti- 22-24 7.8-8.2 400-500 stat 96-h LC50 3.85c Slonim (1977)
culatus) 22-24 6.3-6.9 20-25 stat 96-h LC50 0.61c
Fathead minnow
(Pimephales promelas)
eggs 21 7.0-7.5 150 flow 48-h LOEL 0.1 Henderson et
48-h NOEL 0.001 al. (1981)h
adults 20 192 stat 96-h LC50 4.5c Cowen et al.
(1981)
adults 20 6.9 flow 96-h LC50 5.98 Velte (1984)e
Bluegill sunfish 23-24 7.2-8.4 240-292 stat 96-h LC50 1.08 Fisher et al.
(Lepomis macrochirus) (1980)
23-24 7.8-7.9 164 flow 96-h LOEL 0.43 Fisher et al.
(1980)i
23-24 7.1-7.9 239 stat 96-h LOEL 0.1 Fisher et al.
(1980)i
10 6.7-8.0 160-190 flow 96-h LC50 1.6 Hunt et al.
15.5 1.0 (1981)
21 1.2
Goldfish (Carassius 8.2-8.5 stat 48-h LC50 2.8c Heck et al.
auratus) (1963)j
19 8.1-8.5 135 stat 24-h LC50 0.95 Proteau et al.
(1979)
Roach (Rutilus 19 8.1-8.5 135 stat 24-h LC50 0.54 Proteau et al.
rutilus) (1979)
Zebra fish
(Brachydanio rerio)
5-day-old 26 7.8 110 stat 24-h LC50 0.75 Proteau et al.
(1979)
3-month-old 20 7.6-8.2 110 stat 24-h LC50 2.03 Proteau et al.
(1979)
Table 5. (contd.)
---------------------------------------------------------------------------------------------------------
Organism Tempera- pH Hardness Flow/ Parameter Concentration Reference
ture (mg CaCO3 stata (mg/litre)
(°C) /litre)
---------------------------------------------------------------------------------------------------------
Green sunfish (Leptomis 8.2-8.5 stat 48-h LC50 5.1c Heck et al.
(1963)j
Large mouth bass 8.2-8.5 stat 48-h LC50 3.6c Heck et al.
(1963)j
Channel catfish 8.2-8.5 stat 48-h LC50 1.6c Heck et al.
(1963)j
Fish (marine species)
Stickle back (Gaster- 14- 7.6-8.0 stat 96-h LC50 3.4c Harrah
osteus aculeatus) 15.5 (1978)k
---------------------------------------------------------------------------------------------------------
a Flow-through or static test.
b TT = toxicity threshold.
c No analysis for hydrazine during exposure was reported.
d EC50 and EC100 for 50% and 100% growth inhibition, measured by reading optical density.
e Soft water.
f LOEL = lowest-observed-adverse-effect level for teratogenicity. Exposure of eggs in cleavage stage
until hatching.
g LOEL = lowest-observed-adverse-effect level for lethality. NOEL = no-observed-effect level
for lethality and development.
h LOEL and NOEL = lowest-and-no observed-adverse-effect level for toxicity and teratogenicity.
i LOEL = lowest-observed-adverse-effect level for dorsal light response (at a non-lethal concentration).
j Standard reference water.
k Salinity, 1.8%.
Henderson et al. (1983) also exposed eggs of rainbow trout
(Salmo gairdneri) for 48 h, to hydrazine in continuous-flow
tests at 11.5 - 12 °C, a pH of 7 - 7.5, and a water hardness of
15 mg calcium carbonate/litre. During exposures up to 5 mg/litre
a dose-related increase was observed in the incidence of poorly
fitting jaws, pronounced mouth gape, and absence of body
movement. However, no effects were observed on mortality, heart
beat, hatching rate, or hatching period. Reduced growth and
abnormal development of larvae were observed at 1 and 5
mg/litre. Poor muscular development and poor bone growth were
observed; the authors postulate that this is a result of calcium
binding by hydrazine.
7.2. Microorganisms
The toxicity of hydrazine for a number of species of
bacteria, algae, and protozoa was measured by Bringmann (1975) &
Bringmann & Kuhn (1980, 1981). Some very low toxicity thresholds
were reported, for example, 0.005 mg/litre for a 7-day exposure
for the algae Scenedesmus quadricauda and 0.00008 mg/litre for a
10-day exposure for the blue algae Microcystis aerogenosa. This
is a very sensitive test.
London et al. (1983) described the toxicity of hydrazine for
the soil heterotroph Enterobacter cloacea. Hydrazine caused a
concentration-dependent increase in the lag time of this
organism. In a medium containing 10 mg/litre, this did not
affect the growth rate and final growth yield after the lag
period. At 100 mg/litre, the bacteria were not viable.
Although relatively high concentrations of hydrazine in
water have been recorded as inhibiting, either completely or
partially, the activities of Nitrosomonas, Nitrobacter, and
other bacteria in culture media (Yoshida & Alexander, 1964) in
waste-water treatment (Tomlinson et al., 1966; Farmwald &
MacNaughton, 1981; Kane & Williamson, 1983), the highest
continuously maintained tolerable level in waste water is of the
order of 1 mg/litre, as stated in section 4.3 (Farmwald &
MacNaughton, 1981).
7.3. Plants
Heck et al. (1963) studied the effects of hydrazine on the
germination of seeds and seedling growth after application as a
hydroponic culture contaminant and an air fumigant.
Seeds of summer brush squash (Cucurbita pepo), peanut
(Arachis hypogaea), and corn (Zea mays) were soaked for 48 h in
water containing hydrazine at levels of between 0 and 1000
mg/litre. The temperature was 30 °C. At the highest concentra-
tion, germination of peanut and corn seed was inhibited. Seed-
ling growth was inhibited from 10 mg/litre for squash, 100
mg/litre for corn, and 1000 mg/litre for peanut.
Sixteen-day-old seedlings of cotton (Gossypium hirsutum) in
a hydroponic culture were exposed to hydrazine in the growth
medium for 9 days at concentrations of between 0 and 1000
mg/litre and a temperature of between 22 and 29 °C. Plants died
within 48 h of exposure to 300 mg/litre and within 30 h at the
higher concentrations. Injury was first noted as foliar
dehydration, without chlorosis or necrosis, after 9 days of
exposure to 50 mg/litre or within 24 h of exposure to 300
mg/litre or more.
Several plants were also exposed for 4 h to hydrazine vapour
at concentrations of between 0 and 100 mg/m3 air. Species tested
were soybean (Glycine max), cow pea (Vigna sinensis), pinto bean
(Phaseolus vulgaris), cotton (Gossypium hirsutum), endive
(Cichorium endivia), alfalfa (Medicago sativa), and squash
(Cucurbita pepo). Wilting of leaves in all species was
observed within 2 - 24 h of exposure to 30 mg/m3, followed by
wilting of the whole plant. Death occurred in pinto beans and
endive plants at this exposure level. At higher concentrations,
plants of soybean and alfalfa also died. Six days after
exposure, all surviving plants started to recover.
8. EFFECTS ON EXPERIMENTAL ANIMALS
In section 8, all doses have been expressed in terms of the
free base; however, the form of hydrazine used in each study has
been indicated when possible.
8.1. Single Exposures
The toxicology of hydrazine has been reviewed by Krop
(1954), Clark et al. (1968), and US NIOSH (1978).
LD50 values for rats and mice after oral, iv, and ip expo-
sure were not significantly dependent on the route of exposure
and ranged from 55 to 64 mg/kg body weight for rats and from 57
to 82 mg/kg body weight for mice (free base or hydrazine
hydrate) (Witkin, 1956; O'Brien, 1964; Yaksctat, 1969; Azar et
al., 1970). Oral LD50 values for hydrazine (hydrazine hydrate)
in guinea-pigs and rabbits were 26 and 35 mg/kg body weight,
respectively (Yaksctat, 1969). Dogs and rabbits appeared more
sensitive, LD50 values following iv injection being 25 and 26
mg/kg body weight, respectively. The dermal LD50 for the rabbit
was 93 mg hydrazine (free base)/kg body weight (Rothberg & Cope,
1956; Witkin, 1956). When doses between 96 and 481 mg/kg body
weight (free base) were applied to the skin of dogs, 10 out of
25 animals died within the 6-h observation period; a dose-effect
relationship was not observed (Smith & Clark, 1972). When rats
and mice inhaled hydrazine (free base) for 4 h, the LC50s were
750 and 330 mg/m3, respectively (Jacobson et al., 1955). Death
occurred quickly in both species. Lethal doses of hydrazine
usually induced convulsions, excitement or inactivity, and other
effects on the central nervous system. Rats and mice inhaling
lethal concentrations of hydrazine (free base) also showed
dyspnoea (Comstock et al., 1954; Jacobson et al., 1955; O'Brien,
1964). Spontaneous motor activity depression was noted in rats
at ip doses of 39 and 52 mg/kg body weight (hydrazine sulfate)
(Pradhan & Ziecheck, 1971). Dogs receiving a sublethal iv dose
(free base) did not exhibit convulsions but showed increased
neuromuscular activity, salivation, diarrhoea, vomiting, and
hyperventilation (Wong, 1966).
Few pathological changes have been reported following acute
lethal doses. Some rats that died after inhaling hydrazine (free
base), showed lung oedema with localized damage to the bronchial
mucosa (Comstock et al., 1954). Wells (1908) observed fatty
changes in the liver in 24 h following sublethal doses in many
species. Fatty changes have also been observed in the kidneys of
rats (free base or hydrazine hydrate) (Dominguez et al., 1962;
Scales & Timbrell, 1982). In rats, accumulation of lipid,
swelling of mitochondria, and an increased number of microbodies
were observed in the liver and in the proximal tubules of the
kidneys, 24 h after an ip dose of 20 or 30 mg/kg body weight
(hydrazine hydrate). Similar changes were observed within 1 h,
after doses of 40 or 60 mg/kg body weight (Scales & Timbrell,
1982). In addition, nuclear and nucleolar enlargement and
hypertrophy of the smooth endoplasmic reticulum were observed in
the liver of rats, 2 or more hours after a single
intraperitoneal dose of 64 mg/kg body weight (hydrazine sulfate)
(Ganote & Rosenthal, 1968). Studies on the mechanisms by which
hydrazine causes these effects will be discussed later (section
8.3) together with other effects on the intermediary metabolism,
notably hypoglycaemia and lipid peroxidation, and effects on the
central nervous system, such as an increase in gamma-
aminobutyrate levels in the brain.
In dogs given a sublethal dose of hydrazine (free base),
degeneration of the proximal convoluted tubules of the kidneys
was accompanied by decreased creatinine clearance and increased
glucose reabsorption by the tubules. The glomerular filtration
rate was decreased because of decreased renal blood flow (Van
Stee, 1965; Wong, 1966).
In rhesus monkeys treated intravenously with 2.5 - 9.8 mg
hydrazine (hydrazine sulfate)/kg, liver function tests were
generally within normal limits up to 72 h after dosing. A dose
of 80 mg/kg caused fatty liver, but no necrosis (Warren et al.,
1984).
8.2. Short-Term Exposures
8.2.1. Inhalation exposure
In a 6-month inhalation study, groups of 50 male Sprague
Dawley rats, 40 female ICR mice, 8 male Beagle dogs, and 4
female rhesus monkeys were exposed to 0.26 or 1.3 mg hydrazine
(free base)/m3 air, continuously, or 1.3 or 6.5 mg hydrazine/m3
air, for 6 h/day, 5 days/week. Exposure concentrations were
monitored. Control groups contained the same number of animals.
The exposure regimen was chosen in such a way that the weekly
doses received by the continuously exposed groups were approxi-
mately equal to the weekly doses of hydrazine received by the
intermittently exposed groups. An increased mortality rate was
only seen in mice at the 2 higher exposure levels. In rats,
there was a dose-related decrease in body weight gain, while
body weights of dogs were decreased at the 2 higher exposure
levels. In dogs, the reduced weights were at least partly due to
reduced food consumption. Weights of the exposed monkeys were
comparable with those of controls. Organ weights were unaffected
by the exposure in rats, dogs, and monkeys. Organ and body
weights of mice were not recorded. Central nervous system
effects observed included lethargy in mice at the 2 higher
exposure levels, and tonic convulsions in 1 dog exposed
continuously to 1.3 mg/m3. Monkeys exhibited slight eye irrita-
tion at the 2 higher exposure levels. Fatty changes of the liver
were observed in mice at all exposures and in dogs at the 2
higher exposure levels. The livers of exposed monkeys showed
slight-to-moderate fat accumulation. However, this was also
seen, to some extent, in control animals. Livers of rats were
normal. Finally, dogs exhibited reduced red blood cell counts,
haematocrit, and haemoglobin values at the 2 higher exposure
levels, together with an increased resistance to osmotic haemo-
lysis at all exposure levels. Haematological variables were
normal in rats and monkeys and were not measured in mice. In
dogs, the effects on the liver and the haematological variables
appeared reversible (Haun & Kinkead, 1973).
Decreases in red blood cell count and haematocrit were also
observed in 20 female Swiss mice exposed to 130 mg hydrazine
(free base)/m3 air for 1 h/day, 6 days/week, for 4 weeks. In
this study, a decreased osmotic resistance to haemolysis was
noted in exposed mice (Cier et al., 1967).
Groups of 10 - 30 male Wistar rats were exposed to hydrazine
(free base) at average concentrations of 6, 18, 26, 70, or 295
mg/m3 air, for 5 days/week, 6 h/day, over periods ranging from 5
to 40 days at the 3 highest exposure levels to approximately 6
months at the 2 lowest exposure levels. The control group
consisted of 10 rats. Increased mortality was observed at all
exposure levels but not in controls, and body weights were
decreased at the 3 highest exposure levels. Rats became sluggish
during the 6-month exposure, while at the 3 highest exposure
levels, an initial restlessness was followed by a tendency to
sleep. In some cases, pathological examination revealed lung
oedema with local damage to the bronchial mucosa at the 3
highest exposure levels. Fatty livers were observed in many rats
after 5 days of exposure at 295 mg/m3 (Comstock et al., 1954).
8.2.2. Other routes of exposure
Groups of 25 male Sprague Dawley rats were dosed ip with 10
or 20 mg hydrazine (free base)/kg body weight, 5 times per week,
for 5 weeks. The control group consisted of 15 rats. Mortality
was increased at the dose of 20 mg/kg body weight; 10/25 rats
died after 8 - 21 doses. Body weight was lost in a dose-related
manner; 4.4 and 25.7% of the initial body weight was lost in 10
days in the 10 and 25 mg/kg groups, respectively. At 20 mg/kg
body weight, rats also displayed weakness and lethargy, and 2
rats exhibited convulsions. Pathological examination revealed
hyperaemia and oedema in the lungs of 4 rats and slight fatty
vacuolation in the liver of 7 rats at 20 mg/kg body weight. At
both doses, the haematocrit values were maximally decreased
after 13 injections (Patrick & Back, 1965).
Patrick & Back (1965) treated 12 rhesus monkeys ip with
hydrazine (free base), 5 times per week. Six monkeys received 5
mg/kg body weight for 4 weeks; two of these monkeys received a
further 8 doses of 10 mg/kg body weight, followed by 4 or 5
doses of 20 mg/kg body weight. A group of 6 monkeys received
only 4 or 5 doses of 20 mg/kg body weight. The control group
consisted of 10 monkeys. No monkey died, but all exposed monkeys
showed decreased body weights. Lethargy, weakness, and vomiting
were see in 7 of the 8 monkeys exposed to 20 mg/kg body weight,
while tremors were seen in 1 of these monkeys. Fatty changes
were observed in the liver, proximal tubules of the kidneys,
heart, and skeletal muscles at 20 mg/kg body weight, and
occasionally at 5 mg/kg body weight. Extensive periportal
necrosis was found in the liver of one of the dosed monkeys. The
level of bilirubin was increased and the serum was icteric.
Haematocrit and haemoglobin values, measured at the lower dose
only, dropped slightly, relative to control values (Patrick &
Back, 1965).
The pathological effects on the liver were also investigated
microscopically, in groups of 20 - 29 male DDY mice and 10 male
Wistar rats, after administration of 5, 10, or 20 mg hydrazine
(free base)/kg powdered diet, for 3 - 10 days. No animals died.
Animals of both species exhibited weakness. Megamitochondria or
fatty vacuolation with moderately swollen mitochondria and focal
proliferation of the smooth endoplasmic reticulum were induced
in rats and mice at dose levels of 10 and 20 mg/kg feed. The
induction of megamitochondria was a reversible process (Waka-
bayashi et al., 1983). Noda et al. (1983) observed centrilobular
hepatic necrosis in male rabbits dosed for 5 days with between
14.6 and 32.3 mg hydrazine (hydrazine monohydro-chloride)/kg
body weight per day, iv.
In other studies, hydrazine (hydrazine hydrate) was given
orally in drinking-water to albino rats and guinea-pigs for 7
months at levels providing 0.3, 0.03, 0.003, and 0.0003 mg/kg
body weight per day. At the two highest doses, adverse effects
were observed in the CNS (changes in conditioned reflexes),
liver (increased I131 excretion, changes in enzyme activity,
protein dystrophia), and blood (symptoms of haemoloytic
anaemia). The dose of 0.003 mg/kg body weight was reported to be
the no-observed-adverse-effect level (Yaksctat, 1969).
8.3. Biochemical Effects and Mechanisms of Toxicity
All of the studies described in this section were performed
with doses considered to be toxic.
8.3.1. Effects on lipid metabolism
Hydrazine caused a dose-dependent increase in hepatic
triglyceride levels in rats, the threshold dose for a single ip
injection being 10 - 20 mg/kg body weight. The maximal effect,
an increase of 7 times the control value, was observed after a
dose of 40 or 60 mg hydrazine hydrate/kg body weight. At these
dose levels, the effect was measurable 4 h after injection
(Timbrell et al., 1982). Other authors have also reported
accumulation of triglycerides in the liver of rats exposed to
single doses of hydrazine via injection routes (Amenta &
Dominguez, 1965a; Clark et al., 1970; Lamb & Banks, 1979).
Several mechanisms have been proposed:
1. Increased mobilization of free fatty acids from
adipose tissue (particularly observed at low plasma-
glucose levels) leading to an increased uptake of free
fatty acids, followed by increased triglyceride
synthesis in the liver (Trout, 1965, 1966; Clark et
al., 1970). This mobilization of free fatty acids
might be caused by the effects of hydrazine on the
sympathetic nervous system and on levels of adrenal
steroid hormone, possibly in response to the hypo-
glycaemia induced by hydrazine (Amenta & Dominguez,
1965a). Cooling et al. (1979) found elevated
concentrations of circulating corticosterone and
decreased concentrations of insulin in the serum of
rats exposed to hydrazine. Decreased blood-insulin
levels were also measured in rats by Aleyassine & Lee
(1971).
2. Increased synthesis of triglycerides caused by
increased enzymatic activity of phosphatidate phospho-
hydrolase (EC 3.1.3.4) in hepatocytes both in vivo and
in vitro was reported by Lamb & Banks (1979). It has
been suggested that this was a result of increased
corticosterone levels (Cooling et al., 1979). In
addition, Marshall et al. (1983) found increased fatty
acid synthesis in the liver of rats after hydrazine
administration.
3. Triglycerides could accumulate in hepatocytes as a
result of a decreased secretion of lipoproteins from
liver to plasma (Amenta & Dominguez, 1965a; Clark et
al., 1970). This could be explained by a decreased
lipid-binding capacity of lipoproteins following an
observed alteration in the proportion of phospholipids
and cholesterol (Clark et al., 1970) or by
increased lipid peroxidation (Di Luzio et al., 1973;
Kopylova et al., 1982). The protein moiety of
lipoproteins could also be subject to change (section
8.3.2).
8.3.2. Effects on carbohydrate and protein metabolism
Rats and dogs with starvation-induced depletion of glycogen
stores showed rapidly declining plasma-glucose levels with a
concomitant rise in lactate and pyruvate levels after exposure
to single intravenous hydrazine doses of 64 (free base or hydra-
zine sulfate) and 25 mg (free base)/kg body weight,
respectively. In well-fed dogs, hyperglycaemia and depletion of
glycogen stores preceded hypoglycaemia. Acidosis developed
slowly as a result of an increased lactate-pyruvate ratio
(Fortney, 1966; Fortney et al., 1967; Ray et al., 1970).
It has been postulated that hydrazine inhibits glyconeo-
genesis (Fortney, 1966; Fortney et al., 1967). This could occur
via inhibition of pyridoxal phosphate-dependent aminotrans-
ferases and decarboxylases. It has been shown that hydrazine
interferes with pyridoxal phosphate synthesis in vitro
(McCormick & Snell, 1961) and in vivo (Chatterjee & Sengupta,
1980) (section 8.3.5). Inhibition of transaminases would also
explain the increase in free amino acids observed in the plasma,
liver, brain, and muscle of rats (Cornish & Wilson, 1968; Banks,
1970) and in the plasma and urine of dogs (Korty & Coe, 1968).
It could further explain several observations in rats, such as
the depressed conversion of amino acids to carbon dioxide
(Amenta & Dominguez, 1965b; Dost et al., 1971), the depressed
incorporation of amino acids in plasma-glucose (Fortney et al.,
1967), and the enhanced incorporation of amino-labelled acids in
liver proteins, 24 h following hydrazine exposure (Banks, 1970).
Inhibition of protein synthesis was also observed in rat livers
up to 8.5 h after exposure (Lopez-Mendoza & Villa-Trevino,
1971).
Hydrazine treatment of rats resulted in inhibited activity
of specific aminotransferases and decarboxylases including:
liver aspartate aminotransferase (EC 2.6.1.1) (Stein et al.,
1971), brain gamma-aminobutyrate aminotransferase (EC 2.6.1.19)
and glutamate decarboxylase (EC 4.1.1.15) (Medina, 1963; Perry
et al., 1981), and liver ornithine 2-oxo-acid aminotransferase
(EC 2.6.1.13) (Roberge et al., 1971). The activity of rat liver
ornithine decarboxylase (EC 4.1.1.17) increased following
hydrazine exposure (Springer et al., 1980).
Inhibition of phosphoenolpyruvate carboxykinase (ATP) (EC
4.1.1.49), an enzyme involved in gluconeogenesis, was also
measured in vitro . Hydrazine increased the levels of citrate,
malate, and oxaloacetate in the rat liver (Ray et al., 1970).
Hydrazine affects the urea cycle. Decreased specific
activity of ornithine 2-oxo-acid aminotransferase, caused by
administration of hydrazine to rats (Roberge et al., 1971),
provoked an increase in ornithine in the liver (Banks, 1970),
brain, and plasma (Perry et al., 1981). The concentrations of
citrulline and urea in the liver, kidneys, brain, and blood were
increased, as was the activity of argininosuccinate lyase (EC
4.3.2.1) (Roberge et al., 1971).
8.3.3. Effects on mitochondrial oxidation
Swelling of hepatic mitochondria was observed in rats and
mice following hydrazine administration (Ganote & Rosenthal,
1968; Scales & Timbrell, 1982; Wakabayashi et al., 1983)
(sections 8.1 and 8.2.2). in vitro studies on the effects of
high concentrations of hydrazine on the functional status of
mitochondria showed decreased oxidation of keto-acids (Von
Krulik, 1966). Oxidative phosphorylation measured as the P/O
ratio was either not affected or decreased independently of the
hydrazine concentration (Von Krulik, 1966; Fortney et al.,
1967). Inhibition of beef heart cytochrome a by hydrazine was
also reported (Takemori et al., 1960). After administration of a
single intraperitoneal dose of hydrazine to rats, slightly
stimulated mitochondrial oxidation of succinate and glutamate
was observed with a slight increase in P/O ratio, respiration
control rate, and phosphorylation rate. ATPase (EC 3.6.1.8)
activity was not affected (Higgins & Banks, 1971). When mice
received hydrazine (free base) in the diet (10%) for 3 days and
rats received hydrazine in the diet (20%) for 8 days, oxidation
of succinate and glutamate, coupling efficiency, P/O ratio, and
the activities of ATPase (EC 3.6.1.8) and cytochrome c oxidase
(EC 1.9.3.1) were slightly decreased in liver megamitochondria,
while the activity of monoamine oxidase (EC 1.4.3.4) was
moderately decreased (Wakabayashi et al., 1983).
8.3.4. Effects on microsomal oxidation
Proliferation of the smooth endoplasmic reticulum was
observed in the liver of rats and mice following hydrazine
administration (Ganote & Rosenthal, 1968; Wakabayashi et al.,
1983) (sections 8.1 and 8.2.2). A single dose of 55 mg hydrazine
(free base)/kg body weight in rats decreased the hepatic
cytochrome P-450 content (Gorshtein & Kopylova, 1983). Rats
exposed for 4 days to 12 mg hydrazine (hydrazine sulfate)/kg
body weight per day did not show any effect on cytochrome P-450
levels in the microsomal fraction of the liver, but a slightly
decreased level of cytochrome b 5, inhibition of benzopyrene
hydroxylase (EC 1.14.14.1), and increased parahydroxylation of
aniline (Akin & Norred, 1978).
8.3.5. Effects on the central nervous system
The relationship between the effects of hydrazine on the
central nervous system, especially the occurrence of convul-
sions, and changes in levels of gamma-aminobutyric acid, an
inhibitory neurotransmitter, in the brain of rats and mice has
been investigated. An increase in the level of gamma-amino-
butyric acid was observed in the whole brain of rats after: (a)
a single intraperitoneal dose of 51 mg hydrazine (free base)/kg
body weight (Medina, 1963); (b) a single intravenous dose of 5
mg hydrazine (hydrazine sulfate)/kg body weight (Matsuyama et
al., 1983); or (c) after a daily subcutaneous dose of 2.6 mg
hydrazine (hydrazine monohydrochloride)/kg body weight over 109
days (Perry et al., 1981). In the whole brain of mice, an
increase was observed following a single intramuscular dose of
54 mg hydrazine (free base)/kg body weight (Wood et al., 1980).
The changes in the concentration of this amino acid are caused
by inhibition of pyridoxal phosphate requiring gamma-
aminobutyrate aminotransferase (EC 2.6.1.19) and glutamate
decarboxylase (EC 4.1.1.15) (Medina, 1963; Perry et al., 1981).
Hydrazine treatment of rats also caused a general amino acid
imbalance in the brain (Perry et al., 1981). A relationship was
suggested between the excitable state of the brain and the
gamma-aminobutyric acid contents of nerve endings (decreased)
rather than the whole brain contents of gamma-aminobutyric acid
(increased) (Wood et al., 1980; Geddes & Wood, 1984).
8.4. Reproduction, Embryotoxicity, and Teratogenicity
When groups of 26 Wistar rats were exposed to 0 or 8 mg
hydrazine (hydrazine monohydrochloride)/kg body weight per day,
sc, from day 11 to day 20 of gestation, the exposed dams showed
a 20% decrease in body weight and 2 dams died. Reduced number of
viable fetuses were found in 9 dams killed on day 21 of
gestation (63/172 versus 142/179 in controls), while the number
of implants per litter was not affected. The fetuses had reduced
weights and appeared pale and oedematous, but did not exhibit
any major malformations. In the rats allowed to deliver (12),
perinatal mortality was 100% in treated rats and 20% in controls
(Lee & Aleyassine, 1970).
These results agree with those of another study in which
groups of 6 - 27 Fisher 344 rats received 0, 2.5, 5, or 10 mg
hydrazine (free base)/kg body weight per day, ip, from day 6 to
day 15 of gestation. Body weight gains in dams were decreased
and the number of resorptions per dam were increased in a dose-
related manner. The differences between treated and control
animals were statistically significant at doses of 5 or 10
mg/kg, but not at 2.5 mg/kg for both variables. The numbers of
implants per dam and fetal weights were not affected at any
dose. The incidence of litters or fetuses with abnormalities was
not significantly increased at any dose; however, at 10 mg/kg,
only one out of the 6 females produced a viable litter, and only
6 fetuses were examined. In a subsequent study, 27 rats were
untreated and 11 rats were treated with 10 mg hydrazine/kg per
day, during what appeared to be the most susceptible period of
gestation (days 7 - 9). The incidence of litters or fetuses with
abnormalities in the 10 mg/kg group (6 litters out of 8; 8
fetuses out of 16) was significantly higher than that in the
control group of the preceeding study (8 litters out of 27; 11
fetuses out of 181). The abnormalities observed were mainly
supernumerary and fused ribs, delayed ossification, moderate
hydronephrosis, and moderate dilation of brain ventricles
(Keller et al., 1982).
Subtle postnatal changes were reported in the offspring of
24 female Syrian golden hamsters exposed orally to 0 or 170 mg
hydrazine (hydrazine hydrate)/kg body weight on the 12th day of
gestation. The pups of exposed dams did not exhibit cleft palate
formation, but showed effects on the development of intestinal
brush border enzymes. No other end-points were investigated
(Schiller et al., 1979).
Groups of ICR mice were treated ip with 0, 4, 12, 20, 30, or
40 mg hydrazine (free base)/kg body weight per day, from day 6
to day 9 of gestation. Some dams administered the highest dose
died. Body weights were reduced at doses of 12 mg/kg body weight
or more. While at the lower doses the number of resorptions per
litter was unchanged compared with controls, embryotoxicity was
evident at 30 and 40 mg/kg body weight. At 12 and 20 mg/kg body
weight, 17-day-old fetuses showed reduced weights, and there was
a dose-related increased incidence of litters with abnor-
malities, mainly exencephaly, hydronephrosis, and supernumerary
ribs (Lyng et al., 1980).
Savchenkov & Samoilova (1984) studied the adverse effects on
reproductive function (fertility of females, numbers of newborn,
and resorption of embryos) of female and male albino rats
exposed by gavage to hydrazine (hydrazine nitrate) at a dose of
13 mg/kg body weight, once a day, for 30 days prior to mating.
The development of the surviving litters did not differ from
that of controls.
Albino rats (10/group and 20 controls) of both sexes were
exposed to hydrazine (free base) (99.5% purity) in the drinking-
water at concentrations of 0.82, 0.018, or 0.002 mg/litre (0.016
mg/kg, 0.0014 mg/kg, or 0.00016 mg/kg, respectively, nominal
dose, assuming water consumption 20 ml per day and animal
weights of 250 g). The duration of the study was 6 months. The
number of animals studied and the scheme and time of mating were
not reported. The female rats exposed to the highest concen-
trations had fewer live embryos and more resorptions, as well as
pre- and post-implantation deaths, than the controls. No effects
were observed in animals administered 0.002 mg/litre.
Developmental abnormalities were not reported in any of the 293
embryos from all exposed animals. Destruction of gonadal
epithelium was observed in male rats after 6 months of oral
exposure to hydrazine at concentrations of 0.82 and 0.018
mg/litre (Duamin et al., 1984). In the same study, albino rats
were exposed to hydrazine (free base) at concentrations of 0.85,
0.13, or 0.01 mg/m3 (0.10 mg/kg body weight, 0.016 mg/kg, or
0.0012 mg/kg, respectively, nominal dose, assuming inhalation of
6 litres air per h and animal weights of 200 g), for 5 h per
day, 5 days/week, for 4 months. At the two highest concentra-
tions, embryotoxic effects of the same severity were seen as
when hydrazine was administered orally at the two highest doses.
No abnormalities were observed among 315 embryos. No gonado-
toxic effects occurred in male rats under the conditions of the
study (Duamin et al., 1984).
Additional information relating to reproductive end-points
is presented in section 8.5.1 (Sotomayer et al., 1982).
8.5. Mutagenicity and Related End-Points
8.5.1. DNA damage
Hydrazine was found to react with pyrimidine bases under
non-physiological conditions. These reactions were reviewed by
Kimball (1977). No hydrazine-DNA adducts have been reported to
be formed in vivo. A single oral or intraperitoneal dose of
hydrazine (free base or hydrazine sulfate) administered to rats,
mice, guinea-pigs, and hamsters resulted in the rapid formation
of N7-methylguanine and O6-methylguanine in liver DNA, which was
not detected in controls (Becker et al., 1981; Quintero-Ruiz et
al., 1981; Bosan & Shank, 1983; Shank, 1983). Methylation was
detectable at toxic doses and a sharp increase in the methyl-
ation of guanine in liver-DNA was observed at oral doses
exceeding 60 mg/kg body weight in rats and 45 mg/kg body weight
in hamsters. In both species, maximum methylation approximated
80 N7-methylations and 7 O6-methylations per 100 000 residues of
guanine, 6 h after oral exposure to 90 mg hydrazine/kg body
weight. Elimination of 7-methylguanine from DNA began about 24 h
after exposure with a half-life of 40 - 50 h. Elimination of O6-
methylguanine from DNA began about 24 h after exposure in rats
and about 50 h after exposure in hamsters, with a half-life of
13 h and 17 h, respectively (Becker et al., 1981; Bosan & Shank,
1983). The source of the methyl group was S -adenosyl-methionine
(Becker et al., 1981; Quintero-Ruiz et al., 1981).
Single-strand breaks were detected by the alkaline elution
assay in rat liver cells exposed in vitro (Sina et al., 1983),
and in the liver and lung cells of mice injected ip with 50 or
100 mg hydrazine (hydrazine hydrate)/kg body weight (Parodi et
al., 1981).
Hydrazine was reported to induce lambda phage in Escherichia
coli (Heinemann, 1971); however, no such induction was found by
Thomson (1981), nor did hydrazine induce HPlcl phage in Haemo-
philus influenzae (Balganesh & Setlow, 1984).
Repair of DNA lesions induced by hydrazine was observed in
vitro. In the International Collaborative Programme for the
Evaluation of Short-Term Tests for Carcinogenicity, 5 bacterial
DNA repair tests, using Bacillus subtilis or E. coli, were all
found to produce weak to medium positive responses. Only one
assay required rat liver microsomal fraction. The other positive
responses were reduced in magnitude by microsomal fraction
(Ashby & Kilbey, 1981). Increased unscheduled DNA synthesis was
observed in 1 out of 2 tests using human fibro-blasts (Agrelo &
Amos, 1981; Robinson & Mitchell, 1981).
No increase in unscheduled DNA synthesis was observed in the
germ cells of mice, 16 days after a 5-day exposure to doses of
hydrazine (hydrazine dihydrochloride) of up to 120 mg/kg body
weight per day (Sotomayer et al., 1982).
8.5.2. Mutation and chromosomal effects
The results of tests for gene mutations and chromosome
damage induced by hydrazine or its salts are summarized in Table
6. Hydrazine induces gene mutations and/or chromosome
aberrations in a variety of test systems including plants,
phage phi 80, bacteria, fungi, Drosophila melanogaster, and
mammalian cells in vitro . In a few cases, microsomal activation
was an absolute requirement for a positive effect (Gupta &
Goldstein, 1981; Perry & Thomson, 1981). In most cases, an
effect could be observed, both with and without microsomal
activation, with a stronger effect in some tests with activation
and in other tests without activation. These inconsistencies
were evaluated by Ashby (1981). Some negative results in the
forward mutation tests with mammalian cells invitro can be
traced back to the high locus specificity of hydrazine also
observed in plants. Duamin et al. (1984) observed increased
chromosomal aberrations in bone marrow cells (4.12 ± 0.65%
versus 2.48 ± 0.46% in controls) of albino rats (N = 10) exposed
to hydrazine (free base) at 0.85 mg/m3, 5 h per day, 5
days/week, for 4 months. No increased incidence of nuclear
aberrations, micronuclei, dominant lethals, and sperm-head
abnormalities were observed in hydrazine-treated mice (free base
or hydrazine sulfate).
Table 6. Tests for gene mutations and chromosome damage induced by hydrazine or its salts
---------------------------------------------------------------------------------------------------------
Test description System description Result Reference
Organism Species/strain/cell type
---------------------------------------------------------------------------------------------------------
Forward mutations transforming Bacillus subtilis + Freese et al. (1967)a
G DNA + Bresler et al. (1968)a
E Forward mutations plant tomato + Jain et al. (1968)b
+ Chandra Sekhar & Reddy
(1971)b
N rice + Reddy & Reddy (1972)b
barley + Kak & Kaul (1975)b
E wheat + Khamankar & Jain (1978)b
broad bean + Vishnoi & Gupta (1980)b
Reverse mutations virus phage phi 80 + Chu et al. (1973)c
M Reverse mutations bacteria Salmonella typhimurium TA 1530 + Rosenkranz & Poirier
(1979);Tosk et al.
(1979)
U
Salmonella typhimurium TA 1535 + Purchase et al. (1978);
Herbold (1978);
T Rosenkranz & Poirier
(1979); Bridges et al.
(1981);d,e;
A Parodi et al. (1981);
Rogan et al. (1982);
T Braun et al. (1984);
De Flora et al. (1984)
I Salmonella typhimurium TA 1537 - Bridges et al. (1981)d,e;
Parodi et al. (1981);
O Rogan et al. (1982)
N Salmonella typhimurium TA 1538 + Bridges et al. (1981)d,e
Purchase et al. (1978);
S Herbold (1978);
Rosenkranz & Poirier
(1979); Parodi et al.
(1981)
Salmonella typhimurium TA 100 + Purchase et al. (1978);
Herbold (1978); Bridges
et al. (1981)d,e
---------------------------------------------------------------------------------------------------------
Table 6. (contd.)
---------------------------------------------------------------------------------------------------------
Test description System description Result Reference
Organism Species/strain/cell type
---------------------------------------------------------------------------------------------------------
Reverse mutations bacteria Salmonella typhimurium TA 100 - Bridges et al. (1981)d,e;
G Parodi et al. (1981)
E Salmonella typhimurium TA 98 -, + Herbold (1978); Bridges
et al. (1981)d,e; Parodi
et al. (1981)
N + Purchase et al. (1978)
E Salmonella typhimurium G 46 + Braun et al. (1984)
Reverse mutations bacteria Salmonella typhimurium G 46 + Röhrborn et al. (1972)
(host-mediated (mouse) (NMRI)
assay)
M Reverse mutations bacteria Escherichia coli + Von Wright & Tikkanen
(1980); Bridges et al.
(1981)d,e
U
Haemophilus influenzae + Kimball & Hirsch (1975);
T Kimball (1976)
A Reverse mutations fungi Saccharomyces cerevisiae + Mehta & Von Borstel
(1981)e; Vasudeva &
Vashishat (1985)
T Forward mutations fungi Saccharomyces cerevisiae + Lemontt (1977, 1978)
Saccharomyces pombe + Loprieno (1981)e
I
Sex-linked visibles insect Drosophila melanogaster + Jain & Shukla (1972);
O Shukla (1972);
Vijaykumar & Jain (1979)
N Sex-linked lethals insect Drosophila melanogaster + Shukla (1972)
S Forward mutations hamster ovary cells in vitro + Gupta & Goldstein (1981)e
- Carver et al. (1981)e;
Hsie et al. (1981)e
mouse lymphoma cells in vitro + Rogers & Back (1981)
+ Amacher et al. (1980)
---------------------------------------------------------------------------------------------------------
Table 6. (contd.)
---------------------------------------------------------------------------------------------------------
Test description System description Result Reference
Organism Species/strain/cell type
---------------------------------------------------------------------------------------------------------
C Breaks plant horse bean primary root + Gupta & Grover (1970)
H Breaks, deletions, plant horse bean primary root + Heindorff et al. (1984)
translocations
R
Aberrationsf plant chick pea root + Farook & Nizam (1979)
O
Aberrations rat epithelial liver cells - Dean (1981)e
M in vitro
O Aberrations rat bone marrow cells in vivo + Duamin et al. (1984)
S Breaks, gaps, hamster ovary cells in vitro + Natajaran & Van Kesteren-
exchanges Van Leeuwen (1981)e
O
Sister chromatid hamster ovary cells in vitro + MacRae & Stich (1979);
Perry & Thomson (1981)e
M exchanges
- Natarajan & Van Kesteren-
Van Leeuwen (1981)e; Baker
et al. (1983)
E
lung cells in vitro + Baker et al. (1983)
V-79 cells in vitro + Speit et al. (1980)
Nuclear aberrations mouse epithelial colon cell - Wargovich et al. (1983)
(oral exposure)g
---------------------------------------------------------------------------------------------------------
Table 6. (contd.)
---------------------------------------------------------------------------------------------------------
Test description System description Result Reference
Organism Species/strain/cell type
---------------------------------------------------------------------------------------------------------
D Micronuclei (ip mouse polychromatic erythrocytes + Salomone et al. (1981)e
exposure) - Kirkhart (1981)e;
A Tsuchimoto & Matter
(1981)e
M Dominant lethals mouse germ cells - Epstein et al. (1972)
(ip exposure)
A
G
E
------------------------------------------------------------------------------------------------------------------------
a Inactivation of transforming DNA was observed, which was inhibited by catalase, EDTA, or nitrogen gas treatment.
b Hydrazine appears to be a mutagen that is highly specific for certain loci. Moreover, it not only produced mutants
in the M2 generation but also homozygous recessive mutants in the M1 generation (plants, raised from treated seeds).
c Inactivation of virus observed. Inactivation and, at lower concentrations, mutations were reduced by catalase
treatment.
d Hydrazine sulfate was tested in the International Collaborative Programme for the Evaluation of Short-Term Tests for
Carcinogenicity (de Serres & Ashby, 1981). In the summary on the assay performance of bacterial mutation assays, it
was reported that hydrazine sulfate was mutagenic in most of a total of 20 laboratories. However, there were
inconsistencies in the strains in which the effect was seen and the requirements for S9 mix. Salmonella typhimurium
TA 1535 was the strain in which mutagenic activity was most commonly observed, but it was also seen in all
laboratories that used Escherichia coli strains. In some laboratories, hydrazine sulfate was also mutagenic in
Salmonella typhimurium TA 100. Two laboratories reported a marginal mutagenic activity for strain TA 98, and TA 1538
was positive in another, while no mutagenic activity was observed with TA 1537.
e Tested in the International Collaborative Programme for the Evaluation of Short-Term Tests for Carcinogenicity. In
the final assessment, it was stated that hydrazine was a genotoxic agent in bacteria, yeast, and higher eukaryotes
in vitro . Hydrazine did not appear to be genotoxic in vivo in higher eukaryotic cells (de Serres & Ashby, 1981).
f Stickiness and clumping at metaphase, bridges and fragments at anaphase, laggards, micronuclei, and delayed
cytokinesis at telophase, tripolar spindles, prophase, and metaphase in one cell.
g Micronuclei, pyknotic nuclei, karyorrhetic nuclei, cytolysosomes.
8.5.3. Cell transformation
Hydrazine increased the transformation of baby hamster
kidney cells (Purchase et al., 1978; Daniel & Dehnel, 1981) and
human liver cells (Purchase et al., 1978). While Purchase et al.
(1978) observed an increased transformation of baby hamster
kidney cells, both with and without metabolic activation, Daniel
& Dehnel (1981) found a much lower increased transformation
frequency only with metabolic activation. Hydrazine also caused
enhancement of transformation of mouse 3T3 cells by Herpes
simplex virus in vitro (Johnson, 1982).
Hydrazine induced transformation of human fibroblasts. Transformed
cells were able to induce undifferentiated mesenchymal tumours
after sc injections in pre-irradiated nude mice, while control
cells did not induce tumours (Milo et al., 1981).
8.6. Carcinogenicity
8.6.1. Inhalation exposure
Groups of 100 Fischer 344 rats of each sex, 400 female
C57BL/6 mice, and 200 male golden Syrian hamsters were exposed
to the vapour of hydrazine (free base) for 12 months, 6 h per
day, for 5 days per week, and observed for a further 18, 15, and
12 months, respectively (Table 7). Rats were exposed at 0.06,
0.33, 1.3, or 6.5 mg/m3, mice at 0.06, 0.33, or 1.3 mg/m3, and
hamsters at 0.33, 1.3, or 6.5 mg/m3 air. Control groups
contained 150 rats of each sex, 800 female mice, and 200 male
hamsters, respectively. The hydrazine vapour was monitored
throughout the exposure period. A reduction in body weight gain,
seen in exposed rats throughout the entire study, was greatest
in males at 6.5 mg/m3. The mortality rate was not affected by
the exposures. Inflammatory changes were observed in the upper
respiratory tract of both sexes and in the uterus and oviduct,
especially at the highest exposure. At 6.5 mg/m3, the incidence
of squamous metaplasia was increased in the nose, larynx, and
trachea, while the incidence of epithelial hyperplasia was
increased in the nose and lungs. An increased incidence of
hyperplasia was also observed in the lymph nodes and uterus of
females at 6.5 mg/m3 and in the liver of females at 1.3 and 6.5
mg/m3. A dose-related increased incidence of benign epithelial
tumours of the nose, mainly adenomatous polyps, was observed in
both sexes at the 2 highest exposures, while at the highest
exposure (6.5 mg/m3), the incidence of malignant epithelial
tumours was also increased (2/98 in males and 5/95 in females).
The latency time exceeded 87 weeks in males and 97 weeks in
females. The incidence of lung tumours was not significantly
increased, but, in males, 3 bronchial adenomas and, in one
female, 1 bronchial adenoma, were observed at 6.5 mg/m3 compared
with none in the controls. The total incidence of adenomas and
adenocarcinomas of the thyroid in males was not increased, but
hydrazine exposure at 6.5 mg/m3 increased the fraction of
malignant tumours.
Table 7. Tumour incidence in mice, rats, and hamsters following hydrazine exposure
---------------------------------------------------------------------------------------------------------
Route Species/ Exposure Daily dose Tumour incidenceb Reference
strain period (mg/kg Nose or lung liver
(months) body weight)a Male Female Male Female
(life-time
observation)
---------------------------------------------------------------------------------------------------------
Inhalation mouse 12 0 8/385 - Vernot et al. (1985)
(C57BL/6) 4/378
0.34c 12/379 -
rat 12 0 0/146 0/145 - - Vernot et al. (1985)
(Fischer 0.16c 11/97 4/94 - -
344) 0.8d 75/98 38/95 - -
hamster 12 0 1/181 - Vernot et al. (1985)
(golden 0.93d 16/160 -
Syrian)
Oral mouse 6 - 9 0 1/37 4/47 3/30 1/29 Biancifiori (1969,
(gavage) (CBA) 0.98 2/26 10/25 1/26 0/25 1970a)
2.0 4/25 16/25 7/25 2/25
3.9 5/25 21/24 12/25 16/24
8.0 16/21 19/21 15/25 15/24
rat 17 0 0/28 0/22 0/19 0/14 Severi & Biancifiori
(Cb/Se) 14.6/9.7 3/14 5/18 4/13 0/18 (1968)
Oral mouse 24 - 28 0 11/110 14/110 2/110 3/110 Toth (1969, 1972a)e
(drinking- (Swiss) 2.0/1.6 24/50 27/50 2/50 1/50
water) 5.1/4.6 25/50 24/50 3/50 1/50
---------------------------------------------------------------------------------------------------------
a Calculated doses based on a weight of 300 g and a minute volume of 100 ml for rat, a weight of 35 g
and a minute volume of 25 ml for mice, and a weight of 140 g and a minute volume of 54 ml for hamsters.
b - = not found; open space = not tested (nose in inhalation studies with rats and hamsters, lung in
the other studies.
c 1.3 mg/m3, 6 h/day, for 5 days/week.
d 6.5 mg/m3, 6 h/day, for 5 days/week.
e Liver tumours were hepatomas (1/50 in males at both exposure levels) and angiomas.
A slightly increased mortality rate seen in mice right up to
the end of the study was not related to dose. Body weights were
not affected during exposure, but all exposed groups gained less
weight than controls during the post-exposure period. The
incidence of non-neoplastic lesions was similar in treated and
control groups. The only neoplastic change observed was a
slightly increased incidence of lung adenomas at the highest
exposure. A background incidence of 2 - 3% for pulmonary adenoma
in C57BL/6 mice was reported to be common in the laboratory
concerned.
Almost double the number of deaths occurred during exposure
in each exposed group of hamsters compared with the control
group, but the final mortality rates were similar in all groups,
including the control group. During exposure to 6.5 mg/m3 and
for 10 months after exposure, body weights were depressed. For
several months, an unexplained decrease in body weight occurred
in all groups, including the controls. Dose-related degenerative
changes were observed. These were characterized by amyloidosis
in several organs and issues, mineralizaton in kidneys, and
haemosiderosis in the liver. Increased incidences of senile
atrophy and aspermatogenesis were observed in the testes at the
highest exposure level. In addition, the incidence of bile-duct
hyperplasia was increased at all exposures. The incidence of
benign nasal polyps was increased at 6.5 mg/m3. The only other
striking tumours observed were 3 adenocarcinomas, 1 leiomyoma,
and 1 papilloma in the colon, 1 basal cell carcinoma in the
stomach, and 4 parafollicular cell adenomas in the thyroid at
6.5 mg/m3. No such tumours were found in the controls, but the
increased incidence of each tumour type was not statistically
significant. The authors concluded from these studies "that
hydrazine is capable of inducing nasal tumours, primarily
benign, in rats and hamsters after 1-year intermittent exposure"
(Vernot et al., 1985).
8.6.2. Oral exposure
The oral carcinogenicity studies on hydrazine have been
either preliminary or limited in scope (inadequate limited
histopathology, use of one dose or one drinking-water concentra-
tion, small treatment groups, lack of adequate controls, and
treatment of only one sex); therefore, only the more relevant
studies have been described in this section. Most studies have
concentrated on various mouse strains.
Groups of approximately 25 CBA/Cb/Se mice of each sex
received, by gavage, 150 daily doses of hydrazine sulfate in
water, equivalent to 0.03, 0.07, 0.14, or 0.28 mg hydrazine in
25 weeks (these doses are expressed in Table 7 as 0.98, 2.0,
3.9, or 8.0 mg/kg body weight). The animals were observed for
their life-time. Control groups containing 30 males and 29
females were not treated. Liver, lung, various endocrine glands,
and tissues suspected of having lesions were examined
microscopically. No statistical analysis was reported. No deaths
occurred during the exposure period, but later the mortality
rate was increased in animals that had been exposed daily to
levels of 2.0 mg/kg upwards. Brown degeneration of the adrenals
in treated females was mentioned as a marked non-neoplastic
lesion. An apparent dose-related increased incidence of
hepatocarcinomas was observed in male and female mice. The
average latency time for this tumour did not decrease as the
dose increased (Biancifiori, 1970a). The historical control
incidence of hepatocarcinomas in the laboratory concerned was 8%
(Severi & Biancifiori, 1968). Multiple pulmonary tumours, which
were also observed, are described in another report
(Biancifiori, 1969).
In this report (Biancifiori, 1969), the 3 lowest dose
groups, containing 21 - 26 males and 21 - 25 females, were
treated in the same way as in the study above. The data for the
control group and the highest dose groups were taken from
earlier reports (Biancifiori et al., 1964; Severi & Biancifiori,
1968). The untreated control groups contained 37 males and 47
females. The highest dose group comprising 21 mice of each sex,
received 250 daily doses of hydrazine sulfate in water
equivalent to 0.28 mg hydrazine in 36 weeks. In the 1969 report,
the treatment schedule was reported to be 150 daily doses of
0.28 mg hydrazine in 25 weeks, which was contrary to the
original data. Lungs with trachea, liver, various endocrine
glands, and other tissues suspected of having lesions were
examined microscopically. No deaths occurred during the exposure
period, but the mortality rate after the treatment period was
increased from a dose level of 2.0 mg/kg per day upwards. Common
non-neoplastic lesions in the liver at 0.28 mg per day included
areas of diffuse cell regeneration. Necrosis, nodular
regeneration, sclerosis, and bile-duct proliferation were also
observed. The occurrence of hepatomas has already been
discussed. The incidence and multiplicity of pulmonary tumours
were increased at all dose levels in an apparently dose-related
manner and more so in females than in males (Table 7). The
average latency time for these tumours was also shorter in
females than in males. At the highest dose level, approximately
80% of the tumours were characterized as adenomas in both sexes,
the remainder being described as "anaplastic adenomas and
adenocarcinomas" (Biancifiori, 1969).
Induction of lung tumours by hydrazine sulfate after oral
exposure by gavage was also reported in other mouse strains
(Milia et al., 1965; Roe et al., 1967; Kelly et al., 1969;
Biancifiori, 1970b; Bhide et al., 1976; Maru & Bhide, 1982).
Liver tumour induction was reported in BALB/c/Cb/Se mice
(Biancifiori, 1970b).
Severi & Biancifiori (1968) also exposed 14 male and 18
female Cb/Se rats, by gavage, for 68 weeks, to daily doses of
hydrazine sulfate in water, equivalent to 4.4 mg hydrazine (14.6
mg/kg body weight) per day for males and 2.9 mg hydrazine (9.7
mg/kg) per day for females. Controls (28 males and 22 females)
were not treated. The animals were observed for their life-time.
Lungs, liver, and other organs showing gross lesions were
examined microscopically. No statistical analysis was reported.
Exposed rats showed an increased mortality rate. Pulmonary
tumours, adenomas, and adenocarcinomas were observed in both
sexes (Table 7). Carcinomas (2) and spindle cell sarcomas (2),
observed in the livers of exposed males, were not seen in the
controls.
Groups of 23 and 85 golden hamsters of both sexes received
hydrazine sulfate by gavage as 60 and 100 daily doses, equiv-
alent to 0.74 and 0.68 mg hydrazine over 15 and 20 weeks,
respectively. The control group contained 56 hamsters. Animals
were observed for their life-time and examined as described
above for rats. The mortality rate increased in an apparently
dose-related manner, and toxic effects were observed in treated
animals (liver lesions, reticuloendothelial cell proliferation,
cirrhosis, bile-duct proliferation, degenerative fibrous cells
in hyalinized tissue). No tumours were observed, but, because of
toxic effects, the treated animals were not observed for the
same period of time as the controls (Biancifiori, 1970a).
Groups of 50 Swiss mice of each sex received doses of hydra-
zine sulfate in drinking-water, equivalent to 0.056 mg hydrazine
(2 mg/kg body weight, males; 1.6 mg/kg, females) per day for
their life-time up to a maximum of 113 weeks (Toth, 1972a) or
0.18 mg (5.1 mg/kg body weight) per day (males) and 0.16 mg (4.6
mg/kg) per day (females) for a maximum of 95 weeks (Toth, 1969).
The untreated control group, containing 110 animals of each sex,
was tested concurrently with the groups receiving 0.16 or 0.18
mg per day, but not with those receiving 0.05 mg per day (Toth,
1969). Liver, kidneys, spleen, lungs, and organs with gross
lesions were examined microscopically. No statistical analysis
was reported. Drinking-water solutions containing hydrazine were
prepared 3 times per week. There was no indication that
hydrazine concentrations were measured in the solutions during
the studies. At the highest dose level, body weights were not
affected, but the mortality rate in females was increased.
Numerous lung tumours were observed in both sexes with a
comparable incidence (Table 7) and degree of malignancy at both
dose levels. The average latency time for this tumour was
decreased at both dose levels. At the high dose level, the
latency period for malignant lymphomas, which are characteristic
for the strain, and the incidence of adenocarcinomas of the
mammary gland appeared to be decreased.
Fifty Syrian golden hamsters of each sex also received, for
their life-time, up to a maximum of 110 weeks, doses of hydra-
zine sulfate in drinking-water, equivalent to 0.57 mg hydrazine
per day. Untreated non-concurrent control groups contained 100
hamsters of each sex. The protocol was similar to that described
above for rats. Body weights of exposed hamsters were slightly
reduced, but no tumours or other effects were noted. The authors
considered that there was no treatment-related tumour incidence
and that the mortality rate was not affected by the exposure,
though the mean survival period was slightly reduced (Toth,
1972b).
Groups of 34 male and 29 female Swiss mice were administered
hydrazine sulfate by gastric intubation at doses equivalent to
0.27 mg hydrazine per mouse per day, 5 days per week, for their
lifetime. Lung adenocarcinomas occurred in 30 out of 34 male
mice and in 21 out of 29 female mice compared with 1 out of 20
controls. The earliest tumour appeared after 14 months of
exposure. An increased incidence of lung tumours (20/22)
occurred in F1 animals, which were obtained from treated
mothers, and received hydrazine sulfate post weaning from the
age of 11 weeks. No control mice were mated. An increase in lung
tumours (16/35) was also observed in F2 animals obtained from F1
animals that had been treated with hydrazine throughout
gestation and lactation (Menon & Bhide, 1983).
9. EFFECTS ON MAN
9.1. Poisoning Incidents
Several incidents of systemic poisoning have been reported,
mainly showing effects on the central nervous system, respira-
tory system, and stomach.
After a laboratory technician had drunk 20 - 30 ml of a 6%
aqueous solution of hydrazine (free base), he immediately
vomited. Four hours later, weakness, somnolence, and arrhythmia
were observed. Laboratory findings showed a slight but persis-
tent leukocytosis. The serum-albumin fraction was decreased with
an increase in the fraction of globulin. Two days after
exposure, slightly elevated body temperature and a few red blood
cells in the urine were noted, while the patient showed
irregular breathing. Five days after exposure, the patient had
recovered (Drews et al., 1960).
A case was reported of a man who drank between a mouthful
and a cupful of hydrazine in concentrated solution. He
immediately vomited, became unconscious, and flushed. Vomiting
ceased about 12 h after admission to the hospital. The patient
showed sporadic violent behaviour and later developed ataxia
with a lateral nystagmus, a decreased sense of vibration, and
paraesthesia in the arms and legs. Oliguria was also noted.
Pyridoxine treatment was administered, but it was not clear from
the report whether he benefited from this. The patient's final
condition was not reported (Reid, 1965).
A 24-year-old man accidentally ingested "a mouthful" of
hydrazine and immediately became confused, lethargic, and
restless. On admission to hospital, a complete chemistry profile
was normal, but, 3 - 4 days later, hepatotoxicity was evident as
indicated by elevated levels of aspartate amino-transferase (EC
2.6.1.1), lactate dehydrogenase (EC 1.1.1.27), and total
bilirubin. At this time, the patient was treated with pyridoxine
and recovered completely within 5 days. Peripheral neuropathy,
however, developed subsequently, which resolved over the next 6
months. The authors attributed this neuropathy to the megadose
pyridoxine therapy (Harati & Niakan, 1986).
Another man sustained a chemical burn during an industrial
hydrazine explosion. Exposure occurred probably both via the
skin and by inhalation. The burns on his skin covered 22% of the
body surface. On admission to the hospital, the neuro-
logical status of the patient was normal but, 14 h after
exposure, he became comatose. When the coma persisted for 60 h,
he was treated with pyridoxine, and the neurological disorders
cleared within the next 12 h. Other findings were persistently
elevated glucose levels, haematuria, and respiratory
difficulties. Several biochemical indicators of liver
malfunction were elevated from day 3 after exposure and returned
to normal levels over the next 5 weeks (Kirklin et al., 1976).
Two men were exposed to Aerozine 50 vapour (50% hydrazine
and 50% 1,1-dimethylhydrazine by volume) via a leak in a fuel
line. The first man was exposed for about 90 min via a defective
gas mask. He reported headache, nausea, and a shaky feeling, a
sensation of burning of the face, a sore throat, and tightness
in the chest. The second man had inhaled the vapour 3 times
before evacuation from the contaminated area. He was dyspnoeic,
trembling, and weak. Both men showed neurological disorders
including twitching of extremities and clonic movements in the
first victim and hyperreactive reflexes in both men. All
symptoms cleared after treatment with pyridoxine. From clinical
signs, it was concluded that pulmonary oedema developed in both
men and was treated successfully. Four other cases of poisoning
by Aerozine after a spill were mentioned in this report. These 4
men showed nausea and vomiting. Both symptoms disappeared within
20 min following pyridoxine treatment (Frierson, 1965).
One case of systemic lupus erythaematosus-like disease due
to occupational exposure to hydrazine (free base) has been
described. The patient, a laboratory technician, had recurrent
episodes of joint pain, photosensitive rash, fatigue, and fever
following hydrazine exposure. A challenge patch test suggested
that hydrazine caused the illness. On the basis of detailed
examinations of the patient and her family, it was concluded
that hydrazine could cause the disease in a person with a
genetic predisposition. Some of the genetic factors identified
were slow acetylation phenotype, HLA DR2,3, and immune system
responsiveness such as antinuclear antibodies, antibodies to
single-stranded and native DNA, and inhibition of pokeweed
mitogen-stimulated immunoglobulin G synthesis in lymphocytes
(Reidenberg et al., 1983).
9.2. Occupational Exposure
9.2.1. Inhalation exposure
One case was reported of a man who had handled hydrazine
(hydrazine hydrate) once a week for an unknown number of hours
over a period of 6 months. In simulated conditions, only 0.071
mg hydrazine/m3 air was measured, but probably skin exposure had
also occurred. The man experienced conjunctivitis, tremor, and
lethargy after each exposure. Following the last exposure, he
was feverish, vomited, and showed diarrhoea. In a hospital, 6
days later, many disorders were noted: conjunctivitis,
stomatitis, arrhythmia, upper abdominal pain, enlarged abdomen,
icterus, a tender and palpable liver, black faeces, incoherence,
and oliguria. X-ray examinations showed pleural effusion and
lung shadowing. Laboratory findings comprised elevated bilirubin
and creatinine levels in the blood, and protein and red blood
cells in urine. Treatments administered included haemodialysis
and B-vitamins, which brought only temporary relief. The man
died 21 days after the last exposure. Autopsy revealed
pneumonia, severe renal tubular necrosis and nephritis, and mild
hepatocellular damage (Sotaniemi et al., 1971).
9.2.2. Skin and eye irritation; sensitization
Two cases of skin irritation (Frierson, 1965; Kirklin et
al., 1976) and one case of eye irritation (Sotaniemi et al.,
1971) have already been cited in sections 9.1 and 9.2.1,
respectively. Dermatitis of the hands without systemic effects
was observed in 2 men who were occupationally exposed to hydra-
zine hydrate over a period of 5 months. Both men had had similar
experiences previously, after handling of hydrazine hydrate
(Evans, 1959). No patch tests were carried out, but the allergic
nature of the contact dermatitis that can develop after skin
exposure to hydrazine, has been reported earlier. In 1959, an
outbreak of dermatitis was reported among 12 out of 34 female
workers in a factory where hydrazine hydrochloride was used as a
solder flux. Four months after the use of the solder flux had
stopped, 6 out of 12 workers showed strong positive reactions
towards hydrazine sulfate, while 30 controls did not show any
reaction (Frost & Horth, 1959). In an electronics plant, 35
workers, constituting half the work force, developed contact
dermatitis from a solder flux containing hydrazine
hydrochloride, over a period of 5 years. The symptoms did not
reappear when contact with hydrazine was avoided. Patch tests on
5 female workers of this group were all clearly positive for
hydrazine fluxes, while 3 controls did not react (Wheeler et
al., 1965). Another 7 workers in a chemical workshop, where
solder flux containing hydrazine hydrochloride was used, showed
allergic contact dermatitis towards hydrazine, as did 5 workers
in a pharmaceutical firm producing isoniazid. The results of the
patch tests were strongly positive for the 12 subjects, but a
control group was lacking (Zina & Bonu, 1962). Hydrazine induced
allergic contact dermatitis in 4 men when used as a corrosion
inhibitor (Schultheiss, 1959; Hovding, 1967; Von Keilig & Speer,
1983). A negative control group was tested in only one of these
studies (Hovding, 1967). Allergic contact dermatitis developed
in 15 workers in 2 industries producing hydrazine sulfate. In
one of these industries, patch tests on 15 workers were clearly
positive for hydrazine, while 13 controls from the same industry
did not show any reaction. Symptoms did not reappear when
contact with hydrazine sulfate was avoided (Brandt, 1960). In
the other industry, 10 persons produced a positive reaction
towards hydrazine. No negative controls were tested (Sonneck &
Umlauf, 1961). Two cases of allergic contact dermatitis were
reported following exposure to hydrazine in stain removers with
strong positive reactions towards the chemical in patch tests.
Five controls did not react (Van Ketel, 1964). Systemic effects
were not noted in any of these studies.
Cross sensitization to hydrazine derivatives, such as
isoniazid, phenylhydrazine, and hydralazine, was found by
several investigators (Schultheiss, 1959; Zina & Bonu, 1962; Van
Ketel, 1964; Hovding, 1967; Von Pevny & Peter, 1983).
9.2.3. Mortality studies
The causes of death in a cohort of 427 male workers,
employed for a minimum of 6 months in a hydrazine manufacturing
plant between 1945 and 1971, were studied retrospectively and
followed until July 1982. The plant ceased production in 1971.
The men were divided according to 3 categories of exposure. The
first category contained 78 men who might have been exposed to
concentrations of hydrazine ranging from 1.3 to 13 mg/m3 air.
Categories 2 and 3 (a total of 375 men) included those who might
have been exposed to concentrations between 0.6 and 1.3 mg/m3
and those who had little or no exposure, respectively. A further
subdivision in the first category distinguished between men with
more than 10 years since the first exposure and those with less.
Some men were covered by more than one category. The cohort was
also exposed to other unspecified organic chemicals. The total
number of deaths in 406 men who could be traced was 49 against
61.47 expected. Five men died from lung cancer against an
expected 6.65. Two of these men belonged to the most highly
exposed category. Another 7 cases of cancer were detected (9.27
expected). Further details were not given, except for the remark
that no nasal tumours were involved. The authors noted that the
number of men exposed to hydrazine in this study was small. It
was concluded that the results were encouraging in that no
obvious hazard had yet appeared. A note of caution is introduced
by the observation of 2 deaths from lung cancer in men who had
first been exposed in the heaviest category more than 10 years
previously against 1.61 expected (Wald et al., 1984). The cohort
is being followed up.
A seemingly unusually high incidence of myocardial infarc-
tion prompted further study among workers in a plant manufac-
turing hydrazine. The population at risk was limited to every
on-line hydrazine worker who had worked for 3 months or more
between 1953 and 1978. The number of man years at risk was 534,
and the average employment period was 8 years. Only a
superficial examination of the population at risk and the
working environment was completed. Reference data were provided
by the US Air Force men's rates for the years 1975-77 and by
men's rates from a study over the past 30 years by the US
National Heart Institute (Framingham study). There was a close
compar ison between the 2 sets of reference data. Rates of
occurrence of myocardial infarction (death and non-death) per
thousand men per year within 5-year age groups were calculated.
A total of 5 cases of myocardial infarction, concurrent with
the employment period, were identified among the hydrazine
workers. Their average employment period was 16 years. A total
of 1.2 cases was expected. The difference is highly significant
statistically. Four additional cases were identified in ex-
hydrazine workers. However, as it was impossible to trace all
ex-hydrazine workers, the population at risk was limited to
the "concurrent cases". The author warned that the findings
should be treated with due caution because of the small
numbers involved. He also stated that the strength of the
findings rendered a negative hypothesis almost untenable without
further investigation into the cause of the adverse effect found
(Hamill, 1978).
The Task Group recognized that exposure concentrations were
not reported and that there was no indication that confounding
variables, e.g., smoking history or exercise habits, had been
taken into account.
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1. Evaluation of Human Health Risks
Exposure of human beings to hydrazine may occur occupation-
ally or accidentally, through the ingestion of hydrazine-based
drugs, or through the use of tobacco. Despite the high react-
ivity of this compound and its wide industrial use, few
systematic studies have been carried out on its adverse effects
on man.
