
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
ENVIRONMENTAL HEALTH CRITERIA 153
Carbaryl
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization.
First draft prepared by Professor F. Kaloyanova
(National Center of Hygiene and Medical Ecology
Sofia, Bulgaria) and Dr. P.P. Simeonova
(University of Sofia, Bulgaria)
Published under the joint sponsorship of
the United Nations Environment Programme,
the International Labour Organisation,
and the World Health Organization
World Health Orgnization
Geneva, 1994
The International Programme on Chemical Safety (IPCS) is a joint
venture of the United Nations Environment Programme, the International
Labour Organisation, and the World Health Organization. The main
objective of the IPCS is to carry out and disseminate evaluations of
the effects of chemicals on human health and the quality of the
environment. Supporting activities include the development of
epidemiological, experimental laboratory, and risk-assessment methods
that could produce internationally comparable results, and the
development of manpower in the field of toxicology. Other activities
carried out by the IPCS include the development of know-how for coping
with chemical accidents, coordination of laboratory testing and
epidemiological studies, and promotion of research on the mechanisms
of the biological action of chemicals.
WHO Library Cataloguing in Publication Data
Hexachlorobutadiene.
(Environmental health criteria: 153)
1. Carbaryl - adverse effects 2. Carbaryl - toxicity
3. Environmental exposure I.Series
ISBN 92 4 157153 5 (NLM Classification QU 98)
ISSN 0250-863X
The World Health Organization welcomes requests for permission
to reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland,
Which will be glad to provide the latest information on any changes
made to the text, plans for new editions, and reprints and
translations already available.
(c) World Health Organization 1994
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved.
The designations employed and the presentation of the material in
this publication do not imply the impression of any opinion whatsoever
on the part of the Secretariat of the World Health Organization
concerning the legal status of every country, territory, city, or area
or of its authorities, or concerning the delimitation of its frontiers
or boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar nature
that are not mentioned. Errors and omissions excepted, the names of
proprietary products are distinguished by initial capital letters.
CONTENTS
1. SUMMARY AND EVALUATION, CONCLUSIONS AND RECOMMENDATIONS
1.1. Summary and evaluation
1.1.1. Identity, properties, and analytical methods
1.1.2. Production and uses
1.1.3. Environmental transport, distribution, and
transformation
1.1.4. Environmental levels and human exposure
1.1.5. Kinetics and metabolism
1.1.6. Effects on organisms in the environment.
1.1.7. Effects on experimental animals and in vitro
test systems
1.1.7.1 Reproduction
1.1.7.2 Mutagenicity
1.1.7.3 Carcinogenicity
1.1.7.4 Effects on different organs and systems
1.1.7.5 Primary mechanism of toxicity
1.1.8. Effects on humans
1.2. Conclusions
1.2.1. General population exposure
1.2.2. Subpopulations at high risk
1.2.3. Occupational exposure
1.2.4. Environmental effects
1.3. Recommendations
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1. Identity
2.2. Physical and chemical properties
2.3. Conversion factors
2.4. Analytical methods
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
4.1. Volatilization and air transportation
4.2. Water
4.2.1. Hydrolysis
4.2.2. Photolysis
4.2.3. Degradation by microorganisms
4.2.4. Persistence in surface water
4.2.5. Removal from water
4.2.6. Persistence in sea water
4.2.7. Bioaccumulation/biomagnification
4.3. Soil
4.3.1. Adsorption, desorption
4.3.2. Transformation
4.3.2.1 Photolysis in soil
4.3.3. Biotransformation in soil
4.3.4. Degradation by microorganisms
4.3.5. Persistence in soil
4.3.6. Interaction with other physical, chemical, or
biological factors
4.3.7. Vegetation
4.3.7.1 Uptake and transformation in plants
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1. Environmental levels
5.1.1. Air
5.1.2. Water
5.1.3. Soil
5.1.4. Food and animal feed
5.1.4.1 Fruit, vegetables, and grain
5.1.4.2 Animal products
5.1.4.3 Animal feed crops
5.1.5. Other products
5.1.6. Terrestrial organisms
5.2. General population exposure
5.2.1. Exposure through the food
5.2.2. Exposure during insect control
5.3. Occupational exposure during manufacture, formulation,
or use
6. KINETICS AND METABOLISM
6.1. Absorption
6.2. Distribution
6.3. Metabolism
6.3.1. In vitro studies on animal tissues
6.3.2. In vivo studies on animals
6.3.3. Metabolic transformation in plants
6.3.4. In vitro studies with human tissues
6.3.5. In vivo studies on humans
6.4. Elimination and excretion in expired air, faeces, urine,
milk, and eggs
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
7.1. Microorganisms
7.1.1. Soil microorganisms
7.1.2. Aquatic microorganisms
7.2.1. Aquatic invertebrates
7.2.2. Fish
7.2.2.1 Acute toxicity
7.2.2.2 Short-term
and long-term toxicity
7.2.3. Amphibians
7.3. Terrestrial organisms
7.3.1. Worms
7.3.2. Insects
7.3.3. Birds
7.3.4. Mammals
7.4. Effects on the population and ecosystem
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
8.1. Single exposures
8.1.1. Oral toxicity
8.1.2. Acute inhalation toxicity
8.1.3. Dermal toxicity
8.1.4. Other routes of exposure
8.2. Skin and eye irritation, sensitization
8.2.1. Skin and eye irritation
8.2.2. Sensitization
8.3. Short- and long-term oral exposure
8.4. Short- and long-term inhalation toxicity
8.5. Reproduction and developmental toxicity
8.5.1. Mammalian reproductive
toxicity studies
8.5.1.1 Mouse
8.5.1.2 Rat
8.5.1.3 Gerbil
8.5.2. Mammalian developmental
toxicity studies
8.5.2.1 Mouse
8.5.2.2 Rat
8.5.2.3 Guinea-pig
8.5.2.4 Rabbit
8.5.2.5 Dog
8.5.2.6 Pig
8.5.2.7 Monkey
8.5.3. Reproductive and developmental toxicity studies
in non-mammalian species
8.5.3.1 Fish
8.5.3.2 Amphibian
8.5.3.3 Birds
8.5.4. Appraisal
8.6. Mutagenicity of carbary
and N-nitrosocarbaryl
8.6.1. Genotoxicity assays in vitro
8.6.1.1 Primary DNA damage
8.6.1.2 Gene mutation assay
8.6.1.3 Chromosomal aberration
assays and sister
chromatid exchange
8.6.2. Genotoxicity in vivo
8.6.2.1 Host-mediated assay
8.6.2.2 Drosophila melanogaster and
other insects
8.6.2.3 Chromosomal aberrations
and sister chromatid
exchange
8.6.2.4 Dominant lethal assays
in rodents
8.6.3. Other end-points
8.6.3.1 Cell transformation
8.6.3.2 Aneuploidy induction
8.6.4. Appraisal
8.7. Carcinogenicity
8.7.1. Carcinogenicity studies
of carbaryl in rodents
8.7.1.1 Mouse
8.7.1.2 Rats
8.7.1.3 Overall appraisal of carbaryl
carcinogenicity
8.7.2. Carcinogenicity studies
of N-nitrosocarbaryl
8.7.2.1 Rats
8.7.2.2 Mice
8.7.2.3 Overall evaluation
of the carcinogenicity of
N-nitrosocarbaryl
8.7.3. Carcinogenicity of ß-carbaryl
8.8. Special studies
8.8.1. Neurotoxicity
8.8.2. Effects on the immune system
8.8.2.1 Appraisal on immunotoxicology
8.8.2.2 In vivo studies
8.8.2.3 In vitro studies
8.8.3. Effects in blood
8.8.4. Effects on the liver and other organs
8.8.5. Effects on serum glucose
8.8.6. Interactions with the
drug metabolizing enzyme system
8.8.7. Effects on the endocrine system
8.8.8. Other studies
8.9. Factors modifying toxicity, toxicity of metabolites
8.9.1. Factors modifying toxicity
8.9.2. Toxicity of metabolites
8.9.3. N-nitrosocarbaryl
8.10. Mechanism of toxicity - mode of action
8.10.1. Inhibition of cholineresterase activity
9. EFFECTS ON HUMAN BEINGS
9.1. General population exposure
9.1.1. Acute toxicity, poisoning incidents
9.1.2. Controlled human studies
9.1.3. Long-term exposure
9.2. Occupational exposure
9.2.1. Epidemiological studies
10. PREVIOUS EVALUATION BY INTERNATIONAL BODIES
REFERENCES
APPENDIX
RESUME ET EVALUATION, CONCLUSIONS ET RECOMMANDATIONS
RESUMEN Y EVALUACION, CONCLUSIONES Y RECOMENDACIONES
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR CARBARYL
Members
Dr C.D. Carrington, Food and Drug Administration (FDA) Washington,
DC, USA (Chairman).
Dr N. Chernoff, US Environmental Protection Agency, Research
Triangle Park, North Carolina, USA
Dr T.S.S. Dikshith, VIMTA Labs Ltd, Hyderabad, India
Professor F. Kaloyanova, National Center of Hygiene and Medical
Ecology, Sofia, Bulgaria (Rapporteur)
Professor Yu.I. Kundiev, Institute for Occupational Health, Kiev,
Ukraine (Vice-Chairman)
Dr D. Osborn, Institute of Terrestrial Ecology, Monks Wood
Experimental Station, Huntingdon, United Kingdom
Professor C. Ramel, University of Stockholm, Stockholm, Sweden
Professor Shou-Zheng Xue, Shanghai Medical University, Shanghai, The
People's Republic of China
Observers
Dr S. Kozlen, Rhône-Poulenc, Lyon, France (Representative from
Rhône-Poulenc)
Dr P.G. Pontal, Rhône-Poulenc, Lyon, France (Representative from
ECETOC)
Mr D. Demozay, Rhône-Poulenc, Lyon, France (Representative from
GIFAP)
Secretariat
Dr K.W. Jager, International Programme on Chemical Safety, WHO,
Geneva, Switzerland (Secretary)
NOTE TO READERS OF THE CRITERIA MONOGRAPHS
Every effort has been made to present information in the
criteria monographs as accurately as possible without unduly
delaying their publication. In the interest of all users of the
environmental health criteria monographs, readers are kindly
requested to communicate any errors that may have occurred to the
Director of the International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland, in order that they may be
included in corrigenda, which will appear in subsequent volumes.
* * *
A detailed data profile and a legal file can be obtained from
the international Register of Potentially Toxic Chemicals, Case
postale 356, 1219 Châtelaine, Geneva, Switzerland (Telephone No.
9799111).
* * *
This publication was made possible by grant number 5 U01
ES02617-14 from the National Institute of Environmental Health
Sciences, National Institutes of Health, USA.
* * *
NOTE: The proprietary information contained in this document
cannot replace documentation for registration purposes, because the
latter has to be closely linked to the source, the manufacturing
route, and the purity/impurities of the substance to be registered.
The data should be used in accordance with paragraphs 82-84 and
recommendations paragraph 90 of the Second FAO Government
Consultation (1982).
ENVIRONMENTAL HEALTH CRITERIA FOR CARBARYL
A WHO Task Group on Environmental Health Criteria for Carbaryl
met at the World Health Organization, Geneva, from 21 to 25
September 1992. Dr K.W. Jager, of the IPCS, welcomed the
participants on behalf of the Director IPCS and the three IPCS
cooperating organizations (UNEP/ILO/WHO). The Group reviewed and
revised the draft criteria monograph and made an evaluation of the
risks for human health and the environment from exposure to
carbaryl.
The first draft was prepared by Professor F. Kaloyanova of the
National Center of Hygiene and Medical Ecology and Dr P.P.
Simeonova, Medical Faculty, University of Sofia, Bulgaria, who also
prepared the second draft, incorporating comments received following
circulation of the first drafts to the IPCS contact points for
Environmental Health Criteria monographs.
Dr K.W. Jager of the IPCS Central Unit was responsible for the
scientific content of the document, and Mrs M.O. Head of Oxford,
England, for the editing.
The fact that Rhône-Poulenc Agro, Lyon, France, made available
to the IPCS and the Task Group its proprietary toxicological
information on the product under discussion is gratefully
acknowledged. This allowed the Task Group to make its evaluation on
a more complete data base.
The efforts of all who helped in the preparation and
finalization of this publication are gratefully acknowledged.
1. SUMMARY AND EVALUATION, CONCLUSIONS AND RECOMMENDATIONS
1.1 Summary and evaluation
1.1.1 Identity, properties, and analytical methods
Carbaryl is the common name for the carbamic acid derivative
1-naphthyl N-methylcarbamate. The technical grade product is a white
crystalline solid, with a low volatility; it is poorly soluble in
water, which is stable to light and heat, but easily hydrolysed in
alkaline media. The FAO has established a minimum specification of
98% purity, with an impurity limit of 0.05% for ß-naphthyl
N-methylcarbamate.
Carbaryl and its metabolites are analysed using numerous
analytical procedures, such as thin-layer chromatography,
spectro-photometry, gas chromatography, high pressure liquid
chromatography, and chemical ionization mass spectrometry. Detection
limits of below one nanogram are achievable and recovery is usually
more than 80%.
1.1.2 Production and uses
Carbaryl has been used for about 30 years as a contact and
ingestion insecticide with some systemic properties and controls a
wide range of pests. The principal production plant is in the USA.
Carbaryl is processed by more than 290 formulators into over 1500
different products.
1.1.3 Environmental transport, distribution, and transformation
Under most conditions, carbaryl is not persistent in the
environment. In water, the hydrolysis half-life is dependent on the
temperature, pH, and the initial concentration, and varies from
several minutes to several weeks. The major degradation product is
1-naphthol.
Accumulation of carbaryl, expressed as a bioconcentration
factor in the aquatic environment, has been studied in freshwater
fish and found to be in the range of 14-75. Carbaryl is adsorbed
more readily on soils with a high organic content than on sandy
soils. At the usual application rates, under "good agricultural
practice", dissipation is rapid, with a half-life of 8 days to
1 month under normal conditions. Carbaryl may occasionally be
carried by rainfall and soil cultivation from the surface into the
subsoil (one metre from the surface).
Carbaryl contaminates vegetation, either during spraying, or by
migrating through contaminated soil into plants.
The degradation of carbaryl in the environment is determined by
the extent of the volatilization, photodecomposition, and chemical
and microbial degradation occurring in soil, water, and plants. The
rate of decomposition is more rapid under hot climatic conditions.
1.1.4 Environmental levels and human exposure
Food represents the major source of carbaryl intake for the
general population.
Residues in total dietary samples are relatively low, ranging
from trace amounts to 0.05 mg/kg. In the USA, the daily intake
during the first years of carbaryl application was 0.15 mg/day per
person (in 7.4% of the composites); this decreased to 0.003 mg/day
per person in 1969 (in only 0.8% of the composites). During the
period of application, carbaryl may be found, occasionally, in
surface water and reservoirs.
The general population can be exposed to carbaryl during pest
control operations in both the home and recreation areas.
Workers can be exposed to carbaryl during its manufacture,
formulation, packing, transportation, storage, and during and after
application. Concentrations in the working-air environment during
production varied from <1 mg/m3 to 30 mg/m3. Significant dermal
exposure may occur in industrial and agricultural workers if
protective measures are inadequate.
1.1.5 Kinetics and metabolism
Carbaryl is rapidly absorbed in the lungs and digestive tract.
In human volunteers, dermal absorption of 45% of an applied dose in
acetone occurred in 8 h. However, in vitro dermal penetration data
and toxicity data indicate that dermal absorption usually occurs at
a much lower rate.
The principal metabolic pathways of carbaryl are ring
hydroxylation and hydrolysis. As a result, numerous metabolites are
formed and subjected to conjugation with the formation of
water-soluble sulfates, glucuronides, and mercapturates, excreted in
the urine. Hydrolysis results in the formation of 1-naphthol, carbon
dioxide, and methylamine. Hydroxylation produces 4-hydroxycarbaryl,
5-hydroxycarbaryl, N-hydroxymethylcarbaryl, 5-6-dihydro-5-6-
dihydroxycarbaryl, and 1,4-naphthalendiol. The principal metabolite
in humans is 1-naphthol.
Under normal exposure conditions, the accumulation of carbaryl
in animals is unlikely. Carbaryl is excreted primarily via the
urine, since the product of its hydrolysis, 1-naphthol, is mainly
detoxified to water-soluble conjugates. Enterohepatic cycling of
carbaryl metabolites is also considerable, especially after oral
administration.
The hydrolysis product, N-naphthol carbamic acid, is
spontaneously decomposed to methylamine and carbon dioxide. The
methylamine moiety is later demethylated to carbon dioxide and
formate, the latter being excreted mainly in the urine.
Carbaryl metabolites are also present in a small percentage of
the absorbed doses in saliva and milk.
1.1.6 Effects on organisms in the environment
LC50 values for crustacea vary from 5 to 9 µg/litre (water
fleas, mysid shrimps), 8 to 25 µg/litre (scud), and 500 to
2500 µg/litre (crayfish). Aquatic insects have a similar range of
sensitivity. Plecoptera and Ephemeroptera (stoneflies and mayflies)
are the most sensitive groups. Molluscs are less susceptible with
EC50s in the range of a few mg/litre. For fish, most LC50 values
are between 1 and 30 mg/litre. Salmonids are the most sensitive
group.
The acute toxicity for birds is low. The LD50 for waterfowl
and game birds is >1000 mg/kg. The most susceptible bird tested is
the red-winged blackbird (LD50= 56 mg/kg). There was no evidence
of field effects on birds in forest areas sprayed with 1.1 kg
carbaryl/ha.
Carbaryl is very toxic for honey-bees and earthworms. The oral
LD50 for the former is 0.18 µg/bee (about 1-2 mg/kg).
There are indications that carbaryl may temporarily influence
the species composition of both terrestrial and aquatic ecosystems.
For instance, one study showed that effects on certain terrestrial
invertebrate communities may persist for at least 10 months
following a single application.
1.1.7 Effects on experimental animals and in vitro test systems
The acute toxicity, expressed as the LD50, varies
considerably according to species, formulation, and vehicle.
Estimates of the oral LD50 for the rat range from 200 to
850 mg/kg. Cats are more sensitive with an LD50 of 150 mg/kg. Pigs
and monkeys are less sensitive with an LD50 of >1000 mg/kg.
The maximum achievable aerosol concentration of carbaryl of
792 mg a.i./m3 during a 4-h exposure resulted in the mortality of
one out of five female rats. Carbaryl aerosols, at concentrations of
20 mg/m3, decreased cholinesterase activity (ChEA) in cats during
single 4-h exposures, but this concentration did not have any
observable effects in rats.
Carbaryl is a mild eye irritant and has little or no
sensitizing potential. During long-term studies, the NOEL was
10 mg/kg body weight (200 mg/kg diet) for rats, and 1.8 mg/kg body
weight (100 mg/kg diet) for dogs. The long-term inhalation NOEL for
cats is 0.16 mg/m3. Carbaryl has a low cumulative potential.
1.1.7.1 Reproduction
Carbaryl has been shown to affect mammalian reproduction and
perinatal development adversely in a number of species. Effects on
reproduction include impairment of fertility, decreased litter size,
and reduced postnatal viability. Developmental toxicity is seen as
increased in utero death, reduced fetal weight, and the occurrence
of malformation. With the exception of a small number of studies,
all adverse reproductive and developmental effects were noted only
at doses that caused overt maternal toxicity, and, in a number of
cases, the maternal animal was more sensitive to carbaryl than the
conceptus. The maternal toxic effects included lethality, decreased
growth, and dystocia. Data indicate that the reproductive and
developmental processes of mammals are not especially sensitive to
carbaryl compared with the susceptibility of the adult organism.
1.1.7.2 Mutagenicity
Carbaryl has been evaluated for its potential mutagenicity in a
number of in vitro and in vivo tests, in bacterial, yeast,
plant, insect, and mammalian systems, testing a variety of
end-points.
The available evidence indicates that carbaryl does not have
any DNA-damaging properties. There have been no reports of confirmed
induction of mitotic recombination, gene conversion, and UDS in
prokaryotes ( H. influenzae, B. subtilis) and eukaryotes
( S. cerevisiae, A. nidulans, cultured human lymphocytes, and rat
hepatocytes) in vitro.
Negative results were obtained in tests for gene mutations in a
large number of bacterial assays, with the exception of two cases.
In several studies of gene mutations in mammalian cells in vitro,
carbaryl produced only one equivocal positive result in a cell
culture study. However, the study had several shortcomings and the
result has not been confirmed in any other comparable studies.
Chromosomal damage with high dosages of carbaryl has been
reported in vitro in human, rat, and hamster cells, and in plants.
No such effects have been observed in mammalian tests in vivo,
even at doses as high as 1000 mg/kg.
Carbaryl has been shown to induce disturbances in the spindle
fibre mechanism in plant and mammalian cells in vitro. The
relevance of plant assays for extrapolation to humans is unclear.
It can be concluded that the available data-base does not
support the presumption that carbaryl poses a risk of inducing
genetic changes in either the somatic or the germinal tissue of
humans.
The nitrosated product of carbaryl, N-nitrosocarbaryl, is
capable of inducing mitotic recombination and gene conversion in
prokaryotes ( H. influenzae, B. subtilis) and eukaryotes
( S. cerevisiae) in vitro, and gives positive results in
E. coli spot tests.
Furthermore, experimental results indicate that
N-nitrosocarbaryl binds to DNA, causing alkali-sensitive bonds and
single-strand breakage.
Nitrosocarbaryl has not been established as a clastogen
in vivo (bone marrow and germ cells), even at high toxic doses.
1.1.7.3 Carcinogenicity
Carbaryl has been studied for its carcinogenic potential in
numerous studies on rats and mice. The results of most of these
studies were negative, but the studies were old and did not meet
contemporary standards. However, new studies on mice and rats, which
meet modern standards, are in progress.a
The latest IARC evaluation (IARC, 1987) concluded that there
were no data on cancer in humans and that the evidence of
carcinogenicity in experimental animals was inadequate. Carbaryl
could not be classified as to its carcinogenicity for humans
(Group 3).
N-nitrosocarbaryl has been shown to induce tumours locally in
rats (either sarcoma at the site of injection or forestomach
squamous cell carcinoma, when given by the oral route). Given the
human chemistry of carbaryl, the risk of N-nitrosocarbaryl
carcinogenicity in humans from carbaryl exposure can be judged as
negligible.
aThese studies have not yet been reviewed by the IPCS. The
company performing these studies has indicated that there is a
significant increase in tumors at the highest dose in both species.
1.1.7.4 Effects on different organs and systems
(a) Nervous system
The effects of carbaryl on the nervous system are primarily
related to cholinesterase inhibition and are usually transitory. The
effects on the central nervous system were studied in rats and
monkeys. Oral doses of 10-20 mg carbaryl/kg for 50 days were
reported to disrupt learning and performance in rats.
In a small study on pigs, carbaryl (150 mg/kg body weight in
the diet for 72-82 days) was reported to produce a number of
neuromuscular effects. Reversible leg weakness was noticed in
chickens given high doses of carbaryl. No evidence of demyelination
was observed in the brain, sciatic nerve, or in spinal cord sections
examined microscopically. Similar effects were not observed in
long-term rodent studies.
(b) Immune system
Carbaryl, when administered in vivo, at doses causing overt
clinical signs, has been reported to produce a variety of effects on
the immune system. Many of the effects described were detected at
doses close to the LD50. Most studies on rabbits and mice at doses
permitting survival have not produced significant effects on the
immune system. Shortcomings of several of these studies were a lack
of consistency and, sometimes, overt contradiction between results,
which prevents the description of a defined immunotoxic mechanism.
(c) Blood
Carbaryl has been reported to affect coagulation, but there are
conflicts about the direction of the effect. In glucose-6-phosphate
dehydrogenase-deficient sheep erythrocytes, carbaryl produced a
dose-dependent increase in methaemoglobin (Met-Hb) formation. Human
serum albumin reacted in vitro with the ester group of carbaryl.
Carbaryl binds free blood amino acids.
(d) Liver
Disturbances have been reported in the carbohydrate metabolism
and protein synthesis and detoxification function of the liver in
mammals. Carbaryl is a weak inducer of hepatic microsomal
drug-metabolizing activity. Phenobarbital sleeping time is
shortened. The hepatic levels of cytochrome P-450 and b5 are
increased. Changes in liver metabolism may account in part for the
three-fold increase of the carbaryl LD50 in carbaryl-pretreated
rats.
(e) Gonadotropic function
Carbaryl has been reported to increase the gonadotropic
function of the hypophysis of rats.
1.1.7.5 Primary mechanism of toxicity
Carbaryl is an inhibitor of cholinesterase activity. This
effect is dose-related and quickly reversible. There was no aging of
the carbamylated cholinesterase. All identified metabolites of
carbaryl are appreciably less active cholinesterase inhibitors than
carbaryl itself.
1.1.8 Effects on humans
Carbaryl is easily absorbed through inhalation and via the oral
route and less readily by the dermal route. Since the inhibition of
cholinesterase (ChE) is the principal mechanism of carbaryl action,
the clinical picture of intoxication is dominated by ChE inhibition
symptoms, such as: increased bronchial secretion, excessive
sweating, salivation, and lacrimation; pinpoint pupils,
bronchoconstriction, abdominal cramps (vomiting and diarrhoea);
bradycardia; fasciculation of fine muscles (in severe cases,
diaphragm and respiratory muscles also involved); tachycardia;
headache, dizziness, anxiety, mental confusion, convulsions, and
coma; and depression of the respiratory centre. Signs of
intoxication develop quickly after absorption and disappear rapidly
after exposure ends.
In controlled studies on human volunteers, single doses of less
than 2 mg/kg were well tolerated. A single dose of 250 mg
(2.8 mg/kg) produced moderate ChE inhibition symptoms (epigastric
pain and sweating) within 20 min. Complete recovery occurred within
2 h of treatment with atropine sulfate.
In cases of occupational overexposure to carbaryl, mild
symptoms are observed long before a dangerous dose is absorbed,
which is why severe cases of occupational intoxication with carbaryl
are rare. During agricultural application, dermal exposure may play
an important role. No local irritative effect is usually observed,
however, the appearance of a skin rash after accidental splashing
with carbaryl formulations has been described.
There are conflicting data about the effects of carbaryl on
sperm count and changes in sperm morphology in plant workers. No
adverse effects on reproduction have been reported.
The most sensitive biological indicator of carbaryl exposure is
the appearance of 1-naphthol in the urine and a decrease in ChE
activity in the blood. Levels of 1- naphthol in the urine can be
used as a biological indicator, if there is no 1-naphthol in the
working environment. During occupational exposure, 40% of the urine
samples contained more than 10 mg total 1-naphthol/litre. In one
case of acute intoxication, 31 mg/litre was found in the urine. The
hazard level is >10 mg/litre and the symptomatic level 30 mg
1-naphthol/litre urine ( Data sheet on carbaryl, WHO, 1973,
VBC/DS/75.3).
Measurement of the ChE activity can be a very sensitive test
for monitoring, provided that measurement is carried out soon after
exposure.
1.2 Conclusions
The hazards of carbaryl for human beings are judged to be low,
because of its low vapour pressure, rapid degradation, rapid
spontaneous recovery of inhibited cholinesterase, and the fact that
symptoms usually appear well before a dangerous dose has accumulated
in the body. Good carcinogenicity studies, which meet modern
standards, are not yet available.
1.2.1 General population exposure
Residue levels of carbaryl in food and drinking-water, which
remain after its normal use in agriculture, are far below the
acceptable daily intake (ADI) (0.01 mg/kg body weight per day) and
are not likely to produce health hazards in the general population.
1.2.2 Subpopulations at high risk
Use of carbaryl for public health purposes in the home or in
recreation areas may create overexposure, if the rules for its
application are neglected.
1.2.3 Occupational exposure
By enforcing reasonable work practices, including safety
precautions, personnel protection, and proper supervision,
occupational exposure during the manufacture, formulation, and
application of carbaryl will not create hazards. Undiluted
concentrations must be handled with great care, because improper
work practices may cause skin contamination. Air concentrations in
the workplace should not exceed 5 mg/m3.
1.2.4 Environmental effects
Carbaryl is toxic for honey-bees and earthworms. It should not
be applied to crops during flowering.
With normal use, carbaryl should not cause environmental
concern. Carbaryl is adsorbed on soil to a great extent and does not
readily leach into ground water. It is rapidly degraded in the
environment and therefore is not persistent. Use of carbaryl should
not result in harmful short-term effects on the ecosystem.
1.3 Recommendations
* The handling and application of carbaryl should be
accomplished with the care given to all pesticides.
Instructions for proper usage, provided on the package
containing the chemical, should be carefully followed.
* The manufacture, formulation, use, and disposal of
carbaryl should be carefully managed to minimize
contamination of the environment.
* Regularly exposed worker populations should receive
periodic health evaluations.
* The application of carbaryl should be timed to avoid
effects on non-target species.
* Carcinogenicity studies that meet modern standards should
be conducted.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1 Identity
Structural formula
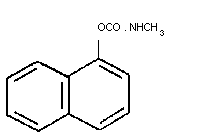 Molecular formula: C12H11N02
Common name: Carbaryl (BSI)
CAS chemical name: 1-naphthalenylmethylcarbamate (9CI)
CAS registry number: 63-25-2
RTECS registry number: FC5950000
Common synonyms:
alpha-naftyl- N-methylkarbamat, alpha-naphthalenyl
methylcarbamate, alpha-naphthyl methylcarbamate, alpha-naphthyl
N-methylcarbamate, carbamic acid, methyl-, 1-naphthyl ester,
N-methyl-alpha-naphthyl-urethan, N-methyl-1-naftyl-
carbamaat, N-methyl-1-naphthyl-naphthyl carbamate,
N-methyl-1-naphthyl-carbamat, N-methylcarbamate de
1-naphtyle, N-metil-1-naftil-carbammato, 1-naphthol
N-methyl-carbamate, 1-naphthyl methylcarbamate, 1
naphthyl- N-methyl-karbamat
The most commonly used chemical name is 1-naphthyl- N-
methyl-carbamate.
Common trade names:
Arilat, Arilate, Arylam, Atoxan, Bercema, Caprolin, Carbacine,
Carbatox, Carbavur, Carbomate, Carpolin, Denapon, Dicarbam,
Dyna-carbyl, Karbaryl, Karbatox, Karbosep, Menaphtam, Monsur,
Mugan, Murvin, Oltitox, Panam, Pomex, Prosevor, Ravyon,
Seffein, Sevimol, Sevin, Vioxan
The most commonly used trade name is Sevin.
Previous codes:
Compound 7744, ENT 23,969, ENT 23969, Experimental insecticide
7744, Germain's HSDB 952, NAC, NMC 50, Union Carbide 7744
Purity:
The technical product is principally manufactured in the USA;
however, there are other minor sources in other parts of the
world.
The technical product manufactured in the USA is produced to a
minimum purity of 99% w/w carbaryl with a <0.05% w/w content
of the 2-naphthyl carbamate isomer (sometimes known as
"beta-carbaryl").
FAO specifies a minimum purity of 98% w/w carbaryl with <0.05%
w/w content of the 2-naphthyl carbamate isomer.
2.2 Physical and chemical properties
Some of the physical properties of carbaryl are listed in
Table 1.
Pure carbaryl is a white crystalline solid without odour.
The explosion limit for dust (finely divided particles) in air
is 20.3 g/m3 (approximately 2500 ppm). It is non-corrosive
(Weston, 1982).
The volatility may increase 4-fold when the relative humidity
is increased from 8 to 80%.
Table 1.Physical properties
Melting point (°C) 142
Boiling point (°C) decomposing
Solubility in water (30 °C) 40 mg/litre
Specific density (20 °C) 1.23
Relative vapour density -
Vapour pressure 1.17 x 10-6-3.1 x 10-7
mmHg at 24-25 °C
Flash point 193 °C
Octanol/water partition 1.59-2.3
coefficient (log Kow)
Flammability (explosive) limits -
Relative molecular mass 201
The solubility of carbaryl increases with temperature (Bowman &
Sans, 1985). In sea water at 18 °C, the solubility is 31 mg/litre
(Karinen et al., 1967). Carbaryl is soluble to some extent in most
organic solvents, and it is soluble in corn oil. It is lipophilic
(Kanazawa, 1981).
It is stable to light and heat (up to 70 °C) and acids, but
easily hydrolysed by alkaline materials (Dittert & Higuchi, 1963).
It is a strong oxidizer.
The quality of carbaryl depends upon the purity of the
precursor, 1-naphthol. The amount of the 2-naphthylcarbamate isomer
found as a contaminant in the final product is directly related to
the purity of this precursor. 1-Naphthol, free of 2-naphthol
(undetectable), is produced in the USA today through the catalytic
conversion of naphthalene. However, 1-naphthol produced by other
manufacturing processes may contain 2-naphthol as a byproduct.
High pressure liquid chromatography (HPLC) has been used to
determine 1- and 2-naphthol in their mixtures in ratios 500:1, in
order to check for traces of contamination in samples of a
commercially important insecticide. The results have been summarized
in Table 2 (Argauer & Warthen, 1975).
Table 2.2-Naphthol recovered from carbaryl samplesa
Sample and size Amount of 2-naphthyl Amount of
methylcarbamate added 2-naphthol
for recovery check found
USA produced
250 mg Union Carbide
99.66% active None Undetectable
500 mg Ortho Sevin
50% wettable powder None Undetectable
570 mg Union Carbide
44% aqueous slurry None Undetectable
310 mg Union Carbide
80% wettable powder None Undetectable
310 mg Union Carbide
80% wettable powder 2.5 mg 1.7 mg
310 mg Union Carbide
80% wettable powder 0.50 mg 0.33 mg
Foreign origin
250 mg Sample A-
technical None 2.3 mg
250 mg Sample B-
technical None 14 mg
500 mg Sample C-
50% wettable powder None 12.4 mg
500 mg Sample D-
50% wettable powder None 1.3 mg
aFrom: Argauer & Warthen (1975).
2.3 Conversion factors
1 ppm = 8.22 mg/m3 of air;
1 mg/m3 = 0.12 ppm.
2.4 Analytical methods
The methods used to determine carbaryl are summarized in
Table 3. They vary considerably in relation to the equipment used.
As an alternative to chemical analysis, Bowman et al. (1982) used
a simple bioassay system procedure to determine foliar residues for
a safe re-entry period and to screen food for residues. Daphnia
and Hyalella were used as highly sensitive test organisms.
The Joint FAO/WHO Codex Alimentarius Commission has given
recommendations for the methods of analysis to be used for the
determination of carbaryl residues (FAO/WHO 1986a).
Table 3. Methods of analysis
Medium Sampling preparation Analytical Detection limit Comments Reference
methods
Meat extraction by paper test based 0.5 mg/kg Filatov & Brytskov
acetone on ChE determination; (1972)
butyryl choline
as a substrate and
phenol red as an
indicator
Air thin-layer 0.2 ng qualitative Wagner (1973)
chromatography 0.5-50 ng
quantitative
Air (carbaryl absorption in UV spectro- 1 mg/kg carbaryl lambda max. Klisenko (1965)
and methanol photometry 281 nm 1-naphthol
1-naphthol) lambda max. 296 nm
Soil plant extraction by thin-layer 0.1 µg recovery 80-95% Kovaleva &
hexane, acetone, chromatography sensitivity 0.02 mg/kg Talanov (1978a)
chloroform
Cow's milk and spectrophotometry 0.002 mg/litre - milk recovery 79-81% Hurwood (1967)
tissues p-nitro-benzene
diazonium fluoborate 0.02 mg/kg - tissues fortification 9.5-5 µg
coupling method
Urine 1-naphthol extraction with gas chromatography 0.02 mg/litre recovery 89-95% Shafik et al. (1971)
benzene tritium detector
Table 3 (continued)
Medium Sampling preparation Analytical Detection limit Comments Reference
methods
Air of working absorption in sodium gas chromatography, Krechniak & Foss
environment hydroxide solution electron capture (1981)
with simultaneous detector
hydrolysis to
1-naphthol;
derivatization by
1-fluoro-2-4
dinitro-benzene
Spinach Chicory sulfuric acid for electron capture 0.2 mg/kg in fortified Tilden & van
hydrolysis to for chromatography crop extract; 20 pg for Middelem (1970)
methylamine salt; pure standards
4-bromobenzoyl
chloride used to
produce derivative
4-bromo-N-methyl
benzamide
Water carbaryl extraction with gas chromatography 0.2 µg/litre recovery for carbaryl, Deuel et al. (1985)
and 1-naphthol dichloromethane H-3 source electron 100%; for naphthol, 90%
capture detector or
63Ni detector
Soil carbaryl extraction with 0.01 mg/kg for recovery for carbaryl,
and 1-naphthol 20% diethylester fortified soil 89.8%; for 1-naphthol,
in dichloromethane samples 79.4%
Table 3 (continued)
Medium Sampling preparation Analytical Detection limit Comments Reference
methods
Air ambient series of gas HPLC with ultraviolet Currier et al. (1982)
scrubbers charged detection at 220 nm
with methanol;
particulate deposit
on Teflon discs
Pads methanol ethanol HPLC 50 ng/pad (103.2 cm2) sensitive, inexpensive Bogus et al. (1985)
extraction; selective procedure
absorption and
elution of reversed
phase solid support
Post-mortem extraction procedure HPLC ultraviolet recovery: blood and Duck & Woolias (1985)
specimens on Extrelut column; detection reversed urine 99%; liver and
protein precipitation phase stomach tissue 95%
with acetonitrile
Foliage surface extraction HPLC recovery: at 5 ng/kg Pieper (1979)
by trichloromethane fortification level
Grass, etc. extraction by CH3CN grass 89.5%;
geranium 86.5%
Water extraction by CH2Cl2 aspen 75%;
Douglas fir 49.8%;
at 0.1 mg/litre level
Table 3 (continued)
Medium Sampling preparation Analytical Detection limit Comments Reference
methods
Soil extraction by soil 103%;
acetonitrile + water stream water 99.7%;
sediment 101%
Water, soil, extraction by thin-layer 0.5 mg/kg qualitative carbaryl and 1-naphthol Klisenko et al. (1972)
cow's milk, benzene ( n-hexane, chromatography
tissue (liver, diethyl ether)
kidney, heart,
lungs, etc.)
Vegetables and extraction by HPLC Recovery 83-97% at Ting et al. (1984)
fruits methanol (10% in 0.5 mg/kg fortification
petroleum ether) or level. Detailed
acetonitrile clean-up description of the
on a florasil column method is given
Water and Serum single extraction by HPLC 0.5 ng/ml Analysis time is Strait et al. (1991)
methanol after 1 ml 10 min Recovery in
of C18 solid-phase water > 99%
extraction columns
Plants methanol and liquid chromatography 0.01 mg/kg Recovery varies from Krause (1985a,b)
mechanical reverse phase; LC 86 to 121%
ultrasonic column using
homogenizer used acetonitrile - water
for extraction mobile phase
Table 3 (continued)
Medium Sampling preparation Analytical Detection limit Comments Reference
methods
Marion-berries extracted by Luke chemical ionization Recommended when GC Cairns et al. (1983);
procedure (Luke et mass spectrometry retention data are
al., 1975, 1981) with both methane inconclusive
cited by Cairns and ammonia as
(1983); eluted reagent gases
through florisil
using 50% mixed
petroleum ethers
Honey-bees extraction by gas chromatography, 0.03-0.10 mg/kg Recovery 95-100% Kendrick et al. (1991)
diethyl ether nitrogen-phosphorous
clean-up using ionization detector
silica cartridge
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
Carbaryl was first synthesized in 1953 and, in 1958, the Union
Carbide Corporation began its commercial production. Carbaryl is now
processed by more than 290 formulators into 1537 different
registered products (Harry, 1977, unpublished report). Carbaryl is
formulated as a 50 and 85% wettable powder, 1.75-50% dust, oil-and
water-based 4% liquid suspension, and 5 and 10% granules and baits.
Annual production is of the order of 10 000 tonnes. It is produced
by the reaction of 1-naphthol with methylisocyanate.
Carbaryl is widely used, in many countries, as a broad spectrum
contact and ingestion insecticide with some systemic properties, and
is recommended for use at 0.25-2.7 kg active ingredient per hectare
to control various insect pests. Up to 10 kg/ha per season can be
used on tree fruits. In the USA, carbaryl is registered for the
control of about 560 different pests. It is used on more than 115
food and fibre crops, trees, and ornamentals. About 40% of the
quantity used is applied to cotton (Kuhr & Dorough, 1976; Mastro &
Cameron, 1976; Payne et al., 1985). In combination with other
substances, or alone, carbaryl is used as a plant regulator for
apple thinning (Looney & McKellar, 1984; Looney & Knight, 1985).
In veterinary practice, carbaryl is used on cattle, poultry,
and pets, especially to control flies, mosquitos, ticks, and lice,
some of which are vectors of disease.
Carbaryl (5% formulation Carbacide) is used to treat human body
louse infestation (Sussman et al., 1969).
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
4.1 Volatilization and air transportation
Carbaryl has a low volatility and a low air-water partition
coefficient. Thus, only limited evaporation can be anticipated after
treatment. Some traces of carbaryl in the air and in fog, resulting
from spray drift, may be detected at a certain distance from the
treated areas. A maximum level of 0.09 mg/m3 in the air has been
reported.
The dimensionless air-water partitioning constant (Henry's Law
Constant) for carbaryl has been evaluated to be 5.3x10-6
(Schomburg et al., 1991).
Deuel et al. (1985) studied the persistence of carbaryl in
paddy water. Results indicated that no measurable dissipation could
occur as a result of volatilization. In contrast to the results from
Deuel, using the BAM model, Lee et al. (1990) calculated that 50
days after treatment, 0.63% of the carbaryl applied to soil could
have been volatilized and 78.84% degraded.
4.2 Water
4.2.1 Hydrolysis
In pure, sterilized water, kept in the dark, the persistence of
carbaryl is pH-dependent. Carbaryl is rather stable in acidic
conditions. Its half-life at pH 7 is 10-16 days, and at pH 8 it is
1.3-1.9 days only. At pH levels higher than 8, its half-life is in
the range of a few hours, or even less (Aly & El Dib, 1971a).
Carbaryl is an example of an N-substituted carbamate that
hydrolyses readily in water. In this mechanism, an acid-base
equilibrium is established and the conjugate carbamate undergoes an
elimination type reaction to give an unstable carbamic acid that
decomposes to the primary amine and CO2. Initially, the principal
non-biological degradation pathway of carbaryl in water, however,
involves base-catalysed hydrolysis to 1-naphthol (Khasawinah, 1977).
Aly & El Dib (1971a,b, 1972) conducted studies to determine the
physical factors that may influence the degradation of carbamates,
including carbaryl, in aquatic systems. Hydrolysis of carbaryl in
alkaline medium was a function of hydroxyl ions in solution and was
first order with respect to these ions. Carbaryl was so sensitive to
hydroxide ions that, at high base concentrations, the liberation of
1-naphthol was too fast (few minutes) to be measured by conventional
methods. Carbaryl was stable to hydrolysis at the acid pH range of
3-6. At pH 7, a rise in the hydrolysis rate was observed, which
increased with increase in pH. Carbaryl was very susceptible towards
hydrolysis in aqueous solutions at neutral and alkaline pH values. A
series of kinetic studies was carried out at different temperatures
(3-33 °C) to study the temperature dependence of the rate of
hydrolysis. Results showed that an increase in temperature resulted
in an increase in the reaction velocity.
Wauchope & Haque (1973) reported that, in weak acidic
solutions, carbaryl and 1-naphthol were stable for several weeks, in
the dark or under laboratory light. In basic solutions, the basic
form of 1-naphthol (1-naphthoxide) was light sensitive.
1-Naphthoxide ion was transformed into 2-hydroxy-1,4-naphthoquinone;
this was confirmed by mass spectrometry.
Khasawinah (1977) carried out the hydrolysis of carbaryl in the
dark, in aqueous buffer solutions at temperatures of 25 and 35 °C.
Increasing the pH and temperature of the buffer accelerated the
disappearance of carbaryl and the formation of 1-naphthol.
Rate constants (at 20 °C) were determined for the purely
chemical hydrolysis of carbaryl in water containing solvents at pH
values ranging from 4 to 8 (Chapman & Cole, 1982). The solvent
composition (water/ethanol = 99/1) was close to pure water and the
solutions were sterilized to ensure that only chemical reactions
were taking place. The half-lives for carbaryl were calculated from
pseudo-first order disappearance rate constants (Table 4).
Fisher & Lohner (1986) conducted tests on the environmental
fate of carbaryl as a function of pH. In both a microcosm and
abiotic studies, greater amounts of carbaryl were detected in water
at pH 4, than at pH 6 or 8.
Hydrolysis of labelled carbaryl in aqueous solution was
conducted by Carpenter (1990) under dark conditions. When
degradation occurred, the major degradation product of significance
was 1-naphthol. No other degradation product accounted for more than
2% of the radioactivity and no volatile products were generated
during the hydrolysis reaction. The test systems were sterile and
the transformation/degradation mechanism was purely chemical
hydrolysis.
Carbaryl half-lives in aqueous solution observed by different
authors are summarized in Table 4.
Table 4. Half-life of carbaryl at different pH values and temperatures in aqueous solution (days)
pH Temperature References
3 4 5 6 7 8 9 10 (°C)
10.5 1.3 0.1 0.01 Aly & El Dib (1971a, 1972)
0.12 0.01 25° Wauchope & Haque (1973)
stable stable 0.14 25° Khasawinah (1977)
stable 29 0.02 35°
1500 15 0.15 27° Wolfe et al. (1978)
2100a 406 14 1.9 20° Chapman & Cole (1982)
104 71.6 1.4 Fisher & Lohner (1986)
171.4 16.5 25° Larkin & Day (1986)
stable 11.6 0.13 Carpenter (1990)
12.5
a pH 4.5
4.2.2 Photolysis
There is sufficient evidence to suggest that photodecomposition
will account for some loss of carbaryl in clear surface waters
exposed to sunlight for long periods. In turbid waters, light
penetration is greatly reduced, thus photolysis will play only a
minor role in the decomposition of carbaryl.
The primary effect of ultraviolet light radiation (UVR) seems
to be cleavage of the ester bond, however, other modifications in
the carbaryl molecule occur. Crosby et al. (1965) studied the
photodecomposition of carbaryl and found several other
cholinesterase inhibitory substances, in addition to 1-naphthol,
indicating that these substances retained the intact carbamate ester
group, and that irradiation resulted in changes at other positions
in the molecule. Both UVR and natural sunlight caused decomposition
of carbaryl, however, the extent of photodecomposition was not the
same under the different conditions of irradiation. Intense UV
irradiation generally resulted in the formation of a greater number
of degradation products. It is expected that the effects of natural
sunlight UVR (292-400 nm) on the photodegradation rate and the
nature of the degradation products would differ from those of the
shorter wavelength irradiations used above.
The effect of UVR on the photodegradation of carbaryl was
studied by Aly & El Dib (1971b, 1972). Generally, the concentration
of carbaryl decreased with time, however, the photolysis rate
gradually decreased. Photodecomposition proceeded at increasing
rates as the pH values of solutions increased. After an exposure
time of 60 min, the decomposition rates of carbaryl at pH 5, 7, and
8 were 50, 57, and 78%, respectively. The half-life at pH 8 was
<20 min while, at pH 5, it was approximately 80 min. The main
decomposition product after 5 min of exposure in all irradiated
solutions was 1-naphthol. However, its concentration also decreased
as the irradiation time was increased, indicating that 1-naphthol
also underwent photolysis, as soon as it appeared in solution. The
photodecomposition of 1-naphthol was also affected by the pH of the
medium. The half-lives of carbaryl and 1-naphthol were 39 and
60 min, respectively, at pH 7 and 8, and 43 min at pH 8.
The photochemistry of carbaryl was studied by Addison et al.
(1975) in aerated and pure ethanol, cyclohexane, isopropyl alcohol,
and tert-butanol. Irradiation of carbaryl produced 1-naphthol and
small amounts of naphthamides, naphthalene, and
ß-napthyl-1-naphthol. In cyclohexane, 1-naphthol was the only
decomposition product.
Khasawinah (1977) conducted a photolysis study on labelled
carbaryl in an aqueous solution buffered at pH 6. The study
terminated before the actual half-life was reached. The half-life
was calculated to be 40-50 days. The author stated that under field
conditions it is expected that carbaryl will photodegrade slowly and
that photodegradation does not play a major role in the
environmental degradation of carbaryl.
The direct photolysis half-life of carbaryl in sunlight was 6.6
days in distilled water (Wolfe et al., 1978). On the basis of the
results of the methods of Zepp et al. (1976) and Zepp & Cline
(1977), the calculated half-life for direct photolysis was about
50 h in a clear water body, near the surface. The annual variation
of the photolysis half-life of carbaryl according to season of the
year was calculated. As the intensity of the sunlight increases, so
do photolysis rates. Carbaryl absorbs UV-B radiation most strongly,
and, thus, can also be photolysed under overcast conditions (cloudy
days). UVR is absorbed by water, and the photolysis rate decreases
as the water deepens. In spring and summer, when carbaryl is
applied, the rate of photolysis is about four times that in winter
months. In distilled water under the June midday sunlight at pH 5.5,
the half-life of carbaryl was 45 h.
Deuel et al. (1985) studied the photodecomposition of
carbaryl in deionized water. They confirmed that carbaryl could be
photodecomposed in an aquatic environment.
The influence of different aqueous systems (rivers, lakes, and
seawater) on the photochemical degradation of some carbamate
insecticides in Greece was studied by Samanidou et al. (1988). In
lake and sea water, carbaryl was almost completely degraded by
sunlight within 4 and 2 days, respectively, in the presence of
oxygen. One day of UV irradiation in river, lake, and sea water,
respectively, resulted in 98%, 87%, and 99% degradation. The high
concentration of suspended matter in river and lake water influences
the absorption of sunlight and consequently the degradation of
carbaryl.
Das (1990a) exposed sterile water, buffered at pH 5, with
labelled carbaryl to artificial sunlight (548.8 watts/m2) for
360 h; 65% of the carbaryl had disappeared by the end of the 360-h
period. The major degradation product was 1-naphthol. In control
test solutions, incubated in the dark, changes in carbaryl
concentrations were insignificant.
4.2.3 Degradation by microorganisms
A review of the chemical and microbial degradation of carbaryl
in aquatic systems has been published by Paris & Lewis (1973).
The rate of hydrolysis of carbaryl in neutral and slightly
basic conditions was so rapid that differences reported between
sterilized and non-sterile water were usually were minor. Thus, it
is generally considered that the microbial degradation of carbaryl
in natural water plays only a secondary role in comparison with
chemical hydrolysis.
The bacterial decomposition of 1-naphthol by ring cleavage was
reported in farm pond water (Hughes & Reuszer, 1970). They studied
bacterial populations in pond water containing drainage water from
carbaryl-treated fields. Their data were the earliest to show that
bacteria can adapt to live on carbaryl and that when this occurs one
strain dominates. They also showed that there may be a minimum
period of time before bacteria can degrade carbaryl and a minimum
concentration below which bacteria will not multiply rapidly enough
to cause degradation.
Ahlrichs et al. (1970) found bacteria that could break the
ring structure of carbaryl, however data concerning the role of
microorganisms in the elimination of insecticides from surface water
were variable.
In a study by Hughes (1971) a bacterium ( Flavobacterium sp.)
was isolated from pond water, which degraded 1-naphthol to
o-hydroxycinnamic acid, salicylic acid, and an unidentified
product. In this study, the bacteria cleaved the naphthalene ring,
since both hydroxycinnamic acid and salicylic acid each have only a
single phenol ring.
Aly & El Dib (1972) studied the biodegradation of carbaryl in
Nile River water in 5-gallon containers, which was buffered to
maintain a pH of 7.2 and held at 25±2 °C under aerobic conditions.
The concentration of carbaryl in water decreased progressively with
time and 89% of the added amount of carbaryl (4.75 mg/litre)
degraded in 6 days. 1-Napthol, which appeared as a degradation
product, did not result only from the chemical hydrolysis of
carbaryl, since a sterile buffered solution showed negligible
hydrolysis. 1-Naphthol was produced mainly as a result of the
biological activity of microorganisms in river water. Subsequent
additions of increasing concentrations of carbaryl disappeared in
shorter periods of time, and there was no build-up of 1-naphthol.
Carbaryl disappeared more rapidly in Nile River water containing
sewage. Thus, the authors considered that natural waters and sewage
contain microorganisms capable of degrading carbaryl and 1-naphthol.
A number of marine microorganisms, including algae, bacteria,
fungi, and yeasts, were tested by Sikka et al. (1973, 1975) for
their ability to metabolize carbaryl or 1-naphthol. None of them was
able to degrade carbaryl to a significant extent. Only a very small
amount of carbaryl was metabolized to form water-soluble metabolites
by the algae Cyclotella nana and Dunaliella tertiolecta.
1-Naphthol was degraded to water- and ether-soluble metabolites by
Culcitalna achraspora, Halosphaeria mediosetigera, Humicola
alopallonella, Aspergillus fumigatus, Serratia marina, Spirillum
sp., and Flavobacterium sp. The organisms differed greatly in
their ability to convert carbaryl to water-soluble products, and
also in their ability to degrade 1-naphthol. Overall, 1-naphthol
appeared more susceptible to degradation than carbaryl, and
filamentous fungi appeared to possess a greater ability to degrade
1-naphthol than bacteria or yeast.
Bacteria isolated from river water were also capable of
degrading 1-naphthol (Bollag et al., 1975; Czaplicki & Bollag,
1975). After 60 h of incubation with 14C-labelled 1-naphthol, it
was possible to trap 44% as CO2 and 22% was recovered. The release
of labelled CO2 clearly indicated that complete biodegradation of
carbaryl had taken place via rupture of the naphthyl ring. However,
15-20% of radioactivity remained in the growth medium. This suggests
that at least 2 different pathways may be involved in the
degradation of 1-naphthol by these bacteria. The radioactivity in
the growth medium was partitioned and the dominant product was
identified as 4-hydroxy-1-tetralone, which suggests an alternative
pathway that involves hydroxylation of the naphthyl ring in the
4-position and conversion of an aromatic ring to an aliphatic cyclic
compound. Walker et al. (1975a) confirmed this pathway with a soil
pseudomonad.
Paris et al. (1975) found that, in heterogeneous bacterial
cultures in water, bacteria did not significantly degrade carbaryl
but did utilize 1-naphthol, produced from hydrolysis, as a carbon
source. Products of the bacterial degradation of the 1-naphthol were
1,4-naphthoquinone and 2 unidentified compounds. Hydrolysis and
photolysis contributed significantly to the degradation of carbaryl,
since half-lives were short compared with those of biolysis.
Within 7 days of incubation in river water, 92% of carbaryl or
1-naphthol disappeared (Prima et al., 1976); 68% was removed by
biochemical degradation, and 24% by a physico-chemical process.
Liu et al. (1981) measured the rate of carbaryl degradation
at pH 6.8 both with, and without, bacteria obtained from lake
sediment. Without the lake sediment inoculum, the half-life of
carbaryl was 8.3 days under aerobic conditions and 15.3 days under
anaerobic conditions. With bacterial metabolism, half-lives of 6.8
and 5.8 days, respectively, were measured. After the addition of
cometabolites (glucose and peptone), the half-lives were further
reduced to 3.8 and 4.2 days, respectively. Thus, bacterial
degradation played a much greater role in the degradation of
carbaryl under anaerobic conditions.
Microbial activity was an important factor in the breakdown of
carbaryl in water from a pond and creek (Szeto et al., 1979).
Autoclaving prior to the addition of carbaryl and incubating for 50
days increased the recovery from 39 to 57% (creek water) and from 28
to 58% (pond samples containing sediment).
Boethling & Alexander (1979) studied the degradation of
carbaryl in stream water (pH 7.5-8.6) at extremely low
concentrations. They reported that, at initial concentrations of 30
and 300 mg/litre, more than 60% of the carbaryl was degraded to
CO2 within 4 days, but 10% or less was converted to CO2 at
0.3 mg/litre and 0.0003 mg/litre. At these two latter
concentrations, CO2 was generated at rates not exceeding 3% of the
starting material per day. The authors concluded that laboratory
tests on bio-degradation are usually conducted with concentrations
of chemicals higher than those found in rivers, lakes, and marine
waters and, therefore, will not accurately predict the environmental
behaviour of microorganisms.
Sharom et al. (1980) compared the persistence of carbaryl in
natural water, distilled water, sterilized natural water, and
sterilized distilled water. Carbaryl disappeared from all four types
of water, which was considered to show that chemical processes
played a major role, and biological processes, a secondary role, in
the degradation of carbaryl in water.
Chaudhry & Wheeler (1988) maintained a Pseudomonas sp.
isolated from a pesticide waste disposal site on a medium containing
normal and radiolabelled carbaryl. Pseudomonas sp. degraded
carbaryl and the authors concluded that Pseudomonas sp. may have
potential as biological treatments for waste and groundwater.
4.2.4 Persistence in surface water
The agricultural use of carbaryl may indirectly produce
residues in surface water and in sediment, following application, as
result of drift or from soil-bound particles. In general, carbaryl
is not expected to persist in the aquatic environment. Although it
is stable to hydrolysis in acidic water, at the pH of most surface
fresh waters (7-8.2), it is highly susceptible to hydrolysis.
Biologically-mediated degradation and photolysis are secondary
mechanisms; sediment and humic substances also influence the
persistence of carbaryl in aquatic systems.
Half-lives of carbaryl in natural water, calculated from
experimental results, are summarized in Table 5. In one case, with
exceptionally cold and acidic conditions, the half-life was in the
range of 70 days. In most cases, half of the carbaryl degraded in a
few days, or even in less than one day.
Table 5. Percentage of carbaryl degraded in water, a certain number of days after treatment,
and the corresponding approximate half-lives
Origin of water Days % Half-life References
after (days)
treatment
laboratory 7 95 1 Eichelberger & Lichtenberg (1971)
pond < 0.5 Romine & Bussian (1971)
containers 6 89 2 Aly & El-Dib (1972)
microcosm < 5 Kanazawa (1975)
river 7 92 2 Prima et al. (1976)
lab. pond water 42 80-82 18 Szeto et al. (1979)
lab. + sediment < 2
lab. creek water 42 60-63 30
lab. + sediment < 7
lab. drainage 28 100 4 Sharom et al. (1980)
field, drainage 6 100 1 Osman & Belal (1980)
brooks, rivers, 1 Stanley & Trial (1980)
streams
stream 17 100 3 Ott et al. (1981)
Table 5. Contd
Origin of water Days % Half-life References
after (days)
treatment
lab. sewage 42 100 11 Odeyemi (1982)
lab. fresh water 60 100 < 15
rice irrigation 10 80 4 Thomas et al. (1982)
pond 1 60 100 8 Gibbs et al. (1981, 1984)
pond 2 138 77 2-3 rice fieldDeuel et al. (1985)
microcosm (pH 4-8) 7 31-73 13-4 Fisher & Lohner (1986)
stream < 0.1 Sundaram & Szeto (1987)
stream 1-3 100 < 1 Springborn (1988b)
rice irrigation < 1 Springborn (1988a)
ponds (18 °C) 4 100 1 Hanazato & Yasuno (1989)
ponds (4 °C) 10 30 20
45 95 10
ponds (4-20 °C) 0.4-0.9 Hanazato & Yasuno
(1990a,b)
aRounded.
The behaviour and persistence of carbaryl in water from a pond
and creek, with and without sediment, were studied under simulated
conditions in the laboratory by Szeto et al. (1979). At 9 °C,
carbaryl was less persistent in pond water (pH 7.5-7.8) than in
creek water (pH 7-7.1). Carbaryl degraded to 18-20% of the initial
amount after 42 days in pond water samples and to 37-40% of the
initial amount in creek water samples after 50 days. The higher pH
of pond water compared with creek water may have contributed to this
effect. The presence of sediment did not affect the rate of loss of
carbaryl, but approximately 50% of the remaining carbaryl was found
in the sediment. Microbial activity was a major factor in the
degradation of carbaryl in this study.
Eichelberger & Lichtenberg (1971) measured the persistence of
carbaryl in river water at room temperature and pH 7.3-8. From an
initial concentration of 10 µg/litre, only 5% could be detected
after 1 week and none was detected at 2 weeks. At the time of
disappearance of carbaryl, 1-naphthol could not be detected.
In Egypt, Osman & Belal (1980) mentioned that residues present
in irrigation and drainage canals following application of carbaryl
disappeared from the water 6 days after spraying.
The persistence of carbaryl was tested by Odeyemi (1982) who
incubated water samples treated wtih 45 mg/kg under tropical
greenhouse conditions (Nigeria). According to colorimetric
measurements, carbaryl disappeared after 42 days of incubation in
sewage water, and after 60 days in fresh water samples.
The impact of an experimental aerial application of carbaryl
(Sevin-4-oil) on woodland ponds in Northern Maine (USA) was studied
by Gibbs et al. (1981, 1984). Carbaryl was applied at the rate of
840 g/ha. Maximum residues levels of 734 µg/litre were detected in
the water, and about 4860 µg/kg (dry weight) in the sediment. In one
pond, carbaryl was not found after 62 days. Data from another pond
showed residues equivalent to 23% of the initial residue after 138
days, and 13% of the initial residues persisting after 375 days.
Investigators noted a rapid movement of carbaryl into bottom
sediments with persistence up to 16 months in this compartment,
compared with 14 months in the water. The anaerobic state of the
organic substrate and acidic conditions in one pond may have
contributed to greater persistence. The lower residue levels in the
other pond were attributed to a greater flow of water.
Hanazato & Yasuno (1990a,b) conducted studies in experimental
outdoor concrete ponds in Japan. Water received three treatments
with carbaryl in order to produce a minimal concentration of
0.5 mg/litre on 12, 19, and 20 October. The water temperature, which
was 20 °C at the start of the experiment, declined steadily to 4 °C
in early December and then did not change. The pH increased from 7.3
on the third day after the start of the experiment to about 9 on the
13th day. It remained between 8-9 until the end of the experiment.
The concentration of carbaryl in water decreased exponentially and
it was no longer detected 4 days after the first and second
treatments and 11 days after the third treatment. The half-lives for
the three treatments were respectively 0.36, 0.40, and 0.86 days.
The corresponding times for 90% degradation were, respectively,
1.18, 1.32, and 2.84 days.
Carbaryl was added to 60 litres of water and 15 kg of soil held
in 110-litre, plastic, garbage containers, buried partially in open
ground (Junk et al., 1984). Carbaryl was studied at the high and
low concentrations of 4 g/litre and 0.2 g/litre, respectively.
Additional variables studied included aeration (1 litre/min) and
peptone nutrients (0.1% by weight). Data obtained from this
experiment demonstrated that soil and water in an inexpensive
container provide satisfactory conditions for the containment of
pesticides so that chemical and biological degradation can occur.
Hydrolysis of carbaryl was rapid.
Deuel et al. (1985) studied the persistence of carbaryl and
the 1-naphthol metabolite in paddy water under flooded rice
cultivation conditions. Persistence was evaluated with respect to
time, application rates (1.1 and 5.6 kg/ha), and irrigation scheme
(intermittent or continuous). Results showed that application rate
and time of sample collection had a significant influence on the
carbaryl residues recovered in paddy water during the 3-year study.
They were found to be greater in plots under intermittent
irrigation, but only in 2 of the 3 years. Carbaryl residues in water
were greatest in years when rainfall occurred within 24 h of foliar
application. Using intermittent treatment data, carbaryl was
determined to dissipate to half the initial residue level within
48-59 h.
4.2.5 Removal from water
As already mentioned, Chaudhry & Wheeler (1988) proposed that
Pseudomonas sp. may have potential as a biological treatment for
waste and groundwater.
According to Miles et al. (1988a,b), the degradation rates of
N-methylcarbamate insecticides, including carbaryl and its
metabolite 1-naphthol, were more rapid in chlorinated water than in
pure water. Half-lives of carbaryl were as follows:
carbaryl control pH 7:10.3 days
carbaryl control pH 8: 1.2 days
carbaryl chlorinated pH 7: 3.5 days
carbaryl chlorinated pH 8: 0.05 days.
A separate experiment with 1-naphthol in chlorinated water
showed that this product is highly unstable with a half-life of the
order of minutes. A water source contaminated with carbaryl and
treated by chlorination will have lower concentrations of the
insecticide in the effluent.
Mason et al. (1990) studied the removal of carbaryl from
drinking-water by the disinfectants, Cl2, ClO2, and O3.
Carbaryl did not react with chlorine or with ClO2, but reacted very
rapidly with O3. Therefore removal/degradation of carbaryl can be
achieved using ozonization.
4.2.6 Persistence in sea water
Under laboratory conditions, the persistence of carbaryl in sea
water is slightly higher than that in freshwater and, according to
temperature, lighting, and microbial presence, its half-life varies
from a few days to about one month. The salt content of natural
waters (ionic strength) may affect the rate of hydrolysis of
carbamates (Christenson, 1964). Thus, carbaryl is expected to be
more stable to hydrolysis in waters with a high salt content than in
freshwater.
Carbaryl may enter marine systems when it is used to control
oyster pests and predators, such as oyster drills, mud shrimp, ghost
shrimp, and star fish (Loosanoff et al., 1960a,b; Lindsay, 1961;
Loosanoff, 1961; Snow & Stewart, 1963; Haydock, 1964; Haven et al.,
1966). However, Hurlburt et al. (1989) stated that tidal action
diluted the insecticide rapidly and that larvae are unlikely to be
subjected to high concentrations of carbaryl for more than several
hours.
The persistence of carbaryl in estuarine water was studied in
the laboratory by Karinen et al. (1967). Samples of water
containing 5, 10, or 25 mg carbaryl/litre and 3% NaCl were buffered
at pH 8 and kept in aquaria at 8 °C. In the absence of mud, the
concentration of carbaryl decreased 50% in 38 days, with most of the
decrease accounted for by the production of 1-naphthol. In another
experiment, naphthyl-14C and carbonyl-14C carbaryl (6.8-8.7 mC)
were used with cold carbaryl at total concentrations of 15 and
25 mg/litre, respectively, and maintained at 20 °C. About 50% of the
carbaryl was hydrolysed in 4 days and, after 17 days, the carbaryl
had almost disappeared with 43% converting to 1-naphthol.
Fluorescent light slightly accelerated the hydrolysis of carbaryl to
1-naphthol. When mud was added to the aquarium system, both carbaryl
and 1-naphthol in sea water declined to less than 10% of the initial
concentration in 10 days. Both compounds were adsorbed by the mud,
where decomposition continued at a slower rate than in sea water.
1-Naphthol persisted in mud for a short period of time, but carbaryl
could be detected for approximately 3 weeks. The naphthol moiety of
the carbaryl molecule was converted to more persistent products. The
experiment with labelled carbaryl demonstrated degradation by
hydrolysis of carbamate and oxidation of the naphthyl ring to
produce 14CO2 and 14CH4. In a preliminary field study,
estuarine mud flats were treated with carbaryl at 11.2 kg/ha (dose
used to control pests in oysters beds); carbaryl could be detected
in the mud for up to 42 days. 1-Naphthol concentrations were found
only after the first day, which may indicate that hydrolysis
proceeds slowly in mud.
The fate of 1-naphthol was studied in simulated marine systems
by Lamberton & Claeys (1970). 1-Naphthol was unstable in the
alkaline environment of sea water, since light and microorganisms
enhanced its degradation to CO2 and other products. 1-Naphthol was
relatively stable in an oxygen-free aqueous solution.
Odeyemi (1982) incubated water samples treated with 45 mg
carbaryl/litre under tropical greenhouse conditions (Nigeria).
According to colorimetric measurements, about 77% of the insecticide
had disappeared after 63 days of incubation in seawater, whereas it
had completely disappeared after 42 days of incubation in sewage
water, and after 60 days in freshwater samples.
4.2.7 Bioaccumulation/biomagnification
Because of its low persistence in water, and its even faster
degradation by living organisms, carbaryl has very low
bio-accumulation properties and presents no risk of biomagnification
under practical conditions.
Kanazawa (1975) studied the uptake and excretion of carbaryl by
Pseudorasbora parva. Fish were reared for 30 days in an aquarium
containing carbaryl at about 1 mg/litre. In water, 95% of the
carbaryl was degraded in 6 days. One day after treatment, residues
of carbaryl in P. parva reached 7.5 mg/kg, which was the maximum
uptake.
Kanazawa et al. (1975) studied the distribution and
metabolism of 14C-1-naphthyl-labelled methylcarbamate (carbaryl)
in an aquatic model ecosystem containing 10 kg soil, 80 litre water,
and catfish ( Ictalurus punctatus), crayfish ( Procambarus sp.),
daphnids ( Daphnia magna), snails (Physa sp.), algae ( Oedogonium
cardiacum) and duckweed ( Lemna minor). After 20 days, 31% of the
radioactivity was lost, 67.85% remained in the soil (about 45%
unextractable residues), 1.1% was present in water, and only 0.1%
was recovered in organisms. Bioaccumulation ratios were calculated
from radioactivity recovery and were likely to be low in the case of
animals. In the case of plants, they were apparently higher, but as
they are based only on radioactivity, including normal synthesis
reutilizing 14CO2 and not on real carbaryl measurement, they
should be considered as an overestimation of the true
bioconcentration factor for carbaryl (Table 6).
A flow-through bioconcentration study was conducted with
bluegill sunfish ( Lepomis macrochirus) exposed to 0.093 mg
carbaryl/litre for 28 days followed by a depuration phase in which
bluegill were held for 14 days in untreated water (Chib, 1986a; Chib
et al., 1986). Analysis of fillet, whole fish, and visceral
portions indicated a rapid uptake of carbaryl. Bioconcentration
factors ranged from 14x to 75x, for fillet and viscera,
respectively. Data suggest that fish stopped accumulating carbaryl
after day 7 of uptake, indicating that a steady state had been
reached.
Table 6. Accumulation of 14C radioactivity expressed as carbaryl
equivalenta by various aquatic organismsb
Carbaryl
Concentration in water 1.66 (1 day)
µg/litre 9.45 (22 days)
Concentration in soil 2.43 (start)
mg/kg 2.55 (22 days)
Organisms mg/kg BARc
Algae 37.9 4000
Duckweed 34.2 3600
Snail 2.81 300
Catfish 1.33 140
Crayfish 2.48 260
aCarbaryl not actually measured.
bFrom: Kanazawa et al. (1975).
cBioaccumulation ratio calculated by dividing mg/kg of dried
tissues by mg/litre in water at harvest.
By the end of the 14-day depuration period, over 90% of the
accumulated carbaryl was eliminated.
The persistence and accumulation of radiolabelled carbaryl
(technical, 99%), administered in either food or water
(flow-through), was studied in catfish ( Ictalurus punctatus) over
a 56-day period (Korn, 1973). Fish were removed periodically for
whole-body residue analysis. Intact carbaryl was not distinguished
from 1-naphthol. Accumulation from dietary carbaryl (2.8 mg/kg per
week) was 11 and 9 mg/kg tissue at 3 and 56 days, respectively. Fish
accumulated 1% or less of the available pesticide via dietary
exposure. The mean total accumulation of carbaryl (and/or metabolite
residues) after 56 days of exposure to water containing 0.25 mg
carbaryl/litre was 11 mg/kg. Fish exposed via the water retained
0.0001% carbaryl. Fish exposed to 2.8 mg/kg per week via diet
eliminated residues rapidly after they were placed on a
carbaryl-free diet for 28 days. However, residues remained constant
for 28 days in fish previously exposed to 0.25 mg carbaryl/litre
water for 56 days. The authors speculated that the greater
persistence of carbaryl residues from the water might be due to the
persistence in fish of 1-naphthol.
The low accumulation and rapid elimination of carbaryl in fish
were confirmed in other laboratory work (Kanazawa, 1975, 1981).
The biodegradation of carbaryl, was studied by Bogacka & Groba
(1980) in a model system simulating the river-water and aqueous
ecosystem conditions. The rate of pesticide decay in water depended
on the initial concentration, temperature, and the kind of model.
Lowering the temperature inhibited this process. The accumulation of
carbaryl in bottom sediments or in aquatic organisms (algae, snails,
and fish) was not observed at concentrations of 10 or 50 µg/litre.
Trace quantities of 1-naphthol could be detected occasionally.
4.3 Soil
4.3.1 Adsorption, desorption
Carbaryl is adsorbed on soil. Most Koc values were in the
range of 100-600, which corresponds to medium to strong adsorption.
Only in some soils from Eastern Australia was carbaryl apparently
less tightly bound (Koc 90-220). Taking into account both the
adsorption/desorption properties and the short persistence of
carbaryl in soil, it can be calculated that the compound has low to
moderate mobility in soil and will remain in the top layers, when
applied under actual agricultural conditions (normal dose rates).
Leenheer & Ahlrichs (1971) stated that fast adsorption rates for
carbaryl on soil particles were related to organic matter (OM) and
were of the order expected for physical adsorption.
Carbaryl desorption and movement in the soil was studied in 1-m
soil columns in glass tubes, which had a drain (LaFleur, 1976a).
Desorption by added water was rectilinear for carbaryl (soil range
1-200 µmol/kg). The movement of carbaryl in the column was a
function of the percentage of the organic matter. In soil containing
5.16% organic matter, carbaryl reached the 40-cm section of the
column only. When the organic matter content of the soil was low
(0.22-0.57%), about one-half of the added carbaryl moved through the
column and was found in the effluent, after addition of a volume of
water that was equal to six months' rainfall. Briggs (1981) also
reported that increasing levels of organic matter in soils resulted
in an increase in the adsorption of carbaryl.
On the basis of the comparative mobility of carbaryl on soil
thin-layer chromatography (TLC) plates using 4 US soils (silt loam,
loam, Norfolk sandy loam, and silty clay loam), Chib & Andrawes
(1985) concluded that the mobility of carbaryl is low to moderate.
With water elution equivalent to about 580 mm of rainfall, the major
portion of carbaryl was in the top 10 cm of soil, thus exhibiting
low mobility. Only 0.6% of the applied 14C was eluted with the
water. Approximately 70% of the applied carbaryl was degraded to
volatile gases during the 30-day incubation.
Aly et al. (1980) studied the adsorption of carbaryl at
different temperatures on Ca-bentonite and on two Egyptian soils
(Nile alluvial and a highly calcareous soil). Carbaryl adsorption
increased as the temperature decreased. Ca-bentonite exhibited the
highest degree of adsorption followed by the Nile alluvial soil and
the calcareous soil. The factors that contributed most to the total
adsorption of carbaryl were the soil contents of clay, calcium
carbonate, and organic matter.
The adsorption-desorption and mobility of carbaryl in 3 soils,
and in a stream sediment were studied by Sharom et al. (1980). The
order of adsorptive capacity was organic soil (75.3% OM, pH 6.1) >
sediment (2.8% OM, pH 6.6) > sandy loam (2.5% OM, pH 6.8) > sand
(0.7% OM, pH 7). Desorption occurred in greatest amounts from sand
> sandy loam > sediment. Leachability studies were consistent with
the adsorption/desorption results and a water solubility of
40 mg/litre. Carbaryl leached more from the sand than from the
organic soil.
In another adsorption/desorption study on carbaryl on sand,
sandy loam, silt loam, silty clay loam, and on aquatic sediment
using batch equilibrium techniques, Chib (1985a) concluded that
carbaryl binds strongly to soil/sediment matrices. This depends on
the carbaryl concentration in solution (the more in solution, the
more on soil) and on the organic content of soil. Desorption
isotherms for carbaryl at low concentrations were nearly flat with
all soils, indicating that carbaryl was tightly bound to the
substrates and difficult to move.
The transport of carbaryl in the soil was also studied under
natural conditions. High-volume rainfall occurring shortly after
carbaryl has been applied to a field can generate the low-level
transport of the pesticide to non-target areas (Caro et al.,
1974). Carbaryl is adsorbed on soil surfaces to a great extent.
Laboratory measurements of absorption isotherms gave a Freundlich k
value of 2.2. Of the 4 kg of carbaryl applied in a watershed, only
5.77 g (0.16%) were found in run-off water and sediment during the
season; (75 and 25% of the seasonal loss, respectively). The
distribution coefficient between sediment and water was 0.33.
Carbaryl had completely disappeared from the sediment by day 70, but
still remained in the water at low concentrations.
Carbaryl could still be found in the soil, 1-2 years after
application at 2 kg 85% WP/ha. It is carried by rainfall and
cultivation from the surface layers of the soil into the deeper
layers. During the first few days after application, it was found in
the root zone. After 3 months, 90% of the carbaryl was present in
the surface layers, and, after 300 days, it was found in the soil at
a depth of 60-70 cm. Rainfall was not specified (Molozhanova, 1968).
Carbaryl was applied to a sandy loam field plot at a dose of
25.4 kg/ha. A shallow water table was present at 1.1 m depth
(LaFleur, 1976b). Rainfall during the following 16 months was
182 cm. After this length of time, the upper 1 m contained 6% of the
applied carbaryl. No carbaryl was found in the 0-20 cm layer after
the fourth month. The half-life of carbaryl in the upper 1 m was >1
month. Carbaryl appeared in the underlying groundwater within 2
months following treatment and could be detected through the eighth
month. The maximum groundwater concentration occurred at the end of
the second month (about 60 µg/litre). The dose rate was more than 10
times the usual one and the persistence was overestimated, as well
as the residues that might be present in shallow groundwater.
Norris (1991) reported a terrestrial field soil dissipation
study conducted with carbaryl under actual use conditions. Carbaryl
was applied to broccoli in California at 2.24 kg/ha, five times, on
a weekly schedule. It was also applied once to sweet corn in North
Carolina at a rate of 7.12 kg a.i./ha. The half-life in soil was
estimated to be 6-12 days at the California site and about 4.8 days
at the North Carolina site. Carbaryl residues were found mainly at a
depth of 0-15 cm, which indicates that carbaryl has a low potential
for leaching through the soil profile under typical agronomic
practices.
4.3.2 Transformation
Degradation of carbaryl in soil occurs as a result of the
activity of microorganisms, and through physical and chemical
effects. It undergoes hydrolysis, oxidation, and other chemical
processes and, on the surface of the soil, is subjected to
photolysis. When applied at the usual doses, carbaryl has a short
persistence in soil. In the field, under temperate and warm climatic
conditions, the half-life of carbaryl in the soil does not exceed
one month (Table 7).
4.3.2.1 Photolysis in soil
Studies have been conducted on the photolysis of carbaryl in
soil. In one study, volatile substances were first identified and
quantified and then soil TLC plates were used over a 30-day period
to quantify degradation products (Chib, 1986b). The calculated
half-life of carbaryl in sandy loam soil on TLC plates irradiated
with a high-pressure mercury vapour lamp (200 watt, Hanovia) at
25 °C was 2.5 days. In controls kept in the dark, labelled carbaryl
degraded with a half-life of >30 days. CO2 was the only major
volatile degradation product from all treatments. The other soil
degradation products identified were 5-hydroxy carbaryl,
N-hydroxymethyl carbaryl and 1-naphthol. Only carbaryl and
1-naphthol were present in control soil extracts. The degradation
products in the irradiated soil extracts suggest that hydrolysis and
oxidation are the main mechanisms in the degradation of carbaryl
under soil photolysis conditions.
Das (1990b) also studied the photolysis of carbaryl in soil.
Labelled carbaryl was applied to a sandy loam on plates
(9.8±0.3 mg/kg) and maintained in artificial sunlight,
intermittently (12 h irradiation + 12 h darkness) for 30 days. The
measured irradiance of the artificial sunlight (502.6 watts/m2)
was comparable to the natural sunlight (545.8 watts/m2). The
evolution of volatile substances was also monitored. Controls were
incubated in the dark. The calculated half-life of irradiated
samples was 41 days (each day 12 h irradiation + 12 h darkness). No
major metabolites were formed under irradiated conditions.
4.3.3 Biotransformation in soil
The persistence and metabolism of 14C-carbaryl (200 mg/kg)
was studied in 5 different soil types by Kazano et al. (1972).
Persistence was influenced by soil type, and the production of
14CO2 varied from 2.2% (loamy sand) to 37.4% (clay loam) of the
initial radioactivity during 32 days of incubation at 25 °C. The
amount of radioactivity left in the soil after extraction was
proportional to the soil organic matter content. A significant
amount (17-57%) of the remaining radiolabel could not be extracted
with organic solvents. Analysis of the extractable residues
indicated the presence of 1-naphthyl N-hydroxymethylcarbamate,
4-hydroxy-1-naphthyl methylcarbamate and 5-hydroxy-1-naphthyl
methylcarbamate, but these were not confirmed. The main pathway of
degradation in soil was probably hydrolysis of the carbamate
linkage, producing CO2 and the corresponding phenol, though it is
possible that hydroxylation of the ring or the methyl carbon
precedes hydrolysis. 1-Naphthol decomposed more rapidly in clay than
in sandy loam. Most of the radioactivity (83-92%) was recovered in
the soils after 60 days of incubation. 1-Naphthol was immobilized on
humic substances in the soil, not by mechanical adsorption, but by
chemical bonding. Four metabolites (coumarin, the others not
identified) were also produced by a soil Pseudomonas.
Gill & Yeoh (1980) studied the degradation of carbaryl in an
extract of flooded paddy field soil (pH 3.7-4.8, organic matter
content 0.5-3%, very high clay content) extract and in the paddy
fish ( Trichogaster pectoralis). Under flooded conditions, the soil
half-life of carbaryl was about 7 weeks. The major significant
metabolite was 1-naphthol. Soil moisture plays an important role in
the extent of degradation since, under flooded conditions and 100%
field capacity, the degradation of carbaryl was more extensive than
under 0 and 50% field capacity, providing more evidence that
microorganisms play an important role in carbaryl degradation in
paddy-field soil. Where sufficient moisture is available, oxidative
mechanisms become important giving rise to ring and N-methyl
hydroxylation in addition to cleavage of the carbonyl moiety, a
common pathway of carbaryl metabolism. Carbaryl was also found to be
more persistent in acidic soil than in alluvial soil.
Rajagopal et al. (1983) measured the residues of carbaryl and
of the 1-naphthol metabolite in 3 flooded soils (organic matter
0.54-1.61%, pH 6.2-9.5) following three applications of the test
substance. Samples received either 1, 2, or 3 applications of
carbaryl in aqueous solution. The concentration of carbaryl
decreased with incubation time in all soils. The disappearance of
the first 50% of the substance in all soils ranged from 10 to 15
days. Subsequent samplings provided evidence for the enrichment of
carbaryl-degrading microorganisms in retreated soils. Thus, the
disappearance time for 75% of the compound was 20-26 days for 3
applications, 28-30 days for 2 applications, and >30 days for 1
application. It appears that carbaryl degradation under flooded
conditions does not follow a clear kinetic pattern (e.g., first
order). However, the first order degradation rates were similar for
the first, second, and third applications for 0-10 days. Degradation
proceeded by hydrolysis with 1-naphthol as the major product, the
amounts formed decreasing with incubation time in 2 out of the 3
soils. The disappearance of 1-naphthol was faster in retreated soils
and may not be attributed only to degradation, because significant
amounts of 1-naphthol may be bound to humic substances, especially
in soils with a high organic matter content.
The persistence of carbaryl was studied in 4 soils under
flooded conditions by Venkateswarlu et al. (1980). They recovered
a substantial portion of carbaryl from all soils 15 days after
application. The recovery ranged from 37% in an alluvial soil to 73%
in an acid sulfate soil. They concluded that flooded conditions
enhance carbaryl degradation.
The metabolism of 14C-carbaryl and 14C-1-naphthol in moist
and flooded soils was studied by Murthy & Raghu (1989) in a
continuous flow-through system, over a period of 28 days, permitting
a 14C-mass balance. The percentage distribution of radiocarbon in
organic volatile compounds, CO2, and extractable and
non-extractable (bound) fractions of soils was determined. Organic
volatile compounds could not be detected in either carbaryl- or
1-naphthol-treated soils. More 14CO2 (25.6%) was evolved from
moist than from flooded soil (15.1%), treated with carbaryl. The
mineralization of14C-1-naphthol was negligible. The level of
extractable radiocarbon was higher (5.5%) in flooded soil treated
with carbaryl. Less than 1% was the parent compound, and carbaryl
was mainly metabolized to 5-hydroxycarbaryl in moist soil and to
4- and 5-hydroxycarbaryl in flooded soil. The extractable
radiocarbon amounted to 18.2% and 24.3% in moist and flooded soils,
respectively, and there was less than 1% of the parent compound with
1-naphthol treatment. Most of the 14C was found as soil-bound
residues, levels being higher with 1-naphthol treatment than with
carbaryl. The humus fraction of the soil organic matter contributed
most to soil-bound residues of both carbaryl and 1-naphthol.
Aerobic and anaerobic soil metabolism studies were conducted
with carbaryl applied to sandy and clay loam soils in the dark
(Wilkes et al., 1977; Khasawinah, 1978). Under aerobic conditions
at room temperatures (23-25 °C), the half-life of carbaryl was
approximately 9-17 days in sandy loam soil (Texas) and 21-27 days in
clay loam (California). Lower temperature (15 °C) nearly doubled its
half-life. The only solvent-extractable apolar material was parent
carbaryl. A major proportion of the radioactivity initially applied
to the soil was lost as CO2. It appears that, under aerobic
conditions, carbaryl was degraded so that 1-naphthol carbon was lost
as CO2, or it was incorporated into the organic matter of the soil
(1-naphthol was not detected). After 112 days, carbaryl levels
declined to 3.6% (average in Texas soil) and 15% (average in
California soil) of the initial application.
In an aerobic, soil study conducted by Miller (1990) with a
sandy loam soil from North Carolina, soil was incubated with
labelled carbaryl in the dark and maintained at 25±1 °C. The
concentration of the parent compound rapidly decreased to a mean
value of 11.9% of the applied dose by day 14. A significant amount
of radioactivity was recovered as CO2. The average maximum value
of CO2 was 59.8% by day 14. The only other major degradation
product was 1-naphthol (maximum concentration of 34.9% of applied
dose by day 1). Base hydrolysis released 41% of the unextractable
residue. Carbaryl rapidly degraded under aerobic conditions with a
half-life of 5.5 days.
Murthy & Raghu (1991) studied the fate of carbaryl in the soil
environment as a function of pH, with respect to the formation of
extractable and non-extractable (soil-bound) residues. Soil samples
(sandy clay, sandy loam, and clay) containing C14-carbaryl
(10 mg/kg) were incubated at 28-30 °C for periods ranging from 7 to
56 days. More 14C-residues could be extracted from sandy clay and
sandy loam than from clay soil, under both moist and flooded
conditions. In general, flooding had no influence on the extractable
14C-residues. Thin-layer chromatography of chloroform extracts
revealed the presence of carbaryl and 1-naphthol. At the end of 56
days, the percentage of carbaryl recovered was 32.1, 6.4, and 1% in
sandy clay (pH 4.2), sandy loam (pH 6.8), and clay soils (pH 8.3),
respectively. The authors considered that there appeared to be a
correlation between soil pH and soil-bound residue formation as an
increase in soil pH was reflected in increased bound residues. The
humin portion of soil organic matter accounted for most of the
14C-residues. Low recoveries in sandy clay and in sandy loam soils
may stem from the possible mineralization of carbaryl.
Carbaryl persistence in the soil was studied under normal
conditions of application (Caro et al., 1974) of 5.03 kg/ha in
granules applied in corn seed furrows. About 135 days were required
for 95% of the carbaryl to disappear (Fig. 1). The pesticide
remained stable in the soil for 25 days (in some cases, more than
116 days) and then decayed rapidly. This decay is an indirect
indication of microbiological degradation.
This hypothesis is sustained by the finding that the stability
of carbaryl in the soil is affected by the nature of the treatments
applied during the period preceding its application. In soil that
had been treated with carbaryl 6 months before sampling, more than
70% of the added radiolabelled carbaryl was degraded after 4 days,
measured by the disappearance of radioactivity. Only 10% was lost in
orchard soil that had been treated for 15 years with various
pesticides, and only 3% was lost in soil that had never been treated
with pesticides. It appeared that soil that was treated with
carbaryl for 6 months increased the ability of the microorganisms to
degrade carbaryl (Rodriguez & Dorough, 1977).
Carbaryl was applied in soil at an agricultural rate and
incorporated to a depth of 6 inches (15 cm) (Heywood, 1975). At 28
days after treatment, 63% of the applied dose appeared as CO2 and
the identifiable residual carbaryl was about 6% of that applied.
Molecular formula: C12H11N02
Common name: Carbaryl (BSI)
CAS chemical name: 1-naphthalenylmethylcarbamate (9CI)
CAS registry number: 63-25-2
RTECS registry number: FC5950000
Common synonyms:
alpha-naftyl- N-methylkarbamat, alpha-naphthalenyl
methylcarbamate, alpha-naphthyl methylcarbamate, alpha-naphthyl
N-methylcarbamate, carbamic acid, methyl-, 1-naphthyl ester,
N-methyl-alpha-naphthyl-urethan, N-methyl-1-naftyl-
carbamaat, N-methyl-1-naphthyl-naphthyl carbamate,
N-methyl-1-naphthyl-carbamat, N-methylcarbamate de
1-naphtyle, N-metil-1-naftil-carbammato, 1-naphthol
N-methyl-carbamate, 1-naphthyl methylcarbamate, 1
naphthyl- N-methyl-karbamat
The most commonly used chemical name is 1-naphthyl- N-
methyl-carbamate.
Common trade names:
Arilat, Arilate, Arylam, Atoxan, Bercema, Caprolin, Carbacine,
Carbatox, Carbavur, Carbomate, Carpolin, Denapon, Dicarbam,
Dyna-carbyl, Karbaryl, Karbatox, Karbosep, Menaphtam, Monsur,
Mugan, Murvin, Oltitox, Panam, Pomex, Prosevor, Ravyon,
Seffein, Sevimol, Sevin, Vioxan
The most commonly used trade name is Sevin.
Previous codes:
Compound 7744, ENT 23,969, ENT 23969, Experimental insecticide
7744, Germain's HSDB 952, NAC, NMC 50, Union Carbide 7744
Purity:
The technical product is principally manufactured in the USA;
however, there are other minor sources in other parts of the
world.
The technical product manufactured in the USA is produced to a
minimum purity of 99% w/w carbaryl with a <0.05% w/w content
of the 2-naphthyl carbamate isomer (sometimes known as
"beta-carbaryl").
FAO specifies a minimum purity of 98% w/w carbaryl with <0.05%
w/w content of the 2-naphthyl carbamate isomer.
2.2 Physical and chemical properties
Some of the physical properties of carbaryl are listed in
Table 1.
Pure carbaryl is a white crystalline solid without odour.
The explosion limit for dust (finely divided particles) in air
is 20.3 g/m3 (approximately 2500 ppm). It is non-corrosive
(Weston, 1982).
The volatility may increase 4-fold when the relative humidity
is increased from 8 to 80%.
Table 1.Physical properties
Melting point (°C) 142
Boiling point (°C) decomposing
Solubility in water (30 °C) 40 mg/litre
Specific density (20 °C) 1.23
Relative vapour density -
Vapour pressure 1.17 x 10-6-3.1 x 10-7
mmHg at 24-25 °C
Flash point 193 °C
Octanol/water partition 1.59-2.3
coefficient (log Kow)
Flammability (explosive) limits -
Relative molecular mass 201
The solubility of carbaryl increases with temperature (Bowman &
Sans, 1985). In sea water at 18 °C, the solubility is 31 mg/litre
(Karinen et al., 1967). Carbaryl is soluble to some extent in most
organic solvents, and it is soluble in corn oil. It is lipophilic
(Kanazawa, 1981).
It is stable to light and heat (up to 70 °C) and acids, but
easily hydrolysed by alkaline materials (Dittert & Higuchi, 1963).
It is a strong oxidizer.
The quality of carbaryl depends upon the purity of the
precursor, 1-naphthol. The amount of the 2-naphthylcarbamate isomer
found as a contaminant in the final product is directly related to
the purity of this precursor. 1-Naphthol, free of 2-naphthol
(undetectable), is produced in the USA today through the catalytic
conversion of naphthalene. However, 1-naphthol produced by other
manufacturing processes may contain 2-naphthol as a byproduct.
High pressure liquid chromatography (HPLC) has been used to
determine 1- and 2-naphthol in their mixtures in ratios 500:1, in
order to check for traces of contamination in samples of a
commercially important insecticide. The results have been summarized
in Table 2 (Argauer & Warthen, 1975).
Table 2.2-Naphthol recovered from carbaryl samplesa
Sample and size Amount of 2-naphthyl Amount of
methylcarbamate added 2-naphthol
for recovery check found
USA produced
250 mg Union Carbide
99.66% active None Undetectable
500 mg Ortho Sevin
50% wettable powder None Undetectable
570 mg Union Carbide
44% aqueous slurry None Undetectable
310 mg Union Carbide
80% wettable powder None Undetectable
310 mg Union Carbide
80% wettable powder 2.5 mg 1.7 mg
310 mg Union Carbide
80% wettable powder 0.50 mg 0.33 mg
Foreign origin
250 mg Sample A-
technical None 2.3 mg
250 mg Sample B-
technical None 14 mg
500 mg Sample C-
50% wettable powder None 12.4 mg
500 mg Sample D-
50% wettable powder None 1.3 mg
aFrom: Argauer & Warthen (1975).
2.3 Conversion factors
1 ppm = 8.22 mg/m3 of air;
1 mg/m3 = 0.12 ppm.
2.4 Analytical methods
The methods used to determine carbaryl are summarized in
Table 3. They vary considerably in relation to the equipment used.
As an alternative to chemical analysis, Bowman et al. (1982) used
a simple bioassay system procedure to determine foliar residues for
a safe re-entry period and to screen food for residues. Daphnia
and Hyalella were used as highly sensitive test organisms.
The Joint FAO/WHO Codex Alimentarius Commission has given
recommendations for the methods of analysis to be used for the
determination of carbaryl residues (FAO/WHO 1986a).
Table 3. Methods of analysis
Medium Sampling preparation Analytical Detection limit Comments Reference
methods
Meat extraction by paper test based 0.5 mg/kg Filatov & Brytskov
acetone on ChE determination; (1972)
butyryl choline
as a substrate and
phenol red as an
indicator
Air thin-layer 0.2 ng qualitative Wagner (1973)
chromatography 0.5-50 ng
quantitative
Air (carbaryl absorption in UV spectro- 1 mg/kg carbaryl lambda max. Klisenko (1965)
and methanol photometry 281 nm 1-naphthol
1-naphthol) lambda max. 296 nm
Soil plant extraction by thin-layer 0.1 µg recovery 80-95% Kovaleva &
hexane, acetone, chromatography sensitivity 0.02 mg/kg Talanov (1978a)
chloroform
Cow's milk and spectrophotometry 0.002 mg/litre - milk recovery 79-81% Hurwood (1967)
tissues p-nitro-benzene
diazonium fluoborate 0.02 mg/kg - tissues fortification 9.5-5 µg
coupling method
Urine 1-naphthol extraction with gas chromatography 0.02 mg/litre recovery 89-95% Shafik et al. (1971)
benzene tritium detector
Table 3 (continued)
Medium Sampling preparation Analytical Detection limit Comments Reference
methods
Air of working absorption in sodium gas chromatography, Krechniak & Foss
environment hydroxide solution electron capture (1981)
with simultaneous detector
hydrolysis to
1-naphthol;
derivatization by
1-fluoro-2-4
dinitro-benzene
Spinach Chicory sulfuric acid for electron capture 0.2 mg/kg in fortified Tilden & van
hydrolysis to for chromatography crop extract; 20 pg for Middelem (1970)
methylamine salt; pure standards
4-bromobenzoyl
chloride used to
produce derivative
4-bromo-N-methyl
benzamide
Water carbaryl extraction with gas chromatography 0.2 µg/litre recovery for carbaryl, Deuel et al. (1985)
and 1-naphthol dichloromethane H-3 source electron 100%; for naphthol, 90%
capture detector or
63Ni detector
Soil carbaryl extraction with 0.01 mg/kg for recovery for carbaryl,
and 1-naphthol 20% diethylester fortified soil 89.8%; for 1-naphthol,
in dichloromethane samples 79.4%
Table 3 (continued)
Medium Sampling preparation Analytical Detection limit Comments Reference
methods
Air ambient series of gas HPLC with ultraviolet Currier et al. (1982)
scrubbers charged detection at 220 nm
with methanol;
particulate deposit
on Teflon discs
Pads methanol ethanol HPLC 50 ng/pad (103.2 cm2) sensitive, inexpensive Bogus et al. (1985)
extraction; selective procedure
absorption and
elution of reversed
phase solid support
Post-mortem extraction procedure HPLC ultraviolet recovery: blood and Duck & Woolias (1985)
specimens on Extrelut column; detection reversed urine 99%; liver and
protein precipitation phase stomach tissue 95%
with acetonitrile
Foliage surface extraction HPLC recovery: at 5 ng/kg Pieper (1979)
by trichloromethane fortification level
Grass, etc. extraction by CH3CN grass 89.5%;
geranium 86.5%
Water extraction by CH2Cl2 aspen 75%;
Douglas fir 49.8%;
at 0.1 mg/litre level
Table 3 (continued)
Medium Sampling preparation Analytical Detection limit Comments Reference
methods
Soil extraction by soil 103%;
acetonitrile + water stream water 99.7%;
sediment 101%
Water, soil, extraction by thin-layer 0.5 mg/kg qualitative carbaryl and 1-naphthol Klisenko et al. (1972)
cow's milk, benzene ( n-hexane, chromatography
tissue (liver, diethyl ether)
kidney, heart,
lungs, etc.)
Vegetables and extraction by HPLC Recovery 83-97% at Ting et al. (1984)
fruits methanol (10% in 0.5 mg/kg fortification
petroleum ether) or level. Detailed
acetonitrile clean-up description of the
on a florasil column method is given
Water and Serum single extraction by HPLC 0.5 ng/ml Analysis time is Strait et al. (1991)
methanol after 1 ml 10 min Recovery in
of C18 solid-phase water > 99%
extraction columns
Plants methanol and liquid chromatography 0.01 mg/kg Recovery varies from Krause (1985a,b)
mechanical reverse phase; LC 86 to 121%
ultrasonic column using
homogenizer used acetonitrile - water
for extraction mobile phase
Table 3 (continued)
Medium Sampling preparation Analytical Detection limit Comments Reference
methods
Marion-berries extracted by Luke chemical ionization Recommended when GC Cairns et al. (1983);
procedure (Luke et mass spectrometry retention data are
al., 1975, 1981) with both methane inconclusive
cited by Cairns and ammonia as
(1983); eluted reagent gases
through florisil
using 50% mixed
petroleum ethers
Honey-bees extraction by gas chromatography, 0.03-0.10 mg/kg Recovery 95-100% Kendrick et al. (1991)
diethyl ether nitrogen-phosphorous
clean-up using ionization detector
silica cartridge
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
Carbaryl was first synthesized in 1953 and, in 1958, the Union
Carbide Corporation began its commercial production. Carbaryl is now
processed by more than 290 formulators into 1537 different
registered products (Harry, 1977, unpublished report). Carbaryl is
formulated as a 50 and 85% wettable powder, 1.75-50% dust, oil-and
water-based 4% liquid suspension, and 5 and 10% granules and baits.
Annual production is of the order of 10 000 tonnes. It is produced
by the reaction of 1-naphthol with methylisocyanate.
Carbaryl is widely used, in many countries, as a broad spectrum
contact and ingestion insecticide with some systemic properties, and
is recommended for use at 0.25-2.7 kg active ingredient per hectare
to control various insect pests. Up to 10 kg/ha per season can be
used on tree fruits. In the USA, carbaryl is registered for the
control of about 560 different pests. It is used on more than 115
food and fibre crops, trees, and ornamentals. About 40% of the
quantity used is applied to cotton (Kuhr & Dorough, 1976; Mastro &
Cameron, 1976; Payne et al., 1985). In combination with other
substances, or alone, carbaryl is used as a plant regulator for
apple thinning (Looney & McKellar, 1984; Looney & Knight, 1985).
In veterinary practice, carbaryl is used on cattle, poultry,
and pets, especially to control flies, mosquitos, ticks, and lice,
some of which are vectors of disease.
Carbaryl (5% formulation Carbacide) is used to treat human body
louse infestation (Sussman et al., 1969).
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
4.1 Volatilization and air transportation
Carbaryl has a low volatility and a low air-water partition
coefficient. Thus, only limited evaporation can be anticipated after
treatment. Some traces of carbaryl in the air and in fog, resulting
from spray drift, may be detected at a certain distance from the
treated areas. A maximum level of 0.09 mg/m3 in the air has been
reported.
The dimensionless air-water partitioning constant (Henry's Law
Constant) for carbaryl has been evaluated to be 5.3x10-6
(Schomburg et al., 1991).
Deuel et al. (1985) studied the persistence of carbaryl in
paddy water. Results indicated that no measurable dissipation could
occur as a result of volatilization. In contrast to the results from
Deuel, using the BAM model, Lee et al. (1990) calculated that 50
days after treatment, 0.63% of the carbaryl applied to soil could
have been volatilized and 78.84% degraded.
4.2 Water
4.2.1 Hydrolysis
In pure, sterilized water, kept in the dark, the persistence of
carbaryl is pH-dependent. Carbaryl is rather stable in acidic
conditions. Its half-life at pH 7 is 10-16 days, and at pH 8 it is
1.3-1.9 days only. At pH levels higher than 8, its half-life is in
the range of a few hours, or even less (Aly & El Dib, 1971a).
Carbaryl is an example of an N-substituted carbamate that
hydrolyses readily in water. In this mechanism, an acid-base
equilibrium is established and the conjugate carbamate undergoes an
elimination type reaction to give an unstable carbamic acid that
decomposes to the primary amine and CO2. Initially, the principal
non-biological degradation pathway of carbaryl in water, however,
involves base-catalysed hydrolysis to 1-naphthol (Khasawinah, 1977).
Aly & El Dib (1971a,b, 1972) conducted studies to determine the
physical factors that may influence the degradation of carbamates,
including carbaryl, in aquatic systems. Hydrolysis of carbaryl in
alkaline medium was a function of hydroxyl ions in solution and was
first order with respect to these ions. Carbaryl was so sensitive to
hydroxide ions that, at high base concentrations, the liberation of
1-naphthol was too fast (few minutes) to be measured by conventional
methods. Carbaryl was stable to hydrolysis at the acid pH range of
3-6. At pH 7, a rise in the hydrolysis rate was observed, which
increased with increase in pH. Carbaryl was very susceptible towards
hydrolysis in aqueous solutions at neutral and alkaline pH values. A
series of kinetic studies was carried out at different temperatures
(3-33 °C) to study the temperature dependence of the rate of
hydrolysis. Results showed that an increase in temperature resulted
in an increase in the reaction velocity.
Wauchope & Haque (1973) reported that, in weak acidic
solutions, carbaryl and 1-naphthol were stable for several weeks, in
the dark or under laboratory light. In basic solutions, the basic
form of 1-naphthol (1-naphthoxide) was light sensitive.
1-Naphthoxide ion was transformed into 2-hydroxy-1,4-naphthoquinone;
this was confirmed by mass spectrometry.
Khasawinah (1977) carried out the hydrolysis of carbaryl in the
dark, in aqueous buffer solutions at temperatures of 25 and 35 °C.
Increasing the pH and temperature of the buffer accelerated the
disappearance of carbaryl and the formation of 1-naphthol.
Rate constants (at 20 °C) were determined for the purely
chemical hydrolysis of carbaryl in water containing solvents at pH
values ranging from 4 to 8 (Chapman & Cole, 1982). The solvent
composition (water/ethanol = 99/1) was close to pure water and the
solutions were sterilized to ensure that only chemical reactions
were taking place. The half-lives for carbaryl were calculated from
pseudo-first order disappearance rate constants (Table 4).
Fisher & Lohner (1986) conducted tests on the environmental
fate of carbaryl as a function of pH. In both a microcosm and
abiotic studies, greater amounts of carbaryl were detected in water
at pH 4, than at pH 6 or 8.
Hydrolysis of labelled carbaryl in aqueous solution was
conducted by Carpenter (1990) under dark conditions. When
degradation occurred, the major degradation product of significance
was 1-naphthol. No other degradation product accounted for more than
2% of the radioactivity and no volatile products were generated
during the hydrolysis reaction. The test systems were sterile and
the transformation/degradation mechanism was purely chemical
hydrolysis.
Carbaryl half-lives in aqueous solution observed by different
authors are summarized in Table 4.
Table 4. Half-life of carbaryl at different pH values and temperatures in aqueous solution (days)
pH Temperature References
3 4 5 6 7 8 9 10 (°C)
10.5 1.3 0.1 0.01 Aly & El Dib (1971a, 1972)
0.12 0.01 25° Wauchope & Haque (1973)
stable stable 0.14 25° Khasawinah (1977)
stable 29 0.02 35°
1500 15 0.15 27° Wolfe et al. (1978)
2100a 406 14 1.9 20° Chapman & Cole (1982)
104 71.6 1.4 Fisher & Lohner (1986)
171.4 16.5 25° Larkin & Day (1986)
stable 11.6 0.13 Carpenter (1990)
12.5
a pH 4.5
4.2.2 Photolysis
There is sufficient evidence to suggest that photodecomposition
will account for some loss of carbaryl in clear surface waters
exposed to sunlight for long periods. In turbid waters, light
penetration is greatly reduced, thus photolysis will play only a
minor role in the decomposition of carbaryl.
The primary effect of ultraviolet light radiation (UVR) seems
to be cleavage of the ester bond, however, other modifications in
the carbaryl molecule occur. Crosby et al. (1965) studied the
photodecomposition of carbaryl and found several other
cholinesterase inhibitory substances, in addition to 1-naphthol,
indicating that these substances retained the intact carbamate ester
group, and that irradiation resulted in changes at other positions
in the molecule. Both UVR and natural sunlight caused decomposition
of carbaryl, however, the extent of photodecomposition was not the
same under the different conditions of irradiation. Intense UV
irradiation generally resulted in the formation of a greater number
of degradation products. It is expected that the effects of natural
sunlight UVR (292-400 nm) on the photodegradation rate and the
nature of the degradation products would differ from those of the
shorter wavelength irradiations used above.
The effect of UVR on the photodegradation of carbaryl was
studied by Aly & El Dib (1971b, 1972). Generally, the concentration
of carbaryl decreased with time, however, the photolysis rate
gradually decreased. Photodecomposition proceeded at increasing
rates as the pH values of solutions increased. After an exposure
time of 60 min, the decomposition rates of carbaryl at pH 5, 7, and
8 were 50, 57, and 78%, respectively. The half-life at pH 8 was
<20 min while, at pH 5, it was approximately 80 min. The main
decomposition product after 5 min of exposure in all irradiated
solutions was 1-naphthol. However, its concentration also decreased
as the irradiation time was increased, indicating that 1-naphthol
also underwent photolysis, as soon as it appeared in solution. The
photodecomposition of 1-naphthol was also affected by the pH of the
medium. The half-lives of carbaryl and 1-naphthol were 39 and
60 min, respectively, at pH 7 and 8, and 43 min at pH 8.
The photochemistry of carbaryl was studied by Addison et al.
(1975) in aerated and pure ethanol, cyclohexane, isopropyl alcohol,
and tert-butanol. Irradiation of carbaryl produced 1-naphthol and
small amounts of naphthamides, naphthalene, and
ß-napthyl-1-naphthol. In cyclohexane, 1-naphthol was the only
decomposition product.
Khasawinah (1977) conducted a photolysis study on labelled
carbaryl in an aqueous solution buffered at pH 6. The study
terminated before the actual half-life was reached. The half-life
was calculated to be 40-50 days. The author stated that under field
conditions it is expected that carbaryl will photodegrade slowly and
that photodegradation does not play a major role in the
environmental degradation of carbaryl.
The direct photolysis half-life of carbaryl in sunlight was 6.6
days in distilled water (Wolfe et al., 1978). On the basis of the
results of the methods of Zepp et al. (1976) and Zepp & Cline
(1977), the calculated half-life for direct photolysis was about
50 h in a clear water body, near the surface. The annual variation
of the photolysis half-life of carbaryl according to season of the
year was calculated. As the intensity of the sunlight increases, so
do photolysis rates. Carbaryl absorbs UV-B radiation most strongly,
and, thus, can also be photolysed under overcast conditions (cloudy
days). UVR is absorbed by water, and the photolysis rate decreases
as the water deepens. In spring and summer, when carbaryl is
applied, the rate of photolysis is about four times that in winter
months. In distilled water under the June midday sunlight at pH 5.5,
the half-life of carbaryl was 45 h.
Deuel et al. (1985) studied the photodecomposition of
carbaryl in deionized water. They confirmed that carbaryl could be
photodecomposed in an aquatic environment.
The influence of different aqueous systems (rivers, lakes, and
seawater) on the photochemical degradation of some carbamate
insecticides in Greece was studied by Samanidou et al. (1988). In
lake and sea water, carbaryl was almost completely degraded by
sunlight within 4 and 2 days, respectively, in the presence of
oxygen. One day of UV irradiation in river, lake, and sea water,
respectively, resulted in 98%, 87%, and 99% degradation. The high
concentration of suspended matter in river and lake water influences
the absorption of sunlight and consequently the degradation of
carbaryl.
Das (1990a) exposed sterile water, buffered at pH 5, with
labelled carbaryl to artificial sunlight (548.8 watts/m2) for
360 h; 65% of the carbaryl had disappeared by the end of the 360-h
period. The major degradation product was 1-naphthol. In control
test solutions, incubated in the dark, changes in carbaryl
concentrations were insignificant.
4.2.3 Degradation by microorganisms
A review of the chemical and microbial degradation of carbaryl
in aquatic systems has been published by Paris & Lewis (1973).
The rate of hydrolysis of carbaryl in neutral and slightly
basic conditions was so rapid that differences reported between
sterilized and non-sterile water were usually were minor. Thus, it
is generally considered that the microbial degradation of carbaryl
in natural water plays only a secondary role in comparison with
chemical hydrolysis.
The bacterial decomposition of 1-naphthol by ring cleavage was
reported in farm pond water (Hughes & Reuszer, 1970). They studied
bacterial populations in pond water containing drainage water from
carbaryl-treated fields. Their data were the earliest to show that
bacteria can adapt to live on carbaryl and that when this occurs one
strain dominates. They also showed that there may be a minimum
period of time before bacteria can degrade carbaryl and a minimum
concentration below which bacteria will not multiply rapidly enough
to cause degradation.
Ahlrichs et al. (1970) found bacteria that could break the
ring structure of carbaryl, however data concerning the role of
microorganisms in the elimination of insecticides from surface water
were variable.
In a study by Hughes (1971) a bacterium ( Flavobacterium sp.)
was isolated from pond water, which degraded 1-naphthol to
o-hydroxycinnamic acid, salicylic acid, and an unidentified
product. In this study, the bacteria cleaved the naphthalene ring,
since both hydroxycinnamic acid and salicylic acid each have only a
single phenol ring.
Aly & El Dib (1972) studied the biodegradation of carbaryl in
Nile River water in 5-gallon containers, which was buffered to
maintain a pH of 7.2 and held at 25±2 °C under aerobic conditions.
The concentration of carbaryl in water decreased progressively with
time and 89% of the added amount of carbaryl (4.75 mg/litre)
degraded in 6 days. 1-Napthol, which appeared as a degradation
product, did not result only from the chemical hydrolysis of
carbaryl, since a sterile buffered solution showed negligible
hydrolysis. 1-Naphthol was produced mainly as a result of the
biological activity of microorganisms in river water. Subsequent
additions of increasing concentrations of carbaryl disappeared in
shorter periods of time, and there was no build-up of 1-naphthol.
Carbaryl disappeared more rapidly in Nile River water containing
sewage. Thus, the authors considered that natural waters and sewage
contain microorganisms capable of degrading carbaryl and 1-naphthol.
A number of marine microorganisms, including algae, bacteria,
fungi, and yeasts, were tested by Sikka et al. (1973, 1975) for
their ability to metabolize carbaryl or 1-naphthol. None of them was
able to degrade carbaryl to a significant extent. Only a very small
amount of carbaryl was metabolized to form water-soluble metabolites
by the algae Cyclotella nana and Dunaliella tertiolecta.
1-Naphthol was degraded to water- and ether-soluble metabolites by
Culcitalna achraspora, Halosphaeria mediosetigera, Humicola
alopallonella, Aspergillus fumigatus, Serratia marina, Spirillum
sp., and Flavobacterium sp. The organisms differed greatly in
their ability to convert carbaryl to water-soluble products, and
also in their ability to degrade 1-naphthol. Overall, 1-naphthol
appeared more susceptible to degradation than carbaryl, and
filamentous fungi appeared to possess a greater ability to degrade
1-naphthol than bacteria or yeast.
Bacteria isolated from river water were also capable of
degrading 1-naphthol (Bollag et al., 1975; Czaplicki & Bollag,
1975). After 60 h of incubation with 14C-labelled 1-naphthol, it
was possible to trap 44% as CO2 and 22% was recovered. The release
of labelled CO2 clearly indicated that complete biodegradation of
carbaryl had taken place via rupture of the naphthyl ring. However,
15-20% of radioactivity remained in the growth medium. This suggests
that at least 2 different pathways may be involved in the
degradation of 1-naphthol by these bacteria. The radioactivity in
the growth medium was partitioned and the dominant product was
identified as 4-hydroxy-1-tetralone, which suggests an alternative
pathway that involves hydroxylation of the naphthyl ring in the
4-position and conversion of an aromatic ring to an aliphatic cyclic
compound. Walker et al. (1975a) confirmed this pathway with a soil
pseudomonad.
Paris et al. (1975) found that, in heterogeneous bacterial
cultures in water, bacteria did not significantly degrade carbaryl
but did utilize 1-naphthol, produced from hydrolysis, as a carbon
source. Products of the bacterial degradation of the 1-naphthol were
1,4-naphthoquinone and 2 unidentified compounds. Hydrolysis and
photolysis contributed significantly to the degradation of carbaryl,
since half-lives were short compared with those of biolysis.
Within 7 days of incubation in river water, 92% of carbaryl or
1-naphthol disappeared (Prima et al., 1976); 68% was removed by
biochemical degradation, and 24% by a physico-chemical process.
Liu et al. (1981) measured the rate of carbaryl degradation
at pH 6.8 both with, and without, bacteria obtained from lake
sediment. Without the lake sediment inoculum, the half-life of
carbaryl was 8.3 days under aerobic conditions and 15.3 days under
anaerobic conditions. With bacterial metabolism, half-lives of 6.8
and 5.8 days, respectively, were measured. After the addition of
cometabolites (glucose and peptone), the half-lives were further
reduced to 3.8 and 4.2 days, respectively. Thus, bacterial
degradation played a much greater role in the degradation of
carbaryl under anaerobic conditions.
Microbial activity was an important factor in the breakdown of
carbaryl in water from a pond and creek (Szeto et al., 1979).
Autoclaving prior to the addition of carbaryl and incubating for 50
days increased the recovery from 39 to 57% (creek water) and from 28
to 58% (pond samples containing sediment).
Boethling & Alexander (1979) studied the degradation of
carbaryl in stream water (pH 7.5-8.6) at extremely low
concentrations. They reported that, at initial concentrations of 30
and 300 mg/litre, more than 60% of the carbaryl was degraded to
CO2 within 4 days, but 10% or less was converted to CO2 at
0.3 mg/litre and 0.0003 mg/litre. At these two latter
concentrations, CO2 was generated at rates not exceeding 3% of the
starting material per day. The authors concluded that laboratory
tests on bio-degradation are usually conducted with concentrations
of chemicals higher than those found in rivers, lakes, and marine
waters and, therefore, will not accurately predict the environmental
behaviour of microorganisms.
Sharom et al. (1980) compared the persistence of carbaryl in
natural water, distilled water, sterilized natural water, and
sterilized distilled water. Carbaryl disappeared from all four types
of water, which was considered to show that chemical processes
played a major role, and biological processes, a secondary role, in
the degradation of carbaryl in water.
Chaudhry & Wheeler (1988) maintained a Pseudomonas sp.
isolated from a pesticide waste disposal site on a medium containing
normal and radiolabelled carbaryl. Pseudomonas sp. degraded
carbaryl and the authors concluded that Pseudomonas sp. may have
potential as biological treatments for waste and groundwater.
4.2.4 Persistence in surface water
The agricultural use of carbaryl may indirectly produce
residues in surface water and in sediment, following application, as
result of drift or from soil-bound particles. In general, carbaryl
is not expected to persist in the aquatic environment. Although it
is stable to hydrolysis in acidic water, at the pH of most surface
fresh waters (7-8.2), it is highly susceptible to hydrolysis.
Biologically-mediated degradation and photolysis are secondary
mechanisms; sediment and humic substances also influence the
persistence of carbaryl in aquatic systems.
Half-lives of carbaryl in natural water, calculated from
experimental results, are summarized in Table 5. In one case, with
exceptionally cold and acidic conditions, the half-life was in the
range of 70 days. In most cases, half of the carbaryl degraded in a
few days, or even in less than one day.
Table 5. Percentage of carbaryl degraded in water, a certain number of days after treatment,
and the corresponding approximate half-lives
Origin of water Days % Half-life References
after (days)
treatment
laboratory 7 95 1 Eichelberger & Lichtenberg (1971)
pond < 0.5 Romine & Bussian (1971)
containers 6 89 2 Aly & El-Dib (1972)
microcosm < 5 Kanazawa (1975)
river 7 92 2 Prima et al. (1976)
lab. pond water 42 80-82 18 Szeto et al. (1979)
lab. + sediment < 2
lab. creek water 42 60-63 30
lab. + sediment < 7
lab. drainage 28 100 4 Sharom et al. (1980)
field, drainage 6 100 1 Osman & Belal (1980)
brooks, rivers, 1 Stanley & Trial (1980)
streams
stream 17 100 3 Ott et al. (1981)
Table 5. Contd
Origin of water Days % Half-life References
after (days)
treatment
lab. sewage 42 100 11 Odeyemi (1982)
lab. fresh water 60 100 < 15
rice irrigation 10 80 4 Thomas et al. (1982)
pond 1 60 100 8 Gibbs et al. (1981, 1984)
pond 2 138 77 2-3 rice fieldDeuel et al. (1985)
microcosm (pH 4-8) 7 31-73 13-4 Fisher & Lohner (1986)
stream < 0.1 Sundaram & Szeto (1987)
stream 1-3 100 < 1 Springborn (1988b)
rice irrigation < 1 Springborn (1988a)
ponds (18 °C) 4 100 1 Hanazato & Yasuno (1989)
ponds (4 °C) 10 30 20
45 95 10
ponds (4-20 °C) 0.4-0.9 Hanazato & Yasuno
(1990a,b)
aRounded.
The behaviour and persistence of carbaryl in water from a pond
and creek, with and without sediment, were studied under simulated
conditions in the laboratory by Szeto et al. (1979). At 9 °C,
carbaryl was less persistent in pond water (pH 7.5-7.8) than in
creek water (pH 7-7.1). Carbaryl degraded to 18-20% of the initial
amount after 42 days in pond water samples and to 37-40% of the
initial amount in creek water samples after 50 days. The higher pH
of pond water compared with creek water may have contributed to this
effect. The presence of sediment did not affect the rate of loss of
carbaryl, but approximately 50% of the remaining carbaryl was found
in the sediment. Microbial activity was a major factor in the
degradation of carbaryl in this study.
Eichelberger & Lichtenberg (1971) measured the persistence of
carbaryl in river water at room temperature and pH 7.3-8. From an
initial concentration of 10 µg/litre, only 5% could be detected
after 1 week and none was detected at 2 weeks. At the time of
disappearance of carbaryl, 1-naphthol could not be detected.
In Egypt, Osman & Belal (1980) mentioned that residues present
in irrigation and drainage canals following application of carbaryl
disappeared from the water 6 days after spraying.
The persistence of carbaryl was tested by Odeyemi (1982) who
incubated water samples treated wtih 45 mg/kg under tropical
greenhouse conditions (Nigeria). According to colorimetric
measurements, carbaryl disappeared after 42 days of incubation in
sewage water, and after 60 days in fresh water samples.
The impact of an experimental aerial application of carbaryl
(Sevin-4-oil) on woodland ponds in Northern Maine (USA) was studied
by Gibbs et al. (1981, 1984). Carbaryl was applied at the rate of
840 g/ha. Maximum residues levels of 734 µg/litre were detected in
the water, and about 4860 µg/kg (dry weight) in the sediment. In one
pond, carbaryl was not found after 62 days. Data from another pond
showed residues equivalent to 23% of the initial residue after 138
days, and 13% of the initial residues persisting after 375 days.
Investigators noted a rapid movement of carbaryl into bottom
sediments with persistence up to 16 months in this compartment,
compared with 14 months in the water. The anaerobic state of the
organic substrate and acidic conditions in one pond may have
contributed to greater persistence. The lower residue levels in the
other pond were attributed to a greater flow of water.
Hanazato & Yasuno (1990a,b) conducted studies in experimental
outdoor concrete ponds in Japan. Water received three treatments
with carbaryl in order to produce a minimal concentration of
0.5 mg/litre on 12, 19, and 20 October. The water temperature, which
was 20 °C at the start of the experiment, declined steadily to 4 °C
in early December and then did not change. The pH increased from 7.3
on the third day after the start of the experiment to about 9 on the
13th day. It remained between 8-9 until the end of the experiment.
The concentration of carbaryl in water decreased exponentially and
it was no longer detected 4 days after the first and second
treatments and 11 days after the third treatment. The half-lives for
the three treatments were respectively 0.36, 0.40, and 0.86 days.
The corresponding times for 90% degradation were, respectively,
1.18, 1.32, and 2.84 days.
Carbaryl was added to 60 litres of water and 15 kg of soil held
in 110-litre, plastic, garbage containers, buried partially in open
ground (Junk et al., 1984). Carbaryl was studied at the high and
low concentrations of 4 g/litre and 0.2 g/litre, respectively.
Additional variables studied included aeration (1 litre/min) and
peptone nutrients (0.1% by weight). Data obtained from this
experiment demonstrated that soil and water in an inexpensive
container provide satisfactory conditions for the containment of
pesticides so that chemical and biological degradation can occur.
Hydrolysis of carbaryl was rapid.
Deuel et al. (1985) studied the persistence of carbaryl and
the 1-naphthol metabolite in paddy water under flooded rice
cultivation conditions. Persistence was evaluated with respect to
time, application rates (1.1 and 5.6 kg/ha), and irrigation scheme
(intermittent or continuous). Results showed that application rate
and time of sample collection had a significant influence on the
carbaryl residues recovered in paddy water during the 3-year study.
They were found to be greater in plots under intermittent
irrigation, but only in 2 of the 3 years. Carbaryl residues in water
were greatest in years when rainfall occurred within 24 h of foliar
application. Using intermittent treatment data, carbaryl was
determined to dissipate to half the initial residue level within
48-59 h.
4.2.5 Removal from water
As already mentioned, Chaudhry & Wheeler (1988) proposed that
Pseudomonas sp. may have potential as a biological treatment for
waste and groundwater.
According to Miles et al. (1988a,b), the degradation rates of
N-methylcarbamate insecticides, including carbaryl and its
metabolite 1-naphthol, were more rapid in chlorinated water than in
pure water. Half-lives of carbaryl were as follows:
carbaryl control pH 7:10.3 days
carbaryl control pH 8: 1.2 days
carbaryl chlorinated pH 7: 3.5 days
carbaryl chlorinated pH 8: 0.05 days.
A separate experiment with 1-naphthol in chlorinated water
showed that this product is highly unstable with a half-life of the
order of minutes. A water source contaminated with carbaryl and
treated by chlorination will have lower concentrations of the
insecticide in the effluent.
Mason et al. (1990) studied the removal of carbaryl from
drinking-water by the disinfectants, Cl2, ClO2, and O3.
Carbaryl did not react with chlorine or with ClO2, but reacted very
rapidly with O3. Therefore removal/degradation of carbaryl can be
achieved using ozonization.
4.2.6 Persistence in sea water
Under laboratory conditions, the persistence of carbaryl in sea
water is slightly higher than that in freshwater and, according to
temperature, lighting, and microbial presence, its half-life varies
from a few days to about one month. The salt content of natural
waters (ionic strength) may affect the rate of hydrolysis of
carbamates (Christenson, 1964). Thus, carbaryl is expected to be
more stable to hydrolysis in waters with a high salt content than in
freshwater.
Carbaryl may enter marine systems when it is used to control
oyster pests and predators, such as oyster drills, mud shrimp, ghost
shrimp, and star fish (Loosanoff et al., 1960a,b; Lindsay, 1961;
Loosanoff, 1961; Snow & Stewart, 1963; Haydock, 1964; Haven et al.,
1966). However, Hurlburt et al. (1989) stated that tidal action
diluted the insecticide rapidly and that larvae are unlikely to be
subjected to high concentrations of carbaryl for more than several
hours.
The persistence of carbaryl in estuarine water was studied in
the laboratory by Karinen et al. (1967). Samples of water
containing 5, 10, or 25 mg carbaryl/litre and 3% NaCl were buffered
at pH 8 and kept in aquaria at 8 °C. In the absence of mud, the
concentration of carbaryl decreased 50% in 38 days, with most of the
decrease accounted for by the production of 1-naphthol. In another
experiment, naphthyl-14C and carbonyl-14C carbaryl (6.8-8.7 mC)
were used with cold carbaryl at total concentrations of 15 and
25 mg/litre, respectively, and maintained at 20 °C. About 50% of the
carbaryl was hydrolysed in 4 days and, after 17 days, the carbaryl
had almost disappeared with 43% converting to 1-naphthol.
Fluorescent light slightly accelerated the hydrolysis of carbaryl to
1-naphthol. When mud was added to the aquarium system, both carbaryl
and 1-naphthol in sea water declined to less than 10% of the initial
concentration in 10 days. Both compounds were adsorbed by the mud,
where decomposition continued at a slower rate than in sea water.
1-Naphthol persisted in mud for a short period of time, but carbaryl
could be detected for approximately 3 weeks. The naphthol moiety of
the carbaryl molecule was converted to more persistent products. The
experiment with labelled carbaryl demonstrated degradation by
hydrolysis of carbamate and oxidation of the naphthyl ring to
produce 14CO2 and 14CH4. In a preliminary field study,
estuarine mud flats were treated with carbaryl at 11.2 kg/ha (dose
used to control pests in oysters beds); carbaryl could be detected
in the mud for up to 42 days. 1-Naphthol concentrations were found
only after the first day, which may indicate that hydrolysis
proceeds slowly in mud.
The fate of 1-naphthol was studied in simulated marine systems
by Lamberton & Claeys (1970). 1-Naphthol was unstable in the
alkaline environment of sea water, since light and microorganisms
enhanced its degradation to CO2 and other products. 1-Naphthol was
relatively stable in an oxygen-free aqueous solution.
Odeyemi (1982) incubated water samples treated with 45 mg
carbaryl/litre under tropical greenhouse conditions (Nigeria).
According to colorimetric measurements, about 77% of the insecticide
had disappeared after 63 days of incubation in seawater, whereas it
had completely disappeared after 42 days of incubation in sewage
water, and after 60 days in freshwater samples.
4.2.7 Bioaccumulation/biomagnification
Because of its low persistence in water, and its even faster
degradation by living organisms, carbaryl has very low
bio-accumulation properties and presents no risk of biomagnification
under practical conditions.
Kanazawa (1975) studied the uptake and excretion of carbaryl by
Pseudorasbora parva. Fish were reared for 30 days in an aquarium
containing carbaryl at about 1 mg/litre. In water, 95% of the
carbaryl was degraded in 6 days. One day after treatment, residues
of carbaryl in P. parva reached 7.5 mg/kg, which was the maximum
uptake.
Kanazawa et al. (1975) studied the distribution and
metabolism of 14C-1-naphthyl-labelled methylcarbamate (carbaryl)
in an aquatic model ecosystem containing 10 kg soil, 80 litre water,
and catfish ( Ictalurus punctatus), crayfish ( Procambarus sp.),
daphnids ( Daphnia magna), snails (Physa sp.), algae ( Oedogonium
cardiacum) and duckweed ( Lemna minor). After 20 days, 31% of the
radioactivity was lost, 67.85% remained in the soil (about 45%
unextractable residues), 1.1% was present in water, and only 0.1%
was recovered in organisms. Bioaccumulation ratios were calculated
from radioactivity recovery and were likely to be low in the case of
animals. In the case of plants, they were apparently higher, but as
they are based only on radioactivity, including normal synthesis
reutilizing 14CO2 and not on real carbaryl measurement, they
should be considered as an overestimation of the true
bioconcentration factor for carbaryl (Table 6).
A flow-through bioconcentration study was conducted with
bluegill sunfish ( Lepomis macrochirus) exposed to 0.093 mg
carbaryl/litre for 28 days followed by a depuration phase in which
bluegill were held for 14 days in untreated water (Chib, 1986a; Chib
et al., 1986). Analysis of fillet, whole fish, and visceral
portions indicated a rapid uptake of carbaryl. Bioconcentration
factors ranged from 14x to 75x, for fillet and viscera,
respectively. Data suggest that fish stopped accumulating carbaryl
after day 7 of uptake, indicating that a steady state had been
reached.
Table 6. Accumulation of 14C radioactivity expressed as carbaryl
equivalenta by various aquatic organismsb
Carbaryl
Concentration in water 1.66 (1 day)
µg/litre 9.45 (22 days)
Concentration in soil 2.43 (start)
mg/kg 2.55 (22 days)
Organisms mg/kg BARc
Algae 37.9 4000
Duckweed 34.2 3600
Snail 2.81 300
Catfish 1.33 140
Crayfish 2.48 260
aCarbaryl not actually measured.
bFrom: Kanazawa et al. (1975).
cBioaccumulation ratio calculated by dividing mg/kg of dried
tissues by mg/litre in water at harvest.
By the end of the 14-day depuration period, over 90% of the
accumulated carbaryl was eliminated.
The persistence and accumulation of radiolabelled carbaryl
(technical, 99%), administered in either food or water
(flow-through), was studied in catfish ( Ictalurus punctatus) over
a 56-day period (Korn, 1973). Fish were removed periodically for
whole-body residue analysis. Intact carbaryl was not distinguished
from 1-naphthol. Accumulation from dietary carbaryl (2.8 mg/kg per
week) was 11 and 9 mg/kg tissue at 3 and 56 days, respectively. Fish
accumulated 1% or less of the available pesticide via dietary
exposure. The mean total accumulation of carbaryl (and/or metabolite
residues) after 56 days of exposure to water containing 0.25 mg
carbaryl/litre was 11 mg/kg. Fish exposed via the water retained
0.0001% carbaryl. Fish exposed to 2.8 mg/kg per week via diet
eliminated residues rapidly after they were placed on a
carbaryl-free diet for 28 days. However, residues remained constant
for 28 days in fish previously exposed to 0.25 mg carbaryl/litre
water for 56 days. The authors speculated that the greater
persistence of carbaryl residues from the water might be due to the
persistence in fish of 1-naphthol.
The low accumulation and rapid elimination of carbaryl in fish
were confirmed in other laboratory work (Kanazawa, 1975, 1981).
The biodegradation of carbaryl, was studied by Bogacka & Groba
(1980) in a model system simulating the river-water and aqueous
ecosystem conditions. The rate of pesticide decay in water depended
on the initial concentration, temperature, and the kind of model.
Lowering the temperature inhibited this process. The accumulation of
carbaryl in bottom sediments or in aquatic organisms (algae, snails,
and fish) was not observed at concentrations of 10 or 50 µg/litre.
Trace quantities of 1-naphthol could be detected occasionally.
4.3 Soil
4.3.1 Adsorption, desorption
Carbaryl is adsorbed on soil. Most Koc values were in the
range of 100-600, which corresponds to medium to strong adsorption.
Only in some soils from Eastern Australia was carbaryl apparently
less tightly bound (Koc 90-220). Taking into account both the
adsorption/desorption properties and the short persistence of
carbaryl in soil, it can be calculated that the compound has low to
moderate mobility in soil and will remain in the top layers, when
applied under actual agricultural conditions (normal dose rates).
Leenheer & Ahlrichs (1971) stated that fast adsorption rates for
carbaryl on soil particles were related to organic matter (OM) and
were of the order expected for physical adsorption.
Carbaryl desorption and movement in the soil was studied in 1-m
soil columns in glass tubes, which had a drain (LaFleur, 1976a).
Desorption by added water was rectilinear for carbaryl (soil range
1-200 µmol/kg). The movement of carbaryl in the column was a
function of the percentage of the organic matter. In soil containing
5.16% organic matter, carbaryl reached the 40-cm section of the
column only. When the organic matter content of the soil was low
(0.22-0.57%), about one-half of the added carbaryl moved through the
column and was found in the effluent, after addition of a volume of
water that was equal to six months' rainfall. Briggs (1981) also
reported that increasing levels of organic matter in soils resulted
in an increase in the adsorption of carbaryl.
On the basis of the comparative mobility of carbaryl on soil
thin-layer chromatography (TLC) plates using 4 US soils (silt loam,
loam, Norfolk sandy loam, and silty clay loam), Chib & Andrawes
(1985) concluded that the mobility of carbaryl is low to moderate.
With water elution equivalent to about 580 mm of rainfall, the major
portion of carbaryl was in the top 10 cm of soil, thus exhibiting
low mobility. Only 0.6% of the applied 14C was eluted with the
water. Approximately 70% of the applied carbaryl was degraded to
volatile gases during the 30-day incubation.
Aly et al. (1980) studied the adsorption of carbaryl at
different temperatures on Ca-bentonite and on two Egyptian soils
(Nile alluvial and a highly calcareous soil). Carbaryl adsorption
increased as the temperature decreased. Ca-bentonite exhibited the
highest degree of adsorption followed by the Nile alluvial soil and
the calcareous soil. The factors that contributed most to the total
adsorption of carbaryl were the soil contents of clay, calcium
carbonate, and organic matter.
The adsorption-desorption and mobility of carbaryl in 3 soils,
and in a stream sediment were studied by Sharom et al. (1980). The
order of adsorptive capacity was organic soil (75.3% OM, pH 6.1) >
sediment (2.8% OM, pH 6.6) > sandy loam (2.5% OM, pH 6.8) > sand
(0.7% OM, pH 7). Desorption occurred in greatest amounts from sand
> sandy loam > sediment. Leachability studies were consistent with
the adsorption/desorption results and a water solubility of
40 mg/litre. Carbaryl leached more from the sand than from the
organic soil.
In another adsorption/desorption study on carbaryl on sand,
sandy loam, silt loam, silty clay loam, and on aquatic sediment
using batch equilibrium techniques, Chib (1985a) concluded that
carbaryl binds strongly to soil/sediment matrices. This depends on
the carbaryl concentration in solution (the more in solution, the
more on soil) and on the organic content of soil. Desorption
isotherms for carbaryl at low concentrations were nearly flat with
all soils, indicating that carbaryl was tightly bound to the
substrates and difficult to move.
The transport of carbaryl in the soil was also studied under
natural conditions. High-volume rainfall occurring shortly after
carbaryl has been applied to a field can generate the low-level
transport of the pesticide to non-target areas (Caro et al.,
1974). Carbaryl is adsorbed on soil surfaces to a great extent.
Laboratory measurements of absorption isotherms gave a Freundlich k
value of 2.2. Of the 4 kg of carbaryl applied in a watershed, only
5.77 g (0.16%) were found in run-off water and sediment during the
season; (75 and 25% of the seasonal loss, respectively). The
distribution coefficient between sediment and water was 0.33.
Carbaryl had completely disappeared from the sediment by day 70, but
still remained in the water at low concentrations.
Carbaryl could still be found in the soil, 1-2 years after
application at 2 kg 85% WP/ha. It is carried by rainfall and
cultivation from the surface layers of the soil into the deeper
layers. During the first few days after application, it was found in
the root zone. After 3 months, 90% of the carbaryl was present in
the surface layers, and, after 300 days, it was found in the soil at
a depth of 60-70 cm. Rainfall was not specified (Molozhanova, 1968).
Carbaryl was applied to a sandy loam field plot at a dose of
25.4 kg/ha. A shallow water table was present at 1.1 m depth
(LaFleur, 1976b). Rainfall during the following 16 months was
182 cm. After this length of time, the upper 1 m contained 6% of the
applied carbaryl. No carbaryl was found in the 0-20 cm layer after
the fourth month. The half-life of carbaryl in the upper 1 m was >1
month. Carbaryl appeared in the underlying groundwater within 2
months following treatment and could be detected through the eighth
month. The maximum groundwater concentration occurred at the end of
the second month (about 60 µg/litre). The dose rate was more than 10
times the usual one and the persistence was overestimated, as well
as the residues that might be present in shallow groundwater.
Norris (1991) reported a terrestrial field soil dissipation
study conducted with carbaryl under actual use conditions. Carbaryl
was applied to broccoli in California at 2.24 kg/ha, five times, on
a weekly schedule. It was also applied once to sweet corn in North
Carolina at a rate of 7.12 kg a.i./ha. The half-life in soil was
estimated to be 6-12 days at the California site and about 4.8 days
at the North Carolina site. Carbaryl residues were found mainly at a
depth of 0-15 cm, which indicates that carbaryl has a low potential
for leaching through the soil profile under typical agronomic
practices.
4.3.2 Transformation
Degradation of carbaryl in soil occurs as a result of the
activity of microorganisms, and through physical and chemical
effects. It undergoes hydrolysis, oxidation, and other chemical
processes and, on the surface of the soil, is subjected to
photolysis. When applied at the usual doses, carbaryl has a short
persistence in soil. In the field, under temperate and warm climatic
conditions, the half-life of carbaryl in the soil does not exceed
one month (Table 7).
4.3.2.1 Photolysis in soil
Studies have been conducted on the photolysis of carbaryl in
soil. In one study, volatile substances were first identified and
quantified and then soil TLC plates were used over a 30-day period
to quantify degradation products (Chib, 1986b). The calculated
half-life of carbaryl in sandy loam soil on TLC plates irradiated
with a high-pressure mercury vapour lamp (200 watt, Hanovia) at
25 °C was 2.5 days. In controls kept in the dark, labelled carbaryl
degraded with a half-life of >30 days. CO2 was the only major
volatile degradation product from all treatments. The other soil
degradation products identified were 5-hydroxy carbaryl,
N-hydroxymethyl carbaryl and 1-naphthol. Only carbaryl and
1-naphthol were present in control soil extracts. The degradation
products in the irradiated soil extracts suggest that hydrolysis and
oxidation are the main mechanisms in the degradation of carbaryl
under soil photolysis conditions.
Das (1990b) also studied the photolysis of carbaryl in soil.
Labelled carbaryl was applied to a sandy loam on plates
(9.8±0.3 mg/kg) and maintained in artificial sunlight,
intermittently (12 h irradiation + 12 h darkness) for 30 days. The
measured irradiance of the artificial sunlight (502.6 watts/m2)
was comparable to the natural sunlight (545.8 watts/m2). The
evolution of volatile substances was also monitored. Controls were
incubated in the dark. The calculated half-life of irradiated
samples was 41 days (each day 12 h irradiation + 12 h darkness). No
major metabolites were formed under irradiated conditions.
4.3.3 Biotransformation in soil
The persistence and metabolism of 14C-carbaryl (200 mg/kg)
was studied in 5 different soil types by Kazano et al. (1972).
Persistence was influenced by soil type, and the production of
14CO2 varied from 2.2% (loamy sand) to 37.4% (clay loam) of the
initial radioactivity during 32 days of incubation at 25 °C. The
amount of radioactivity left in the soil after extraction was
proportional to the soil organic matter content. A significant
amount (17-57%) of the remaining radiolabel could not be extracted
with organic solvents. Analysis of the extractable residues
indicated the presence of 1-naphthyl N-hydroxymethylcarbamate,
4-hydroxy-1-naphthyl methylcarbamate and 5-hydroxy-1-naphthyl
methylcarbamate, but these were not confirmed. The main pathway of
degradation in soil was probably hydrolysis of the carbamate
linkage, producing CO2 and the corresponding phenol, though it is
possible that hydroxylation of the ring or the methyl carbon
precedes hydrolysis. 1-Naphthol decomposed more rapidly in clay than
in sandy loam. Most of the radioactivity (83-92%) was recovered in
the soils after 60 days of incubation. 1-Naphthol was immobilized on
humic substances in the soil, not by mechanical adsorption, but by
chemical bonding. Four metabolites (coumarin, the others not
identified) were also produced by a soil Pseudomonas.
Gill & Yeoh (1980) studied the degradation of carbaryl in an
extract of flooded paddy field soil (pH 3.7-4.8, organic matter
content 0.5-3%, very high clay content) extract and in the paddy
fish ( Trichogaster pectoralis). Under flooded conditions, the soil
half-life of carbaryl was about 7 weeks. The major significant
metabolite was 1-naphthol. Soil moisture plays an important role in
the extent of degradation since, under flooded conditions and 100%
field capacity, the degradation of carbaryl was more extensive than
under 0 and 50% field capacity, providing more evidence that
microorganisms play an important role in carbaryl degradation in
paddy-field soil. Where sufficient moisture is available, oxidative
mechanisms become important giving rise to ring and N-methyl
hydroxylation in addition to cleavage of the carbonyl moiety, a
common pathway of carbaryl metabolism. Carbaryl was also found to be
more persistent in acidic soil than in alluvial soil.
Rajagopal et al. (1983) measured the residues of carbaryl and
of the 1-naphthol metabolite in 3 flooded soils (organic matter
0.54-1.61%, pH 6.2-9.5) following three applications of the test
substance. Samples received either 1, 2, or 3 applications of
carbaryl in aqueous solution. The concentration of carbaryl
decreased with incubation time in all soils. The disappearance of
the first 50% of the substance in all soils ranged from 10 to 15
days. Subsequent samplings provided evidence for the enrichment of
carbaryl-degrading microorganisms in retreated soils. Thus, the
disappearance time for 75% of the compound was 20-26 days for 3
applications, 28-30 days for 2 applications, and >30 days for 1
application. It appears that carbaryl degradation under flooded
conditions does not follow a clear kinetic pattern (e.g., first
order). However, the first order degradation rates were similar for
the first, second, and third applications for 0-10 days. Degradation
proceeded by hydrolysis with 1-naphthol as the major product, the
amounts formed decreasing with incubation time in 2 out of the 3
soils. The disappearance of 1-naphthol was faster in retreated soils
and may not be attributed only to degradation, because significant
amounts of 1-naphthol may be bound to humic substances, especially
in soils with a high organic matter content.
The persistence of carbaryl was studied in 4 soils under
flooded conditions by Venkateswarlu et al. (1980). They recovered
a substantial portion of carbaryl from all soils 15 days after
application. The recovery ranged from 37% in an alluvial soil to 73%
in an acid sulfate soil. They concluded that flooded conditions
enhance carbaryl degradation.
The metabolism of 14C-carbaryl and 14C-1-naphthol in moist
and flooded soils was studied by Murthy & Raghu (1989) in a
continuous flow-through system, over a period of 28 days, permitting
a 14C-mass balance. The percentage distribution of radiocarbon in
organic volatile compounds, CO2, and extractable and
non-extractable (bound) fractions of soils was determined. Organic
volatile compounds could not be detected in either carbaryl- or
1-naphthol-treated soils. More 14CO2 (25.6%) was evolved from
moist than from flooded soil (15.1%), treated with carbaryl. The
mineralization of14C-1-naphthol was negligible. The level of
extractable radiocarbon was higher (5.5%) in flooded soil treated
with carbaryl. Less than 1% was the parent compound, and carbaryl
was mainly metabolized to 5-hydroxycarbaryl in moist soil and to
4- and 5-hydroxycarbaryl in flooded soil. The extractable
radiocarbon amounted to 18.2% and 24.3% in moist and flooded soils,
respectively, and there was less than 1% of the parent compound with
1-naphthol treatment. Most of the 14C was found as soil-bound
residues, levels being higher with 1-naphthol treatment than with
carbaryl. The humus fraction of the soil organic matter contributed
most to soil-bound residues of both carbaryl and 1-naphthol.
Aerobic and anaerobic soil metabolism studies were conducted
with carbaryl applied to sandy and clay loam soils in the dark
(Wilkes et al., 1977; Khasawinah, 1978). Under aerobic conditions
at room temperatures (23-25 °C), the half-life of carbaryl was
approximately 9-17 days in sandy loam soil (Texas) and 21-27 days in
clay loam (California). Lower temperature (15 °C) nearly doubled its
half-life. The only solvent-extractable apolar material was parent
carbaryl. A major proportion of the radioactivity initially applied
to the soil was lost as CO2. It appears that, under aerobic
conditions, carbaryl was degraded so that 1-naphthol carbon was lost
as CO2, or it was incorporated into the organic matter of the soil
(1-naphthol was not detected). After 112 days, carbaryl levels
declined to 3.6% (average in Texas soil) and 15% (average in
California soil) of the initial application.
In an aerobic, soil study conducted by Miller (1990) with a
sandy loam soil from North Carolina, soil was incubated with
labelled carbaryl in the dark and maintained at 25±1 °C. The
concentration of the parent compound rapidly decreased to a mean
value of 11.9% of the applied dose by day 14. A significant amount
of radioactivity was recovered as CO2. The average maximum value
of CO2 was 59.8% by day 14. The only other major degradation
product was 1-naphthol (maximum concentration of 34.9% of applied
dose by day 1). Base hydrolysis released 41% of the unextractable
residue. Carbaryl rapidly degraded under aerobic conditions with a
half-life of 5.5 days.
Murthy & Raghu (1991) studied the fate of carbaryl in the soil
environment as a function of pH, with respect to the formation of
extractable and non-extractable (soil-bound) residues. Soil samples
(sandy clay, sandy loam, and clay) containing C14-carbaryl
(10 mg/kg) were incubated at 28-30 °C for periods ranging from 7 to
56 days. More 14C-residues could be extracted from sandy clay and
sandy loam than from clay soil, under both moist and flooded
conditions. In general, flooding had no influence on the extractable
14C-residues. Thin-layer chromatography of chloroform extracts
revealed the presence of carbaryl and 1-naphthol. At the end of 56
days, the percentage of carbaryl recovered was 32.1, 6.4, and 1% in
sandy clay (pH 4.2), sandy loam (pH 6.8), and clay soils (pH 8.3),
respectively. The authors considered that there appeared to be a
correlation between soil pH and soil-bound residue formation as an
increase in soil pH was reflected in increased bound residues. The
humin portion of soil organic matter accounted for most of the
14C-residues. Low recoveries in sandy clay and in sandy loam soils
may stem from the possible mineralization of carbaryl.
Carbaryl persistence in the soil was studied under normal
conditions of application (Caro et al., 1974) of 5.03 kg/ha in
granules applied in corn seed furrows. About 135 days were required
for 95% of the carbaryl to disappear (Fig. 1). The pesticide
remained stable in the soil for 25 days (in some cases, more than
116 days) and then decayed rapidly. This decay is an indirect
indication of microbiological degradation.
This hypothesis is sustained by the finding that the stability
of carbaryl in the soil is affected by the nature of the treatments
applied during the period preceding its application. In soil that
had been treated with carbaryl 6 months before sampling, more than
70% of the added radiolabelled carbaryl was degraded after 4 days,
measured by the disappearance of radioactivity. Only 10% was lost in
orchard soil that had been treated for 15 years with various
pesticides, and only 3% was lost in soil that had never been treated
with pesticides. It appeared that soil that was treated with
carbaryl for 6 months increased the ability of the microorganisms to
degrade carbaryl (Rodriguez & Dorough, 1977).
Carbaryl was applied in soil at an agricultural rate and
incorporated to a depth of 6 inches (15 cm) (Heywood, 1975). At 28
days after treatment, 63% of the applied dose appeared as CO2 and
the identifiable residual carbaryl was about 6% of that applied.
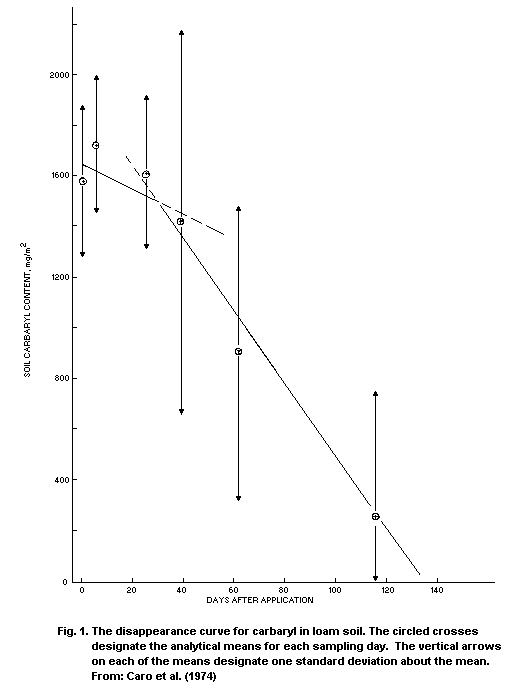 In India, degradation of carbaryl was studied in a greenhouse
by Brahmaprakash & Sethunathan (1984, 1985). Since a soil planted
with crops may be more dynamic and complex than unplanted soil,
because of increased microbial activity, they studied carbaryl
persistence in soil (pH 6.2, organic matter 1.6%) planted with rice
in a greenhouse, under flooded and non-flooded conditions. Carbaryl
disappeared more rapidly from soils planted with rice than from
those without rice, under both flooded and non-flooded conditions.
The amount of carbaryl decreased to between 30.2 and 32.1% of the
original level within 30 days in unplanted soil under both flooded
and non-flooded conditions. During this same period, the carbaryl
concentration decreased to 17-18% of the original level in planted
soil under both water regimes. Degradation occurred by hydrolysis,
but there was no appreciable difference in the rates of degradation
between flooded and non-flooded soils. The rate of degradation of
carbaryl was little affected by moisture. Further degradation of
1-naphthol was slow in both planted and unplanted systems. A
significant portion of the ring-14C accumulated in the soil as
1-naphthol and soil-bound residues. Evolution of 14CO2 from the
labelled side-chain and ring was negligible, even in soil planted to
rice.
4.3.4 Degradation by microorganisms
Many studies have demonstrated the great ability of
microorganisms to degrade carbaryl in the soil, and for some of
them, to use it as a sole source of carbon. The most frequently
identified organisms are bacteria ( Pseudomonas phaseolicola, P.
cepaphia, Rhodococcus sp., Nocardia sp., Xanthomonas sp.,
Achromobacter sp.) and fungi ( Aspergillus niger, A. terreus,
Fusarium solani, Gilocladium roseum, Rhizoctonia solani, R.
practicola, Penicillium sp., Mucor sp., Rhizopus sp.). In many
cases, it was demonstrated that the persistence of carbaryl in the
soil decreased after a first application, which was interpreted as
the selection and build-up of strains more capable of degrading the
product. The same process may also explain that, in some studies,
the rapidity of degradation suddenly rose after a lag period during
which only minor amounts of carbaryl degraded.
Tewfik & Hamdi (1970) mentioned that carbaryl was decomposed
into 4 distinct compounds by a soil bacterium designated as S-1.
They considered that this soil bacterium might utilize carbaryl in a
similar way to Pseudomonas sp., which metabolize naphthalene via
the salicylate pathway.
Zuberi & Zubairi (1971) also reported that carbaryl is degraded
by soil microflora. Pseudomonas phaseolicola and Aspergillus
niger hydrolysed carbaryl to 1-naphthol. In the case of P.
phaseolicola, an unidentified minor metabolite was also detected.
Larkin & Day (1986) reported that 2 bacteria isolated from
garden soil, Pseudomonas sp. (NCIB 12042) and Rhodococcus sp.
(NCIB 12038), could grow on carbaryl as the sole source of carbon
and nitrogen at pH 6.8, but failed to metabolize carbaryl rapidly.
Both could use 1-naphthol as the sole source of carbon and
metabolize it via salicylic acid. Strain NCIB 12038 produced also
gentisic acid. Pseudomonas sp. (NCIB 12043), in a soil perfusion
column enrichment at pH 5.2, metabolized carbaryl rapidly to
1-naphthol and methylamine. 1-Naphthol itself was metabolized via
gentisic acid. A possible pathway for the catabolism of carbaryl and
1-naphthol was proposed.
Walker et al. (1975a), working with Pseudomonas sp.,
observed degradation of 1-naphthol and suggested that carbaryl could
serve as the sole source of nitrogen and carbon for bacteria. It was
also noted that the presence of another nitrogen source in the media
seemed to have a delaying effect on metabolism of carbaryl.
Bacterial communities of at least 12 and 14 members were
selected in continuous culture using carbaryl as the sole source of
carbon and nitrogen at pH 6. These communities were supported by the
slow formation of hydrolysis products and no carbaryl-degrading
bacterium was selected after more than 83 days. When using equimolar
1-naphthol and methylamine as the sole source of carbon and
nitrogen, a bacterial community of at least 8 members was selected.
After a lag of 10-50 days, soil perfusion columns (pH 5.2) and
continuous culture enrichments (pH 5) led to the selection of a
Pseudomonas sp. that could utilize carbaryl as its sole carbon and
nitrogen source (Larkin & Day, 1985).
Sud et al. (1972) showed that Achromobacter sp. also
utilized carbaryl as the sole source of carbon in a salt medium.
Four degradation products of carbaryl were 1-naphthol, hydroquinone,
catechole, and pyruvate. The organism also grew well in the first 3
degradation products.
The rate of degradation of carbaryl after 1, 2, and 3
applications to 3 submerged soils was examined by Rajagopal et al.
(1983). Soils that had been pretreated with carbaryl were able to
degrade carbaryl more rapidly than those without pretreatment. The
enrichment culture was inactivated upon autoclaving. The
concentration of carbaryl decreased in the mineral medium inoculated
with the enrichment cultures from the 3 soils, especially when it
served as the sole source of both carbon and nitrogen.
Rajagopal et al. (1984) studied the metabolism of side-chain
and ring 14C-labelled carbaryl in a mineral salts medium by soil
enrichment cultures. Hydrolysis was the major route of microbial
degradation. During carbaryl degradation by enrichment cultures and
Bacillus sp., 1-naphthol and 1,4-naphthoquinone accumulated in the
medium.
Rajagopal et al. (1986) also observed that carbaryl
disappeared more rapidly from a laterite soil pretreated with
1-naphthol than from a control soil never exposed to 1-naphthol. The
accumulation of 1-naphthol and bound residues formed from
added14C-carbaryl was greater in soils pretreated with 1-naphthol
than in untreated soils.
In experiments with the soil fungus Rhizoctonia practicola,
Bollag et al. (1976) found that it could transform 1-naphthol from
an ether-extractable to a water-soluble product. It was also
observed that, after removal of the fungal cells, the growth medium
possessed the ability to transform 1-naphthol, indicating activity
of an extracellular enzyme. Attempts to analyse the labelled
material in the aqueous phase indicated that the radioactivity was
associated with a compound of comparatively high relative molecular
mass.
Czaplicki & Bollag (1975) exposed 1-naphthol to Rhizoctonia
solani, isolated from soil, and found that it was completely
transformed to a compound not extractable with ether.
Working with the soil fungus Aspergillus terreus, Liu &
Bollag (1971b) investigated the metabolic transformation of carbaryl
through 1-naphthyl- N-hydroxymethylcarbamate and tried to clarify
the pathway of the side-chain. The next intermediate in biological
transformation was 1-naphthyl carbamate, which was further degraded
to 1-naphthol. Within one week, approximately half the amount of
carbaryl was transformed to these other metabolic products. No
attempt was made to clarify whether the formation of 1-naphthol was
the result of the biological or the chemical degradation of
1-naphthylcarbamate, but there were clear indications that
1-naphthol was metabolized further in the presence of A. terreus.
In vitro studies were carried out to investigate the
degradation of carbaryl by soil microorganisms. Three isolates from
soil, including Fusarium solani, a Gram-negative coccus, and a
Gram-positive rod, accelerated the hydrolysis of carbaryl to
1-naphthol and other intermediates. Fusarium solani was the most
effective in decomposing the 14C-labelled compound. Radioactivity
decreased by 24% during the first 5 days and by 82% during 12 days
in a growing culture after inoculation. A mixture of two or three of
the microorganisms was more effective in decomposing carbaryl and
1-naphthol (Bollag & Liu, 1971).
Bollag & Liu (1972a) studied the biological degradation of
14C-1-naphthol during growth, with replacement cultures and cell
extracts of Fusarium solani. The radioactivity of 1-naphthol
disappeared partially during growth, but it was completely
dissipated by cell extract activity. The cell extract of F. solani
degraded more than 80% of the 1-naphthol to form CO2 within 60 min
of incubation, but it was not possible to identify intermediates.
This implies rupture of the naphthol ring. No difference in activity
could be observed between cell-free extracts from the spores or the
mycelium of the fungus at pH 5.7 and 7.2. The enzyme system was
relatively stable since there was no decrease in activity after the
cell extract was stored at -10 °C during 4 months. The enzyme that
participated in the degradation reaction was constitutive (always
present) as shown by cell extracts prepared from cells grown in a
medium with, or without, carbaryl as substrate.
The fungus Gliocladium roseum, isolated from soil by Liu &
Bollag (1971a), metabolized carbaryl to 3 metabolites which were
identified as 1-naphthyl- N-hydroxy methylcarbamate,
4-hydroxy-1-naphthyl methylcarbamate, and 5-hydroxy-1-naphthyl
methyl-carbamate. It was therefore considered that N-alkyl- and
aromatic ring-hydroxylation of carbaryl were important detoxication
reactions of G. roseum. About 70% of the radioactivity was
recovered as carbaryl from an 11-day-old culture. The decrease in
radioactivity from the growth medium containing side-chain labelled
carbaryl indicated that a further degradation of the formed
metabolites occurred, or that an additional pathway was involved in
the degradation of carbaryl by G. roseum.
Bollag & Liu (1972b) reported that most soil fungi
( Penicillium sp., Mucor sp., Rhizopus sp., and Aspergillus
sp. except A. fumigatus), could hydroxylate carbaryl at different
positions, but that the products differed qualitatively as well as
quantitatively with the various fungi.
In a different study, the enzyme or protein fraction
(extracellular phenol oxidase) of the culture filtrate from
Rhizoctonia practicola as chromatographed on a column of Sephadex
G-200 and fractions were obtained that were able to transform
1-naphthol as a substrate (Sjoblad et al., 1976). The enzyme
catalysed the polymerization of 1-naphthol to several products.
Biological oxidation and coupling of phenols are key reactions
in nature, that result in the formation of products such as lignins,
melanins, tannins, alkaloids, and humus compounds. Thus, it can be
assumed that enzymes interact with xenobiotic phenols that are
incorporated into soil or sediment organic matter. Rhizoctonia
practicola (and an extracellular enzyme) was able to polymerize
1-naphthol (dimers, trimer, tetramer) (Bollag et al., 1978).
Rodriguez & Dorough (1977) studied the persistence of carbaryl
in culture media in the presence of mixed and pure cultures of
bacteria and fungi isolated from soil. All fungi ( Fusarium,
Penicillium, Aspergillus, and one unidentified species), isolated
from a soil treated with carbaryl six months before sampling,
produced at least one metabolite from carbaryl. After 14 days of
incubation, 54-79% of the carbaryl was recovered intact, and there
was little formation of carbon dioxide by the fungi. In contrast,
related experiments with bacterial isolates ( Arthrobacter,
Nocardia, Pseudomonas, Xanthomonas, and Bacillus) from the same
soil showed that only 1-9% of the added carbaryl remained
unconverted ( Arthrobacter was the exception with 59.1% remaining
carbaryl). Controls showed that non-biological degradation also
occurred. Bacteria, like fungi, metabolized carbaryl qualitatively
in the manner observed with natural soil populations. However,
quantitative differences were so great that the use of isolates may
be of little value in estimating the degradation rate of carbaryl
and other pesticides in field soil. This is important to note when
conducting microbial degradation studies in the laboratory.
Bollag (1979) summarized the transformations of carbaryl by
microbial activity. It was possible to isolate several
microorganisms capable of hydrolising carbaryl to 1-naphthol. In
addition, Gliocladium roseum showed the formation of:
4-hydroxy-1-naphthyl N-methylcarbamate
5-hydroxy-1-naphthyl N-methylcarbamate
1-naphthyl N-hydroxy-methylcarbamate.
Other terrestrial fungi hydroxylated carbaryl at different
positions, but the products differed quantitatively and
qualitatively with the various fungi. Subsequent metabolic
transformation of the hydroxylated products differed considerably.
With A. terreus, the following products were obtained:
1-naphthyl N-hydroxy-methylcarbamate -->
1-naphthyl-carbamate --> 1-naphthol
Several bacteria isolated from river water were capable of
degrading 1-naphthol. At least two different pathways were involved.
The first was a complete degradation with the release of CO2, the
second produced principally 4-hydroxy-1-tetralone, which may involve
hydroxylation of the naphthyl ring in the 4 position and conversion
to an aliphatic cyclic compound. Such a pathway has also been
described with a soil Pseudomonas by Walker et al. (1975a) and
Davis & Evans (1964).
4.3.5 Persistence in soil
Carbaryl is not usually applied as a soil treatment, therefore,
the amounts of carbaryl that may reach the soil come principally
from spray drift, or from washing off treated crops by rain.
When applied at the usual doses, in the laboratory, carbaryl
has a short persistence (half-life <40 days and usually from 6-20
days), but this may increase when the soil is flooded, or when the
dose is increased. In field studies, the half-life of carbaryl,
applied at the usual dose rates, in warm or temperate climatic
conditions, did not exceed one month in the soil. The results of
laboratory and field studies on the persistence of carbaryl in soil
are summarized in Table 7.
Table 7. Half-life of carbaryl in soil, calculated from experimental laboratory
and field results
Origin of soil Number Degradation Half-life References
of days (%) (days)
Laboratory 40 100 8 Johnson & Stansbury (1965)
Laboratory 0.5 mg/kg 45 77 20 Molozhanova & Kanevskij (1971)
Laboratory 1 mg/kg 45 53 40
Laboratory 10 mg/kg 45 13
Laboratory 30 55-63 < 20 Flores-Ruegg et al. (1980)
Laboratory (flooded) 15 63-27 10-40 Venkatesvarlu et al. (1980)
Laboratory 53 100 < 8 Odeyemi (1982)
Orchard 184 99 30 Ivanova & Molozhanova (1973)
Corn 135 95 30 Caro et al. (1974)
Pretreated soil 4 70 < 3 Rodriguez & Dorough (1977)
Grain cropa 30 53-31 27-55 Gangwar et al. (1978a)
Corn soilb 5 42-56 5 Kavadia et al. (1978)
Potatoes 50 < 99 < 7 Kovaleva & Talamov (1978b)
- 30 96 6
Table 7 (continued)
Origin of soil Number Degradation Half-life References
of days (%) (days)
Unspecified 90 Czaplicki (1979)
Tropical 7 Rajukkannu et al. (1985)
Sesamum 60 56 50 Yadav et al. (1985)
Bare soil < 4 Davis (1986a)
Bare soil 1-13
Forest 1.5 Sundaram & Szeto (1987)
Forest 90 < 78 < 38 Springborn (1988b,c)
Forest 90 < 90 < 24
Flooded rice 180 (56) Springborn (1988a)
Flooded rice 180 (52)
Broccoli 6-12 Norris (1991)
Sweet corn 5
a20-60 kg/ha.
b15-45 kg/ha.
Johnson & Stansbury (1965) calculated a half-life of
approxi-mately 8 days in an agricultural soil (sandy loam, pH 5.5)
treated at 3 concentrations. Residues of carbaryl appeared to be
completely degraded within 40 days.
The considerable influence of the dose rate on the persistence
of carbaryl in soil was demonstrated by Molozhanova & Kanevskij
(1971). The percentages of the initial quantities that were degraded
45 days after treatment are shown in Table 8.
Table 8. Degradation rate of carbaryl in relation to the dose
Initial dose Degradation (%)
(mg/kg)
0.5 77
1 53
1.5 37.3
2 30
2.5 27
3 23
4 20
6 16.7
8 15
10 13.2
The same authors also demonstrated the influence of the type of
soil on the degradation of carbaryl. Forty-five days after
treatment, about 50% degradation was observed in light grey forestry
soil, and only about 20% in peat. The ability of soils to degrade
carbaryl could be ranked as follows: light grey > grey forestry >
turf podzol > southern chernozeme > ordinary chernozeme > meadow
> peat.
Ivanova & Molozhanova (1973) calculated that application of
1.36 kg carbaryl/ha in an orchard resulted in 19.7% being retained
in the soil; 99% of the carbaryl present in soil disappeared within
184 days.
Czaplicki (1979) conducted studies in Poland. The soil
half-life of carbaryl was about 3 months and 90% degradation
occurred within 18 months. From October to March, when the soil
temperature was <5-8 °C, the disappearance of the insecticides in
the soil almost stopped.
Rajukkannu et al. (1985) studied the persistence of 4
products in the red and black soils of Tamil Nadu (India), where the
tropical climate in conjunction with the soil properties shortened
the persistence of insecticides. The half-life of carbaryl was 6.7-7
days and 95% degradation was achieved after 90 days.
It was reported by Odeyemi (1982) that carbaryl disappeared
from soil samples treated with 45 mg/kg after 53 days incubation in
a greenhouse under tropical conditions (Nigeria).
4.3.6 Interaction with other physical, chemical, or biological
factors
Nitrogen fertilizers (ammonium sulfate and urea) increased the
persistence of carbaryl in flooded laterite soil with a low native
content of nitrogen (0.04%). In the alluvial soil with 0.11% total
nitrogen, the persistence of the insecticides was little affected.
The rates of degradation in the two soils treated with nitrogen
fertilizers were almost identical. The authors speculated about the
mechanism of this effect, which was possibly due to the preferential
utilization of inorganic nitrogen by microorganisms, or to the
inhibition of soil hydrolase activity (Rajagopal & Sethunathan,
1984).
4.3.7 Vegetation
4.3.7.1 Uptake and transformation in plants
Carbaryl from soil treated at about 50 times the usual dose
penetrated into apple trees and couch-grass (Molozhanova, 1968).
Three months after application of carbaryl (85%) to soil at a dose
of 100 mg/kg, residues of carbaryl were detected in the roots and
stems in apples (39-13 mg/kg) and in couch-grass (50-38 mg/kg).
Under the same conditions, no residues were detected in tomato
fruits or in wheat grain, but 5.9 mg/kg were detected in potatoes
(Table 9).
Table 9. Migration of carbaryl from the soil into apple trees at
different times following applicationa
Experimental Sampling day Carbaryl content of samplesb
conditions after (mg/kg)
application
Soil Foliage Fruits
85% WP carbaryl 5 9.3 0 -
applied under each
tree at a rate of 12 6.8 0 -
10 mg a.i./kg soil
19 6.0 5.0 -
56 4.4 0.04 4.4
138 2.1 0 -
aFrom: Molozhanova (1968).
bAverage data from 18 samples are given for each case.
Huque (1972) reported that 14C-labelled carbaryl was readily
taken up from granules by 2-week-old rice plants. After 3 days, a
level of about 100 mg/kg was determined in the plant (excluding the
roots). Residues then decreased to about 15 mg/kg after 18 days.
Ferreira & Seiber (1981) studied the uptake and distribution of
carbaryl in rice seedlings following exposure of the roots to the
insecticide. Carbaryl was rapidly absorbed and transported upwards
to the leaves and stems. After exposure was terminated, the major
route of loss of carbaryl was through root exudation (22%) rather
than volatilization from the leaves (4.2%). However, since most of
the remaining carbaryl in the plant was present on the leaf
surfaces, the authors surmised that volatilization might play a
greater role in pesticide loss over longer periods of time.
14C-carbaryl and 14C-1-naphthol from soil-bound residues
were partially released when barley was grown (Murthy & Raghu,
1988). 14C-residues could be detected in both shoots and roots in
the case of carbaryl treatment, while only roots showed
14C-residues in the case of 1-naphthol.
The rate of decomposition of carbaryl in plants depends on the
climatic conditions. It is more rapidly decomposed in hot climates,
at high temperatures, and by intensive ultraviolet radiation. Thus,
residual levels in feed plants were lower in regions with a hot
climate than in other regions (Atabaev, 1972).
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Environmental levels
5.1.1 Air
Ambient air concentrations of carbaryl were measured before and
after a treatment of a large area of forest in Maine, USA, to
control spruce budworm. Concentrations in air ranged from less than
0.0035 to 0.107 µg/m3 several miles away (Shehata et al., 1984).
Room concentrations of airborne carbaryl were measured
following application for pest control as a 5% dust at an
undetermined rate. The levels detected on the day of application,
and 1, 2, or 3 days after application were 1.3, 0.2, 0.1, and 0.01
µg/m3, respectively (Wright et al., 1981).
5.1.2 Water
Carbaryl concentrations in water from different sources in the
USSR were studied by Molozhanova (1970). Data are presented in Table
10.
Table 10. Concentrations of carbaryl in watera
Type of sample Number of samples Mean
Total Carbaryl-containing concentrations
samples (%) (mg/litre)
Well 114 87 0.13
Artesian water 64 70 0.16
Ground water 24 75 0.27
Dam 90 47 0.02
River 77 62 0.044
Lake 84 85 0.03
aFrom: Molozhanova (1970).
According to an earlier report by Molozhanova (1968), carbaryl
was present in trace amounts up to a maximum level of 1 mg/litre in
well water and reservoirs during carbaryl application (6 kg/ha, 85%
WP).
Water irrigation channels, situated at a distance of 200-300 m
from a carbaryl-treated cotton field, contained from 0.01 to
0.25 mg/litre up to 18 days after spraying at a dose of 2kg/ha
(Guseynov, 1970).
Residues of pesticides were monitored in the aquatic system of
Ioannina basin (Greece) and its natural outlet, Kalamas River, for
the period September 1984-October 1985 (Albanis et al., 1986). The
carbaryl concentration in water was found to follow a seasonal
fluctuation with maxima during summer and minima during winter
months. Mean and range values are summarized in Table 11.
Table 11. Mean and range values of carbaryl (ng/litre) on different
sampling dates in the Ioannina basin
Month of sampling River Canal Lake
September 0.5
November NDa NDa
March NDa
May ND
June NDa
July 23.7 21.7
August 2.3
September 1.7 NDa
April NDa
July 8.8
aND = not detected.
An oil-based, carbaryl formulation (Sevin-2-oil) was applied
twice to a coniferous forest in New Brunswick (Canada). At a dose
rate of 280 g/ha, the highest concentration in stream water was
0.314 mg/litre and only 0.123 mg/litre 0.5 h after spraying. More
than 50% of the initial residues dissipated within 1 h (Sundaram &
Szeto, 1987).
Carbaryl was applied aerially as Sevin-4-oil (1.12 kg/ha) to
aforest in Montana. Field samples of water were collected to study
persistence. Residue levels in 4 streams sampled were variable
(3-6, 3-175, 2-260, 4-108 µg/litre) 1.3-2.8 h after application
(Pieper & Roberts, 1978; Pieper, 1979). Local accumulations of
floating oil-based spray may have caused this variation. Grantham
(1980) reported that carbaryl (Sevin-4-oil) levels were generally
highest directly after spraying, but increased at some stream sites
for 1-2 days after application. This was attributed to light rains,
which washed the spray residue off the foliage and into the stream
channel. However, Brown (1980) concluded that carbaryl enters
streams in periodic doses and that rainfall dates are not related to
the presence of residues in streams. Maximum residues of
26.32 µg/litre were found in water 12 h after spraying, with no
residues detectable in samples taken hourly until 36 h. Residues
were found again in samples taken 2.5-3 days and 5.5-6.5 days after
spraying.
In a study where carbaryl (Sevin-4-oil) was applied aerially
(0.84 kg/ha) to a forest in Maine, in 1978, samples were collected
in 9 streams (Stanley & Trial, 1980). Peak concentrations occurred
shortly after spraying and maximum residue levels ranged from 0.93
to 7.8 µg/litre in brooks and from 0.44 to 2 µg/litre in rivers. In
one stream, the maximum concentration was 16 µg/litre. Ott et al.
(1981) reported that carbaryl levels were highest on the day of
spraying (2.7 µg/litre), declined to about 0.7 µg/litre by the third
day, but increased again to about 1.2 µg/litre on day 5. No residues
were detected in stream water samples taken on the 17th day.
Thomas et al. (1982) treated planted rice fields with
carbaryl at rates of 0.63, 0.94, and 1.85 kg a.i./ha, either as a
high volume spray (500 litre/ha) or as a low volume spray (150
litre/ha), and monitored the residues. In irrigation water sampled
one hour after treatment, carbaryl levels amounted to 0.15-0.30 and
0.07-0.18 mg/litre, under high and low volume spraying,
respectively. During the first 24 h after spraying, the
concentration in the irrigation water remained fairly constant, but,
by the fourth day, it had decreased significantly. By the tenth day
after spraying the residues in water had decreased to 0.02-0.05 and
0.03-0.04 mg/litre, respectively.
A rice field dissipation study was conducted in Arkansas (USA)
by Springborn (1988). Carbaryl (Sevin XLR) was applied 3 times at
14-day intervals to an irrigated field plot in which rice was
planted. In irrigation water, carbaryl residues disappeared with a
half-life of <1 day after each treatment. The maximum concentration
in water was 1466 µg/litre and, by day 3 following each treatment,
the concentration was <121 µg/litre. In a similar study conducted
in California, Springborn (1988a) found that carbaryl dissipated
rapidly in irrigation water with a half-life of <1 day. The maximum
concentration in water was 648 µg/litre. By day 3 following each
treatment, the concentration was <27 µg/litre.
Residues of 1-naphthol were found in the water collected from
wells and ponds in and around Bhopal (India). The residues of
1-naphthol in well water (near a manufacturing plant) ranged from
0.002 to 0.024 mg/litre. In pond waters, the levels were found to be
between 0.036-0.098 mg/litre. The level of 1-naphthol in soil
samples was of the order of 0.153-0.656 mg/kg (Dikshith et al.,
1990).
5.1.3 Soil
Contamination of the soil occurs when carbaryl is used in
agriculture. During the treatment period, levels of 1-3 mg
carbaryl/kg were measured in the surface layer of the soil in the
USSR (Molozhanova, 1968). Concentrations of 0.03-0.35 mg/kg were
found in the surface layer of the soil up to 52 days after a single
application of carbaryl at a rate of 2 kg/ha (Guseynov, 1970).
Carbaryl levels in the soil varied depending on the type of soil
(Table 12).
Potatoes were sprayed with carbaryl at 4.25 kg/ha (5 kg Sevin
WP, 85%/ha). One day, 10 days, and 2 months after treatment,
residues in the soil under the potatoes were, respectively, 1.9,
9.55, and 0.02 mg/kg (Kovaleva & Talamov, 1978b). When the soil was
sprayed before sowing, the residues in the soil 1 day, 1 month, 4
months, and 15 months after treatment were 1.5-2, 0.05-0.07,
0.02-0.08, and 0.06 mg/kg, respectively. Within 100 and 150 days
following soil incorporation of carbaryl (Sevin, 10 kg/ha), residues
in non-planted soil decreased to 0.05 and 0.02 mg/kg, respectively
(Kovaleva & Talanov, 1980).
Table 12. Carbaryl content in different types of soil during
harvestinga
Type of soil Number of Carbaryl concentration
samples (mg/kg)
Meadow-chernozem 200 2.22 ± 0.5
Chernozem southern 766 1.01 ± 0.23
Chernozem ordinary 288 1.09 ± 0.26
Chenozem podzol 100 0.15 ± 0.04
Turf-podzol 150 0.04 ± 0.01
Grey-woodland 246 0.0
aFrom: Molozhanova (1970).
Yadav et al. (1985) detected 0.22 mg carbaryl/kg in the soil
at harvest time, when sesamum ( Sesamum indicum) was sprayed twice
with carbaryl (0.2%) on days 45 and 60 of crop growth (21 August and
5 September). As residues in the soil after the first spray were
about 0.65 mg/kg, the rate of reduction of residues in soil was 56%
within 2 months. Shilova et al. (1973) measured about 0.1 mg
carbaryl/kg soil one month after treatment with carbaryl (5 kg/ha)
against blood sucking insects in the subarctic.
Gangwar et al. (1978b) applied a 4% granular formulation to
sandy loam planted with a grain crop (bajra) at very exaggerated
application rates of 20, 40, and 60 kg a.i./ha. Initial residues of
140-354 mg/kg decreased by a total of 31 to 53% within 30 days and
95 to 98% within 90 days. Residues at 90 days were 2.68-17.49 mg/kg.
Application of carbaryl granules at very high doses of 15, 30,
and 45 kg/ha resulted in deposits of 232, 397, and 525 mg/kg,
respectively, in soils cultivated with corn, and in 104-109,
168-217, and 304-422 mg/kg, respectively, in clay loam soils
cultivated with root crops (beet, radish, carrot). The dissipation
rate of carbaryl residues from clay loam soil, in 5 days following
the treatment, was 42-56% during autumn and 55-69% during spring.
The highest residue levels sampled from all plots decreased to
229.7, 142.9, 73.8, 33.8, 6.7, and 1.3 mg/kg on days 5, 10, 15, 30,
60, and 100, respectively. Residues were below detectable levels in
several plots by day 60 and in most plots by day 100 (Kavadia
et al., 1978).
According to Kuhr et al. (1974), the dissipation of carbaryl
in the soil of apple orchards is rapid. Soil residues of carbaryl
had almost completely disappeared from the top 2 inches of soil in 2
weeks. They were 13.8 mg/kg immediately after application and
3 mg/kg, 1-2 days later.
An oil-based carbaryl formulation (Sevin-2-oil) was applied
twice by a fixed-wing aircraft to a coniferous forest in New
Brunswick (Canada). Initial residue levels 1 h post-spray in litter
and soil for both applications were, respectively, 1.21 and
0.86 mg/kg and 0.78 and 0.48 mg/kg (Sundaram & Szeto, 1987).
Relative to the amount sprayed, only a small amount of the chemical
reached the litter and soil, probably because of canopy filtration.
Within 1 day, an average of 40-45% of the initial residues was lost
from litter and soil, respectively, indicating a rapid dissipation
time of 1.5 days for the disappearance of 50% of the initial maximum
concentration. Beyond 5 days, an average of 12% of the initial
concentration remained in both the substrates.
As already reported, two field dissipation studies were
conducted by Springborn (1988b,c) with carbaryl (Sevin-4-oil)
applied twice with a seven-day interval to forest. The first study
was conducted in a coniferous Oregon forest. In soil from the
treated site, carbaryl levels decreased from 0.196-3.877 mg/kg
following the first application to 0.130-1.87 mg/kg, 3 days after
treatment, and from 0.079-5.323 mg/kg following the second
application to 0.242-1.187 mg/kg at 90 days. The second study was
conducted in Pennsylvania. Carbaryl levels in soil decreased from
0.022-0.068 mg/kg following the first application to
<0.012-0.075 mg/kg, 3 days after treatment, and from
0.11-0.932 mg/kg following the second application to
0.01-0.099 mg/kg at 90 days.
A rice field dissipation study was conducted in Arkansas (USA)
by Springborn (1988a). Carbaryl (Sevin XLR) was applied 3 times, at
14-day intervals, to an irrigated field plot in which rice was
planted. In flooded soil, the concentration of carbaryl varied from
<11 to 309 µg/kg throughout the study and was not directly related
to application date. The carbaryl level was <11-309 µg/kg in soil
following the first application and <11-129 µg/kg following the
third application; 180 days later, carbaryl concentrations in the
soil were 11-56 µg/kg.
In a similar study conducted in California, under the same
conditions, Springborn (1988) found that carbaryl dissipated rapidly
in flooded soil. The concentration of carbaryl ranged from <11 to
198 µg/kg throughout the study. Carbaryl was <11-23 µg/kg in soil
at sampling intervals following the first application, and increased
to <11-180 µg/kg following the third application; 180 days after
the third application, carbaryl concentrations in the soil were
11-88 µg/kg.
A soil dissipation field study was conducted in 1985/1986 in
California (sandy loam, pH 6.3) and Iowa (silt loam, pH 6.5) with
carbaryl 80 S formulation (Davis, 1986a). Carbaryl was applied to
strip plots of bare soil, 6 weeks later, 7 applications were made
with intervals of about 5 days between applications. Soil sampling
was performed before treatment, during treatment, and up to 6 months
after the last application. No carbaryl residues were found in
California pretreatment samples. After the last application,
residues reached a maximum of 1.61 and 0.29 mg/kg in the 0-15 and
15-30 cm samples, respectively. Fourteen days after the last
application, carbaryl residues below the 15-cm level were less than
the limit of quantification.
It took 28 days for residues to dissipate completely from the
top 15 cm. The half-life in California soil was calculated to be
2.7-3.8 days.
5.1.4 Food and animal feed
5.1.4.1 Fruit, vegetables, and grain
Contamination of vegetation by carbaryl occurs, either during
spraying, or, by its migration through contaminated soil into the
roots of plants.
Residual levels of carbaryl after the spraying of plants depend
on the type and species of the plants sprayed. Carbaryl levels of up
to 30 mg/kg have been found in plants during the treatment period
(Molozhanova, 1968). It has been found to be rather persistent in
vegetables and fruits. Concentrations of 0.6-3.9 mg/kg were measured
in lettuces 1 week after single or repeated sprayings, and levels up
to 1 mg/kg were found in tomatoes treated according to requirements
(Antonovich, 1970). In cabbage, initial residues after spraying
ranged from 14.8 to 33.9 mg/kg, depending on the concen-tration
used. After 7 days, the residues decreased to 2.5-5.13 mg/kg. In
eggplant, the initial concentrations were 8.3-16.9, and 7 days
later, 3.05-5.4 mg/kg. After washing, the concentrations decreased
considerably to less than 3 mg/kg after day 7 (Mann & Chopra, 1969).
Following application of carbaryl on cauliflower, in the form
of dust (10%) at 1.5-2 kg a.i./ha or wettable powder (50%) at 0.75-1
kg a.i./ha, the residues of carbaryl declined to 3.64-9.59 mg/kg
within 8 days of treatment. The carbaryl deposits on leaves were
between 19.45 and 42.08 mg/kg. Washing of cauliflower with plenty of
water reduced the residues of carbaryl by 36-95%. A waiting period
of 8 days has been suggested for cauliflower (Singh et al., 1978).
Persistence of carbaryl in brinjals and peas after spraying at
1 and 2 kg a.i./ha was found to be 3.42 and 5.03 mg/kg respectively.
Washing of vegetables with water within one hour and after a day of
spraying reduced the level of deposits of carbaryl to about
1.0 mg/kg level. In the case of pea pods, the levels of residues
dropped to 0.20 mg/kg within 5 days. In pea seeds, the residue
levels ranged between 0.03 and 0.09 mg/kg, after 8 days (Krishnaih
et al., 1978).
Measurements of carbaryl in different fruits were taken at
intervals of 5-10 days after plants had been treated with a 0.2%
suspension of carbaryl at 1000 litre/ha during the vegetation period
(Bogomolova, 1968, 1970). The initial amount of carbaryl, 2 days
after the treatment, varied in different fruits from 2.3 to
2.7 mg/kg. Ten days later, the concentrations were reduced by
50-70%, and, after 20 days, the levels were within the range of
0.2-0.8 mg/kg, depending on the species (Table 13). According to
Mann & Chopra (1969), the dissipation of carbaryl from plants after
the first day of spraying was about 40-45% of the initial residue.
In apples, concentrations of 2.5-2.9 mg/kg, 0.4-2.4 mg/kg, and
0.15 mg/kg were found on days 7, 10, and 38, respectively, after
spraying. One to two months after spraying apples with a 0.1%
suspension at a dose of 2000 litre/ha, residues of 0.08-0.10 mg/kg
were measured (Antonovich, 1970). Four months after spraying with a
0.06% suspension at a dose of 9 kg a.i./ha, levels in the range of
0.09-0.24 mg/kg were found in apples, depending on the spraying
procedure. Fine-droplet spraying resulted in residue levels 2.5-3
times lower levels than those with coarse-droplet sprays (Atabaev,
1972).
Table 13. Carbaryl levels in different fruits after spraying
during vegetation (mg/kg)a
Kind of fruit Day after spraying
2 10 20
Strawberries 2.3 1.25
Gooseberries 2.5 0.90 0.6
Blackcurrants 2.4 1.10 0.8
Cherries 2.7 1.90 0.2-0.4
Plums 2.4 1.40 0.2-0.4
aFrom: Bogomolova (1968).
A survey of apples grown in Ontario, Canada, between 1978 and
1986 showed that out of 22% of carbaryl-treated apples (1.67 kg/ha)
sold, 3.6% had detectable carbaryl residues (detection limit of
0.01 mg/kg), and that the average residue level was 0.03 mg/kg, with
a maximum of 0.04 mg/kg (Frank et al., 1989).
Prolonged storage of apples reduced the carbaryl residues only
slightly (Bogomolova, 1968). The initial level (2.4-2.8 mg/kg)
changed little during the first 3 months of storage at a temperature
of +5 to +10 °C. A slight reduction was observed during the fourth
month of storage. During the process of storage, carbaryl migrated
inwards from the surface, so that the concentration in the skin was
reduced, while that in the pulp was increased (Table 14).
Residues on lemon foliage on day 0 of treatment were
4.6±0.3 µg/cm2. Five days after treatment, levels of 2.4 µg/cm2
and 5.6 µg/cm2 were found on lemon and orange foliage,
respectively; on the 60th day, the residual values were 0.41 and
0.36 µg/cm2, respectively. Persistence half-lives were 22 days
(lemons) and 14 days (oranges) (Iwata et al., 1979).
Carbaryl is persistent, particularly in citrus fruits and
grapes. Residues of 3-6 mg/kg were measured in lemons and oranges
2.5-3 months after treatment of trees with 3-10 kg carbaryl/ha, and
amounts of 3.6, 1.9, and 0.4 mg/kg were found in grapes on days 7,
14, and 40, respectively, after repeated spraying of vineyards with
a 0.12% suspension. The half-life of carbaryl in grapes was 29 days,
compared with 2-9 days in cherries and cabbage (Antonovich, 1970).
Washing and peeling reduced carbaryl levels in fruits and
vegetables by 40%; thermal processing by 45-90%, and preparation of
juices by 53% (Molozhanova, 1970). Canning also reduced carbaryl
residues. Thus, canning of different fruits (strawberries, black
currants, gooseberries) containing carbaryl in the range of
0.25-0.45 mg/kg, resulted in a reduction to 0.17-0.29 mg/kg; when
the initial contents were higher (3-7 mg/kg) the levels after
canning dropped to 1.4-2.2 mg/kg and 2.1-2.5 mg/kg, respectively,
i.e., from 30 to 70%. Storage of canned fruit further reduced
carbaryl residues by 2-3 times after 12 months (Bogomolova, 1968).
Boiling vegetables (e.g., cabbage) reduced carbaryl residues by
approximately 50%; however, the residues in pickled cabbage, 5
months after preparation, were only 25% less than the initial amount
(Antonovich, 1970).
Table 14. Carbaryl content in apples during storage (mg/kg)a
Part of apple Day of storage
10 20 30 40 50 70 80 100 110
Skin 2.40 1.80 2.00 1.80 1.92 1.72 1.60 1.45 1.22
Pulp 0.35 1.00 1.02 1.10 1.00 1.10 1.34 1.42 1.30
Total 2.75 2.80 3.02 2.90 2.92 2.82 2.94 2.87 2.52
aFrom: Bogomolova (1968).
Elkins (1989) reported that washing during the commercial
processing of produce removed 97, 87, and 77% of the carbaryl
residues from tomatoes, spinach, and broccoli, respectively.
Blanching (short treatment with hot water) was stated to result in
68% removal of carbaryl from green beans, while blanching, in
addition to washing, resulted in the removal of over 97% of carbaryl
from both spinach and broccoli.
After a single application of carbaryl (Sevin 50 at 2.5 kg/ha),
residues in cauliflower decreased from 16.75 mg/kg on the day of
application to 0.87 mg/kg, 15 days afterwards (Yadav & Jaglan,
1982). Washing further reduced the detectable residues at 15 days to
0.67 mg/kg, while no detectable residues were present after boiling.
Carbaryl was found in vegetation adjacent to treated fields.
Grass growing at a distance of 250 m from a carbaryl-treated cotton
field contained 0.18-0.25 mg carbaryl/kg wet weight up to 45 days
after spraying (Gusseynov, 1970).
The results of the 1989 Pesticide Residue Monitoring Programme
in California showed that carbaryl was detected in 2 samples of
grape from 26 in quantities less than the accepted tolerance level.
It was not found in oranges (Okumura et al., 1991). Summaries of
residue data on different plants are given in Tables 15 and 16.
Carbaryl residues were not detected in 44 samples of wheat in the
United Kingdom analysed by the multiresidue method (Osborne et al.,
1989).
Table 15. Comparison of carbaryl residues with different
formulationsa
Formulation use Plant Mean residues Day after last
(kg a.i./ha) value (mg/kg) application
44% SC 2x0.5 apple 0.25 7
pears 0.16 7
50% WP 2x0.5 apple 0.15 7
pears 0.09 7
44% SC 2x2 spinach 4.44 -
50% WP 2x2 2.38 14
44% SC 2x2 lettuce leaf 2.72 14
50% WP 2x2 1.55
44% SC 2x2 barley 7.87 14
50% WP 2x2 4.63
Table 15 (continued)
44% SC 2x2 wheat 0.96 14
50% WP 2x2 0.99
44% SC 2x2 oats 0.22 14
50% WP 2x2 0.26
aFrom: Davis (1987).
Davis (1987) studied the impact of different types of
formulation on food residues, including carbaryl (44% w/w) oil-based
liquid formulation containing a "sticker", in order to prevent bees
from carrying carbaryl particles back to the beehive. The 50 W
formulation was a "standard" wettable powder formulation. The
results are given in Table 15. This work showed that the addition of
a "sticker" increased levels of food residues to some extent.
Studies on the removal of carbaryl residues from tomatoes,
green beans, spinach, and broccoli by commercial and home
preparation procedures (Elkins et al., 1968; Farrow et al.,
1968, 1969; Lamb et al., 1968) showed a considerable decrease in
the residues with treatment (Table 17).
For pre-harvest use on grain, the rate of application of
carbaryl ranges from 2 to 9.5 kg/ha, depending on the degree of
infestation, density of foliage, and the stage of the life cycle of
the pest. Carbaryl, usually applied at a rate of 5 mg/kg, is also
used to protect stored grain. Studies on stored wheat, barley, oats,
and rice indicated that carbaryl residues on grain have a half-life
of between 26 and 80 weeks, depending on the temperature (FAO/WHO,
1976). Residues of carbaryl in baked bread are then of the order of
1-1.5 mg/kg.
5.1.4.2 Animal products
Technical carbaryl was fed to dairy cows of the Brown Swiss,
Jersey, Holstein, and Ayrshire breeds at 50, 150, and 450 mg/kg of
their average total daily roughage intake (dry weight) for a period
of 2 weeks. Samples of milk were taken at regular intervals and the
cream was analysed for carbaryl by means of the
p-nitrobenzene-diazonium fluoborate coupling method. The
concentration of carbaryl, if present, was below the sensitivity of
the analytical method (0.01 mg/kg) (Gyrisco et al., 1960).
Table 16. Carbaryl residues following different applications
Formulation use Plant Mean value Day after Reference
(kg a.i./ha) residues last
(mg/kg) application
44% SC barley 5.4 14 Davis & Thomas (1987)
44% SC sugar beet 0.07-1.04 14 Thomas (1986)
roots
44% SC pasture 59.7-183 3 Davis (1986b)
aerial ground grass
Carbaryl sweet 0.33 Frank et al. (1987a)
cherries
Carbaryl tomato 1.2 0 Frank et al. (1991)
0.5 3
0.03 6-8
tomato 0.47 0
juice 0.24 3
0.08 6-8
Table 17. Removal of carbaryl after preparation procedures
Vegetable Initial Percentage removal References
residues
(mg/kg) Commercial procedure Home procedure
Tomatoes 5.2 washing 82-99 washing 77 Farrow et al. (1968)
canning 98-99 canning 92
juicing 98 juicing 77
Green beans 7.6 blanching 68-73 washing 52 Elkins et al. (1968)
& canning blanching 81
freezing 94
canning 100
Spinach 20.8 washing 66-88 washing 70 Lamb et al. (1968)
blanching 96-97
Broccoli 12.4 washing 77 washing & cooking 55 Farrow et al. (1968)
blanching 82-97 washing, 90
blanching,
& freezing
Residues resulting from a single application and repeated
applications of carbaryl spray on cattle were rapidly eliminated
from body tissues. On days 1 and 3 after application, carbaryl was
detected in the liver (0.05 mg/kg), muscles (0.04 mg/kg), and
perirenal fat (0.16 mg/kg). Seventy-two hours after treatment, no
residues were found in the tissues studied. The excretion in milk
persisted for at least 69 h after spraying, the highest
concentration being 0.075 mg/litre, 5 h after exposure (Hurwood,
1967).
Data concerning the composition and levels of milk and tissue
residues in cows after continuous feeding with 100 mg
14C-carbaryl/kg diet are shown in Tables 18 and 19.
Table 18. Metabolites in tissues after continuous 28-day feeding
of 100 mg 14C- carbaryl/kg dieta
Metabolite (mg/kg) Kidney Liver Muscle
carbaryl 0.03 0.04 0.02
5,6-dihydrodihydroxycarbaryl 0.05 0.01 0.04
5,6-dihydrodihydroxynaphthol 0.02 0.02 0.0
naphthyl sulfate 0.29 0.02 0.0
water-soluble unknowns 0.43 0.13 0.03
unextractable unknowns 0.18 0.19 0.01
aFrom: Dorough (1971).
Table 19. Composition of milk residues after continuous 28-day
feeding with 100 mg 14C-carbaryl/kg dieta
Percentage mg/kg
Organic phase
5,6-dihydrodihydroxycarbaryl 38.5 0.11
carbaryl 8.4 0.02
3,4-dihydrodihydroxycarbaryl 4.0 0.01
Table 19 (continued)
Percentage mg/kg
5,6-dihydrodihydroxynaphthol 1.0 0.01
Water soluble
5-methoxy-6-hydroxycarbaryl 25.6 0.07
unknowns 10.6 0.03
1-naphthol 5.5 0.02
5-hydroxycarbaryl 3.1 0.01
carbaryl 1.2 0.01
aFrom: Dorough (1970a).
Effective horn fly control for a period of 15 days was obtained
by spreading carbaryl down a cow's back. Results indicated that, if
applications of a 0.5% spray or a 50% dust were made immediately
after the morning or evening milking, no residues of carbaryl would
result in subsequent milkings (Eheart et al., 1962). The results
obtained by Petrovski (1970) were similar. After treating cows with
a 0.85-1% suspension of carbaryl, residues of from 0.1 to
0.3 mg/litre were found in milk, 20 h after application. On the
third day, only trace amounts of carbaryl were found. After
treatment with a 0.5% suspension of carbaryl, no traces were found
in the milk.
5.1.4.3 Animal feed crops
Generally, animal feed crops contain less carbaryl than fruit
and vegetables. Mean levels of 0.82 mg/kg were found in 80 samples
of animal feed plants (Molozhanova, 1970). Levels of 0.3 mg/kg were
found in maize stems at harvest, 70 days after spraying (Antonovich,
1970).
Studies on the carbaryl content of animal feed plants in hot
climates showed that carbaryl can be detected 3-3.5 months after
treatment, depending on the dose and the number of applications.
Thus, after repeated applications of 50% carbaryl dust at a rate of
0.5 g/m2, the carbaryl content in animal feed plants was in the
range of 1-1.8 mg/kg wet weight, but, after a single application of
0.7 g/m2, concentrations of 0.10-0.31 mg/kg were found (Atabaev,
1972).
5.1.5 Other products
Measurement of carbaryl residues in tobacco plants showed that,
15 days after field spraying with 950 g/ha, the content of carbaryl
in green tobacco leaves was 10.1 mg/kg. Drying leaves by hot air
decreased carbaryl residues by only 10% (Antonovich, 1970).
Application of carbaryl at a rate of 2.2 kg/ha resulted in
carbaryl residues on cotton foliage of 7.2 µg/cm2 on the day of
spraying (Estesen et al., 1982). Levels decreased to 5.9, 0.58,
and 0.26 µg/cm2 after 3, 6, and 8 days, respectively.
Carbaryl was found in the foliage of cotton plants in
concentrations of 0.03-1 mg/kg from 10 days to 2.5 months after
treatment. Carbaryl penetrated the plant through foliage and roots
and contaminated the cotton and the seeds. Cotton harvested from
treated fields 2 weeks to 1 month after treatment contained
0.015 mg/kg. Trace amounts of carbaryl were found in cotton oil
(Guseynov, 1970).
After treatment with a 2% suspension of carbaryl against
mountain pine beetle, residues in bark disks after 1 year were
359 mg/kg. In another case, residues in bark disks were
890±146 mg/kg, after spraying, and 531±178 mg/kg 16 months later
(Page et al., 1985).
5.1.6 Terrestrial organisms
The quantities of carbaryl that were absorbed by songbirds,
either by contact or by eating food from a sprayed area, were
studied by Kurtz & Studholme (1974). Towhees (American finches) were
collected 3 days after the forest had been sprayed for gypsy moths
at a rate of 1.1 kg/ha. The amounts of carbaryl found in the towhees
were small, even though the samples were taken close to the spraying
time. Trace amounts of carbaryl were found in three samples out of
five, compared with two samples out of five control birds. The
minimum level of detection was 0.1 µg/g. The low levels of carbaryl
found in "sprayed" birds were explained by the fact that towheeds
are groundfeeders and that only a small amount of carbaryl might
have passed through the trees to the ground.
5.2 General population exposure
5.2.1 Exposure through the food
The daily intake of carbaryl was studied for several years in
the USA (see Table 20).
Table 20. Carbaryl daily intake in the USA
Year Positive samples Daily Intake References
(%) (mg/day
per person)
1964-65 7.4 0.15 Duggan et al. (1971);
1965-66 2.7 0.026 Duggan & Corneliussen
(1972)
1966-67 1.1 0.007
1968-69 0.8 0.003
1969-70 0 -
Infant Toddler
1978 - 0.088 0.05 Gartrell et al. (1986)
1979 - - 0.049
1980 - 0.06 0.035 Reed et al. (1987)
1981-82 - 0.129 0.127
1982 - - 0.012
1982-84 - 0.117 0.017- Gunderson (1988)
0.0315
Carbaryl residues in total diet samples in the USA are
relatively low (Table 21). Carbaryl is found in potatoes, leguminous
vegetables, root vegetables, and fruit. In foods processed in the
usual manner, i.e., by peeling, stripping outer leaves, and cooking,
when appropriate, carbaryl residues usually decreased to
undetectable levels or traces (Manske & Corneliussen, 1974).
Table 21. Carbaryl residues in food in the USA
Number of composites Number of Number of Range in References
analysed composites composites mg/kg
with residues with traces
270, from 30 grocery 20 15 trace to Manske & Corneliussen
stores in 27 cities 0.5 (1974)
June 1970-April 1971
420, from 35 market 6 5 0.02 Manske & Johnson
baskets, 6 June 1971- (1975)
July 1972
360, 12 August 1972- 12 10 0.05-1.10 Johnson & Manske (1976)
July 1973
360, August 1973- 8 2 0.001-0.284 Manske & Johnson (1977)
July 1974
1044, 1978-82 11 (1%) a a Yess et al. (1991a)
3744, 16 market 135 (4%) a a Yess et al. (1991b)
basket collections
1982-86
aNo figures given.
During a 5-year period, from 1982 to 1986, the Los Angeles
District Laboratory analysed 19 851 samples of domestic and imported
food and feed commodities for pesticide residues. A single, rapid,
multiresidue method was used. Carbaryl was detected in 164 samples
from the total 19 851 samples analysed. Not one sample exceeded the
US Federal tolerance levels (Luke et al., 1988). In another
publication, the same authors reported comparative studies on US and
imported food. Carbaryl was found in 21 samples from a total 6391 US
samples in quantities ranging from 0.1 to >2.0 mg/kg, but less than
10 mg/kg. From 12 044 imported agricultural commodities 132 were
contaminated by carbaryl, and levels in 14 of them exceeded the
tolerance levels (Hundley et al., 1988).
5.2.2 Exposure during insect control
Exposure to carbaryl, used to control the gypsy moth in camping
and picnic areas, was monitored on the day of application and then
weekly for 3 weeks. Carbaryl was applied from the air at a rate of
1.12 kg/ha. Exposure was measured using dermal pads.
Extrapolating from the most contaminated group of pads (they
contained an average of 279 µg/pad), the authors calculated a "worst
case" exposure of 0.54 mg/kg on the day of spraying. The authors
concluded that the risk to those who use public areas during, and
after, carbaryl application to trees is negligible (Cameron et al.,
1985).
A 5% spray of carbaryl 85% wettable powder (WP) was applied for
insect control in homes (Vandekar, 1965), as a surface application,
at the rate of 2 g/m2. Urine samples taken from villagers 1 week
after spraying showed a significant increase in 1-naphthol levels
from 30.5 µg/ml baseline to 50.3 µg/ml. Inhibition of plasma ChEA
was found in 48 out of 63 subjects, 1 week after spraying.
During a monitoring study carried out from 1976 to 1980 in the
USA, 1-naphthol was found in 1.4% of the urine samples from persons
between 12 and 74 years old. The source was thought to be carbaryl
and naphthalene (Carey & Kutz, 1985).
5.3 Occupational exposure during manufacture, formulation,
or use
Harry (1977) estimated that about 13 million people in the USA
were potentially exposed to carbaryl during its manufacture,
formulation, packing, transportation, storage, and during and after
application, and while working with treated crops or during harvest.
Best & Murray (1962) published a survey on the exposure of
plant workers during the production of carbaryl. Air concentrations
varied from 0.23 to 31 mg/m3. Urine samples contained an excess of
1 mg total 1-naphthol per 100 ml of urine. During air blast spraying
of orchards, Jegier (1964) found air concentrations of 0.6 mg/m3
(0.18-0.81 mg/m3). The mean respiratory exposure, measured by the
respirator pad technique was 0.29 mg/h (0.24-0.53 mg/h) and mean
dermal exposure, measured by skin pads, was 25.3 mg/h
(18.5-30.3 mg/h). The maximum total exposure was 31 mg/person per h,
or 0.025% of the toxic dose. Simpson (1965) estimated that the
amount of dermal exposure was less than 0.1% of the toxic dose in
orchard sprayers. During cotton spraying by aircraft, Yakim (1965)
found 4 mg carbaryl dust/m3 in the breathing zone of flagmen,
1 mg/m3 during preparation of the solution, and 0.7 mg/m3 in the
pilot's cabin. Adylov (1966) reported the following air
concentrations found during the aerial application of a water
solution: flagmen's breathing zone 1.92 (0.64-2.84) mg/m3; pilot
cabin, trace amounts, workers on the ground (preparation of
solution, etc.,) 6.28 (0.48-19.2) mg/m3. In the urine samples of
carbaryl formulators, 1-naphthol levels varied from 6.2 to
78.8 mg/litre. Agricultural workers who used carbaryl for pest
control excreted from 0.07 to 1.7 mg/litre in their urine (Shafik
et al., 1971).
Exposure studies were completed for pesticide application and
formulating plant workers by the biological monitoring of 1-naphthol
in the urine, dermal pads, and respirator filter pads (total 480
samples of dermal pads and 73 respirator pads). Workers operating
tractor-drawn airblast equipment applied carbaryl at 0.045-0.06% as
spray (Comer et al., 1975). An estimate of the amount of dermal
and respiratory exposure that could occur was made using the
procedures described by Durham & Wolfe (1962). The results are
presented in Table 22.
The concentration of 1-naphthol in the urine varied from 0.2 to
65 mg/litre with a mean of 9 mg/litre. The rate of excretion per
hour varied from 0.004 to 3.4 mg with a mean of 0.5 mg/h. The
maximum level during the working day was reached by late afternoon.
An approximate calculation of the excreted carbaryl (0.5 mg
1-naphthol/h = 0.7 mg/h excretion of carbaryl), and potential
exposure, 75 mg/h, shows great differences. About 1/100 part of
possible absorption occurs.
Exposure to carbaryl during agricultural application was
studied by Leavitt et al. (1982). WP 80 carbaryl was used (0.45 kg
in 200 litre water) to spray trees, lawns, and gardens. The mean
dermal exposure was 128 mg/h and the mean inhalation exposure was
0.1 mg/h. The maximum percentage of the toxic dose that the
applicators received was 0.12%/h. No symptoms or inhibition of ChE
were reported. In another group of applicators, the mean dermal
exposure was 59.4 mg/h and inhalation exposure 0.1 mg/h.
Table 22. Potential dermal and respiratory exposure of formulating
plant workers and field spraymen to carbaryla,b
Subject Route of Exposure Exposure (mg/h)
exposure situations
studied Range Mean
Formulating dermal 48 0.80-1209.30 73.90
plant workersc
respiratory 48 0.03-4.10 1.90
Field dermal 32 1.70-211.80 59.00
spraymend
respiratory 25 0.01-1.08 0.09
aFrom: Comer et al. (1975).
bCalculated on the basis of the worker wearing a short-sleeved,
open-necked shirt, no gloves or hat, with his clothing protecting
the areas covered.
cWorkers on mixing and bagging operations (4 and 5% dust).
dOperating power air-blast spray machines in fruit orchards
(0.045-0.06% solution spray).
Dermal exposure is influenced by the type of spray equipment.
The dermal deposit and respiratory intake of carbaryl in humans was
monitored during home garden spray operations using compressed air
or garden hose-end sprayers (Puech, undated). The greatest mean
dermal deposit in cm2/min was received on the feet. When the spray
target was above shoulder height the next highest dermal spray was
received on the forearm (Table 23). Exposure through inhalation was
insignificant.
A study of the urban application of carbaryl was performed by
Gold et al. (1982). The maximum dermal exposure recorded in this
study was 2.86 mg/kg per h. The maximum air concentration was
0.28 µg/litre. An insignificant decrease in ChEA in serum and
erythrocytes was found in some of the applicators. The mean dermal
carbaryl exposure of the applicator, expressed as a percentage of
toxic dose per h, was 0.01%, with a maximum of 0.08%. This exposure
rate is below the risk rate for applicators. No symptoms of
intoxication were reported by the authors. During the urban
application of carbaryl to trees and ornamental shrubs using
hand-held equipment, air concentrations measured at the breathing
zone in full-shift samples, of 0.010-0.070 mg/m3, were detected in
only 30% of the samples (Leonard & Yearly, 1990).
Table 23. Human exposure during spray operations
Type of spray equipment Mean deposit Total exposure
(µg/cm2 per min) (µg/kg per min)
Compressed air sprayer
below waist 0.030 4.3
above shoulder height 0.096 5.6
Hose-end sprayer
below waist 0.154 18.3
above shoulder height 0.353 24.4
Dermal exposure to carbaryl in harvesters of strawberries was
studied by Zweig et al. (1984, 1985). They observed dermal
exposure on the hands and forearms, and to a much lesser degree, on
the lower legs. The first day, 3 mg carbaryl/h was found on cotton
gloves, 0.66 mg/h on pads on the forearms, and 0.07 on the lower
legs. The following day, the values were 1.23, 0.41, and 0.07 mg/h
for gloves, forearms, and lower legs, respectively. The ratio of
dermal exposure to dislodgeable foliar residues (DFR) was 4.34,
2.82, and 6.17, for 3 consecutive days. The half-life of carbaryl on
strawberry leaves was 4.1 days. Dislodgeable carbaryl residues on
cotton foliage, expressed as µg/cm2 of cotton leaf (one surface
only), following application by man-pulled ground rig were 7.2, 5.9,
0.58, and 0.26 at 0, 72, 144, and 192 h after application,
respectively (Estesen et al., 1982). Approximate dermal exposure
rates may be calculated using the following expression, proposed by
Zweig et al. (1985). Dermal exposure rate (mg/h) = 5x103xDFR
(in µg/cm2). This method is suggested to obtain the exposure rate
of fruit harvesters, in order to establish safe re-entry periods
without human studies.
Factors affecting the levels of exposure during the
agricultural application of pesticides were analysed by Wolfe et
al. (1967). Wind is the most important environmental condition. The
type of activity, equipment used, the duration of exposure,
formulation, individual protection, including attitude, are also
discussed. Carbaryl deposits from air jet application on orchards
were found at 500 m downwind in the presence of inversion, and at
300 m, in its absence. Ground application results gave deposits at
150 and 50 m, respectively (MacCollom et al., 1985, 1986). Air
concentrations were 17.88 µg/m3 during spraying above the orchard
downwind edge, 9.5 µg/m3, 30 min after spraying, and 8.17 µg/m3,
1 h after spraying.
Airborne and deposit levels of carbaryl were measured after
three applications by air to an apple orchard (Currier et al.,
1982). Samples of air were taken at distances of from 12.2 to 3994 m
from the target orchard. At a distance of 12.2 m, the concentration
in air was between 3.3 and 76.2 µg/m3. One hour later it was
between 2.5 and 11.3 µg/m3. At a distance of 3.2-3.3 km, the
initial concentration was between 9 and 28.8 µg/m3 during spraying
and 0.9-14.4 µg/m3, one hour after treatment. Because the area of
the study was one of concentrated agriculture, it is possible that
carbaryl could have been used on other orchards and gardens near to
the sampling point.
6. KINETICS AND METABOLISM
6.1 Absorption
When only 0.1 ml of carbaryl solution (0.025-0.05 mmol/litre)
was administered into the lungs of anaesthetized rats, it was
rapidly absorbed. About 50% of the dose was absorbed in 2.6 min. The
amount of carbaryl absorbed per unit time was directly proportional
to the administered dose (Hwang & Shanker, 1974). Blase & Loomis
(1976) demonstrated that carbaryl could be taken up and metabolized
by the isolated perfused rabbit lung. Retention by rats of
14C-labelled carbaryl, inhaled as vapours during a 1-h exposure
period, was 75.4% of the total dose inhaled, which did not exceed
50 µg (Dorough, 1982).
When a dose of 7.5 µmol radiolabelled carbaryl/kg body weight
was administered intragastrically to fasted, anaesthetized, female
rats, 22 and 67 min after dosing, the proportions absorbed were
52.6±14.1% (n=3) and 81.7±15.7% (n=3), respectively; 89.3% of the
radiolabelled material in the collected portal blood was
[1-naphthyl-1-14C] N-methyl carbarmate (Casper et al., 1973).
Absorption from the small intestine was studied in
anaesthetized rats with a ligature around the pylorus and an
ileocecal junction with major blood vessels not occluded. A
concentration of carbaryl of 0.005-0.1 mmol/litre was used. The
absorption half-time was 6.4 min (Hwang & Schanker, 1974).
On the basis of ligation studies on mice, Ahdaya & Guthrie
(1982) determined that the absorption of carbaryl from the stomach
was relatively low compared with that from the entire
gastrointestinal tract (29%), but was relatively high in comparison
with that of other pesticides.
Variation in the digestive absorption kinetics, according to
the vehicle used, was reported in studies on female Wistar rats
(Cambon et al., 1981). The results indicated that carbaryl was
absorbed more rapidly in the intestine when either DMSO or
tragacanth was used as a vehicle. It seems that milk does not
facilitate carbaryl absorption. Inhibition of ChEA was closely
related to the absorption rate.
The rate of dermal penetration of carbaryl (in acetone
solution) into mammals, birds, amphibia, and insects was studied by
Shah
et al. (1983). The half-time of penetration of 14C-labelled
carbaryl was 317 min in Japanese quail, 12.8 min in mice, 6.4 min in
grass frogs, 4576 min in American roaches, and 791 min in tobacco
horn worms.
In an in vitro study, only about 1% of an applied dose of
carbaryl penetrated through the skin of the rat over an 8-h period
(MacPherson et al., 1991). Using two different methods, Shah &
Guthrie (1983) measured the half-time for penetration of carbaryl,
applied dermally at a rate of 4 µg/cm3, and found 10.34 h and
4.75 h, respectively. Shah et al. (1987) compared the rate of
dermal penetration of carbaryl in young, versus adult, Fischer 344,
female rats and did not find any consistent age-related differences.
Percutaneous absorption of carbaryl in rats was also studied by
Knaak et al. (1984). Carbaryl dissolved in acetone was applied to
back skin (not occluded) at 43.4-48 µg/cm2. Recovery studies
indicated that 57.7% of the applied carbaryl was absorbed.
Approximately 5.8% of carbaryl penetrated the skin within 1 h, the
rate of absorption being 0.18 µg/h per cm2. The half-life of
absorption by blood was 1.26 h, and that for elimination, 67 h.
Carbaryl labelled with radioactive carbon (14C), dissolved in
acetone, was applied to the skin of six volunteers, in order to
study percutaneous penetration (Maibach et al., 1971; Feldmann &
Maibach, 1974). The results showed almost complete penetration of
carbaryl on the forearm and jaw angle. After a 24-h application, the
cumulative urinary excretion over 5 days was 74%. According to other
authors using the same data, the estimated cumulative absorption
over 5 days, as a percentage of the applied dose, was 63% (Fisher
et al., 1985), 45% of this occurring 8 h after the onset of
penetration, which had a lag of 3.5 h.
Comparing the different studies, it is clear that some solvents
can facilitate the dermal penetration of carbaryl.
6.2 Distribution
Plasma levels of carbaryl in rats were compared after iv,
intraduodenal, or hepatic portal administration of 0.5 mg/kg
(Houston et al., 1974). In Fig. 2, it is shown that plasma
concentrations were lower after intraduodenal application, when
carbaryl was subjected to the liver's first pass metabolizing
effect. Plasma concentrations following portal application were also
lower (approximately 80% of the concentration after the systemic
route of application).
In India, degradation of carbaryl was studied in a greenhouse
by Brahmaprakash & Sethunathan (1984, 1985). Since a soil planted
with crops may be more dynamic and complex than unplanted soil,
because of increased microbial activity, they studied carbaryl
persistence in soil (pH 6.2, organic matter 1.6%) planted with rice
in a greenhouse, under flooded and non-flooded conditions. Carbaryl
disappeared more rapidly from soils planted with rice than from
those without rice, under both flooded and non-flooded conditions.
The amount of carbaryl decreased to between 30.2 and 32.1% of the
original level within 30 days in unplanted soil under both flooded
and non-flooded conditions. During this same period, the carbaryl
concentration decreased to 17-18% of the original level in planted
soil under both water regimes. Degradation occurred by hydrolysis,
but there was no appreciable difference in the rates of degradation
between flooded and non-flooded soils. The rate of degradation of
carbaryl was little affected by moisture. Further degradation of
1-naphthol was slow in both planted and unplanted systems. A
significant portion of the ring-14C accumulated in the soil as
1-naphthol and soil-bound residues. Evolution of 14CO2 from the
labelled side-chain and ring was negligible, even in soil planted to
rice.
4.3.4 Degradation by microorganisms
Many studies have demonstrated the great ability of
microorganisms to degrade carbaryl in the soil, and for some of
them, to use it as a sole source of carbon. The most frequently
identified organisms are bacteria ( Pseudomonas phaseolicola, P.
cepaphia, Rhodococcus sp., Nocardia sp., Xanthomonas sp.,
Achromobacter sp.) and fungi ( Aspergillus niger, A. terreus,
Fusarium solani, Gilocladium roseum, Rhizoctonia solani, R.
practicola, Penicillium sp., Mucor sp., Rhizopus sp.). In many
cases, it was demonstrated that the persistence of carbaryl in the
soil decreased after a first application, which was interpreted as
the selection and build-up of strains more capable of degrading the
product. The same process may also explain that, in some studies,
the rapidity of degradation suddenly rose after a lag period during
which only minor amounts of carbaryl degraded.
Tewfik & Hamdi (1970) mentioned that carbaryl was decomposed
into 4 distinct compounds by a soil bacterium designated as S-1.
They considered that this soil bacterium might utilize carbaryl in a
similar way to Pseudomonas sp., which metabolize naphthalene via
the salicylate pathway.
Zuberi & Zubairi (1971) also reported that carbaryl is degraded
by soil microflora. Pseudomonas phaseolicola and Aspergillus
niger hydrolysed carbaryl to 1-naphthol. In the case of P.
phaseolicola, an unidentified minor metabolite was also detected.
Larkin & Day (1986) reported that 2 bacteria isolated from
garden soil, Pseudomonas sp. (NCIB 12042) and Rhodococcus sp.
(NCIB 12038), could grow on carbaryl as the sole source of carbon
and nitrogen at pH 6.8, but failed to metabolize carbaryl rapidly.
Both could use 1-naphthol as the sole source of carbon and
metabolize it via salicylic acid. Strain NCIB 12038 produced also
gentisic acid. Pseudomonas sp. (NCIB 12043), in a soil perfusion
column enrichment at pH 5.2, metabolized carbaryl rapidly to
1-naphthol and methylamine. 1-Naphthol itself was metabolized via
gentisic acid. A possible pathway for the catabolism of carbaryl and
1-naphthol was proposed.
Walker et al. (1975a), working with Pseudomonas sp.,
observed degradation of 1-naphthol and suggested that carbaryl could
serve as the sole source of nitrogen and carbon for bacteria. It was
also noted that the presence of another nitrogen source in the media
seemed to have a delaying effect on metabolism of carbaryl.
Bacterial communities of at least 12 and 14 members were
selected in continuous culture using carbaryl as the sole source of
carbon and nitrogen at pH 6. These communities were supported by the
slow formation of hydrolysis products and no carbaryl-degrading
bacterium was selected after more than 83 days. When using equimolar
1-naphthol and methylamine as the sole source of carbon and
nitrogen, a bacterial community of at least 8 members was selected.
After a lag of 10-50 days, soil perfusion columns (pH 5.2) and
continuous culture enrichments (pH 5) led to the selection of a
Pseudomonas sp. that could utilize carbaryl as its sole carbon and
nitrogen source (Larkin & Day, 1985).
Sud et al. (1972) showed that Achromobacter sp. also
utilized carbaryl as the sole source of carbon in a salt medium.
Four degradation products of carbaryl were 1-naphthol, hydroquinone,
catechole, and pyruvate. The organism also grew well in the first 3
degradation products.
The rate of degradation of carbaryl after 1, 2, and 3
applications to 3 submerged soils was examined by Rajagopal et al.
(1983). Soils that had been pretreated with carbaryl were able to
degrade carbaryl more rapidly than those without pretreatment. The
enrichment culture was inactivated upon autoclaving. The
concentration of carbaryl decreased in the mineral medium inoculated
with the enrichment cultures from the 3 soils, especially when it
served as the sole source of both carbon and nitrogen.
Rajagopal et al. (1984) studied the metabolism of side-chain
and ring 14C-labelled carbaryl in a mineral salts medium by soil
enrichment cultures. Hydrolysis was the major route of microbial
degradation. During carbaryl degradation by enrichment cultures and
Bacillus sp., 1-naphthol and 1,4-naphthoquinone accumulated in the
medium.
Rajagopal et al. (1986) also observed that carbaryl
disappeared more rapidly from a laterite soil pretreated with
1-naphthol than from a control soil never exposed to 1-naphthol. The
accumulation of 1-naphthol and bound residues formed from
added14C-carbaryl was greater in soils pretreated with 1-naphthol
than in untreated soils.
In experiments with the soil fungus Rhizoctonia practicola,
Bollag et al. (1976) found that it could transform 1-naphthol from
an ether-extractable to a water-soluble product. It was also
observed that, after removal of the fungal cells, the growth medium
possessed the ability to transform 1-naphthol, indicating activity
of an extracellular enzyme. Attempts to analyse the labelled
material in the aqueous phase indicated that the radioactivity was
associated with a compound of comparatively high relative molecular
mass.
Czaplicki & Bollag (1975) exposed 1-naphthol to Rhizoctonia
solani, isolated from soil, and found that it was completely
transformed to a compound not extractable with ether.
Working with the soil fungus Aspergillus terreus, Liu &
Bollag (1971b) investigated the metabolic transformation of carbaryl
through 1-naphthyl- N-hydroxymethylcarbamate and tried to clarify
the pathway of the side-chain. The next intermediate in biological
transformation was 1-naphthyl carbamate, which was further degraded
to 1-naphthol. Within one week, approximately half the amount of
carbaryl was transformed to these other metabolic products. No
attempt was made to clarify whether the formation of 1-naphthol was
the result of the biological or the chemical degradation of
1-naphthylcarbamate, but there were clear indications that
1-naphthol was metabolized further in the presence of A. terreus.
In vitro studies were carried out to investigate the
degradation of carbaryl by soil microorganisms. Three isolates from
soil, including Fusarium solani, a Gram-negative coccus, and a
Gram-positive rod, accelerated the hydrolysis of carbaryl to
1-naphthol and other intermediates. Fusarium solani was the most
effective in decomposing the 14C-labelled compound. Radioactivity
decreased by 24% during the first 5 days and by 82% during 12 days
in a growing culture after inoculation. A mixture of two or three of
the microorganisms was more effective in decomposing carbaryl and
1-naphthol (Bollag & Liu, 1971).
Bollag & Liu (1972a) studied the biological degradation of
14C-1-naphthol during growth, with replacement cultures and cell
extracts of Fusarium solani. The radioactivity of 1-naphthol
disappeared partially during growth, but it was completely
dissipated by cell extract activity. The cell extract of F. solani
degraded more than 80% of the 1-naphthol to form CO2 within 60 min
of incubation, but it was not possible to identify intermediates.
This implies rupture of the naphthol ring. No difference in activity
could be observed between cell-free extracts from the spores or the
mycelium of the fungus at pH 5.7 and 7.2. The enzyme system was
relatively stable since there was no decrease in activity after the
cell extract was stored at -10 °C during 4 months. The enzyme that
participated in the degradation reaction was constitutive (always
present) as shown by cell extracts prepared from cells grown in a
medium with, or without, carbaryl as substrate.
The fungus Gliocladium roseum, isolated from soil by Liu &
Bollag (1971a), metabolized carbaryl to 3 metabolites which were
identified as 1-naphthyl- N-hydroxy methylcarbamate,
4-hydroxy-1-naphthyl methylcarbamate, and 5-hydroxy-1-naphthyl
methyl-carbamate. It was therefore considered that N-alkyl- and
aromatic ring-hydroxylation of carbaryl were important detoxication
reactions of G. roseum. About 70% of the radioactivity was
recovered as carbaryl from an 11-day-old culture. The decrease in
radioactivity from the growth medium containing side-chain labelled
carbaryl indicated that a further degradation of the formed
metabolites occurred, or that an additional pathway was involved in
the degradation of carbaryl by G. roseum.
Bollag & Liu (1972b) reported that most soil fungi
( Penicillium sp., Mucor sp., Rhizopus sp., and Aspergillus
sp. except A. fumigatus), could hydroxylate carbaryl at different
positions, but that the products differed qualitatively as well as
quantitatively with the various fungi.
In a different study, the enzyme or protein fraction
(extracellular phenol oxidase) of the culture filtrate from
Rhizoctonia practicola as chromatographed on a column of Sephadex
G-200 and fractions were obtained that were able to transform
1-naphthol as a substrate (Sjoblad et al., 1976). The enzyme
catalysed the polymerization of 1-naphthol to several products.
Biological oxidation and coupling of phenols are key reactions
in nature, that result in the formation of products such as lignins,
melanins, tannins, alkaloids, and humus compounds. Thus, it can be
assumed that enzymes interact with xenobiotic phenols that are
incorporated into soil or sediment organic matter. Rhizoctonia
practicola (and an extracellular enzyme) was able to polymerize
1-naphthol (dimers, trimer, tetramer) (Bollag et al., 1978).
Rodriguez & Dorough (1977) studied the persistence of carbaryl
in culture media in the presence of mixed and pure cultures of
bacteria and fungi isolated from soil. All fungi ( Fusarium,
Penicillium, Aspergillus, and one unidentified species), isolated
from a soil treated with carbaryl six months before sampling,
produced at least one metabolite from carbaryl. After 14 days of
incubation, 54-79% of the carbaryl was recovered intact, and there
was little formation of carbon dioxide by the fungi. In contrast,
related experiments with bacterial isolates ( Arthrobacter,
Nocardia, Pseudomonas, Xanthomonas, and Bacillus) from the same
soil showed that only 1-9% of the added carbaryl remained
unconverted ( Arthrobacter was the exception with 59.1% remaining
carbaryl). Controls showed that non-biological degradation also
occurred. Bacteria, like fungi, metabolized carbaryl qualitatively
in the manner observed with natural soil populations. However,
quantitative differences were so great that the use of isolates may
be of little value in estimating the degradation rate of carbaryl
and other pesticides in field soil. This is important to note when
conducting microbial degradation studies in the laboratory.
Bollag (1979) summarized the transformations of carbaryl by
microbial activity. It was possible to isolate several
microorganisms capable of hydrolising carbaryl to 1-naphthol. In
addition, Gliocladium roseum showed the formation of:
4-hydroxy-1-naphthyl N-methylcarbamate
5-hydroxy-1-naphthyl N-methylcarbamate
1-naphthyl N-hydroxy-methylcarbamate.
Other terrestrial fungi hydroxylated carbaryl at different
positions, but the products differed quantitatively and
qualitatively with the various fungi. Subsequent metabolic
transformation of the hydroxylated products differed considerably.
With A. terreus, the following products were obtained:
1-naphthyl N-hydroxy-methylcarbamate -->
1-naphthyl-carbamate --> 1-naphthol
Several bacteria isolated from river water were capable of
degrading 1-naphthol. At least two different pathways were involved.
The first was a complete degradation with the release of CO2, the
second produced principally 4-hydroxy-1-tetralone, which may involve
hydroxylation of the naphthyl ring in the 4 position and conversion
to an aliphatic cyclic compound. Such a pathway has also been
described with a soil Pseudomonas by Walker et al. (1975a) and
Davis & Evans (1964).
4.3.5 Persistence in soil
Carbaryl is not usually applied as a soil treatment, therefore,
the amounts of carbaryl that may reach the soil come principally
from spray drift, or from washing off treated crops by rain.
When applied at the usual doses, in the laboratory, carbaryl
has a short persistence (half-life <40 days and usually from 6-20
days), but this may increase when the soil is flooded, or when the
dose is increased. In field studies, the half-life of carbaryl,
applied at the usual dose rates, in warm or temperate climatic
conditions, did not exceed one month in the soil. The results of
laboratory and field studies on the persistence of carbaryl in soil
are summarized in Table 7.
Table 7. Half-life of carbaryl in soil, calculated from experimental laboratory
and field results
Origin of soil Number Degradation Half-life References
of days (%) (days)
Laboratory 40 100 8 Johnson & Stansbury (1965)
Laboratory 0.5 mg/kg 45 77 20 Molozhanova & Kanevskij (1971)
Laboratory 1 mg/kg 45 53 40
Laboratory 10 mg/kg 45 13
Laboratory 30 55-63 < 20 Flores-Ruegg et al. (1980)
Laboratory (flooded) 15 63-27 10-40 Venkatesvarlu et al. (1980)
Laboratory 53 100 < 8 Odeyemi (1982)
Orchard 184 99 30 Ivanova & Molozhanova (1973)
Corn 135 95 30 Caro et al. (1974)
Pretreated soil 4 70 < 3 Rodriguez & Dorough (1977)
Grain cropa 30 53-31 27-55 Gangwar et al. (1978a)
Corn soilb 5 42-56 5 Kavadia et al. (1978)
Potatoes 50 < 99 < 7 Kovaleva & Talamov (1978b)
- 30 96 6
Table 7 (continued)
Origin of soil Number Degradation Half-life References
of days (%) (days)
Unspecified 90 Czaplicki (1979)
Tropical 7 Rajukkannu et al. (1985)
Sesamum 60 56 50 Yadav et al. (1985)
Bare soil < 4 Davis (1986a)
Bare soil 1-13
Forest 1.5 Sundaram & Szeto (1987)
Forest 90 < 78 < 38 Springborn (1988b,c)
Forest 90 < 90 < 24
Flooded rice 180 (56) Springborn (1988a)
Flooded rice 180 (52)
Broccoli 6-12 Norris (1991)
Sweet corn 5
a20-60 kg/ha.
b15-45 kg/ha.
Johnson & Stansbury (1965) calculated a half-life of
approxi-mately 8 days in an agricultural soil (sandy loam, pH 5.5)
treated at 3 concentrations. Residues of carbaryl appeared to be
completely degraded within 40 days.
The considerable influence of the dose rate on the persistence
of carbaryl in soil was demonstrated by Molozhanova & Kanevskij
(1971). The percentages of the initial quantities that were degraded
45 days after treatment are shown in Table 8.
Table 8. Degradation rate of carbaryl in relation to the dose
Initial dose Degradation (%)
(mg/kg)
0.5 77
1 53
1.5 37.3
2 30
2.5 27
3 23
4 20
6 16.7
8 15
10 13.2
The same authors also demonstrated the influence of the type of
soil on the degradation of carbaryl. Forty-five days after
treatment, about 50% degradation was observed in light grey forestry
soil, and only about 20% in peat. The ability of soils to degrade
carbaryl could be ranked as follows: light grey > grey forestry >
turf podzol > southern chernozeme > ordinary chernozeme > meadow
> peat.
Ivanova & Molozhanova (1973) calculated that application of
1.36 kg carbaryl/ha in an orchard resulted in 19.7% being retained
in the soil; 99% of the carbaryl present in soil disappeared within
184 days.
Czaplicki (1979) conducted studies in Poland. The soil
half-life of carbaryl was about 3 months and 90% degradation
occurred within 18 months. From October to March, when the soil
temperature was <5-8 °C, the disappearance of the insecticides in
the soil almost stopped.
Rajukkannu et al. (1985) studied the persistence of 4
products in the red and black soils of Tamil Nadu (India), where the
tropical climate in conjunction with the soil properties shortened
the persistence of insecticides. The half-life of carbaryl was 6.7-7
days and 95% degradation was achieved after 90 days.
It was reported by Odeyemi (1982) that carbaryl disappeared
from soil samples treated with 45 mg/kg after 53 days incubation in
a greenhouse under tropical conditions (Nigeria).
4.3.6 Interaction with other physical, chemical, or biological
factors
Nitrogen fertilizers (ammonium sulfate and urea) increased the
persistence of carbaryl in flooded laterite soil with a low native
content of nitrogen (0.04%). In the alluvial soil with 0.11% total
nitrogen, the persistence of the insecticides was little affected.
The rates of degradation in the two soils treated with nitrogen
fertilizers were almost identical. The authors speculated about the
mechanism of this effect, which was possibly due to the preferential
utilization of inorganic nitrogen by microorganisms, or to the
inhibition of soil hydrolase activity (Rajagopal & Sethunathan,
1984).
4.3.7 Vegetation
4.3.7.1 Uptake and transformation in plants
Carbaryl from soil treated at about 50 times the usual dose
penetrated into apple trees and couch-grass (Molozhanova, 1968).
Three months after application of carbaryl (85%) to soil at a dose
of 100 mg/kg, residues of carbaryl were detected in the roots and
stems in apples (39-13 mg/kg) and in couch-grass (50-38 mg/kg).
Under the same conditions, no residues were detected in tomato
fruits or in wheat grain, but 5.9 mg/kg were detected in potatoes
(Table 9).
Table 9. Migration of carbaryl from the soil into apple trees at
different times following applicationa
Experimental Sampling day Carbaryl content of samplesb
conditions after (mg/kg)
application
Soil Foliage Fruits
85% WP carbaryl 5 9.3 0 -
applied under each
tree at a rate of 12 6.8 0 -
10 mg a.i./kg soil
19 6.0 5.0 -
56 4.4 0.04 4.4
138 2.1 0 -
aFrom: Molozhanova (1968).
bAverage data from 18 samples are given for each case.
Huque (1972) reported that 14C-labelled carbaryl was readily
taken up from granules by 2-week-old rice plants. After 3 days, a
level of about 100 mg/kg was determined in the plant (excluding the
roots). Residues then decreased to about 15 mg/kg after 18 days.
Ferreira & Seiber (1981) studied the uptake and distribution of
carbaryl in rice seedlings following exposure of the roots to the
insecticide. Carbaryl was rapidly absorbed and transported upwards
to the leaves and stems. After exposure was terminated, the major
route of loss of carbaryl was through root exudation (22%) rather
than volatilization from the leaves (4.2%). However, since most of
the remaining carbaryl in the plant was present on the leaf
surfaces, the authors surmised that volatilization might play a
greater role in pesticide loss over longer periods of time.
14C-carbaryl and 14C-1-naphthol from soil-bound residues
were partially released when barley was grown (Murthy & Raghu,
1988). 14C-residues could be detected in both shoots and roots in
the case of carbaryl treatment, while only roots showed
14C-residues in the case of 1-naphthol.
The rate of decomposition of carbaryl in plants depends on the
climatic conditions. It is more rapidly decomposed in hot climates,
at high temperatures, and by intensive ultraviolet radiation. Thus,
residual levels in feed plants were lower in regions with a hot
climate than in other regions (Atabaev, 1972).
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Environmental levels
5.1.1 Air
Ambient air concentrations of carbaryl were measured before and
after a treatment of a large area of forest in Maine, USA, to
control spruce budworm. Concentrations in air ranged from less than
0.0035 to 0.107 µg/m3 several miles away (Shehata et al., 1984).
Room concentrations of airborne carbaryl were measured
following application for pest control as a 5% dust at an
undetermined rate. The levels detected on the day of application,
and 1, 2, or 3 days after application were 1.3, 0.2, 0.1, and 0.01
µg/m3, respectively (Wright et al., 1981).
5.1.2 Water
Carbaryl concentrations in water from different sources in the
USSR were studied by Molozhanova (1970). Data are presented in Table
10.
Table 10. Concentrations of carbaryl in watera
Type of sample Number of samples Mean
Total Carbaryl-containing concentrations
samples (%) (mg/litre)
Well 114 87 0.13
Artesian water 64 70 0.16
Ground water 24 75 0.27
Dam 90 47 0.02
River 77 62 0.044
Lake 84 85 0.03
aFrom: Molozhanova (1970).
According to an earlier report by Molozhanova (1968), carbaryl
was present in trace amounts up to a maximum level of 1 mg/litre in
well water and reservoirs during carbaryl application (6 kg/ha, 85%
WP).
Water irrigation channels, situated at a distance of 200-300 m
from a carbaryl-treated cotton field, contained from 0.01 to
0.25 mg/litre up to 18 days after spraying at a dose of 2kg/ha
(Guseynov, 1970).
Residues of pesticides were monitored in the aquatic system of
Ioannina basin (Greece) and its natural outlet, Kalamas River, for
the period September 1984-October 1985 (Albanis et al., 1986). The
carbaryl concentration in water was found to follow a seasonal
fluctuation with maxima during summer and minima during winter
months. Mean and range values are summarized in Table 11.
Table 11. Mean and range values of carbaryl (ng/litre) on different
sampling dates in the Ioannina basin
Month of sampling River Canal Lake
September 0.5
November NDa NDa
March NDa
May ND
June NDa
July 23.7 21.7
August 2.3
September 1.7 NDa
April NDa
July 8.8
aND = not detected.
An oil-based, carbaryl formulation (Sevin-2-oil) was applied
twice to a coniferous forest in New Brunswick (Canada). At a dose
rate of 280 g/ha, the highest concentration in stream water was
0.314 mg/litre and only 0.123 mg/litre 0.5 h after spraying. More
than 50% of the initial residues dissipated within 1 h (Sundaram &
Szeto, 1987).
Carbaryl was applied aerially as Sevin-4-oil (1.12 kg/ha) to
aforest in Montana. Field samples of water were collected to study
persistence. Residue levels in 4 streams sampled were variable
(3-6, 3-175, 2-260, 4-108 µg/litre) 1.3-2.8 h after application
(Pieper & Roberts, 1978; Pieper, 1979). Local accumulations of
floating oil-based spray may have caused this variation. Grantham
(1980) reported that carbaryl (Sevin-4-oil) levels were generally
highest directly after spraying, but increased at some stream sites
for 1-2 days after application. This was attributed to light rains,
which washed the spray residue off the foliage and into the stream
channel. However, Brown (1980) concluded that carbaryl enters
streams in periodic doses and that rainfall dates are not related to
the presence of residues in streams. Maximum residues of
26.32 µg/litre were found in water 12 h after spraying, with no
residues detectable in samples taken hourly until 36 h. Residues
were found again in samples taken 2.5-3 days and 5.5-6.5 days after
spraying.
In a study where carbaryl (Sevin-4-oil) was applied aerially
(0.84 kg/ha) to a forest in Maine, in 1978, samples were collected
in 9 streams (Stanley & Trial, 1980). Peak concentrations occurred
shortly after spraying and maximum residue levels ranged from 0.93
to 7.8 µg/litre in brooks and from 0.44 to 2 µg/litre in rivers. In
one stream, the maximum concentration was 16 µg/litre. Ott et al.
(1981) reported that carbaryl levels were highest on the day of
spraying (2.7 µg/litre), declined to about 0.7 µg/litre by the third
day, but increased again to about 1.2 µg/litre on day 5. No residues
were detected in stream water samples taken on the 17th day.
Thomas et al. (1982) treated planted rice fields with
carbaryl at rates of 0.63, 0.94, and 1.85 kg a.i./ha, either as a
high volume spray (500 litre/ha) or as a low volume spray (150
litre/ha), and monitored the residues. In irrigation water sampled
one hour after treatment, carbaryl levels amounted to 0.15-0.30 and
0.07-0.18 mg/litre, under high and low volume spraying,
respectively. During the first 24 h after spraying, the
concentration in the irrigation water remained fairly constant, but,
by the fourth day, it had decreased significantly. By the tenth day
after spraying the residues in water had decreased to 0.02-0.05 and
0.03-0.04 mg/litre, respectively.
A rice field dissipation study was conducted in Arkansas (USA)
by Springborn (1988). Carbaryl (Sevin XLR) was applied 3 times at
14-day intervals to an irrigated field plot in which rice was
planted. In irrigation water, carbaryl residues disappeared with a
half-life of <1 day after each treatment. The maximum concentration
in water was 1466 µg/litre and, by day 3 following each treatment,
the concentration was <121 µg/litre. In a similar study conducted
in California, Springborn (1988a) found that carbaryl dissipated
rapidly in irrigation water with a half-life of <1 day. The maximum
concentration in water was 648 µg/litre. By day 3 following each
treatment, the concentration was <27 µg/litre.
Residues of 1-naphthol were found in the water collected from
wells and ponds in and around Bhopal (India). The residues of
1-naphthol in well water (near a manufacturing plant) ranged from
0.002 to 0.024 mg/litre. In pond waters, the levels were found to be
between 0.036-0.098 mg/litre. The level of 1-naphthol in soil
samples was of the order of 0.153-0.656 mg/kg (Dikshith et al.,
1990).
5.1.3 Soil
Contamination of the soil occurs when carbaryl is used in
agriculture. During the treatment period, levels of 1-3 mg
carbaryl/kg were measured in the surface layer of the soil in the
USSR (Molozhanova, 1968). Concentrations of 0.03-0.35 mg/kg were
found in the surface layer of the soil up to 52 days after a single
application of carbaryl at a rate of 2 kg/ha (Guseynov, 1970).
Carbaryl levels in the soil varied depending on the type of soil
(Table 12).
Potatoes were sprayed with carbaryl at 4.25 kg/ha (5 kg Sevin
WP, 85%/ha). One day, 10 days, and 2 months after treatment,
residues in the soil under the potatoes were, respectively, 1.9,
9.55, and 0.02 mg/kg (Kovaleva & Talamov, 1978b). When the soil was
sprayed before sowing, the residues in the soil 1 day, 1 month, 4
months, and 15 months after treatment were 1.5-2, 0.05-0.07,
0.02-0.08, and 0.06 mg/kg, respectively. Within 100 and 150 days
following soil incorporation of carbaryl (Sevin, 10 kg/ha), residues
in non-planted soil decreased to 0.05 and 0.02 mg/kg, respectively
(Kovaleva & Talanov, 1980).
Table 12. Carbaryl content in different types of soil during
harvestinga
Type of soil Number of Carbaryl concentration
samples (mg/kg)
Meadow-chernozem 200 2.22 ± 0.5
Chernozem southern 766 1.01 ± 0.23
Chernozem ordinary 288 1.09 ± 0.26
Chenozem podzol 100 0.15 ± 0.04
Turf-podzol 150 0.04 ± 0.01
Grey-woodland 246 0.0
aFrom: Molozhanova (1970).
Yadav et al. (1985) detected 0.22 mg carbaryl/kg in the soil
at harvest time, when sesamum ( Sesamum indicum) was sprayed twice
with carbaryl (0.2%) on days 45 and 60 of crop growth (21 August and
5 September). As residues in the soil after the first spray were
about 0.65 mg/kg, the rate of reduction of residues in soil was 56%
within 2 months. Shilova et al. (1973) measured about 0.1 mg
carbaryl/kg soil one month after treatment with carbaryl (5 kg/ha)
against blood sucking insects in the subarctic.
Gangwar et al. (1978b) applied a 4% granular formulation to
sandy loam planted with a grain crop (bajra) at very exaggerated
application rates of 20, 40, and 60 kg a.i./ha. Initial residues of
140-354 mg/kg decreased by a total of 31 to 53% within 30 days and
95 to 98% within 90 days. Residues at 90 days were 2.68-17.49 mg/kg.
Application of carbaryl granules at very high doses of 15, 30,
and 45 kg/ha resulted in deposits of 232, 397, and 525 mg/kg,
respectively, in soils cultivated with corn, and in 104-109,
168-217, and 304-422 mg/kg, respectively, in clay loam soils
cultivated with root crops (beet, radish, carrot). The dissipation
rate of carbaryl residues from clay loam soil, in 5 days following
the treatment, was 42-56% during autumn and 55-69% during spring.
The highest residue levels sampled from all plots decreased to
229.7, 142.9, 73.8, 33.8, 6.7, and 1.3 mg/kg on days 5, 10, 15, 30,
60, and 100, respectively. Residues were below detectable levels in
several plots by day 60 and in most plots by day 100 (Kavadia
et al., 1978).
According to Kuhr et al. (1974), the dissipation of carbaryl
in the soil of apple orchards is rapid. Soil residues of carbaryl
had almost completely disappeared from the top 2 inches of soil in 2
weeks. They were 13.8 mg/kg immediately after application and
3 mg/kg, 1-2 days later.
An oil-based carbaryl formulation (Sevin-2-oil) was applied
twice by a fixed-wing aircraft to a coniferous forest in New
Brunswick (Canada). Initial residue levels 1 h post-spray in litter
and soil for both applications were, respectively, 1.21 and
0.86 mg/kg and 0.78 and 0.48 mg/kg (Sundaram & Szeto, 1987).
Relative to the amount sprayed, only a small amount of the chemical
reached the litter and soil, probably because of canopy filtration.
Within 1 day, an average of 40-45% of the initial residues was lost
from litter and soil, respectively, indicating a rapid dissipation
time of 1.5 days for the disappearance of 50% of the initial maximum
concentration. Beyond 5 days, an average of 12% of the initial
concentration remained in both the substrates.
As already reported, two field dissipation studies were
conducted by Springborn (1988b,c) with carbaryl (Sevin-4-oil)
applied twice with a seven-day interval to forest. The first study
was conducted in a coniferous Oregon forest. In soil from the
treated site, carbaryl levels decreased from 0.196-3.877 mg/kg
following the first application to 0.130-1.87 mg/kg, 3 days after
treatment, and from 0.079-5.323 mg/kg following the second
application to 0.242-1.187 mg/kg at 90 days. The second study was
conducted in Pennsylvania. Carbaryl levels in soil decreased from
0.022-0.068 mg/kg following the first application to
<0.012-0.075 mg/kg, 3 days after treatment, and from
0.11-0.932 mg/kg following the second application to
0.01-0.099 mg/kg at 90 days.
A rice field dissipation study was conducted in Arkansas (USA)
by Springborn (1988a). Carbaryl (Sevin XLR) was applied 3 times, at
14-day intervals, to an irrigated field plot in which rice was
planted. In flooded soil, the concentration of carbaryl varied from
<11 to 309 µg/kg throughout the study and was not directly related
to application date. The carbaryl level was <11-309 µg/kg in soil
following the first application and <11-129 µg/kg following the
third application; 180 days later, carbaryl concentrations in the
soil were 11-56 µg/kg.
In a similar study conducted in California, under the same
conditions, Springborn (1988) found that carbaryl dissipated rapidly
in flooded soil. The concentration of carbaryl ranged from <11 to
198 µg/kg throughout the study. Carbaryl was <11-23 µg/kg in soil
at sampling intervals following the first application, and increased
to <11-180 µg/kg following the third application; 180 days after
the third application, carbaryl concentrations in the soil were
11-88 µg/kg.