Hydrazine is rapidly absorbed through the skin, lungs, and
gastrointestinal tract and rapidly distributed throughout the
body (section 6).
In cases of acute human poisoning, vomiting, severe irrita-
tion of the respiratory tract with the development of pulmonary
oedema, central nervous system depression, and hepatic and renal
damage have been reported. Data are not available from which the
levels of hydrazine inhaled can be estimated in cases of acute
poisoning by the respiratory route. However, from reports of
poisoning by the oral route, it would appear that ingestion of
amounts of the order of 20 - 50 ml causes severe intoxication
and may be lethal (section 9.1). It is not possible to estimate
a no-observed-adverse-effect level from the available human
data.
Most of the effects in human beings exposed to hydrazine
have also been observed in experimental animals. In addition,
loss of body weight, anaemia, hypoglycaemia, and fatty liver
have frequently been reported. Fatty liver in mice and reduced
growth in rats were reported when the animals were exposed
through inhalation to the lowest (0.26 mg/m3) of three dose
levels. Monkeys and dogs were unaffected at this dose. In only
one study, a no-observed-adverse-effect level of 3 µwg/kg was
reported, when hydrazine was administered by the oral route to
rats. No data are available on the basis of which a no-observed-
adverse-effect level by inhalation can be established (section
8.2).
Embryotoxicity, fetotoxicity, and minor fetal abnormalities
were observed in rats and mice exposed to hydrazine at doses
toxic for the mother. At such doses, perinatal mortality is
high. These effects may also occur at doses just below the
maternally toxic dose (section 8.4). No data are available on
the effects of hydrazine on the human fetus. In the absence of
human data, it would be prudent to assume that hydrazine might
have adverse effects on the human embryo or fetus at exposure
levels approximating those producing toxicity for the mother.
Such levels may occur during accidental spillage but would be
unlikely to occur in the ambient environment because atmospheric
concentrations are low.
Skin and eye irritation have been observed in human beings
who have come into contact with hydrazine, but the data are
insufficient to establish a no-observed-effect level for
irritation. Hydrazine is a strong skin sensitizer in man and
cross-reacts with hydrazine derivatives (section 9.2.2). Slight
eye irritation was observed in monkeys at hydrazine concentra-
tions of 1.3 and 6.5 mg/m3, but none occurred in animals exposed
to 0.26 mg/m3 (section 8.2.1). No skin irritancy studies on
animals have been reported.
The available data indicate that hydrazine induces gene
mutations and chromosome aberrations in a variety of test
systems including plants, phages, bacteria, fungi, Drosophila,
and mammalian cells in vitro. Hydrazine induced indirect
alkylation in the liver DNA of rodents after in vivo exposure to
toxic doses, and it also caused DNA damage in vitro . Hydrazine
transformed human and hamster cells in vitro. No increased
unscheduled DNA synthesis was observed in the germ cells of mice
in vivo . It did not induce chromosome aberrations, micronuclei,
or dominant lethals in mice in vivo , but chromosomal aberrations
were reported in rats in vivo (section 8.5).
Hydrazine vapour induced nasal tumours, most of which were
benign, in F-344 rats and Syrian golden hamsters, after 12
months of treatment and life-time observation. At the exposure
levels at which most of the tumours occurred, there were signs
of severe mucosal irritation. In several studies on mice,
hydrazine induced an increased incidence of pulmonary tumours,
when administered by the oral route. Hepatic tumours were also
reported in two studies. In some of the studies, the pulmonary
tumour incidence was related to the dose administered. Although
in these reports there is little information about the toxic
effects of the compound, it is probable that the doses admin-
istered in some of the studies were at, or near, the toxic
levels. No tumours were observed in hamsters treated orally with
hydrazine (section 8.6). On the basis of the carcinogenicity
studies on experimental animals, there is evidence that
hydrazine is an animal carcinogen. Human data are inadequate to
assess its carcinogenicity in man. In the absence of human data,
and taking into account the mutagenicity data as well as the
carcinogenicity data in animals, it would be prudent to consider
hydrazine as a possible human carcinogen. Thus, exposure to
human beings should be kept as low as feasible.
Since measurable levels are not normally encountered in the
general environment, it can be concluded that, except in cases
of accidental exposure, hydrazine does not pose a significant
practical hazard for the general population.
10.2. Evaluation of Effects on the Environment
Hydrazine can be released into the atmosphere during venting
operations, storage, and transfer. The total emission has been
estimated to be nearly 0.01% of the hydrazine produced
(production > 35 000 tonnes in 1981). Accidental discharges into
water, air, and soil can result from bulk storage, handling,
transport, and improper waste disposal. Evaporation of hydrazine
after a spill can generate an atmospheric concentration as high
as 4 mg/m3, 2 km downwind.
Hydrazine is degraded rapidly in air through reactions with
ozone, hydroxyl radicals, and nitrogen dioxide. In water, it is
degraded rapidly, especially under aerobic conditions, and in
the presence of organic material and/or in alkaline or hard
water. It is more persistent in soft, metal-free water. In soil,
it is adsorbed and decomposed on clay surfaces, under aerobic
conditions. However, available data are inadequate to describe
the nature of hydrazine behaviour in the soil.
Because of the rapid degradation of hydrazine in the
environment, measurable levels are not normally encountered.
Hydrazine can be toxic for aquatic life, even at very low
concentrations. Fish species showed LC50 values of between 0.54
mg/litre (roach) and 5.98 mg/litre (fathead minnow). Nitrifying
bacteria in activated sludge are inhibited by hydrazine levels
higher than 1 mg/litre. Some other microorganisms are more
sensitive and show toxicity thresholds at levels reported to be
as low as 0.00008 mg/litre.
Hydrazine in both air and water is toxic for plants; in
water, it can inhibit plant germination.
On the basis of these data, it can be concluded that hydra-
zine may present a significant hazard for the aquatic environ-
ment and plant life.
11. RECOMMENDATIONS FOR FURTHER STUDIES
1. Dose-response studies pertaining to:
(a) DNA alkylation (adduct identification);
(b) damage of nasal epithelium; and
(c) pulmonary effects.
2. Skin sensitization studies with focus on cross-reactivity
with hydrazine derivatives.
3. Dose-response studies on sensitive, commercially important
fish species and their food supplies.
4. Metabolic studies in conjunction with effects on DNA.
5. Dermal initiation-promotion studies.
6. Reproduction toxicity studies in sensitive rodent species at
continuous low exposure levels.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
The International Agency for Research on Cancer (IARC, 1982)
considered that, while the evidence for the carcinogenicity of
hydrazine for human beings was inadequate, evidence for its
carcinogenicity for animals and for its activity in short-term
tests was sufficient to conclude that hydrazine is probably
carcinogenic for human beings.
REFERENCES
AGRELO, C. & AMOS, H. (1981) DNA repair in human fibroblasts.
In: de Serres, F.J. & Ashby, J., ed. Evaluation of short-term
tests for carcinogens. Report of the International Collaborative
Programme, New York, Amsterdam, Oxford, Elsevier/North Holland,
pp. 528-532 (Progress in Mutation Research Vol. 1).
AKIN, F.J. & NORRED, W.P. (1978) Effects of short-term
administration of maleic hydrazine or hydrazine on rat hepatic
microsomal enzymes. Toxicol. appl. Pharmacol., 43: 287-292.
ALEYASSINE, H. & LEE, S.H. (1971) Inhibition by hydrazine,
phenelzine, and pargyline of insulin release from rat pancreas.
Endocrinology, 89: 125-129.
AMACHER, D.E., PAILLET, S.C., TURNER, G.N., RAY, V.A., &
SALSBURG, D.S. (1980) Point mutations at the thymidine kinase
locus in L5178Y mouse lymphoma cell. II. Test validation and
interpretation. Mutat. Res., 72: 447-474.
AMENTA, J.S. & DOMINGUEZ, A.M. (1965a) Fatty acid flux and
triglyceride secretion in the hydrazine-induced fatty liver.
Exp. mol. Pathol., 4: 282-302.
AMENTA, J.S. & DOMINGUEZ, A.M. (1965b) The effect of hydrazine
and congeners on C1402 respiratory pattern of various metabolic
substrates. Toxicol. appl. Pharmacol., 7: 236-246.
ANDERSEN, M.E. & KELLER, W.C. (1984) Toxicokinetic principles
in relation to percutaneous absorption and cutaneous toxicity.
In: Drill, V.A. & Lazar, P., ed. Cutaneous toxicity, New York,
Raven Press, pp. 9-27.
ASHBY, J. (1981) Overview of study and test chemical
activities. In: De Serres, F. & Ashby, J., ed. Evaluation of
short-term tests for carcinogens. Report of the International
Collaboration Programme, Amsterdam, Elsevier/North Holland, pp.
112-171 (Progress in Mutation Research, Vol. 1).
ASHBY, J. & KILBEY, B. (1981) Summary report on the
performance of bacterial repair, phage induction, degranulation,
and nuclear enlargement assays. In: de Serres, F.J. & Ashby, J.,
ed. Evaluation of short-term tests for carcinogens. Report of
the International Collaborative Programme, New York, Amsterdam,
Oxford, Elsevier/North Holland, pp. 33-48 (Progress in Mutation
Research Vol. 1).
ASTM (1981) Annual book of ASTM standards. Part 31. Water,
Philadelphia, Pennsylvania, American Society for Testing and
Materials, pp. 425-428 (Method D1385: standard test method for
hydrazine in water).
AUDRIETH, L.F. & OGG, B. (1951) The chemistry of hydrazine,
New York, John Wiley and Sons.
AZAR, A., THOMAS, A.A., & SHILLITO, F.H. (1970) Pyridoxine and
phenobarbital as treatment for Aerozine 50 toxicity. Aerosp.
Med., 41: 1-4.
BAKER, R.S.U., MITCHELL, G.A., MEHER-HOMJI, K.M., & PODOBNA, E.
(1983) Sensitivity of two Chinese hamster cell lines to SCE
induction by a variety of chemical mutagens. Mutat. Res., 118:
103-116.
BALGANESH, M. & SETLOW, J.K. (1984) Prophage induction in
Haemophilus influenzae and its relationship to mutation by
chemical and physical agents. Mutat. Res., 125: 15-22.
BANKS, W.L. (1970) Effect of hydrazine treatment on hepatic
protein biosynthesis in vivo. Biochem. Pharmacol., 19: 275-
283.
BECKER, R.A., BARROWS, L.R., & SHANK, R. (1981) Methylation of
liver DNA guanine in hydrazine hepatotoxicity: dose-response and
kinetic characteristics of 7-methylguanine and O6-methylguanine
formation and persistence in rats. Carcinogenesis, 2: 1181-
1188.
BHIDE, S.V., D'SOUZA, R.A., SAWAY, M.M., & RANADIVE, K.J.
(1976) Lung tumour incidence in mice treated with hydrazine
sulfate. Int. J. Cancer, 18: 530-535.
BIANCIFIORI, C. (1969) [Existence of a hormonal factor in the
pulmonary carcinogenicity of hydrazine.] Lav. Ist. Anat. Istol.
Patol. Univ. Studi Perugia, 29: 29-41 (in Italian).
BIANCIFIORI, C. (1970a) Hepatomas in CBA/Cb/Se mice and liver
lesions in golden hamsters induced by hydrazine sulfate. J. Natl
Cancer Inst., 44: 943-953.
BIANCIFIORI, C. (1970b) [Pulmonary and liver tumours from low
doses of hydrazine sulfate in BALB/c/Cb/Se mice.] Lav. Ist.
Anat. Istol. Patol. Univ. Studi Perugia, 30: 89-99 (in
Italian).
BIANCIFIORI, C., BUCCIARELLI, E., CLAYSON, D.B., & SANTILLI,
F.E. (1964) Induction of hepatomas in CBA/Cb/Se mice by
hydrazine sulfate and the lack of effect of croton oil on tumour
induction in BALB/Cb/Se mice. Br. J. Cancer, 18: 543-550.
BLAIR, I.A., MANILLA TINOCO, R., BRODIE, M.J., CLARE, R.A.,
DOLLERY, C.T., TIMBRELL, J.A., & BEEVER, I.A. (1985) Plasma
hydrazine concentrations in man after isoniazid and hydralazine
administration. Hum. Toxicol., 4: 195-202.
BODENSCHATZ, W. (1986) [Hydrazine in drinking-water.] Off.
Gesundheitswes., 48: 205-208 (in German).
BOSAN, W.S. & SHANK, R.C. (1983) Methylation of liver DNA
guanine in hamsters given hydrazine. Toxicol. appl. Pharmacol.,
70: 324-334.
BRANDT, B., VON (1960) [On allergic skin damage by hydrazine
sulfate.] Dermatol. Wochenschr., 141: 376-381 (in German).
BRAUN, R., JAKEL, H.-P., & SCHONEICH, J. (1984) Genetic
effects of isoniazid and the relationships to in vivo and in
vitro biotransformation. Mutat. Res., 137: 61-69.
BRESLER, S.E., KALININ, V.L., & PERUMOV, D.A. (1968)
Inactivation and mutagenesis on isolated DNA. II. Kinetics of
mutagenesis and efficiency of different mutagens. Mutat. Res.,
5: 1-14.
BRIDGES, B.A., MACGREGOR, D., & ZEIGER, E. (1981) Summary
report on the performance of bacterial mutation assays. In: de
Serres, F.J. & Ashby, J., ed. Evaluation of short-term tests for
carcinogens. Report of the International Collaborative
Programme, New York, Amsterdam, Oxford, Elsevier/North Holland,
pp. 45-67 (Progress in Mutation Research Vol. 1).
BRINGMANN, G. (1975) [Determination of the effect of
biological pollutants on the inhibition of cell multiplication
in the blue algae Microcystis. ] Gesundheits-Ingenieur, 96: 238-
241 (in German).
BRINGMANN, G. & KUHN, R. (1980) Comparison of the toxicity
thresholds of water pollutants to bacteria, algae, and protozoa
in the cell multiplication test. Water Res., 14: 231-241.
BRINGMANN, G. & KUHN, R. (1981) [Comparison of the effect of
hazardous substances on flagellated and ciliated, respectively,
holozoic bactivorous and saprozoic protozoa.] GWF Wasser-
Abwasser, 122: 308-313 (in German).
BRINGMANN, G. & KUHN, R. (1982) [Data on the toxicity of water
pollutants to Daphnia magna in an improved standard test.] Z.
Wasser Abwasser Forsch., 15: 1-6 (in German).
BRUGGER, H. (1983) [ Emission factors for 12 selected
substances with probably carcinogenic potential: hydrazine,]
Environment Research Programme of the Federal Ministry of the
Interior, Federal Environment Office, Vol. 3, Part 1, pp. 15-17
(Report No. 104-04-344) (in German).
CARVER, J.H., SALAZAR, E.P., KNIZE, M.G., & WANDRES, D.L.
(1981) Mutation induction at multiple gene loci in Chinese
hamster ovary cells: the genetic activity of 15 coded
carcinogens and noncarcinogens. In: de Serres, F.J. & Ashby, J.,
ed. Evaluation of short-term tests for carcinogens. Report of
the International Collaborative Programme, New York, Amsterdam,
Oxford, Elsevier/North Holland, pp. 594-601 (Progress in
Mutation Research Vol. 1).
CHANDRA SEKHAR, V.S.G. & REDDY, G.M. (1971) Specific locus
mutations in maize by chemical mutagens. Curr. Sci., 40: 136-
137.
CHATTERJEE, A.K. & SENGUPTA, K. (1980) Regulation of formation
in vivo of pyridoxal phosphate in hydrazine-treated rats.
Int. J. Vitam. Nutr. Res., 50: 24-28.
CHU, B.C.F., BROWN, D.M., & BURDON, M.G. (1973) Effect of
nitrogen and of catalase on hydroxylamine and hydrazine
mutagenesis. Mutat. Res., 20: 265-270.
CIER, A., ROUGANNE, J.P., & SCHMITT, M. (1967) Effets
hématologiques d'une intoxication subaiguë à l'hydrazine et à la
dimethyl hydrazine asymétrique chez la souris et le lapin. C.R.
Soc. Biol. (Paris), 161: 854-858.
CLARK, D.A., BAIRRINGTON, J.D., BITTER, H.L., COE, F.L., MEDINA,
M.A., MERRIT, J.H., & SCOTT, W.N. (1968) Pharmacology and
toxicology of propellant hydrazines, Springfield, Virginia, US
Department of Commerce (Aeromedical Reviews No. 11-68) (AD
688500).
CLARK, D.A., LEEDER, L.G., FOULDS, E.L., & TROUT, D.L. (1970)
Changes in lipids of rat liver after hydrazine injection.
Biochem. Pharmacol., 19: 1743-1752.
COMSTOCK, C.C., LAWSON, L.H., GREENE, E.A., & OBERST, F.W.
(1954) Inhalation toxicity of hydrazine vapour. Arch. ind. Hyg.
occup. Med., 10: 476-490.
COOK, L.R., GLENN, R.E., & PODOLAK, G.E. (1979) Monitoring and
analysis of personnel exposures to hydrazines at a rocket
propellant plant. Am. Ind. Hyg. Assoc. J., 40: 69-74.
COOLING, J., BURDITT, S.L., & BRINDLEY, D.N. (1979) Effects of
treating rats with hydrazine on the circulating concentration of
corticosterone and insulin in relation to hepatic triacyl-
glycerol synthesis. Biochem. Soc. Trans., 7: 1501-1503.
CORNISH, H.H. & WILSON, C.E. (1968) Amino acid levels in
hydrazine-treated rats. Toxicol. appl. Pharmacol., 12: 265-272.
DANIEL, M.R. & DEHNEL, J.M. (1981) Cell transformation test
with baby hamster kidney cells. In: de Serres, F.J. & Ashby, J.,
ed. Evaluation of short-term tests for carcinogens. Report of
the International Collaborative Programme, New York, Amsterdam,
Oxford, Elsevier/North Holland, pp. 626-637 (Progress in
Mutation Research Vol. 1).
DAMBRAUSKAS, T. & CORNISH, H.H. (1964) The distribution, meta-
bolism, and excretion of hydrazine in rat and mouse. Toxicol.
appl. Pharmacol., 6: 653-663.
DAVIS, L.C. (1980) Hydrazine as a substrate and inhibitor of
Azobacter vinelandii nitrogenase. Arch. Biochem. Biophys.,
204: 270-276.
DEAN, B.J. (1981) Activity of 27 coded compounds in the R11
chromosome assay. In: de Serres, F.J. & Ashby, J., ed.
Evaluation of short-term tests for carcinogens. Report of the
International Collaborative Programme, New York, Amsterdam,
Oxford, Elsevier/North Holland, pp. 570-579 (Progress in
Mutation Research Vol. 1).
DEE, L.A. (1971) Gas chromatographic determination of aqueous
trace hydrazine and methylhydrazine as corresponding pyrazoles.
Anal. Chem., 43: 1416-1419.
DE FLORA, S., ZANACCHI, P., CAMOIRANO, A., BENNICELLI, C., &
BADOLATI, G.S. (1984) Genotoxic activity and potency of 135
compounds in the Ames reversion test and in a bacterial DNA-
repair test. Mutat. Res., 133: 161-198.
DE SERRES, F.J. & ASHBY, J., ed. (1981) Evaluation of short-
term tests for carcinogens. Report of the International
Collaborative Programme, New York, Amsterdam, Oxford, Elsevier/
North Holland, 827 pp (Progress in Mutation Research, Vol. 1).
DI LUZIO, N.R., STEGE, T.E., & HOFFMAN, E.O. (1973) Protective
influence of diphenyl- p -phenylenediamine on hydrazine-induced
lipid perioxidation and hepatic injury. Exp. mol. Pathol., 19:
284-292.
DOMINGUEZ, A.M., AMENTA, J.S., HILL, C.S., & DOMANSKI, T.J.
(1962) Morphologic and biochemical alterations in the kidney of
the hydrazine-treated rat. Aerosp. Med., 33: 1094-2010.
DOST, F.N., REED, D.J., & WANG, C.H. (1971) Effects of various
hydrazines upon the metabolism of gamma-aminobutyric acid
(GABA)-1-14C by rats. Biochem. Pharmacol., 20: 1702-1707.
DOST, F.N., BRODERICK, D.J., KRIVAK, R.M., & REED, D.J. (1981)
Metabolism of hydrazine, Ohio, Wright-Patterson Air Force Base,
Aerospace Medical Research Laboratory (AFAMRL TR-81-26)
(AD/A101849).
DREWS, A., EVERSMANN, K., & FRITZE, E. (1960) [On oral
poisoning by hydrazine.] Med. Welt, 23: 1295-1297 (in German).
DUAMIN, V.V., DENISOV, V.L., ANDROPOVA, S.N., & MALETIN, V.P.
(1984) [Influence of hydrazine on reproductive function of
animals when administered in organisms by different routes.]
Gig. i Sanit., 9: 25-28 (in Russian).
EPSTEIN, S.S., ARNOLD, E., ANDREA, J., BASS, W., & BISHOP, Y.
(1972) Detection of chemical mutagens by the dominant lethal
assay in the mouse. Toxicol. appl. Pharmacol., 23: 288-325.
EVANS, D.M. (1959) Two cases of hydrazine hydrate dermatitis
without systemic intoxication. Br. J. ind. Med., 16: 126-127.
FARMWALD, J.A. & MACNAUGHTON, M.G.M. (1981) Effects of
hydrazine on the activated sludge process. J. Water Pollut.
Control Fed., 53: 565-575.
FAROOK, S.A.F. & NIZAM, J. (1979) Mutagenic sensitivity of
base-specific chemicals in chick pea. Indian J. Bot., 2: 12-16.
FISHER, J.W., HARRAH, C.B., & BERRY, W.O. (1980) Hydrazine:
acute toxicity to bluegills and sublethal effects on dorsal
light response and aggression. Trans. Am. Fish. Soc., 109: 304-
309.
FLOYD, W.N. (1980) The importance of ammonia in the metabolic
effects of hydrazine. Aviat. Space environ. Med., 51: 899-901.
FORTNEY, S.R. (1966) Effect of hydrazine on liver glycogen,
arterial glucose, lactate, pyruvate, and acid-base balance in
the anaesthetized dog. J. Pharmacol. exp. Ther., 153: 562-568.
FORTNEY, S.R., CLARK, D.A., & STEIN, E. (1967) Inhibition of
gluconeogenesis by hydrazine administration in rats. J.
Pharmacol. exp. Ther., 156: 277-284.
FREESE, E.B., GERSON, J., TABER, H., RHAESE, H.-J., & FREESE, E.
(1967) Inactivating DNA alterations induced by peroxides and
peroxide-producing agents. Mutat. Res., 4: 517-531.
FRIERSON, W.M. (1965) Use of peridoxine HCl in acute hydrazine
and UDMH intoxication. Ind. Med. Surg., 34: 650-651.
FROST, J. & HJORTH, N. (1959) Contact dermatitis from
hydrazine hydrochloride in soldering flux. Cross-sensitization
to Apresoline and isoniazid. Acta dermatovenereol., 39: 82-86.
GANOTE, C.E. & ROSENTHAL, A.S. (1968) Characteristic lesions
of methylazoxymethanol-induced liver damage. A comparative
ultrastructural study with dimethylnitrosamine, hydrazine
sulfate, and carbon tetrachloride. Lab. Invest., 19: 382-398.
GEDDES, J.W. & WOOD, J.D. (1984) Changes in the amino acid
content of nerve ending (synaptosomes) induced by drugs that
alter the metabolism of glutamate and gamma-aminobutyric acid.
J. Neurochem., 42: 16-24.
GORDON, A. & LEWANDOWSKI, P.E. (1980) Control of vapor
emissions from hydrazine-based fuels, Newark, New Jersey,
Institute of Technology, Department of Chemical Engineering
(AFOSR-TR-80-053).
GORMLEY, W.T. & FORD, R.E. (1973) Deoxygenation of
environmental waters by hydrazine-type fuels. In: Proceedings of
the 4th Annual Conference on Environmental Toxicology, Fairborn,
Ohio, 1973, Ohio, Wright-Patterson Air Force Base, Aerospace
Medical Research Laboratory, Paper 28, pp. 387-400 (AMRL-TR-73-
125) (AD/781031).
GORSHTEIN, E.S. & KOPYLOVA, T.N. (1983) [Effect of hydrazine
hydrochloric acid on the hepatic microsomal hydroxylation system
of rats.] Eksp. Med., 15: 22-26 (in Russian).
GREENHOUSE, G. (1976a) Evaluation of the teratogenic effects
of hydrazine, methylhydrazine, and dimethylhydrazine on embryos
of Xenopus laevis, the South African clawed toad. Teratology,
13: 167-178.
GREENHOUSE, G. (1976b) The evaluation of toxic effects of
chemicals in fresh water by using frog embryos and larvae.
Environ. Pollut., 11: 303-315.
GUPTA, A.K. & GROVER, N.S. (1970) Hydrazine-induced breaks in
chromosomes of Vicia faba. Mutat. Res., 10: 519-520.
GUPTA, R.S. & GOLDSTEIN, S. (1981) Mutagen testing in the
human fibroblast diphteria toxin resistance (HF Dipr) system.
In: de Serres, F.J. & Ashby, J., ed. Evaluation of short-term
tests for carcinogens. Report of the International Collaborative
Programme, New York, Amsterdam, Oxford, Elsevier/North Holland,
pp. 614-625 (Progress in Mutation Research Vol. 1).
HACK, W., VON, HOYERMANN, K., & WAGNER, H.G. (1974) [Gas-phase
reactions of hydroxyl radical with ammonia and hydrazine.] Ber.
Bunsen-Ges. Phys. Chem., 78: 386-391 (in German).
HAMILL, P.V.V. (1978) Number of heart attack cases among
workers of the Lake Charles hydrazine plant, 1953-78 (Submitted
to the US EPA by the Olin Corporation, Stamford, Connecticut,
under TSCA, section 8(e)).
HARATI, Y. & NIAKAN, E. (1986) Hydrazine toxicity, pyridoxine
therapy, and peripheral neuropathy. Ann. intern. Med., 104: 728-
729.
HARRAH, C.B. (1978) Biological effects of aqueous hydrazine
solutions. In: Procedings of the Conference on Environmental
Chemistry: Hydrazine Fuels, 1977, Florida, Tyndall Air Force
Base, Civil and Environmental Engineering Development Office,
pp. 167-176 (CEEDO-TR-78-14) (AD/A054194).
HARRIS, G.W., ATKINSON, R., & PITTS, J.N. (1979) Kinetics of
the reactions of the hydroxyl radical with hydrazine and MMH. J.
Phys. Chem., 83: 2557-2559.
HAUN, C.C. & KINKEAD, E.R. (1973) Chronic inhalation toxicity
of hydrazine. In: Proceedings of the 4th Annual Conference on
Environmental Toxicology, Fairborn, Ohio, 1973, Ohio, Wright-
Patterson Air Force Base, Aerospace Medical Research Laboratory,
Paper 25, pp. 351-363.
HECK, W.W., BLOODWORTH, M.E., CLARK, W.J., DARLING, D.R., &
HOOVER, W. (1963) Environmental pollution by missile
propellants, Ohio, Wright-Patterson Air Force Base, Aerospace
Medical Research Laboratory (AMRL-TDR-63-75).
HEINDORFF, K., RIEGER, R., VELEMINSKY, J., & GICHNER, T. (1984)
A comparative study of the clastogenicity of maleic hydrazine
and some of its putative degradation products. Mutat. Res., 140:
123-126.
HEINEMANN, B. (1971) Prophage induction in lysogenic E. coli
with simple hydroxylamine and hydrazine compounds. Appl.
Microbiol., 21: 726-731.
HENDERSON, V., FISHER, J.W., & D'ALLESSANDRIS, R. (1981) Toxic
and teratogenic effects of hydrazine on fathead minnow (Pime-
phales promelas) embryos. Bull. environ. Contam. Toxicol., 26:
807-812.
HENDERSON, V., FISHER, J.W., D'ALLESSANDRIS, R., & LIVINGSTON,
J.M. (1983) Effects of hydrazine on functional morphology of
rainbow trout embryos and larvae. Trans. Am. Fish. Soc., 112:
100-104.
HERBOLD, B.A. (1978) [Mutagenicity testing with the liver
microsome test.] Biol. Zentralbl., 97: 137-152 (in German).
HIGGINS, E.S. & BANKS, W.L. (1971) Cognate effects of ethanol,
hydrazine, and tissue regeneration on hepatic mitochonidrial
activities. Biochem. Pharmacol., 20: 1513-1524.
HOLTZCLAW, J.R., ROSE, S.L., WYATT, J.R., ROUNBEHLER, D.P., &
FINE, D.H. (1984) Simultaneous determination of hydrazine,
methylhydrazine, and 1,1-dimethylhydrazine in air by derivat-
ization/gas chromatography. Anal. Chem., 56: 2952-2956.
HOVDING, G. (1967) Occupational dermatitis from hydrazine
hydrate used in boiler protection. Acta dermatovenereol., 47:
293-297.
HSIE, A.W., O'NEILL, J.P., MACHANOFF, R., SCHENLEY, R.L., &
BRIMER, P.A. (1981) Screening for mutagenic response of four
coded chemical by the CHO/HGPRT system. In: de Serres, F.J. &
Ashby, J., ed. Evaluation of short-term tests for carcinogens.
Report of the International Collaborative Programme, New York,
Amsterdam, Oxford, Elsevier/North Holland, pp. 602-607 (Progress
in Mutation Research Vol. 1).
HUNT, T.P., FISHER, J.W., LIVINGSTON, J.M., & PUTNAM, M.E.
(1981) Temperature effects on hydrazine toxicity to bluegills.
Bull. environ. Contam. Toxicol., 27: 588-595.
IARC (1982) Chemicals, industrial processes, and industries
associated with cancer in humans, Lyons, International Agency
for Research on Cancer, pp. 136-138 (Monographs on the
Evaluation of the Carcinogenic Risk of Chemicals to Humans,
Suppl. 4).
IRPTC (1985) Treatment and disposal methods for waste
chemicals, Geneva, International Register of Potentially Toxic
Chemicals, United Nations Environment Programme, pp. 188-189.
IRPTC (1986) Data profile on hydrazine, Geneva, International
Register of Potentially Toxic Chemicals, United Nations
Environment Programme.
ISAACSON, P.J. & HAYES, M.H.B. (1984) The interaction of
hydrazine hydrate with humic acid preparations at pH 4. J. soil
Sci. , 35(1): 79-92.
JACKSON, E.K., PARSHALL, G.W., & HARDY, R.W.F. (1968) Hydrogen
reactions of nitrogenase. J. biol. Chem., 243: 4952-4958.
JACOBSON, K.H., CLEM, J.H., WHEELWRIGHT, H.J., RINEHART, W.E., &
MAYES, N. (1955) The acute toxicity of the vapours of some
methylated hydrazine derivatives. Am. Med. Assoc. Arch. Ind.
Health, 12: 609-616.
JACOBSON, K.H., RINEHART, W.E., WHEELWRIGHT, H.J., ROSS, M.A.,
PAPIN, J.L., DALY, R.C., GREENE, E.A., & GROFF, W.A. (1958)
The toxicology of an aniline-furfurylalcohol-hydrazine vapour
mixture. J. Am. Ind. Hyg. Assoc., 19: 91-100.
JAIN, H.K. & SHUKLA, P.T. (1972) Locus specificity of mutagens
in Drosophila. Mutat. Res., 14: 440-442.
JAIN, H.K., RAUT, R.N., & KHAMANKAR, Y.G. (1968) Base specific
chemicals and mutation analysis in Lycopersicon. Heredity, 23:
247-256.
JOHNSON, F.B. (1982) Chemical interactions with Herpes
simplex type 2 virus: enhancement of transformation by hydrazine
and 1,2-dimethylhydrazine. Chem.-biol. Interact., 40: 97-112.
KAK, S.N. & KAUL, B.L. (1975) Mutagenic activity of hydrazine
and its combination with maleic hydrazide and X-rays in barley.
Cytobios, 12: 123-128.
KANE, D.A. & WILLIAMSON, K.J. (1983) Bacterial toxicity and
metabolism of hydrazine fuels. Arch. environ. Contam. Toxicol.,
12: 447-453.
KANEO, Y., IGUCHI, S., KUBO, H., IWAGIRI, N., & MATSUYAMA, K.
(1984) Tissue distribution of hydrazine and its metabolites in
rats. J. Pharm. Dyn., 7: 556-562.
KELLER, W.C., OLSON, C.T., BACK, K.C. (1982) Evaluation of the
embryotoxicity of hydrazine in rats, Ohio, Wright-Patterson Air
Force Base, Aerospace Medical Research Laboratory (AFAMRL-TR-82-
29) (AD/A119706).
KELLY, M.G., O'GARA, R.W., YANCEY, S., GADEKAR, K., BOTKIN, C.,
& OLIVERIO, V.T. (1969) Comparative carcinogenicity of N -
isopropyl-alpha-(2-methyl-hydrazino)- p -toluamide. HCl (pro-
carbazine hydrochloride), its degradation products, other hydra-
zines, and isonicotinic acid hydrazide. J. Natl Cancer Inst.,
42: 337-344.
KHAMANKAR, Y.G. & JAIN, H.K. (1978) A comparative study of
chemical and physical mutagens in bread wheat. Indian J. Hered.,
10: 49-57.
KIMBALL, R.F. (1976) Reversions of two proline-requiring
auxotrophs of Haemophilus influenzae by N -methyl- N' -nitro-
N-nitrosoguanidine and hydrazine. Mutat. Res., 36: 29-38.
KIMBALL, R.F. (1977) The mutagenicity of hydrazine and some of
its derivatives. Mutat. Res., 39: 111-126.
KIMBALL, R.F. & HIRSCH, B.F. (1975) Tests for the mutagenic
action of a number of chemicals on Haemophilus influenzae with
special emphasis on hydrazine. Mutat. Res., 30: 9-20.
KIRKHART, B. (1981) Micronucleus test on 21 compounds. In: de
Serres, F.J. & Ashby, J., ed. Evaluation of short-term tests for
carcinogens. Report of the International Collaborative
Programme, New York, Amsterdam, Oxford, Elsevier/North Holland,
pp. 698-704 (Progress in Mutation Research Vol. 1).
KIRKLIN, J.K., WATSON, M., BONDOC, C.C., & BURKE, J.F. (1976)
Treatment of hydrazine-induced coma with pyridoxine. New Engl.
J. Med., 294: 938-939.
KOPYLOVA, T.N., MAJORE, A., ELERTE, D., NOZDRUNOVA, N.A., &
ROZENBERG, I.E. (1982) [Lipid peroxidation in hydrazine
hydrochloride-induced toxicity of the liver.] Eksp. Med., 14:
35-45 (in Russian).
KORTY, P. & COE, F. (1968) The effects of hydrazine on the
concentration of free amino acids of plasma and urine. J.
Pharmacol. exp. Ther., 160 212-216.
KROP, S. (1954) Toxicology of hydrazine: a review. Arch. ind.
Hyg. occup. Med., :9: 199-204.
LAMB, R.G. & BANKS, W.L. (1979) Effect of hydrazine exposure
on hepatic triacylglycerol biosynthesis. Biochim. Biophys. Acta,
574: 440-447.
LEE, S.H. & ALEYASSINE, H. (1970) Hydrazine toxicity in
pregnant rats. Arch. environ. Health, 21: 615-619.
LEMONTT, J.F. (1977) Loss of hydrazine-induced mutability in
wild-type and excision-repair-defective yeast during post-
treatment inhibition of cell division. Mutat. Res., 50: 57-66.
LEMONTT, J.F. (1978) Mutagenesis of yeast by hydrazine:
dependence upon post-treatment cell division. Mutat. Res., 43:
165-178.
LEWALTER, J., PFLANITZER, K., MULLER, G., & SCHALLER, K.H.
(1984) [Hydrazine levels in blood (plasma).] In: [ Analytical
methods,] Vol. 2, pp. D1-D3 (Senate Commission for the Testing
of Health-damaging Work Material in the German Research
Association-Working Group "Analytical Chemistry") (in German).
LIU, Y.Y., SCHMELTZ, I., & HOFFMANN, D. (1974) Chemical
studies on tobacco smoke: quantitative analysis of hydrazine in
tobacco and cigarette smoke. Anal. Chem., 46: 885-889.
LONDON, S.A., MANTEL, C.R., ROBINSON, J.D., & LUKING, S. (1983)
Effects of selected hydrazines on the early death rates of
Enterobacter cloacae. Bull. environ. Contam. Toxicol., 31: 360-
368.
LOPEZ-MENDOZA, D. & VILLA-TREVINO, S. (1971) Hydrazine-
induced inhibition of amino acid incorporation into rat liver
protein. Lab. Invest., 25: 68-72.
LOPRIENO, N. (1981) Screening of coded carcinogenic/non-
carcinogenic chemicals by a forward mutation system with the
yeast Schizosaccharomyces pombe. In: de Serres, F.J. & Ashby,
J., ed. Evaluation of short-term tests for carcinogens. Report
of the International Collaborative Programme, New York,
Amsterdam, Oxford, Elsevier/North Holland, pp. 425-433 (Progress
in Mutation Research Vol. 1).
LYNG, R.D., KELLER, W.C., & BACK, K.C. (1980) Effects of
hydrazine on pregnant ICR mice, Ohio, Wright-Patterson Air Force
Base, Aerospace Medical Research Laboratory (AFAMRL-TR-80-19)
(AD/A084023).
MCCORMICK, D.B. & SNELL, E.E. (1961) Pyridoxal phospho-
kinases. II. Effects of inhibitors. J. biol. Chem., 236: 2085-
2088.
MCKENNIS, H., WEATHERBY, J.H., & WITKIN, L.B. (1955) Studies
on the excretion of hydrazine and metabolites. Am. Med. Assoc.
Arch. Ind. Health, 12: 511-514.
MCKENNIS, H., YARD, A.S., WEATHERBY, J.H., & HAGY, J.A. (1959)
Acetylation of hydrazine and the formation of 1,2-diacetyl-
hydrazine in vivo . J. Pharmacol. exp. Ther., 126: 109-116.
MACNAUGHTON, M.G., STAUFFER, T.B., & STONE, D.A. (1981)
Environmental chemistry and management of hydrazine. Aviat.
Space environ. Med., 52: 149-153.
MACRAE, W.D. & STICH, H.F. (1979) Induction of sister
chromatid exchanges in Chinese hamster ovary cells by thiol and
hydrazine compounds. Mutat. Res., 68: 351-365.
MARSHALL, C.E., WATTS, D.I., & SUGDEN, M.C. (1983) Effects of
hydrazine on liver and brown adipose tissue lipogenesis in 24 h-
starved rats. J. Pharm. Pharmacol., 35: 460-461.
MARU, G.B. & BHIDE, S.V. (1982) Effects of antioxidants and
antitoxicants of isoniazid on the formation of lung tumours in
mice by isoniazid and hydrazine sulfate. Cancer Lett., 17: 75-
80.
MATSUI, F., ROBERTSON, D.L., & LOVERING, E.G. (1983)
Determination of hydrazine in pharmaceuticals. III. Hydralazine
and isoniazid using GLC. J. pharmacol. Sci., 72: 948-951.
MATSUYAMA, K., YAMASHITA, C., SENDOH, T., NODA, A., GOTO, S., &
IGUCHI, S. (1983) Further investigations on brain distribution
of hydrazine and its gamma-aminobutyric acid elevating effect in
rats. J. Pharmacobio-Dyn., 6: 932-937.
MEDINA, M.A. (1963) The in vivo effects of hydrazines and
vitamin B6 on the metabolism of gamma-aminobutyric acid. J.
Pharmacol. exp. Ther., 140: 133-137.
MEHTA, R.D. & VON BORSTEL, R.C. (1981) Mutagenic activity of
42 uncoded compounds in the haploid yeast reversion assay,
strain XV 185-14C. In: de Serres, F.J. & Ashby, J., ed.
Evaluation of short-term tests for carcinogens. Report of the
International Collaborative Programme, New York, Amsterdam,
Oxford, Elsevier/North Holland, pp. 414-423 (Progress in
Mutation Research Vol. 1).
MENON, M.M. & BHIDE, S.V. (1983) Perinatal carcinogenicity of
isoniazide (INH) in Swiss mice. J. cancer Res. clin. Oncol.,
105: 258-261.
MILIA, U., BIANCIFIORI, C., & SANTILLI, F.E.G. (1965) Late
findings in pulmonary carcinogenesis by hydrazine sulfate in
newborn BALB/c/Cb/Se substrain mice. Lav. Ist. Anat. Istol.
Patol. Univ. Studio Perugia, 25: 165-171.
MILO, G.E., OLDHAM, J.W., ZIMMERMAN, R., HATCH, G.G., &
WEISBRODE, S.A. (1981) Characterization of human cells
transformed by chemical and physical carcinogens in vitro . In
vitro, 17: 719-729.
MITCHELL, P.C.H. & SCARLE, R.D. (1972) Role of reactions of
molybdenum compounds with hydrazine in nitrogen fixation.
Nature (Lond.), 240: 417-418.
NATARAJAN, A.T. & VAN KESTEREN-VAN LEEUWEN, A.C. (1981)
Mutagenic activity of 20 coded compounds in chromosome
aberrations/sister chromatid exchanges assay using Chinese
hamster ovary (CHO) cells. In: de Serres, F.J. & Ashby, J., ed.
Evaluation of short-term tests for carcinogens. Report of the
International Collaborative Programme, New York, Amsterdam,
Oxford, Elsevier/North Holland, pp. 551-559 (Progress in
Mutation Research Vol. 1).
NELSON, S.D. & GORDON, W.P. (1982) Metabolic activation of
hydrazines. Adv. exp. Med. Biol., 136B: 971-981.
NEPSS (1975) Disposal of wastewater containing hydrazine. In:
Pollution solution, California, Naval Environmental Protection
Support Service, Navy Environmental Support Office.
NODA, A., GOROMARU, T., MATSUYAMA, K., SOGABE, K., HSU, K.-Y., &
IGUCHI, S. (1978) Quantitative determination of hydrazines
derived from isoniazid in patients. I. J. Pharm. Dyn., 1: 132-
141.
NODA, A., HSU, K., NODA, H., YAMAMOTO, Y.D., & KUROZUMI, T.
(1983) Is isoniazid hepatotoxicity induced by the metabolite
hydrazine? Sangyo Ika Faigaku Zasshi, 5: 183-189.
NODA, A., NODA, H., OHNO, K., SENDO, T., MISAKA, A., KANAZAWA,
Y., ISOBE, R., & HIRATA, M. (1985a) Spin trapping of a free
radical intermediate formed during microsomal metabolism of
hydrazine. Biochem. biophys. Res. Commun., 133: 1086-1091.
NODA, A., SENDO, T., OHNO, K., GOTO, S., NODA, H., & HUS, K.-Y.
(1985b) Effects of rifampicin and phenobarbital on the fate of
isoniazid and hydrazine in vivo in rats. Toxicol. Lett., 25:
313-317.
O'BRIEN, R.D., KIRKPATRICK, M., & MILLER, P.S. (1964)
Poisoning of the rat by hydrazine and alkylhydrazines. Toxicol.
appl. Pharmacol., 6: 371-377.
PARODI, S., DE FLORA, S., CAVANNA, M., PINO, A., ROBBIANI, L.,
BENNICELLI, C., & BRAMBILLA, G. (1981) DNA-damaging activity
in vivo and bacterial mutagenicity of sixteen hydrazine
derivatives as related quantitatively to their carcinogenicity.
Cancer Res., 41: 1469-1482.
PATRICK, R.L. & BACK, K.C. (1965) Pathology and toxicology of
repeated doses of hydrazine and 1,1-dimethylhydrazine in monkeys
and rats. Ind. Med. Surg., 34: 430-435.
PERRY, P.E. & THOMSON, E.J. (1981) Evaluation of the sister
chromatid exchange method in mammalian cells as a screening
system for carcinogens. In: de Serres, F.J. & Ashby, J., ed.
Evaluation of short-term tests for carcinogens. Report of the
International Collaborative Programme, New York, Amsterdam,
Oxford, Elsevier/North Holland, pp. 560-569 (Progress in
Mutation Research Vol. 1).
PERRY, T.L., KISH, S.J., HANSEN, S., WRIGHT, J.M., WALL, R.A.,
DUNN, W.L., & BELLWARD, G.D. (1981) Elevation of brain GABA
content by chronic low-dosage administration of hydrazine, a
metabolite of isoniazid. J. Neurochem., 37: 32-39.
PITTS, J.N., TUAZON, E.C., CARTER, W.P., WINER, A.M., HARRIS,
G.W., ATKINSON, R., & GRAHAM, R.A. (1980) Atmospheric
chemistry of hydrazines: gas phase kinetics and mechanistic
studies, Florida, Tyndall Air Force Base, Air Force Engineering
and Services Centre, Engineering and Services Laboratory
(AFESC/ESL-TR-80-39) (AD/A093486).
PRADHAN, S.N. & ZIECHECK, L.N. (1971) Effect of hydrazine on
behaviour in rats. Toxicol. appl. Pharmacol., 18: 151-157.
PROTEAU, J.P., LIM, P., & LABAT, R. (1979) Toxicité d'un
dérivé bi-azolé, l'hydrate d'hydrazine, pour Carassius
carassius L., Rutilus rutilus L., et différents stades de
développement de Brachydanio rerio H.B. Ann. Limnol., 15: 337-
346.
PURCHASE, I.F.H., LONGSTAFF, E., ASHBY, J., STYLES, J.A.,
ANDERSON, D., LEFEVRE, P.A., & WESTWOOD, F.R. (1978) An
evaluation of 6 short-term tests for detecting organic chemical
carcinogens. Br. J. Cancer, 37: 873 - 935.
RAPHAELIAN, L.A. (1966) Hydrazine. In: Kirk, R.E. & Othmer,
D.F., ed. Encylopaedia of chemical technology, 2nd ed., New
York, John Wiley and Sons, Vol. 11, pp. 164-196.
RAY, P.D., HANSON, R.L., & LARDY, H.A. (1970) Inhibition by
hydrazine of gluconeogenesis in the rat. J. biol. Chem., 245:
690-696.
REDDY, G.M. & REDDY, T.P. (1972) Induction of mutations by
hydrazine in rice. Indian J. Genet. Plant Breed., 32: 388-391.
REID, F.J. (1965) Hydrazine poisoning. Br. med. J., 5472: 1246.
REIDENBERG, M.M., DURANT, P.J., HARRIS, R.A., DE BOCCARDO, G.,
LAHITA, R., & STENZEL, K.H. (1983) Lupus erythaematosus-like
disease due to hydrazine. Am. J. Med., 75: 365-370.
REYNOLDS, B.A. & THOMAS, A.A. (1965) A colorimetric method for
the determination of hydrazine and monomethylhydrazine in blood.
Am. Ind. Hyg. Assoc. J., 26: 527-531.
ROBERGE, A., GOSSELIN, C., & CHARBONNEAU, R. (1971) Effect of
hydrazine on urea cycle enzymes in vitro and in vivo . Biochem.
Pharmacol., 20: 2231-2238.
ROBINSON, D.E. & MITCHELL, A.D. (1981) Unscheduled DNA
synthesis response of human fibroblasts, W1-38 cells, to 20
coded chemicals. In: de Serres, F.J. & Ashby, J., ed.
Evaluation of short-term tests for carcinogens. Report of the
International Collaborative Programme, New York, Amsterdam,
Oxford, Elsevier/North Holland, pp. 517-527 (Progress in
Mutation Research Vol. 1).
ROE, F.J.C., GRANT, G.A., & MILLICAN, D.M. (1967) Carcino-
genicity of hydrazine and 1,1-dimethylhydrazine for mouse lung.
Nature (Lond.), 216: 375-376.
ROGAN, E.G., WALKER, B.A., GINGELL, R., NAGEL, D.L., & TOTH, B.
(1982) Microbial mutagenicity of selected hydrazines. Mutat.
Res., 89(4): 321-328.
ROGERS, A.M. & BACK, K.C. (1981) Comparative mutagenicity of
hydrazine and 3 methylated derivatives in L5178Y mosue lymphomas
cells. Mutat. Res., 16: 189-194.
ROHRBORN, G., PROPPING, P., & BUSELMAIER, W. (1972) Mutagenic
activity of isoniazid and hydrazine in mammalian test systems.
Mutat. Res., 16: 189-194.
ROSENKRANZ, H.S. & POIRIER, L.A. (1979) Evaluation of the
mutagenicity and DNA-modifying activity of carcinogens and non-
carcinogens in microbial systems. J. Natl Cancer Inst., 62: 873-
892.
ROTHBERG, S. & COPE, O.B. (1956) Toxicity studies on hydra-
zine, methylhydrazine, symmetrical dimethylhydrazine, unsym-
metrical dimethylhydrazine, and DMNA, Maryland, Army Chemical
Center (US Army Chemical Warfare Laboratory Report No. 2027).
SALOMONE, M.F., HEDDLE, J.A., & KATZ, M. (1981) Mutagenic
activity of 41 compounds in the in vivo micronucleus assay.
In: de Serres, F.J. & Ashby, J., ed. Evaluation of short-term
tests for carcinogens. Report of the International Collaborative
Programme, New York, Amsterdam, Oxford, Elsevier/North Holland,
pp. 686-697 (Progress in Mutation Research Vol. 1).
SAVCHENKOV, M.F. & SAMOILOVA, T.J. (1984) [Effect of hydrazine
nitrate on reproductive function of albino rats.] In:[ Problems
of limitation of environmental pollutants circulation,]Ufa, pp.
82-84 (in Russian).
SCALES, M.D.C. & TIMBRELL, J.A. (1982) Studies on hydrazine
hepatotoxicity. I. Pathological findings. J. Toxicol. environ.
Health, 10: 941-953.
SCHIESSL, H.W. (1980) Hydrazine and its derivatives. In: Kirk,
R.E. & Othmer, E.F., ed. Encyclopaedia of chemical technology,
3rd ed., New York, John Wiley and Sons, Vol. 12, pp. 734-771.
SCHILLER, C.M., WALDEN, R., & KEE, T.E. (1979) Effects of
hydrazine and its derivatives on the development of intestinal
brush border enzymes. Toxicol. appl. Pharmacol., 49: 305-311.
SCHMIDT, E.W. (1984) Hydrazine and its derivatives: preparation,
properties, and applications, New York, John Wiley and Sons.
SCHULTHEISS, E. (1959) [Hypersensitiveness to hydrazine.]
Berufsdermatosen, 7: 131-136 (in German).
SEVERI, L. & BIANCIFIORI, C. (1968) Hepatic carcinogenesis in
CBA/Cb/Se mice and Cb/Se rats by isonicotinic acid hydrazine and
hydrazine sulfate. J. Natl Cancer Inst., 41: 331-349.
SHANK, R.C. (1983) Evidence for indirect genetic damage as
methylation of DNA guanine in response to cytotoxicity. Dev.
Toxicol. environ. Sci., 11: 145-152.
SHUKLA, P.T. (1972) Analysis of mutagen specificity in
Drosphila melanogaster. Mutat. Res., 16: 363-371.
SINA, J.E., BEAN, C.L., DYSART, G.R., TAYLOR, V.I., & BRADLEY,
M.O. (1983) Evaluation of the alkaline elution/rat hepatocyte
assay as a predictor of carcinogenic/mutagenic potential.
Mutat. Res., 113: 357-391.
SINGH, H.B., LUDWIG, F.L., & JOHNSON, W.B. (1978) Tropospheric
ozone: concentrations and variabilities in clean remote
atmospheres. Atmos. Environ., 12: 2185-2191.
SLONIM, A.R. (1977) Acute toxicity of selected hydrazines to
the common guppy. Water Res., 11: 889-895.
SLONIM, A.R. & GISCLARD, J.B. (1976) Hydrazine degradation in
aquatic systems. Bull. environ. Contam. Toxicol., 16: 301-309.
SMITH, E.B. & CLARK, D.A. (1972) Absorption of hydrazine
through canine skin. Toxicol. appl. Pharmacol., 21: 186-193.
SONNECK, E.B. & UMLAUF, H. (1961) [Occupationally sustained
skin damage by hydrazine.] Haut-Geschlechskr., 31: 179-184 (in
German).
SOTANIEMI, E., HIRVONEN, J., ISOMAKI, H., TAKKUNEN, J., & KAILA,
J. (1971) Hydrazine toxicity in the human. Report of a fatal
case. Am. clin. Res., 3: 30-33.
SOTOMAYER, R.E., CHAUHAN, P.S., & EHLING, U.H. (1982) Induc-
tion of unscheduled DNA synthesis in the germ cells of male mice
after treatment with hydrazine or procarbazine. Toxicology, 25:
201-211.
SPEIT, G., WICK, C., & WOLF, M. (1980) Induction of sister
chromatid exchanges by hydroxylamine, hydrazine, and isoniazid
and their inhibition by cysteine. Hum. Genet., 54: 155-158.
SPINKOVA, (1971) [Determination of small amounts of hydrazine
in isoniazid solutions.] Pharm. Acta Helv., 46: 643-648 (in
German).
SPRINGER, D.L., BRODERICK, D.J., & DOST, F.N. (1980) Effects
of hydrazine and its derivatives on ornithine decarboxylase
synthesis, activity, and inactivation. Toxicol. appl.
Pharmacol., 53: 365-372.
SPRINGER, D.L., KRIVAK, B.M., BRODERICK, D.J., REED, D.J., &
DOST, F.N. (1981) Metabolic fate of hydrazine. J. Toxicol.
environ. Health, 8: 21-29.
STEIN, E.R., CLARK, D.A., & FORTNEY, S.R. (1971) Inhibition of
glutamicoxaloacetic transaminases of rat liver by hydrazine.
Toxicol. appl. Pharmacol., 18: 274-284.
SUGGS, H.J., LUSKUS, L.J., & KILIAN, H.J. (1980) A field
procedure for sampling and analysis of low concentrations of
hydrazine in air. Am. Ind. Hyg. Assoc. J., 41: 879-883.
THOMSON, J.A. (1981) Mutagenic activity of 42 coded compounds
in the lambda induction assay. In: de Serres, F.J. & Ashby, J.,
ed. Evaluation of short-term tests for carcinogens. Report of
the International Collaborative Programme, New York, Amsterdam,
Oxford, Elsevier/North Holland, pp. 224-235 (Progress in
Mutation Research Vol. 1).
THORNELEY, R.N.F., EADY, R.R., & LOWE, D.J. (1978) Biological
nitrogen fixation by way of an enzyme-bound dinitrogen-hydride
intermediate. Nature (Lond.), 272: 557-558.
TIMBRELL, J.A. & HARLAND, S.J. (1979) Identification and
quantitation of hydrazine in the urine of patients treated with
hydralazine. Clin. Pharmacol. Ther., 26: 81-88.
TIMBRELL, J.A., WRIGHT, J.M., & SMITH, C.M. (1977)
Determination of hydrazine metabolites of isoniazid in human
urine by gas chromatography. J. Chromatogr., 138: 165-172.
TIMBRELL, J.A., SCALES, M.D., & STREETER, A.J. (1982) Studies
on hydrazine hepatotoxicity. II. Biochemical findings. J.
Toxicol. environ. Health, 10: 955-968.
TOMLINSON, T.G., BOON, A.G., & TROTMAN, C.N.A. (1966)
Inhibition of nitrification in the activated sludge process of
sewage disposal. J. appl. Bacteriol., 29: 266-291.
TOTH, B. (1969) Lung tumour induction and inhibition of breast
adenocarcinomas by hydrazine sulfate in mice. J. Natl Cancer
Inst., 42: 469-475.
TOTH, B. (1972a) Hydrazine, methylhydrazine, and methylhydra-
zine sulfate carcinogenesis in Swiss mice. Failure of ammonium
hydroxide to interfere in the development of tumours. Int. J.
Cancer, 9: 109-118.
TOTH, B. (1972b) Tumourigenesis studies with 1,2-dimethyl-
hydrazine dihydrochloride, hydrazine sulfate, and isonicotinic
acid in golden hamsters. Cancer Res., 32: 804-807.
TROUT, D.L. (1965) Effects of hydrazine on plasma-free fatty
acid transport. Biochem. Pharmacol., 14: 813-821.
TROUT, D.L. (1966) Effects of hydrazine on fat transport as
affected by blood glucose concentration. J. Pharmacol. exp.
Ther., 152: 529-534.
TSUCHIMOTO, T. & MATTER, B.E. (1981) Activity of coded
compounds in the micronucleus test. In: de Serres, F.J. & Ashby,
J., ed. Evaluation of short-term tests for carcinogens. Report
of the International Collaborative Programme, New York,
Amsterdam, Oxford, Elsevier/North Holland, pp. 705-711 (Progress
in Mutation Research Vol. 1).
TUAZON, E.C., CARTER, W.P.L., BROWN, R.V., ATKINSON, R., WINER,
A.M., & PITTS, J.N. (1982) Atmospheric reaction mechanisms of
amine fuels, Florida, Tyndall Air Force Base, Air Force
Engineering and Service Center, Engineering and Services
Laboratory (AFESC/ESL-TR-82-17) (AD/A118267).
US FDA (1979) Hydrazine: proposed removal from food additive
use. Fed. Reg., 44: 33693-33695.
US NIOSH (1977a) Manual of analytical methods, 2nd ed.,
Cincinnati, Ohio, US National Institute of Occupational Safety
and Health, Vol. 1, pp. S237-1 to S237-6 (US DHEW Publication
No. 77-157A).
US NIOSH (1977b) Manual of analytical methods, 2nd ed.,
Cincinnati, Ohio, US National Institute of Occupational Safety
and Health, Vol. 3, pp. 248-1 to 248-6 (US DHEW Publication No.
77-157A).
US NIOSH (1978) Criteria for a recommended standard:
occupational exposure to hydrazines, Cincinnati, Ohio, US
National Institute for Occupational Safety and Health, Vol. 3,
pp. 248-1 to 248-6 (US DHEW Publication No. 78-172).
US NIOSH (1986) Industrial hygiene study: extent of exposure
to hydrazine, Cincinnati, Ohio, US National Institute for
Occupational Safety and Health, 49 pp (Report No. 136.5;
Contract No. 200-82-2521).
VAN KETEL, W.G. (1964) Contact dermatitis from a hydrazine
derivative in a stain remover. Cross-sensitization to apresoline
and isoniazid. Acta dermatovenereol., 44: 49-53.
VAN STEE, E.W. (1965) Acute effects of exposure to hydrazine
and hydrazine derivatives on renal function in the dog. Aerosp.
Med., 36: 764-767.
VASUDEVA, M. & VASHISHAT, R.K. (1985) Mutagenic and
recombinogenic activity of hydrazine sulfate in Saccharomyces
cerevisiae. Mutat. Res., 155: 113-115.
VELTE, J.S. (1984) Acute toxicity of hydrazine hydrate to the
fathead minnow (Pimephales promelas) and Daphnid (Daphnia
pulex). Bull. environ. Contam. Toxicol., 33: 598-604.
VERNOT, E.H., MACEWEN, J.D., BRUNER, R.H., HAUN, C.C., KINKEAD,
E.R., PRENTICE, D.E., HALL, A., III, SCHMIDT, R.E., EASON, R.L.,
HUBBARD, G.B., & YOUNG, J.T. (1985) Long-term inhalation
toxicity of hydrazine. Fundam. appl. Toxicol., 5: 1050-1064.
VIJAYKUMAR, N.K. & JAIN, H.K. (1979) Comparative studies on
induced mutations with physical and chemical mutagens in
Drosophila melanogaster. Part I. Specificity and spectrum of
mutations. Indian J. exp. Biol., 17: 61-65.
VISHNOI, A.K. & GUPTA, P.K. (1980) Induced mutagenesis in
Vicia faba L. I. Chlorophyll mutations induced by gamma rays,
ethyl methane sulfonate, and hydrazine. Cytobios, 27: 81-87.
VON KEILIG, W. & SPEER, U. (1983) [Occupational allergic
contact dermatitis from hydrazine.] Derm. Beruf Umwelt, 31: 25-
27 (in German).
VON KRULIK, R. (1966) [The influence of hydrazine and
isonicotinic acid hydrazine on certain substances of the sugar
metabolism in vitro. ] Arzneim. Forsch., 16: 1623-1626 (in
German).
VON PEVNY, I. & PETER, G. (1983) [Allergic contact eczema to
derivatives of pyridine and hydrazine.] Dermatosen, 31: 78-83
(in German).
VON WRIGHT, A. & TIKKANEN, L. (1980) The comparative
mutagenicities of hydrazine and its mono- and dimethyl
derivatives in bacterial test systems. Mutat. Res., 78: 17-23.
WAKABAYASHI, T., HORIUCHI, M., SAKAGUCHI, M., ONDA, H., &
MISAWA, K. (1983) Induction of megamitochondria in the mouse
and rat livers by hydrazine. Exp. mol. Pathol., 39: 139-153.
WALD, N., BOREHAM, J., DOLL, R., & BONSALL, J. (1984)
Occupational exposure to hydrazine and subsequent risk of
cancer. Br. J. ind. Med., 41: 31-34.
WARGOVICH, M.J., GOLDBERG, M.T., NEWMARK, H.L., & BRUCE, W.R.
(1983) Nuclear aberrations as a short-term test for geno-
toxicity to the colon: evaluation of 19 agents in mice. J. Natl
Cancer Inst., 71: 133-137.
WARREN, D., CORNELIUS, C., & FORD, B. (1984) Liver function
studies on rhesus monkeys (Maccaca mulatta) following the
administration of hydrazine sulfate. Vet. Human Toxicol., 26:
295-299.
WATJE, W.F. (1978) Potential of a hydrazine-type fuel spill or
emission during movement from supplies to user. In: Proceedings
of the Conference on Environmental Chemistry: Hydrazine Fuels, 1
977, Florida, Tyndall Air Force Base, pp. 19-24 (CEEDO-TR-78-
14).
WELLS, H.G. (1908) The pathological anatomy of hydrazine
poisoning. J. exp. Med., 10: 457-462.
WHEELER, C.E., PENN, S.R., CAWLEY, E.P., & HILL, C. (1965)
Dermatitis from hydrazine hydrobromide solder flux. Arch.
Dermatol., 91: 235-239.
WITKIN, L.B. (1956) Acute toxicity of hydrazine and some of
its methylated derivatives. Arch. ind. Health, 13: 34-36.
WONG, E.T. (1966) Renal functional response to hydrazine and
1,1-dimethylhydrazine. Toxicol. appl. Pharmacol., 8: 51-56.
WOOD, J.D., RUSSELL, M.P., & KURYLO, E. (1980) The gamma-
aminobutyrate content of nerve endings (synaptosomes) in mice
after the intramuscular injection of gamma-aminobutyrate-
elevating agents: a possible role in anticonvulsant activity.
J. Neurochem., 35: 125-130.
WRIGHT, J.M. & TIMBRELL, J.A. (1978) Factors affecting the
metabolism of 14C-acetylhydrazine in rats. Drug Metab. Dispos.,
6: 561-566.
YAKSCTAT, B.Y. (1969) Experimental assessment of hydrazine
hydrate maximum allowable concentration in water bodies. In:
Industrial pollution of water bodies, Moscow, Medicine, pp.
186-200.
YOSHIDA, T. & ALEXANDER, M. (1964) Hydroxylamine formation by
Nitrosomonas europaea. Can. J. Microbiol., 10: 923-926.
ZINA, G. & BONU, G. (1962) [Occupational dermatitis from
hydrazine and derivatives.] Minerva Dermatol., 37: 197-200 (in
Italian).
Approximately 25% of the hydrazine dose remains unaccounted
for. Ammonia was found in the blood of dogs without
significantly elevated blood-urea nitrogen (Floyd, 1980).
Springer et al. (1981) did not find labelled ammonia in the
urine of rats exposed to 15N-hydrazine (Table 4). Therefore,
the ammonia in dogs was probably not derived from hydrazine
(section 8.3.2), but was the result of an effect on metabolic
pathways (Floyd, 1980; Springer et al., 1981).
6.3. Reaction With Body Components
No adduct formation between hydrazine and DNA in vivo has
been reported (Shank, 1983). Under non-physiological conditions,
hydrazine can react with pyrimidine bases. These reactions were
reviewed by Kimball (1977). Indirect methylation of guanines in
DNA following hydrazine exposure has been demonstrated and will
be discussed in section 8.5.1.
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
7.1. Aquatic Organisms
A summary of acute toxicity data is presented in table 5. It
should be realized that the rate of decay of hydrazine in the
aquatic environment depends on the conditions (section 4.2).
When the concentration of hydrazine is not monitored during
exposure, it should be noted that the toxic effects observed
must have occurred at concentrations lower than the nominal
ones, due to degradation of the compound. the increased toxicity
of hydrazine for guppies in soft water at a pH just below 7
compared with the toxicity in hard water at a pH of
approximately 8, as found by Slonim (1977), is at least partly
explained by the increased persistence of hydrazine in soft and
non-alkaline water. Taking into account the decay of hydrazine,
increases in water temperature were found to enhance the
toxicity of the compound for bluegills (hunt et al., 1981).
Teratogenicity and toxicity screening were reported using
the South African clawed toad (Greenhouse, 1976a,b), fathead
minnow (Henderson et al., 1981), and rainbow trout (Henderson et
al., 1983). Eggs of the South African clawed toad in the
cleavage were exposed to hydrazine until hatching. Survival and
development into normal larvae occurred at exposures below 10
mg/litre. At 10 mg/litre, 35% of the embryos were malformed at
hatching. The effect was dose-related. Additional studies
revealed that teratogenic effects appeared during neurulation
(Greenhouse, 1976a). When larvae of the South African clawed
toad were exposed to 1.0 mg hydrazine/litre water, for 120 h,
all died in 24 - 48 h following exposure. No significant effects
on survival and development were observed after exposure to 0.1
mg/litre, the next lower concentration tested (Greenhouse,
1976b).
Eggs of fathead minnows at the mid-cleavage stage were
exposed to hydrazine for 24 or 48 h. Embryos, exposed for 24 h,
to 0.1 mg/litre, showed several defects, such as slightly or
moderately subnormal heart beat, haemoglobin levels, body
movement amount of eye pigment. From 1 mg/litre upwards, the
responses were generally stronger; in addition, body pigment was
absent and developmental arrest was observed. Embryos exposed to
a hydrazine concentration of 1.0 mg/litre for 48 h appeared to
have little chance of survival. Surviving embryos showed severe
deformities and larvae exhibited reduced growth (Henderson et
al., 1981).
Table 5. Acute aquatic toxicity of hydrazine
---------------------------------------------------------------------------------------------------------
Organism Tempera- pH Hardness Flow/ Parameter Concentration Reference
ture (mg CaCO3 stata (mg/litre)
(°C) /litre)
---------------------------------------------------------------------------------------------------------
Bacteria
Pseudomonas putida 20 stat 16-h TT 0.019 Bringmann &
Kühn
(1980)b
Protozoa
Entosiphon sulcatum 25 6.9 stat 72-h TT 0.93 Bringmann &
Kühn (1981)b
Uronema paraduczi 25 6.9 stat 22-h TT 0.24 Bringmann &
Kühn (1981)b
Chilomenas paramecium 20 6.9 stat 48-h TT 0.002 Bringmann &
Kühn (1981)b
Algae
Green algae
(Chlorella pyrenoidosa) 23 6.8 75 stat 48-h EC50 ca.10c Heck et al.
48-h EC100 ca.100c (1963)d
Crustacea
Water flea 20 8.0 stat 24-h EC50 2.3c Bringmann &
(Daphnia pulex) Kühn (1982)
8.2 stat 24-h LC50 1.16c Heck et al.
(1963)
20 7.1-7.2 stat 24-h LC50 0.51 and 1.01 Velte (1984)e
48-h LC50 0.16 and 0.19
Amphibia
South African clawed
toad (Xenopus laevis)
eggs 8.2-8.7 stat LOEL 10c Greenhouse
(1976a)f
larvae stat 120-h LOEL 1.0c Greenhouse
120-h NOEL 0.1c (1976b)g
Table 5. (contd.)
---------------------------------------------------------------------------------------------------------
Organism Tempera- pH Hardness Flow/ Parameter Concentration Reference
ture (mg CaCO3 stata (mg/litre)
(°C) /litre)
---------------------------------------------------------------------------------------------------------
Fish (fresh-water)
Guppy (Lebistes reti- 22-24 7.8-8.2 400-500 stat 96-h LC50 3.85c Slonim (1977)
culatus) 22-24 6.3-6.9 20-25 stat 96-h LC50 0.61c
Fathead minnow
(Pimephales promelas)
eggs 21 7.0-7.5 150 flow 48-h LOEL 0.1 Henderson et
48-h NOEL 0.001 al. (1981)h
adults 20 192 stat 96-h LC50 4.5c Cowen et al.
(1981)
adults 20 6.9 flow 96-h LC50 5.98 Velte (1984)e
Bluegill sunfish 23-24 7.2-8.4 240-292 stat 96-h LC50 1.08 Fisher et al.
(Lepomis macrochirus) (1980)
23-24 7.8-7.9 164 flow 96-h LOEL 0.43 Fisher et al.
(1980)i
23-24 7.1-7.9 239 stat 96-h LOEL 0.1 Fisher et al.
(1980)i
10 6.7-8.0 160-190 flow 96-h LC50 1.6 Hunt et al.
15.5 1.0 (1981)
21 1.2
Goldfish (Carassius 8.2-8.5 stat 48-h LC50 2.8c Heck et al.
auratus) (1963)j
19 8.1-8.5 135 stat 24-h LC50 0.95 Proteau et al.
(1979)
Roach (Rutilus 19 8.1-8.5 135 stat 24-h LC50 0.54 Proteau et al.
rutilus) (1979)
Zebra fish
(Brachydanio rerio)
5-day-old 26 7.8 110 stat 24-h LC50 0.75 Proteau et al.
(1979)
3-month-old 20 7.6-8.2 110 stat 24-h LC50 2.03 Proteau et al.
(1979)
Table 5. (contd.)
---------------------------------------------------------------------------------------------------------
Organism Tempera- pH Hardness Flow/ Parameter Concentration Reference
ture (mg CaCO3 stata (mg/litre)
(°C) /litre)
---------------------------------------------------------------------------------------------------------
Green sunfish (Leptomis 8.2-8.5 stat 48-h LC50 5.1c Heck et al.
(1963)j
Large mouth bass 8.2-8.5 stat 48-h LC50 3.6c Heck et al.
(1963)j
Channel catfish 8.2-8.5 stat 48-h LC50 1.6c Heck et al.
(1963)j
Fish (marine species)
Stickle back (Gaster- 14- 7.6-8.0 stat 96-h LC50 3.4c Harrah
osteus aculeatus) 15.5 (1978)k
---------------------------------------------------------------------------------------------------------
a Flow-through or static test.
b TT = toxicity threshold.
c No analysis for hydrazine during exposure was reported.
d EC50 and EC100 for 50% and 100% growth inhibition, measured by reading optical density.
e Soft water.
f LOEL = lowest-observed-adverse-effect level for teratogenicity. Exposure of eggs in cleavage stage
until hatching.
g LOEL = lowest-observed-adverse-effect level for lethality. NOEL = no-observed-effect level
for lethality and development.
h LOEL and NOEL = lowest-and-no observed-adverse-effect level for toxicity and teratogenicity.
i LOEL = lowest-observed-adverse-effect level for dorsal light response (at a non-lethal concentration).
j Standard reference water.
k Salinity, 1.8%.
Henderson et al. (1983) also exposed eggs of rainbow trout
(Salmo gairdneri) for 48 h, to hydrazine in continuous-flow
tests at 11.5 - 12 °C, a pH of 7 - 7.5, and a water hardness of
15 mg calcium carbonate/litre. During exposures up to 5 mg/litre
a dose-related increase was observed in the incidence of poorly
fitting jaws, pronounced mouth gape, and absence of body
movement. However, no effects were observed on mortality, heart
beat, hatching rate, or hatching period. Reduced growth and
abnormal development of larvae were observed at 1 and 5
mg/litre. Poor muscular development and poor bone growth were
observed; the authors postulate that this is a result of calcium
binding by hydrazine.
7.2. Microorganisms
The toxicity of hydrazine for a number of species of
bacteria, algae, and protozoa was measured by Bringmann (1975) &
Bringmann & Kuhn (1980, 1981). Some very low toxicity thresholds
were reported, for example, 0.005 mg/litre for a 7-day exposure
for the algae Scenedesmus quadricauda and 0.00008 mg/litre for a
10-day exposure for the blue algae Microcystis aerogenosa. This
is a very sensitive test.
London et al. (1983) described the toxicity of hydrazine for
the soil heterotroph Enterobacter cloacea. Hydrazine caused a
concentration-dependent increase in the lag time of this
organism. In a medium containing 10 mg/litre, this did not
affect the growth rate and final growth yield after the lag
period. At 100 mg/litre, the bacteria were not viable.
Although relatively high concentrations of hydrazine in
water have been recorded as inhibiting, either completely or
partially, the activities of Nitrosomonas, Nitrobacter, and
other bacteria in culture media (Yoshida & Alexander, 1964) in
waste-water treatment (Tomlinson et al., 1966; Farmwald &
MacNaughton, 1981; Kane & Williamson, 1983), the highest
continuously maintained tolerable level in waste water is of the
order of 1 mg/litre, as stated in section 4.3 (Farmwald &
MacNaughton, 1981).
7.3. Plants
Heck et al. (1963) studied the effects of hydrazine on the
germination of seeds and seedling growth after application as a
hydroponic culture contaminant and an air fumigant.
Seeds of summer brush squash (Cucurbita pepo), peanut
(Arachis hypogaea), and corn (Zea mays) were soaked for 48 h in
water containing hydrazine at levels of between 0 and 1000
mg/litre. The temperature was 30 °C. At the highest concentra-
tion, germination of peanut and corn seed was inhibited. Seed-
ling growth was inhibited from 10 mg/litre for squash, 100
mg/litre for corn, and 1000 mg/litre for peanut.
Sixteen-day-old seedlings of cotton (Gossypium hirsutum) in
a hydroponic culture were exposed to hydrazine in the growth
medium for 9 days at concentrations of between 0 and 1000
mg/litre and a temperature of between 22 and 29 °C. Plants died
within 48 h of exposure to 300 mg/litre and within 30 h at the
higher concentrations. Injury was first noted as foliar
dehydration, without chlorosis or necrosis, after 9 days of
exposure to 50 mg/litre or within 24 h of exposure to 300
mg/litre or more.
Several plants were also exposed for 4 h to hydrazine vapour
at concentrations of between 0 and 100 mg/m3 air. Species tested
were soybean (Glycine max), cow pea (Vigna sinensis), pinto bean
(Phaseolus vulgaris), cotton (Gossypium hirsutum), endive
(Cichorium endivia), alfalfa (Medicago sativa), and squash
(Cucurbita pepo). Wilting of leaves in all species was
observed within 2 - 24 h of exposure to 30 mg/m3, followed by
wilting of the whole plant. Death occurred in pinto beans and
endive plants at this exposure level. At higher concentrations,
plants of soybean and alfalfa also died. Six days after
exposure, all surviving plants started to recover.
8. EFFECTS ON EXPERIMENTAL ANIMALS
In section 8, all doses have been expressed in terms of the
free base; however, the form of hydrazine used in each study has
been indicated when possible.
8.1. Single Exposures
The toxicology of hydrazine has been reviewed by Krop
(1954), Clark et al. (1968), and US NIOSH (1978).
LD50 values for rats and mice after oral, iv, and ip expo-
sure were not significantly dependent on the route of exposure
and ranged from 55 to 64 mg/kg body weight for rats and from 57
to 82 mg/kg body weight for mice (free base or hydrazine
hydrate) (Witkin, 1956; O'Brien, 1964; Yaksctat, 1969; Azar et
al., 1970). Oral LD50 values for hydrazine (hydrazine hydrate)
in guinea-pigs and rabbits were 26 and 35 mg/kg body weight,
respectively (Yaksctat, 1969). Dogs and rabbits appeared more
sensitive, LD50 values following iv injection being 25 and 26
mg/kg body weight, respectively. The dermal LD50 for the rabbit
was 93 mg hydrazine (free base)/kg body weight (Rothberg & Cope,
1956; Witkin, 1956). When doses between 96 and 481 mg/kg body
weight (free base) were applied to the skin of dogs, 10 out of
25 animals died within the 6-h observation period; a dose-effect
relationship was not observed (Smith & Clark, 1972). When rats
and mice inhaled hydrazine (free base) for 4 h, the LC50s were
750 and 330 mg/m3, respectively (Jacobson et al., 1955). Death
occurred quickly in both species. Lethal doses of hydrazine
usually induced convulsions, excitement or inactivity, and other
effects on the central nervous system. Rats and mice inhaling
lethal concentrations of hydrazine (free base) also showed
dyspnoea (Comstock et al., 1954; Jacobson et al., 1955; O'Brien,
1964). Spontaneous motor activity depression was noted in rats
at ip doses of 39 and 52 mg/kg body weight (hydrazine sulfate)
(Pradhan & Ziecheck, 1971). Dogs receiving a sublethal iv dose
(free base) did not exhibit convulsions but showed increased
neuromuscular activity, salivation, diarrhoea, vomiting, and
hyperventilation (Wong, 1966).
Few pathological changes have been reported following acute
lethal doses. Some rats that died after inhaling hydrazine (free
base), showed lung oedema with localized damage to the bronchial
mucosa (Comstock et al., 1954). Wells (1908) observed fatty
changes in the liver in 24 h following sublethal doses in many
species. Fatty changes have also been observed in the kidneys of
rats (free base or hydrazine hydrate) (Dominguez et al., 1962;
Scales & Timbrell, 1982). In rats, accumulation of lipid,
swelling of mitochondria, and an increased number of microbodies
were observed in the liver and in the proximal tubules of the
kidneys, 24 h after an ip dose of 20 or 30 mg/kg body weight
(hydrazine hydrate). Similar changes were observed within 1 h,
after doses of 40 or 60 mg/kg body weight (Scales & Timbrell,
1982). In addition, nuclear and nucleolar enlargement and
hypertrophy of the smooth endoplasmic reticulum were observed in
the liver of rats, 2 or more hours after a single
intraperitoneal dose of 64 mg/kg body weight (hydrazine sulfate)
(Ganote & Rosenthal, 1968). Studies on the mechanisms by which
hydrazine causes these effects will be discussed later (section
8.3) together with other effects on the intermediary metabolism,
notably hypoglycaemia and lipid peroxidation, and effects on the
central nervous system, such as an increase in gamma-
aminobutyrate levels in the brain.
In dogs given a sublethal dose of hydrazine (free base),
degeneration of the proximal convoluted tubules of the kidneys
was accompanied by decreased creatinine clearance and increased
glucose reabsorption by the tubules. The glomerular filtration
rate was decreased because of decreased renal blood flow (Van
Stee, 1965; Wong, 1966).
In rhesus monkeys treated intravenously with 2.5 - 9.8 mg
hydrazine (hydrazine sulfate)/kg, liver function tests were
generally within normal limits up to 72 h after dosing. A dose
of 80 mg/kg caused fatty liver, but no necrosis (Warren et al.,
1984).
8.2. Short-Term Exposures
8.2.1. Inhalation exposure
In a 6-month inhalation study, groups of 50 male Sprague
Dawley rats, 40 female ICR mice, 8 male Beagle dogs, and 4
female rhesus monkeys were exposed to 0.26 or 1.3 mg hydrazine
(free base)/m3 air, continuously, or 1.3 or 6.5 mg hydrazine/m3
air, for 6 h/day, 5 days/week. Exposure concentrations were
monitored. Control groups contained the same number of animals.
The exposure regimen was chosen in such a way that the weekly
doses received by the continuously exposed groups were approxi-
mately equal to the weekly doses of hydrazine received by the
intermittently exposed groups. An increased mortality rate was
only seen in mice at the 2 higher exposure levels. In rats,
there was a dose-related decrease in body weight gain, while
body weights of dogs were decreased at the 2 higher exposure
levels. In dogs, the reduced weights were at least partly due to
reduced food consumption. Weights of the exposed monkeys were
comparable with those of controls. Organ weights were unaffected
by the exposure in rats, dogs, and monkeys. Organ and body
weights of mice were not recorded. Central nervous system
effects observed included lethargy in mice at the 2 higher
exposure levels, and tonic convulsions in 1 dog exposed
continuously to 1.3 mg/m3. Monkeys exhibited slight eye irrita-
tion at the 2 higher exposure levels. Fatty changes of the liver
were observed in mice at all exposures and in dogs at the 2
higher exposure levels. The livers of exposed monkeys showed
slight-to-moderate fat accumulation. However, this was also
seen, to some extent, in control animals. Livers of rats were
normal. Finally, dogs exhibited reduced red blood cell counts,
haematocrit, and haemoglobin values at the 2 higher exposure
levels, together with an increased resistance to osmotic haemo-
lysis at all exposure levels. Haematological variables were
normal in rats and monkeys and were not measured in mice. In
dogs, the effects on the liver and the haematological variables
appeared reversible (Haun & Kinkead, 1973).
Decreases in red blood cell count and haematocrit were also
observed in 20 female Swiss mice exposed to 130 mg hydrazine
(free base)/m3 air for 1 h/day, 6 days/week, for 4 weeks. In
this study, a decreased osmotic resistance to haemolysis was
noted in exposed mice (Cier et al., 1967).
Groups of 10 - 30 male Wistar rats were exposed to hydrazine
(free base) at average concentrations of 6, 18, 26, 70, or 295
mg/m3 air, for 5 days/week, 6 h/day, over periods ranging from 5
to 40 days at the 3 highest exposure levels to approximately 6
months at the 2 lowest exposure levels. The control group
consisted of 10 rats. Increased mortality was observed at all
exposure levels but not in controls, and body weights were
decreased at the 3 highest exposure levels. Rats became sluggish
during the 6-month exposure, while at the 3 highest exposure
levels, an initial restlessness was followed by a tendency to
sleep. In some cases, pathological examination revealed lung
oedema with local damage to the bronchial mucosa at the 3
highest exposure levels. Fatty livers were observed in many rats
after 5 days of exposure at 295 mg/m3 (Comstock et al., 1954).
8.2.2. Other routes of exposure
Groups of 25 male Sprague Dawley rats were dosed ip with 10
or 20 mg hydrazine (free base)/kg body weight, 5 times per week,
for 5 weeks. The control group consisted of 15 rats. Mortality
was increased at the dose of 20 mg/kg body weight; 10/25 rats
died after 8 - 21 doses. Body weight was lost in a dose-related
manner; 4.4 and 25.7% of the initial body weight was lost in 10
days in the 10 and 25 mg/kg groups, respectively. At 20 mg/kg
body weight, rats also displayed weakness and lethargy, and 2
rats exhibited convulsions. Pathological examination revealed
hyperaemia and oedema in the lungs of 4 rats and slight fatty
vacuolation in the liver of 7 rats at 20 mg/kg body weight. At
both doses, the haematocrit values were maximally decreased
after 13 injections (Patrick & Back, 1965).
Patrick & Back (1965) treated 12 rhesus monkeys ip with
hydrazine (free base), 5 times per week. Six monkeys received 5
mg/kg body weight for 4 weeks; two of these monkeys received a
further 8 doses of 10 mg/kg body weight, followed by 4 or 5
doses of 20 mg/kg body weight. A group of 6 monkeys received
only 4 or 5 doses of 20 mg/kg body weight. The control group
consisted of 10 monkeys. No monkey died, but all exposed monkeys
showed decreased body weights. Lethargy, weakness, and vomiting
were see in 7 of the 8 monkeys exposed to 20 mg/kg body weight,
while tremors were seen in 1 of these monkeys. Fatty changes
were observed in the liver, proximal tubules of the kidneys,
heart, and skeletal muscles at 20 mg/kg body weight, and
occasionally at 5 mg/kg body weight. Extensive periportal
necrosis was found in the liver of one of the dosed monkeys. The
level of bilirubin was increased and the serum was icteric.
Haematocrit and haemoglobin values, measured at the lower dose
only, dropped slightly, relative to control values (Patrick &
Back, 1965).
The pathological effects on the liver were also investigated
microscopically, in groups of 20 - 29 male DDY mice and 10 male
Wistar rats, after administration of 5, 10, or 20 mg hydrazine
(free base)/kg powdered diet, for 3 - 10 days. No animals died.
Animals of both species exhibited weakness. Megamitochondria or
fatty vacuolation with moderately swollen mitochondria and focal
proliferation of the smooth endoplasmic reticulum were induced
in rats and mice at dose levels of 10 and 20 mg/kg feed. The
induction of megamitochondria was a reversible process (Waka-
bayashi et al., 1983). Noda et al. (1983) observed centrilobular
hepatic necrosis in male rabbits dosed for 5 days with between
14.6 and 32.3 mg hydrazine (hydrazine monohydro-chloride)/kg
body weight per day, iv.
In other studies, hydrazine (hydrazine hydrate) was given
orally in drinking-water to albino rats and guinea-pigs for 7
months at levels providing 0.3, 0.03, 0.003, and 0.0003 mg/kg
body weight per day. At the two highest doses, adverse effects
were observed in the CNS (changes in conditioned reflexes),
liver (increased I131 excretion, changes in enzyme activity,
protein dystrophia), and blood (symptoms of haemoloytic
anaemia). The dose of 0.003 mg/kg body weight was reported to be
the no-observed-adverse-effect level (Yaksctat, 1969).
8.3. Biochemical Effects and Mechanisms of Toxicity
All of the studies described in this section were performed
with doses considered to be toxic.
8.3.1. Effects on lipid metabolism
Hydrazine caused a dose-dependent increase in hepatic
triglyceride levels in rats, the threshold dose for a single ip
injection being 10 - 20 mg/kg body weight. The maximal effect,
an increase of 7 times the control value, was observed after a
dose of 40 or 60 mg hydrazine hydrate/kg body weight. At these
dose levels, the effect was measurable 4 h after injection
(Timbrell et al., 1982). Other authors have also reported
accumulation of triglycerides in the liver of rats exposed to
single doses of hydrazine via injection routes (Amenta &
Dominguez, 1965a; Clark et al., 1970; Lamb & Banks, 1979).
Several mechanisms have been proposed:
1. Increased mobilization of free fatty acids from
adipose tissue (particularly observed at low plasma-
glucose levels) leading to an increased uptake of free
fatty acids, followed by increased triglyceride
synthesis in the liver (Trout, 1965, 1966; Clark et
al., 1970). This mobilization of free fatty acids
might be caused by the effects of hydrazine on the
sympathetic nervous system and on levels of adrenal
steroid hormone, possibly in response to the hypo-
glycaemia induced by hydrazine (Amenta & Dominguez,
1965a). Cooling et al. (1979) found elevated
concentrations of circulating corticosterone and
decreased concentrations of insulin in the serum of
rats exposed to hydrazine. Decreased blood-insulin
levels were also measured in rats by Aleyassine & Lee
(1971).
2. Increased synthesis of triglycerides caused by
increased enzymatic activity of phosphatidate phospho-
hydrolase (EC 3.1.3.4) in hepatocytes both in vivo and
in vitro was reported by Lamb & Banks (1979). It has
been suggested that this was a result of increased
corticosterone levels (Cooling et al., 1979). In
addition, Marshall et al. (1983) found increased fatty
acid synthesis in the liver of rats after hydrazine
administration.
3. Triglycerides could accumulate in hepatocytes as a
result of a decreased secretion of lipoproteins from
liver to plasma (Amenta & Dominguez, 1965a; Clark et
al., 1970). This could be explained by a decreased
lipid-binding capacity of lipoproteins following an
observed alteration in the proportion of phospholipids
and cholesterol (Clark et al., 1970) or by
increased lipid peroxidation (Di Luzio et al., 1973;
Kopylova et al., 1982). The protein moiety of
lipoproteins could also be subject to change (section
8.3.2).
8.3.2. Effects on carbohydrate and protein metabolism
Rats and dogs with starvation-induced depletion of glycogen
stores showed rapidly declining plasma-glucose levels with a
concomitant rise in lactate and pyruvate levels after exposure
to single intravenous hydrazine doses of 64 (free base or hydra-
zine sulfate) and 25 mg (free base)/kg body weight,
respectively. In well-fed dogs, hyperglycaemia and depletion of
glycogen stores preceded hypoglycaemia. Acidosis developed
slowly as a result of an increased lactate-pyruvate ratio
(Fortney, 1966; Fortney et al., 1967; Ray et al., 1970).
It has been postulated that hydrazine inhibits glyconeo-
genesis (Fortney, 1966; Fortney et al., 1967). This could occur
via inhibition of pyridoxal phosphate-dependent aminotrans-
ferases and decarboxylases. It has been shown that hydrazine
interferes with pyridoxal phosphate synthesis in vitro
(McCormick & Snell, 1961) and in vivo (Chatterjee & Sengupta,
1980) (section 8.3.5). Inhibition of transaminases would also
explain the increase in free amino acids observed in the plasma,
liver, brain, and muscle of rats (Cornish & Wilson, 1968; Banks,
1970) and in the plasma and urine of dogs (Korty & Coe, 1968).
It could further explain several observations in rats, such as
the depressed conversion of amino acids to carbon dioxide
(Amenta & Dominguez, 1965b; Dost et al., 1971), the depressed
incorporation of amino acids in plasma-glucose (Fortney et al.,
1967), and the enhanced incorporation of amino-labelled acids in
liver proteins, 24 h following hydrazine exposure (Banks, 1970).
Inhibition of protein synthesis was also observed in rat livers
up to 8.5 h after exposure (Lopez-Mendoza & Villa-Trevino,
1971).
Hydrazine treatment of rats resulted in inhibited activity
of specific aminotransferases and decarboxylases including:
liver aspartate aminotransferase (EC 2.6.1.1) (Stein et al.,
1971), brain gamma-aminobutyrate aminotransferase (EC 2.6.1.19)
and glutamate decarboxylase (EC 4.1.1.15) (Medina, 1963; Perry
et al., 1981), and liver ornithine 2-oxo-acid aminotransferase
(EC 2.6.1.13) (Roberge et al., 1971). The activity of rat liver
ornithine decarboxylase (EC 4.1.1.17) increased following
hydrazine exposure (Springer et al., 1980).
Inhibition of phosphoenolpyruvate carboxykinase (ATP) (EC
4.1.1.49), an enzyme involved in gluconeogenesis, was also
measured in vitro . Hydrazine increased the levels of citrate,
malate, and oxaloacetate in the rat liver (Ray et al., 1970).
Hydrazine affects the urea cycle. Decreased specific
activity of ornithine 2-oxo-acid aminotransferase, caused by
administration of hydrazine to rats (Roberge et al., 1971),
provoked an increase in ornithine in the liver (Banks, 1970),
brain, and plasma (Perry et al., 1981). The concentrations of
citrulline and urea in the liver, kidneys, brain, and blood were
increased, as was the activity of argininosuccinate lyase (EC
4.3.2.1) (Roberge et al., 1971).
8.3.3. Effects on mitochondrial oxidation
Swelling of hepatic mitochondria was observed in rats and
mice following hydrazine administration (Ganote & Rosenthal,
1968; Scales & Timbrell, 1982; Wakabayashi et al., 1983)
(sections 8.1 and 8.2.2). in vitro studies on the effects of
high concentrations of hydrazine on the functional status of
mitochondria showed decreased oxidation of keto-acids (Von
Krulik, 1966). Oxidative phosphorylation measured as the P/O
ratio was either not affected or decreased independently of the
hydrazine concentration (Von Krulik, 1966; Fortney et al.,
1967). Inhibition of beef heart cytochrome a by hydrazine was
also reported (Takemori et al., 1960). After administration of a
single intraperitoneal dose of hydrazine to rats, slightly
stimulated mitochondrial oxidation of succinate and glutamate
was observed with a slight increase in P/O ratio, respiration
control rate, and phosphorylation rate. ATPase (EC 3.6.1.8)
activity was not affected (Higgins & Banks, 1971). When mice
received hydrazine (free base) in the diet (10%) for 3 days and
rats received hydrazine in the diet (20%) for 8 days, oxidation
of succinate and glutamate, coupling efficiency, P/O ratio, and
the activities of ATPase (EC 3.6.1.8) and cytochrome c oxidase
(EC 1.9.3.1) were slightly decreased in liver megamitochondria,
while the activity of monoamine oxidase (EC 1.4.3.4) was
moderately decreased (Wakabayashi et al., 1983).
8.3.4. Effects on microsomal oxidation
Proliferation of the smooth endoplasmic reticulum was
observed in the liver of rats and mice following hydrazine
administration (Ganote & Rosenthal, 1968; Wakabayashi et al.,
1983) (sections 8.1 and 8.2.2). A single dose of 55 mg hydrazine
(free base)/kg body weight in rats decreased the hepatic
cytochrome P-450 content (Gorshtein & Kopylova, 1983). Rats
exposed for 4 days to 12 mg hydrazine (hydrazine sulfate)/kg
body weight per day did not show any effect on cytochrome P-450
levels in the microsomal fraction of the liver, but a slightly
decreased level of cytochrome b 5, inhibition of benzopyrene
hydroxylase (EC 1.14.14.1), and increased parahydroxylation of
aniline (Akin & Norred, 1978).
8.3.5. Effects on the central nervous system
The relationship between the effects of hydrazine on the
central nervous system, especially the occurrence of convul-
sions, and changes in levels of gamma-aminobutyric acid, an
inhibitory neurotransmitter, in the brain of rats and mice has
been investigated. An increase in the level of gamma-amino-
butyric acid was observed in the whole brain of rats after: (a)
a single intraperitoneal dose of 51 mg hydrazine (free base)/kg
body weight (Medina, 1963); (b) a single intravenous dose of 5
mg hydrazine (hydrazine sulfate)/kg body weight (Matsuyama et
al., 1983); or (c) after a daily subcutaneous dose of 2.6 mg
hydrazine (hydrazine monohydrochloride)/kg body weight over 109
days (Perry et al., 1981). In the whole brain of mice, an
increase was observed following a single intramuscular dose of
54 mg hydrazine (free base)/kg body weight (Wood et al., 1980).
The changes in the concentration of this amino acid are caused
by inhibition of pyridoxal phosphate requiring gamma-
aminobutyrate aminotransferase (EC 2.6.1.19) and glutamate
decarboxylase (EC 4.1.1.15) (Medina, 1963; Perry et al., 1981).
Hydrazine treatment of rats also caused a general amino acid
imbalance in the brain (Perry et al., 1981). A relationship was
suggested between the excitable state of the brain and the
gamma-aminobutyric acid contents of nerve endings (decreased)
rather than the whole brain contents of gamma-aminobutyric acid
(increased) (Wood et al., 1980; Geddes & Wood, 1984).
8.4. Reproduction, Embryotoxicity, and Teratogenicity
When groups of 26 Wistar rats were exposed to 0 or 8 mg
hydrazine (hydrazine monohydrochloride)/kg body weight per day,
sc, from day 11 to day 20 of gestation, the exposed dams showed
a 20% decrease in body weight and 2 dams died. Reduced number of
viable fetuses were found in 9 dams killed on day 21 of
gestation (63/172 versus 142/179 in controls), while the number
of implants per litter was not affected. The fetuses had reduced
weights and appeared pale and oedematous, but did not exhibit
any major malformations. In the rats allowed to deliver (12),
perinatal mortality was 100% in treated rats and 20% in controls
(Lee & Aleyassine, 1970).
These results agree with those of another study in which
groups of 6 - 27 Fisher 344 rats received 0, 2.5, 5, or 10 mg
hydrazine (free base)/kg body weight per day, ip, from day 6 to
day 15 of gestation. Body weight gains in dams were decreased
and the number of resorptions per dam were increased in a dose-
related manner. The differences between treated and control
animals were statistically significant at doses of 5 or 10
mg/kg, but not at 2.5 mg/kg for both variables. The numbers of
implants per dam and fetal weights were not affected at any
dose. The incidence of litters or fetuses with abnormalities was
not significantly increased at any dose; however, at 10 mg/kg,
only one out of the 6 females produced a viable litter, and only
6 fetuses were examined. In a subsequent study, 27 rats were
untreated and 11 rats were treated with 10 mg hydrazine/kg per
day, during what appeared to be the most susceptible period of
gestation (days 7 - 9). The incidence of litters or fetuses with
abnormalities in the 10 mg/kg group (6 litters out of 8; 8
fetuses out of 16) was significantly higher than that in the
control group of the preceeding study (8 litters out of 27; 11
fetuses out of 181). The abnormalities observed were mainly
supernumerary and fused ribs, delayed ossification, moderate
hydronephrosis, and moderate dilation of brain ventricles
(Keller et al., 1982).
Subtle postnatal changes were reported in the offspring of
24 female Syrian golden hamsters exposed orally to 0 or 170 mg
hydrazine (hydrazine hydrate)/kg body weight on the 12th day of
gestation. The pups of exposed dams did not exhibit cleft palate
formation, but showed effects on the development of intestinal
brush border enzymes. No other end-points were investigated
(Schiller et al., 1979).
Groups of ICR mice were treated ip with 0, 4, 12, 20, 30, or
40 mg hydrazine (free base)/kg body weight per day, from day 6
to day 9 of gestation. Some dams administered the highest dose
died. Body weights were reduced at doses of 12 mg/kg body weight
or more. While at the lower doses the number of resorptions per
litter was unchanged compared with controls, embryotoxicity was
evident at 30 and 40 mg/kg body weight. At 12 and 20 mg/kg body
weight, 17-day-old fetuses showed reduced weights, and there was
a dose-related increased incidence of litters with abnor-
malities, mainly exencephaly, hydronephrosis, and supernumerary
ribs (Lyng et al., 1980).
Savchenkov & Samoilova (1984) studied the adverse effects on
reproductive function (fertility of females, numbers of newborn,
and resorption of embryos) of female and male albino rats
exposed by gavage to hydrazine (hydrazine nitrate) at a dose of
13 mg/kg body weight, once a day, for 30 days prior to mating.
The development of the surviving litters did not differ from
that of controls.
Albino rats (10/group and 20 controls) of both sexes were
exposed to hydrazine (free base) (99.5% purity) in the drinking-
water at concentrations of 0.82, 0.018, or 0.002 mg/litre (0.016
mg/kg, 0.0014 mg/kg, or 0.00016 mg/kg, respectively, nominal
dose, assuming water consumption 20 ml per day and animal
weights of 250 g). The duration of the study was 6 months. The
number of animals studied and the scheme and time of mating were
not reported. The female rats exposed to the highest concen-
trations had fewer live embryos and more resorptions, as well as
pre- and post-implantation deaths, than the controls. No effects
were observed in animals administered 0.002 mg/litre.
Developmental abnormalities were not reported in any of the 293
embryos from all exposed animals. Destruction of gonadal
epithelium was observed in male rats after 6 months of oral
exposure to hydrazine at concentrations of 0.82 and 0.018
mg/litre (Duamin et al., 1984). In the same study, albino rats
were exposed to hydrazine (free base) at concentrations of 0.85,
0.13, or 0.01 mg/m3 (0.10 mg/kg body weight, 0.016 mg/kg, or
0.0012 mg/kg, respectively, nominal dose, assuming inhalation of
6 litres air per h and animal weights of 200 g), for 5 h per
day, 5 days/week, for 4 months. At the two highest concentra-
tions, embryotoxic effects of the same severity were seen as
when hydrazine was administered orally at the two highest doses.
No abnormalities were observed among 315 embryos. No gonado-
toxic effects occurred in male rats under the conditions of the
study (Duamin et al., 1984).
Additional information relating to reproductive end-points
is presented in section 8.5.1 (Sotomayer et al., 1982).
8.5. Mutagenicity and Related End-Points
8.5.1. DNA damage
Hydrazine was found to react with pyrimidine bases under
non-physiological conditions. These reactions were reviewed by
Kimball (1977). No hydrazine-DNA adducts have been reported to
be formed in vivo. A single oral or intraperitoneal dose of
hydrazine (free base or hydrazine sulfate) administered to rats,
mice, guinea-pigs, and hamsters resulted in the rapid formation
of N7-methylguanine and O6-methylguanine in liver DNA, which was
not detected in controls (Becker et al., 1981; Quintero-Ruiz et
al., 1981; Bosan & Shank, 1983; Shank, 1983). Methylation was
detectable at toxic doses and a sharp increase in the methyl-
ation of guanine in liver-DNA was observed at oral doses
exceeding 60 mg/kg body weight in rats and 45 mg/kg body weight
in hamsters. In both species, maximum methylation approximated
80 N7-methylations and 7 O6-methylations per 100 000 residues of
guanine, 6 h after oral exposure to 90 mg hydrazine/kg body
weight. Elimination of 7-methylguanine from DNA began about 24 h
after exposure with a half-life of 40 - 50 h. Elimination of O6-
methylguanine from DNA began about 24 h after exposure in rats
and about 50 h after exposure in hamsters, with a half-life of
13 h and 17 h, respectively (Becker et al., 1981; Bosan & Shank,
1983). The source of the methyl group was S -adenosyl-methionine
(Becker et al., 1981; Quintero-Ruiz et al., 1981).
Single-strand breaks were detected by the alkaline elution
assay in rat liver cells exposed in vitro (Sina et al., 1983),
and in the liver and lung cells of mice injected ip with 50 or
100 mg hydrazine (hydrazine hydrate)/kg body weight (Parodi et
al., 1981).
Hydrazine was reported to induce lambda phage in Escherichia
coli (Heinemann, 1971); however, no such induction was found by
Thomson (1981), nor did hydrazine induce HPlcl phage in Haemo-
philus influenzae (Balganesh & Setlow, 1984).
Repair of DNA lesions induced by hydrazine was observed in
vitro. In the International Collaborative Programme for the
Evaluation of Short-Term Tests for Carcinogenicity, 5 bacterial
DNA repair tests, using Bacillus subtilis or E. coli, were all
found to produce weak to medium positive responses. Only one
assay required rat liver microsomal fraction. The other positive
responses were reduced in magnitude by microsomal fraction
(Ashby & Kilbey, 1981). Increased unscheduled DNA synthesis was
observed in 1 out of 2 tests using human fibro-blasts (Agrelo &
Amos, 1981; Robinson & Mitchell, 1981).
No increase in unscheduled DNA synthesis was observed in the
germ cells of mice, 16 days after a 5-day exposure to doses of
hydrazine (hydrazine dihydrochloride) of up to 120 mg/kg body
weight per day (Sotomayer et al., 1982).
8.5.2. Mutation and chromosomal effects
The results of tests for gene mutations and chromosome
damage induced by hydrazine or its salts are summarized in Table
6. Hydrazine induces gene mutations and/or chromosome
aberrations in a variety of test systems including plants,
phage phi 80, bacteria, fungi, Drosophila melanogaster, and
mammalian cells in vitro . In a few cases, microsomal activation
was an absolute requirement for a positive effect (Gupta &
Goldstein, 1981; Perry & Thomson, 1981). In most cases, an
effect could be observed, both with and without microsomal
activation, with a stronger effect in some tests with activation
and in other tests without activation. These inconsistencies
were evaluated by Ashby (1981). Some negative results in the
forward mutation tests with mammalian cells invitro can be
traced back to the high locus specificity of hydrazine also
observed in plants. Duamin et al. (1984) observed increased
chromosomal aberrations in bone marrow cells (4.12 ± 0.65%
versus 2.48 ± 0.46% in controls) of albino rats (N = 10) exposed
to hydrazine (free base) at 0.85 mg/m3, 5 h per day, 5
days/week, for 4 months. No increased incidence of nuclear
aberrations, micronuclei, dominant lethals, and sperm-head
abnormalities were observed in hydrazine-treated mice (free base
or hydrazine sulfate).
Table 6. Tests for gene mutations and chromosome damage induced by hydrazine or its salts
---------------------------------------------------------------------------------------------------------
Test description System description Result Reference
Organism Species/strain/cell type
---------------------------------------------------------------------------------------------------------
Forward mutations transforming Bacillus subtilis + Freese et al. (1967)a
G DNA + Bresler et al. (1968)a
E Forward mutations plant tomato + Jain et al. (1968)b
+ Chandra Sekhar & Reddy
(1971)b
N rice + Reddy & Reddy (1972)b
barley + Kak & Kaul (1975)b
E wheat + Khamankar & Jain (1978)b
broad bean + Vishnoi & Gupta (1980)b
Reverse mutations virus phage phi 80 + Chu et al. (1973)c
M Reverse mutations bacteria Salmonella typhimurium TA 1530 + Rosenkranz & Poirier
(1979);Tosk et al.
(1979)
U
Salmonella typhimurium TA 1535 + Purchase et al. (1978);
Herbold (1978);
T Rosenkranz & Poirier
(1979); Bridges et al.
(1981);d,e;
A Parodi et al. (1981);
Rogan et al. (1982);
T Braun et al. (1984);
De Flora et al. (1984)
I Salmonella typhimurium TA 1537 - Bridges et al. (1981)d,e;
Parodi et al. (1981);
O Rogan et al. (1982)
N Salmonella typhimurium TA 1538 + Bridges et al. (1981)d,e
Purchase et al. (1978);
S Herbold (1978);
Rosenkranz & Poirier
(1979); Parodi et al.
(1981)
Salmonella typhimurium TA 100 + Purchase et al. (1978);
Herbold (1978); Bridges
et al. (1981)d,e
---------------------------------------------------------------------------------------------------------
Table 6. (contd.)
---------------------------------------------------------------------------------------------------------
Test description System description Result Reference
Organism Species/strain/cell type
---------------------------------------------------------------------------------------------------------
Reverse mutations bacteria Salmonella typhimurium TA 100 - Bridges et al. (1981)d,e;
G Parodi et al. (1981)
E Salmonella typhimurium TA 98 -, + Herbold (1978); Bridges
et al. (1981)d,e; Parodi
et al. (1981)
N + Purchase et al. (1978)
E Salmonella typhimurium G 46 + Braun et al. (1984)
Reverse mutations bacteria Salmonella typhimurium G 46 + Röhrborn et al. (1972)
(host-mediated (mouse) (NMRI)
assay)
M Reverse mutations bacteria Escherichia coli + Von Wright & Tikkanen
(1980); Bridges et al.
(1981)d,e
U
Haemophilus influenzae + Kimball & Hirsch (1975);
T Kimball (1976)
A Reverse mutations fungi Saccharomyces cerevisiae + Mehta & Von Borstel
(1981)e; Vasudeva &
Vashishat (1985)
T Forward mutations fungi Saccharomyces cerevisiae + Lemontt (1977, 1978)
Saccharomyces pombe + Loprieno (1981)e
I
Sex-linked visibles insect Drosophila melanogaster + Jain & Shukla (1972);
O Shukla (1972);
Vijaykumar & Jain (1979)
N Sex-linked lethals insect Drosophila melanogaster + Shukla (1972)
S Forward mutations hamster ovary cells in vitro + Gupta & Goldstein (1981)e
- Carver et al. (1981)e;
Hsie et al. (1981)e
mouse lymphoma cells in vitro + Rogers & Back (1981)
+ Amacher et al. (1980)
---------------------------------------------------------------------------------------------------------
Table 6. (contd.)
---------------------------------------------------------------------------------------------------------
Test description System description Result Reference
Organism Species/strain/cell type
---------------------------------------------------------------------------------------------------------
C Breaks plant horse bean primary root + Gupta & Grover (1970)
H Breaks, deletions, plant horse bean primary root + Heindorff et al. (1984)
translocations
R
Aberrationsf plant chick pea root + Farook & Nizam (1979)
O
Aberrations rat epithelial liver cells - Dean (1981)e
M in vitro
O Aberrations rat bone marrow cells in vivo + Duamin et al. (1984)
S Breaks, gaps, hamster ovary cells in vitro + Natajaran & Van Kesteren-
exchanges Van Leeuwen (1981)e
O
Sister chromatid hamster ovary cells in vitro + MacRae & Stich (1979);
Perry & Thomson (1981)e
M exchanges
- Natarajan & Van Kesteren-
Van Leeuwen (1981)e; Baker
et al. (1983)
E
lung cells in vitro + Baker et al. (1983)
V-79 cells in vitro + Speit et al. (1980)
Nuclear aberrations mouse epithelial colon cell - Wargovich et al. (1983)
(oral exposure)g
---------------------------------------------------------------------------------------------------------
Table 6. (contd.)
---------------------------------------------------------------------------------------------------------
Test description System description Result Reference
Organism Species/strain/cell type
---------------------------------------------------------------------------------------------------------
D Micronuclei (ip mouse polychromatic erythrocytes + Salomone et al. (1981)e
exposure) - Kirkhart (1981)e;
A Tsuchimoto & Matter
(1981)e
M Dominant lethals mouse germ cells - Epstein et al. (1972)
(ip exposure)
A
G
E
------------------------------------------------------------------------------------------------------------------------
a Inactivation of transforming DNA was observed, which was inhibited by catalase, EDTA, or nitrogen gas treatment.
b Hydrazine appears to be a mutagen that is highly specific for certain loci. Moreover, it not only produced mutants
in the M2 generation but also homozygous recessive mutants in the M1 generation (plants, raised from treated seeds).
c Inactivation of virus observed. Inactivation and, at lower concentrations, mutations were reduced by catalase
treatment.
d Hydrazine sulfate was tested in the International Collaborative Programme for the Evaluation of Short-Term Tests for
Carcinogenicity (de Serres & Ashby, 1981). In the summary on the assay performance of bacterial mutation assays, it
was reported that hydrazine sulfate was mutagenic in most of a total of 20 laboratories. However, there were
inconsistencies in the strains in which the effect was seen and the requirements for S9 mix. Salmonella typhimurium
TA 1535 was the strain in which mutagenic activity was most commonly observed, but it was also seen in all
laboratories that used Escherichia coli strains. In some laboratories, hydrazine sulfate was also mutagenic in
Salmonella typhimurium TA 100. Two laboratories reported a marginal mutagenic activity for strain TA 98, and TA 1538
was positive in another, while no mutagenic activity was observed with TA 1537.
e Tested in the International Collaborative Programme for the Evaluation of Short-Term Tests for Carcinogenicity. In
the final assessment, it was stated that hydrazine was a genotoxic agent in bacteria, yeast, and higher eukaryotes
in vitro . Hydrazine did not appear to be genotoxic in vivo in higher eukaryotic cells (de Serres & Ashby, 1981).
f Stickiness and clumping at metaphase, bridges and fragments at anaphase, laggards, micronuclei, and delayed
cytokinesis at telophase, tripolar spindles, prophase, and metaphase in one cell.
g Micronuclei, pyknotic nuclei, karyorrhetic nuclei, cytolysosomes.
8.5.3. Cell transformation
Hydrazine increased the transformation of baby hamster
kidney cells (Purchase et al., 1978; Daniel & Dehnel, 1981) and
human liver cells (Purchase et al., 1978). While Purchase et al.
(1978) observed an increased transformation of baby hamster
kidney cells, both with and without metabolic activation, Daniel
& Dehnel (1981) found a much lower increased transformation
frequency only with metabolic activation. Hydrazine also caused
enhancement of transformation of mouse 3T3 cells by Herpes
simplex virus in vitro (Johnson, 1982).
Hydrazine induced transformation of human fibroblasts. Transformed
cells were able to induce undifferentiated mesenchymal tumours
after sc injections in pre-irradiated nude mice, while control
cells did not induce tumours (Milo et al., 1981).
8.6. Carcinogenicity
8.6.1. Inhalation exposure
Groups of 100 Fischer 344 rats of each sex, 400 female
C57BL/6 mice, and 200 male golden Syrian hamsters were exposed
to the vapour of hydrazine (free base) for 12 months, 6 h per
day, for 5 days per week, and observed for a further 18, 15, and
12 months, respectively (Table 7). Rats were exposed at 0.06,
0.33, 1.3, or 6.5 mg/m3, mice at 0.06, 0.33, or 1.3 mg/m3, and
hamsters at 0.33, 1.3, or 6.5 mg/m3 air. Control groups
contained 150 rats of each sex, 800 female mice, and 200 male
hamsters, respectively. The hydrazine vapour was monitored
throughout the exposure period. A reduction in body weight gain,
seen in exposed rats throughout the entire study, was greatest
in males at 6.5 mg/m3. The mortality rate was not affected by
the exposures. Inflammatory changes were observed in the upper
respiratory tract of both sexes and in the uterus and oviduct,
especially at the highest exposure. At 6.5 mg/m3, the incidence
of squamous metaplasia was increased in the nose, larynx, and
trachea, while the incidence of epithelial hyperplasia was
increased in the nose and lungs. An increased incidence of
hyperplasia was also observed in the lymph nodes and uterus of
females at 6.5 mg/m3 and in the liver of females at 1.3 and 6.5
mg/m3. A dose-related increased incidence of benign epithelial
tumours of the nose, mainly adenomatous polyps, was observed in
both sexes at the 2 highest exposures, while at the highest
exposure (6.5 mg/m3), the incidence of malignant epithelial
tumours was also increased (2/98 in males and 5/95 in females).
The latency time exceeded 87 weeks in males and 97 weeks in
females. The incidence of lung tumours was not significantly
increased, but, in males, 3 bronchial adenomas and, in one
female, 1 bronchial adenoma, were observed at 6.5 mg/m3 compared
with none in the controls. The total incidence of adenomas and
adenocarcinomas of the thyroid in males was not increased, but
hydrazine exposure at 6.5 mg/m3 increased the fraction of
malignant tumours.
Table 7. Tumour incidence in mice, rats, and hamsters following hydrazine exposure
---------------------------------------------------------------------------------------------------------
Route Species/ Exposure Daily dose Tumour incidenceb Reference
strain period (mg/kg Nose or lung liver
(months) body weight)a Male Female Male Female
(life-time
observation)
---------------------------------------------------------------------------------------------------------
Inhalation mouse 12 0 8/385 - Vernot et al. (1985)
(C57BL/6) 4/378
0.34c 12/379 -
rat 12 0 0/146 0/145 - - Vernot et al. (1985)
(Fischer 0.16c 11/97 4/94 - -
344) 0.8d 75/98 38/95 - -
hamster 12 0 1/181 - Vernot et al. (1985)
(golden 0.93d 16/160 -
Syrian)
Oral mouse 6 - 9 0 1/37 4/47 3/30 1/29 Biancifiori (1969,
(gavage) (CBA) 0.98 2/26 10/25 1/26 0/25 1970a)
2.0 4/25 16/25 7/25 2/25
3.9 5/25 21/24 12/25 16/24
8.0 16/21 19/21 15/25 15/24
rat 17 0 0/28 0/22 0/19 0/14 Severi & Biancifiori
(Cb/Se) 14.6/9.7 3/14 5/18 4/13 0/18 (1968)
Oral mouse 24 - 28 0 11/110 14/110 2/110 3/110 Toth (1969, 1972a)e
(drinking- (Swiss) 2.0/1.6 24/50 27/50 2/50 1/50
water) 5.1/4.6 25/50 24/50 3/50 1/50
---------------------------------------------------------------------------------------------------------
a Calculated doses based on a weight of 300 g and a minute volume of 100 ml for rat, a weight of 35 g
and a minute volume of 25 ml for mice, and a weight of 140 g and a minute volume of 54 ml for hamsters.
b - = not found; open space = not tested (nose in inhalation studies with rats and hamsters, lung in
the other studies.
c 1.3 mg/m3, 6 h/day, for 5 days/week.
d 6.5 mg/m3, 6 h/day, for 5 days/week.
e Liver tumours were hepatomas (1/50 in males at both exposure levels) and angiomas.
A slightly increased mortality rate seen in mice right up to
the end of the study was not related to dose. Body weights were
not affected during exposure, but all exposed groups gained less
weight than controls during the post-exposure period. The
incidence of non-neoplastic lesions was similar in treated and
control groups. The only neoplastic change observed was a
slightly increased incidence of lung adenomas at the highest
exposure. A background incidence of 2 - 3% for pulmonary adenoma
in C57BL/6 mice was reported to be common in the laboratory
concerned.
Almost double the number of deaths occurred during exposure
in each exposed group of hamsters compared with the control
group, but the final mortality rates were similar in all groups,
including the control group. During exposure to 6.5 mg/m3 and
for 10 months after exposure, body weights were depressed. For
several months, an unexplained decrease in body weight occurred
in all groups, including the controls. Dose-related degenerative
changes were observed. These were characterized by amyloidosis
in several organs and issues, mineralizaton in kidneys, and
haemosiderosis in the liver. Increased incidences of senile
atrophy and aspermatogenesis were observed in the testes at the
highest exposure level. In addition, the incidence of bile-duct
hyperplasia was increased at all exposures. The incidence of
benign nasal polyps was increased at 6.5 mg/m3. The only other
striking tumours observed were 3 adenocarcinomas, 1 leiomyoma,
and 1 papilloma in the colon, 1 basal cell carcinoma in the
stomach, and 4 parafollicular cell adenomas in the thyroid at
6.5 mg/m3. No such tumours were found in the controls, but the
increased incidence of each tumour type was not statistically
significant. The authors concluded from these studies "that
hydrazine is capable of inducing nasal tumours, primarily
benign, in rats and hamsters after 1-year intermittent exposure"
(Vernot et al., 1985).
8.6.2. Oral exposure
The oral carcinogenicity studies on hydrazine have been
either preliminary or limited in scope (inadequate limited
histopathology, use of one dose or one drinking-water concentra-
tion, small treatment groups, lack of adequate controls, and
treatment of only one sex); therefore, only the more relevant
studies have been described in this section. Most studies have
concentrated on various mouse strains.
Groups of approximately 25 CBA/Cb/Se mice of each sex
received, by gavage, 150 daily doses of hydrazine sulfate in
water, equivalent to 0.03, 0.07, 0.14, or 0.28 mg hydrazine in
25 weeks (these doses are expressed in Table 7 as 0.98, 2.0,
3.9, or 8.0 mg/kg body weight). The animals were observed for
their life-time. Control groups containing 30 males and 29
females were not treated. Liver, lung, various endocrine glands,
and tissues suspected of having lesions were examined
microscopically. No statistical analysis was reported. No deaths
occurred during the exposure period, but later the mortality
rate was increased in animals that had been exposed daily to
levels of 2.0 mg/kg upwards. Brown degeneration of the adrenals
in treated females was mentioned as a marked non-neoplastic
lesion. An apparent dose-related increased incidence of
hepatocarcinomas was observed in male and female mice. The
average latency time for this tumour did not decrease as the
dose increased (Biancifiori, 1970a). The historical control
incidence of hepatocarcinomas in the laboratory concerned was 8%
(Severi & Biancifiori, 1968). Multiple pulmonary tumours, which
were also observed, are described in another report
(Biancifiori, 1969).
In this report (Biancifiori, 1969), the 3 lowest dose
groups, containing 21 - 26 males and 21 - 25 females, were
treated in the same way as in the study above. The data for the
control group and the highest dose groups were taken from
earlier reports (Biancifiori et al., 1964; Severi & Biancifiori,
1968). The untreated control groups contained 37 males and 47
females. The highest dose group comprising 21 mice of each sex,
received 250 daily doses of hydrazine sulfate in water
equivalent to 0.28 mg hydrazine in 36 weeks. In the 1969 report,
the treatment schedule was reported to be 150 daily doses of
0.28 mg hydrazine in 25 weeks, which was contrary to the
original data. Lungs with trachea, liver, various endocrine
glands, and other tissues suspected of having lesions were
examined microscopically. No deaths occurred during the exposure
period, but the mortality rate after the treatment period was
increased from a dose level of 2.0 mg/kg per day upwards. Common
non-neoplastic lesions in the liver at 0.28 mg per day included
areas of diffuse cell regeneration. Necrosis, nodular
regeneration, sclerosis, and bile-duct proliferation were also
observed. The occurrence of hepatomas has already been
discussed. The incidence and multiplicity of pulmonary tumours
were increased at all dose levels in an apparently dose-related
manner and more so in females than in males (Table 7). The
average latency time for these tumours was also shorter in
females than in males. At the highest dose level, approximately
80% of the tumours were characterized as adenomas in both sexes,
the remainder being described as "anaplastic adenomas and
adenocarcinomas" (Biancifiori, 1969).
Induction of lung tumours by hydrazine sulfate after oral
exposure by gavage was also reported in other mouse strains
(Milia et al., 1965; Roe et al., 1967; Kelly et al., 1969;
Biancifiori, 1970b; Bhide et al., 1976; Maru & Bhide, 1982).
Liver tumour induction was reported in BALB/c/Cb/Se mice
(Biancifiori, 1970b).
Severi & Biancifiori (1968) also exposed 14 male and 18
female Cb/Se rats, by gavage, for 68 weeks, to daily doses of
hydrazine sulfate in water, equivalent to 4.4 mg hydrazine (14.6
mg/kg body weight) per day for males and 2.9 mg hydrazine (9.7
mg/kg) per day for females. Controls (28 males and 22 females)
were not treated. The animals were observed for their life-time.
Lungs, liver, and other organs showing gross lesions were
examined microscopically. No statistical analysis was reported.
Exposed rats showed an increased mortality rate. Pulmonary
tumours, adenomas, and adenocarcinomas were observed in both
sexes (Table 7). Carcinomas (2) and spindle cell sarcomas (2),
observed in the livers of exposed males, were not seen in the
controls.
Groups of 23 and 85 golden hamsters of both sexes received
hydrazine sulfate by gavage as 60 and 100 daily doses, equiv-
alent to 0.74 and 0.68 mg hydrazine over 15 and 20 weeks,
respectively. The control group contained 56 hamsters. Animals
were observed for their life-time and examined as described
above for rats. The mortality rate increased in an apparently
dose-related manner, and toxic effects were observed in treated
animals (liver lesions, reticuloendothelial cell proliferation,
cirrhosis, bile-duct proliferation, degenerative fibrous cells
in hyalinized tissue). No tumours were observed, but, because of
toxic effects, the treated animals were not observed for the
same period of time as the controls (Biancifiori, 1970a).
Groups of 50 Swiss mice of each sex received doses of hydra-
zine sulfate in drinking-water, equivalent to 0.056 mg hydrazine
(2 mg/kg body weight, males; 1.6 mg/kg, females) per day for
their life-time up to a maximum of 113 weeks (Toth, 1972a) or
0.18 mg (5.1 mg/kg body weight) per day (males) and 0.16 mg (4.6
mg/kg) per day (females) for a maximum of 95 weeks (Toth, 1969).
The untreated control group, containing 110 animals of each sex,
was tested concurrently with the groups receiving 0.16 or 0.18
mg per day, but not with those receiving 0.05 mg per day (Toth,
1969). Liver, kidneys, spleen, lungs, and organs with gross
lesions were examined microscopically. No statistical analysis
was reported. Drinking-water solutions containing hydrazine were
prepared 3 times per week. There was no indication that
hydrazine concentrations were measured in the solutions during
the studies. At the highest dose level, body weights were not
affected, but the mortality rate in females was increased.
Numerous lung tumours were observed in both sexes with a
comparable incidence (Table 7) and degree of malignancy at both
dose levels. The average latency time for this tumour was
decreased at both dose levels. At the high dose level, the
latency period for malignant lymphomas, which are characteristic
for the strain, and the incidence of adenocarcinomas of the
mammary gland appeared to be decreased.
Fifty Syrian golden hamsters of each sex also received, for
their life-time, up to a maximum of 110 weeks, doses of hydra-
zine sulfate in drinking-water, equivalent to 0.57 mg hydrazine
per day. Untreated non-concurrent control groups contained 100
hamsters of each sex. The protocol was similar to that described
above for rats. Body weights of exposed hamsters were slightly
reduced, but no tumours or other effects were noted. The authors
considered that there was no treatment-related tumour incidence
and that the mortality rate was not affected by the exposure,
though the mean survival period was slightly reduced (Toth,
1972b).
Groups of 34 male and 29 female Swiss mice were administered
hydrazine sulfate by gastric intubation at doses equivalent to
0.27 mg hydrazine per mouse per day, 5 days per week, for their
lifetime. Lung adenocarcinomas occurred in 30 out of 34 male
mice and in 21 out of 29 female mice compared with 1 out of 20
controls. The earliest tumour appeared after 14 months of
exposure. An increased incidence of lung tumours (20/22)
occurred in F1 animals, which were obtained from treated
mothers, and received hydrazine sulfate post weaning from the
age of 11 weeks. No control mice were mated. An increase in lung
tumours (16/35) was also observed in F2 animals obtained from F1
animals that had been treated with hydrazine throughout
gestation and lactation (Menon & Bhide, 1983).
9. EFFECTS ON MAN
9.1. Poisoning Incidents
Several incidents of systemic poisoning have been reported,
mainly showing effects on the central nervous system, respira-
tory system, and stomach.
After a laboratory technician had drunk 20 - 30 ml of a 6%
aqueous solution of hydrazine (free base), he immediately
vomited. Four hours later, weakness, somnolence, and arrhythmia
were observed. Laboratory findings showed a slight but persis-
tent leukocytosis. The serum-albumin fraction was decreased with
an increase in the fraction of globulin. Two days after
exposure, slightly elevated body temperature and a few red blood
cells in the urine were noted, while the patient showed
irregular breathing. Five days after exposure, the patient had
recovered (Drews et al., 1960).
A case was reported of a man who drank between a mouthful
and a cupful of hydrazine in concentrated solution. He
immediately vomited, became unconscious, and flushed. Vomiting
ceased about 12 h after admission to the hospital. The patient
showed sporadic violent behaviour and later developed ataxia
with a lateral nystagmus, a decreased sense of vibration, and
paraesthesia in the arms and legs. Oliguria was also noted.
Pyridoxine treatment was administered, but it was not clear from
the report whether he benefited from this. The patient's final
condition was not reported (Reid, 1965).
A 24-year-old man accidentally ingested "a mouthful" of
hydrazine and immediately became confused, lethargic, and
restless. On admission to hospital, a complete chemistry profile
was normal, but, 3 - 4 days later, hepatotoxicity was evident as
indicated by elevated levels of aspartate amino-transferase (EC
2.6.1.1), lactate dehydrogenase (EC 1.1.1.27), and total
bilirubin. At this time, the patient was treated with pyridoxine
and recovered completely within 5 days. Peripheral neuropathy,
however, developed subsequently, which resolved over the next 6
months. The authors attributed this neuropathy to the megadose
pyridoxine therapy (Harati & Niakan, 1986).
Another man sustained a chemical burn during an industrial
hydrazine explosion. Exposure occurred probably both via the
skin and by inhalation. The burns on his skin covered 22% of the
body surface. On admission to the hospital, the neuro-
logical status of the patient was normal but, 14 h after
exposure, he became comatose. When the coma persisted for 60 h,
he was treated with pyridoxine, and the neurological disorders
cleared within the next 12 h. Other findings were persistently
elevated glucose levels, haematuria, and respiratory
difficulties. Several biochemical indicators of liver
malfunction were elevated from day 3 after exposure and returned
to normal levels over the next 5 weeks (Kirklin et al., 1976).
Two men were exposed to Aerozine 50 vapour (50% hydrazine
and 50% 1,1-dimethylhydrazine by volume) via a leak in a fuel
line. The first man was exposed for about 90 min via a defective
gas mask. He reported headache, nausea, and a shaky feeling, a
sensation of burning of the face, a sore throat, and tightness
in the chest. The second man had inhaled the vapour 3 times
before evacuation from the contaminated area. He was dyspnoeic,
trembling, and weak. Both men showed neurological disorders
including twitching of extremities and clonic movements in the
first victim and hyperreactive reflexes in both men. All
symptoms cleared after treatment with pyridoxine. From clinical
signs, it was concluded that pulmonary oedema developed in both
men and was treated successfully. Four other cases of poisoning
by Aerozine after a spill were mentioned in this report. These 4
men showed nausea and vomiting. Both symptoms disappeared within
20 min following pyridoxine treatment (Frierson, 1965).
One case of systemic lupus erythaematosus-like disease due
to occupational exposure to hydrazine (free base) has been
described. The patient, a laboratory technician, had recurrent
episodes of joint pain, photosensitive rash, fatigue, and fever
following hydrazine exposure. A challenge patch test suggested
that hydrazine caused the illness. On the basis of detailed
examinations of the patient and her family, it was concluded
that hydrazine could cause the disease in a person with a
genetic predisposition. Some of the genetic factors identified
were slow acetylation phenotype, HLA DR2,3, and immune system
responsiveness such as antinuclear antibodies, antibodies to
single-stranded and native DNA, and inhibition of pokeweed
mitogen-stimulated immunoglobulin G synthesis in lymphocytes
(Reidenberg et al., 1983).
9.2. Occupational Exposure
9.2.1. Inhalation exposure
One case was reported of a man who had handled hydrazine
(hydrazine hydrate) once a week for an unknown number of hours
over a period of 6 months. In simulated conditions, only 0.071
mg hydrazine/m3 air was measured, but probably skin exposure had
also occurred. The man experienced conjunctivitis, tremor, and
lethargy after each exposure. Following the last exposure, he
was feverish, vomited, and showed diarrhoea. In a hospital, 6
days later, many disorders were noted: conjunctivitis,
stomatitis, arrhythmia, upper abdominal pain, enlarged abdomen,
icterus, a tender and palpable liver, black faeces, incoherence,
and oliguria. X-ray examinations showed pleural effusion and
lung shadowing. Laboratory findings comprised elevated bilirubin
and creatinine levels in the blood, and protein and red blood
cells in urine. Treatments administered included haemodialysis
and B-vitamins, which brought only temporary relief. The man
died 21 days after the last exposure. Autopsy revealed
pneumonia, severe renal tubular necrosis and nephritis, and mild
hepatocellular damage (Sotaniemi et al., 1971).
9.2.2. Skin and eye irritation; sensitization
Two cases of skin irritation (Frierson, 1965; Kirklin et
al., 1976) and one case of eye irritation (Sotaniemi et al.,
1971) have already been cited in sections 9.1 and 9.2.1,
respectively. Dermatitis of the hands without systemic effects
was observed in 2 men who were occupationally exposed to hydra-
zine hydrate over a period of 5 months. Both men had had similar
experiences previously, after handling of hydrazine hydrate
(Evans, 1959). No patch tests were carried out, but the allergic
nature of the contact dermatitis that can develop after skin
exposure to hydrazine, has been reported earlier. In 1959, an
outbreak of dermatitis was reported among 12 out of 34 female
workers in a factory where hydrazine hydrochloride was used as a
solder flux. Four months after the use of the solder flux had
stopped, 6 out of 12 workers showed strong positive reactions
towards hydrazine sulfate, while 30 controls did not show any
reaction (Frost & Horth, 1959). In an electronics plant, 35
workers, constituting half the work force, developed contact
dermatitis from a solder flux containing hydrazine
hydrochloride, over a period of 5 years. The symptoms did not
reappear when contact with hydrazine was avoided. Patch tests on
5 female workers of this group were all clearly positive for
hydrazine fluxes, while 3 controls did not react (Wheeler et
al., 1965). Another 7 workers in a chemical workshop, where
solder flux containing hydrazine hydrochloride was used, showed
allergic contact dermatitis towards hydrazine, as did 5 workers
in a pharmaceutical firm producing isoniazid. The results of the
patch tests were strongly positive for the 12 subjects, but a
control group was lacking (Zina & Bonu, 1962). Hydrazine induced
allergic contact dermatitis in 4 men when used as a corrosion
inhibitor (Schultheiss, 1959; Hovding, 1967; Von Keilig & Speer,
1983). A negative control group was tested in only one of these
studies (Hovding, 1967). Allergic contact dermatitis developed
in 15 workers in 2 industries producing hydrazine sulfate. In
one of these industries, patch tests on 15 workers were clearly
positive for hydrazine, while 13 controls from the same industry
did not show any reaction. Symptoms did not reappear when
contact with hydrazine sulfate was avoided (Brandt, 1960). In
the other industry, 10 persons produced a positive reaction
towards hydrazine. No negative controls were tested (Sonneck &
Umlauf, 1961). Two cases of allergic contact dermatitis were
reported following exposure to hydrazine in stain removers with
strong positive reactions towards the chemical in patch tests.
Five controls did not react (Van Ketel, 1964). Systemic effects
were not noted in any of these studies.
Cross sensitization to hydrazine derivatives, such as
isoniazid, phenylhydrazine, and hydralazine, was found by
several investigators (Schultheiss, 1959; Zina & Bonu, 1962; Van
Ketel, 1964; Hovding, 1967; Von Pevny & Peter, 1983).
9.2.3. Mortality studies
The causes of death in a cohort of 427 male workers,
employed for a minimum of 6 months in a hydrazine manufacturing
plant between 1945 and 1971, were studied retrospectively and
followed until July 1982. The plant ceased production in 1971.
The men were divided according to 3 categories of exposure. The
first category contained 78 men who might have been exposed to
concentrations of hydrazine ranging from 1.3 to 13 mg/m3 air.
Categories 2 and 3 (a total of 375 men) included those who might
have been exposed to concentrations between 0.6 and 1.3 mg/m3
and those who had little or no exposure, respectively. A further
subdivision in the first category distinguished between men with
more than 10 years since the first exposure and those with less.
Some men were covered by more than one category. The cohort was
also exposed to other unspecified organic chemicals. The total
number of deaths in 406 men who could be traced was 49 against
61.47 expected. Five men died from lung cancer against an
expected 6.65. Two of these men belonged to the most highly
exposed category. Another 7 cases of cancer were detected (9.27
expected). Further details were not given, except for the remark
that no nasal tumours were involved. The authors noted that the
number of men exposed to hydrazine in this study was small. It
was concluded that the results were encouraging in that no
obvious hazard had yet appeared. A note of caution is introduced
by the observation of 2 deaths from lung cancer in men who had
first been exposed in the heaviest category more than 10 years
previously against 1.61 expected (Wald et al., 1984). The cohort
is being followed up.
A seemingly unusually high incidence of myocardial infarc-
tion prompted further study among workers in a plant manufac-
turing hydrazine. The population at risk was limited to every
on-line hydrazine worker who had worked for 3 months or more
between 1953 and 1978. The number of man years at risk was 534,
and the average employment period was 8 years. Only a
superficial examination of the population at risk and the
working environment was completed. Reference data were provided
by the US Air Force men's rates for the years 1975-77 and by
men's rates from a study over the past 30 years by the US
National Heart Institute (Framingham study). There was a close
compar ison between the 2 sets of reference data. Rates of
occurrence of myocardial infarction (death and non-death) per
thousand men per year within 5-year age groups were calculated.