A soil dissipation field study was conducted in 1985/1986 in
California (sandy loam, pH 6.3) and Iowa (silt loam, pH 6.5) with
carbaryl 80 S formulation (Davis, 1986a). Carbaryl was applied to
strip plots of bare soil, 6 weeks later, 7 applications were made
with intervals of about 5 days between applications. Soil sampling
was performed before treatment, during treatment, and up to 6 months
after the last application. No carbaryl residues were found in
California pretreatment samples. After the last application,
residues reached a maximum of 1.61 and 0.29 mg/kg in the 0-15 and
15-30 cm samples, respectively. Fourteen days after the last
application, carbaryl residues below the 15-cm level were less than
the limit of quantification.
It took 28 days for residues to dissipate completely from the
top 15 cm. The half-life in California soil was calculated to be
2.7-3.8 days.
5.1.4 Food and animal feed
5.1.4.1 Fruit, vegetables, and grain
Contamination of vegetation by carbaryl occurs, either during
spraying, or, by its migration through contaminated soil into the
roots of plants.
Residual levels of carbaryl after the spraying of plants depend
on the type and species of the plants sprayed. Carbaryl levels of up
to 30 mg/kg have been found in plants during the treatment period
(Molozhanova, 1968). It has been found to be rather persistent in
vegetables and fruits. Concentrations of 0.6-3.9 mg/kg were measured
in lettuces 1 week after single or repeated sprayings, and levels up
to 1 mg/kg were found in tomatoes treated according to requirements
(Antonovich, 1970). In cabbage, initial residues after spraying
ranged from 14.8 to 33.9 mg/kg, depending on the concen-tration
used. After 7 days, the residues decreased to 2.5-5.13 mg/kg. In
eggplant, the initial concentrations were 8.3-16.9, and 7 days
later, 3.05-5.4 mg/kg. After washing, the concentrations decreased
considerably to less than 3 mg/kg after day 7 (Mann & Chopra, 1969).
Following application of carbaryl on cauliflower, in the form
of dust (10%) at 1.5-2 kg a.i./ha or wettable powder (50%) at 0.75-1
kg a.i./ha, the residues of carbaryl declined to 3.64-9.59 mg/kg
within 8 days of treatment. The carbaryl deposits on leaves were
between 19.45 and 42.08 mg/kg. Washing of cauliflower with plenty of
water reduced the residues of carbaryl by 36-95%. A waiting period
of 8 days has been suggested for cauliflower (Singh et al., 1978).
Persistence of carbaryl in brinjals and peas after spraying at
1 and 2 kg a.i./ha was found to be 3.42 and 5.03 mg/kg respectively.
Washing of vegetables with water within one hour and after a day of
spraying reduced the level of deposits of carbaryl to about
1.0 mg/kg level. In the case of pea pods, the levels of residues
dropped to 0.20 mg/kg within 5 days. In pea seeds, the residue
levels ranged between 0.03 and 0.09 mg/kg, after 8 days (Krishnaih
et al., 1978).
Measurements of carbaryl in different fruits were taken at
intervals of 5-10 days after plants had been treated with a 0.2%
suspension of carbaryl at 1000 litre/ha during the vegetation period
(Bogomolova, 1968, 1970). The initial amount of carbaryl, 2 days
after the treatment, varied in different fruits from 2.3 to
2.7 mg/kg. Ten days later, the concentrations were reduced by
50-70%, and, after 20 days, the levels were within the range of
0.2-0.8 mg/kg, depending on the species (Table 13). According to
Mann & Chopra (1969), the dissipation of carbaryl from plants after
the first day of spraying was about 40-45% of the initial residue.
In apples, concentrations of 2.5-2.9 mg/kg, 0.4-2.4 mg/kg, and
0.15 mg/kg were found on days 7, 10, and 38, respectively, after
spraying. One to two months after spraying apples with a 0.1%
suspension at a dose of 2000 litre/ha, residues of 0.08-0.10 mg/kg
were measured (Antonovich, 1970). Four months after spraying with a
0.06% suspension at a dose of 9 kg a.i./ha, levels in the range of
0.09-0.24 mg/kg were found in apples, depending on the spraying
procedure. Fine-droplet spraying resulted in residue levels 2.5-3
times lower levels than those with coarse-droplet sprays (Atabaev,
1972).
Table 13. Carbaryl levels in different fruits after spraying
during vegetation (mg/kg)a
Kind of fruit Day after spraying
2 10 20
Strawberries 2.3 1.25
Gooseberries 2.5 0.90 0.6
Blackcurrants 2.4 1.10 0.8
Cherries 2.7 1.90 0.2-0.4
Plums 2.4 1.40 0.2-0.4
aFrom: Bogomolova (1968).
A survey of apples grown in Ontario, Canada, between 1978 and
1986 showed that out of 22% of carbaryl-treated apples (1.67 kg/ha)
sold, 3.6% had detectable carbaryl residues (detection limit of
0.01 mg/kg), and that the average residue level was 0.03 mg/kg, with
a maximum of 0.04 mg/kg (Frank et al., 1989).
Prolonged storage of apples reduced the carbaryl residues only
slightly (Bogomolova, 1968). The initial level (2.4-2.8 mg/kg)
changed little during the first 3 months of storage at a temperature
of +5 to +10 °C. A slight reduction was observed during the fourth
month of storage. During the process of storage, carbaryl migrated
inwards from the surface, so that the concentration in the skin was
reduced, while that in the pulp was increased (Table 14).
Residues on lemon foliage on day 0 of treatment were
4.6±0.3 µg/cm2. Five days after treatment, levels of 2.4 µg/cm2
and 5.6 µg/cm2 were found on lemon and orange foliage,
respectively; on the 60th day, the residual values were 0.41 and
0.36 µg/cm2, respectively. Persistence half-lives were 22 days
(lemons) and 14 days (oranges) (Iwata et al., 1979).
Carbaryl is persistent, particularly in citrus fruits and
grapes. Residues of 3-6 mg/kg were measured in lemons and oranges
2.5-3 months after treatment of trees with 3-10 kg carbaryl/ha, and
amounts of 3.6, 1.9, and 0.4 mg/kg were found in grapes on days 7,
14, and 40, respectively, after repeated spraying of vineyards with
a 0.12% suspension. The half-life of carbaryl in grapes was 29 days,
compared with 2-9 days in cherries and cabbage (Antonovich, 1970).
Washing and peeling reduced carbaryl levels in fruits and
vegetables by 40%; thermal processing by 45-90%, and preparation of
juices by 53% (Molozhanova, 1970). Canning also reduced carbaryl
residues. Thus, canning of different fruits (strawberries, black
currants, gooseberries) containing carbaryl in the range of
0.25-0.45 mg/kg, resulted in a reduction to 0.17-0.29 mg/kg; when
the initial contents were higher (3-7 mg/kg) the levels after
canning dropped to 1.4-2.2 mg/kg and 2.1-2.5 mg/kg, respectively,
i.e., from 30 to 70%. Storage of canned fruit further reduced
carbaryl residues by 2-3 times after 12 months (Bogomolova, 1968).
Boiling vegetables (e.g., cabbage) reduced carbaryl residues by
approximately 50%; however, the residues in pickled cabbage, 5
months after preparation, were only 25% less than the initial amount
(Antonovich, 1970).
Table 14. Carbaryl content in apples during storage (mg/kg)a
Part of apple Day of storage
10 20 30 40 50 70 80 100 110
Skin 2.40 1.80 2.00 1.80 1.92 1.72 1.60 1.45 1.22
Pulp 0.35 1.00 1.02 1.10 1.00 1.10 1.34 1.42 1.30
Total 2.75 2.80 3.02 2.90 2.92 2.82 2.94 2.87 2.52
aFrom: Bogomolova (1968).
Elkins (1989) reported that washing during the commercial
processing of produce removed 97, 87, and 77% of the carbaryl
residues from tomatoes, spinach, and broccoli, respectively.
Blanching (short treatment with hot water) was stated to result in
68% removal of carbaryl from green beans, while blanching, in
addition to washing, resulted in the removal of over 97% of carbaryl
from both spinach and broccoli.
After a single application of carbaryl (Sevin 50 at 2.5 kg/ha),
residues in cauliflower decreased from 16.75 mg/kg on the day of
application to 0.87 mg/kg, 15 days afterwards (Yadav & Jaglan,
1982). Washing further reduced the detectable residues at 15 days to
0.67 mg/kg, while no detectable residues were present after boiling.
Carbaryl was found in vegetation adjacent to treated fields.
Grass growing at a distance of 250 m from a carbaryl-treated cotton
field contained 0.18-0.25 mg carbaryl/kg wet weight up to 45 days
after spraying (Gusseynov, 1970).
The results of the 1989 Pesticide Residue Monitoring Programme
in California showed that carbaryl was detected in 2 samples of
grape from 26 in quantities less than the accepted tolerance level.
It was not found in oranges (Okumura et al., 1991). Summaries of
residue data on different plants are given in Tables 15 and 16.
Carbaryl residues were not detected in 44 samples of wheat in the
United Kingdom analysed by the multiresidue method (Osborne et al.,
1989).
Table 15. Comparison of carbaryl residues with different
formulationsa
Formulation use Plant Mean residues Day after last
(kg a.i./ha) value (mg/kg) application
44% SC 2x0.5 apple 0.25 7
pears 0.16 7
50% WP 2x0.5 apple 0.15 7
pears 0.09 7
44% SC 2x2 spinach 4.44 -
50% WP 2x2 2.38 14
44% SC 2x2 lettuce leaf 2.72 14
50% WP 2x2 1.55
44% SC 2x2 barley 7.87 14
50% WP 2x2 4.63
Table 15 (continued)
44% SC 2x2 wheat 0.96 14
50% WP 2x2 0.99
44% SC 2x2 oats 0.22 14
50% WP 2x2 0.26
aFrom: Davis (1987).
Davis (1987) studied the impact of different types of
formulation on food residues, including carbaryl (44% w/w) oil-based
liquid formulation containing a "sticker", in order to prevent bees
from carrying carbaryl particles back to the beehive. The 50 W
formulation was a "standard" wettable powder formulation. The
results are given in Table 15. This work showed that the addition of
a "sticker" increased levels of food residues to some extent.
Studies on the removal of carbaryl residues from tomatoes,
green beans, spinach, and broccoli by commercial and home
preparation procedures (Elkins et al., 1968; Farrow et al.,
1968, 1969; Lamb et al., 1968) showed a considerable decrease in
the residues with treatment (Table 17).
For pre-harvest use on grain, the rate of application of
carbaryl ranges from 2 to 9.5 kg/ha, depending on the degree of
infestation, density of foliage, and the stage of the life cycle of
the pest. Carbaryl, usually applied at a rate of 5 mg/kg, is also
used to protect stored grain. Studies on stored wheat, barley, oats,
and rice indicated that carbaryl residues on grain have a half-life
of between 26 and 80 weeks, depending on the temperature (FAO/WHO,
1976). Residues of carbaryl in baked bread are then of the order of
1-1.5 mg/kg.
5.1.4.2 Animal products
Technical carbaryl was fed to dairy cows of the Brown Swiss,
Jersey, Holstein, and Ayrshire breeds at 50, 150, and 450 mg/kg of
their average total daily roughage intake (dry weight) for a period
of 2 weeks. Samples of milk were taken at regular intervals and the
cream was analysed for carbaryl by means of the
p-nitrobenzene-diazonium fluoborate coupling method. The
concentration of carbaryl, if present, was below the sensitivity of
the analytical method (0.01 mg/kg) (Gyrisco et al., 1960).
Table 16. Carbaryl residues following different applications
Formulation use Plant Mean value Day after Reference
(kg a.i./ha) residues last
(mg/kg) application
44% SC barley 5.4 14 Davis & Thomas (1987)
44% SC sugar beet 0.07-1.04 14 Thomas (1986)
roots
44% SC pasture 59.7-183 3 Davis (1986b)
aerial ground grass
Carbaryl sweet 0.33 Frank et al. (1987a)
cherries
Carbaryl tomato 1.2 0 Frank et al. (1991)
0.5 3
0.03 6-8
tomato 0.47 0
juice 0.24 3
0.08 6-8
Table 17. Removal of carbaryl after preparation procedures
Vegetable Initial Percentage removal References
residues
(mg/kg) Commercial procedure Home procedure
Tomatoes 5.2 washing 82-99 washing 77 Farrow et al. (1968)
canning 98-99 canning 92
juicing 98 juicing 77
Green beans 7.6 blanching 68-73 washing 52 Elkins et al. (1968)
& canning blanching 81
freezing 94
canning 100
Spinach 20.8 washing 66-88 washing 70 Lamb et al. (1968)
blanching 96-97
Broccoli 12.4 washing 77 washing & cooking 55 Farrow et al. (1968)
blanching 82-97 washing, 90
blanching,
& freezing
Residues resulting from a single application and repeated
applications of carbaryl spray on cattle were rapidly eliminated
from body tissues. On days 1 and 3 after application, carbaryl was
detected in the liver (0.05 mg/kg), muscles (0.04 mg/kg), and
perirenal fat (0.16 mg/kg). Seventy-two hours after treatment, no
residues were found in the tissues studied. The excretion in milk
persisted for at least 69 h after spraying, the highest
concentration being 0.075 mg/litre, 5 h after exposure (Hurwood,
1967).
Data concerning the composition and levels of milk and tissue
residues in cows after continuous feeding with 100 mg
14C-carbaryl/kg diet are shown in Tables 18 and 19.
Table 18. Metabolites in tissues after continuous 28-day feeding
of 100 mg 14C- carbaryl/kg dieta
Metabolite (mg/kg) Kidney Liver Muscle
carbaryl 0.03 0.04 0.02
5,6-dihydrodihydroxycarbaryl 0.05 0.01 0.04
5,6-dihydrodihydroxynaphthol 0.02 0.02 0.0
naphthyl sulfate 0.29 0.02 0.0
water-soluble unknowns 0.43 0.13 0.03
unextractable unknowns 0.18 0.19 0.01
aFrom: Dorough (1971).
Table 19. Composition of milk residues after continuous 28-day
feeding with 100 mg 14C-carbaryl/kg dieta
Percentage mg/kg
Organic phase
5,6-dihydrodihydroxycarbaryl 38.5 0.11
carbaryl 8.4 0.02
3,4-dihydrodihydroxycarbaryl 4.0 0.01
Table 19 (continued)
Percentage mg/kg
5,6-dihydrodihydroxynaphthol 1.0 0.01
Water soluble
5-methoxy-6-hydroxycarbaryl 25.6 0.07
unknowns 10.6 0.03
1-naphthol 5.5 0.02
5-hydroxycarbaryl 3.1 0.01
carbaryl 1.2 0.01
aFrom: Dorough (1970a).
Effective horn fly control for a period of 15 days was obtained
by spreading carbaryl down a cow's back. Results indicated that, if
applications of a 0.5% spray or a 50% dust were made immediately
after the morning or evening milking, no residues of carbaryl would
result in subsequent milkings (Eheart et al., 1962). The results
obtained by Petrovski (1970) were similar. After treating cows with
a 0.85-1% suspension of carbaryl, residues of from 0.1 to
0.3 mg/litre were found in milk, 20 h after application. On the
third day, only trace amounts of carbaryl were found. After
treatment with a 0.5% suspension of carbaryl, no traces were found
in the milk.
5.1.4.3 Animal feed crops
Generally, animal feed crops contain less carbaryl than fruit
and vegetables. Mean levels of 0.82 mg/kg were found in 80 samples
of animal feed plants (Molozhanova, 1970). Levels of 0.3 mg/kg were
found in maize stems at harvest, 70 days after spraying (Antonovich,
1970).
Studies on the carbaryl content of animal feed plants in hot
climates showed that carbaryl can be detected 3-3.5 months after
treatment, depending on the dose and the number of applications.
Thus, after repeated applications of 50% carbaryl dust at a rate of
0.5 g/m2, the carbaryl content in animal feed plants was in the
range of 1-1.8 mg/kg wet weight, but, after a single application of
0.7 g/m2, concentrations of 0.10-0.31 mg/kg were found (Atabaev,
1972).
5.1.5 Other products
Measurement of carbaryl residues in tobacco plants showed that,
15 days after field spraying with 950 g/ha, the content of carbaryl
in green tobacco leaves was 10.1 mg/kg. Drying leaves by hot air
decreased carbaryl residues by only 10% (Antonovich, 1970).
Application of carbaryl at a rate of 2.2 kg/ha resulted in
carbaryl residues on cotton foliage of 7.2 µg/cm2 on the day of
spraying (Estesen et al., 1982). Levels decreased to 5.9, 0.58,
and 0.26 µg/cm2 after 3, 6, and 8 days, respectively.
Carbaryl was found in the foliage of cotton plants in
concentrations of 0.03-1 mg/kg from 10 days to 2.5 months after
treatment. Carbaryl penetrated the plant through foliage and roots
and contaminated the cotton and the seeds. Cotton harvested from
treated fields 2 weeks to 1 month after treatment contained
0.015 mg/kg. Trace amounts of carbaryl were found in cotton oil
(Guseynov, 1970).
After treatment with a 2% suspension of carbaryl against
mountain pine beetle, residues in bark disks after 1 year were
359 mg/kg. In another case, residues in bark disks were
890±146 mg/kg, after spraying, and 531±178 mg/kg 16 months later
(Page et al., 1985).
5.1.6 Terrestrial organisms
The quantities of carbaryl that were absorbed by songbirds,
either by contact or by eating food from a sprayed area, were
studied by Kurtz & Studholme (1974). Towhees (American finches) were
collected 3 days after the forest had been sprayed for gypsy moths
at a rate of 1.1 kg/ha. The amounts of carbaryl found in the towhees
were small, even though the samples were taken close to the spraying
time. Trace amounts of carbaryl were found in three samples out of
five, compared with two samples out of five control birds. The
minimum level of detection was 0.1 µg/g. The low levels of carbaryl
found in "sprayed" birds were explained by the fact that towheeds
are groundfeeders and that only a small amount of carbaryl might
have passed through the trees to the ground.
5.2 General population exposure
5.2.1 Exposure through the food
The daily intake of carbaryl was studied for several years in
the USA (see Table 20).
Table 20. Carbaryl daily intake in the USA
Year Positive samples Daily Intake References
(%) (mg/day
per person)
1964-65 7.4 0.15 Duggan et al. (1971);
1965-66 2.7 0.026 Duggan & Corneliussen
(1972)
1966-67 1.1 0.007
1968-69 0.8 0.003
1969-70 0 -
Infant Toddler
1978 - 0.088 0.05 Gartrell et al. (1986)
1979 - - 0.049
1980 - 0.06 0.035 Reed et al. (1987)
1981-82 - 0.129 0.127
1982 - - 0.012
1982-84 - 0.117 0.017- Gunderson (1988)
0.0315
Carbaryl residues in total diet samples in the USA are
relatively low (Table 21). Carbaryl is found in potatoes, leguminous
vegetables, root vegetables, and fruit. In foods processed in the
usual manner, i.e., by peeling, stripping outer leaves, and cooking,
when appropriate, carbaryl residues usually decreased to
undetectable levels or traces (Manske & Corneliussen, 1974).
Table 21. Carbaryl residues in food in the USA
Number of composites Number of Number of Range in References
analysed composites composites mg/kg
with residues with traces
270, from 30 grocery 20 15 trace to Manske & Corneliussen
stores in 27 cities 0.5 (1974)
June 1970-April 1971
420, from 35 market 6 5 0.02 Manske & Johnson
baskets, 6 June 1971- (1975)
July 1972
360, 12 August 1972- 12 10 0.05-1.10 Johnson & Manske (1976)
July 1973
360, August 1973- 8 2 0.001-0.284 Manske & Johnson (1977)
July 1974
1044, 1978-82 11 (1%) a a Yess et al. (1991a)
3744, 16 market 135 (4%) a a Yess et al. (1991b)
basket collections
1982-86
aNo figures given.
During a 5-year period, from 1982 to 1986, the Los Angeles
District Laboratory analysed 19 851 samples of domestic and imported
food and feed commodities for pesticide residues. A single, rapid,
multiresidue method was used. Carbaryl was detected in 164 samples
from the total 19 851 samples analysed. Not one sample exceeded the
US Federal tolerance levels (Luke et al., 1988). In another
publication, the same authors reported comparative studies on US and
imported food. Carbaryl was found in 21 samples from a total 6391 US
samples in quantities ranging from 0.1 to >2.0 mg/kg, but less than
10 mg/kg. From 12 044 imported agricultural commodities 132 were
contaminated by carbaryl, and levels in 14 of them exceeded the
tolerance levels (Hundley et al., 1988).
5.2.2 Exposure during insect control
Exposure to carbaryl, used to control the gypsy moth in camping
and picnic areas, was monitored on the day of application and then
weekly for 3 weeks. Carbaryl was applied from the air at a rate of
1.12 kg/ha. Exposure was measured using dermal pads.
Extrapolating from the most contaminated group of pads (they
contained an average of 279 µg/pad), the authors calculated a "worst
case" exposure of 0.54 mg/kg on the day of spraying. The authors
concluded that the risk to those who use public areas during, and
after, carbaryl application to trees is negligible (Cameron et al.,
1985).
A 5% spray of carbaryl 85% wettable powder (WP) was applied for
insect control in homes (Vandekar, 1965), as a surface application,
at the rate of 2 g/m2. Urine samples taken from villagers 1 week
after spraying showed a significant increase in 1-naphthol levels
from 30.5 µg/ml baseline to 50.3 µg/ml. Inhibition of plasma ChEA
was found in 48 out of 63 subjects, 1 week after spraying.
During a monitoring study carried out from 1976 to 1980 in the
USA, 1-naphthol was found in 1.4% of the urine samples from persons
between 12 and 74 years old. The source was thought to be carbaryl
and naphthalene (Carey & Kutz, 1985).
5.3 Occupational exposure during manufacture, formulation,
or use
Harry (1977) estimated that about 13 million people in the USA
were potentially exposed to carbaryl during its manufacture,
formulation, packing, transportation, storage, and during and after
application, and while working with treated crops or during harvest.
Best & Murray (1962) published a survey on the exposure of
plant workers during the production of carbaryl. Air concentrations
varied from 0.23 to 31 mg/m3. Urine samples contained an excess of
1 mg total 1-naphthol per 100 ml of urine. During air blast spraying
of orchards, Jegier (1964) found air concentrations of 0.6 mg/m3
(0.18-0.81 mg/m3). The mean respiratory exposure, measured by the
respirator pad technique was 0.29 mg/h (0.24-0.53 mg/h) and mean
dermal exposure, measured by skin pads, was 25.3 mg/h
(18.5-30.3 mg/h). The maximum total exposure was 31 mg/person per h,
or 0.025% of the toxic dose. Simpson (1965) estimated that the
amount of dermal exposure was less than 0.1% of the toxic dose in
orchard sprayers. During cotton spraying by aircraft, Yakim (1965)
found 4 mg carbaryl dust/m3 in the breathing zone of flagmen,
1 mg/m3 during preparation of the solution, and 0.7 mg/m3 in the
pilot's cabin. Adylov (1966) reported the following air
concentrations found during the aerial application of a water
solution: flagmen's breathing zone 1.92 (0.64-2.84) mg/m3; pilot
cabin, trace amounts, workers on the ground (preparation of
solution, etc.,) 6.28 (0.48-19.2) mg/m3. In the urine samples of
carbaryl formulators, 1-naphthol levels varied from 6.2 to
78.8 mg/litre. Agricultural workers who used carbaryl for pest
control excreted from 0.07 to 1.7 mg/litre in their urine (Shafik
et al., 1971).
Exposure studies were completed for pesticide application and
formulating plant workers by the biological monitoring of 1-naphthol
in the urine, dermal pads, and respirator filter pads (total 480
samples of dermal pads and 73 respirator pads). Workers operating
tractor-drawn airblast equipment applied carbaryl at 0.045-0.06% as
spray (Comer et al., 1975). An estimate of the amount of dermal
and respiratory exposure that could occur was made using the
procedures described by Durham & Wolfe (1962). The results are
presented in Table 22.
The concentration of 1-naphthol in the urine varied from 0.2 to
65 mg/litre with a mean of 9 mg/litre. The rate of excretion per
hour varied from 0.004 to 3.4 mg with a mean of 0.5 mg/h. The
maximum level during the working day was reached by late afternoon.
An approximate calculation of the excreted carbaryl (0.5 mg
1-naphthol/h = 0.7 mg/h excretion of carbaryl), and potential
exposure, 75 mg/h, shows great differences. About 1/100 part of
possible absorption occurs.
Exposure to carbaryl during agricultural application was
studied by Leavitt et al. (1982). WP 80 carbaryl was used (0.45 kg
in 200 litre water) to spray trees, lawns, and gardens. The mean
dermal exposure was 128 mg/h and the mean inhalation exposure was
0.1 mg/h. The maximum percentage of the toxic dose that the
applicators received was 0.12%/h. No symptoms or inhibition of ChE
were reported. In another group of applicators, the mean dermal
exposure was 59.4 mg/h and inhalation exposure 0.1 mg/h.
Table 22. Potential dermal and respiratory exposure of formulating
plant workers and field spraymen to carbaryla,b
Subject Route of Exposure Exposure (mg/h)
exposure situations
studied Range Mean
Formulating dermal 48 0.80-1209.30 73.90
plant workersc
respiratory 48 0.03-4.10 1.90
Field dermal 32 1.70-211.80 59.00
spraymend
respiratory 25 0.01-1.08 0.09
aFrom: Comer et al. (1975).
bCalculated on the basis of the worker wearing a short-sleeved,
open-necked shirt, no gloves or hat, with his clothing protecting
the areas covered.
cWorkers on mixing and bagging operations (4 and 5% dust).
dOperating power air-blast spray machines in fruit orchards
(0.045-0.06% solution spray).
Dermal exposure is influenced by the type of spray equipment.
The dermal deposit and respiratory intake of carbaryl in humans was
monitored during home garden spray operations using compressed air
or garden hose-end sprayers (Puech, undated). The greatest mean
dermal deposit in cm2/min was received on the feet. When the spray
target was above shoulder height the next highest dermal spray was
received on the forearm (Table 23). Exposure through inhalation was
insignificant.
A study of the urban application of carbaryl was performed by
Gold et al. (1982). The maximum dermal exposure recorded in this
study was 2.86 mg/kg per h. The maximum air concentration was
0.28 µg/litre. An insignificant decrease in ChEA in serum and
erythrocytes was found in some of the applicators. The mean dermal
carbaryl exposure of the applicator, expressed as a percentage of
toxic dose per h, was 0.01%, with a maximum of 0.08%. This exposure
rate is below the risk rate for applicators. No symptoms of
intoxication were reported by the authors. During the urban
application of carbaryl to trees and ornamental shrubs using
hand-held equipment, air concentrations measured at the breathing
zone in full-shift samples, of 0.010-0.070 mg/m3, were detected in
only 30% of the samples (Leonard & Yearly, 1990).
Table 23. Human exposure during spray operations
Type of spray equipment Mean deposit Total exposure
(µg/cm2 per min) (µg/kg per min)
Compressed air sprayer
below waist 0.030 4.3
above shoulder height 0.096 5.6
Hose-end sprayer
below waist 0.154 18.3
above shoulder height 0.353 24.4
Dermal exposure to carbaryl in harvesters of strawberries was
studied by Zweig et al. (1984, 1985). They observed dermal
exposure on the hands and forearms, and to a much lesser degree, on
the lower legs. The first day, 3 mg carbaryl/h was found on cotton
gloves, 0.66 mg/h on pads on the forearms, and 0.07 on the lower
legs. The following day, the values were 1.23, 0.41, and 0.07 mg/h
for gloves, forearms, and lower legs, respectively. The ratio of
dermal exposure to dislodgeable foliar residues (DFR) was 4.34,
2.82, and 6.17, for 3 consecutive days. The half-life of carbaryl on
strawberry leaves was 4.1 days. Dislodgeable carbaryl residues on
cotton foliage, expressed as µg/cm2 of cotton leaf (one surface
only), following application by man-pulled ground rig were 7.2, 5.9,
0.58, and 0.26 at 0, 72, 144, and 192 h after application,
respectively (Estesen et al., 1982). Approximate dermal exposure
rates may be calculated using the following expression, proposed by
Zweig et al. (1985). Dermal exposure rate (mg/h) = 5x103xDFR
(in µg/cm2). This method is suggested to obtain the exposure rate
of fruit harvesters, in order to establish safe re-entry periods
without human studies.
Factors affecting the levels of exposure during the
agricultural application of pesticides were analysed by Wolfe et
al. (1967). Wind is the most important environmental condition. The
type of activity, equipment used, the duration of exposure,
formulation, individual protection, including attitude, are also
discussed. Carbaryl deposits from air jet application on orchards
were found at 500 m downwind in the presence of inversion, and at
300 m, in its absence. Ground application results gave deposits at
150 and 50 m, respectively (MacCollom et al., 1985, 1986). Air
concentrations were 17.88 µg/m3 during spraying above the orchard
downwind edge, 9.5 µg/m3, 30 min after spraying, and 8.17 µg/m3,
1 h after spraying.
Airborne and deposit levels of carbaryl were measured after
three applications by air to an apple orchard (Currier et al.,
1982). Samples of air were taken at distances of from 12.2 to 3994 m
from the target orchard. At a distance of 12.2 m, the concentration
in air was between 3.3 and 76.2 µg/m3. One hour later it was
between 2.5 and 11.3 µg/m3. At a distance of 3.2-3.3 km, the
initial concentration was between 9 and 28.8 µg/m3 during spraying
and 0.9-14.4 µg/m3, one hour after treatment. Because the area of
the study was one of concentrated agriculture, it is possible that
carbaryl could have been used on other orchards and gardens near to
the sampling point.
6. KINETICS AND METABOLISM
6.1 Absorption
When only 0.1 ml of carbaryl solution (0.025-0.05 mmol/litre)
was administered into the lungs of anaesthetized rats, it was
rapidly absorbed. About 50% of the dose was absorbed in 2.6 min. The
amount of carbaryl absorbed per unit time was directly proportional
to the administered dose (Hwang & Shanker, 1974). Blase & Loomis
(1976) demonstrated that carbaryl could be taken up and metabolized
by the isolated perfused rabbit lung. Retention by rats of
14C-labelled carbaryl, inhaled as vapours during a 1-h exposure
period, was 75.4% of the total dose inhaled, which did not exceed
50 µg (Dorough, 1982).
When a dose of 7.5 µmol radiolabelled carbaryl/kg body weight
was administered intragastrically to fasted, anaesthetized, female
rats, 22 and 67 min after dosing, the proportions absorbed were
52.6±14.1% (n=3) and 81.7±15.7% (n=3), respectively; 89.3% of the
radiolabelled material in the collected portal blood was
[1-naphthyl-1-14C] N-methyl carbarmate (Casper et al., 1973).
Absorption from the small intestine was studied in
anaesthetized rats with a ligature around the pylorus and an
ileocecal junction with major blood vessels not occluded. A
concentration of carbaryl of 0.005-0.1 mmol/litre was used. The
absorption half-time was 6.4 min (Hwang & Schanker, 1974).
On the basis of ligation studies on mice, Ahdaya & Guthrie
(1982) determined that the absorption of carbaryl from the stomach
was relatively low compared with that from the entire
gastrointestinal tract (29%), but was relatively high in comparison
with that of other pesticides.
Variation in the digestive absorption kinetics, according to
the vehicle used, was reported in studies on female Wistar rats
(Cambon et al., 1981). The results indicated that carbaryl was
absorbed more rapidly in the intestine when either DMSO or
tragacanth was used as a vehicle. It seems that milk does not
facilitate carbaryl absorption. Inhibition of ChEA was closely
related to the absorption rate.
The rate of dermal penetration of carbaryl (in acetone
solution) into mammals, birds, amphibia, and insects was studied by
Shah
et al. (1983). The half-time of penetration of 14C-labelled
carbaryl was 317 min in Japanese quail, 12.8 min in mice, 6.4 min in
grass frogs, 4576 min in American roaches, and 791 min in tobacco
horn worms.
In an in vitro study, only about 1% of an applied dose of
carbaryl penetrated through the skin of the rat over an 8-h period
(MacPherson et al., 1991). Using two different methods, Shah &
Guthrie (1983) measured the half-time for penetration of carbaryl,
applied dermally at a rate of 4 µg/cm3, and found 10.34 h and
4.75 h, respectively. Shah et al. (1987) compared the rate of
dermal penetration of carbaryl in young, versus adult, Fischer 344,
female rats and did not find any consistent age-related differences.
Percutaneous absorption of carbaryl in rats was also studied by
Knaak et al. (1984). Carbaryl dissolved in acetone was applied to
back skin (not occluded) at 43.4-48 µg/cm2. Recovery studies
indicated that 57.7% of the applied carbaryl was absorbed.
Approximately 5.8% of carbaryl penetrated the skin within 1 h, the
rate of absorption being 0.18 µg/h per cm2. The half-life of
absorption by blood was 1.26 h, and that for elimination, 67 h.
Carbaryl labelled with radioactive carbon (14C), dissolved in
acetone, was applied to the skin of six volunteers, in order to
study percutaneous penetration (Maibach et al., 1971; Feldmann &
Maibach, 1974). The results showed almost complete penetration of
carbaryl on the forearm and jaw angle. After a 24-h application, the
cumulative urinary excretion over 5 days was 74%. According to other
authors using the same data, the estimated cumulative absorption
over 5 days, as a percentage of the applied dose, was 63% (Fisher
et al., 1985), 45% of this occurring 8 h after the onset of
penetration, which had a lag of 3.5 h.
Comparing the different studies, it is clear that some solvents
can facilitate the dermal penetration of carbaryl.
6.2 Distribution
Plasma levels of carbaryl in rats were compared after iv,
intraduodenal, or hepatic portal administration of 0.5 mg/kg
(Houston et al., 1974). In Fig. 2, it is shown that plasma
concentrations were lower after intraduodenal application, when
carbaryl was subjected to the liver's first pass metabolizing
effect. Plasma concentrations following portal application were also
lower (approximately 80% of the concentration after the systemic
route of application).
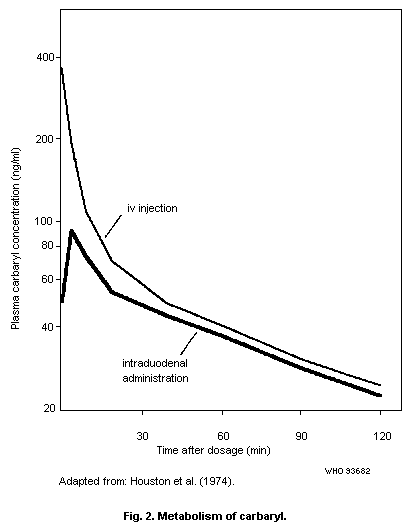 The distribution of carbaryl in rat tissues after a single oral
administration of 144.2 mg/kg body weight (0.2 LD50) was studied
(Klisenko & Yakim, 1966) at 5 and 30 min, and 1, 2, 4, 24, 48, and
72 h. Carbaryl and 1-naphthol were identified by thin-layer
chromatography with a sensitivity of 0.5 µg/g. At 5 min, carbaryl
was found in all organs. Thirty min after administration of
carbaryl, peak concentrations found were: muscle, 35 µg/g, brain,
16 µg/g, spleen, 25 µg/g, and erythrocytes, 120 µg/g; at 60 min, a
level of 45 µg/g was found in the liver. After 24 h, levels of only
1-4 µg carbaryl/g were detected in the liver, kidney, muscles, and
skin. At 48 h, no residues were found. The authors suggested that
carbaryl is rapidly distributed and excreted. 1-Naphthol was found
in the liver, stomach, intestines, kidneys, and lungs. Other
non-identified metabolites were present in the liver and lungs.
Levels of carbaryl in the tissues of rats poisoned with oral
doses of 800 and 1200 mg/kg, respectively, were as follows: liver,
7-58 and 52-80 µg/g; heart, 3.5-31.3 and 40.6-45.9 µg/g; brain,
3-26.8 and 25.9-30.9 µg/g. Higher concentrations were found in
animals that died from poisoning than in animals killed, even though
they were treated with the same doses (Mount et al., 1981).
Yakim (1970) studied the distribution of carbaryl and
1-naphthol after 6-month oral administration of 0.2, 0.1, or 0.05
LD50 carbaryl in rats. Carbaryl was found in the intestines (20-40
µg/g), liver (4-20 µg/g), and kidneys and lungs (in trace amounts);
1-naphthol was found in the kidneys (20-50 µg/g), and in the liver
(5-10 µg/g) in groups treated with 0.2 LD50. The carbaryl level
was <1 µg/g in the organs studied in the group treated with
0.1 LD50; no traces of carbaryl were found in the group treated
with 0.05 LD50. The author suggested that a higher quantity of
carbaryl is found 30 min after a single oral application, which
corresponds to the highest percentage of ChE inhibition.
Two to 6 h after a single dermal application of 500 mg
carbaryl/kg to cats, the compound was found in plasma at 15 µg/ml
and in erythrocytes at 25 µg/ml. Inhalation studies were also
performed on cats (Table 24). The author stressed that carbaryl was
present in smaller amounts in plasma than in erythrocytes because of
the more active metabolism by blood proteins. More 1-naphthol and
other unidentified metabolites were present in plasma. Elimination
of carbaryl and normalization of ChE activities occurred in 48-70 h
with all routes of administration of carbaryl. Carbaryl has a low
cumulation capacity.
In a study in which the abilities of several pesticides to bind
to potential carriers isolated from human blood were compared,
carbaryl was much more effectively bound by albumin than by either
high- or low-density lipoproteins (Maliwal & Guthrie, 1981).
Table 24. Relation between carbaryl inhalation and resulting cholinesterase (ChE) inhibitiona
Experimental Carbaryl and 1-naphthol ChE inhibition in %
conditions Plasma Erythrocytes Plasma Erythrocytes
Single 6 h exposure 2.5-5 µg/ml after 5-10 µg/ml 39-80 (after 4 h) 52-84 (after 4 h)
to 80 mg/m3 exposure; no traces (after exposure)
8 cats 4 h later 10-15 µg/ml (4 h later)
2-2.5 µg/ml (24 h later)
Single 6 h exposure 0-10 µg/ml 28-44 in blood
to 20 mg/m3 in single animal (after 4 h);
8 cats 0 after 48 h 0 (after 48 h)
4-Month exposure to traces in single animal
16 mg/m3 in blood
4 cats
1-Month exposure to 5-15 µg/ml, 0 on the 5-15 µg/ml, 0 on the 44 on the day of 62 on the 7th day of
63 mg/m3 6th day after the end 6th day after the end exposure exposure
4 cats of exposure of exposure
4-Month exposure to 5 µg/ml on the third 10 µg/ml on the third - -
38 mg/m3 day of exposure day of exposure
4 cats
aFrom: Yakin (1979)
Levels of carbaryl in some tissues of male, albino, Fisher
Strain 344 rats, after single and multiple oral administration
(Table 25) were reported by Hassan (1971).
Table 25. Tissue levels of carbaryla
Treatment Time after Concentration of carbaryl
administration Whole blood Heart Brain
(µg/ml) (µg/g) (µg/g)
Single dose 80 mg/kg 2 h 16.2 2.6 3.45
Rats fed 700 mg/kg diet 90 days 4.8 0.85 0.68
Rats fed 100 mg/kg diet 90 days 2.7 < 0.5 < 0.5
aFrom: Hassan (1971).
Andrawes et al. (1972) studied the 14C residues in hen
tissues following the feeding of 1-naphthyl-14C-carbaryl at
70 mg/kg for 4 days (Table 26).
Radiolabelled carbaryl naphthyl 14C (6600 dpm/µg) and
carbaryl carbonyl 14C (4000 dpm/µg) were administered
intratracheally to rats as aerosols for 15 seconds. The maximum
concentration in blood occurred after 2-5 min. Distribution of the
residues in the organs, 1 h after inhalation, was highest in the
lung (9.2-10.5%), liver (4.7-9.2%), bladder (2.7-5.9%), and kidney
(2.5-3.7%) (Nye & Dorough, 1976).
Distribution of 14carbon after intraperitoneal and oral
administration of 7.4 µmol carbaryl/kg body weight to rats was
studied by Krishna & Casida (1966) (Table 27). Carbaryl was found in
all tissues analysed. No marked differences with sex and
administration route were noted. On the basis of the rate of
excretion of radioactivity following an intraperitoneal injection of
labelled carbaryl, Shah & Guthrie (1983) calculated a half-time for
clearance of label of 6.46 h.
Table 26. Concentration of14C residues in hen tissues after
feeding 1-naphthyl-14C carbaryl at the level of
70 mg/kg for 4 daysa
Sample µg/kg at indicated times after
last treatment
16 h 7 days
Brain 17.3 6.8
Heart 47.5 20.9
Kidney 405.5 80.7
Pancreas 69.6 15.6
Skin 86.6 22.4
Fat 25.2 5.2
Gizzard 43.0 13.9
Thigh 28.6 10.1
Breast 25.9 12.7
Leg muscle 29.1 9.0
Blood 197.2 152.2
Lung 138.5 122.5
Liver 332.6 33.4
Spleen 108.7 74.2
Intestine and contents 300.1 < 5.0
Intestinal wall 44.0 11.1
Oviduct 50.5 6.7
Developing egg (small) 534.3 7.4
Developing egg (large) 508.1 35.5
Remaining carcass 35.5 < 5.0
aFrom: Andrawes et al. (1972).
Distribution of carbaryl was studied in 7 Sprague-Dawley rats
and Swiss mice on day 18 of pregnancy. Whole-body auto-radiography
was performed after oral application of 13.5 µCi carbaryl methyl
14C/kg. The transfer to the placenta began in the first hour.
Distribution of the carbaryl occurred in the excretory organs,
fetus, digestive tract, and the bone marrow and brain. The major
portion was quickly eliminated. A more stable localization occurred
in highly active protein-building organs, such as the fetus,
digestive tract, and bone marrow. The 14carbon concentration in the
eye, liver, and brain of the fetus was relatively constant from 8 to
96 h (Declume & Derache, 1976, 1977; Declume & Benard, 1977a,b,
1978).
Table 27. Distribution of 14carbon in various tissues in rats, 48 h after ip administration of carbaryl and its hydrolysis product,
in µmol equivalent/kg of fresh tissue, based on total 14Ca
Labelled position Blood Bone Brain Fat Heart Kidney Liver Lung Muscle Spleen Testes
Corpuscle Plasma
Carbonyl 14C 0.38 0.16 0.26 0.33 0.16 0.39 0.47 0.63 0.32 0.18 0.33
Methyl 14C 0.51 0.22 0.47 0.67 0.19 1.23 1.37 1.78 0.97 0.47 0.97
1-Naphthyl-1-14C 0.02 0.19 0.19 0.03 0.18 0.06 0.17 0.09 0.05 0.03 0.26 0.09
1-Naphthol-1-14C < 0.01 0.06 0.03 0.01 0.04 0.01 0.05 0.14 0.02 1.16 0.13
(hydrolysis product)
aAdapted from: Krishna & Casida (1966).
Fernandez et al. (1982) determined that the elimination of
label following intravenous administration of 20 mg 14C
carbaryl/kg to rats could be best described by a three compartment
model, with 73% of the label excreted within 24 h.
Strother & Wheeler (1976, 1980) reported that 14C-carbaryl
rapidly crossed the rat placenta and was distributed in all fetal
tissues. Fetal brain, heart, and lung contained more 14C on a
weight basis than the maternal counterpart.
6.3 Metabolism
The metabolism of carbaryl has been studied extensively, and
its complexity and the need for additional studies are recognized.
As with other carbamates (WHO, 1986), the principal metabolic
pathways are hydroxylation, hydrolysis, and epoxidation, resulting
in numerous metabolites subjected to conjugation, forming
water-soluble sulfates, glucoronides, and mercapturates (Carpenter
et al., 1961; Dorough et al., 1963; Dorough & Casida, 1964;
Knaak et al., 1965; Menzie, 1969; Bend et al., 1971).
Hydrolysis of carbaryl results in the formation of 1-naphthol,
carbon dioxide, and methylamine (Fig. 3) (Carpenter et al., 1961;
Sakai & Matsumura, 1971).
The first evidence for carbaryl hydroxylation was reported by
Hodgson & Casida (1961). Carbaryl is metabolized by a rat liver
microsome system, requiring NADPH2 and oxygen, to form a
formaldehyde-yielding derivative.
The distribution of carbaryl in rat tissues after a single oral
administration of 144.2 mg/kg body weight (0.2 LD50) was studied
(Klisenko & Yakim, 1966) at 5 and 30 min, and 1, 2, 4, 24, 48, and
72 h. Carbaryl and 1-naphthol were identified by thin-layer
chromatography with a sensitivity of 0.5 µg/g. At 5 min, carbaryl
was found in all organs. Thirty min after administration of
carbaryl, peak concentrations found were: muscle, 35 µg/g, brain,
16 µg/g, spleen, 25 µg/g, and erythrocytes, 120 µg/g; at 60 min, a
level of 45 µg/g was found in the liver. After 24 h, levels of only
1-4 µg carbaryl/g were detected in the liver, kidney, muscles, and
skin. At 48 h, no residues were found. The authors suggested that
carbaryl is rapidly distributed and excreted. 1-Naphthol was found
in the liver, stomach, intestines, kidneys, and lungs. Other
non-identified metabolites were present in the liver and lungs.
Levels of carbaryl in the tissues of rats poisoned with oral
doses of 800 and 1200 mg/kg, respectively, were as follows: liver,
7-58 and 52-80 µg/g; heart, 3.5-31.3 and 40.6-45.9 µg/g; brain,
3-26.8 and 25.9-30.9 µg/g. Higher concentrations were found in
animals that died from poisoning than in animals killed, even though
they were treated with the same doses (Mount et al., 1981).
Yakim (1970) studied the distribution of carbaryl and
1-naphthol after 6-month oral administration of 0.2, 0.1, or 0.05
LD50 carbaryl in rats. Carbaryl was found in the intestines (20-40
µg/g), liver (4-20 µg/g), and kidneys and lungs (in trace amounts);
1-naphthol was found in the kidneys (20-50 µg/g), and in the liver
(5-10 µg/g) in groups treated with 0.2 LD50. The carbaryl level
was <1 µg/g in the organs studied in the group treated with
0.1 LD50; no traces of carbaryl were found in the group treated
with 0.05 LD50. The author suggested that a higher quantity of
carbaryl is found 30 min after a single oral application, which
corresponds to the highest percentage of ChE inhibition.
Two to 6 h after a single dermal application of 500 mg
carbaryl/kg to cats, the compound was found in plasma at 15 µg/ml
and in erythrocytes at 25 µg/ml. Inhalation studies were also
performed on cats (Table 24). The author stressed that carbaryl was
present in smaller amounts in plasma than in erythrocytes because of
the more active metabolism by blood proteins. More 1-naphthol and
other unidentified metabolites were present in plasma. Elimination
of carbaryl and normalization of ChE activities occurred in 48-70 h
with all routes of administration of carbaryl. Carbaryl has a low
cumulation capacity.
In a study in which the abilities of several pesticides to bind
to potential carriers isolated from human blood were compared,
carbaryl was much more effectively bound by albumin than by either
high- or low-density lipoproteins (Maliwal & Guthrie, 1981).
Table 24. Relation between carbaryl inhalation and resulting cholinesterase (ChE) inhibitiona
Experimental Carbaryl and 1-naphthol ChE inhibition in %
conditions Plasma Erythrocytes Plasma Erythrocytes
Single 6 h exposure 2.5-5 µg/ml after 5-10 µg/ml 39-80 (after 4 h) 52-84 (after 4 h)
to 80 mg/m3 exposure; no traces (after exposure)
8 cats 4 h later 10-15 µg/ml (4 h later)
2-2.5 µg/ml (24 h later)
Single 6 h exposure 0-10 µg/ml 28-44 in blood
to 20 mg/m3 in single animal (after 4 h);
8 cats 0 after 48 h 0 (after 48 h)
4-Month exposure to traces in single animal
16 mg/m3 in blood
4 cats
1-Month exposure to 5-15 µg/ml, 0 on the 5-15 µg/ml, 0 on the 44 on the day of 62 on the 7th day of
63 mg/m3 6th day after the end 6th day after the end exposure exposure
4 cats of exposure of exposure
4-Month exposure to 5 µg/ml on the third 10 µg/ml on the third - -
38 mg/m3 day of exposure day of exposure
4 cats
aFrom: Yakin (1979)
Levels of carbaryl in some tissues of male, albino, Fisher
Strain 344 rats, after single and multiple oral administration
(Table 25) were reported by Hassan (1971).
Table 25. Tissue levels of carbaryla
Treatment Time after Concentration of carbaryl
administration Whole blood Heart Brain
(µg/ml) (µg/g) (µg/g)
Single dose 80 mg/kg 2 h 16.2 2.6 3.45
Rats fed 700 mg/kg diet 90 days 4.8 0.85 0.68
Rats fed 100 mg/kg diet 90 days 2.7 < 0.5 < 0.5
aFrom: Hassan (1971).
Andrawes et al. (1972) studied the 14C residues in hen
tissues following the feeding of 1-naphthyl-14C-carbaryl at
70 mg/kg for 4 days (Table 26).
Radiolabelled carbaryl naphthyl 14C (6600 dpm/µg) and
carbaryl carbonyl 14C (4000 dpm/µg) were administered
intratracheally to rats as aerosols for 15 seconds. The maximum
concentration in blood occurred after 2-5 min. Distribution of the
residues in the organs, 1 h after inhalation, was highest in the
lung (9.2-10.5%), liver (4.7-9.2%), bladder (2.7-5.9%), and kidney
(2.5-3.7%) (Nye & Dorough, 1976).
Distribution of 14carbon after intraperitoneal and oral
administration of 7.4 µmol carbaryl/kg body weight to rats was
studied by Krishna & Casida (1966) (Table 27). Carbaryl was found in
all tissues analysed. No marked differences with sex and
administration route were noted. On the basis of the rate of
excretion of radioactivity following an intraperitoneal injection of
labelled carbaryl, Shah & Guthrie (1983) calculated a half-time for
clearance of label of 6.46 h.
Table 26. Concentration of14C residues in hen tissues after
feeding 1-naphthyl-14C carbaryl at the level of
70 mg/kg for 4 daysa
Sample µg/kg at indicated times after
last treatment
16 h 7 days
Brain 17.3 6.8
Heart 47.5 20.9
Kidney 405.5 80.7
Pancreas 69.6 15.6
Skin 86.6 22.4
Fat 25.2 5.2
Gizzard 43.0 13.9
Thigh 28.6 10.1
Breast 25.9 12.7
Leg muscle 29.1 9.0
Blood 197.2 152.2
Lung 138.5 122.5
Liver 332.6 33.4
Spleen 108.7 74.2
Intestine and contents 300.1 < 5.0
Intestinal wall 44.0 11.1
Oviduct 50.5 6.7
Developing egg (small) 534.3 7.4
Developing egg (large) 508.1 35.5
Remaining carcass 35.5 < 5.0
aFrom: Andrawes et al. (1972).
Distribution of carbaryl was studied in 7 Sprague-Dawley rats
and Swiss mice on day 18 of pregnancy. Whole-body auto-radiography
was performed after oral application of 13.5 µCi carbaryl methyl
14C/kg. The transfer to the placenta began in the first hour.
Distribution of the carbaryl occurred in the excretory organs,
fetus, digestive tract, and the bone marrow and brain. The major
portion was quickly eliminated. A more stable localization occurred
in highly active protein-building organs, such as the fetus,
digestive tract, and bone marrow. The 14carbon concentration in the
eye, liver, and brain of the fetus was relatively constant from 8 to
96 h (Declume & Derache, 1976, 1977; Declume & Benard, 1977a,b,
1978).
Table 27. Distribution of 14carbon in various tissues in rats, 48 h after ip administration of carbaryl and its hydrolysis product,
in µmol equivalent/kg of fresh tissue, based on total 14Ca
Labelled position Blood Bone Brain Fat Heart Kidney Liver Lung Muscle Spleen Testes
Corpuscle Plasma
Carbonyl 14C 0.38 0.16 0.26 0.33 0.16 0.39 0.47 0.63 0.32 0.18 0.33
Methyl 14C 0.51 0.22 0.47 0.67 0.19 1.23 1.37 1.78 0.97 0.47 0.97
1-Naphthyl-1-14C 0.02 0.19 0.19 0.03 0.18 0.06 0.17 0.09 0.05 0.03 0.26 0.09
1-Naphthol-1-14C < 0.01 0.06 0.03 0.01 0.04 0.01 0.05 0.14 0.02 1.16 0.13
(hydrolysis product)
aAdapted from: Krishna & Casida (1966).
Fernandez et al. (1982) determined that the elimination of
label following intravenous administration of 20 mg 14C
carbaryl/kg to rats could be best described by a three compartment
model, with 73% of the label excreted within 24 h.
Strother & Wheeler (1976, 1980) reported that 14C-carbaryl
rapidly crossed the rat placenta and was distributed in all fetal
tissues. Fetal brain, heart, and lung contained more 14C on a
weight basis than the maternal counterpart.
6.3 Metabolism
The metabolism of carbaryl has been studied extensively, and
its complexity and the need for additional studies are recognized.
As with other carbamates (WHO, 1986), the principal metabolic
pathways are hydroxylation, hydrolysis, and epoxidation, resulting
in numerous metabolites subjected to conjugation, forming
water-soluble sulfates, glucoronides, and mercapturates (Carpenter
et al., 1961; Dorough et al., 1963; Dorough & Casida, 1964;
Knaak et al., 1965; Menzie, 1969; Bend et al., 1971).
Hydrolysis of carbaryl results in the formation of 1-naphthol,
carbon dioxide, and methylamine (Fig. 3) (Carpenter et al., 1961;
Sakai & Matsumura, 1971).
The first evidence for carbaryl hydroxylation was reported by
Hodgson & Casida (1961). Carbaryl is metabolized by a rat liver
microsome system, requiring NADPH2 and oxygen, to form a
formaldehyde-yielding derivative.
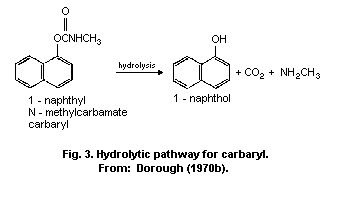 The use of 14carbon-labelled carbaryl (Fig. 4) and thin-layer
chromatography contributed to further studies on carbaryl metabolism
(Skraba & Young, 1959; Krishna et al., 1962). Chin et al. (1974)
used in vitro techniques on tissues from animals and human beings.
The use of 14carbon-labelled carbaryl (Fig. 4) and thin-layer
chromatography contributed to further studies on carbaryl metabolism
(Skraba & Young, 1959; Krishna et al., 1962). Chin et al. (1974)
used in vitro techniques on tissues from animals and human beings.
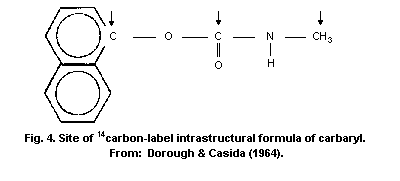 Reviews on carbamate and carbaryl metabolism have been
published (Lykken & Casida, 1969; Kuhr, 1970; Knaak, 1971; Kuhr,
1971; Ryan, 1971; Fukuto, 1973; Dorough, 1973; Kuhr & Dorough,
1976).
6.3.1 In vitro studies on animal tissues
An investigation of the individual metabolism of carbaryl in
the liver, lung, and the kidney of rat was conducted using the
tissue explant maintenance technique. Hepatic tissue of the rat,
incubated with carbaryl, actively performed demethylation,
hydrolysis, hydroxylation, and oxidation, followed by sulfate and
glucuronide conjugations (Chin et al., 1979b).
Rat liver microsomes fortified with reduced nicotineamide-
adenine dinucleotide phosphate were used to study the hydroxylated
products of carbonyl 14C, methyl 14C, and naphthyl 14C
carbaryl (Dorough & Casida, 1964). The metabolites were identified
as N-hydroxymethylcarbaryl, 4-hydroxycarbaryl, and
5-hydroxycarbaryl. At least 2 unidentified metabolites had the
C-O-C(O)-N-C structure intact. 1-Naphthol and at least two
unidentified metabolites without the carbamyl groups were formed as
a product of hydrolysis. The nature of carbaryl metabolites in liver
microsomes in mice, rats, and rabbits was studied by Leeling &
Casida (1966) and in guinea-pigs and rats by Knaak et al. (1965).
Two more metabolites were identified (Table 28): 5,6-dihydro-5,6-
dihydroxycarbaryl, and 1 hydroxy-5,6-dihydro-5,6-dihydroxy-
naphthalene. It was suggested that hydrolysed metabolites are
probably conjugated as glucoronides and sulfates (Knaak et al.,
1965; Hassan et al., 1966; Leeling & Casida, 1966). A study on rat
liver microsomes and small intestine later showed (Mehendale &
Dorough, 1971) that about 90% of 1-naphthol and
N-hydroxymethylcarbaryl and about 40% of 5-hydroxycarbaryl and
4-hydroxycarbaryl were conjugated as glucuronides. The presence of
thioether conjugates in incubation mixtures of mouse liver
homogenates with carbaryl has been confirmed (Bend et al., 1971;
Ryan, 1971).
The in vitro technique for metabolic studies using liver
tissues qualitatively reflects the in vivo metabolic processes of
carbaryl in animals and human beings (Sullivan et al., 1972) and
their similarity (Matsumura & Ward, 1966).
Methylmercury hydroxide pretreatment in rats (10 mg/kg daily
for 2 days) decreased the hepatic microsomal cytochrome P-450
content and aminopyrine demethylase by 50%, as well as the
microsomal hydroxylation reaction in vitro of carbaryl to form
4-hydroxycarbaryl and N-hydroxymethylcarbaryl. Chlordane
pretreatment increased both cytochrome P450 and hydroxylation
(Lucier et al., 1972). However, there were no quantitative changes
in the metabolite pattern.
When the metabolism of carbaryl in rat intestine was studied
in vitro, hydrolysis and the synthesis of 1-naphthyl glucuronide
were reported to occur mainly in the first third of the intestine
(Pekas & Paulson, 1970; Pekas, 1972).
MacPherson et al. (1991) studied the metabolism of carbaryl
using a rat skin preparation in vitro. A post-mitochondrial
fraction was able to catalyse hydrolysis and sulfation and
glucuronidation conjugation reactions, but not ring hydroxylation.
The activities were very small in comparison with activities in
liver microsomes.
The degradation of carbaryl by an esterase of the American
cockroach ( Periplaneta americana) was reported by Matsumura &
Sakai (1968).
Table 28. Carbaryl naphthyl-1-14C metabolism by liver microsomes from mice, rabbits, and rats, in the presence of NADPH2a
Substance determined Total radiocarbon (%) using liver microsomes from:
Mice Rabbits Rats
Ether extract
Carbaryl 32.1 19.4 46.9
Hydroxylated metabolites
1-naphtyl N-hydroxymethylcarbamate 11.9 6.3 11.7
4-hydroxy-1-naphthyl methylcarbamate 6.7 8.1 6.1
5-hydroxy-1-naphthyl methylcarbamate 2.4 1.7 1.3
5,6-dihydro-5,6-dihydroxy-1-naphthyl methylcarbamate 5.3 9.1 3.8
1-hydroxy-5,6-dihydro-5,6-dihydroxy-naphthalene 1.9 2.7 1.5
1-Naphthol 7.2 6.3 5.8
Unidentified metabolites
Metabolite A 4.0 4.5 3.5
Metabolite C 0.6 2.0 0.8
Aqueous fraction 28.5 39.6 19.4
aFrom: Leeling & Casida (1966).
6.3.2 In vivo studies on animals
The metabolism of carbaryl has been studied in a variety of
mammals including rat, rabbit, guinea-pig, monkey, sheep, cow, pig,
and dog. Although many organs have been shown to be able to
metabolize carbaryl, the most important one is the liver.
The metabolism of 1-naphthyl-14C carbaryl was studied in male
and female Beagle dogs following a single, oral administration of
2.5 or 25 mg/kg. The metabolic pathways identified involved
hydrolysis, N-methyl oxidation, ring hydroxylation, and
conjugation. No significant qualitative differences were found
between male and female dogs or between high and low dosage levels.
Faecal elimination accounted for 30-66% of the applied dose and was
found to be primarily the result of incomplete absorption from the
intestinal tract of the solid material and subsequent elimination of
unchanged carbaryl. The metabolic pathway defined is illustrated in
Fig. 5 (Andrawes & Bailey, 1978c).
The metabolism of 1-naphthyl-14C carbaryl was studied in male
and female Sprague-Dawley rats following a single, oral
administration of 2.5 mg/kg. The metabolic pathways identified in
the rat involved hydrolysis, N-methyl oxidation, ring hydroxylation,
and conjugation. New metabolites identified, previously unknown in
the rat, were: 1,5-naphthalenediol, 1,6-naphthalenediol,
3,4-dihydro-3,4-dihydroxy-1-naphthol, and 3-hydroxy-1-naphthyl
methylcarbamate. These new metabolites had been previously
identified in the dog. The metabolic pathway defined is illustrated
in Fig. 5. Faecal elimination accounted for only 2-7% of faecal
14C as carbaryl, indicating more complete absorption of the test
material than in the dog (Andrawes & Bailey (1978a).
Conjugated metabolites of 1-naphthyl-14C carbaryl excreted in
rat and dog urine, after similar single oral treatments of
2.5 mg/kg, were separated and compared as intact conjugates using
gel permeation and thin layer chromatography. The metabolic products
were found to be qualitatively similar in the two animal species,
with evidence of glutathione conjugation in the dog. Urinary
metabolites only differed quantitatively between species. The rat
appeared to be considerably more active in hydrolysing carbaryl to
1-naphthol followed by conjugation, whereas, in the dog, the
principle urinary metabolites were formed through direct conjugation
of carbaryl itself. A significant amount of urinary radioactivity in
both species remained unidentified, i.e., 27 and 34% in the rat and
dog, respectively (Andrawes & Bailey, 1978b). Tables 29 and 30
illustrate the quantitative urinary metabolic differences in rats
and dogs. Note that "Free" refers to unconjugated metabolites
whereas "Acid" and "Enzyme" refer to acid- and enzyme-hydrolysed
conjugates.
Reviews on carbamate and carbaryl metabolism have been
published (Lykken & Casida, 1969; Kuhr, 1970; Knaak, 1971; Kuhr,
1971; Ryan, 1971; Fukuto, 1973; Dorough, 1973; Kuhr & Dorough,
1976).
6.3.1 In vitro studies on animal tissues
An investigation of the individual metabolism of carbaryl in
the liver, lung, and the kidney of rat was conducted using the
tissue explant maintenance technique. Hepatic tissue of the rat,
incubated with carbaryl, actively performed demethylation,
hydrolysis, hydroxylation, and oxidation, followed by sulfate and
glucuronide conjugations (Chin et al., 1979b).
Rat liver microsomes fortified with reduced nicotineamide-
adenine dinucleotide phosphate were used to study the hydroxylated
products of carbonyl 14C, methyl 14C, and naphthyl 14C
carbaryl (Dorough & Casida, 1964). The metabolites were identified
as N-hydroxymethylcarbaryl, 4-hydroxycarbaryl, and
5-hydroxycarbaryl. At least 2 unidentified metabolites had the
C-O-C(O)-N-C structure intact. 1-Naphthol and at least two
unidentified metabolites without the carbamyl groups were formed as
a product of hydrolysis. The nature of carbaryl metabolites in liver
microsomes in mice, rats, and rabbits was studied by Leeling &
Casida (1966) and in guinea-pigs and rats by Knaak et al. (1965).
Two more metabolites were identified (Table 28): 5,6-dihydro-5,6-
dihydroxycarbaryl, and 1 hydroxy-5,6-dihydro-5,6-dihydroxy-
naphthalene. It was suggested that hydrolysed metabolites are
probably conjugated as glucoronides and sulfates (Knaak et al.,
1965; Hassan et al., 1966; Leeling & Casida, 1966). A study on rat
liver microsomes and small intestine later showed (Mehendale &
Dorough, 1971) that about 90% of 1-naphthol and
N-hydroxymethylcarbaryl and about 40% of 5-hydroxycarbaryl and
4-hydroxycarbaryl were conjugated as glucuronides. The presence of
thioether conjugates in incubation mixtures of mouse liver
homogenates with carbaryl has been confirmed (Bend et al., 1971;
Ryan, 1971).
The in vitro technique for metabolic studies using liver
tissues qualitatively reflects the in vivo metabolic processes of
carbaryl in animals and human beings (Sullivan et al., 1972) and
their similarity (Matsumura & Ward, 1966).
Methylmercury hydroxide pretreatment in rats (10 mg/kg daily
for 2 days) decreased the hepatic microsomal cytochrome P-450
content and aminopyrine demethylase by 50%, as well as the
microsomal hydroxylation reaction in vitro of carbaryl to form
4-hydroxycarbaryl and N-hydroxymethylcarbaryl. Chlordane
pretreatment increased both cytochrome P450 and hydroxylation
(Lucier et al., 1972). However, there were no quantitative changes
in the metabolite pattern.
When the metabolism of carbaryl in rat intestine was studied
in vitro, hydrolysis and the synthesis of 1-naphthyl glucuronide
were reported to occur mainly in the first third of the intestine
(Pekas & Paulson, 1970; Pekas, 1972).
MacPherson et al. (1991) studied the metabolism of carbaryl
using a rat skin preparation in vitro. A post-mitochondrial
fraction was able to catalyse hydrolysis and sulfation and
glucuronidation conjugation reactions, but not ring hydroxylation.
The activities were very small in comparison with activities in
liver microsomes.
The degradation of carbaryl by an esterase of the American
cockroach ( Periplaneta americana) was reported by Matsumura &
Sakai (1968).
Table 28. Carbaryl naphthyl-1-14C metabolism by liver microsomes from mice, rabbits, and rats, in the presence of NADPH2a
Substance determined Total radiocarbon (%) using liver microsomes from:
Mice Rabbits Rats
Ether extract
Carbaryl 32.1 19.4 46.9
Hydroxylated metabolites
1-naphtyl N-hydroxymethylcarbamate 11.9 6.3 11.7
4-hydroxy-1-naphthyl methylcarbamate 6.7 8.1 6.1
5-hydroxy-1-naphthyl methylcarbamate 2.4 1.7 1.3
5,6-dihydro-5,6-dihydroxy-1-naphthyl methylcarbamate 5.3 9.1 3.8
1-hydroxy-5,6-dihydro-5,6-dihydroxy-naphthalene 1.9 2.7 1.5
1-Naphthol 7.2 6.3 5.8
Unidentified metabolites
Metabolite A 4.0 4.5 3.5
Metabolite C 0.6 2.0 0.8
Aqueous fraction 28.5 39.6 19.4
aFrom: Leeling & Casida (1966).
6.3.2 In vivo studies on animals
The metabolism of carbaryl has been studied in a variety of
mammals including rat, rabbit, guinea-pig, monkey, sheep, cow, pig,
and dog. Although many organs have been shown to be able to
metabolize carbaryl, the most important one is the liver.
The metabolism of 1-naphthyl-14C carbaryl was studied in male
and female Beagle dogs following a single, oral administration of
2.5 or 25 mg/kg. The metabolic pathways identified involved
hydrolysis, N-methyl oxidation, ring hydroxylation, and
conjugation. No significant qualitative differences were found
between male and female dogs or between high and low dosage levels.
Faecal elimination accounted for 30-66% of the applied dose and was
found to be primarily the result of incomplete absorption from the
intestinal tract of the solid material and subsequent elimination of
unchanged carbaryl. The metabolic pathway defined is illustrated in
Fig. 5 (Andrawes & Bailey, 1978c).
The metabolism of 1-naphthyl-14C carbaryl was studied in male
and female Sprague-Dawley rats following a single, oral
administration of 2.5 mg/kg. The metabolic pathways identified in
the rat involved hydrolysis, N-methyl oxidation, ring hydroxylation,
and conjugation. New metabolites identified, previously unknown in
the rat, were: 1,5-naphthalenediol, 1,6-naphthalenediol,
3,4-dihydro-3,4-dihydroxy-1-naphthol, and 3-hydroxy-1-naphthyl
methylcarbamate. These new metabolites had been previously
identified in the dog. The metabolic pathway defined is illustrated
in Fig. 5. Faecal elimination accounted for only 2-7% of faecal
14C as carbaryl, indicating more complete absorption of the test
material than in the dog (Andrawes & Bailey (1978a).
Conjugated metabolites of 1-naphthyl-14C carbaryl excreted in
rat and dog urine, after similar single oral treatments of
2.5 mg/kg, were separated and compared as intact conjugates using
gel permeation and thin layer chromatography. The metabolic products
were found to be qualitatively similar in the two animal species,
with evidence of glutathione conjugation in the dog. Urinary
metabolites only differed quantitatively between species. The rat
appeared to be considerably more active in hydrolysing carbaryl to
1-naphthol followed by conjugation, whereas, in the dog, the
principle urinary metabolites were formed through direct conjugation
of carbaryl itself. A significant amount of urinary radioactivity in
both species remained unidentified, i.e., 27 and 34% in the rat and
dog, respectively (Andrawes & Bailey, 1978b). Tables 29 and 30
illustrate the quantitative urinary metabolic differences in rats
and dogs. Note that "Free" refers to unconjugated metabolites
whereas "Acid" and "Enzyme" refer to acid- and enzyme-hydrolysed
conjugates.
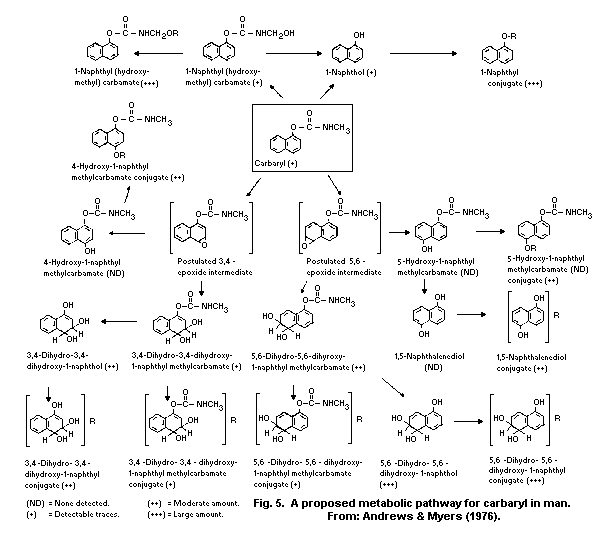 Table 29. Metabolic products present in a 24-h sample of urine of a
rat treated orally with 2.5 mg 1-naphthyl-14C
carbaryl/kg in corn oil
Products Percentage of total radioactivity in urine
Free Enzyme Acid Total
1-Naphthol 0.26 15.86 0.49 16.61
Carbaryl 0.14 0.45 4.22 4.81
Methylol NDa NDa 0.64b 0.64
1,5-Naphthalenediol 0.17 1.12 0.08 1.37
1,6-Naphthalenediol 0.03 0.42 NDa 0.45
5-Hydroxy carbaryl 7.76 3.07 0.24 11.07
5,6-Dihydrodihydroxy
naphthol 1.16 4.99 b 6.15
5,6-Dihydrodihydroxy
carbaryl 5.66 7.75 b 13.41
1,4-Naphthoquinonec 0.03 1.12 0.10 1.25
4-Hydroxy carbaryl 2.30 2.76 0.19 5.25
3-Hydroxy carbaryl NDa NDa 0.06 0.06
3,4-Dihydrodihydroxy
naphthol 0.71 1.53 b 2.24
Unknown 1 0.25 2.95 0.06 3.26
Unknown 2 NDa 3.46 NDa 3.46
Other unknowns 0.37 1.92 0.38 2.67
Highly polar
materials NDa 6.99 20.31 27.30
aND = none detected.
bAcid hydrolysis degrades the methylol to desmethyl carbaryl and
the dihydrodihydroxy derivatives to phenols.
cA decomposition product of 1,4-naphthalenediol during work-up.
Table 30. Metabolic products present in a 24-h sample of urine of a
female dog treated orally with 25 mg 1-naphthyl-14C
carbaryl/kg
Products Percentage of total radioactivity in urine
Free Enzyme Acid Total
1-Naphthol 0.38 2.74 1.47 4.59
Carbaryl 1.71 0.16 9.90 11.77
Methylol NDa NDa 0.22 0.22
1,5-Naphthalenediol 0.21 1.83 1.14 3.18
1,6-Naphthalenediol NDa 1.62 NDa 1.62
5-Hydroxy carbaryl 1.61 1.67 0.20 3.48
5,6-Dihydrodihydroxy
naphthol 1.12 9.79 NDa 10.89
5,6-Dihydrodihydroxy
carbaryl 3.80 3.09 NDa 6.89
1,4-Naphthoquinoneb NDa 1.24 1.36 2.60
4-Hydroxy carbaryl 0.64 5.30 0.44 6.38
3-Hydroxy carbaryl NDa 0.04 0.01 0.05
3,4-Dihydrodihydroxy
naphthol NDa 0.50 NDa 0.50
Unknown 1 NDa 0.20 0.62 0.82
Unknown 2 NDa 1.30 NDa 1.30
Other unknowns NDa 1.26 0.43 1.69
Highly polar materials NDa 9.06 34.96 44.02
aND = none detected.
bA decomposition product of 1,4-naphthalenediol during work-up.
The above work supersedes the work by Knaak & Sullivan (1967),
who mistakenly concluded that the Beagle dog metabolic pathway was
qualitatively different from that of the rat, because of poor
absorption of solid/slurry test material. The marked emphasis on
differences in metabolic pathways between species, together with the
analytical techniques available at the time of the study contributed
to their conclusions.
Studies on the nature of the biliary, water-soluble metabolites
of carbaryl were conducted by Bend et al. (1971) on 19 male Wistar
rats, using a technique with the bile duct cannulated. Water-soluble
conjugates of carbaryl with sulfur-containing amino acid were found
in the urine as well as the bile of treated rats: S-(4-hydroxy-1-
naphthyl) cysteine and S(5-hydroxy-1-naphthyl)-cysteine. Biliary
secretion of 5,6-dihydro-5,6-dihydroxycarbaryl glucuronide following
an infused dose was found to be greatly reduced, from 10% to less
than 1%, by pretreatment with antibiotics (Struble et al., 1983b).
This indicates that bacteria play a role in the enterohepatic
circulation and bilary secretion of this metabolite of carbaryl.
The functional activity of the reticulo-endothelial system
(RES) can influence carbaryl metabolism (Pipy et al., 1980). The
elimination of carbaryl from the blood decreased significantly in
rats (24 male Sprague-Dawley) with RES inhibited by colloidal
carbon, and increased in those in which the RES was activated with
glyceryl trioleate. Correlation of the activity of RES with the
enzyme activity of monoxygenases of the hepatic microsomal fraction
could possibly explain this effect of the RES in the toxicokinetics
of carbaryl.
An intravenous injection of colloidal carbon, which inhibits
liver microsomal metabolism, reduced biliary excretion of an iv dose
of carbaryl given 18 h later (Pipy et al., 1981). Pretreatment of
rats with 75 mg phenobarbital/kg per day, ip, for five days resulted
in an increase in the rate of sulfate conjugation of carbaryl,
following a high dose of carbaryl (16.4 mg/kg), but not a low dose
(1.64 mg/kg) (Knight et al., 1987).
6.3.3 Metabolic transformation in plants
The formation of carbaryl metabolites in plants is primarily
dependent on the hydrolytic, oxidative, and conjugative potential of
the plant tissues, which are similar to the tissues of insects and
mammals. The metabolism in plants is of a shorter duration than that
in insects and mammals, and the tendency to accumulate metabolites
is more pronounced. The carbamate ester bond appears to be quite
stable in plants, which explains the small amount of recovery of
1-naphthol from treated plants. The extent of oxidation is generally
higher in plants and insects than in mammals. Several
hydroxymetabolites are formed by oxidation of the N-methyl group
and naphthalene ring. They are conjugated as glycosides.
Non-enzymatic factors, such as light, heat, and humidity may
contribute to the degradation of carbaryl in plants as well (Kuhr,
1971).
The metabolism of carbaryl has been defined in a wide variety
of plant species (Table 31). Metabolites were isolated in microgram
quantities for mass and ultraviolet spectroscopic analyses (Mumma
et al., 1971).
6.3.4 In vitro studies with human tissues
The metabolism of carbaryl in selected human tissues was
studied in vitro by Chin et al. (1974). On the basis of the
total anionic characteristics of the metabolites derived from each
organ, metabolic activity occurred in the following organs in
descending order: liver, lung, kidney, placenta, vaginal mucosa,
uterus.
The metabolic profiles of carbaryl in human postembryonic fetal
autopsy tissue were determined using 1-naphthyl-14C or
N-methyl-14C-carbaryl. The anionics from fetal liver amounted to
20% of those found with the adult liver. Naphthyl glucuronide and
naphthyl sulfate were produced in the kidney, whereas the lung
produced naphthyl sulfate from carbaryl (Chin et al., 1979a).
Carbaryl was metabolized oxidatively by primary human embryonic
cells in culture (Lin et al., 1975). Complete degradation occurred
after 72 h of incubation. Unconjugated metabolites were identified
as 1-naphthol, 5-hydroxycarbaryl, 4-hydroxycarbaryl, and
5,6-dihydro-5,6-dihydroxycarbaryl. The water-soluble components were
identified as 4-hydroxycarbaryl, 1,4-naphthalenediol, and
5,6-dihydro-5,6-dihydroxycarbaryl. The primary human embryonic lung
cells did not convert carbaryl to carbon dioxide. They may not
possess the enzyme system that is necessary to break down the
naphthalene ring of carbaryl to form carbon dioxide.
Sakai & Matsumura (1971) studied the degradation of carbaryl by
brain esterases. Carbaryl was degraded by band E4 and E6,
whereas, in the mouse brain preparation, the compound was degraded
by band E8, E9, and E6.
Cell culture techniques were used to examine the products of
carbaryl degradation by cultures of an L-132 cell line derived from
normal human embryonic lung. The data indicated that detoxification
was similar to that observed in animals and in in vitro enzyme
systems (Baron & Locke, 1970).
Table 31. The composition of water-soluble metabolites of carbaryl in plants.
(% distribution of released aglycones 21 days after treatment (for apples, 53 days))
Metabolite Beana Beana Wheatb Potatoc Cornd Riced Tomatoc Applese
(mature (shell (seedlings) (mature (seedlings) (seedlings) (mature
foliage) only) foliage) foliage)
5,6-Dihydrodihydroxycarbaryl 2.5 12.7 2.1 NDd 1.3 2.9 ND 2.1
Methylol carbaryle 29.9 47.9 10.5 16.2 13.3 7.8 25.4 2.8
7-Hydroxycarbaryl 24.2 trace ND ND ND ND ND 0.8
4-Hydroxycarbaryl 15.0 4.1 23.6 4.1 8.9 3.7 13.8 13.7
5-Hydroxycarbaryl 7.3 9.4 21.3 4.1 12.9 5.6 3.4 9.2
1-Naphthol 13.8 21.0 1.1 5.8 3.2 3.5 8.1 11.3
5,6-Dihydrodihydroxynaphthol ND ND ND ND 0.5 1.0 ND
Carbaryl 0.3 0.8 20.8 27.0 24.1 42.8 16.8 33.3
Unknown(s) ND ND 3.2 ND 2.9 2.2 5.2 3.0
Unhydrolysed conjugate 7.1 4.1 17.4 42.8 33.0 30.4 27.3 12.6
References: aWiggins & Weiden (1969); bAndrawes & Chancey (1970); cChancey & Andrawes (1971b); dChancey & Andrawes (1971a);
eChancey (1974).
fND = not detected.
Studies on the in vitro metabolism of carbaryl by a human
liver fraction indicated that there was a difference in the
metabolic pattern compared with that in the rat liver. The human
liver produces at least two more metabolites (Strother, 1970).
6.3.5 In vivo studies on humans
The metabolism of carbaryl in humans appears to be
qualitatively similar to that previously reported in other mammals.
Metabolic reactions include: hydroxylation, hydrolysis, and
conjugation. Metabolites of carbaryl were identified in the urine of
human volunteers after ingestion of a 2 mg/kg dose (Andrawes &
Myers, 1976). Only traces of the unchanged carbaryl could be
detected in the urine indicating rapid metabolism. From the spectrum
of the metabolites identified, a metabolic pathway of carbaryl in
humans is proposed and shown in Fig. 6.
The only detectable metabolites in urine samples taken from
workers exposed to carbaryl dust were 1-naphthylglucoronide and
sulfate (Knaak et al., 1965). Later 1-naphthyl and
4-hydroxycarbaryl, as conjugates of glucuronic and sulfuric acid,
were found in a study on volunteers.
Human exposure to carbaryl during aerial application for Gypsy
moth control was assessed by Schulze et al. (1979). The results of
this study indicated a strong positive correlation between carbaryl
exposure and the excretion of urinary 1-naphthol within 24 h of
known exposure and a total lack of 1-naphthol in pre-exposure
samples.
The presence of these metabolites has been confirmed in six
urine samples of workers with high exposures to carbaryl (Andrawes &
Myers, 1976).
One major and three minor interfering chemicals were detected
in no-exposure samples as well as the maximum exposure samples.
These interfering materials developed a colour similar to that of
carbaryl metabolites upon spraying the chromatograms with
p-nitrobenzene diazonium fluoborate. It is estimated that the
major interfering pigment accounted for as much as one-third of the
total colour observed on thin-layer chromatograms. These interfering
chemicals may also be the cause of high blank values often
encountered during the routine analysis of urine samples (Andrawes &
Bailey, 1979).
Table 29. Metabolic products present in a 24-h sample of urine of a
rat treated orally with 2.5 mg 1-naphthyl-14C
carbaryl/kg in corn oil
Products Percentage of total radioactivity in urine
Free Enzyme Acid Total
1-Naphthol 0.26 15.86 0.49 16.61
Carbaryl 0.14 0.45 4.22 4.81
Methylol NDa NDa 0.64b 0.64
1,5-Naphthalenediol 0.17 1.12 0.08 1.37
1,6-Naphthalenediol 0.03 0.42 NDa 0.45
5-Hydroxy carbaryl 7.76 3.07 0.24 11.07
5,6-Dihydrodihydroxy
naphthol 1.16 4.99 b 6.15
5,6-Dihydrodihydroxy
carbaryl 5.66 7.75 b 13.41
1,4-Naphthoquinonec 0.03 1.12 0.10 1.25
4-Hydroxy carbaryl 2.30 2.76 0.19 5.25
3-Hydroxy carbaryl NDa NDa 0.06 0.06
3,4-Dihydrodihydroxy
naphthol 0.71 1.53 b 2.24
Unknown 1 0.25 2.95 0.06 3.26
Unknown 2 NDa 3.46 NDa 3.46
Other unknowns 0.37 1.92 0.38 2.67
Highly polar
materials NDa 6.99 20.31 27.30
aND = none detected.
bAcid hydrolysis degrades the methylol to desmethyl carbaryl and
the dihydrodihydroxy derivatives to phenols.
cA decomposition product of 1,4-naphthalenediol during work-up.
Table 30. Metabolic products present in a 24-h sample of urine of a
female dog treated orally with 25 mg 1-naphthyl-14C
carbaryl/kg
Products Percentage of total radioactivity in urine
Free Enzyme Acid Total
1-Naphthol 0.38 2.74 1.47 4.59
Carbaryl 1.71 0.16 9.90 11.77
Methylol NDa NDa 0.22 0.22
1,5-Naphthalenediol 0.21 1.83 1.14 3.18
1,6-Naphthalenediol NDa 1.62 NDa 1.62
5-Hydroxy carbaryl 1.61 1.67 0.20 3.48
5,6-Dihydrodihydroxy
naphthol 1.12 9.79 NDa 10.89
5,6-Dihydrodihydroxy
carbaryl 3.80 3.09 NDa 6.89
1,4-Naphthoquinoneb NDa 1.24 1.36 2.60
4-Hydroxy carbaryl 0.64 5.30 0.44 6.38
3-Hydroxy carbaryl NDa 0.04 0.01 0.05
3,4-Dihydrodihydroxy
naphthol NDa 0.50 NDa 0.50
Unknown 1 NDa 0.20 0.62 0.82
Unknown 2 NDa 1.30 NDa 1.30
Other unknowns NDa 1.26 0.43 1.69
Highly polar materials NDa 9.06 34.96 44.02
aND = none detected.
bA decomposition product of 1,4-naphthalenediol during work-up.
The above work supersedes the work by Knaak & Sullivan (1967),
who mistakenly concluded that the Beagle dog metabolic pathway was
qualitatively different from that of the rat, because of poor
absorption of solid/slurry test material. The marked emphasis on
differences in metabolic pathways between species, together with the
analytical techniques available at the time of the study contributed
to their conclusions.
Studies on the nature of the biliary, water-soluble metabolites
of carbaryl were conducted by Bend et al. (1971) on 19 male Wistar
rats, using a technique with the bile duct cannulated. Water-soluble
conjugates of carbaryl with sulfur-containing amino acid were found
in the urine as well as the bile of treated rats: S-(4-hydroxy-1-
naphthyl) cysteine and S(5-hydroxy-1-naphthyl)-cysteine. Biliary
secretion of 5,6-dihydro-5,6-dihydroxycarbaryl glucuronide following
an infused dose was found to be greatly reduced, from 10% to less
than 1%, by pretreatment with antibiotics (Struble et al., 1983b).
This indicates that bacteria play a role in the enterohepatic
circulation and bilary secretion of this metabolite of carbaryl.
The functional activity of the reticulo-endothelial system
(RES) can influence carbaryl metabolism (Pipy et al., 1980). The
elimination of carbaryl from the blood decreased significantly in
rats (24 male Sprague-Dawley) with RES inhibited by colloidal
carbon, and increased in those in which the RES was activated with
glyceryl trioleate. Correlation of the activity of RES with the
enzyme activity of monoxygenases of the hepatic microsomal fraction
could possibly explain this effect of the RES in the toxicokinetics
of carbaryl.
An intravenous injection of colloidal carbon, which inhibits
liver microsomal metabolism, reduced biliary excretion of an iv dose
of carbaryl given 18 h later (Pipy et al., 1981). Pretreatment of
rats with 75 mg phenobarbital/kg per day, ip, for five days resulted
in an increase in the rate of sulfate conjugation of carbaryl,
following a high dose of carbaryl (16.4 mg/kg), but not a low dose
(1.64 mg/kg) (Knight et al., 1987).
6.3.3 Metabolic transformation in plants
The formation of carbaryl metabolites in plants is primarily
dependent on the hydrolytic, oxidative, and conjugative potential of
the plant tissues, which are similar to the tissues of insects and
mammals. The metabolism in plants is of a shorter duration than that
in insects and mammals, and the tendency to accumulate metabolites
is more pronounced. The carbamate ester bond appears to be quite
stable in plants, which explains the small amount of recovery of
1-naphthol from treated plants. The extent of oxidation is generally
higher in plants and insects than in mammals. Several
hydroxymetabolites are formed by oxidation of the N-methyl group
and naphthalene ring. They are conjugated as glycosides.
Non-enzymatic factors, such as light, heat, and humidity may
contribute to the degradation of carbaryl in plants as well (Kuhr,
1971).
The metabolism of carbaryl has been defined in a wide variety
of plant species (Table 31). Metabolites were isolated in microgram
quantities for mass and ultraviolet spectroscopic analyses (Mumma
et al., 1971).
6.3.4 In vitro studies with human tissues
The metabolism of carbaryl in selected human tissues was
studied in vitro by Chin et al. (1974). On the basis of the
total anionic characteristics of the metabolites derived from each
organ, metabolic activity occurred in the following organs in
descending order: liver, lung, kidney, placenta, vaginal mucosa,
uterus.
The metabolic profiles of carbaryl in human postembryonic fetal
autopsy tissue were determined using 1-naphthyl-14C or
N-methyl-14C-carbaryl. The anionics from fetal liver amounted to
20% of those found with the adult liver. Naphthyl glucuronide and
naphthyl sulfate were produced in the kidney, whereas the lung
produced naphthyl sulfate from carbaryl (Chin et al., 1979a).
Carbaryl was metabolized oxidatively by primary human embryonic
cells in culture (Lin et al., 1975). Complete degradation occurred
after 72 h of incubation. Unconjugated metabolites were identified
as 1-naphthol, 5-hydroxycarbaryl, 4-hydroxycarbaryl, and
5,6-dihydro-5,6-dihydroxycarbaryl. The water-soluble components were
identified as 4-hydroxycarbaryl, 1,4-naphthalenediol, and
5,6-dihydro-5,6-dihydroxycarbaryl. The primary human embryonic lung
cells did not convert carbaryl to carbon dioxide. They may not
possess the enzyme system that is necessary to break down the
naphthalene ring of carbaryl to form carbon dioxide.
Sakai & Matsumura (1971) studied the degradation of carbaryl by
brain esterases. Carbaryl was degraded by band E4 and E6,
whereas, in the mouse brain preparation, the compound was degraded
by band E8, E9, and E6.
Cell culture techniques were used to examine the products of
carbaryl degradation by cultures of an L-132 cell line derived from
normal human embryonic lung. The data indicated that detoxification
was similar to that observed in animals and in in vitro enzyme
systems (Baron & Locke, 1970).
Table 31. The composition of water-soluble metabolites of carbaryl in plants.
(% distribution of released aglycones 21 days after treatment (for apples, 53 days))
Metabolite Beana Beana Wheatb Potatoc Cornd Riced Tomatoc Applese
(mature (shell (seedlings) (mature (seedlings) (seedlings) (mature
foliage) only) foliage) foliage)
5,6-Dihydrodihydroxycarbaryl 2.5 12.7 2.1 NDd 1.3 2.9 ND 2.1
Methylol carbaryle 29.9 47.9 10.5 16.2 13.3 7.8 25.4 2.8
7-Hydroxycarbaryl 24.2 trace ND ND ND ND ND 0.8
4-Hydroxycarbaryl 15.0 4.1 23.6 4.1 8.9 3.7 13.8 13.7
5-Hydroxycarbaryl 7.3 9.4 21.3 4.1 12.9 5.6 3.4 9.2
1-Naphthol 13.8 21.0 1.1 5.8 3.2 3.5 8.1 11.3
5,6-Dihydrodihydroxynaphthol ND ND ND ND 0.5 1.0 ND
Carbaryl 0.3 0.8 20.8 27.0 24.1 42.8 16.8 33.3
Unknown(s) ND ND 3.2 ND 2.9 2.2 5.2 3.0
Unhydrolysed conjugate 7.1 4.1 17.4 42.8 33.0 30.4 27.3 12.6
References: aWiggins & Weiden (1969); bAndrawes & Chancey (1970); cChancey & Andrawes (1971b); dChancey & Andrawes (1971a);
eChancey (1974).
fND = not detected.
Studies on the in vitro metabolism of carbaryl by a human
liver fraction indicated that there was a difference in the
metabolic pattern compared with that in the rat liver. The human
liver produces at least two more metabolites (Strother, 1970).
6.3.5 In vivo studies on humans
The metabolism of carbaryl in humans appears to be
qualitatively similar to that previously reported in other mammals.
Metabolic reactions include: hydroxylation, hydrolysis, and
conjugation. Metabolites of carbaryl were identified in the urine of
human volunteers after ingestion of a 2 mg/kg dose (Andrawes &
Myers, 1976). Only traces of the unchanged carbaryl could be
detected in the urine indicating rapid metabolism. From the spectrum
of the metabolites identified, a metabolic pathway of carbaryl in
humans is proposed and shown in Fig. 6.
The only detectable metabolites in urine samples taken from
workers exposed to carbaryl dust were 1-naphthylglucoronide and
sulfate (Knaak et al., 1965). Later 1-naphthyl and
4-hydroxycarbaryl, as conjugates of glucuronic and sulfuric acid,
were found in a study on volunteers.
Human exposure to carbaryl during aerial application for Gypsy
moth control was assessed by Schulze et al. (1979). The results of
this study indicated a strong positive correlation between carbaryl
exposure and the excretion of urinary 1-naphthol within 24 h of
known exposure and a total lack of 1-naphthol in pre-exposure
samples.
The presence of these metabolites has been confirmed in six
urine samples of workers with high exposures to carbaryl (Andrawes &
Myers, 1976).
One major and three minor interfering chemicals were detected
in no-exposure samples as well as the maximum exposure samples.
These interfering materials developed a colour similar to that of
carbaryl metabolites upon spraying the chromatograms with
p-nitrobenzene diazonium fluoborate. It is estimated that the
major interfering pigment accounted for as much as one-third of the
total colour observed on thin-layer chromatograms. These interfering
chemicals may also be the cause of high blank values often
encountered during the routine analysis of urine samples (Andrawes &
Bailey, 1979).
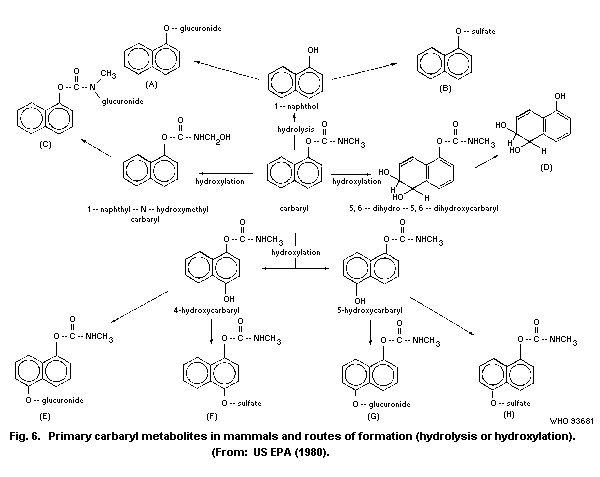 6.4 Elimination and excretion in expired air, faeces, urine,
milk, and eggs
The elimination of metabolized carbaryl is rapid, and
accumulation in animals seems unlikely, under normal exposure
conditions. Carbaryl is generally excreted entirely within 24-96 h
of intake. Elimination takes place mainly through the urine, faeces,
and respiration, and, to a lesser extent, through the milk of dairy
animals and the eggs of poultry.
Carbaryl is mainly excreted as its product of hydrolysis,
1-naphthol, which is detoxified to water-soluble glucuronide
(Carpenter et al., 1961), and sulfate (Whitehurst et al., 1963),
and only in trace amounts as 1-naphthol or unchanged.
The average percentage of metabolites recovered after orally
administered doses of 1-naphthyl 14C, methyl 14C, and carbonyl
14C carbaryl to rats was 94% over a 7-day period (Knaak et al.,
1965). Only residues from carbaryl methyl 14C were detected after
that, since the methyl moiety is incorporated in tissue (2-3%).
Liberated naphthol is conjugated and excreted, while the liberated
carbonyl groups are disposed of as respiratory14CO2 (Knaak
et al., 1965). Forty-seven to 57% of the metabolites excreted
retain the intact C-O-C(O)N-C structure, indicating a nonhydrolytic
pathway for carbaryl. Monkeys and pigs (2 young females) excreted
carbaryl as a conjugate of intact carbaryl and 4-hydroxycarbaryl,
mainly glucuronides. Sheep also excreted 1-naphthyl glucuronide and
sulfate and 4 (methylcarbamoyloxy)1-naphthylsulfate (Knaak et al.,
1968).
Myers (1977) studied the correlation between the amount of
carbaryl ingested and urinary metabolites levels. Metabolites were
measured as three groups:
I. naphthyl sulfate;
II. free and sulfated 5,6-dihydrodihydroxycarbaryl
and dihydrodihydroxynaphthol;
III. other conjugated (glucuronides) of naphthol,
5,6-dihydrodihydroxycarbaryl, and
5,6 dihydrodihydroxynaphthol.
The amounts in groups I and II were 25.5-36.5% of the dose. When all
three groups of metabolites were analysed, the amounts of
metabolites for the same period represented 41.5-52.7% of the dose.
Naphthol was the major metabolite found in Group III (mean 75% of
all metabolites of the group). A good correlation was found between
the amount ingested and urinary metabolites.
Rats treated orally with naphthyl14C carbaryl (2.3 µci)
excreted 90% of the administered radioactivity in the urine within 3
days. During the 72-h sampling period, the faeces contained only
2-5% of the radioactivity (Lucier et al., 1972).
Eighty per cent of 5 mg of 14C naphthyl carbaryl, given
intraperitoneally to rats, (see section 6.3.2) was eliminated in the
24-h urine and 10% in the faeces (Bend et al., 1971). Enzymatic
hydrolysis and reverse isotope dilution showed that 10% of the dose
was excreted as 1-naphthyl-glucoronide, 5%, as 1-naphthylsulfate,
3%, as conjugates of 4-hydroxycarbaryl, 5%, as 1-naphthylsulfate,
3%, as conjugates of 4-hydroxycarbaryl, and 5% of 5-hydroxycarbaryl.
About 2% of the carbaryl appeared unchanged in urine. Other
radioactive metabolites were not identified.
Following a single, ip injection in albino rats of both sexes,
of methyl 14C and carbonyl 14C carbaryl at 30 mg/kg, 75-80% was
recovered after 48 h in the expired air and urine. Liberated by
hydrolysis, N-methyl carbamic acid was spontaneously decomposed to
methylamine and carbon dioxide (43.5%). The methylamine moiety was
later demethylated to 14CO2, which was eliminated in the expired
air and 14C formate (9.2%), which was excreted in the urine
(Hassan et al., 1966).
In the study of Nye & Dorough (1976), 2.5% of the
endotracheally administered carbaryl dose was exhaled as
14C-carbon dioxide. More than 90% of the dose was eliminated in
the urine during 3 days. The faeces contained 2-5%.
A comparative study on the excretion of carbaryl after a
7.5 µmol/kg, ip dose in rats was reported by Krishna & Casida (1966)
who used three types of labelled carbaryl (Table 32).
Table 32. Fate of 14carbon in male Sprague-Dawley rats
after ip administration of carbaryla
Labelled position Administered 14carbon recovered (%)
Expired 14CO2 Urine Faeces
0-24 h 24-48 h
Carbonyl 14C 24.5 62.1 2.4 2.1
Methyl 14C 12.3 54.6 3.4 3.9
Naphthyl 14C 0.2 74.2 2.3 8.9
Hydrolysis product < 0.1 86.4 3.3 1.4
naphthol 14C
aFrom: Krishna & Casida (1966).
Biliary excretion of carbaryl was studied in bile-duct
cannulated rats. Naphthyl 14C and methyl 14C carbaryl (50 µg)
were administered intravenously to rats (see section 6.3.2). During
the first 2 h of collection, 90% of the total biliary radioactivity
was eliminated (Bend et al., 1971). After oral application of
0.01 mg/kg naphthyl-14C carbaryl to rats, 37.5% was excreted via
the bile in the first 3 h and 45.4% (cumulative percentage) of the
dose was eliminated within 48 h. Faecal elimination was 1.4% and
urine elimination was 42.3% (Marshall et al., 1979). The results
of Struble et al. (1983a), who used higher doses of carbaryl (1.5,
31, and 300 mg/kg) on male Sprague-Dawley rats were similar. Twelve
hours after administration, 15-46% of the 14C was excreted in the
bile, 10-40% in the urine, and less than 1% was eliminated in
faeces. Three metabolites were identified from the bile, the major
one being 5-6-dihydro-5-6-dihydroxycarbaryl glucoronide (12-18% of
the biliary 14C).
On the basis of a study on 50, male, Sprague-Dawley rats,
Borzelleca & Skalsky (1980) reported that carbaryl metabolites in
the blood were also present in the saliva, at similar
concentrations.
Leeling & Casida (1966) studied carbaryl metabolites in the
urine of one male and one female New Zealand White rabbit. The
ether-extractable metabolites found in the urine were:
N-hydroxymethyl carbaryl, 4-hydroxycarbaryl, 5-hydroxycarbaryl,
5,6-dihydro-5,6-dihydroxycarbaryl, 5,6-dihydro-5,6-trihydroxy-1-
naphthol, and 1-naphthol.
Thirty-five, adult, female American cockroaches ( Periplaneta
americana) were injected through the central abdominal wall with
5 µg of carbaryl-carbonyl 14C, carbaryl methyl 14C, or 2 µg
carbaryl naphthyl 14C. Metabolites similar to those appearing in
rat liver microsomes were formed. About 19% of the radioactivity of
carbaryl carbonyl 14C was eliminated as 14C-carbon dioxide in
24 h (Dorough & Casida, 1964).
Excretion of carbaryl in milk was studied by Baron (1968).
Application of a total dose of 2 g carbonyl carbaryl 14C to a
lactating cow resulted in approximately 1% of radioactive residues
in the milk. In skim milk, 87% of these residues were water-soluble
and 13% chloroform-soluble. About 90-95% of the14C radioactivity
in the soluble component was removed after crystallization of
lactose.
The metabolism of carbaryl naphthyl 14C was studied in one
lactating Jersey cow (Dorough, 1967). Two consecutive single
treatments at 6-day intervals with 0.25 mg/kg body weight (total
125 mg) and one single dose of 3.05 mg carbaryl naphthyl 14C/kg
body weight added to the feed, resulted in radiolabelled residues in
the milk for as long as 60 h after treatment. The first sample of
milk, taken after 6 h, showed maximum concentrations of chloroform
extractables, water-soluble, and unextractable, residues in whole
milk in the range of 0.063-0.95 mg/litre. Residues of only a
slightly lower magnitude were detected in the 12-h milk samples and
rapidly declined in 24-h samples. Thirty per cent of the residues in
the 6-h sample was a chloroform-extractable metabolite, tentatively
identified as 5,6-dihydro-5,6-dihydroxy-1-naphthyl-
N-methylcarbamate. About 0.35% of the total dose was detected in
the milk in both treatments, 70% in the urine, and 11% in the faeces
in 0.25 mg/kg treatment, and 58% in the urine and 15% in the faeces
in the 3.05 mg/kg treatment. The highest levels in the tissues of
the cow, killed on an unspecified day after treatment, were found in
the liver, kidney, and ovaries.
A single lactating cow was treated orally with a daily total
dose of 518 mg 14C-carbaryl (0.77 mg/kg body weight), given in two
doses, 12 h apart. Results are shown in Table 33 (Dorough, (1969).
Fifty-nine per cent of the radioactivity from a14C-labelled oral
dose of carbaryl administered to a cow was recovered from the urine
within the first 24 h (Saini & Dorough, 1978).
The passage of14C into the milk and its presence in the
suckling neonates has been studied by whole-body autoradiography in
rats fed 14C-methyl carbaryl. The highest concentrations in
newborn offspring, after 48 h, were found in the stomach contents,
the liver, the hair, and the bone marrow (Benard et al., 1979).
Table 33. Distribution and excretion of total-14C equivalents in
milk, urine, faeces, and tissues, 24 h after one-day oral
application of carbaryl in a lactating cow
Sample mg/kg Dose recovered
(%)
Milk 0.04 0.09
Urine 22.4 58.64
Faeces 0.47 1.65
Tissues:
- liver 0.098 0.21
- kidney 0.382 0.17
- leg muscle 0.027 nil
- fat 0.014 nil
A Saanen goat was treated orally with 1.34 mg carbaryl carbonyl
14C/kg. The excretion of radioactivity in the urine was 7.4% at
2 h, 24% at 4 h, and 45% at 24 h. In total, 47% of the radioactivity
was excreted the urine. In the milk, the peak was 0.9 mg/litre at
8 h, dropping to 0.008 mg/litre 60 h after the administration of
carbaryl (Dorough & Casida, 1964).
The excretion of carbaryl was studied in hens. Carbaryl-
carbonyl14C (10 mg/kg body weight) was given orally to Leghorn
hens. Fifty per cent of the 14C was expired within 48 h. When
carbarylnaphthyl 14C was applied at the same dose, no
radioactivity was detected in the expired gases. The urine was the
primary route of excretion. 14Carbon remaining in the carcass,
48 h after dosing, accounted for 1.4 and 7.1% of the dose given as
the ring-labelled and carbonyl-labelled compound, respectively
(Paulson & Feil, 1969). The main metabolites found in the urine of
hens were: 1-naphthol, 1-naphthyl glucoronide, and sulfate esters of
1-naphthol, 4-hydroxycarbaryl, and 5-hydroxycarbaryl. Conjugates
with other metabolites were also found in small quantities (Paulson
et al., 1970). Mature hens were given a single oral dose of
carbaryl naphthyl 14C at 10 mg/kg body weight. Eggs collected for
12 days contained a total dose of 0.33% of the 14C administered
(Paulson & Feil, 1969).
The fate of naphthyl-1-14C carbaryl in laying chickens was
studied by Andrawes et al. (1972). Chickens (3 White Leghorn in
each group) were fed 7, 12, or 70 mg/kg carbaryl in the feed twice
daily for 14 days. They had been pretreated for 17 days with the
same doses of nonlabelled carbaryl in feed. Residues reached maximum
levels within 1 day in excrement, 2 days in egg white, and 6-8 days
in egg yolk. After the end of dosing, the half-life of 14C
residues was <1 day in the excrement and egg white, 2-3 days in the
egg yolk, and 5 days in the carcass. Metabolites and carbaryl found
in the eggs of hens, fed 70 mg carbaryl/kg averaged in total 19.7 µg
of 14C carbaryl equivalent per egg (16.3 µg in yolk and 3.4 µg in
white), collected between the 9th and the 14th day of dosing.
Naphthyl-l-sulfate was the largest single component of egg residues
accounting for the higher concentration of residues in the egg yolk.
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
7.1 Microorganisms
7.1.1 Soil microorganisms
Studies of the effects of insecticides on soil microorganisms
are complex and subject to many sources of variation. It is
therefore difficult to assess the practical significance of the
results of such studies.
Varsheny & Gaur (1972) studied the impact of carbaryl (as
Sevin) on soil fungi. They determined the size of the fungal
population and the species composition of the fungal community after
plating out extracts of soil samples on various media. The authors
suggested that, at very high rates of addition to the soil (up to
5000 mg/kg), there was evidence of a change in species composition
and an increase in the total fungal population.
In laboratory cultures of saltmarsh protozoans (dominated by
Euplotes sp.), Weber et al. (1982) found that both carbaryl and
1-naphthol caused dose-related mortality. For both compounds,
mortality at 1 mg/litre was about 50%. There was almost 100%
mortality at 10 mg/litre.
Carbaryl inhibited the growth of cultures of Bacillus subtilis
(DeGiovanni & Donnelly, 1968).
7.1.2 Aquatic microorganisms
Edmiston et al. (1985) conducted a detailed study of the
effects of carbaryl on Paramecium multimicronucleatum. The 24-h
LC50 was 28 µg/litre in a static plate test. Effects on the oxygen
uptake of cultures and effects on the cell surface were also
reported, but these results were mostly obtained using
concentrations in excess of the 24-h LC50, and must be of limited
value.
Murray & Guthrie (1980) studied the responses of bacterial
cultures originating from Lake Houston. The respiratory rate of
bacterial cultures was increased at water temperatures of between
23-33 °C. Plate counts of bacterial colonies suggested that carbaryl
treated cultures contained more bacteria than control cultures on
most days in a 26-day study.
7.2 Aquatic organisms
Aquatic toxicity studies have been conducted in the laboratory
and field to define the biological effects associated with acute and
long-term exposure to carbaryl and the degradation product,
1-naphthol, of aquatic vertebrates and invertebrates. More data are
available for freshwater organisms than for saltwater organisms. In
addition, actual and simulated field studies have been conducted to
assess the impact of carbaryl exposure on freshwater invertebrates.
7.2.1 Aquatic invertebrates
Studies on the toxicity of carbaryl for non-target aquatic
invertebrates have included investigations on molluscs, crustaceans,
and insects, concentrating on organisms of commercial importance,
organisms most likely to be exposed during a typical application of
carbaryl, or on standardized test species typically used in aquatic
toxicity tests. The acute and long-term laboratory studies under
various environmental conditions (pH, temperature, water hardness,
etc.) are briefly reviewed in Table 34.
Most of the studies on the toxicity of carbaryl for aquatic
invertebrates have been conducted under laboratory conditions and,
consequently, the results only yield data on the relative toxicity
of the substance. They do not reflect realistic exposure in the
environment. Most of the laboratory studies evaluate constant,
short-term (less than 1 week) exposure to carbaryl under static or
flow-through conditions. There are few long-term studies on the
effects of carbaryl on aquatic invertebrates. Exposures in the
environment are typically localized, at much lower concentrations
than in aquatic toxicity tests and are not constant.
The toxicity of carbaryl on the early development of the sea
urchin was studied by Hernandez et al. (1990). Developmental
stages with active cleavage and cellular mobilization (blastula and
gastrula) turned out to be more sensitive. The next two stages
(prism and pluteus) were less sensitive and EC50 values were 8-26
times higher. This effect may be connected with increased
detoxication processes by the cytochrome oxidase system.
Table 34. Acute toxicity of carbaryl for invertebrates
Organism Test Size/age Stat/ Temperature Hardness pH Parameterc Concentration Reference
material Flowa (°C) (mg/litre)b (µg/litre)
Crustacea
(Freshwater)
Water flea
Daphnia magna unknown stat 16 42 7.4 48-h LC50 5.6 Sanders et al. (1983)
Daphnia magna technical 96-h LC50 3280 Lejczak (1977a)
Daphnia magna unknown 48-h EC50 0.26 Rawash et al. (1975)
Daphnia magna technical 21-day MATC 1.5-3.3 Surprenant (1985c)
21-day NOEC 6.0
(survival)
Daphnia pulex technical stat 16 44 7.1 48-h LC50 6.4 Mayer & Ellersieck (1986)
Simocephalus unknown stat 10 44 7.1 48-h LC50 11 Johnson & Finley (1980)
serrulatus stat 16 44 7.1 48-h LC50 7.6
stat 21 44 7.1 48-h LC50 8.1
Ostracod
Cypridopsis
vidua unknown adult stat 21 272 7.4 48-h LC50 115 Mayer & Ellersieck (1986)
Sow bug
Asellus
brevicaudus technical adult stat 18 44 7.1 96-h LC50 280 Johnson & Finley (1980)
Table 34 (continued)
Organism Test Size/age Stat/ Temperature Hardness pH Parameterc Concentration Reference
material Flowa (°C) (mg/litre)b (µg/litre)
Crustacea
(Freshwater)
Amphipod
Gammarus
fasciatus unknown adult stat 21 44 7.1 96-h LC50 26 Johnson & Finley (1980)
Gammarus
lacustris unknown adult stat 21 44 7.1 96-h LC50 22 Johnson & Finley (1980)
Gammarus unknown adult stat 12 40 6.5 48-h LC50 8.13 Mayer & Ellersieck (1986)
pseudolimnaeus adult stat 12 40 7.5 48-h LC50 11.5
adult stat 12 40 8.5 48-h LC50 7.8
Gammarus pulex technical 48-h LC50 29 Bluzat & Seuge (1979)
Gammarus technical 96-h LC50 16 Sanders et al. (1983)
pseudolimnaeus
Gammarus technical 96-h LC50 13 Woodward & Mauck (1980)
pseudolimnaeus
Glass shrimp
Palaemonetes unknown adult stat 21 272 7.4 96-h LC50 5.6 Johnson & Finley (1980)
kadiakensis 25-31 mm stat 96-h LC50 120 Chaiyarach et al. (1975)
Table 34 (continued)
Organism Test Size/age Stat/ Temperature Hardness pH Parameterc Concentration Reference
material Flowa (°C) (mg/litre)b (µg/litre)
Crayfish
Procambarus sp. technical immature stat 12 42 7.5 96-h LC50 1900 Mayer & Ellersieck (1986
Procambarus unknown 60-70 mm stat 96-hLC50 2430 Chaiyarach et al. (1975)
simulans
Procambarus technical 96-h LC50 500 Cheah et al. (1978)
acutus
Crustacea
(Freshwater)
Prawn
Macrobrachium technical 96-h LC50 19 Omkar & Shukla (1985)
lamarrei
Upogebia wettable powderd 48-h EC50 90 Stewart et al. (1967)
pugettensis 1-naphthol 48-h EC50 4400
Callianassa wettable powderd 48-h EC50 80
californiensis 1-naphthol 48-h EC50 3500
Hemigrapsus wettable powderd 24-h EC50 710
oregonensis 1-naphthol 24-h EC50 74 200
Table 34 (continued)
Organism Test Size/age Stat/ Temperature Hardness pH Parameterc Concentration Reference
material Flowa (°C) (mg/litre)b (µg/litre)
Crustacea
(estuarine &
marine)
Blue crab
Callinectes unknown juvenile flow 29 27s 48-h LC50 320 Mayer (1987)
sapidus
Brown shrimp
Penaeus aztecus unknown juvenile flow 30 28s 48-h LC50 1.5
Pink shrimp
Penaeus
duorarum unknown juvenile flow 23 29s 48-h LC50 32
Grass shrimp
Palaemonetes unknown juvenile flow 23 29s 48-h LC50 28
pugio 3-4 days stat 24 28-30s 96-h LC50 22 Thursby & Champhon (1991)
Crustacea
(estuarine &
marine)
Table 34 (continued)
Organism Test Size/age Stat/ Temperature Hardness pH Parameterc Concentration Reference
material Flowa (°C) (mg/litre)b (µg/litre)
Mysid shrimp
Mysidopsis bahia unknown flow 96-h LC50 > 7.7 Nimmo et al. (1981)
Mysidopsis bahia technical 96-h LC50 6.7 Surprenant (1985b)
NOEC 5.1
Mysidopsis bahia 24 h flow 25 31s 96-h LC50 8.46 Thursby & Champhon (1991)
24 h stat 24 30s 96-h LC50 19
24 h flow 26 31-32s 28-day LC50 9.9
Ostracod
Cypretta kawatai technical 72-h LC50 1800 Hansen & Kawatski (1976)
Insects
Stonefly
Isogenus sp. unknown larva stat 7 35 7 96-h LC50 2.8 Mayer & Ellersieck (1986)
Pteronarcys technical 96-h LC50 4.8 Johnson & Finley (1980)
californica
Mosquito
Culex pipiens technical 24-h LC50 75 Rawash et al. (1975)
Table 34 (continued)
Organism Test Size/age Stat/ Temperature Hardness pH Parameterc Concentration Reference
material Flowa (°C) (mg/litre)b (µg/litre)
Insects
Midge
Chironomus technical 24-h LC50 7 Karnak & Collins (1974)
tentans
Chironomus technical 72-h LC50 5900 Hansen & Kawatski (1976)
tentans
Chironomus technical 48-h EC50 10 Sanders et al. (1983)
plumosus
Chironomus unknown larva stat 20 4 24-h LC50 106 Fisher & Lohner (1986)
riparius larva stat 20 6 24-h LC50 133
larva stat 20 8 24-h LC50 127
Back swimmer
Notonecta technical 96-h LC50 200 Federle & Collins (1976)
undulata
Water stick
Ranatra elongata wettable 96-h LC50 624 Shukla et al. (1982)
powder
Table 34 (continued)
Organism Test Size/age Stat/ Temperature Hardness pH Parameterc Concentration Reference
material Flowa (°C) (mg/litre)b (µg/litre)
Diptera
Chaoborus technical 48-h LC50 296 Bluzat & Seuge (1979)
Ephemeroptera
(Mayfly)
Cloeon technical 48-h LC50 480
Crawling water
beetle
Peltodytes sp. technical 96-h LC50 3300 Federle & Collins (1976)
Insects
Stone fly
Pteronarcella unknown larva stat 16 44 7.1 96-h LC50 1.7 Johnson & Finley (1980)
badia larva stat 12 38 6.5 96-h LC50 11 Woodward & Mauck (1980)
larva stat 12 38 7.5 96-h LC50 13 Mayer & Ellersieck (1986)
larva stat 12 38 8.5 96-h LC50 29
Claasenia unknown larva stat 16 44 7.1 96-h LC50 5.6 Johnson & Finley (1980)
sabulosa
Table 34 (continued)
Organism Test Size/age Stat/ Temperature Hardness pH Parameterc Concentration Reference
material Flowa (°C) (mg/litre)b (µg/litre)
Molluscs
Common mussel
Mytilus edulis unknown 48-h EC50 > 30 000 Roberts (1975)
for byssal
attachment
Mytilus edulis wettable larva 48-h EC50 2300 Stewart et al. (1967)
powder 48-h EC50 1300
1-naphthol
Giant oyster
Crassostrea gigas wettable larva 48-h EC50 2200
powder 48-h EC50 800
1-naphthol
Clinocardium wettable adults 24-h EC50 7300
nuttallii powder 24-h EC50 6400
1-naphthol
Molluscs
Eastern Oyster
Crassostrea juvenile flow 29 27s 96-h EC50 > 2000 Mayer (1987)
virginica
Table 34 (continued)
Organism Test Size/age Stat/ Temperature Hardness pH Parameterc Concentration Reference
material Flowa (°C) (mg/litre)b (µg/litre)
Crassostrea technical 48-h EC50 2700 Surprenant (1985a);
virginica Dionne et al. (1985)
Mactrid clam
Rangia cuneata 35-50 mm stat 5s 96-h LC50 125 000 Chaiyarach et al. (1975)
Lymnaea technical 48-h LC50 21 000 Bluzat & Seuge (1979)
stagnalis
aStat = static conditions (water unchanged for the duration of the test).
Flow = flow-through conditions (carbaryl concentration in water continuously maintained).
bHardness = expressed as mg CaCO3/litre.
s = salinity (%).
cEC50s for oyster based on shell deposition.
d50-80% wettable powder.
Carbaryl is slightly toxic for freshwater and marine molluscs
(clams, mussels, oysters). Acute toxicity typically occurs in adult
molluscs at concentrations ranging from 1 mg carbaryl/litre to over
100 mg/litre. The major hydrolytic product of carbaryl, 1-naphthol,
is slightly more acutely toxic for molluscs (mussel, pacific oyster)
than the parent compound (Stewart et al., 1967). In addition,
larvae and young juveniles are more sensitive to carbaryl exposure
than older juveniles and adults.
There have been few studies on the effects of carbaryl on the
growth of molluscs. Davis (1961) and Butler (1962) found that
carbaryl (80% WP) concentrations of 1.0-2 mg/litre reduced the
larval development and growth of oysters ( Crassostrea virginica)
and clams ( Venus mercenaria).
The effects of a 1-h exposure to carbaryl (80% WP) and its
hydrolytic product, 1-naphthol, were studied on six developmental
stages of the mussel ( Mytilus edulis) (Armstrong & Millemann,
1974c). The six stages included unfertilized eggs to the early
veliger stage, 32 h after fertilization. Unfertilized eggs and the
first polar body stage were exposed to carbaryl and 1-naphthol
(separately). The acute toxicity values of carbaryl and 1-naphthol
were similar for the first two stages, ranging from approximately 5
to 25 mg/litre. The stage of development most sensitive to carbaryl
was the first polar body stage. Thereafter, susceptibility decreased
as age of the larvae increased.
LC50 values for crustacea varied from 5 to 9 µg/litre (water
fleas, mysid shrimps), 8 to 25 µg/litre (scud), and 500 to
2500 µg/litre for crayfish. The hydrolytic metabolite 1-naphthol was
less toxic for daphnids and shrimps.
The effects of carbaryl (80% WP) on the life stages of
crustacea have been studied in the Dungeness crab ( Cancer magister)
(Buchanan et al., 1970). Early larvae were more sensitive to
carbaryl than juveniles and adults. Carbaryl (1.0 mg/litre) did not
affect hatching of eggs but prevented moulting of all prezoeae to
zoeae. The concentration that killed 50% of the first zoeae during a
96-h exposure was estimated to be 1 mg/litre.
Young juvenile crabs were more sensitive than older juveniles
or adults. The 24-h EC50s (death or irreversible paralysis) were
estimated to be 0.076 and 0.35-0.62 mg/litre for second and ninth
stage juveniles, respectively. At 0.032 mg/litre, juvenile crabs
were not affected when held in uncontaminated water after exposure.
In adult crabs, the 24-h and 96-h EC50s (death or paralysis) were
0.49 and 0.26 mg/litre, respectively. Post-larval Dungeness crabs
had about the same sensitivity to carbaryl as other crabs. The 24-h
EC50 (death or paralysis) of small stone crabs was 1.0 mg/litre
(Butler, 1962), that of juvenile blue crabs, 0.55 mg/litre (Butler,
1963), and that of adult crabs, 0.06-1.05 mg/litre (Stewart et al.,
1967).
As part of fresh water biota, daphnids have been used as assay
organisms to determine toxic concentrations of a variety of
pesticides. Laboratory bioassays were conducted with carbaryl to
determine its toxicity and immobilization values for two species of
daphnids, Daphnia pulex and Simocephalus serrulatus. The EC50s
for overt effects were 0.0064 mg/litre for Daphnia pulex and
0.0076 mg/litre for Simocephalus serrulatus (Sanders & Cope,
1966).
In a long-term study, Daphnia magna was exposed to technical
carbaryl for 21 days; reproductive performance was the most
sensitive indicator; the MATC was 1.5-3.3 µg/litre (Surprenant,
1985c).
Aquatic insects in the orders Plecoptera (stoneflies) and
Ephemeroptera (mayflies) are generally highly sensitive to low
levels of carbaryl.
The effects of 0.6-50.0 mg carbaryl/litre were studied on
selected aquatic organisms, including: Scanedesmus quadricauda,
Lemna minor, Lebistes reticulatus and Daphnia magna (Bogacka &
Groba, 1980). Carbaryl depressed reproduction, biomass, and
chlorophyll content in Lemna minor (vascular plant) after 24 h
exposure at a concentration >6.6 mg/litre. An almost 100% decrease
occurred at a concentration of 50 mg/litre. Inhibition of
photosynthesis intensity in the alga Scenedesmus quadricauda was
about 50% at a concentration of carbaryl in the water of
32 mg/litre, and 65% at a concentration of 56 mg/litre, after 24 h
exposure. Less than 10% inhibition occurred at a concentration of
1.8 mg/litre. A decrease in the production of chlorophyll was
demonstrated at 4.4 mg/litre.
Carbaryl applications of 5.7 or 11.4 kg/ha were effective in
controlling ghost shrimp ( Callianassa californiensis) as an oyster
pest. The numbers of various clams in the mud were reduced by 22 and
38%, respectively. Clam species differed in susceptibility. For
example, gaper clams (Tresus capax) were reduced by 58 and 69%.
Polychaetes and nemertean worms were not affected during 30 days of
observation (Armstrong & Millemann, 1974a,b).
7.2.2 Fish
7.2.2.1 Acute toxicity
Acute toxicity studies have been conducted with technical
carbaryl, several formulations, and 1-naphthol to determine LC50
values for several freshwater and marine fish (Table 35). The
results indicated that short-term exposure (< 4 days) to carbaryl
and its formulations showed some toxicity for fish (96-h LC50s =
1-30 mg/litre). There were no differences in the toxicity of two
formulations (4-oil-sevin and XLR) for rainbow trout and bluegill.
The LC50s for sheepshead minnow (exposed to technical material)
and rainbow trout (exposed to 4-oil and XLR) were similar and both
were generally more sensitive than bluegill. The toxicity of
1-naphthol for rainbow trout, bluegill, and sheepshead minnow was
determined by Springborn (1988a); 96-h LC50 values for these
species were 0.76, 1.4, and 1.2 mg/litre, respectively. The
corresponding NOECs were < 0.43, 0.55, and 0.47 mg/litre. The
metabolite, 1-naphthol, was more acutely toxic than the formulations
for bluegill. However, the toxicities of 1-naphthol and the
formulations were similar for rainbow trout and sheepshead minnows.
Few studies report acute toxicity values for fish that are
lower than 1 mg/litre. Cold water fish (Salmonidae), such as Coho
Salmon and trout, seem to be susceptible to carbaryl, while the
catfish (Ictaluridae) is tolerant (Macek & McAllister, 1970; Post &
Schroeder, 1971). Irritability, sluggishness, and loss of
equilibrium were classical signs of acute intoxication. Rainbow
trout (Oncorhynchus mykiss), exposed to 1 mg carbaryl/litre for
96 h, exhibited decreased swimming capacity and swimming activity;
the capacity to capture and consume prey was also diminished (Little
et al., 1990).
Other end-point concentrations have been established for
carbaryl for various species. Reference should be made to the
original documentation to obtain a fuller understanding of these
measures of toxicity. The lowest no-observed-effect concentration
(NOEC) was found for rainbow trout at 0.065 mg/litre. Other NOECs
were at least an order of magnitude greater. Maximum acceptable
toxic concentrations (MATCS) have also been calculated. Bansal
et al. (1980) estimated 30-day MATCs for 4 species of carp exposed
to carbaryl (Sevin as a 50% wettable powder). For all species, the
MATC was between 0.052 and 0.078 mg/litre. Verma et al. (1984)
reported a 60-day MATC of between 0.09 and 0.11 mg/litre for another
carp species, exposed to the same type of formulation of Sevin.
Table 35. Acute toxicity of carbaryl for fish
Organism Size/age Stat/ Temperature Hardness pH Formulationc Parameter Concentration Reference
flowa (°C) (mg/litre)b (mg/litre)
Freshwater
Coho salmon 1 g stat 13 44 7.1 96-h LC50 4.3 Mayer & Ellersieck (1986)
(Oncorhynchus stat 13 42 7.1 96-h LC50 2.4
kisutch) 4.6 g stat 13 42 7.1 96-h LC50 1.8
5.1 g stat 13 42 7.1 96-h LC50 2.7
10.6 g stat 13 42 7.1 96-h LC50 1.2
19.1 g CT 96-h LC50 0.8 Macek & McAllister (1970)
CT 96-h LC50 1.3 Post & Schroeder (1971)
Chinook salmon fingerling flow 13 314 7.5 96-h LC50 2.4 Mayer & Ellersieck (1986)
(Oncorhynchus
tshawytscha)
Cutthroat trout 0.5 g stat 12 40 7.5 96-h LC50 7.1
(Salmo clarkii) 0.5 g stat 12 330 7.8 96-h LC50 4.0
0.6 g stat 7 42 7.5 96-h LC50 6.0
0.7 g stat 12 40 6.5 96-h LC50 5.0
0.6 g stat 12 40 8.5 96-h LC50 1.0
CT 96-h LC50 6.0 Woodward & Mauck
C49 96-h LC50 6.7 (1980)
Table 35 (continued)
Organism Size/age Stat/ Temperature Hardness pH Formulationc Parameter Concentration Reference
flowa (°C) (mg/litre)b (mg/litre)
Rainbow trout 1.5 g stat 12 42 7.1 96-h LC50 2.0 Mayer & Ellersieck (1986)
(Oncorynchus 1.5 g stat 12 272 7.4 96-h LC50 1.2
mykiss) 1.2 g stat 12 40 7.4 96-h LC50 3.5
1.2 g stat 12 320 7.4 96-h LC50 3.0
1 g stat 12 40 7.4 96-h LC50 1.6
1 g stat 17 40 7.4 96-h LC50 1.1
1 g stat 12 40 6.5 96-h LC50 1.2
1 g stat 12 40 7.5 96-h LC50 0.8
1 g stat 12 40 8.5 96-h LC50 1.5
0.5 g flow 17 314 7.5 96-h LC50 1.3
CT 96-h LC50 4.3 Macek & McAllister (1970)
XLR 96-h LC50 1.4 Springborn (1985)
S4 96-h LC50 1.3
CT 96-h LC50 2.2 Sanders et al. (1983)
CT 96-h LC50 1.5 Post & Schroeder (1971)
Atlantic salmon 0.2 g stat 7 42 7.5 96-h LC50 0.3 Mayer & Ellersieck (1986)
(Salmo salar) 0.2 g stat 12 42 7.5 96-h LC50 0.9
0.2 g stat 17 42 7.5 96-h LC50 1.0
0.4 g stat 12 42 6.5 96-h LC50 1.3
0.4 g stat 12 42 8.5 96-h LC50 0.9
Brown trout 0.6 g stat 12 42 7.5 96-h LC50 6.3
(Salmo trutta) fingerling flow 12 314 7.5 96-h LC50 2.0
Table 35 (continued)
Organism Size/age Stat/ Temperature Hardness pH Formulationc Parameter Concentration Reference
flowa (°C) (mg/litre)b (mg/litre)
Brook trout 0.8 g stat 12 42 7.5 96-h LC50 2.1 Mayer & Ellersieck (1986)
(Salvelinus 0.8 g stat 7 42 7.5 96-h LC50 3.0
fontinalis) 1 g stat 17 42 7.5 96-h LC50 0.7
0.7 g stat 12 42 6.5 96-h LC50 4.6
0.7 g stat 12 42 8.5 96-h LC50 2.1
0.7 g stat 12 42 9 96-h LC50 1.1
0.8 g stat 12 42 7.5 96-h LC50 1.2
0.8 g stat 12 300 7.5 96-h LC50 1.3
Lake trout 1.7 g stat 12 40 7.5 96-h LC50 0.7
(Salvelinus 1.7 g stat 12 40 6 96-h LC50 0.7
namaycush) 1.7 g stat 12 40 9 96-h LC50 0.9
0.5 g stat 12 162 7.4 96-h LC50 0.9
2.6 g flow 12 162 7.4 96-h LC50 2.3
Gold fish 0.9 g stat 18 40 7.1 96-h LC50 13.2
(Carassius 0.9 g stat 18 272 7.4 96-h LC50 12.8
auratus)
Common carp 0.6 g stat 18 40 7.1 96-h LC50 5.3
(Cyprinus
carpio)
fry CT 96-h LC50 1.7 Chin & Sudderuddin (1979)
Table 35 (continued)
Organism Size/age Stat/ Temperature Hardness pH Formulationc Parameter Concentration Reference
flowa (°C) (mg/litre)b (mg/litre)
Carp C50 WP 72-h LC50 10.4 Toor & Kaur (1974)
(Cyprinus
carpio
communis)
(Catla catla) S 96-h LC50 6.4 Tilak et al. (1981)
(Cirrhina S50 WP 96-h LC50 2.0 Verma et al. (1984)
mrigala)
Fathead 0.5 g stat 12 42 7.5 96-h LC50 14.0 Mayer & Ellersieck (1986)
minnow 0.8 g stat 18 40 7.1 96-h LC50 14.6
(Pimephales 0.8 g stat 18 272 7.4 96-h LC50 7.7
promelas)
Sheepshead CT 96-h LC50 2.2 Springborn (1985)
minnow
(Cyprindon
variegatus)
Black bullhead 1.2 g stat 18 40 7.1 96-h LC50 20.0 Mayer & Ellersieck (1986)
(Ictalurus
malas)
Channel catfish 1.5 g stat 18 40 7.1 96-h LC50 15.8
(Ictalurus 1.5 g stat 18 272 7.4 96-h LC50 7.8
punctatus) fingerling flow 12 314 7.5 96-h LC50 17.31
Table 35 (continued)
Organism Size/age Stat/ Temperature Hardness pH Formulationc Parameter Concentration Reference
flowa (°C) (mg/litre)b (mg/litre)
Freshwater 1.1 g stat 28 S50 WP 72-h LC50 17.5 Arunachalam et al. (1980)
catfish
(Mystus
vittatus)
S 96-h LC50 2.4 Tilak et al. (1981)
(Mystus S 96-h LC50 4.6
cavasius)
Freshwater CT 96-h LC50 46.9 Tripathi & Shukla (1988)
catfish
(Clarias
batrachus)
CT 96-h LC50 107.7
Catfish CT 96-h LC50 9.7 Lejczak (1977a)
(Lebistes
reticulatus)
Green sunfish 1.1 g stat 18 40 7.1 96-h LC50 11.2 Mayer & Ellersieck (1986)
(Lepomis 1.1 g stat 18 272 7.4 96-h LC50 9.5
cyanellus)
Table 35 (continued)
Organism Size/age Stat/ Temperature Hardness pH Formulationc Parameter Concentration Reference
flowa (°C) (mg/litre)b (mg/litre)
Bluegill 1.2 g stat 18 40 7.1 96-h LC50 6.8
(Lepomis 1.2 g stat 18 272 7.4 96-h LC50 5.2
macrochirus) 0.4 g stat 12 44 7.4 96-h LC50 7.4
0.4 g stat 22 44 7.4 96-h LC50 5.2
0.8 g stat 12 40 7.4 96-h LC50 16.0
0.8 g stat 17 40 7.4 96-h LC50 7.0
0.8 g stat 22 40 7.4 96-h LC50 8.2
0.4 g stat 17 320 7.4 96-h LC50 6.2
0.7 g stat 17 40 6.5 96-h LC50 5.4
0.7 g stat 17 40 7.5 96-h LC50 5.2
0.7 g stat 17 40 8.5 96-h LC50 1.8
0.7 g stat 17 40 9.5 96-h LC50 2.6
0.6 g flow 12 314 7.5 96-h LC50 5.1
XLR 96-h LC50 9.8 Springborn (1985)
S4 96-h LC50 10
Mosquito fish 30-40 mm stat 96-h LC50 31.8 Chaiyarach et al. (1975)
(Gambusia
affinis)
Largemouth 0.9 g stat 18 40 7.1 96-h LC50 6.4 Mayer & Ellersieck (1986)
bass
(Micropterus
salmoides)
Black crapple 1 g stat 18 40 7.1 96-h LC50 2.6
(Pomoxis
nigromaculatus)
Table 35 (continued)
Organism Size/age Stat/ Temperature Hardness pH Formulationc Parameter Concentration Reference
flowa (°C) (mg/litre)b (mg/litre)
Yellow perch 1.4 g stat 18 40 7.1 96-h LC50 0.7 Mayer & Ellersieck (1986)
(Perca 0.6 g stat 12 42 7.5 96-h LC50 5.1
flavescens) 1 g stat 7 42 7.5 96-h LC50 13.9
1 g stat 12 42 7.5 96-h LC50 5.4
1 g stat 17 42 7.5 96-h LC50 3.4
1 g stat 22 42 7.5 96-h LC50 1.2
0.9 g stat 12 42 6.5 96-h LC50 4.0
0.9 g stat 12 42 7.5 96-h LC50 4.2
0.9 g stat 12 42 8.5 96-h LC50 0.5
0.9 g stat 12 42 9 96-h LC50 0.4
1 g stat 12 42 8 96-h LC50 3.8
1 g stat 12 170 8 96-h LC50 5.0
1 g stat 12 300 8 96-h LC50 3.8
fingerling flow 12 314 7.5 96-h LC50 1.4
Snakehead fish CT 48-h LC50 8.7 Rao et al. (1985a)
(Channa
punctatus)
C50 WP 96-h LC50 19.5 Singh et al. (1984)
C50 WP 48-h LC50 8.1 Rao et al. (1985b)
(Anabas S 96-h LC50 5.5 Tilak et al. (1981)
testulus)
Table 35 (continued)
Organism Size/age Stat/ Temperature Hardness pH Formulationc Parameter Concentration Reference
flowa (°C) (mg/litre)b (mg/litre)
Tilapid fish S50 WP 72-h LC50 8.0 Koudinya & Ramamurthi
(Sarotherodon (1980)
mosambica)
(Tilapia sp.) C 48-h LC50 5.5 Basha et al. (1983)
Estuarine C 96-h LC50 2.2 Lingaraja & Venugopalan
teleost (1978)
(Therapon
jarbus)
(Hetero-pneustes C50 WP 96-h LC50 20.1 Singh et al. (1984)
fossilis)
(Macropodus) CT 96-h LC50 3.5 Arunachalam &
Palanichamy (1982)
(Cypanus) CT 96-h LC50 4.0
Estuarine &
marine
Longnose juvenile stat 28 19s 48-h LC50 1.6 Mayer (1987)
killifish
(Fundulus
similis)
Table 35 (continued)
Organism Size/age Stat/ Temperature Hardness pH Formulationc Parameter Concentration Reference
flowa (°C) (mg/litre)b (mg/litre)
Striped mullet juvenile stat 24 17s 48-h LC50 2.4
(Mugil
cephalus)
aStat = static conditions (water unchanged for the duration of the test); Flow = flow-through conditions (carbaryl concentration
in water continuously maintained).
bHardness = expressed as mg CaCO3/litre; s = salinity (%).
cFormulation: C = carbaryl; CT = Carbaryl technical (> 95%); C50 WP = Carbaryl 50% wettable powder; S = Sevin; S4 = Sevin-4-oil;
S50 WP = Sevin 50% wettable powder; XLR = Sevin (44% carbaryl); C49 = Carbaryl (49%). Where information on formulation is not given
the carbaryl formulation used was mostly carbaryl technical.
Exposure of C. punctatus to carbaryl at a concentration of
3 mg/litre for 48 h resulted in an increase in free fatty acids,
cholesterol, and lipase activity in the liver (Rao et al., 1985b).
However, the total lipid content was reduced.
The literature indicates that water temperature, hardness, and
pH may influence the toxicity of carbaryl, as well as the size of
the fish (Table 35).
In a study by Post & Schroeder (1971), water was supplied from
a well, classified as very hard and highly alkaline at temperatures
of 13.6-14.6 °C. Carbaryl was more toxic for cutthroat trout
weighing 0.37 g than for those weighing 1-2 g (96-h LC50 1.5 and
2.2 mg/litre, respectively) and more toxic for brook trout weighing
1.15 g than for those weighing 2.04 g (96-h LC50 1.2 and
2.1 mg/litre, respectively).
A comparative toxicity study on carbaryl and 1-naphthol, under
laboratory conditions showed that 1-naphthol was approximately 5
times more toxic than carbaryl for goldfish ( Carassius auratus)
and 2 times more toxic for killifish ( Fundulus heteroclitus) (Shea
& Berry, 1983).
Synergism of the effects of carbaryl and phenthoate on Channa
punctatus was found (Rao et al., 1985a,b). Carbaryl produced
potentiation of the effects of 2,4-D, n-butyl ester, dieldrin,
rotenone, pentachlorophenol, and arecoline on trout (Statham & Lech,
1975).
Application of carbaryl to a forest resulted in a significant
(15-34%) decrease in brain acetylcholinesterase activity in brook
trout from a nearby stream (Haines, 1981). No other effects were
observed.
7.2.2.2 Short-term and long-term toxicity
There is a limited data base on the effects of long-term
carbaryl exposure on fish. In one study, juvenile spot Leiostomus
xanthurus were exposed for five months to carbaryl (technical,
98%) at a level of 0.1 mg/litre in a flow-through system (Lowe,
1967). No carbaryl-related mortality was observed and there was no
effect on growth.
Carlson (1972) conducted the only full life-cycle study on fish
with carbaryl (80%), in which fathead minnows ( Pimephales promelas)
were exposed to five concentrations (0.008-0.68 mg/litre) for 9
months, beginning with the larvae. Survival of fatheads after 6
months at 0.68 mg/litre was lower than that in the controls. After 9
months at 0.68 mg/litre, the mean number of eggs produced per female
was reduced, the mean number eggs per spawning was also affected and
no hatching occurred. No other demonstrable effects were noted at
0.017, 0.062, and 0.21 mg/litre concentrations; thus, the maximum
acceptable toxicant concentration (MATC) for fathead minnows,
exposed to carbaryl in water, was between 0.21 and 0.68 mg/litre.
The lethal threshold concentration for 2-month-old minnows was
9 mg/litre.
Kaur & Toor (1977) exposed different stages of the embryo of
carp ( Cyprinus carpio) to carbaryl through the hatching stage.
There was 100% mortality of carp eggs and embryos at 2.5 mg/litre.
There appeared to be no effect of carbaryl on hatching at
0.01-0.75 mg/litre. However, there was decreased hatching at
1.0 mg/litre and deformed larvae (3.3%) with enlargement of the
pericardial sac and coiling of the posterior region of the embryo.
Thirty days exposure of Channa striatus to 10 or 20 mg
carbaryl/litre retarded oocyte production, with an increase in the
number of immature oocytes and a decrease in the number of mature
oocytes (Kulshrestha & Arora, 1984).
Statistically significant reductions in gonadotropic hormone in
the pituitary gland and plasma in Channa punctatus were observed
following exposure to carbaryl at a concentration of 1.66 mg/litre
(Ghosh et al., 1990). Gonadotropic hormone levels continued to
decrease with continued exposure, with a 30% decrease in the
pituitary gland and a 50% decrease in serum after 30 days of
exposure.
Exposure of the freshwater fish Puntius conchonius to carbaryl
at a concentration of 0.194 mg/litre for 15 days resulted in an
increase in the incidence of lesions in the gill and liver (Gill
et al., 1988). A higher level of exposure also resulted in lesions
in the kidney.
A 27-day exposure to 12.5 mg/litre led to a decrease in the
feeding and growth rate of the catfish ( Mystus rittatus)
(Arunachalam et al., 1980). Long-term toxicity of carbaryl for
fish in most natural surface waters will not occur, since exposure
would not be high enough or constant, because of the substance's
degradation.
7.2.3 Amphibians
The LC50s for bullfrog ( Rana tigrina) 0.1 g tadpoles with
24, 48, 72, or 96 h of exposure were determined to be 12.8, 8.2,
6.7, and 6.3 mg/litre, respectively (Marian et al., 1983). Growth
and feeding were decreased in a dose-dependent manner by doses
ranging from 0.5 to 5 mg/litre.
7.3 Terrestrial organisms
7.3.1 Worms
Carbaryl was classified as extremely toxic for earthworms
(LC50 = 9.1 µg/m2) by Roberts & Dorough (1984). The LC50 value
for the worm Eisenia fetida-Savigny was 14 µg/cm2 (Neuhauser
et al., 1985). The number of earthworms was reduced by 60% when
carbaryl was applied at 0.5 kg (1.12 lb) a.i. per hectare (Thompson,
1971).
Exposure of earthworms to carbaryl (1-8 mg/kg) significantly
increased the burrowing time, which was directly proportional to the
dose and time of exposure, because of the AChE inhibition in the
neural tissue (Gupta & Sundararaman, 1991).
7.3.2 Insects
Administration of carbaryl in sublethal doses produced death in
the early embryonal stage of the silkworm (Kuribayashi, 1981).
The alfalfa leaf-cutting bee ( Megachile pacifica), which is
very important in the pollination of alfalfa, has been reported to
be particularly tolerant to carbaryl (Lee & Brindley, 1974). Waller
(1969) also classified carbaryl as relatively non-toxic for the
alfalfa leaf-cutting bee. The carbaryl tolerance was related to sex
and age. The LD50 was similar for 1-day-old males and 1-and
4-day-old females (240, 245, and 262 µg/g, respectively). However,
four-day-old males were much more susceptible to carbaryl, and had
an LD50 of 51 µg/g. Guirgius & Brindley (1975, 1976) showed that
carbaryl toxicity in alfalfa leaf-cutting bees was controlled by the
activity of mixed function oxidase or microsomal enzymes. This
detoxification system varies with the age and sex of the bees and
results in significantly different carbaryl persistence which, in
turn, leads to differences in carbaryl toxicity. In the more
tolerant groups, carbaryl metabolites were rapidly conjugated and
moved to an aqueous fraction of the bee. Less tolerant insects
(4-day-old males) accumulated these metabolites with time,
indicating that the conjugation mechanisms had deteriorated with
age.
Carbaryl is known to be highly toxic for honey-bees. When
ingested, the LD50 was 0.18 µg/bee (Alvarez et al., 1970). The
contact LD50 (approximately 10-15 mg/kg) for adult bees is
approximately 1.3 µg (Stevenson et al., 1977; Stevenson, 1978).
The carbaryl residue content of bee bread was correlated with
the amount of residue found in the bees and occurred at higher
levels than in honey throughout the 56-day period (Winterlin &
Walker, 1973).
7.3.3 Birds
The toxicity of carbaryl for birds appears to be low
(Table 36). LD50s for six species of waterfowl and game birds were
all greater than 1000 mg/kg (Bart, 1979). There were some
exceptions; thus the red-winged blackbird has an LD50 of 56 mg/kg
(Schafer, 1972). In this study, 180 compounds were found to be toxic
for the red-winged blackbirds and the LD50s ranged between 0.24
and 100 mg/kg.
Food intake, body weight, and locomotor activity were monitored
in adult male bobwhites ( Colinus virginianus), given diets that
contained levels of carbaryl typical of normal exposure under
agricultural conditions in Kansas. No changes were observed in diets
containing 127 or 1235 mg carbaryl/kg (Robel et al., 1982).
Technical carbaryl fed to young Mallard ducks at dietary levels
of 10, 100, 1000, or 3000 mg/kg, revealed dose-correlated signs of
toxicity (reduced intake and/or body weight depression) at the 100,
1000, and 3000 mg/kg level (Fletcher & Leonard, 1986).
There was some tentative evidence that low dosages of carbaryl
may increase the susceptibility of bobwhites ( Colinus virginianus)
to the protozoan parasite Histomonas meleagridis, to which they are
usually resistant (Zeakes et al., 1981).
A study of the effects of carbaryl on forest birds was
conducted in southern New York; plots had been treated 3-4 weeks
previously with carbaryl at the normal rate of 1.1 kg/ha, and at 6
times the normal rate (6.6 kg/ha). In this study, carbaryl had
little, if any, effect on birds. Young birds gained weight normally,
adults continued nesting in the area, no changes were detected in
song frequency, and there was no evidence of birds leaving the area
to forage. This lack of detectable effects, despite the heavy dose
(6.6 kg/ha), indicates that carbaryl applied at the normal rate
(1.1 kg/ha) would have little if any adverse effect on birds (Bart,
1979).
The LC50 of carbaryl for mallard embryos, following field
applications, was determined by Hoffman & Albers (1984) to be
greater than 26.4 kg/ha or 118 µg/g egg. Carbaryl was estimated to
be relatively nontoxic compared with other pesticides.
Inhibition of the brain ChE activity of birds from forests
sprayed with carbaryl 1.13 (kg/ha) was found in 3 out of 12 species
studied (Zinkl et al., 1977) up to 5 days after application.
Table 36. Acute toxicity of carbaryl for birds
Species Age Parametera Concentration Reference
(mg/kg)
Japanese quail 7 days LD50 2290 Hudson et al.
Coturnix coturnix (1984)
japonica 2 months 5 day-LC50 > 10 000 Hill & Camardese
(1986)
Bobwhite quail 23 days 5 day-LC50 > 5000 Hill et al. (1975)
Colinus virginianus
California quail 10 months LD50 > 2000 Hudson et al.
Callipepla californica (1984)
Chukar Alectoris chukar 4 months LD50 1888
Sharp-tailed grouse LD50 < 1000
Tympanuchus
phasianellus
Pheasant 3-4 months LD50 707-> 2000
Phasianus colchicus 23 days LD50 > 5000 Hill et al. (1975)
Mallard 3 months LD50 > 2564 Hudson et al. (1984)
Anas platyrhynchos 24 days 5 day-LC50 > 5000 Hill et al. (1975)
Rock dove LD50 1000-3000 Hudson et al. (1984)
Columbia livia
Canada goose LD50 1790
Branta canadensis
Red-winged blackbird LD50 56 Schafer (1972)
Agelaius phoeniceus
a LD50 = single oral dose expressed as mg/kg body weight.
5 day-LC50 = 5-day dietary exposure (expressed as mg/kg feed) followed by
3 days on a "clean" diet.
7.3.4 Mammals
The effect of carbaryl on wild mice ( Clethrionomys glareolus
and Apodemus sylvaticus) in their natural environment was studied
by Krylov (1970). Carbaryl-containing bait, each consisting of 2 kg
grain treated with 50 g carbaryl in oil suspension, were distributed
twice (in March and June) over 10 ha of woods, at a rate of 1
bait/ha. An adjacent territory of 10 ha was used as a control.
Effects on mice, assessed 1´ months after the last application,
were: reduction of the population by 31.5% compared with the
control; changes in the reproduction system (e.g., decreased number
of embryos in utero, and of corpora lutea, reduced weight of
testicles), and increased weight of adrenal glands.
7.4 Effects on the population and ecosystem
The effects of carbaryl on terrestrial ecosystems have been
studied by Stegeman (1964), Barrett (1968), and Spain (1974).
A large-scale study was performed by Barrett in 1968. It was
designed to determine the effects of carbaryl on an intact ecosystem
in a field that was planted with millet ( Panicum ramosum). The
area was sprayed with a single application of 2.24 kg carbaryl/ha.
There was a highly significant decrease in litter decomposition in
the treated area, 3 weeks after spraying, which was probably because
of a reduction in microarthropods and other decomposers. After 5
weeks, there was a more than 95% reduction in the number of
arthropods and in the total biomass. Phytophagous insects were
severely affected, and predatory insects and spiders were less
affected. The number of species was also reduced, but all species
returned to control levels within 1-2 weeks, except Hemiptera and
Hymenoptera. Reproduction of cotton rats was delayed by 4 weeks.
However, the total mammal population was not affected, because of a
compensating increase in the population of house mice. Old field
mice did not seem to be affected.
The effects of carbaryl on forest soil mites and Collembola,
the two most numerous soil arthropods, were studied by Stegeman
(1964). Mites and Collembola are an important link in the
decomposition process of dead plants and animal matter. They also
are a natural means of decomposing the accumulating litter, and they
provide nutrients for already existing or future crops and fungi.
Application of carbaryl to a test plot in a red-pine plantation, at
doses of 11.2 and 56 kg/ha, reduced the arthropod population
proportional to the severity of the treatment. Neither mites nor
Collembola were totally exterminated by any treatment. The rate of
population increase of the mites, 4-5 months after treatment, was
directly proportional to the dose applied. Collembola were more
vulnerable to treatment than mites and did not recover as rapidly.
The effect of carbaryl at two application rates (0.11 and
1.13 g a.i. per m2) on mixed species population of the litter
fauna of a Corsican pine forest was studied by Spain (1974). He took
a quantitative sampling of the fauna at intervals of 11, 106, 209,
and 315 days after application to record the pattern and the time of
the recovery process (Table 37). The effect of carbaryl varied in
different populations. For Collembola, there was a marked reduction
at the two treatment levels. For Coccoidea and Symphyla, there was a
slight effect at the lower level, and a marked decrease at the
higher level. For Coleoptera, Diptera, Cryptostigmata, Mesostigmata,
and Prostigmata, the effect was small or insignificant. The recovery
process during the period of 315 days was not sufficient to reach
the status of the untreated population.
Tagatz et al. (1979) studied the effects of carbaryl on
animal communities that develop from planktonic larvae in aquaria
containing sand and estuary water. Samples collected after 10 weeks
of exposure were analysed. The numbers of animals and species were
significantly less at 11.1 and 103 µg carbaryl/litre (Table 38)
being decreased to nearly half (from 21 to 12). A carbaryl
concentration of 1.1 µg/litre did not produce significant effects,
except a decrease in the particularly sensitive amphiopod,
Coraphium acerusicum. Carbaryl might have caused changes in
biological interactions that affect the relative abundance of
species. The annelid Polydora ligni increased at a concentration
of 103 µg/litre, and there was a marked decrease in the number of
other annelids and nemerteas.
There have been a number of field studies evaluating the impact
of carbaryl applications on macroinvertebrate populations. In one
study in New York State, carbaryl was applied aerially at a rate of
1.3 kg/ha, in fuel oil with a paraffin oil sticker, and its effects
upon the aquatic fauna of two streams were studied (Burdick et al.,
1960). The data indicated that carbaryl (in oil suspension) was
toxic for mayflies (Ephemeroptera), stoneflies (Plecoptera) and
caddisflies (Trichoptera). Other groups of insects were less
affected and the application did not affect fish. Square-foot
samples, collected before, and shortly after, spraying, showed
reductions of from 50 to 97.2% in the weight of invertebrate
(standing crop) fish food. A progressive effect from upstream to
lower sections was correlated with increased 44-151 exposure time.
No exposure concentrations in water were documented. Owen (1965)
also reported a reduction of 54.2 to 62.8% in the standing crop of
aquatic insects following aerial spraying with carbaryl (80 WP)
(1.3 kg/ha). A control stream showed a 5.9% increase in the same
period.
Table 37. Geometric means for assessed taxa (individuals/m2), post-application sampling of carbaryl-treated plotsa
Sampling day (after treatment) Taxon Control plot High carbaryl Low carbaryl
Coccoidea 272.9 90.8 129.4
Coleoptera (imagines) 425.6 137.7 153.1
Coleoptera (immature) 54.0 47.3 41.4
Collembola 42 771.5 5033.4 9819.7
Diptera 350.9 244.2 243.7
11 Cryptostigmata 5707.3 3933.2 5863.5
Mesostigmata 7239.5 3688.9 5038.2
Prostigmata 1335.6 305.3 564.8
Aranese 238.7 222.7 155.0
Chilopoda 71.1 102.4 37.4
Symphyla 872.1 359.0 388.9
Coccoidea 847.8 157.0 162.9
Coleoptera (imagines) 41.9 379.1 170.7
Coleoptera (immature) 41.9 31.7b 0.0b
Collembola 18 185.1 1621.3 5205.9
Diptera 264.0 173.8 160.5
106 Cryptostigmata 5855.3 6237.9 6623.7
Mesostigmata 8610.3 846.4 595.2
Prostigmata 631.5 846.4 595.2
Aranese 360.6 224.9 253.2
Chilopoda 114.0 157.8 94.3
Symphyla 317.5 47.0b 223.4
Table 37 (continued)
Sampling day (after treatment) Taxon Control plot High carbaryl Low carbaryl
Coccoidea 358.3 181.0 141.0
Coleoptera (imagines) 520.0 86.6 232.6
Coleoptera (immature) 289.6 125.9 74.1
Collembola 41 198.7 3469.4 19 238.9
Diptera 449.9 28.2b 209.5
209 Cryptostigmata 16 298.4 11 184.8 12 014.8
Mesostigmata 16 150.0 7293.9 11 709.0
Prostigmata 2721.6 2407.2 3152.4
Aranese 264.7 108.3 220.2
Chilopoda 241.9 28.2b 150.5
Symphyla 357.3 78.3 174.4
Coccoidea 142.0 207.5 51.5
Coleoptera (imagines) 261.6 64.4 132.5
Coleoptera (immature) 37.0 88.8 23.1b
Collembola 40 139.5 4121.3 10 294.6
Diptera 533.9 291.2 173.1
315 Cryptostigmata 10 889.3 12 743.6 10 506.6
Mesostigmata 11 290.3 7762.7 14 912.0
Prostigmata 2995.0 1844.7 4765.7
Aranese 217.4 157.4 117.8
Chilopoda 159.5 83.4 88.8
Symphyla 349.6 85.4 158.8
aSource:Spain (1974).
b95% confidence limits for the parametric mean include zero.
Table 38. Animals and species, by phylum, collected from control aquaria and aquaria exposed to carbaryla
Control Carbaryl
1.1 µg/litre 11.1 µg/litre 103 µg/litre
Phylum Number Species Number Species Number Species Number Species
Mollusca 1691 3 1563 3 1340 3 1321 5
Arthropoda 380 7 339 8 336 2 269 2
Annelida 102 8 94 7 79 4 200 5
Nemerica 16 1 25 1 20 1 0 0
Coelenterata 3 1 5 1 0 0 0 0
Platyhelmintes 0 0 2 1 0 0 0 0
Echinodermata 0 0 0 0 1 1 0 0
All phyla 2192 20 2086 21 1776 11 1790 12
aFrom:Tagatz et al. (1979).
The effects on stream invertebrates of carbaryl, applied at a
rate of 840 g a.i./ha for spruce budworm suppression, was studied.
Benthos samples showed significant declines among Plecoptera,
Ephemeroptera, and Trichoptera. Plecoptera had not repopulated any
treated stream, 60 days after treatment (Courtemanch & Gibbs, 1980).
Gibbs et al. (1984) conducted a 42-month (1980-83) study on
the occurrence/persistence of carbaryl residues in pond water and
sediment as a result of an application of Sevin-4-oil (840 g
a.i./ha). The immediate and long-term effects on pond
macroinvertebrates and emerging aquatic insects were also evaluated.
A preliminary study by these investigators in 1977 and others
(Coutant, 1964) had shown that there were large increases in the
number of drift organisms, several days after aerial spraying with
carbaryl. Most drift was accompanied by dead Amphipoda,
Ephemeroptera, Plecoptera, and Trichoptera, and carbaryl residues
persisted longer than 30 days in pond sediment. The increase in the
number of dead organisms was accompanied by a reduction in the
standing crop of benthic macroinvertebrates. The most severe and
persistent impact was on Amphipoda with Hyallela azteca and
Crangonyx richmondensis reduced to almost 0/m2; C. richmondensis
failed to recolonize in one of the two treatment ponds, 42 months
after treatment. The numbers of immature Ephemeroptera and
Trichoptera were reduced immediately following spray application,
but this effect did not persist throughout the season or into the
following year. Immediate reduction in numbers of adult
Ephemeroptera and Trichoptera emerging from the ponds was also
found, but recovery of populations was observed. Numbers of immature
Odonata were also reduced following treatment and remained low the
following year. The Chironomidae populations did not appear to be
affected, either as immatures or emerging adults.
The effects of two consecutive years of spraying with
Sevin-4-oil on other aquatic systems appear similar to those
observed in areas treated once (Courtemanch & Gibbs, 1978; Trial,
1978,1979).
In simulated aquatic field studies, 1 mg carbaryl (WP)/litre
was applied to one outdoor concrete pond (4 x 5 x 1 m deep)
(Hanazato & Yasuno, 1987). All zooplankton (Cladocera included) and
Chaoborus larvae were killed. The zooplankton community recovered
rapidly and Cladocera reappeared only two days after application.
Since Chaoborus populations are predators of crustacean zooplankton,
their suppression may also have added to the recovery of increased
populations of Cladocerans. Carbaryl exposure also changed the
community from a rotifer-predominating zooplankton community to that
of Cladocera-predominating. Rotifers in this study were also highly
sensitive to carbaryl. The same phenomenon was observed again after
the second application of carbaryl. Subsequent to this study,
Hanazato & Yasuno (1989) applied carbaryl to simulated ponds in the
same way as in the previous study, but in the spring when the water
temperature was approximately 10 °C lower. Cladocerans never
recovered to the density level of the pre-treatment period. The
rapid recovery of Chaoborus seemed to interfere with the recovery of
Cladoceran populations after treatment. The authors suggested that
the different recovery patterns of the zooplankton community
resulted from different temperatures in the ponds. In another study,
Hanazato & Yasuno (1990a) examined Chaoborus density in relation to
the effects of carbaryl (0.1 and 0.5 mg/litre) on zooplankton
communities in ponds, where the abundance of Chaoborus larvae was
controlled. They concluded that Chaoborus density and/or temperature
may influence the recovery of the zooplankton community following
the effects of carbaryl. The recovery of a zooplankton community may
differ in different aquatic ecosystems (with different community
structures) and under different temperatures, even when the same
treatment is applied.
Hanazato & Yasuno (1990b) also studied the effect of the time
of application of carbaryl (0.5 mg/litre) on recovery patterns of
zooplankton communities in simulated ponds. The loss of carbaryl
from pond water was rapid. The concentration decreased to less than
1% of its initial value, three days after the first or second
application, and six days after the third application. This study
also showed that applications of carbaryl at different times induced
different zooplankton structures (and different recovery patterns),
and, various factors other than toxicity of carbaryl, such as
temperature, competitive interactions between zooplankters, and
trends of zooplankton populations may play important roles in
determining zooplankton community structure after chemical
application. Furthermore, the significance of predators in the
recovery process after chemical treatment was re-emphasized.
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
Reviews of toxicological aspects of carbaryl were prepared by
NIOSH (1976); US EPA (1977, 1980, 1982, 1984); USDHHS/USDL (1978);
US EPA (1980b); Mount & Oehme (1981a); Weston (1982); and Cranmer
(1986). Studies from the Soviet Union were reviewed by IRPTC (1982,
1989).
8.1 Single exposures
8.1.1 Oral toxicity
The oral LD50s for the rat are given in Table 39 and for
other mammals in Table 40. The values varied by about a factor of 4
depending on formulation, route of production, vehicle, and strain
of rat. Interspecies differences were found. Cats are the most
sensitive, with guinea-pigs, rats, mice, and rabbits showing more
resistance in that order. Pigs and monkeys seem to be less sensitive
(Carpenter et al., 1961; Gladenko & Malinin, 1970; Smalley, 1970).
Cattle are more sensitive than pigs. After a single oral
application of carbaryl in cattle, symptoms appeared at a dose level
of 25 mg/kg; 100 mg/kg was the minimum effective dose in pigs
(Gladenko & Malinin, 1970).
Mount & Oehme (1981b) observed that the lethality of carbaryl,
administered to sheep at doses ranging from 300 to 1000 mg/kg, was
highly correlated with concentrations in the brain (>1 mg/kg) and
liver (>3 mg/kg), and with inhibition of acetylcholinesterase
(greater than 50% inhibition).
8.1.2 Acute inhalation toxicity
Acute toxic effects following inhalation at different
concentrations are presented in Table 41.
8.1.3 Dermal toxicity
A dose of 2500 mg/kg, applied as a 40% aqueous suspension of
50% wettable powder, killed 1 out of 4 rabbits (Carpenter et al.,
1961). When 99% technical carbaryl was applied to male and female
rabbits, the LD50 was >2000 mg/kg (Bushy Run, 1983b). In rats,
the LD50 is thought to be >4000 mg/kg (Yakim, 1965; Gaines,
1969).
Table 39. Acute oral LD50s for the rat
Strain (sex) Weight Formulation of Vehicle LD50(mg/kg Symptoms References
(g) carbaryl body weight)
CF-N technical 0.25% agar
(male) 90-120 510 (360-650) - Carpenter et al.
(female) 90-120 610 (490-750) - (1961)
Sherman 850 (600 LD min) Gaines (1969)
(male)
(female) 500 (100 LD min)
Inbred 721 (653-789) Yakim (1965)
Inbred 120-200 sunflower oil 515 Rybakova (1966)
Sprague Dawley 203-246 technical carboxymethyl-cellulose 300 Hamada (1990)
(male, female) in water
Sprague Dawley 112-173 technical 0.25% methyl-cellulose 685 (612-767) death, 4-24 h Field (1980b)
(male, female)
Sprague Dawley 100-160 95% technical 0.25% methyl-cellulose 225 (202-321) death, 2-24 h Field (1980a)
(male, female)
Table 39 (continued)
Strain (sex) Weight Formulation of Vehicle LD50(mg/kg Symptoms References
(g) carbaryl body weight)
Sprague Dawley 200-264 40.38% water tremor, prostration, Hazleton Lab.
(male) 750 (467-1202) laboured respiration, American Inc.
salivation 10 February 1982
(female) 527 (257-977) death, 4-3 days
Harlem Sevin XLR not diluted death, 0.5 h-3 days Kuhn (1991a)
Sprague Dawley plus 44%
(male) 229-286 867 (562-1336)
(female) 182-219 575 (459-721)
Harlem Sevin-4-oil not diluted 658 (456-979) 3 h-3 days Kuhn (1991b)
Sprague Dawley 47% (w/w)
(male) 229-286 963 (802-1160)
(female) 182-219 473 (364-620)
Hilltop Wistar 200-250 98% technical 0.25% methyl-cellulose Bushy Run
(male) 283 death, 1.5-24 h (1983b)
(female) 246
Hilltop Wistar 204-237 80% sprayable water
(male) 406 Bushy Run
(female) 203 death, 1 min-24 h (1983c)
Table 40. Acute oral toxicity for mammals other than rats
Species Number of animals Toxicitya Reference
(mg/kg body weight)
Mouse 6 per dose 363 (294-431) Yakim (1965)
Mouse (female) 80 white 437 ± 70 Rybakova (1966)
Mouse 1310 white 206 (175-480) Bukin (1965)
Guinea-pig 5 per dose 280 Carpenter et al. (1961)
Rabbit 4 per dose 710
Rabbit 56 700 (LD100) Bukin (1965)
Cat (female) 3 250 (LD100) Carpenter et al. (1961)
Cat no data 150 Yakim (1965)
Swine 3 per dose 800-1000 Gladenko & Malinin
(1970)
Swine 1 per dose 1500-2000 Smalley (1970)
Monkey no data > 1000 FAO/WHO (1970)
Duckling 106 500 (LD15) Bukin & Filatov (1965)
6000 (LD100)
Table 40 (continued)
Species Number of animals Toxicitya Reference
(mg/kg body weight)
Chick 48 250 (LD33)
1000 (LD100)
Hen 12 > 1000 (no death)
Hen 12 > 3000 Bukin (1965)
aLD50, unless otherwise stated.
Table 41. Inhalation toxicity single exposure
Species Concentration Effects observed References
Guinea-pig 6 390 mg 50% wettable powder/m3 nasal and ocular irritation after 14 days - Carpenter et al.
(average particle size 15 µm) 4 h haemorrhage areas in the lungs (1961)
Guinea-pig 6 230 mg carbaryl 85S/m3 slight weight decrease; recovered by day 14
(average particle size 5 µm) 4 h
Dog 75 mg carbaryl 85S/m3 5 h typical symptoms for ChE inhibition
Cat 3 groups 82 mg/m3 dust 6 h tremor salivation, muscle fibrillation decreased Yakim (1967, 1968)
of 4 animals ChEA by 39-55% serum; 53-71% red blood
cells; normalization after 72 h
37 mg/m3 dust 6 h decreased ChEA by 23% in serum and 41% in
red blood cells; recovery after 48 h
20 mg/m3 dust 6 h decreased ChEA by 11-27% in serum and
15.28% in red blood cells; recovery after 24 h
Rat 20-23 mg/m3 dust no effect Weil & Carpenter
98% particles (1974)
less than 1.0 µm diameter
Rat 1800 mg/m3 water aerosols lacrimation, tremor with 1.5 h of exposure Myers et al. (1975)
Rat Wistar Albino Aerosols Sevin XLR 44% 792 mg 1/5 females died, tremor, ataxia, increased Faït (1984)
male 5 a.i./m3 per 4 h (the highest respiratory rate; recovery period 6 days
female 5 attainable concentration)
particle size 3.6 ± 2.64 µm
8.1.4 Other routes of exposure
Other routes of exposure are presented in Table 42.
Table 42. Toxicity following parenteral application
Species Weight Vehicle Route of LD50 References
(g) administration mg/kg body
weight)
Rat 10-107 propylene intravenous 18 Mellon Institute
female glycol (1958)
Rat 92-126 polyethylene intravenous 24
glycol 400 intravenous (17-33)
Rat 90-120 95% ethyl intravenous 33
alcohol (26-41)
Rabbit 1686-3544 0.25% agar intraperitoneal 223
(122-407)
Rat 90-120 in lard subcutaneous 1410
Leghorn 10-11 days in alantoic 3.44 mg Tós-Luty et al.
chick old cavity per embryo (1973)
embryos
(250)
8.2 Skin and eye irritation, sensitization
8.2.1 Skin and eye irritation
The results of several studies on skin irritation from carbaryl
were negative (Carpenter et al., 1961; Yakim, 1965). Transient
erythema was noted after an application of 0.5 ml 43.4% carbaryl on
occluded rabbit skin (Bushy Run, 1983a).
Carbaryl is a weak eye irritant (Table 43).
Table 43. Eye irritation
Species Number Formulation Effects observed Reference
Rabbit 5 technical grade mild injury in one Carpenter et al.
10% suspension of five eyes (1961)
in propylene
glycol
25% aqueous no injury
suspension
50 mg dust spots of
corneal-necrosis
Rabbit 6 0.1 ml (90 mg) conjunctival irritation Bushy Run (1983b)
New 99% technical after 2 days recovery
Zealand product
White
0.1 ml transient iritis in Bushy Run
43.4% carbaryl 2 of 6, conjunctival (1983a)
irritation in 6 of 6.
Recovery in 3 days
8.2.2 Sensitization
The sensitization response following topical application of
carbaryl was studied by Myers & Christopher (1987). The induction
which consisted of an application of carbaryl to covered skin, once
a week during 3 weeks, was followed by a 2-week incubation period. A
single challenge dose of 50% (w/v) technical carbaryl in 0.25%
aqueous methyl cellulose solution (0.1-0.3 ml) was administered.
Carbaryl did not produce a positive response in any of the
guinea-pigs tested.
Four out of 16, male, albino guinea-pigs were treated with 8
intravenous injections (3 per week) of 0.1 ml of a 0.1% dispersion
of carbaryl in 3.3% propylene glycol. After a 3-week incubation
period, a challenge dose was given, but did not cause sensitization
(Carpenter et al., 1961). In a more recent study, a similar
procedure, but with 9 doses and a 2-week incubation period, was
used. Although there was some skin irritation, it was considered
that carbaryl had little or no sensitizing potential (Bushy Run,
1983a).
8.3 Short- and long-term oral exposure
Several short- and long-term feeding studies with carbaryl have
been reported (Carpenter et al., 1961; Rybakova, 1967; Orlova &
Zhalbe, 1968; Gladenko & Malinin, 1970; Dikshith et al., 1976;
Hamada, 1991a). Results are shown in Table 44. Doses that do not
show any effects are 200 mg/kg diet equal to 7.9 mg/kg body weight
in rats, and 100 mg/kg diet for mice, and 1.8 mg/kg body weight for
dogs (approximately 100 mg/kg diet).
The cumulation coefficient (LD50 for 3-month exposure/LD50
single application) was 18 (Kassin, 1968), demonstrating a very low
cumulative potential for carbaryl.
8.4 Short- and long-term inhalation toxicity
Short-term and long-term inhalation toxicity data are given in
Table 45.
8.5 Reproduction and developmental toxicity
The reproduction and developmental toxicity of carbaryl has
been studied in many vertebrate species using a wide variety of
study designs.
The data have shown that carbaryl can affect reproduction
(Table 46) and embryo/fetal development (Table 47 and 48) in a
number of species. The relevance of these studies for risk
assessment is influenced by several factors related to experimental
design, dose levels, and the types of effects noted. Shortcomings of
the studies included small sample size; inappropriate dose
selection; the variable degree of maternal toxicity (ranging from no
effect to lethality); and a lack of historical data for some
species. The following sections have been arranged according to
end-point (reproduction and developmental toxicity) and species of
animals studied (mammalian, non-mammalian). Studies that are of
dubious value for risk assessment are listed at the end of the table
sections, and are not discussed in the text. The reasons for such an
evaluation are given below the references to the individual papers.
Table 44. Short- and long-term feeding and oral studies
Species Sex Number Dosage (mg/kg diet Period of Effects observed References
of or mg/kg body weight) exposure
animals
Mouse male 48 100, 400 mg/kg diet 80 weeks no changes in survival rate, pathology, and tumour FAO/WHO (1965)
and incidence
female
Mouse male 10/sex 100, 1000, 7000 mg/kg 53 weeks at 7000 mg/kg diet: decreased survival, body Hamada (1991b)
and per group diet weight gain and erythrocyte count; increased liver
female weight and decreased ovary weight; increased
frequency and severity of chronic nephropathy in
females; cholinesterase (plasma, RBC, and brain)
depressed at 100 and 7000 mg/kg; NOEL
100 mg/kg diet
Rat male 2x10 1500 (58.5 mg/kg 96 days no changes Carpenter et al.
body weight) (1961)
female 2x10 1500 (58.5 mg/kg 96 days kidney weights significantly increased
body weight)
male 2x10 2250 (87.5 mg/kg 96 days increase in liver weight as a % of body weight,
body weight) diffuse cloudy swelling in the kidney tubules in
4 animals (male and female)
female 2x10 2250 (87.5 mg/kg 96 days decrease of body weight, increase in kidney weight
body weight)
Table 44 (continued)
Species Sex Number Dosage (mg/kg diet Period of Effects observed References
of or mg/kg body weight) exposure
animals
Rat male 4x40 50, 100, 200 (2, 4, 2 years no changes Carpenter et al.
CF-N and 7.9 mg/kg body weight) (1961)
female
Rat male 2x40 400 (15.6 mg/kg 2 years weight depression in male, cloudy swelling of the Carpenter et al.
CF-N and body weight) hepatic cords, principally located around the (1961)
female central veins in both sexes; transitory diffuse
cloudy swelling of the epithelial lining of the
primarily convoluted proximal, and loop tubules
Rat 75, 150, 300 mg/kg 3 months cytoplasmatic vacuolization in the proximal tubules FAO/WHO (1970)
body weight
Rat male 4x48 7, 14, 70 mg/kg weight depression at all dose levels; at 70 mg/kg, Rybakova (1967)
and body weight slight morphological liver changes; cloudy swelling
female in convoluted tubules; decreased motility of
spermatocytes at all dose levels, more pronounced
at 70 mg/kg; oedema in the interstitial tissue;
desquamation of spermatogenic epithelium;
destruction of parenchyma; decreased production of
spermatocytes was found at 14 and 70 mg/kg dose
levels; increased estral cycle; increased hypophysis
gonadotropic function; decreased ascorbic acid
contents; cell proliferation, hypertrophy, increased
lipid content in suprarenal glands; decreased thyroid
function; augmentation of follicles, colloid retention,
thickness of follicular epithelium
Table 44 (continued)
Species Sex Number Dosage (mg/kg diet Period of Effects observed References
of or mg/kg body weight) exposure
animals
Rat male 80-90/ 250, 1500, 7500 mg/kg 52 weeks body weight and food consumption lower at middle Hamada (1991c)
Sprague female group diet dose; significantly increased total cholesterol;
Dawley increase in liver and kidney weight; NOEL
250 mg/kg diet
Rat male 876 2, 5, 15 mg/kg 1 year changes in sex glands function at 15 and 5 mg/kg Orlova & Zhalbe
and body weight dose levels; enzymatic activity, spermatozoids and (1968)
female the estral cycle; reduced fecundity; no effect at
2 mg/kg dose level
Rat male total 200 mg/kg 90 days decreased AChE in blood with 33.8%; no Dikshith et al.
local 28 body weight, histological changes (1976)
strain 3 x per week
in peanut oil
Dog male total 0.45, 1.8, 1 year diffuse cloudy swelling of proximal convoluted and Carpenter et al.
Basenji- and 14 7.2 mg/kg body loop tubules of kidney; local sudanophilic granules (1961)
cocker female weight in capsules, in the glomeruli at 400 mg/kg level; the same in
hybrids 5 days/week, to control dogs to a lesser extent; transient hind leg
approximate levels weakness in 1 female after 189th dose at
of 24, 95, 414 mg/kg 0.45 mg/kg body weight
dry diet
Beagle male 24+ 125, 400, 1250 mg/kg 1 year at 400 and 1250 mg/kg diet, decreased AChE in Hamada (1987)
dogs and 24 diet plasma, red blood cells, and in brain; increase in
female leucocyte count and segmented nitrophil count;
increase in liver weight in male; no effect level
125 mg/kg diet
Table 44 (continued)
Species Sex Number Dosage (mg/kg diet Period of Effects observed References
of or mg/kg body weight) exposure
animals
Beagle male 24 0, 20, 45, 125 mg/kg 5 weeks significant inhibition of cholinesterase activity Hamada (1991a)
dogs female 24 diet in plasma at week 2 for males dosed 20 and
125 mg/kg
Monkey 150, 300, 600 mg/kg 38 weeks kidney alterations similar to those found in rats FAO/WHO (1970)
body weight
Swine male 3 150 mg/kg body weight 72-83 progressive myasthenia, incoordination ataxia, Smalley et al.
female 3 in diet days intentional tremor, chronic muscular contraction, (1969)
until terminal paraplegia and prostration;
death myodegeneration
Swine 5 and 10 mg/kg 147-176 no changes Gladenko & Malinin
body weight days (1970)
Cattle 1 and 4 mg/kg 148 days decreased Hb with 20%, and erythrocytes with
(young) body weight 30% occasionally
Table 45. Short- and long-term inhalation toxicity
Species Number of Concentration of carbaryl Days of Effects References
animals exposure
Cat 4 0.06 mg/litre 30 typical cholinergic symptoms, Yakim (1968)
inhibition of ChE plasma 31-40%,
erythrocytes 40-59%
Cat 4 0.03-0.04 mg/litre 30 reaction time increased
Cat 4 0.016 mg/litre 120 no symptoms, fluctuation in
plasma ChE inhibition around 18%
Rat no data 10 mg/m3 dust (85% 90 no mortality no gross visible injury Carpenter et al.
suspension) 7 h/day inhalation (1961)
5 days per week periods
Table 46. Reproduction studies
Species Treatment End-point(s) Reference
(number)
Mouse
Swiss Webster males 30-40 g 0, 8.5, 17, 34 mg/kg body weight weight and uptake of testosterone: Thomas et al. (1974)
(10 + animals/group) orally (5 days) testes and accessory glands
Swiss Webster males 30-40 g 0, 34, 68 mg/kg body weight biotransformation of Dieringer & Thomas
(5 + animals/group) orally (5 days) testosterone-1,2-3H (1974)
C57Bl/6 x C3H 6-8 weeks of 0, 12, 25, 50...800 mg/kg body sperm morphology, testes weight Osterloh et al. (1983)
age (4 animals/group) weight per day 5 days i.p. on day 35 [route of
administration]
Rat
Osborne-Mendel 0, 2000, 5000, 10 000 mg/kg diet fertility, litter size, and viability Collins et al. (1971)
(20 females and 1 male per (3 generations)
group)
Wistar 0, 7, 25, 100, 200 mg/kg body fertility, litter size, and viability Weil et al. (1973)
(13-21 animals/group) weight per day in the diet
0, 3, 7, 25, 100 mg/kg body
weight per day orally
(3 generations)
Male and female 0, 1, 5, 10, 20, 40, 50 mg/kg general toxicity, serum protein, Vashakidze (1975)
body weight per day orally numbers of male reproductive
(1 month) cells, sperm viability, testicular
histology, litter viability
Table 46 (continued)
Species Treatment End-point(s) Reference
(number)
Wistar males 0, 12.5, 25.0, 250.0 mg/kg body sperm morphology Luca & Balan (1987)
(36 animals per group) weight per day
Rats
Male and female 0, 50, 100, 300 mg/kg body fertility indices Vashakidze (1966)
weight per day orally 2 weeks to [insufficient data]
3 months
Male and female 0, 2, 5, 15 mg/kg body weight per fertility indices Orlova & Zhalbe
(total of 876 animals) day orally 12 months for F° (1968); Zhalbe et al.
and 6 months for the F1 (1968) [insufficient
data]
Male and female 0, 2, 5 mg/kg body weight per day fertility indices Shtenberg & Ozhovan
for 6 months (follow-up to Orlova (1971) [insufficient
& Zhable) 2 through 5 generations data]
Male and female 0, 2, 5, 15 mg/kg body weight per fertility indices Shtenberg et al.
day for 12 months (1973) [insufficient
data]
Gerbil
(32-80 animals per group) 0, 2000, 4000, 6000, and fertility, litter size, and viability Collins et al. (1971)
10 000 mg/kg body weight diet
(3 generations)
Table 47. Developmental toxicity studies, mammalian
Species Treatment End-point(s) Reference
(number)
Mouse
CF-1 0, 100, 150 mg/kg body weight fetal examination Murray et al. (1979)
(23-44 in a group) per day orally or 5660 mg/kg diet.
Gestation day 6-15
Swiss albino 0, 100, 150, or 200 mg/kg body fetal examination Mathur & Bhatnagar
(10 per group) weight per day orally. Gestation (1991)
days 8, 12, or 6-15
Charles River 0, 10, 20 mg/kg body weight. fetal examination Benson et al. (1967)
(8 per group) Gestation day 6-parturition [doses too low]
CD-1 100 mg/kg body weight per day postnatal viability Chernoff & Kavlock
(23 animals) orally. Gestation day 8-12 (1982) [teratology
screen test]
CD-1 200 mg/kg body weight per day postnatal viability Kavlock et al. (1987)
(30 animals) orally. Gestation day 8-12 [teratology screen
test]
Rat
Harlan Wistar 0, 20, 100, 500 mg/kg diet. fetal examination Weil & Carpenter
(6 per group) Gestation days 1-7, 5-15, 1-21 (1965); Weil et al.
(1972)
Wistar 0, 200, 350 mg/kg body weight orally fetal examination Golbs et al. (1974)
(10 per group) 40 mg/kg ip. Variety of gestation days.
Table 47 (continued)
Species Treatment End-point(s) Reference
(number)
Sprague-Dawley 0, 1, 10, 100 mg/kg body weight fetal examination Lechner &
(6 or 7 per group) orally. 3 months before and during Abdel-Rahman
gestation (1984)
Sprague-Dawley 0, 20, 37.5 mg/kg body weight fetal examination Hart (1972)
(22 or 23 per group) per day. Gestation day 6-15 [doses too low]
Rats unknown dose (1/50 LD50). fetal examination Dinerman et al.
Gestation days 9, 11, or 13 (1970) [insufficient
data]
Rats 0, 10.6, 106 mg/kg body weight fetal examination Shtenberg et al.
per day. Gestation day 1-20 (1973) [insufficient
data]
Guinea-pigs
HRA/HART 0, 50, 100, 200 mg/kg body fetal examination Weil et al. (1973)
(9-4 per group) weight per day orally. 0, 100,
200, 300 mg/kg body weight per
day in the diet
Coulston strain 300 mg/kg body weight per day. fetal examination Robens (1969)
(26 gestation day treatment Gestation day 11-20 and a variety [insufficient data]
11-20; other treatments of other treatment periods within
unknown) this time
Table 47 (continued)
Species Treatment End-point(s) Reference
(number)
Rabbit
New Zealand White 0, 150, 200 mg/kg body weight fetal examination Murray et al. (1979)
(15-20 per group) per day orally. Gestation day 6-18
New Zealand White 0, 10, 30 mg/kg body weight per fetal examination Shaffer & Levy
(9-12 per group) day. Gestation day 9-16 (1968) [doses too
low]
New Zealand White 0, 50, 100, 200 mg/kg body fetal examination Robens (1969) [small
(4-9 per group) weight per day orally. Gestation number of animals]
day 5-15
Dog
Beagle 0, 3.125, 6.25, 12.5, 25, examination of pups Smalley et al. (1968)
(6-13 per group) 50 mg/kg per day in the diet
throughout gestation
Beagle 0, 2.0, 5.0, 12.5 mg/kg per day in examination of pups Imming et al. (1969)
(7-9 per group) the diet throughout gestation
Pig
Hormel-Hanford 0, 4, 8, 16 mg/kg in the diet 20 examination of fetuses (I) Earl et al. (1973)
(5-16 per group) days before/7 days after breeding or piglets after birth (II)
throughout gestation
Table 47 (continued)
Species Treatment End-point(s) Reference
(number)
Pig (30) up to 30 mg/kg body weight per ? Smalley (1968)
day [insufficient data]
Monkey
Rhesus 0, 2, 20 mg/kg body weight per examination of gestational Dougherty et al.
(4-6 per group) day orally throughout gestation course and new born (1971)
Rhesus 0, 20, 32 mg/kg body weight per examination of gestational Coulston et al. (1974)
(15-16 group) day orally. Gestation day 20-38 course and new born
Sheep
(25 and 26 per group) 159, 297.5 mg/kg diet examination of lambs Panciera (1967)
after birth [significance of
defects impossible to
assess]
Hamster
Golden Syrian 125 mg/kg body weight per day fetal examination Robens (1969)
(6 or 8 per group) on gestation day 6-8; 250 mg/kg [number tested too
body weight per day on gestation small]
day 7 or 8
Table 48. Non-mammalian studies
Species Dosage End-points Reference
Fish
Medaka 0.5, 1.0, 2.5, 5.0, 10.0, and embryonic development Solomon & Weis (1979)
(Oryzias latipes) 20.0 mg/litre, 4-cell through
10 eggs per group blastula
Amphibian
Xenopus laevis 0.1, 1.0, 10.0 mg/litre; embryos to embryonic development, Elliott-Feeley & Armstrong
10-12 per group hatching or tadpoles for 24 h posthatching activity (1982)
Birds
Chicken 0, 0.01, 0.1, 1.0, 10.0 mg/kg embryonic development Olefir & Vinogradova (1968)
4-6 per group body weight
White Leghorn chicken 0, 250, 500 mg/kg diet to pullets egg production, embryonic Lillie (1972)
20 per group for 36 weeks and hatchlings for development, hatchability,
4 weeks posthatching development
White Leghorn chicken 0, 1.0, 2.5, 5.0, and 10.0 mg/egg embryonic development Swartz (1981)
38-40 per group embryonic development injected
into yolk sac after fertilization,
prior to incubation for 5 or 12 days
White Leghorn chicken 10 mg/egg after fertilization prior primordial germ cell Swartz (1985)
8 per group to incubation migration
White Leghorn chicken 1.0, 0.3 mg/egg and lower examination of embryos Eto et al. (1980)
[insufficient data]
Table 48 (continued)
Species Dosage End-points Reference
720 eggs 0, 1, 2, and 4 mg/egg examination of hatchlings Ghadiri & Greenwood (1966)
[insufficient data]
Duck and chicken 10-1000 µg/egg 0, 4, 7, 10, and examination of embryos or Khera (1966)
13 day eggs hatchlings [insufficient data]
Chicken - examination of embryos Dinerman et al. (1970)
[insufficient data]
Quail
Coturnix coturnix 0, 50, 150, 300, 600, 900, growth, reproduction, Bursian & Edens (1977)
japonica 1200 mg/kg diet from hatching post-hatching viability
10 per group through 14 weeks
Colinus virginianus 0, 300, 1000, 3000 mg/kg diet for reproduction, post-hatching Fletcher & Leonard (1986a)
36 per group 22 weeks viability, gross pathology
Duck
Anas platyrhyncos 0, 300, 1000, 3000 mg/kg diet for reproduction, post-hatching Fletcher & Leonard(1986b)
36 per group 22 weeks viability, gross pathology
10-1000 µg/egg 0, 4, 7, 10, and examination of embryos or Khera (1966)
13 days eggs hatchlings [insufficient data]
8.5.1 Mammalian reproductive toxicity studies
8.5.1.1 Mouse
Studies on the effects of carbaryl (8.5-34 mg/kg per day),
given orally for 5 days, to mature male Swiss Webster mice,
indicated no effects on the weights of testes and accessory sex
glands or uptake of C14-labelled testosterone by the prostate
gland, as measured on the day after treatment (Thomas et al.,
1974). These workers also examined the biotransformation of
testosterone-1,2-3H in animals given 34 or 68 mg/kg per day,
orally, for 5 days. They found a significant decrease in
androstenedione synthesis in the high-dose group, indicating
increased hepatic androgen hydroxylase activity (Dieringer & Thomas,
1974).
8.5.1.2 Rat
Collins et al. (1971) studied the effects of carbaryl given
in the diet (0, 2000, 5000, and 10 000 mg/kg) to Osborn-Mendel rats
over 3 generations. The doses actually administered could only be
estimated since there were no measurements of food intake. On the
basis of a 15 g/day food intake and a 235 g rat, it can be estimated
that animals received of the order of 125, 250, or 500 mg/kg per
day. It should be noted, however, that the food intake of lactating
rats greatly increases, so these figures may considerably
under-estimate carbaryl exposure during this critical time period.
The author used the number of animals mated rather than the number
giving birth as the number for litter size and viability, therefore
their calculations are overestimates. Nevertheless, the data do show
impaired fertility in the high-dose group (which also exhibited
growth rate reduction) as well as reduced postnatal survival. The
number of live-born offspring and growth rate were reduced in the
5000 and 10 000 mg/kg diet groups.
A three-generation study on Wistar rats was carried out by Weil
et al. (1973) in which animals were given 0, 7, 25, 100, or
200 mg/kg per day in the diet or 0, 3, 7, 25, or 100 mg/kg per day
orally. Maternal toxicity was seen in the dietary study at 200 mg/kg
per day (decreased weight) and in the gavage study at 100 mg/kg per
day (decreased weight and mortality). Postnatal toxicity was noted
in the 100 mg gavage group (reduced litter size and viability), but
not in lower dose groups. The dietary study indicated fewer effects
in the maternal animals with only an initial loss in weight. No
perinatal effects were noted.
Vashakidze (1975) exposed male and female rats (number and
strain unspecified) to 0, 1, 5, 10, 20, 40, and 50 mg carbaryl/kg,
orally, for 1 month. Dose-related changes were noted in serum
albumin (decrease), globulins (increase), ChE and AChE (decrease),
aspartate transaminase (increase), and alanine transaminase
(decrease). Reductions in stem cells as well as spermatozoa were
noted at doses of 5 mg/kg or more. Adverse litter effects were seen
in treated females. These effects included increased embryo/fetal
death, decreased implantations, and prolonged estrus cycle.
Luca & Balan (1987) administered carbaryl-beta-naphthol to
Wistar rats in the diet for up to 18 months. The treated groups
showed an increase in sperm shape abnormalities, though there were
no clear dose- or time-relationships with effects.
8.5.1.3 Gerbil
Collins et al. (1971) published the results of a 3-generation
reproduction study in which animals were exposed to 0, 2000, 4000,
6000, or 10 000 mg/kg diet. Since all postnatal calculations used
the number of animals mated rather than the number giving birth, the
authors' data must be recalculated. When this is done, the magnitude
of the effects reported is reduced. Adverse effects on various
reproductive parameters are nevertheless seen, though the effects
are not clearly related to dose levels in the 2000-6000 mg/kg diet
groups. These data are difficult to interpret given the lack of
information on maternal effects at doses other than the highest,
where mortality was observed.
8.5.2 Mammalian developmental toxicity studies
8.5.2.1 Mouse
Murray et al. (1979) administered 100 or 150 mg carbaryl/kg
per day, by gavage, or 5660 mg/kg diet (calculated to be 1166 mg/kg
per day) to CF-1 mice on gestation days (g.d.) 6-15. Maternal
toxicity was noted in the 150-mg group (ataxia, lethality). Litter
effects were noted in the 150 mg/kg per day group, where an increase
in entirely resorbed litters was seen, and in the dietary group
where there was decreased fetal weight.
Pregnant mice were given 0, 100, 150, or 200 mg carbaryl/kg per
day, orally, on gestation days 8, 12, or 6-15, and fetuses were
examined at term (Mathur & Bhatnagar, 1991). Maternal death was
noted at the high dose in the group receiving carbaryl on gestation
days 6-15. Fetal weight reductions were seen at the high-dose levels
in all groups, as was reduced ossification, open eyelids, and
enlarged renal pelvis. These effects may be indicative of fetal
growth retardation.
8.5.2.2 Rat
Carbaryl was administered to Harlan Wistar rats at 0, 20, 100,
or 500 mg/kg per day, orally, on gestation days 1-7, 5-15, or 1-21
(Weil & Carpenter, 1965; Weil et al., 1972). Maternal toxicity was
evident as was reduced weight gain in the high-dose groups receiving
the chemical on gestation days 5-15 or 1-2. No adverse fetal effects
were seen.
Golbs et al.(1975) treated Wistar rats with 0, 200, or 350 mg
carbaryl/kg, orally, or 40 mg/kg ip on a variety of single or
multiple gestation days. Reductions in fetal weight were seen in
some groups. No other effects were noted.
Sprague-Dawley rats were treated orally with 0, 1, 10, or
100 mg carbaryl/kg per day, three months before and during
gestation, and litters were examined at term (Lechner &
Abdel-Rahman, 1984). Maternal weight gain was significantly less in
the 100 mg/kg group than in controls. No compound-related effects on
fetuses were noted.
8.5.2.3 Guinea-pig
Weil et al. (1973) administered carbaryl to guinea-pigs at
dose levels of 0, 100, 200, or 300 mg/kg per day in the diet, or 0,
50, 100, or 200 mg/kg per day, orally. These dose levels were
determined to be maximum non-maternally toxic doses in preliminary
studies by the authors, though the 200 mg/kg dose did reduce
maternal weight gain. No significant adverse embryo/fetal effects
were seen in any treatment groups.
8.5.2.4 Rabbit
Murray et al. (1979) tested the effects of carbaryl on New
Zealand White rabbits during gestation. Dams had diarrhoea at the
high dose of 200 mg/kg per day and animals at this dose level as
well as at the lower dose level (150 mg/kg per day) gained less
weight during gestation than controls. A significant increase in
umbilical hernia was noted in fetuses at the 200 mg/kg per day
group.
8.5.2.5 Dog
Smalley et al. (1968) administered 3.125, 6.25, 12.5, 25, or
50 mg carbaryl/kg in the diet, throughout gestation, to beagle dogs.
Maternal toxicity was noted at all dose levels. This toxicity was
described by the authors as dystocia and symptoms included delayed
delivery, anorexia, and restlessness. A variety of birth defects
were found at doses of 6.25 mg/kg or more. The defects included
ectopic intestines, brachygnathia, acaudia, polydactyly, and
intestinal agenesis.
Another study was carried out by Imming et al. (1969) on the
beagle dog. These workers used 0, 2.0, 5.0 or 12.5 mg/kg per day,
orally, throughout gestation. As in the Smalley (1968) study,
treated females exhibited toxicity during labour and single deaths
were recorded at this time in all treated groups. Birth defects
including umbilical hernia and gastrointestinal defects were seen at
the 5.0 and 12.5 mg dose levels only.
8.5.2.6 Pig
Two studies on pigs were carried out by Earl et al. (1973).
In the first study, fetal pigs were examined from dams given 0, 4,
8, or 16 mg carbaryl/kg per day in the diet; in the second, dams
were allowed to farrow after receiving 0, 16, or 32 mg/kg per day in
the diet. Animals were exposed throughout most or all of gestation.
Effects noted were not consistent across the studies and increased
prenatal lethality seen in the first was not noted in the second,
even at a higher dose level. A small number of malformations were
noted, but no characteristic dose-related pattern was evident.
8.5.2.7 Monkey
Dougherty et al. (1971) exposed a small number of female
Rhesus monkeys to 0, 2, or 20 mg carbaryl/kg, throughout gestation.
Of the 8 pregnant, treated females, 5 were reported as having
aborted as opposed only 1 out of 5 in the controls. The Coulston
et al. (1974) study included a larger number of animals per dose,
an additional lower dose level (0.2 mg/kg per day), and a shorter
dosage period (days 20-38 of gestation). No adverse effects were
noted in the second study. These studies are not comparable for the
evaluation of the abortifacient potential of carbaryl, since the
dosing began later in gestation in the second study. The authors
noted that the determination of abortion in the Rhesus monkeys may
not have been reliable.
8.5.3 Reproductive and developmental toxicity studies in
non-mammalian species
8.5.3.1 Fish
Medaka ( Oryzias latipes) embryos were exposed to nominal
carbaryl concentrations of 0.5, 1.0, 2.5, 5.0, 10.0, 20.0, or
30.0 mg/litre in the water from the 4-cell through to the blastula
stages (Solomon & Weis, 1979). Exposure of the eggs resulted in
increased cardiovascular anomalies (heart defects, circulatory
defects, and edema) at dose levels of 5 mg/litre or more.
8.5.3.2 Amphibian
Elliott-Feeley & Armstrong (1982) treated both embryos and
tadpoles of Xenopus laevis with nominal carbaryl concentrations in
the water of 0.1, 1.0, or 10.0 mg/litre. Defects were seen in
embryos exposed to 10 mg/litre, which also resulted in significant
lethality. Embryo growth was reduced at all dose levels.
Carbaryl-exposed tadpoles exhibited decreased activity at all dose
levels.
8.5.3.3 Birds
Chicken eggs have been used by a number of workers to test the
potential of carbaryl to affect avian development. Olefir &
Vinogradova (1968) injected eggs with carbaryl at 0.01, 0.1, 1.0, or
10.0 mg/kg and examined embryos at different times after injection.
Death and anomalies were recorded at 5, 10, 15, and 20 days of
incubation at all doses above 0.01 mg/kg. Lillie (1973) administered
0, 250, or 500 mg/kg diet to pullets for 36 weeks beginning at 32
weeks of age. The adult birds showed reduced weights at both levels
of carbaryl in the adults and at 500 mg in the progeny. No
embryotoxicity or other effects were noted. Swartz (1981) examined
the effects of carbaryl on chicken embryo development. They examined
chick embryos at different days after injection, for 5 or 12 days
post-fertilization. They found vehicle-related increased mortality
(carbaryl was more toxic when administered in sesame oil than in
acetone) for both periods of exposure. Scattered anomalies were
recorded in surviving embryos. In another study (Swartz, 1985)
primordial germ cell migration was followed after injection of 10 mg
carbaryl per egg prior to incubation. Results did not indicate any
significant adverse effects on the primordial germ cells or
reproductive organs.
Bursian & Edens (1977) studied the effects of carbaryl on the
fertility and post hatching viability of Japanese quail ( Coturnix
coturnix japonica). Birds were exposed from hatching to 14 weeks
of age (breeding maturity). Fertility and the hatchability of eggs
were measured. The animals received 0, 50, 150, 300, 600, 900, or
1200 mg carbaryl/kg diet. Growth of the adult birds was reduced at
the 900 and 1200 mg/kg dose levels. There were no significant
effects on any reproductive parameter.
Fletcher & Leonard (1986a) investigated the effects of carbaryl
on reproduction in bobwhite quail ( Colinus virginianus) exposed to
0, 300, 1000, or 3000 mg/kg diet for 22 weeks. No adverse effects
were described in any factors related to hatchability, posthatching
viability, or gross pathology of newly hatched birds. These workers
used a similar protocol to study the effects of carbaryl on mallard
ducks ( Anas platyrhyncos) (Fletcher & Leonard, 1986b). The 3000
mg/kg diet level was toxic with some lethality, decreased numbers of
eggs, and thinner egg shells. No effects were seen at the 300 or
1000 mg/kg diet levels.
8.5.4 Appraisal
In summary, mammalian studies on the reproductive or
developmental toxicity of carbaryl clearly show that this compound
is capable of inducing adverse effects in utero and during the
reproductive process. These effects are always seen only at dose
levels at which there is concurrent maternal toxicity, with the
possible exception of a few studies on the rat which have not been
replicated by other workers. For a number of species, the dams
appear to be more sensitive than their litters. In general, the
adverse effects noted in developmental toxicology studies cannot be
simply attributed to maternal toxicity (Chernoff et al., 1990).
However, the pattern of maternal and fetal toxicity occurring at the
same dose levels indicates that the developing mammalian
embryo/fetus is not especially susceptible to carbaryl.
Carbaryl has been shown to be embryotoxic for fish, amphibians,
and birds, at some exposure concentrations.
8.6 Mutagenicity of carbaryl and N-nitrosocarbaryl
In this section on mutagenicity, and in the section on
carcinogenicity (8.7.1), the two compounds, carbaryl and
nitrosocarbaryl, are discussed (separately), since the formation of
N-nitrosocarbaryl was reported to occur in the stomach of rats and
guinea-pigs, in the presence of sodium nitrite and carbaryl, under
acid conditions (Elespuru & Lijinsky, 1973; Beraud et al., 1979;
Rickard & Dorough, 1984). See also section 8.9.3.
8.6.1 Genotoxicity assays in vitro
8.6.1.1 Primary DNA damage
(a) Carbaryl
Carbaryl did not cause DNA damage in different wild type
strains and DNA recombination lacking strains of Bacillus subtilis
(Table 49). Carbaryl was reported to be non-genotoxic for
B. subtilis Marburg 17A Rec+ and Marburg M45T Rec- strains,
highly sensitive to frameshift mutagens, even at the highest tested
concentration of 10 mg/plate (Uchiyama et al., 1975).
Carbaryl (10-4 mmol/litre) did not affect the sedimentation
profiles of DNA from human skin cells in culture (both normal and
xeroderma pigmentosum), either immediately or 20 h after treatment
(Regan et al., 1976).
There are two controversial reports on the genotoxicity of
carbaryl, evaluated by the induction of mitotic gene conversion in
the diploid strain of Saccharomyces cerevisiae, heteroallelic at
the two different loci ade2 and trp5. Siebert & Eisenbrand (1974)
used an assay system with a 16-h incubation time and a concentration
of carbaryl of 4.97 mmol/litre and did not observe any changes in
the control frequency of mitotic gene conversion. However, Jaszczuk
& Syrowatka (1979), reported a weak positive response with a lower
concentration of carbaryl (2.5 mmol/litre) and shorter (5 h)
incubation time. No converting activity of N-hydroxy carbaryl (2.5
mmol/litre) was found under the same assay conditions.
Table 49. Bacterial assays of genetic toxicity of carbaryl
Test Test organism Concentration Genetic Metabolic Result Reference
(µg/plate) end-point activation
DNA B. subtilis 700 mg/litre Rec assay - negative DeGiovanni-Donnelly et al. (1968)
damage
B. subtilis 20 Rec assay - negative Shirasu et al. (1976)
B. subtilis 0.4, 4, 40, 400 Rec assay - negative Eto et al. (1982)
Gene B. subtilis up to 10 000 Rec assay - negative Uchiyama et al. (1975)
mutation
E. Coli WP2 1000 Try- - negative Ashwood-Smith et al. (1972)
E. Coli WP2 1000-3000 Try- - negative Nagy et al. (1975)
E. Coli WP2 10 000 Try- - negative Uchiyama et al. (1975)
H. influenzae 10 µmol/litre Novobiocim - negative Elespuru et al. (1974)
S. typhimurium
TA 98 up to 2000 His- - negative McCann et al. (1975)
TA 98 up to 2000 His- Rat negative
TA 98 50 nmol/litre His- - negative Blevins et al. (1977)
TA 98 10-1500 His- - negative DeLorenzo et al. (1978)
Table 49 (continued)
Test Test organism Concentration Genetic Metabolic Result Reference
(µg/plate) end-point activation
TA 98 10-1500 His- Rat negative
TA 98 0.25, 2, 5, 50, 1000 His- - negative Jaszczuk et al. (1979)
TA 98 up to 5000 His- - negative Moriya et al. (1983)
TA 98 up to 5000 His- Rat negative
TA 98 0.2, 2, 20 His- - negative Eto et al. (1982)
TA 98 0.2, 2, 20 His- Rat negative
TA 98 5-2000 His- - negative Lawlor (1989)
TA 98 5-2000 His- Rat negative
TA 100 up to 2000 His- - negative McCann et al. (1975)
TA 100 up to 2000 His- Rat negative
TA 100 50 nmol/litre His- - negative Blevins et al. (1977)
TA 100 10-1500 His- - negative DeLorenzo et al. (1978)
TA 100 10-1500 His- Rat negative
TA 100 0.25, 2, 5, 50, 1000 His- - negative Jaszczuk et al. (1979)
TA 100 0.2, 2, 20 His- - negative Eto et al. (1982)
Table 49 (continued)
Test Test organism Concentration Genetic Metabolic Result Reference
(µg/plate) end-point activation
TA 100 0.2, 2, 20 His- Rat negative
TA 100 5-2000 His- - negative Lawlor (1989)
TA 100 5-2000 His- Rat negative
TA 1535 up to 2000 His- - negative McCann et al. (1975)
TA 1535 up to 2000 His- Rat negative
TA 1535 2500 His- - positive Marshall et al. (1976)
TA 1535 1000 His- Rat negative
TA 1535 50 nmol/litre His- - negative Blevins et al. (1977)
TA 1535 10-1500 His- - negative DeLorenzo et al. (1978)
TA 1535 10-1500 His- Rat negative
TA 1535 up to 5000 His- - negative Moriya et al. (1983)
TA 1535 up to 5000 His- Rat negative
TA 1535 5-2000 His- - negative Lawlor (1989)
TA 1535 5-2000 His- Rat negative
Table 49 (continued)
Test Test organism Concentration Genetic Metabolic Result Reference
(µg/plate) end-point activation
TA 1536 2500 His- - negative Marshall et al. (1976)
TA 1536 1000 His- Rat negative
TA 1537 up to 2000 His- - negative McCann et al. (1975)
TA 1537 up to 2000 His- Rat negative
TA 1537 50 nmol/litre His- - negative Blevins et al. (1977)
TA 1537 2500 His- - negative Marshall et al. (1976)
TA 1537 1000 His- Rat negative
TA 1537 10-1500 His- - negative DeLorenzo et al. (1978)
TA 1537 10-1500 His- Rat negative
TA 1537 0.25, 2, 5, 50, 1000 His- - negative Jaszczuk et al. (1979)
TA 1537 up to 5000 His- - negative Moriya et al. (1983)
TA 1537 up to 5000 His- Rat negative
TA 1537 5-2000 His- - negative Lawlor (1989)
TA 1537 5-2000 His- Rat negative
TA 1538 100 His- - positive Egert & Greim (1976)
Table 49 (continued)
Test Test organism Concentration Genetic Metabolic Result Reference
(µg/plate) end-point activation
TA 1538 100 His- Mouse positive
TA 1538 50 nmol/litre His- - negative Blevins et al. (1977)
TA 1538 2500 His- - negative Marshall et al. (1976)
TA 1538 1000 His- Rat negative
TA 1538 10-1500 His- - negative DeLorenzo et al. (1978)
TA 1538 10-1500 His- Rat negative
TA 1538 up to 5000 His- - negative Moriya et al. (1983)
TA 1538 up to 5000 His- Rat negative
TA 1538 5-2000 His- - negative Lawlor (1989)
TA 1538 5-2000 His- Rat negative
Carbaryl was inactive in the rat primary hepatocytes
unscheduled DNA synthesis assay (Cifone, 1989). It did not induce
significant changes in the nuclear labelling of rat primary
hepatocytes in two independent trials with applied concentrations
ranging from 5 to 25 µg/ml.
No DNA-damaging properties of carbaryl, assessed by its
capacity for inducing unscheduled DNA synthesis (UDS) in cultured
human lymphocytes, were reported by Rocchi et al. (1980). However,
the authors did not use a standardized test protocol meeting
established criteria for the UDS assay performance. They tested only
one concentration of carbaryl (50 µg/ml), did not use any metabolic
activation system, did not run negative and adequate positive
controls concomitantly (other than the ultraviolet irradiation), and
did not apply relevant criteria to assess data.
The lack of DNA-damaging properties of carbaryl, as measured by
the induction of UDS, was confirmed by Probst et al. (1981). They
used a standardized protocol and autoradiographic techniques in
metabolically competent, cultured rat hepatocytes with test
concentrations ranging from 0.5 to 1000 nmol/ml. In contrast, Ahmed
et al. (1977a) reported that carbaryl induced the UDS of the
ultraviolet type (long patch repair) in a cultured human fibroblast
VA-4 cell line with, and without, metabolic activation in
concentration ranges of 1, 10, 100, and 1000 µmol/litre. However,
this study had several experimental shortcomings. The protocol, the
control value, and the results of compounds tested were not in
accordance with comparable assays by other authors.
(b) N-nitrosocarbaryl
The DNA-damaging properties of N-nitrosocarbaryl in
B. subtilis have been reported (Table 50).
In one study (Uchiyama et al., 1975), more pronounced
genotoxicity of N-nitrosocarbaryl to B. subtilis was registered
compared with that of N-methyl- N'-nitro- N-nitrosoguanadine.
However, the genotoxicity of N-nitrosocarbaryl was the lowest when
compared with other concomitantly tested nitrocarbamates.
In contrast to carbaryl, N-nitrosocarbaryl showed pronounced
genotoxicity toward S. cerevisiae D4 (Siebert & Eisenbrand, 1974).
N-nitrosocarbaryl, but not carbaryl, reacted with human DNA
in cell culture to form alkaline-sensitive bonds (Regan et al.,
1976). The DNA of nitrosocarbaryl-treated (10-4 mmol/litre) cells
showed a substantial reduction in sedimentation rate immediately,
and up to 20 h, after treatment. Presumably, the effect observed was
related to the induction of numerous single-strand breaks in the DNA
and the formation of DNA adducts. On the basis of selective
labelling, the authors suggested that the methyl-containing moiety
of nitroso-carbaryl was separated from the naphthalene ring in a
human fibro-blast culture and bound irreversibly to DNA (Regan
et al., 1976).
8.6.1.2 Gene mutation assay
(a) Carbaryl
As summarized in Table 49, carbaryl did not exert a mutagenic
effect in studies with E. coli, H. influenzae, or Salmonella.
Among the many reports on Salmonella, only two indicated a
positive effect. Thus, Marshall et al. (1976) observed increased
mutagenicity at 1000 µg/plate with S9 mix for TA1535. An evaluation
of this data is difficult because no negative and positive control
data were presented. Egert & Greim (1976) reported a positive
response for TA1538 by 100 µg/plate. The mutagenicity of carbaryl in
this study greatly increased when a non-standard metabolic
activation system (mouse liver microsome) was used.
Ahmed et al. (1977b) reported a positive mutagenic response
to carbaryl in Chinese hamster V79 cells at a dose level of
0.01 mmol/litre, with no metabolic activation. There was a
concentration-related effect of carbaryl on cell survival; less than
50% of the cells survived at concentrations >0.01 mmol/litre.
Mutation studies carried out with a concentration of 0.01 mmol/litre
showed an approximately 8-fold increase in ouabain resistance
forward mutation in V79 cells as compared with the spontaneous
mutation rate.
However, Wojciechowski et al. (1982) found no
ouabain-resistance with carbaryl in a cell-mediated mutagenesis
assay when they used an exogenous activating system in which
irradiated fetal cells of Syrian hamsters were co-cultivated with
cells of Chinese hamsters V79. No mutagenic response was observed
for carbaryl at concentrations of 0.01, 0.05, 0.1 mmol/litre with,
or without metabolic activation. The toxicity of carbaryl at these
concentrations ranged from 7% at the lowest concentration to 23% at
the highest.
Carbaryl produced a negative result for inducing forward
mutations at the HPRT locus in Chinese hamster ovary cells both
with, and without, metabolic activation (Young, 1990).
Table 50. Bacterial assays of the genetic toxicity of nitrosocarbaryl
Test Test organism Concentration Genetic Metabolic Result Reference
(µg/plate) end-point activation
DNA B. subtilis 0.4, 4, 40 Rec assay - positive Eto et al. (1982)
damage
B. subtilis up to 100 Rec assay - positive Uchiyama et al. (1975)
Gene
mutation E. Coli 30 R 0.1 mmol/litre Try- - positive Elespuru et al. (1974)
E. Coli WP2 5, 10, 50, 100 Try- - positive Uchiyama et al. (1975)
E. Coli K12 100 Try- - positive Egert & Greim (1976)
E. Coli K12 100 Try- Mouse positive
H. influenzae 10 µmol/litre Novobiocin - positive Elespuru et al. (1974)
S. typhimurium
TA 98 0.001-11 His- - positive/ Blevins et al. (1977)
negative
TA 9 10-1500 His- - negative DeLorenzo et al. (1978)
TA 98 10-1500 His- Rat negative
TA 98 0.25-1000 His- - positive Jaszczuk et al. (1979)
Table 50 (continued)
Test Test organism Concentration Genetic Metabolic Result Reference
(µg/plate) end-point activation
TA 98 0.02, 0.2, 2 His- - negative Eto et al. (1982)
TA 98 0.02, 0.2, 2 His- Rat negative
TA 98 0.01-100 His- - negative Rickard et al. (1982)
TA 98 0.1-100 His- Rat negative
TA 100 0.001-11 His- - positive Blevins et al. (1977)
TA 100 0.02, 0.2, 2 His- - positive Eto et al. (1982)
TA 100 0.02, 0.2, 2 His- Rat negative
TA 100 0.1-100 His- - positive Rickard et al. (1982)
TA 100 0.1-100 His- Rat positive
TA 1535 0.5-100 His- - positive Marshall et al. (1976)
TA 1535 50-1000 His- Rat positive
TA 1535 0.001-11 His- - positive Blevins et al. (1977)
TA 1535 0.25-1000 His- - positive Jaszczuk et al. (1979)
TA 1535 0.1-100 His- - positive Rickard et al. (1982)
TA 1535 0.1-100 His- Rat positive
Table 50 (continued)
Test Test organism Concentration Genetic Metabolic Result Reference
(µg/plate) end-point activation
TA 1536 0.5-100 His- - negative Marshall et al. (1976)
TA 1536 50-1000 His- Rat negative
TA 1537 0.001-11 His- - negative Blevins et al. (1977)
TA 1537 0.5-100 His- - positive Marshall et al. (1976)
TA 1537 50-1000 His- Rat positive
TA 1537 0.25-1000 His- - positive Jaszczuk et al. (1979)
TA 1538 100 His- - positive Egert & Greim (1976)
TA 1538 100 His- Rat positive
TA 1538 0.001-11 His- - negative Blevins et al. (1977)
TA 1538 0.5-100 His- - positive Marshall et al. (1976)
TA 1538 50-1000 His- Rat negative
TA 1538 0.25-1000 His- - negative Jaszczuk et al. (1979)
(b) N-nitrosocarbaryl
N-nitrosocarbaryl has shown a positive response in a number
of bacterial assays (Table 50). In Salmonella, N-nitrosocarbaryl
was mutagenic to strains responding to both base substitution
(TA1535, TA100) and frame shift (TA1536, TA1537, TA1538, TA98). The
mutagenic potential of N-nitrosocarbaryl toward Salmonella TA1535
was greatly diminished by the added exogenous activation system
(Marshall et al., 1976). A decrease in mutagenic activity by the
addition of S9 mix on frame shift mutagenesis has also been reported
(Marshall et al., 1976; Jaszczuk et al., 1979).
8.6.1.3 Chromosomal aberration assays and sister chromatid exchange
(a) Carbaryl
Animals
Carbaryl induced a clastogenic response in the three in vitro
bioassays (Ishidate & Odashima, 1977; Kazarnovskaya & Vasilos, 1977;
Önfelt & Klasterska, 1983). All the positive responses were observed
at toxic dose levels (30-80 µg/ml; 50, 100 µmol/litre); exogenous
metabolic activation was not required for activity. Thus, Ishidate &
Odashima (1977) observed a strong positive response for carbaryl
(30 µg/ml) in a chromosomal aberration assay using a Chinese hamster
fibroblast cell line. Carbaryl produced predominantly chromatid type
gaps, breaks, translocations, rings, and fragmentation, 48 h after
treatment.
Carbaryl was negative for inducing chromosomal aberrations in
CHO cells without metabolic activation but was positive under
metabolic activation conditions (Murli, 1989).
Kazarnovskaya & Vasilos (1977) reported a positive response for
carbaryl in cultures of human embryonic fibroblast: levels of 40 and
80 µg/ml caused a 4-fold and 10-fold increase, respectively, in the
frequency of chromosomal aberrations, at 24 h exposure time,
compared with a control rate of 2% (without S9-mix). Again, the
aberrations produced were mainly of a chromatid type; the type most
frequently observed was single fragments. An increased number of
paired fragments was found only at 80 µg carbaryl/ml. Carbaryl
(80 µg/ml) increased the percentage of cells with chromosomal
coiling (9.8% in the control group; 17.9% in the test group) and
aneuploidy (3% in the control group; 29.2% in the test group).
Previously, Kazarnovskaya & Vasilos (1977) had shown that carbaryl
suppressed mitosis, changed the rate of the mitotic phase, and
significantly increased the number of pathological forms of mitosis
in a human embryonic fibroblast culture, with a dose-time response.
Onfelt & Klasterska (1983) observed induction of viable
aneuploid/polyploid cells and multiple chromatid exchanges after
treatment of V79 Chinese hamster cells with carbaryl. The compound
exerted a pronounced chromosome-breaking effect, at a concentration
of 100 µmol/litre, 26 and 50 h after treatment. There was an
increased frequency of multiple chromatid exchanges and fragments,
as well as pulverisation and more diffuse signs of chromosome
damage. The effects of carbaryl on chromosome structure and
distribution were almost abolished by the simultaneous addition of
Aroclor-induced 2 or 10% rat S9-mix and glutathion.
In two in vitro assays for chromosome damage using Chinese
hamster lung fibroblasts, carbaryl was found to be positive
(Ishidate et al., 1981; Onfelt & Klasterska, 1984). In the latter
study, the effect of carbaryl on the incidence of sister chromatid
exchange was decreased by the addition of rat liver microsomes.
Söderpalm & Önfelt (1988) related the mitotic aberrations in V79
Chinese hamster cells, in part, to a reduction in the intracellular
levels of glutathione, and increased lipid peroxidation. They also
hypothesized that the anticholinergic effects of carbaryl may play a
role in the cleavage process.
Plants
A negative clastogenic response for carbaryl in a concentration
range of 50-200 mg/litre was reported by Ma et al. (1984) who used
the Tradescantia micronucleus test. At present, this test is
considered the most established and standardized plant assay system
for the purposes of in situ monitoring of environmental mutagen
pollution (Ma et al., 1984).
Carbaryl induced chromosomal effects in different plant assays
(Wuu & Grant, 1966; Amer & Farah, 1968; Brankovan, 1972). Carbaryl
increased by approximately 10-fold the number of aberrant cells in
the root tips of barley seedings ( Hordeum vulgare) in C1 and C2
generations after the seeds were treated with concentrations of 500,
1000, or 1500 mg carbaryl/litre for 6 and 12 h. The cytogenetic
effects induced included mostly metaphase and anaphase fragments and
anaphase bridges and were time-dependent (Wuu & Grant, 1966).
The mitotic, cytogenetic effects of carbaryl were seen in
Vicia faba (Amer & Farah, 1968) and sugar corn (Brankovan, 1972).
Carbaryl caused chromosomal abnormalities (chromosome lagging;
stickiness, mainly in diakinesis, polyploidy, fragments, anaphase
bridges) in different meiotic states in the pollen mother cells of
Vicia faba after spraying flower buds of different ages (2 weeks,
1 month) with saturated aqueous solutions of a commercial carbaryl
preparation. The percentage of the chromosomal aberrations increased
with increase in the frequency of spraying (every week) or 2 weeks
for l month; daily for 8 days) and then decreased when the recovery
time was increased (Amer & Farah, 1968).
8.6.2 Genotoxicity in vivo
8.6.2.1 Host-mediated assay
(a) Carbaryl
Usha Rani et al. (1980) reported that carbaryl did not have a
gene-mutation potential in vivo, when given orally in a toxic dose
of 438 mg/kg, 3 times daily for 3 days, to 6 Swiss male mice, which
were subsequently injected with Salmonella strain G46. These data
are consistent with those that showed that carbaryl was not
mutagenic for Salmonella in vitro.
8.6.2.2 Drosophila melanogaster and other insects
(a) Carbaryl
There are several reports of bioassays for carbaryl
genotoxicity in which Drosophila melanogaster was used (Brzeskii,
1972; Brzeskii & Vaskov, 1972; Hoque, 1972; Woodruff et al., 1983;
Omer et al., 1986).
In studies by Hoque (1972), carbaryl at 1.5 and 10 mg/litre was
given to female Drosophila. It was reported that the treatment
caused a changed sex ratio, changes in eye colour, and various
chromosomal aberrations in the offspring. The small amounts of
material used and the lack of any detailed presentation of the
findings preclude any evaluation or conclusion.
At high doses (0.3 ml from a 1% suspension of 85% commercial
product in glucose), carbaryl caused a slight increase in mutation
frequency in the F1 generation males of a Drosophila line, which
were studied at different stages of spermatogenesis. There were no
deletions or disturbed fertility (Brzeskii, 1972; Brzeskii & Vaskov,
1972).
Woodruff et al. (1983), who used a sensitive experimental
protocol that incorporated the mating scheme with repair-deficient
females, reported that carbaryl was not mutagenic for Drosophila.
F1 male progeny of males that had ring X chromosomes and double
marked Y chromosomes, were treated with carbaryl at 200 mg/litre and
mated with mus-302, repair-deficient females of Drosophila. The
male progeny did not show any induced complete (ring chromosome) or
partial (Y chromosome markers) chromosome loss.
The mutagenic activity of carbaryl in Drosophila melanogaster
was studied by Omer et al. (1986). The results indicated that
carbaryl does not increase the rate of dominant and sex-linked
recessive lethal mutations.
Carbaryl did not cause chromosome abnormalities in the meiotic
cells of male grasshoppers, when they were given a single toxic dose
(not precisely indicated) of 0.25 ml of the supernatant of an
aqueous suspension/solution of carbaryl by ip injection; however,
there were morphological disturbances in the spermatocytes (Venkat
Reddy et al., 1974).
(b) N-nitrosocarbaryl
Results obtained by Omer et al. (1986) from the
nitrosocarbaryl-treated populations of Drosophila melanogaster
suggested that nitrosocarbaryl increased significantly the
percentage of dominant lethal mutations above the spontaneous
mutation frequency. The results obtained from the 0.05, 0.10, and
0.15% nitrosocarbaryl-treated populations also showed a significant
increase in the percentages of sex-linked recessive mutations.
8.6.2.3 Chromosomal aberrations and sister chromatid exchange
(a) Carbaryl
There are several negative chromosomal studies on carbaryl and
N-nitrosocarbaryl in somatic and germ cells in vivo in mammals
(Venkat Reddy et al., 1974; Degraeve et al., 1976; Seiler, 1977,
Usha Rani et al., 1980; Dzwonkowska & Hübner, 1986). In all these
studies, sufficiently high dose levels of carbaryl were used (up to
the LD50) in order to define the negative cytogenetic responses.
Using the micronucleus test and the cytogenetic analysis of
metaphase chromosomes, Degreave et al. (1976) found no clastogenic
effects in the bone marrow of mice that had been given a single oral
dose of carbaryl (0.2 ml), an intraperitoneal dose (0.5 ml) of
carbaryl, or 7 oral doses of carbaryl solution (1x10-3 mg/litre)
alone, or with sodium nitrite (2x10-3 mg/litre).
A negative response for a high dose of carbaryl (146 mg/kg,
orally, 2 times per 24 h; 30-h sampling time) was reported by Usha
Rani et al. (1980) with an adequate protocol for the mouse bone
marrow micronucleous test.
No increase in the rate of the micronucleated, polychromatic
erythrocytes in the bone marrow of mice was found during in vivo
nitrosation of carbaryl after its oral administration at the maximum
tolerated dose of 100 mg/kg, together with an excess of sodium
nitrite (100 mg/kg) (Seiler, 1977).
A dose of 64 mg carbaryl/kg produced a negative result in an
in vivo assay of chromosomal aberrations in the bone marrow cells
of the Syrian hamster (Dzwonkowska & Hubner, 1986).
No chromosome aberrations were observed in the bone marrow of
Syrian hamsters (6 per dose), given single intraperitoneal
injections of doses up to the LD50 (64, 128, 320, and 640 mg/kg)
of a commercial mixture of carbaryl/lindane (40:10) (Dzwonkowska &
Hubner, 1986).
Daily oral doses of technical carbaryl (10 mg/kg) suspended in
peanut oil were given to male, albino rats for a period of 5 days.
There were no significant chromosomal changes in the bone marrow
cells of the exposed animals (Dikshith, 1991).
8.6.2.4 Dominant lethal assays in rodents
(a) Carbaryl
Epstein et al. (1972) studied the mutagenicity of carbaryl
using the dominant lethal test. In this assay, male ICR/Ha Swiss
mice were treated orally with 1000 or 50 mg carbaryl/kg daily for 5
successive days and then caged with 3 untreated virgin females,
which were replaced weekly for 8 consecutive weeks. The frequency of
early fetal deaths and preimplantation losses in the test groups
were within the limits of the control values. Therefore, dominant
lethal mutations were not induced in mice given sufficiently high
oral doses of carbaryl.
8.6.3 Other end-points
8.6.3.1 Cell transformation
(a) Carbaryl
Transformation of the fibroblast clone A31 of the BALB/3T3
mouse was not induced by carbaryl when given in non-toxic (1.5 and
10 µg/ml) or moderately cytotoxic (20 and 40 µg/ml) doses, over 24 h
(Quarles & Tennant, 1975).
(b) N-nitrosocarbaryl
N-nitrosocarbaryl showed transforming activity, at
concentrations of 10-20 µg/ml, which was cytotoxic for the BALB/3T3,
A31 cells. That 3-10 cell passages are necessary for detecting the
transforming event suggests that the transformation frequency for
N-nitrosocarbaryl in this test system is low. Transformed cells
showed morphological alterations, loss of contact inhibition, and
growth in soft agar, as well as carcinogenic activity in normal
newborn, irradiated, suckling, or athymic BALB/C mice. The tumour
incidence (anaplastic sarcomas) was relatively low; in several
instances, tumours regressed in normal mice, but not in athymic
mice, after 2-3 weeks of growth. N-nitrosocarbaryl did not
activate detectable amounts of the endogenous murine leukaemia
viruses carried by the BALB/3T3 cells or viral protein. However,
viral antigen production in the transformed cells was induced by
iododeoxyuridin, which indicated the presence of the viral genome.
On the basis of this N-nitrosocarbaryl transforming activity in
mammalian cells in culture, Quarles & Tennant (1975) suggested that
the compound may be an active, though weak, carcinogen in vivo.
8.6.3.2 Aneuploidy induction
(a) Carbaryl
Tests of the chemical induction of spindle fibre inactivation
and c-mitosis (colchicine-mitosis) in Allium revealed the
existence of an unspecific physico/chemical mechanism, based on the
partitioning of compounds into hydrophobic compartments of the cell.
This means that chemical compounds, in general, cause c-mitosis
according to their lipophilic characteristics, as indicated by the
octanol/water partition coefficient. Thus, a close correlation
exists between the lipophilicity of compounds and the dose at which
spindle disturbance occurs. However, besides this unspecific effect
on the spindle fibres mechanism, there are some compounds that
exhibit specific effects causing inactivation of the spindle fibre
mechanism at lower doses than indicated by their lipophilicity. This
can occur via different mechanisms, such as specific binding to
actin (colchicine) or binding to sulfhydryl groups (organic
mercury). Onfelt (1987) analysed the c-mitotic effects of 22
compounds in V79 hamster cells. Five of these compounds fell outside
the regression line for lipophilicity/c-mitosis, among them
colchicine, methyl mercury, and carbaryl. Further analyses revealed
that carbaryl owes its pronounced c-mitotic action to its reactivity
with sulfhydryl groups. It is therefore not surprising that several
authors have reported mitotic disturbances caused by carbaryl in
various experimental systems. Onfelt & Klasterska (1983) reported
that a significant increase in the aneuploidy/polyploidy cells was
obtained with both 50 and 100 µmol carbaryl/litre, 26, 59, and 74 h
after treatment. Carbaryl caused mitotic disturbances in Allium
cepa, Vicia faba, Gossypium barbadense, Nigella damascena (Amer,
1965; Amer et al., 1971; Degraeve et al., 1976). Amer (1965)
reported c-mitotic effects of carbaryl in the roots of Allium cepa
that had been treated for 4 or 24 h with different concentrations of
pure (0.5, 0.25%) and commercial products (85% sprayable powder).
The increased rate of abnormal meta-telophases and ana-telophases
depended on the concentration and temperature of the test solutions.
The types of induced abnormal metaphases included star-metaphases
and a few prophase-metaphases. Two types of anaphases, the c-type
and the multipolar type, as well as multinuclear interphase cells,
were observed. Continuous treatment with carbaryl for 24 h nearly
arrested mitosis. There was a full recovery of mitosis in 48 h and
induced signs of polyploidy appeared.
Consistent with these findings are the c-mitotic effects of
carbaryl in Vicia faba and Gossypium barbadense (Amer et al.,
1971). The mitotic index of Vicia faba, after root treatment,
decreased with increased concentrations of carbaryl (25, 50, or
100 mg/litre for 4 h. There was no effect from carbaryl after
seed-soaking for different lengths of time. Mitotic anomalies
(mostly disturbed meta- and anaphases) in the roots of Vicia faba
and Gossypium barbadense showed a concentration-time response.
Carbaryl caused mitotic disturbances (c-mitotic effect,
multipolar anaphases) or cytotoxicity (pycnotic nuclei, tissue
degeneration) after root treatment of Nigella damascena with
concentrations of 2.5x10-4 and 1x10-3 mg/litre. When NaNO2 (2x10-3)
was added to carbaryl solutions of 1x10-2 and 1x10-3 mg/litre, the
effects increased, possibly because of the formation of
nitrosocarbaryl (Degreave et al., 1976). No effect was observed
after grain treatment.
Soaking sugar corn seeds for 48 h with a 0.12 or 0.25% aqueous
solution of 50% commercially prepared carbaryl, resulted in a
dose-response induction of aberrations of anaphase chromosomes of
types not seen in the controls (bridges with, or without, fragments,
dicentrics). Repeated treatment of young plants with a 0.25%
solution of carbaryl for 6 h during meiosis caused typical c-mitotic
effects in metaphase and anaphase through the arrest of cell
division and spindle inactivation. Some of the induced aberrations
persisted until the generative stage. They were fixed and
incorporated through the embryonal and generative development stages
and thus increased pollen sterility (Brankovan, 1972).
Carbaryl was reported to increase the number of aberrant forms
of mitosis (mostly c-mitotic effect) in the small intestine, cornea,
and spleen of rats given oral doses of 400 mg/kg. The authors also
reported that at lower doses of carbaryl (40 or 80 mg/kg), there was
an increased incidence of bridge and chromosome lagging in anaphase
and telophase, in addition to a high percentage of c-mitosis. The
authors reported that there were no effects at 20 mg/kg. Only the
result following the 400 mg/kg dose was fully reported (Vasilos
et al., 1975).
8.6.4 Appraisal
Carbaryl has been evaluated for its potential mutagenicity in a
number of tests in vitro as well as in vivo, in bacterial, yeast,
plant, insect, and mammalian systems, testing a variety of
end-points.
The available evidence indicates that carbaryl has no
DNA-damaging properties. No confirmed induction of mitotic
recombination, gene conversion, and UDS in prokaryotes
( H. influenzae, B. subtilis) and eukaryotes ( S. cerevisiae, A.
nidulans, cultured human lymphocytes, and rat hepatocytes)
in vitro has been reported.
Negative results were obtained in tests for gene mutations in a
number of bacterial assays, with the exception of two cases. In
several studies of gene mutations in mammalian cells in vitro,
carbaryl only produced one equivocal positive result in a cell
culture study. However, the study had several shortcomings and the
result has not been confirmed in any other comparable studies.
Chromosomal damage with high dosages of carbaryl, has been
reported in vitro in human, rat, and hamster cells and in plants.
No such effects have been observed in mammalian tests in vivo, even
at doses as high as 1000 mg/kg.
Carbaryl has been shown to induce disturbances in the spindle
fibre mechanism in plant and mammalian cells in vitro. The
relevance of plant assays for extrapolation to humans is unclear.
It can be concluded that the available data-base does not
support the presumption that carbaryl poses a risk of inducing
genetic changes in either the somatic or the germinal tissue of
humans.
The nitrosated product of carbaryl, N-nitrosocarbaryl, is
capable of inducing mitotic recombination and gene conversion in
prokaryotes (H. influenzae, B. subtilis) and eukaryotes (S.
cerevisiae) in vitro and gave positive results in E. coli spot
tests.
Furthermore, experimental results indicate that
N-nitrosocarbaryl binds to DNA, causing alkali-sensitive bonds and
single-strand breakage.
Nitrosocarbaryl has not been established as a clastogen
in vivo (bone marrow and germ cells), even at high toxic doses.
8.7 Carcinogenicity
8.7.1 Carcinogenicity studies of carbaryl in rodents
8.7.1.1 Mouse
Seven early carcinogenicity studies with carbaryl have been
reported (Table 51) involving different strains of mice,
subcutaneous injection, skin painting, ip and oral routes of
administration, different dose levels and dose schedules, and
various lengths of exposure and observation. None of these negative
studies (one marginal response) had sufficient data or data
reporting and it was not possible to evaluate them.
Table 51. Carcinogenicity studies on carbaryl in rodents
Chemical Species, strain Sex Route and Dose Duration Significant Evaluation Reference
(number) mode of (mg/kg) of study tumour/organ of individual
administration study
Carbaryl Mouse, A/Jax, C3H male SC 1/week 200 mg 20 weeks none inconclusive Carpenter et al.
30 in group (total) (1961)
Carbaryl Mouse, NS not skin painting not 24 months none inconclusive Weil & Carpenter
stated stated (1962)
Carbaryl Mouse, CD-1 male oral, diet 0.01% 80 weeks none inconclusive Mellon Institute
female 0.04% 2 years (1963)
Carbaryl Mouse, A,C3,HA not intraperitoneal 60 2 years none inconclusive Makovskaya et al.
stated 1/week (1965)
Carbaryl Mouse, (C57,B16x male oral gavage daily 4.64 18 months none inconclusive Innes et al.
C5H/Anf)F1 female 7 days-4 weeks 14 mg/kg (1969)
(C57B1/6xAKR)F1 72 of age; in diet body
4 weeks-18 weight
months of age
Carbaryl Mouse, A/He: 16 male intraperitoneal 6 mg 20 weeks marginal (±) inconclusive Shimkin et al.
3/week for (total) lung tumours (1969)
4 weeks response
Carbaryl Mouse, A/J: 124 female oral, diet 1000 10 weeks none inconclusive Triolo et al.
(1982)
Carbaryl Mouse CD-1 male diet 100,1000 53 weeks none no effect Hamada (1991b)
technical female 8000
Table 51 (continued)
Chemical Species, strain Sex Route and Dose Duration Significant Evaluation Reference
(number) mode of (mg/kg) of study tumour/organ of individual
administration study
Carbaryl Rat, CF-N 120 male oral, diet 0.005, 2 years none inconclusive Carpenter et al.
per level female 0.01, 0.02, (1961)
0.04% in
diet
Carbaryl Rat, Sprague-Dawley male diet 250, 1500 52 weeks none negative Hamada (1991b)
technical 80 or 90 per group and 7500
female
ß-Carbaryl Rat, mongrel 60 male oral gavage twice 30 22 months fibrosarcoma, positive but Andrianova &
48 control a week skin; polymorphic inconclusive Alexeev (1970)
cell sarcoma,
stomach;
osteosarcoma
with multiple
mestastases
48 single 20 mg 22 months fibrosarcoma, positive but
48 control subcutaneous skin inconclusive
implantation
Most of these studies involved the lung adenoma test in strain
A mice. This test is no longer considered to be a satisfactory
carcinogenicity bioassay because of the high rate of background
tumours that occur in untreated animals (Clayson, 1987).
Carbaryl did not increase the incidence of lung tumours in two
strains of male mice, A/Jax and C3H, which were given 20
consecutive, weekly, subcutaneous injections of 10 mg carbaryl
(Carpenter et al., 1961). As only one dose of carbaryl was tested
and the initial number of animals entered was insufficient, no
conclusion concerning the carcinogenic potential of carbaryl can be
drawn from this study.
There was no tumour development in mice (unspecified strain and
sex) after carbaryl application by skin painting for 24 months (dose
not specified, 48% water suspension) (Weil & Carpenter, 1962). This
study also lacked details about the experimental procedure and
pathology used and, so, was placed in the inconclusive category.
Carbaryl did not induce tumours in the lungs, liver, kidney,
heart, spleen, pancreas, and the thyroid and adrenal glands in two
strains of mice, A and C3HA, treated ip, once per week for about 2
years, with toxic doses of 60 mg/kg (Makovskaya et al., 1965). The
study involved a sufficient number of animals in the carbaryl group
(400), untreated control group (100), and urethan positive control
group (150), killed at 1, 3, 6, 9, 12, 15, 18, and 24 months after
the beginning of the study and studied histopathologically. However,
no numerical data on the background lung tumours in the untreated
control animals were presented. This lack of data, coupled with the
absence of tumours in the lungs of the carbaryl-treated mice strain
A, which are known for their high rate of naturally occurring lung
adenomas, makes the negative response of little significance.
Carbaryl yielded a marginal tumour response in the pulmonary
tumour induction test on mice (strain A) given a total dose of 6 mg
by ip injections, 3 times per week for 4 weeks (Shimkin et al.,
1969). Again, the lung adenoma test on strain A mice was not
reliable for evaluating possible carcinogenicity and, thus, the
results obtained from this study are not definitive.
No carcinogenic response was obtained with carbaryl
administered orally, by gavage, and/or in the diet to four different
strains of mice at doses ranging from 14 to 1000 mg/kg diet (Melon
Institute, 1963; Innes et al., 1969; Triolo et al., 1982).
There was no increase in the total tumour incidence and no
changes in tumour patterns in either sex of CD-1 mice fed diets
containing doses of 0.01 or 0.04% (100 or 400 mg/kg) carbaryl for 80
weeks and 2 years, respectively (Mellon Institute, 1969). The
survival rate in both test groups was too low for a response to be
observed; no information was given for one-third of the animals;
histopathology was performed only on animals that were suspected of
having tumours. Because of these deficiencies, no conclusion can be
drawn.
Carbaryl did not significantly increase the incidence of any
type of spontaneously occurring tumours (hepatomas, lung adenomas,
lymphoid sarcomas) in either sex of two hybrid strains of mice
(C57B1/6xC3HAnf) F1 and (C57B1/6xAKR) F1, treated orally with
4.64 mg/kg by gavage (mice from 7 days to 4 weeks of age), and in
the diet (mice from 4 weeks to 18 months of age) with 14 mg/kg diet
(Innes et al., 1969).
Another inadequate feeding study of carbaryl carcinogenicity
was conducted by Triolo et al. (1982), using the model of lung
tumour induction in strain A/J female mice. In two studies, the
feeding level of 1000 mg carbaryl/kg, incorporated in the diet of
mice for 20 weeks, did not cause a significant increase in the
incidence of background lung adenomas, nor did it induce tumours in
the glandular stomach or other tissues (spleen, intestinal tract).
However, there were a number of confounding factors in defining the
response in this study including: large variations in the incidence
of lung adenomas in the four separate control groups; in particular,
one such group had no tumours, despite high historical control
levels in strain A mice. In addition, only one dose level was
studied (1000 mg/kg), on the basis of which it is not possible to
establish a dose-response relationship.
In the second study, in which the same feeding level and
exposure period were used, carbaryl increased the lung
benzo( a)pyrene hydroxylase activity, which was associated with a
modest increase in the rate of lung tumours induced by the oral
administration of 3 mg BP twice, on days 7 and 21, respectively of
the study. However, there was great variability in the incidence of
BP-induced lung adenomas in the 3 control groups; the first
(7.2±0.8) was well above the values presented for the test group
carbaryl + BP (5.7±1.4); and the second fell within the limits of
the incidence of spontaneous tumours in the corn-oil control group,
(1.17±1.11), which make the results inconclusive.
A new mouse oncogenicity study is in progress, under
proprietary sponsorship. The study design was as follows: carbaryl
was administered in the diet to male and female CDR-1 mice at
rates of 0, 100, 1000, or 8000 mg/kg diet, for up to 104 weeks.
Groups consisted of 80 mice/sex per group. Ten mice/sex per group
were sacrificed for clinical pathology evaluation after 52 weeks of
treatment. Results of this interim sacrifice are presented in
section 8.3. The remaining animals were designated for continued
exposure to the end of the 104-week treatment period. The study
design and evaluation parameters are consistent with guidelines set
out by the US EPA and OECD, with additional study parameters, for
studies of this nature (Rhône-Poulenc, 1992).a
8.7.1.2 Rats
Two reports by Carpenter et al. (1961) and Andrianova &
Alexeev (1970) are controversial. They studied the carcinogenicity
of carbaryl given to rats by oral gavage, in the diet, or by
subcutaneous implantation. Both studies had insufficiencies in the
protocols used.
Carpenter et al. (1961) noted no significant increase in the
total tumour incidence in either sex of CF-N rats fed a diet
containing 0.005, 0.01, 0.02, or 0.04% carbaryl, for 2 years. Female
mice had more pituitary tumours than male mice, but there was no
significant difference in the incidence of tumours between the
control and test groups. The small initial number of animals used,
their relatively old age (60 days), their low survival rate, and the
lack of detailed pathology are complicating factors in defining a
negative response.
However, Andrianova & Alexeev (1970) reported that carbaryl,
administered by both oral gavage and subcutaneous implantation,
produced positive carcinogenic responses in mongrel rats. Male rats
given oral doses of 30 mg carbaryl/kg, twice weekly for 22 months,
developed skin fibrosarcoma, polymorphic cell sarcoma in the
stomach, and osteosarcoma with multiple metastases. Carbaryl caused
high lethality (80%) during the exposure period. The authors did not
state whether the gross pathology was examined. One fibrosarcoma was
discovered among 46 control animals, at 11 months. No data were
presented on the number of control animals that lived for 22 months,
so a comparison of survival rates in control and test groups could
not be made.
In a parallel study, 20 mg of carbaryl in a purified paraffin
capsule was implanted subcutaneously in 48 male rats. At the end of
the 22-month exposure period, subcutaneous fibrosarcomas, in sites
far from the implantation area, and on the back and neck, were
diagnosed in 2 out of 10 surviving animals. There was no control
group (Andrianova & Alexeev, 1970). The carbaryl used was obtained
from a plant in the USSR and was of technical grade and 97.65%
purity. No information about the chemical composition and impurities
was given. Because of these deficiencies, this study is
inconclusive.
a Information to Task Group. These studies have not yet been
reviewed by the IPCS. The company performing these studies has
indicated that there is a significant increase in tumors at the
highest dose in both species.
A new combined long-term toxicity and oncogenicity study on
rats is in progress, under proprietary sponsorship. The study design
is as follows: carbaryl was administered in the diet to male and
female Sprague-Dawley rats at rates of 0, 250, 1500, or 7500 mg/kg
diet, for up to 104 weeks. Groups consisted of 80 rats/sex per group
for low- and middle-dose groups and 90 rats/sex per group for the
high-dose and control groups. Ten animals/sex per group were
sacrificed after 26 and 52 weeks exposure and evaluated for clinical
pathology and histopathology. In addition, 10 animals/sex from the
control and high-dose groups were used as a recovery group, kept on
a basal control group diet before sacrifice at 56 weeks. The results
of the interim sacrifices are presented in section 8.3. The
remaining animals were designated for continued exposure to the end
of the 104-week treatment period. The study design and evaluation
criteria are consistent with guidelines set out by the US EPA and
OECD, with additional study parameters, for studies of this nature
(Rhône-Poulenc, 1992).a
8.7.1.3 Overall appraisal of carbaryl carcinogenicity
Carbaryl has been studied for its carcinogenic potential in
numerous studies on rats and mice via various routes of
administration. Most of these studies are old and do not meet
contemporary standards.
Only one paper from the existing body of publications clearly
reports a tumorigenic action of carbaryl. Carbaryl induced malignant
tumours in an unidentified strain of rat by the oral and
subcutaneous routes of administration. This study does not meet
contemporary standards, because of insufficient reporting of control
data.
New studies, designed to meet with contemporary standards, are
in progress on rats and mice. Descriptions of the studies are given
in sections 8.7.1.1 and 8.7.1.2.
8.7.2 Carcinogenicity studies of N-nitrosocarbaryl
Carbaryl is a secondary amine and is, therefore, capable of
nitrosation in the presence of nitro donor groups, such as sodium
nitrate, to give a nitrosamide. This nitrosamide, nitrosocarbaryl,
has been proved to be mutagenic and carcinogenic, at high doses in
a Information to Task Group. These studies have not yet been
reviewed by the IPCS. The company performing these studies has
indicated that there is a significant increase in tumors at the
highest dose in both species.
animals (see Table 52). A condition of this nitrosation is an acidic
pH (less than 2), which is comparable to the one found in the human
stomach. However, nitrosocarbaryl is not stable at this pH. Its
maximum stability is between pH 3 and 5, at which pH no significant
amount of carbaryl can be nitrosated. Carbaryl was nitrosated in
several studies, in vitro as well as in vivo, in the guinea-pig,
which has a stomach acidity similar to that of humans. See also
section 8.9.3.
8.7.2.1 Rats
N-nitrosocarbaryl elicited a carcinogenic response in both
sexes of two strains of rats (Wistar and Sprague-Dawley) by two
routes of administration (oral gavage and subcutaneous injection)
(Eisenbrand et al., 1975, 1976; Lijinsky & Taylor, 1976;
Preussmann et al., 1976; Lijinsky & Schmähl, 1978).
A local carcinogenic effect of N-nitrosocarbaryl was reported
by Eisenbrand et al. (1975) in Wistar rats given a single
subcutaneous injection of 1000 mg/kg. Fifteen out of 16 treated
animals developed sarcomas at the injection site; there was no
histopathological evidence of any systemic carcinogenic effects. The
dose of N-nitrosocarbaryl was highly toxic and 14 out of 16
animals had died by day 450. No spontaneous tumours were observed in
control animals. When given to rats orally in single doses of
200-1500 mg/kg, N-nitrosocarbaryl did not induce tumours during 21
months of observation. No signs of toxicity were noted in any of the
test groups.
Further studies involving repeated oral administration of
N-nitrosocarbaryl to Sprague-Dawley rats gave unequivocal evidence
of local carcinogenic effects, produced by both oral and
subcutaneous routes of administration. (Eisenbrand et al., 1976;
Lijinsky & Taylor, 1976, 1977; Preussmann et al., 1976; Lijinsky &
Schmähl, 1978).
The nonglandular part of the stomach (forestomach) was the
target organ of the local carcinogenic action of N-nitrosocarbaryl,
when it was given orally to rats. All stages of malignant
transformation in the forestomach, from hyperplasia to squamous cell
carcinoma, were observed in male Sprague-Dawley rats treated with
oral doses of 130 mg N-nitrosocarbaryl/kg, twice weekly, until
spontaneous death. There was a substantial lowering of the survival
rate of treated animals because of the pronounced toxicity. By the
time of maximum increase of the tumour incidence (after 200 days),
most of the animals were dead. The average survival time of
tumour-bearing animals was 167 days after the onset of the study.
The low background tumour incidence (3 out of 29) in control animals
included lymphosarcomas and leukaemia.