A total of 5 cases of myocardial infarction, concurrent with
the employment period, were identified among the hydrazine
workers. Their average employment period was 16 years. A total
of 1.2 cases was expected. The difference is highly significant
statistically. Four additional cases were identified in ex-
hydrazine workers. However, as it was impossible to trace all
ex-hydrazine workers, the population at risk was limited to
the "concurrent cases". The author warned that the findings
should be treated with due caution because of the small
numbers involved. He also stated that the strength of the
findings rendered a negative hypothesis almost untenable without
further investigation into the cause of the adverse effect found
(Hamill, 1978).
The Task Group recognized that exposure concentrations were
not reported and that there was no indication that confounding
variables, e.g., smoking history or exercise habits, had been
taken into account.
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1. Evaluation of Human Health Risks
Exposure of human beings to hydrazine may occur occupation-
ally or accidentally, through the ingestion of hydrazine-based
drugs, or through the use of tobacco. Despite the high react-
ivity of this compound and its wide industrial use, few
systematic studies have been carried out on its adverse effects
on man.
Hydrazine is rapidly absorbed through the skin, lungs, and
gastrointestinal tract and rapidly distributed throughout the
body (section 6).
In cases of acute human poisoning, vomiting, severe irrita-
tion of the respiratory tract with the development of pulmonary
oedema, central nervous system depression, and hepatic and renal
damage have been reported. Data are not available from which the
levels of hydrazine inhaled can be estimated in cases of acute
poisoning by the respiratory route. However, from reports of
poisoning by the oral route, it would appear that ingestion of
amounts of the order of 20 - 50 ml causes severe intoxication
and may be lethal (section 9.1). It is not possible to estimate
a no-observed-adverse-effect level from the available human
data.
Most of the effects in human beings exposed to hydrazine
have also been observed in experimental animals. In addition,
loss of body weight, anaemia, hypoglycaemia, and fatty liver
have frequently been reported. Fatty liver in mice and reduced
growth in rats were reported when the animals were exposed
through inhalation to the lowest (0.26 mg/m3) of three dose
levels. Monkeys and dogs were unaffected at this dose. In only
one study, a no-observed-adverse-effect level of 3 µwg/kg was
reported, when hydrazine was administered by the oral route to
rats. No data are available on the basis of which a no-observed-
adverse-effect level by inhalation can be established (section
8.2).
Embryotoxicity, fetotoxicity, and minor fetal abnormalities
were observed in rats and mice exposed to hydrazine at doses
toxic for the mother. At such doses, perinatal mortality is
high. These effects may also occur at doses just below the
maternally toxic dose (section 8.4). No data are available on
the effects of hydrazine on the human fetus. In the absence of
human data, it would be prudent to assume that hydrazine might
have adverse effects on the human embryo or fetus at exposure
levels approximating those producing toxicity for the mother.
Such levels may occur during accidental spillage but would be
unlikely to occur in the ambient environment because atmospheric
concentrations are low.
Skin and eye irritation have been observed in human beings
who have come into contact with hydrazine, but the data are
insufficient to establish a no-observed-effect level for
irritation. Hydrazine is a strong skin sensitizer in man and
cross-reacts with hydrazine derivatives (section 9.2.2). Slight
eye irritation was observed in monkeys at hydrazine concentra-
tions of 1.3 and 6.5 mg/m3, but none occurred in animals exposed
to 0.26 mg/m3 (section 8.2.1). No skin irritancy studies on
animals have been reported.
The available data indicate that hydrazine induces gene
mutations and chromosome aberrations in a variety of test
systems including plants, phages, bacteria, fungi, Drosophila,
and mammalian cells in vitro. Hydrazine induced indirect
alkylation in the liver DNA of rodents after in vivo exposure to
toxic doses, and it also caused DNA damage in vitro . Hydrazine
transformed human and hamster cells in vitro. No increased
unscheduled DNA synthesis was observed in the germ cells of mice
in vivo . It did not induce chromosome aberrations, micronuclei,
or dominant lethals in mice in vivo , but chromosomal aberrations
were reported in rats in vivo (section 8.5).
Hydrazine vapour induced nasal tumours, most of which were
benign, in F-344 rats and Syrian golden hamsters, after 12
months of treatment and life-time observation. At the exposure
levels at which most of the tumours occurred, there were signs
of severe mucosal irritation. In several studies on mice,
hydrazine induced an increased incidence of pulmonary tumours,
when administered by the oral route. Hepatic tumours were also
reported in two studies. In some of the studies, the pulmonary
tumour incidence was related to the dose administered. Although
in these reports there is little information about the toxic
effects of the compound, it is probable that the doses admin-
istered in some of the studies were at, or near, the toxic
levels. No tumours were observed in hamsters treated orally with
hydrazine (section 8.6). On the basis of the carcinogenicity
studies on experimental animals, there is evidence that
hydrazine is an animal carcinogen. Human data are inadequate to
assess its carcinogenicity in man. In the absence of human data,
and taking into account the mutagenicity data as well as the
carcinogenicity data in animals, it would be prudent to consider
hydrazine as a possible human carcinogen. Thus, exposure to
human beings should be kept as low as feasible.
Since measurable levels are not normally encountered in the
general environment, it can be concluded that, except in cases
of accidental exposure, hydrazine does not pose a significant
practical hazard for the general population.
10.2. Evaluation of Effects on the Environment
Hydrazine can be released into the atmosphere during venting
operations, storage, and transfer. The total emission has been
estimated to be nearly 0.01% of the hydrazine produced
(production > 35 000 tonnes in 1981). Accidental discharges into
water, air, and soil can result from bulk storage, handling,
transport, and improper waste disposal. Evaporation of hydrazine
after a spill can generate an atmospheric concentration as high
as 4 mg/m3, 2 km downwind.
Hydrazine is degraded rapidly in air through reactions with
ozone, hydroxyl radicals, and nitrogen dioxide. In water, it is
degraded rapidly, especially under aerobic conditions, and in
the presence of organic material and/or in alkaline or hard
water. It is more persistent in soft, metal-free water. In soil,
it is adsorbed and decomposed on clay surfaces, under aerobic
conditions. However, available data are inadequate to describe
the nature of hydrazine behaviour in the soil.
Because of the rapid degradation of hydrazine in the
environment, measurable levels are not normally encountered.
Hydrazine can be toxic for aquatic life, even at very low
concentrations. Fish species showed LC50 values of between 0.54
mg/litre (roach) and 5.98 mg/litre (fathead minnow). Nitrifying
bacteria in activated sludge are inhibited by hydrazine levels
higher than 1 mg/litre. Some other microorganisms are more
sensitive and show toxicity thresholds at levels reported to be
as low as 0.00008 mg/litre.
Hydrazine in both air and water is toxic for plants; in
water, it can inhibit plant germination.
On the basis of these data, it can be concluded that hydra-
zine may present a significant hazard for the aquatic environ-
ment and plant life.
11. RECOMMENDATIONS FOR FURTHER STUDIES
1. Dose-response studies pertaining to:
(a) DNA alkylation (adduct identification);
(b) damage of nasal epithelium; and
(c) pulmonary effects.
2. Skin sensitization studies with focus on cross-reactivity
with hydrazine derivatives.
3. Dose-response studies on sensitive, commercially important
fish species and their food supplies.
4. Metabolic studies in conjunction with effects on DNA.
5. Dermal initiation-promotion studies.
6. Reproduction toxicity studies in sensitive rodent species at
continuous low exposure levels.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
The International Agency for Research on Cancer (IARC, 1982)
considered that, while the evidence for the carcinogenicity of
hydrazine for human beings was inadequate, evidence for its
carcinogenicity for animals and for its activity in short-term
tests was sufficient to conclude that hydrazine is probably
carcinogenic for human beings.
REFERENCES
AGRELO, C. & AMOS, H. (1981) DNA repair in human fibroblasts.
In: de Serres, F.J. & Ashby, J., ed. Evaluation of short-term
tests for carcinogens. Report of the International Collaborative
Programme, New York, Amsterdam, Oxford, Elsevier/North Holland,
pp. 528-532 (Progress in Mutation Research Vol. 1).
AKIN, F.J. & NORRED, W.P. (1978) Effects of short-term
administration of maleic hydrazine or hydrazine on rat hepatic
microsomal enzymes. Toxicol. appl. Pharmacol., 43: 287-292.
ALEYASSINE, H. & LEE, S.H. (1971) Inhibition by hydrazine,
phenelzine, and pargyline of insulin release from rat pancreas.
Endocrinology, 89: 125-129.
AMACHER, D.E., PAILLET, S.C., TURNER, G.N., RAY, V.A., &
SALSBURG, D.S. (1980) Point mutations at the thymidine kinase
locus in L5178Y mouse lymphoma cell. II. Test validation and
interpretation. Mutat. Res., 72: 447-474.
AMENTA, J.S. & DOMINGUEZ, A.M. (1965a) Fatty acid flux and
triglyceride secretion in the hydrazine-induced fatty liver.
Exp. mol. Pathol., 4: 282-302.
AMENTA, J.S. & DOMINGUEZ, A.M. (1965b) The effect of hydrazine
and congeners on C1402 respiratory pattern of various metabolic
substrates. Toxicol. appl. Pharmacol., 7: 236-246.
ANDERSEN, M.E. & KELLER, W.C. (1984) Toxicokinetic principles
in relation to percutaneous absorption and cutaneous toxicity.
In: Drill, V.A. & Lazar, P., ed. Cutaneous toxicity, New York,
Raven Press, pp. 9-27.
ASHBY, J. (1981) Overview of study and test chemical
activities. In: De Serres, F. & Ashby, J., ed. Evaluation of
short-term tests for carcinogens. Report of the International
Collaboration Programme, Amsterdam, Elsevier/North Holland, pp.
112-171 (Progress in Mutation Research, Vol. 1).
ASHBY, J. & KILBEY, B. (1981) Summary report on the
performance of bacterial repair, phage induction, degranulation,
and nuclear enlargement assays. In: de Serres, F.J. & Ashby, J.,
ed. Evaluation of short-term tests for carcinogens. Report of
the International Collaborative Programme, New York, Amsterdam,
Oxford, Elsevier/North Holland, pp. 33-48 (Progress in Mutation
Research Vol. 1).
ASTM (1981) Annual book of ASTM standards. Part 31. Water,
Philadelphia, Pennsylvania, American Society for Testing and
Materials, pp. 425-428 (Method D1385: standard test method for
hydrazine in water).
AUDRIETH, L.F. & OGG, B. (1951) The chemistry of hydrazine,
New York, John Wiley and Sons.
AZAR, A., THOMAS, A.A., & SHILLITO, F.H. (1970) Pyridoxine and
phenobarbital as treatment for Aerozine 50 toxicity. Aerosp.
Med., 41: 1-4.
BAKER, R.S.U., MITCHELL, G.A., MEHER-HOMJI, K.M., & PODOBNA, E.
(1983) Sensitivity of two Chinese hamster cell lines to SCE
induction by a variety of chemical mutagens. Mutat. Res., 118:
103-116.
BALGANESH, M. & SETLOW, J.K. (1984) Prophage induction in
Haemophilus influenzae and its relationship to mutation by
chemical and physical agents. Mutat. Res., 125: 15-22.
BANKS, W.L. (1970) Effect of hydrazine treatment on hepatic
protein biosynthesis in vivo. Biochem. Pharmacol., 19: 275-
283.
BECKER, R.A., BARROWS, L.R., & SHANK, R. (1981) Methylation of
liver DNA guanine in hydrazine hepatotoxicity: dose-response and
kinetic characteristics of 7-methylguanine and O6-methylguanine
formation and persistence in rats. Carcinogenesis, 2: 1181-
1188.
BHIDE, S.V., D'SOUZA, R.A., SAWAY, M.M., & RANADIVE, K.J.
(1976) Lung tumour incidence in mice treated with hydrazine
sulfate. Int. J. Cancer, 18: 530-535.
BIANCIFIORI, C. (1969) [Existence of a hormonal factor in the
pulmonary carcinogenicity of hydrazine.] Lav. Ist. Anat. Istol.
Patol. Univ. Studi Perugia, 29: 29-41 (in Italian).
BIANCIFIORI, C. (1970a) Hepatomas in CBA/Cb/Se mice and liver
lesions in golden hamsters induced by hydrazine sulfate. J. Natl
Cancer Inst., 44: 943-953.
BIANCIFIORI, C. (1970b) [Pulmonary and liver tumours from low
doses of hydrazine sulfate in BALB/c/Cb/Se mice.] Lav. Ist.
Anat. Istol. Patol. Univ. Studi Perugia, 30: 89-99 (in
Italian).
BIANCIFIORI, C., BUCCIARELLI, E., CLAYSON, D.B., & SANTILLI,
F.E. (1964) Induction of hepatomas in CBA/Cb/Se mice by
hydrazine sulfate and the lack of effect of croton oil on tumour
induction in BALB/Cb/Se mice. Br. J. Cancer, 18: 543-550.
BLAIR, I.A., MANILLA TINOCO, R., BRODIE, M.J., CLARE, R.A.,
DOLLERY, C.T., TIMBRELL, J.A., & BEEVER, I.A. (1985) Plasma
hydrazine concentrations in man after isoniazid and hydralazine
administration. Hum. Toxicol., 4: 195-202.
BODENSCHATZ, W. (1986) [Hydrazine in drinking-water.] Off.
Gesundheitswes., 48: 205-208 (in German).
BOSAN, W.S. & SHANK, R.C. (1983) Methylation of liver DNA
guanine in hamsters given hydrazine. Toxicol. appl. Pharmacol.,
70: 324-334.
BRANDT, B., VON (1960) [On allergic skin damage by hydrazine
sulfate.] Dermatol. Wochenschr., 141: 376-381 (in German).
BRAUN, R., JAKEL, H.-P., & SCHONEICH, J. (1984) Genetic
effects of isoniazid and the relationships to in vivo and in
vitro biotransformation. Mutat. Res., 137: 61-69.
BRESLER, S.E., KALININ, V.L., & PERUMOV, D.A. (1968)
Inactivation and mutagenesis on isolated DNA. II. Kinetics of
mutagenesis and efficiency of different mutagens. Mutat. Res.,
5: 1-14.
BRIDGES, B.A., MACGREGOR, D., & ZEIGER, E. (1981) Summary
report on the performance of bacterial mutation assays. In: de
Serres, F.J. & Ashby, J., ed. Evaluation of short-term tests for
carcinogens. Report of the International Collaborative
Programme, New York, Amsterdam, Oxford, Elsevier/North Holland,
pp. 45-67 (Progress in Mutation Research Vol. 1).
BRINGMANN, G. (1975) [Determination of the effect of
biological pollutants on the inhibition of cell multiplication
in the blue algae Microcystis. ] Gesundheits-Ingenieur, 96: 238-
241 (in German).
BRINGMANN, G. & KUHN, R. (1980) Comparison of the toxicity
thresholds of water pollutants to bacteria, algae, and protozoa
in the cell multiplication test. Water Res., 14: 231-241.
BRINGMANN, G. & KUHN, R. (1981) [Comparison of the effect of
hazardous substances on flagellated and ciliated, respectively,
holozoic bactivorous and saprozoic protozoa.] GWF Wasser-
Abwasser, 122: 308-313 (in German).
BRINGMANN, G. & KUHN, R. (1982) [Data on the toxicity of water
pollutants to Daphnia magna in an improved standard test.] Z.
Wasser Abwasser Forsch., 15: 1-6 (in German).
BRUGGER, H. (1983) [ Emission factors for 12 selected
substances with probably carcinogenic potential: hydrazine,]
Environment Research Programme of the Federal Ministry of the
Interior, Federal Environment Office, Vol. 3, Part 1, pp. 15-17
(Report No. 104-04-344) (in German).
CARVER, J.H., SALAZAR, E.P., KNIZE, M.G., & WANDRES, D.L.
(1981) Mutation induction at multiple gene loci in Chinese
hamster ovary cells: the genetic activity of 15 coded
carcinogens and noncarcinogens. In: de Serres, F.J. & Ashby, J.,
ed. Evaluation of short-term tests for carcinogens. Report of
the International Collaborative Programme, New York, Amsterdam,
Oxford, Elsevier/North Holland, pp. 594-601 (Progress in
Mutation Research Vol. 1).
CHANDRA SEKHAR, V.S.G. & REDDY, G.M. (1971) Specific locus
mutations in maize by chemical mutagens. Curr. Sci., 40: 136-
137.
CHATTERJEE, A.K. & SENGUPTA, K. (1980) Regulation of formation
in vivo of pyridoxal phosphate in hydrazine-treated rats.
Int. J. Vitam. Nutr. Res., 50: 24-28.
CHU, B.C.F., BROWN, D.M., & BURDON, M.G. (1973) Effect of
nitrogen and of catalase on hydroxylamine and hydrazine
mutagenesis. Mutat. Res., 20: 265-270.
CIER, A., ROUGANNE, J.P., & SCHMITT, M. (1967) Effets
hématologiques d'une intoxication subaiguë à l'hydrazine et à la
dimethyl hydrazine asymétrique chez la souris et le lapin. C.R.
Soc. Biol. (Paris), 161: 854-858.
CLARK, D.A., BAIRRINGTON, J.D., BITTER, H.L., COE, F.L., MEDINA,
M.A., MERRIT, J.H., & SCOTT, W.N. (1968) Pharmacology and
toxicology of propellant hydrazines, Springfield, Virginia, US
Department of Commerce (Aeromedical Reviews No. 11-68) (AD
688500).
CLARK, D.A., LEEDER, L.G., FOULDS, E.L., & TROUT, D.L. (1970)
Changes in lipids of rat liver after hydrazine injection.
Biochem. Pharmacol., 19: 1743-1752.
COMSTOCK, C.C., LAWSON, L.H., GREENE, E.A., & OBERST, F.W.
(1954) Inhalation toxicity of hydrazine vapour. Arch. ind. Hyg.
occup. Med., 10: 476-490.
COOK, L.R., GLENN, R.E., & PODOLAK, G.E. (1979) Monitoring and
analysis of personnel exposures to hydrazines at a rocket
propellant plant. Am. Ind. Hyg. Assoc. J., 40: 69-74.
COOLING, J., BURDITT, S.L., & BRINDLEY, D.N. (1979) Effects of
treating rats with hydrazine on the circulating concentration of
corticosterone and insulin in relation to hepatic triacyl-
glycerol synthesis. Biochem. Soc. Trans., 7: 1501-1503.
CORNISH, H.H. & WILSON, C.E. (1968) Amino acid levels in
hydrazine-treated rats. Toxicol. appl. Pharmacol., 12: 265-272.
DANIEL, M.R. & DEHNEL, J.M. (1981) Cell transformation test
with baby hamster kidney cells. In: de Serres, F.J. & Ashby, J.,
ed. Evaluation of short-term tests for carcinogens. Report of
the International Collaborative Programme, New York, Amsterdam,
Oxford, Elsevier/North Holland, pp. 626-637 (Progress in
Mutation Research Vol. 1).
DAMBRAUSKAS, T. & CORNISH, H.H. (1964) The distribution, meta-
bolism, and excretion of hydrazine in rat and mouse. Toxicol.
appl. Pharmacol., 6: 653-663.
DAVIS, L.C. (1980) Hydrazine as a substrate and inhibitor of
Azobacter vinelandii nitrogenase. Arch. Biochem. Biophys.,
204: 270-276.
DEAN, B.J. (1981) Activity of 27 coded compounds in the R11
chromosome assay. In: de Serres, F.J. & Ashby, J., ed.
Evaluation of short-term tests for carcinogens. Report of the
International Collaborative Programme, New York, Amsterdam,
Oxford, Elsevier/North Holland, pp. 570-579 (Progress in
Mutation Research Vol. 1).
DEE, L.A. (1971) Gas chromatographic determination of aqueous
trace hydrazine and methylhydrazine as corresponding pyrazoles.
Anal. Chem., 43: 1416-1419.
DE FLORA, S., ZANACCHI, P., CAMOIRANO, A., BENNICELLI, C., &
BADOLATI, G.S. (1984) Genotoxic activity and potency of 135
compounds in the Ames reversion test and in a bacterial DNA-
repair test. Mutat. Res., 133: 161-198.
DE SERRES, F.J. & ASHBY, J., ed. (1981) Evaluation of short-
term tests for carcinogens. Report of the International
Collaborative Programme, New York, Amsterdam, Oxford, Elsevier/
North Holland, 827 pp (Progress in Mutation Research, Vol. 1).
DI LUZIO, N.R., STEGE, T.E., & HOFFMAN, E.O. (1973) Protective
influence of diphenyl- p -phenylenediamine on hydrazine-induced
lipid perioxidation and hepatic injury. Exp. mol. Pathol., 19:
284-292.
DOMINGUEZ, A.M., AMENTA, J.S., HILL, C.S., & DOMANSKI, T.J.
(1962) Morphologic and biochemical alterations in the kidney of
the hydrazine-treated rat. Aerosp. Med., 33: 1094-2010.
DOST, F.N., REED, D.J., & WANG, C.H. (1971) Effects of various
hydrazines upon the metabolism of gamma-aminobutyric acid
(GABA)-1-14C by rats. Biochem. Pharmacol., 20: 1702-1707.
DOST, F.N., BRODERICK, D.J., KRIVAK, R.M., & REED, D.J. (1981)
Metabolism of hydrazine, Ohio, Wright-Patterson Air Force Base,
Aerospace Medical Research Laboratory (AFAMRL TR-81-26)
(AD/A101849).
DREWS, A., EVERSMANN, K., & FRITZE, E. (1960) [On oral
poisoning by hydrazine.] Med. Welt, 23: 1295-1297 (in German).
DUAMIN, V.V., DENISOV, V.L., ANDROPOVA, S.N., & MALETIN, V.P.
(1984) [Influence of hydrazine on reproductive function of
animals when administered in organisms by different routes.]
Gig. i Sanit., 9: 25-28 (in Russian).
EPSTEIN, S.S., ARNOLD, E., ANDREA, J., BASS, W., & BISHOP, Y.
(1972) Detection of chemical mutagens by the dominant lethal
assay in the mouse. Toxicol. appl. Pharmacol., 23: 288-325.
EVANS, D.M. (1959) Two cases of hydrazine hydrate dermatitis
without systemic intoxication. Br. J. ind. Med., 16: 126-127.
FARMWALD, J.A. & MACNAUGHTON, M.G.M. (1981) Effects of
hydrazine on the activated sludge process. J. Water Pollut.
Control Fed., 53: 565-575.
FAROOK, S.A.F. & NIZAM, J. (1979) Mutagenic sensitivity of
base-specific chemicals in chick pea. Indian J. Bot., 2: 12-16.
FISHER, J.W., HARRAH, C.B., & BERRY, W.O. (1980) Hydrazine:
acute toxicity to bluegills and sublethal effects on dorsal
light response and aggression. Trans. Am. Fish. Soc., 109: 304-
309.
FLOYD, W.N. (1980) The importance of ammonia in the metabolic
effects of hydrazine. Aviat. Space environ. Med., 51: 899-901.
FORTNEY, S.R. (1966) Effect of hydrazine on liver glycogen,
arterial glucose, lactate, pyruvate, and acid-base balance in
the anaesthetized dog. J. Pharmacol. exp. Ther., 153: 562-568.
FORTNEY, S.R., CLARK, D.A., & STEIN, E. (1967) Inhibition of
gluconeogenesis by hydrazine administration in rats. J.
Pharmacol. exp. Ther., 156: 277-284.
FREESE, E.B., GERSON, J., TABER, H., RHAESE, H.-J., & FREESE, E.
(1967) Inactivating DNA alterations induced by peroxides and
peroxide-producing agents. Mutat. Res., 4: 517-531.
FRIERSON, W.M. (1965) Use of peridoxine HCl in acute hydrazine
and UDMH intoxication. Ind. Med. Surg., 34: 650-651.
FROST, J. & HJORTH, N. (1959) Contact dermatitis from
hydrazine hydrochloride in soldering flux. Cross-sensitization
to Apresoline and isoniazid. Acta dermatovenereol., 39: 82-86.
GANOTE, C.E. & ROSENTHAL, A.S. (1968) Characteristic lesions
of methylazoxymethanol-induced liver damage. A comparative
ultrastructural study with dimethylnitrosamine, hydrazine
sulfate, and carbon tetrachloride. Lab. Invest., 19: 382-398.
GEDDES, J.W. & WOOD, J.D. (1984) Changes in the amino acid
content of nerve ending (synaptosomes) induced by drugs that
alter the metabolism of glutamate and gamma-aminobutyric acid.
J. Neurochem., 42: 16-24.
GORDON, A. & LEWANDOWSKI, P.E. (1980) Control of vapor
emissions from hydrazine-based fuels, Newark, New Jersey,
Institute of Technology, Department of Chemical Engineering
(AFOSR-TR-80-053).
GORMLEY, W.T. & FORD, R.E. (1973) Deoxygenation of
environmental waters by hydrazine-type fuels. In: Proceedings of
the 4th Annual Conference on Environmental Toxicology, Fairborn,
Ohio, 1973, Ohio, Wright-Patterson Air Force Base, Aerospace
Medical Research Laboratory, Paper 28, pp. 387-400 (AMRL-TR-73-
125) (AD/781031).
GORSHTEIN, E.S. & KOPYLOVA, T.N. (1983) [Effect of hydrazine
hydrochloric acid on the hepatic microsomal hydroxylation system
of rats.] Eksp. Med., 15: 22-26 (in Russian).
GREENHOUSE, G. (1976a) Evaluation of the teratogenic effects
of hydrazine, methylhydrazine, and dimethylhydrazine on embryos
of Xenopus laevis, the South African clawed toad. Teratology,
13: 167-178.
GREENHOUSE, G. (1976b) The evaluation of toxic effects of
chemicals in fresh water by using frog embryos and larvae.
Environ. Pollut., 11: 303-315.
GUPTA, A.K. & GROVER, N.S. (1970) Hydrazine-induced breaks in
chromosomes of Vicia faba. Mutat. Res., 10: 519-520.
GUPTA, R.S. & GOLDSTEIN, S. (1981) Mutagen testing in the
human fibroblast diphteria toxin resistance (HF Dipr) system.
In: de Serres, F.J. & Ashby, J., ed. Evaluation of short-term
tests for carcinogens. Report of the International Collaborative
Programme, New York, Amsterdam, Oxford, Elsevier/North Holland,
pp. 614-625 (Progress in Mutation Research Vol. 1).
HACK, W., VON, HOYERMANN, K., & WAGNER, H.G. (1974) [Gas-phase
reactions of hydroxyl radical with ammonia and hydrazine.] Ber.
Bunsen-Ges. Phys. Chem., 78: 386-391 (in German).
HAMILL, P.V.V. (1978) Number of heart attack cases among
workers of the Lake Charles hydrazine plant, 1953-78 (Submitted
to the US EPA by the Olin Corporation, Stamford, Connecticut,
under TSCA, section 8(e)).
HARATI, Y. & NIAKAN, E. (1986) Hydrazine toxicity, pyridoxine
therapy, and peripheral neuropathy. Ann. intern. Med., 104: 728-
729.
HARRAH, C.B. (1978) Biological effects of aqueous hydrazine
solutions. In: Procedings of the Conference on Environmental
Chemistry: Hydrazine Fuels, 1977, Florida, Tyndall Air Force
Base, Civil and Environmental Engineering Development Office,
pp. 167-176 (CEEDO-TR-78-14) (AD/A054194).
HARRIS, G.W., ATKINSON, R., & PITTS, J.N. (1979) Kinetics of
the reactions of the hydroxyl radical with hydrazine and MMH. J.
Phys. Chem., 83: 2557-2559.
HAUN, C.C. & KINKEAD, E.R. (1973) Chronic inhalation toxicity
of hydrazine. In: Proceedings of the 4th Annual Conference on
Environmental Toxicology, Fairborn, Ohio, 1973, Ohio, Wright-
Patterson Air Force Base, Aerospace Medical Research Laboratory,
Paper 25, pp. 351-363.
HECK, W.W., BLOODWORTH, M.E., CLARK, W.J., DARLING, D.R., &
HOOVER, W. (1963) Environmental pollution by missile
propellants, Ohio, Wright-Patterson Air Force Base, Aerospace
Medical Research Laboratory (AMRL-TDR-63-75).
HEINDORFF, K., RIEGER, R., VELEMINSKY, J., & GICHNER, T. (1984)
A comparative study of the clastogenicity of maleic hydrazine
and some of its putative degradation products. Mutat. Res., 140:
123-126.
HEINEMANN, B. (1971) Prophage induction in lysogenic E. coli
with simple hydroxylamine and hydrazine compounds. Appl.
Microbiol., 21: 726-731.
HENDERSON, V., FISHER, J.W., & D'ALLESSANDRIS, R. (1981) Toxic
and teratogenic effects of hydrazine on fathead minnow (Pime-
phales promelas) embryos. Bull. environ. Contam. Toxicol., 26:
807-812.
HENDERSON, V., FISHER, J.W., D'ALLESSANDRIS, R., & LIVINGSTON,
J.M. (1983) Effects of hydrazine on functional morphology of
rainbow trout embryos and larvae. Trans. Am. Fish. Soc., 112:
100-104.
HERBOLD, B.A. (1978) [Mutagenicity testing with the liver
microsome test.] Biol. Zentralbl., 97: 137-152 (in German).
HIGGINS, E.S. & BANKS, W.L. (1971) Cognate effects of ethanol,
hydrazine, and tissue regeneration on hepatic mitochonidrial
activities. Biochem. Pharmacol., 20: 1513-1524.
HOLTZCLAW, J.R., ROSE, S.L., WYATT, J.R., ROUNBEHLER, D.P., &
FINE, D.H. (1984) Simultaneous determination of hydrazine,
methylhydrazine, and 1,1-dimethylhydrazine in air by derivat-
ization/gas chromatography. Anal. Chem., 56: 2952-2956.
HOVDING, G. (1967) Occupational dermatitis from hydrazine
hydrate used in boiler protection. Acta dermatovenereol., 47:
293-297.
HSIE, A.W., O'NEILL, J.P., MACHANOFF, R., SCHENLEY, R.L., &
BRIMER, P.A. (1981) Screening for mutagenic response of four
coded chemical by the CHO/HGPRT system. In: de Serres, F.J. &
Ashby, J., ed. Evaluation of short-term tests for carcinogens.
Report of the International Collaborative Programme, New York,
Amsterdam, Oxford, Elsevier/North Holland, pp. 602-607 (Progress
in Mutation Research Vol. 1).
HUNT, T.P., FISHER, J.W., LIVINGSTON, J.M., & PUTNAM, M.E.
(1981) Temperature effects on hydrazine toxicity to bluegills.
Bull. environ. Contam. Toxicol., 27: 588-595.
IARC (1982) Chemicals, industrial processes, and industries
associated with cancer in humans, Lyons, International Agency
for Research on Cancer, pp. 136-138 (Monographs on the
Evaluation of the Carcinogenic Risk of Chemicals to Humans,
Suppl. 4).
IRPTC (1985) Treatment and disposal methods for waste
chemicals, Geneva, International Register of Potentially Toxic
Chemicals, United Nations Environment Programme, pp. 188-189.
IRPTC (1986) Data profile on hydrazine, Geneva, International
Register of Potentially Toxic Chemicals, United Nations
Environment Programme.
ISAACSON, P.J. & HAYES, M.H.B. (1984) The interaction of
hydrazine hydrate with humic acid preparations at pH 4. J. soil
Sci. , 35(1): 79-92.
JACKSON, E.K., PARSHALL, G.W., & HARDY, R.W.F. (1968) Hydrogen
reactions of nitrogenase. J. biol. Chem., 243: 4952-4958.
JACOBSON, K.H., CLEM, J.H., WHEELWRIGHT, H.J., RINEHART, W.E., &
MAYES, N. (1955) The acute toxicity of the vapours of some
methylated hydrazine derivatives. Am. Med. Assoc. Arch. Ind.
Health, 12: 609-616.
JACOBSON, K.H., RINEHART, W.E., WHEELWRIGHT, H.J., ROSS, M.A.,
PAPIN, J.L., DALY, R.C., GREENE, E.A., & GROFF, W.A. (1958)
The toxicology of an aniline-furfurylalcohol-hydrazine vapour
mixture. J. Am. Ind. Hyg. Assoc., 19: 91-100.
JAIN, H.K. & SHUKLA, P.T. (1972) Locus specificity of mutagens
in Drosophila. Mutat. Res., 14: 440-442.
JAIN, H.K., RAUT, R.N., & KHAMANKAR, Y.G. (1968) Base specific
chemicals and mutation analysis in Lycopersicon. Heredity, 23:
247-256.
JOHNSON, F.B. (1982) Chemical interactions with Herpes
simplex type 2 virus: enhancement of transformation by hydrazine
and 1,2-dimethylhydrazine. Chem.-biol. Interact., 40: 97-112.
KAK, S.N. & KAUL, B.L. (1975) Mutagenic activity of hydrazine
and its combination with maleic hydrazide and X-rays in barley.
Cytobios, 12: 123-128.
KANE, D.A. & WILLIAMSON, K.J. (1983) Bacterial toxicity and
metabolism of hydrazine fuels. Arch. environ. Contam. Toxicol.,
12: 447-453.
KANEO, Y., IGUCHI, S., KUBO, H., IWAGIRI, N., & MATSUYAMA, K.
(1984) Tissue distribution of hydrazine and its metabolites in
rats. J. Pharm. Dyn., 7: 556-562.
KELLER, W.C., OLSON, C.T., BACK, K.C. (1982) Evaluation of the
embryotoxicity of hydrazine in rats, Ohio, Wright-Patterson Air
Force Base, Aerospace Medical Research Laboratory (AFAMRL-TR-82-
29) (AD/A119706).
KELLY, M.G., O'GARA, R.W., YANCEY, S., GADEKAR, K., BOTKIN, C.,
& OLIVERIO, V.T. (1969) Comparative carcinogenicity of N -
isopropyl-alpha-(2-methyl-hydrazino)- p -toluamide. HCl (pro-
carbazine hydrochloride), its degradation products, other hydra-
zines, and isonicotinic acid hydrazide. J. Natl Cancer Inst.,
42: 337-344.
KHAMANKAR, Y.G. & JAIN, H.K. (1978) A comparative study of
chemical and physical mutagens in bread wheat. Indian J. Hered.,
10: 49-57.
KIMBALL, R.F. (1976) Reversions of two proline-requiring
auxotrophs of Haemophilus influenzae by N -methyl- N' -nitro-
N-nitrosoguanidine and hydrazine. Mutat. Res., 36: 29-38.
KIMBALL, R.F. (1977) The mutagenicity of hydrazine and some of
its derivatives. Mutat. Res., 39: 111-126.
KIMBALL, R.F. & HIRSCH, B.F. (1975) Tests for the mutagenic
action of a number of chemicals on Haemophilus influenzae with
special emphasis on hydrazine. Mutat. Res., 30: 9-20.
KIRKHART, B. (1981) Micronucleus test on 21 compounds. In: de
Serres, F.J. & Ashby, J., ed. Evaluation of short-term tests for
carcinogens. Report of the International Collaborative
Programme, New York, Amsterdam, Oxford, Elsevier/North Holland,
pp. 698-704 (Progress in Mutation Research Vol. 1).
KIRKLIN, J.K., WATSON, M., BONDOC, C.C., & BURKE, J.F. (1976)
Treatment of hydrazine-induced coma with pyridoxine. New Engl.
J. Med., 294: 938-939.
KOPYLOVA, T.N., MAJORE, A., ELERTE, D., NOZDRUNOVA, N.A., &
ROZENBERG, I.E. (1982) [Lipid peroxidation in hydrazine
hydrochloride-induced toxicity of the liver.] Eksp. Med., 14:
35-45 (in Russian).
KORTY, P. & COE, F. (1968) The effects of hydrazine on the
concentration of free amino acids of plasma and urine. J.
Pharmacol. exp. Ther., 160 212-216.
KROP, S. (1954) Toxicology of hydrazine: a review. Arch. ind.
Hyg. occup. Med., :9: 199-204.
LAMB, R.G. & BANKS, W.L. (1979) Effect of hydrazine exposure
on hepatic triacylglycerol biosynthesis. Biochim. Biophys. Acta,
574: 440-447.
LEE, S.H. & ALEYASSINE, H. (1970) Hydrazine toxicity in
pregnant rats. Arch. environ. Health, 21: 615-619.
LEMONTT, J.F. (1977) Loss of hydrazine-induced mutability in
wild-type and excision-repair-defective yeast during post-
treatment inhibition of cell division. Mutat. Res., 50: 57-66.
LEMONTT, J.F. (1978) Mutagenesis of yeast by hydrazine:
dependence upon post-treatment cell division. Mutat. Res., 43:
165-178.
LEWALTER, J., PFLANITZER, K., MULLER, G., & SCHALLER, K.H.
(1984) [Hydrazine levels in blood (plasma).] In: [ Analytical
methods,] Vol. 2, pp. D1-D3 (Senate Commission for the Testing
of Health-damaging Work Material in the German Research
Association-Working Group "Analytical Chemistry") (in German).
LIU, Y.Y., SCHMELTZ, I., & HOFFMANN, D. (1974) Chemical
studies on tobacco smoke: quantitative analysis of hydrazine in
tobacco and cigarette smoke. Anal. Chem., 46: 885-889.
LONDON, S.A., MANTEL, C.R., ROBINSON, J.D., & LUKING, S. (1983)
Effects of selected hydrazines on the early death rates of
Enterobacter cloacae. Bull. environ. Contam. Toxicol., 31: 360-
368.
LOPEZ-MENDOZA, D. & VILLA-TREVINO, S. (1971) Hydrazine-
induced inhibition of amino acid incorporation into rat liver
protein. Lab. Invest., 25: 68-72.
LOPRIENO, N. (1981) Screening of coded carcinogenic/non-
carcinogenic chemicals by a forward mutation system with the
yeast Schizosaccharomyces pombe. In: de Serres, F.J. & Ashby,
J., ed. Evaluation of short-term tests for carcinogens. Report
of the International Collaborative Programme, New York,
Amsterdam, Oxford, Elsevier/North Holland, pp. 425-433 (Progress
in Mutation Research Vol. 1).
LYNG, R.D., KELLER, W.C., & BACK, K.C. (1980) Effects of
hydrazine on pregnant ICR mice, Ohio, Wright-Patterson Air Force
Base, Aerospace Medical Research Laboratory (AFAMRL-TR-80-19)
(AD/A084023).
MCCORMICK, D.B. & SNELL, E.E. (1961) Pyridoxal phospho-
kinases. II. Effects of inhibitors. J. biol. Chem., 236: 2085-
2088.
MCKENNIS, H., WEATHERBY, J.H., & WITKIN, L.B. (1955) Studies
on the excretion of hydrazine and metabolites. Am. Med. Assoc.
Arch. Ind. Health, 12: 511-514.
MCKENNIS, H., YARD, A.S., WEATHERBY, J.H., & HAGY, J.A. (1959)
Acetylation of hydrazine and the formation of 1,2-diacetyl-
hydrazine in vivo . J. Pharmacol. exp. Ther., 126: 109-116.
MACNAUGHTON, M.G., STAUFFER, T.B., & STONE, D.A. (1981)
Environmental chemistry and management of hydrazine. Aviat.
Space environ. Med., 52: 149-153.
MACRAE, W.D. & STICH, H.F. (1979) Induction of sister
chromatid exchanges in Chinese hamster ovary cells by thiol and
hydrazine compounds. Mutat. Res., 68: 351-365.
MARSHALL, C.E., WATTS, D.I., & SUGDEN, M.C. (1983) Effects of
hydrazine on liver and brown adipose tissue lipogenesis in 24 h-
starved rats. J. Pharm. Pharmacol., 35: 460-461.
MARU, G.B. & BHIDE, S.V. (1982) Effects of antioxidants and
antitoxicants of isoniazid on the formation of lung tumours in
mice by isoniazid and hydrazine sulfate. Cancer Lett., 17: 75-
80.
MATSUI, F., ROBERTSON, D.L., & LOVERING, E.G. (1983)
Determination of hydrazine in pharmaceuticals. III. Hydralazine
and isoniazid using GLC. J. pharmacol. Sci., 72: 948-951.
MATSUYAMA, K., YAMASHITA, C., SENDOH, T., NODA, A., GOTO, S., &
IGUCHI, S. (1983) Further investigations on brain distribution
of hydrazine and its gamma-aminobutyric acid elevating effect in
rats. J. Pharmacobio-Dyn., 6: 932-937.
MEDINA, M.A. (1963) The in vivo effects of hydrazines and
vitamin B6 on the metabolism of gamma-aminobutyric acid. J.
Pharmacol. exp. Ther., 140: 133-137.
MEHTA, R.D. & VON BORSTEL, R.C. (1981) Mutagenic activity of
42 uncoded compounds in the haploid yeast reversion assay,
strain XV 185-14C. In: de Serres, F.J. & Ashby, J., ed.
Evaluation of short-term tests for carcinogens. Report of the
International Collaborative Programme, New York, Amsterdam,
Oxford, Elsevier/North Holland, pp. 414-423 (Progress in
Mutation Research Vol. 1).
MENON, M.M. & BHIDE, S.V. (1983) Perinatal carcinogenicity of
isoniazide (INH) in Swiss mice. J. cancer Res. clin. Oncol.,
105: 258-261.
MILIA, U., BIANCIFIORI, C., & SANTILLI, F.E.G. (1965) Late
findings in pulmonary carcinogenesis by hydrazine sulfate in
newborn BALB/c/Cb/Se substrain mice. Lav. Ist. Anat. Istol.
Patol. Univ. Studio Perugia, 25: 165-171.
MILO, G.E., OLDHAM, J.W., ZIMMERMAN, R., HATCH, G.G., &
WEISBRODE, S.A. (1981) Characterization of human cells
transformed by chemical and physical carcinogens in vitro . In
vitro, 17: 719-729.
MITCHELL, P.C.H. & SCARLE, R.D. (1972) Role of reactions of
molybdenum compounds with hydrazine in nitrogen fixation.
Nature (Lond.), 240: 417-418.
NATARAJAN, A.T. & VAN KESTEREN-VAN LEEUWEN, A.C. (1981)
Mutagenic activity of 20 coded compounds in chromosome
aberrations/sister chromatid exchanges assay using Chinese
hamster ovary (CHO) cells. In: de Serres, F.J. & Ashby, J., ed.
Evaluation of short-term tests for carcinogens. Report of the
International Collaborative Programme, New York, Amsterdam,
Oxford, Elsevier/North Holland, pp. 551-559 (Progress in
Mutation Research Vol. 1).
NELSON, S.D. & GORDON, W.P. (1982) Metabolic activation of
hydrazines. Adv. exp. Med. Biol., 136B: 971-981.
NEPSS (1975) Disposal of wastewater containing hydrazine. In:
Pollution solution, California, Naval Environmental Protection
Support Service, Navy Environmental Support Office.
NODA, A., GOROMARU, T., MATSUYAMA, K., SOGABE, K., HSU, K.-Y., &
IGUCHI, S. (1978) Quantitative determination of hydrazines
derived from isoniazid in patients. I. J. Pharm. Dyn., 1: 132-
141.
NODA, A., HSU, K., NODA, H., YAMAMOTO, Y.D., & KUROZUMI, T.
(1983) Is isoniazid hepatotoxicity induced by the metabolite
hydrazine? Sangyo Ika Faigaku Zasshi, 5: 183-189.
NODA, A., NODA, H., OHNO, K., SENDO, T., MISAKA, A., KANAZAWA,
Y., ISOBE, R., & HIRATA, M. (1985a) Spin trapping of a free
radical intermediate formed during microsomal metabolism of
hydrazine. Biochem. biophys. Res. Commun., 133: 1086-1091.
NODA, A., SENDO, T., OHNO, K., GOTO, S., NODA, H., & HUS, K.-Y.
(1985b) Effects of rifampicin and phenobarbital on the fate of
isoniazid and hydrazine in vivo in rats. Toxicol. Lett., 25:
313-317.
O'BRIEN, R.D., KIRKPATRICK, M., & MILLER, P.S. (1964)
Poisoning of the rat by hydrazine and alkylhydrazines. Toxicol.
appl. Pharmacol., 6: 371-377.
PARODI, S., DE FLORA, S., CAVANNA, M., PINO, A., ROBBIANI, L.,
BENNICELLI, C., & BRAMBILLA, G. (1981) DNA-damaging activity
in vivo and bacterial mutagenicity of sixteen hydrazine
derivatives as related quantitatively to their carcinogenicity.
Cancer Res., 41: 1469-1482.
PATRICK, R.L. & BACK, K.C. (1965) Pathology and toxicology of
repeated doses of hydrazine and 1,1-dimethylhydrazine in monkeys
and rats. Ind. Med. Surg., 34: 430-435.
PERRY, P.E. & THOMSON, E.J. (1981) Evaluation of the sister
chromatid exchange method in mammalian cells as a screening
system for carcinogens. In: de Serres, F.J. & Ashby, J., ed.
Evaluation of short-term tests for carcinogens. Report of the
International Collaborative Programme, New York, Amsterdam,
Oxford, Elsevier/North Holland, pp. 560-569 (Progress in
Mutation Research Vol. 1).
PERRY, T.L., KISH, S.J., HANSEN, S., WRIGHT, J.M., WALL, R.A.,
DUNN, W.L., & BELLWARD, G.D. (1981) Elevation of brain GABA
content by chronic low-dosage administration of hydrazine, a
metabolite of isoniazid. J. Neurochem., 37: 32-39.
PITTS, J.N., TUAZON, E.C., CARTER, W.P., WINER, A.M., HARRIS,
G.W., ATKINSON, R., & GRAHAM, R.A. (1980) Atmospheric
chemistry of hydrazines: gas phase kinetics and mechanistic
studies, Florida, Tyndall Air Force Base, Air Force Engineering
and Services Centre, Engineering and Services Laboratory
(AFESC/ESL-TR-80-39) (AD/A093486).
PRADHAN, S.N. & ZIECHECK, L.N. (1971) Effect of hydrazine on
behaviour in rats. Toxicol. appl. Pharmacol., 18: 151-157.
PROTEAU, J.P., LIM, P., & LABAT, R. (1979) Toxicité d'un
dérivé bi-azolé, l'hydrate d'hydrazine, pour Carassius
carassius L., Rutilus rutilus L., et différents stades de
développement de Brachydanio rerio H.B. Ann. Limnol., 15: 337-
346.
PURCHASE, I.F.H., LONGSTAFF, E., ASHBY, J., STYLES, J.A.,
ANDERSON, D., LEFEVRE, P.A., & WESTWOOD, F.R. (1978) An
evaluation of 6 short-term tests for detecting organic chemical
carcinogens. Br. J. Cancer, 37: 873 - 935.
RAPHAELIAN, L.A. (1966) Hydrazine. In: Kirk, R.E. & Othmer,
D.F., ed. Encylopaedia of chemical technology, 2nd ed., New
York, John Wiley and Sons, Vol. 11, pp. 164-196.
RAY, P.D., HANSON, R.L., & LARDY, H.A. (1970) Inhibition by
hydrazine of gluconeogenesis in the rat. J. biol. Chem., 245:
690-696.
REDDY, G.M. & REDDY, T.P. (1972) Induction of mutations by
hydrazine in rice. Indian J. Genet. Plant Breed., 32: 388-391.
REID, F.J. (1965) Hydrazine poisoning. Br. med. J., 5472: 1246.
REIDENBERG, M.M., DURANT, P.J., HARRIS, R.A., DE BOCCARDO, G.,
LAHITA, R., & STENZEL, K.H. (1983) Lupus erythaematosus-like
disease due to hydrazine. Am. J. Med., 75: 365-370.
REYNOLDS, B.A. & THOMAS, A.A. (1965) A colorimetric method for
the determination of hydrazine and monomethylhydrazine in blood.
Am. Ind. Hyg. Assoc. J., 26: 527-531.
ROBERGE, A., GOSSELIN, C., & CHARBONNEAU, R. (1971) Effect of
hydrazine on urea cycle enzymes in vitro and in vivo . Biochem.
Pharmacol., 20: 2231-2238.
ROBINSON, D.E. & MITCHELL, A.D. (1981) Unscheduled DNA
synthesis response of human fibroblasts, W1-38 cells, to 20
coded chemicals. In: de Serres, F.J. & Ashby, J., ed.
Evaluation of short-term tests for carcinogens. Report of the
International Collaborative Programme, New York, Amsterdam,
Oxford, Elsevier/North Holland, pp. 517-527 (Progress in
Mutation Research Vol. 1).
ROE, F.J.C., GRANT, G.A., & MILLICAN, D.M. (1967) Carcino-
genicity of hydrazine and 1,1-dimethylhydrazine for mouse lung.
Nature (Lond.), 216: 375-376.
ROGAN, E.G., WALKER, B.A., GINGELL, R., NAGEL, D.L., & TOTH, B.
(1982) Microbial mutagenicity of selected hydrazines. Mutat.
Res., 89(4): 321-328.
ROGERS, A.M. & BACK, K.C. (1981) Comparative mutagenicity of
hydrazine and 3 methylated derivatives in L5178Y mosue lymphomas
cells. Mutat. Res., 16: 189-194.
ROHRBORN, G., PROPPING, P., & BUSELMAIER, W. (1972) Mutagenic
activity of isoniazid and hydrazine in mammalian test systems.
Mutat. Res., 16: 189-194.
ROSENKRANZ, H.S. & POIRIER, L.A. (1979) Evaluation of the
mutagenicity and DNA-modifying activity of carcinogens and non-
carcinogens in microbial systems. J. Natl Cancer Inst., 62: 873-
892.
ROTHBERG, S. & COPE, O.B. (1956) Toxicity studies on hydra-
zine, methylhydrazine, symmetrical dimethylhydrazine, unsym-
metrical dimethylhydrazine, and DMNA, Maryland, Army Chemical
Center (US Army Chemical Warfare Laboratory Report No. 2027).
SALOMONE, M.F., HEDDLE, J.A., & KATZ, M. (1981) Mutagenic
activity of 41 compounds in the in vivo micronucleus assay.
In: de Serres, F.J. & Ashby, J., ed. Evaluation of short-term
tests for carcinogens. Report of the International Collaborative
Programme, New York, Amsterdam, Oxford, Elsevier/North Holland,
pp. 686-697 (Progress in Mutation Research Vol. 1).
SAVCHENKOV, M.F. & SAMOILOVA, T.J. (1984) [Effect of hydrazine
nitrate on reproductive function of albino rats.] In:[ Problems
of limitation of environmental pollutants circulation,]Ufa, pp.
82-84 (in Russian).
SCALES, M.D.C. & TIMBRELL, J.A. (1982) Studies on hydrazine
hepatotoxicity. I. Pathological findings. J. Toxicol. environ.
Health, 10: 941-953.
SCHIESSL, H.W. (1980) Hydrazine and its derivatives. In: Kirk,
R.E. & Othmer, E.F., ed. Encyclopaedia of chemical technology,
3rd ed., New York, John Wiley and Sons, Vol. 12, pp. 734-771.
SCHILLER, C.M., WALDEN, R., & KEE, T.E. (1979) Effects of
hydrazine and its derivatives on the development of intestinal
brush border enzymes. Toxicol. appl. Pharmacol., 49: 305-311.
SCHMIDT, E.W. (1984) Hydrazine and its derivatives: preparation,
properties, and applications, New York, John Wiley and Sons.
SCHULTHEISS, E. (1959) [Hypersensitiveness to hydrazine.]
Berufsdermatosen, 7: 131-136 (in German).
SEVERI, L. & BIANCIFIORI, C. (1968) Hepatic carcinogenesis in
CBA/Cb/Se mice and Cb/Se rats by isonicotinic acid hydrazine and
hydrazine sulfate. J. Natl Cancer Inst., 41: 331-349.
SHANK, R.C. (1983) Evidence for indirect genetic damage as
methylation of DNA guanine in response to cytotoxicity. Dev.
Toxicol. environ. Sci., 11: 145-152.
SHUKLA, P.T. (1972) Analysis of mutagen specificity in
Drosphila melanogaster. Mutat. Res., 16: 363-371.
SINA, J.E., BEAN, C.L., DYSART, G.R., TAYLOR, V.I., & BRADLEY,
M.O. (1983) Evaluation of the alkaline elution/rat hepatocyte
assay as a predictor of carcinogenic/mutagenic potential.
Mutat. Res., 113: 357-391.
SINGH, H.B., LUDWIG, F.L., & JOHNSON, W.B. (1978) Tropospheric
ozone: concentrations and variabilities in clean remote
atmospheres. Atmos. Environ., 12: 2185-2191.
SLONIM, A.R. (1977) Acute toxicity of selected hydrazines to
the common guppy. Water Res., 11: 889-895.
SLONIM, A.R. & GISCLARD, J.B. (1976) Hydrazine degradation in
aquatic systems. Bull. environ. Contam. Toxicol., 16: 301-309.
SMITH, E.B. & CLARK, D.A. (1972) Absorption of hydrazine
through canine skin. Toxicol. appl. Pharmacol., 21: 186-193.
SONNECK, E.B. & UMLAUF, H. (1961) [Occupationally sustained
skin damage by hydrazine.] Haut-Geschlechskr., 31: 179-184 (in
German).
SOTANIEMI, E., HIRVONEN, J., ISOMAKI, H., TAKKUNEN, J., & KAILA,
J. (1971) Hydrazine toxicity in the human. Report of a fatal
case. Am. clin. Res., 3: 30-33.
SOTOMAYER, R.E., CHAUHAN, P.S., & EHLING, U.H. (1982) Induc-
tion of unscheduled DNA synthesis in the germ cells of male mice
after treatment with hydrazine or procarbazine. Toxicology, 25:
201-211.
SPEIT, G., WICK, C., & WOLF, M. (1980) Induction of sister
chromatid exchanges by hydroxylamine, hydrazine, and isoniazid
and their inhibition by cysteine. Hum. Genet., 54: 155-158.
SPINKOVA, (1971) [Determination of small amounts of hydrazine
in isoniazid solutions.] Pharm. Acta Helv., 46: 643-648 (in
German).
SPRINGER, D.L., BRODERICK, D.J., & DOST, F.N. (1980) Effects
of hydrazine and its derivatives on ornithine decarboxylase
synthesis, activity, and inactivation. Toxicol. appl.
Pharmacol., 53: 365-372.
SPRINGER, D.L., KRIVAK, B.M., BRODERICK, D.J., REED, D.J., &
DOST, F.N. (1981) Metabolic fate of hydrazine. J. Toxicol.
environ. Health, 8: 21-29.
STEIN, E.R., CLARK, D.A., & FORTNEY, S.R. (1971) Inhibition of
glutamicoxaloacetic transaminases of rat liver by hydrazine.
Toxicol. appl. Pharmacol., 18: 274-284.
SUGGS, H.J., LUSKUS, L.J., & KILIAN, H.J. (1980) A field
procedure for sampling and analysis of low concentrations of
hydrazine in air. Am. Ind. Hyg. Assoc. J., 41: 879-883.
THOMSON, J.A. (1981) Mutagenic activity of 42 coded compounds
in the lambda induction assay. In: de Serres, F.J. & Ashby, J.,
ed. Evaluation of short-term tests for carcinogens. Report of
the International Collaborative Programme, New York, Amsterdam,
Oxford, Elsevier/North Holland, pp. 224-235 (Progress in
Mutation Research Vol. 1).
THORNELEY, R.N.F., EADY, R.R., & LOWE, D.J. (1978) Biological
nitrogen fixation by way of an enzyme-bound dinitrogen-hydride
intermediate. Nature (Lond.), 272: 557-558.
TIMBRELL, J.A. & HARLAND, S.J. (1979) Identification and
quantitation of hydrazine in the urine of patients treated with
hydralazine. Clin. Pharmacol. Ther., 26: 81-88.
TIMBRELL, J.A., WRIGHT, J.M., & SMITH, C.M. (1977)
Determination of hydrazine metabolites of isoniazid in human
urine by gas chromatography. J. Chromatogr., 138: 165-172.
TIMBRELL, J.A., SCALES, M.D., & STREETER, A.J. (1982) Studies
on hydrazine hepatotoxicity. II. Biochemical findings. J.
Toxicol. environ. Health, 10: 955-968.
TOMLINSON, T.G., BOON, A.G., & TROTMAN, C.N.A. (1966)
Inhibition of nitrification in the activated sludge process of
sewage disposal. J. appl. Bacteriol., 29: 266-291.
TOTH, B. (1969) Lung tumour induction and inhibition of breast
adenocarcinomas by hydrazine sulfate in mice. J. Natl Cancer
Inst., 42: 469-475.
TOTH, B. (1972a) Hydrazine, methylhydrazine, and methylhydra-
zine sulfate carcinogenesis in Swiss mice. Failure of ammonium
hydroxide to interfere in the development of tumours. Int. J.
Cancer, 9: 109-118.
TOTH, B. (1972b) Tumourigenesis studies with 1,2-dimethyl-
hydrazine dihydrochloride, hydrazine sulfate, and isonicotinic
acid in golden hamsters. Cancer Res., 32: 804-807.
TROUT, D.L. (1965) Effects of hydrazine on plasma-free fatty
acid transport. Biochem. Pharmacol., 14: 813-821.
TROUT, D.L. (1966) Effects of hydrazine on fat transport as
affected by blood glucose concentration. J. Pharmacol. exp.
Ther., 152: 529-534.
TSUCHIMOTO, T. & MATTER, B.E. (1981) Activity of coded
compounds in the micronucleus test. In: de Serres, F.J. & Ashby,
J., ed. Evaluation of short-term tests for carcinogens. Report
of the International Collaborative Programme, New York,
Amsterdam, Oxford, Elsevier/North Holland, pp. 705-711 (Progress
in Mutation Research Vol. 1).
TUAZON, E.C., CARTER, W.P.L., BROWN, R.V., ATKINSON, R., WINER,
A.M., & PITTS, J.N. (1982) Atmospheric reaction mechanisms of
amine fuels, Florida, Tyndall Air Force Base, Air Force
Engineering and Service Center, Engineering and Services
Laboratory (AFESC/ESL-TR-82-17) (AD/A118267).
US FDA (1979) Hydrazine: proposed removal from food additive
use. Fed. Reg., 44: 33693-33695.
US NIOSH (1977a) Manual of analytical methods, 2nd ed.,
Cincinnati, Ohio, US National Institute of Occupational Safety
and Health, Vol. 1, pp. S237-1 to S237-6 (US DHEW Publication
No. 77-157A).
US NIOSH (1977b) Manual of analytical methods, 2nd ed.,
Cincinnati, Ohio, US National Institute of Occupational Safety
and Health, Vol. 3, pp. 248-1 to 248-6 (US DHEW Publication No.
77-157A).
US NIOSH (1978) Criteria for a recommended standard:
occupational exposure to hydrazines, Cincinnati, Ohio, US
National Institute for Occupational Safety and Health, Vol. 3,
pp. 248-1 to 248-6 (US DHEW Publication No. 78-172).
US NIOSH (1986) Industrial hygiene study: extent of exposure
to hydrazine, Cincinnati, Ohio, US National Institute for
Occupational Safety and Health, 49 pp (Report No. 136.5;
Contract No. 200-82-2521).
VAN KETEL, W.G. (1964) Contact dermatitis from a hydrazine
derivative in a stain remover. Cross-sensitization to apresoline
and isoniazid. Acta dermatovenereol., 44: 49-53.
VAN STEE, E.W. (1965) Acute effects of exposure to hydrazine
and hydrazine derivatives on renal function in the dog. Aerosp.
Med., 36: 764-767.
VASUDEVA, M. & VASHISHAT, R.K. (1985) Mutagenic and
recombinogenic activity of hydrazine sulfate in Saccharomyces
cerevisiae. Mutat. Res., 155: 113-115.
VELTE, J.S. (1984) Acute toxicity of hydrazine hydrate to the
fathead minnow (Pimephales promelas) and Daphnid (Daphnia
pulex). Bull. environ. Contam. Toxicol., 33: 598-604.
VERNOT, E.H., MACEWEN, J.D., BRUNER, R.H., HAUN, C.C., KINKEAD,
E.R., PRENTICE, D.E., HALL, A., III, SCHMIDT, R.E., EASON, R.L.,
HUBBARD, G.B., & YOUNG, J.T. (1985) Long-term inhalation
toxicity of hydrazine. Fundam. appl. Toxicol., 5: 1050-1064.
VIJAYKUMAR, N.K. & JAIN, H.K. (1979) Comparative studies on
induced mutations with physical and chemical mutagens in
Drosophila melanogaster. Part I. Specificity and spectrum of
mutations. Indian J. exp. Biol., 17: 61-65.
VISHNOI, A.K. & GUPTA, P.K. (1980) Induced mutagenesis in
Vicia faba L. I. Chlorophyll mutations induced by gamma rays,
ethyl methane sulfonate, and hydrazine. Cytobios, 27: 81-87.
VON KEILIG, W. & SPEER, U. (1983) [Occupational allergic
contact dermatitis from hydrazine.] Derm. Beruf Umwelt, 31: 25-
27 (in German).
VON KRULIK, R. (1966) [The influence of hydrazine and
isonicotinic acid hydrazine on certain substances of the sugar
metabolism in vitro. ] Arzneim. Forsch., 16: 1623-1626 (in
German).
VON PEVNY, I. & PETER, G. (1983) [Allergic contact eczema to
derivatives of pyridine and hydrazine.] Dermatosen, 31: 78-83
(in German).
VON WRIGHT, A. & TIKKANEN, L. (1980) The comparative
mutagenicities of hydrazine and its mono- and dimethyl
derivatives in bacterial test systems. Mutat. Res., 78: 17-23.
WAKABAYASHI, T., HORIUCHI, M., SAKAGUCHI, M., ONDA, H., &
MISAWA, K. (1983) Induction of megamitochondria in the mouse
and rat livers by hydrazine. Exp. mol. Pathol., 39: 139-153.
WALD, N., BOREHAM, J., DOLL, R., & BONSALL, J. (1984)
Occupational exposure to hydrazine and subsequent risk of
cancer. Br. J. ind. Med., 41: 31-34.
WARGOVICH, M.J., GOLDBERG, M.T., NEWMARK, H.L., & BRUCE, W.R.
(1983) Nuclear aberrations as a short-term test for geno-
toxicity to the colon: evaluation of 19 agents in mice. J. Natl
Cancer Inst., 71: 133-137.
WARREN, D., CORNELIUS, C., & FORD, B. (1984) Liver function
studies on rhesus monkeys (Maccaca mulatta) following the
administration of hydrazine sulfate. Vet. Human Toxicol., 26:
295-299.
WATJE, W.F. (1978) Potential of a hydrazine-type fuel spill or
emission during movement from supplies to user. In: Proceedings
of the Conference on Environmental Chemistry: Hydrazine Fuels, 1
977, Florida, Tyndall Air Force Base, pp. 19-24 (CEEDO-TR-78-
14).
WELLS, H.G. (1908) The pathological anatomy of hydrazine
poisoning. J. exp. Med., 10: 457-462.
WHEELER, C.E., PENN, S.R., CAWLEY, E.P., & HILL, C. (1965)
Dermatitis from hydrazine hydrobromide solder flux. Arch.
Dermatol., 91: 235-239.
WITKIN, L.B. (1956) Acute toxicity of hydrazine and some of
its methylated derivatives. Arch. ind. Health, 13: 34-36.
WONG, E.T. (1966) Renal functional response to hydrazine and
1,1-dimethylhydrazine. Toxicol. appl. Pharmacol., 8: 51-56.
WOOD, J.D., RUSSELL, M.P., & KURYLO, E. (1980) The gamma-
aminobutyrate content of nerve endings (synaptosomes) in mice
after the intramuscular injection of gamma-aminobutyrate-
elevating agents: a possible role in anticonvulsant activity.
J. Neurochem., 35: 125-130.
WRIGHT, J.M. & TIMBRELL, J.A. (1978) Factors affecting the
metabolism of 14C-acetylhydrazine in rats. Drug Metab. Dispos.,
6: 561-566.
YAKSCTAT, B.Y. (1969) Experimental assessment of hydrazine
hydrate maximum allowable concentration in water bodies. In:
Industrial pollution of water bodies, Moscow, Medicine, pp.
186-200.
YOSHIDA, T. & ALEXANDER, M. (1964) Hydroxylamine formation by
Nitrosomonas europaea. Can. J. Microbiol., 10: 923-926.
ZINA, G. & BONU, G. (1962) [Occupational dermatitis from
hydrazine and derivatives.] Minerva Dermatol., 37: 197-200 (in
Italian).
 Approximately 25% of the hydrazine dose remains unaccounted
for. Ammonia was found in the blood of dogs without
significantly elevated blood-urea nitrogen (Floyd, 1980).
Springer et al. (1981) did not find labelled ammonia in the
urine of rats exposed to 15N-hydrazine (Table 4). Therefore,
the ammonia in dogs was probably not derived from hydrazine
(section 8.3.2), but was the result of an effect on metabolic
pathways (Floyd, 1980; Springer et al., 1981).
6.3. Reaction With Body Components
No adduct formation between hydrazine and DNA in vivo has
been reported (Shank, 1983). Under non-physiological conditions,
hydrazine can react with pyrimidine bases. These reactions were
reviewed by Kimball (1977). Indirect methylation of guanines in
DNA following hydrazine exposure has been demonstrated and will
be discussed in section 8.5.1.
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
7.1. Aquatic Organisms
A summary of acute toxicity data is presented in table 5. It
should be realized that the rate of decay of hydrazine in the
aquatic environment depends on the conditions (section 4.2).
When the concentration of hydrazine is not monitored during
exposure, it should be noted that the toxic effects observed
must have occurred at concentrations lower than the nominal
ones, due to degradation of the compound. the increased toxicity
of hydrazine for guppies in soft water at a pH just below 7
compared with the toxicity in hard water at a pH of
approximately 8, as found by Slonim (1977), is at least partly
explained by the increased persistence of hydrazine in soft and
non-alkaline water. Taking into account the decay of hydrazine,
increases in water temperature were found to enhance the
toxicity of the compound for bluegills (hunt et al., 1981).
Teratogenicity and toxicity screening were reported using
the South African clawed toad (Greenhouse, 1976a,b), fathead
minnow (Henderson et al., 1981), and rainbow trout (Henderson et
al., 1983). Eggs of the South African clawed toad in the
cleavage were exposed to hydrazine until hatching. Survival and
development into normal larvae occurred at exposures below 10
mg/litre. At 10 mg/litre, 35% of the embryos were malformed at
hatching. The effect was dose-related. Additional studies
revealed that teratogenic effects appeared during neurulation
(Greenhouse, 1976a). When larvae of the South African clawed
toad were exposed to 1.0 mg hydrazine/litre water, for 120 h,
all died in 24 - 48 h following exposure. No significant effects
on survival and development were observed after exposure to 0.1
mg/litre, the next lower concentration tested (Greenhouse,
1976b).
Eggs of fathead minnows at the mid-cleavage stage were
exposed to hydrazine for 24 or 48 h. Embryos, exposed for 24 h,
to 0.1 mg/litre, showed several defects, such as slightly or
moderately subnormal heart beat, haemoglobin levels, body
movement amount of eye pigment. From 1 mg/litre upwards, the
responses were generally stronger; in addition, body pigment was
absent and developmental arrest was observed. Embryos exposed to
a hydrazine concentration of 1.0 mg/litre for 48 h appeared to
have little chance of survival. Surviving embryos showed severe
deformities and larvae exhibited reduced growth (Henderson et
al., 1981).
Table 5. Acute aquatic toxicity of hydrazine
---------------------------------------------------------------------------------------------------------
Organism Tempera- pH Hardness Flow/ Parameter Concentration Reference
ture (mg CaCO3 stata (mg/litre)
(°C) /litre)
---------------------------------------------------------------------------------------------------------
Bacteria
Pseudomonas putida 20 stat 16-h TT 0.019 Bringmann &
Kühn
(1980)b
Protozoa
Entosiphon sulcatum 25 6.9 stat 72-h TT 0.93 Bringmann &
Kühn (1981)b
Uronema paraduczi 25 6.9 stat 22-h TT 0.24 Bringmann &
Kühn (1981)b
Chilomenas paramecium 20 6.9 stat 48-h TT 0.002 Bringmann &
Kühn (1981)b
Algae
Green algae
(Chlorella pyrenoidosa) 23 6.8 75 stat 48-h EC50 ca.10c Heck et al.
48-h EC100 ca.100c (1963)d
Crustacea
Water flea 20 8.0 stat 24-h EC50 2.3c Bringmann &
(Daphnia pulex) Kühn (1982)
8.2 stat 24-h LC50 1.16c Heck et al.
(1963)
20 7.1-7.2 stat 24-h LC50 0.51 and 1.01 Velte (1984)e
48-h LC50 0.16 and 0.19
Amphibia
South African clawed
toad (Xenopus laevis)
eggs 8.2-8.7 stat LOEL 10c Greenhouse
(1976a)f
larvae stat 120-h LOEL 1.0c Greenhouse
120-h NOEL 0.1c (1976b)g
Table 5. (contd.)
---------------------------------------------------------------------------------------------------------
Organism Tempera- pH Hardness Flow/ Parameter Concentration Reference
ture (mg CaCO3 stata (mg/litre)
(°C) /litre)
---------------------------------------------------------------------------------------------------------
Fish (fresh-water)
Guppy (Lebistes reti- 22-24 7.8-8.2 400-500 stat 96-h LC50 3.85c Slonim (1977)
culatus) 22-24 6.3-6.9 20-25 stat 96-h LC50 0.61c
Fathead minnow
(Pimephales promelas)
eggs 21 7.0-7.5 150 flow 48-h LOEL 0.1 Henderson et
48-h NOEL 0.001 al. (1981)h
adults 20 192 stat 96-h LC50 4.5c Cowen et al.
(1981)
adults 20 6.9 flow 96-h LC50 5.98 Velte (1984)e
Bluegill sunfish 23-24 7.2-8.4 240-292 stat 96-h LC50 1.08 Fisher et al.
(Lepomis macrochirus) (1980)
23-24 7.8-7.9 164 flow 96-h LOEL 0.43 Fisher et al.
(1980)i
23-24 7.1-7.9 239 stat 96-h LOEL 0.1 Fisher et al.
(1980)i
10 6.7-8.0 160-190 flow 96-h LC50 1.6 Hunt et al.
15.5 1.0 (1981)
21 1.2
Goldfish (Carassius 8.2-8.5 stat 48-h LC50 2.8c Heck et al.
auratus) (1963)j
19 8.1-8.5 135 stat 24-h LC50 0.95 Proteau et al.
(1979)
Roach (Rutilus 19 8.1-8.5 135 stat 24-h LC50 0.54 Proteau et al.
rutilus) (1979)
Zebra fish
(Brachydanio rerio)
5-day-old 26 7.8 110 stat 24-h LC50 0.75 Proteau et al.
(1979)
3-month-old 20 7.6-8.2 110 stat 24-h LC50 2.03 Proteau et al.
(1979)
Table 5. (contd.)
---------------------------------------------------------------------------------------------------------
Organism Tempera- pH Hardness Flow/ Parameter Concentration Reference
ture (mg CaCO3 stata (mg/litre)
(°C) /litre)
---------------------------------------------------------------------------------------------------------
Green sunfish (Leptomis 8.2-8.5 stat 48-h LC50 5.1c Heck et al.
(1963)j
Large mouth bass 8.2-8.5 stat 48-h LC50 3.6c Heck et al.
(1963)j
Channel catfish 8.2-8.5 stat 48-h LC50 1.6c Heck et al.
(1963)j
Fish (marine species)
Stickle back (Gaster- 14- 7.6-8.0 stat 96-h LC50 3.4c Harrah
osteus aculeatus) 15.5 (1978)k
---------------------------------------------------------------------------------------------------------
a Flow-through or static test.
b TT = toxicity threshold.
c No analysis for hydrazine during exposure was reported.
d EC50 and EC100 for 50% and 100% growth inhibition, measured by reading optical density.
e Soft water.
f LOEL = lowest-observed-adverse-effect level for teratogenicity. Exposure of eggs in cleavage stage
until hatching.
g LOEL = lowest-observed-adverse-effect level for lethality. NOEL = no-observed-effect level
for lethality and development.
h LOEL and NOEL = lowest-and-no observed-adverse-effect level for toxicity and teratogenicity.
i LOEL = lowest-observed-adverse-effect level for dorsal light response (at a non-lethal concentration).
j Standard reference water.
k Salinity, 1.8%.
Henderson et al. (1983) also exposed eggs of rainbow trout
(Salmo gairdneri) for 48 h, to hydrazine in continuous-flow
tests at 11.5 - 12 °C, a pH of 7 - 7.5, and a water hardness of
15 mg calcium carbonate/litre. During exposures up to 5 mg/litre
a dose-related increase was observed in the incidence of poorly
fitting jaws, pronounced mouth gape, and absence of body
movement. However, no effects were observed on mortality, heart
beat, hatching rate, or hatching period. Reduced growth and
abnormal development of larvae were observed at 1 and 5
mg/litre. Poor muscular development and poor bone growth were
observed; the authors postulate that this is a result of calcium
binding by hydrazine.
7.2. Microorganisms
The toxicity of hydrazine for a number of species of
bacteria, algae, and protozoa was measured by Bringmann (1975) &
Bringmann & Kuhn (1980, 1981). Some very low toxicity thresholds
were reported, for example, 0.005 mg/litre for a 7-day exposure
for the algae Scenedesmus quadricauda and 0.00008 mg/litre for a
10-day exposure for the blue algae Microcystis aerogenosa. This
is a very sensitive test.
London et al. (1983) described the toxicity of hydrazine for
the soil heterotroph Enterobacter cloacea. Hydrazine caused a
concentration-dependent increase in the lag time of this
organism. In a medium containing 10 mg/litre, this did not
affect the growth rate and final growth yield after the lag
period. At 100 mg/litre, the bacteria were not viable.
Although relatively high concentrations of hydrazine in
water have been recorded as inhibiting, either completely or
partially, the activities of Nitrosomonas, Nitrobacter, and
other bacteria in culture media (Yoshida & Alexander, 1964) in
waste-water treatment (Tomlinson et al., 1966; Farmwald &
MacNaughton, 1981; Kane & Williamson, 1983), the highest
continuously maintained tolerable level in waste water is of the
order of 1 mg/litre, as stated in section 4.3 (Farmwald &
MacNaughton, 1981).
7.3. Plants
Heck et al. (1963) studied the effects of hydrazine on the
germination of seeds and seedling growth after application as a
hydroponic culture contaminant and an air fumigant.
Seeds of summer brush squash (Cucurbita pepo), peanut
(Arachis hypogaea), and corn (Zea mays) were soaked for 48 h in
water containing hydrazine at levels of between 0 and 1000
mg/litre. The temperature was 30 °C. At the highest concentra-
tion, germination of peanut and corn seed was inhibited. Seed-
ling growth was inhibited from 10 mg/litre for squash, 100
mg/litre for corn, and 1000 mg/litre for peanut.
Sixteen-day-old seedlings of cotton (Gossypium hirsutum) in
a hydroponic culture were exposed to hydrazine in the growth
medium for 9 days at concentrations of between 0 and 1000
mg/litre and a temperature of between 22 and 29 °C. Plants died
within 48 h of exposure to 300 mg/litre and within 30 h at the
higher concentrations. Injury was first noted as foliar
dehydration, without chlorosis or necrosis, after 9 days of
exposure to 50 mg/litre or within 24 h of exposure to 300
mg/litre or more.
Several plants were also exposed for 4 h to hydrazine vapour
at concentrations of between 0 and 100 mg/m3 air. Species tested
were soybean (Glycine max), cow pea (Vigna sinensis), pinto bean
(Phaseolus vulgaris), cotton (Gossypium hirsutum), endive
(Cichorium endivia), alfalfa (Medicago sativa), and squash
(Cucurbita pepo). Wilting of leaves in all species was
observed within 2 - 24 h of exposure to 30 mg/m3, followed by
wilting of the whole plant. Death occurred in pinto beans and
endive plants at this exposure level. At higher concentrations,
plants of soybean and alfalfa also died. Six days after
exposure, all surviving plants started to recover.
8. EFFECTS ON EXPERIMENTAL ANIMALS
In section 8, all doses have been expressed in terms of the
free base; however, the form of hydrazine used in each study has
been indicated when possible.
8.1. Single Exposures
The toxicology of hydrazine has been reviewed by Krop
(1954), Clark et al. (1968), and US NIOSH (1978).
LD50 values for rats and mice after oral, iv, and ip expo-
sure were not significantly dependent on the route of exposure
and ranged from 55 to 64 mg/kg body weight for rats and from 57
to 82 mg/kg body weight for mice (free base or hydrazine
hydrate) (Witkin, 1956; O'Brien, 1964; Yaksctat, 1969; Azar et
al., 1970). Oral LD50 values for hydrazine (hydrazine hydrate)
in guinea-pigs and rabbits were 26 and 35 mg/kg body weight,
respectively (Yaksctat, 1969). Dogs and rabbits appeared more
sensitive, LD50 values following iv injection being 25 and 26
mg/kg body weight, respectively. The dermal LD50 for the rabbit
was 93 mg hydrazine (free base)/kg body weight (Rothberg & Cope,
1956; Witkin, 1956). When doses between 96 and 481 mg/kg body
weight (free base) were applied to the skin of dogs, 10 out of
25 animals died within the 6-h observation period; a dose-effect
relationship was not observed (Smith & Clark, 1972). When rats
and mice inhaled hydrazine (free base) for 4 h, the LC50s were
750 and 330 mg/m3, respectively (Jacobson et al., 1955). Death
occurred quickly in both species. Lethal doses of hydrazine
usually induced convulsions, excitement or inactivity, and other
effects on the central nervous system. Rats and mice inhaling
lethal concentrations of hydrazine (free base) also showed
dyspnoea (Comstock et al., 1954; Jacobson et al., 1955; O'Brien,
1964). Spontaneous motor activity depression was noted in rats
at ip doses of 39 and 52 mg/kg body weight (hydrazine sulfate)
(Pradhan & Ziecheck, 1971). Dogs receiving a sublethal iv dose
(free base) did not exhibit convulsions but showed increased
neuromuscular activity, salivation, diarrhoea, vomiting, and
hyperventilation (Wong, 1966).
Few pathological changes have been reported following acute
lethal doses. Some rats that died after inhaling hydrazine (free
base), showed lung oedema with localized damage to the bronchial
mucosa (Comstock et al., 1954). Wells (1908) observed fatty
changes in the liver in 24 h following sublethal doses in many
species. Fatty changes have also been observed in the kidneys of
rats (free base or hydrazine hydrate) (Dominguez et al., 1962;
Scales & Timbrell, 1982). In rats, accumulation of lipid,
swelling of mitochondria, and an increased number of microbodies
were observed in the liver and in the proximal tubules of the
kidneys, 24 h after an ip dose of 20 or 30 mg/kg body weight
(hydrazine hydrate). Similar changes were observed within 1 h,
after doses of 40 or 60 mg/kg body weight (Scales & Timbrell,
1982). In addition, nuclear and nucleolar enlargement and
hypertrophy of the smooth endoplasmic reticulum were observed in
the liver of rats, 2 or more hours after a single
intraperitoneal dose of 64 mg/kg body weight (hydrazine sulfate)
(Ganote & Rosenthal, 1968). Studies on the mechanisms by which
hydrazine causes these effects will be discussed later (section
8.3) together with other effects on the intermediary metabolism,
notably hypoglycaemia and lipid peroxidation, and effects on the
central nervous system, such as an increase in gamma-
aminobutyrate levels in the brain.
In dogs given a sublethal dose of hydrazine (free base),
degeneration of the proximal convoluted tubules of the kidneys
was accompanied by decreased creatinine clearance and increased
glucose reabsorption by the tubules. The glomerular filtration
rate was decreased because of decreased renal blood flow (Van
Stee, 1965; Wong, 1966).
In rhesus monkeys treated intravenously with 2.5 - 9.8 mg
hydrazine (hydrazine sulfate)/kg, liver function tests were
generally within normal limits up to 72 h after dosing. A dose
of 80 mg/kg caused fatty liver, but no necrosis (Warren et al.,
1984).
8.2. Short-Term Exposures
8.2.1. Inhalation exposure
In a 6-month inhalation study, groups of 50 male Sprague
Dawley rats, 40 female ICR mice, 8 male Beagle dogs, and 4
female rhesus monkeys were exposed to 0.26 or 1.3 mg hydrazine
(free base)/m3 air, continuously, or 1.3 or 6.5 mg hydrazine/m3
air, for 6 h/day, 5 days/week. Exposure concentrations were
monitored. Control groups contained the same number of animals.
The exposure regimen was chosen in such a way that the weekly
doses received by the continuously exposed groups were approxi-
mately equal to the weekly doses of hydrazine received by the
intermittently exposed groups. An increased mortality rate was
only seen in mice at the 2 higher exposure levels. In rats,
there was a dose-related decrease in body weight gain, while
body weights of dogs were decreased at the 2 higher exposure
levels. In dogs, the reduced weights were at least partly due to
reduced food consumption. Weights of the exposed monkeys were
comparable with those of controls. Organ weights were unaffected
by the exposure in rats, dogs, and monkeys. Organ and body
weights of mice were not recorded. Central nervous system
effects observed included lethargy in mice at the 2 higher
exposure levels, and tonic convulsions in 1 dog exposed
continuously to 1.3 mg/m3. Monkeys exhibited slight eye irrita-
tion at the 2 higher exposure levels. Fatty changes of the liver
were observed in mice at all exposures and in dogs at the 2
higher exposure levels. The livers of exposed monkeys showed
slight-to-moderate fat accumulation. However, this was also
seen, to some extent, in control animals. Livers of rats were
normal. Finally, dogs exhibited reduced red blood cell counts,
haematocrit, and haemoglobin values at the 2 higher exposure
levels, together with an increased resistance to osmotic haemo-
lysis at all exposure levels. Haematological variables were
normal in rats and monkeys and were not measured in mice. In
dogs, the effects on the liver and the haematological variables
appeared reversible (Haun & Kinkead, 1973).
Decreases in red blood cell count and haematocrit were also
observed in 20 female Swiss mice exposed to 130 mg hydrazine
(free base)/m3 air for 1 h/day, 6 days/week, for 4 weeks. In
this study, a decreased osmotic resistance to haemolysis was
noted in exposed mice (Cier et al., 1967).
Groups of 10 - 30 male Wistar rats were exposed to hydrazine
(free base) at average concentrations of 6, 18, 26, 70, or 295
mg/m3 air, for 5 days/week, 6 h/day, over periods ranging from 5
to 40 days at the 3 highest exposure levels to approximately 6
months at the 2 lowest exposure levels. The control group
consisted of 10 rats. Increased mortality was observed at all
exposure levels but not in controls, and body weights were
decreased at the 3 highest exposure levels. Rats became sluggish
during the 6-month exposure, while at the 3 highest exposure
levels, an initial restlessness was followed by a tendency to
sleep. In some cases, pathological examination revealed lung
oedema with local damage to the bronchial mucosa at the 3
highest exposure levels. Fatty livers were observed in many rats
after 5 days of exposure at 295 mg/m3 (Comstock et al., 1954).
8.2.2. Other routes of exposure
Groups of 25 male Sprague Dawley rats were dosed ip with 10
or 20 mg hydrazine (free base)/kg body weight, 5 times per week,
for 5 weeks. The control group consisted of 15 rats. Mortality
was increased at the dose of 20 mg/kg body weight; 10/25 rats
died after 8 - 21 doses. Body weight was lost in a dose-related
manner; 4.4 and 25.7% of the initial body weight was lost in 10
days in the 10 and 25 mg/kg groups, respectively. At 20 mg/kg
body weight, rats also displayed weakness and lethargy, and 2
rats exhibited convulsions. Pathological examination revealed
hyperaemia and oedema in the lungs of 4 rats and slight fatty
vacuolation in the liver of 7 rats at 20 mg/kg body weight. At
both doses, the haematocrit values were maximally decreased
after 13 injections (Patrick & Back, 1965).
Patrick & Back (1965) treated 12 rhesus monkeys ip with
hydrazine (free base), 5 times per week. Six monkeys received 5
mg/kg body weight for 4 weeks; two of these monkeys received a
further 8 doses of 10 mg/kg body weight, followed by 4 or 5
doses of 20 mg/kg body weight. A group of 6 monkeys received
only 4 or 5 doses of 20 mg/kg body weight. The control group
consisted of 10 monkeys. No monkey died, but all exposed monkeys
showed decreased body weights. Lethargy, weakness, and vomiting
were see in 7 of the 8 monkeys exposed to 20 mg/kg body weight,
while tremors were seen in 1 of these monkeys. Fatty changes
were observed in the liver, proximal tubules of the kidneys,
heart, and skeletal muscles at 20 mg/kg body weight, and
occasionally at 5 mg/kg body weight. Extensive periportal
necrosis was found in the liver of one of the dosed monkeys. The
level of bilirubin was increased and the serum was icteric.
Haematocrit and haemoglobin values, measured at the lower dose
only, dropped slightly, relative to control values (Patrick &
Back, 1965).
The pathological effects on the liver were also investigated
microscopically, in groups of 20 - 29 male DDY mice and 10 male
Wistar rats, after administration of 5, 10, or 20 mg hydrazine
(free base)/kg powdered diet, for 3 - 10 days. No animals died.
Animals of both species exhibited weakness. Megamitochondria or
fatty vacuolation with moderately swollen mitochondria and focal
proliferation of the smooth endoplasmic reticulum were induced
in rats and mice at dose levels of 10 and 20 mg/kg feed. The
induction of megamitochondria was a reversible process (Waka-
bayashi et al., 1983). Noda et al. (1983) observed centrilobular
hepatic necrosis in male rabbits dosed for 5 days with between
14.6 and 32.3 mg hydrazine (hydrazine monohydro-chloride)/kg
body weight per day, iv.
In other studies, hydrazine (hydrazine hydrate) was given
orally in drinking-water to albino rats and guinea-pigs for 7
months at levels providing 0.3, 0.03, 0.003, and 0.0003 mg/kg
body weight per day. At the two highest doses, adverse effects
were observed in the CNS (changes in conditioned reflexes),
liver (increased I131 excretion, changes in enzyme activity,
protein dystrophia), and blood (symptoms of haemoloytic
anaemia). The dose of 0.003 mg/kg body weight was reported to be
the no-observed-adverse-effect level (Yaksctat, 1969).
8.3. Biochemical Effects and Mechanisms of Toxicity
All of the studies described in this section were performed
with doses considered to be toxic.
8.3.1. Effects on lipid metabolism
Hydrazine caused a dose-dependent increase in hepatic
triglyceride levels in rats, the threshold dose for a single ip
injection being 10 - 20 mg/kg body weight. The maximal effect,
an increase of 7 times the control value, was observed after a
dose of 40 or 60 mg hydrazine hydrate/kg body weight. At these
dose levels, the effect was measurable 4 h after injection
(Timbrell et al., 1982). Other authors have also reported
accumulation of triglycerides in the liver of rats exposed to
single doses of hydrazine via injection routes (Amenta &
Dominguez, 1965a; Clark et al., 1970; Lamb & Banks, 1979).
Several mechanisms have been proposed:
1. Increased mobilization of free fatty acids from
adipose tissue (particularly observed at low plasma-
glucose levels) leading to an increased uptake of free
fatty acids, followed by increased triglyceride
synthesis in the liver (Trout, 1965, 1966; Clark et
al., 1970). This mobilization of free fatty acids
might be caused by the effects of hydrazine on the
sympathetic nervous system and on levels of adrenal
steroid hormone, possibly in response to the hypo-
glycaemia induced by hydrazine (Amenta & Dominguez,
1965a). Cooling et al. (1979) found elevated
concentrations of circulating corticosterone and
decreased concentrations of insulin in the serum of
rats exposed to hydrazine. Decreased blood-insulin
levels were also measured in rats by Aleyassine & Lee
(1971).
2. Increased synthesis of triglycerides caused by
increased enzymatic activity of phosphatidate phospho-
hydrolase (EC 3.1.3.4) in hepatocytes both in vivo and
in vitro was reported by Lamb & Banks (1979). It has
been suggested that this was a result of increased
corticosterone levels (Cooling et al., 1979). In
addition, Marshall et al. (1983) found increased fatty
acid synthesis in the liver of rats after hydrazine
administration.
3. Triglycerides could accumulate in hepatocytes as a
result of a decreased secretion of lipoproteins from
liver to plasma (Amenta & Dominguez, 1965a; Clark et
al., 1970). This could be explained by a decreased
lipid-binding capacity of lipoproteins following an
observed alteration in the proportion of phospholipids
and cholesterol (Clark et al., 1970) or by
increased lipid peroxidation (Di Luzio et al., 1973;
Kopylova et al., 1982). The protein moiety of
lipoproteins could also be subject to change (section
8.3.2).
8.3.2. Effects on carbohydrate and protein metabolism
Rats and dogs with starvation-induced depletion of glycogen
stores showed rapidly declining plasma-glucose levels with a
concomitant rise in lactate and pyruvate levels after exposure
to single intravenous hydrazine doses of 64 (free base or hydra-
zine sulfate) and 25 mg (free base)/kg body weight,
respectively. In well-fed dogs, hyperglycaemia and depletion of
glycogen stores preceded hypoglycaemia. Acidosis developed
slowly as a result of an increased lactate-pyruvate ratio
(Fortney, 1966; Fortney et al., 1967; Ray et al., 1970).
It has been postulated that hydrazine inhibits glyconeo-
genesis (Fortney, 1966; Fortney et al., 1967). This could occur
via inhibition of pyridoxal phosphate-dependent aminotrans-
ferases and decarboxylases. It has been shown that hydrazine
interferes with pyridoxal phosphate synthesis in vitro
(McCormick & Snell, 1961) and in vivo (Chatterjee & Sengupta,
1980) (section 8.3.5). Inhibition of transaminases would also
explain the increase in free amino acids observed in the plasma,
liver, brain, and muscle of rats (Cornish & Wilson, 1968; Banks,
1970) and in the plasma and urine of dogs (Korty & Coe, 1968).
It could further explain several observations in rats, such as
the depressed conversion of amino acids to carbon dioxide
(Amenta & Dominguez, 1965b; Dost et al., 1971), the depressed
incorporation of amino acids in plasma-glucose (Fortney et al.,
1967), and the enhanced incorporation of amino-labelled acids in
liver proteins, 24 h following hydrazine exposure (Banks, 1970).
Inhibition of protein synthesis was also observed in rat livers
up to 8.5 h after exposure (Lopez-Mendoza & Villa-Trevino,
1971).
Hydrazine treatment of rats resulted in inhibited activity
of specific aminotransferases and decarboxylases including:
liver aspartate aminotransferase (EC 2.6.1.1) (Stein et al.,
1971), brain gamma-aminobutyrate aminotransferase (EC 2.6.1.19)
and glutamate decarboxylase (EC 4.1.1.15) (Medina, 1963; Perry
et al., 1981), and liver ornithine 2-oxo-acid aminotransferase
(EC 2.6.1.13) (Roberge et al., 1971). The activity of rat liver
ornithine decarboxylase (EC 4.1.1.17) increased following
hydrazine exposure (Springer et al., 1980).
Inhibition of phosphoenolpyruvate carboxykinase (ATP) (EC
4.1.1.49), an enzyme involved in gluconeogenesis, was also
measured in vitro . Hydrazine increased the levels of citrate,
malate, and oxaloacetate in the rat liver (Ray et al., 1970).
Hydrazine affects the urea cycle. Decreased specific
activity of ornithine 2-oxo-acid aminotransferase, caused by
administration of hydrazine to rats (Roberge et al., 1971),
provoked an increase in ornithine in the liver (Banks, 1970),
brain, and plasma (Perry et al., 1981). The concentrations of
citrulline and urea in the liver, kidneys, brain, and blood were
increased, as was the activity of argininosuccinate lyase (EC
4.3.2.1) (Roberge et al., 1971).
8.3.3. Effects on mitochondrial oxidation
Swelling of hepatic mitochondria was observed in rats and
mice following hydrazine administration (Ganote & Rosenthal,
1968; Scales & Timbrell, 1982; Wakabayashi et al., 1983)
(sections 8.1 and 8.2.2). in vitro studies on the effects of
high concentrations of hydrazine on the functional status of
mitochondria showed decreased oxidation of keto-acids (Von
Krulik, 1966). Oxidative phosphorylation measured as the P/O
ratio was either not affected or decreased independently of the
hydrazine concentration (Von Krulik, 1966; Fortney et al.,
1967). Inhibition of beef heart cytochrome a by hydrazine was
also reported (Takemori et al., 1960). After administration of a
single intraperitoneal dose of hydrazine to rats, slightly
stimulated mitochondrial oxidation of succinate and glutamate
was observed with a slight increase in P/O ratio, respiration
control rate, and phosphorylation rate. ATPase (EC 3.6.1.8)
activity was not affected (Higgins & Banks, 1971). When mice
received hydrazine (free base) in the diet (10%) for 3 days and
rats received hydrazine in the diet (20%) for 8 days, oxidation
of succinate and glutamate, coupling efficiency, P/O ratio, and
the activities of ATPase (EC 3.6.1.8) and cytochrome c oxidase
(EC 1.9.3.1) were slightly decreased in liver megamitochondria,
while the activity of monoamine oxidase (EC 1.4.3.4) was
moderately decreased (Wakabayashi et al., 1983).
8.3.4. Effects on microsomal oxidation
Proliferation of the smooth endoplasmic reticulum was
observed in the liver of rats and mice following hydrazine
administration (Ganote & Rosenthal, 1968; Wakabayashi et al.,
1983) (sections 8.1 and 8.2.2). A single dose of 55 mg hydrazine
(free base)/kg body weight in rats decreased the hepatic
cytochrome P-450 content (Gorshtein & Kopylova, 1983). Rats
exposed for 4 days to 12 mg hydrazine (hydrazine sulfate)/kg
body weight per day did not show any effect on cytochrome P-450
levels in the microsomal fraction of the liver, but a slightly
decreased level of cytochrome b 5, inhibition of benzopyrene
hydroxylase (EC 1.14.14.1), and increased parahydroxylation of
aniline (Akin & Norred, 1978).
8.3.5. Effects on the central nervous system
The relationship between the effects of hydrazine on the
central nervous system, especially the occurrence of convul-
sions, and changes in levels of gamma-aminobutyric acid, an
inhibitory neurotransmitter, in the brain of rats and mice has
been investigated. An increase in the level of gamma-amino-
butyric acid was observed in the whole brain of rats after: (a)
a single intraperitoneal dose of 51 mg hydrazine (free base)/kg
body weight (Medina, 1963); (b) a single intravenous dose of 5
mg hydrazine (hydrazine sulfate)/kg body weight (Matsuyama et
al., 1983); or (c) after a daily subcutaneous dose of 2.6 mg
hydrazine (hydrazine monohydrochloride)/kg body weight over 109
days (Perry et al., 1981). In the whole brain of mice, an
increase was observed following a single intramuscular dose of
54 mg hydrazine (free base)/kg body weight (Wood et al., 1980).
The changes in the concentration of this amino acid are caused
by inhibition of pyridoxal phosphate requiring gamma-
aminobutyrate aminotransferase (EC 2.6.1.19) and glutamate
decarboxylase (EC 4.1.1.15) (Medina, 1963; Perry et al., 1981).
Hydrazine treatment of rats also caused a general amino acid
imbalance in the brain (Perry et al., 1981). A relationship was
suggested between the excitable state of the brain and the
gamma-aminobutyric acid contents of nerve endings (decreased)
rather than the whole brain contents of gamma-aminobutyric acid
(increased) (Wood et al., 1980; Geddes & Wood, 1984).
8.4. Reproduction, Embryotoxicity, and Teratogenicity
When groups of 26 Wistar rats were exposed to 0 or 8 mg
hydrazine (hydrazine monohydrochloride)/kg body weight per day,
sc, from day 11 to day 20 of gestation, the exposed dams showed
a 20% decrease in body weight and 2 dams died. Reduced number of
viable fetuses were found in 9 dams killed on day 21 of
gestation (63/172 versus 142/179 in controls), while the number
of implants per litter was not affected. The fetuses had reduced
weights and appeared pale and oedematous, but did not exhibit
any major malformations. In the rats allowed to deliver (12),
perinatal mortality was 100% in treated rats and 20% in controls
(Lee & Aleyassine, 1970).
These results agree with those of another study in which
groups of 6 - 27 Fisher 344 rats received 0, 2.5, 5, or 10 mg
hydrazine (free base)/kg body weight per day, ip, from day 6 to
day 15 of gestation. Body weight gains in dams were decreased
and the number of resorptions per dam were increased in a dose-
related manner. The differences between treated and control
animals were statistically significant at doses of 5 or 10
mg/kg, but not at 2.5 mg/kg for both variables. The numbers of
implants per dam and fetal weights were not affected at any
dose. The incidence of litters or fetuses with abnormalities was
not significantly increased at any dose; however, at 10 mg/kg,
only one out of the 6 females produced a viable litter, and only
6 fetuses were examined. In a subsequent study, 27 rats were
untreated and 11 rats were treated with 10 mg hydrazine/kg per
day, during what appeared to be the most susceptible period of
gestation (days 7 - 9). The incidence of litters or fetuses with
abnormalities in the 10 mg/kg group (6 litters out of 8; 8
fetuses out of 16) was significantly higher than that in the
control group of the preceeding study (8 litters out of 27; 11
fetuses out of 181). The abnormalities observed were mainly
supernumerary and fused ribs, delayed ossification, moderate
hydronephrosis, and moderate dilation of brain ventricles
(Keller et al., 1982).
Subtle postnatal changes were reported in the offspring of
24 female Syrian golden hamsters exposed orally to 0 or 170 mg
hydrazine (hydrazine hydrate)/kg body weight on the 12th day of
gestation. The pups of exposed dams did not exhibit cleft palate
formation, but showed effects on the development of intestinal
brush border enzymes. No other end-points were investigated
(Schiller et al., 1979).
Groups of ICR mice were treated ip with 0, 4, 12, 20, 30, or
40 mg hydrazine (free base)/kg body weight per day, from day 6
to day 9 of gestation. Some dams administered the highest dose
died. Body weights were reduced at doses of 12 mg/kg body weight
or more. While at the lower doses the number of resorptions per
litter was unchanged compared with controls, embryotoxicity was
evident at 30 and 40 mg/kg body weight. At 12 and 20 mg/kg body
weight, 17-day-old fetuses showed reduced weights, and there was
a dose-related increased incidence of litters with abnor-
malities, mainly exencephaly, hydronephrosis, and supernumerary
ribs (Lyng et al., 1980).
Savchenkov & Samoilova (1984) studied the adverse effects on
reproductive function (fertility of females, numbers of newborn,
and resorption of embryos) of female and male albino rats
exposed by gavage to hydrazine (hydrazine nitrate) at a dose of
13 mg/kg body weight, once a day, for 30 days prior to mating.
The development of the surviving litters did not differ from
that of controls.
Albino rats (10/group and 20 controls) of both sexes were
exposed to hydrazine (free base) (99.5% purity) in the drinking-
water at concentrations of 0.82, 0.018, or 0.002 mg/litre (0.016
mg/kg, 0.0014 mg/kg, or 0.00016 mg/kg, respectively, nominal
dose, assuming water consumption 20 ml per day and animal
weights of 250 g). The duration of the study was 6 months. The
number of animals studied and the scheme and time of mating were
not reported. The female rats exposed to the highest concen-
trations had fewer live embryos and more resorptions, as well as
pre- and post-implantation deaths, than the controls. No effects
were observed in animals administered 0.002 mg/litre.
Developmental abnormalities were not reported in any of the 293
embryos from all exposed animals. Destruction of gonadal
epithelium was observed in male rats after 6 months of oral
exposure to hydrazine at concentrations of 0.82 and 0.018
mg/litre (Duamin et al., 1984). In the same study, albino rats
were exposed to hydrazine (free base) at concentrations of 0.85,
0.13, or 0.01 mg/m3 (0.10 mg/kg body weight, 0.016 mg/kg, or
0.0012 mg/kg, respectively, nominal dose, assuming inhalation of
6 litres air per h and animal weights of 200 g), for 5 h per
day, 5 days/week, for 4 months. At the two highest concentra-
tions, embryotoxic effects of the same severity were seen as
when hydrazine was administered orally at the two highest doses.
No abnormalities were observed among 315 embryos. No gonado-
toxic effects occurred in male rats under the conditions of the
study (Duamin et al., 1984).
Additional information relating to reproductive end-points
is presented in section 8.5.1 (Sotomayer et al., 1982).
8.5. Mutagenicity and Related End-Points
8.5.1. DNA damage
Hydrazine was found to react with pyrimidine bases under
non-physiological conditions. These reactions were reviewed by
Kimball (1977). No hydrazine-DNA adducts have been reported to
be formed in vivo. A single oral or intraperitoneal dose of
hydrazine (free base or hydrazine sulfate) administered to rats,
mice, guinea-pigs, and hamsters resulted in the rapid formation
of N7-methylguanine and O6-methylguanine in liver DNA, which was
not detected in controls (Becker et al., 1981; Quintero-Ruiz et
al., 1981; Bosan & Shank, 1983; Shank, 1983). Methylation was
detectable at toxic doses and a sharp increase in the methyl-
ation of guanine in liver-DNA was observed at oral doses
exceeding 60 mg/kg body weight in rats and 45 mg/kg body weight
in hamsters. In both species, maximum methylation approximated
80 N7-methylations and 7 O6-methylations per 100 000 residues of
guanine, 6 h after oral exposure to 90 mg hydrazine/kg body
weight. Elimination of 7-methylguanine from DNA began about 24 h
after exposure with a half-life of 40 - 50 h. Elimination of O6-
methylguanine from DNA began about 24 h after exposure in rats
and about 50 h after exposure in hamsters, with a half-life of
13 h and 17 h, respectively (Becker et al., 1981; Bosan & Shank,
1983). The source of the methyl group was S -adenosyl-methionine
(Becker et al., 1981; Quintero-Ruiz et al., 1981).
Single-strand breaks were detected by the alkaline elution
assay in rat liver cells exposed in vitro (Sina et al., 1983),
and in the liver and lung cells of mice injected ip with 50 or
100 mg hydrazine (hydrazine hydrate)/kg body weight (Parodi et
al., 1981).
Hydrazine was reported to induce lambda phage in Escherichia
coli (Heinemann, 1971); however, no such induction was found by
Thomson (1981), nor did hydrazine induce HPlcl phage in Haemo-
philus influenzae (Balganesh & Setlow, 1984).
Repair of DNA lesions induced by hydrazine was observed in
vitro. In the International Collaborative Programme for the
Evaluation of Short-Term Tests for Carcinogenicity, 5 bacterial
DNA repair tests, using Bacillus subtilis or E. coli, were all
found to produce weak to medium positive responses. Only one
assay required rat liver microsomal fraction. The other positive
responses were reduced in magnitude by microsomal fraction
(Ashby & Kilbey, 1981). Increased unscheduled DNA synthesis was
observed in 1 out of 2 tests using human fibro-blasts (Agrelo &
Amos, 1981; Robinson & Mitchell, 1981).
No increase in unscheduled DNA synthesis was observed in the
germ cells of mice, 16 days after a 5-day exposure to doses of
hydrazine (hydrazine dihydrochloride) of up to 120 mg/kg body
weight per day (Sotomayer et al., 1982).
8.5.2. Mutation and chromosomal effects
The results of tests for gene mutations and chromosome
damage induced by hydrazine or its salts are summarized in Table
6. Hydrazine induces gene mutations and/or chromosome
aberrations in a variety of test systems including plants,
phage phi 80, bacteria, fungi, Drosophila melanogaster, and
mammalian cells in vitro . In a few cases, microsomal activation
was an absolute requirement for a positive effect (Gupta &
Goldstein, 1981; Perry & Thomson, 1981). In most cases, an
effect could be observed, both with and without microsomal
activation, with a stronger effect in some tests with activation
and in other tests without activation. These inconsistencies
were evaluated by Ashby (1981). Some negative results in the
forward mutation tests with mammalian cells invitro can be
traced back to the high locus specificity of hydrazine also
observed in plants. Duamin et al. (1984) observed increased
chromosomal aberrations in bone marrow cells (4.12 ± 0.65%
versus 2.48 ± 0.46% in controls) of albino rats (N = 10) exposed
to hydrazine (free base) at 0.85 mg/m3, 5 h per day, 5
days/week, for 4 months. No increased incidence of nuclear
aberrations, micronuclei, dominant lethals, and sperm-head
abnormalities were observed in hydrazine-treated mice (free base
or hydrazine sulfate).
Table 6. Tests for gene mutations and chromosome damage induced by hydrazine or its salts
---------------------------------------------------------------------------------------------------------
Test description System description Result Reference
Organism Species/strain/cell type
---------------------------------------------------------------------------------------------------------
Forward mutations transforming Bacillus subtilis + Freese et al. (1967)a
G DNA + Bresler et al. (1968)a
E Forward mutations plant tomato + Jain et al. (1968)b
+ Chandra Sekhar & Reddy
(1971)b
N rice + Reddy & Reddy (1972)b
barley + Kak & Kaul (1975)b
E wheat + Khamankar & Jain (1978)b
broad bean + Vishnoi & Gupta (1980)b
Reverse mutations virus phage phi 80 + Chu et al. (1973)c
M Reverse mutations bacteria Salmonella typhimurium TA 1530 + Rosenkranz & Poirier
(1979);Tosk et al.
(1979)
U
Salmonella typhimurium TA 1535 + Purchase et al. (1978);
Herbold (1978);
T Rosenkranz & Poirier
(1979); Bridges et al.
(1981);d,e;
A Parodi et al. (1981);
Rogan et al. (1982);
T Braun et al. (1984);
De Flora et al. (1984)
I Salmonella typhimurium TA 1537 - Bridges et al. (1981)d,e;
Parodi et al. (1981);
O Rogan et al. (1982)
N Salmonella typhimurium TA 1538 + Bridges et al. (1981)d,e
Purchase et al. (1978);
S Herbold (1978);
Rosenkranz & Poirier
(1979); Parodi et al.
(1981)
Salmonella typhimurium TA 100 + Purchase et al. (1978);
Herbold (1978); Bridges
et al. (1981)d,e
---------------------------------------------------------------------------------------------------------
Table 6. (contd.)
---------------------------------------------------------------------------------------------------------
Test description System description Result Reference
Organism Species/strain/cell type
---------------------------------------------------------------------------------------------------------
Reverse mutations bacteria Salmonella typhimurium TA 100 - Bridges et al. (1981)d,e;
G Parodi et al. (1981)
E Salmonella typhimurium TA 98 -, + Herbold (1978); Bridges
et al. (1981)d,e; Parodi
et al. (1981)
N + Purchase et al. (1978)
E Salmonella typhimurium G 46 + Braun et al. (1984)
Reverse mutations bacteria Salmonella typhimurium G 46 + Röhrborn et al. (1972)
(host-mediated (mouse) (NMRI)
assay)
M Reverse mutations bacteria Escherichia coli + Von Wright & Tikkanen
(1980); Bridges et al.
(1981)d,e
U
Haemophilus influenzae + Kimball & Hirsch (1975);
T Kimball (1976)
A Reverse mutations fungi Saccharomyces cerevisiae + Mehta & Von Borstel
(1981)e; Vasudeva &
Vashishat (1985)
T Forward mutations fungi Saccharomyces cerevisiae + Lemontt (1977, 1978)
Saccharomyces pombe + Loprieno (1981)e
I
Sex-linked visibles insect Drosophila melanogaster + Jain & Shukla (1972);
O Shukla (1972);
Vijaykumar & Jain (1979)
N Sex-linked lethals insect Drosophila melanogaster + Shukla (1972)
S Forward mutations hamster ovary cells in vitro + Gupta & Goldstein (1981)e
- Carver et al. (1981)e;
Hsie et al. (1981)e
mouse lymphoma cells in vitro + Rogers & Back (1981)
+ Amacher et al. (1980)
---------------------------------------------------------------------------------------------------------
Table 6. (contd.)
---------------------------------------------------------------------------------------------------------
Test description System description Result Reference
Organism Species/strain/cell type
---------------------------------------------------------------------------------------------------------
C Breaks plant horse bean primary root + Gupta & Grover (1970)
H Breaks, deletions, plant horse bean primary root + Heindorff et al. (1984)
translocations
R
Aberrationsf plant chick pea root + Farook & Nizam (1979)
O
Aberrations rat epithelial liver cells - Dean (1981)e
M in vitro
O Aberrations rat bone marrow cells in vivo + Duamin et al. (1984)
S Breaks, gaps, hamster ovary cells in vitro + Natajaran & Van Kesteren-
exchanges Van Leeuwen (1981)e
O
Sister chromatid hamster ovary cells in vitro + MacRae & Stich (1979);
Perry & Thomson (1981)e
M exchanges
- Natarajan & Van Kesteren-
Van Leeuwen (1981)e; Baker
et al. (1983)
E
lung cells in vitro + Baker et al. (1983)
V-79 cells in vitro + Speit et al. (1980)
Nuclear aberrations mouse epithelial colon cell - Wargovich et al. (1983)
(oral exposure)g
---------------------------------------------------------------------------------------------------------
Table 6. (contd.)
---------------------------------------------------------------------------------------------------------
Test description System description Result Reference
Organism Species/strain/cell type
---------------------------------------------------------------------------------------------------------
D Micronuclei (ip mouse polychromatic erythrocytes + Salomone et al. (1981)e
exposure) - Kirkhart (1981)e;
A Tsuchimoto & Matter
(1981)e
M Dominant lethals mouse germ cells - Epstein et al. (1972)
(ip exposure)
A
G
E
------------------------------------------------------------------------------------------------------------------------
a Inactivation of transforming DNA was observed, which was inhibited by catalase, EDTA, or nitrogen gas treatment.
b Hydrazine appears to be a mutagen that is highly specific for certain loci. Moreover, it not only produced mutants
in the M2 generation but also homozygous recessive mutants in the M1 generation (plants, raised from treated seeds).
c Inactivation of virus observed. Inactivation and, at lower concentrations, mutations were reduced by catalase
treatment.
d Hydrazine sulfate was tested in the International Collaborative Programme for the Evaluation of Short-Term Tests for
Carcinogenicity (de Serres & Ashby, 1981). In the summary on the assay performance of bacterial mutation assays, it
was reported that hydrazine sulfate was mutagenic in most of a total of 20 laboratories. However, there were
inconsistencies in the strains in which the effect was seen and the requirements for S9 mix. Salmonella typhimurium
TA 1535 was the strain in which mutagenic activity was most commonly observed, but it was also seen in all
laboratories that used Escherichia coli strains. In some laboratories, hydrazine sulfate was also mutagenic in
Salmonella typhimurium TA 100. Two laboratories reported a marginal mutagenic activity for strain TA 98, and TA 1538
was positive in another, while no mutagenic activity was observed with TA 1537.
e Tested in the International Collaborative Programme for the Evaluation of Short-Term Tests for Carcinogenicity. In
the final assessment, it was stated that hydrazine was a genotoxic agent in bacteria, yeast, and higher eukaryotes
in vitro . Hydrazine did not appear to be genotoxic in vivo in higher eukaryotic cells (de Serres & Ashby, 1981).
f Stickiness and clumping at metaphase, bridges and fragments at anaphase, laggards, micronuclei, and delayed
cytokinesis at telophase, tripolar spindles, prophase, and metaphase in one cell.
g Micronuclei, pyknotic nuclei, karyorrhetic nuclei, cytolysosomes.
8.5.3. Cell transformation
Hydrazine increased the transformation of baby hamster
kidney cells (Purchase et al., 1978; Daniel & Dehnel, 1981) and
human liver cells (Purchase et al., 1978). While Purchase et al.
(1978) observed an increased transformation of baby hamster
kidney cells, both with and without metabolic activation, Daniel
& Dehnel (1981) found a much lower increased transformation
frequency only with metabolic activation. Hydrazine also caused
enhancement of transformation of mouse 3T3 cells by Herpes
simplex virus in vitro (Johnson, 1982).
Hydrazine induced transformation of human fibroblasts. Transformed
cells were able to induce undifferentiated mesenchymal tumours
after sc injections in pre-irradiated nude mice, while control
cells did not induce tumours (Milo et al., 1981).
8.6. Carcinogenicity
8.6.1. Inhalation exposure
Groups of 100 Fischer 344 rats of each sex, 400 female
C57BL/6 mice, and 200 male golden Syrian hamsters were exposed
to the vapour of hydrazine (free base) for 12 months, 6 h per
day, for 5 days per week, and observed for a further 18, 15, and
12 months, respectively (Table 7). Rats were exposed at 0.06,
0.33, 1.3, or 6.5 mg/m3, mice at 0.06, 0.33, or 1.3 mg/m3, and
hamsters at 0.33, 1.3, or 6.5 mg/m3 air. Control groups
contained 150 rats of each sex, 800 female mice, and 200 male
hamsters, respectively. The hydrazine vapour was monitored
throughout the exposure period. A reduction in body weight gain,
seen in exposed rats throughout the entire study, was greatest
in males at 6.5 mg/m3. The mortality rate was not affected by
the exposures. Inflammatory changes were observed in the upper
respiratory tract of both sexes and in the uterus and oviduct,
especially at the highest exposure. At 6.5 mg/m3, the incidence
of squamous metaplasia was increased in the nose, larynx, and
trachea, while the incidence of epithelial hyperplasia was
increased in the nose and lungs. An increased incidence of
hyperplasia was also observed in the lymph nodes and uterus of
females at 6.5 mg/m3 and in the liver of females at 1.3 and 6.5
mg/m3. A dose-related increased incidence of benign epithelial
tumours of the nose, mainly adenomatous polyps, was observed in
both sexes at the 2 highest exposures, while at the highest
exposure (6.5 mg/m3), the incidence of malignant epithelial
tumours was also increased (2/98 in males and 5/95 in females).
The latency time exceeded 87 weeks in males and 97 weeks in
females. The incidence of lung tumours was not significantly
increased, but, in males, 3 bronchial adenomas and, in one
female, 1 bronchial adenoma, were observed at 6.5 mg/m3 compared
with none in the controls. The total incidence of adenomas and
adenocarcinomas of the thyroid in males was not increased, but
hydrazine exposure at 6.5 mg/m3 increased the fraction of
malignant tumours.
Table 7. Tumour incidence in mice, rats, and hamsters following hydrazine exposure
---------------------------------------------------------------------------------------------------------
Route Species/ Exposure Daily dose Tumour incidenceb Reference
strain period (mg/kg Nose or lung liver
(months) body weight)a Male Female Male Female
(life-time
observation)
---------------------------------------------------------------------------------------------------------
Inhalation mouse 12 0 8/385 - Vernot et al. (1985)
(C57BL/6) 4/378
0.34c 12/379 -
rat 12 0 0/146 0/145 - - Vernot et al. (1985)
(Fischer 0.16c 11/97 4/94 - -
344) 0.8d 75/98 38/95 - -
hamster 12 0 1/181 - Vernot et al. (1985)
(golden 0.93d 16/160 -
Syrian)
Oral mouse 6 - 9 0 1/37 4/47 3/30 1/29 Biancifiori (1969,
(gavage) (CBA) 0.98 2/26 10/25 1/26 0/25 1970a)
2.0 4/25 16/25 7/25 2/25
3.9 5/25 21/24 12/25 16/24
8.0 16/21 19/21 15/25 15/24
rat 17 0 0/28 0/22 0/19 0/14 Severi & Biancifiori
(Cb/Se) 14.6/9.7 3/14 5/18 4/13 0/18 (1968)
Oral mouse 24 - 28 0 11/110 14/110 2/110 3/110 Toth (1969, 1972a)e
(drinking- (Swiss) 2.0/1.6 24/50 27/50 2/50 1/50
water) 5.1/4.6 25/50 24/50 3/50 1/50
---------------------------------------------------------------------------------------------------------
a Calculated doses based on a weight of 300 g and a minute volume of 100 ml for rat, a weight of 35 g
and a minute volume of 25 ml for mice, and a weight of 140 g and a minute volume of 54 ml for hamsters.
b - = not found; open space = not tested (nose in inhalation studies with rats and hamsters, lung in
the other studies.
c 1.3 mg/m3, 6 h/day, for 5 days/week.
d 6.5 mg/m3, 6 h/day, for 5 days/week.
e Liver tumours were hepatomas (1/50 in males at both exposure levels) and angiomas.
A slightly increased mortality rate seen in mice right up to
the end of the study was not related to dose. Body weights were
not affected during exposure, but all exposed groups gained less
weight than controls during the post-exposure period. The
incidence of non-neoplastic lesions was similar in treated and
control groups. The only neoplastic change observed was a
slightly increased incidence of lung adenomas at the highest
exposure. A background incidence of 2 - 3% for pulmonary adenoma
in C57BL/6 mice was reported to be common in the laboratory
concerned.
Almost double the number of deaths occurred during exposure
in each exposed group of hamsters compared with the control
group, but the final mortality rates were similar in all groups,
including the control group. During exposure to 6.5 mg/m3 and
for 10 months after exposure, body weights were depressed. For
several months, an unexplained decrease in body weight occurred
in all groups, including the controls. Dose-related degenerative
changes were observed. These were characterized by amyloidosis
in several organs and issues, mineralizaton in kidneys, and
haemosiderosis in the liver. Increased incidences of senile
atrophy and aspermatogenesis were observed in the testes at the
highest exposure level. In addition, the incidence of bile-duct
hyperplasia was increased at all exposures. The incidence of
benign nasal polyps was increased at 6.5 mg/m3. The only other
striking tumours observed were 3 adenocarcinomas, 1 leiomyoma,
and 1 papilloma in the colon, 1 basal cell carcinoma in the
stomach, and 4 parafollicular cell adenomas in the thyroid at
6.5 mg/m3. No such tumours were found in the controls, but the
increased incidence of each tumour type was not statistically
significant. The authors concluded from these studies "that
hydrazine is capable of inducing nasal tumours, primarily
benign, in rats and hamsters after 1-year intermittent exposure"
(Vernot et al., 1985).
8.6.2. Oral exposure
The oral carcinogenicity studies on hydrazine have been
either preliminary or limited in scope (inadequate limited
histopathology, use of one dose or one drinking-water concentra-
tion, small treatment groups, lack of adequate controls, and
treatment of only one sex); therefore, only the more relevant
studies have been described in this section. Most studies have
concentrated on various mouse strains.
Groups of approximately 25 CBA/Cb/Se mice of each sex
received, by gavage, 150 daily doses of hydrazine sulfate in
water, equivalent to 0.03, 0.07, 0.14, or 0.28 mg hydrazine in
25 weeks (these doses are expressed in Table 7 as 0.98, 2.0,
3.9, or 8.0 mg/kg body weight). The animals were observed for
their life-time. Control groups containing 30 males and 29
females were not treated. Liver, lung, various endocrine glands,
and tissues suspected of having lesions were examined
microscopically. No statistical analysis was reported. No deaths
occurred during the exposure period, but later the mortality
rate was increased in animals that had been exposed daily to
levels of 2.0 mg/kg upwards. Brown degeneration of the adrenals
in treated females was mentioned as a marked non-neoplastic
lesion. An apparent dose-related increased incidence of
hepatocarcinomas was observed in male and female mice. The
average latency time for this tumour did not decrease as the
dose increased (Biancifiori, 1970a). The historical control
incidence of hepatocarcinomas in the laboratory concerned was 8%
(Severi & Biancifiori, 1968). Multiple pulmonary tumours, which
were also observed, are described in another report
(Biancifiori, 1969).
In this report (Biancifiori, 1969), the 3 lowest dose
groups, containing 21 - 26 males and 21 - 25 females, were
treated in the same way as in the study above. The data for the
control group and the highest dose groups were taken from
earlier reports (Biancifiori et al., 1964; Severi & Biancifiori,
1968). The untreated control groups contained 37 males and 47
females. The highest dose group comprising 21 mice of each sex,
received 250 daily doses of hydrazine sulfate in water
equivalent to 0.28 mg hydrazine in 36 weeks. In the 1969 report,
the treatment schedule was reported to be 150 daily doses of
0.28 mg hydrazine in 25 weeks, which was contrary to the
original data. Lungs with trachea, liver, various endocrine
glands, and other tissues suspected of having lesions were
examined microscopically. No deaths occurred during the exposure
period, but the mortality rate after the treatment period was
increased from a dose level of 2.0 mg/kg per day upwards. Common
non-neoplastic lesions in the liver at 0.28 mg per day included
areas of diffuse cell regeneration. Necrosis, nodular
regeneration, sclerosis, and bile-duct proliferation were also
observed. The occurrence of hepatomas has already been
discussed. The incidence and multiplicity of pulmonary tumours
were increased at all dose levels in an apparently dose-related
manner and more so in females than in males (Table 7). The
average latency time for these tumours was also shorter in
females than in males. At the highest dose level, approximately
80% of the tumours were characterized as adenomas in both sexes,
the remainder being described as "anaplastic adenomas and
adenocarcinomas" (Biancifiori, 1969).
Induction of lung tumours by hydrazine sulfate after oral
exposure by gavage was also reported in other mouse strains
(Milia et al., 1965; Roe et al., 1967; Kelly et al., 1969;
Biancifiori, 1970b; Bhide et al., 1976; Maru & Bhide, 1982).
Liver tumour induction was reported in BALB/c/Cb/Se mice
(Biancifiori, 1970b).
Severi & Biancifiori (1968) also exposed 14 male and 18
female Cb/Se rats, by gavage, for 68 weeks, to daily doses of
hydrazine sulfate in water, equivalent to 4.4 mg hydrazine (14.6
mg/kg body weight) per day for males and 2.9 mg hydrazine (9.7
mg/kg) per day for females. Controls (28 males and 22 females)
were not treated. The animals were observed for their life-time.
Lungs, liver, and other organs showing gross lesions were
examined microscopically. No statistical analysis was reported.
Exposed rats showed an increased mortality rate. Pulmonary
tumours, adenomas, and adenocarcinomas were observed in both
sexes (Table 7). Carcinomas (2) and spindle cell sarcomas (2),
observed in the livers of exposed males, were not seen in the
controls.
Groups of 23 and 85 golden hamsters of both sexes received
hydrazine sulfate by gavage as 60 and 100 daily doses, equiv-
alent to 0.74 and 0.68 mg hydrazine over 15 and 20 weeks,
respectively. The control group contained 56 hamsters. Animals
were observed for their life-time and examined as described
above for rats. The mortality rate increased in an apparently
dose-related manner, and toxic effects were observed in treated
animals (liver lesions, reticuloendothelial cell proliferation,
cirrhosis, bile-duct proliferation, degenerative fibrous cells
in hyalinized tissue). No tumours were observed, but, because of
toxic effects, the treated animals were not observed for the
same period of time as the controls (Biancifiori, 1970a).
Groups of 50 Swiss mice of each sex received doses of hydra-
zine sulfate in drinking-water, equivalent to 0.056 mg hydrazine
(2 mg/kg body weight, males; 1.6 mg/kg, females) per day for
their life-time up to a maximum of 113 weeks (Toth, 1972a) or
0.18 mg (5.1 mg/kg body weight) per day (males) and 0.16 mg (4.6
mg/kg) per day (females) for a maximum of 95 weeks (Toth, 1969).
The untreated control group, containing 110 animals of each sex,
was tested concurrently with the groups receiving 0.16 or 0.18
mg per day, but not with those receiving 0.05 mg per day (Toth,
1969). Liver, kidneys, spleen, lungs, and organs with gross
lesions were examined microscopically. No statistical analysis
was reported. Drinking-water solutions containing hydrazine were
prepared 3 times per week. There was no indication that
hydrazine concentrations were measured in the solutions during
the studies. At the highest dose level, body weights were not
affected, but the mortality rate in females was increased.
Numerous lung tumours were observed in both sexes with a
comparable incidence (Table 7) and degree of malignancy at both
dose levels. The average latency time for this tumour was
decreased at both dose levels. At the high dose level, the
latency period for malignant lymphomas, which are characteristic
for the strain, and the incidence of adenocarcinomas of the
mammary gland appeared to be decreased.
Fifty Syrian golden hamsters of each sex also received, for
their life-time, up to a maximum of 110 weeks, doses of hydra-
zine sulfate in drinking-water, equivalent to 0.57 mg hydrazine
per day. Untreated non-concurrent control groups contained 100
hamsters of each sex. The protocol was similar to that described
above for rats. Body weights of exposed hamsters were slightly
reduced, but no tumours or other effects were noted. The authors
considered that there was no treatment-related tumour incidence
and that the mortality rate was not affected by the exposure,
though the mean survival period was slightly reduced (Toth,
1972b).
Groups of 34 male and 29 female Swiss mice were administered
hydrazine sulfate by gastric intubation at doses equivalent to
0.27 mg hydrazine per mouse per day, 5 days per week, for their
lifetime. Lung adenocarcinomas occurred in 30 out of 34 male
mice and in 21 out of 29 female mice compared with 1 out of 20
controls. The earliest tumour appeared after 14 months of
exposure. An increased incidence of lung tumours (20/22)
occurred in F1 animals, which were obtained from treated
mothers, and received hydrazine sulfate post weaning from the
age of 11 weeks. No control mice were mated. An increase in lung
tumours (16/35) was also observed in F2 animals obtained from F1
animals that had been treated with hydrazine throughout
gestation and lactation (Menon & Bhide, 1983).
9. EFFECTS ON MAN
9.1. Poisoning Incidents
Several incidents of systemic poisoning have been reported,
mainly showing effects on the central nervous system, respira-
tory system, and stomach.
After a laboratory technician had drunk 20 - 30 ml of a 6%
aqueous solution of hydrazine (free base), he immediately
vomited. Four hours later, weakness, somnolence, and arrhythmia
were observed. Laboratory findings showed a slight but persis-
tent leukocytosis. The serum-albumin fraction was decreased with
an increase in the fraction of globulin. Two days after
exposure, slightly elevated body temperature and a few red blood
cells in the urine were noted, while the patient showed
irregular breathing. Five days after exposure, the patient had
recovered (Drews et al., 1960).
A case was reported of a man who drank between a mouthful
and a cupful of hydrazine in concentrated solution. He
immediately vomited, became unconscious, and flushed. Vomiting
ceased about 12 h after admission to the hospital. The patient
showed sporadic violent behaviour and later developed ataxia
with a lateral nystagmus, a decreased sense of vibration, and
paraesthesia in the arms and legs. Oliguria was also noted.
Pyridoxine treatment was administered, but it was not clear from
the report whether he benefited from this. The patient's final
condition was not reported (Reid, 1965).
A 24-year-old man accidentally ingested "a mouthful" of
hydrazine and immediately became confused, lethargic, and
restless. On admission to hospital, a complete chemistry profile
was normal, but, 3 - 4 days later, hepatotoxicity was evident as
indicated by elevated levels of aspartate amino-transferase (EC
2.6.1.1), lactate dehydrogenase (EC 1.1.1.27), and total
bilirubin. At this time, the patient was treated with pyridoxine
and recovered completely within 5 days. Peripheral neuropathy,
however, developed subsequently, which resolved over the next 6
months. The authors attributed this neuropathy to the megadose
pyridoxine therapy (Harati & Niakan, 1986).
Another man sustained a chemical burn during an industrial
hydrazine explosion. Exposure occurred probably both via the
skin and by inhalation. The burns on his skin covered 22% of the
body surface. On admission to the hospital, the neuro-
logical status of the patient was normal but, 14 h after
exposure, he became comatose. When the coma persisted for 60 h,
he was treated with pyridoxine, and the neurological disorders
cleared within the next 12 h. Other findings were persistently
elevated glucose levels, haematuria, and respiratory
difficulties. Several biochemical indicators of liver
malfunction were elevated from day 3 after exposure and returned
to normal levels over the next 5 weeks (Kirklin et al., 1976).
Two men were exposed to Aerozine 50 vapour (50% hydrazine
and 50% 1,1-dimethylhydrazine by volume) via a leak in a fuel
line. The first man was exposed for about 90 min via a defective
gas mask. He reported headache, nausea, and a shaky feeling, a
sensation of burning of the face, a sore throat, and tightness
in the chest. The second man had inhaled the vapour 3 times
before evacuation from the contaminated area. He was dyspnoeic,
trembling, and weak. Both men showed neurological disorders
including twitching of extremities and clonic movements in the
first victim and hyperreactive reflexes in both men. All
symptoms cleared after treatment with pyridoxine. From clinical
signs, it was concluded that pulmonary oedema developed in both
men and was treated successfully. Four other cases of poisoning
by Aerozine after a spill were mentioned in this report. These 4
men showed nausea and vomiting. Both symptoms disappeared within
20 min following pyridoxine treatment (Frierson, 1965).
One case of systemic lupus erythaematosus-like disease due
to occupational exposure to hydrazine (free base) has been
described. The patient, a laboratory technician, had recurrent
episodes of joint pain, photosensitive rash, fatigue, and fever
following hydrazine exposure. A challenge patch test suggested
that hydrazine caused the illness. On the basis of detailed
examinations of the patient and her family, it was concluded
that hydrazine could cause the disease in a person with a
genetic predisposition. Some of the genetic factors identified
were slow acetylation phenotype, HLA DR2,3, and immune system
responsiveness such as antinuclear antibodies, antibodies to
single-stranded and native DNA, and inhibition of pokeweed
mitogen-stimulated immunoglobulin G synthesis in lymphocytes
(Reidenberg et al., 1983).
9.2. Occupational Exposure
9.2.1. Inhalation exposure
One case was reported of a man who had handled hydrazine
(hydrazine hydrate) once a week for an unknown number of hours
over a period of 6 months. In simulated conditions, only 0.071
mg hydrazine/m3 air was measured, but probably skin exposure had
also occurred. The man experienced conjunctivitis, tremor, and
lethargy after each exposure. Following the last exposure, he
was feverish, vomited, and showed diarrhoea. In a hospital, 6
days later, many disorders were noted: conjunctivitis,
stomatitis, arrhythmia, upper abdominal pain, enlarged abdomen,
icterus, a tender and palpable liver, black faeces, incoherence,
and oliguria. X-ray examinations showed pleural effusion and
lung shadowing. Laboratory findings comprised elevated bilirubin
and creatinine levels in the blood, and protein and red blood
cells in urine. Treatments administered included haemodialysis
and B-vitamins, which brought only temporary relief. The man
died 21 days after the last exposure. Autopsy revealed
pneumonia, severe renal tubular necrosis and nephritis, and mild
hepatocellular damage (Sotaniemi et al., 1971).
9.2.2. Skin and eye irritation; sensitization
Two cases of skin irritation (Frierson, 1965; Kirklin et
al., 1976) and one case of eye irritation (Sotaniemi et al.,
1971) have already been cited in sections 9.1 and 9.2.1,
respectively. Dermatitis of the hands without systemic effects
was observed in 2 men who were occupationally exposed to hydra-
zine hydrate over a period of 5 months. Both men had had similar
experiences previously, after handling of hydrazine hydrate
(Evans, 1959). No patch tests were carried out, but the allergic
nature of the contact dermatitis that can develop after skin
exposure to hydrazine, has been reported earlier. In 1959, an
outbreak of dermatitis was reported among 12 out of 34 female
workers in a factory where hydrazine hydrochloride was used as a
solder flux. Four months after the use of the solder flux had
stopped, 6 out of 12 workers showed strong positive reactions
towards hydrazine sulfate, while 30 controls did not show any
reaction (Frost & Horth, 1959). In an electronics plant, 35
workers, constituting half the work force, developed contact
dermatitis from a solder flux containing hydrazine
hydrochloride, over a period of 5 years. The symptoms did not
reappear when contact with hydrazine was avoided. Patch tests on
5 female workers of this group were all clearly positive for
hydrazine fluxes, while 3 controls did not react (Wheeler et
al., 1965). Another 7 workers in a chemical workshop, where
solder flux containing hydrazine hydrochloride was used, showed
allergic contact dermatitis towards hydrazine, as did 5 workers
in a pharmaceutical firm producing isoniazid. The results of the
patch tests were strongly positive for the 12 subjects, but a
control group was lacking (Zina & Bonu, 1962). Hydrazine induced
allergic contact dermatitis in 4 men when used as a corrosion
inhibitor (Schultheiss, 1959; Hovding, 1967; Von Keilig & Speer,
1983). A negative control group was tested in only one of these
studies (Hovding, 1967). Allergic contact dermatitis developed
in 15 workers in 2 industries producing hydrazine sulfate. In
one of these industries, patch tests on 15 workers were clearly
positive for hydrazine, while 13 controls from the same industry
did not show any reaction. Symptoms did not reappear when
contact with hydrazine sulfate was avoided (Brandt, 1960). In
the other industry, 10 persons produced a positive reaction
towards hydrazine. No negative controls were tested (Sonneck &
Umlauf, 1961). Two cases of allergic contact dermatitis were
reported following exposure to hydrazine in stain removers with
strong positive reactions towards the chemical in patch tests.
Five controls did not react (Van Ketel, 1964). Systemic effects
were not noted in any of these studies.
Cross sensitization to hydrazine derivatives, such as
isoniazid, phenylhydrazine, and hydralazine, was found by
several investigators (Schultheiss, 1959; Zina & Bonu, 1962; Van
Ketel, 1964; Hovding, 1967; Von Pevny & Peter, 1983).
9.2.3. Mortality studies
The causes of death in a cohort of 427 male workers,
employed for a minimum of 6 months in a hydrazine manufacturing
plant between 1945 and 1971, were studied retrospectively and
followed until July 1982. The plant ceased production in 1971.
The men were divided according to 3 categories of exposure. The
first category contained 78 men who might have been exposed to
concentrations of hydrazine ranging from 1.3 to 13 mg/m3 air.
Categories 2 and 3 (a total of 375 men) included those who might
have been exposed to concentrations between 0.6 and 1.3 mg/m3
and those who had little or no exposure, respectively. A further
subdivision in the first category distinguished between men with
more than 10 years since the first exposure and those with less.