Table 52. Carcinogenicity studies on N-nitrosocarbaryl in rodents
Chemica Species, strain Sex Route and Dose Duration Significant Evaluation Reference
(number) mode of (mg/kg) of study tumour/organ of individual
administration study
N-nitroso Rat, Wistar male subcutaneous, 1000 450 days polymorphic cell positive Eisenbrand et al.
carbaryl 8: male; female single injection sarcoma at (1975)
8: female injection site;
spindle cell
sarcoma
37 male, oral gavage, 200-1500 21 months none inconclusive
female single
N-nitroso Rat, Sprague-Dawley male oral gavage, 130 (5000 till squamous cell positive Eisenbrand et al.
carbaryl 31 twice per week total) spontaneous sarcoma, (1976)
death papilloma, Preussmann et al.
hyperkeratoses, (1976)
forestomach
N-nitroso Rat, Sprague-Dawley female oral gavage, 50 mg 110 weeks squamous cell positive Lijinsky & Taylor
carbaryl 12 once a week (total) carcinoma (1976)
for 10 weeks forestomach,
papilloma,
oesophagus
12 male oral gavage, 300 mg 90 weeks squamous cell
twice a week (total) carcinoma
for 20 weeks forestomach,
papilloma trachea
N-nitroso Rat, Sprague-Dawley male oral gavage, 600 80 weeks carcinoma positive Lijinsky &
carbaryl 16: male; female once a week (total) forestomach Schmahl (1978)
16: female for 10 weeks
In a comparative study of the carcinogenic potency of
N-nitroso- N-alkylcarbamate esters, Lijinsky & Taylor (1976)
showed that N-nitrosocarbaryl was a strong carcinogen, similar in
action to its highly potent carcinogenic analogue
N-nitrosomethylurethane. A high incidence (75-80%) of
squamous-cell carcinomas in the forestomach was detected in female
Sprague-Dawley rats given 10, weekly, equimolar oral doses
(0.22 mmol) of both compounds. Nitrosomethylurethane was a more
potent carcinogen, because the animals with tumours died earlier
than those in the nitrosocarbaryl group. The same rate of carcinomas
in the forestomach was induced in male rats treated orally with a
considerably higher dose of N-nitrosocarbaryl (1.3 mmol total),
over twice as long a period (20 weeks). The stronger carcinogenic
effect of this dose in male animals was expressed by the shorter
latent period of induced tumours and higher lethality compared with
female animals. The local carcinogenic effect of N-nitrosocarbaryl
in rats, the target organ being the forestomach, was seen in another
oral carcinogenicity study of a series of nitroso- N-methylcarbamate
insecticides carried out by Lijinsky & Schmähl, (1978). The oral
gavage of 10 weekly doses of 60 mg nitrosocarbaryl/kg to both sexes
of Sprague-Dawley rats led to a high incidence (over 70%) of
carcinomas in the forestomach, with no evidence of other systemic
carcinogenic effects.
No carcinogenic response for carbaryl was obtained with the
transplacental carcinogenicity test after in vivo nitrosation in
pregnant Sprague-Dawley rats (Lijinsky & Taylor, 1976). Pregnant
animals were given 30 mg carbaryl/rat, orally, for 10 days (4-18
days of gestation). Two other groups of animals received the same
dose of carbaryl, together with 0.6-1ml of 4% sodium nitrite on days
4-6 and 14-18 of pregnancy. The distribution of tumours in the rats
of various groups was that normally seen in Sprague-Dawley rats. It
is likely that an insufficient amount of nitrosocarbaryl was formed
under the conditions of in vivo nitrosation, or that no
significant amount of nitrosocarbaryl crossed the placenta.
A low rate of in vitro nitrosation of carbaryl under
conditions similar to those that exist in the human stomach was
reported by Eisenbrand et al. (1975). The reaction of
10-3 mol/litre carbaryl with a 5-fold molar excess of sodium nitrite
in 0.1N HC1 (pH, 1) led, after 15-60 min, to yields of only 1.2 and
1.7% of the maximum possible conversion to nitrosocarbaryl.
Reduction of the concentration of carbaryl and sodium nitrite by a
factor of 10 decreased the yield of nitrosocarbaryl by about
one-half. The yields of N-nitrosocarbaryl obtained by the
in vitro nitrosation of carbaryl, under the described conditions,
are low and the potential carcinogenic risk of in vitro nitrosation
and similar nitrosation reactions in vivo is difficult to evaluate
at present.
8.7.2.2 Mice
Nitrosocarbaryl administered for 104 weeks to the skin of
female CFLP mice (65 mice per group) in three doses (12.5, 50, and
200 µg/mouse) was found to be more potent regarding dermal
carcinogenic efficiency than nitrosomethylurea, applied under the
same conditions, though not as effective as benzo- a-pyrene
(Deutsch-Wenzel et al., 1985). Conclusions were drawn on the basis
of the number of animals bearing carcinomas, total cases with local
tumours, observed/expected ratios and dose-response relationships of
incidences of malignant tumours. These results were found to be in
contrast to the ones obtained by Lijinsky & Winter (1981), who used
a single total dose of 23 mg and found that nitrosocarbaryl was a
much less effective inducer of mouse skin tumours than
nitrosomethylurea.
8.7.2.3 Overall evaluation of the carcinogenicity of
N-nitrosocarbaryl
Sufficient evidence of carcinogenicity at the site of
application was seen in multiple studies on both sexes of
Sprague-Dawley rats, by different routes of administration
(subcutaneous and oral gavage), using different dose levels. The
non-glandular stomach was the target tumour site when
nitrosocarbaryl was administered by oral gavage; subcutaneous
injection of nitrosocarbaryl caused sarcomas at the injection site.
The local carcinogenic effect of N-nitrosocarbaryl in rats,
and the lack of any systemic carcinogenicity, characterizes it as a
direct-acting alkylating agent. N-nitrosocarbaryl was active as a
direct bacterial mutagen and interacted with human DNA in vitro.
These data agree with data that show that N-nitrosocarbaryl is an
effective in vivo genotoxin.
8.7.3 Carcinogenicity of ß-carbaryl
Beta-carbaryl (N-methyl ß-naphthyl carbamate) was a component
of technical carbaryl (alpha-carbaryl), suspected of having a
carcinogenic structure and a tumorigenic potential in mice and rats
(Zabezhinski, 1970). In life-time studies on mice, strain CC57W (24
months), and mongrel rats (33 months), using two routes of
administration, oral gavage (10 mg/kg in mice; 25 mg/kg in rats) and
subcutaneous injection (20 mg/kg in mice; 50 mg/kg in rats),
ß-carbaryl showed a weak carcinogenic response. High rates of
lethality (20-50%) were observed in both mice and rats, by the two
routes of administration. No data for survival rates and the
incidence of spontaneous tumours in control animals were presented.
ß-carbaryl caused a low rate of tumours in rats by both routes of
administration. The types of tumours (21%) observed after
subcutaneous injection of ß-carbaryl included: fibrosarcoma at the
injection site, subcutaneous rhabdomyosarcoma, intestinal sarcoma,
leukaemia, reticuloses, and reticulosarcoma. The pattern of
carcinogenic response after orally administered ß-carbaryl (25%)
involved sarcoma in the liver, fibroadenoma and adenocarcinoma in
the mammary glands, carcinoma in the thymus, and granulocellular
carcinoma in the ovaries. No local tumours at the injection site
were observed in control animals after subcutaneous administration
of corn oil. The other tumours (carcinoma in the mammary glands and
thymus, haematopoietic system malignancies) were unusual for control
animals, as well. ß-carbaryl caused a higher tumour incidence in
mice than in rats by both subcutaneous (60%) and oral (30%) routes.
It should be mentioned, however, that most of the tumours observed
(lung adenomas, leukaemia, and liver haemangiomas) occurred
spontaneously in untreated mice. Since no control data were
presented, the carcinogenic response to ß-carbaryl in mice is of
uncertain significance. Because of these deficiencies, this study is
inconclusive.
8.8 Special studies
8.8.1 Neurotoxicity
The effect of carbaryl on the nervous system is primarily
related to ChE inhibition.
Carpenter et al. (1961) studied the delayed neurotoxic
potential of carbaryl in chickens (Rhode Island hens) compared with
that of triorthocresyl-phosphate. Single doses of 250, 500, 1000, or
3000 mg/kg body weight, 25-40% in lard were administered
subcutaneously to chickens. At 2000 mg/kg, weakness was observed on
day 1 or 2 after dosing. In one case, the chicken was unable to walk
for 3 days. No evidence of demyelination was observed in any brain
sciatic nerve or spinal cord section examined microscopically.
According to the authors, there was a transient cholinergic effect
caused by the slow absorption of carbaryl.
The neurotoxic effect of carbaryl was studied in atropinized
chickens (Gaines, 1969) to protect against acute effects of the
subcutaneous injection of 800 or 1600 mg carbaryl/kg body weight.
The higher dose caused leg weakness within 24 h, which recovered by
day 24.
Carbaryl solution in corn oil at a daily dose of 100 mg/kg was
administered orally for 7 consecutive days to 35, 6-day-old, female
broilers, hybrids between Peterson strain roosters and Hubbard hens
(Farage-Elawar, 1989). Altered locomotion and abnormally shortened
gait were observed on the 7th day, and cases of delayed paralysis
20-40 days, after the last treatment. Locomotion changes were found
to have no association with the activities of brain
acetylcholinesterase and neuropathy target esterase, 24 h after the
first, second, third, and fifth doses as well as 1, 3, 6, 10, 20,
30, and 40 days after the last treatment, when no statistical
deviations from the control values of both enzymes were registered.
A similar, but lesser, effect on gait and stride length was
observed when 45 mg carbaryl/kg was injected into chick eggs on day
15 of incubation (Farage-Elawar, 1990). This treatment resulted in
significant inhibition of brain and plasma cholinesterase, and liver
carboxylesterase. Administration of the same dose on day 5 of
incubation resulted in 10% lethality. Brain NTE was not affected.
The cause of this delayed alteration is not known.
Effects of long-term carbaryl exposure on the neuromuscular
system of pigs were reported by Smalley et al. (1969). Six
Yorkshire pigs, 3 male and 3 female, received carbaryl in their diet
(150 mg/kg body weight); the male pigs for 72 days and the female
pigs for 83 days. Three pigs from the same litter were fed carbaryl
at 150 mg/kg body weight daily, for 4 weeks, and then 300 mg/kg body
weight, daily, for 46 days (2 male pigs), and for 85 days (the
female pig). The signs of intoxication started after about 1´
months, and were mostly typical of neuromuscular system damage (see
Table 44; section 8.3). Microscopic examination of the skeletal
muscle revealed myodegeneration. In the myelinated tracts of the
cerebellum, brain stem, and upper spinal cord, moderate to severe
edema was associated with vascular degenerative changes. No
demyelination of nerve tissue was observed. When carbaryl feeding
was stopped and hydrochlorothiazide applied as a diuretic, signs of
toxicity of carbaryl, such as ataxia, and partial paralysis,
disappeared.
Carbaryl disturbed the function of the myoneural synapses. It
produced a decrease in the spontaneous activity and an increase in
the permeability of the muscular fibre membrane for K+ and Na+
after multiple oral administrations of 8.5 mg/kg to rats(Kovtum &
Sokur, 1970).
Kovtun (1970) while analysing the effects of carbaryl on
myoneural formations, pointed out that, at a single intake
(425 mg/kg), multiple intakes during 2 months, or 1/100
LD50-8.5 mg/kg over 6 months, the frequency of tiny potentials of
an edge plate was suppressed by 62-65%. On the basis of the data
obtained it was concluded that carbaryl probably impairs the
function of presynaptic nerve endings. At the same time, carbaryl
does not affect the cholinereceptive membrane substance of muscular
fibres. Carbaryl increases the rest potential of muscular fibres by
11-35%, depending of the carbaryl dose. This increase can be
explained through high potassium ion accumulation inside the
muscular fibres.
Three different laboratories examined the effects of carbaryl
on motor activity in rats. All three reported decreased activity
after intraperitoneal administration, with ED50s that ranged from
13.3 to 17.6 mg/kg (Crofton et al., 1991).
Takahashi et al. (1991) compared the effects of carbaryl on
both young (3 months) and old (12 months) rats. A dose of 50 mg/kg
decreased activity in an open field test, prolonged the duration of
haloperidol-induced catalepsy, decreased body temperature, and
increased the nociception threshold, as measured by a hot-plate
test. A dose of 10 mg also prolonged haloperidol-induced catalepsy.
The effects on body temperature and nociception were significantly
greater in older rats.
Studies were carried out on the mechanical response
characteristics of the soleus muscle in situ. Female Holtzman rats
(6 treated-control pairs) were given 56 mg carbaryl/kg orally.
Carbaryl increased the tension developed during complete tetanus (by
electric stimulus) and decreased the time constant of tension
development (Santolucito & Whitcomb, 1971). The more forceful and
rapid contraction of the skeletal muscle is probably related to an
accelerated catecholamine release.
EEGs on 4 Rhesus monkeys given 0.01 mg carbaryl/kg and on 3
given 1 mg/kg per day, orally, for 18 months, showed only a
reduction in the amount of low-amplitude fast waves and an increased
bilateral synchrony between the right and left hemispheres
(Santolucito & Morrison, 1971). The authors did not relate these EEG
changes to the dose.
Belonozhko & Kuchak (1969) found some changes in the EEG in
rats, such as desynchronisation of rhythms after a single
application of carbaryl at 100 mg/kg. During repeated doses of
35 mg/kg for 90 days, no changes in the EEG were noted, due
(according to the authors) to adaptation of the nervous system. Oral
doses of 72 mg carbaryl/kg, administered to rats for 10 days, caused
a decrease in serotonin and an increase in dopamine levels in the
brain (Kuzminskaya et al., 1984).
Morphological changes in the nervous system caused by carbaryl
were dose-related. One to six months oral treatment of rabbits with
0.01 LD50 caused only haemodynamic disorders and cell
infiltrations, a dose of 0.02 LD50 caused cell dystrophic changes,
and a dose of 0.1 LD50 caused more progressive and serious
disorders (Azizova, 1976).
The behavioural effects of carbaryl were studied in rats and
monkeys. Disturbance of discrete (shock) avoidance behaviour by
carbaryl in rats was reported at ip doses > 2.5 mg/kg, the LD50
being 8 mg/kg (Goldberg et al., 1965). Carbaryl administered at
doses of 8, 16, or 28 mg/kg, ip, decreased maze activity in CD male
rats (10 in each group), whereas doses of 16 and 28 mg/kg reduced
open field activity. After acute exposure, behavioural changes
recovered within 60 min, whereas ChE of the blood and brain
recovered after 240 min (Ruppert et al., 1983).
Carbaryl at 10 mg/kg administered subcutaneously in 16, male,
Long Evans strain rats decreased the number of times they approached
the novel stimuli and explored the exploratory box, and increased
habituation. In familiar situations, carbaryl increased activity
(Albright & Simmel, 1977).
Anger & Setzer (1979) studied the effects of oral and im
administration of carbaryl on repeated chain acquisition in 5 male
monkeys ( Macaccus mascicularis). Oral doses of 50 mg/kg did not
alter the performance of repeated acquisition tasks. The im
injection resulted in consistent changes in performance at 5 and
10 mg/kg, but did not cause any changes at 1 mg/kg.
Carbaryl affected working memory (continuous delayed response
and continuous non-match) in 28 male Sprague-Dawley rats (Heise &
Hudson, 1985a,b). A dose of 10 mg carbaryl/kg, ip, affected
performance of working memory procedures; with increasing dose,
carbaryl nonselectively decreased response.
Administration of 2.24 mg carbaryl/kg, ip for 14 days, did not
affect the performance of rats in activity wheel cages, 24 h after
the last treatment. Acute ip administration of 0.54 or 2.24 mg/kg
significantly decreased the motor activity of rats in activity wheel
cages. This decrease was reversed by atropine sulfate (Singh, 1973).
Doses as low as 7.76 mg/kg ip caused mild tremors.
Oral administration of carbaryl (200 mg/kg, 3 days/week) for a
period of 90 days, though producing inhibition of ChE in blood (33%)
and brain (11%), did not result in any kind of overt signs of
toxicity in male albino rats (Dikshith et al., 1976).
Desi et al. (1974), in long-term studies, investigated the
effects of carbaryl on the learning process, on the performance of
previously learned tasks in mazes, and on the EEG in 40 male Wistar
strain rats. Carbaryl was given at doses of 10 or 20 mg/kg body
weight per day in the diet (100-200 mg/kg food), for 50 days. Mild,
but permanent and increased, functional deviations of the nervous
system were found. Because of increased irritability in the CNS, the
treated rats took less time than the untreated rats to find their
food. Later, the task was performed with difficulty when the
irritability of the CNS was reduced to below the normal level. The
performance of the learned task was impaired. EEG deviations
recorded at the end of the maze studies were slight, but permanent.
They consisted of increased electric activity of the brain,
increased number of moderately slow beta waves, and light flashes of
18 Hz accelerated electrical activity.
Viter (1978) found behavioural changes in rats treated via
inhalation for several months with 12 or 23 mg carbaryl/m3. The
latency period of the conditioned reflex on nutrition was prolonged.
Depression of investigative behaviour and spontaneous motor activity
were noted.
The behavioural effects of carbaryl on rats were examined by
Moser et al. (1988) using a functional observation battery.
Carbaryl was administered at doses of 3-30 mg/kg, ip, and tested
0.5, 3, 24, and 48 h afterwards. Carbaryl decreased spontaneous
activity, CNS excitability, motor and sensory function, and body
temperature and weight. Effects indicative of AChE inhibition were
also observed. All responses were dose dependent.
8.8.2 Effects on the immune system
8.8.2.1 Appraisal on immunotoxicology
The administration of carbaryl, or any other xenobiotic, at
doses resulting in overt toxicity can be expected to result also in
a variety of effects on the immune system. Carbaryl, when
administered in vivo, at a dose not causing overt clinical signs
has been reported to produce a variety of non-life-threatening
effects on the immune system. The effects included cellular as well
as humoral immunity, and several authors have suggested that they
were was due to subtle, treatment-related stress. Many of the
effects described were detected at doses close to the LD50.
Shortcomings of several of these studies were a lack of consistency
and, sometimes, overt contradiction between results, which prevents
the description of a defined immunotoxic mechanism.
Lifetime exposure to carbaryl did not result in increased
occurrence of disease in rats or mice. No enhancement of viral
infections was found with carbaryl, even at dosages close to the
LD50. Most studies on rabbits and mice, at doses permitting
survival, did not produce significant effects on the immune system.
In vitro, a number of researchers have demonstrated viral
enhancement by prior incubation with carbaryl. In vitro, carbaryl
can enhance herpes varicella-zoster, but not herpes simplex. In
goldfish cell culture, carbaryl has been shown to permit viral
enhancement by compromising interferon synthesis. Inhibition of
human serum complement activity as well as interleukine-2 driven
proliferation of large granular lymphocytes have been demonstrated
in vitro. These actions could be mediated through the inhibition
of serine esterases involved in the processes. No increased viral
infections have been seen in long-term in vivo feeding studies,
previously conducted or in progress at present. Carbaryl does not
enhance transformation of BALB/53T fibroblasts in culture or the
expression of endogenous murine leukaemia virus.
8.8.2.2 In vivo studies
Young mice weighing 10-12 g were infected with influenza by
applying 3-4 drops (0.05 g) 1% influenza virus in physiological
solution in the nose. The murine influenza virus strain A.P.R.8 was
used in the primary dilution 1:32. After 2-3 days, this group of 30
mice was treated orally with carbaryl in sunflower oil, at a dose of
500 mg/kg (a dose that killed 3 out of 10 mice). Two control groups
with the same number of mice, one treated with carbaryl alone, and
one infected, were compared with the experimental animals for
survival, blood biochemistry, and pathomorphological changes. The
greatest number of mice dying were in the experimental group. AChE
depression was more pronounced and recovery slower, and there were
more histological changes in the livers of mice infected and
intoxicated by carbaryl (Moreynis & Estrin, 1965). It is clear from
this study that a summation of the effects of both factors occurred,
but, because of the lack of statistical significance, it is
impossible to judge the eventual effect of the enhancement.
The effect of carbaryl on an experimental Erysipelothrix
rhusiopathiae infection in rats was studied by Shabanov et al.
(1983). Rats, treated with doses that increased gradually every 6
days from 2 to 5 mg/rat (mean weight of rats, 50-60 g) during 30
days, were infected iv with Erysipelothrix rhusiopathiae at
1.5-108 cells/rat. The mortality rate in carbaryl-treated rats was
36% versus 14% in control rats. Survival time was halved.
Bacteraemia persisted for 10-12 days in treated groups, and only 5-6
days in the control group. Both the gross and the histopathological
changes were more strongly manifested in the treated group.
Carbaryl, at 0, 2, 20, or 200 mg/kg was administered orally to
rabbits, daily, for 6 months. A decrease in the phagocytic activity
of leukocytes and antibody formation (4-5 times) after immunization
with a murine type of typhoid vaccine, was reported at the dose
level of 200 mg/kg. At the lower dose of 20 mg/kg, there were
different phases of reactivity; at the beginning of the first 2
months, there was an increase and later a decrease in immunological
reactivity (Perelygin et al., 1971).
The effects of oral ingestion of carbaryl on nonreaginic
antibody production in BALB/c mice were studied after pretreatment
with 150 mg carbaryl/kg diet for 10 weeks prior to commencement of
the study. Carbaryl produced significant effects on systemic
antibody production following oral immunization with sheep red blood
cells (SRBC). It significantly increased both IgG1 and IgG2b titres.
No reduction was seen in the synthesis of any antibody class. These
results suggest that, at the doses used, carbaryl increased systemic
antibody responses to orally ingested antigens (André et al.,
1983).
Changes in the immunological structures of lymphatic follicles
in the spleen during carbaryl administration were studied by Dinoeva
(1974, 1982), who used a micrometric method. Albino rats (30
treated-control pair) were given 1.5 mg carbaryl/kg, orally, daily
for 6 months. Plethora in the spleen and retardation in lymphatic
follicles were observed, which were similar to effects seen in the
positive control group treated with cyclophosphamide (2 mg/kg).
There were no changes in the structure and weight of the thymus in
carbaryl-treated rats, while in the cyclophosphamide positive
control group, the weight of the thymus was reduced 2.5 times. The
authors suggested that there is a different mechanism of the
immuno-suppressive effect of carbaryl that needs further research.
A single dose of 500 mg carbaryl/kg inhibited the production of
haemolysins and reduced the number of splenic germinal centres in
White Leghorn chickens (Roszkowski et al., 1976). Dose-dependent
immunosuppressive effects of continued dietary treatment of rabbits
with carbaryl were studied (Street & Sharma, 1975). Rabbits (male
White New Zealand, 7 in each group) were fed 0, 4, 20, 45, or 150 mg
carbaryl/kg diet (corresponding to 0, 0.23, 1.0, 2.3, or 8.38 mg/kg
body weight per day) for 57 days. The treatment reduced the germinal
centres in the spleen and caused atrophy of the thymus cortex. The
antigen-induced increase in serum gamma-globulin was decreased
significantly (no dose relationship was noted), at 10 days. No
changes were detected in the number of plasma cells in the lymph,
haemolysin and haemoglutinin titres, skin sensitivity to tuberculin,
leukocytes count, body weight, etc.
Rats pretreated, orally, with 0.05 LD50 carbaryl, daily, for
2.5 months, were immunized with typhoid antigen. Administration of
carbaryl continued for an additional 2 months. Signs of
insufficiency of the immune system, expressed as a reduction in
specific globulin production, were observed 3.5 months after
carbaryl application began. Carbaryl did not affect immunogenesis at
a dose of 0.001 LD50, daily, under the same exposure conditions.
No overall evaluation was given (Olefir, 1977).
A humoral immunity study in female BALB/C mice after oral
administration of carbaryl was performed by Wiltrout et al. (1978).
The effects of humoral immune competence were measured by the
immunoplaque in gel technique. The number of antibody plaque-forming
cells (PFC) per plate was counted and the PFC/spleen calculated.
Different groups of mice received carbaryl, orally, at about
1 LD50 (153 mg/kg body weight), 5 days before immunization (type
of antigen not specified), on the day of immunization, and 2 days
after. Only the last treatment resulted in significant suppression
of the humoral immune response. The ratio (experimental: control) of
antibody plaque-forming cells was 0.34. The oral administration of
0.1 LD50 for 8 and 28 days did not cause any significant effects
on humoral immune competence. Depression of the PFC/spleen level
corresponds to a decline in the total splenic lymphocyte population.
Akhundov et al. (1981) studied the effects of carbaryl on the
immunological reactivity of 40 rabbits and 40 guinea-pigs given oral
doses of 15 mg carbaryl/kg for 42 days. Heated vaccine Salmonella
typhimurium, at doses of 250 or 500 million microbial cells, was
applied 3 times at 7-day intervals, 21 days after the beginning of
carbaryl administration. The auto-sensitizing effect of the vaccine
was enhanced by carbaryl. Studies on guinea-pigs treated with 15 mg
carbaryl/kg per day for 3 months showed decreased macrophagial
migration activity.
Pipy et al. (1983) evaluated the cellular and humoral
mechanisms of carbaryl-induced reticuloendothelial phagocyte
depression. The function of the reticuloendothelial system (RES)
involved in specific and non-specific aspects of host resistance to
infection, neoplasma, etc., was quantitatively evaluated by the rate
of disappearance of colloids injected into the blood. Colloid
particles are extracted from the blood exclusively by the RES, in
particular liver and spleen macrophages. Plasma or serum factors
called opsonins have a strong stimulatory influence on inert
particle phagocytosis. Male Sprague-Dawley rats (200-240 g) were
given single, iv injections of labelled carbaryl at 0.5-32 mg/kg
body weight (7 different doses). The iv LD50 determined by the
authors was 50 mg/kg body weight. Colloidal carbon was administered
simultaneously. In another group, carbaryl was incubated with serum
from a normal rat for 20 min before the injection. The authors call
this carbaryl "opsonized". The results showed the ability of
carbaryl to induce a state of reticuloendothelial phagocytic
depression, mediated through depletion of opsonins. Carbaryl
inhibits the cell-bound aromatic amino acid esterase of the serine
esterase class. The authors concluded that, apart from a slight
deficiency in plasma opsonins, the inhibitory effect of carbaryl on
the phagocyte function was primarily due to a selective hepatic and
splenic macrophage impairment, which could be related to inhibition
of a cell-bound serine esterase. In another study by Pipy et al.
(1982), more details are given of this possible mechanism of
phagocytoxic blockade by carbaryl. Rats were treated with iv
carbaryl at 8 or 16 mg/kg body weight. The time-dependence of the
effects on phagocytic function and the activities of liver serine
esterases ( N-benzoyl-DL-phenylalanine-ß-naphthyl esterase and
acethylcholinesterase) was studied. Macrophages of the RES had
decreased phagocytic capacities after administration of carbaryl.
Depression of the phagocytosis of colloidal carbon persisted from 1
to 7 h and 1 to 24 h after administration of 8 and 16 mg/kg
carbaryl, respectively. Liver ( N-benzoyl-DL-phenylalanine-ß-
naphthyl esterase (cell-bound serine esterase) was susceptible to
reversible dose-related inhibition by carbaryl. A correlation
between the reactivation kinetics of liver serine esterases and
phagocytic activity was demonstrated.
8.8.2.3 In vitro studies
The replication of goldfish virus 2 (GFV-2) was enhanced in
vitro by pre-treatment of a piscine culture with subtoxic
concentrations (1 mg carbaryl/litre) (Shea, 1983). The mechanism of
enhancement was studied in vitro in goldfish-derived cell lines
and Air Bladder III infected with GFV-2 and pretreated with a
carbaryl solution of 1 µg/ml. Interferon synthesis was studied as a
possible mechanism of virus enhancement (Shea & Berry, 1984). The
authors demonstrated that interferon synthesis was induced in CAR
cells by GFV-2 cells. Antiviral protection provided by supernatants
from infected CAR cultures with carbaryl, against infection of
secondary CAR and Air Bladder III cultures was reduced. The authors
interpreted this phenomenon as a result of general mild suppression
of cellular metabolism, but other possible mechanisms, such as
metabolic interaction, were not excluded. In another study, Shea
(1985) demonstrated quantitative carbaryl inhibition of interferon
synthesis. Supernatants from infected cultures, not treated with
carbaryl, provided 10 times as much antiviral protection as compared
with non-infected cultures. Carbaryl-treated infected culture
provided only twice as much antiviral protection as uninfected
cultures. A similar relationship was observed when comparing the
amount of infectious viral progeny synthesized in the presence of
supernatant from infected cultures not pretreated with carbaryl
(10%), and from infected cultures pretreated with carbaryl (60% of
the amount of virus synthesized in control cultures). The author
speculated about the possibility of superimposition of a viral
infection in a population exposed to pesticides.
Characterization of varicella zoster virus enhancement by
carbaryl was carried out in vitro by Abrahamsen & Jerkofsky (1981)
and Jerkovsky & Abrahamsen (1983). In previous studies, the authors
demonstrated that the replication of varicella zoster virus (a human
herpes virus) is increased 12 to 15-fold by pretreatment of cultures
of human embryonic lung cells (HEL) with carbaryl, and, that
different strains of this virus show differences in sensitivity to
enhancement. Wild-type strains recently isolated from clinical
materials are more sensitive than laboratory adapted strains. In
this study, the authors demonstrated that the maximum enhancement
occurred 48-72 h post-inoculation and that the optimal time for the
pretreatment of monolayers of HEL is 20-24 h. 1-naphthol also
produced increased amounts of virus, but the treated cells cannot
pass on to the daughter cells the ability to enhance virus
replication.
Rodgers et al. (1986) determined that the EC50 for the
inhibition of a T-cell-mediated cytolytic (CTL) response by carbaryl
in vitro was 65.9 µg/ml. The addition of a supernatant containing
liver enzymes reduced the effects of several organophosphorous
pesticides on the CTL response, but had no significant effect on the
potency of carbaryl.
Casale et al. (1989) compared the ability of carbaryl to
interfere with the human serum complement mediated lysis of sheep
blood cells. At a concentration of 3 mmol/litre and with 2-h
preincubation, carbaryl inhibited lysis by 26-45%, depending on the
antibody concentration. Carbaryl was a more potent lysis inhibitor
than diisopropyl phosphorfluoridate or four other anticholinergic
insecticides; the potency was not correlated with anticholinergic
activity.
The effects of carbaryl at concentrations of
0.5-500 µmol/litre on lymphocyte proliferation were studied in
vitro (Bavari et al., 1991). Carbaryl inhibited (3H) thymidine
incorporation, in a concentration-dependent manner, by as much as
50%. Alpha-naphthol also had a slight effect at 50 µmol/litre. The
authors attributed the effect to inhibition of an esterase
responsible for interleukin activation.
8.8.3 Effects in blood
Carbaryl affects the coagulation process. Hyper- and
hypocoagulation were reported in different studies (Hassan & Cueto,
1970; Gapparov, 1974; Lox, 1984; Krug & Berndt, 1985; Krug et al.,
1988).
Gapparov (1974) studied the indices of blood coagulation in
dogs after oral treatment with a daily dose of 2 mg carbaryl/kg body
weight, over 5 months. A clearly manifested hypercoagulation was
established, which was connected with a rise in the general
coagulation activity of the blood, higher thromboplastic activity,
and prothrombin content, increased number of thrombocytes,
accelerated coagulation time, and increased activity of the
fibrinostabilizing factor. Also noted was a decrease in the
recalcification time of blood plasma, fibrinogen concentration, and
free heparin quantity, together with an inhibition of the fibrolytic
activity of the blood. The author interpreted all these changes as
being connected with the arousal of the parasympathetic system.
The blood coagulation time was considerably shortened in
rabbits that were given, orally, a mixture of carbaryl (5 mg/kg),
DDT (5 mg/kg), and parathion (0.5 mg/kg), for 222 days. This effect
corresponded to increased levels of 5-hydroxy-3-indolacetic acid
(5-HIAA) and 4-hydroxy-3-methoxymandelic acid (VMA) in the urine,
indicating an increased rate of metabolism of serotonin and
catecholamine (Hassan & Cueto, 1970). The authors suggested that
this effect was a manifestation of non-specific stress, since
adrenocortical hormones shorten the coagulation time.
Fifteen male Sprague-Dawley rats (250-300 g) were given
drinking-water containing 10 mg carbaryl/litre for 30 days. Platelet
count, prothrombin time, partial thromboplastin time, fibrinogen,
and clotting factor activity for coagulation factors II, V, VII,
VIII, IX, X, and XII were determined. A significant decrease in
platelet count and in the factor VII clotting activity was observed
compared with those in the same number of control rats. Microscopic
evaluation of the liver revealed hepatocyte degeneration, central
vein congestion, some leukocytic infiltration, and vacuolization of
the cytoplasm. The author suggested that carbaryl might have harmful
effects on haemostasis (Lox, 1984).
Carbaryl has an in vitro inhibitory effect on arachidonic
acid-induced platelet aggregation, which corresponds to its
inhibition of thromboxane B2-formation. The results suggest that
carbaryl affected platelet aggregation by inhibition of
cyclooxygenase (the key enzyme of prostaglandin synthesis) by
carbamoylation (Krug & Berndt, 1985). At a concentration of
10 µmol/litre, carbaryl completely blocked platelet aggregation and
cyclo-oxygenase activity (Krug et al., 1988). The carbamoylation
of several platelet proteins, including cyclo-oxygenase, may be
responsible for this effect.
Carbaryl produced, in vitro, a dose-dependent increase in
methaemoglobin (Met Hb) formation at 10 and 100 mg/litre, as well as
decreases in reduced glutathion levels in the erythrocytes of Dorset
sheep with low erythrocyte glucoso-6-phosphate dehydrogenase
(G-6-PD), which is similar to humans who have G-6-PD deficiency.
Carbaryl posed oxidative stress to G-6-PD-deficient red cells,
probably due to its major metabolite alpha-naphthol (Calabrese &
Geiger, 1986). Decreases in the K+ ion concentration in
erythrocytes (with more than 24%) and in haematocrits (from 43.5 to
38%) were found by Sokur (1971) in rats fed carbaryl 0.05 LD50/day
for 2 months.
In an in vitro study, Szczepaniak & Jeleniewicz (1981) found
that carbaryl binds free blood amino acids (plasma and
erythrocytes). These authors also performed a series of in vitro
studies to investigate the effect of carbaryl on amino acids. A
single application of 475 mg carbaryl/kg on 46 treated and 12
control rats produced significantly decreased amino acid values in
the brain, except for valine and phenylalanine. All amino acids
reached the control level after 72 and 120 h. Two hours after
administration, erythrocyte amino acids also decreased >50%
(Szczepaniak & Jeleniewicz, 1980; Jeleniewicz et al., 1984). A
slight decrease in erythrocyte amino acid concentrations was
observed after 30 days with 95 mg carbaryl/kg administered orally
(Jeleniewicz & Szczepaniak, 1980). Blood serum amino acids in 24
rats decreased 4 h after a single application of 189.6 mg/kg. There
was a larger decrease in valine, then in phenylalanine, alanine,
aspergic acid, serine, and glycine (Szczepaniak et al., 1980).
After 15-day dosing of 0.1 LD50(94.8 mg/kg) per day to 12 rats,
there were no changes in levels in serum and erythrocytes, but after
30-day dosing in 8 rats, there was a slight decrease in the alanine
concentration of the erythrocytes (Jeleniewicz & Szczepaniak, 1980).
The effects of carbaryl on the thermoresistance and fractional
content of blood serum proteins was studied by Subbotina &
Belonozhko (1968). A single dose of 150 mg carbaryl/kg administered
to rabbits and multiple applications of 100 mg carbaryl/kg for 2
months showed that, with a single application of carbaryl, there was
an increase in protein thermocoagulation from 28% on day 1 to 67% on
day 7, and with multiple applications of carbaryl there was a
lowering of albumin levels and a rise in globulins (mostly
alpha-globulins) in serum, on day 10. These changes were reversible.
There was also a decrease in the coefficient of thermal dehydration.
The changes in proteins in both studies show lowering of their
lability.
Carbaryl inhibited the incorporation of 3H-uridine and
14C-labelled amino acids into RNA and proteins in cultures of HeLa
cells. The effect was dose-dependent. Incorporation was inhibited by
50% at a 150 µg/ml concentration after 30 min incubation. At a
concentration of 350 µg/ml, only 10% of amino acids and 32% of
uridine incorporation activity were retained (Myhr, 1973). Later,
Blevins & Dunn (1975) showed that carbaryl caused general metabolic
changes in Hela cells. A concentration of 1-2 mg carbaryl/litre
stimulated all divisions and, at 4-8 mg/litre, inhibited growth. A
decrease in cellular protein at 4 mg/litre was noted. Changes in the
phospholipid fraction at 8 mg/litre were probably related to the
alteration of the structure of cellular membranes. Disturbances in
the development of human cells in culture were also reported by
Shpirt (1973). An inhibitory effect of carbaryl on cell development
was demonstrated in Ehrlich ascites tumours in mice. A reduced rate
of incorporation of labelled uridine-5-3H-thymidine methyl-3H
and L-leucine 14C in the RNA, DNA, and proteins in Ehrlich cells
in vitro was also reported (Walker et al., 1975b). The authors
suggested that this effect could be a basis for investigating the
mechanism of the adverse effects that carbaryl has on reproduction.
Carbaryl and N-nitrosocarbaryl appeared to have different
action characteristics with regard to rat liver microsomal membrane
alterations (Beraud et al., 1989b); carbaryl was ineffective on
the lipoperoxidation indices while the nitroso compound had an
inhibitory action on the formation of malonaldehyde and conjugated
dienes as well as on the NADPH-dependent reductase activities.
8.8.4 Effects on the liver and other organs
Several authors reported data on disturbances in the
carbohydrate, protein-forming, and detoxicating functions of the
liver.
A single application of 300 mg carbaryl/kg in rats produced an
increase in albumin and alpha-globulins, and a decrease in ß-and
gamma-globulins (Zapko, 1970).
Kagan et al. (1970) studied the effects of carbaryl on the
liver of 180 rats and 18 rabbits. During an 11-month study, they
gave a daily, oral dose of 38 mg carbaryl/kg to rats. After 1 month,
they observed a rise in the alanine-aminotransferase and
alkaline-phosphatase activities in serum, and a decrease in succinic
dehydrogenase and glycogen in the liver. Doses of 0.76 mg/kg and
0.38 mg/kg, given in the diet to rabbits, caused retention of
bromsulfophthaleine in the blood. The authors also reported a
changed ratio in the protein fractions in serum and an increase in
liver weight. The pathomorphological changes in the liver were
destructive, necrobiotic, and proliferative. An effect on the liver
was also demonstrated in the study of Lox (1987) (see section
8.8.3).
Pavlova et al. (1968) found that, with acute and long-term
exposures, carbaryl affected the oxidative processes in tissues,
because of its direct action on the enzymes of cell respiration and
possible disturbance in the membrane processes. They performed
studies on rats treated with 0.2 LD50 for 3 days and on rats
treated with 0.01 LD50 for 20 weeks. At the higher dose, there
were decreases in the cytochromoxidase and succinedehydrogenase
activities in the liver and brain mitochondria. A histochemical
examination revealed a rather high, irregular activity of the
cytochromoxidase in the heart, as well as increased succinic
dehydrogenase activity. With the long-term dosing, the changes were
not significant, but there was a lowering of the cytochromoxidase
and succinedehydrogenase activities in the heart mitochondria.
Development of experimental cholesterol arteriosclerosis in rabbits
was facilitated by the application of 20 mg carbaryl/kg for 2.5
months (Lukaneva & Rodionov, 1973). Changes were found in the
following indices: general cholesterol, ß-lipoproteins, ECG changes,
and pathomorphological changes in the aorta and coronary vessels.
Carbaryl (100 mg/kg body weight) given to dogs in their diet
for 45 days caused disturbances in the secretion of the intestinal
enzymes. There was an increase in enterokinase secretion, as well as
in the excretion of alkaline phosphates and lipase into the
intestinal juice. The no-observed-effect level (NOEL) for these
effects was 700 µg/kg body weight, which corresponds to 7 mg
carbaryl/kg diet. Three dogs were used in each group (Georgiev,
1967).
8.8.5 Effects on serum glucose
Orzel & Weiss (1966) found that a rise in blood glucose
correlated with the onset and duration of tremors and the degree of
brain ChE inhibition in rats that were treated ip with 5 and 25 mg
carbaryl/kg. The hyperglycaemic response is blocked by
adrenal-ectomy and is unaltered by hypophysectomy. The authors
suggested that the hyperglycaemia was related to increased secretion
of epinephrine. Hyperglycaemia is thought to result from cholinergic
stimulations as it is also found in acute intoxications with
organophosphorous compounds (Kaloyanova, 1963). In their studies,
Orzel & Weiss did not find changes in liver glycogen. Muscle
glycogen was decreased only in non-fasted rats. Elevated levels of
blood glucose and slightly reduced immunoreactive insulin were found
with repeated oral exposure of rats at 3 mg/rat, weekly, for one
year (Wakakura et al., 1978). Glycogen disappeared in most of the
hepatocytes. The cells mainly contained related granular endoplasmic
reticulum with swollen mitochondria. A single application of
30 mg/rat produced transient hypoglycaemia at 20 h followed by
hyperglycaemia at 44 h. The effects of carbaryl on respiration,
glycolysis, and glycogenesis in isolated hepatocytes of male Wistar
rats were studied by Parafita et al. (1984) and Parafita &
Fernandez Otero (1984). The cells were treated with concentrations
of carbaryl of 0.01, 0.1, and 1.0 mmol/litre dissolved in 1%
dimethylsulfoxide (DMSO). DMSO slightly stimulates the respiratory
coefficients. Carbaryl decreased the metabolic output of CO2 at
all concentrations; oxygen consumption was reduced by 40% in
relation to the DMSO-treated groups only at the highest
concentration (1.0 mmol/litre). Glucose remained unchanged in the
presence of carbaryl. Endogenous production of lactic acid was not
affected, and net metabolic production was strongly inhibited by
both DMSO and carbaryl. At a maximum carbaryl concentration
(1 mmol/litre), the net lactic acid production was completely
blocked. Carbaryl inhibited lactate gluconeogenesis, and, to some
extent, gluconeogenesis from fructose pyruvate and alanin. Glycerol
glucogenesis was unaffected. Lactic dehydrogenase activity was
reduced by 38% and glucose 6-phosphate synthetic activity was
increased by 1.0 mmol carbaryl/litre. Aspartate aminotransferase
activities (cytoplasmic and mitochondrial fractions) were inhibited
by 0.1 and 1.0 mmol carbaryl/litre. The results indicated that
carbaryl causes a decrease in glucose production by hepatic cells
and suggested that carbaryl-induced hyperglycaemia in fasted animals
is caused by deficiencies in the peripheral utilization of the
glucose.
8.8.6 Interactions with the drug metabolizing enzyme system
Carbaryl is a weak inducer of hepatic microsomal
drug-metabolizing activity. Cress & Strother (1974) reported
depressed hexobarbital sleeping time in mice given 0.125 or 0.5
LD50 carbaryl, orally, for 10 days. These authors studied the
effects of a 2-week dietary administration of high levels of
carbaryl (10 times higher than the dose given in the earlier study).
Weanling male Swiss-Webster mice, weighing 17-20 g, were divided
into 5 groups of 40 animals each. The daily consumption of carbaryl
by each animal was approximately 119% of the acute LD50. This dose
was well tolerated with no deaths or overt symptoms of
cholinesterase inhibition, probably because carbaryl was
administered over a 24-h period, instead of in a single injection.
Feeding carbaryl resulted in a 44% increase in the rate of in vitro
p-hydroxylation of aniline, and increased in vitro demethylation
of benzphetamine. The hepatic levels of cytochrome P-450 and b5
were increased, but the microsomal protein concentration per gram of
liver was not affected. No changes were reported in
1-naphthol-treated mice. Increased metabolic activity was
demonstrated with phenobarbital and carbaryl. Phenobarbital sleeping
time was shortened to 45 min (control 74 min) in carbaryl-fed mice.
The rate of elimination for the carbaryl-treated animals was twice
as high as that for control animals. The oral LD50 of carbaryl in
carbaryl-pretreated animals (14 days feeding) was three times as
high as in the control animals. 1-Naphthol did not affect the levels
of cytochromes. Sleeping time and the LD50 for carbaryl were not
different in control and 1-naphthol pretreated animals.
Liver weight was low in carbaryl-fed mice, but higher as a
fraction of body weight. The authors concluded that the degree of
the enzyme induction was not high, because most indicators exceeded
the control values by 50% only, except for the lethal dose level.
The pretreatment of rats with 5 daily doses of 10 mg
carbaryl/kg, administered ip, resulted in a 4.2-fold increase in the
rate of benzo( a)pyrene metabolism (Lesca et al., 1984). Total
microsomal P-450 content and benzphetamine demethylase activity were
not significantly affected. The increased ability to hydroxylate
aniline in mice under carbaryl treatment was reported by Guthrie
et al. (1971).
Exposure of murine 3T3 fibroblasts to carbaryl at a
concentration of 10-6 mol/litre was found to increase aryl
hydrocarbon hydroxylase activity (Lahmy et al., 1988). The
magnitude of the effect increased with the duration of exposure.
Carbaryl induction of microsomal enzyme systems in White
Leghorn cockerels was demonstrated by Puyear & Paulson (1972). A
decrease in phenobarbital sleeping time, an increase in liver weight
and elevation of liver aniline hydroxylase activity were found after
oral treatment with carbaryl at 100 mg/kg per day (in gelatine
capsules) for 3 or 6 days. No effect was observed at a dose of
50 mg/kg. Thus, the sleeping time data indicated that the NOEL of
carbaryl for Leghorn cockerels was between 50 and 100 mg/kg per day.
Higher doses caused an increase in liver aminopyrine demethylase
activity (200 or more mg/kg per day) and in liver cytochrome P-450
content (300 or 400 mg/kg per day). Microsomal protein was not
changed. All study parameters returned to control values by day 11
after the treatment was terminated (Puyear & Paulson, 1972).
El-Toukhy et al. (1989) reported that the administration by
gavage of carbaryl to mice at a dose of 166 mg/kg resulted in small,
but statistically significant (15-20%), decreases in liver pyridoxal
phosphokinase and L-tryptophan 2,3-dioxygenase activity.
Administration over a 5-day period of 83 mg carbaryl/kg per day had
no significant effect. Hassan et al. (1990) administered 85 mg/kg,
orally, either once or five times daily. Both the single and
repeated regimens resulted in approximately 40% decreases in
L-tryptophan 2,3-dioxygenase. Carbaryl was also found to be a
competitive inhibitor of this enzyme in vitro.
Inhibition of monoamino-oxidase (MAO) was reported in the
presence of carbaryl in vitro in liver homogenate (Hassan et al.,
1966). The authors suggested that MAO and the catalase system are
involved in oxidative demethylation during carbaryl detoxification.
Exposure of rat liver microsomes to carbaryl in vitro at a
concentration of 0.5 mmol/litre resulted in decreases in the
activities of several monooxygenases (Knight et al., 1986). The
ability of carbaryl to inhibit the N-demethylation of
ethylmorphine appeared to be competitive with a Ki of
2.4x10-4 mol/litre. However, when administered to rats at doses of
up to 25 mg/kg, carbaryl did not have any significant inductive or
inhibitory effect.
Lechner & Abdel-Rahman (1986a) reported that the in vitro
incubation of 2 and 4 mmol carbaryl/litre for 60 min reduced
glutathione levels and increased glutathione S-alkyltransferase in a
rat liver homogenate in a dose- and time-dependent manner. Carbaryl
had no apparent effect on glutathione levels in whole blood with
120 min of incubation. Carbaryl inhibited N-demethylation and
O-demethylation by rat liver microsomes with apparent Ki of 0.1
and 0.7 mmol/litre, respectively (Beraud et al., 1989a).
Carbaryl had an antagonistic action on the release of
microsomal ß-glucuronidase by malathion in a microsomal suspension
in vitro (Lechner & Abdel-Rahman, 1985).
Jacob et al. (1985) studied the effects of carbaryl on the
metabolic activation of the environmental, carcinogenic, polycyclic
aromatic hydrocarbons. Carbaryl was a weak inducer of metabolism of
benz( a)anthracence in the rat liver, and altered its metabolite
profile by shifting from 10, 11-oxidation to 5, 6- and 8,
9-oxidation. Since carbaryl did not induce bay-region activation,
the authors concluded that tumour-promoting activity could not be
expected.
8.8.7 Effects on the endocrine system
The effect of carbaryl on the neuroendocrine system was studied
in rats. Carbaryl was given, orally, at 7, 14, or 70 mg/kg body
weight to male and female rats (each group contained 24 animals
weighing 90-130 g) for a period of 1 year. Growth retardation,
changes in the blood enzymes, and endocrine gland disturbances
varied with the dosage, being especially marked at the end of the
study in the group receiving 70 mg/kg body weight per day.
Acetylcholinesterase and butylcholinesterase activities were
inhibited significantly in these groups. Spermatozoon motility was
reduced progressively with the duration of the exposure. In the
prolonged estrus cycle, the diestrus period was particularly
affected. An increase in the number of corpora lutea and atrophic
follicles in the ovaries was correlated with this disturbance. There
was a dose-dependent increase in the gonadotropic function of the
hypophysis, which was determined by tests on immature mice.
Hypophyseal homogenate from a rat given 70 mg carbaryl/kg increased
the weight of the ovaries by 51.5%, and that of the uterus by 123%,
compared with the weights in control mice. At all doses,
histochemical studies of the hypophysis showed changes indicative of
an increase in the activity of the cells producing a luteinizing
gonadotrophy, i.e., an increase in the size of the cells, loss of
granules, and hyalinization of the cytoplasm. Histological
examination of the adrenal glands revealed an increase in the size
and mitotic activity of cells in the zone glomerulosa. Enlarged
cells with a large nucleus or two nuclei were present in the
fascicular zone. Impairment of thyroid gland functional activity in
the test group was indicated by the reduction in the rate of
absorption and excretion of 131I and its rather low recovery, as
well as by the corresponding histological findings, i.e.,
enlargement of follicles and more dense and basophilic colloid at
all doses. Histological findings were more pronounced in the
70 mg/kg per day groups. It is likely that the effects of carbaryl
on the reproductive organs is mediated by the endocrine glands.
However, the possibility of a direct effect was not excluded by the
authors (Rybakova, 1966; Shtenberg & Rybakova, 1968; Shtenberg
et al., 1970).
The effect of carbaryl on the thyroid gland in rats was studied
by Shtenberg & Hovaeva (1970). Rats were given carbaryl, orally, at
0.7, 2, 5, or 17 mg/kg per day for 6 months. They were fed a normal
iodine diet or an iodine-deficient diet. There was an initial
increase in function in the first 3.5 months of exposure and a
decrease after 6 months of exposure. Recovery was complete 2 months
after the end of the exposure. Decrease in iodine uptake and
reduction of function was 26.5% in rats given 5 mg/kg and 29.5% in
rats given 15 mg/kg. Rats fed an iodine-deficient diet were more
sensitive to carbaryl-induced changes in the thyroid.
The functional states of the thyroid gland and adrenal cortex
were affected by a daily administration of 36 mg carbaryl/kg to rats
for 4 months. The maximum accumulation of 131I occurred after 6 h
and, in the control animals, after 12 h. The 131I absorption rate
normalized after 45 days, but its excretion was delayed. The
concentration of sodium in the blood increased by 36.4% after day 15
and by 74% after 45 days, while the potassium concentrations were
reduced. The daily excretion of 17-corticosteroids in the urine
increased by 35.9%. The changes in the thyroid and adrenal glands
were transient (Dyadicheva, 1971).
Hassan (1971) studied the effects of carbaryl on the synthesis
and degradation of catecholamines in the rat. Rats were given
carbaryl in the diet at concentrations of 100 or 700 mg/kg for 7
months, or in peanut oil at single oral doses of 50, 80, or
250 mg/kg, or 3 single doses of 80 mg/kg body weight. On day 30, the
rats given 700 mg/kg diet eliminated amounts of urinary
4-hydroxy-3-methoxy mandelic acid (VMA) increased by more than 300%;
levels returned to normal after 195 days. A similar effect was
demonstrated after single oral doses with a dose-effect
relationship. The amount of 5-hydroxyindole acetic acid (5-HIAA) in
the urine increased in rats that were treated with a single dose and
3 successive daily doses of 80 mg/kg per day. Adrenalectomy and
hypophysectomy did not change the pattern of VMA excretion produced
by carbaryl. There was an increased turnover rate (68%) of heart
norepinephrine (NE-3H) in rats treated with 80 mg carbaryl/kg, 2 h
before application of NE-3H. The corticosterone level was increased
by about 125% at the same dose level. A carbaryl concentration of
16.2 µg/ml in the blood was enough to trigger maximal corticosterone
secretion. The authors suggested a probable activation of the enzyme
system involved in the catecholamine metabolism, because carbaryl
may also directly affect the adrenergic nerve endings. Activation of
the pituitary adrenal axis and an increase in sympaticoadrenergic
activity with concomitant increased VMA elimination was a
pharmacological effect due to carbaryl treatment.
The effect of a single oral dose of 60 mg carbaryl/kg body
weight on serotonin (5-HT) metabolism in the male Holtzman Albino
rat brain was studied by Hassan & Santolucito (1971); 2, 4, 6, and
24 h after carbaryl application, whole brain samples were analysed
for serotonin, 5-HIAA, and corticosterone. The concentrations of
5-HT and 5-HIAA in the brain increased by 20-30% (5-HT from 0.61 to
0.8; 5-HIAA from 0.25 to 0.31 µg/g) from 2 to 6 h and returned to
normal after 24 h. The plasma corticosterone level was also
increased by about 125%, 1 h after oral administration of carbaryl,
and returned to normal after 20 h. The inhibitory effect of
p-chlorophenylalanine on brain 5-HT formation was diminished by
carbaryl treatment. The serotonin level in the brain of
carbaryl-treated animals pretreated with p-chlorophenylalanine was
72% higher than in the control animals. Increased 5-HIAA levels were
probably a result of an increased rate of synthesis of serotonin.
The authors suggested the possible role of the increased synthesis
of serotonin and tryptophan-5-hydroxylase activity, which could be
related to stress conditions. Carbaryl activation of MAO is probably
related to the increased formation of cerebral 5-HIAA.
The uptake of noradrenaline in vitro by hypothalamic slices,
isolated from rats exposed in vivo to near lethal levels of
carbaryl, was increased in a dose-dependent manner (Jablonska &
Brzezinski, 1990).
Oral administration of 200 mg carbaryl/kg to rats was reported
to raise striatal levels of noradrenaline and homovanillic acid, a
metabolic product of dopamine, when measured 0.5-2 h later (Ray &
Poddar, 1985b).
The mechanism of the stimulation of corticosterone secretion is
not clear.
In vivo studies on male mice receiving 38 and 68 mg
carbaryl/kg showed that carbaryl can increase certain hepatic
androgen hydroxylase activities. In vitro incubation of carbaryl
with prostate tissue and testosterone 1,2-3H stimulated the
formation of dehydrotestosterone-3H, thus, suggesting a direct
action on steroidogenesis. Comparing these results with the effects
of other toxic substances, the authors concluded that carbaryl
cannot exert any major changes in steroid metabolism, nor can it
reduce hormonal disturbances (Dieringer & Thomas, 1974).
Attia et al. (1991) studied the effect of carbaryl on rat
melatonin production as alterations of the latter were found to have
marked consequences in reproduction, immunology, and tumour growth.
Male albino rats ( Rattus rattus), 8 animals in a group, were
exposed to light/dark cycles of 14/10 h. They were treated by
gastric gavage with carbaryl dissolved in corn oil for 6 successive
days. The total doses were 50, 125, and 250 mg/kg. The animals were
killed 2 and 4 h after the onset of darkness, which was 8 or 10 h
after the application of the insecticide. Carbaryl was found to
bring about an augmentation of the pineal melatonin content 4 h
after the onset of darkness, which coincided with the stimulated
N-acetyltransferase levels and hydroxyindole- O-methyltransferase
activity. However, at the same time, carbaryl treatment at all doses
significantly lowered the circulating melatonin titres; this was
supposed to be related to increased hepatic melatonin metabolism due
to the increased activity of the liver drug metabolizing enzyme
system (Gaillard et al., 1977). These results and the established
carbaryl-induced elevated pineal 5-hydroxytryptophan, serotonin, and
5-hydroxyindole acetic acid contents in the course of the night
cycle, support the concept that carbaryl has a significant effect on
pineal melatonin synthesis and secretion.
Kadir & Knowles (1981) reported that carbaryl inhibited rat
brain monamine oxidase activity in vitro.
8.8.8 Other studies
Human serum albumin reacted in vitro with the ester group of
carbaryl and catalysed the hydrolysis and liberation of 1-naphthol.
This reaction is similar to an "esterase type" action (Casida &
Augustinsson, 1959) called carbamoylation (Oonnithan & Casida,
1968).
8.9 Factors modifying toxicity, toxicity of metabolites
8.9.1 Factors modifying toxicity
The toxicity of carbaryl can be modified by altering liver
function by tranylcypromine treatment or 70% hepatectomy. A decrease
in LD50 and increase in ChE activity were more pronounced after
tranylcypromine treatment (Falzon et al., 1983).
The carbaryl LD50 in animals was increased three-fold by
pretreating animals with small doses of carbaryl (Cress & Strother,
1974).
Phenobarbital, administered 24 h before carbaryl treatment,
decreased the acute ip toxicity of carbaryl in mice, while
2-diethyl-amino-ethyl-2,2'-diphenyl-valerate-HC1 (SKF 525-A), given
1 h before carbaryl treatment, increased its acute intraperitoneal
toxicity (Neskovic et al., 1978). Enzyme-mediated binding of
carbaryl to rat hepatic microsomal protein occurred in vitro in
the presence of NADPH and oxygen. Incorporation of radioactivity of
14C-ring labelled carbaryl was studied. A 2- to 3-fold increase in
binding was produced by pretreatment of animals with MFO inducers,
e.g., phenobarbital or 3-methylcholanthrene. SKF-525-A inhibited
binding by approximately 77% of the radioactivity. It is likely that
binding of active carbaryl metabolites formed by MFO occurs. The
radiolabelled metabolic products were covalently bound to amino acid
residues of microsomal protein: 99.3-99.7% of the bound
radioactivity (Miller et al., 1979). A 50% decrease in microsomal
ß-glucuronidase activity was observed 1 h after a single oral
administration of 50 mg carbaryl/kg to female, Sprague-Dawley rats.
The whole liver homogenate ß-glucuronidase content was reduced by
40% after daily treatment with the same dose of carbaryl for 7 days.
This effect was attributed to the decreases in mitochondrial
lysosomal and microsomal ß-glucuronidase. The action of carbaryl in
depleting the endoplasmic reticulum of ß-glucuronidase led to an
increase in serum ß-glucuronidase activity. This was demonstrated in
an in vitro microsomal suspension study which showed that
incubation of 4 mmol carbaryl/litre with microsomal suspensions
effected a release of the ß-glucuronidase enzyme. The effect on the
endoplasmic reticulum was further exemplified by the induction in
microsomal UDP-glucuronyl transferase activity after 7 days of daily
treatment with carbaryl at a dose of 25 mg/kg. Thus, carbaryl
modifies the enzyme activities associated with the endoplasmic
reticulum by decreasing specific activity (90%) towards
ß-glucuronidase enzyme and activating the synthesis of endoplasmic
reticulum protein connected with drug metabolism, such as the
UDP-glucuronyl-transferase enzyme (Abdel-Rahman et al., 1985;
Lechner & Abdel-Rahman, 1985).
Osman & Brindley (1981) conducted bioassays to determine the
carbaryl susceptibility and synergism with piperonyl butoxide in
natural populations of three species of Lobops grassbugs, in order
to estimate monoxygenase detoxification. Pretreatment of the insects
with piperonyl butoxide inhibited the mixed-function oxidase system
and decreased the values of the LC50 for the insects (Osman &
Brindley, 1981). Mixed-function oxydase involvement in the
biochemistry of synergistic insecticides (including carbaryl) has
been reviewed by Casida (1970).
Diet may modify carbaryl toxicity. Boyd & Boulanger (1968)
reported an increased susceptibility to carbaryl toxicity in Albino
rats (272 Wistar strain rats were used) fed a protein-deficient
diet. Boyd & Krijnen (1969) also reported that the LD50 decreased
from 589 mg/kg in rats fed an 81% casein diet to 67 mg/kg in rats
fed a 0% casein diet; 285 male rats were used in this study.
The combined effects of different ambient temperature levels
and carbaryl were studied by Ahdaya et al. (1976). The LD50
values in mice injected ip were 263 mg/kg at 1 °C and 122 mg/kg at
38 °C (588 mg/kg is the LD50 at 27 °C). The thermoregulation
ability of mice treated with carbaryl was affected more than that of
mice treated with parathion which is a stronger inhibitor of ChE.
The authors suggested that this effect was due to an overall
reduction in the basal metabolic rate.
Atropine sulfate decreased signs of parasympathetic stimulation
in a group of pigs acutely poisoned with 1-2 g carbaryl/kg (Smally,
1970). During multiple administrations of carbaryl, drug-induced
(hydrochlorothiazide) diuresis helped in the detoxification
processes. ChE reactivations are contraindicated because they may
aggravate the signs of carbaryl intoxication (Sanderson, 1961;
Podolak & Warchocki, 1980).
Application of atropine or trepazin, 20 min before oral
intoxication with carbaryl in mice, decreased toxicity about 2
times. The results were similar when cholinolytics were applied
2 min after administration of carbaryl (Vyatchanikov & Alexachina,
1968). Atropine administered iv to rats at a dose of 8 mg/kg
increased the carbaryl ip LD50 by a factor of about 7 (70 to
460 mg/kg) (Harris et al., 1989). Co-administration of either of
two different oximes (2-PAM and HI-6) with atropine was found to
provide significantly less protection than that afforded by atropine
alone, indicating that oxime therapy is not a useful treatment for
carbaryl poisoning.
Co-administration of an oral dose of 10 mg malathion/kg in rats
significantly reduced the rates of both absorption and elimination
of an oral dose of 10 mg carbaryl/kg (Lechner & Abdel-Rahman,
1986a). Peak plasma levels of labelled carbaryl were observed about
1 h after the administration of carbaryl alone, and after 2 h when
malathion was administered simultaneously. The terminal rate of
elimination of carbaryl was decreased by a factor of about 4, with
the half-life increasing from 16.96 h to 64.41 h.
Concentrations of cimetidine in the range of 60-240 µg/ml were
observed to decrease the clearance rate of carbaryl by perfused rat
liver in a dose-dependent manner (Ward et al., 1988).
Ray & Poddar (1985a,b, 1990) reported that the intraperitoneal
administration of 1 mg haloperidol/kg, 12.5 to 100 mg
5-hydroxy-tryptamine (5-HTP)/kg, or 100 mg L-tryptophan/kg increased
the incidence of tremors in rats following an oral dose of 50-200 mg
carbaryl/kg. The potentiation of the tremorogenic effect of carbaryl
by 5-HTP was demonstrated to be dose dependent. The potentiating
effects of 5-HTP and haloperidol could be blocked by the
coadministration of the serotonergic antagonist methysergide
(20 mg/kg, ip) or the dopaminergic antagonist bromocriptine
(10 mg/kg, ip), respectively. These results suggest the possible
involvement of a central cholinergic, dopaminergic interaction in
the carbaryl-induced tremor.
The effects of humic acids in the detoxification of carbaryl
during oral application are very slight: only 9.9-18.6% decrease in
toxicity was observed (Golbs et al., 1984).
The combined effects of carbaryl and sodium nitrite have been
studied (Podolak-Majczak & Tyburczyk, 1984, 1986; Tyburczyk &
Podolak-Majczak, 1984a,b; Tyburczyk & Podolak-Majczak, 1986). Dosing
Wistar rats for 3 months with sodium nitrite at 20 mg/kg per day,
and carbaryl at 0.1 LD50/day (60 mg/kg), the brain
gamma-aminobutyryl acid level, methaemoglobin, blood serotonin, free
blood tryptophan, serum and liver alanine amino-transferase activity
levels increased and blood cholinesterase activity decreased.
Decreases were observed in the vitamin E and A levels,
glucose-6-phosphate dehydrogenase activity, and liver aminohexoses
and hydroxyproline levels with a simultaneous increase in the serum
aminohexoses level. This finding may indicate the increasing process
of connective tissue catabolism and lisosomal membranes
labilization.
8.9.2 Toxicity of metabolites
Hydrolysis of the carbamate ester bond of carbaryl results in
detoxification. The carbamate moiety is decomposed to carbon dioxide
and methylamine, and the phenolic part is conjugated and excreted.
Kuhr (1971) summarized the data on the toxicity of carbaryl
metabolites (Table 53).
Table 53. Toxicity of carbaryl and some of its metabolitesa
LD50 (mg/kg) 7-day Molar I50 bovine
NOELb anticholin-esterase
Mouse (mg/kg)
Rat oral Intraperitoneal Rat
Carbaryl 270 29-42 125-250 5 x 10-8
4-Hydroxycarbaryl 1190 74 > 1000 4 x 10-7
5-Hydroxycarbaryl 297 56 > 1000 4.6 x 10-8
7-Hydroxycarbaryl 4760 > 1000
Hydroxymethylcarbaryl > 5000 630-780 250-500 1.4 x 10-5
1-Naphthol 2570 500-1000 1 x 10-3
aFrom: Kuhr (1971).
bNOEL = No-observed-effect level.
Many of the known metabolites are much less toxic than
carbaryl. The pharmacological study of carbaryl in rats showed that
the metabolites with a methylcarbamate moiety are inhibitors of
plasma cholinesterase (Fernandez et al., 1982). None of the
carbaryl metabolites was appreciably more active as a cholinesterase
inhibitor than carbaryl itself (Oonnithan & Casida, 1968).
8.9.3 N-nitrosocarbaryl
Carbaryl is a secondary amine and is, therefore, capable of
nitrosation in the presence of nitro donor groups, such as sodium
nitrate, to give a nitrosamide. This nitrosamide, nitrosocarbaryl,
has been proved to be mutagenic and carcinogenic, at high doses in
animals. Conditions of this nitrosation include a strongly acidic pH
(less than 2), which is comparable with the pH in the human stomach.
However, nitrosocarbaryl is not stable at this pH. Its maximum
stability is between pH 3 and 5, pHs at which no significant amounts
of carbaryl can be nitrosated. Carbaryl has been nitrosated in
several studies, in vitro as well as in vivo, in the guinea-pig,
which has a stomach acidity close to that in man. If significant
yields of nitrosocarbaryl have been shown in these circumstances,
the significance of these findings is still not clear for human risk
evaluation. The pH in the human stomach is variable during food
intake and probably, more importantly, the stomach contains a lot of
food, which will minimize the contact between naturally occurring
nitrite and carbaryl residues, but which will also afford a lot of
nucleophilic sites for nitrosocarbaryl to react with. It is also
noteworthy that all studies that were conducted with jointly
administered carbaryl and nitrite yielded negative results for
oncogenicity. Cranmer (1986) estimated the potential intake of
nitrosocarbaryl at 6x10-9 mg/kg per day. Using different
mathematical models, Cranmer estimated a cancer risk for
nitrosocarbaryl between 10-6 (one-hit model) and 10-9 (probit
model). However, if such oncogenic nitroso-carbamates were to be
found in such quantities in the human stomach, an increased
incidence of stomach cancer would have been expected during the
period when drug and pesticide carbamates were widely used. However,
during this period, the incidence of gastric cancer in the USA
declined considerably.
The bacterial metabolite N-nitrosocarbaryl may act as a
noncompetitive inhibitor of the in vitro metabolism of
aminopyrine, p-nitroanisole, and aniline by rat liver microsomes
(Beraud et al., 1980, 1989a,b). N-nitrosocarbaryl was more
effective as an inhibitor of microsomal activity than the parent
compound.
8.10 Mechanism of toxicity - mode of action
The mechanism of toxicity and the mode of action of carbaryl
and carbamates, in general, have been described in EHC 64 (WHO,
1986).
8.10.1 Inhibition of cholinesterase activity
As a carbamate compound, carbaryl is an inhibitor of
cholinesterase (ChE) activity (for details see Reiner & Aldridge
(1967) and Aldridge (1971)).
A number of studies have been performed by Carpenter et al.
(1961) to assess the extent of ChE inhibition by carbaryl in
mammals. A single oral dose of carbaryl of 560 mg/kg produced a 43%
inhibition of the erythrocyte ChE in rats in 0.5 h, and a 30%
inhibition in brain AChE. However, they returned to normal in rats
that survived 24 h after administration of the dose. Carbaryl did
not depress plasma ChE significantly. Two groups of Beagle dogs were
injected once, iv, with 10 or 15 mg/kg as an 8% solution in 95%
alcohol. No significant effects were found on either erythrocyte or
plasma ChE. On the 5th day, several administrations of the same
doses depressed plasma ChE by 24% and erythrocyte AChE by 40%. It is
doubtful that a long incubation time for the samples (2 h for
plasma) played a role in the slight depression of ChE. Comparative
data on ChE inhibition in the brain, plasma, and erythrocytes of
rats that received single doses of carbaryl were reported by Mount
et al. (1981). As shown in Table 54, there was a dose-dependent
decrease in ChE in the brain, plasma, and erythrocytes.
Brain AChE was significantly different in dead rats and in
surviving rats given 800 mg/kg. AChE depression in the brain was
>70% in the lethally poisoned rats. The inhibition of red blood
cell AChE activity was the same as in plasma.
Table 54. Inhibition of ChE in % in comparison with the controlsa
Sprague-Dawley rats Post-dosing Brain Red blood Plasma
(h) cells
Controls 10 rats 0 0 0
450 mg/kg 0.5 56 44 51
(24 rats sacrificed) 1 74 29 53
2 74 42 68
4 66 48 61
8 55 25 31
24 27 60 38
48 22 23 46
96 6 19 -19
800 mg/kg 0.5 66 32 47
(34 rats sacrificed) 1 70 44 50
2 79 73 69
4 50 72 70
12 50 72 50
28 58 63 68
48 17 26 40
96 16 -6 16
800 mg/kg 3 80 - -
(24 rats died) 4 85 - -
18 88 - -
25 71 - -
29 88 - -
30 84 - -
1200 mg/kg 1-36 88-91 - -
(15 died)
aAdapted from: Mount et al. (1981).
The affinity of AChE of human brain caudate nucleus for
carbaryl was studied in vitro and some inhibition constants were
determined (Patocka & Bajgar, 1971). The value of the I50 affinity
constant was calculated from the dependence of AChE inhibition on
the molar concentration of the inhibitor in probit logarithm
transformation. The Hill coefficient was obtained from Hill plots.
The affinity constant for carbaryl pI50 was 5.59 and the Hill
coefficient was 1.50. According to the authors, some results of this
study suggest that AChE may be an allosteric enzyme.
Intravenous administration of colloidal carbon in the rat,
inhibiting the phagocytic activity of the RES, prolonged the
duration of the anticholinesterase effect of carbaryl (Pipy et al.,
1979). The authors speculated that the metabolism of carbaryl was
decreased by the RES inhibition and, as a result, the reactivation
speed of ChE was slower.
Pregnant rats were treated orally with 1 or 5 mg carbaryl/kg
from day 11 to the last day of gestation (Declume & Benard, 1977b;
Declume et al., 1979). No cholinesterase depression in blood,
brain, and liver were observed in either the dams or the newborn
offspring. However, after administration of 50 mg/kg from day 19 to
the last day of gestation, ChE inhibition was seen in both the
mother and newborn offspring.
Cambon et al. (1978) reported decreased cholinesterase
activity in the blood, brain, and liver in the mother and the fetus
after treatment of the dams at 6.25, 12.5, 25, and 50 mg/kg.
Variability of cholinesterase levels in the controls and
short-comings in the cholinesterase analysis protocol make these
results difficult to interpret.
9. EFFECTS ON HUMAN BEINGS
9.1 General population exposure
9.1.1 Acute toxicity, poisoning incidents
The clinical picture of carbaryl intoxication results from the
accumulation of ACh at nerve endings (WHO, 1986). The signs and
symptoms can be categorized into the following 3 groups:
(a) Muscarinic manifestations
- increased bronchial secretion, excessive sweating,
salivation, and lacrimation;
- pinpoint pupils, bronchoconstriction, abdominal cramps
(vomiting and diarrhoea); and
- bradycardia.
(b) Nicotinic manifestations
- fasciculation of fine muscles (in severe cases, diaphragm
and respiratory muscles also involved); and
- tachycardia.
(c) Central nervous system manifestations
- headache, dizziness, anxiety, mental confusion,
convulsions, and coma; and
- depression of respiratory centre.
All these signs and symptoms can occur in different
combinations and can vary in onset and sequence, depending on the
chemical, dose, and route of exposure. The duration of symptoms is
usually shorter than that observed in organophosphorus poisoning.
Mild poisoning might include muscarinic and nicotinic signs only.
Severe cases always show central nervous system involvement; the
clinical picture is dominated by respiratory failure sometimes
leading to pulmonary oedema, due to the combination of the
above-mentioned syndromes.
A review of carbaryl-related poisoning from 1966 to 1980 was
made by US EPA. During this period, 193 cases of over-exposure to
carbaryl as the sole active ingredient in the poisoning and 144
cases of over-exposure to a combination of carbaryl with other
active ingredients were recorded (Weston, 1982). Not all case
histories have been confirmed. There were 5 deaths. There is no
evidence that carbaryl was involved in these deaths, except for one,
which was acknowledged by the manufacturer.
Hayes (1963) reported two incidents of poisoning: one, a
19-month-old child who swallowed an unknown amount of carbaryl, and
the other, a man who swallowed 250 mg of carbaryl. Both developed
moderately severe ChE inhibition symptoms within 20 min: constricted
pupils, salivation, muscular incoordination in the child, and
epigastric pain, profuse sweating, lassitude, and vomiting in the
man. Both recovered after atropine treatment. Blood cholinesterase
was inhibited.
One death from carbaryl ingestion while drunk was reported by
Farago (1969) who concluded that 2-PAM application hastened the
fatal outcome (due to pulmonary oedema), 6 h after ingestion.
Carbaryl was found in various tissues.
Acute intoxication during the loading of an airplane was
reported by Long (1971).
In a case report of a suicide attempt involving about 25 g
(500 mg/kg) of carbaryl, dicumarin and boric acid, Dickoff et al.
(1987) described the occurrence of a peripheral neuropathy that
resembled the syndrome observed following exposure to some
organophosphorus compounds. Recovery continued for 9 months.
Electrophysiological findings were consistent with axonal peripheral
neuropathy. The cause-effect relationship was confounded because of
combined exposure.
9.1.2 Controlled human studies
Wills et al. (1968) carried out controlled studies in human
volunteers, aged 25-57 years. In a preliminary study, oral doses of
0.5, 1, and 2.0 mg carbaryl/kg in capsules (single application to 2
subjects) were tolerated without any subjective or objective
symptoms. In the main study, one group (5 subjects) took 0.06 mg
carbaryl/kg daily, and one group (6 subjects) took 0.13 mg
carbaryl/kg for 6 weeks. Physical examination, BSP removal from
blood, EEG examination, routine blood and urinalysis were performed,
and ChE of plasma and red blood cells was examined. No changes were
found in the low-dose group. An increase in the ratio of amino acid
nitrogen to creatinine in the urine, at the high dose, may represent
a decrease in the ability of the proximal convoluted tubule to
reabsorb amino acids. This change was reversible.
The urine of some subjects was analysed (Knaak et al., 1968).
The overall recovery (by the fluorometric method) of the carbaryl
equivalents in the urine was 26-28%, and, with the colorimetric
method, 37.8%, in subjects treated with 2 mg carbaryl/kg. The
following metabolites were found chromatographically in a 4-h urine
sample: alpha-naphthol glucuronide (10-15%), and sulfate (6-11%) and
4-(methylcarbamoyloxy)-alpha-naphthyl glucuronide (4%). Another
metabolite, alpha-naphthyl methylimido-carbonate O-glucuronide,
was identified by fluorometry.
A human ingestion study was conducted to determine the
relationship between a single oral dose of carbaryl and the rate of
its urinary excretion as metabolites. Elimination was apparently
first order over the dosage range of the studies (0.25-1 mg/kg). The
model predicts that, 24 h after ingestion, approximately 41% of the
dose can be accounted for as urinary metabolites (Hansen, 1978).
In a study involving one subject, Ward et al. (1988) observed
that pretreatment with a clinical regimen of cimetidine (3x200 mg
over a three-day period) reduced presystemic (first-pass) clearance
of a dose of 1 mg carbaryl/kg by about 46%.
A scientist studying the anthelminthic activity of carbaryl,
tested its human safety by ingesting 250 mg (about 2.8 mg/kg). After
20 min, he experienced epigastric pain and began to sweat profusely.
He was treated with a total dose of 3 mg atropine and recovered
completely 2 h after taking the carbaryl. Another scientist ingested
420 mg carbaryl (4.45 mg/kg), and after 85 min he had vision
troubles, weakness, profuse sweating, and felt lightheaded. He was
treated with a total dose of 4.8 mg atropine and recovered 4 h after
the onset of symptoms (Hayes & Laws, 1991).
9.1.3 Long-term exposure
Branch & Jacqz (1986a,b) reported the case of a 75-year-old man
who was exposed to carbaryl for 8 months, inside his home, after
repeated excessive applications of 10% dust formulation. He
experienced a series of signs and symptoms compatible with
cholinesterase depression in addition to a 40-lb (18-kg) weight
loss. After exposure ceased, the patient's condition improved
markedly. However, a few months later he started to experience
modification of his sleep pattern and peripheral neuropathy and
cerebral atrophy were demonstrated. Other pathologies, such as
recurrent gastric ulcer, cardiac fibrillations, a recent head injury
due to an automobile accident, and other less defined pathologies
were present in this patient which provide other, more likely,
causes for these later symptoms.
The uncertainties associated with long-term exposure to levels
sufficient to result in sustained suppression of plasma
pseudocholinesterase activity and possible brain damage are
discussed by Avashia (1987) and Branch (1987).
9.2 Occupational exposure
9.2.1 Epidemiological studies
The first report on workers exposed to carbaryl was published
by Best & Murray (1962). For 19 months, from the start of carbaryl
production, they studied men working on the production, handling,
and shipping of carbaryl. The most exposed group were bagging
workers occasionally exposed to carbaryl dust under abnormal
conditions (40 mg/m3). These showed a slight depression in blood
ChE activity, but this was below the rate at which clinical symptoms
might be expected. They found that 41% of 689 urine specimens
contained >1000 µg/100 ml total 1-naphthol (>400 µg/100 ml
indicates absorption), with no clinical or subjective symptoms. In
cases of acute intoxication, 3140 µg 1-naphthol/100 ml urine were
found. Knaak et al. (1965) using fluorometric analysis in
conjunction with chromatographic separation, found 5 times more
sulfate (25 mg/litre) than glucuronides (5 mg/litre) of 1-naphthol
in the urine of exposed workers.
Vandekar (1965) in a village-scale trial in Nigeria, assessed
the risk for the population of exposure to carbaryl. A slight
depression of plasma ChE was found in all spraymen, the day after
the spraying. Levels of 1-naphthol derivatives in their urine did
not increase on days 1 and 2 after spraying but increased slightly
on day 6.
In a study on 19 agricultural workers (Yakim, 1967), whole
blood ChE activity was measured before, and after, 3-4 days exposure
to airborne carbaryl. Men exposed to a mean airborne carbaryl
concentration of 2 mg/m3 showed a decrease of 20-24% in ChE
activity. Signalmen exposed to 4 mg/m3 (mean) showed a 13-30%
decrease in ChE activity. No objective signs of ill health were
observed. In the same study, a mean carbaryl concentration of
0.7 mg/m3 was reported in the cabin of an aeroplane used to apply
carbaryl, but no changes were reported in the biological parameters
of the pilots. During the agricultural application of carbaryl dust
at a maximum exposure level of 19 mg/m3 dust on cotton fields,
Adylov (1966) reported a decrease in catalase activity, and a 20%
decrease in ChE activity on day 14.
In cases of occupational overexposure to carbamates, mild
symptoms appear long before a dangerous dose is absorbed, which is
why severe occupational intoxications with carbaryl are rare. Tobin
(1970) gives reasons for the lack of severe intoxications: (1) there
is a very short time (´ h or less) between exposure to carbamates
and the onset of symptoms; (2) lack of symptoms progression, because
of a large margin between the median effective dose and the lethal
dose of carbamates and the early detection of intoxication.
Workers exposed to carbaryl used on pets for flea control,
experienced diarrhoea, increased salivation, cough, difficulty in
breathing, and phlegm (Ames et al., 1989).
Vandekar (1965) reported a skin rash in a sprayman who was
accidentally splashed with a carbaryl formulation. Although
carbamate compounds generally have not caused dermatitis or allergic
skin reactions, Vandekar suggested that they can appear in certain
individuals after unusually heavy exposure.
One out of a group of 30 farmers with contact dermatitis was
identified by a patch test as having a positive allergic reaction to
a 1% solution of carbaryl (Sharma & Kaur, 1990). No allergic
reaction were observed in a control population (No.=20).
To identify possible effects on reproduction, Whorton et al.
(1979) studied a cohort of 47 male workers who had worked for at
least 1 year in the production and packaging of carbaryl. A semen
sample was used for sperm count. Testosterone follicle-stimulating
hormone, and luteinizing hormone were determined by
radioimmunoassay. The range of airborne carbaryl concentrations in
the workplace was 0.03-14.21 mg/m3 with a mean of 4.9 mg/m3.
This cohort showed no seminal or blood abnormalities related to
carbaryl exposure. Although a small excess (not significant at
alpha=0.05) was observed in a small number of oligospermic men in
the exposed group, there was no evidence that testicular function or
fertility in the male workers was affected under these conditions of
exposure.
Wyrobek et al. (1981) used the same cohort of exposed workers
to study sperm abnormalities. Semen was collected from 50 men,
occupationally exposed to carbaryl for 1-18 years. Semen samples
were analysed for changes in sperm motility, sperm count,
morphology, and frequency of sperm-carrying double fluorescent
bodies (YFF). The YFF test represents sperm with two Y chromosomes
due to meiotic nondysjunction. The exposed workers showed changes in
sperm morphology with a higher proportion of sperm with abnormal
head shapes in comparison with the control group of newly hired
unexposed workers. There was no dose dependence as judged by job
classification. A negative correlation between number of years
working in the carbaryl area and the percentage of abnormal sperm
was observed. Workers who had once been exposed to carbaryl, but who
had not been exposed for an average of 6.3 years, showed a
marginally significant elevation in sperm abnormalities, possibly
not reversible. MacLeod (1982) reviewed the Wyrobek et al. (1981)
study and did not find any essential differences in the distribution
of the sperm types in the control and the carbaryl-exposed groups.
No significant changes in sperm count and fertility were reported in
100 workers (Thomas, 1981).
10. PREVIOUS EVALUATION BY INTERNATIONAL BODIES
The latest IARC evaluation of carbaryl was made during 1987
(IARC, 1987). It was concluded that there were no data on cancer in
humans and inadequate evidence of carcinogenicity in experimental
animals. Carbaryl could not be classified with regard to its
carcinogenicity to humans (Group 3).
The FAO/WHO Joint Meeting on Pesticide Residues (JMPR)
evaluated carbaryl at its meetings in 1963, 1965, 1966, 1967, 1968,
1969, 1970, 1971, 1973, 1975, 1976, 1977, 1979 and 1984 (FAO/WHO,
1964, 1965, 1967, 1968, 1969, 1970, 1971, 1972, 1974, 1976, 1977,
1978, 1980, 1985). Since 1973, an acceptable daily intake (ADI) of
0-0.01 mg/kg body weight has been established. This estimate is
based on the following experimental data: no-effect level for rats:
200 mg/kg in the diet = 10 mg/kg per day; for dogs: 100 mg/kg in the
diet = 1.8 mg/kg per day; and for human beings: 0.06 mg/kg per day
(FAO/WHO, 1965, 1967, 1974).
Maximum residue levels (MRLs) for carbaryl were recommended by
FAO/WHO (1986b) (see Table 55). The values recommended for tolerance
levels represent the sum of free carbaryl, combined carbaryl,
conjugated naphthol, and conjugated methylcarbaryl, expressed as
total toxic residues of carbaryl.
A WHO study group on occupational health recommended 5 mg
carbaryl/m3 as a tentative, health-based, maximum permissible
level in the working environment. A biological limit of 30%
inhibition of ChE activity in whole blood, plasma, or red cells with
respect to pre-exposure levels should not be exceeded (WHO, 1982).
In the WHO recommended classification of pesticides by hazard,
technical carbaryl is classified in Class II as moderately hazardous
in normal use (WHO, 1992). WHO/FAO (1975) issued a data sheet on
carbaryl (No. 3).
IRPTC in its series "Scientific reviews of Soviet literature on
toxicity and hazards of chemicals" has published a review on
carbaryl (IRPTC, 1982, 1989).
Table 55. Maximum Residue Limits (MRLs) established by the Codex
Alimentariusa
Commodity MRL
(mg/kg)
Animal feedstuffs (green alfalfa, clover, corn, forage, cow pea 100
foliage, grasses, peanut hay, sorghum forage, soybean vines,
sugarbeet tops, bean and pea vines)
Bran (wheat) 20
Apricots, blackberries, boysenberries, nectarines, peaches, 10
raspberries, asparagus, okra, leafy vegetables (except
brassica), nuts (whole), olives (fresh), sorghum grain, cherries,
plums, kiwi fruit
Blueberries, citrus fruit, cranberries, strawberries 7
Apples, bananas (pulp), grapes, beans, peas (including pod), 5
brassica, tomatoes, peppers, aubergines, pears, poultry skin,
barley, oats, rice (in husk and hulled), rye, wheat
Cucumbers, melons (Cantaloupe), pumpkins, squash 3
Root crop vegetables (beets, carrots, radishes, rutabagas, 2
parsnips), peanuts (ground-nuts, whole), wholemeal flour
Cottonseed (whole), sweet corn (kernels), nuts (shelled), olives 1
(processed), soybeans (dry mature seed), cow-peas
Poultry meat, eggs (without shells) 0.5
Potatoes, meat of cattle, sheep, and goats, sugarbeets, wheat 0.2
flour (white)
Milk and milk products 0.1
aFrom:FAO/WHO (1986b).
REFERENCES
Abdel-Rahman MS, Lechner DWH, & Klein KM (1985) Combination effect
of carbaryl and malathion in rats. Arch Environ Contam Toxicol, 14:
459-464.
Abrahamsen LH & Jerkofsky M (1981) Enhancement of varicella-zoster
virus replication in cultured human embryonic lung cells treated
with the pesticide carbaryl. Appl Environ Microbiol, 41(3): 652-656.
Addison JB, Silk PJ, & Unger I (1975) The photochemistry of
carbamates V. The photodecomposition of Sevin (1-naphthyl- N-methyl
carbamate) and of Phenmec (phenyl- N-methyl carbamate). Int J
Environ Anal Chem, 4: 135-140.
Adylov MA (1966) [Sanitary hygienic assessment of the working
conditions and health state of people working with Sevin in
Uzbekistan.] Gig Primen Toxicol Pestic Klin Otrav, 1866: 271-275 (in
Russian).
Ahdaya SM & Guthrie FE (1982) Stomach absorption of intubated
insecticides in fasted mice. Toxicology, 22(4): 311-317.
Ahdaya SM, Shah PV, & Guthrie FE (1976) Thermoregulation in mice
treated with parathion, carbaryl or DDT. Toxicol Appl Pharmacol, 35:
575.
Ahlrichs JL, Chandler L, Monke EJ, & Reuszler HW (1970) Effect of
pesticide residues and other organotoxicants on the quality of
surface and ground water resources. Lafayette, Indiana, Indiana
Water Research Center, 106 pp (Technical Report No. 10).
Ahmed FE, Hart RW, & Lewis NJ (1977a) Pesticide induced DNA damage
and its repair in cultured human cells. Mutat Res, 42: 161-174.
Ahmed FE, Lewis NJ, & Hart RW (1977b) Pesticide induced ouabain
resistant mutants in Chinese hamster V79 cells. Chem-Biol Interact,
19: 369-374.
Akhundov VY, Lurie LM, & Ismailova IM (1981) [Effect of Sevin on
immunological reactivity.] Gig i Sanit, 46(2): 25-28 (in Russian).
Albanis TA, Pomonis PJ, & Sdoukos AT (1986) Organophosphorous and
carbamates pesticide residues in the aquatic system of Ionnina basin
and Kalamas river (Greece). Chemosphere, 15(8): 1023-1034.
Albright LM & Simmel EC (1977) Behavioural effects of cholinesterase
inhibitor and insecticide carbaryl (Sevin). Paper presented at the
49th Annual Meeting of the Midwestern Physiological Association,
Chicago, 5-7 May 1977.
Aldridge WN (1971) The nature of the reaction of organophosphorous
compounds and carbamates with esterases. Bull World Health Organ,
44: 25.
Alvarez CC, Shimanuki H, & Argauer RJ (1970) Oral toxicity of
carbaryl to adult honey bees. J Econ Entomol, 63(6): 1834-1835.
Aly OM & El Dib MA (1971a) Studies on the persistence of some
carbamate insecticides in the aquatic environment. I. Hydrolysis of
Sevin, Baygon, Pyrolan and Dimetilan in waters. Water Res, 5:
1191-1205.
Aly OM & El Dib MA (1971b) Photodecomposition of some carbamate
insecticides in aquatic environments. In: Faust SJ & Hunter JV ed.
Proceedings of the Rudolfs Research Conference on Organic Compounds
in Aquatic Environments. New York, Basel, Marcel Dekker, Inc., pp
469-493.
Aly OM & El Dib MA (1972) Studies on the persistence of some
carbamate insecticides in the aquatic environment. In: Fate of
organic pesticides in the aquatic environment. Washington, DC,
American Chemical Society, pp 211-241 (Advances in Chemistry Series,
Volume 111).
Aly MI, Bakry N, Kishk F, & El-Sebae AH (1980) Carbaryl adsorption
on calcium-bentonite and soils. J Soil Sci Soc Am, 44(6): 1213-1215.
Amer S (1965) Cytological effects of pesticides. I. Mitotic effects
of N-methyl-1-naphthyl carbamate "Sevin". Cytologia, 30: 175-181.
Amer SM & Farah OR (1968) Cytological effects of pesticides. III.
Meiotic effects of N-methyl-1-naphthyl carbamate "Sevin".
Cytologia, 33: 337-344.
Amer SM, Hammouda MA, & Farah OR (1971) Cytological and
morphological effects of the insecticide N-methyl-1-naphthyl
carbamate "Sevin". Flora (Jena), 160: 433-439.
Ames RG, Brown SK, Rosenberg J, Jackson RJ, Stratton JW, & Quenon SG
(1989) Health symptoms and occupational exposure to flea control
product among California pet handlers. Am Ind Hyg Assoc J, 50(9):
466-472.
Andrawes NR & Bailey RH (1978a) Metabolism of carbaryl in the rat. A
reappraisal (File No. 25051). Research Triangle Park, North
Carolina, Union Carbide Corporation (Unpublished proprietary
information submitted to WHO by Rhône-Poulenc Agro, Lyon).
Andrawes NR & Bailey RH (1978b) Conjugated metabolites of carbaryl:
A comparison between rat and dog. South Charleston, West Virginia,
Union Carbide Research and Development (Project Report No. 811C20)
(Unpublished proprietary information submitted to WHO by
Rhône-Poulenc Agro, Lyon).
Andrawes NR & Bailey RH (1978c) Metabolism of carbaryl in Beagle
dogs. South Charleston, West Virginia, Union Carbide Research and
Development (Project Report No. 811C20) (Unpublished proprietary
information submitted to WHO by Rhône-Poulenc Agro, Lyon).
Andrawes NR & Bailey RH (1979) Carbaryl metabolites detected in the
urine of plant workers (File No. 25957). South Charleston, West
Virginia, Union Carbide Research and Development (Unpublished
proprietary information submitted to WHO by Rhône-Poulenc Agro,
Lyon).
Andrawes NR & Chancey EL (1970) The metabolism of 1-naphthyl-C14
carbaryl in wheat and bean plants. Clayton, North Carolina, Union
Carbide Corporation (Project Report No. 13749) (Unpublished
proprietary information submitted to WHO by Rhône-Poulenc Agro,
Lyon).
Andrawes NR & Myers WR (1976) Identification of carbaryl metabolites
in human urine (File No. 22983). South Charleston, West Virginia,
Union Carbide Corporation (Unpublished proprietary information
submitted to WHO by Rhône-Poulenc Agro, Lyon).
Andrawes NR, Chancey EL, Crabtree RJ, Herrett RA, & Weiden MHJ
(1972) Fate of naphthyl-1-14C carbaryl in laying chickens. J Agric
Food Chem, 20(3): 608-617.
André F, Gillon J, André C, Lafont S, & Jourdan G (1983)
Pesticide-containing diets augment anti-sheep red blood cell
nonreaginic antibody responses in mice but may prolong murine
infection with Giardia muris. Environ Res, 32: 145-150.
Andrianova MM & Alexeev IV (1970) [On the question of the
carcinogenic properties of the pesticides Sevin, Maneb, Ziram and
Zineb.] Vopr Pitan, 6: 71-74 (in Russian).
Anger WK & Setzer JV (1979) Effects of oral and intramuscular
carbaryl administrations on repeated chain acquisition in monkeys. J
Toxicol Environ Health, 5: 793-808.
Antonovich NA (1970) [On the residual quantities of Sevin in food
products.] Gig Primen Toxicol Pestic Klin Otrav, 1970: 176-179 (in
Russian).
Argauer RJ & Warthen JD (1975) Separation of 1- and 2-naphthols and
determination of trace amounts of 2-naphthyl methylcarbamate in
carbaryl formulations by high pressure liquid chromatography with
confirmation by spectrofluorometry. Anal Chem, 47(11): 2472-2474.
Armstrong DA & Millemann RE (1974a) Pathology of acute poisoning
with the insecticide Sevin in the bent-nosed clam, Macom nasuta. J
Invertebr Pathol, 24(2): 201-212.
Armstrong DA & Millemann RE (1974b) Effect of the pesticide carbaryl
on clams and some other intertidal mud flat animals. J Fish Res
Board Can, 31: 466-470.
Armstrong DA & Millemann RE (1974c) Effect of the insecticide Sevin
and its first hydrolytic product 1-naphthol on some developmental
stages of the bay mussel Mytilus edulis. Mar Biol, 28: 11-15.
Arunachalam S & Palanichamy S (1982) Sublethal effects of carbaryl
on surfacing behaviour and food utilization in the air breathing
fish, Macropodus cupanus. Physiol Behav, 29(1): 23-27.
Arunachalam S, Jeyalakshmi K, & Aboobucker S (1980) Toxic and
sublethal effects of carbaryl on a freshwater catfish, Mystus
vittatus (Bloch). Arch Environ Contam Toxicol, 9: 307-316.
Ashwood-Smith M, Trevino J, & Ring R (1972) Mutagenicity of
dichlorvos. Nature (Lond), 240: 418-420.
Atabaev ST (1972) [Carbamate compounds.] In: [Pesticides and hygiene
of the environment in the conditions of warm climate.] Tashkent,
Medizina, pp 114-121 (in Russian).
Attia AM, Reiter RJ, Nonaka KO, Mostafa MH, Soliman SA, & El-Sebae
AH (1991) Carbaryl-induced changes in indoleamine synthesis in the
Pineal gland and its effects on night-time serum melatonin
concentrations. Toxicology, 65: 305-314.
Avashia BH (1987) Is carbaryl safe as its reputation. Letter to the
Editor. Am J Med, 83: 1168-1169.
Azizova OM (1976) [Effect of Sevin and methyl-mercaptophos on
different levels of the nervous system.] In: Medved' LI ed. [Current
problems of the hygiene of application of pesticides in different
climatic and geographical zones. Proceedings of the Itinerary
Scientific Session of Vniigintoks, Erevan.] Kiev, Vniigintoks (in
Russian).
Bansal SK, Verma SR, Gupta AK, & Dalela RC (1980) Predicting
long-term toxicity by subacute screening of pesticides with larvae
and early juveniles of four species of freshwater major carp.
Ecotoxicol Environ Saf, 4: 224-231.
Baron RL (1968) Radioactive lactose in skim milk following
administration of carbonyl-14C-carbaryl to a lactating cow. J
Assoc Off Anal Chem, 51: 1046-1049.
Baron RL & Locke RK (1970) Utilization of cell culture techniques in
carbaryl metabolism studies. Bull Environ Contam Toxicol, 5:
287-291.
Barrett GW (1968) The effect of an acute insecticide stress on a
semi-enclosed grassland ecosystem. Ecology, 49(6): 1019-1035.
Bart J (1979) Effects of acephate and Sevin on forest birds. J Wildl
Manage, 43(2): 544-549.
Basha SM, Rao KSP, Rao KRSS, & Rao KVR (1983) Differential toxicity
of malathion, BHC, and carbaryl to the freshwater fish, Tilapia
mossambica (Peters). Bull Environ Contam Toxicol, 31(5): 543-546.
Bavari S, Casale GP, Gold RE, & Vitzthum EF (1991) Modulation of
interleukin-2 driven proliferation of human large granular
lymphocytes by carbaryl, an anticholinesterase insecticide. Fundam
Appl Toxicol, 17: 61-74.
Belonozhko GA & Kuchak JA (1969) [Electrocortical reactions to
treatment with Sevin in rats.] Gig i Sanit, 34(2): 111-113 (in
Russian).
Benard P, Cambon C, & Declume C (1979) Passage of radioactivity into
the milk of rats treated with [14C] carbaryl. Toxicol Lett, 4:
149-153.
Bend JR, Holder GM, Protos E, & Ryan AJ (1971) Water soluble
metabolites of carbaryl (1-naphthyl N-methylcarbamate) in mouse
liver preparations and in the rat. Aust J Biol Sci, 24: 535-546.
Benson BW, Scott WJ, & Beliles RP (1967) Sevin teratological study
in the mouse (Prepared by Woodard Research Corporation) (Union
Carbide proprietary report, submitted to WHO by Rhône-Poulenc Agro,
Lyon).
Beraud M, Pipy B, Derache R, & Gaillard D (1979) Formation d'un
cancérigène, le N-nitrosocarbaryl, par interaction entre un
insecticide de la série des carbamates, le carbaryl, et le nitrite
de sodium de suc gastrique de rat. Food Cosmet Toxicol, 17(6):
579-583.
Beraud M, Gaillard S, & Derache R (1980) Inhibition of two hepatic
microsomal drug-metabolizing enzymes by a carcinogenic
N-nitrosated pesticide: N-nitrosocarbaryl. Chem-Biol Interact,
31: 103-112.
Beraud M, Forgue M-F, Pipy B, & Derache P (1989a) Interaction of
carbaryl and N-nitrosocarbaryl with microsomal monooxygenase
activities. Toxicol Lett, 45(2-3): 251-260.
Beraud M, Forgue M-F, Pipy B, Didier A, & Carre P (1989b) Lipid
peroxidation in rat liver microsomes: Influence of carcinogenic
N-nitrosocarbaryl. Toxicology, 58: 299-311.
Best EM & Murray BL (1962) Observations on workers exposed to Sevin
insecticide: A preliminary report. J Occup Med, 4(10): 507-517.
Blase W & Loomis TA (1976) The uptake and metabolism of carbaryl by
isolated perfused rabbit lung. Toxicol Appl Pharmacol, 37: 481-490.
Blevins RD & Dunn WC (1975) Effects of carbaryl and dieldrin on the
growth, protein content and phospholipid content of Hela cells. J
Agric Food Chem, 23(3): 377-382.
Blevins RD, Lee M, & Regan JD (1977) Mutagenicity screening of five
methyl carbamate insecticides and their nitroso derivatives using
mutants of Salmonella typhimurium LT2. Mutat Res, 56(1): 1-6.
Bluzat R & Seuge J (1979) Effets de trois insecticides (lindane,
fenthion et carbaryl): toxicité aiguë sur quatre espèces
d'invertébrés limniques; toxicité chronique chez le mollusque
pulmoné lymnea. Environ Pollut, 18: 51-70.
Boethling RS & Alexander M (1979) Effect of concentration of organic
chemicals on their biodegradation by natural microbial communities.
Appl Environ Microbiol, 37(6): 1211-1216.
Bogacka T & Groba J (1980) [Toxicity and biodegradation of
chlorfenvinphos, carbaryl, and propoxur in water environment.]
Bromatol Chem Toksykol, 13(2): 151-158 (in Polish with English
summary).
Bogomolova ZN (1968) [Changes in the content of Sevin in fruitberry
cultures during the process of vegetation, storage and canning.] Gig
Primen Toxicol Pestic Klin Otrav, 1968: 363-367 (in Russian).
Bogomolova ZN (1970) [Content of Sevin in fruit cultures.] Gig
Primen Toxicol Pestic Klin Otrav, 1970: 197-180 (in Russian).
Bogus ER, Gallagher P, Cameron F, & Mumima R (1985) Analysis of
pesticide exposure pads using selective absorption and elution of
reversed-phase solid support. J Agric Food Chem, 33: 1018-1021.
Bollag JM (1979) Transformation of xenobiotics by microbial
activity. In: Proceedings of an EPA Workshop on Microbial
Degradation of Pollutants in Marine Environments. Gulf Breeze,
Florida, US Environmental Protection Agency, Environmental Research
Laboratory, pp 19-27 (EPA 600/9-79-012).
Bollag JM & Liu SY (1971) Degradation of Sevin by soil
microorganisms. Soil Biol Biochem, 3(4): 337-345.
Bollag JM & Liu SY (1972a) Fungal degradation of 1-naphthol. Can J
Microbiol, 18(7): 1113-1117.
Bollag JM & Liu SY (1972b) Hydroxylations of carbaryl by soil fungi.
Nature (Lond), 236: 177-178.
Bollag JM, Czaplicki EJ, & Minard RD (1975) Bacterial metabolism of
1-naphthol. J Agric Food Chem, 23(1): 85-90.
Bollag JM, Sjoblad RD, Czaplicki EJ, & Hoeppel RE (1976)
Transformation of 1-naphthol by the culture filtrate of Rhizoctonia
praticola. Soil Biol Biochem, 8: 7-11.
Bollag JM, Sjoblad RD, & Liu S-Y (1978) Characterization of an
enzyme from Rhizoctonia praticola which polymerizes phenolic
compounds. Can J Microbiol, 25: 229-233.
Borzelleca J & Skalsky HL (1980) The excretion of pesticides in
saliva and its value in assessing exposure. J Environ Sci Health,
B13(6): 843-866.
Bowman BT & Sans WW (1985) Effect of temperature on the water
solubility of insecticides. J Environ Sci Health, B20(6): 625-631.
Bowman MC, Oller W, Kendall D, Dosnell A, & Oliver K (1982) Stressed
bioassay systems for rapid screening of pesticide residues. Part II.
Determination of foliar residues for safe re-entry of agricultural
workers into the field. Arch Environ Contam Toxicol, 11: 447-455.
Boyd EM & Boulanger MA (1968) Insecticide toxicology. Augmented
susceptibility to carbaryl toxicity in albino rats fed purified
casein diets. J Agric Food Chem, 16: 834-838.
Boyd EM & Krijnen CJ (1969) The influence of protein intake on acute
oral toxicity of carbaryl. J Clin Pharmacol, 9(5): 292-297.
Brahmaprakash GP & Sethunathan N (1984) Mineralization of carbaryl
and carbofuran in soil planted to rice. Rice Newsl, 5(3-4): 2.
Brahmaprakash GP & Sethunathan N (1985) Metabolism of carbaryl and
carbofuran in soil planted to rice. Agric Ecosyst Environ, 13(1):
33-42.
Branch RA (1987) The reply. Letter to the Editor. Am J Med, 83:
1169.
Branch RA & Jacqz E (1986a) Is carbaryl as safe as its reputation?
Does it have the potential for causing chronic neurotoxicity in
humans? Am J Med, 80: 659-664.
Branch RA & Jacqz E (1986b) Subacute neurotoxicity following
long-term exposure to carbaryl. Am J Med, 80: 741-745.
Brankovan O (1972) Meiotic effects in corn in embryonic and
generative stages induced by Sevin 50. Arhiv Poljopr Nauke, 90:
125-132.
Briggs GG (1981) Adsorption of pesticides by some Australian soils.
Aust J Soil Res, 19: 61-68.
Brzeskii VV (1972) The study of the mutagenic properties of an
insecticide from the carbamate group - Sevin. Sov Genet, 8: 798-800.
Buchanan DV, Millemann RE, & Stewart NE (1970) Effects of the
insecticide Sevin on various stages of the dungeness crab, Cancer
magister. J Fish Res Board Can, 27: 93-104.
Bukin AL (1965) [Toxicity of Sevin for mammalia and birds.] Gig
Primen Toxicol Pestic Klin Otrav, 1965: 431-435 (in Russian).
Bukin AL & Filatov GV (1965) [Sevin toxicity for mammalia and
birds.] Veterinaria, 42: 93-95 (in Russian).
Burdick GE, Dean HJ, & Harris EJ (1960) Effect of Sevin upon the
aquatic environment. NY Fish Game J, 7(1): 14-25.
Bursian SJ & Edens FW (1977) The prolonged exposure of Japanese
quail to carbaryl and its effect on growth and reproductive
parameters. Bull Environ Contamin Toxicol, 17(3): 360-368.
Bursian SJ & Edens FW (1978) The effect of acute carbaryl
administration on various neurochemical and blood chemical
parameters in the Japanese quail. Toxicol Appl Pharmacol, 46:
463-473.
Bushy Run (1983a) Acute toxicity and irritancy study. Export,
Pennsylvania, Bushy Run Research Center (Project Report No. 46-96)
(Unpublished proprietary information submitted to WHO by
Rhône-Poulenc Agro, Lyon).
Bushy Run (1983b) Sevin 99% technical: Acute toxicity and irritancy
study. Export, Pennsylvania, Bushy Run Research Center (Project
Report No. 46-71) (Unpublished proprietary information submitted to
WHO by Rhône-Poulenc Agro, Lyon).
Bushy Run (1983c) Sevin 80 sprayable. Acute toxicity and irritancy
study. Export, Pennsylvania, Bushy Run Research Center (Project
Report No. 46-97) (Unpublished proprietary information submitted to
WHO by Rhône-Poulenc Agro, Lyon).
Butler PA (1962) Effects on commercial fisheries. In: Effects of
pesticides on fish and wildlife: A review of investigations during
1960. Washington, DC, US Department of the Interior, Fish and
Wildlife Service, pp 11-25 (Circular No. 143).
Butler PA (1963) Commercial fisheries investigations. In:
Pesticide-wildlife studies: A review of Fish and Wildlife Service
investigations during 1961 and 1962. Washington, DC, US Department
of the Interior, Fish and Wildlife Service, pp 11-25 (Circular No.
167).
Cairns T, Siegmund GA, & Doose MG (1983) Potential identification
problems with carbaryl. Bull Environ Contam Toxicol, 30: 93-98.
Calabrese EJ & Geiger CP (1986) Low erythrocyte glusose-6-phosphate
dehydrogenase (G-6-PD) activity and susceptibility to carbaryl
induced methaemoglobin formation and glutathion depletion. Bull
Environ Contam Toxicol, 36(4): 506-509.
Cambon C, Declume Ch, & Derache R (1978) Inhibitions of
acetylcholinesterase from foetal and maternal tissues after oral
intake of carbaryl (1-naphthyl- N-methyl carbamate) by pregnant
rats. Biochem Pharmacol, 27: 2647-2648.
Cambon C, Fernandez J, Falzon M, & Mitjavila S (1981) Variations of
digestive absorption kinetics of carbaryl with the nature of the
vehicle. Toxicology, 22(1): 45-51.
Cameron EA & Mastro VC (1976) Forest insect and disease management /
Evaluation Report: Effectiveness of disparlure in combination with
Sevin 4 oil for Gipsy moth suppression in Pennsylvania 1974. Upper
Darby, Pennsylvania, US Department of Agriculture, Northeastern Area
State and Private Forestry Service.
Cameron EA, Loerch CR, & Mumma RO (1985) Incidental and indirect
exposure to three chemical insecticides used for control of the
Gypsy moth, Lymantria Dispar (L.). Z Angew Entomol, 99: 241-248.
Carey AE & Kutz FW (1985) Trends in ambient concentrations of
agrochemicals in humans and the environment in USA. Environ Monit
Assess, 5(2): 155-164.
Carlson AR (1972) Effects of long-term exposure to carbaryl (Sevin)
on survival, growth and reproduction of the fathead minnow
(Pimephales promelas). J Fish Res Board Can, 29(5): 583-587.
Caro HJ, Freeman HP, & Turner BC (1974) Persistence in soil and
losses in run off of soil incorporated carbaryl in a small
watershed. J Agric Food Chem, 22(5): 860-863.
Carpenter M (1990) Hydrolysis of 14C-carbaryl in aqueous solutions
buffered at pH 5, 7, and 9. Columbia, Missouri, Analytical
Bio-Chemistry Laboratories, Inc. (ABC final report No. 38380)
(Unpublished proprietary information submitted to WHO by
Rhône-Poulenc Agro, Lyon).
Carpenter CP, Weil CW, Palm PH, Woodside MW, Nair JH III, & Smith HF
(1961) Mammalian toxicity of 1-naphthyl- N-methyl carbamate (Sevin
insecticide). J Agric Food Chem, 9: 30-39.
Casale GP, Bavari S, & Connolly JJ (1989) Inhibition of human serum
complement activity by diisopropylfluorophosphate and selected
anticholinesterase insecticides. Fundam Appl Toxicol, 12(3):
460-468.
Casida JE (1970) Mixed-function oxidase involvement in the
biochemistry of insecticide synergists. J Agric Food Chem, 18: 753.
Casida JE & Augustinsson KB (1959) Reaction of plasma albumin with
1-naphthyl- N-methylcarbamate and certain other esters. Biochim
Biophys Acta, 35: 411-426.
Casper HH, Pekas JC, & Dinusson WE (1973) Gastric absorption of a
pesticide (1-naphthyl N-methylcarbamate) in the fasted rat. Pestic
Biochem Physiol, 2: 391-396.
Chaiyarach S, Ratananun V, & Harrel RC (1975) Acute toxicity of the
insecticides toxaphene and carbaryl and the herbicides propanil and
molinate to four species of aquatic organisms. Bull Environ Contam
Toxicol, 14(3): 281-284.
Chancey EL (1974) Sevin carbaryl insecticide: metabolism of carbaryl
in apple fruit (File No. 19496). Clayton, North Carolina, Union
Carbide Corporation (Unpublished proprietary information submitted
to WHO by Rhône-Poulenc Agro, Lyon).
Chancey EL & Andrawes NR (1971a) The absorption and metabolism of
1-naphthyl-C14 carbaryl in corn and rice plants. Clayton, North
Carolina, Union Carbide Corporation (Project Report No. 15712)
(Unpublished proprietary information submitted to WHO by
Rhône-Poulenc Agro, Lyon).
Chancey EL & Andrawes NR (1971b) Metabolism and translocation of
1-naphthyl-C14 carbaryl in tomato fruit and foliage and potato
foliage. Clayton, North Carolina, Union Carbide Corporation (Project
Report No. 15715) (Unpublished proprietary information submitted to
WHO by Rhône-Poulenc Agro, Lyon).
Chapman RA & Cole CM (1982) Observations on the influence of water
and soil pH on the persistence of insecticides. J Environ Sci
Health, B17(5): 487-504.
Chaudhry GR & Wheeler WB (1988) Biodegradation of carbamates. Water
Sci Technol, 20(11/12): 89-94.
Chernoff N & Kavlock RJ (1982) An in vivo teratology screen
utilizing pregnant mice. J Toxicol Environ Health, 10: 541-550.
Chernoff N, Setzer RW, Miller DB, Rosen MB, & Rogers JM (1990)
Effects of chemically induced maternal toxicity on prenatal
development in the rat. Teratology, 42: 651-658.
Chib JS (1985a) The adsorption/desorption of 1-naphthyl
methylcarbamate on five soils (File No. 33820). Research Triangle
Park, North Carolina, Union Carbide Agricultural Products Co., Inc.
(Unpublished proprietary information submitted to WHO by
Rhône-Poulenc Agro, Lyon).
Chib JS (1985b) Sevin: Aerobic aquatic metabolism of carbaryl (File
No. 34234). Research Triangle Park, North Carolina, Union Carbide
Agricultural Products Co., Inc. (Unpublished proprietary information
submitted to WHO by Rhône-Poulenc Agro, Lyon).
Chib JS (1985c) Sevin (1-naphthyl methylcarbamate): Anaerobic
aquatic metabolism of carbaryl (File No. 34086). Research Triangle
Park, North Carolina, Union Carbide Agricultural Products Co., Inc.
(Unpublished proprietary information submitted to WHO by
Rhône-Poulenc Agro, Lyon).
Chib JS (1986a) Sevin brand carbaryl insecticide. Bioconcentration
and fate of carbaryl in bluegill sunfish (Lepomis macrochirus).
Research Triangle Park, North Carolina, Union Carbide Corporation
(Report No. 801R10) (Unpublished proprietary information submitted
to WHO by Rhône-Poulenc Agro, Lyon).
Chib JS (1986b) Sevin brand carbaryl insecticide: Photodegradation
of carbaryl on soil (File No. 34598). Research Triangle Park, North
Carolina, Union Carbide Agricultural Products Co., Inc. (Unpublished
proprietary information submitted to WHO by Rhône-Poulenc Agro,
Lyon).
Chib JS & Andrawes NR (1985) Carbaryl (1-naphthyl methylcarbamate) -
Soil mobility study (File No. 33770). Research Triangle Park, North
Carolina, Union Carbide Agricultural Products Co., Inc. (Unpublished
proprietary information submitted to WHO by Rhône-Poulenc Agro,
Lyon).
Chib JS, Noeth SP, & Smith BK (1986) Sevin brand carbaryl
insecticide. Bioconcentration and fate of carbaryl in Bluegill
Sunfish (Lepomis Macrochirus) (File No 34540). Research Triangle
Park, North Carolina, Union Carbide Agricultural Products Co., Inc.,
Research and Development Department (Unpublished proprietary
information submitted to WHO by Rhône-Poulenc Agro, Lyon).
Chin YN & Sudderuddin KI (1979) Effect of methamidophos on the
growth rate and esterase activity of the common carp Cyprinus
carpio L. Environ Pollut, 18(3): 213-220.
Chin BH, Eldridge JM, & Sullivan LJ (1974) Metabolism of carbaryl by
selected tissues using an organ-maintenance technique. Clin Toxicol,
7: 37-56.
Chin BH, Sullivan LJ, Eldridge JM, & Tallant J (1979a) Metabolism of
carbaryl by kidney, liver, and lung from human postembryonic fetal
autopsy tissue. Clin Toxicol, 14(5): 489-498.
Chin BH, Eldridge JM, Anderson JH, & Sullivan LJ (1979b) Carbaryl
metabolism in the rat. A comparison of in vivo, in vitro (tissue
explant), and liver perfusion techniques. J Agric Food Chem, 27(4):
716-720.
Christenson I (1964) Alkaline hydrolysis of some carbamic esters.
Acta Chem Scand, 18: 904-922.
Cifone MA (1989) Mutagenicity test on carbaryl technical in the in
vitro rat primary hepatocyte unscheduled DNA synthesis assay.
Final report (HLA Study No. 10862-0-435). Kensington, Maryland,
Hazelton Laboratories America, Inc. (Unpublished proprietary
information submitted to WHO by Rhône-Poulenc Agro, Lyon).
Clayson DB (1987) The need for biological risk assessment in
reaching decisions about carcinogens. Mutat Res, 185(3): 243-269.
Collins TFX, Hansen WH, & Keeler HV (1971) The effect of carbaryl
(Sevin) on reproduction of the rat and the gerbil. Toxicol Appl
Pharmacol, 19: 202-216.
Comer SW, Staiff DC, Armstrong JF, & Wolf HR (1975) Exposure of
workers to carbaryl. Bull Environ Contam Toxicol, 13(4): 385-391.
Coulston F, Rosenblum I, & Dougherty WJ (1974) Teratogenic
evaluation of carbaryl in the Rhesus monkey (Macacca mulatta).
Holloman Air Force Base, New Mexico, International Center of
Environmental Safety, Albany Medical College, 54 pp (Unpublished
proprietary information submitted to WHO by Rhône-Poulenc Agro,
Lyon).
Courtemanch DL & Gibbs KE (1978) The effects of Sevin-4-oil on
lenthic communities. In: Environmental monitoring of cooperative
spruce budworm control projects, Maine 1976 and 1977. Augusta,
Maine, Maine Department of Conservation, Forest Service, pp 141-150.
Courtemanch DL & Gibbs KE (1980) Short- and long-term effects of
forest spraying of carbaryl (Sevin-4-oil) on stream invertebrates.
Can Entomol, 112: 271-276.
Coutant CC (1964) Insecticide Sevin: Effect of aerial spraying on
drift of stream insects. Science, 146: 420-421.
Cranmer MF (1986) Carbaryl. A toxicological review and risk
analysis. Neurotoxicology, 7(1): 247-332.
Cress CR & Strother A (1974) Effects on drug metabolism of carbaryl
and 1-naphthol in the mouse. Life Sci, 14: 861-872.
Crofton KM, Howard JL, Moser VC, Gill MW, Reiter LW, Tilson HA, &
MacPhail RC (1991) Interlaboratory comparison of motor activity
experiments: Implications for neurotoxicological assessments.
Neurotoxicol Teratol, 13: 599-609.
Crosby DG, Leitis E, & Winterlin WL (1965) Photodecomposition of
carbamate insecticides. J Agric Food Chem, 13(3): 204-205.
Currier WW, MacCollom GB, & Baumann GI (1982) Drift residues of
air-applied carbaryl in an orchard environment. J Econ Entomol,
75(6): 1062-1068.
Czaplicki E (1979) Persistence in the soil of some more important
insecticides applied in Poland. I. Dynamics of insecticide
disappearance in the soil. Pr Nauk Inst Ochr Rosl, 21(2): 7-40.
Czaplicki EJ & Bollag JM (1975) Microbial degradation of 1-naphthol.
Biul Inst Ochr Rosl, 59: 241-248.
Das YT (1990a) Photodegradation of (1-naphthyl-14C) carbaryl in
aqueous solution buffered at pH 5 under artificial sunlight.
Piscataway, New Jersey, Innovative Scientific Services, Inc. (Report
No. 90060) (Unpublished proprietary information submitted to WHO by
Rhône-Poulenc Agro, Lyon).
Das YT (1990b) Photodegradation of (1-naphthyl-14C) carbaryl on
soil under artificial sunlight. Piscataway, New Jersey, Innovative
Scientific Services, Inc. (Report No. 90061) (Unpublished
proprietary information submitted to WHO by Rhône-Poulenc Agro,
Lyon).
Davies JI & Evans WC (1964) Oxidative metabolism of naphthalene by
soil Pseudomonads. The ring-fission mechanism. Biochem J, 91:
251-261.
Davis HC (1961) Effects of some pesticides on eggs and larvae of
oysters (Crassostrea virginica) and clams (Venus mercenaria).
Commer Fish Rev, 23(12): 8-23.
Davis CW (1986a) Sevin brand carbaryl insecticide: Dissipation of
soil residues under field use conditions (File No. 34620). Research
Triangle Park, North Carolina, Union Carbide Agricultural Products
Co., Inc. (Unpublished proprietary information submitted to WHO by
Rhône-Poulenc Agro, Lyon).
Davis CW (1986b) Sevin brand carbaryl insecticide: Magnitude of
carbaryl residues on rangeland and pasture grasses (File No. 34821).
Research Triangle Park, North Carolina, Union Carbide Agricultural
Products Co., Inc. (Unpublished proprietary information submitted to
WHO by Rhône-Poulenc Agro, Lyon).
Davis CW (1987) Sevin brand carbaryl insecticide: Comparison of the
magnitude of carbaryl residues resulting from applications of XLR
plus and 50 W formulations to tree fruit, leafy vegetables, and
cereals (File No. 35357). Research Triangle Park, North Carolina,
Union Carbide Agricultural Products Co., Inc. (Unpublished
proprietary information submitted to WHO by Rhône-Poulenc Agro,
Lyon).
Davis CW & Thomas SJ (1987) Carbaryl insecticide. Section D-Residues
barley (File No. 40092). Research Triangle Park, North Carolina,
Union Carbide Agricultural Products Co., Inc. (Unpublished
proprietary information submitted to WHO by Rhône-Poulenc Agro,
Lyon).
Declume C & Benard P (1977a) Foetal accumulation of [14C] carbaryl
in rats and mice autoradiographic study. Toxicology, 8: 95-105.
Declume C & Benard P (1977b) Etude autoradiographique de la
distribution d'un agent anticholinestérasique, le 1-naphthyl-
N-methyl [14C] carbamate chez la ratte gestante. Toxicol Appl
Pharmacol, 39: 451-460.
Declume C & Benard P (1978) Etude de la pharmacocinétique du
14C-carbaryl chez la souris gestante. Toxicol Eur Res, 1: 174-180.
Declume C & Derache M (1976) Biodisponibilité du 14C-carbaryl
après l'administration oral à la ratte gestante. C R Acad Sci Paris,
D283: 1799-1801.
Declume C & Derache M (1977) Passage placentaire d'un carbamate
anticholinestérasique à activité insecticide: Le carbaryl.
Chemosphere, 6: 141-146.
Declume C, Cambon C, & Derache R (1979) The effects on new-born rats
of repeated carbaryl administration during gestation. Toxicol Lett,
3: 191-196.
Degraeve N, Moutschen-Dahmen M, Houbrechts N, & Colizzi A (1976) A
propos des risques d'un insecticide: le carbaryl utilisé seul et en
combinaison avec les nitrites. Bull Soc R Sci Liège, 45(1-2): 46-57.
DeGiovanni-Donnelly R, Kolbye SM, & Greeves PD (1968) The effect of
IPC, CIPC, Sevin and Zectran on Bacillus subtilis. Experientia
(Basel), 24: 80-81.
Desi I, Gonczi L, Simon G, Farkas I, & Kneffel Z (1974)
Neurotoxicological studies of two carbamate pesticides in subacute
animal experiments. Toxicol Appl Pharmacol, 27: 456-476.
Deuel LE, Brown KW, Price JD, & Turner FT (1985) Dissipation of
carbaryl and the 1-naphthol metabolite in the flooded rice fields. J
Environ Qual, 14(3): 349-354.
Deutsch-Wenzel P, Brune H, Grimmer G, & Misfeld J (1985) Local
application to mouse skin as a carcinogen specific test system for
non-volatile nitroso compounds. Can Lett, 29: 85-92.
Dickoff DJ, Gerber O, & Turovsky Z (1987) Delayed neurotoxicity
after ingestion of carbamate pesticide. Neurology, 37(7): 1229-1231.
Dieringer CS & Thomas JA (1974) Effects of carbaryl on the
metabolism of androgens in the prostate and liver of the mouse.
Environ Res, 7: 381-386.
Dikshith TSS (1991) Mutagenicity and teratogenicity of pesticides.
In: Dikshith TSS ed. Toxicology of pesticides in animals. Boston,
Boca Raton, Ann Arbor, CRC Press, pp 189-198.
Dikshith TSS, Gupta PK, Gaur JS, Datta KK, & Mathur AK (1976)
Ninety-day toxicity of carbaryl in male rats. Environ Res, 12:
161-170.
Dikshith TSS, Kumar SN, Raizada RB, Srivastava MK, & Ray PK (1990)
Residues of 1-naphthol in soil and water samples in and around
Bhopal, India. Bull Environ Contam Toxicol, 44: 87-91.
Dinerman AA, Lavrent'eva NA, & Il'inskaia NA (1970) [The embryotoxic
action of some pesticides.] Gig i Sanit, 35: 39-42 (in Russian).
Dinoeva SK (1974) [Dynamics of the changes in the immunological
structures of lymphatic follicles in spleen during pesticide
poisoning.] Gig i Sanit, 39(3): 85-87 (in Russian).
Dinoeva S (1982) Humoral immune response in 1st, 2nd and 3rd
generations guinea-pigs in the period of reactivation after
intoxication. In: Immunology of reproduction. Proceedings of the
Fifth International Symposium, Varna, Bulgaria, 26-29 May 1982.
Sofia, Bulgarian Academy of Sciences Press, pp 393-395.
Dionne E, Kendall TB, & Suprenant D (1985) Acute toxicity of
carbaryl technical to embryos-larvae of Eastern oysters
(Crassostrea virginica). Wareham, Massachusetts, Springborn
Bionomics, Inc. (Report No. BW-85-7-1817) (Unpublished proprietary
information submitted to WHO by Rhône-Poulenc Agro, Lyon).
Dittert LW & Higuchi T (1963) Rates of hydrolysis of carbamate and
carbonate esters in alkaline solution. J Pharm Sci, 52: 852-857.
Dorough HW (1967) Carbaryl-C14 metabolism in a lactating cow. J
Agric Food Chem, 15(2): 261-266.
Dorough HW (1969) Carbaryl cow study No. 2. Lyon, Rhône-Poulenc Agro
(Unpublished proprietary information).
Dorough HW (1970a) Continuous feeding of carbaryl naphthyl-C14 to
lactating cows. Progress report. Lyon, Rhône-Poulenc Agro (Document
No. 25, Section C, Part III) (Unpublished proprietary information).
Dorough HW (1970b) Metabolism of insecticidal methylcarbamates in
animals. J Agric Food Chem, 18(6): 1015-1022.
Dorough HW (1971) Carbaryl residues in milk and meat of dairy
animals. Presented at the International Symposium on Pesticide
Terminal Residues, Tel-Aviv, Israel, 17-19 February 1971 (Document
No. 27, Section C, Part III).
Dorough HW (1973) Metabolism of carbamate insecticides. Washington,
DC, US Environmental Protection Agency, Office of Research and
Development, 246 pp (EPA-650/1-74-002).
Dorough HW (1982) Inhalation toxicology: Starting with the
environment. Environ Toxicol Chem, 1(3): 213-220.
Dorough HW & Casida JH ( 1964) Nature of certain carbamate
metabolites of the insecticide Sevin. J Agric Food Chem, 12(4):
294-304.
Dorough HW, Leeling NL, & Casida JE (1963) Nonhydrolytic pathway in
metabolism of N-methylcarbamate insecticides. Science, 140:
170-171.
Dougherty WJ, Goldberg L, & Coulston F (1971) The effect of carbaryl
on reproduction in the monkey (Macacca mulatta). Toxicol Appl
Pharmacol, 19: 365.
Duck BJ & Woolias M (1985) Reverse-phase high performance liquid
chromatographic determination of carbaryl in postmortem specimens. J
Anal Toxicol, 9: 177-179.
Duggan RE & Corneliussen PE (1972) Dietary intake of pesticide
chemicals in the United States (III). June 1968-April 1970. Pestic
Monit J, 5(4): 331-341.
Duggan RE, Lipscomb GQ, Cox EL, Heatwole RE, & Kling RC (1971)
Residues in food and feed. Pesticide residue levels in foods in the
United States from July 1, 1963, to June 30, 1969. Pestic Monit J,
5(2): 73-124.
Durham WF & Wolfe HR (1962) Measurements of the exposure of workers
to pesticides. Bull World Health Organ, 26: 75-91.
Dyadicheva TV (1971) [Experimental data on thyroid and adrenal
cortex function during prolonged effect of carbamine pesticides.]
Vrach Delo, 2: 120-123 (in Russian).
Dzwonkowska A & Hubner H (1986) Induction of chromosomal aberrations
in the Syrian hamster by insecticides tested in vivo. Arch
Toxicol, 58(3): 152-156.
Earl FL, Miller E, & Van Loon EJ (1973) Reproductive teratogenic and
neonatal effects of some pesticides and related compounds in beagle
dogs and miniature swine. In: Deichman W ed. Pesticides and the
environment: A continuing controversy. Proceedings of the 8th
Inter-American Conference on Toxicology and Occupational Medicine,
Miami, Florida, pp 253-266.
Edmiston CE Jr, Goheen M, Malaney GW, & Mills WL (1985) Evaluation
of carbamate toxicity: Acute toxicity in a culture of Paramecium
multimicronucleatum upon exposure to aldicarb, carbaryl, and
mexacarbate as measured by Warburg respirometry and acute plate
assay. Environ Res, 36(2): 338-350.
Egert G & Greim H (1976) Formation of mutagenic N-nitroso
compounds from the pesticides prometryne, dodine and carbaryl in the
presence of nitrite at ph 1. Mutat Res, 37: 179-186.
Eheart JF, Turner EC, & Dickinson J (1962) Residues of Sevin in
whole milk from sprayed and dusted cows. J Entomol, 55(4): 504-505.
Eichelberger J & Lichtenberg J (1971) Persistence of pesticides in
river water. Environ Sci Technol, 5: 541-544.
Eisenbrand G, Ungerer O, & Preussmann R (1975) The reaction of
nitrite with pesticides. II. Formation, chemical properties and
carcinogenic activity of the N-nitroso derivatives of N-methyl-
1-naphthyl carbamate (carbaryl). Food Cosmet Toxicol, 13: 365-367.
Eisenbrand G, Schmähl D, & Preussmann R (1976) Carcinogenicity in
rats of high oral doses of N-nitrosocarbaryl, a nitrosated
pesticide. Can Lett, 1: 281-284.
Elespuru RK & Lijinsky W (1973) The formation of carcinogenic
nitroso compounds from nitrite and some types of agricultural
chemicals. Food Cosmet Toxicol, 11: 807-817.
Elespuru R, Lijinsky W, & Setlow JK (1974) Nitrosocarbaryl as a
potent mutagene of environmental significance. Nature (Lond), 247:
386-387.
Elkins ER (1989) Effect of commercial processing on pesticide
residues in selected fruits and vegetables. J Assoc Off Anal Chem,
72(3): 533-535.
Elkins ER, Lamb FC, Farrow RP, Cook RW, Kawai M, & Kimball JR (1968)
Removal of DDT, malathion and carbaryl from green beans from
commercial and home preparative procedures. J Agric Food Chem,
16(6): 962-966.
Elliot-Feeley E & Armstrong JR (1982) Effects of fenitrothion and
carbaryl on Xenopus laevis development. Toxicology, 22(4):
319-335.
Epstein SS, Arnold E, Andrea J, Bass W, & Bishop Y (1972) Detection
of chemical mutagens by the dominant lethal assay in the mouse.
Toxicol Appl Pharmacol, 23: 288-325.
Estesen BJ, Buck NA, & Ware GW (1982) Dislodgeable insecticide
residues on cotton foliage: carbaryl, cypermethrin, and
methamidophos. Bull Environ Contam Toxicol, 28(4): 490-493.
Eto M, Seifert J, Engel J, & Casida J (1980) Organophosphorous and
methylcarbamate teratogens: Structural requirements for reducing
embryonic abnormalities in chickens and kynurenine formamidase
inhibition in mouse liver. Toxicol Appl Pharmacol, 54: 20-30.
Eto M, Kuwano E, Yoshikawa M, Suiko M, Kohno M, Kamihata M, &
Nakatsu S (1982) Mutagenicity and anticholinesterase activity of
possible metabolites of aryl N-methylcarbamates. J Fac Agric
Kyushu Univ, 26(4): 213-219.
Fait DW (1984) Sevin XLR acute inhalation toxicity test. Export,
Pennsylvania, Bushy Run Research Center (Report No. 47-84)
(Unpublished proprietary information submitted to WHO by
Rhône-Poulenc Agro, Lyon).
Falzon M, Fernandez Y, Cambon C, Gros C, & Mitjavila S (1983)
Influence of experimental hepatic impairment on the toxicokinetics
and the anticholinesterase activity of carbaryl in the rat. J Appl
Toxicol, 3(2): 87-89.
FAO (1982) Second Government Consultation on International
Harmonization of Pesticides Registration Requirements, Rome, 11-15
October 1982. Rome, Food and Agriculture Organization of the United
Nations.
FAO/WHO (1964) Carbaryl. In: Evaluation of the toxicity of
pesticides residues in food. Report of a Joint Meeting of the FAO
Committee on Pesticides in Agriculture and the WHO Expert Committee
on Pesticide Residues, Geneva, 30 September-7 October 1963. Geneva,
World Health Organization, pp 132-135 (FAO Meeting Report No.
PL:1963/13; WHO/Food Add./23).
FAO/WHO (1965) Carbaryl. In: Evaluation of the toxicity of pesticide
residues in food. Geneva, World Health Organization, pp 31-35 (FAO
Meeting Report No. PL:1965/10/1; WHO Food Add./27.65).
FAO/WHO (1967) Carbaryl. In: 1966 Evaluation of some pesticide
residues in food. Geneva, World Health Organization, pp 31-38 (FAO
PL/CP/15; WHO Food Add./67.32)
FAO/WHO (1968) Carbaryl. In: 1967 Evaluation of some pesticide
residues in food. Geneva, World Health Organization, pp 15-25 (FAO
Report No. PL: 1967/M/11/1; WHO Food Add./68.30).
FAO/WHO (1969) Carbaryl. In: 1968 Evaluation of some pesticide
residues in food. Geneva, World Health Organization, pp 35-38 (FAO
PL:1968/M/9/1; WHO Food Add./69.35).
FAO/WHO (1970) Carbaryl. In: 1969 Evaluations of some pesticide
residues in food. Geneva, World Health Organization, pp 45-57 (FAO
PL:1969/M/17/1; WHO Food Add./70.38).
FAO/WHO (1971) Carbaryl. In: 1970 Evaluations of some pesticide
residues in food. Geneva, World Health Organization, pp 3-6 (AGP
1979/M/12/1; WHO Food Add./71.42).
FAO/WHO (1972) 1971 Evaluations of some pesticide residues in food.
Geneva, World Health Organization (WHO Pesticide Residue Series No.
1).
FAO/WHO (1974) Carbaryl. In: 1973 Evaluations of some pesticide
residues in food. Geneva, World Health Organization, pp 141-176 (WHO
Pesticide Residue Series No. 3).
FAO/WHO (1976) Carbaryl. In: 1975 Evaluations of some pesticide
residues in food. Geneva, World Health Organization, pp 83-84 (WHO
Pesticide Residue Series No. 5).
FAO/WHO (1977) Carbaryl. In: 1976 Evaluations of some pesticide
residues in food. Rome, Food and Agriculture Organization of the
United Nations, pp 95-123 (AGP 1977/M/14).
FAO/WHO (1978) Carbaryl. In: Pesticide residues in food. Evaluations
1977. Rome, Food and Agriculture Organization of the United Nations,
pp 47-50 (FAO Plant Production and Protection Paper 10 Sup).
FAO/WHO (1980) Carbaryl. In: Pesticide residues in food. Evaluations
1979. Rome, Food and Agriculture Organization of the United Nations,
pp 101-102 (FAO Plant Production and Protection Paper 20 Sup).
FAO/WHO (1985) Carbaryl. In: Pesticide residues in food - 1984. The
monographs. Rome, Food and Agriculture Organization of the United
Nations, pp 79-83 (FAO Plant Production and Protection Paper 67).
FAO/WHO (1986a) Guide to Codex recommendations concerning pesticide
residues. Part 8. Recommendations for methods of analysis of
pesticide residues, 3rd ed. Rome, Food and Agriculture Organization
of the United Nations, Codex Alimentarius Commission.
FAO/WHO (1986b) Codex maximum limits for pesticide residues, 2nd ed.
Rome, Food and Agriculture Organization of the United Nations, Codex
Alimentarius Commission (CAC/Vol. XIII).
Farage-Elawar M (1989) Enzyme and behavioural changes in young
chicks as a result of carbaryl treatment. J Toxicol Environ Health,
26(1): 119-131.
Farage-Elawar M (1990) Effects of in ovo injection of carbamates
on chick embryo hatchability, esterase enzyme activity and
locomotion of chicks. J Appl Toxicol, 10(3): 197-201.
Farago A (1969) [Fatal suicidal case of Sevin (1-naphthol- N-methyl
carbamate) poisoning.] Arch Toxicol, 24: 309-315 (in German).
Farrow RP, Lamb FC, Cook RW, Kimball JR, & Elkins ER (1968) Removal
of DDT, malathion and carbaryl from tomatoes by commercial and home
preparative methods. J Agric Food Chem, 16(1): 65-71.
Farrow RP, Lamb FC, Elkins ER, Cook RW, Kawai M, & Cortes A (1969)
Effect of commercial and home preparative procedures on parathion
and carbaryl residues in broccoli. J Agric Food Chem, 17(1): 75-79.
Federle PF & Collins WJ (1976) Insecticide toxicity to three insects
from Ohio ponds. Ohio J Sci, 76(1): 19-24.
Feldmann RJ & Maibach HT (1974) Percutaneous penetration of some
pesticides and herbicides in man. Toxicol Appl Pharmacol, 28:
126-132.
Fernandez Y, Falzon M, Cambon C, Gros C, & Mitjavila S (1982)
Carbaryl tricompartmental toxicokinetics and anticholinesterase
activity. Toxicol Lett, 13(3-4): 253-258.
Ferreira GAL & Seiber JN (1981) Volatilization and exudation losses
of 3- N methylcarbamate insecticides applied systemically to rice.
J Agric Food Chem, 29(1): 93-99.
Field WE (1980a) Oral LD50 in rats of carbaryl, technical grade,
made in West Virginia. Clarks Summit, Pennsylvania, Chemicals, Drugs
and Cosmetics Research (Report No. CDC-UC-046-79) (Unpublished
proprietary information submitted to WHO by Rhône-Poulenc Agro,
Lyon).
Field WE (1980b) Oral LD50 in rats of carbaryl, technical grade,
made in India. Clarks Summit, Pennsylvania, Chemicals, Drugs and
Cosmetics Research (Report No. CDC-UC-047-79) (Unpublished
proprietary information submitted to WHO by Rhône-Poulenc Agro,
Lyon).
Filatov DV & Brytskov VE (1972) [Enzymic method of residue
determination of chlorophos and Sevin by means of indicator paper.]
Probl Vet Sanit, XLI: 233-241 (in Russian).
Fisher SW & Lohner TW (1986) Studies on the environmental fate of
carbaryl as a function of pH. Arch Environ Contam Toxicol, 15(6):
661-667.
Fisher HL, Most B, & Hall LL (1985) Dermal absorption of pesticides
calculated by deconvolution. J Appl Toxicol, 5(3): 163-177.
Fletcher DW & Leonard MI (1986a) Toxicity and reproduction study
with carbaryl technical in Bobwhite quail. Neillsville, Wisconsin,
Bio-Life Associate Ltd (Report No. 85-DR-15) (Unpublished
proprietary information submitted to WHO by Rhône-Poulenc Agro,
Lyon).
Fletcher DW & Leonard MI (1986b) 28-day dietary toxicity and
reproduction pilot study with carbaryl technical in Mallard ducks.
Neillsville, Wisconsin, Bio-Life Associate Ltd (Report No. 85 DR
P14) (Unpublished proprietary information submitted to WHO by
Rhône-Poulenc Agro, Lyon).
Flores-Ruegg E, De Andrea MM, Helene CG, & Hirata R (1980)
Persistence of pesticide residues in Brazilian soil samples related
to organic matter and microbiological activity. In: Meeting of the
Advisory Group on Agrochemical Residue Biota Interaction: Proceeding
report. Sao Paulo, Brazil, Biological Institute, pp 181-187.
Frank R, Braun HE, & Ripley BD (1989) Monitoring Ontario grown
apples for pest control chemicals used in their production, 1978-86.
Food Addit Contam, 6(2): 227-234.
Frank R, Braun HE, Ripley BD, & Pitblado R (1991) Residues of nine
insecticides and two fungicides in raw and processed tomatoes. J
Food Prot, 54(1): 41-46.
Fukuto TR (1973) Metabolism of carbamate insecticides. In: Di Carlo
FJ ed. Drug metabolism reviews. New York, Basel, Marcel Dekker,
Inc., vol 1, pp 117-151.
Gaillard D, Chamoiseau G, & Derache R (1977) Interaction du carbaryl
et du nitrite de sodium sur les activités enzymatiques de quelques
mono-oxygénases microsomiques du foie de rat. J Pharmacol, 8: 437.
Gaines TB (1960) The acute toxicity of pesticides to rat. Toxicol
Appl Pharmacol, 2: 88-99.
Gaines TB (1969) Acute toxicity of pesticides to rat. Toxicol Appl
Pharmacol, 14: 514-534.
Gangwar SK, Kavadia VS, Gupta HCL, & Srivastava BP (1978a)
Persistence of carbaryl in sandy loam soil and its uptake in bajra,
Pennistetum typhoides P. Ann Arid Zone, 17(4): 357-362.
Gangwar SK, Kavadia VS, Srivastava BP, & Gupta HCL (1978b)
Persistence of carbaryl residues in sandy loam soil and its uptake
by root crops. Indian J Plant Prot, 8(1): 11-18.
Gapparov Sh (1974) [Haemocoagulation indices in dogs chronically
intoxicated with low doses of Sevin.] [Health Health Care Organ
Uzbekistan], II: 146-149 (in Russian).
Gartrell MJ, Craun JC, Podrebarac DS, & Gunderson EL (1986)
Pesticides, selected elements, and other chemicals in infant and
toddler total diet samples, October 1980-March 1982. J Assoc Off
Anal Chem, 69(1): 123-145.
Georgiev IN (1967) [The effect of toxic and maximal permissible
amounts of Sevin on intestinal secretion.] Gig i Sanit, 32(8): 42-46
(in Russian with English summary).
Ghadiri M & Greenwood DA (1966) Toxicity and biologic effects of
malathion, phosdrin and Sevin in the chick embryo. Toxicol Appl
Pharmacol, 8: 342.
Ghosh P, Bhattacharya S, & Bhattacharya S (1990) Impairment of the
regulation of gonadal function in Channa punctatus by metacid 50
and carbaryl under laboratory and field conditions. Biomed Environ
Sci, 3(1): 106-112.
Gibbs E, Mingo TM, & Courtemanch DL (1981) The effect on pond
macroinvertebrates from forest spraying of carbaryl and its
persistence in water and sediment. Augusta, Maine, Maine Department
of Conservation, Forest Service.
Gibbs KE, Mingo TM, & Courtemanch DL (1984) Persistence of carbaryl
(Sevin-4-oil) in woodland ponds and its effects on ponds macro
invertebrates following forest spraying. Can Entomol, 116: 203-214.
Gill SS & Yeoh CL (1980) Degradation of carbaryl in three components
of the paddy-field ecosystem of Malaysia. In: Meeting of the
Advisory Group on Agrochemical Residue Biota Interaction: Proceeding
report. Sao Paulo, Brazil, Biological Institute, pp 229-243.
Gill TS, Pant JC, & Pant J (1988) Gill, liver, and kidney lesions
associated with experimental exposures to carbaryl and dimethoate in
the fish (Puntius conchonius Ham.). Bull Environ Contam Toxicol,
41(1): 71-78.
Gladenko IN & Malinin OA (1970) [Toxicity of Sevin for livestock.]
Gig Primen Toxicol Pestic Klin Otrav, 1970: 188-193 (in Russian).
Golbs S, Kühnert M, & Leue F (1975) [Prenatal toxicological study of
Sevin (carbaryl) for Wistar rats.] Arch Exp Veterinaermed, 29:
607-614 (in German with English summary).
Golbs S, Kühnert M, & Fuchs V (1984) [Influence on the acute
toxicity of selected pesticides by huminic acids.] Z Gesamte Hyg,
30(12): 720-723 (in German).
Gold RE, Leavitt JR, Holcslaw T, & Tupy D (1982) Exposure of urban
applicators to carbaryl. Arch Environ Contam Toxicol, 11: 63-67.
Goldberg HI, Johnson HE, & Knaak JB (1965) Inhibition of discrete
avoidance behaviour by three anticholinesterase agents.
Psychopharmacologia, 7(1): 72-76.
Guirguis GN & Brindley WA (1975) Carbaryl penetration into and
metabolism by alfalfa leafcutting bees, Megachile pacifica. Agric
Food Chem, 23(2): 274-279.
Guirguis GN & Brindley WA (1976) Effect of chlorcyclizin,
aminopyrine or phenobarbital on carbaryl metabolism in alfalfa
leafcutting bees. Environ Entomol, 5(3): 590-594.
Gunderson EL (1988) FDA total diet study, April 1982-April 1984.
Dietary intake of pesticides, selected elements, and other
chemicals. J Assoc Off Anal Chem, 71(6): 1200-1209.
Gupta SK & Sundararaman V (1991) Correlation between burrowing
capability and AChE activity in the earthworm, Pheretima posthuma,
on exposure to carbaryl. Bull Environ Contam Toxicol, 46: 859-865.
Guseinov MK (1970) [On the question of circulation of Sevin in
environment.] Gig Primen Toxicol Pestic Klin Otrav, 1970: 174-176
(in Russian).
Guthrie FE, Monro RJ, & Abernathy CO (1971) Response of laboratory
mouse to selection for resistance to insecticides. Toxicol Appl
Pharmacol, 18: 92-101.
Gyrisco GG, Lisk DJ, Fertig SN, Huddleston EW, Fox FH, Holland RF, &
Teimberger GW (1960) The effects of feeding high levels of Sevin on
residue, flavour, and odour of the milk of dairy cattle. Agric Food
Chem, 8(5): 409-410.
Haines TA (1981) Effect of an aerial application of carbaryl on
brook trout (Salvelinus fontinalis). Bull Environ Contam Toxicol,
27(4): 534-542.
Hamada NN (1987) One year oral toxicity study in Beagle dogs with
carbaryl technical. Vienna, Virginia, Hazleton Laboratories America,
Inc. (Report No. 400-715) (Unpublished proprietary information
submitted to WHO by Rhône-Poulenc Agro, Lyon).
Hamada NN (1990) Acute oral toxicity study in rats. Carbaryl
technical. Vienna, Virginia, Hazleton Laboratories America, Inc.
(Unpublished proprietary information submitted to WHO by
Rhône-Poulenc Agro, Lyon).
Hamada NN (1991a) Subchronic toxicity study in dogs with carbaryl
technical. Vienna, Virginia, Hazleton Laboratories America, Inc.
(Report No. N656-152) (Unpublished proprietary information submitted
to WHO by Rhône-Poulenc Agro, Lyon).
Hamada NN (1991b) Oncogenicity with carbaryl technical in CD-1(R)
mice. Vienna, Virginia, Hazleton Laboratories America, Inc. (Report
No. N656-138) (Unpublished proprietary information submitted to WHO
by Rhône-Poulenc Agro, Lyon).
Hamada NN (1991c) Combined chronic toxicity and oncogenicity study
with carbaryl technical in Sprague-Dawley rats - 52 week interim
report with 4-week recovery. Vienna, Virginia, Hazleton Laboratories
America, Inc. (Study No. 656-139) (Unpublished proprietary
information submitted to WHO by Rhône-Poulenc Agro, Lyon).
Hanazato T & Yasuno M (1987) Effects of a carbamate insecticide,
carbaryl, on the summer phyto- and zooplankton communities in ponds.
Environ Pollut, 48: 145-159.
Hanazato T & Yasuno M (1989) Influence of over-wintering Daphnia
on spring zooplankton communities: An experimental study. Ecol Res,
4: 323-338.
Hanazato T & Yasuno M (1990a) Influence of Chaoborus density on
the effects of an insecticide on zooplankton communities in ponds.
Hydrobiologia, 194: 183-197.
Hanazato T & Yasuno M (1990b) Influence of time in application of an
insecticide on recovery patterns of a zooplankton community in
experimental ponds. Arch Environ Contam Toxicol, 19: 77-83.
Hansen JL (1978) Carbaryl: Human ingestion study statistical
analysis (File No. 24971). Research Triangle Park, North Carolina,
Union Carbide Corporation (Unpublished proprietary information
submitted to WHO by Rhône-Poulenc Agro, Lyon).
Hansen CR & Kawatski JA (1976) Application of 24-hour post-exposure
observation to acute toxicity studies with invertebrates. J Fish Res
Board Can, 33: 1198-1201.
Harris LW, Talbot BG, Lennox WJ, & Anderson DR (1989) The
relationship between oxime induced reactivation of carbamylated
acetylcholinesterase and antidotal efficacy against carbamate
intoxication. Toxicol Appl Pharmacol, 98(1): 128-133.
Harry JB (1977) A review of human exposure to Sevin carbaryl
insecticide with particular reference to the USA. Research Triangle
Park, North Carolina, Union Carbide Agricultural Products Co., Inc.
(Unpublished proprietary information submitted to WHO by
Rhône-Poulenc Agro, Lyon).
Hart ER (1972) Teratology study. Sevin, vitamin A, aspirin and
malathion. South Charleston, West Virginia, Union Carbide
Corporation, 8 pp (Prepared by Bionetics Research Laboratories)
(Unpublished proprietary information submitted to WHO by
Rhône-Poulenc Agro, Lyon).
Hassan A (1971) Pharmacological effects of carbaryl. The effect of
carbaryl on the synthesis and degradation of catecholamines in the
rat. Biochem Pharmacol, 20: 2299-2308.
Hassan A & Cueto C Jr (1970) Biochemical effects in the rabbit of
repeated administration of a mixture of DDT, carbaryl and parathion.
Z Naturforsch, B25: 521-525.
Hassan A & Santolucito JA (1971) Pharmacological effects of
carbaryl. II. Modification of serotonin metabolism in the rat brain.
Experientia (Basel), 27: 287-288.
Hassan A, Zayed SMAD, & Abdel-Hamid FM (1966) Metabolism of
carbamate drugs. I. Metabolism of 1-naphthyl- N-methylcarbamate
(Sevin) in the rat. Biochem Pharmacol, 15: 2045-2055.
Hassan AA, El Sewedy SM, Minatogawa Y, & Kido R (1990) Effects of
some insecticides on several enzymes of tryptophan metabolism in
rats. J Environ Sci Health, B25(3): 333-346.
Haven D, Castagna M, Chanley P, Wass M, & Whitcomb J (1966) Effects
of the treatment of an oyster bed with polystream and Sevin.
Chesapeake Sci, 7(4): 179-188.
Haydock CI (1964) An experimental study to control oyster drills in
Tomales Bay, California. Calif Fish Game, 50: 11-28.
Hayes WJ (1963) Clinical handbook on economic poisons. Emergency
information for treating poisoning. Atlanta, Georgia, US Department
of Health, Education and Welfare, Communicable Disease Center, pp
44-46.
Hayes WJ Jr & Laws ER Jr ed. (1991) Carbaryl. In: Handbook of
pesticide toxicology. Volume 3 - Classes of pesticides. New York,
San Diego, Academic Press, Inc., pp 1144-1189.
Hazleton Laboratories America, Inc. (1982) Acute oral toxicity study
in rats - Sevin RF. Vienna, Virginia, Hazleton Laboratories America,
Inc. (Unpublished proprietary information submitted to WHO by
Rhône-Poulenc Agro, Lyon).
Heise GA & Hudson JD (1985a) Effects of pesticides and drugs on
working memory in rat: Continuous delayed response. Pharmacol
Biochem Behav, 23: 591-598.
Heise GA & Hudson JD (1985b) Effects of pesticides and drugs on
working memory in rats: Continuous non-match. Pharmacol Biochem
Behav, 23: 599-605.
Hernandez DA, Lombardo RL, Ferrari L, & Tortorelli MC (1990)
Toxicity of ethyl-parathion and carbaryl on early development of sea
urchin. Bull Environ Contam Toxicol, 45: 734-741.
Heywood DL (1975) Degradation of carbamate insecticides in soil.
Environ Qual Saf, 4: 128-133.
Hill EF & Camardese MB (1986) Lethal dietary toxicities of
environmental contaminants and pesticides to Coturnix. Washington,
DC, US Department of the Interior, Fish and Wildlife Service (Fish
and Wildlife Technical Report No. 2).
Hill EF, Heath RG, Spann JW, & Williams JD (1975) Lethal dietary
toxicities of environmental pollutants to birds. Washington, DC, US
Department of the Interior, Fish and Wildlife Service (Special
Scientific Report, Wildlife No. 191).
Hodgson E & Casida JE (1961) Metabolism of N:N-dialkyl carbamate and
related compounds by rat liver. Biochem Pharmacol, 8: 179-191.
Hoffman DJ & Albers PH (1984) Evaluation of potential embryotoxicity
and teratogenicity of 42 herbicides, insecticides, and petroleum
contaminants to mallard eggs. Arch Environ Contam Toxicol, 13:
15-27.
Hoque H (1972) Radio tracer studies on chemical residues in food and
agriculture. Proc. Comb. Panel Res. Coord. Meet. 1971.
Hoque MZ (1972) Carbaryl - A new chemical mutagen. Curr Sci, 41(23):
855-856.
Houston JB, Upshall DG, & Bridges JW (1974) Pharmacokinetics and
metabolism of two carbamates, carbaryl and landrin, in the rat.
Xenobiotica, 5(10): 637-648.
Hudson RH, Tucker RK, & Haegele MA (1984) Handbook of toxicity of
pesticides to wildlife, 2nd ed. Washington, DC, US Department of the
Interior, Fish and Wildlife Service (Resource Publication 153).
Hughes L Jr (1971) A study of the fate of carbaryl insecticide in
surface waters. Lafayette, Indiana, Purdue University (Ph.D
Dissertation) (University microfilms, High Wycombe, England).
Hughes LB & Reuszer HW (1970) A study of bacterial decomposition of
carbaryl in farm pond waters. Lafayette, Indiana, Purdue University,
Water Resources Research Center (Technical Report No. 10).
Hundley HK, Cairns T, Luke AM, & Masumoto HT (1988) Pesticide
residue findings by the Luke method in domestic and imported foods
and animal feeds for fiscal years 1982-1986. J Assoc Off Anal Chem,
71(5): 875-892.
Huque H (1972) Preliminary report on the residues of carbaryl
granules in rice plants. Radiotracer Stud. Chem. Residues Food Agr.,
Proc. Comb. Panel Res. Coord. Meet. 1971. Vienna, International
Atomic Energy Agency, pp 107-109.
Hurlburt E, McMillan R, & Vialle M (1989) Supplemental environmental
impact statement: use of the insecticide carbaryl to control ghosts
and mud shrimp in oyster beds of Willapa Bay and Grays Harbour.
Olympia, Washington, Washington State Department of Fisheries and
Washington State Department of Ecology (Unpublished report submitted
to WHO by Rhône-Poulenc Agro, Lyon).
Hurwood IS (1967) Studies on pesticide residues. 2. Carbaryl
residues in the body tissues and milk of cattle following dermal
application. Qld J Agric Anim Sci, 24: 69-74.
Hwang SW & Schanker S (1974) Absorption of carbaryl from lung and
small intestine of the rat. Environ Res, 7(2): 206-211.
IARC (1987) Overall evaluations of carcinogenicity: An updating of
IARC monographs, volumes 1 to 42. Lyon, International Agency for
Research on Cancer (IARC Monographs on the Evaluation of the
Carcinogenic Risk of Chemicals to Humans, Supplement 7).
Imming RJ, Shaffer BC, & Woodard G (1969) Sevin, safety evaluation
by feeding to female Beagles from day one of gestation through
weaning of the offspring. (Prepared by Woodard Research Corporation
for Union Carbide Agricultural Products Co., Inc., Research Triangle
Park) (Unpublished proprietary information submitted to WHO by
Rhône-Poulenc Agro, Lyon).
Innes JRM, Ulland MB, Valerio MG, Petrucel L, Fishbein L, Hart ER,
Pallotta AJ, Bates RR, Falk HL, Gart JJ, Klein M, Mitchell I, &
Peters H (1969) Bioassay of pesticides and industrial chemicals for
tumorigenicity in mice: a preliminary note. J Natl Cancer Inst, 42:
1101-1114.
IRPTC (1982) In: Izmerov NF ed. IRPTC scientific reviews of Soviet
literature on toxicity and hazard of chemicals No. 7: Carbaryl.
Moscow, Centre of International Projects, GKNT, 13 pp.
IRPTC (1989) In: Izmerov NF ed. [Scientific reviews of Soviet
literature on toxicity and hazard of chemicals No. 7: Carbaryl], 2nd
ed. Moscow, Centre of International Projects, GKNT, 82 pp (in
Russian).
Ishidate M Jr & Odashima S (1977) Chromosome tests with 134
compounds on Chinese hamster cells in vitro: a screening for
chemical carcinogens. Mutat Res,48: 337-354.
Ishidate M Jr, Sofuni T, & Yoshikawa K (1981) Chromosomal aberration
tests in vitro as a primary screening tool for environmental
mutagens and/or carcinogens. Gann Monogr Cancer Res, 27: 95-108.
Ivanova LN & Molozhanova YG (1973) [Static and dynamic distribution
of carbaryl in the environment.] Gig i Sanit, 38(2): 24-28 (in
Russian).
Iwata Y, Dusch ME, Garman GE, & Gunther FA (1979) Worker environment
research: Residues for carbaryl, chlorobenzilate, dimethoate and
trichlorfon applied to citrus trees. J Agric Food Chem, 27(6):
1141-1145.
Jablonska J & Brzezinski J (1990) The influence of carbaryl on the
uptake of (3H) noradrenaline (3H) NA by rat hypothalamic slices.
Arch Toxciol, 64(5): 417-419.
Jacob J, Schmoldt A, Raab J, Hamman M, & Grimmer G (1985)
Monoxygenase induction by various xenobiotics and its influence on
the rat liver microsomal metabolite profile of benza(a)anthracene.
Cancer Lett, 27: 105-113.
Jaszczuk E & Syrowatka T (1979) [Induction of mitotic conversion in
Saccharomyces cerevisiae D4 with propoxur, carbaryl,
dimethylnitrosamine and products of their metabolic activation.]
Rocz Panstw Zakl Hig, 30(4): 323-330 (in Polish).
Jaszczuk E, Syrowatka T, & Cybulski J (1979) [Mutagenic activity of
propoxur, carbaryl and their nitroso derivatives. Induction of
reversion in Salmonella typhimurium.] Rocz Pantsw Zakl Hig, 30(1):
81-88 (in Polish).
Jegier Z (1964) Health hazards in insecticide spraying of crops.
Arch Environ Health, 8: 670-674.
Jeleniewicz KK & Szczepaniak St (1980) [Free aminoacids and amino
nitrogen levels in serum and blood erythrocytes of rat exposed to
subacute intoxication with carbaryl.] Bromatol Chem Toksykol, 13(3):
237-240 (in Polish).
Jeleniewicz KK, Szczepaniak St, & Slenkiewicz E (1984) [Effect of
acute intoxication with carbaryl on brain free aminoacids level in
rat.] Bromatol Chem Toksykol, 17(3): 225-227 (in Polish).
Jerkofsky M & Abrahamsen LH (1983) Variation on human herpes virus
susceptibility to enhancement by the pesticide carbaryl. Appl
Environ Microbiol, 45(5): 1555-1559.
Johnson WW & Finley MT (1980) Handbook of acute toxicity of
chemicals to fish and aquatic invertebrates. Washington, DC, US
Department of the Interior, Fish and Wildlife Service (Resource
Publication 137).
Johnson RD & Manske DD (1976) Pesticide residues in total diet
samples (IX). Pestic Monit J, 9(4): 157-169.
Johnson DP & Stansbury HA (1965) Adaptation of Sevin insecticide
(carbaryl) residue method to various crops. J Agric Food Chem, 13:
235-238.
Junk GA, Richard JJ, & Dahm PA (1984) Degradation of pesticides in
controlled water-soil systems (treatment and disposal of pesticides
wastes). Washington, DC, American Chemical Society, pp 37-67 (ACS
Symposium Series No. 259).
Kadir HA & Knowles CO (1981) Inhibition of rat brain monoamine
oxidase by insecticides, acaricides and related compounds. Gen
Pharmacol, 12(4): 239-247.
Kagan J, Rodionov G, Voronina L, Velitsco L, Kulagin O, & Peremitina
A (1970) [Effect of Sevin on liver function and structure.]
Pharmacol Toxicol, 33: 219-224 (in Russian with English summary).
Kaloyanova F (1963) [Changes in the blood sugar in cases of
intoxications with certain phosphorus organic compounds.] In: [Works
of the Scientific Research Institute on Work Safety and Occupational
Diseases - Volume X.] Sofia, Institute of Hygiene and Occupational
Health, pp 63-67 (in Bulgarian).
Kanazawa J (1975) Uptake and excretion of organophosphorus and
carbamate insecticides by freshwater fish, Motsugo, Pseudorasbora
parva. Bull Environ Contam Toxicol, 14(3): 346-352.
Kanazawa J (1981) Measurement of the bioconcentration factors of
pesticides by freshwater fish and their correlation with
physicochemical properties or acute toxicities. Pestic Sci, 12(4):
417-424.
Kanazawa J, Isensee AR, & Kearney PC (1975) Distribution of carbaryl
and 3,5-xylylmethyl carbamate in an aquatic model ecosystem. J Agric
Food Chem, 23(4): 760-763.
Karinen FJ, Lamberton JG, Stewart NE, & Terrier LC (1967)
Persistence of carbaryl in the marine estuarine environment. J Agric
Food Chem, 15(1): 148-156.
Karnak RE & Collins WJ (1974) The susceptibility to selected
insecticides and acetylcholinesterase activity in a laboratory
colony of midge larvae, Chironomus tentans (Diptera:
chironomidae). Bull Environ Contam Toxicol, 12: 62-69.
Kassin VJ (1968) [Structural changes in endocrine organs under
repeated action of Sevin.] Gig Primen Toxicol Pestic Klin Otrav,
1968: 631-635 (in Russian).
Kaur K & Toor HS (1977) Toxicity of pesticides to embryonic stages
of Cyprinus carpio communis Linn. Indian J Exp Biol, 15: 193-196.
Kavadia VS, Gangwar SK, Srivastava BP, Kathpal TS, & Gupta HCL
(1978) Persistence of carbaryl in clay loam soil and its uptake in
maize and root crops. Indian J Entomol, 40(3): 265-272.
Kavlock RJ, Short RD, & Chernoff N (1987) Further evaluation of an
in vivo teratology screen. Teratog Carcinog Mutagen, 7: 7-16.
Kazano H, Kearney PC, & Kaufman DD (1972) Metabolism of
methylcarbamate insecticides in soil. J Agric Food Chem, 20(5):
975-979.
Kazarnovskaya ML & Vasilos AF (1977) [The effect of Sevin on
chromosomal apparatus of cells in vitro.] Zdravookhranenije,
20(4): 14-16 (in Russian).
Kendrick PN, Trim AJ, Atwal JK, & Brown PM (1991) Direct gas
chromatographic determination of carbaryl residues in honeybees
( Apis mellifera L.) using a nitrogen-phosphorus detector with
confirmation by formation of a chemical derivative. Bull Environ
Contam Toxicol, 46: 654-661.
Khasawinah AM (1977) Hydrolysis of carbaryl in aqueous buffer
solutions (Project No. 111A12, File No. 23092). South Charleston,
West Virginia, Union Carbide Corporation (Prepared in cooperation
with the International Research and Development Corporation)
(Unpublished proprietary information submitted to WHO by
Rhône-Poulenc Agro, Lyon).
Khasawinah AM (1978) Fate of carbaryl in soils (Project No. 285).
Monument, Colorado, Analytical Development Corporation (Unpublished
proprietary information submitted to WHO by Rhône-Poulenc Agro,
Lyon).
Khera K (1966) Toxic and teratogenic effects of insecticides in duck
and chick embryos. Toxicol Appl Pharmacol, 8: 345.
Klisenko MA (1965) [Determination of Sevin in air and biological
material.] J Anal Chim, XX(5): 634-636 (in Russian).
Klisenko MA & Yakim VS (1966) [Accumulation and excretion of Sevin
from the body of warm-blooded animals.] Gig Primen Toxicol Pestic
Klin Otrav, 1966: 146-149 (in Russian).
Klisenko MA, Lebedeva TA, & Yurkova ZF (1972) [Chemical analysis of
micro amounts of pesticides.] Moscow, Medizina, pp 197-201 (in
Russian).
Knaak JB (1971) Biological and nonbiological modifications of
carbamates. Bull World Health Organ, 44: 121-131.
Knaak JB & Sullivan LJ (1967) Metabolism of carbaryl in dog. J Agric
Food Chem, 15(6): 1125-1126.
Knaak JB, Marilyn J, Tallant MJ, Bartley WJ, & Sullivan LF (1965)
The metabolism of carbaryl in rat, guinea-pig, and man. J Agric Food
Chem, 13(6): 537-543.
Knaak JB, Tallant MJ, Kozbelt SJ, & Sullivan LJ (1968) The
metabolism of carbaryl in man, monkey, pig, and sheep. J Agric Food
Chem, 16(3): 465-470.
Knaak JB, Yee K, Ackerman CR, Zweig G, Fry DM, & Wilson BW (1984)
Percutaneous absorption and dermal dose - ChE response studies with
parathion and carbaryl in the rat. Toxicol Appl Pharmacol, 76:
252-263.
Knight EV, Chin BH, Ueng TH, & Alvares AP (1986) Differences in the
in vivo and in vitro effects of the carbamate insecticide,
carbaryl on rat hepatic monooxygenases. Drug Chem Toxicol, 9(3-4):
253-273.
Knight EV, Alvares AP, & Chin BH (1987) Effects of phenobarbital
pre-treatment on the in vivo metabolism of carbaryl in rats. Bull
Environ Contam Toxicol, 39(5): 815-821.
Korn S (1973) The uptake and persistence of carbaryl in channel
catfish. Trans Am Fish Soc, 1: 137-139.
Koundinya PR & Ramamurthi R (1980) Toxicity of Sunithion and Sevin
to the freshwater fish Sarotherodon mossambicus (Peters). Curr
Sci, 49: 875-876.
Kovaleva ES & Talanov GA (1978a) [Methods for determination of Sevin
in soil and plants by means of thin-layer chromatography.] Gig i
Sanit, 7: 69-70 (in Russian).
Kovaleva ES & Talanov GA (1978b) [Residual amounts of Sevin in
plants and soil.] Khim Sel'sk Khoz, 16(8): 77-79 (in Russian).
Kovaleva ES & Talanov GA (1980) [Plant and soil dynamics of Sevin
residues and dithiocarbamic acid derivatives.] In: Bobovnikova TI &
Malakhov SG ed. [Migration of contaminants in soil and contiguous
media. Proceedings of the All-Union Congress, 2nd Meeting, 1978.]
Leningrad, Gidrometeoizdat, pp 155-159 (in Russian).
Kovtun SD (1970) Analysis of the action exerted by Sevine on the
myoneural formations. Pharmacol Toxicol, 35(5): 543-545.
Kovtun SD & Sokur AI (1970) [The effect of Sevin on the spontaneous
electrical activity of myoneural synaps and permeability of the
membrane of skeletal muscle in white rats.] Gig Primen Toxicol
Pestic Klin Otrav, 1970: 211-214 (in Russian).
Krause RT (1985a) Pesticide and industrial chemical residues. Liquid
chromatographic determination of N-methylcarbamate insecticides
and metabolites in crops. I. Collaborative study. J Assoc Off Anal
Chem, 68(4): 726-733.
Krause RT (1985b) Liquid chromatographic determination of
N-methylcarbamate insecticides and metabolites in crops. II.
Analytical characteristics and residue findings. J Assoc Off Anal
Chem, 68(4): 734-740.
Krechniak V & Foss W (1981) Gas chromatography determination of
carbaryl. Pr Cent Inst Ochr Pr, XXXI(110): 183-188.
Krishna JG & Casida JE (1966) Fate in rats of the radiocarbon from
ten variously labelled methyl- and dimethylcarbamate-C14
insecticide chemicals and their hydrolysis products. J Agric Food
Chem, 14(2): 98-105.
Krishna JG, Dorough HW, & Casida JE (1962) Synthesis of
N-methylcarbamates via methyl isocyanate-C14 and chromatographic
purification. J Agric Food Chem, 10(6): 462-466.
Krishnaih K, Prasad VG, & Jagan Mohan N (1978) Carbaryl residues on
brinjal and peas. In: Edwards CA, Veeresh GK, & Krueger HR ed.
Proceedings of the Symposium on Pesticide Residues in the
Environment in India, 7-10 November 1978. Hebbal, Bangalore,
University of Agricultural Sciences, pp 229-231 (UAS Technical
Series No. 32).
Krug HG & Berndt J (1985) Inhibition by pesticides of prostglandin
formation in blood platelets. Blut, 51: 19-23.
Krug HF, Hamm U, & Berndt J (1988) Mechanism of inhibition of
cyclo-oxygenase in human blood platelets by carbamate insecticides.
Biochem. J, 250(1): 103-110.
Krylov DG (1970) [The effect of Sevin on red vole population.] Gig
Primen Toxicol Pestic Klin Otrav, 1970: 218-110 (in Russian).
Kuhn JO (1991a) Acute oral toxicity study in rats with Sevin XLR
plus. Sugar Land, Texas, Stillmeadow, Inc. (Laboratory Study No. IV
8150-91) (Unpublished proprietary information submitted to WHO by
Rhône-Poulenc Agro, Lyon).
Kuhn JO (1991b) Sevin-4-oil 47.0% (w/w). Acute oral toxicity study
in rats . Sugar Land, Texas, Stillmeadow, Inc. (Laboratory Study No.
N 7712-90) (Unpublished proprietary information submitted to WHO by
Rhône-Poulenc Agro, Lyon).
Kuhr RJ (1970) Metabolism of carbamate insecticide chemicals in
plants and insects. J Agric Food Chem, 18(6): 1023-1030.
Kuhr RJ (1971) The formation and importance of carbamate insecticide
metabolites as terminal residues. Pure Appl Chem, Suppl: 199-220.
Kuhr RJ & Dorough HW (1976) Carbamate insecticides: Chemistry,
biochemistry and toxicology. Cleveland, Ohio, CRC Press, Inc., pp
125-200.
Kuhr RJ, Davis AC, & Bourke JB (1974) Dissipation of Guthion, Sevin,
Polyram, Phygon and Systox from apple orchard soil. Bull Environ
Contam Toxicol, 11(3): 224-230.
Kulshrestha SK & Arora N (1984) Impairments induced by sublethal
doses of two pesticides in the ovaries of a freshwater teleost
Channa striatus Bloch. Toxicol Lett, 20(1): 93-98.
Kuribayashi S (1981) Studies on the effect of pesticides on the
reproduction of the silkworm, Bombyx Mori L. I. Effects of
chemicals administered during the larval stage on egg-laying and
hatching. J Toxicol Sci, 6: 169-176.
Kurtz DA & Studholme GA (1974) Recovery of trichlorfon (Dylox) and
carbaryl (Sevin) in songbirds following spraying of forest for Gipsy
moth. Bull Environ Contam Toxicol, 11(1): 78-84.
Kuzminskaya UA, Ivanitskij VA, & Shilina VA (1984) [The influence of
pesticides having anticholinesterase effect upon the content of
biogenic amines in brain.] Gig i Sanit, 5: 80-81 (in Russian).
LaFleur KS (1976a) Carbaryl desorption and movement in soil columns.
Soil Sci, 121(4): 212-216.
LaFleur KS (1976b) Movement of carbaryl through Congaree soil into
ground water. J Environ Qual, 5(1): 91-92.
Lahmy S, Salmon JM, & Viallet P (1988) Influence of long-term
treatment with 3 methylcholanthrene or carbaryl on mixed function
oxidase activity in 3T3 fibroblasts: studies by
microspectro-fluorimetry on single cells. Cell Biochem Funct, 6(4):
275-282.
Lamb FC, Farrow RP, Elkins ER, Kimball JR, & Cook RW (1968) Removal
of DDT, parathion and carbaryl from spinach by commercial and home
preparative methods. J Agric Food Chem, 16: 967-973.
Lamberton JG & Claeys RR (1970) Degradation of 1-naphthol in sea
water. J Agric Food Chem, 18(1): 92-96.
Larkin MJ & Day MJ (1985) The effect of pH on the selection of
carbaryl-degrading bacteria from garden soil. J Appl Bacteriol,
58(2): 175-186.
Larkin MJ & Day MJ (1986) The metabolism of carbaryl by three
bacterial isolates, Pseudomonas spp. (NCIB 12042 & 12043) and
Rodococcus sp. (NCIB 12038) from garden soil. J Appl Bacteriol,
60(3): 233-242.
Lawlor TE (1989) Mutagenicity test on carbaryl (technical) in the
Ames Salmonella/Microsome reverse mutation assay. Final report.
Kensington, Maryland, Hazleton Laboratories America, Inc. (Report
No. 10862-0-401) (Unpublished proprietary information submitted to
WHO by Rhône-Poulenc Agro, Lyon).
Leavitt JRC, Gold RE, Holcslaw T, & Tupy D (1982) Exposure of
professional pesticide applicators to carbaryl. Arch Environ Contam
Toxicol, 11(1): 57-62.
Lechner DMW & Abdel-Rahman MS (1984) A teratology study of carbaryl
and malathion mixtures in rat. J Toxicol Environ Health, 14:
267-278.
Lechner DMW & Abdel-Rahman MS (1985) Alterations in rat liver
microsomal enzymes following exposure to carbaryl and malathion in
combination. Arch Environ Contam Toxicol, 1: 451-457.
Lechner DMW & Abdel-Rahman MS (1986a) The effect of carbaryl and
malathion in combination on gamma glutamyl transpeptidase and
glutathione- S-alkyltransferase activity in vitro. Arch Environ
Contam Toxicol, 15(6): 647-651.
Lechner DMW & Abdel-Rahman MS (1986b) Kinetics of carbaryl and
malathion in combination in the rat. J Toxicol Environ Health,
18(2): 241-256.
Lee RM & Brindley WA (1974) Synergist ratios, EPN detoxication,
lipid and drug-induced changes in carbaryl toxicity in Megachile
pacifica. Environ Entomol, 3: 899-907.
Lee DY, Chen CT, & Houng KH (1990) Behaviour assessment of eight
major pesticides used in Taiwan in soils by mechanistic models. In:
Soils and fertilizers in Taiwan, pp 33-50.
Leeling NC & Casida JE (1966) Metabolites of carbaryl (1-naphthyl
methyl carbamate) in mammals and enzymatic systems for their
formation. J Agric Food Chem, 14(3): 281-290.
Leenheer JA & Ahlrichs (1971) A kinetic and equilibrium study of the
adsorption of carbaryl and parathion upon soil organic matter
surfaces. Soil Sci Soc Am Proc, 35: 700-705.
Lejczak B (1977) Effect of insecticides: chlorfenvinphos, carbaryl
and propoxur on aquatic organisms. Pol Arch Hydrobiol, 24(4):
583-591.
Leonard JA & Yearly RA (1990) Exposure of workers using hand-held
equipment during urban application of pesticides to trees and
ornamental shrubs. Am Ind Hyg Assoc J, 51: 605-609.
Lesca P, Fournier A, Lecointe P, & Cresteil T (1984) A dual assay
for the specific screening of 3 methylcholanthrene and phenobarbital
like chemical inducers of cytochrome P450 monooxygenases. Mutat Res,
129: 229-310.
Lijinsky W & Schmähl D (1978) Carcinogenicity of N-nitroso
derivatives of N-methylcarbamate insecticides in rats. Ecotoxicol
Environ Saf, 2: 413-419.
Lijinsky W & Taylor HW (1976) Carcinogenesis in Sprague-Dawley rats
on N-nitroso- N-alkylcarbamate esters. Cancer Lett, 1: 275-279.
Lijinsky W & Taylor HW (1977) Transplacental chronic toxicity test
of carbaryl with nitrite in rats. Food Cosmet Toxicol, 15: 229-232.
Lijinsky W & Winter C (1981) Skin tumours induced by painting
nitrosoalkylureas on mouse skin. J Cancer Res Clin Oncol, 102:
13-20.
Lillie RJ (1973) Studies on the reproductive performance and progeny
performance of caged white leghorns fed malathion and carbaryl.
Poult Sci, 52: 266-272.
Lin TH, North HH, & Menzer RE (1975) Metabolism of carbaryl
(1-naphthyl N-methylcarbamate) in human embryonic lung cell
cultures. J Agric Food Chem, 23(2): 253-256.
Lindsay CE (1961) Pesticide tests in the marine environment in the
State of Washington. Proc Natl Shellfish Assoc, 52: 87-97.
Lingaraja T & Venugopalan UK (1978) Pesticide induced physiological
and behavioural changes in an estuarine teleost Therapon jarbua
(Forsk). Fish Technol, 15: 115-119.
Little EE, Archeski RD, Flerov BA, & Kozlovskaya VI (1990)
Behavioral indicators of sublethal toxicity in rainbow trout. Arch
Environ Contam Toxicol, 19(3): 380-385.
Liu SY & Bollag JM (1971a) Metabolism of carbaryl by a soil fungus.
J Agric Food Chem, 19(3): 487-490.
Liu SY & Bollag JM (1971b) Carbaryl decomposition to 1-naphthyl
carbamate by Aspergillus terreus. Pestic Biochem Physiol, 1(3-4):
366-372.
Liu D, Thomson K, & Strachan WMJ (1981) Biodegradation of carbaryl
in simulated aquatic environment. Bull Environ Contam Toxicol,
27(3): 412-417.
Long KR (1971) Pesticides and occupational hazards on farms. Am J
Nurs, 71(4): 740-743.
Looney NE & Knight JN (1985) Effects of initial set and carbaryl
treatment in final fruit set on "Greensleeves" apple. Hortic Sci,
20(3): 400-401.
Looney NE & McKellar JE (1984) Thinning Spartan apples with carbaryl
and 1-napthaleneacetic acid: influence of spray volume and
combinations of chemicals. Can J Plant Sci, 64: 161-166.
Loosanoff VL (1961) Recent advances in the control of shellfish
predators and competitors. Proc Gulf Caribb Fish Inst, 13: 113-128.
Loosanoff VL, MacKenzie CL Jr, & Shearer LW (1960a) Use of chemical
barriers to protect shellfish beds from predators. In: Fisheries:
Fish farming. Volume 3 - Fisheries management. Olympia, Washington,
Washington State Department of Fisheries, pp 86-90.
Loosanoff VL, MacKenzie CL Jr, & Shearer LW (1960b) Use of chemical
to control shellfish predators. Science, 131: 1522-1523.
Lowe JI (1967) Effect of prolonged exposure to Sevin on an estuarine
fish, Leiostomus xanthurus Lacépède. Bull Environ Contam Toxicol,
2(3): 147-155.
Lox CD (1984) The effects of acute carbaryl exposure on clotting
factor activity in rat. Ecotoxicol Environ Saf, 8: 280-283.
Luca D & Balan M (1987) Sperm abnormality assay in the evaluation of
the genotoxic potential of carbaryl in rats. Morphol Embryol
(Bucur), 33(1): 19-22.
Lucier GW, McDaniel OS, Williams C, & Klein R (1972) Effects of
chlordane and methylmercury on the metabolism of carbaryl and
carbofuran in rats. Pestic Biochem Physiol, 2: 244-255.
Lukaneva AM & Rodionov GA (1973) [Some experimental data on the
effect of DDT and Sevin upon the development of cholesterin
atherosclerosis.] Gig i Sanit, 9: 41-45 (in Russian).
Luke MA, Masumoto HT, Cairns T, & Hundley HK (1988) Levels and
incidences of pesticide residues in various foods and animal feeds
analyzed by the Luke multiresidue methodology for fiscal years
1982-1986. J Assoc Off Anal Chem, 71(2): 415-420.
Lykken L & Casida JE (1969) Metabolism of organic insecticide
chemicals. Can Med Assoc J, 100: 145-154.
Ma TH, Harris MM, Anderson VA, Ahmed I, Mohammed K, Bare JL, & Lin G
(1984) Tradescantia-Micronucleus (Trad-MCN) tests on 140
health-related agents. Mutat Res, 138: 157-167.
McCann J, Choi E, Yamasaki E, & Ames BM (1975) Detection of
carcinogen as mutagens in the Salmonella/microsome test: Assay of
300 chemicals. Proc Natl Acad Sci (USA), 72(12): 5135-5139.
MacCollom GB, Currier WW, & Baumann GL (1985) Pesticide drift and
quantification from air and ground applications to a single orchard
site. In: Honeycutt RC, Zweg D, & Ragsdale N ed. Dermal exposure
related to pesticide use. Washington, DC, American Chemical Society,
pp 190-198 (ACS Symposium Series No. 273).
MacCollom GB, Currier WW, & Baumann GL (1986) Drift comparisons
between aerial and ground orchard application. J Econ Entomol,
79(2): 459-464.
Macek KJ & McAllister WA (1970) Insecticide susceptibility of some
common fish family representatives. Trans Am Fish Soc, 99(1): 20-27.
MacLeod J (1982) Report on UCC sperm morphology readings submitted
to Union Carbide Agricultural Products Co., Inc. (Unpublished
proprietary information submitted to WHO by Rhône-Poulenc Agro,
Lyon).
MacPherson SE, Scott RC, & Williams FM (1991) Fate of carbaryl in
rat skin. Arch Toxicol, 65: 594-598.
Maibach HI, Feldmann RJ, Milby TH, & Serat WF (1971) Regional
variations in percutaneous penetration in man - Pesticides. Arch
Environ Health, 23: 208-211.
Makovskaya EL, Rapoport MB, & Pintshuk VG (1965) [On the possibility
of carcinogenic effect of some insecticides belonging to the
carbamate group.] Vopr Exp Onkol, 1: 67-74 (in Russian).
Maliwal BP & Guthrie FE (1981) Interaction of insecticides with
human plasma lipoproteins. Chem-Biol Interact, 35(2): 177-188.
Mann GS & Chopra SL (1969) Residues of carbaryl on crops. Pestic
Monit J, 2(4): 163-165.
Manske DD & Corneliussen PE (1974) Pesticides in food and feed.
Pesticide residues in total diet samples (VII). Pestic Monit J,
8(2): 110-124.
Manske DD & Johnson RD (1975) Residues in food and feed. Pesticide
residues in total diet samples (VIII). Pestic Monit J, 9(2): 94-105.
Manske DD & Johnson RD (1977) Residues in food and feed. Pesticide
and other chemical residues in total diet samples (X). Pestic Monit
J, 10: 134-148.
Marian MP, Arul V, & Pandian TJ (1983) Acute and chronic effect of
carbaryl on survival, growth, and metamorphosis in the bullfrog
(Rana tigrina). Arch Environ Contam Toxicol, 12(3): 271-275.
Marshall TC, Dorough HW, & Swin HE (1976) Screening of pesticides
for mutagenic potential using Salmonella typhimurium mutants. J
Agric Food Chem, 24(3): 500-563.
Marshall TC, Dorough HW, & Swin HE (1979) Biliary excretion of
carbamate insecticides in the rat. Pestic Biochem Physiol, 11:
56-63.
Mason Y, Choshen E, & Rav-Acha C (1990) Carbamate insecticides:
Removal from water by chlorination and ozonation. Water Res, 24(1):
11-22.
Mastro VC & Cameron EA (1976) Control of Archips semiferanus on
Quercus, spp with two chemical insecticides. J Econ Entomol,
69(4): 554-556.
Mathur A & Bhatnagar P (1991) A teratogenic study of carbaryl in
Swiss albino mice. Food Chem Toxicol, 29(9): 629-632.
Matsumura F & Sakai K (1968) Degradation of insecticides by
esterases of the American cockroach. J Econ Entomol, 61(3): 598-605.
Matsumura F & Ward CT (1966) Degradation of insecticides by the
human and the rat liver. Arch Environ Health, 13: 257-261.
Mayer FL Jr (1987) Acute toxicity handbook of chemicals to estuarine
organisms. Gulf Breeze, Florida, US Environmental Protection Agency,
Gulf Breeze Laboratory (EPA 600/8-87/017).
Mayer FL & Ellersieck MR (1986) Manual of acute toxicity:
Interpretation and data base for 410 chemicals and 66 species of
freshwater animals. Washington, DC, US Department of the Interior,
Fish and Wildlife Service (Resource Publication 160).
Mehendale HM & Dorough HW (1971) Glucuronidation mechanisms in the
rat and their significance in the metabolism of insecticides. Pestic
Biochem Physiol, 1: 307-318.
Mellon Institute (1958) Acute toxicity of carbaryl. Pittsburgh,
Pennsylvania, Mellon Institute (Unpublished proprietary information
submitted to WHO by Rhône-Poulenc Agro, Lyon).
Mellon Institute (1963) Results of the eighty weeks of inclusion of
Sevin in the diet of mice. Pittsburgh, Pennsylvania, Mellon
Institute, pp 1-12 (Special report No. 26-53) (Unpublished
proprietary information submitted to WHO by Rhône-Poulenc Agro,
Lyon).
Menzie CM (1969) Metabolism of pesticides. Washington, DC, US
Department of the Interior, Bureau of Sport Fisheries and Wildlife,
pp 72-76 (Special Scientific Report, Wildlife No. 127).
Miles CJ, Trehy ML, & Yost RA (1988a) Degradation of
N-methylcarbamate and carbamoyl oximes pesticides in chlorinated
water. Bull Environ Contam Toxicol, 41(6): 838-843.
Miles CJ, Trehy ML, & Yost RA (1988b) Fate of pesticides in
chlorinated water. Paper presented at the 195th Meeting of the
American Chemical Society. Washington, DC, American Chemical Society
(Abstract ENVR 139).
Miller NE (1990) Metabolism of C14-carbaryl under aerobic soil
conditions. Research Triangle Park, North Carolina, Rhône-Poulenc Ag
Company (Unpublished proprietary information submitted to WHO by
Rhône-Poulenc Agro, Lyon).
Miller A III, Henderson MC, & Buhler DR (1979) Covalent binding of
carbaryl (1-naphthyl- N-methylcarbamate) to rat liver microsome in
vitro. Chem-Biol Interact, 24: 1-17.
Molozhanova EG (1968) [Circulation of Sevin in compartments of the
environment.] Gig Primen Toxicol Pestic Klin Otrav, 1968: 355-362
(in Russian).
Molozhanova EG (1970) [Factual content of Sevin in compartments of
the environment and quantities of the formulation apt at getting
into human organism.] Gig Primen Toxicol Pestic Klin Otrav, 1970:
170-174 (in Russian).
Molozhanova EG & Kanevskij AA (1971) [Application of mathematical
analysis to modelling the behaviour of Sevin in soil.] Gig Primen
Toxicol Pestic Klin Otrav, 1971: 77-83 (in Russian).
Moreynis JA & Estrin IM (1965) [Sevin action on animals infected
with the virus of influenza.] Gig Primen Toxicol Pestic Klin Otrav,
1965: 435-442 (in Russian).
Moriya M, Ohta T, Watanabe K, Miyazawa T, Kato K, & Shirasu Y (1983)
Further mutagenicity studies on pesticides in bacterial reversion
assay systems. Mutat Res, 116: 185-216.
Moser VC, McCormick JP, Creason JP, & MacPhail RC (1988) Comparison
of chlordimeform and carbaryl using a functional observational
battery. Fundam Appl Toxicol, 11(2): 189-206.
Mount ME & Oehme FW (1981a) Carbaryl. A literature review. Residue
Rev, 80: 1-64.
Mount ME & Oehme FW (1981b) Diagnostic criteria for carbaryl
poisoning in sheep. Arch Environ Contam Toxicol, 10(4): 483-495.
Mount ME, Dayton AD & Oehme FW (1981) Carbaryl residues in tissues
in cholinesterase activities in brain and blood of rats receiving
carbaryl. Toxicol Appl Pharmacol, 58(2): 282-296.
Mumma RO, Khalifa S, & Hamilton RH (1971) Spectroscopic
identification of metabolites of carbaryl in plants. J Agric Food
Chem, 19(3): 445-451.
Murli H (1989) Mutagenicity test on carbaryl technical in an in
vitro cytogenetic assay measuring chromosomal aberration
frequencies in Chinese hamster ovary (CHO) cells. Kensington,
Maryland, Hazleton Laboratories America, Inc. (Report No.
10862-0-437) (Unpublished proprietary information submitted to WHO
by Rhône-Poulenc Agro, Lyon).
Murray HE & Guthrie RK (1980) Effects of carbaryl, diazinon and
malathion on native aquatic populations of microorganisms. Bull
Environ Contam Toxicol, 24: 535-542.
Murray FJ, Staples RE, & Schwets BA (1979) Teratogenic potential of
carbaryl given to rabbits and mice by gavage or by dietary
inclusion. Toxicol Appl Pharmacol, 51: 81-89.
Murthy NBK & Raghu K (1988) Soil bound residues of carbaryl and
1-naphthol: release and mineralization in soil, and uptake by
plants. J Environ Sci Health, B23(6): 575-585.
Murthy NBK & Raghu K (1989) Metabolism of 14C-carbaryl and
14C-naphthol in moist and flooded soils. J Environ Sci Health,
B24(5): 479-491.
Murthy NBK & Raghu K (1991) Fate of 14C-carbaryl in soils as a
function of pH. Bull Environ Contam Toxicol, 46(3): 374-379.
Myers WF (1977) Carbaryl insecticide. Estimation of carbaryl
exposure to humans. South Charleston, West Virginia, Union Carbide
Corporation (Project Report No. 111A12) (Unpublished proprietary
information submitted to WHO by Rhône-Poulenc Agro, Lyon).
Myers RC & Christopher SM (1987) Sevin technical dermal
sensitization study in the guinea-pig (Project No. ID-49-146).
Export, Pennsylvania, Bushy Run Research Center (Proprietary report
submitted to WHO by Rhône-Poulenc Agro, Lyon).
Myers RC, Weil CS, & Carpenter CP (1975) Sevin liquid, 44% carbaryl,
range-finding toxicity studies. Pittsburgh, Pennsylvania,
Carnegie-Mellon Research Institute (Unpublished proprietary
information submitted to WHO by Rhône-Poulenc Agro, Lyon).
Myhr S (1973) A screen for pesticide toxicity to protein and RNA
synthesis in HeLa cells. J Agric Food Chem, 21(3): 362-367.
Nagy Z, Mile I, & Antony F (1975) Mutagenic effect of pesticides on
Escherichia coli WP2 try. Acta Microbiol Acad Sci Hung, 22:
309-314.
Neskovic N, Terzic M, & Vitorovic S (1978) [Acute toxicity of
carbaryl and propoxur in mice previously treated with phenobarbital
and SKF-525-A.] Arh Hig Rada, 29: 251-256 (in Croatian).
Neuhauser EF, Loehr RC, Malecki MR, Milligan DL, & Durkin PR (1985)
The toxicity of selected organic chemicals to the earthworm Eisenia
fetida. J Environ Qual, 14(3): 383-388.
Nimmo DR, Hamaker TL, Matthews E, & Moore JC (1981) An overview of
the acute and chronic effects of first and second generation
pesticides on an estuarine Mysid. In: Vernberg FJ, Calabrese A,
Thurberg FP, & Vernberg WB ed. Biological monitoring of marine
pollutants. New York, Academic Press, Inc., pp 3-19.
NIOSH (1976) Criteria for a recommended standard ... Occupational
exposure to carbaryl. Cincinnati, Ohio, National Institute for
Occupational Safety and Health, 191 pp (DHEW (NIOSH) Publication No.
77-107).
Norris FA 1991) A terrestrial field soil dissipation study with
carbaryl (Study No. EC 89-080, File No. 40862). Research Triangle
Park, North Carolina, Rhône-Poulenc Ag Company (Unpublished
proprietary information submitted to WHO by Rhône-Poulenc Agro,
Lyon).
Nye DE & Dorough HW (1976) Fate of insecticides administered
endotracheally to rats. Bull Environ Contam Toxicol, 15(3): 291-296.
Odeyemi O (1982) Relative stability of carbaryl in tropical
ecosystems. Environ Pollut, A29(3): 207-213.
Okumura D, Melnicoe R, Jackson T, Drefs C, Maddy K, & Wells J (1991)
Pesticide residues in food crops analyzed by the California
Department of Food and Agriculture in 1989. Rev Environ Contam
Toxicol, 118: 87-150.
Olefir AI (1977) [Effects of pesticides on immunogenesis.] Vrach
Delo, 9: 121-123 (in Russian).
Olefir AI & Vinogradova VKh (1968) [A study of the embryotropic
effect of the pesticides Sevin and Zyram.] Vrach Delo, 11: 103-106
(in Russian).
Omer AA, Youssef MK, Abou-Youssef AY, & Khalil AM (1986)
Mutagenicity of insecticide Sevin and its derivative nitrosocarbaryl
on D. melanogaster. Alex J Agric Res, 31(3): 239-248.
Omkar & Shukla GS (1985) Toxicity of insecticides to Macrobrachium
lamarrei (H. Milne Edwards) (Decapoda, Palaemonidae). Crustaceana,
48: 1-5.
Önfelt A (1987) Spindle disturbances in mammalian cells. III.
Toxicity, c-mitosis and aneuploidy with 22 different compounds.
Specific and unspecific mechanisms. Mutat Res, 182: 135-154.
Önfelt A & Klasterska I (1983) Spindle disturbances in mammalian
cells. II. Induction of viable aneuploid/polyploid cells and
multiple chromatid exchanges after treatment of V-70 Chinese hamster
cells with carbaryl. Modifying effects of glutathion and S9. Mutat
Res, 119: 319-330.
Önfelt A & Klasterska I (1984) Sister chromatid exchanges and
thioguanine resistance in V79 Chinese hamster cells after treatment
with the aneuploidy inducing agent carbaryl +/ S9 mix. Mutat Res,
125: 269-274.
Oonnithan ES & Casida JE (1968) Oxidation of methyl- and
dimethylcarbamate insecticide chemicals by microsomal enzymes and
anticholinesterase activity of the metabolites. J Agric Food Chem,
18(1): 28-44.
Orlova NV & Zhalbe EP (1968) [Experimental material contributive to
the problem of maximal permissible amounts of Sevin in food
products.] Vopr Pitan, 27(6): 49-55 (in Russian).
Orzel RA & Weiss LR (1966) The effect of carbaryl
(1-naphthyl- N-methylcarbamate) on blood glucose, and liver and
muscle glycogen in fasted and nonfasted rats. Biochem Pharmacol, 15:
995-998.
Osborne BG, Barrett GM, Laal-Khoshab A, & Willis KH (1989) The
occurrence of pesticide residues in UK home-grown and imported
wheat. Pestic Sci, 27: 103-109.
Osman MA & Belal MH (1980) Persistence of carbaryl in canal water. J
Environ Sci Health, B15(3): 307-311.
Osman DH & Brindley WA (1981) Estimating monoxygenase detoxification
in field populations: toxicity and distribution of carbaryl in three
species of Labops grassbugs. Environ Entomol, 10(5): 676-680.
Osterloh J, Letz G, Pond S, & Becker Ch (1983) An assessment of the
potential testicular toxicity of 10 pesticides using the mouse sperm
morphology assay. Mutat Res, 116(3-4): 407-415.
Ott FT, McCullogh R, & Stanley JG (1981) Effects of forest spraying
on the ecology of brook trout. Report to the Maine Department of
Conservation, Forest Service, Augusta.
Owen BB Jr (1965) Aquatic insect populations reduced by aerial
spraying of insecticide Sevin. Philadelphia, Pennsylvania,
Pennsylvania Academy of Science, pp 63-69.
Page M, Haverty MJ, & Richmond ChE (1985) Residual activity of
carbaryl protected Lodgepole Pine against Mountain Pine Beetle,
Dillon, Colorado, 1982 and 1983. Berkeley, California, US Department
of Agriculture, Forest Service, Pacific Southwest Forest and Range
Experiment Station (Research Note PSW-375).
Panciera RJ (1967) Determination of teratogenic properties of orally
administrated 1-naphthyl- N-methylcarbamate (Sevin) in sheep.
Research Triangle Park, North Carolina, Union Carbide Agricultural
Products Co., Inc. (Unpublished proprietary information submitted to
WHO by Rhône-Poulenc Agro, Lyon).
Parafita MA & Fernandez-Otero P (1984) The interaction of carbaryl
with the metabolism of isolated hepatocytes. II. Effect on
glycogenesis. Gen Pharmacol, 15(4): 333-337.
Parafita MA, Fernandez-Otero P, & Aldegunde MA (1984) The
interaction of carbaryl with the metabolism of isolated hepatocytes.
I. Effect on respiration and glycolysis. Gen Pharmacol, 15(4):
327-331.
Paris DF & Lewis DL (1973) Chemical and microbiological degradation
of ten selected pesticides in aquatic system. Residue Rev, 45:
95-124.
Paris D, Lewis D, Barnett J, & Baugham G (1975) Microbial
degradation and accumulation of pesticides in aquatic systems.
Corvallis, Oregon, US Environmental Protection Agency, Office of
Research and Development, National Environmental Research Center, pp
1-45 (EPA-660/3-75-007).
Patocka JJ & Bajgar J (1971) Affinity of human brain
acetylcholinesterase to some organophosphates and carbamates in
vitro. J Neurochem, 18: 2545-2546.
Paulson GD & Feil VJ (1969) The fate of a single oral dose of
carbaryl (1-naphthyl- N-methyl-carbamate) in the chicken. Poult
Sci, 48: 1593-1597.
Paulson GD, Zaylskie RG, Zehr MV, Portnoy CE, & Feil VJ (1970)
Metabolites of carbaryl (1-naphthyl N-methyl-carbamate) in chicken
urine. J Agric Food Chem, 18: 110-115.
Pavlova II, Petrovskaya OG, & Ivanova SI (1968) [Biochemical and
histochemical studies on the oxidative-reductive enzymes after an
exposure to Sevin.] Gig Primen Toxicol Pestic Klin Otrav, 1968:
625-630 (in Russian).
Payne JA, Dutcher JD, & Ellis HC (1985) Low pressure soil
application of carbaryl for control of the pecan weevil, Curculio
carvae. J Agric Entomol, 2(1): 1-5.
Pekas JC (1972) Intestinal hydrolysis, metabolism and transport of a
pesticidal carbamate in pH G.5 medium. Toxicol Appl Pharmacol, 23:
62-70.
Pekas JC & Paulson GD (1970) Intestinal hydrolysis and conjugation
of a pesticidal carbamate in vitro. Science, 170: 77-78.
Perelygin VM, Shpirt MB, Aripov OA, & Ershova VI (1971) [Effect of
some pesticides on immunological response reactivity.] Gig i Sanit,
36(12): 29-33 (in Russian).
Petrovski VV (1970) [The level of content and duration of excretion
of Sevin in the milk of cows treated against ticks.] Gig Primen
Toxicol Pestic Klin Otrav, 1970: 193-196 (in Russian).
Pieper GR (1979) Residues analysis of carbaryl on forest foliage and
in stream water using HPLC. Bull Environ Contam Toxicol, 22(1-2):
167-171.
Pieper GR & Roberts RB (1978) Residue analysis of carbaryl on forest
foliage and in creek water. US Department of Agriculture, Forest
Service, Northern Region, pp 1-9 (Report No. 78-5).
Pipy B, Gaillard D, & Derache R (1979) Effect of blockage of the
reticulo-endothelial system on the carbaryl toxicity in the rat.
Toxicology, 12: 135-142.
Pipy B, Gaillard D, Beraud M, & Derache R (1980) Phagocytic activity
of the rat reticuloendothelial system and the pharmakokinetics of an
anticholinesterasic insecticide: carbaryl. Xenobiotica, 10(10):
785-793.
Pipy B, Gaillard D, & Derache R (1981) Phagocytic activity of the
reticuloendothelial system in the rat and rates of in vivo
excretion of metabolites of carbaryl and in vitro microsomal
metabolism. Biochem Pharmacol, 30(6): 669-672.
Pipy B, Gaillard D, Beraud M, & Derache R (1982) Enzymatic
activities of liver serine esterases during the reticuloendothelial
system phagocytosis blockade by carbaryl, an anticholinesterasic
insecticide. Toxicol Appl Pharmacol, 62: 11-18.
Pipy B, de Maroussem D, Beraud M, & Derache R (1983) Evaluation of
cellular and humoral mechanisms of carbaryl-induced
reticuloendothelial phagocytic depression. J Reticuloendothel Soc,
34(5): 395-412.
Podolak M & Warchocki B (1980) [Effect of toxobidin on the kinetics
of reaction of ChE inhibition by chlorfenvinfos and carbaryl.] Rocz
Panstw Zakl Hyg, 31(2): 125-131 (in Polish).
Podolak-Majczak M & Tyburczyk W (1984) Effect of joint action of
sodium nitrite and carbaryl on rats organism. Bromatol Chem
Toksykol, XVII(3): 211-214 (in Polish with English summary).
Podolak-Majczak M & Tyburczyk W (1986) [Effect of joint action of
sodium nitrite and carbaryl on rats organism. Part IV. Hepatotoxic
action.] Bromatol Chem Toksykol, XIX(3): 164-166 (in Polish with
English summary).
Post G & Schroeder TR (1971) The toxicity of four insecticides to
four salmonid species. Bull Environ Contam Toxicol, 6(2): 144-155.
Preussmann RG, Eisenbrand G, & Schmahl D (1976) Carcinogenicity
testing of low doses of nitrosopyrrolidine and of
nitrosobenzo-thiazuron and nitrosocarbaryl in rats. In: Walker EA,
Bogovski P, & Griciute L ed. Environmental N-nitroso compounds
analysis and formation. Proceedings of a Working Conference held at
the Polytechnical Institute, Tallinn, Estonian SSR, 1-3 October
1975. Lyon, International Agency for Research on Cancer, pp 429-433
(IARC Scientific Publications No. 14).
Prima NG, Prima AP, Sem'yan AI, Solov'eva VG, & Bondarenko VF (1976)
[Model studies of the rate of self-purification of river water from
Sevin and 1-naphthol residues.] Probl Okhr Vod, 7: 8-13 (in
Russian).
Probst GS, McMahon RE, Hill LE, Thompson CZ, Epp JK, & Neal SB
(1981) Chemically induced unscheduled DNA synthesis in primary rat
hepatocyte cultures: A comparison with bacterial mutagenicity using
218 compounds. Environ Mutagen, 3(1): 11-32.
Puech AA (undated) A study of the potential for human dermal and
inhalation exposure of Sevin, carbaryl insecticide during normal
garden use. Research Triangle Park, North Carolina, Union Carbide
Corporation (Unpublished proprietary information submitted to WHO by
Rhône-Poulenc Agro, Lyon).
Puyear RL & Paulson GD (1972) Effect of carbaryl (1-naphthyl-
N-methylcarbamate) on phenobarbital-induced sleeping time and some
liver microsomal enzymes in white leghorn cockerels. Toxicol Appl
Pharmacol, 22: 621-627.
Quarles JM & Tennant RW (1975) Effects of nitrosocarbaryl on
Balb/3T3 cells. Cancer Res, 35: 2637-2645.
Rajagopal BS & Sethunathan N (1984) Influence of nitrogen
fertilizers on the persistence of carbaryl and carbofuran in flooded
soils. Pestic Sci, 15: 591-599.
Rajagopal BS, Chendrayan K, Reddy B, Rajasekhar-Reddy B, &
Sethunathan N (1983) Persistence of carbaryl in flooded soils and
its degradation by soil enrichment cultures. Plant Soil, 73(1):
35-45.
Rajagopal BS, Rao VR, Nagendrappa G, & Sethunathan N (1984)
Metabolism of carbaryl and carbofuran by soil-enrichment and
bacterial cultures. Can J Microbiol, 30(12): 1457-1465.
Rajagopal BS, Panda S, & Sethunathan N (1986) Accelerated
degradation of carbaryl and carbofuran in a flooded soil pretreated
with hydrolysis products, 1-naphthol and carbofuran phenol. Bull
Environ Contam Toxicol, 36(6): 827-832.
Rajukkannu K, Basha AA, Habeebullah B, Duraisamy P, &
Balasubramanian M (1985) Degradation and persistence of DDT, BHC,
carbaryl and malathion in soils. Indian J Environ Health, 27(3):
237-243.
Rao KRSS, Rao KSP, Sahib IKA, & Rao KVR (1985a) Combined action of
carbaryl and phenthoate on a freshwater fish ( Carina punctatus
bloch). Ecotoxicol Environ Saf, 10(2): 209-217.
Rao KS, Rao KR, Sahib IK, & Rao KV (1985b) Combined action of
carbaryl and phenthoate on a tissue lipid derivatives of murrel,
Channa punctatus (Bloch). Ecotoxicol Environ Saf, 9(1): 107-111.
Ray SK & Poddar MK (1985a) Central cholinergic dopaminergic
interaction in carbaryl induced tremor. Eur J Pharmacol, 119(3):
251-253.
Ray SK & Poddar MK (1985b) Effect of pentylenetetrazol on carbaryl
induced changes in striatal catecholamines. Biochem Pharmacol,
34(4): 553-557.
Ray SK & Poddar MK (1990) Interaction of central serotonin and
dopamine in the regulation of carbaryl induced tremor. Eur J
Pharmacol, 181(3): 159-166.
Rawash IA, Gaaboub IA, El-Gayar FM, & El-Shazli AY (1975) Standard
curves for Nuvacron, Malathion, Sevin, DDT, and Kelthane tested
against the mosquito Culex pipiens L. and the microcrustacean
Daphnia magna Straus. Toxicology, 4: 133-144.
Regan JD, Seltow RB, Francis AA, & Lijinsky W (1976)
Nitrosocarbaryl: Its effect on human DNA. Mutat Res, 38: 239-302.
Reiner E & Aldridge WN (1967) Effect of pH on inhibition and
spontaneous reactivation of acethylcholinesterase treated with
esters of phosphorus acids and of carbamic acids. Biochem J, 105:
171-179.
Rickard RW & Dorough HW (1984) in vivo formation of
nitrosocarbamates in the stomach of rats and guinea-pigs. J Toxicol
Environ Health, 14(2-3): 279-290.
Rickard RW, Walter-Echols G, Dorough HW, & Lawrence LJ (1982)
Importance of pH in assessing the potential for nitrosocarbamate
formation in the stomach. Pestic Biochem Physiol, 18(3): 325-333.
Robel J, Felthausen RW, & Dayton D (1982) Effects of carbamates on
bobwhite food intake, body weight and locomotor activity. Arch
Environ Contam Toxicol, 11(5): 611-615.
Robens JF (1969) Teratologic studies of carbaryl, diazinon, norea,
disulfiram and thiram in small laboratory animals. Toxicol Appl
Pharmacol, 15: 152-163.
Roberts D (1975) The effect of pesticides on byssus formation in the
common mussel, Mytilus edulis. Environ Pollut, 8(4): 241-254.
Roberts BL & Dorough HW (1984) Relative toxicity of chemicals to the
earthworm Eisenia foetida. Environ Toxicol Chem, 3: 67-78.
Rocchi P, Perocco P, Alberhin W, Fini A, & Prodi G (1980) Effects of
pesticides on scheduled and unscheduled DNA synthesis of rat
thymocytes and human lymphocytes. Arch Toxicol, 45(2): 101-108.
Rodgers KE, Leung N, Imamura T, & Devens BH (1986) Rapid in vitro
screening assay for immunotoxic effects of organophosphorus and
carbamate insecticides in the generation of cytotoxic T-lymphocyte
responses. Pestic Biochem Physiol, 26(3): 292-301.
Rodriguez LD & Dorough HW (1977) Degradation of carbaryl by soil
microorganisms. Arch Environ Contam Toxicol, 6(1): 47-56.
Romine RR & Bussian RA (1971) Sevin Insecticide: The degradation of
carbaryl after surface application to a farm pond (Project No
111A13, File No. 15265). South Charleston, West Virginia, Union
Carbide Corporation (Unpublished proprietary information submitted
to WHO by Rhône-Poulenc Agro, Lyon).
Roszkowski J, Zadura J, & Skwarek P (1976) The effect of carbaryl on
immune response in chickens. Bull Vet Inst Pulawy, 20(3-4): 85-88.
Ruppert P, Cook L, Dean F, & Reiter W (1983) Acute behavioural
toxicity of carbaryl and propoxur in adult rats. Pharmacol Biochem
Behav, 18(4): 579-584.
Ryan AJ (1971) The metabolism of pesticidal carbamates. CRC Crit Rev
Toxicol, 1: 35-54.
Rybakova MN (1966) [Toxic effect of Sevin on animals.] Gig i Sanit,
31(9): 42-47 (in Russian with English summary).
Rybakova MN (1967) [Comparative toxic effect of Sevin and DDT used
in the treatment of food crops.] Vopr Pitan, 26: 9-15 (in Russian
with English summary).
Saini ML & Dorough HW (1978) Metabolism of carbaryl-naphthyl-C14
in a lactating cow, when administered orally. In: Edwards CA,
Veeresh GK, & Krueger HR ed. Proceedings of the Symposium on
Pesticide Residues in the Environment in India, 7-10 November 1978.
Hebbal, Bangalore, University of Agricultural Sciences, pp 74-87
(UAS Technical Series No. 32).
Sakai K & Matsumura F (1971) Degradation of certain organophosphate
and carbamate insecticides by human brain esterases. Toxicol Appl
Pharmacol, 19: 660-666.
Samanidou V, Fytianos K, Pfister G, & Bahadir M (1988) Photochemical
decomposition of carbamate pesticides in natural waters of Northern
Greece. Sci Total Environ, 76(1): 85-92.
Sanders HO & Cope DB (1966) Toxicity of several pesticides in two
species of Cladocerans. Trans Am Fish Soc, 95(2): 165-169.
Sanders HO, Finley MT, & Hunn JB (1983) Acute toxicity of six forest
insecticides to three aquatic invertebrates and four fishes.
Washington, DC, US Department of the Interior, Fish and Wildlife
Service, 5 pp (Technical Paper 110).
Sanderson DM (1961) Treatment of poisoning by anticholinesterase
insecticides in the rat. J Pharm Pharmacol, 13: 435-442.
Santolucito JA & Morrison G (1971) EEC of Rhesus monkeys following
prolonged low level feeding of pesticides. Toxicol Appl Pharmacol,
19: 147-154.
Santolucito JA & Whitcomb E (1971) Mechanical response of skeletal
muscle following oral administration of pesticides. Toxicol Appl
Pharmacol, 20: 66-72.
Schafer EA (1972) The acute oral toxicity of 369 pesticidal,
pharmaceutical and other chemicals to wild birds. Toxicol Appl
Pharmacol, 21: 315-330.
Schomburg CJ, Glottfeldy DE, & Seiber JN (1991) Pesticide occurrence
and distribution in fog collected near Monterey, California. Environ
Sci Technol, 25: 155-160.
Schulze TL, Hauge PN, Mitchell WJ, Remley WJ, Shahied SI, & Marshall
FJ (1979) New Jersey Epidemiologic Studies Program-Pesticides -
Assessment of human exposure to carbaryl during aerial application
for Gypsy moth control in New Jersey. New Jersey State Department of
Health.
Seiler JP (1977) Nitrosation in vivo and in vitro by sodium
nitrate and mutagenicity of nitrogenous pesticides. Mutat Res, 48:
225-236.
Shabanov M, Toshkov A, Georgiev D, & Ibrishimov N (1983) [Effect of
carbaryl on experimental Erysipelothrix rhusiopathiae infection in
rats.] Vet Med Nauk, 20(5-6): 9-15 (in Russian).
Shafik MT, Sullivan HC, & Enos NF (1971) A method for the
determination of 1-naphthol in urine. Bull Environ Contam Toxicol,
6(1): 34-39.
Shah PV & Guthrie FE (1983) Percutaneous penetration of three
insecticides in rats: a comparison of two methods for in vivo
determination. J Invest Dermatol, 80(4): 291-293.
Shah PV, Monroe RV, & Guthrie FE (1983) Comparative penetration of
insecticides in target and nontarget species. Drug Chem Toxicol,
6(2): 155-179.
Shah PV, Fisher HL, Sumler MR, Monroe RJ, Chernoff N, & Hall LL
(1987) Comparison of the penetration of 14 pesticides through the
skin of young and adult rats. J Toxicol Environ Health, 21(3):
353-366.
Sharma VK & Kaur S (1990) Contact sensitization by pesticides in
farmers. Contact Dermatitis, 23(2): 77-80.
Sharom MS, Miles JRW, Harris CR, & McEwen FL (1980) Behaviour of 12
insecticides in soil and aqueous suspensions of soil and sediment.
Water Res, 14(8): 1095-1100.
Shea TB (1985) Carbaryl inhibition of interferon synthesis in
cultured goldfish cells. Bull Environ Contam Toxicol, 34: 109-113.
Shea TB & Berry ES (1983) Toxicity of carbaryl and 1-naphthol to
goldfish (Carassius auratus) and Killifish (Fundulus
heteroclitus). Bull Environ Contam Toxicol, 31: 526-529.
Shea TB & Berry ES (1984) Suppression of interferon synthesis by the
pesticide carbaryl as a mechanism for enhancement of goldfish
virus-2 replication. Appl Environ Microbiol, 47(2): 250-252.
Shehata T, Richardson E, & Cotton E (1984) Assessment of human
population exposure to carbaryl from the 1982 Main Spruce budworm
spray project. J Environ Health, 46(6): 293-297.
Shilova SA, Denisova AV, Sedykh EL, & Efron KM (1973) [After effects
of insecticide treatment in the subartics.] Zool Zh, 52(7):
1008-1012 (in Russian).
Shimkin MB, Wieder R, McDonough M, Fishbein L, & Swern D (1969) Lung
tumour response in strain A mice as a quantitative bioassay of
carcinogenic activity of some carbamates and aziridines. Cancer Res,
29: 2184-2190.
Shpirt MB (1973) [Toxicological assessment of DDT, HCHC, TMTD, Sevin
and Zineb acting on human cell cultures.] Gig Tr Prof Zabol, 14:
32-35 (in Russian).
Shtenberg AI & Hovaeva LA (1970) [The effect of Sevin on the state
of thyroid gland of rats having received artificial full-value
ration with different content of iodine.] Gig i Sanit, 8: 119-122
(in Russian).
Shtenberg AI & Ozhovan MV (1971) [The effect of small doses of Sevin
upon the reproduction function of animals in several generations.]
Vopr Pitan, 1: 42-47 (in Russian).
Shtenberg AI & Rybakova MN (1968) Effect of carbaryl on the
neuroendocrine system in rats. Food Cosmet Toxicol, 6: 461-467.
Shtenberg AI, Orlova NV, Pozdnyakov AL, Tropova GP, Zhalbe JP,
Khovayeva LA, & Akincheva MJ (1970) [Functional and morpho-logical
parallels associated with the state of the sex glands of animals
under the influence of Sevin.] In: [Problems of the hygiene and
toxicology of pesticides.] Moscow, Meditsina, pp 124-129 (in
Russian).
Shtenberg AI, Orlova NV, & Torchinskij AM (1973) [On the effect of
pesticides of different chemical structure on gonads and
embryogenesis in experimental animals.] Gig i Sanit, 8: 16-20 (in
Russian).
Shukla GS, Omkar & Upadhyay VB (1982) Acute toxicity of few
pesticides to an aquatic insect, Ranatra elongata (Fabr.) J Adv
Zool, 3(2): 148-150.
Siboulet R, Grinfeld S, Deparis P, & Jaylet A (1984) Micronuclei in
red blood cells of the newt Pleurodeles waltl Michah: Induction
with X rays and chemicals. Mutat Res, 125(2): 275-281.
Siebert D & Eisenbrand G (1974) Induction of mitotic gene
conversions in Saccharomyces cerevisiae by N-nitrosated
pesticides. Mutat Res, 22: 121-126.
Sikka HC, Miyazaki S, & Rice CP (1973) Metabolism of selected
pesticides by marine microorganisms. Springfield, Virginia, National
Technical Information Service (Syracuse University Annual Report No.
2 - NTIS AS AD-763 410).
Sikka HC, Miyazaki S, & Lynch RS (1975) Degradation of carbaryl and
1-naphthol by marine microorganisms. Bull Environ Contam Toxicol,
13(6): 666-672.
Simpson JR (1965) Exposure to orchard pesticides, dermal and
inhalation exposures. Arch Environ Health, 10: 884-885.
Singh JM (1973) Decreased performance behaviour with carbaryl - an
indication of clinical toxicity. Clin Toxicol, 6(1): 97-108.
Singh KP, Pandey SY, Divekar VV, Surana KC, & Paralikar AB (1978)
Dissipation of carbaryl residues in and on cauliflower. In: Edwards
CA, Veeresh GK, & Krueger HR ed. Proceedings of the Symposium on
Pesticide Residues in the Environment in India, 7-10 November 1978.
Hebbal, Bangalore, University of Agricultural Sciences, pp 210-214
(UAS Technical Series No. 32).
Singh VP, Gupta S, & Saxena PK (1984) Evaluation of acute toxicity
of carbaryl and malathion to freshwater teleosts, Chauna punctatis
(bloch) and Heteropneustes fossilis (bloch). Toxicol Lett, 20:
271-276.
Sjoblad RD, Minard RD, & Bollag JM (1976) Polymerisation of
1-naphthol and related phenolic compounds by an extracellular fungal
enzyme. Pestic Biochem Physiol, 6: 457-463.
Skraba WJ & Young FG (1959) Radioactive Sevin (1-naphthyl-
1-carbon-14 N-methylcarbamate): a convenient synthesis. Agric Food
Chem, 7(9): 612-613.
Smalley HE (1970) Diagnosis and treatment of carbaryl poisoning in
swine. J Am Vet Med Assoc, 156(3): 339-344.
Smalley HE, Curtis JM, & Earl FL (1968) Teratogenic action of
carbaryl in beagle dogs. Toxicol Appl Pharmacol, 13: 392-403.
Smalley HE, O'Hara PJ, Bridges CH, & Radeleef RD (1969) The effect
of chronic carbaryl administration on the neuromuscular system of
swine. Toxicol Appl Pharmacol, 14: 409-419.
Snow CD & Stewart NE (1963) Treatment of Tikamook Bay oyster beds
with MGS-90 (Sevin) (Unpublished report from the Oregon Fish
Commission, submitted to WHO by Rhône-Poulenc Agro, Lyon).
Söderpalm BC & Önfelt A (1988) The action of carbaryl and its
metabolite alpha naphthol on mitosis in V79 Chinese hamster
fibroblasts. Indications of the involvement of some cholinester in
cell division. Mutat Res, 201(2): 349-363.
Sokur AJ (1971) [Hypokaliaemia in erythrocytes after intoxication of
white rats with some pesticides (DDT, Sevin, chlorophos).] Gig
Primen Toxicol Pestic Klin Otrav, 1971: 213-216 (in Russian).
Solomon H & Weis J (1979) Abnormal circulatory development in medaka
caused by the insecticides carbaryl, malathion and parathion.
Toxicology, 19: 51-62.
Spain AV (1974) The effects of carbaryl and DDT on the litter fauna
of a Corsican pine forest (Pinus nigra var. maritima): A
multivariate comparison. J Appl Ecol, 11: 467-481.
Springborn Bionomics, Inc. (1988a) Environmental fate of spray
applications of the rice insecticide Sevin XLR (a.i. carbaryl):
California (Study No. 565-6118/6112). Wareham, Massachusetts,
Springborn Bionomics, Inc. (Unpublished proprietary information
submitted to WHO by Rhône-Poulenc Agro, Lyon).
Springborn Bionomics, Inc. (1988b) Environmental fate of spray
applications of the forest insecticide Sevin-4-oil (a.i. carbaryl):
Oregon (Study No. 565-6120/6114). Wareham, Massachusetts, Springborn
Bionomics, Inc. (Unpublished proprietary information submitted to
WHO by Rhône-Poulenc Agro, Lyon).
Springborn Bionomics, Inc. (1988c) Environmental fate of spray
applications of the forest insecticide Sevin-4-oil (a.i. carbaryl):
Pennsylvania (Study No. 565-6121/6115). Wareham, Massachusetts,
Springborn Bionomics, Inc. (Unpublished proprietary information
submitted to WHO by Rhône-Poulenc Agro, Lyon).
Stanley JG & Trial JG (1980) Disappearance constants of carbaryl
from streams contaminated by forest spraying. Bull Environ Contam
Toxicol, 25(5): 771-776.
Statham CN & Lech JJ (1975) Potentiation of the acute toxicity of
several pesticides and herbicides in trouts by carbaryl. Toxicol
Appl Pharmacol, 34: 83-87.
Stegeman LC (1964) The effects of the carbamate insecticide carbaryl
upon forest soil mites and Collembola. J Econ Entomol, 57(6):
803-808.
Stevenson JH (1978) The acute toxicity of unformulated pesticides to
worker honey bees ( Apis Mellifera L.). Plant Pathol, 27: 38-40.
Stevenson JH, Needham PH, & Walker J (1977) Poisoning of honey bees
by pesticides: Investigations of the changing pattern in Britain
over 20 years. Rothamsted Rep, 2: 55-66.
Stewart NE, Millemann RE, & Breese WP (1967) Acute toxicity of the
insecticide Sevin and its hydrolytic product 1-naphthol to some
marine organisms. Trans Am Fish Soc, 96: 25-30.
Strait JR, Thornwall GC, & Ehrich M (1991) Sensitive
high-performance liquid chromatographic analysis for toxicological
studies with carbaryl. Washington, DC, American Chemical Society.
Street JC & Sharma RP (1975) Alteration of induced cellular and
humoral immune responses by pesticides and chemicals of
environmental concern: Quantitative studies on immunosuppression by
DDT, Aroclor 1254, carbaryl, carbofuran and methylparathion. Toxicol
Appl Pharmacol, 32(5): 587-602.
Strother A (1970) Comparative metabolism of selected
1-methylcarbamates by human and rat liver fractions. Biochem.
Pharmacol, 19: 2525-2529.
Strother A & Wheeler L (1976) Disposition of 14C-carbaryl in
pregnant, nonpregant and fetal tissues of rat. Fed Proc, 35: 327.
Strother A & Wheeler L (1980) Excretion and disposition of
[14C]carbaryl in pregnant, non-pregant and foetal tissues of the
rat after acute administration. Xenobiotica, 10(2): 113-124.
Struble CB, Feil VJ, Pecas JC, & Gerst JW (1983a) Biliary secretion
of 14C and identification of 5,6-dihydro-5,6-dihydroxycarbaryl
glucoronide as a biliary metabolite of (14C) carbaryl in the rat.
Pestic Biochem Physiol, 19: 85-94.
Struble CB, Pecas JC, & Gerst JW (1983b) Enterohepatic circulation
and biliary secretion of 5,6-dihydro-5,6-dihydroxy(14C)carbaryl
glucoronide in the rat. Pestic Biochem Physiol, 19(1): 95-103.
Subbotina SG & Belonozhko GA (1968) [The effect of Sevin on the
thermoresistance and fractionary protein content of blood serum.]
Gig Primen Toxicol Pestic Klin Otrav, 1968: 442-444 (in Russian).
Sud RK, Sud AK, & Gupta KG (1972) Degradation of Sevin (1-naphthyl
N-methylcarbamate) by Achromobacter sp. Arch Microbiol, 87:
353-358.
Sullivan LJ, Chin BH, & Carpenter CP (1972) in vitro vs. in vivo
chromatographic profiles of carbaryl anionic metabolites in man and
lower animals. Toxicol Appl Pharmacol, 22: 161-174.
Sundaram KMS & Szeto SY (1987) Distribution and persistence of
carbaryl in some terrestrial and aquatic components of a forest
environment. J Environ Sci Health, B22(5): 579-599.
Surprenant DC (1985a) Acute toxicity of carbaryl technical to
embryo-larvae of eastern oysters (Crassostrea virginica). Wareham,
Massachusetts, Springborn Bionomics, Inc. (Report No. BW-85-7-1817)
(Unpublished proprietary information submitted to WHO by
Rhône-Poulenc Agro, Lyon).
Surprenant DC (1985b) Acute toxicity of carbaryl technical to mysid
shrimp (Mysidopsis bahia). Wareham, Massachusetts, Springborn
Bionomics, Inc. (Report No. BW-85-6-1790) (Unpublished proprietary
information submitted to WHO by Rhône-Poulenc Agro, Lyon).
Surprenant DC (1985c) The chronic toxicity of carbaryl technical to
Daphnia magna under flow-through conditions. Wareham,
Massachusetts, Springborn Bionomics, Inc. (Report No. BW-85-7-1813)
(Unpublished proprietary information submitted to WHO by
Rhône-Poulenc Agro, Lyon).
Sussman HF, Cooke SB, & Bloch V (1969) Human louse infestation;
treatment with carbacide. Arch Dermatol, 100(1): 82-83.
Swartz WJ (1981) Long- and short-term effects of carbaryl exposure
in chick embryos. Environ Res, 26(2): 463-471.
Swartz WJ (1985) Effects of carbaryl on gonadal development in the
chick embryo. Bull Environ Contam Toxicol, 34: 481-485.
Szczepaniak S & Jeleniewicz K (1980) [Alterations of free amino
acids concentrations in rat blood erythrocytes following the acute
intoxication with carbaryl.] Bromatol Chem Toksykol, XIII(2):
159-163 (in Polish with English summary).
Szczepaniak S & Jeleniewicz K (1981) [ In vitro binding of carbaryl
and alpha naphthol with plasma and erythrocytes amino acids.]
Bromatol Chem Toksykol, XIV(2): 165-168 (in Polish with English
summary).
Szczepaniak S & Jeleniewicz K (1986) [Body amino acids balance in
acute poisoning with carbaryl.] Farm Pol, 9: 475-479 (in Polish).
Szczepaniak S, Siemkievicz E, Jeleniewicz K, & Nowinski H (1980)
[Effects of carbaryl and chlorfenvinfos on the level of free amino
acids in rat blood serum. Part II. Intoxication with a single dose
corresponding to 1/5 LD50.] Bromatol Chem Toksykol, XIII(4):
351-353 (in Polish with English summary).
Szeto SY, MacCarty HR, Oloffs PC, & Shephard RF (1979) The fate of
acephate and carbaryl in water. J Environ Sci Health, B14(6):
635-654.
Tagatz ME, Ivey JM, & Lehman HK (1979) Effects of Sevin on
development of experimental estuarine communities. J Toxicol Environ
Health, 5: 643-651.
Takahashi RN, Poli A, Morato GS, Lima TCM, & Zanin M (1991) Effects
of age on behavioral and physiological responses to carbaryl in
rats. Neurotoxicol Teratol, 13: 21-26.
Tewfik MS & Hamdi YA (1970) Decomposition of Sevin by a soil
bacterium. Acta Microbiol Pol Ser B, 19(2): 133-135.
Thomas A (1981) Introductory remarks: The testes. Environ Health
Perspect, 38: 3-4.
Thomas SJ (1986) Sevin brand carbaryl insecticide: Magnitude of
carbaryl residues in sugar beet roots (File No. 34934). Research
Triangle Park, North Carolina, Union Carbide Agricultural Products
Co., Inc. (Prepared in collaboration with Hazleton Laboratories
America, Inc., Chemical and Biomedical Sciences Division, Madison)
(Unpublished proprietary information submitted to WHO by
Rhône-Poulenc Agro, Lyon.
Thomas JH, Dieringer CS, & Schein L (1974) Effects of carbaryl on
mouse organs of reproduction. Toxicol Appl Pharmacol, 28: 142-145.
Thomas J, Visalakshi A, & Mohandas N (1982) Contamination of water
in rice fields sprayed with carbaryl for insect control. Entomon,
7(3): 379-380.
Thompson AR (1971) Effects of nine insecticides on the numbers and
biomass of earthworms in pasture. Bull Environ Contam Toxicol, 5(6):
577-586.
Tilak KS, Rao DMR, Devi AP, & Murty AS (1981) Toxicity of carbaryl
and 1-naphthol to four species of freshwater fish. J Biosci, 3(4):
457-462.
Tilden RL & Van Middelem CH (1970) Determination of carbaryl as an
amide derivative by electroncapture gas chromatography. J Agric Food
Chem, 18(1): 154-158.
Ting KC, Kho PK, Musselman AS, Root JA, & Tichelaar R (1984) High
performance liquid chromatographic method for determination of six
N-methylcarbamates in vegetables and fruits. Bull Environ Contam
Toxicol, 33: 538-547.
Tobin JS (1970) Carbofuran. A new carbamate insecticide. J Occup
Med, 12(1): 16-19.
Toor HS & Kaur K (1974) Toxicity of pesticides to the fish Cyprinus
carpio communis Linn. Indian J Exp Biol, 12: 334-336.
Tos-Luty S, Puchala-Matysek W, & Latuszynska J (1973) [Carbaryl
toxicity in chick embryo.] Bromatol Chem Toksykol, 6: 409-411 (in
Polish with English summary).
Trial JG (1978) The effects of Sevin-4-oil on aquatic insect
communities of streams: a continuation of 1976 studies. In:
Environmental monitoring of cooperative spruce budworm control
projects, Maine 1976 and 1977. Augusta, Maine, Maine Department of
Conservation, Forest Service, pp 124-140.
Trial JG (1979) The effects of Sevin-4-oil on aquatic insect
communities of stream (1976-1978). In: Environmental moitoring of
cooperative spruce budworm control projects, Maine 1978. Augusta,
Maine, Maine Department of Conservation, Forest Service, pp 6-22.
Triolo AJ, Lang WR, Coon JM, Lindstrom D, & Herr DL (1982) Effect of
the insecticide toxaphene and carbaryl on induction of lung tumors
by benzo(a)pyrene in the mouse. J Toxicol Environ Health, 9(4):
637-649.
Tripathi G & Shukla SP (1988) Toxicity bioassay of technical and
commercial formulations of carbaryl to the freshwater catfish,
Clarias batrachus. Ecotoxicol Environ Saf, 15(3): 277-281.
Tyburczyk W & Podolak-Majczak M (1984a) [Effect of joint action of
sodium nitrite and carbaryl on rats organism.] Bromatol Chem
Toksykol, XVII(2): 125-129 (in Polish with English summary).
Tyburczyk W & Podolak-Majczak M (1984b) [Effect of joint action of
sodium nitrite and carbaryl on rats organism.] Bromatol Chem
Toksykol, XVII(3): 215-219 (in Polish with English summary).
Tyburczyk W & Podolak-Majczak M (1986) [Effect of joint action of
sodium nitrite and carbaryl on rats organism. Part V. The role of
glucose-6-phosphate dehydrogenase and haematologic studies.]
Bromatol Chem Toksykol, XIX(3): 167-173 (in Polish with English
summary).
Uchiyama M, Takeda M, Suzuki T, & Yoshikawa K (1975) Mutagenicity of
nitroso derivatives of N-methylcarbamate insecticides in
microbiological method. Bull Environ Contam Toxicol, 14: 389-394.
US Department of Labour (1978) Occupational health guideline for
carbaryl (Sevin/R). Washington, DC, US Department of Labour, pp 1-5.
US EPA (1977) Aspects of pesticidal uses of carbaryl on man and the
environment. Washington, DC, US Environmental Protection Agency,
Office of Pesticide Programs, 286 pp.
US EPA (1980a) Carbaryl. Decision document. Washington, DC, US
Environmental Protection Agency, Office of Pesticides and Toxic
Substances, 22 pp.
US EPA (1980b) FIFRA Scientific Advisory Panel. Subcommittee
meeting, Arlington, Virginia, 23 July 1980. Washington, DC, US
Environmental Protection Agency, 202 pp.
US EPA (1981) Environmental fates and impacts of major forest use of
pesticides. Washington, DC, US Environmental Protection Agency,
Office of Pesticides and Toxic Substances.
US EPA (1984) Health and environmental profile for carbaryl.
Cincinnati. Ohio, US Environmental Protection Agency.
Usha Rani MV, Reddi OS, & Reddy PP (1980) Mutagenicity studies
involving aldrin, endosulfan, demethoate, phosphamidon, carbaryl and
ceresan. Bull Environ Contam Toxicol, 25(2): 277-282.
Vandekar M (1965) Observations of the toxicity of carbaryl,
folithion and 3-isopropylphenyl N-methylcarbamate in a village
scale trail in Southern Nigeria. Bull World Health Organ, 33:
107-115.
Vandekar M, Plestina R, & Wilhelm M (1971) Toxicity of carbamates
for mammals. Bull World Health Organ, 44: 241-249.
Varshney TN & Gaur AC (1972) Effect of DDT and Sevin on soil fungi.
Acta Microbiol Acad Sci Hung, 19: 97-102.
Vashakidze VI (1966) [On the question of the embryotropic,
gonadotropic and mutagenic effect of some pesticides.] Gig Primen
Toxicol Pestic Klin Otrav, 1966: 80-83 (in Russian).
Vashakidze VI (1975) [Effect of small doses of Sevin on gonad
function following its repeated effect on white rats SbTr NII.] Gig
Tr Prof Zabol, 14: 235-266 (in Russian).
Vasilos AF, Dmitrienko VD, & Shroyt IG (1975) [Disruption of the
mitotic system following acute Sevin poisoning.] Izv Akad Nauk
Moldavskoj SSR Ser Khim Nauk, 3: 64-67 (in Russian).
Venkateswarlu K, Chendrayan K, & Sethunathan N (1980) Persistence
and biodegradation of carbaryl in soils. J Environ Sci Health,
B15(4): 421-429.
Venkat Reddy P, Prem Veer Reddy G, & Subramanyams S (1974)
Cytological effects of the insecticide Sevin on the mitotic cells of
Poecilocerus pictus. Curr Sci, 43(6): 187-189.
Verma SR, Tonk IF, Gupta AK, & Saxena M (1984) Evaluation of an
application factor for determining the safe concentration of
agricultural and industrial chemicals. Water Res, 18(1): 111-115.
Viter VF (1978) [Monotonous and intermittent inhalatory effect of
Sevin upon some behavioural reactions of experimental animals.] Gig
i Sanit, 11: 33-34 (in Russian).
Vyatchanikov KA & Alexachina ZA (1968) [Experimental investigations
for procuring therapeutic and protective means at intoxications by
Sevin.] Gig Primen Toxicol Pestic Klin Otrav, 1968: 442-444 (in
Russian).
Wagner R (1973) [Identification and estimation of organophosphate
insecticides and carbaryl on ready made thin-layer plates.] Z
Gesamte Hyg Grenzgeb, 19(7): 504-510 (in German).
Wakakura M, Ishikawa S, & Uga S (1978) Ultrastructural hepatic
changes by carbamate pesticide (Sevin) in rats. Environ Res, 16:
191-204.
Walker N, Janes NF, Spokes JR, & Van Berkum P (1975a) Degradation of
1-naphthol by a soil pseudomonad. J Appl Bacteriol, 39: 281-286.
Walker M, Gale GR, Atkins LM, & Gadse-Den RH (1975b) Some effects of
carbaryl on Ehrlich ascites tumor cells in vitro and in vivo.
Bull Environ Contam Toxicol, 14(4): 441-448.
Waller GD (1969) Susceptibility of an alfalfa leaf-cutting bee to
residues of insecticides on foliage. J Econ Entomol, 62(1): 189-192.
Ward SA, May DG, Heath AJ, & Branch RA (1988) Carbaryl metabolism is
inhibited by Cimetidine in the isolated perfused rat liver and in
man. J Toxicol Clin Toxicol, 26(5/6): 269-281.
Wauchope RD & Haque R (1973) Effects of pH, light and temperature on
carbaryl in aqueous media. Bull Environ Contam Toxicol, 9(5):
257-260.
Weber FJ, Shea TB, & Berry SE (1982) Toxicity of certain
insecticides to protozoa. Bull Environ Contam Toxicol, 28: 618-631.
Weil CS & Carpenter CP (1962) Studies on carcinogenesis completed in
1962. Pittsburgh, Pennsylvania, Carnegie-Mellon Research Institute
(Special Report 25-122) (Unpublished proprietary information
submitted to WHO by Rhône-Poulenc Agro, Lyon).
Weil CS & Carpenter CP (1965) Results of a three generations
reproductive study on rats fed Sevin in their diet. Pittsburgh,
Pennsylvania, Carnegie-Mellon Research Institute, 18 pp (Report No.
28-53) (Unpublished proprietary information submitted to WHO by
Rhône-Poulenc Agro, Lyon).
Weil CS & Carpenter CP (1974) Sevin: range finding toxicity studies.
Pittsburgh, Pennsylvania, Carnegie Mellon (Report No. 37-53)
(Unpublished proprietary information submitted to WHO by
Rhône-Poulenc Agro, Lyon).
Weil CS, Woodside MD, Carpenter CP, & Smyth HF Jr (1972) Current
studies of tests of carbaryl for reproductive and teratogenic
effect. Toxicol Appl Pharmacol, 21: 390-404.
Weil CS, Woodside MD, Bernard JB, Condra NI, King JM, & Carpenter CP
(1973) Comparative effect of carbaryl on rat reproduction and
guinea-pig teratology when fed either in the diet or by stomach
intubation. Toxicol Appl Pharmacol, 26: 621-638.
Weston RF, Inc. (1982) Carbaryl: A profile on its characteristics as
pesticide. Rockville, Maryland, Roy F. Weston, Inc., Environmental
Health Sciences Centre.
Whitehurst WE, Bishop ET, Critchfield FE, Gyrisco GG, Huddleston EW,
Arnold H, & Lisk OJ (1963) The metabolism of Sevin in dairy cows. J
Agric Food Chem, 11: 167-169.
WHO (1982) Recommended health-based limits in occupational exposure
to pesticides. Report of a WHO study group. Geneva, World Health
Organization, 110 pp (WHO Technical Report Series 677).
WHO (1986) IPCS Environmental Health Criteria 64: Carbamate
pesticides - A general introduction. Geneva, World Health
Organization, 136 pp.
WHO (1992) The WHO recommended classification of pesticides by
hazard and guidelines to classification 1992-1993. Geneva, World
Health Organization, 66 pp (Unpublished document WHO/PCS/92.14).
WHO/FAO (1975) Data sheets on pesticides No. 3: Carbaryl. Geneva,
World Health Organization, 8 pp (Unpublished document VBC/DS/75.3,
Rev. 1).
Whorton MD, Milby TH, Stubbs HA, Avasha BH, & Hull EG (1979)
Testicular function among carbaryl exposed employees. J Toxicol
Environ Health, 5: 925-941.
Wiggins OG & Weiden MHJ (1969) Fate of Sevin applied to the surface
of bean fruit and bean plants under field conditions. Clayton, North
Carolina, Union Carbide Corporation (Report No. 11377) (Unpublished
proprietary information submitted to WHO by Rhône-Poulenc Agro,
Lyon).
Wilkes L, Mulkey N, & Wargo J (1977) Research report laboratory soil
metabolism study of carbaryl (Project No. 285). Monument, Colorado,
Analytical Development Corp. (Unpublished proprietary information
submitted to WHO by Rhône-Poulenc Agro, Lyon).
Wills JH, Jameson E, & Coulston F (1968) Effects of oral doses of
carbaryl on man. Clin Toxicol, 1:(3) 265-271.
Wiltrout RW, Ercegovich CD, & Ceglowski WS (1978) Humoral immunity
in mice following oral administration of selected pesticides. Bull
Environ Contam Toxicol, 20(3): 423-431.
Winterlin W & Walker G (1973) Carbaryl residues in bees, honey, and
bee bread following exposure to carbaryl via the food supply. Arch
Environ Contam Toxicol, 1(4): 362-374.
Wojciechowski JP, Kaur P, & Sabharual PS (1982) Induction of ouabain
resistance in V-79 cells by four carbamate pesticides. Environ Res,
29: 48-53.
Wolfe HP, Durham W, & Armstrong J (1967) Exposure of workers to
pesticides. Arch Environ Health, 14: 622-633.
Wolfe NL, Zepp RG, & Paris DF (1978) Carbaryl, propham and
chlorpropham: A comparison of the rates of hydrolysis and photolysis
with the rate of biolysis. Water Res, 12(8): 565-571.
Woodruff RC, Phillips JP, & Irwin D (1983) Pesticide-induced
complete and partial chromosome loss in screens with
repair-defective females of Drosophila melanogaster. Environ
Mutagen, 5: 835-846.
Woodward DF & Mauck WL (1980) Toxicity of five forest insecticides
to cutthroat trout and two species of aquatic invertebrates. Bull
Environ Contam Toxicol, 25: 846-853.
Wright CG, Leidy RB, & Dupress HE (1981) Insecticides in the ambient
air of rooms following their application for control of pests. Bull
Environ Contam Toxicol, 26(4): 548-553.
Wuu K & Grant W (1966) Morphological and somatic chromosomal
aberrations induced by pesticides in barley (Hordeum vulgare). Can
J Genet Cytol, 8: 481-501.
Wyrobek AJ, Watchmaker G, Gordon L, Wong K, Moore D, & Whorton D
(1981) Sperm shape abnormalities in carbaryl-exposed employees.
Environ Health Perspect, 40: 225-265.
Yadav PR & Jaglan RS (1982) Persistence of carbaryl residues in and
on unprocessed and processed cauliflower curds. Beitr Trop
Landwirtsch Veterinärmed, 20(1): 89-95.
Yadav GS, Kathpal TS, & Singh H (1985) Efficacy of monocrotophos,
carbaryl, and endosulfan against Antigastra catalaunalis Dup. and
their persistence in soil and sesamum seeds. Indian J Agric Sci,
55(6): 438-442.
Yakim VS (1965) [Toxicological hygienic characteristics of Sevin.]
Gig Primen Toxicol Pestic Klin Otrav, 1965: 423-431 (in Russian).
Yakim VS (1967) [Data for substantiating the MAC values for Sevin in
the air of the working zone.] Gig i Sanit, 4: 23-33 (in Russian).
Yakim VS (1968) [The toxicity of Sevin at inhalatory intake.] Gig
Primen Toxicol Pestic Klin Otrav, 1968: 115-117 (in Russian).
Yakim VS (1970) [Content and distribution of Sevin in warm-blooded
animals organism and its effect on ChE activity.] Gig Primen Toxicol
Pestic Klin Otrav, 1970: 214-218 (in Russian).
Yess NJ, Houston MG, & Gunderson EL (1991a) Food and Drug
Administration pesticide residue monitoring of foods: 1978-1982. J
Assoc Off Anal Chem, 75(2): 265-272.
Yess NJ, Houston MG, & Gunderson EL (1991b) Food and Drug
Administration pesticide residue monitoring of foods: 1983-1986. J
Assoc Off Anal Chem, 74(2): 273-280.
Young RR (1990) Mutagenicity test on carbaryl (technical) in the
CHO/HGPRT forward mutation assay. Kensington, Maryland, Hazleton
Laboratories America, Inc. (Study No. 10862-0-435) (Unpublished
proprietary information submitted to WHO by Rhône-Poulenc Agro,
Lyon).
Zabezhinski MA (1970) [Possible carcinogenic effect of ß-Sevin.]
Vopr Onkol, 16: 106-107 (in Russian).
Zapko WG (1970) [Effect on some liver functions of acute
intoxication of experimental animals with cuprosin, Sevin, Eptam and
TMTD.] Gig Primen Toxicol Pestic Klin Otrav, 1970: 237-240 (in
Russian).
Zeakes SJ, Hansen MF, & Robel RJ (1981) Increased susceptibility of
Bobwhites (Colinus virginianus) to Histomonas meleagridis after
exposure to Sevin insecticide. Avian Dis, 25(4): 981-987.
Zepp RG & Cline DM (1977) Rates of direct photolysis in the aquatic
environment. Environ Sci Technol, 11: 359-366.
Zepp RG, Wolfe N, Gordon L, & Fincher RC (1976) Light-induced
transformations of methoxychlor in aquatic systems. J Agric Food
Chem, 24: 727-733.
Zinkl JG, Hennys ChJ, & Dewelse LR (1977) Brain cholinesterase
activity in birds from forest sprayed trichlorfon (Dylox) and
carbaryl (Sevin-4-oil). Bull Environ Contam Toxicol, 17(4):
379-386.
Zuberli R & Zubairi MY (1971) Carbaryl degradation by Pseudomonas
phaseolicola and Aspergillus niger. Pak J Sci Ind Res, 14(4-5):
383-384.
Zweig G, Gao R-Y, Witt JM, Propendorf W, & Bogen K (1984) Dermal
exposure to carbaryl by strawberry harvesters. J Agric Food Chem,
32(6): 1232-1236.
Zweig G, Leffingwell JT, & Propendorf W (1985) The relationship
between dermal pesticide exposure to fruit harvesters and
dislodgeable foliar residues. J Environ Sci Health, B20(1): 27-59.
ANNEX I. TREATMENT OF CARBAMATE PESTICIDE POISONING IN MAN
(From EHC 64: Carbamate Pesticides - A General Introduction)
All cases of carbamate poisoning should be dealt with as an
emergency and the patient should be hospitalized as quickly as
possible.
Extensive descriptions of the treatment of poisoning by
anticholinesterase agents are given in several major references
(Kagan, 1977;Taylor, 1980; Plestina, 1984).
The treatment is based on:
(a) minimizing the absorption;
(b) general supportive treatment; and
(c) specific pharmacological treatment.
I.1 Minimizing the absorption
When dermal exposure occurs, decontamination procedures include
removal of contaminated clothes and washing of the skin with
alkaline soap or with a sodium bicarbonate solution. Particular care
should be taken in the cleaning of the skin area where venupuncture
is performed. Blood might be contaminated with carbamates and
therefore inaccurate measures of ChE inhibition might result.
Extensive eye irrigation with water or saline should also be
performed. In the case of ingestion, vomiting can be induced, if the
patient is conscious, by the administration of ipecacuanha syrup
(10-30 ml) followed by 200 ml of water. However, this treatment is
contraindicated in the case of pesticides dissolved in hydrocarbon
solvents. Gastric lavage (with the addition of bicarbonate solution
or activated charcoal) can also be performed, particularly in
unconscious patients, taking care to prevent aspiration of fluids
into the lungs (i.e., only after a tracheal tube has been put in
place).
The volumes of the fluids introduced in the stomach should be
recorded and samples of gastric lavage frozen and stored for
subsequent chemical analysis. If the formulation of the pesticide
involved is available, it should also be stored for further analysis
(i.e., detection of toxicologically relevant impurities). A purge to
remove the ingested compound can be administered.
I.2 General supportive treatment
Artificial respiration (via a tracheal tube) should be started
at the first sign of respiratory failure and maintained for as long
as necessary.
Cautious administration of fluids is advised as well as general
supportive and symptomatic pharmacological treatment and absolute
rest.
I.3 Specific pharmacological treatment
I.3.1 Atropine
Atropine should be given, beginning with 2 mg iv repeated at 15
to 30-min intervals. The dose and the frequency of atropine
treatment varies from case to case, but should maintain the patient
fully atropinized (dilated pupils, dry mouth, skin flushing, etc.).
I.3.2 Oxime reactivations
Although it might be suspected that oxime cholinesterase
reactivators would be as helpful in carbamate poisoning as they are
in organophosphorous poisoning, this is not the case. There is
experimental evidence that the pyridinium oxime 2-PAM is not
effective in carbamate poisoning and there is some evidence that it
makes poisoning by certain carbamates, including carbaryl, worse.
I.3.3 Diazepam
Diazepam should be included in the therapy of all but the
mildest cases. Besides relieving anxiety it appears to counteract
some aspects of CNS-derived symptoms that are not affected by
atropine. Doses of 10 mg sc or iv are appropriate and may be
repeated as required.
Other centrally acting drugs and drugs that may depress
respiration are not usually recommended in the absence of artificial
respiration procedures.
References to Annex I
KAGAN, J.S. (1977) [The toxicity of organophosphorus pesticides.]
Moscow (in Russian).
PLESTINA, R. (1984) Prevention, diagnosis, and treatment of
insecticide poisoning. Geneva, World Health Organization.
(Unpublished WHO document VBC/84.889).
TAYLOR, P. (1980) Anticholinesterase agents. In: Goodman, L.S. &
Gilman, A., ed. The pharmacological basis of therapeutics. 6th ed.,
New York, Macmillan Publishing Co., pp. 100-119.
RESUME ET EVALUATION, CONCLUSIONS ET RECOMMANDATIONS
1. Résumé et évaluation
1.1 Identité, propriétés et méthodes d'analyse
Carbaryl est la dénomination commune d'un dérivé de l'acide
carbamique, le N-méthylcarbamate de 1-naphtyle. Le produit
technique consiste en un solide cristallin blanc, peut volatil et
peu soluble dans l'eau, qui est stable à la lumière et à la chaleur
mais qui s'hydrolyse facilement en milieu alcalin. Il existe une
norme FAO pour le carbaryl qui stipule un degré de pureté de 98%,
avec une limite pour les impuretés ( N-méthylcarmate de ß-naphtyle)
de 0,05%.
Pour les analyses portant sur le carbaryl et ses métabolites,
il existe de nombreuses méthodes: chromatographie sur couche mince,
spectrophotométrie, chromatographie en phase gazeuse,
chromatographie en phase liquide à haute pression et spectrométrie
de masse à ionisation chimique. La limite de détection peut
descendre en-dessous du nanogramme et le taux de récupération
dépasse généralement 80%.
1.2 Production et usages
On utilise le carbaryl depuis une trentaine d'années comme
insecticide agissant par contact et par ingestion avec certaines
propriétés endothérapiques; il permet de lutter contre de nombreux
vecteurs et ravageurs. La principale unité de production se trouve
aux Etats-Unis d'Amérique. Plus de 290 fabricants proposent une
gamme de formulations de carbaryl qui dépasse les 1500 produits.
1.3 Transport, distribution et transformation dans l'environnement
Dans la plupart des conditions, le carbaryl ne persiste pas
dans l'environnement. Dans l'eau, son temps de demi-hydrolyse dépend
de la température, du pH et de la concentration initiale; il varie
de quelques minutes à plusieurs semaines. Le principal produit de
décomposition est le 1-naphtol.
L'accumulation du carbaryl, exprimée sous la forme d'un facteur
de bioconcentration dans l'environnement aquatique, et plus
précisément chez les poissons d'eau douce, varie de 14 à 75. Le
carbaryl s'adsorbe plus facilement sur les sols à forte teneur
organique que sur les sols sableux. Aux doses d'emploi usuelles, et
lorsqu'il est appliqué conformément "aux bonnes pratiques
agricoles", il se dissipe rapidement, avec une demi-vie de 8 jours à
un mois dans les conditions normales. Il arrive que le carbaryl soit
entraîné par les pluies ou par les travaux agricoles de la surface
vers les couches sous-jacentes (à un mètre de profondeur).
Le carbaryl peut contaminer la végétation, soit au cours de
l'épandage soit par migration à partir d'un sol contaminé.
La décomposition du carbaryl dans l'environnement dépend de son
degré de volatilisation, de photodécomposition et de dégradation
chimique ou microbienne dans le sol, l'eau et les végétaux. la
vitesse de décomposition est plus rapide en climat chaud.
1.4 Concentrations dans l'environnement et exposition humaine
Dans la population générale, le carbaryl est principalement
absorbé par la voie alimentaire.
Les résidus présents dans des échantillons de rations totales
sont relativement faibles, et ils vont de traces à 0,05 mg/kg. Aux
Etats-Unis d'Amérique, on estime que pendant les premières années
d'utilisation du carbaryl, l'ingestion journalière de carbaryl était
de 0,15 mg/personne/jour (dans 7,4% des composites); cette dose est
tombée à 0,003 mg/personne/jour en 1969 (dans seulement 0,8% des
composites). Pendant la période d'épandage, on peut retrouver
parfois du carbaryl dans les eaux de surface et les retenues d'eau.
La population peut être exposée au carbaryl lors d'opérations
de lutte de contre les ravageurs ou les vecteurs tant au domicile
que sur les aires de loisir.
Les travailleurs peuvent être exposés au carbaryl lors de la
fabrication, de la formulation, de l'emballage, du transport et du
stockage du produit ainsi que pendant son épandage. Les
concentrations dans l'atmosphère des lieux de travail au cours de la
production varient de < 1 mg/m3 à 30 mg/m3. Il peut y avoir une
importante exposition cutanée chez les travailleurs de l'industrie
et les ouvriers agricoles en cas de mesures de protection
insuffisantes.
1.5 Cinétique et métabolisme
Le carbaryl est rapidement absorbé au niveau des poumons et des
voies digestives. Une dose de carbaryl dans l'acétone appliquée sur
la peau de volontaires humains a été absorbée par voie percutanée à
raison de 45% en 8 heures. Toutefois, les données obtenues in vitro
concernant la pénétration cutanée ainsi que les données
toxicologiques indiquent que l'absorption percutanée s'effectue
généralement à un rythme beaucoup plus lent.
Les principales voies métaboliques du carbaryl consistent en
une hydroxylation du cycle et une hydrolyse. Il en résulte la
formation de nombreux métabolites qui subissent ensuite une
conjugaison avec formation de sulfates, de glucuronides et de
mercapturates hydrosolubles qui sont excrétés dans l'urine.
L'hydrolyse conduit à la formation de 1-naphtol, de dioxyde de
carbone et de méthylamine. L'hydroxylation produit du
4-hydroxycarbaryl, du 5-hydroxycarbaryl, du
N-hydroxyméthylcarbaryl, du 5,6-dihydro-5,6-dihydroxycarbaryl et
du 1,4-naphtalènediol. Le principal métabolite chez l'homme est le
1-naphtol.
Dans les conditions normales d'exposition, il est improbable
que le carbaryl s'accumule chez les animaux. Il est excrété
principalement par la voie urinaire étant donné que la
détoxification de son produit d'hydrolyse, le 1-naphtol, s'effectue
essentiellement par la formation de conjugués hydrosolubles. Dans le
cas des métabolites du carbaryl, le cycle entérohépatique joue
également un rôle considérable, en particulier après administration
par voie orale.
Le produit d'hydrolyse, l'acide N-naphtolcarbamique, se
décompose spontanément en méthylamine et dioxyde de carbone. La
méthylamine subit ensuite une déméthylation en dioxyde de carbone et
formiate, ce dernier étant ultérieurement excrété, en majeure partie
dans l'urine.
Des métabolites du carbaryl sont également présents en faible
proportion de la dose absorbée dans la salive et le lait.
1.6 Effets sur les êtres vivants dans leur milieu naturel
Les valeurs de la CL50 pour les crustacés varient de 5 à 9
µg/litre (puces d'eau et mysidés), 8 à 25 µg/litre (orchesties) et
500 à 2500 µg/litre (écrevisses). Chez les insectes aquatiques, les
limites de sensibilité sont du même ordre. Les plécoptères et
éphémèroptères (perles et éphémères) en constituent les groupes les
plus sensibles. Les mollusques sont moins sensibles avec des valeurs
de la CE50 de l'ordre de quelques mg/litre. Dans le cas des
poissons, la plupart des valeurs de la CL50 se situent entre 1 et
30 mg/litre. Les salmonidés constituent le groupe le plus sensible.
La toxicité aiguë est faible pour les oiseaux. La DL50 pour
la sauvagine et le gibier à plumes en général est > 1000 mg/kg.
D'après les tests, l'oiseau le plus sensible est un francolin
(Francolinus levaillanti) (DL50 = 56 mg/kg). Rien n'indique que
les oiseaux aient eu à souffrir de l'effet des épandages effectués
sur les zones forestières à la dose de 1,1 kg de carbaryl/ha.
Le carbaryl est très toxique pour les abeilles et les lombrics.
Dans le cas des abeilles, la DL50 par voie orale est de 0,16
µg/insecte (soit 1-2 mg/kg).
On est fondé à penser que le carbaryl puisse avoir une
influence temporaire sur la composition en espèces des écosystèmes
terrestres et aquatiques. Par exemple, une étude a montré que les
effets exercés sur certaines communautés d'invertébrés terrestres
pourraient persister au moins 10 mois après un seul épandage.
1.7 Effets sur les animaux d'expérience et les systèmes d'épreuve
in vitro
La toxicité aiguë, exprimée sous la forme de la DL50, varie
considérablement selon l'espèce, la formulation et le véhicule du
produit. La DL50 estimative par voie orale pour le rat varie de
200 à 850 mg(kg. Les chats sont plus sensibles, avec une DL50 de
150 mg/kg. Les porcs et les singes le sont moins, avec une DL50 >
1000 mg/kg.
Une concentration de 792 mg de matière active par m3, qui
constitue la valeur maximale qu'on puisse obtenir pour un aérosol, a
produit, au cours d'une exposition de 4 heures, une mortalité de 20%
(1/5) chez des rattes. A des concentrations de 20 mg/m3, des
aérosols de carbaryl ont provoqué une réduction de l'activité
cholinestérasique chez des chats lors d'une exposition de 4 heures,
mais cette concentration n'a eu aucun effet observable chez des
rats.
Le carbaryl est légèrement irritant pour l'oeil et n'a que peu
ou pas de pouvoir sensibilisateur. Lors d'études à long terme, la
concentration sans effet nocif observable a été évaluée à 10 mg/kg
de poids corporel (200 mg/kg de nourriture) pour le rat et à 1,8
mg/kg de poids corporel (100 mg/kg de nourriture) pour le chien.
Chez le chat, soumis à une exposition de longue durée par
inhalation, la concentration sans effet nocif observable est de 0,16
mg/m3. Le carbaryl a un faible potentiel d'accumulation.
1.7.1 Reproduction
On a montré que le carbaryl avait des effets indésirables sur
la reproduction des mammifères et le développement périnatal chez un
certain nombre d'espèces. Ces effets sur la reproduction consistent
en une réduction de la fécondité, une diminution de l'effectif des
portées et une réduction de la viabilité postnatale. Les effets
toxiques du carbaryl sur le développement se traduisent pas un
certain nombre de morts foetales, une réduction du poids foetal et
la présence de malformations. A l'exception d'un petit nombre
d'études, les effets nocifs sur la reproduction et le développement
n'ont été constatés en totalité qu'à des doses manifestement
toxiques pour la mère, et dans un certain nombre de cas, la mère
était plus sensible au carbaryl que l'embryon ou le foetus. Ces
effets toxiques sur la femelle gestante consistaient en une
mortalité accrue, une réduction de la croissance et des dystocies.
Les données indiquent que, par rapport à l'organisme adulte, la
fonction de reproduction et le processus de développement des
mammifères n'est pas particulièrement sensible au carbaryl.
1.7.2 Mutagénicité
Un certain nombre de tests in vitro et in vivo ont été
effectués sur la carbaryl afin d'en évaluer le pouvoir mutagène;
divers points d'aboutissement de ces effets ont été étudiés sur
divers systèmes (bactéries, levures, végétaux, insectes et
mammifères).
Les données disponibles montrent que le carbaryl n'a aucune
tendance à endommager l'ADN. On ne dispose d'aucun rapport
confirmant les caractères mutagènes suivants: induction de
recombinaisons mitotiques, conversion génique, et synthèse non
programmée de l'ADN chez les procaryotes (H. influenzae, B.
subtilis) ni chez les eucaryotes ( S. cerevisiae, A. nidulans,
lymphocytes humains en culture et hépatocytes de rat) in vitro.
La recherche de mutations géniques, qui a donné lieu à un grand
nombre d'épreuves sur systèmes bactériens, a toujours donné des
résultats négatifs, sauf dans deux cas. Lors de plusieurs études
portant sur les mutations géniques et effectuées sur des cellules
mammaliennes in vitro, le carbaryl n'a donné qu'un seul résultat
positif équivoque dans une de ces études. Toutefois, cette étude
présentait un certain nombre de défauts et ce résultat n'a pas été
confirmé par ceux d'autres études comparables.
Des lésions chromosomiques ont été observées in vitro sur des
cellules humaines, des cellules de rat et de hamster ainsi que des
cellules végétales exposées à des fortes doses de carbaryl. Aucun
effet de ce genre n'a été observé lors d'épreuves in vivo sur des
systèmes mammaliens, même à des doses atteignant 1000 mg/kg.
On a montré que le carbaryl perturbe le mécanisme d'élongation
des fibres du fuseau chez les cellules végétales et mammaliennes in
vitro. On ne sait pas encore très bien si les résultats des
épreuves effectuées sur des végétaux sont extrapolables à l'homme.
Compte tenu de la base de données sont on dispose actuellement,
on peut conclure que rien n'autorise à soupçonner le carbaryl de
présenter un risque d'effets mutagènes pour les cellules somatiques
ou germinales de l'homme.
Un dérivé nitrosé du carbaryl, le N-nitrosocarbaryl, peut
provoquer des recombinaisons mitotiques et des conversions géniques
chez les procaryotes (H. influenzae, B. subtilis) et les
eucaryotes (S. cerevisiae) in vitro, et il donne des résultats
positifs lors de "spot tests" effectués sur E. coli.
En outre, les résultats expérimentaux indiquent que le
N-nitrosocarbaryl se lie à l'ADN provoquant la formation de
liaisons alcalino-sensibles et des cassures monocaténaires.
Il n'est pas établi que le nitrosocarbaryl soit un agent
clastogène in vivo (cellules de la moelle osseuse et cellules
germinales) même à doses toxiques élevées.
1.7.3 Cancérogénicité
De nombreuses études ont été consacrées au pouvoir cancérogène
du carbaryl chez le rat et la souris. La plupart de ces études ont
donné des résultats négatifs mais il s'agit de travaux anciens qui
ne satisfont pas aux normes actuelles. Quoiqu'il en soit, de
nouvelles études portant sur ces mêmes animaux et qui, elles,
satisfont aux exigences modernes, sont actuellement en coursa. La
dernière évaluation du CIRC (CIRC, 1987) a conclu que l'existence de
cancers imputables au carbaryl n'était pas documentée chez l'homme
et qu'on ne possédait pas de preuves suffisantes d'un pouvoir
cancérogène de cette substance chez les animaux de laboratoire. Il
n'a donc pas été possible de classer le carbaryl en fonction de son
pouvoir cancérogène pour l'homme (Groupe 3).
On a montré que le N-nitrosocarbaryl induisait des tumeurs
locales chez les rats (sarcomes au point d'injection ou carcinomes
spinocellulaires au niveau de la portion cardiaque de l'estomac,
lorsque la substance est administrée par voie orale). Etant donné la
biochimie du carbaryl chez l'homme, on peut considérer comme
négligeable le risque de cancérisation par le N-nitrosocarbaryl
résultant d'une exposition humaine au carbaryl.
1.7.4 Effets sur les différents organes et systèmes
a) Système nerveux
Les effets du carbaryl sur le système nerveux tiennent
essentiellement à l'inhibition de la cholinestérase qui est
généralement passagère. Ces effets ont été étudiés sur des rats et
des singes. Des doses de 10 à 20 mg de carbaryl par kg, administrées
pendant 50 jours par voie orale, ont provoqué des perturbations dans
l'apprentissage et l'exécution de certaines épreuves par les rats
traités.
______________
a Ces études n'ont pas encore fait l'objet d'un examen par le
PISC. La société qui effectue ces travaux indique qu'aux doses
les plus fortes étudiées, on note une augmentation
significative de la fréquence des tumeurs chez les deux
espèces.
Lors d'une petite étude portant sur des porcs, du carbaryl
administré pendant 72 à 82 jours en mélange avec la nourriture à
raison de 150 mg/kg de poids corporel, a produit un certain nombre
d'effets neuromusculaires. On a observé en outre chez des poulets
ayant reçu de fortes doses de carbaryl, une faiblesse réversible des
pattes. Aucun signe de démyélinisation n'a été observé dans le
cerveau, le nerf sciatique ou les coupes de moelle épinière
examinées au microscope. On n'a pas observé d'effets de ce genre à
l'issue d'études à long terme sur des rongeurs.
b) Système immunitaire
Administré in vivo à des doses provoquant des signes
cliniques manifestes, le carbaryl exerce divers effets sur le
système immunitaire. Nombre de ces effets ont été observés à des
doses voisines de la DL50. Dans la plupart des études où les doses
utilisées permettaient la survie des lapins et des souris examinés,
on n'a pas observé d'effets significatifs sur le système
immunitaire. Plusieurs de ces travaux présentaient des défauts, par
exemple un manque de cohérence et parfois des contradictions
manifestes dans les résultats, défauts qui ne permettent pas de
dégager des résultats obtenus un mécanisme immunotoxique bien
défini.
c) Sang
Le carbaryl affecterait l'hémostase mais les résultats
concernant le sens de cet effet sont contradictoires. On a observé
que le carbaryl produisait une augmentation de la formation de
méthémoglobine liée à la dose dans des érythrocytes de moutons
présentant un déficit en glucose-6-phosphate-déshydrogénase. La
sérum-albumine humaine réagit in vitro sur le groupement ester du
carbaryl. Le carbaryl se lie aux acides aminés libres.
d) Foie
On a fait état de troubles affectant le métabolisme des
glucides et la synthèse des protéines au niveau du foie ainsi que
d'une perturbation de la fonction détoxifiante de cet organe chez
les mammifères. Le carbaryl induit faiblement l'activité
pharmacométabolisante des microsomes hépatiques. Il y a
raccourcissement de la durée du sommeil induit par le phénobarbital.
Les taux hépatiques de cytochrome P-450 et b5 sont augmentés. Ces
modifi-cations du métabolisme hépatique pourraient expliquer en
partie le triplement de la DL50 pour le carbaryl observé chez des
rats préalablement traités par cette substance.
e) Fonction gonadotrope
Il a été signalé que le carbaryl augmentait la fonction
gonadotrope de l'hypophyse chez le rat.
1.7.5 Mécanisme fondamental de la toxicité
Le carbaryl est un inhibiteur de l'activité cholinestérasique.
Cet effet est lié à la dose et rapidement réversible. On n'a pas
observé de vieillissement de la cholinestérase carbamylée. Tous les
métabolites reconnus du carbaryl ont une activité
anticholinestérasique sensiblement plus faible que le carbaryl
lui-même.
1.8 Effets sur l'homme
Le carbaryl est facilement absorbé par inhalation et après
administration par voie orale mais moins facilement pas la voie
percutanée. Etant donné que l'inhibition de la cholinestérase est le
principal mécanisme de l'action du carbaryl, le tableau clinique
d'une intoxication par cette substance est dominé par les symptômes
correspondants, à savoir: augmentation de la sécrétion bronchique,
sueurs profuses, salivation et larmoiement; myosis,
bronchoconstriction, crampes abdominales (vomissements et diarrhée);
bradycardie; fasciculation des petits muscles (dans les cas graves
le diaphragme et les muscles respiratoires sont également atteints);
tachycardie, céphalées, vertiges, angoisses, confusion mentale, coma
et dépression des centres respiratoires. Ces signes d'intoxication
se manifestent rapidement après l'absorption et disparaissent aussi
vite une fois que l'exposition a cessé.
Des études contrôlées sur des volontaires humains ont montré
que des doses uniques inférieures à 2 mg/kg étaient bien tolérées.
Une dose unique de 250 mg (2,8 mg/kg) a suscité des symptômes
modérés d'inhibition cholinestérasique (douleurs épigastriques et
sueurs) en l'espace de 20 minutes. Les sujets ont complètement
récupéré dans les 2 heures suivant un traitement par le sulfate
d'atropine.
En cas d'exposition excessive d'origine professionnelle au
carbaryl, on observe des symptômes légers bien avant qu'une dose
dangereuse ne soit absorbée, ce qui explique que les cas graves
d'intoxication professionnelle par le carbaryl soient rares. Lors
des épandages agricoles, l'exposition percutanée peut être
importante. Cependant on n'observe en général aucun effet irritant
local, encore que l'on ait décrit des éruptions cutanées à la suite
l'éclaboussures accidentelles de carbaryl en formulation liquide.
Les données concernant les effets du carbaryl sur le nombre de
spermatozoïdes et la modification de leur morphologie chez les
travailleurs de l'industrie sont contradictoires. Aucun effet
indésirable sur la reproduction n'a été signalé.
L'indicateur biologique le plus sensible de l'exposition au
carbaryl est l'apparition de 1-naphtol dans les urines et la
diminution de l'activité cholinestérasique du sang. On peut donc
utiliser la concentration urinaire du 1-naphtol comme indicateur
biologique à condition qu'il n'y ait pas de 1-naphtol sur le lieu de
travail. Lors de certains cas d'exposition professionnelle, on a
constaté que 40% des échantillons d'urine contenaient plus de 10 mg
de 1-naphtol total par litre. Dans un cas d'intoxication aiguë, on
en a trouvé 31 mg/litre d'urine. On considère qu'il y a danger à
partir de 10 mg/litre et que les symptômes apparaissent à partir de
30 mg de 1-naphtol par litre d'urine (Fiche d'information sur le
carbaryl, OMS, 1973, VBC/DS/75.3).
La mesure de l'activité cholinestérasique peut constituer un
test très sensible pour la surveillance médical des travailleurs, à
condition que le dosage soit effectué peu après l'exposition.
2. Conclusions
On estime que le carbaryl est peu dangereux pour l'homme en
raison de sa faible tension de vapeur, de sa décomposition rapide,
de la désinhibition spontanée également rapide de la cholinestérase
et en raison du fait que les symptômes d'intoxication apparaissent
bien avant qu'une dose dangereuse ne se soit accumulée dans
l'organisme. On ne dispose pas encore de bonnes études de
cancérogénicité qui satisfassent aux normes actuelles en la matière.
2.1 Exposition de la population générale
Les quantités résiduelles de carbaryl qui demeurent dans les
denrées alimentaires et l'eau de boisson après l'utilisation normale
de cet insecticide en agriculture, sont très inférieures à la dose
journalière acceptable (DJA) (0,01 mg/kg de poids corporel/jour) et
il est improbable qu'elles puissent constituer un risque pour la
santé de la population dans son ensemble.
2.2 Sous-groupes de population exposés à un risque élevé
Lorsqu'on utilise du carbaryl à des fins de santé publique,
soit au domicile soit sur des aires de loisir, il y a un risque
d'exposition excessive si l'on ne suit pas les règles concernant son
emploi.
2.3 Exposition professionnelle
En faisant respecter des méthodes de travail raisonnables et
notamment un certain nombre de précautions de sécurité et des
mesures de protection individuelle, avec une surveillance
convenable, il n'existe aucun risque qui puisse résulter d'une
exposition professionnelle au cours de la fabrication, de la
formulation et de l'épandage du carbaryl. Les produits non dilués
doivent être manipulés avec de grandes précautions car toute faute
de manipulation peut entraîner une contamination cutanée. Sur le
lieu de travail, la concentration atmosphérique ne doit pas dépasser
5 mg/m3.
2.4 Effets sur l'environnement
Le carbaryl est toxique pour les abeilles et les lombrics. On
ne doit pas procéder à son épandage pendant la floraison.
En utilisation normale, le carbaryl ne devrait pas poser des
problème écologique. Il est adsorbé en grande partie sur les
particules de sol et il ne passe pas facilement par lessivage dans
les eaux souterraines. Il subit une décomposition rapide dans
l'environnement et n'est donc pas persistant. L'emploi de carbaryl
ne devrait pas entraîner d'effets nocifs à court terme sur
l'écosystème.
3. Recommandations
* La manipulation et l'épandage du carbaryl doivent s'effectuer
en observant les précautions qui s'imposent pour tous les
pesticides. On suivra rigoureusement les instructions
d'utilisation qui figurent sur l'emballage.
* La fabrication, la formulation, l'emploi et l'élimination du
carbaryl doivent se faire avec toutes les précautions voulues
pour réduire au minimum la contamination de l'environnement.
* Les travailleurs qui sont soumis à une exposition régulière
doivent faire l'objet d'un contrôle médical périodique.
* La chronologie des épandages de carbaryl doit être réglée de
manière à éviter tout effet sur les espèces non visées.
* Il importe d'effectuer des études de cancérogénicité
satisfaisant aux normes modernes.
RESUMEN Y EVALUACION, CONCLUSIONES Y RECOMENDACIONES
1. Resumen y evaluación
1.1 Identidad, propiedades y métodos analíticos
El 1-naftil N-metil carbamato, derivado del ácido carbámico,
se conoce por el nombre común de carbarilo. El producto de calidad
técnica es un sólido cristalino blanco, de baja volatilidad y escasa
hidrosolubilidad, y estable a la luz y al calor, pero fácilmente
hidrolizable en medio alcalino. La FAO ha establecido una
especificación mínima de pureza del 98%, con un límite de impureza
del 0,05% para el ß-naftil N-metil carbamato.
Para analizar el carbarilo y sus metabolitos, se pueden
utilizar numerosas técnicas, como la cromatografía de capa fina, la
espectrofotometría, la cromatografía de gases, la cromatografía
líquida de alta presión y la espectrometría de masas con ionización
química. Pueden alcanzarse límites de detección inferiores a un
nanogramo, y la recuperación supera por lo general el 80%.
1.2 Producción y usos
El carbarilo se viene utilizando desde hace unos 30 años como
insecticida de contacto y de ingestión, posee algunas propiedades
sistémicas y permite combatir una amplia serie de plagas. La planta
de fabricación más importante está en los Estados Unidos. Más de 290
fabricantes transforman el carbarilo para integrarlo en más de 1500
productos diferentes.
1.3 Transporte, distribución y transformación en el medio ambiente
En la mayoría de las situaciones, el carbarilo no persiste en
el entorno. En el agua, su semivida por hidrólisis depende de la
temperatura, del pH y de la concentración inicial, y varía entre
varios minutos y varias semanas. El principal producto de
degradación es el 1-naftol.
Se ha estudiado la acumulación de carbarilo en peces de agua
dulce, y expresada como factor de bioconcentración en el medio
acuático se ha cifrado en valores comprendidos entre 14 y 75. El
carbarilo es adsorbido más fácilmente en los suelos que poseen un
alto contenido orgánico que en los suelos arenosos. A un ritmo
habitual de aplicación conforme con unas "prácticas agrícolas
adecuadas", la disipación es rápida, con una semivida de entre 8
días y un mes en condiciones normales. Ocasionalmente, por efecto de
la lluvia y del cultivo agrícola, el carbarilo es transportado de la
superficie al subsuelo (a un metro de la superficie).
El carbarilo contamina la vegetación durante el rociamiento o
por desplazamiento hasta las plantas a través del suelo contaminado.
La degradación del carbarilo en el entorno depende del grado de
volatilización, fotodescomposición y degradación química y
microbiana que se produzca en el suelo, el agua y las plantas. La
descomposición es más rápida cuando el clima es cálido.
1.4 Niveles ambientales y exposición humana
La principal fuente de ingestión de carbarilo entre la
población general son los alimentos.
Los residuos hallados en muestras de la ingesta alimentaria
total son relativamente escasos, pues oscilan entre cantidades
ínfimas y 0,05 mg/kg. En los Estados Unidos, la ingesta diaria
durante los primeros años de aplicación de carbarilo fue de 0,15
mg/día por persona (lo contenían el 7,4% de los compuestos); la
cifra se redujo a 0,003 mg/día por persona en 1969 (sólo lo
contenían un 0,8% de los compuestos). Durante el periodo de
aplicación se encuentra carbarilo ocasionalmente en las aguas
superficiales y los embalses.
La población general puede estar expuesta al carbarilo durante
las operaciones de lucha contra las plagas, en su vivienda o en
zonas recreativas.
Los trabajadores pueden estar expuestos al carbarilo durante su
fabricación, formulación, envasado, transporte y almacenamiento, así
como durante y después de su aplicación. Las concentraciones
halladas en la atmósfera del lugar de trabajo durante su producción
oscilaron entre < 1 mg/m3 y 30 mg/m3. Si las medidas de
protección son inadecuadas, los trabajadores industriales y
agrícolas pueden sufrir exposiciones cutáneas importantes.
1.5 Cinética y metabolismo
El carbarilo es absorbido rápidamente por los pulmones y el
tracto digestivo. En voluntarios se observó una absorción cutánea
del 45% a las 8 horas de aplicar una dosis del producto diluida en
acetona. Sin embargo, los datos referentes a la penetración cutánea
in vitro y a la toxicidad indican que la absorción cutánea se
produce por lo general a una velocidad mucho menor.
Las principales vías metabólicas del carbarilo son la
hidroxilación del anillo y la hidrólisis. El resultado son numerosos
metabolitos que experimentan conjugación, con formación de sulfatos,
glucurónidos y mercapturatos hidrosolubles, que se excretan por la
orina. Como resultado de la hidrólisis se forman 1-naftol, dióxido
de carbono y metilamina. La hidroxilación da lugar a
4-hidroxicarbarilo, 5-hidroxicarbarilo, N-hidroximetilcarbarilo,
5-6-dihidro-5-6-dihidroxicarbarilo y 1,4-naftalendiol. El metabolito
principal en el hombre es el 1-naftol.
En condiciones normales de exposición, el carbarilo rara vez se
acumula en los animales. El producto se excreta principalmente por
la orina, debido a que la detoxificación del producto de su
hidrólisis, el 1-naftol, se produce principalmente por
transformación en conjugados hidrosolubles. La circulación
enterohepática de los metabolitos del carbarilo es también
considerable, sobre todo tras su administración oral.
El producto de la hidrólisis, el ácido carbámico N-naftol, se
descompone espontáneamente en metilamina y dióxido de carbono. La
posterior desmetilación de la metilamina da lugar a dióxido de
carbono y formato, siendo este último excretado principalmente por
la orina.
Un pequeño porcentaje de las dosis de carbarilo absorbidas
aparecen como metabolitos en la saliva y la leche.
1.6 Efectos en otros organismos en el medio ambiente
En los crustáceos, las CL50 oscilan entre 5 y 9 µg/litro
(pulgas de agua, mísidos), 8 y 25 µg/litro (anfípodos), y 500 y 2500
µg/litro (cangrejos de río). El margen de sensibilidad es parecido
en los insectos acuáticos; Plecoptera y Ephemeroptera (gusarapa y
cachipollas) son los grupos más sensibles. Los moluscos son menos
sensibles, situándose su CE50 en niveles de unos pocos mg/litro.
En cuanto a los peces, la mayoría de las CL50 están comprendidas
entre 1 y 30 mg/litro; el grupo más sensible son los salmónidos.
En el caso de las aves la toxicidad aguda es baja. La DL50
para las aves de caza, sean acuáticas o terrestres, es > 1000
mg/kg. El ave más sensible analizada es el mirlo de alas rojas
(DL50 = 56 mg/kg). En zonas forestales rociadas con 1,1 kg de
carbarilo por hectárea no se observó ningún efecto en las aves
locales.
El carbarilo es muy tóxico para las abejas y las lombrices de
tierra. La DL50 oral para las primeras es de 0,18 µg/abeja
(aproximadamente 1-2 mg/kg).
Hay indicios de que el carbarilo puede alterar temporalmente la
composición de especies en los ecosistemas tanto terrestres como
acuáticos. Por ejemplo, un estudio reveló que en determinadas
colonias de invertebrados terrestres sus efectos pueden persistir
durante por lo menos 10 meses tras una sola aplicación.
1.7 Efectos en animales de experimentación y en sistemas de prueba
in vitro
La toxicidad aguda, expresada como DL50, varía
considerablemente según las especies, fórmulas y vehículos. Las
estimaciones de la DL50 oral en la rata oscilan entre 200 y 850
mg/kg. Los gatos son más sensibles, pues presentan una DL50 de 150
mg/kg. Los cerdos y los monos son menos sensibles, pues su DL50 es
> 1000 mg/kg.
La exposición a 792 mg de ingrediente activo de carbarilo
nebulizado, que es la máxima concentración a la que se llegó durante
4 horas, provocó la muerte de una de cinco ratas hembra. Aerosoles
de carbarilo a concentraciones de 20 mg/m3 dieron lugar a una
disminución de la actividad colinesterasa (ChEA) en gatos durante
exposiciones únicas de 4 horas, pero esa misma concentración no tuvo
efectos observables en ratas.
El carbarilo produce leves irritaciones oculares y tiene un
potencial de sensibilización escaso o nulo. Estudios prolongados
revelaron un NOEL de 10 mg/kg de peso corporal (200 mg/kg ingesta
alimentaria) en las ratas, y de 1,8 mg/kg de peso corporal (100
mg/kg dieta) en los perros. El NOEL por inhalación prolongada es de
0,16 mg/m3 en los gatos. El potencial de acumulación de carbarilo
es bajo.
1.7.1 Reproducción
Se ha demostrado que el carbarilo tiene efectos adversos sobre
la reproducción y el desarrollo perinatal en diversas especies de
mamíferos. Los efectos sobre la reproducción comprenden problemas de
infertilidad, una disminución del tamaño de las camadas y una
reducción de la viabilidad postnatal. Los efectos tóxicos sobre el
desarrollo observados son un aumento de la mortalidad in utero,
una disminución del peso del feto y la aparición de malformaciones.
Salvo en un reducido número de estudios, todos los efectos adversos
sobre la reproducción y el desarrollo se observaron sólo a dosis
manifiestamente tóxicas para la madre, y en varios casos ésta
resultó ser más sensible al carbarilo que su prole. Entre los
efectos tóxicos para la madre cabe citar la letalidad, una
disminución del crecimiento y la distocia. Los datos disponibles
indican que los procesos de reproducción y desarrollo de los
mamíferos no son especialmente sensibles al carbarilo en comparación
con la susceptibilidad del organismo adulto.
1.7.2 Mutagenicidad
Se ha evaluado la posible mutagenicidad del carbarilo mediante
diversas pruebas in vitro e in vivo, empleando para ello
bacterias, levadura, plantas, insectos y mamíferos, y analizando
diversos puntos finales.
Según los datos disponibles, el carbarilo no es lesivo para el
ADN. No se ha notificado ningún dato que confirme que ha habido
inducción de la recombinación mitótica, conversión génica o síntesis
imprevista de ADN en procariotas (H. influenzae, B. subtilis) y
eucariotas ( S. cerevisiae, A. nidulans, linfocitos humanos en
cultivo, y hepatocitos de rata) in vitro.
Se obtuvieron resultados negativos en las pruebas de detección
de mutaciones génicas en un gran número de ensayos realizados con
bacterias, salvo en dos casos. En varios estudios de mutagenicidad
del carbarilo llevados a cabo con células de mamífero in vitro, se
obtuvo sólo un resultado positivo equívoco en un estudio de células
en cultivo. Ese estudio, sin embargo, presentaba varias deficiencias
y sus resultados no han sido confirmados en estudios comparables.
Se han notificado lesiones cromosómicas a altas dosis de
carbarilo en células humanas y de rata y hámster in vitro, así
como en plantas. No se han observado efectos de ese tipo en pruebas
in vivo con mamíferos, ni siquiera a dosis de hasta 1000 mg/kg.
Se ha mostrado que el carbarilo altera el mecanismo de las
fibras del huso en células de plantas y mamíferos in vitro. Es
dudoso el interés de realizar ensayos con plantas para extrapolar
sus resultados al hombre.
Cabe concluir que los datos disponibles no corroboran la
suposición de que el carbarilo plantea un riesgo de inducción de
cambios génicos en las células somáticas o germinales del hombre.
El producto nitrosado del carbarilo, el N-nitrosocarbarilo,
puede inducir fenómenos de recombinación mitótica y conversión
génica en los procariotas (H. influenzae, B. subtilis) y
eucariotas (S. cerevisiae) in vitro, y arroja resultados positivos
en las pruebas in situ con E. coli.
Además, resultados experimentales indican que el
N-nitrosocarbarilo se une al ADN, causando la ruptura de los
enlaces alcalisensibles y de las cadenas simples.
No se ha demostrado que el nitrosocarbarilo sea clastógeno in
vivo (médula ósea y células germinales), ni siquiera a dosis
tóxicas elevadas.
1.7.3 Carcinogenicidad
Se han realizado numerosos estudios en la rata y el ratón para
determinar los posibles efectos carcinógenos del carbarilo. Los
resultados de la mayoría de esos estudios fueron negativos, pero se
trata de trabajos realizados hace muchos años y que no satisfacían
los criterios actuales. Se están realizando nuevos estudios
conformes con los actuales criterios en ratones y ratas.a En la
más reciente evaluación del CIIC (CIIC, 1987) se llegaba a la
conclusión de que no había información sobre el cáncer en el hombre
y de que los indicios de carcinogenicidad en animales de
experimentación eran insuficientes. No se podía clasificar el
carbarilo en lo que se refiere a su carcinogenicidad para la especie
humana (Grupo 3).
Se ha mostrado que el N-nitrosocarbarilo induce la aparición
de tumores localmente en la rata (sarcoma en el lugar de la
inyección o carcinoma de células escamosas del antro cardiaco al
administrarlo por vía oral). Teniendo en cuenta las transformaciones
químicas que sufre el carbarilo en el hombre, el riesgo de
carcinogenicidad por N-nitrosocarbarilo como consecuencia de la
exposición a esta sustancia puede considerarse insignificante.
1.7.4 Efectos en distintos órganos y sistemas
a) Sistema nervioso
Los efectos del carbarilo sobre el sistema nervioso se deben
principalmente a la inhibición de la colinesterasa y son por lo
general temporales. En unos estudios sobre los efectos en el sistema
nervioso central de ratas y monos se observó que la administración
oral de 10-20 mg/kg durante 50 días provocaba trastornos del
aprendizaje y del comportamiento en las ratas.
En un pequeño estudio realizado con cerdos, el carbarilo (150
mg/kg de peso corporal en la alimentación durante 72-82 días) tuvo
diversos efectos neuromusculares. Se observó una debilidad
reversible de las patas en pollos sometidos a altas dosis de
carbarilo. No se observaron signos de desmielinización en los cortes
de cerebro, nervio ciático o médula espinal examinados al
microscopio. Tampoco se notaron efectos de esa índole en estudios
prolongados realizados con roedores.
b) Sistema inmunitario
Se ha notificado que el carbarilo administrado in vivo a
dosis que producen claros signos clínicos tiene diversos efectos en
el sistema inmunitario. Muchos de los efectos descritos se
detectaron a dosis cercanas a la DL50. La mayoría de los estudios
llevados a cabo con conejos y ratones a dosis compatibles con la
supervivencia no han revelado efectos de importancia en el sistema
______________
a El IPCS aún no ha examinado esos estudios. La sociedad
encargada de realizarlos ha indicado que se observa un aumento
significativo de tumores a la dosis màs elevada en las dos
especies.
inmunitario. Sin embargo, varios de esos estudios adolecían de
incoherencia y a veces de contradicción patente entre los
resultados, lo cual no permite describir un mecanismo inmunotóxico
bien definido.
c) Sangre
Se ha señalado que el carbarilo afecta a la coagulación, pero
existe cierta controversia en cuanto al tipo de efecto. En
eritrocitos de oveja con déficit de glucosa-6-fosfato
deshidrogenasa, el carbarilo provocó un aumento dosis-dependiente de
la formación de metahemoglobina (Met-Hb). La albúmina sérica humana
reaccionó in vitro con el grupo éster del carbarilo. El carbarilo
se une a los aminoácidos libres de la sangre.
d) Hígado
Se han señalado trastornos del metabolismo de los carbohidratos
y de la síntesis de proteínas, así como de la función de
detoxificación en el hígado de ciertos mamíferos. El carbarilo es un
inductor ligero de la actividad de metabolización de medicamentos
que reside en los microsomas hepáticos. Se observa un acortamiento
del periodo de sueño inducido por el fenobarbital. Los niveles
hepáticos de citocromo P-450 y b5 aumentan. Los cambios del
metabolismo hepático son tal vez parcialmente responsables de la
triplicación de la DL50 por carbarilo en ratas tratadas
previamente con dicho compuesto.
e) Función gonadotrópica
Se ha señalado que el carbarilo estimula la función
gonadotrópica de la hipófisis de la rata.
1.7.5 Mecanismo principal de toxicidad
El carbarilo inhibe la acción de la colinesterasa, efecto que
depende de la dosis y es fácilmente reversible. No se observó ningún
fenómeno de "maduración" de la colinesterasa carbamilada. Todos los
metabolitos identificados del carbarilo son
considerablemente menos activos que éste como inhibidores de la
colinesterasa.
1.8 Efectos en el hombre
El carbarilo es fácilmente absorbido por inhalación y por vía
oral, y menos fácilmente por vía cutánea. Como su principal
mecanismo de acción es la inhibición de la colinesterasa (ChE), en
el cuadro clínico de la intoxicación predominan los síntomas de esa
inhibición, tales como: hipersecreción bronquial, aumento de la
sudación, de la salivación y del lagrimeo; pupilas puntiformes,
broncoconstricción, espasmos abdominales (vómitos y diarrea);
bradicardia; fasciculación de los músculos finos (que también afecta
en los casos graves al diafragma y a los músculos respiratorios);
taquicardia; cefalea, vértigo, ansiedad, confusión mental,
convulsiones y coma; y depresión del centro respiratorio. Los signos
de intoxicación aparecen rápidamente tras la absorción y desaparecen
pronto al cesar la exposición.
En estudios controlados realizados con voluntarios se observó
una buena tolerancia a dosis únicas inferiores a 2 mg/kg. Una dosis
única de 250 mg (2,8 mg/kg) provocó síntomas moderados de inhibición
de la ChE (dolor epigástrico y sudación) al cabo de 20 minutos. La
recuperación completa se produjo tras dos horas de tratamiento con
sulfato de atropina.
En los casos de sobreexposición ocupacional al carbarilo, se
observan síntomas leves mucho antes de que llegue a absorberse una
dosis peligrosa; de ahí que raras veces se produzcan casos graves de
intoxicación ocupacional por este compuesto. Durante las
aplicaciones agrícolas, la exposición cutánea puede desempeñar un
papel importante. No suelen observarse efectos irritantes locales,
pero se ha descrito la aparición de exantema cutáneo tras la
salpicadura accidental con formulaciones de carbarilo.
Los datos acerca de los efectos del carbarilo sobre el número y
la morfología de los espermatozoides en trabajadores industriales
son discordantes. No se han notificado efectos adversos sobre la
reproducción.
El indicador biológico más sensible de la exposición al
carbarilo es la aparición de 1-naftol en la orina y una disminución
de la actividad ChE de la sangre. Si no hay 1-naftol en el entorno
de trabajo, los niveles urinarios de este producto se pueden emplear
como indicador biológico. Durante la exposición ocupacional, el 40%
de las muestras de orina contenían más de 10 mg de 1-naftol/litro.
En un caso de intoxicación aguda se hallaron 31 mg/litro en la
orina. El nivel de riesgo es > 10 mg/litro, y el nivel de aparición
de síntomas, 30 mg 1-naftol/litro de orina (hoja de datos sobre el
carbarilo, OMS, 1973, VBC/DS/75.3).
El análisis de la actividad ChE puede ser una prueba de gran
sensibilidad a efectos de vigilancia, siempre que se realice poco
después de la exposición.
2. Conclusiones
Se considera que los riesgos que plantea el carbarilo para el
ser humano son escasos, debido a su baja presión de vapor, a su
rápida degradación y a la recuperación espontánea y rápida de la
colinesterasa inhibida, así como al hecho de que los síntomas
aparecen normalmente mucho antes de que pueda haberse acumulado en
el organismo una dosis peligrosa. Aún no se dispone de estudios
satisfactorios sobre la carcinogenicidad conformes con los criterios
actuales.
2.1 Exposición de la población general
Los niveles de residuos de carbarilo presentes en los alimentos
y el agua de bebida, resultantes de su uso normal en la agricultura,
son muy inferiores a la ingesta diaria admisible (IDA) (0,01 mg/kg
de peso corporal al día), y difícilmente pueden representar un
riesgo para la salud de la población general.
2.2 Subpoblaciones de alto riesgo
El uso del carbarilo con fines de salud pública en la vivienda
o en zonas recreativas puede ser causa de sobreexposición si se
descuidan las normas aconsejadas para su aplicación.
2.3 Exposición ocupacional
Si se vela por el cumplimiento de unas prácticas laborales
razonables, en particular las medidas de seguridad, la protección
del personal y una supervisión adecuada, la exposición ocupacional
durante la fabricación, formulación y aplicación de carbarilo no
planteará riesgos. Las concentraciones no diluidas se deben manejar
con sumo cuidado, dado que unas prácticas laborales incorrectas
pueden ser causa de contaminación cutánea. Las concentraciones en el
aire del lugar de trabajo no deberían superar los 5 mg/m3.
2.4 Efectos en el medio ambiente
El carbarilo es tóxico para las abejas y las lombrices. No
debería aplicarse a los cultivos durante la floración.
Si se emplea normalmente, el carbarilo no debería suscitar
preocupación desde el punto de vista del medio ambiente. El
carbarilo se adsorbe en buena parte en el suelo y no se lixivia
fácilmente hacia las aguas subterráneas. Se degrada rápidamente en
el entorno, por lo que no tiende a persistir. El uso de carbarilo no
debería tener efectos nocivos a corto plazo en el ecosistema.
3. Recomendaciones
* La manipulación y la aplicación del carbarilo se deberían
realizar adoptando las precauciones previstas para todo
plaguicida y siguiendo minuciosamente las instrucciones
suministradas en el envase para emplear correctamente el
producto.
* Deberían controlarse cuidadosamente la fabricación, la
formulación, el uso y la eliminación del carbarilo, con objeto
de reducir al mínimo la contaminación del medio ambiente.
* Los trabajadores expuestos regularmente al producto deberían
someterse a chequeos periódicos.
* Debería determinarse la época de aplicación del carbarilo de
manera que no afecte a las especies que no se desee combatir.
* Deberían realizarse estudios de carcinogenicidad que satisfagan
los criterios actuales.
6.4 Elimination and excretion in expired air, faeces, urine,
milk, and eggs
The elimination of metabolized carbaryl is rapid, and
accumulation in animals seems unlikely, under normal exposure
conditions. Carbaryl is generally excreted entirely within 24-96 h
of intake. Elimination takes place mainly through the urine, faeces,
and respiration, and, to a lesser extent, through the milk of dairy
animals and the eggs of poultry.
Carbaryl is mainly excreted as its product of hydrolysis,
1-naphthol, which is detoxified to water-soluble glucuronide
(Carpenter et al., 1961), and sulfate (Whitehurst et al., 1963),
and only in trace amounts as 1-naphthol or unchanged.
The average percentage of metabolites recovered after orally
administered doses of 1-naphthyl 14C, methyl 14C, and carbonyl
14C carbaryl to rats was 94% over a 7-day period (Knaak et al.,
1965). Only residues from carbaryl methyl 14C were detected after
that, since the methyl moiety is incorporated in tissue (2-3%).
Liberated naphthol is conjugated and excreted, while the liberated
carbonyl groups are disposed of as respiratory14CO2 (Knaak
et al., 1965). Forty-seven to 57% of the metabolites excreted
retain the intact C-O-C(O)N-C structure, indicating a nonhydrolytic
pathway for carbaryl. Monkeys and pigs (2 young females) excreted
carbaryl as a conjugate of intact carbaryl and 4-hydroxycarbaryl,
mainly glucuronides. Sheep also excreted 1-naphthyl glucuronide and
sulfate and 4 (methylcarbamoyloxy)1-naphthylsulfate (Knaak et al.,
1968).
Myers (1977) studied the correlation between the amount of
carbaryl ingested and urinary metabolites levels. Metabolites were
measured as three groups:
I. naphthyl sulfate;
II. free and sulfated 5,6-dihydrodihydroxycarbaryl
and dihydrodihydroxynaphthol;
III. other conjugated (glucuronides) of naphthol,
5,6-dihydrodihydroxycarbaryl, and
5,6 dihydrodihydroxynaphthol.
The amounts in groups I and II were 25.5-36.5% of the dose. When all
three groups of metabolites were analysed, the amounts of
metabolites for the same period represented 41.5-52.7% of the dose.
Naphthol was the major metabolite found in Group III (mean 75% of
all metabolites of the group). A good correlation was found between
the amount ingested and urinary metabolites.
Rats treated orally with naphthyl14C carbaryl (2.3 µci)
excreted 90% of the administered radioactivity in the urine within 3
days. During the 72-h sampling period, the faeces contained only
2-5% of the radioactivity (Lucier et al., 1972).
Eighty per cent of 5 mg of 14C naphthyl carbaryl, given
intraperitoneally to rats, (see section 6.3.2) was eliminated in the
24-h urine and 10% in the faeces (Bend et al., 1971). Enzymatic
hydrolysis and reverse isotope dilution showed that 10% of the dose
was excreted as 1-naphthyl-glucoronide, 5%, as 1-naphthylsulfate,
3%, as conjugates of 4-hydroxycarbaryl, 5%, as 1-naphthylsulfate,
3%, as conjugates of 4-hydroxycarbaryl, and 5% of 5-hydroxycarbaryl.
About 2% of the carbaryl appeared unchanged in urine. Other
radioactive metabolites were not identified.
Following a single, ip injection in albino rats of both sexes,
of methyl 14C and carbonyl 14C carbaryl at 30 mg/kg, 75-80% was
recovered after 48 h in the expired air and urine. Liberated by
hydrolysis, N-methyl carbamic acid was spontaneously decomposed to
methylamine and carbon dioxide (43.5%). The methylamine moiety was
later demethylated to 14CO2, which was eliminated in the expired
air and 14C formate (9.2%), which was excreted in the urine
(Hassan et al., 1966).
In the study of Nye & Dorough (1976), 2.5% of the
endotracheally administered carbaryl dose was exhaled as
14C-carbon dioxide. More than 90% of the dose was eliminated in
the urine during 3 days. The faeces contained 2-5%.
A comparative study on the excretion of carbaryl after a
7.5 µmol/kg, ip dose in rats was reported by Krishna & Casida (1966)
who used three types of labelled carbaryl (Table 32).
Table 32. Fate of 14carbon in male Sprague-Dawley rats
after ip administration of carbaryla
Labelled position Administered 14carbon recovered (%)
Expired 14CO2 Urine Faeces
0-24 h 24-48 h
Carbonyl 14C 24.5 62.1 2.4 2.1
Methyl 14C 12.3 54.6 3.4 3.9
Naphthyl 14C 0.2 74.2 2.3 8.9
Hydrolysis product < 0.1 86.4 3.3 1.4
naphthol 14C
aFrom: Krishna & Casida (1966).
Biliary excretion of carbaryl was studied in bile-duct
cannulated rats. Naphthyl 14C and methyl 14C carbaryl (50 µg)
were administered intravenously to rats (see section 6.3.2). During
the first 2 h of collection, 90% of the total biliary radioactivity
was eliminated (Bend et al., 1971). After oral application of
0.01 mg/kg naphthyl-14C carbaryl to rats, 37.5% was excreted via
the bile in the first 3 h and 45.4% (cumulative percentage) of the
dose was eliminated within 48 h. Faecal elimination was 1.4% and
urine elimination was 42.3% (Marshall et al., 1979). The results
of Struble et al. (1983a), who used higher doses of carbaryl (1.5,
31, and 300 mg/kg) on male Sprague-Dawley rats were similar. Twelve
hours after administration, 15-46% of the 14C was excreted in the
bile, 10-40% in the urine, and less than 1% was eliminated in
faeces. Three metabolites were identified from the bile, the major
one being 5-6-dihydro-5-6-dihydroxycarbaryl glucoronide (12-18% of
the biliary 14C).
On the basis of a study on 50, male, Sprague-Dawley rats,
Borzelleca & Skalsky (1980) reported that carbaryl metabolites in
the blood were also present in the saliva, at similar
concentrations.
Leeling & Casida (1966) studied carbaryl metabolites in the
urine of one male and one female New Zealand White rabbit. The
ether-extractable metabolites found in the urine were:
N-hydroxymethyl carbaryl, 4-hydroxycarbaryl, 5-hydroxycarbaryl,
5,6-dihydro-5,6-dihydroxycarbaryl, 5,6-dihydro-5,6-trihydroxy-1-
naphthol, and 1-naphthol.
Thirty-five, adult, female American cockroaches ( Periplaneta
americana) were injected through the central abdominal wall with
5 µg of carbaryl-carbonyl 14C, carbaryl methyl 14C, or 2 µg
carbaryl naphthyl 14C. Metabolites similar to those appearing in
rat liver microsomes were formed. About 19% of the radioactivity of
carbaryl carbonyl 14C was eliminated as 14C-carbon dioxide in
24 h (Dorough & Casida, 1964).
Excretion of carbaryl in milk was studied by Baron (1968).
Application of a total dose of 2 g carbonyl carbaryl 14C to a
lactating cow resulted in approximately 1% of radioactive residues
in the milk. In skim milk, 87% of these residues were water-soluble
and 13% chloroform-soluble. About 90-95% of the14C radioactivity
in the soluble component was removed after crystallization of
lactose.
The metabolism of carbaryl naphthyl 14C was studied in one
lactating Jersey cow (Dorough, 1967). Two consecutive single
treatments at 6-day intervals with 0.25 mg/kg body weight (total
125 mg) and one single dose of 3.05 mg carbaryl naphthyl 14C/kg
body weight added to the feed, resulted in radiolabelled residues in
the milk for as long as 60 h after treatment. The first sample of
milk, taken after 6 h, showed maximum concentrations of chloroform
extractables, water-soluble, and unextractable, residues in whole
milk in the range of 0.063-0.95 mg/litre. Residues of only a
slightly lower magnitude were detected in the 12-h milk samples and
rapidly declined in 24-h samples. Thirty per cent of the residues in
the 6-h sample was a chloroform-extractable metabolite, tentatively
identified as 5,6-dihydro-5,6-dihydroxy-1-naphthyl-
N-methylcarbamate. About 0.35% of the total dose was detected in
the milk in both treatments, 70% in the urine, and 11% in the faeces
in 0.25 mg/kg treatment, and 58% in the urine and 15% in the faeces
in the 3.05 mg/kg treatment. The highest levels in the tissues of
the cow, killed on an unspecified day after treatment, were found in
the liver, kidney, and ovaries.
A single lactating cow was treated orally with a daily total
dose of 518 mg 14C-carbaryl (0.77 mg/kg body weight), given in two
doses, 12 h apart. Results are shown in Table 33 (Dorough, (1969).
Fifty-nine per cent of the radioactivity from a14C-labelled oral
dose of carbaryl administered to a cow was recovered from the urine
within the first 24 h (Saini & Dorough, 1978).
The passage of14C into the milk and its presence in the
suckling neonates has been studied by whole-body autoradiography in
rats fed 14C-methyl carbaryl. The highest concentrations in
newborn offspring, after 48 h, were found in the stomach contents,
the liver, the hair, and the bone marrow (Benard et al., 1979).
Table 33. Distribution and excretion of total-14C equivalents in
milk, urine, faeces, and tissues, 24 h after one-day oral
application of carbaryl in a lactating cow
Sample mg/kg Dose recovered
(%)
Milk 0.04 0.09
Urine 22.4 58.64
Faeces 0.47 1.65
Tissues:
- liver 0.098 0.21
- kidney 0.382 0.17
- leg muscle 0.027 nil
- fat 0.014 nil
A Saanen goat was treated orally with 1.34 mg carbaryl carbonyl
14C/kg. The excretion of radioactivity in the urine was 7.4% at
2 h, 24% at 4 h, and 45% at 24 h. In total, 47% of the radioactivity
was excreted the urine. In the milk, the peak was 0.9 mg/litre at
8 h, dropping to 0.008 mg/litre 60 h after the administration of
carbaryl (Dorough & Casida, 1964).
The excretion of carbaryl was studied in hens. Carbaryl-
carbonyl14C (10 mg/kg body weight) was given orally to Leghorn
hens. Fifty per cent of the 14C was expired within 48 h. When
carbarylnaphthyl 14C was applied at the same dose, no
radioactivity was detected in the expired gases. The urine was the
primary route of excretion. 14Carbon remaining in the carcass,
48 h after dosing, accounted for 1.4 and 7.1% of the dose given as
the ring-labelled and carbonyl-labelled compound, respectively
(Paulson & Feil, 1969). The main metabolites found in the urine of
hens were: 1-naphthol, 1-naphthyl glucoronide, and sulfate esters of
1-naphthol, 4-hydroxycarbaryl, and 5-hydroxycarbaryl. Conjugates
with other metabolites were also found in small quantities (Paulson
et al., 1970). Mature hens were given a single oral dose of
carbaryl naphthyl 14C at 10 mg/kg body weight. Eggs collected for
12 days contained a total dose of 0.33% of the 14C administered
(Paulson & Feil, 1969).
The fate of naphthyl-1-14C carbaryl in laying chickens was
studied by Andrawes et al. (1972). Chickens (3 White Leghorn in
each group) were fed 7, 12, or 70 mg/kg carbaryl in the feed twice
daily for 14 days. They had been pretreated for 17 days with the
same doses of nonlabelled carbaryl in feed. Residues reached maximum
levels within 1 day in excrement, 2 days in egg white, and 6-8 days
in egg yolk. After the end of dosing, the half-life of 14C
residues was <1 day in the excrement and egg white, 2-3 days in the
egg yolk, and 5 days in the carcass. Metabolites and carbaryl found
in the eggs of hens, fed 70 mg carbaryl/kg averaged in total 19.7 µg
of 14C carbaryl equivalent per egg (16.3 µg in yolk and 3.4 µg in
white), collected between the 9th and the 14th day of dosing.
Naphthyl-l-sulfate was the largest single component of egg residues
accounting for the higher concentration of residues in the egg yolk.
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
7.1 Microorganisms
7.1.1 Soil microorganisms
Studies of the effects of insecticides on soil microorganisms
are complex and subject to many sources of variation. It is
therefore difficult to assess the practical significance of the
results of such studies.
Varsheny & Gaur (1972) studied the impact of carbaryl (as
Sevin) on soil fungi. They determined the size of the fungal
population and the species composition of the fungal community after
plating out extracts of soil samples on various media. The authors
suggested that, at very high rates of addition to the soil (up to
5000 mg/kg), there was evidence of a change in species composition
and an increase in the total fungal population.
In laboratory cultures of saltmarsh protozoans (dominated by
Euplotes sp.), Weber et al. (1982) found that both carbaryl and
1-naphthol caused dose-related mortality. For both compounds,
mortality at 1 mg/litre was about 50%. There was almost 100%
mortality at 10 mg/litre.
Carbaryl inhibited the growth of cultures of Bacillus subtilis
(DeGiovanni & Donnelly, 1968).
7.1.2 Aquatic microorganisms
Edmiston et al. (1985) conducted a detailed study of the
effects of carbaryl on Paramecium multimicronucleatum. The 24-h
LC50 was 28 µg/litre in a static plate test. Effects on the oxygen
uptake of cultures and effects on the cell surface were also
reported, but these results were mostly obtained using
concentrations in excess of the 24-h LC50, and must be of limited
value.
Murray & Guthrie (1980) studied the responses of bacterial
cultures originating from Lake Houston. The respiratory rate of
bacterial cultures was increased at water temperatures of between
23-33 °C. Plate counts of bacterial colonies suggested that carbaryl
treated cultures contained more bacteria than control cultures on
most days in a 26-day study.
7.2 Aquatic organisms
Aquatic toxicity studies have been conducted in the laboratory
and field to define the biological effects associated with acute and
long-term exposure to carbaryl and the degradation product,
1-naphthol, of aquatic vertebrates and invertebrates. More data are
available for freshwater organisms than for saltwater organisms. In
addition, actual and simulated field studies have been conducted to
assess the impact of carbaryl exposure on freshwater invertebrates.
7.2.1 Aquatic invertebrates
Studies on the toxicity of carbaryl for non-target aquatic
invertebrates have included investigations on molluscs, crustaceans,
and insects, concentrating on organisms of commercial importance,
organisms most likely to be exposed during a typical application of
carbaryl, or on standardized test species typically used in aquatic
toxicity tests. The acute and long-term laboratory studies under
various environmental conditions (pH, temperature, water hardness,
etc.) are briefly reviewed in Table 34.
Most of the studies on the toxicity of carbaryl for aquatic
invertebrates have been conducted under laboratory conditions and,
consequently, the results only yield data on the relative toxicity
of the substance. They do not reflect realistic exposure in the
environment. Most of the laboratory studies evaluate constant,
short-term (less than 1 week) exposure to carbaryl under static or
flow-through conditions. There are few long-term studies on the
effects of carbaryl on aquatic invertebrates. Exposures in the
environment are typically localized, at much lower concentrations
than in aquatic toxicity tests and are not constant.
The toxicity of carbaryl on the early development of the sea
urchin was studied by Hernandez et al. (1990). Developmental
stages with active cleavage and cellular mobilization (blastula and
gastrula) turned out to be more sensitive. The next two stages
(prism and pluteus) were less sensitive and EC50 values were 8-26
times higher. This effect may be connected with increased
detoxication processes by the cytochrome oxidase system.
Table 34. Acute toxicity of carbaryl for invertebrates
Organism Test Size/age Stat/ Temperature Hardness pH Parameterc Concentration Reference
material Flowa (°C) (mg/litre)b (µg/litre)
Crustacea
(Freshwater)
Water flea
Daphnia magna unknown stat 16 42 7.4 48-h LC50 5.6 Sanders et al. (1983)
Daphnia magna technical 96-h LC50 3280 Lejczak (1977a)
Daphnia magna unknown 48-h EC50 0.26 Rawash et al. (1975)
Daphnia magna technical 21-day MATC 1.5-3.3 Surprenant (1985c)
21-day NOEC 6.0
(survival)
Daphnia pulex technical stat 16 44 7.1 48-h LC50 6.4 Mayer & Ellersieck (1986)
Simocephalus unknown stat 10 44 7.1 48-h LC50 11 Johnson & Finley (1980)
serrulatus stat 16 44 7.1 48-h LC50 7.6
stat 21 44 7.1 48-h LC50 8.1
Ostracod
Cypridopsis
vidua unknown adult stat 21 272 7.4 48-h LC50 115 Mayer & Ellersieck (1986)
Sow bug
Asellus
brevicaudus technical adult stat 18 44 7.1 96-h LC50 280 Johnson & Finley (1980)
Table 34 (continued)
Organism Test Size/age Stat/ Temperature Hardness pH Parameterc Concentration Reference
material Flowa (°C) (mg/litre)b (µg/litre)
Crustacea
(Freshwater)
Amphipod
Gammarus
fasciatus unknown adult stat 21 44 7.1 96-h LC50 26 Johnson & Finley (1980)
Gammarus
lacustris unknown adult stat 21 44 7.1 96-h LC50 22 Johnson & Finley (1980)
Gammarus unknown adult stat 12 40 6.5 48-h LC50 8.13 Mayer & Ellersieck (1986)
pseudolimnaeus adult stat 12 40 7.5 48-h LC50 11.5
adult stat 12 40 8.5 48-h LC50 7.8
Gammarus pulex technical 48-h LC50 29 Bluzat & Seuge (1979)
Gammarus technical 96-h LC50 16 Sanders et al. (1983)
pseudolimnaeus
Gammarus technical 96-h LC50 13 Woodward & Mauck (1980)
pseudolimnaeus
Glass shrimp
Palaemonetes unknown adult stat 21 272 7.4 96-h LC50 5.6 Johnson & Finley (1980)
kadiakensis 25-31 mm stat 96-h LC50 120 Chaiyarach et al. (1975)
Table 34 (continued)
Organism Test Size/age Stat/ Temperature Hardness pH Parameterc Concentration Reference
material Flowa (°C) (mg/litre)b (µg/litre)
Crayfish
Procambarus sp. technical immature stat 12 42 7.5 96-h LC50 1900 Mayer & Ellersieck (1986
Procambarus unknown 60-70 mm stat 96-hLC50 2430 Chaiyarach et al. (1975)
simulans
Procambarus technical 96-h LC50 500 Cheah et al. (1978)
acutus
Crustacea
(Freshwater)
Prawn
Macrobrachium technical 96-h LC50 19 Omkar & Shukla (1985)
lamarrei
Upogebia wettable powderd 48-h EC50 90 Stewart et al. (1967)
pugettensis 1-naphthol 48-h EC50 4400
Callianassa wettable powderd 48-h EC50 80
californiensis 1-naphthol 48-h EC50 3500
Hemigrapsus wettable powderd 24-h EC50 710
oregonensis 1-naphthol 24-h EC50 74 200
Table 34 (continued)
Organism Test Size/age Stat/ Temperature Hardness pH Parameterc Concentration Reference
material Flowa (°C) (mg/litre)b (µg/litre)
Crustacea
(estuarine &
marine)
Blue crab
Callinectes unknown juvenile flow 29 27s 48-h LC50 320 Mayer (1987)
sapidus
Brown shrimp
Penaeus aztecus unknown juvenile flow 30 28s 48-h LC50 1.5
Pink shrimp
Penaeus
duorarum unknown juvenile flow 23 29s 48-h LC50 32
Grass shrimp
Palaemonetes unknown juvenile flow 23 29s 48-h LC50 28
pugio 3-4 days stat 24 28-30s 96-h LC50 22 Thursby & Champhon (1991)
Crustacea
(estuarine &
marine)
Table 34 (continued)
Organism Test Size/age Stat/ Temperature Hardness pH Parameterc Concentration Reference
material Flowa (°C) (mg/litre)b (µg/litre)
Mysid shrimp
Mysidopsis bahia unknown flow 96-h LC50 > 7.7 Nimmo et al. (1981)
Mysidopsis bahia technical 96-h LC50 6.7 Surprenant (1985b)
NOEC 5.1
Mysidopsis bahia 24 h flow 25 31s 96-h LC50 8.46 Thursby & Champhon (1991)
24 h stat 24 30s 96-h LC50 19
24 h flow 26 31-32s 28-day LC50 9.9
Ostracod
Cypretta kawatai technical 72-h LC50 1800 Hansen & Kawatski (1976)
Insects
Stonefly
Isogenus sp. unknown larva stat 7 35 7 96-h LC50 2.8 Mayer & Ellersieck (1986)
Pteronarcys technical 96-h LC50 4.8 Johnson & Finley (1980)
californica
Mosquito
Culex pipiens technical 24-h LC50 75 Rawash et al. (1975)
Table 34 (continued)
Organism Test Size/age Stat/ Temperature Hardness pH Parameterc Concentration Reference
material Flowa (°C) (mg/litre)b (µg/litre)
Insects
Midge
Chironomus technical 24-h LC50 7 Karnak & Collins (1974)
tentans
Chironomus technical 72-h LC50 5900 Hansen & Kawatski (1976)
tentans
Chironomus technical 48-h EC50 10 Sanders et al. (1983)
plumosus
Chironomus unknown larva stat 20 4 24-h LC50 106 Fisher & Lohner (1986)
riparius larva stat 20 6 24-h LC50 133
larva stat 20 8 24-h LC50 127
Back swimmer
Notonecta technical 96-h LC50 200 Federle & Collins (1976)
undulata
Water stick
Ranatra elongata wettable 96-h LC50 624 Shukla et al. (1982)
powder
Table 34 (continued)
Organism Test Size/age Stat/ Temperature Hardness pH Parameterc Concentration Reference
material Flowa (°C) (mg/litre)b (µg/litre)
Diptera
Chaoborus technical 48-h LC50 296 Bluzat & Seuge (1979)
Ephemeroptera
(Mayfly)
Cloeon technical 48-h LC50 480
Crawling water
beetle
Peltodytes sp. technical 96-h LC50 3300 Federle & Collins (1976)
Insects
Stone fly
Pteronarcella unknown larva stat 16 44 7.1 96-h LC50 1.7 Johnson & Finley (1980)
badia larva stat 12 38 6.5 96-h LC50 11 Woodward & Mauck (1980)
larva stat 12 38 7.5 96-h LC50 13 Mayer & Ellersieck (1986)
larva stat 12 38 8.5 96-h LC50 29
Claasenia unknown larva stat 16 44 7.1 96-h LC50 5.6 Johnson & Finley (1980)
sabulosa
Table 34 (continued)
Organism Test Size/age Stat/ Temperature Hardness pH Parameterc Concentration Reference
material Flowa (°C) (mg/litre)b (µg/litre)
Molluscs
Common mussel
Mytilus edulis unknown 48-h EC50 > 30 000 Roberts (1975)
for byssal
attachment
Mytilus edulis wettable larva 48-h EC50 2300 Stewart et al. (1967)
powder 48-h EC50 1300
1-naphthol
Giant oyster
Crassostrea gigas wettable larva 48-h EC50 2200
powder 48-h EC50 800
1-naphthol
Clinocardium wettable adults 24-h EC50 7300
nuttallii powder 24-h EC50 6400
1-naphthol
Molluscs
Eastern Oyster
Crassostrea juvenile flow 29 27s 96-h EC50 > 2000 Mayer (1987)
virginica
Table 34 (continued)
Organism Test Size/age Stat/ Temperature Hardness pH Parameterc Concentration Reference
material Flowa (°C) (mg/litre)b (µg/litre)
Crassostrea technical 48-h EC50 2700 Surprenant (1985a);
virginica Dionne et al. (1985)
Mactrid clam
Rangia cuneata 35-50 mm stat 5s 96-h LC50 125 000 Chaiyarach et al. (1975)
Lymnaea technical 48-h LC50 21 000 Bluzat & Seuge (1979)
stagnalis
aStat = static conditions (water unchanged for the duration of the test).
Flow = flow-through conditions (carbaryl concentration in water continuously maintained).
bHardness = expressed as mg CaCO3/litre.
s = salinity (%).
cEC50s for oyster based on shell deposition.
d50-80% wettable powder.
Carbaryl is slightly toxic for freshwater and marine molluscs
(clams, mussels, oysters). Acute toxicity typically occurs in adult
molluscs at concentrations ranging from 1 mg carbaryl/litre to over
100 mg/litre. The major hydrolytic product of carbaryl, 1-naphthol,
is slightly more acutely toxic for molluscs (mussel, pacific oyster)
than the parent compound (Stewart et al., 1967). In addition,
larvae and young juveniles are more sensitive to carbaryl exposure
than older juveniles and adults.
There have been few studies on the effects of carbaryl on the
growth of molluscs. Davis (1961) and Butler (1962) found that
carbaryl (80% WP) concentrations of 1.0-2 mg/litre reduced the
larval development and growth of oysters ( Crassostrea virginica)
and clams ( Venus mercenaria).
The effects of a 1-h exposure to carbaryl (80% WP) and its
hydrolytic product, 1-naphthol, were studied on six developmental
stages of the mussel ( Mytilus edulis) (Armstrong & Millemann,
1974c). The six stages included unfertilized eggs to the early
veliger stage, 32 h after fertilization. Unfertilized eggs and the
first polar body stage were exposed to carbaryl and 1-naphthol
(separately). The acute toxicity values of carbaryl and 1-naphthol
were similar for the first two stages, ranging from approximately 5
to 25 mg/litre. The stage of development most sensitive to carbaryl
was the first polar body stage. Thereafter, susceptibility decreased
as age of the larvae increased.
LC50 values for crustacea varied from 5 to 9 µg/litre (water
fleas, mysid shrimps), 8 to 25 µg/litre (scud), and 500 to
2500 µg/litre for crayfish. The hydrolytic metabolite 1-naphthol was
less toxic for daphnids and shrimps.
The effects of carbaryl (80% WP) on the life stages of
crustacea have been studied in the Dungeness crab ( Cancer magister)
(Buchanan et al., 1970). Early larvae were more sensitive to
carbaryl than juveniles and adults. Carbaryl (1.0 mg/litre) did not
affect hatching of eggs but prevented moulting of all prezoeae to
zoeae. The concentration that killed 50% of the first zoeae during a
96-h exposure was estimated to be 1 mg/litre.
Young juvenile crabs were more sensitive than older juveniles
or adults. The 24-h EC50s (death or irreversible paralysis) were
estimated to be 0.076 and 0.35-0.62 mg/litre for second and ninth
stage juveniles, respectively. At 0.032 mg/litre, juvenile crabs
were not affected when held in uncontaminated water after exposure.
In adult crabs, the 24-h and 96-h EC50s (death or paralysis) were
0.49 and 0.26 mg/litre, respectively. Post-larval Dungeness crabs
had about the same sensitivity to carbaryl as other crabs. The 24-h
EC50 (death or paralysis) of small stone crabs was 1.0 mg/litre
(Butler, 1962), that of juvenile blue crabs, 0.55 mg/litre (Butler,
1963), and that of adult crabs, 0.06-1.05 mg/litre (Stewart et al.,
1967).
As part of fresh water biota, daphnids have been used as assay
organisms to determine toxic concentrations of a variety of
pesticides. Laboratory bioassays were conducted with carbaryl to
determine its toxicity and immobilization values for two species of
daphnids, Daphnia pulex and Simocephalus serrulatus. The EC50s
for overt effects were 0.0064 mg/litre for Daphnia pulex and
0.0076 mg/litre for Simocephalus serrulatus (Sanders & Cope,
1966).
In a long-term study, Daphnia magna was exposed to technical
carbaryl for 21 days; reproductive performance was the most
sensitive indicator; the MATC was 1.5-3.3 µg/litre (Surprenant,
1985c).
Aquatic insects in the orders Plecoptera (stoneflies) and
Ephemeroptera (mayflies) are generally highly sensitive to low
levels of carbaryl.
The effects of 0.6-50.0 mg carbaryl/litre were studied on
selected aquatic organisms, including: Scanedesmus quadricauda,
Lemna minor, Lebistes reticulatus and Daphnia magna (Bogacka &
Groba, 1980). Carbaryl depressed reproduction, biomass, and
chlorophyll content in Lemna minor (vascular plant) after 24 h
exposure at a concentration >6.6 mg/litre. An almost 100% decrease
occurred at a concentration of 50 mg/litre. Inhibition of
photosynthesis intensity in the alga Scenedesmus quadricauda was
about 50% at a concentration of carbaryl in the water of
32 mg/litre, and 65% at a concentration of 56 mg/litre, after 24 h
exposure. Less than 10% inhibition occurred at a concentration of
1.8 mg/litre. A decrease in the production of chlorophyll was
demonstrated at 4.4 mg/litre.
Carbaryl applications of 5.7 or 11.4 kg/ha were effective in
controlling ghost shrimp ( Callianassa californiensis) as an oyster
pest. The numbers of various clams in the mud were reduced by 22 and
38%, respectively. Clam species differed in susceptibility. For
example, gaper clams (Tresus capax) were reduced by 58 and 69%.
Polychaetes and nemertean worms were not affected during 30 days of
observation (Armstrong & Millemann, 1974a,b).
7.2.2 Fish
7.2.2.1 Acute toxicity
Acute toxicity studies have been conducted with technical
carbaryl, several formulations, and 1-naphthol to determine LC50
values for several freshwater and marine fish (Table 35). The
results indicated that short-term exposure (< 4 days) to carbaryl
and its formulations showed some toxicity for fish (96-h LC50s =
1-30 mg/litre). There were no differences in the toxicity of two
formulations (4-oil-sevin and XLR) for rainbow trout and bluegill.
The LC50s for sheepshead minnow (exposed to technical material)
and rainbow trout (exposed to 4-oil and XLR) were similar and both
were generally more sensitive than bluegill. The toxicity of
1-naphthol for rainbow trout, bluegill, and sheepshead minnow was
determined by Springborn (1988a); 96-h LC50 values for these
species were 0.76, 1.4, and 1.2 mg/litre, respectively. The
corresponding NOECs were < 0.43, 0.55, and 0.47 mg/litre. The
metabolite, 1-naphthol, was more acutely toxic than the formulations
for bluegill. However, the toxicities of 1-naphthol and the
formulations were similar for rainbow trout and sheepshead minnows.
Few studies report acute toxicity values for fish that are
lower than 1 mg/litre. Cold water fish (Salmonidae), such as Coho
Salmon and trout, seem to be susceptible to carbaryl, while the
catfish (Ictaluridae) is tolerant (Macek & McAllister, 1970; Post &
Schroeder, 1971). Irritability, sluggishness, and loss of
equilibrium were classical signs of acute intoxication. Rainbow
trout (Oncorhynchus mykiss), exposed to 1 mg carbaryl/litre for
96 h, exhibited decreased swimming capacity and swimming activity;
the capacity to capture and consume prey was also diminished (Little
et al., 1990).
Other end-point concentrations have been established for
carbaryl for various species. Reference should be made to the
original documentation to obtain a fuller understanding of these
measures of toxicity. The lowest no-observed-effect concentration
(NOEC) was found for rainbow trout at 0.065 mg/litre. Other NOECs
were at least an order of magnitude greater. Maximum acceptable
toxic concentrations (MATCS) have also been calculated. Bansal
et al. (1980) estimated 30-day MATCs for 4 species of carp exposed
to carbaryl (Sevin as a 50% wettable powder). For all species, the
MATC was between 0.052 and 0.078 mg/litre. Verma et al. (1984)
reported a 60-day MATC of between 0.09 and 0.11 mg/litre for another
carp species, exposed to the same type of formulation of Sevin.
Table 35. Acute toxicity of carbaryl for fish
Organism Size/age Stat/ Temperature Hardness pH Formulationc Parameter Concentration Reference
flowa (°C) (mg/litre)b (mg/litre)
Freshwater
Coho salmon 1 g stat 13 44 7.1 96-h LC50 4.3 Mayer & Ellersieck (1986)
(Oncorhynchus stat 13 42 7.1 96-h LC50 2.4
kisutch) 4.6 g stat 13 42 7.1 96-h LC50 1.8
5.1 g stat 13 42 7.1 96-h LC50 2.7
10.6 g stat 13 42 7.1 96-h LC50 1.2
19.1 g CT 96-h LC50 0.8 Macek & McAllister (1970)
CT 96-h LC50 1.3 Post & Schroeder (1971)
Chinook salmon fingerling flow 13 314 7.5 96-h LC50 2.4 Mayer & Ellersieck (1986)
(Oncorhynchus
tshawytscha)
Cutthroat trout 0.5 g stat 12 40 7.5 96-h LC50 7.1
(Salmo clarkii) 0.5 g stat 12 330 7.8 96-h LC50 4.0
0.6 g stat 7 42 7.5 96-h LC50 6.0
0.7 g stat 12 40 6.5 96-h LC50 5.0
0.6 g stat 12 40 8.5 96-h LC50 1.0
CT 96-h LC50 6.0 Woodward & Mauck
C49 96-h LC50 6.7 (1980)
Table 35 (continued)
Organism Size/age Stat/ Temperature Hardness pH Formulationc Parameter Concentration Reference
flowa (°C) (mg/litre)b (mg/litre)
Rainbow trout 1.5 g stat 12 42 7.1 96-h LC50 2.0 Mayer & Ellersieck (1986)
(Oncorynchus 1.5 g stat 12 272 7.4 96-h LC50 1.2
mykiss) 1.2 g stat 12 40 7.4 96-h LC50 3.5
1.2 g stat 12 320 7.4 96-h LC50 3.0
1 g stat 12 40 7.4 96-h LC50 1.6
1 g stat 17 40 7.4 96-h LC50 1.1
1 g stat 12 40 6.5 96-h LC50 1.2
1 g stat 12 40 7.5 96-h LC50 0.8
1 g stat 12 40 8.5 96-h LC50 1.5
0.5 g flow 17 314 7.5 96-h LC50 1.3
CT 96-h LC50 4.3 Macek & McAllister (1970)
XLR 96-h LC50 1.4 Springborn (1985)
S4 96-h LC50 1.3
CT 96-h LC50 2.2 Sanders et al. (1983)
CT 96-h LC50 1.5 Post & Schroeder (1971)
Atlantic salmon 0.2 g stat 7 42 7.5 96-h LC50 0.3 Mayer & Ellersieck (1986)
(Salmo salar) 0.2 g stat 12 42 7.5 96-h LC50 0.9
0.2 g stat 17 42 7.5 96-h LC50 1.0
0.4 g stat 12 42 6.5 96-h LC50 1.3
0.4 g stat 12 42 8.5 96-h LC50 0.9
Brown trout 0.6 g stat 12 42 7.5 96-h LC50 6.3
(Salmo trutta) fingerling flow 12 314 7.5 96-h LC50 2.0
Table 35 (continued)
Organism Size/age Stat/ Temperature Hardness pH Formulationc Parameter Concentration Reference
flowa (°C) (mg/litre)b (mg/litre)
Brook trout 0.8 g stat 12 42 7.5 96-h LC50 2.1 Mayer & Ellersieck (1986)
(Salvelinus 0.8 g stat 7 42 7.5 96-h LC50 3.0
fontinalis) 1 g stat 17 42 7.5 96-h LC50 0.7
0.7 g stat 12 42 6.5 96-h LC50 4.6
0.7 g stat 12 42 8.5 96-h LC50 2.1
0.7 g stat 12 42 9 96-h LC50 1.1
0.8 g stat 12 42 7.5 96-h LC50 1.2
0.8 g stat 12 300 7.5 96-h LC50 1.3
Lake trout 1.7 g stat 12 40 7.5 96-h LC50 0.7
(Salvelinus 1.7 g stat 12 40 6 96-h LC50 0.7
namaycush) 1.7 g stat 12 40 9 96-h LC50 0.9
0.5 g stat 12 162 7.4 96-h LC50 0.9
2.6 g flow 12 162 7.4 96-h LC50 2.3
Gold fish 0.9 g stat 18 40 7.1 96-h LC50 13.2
(Carassius 0.9 g stat 18 272 7.4 96-h LC50 12.8
auratus)
Common carp 0.6 g stat 18 40 7.1 96-h LC50 5.3
(Cyprinus
carpio)
fry CT 96-h LC50 1.7 Chin & Sudderuddin (1979)
Table 35 (continued)
Organism Size/age Stat/ Temperature Hardness pH Formulationc Parameter Concentration Reference
flowa (°C) (mg/litre)b (mg/litre)
Carp C50 WP 72-h LC50 10.4 Toor & Kaur (1974)
(Cyprinus
carpio
communis)
(Catla catla) S 96-h LC50 6.4 Tilak et al. (1981)
(Cirrhina S50 WP 96-h LC50 2.0 Verma et al. (1984)
mrigala)
Fathead 0.5 g stat 12 42 7.5 96-h LC50 14.0 Mayer & Ellersieck (1986)
minnow 0.8 g stat 18 40 7.1 96-h LC50 14.6
(Pimephales 0.8 g stat 18 272 7.4 96-h LC50 7.7
promelas)
Sheepshead CT 96-h LC50 2.2 Springborn (1985)
minnow
(Cyprindon
variegatus)
Black bullhead 1.2 g stat 18 40 7.1 96-h LC50 20.0 Mayer & Ellersieck (1986)
(Ictalurus
malas)
Channel catfish 1.5 g stat 18 40 7.1 96-h LC50 15.8
(Ictalurus 1.5 g stat 18 272 7.4 96-h LC50 7.8
punctatus) fingerling flow 12 314 7.5 96-h LC50 17.31
Table 35 (continued)
Organism Size/age Stat/ Temperature Hardness pH Formulationc Parameter Concentration Reference
flowa (°C) (mg/litre)b (mg/litre)
Freshwater 1.1 g stat 28 S50 WP 72-h LC50 17.5 Arunachalam et al. (1980)
catfish
(Mystus
vittatus)
S 96-h LC50 2.4 Tilak et al. (1981)
(Mystus S 96-h LC50 4.6
cavasius)
Freshwater CT 96-h LC50 46.9 Tripathi & Shukla (1988)
catfish
(Clarias
batrachus)
CT 96-h LC50 107.7
Catfish CT 96-h LC50 9.7 Lejczak (1977a)
(Lebistes
reticulatus)
Green sunfish 1.1 g stat 18 40 7.1 96-h LC50 11.2 Mayer & Ellersieck (1986)
(Lepomis 1.1 g stat 18 272 7.4 96-h LC50 9.5
cyanellus)
Table 35 (continued)
Organism Size/age Stat/ Temperature Hardness pH Formulationc Parameter Concentration Reference
flowa (°C) (mg/litre)b (mg/litre)
Bluegill 1.2 g stat 18 40 7.1 96-h LC50 6.8
(Lepomis 1.2 g stat 18 272 7.4 96-h LC50 5.2
macrochirus) 0.4 g stat 12 44 7.4 96-h LC50 7.4
0.4 g stat 22 44 7.4 96-h LC50 5.2
0.8 g stat 12 40 7.4 96-h LC50 16.0
0.8 g stat 17 40 7.4 96-h LC50 7.0
0.8 g stat 22 40 7.4 96-h LC50 8.2
0.4 g stat 17 320 7.4 96-h LC50 6.2
0.7 g stat 17 40 6.5 96-h LC50 5.4
0.7 g stat 17 40 7.5 96-h LC50 5.2
0.7 g stat 17 40 8.5 96-h LC50 1.8
0.7 g stat 17 40 9.5 96-h LC50 2.6
0.6 g flow 12 314 7.5 96-h LC50 5.1
XLR 96-h LC50 9.8 Springborn (1985)
S4 96-h LC50 10
Mosquito fish 30-40 mm stat 96-h LC50 31.8 Chaiyarach et al. (1975)
(Gambusia
affinis)
Largemouth 0.9 g stat 18 40 7.1 96-h LC50 6.4 Mayer & Ellersieck (1986)
bass
(Micropterus
salmoides)
Black crapple 1 g stat 18 40 7.1 96-h LC50 2.6
(Pomoxis
nigromaculatus)
Table 35 (continued)
Organism Size/age Stat/ Temperature Hardness pH Formulationc Parameter Concentration Reference
flowa (°C) (mg/litre)b (mg/litre)
Yellow perch 1.4 g stat 18 40 7.1 96-h LC50 0.7 Mayer & Ellersieck (1986)
(Perca 0.6 g stat 12 42 7.5 96-h LC50 5.1
flavescens) 1 g stat 7 42 7.5 96-h LC50 13.9
1 g stat 12 42 7.5 96-h LC50 5.4
1 g stat 17 42 7.5 96-h LC50 3.4
1 g stat 22 42 7.5 96-h LC50 1.2
0.9 g stat 12 42 6.5 96-h LC50 4.0
0.9 g stat 12 42 7.5 96-h LC50 4.2
0.9 g stat 12 42 8.5 96-h LC50 0.5
0.9 g stat 12 42 9 96-h LC50 0.4
1 g stat 12 42 8 96-h LC50 3.8
1 g stat 12 170 8 96-h LC50 5.0
1 g stat 12 300 8 96-h LC50 3.8
fingerling flow 12 314 7.5 96-h LC50 1.4
Snakehead fish CT 48-h LC50 8.7 Rao et al. (1985a)
(Channa
punctatus)
C50 WP 96-h LC50 19.5 Singh et al. (1984)
C50 WP 48-h LC50 8.1 Rao et al. (1985b)
(Anabas S 96-h LC50 5.5 Tilak et al. (1981)
testulus)
Table 35 (continued)
Organism Size/age Stat/ Temperature Hardness pH Formulationc Parameter Concentration Reference
flowa (°C) (mg/litre)b (mg/litre)
Tilapid fish S50 WP 72-h LC50 8.0 Koudinya & Ramamurthi
(Sarotherodon (1980)
mosambica)
(Tilapia sp.) C 48-h LC50 5.5 Basha et al. (1983)
Estuarine C 96-h LC50 2.2 Lingaraja & Venugopalan
teleost (1978)
(Therapon
jarbus)
(Hetero-pneustes C50 WP 96-h LC50 20.1 Singh et al. (1984)
fossilis)
(Macropodus) CT 96-h LC50 3.5 Arunachalam &
Palanichamy (1982)
(Cypanus) CT 96-h LC50 4.0
Estuarine &
marine
Longnose juvenile stat 28 19s 48-h LC50 1.6 Mayer (1987)
killifish
(Fundulus
similis)
Table 35 (continued)
Organism Size/age Stat/ Temperature Hardness pH Formulationc Parameter Concentration Reference
flowa (°C) (mg/litre)b (mg/litre)
Striped mullet juvenile stat 24 17s 48-h LC50 2.4
(Mugil
cephalus)
aStat = static conditions (water unchanged for the duration of the test); Flow = flow-through conditions (carbaryl concentration
in water continuously maintained).
bHardness = expressed as mg CaCO3/litre; s = salinity (%).
cFormulation: C = carbaryl; CT = Carbaryl technical (> 95%); C50 WP = Carbaryl 50% wettable powder; S = Sevin; S4 = Sevin-4-oil;
S50 WP = Sevin 50% wettable powder; XLR = Sevin (44% carbaryl); C49 = Carbaryl (49%). Where information on formulation is not given
the carbaryl formulation used was mostly carbaryl technical.
Exposure of C. punctatus to carbaryl at a concentration of
3 mg/litre for 48 h resulted in an increase in free fatty acids,
cholesterol, and lipase activity in the liver (Rao et al., 1985b).
However, the total lipid content was reduced.
The literature indicates that water temperature, hardness, and
pH may influence the toxicity of carbaryl, as well as the size of
the fish (Table 35).
In a study by Post & Schroeder (1971), water was supplied from
a well, classified as very hard and highly alkaline at temperatures
of 13.6-14.6 °C. Carbaryl was more toxic for cutthroat trout
weighing 0.37 g than for those weighing 1-2 g (96-h LC50 1.5 and
2.2 mg/litre, respectively) and more toxic for brook trout weighing
1.15 g than for those weighing 2.04 g (96-h LC50 1.2 and
2.1 mg/litre, respectively).
A comparative toxicity study on carbaryl and 1-naphthol, under
laboratory conditions showed that 1-naphthol was approximately 5
times more toxic than carbaryl for goldfish ( Carassius auratus)
and 2 times more toxic for killifish ( Fundulus heteroclitus) (Shea
& Berry, 1983).
Synergism of the effects of carbaryl and phenthoate on Channa
punctatus was found (Rao et al., 1985a,b). Carbaryl produced
potentiation of the effects of 2,4-D, n-butyl ester, dieldrin,
rotenone, pentachlorophenol, and arecoline on trout (Statham & Lech,
1975).
Application of carbaryl to a forest resulted in a significant
(15-34%) decrease in brain acetylcholinesterase activity in brook
trout from a nearby stream (Haines, 1981). No other effects were
observed.
7.2.2.2 Short-term and long-term toxicity
There is a limited data base on the effects of long-term
carbaryl exposure on fish. In one study, juvenile spot Leiostomus
xanthurus were exposed for five months to carbaryl (technical,
98%) at a level of 0.1 mg/litre in a flow-through system (Lowe,
1967). No carbaryl-related mortality was observed and there was no
effect on growth.
Carlson (1972) conducted the only full life-cycle study on fish
with carbaryl (80%), in which fathead minnows ( Pimephales promelas)
were exposed to five concentrations (0.008-0.68 mg/litre) for 9
months, beginning with the larvae. Survival of fatheads after 6
months at 0.68 mg/litre was lower than that in the controls. After 9
months at 0.68 mg/litre, the mean number of eggs produced per female
was reduced, the mean number eggs per spawning was also affected and
no hatching occurred. No other demonstrable effects were noted at
0.017, 0.062, and 0.21 mg/litre concentrations; thus, the maximum
acceptable toxicant concentration (MATC) for fathead minnows,
exposed to carbaryl in water, was between 0.21 and 0.68 mg/litre.
The lethal threshold concentration for 2-month-old minnows was
9 mg/litre.
Kaur & Toor (1977) exposed different stages of the embryo of
carp ( Cyprinus carpio) to carbaryl through the hatching stage.
There was 100% mortality of carp eggs and embryos at 2.5 mg/litre.
There appeared to be no effect of carbaryl on hatching at
0.01-0.75 mg/litre. However, there was decreased hatching at
1.0 mg/litre and deformed larvae (3.3%) with enlargement of the
pericardial sac and coiling of the posterior region of the embryo.
Thirty days exposure of Channa striatus to 10 or 20 mg
carbaryl/litre retarded oocyte production, with an increase in the
number of immature oocytes and a decrease in the number of mature
oocytes (Kulshrestha & Arora, 1984).
Statistically significant reductions in gonadotropic hormone in
the pituitary gland and plasma in Channa punctatus were observed
following exposure to carbaryl at a concentration of 1.66 mg/litre
(Ghosh et al., 1990). Gonadotropic hormone levels continued to
decrease with continued exposure, with a 30% decrease in the
pituitary gland and a 50% decrease in serum after 30 days of
exposure.
Exposure of the freshwater fish Puntius conchonius to carbaryl
at a concentration of 0.194 mg/litre for 15 days resulted in an
increase in the incidence of lesions in the gill and liver (Gill
et al., 1988). A higher level of exposure also resulted in lesions
in the kidney.
A 27-day exposure to 12.5 mg/litre led to a decrease in the
feeding and growth rate of the catfish ( Mystus rittatus)
(Arunachalam et al., 1980). Long-term toxicity of carbaryl for
fish in most natural surface waters will not occur, since exposure
would not be high enough or constant, because of the substance's
degradation.
7.2.3 Amphibians
The LC50s for bullfrog ( Rana tigrina) 0.1 g tadpoles with
24, 48, 72, or 96 h of exposure were determined to be 12.8, 8.2,
6.7, and 6.3 mg/litre, respectively (Marian et al., 1983). Growth
and feeding were decreased in a dose-dependent manner by doses
ranging from 0.5 to 5 mg/litre.
7.3 Terrestrial organisms
7.3.1 Worms
Carbaryl was classified as extremely toxic for earthworms
(LC50 = 9.1 µg/m2) by Roberts & Dorough (1984). The LC50 value
for the worm Eisenia fetida-Savigny was 14 µg/cm2 (Neuhauser
et al., 1985). The number of earthworms was reduced by 60% when
carbaryl was applied at 0.5 kg (1.12 lb) a.i. per hectare (Thompson,
1971).
Exposure of earthworms to carbaryl (1-8 mg/kg) significantly
increased the burrowing time, which was directly proportional to the
dose and time of exposure, because of the AChE inhibition in the
neural tissue (Gupta & Sundararaman, 1991).
7.3.2 Insects
Administration of carbaryl in sublethal doses produced death in
the early embryonal stage of the silkworm (Kuribayashi, 1981).
The alfalfa leaf-cutting bee ( Megachile pacifica), which is
very important in the pollination of alfalfa, has been reported to
be particularly tolerant to carbaryl (Lee & Brindley, 1974). Waller
(1969) also classified carbaryl as relatively non-toxic for the
alfalfa leaf-cutting bee. The carbaryl tolerance was related to sex
and age. The LD50 was similar for 1-day-old males and 1-and
4-day-old females (240, 245, and 262 µg/g, respectively). However,
four-day-old males were much more susceptible to carbaryl, and had
an LD50 of 51 µg/g. Guirgius & Brindley (1975, 1976) showed that
carbaryl toxicity in alfalfa leaf-cutting bees was controlled by the
activity of mixed function oxidase or microsomal enzymes. This
detoxification system varies with the age and sex of the bees and
results in significantly different carbaryl persistence which, in
turn, leads to differences in carbaryl toxicity. In the more
tolerant groups, carbaryl metabolites were rapidly conjugated and
moved to an aqueous fraction of the bee. Less tolerant insects
(4-day-old males) accumulated these metabolites with time,
indicating that the conjugation mechanisms had deteriorated with
age.
Carbaryl is known to be highly toxic for honey-bees. When
ingested, the LD50 was 0.18 µg/bee (Alvarez et al., 1970). The
contact LD50 (approximately 10-15 mg/kg) for adult bees is
approximately 1.3 µg (Stevenson et al., 1977; Stevenson, 1978).
The carbaryl residue content of bee bread was correlated with
the amount of residue found in the bees and occurred at higher
levels than in honey throughout the 56-day period (Winterlin &
Walker, 1973).
7.3.3 Birds
The toxicity of carbaryl for birds appears to be low
(Table 36). LD50s for six species of waterfowl and game birds were
all greater than 1000 mg/kg (Bart, 1979). There were some
exceptions; thus the red-winged blackbird has an LD50 of 56 mg/kg
(Schafer, 1972). In this study, 180 compounds were found to be toxic
for the red-winged blackbirds and the LD50s ranged between 0.24
and 100 mg/kg.
Food intake, body weight, and locomotor activity were monitored
in adult male bobwhites ( Colinus virginianus), given diets that
contained levels of carbaryl typical of normal exposure under
agricultural conditions in Kansas. No changes were observed in diets
containing 127 or 1235 mg carbaryl/kg (Robel et al., 1982).
Technical carbaryl fed to young Mallard ducks at dietary levels
of 10, 100, 1000, or 3000 mg/kg, revealed dose-correlated signs of
toxicity (reduced intake and/or body weight depression) at the 100,
1000, and 3000 mg/kg level (Fletcher & Leonard, 1986).
There was some tentative evidence that low dosages of carbaryl
may increase the susceptibility of bobwhites ( Colinus virginianus)
to the protozoan parasite Histomonas meleagridis, to which they are
usually resistant (Zeakes et al., 1981).
A study of the effects of carbaryl on forest birds was
conducted in southern New York; plots had been treated 3-4 weeks
previously with carbaryl at the normal rate of 1.1 kg/ha, and at 6
times the normal rate (6.6 kg/ha). In this study, carbaryl had
little, if any, effect on birds. Young birds gained weight normally,
adults continued nesting in the area, no changes were detected in
song frequency, and there was no evidence of birds leaving the area
to forage. This lack of detectable effects, despite the heavy dose
(6.6 kg/ha), indicates that carbaryl applied at the normal rate
(1.1 kg/ha) would have little if any adverse effect on birds (Bart,
1979).
The LC50 of carbaryl for mallard embryos, following field
applications, was determined by Hoffman & Albers (1984) to be
greater than 26.4 kg/ha or 118 µg/g egg. Carbaryl was estimated to
be relatively nontoxic compared with other pesticides.
Inhibition of the brain ChE activity of birds from forests
sprayed with carbaryl 1.13 (kg/ha) was found in 3 out of 12 species
studied (Zinkl et al., 1977) up to 5 days after application.
Table 36. Acute toxicity of carbaryl for birds
Species Age Parametera Concentration Reference
(mg/kg)
Japanese quail 7 days LD50 2290 Hudson et al.
Coturnix coturnix (1984)
japonica 2 months 5 day-LC50 > 10 000 Hill & Camardese
(1986)
Bobwhite quail 23 days 5 day-LC50 > 5000 Hill et al. (1975)
Colinus virginianus
California quail 10 months LD50 > 2000 Hudson et al.
Callipepla californica (1984)
Chukar Alectoris chukar 4 months LD50 1888
Sharp-tailed grouse LD50 < 1000
Tympanuchus
phasianellus
Pheasant 3-4 months LD50 707-> 2000
Phasianus colchicus 23 days LD50 > 5000 Hill et al. (1975)
Mallard 3 months LD50 > 2564 Hudson et al. (1984)
Anas platyrhynchos 24 days 5 day-LC50 > 5000 Hill et al. (1975)
Rock dove LD50 1000-3000 Hudson et al. (1984)
Columbia livia
Canada goose LD50 1790
Branta canadensis
Red-winged blackbird LD50 56 Schafer (1972)
Agelaius phoeniceus
a LD50 = single oral dose expressed as mg/kg body weight.
5 day-LC50 = 5-day dietary exposure (expressed as mg/kg feed) followed by
3 days on a "clean" diet.
7.3.4 Mammals
The effect of carbaryl on wild mice ( Clethrionomys glareolus
and Apodemus sylvaticus) in their natural environment was studied
by Krylov (1970). Carbaryl-containing bait, each consisting of 2 kg
grain treated with 50 g carbaryl in oil suspension, were distributed
twice (in March and June) over 10 ha of woods, at a rate of 1
bait/ha. An adjacent territory of 10 ha was used as a control.
Effects on mice, assessed 1´ months after the last application,
were: reduction of the population by 31.5% compared with the
control; changes in the reproduction system (e.g., decreased number
of embryos in utero, and of corpora lutea, reduced weight of
testicles), and increased weight of adrenal glands.
7.4 Effects on the population and ecosystem
The effects of carbaryl on terrestrial ecosystems have been
studied by Stegeman (1964), Barrett (1968), and Spain (1974).
A large-scale study was performed by Barrett in 1968. It was
designed to determine the effects of carbaryl on an intact ecosystem
in a field that was planted with millet ( Panicum ramosum). The
area was sprayed with a single application of 2.24 kg carbaryl/ha.
There was a highly significant decrease in litter decomposition in
the treated area, 3 weeks after spraying, which was probably because
of a reduction in microarthropods and other decomposers. After 5
weeks, there was a more than 95% reduction in the number of
arthropods and in the total biomass. Phytophagous insects were
severely affected, and predatory insects and spiders were less
affected. The number of species was also reduced, but all species
returned to control levels within 1-2 weeks, except Hemiptera and
Hymenoptera. Reproduction of cotton rats was delayed by 4 weeks.
However, the total mammal population was not affected, because of a
compensating increase in the population of house mice. Old field
mice did not seem to be affected.
The effects of carbaryl on forest soil mites and Collembola,
the two most numerous soil arthropods, were studied by Stegeman
(1964). Mites and Collembola are an important link in the
decomposition process of dead plants and animal matter. They also
are a natural means of decomposing the accumulating litter, and they
provide nutrients for already existing or future crops and fungi.
Application of carbaryl to a test plot in a red-pine plantation, at
doses of 11.2 and 56 kg/ha, reduced the arthropod population
proportional to the severity of the treatment. Neither mites nor
Collembola were totally exterminated by any treatment. The rate of
population increase of the mites, 4-5 months after treatment, was
directly proportional to the dose applied. Collembola were more
vulnerable to treatment than mites and did not recover as rapidly.
The effect of carbaryl at two application rates (0.11 and
1.13 g a.i. per m2) on mixed species population of the litter
fauna of a Corsican pine forest was studied by Spain (1974). He took
a quantitative sampling of the fauna at intervals of 11, 106, 209,
and 315 days after application to record the pattern and the time of
the recovery process (Table 37). The effect of carbaryl varied in
different populations. For Collembola, there was a marked reduction
at the two treatment levels. For Coccoidea and Symphyla, there was a
slight effect at the lower level, and a marked decrease at the
higher level. For Coleoptera, Diptera, Cryptostigmata, Mesostigmata,
and Prostigmata, the effect was small or insignificant. The recovery
process during the period of 315 days was not sufficient to reach
the status of the untreated population.
Tagatz et al. (1979) studied the effects of carbaryl on
animal communities that develop from planktonic larvae in aquaria
containing sand and estuary water. Samples collected after 10 weeks
of exposure were analysed. The numbers of animals and species were
significantly less at 11.1 and 103 µg carbaryl/litre (Table 38)
being decreased to nearly half (from 21 to 12). A carbaryl
concentration of 1.1 µg/litre did not produce significant effects,
except a decrease in the particularly sensitive amphiopod,
Coraphium acerusicum. Carbaryl might have caused changes in
biological interactions that affect the relative abundance of
species. The annelid Polydora ligni increased at a concentration
of 103 µg/litre, and there was a marked decrease in the number of
other annelids and nemerteas.
There have been a number of field studies evaluating the impact
of carbaryl applications on macroinvertebrate populations. In one
study in New York State, carbaryl was applied aerially at a rate of
1.3 kg/ha, in fuel oil with a paraffin oil sticker, and its effects
upon the aquatic fauna of two streams were studied (Burdick et al.,
1960). The data indicated that carbaryl (in oil suspension) was
toxic for mayflies (Ephemeroptera), stoneflies (Plecoptera) and
caddisflies (Trichoptera). Other groups of insects were less
affected and the application did not affect fish. Square-foot
samples, collected before, and shortly after, spraying, showed
reductions of from 50 to 97.2% in the weight of invertebrate
(standing crop) fish food. A progressive effect from upstream to
lower sections was correlated with increased 44-151 exposure time.
No exposure concentrations in water were documented. Owen (1965)
also reported a reduction of 54.2 to 62.8% in the standing crop of
aquatic insects following aerial spraying with carbaryl (80 WP)
(1.3 kg/ha). A control stream showed a 5.9% increase in the same
period.
Table 37. Geometric means for assessed taxa (individuals/m2), post-application sampling of carbaryl-treated plotsa
Sampling day (after treatment) Taxon Control plot High carbaryl Low carbaryl
Coccoidea 272.9 90.8 129.4
Coleoptera (imagines) 425.6 137.7 153.1
Coleoptera (immature) 54.0 47.3 41.4
Collembola 42 771.5 5033.4 9819.7
Diptera 350.9 244.2 243.7
11 Cryptostigmata 5707.3 3933.2 5863.5
Mesostigmata 7239.5 3688.9 5038.2
Prostigmata 1335.6 305.3 564.8
Aranese 238.7 222.7 155.0
Chilopoda 71.1 102.4 37.4
Symphyla 872.1 359.0 388.9
Coccoidea 847.8 157.0 162.9
Coleoptera (imagines) 41.9 379.1 170.7
Coleoptera (immature) 41.9 31.7b 0.0b
Collembola 18 185.1 1621.3 5205.9
Diptera 264.0 173.8 160.5
106 Cryptostigmata 5855.3 6237.9 6623.7
Mesostigmata 8610.3 846.4 595.2
Prostigmata 631.5 846.4 595.2
Aranese 360.6 224.9 253.2
Chilopoda 114.0 157.8 94.3
Symphyla 317.5 47.0b 223.4
Table 37 (continued)
Sampling day (after treatment) Taxon Control plot High carbaryl Low carbaryl
Coccoidea 358.3 181.0 141.0
Coleoptera (imagines) 520.0 86.6 232.6
Coleoptera (immature) 289.6 125.9 74.1
Collembola 41 198.7 3469.4 19 238.9
Diptera 449.9 28.2b 209.5
209 Cryptostigmata 16 298.4 11 184.8 12 014.8
Mesostigmata 16 150.0 7293.9 11 709.0
Prostigmata 2721.6 2407.2 3152.4
Aranese 264.7 108.3 220.2
Chilopoda 241.9 28.2b 150.5
Symphyla 357.3 78.3 174.4
Coccoidea 142.0 207.5 51.5
Coleoptera (imagines) 261.6 64.4 132.5
Coleoptera (immature) 37.0 88.8 23.1b
Collembola 40 139.5 4121.3 10 294.6
Diptera 533.9 291.2 173.1
315 Cryptostigmata 10 889.3 12 743.6 10 506.6
Mesostigmata 11 290.3 7762.7 14 912.0
Prostigmata 2995.0 1844.7 4765.7
Aranese 217.4 157.4 117.8
Chilopoda 159.5 83.4 88.8
Symphyla 349.6 85.4 158.8
aSource:Spain (1974).
b95% confidence limits for the parametric mean include zero.
Table 38. Animals and species, by phylum, collected from control aquaria and aquaria exposed to carbaryla
Control Carbaryl
1.1 µg/litre 11.1 µg/litre 103 µg/litre
Phylum Number Species Number Species Number Species Number Species
Mollusca 1691 3 1563 3 1340 3 1321 5
Arthropoda 380 7 339 8 336 2 269 2
Annelida 102 8 94 7 79 4 200 5
Nemerica 16 1 25 1 20 1 0 0
Coelenterata 3 1 5 1 0 0 0 0
Platyhelmintes 0 0 2 1 0 0 0 0
Echinodermata 0 0 0 0 1 1 0 0
All phyla 2192 20 2086 21 1776 11 1790 12
aFrom:Tagatz et al. (1979).
The effects on stream invertebrates of carbaryl, applied at a
rate of 840 g a.i./ha for spruce budworm suppression, was studied.
Benthos samples showed significant declines among Plecoptera,
Ephemeroptera, and Trichoptera. Plecoptera had not repopulated any
treated stream, 60 days after treatment (Courtemanch & Gibbs, 1980).
Gibbs et al. (1984) conducted a 42-month (1980-83) study on
the occurrence/persistence of carbaryl residues in pond water and
sediment as a result of an application of Sevin-4-oil (840 g
a.i./ha). The immediate and long-term effects on pond
macroinvertebrates and emerging aquatic insects were also evaluated.
A preliminary study by these investigators in 1977 and others
(Coutant, 1964) had shown that there were large increases in the
number of drift organisms, several days after aerial spraying with
carbaryl. Most drift was accompanied by dead Amphipoda,
Ephemeroptera, Plecoptera, and Trichoptera, and carbaryl residues
persisted longer than 30 days in pond sediment. The increase in the
number of dead organisms was accompanied by a reduction in the
standing crop of benthic macroinvertebrates. The most severe and
persistent impact was on Amphipoda with Hyallela azteca and
Crangonyx richmondensis reduced to almost 0/m2; C. richmondensis
failed to recolonize in one of the two treatment ponds, 42 months
after treatment. The numbers of immature Ephemeroptera and
Trichoptera were reduced immediately following spray application,
but this effect did not persist throughout the season or into the
following year. Immediate reduction in numbers of adult
Ephemeroptera and Trichoptera emerging from the ponds was also
found, but recovery of populations was observed. Numbers of immature
Odonata were also reduced following treatment and remained low the
following year. The Chironomidae populations did not appear to be
affected, either as immatures or emerging adults.
The effects of two consecutive years of spraying with
Sevin-4-oil on other aquatic systems appear similar to those
observed in areas treated once (Courtemanch & Gibbs, 1978; Trial,
1978,1979).
In simulated aquatic field studies, 1 mg carbaryl (WP)/litre
was applied to one outdoor concrete pond (4 x 5 x 1 m deep)
(Hanazato & Yasuno, 1987). All zooplankton (Cladocera included) and
Chaoborus larvae were killed. The zooplankton community recovered
rapidly and Cladocera reappeared only two days after application.
Since Chaoborus populations are predators of crustacean zooplankton,
their suppression may also have added to the recovery of increased
populations of Cladocerans. Carbaryl exposure also changed the
community from a rotifer-predominating zooplankton community to that
of Cladocera-predominating. Rotifers in this study were also highly
sensitive to carbaryl. The same phenomenon was observed again after
the second application of carbaryl. Subsequent to this study,
Hanazato & Yasuno (1989) applied carbaryl to simulated ponds in the
same way as in the previous study, but in the spring when the water
temperature was approximately 10 °C lower. Cladocerans never
recovered to the density level of the pre-treatment period. The
rapid recovery of Chaoborus seemed to interfere with the recovery of
Cladoceran populations after treatment. The authors suggested that
the different recovery patterns of the zooplankton community
resulted from different temperatures in the ponds. In another study,
Hanazato & Yasuno (1990a) examined Chaoborus density in relation to
the effects of carbaryl (0.1 and 0.5 mg/litre) on zooplankton
communities in ponds, where the abundance of Chaoborus larvae was
controlled. They concluded that Chaoborus density and/or temperature
may influence the recovery of the zooplankton community following
the effects of carbaryl. The recovery of a zooplankton community may
differ in different aquatic ecosystems (with different community
structures) and under different temperatures, even when the same
treatment is applied.
Hanazato & Yasuno (1990b) also studied the effect of the time
of application of carbaryl (0.5 mg/litre) on recovery patterns of
zooplankton communities in simulated ponds. The loss of carbaryl
from pond water was rapid. The concentration decreased to less than
1% of its initial value, three days after the first or second
application, and six days after the third application. This study
also showed that applications of carbaryl at different times induced
different zooplankton structures (and different recovery patterns),
and, various factors other than toxicity of carbaryl, such as
temperature, competitive interactions between zooplankters, and
trends of zooplankton populations may play important roles in
determining zooplankton community structure after chemical
application. Furthermore, the significance of predators in the
recovery process after chemical treatment was re-emphasized.
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
Reviews of toxicological aspects of carbaryl were prepared by
NIOSH (1976); US EPA (1977, 1980, 1982, 1984); USDHHS/USDL (1978);
US EPA (1980b); Mount & Oehme (1981a); Weston (1982); and Cranmer
(1986). Studies from the Soviet Union were reviewed by IRPTC (1982,
1989).
8.1 Single exposures
8.1.1 Oral toxicity
The oral LD50s for the rat are given in Table 39 and for
other mammals in Table 40. The values varied by about a factor of 4
depending on formulation, route of production, vehicle, and strain
of rat. Interspecies differences were found. Cats are the most
sensitive, with guinea-pigs, rats, mice, and rabbits showing more
resistance in that order. Pigs and monkeys seem to be less sensitive
(Carpenter et al., 1961; Gladenko & Malinin, 1970; Smalley, 1970).
Cattle are more sensitive than pigs. After a single oral
application of carbaryl in cattle, symptoms appeared at a dose level
of 25 mg/kg; 100 mg/kg was the minimum effective dose in pigs
(Gladenko & Malinin, 1970).
Mount & Oehme (1981b) observed that the lethality of carbaryl,
administered to sheep at doses ranging from 300 to 1000 mg/kg, was
highly correlated with concentrations in the brain (>1 mg/kg) and
liver (>3 mg/kg), and with inhibition of acetylcholinesterase
(greater than 50% inhibition).
8.1.2 Acute inhalation toxicity
Acute toxic effects following inhalation at different
concentrations are presented in Table 41.
8.1.3 Dermal toxicity
A dose of 2500 mg/kg, applied as a 40% aqueous suspension of
50% wettable powder, killed 1 out of 4 rabbits (Carpenter et al.,
1961). When 99% technical carbaryl was applied to male and female
rabbits, the LD50 was >2000 mg/kg (Bushy Run, 1983b). In rats,
the LD50 is thought to be >4000 mg/kg (Yakim, 1965; Gaines,
1969).
Table 39. Acute oral LD50s for the rat
Strain (sex) Weight Formulation of Vehicle LD50(mg/kg Symptoms References
(g) carbaryl body weight)
CF-N technical 0.25% agar
(male) 90-120 510 (360-650) - Carpenter et al.
(female) 90-120 610 (490-750) - (1961)
Sherman 850 (600 LD min) Gaines (1969)
(male)
(female) 500 (100 LD min)
Inbred 721 (653-789) Yakim (1965)
Inbred 120-200 sunflower oil 515 Rybakova (1966)
Sprague Dawley 203-246 technical carboxymethyl-cellulose 300 Hamada (1990)
(male, female) in water
Sprague Dawley 112-173 technical 0.25% methyl-cellulose 685 (612-767) death, 4-24 h Field (1980b)
(male, female)
Sprague Dawley 100-160 95% technical 0.25% methyl-cellulose 225 (202-321) death, 2-24 h Field (1980a)
(male, female)
Table 39 (continued)
Strain (sex) Weight Formulation of Vehicle LD50(mg/kg Symptoms References
(g) carbaryl body weight)
Sprague Dawley 200-264 40.38% water tremor, prostration, Hazleton Lab.
(male) 750 (467-1202) laboured respiration, American Inc.
salivation 10 February 1982
(female) 527 (257-977) death, 4-3 days
Harlem Sevin XLR not diluted death, 0.5 h-3 days Kuhn (1991a)
Sprague Dawley plus 44%
(male) 229-286 867 (562-1336)
(female) 182-219 575 (459-721)
Harlem Sevin-4-oil not diluted 658 (456-979) 3 h-3 days Kuhn (1991b)
Sprague Dawley 47% (w/w)
(male) 229-286 963 (802-1160)
(female) 182-219 473 (364-620)
Hilltop Wistar 200-250 98% technical 0.25% methyl-cellulose Bushy Run
(male) 283 death, 1.5-24 h (1983b)
(female) 246
Hilltop Wistar 204-237 80% sprayable water
(male) 406 Bushy Run
(female) 203 death, 1 min-24 h (1983c)
Table 40. Acute oral toxicity for mammals other than rats
Species Number of animals Toxicitya Reference
(mg/kg body weight)
Mouse 6 per dose 363 (294-431) Yakim (1965)
Mouse (female) 80 white 437 ± 70 Rybakova (1966)
Mouse 1310 white 206 (175-480) Bukin (1965)
Guinea-pig 5 per dose 280 Carpenter et al. (1961)
Rabbit 4 per dose 710
Rabbit 56 700 (LD100) Bukin (1965)
Cat (female) 3 250 (LD100) Carpenter et al. (1961)
Cat no data 150 Yakim (1965)
Swine 3 per dose 800-1000 Gladenko & Malinin
(1970)
Swine 1 per dose 1500-2000 Smalley (1970)
Monkey no data > 1000 FAO/WHO (1970)
Duckling 106 500 (LD15) Bukin & Filatov (1965)
6000 (LD100)
Table 40 (continued)
Species Number of animals Toxicitya Reference
(mg/kg body weight)
Chick 48 250 (LD33)
1000 (LD100)
Hen 12 > 1000 (no death)
Hen 12 > 3000 Bukin (1965)
aLD50, unless otherwise stated.
Table 41. Inhalation toxicity single exposure
Species Concentration Effects observed References
Guinea-pig 6 390 mg 50% wettable powder/m3 nasal and ocular irritation after 14 days - Carpenter et al.
(average particle size 15 µm) 4 h haemorrhage areas in the lungs (1961)
Guinea-pig 6 230 mg carbaryl 85S/m3 slight weight decrease; recovered by day 14
(average particle size 5 µm) 4 h
Dog 75 mg carbaryl 85S/m3 5 h typical symptoms for ChE inhibition
Cat 3 groups 82 mg/m3 dust 6 h tremor salivation, muscle fibrillation decreased Yakim (1967, 1968)
of 4 animals ChEA by 39-55% serum; 53-71% red blood
cells; normalization after 72 h
37 mg/m3 dust 6 h decreased ChEA by 23% in serum and 41% in
red blood cells; recovery after 48 h
20 mg/m3 dust 6 h decreased ChEA by 11-27% in serum and
15.28% in red blood cells; recovery after 24 h
Rat 20-23 mg/m3 dust no effect Weil & Carpenter
98% particles (1974)
less than 1.0 µm diameter
Rat 1800 mg/m3 water aerosols lacrimation, tremor with 1.5 h of exposure Myers et al. (1975)
Rat Wistar Albino Aerosols Sevin XLR 44% 792 mg 1/5 females died, tremor, ataxia, increased Faït (1984)
male 5 a.i./m3 per 4 h (the highest respiratory rate; recovery period 6 days
female 5 attainable concentration)
particle size 3.6 ± 2.64 µm
8.1.4 Other routes of exposure
Other routes of exposure are presented in Table 42.
Table 42. Toxicity following parenteral application
Species Weight Vehicle Route of LD50 References
(g) administration mg/kg body
weight)
Rat 10-107 propylene intravenous 18 Mellon Institute
female glycol (1958)
Rat 92-126 polyethylene intravenous 24
glycol 400 intravenous (17-33)
Rat 90-120 95% ethyl intravenous 33
alcohol (26-41)
Rabbit 1686-3544 0.25% agar intraperitoneal 223
(122-407)
Rat 90-120 in lard subcutaneous 1410
Leghorn 10-11 days in alantoic 3.44 mg Tós-Luty et al.
chick old cavity per embryo (1973)
embryos
(250)
8.2 Skin and eye irritation, sensitization
8.2.1 Skin and eye irritation
The results of several studies on skin irritation from carbaryl
were negative (Carpenter et al., 1961; Yakim, 1965). Transient
erythema was noted after an application of 0.5 ml 43.4% carbaryl on
occluded rabbit skin (Bushy Run, 1983a).
Carbaryl is a weak eye irritant (Table 43).
Table 43. Eye irritation
Species Number Formulation Effects observed Reference
Rabbit 5 technical grade mild injury in one Carpenter et al.
10% suspension of five eyes (1961)
in propylene
glycol
25% aqueous no injury
suspension
50 mg dust spots of
corneal-necrosis
Rabbit 6 0.1 ml (90 mg) conjunctival irritation Bushy Run (1983b)
New 99% technical after 2 days recovery
Zealand product
White
0.1 ml transient iritis in Bushy Run
43.4% carbaryl 2 of 6, conjunctival (1983a)
irritation in 6 of 6.
Recovery in 3 days
8.2.2 Sensitization
The sensitization response following topical application of
carbaryl was studied by Myers & Christopher (1987). The induction
which consisted of an application of carbaryl to covered skin, once
a week during 3 weeks, was followed by a 2-week incubation period. A
single challenge dose of 50% (w/v) technical carbaryl in 0.25%
aqueous methyl cellulose solution (0.1-0.3 ml) was administered.
Carbaryl did not produce a positive response in any of the
guinea-pigs tested.
Four out of 16, male, albino guinea-pigs were treated with 8
intravenous injections (3 per week) of 0.1 ml of a 0.1% dispersion
of carbaryl in 3.3% propylene glycol. After a 3-week incubation
period, a challenge dose was given, but did not cause sensitization
(Carpenter et al., 1961). In a more recent study, a similar
procedure, but with 9 doses and a 2-week incubation period, was
used. Although there was some skin irritation, it was considered
that carbaryl had little or no sensitizing potential (Bushy Run,
1983a).
8.3 Short- and long-term oral exposure
Several short- and long-term feeding studies with carbaryl have
been reported (Carpenter et al., 1961; Rybakova, 1967; Orlova &
Zhalbe, 1968; Gladenko & Malinin, 1970; Dikshith et al., 1976;
Hamada, 1991a). Results are shown in Table 44. Doses that do not
show any effects are 200 mg/kg diet equal to 7.9 mg/kg body weight
in rats, and 100 mg/kg diet for mice, and 1.8 mg/kg body weight for
dogs (approximately 100 mg/kg diet).
The cumulation coefficient (LD50 for 3-month exposure/LD50
single application) was 18 (Kassin, 1968), demonstrating a very low
cumulative potential for carbaryl.
8.4 Short- and long-term inhalation toxicity
Short-term and long-term inhalation toxicity data are given in
Table 45.
8.5 Reproduction and developmental toxicity
The reproduction and developmental toxicity of carbaryl has
been studied in many vertebrate species using a wide variety of
study designs.
The data have shown that carbaryl can affect reproduction
(Table 46) and embryo/fetal development (Table 47 and 48) in a
number of species. The relevance of these studies for risk
assessment is influenced by several factors related to experimental
design, dose levels, and the types of effects noted. Shortcomings of
the studies included small sample size; inappropriate dose
selection; the variable degree of maternal toxicity (ranging from no
effect to lethality); and a lack of historical data for some
species. The following sections have been arranged according to
end-point (reproduction and developmental toxicity) and species of
animals studied (mammalian, non-mammalian). Studies that are of
dubious value for risk assessment are listed at the end of the table
sections, and are not discussed in the text. The reasons for such an
evaluation are given below the references to the individual papers.
Table 44. Short- and long-term feeding and oral studies
Species Sex Number Dosage (mg/kg diet Period of Effects observed References
of or mg/kg body weight) exposure
animals
Mouse male 48 100, 400 mg/kg diet 80 weeks no changes in survival rate, pathology, and tumour FAO/WHO (1965)
and incidence
female
Mouse male 10/sex 100, 1000, 7000 mg/kg 53 weeks at 7000 mg/kg diet: decreased survival, body Hamada (1991b)
and per group diet weight gain and erythrocyte count; increased liver
female weight and decreased ovary weight; increased
frequency and severity of chronic nephropathy in
females; cholinesterase (plasma, RBC, and brain)
depressed at 100 and 7000 mg/kg; NOEL
100 mg/kg diet
Rat male 2x10 1500 (58.5 mg/kg 96 days no changes Carpenter et al.
body weight) (1961)
female 2x10 1500 (58.5 mg/kg 96 days kidney weights significantly increased
body weight)
male 2x10 2250 (87.5 mg/kg 96 days increase in liver weight as a % of body weight,
body weight) diffuse cloudy swelling in the kidney tubules in
4 animals (male and female)
female 2x10 2250 (87.5 mg/kg 96 days decrease of body weight, increase in kidney weight
body weight)
Table 44 (continued)
Species Sex Number Dosage (mg/kg diet Period of Effects observed References
of or mg/kg body weight) exposure
animals
Rat male 4x40 50, 100, 200 (2, 4, 2 years no changes Carpenter et al.
CF-N and 7.9 mg/kg body weight) (1961)
female
Rat male 2x40 400 (15.6 mg/kg 2 years weight depression in male, cloudy swelling of the Carpenter et al.
CF-N and body weight) hepatic cords, principally located around the (1961)
female central veins in both sexes; transitory diffuse
cloudy swelling of the epithelial lining of the
primarily convoluted proximal, and loop tubules
Rat 75, 150, 300 mg/kg 3 months cytoplasmatic vacuolization in the proximal tubules FAO/WHO (1970)
body weight
Rat male 4x48 7, 14, 70 mg/kg weight depression at all dose levels; at 70 mg/kg, Rybakova (1967)
and body weight slight morphological liver changes; cloudy swelling
female in convoluted tubules; decreased motility of
spermatocytes at all dose levels, more pronounced
at 70 mg/kg; oedema in the interstitial tissue;
desquamation of spermatogenic epithelium;
destruction of parenchyma; decreased production of
spermatocytes was found at 14 and 70 mg/kg dose
levels; increased estral cycle; increased hypophysis
gonadotropic function; decreased ascorbic acid
contents; cell proliferation, hypertrophy, increased
lipid content in suprarenal glands; decreased thyroid
function; augmentation of follicles, colloid retention,
thickness of follicular epithelium
Table 44 (continued)
Species Sex Number Dosage (mg/kg diet Period of Effects observed References
of or mg/kg body weight) exposure
animals
Rat male 80-90/ 250, 1500, 7500 mg/kg 52 weeks body weight and food consumption lower at middle Hamada (1991c)
Sprague female group diet dose; significantly increased total cholesterol;
Dawley increase in liver and kidney weight; NOEL
250 mg/kg diet
Rat male 876 2, 5, 15 mg/kg 1 year changes in sex glands function at 15 and 5 mg/kg Orlova & Zhalbe
and body weight dose levels; enzymatic activity, spermatozoids and (1968)
female the estral cycle; reduced fecundity; no effect at
2 mg/kg dose level
Rat male total 200 mg/kg 90 days decreased AChE in blood with 33.8%; no Dikshith et al.
local 28 body weight, histological changes (1976)
strain 3 x per week
in peanut oil
Dog male total 0.45, 1.8, 1 year diffuse cloudy swelling of proximal convoluted and Carpenter et al.
Basenji- and 14 7.2 mg/kg body loop tubules of kidney; local sudanophilic granules (1961)
cocker female weight in capsules, in the glomeruli at 400 mg/kg level; the same in
hybrids 5 days/week, to control dogs to a lesser extent; transient hind leg
approximate levels weakness in 1 female after 189th dose at
of 24, 95, 414 mg/kg 0.45 mg/kg body weight
dry diet
Beagle male 24+ 125, 400, 1250 mg/kg 1 year at 400 and 1250 mg/kg diet, decreased AChE in Hamada (1987)
dogs and 24 diet plasma, red blood cells, and in brain; increase in
female leucocyte count and segmented nitrophil count;
increase in liver weight in male; no effect level
125 mg/kg diet
Table 44 (continued)
Species Sex Number Dosage (mg/kg diet Period of Effects observed References
of or mg/kg body weight) exposure
animals
Beagle male 24 0, 20, 45, 125 mg/kg 5 weeks significant inhibition of cholinesterase activity Hamada (1991a)
dogs female 24 diet in plasma at week 2 for males dosed 20 and
125 mg/kg
Monkey 150, 300, 600 mg/kg 38 weeks kidney alterations similar to those found in rats FAO/WHO (1970)
body weight
Swine male 3 150 mg/kg body weight 72-83 progressive myasthenia, incoordination ataxia, Smalley et al.
female 3 in diet days intentional tremor, chronic muscular contraction, (1969)
until terminal paraplegia and prostration;
death myodegeneration
Swine 5 and 10 mg/kg 147-176 no changes Gladenko & Malinin
body weight days (1970)
Cattle 1 and 4 mg/kg 148 days decreased Hb with 20%, and erythrocytes with
(young) body weight 30% occasionally
Table 45. Short- and long-term inhalation toxicity
Species Number of Concentration of carbaryl Days of Effects References
animals exposure
Cat 4 0.06 mg/litre 30 typical cholinergic symptoms, Yakim (1968)
inhibition of ChE plasma 31-40%,
erythrocytes 40-59%
Cat 4 0.03-0.04 mg/litre 30 reaction time increased
Cat 4 0.016 mg/litre 120 no symptoms, fluctuation in
plasma ChE inhibition around 18%
Rat no data 10 mg/m3 dust (85% 90 no mortality no gross visible injury Carpenter et al.
suspension) 7 h/day inhalation (1961)
5 days per week periods
Table 46. Reproduction studies
Species Treatment End-point(s) Reference
(number)
Mouse
Swiss Webster males 30-40 g 0, 8.5, 17, 34 mg/kg body weight weight and uptake of testosterone: Thomas et al. (1974)
(10 + animals/group) orally (5 days) testes and accessory glands
Swiss Webster males 30-40 g 0, 34, 68 mg/kg body weight biotransformation of Dieringer & Thomas
(5 + animals/group) orally (5 days) testosterone-1,2-3H (1974)
C57Bl/6 x C3H 6-8 weeks of 0, 12, 25, 50...800 mg/kg body sperm morphology, testes weight Osterloh et al. (1983)
age (4 animals/group) weight per day 5 days i.p. on day 35 [route of
administration]
Rat
Osborne-Mendel 0, 2000, 5000, 10 000 mg/kg diet fertility, litter size, and viability Collins et al. (1971)
(20 females and 1 male per (3 generations)
group)
Wistar 0, 7, 25, 100, 200 mg/kg body fertility, litter size, and viability Weil et al. (1973)
(13-21 animals/group) weight per day in the diet
0, 3, 7, 25, 100 mg/kg body
weight per day orally
(3 generations)
Male and female 0, 1, 5, 10, 20, 40, 50 mg/kg general toxicity, serum protein, Vashakidze (1975)
body weight per day orally numbers of male reproductive
(1 month) cells, sperm viability, testicular
histology, litter viability
Table 46 (continued)
Species Treatment End-point(s) Reference
(number)
Wistar males 0, 12.5, 25.0, 250.0 mg/kg body sperm morphology Luca & Balan (1987)
(36 animals per group) weight per day
Rats
Male and female 0, 50, 100, 300 mg/kg body fertility indices Vashakidze (1966)
weight per day orally 2 weeks to [insufficient data]
3 months
Male and female 0, 2, 5, 15 mg/kg body weight per fertility indices Orlova & Zhalbe
(total of 876 animals) day orally 12 months for F° (1968); Zhalbe et al.
and 6 months for the F1 (1968) [insufficient
data]
Male and female 0, 2, 5 mg/kg body weight per day fertility indices Shtenberg & Ozhovan
for 6 months (follow-up to Orlova (1971) [insufficient
& Zhable) 2 through 5 generations data]
Male and female 0, 2, 5, 15 mg/kg body weight per fertility indices Shtenberg et al.
day for 12 months (1973) [insufficient
data]
Gerbil
(32-80 animals per group) 0, 2000, 4000, 6000, and fertility, litter size, and viability Collins et al. (1971)
10 000 mg/kg body weight diet
(3 generations)
Table 47. Developmental toxicity studies, mammalian
Species Treatment End-point(s) Reference
(number)
Mouse
CF-1 0, 100, 150 mg/kg body weight fetal examination Murray et al. (1979)
(23-44 in a group) per day orally or 5660 mg/kg diet.
Gestation day 6-15
Swiss albino 0, 100, 150, or 200 mg/kg body fetal examination Mathur & Bhatnagar
(10 per group) weight per day orally. Gestation (1991)
days 8, 12, or 6-15
Charles River 0, 10, 20 mg/kg body weight. fetal examination Benson et al. (1967)
(8 per group) Gestation day 6-parturition [doses too low]
CD-1 100 mg/kg body weight per day postnatal viability Chernoff & Kavlock
(23 animals) orally. Gestation day 8-12 (1982) [teratology
screen test]
CD-1 200 mg/kg body weight per day postnatal viability Kavlock et al. (1987)
(30 animals) orally. Gestation day 8-12 [teratology screen
test]
Rat
Harlan Wistar 0, 20, 100, 500 mg/kg diet. fetal examination Weil & Carpenter
(6 per group) Gestation days 1-7, 5-15, 1-21 (1965); Weil et al.
(1972)
Wistar 0, 200, 350 mg/kg body weight orally fetal examination Golbs et al. (1974)
(10 per group) 40 mg/kg ip. Variety of gestation days.
Table 47 (continued)
Species Treatment End-point(s) Reference
(number)
Sprague-Dawley 0, 1, 10, 100 mg/kg body weight fetal examination Lechner &
(6 or 7 per group) orally. 3 months before and during Abdel-Rahman
gestation (1984)
Sprague-Dawley 0, 20, 37.5 mg/kg body weight fetal examination Hart (1972)
(22 or 23 per group) per day. Gestation day 6-15 [doses too low]
Rats unknown dose (1/50 LD50). fetal examination Dinerman et al.
Gestation days 9, 11, or 13 (1970) [insufficient
data]
Rats 0, 10.6, 106 mg/kg body weight fetal examination Shtenberg et al.
per day. Gestation day 1-20 (1973) [insufficient
data]
Guinea-pigs
HRA/HART 0, 50, 100, 200 mg/kg body fetal examination Weil et al. (1973)
(9-4 per group) weight per day orally. 0, 100,
200, 300 mg/kg body weight per
day in the diet
Coulston strain 300 mg/kg body weight per day. fetal examination Robens (1969)
(26 gestation day treatment Gestation day 11-20 and a variety [insufficient data]
11-20; other treatments of other treatment periods within
unknown) this time
Table 47 (continued)
Species Treatment End-point(s) Reference
(number)
Rabbit
New Zealand White 0, 150, 200 mg/kg body weight fetal examination Murray et al. (1979)
(15-20 per group) per day orally. Gestation day 6-18
New Zealand White 0, 10, 30 mg/kg body weight per fetal examination Shaffer & Levy
(9-12 per group) day. Gestation day 9-16 (1968) [doses too
low]
New Zealand White 0, 50, 100, 200 mg/kg body fetal examination Robens (1969) [small
(4-9 per group) weight per day orally. Gestation number of animals]
day 5-15
Dog
Beagle 0, 3.125, 6.25, 12.5, 25, examination of pups Smalley et al. (1968)
(6-13 per group) 50 mg/kg per day in the diet
throughout gestation
Beagle 0, 2.0, 5.0, 12.5 mg/kg per day in examination of pups Imming et al. (1969)
(7-9 per group) the diet throughout gestation
Pig
Hormel-Hanford 0, 4, 8, 16 mg/kg in the diet 20 examination of fetuses (I) Earl et al. (1973)
(5-16 per group) days before/7 days after breeding or piglets after birth (II)
throughout gestation
Table 47 (continued)
Species Treatment End-point(s) Reference
(number)
Pig (30) up to 30 mg/kg body weight per ? Smalley (1968)
day [insufficient data]
Monkey
Rhesus 0, 2, 20 mg/kg body weight per examination of gestational Dougherty et al.
(4-6 per group) day orally throughout gestation course and new born (1971)
Rhesus 0, 20, 32 mg/kg body weight per examination of gestational Coulston et al. (1974)
(15-16 group) day orally. Gestation day 20-38 course and new born
Sheep
(25 and 26 per group) 159, 297.5 mg/kg diet examination of lambs Panciera (1967)
after birth [significance of
defects impossible to
assess]
Hamster
Golden Syrian 125 mg/kg body weight per day fetal examination Robens (1969)
(6 or 8 per group) on gestation day 6-8; 250 mg/kg [number tested too
body weight per day on gestation small]
day 7 or 8
Table 48. Non-mammalian studies
Species Dosage End-points Reference
Fish
Medaka 0.5, 1.0, 2.5, 5.0, 10.0, and embryonic development Solomon & Weis (1979)
(Oryzias latipes) 20.0 mg/litre, 4-cell through
10 eggs per group blastula
Amphibian
Xenopus laevis 0.1, 1.0, 10.0 mg/litre; embryos to embryonic development, Elliott-Feeley & Armstrong
10-12 per group hatching or tadpoles for 24 h posthatching activity (1982)
Birds
Chicken 0, 0.01, 0.1, 1.0, 10.0 mg/kg embryonic development Olefir & Vinogradova (1968)
4-6 per group body weight
White Leghorn chicken 0, 250, 500 mg/kg diet to pullets egg production, embryonic Lillie (1972)
20 per group for 36 weeks and hatchlings for development, hatchability,
4 weeks posthatching development
White Leghorn chicken 0, 1.0, 2.5, 5.0, and 10.0 mg/egg embryonic development Swartz (1981)
38-40 per group embryonic development injected
into yolk sac after fertilization,
prior to incubation for 5 or 12 days
White Leghorn chicken 10 mg/egg after fertilization prior primordial germ cell Swartz (1985)
8 per group to incubation migration
White Leghorn chicken 1.0, 0.3 mg/egg and lower examination of embryos Eto et al. (1980)
[insufficient data]
Table 48 (continued)
Species Dosage End-points Reference
720 eggs 0, 1, 2, and 4 mg/egg examination of hatchlings Ghadiri & Greenwood (1966)
[insufficient data]
Duck and chicken 10-1000 µg/egg 0, 4, 7, 10, and examination of embryos or Khera (1966)
13 day eggs hatchlings [insufficient data]
Chicken - examination of embryos Dinerman et al. (1970)
[insufficient data]
Quail
Coturnix coturnix 0, 50, 150, 300, 600, 900, growth, reproduction, Bursian & Edens (1977)
japonica 1200 mg/kg diet from hatching post-hatching viability
10 per group through 14 weeks
Colinus virginianus 0, 300, 1000, 3000 mg/kg diet for reproduction, post-hatching Fletcher & Leonard (1986a)
36 per group 22 weeks viability, gross pathology
Duck
Anas platyrhyncos 0, 300, 1000, 3000 mg/kg diet for reproduction, post-hatching Fletcher & Leonard(1986b)
36 per group 22 weeks viability, gross pathology
10-1000 µg/egg 0, 4, 7, 10, and examination of embryos or Khera (1966)
13 days eggs hatchlings [insufficient data]
8.5.1 Mammalian reproductive toxicity studies
8.5.1.1 Mouse
Studies on the effects of carbaryl (8.5-34 mg/kg per day),
given orally for 5 days, to mature male Swiss Webster mice,
indicated no effects on the weights of testes and accessory sex
glands or uptake of C14-labelled testosterone by the prostate
gland, as measured on the day after treatment (Thomas et al.,
1974). These workers also examined the biotransformation of
testosterone-1,2-3H in animals given 34 or 68 mg/kg per day,
orally, for 5 days. They found a significant decrease in
androstenedione synthesis in the high-dose group, indicating
increased hepatic androgen hydroxylase activity (Dieringer & Thomas,
1974).
8.5.1.2 Rat
Collins et al. (1971) studied the effects of carbaryl given
in the diet (0, 2000, 5000, and 10 000 mg/kg) to Osborn-Mendel rats
over 3 generations. The doses actually administered could only be
estimated since there were no measurements of food intake. On the
basis of a 15 g/day food intake and a 235 g rat, it can be estimated
that animals received of the order of 125, 250, or 500 mg/kg per
day. It should be noted, however, that the food intake of lactating
rats greatly increases, so these figures may considerably
under-estimate carbaryl exposure during this critical time period.
The author used the number of animals mated rather than the number
giving birth as the number for litter size and viability, therefore
their calculations are overestimates. Nevertheless, the data do show
impaired fertility in the high-dose group (which also exhibited
growth rate reduction) as well as reduced postnatal survival. The
number of live-born offspring and growth rate were reduced in the
5000 and 10 000 mg/kg diet groups.
A three-generation study on Wistar rats was carried out by Weil
et al. (1973) in which animals were given 0, 7, 25, 100, or
200 mg/kg per day in the diet or 0, 3, 7, 25, or 100 mg/kg per day
orally. Maternal toxicity was seen in the dietary study at 200 mg/kg
per day (decreased weight) and in the gavage study at 100 mg/kg per
day (decreased weight and mortality). Postnatal toxicity was noted
in the 100 mg gavage group (reduced litter size and viability), but
not in lower dose groups. The dietary study indicated fewer effects
in the maternal animals with only an initial loss in weight. No
perinatal effects were noted.
Vashakidze (1975) exposed male and female rats (number and
strain unspecified) to 0, 1, 5, 10, 20, 40, and 50 mg carbaryl/kg,
orally, for 1 month. Dose-related changes were noted in serum
albumin (decrease), globulins (increase), ChE and AChE (decrease),
aspartate transaminase (increase), and alanine transaminase
(decrease). Reductions in stem cells as well as spermatozoa were
noted at doses of 5 mg/kg or more. Adverse litter effects were seen
in treated females. These effects included increased embryo/fetal
death, decreased implantations, and prolonged estrus cycle.
Luca & Balan (1987) administered carbaryl-beta-naphthol to
Wistar rats in the diet for up to 18 months. The treated groups
showed an increase in sperm shape abnormalities, though there were
no clear dose- or time-relationships with effects.
8.5.1.3 Gerbil
Collins et al. (1971) published the results of a 3-generation
reproduction study in which animals were exposed to 0, 2000, 4000,
6000, or 10 000 mg/kg diet. Since all postnatal calculations used
the number of animals mated rather than the number giving birth, the
authors' data must be recalculated. When this is done, the magnitude
of the effects reported is reduced. Adverse effects on various
reproductive parameters are nevertheless seen, though the effects
are not clearly related to dose levels in the 2000-6000 mg/kg diet
groups. These data are difficult to interpret given the lack of
information on maternal effects at doses other than the highest,
where mortality was observed.
8.5.2 Mammalian developmental toxicity studies
8.5.2.1 Mouse
Murray et al. (1979) administered 100 or 150 mg carbaryl/kg
per day, by gavage, or 5660 mg/kg diet (calculated to be 1166 mg/kg
per day) to CF-1 mice on gestation days (g.d.) 6-15. Maternal
toxicity was noted in the 150-mg group (ataxia, lethality). Litter
effects were noted in the 150 mg/kg per day group, where an increase
in entirely resorbed litters was seen, and in the dietary group
where there was decreased fetal weight.
Pregnant mice were given 0, 100, 150, or 200 mg carbaryl/kg per
day, orally, on gestation days 8, 12, or 6-15, and fetuses were
examined at term (Mathur & Bhatnagar, 1991). Maternal death was
noted at the high dose in the group receiving carbaryl on gestation
days 6-15. Fetal weight reductions were seen at the high-dose levels
in all groups, as was reduced ossification, open eyelids, and
enlarged renal pelvis. These effects may be indicative of fetal
growth retardation.
8.5.2.2 Rat
Carbaryl was administered to Harlan Wistar rats at 0, 20, 100,
or 500 mg/kg per day, orally, on gestation days 1-7, 5-15, or 1-21
(Weil & Carpenter, 1965; Weil et al., 1972). Maternal toxicity was
evident as was reduced weight gain in the high-dose groups receiving
the chemical on gestation days 5-15 or 1-2. No adverse fetal effects
were seen.
Golbs et al.(1975) treated Wistar rats with 0, 200, or 350 mg
carbaryl/kg, orally, or 40 mg/kg ip on a variety of single or
multiple gestation days. Reductions in fetal weight were seen in
some groups. No other effects were noted.
Sprague-Dawley rats were treated orally with 0, 1, 10, or
100 mg carbaryl/kg per day, three months before and during
gestation, and litters were examined at term (Lechner &
Abdel-Rahman, 1984). Maternal weight gain was significantly less in
the 100 mg/kg group than in controls. No compound-related effects on
fetuses were noted.
8.5.2.3 Guinea-pig
Weil et al. (1973) administered carbaryl to guinea-pigs at
dose levels of 0, 100, 200, or 300 mg/kg per day in the diet, or 0,
50, 100, or 200 mg/kg per day, orally. These dose levels were
determined to be maximum non-maternally toxic doses in preliminary
studies by the authors, though the 200 mg/kg dose did reduce
maternal weight gain. No significant adverse embryo/fetal effects
were seen in any treatment groups.
8.5.2.4 Rabbit
Murray et al. (1979) tested the effects of carbaryl on New
Zealand White rabbits during gestation. Dams had diarrhoea at the
high dose of 200 mg/kg per day and animals at this dose level as
well as at the lower dose level (150 mg/kg per day) gained less
weight during gestation than controls. A significant increase in
umbilical hernia was noted in fetuses at the 200 mg/kg per day
group.
8.5.2.5 Dog
Smalley et al. (1968) administered 3.125, 6.25, 12.5, 25, or
50 mg carbaryl/kg in the diet, throughout gestation, to beagle dogs.
Maternal toxicity was noted at all dose levels. This toxicity was
described by the authors as dystocia and symptoms included delayed
delivery, anorexia, and restlessness. A variety of birth defects
were found at doses of 6.25 mg/kg or more. The defects included
ectopic intestines, brachygnathia, acaudia, polydactyly, and
intestinal agenesis.
Another study was carried out by Imming et al. (1969) on the
beagle dog. These workers used 0, 2.0, 5.0 or 12.5 mg/kg per day,
orally, throughout gestation. As in the Smalley (1968) study,
treated females exhibited toxicity during labour and single deaths
were recorded at this time in all treated groups. Birth defects
including umbilical hernia and gastrointestinal defects were seen at
the 5.0 and 12.5 mg dose levels only.
8.5.2.6 Pig
Two studies on pigs were carried out by Earl et al. (1973).
In the first study, fetal pigs were examined from dams given 0, 4,
8, or 16 mg carbaryl/kg per day in the diet; in the second, dams
were allowed to farrow after receiving 0, 16, or 32 mg/kg per day in
the diet. Animals were exposed throughout most or all of gestation.
Effects noted were not consistent across the studies and increased
prenatal lethality seen in the first was not noted in the second,
even at a higher dose level. A small number of malformations were
noted, but no characteristic dose-related pattern was evident.
8.5.2.7 Monkey
Dougherty et al. (1971) exposed a small number of female
Rhesus monkeys to 0, 2, or 20 mg carbaryl/kg, throughout gestation.
Of the 8 pregnant, treated females, 5 were reported as having
aborted as opposed only 1 out of 5 in the controls. The Coulston
et al. (1974) study included a larger number of animals per dose,
an additional lower dose level (0.2 mg/kg per day), and a shorter
dosage period (days 20-38 of gestation). No adverse effects were
noted in the second study. These studies are not comparable for the
evaluation of the abortifacient potential of carbaryl, since the
dosing began later in gestation in the second study. The authors
noted that the determination of abortion in the Rhesus monkeys may
not have been reliable.
8.5.3 Reproductive and developmental toxicity studies in
non-mammalian species
8.5.3.1 Fish
Medaka ( Oryzias latipes) embryos were exposed to nominal
carbaryl concentrations of 0.5, 1.0, 2.5, 5.0, 10.0, 20.0, or
30.0 mg/litre in the water from the 4-cell through to the blastula
stages (Solomon & Weis, 1979). Exposure of the eggs resulted in
increased cardiovascular anomalies (heart defects, circulatory
defects, and edema) at dose levels of 5 mg/litre or more.
8.5.3.2 Amphibian
Elliott-Feeley & Armstrong (1982) treated both embryos and
tadpoles of Xenopus laevis with nominal carbaryl concentrations in
the water of 0.1, 1.0, or 10.0 mg/litre. Defects were seen in
embryos exposed to 10 mg/litre, which also resulted in significant
lethality. Embryo growth was reduced at all dose levels.
Carbaryl-exposed tadpoles exhibited decreased activity at all dose
levels.
8.5.3.3 Birds
Chicken eggs have been used by a number of workers to test the
potential of carbaryl to affect avian development. Olefir &
Vinogradova (1968) injected eggs with carbaryl at 0.01, 0.1, 1.0, or
10.0 mg/kg and examined embryos at different times after injection.
Death and anomalies were recorded at 5, 10, 15, and 20 days of
incubation at all doses above 0.01 mg/kg. Lillie (1973) administered
0, 250, or 500 mg/kg diet to pullets for 36 weeks beginning at 32
weeks of age. The adult birds showed reduced weights at both levels
of carbaryl in the adults and at 500 mg in the progeny. No
embryotoxicity or other effects were noted. Swartz (1981) examined
the effects of carbaryl on chicken embryo development. They examined
chick embryos at different days after injection, for 5 or 12 days
post-fertilization. They found vehicle-related increased mortality
(carbaryl was more toxic when administered in sesame oil than in
acetone) for both periods of exposure. Scattered anomalies were
recorded in surviving embryos. In another study (Swartz, 1985)
primordial germ cell migration was followed after injection of 10 mg
carbaryl per egg prior to incubation. Results did not indicate any
significant adverse effects on the primordial germ cells or
reproductive organs.
Bursian & Edens (1977) studied the effects of carbaryl on the
fertility and post hatching viability of Japanese quail ( Coturnix
coturnix japonica). Birds were exposed from hatching to 14 weeks
of age (breeding maturity). Fertility and the hatchability of eggs
were measured. The animals received 0, 50, 150, 300, 600, 900, or
1200 mg carbaryl/kg diet. Growth of the adult birds was reduced at
the 900 and 1200 mg/kg dose levels. There were no significant
effects on any reproductive parameter.
Fletcher & Leonard (1986a) investigated the effects of carbaryl
on reproduction in bobwhite quail ( Colinus virginianus) exposed to
0, 300, 1000, or 3000 mg/kg diet for 22 weeks. No adverse effects
were described in any factors related to hatchability, posthatching
viability, or gross pathology of newly hatched birds. These workers
used a similar protocol to study the effects of carbaryl on mallard
ducks ( Anas platyrhyncos) (Fletcher & Leonard, 1986b). The 3000
mg/kg diet level was toxic with some lethality, decreased numbers of
eggs, and thinner egg shells. No effects were seen at the 300 or
1000 mg/kg diet levels.
8.5.4 Appraisal
In summary, mammalian studies on the reproductive or
developmental toxicity of carbaryl clearly show that this compound
is capable of inducing adverse effects in utero and during the
reproductive process. These effects are always seen only at dose
levels at which there is concurrent maternal toxicity, with the
possible exception of a few studies on the rat which have not been
replicated by other workers. For a number of species, the dams
appear to be more sensitive than their litters. In general, the
adverse effects noted in developmental toxicology studies cannot be
simply attributed to maternal toxicity (Chernoff et al., 1990).
However, the pattern of maternal and fetal toxicity occurring at the
same dose levels indicates that the developing mammalian
embryo/fetus is not especially susceptible to carbaryl.
Carbaryl has been shown to be embryotoxic for fish, amphibians,
and birds, at some exposure concentrations.
8.6 Mutagenicity of carbaryl and N-nitrosocarbaryl
In this section on mutagenicity, and in the section on
carcinogenicity (8.7.1), the two compounds, carbaryl and
nitrosocarbaryl, are discussed (separately), since the formation of
N-nitrosocarbaryl was reported to occur in the stomach of rats and
guinea-pigs, in the presence of sodium nitrite and carbaryl, under
acid conditions (Elespuru & Lijinsky, 1973; Beraud et al., 1979;
Rickard & Dorough, 1984). See also section 8.9.3.
8.6.1 Genotoxicity assays in vitro
8.6.1.1 Primary DNA damage
(a) Carbaryl
Carbaryl did not cause DNA damage in different wild type
strains and DNA recombination lacking strains of Bacillus subtilis
(Table 49). Carbaryl was reported to be non-genotoxic for
B. subtilis Marburg 17A Rec+ and Marburg M45T Rec- strains,
highly sensitive to frameshift mutagens, even at the highest tested
concentration of 10 mg/plate (Uchiyama et al., 1975).
Carbaryl (10-4 mmol/litre) did not affect the sedimentation
profiles of DNA from human skin cells in culture (both normal and
xeroderma pigmentosum), either immediately or 20 h after treatment
(Regan et al., 1976).
There are two controversial reports on the genotoxicity of
carbaryl, evaluated by the induction of mitotic gene conversion in
the diploid strain of Saccharomyces cerevisiae, heteroallelic at
the two different loci ade2 and trp5. Siebert & Eisenbrand (1974)
used an assay system with a 16-h incubation time and a concentration
of carbaryl of 4.97 mmol/litre and did not observe any changes in
the control frequency of mitotic gene conversion. However, Jaszczuk
& Syrowatka (1979), reported a weak positive response with a lower
concentration of carbaryl (2.5 mmol/litre) and shorter (5 h)
incubation time. No converting activity of N-hydroxy carbaryl (2.5
mmol/litre) was found under the same assay conditions.
Table 49. Bacterial assays of genetic toxicity of carbaryl
Test Test organism Concentration Genetic Metabolic Result Reference
(µg/plate) end-point activation
DNA B. subtilis 700 mg/litre Rec assay - negative DeGiovanni-Donnelly et al. (1968)
damage
B. subtilis 20 Rec assay - negative Shirasu et al. (1976)
B. subtilis 0.4, 4, 40, 400 Rec assay - negative Eto et al. (1982)
Gene B. subtilis up to 10 000 Rec assay - negative Uchiyama et al. (1975)
mutation
E. Coli WP2 1000 Try- - negative Ashwood-Smith et al. (1972)
E. Coli WP2 1000-3000 Try- - negative Nagy et al. (1975)
E. Coli WP2 10 000 Try- - negative Uchiyama et al. (1975)
H. influenzae 10 µmol/litre Novobiocim - negative Elespuru et al. (1974)
S. typhimurium
TA 98 up to 2000 His- - negative McCann et al. (1975)
TA 98 up to 2000 His- Rat negative
TA 98 50 nmol/litre His- - negative Blevins et al. (1977)
TA 98 10-1500 His- - negative DeLorenzo et al. (1978)
Table 49 (continued)
Test Test organism Concentration Genetic Metabolic Result Reference
(µg/plate) end-point activation
TA 98 10-1500 His- Rat negative
TA 98 0.25, 2, 5, 50, 1000 His- - negative Jaszczuk et al. (1979)
TA 98 up to 5000 His- - negative Moriya et al. (1983)
TA 98 up to 5000 His- Rat negative
TA 98 0.2, 2, 20 His- - negative Eto et al. (1982)
TA 98 0.2, 2, 20 His- Rat negative
TA 98 5-2000 His- - negative Lawlor (1989)
TA 98 5-2000 His- Rat negative
TA 100 up to 2000 His- - negative McCann et al. (1975)
TA 100 up to 2000 His- Rat negative
TA 100 50 nmol/litre His- - negative Blevins et al. (1977)
TA 100 10-1500 His- - negative DeLorenzo et al. (1978)
TA 100 10-1500 His- Rat negative
TA 100 0.25, 2, 5, 50, 1000 His- - negative Jaszczuk et al. (1979)
TA 100 0.2, 2, 20 His- - negative Eto et al. (1982)
Table 49 (continued)
Test Test organism Concentration Genetic Metabolic Result Reference
(µg/plate) end-point activation
TA 100 0.2, 2, 20 His- Rat negative
TA 100 5-2000 His- - negative Lawlor (1989)
TA 100 5-2000 His- Rat negative
TA 1535 up to 2000 His- - negative McCann et al. (1975)
TA 1535 up to 2000 His- Rat negative
TA 1535 2500 His- - positive Marshall et al. (1976)
TA 1535 1000 His- Rat negative
TA 1535 50 nmol/litre His- - negative Blevins et al. (1977)
TA 1535 10-1500 His- - negative DeLorenzo et al. (1978)
TA 1535 10-1500 His- Rat negative
TA 1535 up to 5000 His- - negative Moriya et al. (1983)
TA 1535 up to 5000 His- Rat negative
TA 1535 5-2000 His- - negative Lawlor (1989)
TA 1535 5-2000 His- Rat negative
Table 49 (continued)
Test Test organism Concentration Genetic Metabolic Result Reference
(µg/plate) end-point activation
TA 1536 2500 His- - negative Marshall et al. (1976)
TA 1536 1000 His- Rat negative
TA 1537 up to 2000 His- - negative McCann et al. (1975)
TA 1537 up to 2000 His- Rat negative
TA 1537 50 nmol/litre His- - negative Blevins et al. (1977)
TA 1537 2500 His- - negative Marshall et al. (1976)
TA 1537 1000 His- Rat negative
TA 1537 10-1500 His- - negative DeLorenzo et al. (1978)
TA 1537 10-1500 His- Rat negative
TA 1537 0.25, 2, 5, 50, 1000 His- - negative Jaszczuk et al. (1979)
TA 1537 up to 5000 His- - negative Moriya et al. (1983)
TA 1537 up to 5000 His- Rat negative
TA 1537 5-2000 His- - negative Lawlor (1989)
TA 1537 5-2000 His- Rat negative
TA 1538 100 His- - positive Egert & Greim (1976)
Table 49 (continued)
Test Test organism Concentration Genetic Metabolic Result Reference
(µg/plate) end-point activation
TA 1538 100 His- Mouse positive
TA 1538 50 nmol/litre His- - negative Blevins et al. (1977)
TA 1538 2500 His- - negative Marshall et al. (1976)
TA 1538 1000 His- Rat negative
TA 1538 10-1500 His- - negative DeLorenzo et al. (1978)
TA 1538 10-1500 His- Rat negative
TA 1538 up to 5000 His- - negative Moriya et al. (1983)
TA 1538 up to 5000 His- Rat negative
TA 1538 5-2000 His- - negative Lawlor (1989)
TA 1538 5-2000 His- Rat negative
Carbaryl was inactive in the rat primary hepatocytes
unscheduled DNA synthesis assay (Cifone, 1989). It did not induce
significant changes in the nuclear labelling of rat primary
hepatocytes in two independent trials with applied concentrations
ranging from 5 to 25 µg/ml.
No DNA-damaging properties of carbaryl, assessed by its
capacity for inducing unscheduled DNA synthesis (UDS) in cultured
human lymphocytes, were reported by Rocchi et al. (1980). However,
the authors did not use a standardized test protocol meeting
established criteria for the UDS assay performance. They tested only
one concentration of carbaryl (50 µg/ml), did not use any metabolic
activation system, did not run negative and adequate positive
controls concomitantly (other than the ultraviolet irradiation), and
did not apply relevant criteria to assess data.
The lack of DNA-damaging properties of carbaryl, as measured by
the induction of UDS, was confirmed by Probst et al. (1981). They
used a standardized protocol and autoradiographic techniques in
metabolically competent, cultured rat hepatocytes with test
concentrations ranging from 0.5 to 1000 nmol/ml. In contrast, Ahmed
et al. (1977a) reported that carbaryl induced the UDS of the
ultraviolet type (long patch repair) in a cultured human fibroblast
VA-4 cell line with, and without, metabolic activation in
concentration ranges of 1, 10, 100, and 1000 µmol/litre. However,
this study had several experimental shortcomings. The protocol, the
control value, and the results of compounds tested were not in
accordance with comparable assays by other authors.
(b) N-nitrosocarbaryl
The DNA-damaging properties of N-nitrosocarbaryl in
B. subtilis have been reported (Table 50).
In one study (Uchiyama et al., 1975), more pronounced
genotoxicity of N-nitrosocarbaryl to B. subtilis was registered
compared with that of N-methyl- N'-nitro- N-nitrosoguanadine.
However, the genotoxicity of N-nitrosocarbaryl was the lowest when
compared with other concomitantly tested nitrocarbamates.
In contrast to carbaryl, N-nitrosocarbaryl showed pronounced
genotoxicity toward S. cerevisiae D4 (Siebert & Eisenbrand, 1974).
N-nitrosocarbaryl, but not carbaryl, reacted with human DNA
in cell culture to form alkaline-sensitive bonds (Regan et al.,
1976). The DNA of nitrosocarbaryl-treated (10-4 mmol/litre) cells
showed a substantial reduction in sedimentation rate immediately,
and up to 20 h, after treatment. Presumably, the effect observed was
related to the induction of numerous single-strand breaks in the DNA
and the formation of DNA adducts. On the basis of selective
labelling, the authors suggested that the methyl-containing moiety
of nitroso-carbaryl was separated from the naphthalene ring in a
human fibro-blast culture and bound irreversibly to DNA (Regan
et al., 1976).
8.6.1.2 Gene mutation assay
(a) Carbaryl
As summarized in Table 49, carbaryl did not exert a mutagenic
effect in studies with E. coli, H. influenzae, or Salmonella.
Among the many reports on Salmonella, only two indicated a
positive effect. Thus, Marshall et al. (1976) observed increased
mutagenicity at 1000 µg/plate with S9 mix for TA1535. An evaluation
of this data is difficult because no negative and positive control
data were presented. Egert & Greim (1976) reported a positive
response for TA1538 by 100 µg/plate. The mutagenicity of carbaryl in
this study greatly increased when a non-standard metabolic
activation system (mouse liver microsome) was used.
Ahmed et al. (1977b) reported a positive mutagenic response
to carbaryl in Chinese hamster V79 cells at a dose level of
0.01 mmol/litre, with no metabolic activation. There was a
concentration-related effect of carbaryl on cell survival; less than
50% of the cells survived at concentrations >0.01 mmol/litre.
Mutation studies carried out with a concentration of 0.01 mmol/litre
showed an approximately 8-fold increase in ouabain resistance
forward mutation in V79 cells as compared with the spontaneous
mutation rate.
However, Wojciechowski et al. (1982) found no
ouabain-resistance with carbaryl in a cell-mediated mutagenesis
assay when they used an exogenous activating system in which
irradiated fetal cells of Syrian hamsters were co-cultivated with
cells of Chinese hamsters V79. No mutagenic response was observed
for carbaryl at concentrations of 0.01, 0.05, 0.1 mmol/litre with,
or without metabolic activation. The toxicity of carbaryl at these
concentrations ranged from 7% at the lowest concentration to 23% at
the highest.
Carbaryl produced a negative result for inducing forward
mutations at the HPRT locus in Chinese hamster ovary cells both
with, and without, metabolic activation (Young, 1990).
Table 50. Bacterial assays of the genetic toxicity of nitrosocarbaryl
Test Test organism Concentration Genetic Metabolic Result Reference
(µg/plate) end-point activation
DNA B. subtilis 0.4, 4, 40 Rec assay - positive Eto et al. (1982)
damage
B. subtilis up to 100 Rec assay - positive Uchiyama et al. (1975)
Gene
mutation E. Coli 30 R 0.1 mmol/litre Try- - positive Elespuru et al. (1974)
E. Coli WP2 5, 10, 50, 100 Try- - positive Uchiyama et al. (1975)
E. Coli K12 100 Try- - positive Egert & Greim (1976)
E. Coli K12 100 Try- Mouse positive
H. influenzae 10 µmol/litre Novobiocin - positive Elespuru et al. (1974)
S. typhimurium
TA 98 0.001-11 His- - positive/ Blevins et al. (1977)
negative
TA 9 10-1500 His- - negative DeLorenzo et al. (1978)
TA 98 10-1500 His- Rat negative
TA 98 0.25-1000 His- - positive Jaszczuk et al. (1979)
Table 50 (continued)
Test Test organism Concentration Genetic Metabolic Result Reference
(µg/plate) end-point activation
TA 98 0.02, 0.2, 2 His- - negative Eto et al. (1982)
TA 98 0.02, 0.2, 2 His- Rat negative
TA 98 0.01-100 His- - negative Rickard et al. (1982)
TA 98 0.1-100 His- Rat negative
TA 100 0.001-11 His- - positive Blevins et al. (1977)
TA 100 0.02, 0.2, 2 His- - positive Eto et al. (1982)
TA 100 0.02, 0.2, 2 His- Rat negative
TA 100 0.1-100 His- - positive Rickard et al. (1982)
TA 100 0.1-100 His- Rat positive
TA 1535 0.5-100 His- - positive Marshall et al. (1976)
TA 1535 50-1000 His- Rat positive
TA 1535 0.001-11 His- - positive Blevins et al. (1977)
TA 1535 0.25-1000 His- - positive Jaszczuk et al. (1979)
TA 1535 0.1-100 His- - positive Rickard et al. (1982)
TA 1535 0.1-100 His- Rat positive
Table 50 (continued)
Test Test organism Concentration Genetic Metabolic Result Reference
(µg/plate) end-point activation
TA 1536 0.5-100 His- - negative Marshall et al. (1976)
TA 1536 50-1000 His- Rat negative
TA 1537 0.001-11 His- - negative Blevins et al. (1977)
TA 1537 0.5-100 His- - positive Marshall et al. (1976)
TA 1537 50-1000 His- Rat positive
TA 1537 0.25-1000 His- - positive Jaszczuk et al. (1979)
TA 1538 100 His- - positive Egert & Greim (1976)
TA 1538 100 His- Rat positive
TA 1538 0.001-11 His- - negative Blevins et al. (1977)
TA 1538 0.5-100 His- - positive Marshall et al. (1976)
TA 1538 50-1000 His- Rat negative
TA 1538 0.25-1000 His- - negative Jaszczuk et al. (1979)
(b) N-nitrosocarbaryl
N-nitrosocarbaryl has shown a positive response in a number
of bacterial assays (Table 50). In Salmonella, N-nitrosocarbaryl
was mutagenic to strains responding to both base substitution
(TA1535, TA100) and frame shift (TA1536, TA1537, TA1538, TA98). The
mutagenic potential of N-nitrosocarbaryl toward Salmonella TA1535
was greatly diminished by the added exogenous activation system
(Marshall et al., 1976). A decrease in mutagenic activity by the
addition of S9 mix on frame shift mutagenesis has also been reported
(Marshall et al., 1976; Jaszczuk et al., 1979).
8.6.1.3 Chromosomal aberration assays and sister chromatid exchange
(a) Carbaryl
Animals
Carbaryl induced a clastogenic response in the three in vitro
bioassays (Ishidate & Odashima, 1977; Kazarnovskaya & Vasilos, 1977;
Önfelt & Klasterska, 1983). All the positive responses were observed
at toxic dose levels (30-80 µg/ml; 50, 100 µmol/litre); exogenous
metabolic activation was not required for activity. Thus, Ishidate &
Odashima (1977) observed a strong positive response for carbaryl
(30 µg/ml) in a chromosomal aberration assay using a Chinese hamster
fibroblast cell line. Carbaryl produced predominantly chromatid type
gaps, breaks, translocations, rings, and fragmentation, 48 h after
treatment.
Carbaryl was negative for inducing chromosomal aberrations in
CHO cells without metabolic activation but was positive under
metabolic activation conditions (Murli, 1989).
Kazarnovskaya & Vasilos (1977) reported a positive response for
carbaryl in cultures of human embryonic fibroblast: levels of 40 and
80 µg/ml caused a 4-fold and 10-fold increase, respectively, in the
frequency of chromosomal aberrations, at 24 h exposure time,
compared with a control rate of 2% (without S9-mix). Again, the
aberrations produced were mainly of a chromatid type; the type most
frequently observed was single fragments. An increased number of
paired fragments was found only at 80 µg carbaryl/ml. Carbaryl
(80 µg/ml) increased the percentage of cells with chromosomal
coiling (9.8% in the control group; 17.9% in the test group) and
aneuploidy (3% in the control group; 29.2% in the test group).
Previously, Kazarnovskaya & Vasilos (1977) had shown that carbaryl
suppressed mitosis, changed the rate of the mitotic phase, and
significantly increased the number of pathological forms of mitosis
in a human embryonic fibroblast culture, with a dose-time response.
Onfelt & Klasterska (1983) observed induction of viable
aneuploid/polyploid cells and multiple chromatid exchanges after
treatment of V79 Chinese hamster cells with carbaryl. The compound
exerted a pronounced chromosome-breaking effect, at a concentration
of 100 µmol/litre, 26 and 50 h after treatment. There was an
increased frequency of multiple chromatid exchanges and fragments,
as well as pulverisation and more diffuse signs of chromosome
damage. The effects of carbaryl on chromosome structure and
distribution were almost abolished by the simultaneous addition of
Aroclor-induced 2 or 10% rat S9-mix and glutathion.
In two in vitro assays for chromosome damage using Chinese
hamster lung fibroblasts, carbaryl was found to be positive
(Ishidate et al., 1981; Onfelt & Klasterska, 1984). In the latter
study, the effect of carbaryl on the incidence of sister chromatid
exchange was decreased by the addition of rat liver microsomes.
Söderpalm & Önfelt (1988) related the mitotic aberrations in V79
Chinese hamster cells, in part, to a reduction in the intracellular
levels of glutathione, and increased lipid peroxidation. They also
hypothesized that the anticholinergic effects of carbaryl may play a
role in the cleavage process.
Plants
A negative clastogenic response for carbaryl in a concentration
range of 50-200 mg/litre was reported by Ma et al. (1984) who used
the Tradescantia micronucleus test. At present, this test is
considered the most established and standardized plant assay system
for the purposes of in situ monitoring of environmental mutagen
pollution (Ma et al., 1984).
Carbaryl induced chromosomal effects in different plant assays
(Wuu & Grant, 1966; Amer & Farah, 1968; Brankovan, 1972). Carbaryl
increased by approximately 10-fold the number of aberrant cells in
the root tips of barley seedings ( Hordeum vulgare) in C1 and C2
generations after the seeds were treated with concentrations of 500,
1000, or 1500 mg carbaryl/litre for 6 and 12 h. The cytogenetic
effects induced included mostly metaphase and anaphase fragments and
anaphase bridges and were time-dependent (Wuu & Grant, 1966).
The mitotic, cytogenetic effects of carbaryl were seen in
Vicia faba (Amer & Farah, 1968) and sugar corn (Brankovan, 1972).
Carbaryl caused chromosomal abnormalities (chromosome lagging;
stickiness, mainly in diakinesis, polyploidy, fragments, anaphase
bridges) in different meiotic states in the pollen mother cells of
Vicia faba after spraying flower buds of different ages (2 weeks,
1 month) with saturated aqueous solutions of a commercial carbaryl
preparation. The percentage of the chromosomal aberrations increased
with increase in the frequency of spraying (every week) or 2 weeks
for l month; daily for 8 days) and then decreased when the recovery
time was increased (Amer & Farah, 1968).
8.6.2 Genotoxicity in vivo
8.6.2.1 Host-mediated assay
(a) Carbaryl
Usha Rani et al. (1980) reported that carbaryl did not have a
gene-mutation potential in vivo, when given orally in a toxic dose
of 438 mg/kg, 3 times daily for 3 days, to 6 Swiss male mice, which
were subsequently injected with Salmonella strain G46. These data
are consistent with those that showed that carbaryl was not
mutagenic for Salmonella in vitro.
8.6.2.2 Drosophila melanogaster and other insects
(a) Carbaryl
There are several reports of bioassays for carbaryl
genotoxicity in which Drosophila melanogaster was used (Brzeskii,
1972; Brzeskii & Vaskov, 1972; Hoque, 1972; Woodruff et al., 1983;
Omer et al., 1986).
In studies by Hoque (1972), carbaryl at 1.5 and 10 mg/litre was
given to female Drosophila. It was reported that the treatment
caused a changed sex ratio, changes in eye colour, and various
chromosomal aberrations in the offspring. The small amounts of
material used and the lack of any detailed presentation of the
findings preclude any evaluation or conclusion.
At high doses (0.3 ml from a 1% suspension of 85% commercial
product in glucose), carbaryl caused a slight increase in mutation
frequency in the F1 generation males of a Drosophila line, which
were studied at different stages of spermatogenesis. There were no
deletions or disturbed fertility (Brzeskii, 1972; Brzeskii & Vaskov,
1972).
Woodruff et al. (1983), who used a sensitive experimental
protocol that incorporated the mating scheme with repair-deficient
females, reported that carbaryl was not mutagenic for Drosophila.
F1 male progeny of males that had ring X chromosomes and double
marked Y chromosomes, were treated with carbaryl at 200 mg/litre and
mated with mus-302, repair-deficient females of Drosophila. The
male progeny did not show any induced complete (ring chromosome) or
partial (Y chromosome markers) chromosome loss.
The mutagenic activity of carbaryl in Drosophila melanogaster
was studied by Omer et al. (1986). The results indicated that
carbaryl does not increase the rate of dominant and sex-linked
recessive lethal mutations.
Carbaryl did not cause chromosome abnormalities in the meiotic
cells of male grasshoppers, when they were given a single toxic dose
(not precisely indicated) of 0.25 ml of the supernatant of an
aqueous suspension/solution of carbaryl by ip injection; however,
there were morphological disturbances in the spermatocytes (Venkat
Reddy et al., 1974).
(b) N-nitrosocarbaryl
Results obtained by Omer et al. (1986) from the
nitrosocarbaryl-treated populations of Drosophila melanogaster
suggested that nitrosocarbaryl increased significantly the
percentage of dominant lethal mutations above the spontaneous
mutation frequency. The results obtained from the 0.05, 0.10, and
0.15% nitrosocarbaryl-treated populations also showed a significant
increase in the percentages of sex-linked recessive mutations.
8.6.2.3 Chromosomal aberrations and sister chromatid exchange
(a) Carbaryl
There are several negative chromosomal studies on carbaryl and
N-nitrosocarbaryl in somatic and germ cells in vivo in mammals
(Venkat Reddy et al., 1974; Degraeve et al., 1976; Seiler, 1977,
Usha Rani et al., 1980; Dzwonkowska & Hübner, 1986). In all these
studies, sufficiently high dose levels of carbaryl were used (up to
the LD50) in order to define the negative cytogenetic responses.
Using the micronucleus test and the cytogenetic analysis of
metaphase chromosomes, Degreave et al. (1976) found no clastogenic
effects in the bone marrow of mice that had been given a single oral
dose of carbaryl (0.2 ml), an intraperitoneal dose (0.5 ml) of
carbaryl, or 7 oral doses of carbaryl solution (1x10-3 mg/litre)
alone, or with sodium nitrite (2x10-3 mg/litre).
A negative response for a high dose of carbaryl (146 mg/kg,
orally, 2 times per 24 h; 30-h sampling time) was reported by Usha
Rani et al. (1980) with an adequate protocol for the mouse bone
marrow micronucleous test.
No increase in the rate of the micronucleated, polychromatic
erythrocytes in the bone marrow of mice was found during in vivo
nitrosation of carbaryl after its oral administration at the maximum
tolerated dose of 100 mg/kg, together with an excess of sodium
nitrite (100 mg/kg) (Seiler, 1977).
A dose of 64 mg carbaryl/kg produced a negative result in an
in vivo assay of chromosomal aberrations in the bone marrow cells
of the Syrian hamster (Dzwonkowska & Hubner, 1986).
No chromosome aberrations were observed in the bone marrow of
Syrian hamsters (6 per dose), given single intraperitoneal
injections of doses up to the LD50 (64, 128, 320, and 640 mg/kg)
of a commercial mixture of carbaryl/lindane (40:10) (Dzwonkowska &
Hubner, 1986).
Daily oral doses of technical carbaryl (10 mg/kg) suspended in
peanut oil were given to male, albino rats for a period of 5 days.
There were no significant chromosomal changes in the bone marrow
cells of the exposed animals (Dikshith, 1991).
8.6.2.4 Dominant lethal assays in rodents
(a) Carbaryl
Epstein et al. (1972) studied the mutagenicity of carbaryl
using the dominant lethal test. In this assay, male ICR/Ha Swiss
mice were treated orally with 1000 or 50 mg carbaryl/kg daily for 5
successive days and then caged with 3 untreated virgin females,
which were replaced weekly for 8 consecutive weeks. The frequency of
early fetal deaths and preimplantation losses in the test groups
were within the limits of the control values. Therefore, dominant
lethal mutations were not induced in mice given sufficiently high
oral doses of carbaryl.
8.6.3 Other end-points
8.6.3.1 Cell transformation
(a) Carbaryl
Transformation of the fibroblast clone A31 of the BALB/3T3
mouse was not induced by carbaryl when given in non-toxic (1.5 and
10 µg/ml) or moderately cytotoxic (20 and 40 µg/ml) doses, over 24 h
(Quarles & Tennant, 1975).
(b) N-nitrosocarbaryl
N-nitrosocarbaryl showed transforming activity, at
concentrations of 10-20 µg/ml, which was cytotoxic for the BALB/3T3,
A31 cells. That 3-10 cell passages are necessary for detecting the
transforming event suggests that the transformation frequency for
N-nitrosocarbaryl in this test system is low. Transformed cells
showed morphological alterations, loss of contact inhibition, and
growth in soft agar, as well as carcinogenic activity in normal
newborn, irradiated, suckling, or athymic BALB/C mice. The tumour
incidence (anaplastic sarcomas) was relatively low; in several
instances, tumours regressed in normal mice, but not in athymic
mice, after 2-3 weeks of growth. N-nitrosocarbaryl did not
activate detectable amounts of the endogenous murine leukaemia
viruses carried by the BALB/3T3 cells or viral protein. However,
viral antigen production in the transformed cells was induced by
iododeoxyuridin, which indicated the presence of the viral genome.
On the basis of this N-nitrosocarbaryl transforming activity in
mammalian cells in culture, Quarles & Tennant (1975) suggested that
the compound may be an active, though weak, carcinogen in vivo.
8.6.3.2 Aneuploidy induction
(a) Carbaryl
Tests of the chemical induction of spindle fibre inactivation
and c-mitosis (colchicine-mitosis) in Allium revealed the
existence of an unspecific physico/chemical mechanism, based on the
partitioning of compounds into hydrophobic compartments of the cell.
This means that chemical compounds, in general, cause c-mitosis
according to their lipophilic characteristics, as indicated by the
octanol/water partition coefficient. Thus, a close correlation
exists between the lipophilicity of compounds and the dose at which
spindle disturbance occurs. However, besides this unspecific effect
on the spindle fibres mechanism, there are some compounds that
exhibit specific effects causing inactivation of the spindle fibre
mechanism at lower doses than indicated by their lipophilicity. This
can occur via different mechanisms, such as specific binding to
actin (colchicine) or binding to sulfhydryl groups (organic
mercury). Onfelt (1987) analysed the c-mitotic effects of 22
compounds in V79 hamster cells. Five of these compounds fell outside
the regression line for lipophilicity/c-mitosis, among them
colchicine, methyl mercury, and carbaryl. Further analyses revealed
that carbaryl owes its pronounced c-mitotic action to its reactivity
with sulfhydryl groups. It is therefore not surprising that several
authors have reported mitotic disturbances caused by carbaryl in
various experimental systems. Onfelt & Klasterska (1983) reported
that a significant increase in the aneuploidy/polyploidy cells was
obtained with both 50 and 100 µmol carbaryl/litre, 26, 59, and 74 h
after treatment. Carbaryl caused mitotic disturbances in Allium
cepa, Vicia faba, Gossypium barbadense, Nigella damascena (Amer,
1965; Amer et al., 1971; Degraeve et al., 1976). Amer (1965)
reported c-mitotic effects of carbaryl in the roots of Allium cepa
that had been treated for 4 or 24 h with different concentrations of
pure (0.5, 0.25%) and commercial products (85% sprayable powder).
The increased rate of abnormal meta-telophases and ana-telophases
depended on the concentration and temperature of the test solutions.
The types of induced abnormal metaphases included star-metaphases
and a few prophase-metaphases. Two types of anaphases, the c-type
and the multipolar type, as well as multinuclear interphase cells,
were observed. Continuous treatment with carbaryl for 24 h nearly
arrested mitosis. There was a full recovery of mitosis in 48 h and
induced signs of polyploidy appeared.
Consistent with these findings are the c-mitotic effects of
carbaryl in Vicia faba and Gossypium barbadense (Amer et al.,
1971). The mitotic index of Vicia faba, after root treatment,
decreased with increased concentrations of carbaryl (25, 50, or
100 mg/litre for 4 h. There was no effect from carbaryl after
seed-soaking for different lengths of time. Mitotic anomalies
(mostly disturbed meta- and anaphases) in the roots of Vicia faba
and Gossypium barbadense showed a concentration-time response.
Carbaryl caused mitotic disturbances (c-mitotic effect,
multipolar anaphases) or cytotoxicity (pycnotic nuclei, tissue
degeneration) after root treatment of Nigella damascena with
concentrations of 2.5x10-4 and 1x10-3 mg/litre. When NaNO2 (2x10-3)
was added to carbaryl solutions of 1x10-2 and 1x10-3 mg/litre, the
effects increased, possibly because of the formation of
nitrosocarbaryl (Degreave et al., 1976). No effect was observed
after grain treatment.
Soaking sugar corn seeds for 48 h with a 0.12 or 0.25% aqueous
solution of 50% commercially prepared carbaryl, resulted in a
dose-response induction of aberrations of anaphase chromosomes of
types not seen in the controls (bridges with, or without, fragments,
dicentrics). Repeated treatment of young plants with a 0.25%
solution of carbaryl for 6 h during meiosis caused typical c-mitotic
effects in metaphase and anaphase through the arrest of cell
division and spindle inactivation. Some of the induced aberrations
persisted until the generative stage. They were fixed and
incorporated through the embryonal and generative development stages
and thus increased pollen sterility (Brankovan, 1972).
Carbaryl was reported to increase the number of aberrant forms
of mitosis (mostly c-mitotic effect) in the small intestine, cornea,
and spleen of rats given oral doses of 400 mg/kg. The authors also
reported that at lower doses of carbaryl (40 or 80 mg/kg), there was
an increased incidence of bridge and chromosome lagging in anaphase
and telophase, in addition to a high percentage of c-mitosis. The
authors reported that there were no effects at 20 mg/kg. Only the
result following the 400 mg/kg dose was fully reported (Vasilos
et al., 1975).
8.6.4 Appraisal
Carbaryl has been evaluated for its potential mutagenicity in a
number of tests in vitro as well as in vivo, in bacterial, yeast,
plant, insect, and mammalian systems, testing a variety of
end-points.
The available evidence indicates that carbaryl has no
DNA-damaging properties. No confirmed induction of mitotic
recombination, gene conversion, and UDS in prokaryotes
( H. influenzae, B. subtilis) and eukaryotes ( S. cerevisiae, A.
nidulans, cultured human lymphocytes, and rat hepatocytes)
in vitro has been reported.
Negative results were obtained in tests for gene mutations in a
number of bacterial assays, with the exception of two cases. In
several studies of gene mutations in mammalian cells in vitro,
carbaryl only produced one equivocal positive result in a cell
culture study. However, the study had several shortcomings and the
result has not been confirmed in any other comparable studies.
Chromosomal damage with high dosages of carbaryl, has been
reported in vitro in human, rat, and hamster cells and in plants.
No such effects have been observed in mammalian tests in vivo, even
at doses as high as 1000 mg/kg.
Carbaryl has been shown to induce disturbances in the spindle
fibre mechanism in plant and mammalian cells in vitro. The
relevance of plant assays for extrapolation to humans is unclear.
It can be concluded that the available data-base does not
support the presumption that carbaryl poses a risk of inducing
genetic changes in either the somatic or the germinal tissue of
humans.
The nitrosated product of carbaryl, N-nitrosocarbaryl, is
capable of inducing mitotic recombination and gene conversion in
prokaryotes (H. influenzae, B. subtilis) and eukaryotes (S.
cerevisiae) in vitro and gave positive results in E. coli spot
tests.
Furthermore, experimental results indicate that
N-nitrosocarbaryl binds to DNA, causing alkali-sensitive bonds and
single-strand breakage.
Nitrosocarbaryl has not been established as a clastogen
in vivo (bone marrow and germ cells), even at high toxic doses.
8.7 Carcinogenicity
8.7.1 Carcinogenicity studies of carbaryl in rodents
8.7.1.1 Mouse
Seven early carcinogenicity studies with carbaryl have been
reported (Table 51) involving different strains of mice,
subcutaneous injection, skin painting, ip and oral routes of
administration, different dose levels and dose schedules, and
various lengths of exposure and observation. None of these negative
studies (one marginal response) had sufficient data or data
reporting and it was not possible to evaluate them.
Table 51. Carcinogenicity studies on carbaryl in rodents
Chemical Species, strain Sex Route and Dose Duration Significant Evaluation Reference
(number) mode of (mg/kg) of study tumour/organ of individual
administration study
Carbaryl Mouse, A/Jax, C3H male SC 1/week 200 mg 20 weeks none inconclusive Carpenter et al.
30 in group (total) (1961)
Carbaryl Mouse, NS not skin painting not 24 months none inconclusive Weil & Carpenter
stated stated (1962)
Carbaryl Mouse, CD-1 male oral, diet 0.01% 80 weeks none inconclusive Mellon Institute
female 0.04% 2 years (1963)
Carbaryl Mouse, A,C3,HA not intraperitoneal 60 2 years none inconclusive Makovskaya et al.
stated 1/week (1965)
Carbaryl Mouse, (C57,B16x male oral gavage daily 4.64 18 months none inconclusive Innes et al.
C5H/Anf)F1 female 7 days-4 weeks 14 mg/kg (1969)
(C57B1/6xAKR)F1 72 of age; in diet body
4 weeks-18 weight
months of age
Carbaryl Mouse, A/He: 16 male intraperitoneal 6 mg 20 weeks marginal (±) inconclusive Shimkin et al.
3/week for (total) lung tumours (1969)
4 weeks response
Carbaryl Mouse, A/J: 124 female oral, diet 1000 10 weeks none inconclusive Triolo et al.
(1982)
Carbaryl Mouse CD-1 male diet 100,1000 53 weeks none no effect Hamada (1991b)
technical female 8000
Table 51 (continued)
Chemical Species, strain Sex Route and Dose Duration Significant Evaluation Reference
(number) mode of (mg/kg) of study tumour/organ of individual
administration study
Carbaryl Rat, CF-N 120 male oral, diet 0.005, 2 years none inconclusive Carpenter et al.
per level female 0.01, 0.02, (1961)
0.04% in
diet
Carbaryl Rat, Sprague-Dawley male diet 250, 1500 52 weeks none negative Hamada (1991b)
technical 80 or 90 per group and 7500
female
ß-Carbaryl Rat, mongrel 60 male oral gavage twice 30 22 months fibrosarcoma, positive but Andrianova &
48 control a week skin; polymorphic inconclusive Alexeev (1970)
cell sarcoma,
stomach;
osteosarcoma
with multiple
mestastases
48 single 20 mg 22 months fibrosarcoma, positive but
48 control subcutaneous skin inconclusive
implantation
Most of these studies involved the lung adenoma test in strain
A mice. This test is no longer considered to be a satisfactory
carcinogenicity bioassay because of the high rate of background
tumours that occur in untreated animals (Clayson, 1987).
Carbaryl did not increase the incidence of lung tumours in two
strains of male mice, A/Jax and C3H, which were given 20
consecutive, weekly, subcutaneous injections of 10 mg carbaryl
(Carpenter et al., 1961). As only one dose of carbaryl was tested
and the initial number of animals entered was insufficient, no
conclusion concerning the carcinogenic potential of carbaryl can be
drawn from this study.
There was no tumour development in mice (unspecified strain and
sex) after carbaryl application by skin painting for 24 months (dose
not specified, 48% water suspension) (Weil & Carpenter, 1962). This
study also lacked details about the experimental procedure and
pathology used and, so, was placed in the inconclusive category.
Carbaryl did not induce tumours in the lungs, liver, kidney,
heart, spleen, pancreas, and the thyroid and adrenal glands in two
strains of mice, A and C3HA, treated ip, once per week for about 2
years, with toxic doses of 60 mg/kg (Makovskaya et al., 1965). The
study involved a sufficient number of animals in the carbaryl group
(400), untreated control group (100), and urethan positive control
group (150), killed at 1, 3, 6, 9, 12, 15, 18, and 24 months after
the beginning of the study and studied histopathologically. However,
no numerical data on the background lung tumours in the untreated
control animals were presented. This lack of data, coupled with the
absence of tumours in the lungs of the carbaryl-treated mice strain
A, which are known for their high rate of naturally occurring lung
adenomas, makes the negative response of little significance.
Carbaryl yielded a marginal tumour response in the pulmonary
tumour induction test on mice (strain A) given a total dose of 6 mg
by ip injections, 3 times per week for 4 weeks (Shimkin et al.,
1969). Again, the lung adenoma test on strain A mice was not
reliable for evaluating possible carcinogenicity and, thus, the
results obtained from this study are not definitive.
No carcinogenic response was obtained with carbaryl
administered orally, by gavage, and/or in the diet to four different
strains of mice at doses ranging from 14 to 1000 mg/kg diet (Melon
Institute, 1963; Innes et al., 1969; Triolo et al., 1982).
There was no increase in the total tumour incidence and no
changes in tumour patterns in either sex of CD-1 mice fed diets
containing doses of 0.01 or 0.04% (100 or 400 mg/kg) carbaryl for 80
weeks and 2 years, respectively (Mellon Institute, 1969). The
survival rate in both test groups was too low for a response to be
observed; no information was given for one-third of the animals;
histopathology was performed only on animals that were suspected of
having tumours. Because of these deficiencies, no conclusion can be
drawn.
Carbaryl did not significantly increase the incidence of any
type of spontaneously occurring tumours (hepatomas, lung adenomas,
lymphoid sarcomas) in either sex of two hybrid strains of mice
(C57B1/6xC3HAnf) F1 and (C57B1/6xAKR) F1, treated orally with
4.64 mg/kg by gavage (mice from 7 days to 4 weeks of age), and in
the diet (mice from 4 weeks to 18 months of age) with 14 mg/kg diet
(Innes et al., 1969).
Another inadequate feeding study of carbaryl carcinogenicity
was conducted by Triolo et al. (1982), using the model of lung
tumour induction in strain A/J female mice. In two studies, the
feeding level of 1000 mg carbaryl/kg, incorporated in the diet of
mice for 20 weeks, did not cause a significant increase in the
incidence of background lung adenomas, nor did it induce tumours in
the glandular stomach or other tissues (spleen, intestinal tract).
However, there were a number of confounding factors in defining the
response in this study including: large variations in the incidence
of lung adenomas in the four separate control groups; in particular,
one such group had no tumours, despite high historical control
levels in strain A mice. In addition, only one dose level was
studied (1000 mg/kg), on the basis of which it is not possible to
establish a dose-response relationship.
In the second study, in which the same feeding level and
exposure period were used, carbaryl increased the lung
benzo( a)pyrene hydroxylase activity, which was associated with a
modest increase in the rate of lung tumours induced by the oral
administration of 3 mg BP twice, on days 7 and 21, respectively of
the study. However, there was great variability in the incidence of
BP-induced lung adenomas in the 3 control groups; the first
(7.2±0.8) was well above the values presented for the test group
carbaryl + BP (5.7±1.4); and the second fell within the limits of
the incidence of spontaneous tumours in the corn-oil control group,
(1.17±1.11), which make the results inconclusive.
A new mouse oncogenicity study is in progress, under
proprietary sponsorship. The study design was as follows: carbaryl
was administered in the diet to male and female CDR-1 mice at
rates of 0, 100, 1000, or 8000 mg/kg diet, for up to 104 weeks.
Groups consisted of 80 mice/sex per group. Ten mice/sex per group
were sacrificed for clinical pathology evaluation after 52 weeks of
treatment. Results of this interim sacrifice are presented in
section 8.3. The remaining animals were designated for continued
exposure to the end of the 104-week treatment period. The study
design and evaluation parameters are consistent with guidelines set
out by the US EPA and OECD, with additional study parameters, for
studies of this nature (Rhône-Poulenc, 1992).a
8.7.1.2 Rats
Two reports by Carpenter et al. (1961) and Andrianova &
Alexeev (1970) are controversial. They studied the carcinogenicity
of carbaryl given to rats by oral gavage, in the diet, or by
subcutaneous implantation. Both studies had insufficiencies in the
protocols used.
Carpenter et al. (1961) noted no significant increase in the
total tumour incidence in either sex of CF-N rats fed a diet
containing 0.005, 0.01, 0.02, or 0.04% carbaryl, for 2 years. Female
mice had more pituitary tumours than male mice, but there was no
significant difference in the incidence of tumours between the
control and test groups. The small initial number of animals used,
their relatively old age (60 days), their low survival rate, and the
lack of detailed pathology are complicating factors in defining a
negative response.
However, Andrianova & Alexeev (1970) reported that carbaryl,
administered by both oral gavage and subcutaneous implantation,
produced positive carcinogenic responses in mongrel rats. Male rats
given oral doses of 30 mg carbaryl/kg, twice weekly for 22 months,
developed skin fibrosarcoma, polymorphic cell sarcoma in the
stomach, and osteosarcoma with multiple metastases. Carbaryl caused
high lethality (80%) during the exposure period. The authors did not
state whether the gross pathology was examined. One fibrosarcoma was
discovered among 46 control animals, at 11 months. No data were
presented on the number of control animals that lived for 22 months,
so a comparison of survival rates in control and test groups could
not be made.
In a parallel study, 20 mg of carbaryl in a purified paraffin
capsule was implanted subcutaneously in 48 male rats. At the end of
the 22-month exposure period, subcutaneous fibrosarcomas, in sites
far from the implantation area, and on the back and neck, were
diagnosed in 2 out of 10 surviving animals. There was no control
group (Andrianova & Alexeev, 1970). The carbaryl used was obtained
from a plant in the USSR and was of technical grade and 97.65%
purity. No information about the chemical composition and impurities
was given. Because of these deficiencies, this study is
inconclusive.
a Information to Task Group. These studies have not yet been
reviewed by the IPCS. The company performing these studies has
indicated that there is a significant increase in tumors at the
highest dose in both species.
A new combined long-term toxicity and oncogenicity study on
rats is in progress, under proprietary sponsorship. The study design
is as follows: carbaryl was administered in the diet to male and
female Sprague-Dawley rats at rates of 0, 250, 1500, or 7500 mg/kg
diet, for up to 104 weeks. Groups consisted of 80 rats/sex per group
for low- and middle-dose groups and 90 rats/sex per group for the
high-dose and control groups. Ten animals/sex per group were
sacrificed after 26 and 52 weeks exposure and evaluated for clinical
pathology and histopathology. In addition, 10 animals/sex from the
control and high-dose groups were used as a recovery group, kept on
a basal control group diet before sacrifice at 56 weeks. The results
of the interim sacrifices are presented in section 8.3. The
remaining animals were designated for continued exposure to the end
of the 104-week treatment period. The study design and evaluation
criteria are consistent with guidelines set out by the US EPA and
OECD, with additional study parameters, for studies of this nature
(Rhône-Poulenc, 1992).a
8.7.1.3 Overall appraisal of carbaryl carcinogenicity
Carbaryl has been studied for its carcinogenic potential in
numerous studies on rats and mice via various routes of
administration. Most of these studies are old and do not meet
contemporary standards.
Only one paper from the existing body of publications clearly
reports a tumorigenic action of carbaryl. Carbaryl induced malignant
tumours in an unidentified strain of rat by the oral and
subcutaneous routes of administration. This study does not meet
contemporary standards, because of insufficient reporting of control
data.
New studies, designed to meet with contemporary standards, are
in progress on rats and mice. Descriptions of the studies are given
in sections 8.7.1.1 and 8.7.1.2.
8.7.2 Carcinogenicity studies of N-nitrosocarbaryl
Carbaryl is a secondary amine and is, therefore, capable of
nitrosation in the presence of nitro donor groups, such as sodium
nitrate, to give a nitrosamide. This nitrosamide, nitrosocarbaryl,
has been proved to be mutagenic and carcinogenic, at high doses in
a Information to Task Group. These studies have not yet been
reviewed by the IPCS. The company performing these studies has
indicated that there is a significant increase in tumors at the
highest dose in both species.
animals (see Table 52). A condition of this nitrosation is an acidic
pH (less than 2), which is comparable to the one found in the human
stomach. However, nitrosocarbaryl is not stable at this pH. Its
maximum stability is between pH 3 and 5, at which pH no significant
amount of carbaryl can be nitrosated. Carbaryl was nitrosated in
several studies, in vitro as well as in vivo, in the guinea-pig,
which has a stomach acidity similar to that of humans. See also
section 8.9.3.
8.7.2.1 Rats
N-nitrosocarbaryl elicited a carcinogenic response in both
sexes of two strains of rats (Wistar and Sprague-Dawley) by two
routes of administration (oral gavage and subcutaneous injection)
(Eisenbrand et al., 1975, 1976; Lijinsky & Taylor, 1976;
Preussmann et al., 1976; Lijinsky & Schmähl, 1978).
A local carcinogenic effect of N-nitrosocarbaryl was reported
by Eisenbrand et al. (1975) in Wistar rats given a single
subcutaneous injection of 1000 mg/kg. Fifteen out of 16 treated
animals developed sarcomas at the injection site; there was no
histopathological evidence of any systemic carcinogenic effects. The
dose of N-nitrosocarbaryl was highly toxic and 14 out of 16
animals had died by day 450. No spontaneous tumours were observed in
control animals. When given to rats orally in single doses of
200-1500 mg/kg, N-nitrosocarbaryl did not induce tumours during 21
months of observation. No signs of toxicity were noted in any of the
test groups.
Further studies involving repeated oral administration of
N-nitrosocarbaryl to Sprague-Dawley rats gave unequivocal evidence
of local carcinogenic effects, produced by both oral and
subcutaneous routes of administration. (Eisenbrand et al., 1976;
Lijinsky & Taylor, 1976, 1977; Preussmann et al., 1976; Lijinsky &
Schmähl, 1978).
The nonglandular part of the stomach (forestomach) was the
target organ of the local carcinogenic action of N-nitrosocarbaryl,
when it was given orally to rats. All stages of malignant
transformation in the forestomach, from hyperplasia to squamous cell
carcinoma, were observed in male Sprague-Dawley rats treated with
oral doses of 130 mg N-nitrosocarbaryl/kg, twice weekly, until
spontaneous death. There was a substantial lowering of the survival
rate of treated animals because of the pronounced toxicity. By the
time of maximum increase of the tumour incidence (after 200 days),
most of the animals were dead. The average survival time of
tumour-bearing animals was 167 days after the onset of the study.
The low background tumour incidence (3 out of 29) in control animals
included lymphosarcomas and leukaemia.
Table 52. Carcinogenicity studies on N-nitrosocarbaryl in rodents
Chemica Species, strain Sex Route and Dose Duration Significant Evaluation Reference
(number) mode of (mg/kg) of study tumour/organ of individual
administration study
N-nitroso Rat, Wistar male subcutaneous, 1000 450 days polymorphic cell positive Eisenbrand et al.
carbaryl 8: male; female single injection sarcoma at (1975)
8: female injection site;
spindle cell
sarcoma
37 male, oral gavage, 200-1500 21 months none inconclusive
female single
N-nitroso Rat, Sprague-Dawley male oral gavage, 130 (5000 till squamous cell positive Eisenbrand et al.
carbaryl 31 twice per week total) spontaneous sarcoma, (1976)
death papilloma, Preussmann et al.
hyperkeratoses, (1976)
forestomach
N-nitroso Rat, Sprague-Dawley female oral gavage, 50 mg 110 weeks squamous cell positive Lijinsky & Taylor
carbaryl 12 once a week (total) carcinoma (1976)
for 10 weeks forestomach,
papilloma,
oesophagus
12 male oral gavage, 300 mg 90 weeks squamous cell
twice a week (total) carcinoma
for 20 weeks forestomach,
papilloma trachea
N-nitroso Rat, Sprague-Dawley male oral gavage, 600 80 weeks carcinoma positive Lijinsky &
carbaryl 16: male; female once a week (total) forestomach Schmahl (1978)
16: female for 10 weeks
In a comparative study of the carcinogenic potency of
N-nitroso- N-alkylcarbamate esters, Lijinsky & Taylor (1976)
showed that N-nitrosocarbaryl was a strong carcinogen, similar in
action to its highly potent carcinogenic analogue
N-nitrosomethylurethane. A high incidence (75-80%) of
squamous-cell carcinomas in the forestomach was detected in female
Sprague-Dawley rats given 10, weekly, equimolar oral doses
(0.22 mmol) of both compounds. Nitrosomethylurethane was a more
potent carcinogen, because the animals with tumours died earlier
than those in the nitrosocarbaryl group. The same rate of carcinomas
in the forestomach was induced in male rats treated orally with a
considerably higher dose of N-nitrosocarbaryl (1.3 mmol total),
over twice as long a period (20 weeks). The stronger carcinogenic
effect of this dose in male animals was expressed by the shorter
latent period of induced tumours and higher lethality compared with
female animals. The local carcinogenic effect of N-nitrosocarbaryl
in rats, the target organ being the forestomach, was seen in another
oral carcinogenicity study of a series of nitroso- N-methylcarbamate
insecticides carried out by Lijinsky & Schmähl, (1978). The oral
gavage of 10 weekly doses of 60 mg nitrosocarbaryl/kg to both sexes
of Sprague-Dawley rats led to a high incidence (over 70%) of
carcinomas in the forestomach, with no evidence of other systemic
carcinogenic effects.
No carcinogenic response for carbaryl was obtained with the
transplacental carcinogenicity test after in vivo nitrosation in
pregnant Sprague-Dawley rats (Lijinsky & Taylor, 1976). Pregnant
animals were given 30 mg carbaryl/rat, orally, for 10 days (4-18
days of gestation). Two other groups of animals received the same
dose of carbaryl, together with 0.6-1ml of 4% sodium nitrite on days
4-6 and 14-18 of pregnancy. The distribution of tumours in the rats
of various groups was that normally seen in Sprague-Dawley rats. It
is likely that an insufficient amount of nitrosocarbaryl was formed
under the conditions of in vivo nitrosation, or that no
significant amount of nitrosocarbaryl crossed the placenta.
A low rate of in vitro nitrosation of carbaryl under
conditions similar to those that exist in the human stomach was
reported by Eisenbrand et al. (1975). The reaction of
10-3 mol/litre carbaryl with a 5-fold molar excess of sodium nitrite
in 0.1N HC1 (pH, 1) led, after 15-60 min, to yields of only 1.2 and
1.7% of the maximum possible conversion to nitrosocarbaryl.
Reduction of the concentration of carbaryl and sodium nitrite by a
factor of 10 decreased the yield of nitrosocarbaryl by about
one-half. The yields of N-nitrosocarbaryl obtained by the
in vitro nitrosation of carbaryl, under the described conditions,
are low and the potential carcinogenic risk of in vitro nitrosation
and similar nitrosation reactions in vivo is difficult to evaluate
at present.
8.7.2.2 Mice
Nitrosocarbaryl administered for 104 weeks to the skin of
female CFLP mice (65 mice per group) in three doses (12.5, 50, and
200 µg/mouse) was found to be more potent regarding dermal
carcinogenic efficiency than nitrosomethylurea, applied under the
same conditions, though not as effective as benzo- a-pyrene
(Deutsch-Wenzel et al., 1985). Conclusions were drawn on the basis
of the number of animals bearing carcinomas, total cases with local
tumours, observed/expected ratios and dose-response relationships of
incidences of malignant tumours. These results were found to be in
contrast to the ones obtained by Lijinsky & Winter (1981), who used
a single total dose of 23 mg and found that nitrosocarbaryl was a
much less effective inducer of mouse skin tumours than
nitrosomethylurea.
8.7.2.3 Overall evaluation of the carcinogenicity of
N-nitrosocarbaryl
Sufficient evidence of carcinogenicity at the site of
application was seen in multiple studies on both sexes of
Sprague-Dawley rats, by different routes of administration
(subcutaneous and oral gavage), using different dose levels. The
non-glandular stomach was the target tumour site when
nitrosocarbaryl was administered by oral gavage; subcutaneous
injection of nitrosocarbaryl caused sarcomas at the injection site.
The local carcinogenic effect of N-nitrosocarbaryl in rats,
and the lack of any systemic carcinogenicity, characterizes it as a
direct-acting alkylating agent. N-nitrosocarbaryl was active as a
direct bacterial mutagen and interacted with human DNA in vitro.
These data agree with data that show that N-nitrosocarbaryl is an
effective in vivo genotoxin.
8.7.3 Carcinogenicity of ß-carbaryl
Beta-carbaryl (N-methyl ß-naphthyl carbamate) was a component
of technical carbaryl (alpha-carbaryl), suspected of having a
carcinogenic structure and a tumorigenic potential in mice and rats
(Zabezhinski, 1970). In life-time studies on mice, strain CC57W (24
months), and mongrel rats (33 months), using two routes of
administration, oral gavage (10 mg/kg in mice; 25 mg/kg in rats) and
subcutaneous injection (20 mg/kg in mice; 50 mg/kg in rats),
ß-carbaryl showed a weak carcinogenic response. High rates of
lethality (20-50%) were observed in both mice and rats, by the two
routes of administration. No data for survival rates and the
incidence of spontaneous tumours in control animals were presented.
ß-carbaryl caused a low rate of tumours in rats by both routes of
administration. The types of tumours (21%) observed after
subcutaneous injection of ß-carbaryl included: fibrosarcoma at the
injection site, subcutaneous rhabdomyosarcoma, intestinal sarcoma,
leukaemia, reticuloses, and reticulosarcoma. The pattern of
carcinogenic response after orally administered ß-carbaryl (25%)
involved sarcoma in the liver, fibroadenoma and adenocarcinoma in
the mammary glands, carcinoma in the thymus, and granulocellular
carcinoma in the ovaries. No local tumours at the injection site
were observed in control animals after subcutaneous administration
of corn oil. The other tumours (carcinoma in the mammary glands and
thymus, haematopoietic system malignancies) were unusual for control
animals, as well. ß-carbaryl caused a higher tumour incidence in
mice than in rats by both subcutaneous (60%) and oral (30%) routes.
It should be mentioned, however, that most of the tumours observed
(lung adenomas, leukaemia, and liver haemangiomas) occurred
spontaneously in untreated mice. Since no control data were
presented, the carcinogenic response to ß-carbaryl in mice is of
uncertain significance. Because of these deficiencies, this study is
inconclusive.
8.8 Special studies
8.8.1 Neurotoxicity
The effect of carbaryl on the nervous system is primarily
related to ChE inhibition.
Carpenter et al. (1961) studied the delayed neurotoxic
potential of carbaryl in chickens (Rhode Island hens) compared with
that of triorthocresyl-phosphate. Single doses of 250, 500, 1000, or
3000 mg/kg body weight, 25-40% in lard were administered
subcutaneously to chickens. At 2000 mg/kg, weakness was observed on
day 1 or 2 after dosing. In one case, the chicken was unable to walk
for 3 days. No evidence of demyelination was observed in any brain
sciatic nerve or spinal cord section examined microscopically.
According to the authors, there was a transient cholinergic effect
caused by the slow absorption of carbaryl.
The neurotoxic effect of carbaryl was studied in atropinized
chickens (Gaines, 1969) to protect against acute effects of the
subcutaneous injection of 800 or 1600 mg carbaryl/kg body weight.
The higher dose caused leg weakness within 24 h, which recovered by
day 24.
Carbaryl solution in corn oil at a daily dose of 100 mg/kg was
administered orally for 7 consecutive days to 35, 6-day-old, female
broilers, hybrids between Peterson strain roosters and Hubbard hens
(Farage-Elawar, 1989). Altered locomotion and abnormally shortened
gait were observed on the 7th day, and cases of delayed paralysis
20-40 days, after the last treatment. Locomotion changes were found
to have no association with the activities of brain
acetylcholinesterase and neuropathy target esterase, 24 h after the
first, second, third, and fifth doses as well as 1, 3, 6, 10, 20,
30, and 40 days after the last treatment, when no statistical
deviations from the control values of both enzymes were registered.
A similar, but lesser, effect on gait and stride length was
observed when 45 mg carbaryl/kg was injected into chick eggs on day
15 of incubation (Farage-Elawar, 1990). This treatment resulted in
significant inhibition of brain and plasma cholinesterase, and liver
carboxylesterase. Administration of the same dose on day 5 of
incubation resulted in 10% lethality. Brain NTE was not affected.
The cause of this delayed alteration is not known.
Effects of long-term carbaryl exposure on the neuromuscular
system of pigs were reported by Smalley et al. (1969). Six
Yorkshire pigs, 3 male and 3 female, received carbaryl in their diet
(150 mg/kg body weight); the male pigs for 72 days and the female
pigs for 83 days. Three pigs from the same litter were fed carbaryl
at 150 mg/kg body weight daily, for 4 weeks, and then 300 mg/kg body
weight, daily, for 46 days (2 male pigs), and for 85 days (the
female pig). The signs of intoxication started after about 1´
months, and were mostly typical of neuromuscular system damage (see
Table 44; section 8.3). Microscopic examination of the skeletal
muscle revealed myodegeneration. In the myelinated tracts of the
cerebellum, brain stem, and upper spinal cord, moderate to severe
edema was associated with vascular degenerative changes. No
demyelination of nerve tissue was observed. When carbaryl feeding
was stopped and hydrochlorothiazide applied as a diuretic, signs of
toxicity of carbaryl, such as ataxia, and partial paralysis,
disappeared.
Carbaryl disturbed the function of the myoneural synapses. It
produced a decrease in the spontaneous activity and an increase in
the permeability of the muscular fibre membrane for K+ and Na+
after multiple oral administrations of 8.5 mg/kg to rats(Kovtum &
Sokur, 1970).
Kovtun (1970) while analysing the effects of carbaryl on
myoneural formations, pointed out that, at a single intake
(425 mg/kg), multiple intakes during 2 months, or 1/100
LD50-8.5 mg/kg over 6 months, the frequency of tiny potentials of
an edge plate was suppressed by 62-65%. On the basis of the data
obtained it was concluded that carbaryl probably impairs the
function of presynaptic nerve endings. At the same time, carbaryl
does not affect the cholinereceptive membrane substance of muscular
fibres. Carbaryl increases the rest potential of muscular fibres by
11-35%, depending of the carbaryl dose. This increase can be
explained through high potassium ion accumulation inside the
muscular fibres.
Three different laboratories examined the effects of carbaryl
on motor activity in rats. All three reported decreased activity
after intraperitoneal administration, with ED50s that ranged from
13.3 to 17.6 mg/kg (Crofton et al., 1991).
Takahashi et al. (1991) compared the effects of carbaryl on
both young (3 months) and old (12 months) rats. A dose of 50 mg/kg
decreased activity in an open field test, prolonged the duration of
haloperidol-induced catalepsy, decreased body temperature, and
increased the nociception threshold, as measured by a hot-plate
test. A dose of 10 mg also prolonged haloperidol-induced catalepsy.
The effects on body temperature and nociception were significantly
greater in older rats.
Studies were carried out on the mechanical response
characteristics of the soleus muscle in situ. Female Holtzman rats
(6 treated-control pairs) were given 56 mg carbaryl/kg orally.
Carbaryl increased the tension developed during complete tetanus (by
electric stimulus) and decreased the time constant of tension
development (Santolucito & Whitcomb, 1971). The more forceful and
rapid contraction of the skeletal muscle is probably related to an
accelerated catecholamine release.
EEGs on 4 Rhesus monkeys given 0.01 mg carbaryl/kg and on 3
given 1 mg/kg per day, orally, for 18 months, showed only a
reduction in the amount of low-amplitude fast waves and an increased
bilateral synchrony between the right and left hemispheres
(Santolucito & Morrison, 1971). The authors did not relate these EEG
changes to the dose.
Belonozhko & Kuchak (1969) found some changes in the EEG in
rats, such as desynchronisation of rhythms after a single
application of carbaryl at 100 mg/kg. During repeated doses of
35 mg/kg for 90 days, no changes in the EEG were noted, due
(according to the authors) to adaptation of the nervous system. Oral
doses of 72 mg carbaryl/kg, administered to rats for 10 days, caused
a decrease in serotonin and an increase in dopamine levels in the
brain (Kuzminskaya et al., 1984).
Morphological changes in the nervous system caused by carbaryl
were dose-related. One to six months oral treatment of rabbits with
0.01 LD50 caused only haemodynamic disorders and cell
infiltrations, a dose of 0.02 LD50 caused cell dystrophic changes,
and a dose of 0.1 LD50 caused more progressive and serious
disorders (Azizova, 1976).
The behavioural effects of carbaryl were studied in rats and
monkeys. Disturbance of discrete (shock) avoidance behaviour by
carbaryl in rats was reported at ip doses > 2.5 mg/kg, the LD50
being 8 mg/kg (Goldberg et al., 1965). Carbaryl administered at
doses of 8, 16, or 28 mg/kg, ip, decreased maze activity in CD male
rats (10 in each group), whereas doses of 16 and 28 mg/kg reduced
open field activity. After acute exposure, behavioural changes
recovered within 60 min, whereas ChE of the blood and brain
recovered after 240 min (Ruppert et al., 1983).
Carbaryl at 10 mg/kg administered subcutaneously in 16, male,
Long Evans strain rats decreased the number of times they approached
the novel stimuli and explored the exploratory box, and increased
habituation. In familiar situations, carbaryl increased activity
(Albright & Simmel, 1977).
Anger & Setzer (1979) studied the effects of oral and im
administration of carbaryl on repeated chain acquisition in 5 male
monkeys ( Macaccus mascicularis). Oral doses of 50 mg/kg did not
alter the performance of repeated acquisition tasks. The im
injection resulted in consistent changes in performance at 5 and
10 mg/kg, but did not cause any changes at 1 mg/kg.
Carbaryl affected working memory (continuous delayed response
and continuous non-match) in 28 male Sprague-Dawley rats (Heise &
Hudson, 1985a,b). A dose of 10 mg carbaryl/kg, ip, affected
performance of working memory procedures; with increasing dose,
carbaryl nonselectively decreased response.
Administration of 2.24 mg carbaryl/kg, ip for 14 days, did not
affect the performance of rats in activity wheel cages, 24 h after
the last treatment. Acute ip administration of 0.54 or 2.24 mg/kg
significantly decreased the motor activity of rats in activity wheel
cages. This decrease was reversed by atropine sulfate (Singh, 1973).
Doses as low as 7.76 mg/kg ip caused mild tremors.
Oral administration of carbaryl (200 mg/kg, 3 days/week) for a
period of 90 days, though producing inhibition of ChE in blood (33%)
and brain (11%), did not result in any kind of overt signs of
toxicity in male albino rats (Dikshith et al., 1976).
Desi et al. (1974), in long-term studies, investigated the
effects of carbaryl on the learning process, on the performance of
previously learned tasks in mazes, and on the EEG in 40 male Wistar
strain rats. Carbaryl was given at doses of 10 or 20 mg/kg body
weight per day in the diet (100-200 mg/kg food), for 50 days. Mild,
but permanent and increased, functional deviations of the nervous
system were found. Because of increased irritability in the CNS, the
treated rats took less time than the untreated rats to find their
food. Later, the task was performed with difficulty when the
irritability of the CNS was reduced to below the normal level. The
performance of the learned task was impaired. EEG deviations
recorded at the end of the maze studies were slight, but permanent.
They consisted of increased electric activity of the brain,
increased number of moderately slow beta waves, and light flashes of
18 Hz accelerated electrical activity.
Viter (1978) found behavioural changes in rats treated via
inhalation for several months with 12 or 23 mg carbaryl/m3. The
latency period of the conditioned reflex on nutrition was prolonged.
Depression of investigative behaviour and spontaneous motor activity
were noted.
The behavioural effects of carbaryl on rats were examined by
Moser et al. (1988) using a functional observation battery.
Carbaryl was administered at doses of 3-30 mg/kg, ip, and tested
0.5, 3, 24, and 48 h afterwards. Carbaryl decreased spontaneous
activity, CNS excitability, motor and sensory function, and body
temperature and weight. Effects indicative of AChE inhibition were
also observed. All responses were dose dependent.
8.8.2 Effects on the immune system
8.8.2.1 Appraisal on immunotoxicology
The administration of carbaryl, or any other xenobiotic, at
doses resulting in overt toxicity can be expected to result also in
a variety of effects on the immune system. Carbaryl, when
administered in vivo, at a dose not causing overt clinical signs
has been reported to produce a variety of non-life-threatening
effects on the immune system. The effects included cellular as well
as humoral immunity, and several authors have suggested that they
were was due to subtle, treatment-related stress. Many of the
effects described were detected at doses close to the LD50.
Shortcomings of several of these studies were a lack of consistency
and, sometimes, overt contradiction between results, which prevents
the description of a defined immunotoxic mechanism.
Lifetime exposure to carbaryl did not result in increased
occurrence of disease in rats or mice. No enhancement of viral
infections was found with carbaryl, even at dosages close to the
LD50. Most studies on rabbits and mice, at doses permitting
survival, did not produce significant effects on the immune system.
In vitro, a number of researchers have demonstrated viral
enhancement by prior incubation with carbaryl. In vitro, carbaryl
can enhance herpes varicella-zoster, but not herpes simplex. In
goldfish cell culture, carbaryl has been shown to permit viral
enhancement by compromising interferon synthesis. Inhibition of
human serum complement activity as well as interleukine-2 driven
proliferation of large granular lymphocytes have been demonstrated
in vitro. These actions could be mediated through the inhibition
of serine esterases involved in the processes. No increased viral
infections have been seen in long-term in vivo feeding studies,
previously conducted or in progress at present. Carbaryl does not
enhance transformation of BALB/53T fibroblasts in culture or the
expression of endogenous murine leukaemia virus.
8.8.2.2 In vivo studies
Young mice weighing 10-12 g were infected with influenza by
applying 3-4 drops (0.05 g) 1% influenza virus in physiological
solution in the nose. The murine influenza virus strain A.P.R.8 was
used in the primary dilution 1:32. After 2-3 days, this group of 30
mice was treated orally with carbaryl in sunflower oil, at a dose of
500 mg/kg (a dose that killed 3 out of 10 mice). Two control groups
with the same number of mice, one treated with carbaryl alone, and
one infected, were compared with the experimental animals for
survival, blood biochemistry, and pathomorphological changes. The
greatest number of mice dying were in the experimental group. AChE
depression was more pronounced and recovery slower, and there were
more histological changes in the livers of mice infected and
intoxicated by carbaryl (Moreynis & Estrin, 1965). It is clear from
this study that a summation of the effects of both factors occurred,
but, because of the lack of statistical significance, it is
impossible to judge the eventual effect of the enhancement.
The effect of carbaryl on an experimental Erysipelothrix
rhusiopathiae infection in rats was studied by Shabanov et al.
(1983). Rats, treated with doses that increased gradually every 6
days from 2 to 5 mg/rat (mean weight of rats, 50-60 g) during 30
days, were infected iv with Erysipelothrix rhusiopathiae at
1.5-108 cells/rat. The mortality rate in carbaryl-treated rats was
36% versus 14% in control rats. Survival time was halved.
Bacteraemia persisted for 10-12 days in treated groups, and only 5-6
days in the control group. Both the gross and the histopathological
changes were more strongly manifested in the treated group.
Carbaryl, at 0, 2, 20, or 200 mg/kg was administered orally to
rabbits, daily, for 6 months. A decrease in the phagocytic activity
of leukocytes and antibody formation (4-5 times) after immunization
with a murine type of typhoid vaccine, was reported at the dose
level of 200 mg/kg. At the lower dose of 20 mg/kg, there were
different phases of reactivity; at the beginning of the first 2
months, there was an increase and later a decrease in immunological
reactivity (Perelygin et al., 1971).
The effects of oral ingestion of carbaryl on nonreaginic
antibody production in BALB/c mice were studied after pretreatment
with 150 mg carbaryl/kg diet for 10 weeks prior to commencement of
the study. Carbaryl produced significant effects on systemic
antibody production following oral immunization with sheep red blood
cells (SRBC). It significantly increased both IgG1 and IgG2b titres.
No reduction was seen in the synthesis of any antibody class. These
results suggest that, at the doses used, carbaryl increased systemic
antibody responses to orally ingested antigens (André et al.,
1983).
Changes in the immunological structures of lymphatic follicles
in the spleen during carbaryl administration were studied by Dinoeva
(1974, 1982), who used a micrometric method. Albino rats (30
treated-control pair) were given 1.5 mg carbaryl/kg, orally, daily
for 6 months. Plethora in the spleen and retardation in lymphatic
follicles were observed, which were similar to effects seen in the
positive control group treated with cyclophosphamide (2 mg/kg).
There were no changes in the structure and weight of the thymus in
carbaryl-treated rats, while in the cyclophosphamide positive
control group, the weight of the thymus was reduced 2.5 times. The
authors suggested that there is a different mechanism of the
immuno-suppressive effect of carbaryl that needs further research.
A single dose of 500 mg carbaryl/kg inhibited the production of
haemolysins and reduced the number of splenic germinal centres in
White Leghorn chickens (Roszkowski et al., 1976). Dose-dependent
immunosuppressive effects of continued dietary treatment of rabbits
with carbaryl were studied (Street & Sharma, 1975). Rabbits (male
White New Zealand, 7 in each group) were fed 0, 4, 20, 45, or 150 mg
carbaryl/kg diet (corresponding to 0, 0.23, 1.0, 2.3, or 8.38 mg/kg
body weight per day) for 57 days. The treatment reduced the germinal
centres in the spleen and caused atrophy of the thymus cortex. The
antigen-induced increase in serum gamma-globulin was decreased
significantly (no dose relationship was noted), at 10 days. No
changes were detected in the number of plasma cells in the lymph,
haemolysin and haemoglutinin titres, skin sensitivity to tuberculin,
leukocytes count, body weight, etc.
Rats pretreated, orally, with 0.05 LD50 carbaryl, daily, for
2.5 months, were immunized with typhoid antigen. Administration of
carbaryl continued for an additional 2 months. Signs of
insufficiency of the immune system, expressed as a reduction in
specific globulin production, were observed 3.5 months after
carbaryl application began. Carbaryl did not affect immunogenesis at
a dose of 0.001 LD50, daily, under the same exposure conditions.
No overall evaluation was given (Olefir, 1977).
A humoral immunity study in female BALB/C mice after oral
administration of carbaryl was performed by Wiltrout et al. (1978).
The effects of humoral immune competence were measured by the
immunoplaque in gel technique. The number of antibody plaque-forming
cells (PFC) per plate was counted and the PFC/spleen calculated.
Different groups of mice received carbaryl, orally, at about
1 LD50 (153 mg/kg body weight), 5 days before immunization (type
of antigen not specified), on the day of immunization, and 2 days
after. Only the last treatment resulted in significant suppression
of the humoral immune response. The ratio (experimental: control) of
antibody plaque-forming cells was 0.34. The oral administration of
0.1 LD50 for 8 and 28 days did not cause any significant effects
on humoral immune competence. Depression of the PFC/spleen level
corresponds to a decline in the total splenic lymphocyte population.
Akhundov et al. (1981) studied the effects of carbaryl on the
immunological reactivity of 40 rabbits and 40 guinea-pigs given oral
doses of 15 mg carbaryl/kg for 42 days. Heated vaccine Salmonella
typhimurium, at doses of 250 or 500 million microbial cells, was
applied 3 times at 7-day intervals, 21 days after the beginning of
carbaryl administration. The auto-sensitizing effect of the vaccine
was enhanced by carbaryl. Studies on guinea-pigs treated with 15 mg
carbaryl/kg per day for 3 months showed decreased macrophagial
migration activity.
Pipy et al. (1983) evaluated the cellular and humoral
mechanisms of carbaryl-induced reticuloendothelial phagocyte
depression. The function of the reticuloendothelial system (RES)
involved in specific and non-specific aspects of host resistance to
infection, neoplasma, etc., was quantitatively evaluated by the rate
of disappearance of colloids injected into the blood. Colloid
particles are extracted from the blood exclusively by the RES, in
particular liver and spleen macrophages. Plasma or serum factors
called opsonins have a strong stimulatory influence on inert
particle phagocytosis. Male Sprague-Dawley rats (200-240 g) were
given single, iv injections of labelled carbaryl at 0.5-32 mg/kg
body weight (7 different doses). The iv LD50 determined by the
authors was 50 mg/kg body weight. Colloidal carbon was administered
simultaneously. In another group, carbaryl was incubated with serum
from a normal rat for 20 min before the injection. The authors call
this carbaryl "opsonized". The results showed the ability of
carbaryl to induce a state of reticuloendothelial phagocytic
depression, mediated through depletion of opsonins. Carbaryl
inhibits the cell-bound aromatic amino acid esterase of the serine
esterase class. The authors concluded that, apart from a slight
deficiency in plasma opsonins, the inhibitory effect of carbaryl on
the phagocyte function was primarily due to a selective hepatic and
splenic macrophage impairment, which could be related to inhibition
of a cell-bound serine esterase. In another study by Pipy et al.
(1982), more details are given of this possible mechanism of
phagocytoxic blockade by carbaryl. Rats were treated with iv
carbaryl at 8 or 16 mg/kg body weight. The time-dependence of the
effects on phagocytic function and the activities of liver serine
esterases ( N-benzoyl-DL-phenylalanine-ß-naphthyl esterase and
acethylcholinesterase) was studied. Macrophages of the RES had
decreased phagocytic capacities after administration of carbaryl.
Depression of the phagocytosis of colloidal carbon persisted from 1
to 7 h and 1 to 24 h after administration of 8 and 16 mg/kg
carbaryl, respectively. Liver ( N-benzoyl-DL-phenylalanine-ß-
naphthyl esterase (cell-bound serine esterase) was susceptible to
reversible dose-related inhibition by carbaryl. A correlation
between the reactivation kinetics of liver serine esterases and
phagocytic activity was demonstrated.
8.8.2.3 In vitro studies
The replication of goldfish virus 2 (GFV-2) was enhanced in
vitro by pre-treatment of a piscine culture with subtoxic
concentrations (1 mg carbaryl/litre) (Shea, 1983). The mechanism of
enhancement was studied in vitro in goldfish-derived cell lines
and Air Bladder III infected with GFV-2 and pretreated with a
carbaryl solution of 1 µg/ml. Interferon synthesis was studied as a
possible mechanism of virus enhancement (Shea & Berry, 1984). The
authors demonstrated that interferon synthesis was induced in CAR
cells by GFV-2 cells. Antiviral protection provided by supernatants
from infected CAR cultures with carbaryl, against infection of
secondary CAR and Air Bladder III cultures was reduced. The authors
interpreted this phenomenon as a result of general mild suppression
of cellular metabolism, but other possible mechanisms, such as
metabolic interaction, were not excluded. In another study, Shea
(1985) demonstrated quantitative carbaryl inhibition of interferon
synthesis. Supernatants from infected cultures, not treated with
carbaryl, provided 10 times as much antiviral protection as compared
with non-infected cultures. Carbaryl-treated infected culture
provided only twice as much antiviral protection as uninfected
cultures. A similar relationship was observed when comparing the
amount of infectious viral progeny synthesized in the presence of
supernatant from infected cultures not pretreated with carbaryl
(10%), and from infected cultures pretreated with carbaryl (60% of
the amount of virus synthesized in control cultures). The author
speculated about the possibility of superimposition of a viral
infection in a population exposed to pesticides.
Characterization of varicella zoster virus enhancement by
carbaryl was carried out in vitro by Abrahamsen & Jerkofsky (1981)
and Jerkovsky & Abrahamsen (1983). In previous studies, the authors
demonstrated that the replication of varicella zoster virus (a human
herpes virus) is increased 12 to 15-fold by pretreatment of cultures
of human embryonic lung cells (HEL) with carbaryl, and, that
different strains of this virus show differences in sensitivity to
enhancement. Wild-type strains recently isolated from clinical
materials are more sensitive than laboratory adapted strains. In
this study, the authors demonstrated that the maximum enhancement
occurred 48-72 h post-inoculation and that the optimal time for the
pretreatment of monolayers of HEL is 20-24 h. 1-naphthol also
produced increased amounts of virus, but the treated cells cannot
pass on to the daughter cells the ability to enhance virus
replication.
Rodgers et al. (1986) determined that the EC50 for the
inhibition of a T-cell-mediated cytolytic (CTL) response by carbaryl
in vitro was 65.9 µg/ml. The addition of a supernatant containing
liver enzymes reduced the effects of several organophosphorous
pesticides on the CTL response, but had no significant effect on the
potency of carbaryl.
Casale et al. (1989) compared the ability of carbaryl to
interfere with the human serum complement mediated lysis of sheep
blood cells. At a concentration of 3 mmol/litre and with 2-h
preincubation, carbaryl inhibited lysis by 26-45%, depending on the
antibody concentration. Carbaryl was a more potent lysis inhibitor
than diisopropyl phosphorfluoridate or four other anticholinergic
insecticides; the potency was not correlated with anticholinergic
activity.
The effects of carbaryl at concentrations of
0.5-500 µmol/litre on lymphocyte proliferation were studied in
vitro (Bavari et al., 1991). Carbaryl inhibited (3H) thymidine
incorporation, in a concentration-dependent manner, by as much as
50%. Alpha-naphthol also had a slight effect at 50 µmol/litre. The
authors attributed the effect to inhibition of an esterase
responsible for interleukin activation.
8.8.3 Effects in blood
Carbaryl affects the coagulation process. Hyper- and
hypocoagulation were reported in different studies (Hassan & Cueto,
1970; Gapparov, 1974; Lox, 1984; Krug & Berndt, 1985; Krug et al.,
1988).
Gapparov (1974) studied the indices of blood coagulation in
dogs after oral treatment with a daily dose of 2 mg carbaryl/kg body
weight, over 5 months. A clearly manifested hypercoagulation was
established, which was connected with a rise in the general
coagulation activity of the blood, higher thromboplastic activity,
and prothrombin content, increased number of thrombocytes,
accelerated coagulation time, and increased activity of the
fibrinostabilizing factor. Also noted was a decrease in the
recalcification time of blood plasma, fibrinogen concentration, and
free heparin quantity, together with an inhibition of the fibrolytic
activity of the blood. The author interpreted all these changes as
being connected with the arousal of the parasympathetic system.
The blood coagulation time was considerably shortened in
rabbits that were given, orally, a mixture of carbaryl (5 mg/kg),
DDT (5 mg/kg), and parathion (0.5 mg/kg), for 222 days. This effect
corresponded to increased levels of 5-hydroxy-3-indolacetic acid
(5-HIAA) and 4-hydroxy-3-methoxymandelic acid (VMA) in the urine,
indicating an increased rate of metabolism of serotonin and
catecholamine (Hassan & Cueto, 1970). The authors suggested that
this effect was a manifestation of non-specific stress, since
adrenocortical hormones shorten the coagulation time.
Fifteen male Sprague-Dawley rats (250-300 g) were given
drinking-water containing 10 mg carbaryl/litre for 30 days. Platelet
count, prothrombin time, partial thromboplastin time, fibrinogen,
and clotting factor activity for coagulation factors II, V, VII,
VIII, IX, X, and XII were determined. A significant decrease in
platelet count and in the factor VII clotting activity was observed
compared with those in the same number of control rats. Microscopic
evaluation of the liver revealed hepatocyte degeneration, central
vein congestion, some leukocytic infiltration, and vacuolization of
the cytoplasm. The author suggested that carbaryl might have harmful
effects on haemostasis (Lox, 1984).
Carbaryl has an in vitro inhibitory effect on arachidonic
acid-induced platelet aggregation, which corresponds to its
inhibition of thromboxane B2-formation. The results suggest that
carbaryl affected platelet aggregation by inhibition of
cyclooxygenase (the key enzyme of prostaglandin synthesis) by
carbamoylation (Krug & Berndt, 1985). At a concentration of
10 µmol/litre, carbaryl completely blocked platelet aggregation and
cyclo-oxygenase activity (Krug et al., 1988). The carbamoylation
of several platelet proteins, including cyclo-oxygenase, may be
responsible for this effect.
Carbaryl produced, in vitro, a dose-dependent increase in
methaemoglobin (Met Hb) formation at 10 and 100 mg/litre, as well as
decreases in reduced glutathion levels in the erythrocytes of Dorset
sheep with low erythrocyte glucoso-6-phosphate dehydrogenase
(G-6-PD), which is similar to humans who have G-6-PD deficiency.
Carbaryl posed oxidative stress to G-6-PD-deficient red cells,
probably due to its major metabolite alpha-naphthol (Calabrese &
Geiger, 1986). Decreases in the K+ ion concentration in
erythrocytes (with more than 24%) and in haematocrits (from 43.5 to
38%) were found by Sokur (1971) in rats fed carbaryl 0.05 LD50/day
for 2 months.
In an in vitro study, Szczepaniak & Jeleniewicz (1981) found
that carbaryl binds free blood amino acids (plasma and
erythrocytes). These authors also performed a series of in vitro
studies to investigate the effect of carbaryl on amino acids. A
single application of 475 mg carbaryl/kg on 46 treated and 12
control rats produced significantly decreased amino acid values in
the brain, except for valine and phenylalanine. All amino acids
reached the control level after 72 and 120 h. Two hours after
administration, erythrocyte amino acids also decreased >50%
(Szczepaniak & Jeleniewicz, 1980; Jeleniewicz et al., 1984). A
slight decrease in erythrocyte amino acid concentrations was
observed after 30 days with 95 mg carbaryl/kg administered orally
(Jeleniewicz & Szczepaniak, 1980). Blood serum amino acids in 24
rats decreased 4 h after a single application of 189.6 mg/kg. There
was a larger decrease in valine, then in phenylalanine, alanine,
aspergic acid, serine, and glycine (Szczepaniak et al., 1980).
After 15-day dosing of 0.1 LD50(94.8 mg/kg) per day to 12 rats,
there were no changes in levels in serum and erythrocytes, but after
30-day dosing in 8 rats, there was a slight decrease in the alanine
concentration of the erythrocytes (Jeleniewicz & Szczepaniak, 1980).
The effects of carbaryl on the thermoresistance and fractional
content of blood serum proteins was studied by Subbotina &
Belonozhko (1968). A single dose of 150 mg carbaryl/kg administered
to rabbits and multiple applications of 100 mg carbaryl/kg for 2
months showed that, with a single application of carbaryl, there was
an increase in protein thermocoagulation from 28% on day 1 to 67% on
day 7, and with multiple applications of carbaryl there was a
lowering of albumin levels and a rise in globulins (mostly
alpha-globulins) in serum, on day 10. These changes were reversible.
There was also a decrease in the coefficient of thermal dehydration.
The changes in proteins in both studies show lowering of their
lability.
Carbaryl inhibited the incorporation of 3H-uridine and
14C-labelled amino acids into RNA and proteins in cultures of HeLa
cells. The effect was dose-dependent. Incorporation was inhibited by
50% at a 150 µg/ml concentration after 30 min incubation. At a
concentration of 350 µg/ml, only 10% of amino acids and 32% of
uridine incorporation activity were retained (Myhr, 1973). Later,
Blevins & Dunn (1975) showed that carbaryl caused general metabolic
changes in Hela cells. A concentration of 1-2 mg carbaryl/litre
stimulated all divisions and, at 4-8 mg/litre, inhibited growth. A
decrease in cellular protein at 4 mg/litre was noted. Changes in the
phospholipid fraction at 8 mg/litre were probably related to the
alteration of the structure of cellular membranes. Disturbances in
the development of human cells in culture were also reported by
Shpirt (1973). An inhibitory effect of carbaryl on cell development
was demonstrated in Ehrlich ascites tumours in mice. A reduced rate
of incorporation of labelled uridine-5-3H-thymidine methyl-3H
and L-leucine 14C in the RNA, DNA, and proteins in Ehrlich cells
in vitro was also reported (Walker et al., 1975b). The authors
suggested that this effect could be a basis for investigating the
mechanism of the adverse effects that carbaryl has on reproduction.
Carbaryl and N-nitrosocarbaryl appeared to have different
action characteristics with regard to rat liver microsomal membrane
alterations (Beraud et al., 1989b); carbaryl was ineffective on
the lipoperoxidation indices while the nitroso compound had an
inhibitory action on the formation of malonaldehyde and conjugated
dienes as well as on the NADPH-dependent reductase activities.
8.8.4 Effects on the liver and other organs
Several authors reported data on disturbances in the
carbohydrate, protein-forming, and detoxicating functions of the
liver.
A single application of 300 mg carbaryl/kg in rats produced an
increase in albumin and alpha-globulins, and a decrease in ß-and
gamma-globulins (Zapko, 1970).
Kagan et al. (1970) studied the effects of carbaryl on the
liver of 180 rats and 18 rabbits. During an 11-month study, they
gave a daily, oral dose of 38 mg carbaryl/kg to rats. After 1 month,
they observed a rise in the alanine-aminotransferase and
alkaline-phosphatase activities in serum, and a decrease in succinic
dehydrogenase and glycogen in the liver. Doses of 0.76 mg/kg and
0.38 mg/kg, given in the diet to rabbits, caused retention of
bromsulfophthaleine in the blood. The authors also reported a
changed ratio in the protein fractions in serum and an increase in
liver weight. The pathomorphological changes in the liver were
destructive, necrobiotic, and proliferative. An effect on the liver
was also demonstrated in the study of Lox (1987) (see section
8.8.3).
Pavlova et al. (1968) found that, with acute and long-term
exposures, carbaryl affected the oxidative processes in tissues,
because of its direct action on the enzymes of cell respiration and
possible disturbance in the membrane processes. They performed
studies on rats treated with 0.2 LD50 for 3 days and on rats
treated with 0.01 LD50 for 20 weeks. At the higher dose, there
were decreases in the cytochromoxidase and succinedehydrogenase
activities in the liver and brain mitochondria. A histochemical
examination revealed a rather high, irregular activity of the
cytochromoxidase in the heart, as well as increased succinic
dehydrogenase activity. With the long-term dosing, the changes were
not significant, but there was a lowering of the cytochromoxidase
and succinedehydrogenase activities in the heart mitochondria.
Development of experimental cholesterol arteriosclerosis in rabbits
was facilitated by the application of 20 mg carbaryl/kg for 2.5
months (Lukaneva & Rodionov, 1973). Changes were found in the
following indices: general cholesterol, ß-lipoproteins, ECG changes,
and pathomorphological changes in the aorta and coronary vessels.
Carbaryl (100 mg/kg body weight) given to dogs in their diet
for 45 days caused disturbances in the secretion of the intestinal
enzymes. There was an increase in enterokinase secretion, as well as
in the excretion of alkaline phosphates and lipase into the
intestinal juice. The no-observed-effect level (NOEL) for these
effects was 700 µg/kg body weight, which corresponds to 7 mg
carbaryl/kg diet. Three dogs were used in each group (Georgiev,
1967).
8.8.5 Effects on serum glucose
Orzel & Weiss (1966) found that a rise in blood glucose
correlated with the onset and duration of tremors and the degree of
brain ChE inhibition in rats that were treated ip with 5 and 25 mg
carbaryl/kg. The hyperglycaemic response is blocked by
adrenal-ectomy and is unaltered by hypophysectomy. The authors
suggested that the hyperglycaemia was related to increased secretion
of epinephrine. Hyperglycaemia is thought to result from cholinergic
stimulations as it is also found in acute intoxications with
organophosphorous compounds (Kaloyanova, 1963). In their studies,
Orzel & Weiss did not find changes in liver glycogen. Muscle
glycogen was decreased only in non-fasted rats. Elevated levels of
blood glucose and slightly reduced immunoreactive insulin were found
with repeated oral exposure of rats at 3 mg/rat, weekly, for one
year (Wakakura et al., 1978). Glycogen disappeared in most of the
hepatocytes. The cells mainly contained related granular endoplasmic
reticulum with swollen mitochondria. A single application of
30 mg/rat produced transient hypoglycaemia at 20 h followed by
hyperglycaemia at 44 h. The effects of carbaryl on respiration,
glycolysis, and glycogenesis in isolated hepatocytes of male Wistar
rats were studied by Parafita et al. (1984) and Parafita &
Fernandez Otero (1984). The cells were treated with concentrations
of carbaryl of 0.01, 0.1, and 1.0 mmol/litre dissolved in 1%
dimethylsulfoxide (DMSO). DMSO slightly stimulates the respiratory
coefficients. Carbaryl decreased the metabolic output of CO2 at
all concentrations; oxygen consumption was reduced by 40% in
relation to the DMSO-treated groups only at the highest
concentration (1.0 mmol/litre). Glucose remained unchanged in the
presence of carbaryl. Endogenous production of lactic acid was not
affected, and net metabolic production was strongly inhibited by
both DMSO and carbaryl. At a maximum carbaryl concentration
(1 mmol/litre), the net lactic acid production was completely
blocked. Carbaryl inhibited lactate gluconeogenesis, and, to some
extent, gluconeogenesis from fructose pyruvate and alanin. Glycerol
glucogenesis was unaffected. Lactic dehydrogenase activity was
reduced by 38% and glucose 6-phosphate synthetic activity was
increased by 1.0 mmol carbaryl/litre. Aspartate aminotransferase
activities (cytoplasmic and mitochondrial fractions) were inhibited
by 0.1 and 1.0 mmol carbaryl/litre. The results indicated that
carbaryl causes a decrease in glucose production by hepatic cells
and suggested that carbaryl-induced hyperglycaemia in fasted animals
is caused by deficiencies in the peripheral utilization of the
glucose.
8.8.6 Interactions with the drug metabolizing enzyme system
Carbaryl is a weak inducer of hepatic microsomal
drug-metabolizing activity. Cress & Strother (1974) reported
depressed hexobarbital sleeping time in mice given 0.125 or 0.5
LD50 carbaryl, orally, for 10 days. These authors studied the
effects of a 2-week dietary administration of high levels of
carbaryl (10 times higher than the dose given in the earlier study).
Weanling male Swiss-Webster mice, weighing 17-20 g, were divided
into 5 groups of 40 animals each. The daily consumption of carbaryl
by each animal was approximately 119% of the acute LD50. This dose
was well tolerated with no deaths or overt symptoms of
cholinesterase inhibition, probably because carbaryl was
administered over a 24-h period, instead of in a single injection.
Feeding carbaryl resulted in a 44% increase in the rate of in vitro
p-hydroxylation of aniline, and increased in vitro demethylation
of benzphetamine. The hepatic levels of cytochrome P-450 and b5
were increased, but the microsomal protein concentration per gram of
liver was not affected. No changes were reported in
1-naphthol-treated mice. Increased metabolic activity was
demonstrated with phenobarbital and carbaryl. Phenobarbital sleeping
time was shortened to 45 min (control 74 min) in carbaryl-fed mice.
The rate of elimination for the carbaryl-treated animals was twice
as high as that for control animals. The oral LD50 of carbaryl in
carbaryl-pretreated animals (14 days feeding) was three times as
high as in the control animals. 1-Naphthol did not affect the levels
of cytochromes. Sleeping time and the LD50 for carbaryl were not
different in control and 1-naphthol pretreated animals.
Liver weight was low in carbaryl-fed mice, but higher as a
fraction of body weight. The authors concluded that the degree of
the enzyme induction was not high, because most indicators exceeded
the control values by 50% only, except for the lethal dose level.
The pretreatment of rats with 5 daily doses of 10 mg
carbaryl/kg, administered ip, resulted in a 4.2-fold increase in the
rate of benzo( a)pyrene metabolism (Lesca et al., 1984). Total
microsomal P-450 content and benzphetamine demethylase activity were
not significantly affected. The increased ability to hydroxylate
aniline in mice under carbaryl treatment was reported by Guthrie
et al. (1971).
Exposure of murine 3T3 fibroblasts to carbaryl at a
concentration of 10-6 mol/litre was found to increase aryl
hydrocarbon hydroxylase activity (Lahmy et al., 1988). The
magnitude of the effect increased with the duration of exposure.
Carbaryl induction of microsomal enzyme systems in White
Leghorn cockerels was demonstrated by Puyear & Paulson (1972). A
decrease in phenobarbital sleeping time, an increase in liver weight
and elevation of liver aniline hydroxylase activity were found after
oral treatment with carbaryl at 100 mg/kg per day (in gelatine
capsules) for 3 or 6 days. No effect was observed at a dose of
50 mg/kg. Thus, the sleeping time data indicated that the NOEL of
carbaryl for Leghorn cockerels was between 50 and 100 mg/kg per day.