Some men were covered by more than one category. The cohort was
also exposed to other unspecified organic chemicals. The total
number of deaths in 406 men who could be traced was 49 against
61.47 expected. Five men died from lung cancer against an
expected 6.65. Two of these men belonged to the most highly
exposed category. Another 7 cases of cancer were detected (9.27
expected). Further details were not given, except for the remark
that no nasal tumours were involved. The authors noted that the
number of men exposed to hydrazine in this study was small. It
was concluded that the results were encouraging in that no
obvious hazard had yet appeared. A note of caution is introduced
by the observation of 2 deaths from lung cancer in men who had
first been exposed in the heaviest category more than 10 years
previously against 1.61 expected (Wald et al., 1984). The cohort
is being followed up.
A seemingly unusually high incidence of myocardial infarc-
tion prompted further study among workers in a plant manufac-
turing hydrazine. The population at risk was limited to every
on-line hydrazine worker who had worked for 3 months or more
between 1953 and 1978. The number of man years at risk was 534,
and the average employment period was 8 years. Only a
superficial examination of the population at risk and the
working environment was completed. Reference data were provided
by the US Air Force men's rates for the years 1975-77 and by
men's rates from a study over the past 30 years by the US
National Heart Institute (Framingham study). There was a close
compar ison between the 2 sets of reference data. Rates of
occurrence of myocardial infarction (death and non-death) per
thousand men per year within 5-year age groups were calculated.
A total of 5 cases of myocardial infarction, concurrent with
the employment period, were identified among the hydrazine
workers. Their average employment period was 16 years. A total
of 1.2 cases was expected. The difference is highly significant
statistically. Four additional cases were identified in ex-
hydrazine workers. However, as it was impossible to trace all
ex-hydrazine workers, the population at risk was limited to
the "concurrent cases". The author warned that the findings
should be treated with due caution because of the small
numbers involved. He also stated that the strength of the
findings rendered a negative hypothesis almost untenable without
further investigation into the cause of the adverse effect found
(Hamill, 1978).
The Task Group recognized that exposure concentrations were
not reported and that there was no indication that confounding
variables, e.g., smoking history or exercise habits, had been
taken into account.
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1. Evaluation of Human Health Risks
Exposure of human beings to hydrazine may occur occupation-
ally or accidentally, through the ingestion of hydrazine-based
drugs, or through the use of tobacco. Despite the high react-
ivity of this compound and its wide industrial use, few
systematic studies have been carried out on its adverse effects
on man.
Hydrazine is rapidly absorbed through the skin, lungs, and
gastrointestinal tract and rapidly distributed throughout the
body (section 6).
In cases of acute human poisoning, vomiting, severe irrita-
tion of the respiratory tract with the development of pulmonary
oedema, central nervous system depression, and hepatic and renal
damage have been reported. Data are not available from which the
levels of hydrazine inhaled can be estimated in cases of acute
poisoning by the respiratory route. However, from reports of
poisoning by the oral route, it would appear that ingestion of
amounts of the order of 20 - 50 ml causes severe intoxication
and may be lethal (section 9.1). It is not possible to estimate
a no-observed-adverse-effect level from the available human
data.
Most of the effects in human beings exposed to hydrazine
have also been observed in experimental animals. In addition,
loss of body weight, anaemia, hypoglycaemia, and fatty liver
have frequently been reported. Fatty liver in mice and reduced
growth in rats were reported when the animals were exposed
through inhalation to the lowest (0.26 mg/m3) of three dose
levels. Monkeys and dogs were unaffected at this dose. In only
one study, a no-observed-adverse-effect level of 3 µwg/kg was
reported, when hydrazine was administered by the oral route to
rats. No data are available on the basis of which a no-observed-
adverse-effect level by inhalation can be established (section
8.2).
Embryotoxicity, fetotoxicity, and minor fetal abnormalities
were observed in rats and mice exposed to hydrazine at doses
toxic for the mother. At such doses, perinatal mortality is
high. These effects may also occur at doses just below the
maternally toxic dose (section 8.4). No data are available on
the effects of hydrazine on the human fetus. In the absence of
human data, it would be prudent to assume that hydrazine might
have adverse effects on the human embryo or fetus at exposure
levels approximating those producing toxicity for the mother.
Such levels may occur during accidental spillage but would be
unlikely to occur in the ambient environment because atmospheric
concentrations are low.
Skin and eye irritation have been observed in human beings
who have come into contact with hydrazine, but the data are
insufficient to establish a no-observed-effect level for
irritation. Hydrazine is a strong skin sensitizer in man and
cross-reacts with hydrazine derivatives (section 9.2.2). Slight
eye irritation was observed in monkeys at hydrazine concentra-
tions of 1.3 and 6.5 mg/m3, but none occurred in animals exposed
to 0.26 mg/m3 (section 8.2.1). No skin irritancy studies on
animals have been reported.
The available data indicate that hydrazine induces gene
mutations and chromosome aberrations in a variety of test
systems including plants, phages, bacteria, fungi, Drosophila,
and mammalian cells in vitro. Hydrazine induced indirect
alkylation in the liver DNA of rodents after in vivo exposure to
toxic doses, and it also caused DNA damage in vitro . Hydrazine
transformed human and hamster cells in vitro. No increased
unscheduled DNA synthesis was observed in the germ cells of mice
in vivo . It did not induce chromosome aberrations, micronuclei,
or dominant lethals in mice in vivo , but chromosomal aberrations
were reported in rats in vivo (section 8.5).
Hydrazine vapour induced nasal tumours, most of which were
benign, in F-344 rats and Syrian golden hamsters, after 12
months of treatment and life-time observation. At the exposure
levels at which most of the tumours occurred, there were signs
of severe mucosal irritation. In several studies on mice,
hydrazine induced an increased incidence of pulmonary tumours,
when administered by the oral route. Hepatic tumours were also
reported in two studies. In some of the studies, the pulmonary
tumour incidence was related to the dose administered. Although
in these reports there is little information about the toxic
effects of the compound, it is probable that the doses admin-
istered in some of the studies were at, or near, the toxic
levels. No tumours were observed in hamsters treated orally with
hydrazine (section 8.6). On the basis of the carcinogenicity
studies on experimental animals, there is evidence that
hydrazine is an animal carcinogen. Human data are inadequate to
assess its carcinogenicity in man. In the absence of human data,
and taking into account the mutagenicity data as well as the
carcinogenicity data in animals, it would be prudent to consider
hydrazine as a possible human carcinogen. Thus, exposure to
human beings should be kept as low as feasible.
Since measurable levels are not normally encountered in the
general environment, it can be concluded that, except in cases
of accidental exposure, hydrazine does not pose a significant
practical hazard for the general population.
10.2. Evaluation of Effects on the Environment
Hydrazine can be released into the atmosphere during venting
operations, storage, and transfer. The total emission has been
estimated to be nearly 0.01% of the hydrazine produced
(production > 35 000 tonnes in 1981). Accidental discharges into
water, air, and soil can result from bulk storage, handling,
transport, and improper waste disposal. Evaporation of hydrazine
after a spill can generate an atmospheric concentration as high
as 4 mg/m3, 2 km downwind.
Hydrazine is degraded rapidly in air through reactions with
ozone, hydroxyl radicals, and nitrogen dioxide. In water, it is
degraded rapidly, especially under aerobic conditions, and in
the presence of organic material and/or in alkaline or hard
water. It is more persistent in soft, metal-free water. In soil,
it is adsorbed and decomposed on clay surfaces, under aerobic
conditions. However, available data are inadequate to describe
the nature of hydrazine behaviour in the soil.
Because of the rapid degradation of hydrazine in the
environment, measurable levels are not normally encountered.
Hydrazine can be toxic for aquatic life, even at very low
concentrations. Fish species showed LC50 values of between 0.54
mg/litre (roach) and 5.98 mg/litre (fathead minnow). Nitrifying
bacteria in activated sludge are inhibited by hydrazine levels
higher than 1 mg/litre. Some other microorganisms are more
sensitive and show toxicity thresholds at levels reported to be
as low as 0.00008 mg/litre.
Hydrazine in both air and water is toxic for plants; in
water, it can inhibit plant germination.
On the basis of these data, it can be concluded that hydra-
zine may present a significant hazard for the aquatic environ-
ment and plant life.
11. RECOMMENDATIONS FOR FURTHER STUDIES
1. Dose-response studies pertaining to:
(a) DNA alkylation (adduct identification);
(b) damage of nasal epithelium; and
(c) pulmonary effects.
2. Skin sensitization studies with focus on cross-reactivity
with hydrazine derivatives.
3. Dose-response studies on sensitive, commercially important
fish species and their food supplies.
4. Metabolic studies in conjunction with effects on DNA.
5. Dermal initiation-promotion studies.
6. Reproduction toxicity studies in sensitive rodent species at
continuous low exposure levels.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
The International Agency for Research on Cancer (IARC, 1982)
considered that, while the evidence for the carcinogenicity of
hydrazine for human beings was inadequate, evidence for its
carcinogenicity for animals and for its activity in short-term
tests was sufficient to conclude that hydrazine is probably
carcinogenic for human beings.
REFERENCES
AGRELO, C. & AMOS, H. (1981) DNA repair in human fibroblasts.
In: de Serres, F.J. & Ashby, J., ed. Evaluation of short-term
tests for carcinogens. Report of the International Collaborative
Programme, New York, Amsterdam, Oxford, Elsevier/North Holland,
pp. 528-532 (Progress in Mutation Research Vol. 1).
AKIN, F.J. & NORRED, W.P. (1978) Effects of short-term
administration of maleic hydrazine or hydrazine on rat hepatic
microsomal enzymes. Toxicol. appl. Pharmacol., 43: 287-292.
ALEYASSINE, H. & LEE, S.H. (1971) Inhibition by hydrazine,
phenelzine, and pargyline of insulin release from rat pancreas.
Endocrinology, 89: 125-129.
AMACHER, D.E., PAILLET, S.C., TURNER, G.N., RAY, V.A., &
SALSBURG, D.S. (1980) Point mutations at the thymidine kinase
locus in L5178Y mouse lymphoma cell. II. Test validation and
interpretation. Mutat. Res., 72: 447-474.
AMENTA, J.S. & DOMINGUEZ, A.M. (1965a) Fatty acid flux and
triglyceride secretion in the hydrazine-induced fatty liver.
Exp. mol. Pathol., 4: 282-302.
AMENTA, J.S. & DOMINGUEZ, A.M. (1965b) The effect of hydrazine
and congeners on C1402 respiratory pattern of various metabolic
substrates. Toxicol. appl. Pharmacol., 7: 236-246.
ANDERSEN, M.E. & KELLER, W.C. (1984) Toxicokinetic principles
in relation to percutaneous absorption and cutaneous toxicity.
In: Drill, V.A. & Lazar, P., ed. Cutaneous toxicity, New York,
Raven Press, pp. 9-27.
ASHBY, J. (1981) Overview of study and test chemical
activities. In: De Serres, F. & Ashby, J., ed. Evaluation of
short-term tests for carcinogens. Report of the International
Collaboration Programme, Amsterdam, Elsevier/North Holland, pp.
112-171 (Progress in Mutation Research, Vol. 1).
ASHBY, J. & KILBEY, B. (1981) Summary report on the
performance of bacterial repair, phage induction, degranulation,
and nuclear enlargement assays. In: de Serres, F.J. & Ashby, J.,
ed. Evaluation of short-term tests for carcinogens. Report of
the International Collaborative Programme, New York, Amsterdam,
Oxford, Elsevier/North Holland, pp. 33-48 (Progress in Mutation
Research Vol. 1).
ASTM (1981) Annual book of ASTM standards. Part 31. Water,
Philadelphia, Pennsylvania, American Society for Testing and
Materials, pp. 425-428 (Method D1385: standard test method for
hydrazine in water).
AUDRIETH, L.F. & OGG, B. (1951) The chemistry of hydrazine,
New York, John Wiley and Sons.
AZAR, A., THOMAS, A.A., & SHILLITO, F.H. (1970) Pyridoxine and
phenobarbital as treatment for Aerozine 50 toxicity. Aerosp.
Med., 41: 1-4.
BAKER, R.S.U., MITCHELL, G.A., MEHER-HOMJI, K.M., & PODOBNA, E.
(1983) Sensitivity of two Chinese hamster cell lines to SCE
induction by a variety of chemical mutagens. Mutat. Res., 118:
103-116.
BALGANESH, M. & SETLOW, J.K. (1984) Prophage induction in
Haemophilus influenzae and its relationship to mutation by
chemical and physical agents. Mutat. Res., 125: 15-22.
BANKS, W.L. (1970) Effect of hydrazine treatment on hepatic
protein biosynthesis in vivo. Biochem. Pharmacol., 19: 275-
283.
BECKER, R.A., BARROWS, L.R., & SHANK, R. (1981) Methylation of
liver DNA guanine in hydrazine hepatotoxicity: dose-response and
kinetic characteristics of 7-methylguanine and O6-methylguanine
formation and persistence in rats. Carcinogenesis, 2: 1181-
1188.
BHIDE, S.V., D'SOUZA, R.A., SAWAY, M.M., & RANADIVE, K.J.
(1976) Lung tumour incidence in mice treated with hydrazine
sulfate. Int. J. Cancer, 18: 530-535.
BIANCIFIORI, C. (1969) [Existence of a hormonal factor in the
pulmonary carcinogenicity of hydrazine.] Lav. Ist. Anat. Istol.
Patol. Univ. Studi Perugia, 29: 29-41 (in Italian).
BIANCIFIORI, C. (1970a) Hepatomas in CBA/Cb/Se mice and liver
lesions in golden hamsters induced by hydrazine sulfate. J. Natl
Cancer Inst., 44: 943-953.
BIANCIFIORI, C. (1970b) [Pulmonary and liver tumours from low
doses of hydrazine sulfate in BALB/c/Cb/Se mice.] Lav. Ist.
Anat. Istol. Patol. Univ. Studi Perugia, 30: 89-99 (in
Italian).
BIANCIFIORI, C., BUCCIARELLI, E., CLAYSON, D.B., & SANTILLI,
F.E. (1964) Induction of hepatomas in CBA/Cb/Se mice by
hydrazine sulfate and the lack of effect of croton oil on tumour
induction in BALB/Cb/Se mice. Br. J. Cancer, 18: 543-550.
BLAIR, I.A., MANILLA TINOCO, R., BRODIE, M.J., CLARE, R.A.,
DOLLERY, C.T., TIMBRELL, J.A., & BEEVER, I.A. (1985) Plasma
hydrazine concentrations in man after isoniazid and hydralazine
administration. Hum. Toxicol., 4: 195-202.
BODENSCHATZ, W. (1986) [Hydrazine in drinking-water.] Off.
Gesundheitswes., 48: 205-208 (in German).
BOSAN, W.S. & SHANK, R.C. (1983) Methylation of liver DNA
guanine in hamsters given hydrazine. Toxicol. appl. Pharmacol.,
70: 324-334.
BRANDT, B., VON (1960) [On allergic skin damage by hydrazine
sulfate.] Dermatol. Wochenschr., 141: 376-381 (in German).
BRAUN, R., JAKEL, H.-P., & SCHONEICH, J. (1984) Genetic
effects of isoniazid and the relationships to in vivo and in
vitro biotransformation. Mutat. Res., 137: 61-69.
BRESLER, S.E., KALININ, V.L., & PERUMOV, D.A. (1968)
Inactivation and mutagenesis on isolated DNA. II. Kinetics of
mutagenesis and efficiency of different mutagens. Mutat. Res.,
5: 1-14.
BRIDGES, B.A., MACGREGOR, D., & ZEIGER, E. (1981) Summary
report on the performance of bacterial mutation assays. In: de
Serres, F.J. & Ashby, J., ed. Evaluation of short-term tests for
carcinogens. Report of the International Collaborative
Programme, New York, Amsterdam, Oxford, Elsevier/North Holland,
pp. 45-67 (Progress in Mutation Research Vol. 1).
BRINGMANN, G. (1975) [Determination of the effect of
biological pollutants on the inhibition of cell multiplication
in the blue algae Microcystis. ] Gesundheits-Ingenieur, 96: 238-
241 (in German).
BRINGMANN, G. & KUHN, R. (1980) Comparison of the toxicity
thresholds of water pollutants to bacteria, algae, and protozoa
in the cell multiplication test. Water Res., 14: 231-241.
BRINGMANN, G. & KUHN, R. (1981) [Comparison of the effect of
hazardous substances on flagellated and ciliated, respectively,
holozoic bactivorous and saprozoic protozoa.] GWF Wasser-
Abwasser, 122: 308-313 (in German).
BRINGMANN, G. & KUHN, R. (1982) [Data on the toxicity of water
pollutants to Daphnia magna in an improved standard test.] Z.
Wasser Abwasser Forsch., 15: 1-6 (in German).
BRUGGER, H. (1983) [ Emission factors for 12 selected
substances with probably carcinogenic potential: hydrazine,]
Environment Research Programme of the Federal Ministry of the
Interior, Federal Environment Office, Vol. 3, Part 1, pp. 15-17
(Report No. 104-04-344) (in German).
CARVER, J.H., SALAZAR, E.P., KNIZE, M.G., & WANDRES, D.L.
(1981) Mutation induction at multiple gene loci in Chinese
hamster ovary cells: the genetic activity of 15 coded
carcinogens and noncarcinogens. In: de Serres, F.J. & Ashby, J.,
ed. Evaluation of short-term tests for carcinogens. Report of
the International Collaborative Programme, New York, Amsterdam,
Oxford, Elsevier/North Holland, pp. 594-601 (Progress in
Mutation Research Vol. 1).
CHANDRA SEKHAR, V.S.G. & REDDY, G.M. (1971) Specific locus
mutations in maize by chemical mutagens. Curr. Sci., 40: 136-
137.
CHATTERJEE, A.K. & SENGUPTA, K. (1980) Regulation of formation
in vivo of pyridoxal phosphate in hydrazine-treated rats.
Int. J. Vitam. Nutr. Res., 50: 24-28.
CHU, B.C.F., BROWN, D.M., & BURDON, M.G. (1973) Effect of
nitrogen and of catalase on hydroxylamine and hydrazine
mutagenesis. Mutat. Res., 20: 265-270.
CIER, A., ROUGANNE, J.P., & SCHMITT, M. (1967) Effets
hématologiques d'une intoxication subaiguë à l'hydrazine et à la
dimethyl hydrazine asymétrique chez la souris et le lapin. C.R.
Soc. Biol. (Paris), 161: 854-858.
CLARK, D.A., BAIRRINGTON, J.D., BITTER, H.L., COE, F.L., MEDINA,
M.A., MERRIT, J.H., & SCOTT, W.N. (1968) Pharmacology and
toxicology of propellant hydrazines, Springfield, Virginia, US
Department of Commerce (Aeromedical Reviews No. 11-68) (AD
688500).
CLARK, D.A., LEEDER, L.G., FOULDS, E.L., & TROUT, D.L. (1970)
Changes in lipids of rat liver after hydrazine injection.
Biochem. Pharmacol., 19: 1743-1752.
COMSTOCK, C.C., LAWSON, L.H., GREENE, E.A., & OBERST, F.W.
(1954) Inhalation toxicity of hydrazine vapour. Arch. ind. Hyg.
occup. Med., 10: 476-490.
COOK, L.R., GLENN, R.E., & PODOLAK, G.E. (1979) Monitoring and
analysis of personnel exposures to hydrazines at a rocket
propellant plant. Am. Ind. Hyg. Assoc. J., 40: 69-74.
COOLING, J., BURDITT, S.L., & BRINDLEY, D.N. (1979) Effects of
treating rats with hydrazine on the circulating concentration of
corticosterone and insulin in relation to hepatic triacyl-
glycerol synthesis. Biochem. Soc. Trans., 7: 1501-1503.
CORNISH, H.H. & WILSON, C.E. (1968) Amino acid levels in
hydrazine-treated rats. Toxicol. appl. Pharmacol., 12: 265-272.
DANIEL, M.R. & DEHNEL, J.M. (1981) Cell transformation test
with baby hamster kidney cells. In: de Serres, F.J. & Ashby, J.,
ed. Evaluation of short-term tests for carcinogens. Report of
the International Collaborative Programme, New York, Amsterdam,
Oxford, Elsevier/North Holland, pp. 626-637 (Progress in
Mutation Research Vol. 1).
DAMBRAUSKAS, T. & CORNISH, H.H. (1964) The distribution, meta-
bolism, and excretion of hydrazine in rat and mouse. Toxicol.
appl. Pharmacol., 6: 653-663.
DAVIS, L.C. (1980) Hydrazine as a substrate and inhibitor of
Azobacter vinelandii nitrogenase. Arch. Biochem. Biophys.,
204: 270-276.
DEAN, B.J. (1981) Activity of 27 coded compounds in the R11
chromosome assay. In: de Serres, F.J. & Ashby, J., ed.
Evaluation of short-term tests for carcinogens. Report of the
International Collaborative Programme, New York, Amsterdam,
Oxford, Elsevier/North Holland, pp. 570-579 (Progress in
Mutation Research Vol. 1).
DEE, L.A. (1971) Gas chromatographic determination of aqueous
trace hydrazine and methylhydrazine as corresponding pyrazoles.
Anal. Chem., 43: 1416-1419.
DE FLORA, S., ZANACCHI, P., CAMOIRANO, A., BENNICELLI, C., &
BADOLATI, G.S. (1984) Genotoxic activity and potency of 135
compounds in the Ames reversion test and in a bacterial DNA-
repair test. Mutat. Res., 133: 161-198.
DE SERRES, F.J. & ASHBY, J., ed. (1981) Evaluation of short-
term tests for carcinogens. Report of the International
Collaborative Programme, New York, Amsterdam, Oxford, Elsevier/
North Holland, 827 pp (Progress in Mutation Research, Vol. 1).
DI LUZIO, N.R., STEGE, T.E., & HOFFMAN, E.O. (1973) Protective
influence of diphenyl- p -phenylenediamine on hydrazine-induced
lipid perioxidation and hepatic injury. Exp. mol. Pathol., 19:
284-292.
DOMINGUEZ, A.M., AMENTA, J.S., HILL, C.S., & DOMANSKI, T.J.
(1962) Morphologic and biochemical alterations in the kidney of
the hydrazine-treated rat. Aerosp. Med., 33: 1094-2010.
DOST, F.N., REED, D.J., & WANG, C.H. (1971) Effects of various
hydrazines upon the metabolism of gamma-aminobutyric acid
(GABA)-1-14C by rats. Biochem. Pharmacol., 20: 1702-1707.
DOST, F.N., BRODERICK, D.J., KRIVAK, R.M., & REED, D.J. (1981)
Metabolism of hydrazine, Ohio, Wright-Patterson Air Force Base,
Aerospace Medical Research Laboratory (AFAMRL TR-81-26)
(AD/A101849).
DREWS, A., EVERSMANN, K., & FRITZE, E. (1960) [On oral
poisoning by hydrazine.] Med. Welt, 23: 1295-1297 (in German).
DUAMIN, V.V., DENISOV, V.L., ANDROPOVA, S.N., & MALETIN, V.P.
(1984) [Influence of hydrazine on reproductive function of
animals when administered in organisms by different routes.]
Gig. i Sanit., 9: 25-28 (in Russian).
EPSTEIN, S.S., ARNOLD, E., ANDREA, J., BASS, W., & BISHOP, Y.
(1972) Detection of chemical mutagens by the dominant lethal
assay in the mouse. Toxicol. appl. Pharmacol., 23: 288-325.
EVANS, D.M. (1959) Two cases of hydrazine hydrate dermatitis
without systemic intoxication. Br. J. ind. Med., 16: 126-127.
FARMWALD, J.A. & MACNAUGHTON, M.G.M. (1981) Effects of
hydrazine on the activated sludge process. J. Water Pollut.
Control Fed., 53: 565-575.
FAROOK, S.A.F. & NIZAM, J. (1979) Mutagenic sensitivity of
base-specific chemicals in chick pea. Indian J. Bot., 2: 12-16.
FISHER, J.W., HARRAH, C.B., & BERRY, W.O. (1980) Hydrazine:
acute toxicity to bluegills and sublethal effects on dorsal
light response and aggression. Trans. Am. Fish. Soc., 109: 304-
309.
FLOYD, W.N. (1980) The importance of ammonia in the metabolic
effects of hydrazine. Aviat. Space environ. Med., 51: 899-901.
FORTNEY, S.R. (1966) Effect of hydrazine on liver glycogen,
arterial glucose, lactate, pyruvate, and acid-base balance in
the anaesthetized dog. J. Pharmacol. exp. Ther., 153: 562-568.
FORTNEY, S.R., CLARK, D.A., & STEIN, E. (1967) Inhibition of
gluconeogenesis by hydrazine administration in rats. J.
Pharmacol. exp. Ther., 156: 277-284.
FREESE, E.B., GERSON, J., TABER, H., RHAESE, H.-J., & FREESE, E.
(1967) Inactivating DNA alterations induced by peroxides and
peroxide-producing agents. Mutat. Res., 4: 517-531.
FRIERSON, W.M. (1965) Use of peridoxine HCl in acute hydrazine
and UDMH intoxication. Ind. Med. Surg., 34: 650-651.
FROST, J. & HJORTH, N. (1959) Contact dermatitis from
hydrazine hydrochloride in soldering flux. Cross-sensitization
to Apresoline and isoniazid. Acta dermatovenereol., 39: 82-86.
GANOTE, C.E. & ROSENTHAL, A.S. (1968) Characteristic lesions
of methylazoxymethanol-induced liver damage. A comparative
ultrastructural study with dimethylnitrosamine, hydrazine
sulfate, and carbon tetrachloride. Lab. Invest., 19: 382-398.
GEDDES, J.W. & WOOD, J.D. (1984) Changes in the amino acid
content of nerve ending (synaptosomes) induced by drugs that
alter the metabolism of glutamate and gamma-aminobutyric acid.
J. Neurochem., 42: 16-24.
GORDON, A. & LEWANDOWSKI, P.E. (1980) Control of vapor
emissions from hydrazine-based fuels, Newark, New Jersey,
Institute of Technology, Department of Chemical Engineering
(AFOSR-TR-80-053).
GORMLEY, W.T. & FORD, R.E. (1973) Deoxygenation of
environmental waters by hydrazine-type fuels. In: Proceedings of
the 4th Annual Conference on Environmental Toxicology, Fairborn,
Ohio, 1973, Ohio, Wright-Patterson Air Force Base, Aerospace
Medical Research Laboratory, Paper 28, pp. 387-400 (AMRL-TR-73-
125) (AD/781031).
GORSHTEIN, E.S. & KOPYLOVA, T.N. (1983) [Effect of hydrazine
hydrochloric acid on the hepatic microsomal hydroxylation system
of rats.] Eksp. Med., 15: 22-26 (in Russian).
GREENHOUSE, G. (1976a) Evaluation of the teratogenic effects
of hydrazine, methylhydrazine, and dimethylhydrazine on embryos
of Xenopus laevis, the South African clawed toad. Teratology,
13: 167-178.
GREENHOUSE, G. (1976b) The evaluation of toxic effects of
chemicals in fresh water by using frog embryos and larvae.
Environ. Pollut., 11: 303-315.
GUPTA, A.K. & GROVER, N.S. (1970) Hydrazine-induced breaks in
chromosomes of Vicia faba. Mutat. Res., 10: 519-520.
GUPTA, R.S. & GOLDSTEIN, S. (1981) Mutagen testing in the
human fibroblast diphteria toxin resistance (HF Dipr) system.
In: de Serres, F.J. & Ashby, J., ed. Evaluation of short-term
tests for carcinogens. Report of the International Collaborative
Programme, New York, Amsterdam, Oxford, Elsevier/North Holland,
pp. 614-625 (Progress in Mutation Research Vol. 1).
HACK, W., VON, HOYERMANN, K., & WAGNER, H.G. (1974) [Gas-phase
reactions of hydroxyl radical with ammonia and hydrazine.] Ber.
Bunsen-Ges. Phys. Chem., 78: 386-391 (in German).
HAMILL, P.V.V. (1978) Number of heart attack cases among
workers of the Lake Charles hydrazine plant, 1953-78 (Submitted
to the US EPA by the Olin Corporation, Stamford, Connecticut,
under TSCA, section 8(e)).
HARATI, Y. & NIAKAN, E. (1986) Hydrazine toxicity, pyridoxine
therapy, and peripheral neuropathy. Ann. intern. Med., 104: 728-
729.
HARRAH, C.B. (1978) Biological effects of aqueous hydrazine
solutions. In: Procedings of the Conference on Environmental
Chemistry: Hydrazine Fuels, 1977, Florida, Tyndall Air Force
Base, Civil and Environmental Engineering Development Office,
pp. 167-176 (CEEDO-TR-78-14) (AD/A054194).
HARRIS, G.W., ATKINSON, R., & PITTS, J.N. (1979) Kinetics of
the reactions of the hydroxyl radical with hydrazine and MMH. J.
Phys. Chem., 83: 2557-2559.
HAUN, C.C. & KINKEAD, E.R. (1973) Chronic inhalation toxicity
of hydrazine. In: Proceedings of the 4th Annual Conference on
Environmental Toxicology, Fairborn, Ohio, 1973, Ohio, Wright-
Patterson Air Force Base, Aerospace Medical Research Laboratory,
Paper 25, pp. 351-363.
HECK, W.W., BLOODWORTH, M.E., CLARK, W.J., DARLING, D.R., &
HOOVER, W. (1963) Environmental pollution by missile
propellants, Ohio, Wright-Patterson Air Force Base, Aerospace
Medical Research Laboratory (AMRL-TDR-63-75).
HEINDORFF, K., RIEGER, R., VELEMINSKY, J., & GICHNER, T. (1984)
A comparative study of the clastogenicity of maleic hydrazine
and some of its putative degradation products. Mutat. Res., 140:
123-126.
HEINEMANN, B. (1971) Prophage induction in lysogenic E. coli
with simple hydroxylamine and hydrazine compounds. Appl.
Microbiol., 21: 726-731.
HENDERSON, V., FISHER, J.W., & D'ALLESSANDRIS, R. (1981) Toxic
and teratogenic effects of hydrazine on fathead minnow (Pime-
phales promelas) embryos. Bull. environ. Contam. Toxicol., 26:
807-812.
HENDERSON, V., FISHER, J.W., D'ALLESSANDRIS, R., & LIVINGSTON,
J.M. (1983) Effects of hydrazine on functional morphology of
rainbow trout embryos and larvae. Trans. Am. Fish. Soc., 112:
100-104.
HERBOLD, B.A. (1978) [Mutagenicity testing with the liver
microsome test.] Biol. Zentralbl., 97: 137-152 (in German).
HIGGINS, E.S. & BANKS, W.L. (1971) Cognate effects of ethanol,
hydrazine, and tissue regeneration on hepatic mitochonidrial
activities. Biochem. Pharmacol., 20: 1513-1524.
HOLTZCLAW, J.R., ROSE, S.L., WYATT, J.R., ROUNBEHLER, D.P., &
FINE, D.H. (1984) Simultaneous determination of hydrazine,
methylhydrazine, and 1,1-dimethylhydrazine in air by derivat-
ization/gas chromatography. Anal. Chem., 56: 2952-2956.
HOVDING, G. (1967) Occupational dermatitis from hydrazine
hydrate used in boiler protection. Acta dermatovenereol., 47:
293-297.
HSIE, A.W., O'NEILL, J.P., MACHANOFF, R., SCHENLEY, R.L., &
BRIMER, P.A. (1981) Screening for mutagenic response of four
coded chemical by the CHO/HGPRT system. In: de Serres, F.J. &
Ashby, J., ed. Evaluation of short-term tests for carcinogens.
Report of the International Collaborative Programme, New York,
Amsterdam, Oxford, Elsevier/North Holland, pp. 602-607 (Progress
in Mutation Research Vol. 1).
HUNT, T.P., FISHER, J.W., LIVINGSTON, J.M., & PUTNAM, M.E.
(1981) Temperature effects on hydrazine toxicity to bluegills.
Bull. environ. Contam. Toxicol., 27: 588-595.
IARC (1982) Chemicals, industrial processes, and industries
associated with cancer in humans, Lyons, International Agency
for Research on Cancer, pp. 136-138 (Monographs on the
Evaluation of the Carcinogenic Risk of Chemicals to Humans,
Suppl. 4).
IRPTC (1985) Treatment and disposal methods for waste
chemicals, Geneva, International Register of Potentially Toxic
Chemicals, United Nations Environment Programme, pp. 188-189.
IRPTC (1986) Data profile on hydrazine, Geneva, International
Register of Potentially Toxic Chemicals, United Nations
Environment Programme.
ISAACSON, P.J. & HAYES, M.H.B. (1984) The interaction of
hydrazine hydrate with humic acid preparations at pH 4. J. soil
Sci. , 35(1): 79-92.
JACKSON, E.K., PARSHALL, G.W., & HARDY, R.W.F. (1968) Hydrogen
reactions of nitrogenase. J. biol. Chem., 243: 4952-4958.
JACOBSON, K.H., CLEM, J.H., WHEELWRIGHT, H.J., RINEHART, W.E., &
MAYES, N. (1955) The acute toxicity of the vapours of some
methylated hydrazine derivatives. Am. Med. Assoc. Arch. Ind.
Health, 12: 609-616.
JACOBSON, K.H., RINEHART, W.E., WHEELWRIGHT, H.J., ROSS, M.A.,
PAPIN, J.L., DALY, R.C., GREENE, E.A., & GROFF, W.A. (1958)
The toxicology of an aniline-furfurylalcohol-hydrazine vapour
mixture. J. Am. Ind. Hyg. Assoc., 19: 91-100.
JAIN, H.K. & SHUKLA, P.T. (1972) Locus specificity of mutagens
in Drosophila. Mutat. Res., 14: 440-442.
JAIN, H.K., RAUT, R.N., & KHAMANKAR, Y.G. (1968) Base specific
chemicals and mutation analysis in Lycopersicon. Heredity, 23:
247-256.
JOHNSON, F.B. (1982) Chemical interactions with Herpes
simplex type 2 virus: enhancement of transformation by hydrazine
and 1,2-dimethylhydrazine. Chem.-biol. Interact., 40: 97-112.
KAK, S.N. & KAUL, B.L. (1975) Mutagenic activity of hydrazine
and its combination with maleic hydrazide and X-rays in barley.
Cytobios, 12: 123-128.
KANE, D.A. & WILLIAMSON, K.J. (1983) Bacterial toxicity and
metabolism of hydrazine fuels. Arch. environ. Contam. Toxicol.,
12: 447-453.
KANEO, Y., IGUCHI, S., KUBO, H., IWAGIRI, N., & MATSUYAMA, K.
(1984) Tissue distribution of hydrazine and its metabolites in
rats. J. Pharm. Dyn., 7: 556-562.
KELLER, W.C., OLSON, C.T., BACK, K.C. (1982) Evaluation of the
embryotoxicity of hydrazine in rats, Ohio, Wright-Patterson Air
Force Base, Aerospace Medical Research Laboratory (AFAMRL-TR-82-
29) (AD/A119706).
KELLY, M.G., O'GARA, R.W., YANCEY, S., GADEKAR, K., BOTKIN, C.,
& OLIVERIO, V.T. (1969) Comparative carcinogenicity of N -
isopropyl-alpha-(2-methyl-hydrazino)- p -toluamide. HCl (pro-
carbazine hydrochloride), its degradation products, other hydra-
zines, and isonicotinic acid hydrazide. J. Natl Cancer Inst.,
42: 337-344.
KHAMANKAR, Y.G. & JAIN, H.K. (1978) A comparative study of
chemical and physical mutagens in bread wheat. Indian J. Hered.,
10: 49-57.
KIMBALL, R.F. (1976) Reversions of two proline-requiring
auxotrophs of Haemophilus influenzae by N -methyl- N' -nitro-
N-nitrosoguanidine and hydrazine. Mutat. Res., 36: 29-38.
KIMBALL, R.F. (1977) The mutagenicity of hydrazine and some of
its derivatives. Mutat. Res., 39: 111-126.
KIMBALL, R.F. & HIRSCH, B.F. (1975) Tests for the mutagenic
action of a number of chemicals on Haemophilus influenzae with
special emphasis on hydrazine. Mutat. Res., 30: 9-20.
KIRKHART, B. (1981) Micronucleus test on 21 compounds. In: de
Serres, F.J. & Ashby, J., ed. Evaluation of short-term tests for
carcinogens. Report of the International Collaborative
Programme, New York, Amsterdam, Oxford, Elsevier/North Holland,
pp. 698-704 (Progress in Mutation Research Vol. 1).
KIRKLIN, J.K., WATSON, M., BONDOC, C.C., & BURKE, J.F. (1976)
Treatment of hydrazine-induced coma with pyridoxine. New Engl.
J. Med., 294: 938-939.
KOPYLOVA, T.N., MAJORE, A., ELERTE, D., NOZDRUNOVA, N.A., &
ROZENBERG, I.E. (1982) [Lipid peroxidation in hydrazine
hydrochloride-induced toxicity of the liver.] Eksp. Med., 14:
35-45 (in Russian).
KORTY, P. & COE, F. (1968) The effects of hydrazine on the
concentration of free amino acids of plasma and urine. J.
Pharmacol. exp. Ther., 160 212-216.
KROP, S. (1954) Toxicology of hydrazine: a review. Arch. ind.
Hyg. occup. Med., :9: 199-204.
LAMB, R.G. & BANKS, W.L. (1979) Effect of hydrazine exposure
on hepatic triacylglycerol biosynthesis. Biochim. Biophys. Acta,
574: 440-447.
LEE, S.H. & ALEYASSINE, H. (1970) Hydrazine toxicity in
pregnant rats. Arch. environ. Health, 21: 615-619.
LEMONTT, J.F. (1977) Loss of hydrazine-induced mutability in
wild-type and excision-repair-defective yeast during post-
treatment inhibition of cell division. Mutat. Res., 50: 57-66.
LEMONTT, J.F. (1978) Mutagenesis of yeast by hydrazine:
dependence upon post-treatment cell division. Mutat. Res., 43:
165-178.
LEWALTER, J., PFLANITZER, K., MULLER, G., & SCHALLER, K.H.
(1984) [Hydrazine levels in blood (plasma).] In: [ Analytical
methods,] Vol. 2, pp. D1-D3 (Senate Commission for the Testing
of Health-damaging Work Material in the German Research
Association-Working Group "Analytical Chemistry") (in German).
LIU, Y.Y., SCHMELTZ, I., & HOFFMANN, D. (1974) Chemical
studies on tobacco smoke: quantitative analysis of hydrazine in
tobacco and cigarette smoke. Anal. Chem., 46: 885-889.
LONDON, S.A., MANTEL, C.R., ROBINSON, J.D., & LUKING, S. (1983)
Effects of selected hydrazines on the early death rates of
Enterobacter cloacae. Bull. environ. Contam. Toxicol., 31: 360-
368.
LOPEZ-MENDOZA, D. & VILLA-TREVINO, S. (1971) Hydrazine-
induced inhibition of amino acid incorporation into rat liver
protein. Lab. Invest., 25: 68-72.
LOPRIENO, N. (1981) Screening of coded carcinogenic/non-
carcinogenic chemicals by a forward mutation system with the
yeast Schizosaccharomyces pombe. In: de Serres, F.J. & Ashby,
J., ed. Evaluation of short-term tests for carcinogens. Report
of the International Collaborative Programme, New York,
Amsterdam, Oxford, Elsevier/North Holland, pp. 425-433 (Progress
in Mutation Research Vol. 1).
LYNG, R.D., KELLER, W.C., & BACK, K.C. (1980) Effects of
hydrazine on pregnant ICR mice, Ohio, Wright-Patterson Air Force
Base, Aerospace Medical Research Laboratory (AFAMRL-TR-80-19)
(AD/A084023).
MCCORMICK, D.B. & SNELL, E.E. (1961) Pyridoxal phospho-
kinases. II. Effects of inhibitors. J. biol. Chem., 236: 2085-
2088.
MCKENNIS, H., WEATHERBY, J.H., & WITKIN, L.B. (1955) Studies
on the excretion of hydrazine and metabolites. Am. Med. Assoc.
Arch. Ind. Health, 12: 511-514.
MCKENNIS, H., YARD, A.S., WEATHERBY, J.H., & HAGY, J.A. (1959)
Acetylation of hydrazine and the formation of 1,2-diacetyl-
hydrazine in vivo . J. Pharmacol. exp. Ther., 126: 109-116.
MACNAUGHTON, M.G., STAUFFER, T.B., & STONE, D.A. (1981)
Environmental chemistry and management of hydrazine. Aviat.
Space environ. Med., 52: 149-153.
MACRAE, W.D. & STICH, H.F. (1979) Induction of sister
chromatid exchanges in Chinese hamster ovary cells by thiol and
hydrazine compounds. Mutat. Res., 68: 351-365.
MARSHALL, C.E., WATTS, D.I., & SUGDEN, M.C. (1983) Effects of
hydrazine on liver and brown adipose tissue lipogenesis in 24 h-
starved rats. J. Pharm. Pharmacol., 35: 460-461.
MARU, G.B. & BHIDE, S.V. (1982) Effects of antioxidants and
antitoxicants of isoniazid on the formation of lung tumours in
mice by isoniazid and hydrazine sulfate. Cancer Lett., 17: 75-
80.
MATSUI, F., ROBERTSON, D.L., & LOVERING, E.G. (1983)
Determination of hydrazine in pharmaceuticals. III. Hydralazine
and isoniazid using GLC. J. pharmacol. Sci., 72: 948-951.
MATSUYAMA, K., YAMASHITA, C., SENDOH, T., NODA, A., GOTO, S., &
IGUCHI, S. (1983) Further investigations on brain distribution
of hydrazine and its gamma-aminobutyric acid elevating effect in
rats. J. Pharmacobio-Dyn., 6: 932-937.
MEDINA, M.A. (1963) The in vivo effects of hydrazines and
vitamin B6 on the metabolism of gamma-aminobutyric acid. J.
Pharmacol. exp. Ther., 140: 133-137.
MEHTA, R.D. & VON BORSTEL, R.C. (1981) Mutagenic activity of
42 uncoded compounds in the haploid yeast reversion assay,
strain XV 185-14C. In: de Serres, F.J. & Ashby, J., ed.
Evaluation of short-term tests for carcinogens. Report of the
International Collaborative Programme, New York, Amsterdam,
Oxford, Elsevier/North Holland, pp. 414-423 (Progress in
Mutation Research Vol. 1).
MENON, M.M. & BHIDE, S.V. (1983) Perinatal carcinogenicity of
isoniazide (INH) in Swiss mice. J. cancer Res. clin. Oncol.,
105: 258-261.
MILIA, U., BIANCIFIORI, C., & SANTILLI, F.E.G. (1965) Late
findings in pulmonary carcinogenesis by hydrazine sulfate in
newborn BALB/c/Cb/Se substrain mice. Lav. Ist. Anat. Istol.
Patol. Univ. Studio Perugia, 25: 165-171.
MILO, G.E., OLDHAM, J.W., ZIMMERMAN, R., HATCH, G.G., &
WEISBRODE, S.A. (1981) Characterization of human cells
transformed by chemical and physical carcinogens in vitro . In
vitro, 17: 719-729.
MITCHELL, P.C.H. & SCARLE, R.D. (1972) Role of reactions of
molybdenum compounds with hydrazine in nitrogen fixation.
Nature (Lond.), 240: 417-418.
NATARAJAN, A.T. & VAN KESTEREN-VAN LEEUWEN, A.C. (1981)
Mutagenic activity of 20 coded compounds in chromosome
aberrations/sister chromatid exchanges assay using Chinese
hamster ovary (CHO) cells. In: de Serres, F.J. & Ashby, J., ed.
Evaluation of short-term tests for carcinogens. Report of the
International Collaborative Programme, New York, Amsterdam,
Oxford, Elsevier/North Holland, pp. 551-559 (Progress in
Mutation Research Vol. 1).
NELSON, S.D. & GORDON, W.P. (1982) Metabolic activation of
hydrazines. Adv. exp. Med. Biol., 136B: 971-981.
NEPSS (1975) Disposal of wastewater containing hydrazine. In:
Pollution solution, California, Naval Environmental Protection
Support Service, Navy Environmental Support Office.
NODA, A., GOROMARU, T., MATSUYAMA, K., SOGABE, K., HSU, K.-Y., &
IGUCHI, S. (1978) Quantitative determination of hydrazines
derived from isoniazid in patients. I. J. Pharm. Dyn., 1: 132-
141.
NODA, A., HSU, K., NODA, H., YAMAMOTO, Y.D., & KUROZUMI, T.
(1983) Is isoniazid hepatotoxicity induced by the metabolite
hydrazine? Sangyo Ika Faigaku Zasshi, 5: 183-189.
NODA, A., NODA, H., OHNO, K., SENDO, T., MISAKA, A., KANAZAWA,
Y., ISOBE, R., & HIRATA, M. (1985a) Spin trapping of a free
radical intermediate formed during microsomal metabolism of
hydrazine. Biochem. biophys. Res. Commun., 133: 1086-1091.
NODA, A., SENDO, T., OHNO, K., GOTO, S., NODA, H., & HUS, K.-Y.
(1985b) Effects of rifampicin and phenobarbital on the fate of
isoniazid and hydrazine in vivo in rats. Toxicol. Lett., 25:
313-317.
O'BRIEN, R.D., KIRKPATRICK, M., & MILLER, P.S. (1964)
Poisoning of the rat by hydrazine and alkylhydrazines. Toxicol.
appl. Pharmacol., 6: 371-377.
PARODI, S., DE FLORA, S., CAVANNA, M., PINO, A., ROBBIANI, L.,
BENNICELLI, C., & BRAMBILLA, G. (1981) DNA-damaging activity
in vivo and bacterial mutagenicity of sixteen hydrazine
derivatives as related quantitatively to their carcinogenicity.
Cancer Res., 41: 1469-1482.
PATRICK, R.L. & BACK, K.C. (1965) Pathology and toxicology of
repeated doses of hydrazine and 1,1-dimethylhydrazine in monkeys
and rats. Ind. Med. Surg., 34: 430-435.
PERRY, P.E. & THOMSON, E.J. (1981) Evaluation of the sister
chromatid exchange method in mammalian cells as a screening
system for carcinogens. In: de Serres, F.J. & Ashby, J., ed.
Evaluation of short-term tests for carcinogens. Report of the
International Collaborative Programme, New York, Amsterdam,
Oxford, Elsevier/North Holland, pp. 560-569 (Progress in
Mutation Research Vol. 1).
PERRY, T.L., KISH, S.J., HANSEN, S., WRIGHT, J.M., WALL, R.A.,
DUNN, W.L., & BELLWARD, G.D. (1981) Elevation of brain GABA
content by chronic low-dosage administration of hydrazine, a
metabolite of isoniazid. J. Neurochem., 37: 32-39.
PITTS, J.N., TUAZON, E.C., CARTER, W.P., WINER, A.M., HARRIS,
G.W., ATKINSON, R., & GRAHAM, R.A. (1980) Atmospheric
chemistry of hydrazines: gas phase kinetics and mechanistic
studies, Florida, Tyndall Air Force Base, Air Force Engineering
and Services Centre, Engineering and Services Laboratory
(AFESC/ESL-TR-80-39) (AD/A093486).
PRADHAN, S.N. & ZIECHECK, L.N. (1971) Effect of hydrazine on
behaviour in rats. Toxicol. appl. Pharmacol., 18: 151-157.
PROTEAU, J.P., LIM, P., & LABAT, R. (1979) Toxicité d'un
dérivé bi-azolé, l'hydrate d'hydrazine, pour Carassius
carassius L., Rutilus rutilus L., et différents stades de
développement de Brachydanio rerio H.B. Ann. Limnol., 15: 337-
346.
PURCHASE, I.F.H., LONGSTAFF, E., ASHBY, J., STYLES, J.A.,
ANDERSON, D., LEFEVRE, P.A., & WESTWOOD, F.R. (1978) An
evaluation of 6 short-term tests for detecting organic chemical
carcinogens. Br. J. Cancer, 37: 873 - 935.
RAPHAELIAN, L.A. (1966) Hydrazine. In: Kirk, R.E. & Othmer,
D.F., ed. Encylopaedia of chemical technology, 2nd ed., New
York, John Wiley and Sons, Vol. 11, pp. 164-196.
RAY, P.D., HANSON, R.L., & LARDY, H.A. (1970) Inhibition by
hydrazine of gluconeogenesis in the rat. J. biol. Chem., 245:
690-696.
REDDY, G.M. & REDDY, T.P. (1972) Induction of mutations by
hydrazine in rice. Indian J. Genet. Plant Breed., 32: 388-391.
REID, F.J. (1965) Hydrazine poisoning. Br. med. J., 5472: 1246.
REIDENBERG, M.M., DURANT, P.J., HARRIS, R.A., DE BOCCARDO, G.,
LAHITA, R., & STENZEL, K.H. (1983) Lupus erythaematosus-like
disease due to hydrazine. Am. J. Med., 75: 365-370.
REYNOLDS, B.A. & THOMAS, A.A. (1965) A colorimetric method for
the determination of hydrazine and monomethylhydrazine in blood.
Am. Ind. Hyg. Assoc. J., 26: 527-531.
ROBERGE, A., GOSSELIN, C., & CHARBONNEAU, R. (1971) Effect of
hydrazine on urea cycle enzymes in vitro and in vivo . Biochem.
Pharmacol., 20: 2231-2238.
ROBINSON, D.E. & MITCHELL, A.D. (1981) Unscheduled DNA
synthesis response of human fibroblasts, W1-38 cells, to 20
coded chemicals. In: de Serres, F.J. & Ashby, J., ed.
Evaluation of short-term tests for carcinogens. Report of the
International Collaborative Programme, New York, Amsterdam,
Oxford, Elsevier/North Holland, pp. 517-527 (Progress in
Mutation Research Vol. 1).
ROE, F.J.C., GRANT, G.A., & MILLICAN, D.M. (1967) Carcino-
genicity of hydrazine and 1,1-dimethylhydrazine for mouse lung.
Nature (Lond.), 216: 375-376.
ROGAN, E.G., WALKER, B.A., GINGELL, R., NAGEL, D.L., & TOTH, B.
(1982) Microbial mutagenicity of selected hydrazines. Mutat.
Res., 89(4): 321-328.
ROGERS, A.M. & BACK, K.C. (1981) Comparative mutagenicity of
hydrazine and 3 methylated derivatives in L5178Y mosue lymphomas
cells. Mutat. Res., 16: 189-194.
ROHRBORN, G., PROPPING, P., & BUSELMAIER, W. (1972) Mutagenic
activity of isoniazid and hydrazine in mammalian test systems.
Mutat. Res., 16: 189-194.
ROSENKRANZ, H.S. & POIRIER, L.A. (1979) Evaluation of the
mutagenicity and DNA-modifying activity of carcinogens and non-
carcinogens in microbial systems. J. Natl Cancer Inst., 62: 873-
892.
ROTHBERG, S. & COPE, O.B. (1956) Toxicity studies on hydra-
zine, methylhydrazine, symmetrical dimethylhydrazine, unsym-
metrical dimethylhydrazine, and DMNA, Maryland, Army Chemical
Center (US Army Chemical Warfare Laboratory Report No. 2027).
SALOMONE, M.F., HEDDLE, J.A., & KATZ, M. (1981) Mutagenic
activity of 41 compounds in the in vivo micronucleus assay.
In: de Serres, F.J. & Ashby, J., ed. Evaluation of short-term
tests for carcinogens. Report of the International Collaborative
Programme, New York, Amsterdam, Oxford, Elsevier/North Holland,
pp. 686-697 (Progress in Mutation Research Vol. 1).
SAVCHENKOV, M.F. & SAMOILOVA, T.J. (1984) [Effect of hydrazine
nitrate on reproductive function of albino rats.] In:[ Problems
of limitation of environmental pollutants circulation,]Ufa, pp.
82-84 (in Russian).
SCALES, M.D.C. & TIMBRELL, J.A. (1982) Studies on hydrazine
hepatotoxicity. I. Pathological findings. J. Toxicol. environ.
Health, 10: 941-953.
SCHIESSL, H.W. (1980) Hydrazine and its derivatives. In: Kirk,
R.E. & Othmer, E.F., ed. Encyclopaedia of chemical technology,
3rd ed., New York, John Wiley and Sons, Vol. 12, pp. 734-771.
SCHILLER, C.M., WALDEN, R., & KEE, T.E. (1979) Effects of
hydrazine and its derivatives on the development of intestinal
brush border enzymes. Toxicol. appl. Pharmacol., 49: 305-311.
SCHMIDT, E.W. (1984) Hydrazine and its derivatives: preparation,
properties, and applications, New York, John Wiley and Sons.
SCHULTHEISS, E. (1959) [Hypersensitiveness to hydrazine.]
Berufsdermatosen, 7: 131-136 (in German).
SEVERI, L. & BIANCIFIORI, C. (1968) Hepatic carcinogenesis in
CBA/Cb/Se mice and Cb/Se rats by isonicotinic acid hydrazine and
hydrazine sulfate. J. Natl Cancer Inst., 41: 331-349.
SHANK, R.C. (1983) Evidence for indirect genetic damage as
methylation of DNA guanine in response to cytotoxicity. Dev.
Toxicol. environ. Sci., 11: 145-152.
SHUKLA, P.T. (1972) Analysis of mutagen specificity in
Drosphila melanogaster. Mutat. Res., 16: 363-371.
SINA, J.E., BEAN, C.L., DYSART, G.R., TAYLOR, V.I., & BRADLEY,
M.O. (1983) Evaluation of the alkaline elution/rat hepatocyte
assay as a predictor of carcinogenic/mutagenic potential.
Mutat. Res., 113: 357-391.
SINGH, H.B., LUDWIG, F.L., & JOHNSON, W.B. (1978) Tropospheric
ozone: concentrations and variabilities in clean remote
atmospheres. Atmos. Environ., 12: 2185-2191.
SLONIM, A.R. (1977) Acute toxicity of selected hydrazines to
the common guppy. Water Res., 11: 889-895.
SLONIM, A.R. & GISCLARD, J.B. (1976) Hydrazine degradation in
aquatic systems. Bull. environ. Contam. Toxicol., 16: 301-309.
SMITH, E.B. & CLARK, D.A. (1972) Absorption of hydrazine
through canine skin. Toxicol. appl. Pharmacol., 21: 186-193.
SONNECK, E.B. & UMLAUF, H. (1961) [Occupationally sustained
skin damage by hydrazine.] Haut-Geschlechskr., 31: 179-184 (in
German).
SOTANIEMI, E., HIRVONEN, J., ISOMAKI, H., TAKKUNEN, J., & KAILA,
J. (1971) Hydrazine toxicity in the human. Report of a fatal
case. Am. clin. Res., 3: 30-33.
SOTOMAYER, R.E., CHAUHAN, P.S., & EHLING, U.H. (1982) Induc-
tion of unscheduled DNA synthesis in the germ cells of male mice
after treatment with hydrazine or procarbazine. Toxicology, 25:
201-211.
SPEIT, G., WICK, C., & WOLF, M. (1980) Induction of sister
chromatid exchanges by hydroxylamine, hydrazine, and isoniazid
and their inhibition by cysteine. Hum. Genet., 54: 155-158.
SPINKOVA, (1971) [Determination of small amounts of hydrazine
in isoniazid solutions.] Pharm. Acta Helv., 46: 643-648 (in
German).
SPRINGER, D.L., BRODERICK, D.J., & DOST, F.N. (1980) Effects
of hydrazine and its derivatives on ornithine decarboxylase
synthesis, activity, and inactivation. Toxicol. appl.
Pharmacol., 53: 365-372.
SPRINGER, D.L., KRIVAK, B.M., BRODERICK, D.J., REED, D.J., &
DOST, F.N. (1981) Metabolic fate of hydrazine. J. Toxicol.
environ. Health, 8: 21-29.
STEIN, E.R., CLARK, D.A., & FORTNEY, S.R. (1971) Inhibition of
glutamicoxaloacetic transaminases of rat liver by hydrazine.
Toxicol. appl. Pharmacol., 18: 274-284.
SUGGS, H.J., LUSKUS, L.J., & KILIAN, H.J. (1980) A field
procedure for sampling and analysis of low concentrations of
hydrazine in air. Am. Ind. Hyg. Assoc. J., 41: 879-883.
THOMSON, J.A. (1981) Mutagenic activity of 42 coded compounds
in the lambda induction assay. In: de Serres, F.J. & Ashby, J.,
ed. Evaluation of short-term tests for carcinogens. Report of
the International Collaborative Programme, New York, Amsterdam,
Oxford, Elsevier/North Holland, pp. 224-235 (Progress in
Mutation Research Vol. 1).
THORNELEY, R.N.F., EADY, R.R., & LOWE, D.J. (1978) Biological
nitrogen fixation by way of an enzyme-bound dinitrogen-hydride
intermediate. Nature (Lond.), 272: 557-558.
TIMBRELL, J.A. & HARLAND, S.J. (1979) Identification and
quantitation of hydrazine in the urine of patients treated with
hydralazine. Clin. Pharmacol. Ther., 26: 81-88.
TIMBRELL, J.A., WRIGHT, J.M., & SMITH, C.M. (1977)
Determination of hydrazine metabolites of isoniazid in human
urine by gas chromatography. J. Chromatogr., 138: 165-172.
TIMBRELL, J.A., SCALES, M.D., & STREETER, A.J. (1982) Studies
on hydrazine hepatotoxicity. II. Biochemical findings. J.
Toxicol. environ. Health, 10: 955-968.
TOMLINSON, T.G., BOON, A.G., & TROTMAN, C.N.A. (1966)
Inhibition of nitrification in the activated sludge process of
sewage disposal. J. appl. Bacteriol., 29: 266-291.
TOTH, B. (1969) Lung tumour induction and inhibition of breast
adenocarcinomas by hydrazine sulfate in mice. J. Natl Cancer
Inst., 42: 469-475.
TOTH, B. (1972a) Hydrazine, methylhydrazine, and methylhydra-
zine sulfate carcinogenesis in Swiss mice. Failure of ammonium
hydroxide to interfere in the development of tumours. Int. J.
Cancer, 9: 109-118.
TOTH, B. (1972b) Tumourigenesis studies with 1,2-dimethyl-
hydrazine dihydrochloride, hydrazine sulfate, and isonicotinic
acid in golden hamsters. Cancer Res., 32: 804-807.
TROUT, D.L. (1965) Effects of hydrazine on plasma-free fatty
acid transport. Biochem. Pharmacol., 14: 813-821.
TROUT, D.L. (1966) Effects of hydrazine on fat transport as
affected by blood glucose concentration. J. Pharmacol. exp.
Ther., 152: 529-534.
TSUCHIMOTO, T. & MATTER, B.E. (1981) Activity of coded
compounds in the micronucleus test. In: de Serres, F.J. & Ashby,
J., ed. Evaluation of short-term tests for carcinogens. Report
of the International Collaborative Programme, New York,
Amsterdam, Oxford, Elsevier/North Holland, pp. 705-711 (Progress
in Mutation Research Vol. 1).
TUAZON, E.C., CARTER, W.P.L., BROWN, R.V., ATKINSON, R., WINER,
A.M., & PITTS, J.N. (1982) Atmospheric reaction mechanisms of
amine fuels, Florida, Tyndall Air Force Base, Air Force
Engineering and Service Center, Engineering and Services
Laboratory (AFESC/ESL-TR-82-17) (AD/A118267).
US FDA (1979) Hydrazine: proposed removal from food additive
use. Fed. Reg., 44: 33693-33695.
US NIOSH (1977a) Manual of analytical methods, 2nd ed.,
Cincinnati, Ohio, US National Institute of Occupational Safety
and Health, Vol. 1, pp. S237-1 to S237-6 (US DHEW Publication
No. 77-157A).
US NIOSH (1977b) Manual of analytical methods, 2nd ed.,
Cincinnati, Ohio, US National Institute of Occupational Safety
and Health, Vol. 3, pp. 248-1 to 248-6 (US DHEW Publication No.
77-157A).
US NIOSH (1978) Criteria for a recommended standard:
occupational exposure to hydrazines, Cincinnati, Ohio, US
National Institute for Occupational Safety and Health, Vol. 3,
pp. 248-1 to 248-6 (US DHEW Publication No. 78-172).
US NIOSH (1986) Industrial hygiene study: extent of exposure
to hydrazine, Cincinnati, Ohio, US National Institute for
Occupational Safety and Health, 49 pp (Report No. 136.5;
Contract No. 200-82-2521).
VAN KETEL, W.G. (1964) Contact dermatitis from a hydrazine
derivative in a stain remover. Cross-sensitization to apresoline
and isoniazid. Acta dermatovenereol., 44: 49-53.
VAN STEE, E.W. (1965) Acute effects of exposure to hydrazine
and hydrazine derivatives on renal function in the dog. Aerosp.
Med., 36: 764-767.
VASUDEVA, M. & VASHISHAT, R.K. (1985) Mutagenic and
recombinogenic activity of hydrazine sulfate in Saccharomyces
cerevisiae. Mutat. Res., 155: 113-115.
VELTE, J.S. (1984) Acute toxicity of hydrazine hydrate to the
fathead minnow (Pimephales promelas) and Daphnid (Daphnia
pulex). Bull. environ. Contam. Toxicol., 33: 598-604.
VERNOT, E.H., MACEWEN, J.D., BRUNER, R.H., HAUN, C.C., KINKEAD,
E.R., PRENTICE, D.E., HALL, A., III, SCHMIDT, R.E., EASON, R.L.,
HUBBARD, G.B., & YOUNG, J.T. (1985) Long-term inhalation
toxicity of hydrazine. Fundam. appl. Toxicol., 5: 1050-1064.
VIJAYKUMAR, N.K. & JAIN, H.K. (1979) Comparative studies on
induced mutations with physical and chemical mutagens in
Drosophila melanogaster. Part I. Specificity and spectrum of
mutations. Indian J. exp. Biol., 17: 61-65.
VISHNOI, A.K. & GUPTA, P.K. (1980) Induced mutagenesis in
Vicia faba L. I. Chlorophyll mutations induced by gamma rays,
ethyl methane sulfonate, and hydrazine. Cytobios, 27: 81-87.
VON KEILIG, W. & SPEER, U. (1983) [Occupational allergic
contact dermatitis from hydrazine.] Derm. Beruf Umwelt, 31: 25-
27 (in German).
VON KRULIK, R. (1966) [The influence of hydrazine and
isonicotinic acid hydrazine on certain substances of the sugar
metabolism in vitro. ] Arzneim. Forsch., 16: 1623-1626 (in
German).
VON PEVNY, I. & PETER, G. (1983) [Allergic contact eczema to
derivatives of pyridine and hydrazine.] Dermatosen, 31: 78-83
(in German).
VON WRIGHT, A. & TIKKANEN, L. (1980) The comparative
mutagenicities of hydrazine and its mono- and dimethyl
derivatives in bacterial test systems. Mutat. Res., 78: 17-23.
WAKABAYASHI, T., HORIUCHI, M., SAKAGUCHI, M., ONDA, H., &
MISAWA, K. (1983) Induction of megamitochondria in the mouse
and rat livers by hydrazine. Exp. mol. Pathol., 39: 139-153.
WALD, N., BOREHAM, J., DOLL, R., & BONSALL, J. (1984)
Occupational exposure to hydrazine and subsequent risk of
cancer. Br. J. ind. Med., 41: 31-34.
WARGOVICH, M.J., GOLDBERG, M.T., NEWMARK, H.L., & BRUCE, W.R.
(1983) Nuclear aberrations as a short-term test for geno-
toxicity to the colon: evaluation of 19 agents in mice. J. Natl
Cancer Inst., 71: 133-137.
WARREN, D., CORNELIUS, C., & FORD, B. (1984) Liver function
studies on rhesus monkeys (Maccaca mulatta) following the
administration of hydrazine sulfate. Vet. Human Toxicol., 26:
295-299.
WATJE, W.F. (1978) Potential of a hydrazine-type fuel spill or
emission during movement from supplies to user. In: Proceedings
of the Conference on Environmental Chemistry: Hydrazine Fuels, 1
977, Florida, Tyndall Air Force Base, pp. 19-24 (CEEDO-TR-78-
14).
WELLS, H.G. (1908) The pathological anatomy of hydrazine
poisoning. J. exp. Med., 10: 457-462.
WHEELER, C.E., PENN, S.R., CAWLEY, E.P., & HILL, C. (1965)
Dermatitis from hydrazine hydrobromide solder flux. Arch.
Dermatol., 91: 235-239.
WITKIN, L.B. (1956) Acute toxicity of hydrazine and some of
its methylated derivatives. Arch. ind. Health, 13: 34-36.
WONG, E.T. (1966) Renal functional response to hydrazine and
1,1-dimethylhydrazine. Toxicol. appl. Pharmacol., 8: 51-56.
WOOD, J.D., RUSSELL, M.P., & KURYLO, E. (1980) The gamma-
aminobutyrate content of nerve endings (synaptosomes) in mice
after the intramuscular injection of gamma-aminobutyrate-
elevating agents: a possible role in anticonvulsant activity.
J. Neurochem., 35: 125-130.
WRIGHT, J.M. & TIMBRELL, J.A. (1978) Factors affecting the
metabolism of 14C-acetylhydrazine in rats. Drug Metab. Dispos.,
6: 561-566.
YAKSCTAT, B.Y. (1969) Experimental assessment of hydrazine
hydrate maximum allowable concentration in water bodies. In:
Industrial pollution of water bodies, Moscow, Medicine, pp.
186-200.
YOSHIDA, T. & ALEXANDER, M. (1964) Hydroxylamine formation by
Nitrosomonas europaea. Can. J. Microbiol., 10: 923-926.
ZINA, G. & BONU, G. (1962) [Occupational dermatitis from
hydrazine and derivatives.] Minerva Dermatol., 37: 197-200 (in
Italian).
Approximately 25% of the hydrazine dose remains unaccounted
for. Ammonia was found in the blood of dogs without
significantly elevated blood-urea nitrogen (Floyd, 1980).
Springer et al. (1981) did not find labelled ammonia in the
urine of rats exposed to 15N-hydrazine (Table 4). Therefore,
the ammonia in dogs was probably not derived from hydrazine
(section 8.3.2), but was the result of an effect on metabolic
pathways (Floyd, 1980; Springer et al., 1981).
6.3. Reaction With Body Components
No adduct formation between hydrazine and DNA in vivo has
been reported (Shank, 1983). Under non-physiological conditions,
hydrazine can react with pyrimidine bases. These reactions were
reviewed by Kimball (1977). Indirect methylation of guanines in
DNA following hydrazine exposure has been demonstrated and will
be discussed in section 8.5.1.
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
7.1. Aquatic Organisms
A summary of acute toxicity data is presented in table 5. It
should be realized that the rate of decay of hydrazine in the
aquatic environment depends on the conditions (section 4.2).