Higher doses caused an increase in liver aminopyrine demethylase
activity (200 or more mg/kg per day) and in liver cytochrome P-450
content (300 or 400 mg/kg per day). Microsomal protein was not
changed. All study parameters returned to control values by day 11
after the treatment was terminated (Puyear & Paulson, 1972).
El-Toukhy et al. (1989) reported that the administration by
gavage of carbaryl to mice at a dose of 166 mg/kg resulted in small,
but statistically significant (15-20%), decreases in liver pyridoxal
phosphokinase and L-tryptophan 2,3-dioxygenase activity.
Administration over a 5-day period of 83 mg carbaryl/kg per day had
no significant effect. Hassan et al. (1990) administered 85 mg/kg,
orally, either once or five times daily. Both the single and
repeated regimens resulted in approximately 40% decreases in
L-tryptophan 2,3-dioxygenase. Carbaryl was also found to be a
competitive inhibitor of this enzyme in vitro.
Inhibition of monoamino-oxidase (MAO) was reported in the
presence of carbaryl in vitro in liver homogenate (Hassan et al.,
1966). The authors suggested that MAO and the catalase system are
involved in oxidative demethylation during carbaryl detoxification.
Exposure of rat liver microsomes to carbaryl in vitro at a
concentration of 0.5 mmol/litre resulted in decreases in the
activities of several monooxygenases (Knight et al., 1986). The
ability of carbaryl to inhibit the N-demethylation of
ethylmorphine appeared to be competitive with a Ki of
2.4x10-4 mol/litre. However, when administered to rats at doses of
up to 25 mg/kg, carbaryl did not have any significant inductive or
inhibitory effect.
Lechner & Abdel-Rahman (1986a) reported that the in vitro
incubation of 2 and 4 mmol carbaryl/litre for 60 min reduced
glutathione levels and increased glutathione S-alkyltransferase in a
rat liver homogenate in a dose- and time-dependent manner. Carbaryl
had no apparent effect on glutathione levels in whole blood with
120 min of incubation. Carbaryl inhibited N-demethylation and
O-demethylation by rat liver microsomes with apparent Ki of 0.1
and 0.7 mmol/litre, respectively (Beraud et al., 1989a).
Carbaryl had an antagonistic action on the release of
microsomal ß-glucuronidase by malathion in a microsomal suspension
in vitro (Lechner & Abdel-Rahman, 1985).
Jacob et al. (1985) studied the effects of carbaryl on the
metabolic activation of the environmental, carcinogenic, polycyclic
aromatic hydrocarbons. Carbaryl was a weak inducer of metabolism of
benz( a)anthracence in the rat liver, and altered its metabolite
profile by shifting from 10, 11-oxidation to 5, 6- and 8,
9-oxidation. Since carbaryl did not induce bay-region activation,
the authors concluded that tumour-promoting activity could not be
expected.
8.8.7 Effects on the endocrine system
The effect of carbaryl on the neuroendocrine system was studied
in rats. Carbaryl was given, orally, at 7, 14, or 70 mg/kg body
weight to male and female rats (each group contained 24 animals
weighing 90-130 g) for a period of 1 year. Growth retardation,
changes in the blood enzymes, and endocrine gland disturbances
varied with the dosage, being especially marked at the end of the
study in the group receiving 70 mg/kg body weight per day.
Acetylcholinesterase and butylcholinesterase activities were
inhibited significantly in these groups. Spermatozoon motility was
reduced progressively with the duration of the exposure. In the
prolonged estrus cycle, the diestrus period was particularly
affected. An increase in the number of corpora lutea and atrophic
follicles in the ovaries was correlated with this disturbance. There
was a dose-dependent increase in the gonadotropic function of the
hypophysis, which was determined by tests on immature mice.
Hypophyseal homogenate from a rat given 70 mg carbaryl/kg increased
the weight of the ovaries by 51.5%, and that of the uterus by 123%,
compared with the weights in control mice. At all doses,
histochemical studies of the hypophysis showed changes indicative of
an increase in the activity of the cells producing a luteinizing
gonadotrophy, i.e., an increase in the size of the cells, loss of
granules, and hyalinization of the cytoplasm. Histological
examination of the adrenal glands revealed an increase in the size
and mitotic activity of cells in the zone glomerulosa. Enlarged
cells with a large nucleus or two nuclei were present in the
fascicular zone. Impairment of thyroid gland functional activity in
the test group was indicated by the reduction in the rate of
absorption and excretion of 131I and its rather low recovery, as
well as by the corresponding histological findings, i.e.,
enlargement of follicles and more dense and basophilic colloid at
all doses. Histological findings were more pronounced in the
70 mg/kg per day groups. It is likely that the effects of carbaryl
on the reproductive organs is mediated by the endocrine glands.
However, the possibility of a direct effect was not excluded by the
authors (Rybakova, 1966; Shtenberg & Rybakova, 1968; Shtenberg
et al., 1970).
The effect of carbaryl on the thyroid gland in rats was studied
by Shtenberg & Hovaeva (1970). Rats were given carbaryl, orally, at
0.7, 2, 5, or 17 mg/kg per day for 6 months. They were fed a normal
iodine diet or an iodine-deficient diet. There was an initial
increase in function in the first 3.5 months of exposure and a
decrease after 6 months of exposure. Recovery was complete 2 months
after the end of the exposure. Decrease in iodine uptake and
reduction of function was 26.5% in rats given 5 mg/kg and 29.5% in
rats given 15 mg/kg. Rats fed an iodine-deficient diet were more
sensitive to carbaryl-induced changes in the thyroid.
The functional states of the thyroid gland and adrenal cortex
were affected by a daily administration of 36 mg carbaryl/kg to rats
for 4 months. The maximum accumulation of 131I occurred after 6 h
and, in the control animals, after 12 h. The 131I absorption rate
normalized after 45 days, but its excretion was delayed. The
concentration of sodium in the blood increased by 36.4% after day 15
and by 74% after 45 days, while the potassium concentrations were
reduced. The daily excretion of 17-corticosteroids in the urine
increased by 35.9%. The changes in the thyroid and adrenal glands
were transient (Dyadicheva, 1971).
Hassan (1971) studied the effects of carbaryl on the synthesis
and degradation of catecholamines in the rat. Rats were given
carbaryl in the diet at concentrations of 100 or 700 mg/kg for 7
months, or in peanut oil at single oral doses of 50, 80, or
250 mg/kg, or 3 single doses of 80 mg/kg body weight. On day 30, the
rats given 700 mg/kg diet eliminated amounts of urinary
4-hydroxy-3-methoxy mandelic acid (VMA) increased by more than 300%;
levels returned to normal after 195 days. A similar effect was
demonstrated after single oral doses with a dose-effect
relationship. The amount of 5-hydroxyindole acetic acid (5-HIAA) in
the urine increased in rats that were treated with a single dose and
3 successive daily doses of 80 mg/kg per day. Adrenalectomy and
hypophysectomy did not change the pattern of VMA excretion produced
by carbaryl. There was an increased turnover rate (68%) of heart
norepinephrine (NE-3H) in rats treated with 80 mg carbaryl/kg, 2 h
before application of NE-3H. The corticosterone level was increased
by about 125% at the same dose level. A carbaryl concentration of
16.2 µg/ml in the blood was enough to trigger maximal corticosterone
secretion. The authors suggested a probable activation of the enzyme
system involved in the catecholamine metabolism, because carbaryl
may also directly affect the adrenergic nerve endings. Activation of
the pituitary adrenal axis and an increase in sympaticoadrenergic
activity with concomitant increased VMA elimination was a
pharmacological effect due to carbaryl treatment.
The effect of a single oral dose of 60 mg carbaryl/kg body
weight on serotonin (5-HT) metabolism in the male Holtzman Albino
rat brain was studied by Hassan & Santolucito (1971); 2, 4, 6, and
24 h after carbaryl application, whole brain samples were analysed
for serotonin, 5-HIAA, and corticosterone. The concentrations of
5-HT and 5-HIAA in the brain increased by 20-30% (5-HT from 0.61 to
0.8; 5-HIAA from 0.25 to 0.31 µg/g) from 2 to 6 h and returned to
normal after 24 h. The plasma corticosterone level was also
increased by about 125%, 1 h after oral administration of carbaryl,
and returned to normal after 20 h. The inhibitory effect of
p-chlorophenylalanine on brain 5-HT formation was diminished by
carbaryl treatment. The serotonin level in the brain of
carbaryl-treated animals pretreated with p-chlorophenylalanine was
72% higher than in the control animals. Increased 5-HIAA levels were
probably a result of an increased rate of synthesis of serotonin.
The authors suggested the possible role of the increased synthesis
of serotonin and tryptophan-5-hydroxylase activity, which could be
related to stress conditions. Carbaryl activation of MAO is probably
related to the increased formation of cerebral 5-HIAA.
The uptake of noradrenaline in vitro by hypothalamic slices,
isolated from rats exposed in vivo to near lethal levels of
carbaryl, was increased in a dose-dependent manner (Jablonska &
Brzezinski, 1990).
Oral administration of 200 mg carbaryl/kg to rats was reported
to raise striatal levels of noradrenaline and homovanillic acid, a
metabolic product of dopamine, when measured 0.5-2 h later (Ray &
Poddar, 1985b).
The mechanism of the stimulation of corticosterone secretion is
not clear.
In vivo studies on male mice receiving 38 and 68 mg
carbaryl/kg showed that carbaryl can increase certain hepatic
androgen hydroxylase activities. In vitro incubation of carbaryl
with prostate tissue and testosterone 1,2-3H stimulated the
formation of dehydrotestosterone-3H, thus, suggesting a direct
action on steroidogenesis. Comparing these results with the effects
of other toxic substances, the authors concluded that carbaryl
cannot exert any major changes in steroid metabolism, nor can it
reduce hormonal disturbances (Dieringer & Thomas, 1974).
Attia et al. (1991) studied the effect of carbaryl on rat
melatonin production as alterations of the latter were found to have
marked consequences in reproduction, immunology, and tumour growth.
Male albino rats ( Rattus rattus), 8 animals in a group, were
exposed to light/dark cycles of 14/10 h. They were treated by
gastric gavage with carbaryl dissolved in corn oil for 6 successive
days. The total doses were 50, 125, and 250 mg/kg. The animals were
killed 2 and 4 h after the onset of darkness, which was 8 or 10 h
after the application of the insecticide. Carbaryl was found to
bring about an augmentation of the pineal melatonin content 4 h
after the onset of darkness, which coincided with the stimulated
N-acetyltransferase levels and hydroxyindole- O-methyltransferase
activity. However, at the same time, carbaryl treatment at all doses
significantly lowered the circulating melatonin titres; this was
supposed to be related to increased hepatic melatonin metabolism due
to the increased activity of the liver drug metabolizing enzyme
system (Gaillard et al., 1977). These results and the established
carbaryl-induced elevated pineal 5-hydroxytryptophan, serotonin, and
5-hydroxyindole acetic acid contents in the course of the night
cycle, support the concept that carbaryl has a significant effect on
pineal melatonin synthesis and secretion.
Kadir & Knowles (1981) reported that carbaryl inhibited rat
brain monamine oxidase activity in vitro.
8.8.8 Other studies
Human serum albumin reacted in vitro with the ester group of
carbaryl and catalysed the hydrolysis and liberation of 1-naphthol.
This reaction is similar to an "esterase type" action (Casida &
Augustinsson, 1959) called carbamoylation (Oonnithan & Casida,
1968).
8.9 Factors modifying toxicity, toxicity of metabolites
8.9.1 Factors modifying toxicity
The toxicity of carbaryl can be modified by altering liver
function by tranylcypromine treatment or 70% hepatectomy. A decrease
in LD50 and increase in ChE activity were more pronounced after
tranylcypromine treatment (Falzon et al., 1983).
The carbaryl LD50 in animals was increased three-fold by
pretreating animals with small doses of carbaryl (Cress & Strother,
1974).
Phenobarbital, administered 24 h before carbaryl treatment,
decreased the acute ip toxicity of carbaryl in mice, while
2-diethyl-amino-ethyl-2,2'-diphenyl-valerate-HC1 (SKF 525-A), given
1 h before carbaryl treatment, increased its acute intraperitoneal
toxicity (Neskovic et al., 1978). Enzyme-mediated binding of
carbaryl to rat hepatic microsomal protein occurred in vitro in
the presence of NADPH and oxygen. Incorporation of radioactivity of
14C-ring labelled carbaryl was studied. A 2- to 3-fold increase in
binding was produced by pretreatment of animals with MFO inducers,
e.g., phenobarbital or 3-methylcholanthrene. SKF-525-A inhibited
binding by approximately 77% of the radioactivity. It is likely that
binding of active carbaryl metabolites formed by MFO occurs. The
radiolabelled metabolic products were covalently bound to amino acid
residues of microsomal protein: 99.3-99.7% of the bound
radioactivity (Miller et al., 1979). A 50% decrease in microsomal
ß-glucuronidase activity was observed 1 h after a single oral
administration of 50 mg carbaryl/kg to female, Sprague-Dawley rats.
The whole liver homogenate ß-glucuronidase content was reduced by
40% after daily treatment with the same dose of carbaryl for 7 days.
This effect was attributed to the decreases in mitochondrial
lysosomal and microsomal ß-glucuronidase. The action of carbaryl in
depleting the endoplasmic reticulum of ß-glucuronidase led to an
increase in serum ß-glucuronidase activity. This was demonstrated in
an in vitro microsomal suspension study which showed that
incubation of 4 mmol carbaryl/litre with microsomal suspensions
effected a release of the ß-glucuronidase enzyme. The effect on the
endoplasmic reticulum was further exemplified by the induction in
microsomal UDP-glucuronyl transferase activity after 7 days of daily
treatment with carbaryl at a dose of 25 mg/kg. Thus, carbaryl
modifies the enzyme activities associated with the endoplasmic
reticulum by decreasing specific activity (90%) towards
ß-glucuronidase enzyme and activating the synthesis of endoplasmic
reticulum protein connected with drug metabolism, such as the
UDP-glucuronyl-transferase enzyme (Abdel-Rahman et al., 1985;
Lechner & Abdel-Rahman, 1985).
Osman & Brindley (1981) conducted bioassays to determine the
carbaryl susceptibility and synergism with piperonyl butoxide in
natural populations of three species of Lobops grassbugs, in order
to estimate monoxygenase detoxification. Pretreatment of the insects
with piperonyl butoxide inhibited the mixed-function oxidase system
and decreased the values of the LC50 for the insects (Osman &
Brindley, 1981). Mixed-function oxydase involvement in the
biochemistry of synergistic insecticides (including carbaryl) has
been reviewed by Casida (1970).
Diet may modify carbaryl toxicity. Boyd & Boulanger (1968)
reported an increased susceptibility to carbaryl toxicity in Albino
rats (272 Wistar strain rats were used) fed a protein-deficient
diet. Boyd & Krijnen (1969) also reported that the LD50 decreased
from 589 mg/kg in rats fed an 81% casein diet to 67 mg/kg in rats
fed a 0% casein diet; 285 male rats were used in this study.
The combined effects of different ambient temperature levels
and carbaryl were studied by Ahdaya et al. (1976). The LD50
values in mice injected ip were 263 mg/kg at 1 °C and 122 mg/kg at
38 °C (588 mg/kg is the LD50 at 27 °C). The thermoregulation
ability of mice treated with carbaryl was affected more than that of
mice treated with parathion which is a stronger inhibitor of ChE.
The authors suggested that this effect was due to an overall
reduction in the basal metabolic rate.
Atropine sulfate decreased signs of parasympathetic stimulation
in a group of pigs acutely poisoned with 1-2 g carbaryl/kg (Smally,
1970). During multiple administrations of carbaryl, drug-induced
(hydrochlorothiazide) diuresis helped in the detoxification
processes. ChE reactivations are contraindicated because they may
aggravate the signs of carbaryl intoxication (Sanderson, 1961;
Podolak & Warchocki, 1980).
Application of atropine or trepazin, 20 min before oral
intoxication with carbaryl in mice, decreased toxicity about 2
times. The results were similar when cholinolytics were applied
2 min after administration of carbaryl (Vyatchanikov & Alexachina,
1968). Atropine administered iv to rats at a dose of 8 mg/kg
increased the carbaryl ip LD50 by a factor of about 7 (70 to
460 mg/kg) (Harris et al., 1989). Co-administration of either of
two different oximes (2-PAM and HI-6) with atropine was found to
provide significantly less protection than that afforded by atropine
alone, indicating that oxime therapy is not a useful treatment for
carbaryl poisoning.
Co-administration of an oral dose of 10 mg malathion/kg in rats
significantly reduced the rates of both absorption and elimination
of an oral dose of 10 mg carbaryl/kg (Lechner & Abdel-Rahman,
1986a). Peak plasma levels of labelled carbaryl were observed about
1 h after the administration of carbaryl alone, and after 2 h when
malathion was administered simultaneously. The terminal rate of
elimination of carbaryl was decreased by a factor of about 4, with
the half-life increasing from 16.96 h to 64.41 h.
Concentrations of cimetidine in the range of 60-240 µg/ml were
observed to decrease the clearance rate of carbaryl by perfused rat
liver in a dose-dependent manner (Ward et al., 1988).
Ray & Poddar (1985a,b, 1990) reported that the intraperitoneal
administration of 1 mg haloperidol/kg, 12.5 to 100 mg
5-hydroxy-tryptamine (5-HTP)/kg, or 100 mg L-tryptophan/kg increased
the incidence of tremors in rats following an oral dose of 50-200 mg
carbaryl/kg. The potentiation of the tremorogenic effect of carbaryl
by 5-HTP was demonstrated to be dose dependent. The potentiating
effects of 5-HTP and haloperidol could be blocked by the
coadministration of the serotonergic antagonist methysergide
(20 mg/kg, ip) or the dopaminergic antagonist bromocriptine
(10 mg/kg, ip), respectively. These results suggest the possible
involvement of a central cholinergic, dopaminergic interaction in
the carbaryl-induced tremor.
The effects of humic acids in the detoxification of carbaryl
during oral application are very slight: only 9.9-18.6% decrease in
toxicity was observed (Golbs et al., 1984).
The combined effects of carbaryl and sodium nitrite have been
studied (Podolak-Majczak & Tyburczyk, 1984, 1986; Tyburczyk &
Podolak-Majczak, 1984a,b; Tyburczyk & Podolak-Majczak, 1986). Dosing
Wistar rats for 3 months with sodium nitrite at 20 mg/kg per day,
and carbaryl at 0.1 LD50/day (60 mg/kg), the brain
gamma-aminobutyryl acid level, methaemoglobin, blood serotonin, free
blood tryptophan, serum and liver alanine amino-transferase activity
levels increased and blood cholinesterase activity decreased.
Decreases were observed in the vitamin E and A levels,
glucose-6-phosphate dehydrogenase activity, and liver aminohexoses
and hydroxyproline levels with a simultaneous increase in the serum
aminohexoses level. This finding may indicate the increasing process
of connective tissue catabolism and lisosomal membranes
labilization.
8.9.2 Toxicity of metabolites
Hydrolysis of the carbamate ester bond of carbaryl results in
detoxification. The carbamate moiety is decomposed to carbon dioxide
and methylamine, and the phenolic part is conjugated and excreted.
Kuhr (1971) summarized the data on the toxicity of carbaryl
metabolites (Table 53).
Table 53. Toxicity of carbaryl and some of its metabolitesa
LD50 (mg/kg) 7-day Molar I50 bovine
NOELb anticholin-esterase
Mouse (mg/kg)
Rat oral Intraperitoneal Rat
Carbaryl 270 29-42 125-250 5 x 10-8
4-Hydroxycarbaryl 1190 74 > 1000 4 x 10-7
5-Hydroxycarbaryl 297 56 > 1000 4.6 x 10-8
7-Hydroxycarbaryl 4760 > 1000
Hydroxymethylcarbaryl > 5000 630-780 250-500 1.4 x 10-5
1-Naphthol 2570 500-1000 1 x 10-3
aFrom: Kuhr (1971).
bNOEL = No-observed-effect level.
Many of the known metabolites are much less toxic than
carbaryl. The pharmacological study of carbaryl in rats showed that
the metabolites with a methylcarbamate moiety are inhibitors of
plasma cholinesterase (Fernandez et al., 1982). None of the
carbaryl metabolites was appreciably more active as a cholinesterase
inhibitor than carbaryl itself (Oonnithan & Casida, 1968).
8.9.3 N-nitrosocarbaryl
Carbaryl is a secondary amine and is, therefore, capable of
nitrosation in the presence of nitro donor groups, such as sodium
nitrate, to give a nitrosamide. This nitrosamide, nitrosocarbaryl,
has been proved to be mutagenic and carcinogenic, at high doses in
animals. Conditions of this nitrosation include a strongly acidic pH
(less than 2), which is comparable with the pH in the human stomach.
However, nitrosocarbaryl is not stable at this pH. Its maximum
stability is between pH 3 and 5, pHs at which no significant amounts
of carbaryl can be nitrosated. Carbaryl has been nitrosated in
several studies, in vitro as well as in vivo, in the guinea-pig,
which has a stomach acidity close to that in man. If significant
yields of nitrosocarbaryl have been shown in these circumstances,
the significance of these findings is still not clear for human risk
evaluation. The pH in the human stomach is variable during food
intake and probably, more importantly, the stomach contains a lot of
food, which will minimize the contact between naturally occurring
nitrite and carbaryl residues, but which will also afford a lot of
nucleophilic sites for nitrosocarbaryl to react with. It is also
noteworthy that all studies that were conducted with jointly
administered carbaryl and nitrite yielded negative results for
oncogenicity. Cranmer (1986) estimated the potential intake of
nitrosocarbaryl at 6x10-9 mg/kg per day. Using different
mathematical models, Cranmer estimated a cancer risk for
nitrosocarbaryl between 10-6 (one-hit model) and 10-9 (probit
model). However, if such oncogenic nitroso-carbamates were to be
found in such quantities in the human stomach, an increased
incidence of stomach cancer would have been expected during the
period when drug and pesticide carbamates were widely used. However,
during this period, the incidence of gastric cancer in the USA
declined considerably.
The bacterial metabolite N-nitrosocarbaryl may act as a
noncompetitive inhibitor of the in vitro metabolism of
aminopyrine, p-nitroanisole, and aniline by rat liver microsomes
(Beraud et al., 1980, 1989a,b). N-nitrosocarbaryl was more
effective as an inhibitor of microsomal activity than the parent
compound.
8.10 Mechanism of toxicity - mode of action
The mechanism of toxicity and the mode of action of carbaryl
and carbamates, in general, have been described in EHC 64 (WHO,
1986).
8.10.1 Inhibition of cholinesterase activity
As a carbamate compound, carbaryl is an inhibitor of
cholinesterase (ChE) activity (for details see Reiner & Aldridge
(1967) and Aldridge (1971)).
A number of studies have been performed by Carpenter et al.
(1961) to assess the extent of ChE inhibition by carbaryl in
mammals. A single oral dose of carbaryl of 560 mg/kg produced a 43%
inhibition of the erythrocyte ChE in rats in 0.5 h, and a 30%
inhibition in brain AChE. However, they returned to normal in rats
that survived 24 h after administration of the dose. Carbaryl did
not depress plasma ChE significantly. Two groups of Beagle dogs were
injected once, iv, with 10 or 15 mg/kg as an 8% solution in 95%
alcohol. No significant effects were found on either erythrocyte or
plasma ChE. On the 5th day, several administrations of the same
doses depressed plasma ChE by 24% and erythrocyte AChE by 40%. It is
doubtful that a long incubation time for the samples (2 h for
plasma) played a role in the slight depression of ChE. Comparative
data on ChE inhibition in the brain, plasma, and erythrocytes of
rats that received single doses of carbaryl were reported by Mount
et al. (1981). As shown in Table 54, there was a dose-dependent
decrease in ChE in the brain, plasma, and erythrocytes.
Brain AChE was significantly different in dead rats and in
surviving rats given 800 mg/kg. AChE depression in the brain was
>70% in the lethally poisoned rats. The inhibition of red blood
cell AChE activity was the same as in plasma.
Table 54. Inhibition of ChE in % in comparison with the controlsa
Sprague-Dawley rats Post-dosing Brain Red blood Plasma
(h) cells
Controls 10 rats 0 0 0
450 mg/kg 0.5 56 44 51
(24 rats sacrificed) 1 74 29 53
2 74 42 68
4 66 48 61
8 55 25 31
24 27 60 38
48 22 23 46
96 6 19 -19
800 mg/kg 0.5 66 32 47
(34 rats sacrificed) 1 70 44 50
2 79 73 69
4 50 72 70
12 50 72 50
28 58 63 68
48 17 26 40
96 16 -6 16
800 mg/kg 3 80 - -
(24 rats died) 4 85 - -
18 88 - -
25 71 - -
29 88 - -
30 84 - -
1200 mg/kg 1-36 88-91 - -
(15 died)
aAdapted from: Mount et al. (1981).
The affinity of AChE of human brain caudate nucleus for
carbaryl was studied in vitro and some inhibition constants were
determined (Patocka & Bajgar, 1971). The value of the I50 affinity
constant was calculated from the dependence of AChE inhibition on
the molar concentration of the inhibitor in probit logarithm
transformation. The Hill coefficient was obtained from Hill plots.
The affinity constant for carbaryl pI50 was 5.59 and the Hill
coefficient was 1.50. According to the authors, some results of this
study suggest that AChE may be an allosteric enzyme.
Intravenous administration of colloidal carbon in the rat,
inhibiting the phagocytic activity of the RES, prolonged the
duration of the anticholinesterase effect of carbaryl (Pipy et al.,
1979). The authors speculated that the metabolism of carbaryl was
decreased by the RES inhibition and, as a result, the reactivation
speed of ChE was slower.
Pregnant rats were treated orally with 1 or 5 mg carbaryl/kg
from day 11 to the last day of gestation (Declume & Benard, 1977b;
Declume et al., 1979). No cholinesterase depression in blood,
brain, and liver were observed in either the dams or the newborn
offspring. However, after administration of 50 mg/kg from day 19 to
the last day of gestation, ChE inhibition was seen in both the
mother and newborn offspring.
Cambon et al. (1978) reported decreased cholinesterase
activity in the blood, brain, and liver in the mother and the fetus
after treatment of the dams at 6.25, 12.5, 25, and 50 mg/kg.
Variability of cholinesterase levels in the controls and
short-comings in the cholinesterase analysis protocol make these
results difficult to interpret.
9. EFFECTS ON HUMAN BEINGS
9.1 General population exposure
9.1.1 Acute toxicity, poisoning incidents
The clinical picture of carbaryl intoxication results from the
accumulation of ACh at nerve endings (WHO, 1986). The signs and
symptoms can be categorized into the following 3 groups:
(a) Muscarinic manifestations
- increased bronchial secretion, excessive sweating,
salivation, and lacrimation;
- pinpoint pupils, bronchoconstriction, abdominal cramps
(vomiting and diarrhoea); and
- bradycardia.
(b) Nicotinic manifestations
- fasciculation of fine muscles (in severe cases, diaphragm
and respiratory muscles also involved); and
- tachycardia.
(c) Central nervous system manifestations
- headache, dizziness, anxiety, mental confusion,
convulsions, and coma; and
- depression of respiratory centre.
All these signs and symptoms can occur in different
combinations and can vary in onset and sequence, depending on the
chemical, dose, and route of exposure. The duration of symptoms is
usually shorter than that observed in organophosphorus poisoning.
Mild poisoning might include muscarinic and nicotinic signs only.
Severe cases always show central nervous system involvement; the
clinical picture is dominated by respiratory failure sometimes
leading to pulmonary oedema, due to the combination of the
above-mentioned syndromes.
A review of carbaryl-related poisoning from 1966 to 1980 was
made by US EPA. During this period, 193 cases of over-exposure to
carbaryl as the sole active ingredient in the poisoning and 144
cases of over-exposure to a combination of carbaryl with other
active ingredients were recorded (Weston, 1982). Not all case
histories have been confirmed. There were 5 deaths. There is no
evidence that carbaryl was involved in these deaths, except for one,
which was acknowledged by the manufacturer.
Hayes (1963) reported two incidents of poisoning: one, a
19-month-old child who swallowed an unknown amount of carbaryl, and
the other, a man who swallowed 250 mg of carbaryl. Both developed
moderately severe ChE inhibition symptoms within 20 min: constricted
pupils, salivation, muscular incoordination in the child, and
epigastric pain, profuse sweating, lassitude, and vomiting in the
man. Both recovered after atropine treatment. Blood cholinesterase
was inhibited.
One death from carbaryl ingestion while drunk was reported by
Farago (1969) who concluded that 2-PAM application hastened the
fatal outcome (due to pulmonary oedema), 6 h after ingestion.
Carbaryl was found in various tissues.
Acute intoxication during the loading of an airplane was
reported by Long (1971).
In a case report of a suicide attempt involving about 25 g
(500 mg/kg) of carbaryl, dicumarin and boric acid, Dickoff et al.
(1987) described the occurrence of a peripheral neuropathy that
resembled the syndrome observed following exposure to some
organophosphorus compounds. Recovery continued for 9 months.
Electrophysiological findings were consistent with axonal peripheral
neuropathy. The cause-effect relationship was confounded because of
combined exposure.
9.1.2 Controlled human studies
Wills et al. (1968) carried out controlled studies in human
volunteers, aged 25-57 years. In a preliminary study, oral doses of
0.5, 1, and 2.0 mg carbaryl/kg in capsules (single application to 2
subjects) were tolerated without any subjective or objective
symptoms. In the main study, one group (5 subjects) took 0.06 mg
carbaryl/kg daily, and one group (6 subjects) took 0.13 mg
carbaryl/kg for 6 weeks. Physical examination, BSP removal from
blood, EEG examination, routine blood and urinalysis were performed,
and ChE of plasma and red blood cells was examined. No changes were
found in the low-dose group. An increase in the ratio of amino acid
nitrogen to creatinine in the urine, at the high dose, may represent
a decrease in the ability of the proximal convoluted tubule to
reabsorb amino acids. This change was reversible.
The urine of some subjects was analysed (Knaak et al., 1968).
The overall recovery (by the fluorometric method) of the carbaryl
equivalents in the urine was 26-28%, and, with the colorimetric
method, 37.8%, in subjects treated with 2 mg carbaryl/kg. The
following metabolites were found chromatographically in a 4-h urine
sample: alpha-naphthol glucuronide (10-15%), and sulfate (6-11%) and
4-(methylcarbamoyloxy)-alpha-naphthyl glucuronide (4%). Another
metabolite, alpha-naphthyl methylimido-carbonate O-glucuronide,
was identified by fluorometry.
A human ingestion study was conducted to determine the
relationship between a single oral dose of carbaryl and the rate of
its urinary excretion as metabolites. Elimination was apparently
first order over the dosage range of the studies (0.25-1 mg/kg). The
model predicts that, 24 h after ingestion, approximately 41% of the
dose can be accounted for as urinary metabolites (Hansen, 1978).
In a study involving one subject, Ward et al. (1988) observed
that pretreatment with a clinical regimen of cimetidine (3x200 mg
over a three-day period) reduced presystemic (first-pass) clearance
of a dose of 1 mg carbaryl/kg by about 46%.
A scientist studying the anthelminthic activity of carbaryl,
tested its human safety by ingesting 250 mg (about 2.8 mg/kg). After
20 min, he experienced epigastric pain and began to sweat profusely.
He was treated with a total dose of 3 mg atropine and recovered
completely 2 h after taking the carbaryl. Another scientist ingested
420 mg carbaryl (4.45 mg/kg), and after 85 min he had vision
troubles, weakness, profuse sweating, and felt lightheaded. He was
treated with a total dose of 4.8 mg atropine and recovered 4 h after
the onset of symptoms (Hayes & Laws, 1991).
9.1.3 Long-term exposure
Branch & Jacqz (1986a,b) reported the case of a 75-year-old man
who was exposed to carbaryl for 8 months, inside his home, after
repeated excessive applications of 10% dust formulation. He
experienced a series of signs and symptoms compatible with
cholinesterase depression in addition to a 40-lb (18-kg) weight
loss. After exposure ceased, the patient's condition improved
markedly. However, a few months later he started to experience
modification of his sleep pattern and peripheral neuropathy and
cerebral atrophy were demonstrated. Other pathologies, such as
recurrent gastric ulcer, cardiac fibrillations, a recent head injury
due to an automobile accident, and other less defined pathologies
were present in this patient which provide other, more likely,
causes for these later symptoms.
The uncertainties associated with long-term exposure to levels
sufficient to result in sustained suppression of plasma
pseudocholinesterase activity and possible brain damage are
discussed by Avashia (1987) and Branch (1987).
9.2 Occupational exposure
9.2.1 Epidemiological studies
The first report on workers exposed to carbaryl was published
by Best & Murray (1962). For 19 months, from the start of carbaryl
production, they studied men working on the production, handling,
and shipping of carbaryl. The most exposed group were bagging
workers occasionally exposed to carbaryl dust under abnormal
conditions (40 mg/m3). These showed a slight depression in blood
ChE activity, but this was below the rate at which clinical symptoms
might be expected. They found that 41% of 689 urine specimens
contained >1000 µg/100 ml total 1-naphthol (>400 µg/100 ml
indicates absorption), with no clinical or subjective symptoms. In
cases of acute intoxication, 3140 µg 1-naphthol/100 ml urine were
found. Knaak et al. (1965) using fluorometric analysis in
conjunction with chromatographic separation, found 5 times more
sulfate (25 mg/litre) than glucuronides (5 mg/litre) of 1-naphthol
in the urine of exposed workers.
Vandekar (1965) in a village-scale trial in Nigeria, assessed
the risk for the population of exposure to carbaryl. A slight
depression of plasma ChE was found in all spraymen, the day after
the spraying. Levels of 1-naphthol derivatives in their urine did
not increase on days 1 and 2 after spraying but increased slightly
on day 6.
In a study on 19 agricultural workers (Yakim, 1967), whole
blood ChE activity was measured before, and after, 3-4 days exposure
to airborne carbaryl. Men exposed to a mean airborne carbaryl
concentration of 2 mg/m3 showed a decrease of 20-24% in ChE
activity. Signalmen exposed to 4 mg/m3 (mean) showed a 13-30%
decrease in ChE activity. No objective signs of ill health were
observed. In the same study, a mean carbaryl concentration of
0.7 mg/m3 was reported in the cabin of an aeroplane used to apply
carbaryl, but no changes were reported in the biological parameters
of the pilots. During the agricultural application of carbaryl dust
at a maximum exposure level of 19 mg/m3 dust on cotton fields,
Adylov (1966) reported a decrease in catalase activity, and a 20%
decrease in ChE activity on day 14.
In cases of occupational overexposure to carbamates, mild
symptoms appear long before a dangerous dose is absorbed, which is
why severe occupational intoxications with carbaryl are rare. Tobin
(1970) gives reasons for the lack of severe intoxications: (1) there
is a very short time (´ h or less) between exposure to carbamates
and the onset of symptoms; (2) lack of symptoms progression, because
of a large margin between the median effective dose and the lethal
dose of carbamates and the early detection of intoxication.
Workers exposed to carbaryl used on pets for flea control,
experienced diarrhoea, increased salivation, cough, difficulty in
breathing, and phlegm (Ames et al., 1989).
Vandekar (1965) reported a skin rash in a sprayman who was
accidentally splashed with a carbaryl formulation. Although
carbamate compounds generally have not caused dermatitis or allergic
skin reactions, Vandekar suggested that they can appear in certain
individuals after unusually heavy exposure.
One out of a group of 30 farmers with contact dermatitis was
identified by a patch test as having a positive allergic reaction to
a 1% solution of carbaryl (Sharma & Kaur, 1990). No allergic
reaction were observed in a control population (No.=20).
To identify possible effects on reproduction, Whorton et al.
(1979) studied a cohort of 47 male workers who had worked for at
least 1 year in the production and packaging of carbaryl. A semen
sample was used for sperm count. Testosterone follicle-stimulating
hormone, and luteinizing hormone were determined by
radioimmunoassay. The range of airborne carbaryl concentrations in
the workplace was 0.03-14.21 mg/m3 with a mean of 4.9 mg/m3.
This cohort showed no seminal or blood abnormalities related to
carbaryl exposure. Although a small excess (not significant at
alpha=0.05) was observed in a small number of oligospermic men in
the exposed group, there was no evidence that testicular function or
fertility in the male workers was affected under these conditions of
exposure.
Wyrobek et al. (1981) used the same cohort of exposed workers
to study sperm abnormalities. Semen was collected from 50 men,
occupationally exposed to carbaryl for 1-18 years. Semen samples
were analysed for changes in sperm motility, sperm count,
morphology, and frequency of sperm-carrying double fluorescent
bodies (YFF). The YFF test represents sperm with two Y chromosomes
due to meiotic nondysjunction. The exposed workers showed changes in
sperm morphology with a higher proportion of sperm with abnormal
head shapes in comparison with the control group of newly hired
unexposed workers. There was no dose dependence as judged by job
classification. A negative correlation between number of years
working in the carbaryl area and the percentage of abnormal sperm
was observed. Workers who had once been exposed to carbaryl, but who
had not been exposed for an average of 6.3 years, showed a
marginally significant elevation in sperm abnormalities, possibly
not reversible. MacLeod (1982) reviewed the Wyrobek et al. (1981)
study and did not find any essential differences in the distribution
of the sperm types in the control and the carbaryl-exposed groups.
No significant changes in sperm count and fertility were reported in
100 workers (Thomas, 1981).
10. PREVIOUS EVALUATION BY INTERNATIONAL BODIES
The latest IARC evaluation of carbaryl was made during 1987
(IARC, 1987). It was concluded that there were no data on cancer in
humans and inadequate evidence of carcinogenicity in experimental
animals. Carbaryl could not be classified with regard to its
carcinogenicity to humans (Group 3).
The FAO/WHO Joint Meeting on Pesticide Residues (JMPR)
evaluated carbaryl at its meetings in 1963, 1965, 1966, 1967, 1968,
1969, 1970, 1971, 1973, 1975, 1976, 1977, 1979 and 1984 (FAO/WHO,
1964, 1965, 1967, 1968, 1969, 1970, 1971, 1972, 1974, 1976, 1977,
1978, 1980, 1985). Since 1973, an acceptable daily intake (ADI) of
0-0.01 mg/kg body weight has been established. This estimate is
based on the following experimental data: no-effect level for rats:
200 mg/kg in the diet = 10 mg/kg per day; for dogs: 100 mg/kg in the
diet = 1.8 mg/kg per day; and for human beings: 0.06 mg/kg per day
(FAO/WHO, 1965, 1967, 1974).
Maximum residue levels (MRLs) for carbaryl were recommended by
FAO/WHO (1986b) (see Table 55). The values recommended for tolerance
levels represent the sum of free carbaryl, combined carbaryl,
conjugated naphthol, and conjugated methylcarbaryl, expressed as
total toxic residues of carbaryl.
A WHO study group on occupational health recommended 5 mg
carbaryl/m3 as a tentative, health-based, maximum permissible
level in the working environment. A biological limit of 30%
inhibition of ChE activity in whole blood, plasma, or red cells with
respect to pre-exposure levels should not be exceeded (WHO, 1982).
In the WHO recommended classification of pesticides by hazard,
technical carbaryl is classified in Class II as moderately hazardous
in normal use (WHO, 1992). WHO/FAO (1975) issued a data sheet on
carbaryl (No. 3).
IRPTC in its series "Scientific reviews of Soviet literature on
toxicity and hazards of chemicals" has published a review on
carbaryl (IRPTC, 1982, 1989).
Table 55. Maximum Residue Limits (MRLs) established by the Codex
Alimentariusa
Commodity MRL
(mg/kg)
Animal feedstuffs (green alfalfa, clover, corn, forage, cow pea 100
foliage, grasses, peanut hay, sorghum forage, soybean vines,
sugarbeet tops, bean and pea vines)
Bran (wheat) 20
Apricots, blackberries, boysenberries, nectarines, peaches, 10
raspberries, asparagus, okra, leafy vegetables (except
brassica), nuts (whole), olives (fresh), sorghum grain, cherries,
plums, kiwi fruit
Blueberries, citrus fruit, cranberries, strawberries 7
Apples, bananas (pulp), grapes, beans, peas (including pod), 5
brassica, tomatoes, peppers, aubergines, pears, poultry skin,
barley, oats, rice (in husk and hulled), rye, wheat
Cucumbers, melons (Cantaloupe), pumpkins, squash 3
Root crop vegetables (beets, carrots, radishes, rutabagas, 2
parsnips), peanuts (ground-nuts, whole), wholemeal flour
Cottonseed (whole), sweet corn (kernels), nuts (shelled), olives 1
(processed), soybeans (dry mature seed), cow-peas
Poultry meat, eggs (without shells) 0.5
Potatoes, meat of cattle, sheep, and goats, sugarbeets, wheat 0.2
flour (white)
Milk and milk products 0.1
aFrom:FAO/WHO (1986b).
REFERENCES
Abdel-Rahman MS, Lechner DWH, & Klein KM (1985) Combination effect
of carbaryl and malathion in rats. Arch Environ Contam Toxicol, 14:
459-464.
Abrahamsen LH & Jerkofsky M (1981) Enhancement of varicella-zoster
virus replication in cultured human embryonic lung cells treated
with the pesticide carbaryl. Appl Environ Microbiol, 41(3): 652-656.
Addison JB, Silk PJ, & Unger I (1975) The photochemistry of
carbamates V. The photodecomposition of Sevin (1-naphthyl- N-methyl
carbamate) and of Phenmec (phenyl- N-methyl carbamate). Int J
Environ Anal Chem, 4: 135-140.
Adylov MA (1966) [Sanitary hygienic assessment of the working
conditions and health state of people working with Sevin in
Uzbekistan.] Gig Primen Toxicol Pestic Klin Otrav, 1866: 271-275 (in
Russian).
Ahdaya SM & Guthrie FE (1982) Stomach absorption of intubated
insecticides in fasted mice. Toxicology, 22(4): 311-317.
Ahdaya SM, Shah PV, & Guthrie FE (1976) Thermoregulation in mice
treated with parathion, carbaryl or DDT. Toxicol Appl Pharmacol, 35:
575.
Ahlrichs JL, Chandler L, Monke EJ, & Reuszler HW (1970) Effect of
pesticide residues and other organotoxicants on the quality of
surface and ground water resources. Lafayette, Indiana, Indiana
Water Research Center, 106 pp (Technical Report No. 10).
Ahmed FE, Hart RW, & Lewis NJ (1977a) Pesticide induced DNA damage
and its repair in cultured human cells. Mutat Res, 42: 161-174.
Ahmed FE, Lewis NJ, & Hart RW (1977b) Pesticide induced ouabain
resistant mutants in Chinese hamster V79 cells. Chem-Biol Interact,
19: 369-374.
Akhundov VY, Lurie LM, & Ismailova IM (1981) [Effect of Sevin on
immunological reactivity.] Gig i Sanit, 46(2): 25-28 (in Russian).
Albanis TA, Pomonis PJ, & Sdoukos AT (1986) Organophosphorous and
carbamates pesticide residues in the aquatic system of Ionnina basin
and Kalamas river (Greece). Chemosphere, 15(8): 1023-1034.
Albright LM & Simmel EC (1977) Behavioural effects of cholinesterase
inhibitor and insecticide carbaryl (Sevin). Paper presented at the
49th Annual Meeting of the Midwestern Physiological Association,
Chicago, 5-7 May 1977.
Aldridge WN (1971) The nature of the reaction of organophosphorous
compounds and carbamates with esterases. Bull World Health Organ,
44: 25.
Alvarez CC, Shimanuki H, & Argauer RJ (1970) Oral toxicity of
carbaryl to adult honey bees. J Econ Entomol, 63(6): 1834-1835.
Aly OM & El Dib MA (1971a) Studies on the persistence of some
carbamate insecticides in the aquatic environment. I. Hydrolysis of
Sevin, Baygon, Pyrolan and Dimetilan in waters. Water Res, 5:
1191-1205.
Aly OM & El Dib MA (1971b) Photodecomposition of some carbamate
insecticides in aquatic environments. In: Faust SJ & Hunter JV ed.
Proceedings of the Rudolfs Research Conference on Organic Compounds
in Aquatic Environments. New York, Basel, Marcel Dekker, Inc., pp
469-493.
Aly OM & El Dib MA (1972) Studies on the persistence of some
carbamate insecticides in the aquatic environment. In: Fate of
organic pesticides in the aquatic environment. Washington, DC,
American Chemical Society, pp 211-241 (Advances in Chemistry Series,
Volume 111).
Aly MI, Bakry N, Kishk F, & El-Sebae AH (1980) Carbaryl adsorption
on calcium-bentonite and soils. J Soil Sci Soc Am, 44(6): 1213-1215.
Amer S (1965) Cytological effects of pesticides. I. Mitotic effects
of N-methyl-1-naphthyl carbamate "Sevin". Cytologia, 30: 175-181.
Amer SM & Farah OR (1968) Cytological effects of pesticides. III.
Meiotic effects of N-methyl-1-naphthyl carbamate "Sevin".
Cytologia, 33: 337-344.
Amer SM, Hammouda MA, & Farah OR (1971) Cytological and
morphological effects of the insecticide N-methyl-1-naphthyl
carbamate "Sevin". Flora (Jena), 160: 433-439.
Ames RG, Brown SK, Rosenberg J, Jackson RJ, Stratton JW, & Quenon SG
(1989) Health symptoms and occupational exposure to flea control
product among California pet handlers. Am Ind Hyg Assoc J, 50(9):
466-472.
Andrawes NR & Bailey RH (1978a) Metabolism of carbaryl in the rat. A
reappraisal (File No. 25051). Research Triangle Park, North
Carolina, Union Carbide Corporation (Unpublished proprietary
information submitted to WHO by Rhône-Poulenc Agro, Lyon).
Andrawes NR & Bailey RH (1978b) Conjugated metabolites of carbaryl:
A comparison between rat and dog. South Charleston, West Virginia,
Union Carbide Research and Development (Project Report No. 811C20)
(Unpublished proprietary information submitted to WHO by
Rhône-Poulenc Agro, Lyon).
Andrawes NR & Bailey RH (1978c) Metabolism of carbaryl in Beagle
dogs. South Charleston, West Virginia, Union Carbide Research and
Development (Project Report No. 811C20) (Unpublished proprietary
information submitted to WHO by Rhône-Poulenc Agro, Lyon).
Andrawes NR & Bailey RH (1979) Carbaryl metabolites detected in the
urine of plant workers (File No. 25957). South Charleston, West
Virginia, Union Carbide Research and Development (Unpublished
proprietary information submitted to WHO by Rhône-Poulenc Agro,
Lyon).
Andrawes NR & Chancey EL (1970) The metabolism of 1-naphthyl-C14
carbaryl in wheat and bean plants. Clayton, North Carolina, Union
Carbide Corporation (Project Report No. 13749) (Unpublished
proprietary information submitted to WHO by Rhône-Poulenc Agro,
Lyon).
Andrawes NR & Myers WR (1976) Identification of carbaryl metabolites
in human urine (File No. 22983). South Charleston, West Virginia,
Union Carbide Corporation (Unpublished proprietary information
submitted to WHO by Rhône-Poulenc Agro, Lyon).
Andrawes NR, Chancey EL, Crabtree RJ, Herrett RA, & Weiden MHJ
(1972) Fate of naphthyl-1-14C carbaryl in laying chickens. J Agric
Food Chem, 20(3): 608-617.
André F, Gillon J, André C, Lafont S, & Jourdan G (1983)
Pesticide-containing diets augment anti-sheep red blood cell
nonreaginic antibody responses in mice but may prolong murine
infection with Giardia muris. Environ Res, 32: 145-150.
Andrianova MM & Alexeev IV (1970) [On the question of the
carcinogenic properties of the pesticides Sevin, Maneb, Ziram and
Zineb.] Vopr Pitan, 6: 71-74 (in Russian).
Anger WK & Setzer JV (1979) Effects of oral and intramuscular
carbaryl administrations on repeated chain acquisition in monkeys. J
Toxicol Environ Health, 5: 793-808.
Antonovich NA (1970) [On the residual quantities of Sevin in food
products.] Gig Primen Toxicol Pestic Klin Otrav, 1970: 176-179 (in
Russian).
Argauer RJ & Warthen JD (1975) Separation of 1- and 2-naphthols and
determination of trace amounts of 2-naphthyl methylcarbamate in
carbaryl formulations by high pressure liquid chromatography with
confirmation by spectrofluorometry. Anal Chem, 47(11): 2472-2474.
Armstrong DA & Millemann RE (1974a) Pathology of acute poisoning
with the insecticide Sevin in the bent-nosed clam, Macom nasuta. J
Invertebr Pathol, 24(2): 201-212.
Armstrong DA & Millemann RE (1974b) Effect of the pesticide carbaryl
on clams and some other intertidal mud flat animals. J Fish Res
Board Can, 31: 466-470.
Armstrong DA & Millemann RE (1974c) Effect of the insecticide Sevin
and its first hydrolytic product 1-naphthol on some developmental
stages of the bay mussel Mytilus edulis. Mar Biol, 28: 11-15.
Arunachalam S & Palanichamy S (1982) Sublethal effects of carbaryl
on surfacing behaviour and food utilization in the air breathing
fish, Macropodus cupanus. Physiol Behav, 29(1): 23-27.
Arunachalam S, Jeyalakshmi K, & Aboobucker S (1980) Toxic and
sublethal effects of carbaryl on a freshwater catfish, Mystus
vittatus (Bloch). Arch Environ Contam Toxicol, 9: 307-316.
Ashwood-Smith M, Trevino J, & Ring R (1972) Mutagenicity of
dichlorvos. Nature (Lond), 240: 418-420.
Atabaev ST (1972) [Carbamate compounds.] In: [Pesticides and hygiene
of the environment in the conditions of warm climate.] Tashkent,
Medizina, pp 114-121 (in Russian).
Attia AM, Reiter RJ, Nonaka KO, Mostafa MH, Soliman SA, & El-Sebae
AH (1991) Carbaryl-induced changes in indoleamine synthesis in the
Pineal gland and its effects on night-time serum melatonin
concentrations. Toxicology, 65: 305-314.
Avashia BH (1987) Is carbaryl safe as its reputation. Letter to the
Editor. Am J Med, 83: 1168-1169.
Azizova OM (1976) [Effect of Sevin and methyl-mercaptophos on
different levels of the nervous system.] In: Medved' LI ed. [Current
problems of the hygiene of application of pesticides in different
climatic and geographical zones. Proceedings of the Itinerary
Scientific Session of Vniigintoks, Erevan.] Kiev, Vniigintoks (in
Russian).
Bansal SK, Verma SR, Gupta AK, & Dalela RC (1980) Predicting
long-term toxicity by subacute screening of pesticides with larvae
and early juveniles of four species of freshwater major carp.
Ecotoxicol Environ Saf, 4: 224-231.
Baron RL (1968) Radioactive lactose in skim milk following
administration of carbonyl-14C-carbaryl to a lactating cow. J
Assoc Off Anal Chem, 51: 1046-1049.
Baron RL & Locke RK (1970) Utilization of cell culture techniques in
carbaryl metabolism studies. Bull Environ Contam Toxicol, 5:
287-291.
Barrett GW (1968) The effect of an acute insecticide stress on a
semi-enclosed grassland ecosystem. Ecology, 49(6): 1019-1035.
Bart J (1979) Effects of acephate and Sevin on forest birds. J Wildl
Manage, 43(2): 544-549.
Basha SM, Rao KSP, Rao KRSS, & Rao KVR (1983) Differential toxicity
of malathion, BHC, and carbaryl to the freshwater fish, Tilapia
mossambica (Peters). Bull Environ Contam Toxicol, 31(5): 543-546.
Bavari S, Casale GP, Gold RE, & Vitzthum EF (1991) Modulation of
interleukin-2 driven proliferation of human large granular
lymphocytes by carbaryl, an anticholinesterase insecticide. Fundam
Appl Toxicol, 17: 61-74.
Belonozhko GA & Kuchak JA (1969) [Electrocortical reactions to
treatment with Sevin in rats.] Gig i Sanit, 34(2): 111-113 (in
Russian).
Benard P, Cambon C, & Declume C (1979) Passage of radioactivity into
the milk of rats treated with [14C] carbaryl. Toxicol Lett, 4:
149-153.
Bend JR, Holder GM, Protos E, & Ryan AJ (1971) Water soluble
metabolites of carbaryl (1-naphthyl N-methylcarbamate) in mouse
liver preparations and in the rat. Aust J Biol Sci, 24: 535-546.
Benson BW, Scott WJ, & Beliles RP (1967) Sevin teratological study
in the mouse (Prepared by Woodard Research Corporation) (Union
Carbide proprietary report, submitted to WHO by Rhône-Poulenc Agro,
Lyon).
Beraud M, Pipy B, Derache R, & Gaillard D (1979) Formation d'un
cancérigène, le N-nitrosocarbaryl, par interaction entre un
insecticide de la série des carbamates, le carbaryl, et le nitrite
de sodium de suc gastrique de rat. Food Cosmet Toxicol, 17(6):
579-583.
Beraud M, Gaillard S, & Derache R (1980) Inhibition of two hepatic
microsomal drug-metabolizing enzymes by a carcinogenic
N-nitrosated pesticide: N-nitrosocarbaryl. Chem-Biol Interact,
31: 103-112.
Beraud M, Forgue M-F, Pipy B, & Derache P (1989a) Interaction of
carbaryl and N-nitrosocarbaryl with microsomal monooxygenase
activities. Toxicol Lett, 45(2-3): 251-260.
Beraud M, Forgue M-F, Pipy B, Didier A, & Carre P (1989b) Lipid
peroxidation in rat liver microsomes: Influence of carcinogenic
N-nitrosocarbaryl. Toxicology, 58: 299-311.
Best EM & Murray BL (1962) Observations on workers exposed to Sevin
insecticide: A preliminary report. J Occup Med, 4(10): 507-517.
Blase W & Loomis TA (1976) The uptake and metabolism of carbaryl by
isolated perfused rabbit lung. Toxicol Appl Pharmacol, 37: 481-490.
Blevins RD & Dunn WC (1975) Effects of carbaryl and dieldrin on the
growth, protein content and phospholipid content of Hela cells. J
Agric Food Chem, 23(3): 377-382.
Blevins RD, Lee M, & Regan JD (1977) Mutagenicity screening of five
methyl carbamate insecticides and their nitroso derivatives using
mutants of Salmonella typhimurium LT2. Mutat Res, 56(1): 1-6.
Bluzat R & Seuge J (1979) Effets de trois insecticides (lindane,
fenthion et carbaryl): toxicité aiguë sur quatre espèces
d'invertébrés limniques; toxicité chronique chez le mollusque
pulmoné lymnea. Environ Pollut, 18: 51-70.
Boethling RS & Alexander M (1979) Effect of concentration of organic
chemicals on their biodegradation by natural microbial communities.
Appl Environ Microbiol, 37(6): 1211-1216.
Bogacka T & Groba J (1980) [Toxicity and biodegradation of
chlorfenvinphos, carbaryl, and propoxur in water environment.]
Bromatol Chem Toksykol, 13(2): 151-158 (in Polish with English
summary).
Bogomolova ZN (1968) [Changes in the content of Sevin in fruitberry
cultures during the process of vegetation, storage and canning.] Gig
Primen Toxicol Pestic Klin Otrav, 1968: 363-367 (in Russian).
Bogomolova ZN (1970) [Content of Sevin in fruit cultures.] Gig
Primen Toxicol Pestic Klin Otrav, 1970: 197-180 (in Russian).
Bogus ER, Gallagher P, Cameron F, & Mumima R (1985) Analysis of
pesticide exposure pads using selective absorption and elution of
reversed-phase solid support. J Agric Food Chem, 33: 1018-1021.
Bollag JM (1979) Transformation of xenobiotics by microbial
activity. In: Proceedings of an EPA Workshop on Microbial
Degradation of Pollutants in Marine Environments. Gulf Breeze,
Florida, US Environmental Protection Agency, Environmental Research
Laboratory, pp 19-27 (EPA 600/9-79-012).
Bollag JM & Liu SY (1971) Degradation of Sevin by soil
microorganisms. Soil Biol Biochem, 3(4): 337-345.
Bollag JM & Liu SY (1972a) Fungal degradation of 1-naphthol. Can J
Microbiol, 18(7): 1113-1117.
Bollag JM & Liu SY (1972b) Hydroxylations of carbaryl by soil fungi.
Nature (Lond), 236: 177-178.
Bollag JM, Czaplicki EJ, & Minard RD (1975) Bacterial metabolism of
1-naphthol. J Agric Food Chem, 23(1): 85-90.
Bollag JM, Sjoblad RD, Czaplicki EJ, & Hoeppel RE (1976)
Transformation of 1-naphthol by the culture filtrate of Rhizoctonia
praticola. Soil Biol Biochem, 8: 7-11.
Bollag JM, Sjoblad RD, & Liu S-Y (1978) Characterization of an
enzyme from Rhizoctonia praticola which polymerizes phenolic
compounds. Can J Microbiol, 25: 229-233.
Borzelleca J & Skalsky HL (1980) The excretion of pesticides in
saliva and its value in assessing exposure. J Environ Sci Health,
B13(6): 843-866.
Bowman BT & Sans WW (1985) Effect of temperature on the water
solubility of insecticides. J Environ Sci Health, B20(6): 625-631.
Bowman MC, Oller W, Kendall D, Dosnell A, & Oliver K (1982) Stressed
bioassay systems for rapid screening of pesticide residues. Part II.
Determination of foliar residues for safe re-entry of agricultural
workers into the field. Arch Environ Contam Toxicol, 11: 447-455.
Boyd EM & Boulanger MA (1968) Insecticide toxicology. Augmented
susceptibility to carbaryl toxicity in albino rats fed purified
casein diets. J Agric Food Chem, 16: 834-838.
Boyd EM & Krijnen CJ (1969) The influence of protein intake on acute
oral toxicity of carbaryl. J Clin Pharmacol, 9(5): 292-297.
Brahmaprakash GP & Sethunathan N (1984) Mineralization of carbaryl
and carbofuran in soil planted to rice. Rice Newsl, 5(3-4): 2.
Brahmaprakash GP & Sethunathan N (1985) Metabolism of carbaryl and
carbofuran in soil planted to rice. Agric Ecosyst Environ, 13(1):
33-42.
Branch RA (1987) The reply. Letter to the Editor. Am J Med, 83:
1169.
Branch RA & Jacqz E (1986a) Is carbaryl as safe as its reputation?
Does it have the potential for causing chronic neurotoxicity in
humans? Am J Med, 80: 659-664.
Branch RA & Jacqz E (1986b) Subacute neurotoxicity following
long-term exposure to carbaryl. Am J Med, 80: 741-745.
Brankovan O (1972) Meiotic effects in corn in embryonic and
generative stages induced by Sevin 50. Arhiv Poljopr Nauke, 90:
125-132.
Briggs GG (1981) Adsorption of pesticides by some Australian soils.
Aust J Soil Res, 19: 61-68.
Brzeskii VV (1972) The study of the mutagenic properties of an
insecticide from the carbamate group - Sevin. Sov Genet, 8: 798-800.
Buchanan DV, Millemann RE, & Stewart NE (1970) Effects of the
insecticide Sevin on various stages of the dungeness crab, Cancer
magister. J Fish Res Board Can, 27: 93-104.
Bukin AL (1965) [Toxicity of Sevin for mammalia and birds.] Gig
Primen Toxicol Pestic Klin Otrav, 1965: 431-435 (in Russian).
Bukin AL & Filatov GV (1965) [Sevin toxicity for mammalia and
birds.] Veterinaria, 42: 93-95 (in Russian).
Burdick GE, Dean HJ, & Harris EJ (1960) Effect of Sevin upon the
aquatic environment. NY Fish Game J, 7(1): 14-25.
Bursian SJ & Edens FW (1977) The prolonged exposure of Japanese
quail to carbaryl and its effect on growth and reproductive
parameters. Bull Environ Contamin Toxicol, 17(3): 360-368.
Bursian SJ & Edens FW (1978) The effect of acute carbaryl
administration on various neurochemical and blood chemical
parameters in the Japanese quail. Toxicol Appl Pharmacol, 46:
463-473.
Bushy Run (1983a) Acute toxicity and irritancy study. Export,
Pennsylvania, Bushy Run Research Center (Project Report No. 46-96)
(Unpublished proprietary information submitted to WHO by
Rhône-Poulenc Agro, Lyon).
Bushy Run (1983b) Sevin 99% technical: Acute toxicity and irritancy
study. Export, Pennsylvania, Bushy Run Research Center (Project
Report No. 46-71) (Unpublished proprietary information submitted to
WHO by Rhône-Poulenc Agro, Lyon).
Bushy Run (1983c) Sevin 80 sprayable. Acute toxicity and irritancy
study. Export, Pennsylvania, Bushy Run Research Center (Project
Report No. 46-97) (Unpublished proprietary information submitted to
WHO by Rhône-Poulenc Agro, Lyon).
Butler PA (1962) Effects on commercial fisheries. In: Effects of
pesticides on fish and wildlife: A review of investigations during
1960. Washington, DC, US Department of the Interior, Fish and
Wildlife Service, pp 11-25 (Circular No. 143).
Butler PA (1963) Commercial fisheries investigations. In:
Pesticide-wildlife studies: A review of Fish and Wildlife Service
investigations during 1961 and 1962. Washington, DC, US Department
of the Interior, Fish and Wildlife Service, pp 11-25 (Circular No.
167).
Cairns T, Siegmund GA, & Doose MG (1983) Potential identification
problems with carbaryl. Bull Environ Contam Toxicol, 30: 93-98.
Calabrese EJ & Geiger CP (1986) Low erythrocyte glusose-6-phosphate
dehydrogenase (G-6-PD) activity and susceptibility to carbaryl
induced methaemoglobin formation and glutathion depletion. Bull
Environ Contam Toxicol, 36(4): 506-509.
Cambon C, Declume Ch, & Derache R (1978) Inhibitions of
acetylcholinesterase from foetal and maternal tissues after oral
intake of carbaryl (1-naphthyl- N-methyl carbamate) by pregnant
rats. Biochem Pharmacol, 27: 2647-2648.
Cambon C, Fernandez J, Falzon M, & Mitjavila S (1981) Variations of
digestive absorption kinetics of carbaryl with the nature of the
vehicle. Toxicology, 22(1): 45-51.
Cameron EA & Mastro VC (1976) Forest insect and disease management /
Evaluation Report: Effectiveness of disparlure in combination with
Sevin 4 oil for Gipsy moth suppression in Pennsylvania 1974. Upper
Darby, Pennsylvania, US Department of Agriculture, Northeastern Area
State and Private Forestry Service.
Cameron EA, Loerch CR, & Mumma RO (1985) Incidental and indirect
exposure to three chemical insecticides used for control of the
Gypsy moth, Lymantria Dispar (L.). Z Angew Entomol, 99: 241-248.
Carey AE & Kutz FW (1985) Trends in ambient concentrations of
agrochemicals in humans and the environment in USA. Environ Monit
Assess, 5(2): 155-164.
Carlson AR (1972) Effects of long-term exposure to carbaryl (Sevin)
on survival, growth and reproduction of the fathead minnow
(Pimephales promelas). J Fish Res Board Can, 29(5): 583-587.
Caro HJ, Freeman HP, & Turner BC (1974) Persistence in soil and
losses in run off of soil incorporated carbaryl in a small
watershed. J Agric Food Chem, 22(5): 860-863.
Carpenter M (1990) Hydrolysis of 14C-carbaryl in aqueous solutions
buffered at pH 5, 7, and 9. Columbia, Missouri, Analytical
Bio-Chemistry Laboratories, Inc. (ABC final report No. 38380)
(Unpublished proprietary information submitted to WHO by
Rhône-Poulenc Agro, Lyon).
Carpenter CP, Weil CW, Palm PH, Woodside MW, Nair JH III, & Smith HF
(1961) Mammalian toxicity of 1-naphthyl- N-methyl carbamate (Sevin
insecticide). J Agric Food Chem, 9: 30-39.
Casale GP, Bavari S, & Connolly JJ (1989) Inhibition of human serum
complement activity by diisopropylfluorophosphate and selected
anticholinesterase insecticides. Fundam Appl Toxicol, 12(3):
460-468.
Casida JE (1970) Mixed-function oxidase involvement in the
biochemistry of insecticide synergists. J Agric Food Chem, 18: 753.
Casida JE & Augustinsson KB (1959) Reaction of plasma albumin with
1-naphthyl- N-methylcarbamate and certain other esters. Biochim
Biophys Acta, 35: 411-426.
Casper HH, Pekas JC, & Dinusson WE (1973) Gastric absorption of a
pesticide (1-naphthyl N-methylcarbamate) in the fasted rat. Pestic
Biochem Physiol, 2: 391-396.
Chaiyarach S, Ratananun V, & Harrel RC (1975) Acute toxicity of the
insecticides toxaphene and carbaryl and the herbicides propanil and
molinate to four species of aquatic organisms. Bull Environ Contam
Toxicol, 14(3): 281-284.
Chancey EL (1974) Sevin carbaryl insecticide: metabolism of carbaryl
in apple fruit (File No. 19496). Clayton, North Carolina, Union
Carbide Corporation (Unpublished proprietary information submitted
to WHO by Rhône-Poulenc Agro, Lyon).
Chancey EL & Andrawes NR (1971a) The absorption and metabolism of
1-naphthyl-C14 carbaryl in corn and rice plants. Clayton, North
Carolina, Union Carbide Corporation (Project Report No. 15712)
(Unpublished proprietary information submitted to WHO by
Rhône-Poulenc Agro, Lyon).
Chancey EL & Andrawes NR (1971b) Metabolism and translocation of
1-naphthyl-C14 carbaryl in tomato fruit and foliage and potato
foliage. Clayton, North Carolina, Union Carbide Corporation (Project
Report No. 15715) (Unpublished proprietary information submitted to
WHO by Rhône-Poulenc Agro, Lyon).
Chapman RA & Cole CM (1982) Observations on the influence of water
and soil pH on the persistence of insecticides. J Environ Sci
Health, B17(5): 487-504.
Chaudhry GR & Wheeler WB (1988) Biodegradation of carbamates. Water
Sci Technol, 20(11/12): 89-94.
Chernoff N & Kavlock RJ (1982) An in vivo teratology screen
utilizing pregnant mice. J Toxicol Environ Health, 10: 541-550.
Chernoff N, Setzer RW, Miller DB, Rosen MB, & Rogers JM (1990)
Effects of chemically induced maternal toxicity on prenatal
development in the rat. Teratology, 42: 651-658.
Chib JS (1985a) The adsorption/desorption of 1-naphthyl
methylcarbamate on five soils (File No. 33820). Research Triangle
Park, North Carolina, Union Carbide Agricultural Products Co., Inc.
(Unpublished proprietary information submitted to WHO by
Rhône-Poulenc Agro, Lyon).
Chib JS (1985b) Sevin: Aerobic aquatic metabolism of carbaryl (File
No. 34234). Research Triangle Park, North Carolina, Union Carbide
Agricultural Products Co., Inc. (Unpublished proprietary information
submitted to WHO by Rhône-Poulenc Agro, Lyon).
Chib JS (1985c) Sevin (1-naphthyl methylcarbamate): Anaerobic
aquatic metabolism of carbaryl (File No. 34086). Research Triangle
Park, North Carolina, Union Carbide Agricultural Products Co., Inc.
(Unpublished proprietary information submitted to WHO by
Rhône-Poulenc Agro, Lyon).
Chib JS (1986a) Sevin brand carbaryl insecticide. Bioconcentration
and fate of carbaryl in bluegill sunfish (Lepomis macrochirus).
Research Triangle Park, North Carolina, Union Carbide Corporation
(Report No. 801R10) (Unpublished proprietary information submitted
to WHO by Rhône-Poulenc Agro, Lyon).
Chib JS (1986b) Sevin brand carbaryl insecticide: Photodegradation
of carbaryl on soil (File No. 34598). Research Triangle Park, North
Carolina, Union Carbide Agricultural Products Co., Inc. (Unpublished
proprietary information submitted to WHO by Rhône-Poulenc Agro,
Lyon).
Chib JS & Andrawes NR (1985) Carbaryl (1-naphthyl methylcarbamate) -
Soil mobility study (File No. 33770). Research Triangle Park, North
Carolina, Union Carbide Agricultural Products Co., Inc. (Unpublished
proprietary information submitted to WHO by Rhône-Poulenc Agro,
Lyon).
Chib JS, Noeth SP, & Smith BK (1986) Sevin brand carbaryl
insecticide. Bioconcentration and fate of carbaryl in Bluegill
Sunfish (Lepomis Macrochirus) (File No 34540). Research Triangle
Park, North Carolina, Union Carbide Agricultural Products Co., Inc.,
Research and Development Department (Unpublished proprietary
information submitted to WHO by Rhône-Poulenc Agro, Lyon).
Chin YN & Sudderuddin KI (1979) Effect of methamidophos on the
growth rate and esterase activity of the common carp Cyprinus
carpio L. Environ Pollut, 18(3): 213-220.
Chin BH, Eldridge JM, & Sullivan LJ (1974) Metabolism of carbaryl by
selected tissues using an organ-maintenance technique. Clin Toxicol,
7: 37-56.
Chin BH, Sullivan LJ, Eldridge JM, & Tallant J (1979a) Metabolism of
carbaryl by kidney, liver, and lung from human postembryonic fetal
autopsy tissue. Clin Toxicol, 14(5): 489-498.
Chin BH, Eldridge JM, Anderson JH, & Sullivan LJ (1979b) Carbaryl
metabolism in the rat. A comparison of in vivo, in vitro (tissue
explant), and liver perfusion techniques. J Agric Food Chem, 27(4):
716-720.
Christenson I (1964) Alkaline hydrolysis of some carbamic esters.
Acta Chem Scand, 18: 904-922.
Cifone MA (1989) Mutagenicity test on carbaryl technical in the in
vitro rat primary hepatocyte unscheduled DNA synthesis assay.
Final report (HLA Study No. 10862-0-435). Kensington, Maryland,
Hazelton Laboratories America, Inc. (Unpublished proprietary
information submitted to WHO by Rhône-Poulenc Agro, Lyon).
Clayson DB (1987) The need for biological risk assessment in
reaching decisions about carcinogens. Mutat Res, 185(3): 243-269.
Collins TFX, Hansen WH, & Keeler HV (1971) The effect of carbaryl
(Sevin) on reproduction of the rat and the gerbil. Toxicol Appl
Pharmacol, 19: 202-216.
Comer SW, Staiff DC, Armstrong JF, & Wolf HR (1975) Exposure of
workers to carbaryl. Bull Environ Contam Toxicol, 13(4): 385-391.
Coulston F, Rosenblum I, & Dougherty WJ (1974) Teratogenic
evaluation of carbaryl in the Rhesus monkey (Macacca mulatta).
Holloman Air Force Base, New Mexico, International Center of
Environmental Safety, Albany Medical College, 54 pp (Unpublished
proprietary information submitted to WHO by Rhône-Poulenc Agro,
Lyon).
Courtemanch DL & Gibbs KE (1978) The effects of Sevin-4-oil on
lenthic communities. In: Environmental monitoring of cooperative
spruce budworm control projects, Maine 1976 and 1977. Augusta,
Maine, Maine Department of Conservation, Forest Service, pp 141-150.
Courtemanch DL & Gibbs KE (1980) Short- and long-term effects of
forest spraying of carbaryl (Sevin-4-oil) on stream invertebrates.
Can Entomol, 112: 271-276.
Coutant CC (1964) Insecticide Sevin: Effect of aerial spraying on
drift of stream insects. Science, 146: 420-421.
Cranmer MF (1986) Carbaryl. A toxicological review and risk
analysis. Neurotoxicology, 7(1): 247-332.
Cress CR & Strother A (1974) Effects on drug metabolism of carbaryl
and 1-naphthol in the mouse. Life Sci, 14: 861-872.
Crofton KM, Howard JL, Moser VC, Gill MW, Reiter LW, Tilson HA, &
MacPhail RC (1991) Interlaboratory comparison of motor activity
experiments: Implications for neurotoxicological assessments.
Neurotoxicol Teratol, 13: 599-609.
Crosby DG, Leitis E, & Winterlin WL (1965) Photodecomposition of
carbamate insecticides. J Agric Food Chem, 13(3): 204-205.
Currier WW, MacCollom GB, & Baumann GI (1982) Drift residues of
air-applied carbaryl in an orchard environment. J Econ Entomol,
75(6): 1062-1068.
Czaplicki E (1979) Persistence in the soil of some more important
insecticides applied in Poland. I. Dynamics of insecticide
disappearance in the soil. Pr Nauk Inst Ochr Rosl, 21(2): 7-40.
Czaplicki EJ & Bollag JM (1975) Microbial degradation of 1-naphthol.
Biul Inst Ochr Rosl, 59: 241-248.
Das YT (1990a) Photodegradation of (1-naphthyl-14C) carbaryl in
aqueous solution buffered at pH 5 under artificial sunlight.
Piscataway, New Jersey, Innovative Scientific Services, Inc. (Report
No. 90060) (Unpublished proprietary information submitted to WHO by
Rhône-Poulenc Agro, Lyon).
Das YT (1990b) Photodegradation of (1-naphthyl-14C) carbaryl on
soil under artificial sunlight. Piscataway, New Jersey, Innovative
Scientific Services, Inc. (Report No. 90061) (Unpublished
proprietary information submitted to WHO by Rhône-Poulenc Agro,
Lyon).
Davies JI & Evans WC (1964) Oxidative metabolism of naphthalene by
soil Pseudomonads. The ring-fission mechanism. Biochem J, 91:
251-261.
Davis HC (1961) Effects of some pesticides on eggs and larvae of
oysters (Crassostrea virginica) and clams (Venus mercenaria).
Commer Fish Rev, 23(12): 8-23.
Davis CW (1986a) Sevin brand carbaryl insecticide: Dissipation of
soil residues under field use conditions (File No. 34620). Research
Triangle Park, North Carolina, Union Carbide Agricultural Products
Co., Inc. (Unpublished proprietary information submitted to WHO by
Rhône-Poulenc Agro, Lyon).
Davis CW (1986b) Sevin brand carbaryl insecticide: Magnitude of
carbaryl residues on rangeland and pasture grasses (File No. 34821).
Research Triangle Park, North Carolina, Union Carbide Agricultural
Products Co., Inc. (Unpublished proprietary information submitted to
WHO by Rhône-Poulenc Agro, Lyon).
Davis CW (1987) Sevin brand carbaryl insecticide: Comparison of the
magnitude of carbaryl residues resulting from applications of XLR
plus and 50 W formulations to tree fruit, leafy vegetables, and
cereals (File No. 35357). Research Triangle Park, North Carolina,
Union Carbide Agricultural Products Co., Inc. (Unpublished
proprietary information submitted to WHO by Rhône-Poulenc Agro,
Lyon).
Davis CW & Thomas SJ (1987) Carbaryl insecticide. Section D-Residues
barley (File No. 40092). Research Triangle Park, North Carolina,
Union Carbide Agricultural Products Co., Inc. (Unpublished
proprietary information submitted to WHO by Rhône-Poulenc Agro,
Lyon).
Declume C & Benard P (1977a) Foetal accumulation of [14C] carbaryl
in rats and mice autoradiographic study. Toxicology, 8: 95-105.
Declume C & Benard P (1977b) Etude autoradiographique de la
distribution d'un agent anticholinestérasique, le 1-naphthyl-
N-methyl [14C] carbamate chez la ratte gestante. Toxicol Appl
Pharmacol, 39: 451-460.
Declume C & Benard P (1978) Etude de la pharmacocinétique du
14C-carbaryl chez la souris gestante. Toxicol Eur Res, 1: 174-180.
Declume C & Derache M (1976) Biodisponibilité du 14C-carbaryl
après l'administration oral à la ratte gestante. C R Acad Sci Paris,
D283: 1799-1801.
Declume C & Derache M (1977) Passage placentaire d'un carbamate
anticholinestérasique à activité insecticide: Le carbaryl.
Chemosphere, 6: 141-146.
Declume C, Cambon C, & Derache R (1979) The effects on new-born rats
of repeated carbaryl administration during gestation. Toxicol Lett,
3: 191-196.
Degraeve N, Moutschen-Dahmen M, Houbrechts N, & Colizzi A (1976) A
propos des risques d'un insecticide: le carbaryl utilisé seul et en
combinaison avec les nitrites. Bull Soc R Sci Liège, 45(1-2): 46-57.
DeGiovanni-Donnelly R, Kolbye SM, & Greeves PD (1968) The effect of
IPC, CIPC, Sevin and Zectran on Bacillus subtilis. Experientia
(Basel), 24: 80-81.
Desi I, Gonczi L, Simon G, Farkas I, & Kneffel Z (1974)
Neurotoxicological studies of two carbamate pesticides in subacute
animal experiments. Toxicol Appl Pharmacol, 27: 456-476.
Deuel LE, Brown KW, Price JD, & Turner FT (1985) Dissipation of
carbaryl and the 1-naphthol metabolite in the flooded rice fields. J
Environ Qual, 14(3): 349-354.
Deutsch-Wenzel P, Brune H, Grimmer G, & Misfeld J (1985) Local
application to mouse skin as a carcinogen specific test system for
non-volatile nitroso compounds. Can Lett, 29: 85-92.
Dickoff DJ, Gerber O, & Turovsky Z (1987) Delayed neurotoxicity
after ingestion of carbamate pesticide. Neurology, 37(7): 1229-1231.
Dieringer CS & Thomas JA (1974) Effects of carbaryl on the
metabolism of androgens in the prostate and liver of the mouse.
Environ Res, 7: 381-386.
Dikshith TSS (1991) Mutagenicity and teratogenicity of pesticides.
In: Dikshith TSS ed. Toxicology of pesticides in animals. Boston,
Boca Raton, Ann Arbor, CRC Press, pp 189-198.
Dikshith TSS, Gupta PK, Gaur JS, Datta KK, & Mathur AK (1976)
Ninety-day toxicity of carbaryl in male rats. Environ Res, 12:
161-170.
Dikshith TSS, Kumar SN, Raizada RB, Srivastava MK, & Ray PK (1990)
Residues of 1-naphthol in soil and water samples in and around
Bhopal, India. Bull Environ Contam Toxicol, 44: 87-91.
Dinerman AA, Lavrent'eva NA, & Il'inskaia NA (1970) [The embryotoxic
action of some pesticides.] Gig i Sanit, 35: 39-42 (in Russian).
Dinoeva SK (1974) [Dynamics of the changes in the immunological
structures of lymphatic follicles in spleen during pesticide
poisoning.] Gig i Sanit, 39(3): 85-87 (in Russian).
Dinoeva S (1982) Humoral immune response in 1st, 2nd and 3rd
generations guinea-pigs in the period of reactivation after
intoxication. In: Immunology of reproduction. Proceedings of the
Fifth International Symposium, Varna, Bulgaria, 26-29 May 1982.
Sofia, Bulgarian Academy of Sciences Press, pp 393-395.
Dionne E, Kendall TB, & Suprenant D (1985) Acute toxicity of
carbaryl technical to embryos-larvae of Eastern oysters
(Crassostrea virginica). Wareham, Massachusetts, Springborn
Bionomics, Inc. (Report No. BW-85-7-1817) (Unpublished proprietary
information submitted to WHO by Rhône-Poulenc Agro, Lyon).
Dittert LW & Higuchi T (1963) Rates of hydrolysis of carbamate and
carbonate esters in alkaline solution. J Pharm Sci, 52: 852-857.
Dorough HW (1967) Carbaryl-C14 metabolism in a lactating cow. J
Agric Food Chem, 15(2): 261-266.
Dorough HW (1969) Carbaryl cow study No. 2. Lyon, Rhône-Poulenc Agro
(Unpublished proprietary information).
Dorough HW (1970a) Continuous feeding of carbaryl naphthyl-C14 to
lactating cows. Progress report. Lyon, Rhône-Poulenc Agro (Document
No. 25, Section C, Part III) (Unpublished proprietary information).
Dorough HW (1970b) Metabolism of insecticidal methylcarbamates in
animals. J Agric Food Chem, 18(6): 1015-1022.
Dorough HW (1971) Carbaryl residues in milk and meat of dairy
animals. Presented at the International Symposium on Pesticide
Terminal Residues, Tel-Aviv, Israel, 17-19 February 1971 (Document
No. 27, Section C, Part III).
Dorough HW (1973) Metabolism of carbamate insecticides. Washington,
DC, US Environmental Protection Agency, Office of Research and
Development, 246 pp (EPA-650/1-74-002).
Dorough HW (1982) Inhalation toxicology: Starting with the
environment. Environ Toxicol Chem, 1(3): 213-220.
Dorough HW & Casida JH ( 1964) Nature of certain carbamate
metabolites of the insecticide Sevin. J Agric Food Chem, 12(4):
294-304.
Dorough HW, Leeling NL, & Casida JE (1963) Nonhydrolytic pathway in
metabolism of N-methylcarbamate insecticides. Science, 140:
170-171.
Dougherty WJ, Goldberg L, & Coulston F (1971) The effect of carbaryl
on reproduction in the monkey (Macacca mulatta). Toxicol Appl
Pharmacol, 19: 365.
Duck BJ & Woolias M (1985) Reverse-phase high performance liquid
chromatographic determination of carbaryl in postmortem specimens. J
Anal Toxicol, 9: 177-179.
Duggan RE & Corneliussen PE (1972) Dietary intake of pesticide
chemicals in the United States (III). June 1968-April 1970. Pestic
Monit J, 5(4): 331-341.
Duggan RE, Lipscomb GQ, Cox EL, Heatwole RE, & Kling RC (1971)
Residues in food and feed. Pesticide residue levels in foods in the
United States from July 1, 1963, to June 30, 1969. Pestic Monit J,
5(2): 73-124.
Durham WF & Wolfe HR (1962) Measurements of the exposure of workers
to pesticides. Bull World Health Organ, 26: 75-91.
Dyadicheva TV (1971) [Experimental data on thyroid and adrenal
cortex function during prolonged effect of carbamine pesticides.]
Vrach Delo, 2: 120-123 (in Russian).
Dzwonkowska A & Hubner H (1986) Induction of chromosomal aberrations
in the Syrian hamster by insecticides tested in vivo. Arch
Toxicol, 58(3): 152-156.
Earl FL, Miller E, & Van Loon EJ (1973) Reproductive teratogenic and
neonatal effects of some pesticides and related compounds in beagle
dogs and miniature swine. In: Deichman W ed. Pesticides and the
environment: A continuing controversy. Proceedings of the 8th
Inter-American Conference on Toxicology and Occupational Medicine,
Miami, Florida, pp 253-266.
Edmiston CE Jr, Goheen M, Malaney GW, & Mills WL (1985) Evaluation
of carbamate toxicity: Acute toxicity in a culture of Paramecium
multimicronucleatum upon exposure to aldicarb, carbaryl, and
mexacarbate as measured by Warburg respirometry and acute plate
assay. Environ Res, 36(2): 338-350.
Egert G & Greim H (1976) Formation of mutagenic N-nitroso
compounds from the pesticides prometryne, dodine and carbaryl in the
presence of nitrite at ph 1. Mutat Res, 37: 179-186.
Eheart JF, Turner EC, & Dickinson J (1962) Residues of Sevin in
whole milk from sprayed and dusted cows. J Entomol, 55(4): 504-505.
Eichelberger J & Lichtenberg J (1971) Persistence of pesticides in
river water. Environ Sci Technol, 5: 541-544.
Eisenbrand G, Ungerer O, & Preussmann R (1975) The reaction of
nitrite with pesticides. II. Formation, chemical properties and
carcinogenic activity of the N-nitroso derivatives of N-methyl-
1-naphthyl carbamate (carbaryl). Food Cosmet Toxicol, 13: 365-367.
Eisenbrand G, Schmähl D, & Preussmann R (1976) Carcinogenicity in
rats of high oral doses of N-nitrosocarbaryl, a nitrosated
pesticide. Can Lett, 1: 281-284.
Elespuru RK & Lijinsky W (1973) The formation of carcinogenic
nitroso compounds from nitrite and some types of agricultural
chemicals. Food Cosmet Toxicol, 11: 807-817.
Elespuru R, Lijinsky W, & Setlow JK (1974) Nitrosocarbaryl as a
potent mutagene of environmental significance. Nature (Lond), 247:
386-387.
Elkins ER (1989) Effect of commercial processing on pesticide
residues in selected fruits and vegetables. J Assoc Off Anal Chem,
72(3): 533-535.
Elkins ER, Lamb FC, Farrow RP, Cook RW, Kawai M, & Kimball JR (1968)
Removal of DDT, malathion and carbaryl from green beans from
commercial and home preparative procedures. J Agric Food Chem,
16(6): 962-966.
Elliot-Feeley E & Armstrong JR (1982) Effects of fenitrothion and
carbaryl on Xenopus laevis development. Toxicology, 22(4):
319-335.
Epstein SS, Arnold E, Andrea J, Bass W, & Bishop Y (1972) Detection
of chemical mutagens by the dominant lethal assay in the mouse.
Toxicol Appl Pharmacol, 23: 288-325.
Estesen BJ, Buck NA, & Ware GW (1982) Dislodgeable insecticide
residues on cotton foliage: carbaryl, cypermethrin, and
methamidophos. Bull Environ Contam Toxicol, 28(4): 490-493.
Eto M, Seifert J, Engel J, & Casida J (1980) Organophosphorous and
methylcarbamate teratogens: Structural requirements for reducing
embryonic abnormalities in chickens and kynurenine formamidase
inhibition in mouse liver. Toxicol Appl Pharmacol, 54: 20-30.
Eto M, Kuwano E, Yoshikawa M, Suiko M, Kohno M, Kamihata M, &
Nakatsu S (1982) Mutagenicity and anticholinesterase activity of
possible metabolites of aryl N-methylcarbamates. J Fac Agric
Kyushu Univ, 26(4): 213-219.
Fait DW (1984) Sevin XLR acute inhalation toxicity test. Export,
Pennsylvania, Bushy Run Research Center (Report No. 47-84)
(Unpublished proprietary information submitted to WHO by
Rhône-Poulenc Agro, Lyon).
Falzon M, Fernandez Y, Cambon C, Gros C, & Mitjavila S (1983)
Influence of experimental hepatic impairment on the toxicokinetics
and the anticholinesterase activity of carbaryl in the rat. J Appl
Toxicol, 3(2): 87-89.
FAO (1982) Second Government Consultation on International
Harmonization of Pesticides Registration Requirements, Rome, 11-15
October 1982. Rome, Food and Agriculture Organization of the United
Nations.
FAO/WHO (1964) Carbaryl. In: Evaluation of the toxicity of
pesticides residues in food. Report of a Joint Meeting of the FAO
Committee on Pesticides in Agriculture and the WHO Expert Committee
on Pesticide Residues, Geneva, 30 September-7 October 1963. Geneva,
World Health Organization, pp 132-135 (FAO Meeting Report No.
PL:1963/13; WHO/Food Add./23).
FAO/WHO (1965) Carbaryl. In: Evaluation of the toxicity of pesticide
residues in food. Geneva, World Health Organization, pp 31-35 (FAO
Meeting Report No. PL:1965/10/1; WHO Food Add./27.65).
FAO/WHO (1967) Carbaryl. In: 1966 Evaluation of some pesticide
residues in food. Geneva, World Health Organization, pp 31-38 (FAO
PL/CP/15; WHO Food Add./67.32)
FAO/WHO (1968) Carbaryl. In: 1967 Evaluation of some pesticide
residues in food. Geneva, World Health Organization, pp 15-25 (FAO
Report No. PL: 1967/M/11/1; WHO Food Add./68.30).
FAO/WHO (1969) Carbaryl. In: 1968 Evaluation of some pesticide
residues in food. Geneva, World Health Organization, pp 35-38 (FAO
PL:1968/M/9/1; WHO Food Add./69.35).
FAO/WHO (1970) Carbaryl. In: 1969 Evaluations of some pesticide
residues in food. Geneva, World Health Organization, pp 45-57 (FAO
PL:1969/M/17/1; WHO Food Add./70.38).
FAO/WHO (1971) Carbaryl. In: 1970 Evaluations of some pesticide
residues in food. Geneva, World Health Organization, pp 3-6 (AGP
1979/M/12/1; WHO Food Add./71.42).
FAO/WHO (1972) 1971 Evaluations of some pesticide residues in food.
Geneva, World Health Organization (WHO Pesticide Residue Series No.
1).
FAO/WHO (1974) Carbaryl. In: 1973 Evaluations of some pesticide
residues in food. Geneva, World Health Organization, pp 141-176 (WHO
Pesticide Residue Series No. 3).
FAO/WHO (1976) Carbaryl. In: 1975 Evaluations of some pesticide
residues in food. Geneva, World Health Organization, pp 83-84 (WHO
Pesticide Residue Series No. 5).
FAO/WHO (1977) Carbaryl. In: 1976 Evaluations of some pesticide
residues in food. Rome, Food and Agriculture Organization of the
United Nations, pp 95-123 (AGP 1977/M/14).
FAO/WHO (1978) Carbaryl. In: Pesticide residues in food. Evaluations
1977. Rome, Food and Agriculture Organization of the United Nations,
pp 47-50 (FAO Plant Production and Protection Paper 10 Sup).
FAO/WHO (1980) Carbaryl. In: Pesticide residues in food. Evaluations
1979. Rome, Food and Agriculture Organization of the United Nations,
pp 101-102 (FAO Plant Production and Protection Paper 20 Sup).
FAO/WHO (1985) Carbaryl. In: Pesticide residues in food - 1984. The
monographs. Rome, Food and Agriculture Organization of the United
Nations, pp 79-83 (FAO Plant Production and Protection Paper 67).
FAO/WHO (1986a) Guide to Codex recommendations concerning pesticide
residues. Part 8. Recommendations for methods of analysis of
pesticide residues, 3rd ed. Rome, Food and Agriculture Organization
of the United Nations, Codex Alimentarius Commission.
FAO/WHO (1986b) Codex maximum limits for pesticide residues, 2nd ed.
Rome, Food and Agriculture Organization of the United Nations, Codex
Alimentarius Commission (CAC/Vol. XIII).
Farage-Elawar M (1989) Enzyme and behavioural changes in young
chicks as a result of carbaryl treatment. J Toxicol Environ Health,
26(1): 119-131.
Farage-Elawar M (1990) Effects of in ovo injection of carbamates
on chick embryo hatchability, esterase enzyme activity and
locomotion of chicks. J Appl Toxicol, 10(3): 197-201.
Farago A (1969) [Fatal suicidal case of Sevin (1-naphthol- N-methyl
carbamate) poisoning.] Arch Toxicol, 24: 309-315 (in German).
Farrow RP, Lamb FC, Cook RW, Kimball JR, & Elkins ER (1968) Removal
of DDT, malathion and carbaryl from tomatoes by commercial and home
preparative methods. J Agric Food Chem, 16(1): 65-71.
Farrow RP, Lamb FC, Elkins ER, Cook RW, Kawai M, & Cortes A (1969)
Effect of commercial and home preparative procedures on parathion
and carbaryl residues in broccoli. J Agric Food Chem, 17(1): 75-79.
Federle PF & Collins WJ (1976) Insecticide toxicity to three insects
from Ohio ponds. Ohio J Sci, 76(1): 19-24.
Feldmann RJ & Maibach HT (1974) Percutaneous penetration of some
pesticides and herbicides in man. Toxicol Appl Pharmacol, 28:
126-132.
Fernandez Y, Falzon M, Cambon C, Gros C, & Mitjavila S (1982)
Carbaryl tricompartmental toxicokinetics and anticholinesterase
activity. Toxicol Lett, 13(3-4): 253-258.
Ferreira GAL & Seiber JN (1981) Volatilization and exudation losses
of 3- N methylcarbamate insecticides applied systemically to rice.
J Agric Food Chem, 29(1): 93-99.
Field WE (1980a) Oral LD50 in rats of carbaryl, technical grade,
made in West Virginia. Clarks Summit, Pennsylvania, Chemicals, Drugs
and Cosmetics Research (Report No. CDC-UC-046-79) (Unpublished
proprietary information submitted to WHO by Rhône-Poulenc Agro,
Lyon).
Field WE (1980b) Oral LD50 in rats of carbaryl, technical grade,
made in India. Clarks Summit, Pennsylvania, Chemicals, Drugs and
Cosmetics Research (Report No. CDC-UC-047-79) (Unpublished
proprietary information submitted to WHO by Rhône-Poulenc Agro,
Lyon).
Filatov DV & Brytskov VE (1972) [Enzymic method of residue
determination of chlorophos and Sevin by means of indicator paper.]
Probl Vet Sanit, XLI: 233-241 (in Russian).
Fisher SW & Lohner TW (1986) Studies on the environmental fate of
carbaryl as a function of pH. Arch Environ Contam Toxicol, 15(6):
661-667.
Fisher HL, Most B, & Hall LL (1985) Dermal absorption of pesticides
calculated by deconvolution. J Appl Toxicol, 5(3): 163-177.
Fletcher DW & Leonard MI (1986a) Toxicity and reproduction study
with carbaryl technical in Bobwhite quail. Neillsville, Wisconsin,
Bio-Life Associate Ltd (Report No. 85-DR-15) (Unpublished
proprietary information submitted to WHO by Rhône-Poulenc Agro,
Lyon).
Fletcher DW & Leonard MI (1986b) 28-day dietary toxicity and
reproduction pilot study with carbaryl technical in Mallard ducks.
Neillsville, Wisconsin, Bio-Life Associate Ltd (Report No. 85 DR
P14) (Unpublished proprietary information submitted to WHO by
Rhône-Poulenc Agro, Lyon).
Flores-Ruegg E, De Andrea MM, Helene CG, & Hirata R (1980)
Persistence of pesticide residues in Brazilian soil samples related
to organic matter and microbiological activity. In: Meeting of the
Advisory Group on Agrochemical Residue Biota Interaction: Proceeding
report. Sao Paulo, Brazil, Biological Institute, pp 181-187.
Frank R, Braun HE, & Ripley BD (1989) Monitoring Ontario grown
apples for pest control chemicals used in their production, 1978-86.
Food Addit Contam, 6(2): 227-234.
Frank R, Braun HE, Ripley BD, & Pitblado R (1991) Residues of nine
insecticides and two fungicides in raw and processed tomatoes. J
Food Prot, 54(1): 41-46.
Fukuto TR (1973) Metabolism of carbamate insecticides. In: Di Carlo
FJ ed. Drug metabolism reviews. New York, Basel, Marcel Dekker,
Inc., vol 1, pp 117-151.
Gaillard D, Chamoiseau G, & Derache R (1977) Interaction du carbaryl
et du nitrite de sodium sur les activités enzymatiques de quelques
mono-oxygénases microsomiques du foie de rat. J Pharmacol, 8: 437.
Gaines TB (1960) The acute toxicity of pesticides to rat. Toxicol
Appl Pharmacol, 2: 88-99.
Gaines TB (1969) Acute toxicity of pesticides to rat. Toxicol Appl
Pharmacol, 14: 514-534.
Gangwar SK, Kavadia VS, Gupta HCL, & Srivastava BP (1978a)
Persistence of carbaryl in sandy loam soil and its uptake in bajra,
Pennistetum typhoides P. Ann Arid Zone, 17(4): 357-362.
Gangwar SK, Kavadia VS, Srivastava BP, & Gupta HCL (1978b)
Persistence of carbaryl residues in sandy loam soil and its uptake
by root crops. Indian J Plant Prot, 8(1): 11-18.
Gapparov Sh (1974) [Haemocoagulation indices in dogs chronically
intoxicated with low doses of Sevin.] [Health Health Care Organ
Uzbekistan], II: 146-149 (in Russian).
Gartrell MJ, Craun JC, Podrebarac DS, & Gunderson EL (1986)
Pesticides, selected elements, and other chemicals in infant and
toddler total diet samples, October 1980-March 1982. J Assoc Off
Anal Chem, 69(1): 123-145.
Georgiev IN (1967) [The effect of toxic and maximal permissible
amounts of Sevin on intestinal secretion.] Gig i Sanit, 32(8): 42-46
(in Russian with English summary).
Ghadiri M & Greenwood DA (1966) Toxicity and biologic effects of
malathion, phosdrin and Sevin in the chick embryo. Toxicol Appl
Pharmacol, 8: 342.
Ghosh P, Bhattacharya S, & Bhattacharya S (1990) Impairment of the
regulation of gonadal function in Channa punctatus by metacid 50
and carbaryl under laboratory and field conditions. Biomed Environ
Sci, 3(1): 106-112.
Gibbs E, Mingo TM, & Courtemanch DL (1981) The effect on pond
macroinvertebrates from forest spraying of carbaryl and its
persistence in water and sediment. Augusta, Maine, Maine Department
of Conservation, Forest Service.
Gibbs KE, Mingo TM, & Courtemanch DL (1984) Persistence of carbaryl
(Sevin-4-oil) in woodland ponds and its effects on ponds macro
invertebrates following forest spraying. Can Entomol, 116: 203-214.
Gill SS & Yeoh CL (1980) Degradation of carbaryl in three components
of the paddy-field ecosystem of Malaysia. In: Meeting of the
Advisory Group on Agrochemical Residue Biota Interaction: Proceeding
report. Sao Paulo, Brazil, Biological Institute, pp 229-243.
Gill TS, Pant JC, & Pant J (1988) Gill, liver, and kidney lesions
associated with experimental exposures to carbaryl and dimethoate in
the fish (Puntius conchonius Ham.). Bull Environ Contam Toxicol,
41(1): 71-78.
Gladenko IN & Malinin OA (1970) [Toxicity of Sevin for livestock.]
Gig Primen Toxicol Pestic Klin Otrav, 1970: 188-193 (in Russian).
Golbs S, Kühnert M, & Leue F (1975) [Prenatal toxicological study of
Sevin (carbaryl) for Wistar rats.] Arch Exp Veterinaermed, 29:
607-614 (in German with English summary).
Golbs S, Kühnert M, & Fuchs V (1984) [Influence on the acute
toxicity of selected pesticides by huminic acids.] Z Gesamte Hyg,
30(12): 720-723 (in German).
Gold RE, Leavitt JR, Holcslaw T, & Tupy D (1982) Exposure of urban
applicators to carbaryl. Arch Environ Contam Toxicol, 11: 63-67.
Goldberg HI, Johnson HE, & Knaak JB (1965) Inhibition of discrete
avoidance behaviour by three anticholinesterase agents.
Psychopharmacologia, 7(1): 72-76.
Guirguis GN & Brindley WA (1975) Carbaryl penetration into and
metabolism by alfalfa leafcutting bees, Megachile pacifica. Agric
Food Chem, 23(2): 274-279.
Guirguis GN & Brindley WA (1976) Effect of chlorcyclizin,
aminopyrine or phenobarbital on carbaryl metabolism in alfalfa
leafcutting bees. Environ Entomol, 5(3): 590-594.
Gunderson EL (1988) FDA total diet study, April 1982-April 1984.
Dietary intake of pesticides, selected elements, and other
chemicals. J Assoc Off Anal Chem, 71(6): 1200-1209.
Gupta SK & Sundararaman V (1991) Correlation between burrowing
capability and AChE activity in the earthworm, Pheretima posthuma,
on exposure to carbaryl. Bull Environ Contam Toxicol, 46: 859-865.
Guseinov MK (1970) [On the question of circulation of Sevin in
environment.] Gig Primen Toxicol Pestic Klin Otrav, 1970: 174-176
(in Russian).
Guthrie FE, Monro RJ, & Abernathy CO (1971) Response of laboratory
mouse to selection for resistance to insecticides. Toxicol Appl
Pharmacol, 18: 92-101.
Gyrisco GG, Lisk DJ, Fertig SN, Huddleston EW, Fox FH, Holland RF, &
Teimberger GW (1960) The effects of feeding high levels of Sevin on
residue, flavour, and odour of the milk of dairy cattle. Agric Food
Chem, 8(5): 409-410.
Haines TA (1981) Effect of an aerial application of carbaryl on
brook trout (Salvelinus fontinalis). Bull Environ Contam Toxicol,
27(4): 534-542.
Hamada NN (1987) One year oral toxicity study in Beagle dogs with
carbaryl technical. Vienna, Virginia, Hazleton Laboratories America,
Inc. (Report No. 400-715) (Unpublished proprietary information
submitted to WHO by Rhône-Poulenc Agro, Lyon).
Hamada NN (1990) Acute oral toxicity study in rats. Carbaryl
technical. Vienna, Virginia, Hazleton Laboratories America, Inc.
(Unpublished proprietary information submitted to WHO by
Rhône-Poulenc Agro, Lyon).
Hamada NN (1991a) Subchronic toxicity study in dogs with carbaryl
technical. Vienna, Virginia, Hazleton Laboratories America, Inc.
(Report No. N656-152) (Unpublished proprietary information submitted
to WHO by Rhône-Poulenc Agro, Lyon).
Hamada NN (1991b) Oncogenicity with carbaryl technical in CD-1(R)
mice. Vienna, Virginia, Hazleton Laboratories America, Inc. (Report
No. N656-138) (Unpublished proprietary information submitted to WHO
by Rhône-Poulenc Agro, Lyon).
Hamada NN (1991c) Combined chronic toxicity and oncogenicity study
with carbaryl technical in Sprague-Dawley rats - 52 week interim
report with 4-week recovery. Vienna, Virginia, Hazleton Laboratories
America, Inc. (Study No. 656-139) (Unpublished proprietary
information submitted to WHO by Rhône-Poulenc Agro, Lyon).
Hanazato T & Yasuno M (1987) Effects of a carbamate insecticide,
carbaryl, on the summer phyto- and zooplankton communities in ponds.
Environ Pollut, 48: 145-159.
Hanazato T & Yasuno M (1989) Influence of over-wintering Daphnia
on spring zooplankton communities: An experimental study. Ecol Res,
4: 323-338.
Hanazato T & Yasuno M (1990a) Influence of Chaoborus density on
the effects of an insecticide on zooplankton communities in ponds.
Hydrobiologia, 194: 183-197.
Hanazato T & Yasuno M (1990b) Influence of time in application of an
insecticide on recovery patterns of a zooplankton community in
experimental ponds. Arch Environ Contam Toxicol, 19: 77-83.
Hansen JL (1978) Carbaryl: Human ingestion study statistical
analysis (File No. 24971). Research Triangle Park, North Carolina,
Union Carbide Corporation (Unpublished proprietary information
submitted to WHO by Rhône-Poulenc Agro, Lyon).
Hansen CR & Kawatski JA (1976) Application of 24-hour post-exposure
observation to acute toxicity studies with invertebrates. J Fish Res
Board Can, 33: 1198-1201.
Harris LW, Talbot BG, Lennox WJ, & Anderson DR (1989) The
relationship between oxime induced reactivation of carbamylated
acetylcholinesterase and antidotal efficacy against carbamate
intoxication. Toxicol Appl Pharmacol, 98(1): 128-133.
Harry JB (1977) A review of human exposure to Sevin carbaryl
insecticide with particular reference to the USA. Research Triangle
Park, North Carolina, Union Carbide Agricultural Products Co., Inc.
(Unpublished proprietary information submitted to WHO by
Rhône-Poulenc Agro, Lyon).
Hart ER (1972) Teratology study. Sevin, vitamin A, aspirin and
malathion. South Charleston, West Virginia, Union Carbide
Corporation, 8 pp (Prepared by Bionetics Research Laboratories)
(Unpublished proprietary information submitted to WHO by
Rhône-Poulenc Agro, Lyon).
Hassan A (1971) Pharmacological effects of carbaryl. The effect of
carbaryl on the synthesis and degradation of catecholamines in the
rat. Biochem Pharmacol, 20: 2299-2308.
Hassan A & Cueto C Jr (1970) Biochemical effects in the rabbit of
repeated administration of a mixture of DDT, carbaryl and parathion.
Z Naturforsch, B25: 521-525.
Hassan A & Santolucito JA (1971) Pharmacological effects of
carbaryl. II. Modification of serotonin metabolism in the rat brain.
Experientia (Basel), 27: 287-288.
Hassan A, Zayed SMAD, & Abdel-Hamid FM (1966) Metabolism of
carbamate drugs. I. Metabolism of 1-naphthyl- N-methylcarbamate
(Sevin) in the rat. Biochem Pharmacol, 15: 2045-2055.
Hassan AA, El Sewedy SM, Minatogawa Y, & Kido R (1990) Effects of
some insecticides on several enzymes of tryptophan metabolism in
rats. J Environ Sci Health, B25(3): 333-346.
Haven D, Castagna M, Chanley P, Wass M, & Whitcomb J (1966) Effects
of the treatment of an oyster bed with polystream and Sevin.
Chesapeake Sci, 7(4): 179-188.
Haydock CI (1964) An experimental study to control oyster drills in
Tomales Bay, California. Calif Fish Game, 50: 11-28.
Hayes WJ (1963) Clinical handbook on economic poisons. Emergency
information for treating poisoning. Atlanta, Georgia, US Department
of Health, Education and Welfare, Communicable Disease Center, pp
44-46.
Hayes WJ Jr & Laws ER Jr ed. (1991) Carbaryl. In: Handbook of
pesticide toxicology. Volume 3 - Classes of pesticides. New York,
San Diego, Academic Press, Inc., pp 1144-1189.
Hazleton Laboratories America, Inc. (1982) Acute oral toxicity study
in rats - Sevin RF. Vienna, Virginia, Hazleton Laboratories America,
Inc. (Unpublished proprietary information submitted to WHO by
Rhône-Poulenc Agro, Lyon).
Heise GA & Hudson JD (1985a) Effects of pesticides and drugs on
working memory in rat: Continuous delayed response. Pharmacol
Biochem Behav, 23: 591-598.
Heise GA & Hudson JD (1985b) Effects of pesticides and drugs on
working memory in rats: Continuous non-match. Pharmacol Biochem
Behav, 23: 599-605.
Hernandez DA, Lombardo RL, Ferrari L, & Tortorelli MC (1990)
Toxicity of ethyl-parathion and carbaryl on early development of sea
urchin. Bull Environ Contam Toxicol, 45: 734-741.
Heywood DL (1975) Degradation of carbamate insecticides in soil.
Environ Qual Saf, 4: 128-133.
Hill EF & Camardese MB (1986) Lethal dietary toxicities of
environmental contaminants and pesticides to Coturnix. Washington,
DC, US Department of the Interior, Fish and Wildlife Service (Fish
and Wildlife Technical Report No. 2).
Hill EF, Heath RG, Spann JW, & Williams JD (1975) Lethal dietary
toxicities of environmental pollutants to birds. Washington, DC, US
Department of the Interior, Fish and Wildlife Service (Special
Scientific Report, Wildlife No. 191).
Hodgson E & Casida JE (1961) Metabolism of N:N-dialkyl carbamate and
related compounds by rat liver. Biochem Pharmacol, 8: 179-191.
Hoffman DJ & Albers PH (1984) Evaluation of potential embryotoxicity
and teratogenicity of 42 herbicides, insecticides, and petroleum
contaminants to mallard eggs. Arch Environ Contam Toxicol, 13:
15-27.
Hoque H (1972) Radio tracer studies on chemical residues in food and
agriculture. Proc. Comb. Panel Res. Coord. Meet. 1971.
Hoque MZ (1972) Carbaryl - A new chemical mutagen. Curr Sci, 41(23):
855-856.
Houston JB, Upshall DG, & Bridges JW (1974) Pharmacokinetics and
metabolism of two carbamates, carbaryl and landrin, in the rat.
Xenobiotica, 5(10): 637-648.
Hudson RH, Tucker RK, & Haegele MA (1984) Handbook of toxicity of
pesticides to wildlife, 2nd ed. Washington, DC, US Department of the
Interior, Fish and Wildlife Service (Resource Publication 153).
Hughes L Jr (1971) A study of the fate of carbaryl insecticide in
surface waters. Lafayette, Indiana, Purdue University (Ph.D
Dissertation) (University microfilms, High Wycombe, England).
Hughes LB & Reuszer HW (1970) A study of bacterial decomposition of
carbaryl in farm pond waters. Lafayette, Indiana, Purdue University,
Water Resources Research Center (Technical Report No. 10).
Hundley HK, Cairns T, Luke AM, & Masumoto HT (1988) Pesticide
residue findings by the Luke method in domestic and imported foods
and animal feeds for fiscal years 1982-1986. J Assoc Off Anal Chem,
71(5): 875-892.
Huque H (1972) Preliminary report on the residues of carbaryl
granules in rice plants. Radiotracer Stud. Chem. Residues Food Agr.,
Proc. Comb. Panel Res. Coord. Meet. 1971. Vienna, International
Atomic Energy Agency, pp 107-109.
Hurlburt E, McMillan R, & Vialle M (1989) Supplemental environmental
impact statement: use of the insecticide carbaryl to control ghosts
and mud shrimp in oyster beds of Willapa Bay and Grays Harbour.
Olympia, Washington, Washington State Department of Fisheries and
Washington State Department of Ecology (Unpublished report submitted
to WHO by Rhône-Poulenc Agro, Lyon).
Hurwood IS (1967) Studies on pesticide residues. 2. Carbaryl
residues in the body tissues and milk of cattle following dermal
application. Qld J Agric Anim Sci, 24: 69-74.
Hwang SW & Schanker S (1974) Absorption of carbaryl from lung and
small intestine of the rat. Environ Res, 7(2): 206-211.
IARC (1987) Overall evaluations of carcinogenicity: An updating of
IARC monographs, volumes 1 to 42. Lyon, International Agency for
Research on Cancer (IARC Monographs on the Evaluation of the
Carcinogenic Risk of Chemicals to Humans, Supplement 7).
Imming RJ, Shaffer BC, & Woodard G (1969) Sevin, safety evaluation
by feeding to female Beagles from day one of gestation through
weaning of the offspring. (Prepared by Woodard Research Corporation
for Union Carbide Agricultural Products Co., Inc., Research Triangle
Park) (Unpublished proprietary information submitted to WHO by
Rhône-Poulenc Agro, Lyon).
Innes JRM, Ulland MB, Valerio MG, Petrucel L, Fishbein L, Hart ER,
Pallotta AJ, Bates RR, Falk HL, Gart JJ, Klein M, Mitchell I, &
Peters H (1969) Bioassay of pesticides and industrial chemicals for
tumorigenicity in mice: a preliminary note. J Natl Cancer Inst, 42:
1101-1114.
IRPTC (1982) In: Izmerov NF ed. IRPTC scientific reviews of Soviet
literature on toxicity and hazard of chemicals No. 7: Carbaryl.
Moscow, Centre of International Projects, GKNT, 13 pp.
IRPTC (1989) In: Izmerov NF ed. [Scientific reviews of Soviet
literature on toxicity and hazard of chemicals No. 7: Carbaryl], 2nd
ed. Moscow, Centre of International Projects, GKNT, 82 pp (in
Russian).
Ishidate M Jr & Odashima S (1977) Chromosome tests with 134
compounds on Chinese hamster cells in vitro: a screening for
chemical carcinogens. Mutat Res,48: 337-354.
Ishidate M Jr, Sofuni T, & Yoshikawa K (1981) Chromosomal aberration
tests in vitro as a primary screening tool for environmental
mutagens and/or carcinogens. Gann Monogr Cancer Res, 27: 95-108.
Ivanova LN & Molozhanova YG (1973) [Static and dynamic distribution
of carbaryl in the environment.] Gig i Sanit, 38(2): 24-28 (in
Russian).
Iwata Y, Dusch ME, Garman GE, & Gunther FA (1979) Worker environment
research: Residues for carbaryl, chlorobenzilate, dimethoate and
trichlorfon applied to citrus trees. J Agric Food Chem, 27(6):
1141-1145.
Jablonska J & Brzezinski J (1990) The influence of carbaryl on the
uptake of (3H) noradrenaline (3H) NA by rat hypothalamic slices.
Arch Toxciol, 64(5): 417-419.
Jacob J, Schmoldt A, Raab J, Hamman M, & Grimmer G (1985)
Monoxygenase induction by various xenobiotics and its influence on
the rat liver microsomal metabolite profile of benza(a)anthracene.
Cancer Lett, 27: 105-113.
Jaszczuk E & Syrowatka T (1979) [Induction of mitotic conversion in
Saccharomyces cerevisiae D4 with propoxur, carbaryl,
dimethylnitrosamine and products of their metabolic activation.]
Rocz Panstw Zakl Hig, 30(4): 323-330 (in Polish).
Jaszczuk E, Syrowatka T, & Cybulski J (1979) [Mutagenic activity of
propoxur, carbaryl and their nitroso derivatives. Induction of
reversion in Salmonella typhimurium.] Rocz Pantsw Zakl Hig, 30(1):
81-88 (in Polish).
Jegier Z (1964) Health hazards in insecticide spraying of crops.
Arch Environ Health, 8: 670-674.
Jeleniewicz KK & Szczepaniak St (1980) [Free aminoacids and amino
nitrogen levels in serum and blood erythrocytes of rat exposed to
subacute intoxication with carbaryl.] Bromatol Chem Toksykol, 13(3):
237-240 (in Polish).
Jeleniewicz KK, Szczepaniak St, & Slenkiewicz E (1984) [Effect of
acute intoxication with carbaryl on brain free aminoacids level in
rat.] Bromatol Chem Toksykol, 17(3): 225-227 (in Polish).
Jerkofsky M & Abrahamsen LH (1983) Variation on human herpes virus
susceptibility to enhancement by the pesticide carbaryl. Appl
Environ Microbiol, 45(5): 1555-1559.
Johnson WW & Finley MT (1980) Handbook of acute toxicity of
chemicals to fish and aquatic invertebrates. Washington, DC, US
Department of the Interior, Fish and Wildlife Service (Resource
Publication 137).
Johnson RD & Manske DD (1976) Pesticide residues in total diet
samples (IX). Pestic Monit J, 9(4): 157-169.
Johnson DP & Stansbury HA (1965) Adaptation of Sevin insecticide
(carbaryl) residue method to various crops. J Agric Food Chem, 13:
235-238.
Junk GA, Richard JJ, & Dahm PA (1984) Degradation of pesticides in
controlled water-soil systems (treatment and disposal of pesticides
wastes). Washington, DC, American Chemical Society, pp 37-67 (ACS
Symposium Series No. 259).
Kadir HA & Knowles CO (1981) Inhibition of rat brain monoamine
oxidase by insecticides, acaricides and related compounds. Gen
Pharmacol, 12(4): 239-247.
Kagan J, Rodionov G, Voronina L, Velitsco L, Kulagin O, & Peremitina
A (1970) [Effect of Sevin on liver function and structure.]
Pharmacol Toxicol, 33: 219-224 (in Russian with English summary).
Kaloyanova F (1963) [Changes in the blood sugar in cases of
intoxications with certain phosphorus organic compounds.] In: [Works
of the Scientific Research Institute on Work Safety and Occupational
Diseases - Volume X.] Sofia, Institute of Hygiene and Occupational
Health, pp 63-67 (in Bulgarian).
Kanazawa J (1975) Uptake and excretion of organophosphorus and
carbamate insecticides by freshwater fish, Motsugo, Pseudorasbora
parva. Bull Environ Contam Toxicol, 14(3): 346-352.
Kanazawa J (1981) Measurement of the bioconcentration factors of
pesticides by freshwater fish and their correlation with
physicochemical properties or acute toxicities. Pestic Sci, 12(4):
417-424.
Kanazawa J, Isensee AR, & Kearney PC (1975) Distribution of carbaryl
and 3,5-xylylmethyl carbamate in an aquatic model ecosystem. J Agric
Food Chem, 23(4): 760-763.
Karinen FJ, Lamberton JG, Stewart NE, & Terrier LC (1967)
Persistence of carbaryl in the marine estuarine environment. J Agric
Food Chem, 15(1): 148-156.
Karnak RE & Collins WJ (1974) The susceptibility to selected
insecticides and acetylcholinesterase activity in a laboratory
colony of midge larvae, Chironomus tentans (Diptera:
chironomidae). Bull Environ Contam Toxicol, 12: 62-69.
Kassin VJ (1968) [Structural changes in endocrine organs under
repeated action of Sevin.] Gig Primen Toxicol Pestic Klin Otrav,
1968: 631-635 (in Russian).
Kaur K & Toor HS (1977) Toxicity of pesticides to embryonic stages
of Cyprinus carpio communis Linn. Indian J Exp Biol, 15: 193-196.
Kavadia VS, Gangwar SK, Srivastava BP, Kathpal TS, & Gupta HCL
(1978) Persistence of carbaryl in clay loam soil and its uptake in
maize and root crops. Indian J Entomol, 40(3): 265-272.
Kavlock RJ, Short RD, & Chernoff N (1987) Further evaluation of an
in vivo teratology screen. Teratog Carcinog Mutagen, 7: 7-16.
Kazano H, Kearney PC, & Kaufman DD (1972) Metabolism of
methylcarbamate insecticides in soil. J Agric Food Chem, 20(5):
975-979.
Kazarnovskaya ML & Vasilos AF (1977) [The effect of Sevin on
chromosomal apparatus of cells in vitro.] Zdravookhranenije,
20(4): 14-16 (in Russian).
Kendrick PN, Trim AJ, Atwal JK, & Brown PM (1991) Direct gas
chromatographic determination of carbaryl residues in honeybees
( Apis mellifera L.) using a nitrogen-phosphorus detector with
confirmation by formation of a chemical derivative. Bull Environ
Contam Toxicol, 46: 654-661.
Khasawinah AM (1977) Hydrolysis of carbaryl in aqueous buffer
solutions (Project No. 111A12, File No. 23092). South Charleston,
West Virginia, Union Carbide Corporation (Prepared in cooperation
with the International Research and Development Corporation)
(Unpublished proprietary information submitted to WHO by
Rhône-Poulenc Agro, Lyon).
Khasawinah AM (1978) Fate of carbaryl in soils (Project No. 285).
Monument, Colorado, Analytical Development Corporation (Unpublished
proprietary information submitted to WHO by Rhône-Poulenc Agro,
Lyon).
Khera K (1966) Toxic and teratogenic effects of insecticides in duck
and chick embryos. Toxicol Appl Pharmacol, 8: 345.
Klisenko MA (1965) [Determination of Sevin in air and biological
material.] J Anal Chim, XX(5): 634-636 (in Russian).
Klisenko MA & Yakim VS (1966) [Accumulation and excretion of Sevin
from the body of warm-blooded animals.] Gig Primen Toxicol Pestic
Klin Otrav, 1966: 146-149 (in Russian).
Klisenko MA, Lebedeva TA, & Yurkova ZF (1972) [Chemical analysis of
micro amounts of pesticides.] Moscow, Medizina, pp 197-201 (in
Russian).
Knaak JB (1971) Biological and nonbiological modifications of
carbamates. Bull World Health Organ, 44: 121-131.
Knaak JB & Sullivan LJ (1967) Metabolism of carbaryl in dog. J Agric
Food Chem, 15(6): 1125-1126.
Knaak JB, Marilyn J, Tallant MJ, Bartley WJ, & Sullivan LF (1965)
The metabolism of carbaryl in rat, guinea-pig, and man. J Agric Food
Chem, 13(6): 537-543.
Knaak JB, Tallant MJ, Kozbelt SJ, & Sullivan LJ (1968) The
metabolism of carbaryl in man, monkey, pig, and sheep. J Agric Food
Chem, 16(3): 465-470.
Knaak JB, Yee K, Ackerman CR, Zweig G, Fry DM, & Wilson BW (1984)
Percutaneous absorption and dermal dose - ChE response studies with
parathion and carbaryl in the rat. Toxicol Appl Pharmacol, 76:
252-263.
Knight EV, Chin BH, Ueng TH, & Alvares AP (1986) Differences in the
in vivo and in vitro effects of the carbamate insecticide,
carbaryl on rat hepatic monooxygenases. Drug Chem Toxicol, 9(3-4):
253-273.
Knight EV, Alvares AP, & Chin BH (1987) Effects of phenobarbital
pre-treatment on the in vivo metabolism of carbaryl in rats. Bull
Environ Contam Toxicol, 39(5): 815-821.
Korn S (1973) The uptake and persistence of carbaryl in channel
catfish. Trans Am Fish Soc, 1: 137-139.
Koundinya PR & Ramamurthi R (1980) Toxicity of Sunithion and Sevin
to the freshwater fish Sarotherodon mossambicus (Peters). Curr
Sci, 49: 875-876.
Kovaleva ES & Talanov GA (1978a) [Methods for determination of Sevin
in soil and plants by means of thin-layer chromatography.] Gig i
Sanit, 7: 69-70 (in Russian).
Kovaleva ES & Talanov GA (1978b) [Residual amounts of Sevin in
plants and soil.] Khim Sel'sk Khoz, 16(8): 77-79 (in Russian).
Kovaleva ES & Talanov GA (1980) [Plant and soil dynamics of Sevin
residues and dithiocarbamic acid derivatives.] In: Bobovnikova TI &
Malakhov SG ed. [Migration of contaminants in soil and contiguous
media. Proceedings of the All-Union Congress, 2nd Meeting, 1978.]
Leningrad, Gidrometeoizdat, pp 155-159 (in Russian).
Kovtun SD (1970) Analysis of the action exerted by Sevine on the
myoneural formations. Pharmacol Toxicol, 35(5): 543-545.
Kovtun SD & Sokur AI (1970) [The effect of Sevin on the spontaneous
electrical activity of myoneural synaps and permeability of the
membrane of skeletal muscle in white rats.] Gig Primen Toxicol
Pestic Klin Otrav, 1970: 211-214 (in Russian).
Krause RT (1985a) Pesticide and industrial chemical residues. Liquid
chromatographic determination of N-methylcarbamate insecticides
and metabolites in crops. I. Collaborative study. J Assoc Off Anal
Chem, 68(4): 726-733.
Krause RT (1985b) Liquid chromatographic determination of
N-methylcarbamate insecticides and metabolites in crops. II.
Analytical characteristics and residue findings. J Assoc Off Anal
Chem, 68(4): 734-740.
Krechniak V & Foss W (1981) Gas chromatography determination of
carbaryl. Pr Cent Inst Ochr Pr, XXXI(110): 183-188.
Krishna JG & Casida JE (1966) Fate in rats of the radiocarbon from
ten variously labelled methyl- and dimethylcarbamate-C14
insecticide chemicals and their hydrolysis products. J Agric Food
Chem, 14(2): 98-105.
Krishna JG, Dorough HW, & Casida JE (1962) Synthesis of
N-methylcarbamates via methyl isocyanate-C14 and chromatographic
purification. J Agric Food Chem, 10(6): 462-466.
Krishnaih K, Prasad VG, & Jagan Mohan N (1978) Carbaryl residues on
brinjal and peas. In: Edwards CA, Veeresh GK, & Krueger HR ed.
Proceedings of the Symposium on Pesticide Residues in the
Environment in India, 7-10 November 1978. Hebbal, Bangalore,
University of Agricultural Sciences, pp 229-231 (UAS Technical
Series No. 32).
Krug HG & Berndt J (1985) Inhibition by pesticides of prostglandin
formation in blood platelets. Blut, 51: 19-23.
Krug HF, Hamm U, & Berndt J (1988) Mechanism of inhibition of
cyclo-oxygenase in human blood platelets by carbamate insecticides.
Biochem. J, 250(1): 103-110.
Krylov DG (1970) [The effect of Sevin on red vole population.] Gig
Primen Toxicol Pestic Klin Otrav, 1970: 218-110 (in Russian).
Kuhn JO (1991a) Acute oral toxicity study in rats with Sevin XLR
plus. Sugar Land, Texas, Stillmeadow, Inc. (Laboratory Study No. IV
8150-91) (Unpublished proprietary information submitted to WHO by
Rhône-Poulenc Agro, Lyon).
Kuhn JO (1991b) Sevin-4-oil 47.0% (w/w). Acute oral toxicity study
in rats . Sugar Land, Texas, Stillmeadow, Inc. (Laboratory Study No.
N 7712-90) (Unpublished proprietary information submitted to WHO by
Rhône-Poulenc Agro, Lyon).
Kuhr RJ (1970) Metabolism of carbamate insecticide chemicals in
plants and insects. J Agric Food Chem, 18(6): 1023-1030.
Kuhr RJ (1971) The formation and importance of carbamate insecticide
metabolites as terminal residues. Pure Appl Chem, Suppl: 199-220.
Kuhr RJ & Dorough HW (1976) Carbamate insecticides: Chemistry,
biochemistry and toxicology. Cleveland, Ohio, CRC Press, Inc., pp
125-200.
Kuhr RJ, Davis AC, & Bourke JB (1974) Dissipation of Guthion, Sevin,
Polyram, Phygon and Systox from apple orchard soil. Bull Environ
Contam Toxicol, 11(3): 224-230.
Kulshrestha SK & Arora N (1984) Impairments induced by sublethal
doses of two pesticides in the ovaries of a freshwater teleost
Channa striatus Bloch. Toxicol Lett, 20(1): 93-98.
Kuribayashi S (1981) Studies on the effect of pesticides on the
reproduction of the silkworm, Bombyx Mori L. I. Effects of
chemicals administered during the larval stage on egg-laying and
hatching. J Toxicol Sci, 6: 169-176.
Kurtz DA & Studholme GA (1974) Recovery of trichlorfon (Dylox) and
carbaryl (Sevin) in songbirds following spraying of forest for Gipsy
moth. Bull Environ Contam Toxicol, 11(1): 78-84.
Kuzminskaya UA, Ivanitskij VA, & Shilina VA (1984) [The influence of
pesticides having anticholinesterase effect upon the content of
biogenic amines in brain.] Gig i Sanit, 5: 80-81 (in Russian).
LaFleur KS (1976a) Carbaryl desorption and movement in soil columns.
Soil Sci, 121(4): 212-216.
LaFleur KS (1976b) Movement of carbaryl through Congaree soil into
ground water. J Environ Qual, 5(1): 91-92.
Lahmy S, Salmon JM, & Viallet P (1988) Influence of long-term
treatment with 3 methylcholanthrene or carbaryl on mixed function
oxidase activity in 3T3 fibroblasts: studies by
microspectro-fluorimetry on single cells. Cell Biochem Funct, 6(4):
275-282.
Lamb FC, Farrow RP, Elkins ER, Kimball JR, & Cook RW (1968) Removal
of DDT, parathion and carbaryl from spinach by commercial and home
preparative methods. J Agric Food Chem, 16: 967-973.
Lamberton JG & Claeys RR (1970) Degradation of 1-naphthol in sea
water. J Agric Food Chem, 18(1): 92-96.
Larkin MJ & Day MJ (1985) The effect of pH on the selection of
carbaryl-degrading bacteria from garden soil. J Appl Bacteriol,
58(2): 175-186.
Larkin MJ & Day MJ (1986) The metabolism of carbaryl by three
bacterial isolates, Pseudomonas spp. (NCIB 12042 & 12043) and
Rodococcus sp. (NCIB 12038) from garden soil. J Appl Bacteriol,
60(3): 233-242.
Lawlor TE (1989) Mutagenicity test on carbaryl (technical) in the
Ames Salmonella/Microsome reverse mutation assay. Final report.
Kensington, Maryland, Hazleton Laboratories America, Inc. (Report
No. 10862-0-401) (Unpublished proprietary information submitted to
WHO by Rhône-Poulenc Agro, Lyon).
Leavitt JRC, Gold RE, Holcslaw T, & Tupy D (1982) Exposure of
professional pesticide applicators to carbaryl. Arch Environ Contam
Toxicol, 11(1): 57-62.
Lechner DMW & Abdel-Rahman MS (1984) A teratology study of carbaryl
and malathion mixtures in rat. J Toxicol Environ Health, 14:
267-278.
Lechner DMW & Abdel-Rahman MS (1985) Alterations in rat liver
microsomal enzymes following exposure to carbaryl and malathion in
combination. Arch Environ Contam Toxicol, 1: 451-457.
Lechner DMW & Abdel-Rahman MS (1986a) The effect of carbaryl and
malathion in combination on gamma glutamyl transpeptidase and
glutathione- S-alkyltransferase activity in vitro. Arch Environ
Contam Toxicol, 15(6): 647-651.
Lechner DMW & Abdel-Rahman MS (1986b) Kinetics of carbaryl and
malathion in combination in the rat. J Toxicol Environ Health,
18(2): 241-256.
Lee RM & Brindley WA (1974) Synergist ratios, EPN detoxication,
lipid and drug-induced changes in carbaryl toxicity in Megachile
pacifica. Environ Entomol, 3: 899-907.
Lee DY, Chen CT, & Houng KH (1990) Behaviour assessment of eight
major pesticides used in Taiwan in soils by mechanistic models. In:
Soils and fertilizers in Taiwan, pp 33-50.
Leeling NC & Casida JE (1966) Metabolites of carbaryl (1-naphthyl
methyl carbamate) in mammals and enzymatic systems for their
formation. J Agric Food Chem, 14(3): 281-290.
Leenheer JA & Ahlrichs (1971) A kinetic and equilibrium study of the
adsorption of carbaryl and parathion upon soil organic matter
surfaces. Soil Sci Soc Am Proc, 35: 700-705.
Lejczak B (1977) Effect of insecticides: chlorfenvinphos, carbaryl
and propoxur on aquatic organisms. Pol Arch Hydrobiol, 24(4):
583-591.
Leonard JA & Yearly RA (1990) Exposure of workers using hand-held
equipment during urban application of pesticides to trees and
ornamental shrubs. Am Ind Hyg Assoc J, 51: 605-609.
Lesca P, Fournier A, Lecointe P, & Cresteil T (1984) A dual assay
for the specific screening of 3 methylcholanthrene and phenobarbital
like chemical inducers of cytochrome P450 monooxygenases. Mutat Res,
129: 229-310.
Lijinsky W & Schmähl D (1978) Carcinogenicity of N-nitroso
derivatives of N-methylcarbamate insecticides in rats. Ecotoxicol
Environ Saf, 2: 413-419.
Lijinsky W & Taylor HW (1976) Carcinogenesis in Sprague-Dawley rats
on N-nitroso- N-alkylcarbamate esters. Cancer Lett, 1: 275-279.
Lijinsky W & Taylor HW (1977) Transplacental chronic toxicity test
of carbaryl with nitrite in rats. Food Cosmet Toxicol, 15: 229-232.
Lijinsky W & Winter C (1981) Skin tumours induced by painting
nitrosoalkylureas on mouse skin. J Cancer Res Clin Oncol, 102:
13-20.
Lillie RJ (1973) Studies on the reproductive performance and progeny
performance of caged white leghorns fed malathion and carbaryl.
Poult Sci, 52: 266-272.
Lin TH, North HH, & Menzer RE (1975) Metabolism of carbaryl
(1-naphthyl N-methylcarbamate) in human embryonic lung cell
cultures. J Agric Food Chem, 23(2): 253-256.
Lindsay CE (1961) Pesticide tests in the marine environment in the
State of Washington. Proc Natl Shellfish Assoc, 52: 87-97.
Lingaraja T & Venugopalan UK (1978) Pesticide induced physiological
and behavioural changes in an estuarine teleost Therapon jarbua
(Forsk). Fish Technol, 15: 115-119.
Little EE, Archeski RD, Flerov BA, & Kozlovskaya VI (1990)
Behavioral indicators of sublethal toxicity in rainbow trout. Arch
Environ Contam Toxicol, 19(3): 380-385.
Liu SY & Bollag JM (1971a) Metabolism of carbaryl by a soil fungus.
J Agric Food Chem, 19(3): 487-490.
Liu SY & Bollag JM (1971b) Carbaryl decomposition to 1-naphthyl
carbamate by Aspergillus terreus. Pestic Biochem Physiol, 1(3-4):
366-372.
Liu D, Thomson K, & Strachan WMJ (1981) Biodegradation of carbaryl
in simulated aquatic environment. Bull Environ Contam Toxicol,
27(3): 412-417.
Long KR (1971) Pesticides and occupational hazards on farms. Am J
Nurs, 71(4): 740-743.
Looney NE & Knight JN (1985) Effects of initial set and carbaryl
treatment in final fruit set on "Greensleeves" apple. Hortic Sci,
20(3): 400-401.
Looney NE & McKellar JE (1984) Thinning Spartan apples with carbaryl
and 1-napthaleneacetic acid: influence of spray volume and
combinations of chemicals. Can J Plant Sci, 64: 161-166.
Loosanoff VL (1961) Recent advances in the control of shellfish
predators and competitors. Proc Gulf Caribb Fish Inst, 13: 113-128.
Loosanoff VL, MacKenzie CL Jr, & Shearer LW (1960a) Use of chemical
barriers to protect shellfish beds from predators. In: Fisheries:
Fish farming. Volume 3 - Fisheries management. Olympia, Washington,
Washington State Department of Fisheries, pp 86-90.
Loosanoff VL, MacKenzie CL Jr, & Shearer LW (1960b) Use of chemical
to control shellfish predators. Science, 131: 1522-1523.
Lowe JI (1967) Effect of prolonged exposure to Sevin on an estuarine
fish, Leiostomus xanthurus Lacépède. Bull Environ Contam Toxicol,
2(3): 147-155.
Lox CD (1984) The effects of acute carbaryl exposure on clotting
factor activity in rat. Ecotoxicol Environ Saf, 8: 280-283.
Luca D & Balan M (1987) Sperm abnormality assay in the evaluation of
the genotoxic potential of carbaryl in rats. Morphol Embryol
(Bucur), 33(1): 19-22.
Lucier GW, McDaniel OS, Williams C, & Klein R (1972) Effects of
chlordane and methylmercury on the metabolism of carbaryl and
carbofuran in rats. Pestic Biochem Physiol, 2: 244-255.
Lukaneva AM & Rodionov GA (1973) [Some experimental data on the
effect of DDT and Sevin upon the development of cholesterin
atherosclerosis.] Gig i Sanit, 9: 41-45 (in Russian).
Luke MA, Masumoto HT, Cairns T, & Hundley HK (1988) Levels and
incidences of pesticide residues in various foods and animal feeds
analyzed by the Luke multiresidue methodology for fiscal years
1982-1986. J Assoc Off Anal Chem, 71(2): 415-420.
Lykken L & Casida JE (1969) Metabolism of organic insecticide
chemicals. Can Med Assoc J, 100: 145-154.
Ma TH, Harris MM, Anderson VA, Ahmed I, Mohammed K, Bare JL, & Lin G
(1984) Tradescantia-Micronucleus (Trad-MCN) tests on 140
health-related agents. Mutat Res, 138: 157-167.
McCann J, Choi E, Yamasaki E, & Ames BM (1975) Detection of
carcinogen as mutagens in the Salmonella/microsome test: Assay of
300 chemicals. Proc Natl Acad Sci (USA), 72(12): 5135-5139.
MacCollom GB, Currier WW, & Baumann GL (1985) Pesticide drift and
quantification from air and ground applications to a single orchard
site. In: Honeycutt RC, Zweg D, & Ragsdale N ed. Dermal exposure
related to pesticide use. Washington, DC, American Chemical Society,
pp 190-198 (ACS Symposium Series No. 273).
MacCollom GB, Currier WW, & Baumann GL (1986) Drift comparisons
between aerial and ground orchard application. J Econ Entomol,
79(2): 459-464.
Macek KJ & McAllister WA (1970) Insecticide susceptibility of some
common fish family representatives. Trans Am Fish Soc, 99(1): 20-27.
MacLeod J (1982) Report on UCC sperm morphology readings submitted
to Union Carbide Agricultural Products Co., Inc. (Unpublished
proprietary information submitted to WHO by Rhône-Poulenc Agro,
Lyon).
MacPherson SE, Scott RC, & Williams FM (1991) Fate of carbaryl in
rat skin. Arch Toxicol, 65: 594-598.
Maibach HI, Feldmann RJ, Milby TH, & Serat WF (1971) Regional
variations in percutaneous penetration in man - Pesticides. Arch
Environ Health, 23: 208-211.
Makovskaya EL, Rapoport MB, & Pintshuk VG (1965) [On the possibility
of carcinogenic effect of some insecticides belonging to the
carbamate group.] Vopr Exp Onkol, 1: 67-74 (in Russian).
Maliwal BP & Guthrie FE (1981) Interaction of insecticides with
human plasma lipoproteins. Chem-Biol Interact, 35(2): 177-188.
Mann GS & Chopra SL (1969) Residues of carbaryl on crops. Pestic
Monit J, 2(4): 163-165.
Manske DD & Corneliussen PE (1974) Pesticides in food and feed.
Pesticide residues in total diet samples (VII). Pestic Monit J,
8(2): 110-124.
Manske DD & Johnson RD (1975) Residues in food and feed. Pesticide
residues in total diet samples (VIII). Pestic Monit J, 9(2): 94-105.
Manske DD & Johnson RD (1977) Residues in food and feed. Pesticide
and other chemical residues in total diet samples (X). Pestic Monit
J, 10: 134-148.
Marian MP, Arul V, & Pandian TJ (1983) Acute and chronic effect of
carbaryl on survival, growth, and metamorphosis in the bullfrog
(Rana tigrina). Arch Environ Contam Toxicol, 12(3): 271-275.
Marshall TC, Dorough HW, & Swin HE (1976) Screening of pesticides
for mutagenic potential using Salmonella typhimurium mutants. J
Agric Food Chem, 24(3): 500-563.
Marshall TC, Dorough HW, & Swin HE (1979) Biliary excretion of
carbamate insecticides in the rat. Pestic Biochem Physiol, 11:
56-63.
Mason Y, Choshen E, & Rav-Acha C (1990) Carbamate insecticides:
Removal from water by chlorination and ozonation. Water Res, 24(1):
11-22.
Mastro VC & Cameron EA (1976) Control of Archips semiferanus on
Quercus, spp with two chemical insecticides. J Econ Entomol,
69(4): 554-556.
Mathur A & Bhatnagar P (1991) A teratogenic study of carbaryl in
Swiss albino mice. Food Chem Toxicol, 29(9): 629-632.
Matsumura F & Sakai K (1968) Degradation of insecticides by
esterases of the American cockroach. J Econ Entomol, 61(3): 598-605.
Matsumura F & Ward CT (1966) Degradation of insecticides by the
human and the rat liver. Arch Environ Health, 13: 257-261.
Mayer FL Jr (1987) Acute toxicity handbook of chemicals to estuarine
organisms. Gulf Breeze, Florida, US Environmental Protection Agency,
Gulf Breeze Laboratory (EPA 600/8-87/017).
Mayer FL & Ellersieck MR (1986) Manual of acute toxicity:
Interpretation and data base for 410 chemicals and 66 species of
freshwater animals. Washington, DC, US Department of the Interior,
Fish and Wildlife Service (Resource Publication 160).
Mehendale HM & Dorough HW (1971) Glucuronidation mechanisms in the
rat and their significance in the metabolism of insecticides. Pestic
Biochem Physiol, 1: 307-318.
Mellon Institute (1958) Acute toxicity of carbaryl. Pittsburgh,
Pennsylvania, Mellon Institute (Unpublished proprietary information
submitted to WHO by Rhône-Poulenc Agro, Lyon).
Mellon Institute (1963) Results of the eighty weeks of inclusion of
Sevin in the diet of mice. Pittsburgh, Pennsylvania, Mellon
Institute, pp 1-12 (Special report No. 26-53) (Unpublished
proprietary information submitted to WHO by Rhône-Poulenc Agro,
Lyon).
Menzie CM (1969) Metabolism of pesticides. Washington, DC, US
Department of the Interior, Bureau of Sport Fisheries and Wildlife,
pp 72-76 (Special Scientific Report, Wildlife No. 127).
Miles CJ, Trehy ML, & Yost RA (1988a) Degradation of
N-methylcarbamate and carbamoyl oximes pesticides in chlorinated
water. Bull Environ Contam Toxicol, 41(6): 838-843.
Miles CJ, Trehy ML, & Yost RA (1988b) Fate of pesticides in
chlorinated water. Paper presented at the 195th Meeting of the
American Chemical Society. Washington, DC, American Chemical Society
(Abstract ENVR 139).
Miller NE (1990) Metabolism of C14-carbaryl under aerobic soil
conditions. Research Triangle Park, North Carolina, Rhône-Poulenc Ag
Company (Unpublished proprietary information submitted to WHO by
Rhône-Poulenc Agro, Lyon).
Miller A III, Henderson MC, & Buhler DR (1979) Covalent binding of
carbaryl (1-naphthyl- N-methylcarbamate) to rat liver microsome in
vitro. Chem-Biol Interact, 24: 1-17.
Molozhanova EG (1968) [Circulation of Sevin in compartments of the
environment.] Gig Primen Toxicol Pestic Klin Otrav, 1968: 355-362
(in Russian).
Molozhanova EG (1970) [Factual content of Sevin in compartments of
the environment and quantities of the formulation apt at getting
into human organism.] Gig Primen Toxicol Pestic Klin Otrav, 1970:
170-174 (in Russian).
Molozhanova EG & Kanevskij AA (1971) [Application of mathematical
analysis to modelling the behaviour of Sevin in soil.] Gig Primen
Toxicol Pestic Klin Otrav, 1971: 77-83 (in Russian).
Moreynis JA & Estrin IM (1965) [Sevin action on animals infected
with the virus of influenza.] Gig Primen Toxicol Pestic Klin Otrav,
1965: 435-442 (in Russian).
Moriya M, Ohta T, Watanabe K, Miyazawa T, Kato K, & Shirasu Y (1983)
Further mutagenicity studies on pesticides in bacterial reversion
assay systems. Mutat Res, 116: 185-216.
Moser VC, McCormick JP, Creason JP, & MacPhail RC (1988) Comparison
of chlordimeform and carbaryl using a functional observational
battery. Fundam Appl Toxicol, 11(2): 189-206.
Mount ME & Oehme FW (1981a) Carbaryl. A literature review. Residue
Rev, 80: 1-64.
Mount ME & Oehme FW (1981b) Diagnostic criteria for carbaryl
poisoning in sheep. Arch Environ Contam Toxicol, 10(4): 483-495.
Mount ME, Dayton AD & Oehme FW (1981) Carbaryl residues in tissues
in cholinesterase activities in brain and blood of rats receiving
carbaryl. Toxicol Appl Pharmacol, 58(2): 282-296.
Mumma RO, Khalifa S, & Hamilton RH (1971) Spectroscopic
identification of metabolites of carbaryl in plants. J Agric Food
Chem, 19(3): 445-451.
Murli H (1989) Mutagenicity test on carbaryl technical in an in
vitro cytogenetic assay measuring chromosomal aberration
frequencies in Chinese hamster ovary (CHO) cells. Kensington,
Maryland, Hazleton Laboratories America, Inc. (Report No.
10862-0-437) (Unpublished proprietary information submitted to WHO
by Rhône-Poulenc Agro, Lyon).
Murray HE & Guthrie RK (1980) Effects of carbaryl, diazinon and
malathion on native aquatic populations of microorganisms. Bull
Environ Contam Toxicol, 24: 535-542.
Murray FJ, Staples RE, & Schwets BA (1979) Teratogenic potential of
carbaryl given to rabbits and mice by gavage or by dietary
inclusion. Toxicol Appl Pharmacol, 51: 81-89.
Murthy NBK & Raghu K (1988) Soil bound residues of carbaryl and
1-naphthol: release and mineralization in soil, and uptake by
plants. J Environ Sci Health, B23(6): 575-585.
Murthy NBK & Raghu K (1989) Metabolism of 14C-carbaryl and
14C-naphthol in moist and flooded soils. J Environ Sci Health,
B24(5): 479-491.
Murthy NBK & Raghu K (1991) Fate of 14C-carbaryl in soils as a
function of pH. Bull Environ Contam Toxicol, 46(3): 374-379.
Myers WF (1977) Carbaryl insecticide. Estimation of carbaryl
exposure to humans. South Charleston, West Virginia, Union Carbide
Corporation (Project Report No. 111A12) (Unpublished proprietary
information submitted to WHO by Rhône-Poulenc Agro, Lyon).
Myers RC & Christopher SM (1987) Sevin technical dermal
sensitization study in the guinea-pig (Project No. ID-49-146).
Export, Pennsylvania, Bushy Run Research Center (Proprietary report
submitted to WHO by Rhône-Poulenc Agro, Lyon).
Myers RC, Weil CS, & Carpenter CP (1975) Sevin liquid, 44% carbaryl,
range-finding toxicity studies. Pittsburgh, Pennsylvania,
Carnegie-Mellon Research Institute (Unpublished proprietary
information submitted to WHO by Rhône-Poulenc Agro, Lyon).
Myhr S (1973) A screen for pesticide toxicity to protein and RNA
synthesis in HeLa cells. J Agric Food Chem, 21(3): 362-367.
Nagy Z, Mile I, & Antony F (1975) Mutagenic effect of pesticides on
Escherichia coli WP2 try. Acta Microbiol Acad Sci Hung, 22:
309-314.
Neskovic N, Terzic M, & Vitorovic S (1978) [Acute toxicity of
carbaryl and propoxur in mice previously treated with phenobarbital
and SKF-525-A.] Arh Hig Rada, 29: 251-256 (in Croatian).
Neuhauser EF, Loehr RC, Malecki MR, Milligan DL, & Durkin PR (1985)
The toxicity of selected organic chemicals to the earthworm Eisenia
fetida. J Environ Qual, 14(3): 383-388.
Nimmo DR, Hamaker TL, Matthews E, & Moore JC (1981) An overview of
the acute and chronic effects of first and second generation
pesticides on an estuarine Mysid. In: Vernberg FJ, Calabrese A,
Thurberg FP, & Vernberg WB ed. Biological monitoring of marine
pollutants. New York, Academic Press, Inc., pp 3-19.
NIOSH (1976) Criteria for a recommended standard ... Occupational
exposure to carbaryl. Cincinnati, Ohio, National Institute for
Occupational Safety and Health, 191 pp (DHEW (NIOSH) Publication No.
77-107).
Norris FA 1991) A terrestrial field soil dissipation study with
carbaryl (Study No. EC 89-080, File No. 40862). Research Triangle
Park, North Carolina, Rhône-Poulenc Ag Company (Unpublished
proprietary information submitted to WHO by Rhône-Poulenc Agro,
Lyon).
Nye DE & Dorough HW (1976) Fate of insecticides administered
endotracheally to rats. Bull Environ Contam Toxicol, 15(3): 291-296.
Odeyemi O (1982) Relative stability of carbaryl in tropical
ecosystems. Environ Pollut, A29(3): 207-213.
Okumura D, Melnicoe R, Jackson T, Drefs C, Maddy K, & Wells J (1991)
Pesticide residues in food crops analyzed by the California
Department of Food and Agriculture in 1989. Rev Environ Contam
Toxicol, 118: 87-150.
Olefir AI (1977) [Effects of pesticides on immunogenesis.] Vrach
Delo, 9: 121-123 (in Russian).
Olefir AI & Vinogradova VKh (1968) [A study of the embryotropic
effect of the pesticides Sevin and Zyram.] Vrach Delo, 11: 103-106
(in Russian).
Omer AA, Youssef MK, Abou-Youssef AY, & Khalil AM (1986)
Mutagenicity of insecticide Sevin and its derivative nitrosocarbaryl
on D. melanogaster. Alex J Agric Res, 31(3): 239-248.
Omkar & Shukla GS (1985) Toxicity of insecticides to Macrobrachium
lamarrei (H. Milne Edwards) (Decapoda, Palaemonidae). Crustaceana,
48: 1-5.
Önfelt A (1987) Spindle disturbances in mammalian cells. III.
Toxicity, c-mitosis and aneuploidy with 22 different compounds.
Specific and unspecific mechanisms. Mutat Res, 182: 135-154.
Önfelt A & Klasterska I (1983) Spindle disturbances in mammalian
cells. II. Induction of viable aneuploid/polyploid cells and
multiple chromatid exchanges after treatment of V-70 Chinese hamster
cells with carbaryl. Modifying effects of glutathion and S9. Mutat
Res, 119: 319-330.
Önfelt A & Klasterska I (1984) Sister chromatid exchanges and
thioguanine resistance in V79 Chinese hamster cells after treatment
with the aneuploidy inducing agent carbaryl +/ S9 mix. Mutat Res,
125: 269-274.
Oonnithan ES & Casida JE (1968) Oxidation of methyl- and
dimethylcarbamate insecticide chemicals by microsomal enzymes and
anticholinesterase activity of the metabolites. J Agric Food Chem,
18(1): 28-44.
Orlova NV & Zhalbe EP (1968) [Experimental material contributive to
the problem of maximal permissible amounts of Sevin in food
products.] Vopr Pitan, 27(6): 49-55 (in Russian).
Orzel RA & Weiss LR (1966) The effect of carbaryl
(1-naphthyl- N-methylcarbamate) on blood glucose, and liver and
muscle glycogen in fasted and nonfasted rats. Biochem Pharmacol, 15:
995-998.
Osborne BG, Barrett GM, Laal-Khoshab A, & Willis KH (1989) The
occurrence of pesticide residues in UK home-grown and imported
wheat. Pestic Sci, 27: 103-109.
Osman MA & Belal MH (1980) Persistence of carbaryl in canal water. J
Environ Sci Health, B15(3): 307-311.
Osman DH & Brindley WA (1981) Estimating monoxygenase detoxification
in field populations: toxicity and distribution of carbaryl in three
species of Labops grassbugs. Environ Entomol, 10(5): 676-680.
Osterloh J, Letz G, Pond S, & Becker Ch (1983) An assessment of the
potential testicular toxicity of 10 pesticides using the mouse sperm
morphology assay. Mutat Res, 116(3-4): 407-415.
Ott FT, McCullogh R, & Stanley JG (1981) Effects of forest spraying
on the ecology of brook trout. Report to the Maine Department of
Conservation, Forest Service, Augusta.
Owen BB Jr (1965) Aquatic insect populations reduced by aerial
spraying of insecticide Sevin. Philadelphia, Pennsylvania,
Pennsylvania Academy of Science, pp 63-69.
Page M, Haverty MJ, & Richmond ChE (1985) Residual activity of
carbaryl protected Lodgepole Pine against Mountain Pine Beetle,
Dillon, Colorado, 1982 and 1983. Berkeley, California, US Department
of Agriculture, Forest Service, Pacific Southwest Forest and Range
Experiment Station (Research Note PSW-375).
Panciera RJ (1967) Determination of teratogenic properties of orally
administrated 1-naphthyl- N-methylcarbamate (Sevin) in sheep.
Research Triangle Park, North Carolina, Union Carbide Agricultural
Products Co., Inc. (Unpublished proprietary information submitted to
WHO by Rhône-Poulenc Agro, Lyon).
Parafita MA & Fernandez-Otero P (1984) The interaction of carbaryl
with the metabolism of isolated hepatocytes. II. Effect on
glycogenesis. Gen Pharmacol, 15(4): 333-337.
Parafita MA, Fernandez-Otero P, & Aldegunde MA (1984) The
interaction of carbaryl with the metabolism of isolated hepatocytes.
I. Effect on respiration and glycolysis. Gen Pharmacol, 15(4):
327-331.
Paris DF & Lewis DL (1973) Chemical and microbiological degradation
of ten selected pesticides in aquatic system. Residue Rev, 45:
95-124.
Paris D, Lewis D, Barnett J, & Baugham G (1975) Microbial
degradation and accumulation of pesticides in aquatic systems.
Corvallis, Oregon, US Environmental Protection Agency, Office of
Research and Development, National Environmental Research Center, pp
1-45 (EPA-660/3-75-007).
Patocka JJ & Bajgar J (1971) Affinity of human brain
acetylcholinesterase to some organophosphates and carbamates in
vitro. J Neurochem, 18: 2545-2546.
Paulson GD & Feil VJ (1969) The fate of a single oral dose of
carbaryl (1-naphthyl- N-methyl-carbamate) in the chicken. Poult
Sci, 48: 1593-1597.
Paulson GD, Zaylskie RG, Zehr MV, Portnoy CE, & Feil VJ (1970)
Metabolites of carbaryl (1-naphthyl N-methyl-carbamate) in chicken
urine. J Agric Food Chem, 18: 110-115.
Pavlova II, Petrovskaya OG, & Ivanova SI (1968) [Biochemical and
histochemical studies on the oxidative-reductive enzymes after an
exposure to Sevin.] Gig Primen Toxicol Pestic Klin Otrav, 1968:
625-630 (in Russian).
Payne JA, Dutcher JD, & Ellis HC (1985) Low pressure soil
application of carbaryl for control of the pecan weevil, Curculio
carvae. J Agric Entomol, 2(1): 1-5.
Pekas JC (1972) Intestinal hydrolysis, metabolism and transport of a
pesticidal carbamate in pH G.5 medium. Toxicol Appl Pharmacol, 23:
62-70.
Pekas JC & Paulson GD (1970) Intestinal hydrolysis and conjugation
of a pesticidal carbamate in vitro. Science, 170: 77-78.
Perelygin VM, Shpirt MB, Aripov OA, & Ershova VI (1971) [Effect of
some pesticides on immunological response reactivity.] Gig i Sanit,
36(12): 29-33 (in Russian).
Petrovski VV (1970) [The level of content and duration of excretion
of Sevin in the milk of cows treated against ticks.] Gig Primen
Toxicol Pestic Klin Otrav, 1970: 193-196 (in Russian).
Pieper GR (1979) Residues analysis of carbaryl on forest foliage and
in stream water using HPLC. Bull Environ Contam Toxicol, 22(1-2):
167-171.
Pieper GR & Roberts RB (1978) Residue analysis of carbaryl on forest
foliage and in creek water. US Department of Agriculture, Forest
Service, Northern Region, pp 1-9 (Report No. 78-5).
Pipy B, Gaillard D, & Derache R (1979) Effect of blockage of the
reticulo-endothelial system on the carbaryl toxicity in the rat.
Toxicology, 12: 135-142.
Pipy B, Gaillard D, Beraud M, & Derache R (1980) Phagocytic activity
of the rat reticuloendothelial system and the pharmakokinetics of an
anticholinesterasic insecticide: carbaryl. Xenobiotica, 10(10):
785-793.
Pipy B, Gaillard D, & Derache R (1981) Phagocytic activity of the
reticuloendothelial system in the rat and rates of in vivo
excretion of metabolites of carbaryl and in vitro microsomal
metabolism. Biochem Pharmacol, 30(6): 669-672.
Pipy B, Gaillard D, Beraud M, & Derache R (1982) Enzymatic
activities of liver serine esterases during the reticuloendothelial
system phagocytosis blockade by carbaryl, an anticholinesterasic
insecticide. Toxicol Appl Pharmacol, 62: 11-18.
Pipy B, de Maroussem D, Beraud M, & Derache R (1983) Evaluation of
cellular and humoral mechanisms of carbaryl-induced
reticuloendothelial phagocytic depression. J Reticuloendothel Soc,
34(5): 395-412.
Podolak M & Warchocki B (1980) [Effect of toxobidin on the kinetics
of reaction of ChE inhibition by chlorfenvinfos and carbaryl.] Rocz
Panstw Zakl Hyg, 31(2): 125-131 (in Polish).
Podolak-Majczak M & Tyburczyk W (1984) Effect of joint action of
sodium nitrite and carbaryl on rats organism. Bromatol Chem
Toksykol, XVII(3): 211-214 (in Polish with English summary).
Podolak-Majczak M & Tyburczyk W (1986) [Effect of joint action of
sodium nitrite and carbaryl on rats organism. Part IV. Hepatotoxic
action.] Bromatol Chem Toksykol, XIX(3): 164-166 (in Polish with
English summary).
Post G & Schroeder TR (1971) The toxicity of four insecticides to
four salmonid species. Bull Environ Contam Toxicol, 6(2): 144-155.
Preussmann RG, Eisenbrand G, & Schmahl D (1976) Carcinogenicity
testing of low doses of nitrosopyrrolidine and of
nitrosobenzo-thiazuron and nitrosocarbaryl in rats. In: Walker EA,
Bogovski P, & Griciute L ed. Environmental N-nitroso compounds
analysis and formation. Proceedings of a Working Conference held at
the Polytechnical Institute, Tallinn, Estonian SSR, 1-3 October
1975. Lyon, International Agency for Research on Cancer, pp 429-433
(IARC Scientific Publications No. 14).
Prima NG, Prima AP, Sem'yan AI, Solov'eva VG, & Bondarenko VF (1976)
[Model studies of the rate of self-purification of river water from
Sevin and 1-naphthol residues.] Probl Okhr Vod, 7: 8-13 (in
Russian).
Probst GS, McMahon RE, Hill LE, Thompson CZ, Epp JK, & Neal SB
(1981) Chemically induced unscheduled DNA synthesis in primary rat
hepatocyte cultures: A comparison with bacterial mutagenicity using
218 compounds. Environ Mutagen, 3(1): 11-32.
Puech AA (undated) A study of the potential for human dermal and
inhalation exposure of Sevin, carbaryl insecticide during normal
garden use. Research Triangle Park, North Carolina, Union Carbide
Corporation (Unpublished proprietary information submitted to WHO by
Rhône-Poulenc Agro, Lyon).
Puyear RL & Paulson GD (1972) Effect of carbaryl (1-naphthyl-
N-methylcarbamate) on phenobarbital-induced sleeping time and some
liver microsomal enzymes in white leghorn cockerels. Toxicol Appl
Pharmacol, 22: 621-627.
Quarles JM & Tennant RW (1975) Effects of nitrosocarbaryl on
Balb/3T3 cells. Cancer Res, 35: 2637-2645.
Rajagopal BS & Sethunathan N (1984) Influence of nitrogen
fertilizers on the persistence of carbaryl and carbofuran in flooded
soils. Pestic Sci, 15: 591-599.
Rajagopal BS, Chendrayan K, Reddy B, Rajasekhar-Reddy B, &
Sethunathan N (1983) Persistence of carbaryl in flooded soils and
its degradation by soil enrichment cultures. Plant Soil, 73(1):
35-45.
Rajagopal BS, Rao VR, Nagendrappa G, & Sethunathan N (1984)
Metabolism of carbaryl and carbofuran by soil-enrichment and
bacterial cultures. Can J Microbiol, 30(12): 1457-1465.
Rajagopal BS, Panda S, & Sethunathan N (1986) Accelerated
degradation of carbaryl and carbofuran in a flooded soil pretreated
with hydrolysis products, 1-naphthol and carbofuran phenol. Bull
Environ Contam Toxicol, 36(6): 827-832.
Rajukkannu K, Basha AA, Habeebullah B, Duraisamy P, &
Balasubramanian M (1985) Degradation and persistence of DDT, BHC,
carbaryl and malathion in soils. Indian J Environ Health, 27(3):
237-243.
Rao KRSS, Rao KSP, Sahib IKA, & Rao KVR (1985a) Combined action of
carbaryl and phenthoate on a freshwater fish ( Carina punctatus
bloch). Ecotoxicol Environ Saf, 10(2): 209-217.
Rao KS, Rao KR, Sahib IK, & Rao KV (1985b) Combined action of
carbaryl and phenthoate on a tissue lipid derivatives of murrel,
Channa punctatus (Bloch). Ecotoxicol Environ Saf, 9(1): 107-111.
Ray SK & Poddar MK (1985a) Central cholinergic dopaminergic
interaction in carbaryl induced tremor. Eur J Pharmacol, 119(3):
251-253.
Ray SK & Poddar MK (1985b) Effect of pentylenetetrazol on carbaryl
induced changes in striatal catecholamines. Biochem Pharmacol,
34(4): 553-557.
Ray SK & Poddar MK (1990) Interaction of central serotonin and
dopamine in the regulation of carbaryl induced tremor. Eur J
Pharmacol, 181(3): 159-166.
Rawash IA, Gaaboub IA, El-Gayar FM, & El-Shazli AY (1975) Standard
curves for Nuvacron, Malathion, Sevin, DDT, and Kelthane tested
against the mosquito Culex pipiens L. and the microcrustacean
Daphnia magna Straus. Toxicology, 4: 133-144.
Regan JD, Seltow RB, Francis AA, & Lijinsky W (1976)
Nitrosocarbaryl: Its effect on human DNA. Mutat Res, 38: 239-302.
Reiner E & Aldridge WN (1967) Effect of pH on inhibition and
spontaneous reactivation of acethylcholinesterase treated with
esters of phosphorus acids and of carbamic acids. Biochem J, 105:
171-179.
Rickard RW & Dorough HW (1984) in vivo formation of
nitrosocarbamates in the stomach of rats and guinea-pigs. J Toxicol
Environ Health, 14(2-3): 279-290.
Rickard RW, Walter-Echols G, Dorough HW, & Lawrence LJ (1982)
Importance of pH in assessing the potential for nitrosocarbamate
formation in the stomach. Pestic Biochem Physiol, 18(3): 325-333.
Robel J, Felthausen RW, & Dayton D (1982) Effects of carbamates on
bobwhite food intake, body weight and locomotor activity. Arch
Environ Contam Toxicol, 11(5): 611-615.
Robens JF (1969) Teratologic studies of carbaryl, diazinon, norea,
disulfiram and thiram in small laboratory animals. Toxicol Appl
Pharmacol, 15: 152-163.
Roberts D (1975) The effect of pesticides on byssus formation in the
common mussel, Mytilus edulis. Environ Pollut, 8(4): 241-254.
Roberts BL & Dorough HW (1984) Relative toxicity of chemicals to the
earthworm Eisenia foetida. Environ Toxicol Chem, 3: 67-78.
Rocchi P, Perocco P, Alberhin W, Fini A, & Prodi G (1980) Effects of
pesticides on scheduled and unscheduled DNA synthesis of rat
thymocytes and human lymphocytes. Arch Toxicol, 45(2): 101-108.
Rodgers KE, Leung N, Imamura T, & Devens BH (1986) Rapid in vitro
screening assay for immunotoxic effects of organophosphorus and
carbamate insecticides in the generation of cytotoxic T-lymphocyte
responses. Pestic Biochem Physiol, 26(3): 292-301.
Rodriguez LD & Dorough HW (1977) Degradation of carbaryl by soil
microorganisms. Arch Environ Contam Toxicol, 6(1): 47-56.
Romine RR & Bussian RA (1971) Sevin Insecticide: The degradation of
carbaryl after surface application to a farm pond (Project No
111A13, File No. 15265). South Charleston, West Virginia, Union
Carbide Corporation (Unpublished proprietary information submitted
to WHO by Rhône-Poulenc Agro, Lyon).
Roszkowski J, Zadura J, & Skwarek P (1976) The effect of carbaryl on
immune response in chickens. Bull Vet Inst Pulawy, 20(3-4): 85-88.
Ruppert P, Cook L, Dean F, & Reiter W (1983) Acute behavioural
toxicity of carbaryl and propoxur in adult rats. Pharmacol Biochem
Behav, 18(4): 579-584.
Ryan AJ (1971) The metabolism of pesticidal carbamates. CRC Crit Rev
Toxicol, 1: 35-54.
Rybakova MN (1966) [Toxic effect of Sevin on animals.] Gig i Sanit,
31(9): 42-47 (in Russian with English summary).
Rybakova MN (1967) [Comparative toxic effect of Sevin and DDT used
in the treatment of food crops.] Vopr Pitan, 26: 9-15 (in Russian
with English summary).
Saini ML & Dorough HW (1978) Metabolism of carbaryl-naphthyl-C14
in a lactating cow, when administered orally. In: Edwards CA,
Veeresh GK, & Krueger HR ed. Proceedings of the Symposium on
Pesticide Residues in the Environment in India, 7-10 November 1978.
Hebbal, Bangalore, University of Agricultural Sciences, pp 74-87
(UAS Technical Series No. 32).
Sakai K & Matsumura F (1971) Degradation of certain organophosphate
and carbamate insecticides by human brain esterases. Toxicol Appl
Pharmacol, 19: 660-666.
Samanidou V, Fytianos K, Pfister G, & Bahadir M (1988) Photochemical
decomposition of carbamate pesticides in natural waters of Northern
Greece. Sci Total Environ, 76(1): 85-92.
Sanders HO & Cope DB (1966) Toxicity of several pesticides in two
species of Cladocerans. Trans Am Fish Soc, 95(2): 165-169.
Sanders HO, Finley MT, & Hunn JB (1983) Acute toxicity of six forest
insecticides to three aquatic invertebrates and four fishes.
Washington, DC, US Department of the Interior, Fish and Wildlife
Service, 5 pp (Technical Paper 110).
Sanderson DM (1961) Treatment of poisoning by anticholinesterase
insecticides in the rat. J Pharm Pharmacol, 13: 435-442.
Santolucito JA & Morrison G (1971) EEC of Rhesus monkeys following
prolonged low level feeding of pesticides. Toxicol Appl Pharmacol,
19: 147-154.
Santolucito JA & Whitcomb E (1971) Mechanical response of skeletal
muscle following oral administration of pesticides. Toxicol Appl
Pharmacol, 20: 66-72.
Schafer EA (1972) The acute oral toxicity of 369 pesticidal,
pharmaceutical and other chemicals to wild birds. Toxicol Appl
Pharmacol, 21: 315-330.
Schomburg CJ, Glottfeldy DE, & Seiber JN (1991) Pesticide occurrence
and distribution in fog collected near Monterey, California. Environ
Sci Technol, 25: 155-160.
Schulze TL, Hauge PN, Mitchell WJ, Remley WJ, Shahied SI, & Marshall
FJ (1979) New Jersey Epidemiologic Studies Program-Pesticides -
Assessment of human exposure to carbaryl during aerial application
for Gypsy moth control in New Jersey. New Jersey State Department of
Health.
Seiler JP (1977) Nitrosation in vivo and in vitro by sodium
nitrate and mutagenicity of nitrogenous pesticides. Mutat Res, 48:
225-236.
Shabanov M, Toshkov A, Georgiev D, & Ibrishimov N (1983) [Effect of
carbaryl on experimental Erysipelothrix rhusiopathiae infection in
rats.] Vet Med Nauk, 20(5-6): 9-15 (in Russian).
Shafik MT, Sullivan HC, & Enos NF (1971) A method for the
determination of 1-naphthol in urine. Bull Environ Contam Toxicol,
6(1): 34-39.
Shah PV & Guthrie FE (1983) Percutaneous penetration of three
insecticides in rats: a comparison of two methods for in vivo
determination. J Invest Dermatol, 80(4): 291-293.
Shah PV, Monroe RV, & Guthrie FE (1983) Comparative penetration of
insecticides in target and nontarget species. Drug Chem Toxicol,
6(2): 155-179.
Shah PV, Fisher HL, Sumler MR, Monroe RJ, Chernoff N, & Hall LL
(1987) Comparison of the penetration of 14 pesticides through the
skin of young and adult rats. J Toxicol Environ Health, 21(3):
353-366.
Sharma VK & Kaur S (1990) Contact sensitization by pesticides in
farmers. Contact Dermatitis, 23(2): 77-80.
Sharom MS, Miles JRW, Harris CR, & McEwen FL (1980) Behaviour of 12
insecticides in soil and aqueous suspensions of soil and sediment.
Water Res, 14(8): 1095-1100.
Shea TB (1985) Carbaryl inhibition of interferon synthesis in
cultured goldfish cells. Bull Environ Contam Toxicol, 34: 109-113.
Shea TB & Berry ES (1983) Toxicity of carbaryl and 1-naphthol to
goldfish (Carassius auratus) and Killifish (Fundulus
heteroclitus). Bull Environ Contam Toxicol, 31: 526-529.
Shea TB & Berry ES (1984) Suppression of interferon synthesis by the
pesticide carbaryl as a mechanism for enhancement of goldfish
virus-2 replication. Appl Environ Microbiol, 47(2): 250-252.
Shehata T, Richardson E, & Cotton E (1984) Assessment of human
population exposure to carbaryl from the 1982 Main Spruce budworm
spray project. J Environ Health, 46(6): 293-297.
Shilova SA, Denisova AV, Sedykh EL, & Efron KM (1973) [After effects
of insecticide treatment in the subartics.] Zool Zh, 52(7):
1008-1012 (in Russian).
Shimkin MB, Wieder R, McDonough M, Fishbein L, & Swern D (1969) Lung
tumour response in strain A mice as a quantitative bioassay of
carcinogenic activity of some carbamates and aziridines. Cancer Res,
29: 2184-2190.
Shpirt MB (1973) [Toxicological assessment of DDT, HCHC, TMTD, Sevin
and Zineb acting on human cell cultures.] Gig Tr Prof Zabol, 14:
32-35 (in Russian).
Shtenberg AI & Hovaeva LA (1970) [The effect of Sevin on the state
of thyroid gland of rats having received artificial full-value
ration with different content of iodine.] Gig i Sanit, 8: 119-122
(in Russian).
Shtenberg AI & Ozhovan MV (1971) [The effect of small doses of Sevin
upon the reproduction function of animals in several generations.]
Vopr Pitan, 1: 42-47 (in Russian).
Shtenberg AI & Rybakova MN (1968) Effect of carbaryl on the
neuroendocrine system in rats. Food Cosmet Toxicol, 6: 461-467.
Shtenberg AI, Orlova NV, Pozdnyakov AL, Tropova GP, Zhalbe JP,
Khovayeva LA, & Akincheva MJ (1970) [Functional and morpho-logical
parallels associated with the state of the sex glands of animals
under the influence of Sevin.] In: [Problems of the hygiene and
toxicology of pesticides.] Moscow, Meditsina, pp 124-129 (in
Russian).
Shtenberg AI, Orlova NV, & Torchinskij AM (1973) [On the effect of
pesticides of different chemical structure on gonads and
embryogenesis in experimental animals.] Gig i Sanit, 8: 16-20 (in
Russian).
Shukla GS, Omkar & Upadhyay VB (1982) Acute toxicity of few
pesticides to an aquatic insect, Ranatra elongata (Fabr.) J Adv
Zool, 3(2): 148-150.
Siboulet R, Grinfeld S, Deparis P, & Jaylet A (1984) Micronuclei in
red blood cells of the newt Pleurodeles waltl Michah: Induction
with X rays and chemicals. Mutat Res, 125(2): 275-281.
Siebert D & Eisenbrand G (1974) Induction of mitotic gene
conversions in Saccharomyces cerevisiae by N-nitrosated
pesticides. Mutat Res, 22: 121-126.
Sikka HC, Miyazaki S, & Rice CP (1973) Metabolism of selected
pesticides by marine microorganisms. Springfield, Virginia, National
Technical Information Service (Syracuse University Annual Report No.
2 - NTIS AS AD-763 410).
Sikka HC, Miyazaki S, & Lynch RS (1975) Degradation of carbaryl and
1-naphthol by marine microorganisms. Bull Environ Contam Toxicol,
13(6): 666-672.
Simpson JR (1965) Exposure to orchard pesticides, dermal and
inhalation exposures. Arch Environ Health, 10: 884-885.
Singh JM (1973) Decreased performance behaviour with carbaryl - an
indication of clinical toxicity. Clin Toxicol, 6(1): 97-108.
Singh KP, Pandey SY, Divekar VV, Surana KC, & Paralikar AB (1978)
Dissipation of carbaryl residues in and on cauliflower. In: Edwards
CA, Veeresh GK, & Krueger HR ed. Proceedings of the Symposium on
Pesticide Residues in the Environment in India, 7-10 November 1978.
Hebbal, Bangalore, University of Agricultural Sciences, pp 210-214
(UAS Technical Series No. 32).
Singh VP, Gupta S, & Saxena PK (1984) Evaluation of acute toxicity
of carbaryl and malathion to freshwater teleosts, Chauna punctatis
(bloch) and Heteropneustes fossilis (bloch). Toxicol Lett, 20:
271-276.
Sjoblad RD, Minard RD, & Bollag JM (1976) Polymerisation of
1-naphthol and related phenolic compounds by an extracellular fungal
enzyme. Pestic Biochem Physiol, 6: 457-463.
Skraba WJ & Young FG (1959) Radioactive Sevin (1-naphthyl-
1-carbon-14 N-methylcarbamate): a convenient synthesis. Agric Food
Chem, 7(9): 612-613.
Smalley HE (1970) Diagnosis and treatment of carbaryl poisoning in
swine. J Am Vet Med Assoc, 156(3): 339-344.
Smalley HE, Curtis JM, & Earl FL (1968) Teratogenic action of
carbaryl in beagle dogs. Toxicol Appl Pharmacol, 13: 392-403.
Smalley HE, O'Hara PJ, Bridges CH, & Radeleef RD (1969) The effect
of chronic carbaryl administration on the neuromuscular system of
swine. Toxicol Appl Pharmacol, 14: 409-419.
Snow CD & Stewart NE (1963) Treatment of Tikamook Bay oyster beds
with MGS-90 (Sevin) (Unpublished report from the Oregon Fish
Commission, submitted to WHO by Rhône-Poulenc Agro, Lyon).
Söderpalm BC & Önfelt A (1988) The action of carbaryl and its
metabolite alpha naphthol on mitosis in V79 Chinese hamster
fibroblasts. Indications of the involvement of some cholinester in
cell division. Mutat Res, 201(2): 349-363.
Sokur AJ (1971) [Hypokaliaemia in erythrocytes after intoxication of
white rats with some pesticides (DDT, Sevin, chlorophos).] Gig
Primen Toxicol Pestic Klin Otrav, 1971: 213-216 (in Russian).
Solomon H & Weis J (1979) Abnormal circulatory development in medaka
caused by the insecticides carbaryl, malathion and parathion.
Toxicology, 19: 51-62.
Spain AV (1974) The effects of carbaryl and DDT on the litter fauna
of a Corsican pine forest (Pinus nigra var. maritima): A
multivariate comparison. J Appl Ecol, 11: 467-481.
Springborn Bionomics, Inc. (1988a) Environmental fate of spray
applications of the rice insecticide Sevin XLR (a.i. carbaryl):
California (Study No. 565-6118/6112). Wareham, Massachusetts,
Springborn Bionomics, Inc. (Unpublished proprietary information
submitted to WHO by Rhône-Poulenc Agro, Lyon).
Springborn Bionomics, Inc. (1988b) Environmental fate of spray
applications of the forest insecticide Sevin-4-oil (a.i. carbaryl):
Oregon (Study No. 565-6120/6114). Wareham, Massachusetts, Springborn
Bionomics, Inc. (Unpublished proprietary information submitted to
WHO by Rhône-Poulenc Agro, Lyon).
Springborn Bionomics, Inc. (1988c) Environmental fate of spray
applications of the forest insecticide Sevin-4-oil (a.i. carbaryl):
Pennsylvania (Study No. 565-6121/6115). Wareham, Massachusetts,
Springborn Bionomics, Inc. (Unpublished proprietary information
submitted to WHO by Rhône-Poulenc Agro, Lyon).
Stanley JG & Trial JG (1980) Disappearance constants of carbaryl
from streams contaminated by forest spraying. Bull Environ Contam
Toxicol, 25(5): 771-776.
Statham CN & Lech JJ (1975) Potentiation of the acute toxicity of
several pesticides and herbicides in trouts by carbaryl. Toxicol
Appl Pharmacol, 34: 83-87.
Stegeman LC (1964) The effects of the carbamate insecticide carbaryl
upon forest soil mites and Collembola. J Econ Entomol, 57(6):
803-808.
Stevenson JH (1978) The acute toxicity of unformulated pesticides to
worker honey bees ( Apis Mellifera L.). Plant Pathol, 27: 38-40.
Stevenson JH, Needham PH, & Walker J (1977) Poisoning of honey bees
by pesticides: Investigations of the changing pattern in Britain
over 20 years. Rothamsted Rep, 2: 55-66.
Stewart NE, Millemann RE, & Breese WP (1967) Acute toxicity of the
insecticide Sevin and its hydrolytic product 1-naphthol to some
marine organisms. Trans Am Fish Soc, 96: 25-30.
Strait JR, Thornwall GC, & Ehrich M (1991) Sensitive
high-performance liquid chromatographic analysis for toxicological
studies with carbaryl. Washington, DC, American Chemical Society.
Street JC & Sharma RP (1975) Alteration of induced cellular and
humoral immune responses by pesticides and chemicals of
environmental concern: Quantitative studies on immunosuppression by
DDT, Aroclor 1254, carbaryl, carbofuran and methylparathion. Toxicol
Appl Pharmacol, 32(5): 587-602.
Strother A (1970) Comparative metabolism of selected
1-methylcarbamates by human and rat liver fractions. Biochem.
Pharmacol, 19: 2525-2529.
Strother A & Wheeler L (1976) Disposition of 14C-carbaryl in
pregnant, nonpregant and fetal tissues of rat. Fed Proc, 35: 327.
Strother A & Wheeler L (1980) Excretion and disposition of
[14C]carbaryl in pregnant, non-pregant and foetal tissues of the
rat after acute administration. Xenobiotica, 10(2): 113-124.
Struble CB, Feil VJ, Pecas JC, & Gerst JW (1983a) Biliary secretion
of 14C and identification of 5,6-dihydro-5,6-dihydroxycarbaryl
glucoronide as a biliary metabolite of (14C) carbaryl in the rat.
Pestic Biochem Physiol, 19: 85-94.
Struble CB, Pecas JC, & Gerst JW (1983b) Enterohepatic circulation
and biliary secretion of 5,6-dihydro-5,6-dihydroxy(14C)carbaryl
glucoronide in the rat. Pestic Biochem Physiol, 19(1): 95-103.
Subbotina SG & Belonozhko GA (1968) [The effect of Sevin on the
thermoresistance and fractionary protein content of blood serum.]
Gig Primen Toxicol Pestic Klin Otrav, 1968: 442-444 (in Russian).
Sud RK, Sud AK, & Gupta KG (1972) Degradation of Sevin (1-naphthyl
N-methylcarbamate) by Achromobacter sp. Arch Microbiol, 87:
353-358.
Sullivan LJ, Chin BH, & Carpenter CP (1972) in vitro vs. in vivo
chromatographic profiles of carbaryl anionic metabolites in man and
lower animals. Toxicol Appl Pharmacol, 22: 161-174.
Sundaram KMS & Szeto SY (1987) Distribution and persistence of
carbaryl in some terrestrial and aquatic components of a forest
environment. J Environ Sci Health, B22(5): 579-599.
Surprenant DC (1985a) Acute toxicity of carbaryl technical to
embryo-larvae of eastern oysters (Crassostrea virginica). Wareham,
Massachusetts, Springborn Bionomics, Inc. (Report No. BW-85-7-1817)
(Unpublished proprietary information submitted to WHO by
Rhône-Poulenc Agro, Lyon).
Surprenant DC (1985b) Acute toxicity of carbaryl technical to mysid
shrimp (Mysidopsis bahia). Wareham, Massachusetts, Springborn
Bionomics, Inc. (Report No. BW-85-6-1790) (Unpublished proprietary
information submitted to WHO by Rhône-Poulenc Agro, Lyon).
Surprenant DC (1985c) The chronic toxicity of carbaryl technical to
Daphnia magna under flow-through conditions. Wareham,
Massachusetts, Springborn Bionomics, Inc. (Report No. BW-85-7-1813)
(Unpublished proprietary information submitted to WHO by
Rhône-Poulenc Agro, Lyon).
Sussman HF, Cooke SB, & Bloch V (1969) Human louse infestation;
treatment with carbacide. Arch Dermatol, 100(1): 82-83.
Swartz WJ (1981) Long- and short-term effects of carbaryl exposure
in chick embryos. Environ Res, 26(2): 463-471.
Swartz WJ (1985) Effects of carbaryl on gonadal development in the
chick embryo. Bull Environ Contam Toxicol, 34: 481-485.
Szczepaniak S & Jeleniewicz K (1980) [Alterations of free amino
acids concentrations in rat blood erythrocytes following the acute
intoxication with carbaryl.] Bromatol Chem Toksykol, XIII(2):
159-163 (in Polish with English summary).
Szczepaniak S & Jeleniewicz K (1981) [ In vitro binding of carbaryl
and alpha naphthol with plasma and erythrocytes amino acids.]
Bromatol Chem Toksykol, XIV(2): 165-168 (in Polish with English
summary).
Szczepaniak S & Jeleniewicz K (1986) [Body amino acids balance in
acute poisoning with carbaryl.] Farm Pol, 9: 475-479 (in Polish).
Szczepaniak S, Siemkievicz E, Jeleniewicz K, & Nowinski H (1980)
[Effects of carbaryl and chlorfenvinfos on the level of free amino
acids in rat blood serum. Part II. Intoxication with a single dose
corresponding to 1/5 LD50.] Bromatol Chem Toksykol, XIII(4):
351-353 (in Polish with English summary).
Szeto SY, MacCarty HR, Oloffs PC, & Shephard RF (1979) The fate of
acephate and carbaryl in water. J Environ Sci Health, B14(6):
635-654.
Tagatz ME, Ivey JM, & Lehman HK (1979) Effects of Sevin on
development of experimental estuarine communities. J Toxicol Environ
Health, 5: 643-651.
Takahashi RN, Poli A, Morato GS, Lima TCM, & Zanin M (1991) Effects
of age on behavioral and physiological responses to carbaryl in
rats. Neurotoxicol Teratol, 13: 21-26.
Tewfik MS & Hamdi YA (1970) Decomposition of Sevin by a soil
bacterium. Acta Microbiol Pol Ser B, 19(2): 133-135.
Thomas A (1981) Introductory remarks: The testes. Environ Health
Perspect, 38: 3-4.
Thomas SJ (1986) Sevin brand carbaryl insecticide: Magnitude of
carbaryl residues in sugar beet roots (File No. 34934). Research
Triangle Park, North Carolina, Union Carbide Agricultural Products
Co., Inc. (Prepared in collaboration with Hazleton Laboratories
America, Inc., Chemical and Biomedical Sciences Division, Madison)
(Unpublished proprietary information submitted to WHO by
Rhône-Poulenc Agro, Lyon.
Thomas JH, Dieringer CS, & Schein L (1974) Effects of carbaryl on
mouse organs of reproduction. Toxicol Appl Pharmacol, 28: 142-145.
Thomas J, Visalakshi A, & Mohandas N (1982) Contamination of water
in rice fields sprayed with carbaryl for insect control. Entomon,
7(3): 379-380.
Thompson AR (1971) Effects of nine insecticides on the numbers and
biomass of earthworms in pasture. Bull Environ Contam Toxicol, 5(6):
577-586.
Tilak KS, Rao DMR, Devi AP, & Murty AS (1981) Toxicity of carbaryl
and 1-naphthol to four species of freshwater fish. J Biosci, 3(4):
457-462.
Tilden RL & Van Middelem CH (1970) Determination of carbaryl as an
amide derivative by electroncapture gas chromatography. J Agric Food
Chem, 18(1): 154-158.
Ting KC, Kho PK, Musselman AS, Root JA, & Tichelaar R (1984) High
performance liquid chromatographic method for determination of six
N-methylcarbamates in vegetables and fruits. Bull Environ Contam
Toxicol, 33: 538-547.
Tobin JS (1970) Carbofuran. A new carbamate insecticide. J Occup
Med, 12(1): 16-19.
Toor HS & Kaur K (1974) Toxicity of pesticides to the fish Cyprinus
carpio communis Linn. Indian J Exp Biol, 12: 334-336.
Tos-Luty S, Puchala-Matysek W, & Latuszynska J (1973) [Carbaryl
toxicity in chick embryo.] Bromatol Chem Toksykol, 6: 409-411 (in
Polish with English summary).
Trial JG (1978) The effects of Sevin-4-oil on aquatic insect
communities of streams: a continuation of 1976 studies. In:
Environmental monitoring of cooperative spruce budworm control
projects, Maine 1976 and 1977. Augusta, Maine, Maine Department of
Conservation, Forest Service, pp 124-140.
Trial JG (1979) The effects of Sevin-4-oil on aquatic insect
communities of stream (1976-1978). In: Environmental moitoring of
cooperative spruce budworm control projects, Maine 1978. Augusta,
Maine, Maine Department of Conservation, Forest Service, pp 6-22.
Triolo AJ, Lang WR, Coon JM, Lindstrom D, & Herr DL (1982) Effect of
the insecticide toxaphene and carbaryl on induction of lung tumors
by benzo(a)pyrene in the mouse. J Toxicol Environ Health, 9(4):
637-649.
Tripathi G & Shukla SP (1988) Toxicity bioassay of technical and
commercial formulations of carbaryl to the freshwater catfish,
Clarias batrachus. Ecotoxicol Environ Saf, 15(3): 277-281.
Tyburczyk W & Podolak-Majczak M (1984a) [Effect of joint action of
sodium nitrite and carbaryl on rats organism.] Bromatol Chem
Toksykol, XVII(2): 125-129 (in Polish with English summary).
Tyburczyk W & Podolak-Majczak M (1984b) [Effect of joint action of
sodium nitrite and carbaryl on rats organism.] Bromatol Chem
Toksykol, XVII(3): 215-219 (in Polish with English summary).
Tyburczyk W & Podolak-Majczak M (1986) [Effect of joint action of
sodium nitrite and carbaryl on rats organism. Part V. The role of
glucose-6-phosphate dehydrogenase and haematologic studies.]
Bromatol Chem Toksykol, XIX(3): 167-173 (in Polish with English
summary).
Uchiyama M, Takeda M, Suzuki T, & Yoshikawa K (1975) Mutagenicity of
nitroso derivatives of N-methylcarbamate insecticides in
microbiological method. Bull Environ Contam Toxicol, 14: 389-394.
US Department of Labour (1978) Occupational health guideline for
carbaryl (Sevin/R). Washington, DC, US Department of Labour, pp 1-5.
US EPA (1977) Aspects of pesticidal uses of carbaryl on man and the
environment. Washington, DC, US Environmental Protection Agency,
Office of Pesticide Programs, 286 pp.
US EPA (1980a) Carbaryl. Decision document. Washington, DC, US
Environmental Protection Agency, Office of Pesticides and Toxic
Substances, 22 pp.
US EPA (1980b) FIFRA Scientific Advisory Panel. Subcommittee
meeting, Arlington, Virginia, 23 July 1980. Washington, DC, US
Environmental Protection Agency, 202 pp.
US EPA (1981) Environmental fates and impacts of major forest use of
pesticides. Washington, DC, US Environmental Protection Agency,
Office of Pesticides and Toxic Substances.
US EPA (1984) Health and environmental profile for carbaryl.
Cincinnati. Ohio, US Environmental Protection Agency.
Usha Rani MV, Reddi OS, & Reddy PP (1980) Mutagenicity studies
involving aldrin, endosulfan, demethoate, phosphamidon, carbaryl and
ceresan. Bull Environ Contam Toxicol, 25(2): 277-282.
Vandekar M (1965) Observations of the toxicity of carbaryl,
folithion and 3-isopropylphenyl N-methylcarbamate in a village
scale trail in Southern Nigeria. Bull World Health Organ, 33:
107-115.
Vandekar M, Plestina R, & Wilhelm M (1971) Toxicity of carbamates
for mammals. Bull World Health Organ, 44: 241-249.
Varshney TN & Gaur AC (1972) Effect of DDT and Sevin on soil fungi.
Acta Microbiol Acad Sci Hung, 19: 97-102.
Vashakidze VI (1966) [On the question of the embryotropic,
gonadotropic and mutagenic effect of some pesticides.] Gig Primen
Toxicol Pestic Klin Otrav, 1966: 80-83 (in Russian).
Vashakidze VI (1975) [Effect of small doses of Sevin on gonad
function following its repeated effect on white rats SbTr NII.] Gig
Tr Prof Zabol, 14: 235-266 (in Russian).
Vasilos AF, Dmitrienko VD, & Shroyt IG (1975) [Disruption of the
mitotic system following acute Sevin poisoning.] Izv Akad Nauk
Moldavskoj SSR Ser Khim Nauk, 3: 64-67 (in Russian).
Venkateswarlu K, Chendrayan K, & Sethunathan N (1980) Persistence
and biodegradation of carbaryl in soils. J Environ Sci Health,
B15(4): 421-429.
Venkat Reddy P, Prem Veer Reddy G, & Subramanyams S (1974)
Cytological effects of the insecticide Sevin on the mitotic cells of
Poecilocerus pictus. Curr Sci, 43(6): 187-189.
Verma SR, Tonk IF, Gupta AK, & Saxena M (1984) Evaluation of an
application factor for determining the safe concentration of
agricultural and industrial chemicals. Water Res, 18(1): 111-115.
Viter VF (1978) [Monotonous and intermittent inhalatory effect of
Sevin upon some behavioural reactions of experimental animals.] Gig
i Sanit, 11: 33-34 (in Russian).
Vyatchanikov KA & Alexachina ZA (1968) [Experimental investigations
for procuring therapeutic and protective means at intoxications by
Sevin.] Gig Primen Toxicol Pestic Klin Otrav, 1968: 442-444 (in
Russian).
Wagner R (1973) [Identification and estimation of organophosphate
insecticides and carbaryl on ready made thin-layer plates.] Z
Gesamte Hyg Grenzgeb, 19(7): 504-510 (in German).
Wakakura M, Ishikawa S, & Uga S (1978) Ultrastructural hepatic
changes by carbamate pesticide (Sevin) in rats. Environ Res, 16:
191-204.
Walker N, Janes NF, Spokes JR, & Van Berkum P (1975a) Degradation of
1-naphthol by a soil pseudomonad. J Appl Bacteriol, 39: 281-286.
Walker M, Gale GR, Atkins LM, & Gadse-Den RH (1975b) Some effects of
carbaryl on Ehrlich ascites tumor cells in vitro and in vivo.
Bull Environ Contam Toxicol, 14(4): 441-448.
Waller GD (1969) Susceptibility of an alfalfa leaf-cutting bee to
residues of insecticides on foliage. J Econ Entomol, 62(1): 189-192.
Ward SA, May DG, Heath AJ, & Branch RA (1988) Carbaryl metabolism is
inhibited by Cimetidine in the isolated perfused rat liver and in
man. J Toxicol Clin Toxicol, 26(5/6): 269-281.
Wauchope RD & Haque R (1973) Effects of pH, light and temperature on
carbaryl in aqueous media. Bull Environ Contam Toxicol, 9(5):
257-260.
Weber FJ, Shea TB, & Berry SE (1982) Toxicity of certain
insecticides to protozoa. Bull Environ Contam Toxicol, 28: 618-631.
Weil CS & Carpenter CP (1962) Studies on carcinogenesis completed in
1962. Pittsburgh, Pennsylvania, Carnegie-Mellon Research Institute
(Special Report 25-122) (Unpublished proprietary information
submitted to WHO by Rhône-Poulenc Agro, Lyon).
Weil CS & Carpenter CP (1965) Results of a three generations
reproductive study on rats fed Sevin in their diet. Pittsburgh,
Pennsylvania, Carnegie-Mellon Research Institute, 18 pp (Report No.
28-53) (Unpublished proprietary information submitted to WHO by
Rhône-Poulenc Agro, Lyon).
Weil CS & Carpenter CP (1974) Sevin: range finding toxicity studies.
Pittsburgh, Pennsylvania, Carnegie Mellon (Report No. 37-53)
(Unpublished proprietary information submitted to WHO by
Rhône-Poulenc Agro, Lyon).
Weil CS, Woodside MD, Carpenter CP, & Smyth HF Jr (1972) Current
studies of tests of carbaryl for reproductive and teratogenic
effect. Toxicol Appl Pharmacol, 21: 390-404.
Weil CS, Woodside MD, Bernard JB, Condra NI, King JM, & Carpenter CP
(1973) Comparative effect of carbaryl on rat reproduction and
guinea-pig teratology when fed either in the diet or by stomach
intubation. Toxicol Appl Pharmacol, 26: 621-638.
Weston RF, Inc. (1982) Carbaryl: A profile on its characteristics as
pesticide. Rockville, Maryland, Roy F. Weston, Inc., Environmental
Health Sciences Centre.
Whitehurst WE, Bishop ET, Critchfield FE, Gyrisco GG, Huddleston EW,
Arnold H, & Lisk OJ (1963) The metabolism of Sevin in dairy cows. J
Agric Food Chem, 11: 167-169.
WHO (1982) Recommended health-based limits in occupational exposure
to pesticides. Report of a WHO study group. Geneva, World Health
Organization, 110 pp (WHO Technical Report Series 677).
WHO (1986) IPCS Environmental Health Criteria 64: Carbamate
pesticides - A general introduction. Geneva, World Health
Organization, 136 pp.
WHO (1992) The WHO recommended classification of pesticides by
hazard and guidelines to classification 1992-1993. Geneva, World
Health Organization, 66 pp (Unpublished document WHO/PCS/92.14).
WHO/FAO (1975) Data sheets on pesticides No. 3: Carbaryl. Geneva,
World Health Organization, 8 pp (Unpublished document VBC/DS/75.3,
Rev. 1).
Whorton MD, Milby TH, Stubbs HA, Avasha BH, & Hull EG (1979)
Testicular function among carbaryl exposed employees. J Toxicol
Environ Health, 5: 925-941.
Wiggins OG & Weiden MHJ (1969) Fate of Sevin applied to the surface
of bean fruit and bean plants under field conditions. Clayton, North
Carolina, Union Carbide Corporation (Report No. 11377) (Unpublished
proprietary information submitted to WHO by Rhône-Poulenc Agro,
Lyon).
Wilkes L, Mulkey N, & Wargo J (1977) Research report laboratory soil
metabolism study of carbaryl (Project No. 285). Monument, Colorado,
Analytical Development Corp. (Unpublished proprietary information
submitted to WHO by Rhône-Poulenc Agro, Lyon).
Wills JH, Jameson E, & Coulston F (1968) Effects of oral doses of
carbaryl on man. Clin Toxicol, 1:(3) 265-271.
Wiltrout RW, Ercegovich CD, & Ceglowski WS (1978) Humoral immunity
in mice following oral administration of selected pesticides. Bull
Environ Contam Toxicol, 20(3): 423-431.
Winterlin W & Walker G (1973) Carbaryl residues in bees, honey, and
bee bread following exposure to carbaryl via the food supply. Arch
Environ Contam Toxicol, 1(4): 362-374.
Wojciechowski JP, Kaur P, & Sabharual PS (1982) Induction of ouabain
resistance in V-79 cells by four carbamate pesticides. Environ Res,
29: 48-53.
Wolfe HP, Durham W, & Armstrong J (1967) Exposure of workers to
pesticides. Arch Environ Health, 14: 622-633.
Wolfe NL, Zepp RG, & Paris DF (1978) Carbaryl, propham and
chlorpropham: A comparison of the rates of hydrolysis and photolysis
with the rate of biolysis. Water Res, 12(8): 565-571.
Woodruff RC, Phillips JP, & Irwin D (1983) Pesticide-induced
complete and partial chromosome loss in screens with
repair-defective females of Drosophila melanogaster. Environ
Mutagen, 5: 835-846.
Woodward DF & Mauck WL (1980) Toxicity of five forest insecticides
to cutthroat trout and two species of aquatic invertebrates. Bull
Environ Contam Toxicol, 25: 846-853.
Wright CG, Leidy RB, & Dupress HE (1981) Insecticides in the ambient
air of rooms following their application for control of pests. Bull
Environ Contam Toxicol, 26(4): 548-553.
Wuu K & Grant W (1966) Morphological and somatic chromosomal
aberrations induced by pesticides in barley (Hordeum vulgare). Can
J Genet Cytol, 8: 481-501.
Wyrobek AJ, Watchmaker G, Gordon L, Wong K, Moore D, & Whorton D
(1981) Sperm shape abnormalities in carbaryl-exposed employees.
Environ Health Perspect, 40: 225-265.
Yadav PR & Jaglan RS (1982) Persistence of carbaryl residues in and
on unprocessed and processed cauliflower curds. Beitr Trop
Landwirtsch Veterinärmed, 20(1): 89-95.
Yadav GS, Kathpal TS, & Singh H (1985) Efficacy of monocrotophos,
carbaryl, and endosulfan against Antigastra catalaunalis Dup. and
their persistence in soil and sesamum seeds. Indian J Agric Sci,
55(6): 438-442.
Yakim VS (1965) [Toxicological hygienic characteristics of Sevin.]
Gig Primen Toxicol Pestic Klin Otrav, 1965: 423-431 (in Russian).
Yakim VS (1967) [Data for substantiating the MAC values for Sevin in
the air of the working zone.] Gig i Sanit, 4: 23-33 (in Russian).
Yakim VS (1968) [The toxicity of Sevin at inhalatory intake.] Gig
Primen Toxicol Pestic Klin Otrav, 1968: 115-117 (in Russian).
Yakim VS (1970) [Content and distribution of Sevin in warm-blooded
animals organism and its effect on ChE activity.] Gig Primen Toxicol
Pestic Klin Otrav, 1970: 214-218 (in Russian).
Yess NJ, Houston MG, & Gunderson EL (1991a) Food and Drug
Administration pesticide residue monitoring of foods: 1978-1982. J
Assoc Off Anal Chem, 75(2): 265-272.
Yess NJ, Houston MG, & Gunderson EL (1991b) Food and Drug
Administration pesticide residue monitoring of foods: 1983-1986. J
Assoc Off Anal Chem, 74(2): 273-280.
Young RR (1990) Mutagenicity test on carbaryl (technical) in the
CHO/HGPRT forward mutation assay. Kensington, Maryland, Hazleton
Laboratories America, Inc. (Study No. 10862-0-435) (Unpublished
proprietary information submitted to WHO by Rhône-Poulenc Agro,
Lyon).
Zabezhinski MA (1970) [Possible carcinogenic effect of ß-Sevin.]
Vopr Onkol, 16: 106-107 (in Russian).
Zapko WG (1970) [Effect on some liver functions of acute
intoxication of experimental animals with cuprosin, Sevin, Eptam and
TMTD.] Gig Primen Toxicol Pestic Klin Otrav, 1970: 237-240 (in
Russian).
Zeakes SJ, Hansen MF, & Robel RJ (1981) Increased susceptibility of
Bobwhites (Colinus virginianus) to Histomonas meleagridis after
exposure to Sevin insecticide. Avian Dis, 25(4): 981-987.
Zepp RG & Cline DM (1977) Rates of direct photolysis in the aquatic
environment. Environ Sci Technol, 11: 359-366.
Zepp RG, Wolfe N, Gordon L, & Fincher RC (1976) Light-induced
transformations of methoxychlor in aquatic systems. J Agric Food
Chem, 24: 727-733.
Zinkl JG, Hennys ChJ, & Dewelse LR (1977) Brain cholinesterase
activity in birds from forest sprayed trichlorfon (Dylox) and
carbaryl (Sevin-4-oil). Bull Environ Contam Toxicol, 17(4):
379-386.
Zuberli R & Zubairi MY (1971) Carbaryl degradation by Pseudomonas
phaseolicola and Aspergillus niger. Pak J Sci Ind Res, 14(4-5):
383-384.
Zweig G, Gao R-Y, Witt JM, Propendorf W, & Bogen K (1984) Dermal
exposure to carbaryl by strawberry harvesters. J Agric Food Chem,
32(6): 1232-1236.
Zweig G, Leffingwell JT, & Propendorf W (1985) The relationship
between dermal pesticide exposure to fruit harvesters and
dislodgeable foliar residues. J Environ Sci Health, B20(1): 27-59.
ANNEX I. TREATMENT OF CARBAMATE PESTICIDE POISONING IN MAN
(From EHC 64: Carbamate Pesticides - A General Introduction)
All cases of carbamate poisoning should be dealt with as an
emergency and the patient should be hospitalized as quickly as
possible.
Extensive descriptions of the treatment of poisoning by
anticholinesterase agents are given in several major references
(Kagan, 1977;Taylor, 1980; Plestina, 1984).
The treatment is based on:
(a) minimizing the absorption;
(b) general supportive treatment; and
(c) specific pharmacological treatment.
I.1 Minimizing the absorption
When dermal exposure occurs, decontamination procedures include
removal of contaminated clothes and washing of the skin with
alkaline soap or with a sodium bicarbonate solution. Particular care
should be taken in the cleaning of the skin area where venupuncture
is performed. Blood might be contaminated with carbamates and
therefore inaccurate measures of ChE inhibition might result.
Extensive eye irrigation with water or saline should also be
performed. In the case of ingestion, vomiting can be induced, if the
patient is conscious, by the administration of ipecacuanha syrup
(10-30 ml) followed by 200 ml of water. However, this treatment is
contraindicated in the case of pesticides dissolved in hydrocarbon
solvents. Gastric lavage (with the addition of bicarbonate solution
or activated charcoal) can also be performed, particularly in
unconscious patients, taking care to prevent aspiration of fluids
into the lungs (i.e., only after a tracheal tube has been put in
place).
The volumes of the fluids introduced in the stomach should be
recorded and samples of gastric lavage frozen and stored for
subsequent chemical analysis. If the formulation of the pesticide
involved is available, it should also be stored for further analysis
(i.e., detection of toxicologically relevant impurities). A purge to
remove the ingested compound can be administered.
I.2 General supportive treatment
Artificial respiration (via a tracheal tube) should be started
at the first sign of respiratory failure and maintained for as long
as necessary.
Cautious administration of fluids is advised as well as general
supportive and symptomatic pharmacological treatment and absolute
rest.
I.3 Specific pharmacological treatment
I.3.1 Atropine
Atropine should be given, beginning with 2 mg iv repeated at 15
to 30-min intervals. The dose and the frequency of atropine
treatment varies from case to case, but should maintain the patient
fully atropinized (dilated pupils, dry mouth, skin flushing, etc.).
I.3.2 Oxime reactivations
Although it might be suspected that oxime cholinesterase
reactivators would be as helpful in carbamate poisoning as they are
in organophosphorous poisoning, this is not the case. There is
experimental evidence that the pyridinium oxime 2-PAM is not
effective in carbamate poisoning and there is some evidence that it
makes poisoning by certain carbamates, including carbaryl, worse.
I.3.3 Diazepam
Diazepam should be included in the therapy of all but the
mildest cases. Besides relieving anxiety it appears to counteract
some aspects of CNS-derived symptoms that are not affected by
atropine. Doses of 10 mg sc or iv are appropriate and may be
repeated as required.
Other centrally acting drugs and drugs that may depress
respiration are not usually recommended in the absence of artificial
respiration procedures.
References to Annex I
KAGAN, J.S. (1977) [The toxicity of organophosphorus pesticides.]
Moscow (in Russian).
PLESTINA, R. (1984) Prevention, diagnosis, and treatment of
insecticide poisoning. Geneva, World Health Organization.
(Unpublished WHO document VBC/84.889).
TAYLOR, P. (1980) Anticholinesterase agents. In: Goodman, L.S. &
Gilman, A., ed. The pharmacological basis of therapeutics. 6th ed.,
New York, Macmillan Publishing Co., pp. 100-119.
RESUME ET EVALUATION, CONCLUSIONS ET RECOMMANDATIONS
1. Résumé et évaluation
1.1 Identité, propriétés et méthodes d'analyse
Carbaryl est la dénomination commune d'un dérivé de l'acide
carbamique, le N-méthylcarbamate de 1-naphtyle. Le produit
technique consiste en un solide cristallin blanc, peut volatil et
peu soluble dans l'eau, qui est stable à la lumière et à la chaleur
mais qui s'hydrolyse facilement en milieu alcalin. Il existe une
norme FAO pour le carbaryl qui stipule un degré de pureté de 98%,
avec une limite pour les impuretés ( N-méthylcarmate de ß-naphtyle)
de 0,05%.
Pour les analyses portant sur le carbaryl et ses métabolites,
il existe de nombreuses méthodes: chromatographie sur couche mince,
spectrophotométrie, chromatographie en phase gazeuse,
chromatographie en phase liquide à haute pression et spectrométrie
de masse à ionisation chimique. La limite de détection peut
descendre en-dessous du nanogramme et le taux de récupération
dépasse généralement 80%.
1.2 Production et usages
On utilise le carbaryl depuis une trentaine d'années comme
insecticide agissant par contact et par ingestion avec certaines
propriétés endothérapiques; il permet de lutter contre de nombreux
vecteurs et ravageurs. La principale unité de production se trouve
aux Etats-Unis d'Amérique. Plus de 290 fabricants proposent une
gamme de formulations de carbaryl qui dépasse les 1500 produits.
1.3 Transport, distribution et transformation dans l'environnement
Dans la plupart des conditions, le carbaryl ne persiste pas
dans l'environnement. Dans l'eau, son temps de demi-hydrolyse dépend
de la température, du pH et de la concentration initiale; il varie
de quelques minutes à plusieurs semaines. Le principal produit de
décomposition est le 1-naphtol.
L'accumulation du carbaryl, exprimée sous la forme d'un facteur
de bioconcentration dans l'environnement aquatique, et plus
précisément chez les poissons d'eau douce, varie de 14 à 75. Le
carbaryl s'adsorbe plus facilement sur les sols à forte teneur
organique que sur les sols sableux. Aux doses d'emploi usuelles, et
lorsqu'il est appliqué conformément "aux bonnes pratiques
agricoles", il se dissipe rapidement, avec une demi-vie de 8 jours à
un mois dans les conditions normales. Il arrive que le carbaryl soit
entraîné par les pluies ou par les travaux agricoles de la surface
vers les couches sous-jacentes (à un mètre de profondeur).
Le carbaryl peut contaminer la végétation, soit au cours de
l'épandage soit par migration à partir d'un sol contaminé.
La décomposition du carbaryl dans l'environnement dépend de son
degré de volatilisation, de photodécomposition et de dégradation
chimique ou microbienne dans le sol, l'eau et les végétaux. la
vitesse de décomposition est plus rapide en climat chaud.
1.4 Concentrations dans l'environnement et exposition humaine
Dans la population générale, le carbaryl est principalement
absorbé par la voie alimentaire.
Les résidus présents dans des échantillons de rations totales
sont relativement faibles, et ils vont de traces à 0,05 mg/kg. Aux
Etats-Unis d'Amérique, on estime que pendant les premières années
d'utilisation du carbaryl, l'ingestion journalière de carbaryl était
de 0,15 mg/personne/jour (dans 7,4% des composites); cette dose est
tombée à 0,003 mg/personne/jour en 1969 (dans seulement 0,8% des
composites). Pendant la période d'épandage, on peut retrouver
parfois du carbaryl dans les eaux de surface et les retenues d'eau.
La population peut être exposée au carbaryl lors d'opérations
de lutte de contre les ravageurs ou les vecteurs tant au domicile
que sur les aires de loisir.
Les travailleurs peuvent être exposés au carbaryl lors de la
fabrication, de la formulation, de l'emballage, du transport et du
stockage du produit ainsi que pendant son épandage. Les
concentrations dans l'atmosphère des lieux de travail au cours de la
production varient de < 1 mg/m3 à 30 mg/m3. Il peut y avoir une
importante exposition cutanée chez les travailleurs de l'industrie
et les ouvriers agricoles en cas de mesures de protection
insuffisantes.
1.5 Cinétique et métabolisme
Le carbaryl est rapidement absorbé au niveau des poumons et des
voies digestives. Une dose de carbaryl dans l'acétone appliquée sur
la peau de volontaires humains a été absorbée par voie percutanée à
raison de 45% en 8 heures. Toutefois, les données obtenues in vitro
concernant la pénétration cutanée ainsi que les données
toxicologiques indiquent que l'absorption percutanée s'effectue
généralement à un rythme beaucoup plus lent.
Les principales voies métaboliques du carbaryl consistent en
une hydroxylation du cycle et une hydrolyse. Il en résulte la
formation de nombreux métabolites qui subissent ensuite une
conjugaison avec formation de sulfates, de glucuronides et de
mercapturates hydrosolubles qui sont excrétés dans l'urine.
L'hydrolyse conduit à la formation de 1-naphtol, de dioxyde de
carbone et de méthylamine. L'hydroxylation produit du
4-hydroxycarbaryl, du 5-hydroxycarbaryl, du
N-hydroxyméthylcarbaryl, du 5,6-dihydro-5,6-dihydroxycarbaryl et
du 1,4-naphtalènediol. Le principal métabolite chez l'homme est le
1-naphtol.
Dans les conditions normales d'exposition, il est improbable
que le carbaryl s'accumule chez les animaux. Il est excrété
principalement par la voie urinaire étant donné que la
détoxification de son produit d'hydrolyse, le 1-naphtol, s'effectue
essentiellement par la formation de conjugués hydrosolubles. Dans le
cas des métabolites du carbaryl, le cycle entérohépatique joue
également un rôle considérable, en particulier après administration
par voie orale.
Le produit d'hydrolyse, l'acide N-naphtolcarbamique, se
décompose spontanément en méthylamine et dioxyde de carbone. La
méthylamine subit ensuite une déméthylation en dioxyde de carbone et
formiate, ce dernier étant ultérieurement excrété, en majeure partie
dans l'urine.
Des métabolites du carbaryl sont également présents en faible
proportion de la dose absorbée dans la salive et le lait.
1.6 Effets sur les êtres vivants dans leur milieu naturel
Les valeurs de la CL50 pour les crustacés varient de 5 à 9
µg/litre (puces d'eau et mysidés), 8 à 25 µg/litre (orchesties) et
500 à 2500 µg/litre (écrevisses). Chez les insectes aquatiques, les
limites de sensibilité sont du même ordre. Les plécoptères et
éphémèroptères (perles et éphémères) en constituent les groupes les
plus sensibles. Les mollusques sont moins sensibles avec des valeurs
de la CE50 de l'ordre de quelques mg/litre. Dans le cas des
poissons, la plupart des valeurs de la CL50 se situent entre 1 et
30 mg/litre. Les salmonidés constituent le groupe le plus sensible.
La toxicité aiguë est faible pour les oiseaux. La DL50 pour
la sauvagine et le gibier à plumes en général est > 1000 mg/kg.
D'après les tests, l'oiseau le plus sensible est un francolin
(Francolinus levaillanti) (DL50 = 56 mg/kg). Rien n'indique que
les oiseaux aient eu à souffrir de l'effet des épandages effectués
sur les zones forestières à la dose de 1,1 kg de carbaryl/ha.
Le carbaryl est très toxique pour les abeilles et les lombrics.
Dans le cas des abeilles, la DL50 par voie orale est de 0,16
µg/insecte (soit 1-2 mg/kg).
On est fondé à penser que le carbaryl puisse avoir une
influence temporaire sur la composition en espèces des écosystèmes
terrestres et aquatiques. Par exemple, une étude a montré que les
effets exercés sur certaines communautés d'invertébrés terrestres
pourraient persister au moins 10 mois après un seul épandage.
1.7 Effets sur les animaux d'expérience et les systèmes d'épreuve
in vitro
La toxicité aiguë, exprimée sous la forme de la DL50, varie
considérablement selon l'espèce, la formulation et le véhicule du
produit. La DL50 estimative par voie orale pour le rat varie de
200 à 850 mg(kg. Les chats sont plus sensibles, avec une DL50 de
150 mg/kg. Les porcs et les singes le sont moins, avec une DL50 >
1000 mg/kg.
Une concentration de 792 mg de matière active par m3, qui
constitue la valeur maximale qu'on puisse obtenir pour un aérosol, a
produit, au cours d'une exposition de 4 heures, une mortalité de 20%
(1/5) chez des rattes. A des concentrations de 20 mg/m3, des
aérosols de carbaryl ont provoqué une réduction de l'activité
cholinestérasique chez des chats lors d'une exposition de 4 heures,
mais cette concentration n'a eu aucun effet observable chez des
rats.
Le carbaryl est légèrement irritant pour l'oeil et n'a que peu
ou pas de pouvoir sensibilisateur. Lors d'études à long terme, la
concentration sans effet nocif observable a été évaluée à 10 mg/kg
de poids corporel (200 mg/kg de nourriture) pour le rat et à 1,8
mg/kg de poids corporel (100 mg/kg de nourriture) pour le chien.
Chez le chat, soumis à une exposition de longue durée par
inhalation, la concentration sans effet nocif observable est de 0,16
mg/m3. Le carbaryl a un faible potentiel d'accumulation.
1.7.1 Reproduction
On a montré que le carbaryl avait des effets indésirables sur
la reproduction des mammifères et le développement périnatal chez un
certain nombre d'espèces. Ces effets sur la reproduction consistent
en une réduction de la fécondité, une diminution de l'effectif des
portées et une réduction de la viabilité postnatale. Les effets
toxiques du carbaryl sur le développement se traduisent pas un
certain nombre de morts foetales, une réduction du poids foetal et
la présence de malformations. A l'exception d'un petit nombre
d'études, les effets nocifs sur la reproduction et le développement
n'ont été constatés en totalité qu'à des doses manifestement
toxiques pour la mère, et dans un certain nombre de cas, la mère
était plus sensible au carbaryl que l'embryon ou le foetus. Ces
effets toxiques sur la femelle gestante consistaient en une
mortalité accrue, une réduction de la croissance et des dystocies.
Les données indiquent que, par rapport à l'organisme adulte, la
fonction de reproduction et le processus de développement des
mammifères n'est pas particulièrement sensible au carbaryl.
1.7.2 Mutagénicité
Un certain nombre de tests in vitro et in vivo ont été
effectués sur la carbaryl afin d'en évaluer le pouvoir mutagène;
divers points d'aboutissement de ces effets ont été étudiés sur
divers systèmes (bactéries, levures, végétaux, insectes et
mammifères).
Les données disponibles montrent que le carbaryl n'a aucune
tendance à endommager l'ADN. On ne dispose d'aucun rapport
confirmant les caractères mutagènes suivants: induction de
recombinaisons mitotiques, conversion génique, et synthèse non
programmée de l'ADN chez les procaryotes (H. influenzae, B.
subtilis) ni chez les eucaryotes ( S. cerevisiae, A. nidulans,
lymphocytes humains en culture et hépatocytes de rat) in vitro.
La recherche de mutations géniques, qui a donné lieu à un grand
nombre d'épreuves sur systèmes bactériens, a toujours donné des
résultats négatifs, sauf dans deux cas. Lors de plusieurs études
portant sur les mutations géniques et effectuées sur des cellules
mammaliennes in vitro, le carbaryl n'a donné qu'un seul résultat
positif équivoque dans une de ces études. Toutefois, cette étude
présentait un certain nombre de défauts et ce résultat n'a pas été
confirmé par ceux d'autres études comparables.
Des lésions chromosomiques ont été observées in vitro sur des
cellules humaines, des cellules de rat et de hamster ainsi que des
cellules végétales exposées à des fortes doses de carbaryl. Aucun
effet de ce genre n'a été observé lors d'épreuves in vivo sur des
systèmes mammaliens, même à des doses atteignant 1000 mg/kg.
On a montré que le carbaryl perturbe le mécanisme d'élongation
des fibres du fuseau chez les cellules végétales et mammaliennes in
vitro. On ne sait pas encore très bien si les résultats des
épreuves effectuées sur des végétaux sont extrapolables à l'homme.
Compte tenu de la base de données sont on dispose actuellement,
on peut conclure que rien n'autorise à soupçonner le carbaryl de
présenter un risque d'effets mutagènes pour les cellules somatiques
ou germinales de l'homme.
Un dérivé nitrosé du carbaryl, le N-nitrosocarbaryl, peut
provoquer des recombinaisons mitotiques et des conversions géniques
chez les procaryotes (H. influenzae, B. subtilis) et les
eucaryotes (S. cerevisiae) in vitro, et il donne des résultats
positifs lors de "spot tests" effectués sur E. coli.
En outre, les résultats expérimentaux indiquent que le
N-nitrosocarbaryl se lie à l'ADN provoquant la formation de
liaisons alcalino-sensibles et des cassures monocaténaires.
Il n'est pas établi que le nitrosocarbaryl soit un agent
clastogène in vivo (cellules de la moelle osseuse et cellules
germinales) même à doses toxiques élevées.
1.7.3 Cancérogénicité
De nombreuses études ont été consacrées au pouvoir cancérogène
du carbaryl chez le rat et la souris. La plupart de ces études ont
donné des résultats négatifs mais il s'agit de travaux anciens qui
ne satisfont pas aux normes actuelles. Quoiqu'il en soit, de
nouvelles études portant sur ces mêmes animaux et qui, elles,
satisfont aux exigences modernes, sont actuellement en coursa. La
dernière évaluation du CIRC (CIRC, 1987) a conclu que l'existence de
cancers imputables au carbaryl n'était pas documentée chez l'homme
et qu'on ne possédait pas de preuves suffisantes d'un pouvoir
cancérogène de cette substance chez les animaux de laboratoire. Il
n'a donc pas été possible de classer le carbaryl en fonction de son
pouvoir cancérogène pour l'homme (Groupe 3).
On a montré que le N-nitrosocarbaryl induisait des tumeurs
locales chez les rats (sarcomes au point d'injection ou carcinomes
spinocellulaires au niveau de la portion cardiaque de l'estomac,
lorsque la substance est administrée par voie orale). Etant donné la
biochimie du carbaryl chez l'homme, on peut considérer comme
négligeable le risque de cancérisation par le N-nitrosocarbaryl
résultant d'une exposition humaine au carbaryl.
1.7.4 Effets sur les différents organes et systèmes
a) Système nerveux
Les effets du carbaryl sur le système nerveux tiennent
essentiellement à l'inhibition de la cholinestérase qui est
généralement passagère. Ces effets ont été étudiés sur des rats et
des singes. Des doses de 10 à 20 mg de carbaryl par kg, administrées
pendant 50 jours par voie orale, ont provoqué des perturbations dans
l'apprentissage et l'exécution de certaines épreuves par les rats
traités.
______________
a Ces études n'ont pas encore fait l'objet d'un examen par le
PISC. La société qui effectue ces travaux indique qu'aux doses
les plus fortes étudiées, on note une augmentation
significative de la fréquence des tumeurs chez les deux
espèces.
Lors d'une petite étude portant sur des porcs, du carbaryl
administré pendant 72 à 82 jours en mélange avec la nourriture à
raison de 150 mg/kg de poids corporel, a produit un certain nombre
d'effets neuromusculaires. On a observé en outre chez des poulets
ayant reçu de fortes doses de carbaryl, une faiblesse réversible des
pattes. Aucun signe de démyélinisation n'a été observé dans le
cerveau, le nerf sciatique ou les coupes de moelle épinière
examinées au microscope. On n'a pas observé d'effets de ce genre à
l'issue d'études à long terme sur des rongeurs.
b) Système immunitaire
Administré in vivo à des doses provoquant des signes
cliniques manifestes, le carbaryl exerce divers effets sur le
système immunitaire. Nombre de ces effets ont été observés à des
doses voisines de la DL50. Dans la plupart des études où les doses
utilisées permettaient la survie des lapins et des souris examinés,
on n'a pas observé d'effets significatifs sur le système
immunitaire. Plusieurs de ces travaux présentaient des défauts, par
exemple un manque de cohérence et parfois des contradictions
manifestes dans les résultats, défauts qui ne permettent pas de
dégager des résultats obtenus un mécanisme immunotoxique bien
défini.
c) Sang
Le carbaryl affecterait l'hémostase mais les résultats
concernant le sens de cet effet sont contradictoires. On a observé
que le carbaryl produisait une augmentation de la formation de
méthémoglobine liée à la dose dans des érythrocytes de moutons
présentant un déficit en glucose-6-phosphate-déshydrogénase. La
sérum-albumine humaine réagit in vitro sur le groupement ester du
carbaryl. Le carbaryl se lie aux acides aminés libres.
d) Foie
On a fait état de troubles affectant le métabolisme des
glucides et la synthèse des protéines au niveau du foie ainsi que
d'une perturbation de la fonction détoxifiante de cet organe chez
les mammifères. Le carbaryl induit faiblement l'activité
pharmacométabolisante des microsomes hépatiques. Il y a
raccourcissement de la durée du sommeil induit par le phénobarbital.
Les taux hépatiques de cytochrome P-450 et b5 sont augmentés. Ces
modifi-cations du métabolisme hépatique pourraient expliquer en
partie le triplement de la DL50 pour le carbaryl observé chez des
rats préalablement traités par cette substance.
e) Fonction gonadotrope
Il a été signalé que le carbaryl augmentait la fonction
gonadotrope de l'hypophyse chez le rat.
1.7.5 Mécanisme fondamental de la toxicité
Le carbaryl est un inhibiteur de l'activité cholinestérasique.
Cet effet est lié à la dose et rapidement réversible. On n'a pas
observé de vieillissement de la cholinestérase carbamylée. Tous les
métabolites reconnus du carbaryl ont une activité
anticholinestérasique sensiblement plus faible que le carbaryl
lui-même.
1.8 Effets sur l'homme
Le carbaryl est facilement absorbé par inhalation et après
administration par voie orale mais moins facilement pas la voie
percutanée. Etant donné que l'inhibition de la cholinestérase est le
principal mécanisme de l'action du carbaryl, le tableau clinique
d'une intoxication par cette substance est dominé par les symptômes
correspondants, à savoir: augmentation de la sécrétion bronchique,
sueurs profuses, salivation et larmoiement; myosis,
bronchoconstriction, crampes abdominales (vomissements et diarrhée);
bradycardie; fasciculation des petits muscles (dans les cas graves
le diaphragme et les muscles respiratoires sont également atteints);
tachycardie, céphalées, vertiges, angoisses, confusion mentale, coma
et dépression des centres respiratoires. Ces signes d'intoxication
se manifestent rapidement après l'absorption et disparaissent aussi
vite une fois que l'exposition a cessé.
Des études contrôlées sur des volontaires humains ont montré
que des doses uniques inférieures à 2 mg/kg étaient bien tolérées.
Une dose unique de 250 mg (2,8 mg/kg) a suscité des symptômes
modérés d'inhibition cholinestérasique (douleurs épigastriques et
sueurs) en l'espace de 20 minutes. Les sujets ont complètement
récupéré dans les 2 heures suivant un traitement par le sulfate
d'atropine.
En cas d'exposition excessive d'origine professionnelle au
carbaryl, on observe des symptômes légers bien avant qu'une dose
dangereuse ne soit absorbée, ce qui explique que les cas graves
d'intoxication professionnelle par le carbaryl soient rares. Lors
des épandages agricoles, l'exposition percutanée peut être
importante. Cependant on n'observe en général aucun effet irritant
local, encore que l'on ait décrit des éruptions cutanées à la suite
l'éclaboussures accidentelles de carbaryl en formulation liquide.
Les données concernant les effets du carbaryl sur le nombre de
spermatozoïdes et la modification de leur morphologie chez les
travailleurs de l'industrie sont contradictoires. Aucun effet
indésirable sur la reproduction n'a été signalé.
L'indicateur biologique le plus sensible de l'exposition au
carbaryl est l'apparition de 1-naphtol dans les urines et la
diminution de l'activité cholinestérasique du sang. On peut donc
utiliser la concentration urinaire du 1-naphtol comme indicateur
biologique à condition qu'il n'y ait pas de 1-naphtol sur le lieu de
travail. Lors de certains cas d'exposition professionnelle, on a
constaté que 40% des échantillons d'urine contenaient plus de 10 mg
de 1-naphtol total par litre. Dans un cas d'intoxication aiguë, on
en a trouvé 31 mg/litre d'urine. On considère qu'il y a danger à
partir de 10 mg/litre et que les symptômes apparaissent à partir de
30 mg de 1-naphtol par litre d'urine (Fiche d'information sur le
carbaryl, OMS, 1973, VBC/DS/75.3).
La mesure de l'activité cholinestérasique peut constituer un
test très sensible pour la surveillance médical des travailleurs, à
condition que le dosage soit effectué peu après l'exposition.
2. Conclusions
On estime que le carbaryl est peu dangereux pour l'homme en
raison de sa faible tension de vapeur, de sa décomposition rapide,
de la désinhibition spontanée également rapide de la cholinestérase
et en raison du fait que les symptômes d'intoxication apparaissent
bien avant qu'une dose dangereuse ne se soit accumulée dans
l'organisme. On ne dispose pas encore de bonnes études de
cancérogénicité qui satisfassent aux normes actuelles en la matière.
2.1 Exposition de la population générale
Les quantités résiduelles de carbaryl qui demeurent dans les
denrées alimentaires et l'eau de boisson après l'utilisation normale
de cet insecticide en agriculture, sont très inférieures à la dose
journalière acceptable (DJA) (0,01 mg/kg de poids corporel/jour) et
il est improbable qu'elles puissent constituer un risque pour la
santé de la population dans son ensemble.
2.2 Sous-groupes de population exposés à un risque élevé
Lorsqu'on utilise du carbaryl à des fins de santé publique,
soit au domicile soit sur des aires de loisir, il y a un risque
d'exposition excessive si l'on ne suit pas les règles concernant son
emploi.
2.3 Exposition professionnelle
En faisant respecter des méthodes de travail raisonnables et
notamment un certain nombre de précautions de sécurité et des
mesures de protection individuelle, avec une surveillance
convenable, il n'existe aucun risque qui puisse résulter d'une
exposition professionnelle au cours de la fabrication, de la
formulation et de l'épandage du carbaryl. Les produits non dilués
doivent être manipulés avec de grandes précautions car toute faute
de manipulation peut entraîner une contamination cutanée. Sur le
lieu de travail, la concentration atmosphérique ne doit pas dépasser
5 mg/m3.
2.4 Effets sur l'environnement
Le carbaryl est toxique pour les abeilles et les lombrics. On
ne doit pas procéder à son épandage pendant la floraison.
En utilisation normale, le carbaryl ne devrait pas poser des
problème écologique. Il est adsorbé en grande partie sur les
particules de sol et il ne passe pas facilement par lessivage dans
les eaux souterraines. Il subit une décomposition rapide dans
l'environnement et n'est donc pas persistant. L'emploi de carbaryl
ne devrait pas entraîner d'effets nocifs à court terme sur
l'écosystème.
3. Recommandations
* La manipulation et l'épandage du carbaryl doivent s'effectuer
en observant les précautions qui s'imposent pour tous les
pesticides. On suivra rigoureusement les instructions
d'utilisation qui figurent sur l'emballage.
* La fabrication, la formulation, l'emploi et l'élimination du
carbaryl doivent se faire avec toutes les précautions voulues
pour réduire au minimum la contamination de l'environnement.
* Les travailleurs qui sont soumis à une exposition régulière
doivent faire l'objet d'un contrôle médical périodique.
* La chronologie des épandages de carbaryl doit être réglée de
manière à éviter tout effet sur les espèces non visées.
* Il importe d'effectuer des études de cancérogénicité
satisfaisant aux normes modernes.
RESUMEN Y EVALUACION, CONCLUSIONES Y RECOMENDACIONES
1. Resumen y evaluación
1.1 Identidad, propiedades y métodos analíticos
El 1-naftil N-metil carbamato, derivado del ácido carbámico,
se conoce por el nombre común de carbarilo. El producto de calidad
técnica es un sólido cristalino blanco, de baja volatilidad y escasa
hidrosolubilidad, y estable a la luz y al calor, pero fácilmente
hidrolizable en medio alcalino. La FAO ha establecido una
especificación mínima de pureza del 98%, con un límite de impureza
del 0,05% para el ß-naftil N-metil carbamato.
Para analizar el carbarilo y sus metabolitos, se pueden
utilizar numerosas técnicas, como la cromatografía de capa fina, la
espectrofotometría, la cromatografía de gases, la cromatografía
líquida de alta presión y la espectrometría de masas con ionización
química. Pueden alcanzarse límites de detección inferiores a un
nanogramo, y la recuperación supera por lo general el 80%.
1.2 Producción y usos
El carbarilo se viene utilizando desde hace unos 30 años como
insecticida de contacto y de ingestión, posee algunas propiedades
sistémicas y permite combatir una amplia serie de plagas. La planta
de fabricación más importante está en los Estados Unidos. Más de 290
fabricantes transforman el carbarilo para integrarlo en más de 1500
productos diferentes.
1.3 Transporte, distribución y transformación en el medio ambiente
En la mayoría de las situaciones, el carbarilo no persiste en
el entorno. En el agua, su semivida por hidrólisis depende de la
temperatura, del pH y de la concentración inicial, y varía entre
varios minutos y varias semanas. El principal producto de
degradación es el 1-naftol.
Se ha estudiado la acumulación de carbarilo en peces de agua
dulce, y expresada como factor de bioconcentración en el medio
acuático se ha cifrado en valores comprendidos entre 14 y 75. El
carbarilo es adsorbido más fácilmente en los suelos que poseen un
alto contenido orgánico que en los suelos arenosos. A un ritmo
habitual de aplicación conforme con unas "prácticas agrícolas
adecuadas", la disipación es rápida, con una semivida de entre 8
días y un mes en condiciones normales. Ocasionalmente, por efecto de
la lluvia y del cultivo agrícola, el carbarilo es transportado de la
superficie al subsuelo (a un metro de la superficie).
El carbarilo contamina la vegetación durante el rociamiento o
por desplazamiento hasta las plantas a través del suelo contaminado.
La degradación del carbarilo en el entorno depende del grado de
volatilización, fotodescomposición y degradación química y
microbiana que se produzca en el suelo, el agua y las plantas. La
descomposición es más rápida cuando el clima es cálido.
1.4 Niveles ambientales y exposición humana
La principal fuente de ingestión de carbarilo entre la
población general son los alimentos.
Los residuos hallados en muestras de la ingesta alimentaria
total son relativamente escasos, pues oscilan entre cantidades
ínfimas y 0,05 mg/kg. En los Estados Unidos, la ingesta diaria
durante los primeros años de aplicación de carbarilo fue de 0,15
mg/día por persona (lo contenían el 7,4% de los compuestos); la
cifra se redujo a 0,003 mg/día por persona en 1969 (sólo lo
contenían un 0,8% de los compuestos). Durante el periodo de
aplicación se encuentra carbarilo ocasionalmente en las aguas
superficiales y los embalses.
La población general puede estar expuesta al carbarilo durante
las operaciones de lucha contra las plagas, en su vivienda o en
zonas recreativas.
Los trabajadores pueden estar expuestos al carbarilo durante su
fabricación, formulación, envasado, transporte y almacenamiento, así
como durante y después de su aplicación. Las concentraciones
halladas en la atmósfera del lugar de trabajo durante su producción
oscilaron entre < 1 mg/m3 y 30 mg/m3. Si las medidas de
protección son inadecuadas, los trabajadores industriales y
agrícolas pueden sufrir exposiciones cutáneas importantes.
1.5 Cinética y metabolismo
El carbarilo es absorbido rápidamente por los pulmones y el
tracto digestivo. En voluntarios se observó una absorción cutánea
del 45% a las 8 horas de aplicar una dosis del producto diluida en
acetona. Sin embargo, los datos referentes a la penetración cutánea
in vitro y a la toxicidad indican que la absorción cutánea se
produce por lo general a una velocidad mucho menor.
Las principales vías metabólicas del carbarilo son la
hidroxilación del anillo y la hidrólisis. El resultado son numerosos
metabolitos que experimentan conjugación, con formación de sulfatos,
glucurónidos y mercapturatos hidrosolubles, que se excretan por la
orina. Como resultado de la hidrólisis se forman 1-naftol, dióxido
de carbono y metilamina. La hidroxilación da lugar a
4-hidroxicarbarilo, 5-hidroxicarbarilo, N-hidroximetilcarbarilo,
5-6-dihidro-5-6-dihidroxicarbarilo y 1,4-naftalendiol. El metabolito
principal en el hombre es el 1-naftol.
En condiciones normales de exposición, el carbarilo rara vez se
acumula en los animales. El producto se excreta principalmente por
la orina, debido a que la detoxificación del producto de su
hidrólisis, el 1-naftol, se produce principalmente por
transformación en conjugados hidrosolubles. La circulación
enterohepática de los metabolitos del carbarilo es también
considerable, sobre todo tras su administración oral.
El producto de la hidrólisis, el ácido carbámico N-naftol, se
descompone espontáneamente en metilamina y dióxido de carbono. La
posterior desmetilación de la metilamina da lugar a dióxido de
carbono y formato, siendo este último excretado principalmente por
la orina.
Un pequeño porcentaje de las dosis de carbarilo absorbidas
aparecen como metabolitos en la saliva y la leche.
1.6 Efectos en otros organismos en el medio ambiente
En los crustáceos, las CL50 oscilan entre 5 y 9 µg/litro
(pulgas de agua, mísidos), 8 y 25 µg/litro (anfípodos), y 500 y 2500
µg/litro (cangrejos de río). El margen de sensibilidad es parecido
en los insectos acuáticos; Plecoptera y Ephemeroptera (gusarapa y
cachipollas) son los grupos más sensibles. Los moluscos son menos
sensibles, situándose su CE50 en niveles de unos pocos mg/litro.
En cuanto a los peces, la mayoría de las CL50 están comprendidas
entre 1 y 30 mg/litro; el grupo más sensible son los salmónidos.
En el caso de las aves la toxicidad aguda es baja. La DL50
para las aves de caza, sean acuáticas o terrestres, es > 1000
mg/kg. El ave más sensible analizada es el mirlo de alas rojas
(DL50 = 56 mg/kg). En zonas forestales rociadas con 1,1 kg de
carbarilo por hectárea no se observó ningún efecto en las aves
locales.
El carbarilo es muy tóxico para las abejas y las lombrices de
tierra. La DL50 oral para las primeras es de 0,18 µg/abeja
(aproximadamente 1-2 mg/kg).
Hay indicios de que el carbarilo puede alterar temporalmente la
composición de especies en los ecosistemas tanto terrestres como
acuáticos. Por ejemplo, un estudio reveló que en determinadas
colonias de invertebrados terrestres sus efectos pueden persistir
durante por lo menos 10 meses tras una sola aplicación.
1.7 Efectos en animales de experimentación y en sistemas de prueba
in vitro
La toxicidad aguda, expresada como DL50, varía
considerablemente según las especies, fórmulas y vehículos. Las
estimaciones de la DL50 oral en la rata oscilan entre 200 y 850
mg/kg. Los gatos son más sensibles, pues presentan una DL50 de 150
mg/kg. Los cerdos y los monos son menos sensibles, pues su DL50 es
> 1000 mg/kg.
La exposición a 792 mg de ingrediente activo de carbarilo
nebulizado, que es la máxima concentración a la que se llegó durante
4 horas, provocó la muerte de una de cinco ratas hembra. Aerosoles
de carbarilo a concentraciones de 20 mg/m3 dieron lugar a una
disminución de la actividad colinesterasa (ChEA) en gatos durante
exposiciones únicas de 4 horas, pero esa misma concentración no tuvo
efectos observables en ratas.
El carbarilo produce leves irritaciones oculares y tiene un
potencial de sensibilización escaso o nulo. Estudios prolongados
revelaron un NOEL de 10 mg/kg de peso corporal (200 mg/kg ingesta
alimentaria) en las ratas, y de 1,8 mg/kg de peso corporal (100
mg/kg dieta) en los perros. El NOEL por inhalación prolongada es de
0,16 mg/m3 en los gatos. El potencial de acumulación de carbarilo
es bajo.
1.7.1 Reproducción
Se ha demostrado que el carbarilo tiene efectos adversos sobre
la reproducción y el desarrollo perinatal en diversas especies de
mamíferos. Los efectos sobre la reproducción comprenden problemas de
infertilidad, una disminución del tamaño de las camadas y una
reducción de la viabilidad postnatal. Los efectos tóxicos sobre el
desarrollo observados son un aumento de la mortalidad in utero,
una disminución del peso del feto y la aparición de malformaciones.
Salvo en un reducido número de estudios, todos los efectos adversos
sobre la reproducción y el desarrollo se observaron sólo a dosis
manifiestamente tóxicas para la madre, y en varios casos ésta
resultó ser más sensible al carbarilo que su prole. Entre los
efectos tóxicos para la madre cabe citar la letalidad, una
disminución del crecimiento y la distocia. Los datos disponibles
indican que los procesos de reproducción y desarrollo de los
mamíferos no son especialmente sensibles al carbarilo en comparación
con la susceptibilidad del organismo adulto.
1.7.2 Mutagenicidad
Se ha evaluado la posible mutagenicidad del carbarilo mediante
diversas pruebas in vitro e in vivo, empleando para ello
bacterias, levadura, plantas, insectos y mamíferos, y analizando
diversos puntos finales.
Según los datos disponibles, el carbarilo no es lesivo para el
ADN. No se ha notificado ningún dato que confirme que ha habido
inducción de la recombinación mitótica, conversión génica o síntesis
imprevista de ADN en procariotas (H. influenzae, B. subtilis) y
eucariotas ( S. cerevisiae, A. nidulans, linfocitos humanos en
cultivo, y hepatocitos de rata) in vitro.
Se obtuvieron resultados negativos en las pruebas de detección
de mutaciones génicas en un gran número de ensayos realizados con
bacterias, salvo en dos casos. En varios estudios de mutagenicidad
del carbarilo llevados a cabo con células de mamífero in vitro, se
obtuvo sólo un resultado positivo equívoco en un estudio de células
en cultivo. Ese estudio, sin embargo, presentaba varias deficiencias
y sus resultados no han sido confirmados en estudios comparables.
Se han notificado lesiones cromosómicas a altas dosis de
carbarilo en células humanas y de rata y hámster in vitro, así
como en plantas. No se han observado efectos de ese tipo en pruebas
in vivo con mamíferos, ni siquiera a dosis de hasta 1000 mg/kg.
Se ha mostrado que el carbarilo altera el mecanismo de las
fibras del huso en células de plantas y mamíferos in vitro. Es
dudoso el interés de realizar ensayos con plantas para extrapolar
sus resultados al hombre.
Cabe concluir que los datos disponibles no corroboran la
suposición de que el carbarilo plantea un riesgo de inducción de
cambios génicos en las células somáticas o germinales del hombre.
El producto nitrosado del carbarilo, el N-nitrosocarbarilo,
puede inducir fenómenos de recombinación mitótica y conversión
génica en los procariotas (H. influenzae, B. subtilis) y
eucariotas (S. cerevisiae) in vitro, y arroja resultados positivos
en las pruebas in situ con E. coli.
Además, resultados experimentales indican que el
N-nitrosocarbarilo se une al ADN, causando la ruptura de los
enlaces alcalisensibles y de las cadenas simples.
No se ha demostrado que el nitrosocarbarilo sea clastógeno in
vivo (médula ósea y células germinales), ni siquiera a dosis
tóxicas elevadas.
1.7.3 Carcinogenicidad
Se han realizado numerosos estudios en la rata y el ratón para
determinar los posibles efectos carcinógenos del carbarilo. Los
resultados de la mayoría de esos estudios fueron negativos, pero se
trata de trabajos realizados hace muchos años y que no satisfacían
los criterios actuales. Se están realizando nuevos estudios
conformes con los actuales criterios en ratones y ratas.a En la
más reciente evaluación del CIIC (CIIC, 1987) se llegaba a la
conclusión de que no había información sobre el cáncer en el hombre
y de que los indicios de carcinogenicidad en animales de
experimentación eran insuficientes. No se podía clasificar el
carbarilo en lo que se refiere a su carcinogenicidad para la especie
humana (Grupo 3).
Se ha mostrado que el N-nitrosocarbarilo induce la aparición
de tumores localmente en la rata (sarcoma en el lugar de la
inyección o carcinoma de células escamosas del antro cardiaco al
administrarlo por vía oral). Teniendo en cuenta las transformaciones
químicas que sufre el carbarilo en el hombre, el riesgo de
carcinogenicidad por N-nitrosocarbarilo como consecuencia de la
exposición a esta sustancia puede considerarse insignificante.
1.7.4 Efectos en distintos órganos y sistemas
a) Sistema nervioso
Los efectos del carbarilo sobre el sistema nervioso se deben
principalmente a la inhibición de la colinesterasa y son por lo
general temporales. En unos estudios sobre los efectos en el sistema
nervioso central de ratas y monos se observó que la administración
oral de 10-20 mg/kg durante 50 días provocaba trastornos del
aprendizaje y del comportamiento en las ratas.
En un pequeño estudio realizado con cerdos, el carbarilo (150
mg/kg de peso corporal en la alimentación durante 72-82 días) tuvo
diversos efectos neuromusculares. Se observó una debilidad
reversible de las patas en pollos sometidos a altas dosis de
carbarilo. No se observaron signos de desmielinización en los cortes
de cerebro, nervio ciático o médula espinal examinados al
microscopio. Tampoco se notaron efectos de esa índole en estudios
prolongados realizados con roedores.
b) Sistema inmunitario
Se ha notificado que el carbarilo administrado in vivo a
dosis que producen claros signos clínicos tiene diversos efectos en
el sistema inmunitario. Muchos de los efectos descritos se
detectaron a dosis cercanas a la DL50. La mayoría de los estudios
llevados a cabo con conejos y ratones a dosis compatibles con la
supervivencia no han revelado efectos de importancia en el sistema
______________
a El IPCS aún no ha examinado esos estudios. La sociedad
encargada de realizarlos ha indicado que se observa un aumento
significativo de tumores a la dosis màs elevada en las dos
especies.
inmunitario. Sin embargo, varios de esos estudios adolecían de
incoherencia y a veces de contradicción patente entre los
resultados, lo cual no permite describir un mecanismo inmunotóxico
bien definido.
c) Sangre
Se ha señalado que el carbarilo afecta a la coagulación, pero
existe cierta controversia en cuanto al tipo de efecto. En
eritrocitos de oveja con déficit de glucosa-6-fosfato
deshidrogenasa, el carbarilo provocó un aumento dosis-dependiente de
la formación de metahemoglobina (Met-Hb). La albúmina sérica humana
reaccionó in vitro con el grupo éster del carbarilo. El carbarilo
se une a los aminoácidos libres de la sangre.
d) Hígado
Se han señalado trastornos del metabolismo de los carbohidratos
y de la síntesis de proteínas, así como de la función de
detoxificación en el hígado de ciertos mamíferos. El carbarilo es un
inductor ligero de la actividad de metabolización de medicamentos
que reside en los microsomas hepáticos. Se observa un acortamiento
del periodo de sueño inducido por el fenobarbital. Los niveles
hepáticos de citocromo P-450 y b5 aumentan. Los cambios del
metabolismo hepático son tal vez parcialmente responsables de la
triplicación de la DL50 por carbarilo en ratas tratadas
previamente con dicho compuesto.
e) Función gonadotrópica
Se ha señalado que el carbarilo estimula la función
gonadotrópica de la hipófisis de la rata.
1.7.5 Mecanismo principal de toxicidad
El carbarilo inhibe la acción de la colinesterasa, efecto que
depende de la dosis y es fácilmente reversible. No se observó ningún
fenómeno de "maduración" de la colinesterasa carbamilada. Todos los
metabolitos identificados del carbarilo son
considerablemente menos activos que éste como inhibidores de la
colinesterasa.
1.8 Efectos en el hombre
El carbarilo es fácilmente absorbido por inhalación y por vía
oral, y menos fácilmente por vía cutánea. Como su principal
mecanismo de acción es la inhibición de la colinesterasa (ChE), en
el cuadro clínico de la intoxicación predominan los síntomas de esa
inhibición, tales como: hipersecreción bronquial, aumento de la
sudación, de la salivación y del lagrimeo; pupilas puntiformes,
broncoconstricción, espasmos abdominales (vómitos y diarrea);
bradicardia; fasciculación de los músculos finos (que también afecta
en los casos graves al diafragma y a los músculos respiratorios);
taquicardia; cefalea, vértigo, ansiedad, confusión mental,
convulsiones y coma; y depresión del centro respiratorio. Los signos
de intoxicación aparecen rápidamente tras la absorción y desaparecen
pronto al cesar la exposición.
En estudios controlados realizados con voluntarios se observó
una buena tolerancia a dosis únicas inferiores a 2 mg/kg. Una dosis
única de 250 mg (2,8 mg/kg) provocó síntomas moderados de inhibición
de la ChE (dolor epigástrico y sudación) al cabo de 20 minutos. La
recuperación completa se produjo tras dos horas de tratamiento con
sulfato de atropina.
En los casos de sobreexposición ocupacional al carbarilo, se
observan síntomas leves mucho antes de que llegue a absorberse una
dosis peligrosa; de ahí que raras veces se produzcan casos graves de
intoxicación ocupacional por este compuesto. Durante las
aplicaciones agrícolas, la exposición cutánea puede desempeñar un
papel importante. No suelen observarse efectos irritantes locales,
pero se ha descrito la aparición de exantema cutáneo tras la
salpicadura accidental con formulaciones de carbarilo.
Los datos acerca de los efectos del carbarilo sobre el número y
la morfología de los espermatozoides en trabajadores industriales
son discordantes. No se han notificado efectos adversos sobre la
reproducción.
El indicador biológico más sensible de la exposición al
carbarilo es la aparición de 1-naftol en la orina y una disminución
de la actividad ChE de la sangre. Si no hay 1-naftol en el entorno
de trabajo, los niveles urinarios de este producto se pueden emplear
como indicador biológico. Durante la exposición ocupacional, el 40%
de las muestras de orina contenían más de 10 mg de 1-naftol/litro.
En un caso de intoxicación aguda se hallaron 31 mg/litro en la
orina. El nivel de riesgo es > 10 mg/litro, y el nivel de aparición
de síntomas, 30 mg 1-naftol/litro de orina (hoja de datos sobre el
carbarilo, OMS, 1973, VBC/DS/75.3).
El análisis de la actividad ChE puede ser una prueba de gran
sensibilidad a efectos de vigilancia, siempre que se realice poco
después de la exposición.
2. Conclusiones
Se considera que los riesgos que plantea el carbarilo para el
ser humano son escasos, debido a su baja presión de vapor, a su
rápida degradación y a la recuperación espontánea y rápida de la
colinesterasa inhibida, así como al hecho de que los síntomas
aparecen normalmente mucho antes de que pueda haberse acumulado en
el organismo una dosis peligrosa. Aún no se dispone de estudios
satisfactorios sobre la carcinogenicidad conformes con los criterios
actuales.
2.1 Exposición de la población general
Los niveles de residuos de carbarilo presentes en los alimentos
y el agua de bebida, resultantes de su uso normal en la agricultura,
son muy inferiores a la ingesta diaria admisible (IDA) (0,01 mg/kg
de peso corporal al día), y difícilmente pueden representar un
riesgo para la salud de la población general.
2.2 Subpoblaciones de alto riesgo
El uso del carbarilo con fines de salud pública en la vivienda
o en zonas recreativas puede ser causa de sobreexposición si se
descuidan las normas aconsejadas para su aplicación.
2.3 Exposición ocupacional
Si se vela por el cumplimiento de unas prácticas laborales
razonables, en particular las medidas de seguridad, la protección
del personal y una supervisión adecuada, la exposición ocupacional
durante la fabricación, formulación y aplicación de carbarilo no
planteará riesgos. Las concentraciones no diluidas se deben manejar
con sumo cuidado, dado que unas prácticas laborales incorrectas
pueden ser causa de contaminación cutánea. Las concentraciones en el
aire del lugar de trabajo no deberían superar los 5 mg/m3.
2.4 Efectos en el medio ambiente
El carbarilo es tóxico para las abejas y las lombrices. No
debería aplicarse a los cultivos durante la floración.
Si se emplea normalmente, el carbarilo no debería suscitar
preocupación desde el punto de vista del medio ambiente. El
carbarilo se adsorbe en buena parte en el suelo y no se lixivia
fácilmente hacia las aguas subterráneas. Se degrada rápidamente en
el entorno, por lo que no tiende a persistir. El uso de carbarilo no
debería tener efectos nocivos a corto plazo en el ecosistema.
3. Recomendaciones
* La manipulación y la aplicación del carbarilo se deberían
realizar adoptando las precauciones previstas para todo
plaguicida y siguiendo minuciosamente las instrucciones
suministradas en el envase para emplear correctamente el
producto.
* Deberían controlarse cuidadosamente la fabricación, la
formulación, el uso y la eliminación del carbarilo, con objeto
de reducir al mínimo la contaminación del medio ambiente.
* Los trabajadores expuestos regularmente al producto deberían
someterse a chequeos periódicos.
* Debería determinarse la época de aplicación del carbarilo de
manera que no afecte a las especies que no se desee combatir.
* Deberían realizarse estudios de carcinogenicidad que satisfagan
los criterios actuales.
 Molecular formula: C12H11N02
Common name: Carbaryl (BSI)
CAS chemical name: 1-naphthalenylmethylcarbamate (9CI)
CAS registry number:
Molecular formula: C12H11N02
Common name: Carbaryl (BSI)
CAS chemical name: 1-naphthalenylmethylcarbamate (9CI)
CAS registry number:  In India, degradation of carbaryl was studied in a greenhouse
by Brahmaprakash & Sethunathan (1984, 1985). Since a soil planted
with crops may be more dynamic and complex than unplanted soil,
because of increased microbial activity, they studied carbaryl
persistence in soil (pH 6.2, organic matter 1.6%) planted with rice
in a greenhouse, under flooded and non-flooded conditions. Carbaryl
disappeared more rapidly from soils planted with rice than from
those without rice, under both flooded and non-flooded conditions.
The amount of carbaryl decreased to between 30.2 and 32.1% of the
original level within 30 days in unplanted soil under both flooded
and non-flooded conditions. During this same period, the carbaryl
concentration decreased to 17-18% of the original level in planted
soil under both water regimes. Degradation occurred by hydrolysis,
but there was no appreciable difference in the rates of degradation
between flooded and non-flooded soils. The rate of degradation of
carbaryl was little affected by moisture. Further degradation of
1-naphthol was slow in both planted and unplanted systems. A
significant portion of the ring-14C accumulated in the soil as
1-naphthol and soil-bound residues. Evolution of 14CO2 from the
labelled side-chain and ring was negligible, even in soil planted to
rice.
4.3.4 Degradation by microorganisms
Many studies have demonstrated the great ability of
microorganisms to degrade carbaryl in the soil, and for some of
them, to use it as a sole source of carbon. The most frequently
identified organisms are bacteria ( Pseudomonas phaseolicola, P.
cepaphia, Rhodococcus sp., Nocardia sp., Xanthomonas sp.,
Achromobacter sp.) and fungi ( Aspergillus niger, A. terreus,
Fusarium solani, Gilocladium roseum, Rhizoctonia solani, R.
practicola, Penicillium sp., Mucor sp., Rhizopus sp.). In many
cases, it was demonstrated that the persistence of carbaryl in the
soil decreased after a first application, which was interpreted as
the selection and build-up of strains more capable of degrading the
product. The same process may also explain that, in some studies,
the rapidity of degradation suddenly rose after a lag period during
which only minor amounts of carbaryl degraded.
Tewfik & Hamdi (1970) mentioned that carbaryl was decomposed
into 4 distinct compounds by a soil bacterium designated as S-1.
They considered that this soil bacterium might utilize carbaryl in a
similar way to Pseudomonas sp., which metabolize naphthalene via
the salicylate pathway.
Zuberi & Zubairi (1971) also reported that carbaryl is degraded
by soil microflora. Pseudomonas phaseolicola and Aspergillus
niger hydrolysed carbaryl to 1-naphthol. In the case of P.
phaseolicola, an unidentified minor metabolite was also detected.
Larkin & Day (1986) reported that 2 bacteria isolated from
garden soil, Pseudomonas sp. (NCIB 12042) and Rhodococcus sp.
(NCIB 12038), could grow on carbaryl as the sole source of carbon
and nitrogen at pH 6.8, but failed to metabolize carbaryl rapidly.
Both could use 1-naphthol as the sole source of carbon and
metabolize it via salicylic acid. Strain NCIB 12038 produced also
gentisic acid. Pseudomonas sp. (NCIB 12043), in a soil perfusion
column enrichment at pH 5.2, metabolized carbaryl rapidly to
1-naphthol and methylamine. 1-Naphthol itself was metabolized via
gentisic acid. A possible pathway for the catabolism of carbaryl and
1-naphthol was proposed.
Walker et al. (1975a), working with Pseudomonas sp.,
observed degradation of 1-naphthol and suggested that carbaryl could
serve as the sole source of nitrogen and carbon for bacteria. It was
also noted that the presence of another nitrogen source in the media
seemed to have a delaying effect on metabolism of carbaryl.
Bacterial communities of at least 12 and 14 members were
selected in continuous culture using carbaryl as the sole source of
carbon and nitrogen at pH 6. These communities were supported by the
slow formation of hydrolysis products and no carbaryl-degrading
bacterium was selected after more than 83 days. When using equimolar
1-naphthol and methylamine as the sole source of carbon and
nitrogen, a bacterial community of at least 8 members was selected.
After a lag of 10-50 days, soil perfusion columns (pH 5.2) and
continuous culture enrichments (pH 5) led to the selection of a
Pseudomonas sp. that could utilize carbaryl as its sole carbon and
nitrogen source (Larkin & Day, 1985).
Sud et al. (1972) showed that Achromobacter sp. also
utilized carbaryl as the sole source of carbon in a salt medium.
Four degradation products of carbaryl were 1-naphthol, hydroquinone,
catechole, and pyruvate. The organism also grew well in the first 3
degradation products.
The rate of degradation of carbaryl after 1, 2, and 3
applications to 3 submerged soils was examined by Rajagopal et al.
(1983). Soils that had been pretreated with carbaryl were able to
degrade carbaryl more rapidly than those without pretreatment. The
enrichment culture was inactivated upon autoclaving. The
concentration of carbaryl decreased in the mineral medium inoculated
with the enrichment cultures from the 3 soils, especially when it
served as the sole source of both carbon and nitrogen.
Rajagopal et al. (1984) studied the metabolism of side-chain
and ring 14C-labelled carbaryl in a mineral salts medium by soil
enrichment cultures. Hydrolysis was the major route of microbial
degradation. During carbaryl degradation by enrichment cultures and
Bacillus sp., 1-naphthol and 1,4-naphthoquinone accumulated in the
medium.
Rajagopal et al. (1986) also observed that carbaryl
disappeared more rapidly from a laterite soil pretreated with
1-naphthol than from a control soil never exposed to 1-naphthol. The
accumulation of 1-naphthol and bound residues formed from
added14C-carbaryl was greater in soils pretreated with 1-naphthol
than in untreated soils.
In experiments with the soil fungus Rhizoctonia practicola,
Bollag et al. (1976) found that it could transform 1-naphthol from
an ether-extractable to a water-soluble product. It was also
observed that, after removal of the fungal cells, the growth medium
possessed the ability to transform 1-naphthol, indicating activity
of an extracellular enzyme. Attempts to analyse the labelled
material in the aqueous phase indicated that the radioactivity was
associated with a compound of comparatively high relative molecular
mass.
Czaplicki & Bollag (1975) exposed 1-naphthol to Rhizoctonia
solani, isolated from soil, and found that it was completely
transformed to a compound not extractable with ether.
Working with the soil fungus Aspergillus terreus, Liu &
Bollag (1971b) investigated the metabolic transformation of carbaryl
through 1-naphthyl- N-hydroxymethylcarbamate and tried to clarify
the pathway of the side-chain. The next intermediate in biological
transformation was 1-naphthyl carbamate, which was further degraded
to 1-naphthol. Within one week, approximately half the amount of
carbaryl was transformed to these other metabolic products. No
attempt was made to clarify whether the formation of 1-naphthol was
the result of the biological or the chemical degradation of
1-naphthylcarbamate, but there were clear indications that
1-naphthol was metabolized further in the presence of A. terreus.
In vitro studies were carried out to investigate the
degradation of carbaryl by soil microorganisms. Three isolates from
soil, including Fusarium solani, a Gram-negative coccus, and a
Gram-positive rod, accelerated the hydrolysis of carbaryl to
1-naphthol and other intermediates. Fusarium solani was the most
effective in decomposing the 14C-labelled compound. Radioactivity
decreased by 24% during the first 5 days and by 82% during 12 days
in a growing culture after inoculation. A mixture of two or three of
the microorganisms was more effective in decomposing carbaryl and
1-naphthol (Bollag & Liu, 1971).
Bollag & Liu (1972a) studied the biological degradation of
14C-1-naphthol during growth, with replacement cultures and cell
extracts of Fusarium solani. The radioactivity of 1-naphthol
disappeared partially during growth, but it was completely
dissipated by cell extract activity. The cell extract of F. solani
degraded more than 80% of the 1-naphthol to form CO2 within 60 min
of incubation, but it was not possible to identify intermediates.
This implies rupture of the naphthol ring. No difference in activity
could be observed between cell-free extracts from the spores or the
mycelium of the fungus at pH 5.7 and 7.2. The enzyme system was
relatively stable since there was no decrease in activity after the
cell extract was stored at -10 °C during 4 months. The enzyme that
participated in the degradation reaction was constitutive (always
present) as shown by cell extracts prepared from cells grown in a
medium with, or without, carbaryl as substrate.
The fungus Gliocladium roseum, isolated from soil by Liu &
Bollag (1971a), metabolized carbaryl to 3 metabolites which were
identified as 1-naphthyl- N-hydroxy methylcarbamate,
4-hydroxy-1-naphthyl methylcarbamate, and 5-hydroxy-1-naphthyl
methyl-carbamate. It was therefore considered that N-alkyl- and
aromatic ring-hydroxylation of carbaryl were important detoxication
reactions of G. roseum. About 70% of the radioactivity was
recovered as carbaryl from an 11-day-old culture. The decrease in
radioactivity from the growth medium containing side-chain labelled
carbaryl indicated that a further degradation of the formed
metabolites occurred, or that an additional pathway was involved in
the degradation of carbaryl by G. roseum.
Bollag & Liu (1972b) reported that most soil fungi
( Penicillium sp., Mucor sp., Rhizopus sp., and Aspergillus
sp. except A. fumigatus), could hydroxylate carbaryl at different
positions, but that the products differed qualitatively as well as
quantitatively with the various fungi.
In a different study, the enzyme or protein fraction
(extracellular phenol oxidase) of the culture filtrate from
Rhizoctonia practicola as chromatographed on a column of Sephadex
G-200 and fractions were obtained that were able to transform
1-naphthol as a substrate (Sjoblad et al., 1976). The enzyme
catalysed the polymerization of 1-naphthol to several products.
Biological oxidation and coupling of phenols are key reactions
in nature, that result in the formation of products such as lignins,
melanins, tannins, alkaloids, and humus compounds. Thus, it can be
assumed that enzymes interact with xenobiotic phenols that are
incorporated into soil or sediment organic matter. Rhizoctonia
practicola (and an extracellular enzyme) was able to polymerize
1-naphthol (dimers, trimer, tetramer) (Bollag et al., 1978).
Rodriguez & Dorough (1977) studied the persistence of carbaryl
in culture media in the presence of mixed and pure cultures of
bacteria and fungi isolated from soil. All fungi ( Fusarium,
Penicillium, Aspergillus, and one unidentified species), isolated
from a soil treated with carbaryl six months before sampling,
produced at least one metabolite from carbaryl. After 14 days of
incubation, 54-79% of the carbaryl was recovered intact, and there
was little formation of carbon dioxide by the fungi. In contrast,
related experiments with bacterial isolates ( Arthrobacter,
Nocardia, Pseudomonas, Xanthomonas, and Bacillus) from the same
soil showed that only 1-9% of the added carbaryl remained
unconverted ( Arthrobacter was the exception with 59.1% remaining
carbaryl). Controls showed that non-biological degradation also
occurred. Bacteria, like fungi, metabolized carbaryl qualitatively
in the manner observed with natural soil populations. However,
quantitative differences were so great that the use of isolates may
be of little value in estimating the degradation rate of carbaryl
and other pesticides in field soil. This is important to note when
conducting microbial degradation studies in the laboratory.
Bollag (1979) summarized the transformations of carbaryl by
microbial activity. It was possible to isolate several
microorganisms capable of hydrolising carbaryl to 1-naphthol. In
addition, Gliocladium roseum showed the formation of:
4-hydroxy-1-naphthyl N-methylcarbamate
5-hydroxy-1-naphthyl N-methylcarbamate
1-naphthyl N-hydroxy-methylcarbamate.
Other terrestrial fungi hydroxylated carbaryl at different
positions, but the products differed quantitatively and
qualitatively with the various fungi. Subsequent metabolic
transformation of the hydroxylated products differed considerably.
With A. terreus, the following products were obtained:
1-naphthyl N-hydroxy-methylcarbamate -->
1-naphthyl-carbamate --> 1-naphthol
Several bacteria isolated from river water were capable of
degrading 1-naphthol. At least two different pathways were involved.
The first was a complete degradation with the release of CO2, the
second produced principally 4-hydroxy-1-tetralone, which may involve
hydroxylation of the naphthyl ring in the 4 position and conversion
to an aliphatic cyclic compound. Such a pathway has also been
described with a soil Pseudomonas by Walker et al. (1975a) and
Davis & Evans (1964).
4.3.5 Persistence in soil
Carbaryl is not usually applied as a soil treatment, therefore,
the amounts of carbaryl that may reach the soil come principally
from spray drift, or from washing off treated crops by rain.
When applied at the usual doses, in the laboratory, carbaryl
has a short persistence (half-life <40 days and usually from 6-20
days), but this may increase when the soil is flooded, or when the
dose is increased. In field studies, the half-life of carbaryl,
applied at the usual dose rates, in warm or temperate climatic
conditions, did not exceed one month in the soil. The results of
laboratory and field studies on the persistence of carbaryl in soil
are summarized in Table 7.
Table 7. Half-life of carbaryl in soil, calculated from experimental laboratory
and field results
Origin of soil Number Degradation Half-life References
of days (%) (days)
Laboratory 40 100 8 Johnson & Stansbury (1965)
Laboratory 0.5 mg/kg 45 77 20 Molozhanova & Kanevskij (1971)
Laboratory 1 mg/kg 45 53 40
Laboratory 10 mg/kg 45 13
Laboratory 30 55-63 < 20 Flores-Ruegg et al. (1980)
Laboratory (flooded) 15 63-27 10-40 Venkatesvarlu et al. (1980)
Laboratory 53 100 < 8 Odeyemi (1982)
Orchard 184 99 30 Ivanova & Molozhanova (1973)
Corn 135 95 30 Caro et al. (1974)
Pretreated soil 4 70 < 3 Rodriguez & Dorough (1977)
Grain cropa 30 53-31 27-55 Gangwar et al. (1978a)
Corn soilb 5 42-56 5 Kavadia et al. (1978)
Potatoes 50 < 99 < 7 Kovaleva & Talamov (1978b)
- 30 96 6
Table 7 (continued)
Origin of soil Number Degradation Half-life References
of days (%) (days)
Unspecified 90 Czaplicki (1979)
Tropical 7 Rajukkannu et al. (1985)
Sesamum 60 56 50 Yadav et al. (1985)
Bare soil < 4 Davis (1986a)
Bare soil 1-13
Forest 1.5 Sundaram & Szeto (1987)
Forest 90 < 78 < 38 Springborn (1988b,c)
Forest 90 < 90 < 24
Flooded rice 180 (56) Springborn (1988a)
Flooded rice 180 (52)
Broccoli 6-12 Norris (1991)
Sweet corn 5
a20-60 kg/ha.
b15-45 kg/ha.
Johnson & Stansbury (1965) calculated a half-life of
approxi-mately 8 days in an agricultural soil (sandy loam, pH 5.5)
treated at 3 concentrations. Residues of carbaryl appeared to be
completely degraded within 40 days.
The considerable influence of the dose rate on the persistence
of carbaryl in soil was demonstrated by Molozhanova & Kanevskij
(1971). The percentages of the initial quantities that were degraded
45 days after treatment are shown in Table 8.
Table 8. Degradation rate of carbaryl in relation to the dose
Initial dose Degradation (%)
(mg/kg)
0.5 77
1 53
1.5 37.3
2 30
2.5 27
3 23
4 20
6 16.7
8 15
10 13.2
The same authors also demonstrated the influence of the type of
soil on the degradation of carbaryl. Forty-five days after
treatment, about 50% degradation was observed in light grey forestry
soil, and only about 20% in peat. The ability of soils to degrade
carbaryl could be ranked as follows: light grey > grey forestry >
turf podzol > southern chernozeme > ordinary chernozeme > meadow
> peat.
Ivanova & Molozhanova (1973) calculated that application of
1.36 kg carbaryl/ha in an orchard resulted in 19.7% being retained
in the soil; 99% of the carbaryl present in soil disappeared within
184 days.
Czaplicki (1979) conducted studies in Poland. The soil
half-life of carbaryl was about 3 months and 90% degradation
occurred within 18 months. From October to March, when the soil
temperature was <5-8 °C, the disappearance of the insecticides in
the soil almost stopped.
Rajukkannu et al. (1985) studied the persistence of 4
products in the red and black soils of Tamil Nadu (India), where the
tropical climate in conjunction with the soil properties shortened
the persistence of insecticides. The half-life of carbaryl was 6.7-7
days and 95% degradation was achieved after 90 days.
It was reported by Odeyemi (1982) that carbaryl disappeared
from soil samples treated with 45 mg/kg after 53 days incubation in
a greenhouse under tropical conditions (Nigeria).
4.3.6 Interaction with other physical, chemical, or biological
factors
Nitrogen fertilizers (ammonium sulfate and urea) increased the
persistence of carbaryl in flooded laterite soil with a low native
content of nitrogen (0.04%). In the alluvial soil with 0.11% total
nitrogen, the persistence of the insecticides was little affected.
The rates of degradation in the two soils treated with nitrogen
fertilizers were almost identical. The authors speculated about the
mechanism of this effect, which was possibly due to the preferential
utilization of inorganic nitrogen by microorganisms, or to the
inhibition of soil hydrolase activity (Rajagopal & Sethunathan,
1984).
4.3.7 Vegetation
4.3.7.1 Uptake and transformation in plants
Carbaryl from soil treated at about 50 times the usual dose
penetrated into apple trees and couch-grass (Molozhanova, 1968).
Three months after application of carbaryl (85%) to soil at a dose
of 100 mg/kg, residues of carbaryl were detected in the roots and
stems in apples (39-13 mg/kg) and in couch-grass (50-38 mg/kg).
Under the same conditions, no residues were detected in tomato
fruits or in wheat grain, but 5.9 mg/kg were detected in potatoes
(Table 9).
Table 9. Migration of carbaryl from the soil into apple trees at
different times following applicationa
Experimental Sampling day Carbaryl content of samplesb
conditions after (mg/kg)
application
Soil Foliage Fruits
85% WP carbaryl 5 9.3 0 -
applied under each
tree at a rate of 12 6.8 0 -
10 mg a.i./kg soil
19 6.0 5.0 -
56 4.4 0.04 4.4
138 2.1 0 -
aFrom: Molozhanova (1968).
bAverage data from 18 samples are given for each case.
Huque (1972) reported that 14C-labelled carbaryl was readily
taken up from granules by 2-week-old rice plants. After 3 days, a
level of about 100 mg/kg was determined in the plant (excluding the
roots). Residues then decreased to about 15 mg/kg after 18 days.
Ferreira & Seiber (1981) studied the uptake and distribution of
carbaryl in rice seedlings following exposure of the roots to the
insecticide. Carbaryl was rapidly absorbed and transported upwards
to the leaves and stems. After exposure was terminated, the major
route of loss of carbaryl was through root exudation (22%) rather
than volatilization from the leaves (4.2%). However, since most of
the remaining carbaryl in the plant was present on the leaf
surfaces, the authors surmised that volatilization might play a
greater role in pesticide loss over longer periods of time.
14C-carbaryl and 14C-1-naphthol from soil-bound residues
were partially released when barley was grown (Murthy & Raghu,
1988). 14C-residues could be detected in both shoots and roots in
the case of carbaryl treatment, while only roots showed
14C-residues in the case of 1-naphthol.
The rate of decomposition of carbaryl in plants depends on the
climatic conditions. It is more rapidly decomposed in hot climates,
at high temperatures, and by intensive ultraviolet radiation. Thus,
residual levels in feed plants were lower in regions with a hot
climate than in other regions (Atabaev, 1972).
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Environmental levels
5.1.1 Air
Ambient air concentrations of carbaryl were measured before and
after a treatment of a large area of forest in Maine, USA, to
control spruce budworm. Concentrations in air ranged from less than
0.0035 to 0.107 µg/m3 several miles away (Shehata et al., 1984).
Room concentrations of airborne carbaryl were measured
following application for pest control as a 5% dust at an
undetermined rate. The levels detected on the day of application,
and 1, 2, or 3 days after application were 1.3, 0.2, 0.1, and 0.01
µg/m3, respectively (Wright et al., 1981).
5.1.2 Water
Carbaryl concentrations in water from different sources in the
USSR were studied by Molozhanova (1970). Data are presented in Table
10.
Table 10. Concentrations of carbaryl in watera
Type of sample Number of samples Mean
Total Carbaryl-containing concentrations
samples (%) (mg/litre)
Well 114 87 0.13
Artesian water 64 70 0.16
Ground water 24 75 0.27
Dam 90 47 0.02
River 77 62 0.044
Lake 84 85 0.03
aFrom: Molozhanova (1970).
According to an earlier report by Molozhanova (1968), carbaryl
was present in trace amounts up to a maximum level of 1 mg/litre in
well water and reservoirs during carbaryl application (6 kg/ha, 85%
WP).
Water irrigation channels, situated at a distance of 200-300 m
from a carbaryl-treated cotton field, contained from 0.01 to
0.25 mg/litre up to 18 days after spraying at a dose of 2kg/ha
(Guseynov, 1970).
Residues of pesticides were monitored in the aquatic system of
Ioannina basin (Greece) and its natural outlet, Kalamas River, for
the period September 1984-October 1985 (Albanis et al., 1986). The
carbaryl concentration in water was found to follow a seasonal
fluctuation with maxima during summer and minima during winter
months. Mean and range values are summarized in Table 11.
Table 11. Mean and range values of carbaryl (ng/litre) on different
sampling dates in the Ioannina basin
Month of sampling River Canal Lake
September 0.5
November NDa NDa
March NDa
May ND
June NDa
July 23.7 21.7
August 2.3
September 1.7 NDa
April NDa
July 8.8
aND = not detected.
An oil-based, carbaryl formulation (Sevin-2-oil) was applied
twice to a coniferous forest in New Brunswick (Canada). At a dose
rate of 280 g/ha, the highest concentration in stream water was
0.314 mg/litre and only 0.123 mg/litre 0.5 h after spraying. More
than 50% of the initial residues dissipated within 1 h (Sundaram &
Szeto, 1987).
Carbaryl was applied aerially as Sevin-4-oil (1.12 kg/ha) to
aforest in Montana. Field samples of water were collected to study
persistence. Residue levels in 4 streams sampled were variable
(3-6, 3-175, 2-260, 4-108 µg/litre) 1.3-2.8 h after application
(Pieper & Roberts, 1978; Pieper, 1979). Local accumulations of
floating oil-based spray may have caused this variation. Grantham
(1980) reported that carbaryl (Sevin-4-oil) levels were generally
highest directly after spraying, but increased at some stream sites
for 1-2 days after application. This was attributed to light rains,
which washed the spray residue off the foliage and into the stream
channel. However, Brown (1980) concluded that carbaryl enters
streams in periodic doses and that rainfall dates are not related to
the presence of residues in streams. Maximum residues of
26.32 µg/litre were found in water 12 h after spraying, with no
residues detectable in samples taken hourly until 36 h. Residues
were found again in samples taken 2.5-3 days and 5.5-6.5 days after
spraying.
In a study where carbaryl (Sevin-4-oil) was applied aerially
(0.84 kg/ha) to a forest in Maine, in 1978, samples were collected
in 9 streams (Stanley & Trial, 1980). Peak concentrations occurred
shortly after spraying and maximum residue levels ranged from 0.93
to 7.8 µg/litre in brooks and from 0.44 to 2 µg/litre in rivers. In
one stream, the maximum concentration was 16 µg/litre. Ott et al.
(1981) reported that carbaryl levels were highest on the day of
spraying (2.7 µg/litre), declined to about 0.7 µg/litre by the third
day, but increased again to about 1.2 µg/litre on day 5. No residues
were detected in stream water samples taken on the 17th day.
Thomas et al. (1982) treated planted rice fields with
carbaryl at rates of 0.63, 0.94, and 1.85 kg a.i./ha, either as a
high volume spray (500 litre/ha) or as a low volume spray (150
litre/ha), and monitored the residues. In irrigation water sampled
one hour after treatment, carbaryl levels amounted to 0.15-0.30 and
0.07-0.18 mg/litre, under high and low volume spraying,
respectively. During the first 24 h after spraying, the
concentration in the irrigation water remained fairly constant, but,
by the fourth day, it had decreased significantly. By the tenth day
after spraying the residues in water had decreased to 0.02-0.05 and
0.03-0.04 mg/litre, respectively.
A rice field dissipation study was conducted in Arkansas (USA)
by Springborn (1988). Carbaryl (Sevin XLR) was applied 3 times at
14-day intervals to an irrigated field plot in which rice was
planted. In irrigation water, carbaryl residues disappeared with a
half-life of <1 day after each treatment. The maximum concentration
in water was 1466 µg/litre and, by day 3 following each treatment,
the concentration was <121 µg/litre. In a similar study conducted
in California, Springborn (1988a) found that carbaryl dissipated
rapidly in irrigation water with a half-life of <1 day. The maximum
concentration in water was 648 µg/litre. By day 3 following each
treatment, the concentration was <27 µg/litre.
Residues of 1-naphthol were found in the water collected from
wells and ponds in and around Bhopal (India). The residues of
1-naphthol in well water (near a manufacturing plant) ranged from
0.002 to 0.024 mg/litre. In pond waters, the levels were found to be
between 0.036-0.098 mg/litre. The level of 1-naphthol in soil
samples was of the order of 0.153-0.656 mg/kg (Dikshith et al.,
1990).
5.1.3 Soil
Contamination of the soil occurs when carbaryl is used in
agriculture. During the treatment period, levels of 1-3 mg
carbaryl/kg were measured in the surface layer of the soil in the
USSR (Molozhanova, 1968). Concentrations of 0.03-0.35 mg/kg were
found in the surface layer of the soil up to 52 days after a single
application of carbaryl at a rate of 2 kg/ha (Guseynov, 1970).
Carbaryl levels in the soil varied depending on the type of soil
(Table 12).
Potatoes were sprayed with carbaryl at 4.25 kg/ha (5 kg Sevin
WP, 85%/ha). One day, 10 days, and 2 months after treatment,
residues in the soil under the potatoes were, respectively, 1.9,
9.55, and 0.02 mg/kg (Kovaleva & Talamov, 1978b). When the soil was
sprayed before sowing, the residues in the soil 1 day, 1 month, 4
months, and 15 months after treatment were 1.5-2, 0.05-0.07,
0.02-0.08, and 0.06 mg/kg, respectively. Within 100 and 150 days
following soil incorporation of carbaryl (Sevin, 10 kg/ha), residues
in non-planted soil decreased to 0.05 and 0.02 mg/kg, respectively
(Kovaleva & Talanov, 1980).
Table 12. Carbaryl content in different types of soil during
harvestinga
Type of soil Number of Carbaryl concentration
samples (mg/kg)
Meadow-chernozem 200 2.22 ± 0.5
Chernozem southern 766 1.01 ± 0.23
Chernozem ordinary 288 1.09 ± 0.26
Chenozem podzol 100 0.15 ± 0.04
Turf-podzol 150 0.04 ± 0.01
Grey-woodland 246 0.0
aFrom: Molozhanova (1970).
Yadav et al. (1985) detected 0.22 mg carbaryl/kg in the soil
at harvest time, when sesamum ( Sesamum indicum) was sprayed twice
with carbaryl (0.2%) on days 45 and 60 of crop growth (21 August and
5 September). As residues in the soil after the first spray were
about 0.65 mg/kg, the rate of reduction of residues in soil was 56%
within 2 months. Shilova et al. (1973) measured about 0.1 mg
carbaryl/kg soil one month after treatment with carbaryl (5 kg/ha)
against blood sucking insects in the subarctic.
Gangwar et al. (1978b) applied a 4% granular formulation to
sandy loam planted with a grain crop (bajra) at very exaggerated
application rates of 20, 40, and 60 kg a.i./ha. Initial residues of
140-354 mg/kg decreased by a total of 31 to 53% within 30 days and
95 to 98% within 90 days. Residues at 90 days were 2.68-17.49 mg/kg.
Application of carbaryl granules at very high doses of 15, 30,
and 45 kg/ha resulted in deposits of 232, 397, and 525 mg/kg,
respectively, in soils cultivated with corn, and in 104-109,
168-217, and 304-422 mg/kg, respectively, in clay loam soils
cultivated with root crops (beet, radish, carrot). The dissipation
rate of carbaryl residues from clay loam soil, in 5 days following
the treatment, was 42-56% during autumn and 55-69% during spring.
The highest residue levels sampled from all plots decreased to
229.7, 142.9, 73.8, 33.8, 6.7, and 1.3 mg/kg on days 5, 10, 15, 30,
60, and 100, respectively. Residues were below detectable levels in
several plots by day 60 and in most plots by day 100 (Kavadia
et al., 1978).
According to Kuhr et al. (1974), the dissipation of carbaryl
in the soil of apple orchards is rapid. Soil residues of carbaryl
had almost completely disappeared from the top 2 inches of soil in 2
weeks. They were 13.8 mg/kg immediately after application and
3 mg/kg, 1-2 days later.
An oil-based carbaryl formulation (Sevin-2-oil) was applied
twice by a fixed-wing aircraft to a coniferous forest in New
Brunswick (Canada). Initial residue levels 1 h post-spray in litter
and soil for both applications were, respectively, 1.21 and
0.86 mg/kg and 0.78 and 0.48 mg/kg (Sundaram & Szeto, 1987).
Relative to the amount sprayed, only a small amount of the chemical
reached the litter and soil, probably because of canopy filtration.
Within 1 day, an average of 40-45% of the initial residues was lost
from litter and soil, respectively, indicating a rapid dissipation
time of 1.5 days for the disappearance of 50% of the initial maximum
concentration. Beyond 5 days, an average of 12% of the initial
concentration remained in both the substrates.
As already reported, two field dissipation studies were
conducted by Springborn (1988b,c) with carbaryl (Sevin-4-oil)
applied twice with a seven-day interval to forest. The first study
was conducted in a coniferous Oregon forest. In soil from the
treated site, carbaryl levels decreased from 0.196-3.877 mg/kg
following the first application to 0.130-1.87 mg/kg, 3 days after
treatment, and from 0.079-5.323 mg/kg following the second
application to 0.242-1.187 mg/kg at 90 days. The second study was
conducted in Pennsylvania. Carbaryl levels in soil decreased from
0.022-0.068 mg/kg following the first application to
<0.012-0.075 mg/kg, 3 days after treatment, and from
0.11-0.932 mg/kg following the second application to
0.01-0.099 mg/kg at 90 days.
A rice field dissipation study was conducted in Arkansas (USA)
by Springborn (1988a). Carbaryl (Sevin XLR) was applied 3 times, at
14-day intervals, to an irrigated field plot in which rice was
planted. In flooded soil, the concentration of carbaryl varied from
<11 to 309 µg/kg throughout the study and was not directly related
to application date. The carbaryl level was <11-309 µg/kg in soil
following the first application and <11-129 µg/kg following the
third application; 180 days later, carbaryl concentrations in the
soil were 11-56 µg/kg.
In a similar study conducted in California, under the same
conditions, Springborn (1988) found that carbaryl dissipated rapidly
in flooded soil. The concentration of carbaryl ranged from <11 to
198 µg/kg throughout the study. Carbaryl was <11-23 µg/kg in soil
at sampling intervals following the first application, and increased
to <11-180 µg/kg following the third application; 180 days after
the third application, carbaryl concentrations in the soil were
11-88 µg/kg.
A soil dissipation field study was conducted in 1985/1986 in
California (sandy loam, pH 6.3) and Iowa (silt loam, pH 6.5) with
carbaryl 80 S formulation (Davis, 1986a). Carbaryl was applied to
strip plots of bare soil, 6 weeks later, 7 applications were made
with intervals of about 5 days between applications. Soil sampling
was performed before treatment, during treatment, and up to 6 months
after the last application. No carbaryl residues were found in
California pretreatment samples. After the last application,
residues reached a maximum of 1.61 and 0.29 mg/kg in the 0-15 and
15-30 cm samples, respectively. Fourteen days after the last
application, carbaryl residues below the 15-cm level were less than
the limit of quantification.
It took 28 days for residues to dissipate completely from the
top 15 cm. The half-life in California soil was calculated to be
2.7-3.8 days.
5.1.4 Food and animal feed
5.1.4.1 Fruit, vegetables, and grain
Contamination of vegetation by carbaryl occurs, either during
spraying, or, by its migration through contaminated soil into the
roots of plants.
Residual levels of carbaryl after the spraying of plants depend
on the type and species of the plants sprayed. Carbaryl levels of up
to 30 mg/kg have been found in plants during the treatment period
(Molozhanova, 1968). It has been found to be rather persistent in
vegetables and fruits. Concentrations of 0.6-3.9 mg/kg were measured
in lettuces 1 week after single or repeated sprayings, and levels up
to 1 mg/kg were found in tomatoes treated according to requirements
(Antonovich, 1970). In cabbage, initial residues after spraying
ranged from 14.8 to 33.9 mg/kg, depending on the concen-tration
used. After 7 days, the residues decreased to 2.5-5.13 mg/kg. In
eggplant, the initial concentrations were 8.3-16.9, and 7 days
later, 3.05-5.4 mg/kg. After washing, the concentrations decreased
considerably to less than 3 mg/kg after day 7 (Mann & Chopra, 1969).
Following application of carbaryl on cauliflower, in the form
of dust (10%) at 1.5-2 kg a.i./ha or wettable powder (50%) at 0.75-1
kg a.i./ha, the residues of carbaryl declined to 3.64-9.59 mg/kg
within 8 days of treatment. The carbaryl deposits on leaves were
between 19.45 and 42.08 mg/kg. Washing of cauliflower with plenty of
water reduced the residues of carbaryl by 36-95%. A waiting period
of 8 days has been suggested for cauliflower (Singh et al., 1978).
Persistence of carbaryl in brinjals and peas after spraying at
1 and 2 kg a.i./ha was found to be 3.42 and 5.03 mg/kg respectively.
Washing of vegetables with water within one hour and after a day of
spraying reduced the level of deposits of carbaryl to about
1.0 mg/kg level. In the case of pea pods, the levels of residues
dropped to 0.20 mg/kg within 5 days. In pea seeds, the residue
levels ranged between 0.03 and 0.09 mg/kg, after 8 days (Krishnaih
et al., 1978).
Measurements of carbaryl in different fruits were taken at
intervals of 5-10 days after plants had been treated with a 0.2%
suspension of carbaryl at 1000 litre/ha during the vegetation period
(Bogomolova, 1968, 1970). The initial amount of carbaryl, 2 days
after the treatment, varied in different fruits from 2.3 to
2.7 mg/kg. Ten days later, the concentrations were reduced by
50-70%, and, after 20 days, the levels were within the range of
0.2-0.8 mg/kg, depending on the species (Table 13). According to
Mann & Chopra (1969), the dissipation of carbaryl from plants after
the first day of spraying was about 40-45% of the initial residue.
In apples, concentrations of 2.5-2.9 mg/kg, 0.4-2.4 mg/kg, and
0.15 mg/kg were found on days 7, 10, and 38, respectively, after
spraying. One to two months after spraying apples with a 0.1%
suspension at a dose of 2000 litre/ha, residues of 0.08-0.10 mg/kg
were measured (Antonovich, 1970). Four months after spraying with a
0.06% suspension at a dose of 9 kg a.i./ha, levels in the range of
0.09-0.24 mg/kg were found in apples, depending on the spraying
procedure. Fine-droplet spraying resulted in residue levels 2.5-3
times lower levels than those with coarse-droplet sprays (Atabaev,
1972).
Table 13. Carbaryl levels in different fruits after spraying
during vegetation (mg/kg)a
Kind of fruit Day after spraying
2 10 20
Strawberries 2.3 1.25
Gooseberries 2.5 0.90 0.6
Blackcurrants 2.4 1.10 0.8
Cherries 2.7 1.90 0.2-0.4
Plums 2.4 1.40 0.2-0.4
aFrom: Bogomolova (1968).
A survey of apples grown in Ontario, Canada, between 1978 and
1986 showed that out of 22% of carbaryl-treated apples (1.67 kg/ha)
sold, 3.6% had detectable carbaryl residues (detection limit of
0.01 mg/kg), and that the average residue level was 0.03 mg/kg, with
a maximum of 0.04 mg/kg (Frank et al., 1989).
Prolonged storage of apples reduced the carbaryl residues only
slightly (Bogomolova, 1968). The initial level (2.4-2.8 mg/kg)
changed little during the first 3 months of storage at a temperature
of +5 to +10 °C. A slight reduction was observed during the fourth
month of storage. During the process of storage, carbaryl migrated
inwards from the surface, so that the concentration in the skin was
reduced, while that in the pulp was increased (Table 14).
Residues on lemon foliage on day 0 of treatment were
4.6±0.3 µg/cm2. Five days after treatment, levels of 2.4 µg/cm2
and 5.6 µg/cm2 were found on lemon and orange foliage,
respectively; on the 60th day, the residual values were 0.41 and
0.36 µg/cm2, respectively. Persistence half-lives were 22 days
(lemons) and 14 days (oranges) (Iwata et al., 1979).
Carbaryl is persistent, particularly in citrus fruits and
grapes. Residues of 3-6 mg/kg were measured in lemons and oranges
2.5-3 months after treatment of trees with 3-10 kg carbaryl/ha, and
amounts of 3.6, 1.9, and 0.4 mg/kg were found in grapes on days 7,
14, and 40, respectively, after repeated spraying of vineyards with
a 0.12% suspension. The half-life of carbaryl in grapes was 29 days,
compared with 2-9 days in cherries and cabbage (Antonovich, 1970).
Washing and peeling reduced carbaryl levels in fruits and
vegetables by 40%; thermal processing by 45-90%, and preparation of
juices by 53% (Molozhanova, 1970). Canning also reduced carbaryl
residues. Thus, canning of different fruits (strawberries, black
currants, gooseberries) containing carbaryl in the range of
0.25-0.45 mg/kg, resulted in a reduction to 0.17-0.29 mg/kg; when
the initial contents were higher (3-7 mg/kg) the levels after
canning dropped to 1.4-2.2 mg/kg and 2.1-2.5 mg/kg, respectively,
i.e., from 30 to 70%. Storage of canned fruit further reduced
carbaryl residues by 2-3 times after 12 months (Bogomolova, 1968).
Boiling vegetables (e.g., cabbage) reduced carbaryl residues by
approximately 50%; however, the residues in pickled cabbage, 5
months after preparation, were only 25% less than the initial amount
(Antonovich, 1970).
Table 14. Carbaryl content in apples during storage (mg/kg)a
Part of apple Day of storage
10 20 30 40 50 70 80 100 110
Skin 2.40 1.80 2.00 1.80 1.92 1.72 1.60 1.45 1.22
Pulp 0.35 1.00 1.02 1.10 1.00 1.10 1.34 1.42 1.30
Total 2.75 2.80 3.02 2.90 2.92 2.82 2.94 2.87 2.52
aFrom: Bogomolova (1968).
Elkins (1989) reported that washing during the commercial
processing of produce removed 97, 87, and 77% of the carbaryl
residues from tomatoes, spinach, and broccoli, respectively.
Blanching (short treatment with hot water) was stated to result in
68% removal of carbaryl from green beans, while blanching, in
addition to washing, resulted in the removal of over 97% of carbaryl
from both spinach and broccoli.
After a single application of carbaryl (Sevin 50 at 2.5 kg/ha),
residues in cauliflower decreased from 16.75 mg/kg on the day of
application to 0.87 mg/kg, 15 days afterwards (Yadav & Jaglan,
1982). Washing further reduced the detectable residues at 15 days to
0.67 mg/kg, while no detectable residues were present after boiling.
Carbaryl was found in vegetation adjacent to treated fields.
Grass growing at a distance of 250 m from a carbaryl-treated cotton
field contained 0.18-0.25 mg carbaryl/kg wet weight up to 45 days
after spraying (Gusseynov, 1970).
The results of the 1989 Pesticide Residue Monitoring Programme
in California showed that carbaryl was detected in 2 samples of
grape from 26 in quantities less than the accepted tolerance level.
It was not found in oranges (Okumura et al., 1991). Summaries of
residue data on different plants are given in Tables 15 and 16.
Carbaryl residues were not detected in 44 samples of wheat in the
United Kingdom analysed by the multiresidue method (Osborne et al.,
1989).
Table 15. Comparison of carbaryl residues with different
formulationsa
Formulation use Plant Mean residues Day after last
(kg a.i./ha) value (mg/kg) application
44% SC 2x0.5 apple 0.25 7
pears 0.16 7
50% WP 2x0.5 apple 0.15 7
pears 0.09 7
44% SC 2x2 spinach 4.44 -
50% WP 2x2 2.38 14
44% SC 2x2 lettuce leaf 2.72 14
50% WP 2x2 1.55
44% SC 2x2 barley 7.87 14
50% WP 2x2 4.63
Table 15 (continued)
44% SC 2x2 wheat 0.96 14
50% WP 2x2 0.99
44% SC 2x2 oats 0.22 14
50% WP 2x2 0.26
aFrom: Davis (1987).
Davis (1987) studied the impact of different types of
formulation on food residues, including carbaryl (44% w/w) oil-based
liquid formulation containing a "sticker", in order to prevent bees
from carrying carbaryl particles back to the beehive. The 50 W
formulation was a "standard" wettable powder formulation. The
results are given in Table 15. This work showed that the addition of
a "sticker" increased levels of food residues to some extent.
Studies on the removal of carbaryl residues from tomatoes,
green beans, spinach, and broccoli by commercial and home
preparation procedures (Elkins et al., 1968; Farrow et al.,
1968, 1969; Lamb et al., 1968) showed a considerable decrease in
the residues with treatment (Table 17).
For pre-harvest use on grain, the rate of application of
carbaryl ranges from 2 to 9.5 kg/ha, depending on the degree of
infestation, density of foliage, and the stage of the life cycle of
the pest. Carbaryl, usually applied at a rate of 5 mg/kg, is also
used to protect stored grain. Studies on stored wheat, barley, oats,
and rice indicated that carbaryl residues on grain have a half-life
of between 26 and 80 weeks, depending on the temperature (FAO/WHO,
1976). Residues of carbaryl in baked bread are then of the order of
1-1.5 mg/kg.
5.1.4.2 Animal products
Technical carbaryl was fed to dairy cows of the Brown Swiss,
Jersey, Holstein, and Ayrshire breeds at 50, 150, and 450 mg/kg of
their average total daily roughage intake (dry weight) for a period
of 2 weeks. Samples of milk were taken at regular intervals and the
cream was analysed for carbaryl by means of the
p-nitrobenzene-diazonium fluoborate coupling method. The
concentration of carbaryl, if present, was below the sensitivity of
the analytical method (0.01 mg/kg) (Gyrisco et al., 1960).
Table 16. Carbaryl residues following different applications
Formulation use Plant Mean value Day after Reference
(kg a.i./ha) residues last
(mg/kg) application
44% SC barley 5.4 14 Davis & Thomas (1987)
44% SC sugar beet 0.07-1.04 14 Thomas (1986)
roots
44% SC pasture 59.7-183 3 Davis (1986b)
aerial ground grass
Carbaryl sweet 0.33 Frank et al. (1987a)
cherries
Carbaryl tomato 1.2 0 Frank et al. (1991)
0.5 3
0.03 6-8
tomato 0.47 0
juice 0.24 3
0.08 6-8
Table 17. Removal of carbaryl after preparation procedures
Vegetable Initial Percentage removal References
residues
(mg/kg) Commercial procedure Home procedure
Tomatoes 5.2 washing 82-99 washing 77 Farrow et al. (1968)
canning 98-99 canning 92
juicing 98 juicing 77
Green beans 7.6 blanching 68-73 washing 52 Elkins et al. (1968)
& canning blanching 81
freezing 94
canning 100
Spinach 20.8 washing 66-88 washing 70 Lamb et al. (1968)
blanching 96-97
Broccoli 12.4 washing 77 washing & cooking 55 Farrow et al. (1968)
blanching 82-97 washing, 90
blanching,
& freezing
Residues resulting from a single application and repeated
applications of carbaryl spray on cattle were rapidly eliminated
from body tissues. On days 1 and 3 after application, carbaryl was
detected in the liver (0.05 mg/kg), muscles (0.04 mg/kg), and
perirenal fat (0.16 mg/kg). Seventy-two hours after treatment, no
residues were found in the tissues studied. The excretion in milk
persisted for at least 69 h after spraying, the highest
concentration being 0.075 mg/litre, 5 h after exposure (Hurwood,
1967).
Data concerning the composition and levels of milk and tissue
residues in cows after continuous feeding with 100 mg
14C-carbaryl/kg diet are shown in Tables 18 and 19.
Table 18. Metabolites in tissues after continuous 28-day feeding
of 100 mg 14C- carbaryl/kg dieta
Metabolite (mg/kg) Kidney Liver Muscle
carbaryl 0.03 0.04 0.02
5,6-dihydrodihydroxycarbaryl 0.05 0.01 0.04
5,6-dihydrodihydroxynaphthol 0.02 0.02 0.0
naphthyl sulfate 0.29 0.02 0.0
water-soluble unknowns 0.43 0.13 0.03
unextractable unknowns 0.18 0.19 0.01
aFrom: Dorough (1971).
Table 19. Composition of milk residues after continuous 28-day
feeding with 100 mg 14C-carbaryl/kg dieta
Percentage mg/kg
Organic phase
5,6-dihydrodihydroxycarbaryl 38.5 0.11
carbaryl 8.4 0.02
3,4-dihydrodihydroxycarbaryl 4.0 0.01
Table 19 (continued)
Percentage mg/kg
5,6-dihydrodihydroxynaphthol 1.0 0.01
Water soluble
5-methoxy-6-hydroxycarbaryl 25.6 0.07
unknowns 10.6 0.03
1-naphthol 5.5 0.02
5-hydroxycarbaryl 3.1 0.01
carbaryl 1.2 0.01
aFrom: Dorough (1970a).
Effective horn fly control for a period of 15 days was obtained
by spreading carbaryl down a cow's back. Results indicated that, if
applications of a 0.5% spray or a 50% dust were made immediately
after the morning or evening milking, no residues of carbaryl would
result in subsequent milkings (Eheart et al., 1962). The results
obtained by Petrovski (1970) were similar. After treating cows with
a 0.85-1% suspension of carbaryl, residues of from 0.1 to
0.3 mg/litre were found in milk, 20 h after application. On the
third day, only trace amounts of carbaryl were found. After
treatment with a 0.5% suspension of carbaryl, no traces were found
in the milk.
5.1.4.3 Animal feed crops
Generally, animal feed crops contain less carbaryl than fruit
and vegetables. Mean levels of 0.82 mg/kg were found in 80 samples
of animal feed plants (Molozhanova, 1970). Levels of 0.3 mg/kg were
found in maize stems at harvest, 70 days after spraying (Antonovich,
1970).
Studies on the carbaryl content of animal feed plants in hot
climates showed that carbaryl can be detected 3-3.5 months after
treatment, depending on the dose and the number of applications.
Thus, after repeated applications of 50% carbaryl dust at a rate of
0.5 g/m2, the carbaryl content in animal feed plants was in the
range of 1-1.8 mg/kg wet weight, but, after a single application of
0.7 g/m2, concentrations of 0.10-0.31 mg/kg were found (Atabaev,
1972).
5.1.5 Other products
Measurement of carbaryl residues in tobacco plants showed that,
15 days after field spraying with 950 g/ha, the content of carbaryl
in green tobacco leaves was 10.1 mg/kg. Drying leaves by hot air
decreased carbaryl residues by only 10% (Antonovich, 1970).
Application of carbaryl at a rate of 2.2 kg/ha resulted in
carbaryl residues on cotton foliage of 7.2 µg/cm2 on the day of
spraying (Estesen et al., 1982). Levels decreased to 5.9, 0.58,
and 0.26 µg/cm2 after 3, 6, and 8 days, respectively.
Carbaryl was found in the foliage of cotton plants in
concentrations of 0.03-1 mg/kg from 10 days to 2.5 months after
treatment. Carbaryl penetrated the plant through foliage and roots
and contaminated the cotton and the seeds. Cotton harvested from
treated fields 2 weeks to 1 month after treatment contained
0.015 mg/kg. Trace amounts of carbaryl were found in cotton oil
(Guseynov, 1970).
After treatment with a 2% suspension of carbaryl against
mountain pine beetle, residues in bark disks after 1 year were
359 mg/kg. In another case, residues in bark disks were
890±146 mg/kg, after spraying, and 531±178 mg/kg 16 months later
(Page et al., 1985).
5.1.6 Terrestrial organisms
The quantities of carbaryl that were absorbed by songbirds,
either by contact or by eating food from a sprayed area, were
studied by Kurtz & Studholme (1974). Towhees (American finches) were
collected 3 days after the forest had been sprayed for gypsy moths
at a rate of 1.1 kg/ha. The amounts of carbaryl found in the towhees
were small, even though the samples were taken close to the spraying
time. Trace amounts of carbaryl were found in three samples out of
five, compared with two samples out of five control birds. The
minimum level of detection was 0.1 µg/g. The low levels of carbaryl
found in "sprayed" birds were explained by the fact that towheeds
are groundfeeders and that only a small amount of carbaryl might
have passed through the trees to the ground.
5.2 General population exposure
5.2.1 Exposure through the food
The daily intake of carbaryl was studied for several years in
the USA (see Table 20).
Table 20. Carbaryl daily intake in the USA
Year Positive samples Daily Intake References
(%) (mg/day
per person)
1964-65 7.4 0.15 Duggan et al. (1971);
1965-66 2.7 0.026 Duggan & Corneliussen
(1972)
1966-67 1.1 0.007
1968-69 0.8 0.003
1969-70 0 -
Infant Toddler
1978 - 0.088 0.05 Gartrell et al. (1986)
1979 - - 0.049
1980 - 0.06 0.035 Reed et al. (1987)
1981-82 - 0.129 0.127
1982 - - 0.012
1982-84 - 0.117 0.017- Gunderson (1988)
0.0315
Carbaryl residues in total diet samples in the USA are
relatively low (Table 21). Carbaryl is found in potatoes, leguminous
vegetables, root vegetables, and fruit. In foods processed in the
usual manner, i.e., by peeling, stripping outer leaves, and cooking,
when appropriate, carbaryl residues usually decreased to
undetectable levels or traces (Manske & Corneliussen, 1974).
Table 21. Carbaryl residues in food in the USA
Number of composites Number of Number of Range in References
analysed composites composites mg/kg
with residues with traces
270, from 30 grocery 20 15 trace to Manske & Corneliussen
stores in 27 cities 0.5 (1974)
June 1970-April 1971
420, from 35 market 6 5 0.02 Manske & Johnson
baskets, 6 June 1971- (1975)
July 1972
360, 12 August 1972- 12 10 0.05-1.10 Johnson & Manske (1976)
July 1973
360, August 1973- 8 2 0.001-0.284 Manske & Johnson (1977)
July 1974
1044, 1978-82 11 (1%) a a Yess et al. (1991a)
3744, 16 market 135 (4%) a a Yess et al. (1991b)
basket collections
1982-86
aNo figures given.
During a 5-year period, from 1982 to 1986, the Los Angeles
District Laboratory analysed 19 851 samples of domestic and imported
food and feed commodities for pesticide residues. A single, rapid,
multiresidue method was used. Carbaryl was detected in 164 samples
from the total 19 851 samples analysed. Not one sample exceeded the
US Federal tolerance levels (Luke et al., 1988). In another
publication, the same authors reported comparative studies on US and
imported food. Carbaryl was found in 21 samples from a total 6391 US
samples in quantities ranging from 0.1 to >2.0 mg/kg, but less than
10 mg/kg. From 12 044 imported agricultural commodities 132 were
contaminated by carbaryl, and levels in 14 of them exceeded the
tolerance levels (Hundley et al., 1988).
5.2.2 Exposure during insect control
Exposure to carbaryl, used to control the gypsy moth in camping
and picnic areas, was monitored on the day of application and then
weekly for 3 weeks. Carbaryl was applied from the air at a rate of
1.12 kg/ha. Exposure was measured using dermal pads.
Extrapolating from the most contaminated group of pads (they
contained an average of 279 µg/pad), the authors calculated a "worst
case" exposure of 0.54 mg/kg on the day of spraying. The authors
concluded that the risk to those who use public areas during, and
after, carbaryl application to trees is negligible (Cameron et al.,
1985).
A 5% spray of carbaryl 85% wettable powder (WP) was applied for
insect control in homes (Vandekar, 1965), as a surface application,
at the rate of 2 g/m2. Urine samples taken from villagers 1 week
after spraying showed a significant increase in 1-naphthol levels
from 30.5 µg/ml baseline to 50.3 µg/ml. Inhibition of plasma ChEA
was found in 48 out of 63 subjects, 1 week after spraying.
During a monitoring study carried out from 1976 to 1980 in the
USA, 1-naphthol was found in 1.4% of the urine samples from persons
between 12 and 74 years old. The source was thought to be carbaryl
and naphthalene (Carey & Kutz, 1985).
5.3 Occupational exposure during manufacture, formulation,
or use
Harry (1977) estimated that about 13 million people in the USA
were potentially exposed to carbaryl during its manufacture,
formulation, packing, transportation, storage, and during and after
application, and while working with treated crops or during harvest.
Best & Murray (1962) published a survey on the exposure of
plant workers during the production of carbaryl. Air concentrations
varied from 0.23 to 31 mg/m3. Urine samples contained an excess of
1 mg total 1-naphthol per 100 ml of urine. During air blast spraying
of orchards, Jegier (1964) found air concentrations of 0.6 mg/m3
(0.18-0.81 mg/m3). The mean respiratory exposure, measured by the
respirator pad technique was 0.29 mg/h (0.24-0.53 mg/h) and mean
dermal exposure, measured by skin pads, was 25.3 mg/h
(18.5-30.3 mg/h). The maximum total exposure was 31 mg/person per h,
or 0.025% of the toxic dose. Simpson (1965) estimated that the
amount of dermal exposure was less than 0.1% of the toxic dose in
orchard sprayers. During cotton spraying by aircraft, Yakim (1965)
found 4 mg carbaryl dust/m3 in the breathing zone of flagmen,
1 mg/m3 during preparation of the solution, and 0.7 mg/m3 in the
pilot's cabin. Adylov (1966) reported the following air
concentrations found during the aerial application of a water
solution: flagmen's breathing zone 1.92 (0.64-2.84) mg/m3; pilot
cabin, trace amounts, workers on the ground (preparation of
solution, etc.,) 6.28 (0.48-19.2) mg/m3. In the urine samples of
carbaryl formulators, 1-naphthol levels varied from 6.2 to
78.8 mg/litre. Agricultural workers who used carbaryl for pest
control excreted from 0.07 to 1.7 mg/litre in their urine (Shafik
et al., 1971).
Exposure studies were completed for pesticide application and
formulating plant workers by the biological monitoring of 1-naphthol
in the urine, dermal pads, and respirator filter pads (total 480
samples of dermal pads and 73 respirator pads). Workers operating
tractor-drawn airblast equipment applied carbaryl at 0.045-0.06% as
spray (Comer et al., 1975). An estimate of the amount of dermal
and respiratory exposure that could occur was made using the
procedures described by Durham & Wolfe (1962). The results are
presented in Table 22.
The concentration of 1-naphthol in the urine varied from 0.2 to
65 mg/litre with a mean of 9 mg/litre. The rate of excretion per
hour varied from 0.004 to 3.4 mg with a mean of 0.5 mg/h. The
maximum level during the working day was reached by late afternoon.
An approximate calculation of the excreted carbaryl (0.5 mg
1-naphthol/h = 0.7 mg/h excretion of carbaryl), and potential
exposure, 75 mg/h, shows great differences. About 1/100 part of
possible absorption occurs.
Exposure to carbaryl during agricultural application was
studied by Leavitt et al. (1982). WP 80 carbaryl was used (0.45 kg
in 200 litre water) to spray trees, lawns, and gardens. The mean
dermal exposure was 128 mg/h and the mean inhalation exposure was
0.1 mg/h. The maximum percentage of the toxic dose that the
applicators received was 0.12%/h. No symptoms or inhibition of ChE
were reported. In another group of applicators, the mean dermal
exposure was 59.4 mg/h and inhalation exposure 0.1 mg/h.
Table 22. Potential dermal and respiratory exposure of formulating
plant workers and field spraymen to carbaryla,b
Subject Route of Exposure Exposure (mg/h)
exposure situations
studied Range Mean
Formulating dermal 48 0.80-1209.30 73.90
plant workersc
respiratory 48 0.03-4.10 1.90
Field dermal 32 1.70-211.80 59.00
spraymend
respiratory 25 0.01-1.08 0.09
aFrom: Comer et al. (1975).
bCalculated on the basis of the worker wearing a short-sleeved,
open-necked shirt, no gloves or hat, with his clothing protecting
the areas covered.
cWorkers on mixing and bagging operations (4 and 5% dust).
dOperating power air-blast spray machines in fruit orchards
(0.045-0.06% solution spray).
Dermal exposure is influenced by the type of spray equipment.
The dermal deposit and respiratory intake of carbaryl in humans was
monitored during home garden spray operations using compressed air
or garden hose-end sprayers (Puech, undated). The greatest mean
dermal deposit in cm2/min was received on the feet. When the spray
target was above shoulder height the next highest dermal spray was
received on the forearm (Table 23). Exposure through inhalation was
insignificant.
A study of the urban application of carbaryl was performed by
Gold et al. (1982). The maximum dermal exposure recorded in this
study was 2.86 mg/kg per h. The maximum air concentration was
0.28 µg/litre. An insignificant decrease in ChEA in serum and
erythrocytes was found in some of the applicators. The mean dermal
carbaryl exposure of the applicator, expressed as a percentage of
toxic dose per h, was 0.01%, with a maximum of 0.08%. This exposure
rate is below the risk rate for applicators. No symptoms of
intoxication were reported by the authors. During the urban
application of carbaryl to trees and ornamental shrubs using
hand-held equipment, air concentrations measured at the breathing
zone in full-shift samples, of 0.010-0.070 mg/m3, were detected in
only 30% of the samples (Leonard & Yearly, 1990).
Table 23. Human exposure during spray operations
Type of spray equipment Mean deposit Total exposure
(µg/cm2 per min) (µg/kg per min)
Compressed air sprayer
below waist 0.030 4.3
above shoulder height 0.096 5.6
Hose-end sprayer
below waist 0.154 18.3
above shoulder height 0.353 24.4
Dermal exposure to carbaryl in harvesters of strawberries was
studied by Zweig et al. (1984, 1985). They observed dermal
exposure on the hands and forearms, and to a much lesser degree, on
the lower legs. The first day, 3 mg carbaryl/h was found on cotton
gloves, 0.66 mg/h on pads on the forearms, and 0.07 on the lower
legs. The following day, the values were 1.23, 0.41, and 0.07 mg/h
for gloves, forearms, and lower legs, respectively. The ratio of
dermal exposure to dislodgeable foliar residues (DFR) was 4.34,
2.82, and 6.17, for 3 consecutive days. The half-life of carbaryl on
strawberry leaves was 4.1 days. Dislodgeable carbaryl residues on
cotton foliage, expressed as µg/cm2 of cotton leaf (one surface
only), following application by man-pulled ground rig were 7.2, 5.9,
0.58, and 0.26 at 0, 72, 144, and 192 h after application,
respectively (Estesen et al., 1982). Approximate dermal exposure
rates may be calculated using the following expression, proposed by
Zweig et al. (1985). Dermal exposure rate (mg/h) = 5x103xDFR
(in µg/cm2). This method is suggested to obtain the exposure rate
of fruit harvesters, in order to establish safe re-entry periods
without human studies.
Factors affecting the levels of exposure during the
agricultural application of pesticides were analysed by Wolfe et
al. (1967). Wind is the most important environmental condition. The
type of activity, equipment used, the duration of exposure,
formulation, individual protection, including attitude, are also
discussed. Carbaryl deposits from air jet application on orchards
were found at 500 m downwind in the presence of inversion, and at
300 m, in its absence. Ground application results gave deposits at
150 and 50 m, respectively (MacCollom et al., 1985, 1986). Air
concentrations were 17.88 µg/m3 during spraying above the orchard
downwind edge, 9.5 µg/m3, 30 min after spraying, and 8.17 µg/m3,
1 h after spraying.
Airborne and deposit levels of carbaryl were measured after
three applications by air to an apple orchard (Currier et al.,
1982). Samples of air were taken at distances of from 12.2 to 3994 m
from the target orchard. At a distance of 12.2 m, the concentration
in air was between 3.3 and 76.2 µg/m3. One hour later it was
between 2.5 and 11.3 µg/m3. At a distance of 3.2-3.3 km, the
initial concentration was between 9 and 28.8 µg/m3 during spraying
and 0.9-14.4 µg/m3, one hour after treatment. Because the area of
the study was one of concentrated agriculture, it is possible that
carbaryl could have been used on other orchards and gardens near to
the sampling point.
6. KINETICS AND METABOLISM
6.1 Absorption
When only 0.1 ml of carbaryl solution (0.025-0.05 mmol/litre)
was administered into the lungs of anaesthetized rats, it was
rapidly absorbed. About 50% of the dose was absorbed in 2.6 min. The
amount of carbaryl absorbed per unit time was directly proportional
to the administered dose (Hwang & Shanker, 1974). Blase & Loomis
(1976) demonstrated that carbaryl could be taken up and metabolized
by the isolated perfused rabbit lung. Retention by rats of
14C-labelled carbaryl, inhaled as vapours during a 1-h exposure
period, was 75.4% of the total dose inhaled, which did not exceed
50 µg (Dorough, 1982).
When a dose of 7.5 µmol radiolabelled carbaryl/kg body weight
was administered intragastrically to fasted, anaesthetized, female
rats, 22 and 67 min after dosing, the proportions absorbed were
52.6±14.1% (n=3) and 81.7±15.7% (n=3), respectively; 89.3% of the
radiolabelled material in the collected portal blood was
[1-naphthyl-1-14C] N-methyl carbarmate (Casper et al., 1973).
Absorption from the small intestine was studied in
anaesthetized rats with a ligature around the pylorus and an
ileocecal junction with major blood vessels not occluded. A
concentration of carbaryl of 0.005-0.1 mmol/litre was used. The
absorption half-time was 6.4 min (Hwang & Schanker, 1974).
On the basis of ligation studies on mice, Ahdaya & Guthrie
(1982) determined that the absorption of carbaryl from the stomach
was relatively low compared with that from the entire
gastrointestinal tract (29%), but was relatively high in comparison
with that of other pesticides.
Variation in the digestive absorption kinetics, according to
the vehicle used, was reported in studies on female Wistar rats
(Cambon et al., 1981). The results indicated that carbaryl was
absorbed more rapidly in the intestine when either DMSO or
tragacanth was used as a vehicle. It seems that milk does not
facilitate carbaryl absorption. Inhibition of ChEA was closely
related to the absorption rate.
The rate of dermal penetration of carbaryl (in acetone
solution) into mammals, birds, amphibia, and insects was studied by
Shah
et al. (1983). The half-time of penetration of 14C-labelled
carbaryl was 317 min in Japanese quail, 12.8 min in mice, 6.4 min in
grass frogs, 4576 min in American roaches, and 791 min in tobacco
horn worms.
In an in vitro study, only about 1% of an applied dose of
carbaryl penetrated through the skin of the rat over an 8-h period
(MacPherson et al., 1991). Using two different methods, Shah &
Guthrie (1983) measured the half-time for penetration of carbaryl,
applied dermally at a rate of 4 µg/cm3, and found 10.34 h and
4.75 h, respectively. Shah et al. (1987) compared the rate of
dermal penetration of carbaryl in young, versus adult, Fischer 344,
female rats and did not find any consistent age-related differences.
Percutaneous absorption of carbaryl in rats was also studied by
Knaak et al. (1984). Carbaryl dissolved in acetone was applied to
back skin (not occluded) at 43.4-48 µg/cm2. Recovery studies
indicated that 57.7% of the applied carbaryl was absorbed.
Approximately 5.8% of carbaryl penetrated the skin within 1 h, the
rate of absorption being 0.18 µg/h per cm2. The half-life of
absorption by blood was 1.26 h, and that for elimination, 67 h.
Carbaryl labelled with radioactive carbon (14C), dissolved in
acetone, was applied to the skin of six volunteers, in order to
study percutaneous penetration (Maibach et al., 1971; Feldmann &
Maibach, 1974). The results showed almost complete penetration of
carbaryl on the forearm and jaw angle. After a 24-h application, the
cumulative urinary excretion over 5 days was 74%. According to other
authors using the same data, the estimated cumulative absorption
over 5 days, as a percentage of the applied dose, was 63% (Fisher
et al., 1985), 45% of this occurring 8 h after the onset of
penetration, which had a lag of 3.5 h.
Comparing the different studies, it is clear that some solvents
can facilitate the dermal penetration of carbaryl.
6.2 Distribution
Plasma levels of carbaryl in rats were compared after iv,
intraduodenal, or hepatic portal administration of 0.5 mg/kg
(Houston et al., 1974). In Fig. 2, it is shown that plasma
concentrations were lower after intraduodenal application, when
carbaryl was subjected to the liver's first pass metabolizing
effect. Plasma concentrations following portal application were also
lower (approximately 80% of the concentration after the systemic
route of application).
In India, degradation of carbaryl was studied in a greenhouse
by Brahmaprakash & Sethunathan (1984, 1985). Since a soil planted
with crops may be more dynamic and complex than unplanted soil,
because of increased microbial activity, they studied carbaryl
persistence in soil (pH 6.2, organic matter 1.6%) planted with rice
in a greenhouse, under flooded and non-flooded conditions. Carbaryl
disappeared more rapidly from soils planted with rice than from
those without rice, under both flooded and non-flooded conditions.
The amount of carbaryl decreased to between 30.2 and 32.1% of the
original level within 30 days in unplanted soil under both flooded
and non-flooded conditions. During this same period, the carbaryl
concentration decreased to 17-18% of the original level in planted
soil under both water regimes. Degradation occurred by hydrolysis,
but there was no appreciable difference in the rates of degradation
between flooded and non-flooded soils. The rate of degradation of
carbaryl was little affected by moisture. Further degradation of
1-naphthol was slow in both planted and unplanted systems. A
significant portion of the ring-14C accumulated in the soil as
1-naphthol and soil-bound residues. Evolution of 14CO2 from the
labelled side-chain and ring was negligible, even in soil planted to
rice.
4.3.4 Degradation by microorganisms
Many studies have demonstrated the great ability of
microorganisms to degrade carbaryl in the soil, and for some of
them, to use it as a sole source of carbon. The most frequently
identified organisms are bacteria ( Pseudomonas phaseolicola, P.
cepaphia, Rhodococcus sp., Nocardia sp., Xanthomonas sp.,
Achromobacter sp.) and fungi ( Aspergillus niger, A. terreus,
Fusarium solani, Gilocladium roseum, Rhizoctonia solani, R.
practicola, Penicillium sp., Mucor sp., Rhizopus sp.). In many
cases, it was demonstrated that the persistence of carbaryl in the
soil decreased after a first application, which was interpreted as
the selection and build-up of strains more capable of degrading the
product. The same process may also explain that, in some studies,
the rapidity of degradation suddenly rose after a lag period during
which only minor amounts of carbaryl degraded.
Tewfik & Hamdi (1970) mentioned that carbaryl was decomposed
into 4 distinct compounds by a soil bacterium designated as S-1.
They considered that this soil bacterium might utilize carbaryl in a
similar way to Pseudomonas sp., which metabolize naphthalene via
the salicylate pathway.
Zuberi & Zubairi (1971) also reported that carbaryl is degraded
by soil microflora. Pseudomonas phaseolicola and Aspergillus
niger hydrolysed carbaryl to 1-naphthol. In the case of P.
phaseolicola, an unidentified minor metabolite was also detected.
Larkin & Day (1986) reported that 2 bacteria isolated from
garden soil, Pseudomonas sp. (NCIB 12042) and Rhodococcus sp.
(NCIB 12038), could grow on carbaryl as the sole source of carbon
and nitrogen at pH 6.8, but failed to metabolize carbaryl rapidly.
Both could use 1-naphthol as the sole source of carbon and
metabolize it via salicylic acid. Strain NCIB 12038 produced also
gentisic acid. Pseudomonas sp. (NCIB 12043), in a soil perfusion
column enrichment at pH 5.2, metabolized carbaryl rapidly to
1-naphthol and methylamine. 1-Naphthol itself was metabolized via
gentisic acid. A possible pathway for the catabolism of carbaryl and
1-naphthol was proposed.
Walker et al. (1975a), working with Pseudomonas sp.,
observed degradation of 1-naphthol and suggested that carbaryl could
serve as the sole source of nitrogen and carbon for bacteria. It was
also noted that the presence of another nitrogen source in the media
seemed to have a delaying effect on metabolism of carbaryl.
Bacterial communities of at least 12 and 14 members were
selected in continuous culture using carbaryl as the sole source of
carbon and nitrogen at pH 6. These communities were supported by the
slow formation of hydrolysis products and no carbaryl-degrading
bacterium was selected after more than 83 days. When using equimolar
1-naphthol and methylamine as the sole source of carbon and
nitrogen, a bacterial community of at least 8 members was selected.
After a lag of 10-50 days, soil perfusion columns (pH 5.2) and
continuous culture enrichments (pH 5) led to the selection of a
Pseudomonas sp. that could utilize carbaryl as its sole carbon and
nitrogen source (Larkin & Day, 1985).
Sud et al. (1972) showed that Achromobacter sp. also
utilized carbaryl as the sole source of carbon in a salt medium.
Four degradation products of carbaryl were 1-naphthol, hydroquinone,
catechole, and pyruvate. The organism also grew well in the first 3
degradation products.
The rate of degradation of carbaryl after 1, 2, and 3
applications to 3 submerged soils was examined by Rajagopal et al.
(1983). Soils that had been pretreated with carbaryl were able to
degrade carbaryl more rapidly than those without pretreatment. The
enrichment culture was inactivated upon autoclaving. The
concentration of carbaryl decreased in the mineral medium inoculated
with the enrichment cultures from the 3 soils, especially when it
served as the sole source of both carbon and nitrogen.
Rajagopal et al. (1984) studied the metabolism of side-chain
and ring 14C-labelled carbaryl in a mineral salts medium by soil
enrichment cultures. Hydrolysis was the major route of microbial
degradation. During carbaryl degradation by enrichment cultures and
Bacillus sp., 1-naphthol and 1,4-naphthoquinone accumulated in the
medium.
Rajagopal et al. (1986) also observed that carbaryl
disappeared more rapidly from a laterite soil pretreated with
1-naphthol than from a control soil never exposed to 1-naphthol. The
accumulation of 1-naphthol and bound residues formed from
added14C-carbaryl was greater in soils pretreated with 1-naphthol
than in untreated soils.
In experiments with the soil fungus Rhizoctonia practicola,
Bollag et al. (1976) found that it could transform 1-naphthol from
an ether-extractable to a water-soluble product. It was also
observed that, after removal of the fungal cells, the growth medium
possessed the ability to transform 1-naphthol, indicating activity
of an extracellular enzyme. Attempts to analyse the labelled
material in the aqueous phase indicated that the radioactivity was
associated with a compound of comparatively high relative molecular
mass.
Czaplicki & Bollag (1975) exposed 1-naphthol to Rhizoctonia
solani, isolated from soil, and found that it was completely
transformed to a compound not extractable with ether.
Working with the soil fungus Aspergillus terreus, Liu &
Bollag (1971b) investigated the metabolic transformation of carbaryl
through 1-naphthyl- N-hydroxymethylcarbamate and tried to clarify
the pathway of the side-chain. The next intermediate in biological
transformation was 1-naphthyl carbamate, which was further degraded
to 1-naphthol. Within one week, approximately half the amount of
carbaryl was transformed to these other metabolic products. No
attempt was made to clarify whether the formation of 1-naphthol was
the result of the biological or the chemical degradation of
1-naphthylcarbamate, but there were clear indications that
1-naphthol was metabolized further in the presence of A. terreus.
In vitro studies were carried out to investigate the
degradation of carbaryl by soil microorganisms. Three isolates from
soil, including Fusarium solani, a Gram-negative coccus, and a
Gram-positive rod, accelerated the hydrolysis of carbaryl to
1-naphthol and other intermediates. Fusarium solani was the most
effective in decomposing the 14C-labelled compound. Radioactivity
decreased by 24% during the first 5 days and by 82% during 12 days
in a growing culture after inoculation. A mixture of two or three of
the microorganisms was more effective in decomposing carbaryl and
1-naphthol (Bollag & Liu, 1971).
Bollag & Liu (1972a) studied the biological degradation of
14C-1-naphthol during growth, with replacement cultures and cell
extracts of Fusarium solani. The radioactivity of 1-naphthol
disappeared partially during growth, but it was completely
dissipated by cell extract activity. The cell extract of F. solani
degraded more than 80% of the 1-naphthol to form CO2 within 60 min
of incubation, but it was not possible to identify intermediates.
This implies rupture of the naphthol ring. No difference in activity
could be observed between cell-free extracts from the spores or the
mycelium of the fungus at pH 5.7 and 7.2. The enzyme system was
relatively stable since there was no decrease in activity after the
cell extract was stored at -10 °C during 4 months. The enzyme that
participated in the degradation reaction was constitutive (always
present) as shown by cell extracts prepared from cells grown in a
medium with, or without, carbaryl as substrate.
The fungus Gliocladium roseum, isolated from soil by Liu &
Bollag (1971a), metabolized carbaryl to 3 metabolites which were
identified as 1-naphthyl- N-hydroxy methylcarbamate,
4-hydroxy-1-naphthyl methylcarbamate, and 5-hydroxy-1-naphthyl
methyl-carbamate. It was therefore considered that N-alkyl- and
aromatic ring-hydroxylation of carbaryl were important detoxication
reactions of G. roseum. About 70% of the radioactivity was
recovered as carbaryl from an 11-day-old culture. The decrease in
radioactivity from the growth medium containing side-chain labelled
carbaryl indicated that a further degradation of the formed
metabolites occurred, or that an additional pathway was involved in
the degradation of carbaryl by G. roseum.
Bollag & Liu (1972b) reported that most soil fungi
( Penicillium sp., Mucor sp., Rhizopus sp., and Aspergillus
sp. except A. fumigatus), could hydroxylate carbaryl at different
positions, but that the products differed qualitatively as well as
quantitatively with the various fungi.
In a different study, the enzyme or protein fraction
(extracellular phenol oxidase) of the culture filtrate from
Rhizoctonia practicola as chromatographed on a column of Sephadex
G-200 and fractions were obtained that were able to transform
1-naphthol as a substrate (Sjoblad et al., 1976). The enzyme
catalysed the polymerization of 1-naphthol to several products.
Biological oxidation and coupling of phenols are key reactions
in nature, that result in the formation of products such as lignins,
melanins, tannins, alkaloids, and humus compounds. Thus, it can be
assumed that enzymes interact with xenobiotic phenols that are
incorporated into soil or sediment organic matter. Rhizoctonia
practicola (and an extracellular enzyme) was able to polymerize
1-naphthol (dimers, trimer, tetramer) (Bollag et al., 1978).
Rodriguez & Dorough (1977) studied the persistence of carbaryl
in culture media in the presence of mixed and pure cultures of
bacteria and fungi isolated from soil. All fungi ( Fusarium,
Penicillium, Aspergillus, and one unidentified species), isolated
from a soil treated with carbaryl six months before sampling,
produced at least one metabolite from carbaryl. After 14 days of
incubation, 54-79% of the carbaryl was recovered intact, and there
was little formation of carbon dioxide by the fungi. In contrast,
related experiments with bacterial isolates ( Arthrobacter,
Nocardia, Pseudomonas, Xanthomonas, and Bacillus) from the same
soil showed that only 1-9% of the added carbaryl remained
unconverted ( Arthrobacter was the exception with 59.1% remaining
carbaryl). Controls showed that non-biological degradation also
occurred. Bacteria, like fungi, metabolized carbaryl qualitatively
in the manner observed with natural soil populations. However,
quantitative differences were so great that the use of isolates may
be of little value in estimating the degradation rate of carbaryl
and other pesticides in field soil. This is important to note when
conducting microbial degradation studies in the laboratory.
Bollag (1979) summarized the transformations of carbaryl by
microbial activity. It was possible to isolate several
microorganisms capable of hydrolising carbaryl to 1-naphthol. In
addition, Gliocladium roseum showed the formation of:
4-hydroxy-1-naphthyl N-methylcarbamate
5-hydroxy-1-naphthyl N-methylcarbamate
1-naphthyl N-hydroxy-methylcarbamate.
Other terrestrial fungi hydroxylated carbaryl at different
positions, but the products differed quantitatively and
qualitatively with the various fungi. Subsequent metabolic
transformation of the hydroxylated products differed considerably.
With A. terreus, the following products were obtained:
1-naphthyl N-hydroxy-methylcarbamate -->
1-naphthyl-carbamate --> 1-naphthol
Several bacteria isolated from river water were capable of
degrading 1-naphthol. At least two different pathways were involved.
The first was a complete degradation with the release of CO2, the
second produced principally 4-hydroxy-1-tetralone, which may involve
hydroxylation of the naphthyl ring in the 4 position and conversion
to an aliphatic cyclic compound. Such a pathway has also been
described with a soil Pseudomonas by Walker et al. (1975a) and
Davis & Evans (1964).
4.3.5 Persistence in soil
Carbaryl is not usually applied as a soil treatment, therefore,
the amounts of carbaryl that may reach the soil come principally
from spray drift, or from washing off treated crops by rain.
When applied at the usual doses, in the laboratory, carbaryl
has a short persistence (half-life <40 days and usually from 6-20
days), but this may increase when the soil is flooded, or when the
dose is increased. In field studies, the half-life of carbaryl,
applied at the usual dose rates, in warm or temperate climatic
conditions, did not exceed one month in the soil. The results of
laboratory and field studies on the persistence of carbaryl in soil
are summarized in Table 7.
Table 7. Half-life of carbaryl in soil, calculated from experimental laboratory
and field results
Origin of soil Number Degradation Half-life References
of days (%) (days)
Laboratory 40 100 8 Johnson & Stansbury (1965)
Laboratory 0.5 mg/kg 45 77 20 Molozhanova & Kanevskij (1971)
Laboratory 1 mg/kg 45 53 40
Laboratory 10 mg/kg 45 13
Laboratory 30 55-63 < 20 Flores-Ruegg et al. (1980)
Laboratory (flooded) 15 63-27 10-40 Venkatesvarlu et al. (1980)
Laboratory 53 100 < 8 Odeyemi (1982)
Orchard 184 99 30 Ivanova & Molozhanova (1973)
Corn 135 95 30 Caro et al. (1974)
Pretreated soil 4 70 < 3 Rodriguez & Dorough (1977)
Grain cropa 30 53-31 27-55 Gangwar et al. (1978a)
Corn soilb 5 42-56 5 Kavadia et al. (1978)
Potatoes 50 < 99 < 7 Kovaleva & Talamov (1978b)
- 30 96 6
Table 7 (continued)
Origin of soil Number Degradation Half-life References
of days (%) (days)
Unspecified 90 Czaplicki (1979)
Tropical 7 Rajukkannu et al. (1985)
Sesamum 60 56 50 Yadav et al. (1985)
Bare soil < 4 Davis (1986a)
Bare soil 1-13
Forest 1.5 Sundaram & Szeto (1987)
Forest 90 < 78 < 38 Springborn (1988b,c)
Forest 90 < 90 < 24
Flooded rice 180 (56) Springborn (1988a)
Flooded rice 180 (52)
Broccoli 6-12 Norris (1991)
Sweet corn 5
a20-60 kg/ha.
b15-45 kg/ha.
Johnson & Stansbury (1965) calculated a half-life of
approxi-mately 8 days in an agricultural soil (sandy loam, pH 5.5)
treated at 3 concentrations. Residues of carbaryl appeared to be
completely degraded within 40 days.
The considerable influence of the dose rate on the persistence
of carbaryl in soil was demonstrated by Molozhanova & Kanevskij
(1971). The percentages of the initial quantities that were degraded
45 days after treatment are shown in Table 8.
Table 8. Degradation rate of carbaryl in relation to the dose
Initial dose Degradation (%)
(mg/kg)
0.5 77
1 53
1.5 37.3
2 30
2.5 27
3 23
4 20
6 16.7
8 15
10 13.2
The same authors also demonstrated the influence of the type of
soil on the degradation of carbaryl. Forty-five days after
treatment, about 50% degradation was observed in light grey forestry
soil, and only about 20% in peat. The ability of soils to degrade
carbaryl could be ranked as follows: light grey > grey forestry >
turf podzol > southern chernozeme > ordinary chernozeme > meadow
> peat.
Ivanova & Molozhanova (1973) calculated that application of
1.36 kg carbaryl/ha in an orchard resulted in 19.7% being retained
in the soil; 99% of the carbaryl present in soil disappeared within
184 days.
Czaplicki (1979) conducted studies in Poland. The soil
half-life of carbaryl was about 3 months and 90% degradation
occurred within 18 months. From October to March, when the soil
temperature was <5-8 °C, the disappearance of the insecticides in
the soil almost stopped.
Rajukkannu et al. (1985) studied the persistence of 4
products in the red and black soils of Tamil Nadu (India), where the
tropical climate in conjunction with the soil properties shortened
the persistence of insecticides. The half-life of carbaryl was 6.7-7
days and 95% degradation was achieved after 90 days.
It was reported by Odeyemi (1982) that carbaryl disappeared
from soil samples treated with 45 mg/kg after 53 days incubation in
a greenhouse under tropical conditions (Nigeria).
4.3.6 Interaction with other physical, chemical, or biological
factors
Nitrogen fertilizers (ammonium sulfate and urea) increased the
persistence of carbaryl in flooded laterite soil with a low native
content of nitrogen (0.04%). In the alluvial soil with 0.11% total
nitrogen, the persistence of the insecticides was little affected.
The rates of degradation in the two soils treated with nitrogen
fertilizers were almost identical. The authors speculated about the
mechanism of this effect, which was possibly due to the preferential
utilization of inorganic nitrogen by microorganisms, or to the
inhibition of soil hydrolase activity (Rajagopal & Sethunathan,
1984).
4.3.7 Vegetation
4.3.7.1 Uptake and transformation in plants
Carbaryl from soil treated at about 50 times the usual dose
penetrated into apple trees and couch-grass (Molozhanova, 1968).
Three months after application of carbaryl (85%) to soil at a dose
of 100 mg/kg, residues of carbaryl were detected in the roots and
stems in apples (39-13 mg/kg) and in couch-grass (50-38 mg/kg).
Under the same conditions, no residues were detected in tomato
fruits or in wheat grain, but 5.9 mg/kg were detected in potatoes
(Table 9).
Table 9. Migration of carbaryl from the soil into apple trees at
different times following applicationa
Experimental Sampling day Carbaryl content of samplesb
conditions after (mg/kg)
application
Soil Foliage Fruits
85% WP carbaryl 5 9.3 0 -
applied under each
tree at a rate of 12 6.8 0 -
10 mg a.i./kg soil
19 6.0 5.0 -
56 4.4 0.04 4.4
138 2.1 0 -
aFrom: Molozhanova (1968).
bAverage data from 18 samples are given for each case.
Huque (1972) reported that 14C-labelled carbaryl was readily
taken up from granules by 2-week-old rice plants. After 3 days, a
level of about 100 mg/kg was determined in the plant (excluding the
roots). Residues then decreased to about 15 mg/kg after 18 days.
Ferreira & Seiber (1981) studied the uptake and distribution of
carbaryl in rice seedlings following exposure of the roots to the
insecticide. Carbaryl was rapidly absorbed and transported upwards
to the leaves and stems. After exposure was terminated, the major
route of loss of carbaryl was through root exudation (22%) rather
than volatilization from the leaves (4.2%). However, since most of
the remaining carbaryl in the plant was present on the leaf
surfaces, the authors surmised that volatilization might play a
greater role in pesticide loss over longer periods of time.
14C-carbaryl and 14C-1-naphthol from soil-bound residues
were partially released when barley was grown (Murthy & Raghu,
1988). 14C-residues could be detected in both shoots and roots in
the case of carbaryl treatment, while only roots showed
14C-residues in the case of 1-naphthol.
The rate of decomposition of carbaryl in plants depends on the
climatic conditions. It is more rapidly decomposed in hot climates,
at high temperatures, and by intensive ultraviolet radiation. Thus,
residual levels in feed plants were lower in regions with a hot
climate than in other regions (Atabaev, 1972).
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Environmental levels
5.1.1 Air
Ambient air concentrations of carbaryl were measured before and
after a treatment of a large area of forest in Maine, USA, to
control spruce budworm. Concentrations in air ranged from less than
0.0035 to 0.107 µg/m3 several miles away (Shehata et al., 1984).
Room concentrations of airborne carbaryl were measured
following application for pest control as a 5% dust at an
undetermined rate. The levels detected on the day of application,
and 1, 2, or 3 days after application were 1.3, 0.2, 0.1, and 0.01
µg/m3, respectively (Wright et al., 1981).
5.1.2 Water
Carbaryl concentrations in water from different sources in the
USSR were studied by Molozhanova (1970). Data are presented in Table
10.
Table 10. Concentrations of carbaryl in watera
Type of sample Number of samples Mean
Total Carbaryl-containing concentrations
samples (%) (mg/litre)
Well 114 87 0.13
Artesian water 64 70 0.16
Ground water 24 75 0.27
Dam 90 47 0.02
River 77 62 0.044
Lake 84 85 0.03
aFrom: Molozhanova (1970).
According to an earlier report by Molozhanova (1968), carbaryl
was present in trace amounts up to a maximum level of 1 mg/litre in
well water and reservoirs during carbaryl application (6 kg/ha, 85%
WP).
Water irrigation channels, situated at a distance of 200-300 m
from a carbaryl-treated cotton field, contained from 0.01 to
0.25 mg/litre up to 18 days after spraying at a dose of 2kg/ha
(Guseynov, 1970).
Residues of pesticides were monitored in the aquatic system of
Ioannina basin (Greece) and its natural outlet, Kalamas River, for
the period September 1984-October 1985 (Albanis et al., 1986). The
carbaryl concentration in water was found to follow a seasonal
fluctuation with maxima during summer and minima during winter
months. Mean and range values are summarized in Table 11.
Table 11. Mean and range values of carbaryl (ng/litre) on different
sampling dates in the Ioannina basin
Month of sampling River Canal Lake
September 0.5
November NDa NDa
March NDa
May ND
June NDa
July 23.7 21.7
August 2.3
September 1.7 NDa
April NDa
July 8.8
aND = not detected.
An oil-based, carbaryl formulation (Sevin-2-oil) was applied
twice to a coniferous forest in New Brunswick (Canada). At a dose
rate of 280 g/ha, the highest concentration in stream water was
0.314 mg/litre and only 0.123 mg/litre 0.5 h after spraying. More
than 50% of the initial residues dissipated within 1 h (Sundaram &
Szeto, 1987).
Carbaryl was applied aerially as Sevin-4-oil (1.12 kg/ha) to
aforest in Montana. Field samples of water were collected to study
persistence. Residue levels in 4 streams sampled were variable
(3-6, 3-175, 2-260, 4-108 µg/litre) 1.3-2.8 h after application
(Pieper & Roberts, 1978; Pieper, 1979). Local accumulations of
floating oil-based spray may have caused this variation. Grantham
(1980) reported that carbaryl (Sevin-4-oil) levels were generally
highest directly after spraying, but increased at some stream sites
for 1-2 days after application. This was attributed to light rains,
which washed the spray residue off the foliage and into the stream
channel. However, Brown (1980) concluded that carbaryl enters
streams in periodic doses and that rainfall dates are not related to
the presence of residues in streams. Maximum residues of
26.32 µg/litre were found in water 12 h after spraying, with no
residues detectable in samples taken hourly until 36 h. Residues
were found again in samples taken 2.5-3 days and 5.5-6.5 days after
spraying.
In a study where carbaryl (Sevin-4-oil) was applied aerially
(0.84 kg/ha) to a forest in Maine, in 1978, samples were collected
in 9 streams (Stanley & Trial, 1980). Peak concentrations occurred
shortly after spraying and maximum residue levels ranged from 0.93
to 7.8 µg/litre in brooks and from 0.44 to 2 µg/litre in rivers. In
one stream, the maximum concentration was 16 µg/litre. Ott et al.
(1981) reported that carbaryl levels were highest on the day of
spraying (2.7 µg/litre), declined to about 0.7 µg/litre by the third
day, but increased again to about 1.2 µg/litre on day 5. No residues
were detected in stream water samples taken on the 17th day.
Thomas et al. (1982) treated planted rice fields with
carbaryl at rates of 0.63, 0.94, and 1.85 kg a.i./ha, either as a
high volume spray (500 litre/ha) or as a low volume spray (150
litre/ha), and monitored the residues. In irrigation water sampled
one hour after treatment, carbaryl levels amounted to 0.15-0.30 and
0.07-0.18 mg/litre, under high and low volume spraying,
respectively. During the first 24 h after spraying, the
concentration in the irrigation water remained fairly constant, but,
by the fourth day, it had decreased significantly. By the tenth day
after spraying the residues in water had decreased to 0.02-0.05 and
0.03-0.04 mg/litre, respectively.
A rice field dissipation study was conducted in Arkansas (USA)
by Springborn (1988). Carbaryl (Sevin XLR) was applied 3 times at
14-day intervals to an irrigated field plot in which rice was
planted. In irrigation water, carbaryl residues disappeared with a
half-life of <1 day after each treatment. The maximum concentration
in water was 1466 µg/litre and, by day 3 following each treatment,
the concentration was <121 µg/litre. In a similar study conducted
in California, Springborn (1988a) found that carbaryl dissipated
rapidly in irrigation water with a half-life of <1 day. The maximum
concentration in water was 648 µg/litre. By day 3 following each
treatment, the concentration was <27 µg/litre.
Residues of 1-naphthol were found in the water collected from
wells and ponds in and around Bhopal (India). The residues of
1-naphthol in well water (near a manufacturing plant) ranged from
0.002 to 0.024 mg/litre. In pond waters, the levels were found to be
between 0.036-0.098 mg/litre. The level of 1-naphthol in soil
samples was of the order of 0.153-0.656 mg/kg (Dikshith et al.,
1990).
5.1.3 Soil
Contamination of the soil occurs when carbaryl is used in
agriculture. During the treatment period, levels of 1-3 mg
carbaryl/kg were measured in the surface layer of the soil in the
USSR (Molozhanova, 1968). Concentrations of 0.03-0.35 mg/kg were
found in the surface layer of the soil up to 52 days after a single
application of carbaryl at a rate of 2 kg/ha (Guseynov, 1970).
Carbaryl levels in the soil varied depending on the type of soil
(Table 12).
Potatoes were sprayed with carbaryl at 4.25 kg/ha (5 kg Sevin
WP, 85%/ha). One day, 10 days, and 2 months after treatment,
residues in the soil under the potatoes were, respectively, 1.9,
9.55, and 0.02 mg/kg (Kovaleva & Talamov, 1978b). When the soil was
sprayed before sowing, the residues in the soil 1 day, 1 month, 4
months, and 15 months after treatment were 1.5-2, 0.05-0.07,
0.02-0.08, and 0.06 mg/kg, respectively. Within 100 and 150 days
following soil incorporation of carbaryl (Sevin, 10 kg/ha), residues
in non-planted soil decreased to 0.05 and 0.02 mg/kg, respectively
(Kovaleva & Talanov, 1980).
Table 12. Carbaryl content in different types of soil during
harvestinga
Type of soil Number of Carbaryl concentration
samples (mg/kg)
Meadow-chernozem 200 2.22 ± 0.5
Chernozem southern 766 1.01 ± 0.23
Chernozem ordinary 288 1.09 ± 0.26
Chenozem podzol 100 0.15 ± 0.04
Turf-podzol 150 0.04 ± 0.01
Grey-woodland 246 0.0
aFrom: Molozhanova (1970).
Yadav et al. (1985) detected 0.22 mg carbaryl/kg in the soil
at harvest time, when sesamum ( Sesamum indicum) was sprayed twice
with carbaryl (0.2%) on days 45 and 60 of crop growth (21 August and
5 September). As residues in the soil after the first spray were
about 0.65 mg/kg, the rate of reduction of residues in soil was 56%
within 2 months. Shilova et al. (1973) measured about 0.1 mg
carbaryl/kg soil one month after treatment with carbaryl (5 kg/ha)
against blood sucking insects in the subarctic.
Gangwar et al. (1978b) applied a 4% granular formulation to
sandy loam planted with a grain crop (bajra) at very exaggerated
application rates of 20, 40, and 60 kg a.i./ha. Initial residues of
140-354 mg/kg decreased by a total of 31 to 53% within 30 days and
95 to 98% within 90 days. Residues at 90 days were 2.68-17.49 mg/kg.
Application of carbaryl granules at very high doses of 15, 30,
and 45 kg/ha resulted in deposits of 232, 397, and 525 mg/kg,
respectively, in soils cultivated with corn, and in 104-109,
168-217, and 304-422 mg/kg, respectively, in clay loam soils
cultivated with root crops (beet, radish, carrot). The dissipation
rate of carbaryl residues from clay loam soil, in 5 days following
the treatment, was 42-56% during autumn and 55-69% during spring.
The highest residue levels sampled from all plots decreased to
229.7, 142.9, 73.8, 33.8, 6.7, and 1.3 mg/kg on days 5, 10, 15, 30,
60, and 100, respectively. Residues were below detectable levels in
several plots by day 60 and in most plots by day 100 (Kavadia
et al., 1978).
According to Kuhr et al. (1974), the dissipation of carbaryl
in the soil of apple orchards is rapid. Soil residues of carbaryl
had almost completely disappeared from the top 2 inches of soil in 2
weeks. They were 13.8 mg/kg immediately after application and
3 mg/kg, 1-2 days later.
An oil-based carbaryl formulation (Sevin-2-oil) was applied
twice by a fixed-wing aircraft to a coniferous forest in New
Brunswick (Canada). Initial residue levels 1 h post-spray in litter
and soil for both applications were, respectively, 1.21 and
0.86 mg/kg and 0.78 and 0.48 mg/kg (Sundaram & Szeto, 1987).
Relative to the amount sprayed, only a small amount of the chemical
reached the litter and soil, probably because of canopy filtration.
Within 1 day, an average of 40-45% of the initial residues was lost
from litter and soil, respectively, indicating a rapid dissipation
time of 1.5 days for the disappearance of 50% of the initial maximum
concentration. Beyond 5 days, an average of 12% of the initial
concentration remained in both the substrates.
As already reported, two field dissipation studies were
conducted by Springborn (1988b,c) with carbaryl (Sevin-4-oil)
applied twice with a seven-day interval to forest. The first study
was conducted in a coniferous Oregon forest. In soil from the
treated site, carbaryl levels decreased from 0.196-3.877 mg/kg
following the first application to 0.130-1.87 mg/kg, 3 days after
treatment, and from 0.079-5.323 mg/kg following the second
application to 0.242-1.187 mg/kg at 90 days. The second study was
conducted in Pennsylvania. Carbaryl levels in soil decreased from
0.022-0.068 mg/kg following the first application to
<0.012-0.075 mg/kg, 3 days after treatment, and from
0.11-0.932 mg/kg following the second application to
0.01-0.099 mg/kg at 90 days.
A rice field dissipation study was conducted in Arkansas (USA)
by Springborn (1988a). Carbaryl (Sevin XLR) was applied 3 times, at
14-day intervals, to an irrigated field plot in which rice was
planted. In flooded soil, the concentration of carbaryl varied from
<11 to 309 µg/kg throughout the study and was not directly related
to application date. The carbaryl level was <11-309 µg/kg in soil
following the first application and <11-129 µg/kg following the
third application; 180 days later, carbaryl concentrations in the
soil were 11-56 µg/kg.
In a similar study conducted in California, under the same
conditions, Springborn (1988) found that carbaryl dissipated rapidly
in flooded soil. The concentration of carbaryl ranged from <11 to
198 µg/kg throughout the study. Carbaryl was <11-23 µg/kg in soil
at sampling intervals following the first application, and increased
to <11-180 µg/kg following the third application; 180 days after
the third application, carbaryl concentrations in the soil were
11-88 µg/kg.
A soil dissipation field study was conducted in 1985/1986 in
California (sandy loam, pH 6.3) and Iowa (silt loam, pH 6.5) with
carbaryl 80 S formulation (Davis, 1986a). Carbaryl was applied to
strip plots of bare soil, 6 weeks later, 7 applications were made
with intervals of about 5 days between applications. Soil sampling
was performed before treatment, during treatment, and up to 6 months
after the last application. No carbaryl residues were found in
California pretreatment samples. After the last application,
residues reached a maximum of 1.61 and 0.29 mg/kg in the 0-15 and
15-30 cm samples, respectively. Fourteen days after the last
application, carbaryl residues below the 15-cm level were less than
the limit of quantification.
It took 28 days for residues to dissipate completely from the
top 15 cm. The half-life in California soil was calculated to be
2.7-3.8 days.
5.1.4 Food and animal feed
5.1.4.1 Fruit, vegetables, and grain
Contamination of vegetation by carbaryl occurs, either during
spraying, or, by its migration through contaminated soil into the
roots of plants.
Residual levels of carbaryl after the spraying of plants depend
on the type and species of the plants sprayed. Carbaryl levels of up
to 30 mg/kg have been found in plants during the treatment period
(Molozhanova, 1968). It has been found to be rather persistent in
vegetables and fruits. Concentrations of 0.6-3.9 mg/kg were measured
in lettuces 1 week after single or repeated sprayings, and levels up
to 1 mg/kg were found in tomatoes treated according to requirements
(Antonovich, 1970). In cabbage, initial residues after spraying
ranged from 14.8 to 33.9 mg/kg, depending on the concen-tration
used. After 7 days, the residues decreased to 2.5-5.13 mg/kg. In
eggplant, the initial concentrations were 8.3-16.9, and 7 days
later, 3.05-5.4 mg/kg. After washing, the concentrations decreased
considerably to less than 3 mg/kg after day 7 (Mann & Chopra, 1969).
Following application of carbaryl on cauliflower, in the form
of dust (10%) at 1.5-2 kg a.i./ha or wettable powder (50%) at 0.75-1
kg a.i./ha, the residues of carbaryl declined to 3.64-9.59 mg/kg
within 8 days of treatment. The carbaryl deposits on leaves were
between 19.45 and 42.08 mg/kg. Washing of cauliflower with plenty of
water reduced the residues of carbaryl by 36-95%. A waiting period
of 8 days has been suggested for cauliflower (Singh et al., 1978).
Persistence of carbaryl in brinjals and peas after spraying at
1 and 2 kg a.i./ha was found to be 3.42 and 5.03 mg/kg respectively.
Washing of vegetables with water within one hour and after a day of
spraying reduced the level of deposits of carbaryl to about
1.0 mg/kg level. In the case of pea pods, the levels of residues
dropped to 0.20 mg/kg within 5 days. In pea seeds, the residue
levels ranged between 0.03 and 0.09 mg/kg, after 8 days (Krishnaih
et al., 1978).
Measurements of carbaryl in different fruits were taken at
intervals of 5-10 days after plants had been treated with a 0.2%
suspension of carbaryl at 1000 litre/ha during the vegetation period
(Bogomolova, 1968, 1970). The initial amount of carbaryl, 2 days
after the treatment, varied in different fruits from 2.3 to
2.7 mg/kg. Ten days later, the concentrations were reduced by
50-70%, and, after 20 days, the levels were within the range of
0.2-0.8 mg/kg, depending on the species (Table 13). According to
Mann & Chopra (1969), the dissipation of carbaryl from plants after
the first day of spraying was about 40-45% of the initial residue.
In apples, concentrations of 2.5-2.9 mg/kg, 0.4-2.4 mg/kg, and
0.15 mg/kg were found on days 7, 10, and 38, respectively, after
spraying. One to two months after spraying apples with a 0.1%
suspension at a dose of 2000 litre/ha, residues of 0.08-0.10 mg/kg
were measured (Antonovich, 1970). Four months after spraying with a
0.06% suspension at a dose of 9 kg a.i./ha, levels in the range of
0.09-0.24 mg/kg were found in apples, depending on the spraying
procedure. Fine-droplet spraying resulted in residue levels 2.5-3
times lower levels than those with coarse-droplet sprays (Atabaev,
1972).
Table 13. Carbaryl levels in different fruits after spraying
during vegetation (mg/kg)a
Kind of fruit Day after spraying
2 10 20
Strawberries 2.3 1.25
Gooseberries 2.5 0.90 0.6
Blackcurrants 2.4 1.10 0.8
Cherries 2.7 1.90 0.2-0.4
Plums 2.4 1.40 0.2-0.4
aFrom: Bogomolova (1968).
A survey of apples grown in Ontario, Canada, between 1978 and
1986 showed that out of 22% of carbaryl-treated apples (1.67 kg/ha)
sold, 3.6% had detectable carbaryl residues (detection limit of
0.01 mg/kg), and that the average residue level was 0.03 mg/kg, with
a maximum of 0.04 mg/kg (Frank et al., 1989).
Prolonged storage of apples reduced the carbaryl residues only
slightly (Bogomolova, 1968). The initial level (2.4-2.8 mg/kg)
changed little during the first 3 months of storage at a temperature
of +5 to +10 °C. A slight reduction was observed during the fourth
month of storage. During the process of storage, carbaryl migrated
inwards from the surface, so that the concentration in the skin was
reduced, while that in the pulp was increased (Table 14).
Residues on lemon foliage on day 0 of treatment were
4.6±0.3 µg/cm2. Five days after treatment, levels of 2.4 µg/cm2
and 5.6 µg/cm2 were found on lemon and orange foliage,
respectively; on the 60th day, the residual values were 0.41 and
0.36 µg/cm2, respectively. Persistence half-lives were 22 days
(lemons) and 14 days (oranges) (Iwata et al., 1979).
Carbaryl is persistent, particularly in citrus fruits and
grapes. Residues of 3-6 mg/kg were measured in lemons and oranges
2.5-3 months after treatment of trees with 3-10 kg carbaryl/ha, and
amounts of 3.6, 1.9, and 0.4 mg/kg were found in grapes on days 7,
14, and 40, respectively, after repeated spraying of vineyards with
a 0.12% suspension. The half-life of carbaryl in grapes was 29 days,
compared with 2-9 days in cherries and cabbage (Antonovich, 1970).
Washing and peeling reduced carbaryl levels in fruits and
vegetables by 40%; thermal processing by 45-90%, and preparation of
juices by 53% (Molozhanova, 1970). Canning also reduced carbaryl
residues. Thus, canning of different fruits (strawberries, black
currants, gooseberries) containing carbaryl in the range of
0.25-0.45 mg/kg, resulted in a reduction to 0.17-0.29 mg/kg; when
the initial contents were higher (3-7 mg/kg) the levels after
canning dropped to 1.4-2.2 mg/kg and 2.1-2.5 mg/kg, respectively,
i.e., from 30 to 70%. Storage of canned fruit further reduced
carbaryl residues by 2-3 times after 12 months (Bogomolova, 1968).
Boiling vegetables (e.g., cabbage) reduced carbaryl residues by
approximately 50%; however, the residues in pickled cabbage, 5
months after preparation, were only 25% less than the initial amount
(Antonovich, 1970).
Table 14. Carbaryl content in apples during storage (mg/kg)a
Part of apple Day of storage
10 20 30 40 50 70 80 100 110
Skin 2.40 1.80 2.00 1.80 1.92 1.72 1.60 1.45 1.22
Pulp 0.35 1.00 1.02 1.10 1.00 1.10 1.34 1.42 1.30
Total 2.75 2.80 3.02 2.90 2.92 2.82 2.94 2.87 2.52
aFrom: Bogomolova (1968).
Elkins (1989) reported that washing during the commercial
processing of produce removed 97, 87, and 77% of the carbaryl
residues from tomatoes, spinach, and broccoli, respectively.
Blanching (short treatment with hot water) was stated to result in
68% removal of carbaryl from green beans, while blanching, in
addition to washing, resulted in the removal of over 97% of carbaryl
from both spinach and broccoli.
After a single application of carbaryl (Sevin 50 at 2.5 kg/ha),
residues in cauliflower decreased from 16.75 mg/kg on the day of
application to 0.87 mg/kg, 15 days afterwards (Yadav & Jaglan,
1982). Washing further reduced the detectable residues at 15 days to
0.67 mg/kg, while no detectable residues were present after boiling.
Carbaryl was found in vegetation adjacent to treated fields.
Grass growing at a distance of 250 m from a carbaryl-treated cotton
field contained 0.18-0.25 mg carbaryl/kg wet weight up to 45 days
after spraying (Gusseynov, 1970).
The results of the 1989 Pesticide Residue Monitoring Programme
in California showed that carbaryl was detected in 2 samples of
grape from 26 in quantities less than the accepted tolerance level.
It was not found in oranges (Okumura et al., 1991). Summaries of
residue data on different plants are given in Tables 15 and 16.
Carbaryl residues were not detected in 44 samples of wheat in the
United Kingdom analysed by the multiresidue method (Osborne et al.,
1989).
Table 15. Comparison of carbaryl residues with different
formulationsa
Formulation use Plant Mean residues Day after last
(kg a.i./ha) value (mg/kg) application
44% SC 2x0.5 apple 0.25 7
pears 0.16 7
50% WP 2x0.5 apple 0.15 7
pears 0.09 7
44% SC 2x2 spinach 4.44 -
50% WP 2x2 2.38 14
44% SC 2x2 lettuce leaf 2.72 14
50% WP 2x2 1.55
44% SC 2x2 barley 7.87 14
50% WP 2x2 4.63
Table 15 (continued)
44% SC 2x2 wheat 0.96 14
50% WP 2x2 0.99
44% SC 2x2 oats 0.22 14
50% WP 2x2 0.26
aFrom: Davis (1987).
Davis (1987) studied the impact of different types of
formulation on food residues, including carbaryl (44% w/w) oil-based
liquid formulation containing a "sticker", in order to prevent bees
from carrying carbaryl particles back to the beehive. The 50 W
formulation was a "standard" wettable powder formulation. The
results are given in Table 15. This work showed that the addition of
a "sticker" increased levels of food residues to some extent.
Studies on the removal of carbaryl residues from tomatoes,
green beans, spinach, and broccoli by commercial and home
preparation procedures (Elkins et al., 1968; Farrow et al.,
1968, 1969; Lamb et al., 1968) showed a considerable decrease in
the residues with treatment (Table 17).
For pre-harvest use on grain, the rate of application of
carbaryl ranges from 2 to 9.5 kg/ha, depending on the degree of
infestation, density of foliage, and the stage of the life cycle of
the pest. Carbaryl, usually applied at a rate of 5 mg/kg, is also
used to protect stored grain. Studies on stored wheat, barley, oats,
and rice indicated that carbaryl residues on grain have a half-life
of between 26 and 80 weeks, depending on the temperature (FAO/WHO,
1976). Residues of carbaryl in baked bread are then of the order of
1-1.5 mg/kg.
5.1.4.2 Animal products
Technical carbaryl was fed to dairy cows of the Brown Swiss,
Jersey, Holstein, and Ayrshire breeds at 50, 150, and 450 mg/kg of
their average total daily roughage intake (dry weight) for a period
of 2 weeks. Samples of milk were taken at regular intervals and the
cream was analysed for carbaryl by means of the
p-nitrobenzene-diazonium fluoborate coupling method. The
concentration of carbaryl, if present, was below the sensitivity of
the analytical method (0.01 mg/kg) (Gyrisco et al., 1960).
Table 16. Carbaryl residues following different applications
Formulation use Plant Mean value Day after Reference
(kg a.i./ha) residues last
(mg/kg) application
44% SC barley 5.4 14 Davis & Thomas (1987)
44% SC sugar beet 0.07-1.04 14 Thomas (1986)
roots
44% SC pasture 59.7-183 3 Davis (1986b)
aerial ground grass
Carbaryl sweet 0.33 Frank et al. (1987a)
cherries
Carbaryl tomato 1.2 0 Frank et al. (1991)
0.5 3
0.03 6-8
tomato 0.47 0
juice 0.24 3
0.08 6-8
Table 17. Removal of carbaryl after preparation procedures
Vegetable Initial Percentage removal References
residues
(mg/kg) Commercial procedure Home procedure
Tomatoes 5.2 washing 82-99 washing 77 Farrow et al. (1968)
canning 98-99 canning 92
juicing 98 juicing 77
Green beans 7.6 blanching 68-73 washing 52 Elkins et al. (1968)
& canning blanching 81
freezing 94
canning 100
Spinach 20.8 washing 66-88 washing 70 Lamb et al. (1968)
blanching 96-97
Broccoli 12.4 washing 77 washing & cooking 55 Farrow et al. (1968)
blanching 82-97 washing, 90
blanching,
& freezing
Residues resulting from a single application and repeated
applications of carbaryl spray on cattle were rapidly eliminated
from body tissues. On days 1 and 3 after application, carbaryl was
detected in the liver (0.05 mg/kg), muscles (0.04 mg/kg), and
perirenal fat (0.16 mg/kg). Seventy-two hours after treatment, no
residues were found in the tissues studied. The excretion in milk
persisted for at least 69 h after spraying, the highest
concentration being 0.075 mg/litre, 5 h after exposure (Hurwood,
1967).
Data concerning the composition and levels of milk and tissue
residues in cows after continuous feeding with 100 mg
14C-carbaryl/kg diet are shown in Tables 18 and 19.
Table 18. Metabolites in tissues after continuous 28-day feeding
of 100 mg 14C- carbaryl/kg dieta
Metabolite (mg/kg) Kidney Liver Muscle
carbaryl 0.03 0.04 0.02
5,6-dihydrodihydroxycarbaryl 0.05 0.01 0.04
5,6-dihydrodihydroxynaphthol 0.02 0.02 0.0
naphthyl sulfate 0.29 0.02 0.0
water-soluble unknowns 0.43 0.13 0.03
unextractable unknowns 0.18 0.19 0.01
aFrom: Dorough (1971).
Table 19. Composition of milk residues after continuous 28-day
feeding with 100 mg 14C-carbaryl/kg dieta
Percentage mg/kg
Organic phase
5,6-dihydrodihydroxycarbaryl 38.5 0.11
carbaryl 8.4 0.02
3,4-dihydrodihydroxycarbaryl 4.0 0.01
Table 19 (continued)
Percentage mg/kg
5,6-dihydrodihydroxynaphthol 1.0 0.01
Water soluble
5-methoxy-6-hydroxycarbaryl 25.6 0.07
unknowns 10.6 0.03
1-naphthol 5.5 0.02
5-hydroxycarbaryl 3.1 0.01
carbaryl 1.2 0.01
aFrom: Dorough (1970a).
Effective horn fly control for a period of 15 days was obtained
by spreading carbaryl down a cow's back. Results indicated that, if
applications of a 0.5% spray or a 50% dust were made immediately
after the morning or evening milking, no residues of carbaryl would
result in subsequent milkings (Eheart et al., 1962). The results
obtained by Petrovski (1970) were similar. After treating cows with
a 0.85-1% suspension of carbaryl, residues of from 0.1 to
0.3 mg/litre were found in milk, 20 h after application. On the
third day, only trace amounts of carbaryl were found. After
treatment with a 0.5% suspension of carbaryl, no traces were found
in the milk.
5.1.4.3 Animal feed crops
Generally, animal feed crops contain less carbaryl than fruit
and vegetables. Mean levels of 0.82 mg/kg were found in 80 samples
of animal feed plants (Molozhanova, 1970). Levels of 0.3 mg/kg were
found in maize stems at harvest, 70 days after spraying (Antonovich,
1970).
Studies on the carbaryl content of animal feed plants in hot
climates showed that carbaryl can be detected 3-3.5 months after
treatment, depending on the dose and the number of applications.
Thus, after repeated applications of 50% carbaryl dust at a rate of
0.5 g/m2, the carbaryl content in animal feed plants was in the
range of 1-1.8 mg/kg wet weight, but, after a single application of
0.7 g/m2, concentrations of 0.10-0.31 mg/kg were found (Atabaev,
1972).
5.1.5 Other products
Measurement of carbaryl residues in tobacco plants showed that,
15 days after field spraying with 950 g/ha, the content of carbaryl
in green tobacco leaves was 10.1 mg/kg. Drying leaves by hot air
decreased carbaryl residues by only 10% (Antonovich, 1970).
Application of carbaryl at a rate of 2.2 kg/ha resulted in
carbaryl residues on cotton foliage of 7.2 µg/cm2 on the day of
spraying (Estesen et al., 1982). Levels decreased to 5.9, 0.58,
and 0.26 µg/cm2 after 3, 6, and 8 days, respectively.
Carbaryl was found in the foliage of cotton plants in
concentrations of 0.03-1 mg/kg from 10 days to 2.5 months after
treatment. Carbaryl penetrated the plant through foliage and roots
and contaminated the cotton and the seeds. Cotton harvested from
treated fields 2 weeks to 1 month after treatment contained
0.015 mg/kg. Trace amounts of carbaryl were found in cotton oil
(Guseynov, 1970).
After treatment with a 2% suspension of carbaryl against
mountain pine beetle, residues in bark disks after 1 year were
359 mg/kg. In another case, residues in bark disks were
890±146 mg/kg, after spraying, and 531±178 mg/kg 16 months later
(Page et al., 1985).
5.1.6 Terrestrial organisms
The quantities of carbaryl that were absorbed by songbirds,
either by contact or by eating food from a sprayed area, were
studied by Kurtz & Studholme (1974). Towhees (American finches) were
collected 3 days after the forest had been sprayed for gypsy moths
at a rate of 1.1 kg/ha. The amounts of carbaryl found in the towhees
were small, even though the samples were taken close to the spraying
time. Trace amounts of carbaryl were found in three samples out of
five, compared with two samples out of five control birds. The
minimum level of detection was 0.1 µg/g. The low levels of carbaryl
found in "sprayed" birds were explained by the fact that towheeds
are groundfeeders and that only a small amount of carbaryl might
have passed through the trees to the ground.
5.2 General population exposure
5.2.1 Exposure through the food
The daily intake of carbaryl was studied for several years in
the USA (see Table 20).
Table 20. Carbaryl daily intake in the USA
Year Positive samples Daily Intake References
(%) (mg/day
per person)
1964-65 7.4 0.15 Duggan et al. (1971);
1965-66 2.7 0.026 Duggan & Corneliussen
(1972)
1966-67 1.1 0.007
1968-69 0.8 0.003
1969-70 0 -
Infant Toddler
1978 - 0.088 0.05 Gartrell et al. (1986)
1979 - - 0.049
1980 - 0.06 0.035 Reed et al. (1987)
1981-82 - 0.129 0.127
1982 - - 0.012
1982-84 - 0.117 0.017- Gunderson (1988)
0.0315
Carbaryl residues in total diet samples in the USA are
relatively low (Table 21). Carbaryl is found in potatoes, leguminous
vegetables, root vegetables, and fruit. In foods processed in the
usual manner, i.e., by peeling, stripping outer leaves, and cooking,
when appropriate, carbaryl residues usually decreased to
undetectable levels or traces (Manske & Corneliussen, 1974).
Table 21. Carbaryl residues in food in the USA
Number of composites Number of Number of Range in References
analysed composites composites mg/kg
with residues with traces
270, from 30 grocery 20 15 trace to Manske & Corneliussen
stores in 27 cities 0.5 (1974)
June 1970-April 1971
420, from 35 market 6 5 0.02 Manske & Johnson
baskets, 6 June 1971- (1975)
July 1972
360, 12 August 1972- 12 10 0.05-1.10 Johnson & Manske (1976)
July 1973
360, August 1973- 8 2 0.001-0.284 Manske & Johnson (1977)
July 1974
1044, 1978-82 11 (1%) a a Yess et al. (1991a)
3744, 16 market 135 (4%) a a Yess et al. (1991b)
basket collections
1982-86
aNo figures given.
During a 5-year period, from 1982 to 1986, the Los Angeles
District Laboratory analysed 19 851 samples of domestic and imported
food and feed commodities for pesticide residues. A single, rapid,
multiresidue method was used. Carbaryl was detected in 164 samples
from the total 19 851 samples analysed. Not one sample exceeded the
US Federal tolerance levels (Luke et al., 1988). In another
publication, the same authors reported comparative studies on US and
imported food. Carbaryl was found in 21 samples from a total 6391 US
samples in quantities ranging from 0.1 to >2.0 mg/kg, but less than
10 mg/kg. From 12 044 imported agricultural commodities 132 were
contaminated by carbaryl, and levels in 14 of them exceeded the
tolerance levels (Hundley et al., 1988).
5.2.2 Exposure during insect control
Exposure to carbaryl, used to control the gypsy moth in camping
and picnic areas, was monitored on the day of application and then
weekly for 3 weeks. Carbaryl was applied from the air at a rate of
1.12 kg/ha. Exposure was measured using dermal pads.
Extrapolating from the most contaminated group of pads (they
contained an average of 279 µg/pad), the authors calculated a "worst
case" exposure of 0.54 mg/kg on the day of spraying. The authors
concluded that the risk to those who use public areas during, and
after, carbaryl application to trees is negligible (Cameron et al.,
1985).
A 5% spray of carbaryl 85% wettable powder (WP) was applied for
insect control in homes (Vandekar, 1965), as a surface application,
at the rate of 2 g/m2. Urine samples taken from villagers 1 week
after spraying showed a significant increase in 1-naphthol levels
from 30.5 µg/ml baseline to 50.3 µg/ml. Inhibition of plasma ChEA
was found in 48 out of 63 subjects, 1 week after spraying.
During a monitoring study carried out from 1976 to 1980 in the
USA, 1-naphthol was found in 1.4% of the urine samples from persons
between 12 and 74 years old. The source was thought to be carbaryl
and naphthalene (Carey & Kutz, 1985).
5.3 Occupational exposure during manufacture, formulation,
or use
Harry (1977) estimated that about 13 million people in the USA
were potentially exposed to carbaryl during its manufacture,
formulation, packing, transportation, storage, and during and after
application, and while working with treated crops or during harvest.
Best & Murray (1962) published a survey on the exposure of
plant workers during the production of carbaryl. Air concentrations
varied from 0.23 to 31 mg/m3. Urine samples contained an excess of
1 mg total 1-naphthol per 100 ml of urine. During air blast spraying
of orchards, Jegier (1964) found air concentrations of 0.6 mg/m3
(0.18-0.81 mg/m3). The mean respiratory exposure, measured by the
respirator pad technique was 0.29 mg/h (0.24-0.53 mg/h) and mean
dermal exposure, measured by skin pads, was 25.3 mg/h
(18.5-30.3 mg/h). The maximum total exposure was 31 mg/person per h,
or 0.025% of the toxic dose. Simpson (1965) estimated that the
amount of dermal exposure was less than 0.1% of the toxic dose in
orchard sprayers. During cotton spraying by aircraft, Yakim (1965)
found 4 mg carbaryl dust/m3 in the breathing zone of flagmen,
1 mg/m3 during preparation of the solution, and 0.7 mg/m3 in the
pilot's cabin. Adylov (1966) reported the following air
concentrations found during the aerial application of a water
solution: flagmen's breathing zone 1.92 (0.64-2.84) mg/m3; pilot
cabin, trace amounts, workers on the ground (preparation of
solution, etc.,) 6.28 (0.48-19.2) mg/m3. In the urine samples of
carbaryl formulators, 1-naphthol levels varied from 6.2 to
78.8 mg/litre. Agricultural workers who used carbaryl for pest
control excreted from 0.07 to 1.7 mg/litre in their urine (Shafik
et al., 1971).
Exposure studies were completed for pesticide application and
formulating plant workers by the biological monitoring of 1-naphthol
in the urine, dermal pads, and respirator filter pads (total 480
samples of dermal pads and 73 respirator pads). Workers operating
tractor-drawn airblast equipment applied carbaryl at 0.045-0.06% as
spray (Comer et al., 1975). An estimate of the amount of dermal
and respiratory exposure that could occur was made using the
procedures described by Durham & Wolfe (1962). The results are
presented in Table 22.
The concentration of 1-naphthol in the urine varied from 0.2 to
65 mg/litre with a mean of 9 mg/litre. The rate of excretion per
hour varied from 0.004 to 3.4 mg with a mean of 0.5 mg/h. The
maximum level during the working day was reached by late afternoon.
An approximate calculation of the excreted carbaryl (0.5 mg
1-naphthol/h = 0.7 mg/h excretion of carbaryl), and potential
exposure, 75 mg/h, shows great differences. About 1/100 part of
possible absorption occurs.
Exposure to carbaryl during agricultural application was
studied by Leavitt et al. (1982). WP 80 carbaryl was used (0.45 kg
in 200 litre water) to spray trees, lawns, and gardens. The mean
dermal exposure was 128 mg/h and the mean inhalation exposure was
0.1 mg/h. The maximum percentage of the toxic dose that the
applicators received was 0.12%/h. No symptoms or inhibition of ChE
were reported. In another group of applicators, the mean dermal
exposure was 59.4 mg/h and inhalation exposure 0.1 mg/h.
Table 22. Potential dermal and respiratory exposure of formulating
plant workers and field spraymen to carbaryla,b
Subject Route of Exposure Exposure (mg/h)
exposure situations
studied Range Mean
Formulating dermal 48 0.80-1209.30 73.90
plant workersc
respiratory 48 0.03-4.10 1.90
Field dermal 32 1.70-211.80 59.00
spraymend
respiratory 25 0.01-1.08 0.09
aFrom: Comer et al. (1975).
bCalculated on the basis of the worker wearing a short-sleeved,
open-necked shirt, no gloves or hat, with his clothing protecting
the areas covered.
cWorkers on mixing and bagging operations (4 and 5% dust).
dOperating power air-blast spray machines in fruit orchards
(0.045-0.06% solution spray).
Dermal exposure is influenced by the type of spray equipment.
The dermal deposit and respiratory intake of carbaryl in humans was
monitored during home garden spray operations using compressed air
or garden hose-end sprayers (Puech, undated). The greatest mean
dermal deposit in cm2/min was received on the feet. When the spray
target was above shoulder height the next highest dermal spray was
received on the forearm (Table 23). Exposure through inhalation was
insignificant.
A study of the urban application of carbaryl was performed by
Gold et al. (1982). The maximum dermal exposure recorded in this
study was 2.86 mg/kg per h. The maximum air concentration was
0.28 µg/litre. An insignificant decrease in ChEA in serum and
erythrocytes was found in some of the applicators. The mean dermal
carbaryl exposure of the applicator, expressed as a percentage of
toxic dose per h, was 0.01%, with a maximum of 0.08%. This exposure
rate is below the risk rate for applicators. No symptoms of
intoxication were reported by the authors. During the urban
application of carbaryl to trees and ornamental shrubs using
hand-held equipment, air concentrations measured at the breathing
zone in full-shift samples, of 0.010-0.070 mg/m3, were detected in
only 30% of the samples (Leonard & Yearly, 1990).
Table 23. Human exposure during spray operations
Type of spray equipment Mean deposit Total exposure
(µg/cm2 per min) (µg/kg per min)
Compressed air sprayer
below waist 0.030 4.3
above shoulder height 0.096 5.6
Hose-end sprayer
below waist 0.154 18.3
above shoulder height 0.353 24.4
Dermal exposure to carbaryl in harvesters of strawberries was
studied by Zweig et al. (1984, 1985). They observed dermal
exposure on the hands and forearms, and to a much lesser degree, on
the lower legs. The first day, 3 mg carbaryl/h was found on cotton
gloves, 0.66 mg/h on pads on the forearms, and 0.07 on the lower
legs. The following day, the values were 1.23, 0.41, and 0.07 mg/h
for gloves, forearms, and lower legs, respectively. The ratio of
dermal exposure to dislodgeable foliar residues (DFR) was 4.34,
2.82, and 6.17, for 3 consecutive days. The half-life of carbaryl on
strawberry leaves was 4.1 days. Dislodgeable carbaryl residues on
cotton foliage, expressed as µg/cm2 of cotton leaf (one surface
only), following application by man-pulled ground rig were 7.2, 5.9,
0.58, and 0.26 at 0, 72, 144, and 192 h after application,
respectively (Estesen et al., 1982). Approximate dermal exposure
rates may be calculated using the following expression, proposed by
Zweig et al. (1985). Dermal exposure rate (mg/h) = 5x103xDFR
(in µg/cm2). This method is suggested to obtain the exposure rate
of fruit harvesters, in order to establish safe re-entry periods
without human studies.
Factors affecting the levels of exposure during the
agricultural application of pesticides were analysed by Wolfe et
al. (1967). Wind is the most important environmental condition. The
type of activity, equipment used, the duration of exposure,
formulation, individual protection, including attitude, are also
discussed. Carbaryl deposits from air jet application on orchards
were found at 500 m downwind in the presence of inversion, and at
300 m, in its absence. Ground application results gave deposits at
150 and 50 m, respectively (MacCollom et al., 1985, 1986). Air
concentrations were 17.88 µg/m3 during spraying above the orchard
downwind edge, 9.5 µg/m3, 30 min after spraying, and 8.17 µg/m3,
1 h after spraying.
Airborne and deposit levels of carbaryl were measured after
three applications by air to an apple orchard (Currier et al.,
1982). Samples of air were taken at distances of from 12.2 to 3994 m
from the target orchard. At a distance of 12.2 m, the concentration
in air was between 3.3 and 76.2 µg/m3. One hour later it was
between 2.5 and 11.3 µg/m3. At a distance of 3.2-3.3 km, the
initial concentration was between 9 and 28.8 µg/m3 during spraying
and 0.9-14.4 µg/m3, one hour after treatment. Because the area of
the study was one of concentrated agriculture, it is possible that
carbaryl could have been used on other orchards and gardens near to
the sampling point.
6. KINETICS AND METABOLISM
6.1 Absorption
When only 0.1 ml of carbaryl solution (0.025-0.05 mmol/litre)
was administered into the lungs of anaesthetized rats, it was
rapidly absorbed. About 50% of the dose was absorbed in 2.6 min. The
amount of carbaryl absorbed per unit time was directly proportional
to the administered dose (Hwang & Shanker, 1974). Blase & Loomis
(1976) demonstrated that carbaryl could be taken up and metabolized
by the isolated perfused rabbit lung. Retention by rats of
14C-labelled carbaryl, inhaled as vapours during a 1-h exposure
period, was 75.4% of the total dose inhaled, which did not exceed
50 µg (Dorough, 1982).
When a dose of 7.5 µmol radiolabelled carbaryl/kg body weight
was administered intragastrically to fasted, anaesthetized, female
rats, 22 and 67 min after dosing, the proportions absorbed were
52.6±14.1% (n=3) and 81.7±15.7% (n=3), respectively; 89.3% of the
radiolabelled material in the collected portal blood was
[1-naphthyl-1-14C] N-methyl carbarmate (Casper et al., 1973).
Absorption from the small intestine was studied in
anaesthetized rats with a ligature around the pylorus and an
ileocecal junction with major blood vessels not occluded. A
concentration of carbaryl of 0.005-0.1 mmol/litre was used. The
absorption half-time was 6.4 min (Hwang & Schanker, 1974).
On the basis of ligation studies on mice, Ahdaya & Guthrie
(1982) determined that the absorption of carbaryl from the stomach
was relatively low compared with that from the entire
gastrointestinal tract (29%), but was relatively high in comparison
with that of other pesticides.
Variation in the digestive absorption kinetics, according to
the vehicle used, was reported in studies on female Wistar rats
(Cambon et al., 1981). The results indicated that carbaryl was
absorbed more rapidly in the intestine when either DMSO or
tragacanth was used as a vehicle. It seems that milk does not
facilitate carbaryl absorption. Inhibition of ChEA was closely
related to the absorption rate.
The rate of dermal penetration of carbaryl (in acetone
solution) into mammals, birds, amphibia, and insects was studied by
Shah
et al. (1983). The half-time of penetration of 14C-labelled
carbaryl was 317 min in Japanese quail, 12.8 min in mice, 6.4 min in
grass frogs, 4576 min in American roaches, and 791 min in tobacco
horn worms.
In an in vitro study, only about 1% of an applied dose of
carbaryl penetrated through the skin of the rat over an 8-h period
(MacPherson et al., 1991). Using two different methods, Shah &
Guthrie (1983) measured the half-time for penetration of carbaryl,
applied dermally at a rate of 4 µg/cm3, and found 10.34 h and
4.75 h, respectively. Shah et al. (1987) compared the rate of
dermal penetration of carbaryl in young, versus adult, Fischer 344,
female rats and did not find any consistent age-related differences.
Percutaneous absorption of carbaryl in rats was also studied by
Knaak et al. (1984). Carbaryl dissolved in acetone was applied to
back skin (not occluded) at 43.4-48 µg/cm2. Recovery studies
indicated that 57.7% of the applied carbaryl was absorbed.
Approximately 5.8% of carbaryl penetrated the skin within 1 h, the
rate of absorption being 0.18 µg/h per cm2. The half-life of
absorption by blood was 1.26 h, and that for elimination, 67 h.
Carbaryl labelled with radioactive carbon (14C), dissolved in
acetone, was applied to the skin of six volunteers, in order to
study percutaneous penetration (Maibach et al., 1971; Feldmann &
Maibach, 1974). The results showed almost complete penetration of
carbaryl on the forearm and jaw angle. After a 24-h application, the
cumulative urinary excretion over 5 days was 74%. According to other
authors using the same data, the estimated cumulative absorption
over 5 days, as a percentage of the applied dose, was 63% (Fisher
et al., 1985), 45% of this occurring 8 h after the onset of
penetration, which had a lag of 3.5 h.
Comparing the different studies, it is clear that some solvents
can facilitate the dermal penetration of carbaryl.
6.2 Distribution
Plasma levels of carbaryl in rats were compared after iv,
intraduodenal, or hepatic portal administration of 0.5 mg/kg
(Houston et al., 1974). In Fig. 2, it is shown that plasma
concentrations were lower after intraduodenal application, when
carbaryl was subjected to the liver's first pass metabolizing
effect. Plasma concentrations following portal application were also
lower (approximately 80% of the concentration after the systemic
route of application).
 The distribution of carbaryl in rat tissues after a single oral
administration of 144.2 mg/kg body weight (0.2 LD50) was studied
(Klisenko & Yakim, 1966) at 5 and 30 min, and 1, 2, 4, 24, 48, and
72 h. Carbaryl and 1-naphthol were identified by thin-layer
chromatography with a sensitivity of 0.5 µg/g. At 5 min, carbaryl
was found in all organs. Thirty min after administration of
carbaryl, peak concentrations found were: muscle, 35 µg/g, brain,
16 µg/g, spleen, 25 µg/g, and erythrocytes, 120 µg/g; at 60 min, a
level of 45 µg/g was found in the liver. After 24 h, levels of only
1-4 µg carbaryl/g were detected in the liver, kidney, muscles, and
skin. At 48 h, no residues were found. The authors suggested that
carbaryl is rapidly distributed and excreted. 1-Naphthol was found
in the liver, stomach, intestines, kidneys, and lungs. Other
non-identified metabolites were present in the liver and lungs.
Levels of carbaryl in the tissues of rats poisoned with oral
doses of 800 and 1200 mg/kg, respectively, were as follows: liver,
7-58 and 52-80 µg/g; heart, 3.5-31.3 and 40.6-45.9 µg/g; brain,
3-26.8 and 25.9-30.9 µg/g. Higher concentrations were found in
animals that died from poisoning than in animals killed, even though
they were treated with the same doses (Mount et al., 1981).
Yakim (1970) studied the distribution of carbaryl and
1-naphthol after 6-month oral administration of 0.2, 0.1, or 0.05
LD50 carbaryl in rats. Carbaryl was found in the intestines (20-40
µg/g), liver (4-20 µg/g), and kidneys and lungs (in trace amounts);
1-naphthol was found in the kidneys (20-50 µg/g), and in the liver
(5-10 µg/g) in groups treated with 0.2 LD50. The carbaryl level
was <1 µg/g in the organs studied in the group treated with
0.1 LD50; no traces of carbaryl were found in the group treated
with 0.05 LD50. The author suggested that a higher quantity of
carbaryl is found 30 min after a single oral application, which
corresponds to the highest percentage of ChE inhibition.
Two to 6 h after a single dermal application of 500 mg
carbaryl/kg to cats, the compound was found in plasma at 15 µg/ml
and in erythrocytes at 25 µg/ml. Inhalation studies were also
performed on cats (Table 24). The author stressed that carbaryl was
present in smaller amounts in plasma than in erythrocytes because of
the more active metabolism by blood proteins. More 1-naphthol and
other unidentified metabolites were present in plasma. Elimination
of carbaryl and normalization of ChE activities occurred in 48-70 h
with all routes of administration of carbaryl. Carbaryl has a low
cumulation capacity.
In a study in which the abilities of several pesticides to bind
to potential carriers isolated from human blood were compared,
carbaryl was much more effectively bound by albumin than by either
high- or low-density lipoproteins (Maliwal & Guthrie, 1981).
Table 24. Relation between carbaryl inhalation and resulting cholinesterase (ChE) inhibitiona
Experimental Carbaryl and 1-naphthol ChE inhibition in %
conditions Plasma Erythrocytes Plasma Erythrocytes
Single 6 h exposure 2.5-5 µg/ml after 5-10 µg/ml 39-80 (after 4 h) 52-84 (after 4 h)
to 80 mg/m3 exposure; no traces (after exposure)
8 cats 4 h later 10-15 µg/ml (4 h later)
2-2.5 µg/ml (24 h later)
Single 6 h exposure 0-10 µg/ml 28-44 in blood
to 20 mg/m3 in single animal (after 4 h);
8 cats 0 after 48 h 0 (after 48 h)
4-Month exposure to traces in single animal
16 mg/m3 in blood
4 cats
1-Month exposure to 5-15 µg/ml, 0 on the 5-15 µg/ml, 0 on the 44 on the day of 62 on the 7th day of
63 mg/m3 6th day after the end 6th day after the end exposure exposure
4 cats of exposure of exposure
4-Month exposure to 5 µg/ml on the third 10 µg/ml on the third - -
38 mg/m3 day of exposure day of exposure
4 cats
aFrom: Yakin (1979)
Levels of carbaryl in some tissues of male, albino, Fisher
Strain 344 rats, after single and multiple oral administration
(Table 25) were reported by Hassan (1971).
Table 25. Tissue levels of carbaryla
Treatment Time after Concentration of carbaryl
administration Whole blood Heart Brain
(µg/ml) (µg/g) (µg/g)
Single dose 80 mg/kg 2 h 16.2 2.6 3.45
Rats fed 700 mg/kg diet 90 days 4.8 0.85 0.68
Rats fed 100 mg/kg diet 90 days 2.7 < 0.5 < 0.5
aFrom: Hassan (1971).
Andrawes et al. (1972) studied the 14C residues in hen
tissues following the feeding of 1-naphthyl-14C-carbaryl at
70 mg/kg for 4 days (Table 26).
Radiolabelled carbaryl naphthyl 14C (6600 dpm/µg) and
carbaryl carbonyl 14C (4000 dpm/µg) were administered
intratracheally to rats as aerosols for 15 seconds. The maximum
concentration in blood occurred after 2-5 min. Distribution of the
residues in the organs, 1 h after inhalation, was highest in the
lung (9.2-10.5%), liver (4.7-9.2%), bladder (2.7-5.9%), and kidney
(2.5-3.7%) (Nye & Dorough, 1976).
Distribution of 14carbon after intraperitoneal and oral
administration of 7.4 µmol carbaryl/kg body weight to rats was
studied by Krishna & Casida (1966) (Table 27). Carbaryl was found in
all tissues analysed. No marked differences with sex and
administration route were noted. On the basis of the rate of
excretion of radioactivity following an intraperitoneal injection of
labelled carbaryl, Shah & Guthrie (1983) calculated a half-time for
clearance of label of 6.46 h.
Table 26. Concentration of14C residues in hen tissues after
feeding 1-naphthyl-14C carbaryl at the level of
70 mg/kg for 4 daysa
Sample µg/kg at indicated times after
last treatment
16 h 7 days
Brain 17.3 6.8
Heart 47.5 20.9
Kidney 405.5 80.7
Pancreas 69.6 15.6
Skin 86.6 22.4
Fat 25.2 5.2
Gizzard 43.0 13.9
Thigh 28.6 10.1
Breast 25.9 12.7
Leg muscle 29.1 9.0
Blood 197.2 152.2
Lung 138.5 122.5
Liver 332.6 33.4
Spleen 108.7 74.2
Intestine and contents 300.1 < 5.0
Intestinal wall 44.0 11.1
Oviduct 50.5 6.7
Developing egg (small) 534.3 7.4
Developing egg (large) 508.1 35.5
Remaining carcass 35.5 < 5.0
aFrom: Andrawes et al. (1972).
Distribution of carbaryl was studied in 7 Sprague-Dawley rats
and Swiss mice on day 18 of pregnancy. Whole-body auto-radiography
was performed after oral application of 13.5 µCi carbaryl methyl
14C/kg. The transfer to the placenta began in the first hour.
Distribution of the carbaryl occurred in the excretory organs,
fetus, digestive tract, and the bone marrow and brain. The major
portion was quickly eliminated. A more stable localization occurred
in highly active protein-building organs, such as the fetus,
digestive tract, and bone marrow. The 14carbon concentration in the
eye, liver, and brain of the fetus was relatively constant from 8 to
96 h (Declume & Derache, 1976, 1977; Declume & Benard, 1977a,b,
1978).
Table 27. Distribution of 14carbon in various tissues in rats, 48 h after ip administration of carbaryl and its hydrolysis product,
in µmol equivalent/kg of fresh tissue, based on total 14Ca
Labelled position Blood Bone Brain Fat Heart Kidney Liver Lung Muscle Spleen Testes
Corpuscle Plasma
Carbonyl 14C 0.38 0.16 0.26 0.33 0.16 0.39 0.47 0.63 0.32 0.18 0.33
Methyl 14C 0.51 0.22 0.47 0.67 0.19 1.23 1.37 1.78 0.97 0.47 0.97
1-Naphthyl-1-14C 0.02 0.19 0.19 0.03 0.18 0.06 0.17 0.09 0.05 0.03 0.26 0.09
1-Naphthol-1-14C < 0.01 0.06 0.03 0.01 0.04 0.01 0.05 0.14 0.02 1.16 0.13
(hydrolysis product)
aAdapted from: Krishna & Casida (1966).
Fernandez et al. (1982) determined that the elimination of
label following intravenous administration of 20 mg 14C
carbaryl/kg to rats could be best described by a three compartment
model, with 73% of the label excreted within 24 h.
Strother & Wheeler (1976, 1980) reported that 14C-carbaryl
rapidly crossed the rat placenta and was distributed in all fetal
tissues. Fetal brain, heart, and lung contained more 14C on a
weight basis than the maternal counterpart.
6.3 Metabolism
The metabolism of carbaryl has been studied extensively, and
its complexity and the need for additional studies are recognized.
As with other carbamates (WHO, 1986), the principal metabolic
pathways are hydroxylation, hydrolysis, and epoxidation, resulting
in numerous metabolites subjected to conjugation, forming
water-soluble sulfates, glucoronides, and mercapturates (Carpenter
et al., 1961; Dorough et al., 1963; Dorough & Casida, 1964;
Knaak et al., 1965; Menzie, 1969; Bend et al., 1971).
Hydrolysis of carbaryl results in the formation of 1-naphthol,
carbon dioxide, and methylamine (Fig. 3) (Carpenter et al., 1961;
Sakai & Matsumura, 1971).
The first evidence for carbaryl hydroxylation was reported by
Hodgson & Casida (1961). Carbaryl is metabolized by a rat liver
microsome system, requiring NADPH2 and oxygen, to form a
formaldehyde-yielding derivative.
The distribution of carbaryl in rat tissues after a single oral
administration of 144.2 mg/kg body weight (0.2 LD50) was studied
(Klisenko & Yakim, 1966) at 5 and 30 min, and 1, 2, 4, 24, 48, and
72 h. Carbaryl and 1-naphthol were identified by thin-layer
chromatography with a sensitivity of 0.5 µg/g. At 5 min, carbaryl
was found in all organs. Thirty min after administration of
carbaryl, peak concentrations found were: muscle, 35 µg/g, brain,
16 µg/g, spleen, 25 µg/g, and erythrocytes, 120 µg/g; at 60 min, a
level of 45 µg/g was found in the liver. After 24 h, levels of only
1-4 µg carbaryl/g were detected in the liver, kidney, muscles, and
skin. At 48 h, no residues were found. The authors suggested that
carbaryl is rapidly distributed and excreted. 1-Naphthol was found
in the liver, stomach, intestines, kidneys, and lungs. Other
non-identified metabolites were present in the liver and lungs.
Levels of carbaryl in the tissues of rats poisoned with oral
doses of 800 and 1200 mg/kg, respectively, were as follows: liver,
7-58 and 52-80 µg/g; heart, 3.5-31.3 and 40.6-45.9 µg/g; brain,
3-26.8 and 25.9-30.9 µg/g. Higher concentrations were found in
animals that died from poisoning than in animals killed, even though
they were treated with the same doses (Mount et al., 1981).
Yakim (1970) studied the distribution of carbaryl and
1-naphthol after 6-month oral administration of 0.2, 0.1, or 0.05
LD50 carbaryl in rats. Carbaryl was found in the intestines (20-40
µg/g), liver (4-20 µg/g), and kidneys and lungs (in trace amounts);
1-naphthol was found in the kidneys (20-50 µg/g), and in the liver
(5-10 µg/g) in groups treated with 0.2 LD50. The carbaryl level
was <1 µg/g in the organs studied in the group treated with
0.1 LD50; no traces of carbaryl were found in the group treated
with 0.05 LD50. The author suggested that a higher quantity of
carbaryl is found 30 min after a single oral application, which
corresponds to the highest percentage of ChE inhibition.
Two to 6 h after a single dermal application of 500 mg
carbaryl/kg to cats, the compound was found in plasma at 15 µg/ml
and in erythrocytes at 25 µg/ml. Inhalation studies were also
performed on cats (Table 24). The author stressed that carbaryl was
present in smaller amounts in plasma than in erythrocytes because of
the more active metabolism by blood proteins. More 1-naphthol and
other unidentified metabolites were present in plasma. Elimination
of carbaryl and normalization of ChE activities occurred in 48-70 h
with all routes of administration of carbaryl. Carbaryl has a low
cumulation capacity.
In a study in which the abilities of several pesticides to bind
to potential carriers isolated from human blood were compared,
carbaryl was much more effectively bound by albumin than by either
high- or low-density lipoproteins (Maliwal & Guthrie, 1981).
Table 24. Relation between carbaryl inhalation and resulting cholinesterase (ChE) inhibitiona
Experimental Carbaryl and 1-naphthol ChE inhibition in %
conditions Plasma Erythrocytes Plasma Erythrocytes
Single 6 h exposure 2.5-5 µg/ml after 5-10 µg/ml 39-80 (after 4 h) 52-84 (after 4 h)
to 80 mg/m3 exposure; no traces (after exposure)
8 cats 4 h later 10-15 µg/ml (4 h later)
2-2.5 µg/ml (24 h later)
Single 6 h exposure 0-10 µg/ml 28-44 in blood
to 20 mg/m3 in single animal (after 4 h);
8 cats 0 after 48 h 0 (after 48 h)
4-Month exposure to traces in single animal
16 mg/m3 in blood
4 cats
1-Month exposure to 5-15 µg/ml, 0 on the 5-15 µg/ml, 0 on the 44 on the day of 62 on the 7th day of
63 mg/m3 6th day after the end 6th day after the end exposure exposure
4 cats of exposure of exposure
4-Month exposure to 5 µg/ml on the third 10 µg/ml on the third - -
38 mg/m3 day of exposure day of exposure
4 cats
aFrom: Yakin (1979)
Levels of carbaryl in some tissues of male, albino, Fisher
Strain 344 rats, after single and multiple oral administration
(Table 25) were reported by Hassan (1971).
Table 25. Tissue levels of carbaryla
Treatment Time after Concentration of carbaryl
administration Whole blood Heart Brain
(µg/ml) (µg/g) (µg/g)
Single dose 80 mg/kg 2 h 16.2 2.6 3.45
Rats fed 700 mg/kg diet 90 days 4.8 0.85 0.68
Rats fed 100 mg/kg diet 90 days 2.7 < 0.5 < 0.5
aFrom: Hassan (1971).
Andrawes et al. (1972) studied the 14C residues in hen
tissues following the feeding of 1-naphthyl-14C-carbaryl at
70 mg/kg for 4 days (Table 26).
Radiolabelled carbaryl naphthyl 14C (6600 dpm/µg) and
carbaryl carbonyl 14C (4000 dpm/µg) were administered
intratracheally to rats as aerosols for 15 seconds. The maximum
concentration in blood occurred after 2-5 min. Distribution of the
residues in the organs, 1 h after inhalation, was highest in the
lung (9.2-10.5%), liver (4.7-9.2%), bladder (2.7-5.9%), and kidney
(2.5-3.7%) (Nye & Dorough, 1976).
Distribution of 14carbon after intraperitoneal and oral
administration of 7.4 µmol carbaryl/kg body weight to rats was
studied by Krishna & Casida (1966) (Table 27). Carbaryl was found in
all tissues analysed. No marked differences with sex and
administration route were noted. On the basis of the rate of
excretion of radioactivity following an intraperitoneal injection of
labelled carbaryl, Shah & Guthrie (1983) calculated a half-time for
clearance of label of 6.46 h.
Table 26. Concentration of14C residues in hen tissues after
feeding 1-naphthyl-14C carbaryl at the level of
70 mg/kg for 4 daysa
Sample µg/kg at indicated times after
last treatment
16 h 7 days
Brain 17.3 6.8
Heart 47.5 20.9
Kidney 405.5 80.7
Pancreas 69.6 15.6
Skin 86.6 22.4
Fat 25.2 5.2
Gizzard 43.0 13.9
Thigh 28.6 10.1
Breast 25.9 12.7
Leg muscle 29.1 9.0
Blood 197.2 152.2
Lung 138.5 122.5
Liver 332.6 33.4
Spleen 108.7 74.2
Intestine and contents 300.1 < 5.0
Intestinal wall 44.0 11.1
Oviduct 50.5 6.7
Developing egg (small) 534.3 7.4
Developing egg (large) 508.1 35.5
Remaining carcass 35.5 < 5.0
aFrom: Andrawes et al. (1972).
Distribution of carbaryl was studied in 7 Sprague-Dawley rats
and Swiss mice on day 18 of pregnancy. Whole-body auto-radiography
was performed after oral application of 13.5 µCi carbaryl methyl
14C/kg. The transfer to the placenta began in the first hour.
Distribution of the carbaryl occurred in the excretory organs,
fetus, digestive tract, and the bone marrow and brain. The major
portion was quickly eliminated. A more stable localization occurred
in highly active protein-building organs, such as the fetus,
digestive tract, and bone marrow. The 14carbon concentration in the
eye, liver, and brain of the fetus was relatively constant from 8 to
96 h (Declume & Derache, 1976, 1977; Declume & Benard, 1977a,b,
1978).
Table 27. Distribution of 14carbon in various tissues in rats, 48 h after ip administration of carbaryl and its hydrolysis product,
in µmol equivalent/kg of fresh tissue, based on total 14Ca
Labelled position Blood Bone Brain Fat Heart Kidney Liver Lung Muscle Spleen Testes
Corpuscle Plasma
Carbonyl 14C 0.38 0.16 0.26 0.33 0.16 0.39 0.47 0.63 0.32 0.18 0.33
Methyl 14C 0.51 0.22 0.47 0.67 0.19 1.23 1.37 1.78 0.97 0.47 0.97
1-Naphthyl-1-14C 0.02 0.19 0.19 0.03 0.18 0.06 0.17 0.09 0.05 0.03 0.26 0.09
1-Naphthol-1-14C < 0.01 0.06 0.03 0.01 0.04 0.01 0.05 0.14 0.02 1.16 0.13
(hydrolysis product)
aAdapted from: Krishna & Casida (1966).
Fernandez et al. (1982) determined that the elimination of
label following intravenous administration of 20 mg 14C
carbaryl/kg to rats could be best described by a three compartment
model, with 73% of the label excreted within 24 h.
Strother & Wheeler (1976, 1980) reported that 14C-carbaryl
rapidly crossed the rat placenta and was distributed in all fetal
tissues. Fetal brain, heart, and lung contained more 14C on a
weight basis than the maternal counterpart.
6.3 Metabolism
The metabolism of carbaryl has been studied extensively, and
its complexity and the need for additional studies are recognized.
As with other carbamates (WHO, 1986), the principal metabolic
pathways are hydroxylation, hydrolysis, and epoxidation, resulting
in numerous metabolites subjected to conjugation, forming
water-soluble sulfates, glucoronides, and mercapturates (Carpenter
et al., 1961; Dorough et al., 1963; Dorough & Casida, 1964;
Knaak et al., 1965; Menzie, 1969; Bend et al., 1971).
Hydrolysis of carbaryl results in the formation of 1-naphthol,
carbon dioxide, and methylamine (Fig. 3) (Carpenter et al., 1961;
Sakai & Matsumura, 1971).
The first evidence for carbaryl hydroxylation was reported by
Hodgson & Casida (1961). Carbaryl is metabolized by a rat liver
microsome system, requiring NADPH2 and oxygen, to form a
formaldehyde-yielding derivative.
 The use of 14carbon-labelled carbaryl (Fig. 4) and thin-layer
chromatography contributed to further studies on carbaryl metabolism
(Skraba & Young, 1959; Krishna et al., 1962). Chin et al. (1974)
used in vitro techniques on tissues from animals and human beings.
The use of 14carbon-labelled carbaryl (Fig. 4) and thin-layer
chromatography contributed to further studies on carbaryl metabolism
(Skraba & Young, 1959; Krishna et al., 1962). Chin et al. (1974)
used in vitro techniques on tissues from animals and human beings.
 Reviews on carbamate and carbaryl metabolism have been
published (Lykken & Casida, 1969; Kuhr, 1970; Knaak, 1971; Kuhr,
1971; Ryan, 1971; Fukuto, 1973; Dorough, 1973; Kuhr & Dorough,
1976).
6.3.1 In vitro studies on animal tissues
An investigation of the individual metabolism of carbaryl in
the liver, lung, and the kidney of rat was conducted using the
tissue explant maintenance technique. Hepatic tissue of the rat,
incubated with carbaryl, actively performed demethylation,
hydrolysis, hydroxylation, and oxidation, followed by sulfate and
glucuronide conjugations (Chin et al., 1979b).
Rat liver microsomes fortified with reduced nicotineamide-
adenine dinucleotide phosphate were used to study the hydroxylated
products of carbonyl 14C, methyl 14C, and naphthyl 14C
carbaryl (Dorough & Casida, 1964). The metabolites were identified
as N-hydroxymethylcarbaryl, 4-hydroxycarbaryl, and
5-hydroxycarbaryl. At least 2 unidentified metabolites had the
C-O-C(O)-N-C structure intact. 1-Naphthol and at least two
unidentified metabolites without the carbamyl groups were formed as
a product of hydrolysis. The nature of carbaryl metabolites in liver
microsomes in mice, rats, and rabbits was studied by Leeling &
Casida (1966) and in guinea-pigs and rats by Knaak et al. (1965).
Two more metabolites were identified (Table 28): 5,6-dihydro-5,6-
dihydroxycarbaryl, and 1 hydroxy-5,6-dihydro-5,6-dihydroxy-
naphthalene. It was suggested that hydrolysed metabolites are
probably conjugated as glucoronides and sulfates (Knaak et al.,
1965; Hassan et al., 1966; Leeling & Casida, 1966). A study on rat
liver microsomes and small intestine later showed (Mehendale &
Dorough, 1971) that about 90% of 1-naphthol and
N-hydroxymethylcarbaryl and about 40% of 5-hydroxycarbaryl and
4-hydroxycarbaryl were conjugated as glucuronides. The presence of
thioether conjugates in incubation mixtures of mouse liver
homogenates with carbaryl has been confirmed (Bend et al., 1971;
Ryan, 1971).
The in vitro technique for metabolic studies using liver
tissues qualitatively reflects the in vivo metabolic processes of
carbaryl in animals and human beings (Sullivan et al., 1972) and
their similarity (Matsumura & Ward, 1966).
Methylmercury hydroxide pretreatment in rats (10 mg/kg daily
for 2 days) decreased the hepatic microsomal cytochrome P-450
content and aminopyrine demethylase by 50%, as well as the
microsomal hydroxylation reaction in vitro of carbaryl to form
4-hydroxycarbaryl and N-hydroxymethylcarbaryl. Chlordane
pretreatment increased both cytochrome P450 and hydroxylation
(Lucier et al., 1972). However, there were no quantitative changes
in the metabolite pattern.
When the metabolism of carbaryl in rat intestine was studied
in vitro, hydrolysis and the synthesis of 1-naphthyl glucuronide
were reported to occur mainly in the first third of the intestine
(Pekas & Paulson, 1970; Pekas, 1972).
MacPherson et al. (1991) studied the metabolism of carbaryl
using a rat skin preparation in vitro. A post-mitochondrial
fraction was able to catalyse hydrolysis and sulfation and
glucuronidation conjugation reactions, but not ring hydroxylation.
The activities were very small in comparison with activities in
liver microsomes.
The degradation of carbaryl by an esterase of the American
cockroach ( Periplaneta americana) was reported by Matsumura &
Sakai (1968).
Table 28. Carbaryl naphthyl-1-14C metabolism by liver microsomes from mice, rabbits, and rats, in the presence of NADPH2a
Substance determined Total radiocarbon (%) using liver microsomes from:
Mice Rabbits Rats
Ether extract
Carbaryl 32.1 19.4 46.9
Hydroxylated metabolites
1-naphtyl N-hydroxymethylcarbamate 11.9 6.3 11.7
4-hydroxy-1-naphthyl methylcarbamate 6.7 8.1 6.1
5-hydroxy-1-naphthyl methylcarbamate 2.4 1.7 1.3
5,6-dihydro-5,6-dihydroxy-1-naphthyl methylcarbamate 5.3 9.1 3.8
1-hydroxy-5,6-dihydro-5,6-dihydroxy-naphthalene 1.9 2.7 1.5
1-Naphthol 7.2 6.3 5.8
Unidentified metabolites
Metabolite A 4.0 4.5 3.5
Metabolite C 0.6 2.0 0.8
Aqueous fraction 28.5 39.6 19.4
aFrom: Leeling & Casida (1966).
6.3.2 In vivo studies on animals
The metabolism of carbaryl has been studied in a variety of
mammals including rat, rabbit, guinea-pig, monkey, sheep, cow, pig,
and dog. Although many organs have been shown to be able to
metabolize carbaryl, the most important one is the liver.
The metabolism of 1-naphthyl-14C carbaryl was studied in male
and female Beagle dogs following a single, oral administration of
2.5 or 25 mg/kg. The metabolic pathways identified involved
hydrolysis, N-methyl oxidation, ring hydroxylation, and
conjugation. No significant qualitative differences were found
between male and female dogs or between high and low dosage levels.
Faecal elimination accounted for 30-66% of the applied dose and was
found to be primarily the result of incomplete absorption from the
intestinal tract of the solid material and subsequent elimination of
unchanged carbaryl. The metabolic pathway defined is illustrated in
Fig. 5 (Andrawes & Bailey, 1978c).
The metabolism of 1-naphthyl-14C carbaryl was studied in male
and female Sprague-Dawley rats following a single, oral
administration of 2.5 mg/kg. The metabolic pathways identified in
the rat involved hydrolysis, N-methyl oxidation, ring hydroxylation,
and conjugation. New metabolites identified, previously unknown in
the rat, were: 1,5-naphthalenediol, 1,6-naphthalenediol,
3,4-dihydro-3,4-dihydroxy-1-naphthol, and 3-hydroxy-1-naphthyl
methylcarbamate. These new metabolites had been previously
identified in the dog. The metabolic pathway defined is illustrated
in Fig. 5. Faecal elimination accounted for only 2-7% of faecal
14C as carbaryl, indicating more complete absorption of the test
material than in the dog (Andrawes & Bailey (1978a).
Conjugated metabolites of 1-naphthyl-14C carbaryl excreted in
rat and dog urine, after similar single oral treatments of
2.5 mg/kg, were separated and compared as intact conjugates using
gel permeation and thin layer chromatography. The metabolic products
were found to be qualitatively similar in the two animal species,
with evidence of glutathione conjugation in the dog. Urinary
metabolites only differed quantitatively between species. The rat
appeared to be considerably more active in hydrolysing carbaryl to
1-naphthol followed by conjugation, whereas, in the dog, the
principle urinary metabolites were formed through direct conjugation
of carbaryl itself. A significant amount of urinary radioactivity in
both species remained unidentified, i.e., 27 and 34% in the rat and
dog, respectively (Andrawes & Bailey, 1978b). Tables 29 and 30
illustrate the quantitative urinary metabolic differences in rats
and dogs. Note that "Free" refers to unconjugated metabolites
whereas "Acid" and "Enzyme" refer to acid- and enzyme-hydrolysed
conjugates.
Reviews on carbamate and carbaryl metabolism have been
published (Lykken & Casida, 1969; Kuhr, 1970; Knaak, 1971; Kuhr,
1971; Ryan, 1971; Fukuto, 1973; Dorough, 1973; Kuhr & Dorough,
1976).
6.3.1 In vitro studies on animal tissues
An investigation of the individual metabolism of carbaryl in
the liver, lung, and the kidney of rat was conducted using the
tissue explant maintenance technique. Hepatic tissue of the rat,
incubated with carbaryl, actively performed demethylation,
hydrolysis, hydroxylation, and oxidation, followed by sulfate and
glucuronide conjugations (Chin et al., 1979b).
Rat liver microsomes fortified with reduced nicotineamide-
adenine dinucleotide phosphate were used to study the hydroxylated
products of carbonyl 14C, methyl 14C, and naphthyl 14C
carbaryl (Dorough & Casida, 1964). The metabolites were identified
as N-hydroxymethylcarbaryl, 4-hydroxycarbaryl, and
5-hydroxycarbaryl. At least 2 unidentified metabolites had the
C-O-C(O)-N-C structure intact. 1-Naphthol and at least two
unidentified metabolites without the carbamyl groups were formed as
a product of hydrolysis. The nature of carbaryl metabolites in liver
microsomes in mice, rats, and rabbits was studied by Leeling &
Casida (1966) and in guinea-pigs and rats by Knaak et al. (1965).
Two more metabolites were identified (Table 28): 5,6-dihydro-5,6-
dihydroxycarbaryl, and 1 hydroxy-5,6-dihydro-5,6-dihydroxy-
naphthalene. It was suggested that hydrolysed metabolites are
probably conjugated as glucoronides and sulfates (Knaak et al.,
1965; Hassan et al., 1966; Leeling & Casida, 1966). A study on rat
liver microsomes and small intestine later showed (Mehendale &
Dorough, 1971) that about 90% of 1-naphthol and
N-hydroxymethylcarbaryl and about 40% of 5-hydroxycarbaryl and
4-hydroxycarbaryl were conjugated as glucuronides. The presence of
thioether conjugates in incubation mixtures of mouse liver
homogenates with carbaryl has been confirmed (Bend et al., 1971;
Ryan, 1971).
The in vitro technique for metabolic studies using liver
tissues qualitatively reflects the in vivo metabolic processes of
carbaryl in animals and human beings (Sullivan et al., 1972) and
their similarity (Matsumura & Ward, 1966).
Methylmercury hydroxide pretreatment in rats (10 mg/kg daily
for 2 days) decreased the hepatic microsomal cytochrome P-450
content and aminopyrine demethylase by 50%, as well as the
microsomal hydroxylation reaction in vitro of carbaryl to form
4-hydroxycarbaryl and N-hydroxymethylcarbaryl. Chlordane
pretreatment increased both cytochrome P450 and hydroxylation
(Lucier et al., 1972). However, there were no quantitative changes
in the metabolite pattern.
When the metabolism of carbaryl in rat intestine was studied
in vitro, hydrolysis and the synthesis of 1-naphthyl glucuronide
were reported to occur mainly in the first third of the intestine
(Pekas & Paulson, 1970; Pekas, 1972).
MacPherson et al. (1991) studied the metabolism of carbaryl
using a rat skin preparation in vitro. A post-mitochondrial
fraction was able to catalyse hydrolysis and sulfation and
glucuronidation conjugation reactions, but not ring hydroxylation.
The activities were very small in comparison with activities in
liver microsomes.
The degradation of carbaryl by an esterase of the American
cockroach ( Periplaneta americana) was reported by Matsumura &
Sakai (1968).
Table 28. Carbaryl naphthyl-1-14C metabolism by liver microsomes from mice, rabbits, and rats, in the presence of NADPH2a
Substance determined Total radiocarbon (%) using liver microsomes from:
Mice Rabbits Rats
Ether extract
Carbaryl 32.1 19.4 46.9
Hydroxylated metabolites
1-naphtyl N-hydroxymethylcarbamate 11.9 6.3 11.7
4-hydroxy-1-naphthyl methylcarbamate 6.7 8.1 6.1
5-hydroxy-1-naphthyl methylcarbamate 2.4 1.7 1.3
5,6-dihydro-5,6-dihydroxy-1-naphthyl methylcarbamate 5.3 9.1 3.8
1-hydroxy-5,6-dihydro-5,6-dihydroxy-naphthalene 1.9 2.7 1.5
1-Naphthol 7.2 6.3 5.8
Unidentified metabolites
Metabolite A 4.0 4.5 3.5
Metabolite C 0.6 2.0 0.8
Aqueous fraction 28.5 39.6 19.4
aFrom: Leeling & Casida (1966).
6.3.2 In vivo studies on animals
The metabolism of carbaryl has been studied in a variety of
mammals including rat, rabbit, guinea-pig, monkey, sheep, cow, pig,
and dog. Although many organs have been shown to be able to
metabolize carbaryl, the most important one is the liver.
The metabolism of 1-naphthyl-14C carbaryl was studied in male
and female Beagle dogs following a single, oral administration of
2.5 or 25 mg/kg. The metabolic pathways identified involved
hydrolysis, N-methyl oxidation, ring hydroxylation, and
conjugation. No significant qualitative differences were found
between male and female dogs or between high and low dosage levels.
Faecal elimination accounted for 30-66% of the applied dose and was
found to be primarily the result of incomplete absorption from the
intestinal tract of the solid material and subsequent elimination of
unchanged carbaryl. The metabolic pathway defined is illustrated in
Fig. 5 (Andrawes & Bailey, 1978c).
The metabolism of 1-naphthyl-14C carbaryl was studied in male
and female Sprague-Dawley rats following a single, oral
administration of 2.5 mg/kg. The metabolic pathways identified in
the rat involved hydrolysis, N-methyl oxidation, ring hydroxylation,
and conjugation. New metabolites identified, previously unknown in
the rat, were: 1,5-naphthalenediol, 1,6-naphthalenediol,
3,4-dihydro-3,4-dihydroxy-1-naphthol, and 3-hydroxy-1-naphthyl
methylcarbamate. These new metabolites had been previously
identified in the dog. The metabolic pathway defined is illustrated
in Fig. 5. Faecal elimination accounted for only 2-7% of faecal
14C as carbaryl, indicating more complete absorption of the test
material than in the dog (Andrawes & Bailey (1978a).
Conjugated metabolites of 1-naphthyl-14C carbaryl excreted in
rat and dog urine, after similar single oral treatments of
2.5 mg/kg, were separated and compared as intact conjugates using
gel permeation and thin layer chromatography. The metabolic products
were found to be qualitatively similar in the two animal species,
with evidence of glutathione conjugation in the dog. Urinary
metabolites only differed quantitatively between species. The rat
appeared to be considerably more active in hydrolysing carbaryl to
1-naphthol followed by conjugation, whereas, in the dog, the
principle urinary metabolites were formed through direct conjugation
of carbaryl itself. A significant amount of urinary radioactivity in
both species remained unidentified, i.e., 27 and 34% in the rat and
dog, respectively (Andrawes & Bailey, 1978b). Tables 29 and 30
illustrate the quantitative urinary metabolic differences in rats
and dogs. Note that "Free" refers to unconjugated metabolites
whereas "Acid" and "Enzyme" refer to acid- and enzyme-hydrolysed
conjugates.
 Table 29. Metabolic products present in a 24-h sample of urine of a
rat treated orally with 2.5 mg 1-naphthyl-14C
carbaryl/kg in corn oil
Products Percentage of total radioactivity in urine
Free Enzyme Acid Total
1-Naphthol 0.26 15.86 0.49 16.61
Carbaryl 0.14 0.45 4.22 4.81
Methylol NDa NDa 0.64b 0.64
1,5-Naphthalenediol 0.17 1.12 0.08 1.37
1,6-Naphthalenediol 0.03 0.42 NDa 0.45
5-Hydroxy carbaryl 7.76 3.07 0.24 11.07
5,6-Dihydrodihydroxy
naphthol 1.16 4.99 b 6.15
5,6-Dihydrodihydroxy
carbaryl 5.66 7.75 b 13.41
1,4-Naphthoquinonec 0.03 1.12 0.10 1.25
4-Hydroxy carbaryl 2.30 2.76 0.19 5.25
3-Hydroxy carbaryl NDa NDa 0.06 0.06
3,4-Dihydrodihydroxy
naphthol 0.71 1.53 b 2.24
Unknown 1 0.25 2.95 0.06 3.26
Unknown 2 NDa 3.46 NDa 3.46
Other unknowns 0.37 1.92 0.38 2.67
Highly polar
materials NDa 6.99 20.31 27.30
aND = none detected.
bAcid hydrolysis degrades the methylol to desmethyl carbaryl and
the dihydrodihydroxy derivatives to phenols.
cA decomposition product of 1,4-naphthalenediol during work-up.
Table 30. Metabolic products present in a 24-h sample of urine of a
female dog treated orally with 25 mg 1-naphthyl-14C
carbaryl/kg
Products Percentage of total radioactivity in urine
Free Enzyme Acid Total
1-Naphthol 0.38 2.74 1.47 4.59
Carbaryl 1.71 0.16 9.90 11.77
Methylol NDa NDa 0.22 0.22
1,5-Naphthalenediol 0.21 1.83 1.14 3.18
1,6-Naphthalenediol NDa 1.62 NDa 1.62
5-Hydroxy carbaryl 1.61 1.67 0.20 3.48
5,6-Dihydrodihydroxy
naphthol 1.12 9.79 NDa 10.89
5,6-Dihydrodihydroxy
carbaryl 3.80 3.09 NDa 6.89
1,4-Naphthoquinoneb NDa 1.24 1.36 2.60
4-Hydroxy carbaryl 0.64 5.30 0.44 6.38
3-Hydroxy carbaryl NDa 0.04 0.01 0.05
3,4-Dihydrodihydroxy
naphthol NDa 0.50 NDa 0.50
Unknown 1 NDa 0.20 0.62 0.82
Unknown 2 NDa 1.30 NDa 1.30
Other unknowns NDa 1.26 0.43 1.69
Highly polar materials NDa 9.06 34.96 44.02
aND = none detected.
bA decomposition product of 1,4-naphthalenediol during work-up.
The above work supersedes the work by Knaak & Sullivan (1967),
who mistakenly concluded that the Beagle dog metabolic pathway was
qualitatively different from that of the rat, because of poor
absorption of solid/slurry test material. The marked emphasis on
differences in metabolic pathways between species, together with the
analytical techniques available at the time of the study contributed
to their conclusions.
Studies on the nature of the biliary, water-soluble metabolites
of carbaryl were conducted by Bend et al. (1971) on 19 male Wistar
rats, using a technique with the bile duct cannulated. Water-soluble
conjugates of carbaryl with sulfur-containing amino acid were found
in the urine as well as the bile of treated rats: S-(4-hydroxy-1-
naphthyl) cysteine and S(5-hydroxy-1-naphthyl)-cysteine. Biliary
secretion of 5,6-dihydro-5,6-dihydroxycarbaryl glucuronide following
an infused dose was found to be greatly reduced, from 10% to less
than 1%, by pretreatment with antibiotics (Struble et al., 1983b).
This indicates that bacteria play a role in the enterohepatic
circulation and bilary secretion of this metabolite of carbaryl.
The functional activity of the reticulo-endothelial system
(RES) can influence carbaryl metabolism (Pipy et al., 1980). The
elimination of carbaryl from the blood decreased significantly in
rats (24 male Sprague-Dawley) with RES inhibited by colloidal
carbon, and increased in those in which the RES was activated with
glyceryl trioleate. Correlation of the activity of RES with the
enzyme activity of monoxygenases of the hepatic microsomal fraction
could possibly explain this effect of the RES in the toxicokinetics
of carbaryl.
An intravenous injection of colloidal carbon, which inhibits
liver microsomal metabolism, reduced biliary excretion of an iv dose
of carbaryl given 18 h later (Pipy et al., 1981). Pretreatment of
rats with 75 mg phenobarbital/kg per day, ip, for five days resulted
in an increase in the rate of sulfate conjugation of carbaryl,
following a high dose of carbaryl (16.4 mg/kg), but not a low dose
(1.64 mg/kg) (Knight et al., 1987).
6.3.3 Metabolic transformation in plants
The formation of carbaryl metabolites in plants is primarily
dependent on the hydrolytic, oxidative, and conjugative potential of
the plant tissues, which are similar to the tissues of insects and
mammals. The metabolism in plants is of a shorter duration than that
in insects and mammals, and the tendency to accumulate metabolites
is more pronounced. The carbamate ester bond appears to be quite
stable in plants, which explains the small amount of recovery of
1-naphthol from treated plants. The extent of oxidation is generally
higher in plants and insects than in mammals. Several
hydroxymetabolites are formed by oxidation of the N-methyl group
and naphthalene ring. They are conjugated as glycosides.
Non-enzymatic factors, such as light, heat, and humidity may
contribute to the degradation of carbaryl in plants as well (Kuhr,
1971).
The metabolism of carbaryl has been defined in a wide variety
of plant species (Table 31). Metabolites were isolated in microgram
quantities for mass and ultraviolet spectroscopic analyses (Mumma
et al., 1971).
6.3.4 In vitro studies with human tissues
The metabolism of carbaryl in selected human tissues was
studied in vitro by Chin et al. (1974). On the basis of the
total anionic characteristics of the metabolites derived from each
organ, metabolic activity occurred in the following organs in
descending order: liver, lung, kidney, placenta, vaginal mucosa,
uterus.
The metabolic profiles of carbaryl in human postembryonic fetal
autopsy tissue were determined using 1-naphthyl-14C or
N-methyl-14C-carbaryl. The anionics from fetal liver amounted to
20% of those found with the adult liver. Naphthyl glucuronide and
naphthyl sulfate were produced in the kidney, whereas the lung
produced naphthyl sulfate from carbaryl (Chin et al., 1979a).
Carbaryl was metabolized oxidatively by primary human embryonic
cells in culture (Lin et al., 1975). Complete degradation occurred
after 72 h of incubation. Unconjugated metabolites were identified
as 1-naphthol, 5-hydroxycarbaryl, 4-hydroxycarbaryl, and
5,6-dihydro-5,6-dihydroxycarbaryl. The water-soluble components were
identified as 4-hydroxycarbaryl, 1,4-naphthalenediol, and
5,6-dihydro-5,6-dihydroxycarbaryl. The primary human embryonic lung
cells did not convert carbaryl to carbon dioxide. They may not
possess the enzyme system that is necessary to break down the
naphthalene ring of carbaryl to form carbon dioxide.
Sakai & Matsumura (1971) studied the degradation of carbaryl by
brain esterases. Carbaryl was degraded by band E4 and E6,
whereas, in the mouse brain preparation, the compound was degraded
by band E8, E9, and E6.
Cell culture techniques were used to examine the products of
carbaryl degradation by cultures of an L-132 cell line derived from
normal human embryonic lung. The data indicated that detoxification
was similar to that observed in animals and in in vitro enzyme
systems (Baron & Locke, 1970).
Table 31. The composition of water-soluble metabolites of carbaryl in plants.
(% distribution of released aglycones 21 days after treatment (for apples, 53 days))
Metabolite Beana Beana Wheatb Potatoc Cornd Riced Tomatoc Applese
(mature (shell (seedlings) (mature (seedlings) (seedlings) (mature
foliage) only) foliage) foliage)
5,6-Dihydrodihydroxycarbaryl 2.5 12.7 2.1 NDd 1.3 2.9 ND 2.1
Methylol carbaryle 29.9 47.9 10.5 16.2 13.3 7.8 25.4 2.8
7-Hydroxycarbaryl 24.2 trace ND ND ND ND ND 0.8
4-Hydroxycarbaryl 15.0 4.1 23.6 4.1 8.9 3.7 13.8 13.7
5-Hydroxycarbaryl 7.3 9.4 21.3 4.1 12.9 5.6 3.4 9.2
1-Naphthol 13.8 21.0 1.1 5.8 3.2 3.5 8.1 11.3
5,6-Dihydrodihydroxynaphthol ND ND ND ND 0.5 1.0 ND
Carbaryl 0.3 0.8 20.8 27.0 24.1 42.8 16.8 33.3
Unknown(s) ND ND 3.2 ND 2.9 2.2 5.2 3.0
Unhydrolysed conjugate 7.1 4.1 17.4 42.8 33.0 30.4 27.3 12.6
References: aWiggins & Weiden (1969); bAndrawes & Chancey (1970); cChancey & Andrawes (1971b); dChancey & Andrawes (1971a);
eChancey (1974).
fND = not detected.
Studies on the in vitro metabolism of carbaryl by a human
liver fraction indicated that there was a difference in the
metabolic pattern compared with that in the rat liver. The human
liver produces at least two more metabolites (Strother, 1970).
6.3.5 In vivo studies on humans
The metabolism of carbaryl in humans appears to be
qualitatively similar to that previously reported in other mammals.
Metabolic reactions include: hydroxylation, hydrolysis, and
conjugation. Metabolites of carbaryl were identified in the urine of
human volunteers after ingestion of a 2 mg/kg dose (Andrawes &
Myers, 1976). Only traces of the unchanged carbaryl could be
detected in the urine indicating rapid metabolism. From the spectrum
of the metabolites identified, a metabolic pathway of carbaryl in
humans is proposed and shown in Fig. 6.
The only detectable metabolites in urine samples taken from
workers exposed to carbaryl dust were 1-naphthylglucoronide and
sulfate (Knaak et al., 1965). Later 1-naphthyl and
4-hydroxycarbaryl, as conjugates of glucuronic and sulfuric acid,
were found in a study on volunteers.
Human exposure to carbaryl during aerial application for Gypsy
moth control was assessed by Schulze et al. (1979). The results of
this study indicated a strong positive correlation between carbaryl
exposure and the excretion of urinary 1-naphthol within 24 h of
known exposure and a total lack of 1-naphthol in pre-exposure
samples.
The presence of these metabolites has been confirmed in six
urine samples of workers with high exposures to carbaryl (Andrawes &
Myers, 1976).
One major and three minor interfering chemicals were detected
in no-exposure samples as well as the maximum exposure samples.
These interfering materials developed a colour similar to that of
carbaryl metabolites upon spraying the chromatograms with
p-nitrobenzene diazonium fluoborate. It is estimated that the
major interfering pigment accounted for as much as one-third of the
total colour observed on thin-layer chromatograms. These interfering
chemicals may also be the cause of high blank values often
encountered during the routine analysis of urine samples (Andrawes &
Bailey, 1979).
Table 29. Metabolic products present in a 24-h sample of urine of a
rat treated orally with 2.5 mg 1-naphthyl-14C
carbaryl/kg in corn oil
Products Percentage of total radioactivity in urine
Free Enzyme Acid Total
1-Naphthol 0.26 15.86 0.49 16.61
Carbaryl 0.14 0.45 4.22 4.81
Methylol NDa NDa 0.64b 0.64
1,5-Naphthalenediol 0.17 1.12 0.08 1.37
1,6-Naphthalenediol 0.03 0.42 NDa 0.45
5-Hydroxy carbaryl 7.76 3.07 0.24 11.07
5,6-Dihydrodihydroxy
naphthol 1.16 4.99 b 6.15
5,6-Dihydrodihydroxy
carbaryl 5.66 7.75 b 13.41
1,4-Naphthoquinonec 0.03 1.12 0.10 1.25
4-Hydroxy carbaryl 2.30 2.76 0.19 5.25
3-Hydroxy carbaryl NDa NDa 0.06 0.06
3,4-Dihydrodihydroxy
naphthol 0.71 1.53 b 2.24
Unknown 1 0.25 2.95 0.06 3.26
Unknown 2 NDa 3.46 NDa 3.46
Other unknowns 0.37 1.92 0.38 2.67
Highly polar
materials NDa 6.99 20.31 27.30
aND = none detected.
bAcid hydrolysis degrades the methylol to desmethyl carbaryl and
the dihydrodihydroxy derivatives to phenols.
cA decomposition product of 1,4-naphthalenediol during work-up.
Table 30. Metabolic products present in a 24-h sample of urine of a
female dog treated orally with 25 mg 1-naphthyl-14C
carbaryl/kg
Products Percentage of total radioactivity in urine
Free Enzyme Acid Total
1-Naphthol 0.38 2.74 1.47 4.59
Carbaryl 1.71 0.16 9.90 11.77
Methylol NDa NDa 0.22 0.22
1,5-Naphthalenediol 0.21 1.83 1.14 3.18
1,6-Naphthalenediol NDa 1.62 NDa 1.62
5-Hydroxy carbaryl 1.61 1.67 0.20 3.48
5,6-Dihydrodihydroxy
naphthol 1.12 9.79 NDa 10.89
5,6-Dihydrodihydroxy
carbaryl 3.80 3.09 NDa 6.89
1,4-Naphthoquinoneb NDa 1.24 1.36 2.60
4-Hydroxy carbaryl 0.64 5.30 0.44 6.38
3-Hydroxy carbaryl NDa 0.04 0.01 0.05
3,4-Dihydrodihydroxy
naphthol NDa 0.50 NDa 0.50
Unknown 1 NDa 0.20 0.62 0.82
Unknown 2 NDa 1.30 NDa 1.30
Other unknowns NDa 1.26 0.43 1.69
Highly polar materials NDa 9.06 34.96 44.02
aND = none detected.
bA decomposition product of 1,4-naphthalenediol during work-up.
The above work supersedes the work by Knaak & Sullivan (1967),
who mistakenly concluded that the Beagle dog metabolic pathway was
qualitatively different from that of the rat, because of poor
absorption of solid/slurry test material. The marked emphasis on
differences in metabolic pathways between species, together with the
analytical techniques available at the time of the study contributed
to their conclusions.
Studies on the nature of the biliary, water-soluble metabolites
of carbaryl were conducted by Bend et al. (1971) on 19 male Wistar
rats, using a technique with the bile duct cannulated. Water-soluble
conjugates of carbaryl with sulfur-containing amino acid were found
in the urine as well as the bile of treated rats: S-(4-hydroxy-1-
naphthyl) cysteine and S(5-hydroxy-1-naphthyl)-cysteine. Biliary
secretion of 5,6-dihydro-5,6-dihydroxycarbaryl glucuronide following
an infused dose was found to be greatly reduced, from 10% to less
than 1%, by pretreatment with antibiotics (Struble et al., 1983b).
This indicates that bacteria play a role in the enterohepatic
circulation and bilary secretion of this metabolite of carbaryl.
The functional activity of the reticulo-endothelial system
(RES) can influence carbaryl metabolism (Pipy et al., 1980). The
elimination of carbaryl from the blood decreased significantly in
rats (24 male Sprague-Dawley) with RES inhibited by colloidal
carbon, and increased in those in which the RES was activated with
glyceryl trioleate. Correlation of the activity of RES with the
enzyme activity of monoxygenases of the hepatic microsomal fraction
could possibly explain this effect of the RES in the toxicokinetics
of carbaryl.
An intravenous injection of colloidal carbon, which inhibits
liver microsomal metabolism, reduced biliary excretion of an iv dose
of carbaryl given 18 h later (Pipy et al., 1981). Pretreatment of
rats with 75 mg phenobarbital/kg per day, ip, for five days resulted
in an increase in the rate of sulfate conjugation of carbaryl,
following a high dose of carbaryl (16.4 mg/kg), but not a low dose
(1.64 mg/kg) (Knight et al., 1987).
6.3.3 Metabolic transformation in plants
The formation of carbaryl metabolites in plants is primarily
dependent on the hydrolytic, oxidative, and conjugative potential of
the plant tissues, which are similar to the tissues of insects and
mammals. The metabolism in plants is of a shorter duration than that
in insects and mammals, and the tendency to accumulate metabolites
is more pronounced. The carbamate ester bond appears to be quite
stable in plants, which explains the small amount of recovery of
1-naphthol from treated plants. The extent of oxidation is generally
higher in plants and insects than in mammals. Several
hydroxymetabolites are formed by oxidation of the N-methyl group
and naphthalene ring. They are conjugated as glycosides.
Non-enzymatic factors, such as light, heat, and humidity may
contribute to the degradation of carbaryl in plants as well (Kuhr,
1971).
The metabolism of carbaryl has been defined in a wide variety
of plant species (Table 31). Metabolites were isolated in microgram
quantities for mass and ultraviolet spectroscopic analyses (Mumma
et al., 1971).
6.3.4 In vitro studies with human tissues
The metabolism of carbaryl in selected human tissues was
studied in vitro by Chin et al. (1974). On the basis of the
total anionic characteristics of the metabolites derived from each
organ, metabolic activity occurred in the following organs in
descending order: liver, lung, kidney, placenta, vaginal mucosa,
uterus.
The metabolic profiles of carbaryl in human postembryonic fetal
autopsy tissue were determined using 1-naphthyl-14C or
N-methyl-14C-carbaryl. The anionics from fetal liver amounted to
20% of those found with the adult liver. Naphthyl glucuronide and
naphthyl sulfate were produced in the kidney, whereas the lung
produced naphthyl sulfate from carbaryl (Chin et al., 1979a).
Carbaryl was metabolized oxidatively by primary human embryonic
cells in culture (Lin et al., 1975). Complete degradation occurred
after 72 h of incubation. Unconjugated metabolites were identified
as 1-naphthol, 5-hydroxycarbaryl, 4-hydroxycarbaryl, and
5,6-dihydro-5,6-dihydroxycarbaryl. The water-soluble components were
identified as 4-hydroxycarbaryl, 1,4-naphthalenediol, and
5,6-dihydro-5,6-dihydroxycarbaryl. The primary human embryonic lung
cells did not convert carbaryl to carbon dioxide. They may not
possess the enzyme system that is necessary to break down the
naphthalene ring of carbaryl to form carbon dioxide.
Sakai & Matsumura (1971) studied the degradation of carbaryl by
brain esterases. Carbaryl was degraded by band E4 and E6,
whereas, in the mouse brain preparation, the compound was degraded
by band E8, E9, and E6.
Cell culture techniques were used to examine the products of
carbaryl degradation by cultures of an L-132 cell line derived from
normal human embryonic lung. The data indicated that detoxification
was similar to that observed in animals and in in vitro enzyme
systems (Baron & Locke, 1970).
Table 31. The composition of water-soluble metabolites of carbaryl in plants.
(% distribution of released aglycones 21 days after treatment (for apples, 53 days))
Metabolite Beana Beana Wheatb Potatoc Cornd Riced Tomatoc Applese
(mature (shell (seedlings) (mature (seedlings) (seedlings) (mature
foliage) only) foliage) foliage)
5,6-Dihydrodihydroxycarbaryl 2.5 12.7 2.1 NDd 1.3 2.9 ND 2.1
Methylol carbaryle 29.9 47.9 10.5 16.2 13.3 7.8 25.4 2.8
7-Hydroxycarbaryl 24.2 trace ND ND ND ND ND 0.8
4-Hydroxycarbaryl 15.0 4.1 23.6 4.1 8.9 3.7 13.8 13.7
5-Hydroxycarbaryl 7.3 9.4 21.3 4.1 12.9 5.6 3.4 9.2
1-Naphthol 13.8 21.0 1.1 5.8 3.2 3.5 8.1 11.3
5,6-Dihydrodihydroxynaphthol ND ND ND ND 0.5 1.0 ND
Carbaryl 0.3 0.8 20.8 27.0 24.1 42.8 16.8 33.3
Unknown(s) ND ND 3.2 ND 2.9 2.2 5.2 3.0
Unhydrolysed conjugate 7.1 4.1 17.4 42.8 33.0 30.4 27.3 12.6
References: aWiggins & Weiden (1969); bAndrawes & Chancey (1970); cChancey & Andrawes (1971b); dChancey & Andrawes (1971a);
eChancey (1974).
fND = not detected.
Studies on the in vitro metabolism of carbaryl by a human
liver fraction indicated that there was a difference in the
metabolic pattern compared with that in the rat liver. The human
liver produces at least two more metabolites (Strother, 1970).
6.3.5 In vivo studies on humans
The metabolism of carbaryl in humans appears to be
qualitatively similar to that previously reported in other mammals.
Metabolic reactions include: hydroxylation, hydrolysis, and
conjugation. Metabolites of carbaryl were identified in the urine of
human volunteers after ingestion of a 2 mg/kg dose (Andrawes &
Myers, 1976). Only traces of the unchanged carbaryl could be
detected in the urine indicating rapid metabolism. From the spectrum
of the metabolites identified, a metabolic pathway of carbaryl in
humans is proposed and shown in Fig. 6.
The only detectable metabolites in urine samples taken from
workers exposed to carbaryl dust were 1-naphthylglucoronide and
sulfate (Knaak et al., 1965). Later 1-naphthyl and
4-hydroxycarbaryl, as conjugates of glucuronic and sulfuric acid,
were found in a study on volunteers.
Human exposure to carbaryl during aerial application for Gypsy
moth control was assessed by Schulze et al. (1979). The results of
this study indicated a strong positive correlation between carbaryl
exposure and the excretion of urinary 1-naphthol within 24 h of
known exposure and a total lack of 1-naphthol in pre-exposure
samples.
The presence of these metabolites has been confirmed in six
urine samples of workers with high exposures to carbaryl (Andrawes &
Myers, 1976).
One major and three minor interfering chemicals were detected
in no-exposure samples as well as the maximum exposure samples.
These interfering materials developed a colour similar to that of
carbaryl metabolites upon spraying the chromatograms with
p-nitrobenzene diazonium fluoborate. It is estimated that the
major interfering pigment accounted for as much as one-third of the
total colour observed on thin-layer chromatograms. These interfering
chemicals may also be the cause of high blank values often
encountered during the routine analysis of urine samples (Andrawes &
Bailey, 1979).
 6.4 Elimination and excretion in expired air, faeces, urine,
milk, and eggs
The elimination of metabolized carbaryl is rapid, and
accumulation in animals seems unlikely, under normal exposure
conditions. Carbaryl is generally excreted entirely within 24-96 h
of intake. Elimination takes place mainly through the urine, faeces,
and respiration, and, to a lesser extent, through the milk of dairy
animals and the eggs of poultry.
Carbaryl is mainly excreted as its product of hydrolysis,
1-naphthol, which is detoxified to water-soluble glucuronide
(Carpenter et al., 1961), and sulfate (Whitehurst et al., 1963),
and only in trace amounts as 1-naphthol or unchanged.
The average percentage of metabolites recovered after orally
administered doses of 1-naphthyl 14C, methyl 14C, and carbonyl
14C carbaryl to rats was 94% over a 7-day period (Knaak et al.,
1965). Only residues from carbaryl methyl 14C were detected after
that, since the methyl moiety is incorporated in tissue (2-3%).
Liberated naphthol is conjugated and excreted, while the liberated
carbonyl groups are disposed of as respiratory14CO2 (Knaak
et al., 1965). Forty-seven to 57% of the metabolites excreted
retain the intact C-O-C(O)N-C structure, indicating a nonhydrolytic
pathway for carbaryl. Monkeys and pigs (2 young females) excreted
carbaryl as a conjugate of intact carbaryl and 4-hydroxycarbaryl,
mainly glucuronides. Sheep also excreted 1-naphthyl glucuronide and
sulfate and 4 (methylcarbamoyloxy)1-naphthylsulfate (Knaak et al.,
1968).
Myers (1977) studied the correlation between the amount of
carbaryl ingested and urinary metabolites levels. Metabolites were
measured as three groups:
I. naphthyl sulfate;
II. free and sulfated 5,6-dihydrodihydroxycarbaryl
and dihydrodihydroxynaphthol;
III. other conjugated (glucuronides) of naphthol,
5,6-dihydrodihydroxycarbaryl, and
5,6 dihydrodihydroxynaphthol.
The amounts in groups I and II were 25.5-36.5% of the dose. When all
three groups of metabolites were analysed, the amounts of
metabolites for the same period represented 41.5-52.7% of the dose.
Naphthol was the major metabolite found in Group III (mean 75% of
all metabolites of the group). A good correlation was found between
the amount ingested and urinary metabolites.
Rats treated orally with naphthyl14C carbaryl (2.3 µci)
excreted 90% of the administered radioactivity in the urine within 3
days. During the 72-h sampling period, the faeces contained only
2-5% of the radioactivity (Lucier et al., 1972).
Eighty per cent of 5 mg of 14C naphthyl carbaryl, given
intraperitoneally to rats, (see section 6.3.2) was eliminated in the
24-h urine and 10% in the faeces (Bend et al., 1971). Enzymatic
hydrolysis and reverse isotope dilution showed that 10% of the dose
was excreted as 1-naphthyl-glucoronide, 5%, as 1-naphthylsulfate,
3%, as conjugates of 4-hydroxycarbaryl, 5%, as 1-naphthylsulfate,
3%, as conjugates of 4-hydroxycarbaryl, and 5% of 5-hydroxycarbaryl.
About 2% of the carbaryl appeared unchanged in urine. Other
radioactive metabolites were not identified.
Following a single, ip injection in albino rats of both sexes,
of methyl 14C and carbonyl 14C carbaryl at 30 mg/kg, 75-80% was
recovered after 48 h in the expired air and urine. Liberated by
hydrolysis, N-methyl carbamic acid was spontaneously decomposed to
methylamine and carbon dioxide (43.5%). The methylamine moiety was
later demethylated to 14CO2, which was eliminated in the expired
air and 14C formate (9.2%), which was excreted in the urine
(Hassan et al., 1966).
In the study of Nye & Dorough (1976), 2.5% of the
endotracheally administered carbaryl dose was exhaled as
14C-carbon dioxide. More than 90% of the dose was eliminated in
the urine during 3 days. The faeces contained 2-5%.
A comparative study on the excretion of carbaryl after a
7.5 µmol/kg, ip dose in rats was reported by Krishna & Casida (1966)
who used three types of labelled carbaryl (Table 32).
Table 32. Fate of 14carbon in male Sprague-Dawley rats
after ip administration of carbaryla
Labelled position Administered 14carbon recovered (%)
Expired 14CO2 Urine Faeces
0-24 h 24-48 h
Carbonyl 14C 24.5 62.1 2.4 2.1
Methyl 14C 12.3 54.6 3.4 3.9
Naphthyl 14C 0.2 74.2 2.3 8.9
Hydrolysis product < 0.1 86.4 3.3 1.4
naphthol 14C
aFrom: Krishna & Casida (1966).
Biliary excretion of carbaryl was studied in bile-duct
cannulated rats. Naphthyl 14C and methyl 14C carbaryl (50 µg)
were administered intravenously to rats (see section 6.3.2). During
the first 2 h of collection, 90% of the total biliary radioactivity
was eliminated (Bend et al., 1971). After oral application of
0.01 mg/kg naphthyl-14C carbaryl to rats, 37.5% was excreted via
the bile in the first 3 h and 45.4% (cumulative percentage) of the
dose was eliminated within 48 h. Faecal elimination was 1.4% and
urine elimination was 42.3% (Marshall et al., 1979). The results
of Struble et al. (1983a), who used higher doses of carbaryl (1.5,
31, and 300 mg/kg) on male Sprague-Dawley rats were similar. Twelve
hours after administration, 15-46% of the 14C was excreted in the
bile, 10-40% in the urine, and less than 1% was eliminated in
faeces. Three metabolites were identified from the bile, the major
one being 5-6-dihydro-5-6-dihydroxycarbaryl glucoronide (12-18% of
the biliary 14C).
On the basis of a study on 50, male, Sprague-Dawley rats,
Borzelleca & Skalsky (1980) reported that carbaryl metabolites in
the blood were also present in the saliva, at similar
concentrations.
Leeling & Casida (1966) studied carbaryl metabolites in the
urine of one male and one female New Zealand White rabbit. The
ether-extractable metabolites found in the urine were:
N-hydroxymethyl carbaryl, 4-hydroxycarbaryl, 5-hydroxycarbaryl,
5,6-dihydro-5,6-dihydroxycarbaryl, 5,6-dihydro-5,6-trihydroxy-1-
naphthol, and 1-naphthol.
Thirty-five, adult, female American cockroaches ( Periplaneta
americana) were injected through the central abdominal wall with
5 µg of carbaryl-carbonyl 14C, carbaryl methyl 14C, or 2 µg
carbaryl naphthyl 14C. Metabolites similar to those appearing in
rat liver microsomes were formed. About 19% of the radioactivity of
carbaryl carbonyl 14C was eliminated as 14C-carbon dioxide in
24 h (Dorough & Casida, 1964).
Excretion of carbaryl in milk was studied by Baron (1968).
Application of a total dose of 2 g carbonyl carbaryl 14C to a
lactating cow resulted in approximately 1% of radioactive residues
in the milk. In skim milk, 87% of these residues were water-soluble
and 13% chloroform-soluble. About 90-95% of the14C radioactivity
in the soluble component was removed after crystallization of
lactose.
The metabolism of carbaryl naphthyl 14C was studied in one
lactating Jersey cow (Dorough, 1967). Two consecutive single
treatments at 6-day intervals with 0.25 mg/kg body weight (total
125 mg) and one single dose of 3.05 mg carbaryl naphthyl 14C/kg
body weight added to the feed, resulted in radiolabelled residues in
the milk for as long as 60 h after treatment. The first sample of
milk, taken after 6 h, showed maximum concentrations of chloroform
extractables, water-soluble, and unextractable, residues in whole
milk in the range of 0.063-0.95 mg/litre. Residues of only a
slightly lower magnitude were detected in the 12-h milk samples and
rapidly declined in 24-h samples. Thirty per cent of the residues in
the 6-h sample was a chloroform-extractable metabolite, tentatively
identified as 5,6-dihydro-5,6-dihydroxy-1-naphthyl-
N-methylcarbamate. About 0.35% of the total dose was detected in
the milk in both treatments, 70% in the urine, and 11% in the faeces
in 0.25 mg/kg treatment, and 58% in the urine and 15% in the faeces
in the 3.05 mg/kg treatment. The highest levels in the tissues of
the cow, killed on an unspecified day after treatment, were found in
the liver, kidney, and ovaries.
A single lactating cow was treated orally with a daily total
dose of 518 mg 14C-carbaryl (0.77 mg/kg body weight), given in two
doses, 12 h apart. Results are shown in Table 33 (Dorough, (1969).
Fifty-nine per cent of the radioactivity from a14C-labelled oral
dose of carbaryl administered to a cow was recovered from the urine
within the first 24 h (Saini & Dorough, 1978).
The passage of14C into the milk and its presence in the
suckling neonates has been studied by whole-body autoradiography in
rats fed 14C-methyl carbaryl. The highest concentrations in
newborn offspring, after 48 h, were found in the stomach contents,
the liver, the hair, and the bone marrow (Benard et al., 1979).
Table 33. Distribution and excretion of total-14C equivalents in
milk, urine, faeces, and tissues, 24 h after one-day oral
application of carbaryl in a lactating cow
Sample mg/kg Dose recovered
(%)
Milk 0.04 0.09
Urine 22.4 58.64
Faeces 0.47 1.65
Tissues:
- liver 0.098 0.21
- kidney 0.382 0.17
- leg muscle 0.027 nil
- fat 0.014 nil
A Saanen goat was treated orally with 1.34 mg carbaryl carbonyl
14C/kg. The excretion of radioactivity in the urine was 7.4% at
2 h, 24% at 4 h, and 45% at 24 h. In total, 47% of the radioactivity
was excreted the urine. In the milk, the peak was 0.9 mg/litre at
8 h, dropping to 0.008 mg/litre 60 h after the administration of
carbaryl (Dorough & Casida, 1964).
The excretion of carbaryl was studied in hens. Carbaryl-
carbonyl14C (10 mg/kg body weight) was given orally to Leghorn
hens. Fifty per cent of the 14C was expired within 48 h. When
carbarylnaphthyl 14C was applied at the same dose, no
radioactivity was detected in the expired gases. The urine was the
primary route of excretion. 14Carbon remaining in the carcass,
48 h after dosing, accounted for 1.4 and 7.1% of the dose given as
the ring-labelled and carbonyl-labelled compound, respectively
(Paulson & Feil, 1969). The main metabolites found in the urine of
hens were: 1-naphthol, 1-naphthyl glucoronide, and sulfate esters of
1-naphthol, 4-hydroxycarbaryl, and 5-hydroxycarbaryl. Conjugates
with other metabolites were also found in small quantities (Paulson
et al., 1970). Mature hens were given a single oral dose of
carbaryl naphthyl 14C at 10 mg/kg body weight. Eggs collected for
12 days contained a total dose of 0.33% of the 14C administered
(Paulson & Feil, 1969).
The fate of naphthyl-1-14C carbaryl in laying chickens was
studied by Andrawes et al. (1972). Chickens (3 White Leghorn in
each group) were fed 7, 12, or 70 mg/kg carbaryl in the feed twice
daily for 14 days. They had been pretreated for 17 days with the
same doses of nonlabelled carbaryl in feed. Residues reached maximum
levels within 1 day in excrement, 2 days in egg white, and 6-8 days
in egg yolk. After the end of dosing, the half-life of 14C
residues was <1 day in the excrement and egg white, 2-3 days in the
egg yolk, and 5 days in the carcass. Metabolites and carbaryl found
in the eggs of hens, fed 70 mg carbaryl/kg averaged in total 19.7 µg
of 14C carbaryl equivalent per egg (16.3 µg in yolk and 3.4 µg in
white), collected between the 9th and the 14th day of dosing.
Naphthyl-l-sulfate was the largest single component of egg residues
accounting for the higher concentration of residues in the egg yolk.
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
7.1 Microorganisms
7.1.1 Soil microorganisms
Studies of the effects of insecticides on soil microorganisms
are complex and subject to many sources of variation. It is
therefore difficult to assess the practical significance of the
results of such studies.
Varsheny & Gaur (1972) studied the impact of carbaryl (as
Sevin) on soil fungi. They determined the size of the fungal
population and the species composition of the fungal community after
plating out extracts of soil samples on various media. The authors
suggested that, at very high rates of addition to the soil (up to
5000 mg/kg), there was evidence of a change in species composition
and an increase in the total fungal population.
In laboratory cultures of saltmarsh protozoans (dominated by
Euplotes sp.), Weber et al. (1982) found that both carbaryl and
1-naphthol caused dose-related mortality. For both compounds,
mortality at 1 mg/litre was about 50%. There was almost 100%
mortality at 10 mg/litre.
Carbaryl inhibited the growth of cultures of Bacillus subtilis
(DeGiovanni & Donnelly, 1968).
7.1.2 Aquatic microorganisms
Edmiston et al. (1985) conducted a detailed study of the
effects of carbaryl on Paramecium multimicronucleatum. The 24-h
LC50 was 28 µg/litre in a static plate test. Effects on the oxygen
uptake of cultures and effects on the cell surface were also
reported, but these results were mostly obtained using
concentrations in excess of the 24-h LC50, and must be of limited
value.
Murray & Guthrie (1980) studied the responses of bacterial
cultures originating from Lake Houston. The respiratory rate of
bacterial cultures was increased at water temperatures of between
23-33 °C. Plate counts of bacterial colonies suggested that carbaryl
treated cultures contained more bacteria than control cultures on
most days in a 26-day study.
7.2 Aquatic organisms
Aquatic toxicity studies have been conducted in the laboratory
and field to define the biological effects associated with acute and
long-term exposure to carbaryl and the degradation product,
1-naphthol, of aquatic vertebrates and invertebrates. More data are
available for freshwater organisms than for saltwater organisms. In
addition, actual and simulated field studies have been conducted to
assess the impact of carbaryl exposure on freshwater invertebrates.
7.2.1 Aquatic invertebrates
Studies on the toxicity of carbaryl for non-target aquatic
invertebrates have included investigations on molluscs, crustaceans,
and insects, concentrating on organisms of commercial importance,
organisms most likely to be exposed during a typical application of
carbaryl, or on standardized test species typically used in aquatic
toxicity tests. The acute and long-term laboratory studies under
various environmental conditions (pH, temperature, water hardness,
etc.) are briefly reviewed in Table 34.
Most of the studies on the toxicity of carbaryl for aquatic
invertebrates have been conducted under laboratory conditions and,
consequently, the results only yield data on the relative toxicity
of the substance. They do not reflect realistic exposure in the
environment. Most of the laboratory studies evaluate constant,
short-term (less than 1 week) exposure to carbaryl under static or
flow-through conditions. There are few long-term studies on the
effects of carbaryl on aquatic invertebrates. Exposures in the
environment are typically localized, at much lower concentrations
than in aquatic toxicity tests and are not constant.
The toxicity of carbaryl on the early development of the sea
urchin was studied by Hernandez et al. (1990). Developmental
stages with active cleavage and cellular mobilization (blastula and
gastrula) turned out to be more sensitive. The next two stages
(prism and pluteus) were less sensitive and EC50 values were 8-26
times higher. This effect may be connected with increased
detoxication processes by the cytochrome oxidase system.
Table 34. Acute toxicity of carbaryl for invertebrates
Organism Test Size/age Stat/ Temperature Hardness pH Parameterc Concentration Reference
material Flowa (°C) (mg/litre)b (µg/litre)
Crustacea
(Freshwater)
Water flea
Daphnia magna unknown stat 16 42 7.4 48-h LC50 5.6 Sanders et al. (1983)
Daphnia magna technical 96-h LC50 3280 Lejczak (1977a)
Daphnia magna unknown 48-h EC50 0.26 Rawash et al. (1975)
Daphnia magna technical 21-day MATC 1.5-3.3 Surprenant (1985c)
21-day NOEC 6.0
(survival)
Daphnia pulex technical stat 16 44 7.1 48-h LC50 6.4 Mayer & Ellersieck (1986)
Simocephalus unknown stat 10 44 7.1 48-h LC50 11 Johnson & Finley (1980)
serrulatus stat 16 44 7.1 48-h LC50 7.6
stat 21 44 7.1 48-h LC50 8.1
Ostracod
Cypridopsis
vidua unknown adult stat 21 272 7.4 48-h LC50 115 Mayer & Ellersieck (1986)
Sow bug
Asellus
brevicaudus technical adult stat 18 44 7.1 96-h LC50 280 Johnson & Finley (1980)
Table 34 (continued)
Organism Test Size/age Stat/ Temperature Hardness pH Parameterc Concentration Reference
material Flowa (°C) (mg/litre)b (µg/litre)
Crustacea
(Freshwater)
Amphipod
Gammarus
fasciatus unknown adult stat 21 44 7.1 96-h LC50 26 Johnson & Finley (1980)
Gammarus
lacustris unknown adult stat 21 44 7.1 96-h LC50 22 Johnson & Finley (1980)
Gammarus unknown adult stat 12 40 6.5 48-h LC50 8.13 Mayer & Ellersieck (1986)
pseudolimnaeus adult stat 12 40 7.5 48-h LC50 11.5
adult stat 12 40 8.5 48-h LC50 7.8
Gammarus pulex technical 48-h LC50 29 Bluzat & Seuge (1979)
Gammarus technical 96-h LC50 16 Sanders et al. (1983)
pseudolimnaeus
Gammarus technical 96-h LC50 13 Woodward & Mauck (1980)
pseudolimnaeus
Glass shrimp
Palaemonetes unknown adult stat 21 272 7.4 96-h LC50 5.6 Johnson & Finley (1980)
kadiakensis 25-31 mm stat 96-h LC50 120 Chaiyarach et al. (1975)
Table 34 (continued)
Organism Test Size/age Stat/ Temperature Hardness pH Parameterc Concentration Reference
material Flowa (°C) (mg/litre)b (µg/litre)
Crayfish
Procambarus sp. technical immature stat 12 42 7.5 96-h LC50 1900 Mayer & Ellersieck (1986
Procambarus unknown 60-70 mm stat 96-hLC50 2430 Chaiyarach et al. (1975)
simulans
Procambarus technical 96-h LC50 500 Cheah et al. (1978)
acutus
Crustacea
(Freshwater)
Prawn
Macrobrachium technical 96-h LC50 19 Omkar & Shukla (1985)
lamarrei
Upogebia wettable powderd 48-h EC50 90 Stewart et al. (1967)
pugettensis 1-naphthol 48-h EC50 4400
Callianassa wettable powderd 48-h EC50 80
californiensis 1-naphthol 48-h EC50 3500
Hemigrapsus wettable powderd 24-h EC50 710
oregonensis 1-naphthol 24-h EC50 74 200
Table 34 (continued)
Organism Test Size/age Stat/ Temperature Hardness pH Parameterc Concentration Reference
material Flowa (°C) (mg/litre)b (µg/litre)
Crustacea
(estuarine &
marine)
Blue crab
Callinectes unknown juvenile flow 29 27s 48-h LC50 320 Mayer (1987)
sapidus
Brown shrimp
Penaeus aztecus unknown juvenile flow 30 28s 48-h LC50 1.5
Pink shrimp
Penaeus
duorarum unknown juvenile flow 23 29s 48-h LC50 32
Grass shrimp
Palaemonetes unknown juvenile flow 23 29s 48-h LC50 28
pugio 3-4 days stat 24 28-30s 96-h LC50 22 Thursby & Champhon (1991)
Crustacea
(estuarine &
marine)
Table 34 (continued)
Organism Test Size/age Stat/ Temperature Hardness pH Parameterc Concentration Reference
material Flowa (°C) (mg/litre)b (µg/litre)
Mysid shrimp
Mysidopsis bahia unknown flow 96-h LC50 > 7.7 Nimmo et al. (1981)
Mysidopsis bahia technical 96-h LC50 6.7 Surprenant (1985b)
NOEC 5.1
Mysidopsis bahia 24 h flow 25 31s 96-h LC50 8.46 Thursby & Champhon (1991)
24 h stat 24 30s 96-h LC50 19
24 h flow 26 31-32s 28-day LC50 9.9
Ostracod
Cypretta kawatai technical 72-h LC50 1800 Hansen & Kawatski (1976)
Insects
Stonefly
Isogenus sp. unknown larva stat 7 35 7 96-h LC50 2.8 Mayer & Ellersieck (1986)
Pteronarcys technical 96-h LC50 4.8 Johnson & Finley (1980)
californica
Mosquito
Culex pipiens technical 24-h LC50 75 Rawash et al. (1975)
Table 34 (continued)
Organism Test Size/age Stat/ Temperature Hardness pH Parameterc Concentration Reference
material Flowa (°C) (mg/litre)b (µg/litre)
Insects
Midge
Chironomus technical 24-h LC50 7 Karnak & Collins (1974)
tentans
Chironomus technical 72-h LC50 5900 Hansen & Kawatski (1976)
tentans
Chironomus technical 48-h EC50 10 Sanders et al. (1983)
plumosus
Chironomus unknown larva stat 20 4 24-h LC50 106 Fisher & Lohner (1986)
riparius larva stat 20 6 24-h LC50 133
larva stat 20 8 24-h LC50 127
Back swimmer
Notonecta technical 96-h LC50 200 Federle & Collins (1976)
undulata
Water stick
Ranatra elongata wettable 96-h LC50 624 Shukla et al. (1982)
powder
Table 34 (continued)
Organism Test Size/age Stat/ Temperature Hardness pH Parameterc Concentration Reference
material Flowa (°C) (mg/litre)b (µg/litre)
Diptera
Chaoborus technical 48-h LC50 296 Bluzat & Seuge (1979)
Ephemeroptera
(Mayfly)
Cloeon technical 48-h LC50 480
Crawling water
beetle
Peltodytes sp. technical 96-h LC50 3300 Federle & Collins (1976)
Insects
Stone fly
Pteronarcella unknown larva stat 16 44 7.1 96-h LC50 1.7 Johnson & Finley (1980)
badia larva stat 12 38 6.5 96-h LC50 11 Woodward & Mauck (1980)
larva stat 12 38 7.5 96-h LC50 13 Mayer & Ellersieck (1986)
larva stat 12 38 8.5 96-h LC50 29
Claasenia unknown larva stat 16 44 7.1 96-h LC50 5.6 Johnson & Finley (1980)
sabulosa
Table 34 (continued)
Organism Test Size/age Stat/ Temperature Hardness pH Parameterc Concentration Reference
material Flowa (°C) (mg/litre)b (µg/litre)
Molluscs
Common mussel
Mytilus edulis unknown 48-h EC50 > 30 000 Roberts (1975)
for byssal
attachment
Mytilus edulis wettable larva 48-h EC50 2300 Stewart et al. (1967)
powder 48-h EC50 1300
1-naphthol
Giant oyster
Crassostrea gigas wettable larva 48-h EC50 2200
powder 48-h EC50 800
1-naphthol
Clinocardium wettable adults 24-h EC50 7300
nuttallii powder 24-h EC50 6400
1-naphthol
Molluscs
Eastern Oyster
Crassostrea juvenile flow 29 27s 96-h EC50 > 2000 Mayer (1987)
virginica
Table 34 (continued)
Organism Test Size/age Stat/ Temperature Hardness pH Parameterc Concentration Reference
material Flowa (°C) (mg/litre)b (µg/litre)
Crassostrea technical 48-h EC50 2700 Surprenant (1985a);
virginica Dionne et al. (1985)
Mactrid clam
Rangia cuneata 35-50 mm stat 5s 96-h LC50 125 000 Chaiyarach et al. (1975)
Lymnaea technical 48-h LC50 21 000 Bluzat & Seuge (1979)
stagnalis
aStat = static conditions (water unchanged for the duration of the test).
Flow = flow-through conditions (carbaryl concentration in water continuously maintained).
bHardness = expressed as mg CaCO3/litre.
s = salinity (%).
cEC50s for oyster based on shell deposition.
d50-80% wettable powder.
Carbaryl is slightly toxic for freshwater and marine molluscs
(clams, mussels, oysters). Acute toxicity typically occurs in adult
molluscs at concentrations ranging from 1 mg carbaryl/litre to over
100 mg/litre. The major hydrolytic product of carbaryl, 1-naphthol,
is slightly more acutely toxic for molluscs (mussel, pacific oyster)
than the parent compound (Stewart et al., 1967). In addition,
larvae and young juveniles are more sensitive to carbaryl exposure
than older juveniles and adults.
There have been few studies on the effects of carbaryl on the
growth of molluscs. Davis (1961) and Butler (1962) found that
carbaryl (80% WP) concentrations of 1.0-2 mg/litre reduced the
larval development and growth of oysters ( Crassostrea virginica)
and clams ( Venus mercenaria).
The effects of a 1-h exposure to carbaryl (80% WP) and its
hydrolytic product, 1-naphthol, were studied on six developmental
stages of the mussel ( Mytilus edulis) (Armstrong & Millemann,
1974c). The six stages included unfertilized eggs to the early
veliger stage, 32 h after fertilization. Unfertilized eggs and the
first polar body stage were exposed to carbaryl and 1-naphthol
(separately). The acute toxicity values of carbaryl and 1-naphthol
were similar for the first two stages, ranging from approximately 5
to 25 mg/litre. The stage of development most sensitive to carbaryl
was the first polar body stage. Thereafter, susceptibility decreased
as age of the larvae increased.
LC50 values for crustacea varied from 5 to 9 µg/litre (water
fleas, mysid shrimps), 8 to 25 µg/litre (scud), and 500 to
2500 µg/litre for crayfish. The hydrolytic metabolite 1-naphthol was
less toxic for daphnids and shrimps.
The effects of carbaryl (80% WP) on the life stages of
crustacea have been studied in the Dungeness crab ( Cancer magister)
(Buchanan et al., 1970). Early larvae were more sensitive to
carbaryl than juveniles and adults. Carbaryl (1.0 mg/litre) did not
affect hatching of eggs but prevented moulting of all prezoeae to
zoeae. The concentration that killed 50% of the first zoeae during a
96-h exposure was estimated to be 1 mg/litre.
Young juvenile crabs were more sensitive than older juveniles
or adults. The 24-h EC50s (death or irreversible paralysis) were
estimated to be 0.076 and 0.35-0.62 mg/litre for second and ninth
stage juveniles, respectively. At 0.032 mg/litre, juvenile crabs
were not affected when held in uncontaminated water after exposure.
In adult crabs, the 24-h and 96-h EC50s (death or paralysis) were
0.49 and 0.26 mg/litre, respectively. Post-larval Dungeness crabs
had about the same sensitivity to carbaryl as other crabs. The 24-h
EC50 (death or paralysis) of small stone crabs was 1.0 mg/litre
(Butler, 1962), that of juvenile blue crabs, 0.55 mg/litre (Butler,
1963), and that of adult crabs, 0.06-1.05 mg/litre (Stewart et al.,
1967).
As part of fresh water biota, daphnids have been used as assay
organisms to determine toxic concentrations of a variety of
pesticides. Laboratory bioassays were conducted with carbaryl to
determine its toxicity and immobilization values for two species of
daphnids, Daphnia pulex and Simocephalus serrulatus. The EC50s
for overt effects were 0.0064 mg/litre for Daphnia pulex and
0.0076 mg/litre for Simocephalus serrulatus (Sanders & Cope,
1966).
In a long-term study, Daphnia magna was exposed to technical
carbaryl for 21 days; reproductive performance was the most
sensitive indicator; the MATC was 1.5-3.3 µg/litre (Surprenant,
1985c).
Aquatic insects in the orders Plecoptera (stoneflies) and
Ephemeroptera (mayflies) are generally highly sensitive to low
levels of carbaryl.
The effects of 0.6-50.0 mg carbaryl/litre were studied on
selected aquatic organisms, including: Scanedesmus quadricauda,
Lemna minor, Lebistes reticulatus and Daphnia magna (Bogacka &
Groba, 1980). Carbaryl depressed reproduction, biomass, and
chlorophyll content in Lemna minor (vascular plant) after 24 h
exposure at a concentration >6.6 mg/litre. An almost 100% decrease
occurred at a concentration of 50 mg/litre. Inhibition of
photosynthesis intensity in the alga Scenedesmus quadricauda was
about 50% at a concentration of carbaryl in the water of
32 mg/litre, and 65% at a concentration of 56 mg/litre, after 24 h
exposure. Less than 10% inhibition occurred at a concentration of
1.8 mg/litre. A decrease in the production of chlorophyll was
demonstrated at 4.4 mg/litre.
Carbaryl applications of 5.7 or 11.4 kg/ha were effective in
controlling ghost shrimp ( Callianassa californiensis) as an oyster
pest. The numbers of various clams in the mud were reduced by 22 and
38%, respectively. Clam species differed in susceptibility. For
example, gaper clams (Tresus capax) were reduced by 58 and 69%.
Polychaetes and nemertean worms were not affected during 30 days of
observation (Armstrong & Millemann, 1974a,b).
7.2.2 Fish
7.2.2.1 Acute toxicity
Acute toxicity studies have been conducted with technical
carbaryl, several formulations, and 1-naphthol to determine LC50
values for several freshwater and marine fish (Table 35). The
results indicated that short-term exposure (< 4 days) to carbaryl
and its formulations showed some toxicity for fish (96-h LC50s =
1-30 mg/litre). There were no differences in the toxicity of two
formulations (4-oil-sevin and XLR) for rainbow trout and bluegill.
The LC50s for sheepshead minnow (exposed to technical material)
and rainbow trout (exposed to 4-oil and XLR) were similar and both
were generally more sensitive than bluegill. The toxicity of
1-naphthol for rainbow trout, bluegill, and sheepshead minnow was
determined by Springborn (1988a); 96-h LC50 values for these
species were 0.76, 1.4, and 1.2 mg/litre, respectively. The
corresponding NOECs were < 0.43, 0.55, and 0.47 mg/litre. The
metabolite, 1-naphthol, was more acutely toxic than the formulations
for bluegill. However, the toxicities of 1-naphthol and the
formulations were similar for rainbow trout and sheepshead minnows.
Few studies report acute toxicity values for fish that are
lower than 1 mg/litre. Cold water fish (Salmonidae), such as Coho
Salmon and trout, seem to be susceptible to carbaryl, while the
catfish (Ictaluridae) is tolerant (Macek & McAllister, 1970; Post &
Schroeder, 1971). Irritability, sluggishness, and loss of
equilibrium were classical signs of acute intoxication. Rainbow
trout (Oncorhynchus mykiss), exposed to 1 mg carbaryl/litre for
96 h, exhibited decreased swimming capacity and swimming activity;
the capacity to capture and consume prey was also diminished (Little
et al., 1990).
Other end-point concentrations have been established for
carbaryl for various species. Reference should be made to the
original documentation to obtain a fuller understanding of these
measures of toxicity. The lowest no-observed-effect concentration
(NOEC) was found for rainbow trout at 0.065 mg/litre. Other NOECs
were at least an order of magnitude greater. Maximum acceptable
toxic concentrations (MATCS) have also been calculated. Bansal
et al. (1980) estimated 30-day MATCs for 4 species of carp exposed
to carbaryl (Sevin as a 50% wettable powder). For all species, the
MATC was between 0.052 and 0.078 mg/litre. Verma et al. (1984)
reported a 60-day MATC of between 0.09 and 0.11 mg/litre for another
carp species, exposed to the same type of formulation of Sevin.
Table 35. Acute toxicity of carbaryl for fish
Organism Size/age Stat/ Temperature Hardness pH Formulationc Parameter Concentration Reference
flowa (°C) (mg/litre)b (mg/litre)
Freshwater
Coho salmon 1 g stat 13 44 7.1 96-h LC50 4.3 Mayer & Ellersieck (1986)
(Oncorhynchus stat 13 42 7.1 96-h LC50 2.4
kisutch) 4.6 g stat 13 42 7.1 96-h LC50 1.8
5.1 g stat 13 42 7.1 96-h LC50 2.7
10.6 g stat 13 42 7.1 96-h LC50 1.2
19.1 g CT 96-h LC50 0.8 Macek & McAllister (1970)
CT 96-h LC50 1.3 Post & Schroeder (1971)
Chinook salmon fingerling flow 13 314 7.5 96-h LC50 2.4 Mayer & Ellersieck (1986)
(Oncorhynchus
tshawytscha)
Cutthroat trout 0.5 g stat 12 40 7.5 96-h LC50 7.1
(Salmo clarkii) 0.5 g stat 12 330 7.8 96-h LC50 4.0
0.6 g stat 7 42 7.5 96-h LC50 6.0
0.7 g stat 12 40 6.5 96-h LC50 5.0
0.6 g stat 12 40 8.5 96-h LC50 1.0
CT 96-h LC50 6.0 Woodward & Mauck
C49 96-h LC50 6.7 (1980)
Table 35 (continued)
Organism Size/age Stat/ Temperature Hardness pH Formulationc Parameter Concentration Reference
flowa (°C) (mg/litre)b (mg/litre)
Rainbow trout 1.5 g stat 12 42 7.1 96-h LC50 2.0 Mayer & Ellersieck (1986)
(Oncorynchus 1.5 g stat 12 272 7.4 96-h LC50 1.2
mykiss) 1.2 g stat 12 40 7.4 96-h LC50 3.5
1.2 g stat 12 320 7.4 96-h LC50 3.0
1 g stat 12 40 7.4 96-h LC50 1.6
1 g stat 17 40 7.4 96-h LC50 1.1
1 g stat 12 40 6.5 96-h LC50 1.2
1 g stat 12 40 7.5 96-h LC50 0.8
1 g stat 12 40 8.5 96-h LC50 1.5
0.5 g flow 17 314 7.5 96-h LC50 1.3
CT 96-h LC50 4.3 Macek & McAllister (1970)
XLR 96-h LC50 1.4 Springborn (1985)
S4 96-h LC50 1.3
CT 96-h LC50 2.2 Sanders et al. (1983)
CT 96-h LC50 1.5 Post & Schroeder (1971)
Atlantic salmon 0.2 g stat 7 42 7.5 96-h LC50 0.3 Mayer & Ellersieck (1986)
(Salmo salar) 0.2 g stat 12 42 7.5 96-h LC50 0.9
0.2 g stat 17 42 7.5 96-h LC50 1.0
0.4 g stat 12 42 6.5 96-h LC50 1.3
0.4 g stat 12 42 8.5 96-h LC50 0.9
Brown trout 0.6 g stat 12 42 7.5 96-h LC50 6.3
(Salmo trutta) fingerling flow 12 314 7.5 96-h LC50 2.0
Table 35 (continued)
Organism Size/age Stat/ Temperature Hardness pH Formulationc Parameter Concentration Reference
flowa (°C) (mg/litre)b (mg/litre)
Brook trout 0.8 g stat 12 42 7.5 96-h LC50 2.1 Mayer & Ellersieck (1986)
(Salvelinus 0.8 g stat 7 42 7.5 96-h LC50 3.0
fontinalis) 1 g stat 17 42 7.5 96-h LC50 0.7
0.7 g stat 12 42 6.5 96-h LC50 4.6
0.7 g stat 12 42 8.5 96-h LC50 2.1
0.7 g stat 12 42 9 96-h LC50 1.1
0.8 g stat 12 42 7.5 96-h LC50 1.2
0.8 g stat 12 300 7.5 96-h LC50 1.3
Lake trout 1.7 g stat 12 40 7.5 96-h LC50 0.7
(Salvelinus 1.7 g stat 12 40 6 96-h LC50 0.7
namaycush) 1.7 g stat 12 40 9 96-h LC50 0.9
0.5 g stat 12 162 7.4 96-h LC50 0.9
2.6 g flow 12 162 7.4 96-h LC50 2.3
Gold fish 0.9 g stat 18 40 7.1 96-h LC50 13.2
(Carassius 0.9 g stat 18 272 7.4 96-h LC50 12.8
auratus)
Common carp 0.6 g stat 18 40 7.1 96-h LC50 5.3
(Cyprinus
carpio)
fry CT 96-h LC50 1.7 Chin & Sudderuddin (1979)
Table 35 (continued)
Organism Size/age Stat/ Temperature Hardness pH Formulationc Parameter Concentration Reference
flowa (°C) (mg/litre)b (mg/litre)
Carp C50 WP 72-h LC50 10.4 Toor & Kaur (1974)
(Cyprinus
carpio
communis)
(Catla catla) S 96-h LC50 6.4 Tilak et al. (1981)
(Cirrhina S50 WP 96-h LC50 2.0 Verma et al. (1984)
mrigala)
Fathead 0.5 g stat 12 42 7.5 96-h LC50 14.0 Mayer & Ellersieck (1986)
minnow 0.8 g stat 18 40 7.1 96-h LC50 14.6
(Pimephales 0.8 g stat 18 272 7.4 96-h LC50 7.7
promelas)
Sheepshead CT 96-h LC50 2.2 Springborn (1985)
minnow
(Cyprindon
variegatus)
Black bullhead 1.2 g stat 18 40 7.1 96-h LC50 20.0 Mayer & Ellersieck (1986)
(Ictalurus
malas)
Channel catfish 1.5 g stat 18 40 7.1 96-h LC50 15.8
(Ictalurus 1.5 g stat 18 272 7.4 96-h LC50 7.8
punctatus) fingerling flow 12 314 7.5 96-h LC50 17.31
Table 35 (continued)
Organism Size/age Stat/ Temperature Hardness pH Formulationc Parameter Concentration Reference
flowa (°C) (mg/litre)b (mg/litre)
Freshwater 1.1 g stat 28 S50 WP 72-h LC50 17.5 Arunachalam et al. (1980)
catfish
(Mystus
vittatus)
S 96-h LC50 2.4 Tilak et al. (1981)
(Mystus S 96-h LC50 4.6
cavasius)
Freshwater CT 96-h LC50 46.9 Tripathi & Shukla (1988)
catfish
(Clarias
batrachus)
CT 96-h LC50 107.7
Catfish CT 96-h LC50 9.7 Lejczak (1977a)
(Lebistes
reticulatus)
Green sunfish 1.1 g stat 18 40 7.1 96-h LC50 11.2 Mayer & Ellersieck (1986)
(Lepomis 1.1 g stat 18 272 7.4 96-h LC50 9.5
cyanellus)
Table 35 (continued)
Organism Size/age Stat/ Temperature Hardness pH Formulationc Parameter Concentration Reference
flowa (°C) (mg/litre)b (mg/litre)
Bluegill 1.2 g stat 18 40 7.1 96-h LC50 6.8
(Lepomis 1.2 g stat 18 272 7.4 96-h LC50 5.2
macrochirus) 0.4 g stat 12 44 7.4 96-h LC50 7.4
0.4 g stat 22 44 7.4 96-h LC50 5.2
0.8 g stat 12 40 7.4 96-h LC50 16.0
0.8 g stat 17 40 7.4 96-h LC50 7.0
0.8 g stat 22 40 7.4 96-h LC50 8.2
0.4 g stat 17 320 7.4 96-h LC50 6.2
0.7 g stat 17 40 6.5 96-h LC50 5.4
0.7 g stat 17 40 7.5 96-h LC50 5.2
0.7 g stat 17 40 8.5 96-h LC50 1.8
0.7 g stat 17 40 9.5 96-h LC50 2.6
0.6 g flow 12 314 7.5 96-h LC50 5.1
XLR 96-h LC50 9.8 Springborn (1985)
S4 96-h LC50 10
Mosquito fish 30-40 mm stat 96-h LC50 31.8 Chaiyarach et al. (1975)
(Gambusia
affinis)
Largemouth 0.9 g stat 18 40 7.1 96-h LC50 6.4 Mayer & Ellersieck (1986)
bass
(Micropterus
salmoides)
Black crapple 1 g stat 18 40 7.1 96-h LC50 2.6
(Pomoxis
nigromaculatus)
Table 35 (continued)
Organism Size/age Stat/ Temperature Hardness pH Formulationc Parameter Concentration Reference
flowa (°C) (mg/litre)b (mg/litre)
Yellow perch 1.4 g stat 18 40 7.1 96-h LC50 0.7 Mayer & Ellersieck (1986)
(Perca 0.6 g stat 12 42 7.5 96-h LC50 5.1
flavescens) 1 g stat 7 42 7.5 96-h LC50 13.9
1 g stat 12 42 7.5 96-h LC50 5.4
1 g stat 17 42 7.5 96-h LC50 3.4
1 g stat 22 42 7.5 96-h LC50 1.2
0.9 g stat 12 42 6.5 96-h LC50 4.0
0.9 g stat 12 42 7.5 96-h LC50 4.2
0.9 g stat 12 42 8.5 96-h LC50 0.5
0.9 g stat 12 42 9 96-h LC50 0.4
1 g stat 12 42 8 96-h LC50 3.8
1 g stat 12 170 8 96-h LC50 5.0
1 g stat 12 300 8 96-h LC50 3.8
fingerling flow 12 314 7.5 96-h LC50 1.4
Snakehead fish CT 48-h LC50 8.7 Rao et al. (1985a)
(Channa
punctatus)
C50 WP 96-h LC50 19.5 Singh et al. (1984)
C50 WP 48-h LC50 8.1 Rao et al. (1985b)
(Anabas S 96-h LC50 5.5 Tilak et al. (1981)
testulus)
Table 35 (continued)
Organism Size/age Stat/ Temperature Hardness pH Formulationc Parameter Concentration Reference
flowa (°C) (mg/litre)b (mg/litre)
Tilapid fish S50 WP 72-h LC50 8.0 Koudinya & Ramamurthi
(Sarotherodon (1980)
mosambica)
(Tilapia sp.) C 48-h LC50 5.5 Basha et al. (1983)
Estuarine C 96-h LC50 2.2 Lingaraja & Venugopalan
teleost (1978)
(Therapon
jarbus)
(Hetero-pneustes C50 WP 96-h LC50 20.1 Singh et al. (1984)
fossilis)
(Macropodus) CT 96-h LC50 3.5 Arunachalam &
Palanichamy (1982)
(Cypanus) CT 96-h LC50 4.0
Estuarine &
marine
Longnose juvenile stat 28 19s 48-h LC50 1.6 Mayer (1987)
killifish
(Fundulus
similis)
Table 35 (continued)
Organism Size/age Stat/ Temperature Hardness pH Formulationc Parameter Concentration Reference
flowa (°C) (mg/litre)b (mg/litre)
Striped mullet juvenile stat 24 17s 48-h LC50 2.4
(Mugil
cephalus)
aStat = static conditions (water unchanged for the duration of the test); Flow = flow-through conditions (carbaryl concentration
in water continuously maintained).
bHardness = expressed as mg CaCO3/litre; s = salinity (%).
cFormulation: C = carbaryl; CT = Carbaryl technical (> 95%); C50 WP = Carbaryl 50% wettable powder; S = Sevin; S4 = Sevin-4-oil;
S50 WP = Sevin 50% wettable powder; XLR = Sevin (44% carbaryl); C49 = Carbaryl (49%). Where information on formulation is not given
the carbaryl formulation used was mostly carbaryl technical.
Exposure of C. punctatus to carbaryl at a concentration of
3 mg/litre for 48 h resulted in an increase in free fatty acids,
cholesterol, and lipase activity in the liver (Rao et al., 1985b).
However, the total lipid content was reduced.
The literature indicates that water temperature, hardness, and
pH may influence the toxicity of carbaryl, as well as the size of
the fish (Table 35).
In a study by Post & Schroeder (1971), water was supplied from
a well, classified as very hard and highly alkaline at temperatures
of 13.6-14.6 °C. Carbaryl was more toxic for cutthroat trout
weighing 0.37 g than for those weighing 1-2 g (96-h LC50 1.5 and
2.2 mg/litre, respectively) and more toxic for brook trout weighing
1.15 g than for those weighing 2.04 g (96-h LC50 1.2 and
2.1 mg/litre, respectively).
A comparative toxicity study on carbaryl and 1-naphthol, under
laboratory conditions showed that 1-naphthol was approximately 5
times more toxic than carbaryl for goldfish ( Carassius auratus)
and 2 times more toxic for killifish ( Fundulus heteroclitus) (Shea
& Berry, 1983).
Synergism of the effects of carbaryl and phenthoate on Channa
punctatus was found (Rao et al., 1985a,b). Carbaryl produced
potentiation of the effects of 2,4-D, n-butyl ester, dieldrin,
rotenone, pentachlorophenol, and arecoline on trout (Statham & Lech,
1975).
Application of carbaryl to a forest resulted in a significant
(15-34%) decrease in brain acetylcholinesterase activity in brook
trout from a nearby stream (Haines, 1981). No other effects were
observed.
7.2.2.2 Short-term and long-term toxicity
There is a limited data base on the effects of long-term
carbaryl exposure on fish. In one study, juvenile spot Leiostomus
xanthurus were exposed for five months to carbaryl (technical,
98%) at a level of 0.1 mg/litre in a flow-through system (Lowe,
1967). No carbaryl-related mortality was observed and there was no
effect on growth.
Carlson (1972) conducted the only full life-cycle study on fish
with carbaryl (80%), in which fathead minnows ( Pimephales promelas)
were exposed to five concentrations (0.008-0.68 mg/litre) for 9
months, beginning with the larvae. Survival of fatheads after 6
months at 0.68 mg/litre was lower than that in the controls. After 9
months at 0.68 mg/litre, the mean number of eggs produced per female
was reduced, the mean number eggs per spawning was also affected and
no hatching occurred. No other demonstrable effects were noted at
0.017, 0.062, and 0.21 mg/litre concentrations; thus, the maximum
acceptable toxicant concentration (MATC) for fathead minnows,
exposed to carbaryl in water, was between 0.21 and 0.68 mg/litre.
The lethal threshold concentration for 2-month-old minnows was
9 mg/litre.
Kaur & Toor (1977) exposed different stages of the embryo of
carp ( Cyprinus carpio) to carbaryl through the hatching stage.
There was 100% mortality of carp eggs and embryos at 2.5 mg/litre.
There appeared to be no effect of carbaryl on hatching at
0.01-0.75 mg/litre. However, there was decreased hatching at
1.0 mg/litre and deformed larvae (3.3%) with enlargement of the
pericardial sac and coiling of the posterior region of the embryo.
Thirty days exposure of Channa striatus to 10 or 20 mg
carbaryl/litre retarded oocyte production, with an increase in the
number of immature oocytes and a decrease in the number of mature
oocytes (Kulshrestha & Arora, 1984).
Statistically significant reductions in gonadotropic hormone in
the pituitary gland and plasma in Channa punctatus were observed
following exposure to carbaryl at a concentration of 1.66 mg/litre
(Ghosh et al., 1990). Gonadotropic hormone levels continued to
decrease with continued exposure, with a 30% decrease in the
pituitary gland and a 50% decrease in serum after 30 days of
exposure.
Exposure of the freshwater fish Puntius conchonius to carbaryl
at a concentration of 0.194 mg/litre for 15 days resulted in an
increase in the incidence of lesions in the gill and liver (Gill
et al., 1988). A higher level of exposure also resulted in lesions
in the kidney.
A 27-day exposure to 12.5 mg/litre led to a decrease in the
feeding and growth rate of the catfish ( Mystus rittatus)
(Arunachalam et al., 1980). Long-term toxicity of carbaryl for
fish in most natural surface waters will not occur, since exposure
would not be high enough or constant, because of the substance's
degradation.
7.2.3 Amphibians
The LC50s for bullfrog ( Rana tigrina) 0.1 g tadpoles with
24, 48, 72, or 96 h of exposure were determined to be 12.8, 8.2,
6.7, and 6.3 mg/litre, respectively (Marian et al., 1983). Growth
and feeding were decreased in a dose-dependent manner by doses
ranging from 0.5 to 5 mg/litre.
7.3 Terrestrial organisms
7.3.1 Worms
Carbaryl was classified as extremely toxic for earthworms
(LC50 = 9.1 µg/m2) by Roberts & Dorough (1984). The LC50 value
for the worm Eisenia fetida-Savigny was 14 µg/cm2 (Neuhauser
et al., 1985). The number of earthworms was reduced by 60% when
carbaryl was applied at 0.5 kg (1.12 lb) a.i. per hectare (Thompson,
1971).
Exposure of earthworms to carbaryl (1-8 mg/kg) significantly
increased the burrowing time, which was directly proportional to the
dose and time of exposure, because of the AChE inhibition in the
neural tissue (Gupta & Sundararaman, 1991).
7.3.2 Insects
Administration of carbaryl in sublethal doses produced death in
the early embryonal stage of the silkworm (Kuribayashi, 1981).
The alfalfa leaf-cutting bee ( Megachile pacifica), which is
very important in the pollination of alfalfa, has been reported to
be particularly tolerant to carbaryl (Lee & Brindley, 1974). Waller
(1969) also classified carbaryl as relatively non-toxic for the
alfalfa leaf-cutting bee. The carbaryl tolerance was related to sex
and age. The LD50 was similar for 1-day-old males and 1-and
4-day-old females (240, 245, and 262 µg/g, respectively). However,
four-day-old males were much more susceptible to carbaryl, and had
an LD50 of 51 µg/g. Guirgius & Brindley (1975, 1976) showed that
carbaryl toxicity in alfalfa leaf-cutting bees was controlled by the
activity of mixed function oxidase or microsomal enzymes. This
detoxification system varies with the age and sex of the bees and
results in significantly different carbaryl persistence which, in
turn, leads to differences in carbaryl toxicity. In the more
tolerant groups, carbaryl metabolites were rapidly conjugated and
moved to an aqueous fraction of the bee. Less tolerant insects
(4-day-old males) accumulated these metabolites with time,
indicating that the conjugation mechanisms had deteriorated with
age.
Carbaryl is known to be highly toxic for honey-bees. When
ingested, the LD50 was 0.18 µg/bee (Alvarez et al., 1970). The
contact LD50 (approximately 10-15 mg/kg) for adult bees is
approximately 1.3 µg (Stevenson et al., 1977; Stevenson, 1978).
The carbaryl residue content of bee bread was correlated with
the amount of residue found in the bees and occurred at higher
levels than in honey throughout the 56-day period (Winterlin &
Walker, 1973).
7.3.3 Birds
The toxicity of carbaryl for birds appears to be low
(Table 36). LD50s for six species of waterfowl and game birds were
all greater than 1000 mg/kg (Bart, 1979). There were some
exceptions; thus the red-winged blackbird has an LD50 of 56 mg/kg
(Schafer, 1972). In this study, 180 compounds were found to be toxic
for the red-winged blackbirds and the LD50s ranged between 0.24
and 100 mg/kg.
Food intake, body weight, and locomotor activity were monitored
in adult male bobwhites ( Colinus virginianus), given diets that
contained levels of carbaryl typical of normal exposure under
agricultural conditions in Kansas. No changes were observed in diets
containing 127 or 1235 mg carbaryl/kg (Robel et al., 1982).
Technical carbaryl fed to young Mallard ducks at dietary levels
of 10, 100, 1000, or 3000 mg/kg, revealed dose-correlated signs of
toxicity (reduced intake and/or body weight depression) at the 100,
1000, and 3000 mg/kg level (Fletcher & Leonard, 1986).
There was some tentative evidence that low dosages of carbaryl
may increase the susceptibility of bobwhites ( Colinus virginianus)
to the protozoan parasite Histomonas meleagridis, to which they are
usually resistant (Zeakes et al., 1981).
A study of the effects of carbaryl on forest birds was
conducted in southern New York; plots had been treated 3-4 weeks
previously with carbaryl at the normal rate of 1.1 kg/ha, and at 6
times the normal rate (6.6 kg/ha). In this study, carbaryl had
little, if any, effect on birds. Young birds gained weight normally,
adults continued nesting in the area, no changes were detected in
song frequency, and there was no evidence of birds leaving the area
to forage. This lack of detectable effects, despite the heavy dose
(6.6 kg/ha), indicates that carbaryl applied at the normal rate
(1.1 kg/ha) would have little if any adverse effect on birds (Bart,
1979).
The LC50 of carbaryl for mallard embryos, following field
applications, was determined by Hoffman & Albers (1984) to be
greater than 26.4 kg/ha or 118 µg/g egg. Carbaryl was estimated to
be relatively nontoxic compared with other pesticides.
Inhibition of the brain ChE activity of birds from forests
sprayed with carbaryl 1.13 (kg/ha) was found in 3 out of 12 species
studied (Zinkl et al., 1977) up to 5 days after application.
Table 36. Acute toxicity of carbaryl for birds
Species Age Parametera Concentration Reference
(mg/kg)
Japanese quail 7 days LD50 2290 Hudson et al.
Coturnix coturnix (1984)
japonica 2 months 5 day-LC50 > 10 000 Hill & Camardese
(1986)
Bobwhite quail 23 days 5 day-LC50 > 5000 Hill et al. (1975)
Colinus virginianus
California quail 10 months LD50 > 2000 Hudson et al.
Callipepla californica (1984)
Chukar Alectoris chukar 4 months LD50 1888
Sharp-tailed grouse LD50 < 1000
Tympanuchus
phasianellus
Pheasant 3-4 months LD50 707-> 2000
Phasianus colchicus 23 days LD50 > 5000 Hill et al. (1975)
Mallard 3 months LD50 > 2564 Hudson et al. (1984)
Anas platyrhynchos 24 days 5 day-LC50 > 5000 Hill et al. (1975)
Rock dove LD50 1000-3000 Hudson et al. (1984)
Columbia livia
Canada goose LD50 1790
Branta canadensis
Red-winged blackbird LD50 56 Schafer (1972)
Agelaius phoeniceus
a LD50 = single oral dose expressed as mg/kg body weight.
5 day-LC50 = 5-day dietary exposure (expressed as mg/kg feed) followed by
3 days on a "clean" diet.
7.3.4 Mammals
The effect of carbaryl on wild mice ( Clethrionomys glareolus
and Apodemus sylvaticus) in their natural environment was studied
by Krylov (1970). Carbaryl-containing bait, each consisting of 2 kg
grain treated with 50 g carbaryl in oil suspension, were distributed
twice (in March and June) over 10 ha of woods, at a rate of 1
bait/ha. An adjacent territory of 10 ha was used as a control.
Effects on mice, assessed 1´ months after the last application,
were: reduction of the population by 31.5% compared with the
control; changes in the reproduction system (e.g., decreased number
of embryos in utero, and of corpora lutea, reduced weight of
testicles), and increased weight of adrenal glands.
7.4 Effects on the population and ecosystem
The effects of carbaryl on terrestrial ecosystems have been
studied by Stegeman (1964), Barrett (1968), and Spain (1974).
A large-scale study was performed by Barrett in 1968. It was
designed to determine the effects of carbaryl on an intact ecosystem
in a field that was planted with millet ( Panicum ramosum). The
area was sprayed with a single application of 2.24 kg carbaryl/ha.
There was a highly significant decrease in litter decomposition in
the treated area, 3 weeks after spraying, which was probably because
of a reduction in microarthropods and other decomposers. After 5
weeks, there was a more than 95% reduction in the number of
arthropods and in the total biomass. Phytophagous insects were
severely affected, and predatory insects and spiders were less
affected. The number of species was also reduced, but all species
returned to control levels within 1-2 weeks, except Hemiptera and
Hymenoptera. Reproduction of cotton rats was delayed by 4 weeks.
However, the total mammal population was not affected, because of a
compensating increase in the population of house mice. Old field
mice did not seem to be affected.
The effects of carbaryl on forest soil mites and Collembola,
the two most numerous soil arthropods, were studied by Stegeman
(1964). Mites and Collembola are an important link in the
decomposition process of dead plants and animal matter. They also
are a natural means of decomposing the accumulating litter, and they
provide nutrients for already existing or future crops and fungi.
Application of carbaryl to a test plot in a red-pine plantation, at
doses of 11.2 and 56 kg/ha, reduced the arthropod population
proportional to the severity of the treatment. Neither mites nor
Collembola were totally exterminated by any treatment. The rate of
population increase of the mites, 4-5 months after treatment, was
directly proportional to the dose applied. Collembola were more
vulnerable to treatment than mites and did not recover as rapidly.