When the concentration of hydrazine is not monitored during
exposure, it should be noted that the toxic effects observed
must have occurred at concentrations lower than the nominal
ones, due to degradation of the compound. the increased toxicity
of hydrazine for guppies in soft water at a pH just below 7
compared with the toxicity in hard water at a pH of
approximately 8, as found by Slonim (1977), is at least partly
explained by the increased persistence of hydrazine in soft and
non-alkaline water. Taking into account the decay of hydrazine,
increases in water temperature were found to enhance the
toxicity of the compound for bluegills (hunt et al., 1981).
Teratogenicity and toxicity screening were reported using
the South African clawed toad (Greenhouse, 1976a,b), fathead
minnow (Henderson et al., 1981), and rainbow trout (Henderson et
al., 1983). Eggs of the South African clawed toad in the
cleavage were exposed to hydrazine until hatching. Survival and
development into normal larvae occurred at exposures below 10
mg/litre. At 10 mg/litre, 35% of the embryos were malformed at
hatching. The effect was dose-related. Additional studies
revealed that teratogenic effects appeared during neurulation
(Greenhouse, 1976a). When larvae of the South African clawed
toad were exposed to 1.0 mg hydrazine/litre water, for 120 h,
all died in 24 - 48 h following exposure. No significant effects
on survival and development were observed after exposure to 0.1
mg/litre, the next lower concentration tested (Greenhouse,
1976b).
Eggs of fathead minnows at the mid-cleavage stage were
exposed to hydrazine for 24 or 48 h. Embryos, exposed for 24 h,
to 0.1 mg/litre, showed several defects, such as slightly or
moderately subnormal heart beat, haemoglobin levels, body
movement amount of eye pigment. From 1 mg/litre upwards, the
responses were generally stronger; in addition, body pigment was
absent and developmental arrest was observed. Embryos exposed to
a hydrazine concentration of 1.0 mg/litre for 48 h appeared to
have little chance of survival. Surviving embryos showed severe
deformities and larvae exhibited reduced growth (Henderson et
al., 1981).
Table 5. Acute aquatic toxicity of hydrazine
---------------------------------------------------------------------------------------------------------
Organism Tempera- pH Hardness Flow/ Parameter Concentration Reference
ture (mg CaCO3 stata (mg/litre)
(°C) /litre)
---------------------------------------------------------------------------------------------------------
Bacteria
Pseudomonas putida 20 stat 16-h TT 0.019 Bringmann &
Kühn
(1980)b
Protozoa
Entosiphon sulcatum 25 6.9 stat 72-h TT 0.93 Bringmann &
Kühn (1981)b
Uronema paraduczi 25 6.9 stat 22-h TT 0.24 Bringmann &
Kühn (1981)b
Chilomenas paramecium 20 6.9 stat 48-h TT 0.002 Bringmann &
Kühn (1981)b
Algae
Green algae
(Chlorella pyrenoidosa) 23 6.8 75 stat 48-h EC50 ca.10c Heck et al.
48-h EC100 ca.100c (1963)d
Crustacea
Water flea 20 8.0 stat 24-h EC50 2.3c Bringmann &
(Daphnia pulex) Kühn (1982)
8.2 stat 24-h LC50 1.16c Heck et al.
(1963)
20 7.1-7.2 stat 24-h LC50 0.51 and 1.01 Velte (1984)e
48-h LC50 0.16 and 0.19
Amphibia
South African clawed
toad (Xenopus laevis)
eggs 8.2-8.7 stat LOEL 10c Greenhouse
(1976a)f
larvae stat 120-h LOEL 1.0c Greenhouse
120-h NOEL 0.1c (1976b)g
Table 5. (contd.)
---------------------------------------------------------------------------------------------------------
Organism Tempera- pH Hardness Flow/ Parameter Concentration Reference
ture (mg CaCO3 stata (mg/litre)
(°C) /litre)
---------------------------------------------------------------------------------------------------------
Fish (fresh-water)
Guppy (Lebistes reti- 22-24 7.8-8.2 400-500 stat 96-h LC50 3.85c Slonim (1977)
culatus) 22-24 6.3-6.9 20-25 stat 96-h LC50 0.61c
Fathead minnow
(Pimephales promelas)
eggs 21 7.0-7.5 150 flow 48-h LOEL 0.1 Henderson et
48-h NOEL 0.001 al. (1981)h
adults 20 192 stat 96-h LC50 4.5c Cowen et al.
(1981)
adults 20 6.9 flow 96-h LC50 5.98 Velte (1984)e
Bluegill sunfish 23-24 7.2-8.4 240-292 stat 96-h LC50 1.08 Fisher et al.
(Lepomis macrochirus) (1980)
23-24 7.8-7.9 164 flow 96-h LOEL 0.43 Fisher et al.
(1980)i
23-24 7.1-7.9 239 stat 96-h LOEL 0.1 Fisher et al.
(1980)i
10 6.7-8.0 160-190 flow 96-h LC50 1.6 Hunt et al.
15.5 1.0 (1981)
21 1.2
Goldfish (Carassius 8.2-8.5 stat 48-h LC50 2.8c Heck et al.
auratus) (1963)j
19 8.1-8.5 135 stat 24-h LC50 0.95 Proteau et al.
(1979)
Roach (Rutilus 19 8.1-8.5 135 stat 24-h LC50 0.54 Proteau et al.
rutilus) (1979)
Zebra fish
(Brachydanio rerio)
5-day-old 26 7.8 110 stat 24-h LC50 0.75 Proteau et al.
(1979)
3-month-old 20 7.6-8.2 110 stat 24-h LC50 2.03 Proteau et al.
(1979)
Table 5. (contd.)
---------------------------------------------------------------------------------------------------------
Organism Tempera- pH Hardness Flow/ Parameter Concentration Reference
ture (mg CaCO3 stata (mg/litre)
(°C) /litre)
---------------------------------------------------------------------------------------------------------
Green sunfish (Leptomis 8.2-8.5 stat 48-h LC50 5.1c Heck et al.
(1963)j
Large mouth bass 8.2-8.5 stat 48-h LC50 3.6c Heck et al.
(1963)j
Channel catfish 8.2-8.5 stat 48-h LC50 1.6c Heck et al.
(1963)j
Fish (marine species)
Stickle back (Gaster- 14- 7.6-8.0 stat 96-h LC50 3.4c Harrah
osteus aculeatus) 15.5 (1978)k
---------------------------------------------------------------------------------------------------------
a Flow-through or static test.
b TT = toxicity threshold.
c No analysis for hydrazine during exposure was reported.
d EC50 and EC100 for 50% and 100% growth inhibition, measured by reading optical density.
e Soft water.
f LOEL = lowest-observed-adverse-effect level for teratogenicity. Exposure of eggs in cleavage stage
until hatching.
g LOEL = lowest-observed-adverse-effect level for lethality. NOEL = no-observed-effect level
for lethality and development.
h LOEL and NOEL = lowest-and-no observed-adverse-effect level for toxicity and teratogenicity.
i LOEL = lowest-observed-adverse-effect level for dorsal light response (at a non-lethal concentration).
j Standard reference water.
k Salinity, 1.8%.
Henderson et al. (1983) also exposed eggs of rainbow trout
(Salmo gairdneri) for 48 h, to hydrazine in continuous-flow
tests at 11.5 - 12 °C, a pH of 7 - 7.5, and a water hardness of
15 mg calcium carbonate/litre. During exposures up to 5 mg/litre
a dose-related increase was observed in the incidence of poorly
fitting jaws, pronounced mouth gape, and absence of body
movement. However, no effects were observed on mortality, heart
beat, hatching rate, or hatching period. Reduced growth and
abnormal development of larvae were observed at 1 and 5
mg/litre. Poor muscular development and poor bone growth were
observed; the authors postulate that this is a result of calcium
binding by hydrazine.
7.2. Microorganisms
The toxicity of hydrazine for a number of species of
bacteria, algae, and protozoa was measured by Bringmann (1975) &
Bringmann & Kuhn (1980, 1981). Some very low toxicity thresholds
were reported, for example, 0.005 mg/litre for a 7-day exposure
for the algae Scenedesmus quadricauda and 0.00008 mg/litre for a
10-day exposure for the blue algae Microcystis aerogenosa. This
is a very sensitive test.
London et al. (1983) described the toxicity of hydrazine for
the soil heterotroph Enterobacter cloacea. Hydrazine caused a
concentration-dependent increase in the lag time of this
organism. In a medium containing 10 mg/litre, this did not
affect the growth rate and final growth yield after the lag
period. At 100 mg/litre, the bacteria were not viable.
Although relatively high concentrations of hydrazine in
water have been recorded as inhibiting, either completely or
partially, the activities of Nitrosomonas, Nitrobacter, and
other bacteria in culture media (Yoshida & Alexander, 1964) in
waste-water treatment (Tomlinson et al., 1966; Farmwald &
MacNaughton, 1981; Kane & Williamson, 1983), the highest
continuously maintained tolerable level in waste water is of the
order of 1 mg/litre, as stated in section 4.3 (Farmwald &
MacNaughton, 1981).
7.3. Plants
Heck et al. (1963) studied the effects of hydrazine on the
germination of seeds and seedling growth after application as a
hydroponic culture contaminant and an air fumigant.
Seeds of summer brush squash (Cucurbita pepo), peanut
(Arachis hypogaea), and corn (Zea mays) were soaked for 48 h in
water containing hydrazine at levels of between 0 and 1000
mg/litre. The temperature was 30 °C. At the highest concentra-
tion, germination of peanut and corn seed was inhibited. Seed-
ling growth was inhibited from 10 mg/litre for squash, 100
mg/litre for corn, and 1000 mg/litre for peanut.
Sixteen-day-old seedlings of cotton (Gossypium hirsutum) in
a hydroponic culture were exposed to hydrazine in the growth
medium for 9 days at concentrations of between 0 and 1000
mg/litre and a temperature of between 22 and 29 °C. Plants died
within 48 h of exposure to 300 mg/litre and within 30 h at the
higher concentrations. Injury was first noted as foliar
dehydration, without chlorosis or necrosis, after 9 days of
exposure to 50 mg/litre or within 24 h of exposure to 300
mg/litre or more.
Several plants were also exposed for 4 h to hydrazine vapour
at concentrations of between 0 and 100 mg/m3 air. Species tested
were soybean (Glycine max), cow pea (Vigna sinensis), pinto bean
(Phaseolus vulgaris), cotton (Gossypium hirsutum), endive
(Cichorium endivia), alfalfa (Medicago sativa), and squash
(Cucurbita pepo). Wilting of leaves in all species was
observed within 2 - 24 h of exposure to 30 mg/m3, followed by
wilting of the whole plant. Death occurred in pinto beans and
endive plants at this exposure level. At higher concentrations,
plants of soybean and alfalfa also died. Six days after
exposure, all surviving plants started to recover.
8. EFFECTS ON EXPERIMENTAL ANIMALS
In section 8, all doses have been expressed in terms of the
free base; however, the form of hydrazine used in each study has
been indicated when possible.
8.1. Single Exposures
The toxicology of hydrazine has been reviewed by Krop
(1954), Clark et al. (1968), and US NIOSH (1978).
LD50 values for rats and mice after oral, iv, and ip expo-
sure were not significantly dependent on the route of exposure
and ranged from 55 to 64 mg/kg body weight for rats and from 57
to 82 mg/kg body weight for mice (free base or hydrazine
hydrate) (Witkin, 1956; O'Brien, 1964; Yaksctat, 1969; Azar et
al., 1970). Oral LD50 values for hydrazine (hydrazine hydrate)
in guinea-pigs and rabbits were 26 and 35 mg/kg body weight,
respectively (Yaksctat, 1969). Dogs and rabbits appeared more
sensitive, LD50 values following iv injection being 25 and 26
mg/kg body weight, respectively. The dermal LD50 for the rabbit
was 93 mg hydrazine (free base)/kg body weight (Rothberg & Cope,
1956; Witkin, 1956). When doses between 96 and 481 mg/kg body
weight (free base) were applied to the skin of dogs, 10 out of
25 animals died within the 6-h observation period; a dose-effect
relationship was not observed (Smith & Clark, 1972). When rats
and mice inhaled hydrazine (free base) for 4 h, the LC50s were
750 and 330 mg/m3, respectively (Jacobson et al., 1955). Death
occurred quickly in both species. Lethal doses of hydrazine
usually induced convulsions, excitement or inactivity, and other
effects on the central nervous system. Rats and mice inhaling
lethal concentrations of hydrazine (free base) also showed
dyspnoea (Comstock et al., 1954; Jacobson et al., 1955; O'Brien,
1964). Spontaneous motor activity depression was noted in rats
at ip doses of 39 and 52 mg/kg body weight (hydrazine sulfate)
(Pradhan & Ziecheck, 1971). Dogs receiving a sublethal iv dose
(free base) did not exhibit convulsions but showed increased
neuromuscular activity, salivation, diarrhoea, vomiting, and
hyperventilation (Wong, 1966).
Few pathological changes have been reported following acute
lethal doses. Some rats that died after inhaling hydrazine (free
base), showed lung oedema with localized damage to the bronchial
mucosa (Comstock et al., 1954). Wells (1908) observed fatty
changes in the liver in 24 h following sublethal doses in many
species. Fatty changes have also been observed in the kidneys of
rats (free base or hydrazine hydrate) (Dominguez et al., 1962;
Scales & Timbrell, 1982). In rats, accumulation of lipid,
swelling of mitochondria, and an increased number of microbodies
were observed in the liver and in the proximal tubules of the
kidneys, 24 h after an ip dose of 20 or 30 mg/kg body weight
(hydrazine hydrate). Similar changes were observed within 1 h,
after doses of 40 or 60 mg/kg body weight (Scales & Timbrell,
1982). In addition, nuclear and nucleolar enlargement and
hypertrophy of the smooth endoplasmic reticulum were observed in
the liver of rats, 2 or more hours after a single
intraperitoneal dose of 64 mg/kg body weight (hydrazine sulfate)
(Ganote & Rosenthal, 1968). Studies on the mechanisms by which
hydrazine causes these effects will be discussed later (section
8.3) together with other effects on the intermediary metabolism,
notably hypoglycaemia and lipid peroxidation, and effects on the
central nervous system, such as an increase in gamma-
aminobutyrate levels in the brain.
In dogs given a sublethal dose of hydrazine (free base),
degeneration of the proximal convoluted tubules of the kidneys
was accompanied by decreased creatinine clearance and increased
glucose reabsorption by the tubules. The glomerular filtration
rate was decreased because of decreased renal blood flow (Van
Stee, 1965; Wong, 1966).
In rhesus monkeys treated intravenously with 2.5 - 9.8 mg
hydrazine (hydrazine sulfate)/kg, liver function tests were
generally within normal limits up to 72 h after dosing. A dose
of 80 mg/kg caused fatty liver, but no necrosis (Warren et al.,
1984).
8.2. Short-Term Exposures
8.2.1. Inhalation exposure
In a 6-month inhalation study, groups of 50 male Sprague
Dawley rats, 40 female ICR mice, 8 male Beagle dogs, and 4
female rhesus monkeys were exposed to 0.26 or 1.3 mg hydrazine
(free base)/m3 air, continuously, or 1.3 or 6.5 mg hydrazine/m3
air, for 6 h/day, 5 days/week. Exposure concentrations were
monitored. Control groups contained the same number of animals.
The exposure regimen was chosen in such a way that the weekly
doses received by the continuously exposed groups were approxi-
mately equal to the weekly doses of hydrazine received by the
intermittently exposed groups. An increased mortality rate was
only seen in mice at the 2 higher exposure levels. In rats,
there was a dose-related decrease in body weight gain, while
body weights of dogs were decreased at the 2 higher exposure
levels. In dogs, the reduced weights were at least partly due to
reduced food consumption. Weights of the exposed monkeys were
comparable with those of controls. Organ weights were unaffected
by the exposure in rats, dogs, and monkeys. Organ and body
weights of mice were not recorded. Central nervous system
effects observed included lethargy in mice at the 2 higher
exposure levels, and tonic convulsions in 1 dog exposed
continuously to 1.3 mg/m3. Monkeys exhibited slight eye irrita-
tion at the 2 higher exposure levels. Fatty changes of the liver
were observed in mice at all exposures and in dogs at the 2
higher exposure levels. The livers of exposed monkeys showed
slight-to-moderate fat accumulation. However, this was also
seen, to some extent, in control animals. Livers of rats were
normal. Finally, dogs exhibited reduced red blood cell counts,
haematocrit, and haemoglobin values at the 2 higher exposure
levels, together with an increased resistance to osmotic haemo-
lysis at all exposure levels. Haematological variables were
normal in rats and monkeys and were not measured in mice. In
dogs, the effects on the liver and the haematological variables
appeared reversible (Haun & Kinkead, 1973).
Decreases in red blood cell count and haematocrit were also
observed in 20 female Swiss mice exposed to 130 mg hydrazine
(free base)/m3 air for 1 h/day, 6 days/week, for 4 weeks. In
this study, a decreased osmotic resistance to haemolysis was
noted in exposed mice (Cier et al., 1967).
Groups of 10 - 30 male Wistar rats were exposed to hydrazine
(free base) at average concentrations of 6, 18, 26, 70, or 295
mg/m3 air, for 5 days/week, 6 h/day, over periods ranging from 5
to 40 days at the 3 highest exposure levels to approximately 6
months at the 2 lowest exposure levels. The control group
consisted of 10 rats. Increased mortality was observed at all
exposure levels but not in controls, and body weights were
decreased at the 3 highest exposure levels. Rats became sluggish
during the 6-month exposure, while at the 3 highest exposure
levels, an initial restlessness was followed by a tendency to
sleep. In some cases, pathological examination revealed lung
oedema with local damage to the bronchial mucosa at the 3
highest exposure levels. Fatty livers were observed in many rats
after 5 days of exposure at 295 mg/m3 (Comstock et al., 1954).
8.2.2. Other routes of exposure
Groups of 25 male Sprague Dawley rats were dosed ip with 10
or 20 mg hydrazine (free base)/kg body weight, 5 times per week,
for 5 weeks. The control group consisted of 15 rats. Mortality
was increased at the dose of 20 mg/kg body weight; 10/25 rats
died after 8 - 21 doses. Body weight was lost in a dose-related
manner; 4.4 and 25.7% of the initial body weight was lost in 10
days in the 10 and 25 mg/kg groups, respectively. At 20 mg/kg
body weight, rats also displayed weakness and lethargy, and 2
rats exhibited convulsions. Pathological examination revealed
hyperaemia and oedema in the lungs of 4 rats and slight fatty
vacuolation in the liver of 7 rats at 20 mg/kg body weight. At
both doses, the haematocrit values were maximally decreased
after 13 injections (Patrick & Back, 1965).
Patrick & Back (1965) treated 12 rhesus monkeys ip with
hydrazine (free base), 5 times per week. Six monkeys received 5
mg/kg body weight for 4 weeks; two of these monkeys received a
further 8 doses of 10 mg/kg body weight, followed by 4 or 5
doses of 20 mg/kg body weight. A group of 6 monkeys received
only 4 or 5 doses of 20 mg/kg body weight. The control group
consisted of 10 monkeys. No monkey died, but all exposed monkeys
showed decreased body weights. Lethargy, weakness, and vomiting
were see in 7 of the 8 monkeys exposed to 20 mg/kg body weight,
while tremors were seen in 1 of these monkeys. Fatty changes
were observed in the liver, proximal tubules of the kidneys,
heart, and skeletal muscles at 20 mg/kg body weight, and
occasionally at 5 mg/kg body weight. Extensive periportal
necrosis was found in the liver of one of the dosed monkeys. The
level of bilirubin was increased and the serum was icteric.
Haematocrit and haemoglobin values, measured at the lower dose
only, dropped slightly, relative to control values (Patrick &
Back, 1965).
The pathological effects on the liver were also investigated
microscopically, in groups of 20 - 29 male DDY mice and 10 male
Wistar rats, after administration of 5, 10, or 20 mg hydrazine
(free base)/kg powdered diet, for 3 - 10 days. No animals died.
Animals of both species exhibited weakness. Megamitochondria or
fatty vacuolation with moderately swollen mitochondria and focal
proliferation of the smooth endoplasmic reticulum were induced
in rats and mice at dose levels of 10 and 20 mg/kg feed. The
induction of megamitochondria was a reversible process (Waka-
bayashi et al., 1983). Noda et al. (1983) observed centrilobular
hepatic necrosis in male rabbits dosed for 5 days with between
14.6 and 32.3 mg hydrazine (hydrazine monohydro-chloride)/kg
body weight per day, iv.
In other studies, hydrazine (hydrazine hydrate) was given
orally in drinking-water to albino rats and guinea-pigs for 7
months at levels providing 0.3, 0.03, 0.003, and 0.0003 mg/kg
body weight per day. At the two highest doses, adverse effects
were observed in the CNS (changes in conditioned reflexes),
liver (increased I131 excretion, changes in enzyme activity,
protein dystrophia), and blood (symptoms of haemoloytic
anaemia). The dose of 0.003 mg/kg body weight was reported to be
the no-observed-adverse-effect level (Yaksctat, 1969).
8.3. Biochemical Effects and Mechanisms of Toxicity
All of the studies described in this section were performed
with doses considered to be toxic.
8.3.1. Effects on lipid metabolism
Hydrazine caused a dose-dependent increase in hepatic
triglyceride levels in rats, the threshold dose for a single ip
injection being 10 - 20 mg/kg body weight. The maximal effect,
an increase of 7 times the control value, was observed after a
dose of 40 or 60 mg hydrazine hydrate/kg body weight. At these
dose levels, the effect was measurable 4 h after injection
(Timbrell et al., 1982). Other authors have also reported
accumulation of triglycerides in the liver of rats exposed to
single doses of hydrazine via injection routes (Amenta &
Dominguez, 1965a; Clark et al., 1970; Lamb & Banks, 1979).
Several mechanisms have been proposed:
1. Increased mobilization of free fatty acids from
adipose tissue (particularly observed at low plasma-
glucose levels) leading to an increased uptake of free
fatty acids, followed by increased triglyceride
synthesis in the liver (Trout, 1965, 1966; Clark et
al., 1970). This mobilization of free fatty acids
might be caused by the effects of hydrazine on the
sympathetic nervous system and on levels of adrenal
steroid hormone, possibly in response to the hypo-
glycaemia induced by hydrazine (Amenta & Dominguez,
1965a). Cooling et al. (1979) found elevated
concentrations of circulating corticosterone and
decreased concentrations of insulin in the serum of
rats exposed to hydrazine. Decreased blood-insulin
levels were also measured in rats by Aleyassine & Lee
(1971).
2. Increased synthesis of triglycerides caused by
increased enzymatic activity of phosphatidate phospho-
hydrolase (EC 3.1.3.4) in hepatocytes both in vivo and
in vitro was reported by Lamb & Banks (1979). It has
been suggested that this was a result of increased
corticosterone levels (Cooling et al., 1979). In
addition, Marshall et al. (1983) found increased fatty
acid synthesis in the liver of rats after hydrazine
administration.
3. Triglycerides could accumulate in hepatocytes as a
result of a decreased secretion of lipoproteins from
liver to plasma (Amenta & Dominguez, 1965a; Clark et
al., 1970). This could be explained by a decreased
lipid-binding capacity of lipoproteins following an
observed alteration in the proportion of phospholipids
and cholesterol (Clark et al., 1970) or by
increased lipid peroxidation (Di Luzio et al., 1973;
Kopylova et al., 1982). The protein moiety of
lipoproteins could also be subject to change (section
8.3.2).
8.3.2. Effects on carbohydrate and protein metabolism
Rats and dogs with starvation-induced depletion of glycogen
stores showed rapidly declining plasma-glucose levels with a
concomitant rise in lactate and pyruvate levels after exposure
to single intravenous hydrazine doses of 64 (free base or hydra-
zine sulfate) and 25 mg (free base)/kg body weight,
respectively. In well-fed dogs, hyperglycaemia and depletion of
glycogen stores preceded hypoglycaemia. Acidosis developed
slowly as a result of an increased lactate-pyruvate ratio
(Fortney, 1966; Fortney et al., 1967; Ray et al., 1970).
It has been postulated that hydrazine inhibits glyconeo-
genesis (Fortney, 1966; Fortney et al., 1967). This could occur
via inhibition of pyridoxal phosphate-dependent aminotrans-
ferases and decarboxylases. It has been shown that hydrazine
interferes with pyridoxal phosphate synthesis in vitro
(McCormick & Snell, 1961) and in vivo (Chatterjee & Sengupta,
1980) (section 8.3.5). Inhibition of transaminases would also
explain the increase in free amino acids observed in the plasma,
liver, brain, and muscle of rats (Cornish & Wilson, 1968; Banks,
1970) and in the plasma and urine of dogs (Korty & Coe, 1968).
It could further explain several observations in rats, such as
the depressed conversion of amino acids to carbon dioxide
(Amenta & Dominguez, 1965b; Dost et al., 1971), the depressed
incorporation of amino acids in plasma-glucose (Fortney et al.,
1967), and the enhanced incorporation of amino-labelled acids in
liver proteins, 24 h following hydrazine exposure (Banks, 1970).
Inhibition of protein synthesis was also observed in rat livers
up to 8.5 h after exposure (Lopez-Mendoza & Villa-Trevino,
1971).
Hydrazine treatment of rats resulted in inhibited activity
of specific aminotransferases and decarboxylases including:
liver aspartate aminotransferase (EC 2.6.1.1) (Stein et al.,
1971), brain gamma-aminobutyrate aminotransferase (EC 2.6.1.19)
and glutamate decarboxylase (EC 4.1.1.15) (Medina, 1963; Perry
et al., 1981), and liver ornithine 2-oxo-acid aminotransferase
(EC 2.6.1.13) (Roberge et al., 1971). The activity of rat liver
ornithine decarboxylase (EC 4.1.1.17) increased following
hydrazine exposure (Springer et al., 1980).
Inhibition of phosphoenolpyruvate carboxykinase (ATP) (EC
4.1.1.49), an enzyme involved in gluconeogenesis, was also
measured in vitro . Hydrazine increased the levels of citrate,
malate, and oxaloacetate in the rat liver (Ray et al., 1970).
Hydrazine affects the urea cycle. Decreased specific
activity of ornithine 2-oxo-acid aminotransferase, caused by
administration of hydrazine to rats (Roberge et al., 1971),
provoked an increase in ornithine in the liver (Banks, 1970),
brain, and plasma (Perry et al., 1981). The concentrations of
citrulline and urea in the liver, kidneys, brain, and blood were
increased, as was the activity of argininosuccinate lyase (EC
4.3.2.1) (Roberge et al., 1971).
8.3.3. Effects on mitochondrial oxidation
Swelling of hepatic mitochondria was observed in rats and
mice following hydrazine administration (Ganote & Rosenthal,
1968; Scales & Timbrell, 1982; Wakabayashi et al., 1983)
(sections 8.1 and 8.2.2). in vitro studies on the effects of
high concentrations of hydrazine on the functional status of
mitochondria showed decreased oxidation of keto-acids (Von
Krulik, 1966). Oxidative phosphorylation measured as the P/O
ratio was either not affected or decreased independently of the
hydrazine concentration (Von Krulik, 1966; Fortney et al.,
1967). Inhibition of beef heart cytochrome a by hydrazine was
also reported (Takemori et al., 1960). After administration of a
single intraperitoneal dose of hydrazine to rats, slightly
stimulated mitochondrial oxidation of succinate and glutamate
was observed with a slight increase in P/O ratio, respiration
control rate, and phosphorylation rate. ATPase (EC 3.6.1.8)
activity was not affected (Higgins & Banks, 1971). When mice
received hydrazine (free base) in the diet (10%) for 3 days and
rats received hydrazine in the diet (20%) for 8 days, oxidation
of succinate and glutamate, coupling efficiency, P/O ratio, and
the activities of ATPase (EC 3.6.1.8) and cytochrome c oxidase
(EC 1.9.3.1) were slightly decreased in liver megamitochondria,
while the activity of monoamine oxidase (EC 1.4.3.4) was
moderately decreased (Wakabayashi et al., 1983).
8.3.4. Effects on microsomal oxidation
Proliferation of the smooth endoplasmic reticulum was
observed in the liver of rats and mice following hydrazine
administration (Ganote & Rosenthal, 1968; Wakabayashi et al.,
1983) (sections 8.1 and 8.2.2). A single dose of 55 mg hydrazine
(free base)/kg body weight in rats decreased the hepatic
cytochrome P-450 content (Gorshtein & Kopylova, 1983). Rats
exposed for 4 days to 12 mg hydrazine (hydrazine sulfate)/kg
body weight per day did not show any effect on cytochrome P-450
levels in the microsomal fraction of the liver, but a slightly
decreased level of cytochrome b 5, inhibition of benzopyrene
hydroxylase (EC 1.14.14.1), and increased parahydroxylation of
aniline (Akin & Norred, 1978).
8.3.5. Effects on the central nervous system
The relationship between the effects of hydrazine on the
central nervous system, especially the occurrence of convul-
sions, and changes in levels of gamma-aminobutyric acid, an
inhibitory neurotransmitter, in the brain of rats and mice has
been investigated. An increase in the level of gamma-amino-
butyric acid was observed in the whole brain of rats after: (a)
a single intraperitoneal dose of 51 mg hydrazine (free base)/kg
body weight (Medina, 1963); (b) a single intravenous dose of 5
mg hydrazine (hydrazine sulfate)/kg body weight (Matsuyama et
al., 1983); or (c) after a daily subcutaneous dose of 2.6 mg
hydrazine (hydrazine monohydrochloride)/kg body weight over 109
days (Perry et al., 1981). In the whole brain of mice, an
increase was observed following a single intramuscular dose of
54 mg hydrazine (free base)/kg body weight (Wood et al., 1980).
The changes in the concentration of this amino acid are caused
by inhibition of pyridoxal phosphate requiring gamma-
aminobutyrate aminotransferase (EC 2.6.1.19) and glutamate
decarboxylase (EC 4.1.1.15) (Medina, 1963; Perry et al., 1981).
Hydrazine treatment of rats also caused a general amino acid
imbalance in the brain (Perry et al., 1981). A relationship was
suggested between the excitable state of the brain and the
gamma-aminobutyric acid contents of nerve endings (decreased)
rather than the whole brain contents of gamma-aminobutyric acid
(increased) (Wood et al., 1980; Geddes & Wood, 1984).
8.4. Reproduction, Embryotoxicity, and Teratogenicity
When groups of 26 Wistar rats were exposed to 0 or 8 mg
hydrazine (hydrazine monohydrochloride)/kg body weight per day,
sc, from day 11 to day 20 of gestation, the exposed dams showed
a 20% decrease in body weight and 2 dams died. Reduced number of
viable fetuses were found in 9 dams killed on day 21 of
gestation (63/172 versus 142/179 in controls), while the number
of implants per litter was not affected. The fetuses had reduced
weights and appeared pale and oedematous, but did not exhibit
any major malformations. In the rats allowed to deliver (12),
perinatal mortality was 100% in treated rats and 20% in controls
(Lee & Aleyassine, 1970).
These results agree with those of another study in which
groups of 6 - 27 Fisher 344 rats received 0, 2.5, 5, or 10 mg
hydrazine (free base)/kg body weight per day, ip, from day 6 to
day 15 of gestation. Body weight gains in dams were decreased
and the number of resorptions per dam were increased in a dose-
related manner. The differences between treated and control
animals were statistically significant at doses of 5 or 10
mg/kg, but not at 2.5 mg/kg for both variables. The numbers of
implants per dam and fetal weights were not affected at any
dose. The incidence of litters or fetuses with abnormalities was
not significantly increased at any dose; however, at 10 mg/kg,
only one out of the 6 females produced a viable litter, and only
6 fetuses were examined. In a subsequent study, 27 rats were
untreated and 11 rats were treated with 10 mg hydrazine/kg per
day, during what appeared to be the most susceptible period of
gestation (days 7 - 9). The incidence of litters or fetuses with
abnormalities in the 10 mg/kg group (6 litters out of 8; 8
fetuses out of 16) was significantly higher than that in the
control group of the preceeding study (8 litters out of 27; 11
fetuses out of 181). The abnormalities observed were mainly
supernumerary and fused ribs, delayed ossification, moderate
hydronephrosis, and moderate dilation of brain ventricles
(Keller et al., 1982).
Subtle postnatal changes were reported in the offspring of
24 female Syrian golden hamsters exposed orally to 0 or 170 mg
hydrazine (hydrazine hydrate)/kg body weight on the 12th day of
gestation. The pups of exposed dams did not exhibit cleft palate
formation, but showed effects on the development of intestinal
brush border enzymes. No other end-points were investigated
(Schiller et al., 1979).
Groups of ICR mice were treated ip with 0, 4, 12, 20, 30, or
40 mg hydrazine (free base)/kg body weight per day, from day 6
to day 9 of gestation. Some dams administered the highest dose
died. Body weights were reduced at doses of 12 mg/kg body weight
or more. While at the lower doses the number of resorptions per
litter was unchanged compared with controls, embryotoxicity was
evident at 30 and 40 mg/kg body weight. At 12 and 20 mg/kg body
weight, 17-day-old fetuses showed reduced weights, and there was
a dose-related increased incidence of litters with abnor-
malities, mainly exencephaly, hydronephrosis, and supernumerary
ribs (Lyng et al., 1980).
Savchenkov & Samoilova (1984) studied the adverse effects on
reproductive function (fertility of females, numbers of newborn,
and resorption of embryos) of female and male albino rats
exposed by gavage to hydrazine (hydrazine nitrate) at a dose of
13 mg/kg body weight, once a day, for 30 days prior to mating.
The development of the surviving litters did not differ from
that of controls.
Albino rats (10/group and 20 controls) of both sexes were
exposed to hydrazine (free base) (99.5% purity) in the drinking-
water at concentrations of 0.82, 0.018, or 0.002 mg/litre (0.016
mg/kg, 0.0014 mg/kg, or 0.00016 mg/kg, respectively, nominal
dose, assuming water consumption 20 ml per day and animal
weights of 250 g). The duration of the study was 6 months. The
number of animals studied and the scheme and time of mating were
not reported. The female rats exposed to the highest concen-
trations had fewer live embryos and more resorptions, as well as
pre- and post-implantation deaths, than the controls. No effects
were observed in animals administered 0.002 mg/litre.
Developmental abnormalities were not reported in any of the 293
embryos from all exposed animals. Destruction of gonadal
epithelium was observed in male rats after 6 months of oral
exposure to hydrazine at concentrations of 0.82 and 0.018
mg/litre (Duamin et al., 1984). In the same study, albino rats
were exposed to hydrazine (free base) at concentrations of 0.85,
0.13, or 0.01 mg/m3 (0.10 mg/kg body weight, 0.016 mg/kg, or
0.0012 mg/kg, respectively, nominal dose, assuming inhalation of
6 litres air per h and animal weights of 200 g), for 5 h per
day, 5 days/week, for 4 months. At the two highest concentra-
tions, embryotoxic effects of the same severity were seen as
when hydrazine was administered orally at the two highest doses.
No abnormalities were observed among 315 embryos. No gonado-
toxic effects occurred in male rats under the conditions of the
study (Duamin et al., 1984).
Additional information relating to reproductive end-points
is presented in section 8.5.1 (Sotomayer et al., 1982).
8.5. Mutagenicity and Related End-Points
8.5.1. DNA damage
Hydrazine was found to react with pyrimidine bases under
non-physiological conditions. These reactions were reviewed by
Kimball (1977). No hydrazine-DNA adducts have been reported to
be formed in vivo. A single oral or intraperitoneal dose of
hydrazine (free base or hydrazine sulfate) administered to rats,
mice, guinea-pigs, and hamsters resulted in the rapid formation
of N7-methylguanine and O6-methylguanine in liver DNA, which was
not detected in controls (Becker et al., 1981; Quintero-Ruiz et
al., 1981; Bosan & Shank, 1983; Shank, 1983). Methylation was
detectable at toxic doses and a sharp increase in the methyl-
ation of guanine in liver-DNA was observed at oral doses
exceeding 60 mg/kg body weight in rats and 45 mg/kg body weight
in hamsters. In both species, maximum methylation approximated
80 N7-methylations and 7 O6-methylations per 100 000 residues of
guanine, 6 h after oral exposure to 90 mg hydrazine/kg body
weight. Elimination of 7-methylguanine from DNA began about 24 h
after exposure with a half-life of 40 - 50 h. Elimination of O6-
methylguanine from DNA began about 24 h after exposure in rats
and about 50 h after exposure in hamsters, with a half-life of
13 h and 17 h, respectively (Becker et al., 1981; Bosan & Shank,
1983). The source of the methyl group was S -adenosyl-methionine
(Becker et al., 1981; Quintero-Ruiz et al., 1981).
Single-strand breaks were detected by the alkaline elution
assay in rat liver cells exposed in vitro (Sina et al., 1983),
and in the liver and lung cells of mice injected ip with 50 or
100 mg hydrazine (hydrazine hydrate)/kg body weight (Parodi et
al., 1981).
Hydrazine was reported to induce lambda phage in Escherichia
coli (Heinemann, 1971); however, no such induction was found by
Thomson (1981), nor did hydrazine induce HPlcl phage in Haemo-
philus influenzae (Balganesh & Setlow, 1984).
Repair of DNA lesions induced by hydrazine was observed in
vitro. In the International Collaborative Programme for the
Evaluation of Short-Term Tests for Carcinogenicity, 5 bacterial
DNA repair tests, using Bacillus subtilis or E. coli, were all
found to produce weak to medium positive responses. Only one
assay required rat liver microsomal fraction. The other positive
responses were reduced in magnitude by microsomal fraction
(Ashby & Kilbey, 1981). Increased unscheduled DNA synthesis was
observed in 1 out of 2 tests using human fibro-blasts (Agrelo &
Amos, 1981; Robinson & Mitchell, 1981).
No increase in unscheduled DNA synthesis was observed in the
germ cells of mice, 16 days after a 5-day exposure to doses of
hydrazine (hydrazine dihydrochloride) of up to 120 mg/kg body
weight per day (Sotomayer et al., 1982).
8.5.2. Mutation and chromosomal effects
The results of tests for gene mutations and chromosome
damage induced by hydrazine or its salts are summarized in Table
6. Hydrazine induces gene mutations and/or chromosome
aberrations in a variety of test systems including plants,
phage phi 80, bacteria, fungi, Drosophila melanogaster, and
mammalian cells in vitro . In a few cases, microsomal activation
was an absolute requirement for a positive effect (Gupta &
Goldstein, 1981; Perry & Thomson, 1981). In most cases, an
effect could be observed, both with and without microsomal
activation, with a stronger effect in some tests with activation
and in other tests without activation. These inconsistencies
were evaluated by Ashby (1981). Some negative results in the
forward mutation tests with mammalian cells invitro can be
traced back to the high locus specificity of hydrazine also
observed in plants. Duamin et al. (1984) observed increased
chromosomal aberrations in bone marrow cells (4.12 ± 0.65%
versus 2.48 ± 0.46% in controls) of albino rats (N = 10) exposed
to hydrazine (free base) at 0.85 mg/m3, 5 h per day, 5
days/week, for 4 months. No increased incidence of nuclear
aberrations, micronuclei, dominant lethals, and sperm-head
abnormalities were observed in hydrazine-treated mice (free base
or hydrazine sulfate).
Table 6. Tests for gene mutations and chromosome damage induced by hydrazine or its salts
---------------------------------------------------------------------------------------------------------
Test description System description Result Reference
Organism Species/strain/cell type
---------------------------------------------------------------------------------------------------------
Forward mutations transforming Bacillus subtilis + Freese et al. (1967)a
G DNA + Bresler et al. (1968)a
E Forward mutations plant tomato + Jain et al. (1968)b
+ Chandra Sekhar & Reddy
(1971)b
N rice + Reddy & Reddy (1972)b
barley + Kak & Kaul (1975)b
E wheat + Khamankar & Jain (1978)b
broad bean + Vishnoi & Gupta (1980)b
Reverse mutations virus phage phi 80 + Chu et al. (1973)c
M Reverse mutations bacteria Salmonella typhimurium TA 1530 + Rosenkranz & Poirier
(1979);Tosk et al.
(1979)
U
Salmonella typhimurium TA 1535 + Purchase et al. (1978);
Herbold (1978);
T Rosenkranz & Poirier
(1979); Bridges et al.
(1981);d,e;
A Parodi et al. (1981);
Rogan et al. (1982);
T Braun et al. (1984);
De Flora et al. (1984)
I Salmonella typhimurium TA 1537 - Bridges et al. (1981)d,e;
Parodi et al. (1981);
O Rogan et al. (1982)
N Salmonella typhimurium TA 1538 + Bridges et al. (1981)d,e
Purchase et al. (1978);
S Herbold (1978);
Rosenkranz & Poirier
(1979); Parodi et al.
(1981)
Salmonella typhimurium TA 100 + Purchase et al. (1978);
Herbold (1978); Bridges
et al. (1981)d,e
---------------------------------------------------------------------------------------------------------
Table 6. (contd.)
---------------------------------------------------------------------------------------------------------
Test description System description Result Reference
Organism Species/strain/cell type
---------------------------------------------------------------------------------------------------------
Reverse mutations bacteria Salmonella typhimurium TA 100 - Bridges et al. (1981)d,e;
G Parodi et al. (1981)
E Salmonella typhimurium TA 98 -, + Herbold (1978); Bridges
et al. (1981)d,e; Parodi
et al. (1981)
N + Purchase et al. (1978)
E Salmonella typhimurium G 46 + Braun et al. (1984)
Reverse mutations bacteria Salmonella typhimurium G 46 + Röhrborn et al. (1972)
(host-mediated (mouse) (NMRI)
assay)
M Reverse mutations bacteria Escherichia coli + Von Wright & Tikkanen
(1980); Bridges et al.
(1981)d,e
U
Haemophilus influenzae + Kimball & Hirsch (1975);
T Kimball (1976)
A Reverse mutations fungi Saccharomyces cerevisiae + Mehta & Von Borstel
(1981)e; Vasudeva &
Vashishat (1985)
T Forward mutations fungi Saccharomyces cerevisiae + Lemontt (1977, 1978)
Saccharomyces pombe + Loprieno (1981)e
I
Sex-linked visibles insect Drosophila melanogaster + Jain & Shukla (1972);
O Shukla (1972);
Vijaykumar & Jain (1979)
N Sex-linked lethals insect Drosophila melanogaster + Shukla (1972)
S Forward mutations hamster ovary cells in vitro + Gupta & Goldstein (1981)e
- Carver et al. (1981)e;
Hsie et al. (1981)e
mouse lymphoma cells in vitro + Rogers & Back (1981)
+ Amacher et al. (1980)
---------------------------------------------------------------------------------------------------------
Table 6. (contd.)
---------------------------------------------------------------------------------------------------------
Test description System description Result Reference
Organism Species/strain/cell type
---------------------------------------------------------------------------------------------------------
C Breaks plant horse bean primary root + Gupta & Grover (1970)
H Breaks, deletions, plant horse bean primary root + Heindorff et al. (1984)
translocations
R
Aberrationsf plant chick pea root + Farook & Nizam (1979)
O
Aberrations rat epithelial liver cells - Dean (1981)e
M in vitro
O Aberrations rat bone marrow cells in vivo + Duamin et al. (1984)
S Breaks, gaps, hamster ovary cells in vitro + Natajaran & Van Kesteren-
exchanges Van Leeuwen (1981)e
O
Sister chromatid hamster ovary cells in vitro + MacRae & Stich (1979);
Perry & Thomson (1981)e
M exchanges
- Natarajan & Van Kesteren-
Van Leeuwen (1981)e; Baker
et al. (1983)
E
lung cells in vitro + Baker et al. (1983)
V-79 cells in vitro + Speit et al. (1980)
Nuclear aberrations mouse epithelial colon cell - Wargovich et al. (1983)
(oral exposure)g
---------------------------------------------------------------------------------------------------------
Table 6. (contd.)
---------------------------------------------------------------------------------------------------------
Test description System description Result Reference
Organism Species/strain/cell type
---------------------------------------------------------------------------------------------------------
D Micronuclei (ip mouse polychromatic erythrocytes + Salomone et al. (1981)e
exposure) - Kirkhart (1981)e;
A Tsuchimoto & Matter
(1981)e
M Dominant lethals mouse germ cells - Epstein et al. (1972)
(ip exposure)
A
G
E
------------------------------------------------------------------------------------------------------------------------
a Inactivation of transforming DNA was observed, which was inhibited by catalase, EDTA, or nitrogen gas treatment.
b Hydrazine appears to be a mutagen that is highly specific for certain loci. Moreover, it not only produced mutants
in the M2 generation but also homozygous recessive mutants in the M1 generation (plants, raised from treated seeds).
c Inactivation of virus observed. Inactivation and, at lower concentrations, mutations were reduced by catalase
treatment.
d Hydrazine sulfate was tested in the International Collaborative Programme for the Evaluation of Short-Term Tests for
Carcinogenicity (de Serres & Ashby, 1981). In the summary on the assay performance of bacterial mutation assays, it
was reported that hydrazine sulfate was mutagenic in most of a total of 20 laboratories. However, there were
inconsistencies in the strains in which the effect was seen and the requirements for S9 mix. Salmonella typhimurium
TA 1535 was the strain in which mutagenic activity was most commonly observed, but it was also seen in all
laboratories that used Escherichia coli strains. In some laboratories, hydrazine sulfate was also mutagenic in
Salmonella typhimurium TA 100. Two laboratories reported a marginal mutagenic activity for strain TA 98, and TA 1538
was positive in another, while no mutagenic activity was observed with TA 1537.
e Tested in the International Collaborative Programme for the Evaluation of Short-Term Tests for Carcinogenicity. In
the final assessment, it was stated that hydrazine was a genotoxic agent in bacteria, yeast, and higher eukaryotes
in vitro . Hydrazine did not appear to be genotoxic in vivo in higher eukaryotic cells (de Serres & Ashby, 1981).
f Stickiness and clumping at metaphase, bridges and fragments at anaphase, laggards, micronuclei, and delayed
cytokinesis at telophase, tripolar spindles, prophase, and metaphase in one cell.
g Micronuclei, pyknotic nuclei, karyorrhetic nuclei, cytolysosomes.
8.5.3. Cell transformation
Hydrazine increased the transformation of baby hamster
kidney cells (Purchase et al., 1978; Daniel & Dehnel, 1981) and
human liver cells (Purchase et al., 1978). While Purchase et al.
(1978) observed an increased transformation of baby hamster
kidney cells, both with and without metabolic activation, Daniel
& Dehnel (1981) found a much lower increased transformation
frequency only with metabolic activation. Hydrazine also caused
enhancement of transformation of mouse 3T3 cells by Herpes
simplex virus in vitro (Johnson, 1982).
Hydrazine induced transformation of human fibroblasts. Transformed
cells were able to induce undifferentiated mesenchymal tumours
after sc injections in pre-irradiated nude mice, while control
cells did not induce tumours (Milo et al., 1981).
8.6. Carcinogenicity
8.6.1. Inhalation exposure
Groups of 100 Fischer 344 rats of each sex, 400 female
C57BL/6 mice, and 200 male golden Syrian hamsters were exposed
to the vapour of hydrazine (free base) for 12 months, 6 h per
day, for 5 days per week, and observed for a further 18, 15, and
12 months, respectively (Table 7). Rats were exposed at 0.06,
0.33, 1.3, or 6.5 mg/m3, mice at 0.06, 0.33, or 1.3 mg/m3, and
hamsters at 0.33, 1.3, or 6.5 mg/m3 air. Control groups
contained 150 rats of each sex, 800 female mice, and 200 male
hamsters, respectively. The hydrazine vapour was monitored
throughout the exposure period. A reduction in body weight gain,
seen in exposed rats throughout the entire study, was greatest
in males at 6.5 mg/m3. The mortality rate was not affected by
the exposures. Inflammatory changes were observed in the upper
respiratory tract of both sexes and in the uterus and oviduct,
especially at the highest exposure. At 6.5 mg/m3, the incidence
of squamous metaplasia was increased in the nose, larynx, and
trachea, while the incidence of epithelial hyperplasia was
increased in the nose and lungs. An increased incidence of
hyperplasia was also observed in the lymph nodes and uterus of
females at 6.5 mg/m3 and in the liver of females at 1.3 and 6.5
mg/m3. A dose-related increased incidence of benign epithelial
tumours of the nose, mainly adenomatous polyps, was observed in
both sexes at the 2 highest exposures, while at the highest
exposure (6.5 mg/m3), the incidence of malignant epithelial
tumours was also increased (2/98 in males and 5/95 in females).
The latency time exceeded 87 weeks in males and 97 weeks in
females. The incidence of lung tumours was not significantly
increased, but, in males, 3 bronchial adenomas and, in one
female, 1 bronchial adenoma, were observed at 6.5 mg/m3 compared
with none in the controls. The total incidence of adenomas and
adenocarcinomas of the thyroid in males was not increased, but
hydrazine exposure at 6.5 mg/m3 increased the fraction of
malignant tumours.
Table 7. Tumour incidence in mice, rats, and hamsters following hydrazine exposure
---------------------------------------------------------------------------------------------------------
Route Species/ Exposure Daily dose Tumour incidenceb Reference
strain period (mg/kg Nose or lung liver
(months) body weight)a Male Female Male Female
(life-time
observation)
---------------------------------------------------------------------------------------------------------
Inhalation mouse 12 0 8/385 - Vernot et al. (1985)
(C57BL/6) 4/378
0.34c 12/379 -
rat 12 0 0/146 0/145 - - Vernot et al. (1985)
(Fischer 0.16c 11/97 4/94 - -
344) 0.8d 75/98 38/95 - -
hamster 12 0 1/181 - Vernot et al. (1985)
(golden 0.93d 16/160 -
Syrian)
Oral mouse 6 - 9 0 1/37 4/47 3/30 1/29 Biancifiori (1969,
(gavage) (CBA) 0.98 2/26 10/25 1/26 0/25 1970a)
2.0 4/25 16/25 7/25 2/25
3.9 5/25 21/24 12/25 16/24
8.0 16/21 19/21 15/25 15/24
rat 17 0 0/28 0/22 0/19 0/14 Severi & Biancifiori
(Cb/Se) 14.6/9.7 3/14 5/18 4/13 0/18 (1968)
Oral mouse 24 - 28 0 11/110 14/110 2/110 3/110 Toth (1969, 1972a)e
(drinking- (Swiss) 2.0/1.6 24/50 27/50 2/50 1/50
water) 5.1/4.6 25/50 24/50 3/50 1/50
---------------------------------------------------------------------------------------------------------
a Calculated doses based on a weight of 300 g and a minute volume of 100 ml for rat, a weight of 35 g
and a minute volume of 25 ml for mice, and a weight of 140 g and a minute volume of 54 ml for hamsters.
b - = not found; open space = not tested (nose in inhalation studies with rats and hamsters, lung in
the other studies.
c 1.3 mg/m3, 6 h/day, for 5 days/week.
d 6.5 mg/m3, 6 h/day, for 5 days/week.
e Liver tumours were hepatomas (1/50 in males at both exposure levels) and angiomas.
A slightly increased mortality rate seen in mice right up to
the end of the study was not related to dose. Body weights were
not affected during exposure, but all exposed groups gained less
weight than controls during the post-exposure period. The
incidence of non-neoplastic lesions was similar in treated and
control groups. The only neoplastic change observed was a
slightly increased incidence of lung adenomas at the highest
exposure. A background incidence of 2 - 3% for pulmonary adenoma
in C57BL/6 mice was reported to be common in the laboratory
concerned.
Almost double the number of deaths occurred during exposure
in each exposed group of hamsters compared with the control
group, but the final mortality rates were similar in all groups,
including the control group. During exposure to 6.5 mg/m3 and
for 10 months after exposure, body weights were depressed. For
several months, an unexplained decrease in body weight occurred
in all groups, including the controls. Dose-related degenerative
changes were observed. These were characterized by amyloidosis
in several organs and issues, mineralizaton in kidneys, and
haemosiderosis in the liver. Increased incidences of senile
atrophy and aspermatogenesis were observed in the testes at the
highest exposure level. In addition, the incidence of bile-duct
hyperplasia was increased at all exposures. The incidence of
benign nasal polyps was increased at 6.5 mg/m3. The only other
striking tumours observed were 3 adenocarcinomas, 1 leiomyoma,
and 1 papilloma in the colon, 1 basal cell carcinoma in the
stomach, and 4 parafollicular cell adenomas in the thyroid at
6.5 mg/m3. No such tumours were found in the controls, but the
increased incidence of each tumour type was not statistically
significant. The authors concluded from these studies "that
hydrazine is capable of inducing nasal tumours, primarily
benign, in rats and hamsters after 1-year intermittent exposure"
(Vernot et al., 1985).
8.6.2. Oral exposure
The oral carcinogenicity studies on hydrazine have been
either preliminary or limited in scope (inadequate limited
histopathology, use of one dose or one drinking-water concentra-
tion, small treatment groups, lack of adequate controls, and
treatment of only one sex); therefore, only the more relevant
studies have been described in this section. Most studies have
concentrated on various mouse strains.
Groups of approximately 25 CBA/Cb/Se mice of each sex
received, by gavage, 150 daily doses of hydrazine sulfate in
water, equivalent to 0.03, 0.07, 0.14, or 0.28 mg hydrazine in
25 weeks (these doses are expressed in Table 7 as 0.98, 2.0,
3.9, or 8.0 mg/kg body weight). The animals were observed for
their life-time. Control groups containing 30 males and 29
females were not treated. Liver, lung, various endocrine glands,
and tissues suspected of having lesions were examined
microscopically. No statistical analysis was reported. No deaths
occurred during the exposure period, but later the mortality
rate was increased in animals that had been exposed daily to
levels of 2.0 mg/kg upwards. Brown degeneration of the adrenals
in treated females was mentioned as a marked non-neoplastic
lesion. An apparent dose-related increased incidence of
hepatocarcinomas was observed in male and female mice. The
average latency time for this tumour did not decrease as the
dose increased (Biancifiori, 1970a). The historical control
incidence of hepatocarcinomas in the laboratory concerned was 8%
(Severi & Biancifiori, 1968). Multiple pulmonary tumours, which
were also observed, are described in another report
(Biancifiori, 1969).
In this report (Biancifiori, 1969), the 3 lowest dose
groups, containing 21 - 26 males and 21 - 25 females, were
treated in the same way as in the study above. The data for the
control group and the highest dose groups were taken from
earlier reports (Biancifiori et al., 1964; Severi & Biancifiori,
1968). The untreated control groups contained 37 males and 47
females. The highest dose group comprising 21 mice of each sex,
received 250 daily doses of hydrazine sulfate in water
equivalent to 0.28 mg hydrazine in 36 weeks. In the 1969 report,
the treatment schedule was reported to be 150 daily doses of
0.28 mg hydrazine in 25 weeks, which was contrary to the
original data. Lungs with trachea, liver, various endocrine
glands, and other tissues suspected of having lesions were
examined microscopically. No deaths occurred during the exposure
period, but the mortality rate after the treatment period was
increased from a dose level of 2.0 mg/kg per day upwards. Common
non-neoplastic lesions in the liver at 0.28 mg per day included
areas of diffuse cell regeneration. Necrosis, nodular
regeneration, sclerosis, and bile-duct proliferation were also
observed. The occurrence of hepatomas has already been
discussed. The incidence and multiplicity of pulmonary tumours
were increased at all dose levels in an apparently dose-related
manner and more so in females than in males (Table 7). The
average latency time for these tumours was also shorter in
females than in males. At the highest dose level, approximately
80% of the tumours were characterized as adenomas in both sexes,
the remainder being described as "anaplastic adenomas and
adenocarcinomas" (Biancifiori, 1969).
Induction of lung tumours by hydrazine sulfate after oral
exposure by gavage was also reported in other mouse strains
(Milia et al., 1965; Roe et al., 1967; Kelly et al., 1969;
Biancifiori, 1970b; Bhide et al., 1976; Maru & Bhide, 1982).
Liver tumour induction was reported in BALB/c/Cb/Se mice
(Biancifiori, 1970b).
Severi & Biancifiori (1968) also exposed 14 male and 18
female Cb/Se rats, by gavage, for 68 weeks, to daily doses of
hydrazine sulfate in water, equivalent to 4.4 mg hydrazine (14.6
mg/kg body weight) per day for males and 2.9 mg hydrazine (9.7
mg/kg) per day for females. Controls (28 males and 22 females)
were not treated. The animals were observed for their life-time.
Lungs, liver, and other organs showing gross lesions were
examined microscopically. No statistical analysis was reported.
Exposed rats showed an increased mortality rate. Pulmonary
tumours, adenomas, and adenocarcinomas were observed in both
sexes (Table 7). Carcinomas (2) and spindle cell sarcomas (2),
observed in the livers of exposed males, were not seen in the
controls.
Groups of 23 and 85 golden hamsters of both sexes received
hydrazine sulfate by gavage as 60 and 100 daily doses, equiv-
alent to 0.74 and 0.68 mg hydrazine over 15 and 20 weeks,
respectively. The control group contained 56 hamsters. Animals
were observed for their life-time and examined as described
above for rats. The mortality rate increased in an apparently
dose-related manner, and toxic effects were observed in treated
animals (liver lesions, reticuloendothelial cell proliferation,
cirrhosis, bile-duct proliferation, degenerative fibrous cells
in hyalinized tissue). No tumours were observed, but, because of
toxic effects, the treated animals were not observed for the
same period of time as the controls (Biancifiori, 1970a).
Groups of 50 Swiss mice of each sex received doses of hydra-
zine sulfate in drinking-water, equivalent to 0.056 mg hydrazine
(2 mg/kg body weight, males; 1.6 mg/kg, females) per day for
their life-time up to a maximum of 113 weeks (Toth, 1972a) or
0.18 mg (5.1 mg/kg body weight) per day (males) and 0.16 mg (4.6
mg/kg) per day (females) for a maximum of 95 weeks (Toth, 1969).
The untreated control group, containing 110 animals of each sex,
was tested concurrently with the groups receiving 0.16 or 0.18
mg per day, but not with those receiving 0.05 mg per day (Toth,
1969). Liver, kidneys, spleen, lungs, and organs with gross
lesions were examined microscopically. No statistical analysis
was reported. Drinking-water solutions containing hydrazine were
prepared 3 times per week. There was no indication that
hydrazine concentrations were measured in the solutions during
the studies. At the highest dose level, body weights were not
affected, but the mortality rate in females was increased.
Numerous lung tumours were observed in both sexes with a
comparable incidence (Table 7) and degree of malignancy at both
dose levels. The average latency time for this tumour was
decreased at both dose levels. At the high dose level, the
latency period for malignant lymphomas, which are characteristic
for the strain, and the incidence of adenocarcinomas of the
mammary gland appeared to be decreased.
Fifty Syrian golden hamsters of each sex also received, for
their life-time, up to a maximum of 110 weeks, doses of hydra-
zine sulfate in drinking-water, equivalent to 0.57 mg hydrazine
per day. Untreated non-concurrent control groups contained 100
hamsters of each sex. The protocol was similar to that described
above for rats. Body weights of exposed hamsters were slightly
reduced, but no tumours or other effects were noted. The authors
considered that there was no treatment-related tumour incidence
and that the mortality rate was not affected by the exposure,
though the mean survival period was slightly reduced (Toth,
1972b).
Groups of 34 male and 29 female Swiss mice were administered
hydrazine sulfate by gastric intubation at doses equivalent to
0.27 mg hydrazine per mouse per day, 5 days per week, for their
lifetime. Lung adenocarcinomas occurred in 30 out of 34 male
mice and in 21 out of 29 female mice compared with 1 out of 20
controls. The earliest tumour appeared after 14 months of
exposure. An increased incidence of lung tumours (20/22)
occurred in F1 animals, which were obtained from treated
mothers, and received hydrazine sulfate post weaning from the
age of 11 weeks. No control mice were mated. An increase in lung
tumours (16/35) was also observed in F2 animals obtained from F1
animals that had been treated with hydrazine throughout
gestation and lactation (Menon & Bhide, 1983).
9. EFFECTS ON MAN
9.1. Poisoning Incidents
Several incidents of systemic poisoning have been reported,
mainly showing effects on the central nervous system, respira-
tory system, and stomach.
After a laboratory technician had drunk 20 - 30 ml of a 6%
aqueous solution of hydrazine (free base), he immediately
vomited. Four hours later, weakness, somnolence, and arrhythmia
were observed. Laboratory findings showed a slight but persis-
tent leukocytosis. The serum-albumin fraction was decreased with
an increase in the fraction of globulin. Two days after
exposure, slightly elevated body temperature and a few red blood
cells in the urine were noted, while the patient showed
irregular breathing. Five days after exposure, the patient had
recovered (Drews et al., 1960).
A case was reported of a man who drank between a mouthful
and a cupful of hydrazine in concentrated solution. He
immediately vomited, became unconscious, and flushed. Vomiting
ceased about 12 h after admission to the hospital. The patient
showed sporadic violent behaviour and later developed ataxia
with a lateral nystagmus, a decreased sense of vibration, and
paraesthesia in the arms and legs. Oliguria was also noted.
Pyridoxine treatment was administered, but it was not clear from
the report whether he benefited from this. The patient's final
condition was not reported (Reid, 1965).
A 24-year-old man accidentally ingested "a mouthful" of
hydrazine and immediately became confused, lethargic, and
restless. On admission to hospital, a complete chemistry profile
was normal, but, 3 - 4 days later, hepatotoxicity was evident as
indicated by elevated levels of aspartate amino-transferase (EC
2.6.1.1), lactate dehydrogenase (EC 1.1.1.27), and total
bilirubin. At this time, the patient was treated with pyridoxine
and recovered completely within 5 days. Peripheral neuropathy,
however, developed subsequently, which resolved over the next 6
months. The authors attributed this neuropathy to the megadose
pyridoxine therapy (Harati & Niakan, 1986).
Another man sustained a chemical burn during an industrial
hydrazine explosion. Exposure occurred probably both via the
skin and by inhalation. The burns on his skin covered 22% of the
body surface. On admission to the hospital, the neuro-
logical status of the patient was normal but, 14 h after
exposure, he became comatose. When the coma persisted for 60 h,
he was treated with pyridoxine, and the neurological disorders
cleared within the next 12 h. Other findings were persistently
elevated glucose levels, haematuria, and respiratory
difficulties. Several biochemical indicators of liver
malfunction were elevated from day 3 after exposure and returned
to normal levels over the next 5 weeks (Kirklin et al., 1976).
Two men were exposed to Aerozine 50 vapour (50% hydrazine
and 50% 1,1-dimethylhydrazine by volume) via a leak in a fuel
line. The first man was exposed for about 90 min via a defective
gas mask. He reported headache, nausea, and a shaky feeling, a
sensation of burning of the face, a sore throat, and tightness
in the chest. The second man had inhaled the vapour 3 times
before evacuation from the contaminated area. He was dyspnoeic,
trembling, and weak. Both men showed neurological disorders
including twitching of extremities and clonic movements in the
first victim and hyperreactive reflexes in both men. All
symptoms cleared after treatment with pyridoxine. From clinical
signs, it was concluded that pulmonary oedema developed in both
men and was treated successfully. Four other cases of poisoning
by Aerozine after a spill were mentioned in this report. These 4
men showed nausea and vomiting. Both symptoms disappeared within
20 min following pyridoxine treatment (Frierson, 1965).
One case of systemic lupus erythaematosus-like disease due
to occupational exposure to hydrazine (free base) has been
described. The patient, a laboratory technician, had recurrent
episodes of joint pain, photosensitive rash, fatigue, and fever
following hydrazine exposure. A challenge patch test suggested
that hydrazine caused the illness. On the basis of detailed
examinations of the patient and her family, it was concluded
that hydrazine could cause the disease in a person with a
genetic predisposition. Some of the genetic factors identified
were slow acetylation phenotype, HLA DR2,3, and immune system
responsiveness such as antinuclear antibodies, antibodies to
single-stranded and native DNA, and inhibition of pokeweed
mitogen-stimulated immunoglobulin G synthesis in lymphocytes
(Reidenberg et al., 1983).
9.2. Occupational Exposure
9.2.1. Inhalation exposure
One case was reported of a man who had handled hydrazine
(hydrazine hydrate) once a week for an unknown number of hours
over a period of 6 months. In simulated conditions, only 0.071
mg hydrazine/m3 air was measured, but probably skin exposure had
also occurred. The man experienced conjunctivitis, tremor, and
lethargy after each exposure. Following the last exposure, he
was feverish, vomited, and showed diarrhoea. In a hospital, 6
days later, many disorders were noted: conjunctivitis,
stomatitis, arrhythmia, upper abdominal pain, enlarged abdomen,
icterus, a tender and palpable liver, black faeces, incoherence,
and oliguria. X-ray examinations showed pleural effusion and
lung shadowing. Laboratory findings comprised elevated bilirubin
and creatinine levels in the blood, and protein and red blood
cells in urine. Treatments administered included haemodialysis
and B-vitamins, which brought only temporary relief. The man
died 21 days after the last exposure. Autopsy revealed
pneumonia, severe renal tubular necrosis and nephritis, and mild
hepatocellular damage (Sotaniemi et al., 1971).
9.2.2. Skin and eye irritation; sensitization
Two cases of skin irritation (Frierson, 1965; Kirklin et
al., 1976) and one case of eye irritation (Sotaniemi et al.,
1971) have already been cited in sections 9.1 and 9.2.1,
respectively. Dermatitis of the hands without systemic effects
was observed in 2 men who were occupationally exposed to hydra-
zine hydrate over a period of 5 months. Both men had had similar
experiences previously, after handling of hydrazine hydrate
(Evans, 1959). No patch tests were carried out, but the allergic
nature of the contact dermatitis that can develop after skin
exposure to hydrazine, has been reported earlier. In 1959, an
outbreak of dermatitis was reported among 12 out of 34 female
workers in a factory where hydrazine hydrochloride was used as a
solder flux. Four months after the use of the solder flux had
stopped, 6 out of 12 workers showed strong positive reactions
towards hydrazine sulfate, while 30 controls did not show any
reaction (Frost & Horth, 1959). In an electronics plant, 35
workers, constituting half the work force, developed contact
dermatitis from a solder flux containing hydrazine
hydrochloride, over a period of 5 years. The symptoms did not
reappear when contact with hydrazine was avoided. Patch tests on
5 female workers of this group were all clearly positive for
hydrazine fluxes, while 3 controls did not react (Wheeler et
al., 1965). Another 7 workers in a chemical workshop, where
solder flux containing hydrazine hydrochloride was used, showed
allergic contact dermatitis towards hydrazine, as did 5 workers
in a pharmaceutical firm producing isoniazid. The results of the
patch tests were strongly positive for the 12 subjects, but a
control group was lacking (Zina & Bonu, 1962). Hydrazine induced
allergic contact dermatitis in 4 men when used as a corrosion
inhibitor (Schultheiss, 1959; Hovding, 1967; Von Keilig & Speer,
1983). A negative control group was tested in only one of these
studies (Hovding, 1967). Allergic contact dermatitis developed
in 15 workers in 2 industries producing hydrazine sulfate. In
one of these industries, patch tests on 15 workers were clearly
positive for hydrazine, while 13 controls from the same industry
did not show any reaction. Symptoms did not reappear when
contact with hydrazine sulfate was avoided (Brandt, 1960). In
the other industry, 10 persons produced a positive reaction
towards hydrazine. No negative controls were tested (Sonneck &
Umlauf, 1961). Two cases of allergic contact dermatitis were
reported following exposure to hydrazine in stain removers with
strong positive reactions towards the chemical in patch tests.
Five controls did not react (Van Ketel, 1964). Systemic effects
were not noted in any of these studies.
Cross sensitization to hydrazine derivatives, such as
isoniazid, phenylhydrazine, and hydralazine, was found by
several investigators (Schultheiss, 1959; Zina & Bonu, 1962; Van
Ketel, 1964; Hovding, 1967; Von Pevny & Peter, 1983).
9.2.3. Mortality studies
The causes of death in a cohort of 427 male workers,
employed for a minimum of 6 months in a hydrazine manufacturing
plant between 1945 and 1971, were studied retrospectively and
followed until July 1982. The plant ceased production in 1971.
The men were divided according to 3 categories of exposure. The
first category contained 78 men who might have been exposed to
concentrations of hydrazine ranging from 1.3 to 13 mg/m3 air.
Categories 2 and 3 (a total of 375 men) included those who might
have been exposed to concentrations between 0.6 and 1.3 mg/m3
and those who had little or no exposure, respectively. A further
subdivision in the first category distinguished between men with
more than 10 years since the first exposure and those with less.
Some men were covered by more than one category. The cohort was
also exposed to other unspecified organic chemicals. The total
number of deaths in 406 men who could be traced was 49 against
61.47 expected. Five men died from lung cancer against an
expected 6.65. Two of these men belonged to the most highly
exposed category. Another 7 cases of cancer were detected (9.27
expected). Further details were not given, except for the remark
that no nasal tumours were involved. The authors noted that the
number of men exposed to hydrazine in this study was small. It
was concluded that the results were encouraging in that no
obvious hazard had yet appeared. A note of caution is introduced
by the observation of 2 deaths from lung cancer in men who had
first been exposed in the heaviest category more than 10 years
previously against 1.61 expected (Wald et al., 1984). The cohort
is being followed up.
A seemingly unusually high incidence of myocardial infarc-
tion prompted further study among workers in a plant manufac-
turing hydrazine. The population at risk was limited to every
on-line hydrazine worker who had worked for 3 months or more
between 1953 and 1978. The number of man years at risk was 534,
and the average employment period was 8 years. Only a
superficial examination of the population at risk and the
working environment was completed. Reference data were provided
by the US Air Force men's rates for the years 1975-77 and by
men's rates from a study over the past 30 years by the US
National Heart Institute (Framingham study). There was a close
compar ison between the 2 sets of reference data. Rates of
occurrence of myocardial infarction (death and non-death) per
thousand men per year within 5-year age groups were calculated.
A total of 5 cases of myocardial infarction, concurrent with
the employment period, were identified among the hydrazine
workers. Their average employment period was 16 years. A total
of 1.2 cases was expected. The difference is highly significant
statistically. Four additional cases were identified in ex-
hydrazine workers. However, as it was impossible to trace all
ex-hydrazine workers, the population at risk was limited to
the "concurrent cases". The author warned that the findings
should be treated with due caution because of the small
numbers involved. He also stated that the strength of the
findings rendered a negative hypothesis almost untenable without
further investigation into the cause of the adverse effect found
(Hamill, 1978).
The Task Group recognized that exposure concentrations were
not reported and that there was no indication that confounding
variables, e.g., smoking history or exercise habits, had been
taken into account.
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1. Evaluation of Human Health Risks
Exposure of human beings to hydrazine may occur occupation-
ally or accidentally, through the ingestion of hydrazine-based
drugs, or through the use of tobacco. Despite the high react-
ivity of this compound and its wide industrial use, few
systematic studies have been carried out on its adverse effects
on man.
Hydrazine is rapidly absorbed through the skin, lungs, and
gastrointestinal tract and rapidly distributed throughout the
body (section 6).
In cases of acute human poisoning, vomiting, severe irrita-
tion of the respiratory tract with the development of pulmonary
oedema, central nervous system depression, and hepatic and renal
damage have been reported. Data are not available from which the
levels of hydrazine inhaled can be estimated in cases of acute
poisoning by the respiratory route. However, from reports of
poisoning by the oral route, it would appear that ingestion of
amounts of the order of 20 - 50 ml causes severe intoxication
and may be lethal (section 9.1). It is not possible to estimate
a no-observed-adverse-effect level from the available human
data.
Most of the effects in human beings exposed to hydrazine
have also been observed in experimental animals. In addition,
loss of body weight, anaemia, hypoglycaemia, and fatty liver
have frequently been reported. Fatty liver in mice and reduced
growth in rats were reported when the animals were exposed
through inhalation to the lowest (0.26 mg/m3) of three dose
levels. Monkeys and dogs were unaffected at this dose. In only
one study, a no-observed-adverse-effect level of 3 µwg/kg was
reported, when hydrazine was administered by the oral route to
rats. No data are available on the basis of which a no-observed-
adverse-effect level by inhalation can be established (section
8.2).
Embryotoxicity, fetotoxicity, and minor fetal abnormalities
were observed in rats and mice exposed to hydrazine at doses
toxic for the mother. At such doses, perinatal mortality is
high. These effects may also occur at doses just below the
maternally toxic dose (section 8.4). No data are available on
the effects of hydrazine on the human fetus. In the absence of
human data, it would be prudent to assume that hydrazine might
have adverse effects on the human embryo or fetus at exposure
levels approximating those producing toxicity for the mother.
Such levels may occur during accidental spillage but would be
unlikely to occur in the ambient environment because atmospheric
concentrations are low.
Skin and eye irritation have been observed in human beings
who have come into contact with hydrazine, but the data are
insufficient to establish a no-observed-effect level for
irritation. Hydrazine is a strong skin sensitizer in man and
cross-reacts with hydrazine derivatives (section 9.2.2). Slight
eye irritation was observed in monkeys at hydrazine concentra-
tions of 1.3 and 6.5 mg/m3, but none occurred in animals exposed
to 0.26 mg/m3 (section 8.2.1). No skin irritancy studies on
animals have been reported.
The available data indicate that hydrazine induces gene
mutations and chromosome aberrations in a variety of test
systems including plants, phages, bacteria, fungi, Drosophila,
and mammalian cells in vitro. Hydrazine induced indirect
alkylation in the liver DNA of rodents after in vivo exposure to
toxic doses, and it also caused DNA damage in vitro . Hydrazine
transformed human and hamster cells in vitro. No increased
unscheduled DNA synthesis was observed in the germ cells of mice
in vivo . It did not induce chromosome aberrations, micronuclei,
or dominant lethals in mice in vivo , but chromosomal aberrations
were reported in rats in vivo (section 8.5).
Hydrazine vapour induced nasal tumours, most of which were
benign, in F-344 rats and Syrian golden hamsters, after 12
months of treatment and life-time observation. At the exposure
levels at which most of the tumours occurred, there were signs
of severe mucosal irritation. In several studies on mice,
hydrazine induced an increased incidence of pulmonary tumours,
when administered by the oral route. Hepatic tumours were also
reported in two studies. In some of the studies, the pulmonary
tumour incidence was related to the dose administered. Although
in these reports there is little information about the toxic
effects of the compound, it is probable that the doses admin-
istered in some of the studies were at, or near, the toxic
levels. No tumours were observed in hamsters treated orally with
hydrazine (section 8.6). On the basis of the carcinogenicity
studies on experimental animals, there is evidence that
hydrazine is an animal carcinogen. Human data are inadequate to
assess its carcinogenicity in man. In the absence of human data,
and taking into account the mutagenicity data as well as the
carcinogenicity data in animals, it would be prudent to consider
hydrazine as a possible human carcinogen. Thus, exposure to
human beings should be kept as low as feasible.
Since measurable levels are not normally encountered in the
general environment, it can be concluded that, except in cases
of accidental exposure, hydrazine does not pose a significant
practical hazard for the general population.
10.2. Evaluation of Effects on the Environment
Hydrazine can be released into the atmosphere during venting
operations, storage, and transfer. The total emission has been
estimated to be nearly 0.01% of the hydrazine produced
(production > 35 000 tonnes in 1981). Accidental discharges into
water, air, and soil can result from bulk storage, handling,
transport, and improper waste disposal. Evaporation of hydrazine
after a spill can generate an atmospheric concentration as high
as 4 mg/m3, 2 km downwind.
Hydrazine is degraded rapidly in air through reactions with
ozone, hydroxyl radicals, and nitrogen dioxide. In water, it is
degraded rapidly, especially under aerobic conditions, and in
the presence of organic material and/or in alkaline or hard
water. It is more persistent in soft, metal-free water. In soil,
it is adsorbed and decomposed on clay surfaces, under aerobic
conditions. However, available data are inadequate to describe
the nature of hydrazine behaviour in the soil.
Because of the rapid degradation of hydrazine in the
environment, measurable levels are not normally encountered.
Hydrazine can be toxic for aquatic life, even at very low
concentrations. Fish species showed LC50 values of between 0.54
mg/litre (roach) and 5.98 mg/litre (fathead minnow). Nitrifying
bacteria in activated sludge are inhibited by hydrazine levels
higher than 1 mg/litre. Some other microorganisms are more
sensitive and show toxicity thresholds at levels reported to be
as low as 0.00008 mg/litre.
Hydrazine in both air and water is toxic for plants; in
water, it can inhibit plant germination.
On the basis of these data, it can be concluded that hydra-
zine may present a significant hazard for the aquatic environ-
ment and plant life.
11. RECOMMENDATIONS FOR FURTHER STUDIES
1. Dose-response studies pertaining to:
(a) DNA alkylation (adduct identification);
(b) damage of nasal epithelium; and
(c) pulmonary effects.
2. Skin sensitization studies with focus on cross-reactivity
with hydrazine derivatives.
3. Dose-response studies on sensitive, commercially important
fish species and their food supplies.
4. Metabolic studies in conjunction with effects on DNA.
5. Dermal initiation-promotion studies.
6. Reproduction toxicity studies in sensitive rodent species at
continuous low exposure levels.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
The International Agency for Research on Cancer (IARC, 1982)
considered that, while the evidence for the carcinogenicity of
hydrazine for human beings was inadequate, evidence for its
carcinogenicity for animals and for its activity in short-term
tests was sufficient to conclude that hydrazine is probably
carcinogenic for human beings.
REFERENCES
AGRELO, C. & AMOS, H. (1981) DNA repair in human fibroblasts.
In: de Serres, F.J. & Ashby, J., ed. Evaluation of short-term
tests for carcinogens. Report of the International Collaborative
Programme, New York, Amsterdam, Oxford, Elsevier/North Holland,
pp. 528-532 (Progress in Mutation Research Vol. 1).
AKIN, F.J. & NORRED, W.P. (1978) Effects of short-term
administration of maleic hydrazine or hydrazine on rat hepatic
microsomal enzymes. Toxicol. appl. Pharmacol., 43: 287-292.
ALEYASSINE, H. & LEE, S.H. (1971) Inhibition by hydrazine,
phenelzine, and pargyline of insulin release from rat pancreas.
Endocrinology, 89: 125-129.
AMACHER, D.E., PAILLET, S.C., TURNER, G.N., RAY, V.A., &
SALSBURG, D.S. (1980) Point mutations at the thymidine kinase
locus in L5178Y mouse lymphoma cell. II. Test validation and
interpretation. Mutat. Res., 72: 447-474.
AMENTA, J.S. & DOMINGUEZ, A.M. (1965a) Fatty acid flux and
triglyceride secretion in the hydrazine-induced fatty liver.
Exp. mol. Pathol., 4: 282-302.
AMENTA, J.S. & DOMINGUEZ, A.M. (1965b) The effect of hydrazine
and congeners on C1402 respiratory pattern of various metabolic
substrates. Toxicol. appl. Pharmacol., 7: 236-246.
ANDERSEN, M.E. & KELLER, W.C. (1984) Toxicokinetic principles
in relation to percutaneous absorption and cutaneous toxicity.
In: Drill, V.A. & Lazar, P., ed. Cutaneous toxicity, New York,
Raven Press, pp. 9-27.
ASHBY, J. (1981) Overview of study and test chemical
activities. In: De Serres, F. & Ashby, J., ed. Evaluation of
short-term tests for carcinogens. Report of the International
Collaboration Programme, Amsterdam, Elsevier/North Holland, pp.
112-171 (Progress in Mutation Research, Vol. 1).
ASHBY, J. & KILBEY, B. (1981) Summary report on the
performance of bacterial repair, phage induction, degranulation,
and nuclear enlargement assays. In: de Serres, F.J. & Ashby, J.,
ed. Evaluation of short-term tests for carcinogens. Report of
the International Collaborative Programme, New York, Amsterdam,
Oxford, Elsevier/North Holland, pp. 33-48 (Progress in Mutation
Research Vol. 1).
ASTM (1981) Annual book of ASTM standards. Part 31. Water,
Philadelphia, Pennsylvania, American Society for Testing and
Materials, pp. 425-428 (Method D1385: standard test method for
hydrazine in water).
AUDRIETH, L.F. & OGG, B. (1951) The chemistry of hydrazine,
New York, John Wiley and Sons.
AZAR, A., THOMAS, A.A., & SHILLITO, F.H. (1970) Pyridoxine and
phenobarbital as treatment for Aerozine 50 toxicity. Aerosp.
Med., 41: 1-4.
BAKER, R.S.U., MITCHELL, G.A., MEHER-HOMJI, K.M., & PODOBNA, E.
(1983) Sensitivity of two Chinese hamster cell lines to SCE
induction by a variety of chemical mutagens. Mutat. Res., 118:
103-116.
BALGANESH, M. & SETLOW, J.K. (1984) Prophage induction in
Haemophilus influenzae and its relationship to mutation by
chemical and physical agents. Mutat. Res., 125: 15-22.
BANKS, W.L. (1970) Effect of hydrazine treatment on hepatic
protein biosynthesis in vivo. Biochem. Pharmacol., 19: 275-
283.
BECKER, R.A., BARROWS, L.R., & SHANK, R. (1981) Methylation of
liver DNA guanine in hydrazine hepatotoxicity: dose-response and
kinetic characteristics of 7-methylguanine and O6-methylguanine
formation and persistence in rats. Carcinogenesis, 2: 1181-
1188.
BHIDE, S.V., D'SOUZA, R.A., SAWAY, M.M., & RANADIVE, K.J.
(1976) Lung tumour incidence in mice treated with hydrazine
sulfate. Int. J. Cancer, 18: 530-535.
BIANCIFIORI, C. (1969) [Existence of a hormonal factor in the
pulmonary carcinogenicity of hydrazine.] Lav. Ist. Anat. Istol.
Patol. Univ. Studi Perugia, 29: 29-41 (in Italian).
BIANCIFIORI, C. (1970a) Hepatomas in CBA/Cb/Se mice and liver
lesions in golden hamsters induced by hydrazine sulfate. J. Natl
Cancer Inst., 44: 943-953.
BIANCIFIORI, C. (1970b) [Pulmonary and liver tumours from low
doses of hydrazine sulfate in BALB/c/Cb/Se mice.] Lav. Ist.
Anat. Istol. Patol. Univ. Studi Perugia, 30: 89-99 (in
Italian).
BIANCIFIORI, C., BUCCIARELLI, E., CLAYSON, D.B., & SANTILLI,
F.E. (1964) Induction of hepatomas in CBA/Cb/Se mice by
hydrazine sulfate and the lack of effect of croton oil on tumour
induction in BALB/Cb/Se mice. Br. J. Cancer, 18: 543-550.
BLAIR, I.A., MANILLA TINOCO, R., BRODIE, M.J., CLARE, R.A.,
DOLLERY, C.T., TIMBRELL, J.A., & BEEVER, I.A. (1985) Plasma
hydrazine concentrations in man after isoniazid and hydralazine
administration. Hum. Toxicol., 4: 195-202.
BODENSCHATZ, W. (1986) [Hydrazine in drinking-water.] Off.
Gesundheitswes., 48: 205-208 (in German).
BOSAN, W.S. & SHANK, R.C. (1983) Methylation of liver DNA
guanine in hamsters given hydrazine. Toxicol. appl. Pharmacol.,
70: 324-334.
BRANDT, B., VON (1960) [On allergic skin damage by hydrazine
sulfate.] Dermatol. Wochenschr., 141: 376-381 (in German).
BRAUN, R., JAKEL, H.-P., & SCHONEICH, J. (1984) Genetic
effects of isoniazid and the relationships to in vivo and in
vitro biotransformation. Mutat. Res., 137: 61-69.
BRESLER, S.E., KALININ, V.L., & PERUMOV, D.A. (1968)
Inactivation and mutagenesis on isolated DNA. II. Kinetics of
mutagenesis and efficiency of different mutagens. Mutat. Res.,
5: 1-14.
BRIDGES, B.A., MACGREGOR, D., & ZEIGER, E. (1981) Summary
report on the performance of bacterial mutation assays. In: de
Serres, F.J. & Ashby, J., ed. Evaluation of short-term tests for
carcinogens. Report of the International Collaborative
Programme, New York, Amsterdam, Oxford, Elsevier/North Holland,
pp. 45-67 (Progress in Mutation Research Vol. 1).
BRINGMANN, G. (1975) [Determination of the effect of
biological pollutants on the inhibition of cell multiplication
in the blue algae Microcystis. ] Gesundheits-Ingenieur, 96: 238-
241 (in German).
BRINGMANN, G. & KUHN, R. (1980) Comparison of the toxicity
thresholds of water pollutants to bacteria, algae, and protozoa
in the cell multiplication test. Water Res., 14: 231-241.
BRINGMANN, G. & KUHN, R. (1981) [Comparison of the effect of
hazardous substances on flagellated and ciliated, respectively,
holozoic bactivorous and saprozoic protozoa.] GWF Wasser-
Abwasser, 122: 308-313 (in German).
BRINGMANN, G. & KUHN, R. (1982) [Data on the toxicity of water
pollutants to Daphnia magna in an improved standard test.] Z.
Wasser Abwasser Forsch., 15: 1-6 (in German).
BRUGGER, H. (1983) [ Emission factors for 12 selected
substances with probably carcinogenic potential: hydrazine,]
Environment Research Programme of the Federal Ministry of the
Interior, Federal Environment Office, Vol. 3, Part 1, pp. 15-17
(Report No. 104-04-344) (in German).
CARVER, J.H., SALAZAR, E.P., KNIZE, M.G., & WANDRES, D.L.
(1981) Mutation induction at multiple gene loci in Chinese
hamster ovary cells: the genetic activity of 15 coded
carcinogens and noncarcinogens. In: de Serres, F.J. & Ashby, J.,
ed. Evaluation of short-term tests for carcinogens. Report of
the International Collaborative Programme, New York, Amsterdam,
Oxford, Elsevier/North Holland, pp. 594-601 (Progress in
Mutation Research Vol. 1).
CHANDRA SEKHAR, V.S.G. & REDDY, G.M. (1971) Specific locus
mutations in maize by chemical mutagens. Curr. Sci., 40: 136-
137.
CHATTERJEE, A.K. & SENGUPTA, K. (1980) Regulation of formation
in vivo of pyridoxal phosphate in hydrazine-treated rats.
Int. J. Vitam. Nutr. Res., 50: 24-28.
CHU, B.C.F., BROWN, D.M., & BURDON, M.G. (1973) Effect of
nitrogen and of catalase on hydroxylamine and hydrazine
mutagenesis. Mutat. Res., 20: 265-270.
CIER, A., ROUGANNE, J.P., & SCHMITT, M. (1967) Effets
hématologiques d'une intoxication subaiguë à l'hydrazine et à la
dimethyl hydrazine asymétrique chez la souris et le lapin. C.R.
Soc. Biol. (Paris), 161: 854-858.
CLARK, D.A., BAIRRINGTON, J.D., BITTER, H.L., COE, F.L., MEDINA,
M.A., MERRIT, J.H., & SCOTT, W.N. (1968) Pharmacology and
toxicology of propellant hydrazines, Springfield, Virginia, US
Department of Commerce (Aeromedical Reviews No. 11-68) (AD
688500).
CLARK, D.A., LEEDER, L.G., FOULDS, E.L., & TROUT, D.L. (1970)
Changes in lipids of rat liver after hydrazine injection.
Biochem. Pharmacol., 19: 1743-1752.
COMSTOCK, C.C., LAWSON, L.H., GREENE, E.A., & OBERST, F.W.
(1954) Inhalation toxicity of hydrazine vapour. Arch. ind. Hyg.
occup. Med., 10: 476-490.
COOK, L.R., GLENN, R.E., & PODOLAK, G.E. (1979) Monitoring and
analysis of personnel exposures to hydrazines at a rocket
propellant plant. Am. Ind. Hyg. Assoc. J., 40: 69-74.
COOLING, J., BURDITT, S.L., & BRINDLEY, D.N. (1979) Effects of
treating rats with hydrazine on the circulating concentration of
corticosterone and insulin in relation to hepatic triacyl-
glycerol synthesis. Biochem. Soc. Trans., 7: 1501-1503.
CORNISH, H.H. & WILSON, C.E. (1968) Amino acid levels in
hydrazine-treated rats. Toxicol. appl. Pharmacol., 12: 265-272.
DANIEL, M.R. & DEHNEL, J.M. (1981) Cell transformation test
with baby hamster kidney cells. In: de Serres, F.J. & Ashby, J.,
ed. Evaluation of short-term tests for carcinogens. Report of
the International Collaborative Programme, New York, Amsterdam,
Oxford, Elsevier/North Holland, pp. 626-637 (Progress in
Mutation Research Vol. 1).
DAMBRAUSKAS, T. & CORNISH, H.H. (1964) The distribution, meta-
bolism, and excretion of hydrazine in rat and mouse. Toxicol.
appl. Pharmacol., 6: 653-663.
DAVIS, L.C. (1980) Hydrazine as a substrate and inhibitor of
Azobacter vinelandii nitrogenase. Arch. Biochem. Biophys.,
204: 270-276.
DEAN, B.J. (1981) Activity of 27 coded compounds in the R11
chromosome assay. In: de Serres, F.J. & Ashby, J., ed.
Evaluation of short-term tests for carcinogens. Report of the
International Collaborative Programme, New York, Amsterdam,
Oxford, Elsevier/North Holland, pp. 570-579 (Progress in
Mutation Research Vol. 1).
DEE, L.A. (1971) Gas chromatographic determination of aqueous
trace hydrazine and methylhydrazine as corresponding pyrazoles.
Anal. Chem., 43: 1416-1419.
DE FLORA, S., ZANACCHI, P., CAMOIRANO, A., BENNICELLI, C., &
BADOLATI, G.S. (1984) Genotoxic activity and potency of 135
compounds in the Ames reversion test and in a bacterial DNA-
repair test. Mutat. Res., 133: 161-198.
DE SERRES, F.J. & ASHBY, J., ed. (1981) Evaluation of short-
term tests for carcinogens. Report of the International
Collaborative Programme, New York, Amsterdam, Oxford, Elsevier/
North Holland, 827 pp (Progress in Mutation Research, Vol. 1).
DI LUZIO, N.R., STEGE, T.E., & HOFFMAN, E.O. (1973) Protective
influence of diphenyl- p -phenylenediamine on hydrazine-induced
lipid perioxidation and hepatic injury. Exp. mol. Pathol., 19:
284-292.
DOMINGUEZ, A.M., AMENTA, J.S., HILL, C.S., & DOMANSKI, T.J.
(1962) Morphologic and biochemical alterations in the kidney of
the hydrazine-treated rat. Aerosp. Med., 33: 1094-2010.
DOST, F.N., REED, D.J., & WANG, C.H. (1971) Effects of various
hydrazines upon the metabolism of gamma-aminobutyric acid
(GABA)-1-14C by rats. Biochem. Pharmacol., 20: 1702-1707.
DOST, F.N., BRODERICK, D.J., KRIVAK, R.M., & REED, D.J. (1981)
Metabolism of hydrazine, Ohio, Wright-Patterson Air Force Base,
Aerospace Medical Research Laboratory (AFAMRL TR-81-26)
(AD/A101849).
DREWS, A., EVERSMANN, K., & FRITZE, E. (1960) [On oral
poisoning by hydrazine.] Med. Welt, 23: 1295-1297 (in German).
DUAMIN, V.V., DENISOV, V.L., ANDROPOVA, S.N., & MALETIN, V.P.
(1984) [Influence of hydrazine on reproductive function of
animals when administered in organisms by different routes.]
Gig. i Sanit., 9: 25-28 (in Russian).
EPSTEIN, S.S., ARNOLD, E., ANDREA, J., BASS, W., & BISHOP, Y.
(1972) Detection of chemical mutagens by the dominant lethal
assay in the mouse. Toxicol. appl. Pharmacol., 23: 288-325.
EVANS, D.M. (1959) Two cases of hydrazine hydrate dermatitis
without systemic intoxication. Br. J. ind. Med., 16: 126-127.
FARMWALD, J.A. & MACNAUGHTON, M.G.M. (1981) Effects of
hydrazine on the activated sludge process. J. Water Pollut.
Control Fed., 53: 565-575.
FAROOK, S.A.F. & NIZAM, J. (1979) Mutagenic sensitivity of
base-specific chemicals in chick pea. Indian J. Bot., 2: 12-16.
FISHER, J.W., HARRAH, C.B., & BERRY, W.O. (1980) Hydrazine:
acute toxicity to bluegills and sublethal effects on dorsal
light response and aggression. Trans. Am. Fish. Soc., 109: 304-
309.
FLOYD, W.N. (1980) The importance of ammonia in the metabolic
effects of hydrazine. Aviat. Space environ. Med., 51: 899-901.
FORTNEY, S.R. (1966) Effect of hydrazine on liver glycogen,
arterial glucose, lactate, pyruvate, and acid-base balance in
the anaesthetized dog. J. Pharmacol. exp. Ther., 153: 562-568.
FORTNEY, S.R., CLARK, D.A., & STEIN, E. (1967) Inhibition of
gluconeogenesis by hydrazine administration in rats. J.
Pharmacol. exp. Ther., 156: 277-284.
FREESE, E.B., GERSON, J., TABER, H., RHAESE, H.-J., & FREESE, E.
(1967) Inactivating DNA alterations induced by peroxides and
peroxide-producing agents. Mutat. Res., 4: 517-531.
FRIERSON, W.M. (1965) Use of peridoxine HCl in acute hydrazine
and UDMH intoxication. Ind. Med. Surg., 34: 650-651.
FROST, J. & HJORTH, N. (1959) Contact dermatitis from
hydrazine hydrochloride in soldering flux. Cross-sensitization
to Apresoline and isoniazid. Acta dermatovenereol., 39: 82-86.
GANOTE, C.E. & ROSENTHAL, A.S. (1968) Characteristic lesions
of methylazoxymethanol-induced liver damage. A comparative
ultrastructural study with dimethylnitrosamine, hydrazine
sulfate, and carbon tetrachloride. Lab. Invest., 19: 382-398.
GEDDES, J.W. & WOOD, J.D. (1984) Changes in the amino acid
content of nerve ending (synaptosomes) induced by drugs that
alter the metabolism of glutamate and gamma-aminobutyric acid.
J. Neurochem., 42: 16-24.
GORDON, A. & LEWANDOWSKI, P.E. (1980) Control of vapor
emissions from hydrazine-based fuels, Newark, New Jersey,
Institute of Technology, Department of Chemical Engineering
(AFOSR-TR-80-053).
GORMLEY, W.T. & FORD, R.E. (1973) Deoxygenation of
environmental waters by hydrazine-type fuels. In: Proceedings of
the 4th Annual Conference on Environmental Toxicology, Fairborn,
Ohio, 1973, Ohio, Wright-Patterson Air Force Base, Aerospace
Medical Research Laboratory, Paper 28, pp. 387-400 (AMRL-TR-73-
125) (AD/781031).
GORSHTEIN, E.S. & KOPYLOVA, T.N. (1983) [Effect of hydrazine
hydrochloric acid on the hepatic microsomal hydroxylation system
of rats.] Eksp. Med., 15: 22-26 (in Russian).
GREENHOUSE, G. (1976a) Evaluation of the teratogenic effects
of hydrazine, methylhydrazine, and dimethylhydrazine on embryos
of Xenopus laevis, the South African clawed toad. Teratology,
13: 167-178.
GREENHOUSE, G. (1976b) The evaluation of toxic effects of
chemicals in fresh water by using frog embryos and larvae.
Environ. Pollut., 11: 303-315.
GUPTA, A.K. & GROVER, N.S. (1970) Hydrazine-induced breaks in
chromosomes of Vicia faba. Mutat. Res., 10: 519-520.
GUPTA, R.S. & GOLDSTEIN, S. (1981) Mutagen testing in the
human fibroblast diphteria toxin resistance (HF Dipr) system.
In: de Serres, F.J. & Ashby, J., ed. Evaluation of short-term
tests for carcinogens. Report of the International Collaborative
Programme, New York, Amsterdam, Oxford, Elsevier/North Holland,
pp. 614-625 (Progress in Mutation Research Vol. 1).
HACK, W., VON, HOYERMANN, K., & WAGNER, H.G. (1974) [Gas-phase
reactions of hydroxyl radical with ammonia and hydrazine.] Ber.
Bunsen-Ges. Phys. Chem., 78: 386-391 (in German).
HAMILL, P.V.V. (1978) Number of heart attack cases among
workers of the Lake Charles hydrazine plant, 1953-78 (Submitted
to the US EPA by the Olin Corporation, Stamford, Connecticut,
under TSCA, section 8(e)).
HARATI, Y. & NIAKAN, E. (1986) Hydrazine toxicity, pyridoxine
therapy, and peripheral neuropathy. Ann. intern. Med., 104: 728-
729.
HARRAH, C.B. (1978) Biological effects of aqueous hydrazine
solutions. In: Procedings of the Conference on Environmental
Chemistry: Hydrazine Fuels, 1977, Florida, Tyndall Air Force
Base, Civil and Environmental Engineering Development Office,
pp. 167-176 (CEEDO-TR-78-14) (AD/A054194).
HARRIS, G.W., ATKINSON, R., & PITTS, J.N. (1979) Kinetics of
the reactions of the hydroxyl radical with hydrazine and MMH. J.
Phys. Chem., 83: 2557-2559.
HAUN, C.C. & KINKEAD, E.R. (1973) Chronic inhalation toxicity
of hydrazine. In: Proceedings of the 4th Annual Conference on
Environmental Toxicology, Fairborn, Ohio, 1973, Ohio, Wright-
Patterson Air Force Base, Aerospace Medical Research Laboratory,
Paper 25, pp. 351-363.
HECK, W.W., BLOODWORTH, M.E., CLARK, W.J., DARLING, D.R., &
HOOVER, W. (1963) Environmental pollution by missile
propellants, Ohio, Wright-Patterson Air Force Base, Aerospace
Medical Research Laboratory (AMRL-TDR-63-75).
HEINDORFF, K., RIEGER, R., VELEMINSKY, J., & GICHNER, T. (1984)
A comparative study of the clastogenicity of maleic hydrazine
and some of its putative degradation products. Mutat. Res., 140:
123-126.
HEINEMANN, B. (1971) Prophage induction in lysogenic E. coli
with simple hydroxylamine and hydrazine compounds. Appl.
Microbiol., 21: 726-731.
HENDERSON, V., FISHER, J.W., & D'ALLESSANDRIS, R. (1981) Toxic
and teratogenic effects of hydrazine on fathead minnow (Pime-
phales promelas) embryos. Bull. environ. Contam. Toxicol., 26:
807-812.
HENDERSON, V., FISHER, J.W., D'ALLESSANDRIS, R., & LIVINGSTON,
J.M. (1983) Effects of hydrazine on functional morphology of
rainbow trout embryos and larvae. Trans. Am. Fish. Soc., 112:
100-104.
HERBOLD, B.A. (1978) [Mutagenicity testing with the liver
microsome test.] Biol. Zentralbl., 97: 137-152 (in German).
HIGGINS, E.S. & BANKS, W.L. (1971) Cognate effects of ethanol,
hydrazine, and tissue regeneration on hepatic mitochonidrial
activities. Biochem. Pharmacol., 20: 1513-1524.
HOLTZCLAW, J.R., ROSE, S.L., WYATT, J.R., ROUNBEHLER, D.P., &
FINE, D.H. (1984) Simultaneous determination of hydrazine,
methylhydrazine, and 1,1-dimethylhydrazine in air by derivat-
ization/gas chromatography. Anal. Chem., 56: 2952-2956.
HOVDING, G. (1967) Occupational dermatitis from hydrazine
hydrate used in boiler protection. Acta dermatovenereol., 47:
293-297.
HSIE, A.W., O'NEILL, J.P., MACHANOFF, R., SCHENLEY, R.L., &
BRIMER, P.A. (1981) Screening for mutagenic response of four
coded chemical by the CHO/HGPRT system. In: de Serres, F.J. &
Ashby, J., ed. Evaluation of short-term tests for carcinogens.
Report of the International Collaborative Programme, New York,
Amsterdam, Oxford, Elsevier/North Holland, pp. 602-607 (Progress
in Mutation Research Vol. 1).
HUNT, T.P., FISHER, J.W., LIVINGSTON, J.M., & PUTNAM, M.E.
(1981) Temperature effects on hydrazine toxicity to bluegills.
Bull. environ. Contam. Toxicol., 27: 588-595.
IARC (1982) Chemicals, industrial processes, and industries
associated with cancer in humans, Lyons, International Agency
for Research on Cancer, pp. 136-138 (Monographs on the
Evaluation of the Carcinogenic Risk of Chemicals to Humans,
Suppl. 4).
IRPTC (1985) Treatment and disposal methods for waste
chemicals, Geneva, International Register of Potentially Toxic
Chemicals, United Nations Environment Programme, pp. 188-189.
IRPTC (1986) Data profile on hydrazine, Geneva, International
Register of Potentially Toxic Chemicals, United Nations
Environment Programme.
ISAACSON, P.J. & HAYES, M.H.B. (1984) The interaction of
hydrazine hydrate with humic acid preparations at pH 4. J. soil
Sci. , 35(1): 79-92.
JACKSON, E.K., PARSHALL, G.W., & HARDY, R.W.F. (1968) Hydrogen
reactions of nitrogenase. J. biol. Chem., 243: 4952-4958.
JACOBSON, K.H., CLEM, J.H., WHEELWRIGHT, H.J., RINEHART, W.E., &
MAYES, N. (1955) The acute toxicity of the vapours of some
methylated hydrazine derivatives. Am. Med. Assoc. Arch. Ind.
Health, 12: 609-616.
JACOBSON, K.H., RINEHART, W.E., WHEELWRIGHT, H.J., ROSS, M.A.,
PAPIN, J.L., DALY, R.C., GREENE, E.A., & GROFF, W.A. (1958)
The toxicology of an aniline-furfurylalcohol-hydrazine vapour
mixture. J. Am. Ind. Hyg. Assoc., 19: 91-100.
JAIN, H.K. & SHUKLA, P.T. (1972) Locus specificity of mutagens
in Drosophila. Mutat. Res., 14: 440-442.
JAIN, H.K., RAUT, R.N., & KHAMANKAR, Y.G. (1968) Base specific
chemicals and mutation analysis in Lycopersicon. Heredity, 23:
247-256.
JOHNSON, F.B. (1982) Chemical interactions with Herpes
simplex type 2 virus: enhancement of transformation by hydrazine
and 1,2-dimethylhydrazine. Chem.-biol. Interact., 40: 97-112.
KAK, S.N. & KAUL, B.L. (1975) Mutagenic activity of hydrazine
and its combination with maleic hydrazide and X-rays in barley.
Cytobios, 12: 123-128.
KANE, D.A. & WILLIAMSON, K.J. (1983) Bacterial toxicity and
metabolism of hydrazine fuels. Arch. environ. Contam. Toxicol.,
12: 447-453.
KANEO, Y., IGUCHI, S., KUBO, H., IWAGIRI, N., & MATSUYAMA, K.
(1984) Tissue distribution of hydrazine and its metabolites in
rats. J. Pharm. Dyn., 7: 556-562.
KELLER, W.C., OLSON, C.T., BACK, K.C. (1982) Evaluation of the
embryotoxicity of hydrazine in rats, Ohio, Wright-Patterson Air
Force Base, Aerospace Medical Research Laboratory (AFAMRL-TR-82-
29) (AD/A119706).
KELLY, M.G., O'GARA, R.W., YANCEY, S., GADEKAR, K., BOTKIN, C.,
& OLIVERIO, V.T. (1969) Comparative carcinogenicity of N -
isopropyl-alpha-(2-methyl-hydrazino)- p -toluamide. HCl (pro-
carbazine hydrochloride), its degradation products, other hydra-
zines, and isonicotinic acid hydrazide. J. Natl Cancer Inst.,
42: 337-344.
KHAMANKAR, Y.G. & JAIN, H.K. (1978) A comparative study of
chemical and physical mutagens in bread wheat. Indian J. Hered.,
10: 49-57.
KIMBALL, R.F. (1976) Reversions of two proline-requiring
auxotrophs of Haemophilus influenzae by N -methyl- N' -nitro-
N-nitrosoguanidine and hydrazine. Mutat. Res., 36: 29-38.
KIMBALL, R.F. (1977) The mutagenicity of hydrazine and some of
its derivatives. Mutat. Res., 39: 111-126.
KIMBALL, R.F. & HIRSCH, B.F. (1975) Tests for the mutagenic
action of a number of chemicals on Haemophilus influenzae with
special emphasis on hydrazine. Mutat. Res., 30: 9-20.
KIRKHART, B. (1981) Micronucleus test on 21 compounds. In: de
Serres, F.J. & Ashby, J., ed. Evaluation of short-term tests for
carcinogens. Report of the International Collaborative
Programme, New York, Amsterdam, Oxford, Elsevier/North Holland,
pp. 698-704 (Progress in Mutation Research Vol. 1).
KIRKLIN, J.K., WATSON, M., BONDOC, C.C., & BURKE, J.F. (1976)
Treatment of hydrazine-induced coma with pyridoxine. New Engl.
J. Med., 294: 938-939.
KOPYLOVA, T.N., MAJORE, A., ELERTE, D., NOZDRUNOVA, N.A., &
ROZENBERG, I.E. (1982) [Lipid peroxidation in hydrazine
hydrochloride-induced toxicity of the liver.] Eksp. Med., 14:
35-45 (in Russian).
KORTY, P. & COE, F. (1968) The effects of hydrazine on the
concentration of free amino acids of plasma and urine. J.
Pharmacol. exp. Ther., 160 212-216.
KROP, S. (1954) Toxicology of hydrazine: a review. Arch. ind.
Hyg. occup. Med., :9: 199-204.
LAMB, R.G. & BANKS, W.L. (1979) Effect of hydrazine exposure
on hepatic triacylglycerol biosynthesis. Biochim. Biophys. Acta,
574: 440-447.
LEE, S.H. & ALEYASSINE, H. (1970) Hydrazine toxicity in
pregnant rats. Arch. environ. Health, 21: 615-619.
LEMONTT, J.F. (1977) Loss of hydrazine-induced mutability in
wild-type and excision-repair-defective yeast during post-
treatment inhibition of cell division. Mutat. Res., 50: 57-66.
LEMONTT, J.F. (1978) Mutagenesis of yeast by hydrazine:
dependence upon post-treatment cell division. Mutat. Res., 43:
165-178.
LEWALTER, J., PFLANITZER, K., MULLER, G., & SCHALLER, K.H.
(1984) [Hydrazine levels in blood (plasma).] In: [ Analytical
methods,] Vol. 2, pp. D1-D3 (Senate Commission for the Testing
of Health-damaging Work Material in the German Research
Association-Working Group "Analytical Chemistry") (in German).
LIU, Y.Y., SCHMELTZ, I., & HOFFMANN, D. (1974) Chemical
studies on tobacco smoke: quantitative analysis of hydrazine in
tobacco and cigarette smoke. Anal. Chem., 46: 885-889.
LONDON, S.A., MANTEL, C.R., ROBINSON, J.D., & LUKING, S. (1983)
Effects of selected hydrazines on the early death rates of
Enterobacter cloacae. Bull. environ. Contam. Toxicol., 31: 360-
368.
LOPEZ-MENDOZA, D. & VILLA-TREVINO, S. (1971) Hydrazine-
induced inhibition of amino acid incorporation into rat liver
protein. Lab. Invest., 25: 68-72.
LOPRIENO, N. (1981) Screening of coded carcinogenic/non-
carcinogenic chemicals by a forward mutation system with the
yeast Schizosaccharomyces pombe. In: de Serres, F.J. & Ashby,
J., ed. Evaluation of short-term tests for carcinogens. Report
of the International Collaborative Programme, New York,
Amsterdam, Oxford, Elsevier/North Holland, pp. 425-433 (Progress
in Mutation Research Vol. 1).
LYNG, R.D., KELLER, W.C., & BACK, K.C. (1980) Effects of
hydrazine on pregnant ICR mice, Ohio, Wright-Patterson Air Force
Base, Aerospace Medical Research Laboratory (AFAMRL-TR-80-19)
(AD/A084023).
MCCORMICK, D.B. & SNELL, E.E. (1961) Pyridoxal phospho-
kinases. II. Effects of inhibitors. J. biol. Chem., 236: 2085-
2088.
MCKENNIS, H., WEATHERBY, J.H., & WITKIN, L.B. (1955) Studies
on the excretion of hydrazine and metabolites. Am. Med. Assoc.
Arch. Ind. Health, 12: 511-514.
MCKENNIS, H., YARD, A.S., WEATHERBY, J.H., & HAGY, J.A. (1959)
Acetylation of hydrazine and the formation of 1,2-diacetyl-
hydrazine in vivo . J. Pharmacol. exp. Ther., 126: 109-116.
MACNAUGHTON, M.G., STAUFFER, T.B., & STONE, D.A. (1981)
Environmental chemistry and management of hydrazine. Aviat.
Space environ. Med., 52: 149-153.
MACRAE, W.D. & STICH, H.F. (1979) Induction of sister
chromatid exchanges in Chinese hamster ovary cells by thiol and
hydrazine compounds. Mutat. Res., 68: 351-365.
MARSHALL, C.E., WATTS, D.I., & SUGDEN, M.C. (1983) Effects of
hydrazine on liver and brown adipose tissue lipogenesis in 24 h-
starved rats. J. Pharm. Pharmacol., 35: 460-461.
MARU, G.B. & BHIDE, S.V. (1982) Effects of antioxidants and
antitoxicants of isoniazid on the formation of lung tumours in
mice by isoniazid and hydrazine sulfate. Cancer Lett., 17: 75-
80.
MATSUI, F., ROBERTSON, D.L., & LOVERING, E.G. (1983)
Determination of hydrazine in pharmaceuticals. III. Hydralazine
and isoniazid using GLC. J. pharmacol. Sci., 72: 948-951.
MATSUYAMA, K., YAMASHITA, C., SENDOH, T., NODA, A., GOTO, S., &
IGUCHI, S. (1983) Further investigations on brain distribution
of hydrazine and its gamma-aminobutyric acid elevating effect in
rats. J. Pharmacobio-Dyn., 6: 932-937.
MEDINA, M.A. (1963) The in vivo effects of hydrazines and
vitamin B6 on the metabolism of gamma-aminobutyric acid. J.
Pharmacol. exp. Ther., 140: 133-137.
MEHTA, R.D. & VON BORSTEL, R.C. (1981) Mutagenic activity of
42 uncoded compounds in the haploid yeast reversion assay,
strain XV 185-14C. In: de Serres, F.J. & Ashby, J., ed.
Evaluation of short-term tests for carcinogens. Report of the
International Collaborative Programme, New York, Amsterdam,
Oxford, Elsevier/North Holland, pp. 414-423 (Progress in
Mutation Research Vol. 1).
MENON, M.M. & BHIDE, S.V. (1983) Perinatal carcinogenicity of
isoniazide (INH) in Swiss mice. J. cancer Res. clin. Oncol.,
105: 258-261.
MILIA, U., BIANCIFIORI, C., & SANTILLI, F.E.G. (1965) Late
findings in pulmonary carcinogenesis by hydrazine sulfate in
newborn BALB/c/Cb/Se substrain mice. Lav. Ist. Anat. Istol.
Patol. Univ. Studio Perugia, 25: 165-171.
MILO, G.E., OLDHAM, J.W., ZIMMERMAN, R., HATCH, G.G., &
WEISBRODE, S.A. (1981) Characterization of human cells
transformed by chemical and physical carcinogens in vitro . In
vitro, 17: 719-729.
MITCHELL, P.C.H. & SCARLE, R.D. (1972) Role of reactions of
molybdenum compounds with hydrazine in nitrogen fixation.
Nature (Lond.), 240: 417-418.
NATARAJAN, A.T. & VAN KESTEREN-VAN LEEUWEN, A.C. (1981)
Mutagenic activity of 20 coded compounds in chromosome
aberrations/sister chromatid exchanges assay using Chinese
hamster ovary (CHO) cells. In: de Serres, F.J. & Ashby, J., ed.
Evaluation of short-term tests for carcinogens. Report of the
International Collaborative Programme, New York, Amsterdam,
Oxford, Elsevier/North Holland, pp. 551-559 (Progress in
Mutation Research Vol. 1).
NELSON, S.D. & GORDON, W.P. (1982) Metabolic activation of
hydrazines. Adv. exp. Med. Biol., 136B: 971-981.
NEPSS (1975) Disposal of wastewater containing hydrazine. In:
Pollution solution, California, Naval Environmental Protection
Support Service, Navy Environmental Support Office.
NODA, A., GOROMARU, T., MATSUYAMA, K., SOGABE, K., HSU, K.-Y., &
IGUCHI, S. (1978) Quantitative determination of hydrazines
derived from isoniazid in patients. I. J. Pharm. Dyn., 1: 132-
141.
NODA, A., HSU, K., NODA, H., YAMAMOTO, Y.D., & KUROZUMI, T.
(1983) Is isoniazid hepatotoxicity induced by the metabolite
hydrazine? Sangyo Ika Faigaku Zasshi, 5: 183-189.
NODA, A., NODA, H., OHNO, K., SENDO, T., MISAKA, A., KANAZAWA,
Y., ISOBE, R., & HIRATA, M. (1985a) Spin trapping of a free
radical intermediate formed during microsomal metabolism of
hydrazine. Biochem. biophys. Res. Commun., 133: 1086-1091.
NODA, A., SENDO, T., OHNO, K., GOTO, S., NODA, H., & HUS, K.-Y.
(1985b) Effects of rifampicin and phenobarbital on the fate of
isoniazid and hydrazine in vivo in rats. Toxicol. Lett., 25:
313-317.
O'BRIEN, R.D., KIRKPATRICK, M., & MILLER, P.S. (1964)
Poisoning of the rat by hydrazine and alkylhydrazines. Toxicol.
appl. Pharmacol., 6: 371-377.
PARODI, S., DE FLORA, S., CAVANNA, M., PINO, A., ROBBIANI, L.,
BENNICELLI, C., & BRAMBILLA, G. (1981) DNA-damaging activity
in vivo and bacterial mutagenicity of sixteen hydrazine
derivatives as related quantitatively to their carcinogenicity.
Cancer Res., 41: 1469-1482.
PATRICK, R.L. & BACK, K.C. (1965) Pathology and toxicology of
repeated doses of hydrazine and 1,1-dimethylhydrazine in monkeys
and rats. Ind. Med. Surg., 34: 430-435.
PERRY, P.E. & THOMSON, E.J. (1981) Evaluation of the sister
chromatid exchange method in mammalian cells as a screening
system for carcinogens. In: de Serres, F.J. & Ashby, J., ed.
Evaluation of short-term tests for carcinogens. Report of the
International Collaborative Programme, New York, Amsterdam,
Oxford, Elsevier/North Holland, pp. 560-569 (Progress in
Mutation Research Vol. 1).
PERRY, T.L., KISH, S.J., HANSEN, S., WRIGHT, J.M., WALL, R.A.,
DUNN, W.L., & BELLWARD, G.D. (1981) Elevation of brain GABA
content by chronic low-dosage administration of hydrazine, a
metabolite of isoniazid. J. Neurochem., 37: 32-39.
PITTS, J.N., TUAZON, E.C., CARTER, W.P., WINER, A.M., HARRIS,
G.W., ATKINSON, R., & GRAHAM, R.A. (1980) Atmospheric
chemistry of hydrazines: gas phase kinetics and mechanistic
studies, Florida, Tyndall Air Force Base, Air Force Engineering
and Services Centre, Engineering and Services Laboratory
(AFESC/ESL-TR-80-39) (AD/A093486).
PRADHAN, S.N. & ZIECHECK, L.N. (1971) Effect of hydrazine on
behaviour in rats. Toxicol. appl. Pharmacol., 18: 151-157.
PROTEAU, J.P., LIM, P., & LABAT, R. (1979) Toxicité d'un
dérivé bi-azolé, l'hydrate d'hydrazine, pour Carassius
carassius L., Rutilus rutilus L., et différents stades de
développement de Brachydanio rerio H.B. Ann. Limnol., 15: 337-
346.
PURCHASE, I.F.H., LONGSTAFF, E., ASHBY, J., STYLES, J.A.,
ANDERSON, D., LEFEVRE, P.A., & WESTWOOD, F.R. (1978) An
evaluation of 6 short-term tests for detecting organic chemical
carcinogens. Br. J. Cancer, 37: 873 - 935.
RAPHAELIAN, L.A. (1966) Hydrazine. In: Kirk, R.E. & Othmer,
D.F., ed. Encylopaedia of chemical technology, 2nd ed., New
York, John Wiley and Sons, Vol. 11, pp. 164-196.
RAY, P.D., HANSON, R.L., & LARDY, H.A. (1970) Inhibition by
hydrazine of gluconeogenesis in the rat. J. biol. Chem., 245:
690-696.
REDDY, G.M. & REDDY, T.P. (1972) Induction of mutations by
hydrazine in rice. Indian J. Genet. Plant Breed., 32: 388-391.
REID, F.J. (1965) Hydrazine poisoning. Br. med. J., 5472: 1246.
REIDENBERG, M.M., DURANT, P.J., HARRIS, R.A., DE BOCCARDO, G.,
LAHITA, R., & STENZEL, K.H. (1983) Lupus erythaematosus-like
disease due to hydrazine. Am. J. Med., 75: 365-370.
REYNOLDS, B.A. & THOMAS, A.A. (1965) A colorimetric method for
the determination of hydrazine and monomethylhydrazine in blood.
Am. Ind. Hyg. Assoc. J., 26: 527-531.
ROBERGE, A., GOSSELIN, C., & CHARBONNEAU, R. (1971) Effect of
hydrazine on urea cycle enzymes in vitro and in vivo . Biochem.
Pharmacol., 20: 2231-2238.
ROBINSON, D.E. & MITCHELL, A.D. (1981) Unscheduled DNA
synthesis response of human fibroblasts, W1-38 cells, to 20
coded chemicals. In: de Serres, F.J. & Ashby, J., ed.
Evaluation of short-term tests for carcinogens. Report of the
International Collaborative Programme, New York, Amsterdam,
Oxford, Elsevier/North Holland, pp. 517-527 (Progress in
Mutation Research Vol. 1).
ROE, F.J.C., GRANT, G.A., & MILLICAN, D.M. (1967) Carcino-
genicity of hydrazine and 1,1-dimethylhydrazine for mouse lung.
Nature (Lond.), 216: 375-376.
ROGAN, E.G., WALKER, B.A., GINGELL, R., NAGEL, D.L., & TOTH, B.
(1982) Microbial mutagenicity of selected hydrazines. Mutat.
Res., 89(4): 321-328.
ROGERS, A.M. & BACK, K.C. (1981) Comparative mutagenicity of
hydrazine and 3 methylated derivatives in L5178Y mosue lymphomas
cells. Mutat. Res., 16: 189-194.
ROHRBORN, G., PROPPING, P., & BUSELMAIER, W. (1972) Mutagenic
activity of isoniazid and hydrazine in mammalian test systems.
Mutat. Res., 16: 189-194.
ROSENKRANZ, H.S. & POIRIER, L.A. (1979) Evaluation of the
mutagenicity and DNA-modifying activity of carcinogens and non-
carcinogens in microbial systems. J. Natl Cancer Inst., 62: 873-
892.
ROTHBERG, S. & COPE, O.B. (1956) Toxicity studies on hydra-
zine, methylhydrazine, symmetrical dimethylhydrazine, unsym-
metrical dimethylhydrazine, and DMNA, Maryland, Army Chemical
Center (US Army Chemical Warfare Laboratory Report No. 2027).
SALOMONE, M.F., HEDDLE, J.A., & KATZ, M. (1981) Mutagenic
activity of 41 compounds in the in vivo micronucleus assay.
In: de Serres, F.J. & Ashby, J., ed. Evaluation of short-term
tests for carcinogens. Report of the International Collaborative
Programme, New York, Amsterdam, Oxford, Elsevier/North Holland,
pp. 686-697 (Progress in Mutation Research Vol. 1).
SAVCHENKOV, M.F. & SAMOILOVA, T.J. (1984) [Effect of hydrazine
nitrate on reproductive function of albino rats.] In:[ Problems
of limitation of environmental pollutants circulation,]Ufa, pp.
82-84 (in Russian).
SCALES, M.D.C. & TIMBRELL, J.A. (1982) Studies on hydrazine
hepatotoxicity. I. Pathological findings. J. Toxicol. environ.
Health, 10: 941-953.
SCHIESSL, H.W. (1980) Hydrazine and its derivatives. In: Kirk,
R.E. & Othmer, E.F., ed. Encyclopaedia of chemical technology,
3rd ed., New York, John Wiley and Sons, Vol. 12, pp. 734-771.
SCHILLER, C.M., WALDEN, R., & KEE, T.E. (1979) Effects of
hydrazine and its derivatives on the development of intestinal
brush border enzymes. Toxicol. appl. Pharmacol., 49: 305-311.
SCHMIDT, E.W. (1984) Hydrazine and its derivatives: preparation,
properties, and applications, New York, John Wiley and Sons.
SCHULTHEISS, E. (1959) [Hypersensitiveness to hydrazine.]
Berufsdermatosen, 7: 131-136 (in German).
SEVERI, L. & BIANCIFIORI, C. (1968) Hepatic carcinogenesis in
CBA/Cb/Se mice and Cb/Se rats by isonicotinic acid hydrazine and
hydrazine sulfate. J. Natl Cancer Inst., 41: 331-349.
SHANK, R.C. (1983) Evidence for indirect genetic damage as
methylation of DNA guanine in response to cytotoxicity. Dev.
Toxicol. environ. Sci., 11: 145-152.
SHUKLA, P.T. (1972) Analysis of mutagen specificity in
Drosphila melanogaster. Mutat. Res., 16: 363-371.
SINA, J.E., BEAN, C.L., DYSART, G.R., TAYLOR, V.I., & BRADLEY,
M.O. (1983) Evaluation of the alkaline elution/rat hepatocyte
assay as a predictor of carcinogenic/mutagenic potential.
Mutat. Res., 113: 357-391.
SINGH, H.B., LUDWIG, F.L., & JOHNSON, W.B. (1978) Tropospheric
ozone: concentrations and variabilities in clean remote
atmospheres. Atmos. Environ., 12: 2185-2191.
SLONIM, A.R. (1977) Acute toxicity of selected hydrazines to
the common guppy. Water Res., 11: 889-895.
SLONIM, A.R. & GISCLARD, J.B. (1976) Hydrazine degradation in
aquatic systems. Bull. environ. Contam. Toxicol., 16: 301-309.
SMITH, E.B. & CLARK, D.A. (1972) Absorption of hydrazine
through canine skin. Toxicol. appl. Pharmacol., 21: 186-193.
SONNECK, E.B. & UMLAUF, H. (1961) [Occupationally sustained
skin damage by hydrazine.] Haut-Geschlechskr., 31: 179-184 (in
German).
SOTANIEMI, E., HIRVONEN, J., ISOMAKI, H., TAKKUNEN, J., & KAILA,
J. (1971) Hydrazine toxicity in the human. Report of a fatal
case. Am. clin. Res., 3: 30-33.
SOTOMAYER, R.E., CHAUHAN, P.S., & EHLING, U.H. (1982) Induc-
tion of unscheduled DNA synthesis in the germ cells of male mice
after treatment with hydrazine or procarbazine. Toxicology, 25:
201-211.
SPEIT, G., WICK, C., & WOLF, M. (1980) Induction of sister
chromatid exchanges by hydroxylamine, hydrazine, and isoniazid
and their inhibition by cysteine. Hum. Genet., 54: 155-158.
SPINKOVA, (1971) [Determination of small amounts of hydrazine
in isoniazid solutions.] Pharm. Acta Helv., 46: 643-648 (in
German).
SPRINGER, D.L., BRODERICK, D.J., & DOST, F.N. (1980) Effects
of hydrazine and its derivatives on ornithine decarboxylase
synthesis, activity, and inactivation. Toxicol. appl.
Pharmacol., 53: 365-372.
SPRINGER, D.L., KRIVAK, B.M., BRODERICK, D.J., REED, D.J., &
DOST, F.N. (1981) Metabolic fate of hydrazine. J. Toxicol.
environ. Health, 8: 21-29.
STEIN, E.R., CLARK, D.A., & FORTNEY, S.R. (1971) Inhibition of
glutamicoxaloacetic transaminases of rat liver by hydrazine.
Toxicol. appl. Pharmacol., 18: 274-284.
SUGGS, H.J., LUSKUS, L.J., & KILIAN, H.J. (1980) A field
procedure for sampling and analysis of low concentrations of
hydrazine in air. Am. Ind. Hyg. Assoc. J., 41: 879-883.
THOMSON, J.A. (1981) Mutagenic activity of 42 coded compounds
in the lambda induction assay. In: de Serres, F.J. & Ashby, J.,
ed. Evaluation of short-term tests for carcinogens. Report of
the International Collaborative Programme, New York, Amsterdam,
Oxford, Elsevier/North Holland, pp. 224-235 (Progress in
Mutation Research Vol. 1).
THORNELEY, R.N.F., EADY, R.R., & LOWE, D.J. (1978) Biological
nitrogen fixation by way of an enzyme-bound dinitrogen-hydride
intermediate. Nature (Lond.), 272: 557-558.
TIMBRELL, J.A. & HARLAND, S.J. (1979) Identification and
quantitation of hydrazine in the urine of patients treated with
hydralazine. Clin. Pharmacol. Ther., 26: 81-88.
TIMBRELL, J.A., WRIGHT, J.M., & SMITH, C.M. (1977)
Determination of hydrazine metabolites of isoniazid in human
urine by gas chromatography. J. Chromatogr., 138: 165-172.
TIMBRELL, J.A., SCALES, M.D., & STREETER, A.J. (1982) Studies
on hydrazine hepatotoxicity. II. Biochemical findings. J.
Toxicol. environ. Health, 10: 955-968.
TOMLINSON, T.G., BOON, A.G., & TROTMAN, C.N.A. (1966)
Inhibition of nitrification in the activated sludge process of
sewage disposal. J. appl. Bacteriol., 29: 266-291.
TOTH, B. (1969) Lung tumour induction and inhibition of breast
adenocarcinomas by hydrazine sulfate in mice. J. Natl Cancer
Inst., 42: 469-475.
TOTH, B. (1972a) Hydrazine, methylhydrazine, and methylhydra-
zine sulfate carcinogenesis in Swiss mice. Failure of ammonium
hydroxide to interfere in the development of tumours. Int. J.
Cancer, 9: 109-118.
TOTH, B. (1972b) Tumourigenesis studies with 1,2-dimethyl-
hydrazine dihydrochloride, hydrazine sulfate, and isonicotinic
acid in golden hamsters. Cancer Res., 32: 804-807.
TROUT, D.L. (1965) Effects of hydrazine on plasma-free fatty
acid transport. Biochem. Pharmacol., 14: 813-821.
TROUT, D.L. (1966) Effects of hydrazine on fat transport as
affected by blood glucose concentration. J. Pharmacol. exp.
Ther., 152: 529-534.
TSUCHIMOTO, T. & MATTER, B.E. (1981) Activity of coded
compounds in the micronucleus test. In: de Serres, F.J. & Ashby,
J., ed. Evaluation of short-term tests for carcinogens. Report
of the International Collaborative Programme, New York,
Amsterdam, Oxford, Elsevier/North Holland, pp. 705-711 (Progress
in Mutation Research Vol. 1).
TUAZON, E.C., CARTER, W.P.L., BROWN, R.V., ATKINSON, R., WINER,
A.M., & PITTS, J.N. (1982) Atmospheric reaction mechanisms of
amine fuels, Florida, Tyndall Air Force Base, Air Force
Engineering and Service Center, Engineering and Services
Laboratory (AFESC/ESL-TR-82-17) (AD/A118267).
US FDA (1979) Hydrazine: proposed removal from food additive
use. Fed. Reg., 44: 33693-33695.
US NIOSH (1977a) Manual of analytical methods, 2nd ed.,
Cincinnati, Ohio, US National Institute of Occupational Safety
and Health, Vol. 1, pp. S237-1 to S237-6 (US DHEW Publication
No. 77-157A).
US NIOSH (1977b) Manual of analytical methods, 2nd ed.,
Cincinnati, Ohio, US National Institute of Occupational Safety
and Health, Vol. 3, pp. 248-1 to 248-6 (US DHEW Publication No.
77-157A).
US NIOSH (1978) Criteria for a recommended standard:
occupational exposure to hydrazines, Cincinnati, Ohio, US
National Institute for Occupational Safety and Health, Vol. 3,
pp. 248-1 to 248-6 (US DHEW Publication No. 78-172).
US NIOSH (1986) Industrial hygiene study: extent of exposure
to hydrazine, Cincinnati, Ohio, US National Institute for
Occupational Safety and Health, 49 pp (Report No. 136.5;
Contract No. 200-82-2521).
VAN KETEL, W.G. (1964) Contact dermatitis from a hydrazine
derivative in a stain remover. Cross-sensitization to apresoline
and isoniazid. Acta dermatovenereol., 44: 49-53.
VAN STEE, E.W. (1965) Acute effects of exposure to hydrazine
and hydrazine derivatives on renal function in the dog. Aerosp.
Med., 36: 764-767.
VASUDEVA, M. & VASHISHAT, R.K. (1985) Mutagenic and
recombinogenic activity of hydrazine sulfate in Saccharomyces
cerevisiae. Mutat. Res., 155: 113-115.
VELTE, J.S. (1984) Acute toxicity of hydrazine hydrate to the
fathead minnow (Pimephales promelas) and Daphnid (Daphnia
pulex). Bull. environ. Contam. Toxicol., 33: 598-604.
VERNOT, E.H., MACEWEN, J.D., BRUNER, R.H., HAUN, C.C., KINKEAD,
E.R., PRENTICE, D.E., HALL, A., III, SCHMIDT, R.E., EASON, R.L.,
HUBBARD, G.B., & YOUNG, J.T. (1985) Long-term inhalation
toxicity of hydrazine. Fundam. appl. Toxicol., 5: 1050-1064.
VIJAYKUMAR, N.K. & JAIN, H.K. (1979) Comparative studies on
induced mutations with physical and chemical mutagens in
Drosophila melanogaster. Part I. Specificity and spectrum of
mutations. Indian J. exp. Biol., 17: 61-65.
VISHNOI, A.K. & GUPTA, P.K. (1980) Induced mutagenesis in
Vicia faba L. I. Chlorophyll mutations induced by gamma rays,
ethyl methane sulfonate, and hydrazine. Cytobios, 27: 81-87.
VON KEILIG, W. & SPEER, U. (1983) [Occupational allergic
contact dermatitis from hydrazine.] Derm. Beruf Umwelt, 31: 25-
27 (in German).
VON KRULIK, R. (1966) [The influence of hydrazine and
isonicotinic acid hydrazine on certain substances of the sugar
metabolism in vitro. ] Arzneim. Forsch., 16: 1623-1626 (in
German).
VON PEVNY, I. & PETER, G. (1983) [Allergic contact eczema to
derivatives of pyridine and hydrazine.] Dermatosen, 31: 78-83
(in German).
VON WRIGHT, A. & TIKKANEN, L. (1980) The comparative
mutagenicities of hydrazine and its mono- and dimethyl
derivatives in bacterial test systems. Mutat. Res., 78: 17-23.
WAKABAYASHI, T., HORIUCHI, M., SAKAGUCHI, M., ONDA, H., &
MISAWA, K. (1983) Induction of megamitochondria in the mouse
and rat livers by hydrazine. Exp. mol. Pathol., 39: 139-153.
WALD, N., BOREHAM, J., DOLL, R., & BONSALL, J. (1984)
Occupational exposure to hydrazine and subsequent risk of
cancer. Br. J. ind. Med., 41: 31-34.
WARGOVICH, M.J., GOLDBERG, M.T., NEWMARK, H.L., & BRUCE, W.R.
(1983) Nuclear aberrations as a short-term test for geno-
toxicity to the colon: evaluation of 19 agents in mice. J. Natl
Cancer Inst., 71: 133-137.
WARREN, D., CORNELIUS, C., & FORD, B. (1984) Liver function
studies on rhesus monkeys (Maccaca mulatta) following the
administration of hydrazine sulfate. Vet. Human Toxicol., 26:
295-299.
WATJE, W.F. (1978) Potential of a hydrazine-type fuel spill or
emission during movement from supplies to user. In: Proceedings
of the Conference on Environmental Chemistry: Hydrazine Fuels, 1
977, Florida, Tyndall Air Force Base, pp. 19-24 (CEEDO-TR-78-
14).
WELLS, H.G. (1908) The pathological anatomy of hydrazine
poisoning. J. exp. Med., 10: 457-462.
WHEELER, C.E., PENN, S.R., CAWLEY, E.P., & HILL, C. (1965)
Dermatitis from hydrazine hydrobromide solder flux. Arch.
Dermatol., 91: 235-239.
WITKIN, L.B. (1956) Acute toxicity of hydrazine and some of
its methylated derivatives. Arch. ind. Health, 13: 34-36.
WONG, E.T. (1966) Renal functional response to hydrazine and
1,1-dimethylhydrazine. Toxicol. appl. Pharmacol., 8: 51-56.
WOOD, J.D., RUSSELL, M.P., & KURYLO, E. (1980) The gamma-
aminobutyrate content of nerve endings (synaptosomes) in mice
after the intramuscular injection of gamma-aminobutyrate-
elevating agents: a possible role in anticonvulsant activity.
J. Neurochem., 35: 125-130.
WRIGHT, J.M. & TIMBRELL, J.A. (1978) Factors affecting the
metabolism of 14C-acetylhydrazine in rats. Drug Metab. Dispos.,
6: 561-566.
YAKSCTAT, B.Y. (1969) Experimental assessment of hydrazine
hydrate maximum allowable concentration in water bodies. In:
Industrial pollution of water bodies, Moscow, Medicine, pp.
186-200.
YOSHIDA, T. & ALEXANDER, M. (1964) Hydroxylamine formation by
Nitrosomonas europaea. Can. J. Microbiol., 10: 923-926.
ZINA, G. & BONU, G. (1962) [Occupational dermatitis from
hydrazine and derivatives.] Minerva Dermatol., 37: 197-200 (in
Italian).