The effect of carbaryl at two application rates (0.11 and
1.13 g a.i. per m2) on mixed species population of the litter
fauna of a Corsican pine forest was studied by Spain (1974). He took
a quantitative sampling of the fauna at intervals of 11, 106, 209,
and 315 days after application to record the pattern and the time of
the recovery process (Table 37). The effect of carbaryl varied in
different populations. For Collembola, there was a marked reduction
at the two treatment levels. For Coccoidea and Symphyla, there was a
slight effect at the lower level, and a marked decrease at the
higher level. For Coleoptera, Diptera, Cryptostigmata, Mesostigmata,
and Prostigmata, the effect was small or insignificant. The recovery
process during the period of 315 days was not sufficient to reach
the status of the untreated population.
Tagatz et al. (1979) studied the effects of carbaryl on
animal communities that develop from planktonic larvae in aquaria
containing sand and estuary water. Samples collected after 10 weeks
of exposure were analysed. The numbers of animals and species were
significantly less at 11.1 and 103 µg carbaryl/litre (Table 38)
being decreased to nearly half (from 21 to 12). A carbaryl
concentration of 1.1 µg/litre did not produce significant effects,
except a decrease in the particularly sensitive amphiopod,
Coraphium acerusicum. Carbaryl might have caused changes in
biological interactions that affect the relative abundance of
species. The annelid Polydora ligni increased at a concentration
of 103 µg/litre, and there was a marked decrease in the number of
other annelids and nemerteas.
There have been a number of field studies evaluating the impact
of carbaryl applications on macroinvertebrate populations. In one
study in New York State, carbaryl was applied aerially at a rate of
1.3 kg/ha, in fuel oil with a paraffin oil sticker, and its effects
upon the aquatic fauna of two streams were studied (Burdick et al.,
1960). The data indicated that carbaryl (in oil suspension) was
toxic for mayflies (Ephemeroptera), stoneflies (Plecoptera) and
caddisflies (Trichoptera). Other groups of insects were less
affected and the application did not affect fish. Square-foot
samples, collected before, and shortly after, spraying, showed
reductions of from 50 to 97.2% in the weight of invertebrate
(standing crop) fish food. A progressive effect from upstream to
lower sections was correlated with increased 44-151 exposure time.
No exposure concentrations in water were documented. Owen (1965)
also reported a reduction of 54.2 to 62.8% in the standing crop of
aquatic insects following aerial spraying with carbaryl (80 WP)
(1.3 kg/ha). A control stream showed a 5.9% increase in the same
period.
Table 37. Geometric means for assessed taxa (individuals/m2), post-application sampling of carbaryl-treated plotsa
Sampling day (after treatment) Taxon Control plot High carbaryl Low carbaryl
Coccoidea 272.9 90.8 129.4
Coleoptera (imagines) 425.6 137.7 153.1
Coleoptera (immature) 54.0 47.3 41.4
Collembola 42 771.5 5033.4 9819.7
Diptera 350.9 244.2 243.7
11 Cryptostigmata 5707.3 3933.2 5863.5
Mesostigmata 7239.5 3688.9 5038.2
Prostigmata 1335.6 305.3 564.8
Aranese 238.7 222.7 155.0
Chilopoda 71.1 102.4 37.4
Symphyla 872.1 359.0 388.9
Coccoidea 847.8 157.0 162.9
Coleoptera (imagines) 41.9 379.1 170.7
Coleoptera (immature) 41.9 31.7b 0.0b
Collembola 18 185.1 1621.3 5205.9
Diptera 264.0 173.8 160.5
106 Cryptostigmata 5855.3 6237.9 6623.7
Mesostigmata 8610.3 846.4 595.2
Prostigmata 631.5 846.4 595.2
Aranese 360.6 224.9 253.2
Chilopoda 114.0 157.8 94.3
Symphyla 317.5 47.0b 223.4
Table 37 (continued)
Sampling day (after treatment) Taxon Control plot High carbaryl Low carbaryl
Coccoidea 358.3 181.0 141.0
Coleoptera (imagines) 520.0 86.6 232.6
Coleoptera (immature) 289.6 125.9 74.1
Collembola 41 198.7 3469.4 19 238.9
Diptera 449.9 28.2b 209.5
209 Cryptostigmata 16 298.4 11 184.8 12 014.8
Mesostigmata 16 150.0 7293.9 11 709.0
Prostigmata 2721.6 2407.2 3152.4
Aranese 264.7 108.3 220.2
Chilopoda 241.9 28.2b 150.5
Symphyla 357.3 78.3 174.4
Coccoidea 142.0 207.5 51.5
Coleoptera (imagines) 261.6 64.4 132.5
Coleoptera (immature) 37.0 88.8 23.1b
Collembola 40 139.5 4121.3 10 294.6
Diptera 533.9 291.2 173.1
315 Cryptostigmata 10 889.3 12 743.6 10 506.6
Mesostigmata 11 290.3 7762.7 14 912.0
Prostigmata 2995.0 1844.7 4765.7
Aranese 217.4 157.4 117.8
Chilopoda 159.5 83.4 88.8
Symphyla 349.6 85.4 158.8
aSource:Spain (1974).
b95% confidence limits for the parametric mean include zero.
Table 38. Animals and species, by phylum, collected from control aquaria and aquaria exposed to carbaryla
Control Carbaryl
1.1 µg/litre 11.1 µg/litre 103 µg/litre
Phylum Number Species Number Species Number Species Number Species
Mollusca 1691 3 1563 3 1340 3 1321 5
Arthropoda 380 7 339 8 336 2 269 2
Annelida 102 8 94 7 79 4 200 5
Nemerica 16 1 25 1 20 1 0 0
Coelenterata 3 1 5 1 0 0 0 0
Platyhelmintes 0 0 2 1 0 0 0 0
Echinodermata 0 0 0 0 1 1 0 0
All phyla 2192 20 2086 21 1776 11 1790 12
aFrom:Tagatz et al. (1979).
The effects on stream invertebrates of carbaryl, applied at a
rate of 840 g a.i./ha for spruce budworm suppression, was studied.
Benthos samples showed significant declines among Plecoptera,
Ephemeroptera, and Trichoptera. Plecoptera had not repopulated any
treated stream, 60 days after treatment (Courtemanch & Gibbs, 1980).
Gibbs et al. (1984) conducted a 42-month (1980-83) study on
the occurrence/persistence of carbaryl residues in pond water and
sediment as a result of an application of Sevin-4-oil (840 g
a.i./ha). The immediate and long-term effects on pond
macroinvertebrates and emerging aquatic insects were also evaluated.
A preliminary study by these investigators in 1977 and others
(Coutant, 1964) had shown that there were large increases in the
number of drift organisms, several days after aerial spraying with
carbaryl. Most drift was accompanied by dead Amphipoda,
Ephemeroptera, Plecoptera, and Trichoptera, and carbaryl residues
persisted longer than 30 days in pond sediment. The increase in the
number of dead organisms was accompanied by a reduction in the
standing crop of benthic macroinvertebrates. The most severe and
persistent impact was on Amphipoda with Hyallela azteca and
Crangonyx richmondensis reduced to almost 0/m2; C. richmondensis
failed to recolonize in one of the two treatment ponds, 42 months
after treatment. The numbers of immature Ephemeroptera and
Trichoptera were reduced immediately following spray application,
but this effect did not persist throughout the season or into the
following year. Immediate reduction in numbers of adult
Ephemeroptera and Trichoptera emerging from the ponds was also
found, but recovery of populations was observed. Numbers of immature
Odonata were also reduced following treatment and remained low the
following year. The Chironomidae populations did not appear to be
affected, either as immatures or emerging adults.
The effects of two consecutive years of spraying with
Sevin-4-oil on other aquatic systems appear similar to those
observed in areas treated once (Courtemanch & Gibbs, 1978; Trial,
1978,1979).
In simulated aquatic field studies, 1 mg carbaryl (WP)/litre
was applied to one outdoor concrete pond (4 x 5 x 1 m deep)
(Hanazato & Yasuno, 1987). All zooplankton (Cladocera included) and
Chaoborus larvae were killed. The zooplankton community recovered
rapidly and Cladocera reappeared only two days after application.
Since Chaoborus populations are predators of crustacean zooplankton,
their suppression may also have added to the recovery of increased
populations of Cladocerans. Carbaryl exposure also changed the
community from a rotifer-predominating zooplankton community to that
of Cladocera-predominating. Rotifers in this study were also highly
sensitive to carbaryl. The same phenomenon was observed again after
the second application of carbaryl. Subsequent to this study,
Hanazato & Yasuno (1989) applied carbaryl to simulated ponds in the
same way as in the previous study, but in the spring when the water
temperature was approximately 10 °C lower. Cladocerans never
recovered to the density level of the pre-treatment period. The
rapid recovery of Chaoborus seemed to interfere with the recovery of
Cladoceran populations after treatment. The authors suggested that
the different recovery patterns of the zooplankton community
resulted from different temperatures in the ponds. In another study,
Hanazato & Yasuno (1990a) examined Chaoborus density in relation to
the effects of carbaryl (0.1 and 0.5 mg/litre) on zooplankton
communities in ponds, where the abundance of Chaoborus larvae was
controlled. They concluded that Chaoborus density and/or temperature
may influence the recovery of the zooplankton community following
the effects of carbaryl. The recovery of a zooplankton community may
differ in different aquatic ecosystems (with different community
structures) and under different temperatures, even when the same
treatment is applied.
Hanazato & Yasuno (1990b) also studied the effect of the time
of application of carbaryl (0.5 mg/litre) on recovery patterns of
zooplankton communities in simulated ponds. The loss of carbaryl
from pond water was rapid. The concentration decreased to less than
1% of its initial value, three days after the first or second
application, and six days after the third application. This study
also showed that applications of carbaryl at different times induced
different zooplankton structures (and different recovery patterns),
and, various factors other than toxicity of carbaryl, such as
temperature, competitive interactions between zooplankters, and
trends of zooplankton populations may play important roles in
determining zooplankton community structure after chemical
application. Furthermore, the significance of predators in the
recovery process after chemical treatment was re-emphasized.
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
Reviews of toxicological aspects of carbaryl were prepared by
NIOSH (1976); US EPA (1977, 1980, 1982, 1984); USDHHS/USDL (1978);
US EPA (1980b); Mount & Oehme (1981a); Weston (1982); and Cranmer
(1986). Studies from the Soviet Union were reviewed by IRPTC (1982,
1989).
8.1 Single exposures
8.1.1 Oral toxicity
The oral LD50s for the rat are given in Table 39 and for
other mammals in Table 40. The values varied by about a factor of 4
depending on formulation, route of production, vehicle, and strain
of rat. Interspecies differences were found. Cats are the most
sensitive, with guinea-pigs, rats, mice, and rabbits showing more
resistance in that order. Pigs and monkeys seem to be less sensitive
(Carpenter et al., 1961; Gladenko & Malinin, 1970; Smalley, 1970).
Cattle are more sensitive than pigs. After a single oral
application of carbaryl in cattle, symptoms appeared at a dose level
of 25 mg/kg; 100 mg/kg was the minimum effective dose in pigs
(Gladenko & Malinin, 1970).
Mount & Oehme (1981b) observed that the lethality of carbaryl,
administered to sheep at doses ranging from 300 to 1000 mg/kg, was
highly correlated with concentrations in the brain (>1 mg/kg) and
liver (>3 mg/kg), and with inhibition of acetylcholinesterase
(greater than 50% inhibition).
8.1.2 Acute inhalation toxicity
Acute toxic effects following inhalation at different
concentrations are presented in Table 41.
8.1.3 Dermal toxicity
A dose of 2500 mg/kg, applied as a 40% aqueous suspension of
50% wettable powder, killed 1 out of 4 rabbits (Carpenter et al.,
1961). When 99% technical carbaryl was applied to male and female
rabbits, the LD50 was >2000 mg/kg (Bushy Run, 1983b). In rats,
the LD50 is thought to be >4000 mg/kg (Yakim, 1965; Gaines,
1969).
Table 39. Acute oral LD50s for the rat
Strain (sex) Weight Formulation of Vehicle LD50(mg/kg Symptoms References
(g) carbaryl body weight)
CF-N technical 0.25% agar
(male) 90-120 510 (360-650) - Carpenter et al.
(female) 90-120 610 (490-750) - (1961)
Sherman 850 (600 LD min) Gaines (1969)
(male)
(female) 500 (100 LD min)
Inbred 721 (653-789) Yakim (1965)
Inbred 120-200 sunflower oil 515 Rybakova (1966)
Sprague Dawley 203-246 technical carboxymethyl-cellulose 300 Hamada (1990)
(male, female) in water
Sprague Dawley 112-173 technical 0.25% methyl-cellulose 685 (612-767) death, 4-24 h Field (1980b)
(male, female)
Sprague Dawley 100-160 95% technical 0.25% methyl-cellulose 225 (202-321) death, 2-24 h Field (1980a)
(male, female)
Table 39 (continued)
Strain (sex) Weight Formulation of Vehicle LD50(mg/kg Symptoms References
(g) carbaryl body weight)
Sprague Dawley 200-264 40.38% water tremor, prostration, Hazleton Lab.
(male) 750 (467-1202) laboured respiration, American Inc.
salivation 10 February 1982
(female) 527 (257-977) death, 4-3 days
Harlem Sevin XLR not diluted death, 0.5 h-3 days Kuhn (1991a)
Sprague Dawley plus 44%
(male) 229-286 867 (562-1336)
(female) 182-219 575 (459-721)
Harlem Sevin-4-oil not diluted 658 (456-979) 3 h-3 days Kuhn (1991b)
Sprague Dawley 47% (w/w)
(male) 229-286 963 (802-1160)
(female) 182-219 473 (364-620)
Hilltop Wistar 200-250 98% technical 0.25% methyl-cellulose Bushy Run
(male) 283 death, 1.5-24 h (1983b)
(female) 246
Hilltop Wistar 204-237 80% sprayable water
(male) 406 Bushy Run
(female) 203 death, 1 min-24 h (1983c)
Table 40. Acute oral toxicity for mammals other than rats
Species Number of animals Toxicitya Reference
(mg/kg body weight)
Mouse 6 per dose 363 (294-431) Yakim (1965)
Mouse (female) 80 white 437 ± 70 Rybakova (1966)
Mouse 1310 white 206 (175-480) Bukin (1965)
Guinea-pig 5 per dose 280 Carpenter et al. (1961)
Rabbit 4 per dose 710
Rabbit 56 700 (LD100) Bukin (1965)
Cat (female) 3 250 (LD100) Carpenter et al. (1961)
Cat no data 150 Yakim (1965)
Swine 3 per dose 800-1000 Gladenko & Malinin
(1970)
Swine 1 per dose 1500-2000 Smalley (1970)
Monkey no data > 1000 FAO/WHO (1970)
Duckling 106 500 (LD15) Bukin & Filatov (1965)
6000 (LD100)
Table 40 (continued)
Species Number of animals Toxicitya Reference
(mg/kg body weight)
Chick 48 250 (LD33)
1000 (LD100)
Hen 12 > 1000 (no death)
Hen 12 > 3000 Bukin (1965)
aLD50, unless otherwise stated.
Table 41. Inhalation toxicity single exposure
Species Concentration Effects observed References
Guinea-pig 6 390 mg 50% wettable powder/m3 nasal and ocular irritation after 14 days - Carpenter et al.
(average particle size 15 µm) 4 h haemorrhage areas in the lungs (1961)
Guinea-pig 6 230 mg carbaryl 85S/m3 slight weight decrease; recovered by day 14
(average particle size 5 µm) 4 h
Dog 75 mg carbaryl 85S/m3 5 h typical symptoms for ChE inhibition
Cat 3 groups 82 mg/m3 dust 6 h tremor salivation, muscle fibrillation decreased Yakim (1967, 1968)
of 4 animals ChEA by 39-55% serum; 53-71% red blood
cells; normalization after 72 h
37 mg/m3 dust 6 h decreased ChEA by 23% in serum and 41% in
red blood cells; recovery after 48 h
20 mg/m3 dust 6 h decreased ChEA by 11-27% in serum and
15.28% in red blood cells; recovery after 24 h
Rat 20-23 mg/m3 dust no effect Weil & Carpenter
98% particles (1974)
less than 1.0 µm diameter
Rat 1800 mg/m3 water aerosols lacrimation, tremor with 1.5 h of exposure Myers et al. (1975)
Rat Wistar Albino Aerosols Sevin XLR 44% 792 mg 1/5 females died, tremor, ataxia, increased Faït (1984)
male 5 a.i./m3 per 4 h (the highest respiratory rate; recovery period 6 days
female 5 attainable concentration)
particle size 3.6 ± 2.64 µm
8.1.4 Other routes of exposure
Other routes of exposure are presented in Table 42.
Table 42. Toxicity following parenteral application
Species Weight Vehicle Route of LD50 References
(g) administration mg/kg body
weight)
Rat 10-107 propylene intravenous 18 Mellon Institute
female glycol (1958)
Rat 92-126 polyethylene intravenous 24
glycol 400 intravenous (17-33)
Rat 90-120 95% ethyl intravenous 33
alcohol (26-41)
Rabbit 1686-3544 0.25% agar intraperitoneal 223
(122-407)
Rat 90-120 in lard subcutaneous 1410
Leghorn 10-11 days in alantoic 3.44 mg Tós-Luty et al.
chick old cavity per embryo (1973)
embryos
(250)
8.2 Skin and eye irritation, sensitization
8.2.1 Skin and eye irritation
The results of several studies on skin irritation from carbaryl
were negative (Carpenter et al., 1961; Yakim, 1965). Transient
erythema was noted after an application of 0.5 ml 43.4% carbaryl on
occluded rabbit skin (Bushy Run, 1983a).
Carbaryl is a weak eye irritant (Table 43).
Table 43. Eye irritation
Species Number Formulation Effects observed Reference
Rabbit 5 technical grade mild injury in one Carpenter et al.
10% suspension of five eyes (1961)
in propylene
glycol
25% aqueous no injury
suspension
50 mg dust spots of
corneal-necrosis
Rabbit 6 0.1 ml (90 mg) conjunctival irritation Bushy Run (1983b)
New 99% technical after 2 days recovery
Zealand product
White
0.1 ml transient iritis in Bushy Run
43.4% carbaryl 2 of 6, conjunctival (1983a)
irritation in 6 of 6.
Recovery in 3 days
8.2.2 Sensitization
The sensitization response following topical application of
carbaryl was studied by Myers & Christopher (1987). The induction
which consisted of an application of carbaryl to covered skin, once
a week during 3 weeks, was followed by a 2-week incubation period. A
single challenge dose of 50% (w/v) technical carbaryl in 0.25%
aqueous methyl cellulose solution (0.1-0.3 ml) was administered.
Carbaryl did not produce a positive response in any of the
guinea-pigs tested.
Four out of 16, male, albino guinea-pigs were treated with 8
intravenous injections (3 per week) of 0.1 ml of a 0.1% dispersion
of carbaryl in 3.3% propylene glycol. After a 3-week incubation
period, a challenge dose was given, but did not cause sensitization
(Carpenter et al., 1961). In a more recent study, a similar
procedure, but with 9 doses and a 2-week incubation period, was
used. Although there was some skin irritation, it was considered
that carbaryl had little or no sensitizing potential (Bushy Run,
1983a).
8.3 Short- and long-term oral exposure
Several short- and long-term feeding studies with carbaryl have
been reported (Carpenter et al., 1961; Rybakova, 1967; Orlova &
Zhalbe, 1968; Gladenko & Malinin, 1970; Dikshith et al., 1976;
Hamada, 1991a). Results are shown in Table 44. Doses that do not
show any effects are 200 mg/kg diet equal to 7.9 mg/kg body weight
in rats, and 100 mg/kg diet for mice, and 1.8 mg/kg body weight for
dogs (approximately 100 mg/kg diet).
The cumulation coefficient (LD50 for 3-month exposure/LD50
single application) was 18 (Kassin, 1968), demonstrating a very low
cumulative potential for carbaryl.
8.4 Short- and long-term inhalation toxicity
Short-term and long-term inhalation toxicity data are given in
Table 45.
8.5 Reproduction and developmental toxicity
The reproduction and developmental toxicity of carbaryl has
been studied in many vertebrate species using a wide variety of
study designs.
The data have shown that carbaryl can affect reproduction
(Table 46) and embryo/fetal development (Table 47 and 48) in a
number of species. The relevance of these studies for risk
assessment is influenced by several factors related to experimental
design, dose levels, and the types of effects noted. Shortcomings of
the studies included small sample size; inappropriate dose
selection; the variable degree of maternal toxicity (ranging from no
effect to lethality); and a lack of historical data for some
species. The following sections have been arranged according to
end-point (reproduction and developmental toxicity) and species of
animals studied (mammalian, non-mammalian). Studies that are of
dubious value for risk assessment are listed at the end of the table
sections, and are not discussed in the text. The reasons for such an
evaluation are given below the references to the individual papers.
Table 44. Short- and long-term feeding and oral studies
Species Sex Number Dosage (mg/kg diet Period of Effects observed References
of or mg/kg body weight) exposure
animals
Mouse male 48 100, 400 mg/kg diet 80 weeks no changes in survival rate, pathology, and tumour FAO/WHO (1965)
and incidence
female
Mouse male 10/sex 100, 1000, 7000 mg/kg 53 weeks at 7000 mg/kg diet: decreased survival, body Hamada (1991b)
and per group diet weight gain and erythrocyte count; increased liver
female weight and decreased ovary weight; increased
frequency and severity of chronic nephropathy in
females; cholinesterase (plasma, RBC, and brain)
depressed at 100 and 7000 mg/kg; NOEL
100 mg/kg diet
Rat male 2x10 1500 (58.5 mg/kg 96 days no changes Carpenter et al.
body weight) (1961)
female 2x10 1500 (58.5 mg/kg 96 days kidney weights significantly increased
body weight)
male 2x10 2250 (87.5 mg/kg 96 days increase in liver weight as a % of body weight,
body weight) diffuse cloudy swelling in the kidney tubules in
4 animals (male and female)
female 2x10 2250 (87.5 mg/kg 96 days decrease of body weight, increase in kidney weight
body weight)
Table 44 (continued)
Species Sex Number Dosage (mg/kg diet Period of Effects observed References
of or mg/kg body weight) exposure
animals
Rat male 4x40 50, 100, 200 (2, 4, 2 years no changes Carpenter et al.
CF-N and 7.9 mg/kg body weight) (1961)
female
Rat male 2x40 400 (15.6 mg/kg 2 years weight depression in male, cloudy swelling of the Carpenter et al.
CF-N and body weight) hepatic cords, principally located around the (1961)
female central veins in both sexes; transitory diffuse
cloudy swelling of the epithelial lining of the
primarily convoluted proximal, and loop tubules
Rat 75, 150, 300 mg/kg 3 months cytoplasmatic vacuolization in the proximal tubules FAO/WHO (1970)
body weight
Rat male 4x48 7, 14, 70 mg/kg weight depression at all dose levels; at 70 mg/kg, Rybakova (1967)
and body weight slight morphological liver changes; cloudy swelling
female in convoluted tubules; decreased motility of
spermatocytes at all dose levels, more pronounced
at 70 mg/kg; oedema in the interstitial tissue;
desquamation of spermatogenic epithelium;
destruction of parenchyma; decreased production of
spermatocytes was found at 14 and 70 mg/kg dose
levels; increased estral cycle; increased hypophysis
gonadotropic function; decreased ascorbic acid
contents; cell proliferation, hypertrophy, increased
lipid content in suprarenal glands; decreased thyroid
function; augmentation of follicles, colloid retention,
thickness of follicular epithelium
Table 44 (continued)
Species Sex Number Dosage (mg/kg diet Period of Effects observed References
of or mg/kg body weight) exposure
animals
Rat male 80-90/ 250, 1500, 7500 mg/kg 52 weeks body weight and food consumption lower at middle Hamada (1991c)
Sprague female group diet dose; significantly increased total cholesterol;
Dawley increase in liver and kidney weight; NOEL
250 mg/kg diet
Rat male 876 2, 5, 15 mg/kg 1 year changes in sex glands function at 15 and 5 mg/kg Orlova & Zhalbe
and body weight dose levels; enzymatic activity, spermatozoids and (1968)
female the estral cycle; reduced fecundity; no effect at
2 mg/kg dose level
Rat male total 200 mg/kg 90 days decreased AChE in blood with 33.8%; no Dikshith et al.
local 28 body weight, histological changes (1976)
strain 3 x per week
in peanut oil
Dog male total 0.45, 1.8, 1 year diffuse cloudy swelling of proximal convoluted and Carpenter et al.
Basenji- and 14 7.2 mg/kg body loop tubules of kidney; local sudanophilic granules (1961)
cocker female weight in capsules, in the glomeruli at 400 mg/kg level; the same in
hybrids 5 days/week, to control dogs to a lesser extent; transient hind leg
approximate levels weakness in 1 female after 189th dose at
of 24, 95, 414 mg/kg 0.45 mg/kg body weight
dry diet
Beagle male 24+ 125, 400, 1250 mg/kg 1 year at 400 and 1250 mg/kg diet, decreased AChE in Hamada (1987)
dogs and 24 diet plasma, red blood cells, and in brain; increase in
female leucocyte count and segmented nitrophil count;
increase in liver weight in male; no effect level
125 mg/kg diet
Table 44 (continued)
Species Sex Number Dosage (mg/kg diet Period of Effects observed References
of or mg/kg body weight) exposure
animals
Beagle male 24 0, 20, 45, 125 mg/kg 5 weeks significant inhibition of cholinesterase activity Hamada (1991a)
dogs female 24 diet in plasma at week 2 for males dosed 20 and
125 mg/kg
Monkey 150, 300, 600 mg/kg 38 weeks kidney alterations similar to those found in rats FAO/WHO (1970)
body weight
Swine male 3 150 mg/kg body weight 72-83 progressive myasthenia, incoordination ataxia, Smalley et al.
female 3 in diet days intentional tremor, chronic muscular contraction, (1969)
until terminal paraplegia and prostration;
death myodegeneration
Swine 5 and 10 mg/kg 147-176 no changes Gladenko & Malinin
body weight days (1970)
Cattle 1 and 4 mg/kg 148 days decreased Hb with 20%, and erythrocytes with
(young) body weight 30% occasionally
Table 45. Short- and long-term inhalation toxicity
Species Number of Concentration of carbaryl Days of Effects References
animals exposure
Cat 4 0.06 mg/litre 30 typical cholinergic symptoms, Yakim (1968)
inhibition of ChE plasma 31-40%,
erythrocytes 40-59%
Cat 4 0.03-0.04 mg/litre 30 reaction time increased
Cat 4 0.016 mg/litre 120 no symptoms, fluctuation in
plasma ChE inhibition around 18%
Rat no data 10 mg/m3 dust (85% 90 no mortality no gross visible injury Carpenter et al.
suspension) 7 h/day inhalation (1961)
5 days per week periods
Table 46. Reproduction studies
Species Treatment End-point(s) Reference
(number)
Mouse
Swiss Webster males 30-40 g 0, 8.5, 17, 34 mg/kg body weight weight and uptake of testosterone: Thomas et al. (1974)
(10 + animals/group) orally (5 days) testes and accessory glands
Swiss Webster males 30-40 g 0, 34, 68 mg/kg body weight biotransformation of Dieringer & Thomas
(5 + animals/group) orally (5 days) testosterone-1,2-3H (1974)
C57Bl/6 x C3H 6-8 weeks of 0, 12, 25, 50...800 mg/kg body sperm morphology, testes weight Osterloh et al. (1983)
age (4 animals/group) weight per day 5 days i.p. on day 35 [route of
administration]
Rat
Osborne-Mendel 0, 2000, 5000, 10 000 mg/kg diet fertility, litter size, and viability Collins et al. (1971)
(20 females and 1 male per (3 generations)
group)
Wistar 0, 7, 25, 100, 200 mg/kg body fertility, litter size, and viability Weil et al. (1973)
(13-21 animals/group) weight per day in the diet
0, 3, 7, 25, 100 mg/kg body
weight per day orally
(3 generations)
Male and female 0, 1, 5, 10, 20, 40, 50 mg/kg general toxicity, serum protein, Vashakidze (1975)
body weight per day orally numbers of male reproductive
(1 month) cells, sperm viability, testicular
histology, litter viability
Table 46 (continued)
Species Treatment End-point(s) Reference
(number)
Wistar males 0, 12.5, 25.0, 250.0 mg/kg body sperm morphology Luca & Balan (1987)
(36 animals per group) weight per day
Rats
Male and female 0, 50, 100, 300 mg/kg body fertility indices Vashakidze (1966)
weight per day orally 2 weeks to [insufficient data]
3 months
Male and female 0, 2, 5, 15 mg/kg body weight per fertility indices Orlova & Zhalbe
(total of 876 animals) day orally 12 months for F° (1968); Zhalbe et al.
and 6 months for the F1 (1968) [insufficient
data]
Male and female 0, 2, 5 mg/kg body weight per day fertility indices Shtenberg & Ozhovan
for 6 months (follow-up to Orlova (1971) [insufficient
& Zhable) 2 through 5 generations data]
Male and female 0, 2, 5, 15 mg/kg body weight per fertility indices Shtenberg et al.
day for 12 months (1973) [insufficient
data]
Gerbil
(32-80 animals per group) 0, 2000, 4000, 6000, and fertility, litter size, and viability Collins et al. (1971)
10 000 mg/kg body weight diet
(3 generations)
Table 47. Developmental toxicity studies, mammalian
Species Treatment End-point(s) Reference
(number)
Mouse
CF-1 0, 100, 150 mg/kg body weight fetal examination Murray et al. (1979)
(23-44 in a group) per day orally or 5660 mg/kg diet.
Gestation day 6-15
Swiss albino 0, 100, 150, or 200 mg/kg body fetal examination Mathur & Bhatnagar
(10 per group) weight per day orally. Gestation (1991)
days 8, 12, or 6-15
Charles River 0, 10, 20 mg/kg body weight. fetal examination Benson et al. (1967)
(8 per group) Gestation day 6-parturition [doses too low]
CD-1 100 mg/kg body weight per day postnatal viability Chernoff & Kavlock
(23 animals) orally. Gestation day 8-12 (1982) [teratology
screen test]
CD-1 200 mg/kg body weight per day postnatal viability Kavlock et al. (1987)
(30 animals) orally. Gestation day 8-12 [teratology screen
test]
Rat
Harlan Wistar 0, 20, 100, 500 mg/kg diet. fetal examination Weil & Carpenter
(6 per group) Gestation days 1-7, 5-15, 1-21 (1965); Weil et al.
(1972)
Wistar 0, 200, 350 mg/kg body weight orally fetal examination Golbs et al. (1974)
(10 per group) 40 mg/kg ip. Variety of gestation days.
Table 47 (continued)
Species Treatment End-point(s) Reference
(number)
Sprague-Dawley 0, 1, 10, 100 mg/kg body weight fetal examination Lechner &
(6 or 7 per group) orally. 3 months before and during Abdel-Rahman
gestation (1984)
Sprague-Dawley 0, 20, 37.5 mg/kg body weight fetal examination Hart (1972)
(22 or 23 per group) per day. Gestation day 6-15 [doses too low]
Rats unknown dose (1/50 LD50). fetal examination Dinerman et al.
Gestation days 9, 11, or 13 (1970) [insufficient
data]
Rats 0, 10.6, 106 mg/kg body weight fetal examination Shtenberg et al.
per day. Gestation day 1-20 (1973) [insufficient
data]
Guinea-pigs
HRA/HART 0, 50, 100, 200 mg/kg body fetal examination Weil et al. (1973)
(9-4 per group) weight per day orally. 0, 100,
200, 300 mg/kg body weight per
day in the diet
Coulston strain 300 mg/kg body weight per day. fetal examination Robens (1969)
(26 gestation day treatment Gestation day 11-20 and a variety [insufficient data]
11-20; other treatments of other treatment periods within
unknown) this time
Table 47 (continued)
Species Treatment End-point(s) Reference
(number)
Rabbit
New Zealand White 0, 150, 200 mg/kg body weight fetal examination Murray et al. (1979)
(15-20 per group) per day orally. Gestation day 6-18
New Zealand White 0, 10, 30 mg/kg body weight per fetal examination Shaffer & Levy
(9-12 per group) day. Gestation day 9-16 (1968) [doses too
low]
New Zealand White 0, 50, 100, 200 mg/kg body fetal examination Robens (1969) [small
(4-9 per group) weight per day orally. Gestation number of animals]
day 5-15
Dog
Beagle 0, 3.125, 6.25, 12.5, 25, examination of pups Smalley et al. (1968)
(6-13 per group) 50 mg/kg per day in the diet
throughout gestation
Beagle 0, 2.0, 5.0, 12.5 mg/kg per day in examination of pups Imming et al. (1969)
(7-9 per group) the diet throughout gestation
Pig
Hormel-Hanford 0, 4, 8, 16 mg/kg in the diet 20 examination of fetuses (I) Earl et al. (1973)
(5-16 per group) days before/7 days after breeding or piglets after birth (II)
throughout gestation
Table 47 (continued)
Species Treatment End-point(s) Reference
(number)
Pig (30) up to 30 mg/kg body weight per ? Smalley (1968)
day [insufficient data]
Monkey
Rhesus 0, 2, 20 mg/kg body weight per examination of gestational Dougherty et al.
(4-6 per group) day orally throughout gestation course and new born (1971)
Rhesus 0, 20, 32 mg/kg body weight per examination of gestational Coulston et al. (1974)
(15-16 group) day orally. Gestation day 20-38 course and new born
Sheep
(25 and 26 per group) 159, 297.5 mg/kg diet examination of lambs Panciera (1967)
after birth [significance of
defects impossible to
assess]
Hamster
Golden Syrian 125 mg/kg body weight per day fetal examination Robens (1969)
(6 or 8 per group) on gestation day 6-8; 250 mg/kg [number tested too
body weight per day on gestation small]
day 7 or 8
Table 48. Non-mammalian studies
Species Dosage End-points Reference
Fish
Medaka 0.5, 1.0, 2.5, 5.0, 10.0, and embryonic development Solomon & Weis (1979)
(Oryzias latipes) 20.0 mg/litre, 4-cell through
10 eggs per group blastula
Amphibian
Xenopus laevis 0.1, 1.0, 10.0 mg/litre; embryos to embryonic development, Elliott-Feeley & Armstrong
10-12 per group hatching or tadpoles for 24 h posthatching activity (1982)
Birds
Chicken 0, 0.01, 0.1, 1.0, 10.0 mg/kg embryonic development Olefir & Vinogradova (1968)
4-6 per group body weight
White Leghorn chicken 0, 250, 500 mg/kg diet to pullets egg production, embryonic Lillie (1972)
20 per group for 36 weeks and hatchlings for development, hatchability,
4 weeks posthatching development
White Leghorn chicken 0, 1.0, 2.5, 5.0, and 10.0 mg/egg embryonic development Swartz (1981)
38-40 per group embryonic development injected
into yolk sac after fertilization,
prior to incubation for 5 or 12 days
White Leghorn chicken 10 mg/egg after fertilization prior primordial germ cell Swartz (1985)
8 per group to incubation migration
White Leghorn chicken 1.0, 0.3 mg/egg and lower examination of embryos Eto et al. (1980)
[insufficient data]
Table 48 (continued)
Species Dosage End-points Reference
720 eggs 0, 1, 2, and 4 mg/egg examination of hatchlings Ghadiri & Greenwood (1966)
[insufficient data]
Duck and chicken 10-1000 µg/egg 0, 4, 7, 10, and examination of embryos or Khera (1966)
13 day eggs hatchlings [insufficient data]
Chicken - examination of embryos Dinerman et al. (1970)
[insufficient data]
Quail
Coturnix coturnix 0, 50, 150, 300, 600, 900, growth, reproduction, Bursian & Edens (1977)
japonica 1200 mg/kg diet from hatching post-hatching viability
10 per group through 14 weeks
Colinus virginianus 0, 300, 1000, 3000 mg/kg diet for reproduction, post-hatching Fletcher & Leonard (1986a)
36 per group 22 weeks viability, gross pathology
Duck
Anas platyrhyncos 0, 300, 1000, 3000 mg/kg diet for reproduction, post-hatching Fletcher & Leonard(1986b)
36 per group 22 weeks viability, gross pathology
10-1000 µg/egg 0, 4, 7, 10, and examination of embryos or Khera (1966)
13 days eggs hatchlings [insufficient data]
8.5.1 Mammalian reproductive toxicity studies
8.5.1.1 Mouse
Studies on the effects of carbaryl (8.5-34 mg/kg per day),
given orally for 5 days, to mature male Swiss Webster mice,
indicated no effects on the weights of testes and accessory sex
glands or uptake of C14-labelled testosterone by the prostate
gland, as measured on the day after treatment (Thomas et al.,
1974). These workers also examined the biotransformation of
testosterone-1,2-3H in animals given 34 or 68 mg/kg per day,
orally, for 5 days. They found a significant decrease in
androstenedione synthesis in the high-dose group, indicating
increased hepatic androgen hydroxylase activity (Dieringer & Thomas,
1974).
8.5.1.2 Rat
Collins et al. (1971) studied the effects of carbaryl given
in the diet (0, 2000, 5000, and 10 000 mg/kg) to Osborn-Mendel rats
over 3 generations. The doses actually administered could only be
estimated since there were no measurements of food intake. On the
basis of a 15 g/day food intake and a 235 g rat, it can be estimated
that animals received of the order of 125, 250, or 500 mg/kg per
day. It should be noted, however, that the food intake of lactating
rats greatly increases, so these figures may considerably
under-estimate carbaryl exposure during this critical time period.
The author used the number of animals mated rather than the number
giving birth as the number for litter size and viability, therefore
their calculations are overestimates. Nevertheless, the data do show
impaired fertility in the high-dose group (which also exhibited
growth rate reduction) as well as reduced postnatal survival. The
number of live-born offspring and growth rate were reduced in the
5000 and 10 000 mg/kg diet groups.
A three-generation study on Wistar rats was carried out by Weil
et al. (1973) in which animals were given 0, 7, 25, 100, or
200 mg/kg per day in the diet or 0, 3, 7, 25, or 100 mg/kg per day
orally. Maternal toxicity was seen in the dietary study at 200 mg/kg
per day (decreased weight) and in the gavage study at 100 mg/kg per
day (decreased weight and mortality). Postnatal toxicity was noted
in the 100 mg gavage group (reduced litter size and viability), but
not in lower dose groups. The dietary study indicated fewer effects
in the maternal animals with only an initial loss in weight. No
perinatal effects were noted.
Vashakidze (1975) exposed male and female rats (number and
strain unspecified) to 0, 1, 5, 10, 20, 40, and 50 mg carbaryl/kg,
orally, for 1 month. Dose-related changes were noted in serum
albumin (decrease), globulins (increase), ChE and AChE (decrease),
aspartate transaminase (increase), and alanine transaminase
(decrease). Reductions in stem cells as well as spermatozoa were
noted at doses of 5 mg/kg or more. Adverse litter effects were seen
in treated females. These effects included increased embryo/fetal
death, decreased implantations, and prolonged estrus cycle.
Luca & Balan (1987) administered carbaryl-beta-naphthol to
Wistar rats in the diet for up to 18 months. The treated groups
showed an increase in sperm shape abnormalities, though there were
no clear dose- or time-relationships with effects.
8.5.1.3 Gerbil
Collins et al. (1971) published the results of a 3-generation
reproduction study in which animals were exposed to 0, 2000, 4000,
6000, or 10 000 mg/kg diet. Since all postnatal calculations used
the number of animals mated rather than the number giving birth, the
authors' data must be recalculated. When this is done, the magnitude
of the effects reported is reduced. Adverse effects on various
reproductive parameters are nevertheless seen, though the effects
are not clearly related to dose levels in the 2000-6000 mg/kg diet
groups. These data are difficult to interpret given the lack of
information on maternal effects at doses other than the highest,
where mortality was observed.
8.5.2 Mammalian developmental toxicity studies
8.5.2.1 Mouse
Murray et al. (1979) administered 100 or 150 mg carbaryl/kg
per day, by gavage, or 5660 mg/kg diet (calculated to be 1166 mg/kg
per day) to CF-1 mice on gestation days (g.d.) 6-15. Maternal
toxicity was noted in the 150-mg group (ataxia, lethality). Litter
effects were noted in the 150 mg/kg per day group, where an increase
in entirely resorbed litters was seen, and in the dietary group
where there was decreased fetal weight.
Pregnant mice were given 0, 100, 150, or 200 mg carbaryl/kg per
day, orally, on gestation days 8, 12, or 6-15, and fetuses were
examined at term (Mathur & Bhatnagar, 1991). Maternal death was
noted at the high dose in the group receiving carbaryl on gestation
days 6-15. Fetal weight reductions were seen at the high-dose levels
in all groups, as was reduced ossification, open eyelids, and
enlarged renal pelvis. These effects may be indicative of fetal
growth retardation.
8.5.2.2 Rat
Carbaryl was administered to Harlan Wistar rats at 0, 20, 100,
or 500 mg/kg per day, orally, on gestation days 1-7, 5-15, or 1-21
(Weil & Carpenter, 1965; Weil et al., 1972). Maternal toxicity was
evident as was reduced weight gain in the high-dose groups receiving
the chemical on gestation days 5-15 or 1-2. No adverse fetal effects
were seen.
Golbs et al.(1975) treated Wistar rats with 0, 200, or 350 mg
carbaryl/kg, orally, or 40 mg/kg ip on a variety of single or
multiple gestation days. Reductions in fetal weight were seen in
some groups. No other effects were noted.
Sprague-Dawley rats were treated orally with 0, 1, 10, or
100 mg carbaryl/kg per day, three months before and during
gestation, and litters were examined at term (Lechner &
Abdel-Rahman, 1984). Maternal weight gain was significantly less in
the 100 mg/kg group than in controls. No compound-related effects on
fetuses were noted.
8.5.2.3 Guinea-pig
Weil et al. (1973) administered carbaryl to guinea-pigs at
dose levels of 0, 100, 200, or 300 mg/kg per day in the diet, or 0,
50, 100, or 200 mg/kg per day, orally. These dose levels were
determined to be maximum non-maternally toxic doses in preliminary
studies by the authors, though the 200 mg/kg dose did reduce
maternal weight gain. No significant adverse embryo/fetal effects
were seen in any treatment groups.
8.5.2.4 Rabbit
Murray et al. (1979) tested the effects of carbaryl on New
Zealand White rabbits during gestation. Dams had diarrhoea at the
high dose of 200 mg/kg per day and animals at this dose level as
well as at the lower dose level (150 mg/kg per day) gained less
weight during gestation than controls. A significant increase in
umbilical hernia was noted in fetuses at the 200 mg/kg per day
group.
8.5.2.5 Dog
Smalley et al. (1968) administered 3.125, 6.25, 12.5, 25, or
50 mg carbaryl/kg in the diet, throughout gestation, to beagle dogs.
Maternal toxicity was noted at all dose levels. This toxicity was
described by the authors as dystocia and symptoms included delayed
delivery, anorexia, and restlessness. A variety of birth defects
were found at doses of 6.25 mg/kg or more. The defects included
ectopic intestines, brachygnathia, acaudia, polydactyly, and
intestinal agenesis.
Another study was carried out by Imming et al. (1969) on the
beagle dog. These workers used 0, 2.0, 5.0 or 12.5 mg/kg per day,
orally, throughout gestation. As in the Smalley (1968) study,
treated females exhibited toxicity during labour and single deaths
were recorded at this time in all treated groups. Birth defects
including umbilical hernia and gastrointestinal defects were seen at
the 5.0 and 12.5 mg dose levels only.
8.5.2.6 Pig
Two studies on pigs were carried out by Earl et al. (1973).
In the first study, fetal pigs were examined from dams given 0, 4,
8, or 16 mg carbaryl/kg per day in the diet; in the second, dams
were allowed to farrow after receiving 0, 16, or 32 mg/kg per day in
the diet. Animals were exposed throughout most or all of gestation.
Effects noted were not consistent across the studies and increased
prenatal lethality seen in the first was not noted in the second,
even at a higher dose level. A small number of malformations were
noted, but no characteristic dose-related pattern was evident.
8.5.2.7 Monkey
Dougherty et al. (1971) exposed a small number of female
Rhesus monkeys to 0, 2, or 20 mg carbaryl/kg, throughout gestation.
Of the 8 pregnant, treated females, 5 were reported as having
aborted as opposed only 1 out of 5 in the controls. The Coulston
et al. (1974) study included a larger number of animals per dose,
an additional lower dose level (0.2 mg/kg per day), and a shorter
dosage period (days 20-38 of gestation). No adverse effects were
noted in the second study. These studies are not comparable for the
evaluation of the abortifacient potential of carbaryl, since the
dosing began later in gestation in the second study. The authors
noted that the determination of abortion in the Rhesus monkeys may
not have been reliable.
8.5.3 Reproductive and developmental toxicity studies in
non-mammalian species
8.5.3.1 Fish
Medaka ( Oryzias latipes) embryos were exposed to nominal
carbaryl concentrations of 0.5, 1.0, 2.5, 5.0, 10.0, 20.0, or
30.0 mg/litre in the water from the 4-cell through to the blastula
stages (Solomon & Weis, 1979). Exposure of the eggs resulted in
increased cardiovascular anomalies (heart defects, circulatory
defects, and edema) at dose levels of 5 mg/litre or more.
8.5.3.2 Amphibian
Elliott-Feeley & Armstrong (1982) treated both embryos and
tadpoles of Xenopus laevis with nominal carbaryl concentrations in
the water of 0.1, 1.0, or 10.0 mg/litre. Defects were seen in
embryos exposed to 10 mg/litre, which also resulted in significant
lethality. Embryo growth was reduced at all dose levels.
Carbaryl-exposed tadpoles exhibited decreased activity at all dose
levels.
8.5.3.3 Birds
Chicken eggs have been used by a number of workers to test the
potential of carbaryl to affect avian development. Olefir &
Vinogradova (1968) injected eggs with carbaryl at 0.01, 0.1, 1.0, or
10.0 mg/kg and examined embryos at different times after injection.
Death and anomalies were recorded at 5, 10, 15, and 20 days of
incubation at all doses above 0.01 mg/kg. Lillie (1973) administered
0, 250, or 500 mg/kg diet to pullets for 36 weeks beginning at 32
weeks of age. The adult birds showed reduced weights at both levels
of carbaryl in the adults and at 500 mg in the progeny. No
embryotoxicity or other effects were noted. Swartz (1981) examined
the effects of carbaryl on chicken embryo development. They examined
chick embryos at different days after injection, for 5 or 12 days
post-fertilization. They found vehicle-related increased mortality
(carbaryl was more toxic when administered in sesame oil than in
acetone) for both periods of exposure. Scattered anomalies were
recorded in surviving embryos. In another study (Swartz, 1985)
primordial germ cell migration was followed after injection of 10 mg
carbaryl per egg prior to incubation. Results did not indicate any
significant adverse effects on the primordial germ cells or
reproductive organs.
Bursian & Edens (1977) studied the effects of carbaryl on the
fertility and post hatching viability of Japanese quail ( Coturnix
coturnix japonica). Birds were exposed from hatching to 14 weeks
of age (breeding maturity). Fertility and the hatchability of eggs
were measured. The animals received 0, 50, 150, 300, 600, 900, or
1200 mg carbaryl/kg diet. Growth of the adult birds was reduced at
the 900 and 1200 mg/kg dose levels. There were no significant
effects on any reproductive parameter.
Fletcher & Leonard (1986a) investigated the effects of carbaryl
on reproduction in bobwhite quail ( Colinus virginianus) exposed to
0, 300, 1000, or 3000 mg/kg diet for 22 weeks. No adverse effects
were described in any factors related to hatchability, posthatching
viability, or gross pathology of newly hatched birds. These workers
used a similar protocol to study the effects of carbaryl on mallard
ducks ( Anas platyrhyncos) (Fletcher & Leonard, 1986b). The 3000
mg/kg diet level was toxic with some lethality, decreased numbers of
eggs, and thinner egg shells. No effects were seen at the 300 or
1000 mg/kg diet levels.
8.5.4 Appraisal
In summary, mammalian studies on the reproductive or
developmental toxicity of carbaryl clearly show that this compound
is capable of inducing adverse effects in utero and during the
reproductive process. These effects are always seen only at dose
levels at which there is concurrent maternal toxicity, with the
possible exception of a few studies on the rat which have not been
replicated by other workers. For a number of species, the dams
appear to be more sensitive than their litters. In general, the
adverse effects noted in developmental toxicology studies cannot be
simply attributed to maternal toxicity (Chernoff et al., 1990).
However, the pattern of maternal and fetal toxicity occurring at the
same dose levels indicates that the developing mammalian
embryo/fetus is not especially susceptible to carbaryl.
Carbaryl has been shown to be embryotoxic for fish, amphibians,
and birds, at some exposure concentrations.
8.6 Mutagenicity of carbaryl and N-nitrosocarbaryl
In this section on mutagenicity, and in the section on
carcinogenicity (8.7.1), the two compounds, carbaryl and
nitrosocarbaryl, are discussed (separately), since the formation of
N-nitrosocarbaryl was reported to occur in the stomach of rats and
guinea-pigs, in the presence of sodium nitrite and carbaryl, under
acid conditions (Elespuru & Lijinsky, 1973; Beraud et al., 1979;
Rickard & Dorough, 1984). See also section 8.9.3.
8.6.1 Genotoxicity assays in vitro
8.6.1.1 Primary DNA damage
(a) Carbaryl
Carbaryl did not cause DNA damage in different wild type
strains and DNA recombination lacking strains of Bacillus subtilis
(Table 49). Carbaryl was reported to be non-genotoxic for
B. subtilis Marburg 17A Rec+ and Marburg M45T Rec- strains,
highly sensitive to frameshift mutagens, even at the highest tested
concentration of 10 mg/plate (Uchiyama et al., 1975).
Carbaryl (10-4 mmol/litre) did not affect the sedimentation
profiles of DNA from human skin cells in culture (both normal and
xeroderma pigmentosum), either immediately or 20 h after treatment
(Regan et al., 1976).
There are two controversial reports on the genotoxicity of
carbaryl, evaluated by the induction of mitotic gene conversion in
the diploid strain of Saccharomyces cerevisiae, heteroallelic at
the two different loci ade2 and trp5. Siebert & Eisenbrand (1974)
used an assay system with a 16-h incubation time and a concentration
of carbaryl of 4.97 mmol/litre and did not observe any changes in
the control frequency of mitotic gene conversion. However, Jaszczuk
& Syrowatka (1979), reported a weak positive response with a lower
concentration of carbaryl (2.5 mmol/litre) and shorter (5 h)
incubation time. No converting activity of N-hydroxy carbaryl (2.5
mmol/litre) was found under the same assay conditions.
Table 49. Bacterial assays of genetic toxicity of carbaryl
Test Test organism Concentration Genetic Metabolic Result Reference
(µg/plate) end-point activation
DNA B. subtilis 700 mg/litre Rec assay - negative DeGiovanni-Donnelly et al. (1968)
damage
B. subtilis 20 Rec assay - negative Shirasu et al. (1976)
B. subtilis 0.4, 4, 40, 400 Rec assay - negative Eto et al. (1982)
Gene B. subtilis up to 10 000 Rec assay - negative Uchiyama et al. (1975)
mutation
E. Coli WP2 1000 Try- - negative Ashwood-Smith et al. (1972)
E. Coli WP2 1000-3000 Try- - negative Nagy et al. (1975)
E. Coli WP2 10 000 Try- - negative Uchiyama et al. (1975)
H. influenzae 10 µmol/litre Novobiocim - negative Elespuru et al. (1974)
S. typhimurium
TA 98 up to 2000 His- - negative McCann et al. (1975)
TA 98 up to 2000 His- Rat negative
TA 98 50 nmol/litre His- - negative Blevins et al. (1977)
TA 98 10-1500 His- - negative DeLorenzo et al. (1978)
Table 49 (continued)
Test Test organism Concentration Genetic Metabolic Result Reference
(µg/plate) end-point activation
TA 98 10-1500 His- Rat negative
TA 98 0.25, 2, 5, 50, 1000 His- - negative Jaszczuk et al. (1979)
TA 98 up to 5000 His- - negative Moriya et al. (1983)
TA 98 up to 5000 His- Rat negative
TA 98 0.2, 2, 20 His- - negative Eto et al. (1982)
TA 98 0.2, 2, 20 His- Rat negative
TA 98 5-2000 His- - negative Lawlor (1989)
TA 98 5-2000 His- Rat negative
TA 100 up to 2000 His- - negative McCann et al. (1975)
TA 100 up to 2000 His- Rat negative
TA 100 50 nmol/litre His- - negative Blevins et al. (1977)
TA 100 10-1500 His- - negative DeLorenzo et al. (1978)
TA 100 10-1500 His- Rat negative
TA 100 0.25, 2, 5, 50, 1000 His- - negative Jaszczuk et al. (1979)
TA 100 0.2, 2, 20 His- - negative Eto et al. (1982)
Table 49 (continued)
Test Test organism Concentration Genetic Metabolic Result Reference
(µg/plate) end-point activation
TA 100 0.2, 2, 20 His- Rat negative
TA 100 5-2000 His- - negative Lawlor (1989)
TA 100 5-2000 His- Rat negative
TA 1535 up to 2000 His- - negative McCann et al. (1975)
TA 1535 up to 2000 His- Rat negative
TA 1535 2500 His- - positive Marshall et al. (1976)
TA 1535 1000 His- Rat negative
TA 1535 50 nmol/litre His- - negative Blevins et al. (1977)
TA 1535 10-1500 His- - negative DeLorenzo et al. (1978)
TA 1535 10-1500 His- Rat negative
TA 1535 up to 5000 His- - negative Moriya et al. (1983)
TA 1535 up to 5000 His- Rat negative
TA 1535 5-2000 His- - negative Lawlor (1989)
TA 1535 5-2000 His- Rat negative
Table 49 (continued)
Test Test organism Concentration Genetic Metabolic Result Reference
(µg/plate) end-point activation
TA 1536 2500 His- - negative Marshall et al. (1976)
TA 1536 1000 His- Rat negative
TA 1537 up to 2000 His- - negative McCann et al. (1975)
TA 1537 up to 2000 His- Rat negative
TA 1537 50 nmol/litre His- - negative Blevins et al. (1977)
TA 1537 2500 His- - negative Marshall et al. (1976)
TA 1537 1000 His- Rat negative
TA 1537 10-1500 His- - negative DeLorenzo et al. (1978)
TA 1537 10-1500 His- Rat negative
TA 1537 0.25, 2, 5, 50, 1000 His- - negative Jaszczuk et al. (1979)
TA 1537 up to 5000 His- - negative Moriya et al. (1983)
TA 1537 up to 5000 His- Rat negative
TA 1537 5-2000 His- - negative Lawlor (1989)
TA 1537 5-2000 His- Rat negative
TA 1538 100 His- - positive Egert & Greim (1976)
Table 49 (continued)
Test Test organism Concentration Genetic Metabolic Result Reference
(µg/plate) end-point activation
TA 1538 100 His- Mouse positive
TA 1538 50 nmol/litre His- - negative Blevins et al. (1977)
TA 1538 2500 His- - negative Marshall et al. (1976)
TA 1538 1000 His- Rat negative
TA 1538 10-1500 His- - negative DeLorenzo et al. (1978)
TA 1538 10-1500 His- Rat negative
TA 1538 up to 5000 His- - negative Moriya et al. (1983)
TA 1538 up to 5000 His- Rat negative
TA 1538 5-2000 His- - negative Lawlor (1989)
TA 1538 5-2000 His- Rat negative
Carbaryl was inactive in the rat primary hepatocytes
unscheduled DNA synthesis assay (Cifone, 1989). It did not induce
significant changes in the nuclear labelling of rat primary
hepatocytes in two independent trials with applied concentrations
ranging from 5 to 25 µg/ml.
No DNA-damaging properties of carbaryl, assessed by its
capacity for inducing unscheduled DNA synthesis (UDS) in cultured
human lymphocytes, were reported by Rocchi et al. (1980). However,
the authors did not use a standardized test protocol meeting
established criteria for the UDS assay performance. They tested only
one concentration of carbaryl (50 µg/ml), did not use any metabolic
activation system, did not run negative and adequate positive
controls concomitantly (other than the ultraviolet irradiation), and
did not apply relevant criteria to assess data.
The lack of DNA-damaging properties of carbaryl, as measured by
the induction of UDS, was confirmed by Probst et al. (1981). They
used a standardized protocol and autoradiographic techniques in
metabolically competent, cultured rat hepatocytes with test
concentrations ranging from 0.5 to 1000 nmol/ml. In contrast, Ahmed
et al. (1977a) reported that carbaryl induced the UDS of the
ultraviolet type (long patch repair) in a cultured human fibroblast
VA-4 cell line with, and without, metabolic activation in
concentration ranges of 1, 10, 100, and 1000 µmol/litre. However,
this study had several experimental shortcomings. The protocol, the
control value, and the results of compounds tested were not in
accordance with comparable assays by other authors.
(b) N-nitrosocarbaryl
The DNA-damaging properties of N-nitrosocarbaryl in
B. subtilis have been reported (Table 50).
In one study (Uchiyama et al., 1975), more pronounced
genotoxicity of N-nitrosocarbaryl to B. subtilis was registered
compared with that of N-methyl- N'-nitro- N-nitrosoguanadine.
However, the genotoxicity of N-nitrosocarbaryl was the lowest when
compared with other concomitantly tested nitrocarbamates.
In contrast to carbaryl, N-nitrosocarbaryl showed pronounced
genotoxicity toward S. cerevisiae D4 (Siebert & Eisenbrand, 1974).
N-nitrosocarbaryl, but not carbaryl, reacted with human DNA
in cell culture to form alkaline-sensitive bonds (Regan et al.,
1976). The DNA of nitrosocarbaryl-treated (10-4 mmol/litre) cells
showed a substantial reduction in sedimentation rate immediately,
and up to 20 h, after treatment. Presumably, the effect observed was
related to the induction of numerous single-strand breaks in the DNA
and the formation of DNA adducts. On the basis of selective
labelling, the authors suggested that the methyl-containing moiety
of nitroso-carbaryl was separated from the naphthalene ring in a
human fibro-blast culture and bound irreversibly to DNA (Regan
et al., 1976).
8.6.1.2 Gene mutation assay
(a) Carbaryl
As summarized in Table 49, carbaryl did not exert a mutagenic
effect in studies with E. coli, H. influenzae, or Salmonella.
Among the many reports on Salmonella, only two indicated a
positive effect. Thus, Marshall et al. (1976) observed increased
mutagenicity at 1000 µg/plate with S9 mix for TA1535. An evaluation
of this data is difficult because no negative and positive control
data were presented. Egert & Greim (1976) reported a positive
response for TA1538 by 100 µg/plate. The mutagenicity of carbaryl in
this study greatly increased when a non-standard metabolic
activation system (mouse liver microsome) was used.
Ahmed et al. (1977b) reported a positive mutagenic response
to carbaryl in Chinese hamster V79 cells at a dose level of
0.01 mmol/litre, with no metabolic activation. There was a
concentration-related effect of carbaryl on cell survival; less than
50% of the cells survived at concentrations >0.01 mmol/litre.
Mutation studies carried out with a concentration of 0.01 mmol/litre
showed an approximately 8-fold increase in ouabain resistance
forward mutation in V79 cells as compared with the spontaneous
mutation rate.
However, Wojciechowski et al. (1982) found no
ouabain-resistance with carbaryl in a cell-mediated mutagenesis
assay when they used an exogenous activating system in which
irradiated fetal cells of Syrian hamsters were co-cultivated with
cells of Chinese hamsters V79. No mutagenic response was observed
for carbaryl at concentrations of 0.01, 0.05, 0.1 mmol/litre with,
or without metabolic activation. The toxicity of carbaryl at these
concentrations ranged from 7% at the lowest concentration to 23% at
the highest.
Carbaryl produced a negative result for inducing forward
mutations at the HPRT locus in Chinese hamster ovary cells both
with, and without, metabolic activation (Young, 1990).
Table 50. Bacterial assays of the genetic toxicity of nitrosocarbaryl
Test Test organism Concentration Genetic Metabolic Result Reference
(µg/plate) end-point activation
DNA B. subtilis 0.4, 4, 40 Rec assay - positive Eto et al. (1982)
damage
B. subtilis up to 100 Rec assay - positive Uchiyama et al. (1975)
Gene
mutation E. Coli 30 R 0.1 mmol/litre Try- - positive Elespuru et al. (1974)
E. Coli WP2 5, 10, 50, 100 Try- - positive Uchiyama et al. (1975)
E. Coli K12 100 Try- - positive Egert & Greim (1976)
E. Coli K12 100 Try- Mouse positive
H. influenzae 10 µmol/litre Novobiocin - positive Elespuru et al. (1974)
S. typhimurium
TA 98 0.001-11 His- - positive/ Blevins et al. (1977)
negative
TA 9 10-1500 His- - negative DeLorenzo et al. (1978)
TA 98 10-1500 His- Rat negative
TA 98 0.25-1000 His- - positive Jaszczuk et al. (1979)
Table 50 (continued)
Test Test organism Concentration Genetic Metabolic Result Reference
(µg/plate) end-point activation
TA 98 0.02, 0.2, 2 His- - negative Eto et al. (1982)
TA 98 0.02, 0.2, 2 His- Rat negative
TA 98 0.01-100 His- - negative Rickard et al. (1982)
TA 98 0.1-100 His- Rat negative
TA 100 0.001-11 His- - positive Blevins et al. (1977)
TA 100 0.02, 0.2, 2 His- - positive Eto et al. (1982)
TA 100 0.02, 0.2, 2 His- Rat negative
TA 100 0.1-100 His- - positive Rickard et al. (1982)
TA 100 0.1-100 His- Rat positive
TA 1535 0.5-100 His- - positive Marshall et al. (1976)
TA 1535 50-1000 His- Rat positive
TA 1535 0.001-11 His- - positive Blevins et al. (1977)
TA 1535 0.25-1000 His- - positive Jaszczuk et al. (1979)
TA 1535 0.1-100 His- - positive Rickard et al. (1982)
TA 1535 0.1-100 His- Rat positive
Table 50 (continued)
Test Test organism Concentration Genetic Metabolic Result Reference
(µg/plate) end-point activation
TA 1536 0.5-100 His- - negative Marshall et al. (1976)
TA 1536 50-1000 His- Rat negative
TA 1537 0.001-11 His- - negative Blevins et al. (1977)
TA 1537 0.5-100 His- - positive Marshall et al. (1976)
TA 1537 50-1000 His- Rat positive
TA 1537 0.25-1000 His- - positive Jaszczuk et al. (1979)
TA 1538 100 His- - positive Egert & Greim (1976)
TA 1538 100 His- Rat positive
TA 1538 0.001-11 His- - negative Blevins et al. (1977)
TA 1538 0.5-100 His- - positive Marshall et al. (1976)
TA 1538 50-1000 His- Rat negative
TA 1538 0.25-1000 His- - negative Jaszczuk et al. (1979)
(b) N-nitrosocarbaryl
N-nitrosocarbaryl has shown a positive response in a number
of bacterial assays (Table 50). In Salmonella, N-nitrosocarbaryl
was mutagenic to strains responding to both base substitution
(TA1535, TA100) and frame shift (TA1536, TA1537, TA1538, TA98). The
mutagenic potential of N-nitrosocarbaryl toward Salmonella TA1535
was greatly diminished by the added exogenous activation system
(Marshall et al., 1976). A decrease in mutagenic activity by the
addition of S9 mix on frame shift mutagenesis has also been reported
(Marshall et al., 1976; Jaszczuk et al., 1979).
8.6.1.3 Chromosomal aberration assays and sister chromatid exchange
(a) Carbaryl
Animals
Carbaryl induced a clastogenic response in the three in vitro
bioassays (Ishidate & Odashima, 1977; Kazarnovskaya & Vasilos, 1977;
Önfelt & Klasterska, 1983). All the positive responses were observed
at toxic dose levels (30-80 µg/ml; 50, 100 µmol/litre); exogenous
metabolic activation was not required for activity. Thus, Ishidate &
Odashima (1977) observed a strong positive response for carbaryl
(30 µg/ml) in a chromosomal aberration assay using a Chinese hamster
fibroblast cell line. Carbaryl produced predominantly chromatid type
gaps, breaks, translocations, rings, and fragmentation, 48 h after
treatment.
Carbaryl was negative for inducing chromosomal aberrations in
CHO cells without metabolic activation but was positive under
metabolic activation conditions (Murli, 1989).
Kazarnovskaya & Vasilos (1977) reported a positive response for
carbaryl in cultures of human embryonic fibroblast: levels of 40 and
80 µg/ml caused a 4-fold and 10-fold increase, respectively, in the
frequency of chromosomal aberrations, at 24 h exposure time,
compared with a control rate of 2% (without S9-mix). Again, the
aberrations produced were mainly of a chromatid type; the type most
frequently observed was single fragments. An increased number of
paired fragments was found only at 80 µg carbaryl/ml. Carbaryl
(80 µg/ml) increased the percentage of cells with chromosomal
coiling (9.8% in the control group; 17.9% in the test group) and
aneuploidy (3% in the control group; 29.2% in the test group).
Previously, Kazarnovskaya & Vasilos (1977) had shown that carbaryl
suppressed mitosis, changed the rate of the mitotic phase, and
significantly increased the number of pathological forms of mitosis
in a human embryonic fibroblast culture, with a dose-time response.
Onfelt & Klasterska (1983) observed induction of viable
aneuploid/polyploid cells and multiple chromatid exchanges after
treatment of V79 Chinese hamster cells with carbaryl. The compound
exerted a pronounced chromosome-breaking effect, at a concentration
of 100 µmol/litre, 26 and 50 h after treatment. There was an
increased frequency of multiple chromatid exchanges and fragments,
as well as pulverisation and more diffuse signs of chromosome
damage. The effects of carbaryl on chromosome structure and
distribution were almost abolished by the simultaneous addition of
Aroclor-induced 2 or 10% rat S9-mix and glutathion.
In two in vitro assays for chromosome damage using Chinese
hamster lung fibroblasts, carbaryl was found to be positive
(Ishidate et al., 1981; Onfelt & Klasterska, 1984). In the latter
study, the effect of carbaryl on the incidence of sister chromatid
exchange was decreased by the addition of rat liver microsomes.
Söderpalm & Önfelt (1988) related the mitotic aberrations in V79
Chinese hamster cells, in part, to a reduction in the intracellular
levels of glutathione, and increased lipid peroxidation. They also
hypothesized that the anticholinergic effects of carbaryl may play a
role in the cleavage process.
Plants
A negative clastogenic response for carbaryl in a concentration
range of 50-200 mg/litre was reported by Ma et al. (1984) who used
the Tradescantia micronucleus test. At present, this test is
considered the most established and standardized plant assay system
for the purposes of in situ monitoring of environmental mutagen
pollution (Ma et al., 1984).
Carbaryl induced chromosomal effects in different plant assays
(Wuu & Grant, 1966; Amer & Farah, 1968; Brankovan, 1972). Carbaryl
increased by approximately 10-fold the number of aberrant cells in
the root tips of barley seedings ( Hordeum vulgare) in C1 and C2
generations after the seeds were treated with concentrations of 500,
1000, or 1500 mg carbaryl/litre for 6 and 12 h. The cytogenetic
effects induced included mostly metaphase and anaphase fragments and
anaphase bridges and were time-dependent (Wuu & Grant, 1966).
The mitotic, cytogenetic effects of carbaryl were seen in
Vicia faba (Amer & Farah, 1968) and sugar corn (Brankovan, 1972).
Carbaryl caused chromosomal abnormalities (chromosome lagging;
stickiness, mainly in diakinesis, polyploidy, fragments, anaphase
bridges) in different meiotic states in the pollen mother cells of
Vicia faba after spraying flower buds of different ages (2 weeks,
1 month) with saturated aqueous solutions of a commercial carbaryl
preparation. The percentage of the chromosomal aberrations increased
with increase in the frequency of spraying (every week) or 2 weeks
for l month; daily for 8 days) and then decreased when the recovery
time was increased (Amer & Farah, 1968).
8.6.2 Genotoxicity in vivo
8.6.2.1 Host-mediated assay
(a) Carbaryl
Usha Rani et al. (1980) reported that carbaryl did not have a
gene-mutation potential in vivo, when given orally in a toxic dose
of 438 mg/kg, 3 times daily for 3 days, to 6 Swiss male mice, which
were subsequently injected with Salmonella strain G46. These data
are consistent with those that showed that carbaryl was not
mutagenic for Salmonella in vitro.
8.6.2.2 Drosophila melanogaster and other insects
(a) Carbaryl
There are several reports of bioassays for carbaryl
genotoxicity in which Drosophila melanogaster was used (Brzeskii,
1972; Brzeskii & Vaskov, 1972; Hoque, 1972; Woodruff et al., 1983;
Omer et al., 1986).
In studies by Hoque (1972), carbaryl at 1.5 and 10 mg/litre was
given to female Drosophila. It was reported that the treatment
caused a changed sex ratio, changes in eye colour, and various
chromosomal aberrations in the offspring. The small amounts of
material used and the lack of any detailed presentation of the
findings preclude any evaluation or conclusion.
At high doses (0.3 ml from a 1% suspension of 85% commercial
product in glucose), carbaryl caused a slight increase in mutation
frequency in the F1 generation males of a Drosophila line, which
were studied at different stages of spermatogenesis. There were no
deletions or disturbed fertility (Brzeskii, 1972; Brzeskii & Vaskov,
1972).
Woodruff et al. (1983), who used a sensitive experimental
protocol that incorporated the mating scheme with repair-deficient
females, reported that carbaryl was not mutagenic for Drosophila.
F1 male progeny of males that had ring X chromosomes and double
marked Y chromosomes, were treated with carbaryl at 200 mg/litre and
mated with mus-302, repair-deficient females of Drosophila. The
male progeny did not show any induced complete (ring chromosome) or
partial (Y chromosome markers) chromosome loss.
The mutagenic activity of carbaryl in Drosophila melanogaster
was studied by Omer et al. (1986). The results indicated that
carbaryl does not increase the rate of dominant and sex-linked
recessive lethal mutations.
Carbaryl did not cause chromosome abnormalities in the meiotic
cells of male grasshoppers, when they were given a single toxic dose
(not precisely indicated) of 0.25 ml of the supernatant of an
aqueous suspension/solution of carbaryl by ip injection; however,
there were morphological disturbances in the spermatocytes (Venkat
Reddy et al., 1974).
(b) N-nitrosocarbaryl
Results obtained by Omer et al. (1986) from the
nitrosocarbaryl-treated populations of Drosophila melanogaster
suggested that nitrosocarbaryl increased significantly the
percentage of dominant lethal mutations above the spontaneous
mutation frequency. The results obtained from the 0.05, 0.10, and
0.15% nitrosocarbaryl-treated populations also showed a significant
increase in the percentages of sex-linked recessive mutations.
8.6.2.3 Chromosomal aberrations and sister chromatid exchange
(a) Carbaryl
There are several negative chromosomal studies on carbaryl and
N-nitrosocarbaryl in somatic and germ cells in vivo in mammals
(Venkat Reddy et al., 1974; Degraeve et al., 1976; Seiler, 1977,
Usha Rani et al., 1980; Dzwonkowska & Hübner, 1986). In all these
studies, sufficiently high dose levels of carbaryl were used (up to
the LD50) in order to define the negative cytogenetic responses.
Using the micronucleus test and the cytogenetic analysis of
metaphase chromosomes, Degreave et al. (1976) found no clastogenic
effects in the bone marrow of mice that had been given a single oral
dose of carbaryl (0.2 ml), an intraperitoneal dose (0.5 ml) of
carbaryl, or 7 oral doses of carbaryl solution (1x10-3 mg/litre)
alone, or with sodium nitrite (2x10-3 mg/litre).
A negative response for a high dose of carbaryl (146 mg/kg,
orally, 2 times per 24 h; 30-h sampling time) was reported by Usha
Rani et al. (1980) with an adequate protocol for the mouse bone
marrow micronucleous test.
No increase in the rate of the micronucleated, polychromatic
erythrocytes in the bone marrow of mice was found during in vivo
nitrosation of carbaryl after its oral administration at the maximum
tolerated dose of 100 mg/kg, together with an excess of sodium
nitrite (100 mg/kg) (Seiler, 1977).
A dose of 64 mg carbaryl/kg produced a negative result in an
in vivo assay of chromosomal aberrations in the bone marrow cells
of the Syrian hamster (Dzwonkowska & Hubner, 1986).
No chromosome aberrations were observed in the bone marrow of
Syrian hamsters (6 per dose), given single intraperitoneal
injections of doses up to the LD50 (64, 128, 320, and 640 mg/kg)
of a commercial mixture of carbaryl/lindane (40:10) (Dzwonkowska &
Hubner, 1986).
Daily oral doses of technical carbaryl (10 mg/kg) suspended in
peanut oil were given to male, albino rats for a period of 5 days.
There were no significant chromosomal changes in the bone marrow
cells of the exposed animals (Dikshith, 1991).
8.6.2.4 Dominant lethal assays in rodents
(a) Carbaryl
Epstein et al. (1972) studied the mutagenicity of carbaryl
using the dominant lethal test. In this assay, male ICR/Ha Swiss
mice were treated orally with 1000 or 50 mg carbaryl/kg daily for 5
successive days and then caged with 3 untreated virgin females,
which were replaced weekly for 8 consecutive weeks. The frequency of
early fetal deaths and preimplantation losses in the test groups
were within the limits of the control values. Therefore, dominant
lethal mutations were not induced in mice given sufficiently high
oral doses of carbaryl.
8.6.3 Other end-points
8.6.3.1 Cell transformation
(a) Carbaryl
Transformation of the fibroblast clone A31 of the BALB/3T3
mouse was not induced by carbaryl when given in non-toxic (1.5 and
10 µg/ml) or moderately cytotoxic (20 and 40 µg/ml) doses, over 24 h
(Quarles & Tennant, 1975).
(b) N-nitrosocarbaryl
N-nitrosocarbaryl showed transforming activity, at
concentrations of 10-20 µg/ml, which was cytotoxic for the BALB/3T3,
A31 cells. That 3-10 cell passages are necessary for detecting the
transforming event suggests that the transformation frequency for
N-nitrosocarbaryl in this test system is low. Transformed cells
showed morphological alterations, loss of contact inhibition, and
growth in soft agar, as well as carcinogenic activity in normal
newborn, irradiated, suckling, or athymic BALB/C mice. The tumour
incidence (anaplastic sarcomas) was relatively low; in several
instances, tumours regressed in normal mice, but not in athymic
mice, after 2-3 weeks of growth. N-nitrosocarbaryl did not
activate detectable amounts of the endogenous murine leukaemia
viruses carried by the BALB/3T3 cells or viral protein. However,
viral antigen production in the transformed cells was induced by
iododeoxyuridin, which indicated the presence of the viral genome.
On the basis of this N-nitrosocarbaryl transforming activity in
mammalian cells in culture, Quarles & Tennant (1975) suggested that
the compound may be an active, though weak, carcinogen in vivo.
8.6.3.2 Aneuploidy induction
(a) Carbaryl
Tests of the chemical induction of spindle fibre inactivation
and c-mitosis (colchicine-mitosis) in Allium revealed the
existence of an unspecific physico/chemical mechanism, based on the
partitioning of compounds into hydrophobic compartments of the cell.
This means that chemical compounds, in general, cause c-mitosis
according to their lipophilic characteristics, as indicated by the
octanol/water partition coefficient. Thus, a close correlation
exists between the lipophilicity of compounds and the dose at which
spindle disturbance occurs. However, besides this unspecific effect
on the spindle fibres mechanism, there are some compounds that
exhibit specific effects causing inactivation of the spindle fibre
mechanism at lower doses than indicated by their lipophilicity. This
can occur via different mechanisms, such as specific binding to
actin (colchicine) or binding to sulfhydryl groups (organic
mercury). Onfelt (1987) analysed the c-mitotic effects of 22
compounds in V79 hamster cells. Five of these compounds fell outside
the regression line for lipophilicity/c-mitosis, among them
colchicine, methyl mercury, and carbaryl. Further analyses revealed
that carbaryl owes its pronounced c-mitotic action to its reactivity
with sulfhydryl groups. It is therefore not surprising that several
authors have reported mitotic disturbances caused by carbaryl in
various experimental systems. Onfelt & Klasterska (1983) reported
that a significant increase in the aneuploidy/polyploidy cells was
obtained with both 50 and 100 µmol carbaryl/litre, 26, 59, and 74 h
after treatment. Carbaryl caused mitotic disturbances in Allium
cepa, Vicia faba, Gossypium barbadense, Nigella damascena (Amer,
1965; Amer et al., 1971; Degraeve et al., 1976). Amer (1965)
reported c-mitotic effects of carbaryl in the roots of Allium cepa
that had been treated for 4 or 24 h with different concentrations of
pure (0.5, 0.25%) and commercial products (85% sprayable powder).
The increased rate of abnormal meta-telophases and ana-telophases
depended on the concentration and temperature of the test solutions.
The types of induced abnormal metaphases included star-metaphases
and a few prophase-metaphases. Two types of anaphases, the c-type
and the multipolar type, as well as multinuclear interphase cells,
were observed. Continuous treatment with carbaryl for 24 h nearly
arrested mitosis. There was a full recovery of mitosis in 48 h and
induced signs of polyploidy appeared.
Consistent with these findings are the c-mitotic effects of
carbaryl in Vicia faba and Gossypium barbadense (Amer et al.,
1971). The mitotic index of Vicia faba, after root treatment,
decreased with increased concentrations of carbaryl (25, 50, or
100 mg/litre for 4 h. There was no effect from carbaryl after
seed-soaking for different lengths of time. Mitotic anomalies
(mostly disturbed meta- and anaphases) in the roots of Vicia faba
and Gossypium barbadense showed a concentration-time response.
Carbaryl caused mitotic disturbances (c-mitotic effect,
multipolar anaphases) or cytotoxicity (pycnotic nuclei, tissue
degeneration) after root treatment of Nigella damascena with
concentrations of 2.5x10-4 and 1x10-3 mg/litre. When NaNO2 (2x10-3)
was added to carbaryl solutions of 1x10-2 and 1x10-3 mg/litre, the
effects increased, possibly because of the formation of
nitrosocarbaryl (Degreave et al., 1976). No effect was observed
after grain treatment.
Soaking sugar corn seeds for 48 h with a 0.12 or 0.25% aqueous
solution of 50% commercially prepared carbaryl, resulted in a
dose-response induction of aberrations of anaphase chromosomes of
types not seen in the controls (bridges with, or without, fragments,
dicentrics). Repeated treatment of young plants with a 0.25%
solution of carbaryl for 6 h during meiosis caused typical c-mitotic
effects in metaphase and anaphase through the arrest of cell
division and spindle inactivation. Some of the induced aberrations
persisted until the generative stage. They were fixed and
incorporated through the embryonal and generative development stages
and thus increased pollen sterility (Brankovan, 1972).
Carbaryl was reported to increase the number of aberrant forms
of mitosis (mostly c-mitotic effect) in the small intestine, cornea,
and spleen of rats given oral doses of 400 mg/kg. The authors also
reported that at lower doses of carbaryl (40 or 80 mg/kg), there was
an increased incidence of bridge and chromosome lagging in anaphase
and telophase, in addition to a high percentage of c-mitosis. The
authors reported that there were no effects at 20 mg/kg. Only the
result following the 400 mg/kg dose was fully reported (Vasilos
et al., 1975).
8.6.4 Appraisal
Carbaryl has been evaluated for its potential mutagenicity in a
number of tests in vitro as well as in vivo, in bacterial, yeast,
plant, insect, and mammalian systems, testing a variety of
end-points.
The available evidence indicates that carbaryl has no
DNA-damaging properties. No confirmed induction of mitotic
recombination, gene conversion, and UDS in prokaryotes
( H. influenzae, B. subtilis) and eukaryotes ( S. cerevisiae, A.
nidulans, cultured human lymphocytes, and rat hepatocytes)
in vitro has been reported.
Negative results were obtained in tests for gene mutations in a
number of bacterial assays, with the exception of two cases. In
several studies of gene mutations in mammalian cells in vitro,
carbaryl only produced one equivocal positive result in a cell
culture study. However, the study had several shortcomings and the
result has not been confirmed in any other comparable studies.
Chromosomal damage with high dosages of carbaryl, has been
reported in vitro in human, rat, and hamster cells and in plants.
No such effects have been observed in mammalian tests in vivo, even
at doses as high as 1000 mg/kg.
Carbaryl has been shown to induce disturbances in the spindle
fibre mechanism in plant and mammalian cells in vitro. The
relevance of plant assays for extrapolation to humans is unclear.
It can be concluded that the available data-base does not
support the presumption that carbaryl poses a risk of inducing
genetic changes in either the somatic or the germinal tissue of
humans.
The nitrosated product of carbaryl, N-nitrosocarbaryl, is
capable of inducing mitotic recombination and gene conversion in
prokaryotes (H. influenzae, B. subtilis) and eukaryotes (S.
cerevisiae) in vitro and gave positive results in E. coli spot
tests.
Furthermore, experimental results indicate that
N-nitrosocarbaryl binds to DNA, causing alkali-sensitive bonds and
single-strand breakage.
Nitrosocarbaryl has not been established as a clastogen
in vivo (bone marrow and germ cells), even at high toxic doses.
8.7 Carcinogenicity
8.7.1 Carcinogenicity studies of carbaryl in rodents
8.7.1.1 Mouse
Seven early carcinogenicity studies with carbaryl have been
reported (Table 51) involving different strains of mice,
subcutaneous injection, skin painting, ip and oral routes of
administration, different dose levels and dose schedules, and
various lengths of exposure and observation. None of these negative
studies (one marginal response) had sufficient data or data
reporting and it was not possible to evaluate them.
Table 51. Carcinogenicity studies on carbaryl in rodents
Chemical Species, strain Sex Route and Dose Duration Significant Evaluation Reference
(number) mode of (mg/kg) of study tumour/organ of individual
administration study
Carbaryl Mouse, A/Jax, C3H male SC 1/week 200 mg 20 weeks none inconclusive Carpenter et al.
30 in group (total) (1961)
Carbaryl Mouse, NS not skin painting not 24 months none inconclusive Weil & Carpenter
stated stated (1962)
Carbaryl Mouse, CD-1 male oral, diet 0.01% 80 weeks none inconclusive Mellon Institute
female 0.04% 2 years (1963)
Carbaryl Mouse, A,C3,HA not intraperitoneal 60 2 years none inconclusive Makovskaya et al.
stated 1/week (1965)
Carbaryl Mouse, (C57,B16x male oral gavage daily 4.64 18 months none inconclusive Innes et al.
C5H/Anf)F1 female 7 days-4 weeks 14 mg/kg (1969)
(C57B1/6xAKR)F1 72 of age; in diet body
4 weeks-18 weight
months of age
Carbaryl Mouse, A/He: 16 male intraperitoneal 6 mg 20 weeks marginal (±) inconclusive Shimkin et al.
3/week for (total) lung tumours (1969)
4 weeks response
Carbaryl Mouse, A/J: 124 female oral, diet 1000 10 weeks none inconclusive Triolo et al.
(1982)
Carbaryl Mouse CD-1 male diet 100,1000 53 weeks none no effect Hamada (1991b)
technical female 8000
Table 51 (continued)
Chemical Species, strain Sex Route and Dose Duration Significant Evaluation Reference
(number) mode of (mg/kg) of study tumour/organ of individual
administration study
Carbaryl Rat, CF-N 120 male oral, diet 0.005, 2 years none inconclusive Carpenter et al.
per level female 0.01, 0.02, (1961)
0.04% in
diet
Carbaryl Rat, Sprague-Dawley male diet 250, 1500 52 weeks none negative Hamada (1991b)
technical 80 or 90 per group and 7500
female
ß-Carbaryl Rat, mongrel 60 male oral gavage twice 30 22 months fibrosarcoma, positive but Andrianova &
48 control a week skin; polymorphic inconclusive Alexeev (1970)
cell sarcoma,
stomach;
osteosarcoma
with multiple
mestastases
48 single 20 mg 22 months fibrosarcoma, positive but
48 control subcutaneous skin inconclusive
implantation
Most of these studies involved the lung adenoma test in strain
A mice. This test is no longer considered to be a satisfactory
carcinogenicity bioassay because of the high rate of background
tumours that occur in untreated animals (Clayson, 1987).
Carbaryl did not increase the incidence of lung tumours in two
strains of male mice, A/Jax and C3H, which were given 20
consecutive, weekly, subcutaneous injections of 10 mg carbaryl
(Carpenter et al., 1961). As only one dose of carbaryl was tested
and the initial number of animals entered was insufficient, no
conclusion concerning the carcinogenic potential of carbaryl can be
drawn from this study.
There was no tumour development in mice (unspecified strain and
sex) after carbaryl application by skin painting for 24 months (dose
not specified, 48% water suspension) (Weil & Carpenter, 1962). This
study also lacked details about the experimental procedure and
pathology used and, so, was placed in the inconclusive category.
Carbaryl did not induce tumours in the lungs, liver, kidney,
heart, spleen, pancreas, and the thyroid and adrenal glands in two
strains of mice, A and C3HA, treated ip, once per week for about 2
years, with toxic doses of 60 mg/kg (Makovskaya et al., 1965). The
study involved a sufficient number of animals in the carbaryl group
(400), untreated control group (100), and urethan positive control
group (150), killed at 1, 3, 6, 9, 12, 15, 18, and 24 months after
the beginning of the study and studied histopathologically. However,
no numerical data on the background lung tumours in the untreated
control animals were presented. This lack of data, coupled with the
absence of tumours in the lungs of the carbaryl-treated mice strain
A, which are known for their high rate of naturally occurring lung
adenomas, makes the negative response of little significance.
Carbaryl yielded a marginal tumour response in the pulmonary
tumour induction test on mice (strain A) given a total dose of 6 mg
by ip injections, 3 times per week for 4 weeks (Shimkin et al.,
1969). Again, the lung adenoma test on strain A mice was not
reliable for evaluating possible carcinogenicity and, thus, the
results obtained from this study are not definitive.
No carcinogenic response was obtained with carbaryl
administered orally, by gavage, and/or in the diet to four different
strains of mice at doses ranging from 14 to 1000 mg/kg diet (Melon
Institute, 1963; Innes et al., 1969; Triolo et al., 1982).
There was no increase in the total tumour incidence and no
changes in tumour patterns in either sex of CD-1 mice fed diets
containing doses of 0.01 or 0.04% (100 or 400 mg/kg) carbaryl for 80
weeks and 2 years, respectively (Mellon Institute, 1969). The
survival rate in both test groups was too low for a response to be
observed; no information was given for one-third of the animals;
histopathology was performed only on animals that were suspected of
having tumours. Because of these deficiencies, no conclusion can be
drawn.
Carbaryl did not significantly increase the incidence of any
type of spontaneously occurring tumours (hepatomas, lung adenomas,
lymphoid sarcomas) in either sex of two hybrid strains of mice
(C57B1/6xC3HAnf) F1 and (C57B1/6xAKR) F1, treated orally with
4.64 mg/kg by gavage (mice from 7 days to 4 weeks of age), and in
the diet (mice from 4 weeks to 18 months of age) with 14 mg/kg diet
(Innes et al., 1969).
Another inadequate feeding study of carbaryl carcinogenicity
was conducted by Triolo et al. (1982), using the model of lung
tumour induction in strain A/J female mice. In two studies, the
feeding level of 1000 mg carbaryl/kg, incorporated in the diet of
mice for 20 weeks, did not cause a significant increase in the
incidence of background lung adenomas, nor did it induce tumours in
the glandular stomach or other tissues (spleen, intestinal tract).
However, there were a number of confounding factors in defining the
response in this study including: large variations in the incidence
of lung adenomas in the four separate control groups; in particular,
one such group had no tumours, despite high historical control
levels in strain A mice. In addition, only one dose level was
studied (1000 mg/kg), on the basis of which it is not possible to
establish a dose-response relationship.
In the second study, in which the same feeding level and
exposure period were used, carbaryl increased the lung
benzo( a)pyrene hydroxylase activity, which was associated with a
modest increase in the rate of lung tumours induced by the oral
administration of 3 mg BP twice, on days 7 and 21, respectively of
the study. However, there was great variability in the incidence of
BP-induced lung adenomas in the 3 control groups; the first
(7.2±0.8) was well above the values presented for the test group
carbaryl + BP (5.7±1.4); and the second fell within the limits of
the incidence of spontaneous tumours in the corn-oil control group,
(1.17±1.11), which make the results inconclusive.
A new mouse oncogenicity study is in progress, under
proprietary sponsorship. The study design was as follows: carbaryl
was administered in the diet to male and female CDR-1 mice at
rates of 0, 100, 1000, or 8000 mg/kg diet, for up to 104 weeks.
Groups consisted of 80 mice/sex per group. Ten mice/sex per group
were sacrificed for clinical pathology evaluation after 52 weeks of
treatment. Results of this interim sacrifice are presented in
section 8.3. The remaining animals were designated for continued
exposure to the end of the 104-week treatment period. The study
design and evaluation parameters are consistent with guidelines set
out by the US EPA and OECD, with additional study parameters, for
studies of this nature (Rhône-Poulenc, 1992).a
8.7.1.2 Rats
Two reports by Carpenter et al. (1961) and Andrianova &
Alexeev (1970) are controversial. They studied the carcinogenicity
of carbaryl given to rats by oral gavage, in the diet, or by
subcutaneous implantation. Both studies had insufficiencies in the
protocols used.
Carpenter et al. (1961) noted no significant increase in the
total tumour incidence in either sex of CF-N rats fed a diet
containing 0.005, 0.01, 0.02, or 0.04% carbaryl, for 2 years. Female
mice had more pituitary tumours than male mice, but there was no
significant difference in the incidence of tumours between the
control and test groups. The small initial number of animals used,
their relatively old age (60 days), their low survival rate, and the
lack of detailed pathology are complicating factors in defining a
negative response.
However, Andrianova & Alexeev (1970) reported that carbaryl,
administered by both oral gavage and subcutaneous implantation,
produced positive carcinogenic responses in mongrel rats. Male rats
given oral doses of 30 mg carbaryl/kg, twice weekly for 22 months,
developed skin fibrosarcoma, polymorphic cell sarcoma in the
stomach, and osteosarcoma with multiple metastases. Carbaryl caused
high lethality (80%) during the exposure period. The authors did not
state whether the gross pathology was examined. One fibrosarcoma was
discovered among 46 control animals, at 11 months. No data were
presented on the number of control animals that lived for 22 months,
so a comparison of survival rates in control and test groups could
not be made.
In a parallel study, 20 mg of carbaryl in a purified paraffin
capsule was implanted subcutaneously in 48 male rats. At the end of
the 22-month exposure period, subcutaneous fibrosarcomas, in sites
far from the implantation area, and on the back and neck, were
diagnosed in 2 out of 10 surviving animals. There was no control
group (Andrianova & Alexeev, 1970). The carbaryl used was obtained
from a plant in the USSR and was of technical grade and 97.65%
purity. No information about the chemical composition and impurities
was given. Because of these deficiencies, this study is
inconclusive.
a Information to Task Group. These studies have not yet been
reviewed by the IPCS. The company performing these studies has
indicated that there is a significant increase in tumors at the
highest dose in both species.
A new combined long-term toxicity and oncogenicity study on
rats is in progress, under proprietary sponsorship. The study design
is as follows: carbaryl was administered in the diet to male and
female Sprague-Dawley rats at rates of 0, 250, 1500, or 7500 mg/kg
diet, for up to 104 weeks. Groups consisted of 80 rats/sex per group
for low- and middle-dose groups and 90 rats/sex per group for the
high-dose and control groups. Ten animals/sex per group were
sacrificed after 26 and 52 weeks exposure and evaluated for clinical
pathology and histopathology. In addition, 10 animals/sex from the
control and high-dose groups were used as a recovery group, kept on
a basal control group diet before sacrifice at 56 weeks. The results
of the interim sacrifices are presented in section 8.3. The
remaining animals were designated for continued exposure to the end
of the 104-week treatment period. The study design and evaluation
criteria are consistent with guidelines set out by the US EPA and
OECD, with additional study parameters, for studies of this nature
(Rhône-Poulenc, 1992).a
8.7.1.3 Overall appraisal of carbaryl carcinogenicity
Carbaryl has been studied for its carcinogenic potential in
numerous studies on rats and mice via various routes of
administration. Most of these studies are old and do not meet
contemporary standards.
Only one paper from the existing body of publications clearly
reports a tumorigenic action of carbaryl. Carbaryl induced malignant
tumours in an unidentified strain of rat by the oral and
subcutaneous routes of administration. This study does not meet
contemporary standards, because of insufficient reporting of control
data.
New studies, designed to meet with contemporary standards, are
in progress on rats and mice. Descriptions of the studies are given
in sections 8.7.1.1 and 8.7.1.2.
8.7.2 Carcinogenicity studies of N-nitrosocarbaryl
Carbaryl is a secondary amine and is, therefore, capable of
nitrosation in the presence of nitro donor groups, such as sodium
nitrate, to give a nitrosamide. This nitrosamide, nitrosocarbaryl,
has been proved to be mutagenic and carcinogenic, at high doses in
a Information to Task Group. These studies have not yet been
reviewed by the IPCS. The company performing these studies has
indicated that there is a significant increase in tumors at the
highest dose in both species.
animals (see Table 52). A condition of this nitrosation is an acidic
pH (less than 2), which is comparable to the one found in the human
stomach. However, nitrosocarbaryl is not stable at this pH. Its
maximum stability is between pH 3 and 5, at which pH no significant
amount of carbaryl can be nitrosated. Carbaryl was nitrosated in
several studies, in vitro as well as in vivo, in the guinea-pig,
which has a stomach acidity similar to that of humans. See also
section 8.9.3.
8.7.2.1 Rats
N-nitrosocarbaryl elicited a carcinogenic response in both
sexes of two strains of rats (Wistar and Sprague-Dawley) by two
routes of administration (oral gavage and subcutaneous injection)
(Eisenbrand et al., 1975, 1976; Lijinsky & Taylor, 1976;
Preussmann et al., 1976; Lijinsky & Schmähl, 1978).
A local carcinogenic effect of N-nitrosocarbaryl was reported
by Eisenbrand et al. (1975) in Wistar rats given a single
subcutaneous injection of 1000 mg/kg. Fifteen out of 16 treated
animals developed sarcomas at the injection site; there was no
histopathological evidence of any systemic carcinogenic effects. The
dose of N-nitrosocarbaryl was highly toxic and 14 out of 16
animals had died by day 450. No spontaneous tumours were observed in
control animals. When given to rats orally in single doses of
200-1500 mg/kg, N-nitrosocarbaryl did not induce tumours during 21
months of observation. No signs of toxicity were noted in any of the
test groups.
Further studies involving repeated oral administration of
N-nitrosocarbaryl to Sprague-Dawley rats gave unequivocal evidence
of local carcinogenic effects, produced by both oral and
subcutaneous routes of administration. (Eisenbrand et al., 1976;
Lijinsky & Taylor, 1976, 1977; Preussmann et al., 1976; Lijinsky &
Schmähl, 1978).
The nonglandular part of the stomach (forestomach) was the
target organ of the local carcinogenic action of N-nitrosocarbaryl,
when it was given orally to rats. All stages of malignant
transformation in the forestomach, from hyperplasia to squamous cell
carcinoma, were observed in male Sprague-Dawley rats treated with
oral doses of 130 mg N-nitrosocarbaryl/kg, twice weekly, until
spontaneous death. There was a substantial lowering of the survival
rate of treated animals because of the pronounced toxicity. By the
time of maximum increase of the tumour incidence (after 200 days),
most of the animals were dead. The average survival time of
tumour-bearing animals was 167 days after the onset of the study.
The low background tumour incidence (3 out of 29) in control animals
included lymphosarcomas and leukaemia.
Table 52. Carcinogenicity studies on N-nitrosocarbaryl in rodents
Chemica Species, strain Sex Route and Dose Duration Significant Evaluation Reference
(number) mode of (mg/kg) of study tumour/organ of individual
administration study
N-nitroso Rat, Wistar male subcutaneous, 1000 450 days polymorphic cell positive Eisenbrand et al.
carbaryl 8: male; female single injection sarcoma at (1975)
8: female injection site;
spindle cell
sarcoma
37 male, oral gavage, 200-1500 21 months none inconclusive
female single
N-nitroso Rat, Sprague-Dawley male oral gavage, 130 (5000 till squamous cell positive Eisenbrand et al.
carbaryl 31 twice per week total) spontaneous sarcoma, (1976)
death papilloma, Preussmann et al.
hyperkeratoses, (1976)
forestomach
N-nitroso Rat, Sprague-Dawley female oral gavage, 50 mg 110 weeks squamous cell positive Lijinsky & Taylor
carbaryl 12 once a week (total) carcinoma (1976)
for 10 weeks forestomach,
papilloma,
oesophagus
12 male oral gavage, 300 mg 90 weeks squamous cell
twice a week (total) carcinoma
for 20 weeks forestomach,
papilloma trachea
N-nitroso Rat, Sprague-Dawley male oral gavage, 600 80 weeks carcinoma positive Lijinsky &
carbaryl 16: male; female once a week (total) forestomach Schmahl (1978)
16: female for 10 weeks
In a comparative study of the carcinogenic potency of
N-nitroso- N-alkylcarbamate esters, Lijinsky & Taylor (1976)
showed that N-nitrosocarbaryl was a strong carcinogen, similar in
action to its highly potent carcinogenic analogue
N-nitrosomethylurethane. A high incidence (75-80%) of
squamous-cell carcinomas in the forestomach was detected in female
Sprague-Dawley rats given 10, weekly, equimolar oral doses
(0.22 mmol) of both compounds. Nitrosomethylurethane was a more
potent carcinogen, because the animals with tumours died earlier
than those in the nitrosocarbaryl group. The same rate of carcinomas
in the forestomach was induced in male rats treated orally with a
considerably higher dose of N-nitrosocarbaryl (1.3 mmol total),
over twice as long a period (20 weeks). The stronger carcinogenic
effect of this dose in male animals was expressed by the shorter
latent period of induced tumours and higher lethality compared with
female animals. The local carcinogenic effect of N-nitrosocarbaryl
in rats, the target organ being the forestomach, was seen in another
oral carcinogenicity study of a series of nitroso- N-methylcarbamate
insecticides carried out by Lijinsky & Schmähl, (1978). The oral
gavage of 10 weekly doses of 60 mg nitrosocarbaryl/kg to both sexes
of Sprague-Dawley rats led to a high incidence (over 70%) of
carcinomas in the forestomach, with no evidence of other systemic
carcinogenic effects.
No carcinogenic response for carbaryl was obtained with the
transplacental carcinogenicity test after in vivo nitrosation in
pregnant Sprague-Dawley rats (Lijinsky & Taylor, 1976). Pregnant
animals were given 30 mg carbaryl/rat, orally, for 10 days (4-18
days of gestation). Two other groups of animals received the same
dose of carbaryl, together with 0.6-1ml of 4% sodium nitrite on days
4-6 and 14-18 of pregnancy. The distribution of tumours in the rats
of various groups was that normally seen in Sprague-Dawley rats. It
is likely that an insufficient amount of nitrosocarbaryl was formed
under the conditions of in vivo nitrosation, or that no
significant amount of nitrosocarbaryl crossed the placenta.
A low rate of in vitro nitrosation of carbaryl under
conditions similar to those that exist in the human stomach was
reported by Eisenbrand et al. (1975). The reaction of
10-3 mol/litre carbaryl with a 5-fold molar excess of sodium nitrite
in 0.1N HC1 (pH, 1) led, after 15-60 min, to yields of only 1.2 and
1.7% of the maximum possible conversion to nitrosocarbaryl.
Reduction of the concentration of carbaryl and sodium nitrite by a
factor of 10 decreased the yield of nitrosocarbaryl by about
one-half. The yields of N-nitrosocarbaryl obtained by the
in vitro nitrosation of carbaryl, under the described conditions,
are low and the potential carcinogenic risk of in vitro nitrosation
and similar nitrosation reactions in vivo is difficult to evaluate
at present.
8.7.2.2 Mice
Nitrosocarbaryl administered for 104 weeks to the skin of
female CFLP mice (65 mice per group) in three doses (12.5, 50, and
200 µg/mouse) was found to be more potent regarding dermal
carcinogenic efficiency than nitrosomethylurea, applied under the
same conditions, though not as effective as benzo- a-pyrene
(Deutsch-Wenzel et al., 1985). Conclusions were drawn on the basis
of the number of animals bearing carcinomas, total cases with local
tumours, observed/expected ratios and dose-response relationships of
incidences of malignant tumours. These results were found to be in
contrast to the ones obtained by Lijinsky & Winter (1981), who used
a single total dose of 23 mg and found that nitrosocarbaryl was a
much less effective inducer of mouse skin tumours than
nitrosomethylurea.
8.7.2.3 Overall evaluation of the carcinogenicity of
N-nitrosocarbaryl
Sufficient evidence of carcinogenicity at the site of
application was seen in multiple studies on both sexes of
Sprague-Dawley rats, by different routes of administration
(subcutaneous and oral gavage), using different dose levels. The
non-glandular stomach was the target tumour site when
nitrosocarbaryl was administered by oral gavage; subcutaneous
injection of nitrosocarbaryl caused sarcomas at the injection site.
The local carcinogenic effect of N-nitrosocarbaryl in rats,
and the lack of any systemic carcinogenicity, characterizes it as a
direct-acting alkylating agent. N-nitrosocarbaryl was active as a
direct bacterial mutagen and interacted with human DNA in vitro.
These data agree with data that show that N-nitrosocarbaryl is an
effective in vivo genotoxin.
8.7.3 Carcinogenicity of ß-carbaryl
Beta-carbaryl (N-methyl ß-naphthyl carbamate) was a component
of technical carbaryl (alpha-carbaryl), suspected of having a
carcinogenic structure and a tumorigenic potential in mice and rats
(Zabezhinski, 1970). In life-time studies on mice, strain CC57W (24
months), and mongrel rats (33 months), using two routes of
administration, oral gavage (10 mg/kg in mice; 25 mg/kg in rats) and
subcutaneous injection (20 mg/kg in mice; 50 mg/kg in rats),
ß-carbaryl showed a weak carcinogenic response. High rates of
lethality (20-50%) were observed in both mice and rats, by the two
routes of administration. No data for survival rates and the
incidence of spontaneous tumours in control animals were presented.
ß-carbaryl caused a low rate of tumours in rats by both routes of
administration. The types of tumours (21%) observed after
subcutaneous injection of ß-carbaryl included: fibrosarcoma at the
injection site, subcutaneous rhabdomyosarcoma, intestinal sarcoma,
leukaemia, reticuloses, and reticulosarcoma. The pattern of
carcinogenic response after orally administered ß-carbaryl (25%)
involved sarcoma in the liver, fibroadenoma and adenocarcinoma in
the mammary glands, carcinoma in the thymus, and granulocellular
carcinoma in the ovaries. No local tumours at the injection site
were observed in control animals after subcutaneous administration
of corn oil. The other tumours (carcinoma in the mammary glands and
thymus, haematopoietic system malignancies) were unusual for control
animals, as well. ß-carbaryl caused a higher tumour incidence in
mice than in rats by both subcutaneous (60%) and oral (30%) routes.
It should be mentioned, however, that most of the tumours observed
(lung adenomas, leukaemia, and liver haemangiomas) occurred
spontaneously in untreated mice. Since no control data were
presented, the carcinogenic response to ß-carbaryl in mice is of
uncertain significance. Because of these deficiencies, this study is
inconclusive.
8.8 Special studies
8.8.1 Neurotoxicity
The effect of carbaryl on the nervous system is primarily
related to ChE inhibition.
Carpenter et al. (1961) studied the delayed neurotoxic
potential of carbaryl in chickens (Rhode Island hens) compared with
that of triorthocresyl-phosphate. Single doses of 250, 500, 1000, or
3000 mg/kg body weight, 25-40% in lard were administered
subcutaneously to chickens. At 2000 mg/kg, weakness was observed on
day 1 or 2 after dosing. In one case, the chicken was unable to walk
for 3 days. No evidence of demyelination was observed in any brain
sciatic nerve or spinal cord section examined microscopically.
According to the authors, there was a transient cholinergic effect
caused by the slow absorption of carbaryl.
The neurotoxic effect of carbaryl was studied in atropinized
chickens (Gaines, 1969) to protect against acute effects of the
subcutaneous injection of 800 or 1600 mg carbaryl/kg body weight.
The higher dose caused leg weakness within 24 h, which recovered by
day 24.
Carbaryl solution in corn oil at a daily dose of 100 mg/kg was
administered orally for 7 consecutive days to 35, 6-day-old, female
broilers, hybrids between Peterson strain roosters and Hubbard hens
(Farage-Elawar, 1989). Altered locomotion and abnormally shortened
gait were observed on the 7th day, and cases of delayed paralysis
20-40 days, after the last treatment. Locomotion changes were found
to have no association with the activities of brain
acetylcholinesterase and neuropathy target esterase, 24 h after the
first, second, third, and fifth doses as well as 1, 3, 6, 10, 20,
30, and 40 days after the last treatment, when no statistical
deviations from the control values of both enzymes were registered.
A similar, but lesser, effect on gait and stride length was
observed when 45 mg carbaryl/kg was injected into chick eggs on day
15 of incubation (Farage-Elawar, 1990). This treatment resulted in
significant inhibition of brain and plasma cholinesterase, and liver
carboxylesterase. Administration of the same dose on day 5 of
incubation resulted in 10% lethality. Brain NTE was not affected.
The cause of this delayed alteration is not known.
Effects of long-term carbaryl exposure on the neuromuscular
system of pigs were reported by Smalley et al. (1969). Six
Yorkshire pigs, 3 male and 3 female, received carbaryl in their diet
(150 mg/kg body weight); the male pigs for 72 days and the female
pigs for 83 days. Three pigs from the same litter were fed carbaryl
at 150 mg/kg body weight daily, for 4 weeks, and then 300 mg/kg body
weight, daily, for 46 days (2 male pigs), and for 85 days (the
female pig). The signs of intoxication started after about 1´
months, and were mostly typical of neuromuscular system damage (see
Table 44; section 8.3). Microscopic examination of the skeletal
muscle revealed myodegeneration. In the myelinated tracts of the
cerebellum, brain stem, and upper spinal cord, moderate to severe
edema was associated with vascular degenerative changes. No
demyelination of nerve tissue was observed. When carbaryl feeding
was stopped and hydrochlorothiazide applied as a diuretic, signs of
toxicity of carbaryl, such as ataxia, and partial paralysis,
disappeared.
Carbaryl disturbed the function of the myoneural synapses. It
produced a decrease in the spontaneous activity and an increase in
the permeability of the muscular fibre membrane for K+ and Na+
after multiple oral administrations of 8.5 mg/kg to rats(Kovtum &
Sokur, 1970).
Kovtun (1970) while analysing the effects of carbaryl on
myoneural formations, pointed out that, at a single intake
(425 mg/kg), multiple intakes during 2 months, or 1/100
LD50-8.5 mg/kg over 6 months, the frequency of tiny potentials of
an edge plate was suppressed by 62-65%. On the basis of the data
obtained it was concluded that carbaryl probably impairs the
function of presynaptic nerve endings. At the same time, carbaryl
does not affect the cholinereceptive membrane substance of muscular
fibres. Carbaryl increases the rest potential of muscular fibres by
11-35%, depending of the carbaryl dose. This increase can be
explained through high potassium ion accumulation inside the
muscular fibres.
Three different laboratories examined the effects of carbaryl
on motor activity in rats. All three reported decreased activity
after intraperitoneal administration, with ED50s that ranged from
13.3 to 17.6 mg/kg (Crofton et al., 1991).
Takahashi et al. (1991) compared the effects of carbaryl on
both young (3 months) and old (12 months) rats. A dose of 50 mg/kg
decreased activity in an open field test, prolonged the duration of
haloperidol-induced catalepsy, decreased body temperature, and
increased the nociception threshold, as measured by a hot-plate
test. A dose of 10 mg also prolonged haloperidol-induced catalepsy.
The effects on body temperature and nociception were significantly
greater in older rats.
Studies were carried out on the mechanical response
characteristics of the soleus muscle in situ. Female Holtzman rats
(6 treated-control pairs) were given 56 mg carbaryl/kg orally.
Carbaryl increased the tension developed during complete tetanus (by
electric stimulus) and decreased the time constant of tension
development (Santolucito & Whitcomb, 1971). The more forceful and
rapid contraction of the skeletal muscle is probably related to an
accelerated catecholamine release.
EEGs on 4 Rhesus monkeys given 0.01 mg carbaryl/kg and on 3
given 1 mg/kg per day, orally, for 18 months, showed only a
reduction in the amount of low-amplitude fast waves and an increased
bilateral synchrony between the right and left hemispheres
(Santolucito & Morrison, 1971). The authors did not relate these EEG
changes to the dose.
Belonozhko & Kuchak (1969) found some changes in the EEG in
rats, such as desynchronisation of rhythms after a single
application of carbaryl at 100 mg/kg. During repeated doses of
35 mg/kg for 90 days, no changes in the EEG were noted, due
(according to the authors) to adaptation of the nervous system. Oral
doses of 72 mg carbaryl/kg, administered to rats for 10 days, caused
a decrease in serotonin and an increase in dopamine levels in the
brain (Kuzminskaya et al., 1984).
Morphological changes in the nervous system caused by carbaryl
were dose-related. One to six months oral treatment of rabbits with
0.01 LD50 caused only haemodynamic disorders and cell
infiltrations, a dose of 0.02 LD50 caused cell dystrophic changes,
and a dose of 0.1 LD50 caused more progressive and serious
disorders (Azizova, 1976).
The behavioural effects of carbaryl were studied in rats and
monkeys. Disturbance of discrete (shock) avoidance behaviour by
carbaryl in rats was reported at ip doses > 2.5 mg/kg, the LD50
being 8 mg/kg (Goldberg et al., 1965). Carbaryl administered at
doses of 8, 16, or 28 mg/kg, ip, decreased maze activity in CD male
rats (10 in each group), whereas doses of 16 and 28 mg/kg reduced
open field activity. After acute exposure, behavioural changes
recovered within 60 min, whereas ChE of the blood and brain
recovered after 240 min (Ruppert et al., 1983).
Carbaryl at 10 mg/kg administered subcutaneously in 16, male,
Long Evans strain rats decreased the number of times they approached
the novel stimuli and explored the exploratory box, and increased
habituation. In familiar situations, carbaryl increased activity
(Albright & Simmel, 1977).
Anger & Setzer (1979) studied the effects of oral and im
administration of carbaryl on repeated chain acquisition in 5 male
monkeys ( Macaccus mascicularis). Oral doses of 50 mg/kg did not
alter the performance of repeated acquisition tasks. The im
injection resulted in consistent changes in performance at 5 and
10 mg/kg, but did not cause any changes at 1 mg/kg.
Carbaryl affected working memory (continuous delayed response
and continuous non-match) in 28 male Sprague-Dawley rats (Heise &
Hudson, 1985a,b). A dose of 10 mg carbaryl/kg, ip, affected
performance of working memory procedures; with increasing dose,
carbaryl nonselectively decreased response.
Administration of 2.24 mg carbaryl/kg, ip for 14 days, did not
affect the performance of rats in activity wheel cages, 24 h after
the last treatment. Acute ip administration of 0.54 or 2.24 mg/kg
significantly decreased the motor activity of rats in activity wheel
cages. This decrease was reversed by atropine sulfate (Singh, 1973).
Doses as low as 7.76 mg/kg ip caused mild tremors.
Oral administration of carbaryl (200 mg/kg, 3 days/week) for a
period of 90 days, though producing inhibition of ChE in blood (33%)
and brain (11%), did not result in any kind of overt signs of
toxicity in male albino rats (Dikshith et al., 1976).
Desi et al. (1974), in long-term studies, investigated the
effects of carbaryl on the learning process, on the performance of
previously learned tasks in mazes, and on the EEG in 40 male Wistar
strain rats. Carbaryl was given at doses of 10 or 20 mg/kg body
weight per day in the diet (100-200 mg/kg food), for 50 days. Mild,
but permanent and increased, functional deviations of the nervous
system were found. Because of increased irritability in the CNS, the
treated rats took less time than the untreated rats to find their
food. Later, the task was performed with difficulty when the
irritability of the CNS was reduced to below the normal level. The
performance of the learned task was impaired. EEG deviations
recorded at the end of the maze studies were slight, but permanent.
They consisted of increased electric activity of the brain,
increased number of moderately slow beta waves, and light flashes of
18 Hz accelerated electrical activity.
Viter (1978) found behavioural changes in rats treated via
inhalation for several months with 12 or 23 mg carbaryl/m3. The
latency period of the conditioned reflex on nutrition was prolonged.
Depression of investigative behaviour and spontaneous motor activity
were noted.
The behavioural effects of carbaryl on rats were examined by
Moser et al. (1988) using a functional observation battery.
Carbaryl was administered at doses of 3-30 mg/kg, ip, and tested
0.5, 3, 24, and 48 h afterwards. Carbaryl decreased spontaneous
activity, CNS excitability, motor and sensory function, and body
temperature and weight. Effects indicative of AChE inhibition were
also observed. All responses were dose dependent.
8.8.2 Effects on the immune system
8.8.2.1 Appraisal on immunotoxicology
The administration of carbaryl, or any other xenobiotic, at
doses resulting in overt toxicity can be expected to result also in
a variety of effects on the immune system. Carbaryl, when
administered in vivo, at a dose not causing overt clinical signs
has been reported to produce a variety of non-life-threatening
effects on the immune system. The effects included cellular as well
as humoral immunity, and several authors have suggested that they
were was due to subtle, treatment-related stress. Many of the
effects described were detected at doses close to the LD50.
Shortcomings of several of these studies were a lack of consistency
and, sometimes, overt contradiction between results, which prevents
the description of a defined immunotoxic mechanism.
Lifetime exposure to carbaryl did not result in increased
occurrence of disease in rats or mice. No enhancement of viral
infections was found with carbaryl, even at dosages close to the
LD50. Most studies on rabbits and mice, at doses permitting
survival, did not produce significant effects on the immune system.
In vitro, a number of researchers have demonstrated viral
enhancement by prior incubation with carbaryl. In vitro, carbaryl
can enhance herpes varicella-zoster, but not herpes simplex. In
goldfish cell culture, carbaryl has been shown to permit viral
enhancement by compromising interferon synthesis. Inhibition of
human serum complement activity as well as interleukine-2 driven
proliferation of large granular lymphocytes have been demonstrated
in vitro. These actions could be mediated through the inhibition
of serine esterases involved in the processes. No increased viral
infections have been seen in long-term in vivo feeding studies,
previously conducted or in progress at present. Carbaryl does not
enhance transformation of BALB/53T fibroblasts in culture or the
expression of endogenous murine leukaemia virus.
8.8.2.2 In vivo studies
Young mice weighing 10-12 g were infected with influenza by
applying 3-4 drops (0.05 g) 1% influenza virus in physiological
solution in the nose. The murine influenza virus strain A.P.R.8 was
used in the primary dilution 1:32. After 2-3 days, this group of 30
mice was treated orally with carbaryl in sunflower oil, at a dose of
500 mg/kg (a dose that killed 3 out of 10 mice). Two control groups
with the same number of mice, one treated with carbaryl alone, and
one infected, were compared with the experimental animals for
survival, blood biochemistry, and pathomorphological changes. The
greatest number of mice dying were in the experimental group. AChE
depression was more pronounced and recovery slower, and there were
more histological changes in the livers of mice infected and
intoxicated by carbaryl (Moreynis & Estrin, 1965). It is clear from
this study that a summation of the effects of both factors occurred,
but, because of the lack of statistical significance, it is
impossible to judge the eventual effect of the enhancement.
The effect of carbaryl on an experimental Erysipelothrix
rhusiopathiae infection in rats was studied by Shabanov et al.
(1983). Rats, treated with doses that increased gradually every 6
days from 2 to 5 mg/rat (mean weight of rats, 50-60 g) during 30
days, were infected iv with Erysipelothrix rhusiopathiae at
1.5-108 cells/rat. The mortality rate in carbaryl-treated rats was
36% versus 14% in control rats. Survival time was halved.
Bacteraemia persisted for 10-12 days in treated groups, and only 5-6
days in the control group. Both the gross and the histopathological
changes were more strongly manifested in the treated group.
Carbaryl, at 0, 2, 20, or 200 mg/kg was administered orally to
rabbits, daily, for 6 months. A decrease in the phagocytic activity
of leukocytes and antibody formation (4-5 times) after immunization
with a murine type of typhoid vaccine, was reported at the dose
level of 200 mg/kg. At the lower dose of 20 mg/kg, there were
different phases of reactivity; at the beginning of the first 2
months, there was an increase and later a decrease in immunological
reactivity (Perelygin et al., 1971).
The effects of oral ingestion of carbaryl on nonreaginic
antibody production in BALB/c mice were studied after pretreatment
with 150 mg carbaryl/kg diet for 10 weeks prior to commencement of
the study. Carbaryl produced significant effects on systemic
antibody production following oral immunization with sheep red blood
cells (SRBC). It significantly increased both IgG1 and IgG2b titres.
No reduction was seen in the synthesis of any antibody class. These
results suggest that, at the doses used, carbaryl increased systemic
antibody responses to orally ingested antigens (André et al.,
1983).
Changes in the immunological structures of lymphatic follicles
in the spleen during carbaryl administration were studied by Dinoeva
(1974, 1982), who used a micrometric method. Albino rats (30
treated-control pair) were given 1.5 mg carbaryl/kg, orally, daily
for 6 months. Plethora in the spleen and retardation in lymphatic
follicles were observed, which were similar to effects seen in the
positive control group treated with cyclophosphamide (2 mg/kg).
There were no changes in the structure and weight of the thymus in
carbaryl-treated rats, while in the cyclophosphamide positive
control group, the weight of the thymus was reduced 2.5 times. The
authors suggested that there is a different mechanism of the
immuno-suppressive effect of carbaryl that needs further research.
A single dose of 500 mg carbaryl/kg inhibited the production of
haemolysins and reduced the number of splenic germinal centres in
White Leghorn chickens (Roszkowski et al., 1976). Dose-dependent
immunosuppressive effects of continued dietary treatment of rabbits
with carbaryl were studied (Street & Sharma, 1975). Rabbits (male
White New Zealand, 7 in each group) were fed 0, 4, 20, 45, or 150 mg
carbaryl/kg diet (corresponding to 0, 0.23, 1.0, 2.3, or 8.38 mg/kg
body weight per day) for 57 days. The treatment reduced the germinal
centres in the spleen and caused atrophy of the thymus cortex. The
antigen-induced increase in serum gamma-globulin was decreased
significantly (no dose relationship was noted), at 10 days. No
changes were detected in the number of plasma cells in the lymph,
haemolysin and haemoglutinin titres, skin sensitivity to tuberculin,
leukocytes count, body weight, etc.
Rats pretreated, orally, with 0.05 LD50 carbaryl, daily, for
2.5 months, were immunized with typhoid antigen. Administration of
carbaryl continued for an additional 2 months. Signs of
insufficiency of the immune system, expressed as a reduction in
specific globulin production, were observed 3.5 months after
carbaryl application began. Carbaryl did not affect immunogenesis at
a dose of 0.001 LD50, daily, under the same exposure conditions.
No overall evaluation was given (Olefir, 1977).
A humoral immunity study in female BALB/C mice after oral
administration of carbaryl was performed by Wiltrout et al. (1978).
The effects of humoral immune competence were measured by the
immunoplaque in gel technique. The number of antibody plaque-forming
cells (PFC) per plate was counted and the PFC/spleen calculated.
Different groups of mice received carbaryl, orally, at about
1 LD50 (153 mg/kg body weight), 5 days before immunization (type
of antigen not specified), on the day of immunization, and 2 days
after. Only the last treatment resulted in significant suppression
of the humoral immune response. The ratio (experimental: control) of
antibody plaque-forming cells was 0.34. The oral administration of
0.1 LD50 for 8 and 28 days did not cause any significant effects
on humoral immune competence. Depression of the PFC/spleen level
corresponds to a decline in the total splenic lymphocyte population.
Akhundov et al. (1981) studied the effects of carbaryl on the
immunological reactivity of 40 rabbits and 40 guinea-pigs given oral
doses of 15 mg carbaryl/kg for 42 days. Heated vaccine Salmonella
typhimurium, at doses of 250 or 500 million microbial cells, was
applied 3 times at 7-day intervals, 21 days after the beginning of
carbaryl administration. The auto-sensitizing effect of the vaccine
was enhanced by carbaryl. Studies on guinea-pigs treated with 15 mg
carbaryl/kg per day for 3 months showed decreased macrophagial
migration activity.
Pipy et al. (1983) evaluated the cellular and humoral
mechanisms of carbaryl-induced reticuloendothelial phagocyte
depression. The function of the reticuloendothelial system (RES)
involved in specific and non-specific aspects of host resistance to
infection, neoplasma, etc., was quantitatively evaluated by the rate
of disappearance of colloids injected into the blood. Colloid
particles are extracted from the blood exclusively by the RES, in
particular liver and spleen macrophages. Plasma or serum factors
called opsonins have a strong stimulatory influence on inert
particle phagocytosis. Male Sprague-Dawley rats (200-240 g) were
given single, iv injections of labelled carbaryl at 0.5-32 mg/kg
body weight (7 different doses). The iv LD50 determined by the
authors was 50 mg/kg body weight. Colloidal carbon was administered
simultaneously. In another group, carbaryl was incubated with serum
from a normal rat for 20 min before the injection. The authors call
this carbaryl "opsonized". The results showed the ability of
carbaryl to induce a state of reticuloendothelial phagocytic
depression, mediated through depletion of opsonins. Carbaryl
inhibits the cell-bound aromatic amino acid esterase of the serine
esterase class. The authors concluded that, apart from a slight
deficiency in plasma opsonins, the inhibitory effect of carbaryl on
the phagocyte function was primarily due to a selective hepatic and
splenic macrophage impairment, which could be related to inhibition
of a cell-bound serine esterase. In another study by Pipy et al.
(1982), more details are given of this possible mechanism of
phagocytoxic blockade by carbaryl. Rats were treated with iv
carbaryl at 8 or 16 mg/kg body weight. The time-dependence of the
effects on phagocytic function and the activities of liver serine
esterases ( N-benzoyl-DL-phenylalanine-ß-naphthyl esterase and
acethylcholinesterase) was studied. Macrophages of the RES had
decreased phagocytic capacities after administration of carbaryl.
Depression of the phagocytosis of colloidal carbon persisted from 1
to 7 h and 1 to 24 h after administration of 8 and 16 mg/kg
carbaryl, respectively. Liver ( N-benzoyl-DL-phenylalanine-ß-
naphthyl esterase (cell-bound serine esterase) was susceptible to
reversible dose-related inhibition by carbaryl. A correlation
between the reactivation kinetics of liver serine esterases and
phagocytic activity was demonstrated.
8.8.2.3 In vitro studies
The replication of goldfish virus 2 (GFV-2) was enhanced in
vitro by pre-treatment of a piscine culture with subtoxic
concentrations (1 mg carbaryl/litre) (Shea, 1983). The mechanism of
enhancement was studied in vitro in goldfish-derived cell lines
and Air Bladder III infected with GFV-2 and pretreated with a
carbaryl solution of 1 µg/ml. Interferon synthesis was studied as a
possible mechanism of virus enhancement (Shea & Berry, 1984). The
authors demonstrated that interferon synthesis was induced in CAR
cells by GFV-2 cells. Antiviral protection provided by supernatants
from infected CAR cultures with carbaryl, against infection of
secondary CAR and Air Bladder III cultures was reduced. The authors
interpreted this phenomenon as a result of general mild suppression
of cellular metabolism, but other possible mechanisms, such as
metabolic interaction, were not excluded. In another study, Shea
(1985) demonstrated quantitative carbaryl inhibition of interferon
synthesis. Supernatants from infected cultures, not treated with
carbaryl, provided 10 times as much antiviral protection as compared
with non-infected cultures. Carbaryl-treated infected culture
provided only twice as much antiviral protection as uninfected
cultures. A similar relationship was observed when comparing the
amount of infectious viral progeny synthesized in the presence of
supernatant from infected cultures not pretreated with carbaryl
(10%), and from infected cultures pretreated with carbaryl (60% of
the amount of virus synthesized in control cultures). The author
speculated about the possibility of superimposition of a viral
infection in a population exposed to pesticides.
Characterization of varicella zoster virus enhancement by
carbaryl was carried out in vitro by Abrahamsen & Jerkofsky (1981)
and Jerkovsky & Abrahamsen (1983). In previous studies, the authors
demonstrated that the replication of varicella zoster virus (a human
herpes virus) is increased 12 to 15-fold by pretreatment of cultures
of human embryonic lung cells (HEL) with carbaryl, and, that
different strains of this virus show differences in sensitivity to
enhancement. Wild-type strains recently isolated from clinical
materials are more sensitive than laboratory adapted strains. In
this study, the authors demonstrated that the maximum enhancement
occurred 48-72 h post-inoculation and that the optimal time for the
pretreatment of monolayers of HEL is 20-24 h. 1-naphthol also
produced increased amounts of virus, but the treated cells cannot
pass on to the daughter cells the ability to enhance virus
replication.
Rodgers et al. (1986) determined that the EC50 for the
inhibition of a T-cell-mediated cytolytic (CTL) response by carbaryl
in vitro was 65.9 µg/ml. The addition of a supernatant containing
liver enzymes reduced the effects of several organophosphorous
pesticides on the CTL response, but had no significant effect on the
potency of carbaryl.
Casale et al. (1989) compared the ability of carbaryl to
interfere with the human serum complement mediated lysis of sheep
blood cells. At a concentration of 3 mmol/litre and with 2-h
preincubation, carbaryl inhibited lysis by 26-45%, depending on the
antibody concentration. Carbaryl was a more potent lysis inhibitor
than diisopropyl phosphorfluoridate or four other anticholinergic
insecticides; the potency was not correlated with anticholinergic
activity.
The effects of carbaryl at concentrations of
0.5-500 µmol/litre on lymphocyte proliferation were studied in
vitro (Bavari et al., 1991). Carbaryl inhibited (3H) thymidine
incorporation, in a concentration-dependent manner, by as much as
50%. Alpha-naphthol also had a slight effect at 50 µmol/litre. The
authors attributed the effect to inhibition of an esterase
responsible for interleukin activation.
8.8.3 Effects in blood
Carbaryl affects the coagulation process. Hyper- and
hypocoagulation were reported in different studies (Hassan & Cueto,
1970; Gapparov, 1974; Lox, 1984; Krug & Berndt, 1985; Krug et al.,
1988).
Gapparov (1974) studied the indices of blood coagulation in
dogs after oral treatment with a daily dose of 2 mg carbaryl/kg body
weight, over 5 months. A clearly manifested hypercoagulation was
established, which was connected with a rise in the general
coagulation activity of the blood, higher thromboplastic activity,
and prothrombin content, increased number of thrombocytes,
accelerated coagulation time, and increased activity of the
fibrinostabilizing factor. Also noted was a decrease in the
recalcification time of blood plasma, fibrinogen concentration, and
free heparin quantity, together with an inhibition of the fibrolytic
activity of the blood. The author interpreted all these changes as
being connected with the arousal of the parasympathetic system.
The blood coagulation time was considerably shortened in
rabbits that were given, orally, a mixture of carbaryl (5 mg/kg),
DDT (5 mg/kg), and parathion (0.5 mg/kg), for 222 days. This effect
corresponded to increased levels of 5-hydroxy-3-indolacetic acid
(5-HIAA) and 4-hydroxy-3-methoxymandelic acid (VMA) in the urine,
indicating an increased rate of metabolism of serotonin and
catecholamine (Hassan & Cueto, 1970). The authors suggested that
this effect was a manifestation of non-specific stress, since
adrenocortical hormones shorten the coagulation time.
Fifteen male Sprague-Dawley rats (250-300 g) were given
drinking-water containing 10 mg carbaryl/litre for 30 days. Platelet
count, prothrombin time, partial thromboplastin time, fibrinogen,
and clotting factor activity for coagulation factors II, V, VII,
VIII, IX, X, and XII were determined. A significant decrease in
platelet count and in the factor VII clotting activity was observed
compared with those in the same number of control rats. Microscopic
evaluation of the liver revealed hepatocyte degeneration, central
vein congestion, some leukocytic infiltration, and vacuolization of
the cytoplasm. The author suggested that carbaryl might have harmful
effects on haemostasis (Lox, 1984).
Carbaryl has an in vitro inhibitory effect on arachidonic
acid-induced platelet aggregation, which corresponds to its
inhibition of thromboxane B2-formation. The results suggest that
carbaryl affected platelet aggregation by inhibition of
cyclooxygenase (the key enzyme of prostaglandin synthesis) by
carbamoylation (Krug & Berndt, 1985). At a concentration of
10 µmol/litre, carbaryl completely blocked platelet aggregation and
cyclo-oxygenase activity (Krug et al., 1988). The carbamoylation
of several platelet proteins, including cyclo-oxygenase, may be
responsible for this effect.
Carbaryl produced, in vitro, a dose-dependent increase in
methaemoglobin (Met Hb) formation at 10 and 100 mg/litre, as well as
decreases in reduced glutathion levels in the erythrocytes of Dorset
sheep with low erythrocyte glucoso-6-phosphate dehydrogenase
(G-6-PD), which is similar to humans who have G-6-PD deficiency.
Carbaryl posed oxidative stress to G-6-PD-deficient red cells,
probably due to its major metabolite alpha-naphthol (Calabrese &
Geiger, 1986). Decreases in the K+ ion concentration in
erythrocytes (with more than 24%) and in haematocrits (from 43.5 to
38%) were found by Sokur (1971) in rats fed carbaryl 0.05 LD50/day
for 2 months.
In an in vitro study, Szczepaniak & Jeleniewicz (1981) found
that carbaryl binds free blood amino acids (plasma and
erythrocytes). These authors also performed a series of in vitro
studies to investigate the effect of carbaryl on amino acids. A
single application of 475 mg carbaryl/kg on 46 treated and 12
control rats produced significantly decreased amino acid values in
the brain, except for valine and phenylalanine. All amino acids
reached the control level after 72 and 120 h. Two hours after
administration, erythrocyte amino acids also decreased >50%
(Szczepaniak & Jeleniewicz, 1980; Jeleniewicz et al., 1984). A
slight decrease in erythrocyte amino acid concentrations was
observed after 30 days with 95 mg carbaryl/kg administered orally
(Jeleniewicz & Szczepaniak, 1980). Blood serum amino acids in 24
rats decreased 4 h after a single application of 189.6 mg/kg. There
was a larger decrease in valine, then in phenylalanine, alanine,
aspergic acid, serine, and glycine (Szczepaniak et al., 1980).
After 15-day dosing of 0.1 LD50(94.8 mg/kg) per day to 12 rats,
there were no changes in levels in serum and erythrocytes, but after
30-day dosing in 8 rats, there was a slight decrease in the alanine
concentration of the erythrocytes (Jeleniewicz & Szczepaniak, 1980).
The effects of carbaryl on the thermoresistance and fractional
content of blood serum proteins was studied by Subbotina &
Belonozhko (1968). A single dose of 150 mg carbaryl/kg administered
to rabbits and multiple applications of 100 mg carbaryl/kg for 2
months showed that, with a single application of carbaryl, there was
an increase in protein thermocoagulation from 28% on day 1 to 67% on
day 7, and with multiple applications of carbaryl there was a
lowering of albumin levels and a rise in globulins (mostly
alpha-globulins) in serum, on day 10. These changes were reversible.
There was also a decrease in the coefficient of thermal dehydration.
The changes in proteins in both studies show lowering of their
lability.
Carbaryl inhibited the incorporation of 3H-uridine and
14C-labelled amino acids into RNA and proteins in cultures of HeLa
cells. The effect was dose-dependent. Incorporation was inhibited by
50% at a 150 µg/ml concentration after 30 min incubation. At a
concentration of 350 µg/ml, only 10% of amino acids and 32% of
uridine incorporation activity were retained (Myhr, 1973). Later,
Blevins & Dunn (1975) showed that carbaryl caused general metabolic
changes in Hela cells. A concentration of 1-2 mg carbaryl/litre
stimulated all divisions and, at 4-8 mg/litre, inhibited growth. A
decrease in cellular protein at 4 mg/litre was noted. Changes in the
phospholipid fraction at 8 mg/litre were probably related to the
alteration of the structure of cellular membranes. Disturbances in
the development of human cells in culture were also reported by
Shpirt (1973). An inhibitory effect of carbaryl on cell development
was demonstrated in Ehrlich ascites tumours in mice. A reduced rate
of incorporation of labelled uridine-5-3H-thymidine methyl-3H
and L-leucine 14C in the RNA, DNA, and proteins in Ehrlich cells
in vitro was also reported (Walker et al., 1975b). The authors
suggested that this effect could be a basis for investigating the
mechanism of the adverse effects that carbaryl has on reproduction.
Carbaryl and N-nitrosocarbaryl appeared to have different
action characteristics with regard to rat liver microsomal membrane
alterations (Beraud et al., 1989b); carbaryl was ineffective on
the lipoperoxidation indices while the nitroso compound had an
inhibitory action on the formation of malonaldehyde and conjugated
dienes as well as on the NADPH-dependent reductase activities.
8.8.4 Effects on the liver and other organs
Several authors reported data on disturbances in the
carbohydrate, protein-forming, and detoxicating functions of the
liver.
A single application of 300 mg carbaryl/kg in rats produced an
increase in albumin and alpha-globulins, and a decrease in ß-and
gamma-globulins (Zapko, 1970).
Kagan et al. (1970) studied the effects of carbaryl on the
liver of 180 rats and 18 rabbits. During an 11-month study, they
gave a daily, oral dose of 38 mg carbaryl/kg to rats. After 1 month,
they observed a rise in the alanine-aminotransferase and
alkaline-phosphatase activities in serum, and a decrease in succinic
dehydrogenase and glycogen in the liver. Doses of 0.76 mg/kg and
0.38 mg/kg, given in the diet to rabbits, caused retention of
bromsulfophthaleine in the blood. The authors also reported a
changed ratio in the protein fractions in serum and an increase in
liver weight. The pathomorphological changes in the liver were
destructive, necrobiotic, and proliferative. An effect on the liver
was also demonstrated in the study of Lox (1987) (see section
8.8.3).
Pavlova et al. (1968) found that, with acute and long-term
exposures, carbaryl affected the oxidative processes in tissues,
because of its direct action on the enzymes of cell respiration and
possible disturbance in the membrane processes. They performed
studies on rats treated with 0.2 LD50 for 3 days and on rats
treated with 0.01 LD50 for 20 weeks. At the higher dose, there
were decreases in the cytochromoxidase and succinedehydrogenase
activities in the liver and brain mitochondria. A histochemical
examination revealed a rather high, irregular activity of the
cytochromoxidase in the heart, as well as increased succinic
dehydrogenase activity. With the long-term dosing, the changes were
not significant, but there was a lowering of the cytochromoxidase
and succinedehydrogenase activities in the heart mitochondria.
Development of experimental cholesterol arteriosclerosis in rabbits
was facilitated by the application of 20 mg carbaryl/kg for 2.5
months (Lukaneva & Rodionov, 1973). Changes were found in the
following indices: general cholesterol, ß-lipoproteins, ECG changes,
and pathomorphological changes in the aorta and coronary vessels.
Carbaryl (100 mg/kg body weight) given to dogs in their diet
for 45 days caused disturbances in the secretion of the intestinal
enzymes. There was an increase in enterokinase secretion, as well as
in the excretion of alkaline phosphates and lipase into the
intestinal juice. The no-observed-effect level (NOEL) for these
effects was 700 µg/kg body weight, which corresponds to 7 mg
carbaryl/kg diet. Three dogs were used in each group (Georgiev,
1967).
8.8.5 Effects on serum glucose
Orzel & Weiss (1966) found that a rise in blood glucose
correlated with the onset and duration of tremors and the degree of
brain ChE inhibition in rats that were treated ip with 5 and 25 mg
carbaryl/kg. The hyperglycaemic response is blocked by
adrenal-ectomy and is unaltered by hypophysectomy. The authors
suggested that the hyperglycaemia was related to increased secretion
of epinephrine. Hyperglycaemia is thought to result from cholinergic
stimulations as it is also found in acute intoxications with
organophosphorous compounds (Kaloyanova, 1963). In their studies,
Orzel & Weiss did not find changes in liver glycogen. Muscle
glycogen was decreased only in non-fasted rats. Elevated levels of
blood glucose and slightly reduced immunoreactive insulin were found
with repeated oral exposure of rats at 3 mg/rat, weekly, for one
year (Wakakura et al., 1978). Glycogen disappeared in most of the
hepatocytes. The cells mainly contained related granular endoplasmic
reticulum with swollen mitochondria. A single application of
30 mg/rat produced transient hypoglycaemia at 20 h followed by
hyperglycaemia at 44 h. The effects of carbaryl on respiration,
glycolysis, and glycogenesis in isolated hepatocytes of male Wistar
rats were studied by Parafita et al. (1984) and Parafita &
Fernandez Otero (1984). The cells were treated with concentrations
of carbaryl of 0.01, 0.1, and 1.0 mmol/litre dissolved in 1%
dimethylsulfoxide (DMSO). DMSO slightly stimulates the respiratory
coefficients. Carbaryl decreased the metabolic output of CO2 at
all concentrations; oxygen consumption was reduced by 40% in
relation to the DMSO-treated groups only at the highest
concentration (1.0 mmol/litre). Glucose remained unchanged in the
presence of carbaryl. Endogenous production of lactic acid was not
affected, and net metabolic production was strongly inhibited by
both DMSO and carbaryl. At a maximum carbaryl concentration
(1 mmol/litre), the net lactic acid production was completely
blocked. Carbaryl inhibited lactate gluconeogenesis, and, to some
extent, gluconeogenesis from fructose pyruvate and alanin. Glycerol
glucogenesis was unaffected. Lactic dehydrogenase activity was
reduced by 38% and glucose 6-phosphate synthetic activity was
increased by 1.0 mmol carbaryl/litre. Aspartate aminotransferase
activities (cytoplasmic and mitochondrial fractions) were inhibited
by 0.1 and 1.0 mmol carbaryl/litre. The results indicated that
carbaryl causes a decrease in glucose production by hepatic cells
and suggested that carbaryl-induced hyperglycaemia in fasted animals
is caused by deficiencies in the peripheral utilization of the
glucose.
8.8.6 Interactions with the drug metabolizing enzyme system
Carbaryl is a weak inducer of hepatic microsomal
drug-metabolizing activity. Cress & Strother (1974) reported
depressed hexobarbital sleeping time in mice given 0.125 or 0.5
LD50 carbaryl, orally, for 10 days. These authors studied the
effects of a 2-week dietary administration of high levels of
carbaryl (10 times higher than the dose given in the earlier study).
Weanling male Swiss-Webster mice, weighing 17-20 g, were divided
into 5 groups of 40 animals each. The daily consumption of carbaryl
by each animal was approximately 119% of the acute LD50. This dose
was well tolerated with no deaths or overt symptoms of
cholinesterase inhibition, probably because carbaryl was
administered over a 24-h period, instead of in a single injection.
Feeding carbaryl resulted in a 44% increase in the rate of in vitro
p-hydroxylation of aniline, and increased in vitro demethylation
of benzphetamine. The hepatic levels of cytochrome P-450 and b5
were increased, but the microsomal protein concentration per gram of
liver was not affected. No changes were reported in
1-naphthol-treated mice. Increased metabolic activity was
demonstrated with phenobarbital and carbaryl. Phenobarbital sleeping
time was shortened to 45 min (control 74 min) in carbaryl-fed mice.
The rate of elimination for the carbaryl-treated animals was twice
as high as that for control animals. The oral LD50 of carbaryl in
carbaryl-pretreated animals (14 days feeding) was three times as
high as in the control animals. 1-Naphthol did not affect the levels
of cytochromes. Sleeping time and the LD50 for carbaryl were not
different in control and 1-naphthol pretreated animals.
Liver weight was low in carbaryl-fed mice, but higher as a
fraction of body weight. The authors concluded that the degree of
the enzyme induction was not high, because most indicators exceeded
the control values by 50% only, except for the lethal dose level.
The pretreatment of rats with 5 daily doses of 10 mg
carbaryl/kg, administered ip, resulted in a 4.2-fold increase in the
rate of benzo( a)pyrene metabolism (Lesca et al., 1984). Total
microsomal P-450 content and benzphetamine demethylase activity were
not significantly affected. The increased ability to hydroxylate
aniline in mice under carbaryl treatment was reported by Guthrie
et al. (1971).
Exposure of murine 3T3 fibroblasts to carbaryl at a
concentration of 10-6 mol/litre was found to increase aryl
hydrocarbon hydroxylase activity (Lahmy et al., 1988). The
magnitude of the effect increased with the duration of exposure.
Carbaryl induction of microsomal enzyme systems in White
Leghorn cockerels was demonstrated by Puyear & Paulson (1972). A
decrease in phenobarbital sleeping time, an increase in liver weight
and elevation of liver aniline hydroxylase activity were found after
oral treatment with carbaryl at 100 mg/kg per day (in gelatine
capsules) for 3 or 6 days. No effect was observed at a dose of
50 mg/kg. Thus, the sleeping time data indicated that the NOEL of
carbaryl for Leghorn cockerels was between 50 and 100 mg/kg per day.
Higher doses caused an increase in liver aminopyrine demethylase
activity (200 or more mg/kg per day) and in liver cytochrome P-450
content (300 or 400 mg/kg per day). Microsomal protein was not
changed. All study parameters returned to control values by day 11
after the treatment was terminated (Puyear & Paulson, 1972).
El-Toukhy et al. (1989) reported that the administration by
gavage of carbaryl to mice at a dose of 166 mg/kg resulted in small,
but statistically significant (15-20%), decreases in liver pyridoxal
phosphokinase and L-tryptophan 2,3-dioxygenase activity.
Administration over a 5-day period of 83 mg carbaryl/kg per day had
no significant effect. Hassan et al. (1990) administered 85 mg/kg,
orally, either once or five times daily. Both the single and
repeated regimens resulted in approximately 40% decreases in
L-tryptophan 2,3-dioxygenase. Carbaryl was also found to be a
competitive inhibitor of this enzyme in vitro.
Inhibition of monoamino-oxidase (MAO) was reported in the
presence of carbaryl in vitro in liver homogenate (Hassan et al.,
1966). The authors suggested that MAO and the catalase system are
involved in oxidative demethylation during carbaryl detoxification.
Exposure of rat liver microsomes to carbaryl in vitro at a
concentration of 0.5 mmol/litre resulted in decreases in the
activities of several monooxygenases (Knight et al., 1986). The
ability of carbaryl to inhibit the N-demethylation of
ethylmorphine appeared to be competitive with a Ki of
2.4x10-4 mol/litre. However, when administered to rats at doses of
up to 25 mg/kg, carbaryl did not have any significant inductive or
inhibitory effect.
Lechner & Abdel-Rahman (1986a) reported that the in vitro
incubation of 2 and 4 mmol carbaryl/litre for 60 min reduced
glutathione levels and increased glutathione S-alkyltransferase in a
rat liver homogenate in a dose- and time-dependent manner. Carbaryl
had no apparent effect on glutathione levels in whole blood with
120 min of incubation. Carbaryl inhibited N-demethylation and
O-demethylation by rat liver microsomes with apparent Ki of 0.1
and 0.7 mmol/litre, respectively (Beraud et al., 1989a).
Carbaryl had an antagonistic action on the release of
microsomal ß-glucuronidase by malathion in a microsomal suspension
in vitro (Lechner & Abdel-Rahman, 1985).
Jacob et al. (1985) studied the effects of carbaryl on the
metabolic activation of the environmental, carcinogenic, polycyclic
aromatic hydrocarbons. Carbaryl was a weak inducer of metabolism of
benz( a)anthracence in the rat liver, and altered its metabolite
profile by shifting from 10, 11-oxidation to 5, 6- and 8,
9-oxidation. Since carbaryl did not induce bay-region activation,
the authors concluded that tumour-promoting activity could not be
expected.
8.8.7 Effects on the endocrine system
The effect of carbaryl on the neuroendocrine system was studied
in rats. Carbaryl was given, orally, at 7, 14, or 70 mg/kg body
weight to male and female rats (each group contained 24 animals
weighing 90-130 g) for a period of 1 year. Growth retardation,
changes in the blood enzymes, and endocrine gland disturbances
varied with the dosage, being especially marked at the end of the
study in the group receiving 70 mg/kg body weight per day.
Acetylcholinesterase and butylcholinesterase activities were
inhibited significantly in these groups. Spermatozoon motility was
reduced progressively with the duration of the exposure. In the
prolonged estrus cycle, the diestrus period was particularly
affected. An increase in the number of corpora lutea and atrophic
follicles in the ovaries was correlated with this disturbance. There
was a dose-dependent increase in the gonadotropic function of the
hypophysis, which was determined by tests on immature mice.
Hypophyseal homogenate from a rat given 70 mg carbaryl/kg increased
the weight of the ovaries by 51.5%, and that of the uterus by 123%,
compared with the weights in control mice. At all doses,
histochemical studies of the hypophysis showed changes indicative of
an increase in the activity of the cells producing a luteinizing
gonadotrophy, i.e., an increase in the size of the cells, loss of
granules, and hyalinization of the cytoplasm. Histological
examination of the adrenal glands revealed an increase in the size
and mitotic activity of cells in the zone glomerulosa. Enlarged
cells with a large nucleus or two nuclei were present in the
fascicular zone. Impairment of thyroid gland functional activity in
the test group was indicated by the reduction in the rate of
absorption and excretion of 131I and its rather low recovery, as
well as by the corresponding histological findings, i.e.,
enlargement of follicles and more dense and basophilic colloid at
all doses. Histological findings were more pronounced in the
70 mg/kg per day groups. It is likely that the effects of carbaryl
on the reproductive organs is mediated by the endocrine glands.
However, the possibility of a direct effect was not excluded by the
authors (Rybakova, 1966; Shtenberg & Rybakova, 1968; Shtenberg
et al., 1970).
The effect of carbaryl on the thyroid gland in rats was studied
by Shtenberg & Hovaeva (1970). Rats were given carbaryl, orally, at
0.7, 2, 5, or 17 mg/kg per day for 6 months. They were fed a normal
iodine diet or an iodine-deficient diet. There was an initial
increase in function in the first 3.5 months of exposure and a
decrease after 6 months of exposure. Recovery was complete 2 months
after the end of the exposure. Decrease in iodine uptake and
reduction of function was 26.5% in rats given 5 mg/kg and 29.5% in
rats given 15 mg/kg. Rats fed an iodine-deficient diet were more
sensitive to carbaryl-induced changes in the thyroid.
The functional states of the thyroid gland and adrenal cortex
were affected by a daily administration of 36 mg carbaryl/kg to rats
for 4 months. The maximum accumulation of 131I occurred after 6 h
and, in the control animals, after 12 h. The 131I absorption rate
normalized after 45 days, but its excretion was delayed. The
concentration of sodium in the blood increased by 36.4% after day 15
and by 74% after 45 days, while the potassium concentrations were
reduced. The daily excretion of 17-corticosteroids in the urine
increased by 35.9%. The changes in the thyroid and adrenal glands
were transient (Dyadicheva, 1971).
Hassan (1971) studied the effects of carbaryl on the synthesis
and degradation of catecholamines in the rat. Rats were given
carbaryl in the diet at concentrations of 100 or 700 mg/kg for 7
months, or in peanut oil at single oral doses of 50, 80, or
250 mg/kg, or 3 single doses of 80 mg/kg body weight. On day 30, the
rats given 700 mg/kg diet eliminated amounts of urinary
4-hydroxy-3-methoxy mandelic acid (VMA) increased by more than 300%;
levels returned to normal after 195 days. A similar effect was
demonstrated after single oral doses with a dose-effect
relationship. The amount of 5-hydroxyindole acetic acid (5-HIAA) in
the urine increased in rats that were treated with a single dose and
3 successive daily doses of 80 mg/kg per day. Adrenalectomy and
hypophysectomy did not change the pattern of VMA excretion produced
by carbaryl. There was an increased turnover rate (68%) of heart
norepinephrine (NE-3H) in rats treated with 80 mg carbaryl/kg, 2 h
before application of NE-3H. The corticosterone level was increased
by about 125% at the same dose level. A carbaryl concentration of
16.2 µg/ml in the blood was enough to trigger maximal corticosterone
secretion. The authors suggested a probable activation of the enzyme
system involved in the catecholamine metabolism, because carbaryl
may also directly affect the adrenergic nerve endings. Activation of
the pituitary adrenal axis and an increase in sympaticoadrenergic
activity with concomitant increased VMA elimination was a
pharmacological effect due to carbaryl treatment.
The effect of a single oral dose of 60 mg carbaryl/kg body
weight on serotonin (5-HT) metabolism in the male Holtzman Albino
rat brain was studied by Hassan & Santolucito (1971); 2, 4, 6, and
24 h after carbaryl application, whole brain samples were analysed
for serotonin, 5-HIAA, and corticosterone. The concentrations of
5-HT and 5-HIAA in the brain increased by 20-30% (5-HT from 0.61 to
0.8; 5-HIAA from 0.25 to 0.31 µg/g) from 2 to 6 h and returned to
normal after 24 h. The plasma corticosterone level was also
increased by about 125%, 1 h after oral administration of carbaryl,
and returned to normal after 20 h. The inhibitory effect of
p-chlorophenylalanine on brain 5-HT formation was diminished by
carbaryl treatment. The serotonin level in the brain of
carbaryl-treated animals pretreated with p-chlorophenylalanine was
72% higher than in the control animals. Increased 5-HIAA levels were
probably a result of an increased rate of synthesis of serotonin.
The authors suggested the possible role of the increased synthesis
of serotonin and tryptophan-5-hydroxylase activity, which could be
related to stress conditions. Carbaryl activation of MAO is probably
related to the increased formation of cerebral 5-HIAA.
The uptake of noradrenaline in vitro by hypothalamic slices,
isolated from rats exposed in vivo to near lethal levels of
carbaryl, was increased in a dose-dependent manner (Jablonska &
Brzezinski, 1990).
Oral administration of 200 mg carbaryl/kg to rats was reported
to raise striatal levels of noradrenaline and homovanillic acid, a
metabolic product of dopamine, when measured 0.5-2 h later (Ray &
Poddar, 1985b).
The mechanism of the stimulation of corticosterone secretion is
not clear.
In vivo studies on male mice receiving 38 and 68 mg
carbaryl/kg showed that carbaryl can increase certain hepatic
androgen hydroxylase activities. In vitro incubation of carbaryl
with prostate tissue and testosterone 1,2-3H stimulated the
formation of dehydrotestosterone-3H, thus, suggesting a direct
action on steroidogenesis. Comparing these results with the effects
of other toxic substances, the authors concluded that carbaryl
cannot exert any major changes in steroid metabolism, nor can it
reduce hormonal disturbances (Dieringer & Thomas, 1974).
Attia et al. (1991) studied the effect of carbaryl on rat
melatonin production as alterations of the latter were found to have
marked consequences in reproduction, immunology, and tumour growth.
Male albino rats ( Rattus rattus), 8 animals in a group, were
exposed to light/dark cycles of 14/10 h. They were treated by
gastric gavage with carbaryl dissolved in corn oil for 6 successive
days. The total doses were 50, 125, and 250 mg/kg. The animals were
killed 2 and 4 h after the onset of darkness, which was 8 or 10 h
after the application of the insecticide. Carbaryl was found to
bring about an augmentation of the pineal melatonin content 4 h
after the onset of darkness, which coincided with the stimulated
N-acetyltransferase levels and hydroxyindole- O-methyltransferase
activity. However, at the same time, carbaryl treatment at all doses
significantly lowered the circulating melatonin titres; this was
supposed to be related to increased hepatic melatonin metabolism due
to the increased activity of the liver drug metabolizing enzyme
system (Gaillard et al., 1977). These results and the established
carbaryl-induced elevated pineal 5-hydroxytryptophan, serotonin, and
5-hydroxyindole acetic acid contents in the course of the night
cycle, support the concept that carbaryl has a significant effect on
pineal melatonin synthesis and secretion.
Kadir & Knowles (1981) reported that carbaryl inhibited rat
brain monamine oxidase activity in vitro.
8.8.8 Other studies
Human serum albumin reacted in vitro with the ester group of
carbaryl and catalysed the hydrolysis and liberation of 1-naphthol.
This reaction is similar to an "esterase type" action (Casida &
Augustinsson, 1959) called carbamoylation (Oonnithan & Casida,
1968).
8.9 Factors modifying toxicity, toxicity of metabolites
8.9.1 Factors modifying toxicity
The toxicity of carbaryl can be modified by altering liver
function by tranylcypromine treatment or 70% hepatectomy. A decrease
in LD50 and increase in ChE activity were more pronounced after
tranylcypromine treatment (Falzon et al., 1983).
The carbaryl LD50 in animals was increased three-fold by
pretreating animals with small doses of carbaryl (Cress & Strother,
1974).
Phenobarbital, administered 24 h before carbaryl treatment,
decreased the acute ip toxicity of carbaryl in mice, while
2-diethyl-amino-ethyl-2,2'-diphenyl-valerate-HC1 (SKF 525-A), given
1 h before carbaryl treatment, increased its acute intraperitoneal
toxicity (Neskovic et al., 1978). Enzyme-mediated binding of
carbaryl to rat hepatic microsomal protein occurred in vitro in
the presence of NADPH and oxygen. Incorporation of radioactivity of
14C-ring labelled carbaryl was studied. A 2- to 3-fold increase in
binding was produced by pretreatment of animals with MFO inducers,
e.g., phenobarbital or 3-methylcholanthrene. SKF-525-A inhibited
binding by approximately 77% of the radioactivity. It is likely that
binding of active carbaryl metabolites formed by MFO occurs. The
radiolabelled metabolic products were covalently bound to amino acid
residues of microsomal protein: 99.3-99.7% of the bound
radioactivity (Miller et al., 1979). A 50% decrease in microsomal
ß-glucuronidase activity was observed 1 h after a single oral
administration of 50 mg carbaryl/kg to female, Sprague-Dawley rats.
The whole liver homogenate ß-glucuronidase content was reduced by
40% after daily treatment with the same dose of carbaryl for 7 days.
This effect was attributed to the decreases in mitochondrial
lysosomal and microsomal ß-glucuronidase. The action of carbaryl in
depleting the endoplasmic reticulum of ß-glucuronidase led to an
increase in serum ß-glucuronidase activity. This was demonstrated in
an in vitro microsomal suspension study which showed that
incubation of 4 mmol carbaryl/litre with microsomal suspensions
effected a release of the ß-glucuronidase enzyme. The effect on the
endoplasmic reticulum was further exemplified by the induction in
microsomal UDP-glucuronyl transferase activity after 7 days of daily
treatment with carbaryl at a dose of 25 mg/kg. Thus, carbaryl
modifies the enzyme activities associated with the endoplasmic
reticulum by decreasing specific activity (90%) towards
ß-glucuronidase enzyme and activating the synthesis of endoplasmic
reticulum protein connected with drug metabolism, such as the
UDP-glucuronyl-transferase enzyme (Abdel-Rahman et al., 1985;
Lechner & Abdel-Rahman, 1985).
Osman & Brindley (1981) conducted bioassays to determine the
carbaryl susceptibility and synergism with piperonyl butoxide in
natural populations of three species of Lobops grassbugs, in order
to estimate monoxygenase detoxification. Pretreatment of the insects
with piperonyl butoxide inhibited the mixed-function oxidase system
and decreased the values of the LC50 for the insects (Osman &
Brindley, 1981). Mixed-function oxydase involvement in the
biochemistry of synergistic insecticides (including carbaryl) has
been reviewed by Casida (1970).
Diet may modify carbaryl toxicity. Boyd & Boulanger (1968)
reported an increased susceptibility to carbaryl toxicity in Albino
rats (272 Wistar strain rats were used) fed a protein-deficient
diet. Boyd & Krijnen (1969) also reported that the LD50 decreased
from 589 mg/kg in rats fed an 81% casein diet to 67 mg/kg in rats
fed a 0% casein diet; 285 male rats were used in this study.
The combined effects of different ambient temperature levels
and carbaryl were studied by Ahdaya et al. (1976). The LD50
values in mice injected ip were 263 mg/kg at 1 °C and 122 mg/kg at
38 °C (588 mg/kg is the LD50 at 27 °C). The thermoregulation
ability of mice treated with carbaryl was affected more than that of
mice treated with parathion which is a stronger inhibitor of ChE.
The authors suggested that this effect was due to an overall
reduction in the basal metabolic rate.
Atropine sulfate decreased signs of parasympathetic stimulation
in a group of pigs acutely poisoned with 1-2 g carbaryl/kg (Smally,
1970). During multiple administrations of carbaryl, drug-induced
(hydrochlorothiazide) diuresis helped in the detoxification
processes. ChE reactivations are contraindicated because they may
aggravate the signs of carbaryl intoxication (Sanderson, 1961;
Podolak & Warchocki, 1980).
Application of atropine or trepazin, 20 min before oral
intoxication with carbaryl in mice, decreased toxicity about 2
times. The results were similar when cholinolytics were applied
2 min after administration of carbaryl (Vyatchanikov & Alexachina,
1968). Atropine administered iv to rats at a dose of 8 mg/kg
increased the carbaryl ip LD50 by a factor of about 7 (70 to
460 mg/kg) (Harris et al., 1989). Co-administration of either of
two different oximes (2-PAM and HI-6) with atropine was found to
provide significantly less protection than that afforded by atropine
alone, indicating that oxime therapy is not a useful treatment for
carbaryl poisoning.
Co-administration of an oral dose of 10 mg malathion/kg in rats
significantly reduced the rates of both absorption and elimination
of an oral dose of 10 mg carbaryl/kg (Lechner & Abdel-Rahman,
1986a). Peak plasma levels of labelled carbaryl were observed about
1 h after the administration of carbaryl alone, and after 2 h when
malathion was administered simultaneously. The terminal rate of
elimination of carbaryl was decreased by a factor of about 4, with
the half-life increasing from 16.96 h to 64.41 h.
Concentrations of cimetidine in the range of 60-240 µg/ml were
observed to decrease the clearance rate of carbaryl by perfused rat
liver in a dose-dependent manner (Ward et al., 1988).
Ray & Poddar (1985a,b, 1990) reported that the intraperitoneal
administration of 1 mg haloperidol/kg, 12.5 to 100 mg
5-hydroxy-tryptamine (5-HTP)/kg, or 100 mg L-tryptophan/kg increased
the incidence of tremors in rats following an oral dose of 50-200 mg
carbaryl/kg. The potentiation of the tremorogenic effect of carbaryl
by 5-HTP was demonstrated to be dose dependent. The potentiating
effects of 5-HTP and haloperidol could be blocked by the
coadministration of the serotonergic antagonist methysergide
(20 mg/kg, ip) or the dopaminergic antagonist bromocriptine
(10 mg/kg, ip), respectively. These results suggest the possible
involvement of a central cholinergic, dopaminergic interaction in
the carbaryl-induced tremor.
The effects of humic acids in the detoxification of carbaryl
during oral application are very slight: only 9.9-18.6% decrease in
toxicity was observed (Golbs et al., 1984).
The combined effects of carbaryl and sodium nitrite have been
studied (Podolak-Majczak & Tyburczyk, 1984, 1986; Tyburczyk &
Podolak-Majczak, 1984a,b; Tyburczyk & Podolak-Majczak, 1986). Dosing
Wistar rats for 3 months with sodium nitrite at 20 mg/kg per day,
and carbaryl at 0.1 LD50/day (60 mg/kg), the brain
gamma-aminobutyryl acid level, methaemoglobin, blood serotonin, free
blood tryptophan, serum and liver alanine amino-transferase activity
levels increased and blood cholinesterase activity decreased.
Decreases were observed in the vitamin E and A levels,
glucose-6-phosphate dehydrogenase activity, and liver aminohexoses
and hydroxyproline levels with a simultaneous increase in the serum
aminohexoses level. This finding may indicate the increasing process
of connective tissue catabolism and lisosomal membranes
labilization.
8.9.2 Toxicity of metabolites
Hydrolysis of the carbamate ester bond of carbaryl results in
detoxification. The carbamate moiety is decomposed to carbon dioxide
and methylamine, and the phenolic part is conjugated and excreted.
Kuhr (1971) summarized the data on the toxicity of carbaryl
metabolites (Table 53).
Table 53. Toxicity of carbaryl and some of its metabolitesa
LD50 (mg/kg) 7-day Molar I50 bovine
NOELb anticholin-esterase
Mouse (mg/kg)
Rat oral Intraperitoneal Rat
Carbaryl 270 29-42 125-250 5 x 10-8
4-Hydroxycarbaryl 1190 74 > 1000 4 x 10-7
5-Hydroxycarbaryl 297 56 > 1000 4.6 x 10-8
7-Hydroxycarbaryl 4760 > 1000
Hydroxymethylcarbaryl > 5000 630-780 250-500 1.4 x 10-5
1-Naphthol 2570 500-1000 1 x 10-3
aFrom: Kuhr (1971).
bNOEL = No-observed-effect level.
Many of the known metabolites are much less toxic than
carbaryl. The pharmacological study of carbaryl in rats showed that
the metabolites with a methylcarbamate moiety are inhibitors of
plasma cholinesterase (Fernandez et al., 1982). None of the
carbaryl metabolites was appreciably more active as a cholinesterase
inhibitor than carbaryl itself (Oonnithan & Casida, 1968).
8.9.3 N-nitrosocarbaryl
Carbaryl is a secondary amine and is, therefore, capable of
nitrosation in the presence of nitro donor groups, such as sodium
nitrate, to give a nitrosamide. This nitrosamide, nitrosocarbaryl,
has been proved to be mutagenic and carcinogenic, at high doses in
animals. Conditions of this nitrosation include a strongly acidic pH
(less than 2), which is comparable with the pH in the human stomach.
However, nitrosocarbaryl is not stable at this pH. Its maximum
stability is between pH 3 and 5, pHs at which no significant amounts
of carbaryl can be nitrosated. Carbaryl has been nitrosated in
several studies, in vitro as well as in vivo, in the guinea-pig,
which has a stomach acidity close to that in man. If significant
yields of nitrosocarbaryl have been shown in these circumstances,
the significance of these findings is still not clear for human risk
evaluation. The pH in the human stomach is variable during food
intake and probably, more importantly, the stomach contains a lot of
food, which will minimize the contact between naturally occurring
nitrite and carbaryl residues, but which will also afford a lot of
nucleophilic sites for nitrosocarbaryl to react with. It is also
noteworthy that all studies that were conducted with jointly
administered carbaryl and nitrite yielded negative results for
oncogenicity. Cranmer (1986) estimated the potential intake of
nitrosocarbaryl at 6x10-9 mg/kg per day. Using different
mathematical models, Cranmer estimated a cancer risk for
nitrosocarbaryl between 10-6 (one-hit model) and 10-9 (probit
model). However, if such oncogenic nitroso-carbamates were to be
found in such quantities in the human stomach, an increased
incidence of stomach cancer would have been expected during the
period when drug and pesticide carbamates were widely used. However,
during this period, the incidence of gastric cancer in the USA
declined considerably.
The bacterial metabolite N-nitrosocarbaryl may act as a
noncompetitive inhibitor of the in vitro metabolism of
aminopyrine, p-nitroanisole, and aniline by rat liver microsomes
(Beraud et al., 1980, 1989a,b). N-nitrosocarbaryl was more
effective as an inhibitor of microsomal activity than the parent
compound.
8.10 Mechanism of toxicity - mode of action
The mechanism of toxicity and the mode of action of carbaryl
and carbamates, in general, have been described in EHC 64 (WHO,
1986).
8.10.1 Inhibition of cholinesterase activity
As a carbamate compound, carbaryl is an inhibitor of
cholinesterase (ChE) activity (for details see Reiner & Aldridge
(1967) and Aldridge (1971)).
A number of studies have been performed by Carpenter et al.
(1961) to assess the extent of ChE inhibition by carbaryl in
mammals. A single oral dose of carbaryl of 560 mg/kg produced a 43%
inhibition of the erythrocyte ChE in rats in 0.5 h, and a 30%
inhibition in brain AChE. However, they returned to normal in rats
that survived 24 h after administration of the dose. Carbaryl did
not depress plasma ChE significantly. Two groups of Beagle dogs were
injected once, iv, with 10 or 15 mg/kg as an 8% solution in 95%
alcohol. No significant effects were found on either erythrocyte or
plasma ChE. On the 5th day, several administrations of the same
doses depressed plasma ChE by 24% and erythrocyte AChE by 40%. It is
doubtful that a long incubation time for the samples (2 h for
plasma) played a role in the slight depression of ChE. Comparative
data on ChE inhibition in the brain, plasma, and erythrocytes of
rats that received single doses of carbaryl were reported by Mount
et al. (1981). As shown in Table 54, there was a dose-dependent
decrease in ChE in the brain, plasma, and erythrocytes.
Brain AChE was significantly different in dead rats and in
surviving rats given 800 mg/kg. AChE depression in the brain was
>70% in the lethally poisoned rats. The inhibition of red blood
cell AChE activity was the same as in plasma.
Table 54. Inhibition of ChE in % in comparison with the controlsa
Sprague-Dawley rats Post-dosing Brain Red blood Plasma
(h) cells
Controls 10 rats 0 0 0
450 mg/kg 0.5 56 44 51
(24 rats sacrificed) 1 74 29 53
2 74 42 68
4 66 48 61
8 55 25 31
24 27 60 38
48 22 23 46
96 6 19 -19
800 mg/kg 0.5 66 32 47
(34 rats sacrificed) 1 70 44 50
2 79 73 69
4 50 72 70
12 50 72 50
28 58 63 68
48 17 26 40
96 16 -6 16
800 mg/kg 3 80 - -
(24 rats died) 4 85 - -
18 88 - -
25 71 - -
29 88 - -
30 84 - -
1200 mg/kg 1-36 88-91 - -
(15 died)
aAdapted from: Mount et al. (1981).
The affinity of AChE of human brain caudate nucleus for
carbaryl was studied in vitro and some inhibition constants were
determined (Patocka & Bajgar, 1971). The value of the I50 affinity
constant was calculated from the dependence of AChE inhibition on
the molar concentration of the inhibitor in probit logarithm
transformation. The Hill coefficient was obtained from Hill plots.
The affinity constant for carbaryl pI50 was 5.59 and the Hill
coefficient was 1.50. According to the authors, some results of this
study suggest that AChE may be an allosteric enzyme.
Intravenous administration of colloidal carbon in the rat,
inhibiting the phagocytic activity of the RES, prolonged the
duration of the anticholinesterase effect of carbaryl (Pipy et al.,
1979). The authors speculated that the metabolism of carbaryl was
decreased by the RES inhibition and, as a result, the reactivation
speed of ChE was slower.
Pregnant rats were treated orally with 1 or 5 mg carbaryl/kg
from day 11 to the last day of gestation (Declume & Benard, 1977b;
Declume et al., 1979). No cholinesterase depression in blood,
brain, and liver were observed in either the dams or the newborn
offspring. However, after administration of 50 mg/kg from day 19 to
the last day of gestation, ChE inhibition was seen in both the
mother and newborn offspring.
Cambon et al. (1978) reported decreased cholinesterase
activity in the blood, brain, and liver in the mother and the fetus
after treatment of the dams at 6.25, 12.5, 25, and 50 mg/kg.
Variability of cholinesterase levels in the controls and
short-comings in the cholinesterase analysis protocol make these
results difficult to interpret.
9. EFFECTS ON HUMAN BEINGS
9.1 General population exposure
9.1.1 Acute toxicity, poisoning incidents
The clinical picture of carbaryl intoxication results from the
accumulation of ACh at nerve endings (WHO, 1986). The signs and
symptoms can be categorized into the following 3 groups:
(a) Muscarinic manifestations
- increased bronchial secretion, excessive sweating,
salivation, and lacrimation;
- pinpoint pupils, bronchoconstriction, abdominal cramps
(vomiting and diarrhoea); and
- bradycardia.
(b) Nicotinic manifestations
- fasciculation of fine muscles (in severe cases, diaphragm
and respiratory muscles also involved); and
- tachycardia.
(c) Central nervous system manifestations
- headache, dizziness, anxiety, mental confusion,
convulsions, and coma; and
- depression of respiratory centre.
All these signs and symptoms can occur in different
combinations and can vary in onset and sequence, depending on the
chemical, dose, and route of exposure. The duration of symptoms is
usually shorter than that observed in organophosphorus poisoning.
Mild poisoning might include muscarinic and nicotinic signs only.
Severe cases always show central nervous system involvement; the
clinical picture is dominated by respiratory failure sometimes
leading to pulmonary oedema, due to the combination of the
above-mentioned syndromes.
A review of carbaryl-related poisoning from 1966 to 1980 was
made by US EPA. During this period, 193 cases of over-exposure to
carbaryl as the sole active ingredient in the poisoning and 144
cases of over-exposure to a combination of carbaryl with other
active ingredients were recorded (Weston, 1982). Not all case
histories have been confirmed. There were 5 deaths. There is no
evidence that carbaryl was involved in these deaths, except for one,
which was acknowledged by the manufacturer.
Hayes (1963) reported two incidents of poisoning: one, a
19-month-old child who swallowed an unknown amount of carbaryl, and
the other, a man who swallowed 250 mg of carbaryl. Both developed
moderately severe ChE inhibition symptoms within 20 min: constricted
pupils, salivation, muscular incoordination in the child, and
epigastric pain, profuse sweating, lassitude, and vomiting in the
man. Both recovered after atropine treatment. Blood cholinesterase
was inhibited.
One death from carbaryl ingestion while drunk was reported by
Farago (1969) who concluded that 2-PAM application hastened the
fatal outcome (due to pulmonary oedema), 6 h after ingestion.
Carbaryl was found in various tissues.
Acute intoxication during the loading of an airplane was
reported by Long (1971).
In a case report of a suicide attempt involving about 25 g
(500 mg/kg) of carbaryl, dicumarin and boric acid, Dickoff et al.
(1987) described the occurrence of a peripheral neuropathy that
resembled the syndrome observed following exposure to some
organophosphorus compounds. Recovery continued for 9 months.
Electrophysiological findings were consistent with axonal peripheral
neuropathy. The cause-effect relationship was confounded because of
combined exposure.
9.1.2 Controlled human studies
Wills et al. (1968) carried out controlled studies in human
volunteers, aged 25-57 years. In a preliminary study, oral doses of
0.5, 1, and 2.0 mg carbaryl/kg in capsules (single application to 2
subjects) were tolerated without any subjective or objective
symptoms. In the main study, one group (5 subjects) took 0.06 mg
carbaryl/kg daily, and one group (6 subjects) took 0.13 mg
carbaryl/kg for 6 weeks. Physical examination, BSP removal from
blood, EEG examination, routine blood and urinalysis were performed,
and ChE of plasma and red blood cells was examined. No changes were
found in the low-dose group. An increase in the ratio of amino acid
nitrogen to creatinine in the urine, at the high dose, may represent
a decrease in the ability of the proximal convoluted tubule to
reabsorb amino acids. This change was reversible.
The urine of some subjects was analysed (Knaak et al., 1968).
The overall recovery (by the fluorometric method) of the carbaryl
equivalents in the urine was 26-28%, and, with the colorimetric
method, 37.8%, in subjects treated with 2 mg carbaryl/kg. The
following metabolites were found chromatographically in a 4-h urine
sample: alpha-naphthol glucuronide (10-15%), and sulfate (6-11%) and
4-(methylcarbamoyloxy)-alpha-naphthyl glucuronide (4%). Another
metabolite, alpha-naphthyl methylimido-carbonate O-glucuronide,
was identified by fluorometry.
A human ingestion study was conducted to determine the
relationship between a single oral dose of carbaryl and the rate of
its urinary excretion as metabolites. Elimination was apparently
first order over the dosage range of the studies (0.25-1 mg/kg). The
model predicts that, 24 h after ingestion, approximately 41% of the
dose can be accounted for as urinary metabolites (Hansen, 1978).
In a study involving one subject, Ward et al. (1988) observed
that pretreatment with a clinical regimen of cimetidine (3x200 mg
over a three-day period) reduced presystemic (first-pass) clearance
of a dose of 1 mg carbaryl/kg by about 46%.
A scientist studying the anthelminthic activity of carbaryl,
tested its human safety by ingesting 250 mg (about 2.8 mg/kg). After
20 min, he experienced epigastric pain and began to sweat profusely.
He was treated with a total dose of 3 mg atropine and recovered
completely 2 h after taking the carbaryl. Another scientist ingested
420 mg carbaryl (4.45 mg/kg), and after 85 min he had vision
troubles, weakness, profuse sweating, and felt lightheaded. He was
treated with a total dose of 4.8 mg atropine and recovered 4 h after
the onset of symptoms (Hayes & Laws, 1991).
9.1.3 Long-term exposure
Branch & Jacqz (1986a,b) reported the case of a 75-year-old man
who was exposed to carbaryl for 8 months, inside his home, after
repeated excessive applications of 10% dust formulation. He
experienced a series of signs and symptoms compatible with
cholinesterase depression in addition to a 40-lb (18-kg) weight
loss. After exposure ceased, the patient's condition improved
markedly. However, a few months later he started to experience
modification of his sleep pattern and peripheral neuropathy and
cerebral atrophy were demonstrated. Other pathologies, such as
recurrent gastric ulcer, cardiac fibrillations, a recent head injury
due to an automobile accident, and other less defined pathologies
were present in this patient which provide other, more likely,
causes for these later symptoms.
The uncertainties associated with long-term exposure to levels
sufficient to result in sustained suppression of plasma
pseudocholinesterase activity and possible brain damage are
discussed by Avashia (1987) and Branch (1987).
9.2 Occupational exposure
9.2.1 Epidemiological studies
The first report on workers exposed to carbaryl was published
by Best & Murray (1962). For 19 months, from the start of carbaryl
production, they studied men working on the production, handling,
and shipping of carbaryl. The most exposed group were bagging
workers occasionally exposed to carbaryl dust under abnormal
conditions (40 mg/m3). These showed a slight depression in blood
ChE activity, but this was below the rate at which clinical symptoms
might be expected. They found that 41% of 689 urine specimens
contained >1000 µg/100 ml total 1-naphthol (>400 µg/100 ml
indicates absorption), with no clinical or subjective symptoms. In
cases of acute intoxication, 3140 µg 1-naphthol/100 ml urine were
found. Knaak et al. (1965) using fluorometric analysis in
conjunction with chromatographic separation, found 5 times more
sulfate (25 mg/litre) than glucuronides (5 mg/litre) of 1-naphthol
in the urine of exposed workers.
Vandekar (1965) in a village-scale trial in Nigeria, assessed
the risk for the population of exposure to carbaryl. A slight
depression of plasma ChE was found in all spraymen, the day after
the spraying. Levels of 1-naphthol derivatives in their urine did
not increase on days 1 and 2 after spraying but increased slightly
on day 6.
In a study on 19 agricultural workers (Yakim, 1967), whole
blood ChE activity was measured before, and after, 3-4 days exposure
to airborne carbaryl. Men exposed to a mean airborne carbaryl
concentration of 2 mg/m3 showed a decrease of 20-24% in ChE
activity. Signalmen exposed to 4 mg/m3 (mean) showed a 13-30%
decrease in ChE activity. No objective signs of ill health were
observed. In the same study, a mean carbaryl concentration of
0.7 mg/m3 was reported in the cabin of an aeroplane used to apply
carbaryl, but no changes were reported in the biological parameters
of the pilots. During the agricultural application of carbaryl dust
at a maximum exposure level of 19 mg/m3 dust on cotton fields,
Adylov (1966) reported a decrease in catalase activity, and a 20%
decrease in ChE activity on day 14.
In cases of occupational overexposure to carbamates, mild
symptoms appear long before a dangerous dose is absorbed, which is
why severe occupational intoxications with carbaryl are rare. Tobin
(1970) gives reasons for the lack of severe intoxications: (1) there
is a very short time (´ h or less) between exposure to carbamates
and the onset of symptoms; (2) lack of symptoms progression, because
of a large margin between the median effective dose and the lethal
dose of carbamates and the early detection of intoxication.
Workers exposed to carbaryl used on pets for flea control,
experienced diarrhoea, increased salivation, cough, difficulty in
breathing, and phlegm (Ames et al., 1989).
Vandekar (1965) reported a skin rash in a sprayman who was
accidentally splashed with a carbaryl formulation. Although
carbamate compounds generally have not caused dermatitis or allergic
skin reactions, Vandekar suggested that they can appear in certain
individuals after unusually heavy exposure.
One out of a group of 30 farmers with contact dermatitis was
identified by a patch test as having a positive allergic reaction to
a 1% solution of carbaryl (Sharma & Kaur, 1990). No allergic
reaction were observed in a control population (No.=20).
To identify possible effects on reproduction, Whorton et al.
(1979) studied a cohort of 47 male workers who had worked for at
least 1 year in the production and packaging of carbaryl. A semen
sample was used for sperm count. Testosterone follicle-stimulating
hormone, and luteinizing hormone were determined by
radioimmunoassay. The range of airborne carbaryl concentrations in
the workplace was 0.03-14.21 mg/m3 with a mean of 4.9 mg/m3.
This cohort showed no seminal or blood abnormalities related to
carbaryl exposure. Although a small excess (not significant at
alpha=0.05) was observed in a small number of oligospermic men in
the exposed group, there was no evidence that testicular function or
fertility in the male workers was affected under these conditions of
exposure.
Wyrobek et al. (1981) used the same cohort of exposed workers
to study sperm abnormalities. Semen was collected from 50 men,
occupationally exposed to carbaryl for 1-18 years. Semen samples
were analysed for changes in sperm motility, sperm count,
morphology, and frequency of sperm-carrying double fluorescent
bodies (YFF). The YFF test represents sperm with two Y chromosomes
due to meiotic nondysjunction. The exposed workers showed changes in
sperm morphology with a higher proportion of sperm with abnormal
head shapes in comparison with the control group of newly hired
unexposed workers. There was no dose dependence as judged by job
classification. A negative correlation between number of years
working in the carbaryl area and the percentage of abnormal sperm
was observed. Workers who had once been exposed to carbaryl, but who
had not been exposed for an average of 6.3 years, showed a
marginally significant elevation in sperm abnormalities, possibly
not reversible. MacLeod (1982) reviewed the Wyrobek et al. (1981)
study and did not find any essential differences in the distribution
of the sperm types in the control and the carbaryl-exposed groups.
No significant changes in sperm count and fertility were reported in
100 workers (Thomas, 1981).
10. PREVIOUS EVALUATION BY INTERNATIONAL BODIES
The latest IARC evaluation of carbaryl was made during 1987
(IARC, 1987). It was concluded that there were no data on cancer in
humans and inadequate evidence of carcinogenicity in experimental
animals. Carbaryl could not be classified with regard to its
carcinogenicity to humans (Group 3).
The FAO/WHO Joint Meeting on Pesticide Residues (JMPR)
evaluated carbaryl at its meetings in 1963, 1965, 1966, 1967, 1968,
1969, 1970, 1971, 1973, 1975, 1976, 1977, 1979 and 1984 (FAO/WHO,
1964, 1965, 1967, 1968, 1969, 1970, 1971, 1972, 1974, 1976, 1977,
1978, 1980, 1985). Since 1973, an acceptable daily intake (ADI) of
0-0.01 mg/kg body weight has been established. This estimate is
based on the following experimental data: no-effect level for rats:
200 mg/kg in the diet = 10 mg/kg per day; for dogs: 100 mg/kg in the
diet = 1.8 mg/kg per day; and for human beings: 0.06 mg/kg per day
(FAO/WHO, 1965, 1967, 1974).
Maximum residue levels (MRLs) for carbaryl were recommended by
FAO/WHO (1986b) (see Table 55). The values recommended for tolerance
levels represent the sum of free carbaryl, combined carbaryl,
conjugated naphthol, and conjugated methylcarbaryl, expressed as
total toxic residues of carbaryl.
A WHO study group on occupational health recommended 5 mg
carbaryl/m3 as a tentative, health-based, maximum permissible
level in the working environment. A biological limit of 30%
inhibition of ChE activity in whole blood, plasma, or red cells with
respect to pre-exposure levels should not be exceeded (WHO, 1982).
In the WHO recommended classification of pesticides by hazard,
technical carbaryl is classified in Class II as moderately hazardous
in normal use (WHO, 1992). WHO/FAO (1975) issued a data sheet on
carbaryl (No. 3).
IRPTC in its series "Scientific reviews of Soviet literature on
toxicity and hazards of chemicals" has published a review on
carbaryl (IRPTC, 1982, 1989).
Table 55. Maximum Residue Limits (MRLs) established by the Codex
Alimentariusa
Commodity MRL
(mg/kg)
Animal feedstuffs (green alfalfa, clover, corn, forage, cow pea 100
foliage, grasses, peanut hay, sorghum forage, soybean vines,
sugarbeet tops, bean and pea vines)
Bran (wheat) 20
Apricots, blackberries, boysenberries, nectarines, peaches, 10
raspberries, asparagus, okra, leafy vegetables (except
brassica), nuts (whole), olives (fresh), sorghum grain, cherries,
plums, kiwi fruit
Blueberries, citrus fruit, cranberries, strawberries 7
Apples, bananas (pulp), grapes, beans, peas (including pod), 5
brassica, tomatoes, peppers, aubergines, pears, poultry skin,
barley, oats, rice (in husk and hulled), rye, wheat
Cucumbers, melons (Cantaloupe), pumpkins, squash 3
Root crop vegetables (beets, carrots, radishes, rutabagas, 2
parsnips), peanuts (ground-nuts, whole), wholemeal flour
Cottonseed (whole), sweet corn (kernels), nuts (shelled), olives 1
(processed), soybeans (dry mature seed), cow-peas
Poultry meat, eggs (without shells) 0.5
Potatoes, meat of cattle, sheep, and goats, sugarbeets, wheat 0.2
flour (white)
Milk and milk products 0.1
aFrom:FAO/WHO (1986b).
REFERENCES
Abdel-Rahman MS, Lechner DWH, & Klein KM (1985) Combination effect
of carbaryl and malathion in rats. Arch Environ Contam Toxicol, 14:
459-464.
Abrahamsen LH & Jerkofsky M (1981) Enhancement of varicella-zoster
virus replication in cultured human embryonic lung cells treated
with the pesticide carbaryl. Appl Environ Microbiol, 41(3): 652-656.
Addison JB, Silk PJ, & Unger I (1975) The photochemistry of
carbamates V. The photodecomposition of Sevin (1-naphthyl- N-methyl
carbamate) and of Phenmec (phenyl- N-methyl carbamate). Int J
Environ Anal Chem, 4: 135-140.
Adylov MA (1966) [Sanitary hygienic assessment of the working
conditions and health state of people working with Sevin in
Uzbekistan.] Gig Primen Toxicol Pestic Klin Otrav, 1866: 271-275 (in
Russian).
Ahdaya SM & Guthrie FE (1982) Stomach absorption of intubated
insecticides in fasted mice. Toxicology, 22(4): 311-317.
Ahdaya SM, Shah PV, & Guthrie FE (1976) Thermoregulation in mice
treated with parathion, carbaryl or DDT. Toxicol Appl Pharmacol, 35:
575.
Ahlrichs JL, Chandler L, Monke EJ, & Reuszler HW (1970) Effect of
pesticide residues and other organotoxicants on the quality of
surface and ground water resources. Lafayette, Indiana, Indiana
Water Research Center, 106 pp (Technical Report No. 10).
Ahmed FE, Hart RW, & Lewis NJ (1977a) Pesticide induced DNA damage
and its repair in cultured human cells. Mutat Res, 42: 161-174.
Ahmed FE, Lewis NJ, & Hart RW (1977b) Pesticide induced ouabain
resistant mutants in Chinese hamster V79 cells. Chem-Biol Interact,
19: 369-374.
Akhundov VY, Lurie LM, & Ismailova IM (1981) [Effect of Sevin on
immunological reactivity.] Gig i Sanit, 46(2): 25-28 (in Russian).
Albanis TA, Pomonis PJ, & Sdoukos AT (1986) Organophosphorous and
carbamates pesticide residues in the aquatic system of Ionnina basin
and Kalamas river (Greece). Chemosphere, 15(8): 1023-1034.
Albright LM & Simmel EC (1977) Behavioural effects of cholinesterase
inhibitor and insecticide carbaryl (Sevin). Paper presented at the
49th Annual Meeting of the Midwestern Physiological Association,
Chicago, 5-7 May 1977.
Aldridge WN (1971) The nature of the reaction of organophosphorous
compounds and carbamates with esterases. Bull World Health Organ,
44: 25.
Alvarez CC, Shimanuki H, & Argauer RJ (1970) Oral toxicity of
carbaryl to adult honey bees. J Econ Entomol, 63(6): 1834-1835.
Aly OM & El Dib MA (1971a) Studies on the persistence of some
carbamate insecticides in the aquatic environment. I. Hydrolysis of
Sevin, Baygon, Pyrolan and Dimetilan in waters. Water Res, 5:
1191-1205.
Aly OM & El Dib MA (1971b) Photodecomposition of some carbamate
insecticides in aquatic environments. In: Faust SJ & Hunter JV ed.
Proceedings of the Rudolfs Research Conference on Organic Compounds
in Aquatic Environments. New York, Basel, Marcel Dekker, Inc., pp
469-493.
Aly OM & El Dib MA (1972) Studies on the persistence of some
carbamate insecticides in the aquatic environment. In: Fate of
organic pesticides in the aquatic environment. Washington, DC,
American Chemical Society, pp 211-241 (Advances in Chemistry Series,
Volume 111).
Aly MI, Bakry N, Kishk F, & El-Sebae AH (1980) Carbaryl adsorption
on calcium-bentonite and soils. J Soil Sci Soc Am, 44(6): 1213-1215.
Amer S (1965) Cytological effects of pesticides. I. Mitotic effects
of N-methyl-1-naphthyl carbamate "Sevin". Cytologia, 30: 175-181.
Amer SM & Farah OR (1968) Cytological effects of pesticides. III.
Meiotic effects of N-methyl-1-naphthyl carbamate "Sevin".
Cytologia, 33: 337-344.
Amer SM, Hammouda MA, & Farah OR (1971) Cytological and
morphological effects of the insecticide N-methyl-1-naphthyl
carbamate "Sevin". Flora (Jena), 160: 433-439.
Ames RG, Brown SK, Rosenberg J, Jackson RJ, Stratton JW, & Quenon SG
(1989) Health symptoms and occupational exposure to flea control
product among California pet handlers. Am Ind Hyg Assoc J, 50(9):
466-472.
Andrawes NR & Bailey RH (1978a) Metabolism of carbaryl in the rat. A
reappraisal (File No. 25051). Research Triangle Park, North
Carolina, Union Carbide Corporation (Unpublished proprietary
information submitted to WHO by Rhône-Poulenc Agro, Lyon).
Andrawes NR & Bailey RH (1978b) Conjugated metabolites of carbaryl:
A comparison between rat and dog. South Charleston, West Virginia,
Union Carbide Research and Development (Project Report No. 811C20)
(Unpublished proprietary information submitted to WHO by
Rhône-Poulenc Agro, Lyon).
Andrawes NR & Bailey RH (1978c) Metabolism of carbaryl in Beagle
dogs. South Charleston, West Virginia, Union Carbide Research and
Development (Project Report No. 811C20) (Unpublished proprietary
information submitted to WHO by Rhône-Poulenc Agro, Lyon).
Andrawes NR & Bailey RH (1979) Carbaryl metabolites detected in the
urine of plant workers (File No. 25957). South Charleston, West
Virginia, Union Carbide Research and Development (Unpublished
proprietary information submitted to WHO by Rhône-Poulenc Agro,
Lyon).
Andrawes NR & Chancey EL (1970) The metabolism of 1-naphthyl-C14
carbaryl in wheat and bean plants. Clayton, North Carolina, Union
Carbide Corporation (Project Report No. 13749) (Unpublished
proprietary information submitted to WHO by Rhône-Poulenc Agro,
Lyon).
Andrawes NR & Myers WR (1976) Identification of carbaryl metabolites
in human urine (File No. 22983). South Charleston, West Virginia,
Union Carbide Corporation (Unpublished proprietary information
submitted to WHO by Rhône-Poulenc Agro, Lyon).
Andrawes NR, Chancey EL, Crabtree RJ, Herrett RA, & Weiden MHJ
(1972) Fate of naphthyl-1-14C carbaryl in laying chickens. J Agric
Food Chem, 20(3): 608-617.
André F, Gillon J, André C, Lafont S, & Jourdan G (1983)
Pesticide-containing diets augment anti-sheep red blood cell
nonreaginic antibody responses in mice but may prolong murine
infection with Giardia muris. Environ Res, 32: 145-150.
Andrianova MM & Alexeev IV (1970) [On the question of the
carcinogenic properties of the pesticides Sevin, Maneb, Ziram and
Zineb.] Vopr Pitan, 6: 71-74 (in Russian).
Anger WK & Setzer JV (1979) Effects of oral and intramuscular
carbaryl administrations on repeated chain acquisition in monkeys. J
Toxicol Environ Health, 5: 793-808.
Antonovich NA (1970) [On the residual quantities of Sevin in food
products.] Gig Primen Toxicol Pestic Klin Otrav, 1970: 176-179 (in
Russian).
Argauer RJ & Warthen JD (1975) Separation of 1- and 2-naphthols and
determination of trace amounts of 2-naphthyl methylcarbamate in
carbaryl formulations by high pressure liquid chromatography with
confirmation by spectrofluorometry. Anal Chem, 47(11): 2472-2474.
Armstrong DA & Millemann RE (1974a) Pathology of acute poisoning
with the insecticide Sevin in the bent-nosed clam, Macom nasuta. J
Invertebr Pathol, 24(2): 201-212.
Armstrong DA & Millemann RE (1974b) Effect of the pesticide carbaryl
on clams and some other intertidal mud flat animals. J Fish Res
Board Can, 31: 466-470.
Armstrong DA & Millemann RE (1974c) Effect of the insecticide Sevin
and its first hydrolytic product 1-naphthol on some developmental
stages of the bay mussel Mytilus edulis. Mar Biol, 28: 11-15.
Arunachalam S & Palanichamy S (1982) Sublethal effects of carbaryl
on surfacing behaviour and food utilization in the air breathing
fish, Macropodus cupanus. Physiol Behav, 29(1): 23-27.
Arunachalam S, Jeyalakshmi K, & Aboobucker S (1980) Toxic and
sublethal effects of carbaryl on a freshwater catfish, Mystus
vittatus (Bloch). Arch Environ Contam Toxicol, 9: 307-316.
Ashwood-Smith M, Trevino J, & Ring R (1972) Mutagenicity of
dichlorvos. Nature (Lond), 240: 418-420.
Atabaev ST (1972) [Carbamate compounds.] In: [Pesticides and hygiene
of the environment in the conditions of warm climate.] Tashkent,
Medizina, pp 114-121 (in Russian).
Attia AM, Reiter RJ, Nonaka KO, Mostafa MH, Soliman SA, & El-Sebae
AH (1991) Carbaryl-induced changes in indoleamine synthesis in the
Pineal gland and its effects on night-time serum melatonin
concentrations. Toxicology, 65: 305-314.
Avashia BH (1987) Is carbaryl safe as its reputation. Letter to the
Editor. Am J Med, 83: 1168-1169.
Azizova OM (1976) [Effect of Sevin and methyl-mercaptophos on
different levels of the nervous system.] In: Medved' LI ed. [Current
problems of the hygiene of application of pesticides in different
climatic and geographical zones. Proceedings of the Itinerary
Scientific Session of Vniigintoks, Erevan.] Kiev, Vniigintoks (in
Russian).
Bansal SK, Verma SR, Gupta AK, & Dalela RC (1980) Predicting
long-term toxicity by subacute screening of pesticides with larvae
and early juveniles of four species of freshwater major carp.
Ecotoxicol Environ Saf, 4: 224-231.
Baron RL (1968) Radioactive lactose in skim milk following
administration of carbonyl-14C-carbaryl to a lactating cow. J
Assoc Off Anal Chem, 51: 1046-1049.
Baron RL & Locke RK (1970) Utilization of cell culture techniques in
carbaryl metabolism studies. Bull Environ Contam Toxicol, 5:
287-291.
Barrett GW (1968) The effect of an acute insecticide stress on a
semi-enclosed grassland ecosystem. Ecology, 49(6): 1019-1035.
Bart J (1979) Effects of acephate and Sevin on forest birds. J Wildl
Manage, 43(2): 544-549.
Basha SM, Rao KSP, Rao KRSS, & Rao KVR (1983) Differential toxicity
of malathion, BHC, and carbaryl to the freshwater fish, Tilapia
mossambica (Peters). Bull Environ Contam Toxicol, 31(5): 543-546.
Bavari S, Casale GP, Gold RE, & Vitzthum EF (1991) Modulation of
interleukin-2 driven proliferation of human large granular
lymphocytes by carbaryl, an anticholinesterase insecticide. Fundam
Appl Toxicol, 17: 61-74.
Belonozhko GA & Kuchak JA (1969) [Electrocortical reactions to
treatment with Sevin in rats.] Gig i Sanit, 34(2): 111-113 (in
Russian).
Benard P, Cambon C, & Declume C (1979) Passage of radioactivity into
the milk of rats treated with [14C] carbaryl. Toxicol Lett, 4:
149-153.
Bend JR, Holder GM, Protos E, & Ryan AJ (1971) Water soluble
metabolites of carbaryl (1-naphthyl N-methylcarbamate) in mouse
liver preparations and in the rat. Aust J Biol Sci, 24: 535-546.
Benson BW, Scott WJ, & Beliles RP (1967) Sevin teratological study
in the mouse (Prepared by Woodard Research Corporation) (Union
Carbide proprietary report, submitted to WHO by Rhône-Poulenc Agro,
Lyon).
Beraud M, Pipy B, Derache R, & Gaillard D (1979) Formation d'un
cancérigène, le N-nitrosocarbaryl, par interaction entre un
insecticide de la série des carbamates, le carbaryl, et le nitrite
de sodium de suc gastrique de rat. Food Cosmet Toxicol, 17(6):
579-583.
Beraud M, Gaillard S, & Derache R (1980) Inhibition of two hepatic
microsomal drug-metabolizing enzymes by a carcinogenic
N-nitrosated pesticide: N-nitrosocarbaryl. Chem-Biol Interact,
31: 103-112.
Beraud M, Forgue M-F, Pipy B, & Derache P (1989a) Interaction of
carbaryl and N-nitrosocarbaryl with microsomal monooxygenase
activities. Toxicol Lett, 45(2-3): 251-260.
Beraud M, Forgue M-F, Pipy B, Didier A, & Carre P (1989b) Lipid
peroxidation in rat liver microsomes: Influence of carcinogenic
N-nitrosocarbaryl. Toxicology, 58: 299-311.
Best EM & Murray BL (1962) Observations on workers exposed to Sevin
insecticide: A preliminary report. J Occup Med, 4(10): 507-517.
Blase W & Loomis TA (1976) The uptake and metabolism of carbaryl by
isolated perfused rabbit lung. Toxicol Appl Pharmacol, 37: 481-490.
Blevins RD & Dunn WC (1975) Effects of carbaryl and dieldrin on the
growth, protein content and phospholipid content of Hela cells. J
Agric Food Chem, 23(3): 377-382.
Blevins RD, Lee M, & Regan JD (1977) Mutagenicity screening of five
methyl carbamate insecticides and their nitroso derivatives using
mutants of Salmonella typhimurium LT2. Mutat Res, 56(1): 1-6.
Bluzat R & Seuge J (1979) Effets de trois insecticides (lindane,
fenthion et carbaryl): toxicité aiguë sur quatre espèces
d'invertébrés limniques; toxicité chronique chez le mollusque
pulmoné lymnea. Environ Pollut, 18: 51-70.
Boethling RS & Alexander M (1979) Effect of concentration of organic
chemicals on their biodegradation by natural microbial communities.
Appl Environ Microbiol, 37(6): 1211-1216.
Bogacka T & Groba J (1980) [Toxicity and biodegradation of
chlorfenvinphos, carbaryl, and propoxur in water environment.]
Bromatol Chem Toksykol, 13(2): 151-158 (in Polish with English
summary).
Bogomolova ZN (1968) [Changes in the content of Sevin in fruitberry
cultures during the process of vegetation, storage and canning.] Gig
Primen Toxicol Pestic Klin Otrav, 1968: 363-367 (in Russian).
Bogomolova ZN (1970) [Content of Sevin in fruit cultures.] Gig
Primen Toxicol Pestic Klin Otrav, 1970: 197-180 (in Russian).
Bogus ER, Gallagher P, Cameron F, & Mumima R (1985) Analysis of
pesticide exposure pads using selective absorption and elution of
reversed-phase solid support. J Agric Food Chem, 33: 1018-1021.
Bollag JM (1979) Transformation of xenobiotics by microbial
activity. In: Proceedings of an EPA Workshop on Microbial
Degradation of Pollutants in Marine Environments. Gulf Breeze,
Florida, US Environmental Protection Agency, Environmental Research
Laboratory, pp 19-27 (EPA 600/9-79-012).
Bollag JM & Liu SY (1971) Degradation of Sevin by soil
microorganisms. Soil Biol Biochem, 3(4): 337-345.
Bollag JM & Liu SY (1972a) Fungal degradation of 1-naphthol. Can J
Microbiol, 18(7): 1113-1117.
Bollag JM & Liu SY (1972b) Hydroxylations of carbaryl by soil fungi.
Nature (Lond), 236: 177-178.
Bollag JM, Czaplicki EJ, & Minard RD (1975) Bacterial metabolism of
1-naphthol. J Agric Food Chem, 23(1): 85-90.
Bollag JM, Sjoblad RD, Czaplicki EJ, & Hoeppel RE (1976)
Transformation of 1-naphthol by the culture filtrate of Rhizoctonia
praticola. Soil Biol Biochem, 8: 7-11.
Bollag JM, Sjoblad RD, & Liu S-Y (1978) Characterization of an
enzyme from Rhizoctonia praticola which polymerizes phenolic
compounds. Can J Microbiol, 25: 229-233.
Borzelleca J & Skalsky HL (1980) The excretion of pesticides in
saliva and its value in assessing exposure. J Environ Sci Health,
B13(6): 843-866.
Bowman BT & Sans WW (1985) Effect of temperature on the water
solubility of insecticides. J Environ Sci Health, B20(6): 625-631.
Bowman MC, Oller W, Kendall D, Dosnell A, & Oliver K (1982) Stressed
bioassay systems for rapid screening of pesticide residues. Part II.
Determination of foliar residues for safe re-entry of agricultural
workers into the field. Arch Environ Contam Toxicol, 11: 447-455.
Boyd EM & Boulanger MA (1968) Insecticide toxicology. Augmented
susceptibility to carbaryl toxicity in albino rats fed purified
casein diets. J Agric Food Chem, 16: 834-838.
Boyd EM & Krijnen CJ (1969) The influence of protein intake on acute
oral toxicity of carbaryl. J Clin Pharmacol, 9(5): 292-297.
Brahmaprakash GP & Sethunathan N (1984) Mineralization of carbaryl
and carbofuran in soil planted to rice. Rice Newsl, 5(3-4): 2.
Brahmaprakash GP & Sethunathan N (1985) Metabolism of carbaryl and
carbofuran in soil planted to rice. Agric Ecosyst Environ, 13(1):
33-42.
Branch RA (1987) The reply. Letter to the Editor. Am J Med, 83:
1169.
Branch RA & Jacqz E (1986a) Is carbaryl as safe as its reputation?
Does it have the potential for causing chronic neurotoxicity in
humans? Am J Med, 80: 659-664.
Branch RA & Jacqz E (1986b) Subacute neurotoxicity following
long-term exposure to carbaryl. Am J Med, 80: 741-745.
Brankovan O (1972) Meiotic effects in corn in embryonic and
generative stages induced by Sevin 50. Arhiv Poljopr Nauke, 90:
125-132.
Briggs GG (1981) Adsorption of pesticides by some Australian soils.
Aust J Soil Res, 19: 61-68.
Brzeskii VV (1972) The study of the mutagenic properties of an
insecticide from the carbamate group - Sevin. Sov Genet, 8: 798-800.
Buchanan DV, Millemann RE, & Stewart NE (1970) Effects of the
insecticide Sevin on various stages of the dungeness crab, Cancer
magister. J Fish Res Board Can, 27: 93-104.
Bukin AL (1965) [Toxicity of Sevin for mammalia and birds.] Gig
Primen Toxicol Pestic Klin Otrav, 1965: 431-435 (in Russian).
Bukin AL & Filatov GV (1965) [Sevin toxicity for mammalia and
birds.] Veterinaria, 42: 93-95 (in Russian).
Burdick GE, Dean HJ, & Harris EJ (1960) Effect of Sevin upon the
aquatic environment. NY Fish Game J, 7(1): 14-25.
Bursian SJ & Edens FW (1977) The prolonged exposure of Japanese
quail to carbaryl and its effect on growth and reproductive
parameters. Bull Environ Contamin Toxicol, 17(3): 360-368.
Bursian SJ & Edens FW (1978) The effect of acute carbaryl
administration on various neurochemical and blood chemical
parameters in the Japanese quail. Toxicol Appl Pharmacol, 46:
463-473.
Bushy Run (1983a) Acute toxicity and irritancy study. Export,
Pennsylvania, Bushy Run Research Center (Project Report No. 46-96)
(Unpublished proprietary information submitted to WHO by
Rhône-Poulenc Agro, Lyon).
Bushy Run (1983b) Sevin 99% technical: Acute toxicity and irritancy
study. Export, Pennsylvania, Bushy Run Research Center (Project
Report No. 46-71) (Unpublished proprietary information submitted to
WHO by Rhône-Poulenc Agro, Lyon).
Bushy Run (1983c) Sevin 80 sprayable. Acute toxicity and irritancy
study. Export, Pennsylvania, Bushy Run Research Center (Project
Report No. 46-97) (Unpublished proprietary information submitted to
WHO by Rhône-Poulenc Agro, Lyon).
Butler PA (1962) Effects on commercial fisheries. In: Effects of
pesticides on fish and wildlife: A review of investigations during
1960. Washington, DC, US Department of the Interior, Fish and
Wildlife Service, pp 11-25 (Circular No. 143).
Butler PA (1963) Commercial fisheries investigations. In:
Pesticide-wildlife studies: A review of Fish and Wildlife Service
investigations during 1961 and 1962. Washington, DC, US Department
of the Interior, Fish and Wildlife Service, pp 11-25 (Circular No.
167).
Cairns T, Siegmund GA, & Doose MG (1983) Potential identification
problems with carbaryl. Bull Environ Contam Toxicol, 30: 93-98.
Calabrese EJ & Geiger CP (1986) Low erythrocyte glusose-6-phosphate
dehydrogenase (G-6-PD) activity and susceptibility to carbaryl
induced methaemoglobin formation and glutathion depletion. Bull
Environ Contam Toxicol, 36(4): 506-509.
Cambon C, Declume Ch, & Derache R (1978) Inhibitions of
acetylcholinesterase from foetal and maternal tissues after oral
intake of carbaryl (1-naphthyl- N-methyl carbamate) by pregnant
rats. Biochem Pharmacol, 27: 2647-2648.
Cambon C, Fernandez J, Falzon M, & Mitjavila S (1981) Variations of
digestive absorption kinetics of carbaryl with the nature of the
vehicle. Toxicology, 22(1): 45-51.
Cameron EA & Mastro VC (1976) Forest insect and disease management /
Evaluation Report: Effectiveness of disparlure in combination with
Sevin 4 oil for Gipsy moth suppression in Pennsylvania 1974. Upper
Darby, Pennsylvania, US Department of Agriculture, Northeastern Area
State and Private Forestry Service.
Cameron EA, Loerch CR, & Mumma RO (1985) Incidental and indirect
exposure to three chemical insecticides used for control of the
Gypsy moth, Lymantria Dispar (L.). Z Angew Entomol, 99: 241-248.
Carey AE & Kutz FW (1985) Trends in ambient concentrations of
agrochemicals in humans and the environment in USA. Environ Monit
Assess, 5(2): 155-164.
Carlson AR (1972) Effects of long-term exposure to carbaryl (Sevin)
on survival, growth and reproduction of the fathead minnow
(Pimephales promelas). J Fish Res Board Can, 29(5): 583-587.
Caro HJ, Freeman HP, & Turner BC (1974) Persistence in soil and
losses in run off of soil incorporated carbaryl in a small
watershed. J Agric Food Chem, 22(5): 860-863.
Carpenter M (1990) Hydrolysis of 14C-carbaryl in aqueous solutions
buffered at pH 5, 7, and 9. Columbia, Missouri, Analytical
Bio-Chemistry Laboratories, Inc. (ABC final report No. 38380)
(Unpublished proprietary information submitted to WHO by
Rhône-Poulenc Agro, Lyon).
Carpenter CP, Weil CW, Palm PH, Woodside MW, Nair JH III, & Smith HF
(1961) Mammalian toxicity of 1-naphthyl- N-methyl carbamate (Sevin
insecticide). J Agric Food Chem, 9: 30-39.
Casale GP, Bavari S, & Connolly JJ (1989) Inhibition of human serum
complement activity by diisopropylfluorophosphate and selected
anticholinesterase insecticides. Fundam Appl Toxicol, 12(3):
460-468.
Casida JE (1970) Mixed-function oxidase involvement in the
biochemistry of insecticide synergists. J Agric Food Chem, 18: 753.
Casida JE & Augustinsson KB (1959) Reaction of plasma albumin with
1-naphthyl- N-methylcarbamate and certain other esters. Biochim
Biophys Acta, 35: 411-426.
Casper HH, Pekas JC, & Dinusson WE (1973) Gastric absorption of a
pesticide (1-naphthyl N-methylcarbamate) in the fasted rat. Pestic
Biochem Physiol, 2: 391-396.
Chaiyarach S, Ratananun V, & Harrel RC (1975) Acute toxicity of the
insecticides toxaphene and carbaryl and the herbicides propanil and
molinate to four species of aquatic organisms. Bull Environ Contam
Toxicol, 14(3): 281-284.
Chancey EL (1974) Sevin carbaryl insecticide: metabolism of carbaryl
in apple fruit (File No. 19496). Clayton, North Carolina, Union
Carbide Corporation (Unpublished proprietary information submitted
to WHO by Rhône-Poulenc Agro, Lyon).
Chancey EL & Andrawes NR (1971a) The absorption and metabolism of
1-naphthyl-C14 carbaryl in corn and rice plants. Clayton, North
Carolina, Union Carbide Corporation (Project Report No. 15712)
(Unpublished proprietary information submitted to WHO by
Rhône-Poulenc Agro, Lyon).
Chancey EL & Andrawes NR (1971b) Metabolism and translocation of
1-naphthyl-C14 carbaryl in tomato fruit and foliage and potato
foliage. Clayton, North Carolina, Union Carbide Corporation (Project
Report No. 15715) (Unpublished proprietary information submitted to
WHO by Rhône-Poulenc Agro, Lyon).
Chapman RA & Cole CM (1982) Observations on the influence of water
and soil pH on the persistence of insecticides. J Environ Sci
Health, B17(5): 487-504.
Chaudhry GR & Wheeler WB (1988) Biodegradation of carbamates. Water
Sci Technol, 20(11/12): 89-94.
Chernoff N & Kavlock RJ (1982) An in vivo teratology screen
utilizing pregnant mice. J Toxicol Environ Health, 10: 541-550.
Chernoff N, Setzer RW, Miller DB, Rosen MB, & Rogers JM (1990)
Effects of chemically induced maternal toxicity on prenatal
development in the rat. Teratology, 42: 651-658.
Chib JS (1985a) The adsorption/desorption of 1-naphthyl
methylcarbamate on five soils (File No. 33820). Research Triangle
Park, North Carolina, Union Carbide Agricultural Products Co., Inc.
(Unpublished proprietary information submitted to WHO by
Rhône-Poulenc Agro, Lyon).
Chib JS (1985b) Sevin: Aerobic aquatic metabolism of carbaryl (File
No. 34234). Research Triangle Park, North Carolina, Union Carbide
Agricultural Products Co., Inc. (Unpublished proprietary information
submitted to WHO by Rhône-Poulenc Agro, Lyon).
Chib JS (1985c) Sevin (1-naphthyl methylcarbamate): Anaerobic
aquatic metabolism of carbaryl (File No. 34086). Research Triangle
Park, North Carolina, Union Carbide Agricultural Products Co., Inc.
(Unpublished proprietary information submitted to WHO by
Rhône-Poulenc Agro, Lyon).
Chib JS (1986a) Sevin brand carbaryl insecticide. Bioconcentration
and fate of carbaryl in bluegill sunfish (Lepomis macrochirus).
Research Triangle Park, North Carolina, Union Carbide Corporation
(Report No. 801R10) (Unpublished proprietary information submitted
to WHO by Rhône-Poulenc Agro, Lyon).
Chib JS (1986b) Sevin brand carbaryl insecticide: Photodegradation
of carbaryl on soil (File No. 34598). Research Triangle Park, North
Carolina, Union Carbide Agricultural Products Co., Inc. (Unpublished
proprietary information submitted to WHO by Rhône-Poulenc Agro,
Lyon).
Chib JS & Andrawes NR (1985) Carbaryl (1-naphthyl methylcarbamate) -
Soil mobility study (File No. 33770). Research Triangle Park, North
Carolina, Union Carbide Agricultural Products Co., Inc. (Unpublished
proprietary information submitted to WHO by Rhône-Poulenc Agro,
Lyon).
Chib JS, Noeth SP, & Smith BK (1986) Sevin brand carbaryl
insecticide. Bioconcentration and fate of carbaryl in Bluegill
Sunfish (Lepomis Macrochirus) (File No 34540). Research Triangle
Park, North Carolina, Union Carbide Agricultural Products Co., Inc.,
Research and Development Department (Unpublished proprietary
information submitted to WHO by Rhône-Poulenc Agro, Lyon).
Chin YN & Sudderuddin KI (1979) Effect of methamidophos on the
growth rate and esterase activity of the common carp Cyprinus
carpio L. Environ Pollut, 18(3): 213-220.
Chin BH, Eldridge JM, & Sullivan LJ (1974) Metabolism of carbaryl by
selected tissues using an organ-maintenance technique. Clin Toxicol,
7: 37-56.
Chin BH, Sullivan LJ, Eldridge JM, & Tallant J (1979a) Metabolism of
carbaryl by kidney, liver, and lung from human postembryonic fetal
autopsy tissue. Clin Toxicol, 14(5): 489-498.
Chin BH, Eldridge JM, Anderson JH, & Sullivan LJ (1979b) Carbaryl
metabolism in the rat. A comparison of in vivo, in vitro (tissue
explant), and liver perfusion techniques. J Agric Food Chem, 27(4):
716-720.
Christenson I (1964) Alkaline hydrolysis of some carbamic esters.
Acta Chem Scand, 18: 904-922.
Cifone MA (1989) Mutagenicity test on carbaryl technical in the in
vitro rat primary hepatocyte unscheduled DNA synthesis assay.
Final report (HLA Study No. 10862-0-435). Kensington, Maryland,
Hazelton Laboratories America, Inc. (Unpublished proprietary
information submitted to WHO by Rhône-Poulenc Agro, Lyon).
Clayson DB (1987) The need for biological risk assessment in
reaching decisions about carcinogens. Mutat Res, 185(3): 243-269.
Collins TFX, Hansen WH, & Keeler HV (1971) The effect of carbaryl
(Sevin) on reproduction of the rat and the gerbil. Toxicol Appl
Pharmacol, 19: 202-216.
Comer SW, Staiff DC, Armstrong JF, & Wolf HR (1975) Exposure of
workers to carbaryl. Bull Environ Contam Toxicol, 13(4): 385-391.
Coulston F, Rosenblum I, & Dougherty WJ (1974) Teratogenic
evaluation of carbaryl in the Rhesus monkey (Macacca mulatta).
Holloman Air Force Base, New Mexico, International Center of
Environmental Safety, Albany Medical College, 54 pp (Unpublished
proprietary information submitted to WHO by Rhône-Poulenc Agro,
Lyon).
Courtemanch DL & Gibbs KE (1978) The effects of Sevin-4-oil on
lenthic communities. In: Environmental monitoring of cooperative
spruce budworm control projects, Maine 1976 and 1977. Augusta,
Maine, Maine Department of Conservation, Forest Service, pp 141-150.
Courtemanch DL & Gibbs KE (1980) Short- and long-term effects of
forest spraying of carbaryl (Sevin-4-oil) on stream invertebrates.
Can Entomol, 112: 271-276.
Coutant CC (1964) Insecticide Sevin: Effect of aerial spraying on
drift of stream insects. Science, 146: 420-421.
Cranmer MF (1986) Carbaryl. A toxicological review and risk
analysis. Neurotoxicology, 7(1): 247-332.
Cress CR & Strother A (1974) Effects on drug metabolism of carbaryl
and 1-naphthol in the mouse. Life Sci, 14: 861-872.
Crofton KM, Howard JL, Moser VC, Gill MW, Reiter LW, Tilson HA, &
MacPhail RC (1991) Interlaboratory comparison of motor activity
experiments: Implications for neurotoxicological assessments.
Neurotoxicol Teratol, 13: 599-609.
Crosby DG, Leitis E, & Winterlin WL (1965) Photodecomposition of
carbamate insecticides. J Agric Food Chem, 13(3): 204-205.
Currier WW, MacCollom GB, & Baumann GI (1982) Drift residues of
air-applied carbaryl in an orchard environment. J Econ Entomol,
75(6): 1062-1068.
Czaplicki E (1979) Persistence in the soil of some more important
insecticides applied in Poland. I. Dynamics of insecticide
disappearance in the soil. Pr Nauk Inst Ochr Rosl, 21(2): 7-40.
Czaplicki EJ & Bollag JM (1975) Microbial degradation of 1-naphthol.
Biul Inst Ochr Rosl, 59: 241-248.
Das YT (1990a) Photodegradation of (1-naphthyl-14C) carbaryl in
aqueous solution buffered at pH 5 under artificial sunlight.
Piscataway, New Jersey, Innovative Scientific Services, Inc. (Report
No. 90060) (Unpublished proprietary information submitted to WHO by
Rhône-Poulenc Agro, Lyon).
Das YT (1990b) Photodegradation of (1-naphthyl-14C) carbaryl on
soil under artificial sunlight. Piscataway, New Jersey, Innovative
Scientific Services, Inc. (Report No. 90061) (Unpublished
proprietary information submitted to WHO by Rhône-Poulenc Agro,
Lyon).
Davies JI & Evans WC (1964) Oxidative metabolism of naphthalene by
soil Pseudomonads. The ring-fission mechanism. Biochem J, 91:
251-261.
Davis HC (1961) Effects of some pesticides on eggs and larvae of
oysters (Crassostrea virginica) and clams (Venus mercenaria).
Commer Fish Rev, 23(12): 8-23.
Davis CW (1986a) Sevin brand carbaryl insecticide: Dissipation of
soil residues under field use conditions (File No. 34620). Research
Triangle Park, North Carolina, Union Carbide Agricultural Products
Co., Inc. (Unpublished proprietary information submitted to WHO by
Rhône-Poulenc Agro, Lyon).
Davis CW (1986b) Sevin brand carbaryl insecticide: Magnitude of
carbaryl residues on rangeland and pasture grasses (File No. 34821).
Research Triangle Park, North Carolina, Union Carbide Agricultural
Products Co., Inc. (Unpublished proprietary information submitted to
WHO by Rhône-Poulenc Agro, Lyon).
Davis CW (1987) Sevin brand carbaryl insecticide: Comparison of the
magnitude of carbaryl residues resulting from applications of XLR
plus and 50 W formulations to tree fruit, leafy vegetables, and
cereals (File No. 35357). Research Triangle Park, North Carolina,
Union Carbide Agricultural Products Co., Inc. (Unpublished
proprietary information submitted to WHO by Rhône-Poulenc Agro,
Lyon).
Davis CW & Thomas SJ (1987) Carbaryl insecticide. Section D-Residues
barley (File No. 40092). Research Triangle Park, North Carolina,
Union Carbide Agricultural Products Co., Inc. (Unpublished
proprietary information submitted to WHO by Rhône-Poulenc Agro,
Lyon).
Declume C & Benard P (1977a) Foetal accumulation of [14C] carbaryl
in rats and mice autoradiographic study. Toxicology, 8: 95-105.
Declume C & Benard P (1977b) Etude autoradiographique de la
distribution d'un agent anticholinestérasique, le 1-naphthyl-
N-methyl [14C] carbamate chez la ratte gestante. Toxicol Appl
Pharmacol, 39: 451-460.
Declume C & Benard P (1978) Etude de la pharmacocinétique du
14C-carbaryl chez la souris gestante. Toxicol Eur Res, 1: 174-180.
Declume C & Derache M (1976) Biodisponibilité du 14C-carbaryl
après l'administration oral à la ratte gestante. C R Acad Sci Paris,
D283: 1799-1801.
Declume C & Derache M (1977) Passage placentaire d'un carbamate
anticholinestérasique à activité insecticide: Le carbaryl.
Chemosphere, 6: 141-146.
Declume C, Cambon C, & Derache R (1979) The effects on new-born rats
of repeated carbaryl administration during gestation. Toxicol Lett,
3: 191-196.
Degraeve N, Moutschen-Dahmen M, Houbrechts N, & Colizzi A (1976) A
propos des risques d'un insecticide: le carbaryl utilisé seul et en
combinaison avec les nitrites. Bull Soc R Sci Liège, 45(1-2): 46-57.
DeGiovanni-Donnelly R, Kolbye SM, & Greeves PD (1968) The effect of
IPC, CIPC, Sevin and Zectran on Bacillus subtilis. Experientia
(Basel), 24: 80-81.
Desi I, Gonczi L, Simon G, Farkas I, & Kneffel Z (1974)
Neurotoxicological studies of two carbamate pesticides in subacute
animal experiments. Toxicol Appl Pharmacol, 27: 456-476.
Deuel LE, Brown KW, Price JD, & Turner FT (1985) Dissipation of
carbaryl and the 1-naphthol metabolite in the flooded rice fields. J
Environ Qual, 14(3): 349-354.
Deutsch-Wenzel P, Brune H, Grimmer G, & Misfeld J (1985) Local
application to mouse skin as a carcinogen specific test system for
non-volatile nitroso compounds. Can Lett, 29: 85-92.
Dickoff DJ, Gerber O, & Turovsky Z (1987) Delayed neurotoxicity
after ingestion of carbamate pesticide. Neurology, 37(7): 1229-1231.
Dieringer CS & Thomas JA (1974) Effects of carbaryl on the
metabolism of androgens in the prostate and liver of the mouse.
Environ Res, 7: 381-386.
Dikshith TSS (1991) Mutagenicity and teratogenicity of pesticides.
In: Dikshith TSS ed. Toxicology of pesticides in animals. Boston,
Boca Raton, Ann Arbor, CRC Press, pp 189-198.
Dikshith TSS, Gupta PK, Gaur JS, Datta KK, & Mathur AK (1976)
Ninety-day toxicity of carbaryl in male rats. Environ Res, 12:
161-170.
Dikshith TSS, Kumar SN, Raizada RB, Srivastava MK, & Ray PK (1990)
Residues of 1-naphthol in soil and water samples in and around
Bhopal, India. Bull Environ Contam Toxicol, 44: 87-91.
Dinerman AA, Lavrent'eva NA, & Il'inskaia NA (1970) [The embryotoxic
action of some pesticides.] Gig i Sanit, 35: 39-42 (in Russian).
Dinoeva SK (1974) [Dynamics of the changes in the immunological
structures of lymphatic follicles in spleen during pesticide
poisoning.] Gig i Sanit, 39(3): 85-87 (in Russian).
Dinoeva S (1982) Humoral immune response in 1st, 2nd and 3rd
generations guinea-pigs in the period of reactivation after
intoxication. In: Immunology of reproduction. Proceedings of the
Fifth International Symposium, Varna, Bulgaria, 26-29 May 1982.
Sofia, Bulgarian Academy of Sciences Press, pp 393-395.
Dionne E, Kendall TB, & Suprenant D (1985) Acute toxicity of
carbaryl technical to embryos-larvae of Eastern oysters
(Crassostrea virginica). Wareham, Massachusetts, Springborn
Bionomics, Inc. (Report No. BW-85-7-1817) (Unpublished proprietary
information submitted to WHO by Rhône-Poulenc Agro, Lyon).
Dittert LW & Higuchi T (1963) Rates of hydrolysis of carbamate and
carbonate esters in alkaline solution. J Pharm Sci, 52: 852-857.
Dorough HW (1967) Carbaryl-C14 metabolism in a lactating cow. J
Agric Food Chem, 15(2): 261-266.
Dorough HW (1969) Carbaryl cow study No. 2. Lyon, Rhône-Poulenc Agro
(Unpublished proprietary information).
Dorough HW (1970a) Continuous feeding of carbaryl naphthyl-C14 to
lactating cows. Progress report. Lyon, Rhône-Poulenc Agro (Document
No. 25, Section C, Part III) (Unpublished proprietary information).
Dorough HW (1970b) Metabolism of insecticidal methylcarbamates in
animals. J Agric Food Chem, 18(6): 1015-1022.
Dorough HW (1971) Carbaryl residues in milk and meat of dairy
animals. Presented at the International Symposium on Pesticide
Terminal Residues, Tel-Aviv, Israel, 17-19 February 1971 (Document
No. 27, Section C, Part III).
Dorough HW (1973) Metabolism of carbamate insecticides. Washington,
DC, US Environmental Protection Agency, Office of Research and
Development, 246 pp (EPA-650/1-74-002).
Dorough HW (1982) Inhalation toxicology: Starting with the
environment. Environ Toxicol Chem, 1(3): 213-220.
Dorough HW & Casida JH ( 1964) Nature of certain carbamate
metabolites of the insecticide Sevin. J Agric Food Chem, 12(4):
294-304.
Dorough HW, Leeling NL, & Casida JE (1963) Nonhydrolytic pathway in
metabolism of N-methylcarbamate insecticides. Science, 140:
170-171.
Dougherty WJ, Goldberg L, & Coulston F (1971) The effect of carbaryl
on reproduction in the monkey (Macacca mulatta). Toxicol Appl
Pharmacol, 19: 365.
Duck BJ & Woolias M (1985) Reverse-phase high performance liquid
chromatographic determination of carbaryl in postmortem specimens. J
Anal Toxicol, 9: 177-179.
Duggan RE & Corneliussen PE (1972) Dietary intake of pesticide
chemicals in the United States (III). June 1968-April 1970. Pestic
Monit J, 5(4): 331-341.
Duggan RE, Lipscomb GQ, Cox EL, Heatwole RE, & Kling RC (1971)
Residues in food and feed. Pesticide residue levels in foods in the
United States from July 1, 1963, to June 30, 1969. Pestic Monit J,
5(2): 73-124.
Durham WF & Wolfe HR (1962) Measurements of the exposure of workers
to pesticides. Bull World Health Organ, 26: 75-91.
Dyadicheva TV (1971) [Experimental data on thyroid and adrenal
cortex function during prolonged effect of carbamine pesticides.]
Vrach Delo, 2: 120-123 (in Russian).
Dzwonkowska A & Hubner H (1986) Induction of chromosomal aberrations
in the Syrian hamster by insecticides tested in vivo. Arch
Toxicol, 58(3): 152-156.
Earl FL, Miller E, & Van Loon EJ (1973) Reproductive teratogenic and
neonatal effects of some pesticides and related compounds in beagle
dogs and miniature swine. In: Deichman W ed. Pesticides and the
environment: A continuing controversy. Proceedings of the 8th
Inter-American Conference on Toxicology and Occupational Medicine,
Miami, Florida, pp 253-266.
Edmiston CE Jr, Goheen M, Malaney GW, & Mills WL (1985) Evaluation
of carbamate toxicity: Acute toxicity in a culture of Paramecium
multimicronucleatum upon exposure to aldicarb, carbaryl, and
mexacarbate as measured by Warburg respirometry and acute plate
assay. Environ Res, 36(2): 338-350.
Egert G & Greim H (1976) Formation of mutagenic N-nitroso
compounds from the pesticides prometryne, dodine and carbaryl in the
presence of nitrite at ph 1. Mutat Res, 37: 179-186.
Eheart JF, Turner EC, & Dickinson J (1962) Residues of Sevin in
whole milk from sprayed and dusted cows. J Entomol, 55(4): 504-505.
Eichelberger J & Lichtenberg J (1971) Persistence of pesticides in
river water. Environ Sci Technol, 5: 541-544.
Eisenbrand G, Ungerer O, & Preussmann R (1975) The reaction of
nitrite with pesticides. II. Formation, chemical properties and
carcinogenic activity of the N-nitroso derivatives of N-methyl-
1-naphthyl carbamate (carbaryl). Food Cosmet Toxicol, 13: 365-367.
Eisenbrand G, Schmähl D, & Preussmann R (1976) Carcinogenicity in
rats of high oral doses of N-nitrosocarbaryl, a nitrosated
pesticide. Can Lett, 1: 281-284.
Elespuru RK & Lijinsky W (1973) The formation of carcinogenic
nitroso compounds from nitrite and some types of agricultural
chemicals. Food Cosmet Toxicol, 11: 807-817.
Elespuru R, Lijinsky W, & Setlow JK (1974) Nitrosocarbaryl as a
potent mutagene of environmental significance. Nature (Lond), 247:
386-387.
Elkins ER (1989) Effect of commercial processing on pesticide
residues in selected fruits and vegetables. J Assoc Off Anal Chem,
72(3): 533-535.
Elkins ER, Lamb FC, Farrow RP, Cook RW, Kawai M, & Kimball JR (1968)
Removal of DDT, malathion and carbaryl from green beans from
commercial and home preparative procedures. J Agric Food Chem,
16(6): 962-966.
Elliot-Feeley E & Armstrong JR (1982) Effects of fenitrothion and
carbaryl on Xenopus laevis development. Toxicology, 22(4):
319-335.
Epstein SS, Arnold E, Andrea J, Bass W, & Bishop Y (1972) Detection
of chemical mutagens by the dominant lethal assay in the mouse.
Toxicol Appl Pharmacol, 23: 288-325.
Estesen BJ, Buck NA, & Ware GW (1982) Dislodgeable insecticide
residues on cotton foliage: carbaryl, cypermethrin, and
methamidophos. Bull Environ Contam Toxicol, 28(4): 490-493.
Eto M, Seifert J, Engel J, & Casida J (1980) Organophosphorous and
methylcarbamate teratogens: Structural requirements for reducing
embryonic abnormalities in chickens and kynurenine formamidase
inhibition in mouse liver. Toxicol Appl Pharmacol, 54: 20-30.
Eto M, Kuwano E, Yoshikawa M, Suiko M, Kohno M, Kamihata M, &
Nakatsu S (1982) Mutagenicity and anticholinesterase activity of
possible metabolites of aryl N-methylcarbamates. J Fac Agric
Kyushu Univ, 26(4): 213-219.
Fait DW (1984) Sevin XLR acute inhalation toxicity test. Export,
Pennsylvania, Bushy Run Research Center (Report No. 47-84)
(Unpublished proprietary information submitted to WHO by
Rhône-Poulenc Agro, Lyon).
Falzon M, Fernandez Y, Cambon C, Gros C, & Mitjavila S (1983)
Influence of experimental hepatic impairment on the toxicokinetics
and the anticholinesterase activity of carbaryl in the rat. J Appl
Toxicol, 3(2): 87-89.
FAO (1982) Second Government Consultation on International
Harmonization of Pesticides Registration Requirements, Rome, 11-15
October 1982. Rome, Food and Agriculture Organization of the United
Nations.
FAO/WHO (1964) Carbaryl. In: Evaluation of the toxicity of
pesticides residues in food. Report of a Joint Meeting of the FAO
Committee on Pesticides in Agriculture and the WHO Expert Committee
on Pesticide Residues, Geneva, 30 September-7 October 1963. Geneva,
World Health Organization, pp 132-135 (FAO Meeting Report No.
PL:1963/13; WHO/Food Add./23).
FAO/WHO (1965) Carbaryl. In: Evaluation of the toxicity of pesticide
residues in food. Geneva, World Health Organization, pp 31-35 (FAO
Meeting Report No. PL:1965/10/1; WHO Food Add./27.65).
FAO/WHO (1967) Carbaryl. In: 1966 Evaluation of some pesticide
residues in food. Geneva, World Health Organization, pp 31-38 (FAO
PL/CP/15; WHO Food Add./67.32)
FAO/WHO (1968) Carbaryl. In: 1967 Evaluation of some pesticide
residues in food. Geneva, World Health Organization, pp 15-25 (FAO
Report No. PL: 1967/M/11/1; WHO Food Add./68.30).
FAO/WHO (1969) Carbaryl. In: 1968 Evaluation of some pesticide
residues in food. Geneva, World Health Organization, pp 35-38 (FAO
PL:1968/M/9/1; WHO Food Add./69.35).
FAO/WHO (1970) Carbaryl. In: 1969 Evaluations of some pesticide
residues in food. Geneva, World Health Organization, pp 45-57 (FAO
PL:1969/M/17/1; WHO Food Add./70.38).
FAO/WHO (1971) Carbaryl. In: 1970 Evaluations of some pesticide
residues in food. Geneva, World Health Organization, pp 3-6 (AGP
1979/M/12/1; WHO Food Add./71.42).
FAO/WHO (1972) 1971 Evaluations of some pesticide residues in food.
Geneva, World Health Organization (WHO Pesticide Residue Series No.
1).
FAO/WHO (1974) Carbaryl. In: 1973 Evaluations of some pesticide
residues in food. Geneva, World Health Organization, pp 141-176 (WHO
Pesticide Residue Series No. 3).
FAO/WHO (1976) Carbaryl. In: 1975 Evaluations of some pesticide
residues in food. Geneva, World Health Organization, pp 83-84 (WHO
Pesticide Residue Series No. 5).
FAO/WHO (1977) Carbaryl. In: 1976 Evaluations of some pesticide
residues in food. Rome, Food and Agriculture Organization of the
United Nations, pp 95-123 (AGP 1977/M/14).
FAO/WHO (1978) Carbaryl. In: Pesticide residues in food. Evaluations
1977. Rome, Food and Agriculture Organization of the United Nations,
pp 47-50 (FAO Plant Production and Protection Paper 10 Sup).
FAO/WHO (1980) Carbaryl. In: Pesticide residues in food. Evaluations
1979. Rome, Food and Agriculture Organization of the United Nations,
pp 101-102 (FAO Plant Production and Protection Paper 20 Sup).
FAO/WHO (1985) Carbaryl. In: Pesticide residues in food - 1984. The
monographs. Rome, Food and Agriculture Organization of the United
Nations, pp 79-83 (FAO Plant Production and Protection Paper 67).
FAO/WHO (1986a) Guide to Codex recommendations concerning pesticide
residues. Part 8. Recommendations for methods of analysis of
pesticide residues, 3rd ed. Rome, Food and Agriculture Organization
of the United Nations, Codex Alimentarius Commission.
FAO/WHO (1986b) Codex maximum limits for pesticide residues, 2nd ed.
Rome, Food and Agriculture Organization of the United Nations, Codex
Alimentarius Commission (CAC/Vol. XIII).
Farage-Elawar M (1989) Enzyme and behavioural changes in young
chicks as a result of carbaryl treatment. J Toxicol Environ Health,
26(1): 119-131.
Farage-Elawar M (1990) Effects of in ovo injection of carbamates
on chick embryo hatchability, esterase enzyme activity and
locomotion of chicks. J Appl Toxicol, 10(3): 197-201.
Farago A (1969) [Fatal suicidal case of Sevin (1-naphthol- N-methyl
carbamate) poisoning.] Arch Toxicol, 24: 309-315 (in German).
Farrow RP, Lamb FC, Cook RW, Kimball JR, & Elkins ER (1968) Removal
of DDT, malathion and carbaryl from tomatoes by commercial and home
preparative methods. J Agric Food Chem, 16(1): 65-71.
Farrow RP, Lamb FC, Elkins ER, Cook RW, Kawai M, & Cortes A (1969)
Effect of commercial and home preparative procedures on parathion
and carbaryl residues in broccoli. J Agric Food Chem, 17(1): 75-79.
Federle PF & Collins WJ (1976) Insecticide toxicity to three insects
from Ohio ponds. Ohio J Sci, 76(1): 19-24.
Feldmann RJ & Maibach HT (1974) Percutaneous penetration of some
pesticides and herbicides in man. Toxicol Appl Pharmacol, 28:
126-132.
Fernandez Y, Falzon M, Cambon C, Gros C, & Mitjavila S (1982)
Carbaryl tricompartmental toxicokinetics and anticholinesterase
activity. Toxicol Lett, 13(3-4): 253-258.
Ferreira GAL & Seiber JN (1981) Volatilization and exudation losses
of 3- N methylcarbamate insecticides applied systemically to rice.
J Agric Food Chem, 29(1): 93-99.
Field WE (1980a) Oral LD50 in rats of carbaryl, technical grade,
made in West Virginia. Clarks Summit, Pennsylvania, Chemicals, Drugs
and Cosmetics Research (Report No. CDC-UC-046-79) (Unpublished
proprietary information submitted to WHO by Rhône-Poulenc Agro,
Lyon).
Field WE (1980b) Oral LD50 in rats of carbaryl, technical grade,
made in India. Clarks Summit, Pennsylvania, Chemicals, Drugs and
Cosmetics Research (Report No. CDC-UC-047-79) (Unpublished
proprietary information submitted to WHO by Rhône-Poulenc Agro,
Lyon).
Filatov DV & Brytskov VE (1972) [Enzymic method of residue
determination of chlorophos and Sevin by means of indicator paper.]
Probl Vet Sanit, XLI: 233-241 (in Russian).
Fisher SW & Lohner TW (1986) Studies on the environmental fate of
carbaryl as a function of pH. Arch Environ Contam Toxicol, 15(6):
661-667.
Fisher HL, Most B, & Hall LL (1985) Dermal absorption of pesticides
calculated by deconvolution. J Appl Toxicol, 5(3): 163-177.
Fletcher DW & Leonard MI (1986a) Toxicity and reproduction study
with carbaryl technical in Bobwhite quail. Neillsville, Wisconsin,
Bio-Life Associate Ltd (Report No. 85-DR-15) (Unpublished
proprietary information submitted to WHO by Rhône-Poulenc Agro,
Lyon).
Fletcher DW & Leonard MI (1986b) 28-day dietary toxicity and
reproduction pilot study with carbaryl technical in Mallard ducks.
Neillsville, Wisconsin, Bio-Life Associate Ltd (Report No. 85 DR
P14) (Unpublished proprietary information submitted to WHO by
Rhône-Poulenc Agro, Lyon).
Flores-Ruegg E, De Andrea MM, Helene CG, & Hirata R (1980)
Persistence of pesticide residues in Brazilian soil samples related
to organic matter and microbiological activity. In: Meeting of the
Advisory Group on Agrochemical Residue Biota Interaction: Proceeding
report. Sao Paulo, Brazil, Biological Institute, pp 181-187.
Frank R, Braun HE, & Ripley BD (1989) Monitoring Ontario grown
apples for pest control chemicals used in their production, 1978-86.
Food Addit Contam, 6(2): 227-234.
Frank R, Braun HE, Ripley BD, & Pitblado R (1991) Residues of nine
insecticides and two fungicides in raw and processed tomatoes. J
Food Prot, 54(1): 41-46.
Fukuto TR (1973) Metabolism of carbamate insecticides. In: Di Carlo
FJ ed. Drug metabolism reviews. New York, Basel, Marcel Dekker,
Inc., vol 1, pp 117-151.
Gaillard D, Chamoiseau G, & Derache R (1977) Interaction du carbaryl
et du nitrite de sodium sur les activités enzymatiques de quelques
mono-oxygénases microsomiques du foie de rat. J Pharmacol, 8: 437.
Gaines TB (1960) The acute toxicity of pesticides to rat. Toxicol
Appl Pharmacol, 2: 88-99.
Gaines TB (1969) Acute toxicity of pesticides to rat. Toxicol Appl
Pharmacol, 14: 514-534.
Gangwar SK, Kavadia VS, Gupta HCL, & Srivastava BP (1978a)
Persistence of carbaryl in sandy loam soil and its uptake in bajra,
Pennistetum typhoides P. Ann Arid Zone, 17(4): 357-362.
Gangwar SK, Kavadia VS, Srivastava BP, & Gupta HCL (1978b)
Persistence of carbaryl residues in sandy loam soil and its uptake
by root crops. Indian J Plant Prot, 8(1): 11-18.
Gapparov Sh (1974) [Haemocoagulation indices in dogs chronically
intoxicated with low doses of Sevin.] [Health Health Care Organ
Uzbekistan], II: 146-149 (in Russian).
Gartrell MJ, Craun JC, Podrebarac DS, & Gunderson EL (1986)
Pesticides, selected elements, and other chemicals in infant and
toddler total diet samples, October 1980-March 1982. J Assoc Off
Anal Chem, 69(1): 123-145.
Georgiev IN (1967) [The effect of toxic and maximal permissible
amounts of Sevin on intestinal secretion.] Gig i Sanit, 32(8): 42-46
(in Russian with English summary).
Ghadiri M & Greenwood DA (1966) Toxicity and biologic effects of
malathion, phosdrin and Sevin in the chick embryo. Toxicol Appl
Pharmacol, 8: 342.
Ghosh P, Bhattacharya S, & Bhattacharya S (1990) Impairment of the
regulation of gonadal function in Channa punctatus by metacid 50
and carbaryl under laboratory and field conditions. Biomed Environ
Sci, 3(1): 106-112.
Gibbs E, Mingo TM, & Courtemanch DL (1981) The effect on pond
macroinvertebrates from forest spraying of carbaryl and its
persistence in water and sediment. Augusta, Maine, Maine Department
of Conservation, Forest Service.
Gibbs KE, Mingo TM, & Courtemanch DL (1984) Persistence of carbaryl
(Sevin-4-oil) in woodland ponds and its effects on ponds macro
invertebrates following forest spraying. Can Entomol, 116: 203-214.
Gill SS & Yeoh CL (1980) Degradation of carbaryl in three components
of the paddy-field ecosystem of Malaysia. In: Meeting of the
Advisory Group on Agrochemical Residue Biota Interaction: Proceeding
report. Sao Paulo, Brazil, Biological Institute, pp 229-243.
Gill TS, Pant JC, & Pant J (1988) Gill, liver, and kidney lesions
associated with experimental exposures to carbaryl and dimethoate in
the fish (Puntius conchonius Ham.). Bull Environ Contam Toxicol,
41(1): 71-78.
Gladenko IN & Malinin OA (1970) [Toxicity of Sevin for livestock.]
Gig Primen Toxicol Pestic Klin Otrav, 1970: 188-193 (in Russian).
Golbs S, Kühnert M, & Leue F (1975) [Prenatal toxicological study of
Sevin (carbaryl) for Wistar rats.] Arch Exp Veterinaermed, 29:
607-614 (in German with English summary).
Golbs S, Kühnert M, & Fuchs V (1984) [Influence on the acute
toxicity of selected pesticides by huminic acids.] Z Gesamte Hyg,
30(12): 720-723 (in German).
Gold RE, Leavitt JR, Holcslaw T, & Tupy D (1982) Exposure of urban
applicators to carbaryl. Arch Environ Contam Toxicol, 11: 63-67.
Goldberg HI, Johnson HE, & Knaak JB (1965) Inhibition of discrete
avoidance behaviour by three anticholinesterase agents.
Psychopharmacologia, 7(1): 72-76.
Guirguis GN & Brindley WA (1975) Carbaryl penetration into and
metabolism by alfalfa leafcutting bees, Megachile pacifica. Agric
Food Chem, 23(2): 274-279.
Guirguis GN & Brindley WA (1976) Effect of chlorcyclizin,
aminopyrine or phenobarbital on carbaryl metabolism in alfalfa
leafcutting bees. Environ Entomol, 5(3): 590-594.
Gunderson EL (1988) FDA total diet study, April 1982-April 1984.
Dietary intake of pesticides, selected elements, and other
chemicals. J Assoc Off Anal Chem, 71(6): 1200-1209.
Gupta SK & Sundararaman V (1991) Correlation between burrowing
capability and AChE activity in the earthworm, Pheretima posthuma,
on exposure to carbaryl. Bull Environ Contam Toxicol, 46: 859-865.
Guseinov MK (1970) [On the question of circulation of Sevin in
environment.] Gig Primen Toxicol Pestic Klin Otrav, 1970: 174-176
(in Russian).
Guthrie FE, Monro RJ, & Abernathy CO (1971) Response of laboratory
mouse to selection for resistance to insecticides. Toxicol Appl
Pharmacol, 18: 92-101.
Gyrisco GG, Lisk DJ, Fertig SN, Huddleston EW, Fox FH, Holland RF, &
Teimberger GW (1960) The effects of feeding high levels of Sevin on
residue, flavour, and odour of the milk of dairy cattle. Agric Food
Chem, 8(5): 409-410.
Haines TA (1981) Effect of an aerial application of carbaryl on
brook trout (Salvelinus fontinalis). Bull Environ Contam Toxicol,
27(4): 534-542.
Hamada NN (1987) One year oral toxicity study in Beagle dogs with
carbaryl technical. Vienna, Virginia, Hazleton Laboratories America,
Inc. (Report No. 400-715) (Unpublished proprietary information
submitted to WHO by Rhône-Poulenc Agro, Lyon).
Hamada NN (1990) Acute oral toxicity study in rats. Carbaryl
technical. Vienna, Virginia, Hazleton Laboratories America, Inc.
(Unpublished proprietary information submitted to WHO by
Rhône-Poulenc Agro, Lyon).
Hamada NN (1991a) Subchronic toxicity study in dogs with carbaryl
technical. Vienna, Virginia, Hazleton Laboratories America, Inc.
(Report No. N656-152) (Unpublished proprietary information submitted
to WHO by Rhône-Poulenc Agro, Lyon).
Hamada NN (1991b) Oncogenicity with carbaryl technical in CD-1(R)
mice. Vienna, Virginia, Hazleton Laboratories America, Inc. (Report
No. N656-138) (Unpublished proprietary information submitted to WHO
by Rhône-Poulenc Agro, Lyon).
Hamada NN (1991c) Combined chronic toxicity and oncogenicity study
with carbaryl technical in Sprague-Dawley rats - 52 week interim
report with 4-week recovery. Vienna, Virginia, Hazleton Laboratories
America, Inc. (Study No. 656-139) (Unpublished proprietary
information submitted to WHO by Rhône-Poulenc Agro, Lyon).
Hanazato T & Yasuno M (1987) Effects of a carbamate insecticide,
carbaryl, on the summer phyto- and zooplankton communities in ponds.
Environ Pollut, 48: 145-159.
Hanazato T & Yasuno M (1989) Influence of over-wintering Daphnia
on spring zooplankton communities: An experimental study. Ecol Res,
4: 323-338.
Hanazato T & Yasuno M (1990a) Influence of Chaoborus density on
the effects of an insecticide on zooplankton communities in ponds.
Hydrobiologia, 194: 183-197.
Hanazato T & Yasuno M (1990b) Influence of time in application of an
insecticide on recovery patterns of a zooplankton community in
experimental ponds. Arch Environ Contam Toxicol, 19: 77-83.
Hansen JL (1978) Carbaryl: Human ingestion study statistical
analysis (File No. 24971). Research Triangle Park, North Carolina,
Union Carbide Corporation (Unpublished proprietary information
submitted to WHO by Rhône-Poulenc Agro, Lyon).
Hansen CR & Kawatski JA (1976) Application of 24-hour post-exposure
observation to acute toxicity studies with invertebrates. J Fish Res
Board Can, 33: 1198-1201.
Harris LW, Talbot BG, Lennox WJ, & Anderson DR (1989) The
relationship between oxime induced reactivation of carbamylated
acetylcholinesterase and antidotal efficacy against carbamate
intoxication. Toxicol Appl Pharmacol, 98(1): 128-133.
Harry JB (1977) A review of human exposure to Sevin carbaryl
insecticide with particular reference to the USA. Research Triangle
Park, North Carolina, Union Carbide Agricultural Products Co., Inc.
(Unpublished proprietary information submitted to WHO by
Rhône-Poulenc Agro, Lyon).
Hart ER (1972) Teratology study. Sevin, vitamin A, aspirin and
malathion. South Charleston, West Virginia, Union Carbide
Corporation, 8 pp (Prepared by Bionetics Research Laboratories)
(Unpublished proprietary information submitted to WHO by
Rhône-Poulenc Agro, Lyon).
Hassan A (1971) Pharmacological effects of carbaryl. The effect of
carbaryl on the synthesis and degradation of catecholamines in the
rat. Biochem Pharmacol, 20: 2299-2308.
Hassan A & Cueto C Jr (1970) Biochemical effects in the rabbit of
repeated administration of a mixture of DDT, carbaryl and parathion.
Z Naturforsch, B25: 521-525.
Hassan A & Santolucito JA (1971) Pharmacological effects of
carbaryl. II. Modification of serotonin metabolism in the rat brain.
Experientia (Basel), 27: 287-288.
Hassan A, Zayed SMAD, & Abdel-Hamid FM (1966) Metabolism of
carbamate drugs. I. Metabolism of 1-naphthyl- N-methylcarbamate
(Sevin) in the rat. Biochem Pharmacol, 15: 2045-2055.
Hassan AA, El Sewedy SM, Minatogawa Y, & Kido R (1990) Effects of
some insecticides on several enzymes of tryptophan metabolism in
rats. J Environ Sci Health, B25(3): 333-346.
Haven D, Castagna M, Chanley P, Wass M, & Whitcomb J (1966) Effects
of the treatment of an oyster bed with polystream and Sevin.
Chesapeake Sci, 7(4): 179-188.
Haydock CI (1964) An experimental study to control oyster drills in
Tomales Bay, California. Calif Fish Game, 50: 11-28.
Hayes WJ (1963) Clinical handbook on economic poisons. Emergency
information for treating poisoning. Atlanta, Georgia, US Department
of Health, Education and Welfare, Communicable Disease Center, pp
44-46.
Hayes WJ Jr & Laws ER Jr ed. (1991) Carbaryl. In: Handbook of
pesticide toxicology. Volume 3 - Classes of pesticides. New York,
San Diego, Academic Press, Inc., pp 1144-1189.
Hazleton Laboratories America, Inc. (1982) Acute oral toxicity study
in rats - Sevin RF. Vienna, Virginia, Hazleton Laboratories America,
Inc. (Unpublished proprietary information submitted to WHO by
Rhône-Poulenc Agro, Lyon).
Heise GA & Hudson JD (1985a) Effects of pesticides and drugs on
working memory in rat: Continuous delayed response. Pharmacol
Biochem Behav, 23: 591-598.
Heise GA & Hudson JD (1985b) Effects of pesticides and drugs on
working memory in rats: Continuous non-match. Pharmacol Biochem
Behav, 23: 599-605.
Hernandez DA, Lombardo RL, Ferrari L, & Tortorelli MC (1990)
Toxicity of ethyl-parathion and carbaryl on early development of sea
urchin. Bull Environ Contam Toxicol, 45: 734-741.
Heywood DL (1975) Degradation of carbamate insecticides in soil.
Environ Qual Saf, 4: 128-133.
Hill EF & Camardese MB (1986) Lethal dietary toxicities of
environmental contaminants and pesticides to Coturnix. Washington,
DC, US Department of the Interior, Fish and Wildlife Service (Fish
and Wildlife Technical Report No. 2).
Hill EF, Heath RG, Spann JW, & Williams JD (1975) Lethal dietary
toxicities of environmental pollutants to birds. Washington, DC, US
Department of the Interior, Fish and Wildlife Service (Special
Scientific Report, Wildlife No. 191).
Hodgson E & Casida JE (1961) Metabolism of N:N-dialkyl carbamate and
related compounds by rat liver. Biochem Pharmacol, 8: 179-191.
Hoffman DJ & Albers PH (1984) Evaluation of potential embryotoxicity
and teratogenicity of 42 herbicides, insecticides, and petroleum
contaminants to mallard eggs. Arch Environ Contam Toxicol, 13:
15-27.
Hoque H (1972) Radio tracer studies on chemical residues in food and
agriculture. Proc. Comb. Panel Res. Coord. Meet. 1971.
Hoque MZ (1972) Carbaryl - A new chemical mutagen. Curr Sci, 41(23):
855-856.
Houston JB, Upshall DG, & Bridges JW (1974) Pharmacokinetics and
metabolism of two carbamates, carbaryl and landrin, in the rat.
Xenobiotica, 5(10): 637-648.
Hudson RH, Tucker RK, & Haegele MA (1984) Handbook of toxicity of
pesticides to wildlife, 2nd ed. Washington, DC, US Department of the
Interior, Fish and Wildlife Service (Resource Publication 153).
Hughes L Jr (1971) A study of the fate of carbaryl insecticide in
surface waters. Lafayette, Indiana, Purdue University (Ph.D
Dissertation) (University microfilms, High Wycombe, England).
Hughes LB & Reuszer HW (1970) A study of bacterial decomposition of
carbaryl in farm pond waters. Lafayette, Indiana, Purdue University,
Water Resources Research Center (Technical Report No. 10).
Hundley HK, Cairns T, Luke AM, & Masumoto HT (1988) Pesticide
residue findings by the Luke method in domestic and imported foods
and animal feeds for fiscal years 1982-1986. J Assoc Off Anal Chem,
71(5): 875-892.
Huque H (1972) Preliminary report on the residues of carbaryl
granules in rice plants. Radiotracer Stud. Chem. Residues Food Agr.,
Proc. Comb. Panel Res. Coord. Meet. 1971. Vienna, International
Atomic Energy Agency, pp 107-109.
Hurlburt E, McMillan R, & Vialle M (1989) Supplemental environmental
impact statement: use of the insecticide carbaryl to control ghosts
and mud shrimp in oyster beds of Willapa Bay and Grays Harbour.
Olympia, Washington, Washington State Department of Fisheries and
Washington State Department of Ecology (Unpublished report submitted
to WHO by Rhône-Poulenc Agro, Lyon).
Hurwood IS (1967) Studies on pesticide residues. 2. Carbaryl
residues in the body tissues and milk of cattle following dermal
application. Qld J Agric Anim Sci, 24: 69-74.
Hwang SW & Schanker S (1974) Absorption of carbaryl from lung and
small intestine of the rat. Environ Res, 7(2): 206-211.
IARC (1987) Overall evaluations of carcinogenicity: An updating of
IARC monographs, volumes 1 to 42. Lyon, International Agency for
Research on Cancer (IARC Monographs on the Evaluation of the
Carcinogenic Risk of Chemicals to Humans, Supplement 7).
Imming RJ, Shaffer BC, & Woodard G (1969) Sevin, safety evaluation
by feeding to female Beagles from day one of gestation through
weaning of the offspring. (Prepared by Woodard Research Corporation
for Union Carbide Agricultural Products Co., Inc., Research Triangle
Park) (Unpublished proprietary information submitted to WHO by
Rhône-Poulenc Agro, Lyon).
Innes JRM, Ulland MB, Valerio MG, Petrucel L, Fishbein L, Hart ER,
Pallotta AJ, Bates RR, Falk HL, Gart JJ, Klein M, Mitchell I, &
Peters H (1969) Bioassay of pesticides and industrial chemicals for
tumorigenicity in mice: a preliminary note. J Natl Cancer Inst, 42:
1101-1114.
IRPTC (1982) In: Izmerov NF ed. IRPTC scientific reviews of Soviet
literature on toxicity and hazard of chemicals No. 7: Carbaryl.
Moscow, Centre of International Projects, GKNT, 13 pp.
IRPTC (1989) In: Izmerov NF ed. [Scientific reviews of Soviet
literature on toxicity and hazard of chemicals No. 7: Carbaryl], 2nd
ed. Moscow, Centre of International Projects, GKNT, 82 pp (in
Russian).
Ishidate M Jr & Odashima S (1977) Chromosome tests with 134
compounds on Chinese hamster cells in vitro: a screening for
chemical carcinogens. Mutat Res,48: 337-354.
Ishidate M Jr, Sofuni T, & Yoshikawa K (1981) Chromosomal aberration
tests in vitro as a primary screening tool for environmental
mutagens and/or carcinogens. Gann Monogr Cancer Res, 27: 95-108.
Ivanova LN & Molozhanova YG (1973) [Static and dynamic distribution
of carbaryl in the environment.] Gig i Sanit, 38(2): 24-28 (in
Russian).
Iwata Y, Dusch ME, Garman GE, & Gunther FA (1979) Worker environment
research: Residues for carbaryl, chlorobenzilate, dimethoate and
trichlorfon applied to citrus trees. J Agric Food Chem, 27(6):
1141-1145.
Jablonska J & Brzezinski J (1990) The influence of carbaryl on the
uptake of (3H) noradrenaline (3H) NA by rat hypothalamic slices.
Arch Toxciol, 64(5): 417-419.
Jacob J, Schmoldt A, Raab J, Hamman M, & Grimmer G (1985)
Monoxygenase induction by various xenobiotics and its influence on
the rat liver microsomal metabolite profile of benza(a)anthracene.
Cancer Lett, 27: 105-113.
Jaszczuk E & Syrowatka T (1979) [Induction of mitotic conversion in
Saccharomyces cerevisiae D4 with propoxur, carbaryl,
dimethylnitrosamine and products of their metabolic activation.]
Rocz Panstw Zakl Hig, 30(4): 323-330 (in Polish).
Jaszczuk E, Syrowatka T, & Cybulski J (1979) [Mutagenic activity of
propoxur, carbaryl and their nitroso derivatives. Induction of
reversion in Salmonella typhimurium.] Rocz Pantsw Zakl Hig, 30(1):
81-88 (in Polish).
Jegier Z (1964) Health hazards in insecticide spraying of crops.
Arch Environ Health, 8: 670-674.
Jeleniewicz KK & Szczepaniak St (1980) [Free aminoacids and amino
nitrogen levels in serum and blood erythrocytes of rat exposed to
subacute intoxication with carbaryl.] Bromatol Chem Toksykol, 13(3):
237-240 (in Polish).
Jeleniewicz KK, Szczepaniak St, & Slenkiewicz E (1984) [Effect of
acute intoxication with carbaryl on brain free aminoacids level in
rat.] Bromatol Chem Toksykol, 17(3): 225-227 (in Polish).
Jerkofsky M & Abrahamsen LH (1983) Variation on human herpes virus
susceptibility to enhancement by the pesticide carbaryl. Appl
Environ Microbiol, 45(5): 1555-1559.
Johnson WW & Finley MT (1980) Handbook of acute toxicity of
chemicals to fish and aquatic invertebrates. Washington, DC, US
Department of the Interior, Fish and Wildlife Service (Resource
Publication 137).
Johnson RD & Manske DD (1976) Pesticide residues in total diet
samples (IX). Pestic Monit J, 9(4): 157-169.
Johnson DP & Stansbury HA (1965) Adaptation of Sevin insecticide
(carbaryl) residue method to various crops. J Agric Food Chem, 13:
235-238.
Junk GA, Richard JJ, & Dahm PA (1984) Degradation of pesticides in
controlled water-soil systems (treatment and disposal of pesticides
wastes). Washington, DC, American Chemical Society, pp 37-67 (ACS
Symposium Series No. 259).
Kadir HA & Knowles CO (1981) Inhibition of rat brain monoamine
oxidase by insecticides, acaricides and related compounds. Gen
Pharmacol, 12(4): 239-247.
Kagan J, Rodionov G, Voronina L, Velitsco L, Kulagin O, & Peremitina
A (1970) [Effect of Sevin on liver function and structure.]
Pharmacol Toxicol, 33: 219-224 (in Russian with English summary).
Kaloyanova F (1963) [Changes in the blood sugar in cases of
intoxications with certain phosphorus organic compounds.] In: [Works
of the Scientific Research Institute on Work Safety and Occupational
Diseases - Volume X.] Sofia, Institute of Hygiene and Occupational
Health, pp 63-67 (in Bulgarian).
Kanazawa J (1975) Uptake and excretion of organophosphorus and
carbamate insecticides by freshwater fish, Motsugo, Pseudorasbora
parva. Bull Environ Contam Toxicol, 14(3): 346-352.
Kanazawa J (1981) Measurement of the bioconcentration factors of
pesticides by freshwater fish and their correlation with
physicochemical properties or acute toxicities. Pestic Sci, 12(4):
417-424.
Kanazawa J, Isensee AR, & Kearney PC (1975) Distribution of carbaryl
and 3,5-xylylmethyl carbamate in an aquatic model ecosystem. J Agric
Food Chem, 23(4): 760-763.
Karinen FJ, Lamberton JG, Stewart NE, & Terrier LC (1967)
Persistence of carbaryl in the marine estuarine environment. J Agric
Food Chem, 15(1): 148-156.
Karnak RE & Collins WJ (1974) The susceptibility to selected
insecticides and acetylcholinesterase activity in a laboratory
colony of midge larvae, Chironomus tentans (Diptera:
chironomidae). Bull Environ Contam Toxicol, 12: 62-69.
Kassin VJ (1968) [Structural changes in endocrine organs under
repeated action of Sevin.] Gig Primen Toxicol Pestic Klin Otrav,
1968: 631-635 (in Russian).
Kaur K & Toor HS (1977) Toxicity of pesticides to embryonic stages
of Cyprinus carpio communis Linn. Indian J Exp Biol, 15: 193-196.
Kavadia VS, Gangwar SK, Srivastava BP, Kathpal TS, & Gupta HCL
(1978) Persistence of carbaryl in clay loam soil and its uptake in
maize and root crops. Indian J Entomol, 40(3): 265-272.
Kavlock RJ, Short RD, & Chernoff N (1987) Further evaluation of an
in vivo teratology screen. Teratog Carcinog Mutagen, 7: 7-16.
Kazano H, Kearney PC, & Kaufman DD (1972) Metabolism of
methylcarbamate insecticides in soil. J Agric Food Chem, 20(5):
975-979.
Kazarnovskaya ML & Vasilos AF (1977) [The effect of Sevin on
chromosomal apparatus of cells in vitro.] Zdravookhranenije,
20(4): 14-16 (in Russian).
Kendrick PN, Trim AJ, Atwal JK, & Brown PM (1991) Direct gas
chromatographic determination of carbaryl residues in honeybees
( Apis mellifera L.) using a nitrogen-phosphorus detector with
confirmation by formation of a chemical derivative. Bull Environ
Contam Toxicol, 46: 654-661.
Khasawinah AM (1977) Hydrolysis of carbaryl in aqueous buffer
solutions (Project No. 111A12, File No. 23092). South Charleston,
West Virginia, Union Carbide Corporation (Prepared in cooperation
with the International Research and Development Corporation)
(Unpublished proprietary information submitted to WHO by
Rhône-Poulenc Agro, Lyon).
Khasawinah AM (1978) Fate of carbaryl in soils (Project No. 285).
Monument, Colorado, Analytical Development Corporation (Unpublished
proprietary information submitted to WHO by Rhône-Poulenc Agro,
Lyon).
Khera K (1966) Toxic and teratogenic effects of insecticides in duck
and chick embryos. Toxicol Appl Pharmacol, 8: 345.
Klisenko MA (1965) [Determination of Sevin in air and biological
material.] J Anal Chim, XX(5): 634-636 (in Russian).
Klisenko MA & Yakim VS (1966) [Accumulation and excretion of Sevin
from the body of warm-blooded animals.] Gig Primen Toxicol Pestic
Klin Otrav, 1966: 146-149 (in Russian).
Klisenko MA, Lebedeva TA, & Yurkova ZF (1972) [Chemical analysis of
micro amounts of pesticides.] Moscow, Medizina, pp 197-201 (in
Russian).
Knaak JB (1971) Biological and nonbiological modifications of
carbamates. Bull World Health Organ, 44: 121-131.
Knaak JB & Sullivan LJ (1967) Metabolism of carbaryl in dog. J Agric
Food Chem, 15(6): 1125-1126.
Knaak JB, Marilyn J, Tallant MJ, Bartley WJ, & Sullivan LF (1965)
The metabolism of carbaryl in rat, guinea-pig, and man. J Agric Food
Chem, 13(6): 537-543.
Knaak JB, Tallant MJ, Kozbelt SJ, & Sullivan LJ (1968) The
metabolism of carbaryl in man, monkey, pig, and sheep. J Agric Food
Chem, 16(3): 465-470.
Knaak JB, Yee K, Ackerman CR, Zweig G, Fry DM, & Wilson BW (1984)
Percutaneous absorption and dermal dose - ChE response studies with
parathion and carbaryl in the rat. Toxicol Appl Pharmacol, 76:
252-263.
Knight EV, Chin BH, Ueng TH, & Alvares AP (1986) Differences in the
in vivo and in vitro effects of the carbamate insecticide,
carbaryl on rat hepatic monooxygenases. Drug Chem Toxicol, 9(3-4):
253-273.
Knight EV, Alvares AP, & Chin BH (1987) Effects of phenobarbital
pre-treatment on the in vivo metabolism of carbaryl in rats. Bull
Environ Contam Toxicol, 39(5): 815-821.
Korn S (1973) The uptake and persistence of carbaryl in channel
catfish. Trans Am Fish Soc, 1: 137-139.
Koundinya PR & Ramamurthi R (1980) Toxicity of Sunithion and Sevin
to the freshwater fish Sarotherodon mossambicus (Peters). Curr
Sci, 49: 875-876.
Kovaleva ES & Talanov GA (1978a) [Methods for determination of Sevin
in soil and plants by means of thin-layer chromatography.] Gig i
Sanit, 7: 69-70 (in Russian).
Kovaleva ES & Talanov GA (1978b) [Residual amounts of Sevin in
plants and soil.] Khim Sel'sk Khoz, 16(8): 77-79 (in Russian).
Kovaleva ES & Talanov GA (1980) [Plant and soil dynamics of Sevin
residues and dithiocarbamic acid derivatives.] In: Bobovnikova TI &
Malakhov SG ed. [Migration of contaminants in soil and contiguous
media. Proceedings of the All-Union Congress, 2nd Meeting, 1978.]
Leningrad, Gidrometeoizdat, pp 155-159 (in Russian).
Kovtun SD (1970) Analysis of the action exerted by Sevine on the
myoneural formations. Pharmacol Toxicol, 35(5): 543-545.
Kovtun SD & Sokur AI (1970) [The effect of Sevin on the spontaneous
electrical activity of myoneural synaps and permeability of the
membrane of skeletal muscle in white rats.] Gig Primen Toxicol
Pestic Klin Otrav, 1970: 211-214 (in Russian).
Krause RT (1985a) Pesticide and industrial chemical residues. Liquid
chromatographic determination of N-methylcarbamate insecticides
and metabolites in crops. I. Collaborative study. J Assoc Off Anal
Chem, 68(4): 726-733.
Krause RT (1985b) Liquid chromatographic determination of
N-methylcarbamate insecticides and metabolites in crops. II.
Analytical characteristics and residue findings. J Assoc Off Anal
Chem, 68(4): 734-740.
Krechniak V & Foss W (1981) Gas chromatography determination of
carbaryl. Pr Cent Inst Ochr Pr, XXXI(110): 183-188.
Krishna JG & Casida JE (1966) Fate in rats of the radiocarbon from
ten variously labelled methyl- and dimethylcarbamate-C14
insecticide chemicals and their hydrolysis products. J Agric Food
Chem, 14(2): 98-105.
Krishna JG, Dorough HW, & Casida JE (1962) Synthesis of
N-methylcarbamates via methyl isocyanate-C14 and chromatographic
purification. J Agric Food Chem, 10(6): 462-466.
Krishnaih K, Prasad VG, & Jagan Mohan N (1978) Carbaryl residues on
brinjal and peas. In: Edwards CA, Veeresh GK, & Krueger HR ed.
Proceedings of the Symposium on Pesticide Residues in the
Environment in India, 7-10 November 1978. Hebbal, Bangalore,
University of Agricultural Sciences, pp 229-231 (UAS Technical
Series No. 32).
Krug HG & Berndt J (1985) Inhibition by pesticides of prostglandin
formation in blood platelets. Blut, 51: 19-23.
Krug HF, Hamm U, & Berndt J (1988) Mechanism of inhibition of
cyclo-oxygenase in human blood platelets by carbamate insecticides.
Biochem. J, 250(1): 103-110.
Krylov DG (1970) [The effect of Sevin on red vole population.] Gig
Primen Toxicol Pestic Klin Otrav, 1970: 218-110 (in Russian).
Kuhn JO (1991a) Acute oral toxicity study in rats with Sevin XLR
plus. Sugar Land, Texas, Stillmeadow, Inc. (Laboratory Study No. IV
8150-91) (Unpublished proprietary information submitted to WHO by
Rhône-Poulenc Agro, Lyon).
Kuhn JO (1991b) Sevin-4-oil 47.0% (w/w). Acute oral toxicity study
in rats . Sugar Land, Texas, Stillmeadow, Inc. (Laboratory Study No.
N 7712-90) (Unpublished proprietary information submitted to WHO by
Rhône-Poulenc Agro, Lyon).
Kuhr RJ (1970) Metabolism of carbamate insecticide chemicals in
plants and insects. J Agric Food Chem, 18(6): 1023-1030.
Kuhr RJ (1971) The formation and importance of carbamate insecticide
metabolites as terminal residues. Pure Appl Chem, Suppl: 199-220.
Kuhr RJ & Dorough HW (1976) Carbamate insecticides: Chemistry,
biochemistry and toxicology. Cleveland, Ohio, CRC Press, Inc., pp
125-200.
Kuhr RJ, Davis AC, & Bourke JB (1974) Dissipation of Guthion, Sevin,
Polyram, Phygon and Systox from apple orchard soil. Bull Environ
Contam Toxicol, 11(3): 224-230.
Kulshrestha SK & Arora N (1984) Impairments induced by sublethal
doses of two pesticides in the ovaries of a freshwater teleost
Channa striatus Bloch. Toxicol Lett, 20(1): 93-98.
Kuribayashi S (1981) Studies on the effect of pesticides on the
reproduction of the silkworm, Bombyx Mori L. I. Effects of
chemicals administered during the larval stage on egg-laying and
hatching. J Toxicol Sci, 6: 169-176.
Kurtz DA & Studholme GA (1974) Recovery of trichlorfon (Dylox) and
carbaryl (Sevin) in songbirds following spraying of forest for Gipsy
moth. Bull Environ Contam Toxicol, 11(1): 78-84.
Kuzminskaya UA, Ivanitskij VA, & Shilina VA (1984) [The influence of
pesticides having anticholinesterase effect upon the content of
biogenic amines in brain.] Gig i Sanit, 5: 80-81 (in Russian).
LaFleur KS (1976a) Carbaryl desorption and movement in soil columns.
Soil Sci, 121(4): 212-216.
LaFleur KS (1976b) Movement of carbaryl through Congaree soil into
ground water. J Environ Qual, 5(1): 91-92.
Lahmy S, Salmon JM, & Viallet P (1988) Influence of long-term
treatment with 3 methylcholanthrene or carbaryl on mixed function
oxidase activity in 3T3 fibroblasts: studies by
microspectro-fluorimetry on single cells. Cell Biochem Funct, 6(4):
275-282.
Lamb FC, Farrow RP, Elkins ER, Kimball JR, & Cook RW (1968) Removal
of DDT, parathion and carbaryl from spinach by commercial and home
preparative methods. J Agric Food Chem, 16: 967-973.
Lamberton JG & Claeys RR (1970) Degradation of 1-naphthol in sea
water. J Agric Food Chem, 18(1): 92-96.
Larkin MJ & Day MJ (1985) The effect of pH on the selection of
carbaryl-degrading bacteria from garden soil. J Appl Bacteriol,
58(2): 175-186.
Larkin MJ & Day MJ (1986) The metabolism of carbaryl by three
bacterial isolates, Pseudomonas spp. (NCIB 12042 & 12043) and
Rodococcus sp. (NCIB 12038) from garden soil. J Appl Bacteriol,
60(3): 233-242.
Lawlor TE (1989) Mutagenicity test on carbaryl (technical) in the
Ames Salmonella/Microsome reverse mutation assay. Final report.
Kensington, Maryland, Hazleton Laboratories America, Inc. (Report
No. 10862-0-401) (Unpublished proprietary information submitted to
WHO by Rhône-Poulenc Agro, Lyon).
Leavitt JRC, Gold RE, Holcslaw T, & Tupy D (1982) Exposure of
professional pesticide applicators to carbaryl. Arch Environ Contam
Toxicol, 11(1): 57-62.
Lechner DMW & Abdel-Rahman MS (1984) A teratology study of carbaryl
and malathion mixtures in rat. J Toxicol Environ Health, 14:
267-278.
Lechner DMW & Abdel-Rahman MS (1985) Alterations in rat liver
microsomal enzymes following exposure to carbaryl and malathion in
combination. Arch Environ Contam Toxicol, 1: 451-457.
Lechner DMW & Abdel-Rahman MS (1986a) The effect of carbaryl and
malathion in combination on gamma glutamyl transpeptidase and
glutathione- S-alkyltransferase activity in vitro. Arch Environ
Contam Toxicol, 15(6): 647-651.
Lechner DMW & Abdel-Rahman MS (1986b) Kinetics of carbaryl and
malathion in combination in the rat. J Toxicol Environ Health,
18(2): 241-256.
Lee RM & Brindley WA (1974) Synergist ratios, EPN detoxication,
lipid and drug-induced changes in carbaryl toxicity in Megachile
pacifica. Environ Entomol, 3: 899-907.
Lee DY, Chen CT, & Houng KH (1990) Behaviour assessment of eight
major pesticides used in Taiwan in soils by mechanistic models. In:
Soils and fertilizers in Taiwan, pp 33-50.
Leeling NC & Casida JE (1966) Metabolites of carbaryl (1-naphthyl
methyl carbamate) in mammals and enzymatic systems for their
formation. J Agric Food Chem, 14(3): 281-290.
Leenheer JA & Ahlrichs (1971) A kinetic and equilibrium study of the
adsorption of carbaryl and parathion upon soil organic matter
surfaces. Soil Sci Soc Am Proc, 35: 700-705.
Lejczak B (1977) Effect of insecticides: chlorfenvinphos, carbaryl
and propoxur on aquatic organisms. Pol Arch Hydrobiol, 24(4):
583-591.
Leonard JA & Yearly RA (1990) Exposure of workers using hand-held
equipment during urban application of pesticides to trees and
ornamental shrubs. Am Ind Hyg Assoc J, 51: 605-609.
Lesca P, Fournier A, Lecointe P, & Cresteil T (1984) A dual assay
for the specific screening of 3 methylcholanthrene and phenobarbital
like chemical inducers of cytochrome P450 monooxygenases. Mutat Res,
129: 229-310.
Lijinsky W & Schmähl D (1978) Carcinogenicity of N-nitroso
derivatives of N-methylcarbamate insecticides in rats. Ecotoxicol
Environ Saf, 2: 413-419.
Lijinsky W & Taylor HW (1976) Carcinogenesis in Sprague-Dawley rats
on N-nitroso- N-alkylcarbamate esters. Cancer Lett, 1: 275-279.
Lijinsky W & Taylor HW (1977) Transplacental chronic toxicity test
of carbaryl with nitrite in rats. Food Cosmet Toxicol, 15: 229-232.
Lijinsky W & Winter C (1981) Skin tumours induced by painting
nitrosoalkylureas on mouse skin. J Cancer Res Clin Oncol, 102:
13-20.
Lillie RJ (1973) Studies on the reproductive performance and progeny
performance of caged white leghorns fed malathion and carbaryl.
Poult Sci, 52: 266-272.
Lin TH, North HH, & Menzer RE (1975) Metabolism of carbaryl
(1-naphthyl N-methylcarbamate) in human embryonic lung cell
cultures. J Agric Food Chem, 23(2): 253-256.
Lindsay CE (1961) Pesticide tests in the marine environment in the
State of Washington. Proc Natl Shellfish Assoc, 52: 87-97.
Lingaraja T & Venugopalan UK (1978) Pesticide induced physiological
and behavioural changes in an estuarine teleost Therapon jarbua
(Forsk). Fish Technol, 15: 115-119.
Little EE, Archeski RD, Flerov BA, & Kozlovskaya VI (1990)
Behavioral indicators of sublethal toxicity in rainbow trout. Arch
Environ Contam Toxicol, 19(3): 380-385.
Liu SY & Bollag JM (1971a) Metabolism of carbaryl by a soil fungus.
J Agric Food Chem, 19(3): 487-490.
Liu SY & Bollag JM (1971b) Carbaryl decomposition to 1-naphthyl
carbamate by Aspergillus terreus. Pestic Biochem Physiol, 1(3-4):
366-372.
Liu D, Thomson K, & Strachan WMJ (1981) Biodegradation of carbaryl
in simulated aquatic environment. Bull Environ Contam Toxicol,
27(3): 412-417.
Long KR (1971) Pesticides and occupational hazards on farms. Am J
Nurs, 71(4): 740-743.
Looney NE & Knight JN (1985) Effects of initial set and carbaryl
treatment in final fruit set on "Greensleeves" apple. Hortic Sci,
20(3): 400-401.
Looney NE & McKellar JE (1984) Thinning Spartan apples with carbaryl
and 1-napthaleneacetic acid: influence of spray volume and
combinations of chemicals. Can J Plant Sci, 64: 161-166.
Loosanoff VL (1961) Recent advances in the control of shellfish
predators and competitors. Proc Gulf Caribb Fish Inst, 13: 113-128.
Loosanoff VL, MacKenzie CL Jr, & Shearer LW (1960a) Use of chemical
barriers to protect shellfish beds from predators. In: Fisheries:
Fish farming. Volume 3 - Fisheries management. Olympia, Washington,
Washington State Department of Fisheries, pp 86-90.
Loosanoff VL, MacKenzie CL Jr, & Shearer LW (1960b) Use of chemical
to control shellfish predators. Science, 131: 1522-1523.
Lowe JI (1967) Effect of prolonged exposure to Sevin on an estuarine
fish, Leiostomus xanthurus Lacépède. Bull Environ Contam Toxicol,
2(3): 147-155.
Lox CD (1984) The effects of acute carbaryl exposure on clotting
factor activity in rat. Ecotoxicol Environ Saf, 8: 280-283.
Luca D & Balan M (1987) Sperm abnormality assay in the evaluation of
the genotoxic potential of carbaryl in rats. Morphol Embryol
(Bucur), 33(1): 19-22.
Lucier GW, McDaniel OS, Williams C, & Klein R (1972) Effects of
chlordane and methylmercury on the metabolism of carbaryl and
carbofuran in rats. Pestic Biochem Physiol, 2: 244-255.
Lukaneva AM & Rodionov GA (1973) [Some experimental data on the
effect of DDT and Sevin upon the development of cholesterin
atherosclerosis.] Gig i Sanit, 9: 41-45 (in Russian).
Luke MA, Masumoto HT, Cairns T, & Hundley HK (1988) Levels and
incidences of pesticide residues in various foods and animal feeds
analyzed by the Luke multiresidue methodology for fiscal years
1982-1986. J Assoc Off Anal Chem, 71(2): 415-420.
Lykken L & Casida JE (1969) Metabolism of organic insecticide
chemicals. Can Med Assoc J, 100: 145-154.
Ma TH, Harris MM, Anderson VA, Ahmed I, Mohammed K, Bare JL, & Lin G
(1984) Tradescantia-Micronucleus (Trad-MCN) tests on 140
health-related agents. Mutat Res, 138: 157-167.
McCann J, Choi E, Yamasaki E, & Ames BM (1975) Detection of
carcinogen as mutagens in the Salmonella/microsome test: Assay of
300 chemicals. Proc Natl Acad Sci (USA), 72(12): 5135-5139.
MacCollom GB, Currier WW, & Baumann GL (1985) Pesticide drift and
quantification from air and ground applications to a single orchard
site. In: Honeycutt RC, Zweg D, & Ragsdale N ed. Dermal exposure
related to pesticide use. Washington, DC, American Chemical Society,
pp 190-198 (ACS Symposium Series No. 273).
MacCollom GB, Currier WW, & Baumann GL (1986) Drift comparisons
between aerial and ground orchard application. J Econ Entomol,
79(2): 459-464.
Macek KJ & McAllister WA (1970) Insecticide susceptibility of some
common fish family representatives. Trans Am Fish Soc, 99(1): 20-27.
MacLeod J (1982) Report on UCC sperm morphology readings submitted
to Union Carbide Agricultural Products Co., Inc. (Unpublished
proprietary information submitted to WHO by Rhône-Poulenc Agro,
Lyon).
MacPherson SE, Scott RC, & Williams FM (1991) Fate of carbaryl in
rat skin. Arch Toxicol, 65: 594-598.
Maibach HI, Feldmann RJ, Milby TH, & Serat WF (1971) Regional
variations in percutaneous penetration in man - Pesticides. Arch
Environ Health, 23: 208-211.
Makovskaya EL, Rapoport MB, & Pintshuk VG (1965) [On the possibility
of carcinogenic effect of some insecticides belonging to the
carbamate group.] Vopr Exp Onkol, 1: 67-74 (in Russian).
Maliwal BP & Guthrie FE (1981) Interaction of insecticides with
human plasma lipoproteins. Chem-Biol Interact, 35(2): 177-188.
Mann GS & Chopra SL (1969) Residues of carbaryl on crops. Pestic
Monit J, 2(4): 163-165.
Manske DD & Corneliussen PE (1974) Pesticides in food and feed.
Pesticide residues in total diet samples (VII). Pestic Monit J,
8(2): 110-124.
Manske DD & Johnson RD (1975) Residues in food and feed. Pesticide
residues in total diet samples (VIII). Pestic Monit J, 9(2): 94-105.
Manske DD & Johnson RD (1977) Residues in food and feed. Pesticide
and other chemical residues in total diet samples (X). Pestic Monit
J, 10: 134-148.
Marian MP, Arul V, & Pandian TJ (1983) Acute and chronic effect of
carbaryl on survival, growth, and metamorphosis in the bullfrog
(Rana tigrina). Arch Environ Contam Toxicol, 12(3): 271-275.
Marshall TC, Dorough HW, & Swin HE (1976) Screening of pesticides
for mutagenic potential using Salmonella typhimurium mutants. J
Agric Food Chem, 24(3): 500-563.
Marshall TC, Dorough HW, & Swin HE (1979) Biliary excretion of
carbamate insecticides in the rat. Pestic Biochem Physiol, 11:
56-63.
Mason Y, Choshen E, & Rav-Acha C (1990) Carbamate insecticides:
Removal from water by chlorination and ozonation. Water Res, 24(1):
11-22.
Mastro VC & Cameron EA (1976) Control of Archips semiferanus on
Quercus, spp with two chemical insecticides. J Econ Entomol,
69(4): 554-556.
Mathur A & Bhatnagar P (1991) A teratogenic study of carbaryl in
Swiss albino mice. Food Chem Toxicol, 29(9): 629-632.
Matsumura F & Sakai K (1968) Degradation of insecticides by
esterases of the American cockroach. J Econ Entomol, 61(3): 598-605.
Matsumura F & Ward CT (1966) Degradation of insecticides by the
human and the rat liver. Arch Environ Health, 13: 257-261.
Mayer FL Jr (1987) Acute toxicity handbook of chemicals to estuarine
organisms. Gulf Breeze, Florida, US Environmental Protection Agency,
Gulf Breeze Laboratory (EPA 600/8-87/017).
Mayer FL & Ellersieck MR (1986) Manual of acute toxicity:
Interpretation and data base for 410 chemicals and 66 species of
freshwater animals. Washington, DC, US Department of the Interior,
Fish and Wildlife Service (Resource Publication 160).
Mehendale HM & Dorough HW (1971) Glucuronidation mechanisms in the
rat and their significance in the metabolism of insecticides. Pestic
Biochem Physiol, 1: 307-318.
Mellon Institute (1958) Acute toxicity of carbaryl. Pittsburgh,
Pennsylvania, Mellon Institute (Unpublished proprietary information
submitted to WHO by Rhône-Poulenc Agro, Lyon).
Mellon Institute (1963) Results of the eighty weeks of inclusion of
Sevin in the diet of mice. Pittsburgh, Pennsylvania, Mellon
Institute, pp 1-12 (Special report No. 26-53) (Unpublished
proprietary information submitted to WHO by Rhône-Poulenc Agro,
Lyon).
Menzie CM (1969) Metabolism of pesticides. Washington, DC, US
Department of the Interior, Bureau of Sport Fisheries and Wildlife,
pp 72-76 (Special Scientific Report, Wildlife No. 127).
Miles CJ, Trehy ML, & Yost RA (1988a) Degradation of
N-methylcarbamate and carbamoyl oximes pesticides in chlorinated
water. Bull Environ Contam Toxicol, 41(6): 838-843.
Miles CJ, Trehy ML, & Yost RA (1988b) Fate of pesticides in
chlorinated water. Paper presented at the 195th Meeting of the
American Chemical Society. Washington, DC, American Chemical Society
(Abstract ENVR 139).
Miller NE (1990) Metabolism of C14-carbaryl under aerobic soil
conditions. Research Triangle Park, North Carolina, Rhône-Poulenc Ag
Company (Unpublished proprietary information submitted to WHO by
Rhône-Poulenc Agro, Lyon).
Miller A III, Henderson MC, & Buhler DR (1979) Covalent binding of
carbaryl (1-naphthyl- N-methylcarbamate) to rat liver microsome in
vitro. Chem-Biol Interact, 24: 1-17.
Molozhanova EG (1968) [Circulation of Sevin in compartments of the
environment.] Gig Primen Toxicol Pestic Klin Otrav, 1968: 355-362
(in Russian).
Molozhanova EG (1970) [Factual content of Sevin in compartments of
the environment and quantities of the formulation apt at getting
into human organism.] Gig Primen Toxicol Pestic Klin Otrav, 1970:
170-174 (in Russian).
Molozhanova EG & Kanevskij AA (1971) [Application of mathematical
analysis to modelling the behaviour of Sevin in soil.] Gig Primen
Toxicol Pestic Klin Otrav, 1971: 77-83 (in Russian).
Moreynis JA & Estrin IM (1965) [Sevin action on animals infected
with the virus of influenza.] Gig Primen Toxicol Pestic Klin Otrav,
1965: 435-442 (in Russian).
Moriya M, Ohta T, Watanabe K, Miyazawa T, Kato K, & Shirasu Y (1983)
Further mutagenicity studies on pesticides in bacterial reversion
assay systems. Mutat Res, 116: 185-216.
Moser VC, McCormick JP, Creason JP, & MacPhail RC (1988) Comparison
of chlordimeform and carbaryl using a functional observational
battery. Fundam Appl Toxicol, 11(2): 189-206.
Mount ME & Oehme FW (1981a) Carbaryl. A literature review. Residue
Rev, 80: 1-64.
Mount ME & Oehme FW (1981b) Diagnostic criteria for carbaryl
poisoning in sheep. Arch Environ Contam Toxicol, 10(4): 483-495.
Mount ME, Dayton AD & Oehme FW (1981) Carbaryl residues in tissues
in cholinesterase activities in brain and blood of rats receiving
carbaryl. Toxicol Appl Pharmacol, 58(2): 282-296.
Mumma RO, Khalifa S, & Hamilton RH (1971) Spectroscopic
identification of metabolites of carbaryl in plants. J Agric Food
Chem, 19(3): 445-451.
Murli H (1989) Mutagenicity test on carbaryl technical in an in
vitro cytogenetic assay measuring chromosomal aberration
frequencies in Chinese hamster ovary (CHO) cells. Kensington,
Maryland, Hazleton Laboratories America, Inc. (Report No.
10862-0-437) (Unpublished proprietary information submitted to WHO
by Rhône-Poulenc Agro, Lyon).
Murray HE & Guthrie RK (1980) Effects of carbaryl, diazinon and
malathion on native aquatic populations of microorganisms. Bull
Environ Contam Toxicol, 24: 535-542.
Murray FJ, Staples RE, & Schwets BA (1979) Teratogenic potential of
carbaryl given to rabbits and mice by gavage or by dietary
inclusion. Toxicol Appl Pharmacol, 51: 81-89.
Murthy NBK & Raghu K (1988) Soil bound residues of carbaryl and
1-naphthol: release and mineralization in soil, and uptake by
plants. J Environ Sci Health, B23(6): 575-585.
Murthy NBK & Raghu K (1989) Metabolism of 14C-carbaryl and
14C-naphthol in moist and flooded soils. J Environ Sci Health,
B24(5): 479-491.
Murthy NBK & Raghu K (1991) Fate of 14C-carbaryl in soils as a
function of pH. Bull Environ Contam Toxicol, 46(3): 374-379.
Myers WF (1977) Carbaryl insecticide. Estimation of carbaryl
exposure to humans. South Charleston, West Virginia, Union Carbide
Corporation (Project Report No. 111A12) (Unpublished proprietary
information submitted to WHO by Rhône-Poulenc Agro, Lyon).
Myers RC & Christopher SM (1987) Sevin technical dermal
sensitization study in the guinea-pig (Project No. ID-49-146).
Export, Pennsylvania, Bushy Run Research Center (Proprietary report
submitted to WHO by Rhône-Poulenc Agro, Lyon).
Myers RC, Weil CS, & Carpenter CP (1975) Sevin liquid, 44% carbaryl,
range-finding toxicity studies. Pittsburgh, Pennsylvania,
Carnegie-Mellon Research Institute (Unpublished proprietary
information submitted to WHO by Rhône-Poulenc Agro, Lyon).
Myhr S (1973) A screen for pesticide toxicity to protein and RNA
synthesis in HeLa cells. J Agric Food Chem, 21(3): 362-367.
Nagy Z, Mile I, & Antony F (1975) Mutagenic effect of pesticides on
Escherichia coli WP2 try. Acta Microbiol Acad Sci Hung, 22:
309-314.
Neskovic N, Terzic M, & Vitorovic S (1978) [Acute toxicity of
carbaryl and propoxur in mice previously treated with phenobarbital
and SKF-525-A.] Arh Hig Rada, 29: 251-256 (in Croatian).
Neuhauser EF, Loehr RC, Malecki MR, Milligan DL, & Durkin PR (1985)
The toxicity of selected organic chemicals to the earthworm Eisenia
fetida. J Environ Qual, 14(3): 383-388.
Nimmo DR, Hamaker TL, Matthews E, & Moore JC (1981) An overview of
the acute and chronic effects of first and second generation
pesticides on an estuarine Mysid. In: Vernberg FJ, Calabrese A,
Thurberg FP, & Vernberg WB ed. Biological monitoring of marine
pollutants. New York, Academic Press, Inc., pp 3-19.
NIOSH (1976) Criteria for a recommended standard ... Occupational
exposure to carbaryl. Cincinnati, Ohio, National Institute for
Occupational Safety and Health, 191 pp (DHEW (NIOSH) Publication No.
77-107).
Norris FA 1991) A terrestrial field soil dissipation study with
carbaryl (Study No. EC 89-080, File No. 40862). Research Triangle
Park, North Carolina, Rhône-Poulenc Ag Company (Unpublished
proprietary information submitted to WHO by Rhône-Poulenc Agro,
Lyon).
Nye DE & Dorough HW (1976) Fate of insecticides administered
endotracheally to rats. Bull Environ Contam Toxicol, 15(3): 291-296.
Odeyemi O (1982) Relative stability of carbaryl in tropical
ecosystems. Environ Pollut, A29(3): 207-213.
Okumura D, Melnicoe R, Jackson T, Drefs C, Maddy K, & Wells J (1991)
Pesticide residues in food crops analyzed by the California
Department of Food and Agriculture in 1989. Rev Environ Contam
Toxicol, 118: 87-150.
Olefir AI (1977) [Effects of pesticides on immunogenesis.] Vrach
Delo, 9: 121-123 (in Russian).
Olefir AI & Vinogradova VKh (1968) [A study of the embryotropic
effect of the pesticides Sevin and Zyram.] Vrach Delo, 11: 103-106
(in Russian).
Omer AA, Youssef MK, Abou-Youssef AY, & Khalil AM (1986)
Mutagenicity of insecticide Sevin and its derivative nitrosocarbaryl
on D. melanogaster. Alex J Agric Res, 31(3): 239-248.
Omkar & Shukla GS (1985) Toxicity of insecticides to Macrobrachium
lamarrei (H. Milne Edwards) (Decapoda, Palaemonidae). Crustaceana,
48: 1-5.
Önfelt A (1987) Spindle disturbances in mammalian cells. III.
Toxicity, c-mitosis and aneuploidy with 22 different compounds.
Specific and unspecific mechanisms. Mutat Res, 182: 135-154.
Önfelt A & Klasterska I (1983) Spindle disturbances in mammalian
cells. II. Induction of viable aneuploid/polyploid cells and
multiple chromatid exchanges after treatment of V-70 Chinese hamster
cells with carbaryl. Modifying effects of glutathion and S9. Mutat
Res, 119: 319-330.
Önfelt A & Klasterska I (1984) Sister chromatid exchanges and
thioguanine resistance in V79 Chinese hamster cells after treatment
with the aneuploidy inducing agent carbaryl +/ S9 mix. Mutat Res,
125: 269-274.
Oonnithan ES & Casida JE (1968) Oxidation of methyl- and
dimethylcarbamate insecticide chemicals by microsomal enzymes and
anticholinesterase activity of the metabolites. J Agric Food Chem,
18(1): 28-44.
Orlova NV & Zhalbe EP (1968) [Experimental material contributive to
the problem of maximal permissible amounts of Sevin in food
products.] Vopr Pitan, 27(6): 49-55 (in Russian).
Orzel RA & Weiss LR (1966) The effect of carbaryl
(1-naphthyl- N-methylcarbamate) on blood glucose, and liver and
muscle glycogen in fasted and nonfasted rats. Biochem Pharmacol, 15:
995-998.
Osborne BG, Barrett GM, Laal-Khoshab A, & Willis KH (1989) The
occurrence of pesticide residues in UK home-grown and imported
wheat. Pestic Sci, 27: 103-109.
Osman MA & Belal MH (1980) Persistence of carbaryl in canal water. J
Environ Sci Health, B15(3): 307-311.
Osman DH & Brindley WA (1981) Estimating monoxygenase detoxification
in field populations: toxicity and distribution of carbaryl in three
species of Labops grassbugs. Environ Entomol, 10(5): 676-680.
Osterloh J, Letz G, Pond S, & Becker Ch (1983) An assessment of the
potential testicular toxicity of 10 pesticides using the mouse sperm
morphology assay. Mutat Res, 116(3-4): 407-415.
Ott FT, McCullogh R, & Stanley JG (1981) Effects of forest spraying
on the ecology of brook trout. Report to the Maine Department of
Conservation, Forest Service, Augusta.
Owen BB Jr (1965) Aquatic insect populations reduced by aerial
spraying of insecticide Sevin. Philadelphia, Pennsylvania,
Pennsylvania Academy of Science, pp 63-69.
Page M, Haverty MJ, & Richmond ChE (1985) Residual activity of
carbaryl protected Lodgepole Pine against Mountain Pine Beetle,
Dillon, Colorado, 1982 and 1983. Berkeley, California, US Department
of Agriculture, Forest Service, Pacific Southwest Forest and Range
Experiment Station (Research Note PSW-375).
Panciera RJ (1967) Determination of teratogenic properties of orally
administrated 1-naphthyl- N-methylcarbamate (Sevin) in sheep.
Research Triangle Park, North Carolina, Union Carbide Agricultural
Products Co., Inc. (Unpublished proprietary information submitted to
WHO by Rhône-Poulenc Agro, Lyon).
Parafita MA & Fernandez-Otero P (1984) The interaction of carbaryl
with the metabolism of isolated hepatocytes. II. Effect on
glycogenesis. Gen Pharmacol, 15(4): 333-337.
Parafita MA, Fernandez-Otero P, & Aldegunde MA (1984) The
interaction of carbaryl with the metabolism of isolated hepatocytes.
I. Effect on respiration and glycolysis. Gen Pharmacol, 15(4):
327-331.
Paris DF & Lewis DL (1973) Chemical and microbiological degradation
of ten selected pesticides in aquatic system. Residue Rev, 45:
95-124.
Paris D, Lewis D, Barnett J, & Baugham G (1975) Microbial
degradation and accumulation of pesticides in aquatic systems.
Corvallis, Oregon, US Environmental Protection Agency, Office of
Research and Development, National Environmental Research Center, pp
1-45 (EPA-660/3-75-007).
Patocka JJ & Bajgar J (1971) Affinity of human brain
acetylcholinesterase to some organophosphates and carbamates in
vitro. J Neurochem, 18: 2545-2546.
Paulson GD & Feil VJ (1969) The fate of a single oral dose of
carbaryl (1-naphthyl- N-methyl-carbamate) in the chicken. Poult
Sci, 48: 1593-1597.
Paulson GD, Zaylskie RG, Zehr MV, Portnoy CE, & Feil VJ (1970)
Metabolites of carbaryl (1-naphthyl N-methyl-carbamate) in chicken
urine. J Agric Food Chem, 18: 110-115.
Pavlova II, Petrovskaya OG, & Ivanova SI (1968) [Biochemical and
histochemical studies on the oxidative-reductive enzymes after an
exposure to Sevin.] Gig Primen Toxicol Pestic Klin Otrav, 1968:
625-630 (in Russian).
Payne JA, Dutcher JD, & Ellis HC (1985) Low pressure soil
application of carbaryl for control of the pecan weevil, Curculio
carvae. J Agric Entomol, 2(1): 1-5.
Pekas JC (1972) Intestinal hydrolysis, metabolism and transport of a
pesticidal carbamate in pH G.5 medium. Toxicol Appl Pharmacol, 23:
62-70.
Pekas JC & Paulson GD (1970) Intestinal hydrolysis and conjugation
of a pesticidal carbamate in vitro. Science, 170: 77-78.
Perelygin VM, Shpirt MB, Aripov OA, & Ershova VI (1971) [Effect of
some pesticides on immunological response reactivity.] Gig i Sanit,
36(12): 29-33 (in Russian).
Petrovski VV (1970) [The level of content and duration of excretion
of Sevin in the milk of cows treated against ticks.] Gig Primen
Toxicol Pestic Klin Otrav, 1970: 193-196 (in Russian).
Pieper GR (1979) Residues analysis of carbaryl on forest foliage and
in stream water using HPLC. Bull Environ Contam Toxicol, 22(1-2):
167-171.
Pieper GR & Roberts RB (1978) Residue analysis of carbaryl on forest
foliage and in creek water. US Department of Agriculture, Forest
Service, Northern Region, pp 1-9 (Report No. 78-5).
Pipy B, Gaillard D, & Derache R (1979) Effect of blockage of the
reticulo-endothelial system on the carbaryl toxicity in the rat.
Toxicology, 12: 135-142.
Pipy B, Gaillard D, Beraud M, & Derache R (1980) Phagocytic activity
of the rat reticuloendothelial system and the pharmakokinetics of an
anticholinesterasic insecticide: carbaryl. Xenobiotica, 10(10):
785-793.
Pipy B, Gaillard D, & Derache R (1981) Phagocytic activity of the
reticuloendothelial system in the rat and rates of in vivo
excretion of metabolites of carbaryl and in vitro microsomal
metabolism. Biochem Pharmacol, 30(6): 669-672.
Pipy B, Gaillard D, Beraud M, & Derache R (1982) Enzymatic
activities of liver serine esterases during the reticuloendothelial
system phagocytosis blockade by carbaryl, an anticholinesterasic
insecticide. Toxicol Appl Pharmacol, 62: 11-18.
Pipy B, de Maroussem D, Beraud M, & Derache R (1983) Evaluation of
cellular and humoral mechanisms of carbaryl-induced
reticuloendothelial phagocytic depression. J Reticuloendothel Soc,
34(5): 395-412.
Podolak M & Warchocki B (1980) [Effect of toxobidin on the kinetics
of reaction of ChE inhibition by chlorfenvinfos and carbaryl.] Rocz
Panstw Zakl Hyg, 31(2): 125-131 (in Polish).
Podolak-Majczak M & Tyburczyk W (1984) Effect of joint action of
sodium nitrite and carbaryl on rats organism. Bromatol Chem
Toksykol, XVII(3): 211-214 (in Polish with English summary).
Podolak-Majczak M & Tyburczyk W (1986) [Effect of joint action of
sodium nitrite and carbaryl on rats organism. Part IV. Hepatotoxic
action.] Bromatol Chem Toksykol, XIX(3): 164-166 (in Polish with
English summary).
Post G & Schroeder TR (1971) The toxicity of four insecticides to
four salmonid species. Bull Environ Contam Toxicol, 6(2): 144-155.
Preussmann RG, Eisenbrand G, & Schmahl D (1976) Carcinogenicity
testing of low doses of nitrosopyrrolidine and of
nitrosobenzo-thiazuron and nitrosocarbaryl in rats. In: Walker EA,
Bogovski P, & Griciute L ed. Environmental N-nitroso compounds
analysis and formation. Proceedings of a Working Conference held at
the Polytechnical Institute, Tallinn, Estonian SSR, 1-3 October
1975. Lyon, International Agency for Research on Cancer, pp 429-433
(IARC Scientific Publications No. 14).
Prima NG, Prima AP, Sem'yan AI, Solov'eva VG, & Bondarenko VF (1976)
[Model studies of the rate of self-purification of river water from
Sevin and 1-naphthol residues.] Probl Okhr Vod, 7: 8-13 (in
Russian).
Probst GS, McMahon RE, Hill LE, Thompson CZ, Epp JK, & Neal SB
(1981) Chemically induced unscheduled DNA synthesis in primary rat
hepatocyte cultures: A comparison with bacterial mutagenicity using
218 compounds. Environ Mutagen, 3(1): 11-32.
Puech AA (undated) A study of the potential for human dermal and
inhalation exposure of Sevin, carbaryl insecticide during normal
garden use. Research Triangle Park, North Carolina, Union Carbide
Corporation (Unpublished proprietary information submitted to WHO by
Rhône-Poulenc Agro, Lyon).
Puyear RL & Paulson GD (1972) Effect of carbaryl (1-naphthyl-
N-methylcarbamate) on phenobarbital-induced sleeping time and some
liver microsomal enzymes in white leghorn cockerels. Toxicol Appl
Pharmacol, 22: 621-627.
Quarles JM & Tennant RW (1975) Effects of nitrosocarbaryl on
Balb/3T3 cells. Cancer Res, 35: 2637-2645.
Rajagopal BS & Sethunathan N (1984) Influence of nitrogen
fertilizers on the persistence of carbaryl and carbofuran in flooded
soils. Pestic Sci, 15: 591-599.
Rajagopal BS, Chendrayan K, Reddy B, Rajasekhar-Reddy B, &
Sethunathan N (1983) Persistence of carbaryl in flooded soils and
its degradation by soil enrichment cultures. Plant Soil, 73(1):
35-45.
Rajagopal BS, Rao VR, Nagendrappa G, & Sethunathan N (1984)
Metabolism of carbaryl and carbofuran by soil-enrichment and
bacterial cultures. Can J Microbiol, 30(12): 1457-1465.
Rajagopal BS, Panda S, & Sethunathan N (1986) Accelerated
degradation of carbaryl and carbofuran in a flooded soil pretreated
with hydrolysis products, 1-naphthol and carbofuran phenol. Bull
Environ Contam Toxicol, 36(6): 827-832.
Rajukkannu K, Basha AA, Habeebullah B, Duraisamy P, &
Balasubramanian M (1985) Degradation and persistence of DDT, BHC,
carbaryl and malathion in soils. Indian J Environ Health, 27(3):
237-243.
Rao KRSS, Rao KSP, Sahib IKA, & Rao KVR (1985a) Combined action of
carbaryl and phenthoate on a freshwater fish ( Carina punctatus
bloch). Ecotoxicol Environ Saf, 10(2): 209-217.
Rao KS, Rao KR, Sahib IK, & Rao KV (1985b) Combined action of
carbaryl and phenthoate on a tissue lipid derivatives of murrel,
Channa punctatus (Bloch). Ecotoxicol Environ Saf, 9(1): 107-111.
Ray SK & Poddar MK (1985a) Central cholinergic dopaminergic
interaction in carbaryl induced tremor. Eur J Pharmacol, 119(3):
251-253.
Ray SK & Poddar MK (1985b) Effect of pentylenetetrazol on carbaryl
induced changes in striatal catecholamines. Biochem Pharmacol,
34(4): 553-557.
Ray SK & Poddar MK (1990) Interaction of central serotonin and
dopamine in the regulation of carbaryl induced tremor. Eur J
Pharmacol, 181(3): 159-166.
Rawash IA, Gaaboub IA, El-Gayar FM, & El-Shazli AY (1975) Standard
curves for Nuvacron, Malathion, Sevin, DDT, and Kelthane tested
against the mosquito Culex pipiens L. and the microcrustacean
Daphnia magna Straus. Toxicology, 4: 133-144.
Regan JD, Seltow RB, Francis AA, & Lijinsky W (1976)
Nitrosocarbaryl: Its effect on human DNA. Mutat Res, 38: 239-302.
Reiner E & Aldridge WN (1967) Effect of pH on inhibition and
spontaneous reactivation of acethylcholinesterase treated with
esters of phosphorus acids and of carbamic acids. Biochem J, 105:
171-179.
Rickard RW & Dorough HW (1984) in vivo formation of
nitrosocarbamates in the stomach of rats and guinea-pigs. J Toxicol
Environ Health, 14(2-3): 279-290.
Rickard RW, Walter-Echols G, Dorough HW, & Lawrence LJ (1982)
Importance of pH in assessing the potential for nitrosocarbamate
formation in the stomach. Pestic Biochem Physiol, 18(3): 325-333.
Robel J, Felthausen RW, & Dayton D (1982) Effects of carbamates on
bobwhite food intake, body weight and locomotor activity. Arch
Environ Contam Toxicol, 11(5): 611-615.
Robens JF (1969) Teratologic studies of carbaryl, diazinon, norea,
disulfiram and thiram in small laboratory animals. Toxicol Appl
Pharmacol, 15: 152-163.
Roberts D (1975) The effect of pesticides on byssus formation in the
common mussel, Mytilus edulis. Environ Pollut, 8(4): 241-254.
Roberts BL & Dorough HW (1984) Relative toxicity of chemicals to the
earthworm Eisenia foetida. Environ Toxicol Chem, 3: 67-78.
Rocchi P, Perocco P, Alberhin W, Fini A, & Prodi G (1980) Effects of
pesticides on scheduled and unscheduled DNA synthesis of rat
thymocytes and human lymphocytes. Arch Toxicol, 45(2): 101-108.
Rodgers KE, Leung N, Imamura T, & Devens BH (1986) Rapid in vitro
screening assay for immunotoxic effects of organophosphorus and
carbamate insecticides in the generation of cytotoxic T-lymphocyte
responses. Pestic Biochem Physiol, 26(3): 292-301.
Rodriguez LD & Dorough HW (1977) Degradation of carbaryl by soil
microorganisms. Arch Environ Contam Toxicol, 6(1): 47-56.
Romine RR & Bussian RA (1971) Sevin Insecticide: The degradation of
carbaryl after surface application to a farm pond (Project No
111A13, File No. 15265). South Charleston, West Virginia, Union
Carbide Corporation (Unpublished proprietary information submitted
to WHO by Rhône-Poulenc Agro, Lyon).
Roszkowski J, Zadura J, & Skwarek P (1976) The effect of carbaryl on
immune response in chickens. Bull Vet Inst Pulawy, 20(3-4): 85-88.
Ruppert P, Cook L, Dean F, & Reiter W (1983) Acute behavioural
toxicity of carbaryl and propoxur in adult rats. Pharmacol Biochem
Behav, 18(4): 579-584.
Ryan AJ (1971) The metabolism of pesticidal carbamates. CRC Crit Rev
Toxicol, 1: 35-54.
Rybakova MN (1966) [Toxic effect of Sevin on animals.] Gig i Sanit,
31(9): 42-47 (in Russian with English summary).
Rybakova MN (1967) [Comparative toxic effect of Sevin and DDT used
in the treatment of food crops.] Vopr Pitan, 26: 9-15 (in Russian
with English summary).
Saini ML & Dorough HW (1978) Metabolism of carbaryl-naphthyl-C14
in a lactating cow, when administered orally. In: Edwards CA,
Veeresh GK, & Krueger HR ed. Proceedings of the Symposium on
Pesticide Residues in the Environment in India, 7-10 November 1978.
Hebbal, Bangalore, University of Agricultural Sciences, pp 74-87
(UAS Technical Series No. 32).
Sakai K & Matsumura F (1971) Degradation of certain organophosphate
and carbamate insecticides by human brain esterases. Toxicol Appl
Pharmacol, 19: 660-666.
Samanidou V, Fytianos K, Pfister G, & Bahadir M (1988) Photochemical
decomposition of carbamate pesticides in natural waters of Northern
Greece. Sci Total Environ, 76(1): 85-92.
Sanders HO & Cope DB (1966) Toxicity of several pesticides in two
species of Cladocerans. Trans Am Fish Soc, 95(2): 165-169.
Sanders HO, Finley MT, & Hunn JB (1983) Acute toxicity of six forest
insecticides to three aquatic invertebrates and four fishes.
Washington, DC, US Department of the Interior, Fish and Wildlife
Service, 5 pp (Technical Paper 110).
Sanderson DM (1961) Treatment of poisoning by anticholinesterase
insecticides in the rat. J Pharm Pharmacol, 13: 435-442.
Santolucito JA & Morrison G (1971) EEC of Rhesus monkeys following
prolonged low level feeding of pesticides. Toxicol Appl Pharmacol,
19: 147-154.
Santolucito JA & Whitcomb E (1971) Mechanical response of skeletal
muscle following oral administration of pesticides. Toxicol Appl
Pharmacol, 20: 66-72.
Schafer EA (1972) The acute oral toxicity of 369 pesticidal,
pharmaceutical and other chemicals to wild birds. Toxicol Appl
Pharmacol, 21: 315-330.
Schomburg CJ, Glottfeldy DE, & Seiber JN (1991) Pesticide occurrence
and distribution in fog collected near Monterey, California. Environ
Sci Technol, 25: 155-160.
Schulze TL, Hauge PN, Mitchell WJ, Remley WJ, Shahied SI, & Marshall
FJ (1979) New Jersey Epidemiologic Studies Program-Pesticides -
Assessment of human exposure to carbaryl during aerial application
for Gypsy moth control in New Jersey. New Jersey State Department of
Health.
Seiler JP (1977) Nitrosation in vivo and in vitro by sodium
nitrate and mutagenicity of nitrogenous pesticides. Mutat Res, 48:
225-236.
Shabanov M, Toshkov A, Georgiev D, & Ibrishimov N (1983) [Effect of
carbaryl on experimental Erysipelothrix rhusiopathiae infection in
rats.] Vet Med Nauk, 20(5-6): 9-15 (in Russian).
Shafik MT, Sullivan HC, & Enos NF (1971) A method for the
determination of 1-naphthol in urine. Bull Environ Contam Toxicol,
6(1): 34-39.
Shah PV & Guthrie FE (1983) Percutaneous penetration of three
insecticides in rats: a comparison of two methods for in vivo
determination. J Invest Dermatol, 80(4): 291-293.
Shah PV, Monroe RV, & Guthrie FE (1983) Comparative penetration of
insecticides in target and nontarget species. Drug Chem Toxicol,
6(2): 155-179.
Shah PV, Fisher HL, Sumler MR, Monroe RJ, Chernoff N, & Hall LL
(1987) Comparison of the penetration of 14 pesticides through the
skin of young and adult rats. J Toxicol Environ Health, 21(3):
353-366.
Sharma VK & Kaur S (1990) Contact sensitization by pesticides in
farmers. Contact Dermatitis, 23(2): 77-80.
Sharom MS, Miles JRW, Harris CR, & McEwen FL (1980) Behaviour of 12
insecticides in soil and aqueous suspensions of soil and sediment.
Water Res, 14(8): 1095-1100.
Shea TB (1985) Carbaryl inhibition of interferon synthesis in
cultured goldfish cells. Bull Environ Contam Toxicol, 34: 109-113.
Shea TB & Berry ES (1983) Toxicity of carbaryl and 1-naphthol to
goldfish (Carassius auratus) and Killifish (Fundulus
heteroclitus). Bull Environ Contam Toxicol, 31: 526-529.
Shea TB & Berry ES (1984) Suppression of interferon synthesis by the
pesticide carbaryl as a mechanism for enhancement of goldfish
virus-2 replication. Appl Environ Microbiol, 47(2): 250-252.
Shehata T, Richardson E, & Cotton E (1984) Assessment of human
population exposure to carbaryl from the 1982 Main Spruce budworm
spray project. J Environ Health, 46(6): 293-297.
Shilova SA, Denisova AV, Sedykh EL, & Efron KM (1973) [After effects
of insecticide treatment in the subartics.] Zool Zh, 52(7):
1008-1012 (in Russian).
Shimkin MB, Wieder R, McDonough M, Fishbein L, & Swern D (1969) Lung
tumour response in strain A mice as a quantitative bioassay of
carcinogenic activity of some carbamates and aziridines. Cancer Res,
29: 2184-2190.
Shpirt MB (1973) [Toxicological assessment of DDT, HCHC, TMTD, Sevin
and Zineb acting on human cell cultures.] Gig Tr Prof Zabol, 14:
32-35 (in Russian).
Shtenberg AI & Hovaeva LA (1970) [The effect of Sevin on the state
of thyroid gland of rats having received artificial full-value
ration with different content of iodine.] Gig i Sanit, 8: 119-122
(in Russian).
Shtenberg AI & Ozhovan MV (1971) [The effect of small doses of Sevin
upon the reproduction function of animals in several generations.]
Vopr Pitan, 1: 42-47 (in Russian).
Shtenberg AI & Rybakova MN (1968) Effect of carbaryl on the
neuroendocrine system in rats. Food Cosmet Toxicol, 6: 461-467.
Shtenberg AI, Orlova NV, Pozdnyakov AL, Tropova GP, Zhalbe JP,
Khovayeva LA, & Akincheva MJ (1970) [Functional and morpho-logical
parallels associated with the state of the sex glands of animals
under the influence of Sevin.] In: [Problems of the hygiene and
toxicology of pesticides.] Moscow, Meditsina, pp 124-129 (in
Russian).
Shtenberg AI, Orlova NV, & Torchinskij AM (1973) [On the effect of
pesticides of different chemical structure on gonads and
embryogenesis in experimental animals.] Gig i Sanit, 8: 16-20 (in
Russian).
Shukla GS, Omkar & Upadhyay VB (1982) Acute toxicity of few
pesticides to an aquatic insect, Ranatra elongata (Fabr.) J Adv
Zool, 3(2): 148-150.
Siboulet R, Grinfeld S, Deparis P, & Jaylet A (1984) Micronuclei in
red blood cells of the newt Pleurodeles waltl Michah: Induction
with X rays and chemicals. Mutat Res, 125(2): 275-281.
Siebert D & Eisenbrand G (1974) Induction of mitotic gene
conversions in Saccharomyces cerevisiae by N-nitrosated
pesticides. Mutat Res, 22: 121-126.
Sikka HC, Miyazaki S, & Rice CP (1973) Metabolism of selected
pesticides by marine microorganisms. Springfield, Virginia, National
Technical Information Service (Syracuse University Annual Report No.
2 - NTIS AS AD-763 410).
Sikka HC, Miyazaki S, & Lynch RS (1975) Degradation of carbaryl and
1-naphthol by marine microorganisms. Bull Environ Contam Toxicol,
13(6): 666-672.
Simpson JR (1965) Exposure to orchard pesticides, dermal and
inhalation exposures. Arch Environ Health, 10: 884-885.
Singh JM (1973) Decreased performance behaviour with carbaryl - an
indication of clinical toxicity. Clin Toxicol, 6(1): 97-108.
Singh KP, Pandey SY, Divekar VV, Surana KC, & Paralikar AB (1978)
Dissipation of carbaryl residues in and on cauliflower. In: Edwards
CA, Veeresh GK, & Krueger HR ed. Proceedings of the Symposium on
Pesticide Residues in the Environment in India, 7-10 November 1978.
Hebbal, Bangalore, University of Agricultural Sciences, pp 210-214
(UAS Technical Series No. 32).
Singh VP, Gupta S, & Saxena PK (1984) Evaluation of acute toxicity
of carbaryl and malathion to freshwater teleosts, Chauna punctatis
(bloch) and Heteropneustes fossilis (bloch). Toxicol Lett, 20:
271-276.
Sjoblad RD, Minard RD, & Bollag JM (1976) Polymerisation of
1-naphthol and related phenolic compounds by an extracellular fungal
enzyme. Pestic Biochem Physiol, 6: 457-463.
Skraba WJ & Young FG (1959) Radioactive Sevin (1-naphthyl-
1-carbon-14 N-methylcarbamate): a convenient synthesis. Agric Food
Chem, 7(9): 612-613.
Smalley HE (1970) Diagnosis and treatment of carbaryl poisoning in
swine. J Am Vet Med Assoc, 156(3): 339-344.
Smalley HE, Curtis JM, & Earl FL (1968) Teratogenic action of
carbaryl in beagle dogs. Toxicol Appl Pharmacol, 13: 392-403.
Smalley HE, O'Hara PJ, Bridges CH, & Radeleef RD (1969) The effect
of chronic carbaryl administration on the neuromuscular system of
swine. Toxicol Appl Pharmacol, 14: 409-419.
Snow CD & Stewart NE (1963) Treatment of Tikamook Bay oyster beds
with MGS-90 (Sevin) (Unpublished report from the Oregon Fish
Commission, submitted to WHO by Rhône-Poulenc Agro, Lyon).
Söderpalm BC & Önfelt A (1988) The action of carbaryl and its
metabolite alpha naphthol on mitosis in V79 Chinese hamster
fibroblasts. Indications of the involvement of some cholinester in
cell division. Mutat Res, 201(2): 349-363.
Sokur AJ (1971) [Hypokaliaemia in erythrocytes after intoxication of
white rats with some pesticides (DDT, Sevin, chlorophos).] Gig
Primen Toxicol Pestic Klin Otrav, 1971: 213-216 (in Russian).
Solomon H & Weis J (1979) Abnormal circulatory development in medaka
caused by the insecticides carbaryl, malathion and parathion.
Toxicology, 19: 51-62.
Spain AV (1974) The effects of carbaryl and DDT on the litter fauna
of a Corsican pine forest (Pinus nigra var. maritima): A
multivariate comparison. J Appl Ecol, 11: 467-481.
Springborn Bionomics, Inc. (1988a) Environmental fate of spray
applications of the rice insecticide Sevin XLR (a.i. carbaryl):
California (Study No. 565-6118/6112). Wareham, Massachusetts,
Springborn Bionomics, Inc. (Unpublished proprietary information
submitted to WHO by Rhône-Poulenc Agro, Lyon).
Springborn Bionomics, Inc. (1988b) Environmental fate of spray
applications of the forest insecticide Sevin-4-oil (a.i. carbaryl):
Oregon (Study No. 565-6120/6114). Wareham, Massachusetts, Springborn
Bionomics, Inc. (Unpublished proprietary information submitted to
WHO by Rhône-Poulenc Agro, Lyon).
Springborn Bionomics, Inc. (1988c) Environmental fate of spray
applications of the forest insecticide Sevin-4-oil (a.i. carbaryl):
Pennsylvania (Study No. 565-6121/6115). Wareham, Massachusetts,
Springborn Bionomics, Inc. (Unpublished proprietary information
submitted to WHO by Rhône-Poulenc Agro, Lyon).
Stanley JG & Trial JG (1980) Disappearance constants of carbaryl
from streams contaminated by forest spraying. Bull Environ Contam
Toxicol, 25(5): 771-776.
Statham CN & Lech JJ (1975) Potentiation of the acute toxicity of
several pesticides and herbicides in trouts by carbaryl. Toxicol
Appl Pharmacol, 34: 83-87.
Stegeman LC (1964) The effects of the carbamate insecticide carbaryl
upon forest soil mites and Collembola. J Econ Entomol, 57(6):
803-808.
Stevenson JH (1978) The acute toxicity of unformulated pesticides to
worker honey bees ( Apis Mellifera L.). Plant Pathol, 27: 38-40.
Stevenson JH, Needham PH, & Walker J (1977) Poisoning of honey bees
by pesticides: Investigations of the changing pattern in Britain
over 20 years. Rothamsted Rep, 2: 55-66.
Stewart NE, Millemann RE, & Breese WP (1967) Acute toxicity of the
insecticide Sevin and its hydrolytic product 1-naphthol to some
marine organisms. Trans Am Fish Soc, 96: 25-30.
Strait JR, Thornwall GC, & Ehrich M (1991) Sensitive
high-performance liquid chromatographic analysis for toxicological
studies with carbaryl. Washington, DC, American Chemical Society.
Street JC & Sharma RP (1975) Alteration of induced cellular and
humoral immune responses by pesticides and chemicals of
environmental concern: Quantitative studies on immunosuppression by
DDT, Aroclor 1254, carbaryl, carbofuran and methylparathion. Toxicol
Appl Pharmacol, 32(5): 587-602.
Strother A (1970) Comparative metabolism of selected
1-methylcarbamates by human and rat liver fractions. Biochem.
Pharmacol, 19: 2525-2529.
Strother A & Wheeler L (1976) Disposition of 14C-carbaryl in
pregnant, nonpregant and fetal tissues of rat. Fed Proc, 35: 327.
Strother A & Wheeler L (1980) Excretion and disposition of
[14C]carbaryl in pregnant, non-pregant and foetal tissues of the
rat after acute administration. Xenobiotica, 10(2): 113-124.
Struble CB, Feil VJ, Pecas JC, & Gerst JW (1983a) Biliary secretion
of 14C and identification of 5,6-dihydro-5,6-dihydroxycarbaryl
glucoronide as a biliary metabolite of (14C) carbaryl in the rat.
Pestic Biochem Physiol, 19: 85-94.
Struble CB, Pecas JC, & Gerst JW (1983b) Enterohepatic circulation
and biliary secretion of 5,6-dihydro-5,6-dihydroxy(14C)carbaryl
glucoronide in the rat. Pestic Biochem Physiol, 19(1): 95-103.
Subbotina SG & Belonozhko GA (1968) [The effect of Sevin on the
thermoresistance and fractionary protein content of blood serum.]
Gig Primen Toxicol Pestic Klin Otrav, 1968: 442-444 (in Russian).
Sud RK, Sud AK, & Gupta KG (1972) Degradation of Sevin (1-naphthyl
N-methylcarbamate) by Achromobacter sp. Arch Microbiol, 87:
353-358.
Sullivan LJ, Chin BH, & Carpenter CP (1972) in vitro vs. in vivo
chromatographic profiles of carbaryl anionic metabolites in man and
lower animals. Toxicol Appl Pharmacol, 22: 161-174.
Sundaram KMS & Szeto SY (1987) Distribution and persistence of
carbaryl in some terrestrial and aquatic components of a forest
environment. J Environ Sci Health, B22(5): 579-599.
Surprenant DC (1985a) Acute toxicity of carbaryl technical to
embryo-larvae of eastern oysters (Crassostrea virginica). Wareham,
Massachusetts, Springborn Bionomics, Inc. (Report No. BW-85-7-1817)
(Unpublished proprietary information submitted to WHO by
Rhône-Poulenc Agro, Lyon).
Surprenant DC (1985b) Acute toxicity of carbaryl technical to mysid
shrimp (Mysidopsis bahia). Wareham, Massachusetts, Springborn
Bionomics, Inc. (Report No. BW-85-6-1790) (Unpublished proprietary
information submitted to WHO by Rhône-Poulenc Agro, Lyon).
Surprenant DC (1985c) The chronic toxicity of carbaryl technical to
Daphnia magna under flow-through conditions. Wareham,
Massachusetts, Springborn Bionomics, Inc. (Report No. BW-85-7-1813)
(Unpublished proprietary information submitted to WHO by
Rhône-Poulenc Agro, Lyon).
Sussman HF, Cooke SB, & Bloch V (1969) Human louse infestation;
treatment with carbacide. Arch Dermatol, 100(1): 82-83.
Swartz WJ (1981) Long- and short-term effects of carbaryl exposure
in chick embryos. Environ Res, 26(2): 463-471.
Swartz WJ (1985) Effects of carbaryl on gonadal development in the
chick embryo. Bull Environ Contam Toxicol, 34: 481-485.
Szczepaniak S & Jeleniewicz K (1980) [Alterations of free amino
acids concentrations in rat blood erythrocytes following the acute
intoxication with carbaryl.] Bromatol Chem Toksykol, XIII(2):
159-163 (in Polish with English summary).
Szczepaniak S & Jeleniewicz K (1981) [ In vitro binding of carbaryl
and alpha naphthol with plasma and erythrocytes amino acids.]
Bromatol Chem Toksykol, XIV(2): 165-168 (in Polish with English
summary).
Szczepaniak S & Jeleniewicz K (1986) [Body amino acids balance in
acute poisoning with carbaryl.] Farm Pol, 9: 475-479 (in Polish).
Szczepaniak S, Siemkievicz E, Jeleniewicz K, & Nowinski H (1980)
[Effects of carbaryl and chlorfenvinfos on the level of free amino
acids in rat blood serum. Part II. Intoxication with a single dose
corresponding to 1/5 LD50.] Bromatol Chem Toksykol, XIII(4):
351-353 (in Polish with English summary).
Szeto SY, MacCarty HR, Oloffs PC, & Shephard RF (1979) The fate of
acephate and carbaryl in water. J Environ Sci Health, B14(6):
635-654.
Tagatz ME, Ivey JM, & Lehman HK (1979) Effects of Sevin on
development of experimental estuarine communities. J Toxicol Environ
Health, 5: 643-651.
Takahashi RN, Poli A, Morato GS, Lima TCM, & Zanin M (1991) Effects
of age on behavioral and physiological responses to carbaryl in
rats. Neurotoxicol Teratol, 13: 21-26.
Tewfik MS & Hamdi YA (1970) Decomposition of Sevin by a soil
bacterium. Acta Microbiol Pol Ser B, 19(2): 133-135.
Thomas A (1981) Introductory remarks: The testes. Environ Health
Perspect, 38: 3-4.
Thomas SJ (1986) Sevin brand carbaryl insecticide: Magnitude of
carbaryl residues in sugar beet roots (File No. 34934). Research
Triangle Park, North Carolina, Union Carbide Agricultural Products
Co., Inc. (Prepared in collaboration with Hazleton Laboratories
America, Inc., Chemical and Biomedical Sciences Division, Madison)
(Unpublished proprietary information submitted to WHO by
Rhône-Poulenc Agro, Lyon.
Thomas JH, Dieringer CS, & Schein L (1974) Effects of carbaryl on
mouse organs of reproduction. Toxicol Appl Pharmacol, 28: 142-145.
Thomas J, Visalakshi A, & Mohandas N (1982) Contamination of water
in rice fields sprayed with carbaryl for insect control. Entomon,
7(3): 379-380.
Thompson AR (1971) Effects of nine insecticides on the numbers and
biomass of earthworms in pasture. Bull Environ Contam Toxicol, 5(6):
577-586.
Tilak KS, Rao DMR, Devi AP, & Murty AS (1981) Toxicity of carbaryl
and 1-naphthol to four species of freshwater fish. J Biosci, 3(4):
457-462.
Tilden RL & Van Middelem CH (1970) Determination of carbaryl as an
amide derivative by electroncapture gas chromatography. J Agric Food
Chem, 18(1): 154-158.
Ting KC, Kho PK, Musselman AS, Root JA, & Tichelaar R (1984) High
performance liquid chromatographic method for determination of six
N-methylcarbamates in vegetables and fruits. Bull Environ Contam
Toxicol, 33: 538-547.
Tobin JS (1970) Carbofuran. A new carbamate insecticide. J Occup
Med, 12(1): 16-19.
Toor HS & Kaur K (1974) Toxicity of pesticides to the fish Cyprinus
carpio communis Linn. Indian J Exp Biol, 12: 334-336.
Tos-Luty S, Puchala-Matysek W, & Latuszynska J (1973) [Carbaryl
toxicity in chick embryo.] Bromatol Chem Toksykol, 6: 409-411 (in
Polish with English summary).
Trial JG (1978) The effects of Sevin-4-oil on aquatic insect
communities of streams: a continuation of 1976 studies. In:
Environmental monitoring of cooperative spruce budworm control
projects, Maine 1976 and 1977. Augusta, Maine, Maine Department of
Conservation, Forest Service, pp 124-140.
Trial JG (1979) The effects of Sevin-4-oil on aquatic insect
communities of stream (1976-1978). In: Environmental moitoring of
cooperative spruce budworm control projects, Maine 1978. Augusta,
Maine, Maine Department of Conservation, Forest Service, pp 6-22.
Triolo AJ, Lang WR, Coon JM, Lindstrom D, & Herr DL (1982) Effect of
the insecticide toxaphene and carbaryl on induction of lung tumors
by benzo(a)pyrene in the mouse. J Toxicol Environ Health, 9(4):
637-649.
Tripathi G & Shukla SP (1988) Toxicity bioassay of technical and
commercial formulations of carbaryl to the freshwater catfish,
Clarias batrachus. Ecotoxicol Environ Saf, 15(3): 277-281.
Tyburczyk W & Podolak-Majczak M (1984a) [Effect of joint action of
sodium nitrite and carbaryl on rats organism.] Bromatol Chem
Toksykol, XVII(2): 125-129 (in Polish with English summary).
Tyburczyk W & Podolak-Majczak M (1984b) [Effect of joint action of
sodium nitrite and carbaryl on rats organism.] Bromatol Chem
Toksykol, XVII(3): 215-219 (in Polish with English summary).
Tyburczyk W & Podolak-Majczak M (1986) [Effect of joint action of
sodium nitrite and carbaryl on rats organism. Part V. The role of
glucose-6-phosphate dehydrogenase and haematologic studies.]
Bromatol Chem Toksykol, XIX(3): 167-173 (in Polish with English
summary).
Uchiyama M, Takeda M, Suzuki T, & Yoshikawa K (1975) Mutagenicity of
nitroso derivatives of N-methylcarbamate insecticides in
microbiological method. Bull Environ Contam Toxicol, 14: 389-394.
US Department of Labour (1978) Occupational health guideline for
carbaryl (Sevin/R). Washington, DC, US Department of Labour, pp 1-5.
US EPA (1977) Aspects of pesticidal uses of carbaryl on man and the
environment. Washington, DC, US Environmental Protection Agency,
Office of Pesticide Programs, 286 pp.
US EPA (1980a) Carbaryl. Decision document. Washington, DC, US
Environmental Protection Agency, Office of Pesticides and Toxic
Substances, 22 pp.
US EPA (1980b) FIFRA Scientific Advisory Panel. Subcommittee
meeting, Arlington, Virginia, 23 July 1980. Washington, DC, US
Environmental Protection Agency, 202 pp.
US EPA (1981) Environmental fates and impacts of major forest use of
pesticides. Washington, DC, US Environmental Protection Agency,
Office of Pesticides and Toxic Substances.
US EPA (1984) Health and environmental profile for carbaryl.
Cincinnati. Ohio, US Environmental Protection Agency.
Usha Rani MV, Reddi OS, & Reddy PP (1980) Mutagenicity studies
involving aldrin, endosulfan, demethoate, phosphamidon, carbaryl and
ceresan. Bull Environ Contam Toxicol, 25(2): 277-282.
Vandekar M (1965) Observations of the toxicity of carbaryl,
folithion and 3-isopropylphenyl N-methylcarbamate in a village
scale trail in Southern Nigeria. Bull World Health Organ, 33:
107-115.
Vandekar M, Plestina R, & Wilhelm M (1971) Toxicity of carbamates
for mammals. Bull World Health Organ, 44: 241-249.
Varshney TN & Gaur AC (1972) Effect of DDT and Sevin on soil fungi.
Acta Microbiol Acad Sci Hung, 19: 97-102.
Vashakidze VI (1966) [On the question of the embryotropic,
gonadotropic and mutagenic effect of some pesticides.] Gig Primen
Toxicol Pestic Klin Otrav, 1966: 80-83 (in Russian).
Vashakidze VI (1975) [Effect of small doses of Sevin on gonad
function following its repeated effect on white rats SbTr NII.] Gig
Tr Prof Zabol, 14: 235-266 (in Russian).
Vasilos AF, Dmitrienko VD, & Shroyt IG (1975) [Disruption of the
mitotic system following acute Sevin poisoning.] Izv Akad Nauk
Moldavskoj SSR Ser Khim Nauk, 3: 64-67 (in Russian).
Venkateswarlu K, Chendrayan K, & Sethunathan N (1980) Persistence
and biodegradation of carbaryl in soils. J Environ Sci Health,
B15(4): 421-429.
Venkat Reddy P, Prem Veer Reddy G, & Subramanyams S (1974)
Cytological effects of the insecticide Sevin on the mitotic cells of
Poecilocerus pictus. Curr Sci, 43(6): 187-189.
Verma SR, Tonk IF, Gupta AK, & Saxena M (1984) Evaluation of an
application factor for determining the safe concentration of
agricultural and industrial chemicals. Water Res, 18(1): 111-115.
Viter VF (1978) [Monotonous and intermittent inhalatory effect of
Sevin upon some behavioural reactions of experimental animals.] Gig
i Sanit, 11: 33-34 (in Russian).
Vyatchanikov KA & Alexachina ZA (1968) [Experimental investigations
for procuring therapeutic and protective means at intoxications by
Sevin.] Gig Primen Toxicol Pestic Klin Otrav, 1968: 442-444 (in
Russian).
Wagner R (1973) [Identification and estimation of organophosphate
insecticides and carbaryl on ready made thin-layer plates.] Z
Gesamte Hyg Grenzgeb, 19(7): 504-510 (in German).
Wakakura M, Ishikawa S, & Uga S (1978) Ultrastructural hepatic
changes by carbamate pesticide (Sevin) in rats. Environ Res, 16:
191-204.
Walker N, Janes NF, Spokes JR, & Van Berkum P (1975a) Degradation of
1-naphthol by a soil pseudomonad. J Appl Bacteriol, 39: 281-286.
Walker M, Gale GR, Atkins LM, & Gadse-Den RH (1975b) Some effects of
carbaryl on Ehrlich ascites tumor cells in vitro and in vivo.
Bull Environ Contam Toxicol, 14(4): 441-448.
Waller GD (1969) Susceptibility of an alfalfa leaf-cutting bee to
residues of insecticides on foliage. J Econ Entomol, 62(1): 189-192.
Ward SA, May DG, Heath AJ, & Branch RA (1988) Carbaryl metabolism is
inhibited by Cimetidine in the isolated perfused rat liver and in
man. J Toxicol Clin Toxicol, 26(5/6): 269-281.
Wauchope RD & Haque R (1973) Effects of pH, light and temperature on
carbaryl in aqueous media. Bull Environ Contam Toxicol, 9(5):
257-260.
Weber FJ, Shea TB, & Berry SE (1982) Toxicity of certain
insecticides to protozoa. Bull Environ Contam Toxicol, 28: 618-631.
Weil CS & Carpenter CP (1962) Studies on carcinogenesis completed in
1962. Pittsburgh, Pennsylvania, Carnegie-Mellon Research Institute
(Special Report 25-122) (Unpublished proprietary information
submitted to WHO by Rhône-Poulenc Agro, Lyon).
Weil CS & Carpenter CP (1965) Results of a three generations
reproductive study on rats fed Sevin in their diet. Pittsburgh,
Pennsylvania, Carnegie-Mellon Research Institute, 18 pp (Report No.
28-53) (Unpublished proprietary information submitted to WHO by
Rhône-Poulenc Agro, Lyon).
Weil CS & Carpenter CP (1974) Sevin: range finding toxicity studies.
Pittsburgh, Pennsylvania, Carnegie Mellon (Report No. 37-53)
(Unpublished proprietary information submitted to WHO by
Rhône-Poulenc Agro, Lyon).
Weil CS, Woodside MD, Carpenter CP, & Smyth HF Jr (1972) Current
studies of tests of carbaryl for reproductive and teratogenic
effect. Toxicol Appl Pharmacol, 21: 390-404.
Weil CS, Woodside MD, Bernard JB, Condra NI, King JM, & Carpenter CP
(1973) Comparative effect of carbaryl on rat reproduction and
guinea-pig teratology when fed either in the diet or by stomach
intubation. Toxicol Appl Pharmacol, 26: 621-638.
Weston RF, Inc. (1982) Carbaryl: A profile on its characteristics as
pesticide. Rockville, Maryland, Roy F. Weston, Inc., Environmental
Health Sciences Centre.
Whitehurst WE, Bishop ET, Critchfield FE, Gyrisco GG, Huddleston EW,
Arnold H, & Lisk OJ (1963) The metabolism of Sevin in dairy cows. J
Agric Food Chem, 11: 167-169.
WHO (1982) Recommended health-based limits in occupational exposure
to pesticides. Report of a WHO study group. Geneva, World Health
Organization, 110 pp (WHO Technical Report Series 677).
WHO (1986) IPCS Environmental Health Criteria 64: Carbamate
pesticides - A general introduction. Geneva, World Health
Organization, 136 pp.
WHO (1992) The WHO recommended classification of pesticides by
hazard and guidelines to classification 1992-1993. Geneva, World
Health Organization, 66 pp (Unpublished document WHO/PCS/92.14).
WHO/FAO (1975) Data sheets on pesticides No. 3: Carbaryl. Geneva,
World Health Organization, 8 pp (Unpublished document VBC/DS/75.3,
Rev. 1).
Whorton MD, Milby TH, Stubbs HA, Avasha BH, & Hull EG (1979)
Testicular function among carbaryl exposed employees. J Toxicol
Environ Health, 5: 925-941.
Wiggins OG & Weiden MHJ (1969) Fate of Sevin applied to the surface
of bean fruit and bean plants under field conditions. Clayton, North
Carolina, Union Carbide Corporation (Report No. 11377) (Unpublished
proprietary information submitted to WHO by Rhône-Poulenc Agro,
Lyon).
Wilkes L, Mulkey N, & Wargo J (1977) Research report laboratory soil
metabolism study of carbaryl (Project No. 285). Monument, Colorado,
Analytical Development Corp. (Unpublished proprietary information
submitted to WHO by Rhône-Poulenc Agro, Lyon).
Wills JH, Jameson E, & Coulston F (1968) Effects of oral doses of
carbaryl on man. Clin Toxicol, 1:(3) 265-271.
Wiltrout RW, Ercegovich CD, & Ceglowski WS (1978) Humoral immunity
in mice following oral administration of selected pesticides. Bull
Environ Contam Toxicol, 20(3): 423-431.
Winterlin W & Walker G (1973) Carbaryl residues in bees, honey, and
bee bread following exposure to carbaryl via the food supply. Arch
Environ Contam Toxicol, 1(4): 362-374.
Wojciechowski JP, Kaur P, & Sabharual PS (1982) Induction of ouabain
resistance in V-79 cells by four carbamate pesticides. Environ Res,
29: 48-53.
Wolfe HP, Durham W, & Armstrong J (1967) Exposure of workers to
pesticides. Arch Environ Health, 14: 622-633.
Wolfe NL, Zepp RG, & Paris DF (1978) Carbaryl, propham and
chlorpropham: A comparison of the rates of hydrolysis and photolysis
with the rate of biolysis. Water Res, 12(8): 565-571.
Woodruff RC, Phillips JP, & Irwin D (1983) Pesticide-induced
complete and partial chromosome loss in screens with
repair-defective females of Drosophila melanogaster. Environ
Mutagen, 5: 835-846.
Woodward DF & Mauck WL (1980) Toxicity of five forest insecticides
to cutthroat trout and two species of aquatic invertebrates. Bull
Environ Contam Toxicol, 25: 846-853.
Wright CG, Leidy RB, & Dupress HE (1981) Insecticides in the ambient
air of rooms following their application for control of pests. Bull
Environ Contam Toxicol, 26(4): 548-553.
Wuu K & Grant W (1966) Morphological and somatic chromosomal
aberrations induced by pesticides in barley (Hordeum vulgare). Can
J Genet Cytol, 8: 481-501.
Wyrobek AJ, Watchmaker G, Gordon L, Wong K, Moore D, & Whorton D
(1981) Sperm shape abnormalities in carbaryl-exposed employees.
Environ Health Perspect, 40: 225-265.
Yadav PR & Jaglan RS (1982) Persistence of carbaryl residues in and
on unprocessed and processed cauliflower curds. Beitr Trop
Landwirtsch Veterinärmed, 20(1): 89-95.
Yadav GS, Kathpal TS, & Singh H (1985) Efficacy of monocrotophos,
carbaryl, and endosulfan against Antigastra catalaunalis Dup. and
their persistence in soil and sesamum seeds. Indian J Agric Sci,
55(6): 438-442.
Yakim VS (1965) [Toxicological hygienic characteristics of Sevin.]
Gig Primen Toxicol Pestic Klin Otrav, 1965: 423-431 (in Russian).
Yakim VS (1967) [Data for substantiating the MAC values for Sevin in
the air of the working zone.] Gig i Sanit, 4: 23-33 (in Russian).
Yakim VS (1968) [The toxicity of Sevin at inhalatory intake.] Gig
Primen Toxicol Pestic Klin Otrav, 1968: 115-117 (in Russian).
Yakim VS (1970) [Content and distribution of Sevin in warm-blooded
animals organism and its effect on ChE activity.] Gig Primen Toxicol
Pestic Klin Otrav, 1970: 214-218 (in Russian).
Yess NJ, Houston MG, & Gunderson EL (1991a) Food and Drug
Administration pesticide residue monitoring of foods: 1978-1982. J
Assoc Off Anal Chem, 75(2): 265-272.
Yess NJ, Houston MG, & Gunderson EL (1991b) Food and Drug
Administration pesticide residue monitoring of foods: 1983-1986. J
Assoc Off Anal Chem, 74(2): 273-280.
Young RR (1990) Mutagenicity test on carbaryl (technical) in the
CHO/HGPRT forward mutation assay. Kensington, Maryland, Hazleton
Laboratories America, Inc. (Study No. 10862-0-435) (Unpublished
proprietary information submitted to WHO by Rhône-Poulenc Agro,
Lyon).
Zabezhinski MA (1970) [Possible carcinogenic effect of ß-Sevin.]
Vopr Onkol, 16: 106-107 (in Russian).
Zapko WG (1970) [Effect on some liver functions of acute
intoxication of experimental animals with cuprosin, Sevin, Eptam and
TMTD.] Gig Primen Toxicol Pestic Klin Otrav, 1970: 237-240 (in
Russian).
Zeakes SJ, Hansen MF, & Robel RJ (1981) Increased susceptibility of
Bobwhites (Colinus virginianus) to Histomonas meleagridis after
exposure to Sevin insecticide. Avian Dis, 25(4): 981-987.
Zepp RG & Cline DM (1977) Rates of direct photolysis in the aquatic
environment. Environ Sci Technol, 11: 359-366.
Zepp RG, Wolfe N, Gordon L, & Fincher RC (1976) Light-induced
transformations of methoxychlor in aquatic systems. J Agric Food
Chem, 24: 727-733.
Zinkl JG, Hennys ChJ, & Dewelse LR (1977) Brain cholinesterase
activity in birds from forest sprayed trichlorfon (Dylox) and
carbaryl (Sevin-4-oil). Bull Environ Contam Toxicol, 17(4):
379-386.
Zuberli R & Zubairi MY (1971) Carbaryl degradation by Pseudomonas
phaseolicola and Aspergillus niger. Pak J Sci Ind Res, 14(4-5):
383-384.
Zweig G, Gao R-Y, Witt JM, Propendorf W, & Bogen K (1984) Dermal
exposure to carbaryl by strawberry harvesters. J Agric Food Chem,
32(6): 1232-1236.
Zweig G, Leffingwell JT, & Propendorf W (1985) The relationship
between dermal pesticide exposure to fruit harvesters and
dislodgeable foliar residues. J Environ Sci Health, B20(1): 27-59.
ANNEX I. TREATMENT OF CARBAMATE PESTICIDE POISONING IN MAN
(From EHC 64: Carbamate Pesticides - A General Introduction)
All cases of carbamate poisoning should be dealt with as an
emergency and the patient should be hospitalized as quickly as
possible.
Extensive descriptions of the treatment of poisoning by
anticholinesterase agents are given in several major references
(Kagan, 1977;Taylor, 1980; Plestina, 1984).
The treatment is based on:
(a) minimizing the absorption;
(b) general supportive treatment; and
(c) specific pharmacological treatment.
I.1 Minimizing the absorption
When dermal exposure occurs, decontamination procedures include
removal of contaminated clothes and washing of the skin with
alkaline soap or with a sodium bicarbonate solution. Particular care
should be taken in the cleaning of the skin area where venupuncture
is performed. Blood might be contaminated with carbamates and
therefore inaccurate measures of ChE inhibition might result.
Extensive eye irrigation with water or saline should also be
performed. In the case of ingestion, vomiting can be induced, if the
patient is conscious, by the administration of ipecacuanha syrup
(10-30 ml) followed by 200 ml of water. However, this treatment is
contraindicated in the case of pesticides dissolved in hydrocarbon
solvents. Gastric lavage (with the addition of bicarbonate solution
or activated charcoal) can also be performed, particularly in
unconscious patients, taking care to prevent aspiration of fluids
into the lungs (i.e., only after a tracheal tube has been put in
place).
The volumes of the fluids introduced in the stomach should be
recorded and samples of gastric lavage frozen and stored for
subsequent chemical analysis. If the formulation of the pesticide
involved is available, it should also be stored for further analysis
(i.e., detection of toxicologically relevant impurities). A purge to
remove the ingested compound can be administered.
I.2 General supportive treatment
Artificial respiration (via a tracheal tube) should be started
at the first sign of respiratory failure and maintained for as long
as necessary.
Cautious administration of fluids is advised as well as general
supportive and symptomatic pharmacological treatment and absolute
rest.
I.3 Specific pharmacological treatment
I.3.1 Atropine
Atropine should be given, beginning with 2 mg iv repeated at 15
to 30-min intervals. The dose and the frequency of atropine
treatment varies from case to case, but should maintain the patient
fully atropinized (dilated pupils, dry mouth, skin flushing, etc.).
I.3.2 Oxime reactivations
Although it might be suspected that oxime cholinesterase
reactivators would be as helpful in carbamate poisoning as they are
in organophosphorous poisoning, this is not the case. There is
experimental evidence that the pyridinium oxime 2-PAM is not
effective in carbamate poisoning and there is some evidence that it
makes poisoning by certain carbamates, including carbaryl, worse.
I.3.3 Diazepam
Diazepam should be included in the therapy of all but the
mildest cases. Besides relieving anxiety it appears to counteract
some aspects of CNS-derived symptoms that are not affected by
atropine. Doses of 10 mg sc or iv are appropriate and may be
repeated as required.
Other centrally acting drugs and drugs that may depress
respiration are not usually recommended in the absence of artificial
respiration procedures.
References to Annex I
KAGAN, J.S. (1977) [The toxicity of organophosphorus pesticides.]
Moscow (in Russian).
PLESTINA, R. (1984) Prevention, diagnosis, and treatment of
insecticide poisoning. Geneva, World Health Organization.
(Unpublished WHO document VBC/84.889).
TAYLOR, P. (1980) Anticholinesterase agents. In: Goodman, L.S. &
Gilman, A., ed. The pharmacological basis of therapeutics. 6th ed.,
New York, Macmillan Publishing Co., pp. 100-119.
RESUME ET EVALUATION, CONCLUSIONS ET RECOMMANDATIONS
1. Résumé et évaluation
1.1 Identité, propriétés et méthodes d'analyse
Carbaryl est la dénomination commune d'un dérivé de l'acide
carbamique, le N-méthylcarbamate de 1-naphtyle. Le produit
technique consiste en un solide cristallin blanc, peut volatil et
peu soluble dans l'eau, qui est stable à la lumière et à la chaleur
mais qui s'hydrolyse facilement en milieu alcalin. Il existe une
norme FAO pour le carbaryl qui stipule un degré de pureté de 98%,
avec une limite pour les impuretés ( N-méthylcarmate de ß-naphtyle)
de 0,05%.
Pour les analyses portant sur le carbaryl et ses métabolites,
il existe de nombreuses méthodes: chromatographie sur couche mince,
spectrophotométrie, chromatographie en phase gazeuse,
chromatographie en phase liquide à haute pression et spectrométrie
de masse à ionisation chimique. La limite de détection peut
descendre en-dessous du nanogramme et le taux de récupération
dépasse généralement 80%.
1.2 Production et usages
On utilise le carbaryl depuis une trentaine d'années comme
insecticide agissant par contact et par ingestion avec certaines
propriétés endothérapiques; il permet de lutter contre de nombreux
vecteurs et ravageurs. La principale unité de production se trouve
aux Etats-Unis d'Amérique. Plus de 290 fabricants proposent une
gamme de formulations de carbaryl qui dépasse les 1500 produits.
1.3 Transport, distribution et transformation dans l'environnement
Dans la plupart des conditions, le carbaryl ne persiste pas
dans l'environnement. Dans l'eau, son temps de demi-hydrolyse dépend
de la température, du pH et de la concentration initiale; il varie
de quelques minutes à plusieurs semaines. Le principal produit de
décomposition est le 1-naphtol.
L'accumulation du carbaryl, exprimée sous la forme d'un facteur
de bioconcentration dans l'environnement aquatique, et plus
précisément chez les poissons d'eau douce, varie de 14 à 75. Le
carbaryl s'adsorbe plus facilement sur les sols à forte teneur
organique que sur les sols sableux. Aux doses d'emploi usuelles, et
lorsqu'il est appliqué conformément "aux bonnes pratiques
agricoles", il se dissipe rapidement, avec une demi-vie de 8 jours à
un mois dans les conditions normales. Il arrive que le carbaryl soit
entraîné par les pluies ou par les travaux agricoles de la surface
vers les couches sous-jacentes (à un mètre de profondeur).
Le carbaryl peut contaminer la végétation, soit au cours de
l'épandage soit par migration à partir d'un sol contaminé.
La décomposition du carbaryl dans l'environnement dépend de son
degré de volatilisation, de photodécomposition et de dégradation
chimique ou microbienne dans le sol, l'eau et les végétaux. la
vitesse de décomposition est plus rapide en climat chaud.
1.4 Concentrations dans l'environnement et exposition humaine
Dans la population générale, le carbaryl est principalement
absorbé par la voie alimentaire.
Les résidus présents dans des échantillons de rations totales
sont relativement faibles, et ils vont de traces à 0,05 mg/kg. Aux
Etats-Unis d'Amérique, on estime que pendant les premières années
d'utilisation du carbaryl, l'ingestion journalière de carbaryl était
de 0,15 mg/personne/jour (dans 7,4% des composites); cette dose est
tombée à 0,003 mg/personne/jour en 1969 (dans seulement 0,8% des
composites). Pendant la période d'épandage, on peut retrouver
parfois du carbaryl dans les eaux de surface et les retenues d'eau.
La population peut être exposée au carbaryl lors d'opérations
de lutte de contre les ravageurs ou les vecteurs tant au domicile
que sur les aires de loisir.
Les travailleurs peuvent être exposés au carbaryl lors de la
fabrication, de la formulation, de l'emballage, du transport et du
stockage du produit ainsi que pendant son épandage. Les
concentrations dans l'atmosphère des lieux de travail au cours de la
production varient de < 1 mg/m3 à 30 mg/m3. Il peut y avoir une
importante exposition cutanée chez les travailleurs de l'industrie
et les ouvriers agricoles en cas de mesures de protection
insuffisantes.
1.5 Cinétique et métabolisme
Le carbaryl est rapidement absorbé au niveau des poumons et des
voies digestives. Une dose de carbaryl dans l'acétone appliquée sur
la peau de volontaires humains a été absorbée par voie percutanée à
raison de 45% en 8 heures. Toutefois, les données obtenues in vitro
concernant la pénétration cutanée ainsi que les données
toxicologiques indiquent que l'absorption percutanée s'effectue
généralement à un rythme beaucoup plus lent.
Les principales voies métaboliques du carbaryl consistent en
une hydroxylation du cycle et une hydrolyse. Il en résulte la
formation de nombreux métabolites qui subissent ensuite une
conjugaison avec formation de sulfates, de glucuronides et de
mercapturates hydrosolubles qui sont excrétés dans l'urine.
L'hydrolyse conduit à la formation de 1-naphtol, de dioxyde de
carbone et de méthylamine. L'hydroxylation produit du
4-hydroxycarbaryl, du 5-hydroxycarbaryl, du
N-hydroxyméthylcarbaryl, du 5,6-dihydro-5,6-dihydroxycarbaryl et
du 1,4-naphtalènediol. Le principal métabolite chez l'homme est le
1-naphtol.
Dans les conditions normales d'exposition, il est improbable
que le carbaryl s'accumule chez les animaux. Il est excrété
principalement par la voie urinaire étant donné que la
détoxification de son produit d'hydrolyse, le 1-naphtol, s'effectue
essentiellement par la formation de conjugués hydrosolubles. Dans le
cas des métabolites du carbaryl, le cycle entérohépatique joue
également un rôle considérable, en particulier après administration
par voie orale.
Le produit d'hydrolyse, l'acide N-naphtolcarbamique, se
décompose spontanément en méthylamine et dioxyde de carbone. La
méthylamine subit ensuite une déméthylation en dioxyde de carbone et
formiate, ce dernier étant ultérieurement excrété, en majeure partie
dans l'urine.
Des métabolites du carbaryl sont également présents en faible
proportion de la dose absorbée dans la salive et le lait.
1.6 Effets sur les êtres vivants dans leur milieu naturel
Les valeurs de la CL50 pour les crustacés varient de 5 à 9
µg/litre (puces d'eau et mysidés), 8 à 25 µg/litre (orchesties) et
500 à 2500 µg/litre (écrevisses). Chez les insectes aquatiques, les
limites de sensibilité sont du même ordre. Les plécoptères et
éphémèroptères (perles et éphémères) en constituent les groupes les
plus sensibles. Les mollusques sont moins sensibles avec des valeurs
de la CE50 de l'ordre de quelques mg/litre. Dans le cas des
poissons, la plupart des valeurs de la CL50 se situent entre 1 et
30 mg/litre. Les salmonidés constituent le groupe le plus sensible.
La toxicité aiguë est faible pour les oiseaux. La DL50 pour
la sauvagine et le gibier à plumes en général est > 1000 mg/kg.
D'après les tests, l'oiseau le plus sensible est un francolin
(Francolinus levaillanti) (DL50 = 56 mg/kg). Rien n'indique que
les oiseaux aient eu à souffrir de l'effet des épandages effectués
sur les zones forestières à la dose de 1,1 kg de carbaryl/ha.
Le carbaryl est très toxique pour les abeilles et les lombrics.
Dans le cas des abeilles, la DL50 par voie orale est de 0,16
µg/insecte (soit 1-2 mg/kg).
On est fondé à penser que le carbaryl puisse avoir une
influence temporaire sur la composition en espèces des écosystèmes
terrestres et aquatiques. Par exemple, une étude a montré que les
effets exercés sur certaines communautés d'invertébrés terrestres
pourraient persister au moins 10 mois après un seul épandage.
1.7 Effets sur les animaux d'expérience et les systèmes d'épreuve
in vitro
La toxicité aiguë, exprimée sous la forme de la DL50, varie
considérablement selon l'espèce, la formulation et le véhicule du
produit. La DL50 estimative par voie orale pour le rat varie de
200 à 850 mg(kg. Les chats sont plus sensibles, avec une DL50 de
150 mg/kg. Les porcs et les singes le sont moins, avec une DL50 >
1000 mg/kg.
Une concentration de 792 mg de matière active par m3, qui
constitue la valeur maximale qu'on puisse obtenir pour un aérosol, a
produit, au cours d'une exposition de 4 heures, une mortalité de 20%
(1/5) chez des rattes. A des concentrations de 20 mg/m3, des
aérosols de carbaryl ont provoqué une réduction de l'activité
cholinestérasique chez des chats lors d'une exposition de 4 heures,
mais cette concentration n'a eu aucun effet observable chez des
rats.
Le carbaryl est légèrement irritant pour l'oeil et n'a que peu
ou pas de pouvoir sensibilisateur. Lors d'études à long terme, la
concentration sans effet nocif observable a été évaluée à 10 mg/kg
de poids corporel (200 mg/kg de nourriture) pour le rat et à 1,8
mg/kg de poids corporel (100 mg/kg de nourriture) pour le chien.
Chez le chat, soumis à une exposition de longue durée par
inhalation, la concentration sans effet nocif observable est de 0,16
mg/m3. Le carbaryl a un faible potentiel d'accumulation.
1.7.1 Reproduction
On a montré que le carbaryl avait des effets indésirables sur
la reproduction des mammifères et le développement périnatal chez un
certain nombre d'espèces. Ces effets sur la reproduction consistent
en une réduction de la fécondité, une diminution de l'effectif des
portées et une réduction de la viabilité postnatale. Les effets
toxiques du carbaryl sur le développement se traduisent pas un
certain nombre de morts foetales, une réduction du poids foetal et
la présence de malformations. A l'exception d'un petit nombre
d'études, les effets nocifs sur la reproduction et le développement
n'ont été constatés en totalité qu'à des doses manifestement
toxiques pour la mère, et dans un certain nombre de cas, la mère
était plus sensible au carbaryl que l'embryon ou le foetus. Ces
effets toxiques sur la femelle gestante consistaient en une
mortalité accrue, une réduction de la croissance et des dystocies.
Les données indiquent que, par rapport à l'organisme adulte, la
fonction de reproduction et le processus de développement des
mammifères n'est pas particulièrement sensible au carbaryl.
1.7.2 Mutagénicité
Un certain nombre de tests in vitro et in vivo ont été
effectués sur la carbaryl afin d'en évaluer le pouvoir mutagène;
divers points d'aboutissement de ces effets ont été étudiés sur
divers systèmes (bactéries, levures, végétaux, insectes et
mammifères).
Les données disponibles montrent que le carbaryl n'a aucune
tendance à endommager l'ADN. On ne dispose d'aucun rapport
confirmant les caractères mutagènes suivants: induction de
recombinaisons mitotiques, conversion génique, et synthèse non
programmée de l'ADN chez les procaryotes (H. influenzae, B.
subtilis) ni chez les eucaryotes ( S. cerevisiae, A. nidulans,
lymphocytes humains en culture et hépatocytes de rat) in vitro.
La recherche de mutations géniques, qui a donné lieu à un grand
nombre d'épreuves sur systèmes bactériens, a toujours donné des
résultats négatifs, sauf dans deux cas. Lors de plusieurs études
portant sur les mutations géniques et effectuées sur des cellules
mammaliennes in vitro, le carbaryl n'a donné qu'un seul résultat
positif équivoque dans une de ces études. Toutefois, cette étude
présentait un certain nombre de défauts et ce résultat n'a pas été
confirmé par ceux d'autres études comparables.
Des lésions chromosomiques ont été observées in vitro sur des
cellules humaines, des cellules de rat et de hamster ainsi que des
cellules végétales exposées à des fortes doses de carbaryl. Aucun
effet de ce genre n'a été observé lors d'épreuves in vivo sur des
systèmes mammaliens, même à des doses atteignant 1000 mg/kg.
On a montré que le carbaryl perturbe le mécanisme d'élongation
des fibres du fuseau chez les cellules végétales et mammaliennes in
vitro. On ne sait pas encore très bien si les résultats des
épreuves effectuées sur des végétaux sont extrapolables à l'homme.
Compte tenu de la base de données sont on dispose actuellement,
on peut conclure que rien n'autorise à soupçonner le carbaryl de
présenter un risque d'effets mutagènes pour les cellules somatiques
ou germinales de l'homme.
Un dérivé nitrosé du carbaryl, le N-nitrosocarbaryl, peut
provoquer des recombinaisons mitotiques et des conversions géniques
chez les procaryotes (H. influenzae, B. subtilis) et les
eucaryotes (S. cerevisiae) in vitro, et il donne des résultats
positifs lors de "spot tests" effectués sur E. coli.
En outre, les résultats expérimentaux indiquent que le
N-nitrosocarbaryl se lie à l'ADN provoquant la formation de
liaisons alcalino-sensibles et des cassures monocaténaires.
Il n'est pas établi que le nitrosocarbaryl soit un agent
clastogène in vivo (cellules de la moelle osseuse et cellules
germinales) même à doses toxiques élevées.
1.7.3 Cancérogénicité
De nombreuses études ont été consacrées au pouvoir cancérogène
du carbaryl chez le rat et la souris. La plupart de ces études ont
donné des résultats négatifs mais il s'agit de travaux anciens qui
ne satisfont pas aux normes actuelles. Quoiqu'il en soit, de
nouvelles études portant sur ces mêmes animaux et qui, elles,
satisfont aux exigences modernes, sont actuellement en coursa. La
dernière évaluation du CIRC (CIRC, 1987) a conclu que l'existence de
cancers imputables au carbaryl n'était pas documentée chez l'homme
et qu'on ne possédait pas de preuves suffisantes d'un pouvoir
cancérogène de cette substance chez les animaux de laboratoire. Il
n'a donc pas été possible de classer le carbaryl en fonction de son
pouvoir cancérogène pour l'homme (Groupe 3).
On a montré que le N-nitrosocarbaryl induisait des tumeurs
locales chez les rats (sarcomes au point d'injection ou carcinomes
spinocellulaires au niveau de la portion cardiaque de l'estomac,
lorsque la substance est administrée par voie orale). Etant donné la
biochimie du carbaryl chez l'homme, on peut considérer comme
négligeable le risque de cancérisation par le N-nitrosocarbaryl
résultant d'une exposition humaine au carbaryl.
1.7.4 Effets sur les différents organes et systèmes
a) Système nerveux
Les effets du carbaryl sur le système nerveux tiennent
essentiellement à l'inhibition de la cholinestérase qui est
généralement passagère. Ces effets ont été étudiés sur des rats et
des singes. Des doses de 10 à 20 mg de carbaryl par kg, administrées
pendant 50 jours par voie orale, ont provoqué des perturbations dans
l'apprentissage et l'exécution de certaines épreuves par les rats
traités.
______________
a Ces études n'ont pas encore fait l'objet d'un examen par le
PISC. La société qui effectue ces travaux indique qu'aux doses
les plus fortes étudiées, on note une augmentation
significative de la fréquence des tumeurs chez les deux
espèces.
Lors d'une petite étude portant sur des porcs, du carbaryl
administré pendant 72 à 82 jours en mélange avec la nourriture à
raison de 150 mg/kg de poids corporel, a produit un certain nombre
d'effets neuromusculaires. On a observé en outre chez des poulets
ayant reçu de fortes doses de carbaryl, une faiblesse réversible des
pattes. Aucun signe de démyélinisation n'a été observé dans le
cerveau, le nerf sciatique ou les coupes de moelle épinière
examinées au microscope. On n'a pas observé d'effets de ce genre à
l'issue d'études à long terme sur des rongeurs.
b) Système immunitaire
Administré in vivo à des doses provoquant des signes
cliniques manifestes, le carbaryl exerce divers effets sur le
système immunitaire. Nombre de ces effets ont été observés à des
doses voisines de la DL50. Dans la plupart des études où les doses
utilisées permettaient la survie des lapins et des souris examinés,
on n'a pas observé d'effets significatifs sur le système
immunitaire. Plusieurs de ces travaux présentaient des défauts, par
exemple un manque de cohérence et parfois des contradictions
manifestes dans les résultats, défauts qui ne permettent pas de
dégager des résultats obtenus un mécanisme immunotoxique bien
défini.
c) Sang
Le carbaryl affecterait l'hémostase mais les résultats
concernant le sens de cet effet sont contradictoires. On a observé
que le carbaryl produisait une augmentation de la formation de
méthémoglobine liée à la dose dans des érythrocytes de moutons
présentant un déficit en glucose-6-phosphate-déshydrogénase. La
sérum-albumine humaine réagit in vitro sur le groupement ester du
carbaryl. Le carbaryl se lie aux acides aminés libres.
d) Foie
On a fait état de troubles affectant le métabolisme des
glucides et la synthèse des protéines au niveau du foie ainsi que
d'une perturbation de la fonction détoxifiante de cet organe chez
les mammifères. Le carbaryl induit faiblement l'activité
pharmacométabolisante des microsomes hépatiques. Il y a
raccourcissement de la durée du sommeil induit par le phénobarbital.
Les taux hépatiques de cytochrome P-450 et b5 sont augmentés. Ces
modifi-cations du métabolisme hépatique pourraient expliquer en
partie le triplement de la DL50 pour le carbaryl observé chez des
rats préalablement traités par cette substance.
e) Fonction gonadotrope
Il a été signalé que le carbaryl augmentait la fonction
gonadotrope de l'hypophyse chez le rat.
1.7.5 Mécanisme fondamental de la toxicité
Le carbaryl est un inhibiteur de l'activité cholinestérasique.
Cet effet est lié à la dose et rapidement réversible. On n'a pas
observé de vieillissement de la cholinestérase carbamylée. Tous les
métabolites reconnus du carbaryl ont une activité
anticholinestérasique sensiblement plus faible que le carbaryl
lui-même.
1.8 Effets sur l'homme
Le carbaryl est facilement absorbé par inhalation et après
administration par voie orale mais moins facilement pas la voie
percutanée. Etant donné que l'inhibition de la cholinestérase est le
principal mécanisme de l'action du carbaryl, le tableau clinique
d'une intoxication par cette substance est dominé par les symptômes
correspondants, à savoir: augmentation de la sécrétion bronchique,
sueurs profuses, salivation et larmoiement; myosis,
bronchoconstriction, crampes abdominales (vomissements et diarrhée);
bradycardie; fasciculation des petits muscles (dans les cas graves
le diaphragme et les muscles respiratoires sont également atteints);
tachycardie, céphalées, vertiges, angoisses, confusion mentale, coma
et dépression des centres respiratoires. Ces signes d'intoxication
se manifestent rapidement après l'absorption et disparaissent aussi
vite une fois que l'exposition a cessé.
Des études contrôlées sur des volontaires humains ont montré
que des doses uniques inférieures à 2 mg/kg étaient bien tolérées.
Une dose unique de 250 mg (2,8 mg/kg) a suscité des symptômes
modérés d'inhibition cholinestérasique (douleurs épigastriques et
sueurs) en l'espace de 20 minutes. Les sujets ont complètement
récupéré dans les 2 heures suivant un traitement par le sulfate
d'atropine.
En cas d'exposition excessive d'origine professionnelle au
carbaryl, on observe des symptômes légers bien avant qu'une dose
dangereuse ne soit absorbée, ce qui explique que les cas graves
d'intoxication professionnelle par le carbaryl soient rares. Lors
des épandages agricoles, l'exposition percutanée peut être
importante. Cependant on n'observe en général aucun effet irritant
local, encore que l'on ait décrit des éruptions cutanées à la suite
l'éclaboussures accidentelles de carbaryl en formulation liquide.
Les données concernant les effets du carbaryl sur le nombre de
spermatozoïdes et la modification de leur morphologie chez les
travailleurs de l'industrie sont contradictoires. Aucun effet
indésirable sur la reproduction n'a été signalé.
L'indicateur biologique le plus sensible de l'exposition au
carbaryl est l'apparition de 1-naphtol dans les urines et la
diminution de l'activité cholinestérasique du sang. On peut donc
utiliser la concentration urinaire du 1-naphtol comme indicateur
biologique à condition qu'il n'y ait pas de 1-naphtol sur le lieu de
travail. Lors de certains cas d'exposition professionnelle, on a
constaté que 40% des échantillons d'urine contenaient plus de 10 mg
de 1-naphtol total par litre. Dans un cas d'intoxication aiguë, on
en a trouvé 31 mg/litre d'urine. On considère qu'il y a danger à
partir de 10 mg/litre et que les symptômes apparaissent à partir de
30 mg de 1-naphtol par litre d'urine (Fiche d'information sur le
carbaryl, OMS, 1973, VBC/DS/75.3).
La mesure de l'activité cholinestérasique peut constituer un
test très sensible pour la surveillance médical des travailleurs, à
condition que le dosage soit effectué peu après l'exposition.
2. Conclusions
On estime que le carbaryl est peu dangereux pour l'homme en
raison de sa faible tension de vapeur, de sa décomposition rapide,
de la désinhibition spontanée également rapide de la cholinestérase
et en raison du fait que les symptômes d'intoxication apparaissent
bien avant qu'une dose dangereuse ne se soit accumulée dans
l'organisme. On ne dispose pas encore de bonnes études de
cancérogénicité qui satisfassent aux normes actuelles en la matière.
2.1 Exposition de la population générale
Les quantités résiduelles de carbaryl qui demeurent dans les
denrées alimentaires et l'eau de boisson après l'utilisation normale
de cet insecticide en agriculture, sont très inférieures à la dose
journalière acceptable (DJA) (0,01 mg/kg de poids corporel/jour) et
il est improbable qu'elles puissent constituer un risque pour la
santé de la population dans son ensemble.
2.2 Sous-groupes de population exposés à un risque élevé
Lorsqu'on utilise du carbaryl à des fins de santé publique,
soit au domicile soit sur des aires de loisir, il y a un risque
d'exposition excessive si l'on ne suit pas les règles concernant son
emploi.
2.3 Exposition professionnelle
En faisant respecter des méthodes de travail raisonnables et
notamment un certain nombre de précautions de sécurité et des
mesures de protection individuelle, avec une surveillance
convenable, il n'existe aucun risque qui puisse résulter d'une
exposition professionnelle au cours de la fabrication, de la
formulation et de l'épandage du carbaryl. Les produits non dilués
doivent être manipulés avec de grandes précautions car toute faute
de manipulation peut entraîner une contamination cutanée. Sur le
lieu de travail, la concentration atmosphérique ne doit pas dépasser
5 mg/m3.
2.4 Effets sur l'environnement
Le carbaryl est toxique pour les abeilles et les lombrics. On
ne doit pas procéder à son épandage pendant la floraison.
En utilisation normale, le carbaryl ne devrait pas poser des
problème écologique. Il est adsorbé en grande partie sur les
particules de sol et il ne passe pas facilement par lessivage dans
les eaux souterraines. Il subit une décomposition rapide dans
l'environnement et n'est donc pas persistant. L'emploi de carbaryl
ne devrait pas entraîner d'effets nocifs à court terme sur
l'écosystème.
3. Recommandations
* La manipulation et l'épandage du carbaryl doivent s'effectuer
en observant les précautions qui s'imposent pour tous les
pesticides. On suivra rigoureusement les instructions
d'utilisation qui figurent sur l'emballage.
* La fabrication, la formulation, l'emploi et l'élimination du
carbaryl doivent se faire avec toutes les précautions voulues
pour réduire au minimum la contamination de l'environnement.
* Les travailleurs qui sont soumis à une exposition régulière
doivent faire l'objet d'un contrôle médical périodique.
* La chronologie des épandages de carbaryl doit être réglée de
manière à éviter tout effet sur les espèces non visées.
* Il importe d'effectuer des études de cancérogénicité
satisfaisant aux normes modernes.
RESUMEN Y EVALUACION, CONCLUSIONES Y RECOMENDACIONES
1. Resumen y evaluación
1.1 Identidad, propiedades y métodos analíticos
El 1-naftil N-metil carbamato, derivado del ácido carbámico,
se conoce por el nombre común de carbarilo. El producto de calidad
técnica es un sólido cristalino blanco, de baja volatilidad y escasa
hidrosolubilidad, y estable a la luz y al calor, pero fácilmente
hidrolizable en medio alcalino. La FAO ha establecido una
especificación mínima de pureza del 98%, con un límite de impureza
del 0,05% para el ß-naftil N-metil carbamato.
Para analizar el carbarilo y sus metabolitos, se pueden
utilizar numerosas técnicas, como la cromatografía de capa fina, la
espectrofotometría, la cromatografía de gases, la cromatografía
líquida de alta presión y la espectrometría de masas con ionización
química. Pueden alcanzarse límites de detección inferiores a un
nanogramo, y la recuperación supera por lo general el 80%.
1.2 Producción y usos
El carbarilo se viene utilizando desde hace unos 30 años como
insecticida de contacto y de ingestión, posee algunas propiedades
sistémicas y permite combatir una amplia serie de plagas. La planta
de fabricación más importante está en los Estados Unidos. Más de 290
fabricantes transforman el carbarilo para integrarlo en más de 1500
productos diferentes.
1.3 Transporte, distribución y transformación en el medio ambiente
En la mayoría de las situaciones, el carbarilo no persiste en
el entorno. En el agua, su semivida por hidrólisis depende de la
temperatura, del pH y de la concentración inicial, y varía entre
varios minutos y varias semanas. El principal producto de
degradación es el 1-naftol.
Se ha estudiado la acumulación de carbarilo en peces de agua
dulce, y expresada como factor de bioconcentración en el medio
acuático se ha cifrado en valores comprendidos entre 14 y 75. El
carbarilo es adsorbido más fácilmente en los suelos que poseen un
alto contenido orgánico que en los suelos arenosos. A un ritmo
habitual de aplicación conforme con unas "prácticas agrícolas
adecuadas", la disipación es rápida, con una semivida de entre 8
días y un mes en condiciones normales. Ocasionalmente, por efecto de
la lluvia y del cultivo agrícola, el carbarilo es transportado de la
superficie al subsuelo (a un metro de la superficie).
El carbarilo contamina la vegetación durante el rociamiento o
por desplazamiento hasta las plantas a través del suelo contaminado.
La degradación del carbarilo en el entorno depende del grado de
volatilización, fotodescomposición y degradación química y
microbiana que se produzca en el suelo, el agua y las plantas. La
descomposición es más rápida cuando el clima es cálido.
1.4 Niveles ambientales y exposición humana
La principal fuente de ingestión de carbarilo entre la
población general son los alimentos.
Los residuos hallados en muestras de la ingesta alimentaria
total son relativamente escasos, pues oscilan entre cantidades
ínfimas y 0,05 mg/kg. En los Estados Unidos, la ingesta diaria
durante los primeros años de aplicación de carbarilo fue de 0,15
mg/día por persona (lo contenían el 7,4% de los compuestos); la
cifra se redujo a 0,003 mg/día por persona en 1969 (sólo lo
contenían un 0,8% de los compuestos). Durante el periodo de
aplicación se encuentra carbarilo ocasionalmente en las aguas
superficiales y los embalses.
La población general puede estar expuesta al carbarilo durante
las operaciones de lucha contra las plagas, en su vivienda o en
zonas recreativas.
Los trabajadores pueden estar expuestos al carbarilo durante su
fabricación, formulación, envasado, transporte y almacenamiento, así
como durante y después de su aplicación. Las concentraciones
halladas en la atmósfera del lugar de trabajo durante su producción
oscilaron entre < 1 mg/m3 y 30 mg/m3. Si las medidas de
protección son inadecuadas, los trabajadores industriales y
agrícolas pueden sufrir exposiciones cutáneas importantes.
1.5 Cinética y metabolismo
El carbarilo es absorbido rápidamente por los pulmones y el
tracto digestivo. En voluntarios se observó una absorción cutánea
del 45% a las 8 horas de aplicar una dosis del producto diluida en
acetona. Sin embargo, los datos referentes a la penetración cutánea
in vitro y a la toxicidad indican que la absorción cutánea se
produce por lo general a una velocidad mucho menor.
Las principales vías metabólicas del carbarilo son la
hidroxilación del anillo y la hidrólisis. El resultado son numerosos
metabolitos que experimentan conjugación, con formación de sulfatos,
glucurónidos y mercapturatos hidrosolubles, que se excretan por la
orina. Como resultado de la hidrólisis se forman 1-naftol, dióxido
de carbono y metilamina. La hidroxilación da lugar a
4-hidroxicarbarilo, 5-hidroxicarbarilo, N-hidroximetilcarbarilo,
5-6-dihidro-5-6-dihidroxicarbarilo y 1,4-naftalendiol. El metabolito
principal en el hombre es el 1-naftol.
En condiciones normales de exposición, el carbarilo rara vez se
acumula en los animales. El producto se excreta principalmente por
la orina, debido a que la detoxificación del producto de su
hidrólisis, el 1-naftol, se produce principalmente por
transformación en conjugados hidrosolubles. La circulación
enterohepática de los metabolitos del carbarilo es también
considerable, sobre todo tras su administración oral.
El producto de la hidrólisis, el ácido carbámico N-naftol, se
descompone espontáneamente en metilamina y dióxido de carbono. La
posterior desmetilación de la metilamina da lugar a dióxido de
carbono y formato, siendo este último excretado principalmente por
la orina.
Un pequeño porcentaje de las dosis de carbarilo absorbidas
aparecen como metabolitos en la saliva y la leche.
1.6 Efectos en otros organismos en el medio ambiente
En los crustáceos, las CL50 oscilan entre 5 y 9 µg/litro
(pulgas de agua, mísidos), 8 y 25 µg/litro (anfípodos), y 500 y 2500
µg/litro (cangrejos de río). El margen de sensibilidad es parecido
en los insectos acuáticos; Plecoptera y Ephemeroptera (gusarapa y
cachipollas) son los grupos más sensibles. Los moluscos son menos
sensibles, situándose su CE50 en niveles de unos pocos mg/litro.
En cuanto a los peces, la mayoría de las CL50 están comprendidas
entre 1 y 30 mg/litro; el grupo más sensible son los salmónidos.
En el caso de las aves la toxicidad aguda es baja. La DL50
para las aves de caza, sean acuáticas o terrestres, es > 1000
mg/kg. El ave más sensible analizada es el mirlo de alas rojas
(DL50 = 56 mg/kg). En zonas forestales rociadas con 1,1 kg de
carbarilo por hectárea no se observó ningún efecto en las aves
locales.
El carbarilo es muy tóxico para las abejas y las lombrices de
tierra. La DL50 oral para las primeras es de 0,18 µg/abeja
(aproximadamente 1-2 mg/kg).
Hay indicios de que el carbarilo puede alterar temporalmente la
composición de especies en los ecosistemas tanto terrestres como
acuáticos. Por ejemplo, un estudio reveló que en determinadas
colonias de invertebrados terrestres sus efectos pueden persistir
durante por lo menos 10 meses tras una sola aplicación.
1.7 Efectos en animales de experimentación y en sistemas de prueba
in vitro
La toxicidad aguda, expresada como DL50, varía
considerablemente según las especies, fórmulas y vehículos. Las
estimaciones de la DL50 oral en la rata oscilan entre 200 y 850
mg/kg. Los gatos son más sensibles, pues presentan una DL50 de 150
mg/kg. Los cerdos y los monos son menos sensibles, pues su DL50 es
> 1000 mg/kg.
La exposición a 792 mg de ingrediente activo de carbarilo
nebulizado, que es la máxima concentración a la que se llegó durante
4 horas, provocó la muerte de una de cinco ratas hembra. Aerosoles
de carbarilo a concentraciones de 20 mg/m3 dieron lugar a una
disminución de la actividad colinesterasa (ChEA) en gatos durante
exposiciones únicas de 4 horas, pero esa misma concentración no tuvo
efectos observables en ratas.
El carbarilo produce leves irritaciones oculares y tiene un
potencial de sensibilización escaso o nulo. Estudios prolongados
revelaron un NOEL de 10 mg/kg de peso corporal (200 mg/kg ingesta
alimentaria) en las ratas, y de 1,8 mg/kg de peso corporal (100
mg/kg dieta) en los perros. El NOEL por inhalación prolongada es de
0,16 mg/m3 en los gatos. El potencial de acumulación de carbarilo
es bajo.
1.7.1 Reproducción
Se ha demostrado que el carbarilo tiene efectos adversos sobre
la reproducción y el desarrollo perinatal en diversas especies de
mamíferos. Los efectos sobre la reproducción comprenden problemas de
infertilidad, una disminución del tamaño de las camadas y una
reducción de la viabilidad postnatal. Los efectos tóxicos sobre el
desarrollo observados son un aumento de la mortalidad in utero,
una disminución del peso del feto y la aparición de malformaciones.
Salvo en un reducido número de estudios, todos los efectos adversos
sobre la reproducción y el desarrollo se observaron sólo a dosis
manifiestamente tóxicas para la madre, y en varios casos ésta
resultó ser más sensible al carbarilo que su prole. Entre los
efectos tóxicos para la madre cabe citar la letalidad, una
disminución del crecimiento y la distocia. Los datos disponibles
indican que los procesos de reproducción y desarrollo de los
mamíferos no son especialmente sensibles al carbarilo en comparación
con la susceptibilidad del organismo adulto.
1.7.2 Mutagenicidad
Se ha evaluado la posible mutagenicidad del carbarilo mediante
diversas pruebas in vitro e in vivo, empleando para ello
bacterias, levadura, plantas, insectos y mamíferos, y analizando
diversos puntos finales.
Según los datos disponibles, el carbarilo no es lesivo para el
ADN. No se ha notificado ningún dato que confirme que ha habido
inducción de la recombinación mitótica, conversión génica o síntesis
imprevista de ADN en procariotas (H. influenzae, B. subtilis) y
eucariotas ( S. cerevisiae, A. nidulans, linfocitos humanos en
cultivo, y hepatocitos de rata) in vitro.
Se obtuvieron resultados negativos en las pruebas de detección
de mutaciones génicas en un gran número de ensayos realizados con
bacterias, salvo en dos casos. En varios estudios de mutagenicidad
del carbarilo llevados a cabo con células de mamífero in vitro, se
obtuvo sólo un resultado positivo equívoco en un estudio de células
en cultivo. Ese estudio, sin embargo, presentaba varias deficiencias
y sus resultados no han sido confirmados en estudios comparables.
Se han notificado lesiones cromosómicas a altas dosis de
carbarilo en células humanas y de rata y hámster in vitro, así
como en plantas. No se han observado efectos de ese tipo en pruebas
in vivo con mamíferos, ni siquiera a dosis de hasta 1000 mg/kg.
Se ha mostrado que el carbarilo altera el mecanismo de las
fibras del huso en células de plantas y mamíferos in vitro. Es
dudoso el interés de realizar ensayos con plantas para extrapolar
sus resultados al hombre.
Cabe concluir que los datos disponibles no corroboran la
suposición de que el carbarilo plantea un riesgo de inducción de
cambios génicos en las células somáticas o germinales del hombre.
El producto nitrosado del carbarilo, el N-nitrosocarbarilo,
puede inducir fenómenos de recombinación mitótica y conversión
génica en los procariotas (H. influenzae, B. subtilis) y
eucariotas (S. cerevisiae) in vitro, y arroja resultados positivos
en las pruebas in situ con E. coli.
Además, resultados experimentales indican que el
N-nitrosocarbarilo se une al ADN, causando la ruptura de los
enlaces alcalisensibles y de las cadenas simples.
No se ha demostrado que el nitrosocarbarilo sea clastógeno in
vivo (médula ósea y células germinales), ni siquiera a dosis
tóxicas elevadas.
1.7.3 Carcinogenicidad
Se han realizado numerosos estudios en la rata y el ratón para
determinar los posibles efectos carcinógenos del carbarilo. Los
resultados de la mayoría de esos estudios fueron negativos, pero se
trata de trabajos realizados hace muchos años y que no satisfacían
los criterios actuales. Se están realizando nuevos estudios
conformes con los actuales criterios en ratones y ratas.a En la
más reciente evaluación del CIIC (CIIC, 1987) se llegaba a la
conclusión de que no había información sobre el cáncer en el hombre
y de que los indicios de carcinogenicidad en animales de
experimentación eran insuficientes. No se podía clasificar el
carbarilo en lo que se refiere a su carcinogenicidad para la especie
humana (Grupo 3).
Se ha mostrado que el N-nitrosocarbarilo induce la aparición
de tumores localmente en la rata (sarcoma en el lugar de la
inyección o carcinoma de células escamosas del antro cardiaco al
administrarlo por vía oral). Teniendo en cuenta las transformaciones
químicas que sufre el carbarilo en el hombre, el riesgo de
carcinogenicidad por N-nitrosocarbarilo como consecuencia de la
exposición a esta sustancia puede considerarse insignificante.
1.7.4 Efectos en distintos órganos y sistemas
a) Sistema nervioso
Los efectos del carbarilo sobre el sistema nervioso se deben
principalmente a la inhibición de la colinesterasa y son por lo
general temporales. En unos estudios sobre los efectos en el sistema
nervioso central de ratas y monos se observó que la administración
oral de 10-20 mg/kg durante 50 días provocaba trastornos del
aprendizaje y del comportamiento en las ratas.
En un pequeño estudio realizado con cerdos, el carbarilo (150
mg/kg de peso corporal en la alimentación durante 72-82 días) tuvo
diversos efectos neuromusculares. Se observó una debilidad
reversible de las patas en pollos sometidos a altas dosis de
carbarilo. No se observaron signos de desmielinización en los cortes
de cerebro, nervio ciático o médula espinal examinados al
microscopio. Tampoco se notaron efectos de esa índole en estudios
prolongados realizados con roedores.
b) Sistema inmunitario
Se ha notificado que el carbarilo administrado in vivo a
dosis que producen claros signos clínicos tiene diversos efectos en
el sistema inmunitario. Muchos de los efectos descritos se
detectaron a dosis cercanas a la DL50. La mayoría de los estudios
llevados a cabo con conejos y ratones a dosis compatibles con la
supervivencia no han revelado efectos de importancia en el sistema
______________
a El IPCS aún no ha examinado esos estudios. La sociedad
encargada de realizarlos ha indicado que se observa un aumento
significativo de tumores a la dosis màs elevada en las dos
especies.
inmunitario. Sin embargo, varios de esos estudios adolecían de
incoherencia y a veces de contradicción patente entre los
resultados, lo cual no permite describir un mecanismo inmunotóxico
bien definido.
c) Sangre
Se ha señalado que el carbarilo afecta a la coagulación, pero
existe cierta controversia en cuanto al tipo de efecto. En
eritrocitos de oveja con déficit de glucosa-6-fosfato
deshidrogenasa, el carbarilo provocó un aumento dosis-dependiente de
la formación de metahemoglobina (Met-Hb). La albúmina sérica humana
reaccionó in vitro con el grupo éster del carbarilo. El carbarilo
se une a los aminoácidos libres de la sangre.
d) Hígado
Se han señalado trastornos del metabolismo de los carbohidratos
y de la síntesis de proteínas, así como de la función de
detoxificación en el hígado de ciertos mamíferos. El carbarilo es un
inductor ligero de la actividad de metabolización de medicamentos
que reside en los microsomas hepáticos. Se observa un acortamiento
del periodo de sueño inducido por el fenobarbital. Los niveles
hepáticos de citocromo P-450 y b5 aumentan. Los cambios del
metabolismo hepático son tal vez parcialmente responsables de la
triplicación de la DL50 por carbarilo en ratas tratadas
previamente con dicho compuesto.
e) Función gonadotrópica
Se ha señalado que el carbarilo estimula la función
gonadotrópica de la hipófisis de la rata.
1.7.5 Mecanismo principal de toxicidad
El carbarilo inhibe la acción de la colinesterasa, efecto que
depende de la dosis y es fácilmente reversible. No se observó ningún
fenómeno de "maduración" de la colinesterasa carbamilada. Todos los
metabolitos identificados del carbarilo son
considerablemente menos activos que éste como inhibidores de la
colinesterasa.
1.8 Efectos en el hombre
El carbarilo es fácilmente absorbido por inhalación y por vía
oral, y menos fácilmente por vía cutánea. Como su principal
mecanismo de acción es la inhibición de la colinesterasa (ChE), en
el cuadro clínico de la intoxicación predominan los síntomas de esa
inhibición, tales como: hipersecreción bronquial, aumento de la
sudación, de la salivación y del lagrimeo; pupilas puntiformes,
broncoconstricción, espasmos abdominales (vómitos y diarrea);
bradicardia; fasciculación de los músculos finos (que también afecta
en los casos graves al diafragma y a los músculos respiratorios);
taquicardia; cefalea, vértigo, ansiedad, confusión mental,
convulsiones y coma; y depresión del centro respiratorio. Los signos
de intoxicación aparecen rápidamente tras la absorción y desaparecen
pronto al cesar la exposición.
En estudios controlados realizados con voluntarios se observó
una buena tolerancia a dosis únicas inferiores a 2 mg/kg. Una dosis
única de 250 mg (2,8 mg/kg) provocó síntomas moderados de inhibición
de la ChE (dolor epigástrico y sudación) al cabo de 20 minutos. La
recuperación completa se produjo tras dos horas de tratamiento con
sulfato de atropina.
En los casos de sobreexposición ocupacional al carbarilo, se
observan síntomas leves mucho antes de que llegue a absorberse una
dosis peligrosa; de ahí que raras veces se produzcan casos graves de
intoxicación ocupacional por este compuesto. Durante las
aplicaciones agrícolas, la exposición cutánea puede desempeñar un
papel importante. No suelen observarse efectos irritantes locales,
pero se ha descrito la aparición de exantema cutáneo tras la
salpicadura accidental con formulaciones de carbarilo.
Los datos acerca de los efectos del carbarilo sobre el número y
la morfología de los espermatozoides en trabajadores industriales
son discordantes. No se han notificado efectos adversos sobre la
reproducción.
El indicador biológico más sensible de la exposición al
carbarilo es la aparición de 1-naftol en la orina y una disminución
de la actividad ChE de la sangre. Si no hay 1-naftol en el entorno
de trabajo, los niveles urinarios de este producto se pueden emplear
como indicador biológico. Durante la exposición ocupacional, el 40%
de las muestras de orina contenían más de 10 mg de 1-naftol/litro.
En un caso de intoxicación aguda se hallaron 31 mg/litro en la
orina. El nivel de riesgo es > 10 mg/litro, y el nivel de aparición
de síntomas, 30 mg 1-naftol/litro de orina (hoja de datos sobre el
carbarilo, OMS, 1973, VBC/DS/75.3).
El análisis de la actividad ChE puede ser una prueba de gran
sensibilidad a efectos de vigilancia, siempre que se realice poco
después de la exposición.
2. Conclusiones
Se considera que los riesgos que plantea el carbarilo para el
ser humano son escasos, debido a su baja presión de vapor, a su
rápida degradación y a la recuperación espontánea y rápida de la
colinesterasa inhibida, así como al hecho de que los síntomas
aparecen normalmente mucho antes de que pueda haberse acumulado en
el organismo una dosis peligrosa. Aún no se dispone de estudios
satisfactorios sobre la carcinogenicidad conformes con los criterios
actuales.
2.1 Exposición de la población general
Los niveles de residuos de carbarilo presentes en los alimentos
y el agua de bebida, resultantes de su uso normal en la agricultura,
son muy inferiores a la ingesta diaria admisible (IDA) (0,01 mg/kg
de peso corporal al día), y difícilmente pueden representar un
riesgo para la salud de la población general.
2.2 Subpoblaciones de alto riesgo
El uso del carbarilo con fines de salud pública en la vivienda
o en zonas recreativas puede ser causa de sobreexposición si se
descuidan las normas aconsejadas para su aplicación.
2.3 Exposición ocupacional
Si se vela por el cumplimiento de unas prácticas laborales
razonables, en particular las medidas de seguridad, la protección
del personal y una supervisión adecuada, la exposición ocupacional
durante la fabricación, formulación y aplicación de carbarilo no
planteará riesgos. Las concentraciones no diluidas se deben manejar
con sumo cuidado, dado que unas prácticas laborales incorrectas
pueden ser causa de contaminación cutánea. Las concentraciones en el
aire del lugar de trabajo no deberían superar los 5 mg/m3.
2.4 Efectos en el medio ambiente
El carbarilo es tóxico para las abejas y las lombrices. No
debería aplicarse a los cultivos durante la floración.
Si se emplea normalmente, el carbarilo no debería suscitar
preocupación desde el punto de vista del medio ambiente. El
carbarilo se adsorbe en buena parte en el suelo y no se lixivia
fácilmente hacia las aguas subterráneas. Se degrada rápidamente en
el entorno, por lo que no tiende a persistir. El uso de carbarilo no
debería tener efectos nocivos a corto plazo en el ecosistema.
3. Recomendaciones
* La manipulación y la aplicación del carbarilo se deberían
realizar adoptando las precauciones previstas para todo
plaguicida y siguiendo minuciosamente las instrucciones
suministradas en el envase para emplear correctamente el
producto.
* Deberían controlarse cuidadosamente la fabricación, la
formulación, el uso y la eliminación del carbarilo, con objeto
de reducir al mínimo la contaminación del medio ambiente.
* Los trabajadores expuestos regularmente al producto deberían
someterse a chequeos periódicos.
* Debería determinarse la época de aplicación del carbarilo de
manera que no afecte a las especies que no se desee combatir.
* Deberían realizarse estudios de carcinogenicidad que satisfagan
los criterios actuales.
6.4 Elimination and excretion in expired air, faeces, urine,
milk, and eggs
The elimination of metabolized carbaryl is rapid, and
accumulation in animals seems unlikely, under normal exposure
conditions. Carbaryl is generally excreted entirely within 24-96 h
of intake. Elimination takes place mainly through the urine, faeces,
and respiration, and, to a lesser extent, through the milk of dairy
animals and the eggs of poultry.
Carbaryl is mainly excreted as its product of hydrolysis,
1-naphthol, which is detoxified to water-soluble glucuronide
(Carpenter et al., 1961), and sulfate (Whitehurst et al., 1963),
and only in trace amounts as 1-naphthol or unchanged.
The average percentage of metabolites recovered after orally
administered doses of 1-naphthyl 14C, methyl 14C, and carbonyl
14C carbaryl to rats was 94% over a 7-day period (Knaak et al.,
1965). Only residues from carbaryl methyl 14C were detected after
that, since the methyl moiety is incorporated in tissue (2-3%).
Liberated naphthol is conjugated and excreted, while the liberated
carbonyl groups are disposed of as respiratory14CO2 (Knaak
et al., 1965). Forty-seven to 57% of the metabolites excreted
retain the intact C-O-C(O)N-C structure, indicating a nonhydrolytic
pathway for carbaryl. Monkeys and pigs (2 young females) excreted
carbaryl as a conjugate of intact carbaryl and 4-hydroxycarbaryl,
mainly glucuronides. Sheep also excreted 1-naphthyl glucuronide and
sulfate and 4 (methylcarbamoyloxy)1-naphthylsulfate (Knaak et al.,
1968).
Myers (1977) studied the correlation between the amount of
carbaryl ingested and urinary metabolites levels. Metabolites were
measured as three groups:
I. naphthyl sulfate;
II. free and sulfated 5,6-dihydrodihydroxycarbaryl
and dihydrodihydroxynaphthol;
III. other conjugated (glucuronides) of naphthol,
5,6-dihydrodihydroxycarbaryl, and
5,6 dihydrodihydroxynaphthol.
The amounts in groups I and II were 25.5-36.5% of the dose. When all
three groups of metabolites were analysed, the amounts of
metabolites for the same period represented 41.5-52.7% of the dose.
Naphthol was the major metabolite found in Group III (mean 75% of
all metabolites of the group). A good correlation was found between
the amount ingested and urinary metabolites.
Rats treated orally with naphthyl14C carbaryl (2.3 µci)
excreted 90% of the administered radioactivity in the urine within 3
days. During the 72-h sampling period, the faeces contained only
2-5% of the radioactivity (Lucier et al., 1972).
Eighty per cent of 5 mg of 14C naphthyl carbaryl, given
intraperitoneally to rats, (see section 6.3.2) was eliminated in the
24-h urine and 10% in the faeces (Bend et al., 1971). Enzymatic
hydrolysis and reverse isotope dilution showed that 10% of the dose
was excreted as 1-naphthyl-glucoronide, 5%, as 1-naphthylsulfate,
3%, as conjugates of 4-hydroxycarbaryl, 5%, as 1-naphthylsulfate,
3%, as conjugates of 4-hydroxycarbaryl, and 5% of 5-hydroxycarbaryl.
About 2% of the carbaryl appeared unchanged in urine. Other
radioactive metabolites were not identified.
Following a single, ip injection in albino rats of both sexes,
of methyl 14C and carbonyl 14C carbaryl at 30 mg/kg, 75-80% was
recovered after 48 h in the expired air and urine. Liberated by
hydrolysis, N-methyl carbamic acid was spontaneously decomposed to
methylamine and carbon dioxide (43.5%). The methylamine moiety was
later demethylated to 14CO2, which was eliminated in the expired
air and 14C formate (9.2%), which was excreted in the urine
(Hassan et al., 1966).
In the study of Nye & Dorough (1976), 2.5% of the
endotracheally administered carbaryl dose was exhaled as
14C-carbon dioxide. More than 90% of the dose was eliminated in
the urine during 3 days. The faeces contained 2-5%.
A comparative study on the excretion of carbaryl after a
7.5 µmol/kg, ip dose in rats was reported by Krishna & Casida (1966)
who used three types of labelled carbaryl (Table 32).
Table 32. Fate of 14carbon in male Sprague-Dawley rats
after ip administration of carbaryla
Labelled position Administered 14carbon recovered (%)
Expired 14CO2 Urine Faeces
0-24 h 24-48 h
Carbonyl 14C 24.5 62.1 2.4 2.1
Methyl 14C 12.3 54.6 3.4 3.9
Naphthyl 14C 0.2 74.2 2.3 8.9
Hydrolysis product < 0.1 86.4 3.3 1.4
naphthol 14C
aFrom: Krishna & Casida (1966).
Biliary excretion of carbaryl was studied in bile-duct
cannulated rats. Naphthyl 14C and methyl 14C carbaryl (50 µg)
were administered intravenously to rats (see section 6.3.2). During
the first 2 h of collection, 90% of the total biliary radioactivity
was eliminated (Bend et al., 1971). After oral application of
0.01 mg/kg naphthyl-14C carbaryl to rats, 37.5% was excreted via
the bile in the first 3 h and 45.4% (cumulative percentage) of the
dose was eliminated within 48 h. Faecal elimination was 1.4% and
urine elimination was 42.3% (Marshall et al., 1979). The results
of Struble et al. (1983a), who used higher doses of carbaryl (1.5,
31, and 300 mg/kg) on male Sprague-Dawley rats were similar. Twelve
hours after administration, 15-46% of the 14C was excreted in the
bile, 10-40% in the urine, and less than 1% was eliminated in
faeces. Three metabolites were identified from the bile, the major
one being 5-6-dihydro-5-6-dihydroxycarbaryl glucoronide (12-18% of
the biliary 14C).
On the basis of a study on 50, male, Sprague-Dawley rats,
Borzelleca & Skalsky (1980) reported that carbaryl metabolites in
the blood were also present in the saliva, at similar
concentrations.
Leeling & Casida (1966) studied carbaryl metabolites in the
urine of one male and one female New Zealand White rabbit. The
ether-extractable metabolites found in the urine were:
N-hydroxymethyl carbaryl, 4-hydroxycarbaryl, 5-hydroxycarbaryl,
5,6-dihydro-5,6-dihydroxycarbaryl, 5,6-dihydro-5,6-trihydroxy-1-
naphthol, and 1-naphthol.
Thirty-five, adult, female American cockroaches ( Periplaneta
americana) were injected through the central abdominal wall with
5 µg of carbaryl-carbonyl 14C, carbaryl methyl 14C, or 2 µg
carbaryl naphthyl 14C. Metabolites similar to those appearing in
rat liver microsomes were formed. About 19% of the radioactivity of
carbaryl carbonyl 14C was eliminated as 14C-carbon dioxide in
24 h (Dorough & Casida, 1964).
Excretion of carbaryl in milk was studied by Baron (1968).
Application of a total dose of 2 g carbonyl carbaryl 14C to a
lactating cow resulted in approximately 1% of radioactive residues
in the milk. In skim milk, 87% of these residues were water-soluble
and 13% chloroform-soluble. About 90-95% of the14C radioactivity
in the soluble component was removed after crystallization of
lactose.
The metabolism of carbaryl naphthyl 14C was studied in one
lactating Jersey cow (Dorough, 1967). Two consecutive single
treatments at 6-day intervals with 0.25 mg/kg body weight (total
125 mg) and one single dose of 3.05 mg carbaryl naphthyl 14C/kg
body weight added to the feed, resulted in radiolabelled residues in
the milk for as long as 60 h after treatment. The first sample of
milk, taken after 6 h, showed maximum concentrations of chloroform
extractables, water-soluble, and unextractable, residues in whole
milk in the range of 0.063-0.95 mg/litre. Residues of only a
slightly lower magnitude were detected in the 12-h milk samples and
rapidly declined in 24-h samples. Thirty per cent of the residues in
the 6-h sample was a chloroform-extractable metabolite, tentatively
identified as 5,6-dihydro-5,6-dihydroxy-1-naphthyl-
N-methylcarbamate. About 0.35% of the total dose was detected in
the milk in both treatments, 70% in the urine, and 11% in the faeces
in 0.25 mg/kg treatment, and 58% in the urine and 15% in the faeces
in the 3.05 mg/kg treatment. The highest levels in the tissues of
the cow, killed on an unspecified day after treatment, were found in
the liver, kidney, and ovaries.
A single lactating cow was treated orally with a daily total
dose of 518 mg 14C-carbaryl (0.77 mg/kg body weight), given in two
doses, 12 h apart. Results are shown in Table 33 (Dorough, (1969).
Fifty-nine per cent of the radioactivity from a14C-labelled oral
dose of carbaryl administered to a cow was recovered from the urine
within the first 24 h (Saini & Dorough, 1978).
The passage of14C into the milk and its presence in the
suckling neonates has been studied by whole-body autoradiography in
rats fed 14C-methyl carbaryl. The highest concentrations in
newborn offspring, after 48 h, were found in the stomach contents,
the liver, the hair, and the bone marrow (Benard et al., 1979).
Table 33. Distribution and excretion of total-14C equivalents in
milk, urine, faeces, and tissues, 24 h after one-day oral
application of carbaryl in a lactating cow
Sample mg/kg Dose recovered
(%)
Milk 0.04 0.09
Urine 22.4 58.64
Faeces 0.47 1.65
Tissues:
- liver 0.098 0.21
- kidney 0.382 0.17
- leg muscle 0.027 nil
- fat 0.014 nil
A Saanen goat was treated orally with 1.34 mg carbaryl carbonyl
14C/kg. The excretion of radioactivity in the urine was 7.4% at
2 h, 24% at 4 h, and 45% at 24 h. In total, 47% of the radioactivity
was excreted the urine. In the milk, the peak was 0.9 mg/litre at
8 h, dropping to 0.008 mg/litre 60 h after the administration of
carbaryl (Dorough & Casida, 1964).
The excretion of carbaryl was studied in hens. Carbaryl-
carbonyl14C (10 mg/kg body weight) was given orally to Leghorn
hens. Fifty per cent of the 14C was expired within 48 h. When
carbarylnaphthyl 14C was applied at the same dose, no
radioactivity was detected in the expired gases. The urine was the
primary route of excretion. 14Carbon remaining in the carcass,
48 h after dosing, accounted for 1.4 and 7.1% of the dose given as
the ring-labelled and carbonyl-labelled compound, respectively
(Paulson & Feil, 1969). The main metabolites found in the urine of
hens were: 1-naphthol, 1-naphthyl glucoronide, and sulfate esters of
1-naphthol, 4-hydroxycarbaryl, and 5-hydroxycarbaryl. Conjugates
with other metabolites were also found in small quantities (Paulson
et al., 1970). Mature hens were given a single oral dose of
carbaryl naphthyl 14C at 10 mg/kg body weight. Eggs collected for
12 days contained a total dose of 0.33% of the 14C administered
(Paulson & Feil, 1969).
The fate of naphthyl-1-14C carbaryl in laying chickens was
studied by Andrawes et al. (1972). Chickens (3 White Leghorn in
each group) were fed 7, 12, or 70 mg/kg carbaryl in the feed twice
daily for 14 days. They had been pretreated for 17 days with the
same doses of nonlabelled carbaryl in feed. Residues reached maximum
levels within 1 day in excrement, 2 days in egg white, and 6-8 days
in egg yolk. After the end of dosing, the half-life of 14C
residues was <1 day in the excrement and egg white, 2-3 days in the
egg yolk, and 5 days in the carcass. Metabolites and carbaryl found
in the eggs of hens, fed 70 mg carbaryl/kg averaged in total 19.7 µg
of 14C carbaryl equivalent per egg (16.3 µg in yolk and 3.4 µg in
white), collected between the 9th and the 14th day of dosing.
Naphthyl-l-sulfate was the largest single component of egg residues
accounting for the higher concentration of residues in the egg yolk.
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
7.1 Microorganisms
7.1.1 Soil microorganisms
Studies of the effects of insecticides on soil microorganisms
are complex and subject to many sources of variation. It is
therefore difficult to assess the practical significance of the
results of such studies.
Varsheny & Gaur (1972) studied the impact of carbaryl (as
Sevin) on soil fungi. They determined the size of the fungal
population and the species composition of the fungal community after
plating out extracts of soil samples on various media. The authors
suggested that, at very high rates of addition to the soil (up to
5000 mg/kg), there was evidence of a change in species composition
and an increase in the total fungal population.
In laboratory cultures of saltmarsh protozoans (dominated by
Euplotes sp.), Weber et al. (1982) found that both carbaryl and
1-naphthol caused dose-related mortality. For both compounds,
mortality at 1 mg/litre was about 50%. There was almost 100%
mortality at 10 mg/litre.
Carbaryl inhibited the growth of cultures of Bacillus subtilis
(DeGiovanni & Donnelly, 1968).
7.1.2 Aquatic microorganisms
Edmiston et al. (1985) conducted a detailed study of the
effects of carbaryl on Paramecium multimicronucleatum. The 24-h
LC50 was 28 µg/litre in a static plate test. Effects on the oxygen
uptake of cultures and effects on the cell surface were also
reported, but these results were mostly obtained using
concentrations in excess of the 24-h LC50, and must be of limited
value.
Murray & Guthrie (1980) studied the responses of bacterial
cultures originating from Lake Houston. The respiratory rate of
bacterial cultures was increased at water temperatures of between
23-33 °C. Plate counts of bacterial colonies suggested that carbaryl
treated cultures contained more bacteria than control cultures on
most days in a 26-day study.
7.2 Aquatic organisms
Aquatic toxicity studies have been conducted in the laboratory
and field to define the biological effects associated with acute and
long-term exposure to carbaryl and the degradation product,
1-naphthol, of aquatic vertebrates and invertebrates. More data are
available for freshwater organisms than for saltwater organisms. In
addition, actual and simulated field studies have been conducted to
assess the impact of carbaryl exposure on freshwater invertebrates.
7.2.1 Aquatic invertebrates
Studies on the toxicity of carbaryl for non-target aquatic
invertebrates have included investigations on molluscs, crustaceans,
and insects, concentrating on organisms of commercial importance,
organisms most likely to be exposed during a typical application of
carbaryl, or on standardized test species typically used in aquatic
toxicity tests. The acute and long-term laboratory studies under
various environmental conditions (pH, temperature, water hardness,
etc.) are briefly reviewed in Table 34.
Most of the studies on the toxicity of carbaryl for aquatic
invertebrates have been conducted under laboratory conditions and,
consequently, the results only yield data on the relative toxicity
of the substance. They do not reflect realistic exposure in the
environment. Most of the laboratory studies evaluate constant,
short-term (less than 1 week) exposure to carbaryl under static or
flow-through conditions. There are few long-term studies on the
effects of carbaryl on aquatic invertebrates. Exposures in the
environment are typically localized, at much lower concentrations
than in aquatic toxicity tests and are not constant.
The toxicity of carbaryl on the early development of the sea
urchin was studied by Hernandez et al. (1990). Developmental
stages with active cleavage and cellular mobilization (blastula and
gastrula) turned out to be more sensitive. The next two stages
(prism and pluteus) were less sensitive and EC50 values were 8-26
times higher. This effect may be connected with increased
detoxication processes by the cytochrome oxidase system.
Table 34. Acute toxicity of carbaryl for invertebrates
Organism Test Size/age Stat/ Temperature Hardness pH Parameterc Concentration Reference
material Flowa (°C) (mg/litre)b (µg/litre)
Crustacea
(Freshwater)
Water flea
Daphnia magna unknown stat 16 42 7.4 48-h LC50 5.6 Sanders et al. (1983)
Daphnia magna technical 96-h LC50 3280 Lejczak (1977a)
Daphnia magna unknown 48-h EC50 0.26 Rawash et al. (1975)
Daphnia magna technical 21-day MATC 1.5-3.3 Surprenant (1985c)
21-day NOEC 6.0
(survival)
Daphnia pulex technical stat 16 44 7.1 48-h LC50 6.4 Mayer & Ellersieck (1986)
Simocephalus unknown stat 10 44 7.1 48-h LC50 11 Johnson & Finley (1980)
serrulatus stat 16 44 7.1 48-h LC50 7.6
stat 21 44 7.1 48-h LC50 8.1
Ostracod
Cypridopsis
vidua unknown adult stat 21 272 7.4 48-h LC50 115 Mayer & Ellersieck (1986)
Sow bug
Asellus
brevicaudus technical adult stat 18 44 7.1 96-h LC50 280 Johnson & Finley (1980)
Table 34 (continued)
Organism Test Size/age Stat/ Temperature Hardness pH Parameterc Concentration Reference
material Flowa (°C) (mg/litre)b (µg/litre)
Crustacea
(Freshwater)
Amphipod
Gammarus
fasciatus unknown adult stat 21 44 7.1 96-h LC50 26 Johnson & Finley (1980)
Gammarus
lacustris unknown adult stat 21 44 7.1 96-h LC50 22 Johnson & Finley (1980)
Gammarus unknown adult stat 12 40 6.5 48-h LC50 8.13 Mayer & Ellersieck (1986)
pseudolimnaeus adult stat 12 40 7.5 48-h LC50 11.5
adult stat 12 40 8.5 48-h LC50 7.8
Gammarus pulex technical 48-h LC50 29 Bluzat & Seuge (1979)
Gammarus technical 96-h LC50 16 Sanders et al. (1983)
pseudolimnaeus
Gammarus technical 96-h LC50 13 Woodward & Mauck (1980)
pseudolimnaeus
Glass shrimp
Palaemonetes unknown adult stat 21 272 7.4 96-h LC50 5.6 Johnson & Finley (1980)
kadiakensis 25-31 mm stat 96-h LC50 120 Chaiyarach et al. (1975)
Table 34 (continued)
Organism Test Size/age Stat/ Temperature Hardness pH Parameterc Concentration Reference
material Flowa (°C) (mg/litre)b (µg/litre)
Crayfish
Procambarus sp. technical immature stat 12 42 7.5 96-h LC50 1900 Mayer & Ellersieck (1986
Procambarus unknown 60-70 mm stat 96-hLC50 2430 Chaiyarach et al. (1975)
simulans
Procambarus technical 96-h LC50 500 Cheah et al. (1978)
acutus
Crustacea
(Freshwater)
Prawn
Macrobrachium technical 96-h LC50 19 Omkar & Shukla (1985)
lamarrei
Upogebia wettable powderd 48-h EC50 90 Stewart et al. (1967)
pugettensis 1-naphthol 48-h EC50 4400
Callianassa wettable powderd 48-h EC50 80
californiensis 1-naphthol 48-h EC50 3500
Hemigrapsus wettable powderd 24-h EC50 710
oregonensis 1-naphthol 24-h EC50 74 200
Table 34 (continued)
Organism Test Size/age Stat/ Temperature Hardness pH Parameterc Concentration Reference
material Flowa (°C) (mg/litre)b (µg/litre)
Crustacea
(estuarine &
marine)
Blue crab
Callinectes unknown juvenile flow 29 27s 48-h LC50 320 Mayer (1987)
sapidus
Brown shrimp
Penaeus aztecus unknown juvenile flow 30 28s 48-h LC50 1.5
Pink shrimp
Penaeus
duorarum unknown juvenile flow 23 29s 48-h LC50 32
Grass shrimp
Palaemonetes unknown juvenile flow 23 29s 48-h LC50 28
pugio 3-4 days stat 24 28-30s 96-h LC50 22 Thursby & Champhon (1991)
Crustacea
(estuarine &
marine)
Table 34 (continued)
Organism Test Size/age Stat/ Temperature Hardness pH Parameterc Concentration Reference
material Flowa (°C) (mg/litre)b (µg/litre)
Mysid shrimp
Mysidopsis bahia unknown flow 96-h LC50 > 7.7 Nimmo et al. (1981)
Mysidopsis bahia technical 96-h LC50 6.7 Surprenant (1985b)
NOEC 5.1
Mysidopsis bahia 24 h flow 25 31s 96-h LC50 8.46 Thursby & Champhon (1991)
24 h stat 24 30s 96-h LC50 19
24 h flow 26 31-32s 28-day LC50 9.9
Ostracod
Cypretta kawatai technical 72-h LC50 1800 Hansen & Kawatski (1976)
Insects
Stonefly
Isogenus sp. unknown larva stat 7 35 7 96-h LC50 2.8 Mayer & Ellersieck (1986)
Pteronarcys technical 96-h LC50 4.8 Johnson & Finley (1980)
californica
Mosquito
Culex pipiens technical 24-h LC50 75 Rawash et al. (1975)
Table 34 (continued)
Organism Test Size/age Stat/ Temperature Hardness pH Parameterc Concentration Reference
material Flowa (°C) (mg/litre)b (µg/litre)
Insects
Midge
Chironomus technical 24-h LC50 7 Karnak & Collins (1974)
tentans
Chironomus technical 72-h LC50 5900 Hansen & Kawatski (1976)
tentans
Chironomus technical 48-h EC50 10 Sanders et al. (1983)
plumosus
Chironomus unknown larva stat 20 4 24-h LC50 106 Fisher & Lohner (1986)
riparius larva stat 20 6 24-h LC50 133
larva stat 20 8 24-h LC50 127
Back swimmer
Notonecta technical 96-h LC50 200 Federle & Collins (1976)
undulata
Water stick
Ranatra elongata wettable 96-h LC50 624 Shukla et al. (1982)
powder
Table 34 (continued)
Organism Test Size/age Stat/ Temperature Hardness pH Parameterc Concentration Reference
material Flowa (°C) (mg/litre)b (µg/litre)
Diptera
Chaoborus technical 48-h LC50 296 Bluzat & Seuge (1979)
Ephemeroptera
(Mayfly)
Cloeon technical 48-h LC50 480
Crawling water
beetle
Peltodytes sp. technical 96-h LC50 3300 Federle & Collins (1976)
Insects
Stone fly
Pteronarcella unknown larva stat 16 44 7.1 96-h LC50 1.7 Johnson & Finley (1980)
badia larva stat 12 38 6.5 96-h LC50 11 Woodward & Mauck (1980)
larva stat 12 38 7.5 96-h LC50 13 Mayer & Ellersieck (1986)
larva stat 12 38 8.5 96-h LC50 29
Claasenia unknown larva stat 16 44 7.1 96-h LC50 5.6 Johnson & Finley (1980)
sabulosa
Table 34 (continued)
Organism Test Size/age Stat/ Temperature Hardness pH Parameterc Concentration Reference
material Flowa (°C) (mg/litre)b (µg/litre)
Molluscs
Common mussel
Mytilus edulis unknown 48-h EC50 > 30 000 Roberts (1975)
for byssal
attachment
Mytilus edulis wettable larva 48-h EC50 2300 Stewart et al. (1967)
powder 48-h EC50 1300
1-naphthol
Giant oyster
Crassostrea gigas wettable larva 48-h EC50 2200
powder 48-h EC50 800
1-naphthol
Clinocardium wettable adults 24-h EC50 7300
nuttallii powder 24-h EC50 6400
1-naphthol
Molluscs
Eastern Oyster
Crassostrea juvenile flow 29 27s 96-h EC50 > 2000 Mayer (1987)
virginica
Table 34 (continued)
Organism Test Size/age Stat/ Temperature Hardness pH Parameterc Concentration Reference
material Flowa (°C) (mg/litre)b (µg/litre)
Crassostrea technical 48-h EC50 2700 Surprenant (1985a);
virginica Dionne et al. (1985)
Mactrid clam
Rangia cuneata 35-50 mm stat 5s 96-h LC50 125 000 Chaiyarach et al. (1975)
Lymnaea technical 48-h LC50 21 000 Bluzat & Seuge (1979)
stagnalis
aStat = static conditions (water unchanged for the duration of the test).
Flow = flow-through conditions (carbaryl concentration in water continuously maintained).
bHardness = expressed as mg CaCO3/litre.
s = salinity (%).
cEC50s for oyster based on shell deposition.
d50-80% wettable powder.
Carbaryl is slightly toxic for freshwater and marine molluscs
(clams, mussels, oysters). Acute toxicity typically occurs in adult
molluscs at concentrations ranging from 1 mg carbaryl/litre to over
100 mg/litre. The major hydrolytic product of carbaryl, 1-naphthol,
is slightly more acutely toxic for molluscs (mussel, pacific oyster)
than the parent compound (Stewart et al., 1967). In addition,
larvae and young juveniles are more sensitive to carbaryl exposure
than older juveniles and adults.
There have been few studies on the effects of carbaryl on the
growth of molluscs. Davis (1961) and Butler (1962) found that
carbaryl (80% WP) concentrations of 1.0-2 mg/litre reduced the
larval development and growth of oysters ( Crassostrea virginica)
and clams ( Venus mercenaria).
The effects of a 1-h exposure to carbaryl (80% WP) and its
hydrolytic product, 1-naphthol, were studied on six developmental
stages of the mussel ( Mytilus edulis) (Armstrong & Millemann,
1974c). The six stages included unfertilized eggs to the early
veliger stage, 32 h after fertilization. Unfertilized eggs and the
first polar body stage were exposed to carbaryl and 1-naphthol
(separately). The acute toxicity values of carbaryl and 1-naphthol
were similar for the first two stages, ranging from approximately 5
to 25 mg/litre. The stage of development most sensitive to carbaryl
was the first polar body stage. Thereafter, susceptibility decreased
as age of the larvae increased.
LC50 values for crustacea varied from 5 to 9 µg/litre (water
fleas, mysid shrimps), 8 to 25 µg/litre (scud), and 500 to
2500 µg/litre for crayfish. The hydrolytic metabolite 1-naphthol was
less toxic for daphnids and shrimps.
The effects of carbaryl (80% WP) on the life stages of
crustacea have been studied in the Dungeness crab ( Cancer magister)
(Buchanan et al., 1970). Early larvae were more sensitive to
carbaryl than juveniles and adults. Carbaryl (1.0 mg/litre) did not
affect hatching of eggs but prevented moulting of all prezoeae to
zoeae. The concentration that killed 50% of the first zoeae during a
96-h exposure was estimated to be 1 mg/litre.
Young juvenile crabs were more sensitive than older juveniles
or adults. The 24-h EC50s (death or irreversible paralysis) were
estimated to be 0.076 and 0.35-0.62 mg/litre for second and ninth
stage juveniles, respectively. At 0.032 mg/litre, juvenile crabs
were not affected when held in uncontaminated water after exposure.
In adult crabs, the 24-h and 96-h EC50s (death or paralysis) were
0.49 and 0.26 mg/litre, respectively. Post-larval Dungeness crabs
had about the same sensitivity to carbaryl as other crabs. The 24-h
EC50 (death or paralysis) of small stone crabs was 1.0 mg/litre
(Butler, 1962), that of juvenile blue crabs, 0.55 mg/litre (Butler,
1963), and that of adult crabs, 0.06-1.05 mg/litre (Stewart et al.,
1967).
As part of fresh water biota, daphnids have been used as assay
organisms to determine toxic concentrations of a variety of
pesticides. Laboratory bioassays were conducted with carbaryl to
determine its toxicity and immobilization values for two species of
daphnids, Daphnia pulex and Simocephalus serrulatus. The EC50s
for overt effects were 0.0064 mg/litre for Daphnia pulex and
0.0076 mg/litre for Simocephalus serrulatus (Sanders & Cope,
1966).
In a long-term study, Daphnia magna was exposed to technical
carbaryl for 21 days; reproductive performance was the most
sensitive indicator; the MATC was 1.5-3.3 µg/litre (Surprenant,
1985c).
Aquatic insects in the orders Plecoptera (stoneflies) and
Ephemeroptera (mayflies) are generally highly sensitive to low
levels of carbaryl.
The effects of 0.6-50.0 mg carbaryl/litre were studied on
selected aquatic organisms, including: Scanedesmus quadricauda,
Lemna minor, Lebistes reticulatus and Daphnia magna (Bogacka &
Groba, 1980). Carbaryl depressed reproduction, biomass, and
chlorophyll content in Lemna minor (vascular plant) after 24 h
exposure at a concentration >6.6 mg/litre. An almost 100% decrease
occurred at a concentration of 50 mg/litre. Inhibition of
photosynthesis intensity in the alga Scenedesmus quadricauda was
about 50% at a concentration of carbaryl in the water of
32 mg/litre, and 65% at a concentration of 56 mg/litre, after 24 h
exposure. Less than 10% inhibition occurred at a concentration of
1.8 mg/litre. A decrease in the production of chlorophyll was
demonstrated at 4.4 mg/litre.
Carbaryl applications of 5.7 or 11.4 kg/ha were effective in
controlling ghost shrimp ( Callianassa californiensis) as an oyster
pest. The numbers of various clams in the mud were reduced by 22 and
38%, respectively. Clam species differed in susceptibility. For
example, gaper clams (Tresus capax) were reduced by 58 and 69%.
Polychaetes and nemertean worms were not affected during 30 days of
observation (Armstrong & Millemann, 1974a,b).
7.2.2 Fish
7.2.2.1 Acute toxicity
Acute toxicity studies have been conducted with technical
carbaryl, several formulations, and 1-naphthol to determine LC50
values for several freshwater and marine fish (Table 35). The
results indicated that short-term exposure (< 4 days) to carbaryl
and its formulations showed some toxicity for fish (96-h LC50s =
1-30 mg/litre). There were no differences in the toxicity of two
formulations (4-oil-sevin and XLR) for rainbow trout and bluegill.
The LC50s for sheepshead minnow (exposed to technical material)
and rainbow trout (exposed to 4-oil and XLR) were similar and both
were generally more sensitive than bluegill. The toxicity of
1-naphthol for rainbow trout, bluegill, and sheepshead minnow was
determined by Springborn (1988a); 96-h LC50 values for these
species were 0.76, 1.4, and 1.2 mg/litre, respectively. The
corresponding NOECs were < 0.43, 0.55, and 0.47 mg/litre. The
metabolite, 1-naphthol, was more acutely toxic than the formulations
for bluegill. However, the toxicities of 1-naphthol and the
formulations were similar for rainbow trout and sheepshead minnows.
Few studies report acute toxicity values for fish that are
lower than 1 mg/litre. Cold water fish (Salmonidae), such as Coho
Salmon and trout, seem to be susceptible to carbaryl, while the
catfish (Ictaluridae) is tolerant (Macek & McAllister, 1970; Post &
Schroeder, 1971). Irritability, sluggishness, and loss of
equilibrium were classical signs of acute intoxication. Rainbow
trout (Oncorhynchus mykiss), exposed to 1 mg carbaryl/litre for
96 h, exhibited decreased swimming capacity and swimming activity;
the capacity to capture and consume prey was also diminished (Little
et al., 1990).
Other end-point concentrations have been established for
carbaryl for various species. Reference should be made to the
original documentation to obtain a fuller understanding of these
measures of toxicity. The lowest no-observed-effect concentration
(NOEC) was found for rainbow trout at 0.065 mg/litre. Other NOECs
were at least an order of magnitude greater. Maximum acceptable
toxic concentrations (MATCS) have also been calculated. Bansal
et al. (1980) estimated 30-day MATCs for 4 species of carp exposed
to carbaryl (Sevin as a 50% wettable powder). For all species, the
MATC was between 0.052 and 0.078 mg/litre. Verma et al. (1984)
reported a 60-day MATC of between 0.09 and 0.11 mg/litre for another
carp species, exposed to the same type of formulation of Sevin.
Table 35. Acute toxicity of carbaryl for fish
Organism Size/age Stat/ Temperature Hardness pH Formulationc Parameter Concentration Reference
flowa (°C) (mg/litre)b (mg/litre)
Freshwater
Coho salmon 1 g stat 13 44 7.1 96-h LC50 4.3 Mayer & Ellersieck (1986)
(Oncorhynchus stat 13 42 7.1 96-h LC50 2.4
kisutch) 4.6 g stat 13 42 7.1 96-h LC50 1.8
5.1 g stat 13 42 7.1 96-h LC50 2.7
10.6 g stat 13 42 7.1 96-h LC50 1.2
19.1 g CT 96-h LC50 0.8 Macek & McAllister (1970)
CT 96-h LC50 1.3 Post & Schroeder (1971)
Chinook salmon fingerling flow 13 314 7.5 96-h LC50 2.4 Mayer & Ellersieck (1986)
(Oncorhynchus
tshawytscha)
Cutthroat trout 0.5 g stat 12 40 7.5 96-h LC50 7.1
(Salmo clarkii) 0.5 g stat 12 330 7.8 96-h LC50 4.0
0.6 g stat 7 42 7.5 96-h LC50 6.0
0.7 g stat 12 40 6.5 96-h LC50 5.0
0.6 g stat 12 40 8.5 96-h LC50 1.0
CT 96-h LC50 6.0 Woodward & Mauck
C49 96-h LC50 6.7 (1980)
Table 35 (continued)
Organism Size/age Stat/ Temperature Hardness pH Formulationc Parameter Concentration Reference
flowa (°C) (mg/litre)b (mg/litre)
Rainbow trout 1.5 g stat 12 42 7.1 96-h LC50 2.0 Mayer & Ellersieck (1986)
(Oncorynchus 1.5 g stat 12 272 7.4 96-h LC50 1.2
mykiss) 1.2 g stat 12 40 7.4 96-h LC50 3.5
1.2 g stat 12 320 7.4 96-h LC50 3.0
1 g stat 12 40 7.4 96-h LC50 1.6
1 g stat 17 40 7.4 96-h LC50 1.1
1 g stat 12 40 6.5 96-h LC50 1.2
1 g stat 12 40 7.5 96-h LC50 0.8
1 g stat 12 40 8.5 96-h LC50 1.5
0.5 g flow 17 314 7.5 96-h LC50 1.3
CT 96-h LC50 4.3 Macek & McAllister (1970)
XLR 96-h LC50 1.4 Springborn (1985)
S4 96-h LC50 1.3
CT 96-h LC50 2.2 Sanders et al. (1983)
CT 96-h LC50 1.5 Post & Schroeder (1971)
Atlantic salmon 0.2 g stat 7 42 7.5 96-h LC50 0.3 Mayer & Ellersieck (1986)
(Salmo salar) 0.2 g stat 12 42 7.5 96-h LC50 0.9
0.2 g stat 17 42 7.5 96-h LC50 1.0
0.4 g stat 12 42 6.5 96-h LC50 1.3
0.4 g stat 12 42 8.5 96-h LC50 0.9
Brown trout 0.6 g stat 12 42 7.5 96-h LC50 6.3
(Salmo trutta) fingerling flow 12 314 7.5 96-h LC50 2.0
Table 35 (continued)
Organism Size/age Stat/ Temperature Hardness pH Formulationc Parameter Concentration Reference
flowa (°C) (mg/litre)b (mg/litre)
Brook trout 0.8 g stat 12 42 7.5 96-h LC50 2.1 Mayer & Ellersieck (1986)
(Salvelinus 0.8 g stat 7 42 7.5 96-h LC50 3.0
fontinalis) 1 g stat 17 42 7.5 96-h LC50 0.7
0.7 g stat 12 42 6.5 96-h LC50 4.6
0.7 g stat 12 42 8.5 96-h LC50 2.1
0.7 g stat 12 42 9 96-h LC50 1.1
0.8 g stat 12 42 7.5 96-h LC50 1.2
0.8 g stat 12 300 7.5 96-h LC50 1.3
Lake trout 1.7 g stat 12 40 7.5 96-h LC50 0.7
(Salvelinus 1.7 g stat 12 40 6 96-h LC50 0.7
namaycush) 1.7 g stat 12 40 9 96-h LC50 0.9
0.5 g stat 12 162 7.4 96-h LC50 0.9
2.6 g flow 12 162 7.4 96-h LC50 2.3
Gold fish 0.9 g stat 18 40 7.1 96-h LC50 13.2
(Carassius 0.9 g stat 18 272 7.4 96-h LC50 12.8
auratus)
Common carp 0.6 g stat 18 40 7.1 96-h LC50 5.3
(Cyprinus
carpio)
fry CT 96-h LC50 1.7 Chin & Sudderuddin (1979)
Table 35 (continued)
Organism Size/age Stat/ Temperature Hardness pH Formulationc Parameter Concentration Reference
flowa (°C) (mg/litre)b (mg/litre)
Carp C50 WP 72-h LC50 10.4 Toor & Kaur (1974)
(Cyprinus
carpio
communis)
(Catla catla) S 96-h LC50 6.4 Tilak et al. (1981)
(Cirrhina S50 WP 96-h LC50 2.0 Verma et al. (1984)
mrigala)
Fathead 0.5 g stat 12 42 7.5 96-h LC50 14.0 Mayer & Ellersieck (1986)
minnow 0.8 g stat 18 40 7.1 96-h LC50 14.6
(Pimephales 0.8 g stat 18 272 7.4 96-h LC50 7.7
promelas)
Sheepshead CT 96-h LC50 2.2 Springborn (1985)
minnow
(Cyprindon
variegatus)
Black bullhead 1.2 g stat 18 40 7.1 96-h LC50 20.0 Mayer & Ellersieck (1986)
(Ictalurus
malas)
Channel catfish 1.5 g stat 18 40 7.1 96-h LC50 15.8
(Ictalurus 1.5 g stat 18 272 7.4 96-h LC50 7.8
punctatus) fingerling flow 12 314 7.5 96-h LC50 17.31
Table 35 (continued)
Organism Size/age Stat/ Temperature Hardness pH Formulationc Parameter Concentration Reference
flowa (°C) (mg/litre)b (mg/litre)
Freshwater 1.1 g stat 28 S50 WP 72-h LC50 17.5 Arunachalam et al. (1980)
catfish
(Mystus
vittatus)
S 96-h LC50 2.4 Tilak et al. (1981)
(Mystus S 96-h LC50 4.6
cavasius)
Freshwater CT 96-h LC50 46.9 Tripathi & Shukla (1988)
catfish
(Clarias
batrachus)
CT 96-h LC50 107.7
Catfish CT 96-h LC50 9.7 Lejczak (1977a)
(Lebistes
reticulatus)
Green sunfish 1.1 g stat 18 40 7.1 96-h LC50 11.2 Mayer & Ellersieck (1986)
(Lepomis 1.1 g stat 18 272 7.4 96-h LC50 9.5
cyanellus)
Table 35 (continued)
Organism Size/age Stat/ Temperature Hardness pH Formulationc Parameter Concentration Reference
flowa (°C) (mg/litre)b (mg/litre)
Bluegill 1.2 g stat 18 40 7.1 96-h LC50 6.8
(Lepomis 1.2 g stat 18 272 7.4 96-h LC50 5.2
macrochirus) 0.4 g stat 12 44 7.4 96-h LC50 7.4
0.4 g stat 22 44 7.4 96-h LC50 5.2
0.8 g stat 12 40 7.4 96-h LC50 16.0
0.8 g stat 17 40 7.4 96-h LC50 7.0
0.8 g stat 22 40 7.4 96-h LC50 8.2
0.4 g stat 17 320 7.4 96-h LC50 6.2
0.7 g stat 17 40 6.5 96-h LC50 5.4
0.7 g stat 17 40 7.5 96-h LC50 5.2
0.7 g stat 17 40 8.5 96-h LC50 1.8
0.7 g stat 17 40 9.5 96-h LC50 2.6
0.6 g flow 12 314 7.5 96-h LC50 5.1
XLR 96-h LC50 9.8 Springborn (1985)
S4 96-h LC50 10
Mosquito fish 30-40 mm stat 96-h LC50 31.8 Chaiyarach et al. (1975)
(Gambusia
affinis)
Largemouth 0.9 g stat 18 40 7.1 96-h LC50 6.4 Mayer & Ellersieck (1986)
bass
(Micropterus
salmoides)
Black crapple 1 g stat 18 40 7.1 96-h LC50 2.6
(Pomoxis
nigromaculatus)
Table 35 (continued)
Organism Size/age Stat/ Temperature Hardness pH Formulationc Parameter Concentration Reference
flowa (°C) (mg/litre)b (mg/litre)
Yellow perch 1.4 g stat 18 40 7.1 96-h LC50 0.7 Mayer & Ellersieck (1986)
(Perca 0.6 g stat 12 42 7.5 96-h LC50 5.1
flavescens) 1 g stat 7 42 7.5 96-h LC50 13.9
1 g stat 12 42 7.5 96-h LC50 5.4
1 g stat 17 42 7.5 96-h LC50 3.4
1 g stat 22 42 7.5 96-h LC50 1.2
0.9 g stat 12 42 6.5 96-h LC50 4.0
0.9 g stat 12 42 7.5 96-h LC50 4.2
0.9 g stat 12 42 8.5 96-h LC50 0.5
0.9 g stat 12 42 9 96-h LC50 0.4
1 g stat 12 42 8 96-h LC50 3.8
1 g stat 12 170 8 96-h LC50 5.0
1 g stat 12 300 8 96-h LC50 3.8
fingerling flow 12 314 7.5 96-h LC50 1.4
Snakehead fish CT 48-h LC50 8.7 Rao et al. (1985a)
(Channa
punctatus)
C50 WP 96-h LC50 19.5 Singh et al. (1984)
C50 WP 48-h LC50 8.1 Rao et al. (1985b)
(Anabas S 96-h LC50 5.5 Tilak et al. (1981)
testulus)
Table 35 (continued)
Organism Size/age Stat/ Temperature Hardness pH Formulationc Parameter Concentration Reference
flowa (°C) (mg/litre)b (mg/litre)
Tilapid fish S50 WP 72-h LC50 8.0 Koudinya & Ramamurthi
(Sarotherodon (1980)
mosambica)
(Tilapia sp.) C 48-h LC50 5.5 Basha et al. (1983)
Estuarine C 96-h LC50 2.2 Lingaraja & Venugopalan
teleost (1978)
(Therapon
jarbus)
(Hetero-pneustes C50 WP 96-h LC50 20.1 Singh et al. (1984)
fossilis)
(Macropodus) CT 96-h LC50 3.5 Arunachalam &
Palanichamy (1982)
(Cypanus) CT 96-h LC50 4.0
Estuarine &
marine
Longnose juvenile stat 28 19s 48-h LC50 1.6 Mayer (1987)
killifish
(Fundulus
similis)
Table 35 (continued)
Organism Size/age Stat/ Temperature Hardness pH Formulationc Parameter Concentration Reference
flowa (°C) (mg/litre)b (mg/litre)
Striped mullet juvenile stat 24 17s 48-h LC50 2.4
(Mugil
cephalus)
aStat = static conditions (water unchanged for the duration of the test); Flow = flow-through conditions (carbaryl concentration
in water continuously maintained).
bHardness = expressed as mg CaCO3/litre; s = salinity (%).
cFormulation: C = carbaryl; CT = Carbaryl technical (> 95%); C50 WP = Carbaryl 50% wettable powder; S = Sevin; S4 = Sevin-4-oil;
S50 WP = Sevin 50% wettable powder; XLR = Sevin (44% carbaryl); C49 = Carbaryl (49%). Where information on formulation is not given
the carbaryl formulation used was mostly carbaryl technical.
Exposure of C. punctatus to carbaryl at a concentration of
3 mg/litre for 48 h resulted in an increase in free fatty acids,
cholesterol, and lipase activity in the liver (Rao et al., 1985b).
However, the total lipid content was reduced.
The literature indicates that water temperature, hardness, and
pH may influence the toxicity of carbaryl, as well as the size of
the fish (Table 35).
In a study by Post & Schroeder (1971), water was supplied from
a well, classified as very hard and highly alkaline at temperatures
of 13.6-14.6 °C. Carbaryl was more toxic for cutthroat trout
weighing 0.37 g than for those weighing 1-2 g (96-h LC50 1.5 and
2.2 mg/litre, respectively) and more toxic for brook trout weighing
1.15 g than for those weighing 2.04 g (96-h LC50 1.2 and
2.1 mg/litre, respectively).
A comparative toxicity study on carbaryl and 1-naphthol, under
laboratory conditions showed that 1-naphthol was approximately 5
times more toxic than carbaryl for goldfish ( Carassius auratus)
and 2 times more toxic for killifish ( Fundulus heteroclitus) (Shea
& Berry, 1983).
Synergism of the effects of carbaryl and phenthoate on Channa
punctatus was found (Rao et al., 1985a,b). Carbaryl produced
potentiation of the effects of 2,4-D, n-butyl ester, dieldrin,
rotenone, pentachlorophenol, and arecoline on trout (Statham & Lech,
1975).
Application of carbaryl to a forest resulted in a significant
(15-34%) decrease in brain acetylcholinesterase activity in brook
trout from a nearby stream (Haines, 1981). No other effects were
observed.
7.2.2.2 Short-term and long-term toxicity
There is a limited data base on the effects of long-term
carbaryl exposure on fish. In one study, juvenile spot Leiostomus
xanthurus were exposed for five months to carbaryl (technical,
98%) at a level of 0.1 mg/litre in a flow-through system (Lowe,
1967). No carbaryl-related mortality was observed and there was no
effect on growth.
Carlson (1972) conducted the only full life-cycle study on fish
with carbaryl (80%), in which fathead minnows ( Pimephales promelas)
were exposed to five concentrations (0.008-0.68 mg/litre) for 9
months, beginning with the larvae. Survival of fatheads after 6
months at 0.68 mg/litre was lower than that in the controls. After 9
months at 0.68 mg/litre, the mean number of eggs produced per female
was reduced, the mean number eggs per spawning was also affected and
no hatching occurred. No other demonstrable effects were noted at
0.017, 0.062, and 0.21 mg/litre concentrations; thus, the maximum
acceptable toxicant concentration (MATC) for fathead minnows,
exposed to carbaryl in water, was between 0.21 and 0.68 mg/litre.
The lethal threshold concentration for 2-month-old minnows was
9 mg/litre.
Kaur & Toor (1977) exposed different stages of the embryo of
carp ( Cyprinus carpio) to carbaryl through the hatching stage.
There was 100% mortality of carp eggs and embryos at 2.5 mg/litre.
There appeared to be no effect of carbaryl on hatching at
0.01-0.75 mg/litre. However, there was decreased hatching at
1.0 mg/litre and deformed larvae (3.3%) with enlargement of the
pericardial sac and coiling of the posterior region of the embryo.
Thirty days exposure of Channa striatus to 10 or 20 mg
carbaryl/litre retarded oocyte production, with an increase in the
number of immature oocytes and a decrease in the number of mature
oocytes (Kulshrestha & Arora, 1984).
Statistically significant reductions in gonadotropic hormone in
the pituitary gland and plasma in Channa punctatus were observed
following exposure to carbaryl at a concentration of 1.66 mg/litre
(Ghosh et al., 1990). Gonadotropic hormone levels continued to
decrease with continued exposure, with a 30% decrease in the
pituitary gland and a 50% decrease in serum after 30 days of
exposure.
Exposure of the freshwater fish Puntius conchonius to carbaryl
at a concentration of 0.194 mg/litre for 15 days resulted in an
increase in the incidence of lesions in the gill and liver (Gill
et al., 1988). A higher level of exposure also resulted in lesions
in the kidney.
A 27-day exposure to 12.5 mg/litre led to a decrease in the
feeding and growth rate of the catfish ( Mystus rittatus)
(Arunachalam et al., 1980). Long-term toxicity of carbaryl for
fish in most natural surface waters will not occur, since exposure
would not be high enough or constant, because of the substance's
degradation.
7.2.3 Amphibians
The LC50s for bullfrog ( Rana tigrina) 0.1 g tadpoles with
24, 48, 72, or 96 h of exposure were determined to be 12.8, 8.2,
6.7, and 6.3 mg/litre, respectively (Marian et al., 1983). Growth
and feeding were decreased in a dose-dependent manner by doses
ranging from 0.5 to 5 mg/litre.
7.3 Terrestrial organisms
7.3.1 Worms
Carbaryl was classified as extremely toxic for earthworms
(LC50 = 9.1 µg/m2) by Roberts & Dorough (1984). The LC50 value
for the worm Eisenia fetida-Savigny was 14 µg/cm2 (Neuhauser
et al., 1985). The number of earthworms was reduced by 60% when
carbaryl was applied at 0.5 kg (1.12 lb) a.i. per hectare (Thompson,
1971).
Exposure of earthworms to carbaryl (1-8 mg/kg) significantly
increased the burrowing time, which was directly proportional to the
dose and time of exposure, because of the AChE inhibition in the
neural tissue (Gupta & Sundararaman, 1991).
7.3.2 Insects
Administration of carbaryl in sublethal doses produced death in
the early embryonal stage of the silkworm (Kuribayashi, 1981).
The alfalfa leaf-cutting bee ( Megachile pacifica), which is
very important in the pollination of alfalfa, has been reported to
be particularly tolerant to carbaryl (Lee & Brindley, 1974). Waller
(1969) also classified carbaryl as relatively non-toxic for the
alfalfa leaf-cutting bee. The carbaryl tolerance was related to sex
and age. The LD50 was similar for 1-day-old males and 1-and
4-day-old females (240, 245, and 262 µg/g, respectively). However,
four-day-old males were much more susceptible to carbaryl, and had
an LD50 of 51 µg/g. Guirgius & Brindley (1975, 1976) showed that
carbaryl toxicity in alfalfa leaf-cutting bees was controlled by the
activity of mixed function oxidase or microsomal enzymes. This
detoxification system varies with the age and sex of the bees and
results in significantly different carbaryl persistence which, in
turn, leads to differences in carbaryl toxicity. In the more
tolerant groups, carbaryl metabolites were rapidly conjugated and
moved to an aqueous fraction of the bee. Less tolerant insects
(4-day-old males) accumulated these metabolites with time,
indicating that the conjugation mechanisms had deteriorated with
age.
Carbaryl is known to be highly toxic for honey-bees. When
ingested, the LD50 was 0.18 µg/bee (Alvarez et al., 1970). The
contact LD50 (approximately 10-15 mg/kg) for adult bees is
approximately 1.3 µg (Stevenson et al., 1977; Stevenson, 1978).
The carbaryl residue content of bee bread was correlated with
the amount of residue found in the bees and occurred at higher
levels than in honey throughout the 56-day period (Winterlin &
Walker, 1973).
7.3.3 Birds
The toxicity of carbaryl for birds appears to be low
(Table 36). LD50s for six species of waterfowl and game birds were
all greater than 1000 mg/kg (Bart, 1979). There were some
exceptions; thus the red-winged blackbird has an LD50 of 56 mg/kg
(Schafer, 1972). In this study, 180 compounds were found to be toxic
for the red-winged blackbirds and the LD50s ranged between 0.24
and 100 mg/kg.
Food intake, body weight, and locomotor activity were monitored
in adult male bobwhites ( Colinus virginianus), given diets that
contained levels of carbaryl typical of normal exposure under
agricultural conditions in Kansas. No changes were observed in diets
containing 127 or 1235 mg carbaryl/kg (Robel et al., 1982).
Technical carbaryl fed to young Mallard ducks at dietary levels
of 10, 100, 1000, or 3000 mg/kg, revealed dose-correlated signs of
toxicity (reduced intake and/or body weight depression) at the 100,
1000, and 3000 mg/kg level (Fletcher & Leonard, 1986).
There was some tentative evidence that low dosages of carbaryl
may increase the susceptibility of bobwhites ( Colinus virginianus)
to the protozoan parasite Histomonas meleagridis, to which they are
usually resistant (Zeakes et al., 1981).
A study of the effects of carbaryl on forest birds was
conducted in southern New York; plots had been treated 3-4 weeks
previously with carbaryl at the normal rate of 1.1 kg/ha, and at 6
times the normal rate (6.6 kg/ha). In this study, carbaryl had
little, if any, effect on birds. Young birds gained weight normally,
adults continued nesting in the area, no changes were detected in
song frequency, and there was no evidence of birds leaving the area
to forage. This lack of detectable effects, despite the heavy dose
(6.6 kg/ha), indicates that carbaryl applied at the normal rate
(1.1 kg/ha) would have little if any adverse effect on birds (Bart,
1979).
The LC50 of carbaryl for mallard embryos, following field
applications, was determined by Hoffman & Albers (1984) to be
greater than 26.4 kg/ha or 118 µg/g egg. Carbaryl was estimated to
be relatively nontoxic compared with other pesticides.
Inhibition of the brain ChE activity of birds from forests
sprayed with carbaryl 1.13 (kg/ha) was found in 3 out of 12 species
studied (Zinkl et al., 1977) up to 5 days after application.
Table 36. Acute toxicity of carbaryl for birds
Species Age Parametera Concentration Reference
(mg/kg)
Japanese quail 7 days LD50 2290 Hudson et al.
Coturnix coturnix (1984)
japonica 2 months 5 day-LC50 > 10 000 Hill & Camardese
(1986)
Bobwhite quail 23 days 5 day-LC50 > 5000 Hill et al. (1975)
Colinus virginianus
California quail 10 months LD50 > 2000 Hudson et al.
Callipepla californica (1984)
Chukar Alectoris chukar 4 months LD50 1888
Sharp-tailed grouse LD50 < 1000
Tympanuchus
phasianellus
Pheasant 3-4 months LD50 707-> 2000
Phasianus colchicus 23 days LD50 > 5000 Hill et al. (1975)
Mallard 3 months LD50 > 2564 Hudson et al. (1984)
Anas platyrhynchos 24 days 5 day-LC50 > 5000 Hill et al. (1975)
Rock dove LD50 1000-3000 Hudson et al. (1984)
Columbia livia
Canada goose LD50 1790
Branta canadensis
Red-winged blackbird LD50 56 Schafer (1972)
Agelaius phoeniceus
a LD50 = single oral dose expressed as mg/kg body weight.
5 day-LC50 = 5-day dietary exposure (expressed as mg/kg feed) followed by
3 days on a "clean" diet.
7.3.4 Mammals
The effect of carbaryl on wild mice ( Clethrionomys glareolus
and Apodemus sylvaticus) in their natural environment was studied
by Krylov (1970). Carbaryl-containing bait, each consisting of 2 kg
grain treated with 50 g carbaryl in oil suspension, were distributed
twice (in March and June) over 10 ha of woods, at a rate of 1
bait/ha. An adjacent territory of 10 ha was used as a control.
Effects on mice, assessed 1´ months after the last application,
were: reduction of the population by 31.5% compared with the
control; changes in the reproduction system (e.g., decreased number
of embryos in utero, and of corpora lutea, reduced weight of
testicles), and increased weight of adrenal glands.
7.4 Effects on the population and ecosystem
The effects of carbaryl on terrestrial ecosystems have been
studied by Stegeman (1964), Barrett (1968), and Spain (1974).
A large-scale study was performed by Barrett in 1968. It was
designed to determine the effects of carbaryl on an intact ecosystem
in a field that was planted with millet ( Panicum ramosum). The
area was sprayed with a single application of 2.24 kg carbaryl/ha.
There was a highly significant decrease in litter decomposition in
the treated area, 3 weeks after spraying, which was probably because
of a reduction in microarthropods and other decomposers. After 5
weeks, there was a more than 95% reduction in the number of
arthropods and in the total biomass. Phytophagous insects were
severely affected, and predatory insects and spiders were less
affected. The number of species was also reduced, but all species
returned to control levels within 1-2 weeks, except Hemiptera and
Hymenoptera. Reproduction of cotton rats was delayed by 4 weeks.
However, the total mammal population was not affected, because of a
compensating increase in the population of house mice. Old field
mice did not seem to be affected.
The effects of carbaryl on forest soil mites and Collembola,
the two most numerous soil arthropods, were studied by Stegeman
(1964). Mites and Collembola are an important link in the
decomposition process of dead plants and animal matter. They also
are a natural means of decomposing the accumulating litter, and they
provide nutrients for already existing or future crops and fungi.
Application of carbaryl to a test plot in a red-pine plantation, at
doses of 11.2 and 56 kg/ha, reduced the arthropod population
proportional to the severity of the treatment. Neither mites nor
Collembola were totally exterminated by any treatment. The rate of
population increase of the mites, 4-5 months after treatment, was
directly proportional to the dose applied. Collembola were more
vulnerable to treatment than mites and did not recover as rapidly.
The effect of carbaryl at two application rates (0.11 and
1.13 g a.i. per m2) on mixed species population of the litter
fauna of a Corsican pine forest was studied by Spain (1974). He took
a quantitative sampling of the fauna at intervals of 11, 106, 209,
and 315 days after application to record the pattern and the time of
the recovery process (Table 37). The effect of carbaryl varied in
different populations. For Collembola, there was a marked reduction
at the two treatment levels. For Coccoidea and Symphyla, there was a
slight effect at the lower level, and a marked decrease at the
higher level. For Coleoptera, Diptera, Cryptostigmata, Mesostigmata,
and Prostigmata, the effect was small or insignificant. The recovery
process during the period of 315 days was not sufficient to reach
the status of the untreated population.
Tagatz et al. (1979) studied the effects of carbaryl on
animal communities that develop from planktonic larvae in aquaria
containing sand and estuary water. Samples collected after 10 weeks
of exposure were analysed. The numbers of animals and species were
significantly less at 11.1 and 103 µg carbaryl/litre (Table 38)
being decreased to nearly half (from 21 to 12). A carbaryl
concentration of 1.1 µg/litre did not produce significant effects,
except a decrease in the particularly sensitive amphiopod,
Coraphium acerusicum. Carbaryl might have caused changes in
biological interactions that affect the relative abundance of
species. The annelid Polydora ligni increased at a concentration
of 103 µg/litre, and there was a marked decrease in the number of
other annelids and nemerteas.
There have been a number of field studies evaluating the impact
of carbaryl applications on macroinvertebrate populations. In one
study in New York State, carbaryl was applied aerially at a rate of
1.3 kg/ha, in fuel oil with a paraffin oil sticker, and its effects
upon the aquatic fauna of two streams were studied (Burdick et al.,
1960). The data indicated that carbaryl (in oil suspension) was
toxic for mayflies (Ephemeroptera), stoneflies (Plecoptera) and
caddisflies (Trichoptera). Other groups of insects were less
affected and the application did not affect fish. Square-foot
samples, collected before, and shortly after, spraying, showed
reductions of from 50 to 97.2% in the weight of invertebrate
(standing crop) fish food. A progressive effect from upstream to
lower sections was correlated with increased 44-151 exposure time.
No exposure concentrations in water were documented. Owen (1965)
also reported a reduction of 54.2 to 62.8% in the standing crop of
aquatic insects following aerial spraying with carbaryl (80 WP)
(1.3 kg/ha). A control stream showed a 5.9% increase in the same
period.
Table 37. Geometric means for assessed taxa (individuals/m2), post-application sampling of carbaryl-treated plotsa
Sampling day (after treatment) Taxon Control plot High carbaryl Low carbaryl
Coccoidea 272.9 90.8 129.4
Coleoptera (imagines) 425.6 137.7 153.1
Coleoptera (immature) 54.0 47.3 41.4
Collembola 42 771.5 5033.4 9819.7
Diptera 350.9 244.2 243.7
11 Cryptostigmata 5707.3 3933.2 5863.5
Mesostigmata 7239.5 3688.9 5038.2
Prostigmata 1335.6 305.3 564.8
Aranese 238.7 222.7 155.0
Chilopoda 71.1 102.4 37.4
Symphyla 872.1 359.0 388.9
Coccoidea 847.8 157.0 162.9
Coleoptera (imagines) 41.9 379.1 170.7
Coleoptera (immature) 41.9 31.7b 0.0b
Collembola 18 185.1 1621.3 5205.9
Diptera 264.0 173.8 160.5
106 Cryptostigmata 5855.3 6237.9 6623.7
Mesostigmata 8610.3 846.4 595.2
Prostigmata 631.5 846.4 595.2
Aranese 360.6 224.9 253.2
Chilopoda 114.0 157.8 94.3
Symphyla 317.5 47.0b 223.4
Table 37 (continued)
Sampling day (after treatment) Taxon Control plot High carbaryl Low carbaryl
Coccoidea 358.3 181.0 141.0
Coleoptera (imagines) 520.0 86.6 232.6
Coleoptera (immature) 289.6 125.9 74.1
Collembola 41 198.7 3469.4 19 238.9
Diptera 449.9 28.2b 209.5
209 Cryptostigmata 16 298.4 11 184.8 12 014.8
Mesostigmata 16 150.0 7293.9 11 709.0
Prostigmata 2721.6 2407.2 3152.4
Aranese 264.7 108.3 220.2
Chilopoda 241.9 28.2b 150.5
Symphyla 357.3 78.3 174.4
Coccoidea 142.0 207.5 51.5
Coleoptera (imagines) 261.6 64.4 132.5
Coleoptera (immature) 37.0 88.8 23.1b
Collembola 40 139.5 4121.3 10 294.6
Diptera 533.9 291.2 173.1
315 Cryptostigmata 10 889.3 12 743.6 10 506.6
Mesostigmata 11 290.3 7762.7 14 912.0
Prostigmata 2995.0 1844.7 4765.7
Aranese 217.4 157.4 117.8
Chilopoda 159.5 83.4 88.8
Symphyla 349.6 85.4 158.8
aSource:Spain (1974).
b95% confidence limits for the parametric mean include zero.
Table 38. Animals and species, by phylum, collected from control aquaria and aquaria exposed to carbaryla
Control Carbaryl
1.1 µg/litre 11.1 µg/litre 103 µg/litre
Phylum Number Species Number Species Number Species Number Species
Mollusca 1691 3 1563 3 1340 3 1321 5
Arthropoda 380 7 339 8 336 2 269 2
Annelida 102 8 94 7 79 4 200 5
Nemerica 16 1 25 1 20 1 0 0
Coelenterata 3 1 5 1 0 0 0 0
Platyhelmintes 0 0 2 1 0 0 0 0
Echinodermata 0 0 0 0 1 1 0 0
All phyla 2192 20 2086 21 1776 11 1790 12
aFrom:Tagatz et al. (1979).
The effects on stream invertebrates of carbaryl, applied at a
rate of 840 g a.i./ha for spruce budworm suppression, was studied.
Benthos samples showed significant declines among Plecoptera,
Ephemeroptera, and Trichoptera. Plecoptera had not repopulated any
treated stream, 60 days after treatment (Courtemanch & Gibbs, 1980).
Gibbs et al. (1984) conducted a 42-month (1980-83) study on
the occurrence/persistence of carbaryl residues in pond water and
sediment as a result of an application of Sevin-4-oil (840 g
a.i./ha). The immediate and long-term effects on pond
macroinvertebrates and emerging aquatic insects were also evaluated.
A preliminary study by these investigators in 1977 and others
(Coutant, 1964) had shown that there were large increases in the
number of drift organisms, several days after aerial spraying with
carbaryl. Most drift was accompanied by dead Amphipoda,
Ephemeroptera, Plecoptera, and Trichoptera, and carbaryl residues
persisted longer than 30 days in pond sediment. The increase in the
number of dead organisms was accompanied by a reduction in the
standing crop of benthic macroinvertebrates. The most severe and
persistent impact was on Amphipoda with Hyallela azteca and
Crangonyx richmondensis reduced to almost 0/m2; C. richmondensis
failed to recolonize in one of the two treatment ponds, 42 months
after treatment. The numbers of immature Ephemeroptera and
Trichoptera were reduced immediately following spray application,
but this effect did not persist throughout the season or into the
following year. Immediate reduction in numbers of adult
Ephemeroptera and Trichoptera emerging from the ponds was also
found, but recovery of populations was observed. Numbers of immature
Odonata were also reduced following treatment and remained low the
following year. The Chironomidae populations did not appear to be
affected, either as immatures or emerging adults.
The effects of two consecutive years of spraying with
Sevin-4-oil on other aquatic systems appear similar to those
observed in areas treated once (Courtemanch & Gibbs, 1978; Trial,
1978,1979).
In simulated aquatic field studies, 1 mg carbaryl (WP)/litre
was applied to one outdoor concrete pond (4 x 5 x 1 m deep)
(Hanazato & Yasuno, 1987). All zooplankton (Cladocera included) and
Chaoborus larvae were killed. The zooplankton community recovered
rapidly and Cladocera reappeared only two days after application.
Since Chaoborus populations are predators of crustacean zooplankton,
their suppression may also have added to the recovery of increased
populations of Cladocerans. Carbaryl exposure also changed the
community from a rotifer-predominating zooplankton community to that
of Cladocera-predominating. Rotifers in this study were also highly
sensitive to carbaryl. The same phenomenon was observed again after
the second application of carbaryl. Subsequent to this study,
Hanazato & Yasuno (1989) applied carbaryl to simulated ponds in the
same way as in the previous study, but in the spring when the water
temperature was approximately 10 °C lower. Cladocerans never
recovered to the density level of the pre-treatment period. The
rapid recovery of Chaoborus seemed to interfere with the recovery of
Cladoceran populations after treatment. The authors suggested that
the different recovery patterns of the zooplankton community
resulted from different temperatures in the ponds. In another study,
Hanazato & Yasuno (1990a) examined Chaoborus density in relation to
the effects of carbaryl (0.1 and 0.5 mg/litre) on zooplankton
communities in ponds, where the abundance of Chaoborus larvae was
controlled. They concluded that Chaoborus density and/or temperature
may influence the recovery of the zooplankton community following
the effects of carbaryl. The recovery of a zooplankton community may
differ in different aquatic ecosystems (with different community
structures) and under different temperatures, even when the same
treatment is applied.
Hanazato & Yasuno (1990b) also studied the effect of the time
of application of carbaryl (0.5 mg/litre) on recovery patterns of
zooplankton communities in simulated ponds. The loss of carbaryl
from pond water was rapid. The concentration decreased to less than
1% of its initial value, three days after the first or second
application, and six days after the third application. This study
also showed that applications of carbaryl at different times induced
different zooplankton structures (and different recovery patterns),
and, various factors other than toxicity of carbaryl, such as
temperature, competitive interactions between zooplankters, and
trends of zooplankton populations may play important roles in
determining zooplankton community structure after chemical
application. Furthermore, the significance of predators in the
recovery process after chemical treatment was re-emphasized.
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
Reviews of toxicological aspects of carbaryl were prepared by
NIOSH (1976); US EPA (1977, 1980, 1982, 1984); USDHHS/USDL (1978);
US EPA (1980b); Mount & Oehme (1981a); Weston (1982); and Cranmer
(1986). Studies from the Soviet Union were reviewed by IRPTC (1982,
1989).
8.1 Single exposures
8.1.1 Oral toxicity
The oral LD50s for the rat are given in Table 39 and for
other mammals in Table 40. The values varied by about a factor of 4
depending on formulation, route of production, vehicle, and strain
of rat. Interspecies differences were found. Cats are the most
sensitive, with guinea-pigs, rats, mice, and rabbits showing more
resistance in that order. Pigs and monkeys seem to be less sensitive
(Carpenter et al., 1961; Gladenko & Malinin, 1970; Smalley, 1970).
Cattle are more sensitive than pigs. After a single oral
application of carbaryl in cattle, symptoms appeared at a dose level
of 25 mg/kg; 100 mg/kg was the minimum effective dose in pigs
(Gladenko & Malinin, 1970).
Mount & Oehme (1981b) observed that the lethality of carbaryl,
administered to sheep at doses ranging from 300 to 1000 mg/kg, was
highly correlated with concentrations in the brain (>1 mg/kg) and
liver (>3 mg/kg), and with inhibition of acetylcholinesterase
(greater than 50% inhibition).
8.1.2 Acute inhalation toxicity
Acute toxic effects following inhalation at different
concentrations are presented in Table 41.
8.1.3 Dermal toxicity
A dose of 2500 mg/kg, applied as a 40% aqueous suspension of
50% wettable powder, killed 1 out of 4 rabbits (Carpenter et al.,
1961). When 99% technical carbaryl was applied to male and female
rabbits, the LD50 was >2000 mg/kg (Bushy Run, 1983b). In rats,
the LD50 is thought to be >4000 mg/kg (Yakim, 1965; Gaines,
1969).
Table 39. Acute oral LD50s for the rat
Strain (sex) Weight Formulation of Vehicle LD50(mg/kg Symptoms References
(g) carbaryl body weight)
CF-N technical 0.25% agar
(male) 90-120 510 (360-650) - Carpenter et al.
(female) 90-120 610 (490-750) - (1961)
Sherman 850 (600 LD min) Gaines (1969)
(male)
(female) 500 (100 LD min)
Inbred 721 (653-789) Yakim (1965)
Inbred 120-200 sunflower oil 515 Rybakova (1966)
Sprague Dawley 203-246 technical carboxymethyl-cellulose 300 Hamada (1990)
(male, female) in water
Sprague Dawley 112-173 technical 0.25% methyl-cellulose 685 (612-767) death, 4-24 h Field (1980b)
(male, female)
Sprague Dawley 100-160 95% technical 0.25% methyl-cellulose 225 (202-321) death, 2-24 h Field (1980a)
(male, female)
Table 39 (continued)
Strain (sex) Weight Formulation of Vehicle LD50(mg/kg Symptoms References
(g) carbaryl body weight)
Sprague Dawley 200-264 40.38% water tremor, prostration, Hazleton Lab.
(male) 750 (467-1202) laboured respiration, American Inc.
salivation 10 February 1982
(female) 527 (257-977) death, 4-3 days
Harlem Sevin XLR not diluted death, 0.5 h-3 days Kuhn (1991a)
Sprague Dawley plus 44%
(male) 229-286 867 (562-1336)
(female) 182-219 575 (459-721)
Harlem Sevin-4-oil not diluted 658 (456-979) 3 h-3 days Kuhn (1991b)
Sprague Dawley 47% (w/w)
(male) 229-286 963 (802-1160)
(female) 182-219 473 (364-620)
Hilltop Wistar 200-250 98% technical 0.25% methyl-cellulose Bushy Run
(male) 283 death, 1.5-24 h (1983b)
(female) 246
Hilltop Wistar 204-237 80% sprayable water
(male) 406 Bushy Run
(female) 203 death, 1 min-24 h (1983c)
Table 40. Acute oral toxicity for mammals other than rats
Species Number of animals Toxicitya Reference
(mg/kg body weight)
Mouse 6 per dose 363 (294-431) Yakim (1965)
Mouse (female) 80 white 437 ± 70 Rybakova (1966)
Mouse 1310 white 206 (175-480) Bukin (1965)
Guinea-pig 5 per dose 280 Carpenter et al. (1961)
Rabbit 4 per dose 710
Rabbit 56 700 (LD100) Bukin (1965)
Cat (female) 3 250 (LD100) Carpenter et al. (1961)
Cat no data 150 Yakim (1965)
Swine 3 per dose 800-1000 Gladenko & Malinin
(1970)
Swine 1 per dose 1500-2000 Smalley (1970)
Monkey no data > 1000 FAO/WHO (1970)
Duckling 106 500 (LD15) Bukin & Filatov (1965)
6000 (LD100)
Table 40 (continued)
Species Number of animals Toxicitya Reference
(mg/kg body weight)
Chick 48 250 (LD33)
1000 (LD100)
Hen 12 > 1000 (no death)
Hen 12 > 3000 Bukin (1965)
aLD50, unless otherwise stated.
Table 41. Inhalation toxicity single exposure
Species Concentration Effects observed References
Guinea-pig 6 390 mg 50% wettable powder/m3 nasal and ocular irritation after 14 days - Carpenter et al.
(average particle size 15 µm) 4 h haemorrhage areas in the lungs (1961)
Guinea-pig 6 230 mg carbaryl 85S/m3 slight weight decrease; recovered by day 14
(average particle size 5 µm) 4 h
Dog 75 mg carbaryl 85S/m3 5 h typical symptoms for ChE inhibition
Cat 3 groups 82 mg/m3 dust 6 h tremor salivation, muscle fibrillation decreased Yakim (1967, 1968)
of 4 animals ChEA by 39-55% serum; 53-71% red blood
cells; normalization after 72 h
37 mg/m3 dust 6 h decreased ChEA by 23% in serum and 41% in
red blood cells; recovery after 48 h
20 mg/m3 dust 6 h decreased ChEA by 11-27% in serum and
15.28% in red blood cells; recovery after 24 h
Rat 20-23 mg/m3 dust no effect Weil & Carpenter
98% particles (1974)
less than 1.0 µm diameter
Rat 1800 mg/m3 water aerosols lacrimation, tremor with 1.5 h of exposure Myers et al. (1975)
Rat Wistar Albino Aerosols Sevin XLR 44% 792 mg 1/5 females died, tremor, ataxia, increased Faït (1984)
male 5 a.i./m3 per 4 h (the highest respiratory rate; recovery period 6 days
female 5 attainable concentration)
particle size 3.6 ± 2.64 µm
8.1.4 Other routes of exposure
Other routes of exposure are presented in Table 42.
Table 42. Toxicity following parenteral application
Species Weight Vehicle Route of LD50 References
(g) administration mg/kg body
weight)
Rat 10-107 propylene intravenous 18 Mellon Institute
female glycol (1958)
Rat 92-126 polyethylene intravenous 24
glycol 400 intravenous (17-33)
Rat 90-120 95% ethyl intravenous 33
alcohol (26-41)
Rabbit 1686-3544 0.25% agar intraperitoneal 223
(122-407)
Rat 90-120 in lard subcutaneous 1410
Leghorn 10-11 days in alantoic 3.44 mg Tós-Luty et al.
chick old cavity per embryo (1973)
embryos
(250)
8.2 Skin and eye irritation, sensitization
8.2.1 Skin and eye irritation
The results of several studies on skin irritation from carbaryl
were negative (Carpenter et al., 1961; Yakim, 1965). Transient
erythema was noted after an application of 0.5 ml 43.4% carbaryl on
occluded rabbit skin (Bushy Run, 1983a).
Carbaryl is a weak eye irritant (Table 43).
Table 43. Eye irritation
Species Number Formulation Effects observed Reference
Rabbit 5 technical grade mild injury in one Carpenter et al.
10% suspension of five eyes (1961)
in propylene
glycol
25% aqueous no injury
suspension
50 mg dust spots of
corneal-necrosis
Rabbit 6 0.1 ml (90 mg) conjunctival irritation Bushy Run (1983b)
New 99% technical after 2 days recovery
Zealand product
White
0.1 ml transient iritis in Bushy Run
43.4% carbaryl 2 of 6, conjunctival (1983a)
irritation in 6 of 6.
Recovery in 3 days
8.2.2 Sensitization
The sensitization response following topical application of
carbaryl was studied by Myers & Christopher (1987). The induction
which consisted of an application of carbaryl to covered skin, once
a week during 3 weeks, was followed by a 2-week incubation period. A
single challenge dose of 50% (w/v) technical carbaryl in 0.25%
aqueous methyl cellulose solution (0.1-0.3 ml) was administered.
Carbaryl did not produce a positive response in any of the
guinea-pigs tested.
Four out of 16, male, albino guinea-pigs were treated with 8
intravenous injections (3 per week) of 0.1 ml of a 0.1% dispersion
of carbaryl in 3.3% propylene glycol. After a 3-week incubation
period, a challenge dose was given, but did not cause sensitization
(Carpenter et al., 1961). In a more recent study, a similar
procedure, but with 9 doses and a 2-week incubation period, was
used. Although there was some skin irritation, it was considered
that carbaryl had little or no sensitizing potential (Bushy Run,
1983a).
8.3 Short- and long-term oral exposure
Several short- and long-term feeding studies with carbaryl have
been reported (Carpenter et al., 1961; Rybakova, 1967; Orlova &
Zhalbe, 1968; Gladenko & Malinin, 1970; Dikshith et al., 1976;
Hamada, 1991a). Results are shown in Table 44. Doses that do not
show any effects are 200 mg/kg diet equal to 7.9 mg/kg body weight
in rats, and 100 mg/kg diet for mice, and 1.8 mg/kg body weight for
dogs (approximately 100 mg/kg diet).
The cumulation coefficient (LD50 for 3-month exposure/LD50
single application) was 18 (Kassin, 1968), demonstrating a very low
cumulative potential for carbaryl.
8.4 Short- and long-term inhalation toxicity
Short-term and long-term inhalation toxicity data are given in
Table 45.
8.5 Reproduction and developmental toxicity
The reproduction and developmental toxicity of carbaryl has
been studied in many vertebrate species using a wide variety of
study designs.
The data have shown that carbaryl can affect reproduction
(Table 46) and embryo/fetal development (Table 47 and 48) in a
number of species. The relevance of these studies for risk
assessment is influenced by several factors related to experimental
design, dose levels, and the types of effects noted. Shortcomings of
the studies included small sample size; inappropriate dose
selection; the variable degree of maternal toxicity (ranging from no
effect to lethality); and a lack of historical data for some
species. The following sections have been arranged according to
end-point (reproduction and developmental toxicity) and species of
animals studied (mammalian, non-mammalian). Studies that are of
dubious value for risk assessment are listed at the end of the table
sections, and are not discussed in the text. The reasons for such an
evaluation are given below the references to the individual papers.
Table 44. Short- and long-term feeding and oral studies
Species Sex Number Dosage (mg/kg diet Period of Effects observed References
of or mg/kg body weight) exposure
animals
Mouse male 48 100, 400 mg/kg diet 80 weeks no changes in survival rate, pathology, and tumour FAO/WHO (1965)
and incidence
female
Mouse male 10/sex 100, 1000, 7000 mg/kg 53 weeks at 7000 mg/kg diet: decreased survival, body Hamada (1991b)
and per group diet weight gain and erythrocyte count; increased liver
female weight and decreased ovary weight; increased
frequency and severity of chronic nephropathy in
females; cholinesterase (plasma, RBC, and brain)
depressed at 100 and 7000 mg/kg; NOEL
100 mg/kg diet
Rat male 2x10 1500 (58.5 mg/kg 96 days no changes Carpenter et al.
body weight) (1961)
female 2x10 1500 (58.5 mg/kg 96 days kidney weights significantly increased
body weight)
male 2x10 2250 (87.5 mg/kg 96 days increase in liver weight as a % of body weight,
body weight) diffuse cloudy swelling in the kidney tubules in
4 animals (male and female)
female 2x10 2250 (87.5 mg/kg 96 days decrease of body weight, increase in kidney weight
body weight)
Table 44 (continued)
Species Sex Number Dosage (mg/kg diet Period of Effects observed References
of or mg/kg body weight) exposure
animals
Rat male 4x40 50, 100, 200 (2, 4, 2 years no changes Carpenter et al.
CF-N and 7.9 mg/kg body weight) (1961)
female
Rat male 2x40 400 (15.6 mg/kg 2 years weight depression in male, cloudy swelling of the Carpenter et al.
CF-N and body weight) hepatic cords, principally located around the (1961)
female central veins in both sexes; transitory diffuse
cloudy swelling of the epithelial lining of the
primarily convoluted proximal, and loop tubules
Rat 75, 150, 300 mg/kg 3 months cytoplasmatic vacuolization in the proximal tubules FAO/WHO (1970)
body weight
Rat male 4x48 7, 14, 70 mg/kg weight depression at all dose levels; at 70 mg/kg, Rybakova (1967)
and body weight slight morphological liver changes; cloudy swelling
female in convoluted tubules; decreased motility of
spermatocytes at all dose levels, more pronounced
at 70 mg/kg; oedema in the interstitial tissue;
desquamation of spermatogenic epithelium;
destruction of parenchyma; decreased production of
spermatocytes was found at 14 and 70 mg/kg dose
levels; increased estral cycle; increased hypophysis
gonadotropic function; decreased ascorbic acid
contents; cell proliferation, hypertrophy, increased
lipid content in suprarenal glands; decreased thyroid
function; augmentation of follicles, colloid retention,
thickness of follicular epithelium
Table 44 (continued)
Species Sex Number Dosage (mg/kg diet Period of Effects observed References
of or mg/kg body weight) exposure
animals
Rat male 80-90/ 250, 1500, 7500 mg/kg 52 weeks body weight and food consumption lower at middle Hamada (1991c)
Sprague female group diet dose; significantly increased total cholesterol;
Dawley increase in liver and kidney weight; NOEL
250 mg/kg diet
Rat male 876 2, 5, 15 mg/kg 1 year changes in sex glands function at 15 and 5 mg/kg Orlova & Zhalbe
and body weight dose levels; enzymatic activity, spermatozoids and (1968)
female the estral cycle; reduced fecundity; no effect at
2 mg/kg dose level
Rat male total 200 mg/kg 90 days decreased AChE in blood with 33.8%; no Dikshith et al.
local 28 body weight, histological changes (1976)
strain 3 x per week
in peanut oil
Dog male total 0.45, 1.8, 1 year diffuse cloudy swelling of proximal convoluted and Carpenter et al.
Basenji- and 14 7.2 mg/kg body loop tubules of kidney; local sudanophilic granules (1961)
cocker female weight in capsules, in the glomeruli at 400 mg/kg level; the same in
hybrids 5 days/week, to control dogs to a lesser extent; transient hind leg
approximate levels weakness in 1 female after 189th dose at
of 24, 95, 414 mg/kg 0.45 mg/kg body weight
dry diet
Beagle male 24+ 125, 400, 1250 mg/kg 1 year at 400 and 1250 mg/kg diet, decreased AChE in Hamada (1987)
dogs and 24 diet plasma, red blood cells, and in brain; increase in
female leucocyte count and segmented nitrophil count;
increase in liver weight in male; no effect level
125 mg/kg diet
Table 44 (continued)
Species Sex Number Dosage (mg/kg diet Period of Effects observed References
of or mg/kg body weight) exposure
animals
Beagle male 24 0, 20, 45, 125 mg/kg 5 weeks significant inhibition of cholinesterase activity Hamada (1991a)
dogs female 24 diet in plasma at week 2 for males dosed 20 and
125 mg/kg
Monkey 150, 300, 600 mg/kg 38 weeks kidney alterations similar to those found in rats FAO/WHO (1970)
body weight
Swine male 3 150 mg/kg body weight 72-83 progressive myasthenia, incoordination ataxia, Smalley et al.
female 3 in diet days intentional tremor, chronic muscular contraction, (1969)
until terminal paraplegia and prostration;
death myodegeneration
Swine 5 and 10 mg/kg 147-176 no changes Gladenko & Malinin
body weight days (1970)
Cattle 1 and 4 mg/kg 148 days decreased Hb with 20%, and erythrocytes with
(young) body weight 30% occasionally
Table 45. Short- and long-term inhalation toxicity
Species Number of Concentration of carbaryl Days of Effects References
animals exposure
Cat 4 0.06 mg/litre 30 typical cholinergic symptoms, Yakim (1968)
inhibition of ChE plasma 31-40%,
erythrocytes 40-59%
Cat 4 0.03-0.04 mg/litre 30 reaction time increased
Cat 4 0.016 mg/litre 120 no symptoms, fluctuation in
plasma ChE inhibition around 18%
Rat no data 10 mg/m3 dust (85% 90 no mortality no gross visible injury Carpenter et al.
suspension) 7 h/day inhalation (1961)
5 days per week periods
Table 46. Reproduction studies
Species Treatment End-point(s) Reference
(number)
Mouse
Swiss Webster males 30-40 g 0, 8.5, 17, 34 mg/kg body weight weight and uptake of testosterone: Thomas et al. (1974)
(10 + animals/group) orally (5 days) testes and accessory glands
Swiss Webster males 30-40 g 0, 34, 68 mg/kg body weight biotransformation of Dieringer & Thomas
(5 + animals/group) orally (5 days) testosterone-1,2-3H (1974)
C57Bl/6 x C3H 6-8 weeks of 0, 12, 25, 50...800 mg/kg body sperm morphology, testes weight Osterloh et al. (1983)
age (4 animals/group) weight per day 5 days i.p. on day 35 [route of
administration]
Rat
Osborne-Mendel 0, 2000, 5000, 10 000 mg/kg diet fertility, litter size, and viability Collins et al. (1971)
(20 females and 1 male per (3 generations)
group)
Wistar 0, 7, 25, 100, 200 mg/kg body fertility, litter size, and viability Weil et al. (1973)
(13-21 animals/group) weight per day in the diet
0, 3, 7, 25, 100 mg/kg body
weight per day orally
(3 generations)
Male and female 0, 1, 5, 10, 20, 40, 50 mg/kg general toxicity, serum protein, Vashakidze (1975)
body weight per day orally numbers of male reproductive
(1 month) cells, sperm viability, testicular
histology, litter viability
Table 46 (continued)
Species Treatment End-point(s) Reference
(number)
Wistar males 0, 12.5, 25.0, 250.0 mg/kg body sperm morphology Luca & Balan (1987)
(36 animals per group) weight per day
Rats
Male and female 0, 50, 100, 300 mg/kg body fertility indices Vashakidze (1966)
weight per day orally 2 weeks to [insufficient data]
3 months
Male and female 0, 2, 5, 15 mg/kg body weight per fertility indices Orlova & Zhalbe
(total of 876 animals) day orally 12 months for F° (1968); Zhalbe et al.
and 6 months for the F1 (1968) [insufficient
data]
Male and female 0, 2, 5 mg/kg body weight per day fertility indices Shtenberg & Ozhovan
for 6 months (follow-up to Orlova (1971) [insufficient
& Zhable) 2 through 5 generations data]
Male and female 0, 2, 5, 15 mg/kg body weight per fertility indices Shtenberg et al.
day for 12 months (1973) [insufficient
data]
Gerbil
(32-80 animals per group) 0, 2000, 4000, 6000, and fertility, litter size, and viability Collins et al. (1971)
10 000 mg/kg body weight diet
(3 generations)
Table 47. Developmental toxicity studies, mammalian
Species Treatment End-point(s) Reference
(number)
Mouse
CF-1 0, 100, 150 mg/kg body weight fetal examination Murray et al. (1979)
(23-44 in a group) per day orally or 5660 mg/kg diet.
Gestation day 6-15
Swiss albino 0, 100, 150, or 200 mg/kg body fetal examination Mathur & Bhatnagar
(10 per group) weight per day orally. Gestation (1991)
days 8, 12, or 6-15
Charles River 0, 10, 20 mg/kg body weight. fetal examination Benson et al. (1967)
(8 per group) Gestation day 6-parturition [doses too low]
CD-1 100 mg/kg body weight per day postnatal viability Chernoff & Kavlock
(23 animals) orally. Gestation day 8-12 (1982) [teratology
screen test]
CD-1 200 mg/kg body weight per day postnatal viability Kavlock et al. (1987)
(30 animals) orally. Gestation day 8-12 [teratology screen
test]
Rat
Harlan Wistar 0, 20, 100, 500 mg/kg diet. fetal examination Weil & Carpenter
(6 per group) Gestation days 1-7, 5-15, 1-21 (1965); Weil et al.
(1972)
Wistar 0, 200, 350 mg/kg body weight orally fetal examination Golbs et al. (1974)
(10 per group) 40 mg/kg ip. Variety of gestation days.
Table 47 (continued)
Species Treatment End-point(s) Reference
(number)
Sprague-Dawley 0, 1, 10, 100 mg/kg body weight fetal examination Lechner &
(6 or 7 per group) orally. 3 months before and during Abdel-Rahman
gestation (1984)
Sprague-Dawley 0, 20, 37.5 mg/kg body weight fetal examination Hart (1972)
(22 or 23 per group) per day. Gestation day 6-15 [doses too low]
Rats unknown dose (1/50 LD50). fetal examination Dinerman et al.
Gestation days 9, 11, or 13 (1970) [insufficient
data]
Rats 0, 10.6, 106 mg/kg body weight fetal examination Shtenberg et al.
per day. Gestation day 1-20 (1973) [insufficient
data]
Guinea-pigs
HRA/HART 0, 50, 100, 200 mg/kg body fetal examination Weil et al. (1973)
(9-4 per group) weight per day orally. 0, 100,
200, 300 mg/kg body weight per
day in the diet
Coulston strain 300 mg/kg body weight per day. fetal examination Robens (1969)
(26 gestation day treatment Gestation day 11-20 and a variety [insufficient data]
11-20; other treatments of other treatment periods within
unknown) this time
Table 47 (continued)
Species Treatment End-point(s) Reference
(number)
Rabbit
New Zealand White 0, 150, 200 mg/kg body weight fetal examination Murray et al. (1979)
(15-20 per group) per day orally. Gestation day 6-18
New Zealand White 0, 10, 30 mg/kg body weight per fetal examination Shaffer & Levy
(9-12 per group) day. Gestation day 9-16 (1968) [doses too
low]
New Zealand White 0, 50, 100, 200 mg/kg body fetal examination Robens (1969) [small
(4-9 per group) weight per day orally. Gestation number of animals]
day 5-15
Dog
Beagle 0, 3.125, 6.25, 12.5, 25, examination of pups Smalley et al. (1968)
(6-13 per group) 50 mg/kg per day in the diet
throughout gestation
Beagle 0, 2.0, 5.0, 12.5 mg/kg per day in examination of pups Imming et al. (1969)
(7-9 per group) the diet throughout gestation
Pig
Hormel-Hanford 0, 4, 8, 16 mg/kg in the diet 20 examination of fetuses (I) Earl et al. (1973)
(5-16 per group) days before/7 days after breeding or piglets after birth (II)
throughout gestation
Table 47 (continued)
Species Treatment End-point(s) Reference
(number)
Pig (30) up to 30 mg/kg body weight per ? Smalley (1968)
day [insufficient data]
Monkey
Rhesus 0, 2, 20 mg/kg body weight per examination of gestational Dougherty et al.
(4-6 per group) day orally throughout gestation course and new born (1971)
Rhesus 0, 20, 32 mg/kg body weight per examination of gestational Coulston et al. (1974)
(15-16 group) day orally. Gestation day 20-38 course and new born
Sheep
(25 and 26 per group) 159, 297.5 mg/kg diet examination of lambs Panciera (1967)
after birth [significance of
defects impossible to
assess]
Hamster
Golden Syrian 125 mg/kg body weight per day fetal examination Robens (1969)
(6 or 8 per group) on gestation day 6-8; 250 mg/kg [number tested too
body weight per day on gestation small]
day 7 or 8
Table 48. Non-mammalian studies
Species Dosage End-points Reference
Fish
Medaka 0.5, 1.0, 2.5, 5.0, 10.0, and embryonic development Solomon & Weis (1979)
(Oryzias latipes) 20.0 mg/litre, 4-cell through
10 eggs per group blastula
Amphibian
Xenopus laevis 0.1, 1.0, 10.0 mg/litre; embryos to embryonic development, Elliott-Feeley & Armstrong
10-12 per group hatching or tadpoles for 24 h posthatching activity (1982)
Birds
Chicken 0, 0.01, 0.1, 1.0, 10.0 mg/kg embryonic development Olefir & Vinogradova (1968)
4-6 per group body weight
White Leghorn chicken 0, 250, 500 mg/kg diet to pullets egg production, embryonic Lillie (1972)
20 per group for 36 weeks and hatchlings for development, hatchability,
4 weeks posthatching development
White Leghorn chicken 0, 1.0, 2.5, 5.0, and 10.0 mg/egg embryonic development Swartz (1981)
38-40 per group embryonic development injected
into yolk sac after fertilization,
prior to incubation for 5 or 12 days
White Leghorn chicken 10 mg/egg after fertilization prior primordial germ cell Swartz (1985)
8 per group to incubation migration
White Leghorn chicken 1.0, 0.3 mg/egg and lower examination of embryos Eto et al. (1980)
[insufficient data]
Table 48 (continued)
Species Dosage End-points Reference
720 eggs 0, 1, 2, and 4 mg/egg examination of hatchlings Ghadiri & Greenwood (1966)
[insufficient data]
Duck and chicken 10-1000 µg/egg 0, 4, 7, 10, and examination of embryos or Khera (1966)
13 day eggs hatchlings [insufficient data]
Chicken - examination of embryos Dinerman et al. (1970)
[insufficient data]
Quail
Coturnix coturnix 0, 50, 150, 300, 600, 900, growth, reproduction, Bursian & Edens (1977)
japonica 1200 mg/kg diet from hatching post-hatching viability
10 per group through 14 weeks
Colinus virginianus 0, 300, 1000, 3000 mg/kg diet for reproduction, post-hatching Fletcher & Leonard (1986a)
36 per group 22 weeks viability, gross pathology
Duck
Anas platyrhyncos 0, 300, 1000, 3000 mg/kg diet for reproduction, post-hatching Fletcher & Leonard(1986b)
36 per group 22 weeks viability, gross pathology
10-1000 µg/egg 0, 4, 7, 10, and examination of embryos or Khera (1966)
13 days eggs hatchlings [insufficient data]
8.5.1 Mammalian reproductive toxicity studies
8.5.1.1 Mouse
Studies on the effects of carbaryl (8.5-34 mg/kg per day),
given orally for 5 days, to mature male Swiss Webster mice,
indicated no effects on the weights of testes and accessory sex
glands or uptake of C14-labelled testosterone by the prostate
gland, as measured on the day after treatment (Thomas et al.,
1974). These workers also examined the biotransformation of
testosterone-1,2-3H in animals given 34 or 68 mg/kg per day,
orally, for 5 days. They found a significant decrease in
androstenedione synthesis in the high-dose group, indicating
increased hepatic androgen hydroxylase activity (Dieringer & Thomas,
1974).
8.5.1.2 Rat
Collins et al. (1971) studied the effects of carbaryl given
in the diet (0, 2000, 5000, and 10 000 mg/kg) to Osborn-Mendel rats
over 3 generations. The doses actually administered could only be
estimated since there were no measurements of food intake. On the
basis of a 15 g/day food intake and a 235 g rat, it can be estimated
that animals received of the order of 125, 250, or 500 mg/kg per
day. It should be noted, however, that the food intake of lactating
rats greatly increases, so these figures may considerably
under-estimate carbaryl exposure during this critical time period.
The author used the number of animals mated rather than the number
giving birth as the number for litter size and viability, therefore
their calculations are overestimates. Nevertheless, the data do show
impaired fertility in the high-dose group (which also exhibited
growth rate reduction) as well as reduced postnatal survival. The
number of live-born offspring and growth rate were reduced in the
5000 and 10 000 mg/kg diet groups.
A three-generation study on Wistar rats was carried out by Weil
et al. (1973) in which animals were given 0, 7, 25, 100, or
200 mg/kg per day in the diet or 0, 3, 7, 25, or 100 mg/kg per day
orally. Maternal toxicity was seen in the dietary study at 200 mg/kg
per day (decreased weight) and in the gavage study at 100 mg/kg per
day (decreased weight and mortality). Postnatal toxicity was noted
in the 100 mg gavage group (reduced litter size and viability), but
not in lower dose groups. The dietary study indicated fewer effects
in the maternal animals with only an initial loss in weight. No
perinatal effects were noted.
Vashakidze (1975) exposed male and female rats (number and
strain unspecified) to 0, 1, 5, 10, 20, 40, and 50 mg carbaryl/kg,
orally, for 1 month. Dose-related changes were noted in serum
albumin (decrease), globulins (increase), ChE and AChE (decrease),
aspartate transaminase (increase), and alanine transaminase
(decrease). Reductions in stem cells as well as spermatozoa were
noted at doses of 5 mg/kg or more. Adverse litter effects were seen
in treated females. These effects included increased embryo/fetal
death, decreased implantations, and prolonged estrus cycle.
Luca & Balan (1987) administered carbaryl-beta-naphthol to
Wistar rats in the diet for up to 18 months. The treated groups
showed an increase in sperm shape abnormalities, though there were
no clear dose- or time-relationships with effects.
8.5.1.3 Gerbil
Collins et al. (1971) published the results of a 3-generation
reproduction study in which animals were exposed to 0, 2000, 4000,
6000, or 10 000 mg/kg diet. Since all postnatal calculations used
the number of animals mated rather than the number giving birth, the
authors' data must be recalculated. When this is done, the magnitude
of the effects reported is reduced. Adverse effects on various
reproductive parameters are nevertheless seen, though the effects
are not clearly related to dose levels in the 2000-6000 mg/kg diet
groups. These data are difficult to interpret given the lack of
information on maternal effects at doses other than the highest,
where mortality was observed.
8.5.2 Mammalian developmental toxicity studies
8.5.2.1 Mouse
Murray et al. (1979) administered 100 or 150 mg carbaryl/kg
per day, by gavage, or 5660 mg/kg diet (calculated to be 1166 mg/kg
per day) to CF-1 mice on gestation days (g.d.) 6-15. Maternal
toxicity was noted in the 150-mg group (ataxia, lethality). Litter
effects were noted in the 150 mg/kg per day group, where an increase
in entirely resorbed litters was seen, and in the dietary group
where there was decreased fetal weight.
Pregnant mice were given 0, 100, 150, or 200 mg carbaryl/kg per
day, orally, on gestation days 8, 12, or 6-15, and fetuses were
examined at term (Mathur & Bhatnagar, 1991). Maternal death was
noted at the high dose in the group receiving carbaryl on gestation
days 6-15. Fetal weight reductions were seen at the high-dose levels
in all groups, as was reduced ossification, open eyelids, and
enlarged renal pelvis. These effects may be indicative of fetal
growth retardation.
8.5.2.2 Rat
Carbaryl was administered to Harlan Wistar rats at 0, 20, 100,
or 500 mg/kg per day, orally, on gestation days 1-7, 5-15, or 1-21
(Weil & Carpenter, 1965; Weil et al., 1972). Maternal toxicity was
evident as was reduced weight gain in the high-dose groups receiving
the chemical on gestation days 5-15 or 1-2. No adverse fetal effects
were seen.
Golbs et al.(1975) treated Wistar rats with 0, 200, or 350 mg
carbaryl/kg, orally, or 40 mg/kg ip on a variety of single or
multiple gestation days. Reductions in fetal weight were seen in
some groups. No other effects were noted.
Sprague-Dawley rats were treated orally with 0, 1, 10, or
100 mg carbaryl/kg per day, three months before and during
gestation, and litters were examined at term (Lechner &
Abdel-Rahman, 1984). Maternal weight gain was significantly less in
the 100 mg/kg group than in controls. No compound-related effects on
fetuses were noted.
8.5.2.3 Guinea-pig
Weil et al. (1973) administered carbaryl to guinea-pigs at
dose levels of 0, 100, 200, or 300 mg/kg per day in the diet, or 0,
50, 100, or 200 mg/kg per day, orally. These dose levels were
determined to be maximum non-maternally toxic doses in preliminary
studies by the authors, though the 200 mg/kg dose did reduce
maternal weight gain. No significant adverse embryo/fetal effects
were seen in any treatment groups.
8.5.2.4 Rabbit
Murray et al. (1979) tested the effects of carbaryl on New
Zealand White rabbits during gestation. Dams had diarrhoea at the
high dose of 200 mg/kg per day and animals at this dose level as
well as at the lower dose level (150 mg/kg per day) gained less
weight during gestation than controls. A significant increase in
umbilical hernia was noted in fetuses at the 200 mg/kg per day
group.
8.5.2.5 Dog
Smalley et al. (1968) administered 3.125, 6.25, 12.5, 25, or
50 mg carbaryl/kg in the diet, throughout gestation, to beagle dogs.
Maternal toxicity was noted at all dose levels. This toxicity was
described by the authors as dystocia and symptoms included delayed
delivery, anorexia, and restlessness. A variety of birth defects
were found at doses of 6.25 mg/kg or more. The defects included
ectopic intestines, brachygnathia, acaudia, polydactyly, and
intestinal agenesis.
Another study was carried out by Imming et al. (1969) on the
beagle dog. These workers used 0, 2.0, 5.0 or 12.5 mg/kg per day,
orally, throughout gestation. As in the Smalley (1968) study,
treated females exhibited toxicity during labour and single deaths
were recorded at this time in all treated groups. Birth defects
including umbilical hernia and gastrointestinal defects were seen at
the 5.0 and 12.5 mg dose levels only.
8.5.2.6 Pig
Two studies on pigs were carried out by Earl et al. (1973).
In the first study, fetal pigs were examined from dams given 0, 4,
8, or 16 mg carbaryl/kg per day in the diet; in the second, dams
were allowed to farrow after receiving 0, 16, or 32 mg/kg per day in
the diet. Animals were exposed throughout most or all of gestation.
Effects noted were not consistent across the studies and increased
prenatal lethality seen in the first was not noted in the second,
even at a higher dose level. A small number of malformations were
noted, but no characteristic dose-related pattern was evident.
8.5.2.7 Monkey
Dougherty et al. (1971) exposed a small number of female
Rhesus monkeys to 0, 2, or 20 mg carbaryl/kg, throughout gestation.
Of the 8 pregnant, treated females, 5 were reported as having
aborted as opposed only 1 out of 5 in the controls. The Coulston
et al. (1974) study included a larger number of animals per dose,
an additional lower dose level (0.2 mg/kg per day), and a shorter
dosage period (days 20-38 of gestation). No adverse effects were
noted in the second study. These studies are not comparable for the
evaluation of the abortifacient potential of carbaryl, since the
dosing began later in gestation in the second study. The authors
noted that the determination of abortion in the Rhesus monkeys may
not have been reliable.
8.5.3 Reproductive and developmental toxicity studies in
non-mammalian species
8.5.3.1 Fish
Medaka ( Oryzias latipes) embryos were exposed to nominal
carbaryl concentrations of 0.5, 1.0, 2.5, 5.0, 10.0, 20.0, or
30.0 mg/litre in the water from the 4-cell through to the blastula
stages (Solomon & Weis, 1979). Exposure of the eggs resulted in
increased cardiovascular anomalies (heart defects, circulatory
defects, and edema) at dose levels of 5 mg/litre or more.
8.5.3.2 Amphibian
Elliott-Feeley & Armstrong (1982) treated both embryos and
tadpoles of Xenopus laevis with nominal carbaryl concentrations in
the water of 0.1, 1.0, or 10.0 mg/litre. Defects were seen in
embryos exposed to 10 mg/litre, which also resulted in significant
lethality. Embryo growth was reduced at all dose levels.
Carbaryl-exposed tadpoles exhibited decreased activity at all dose
levels.
8.5.3.3 Birds
Chicken eggs have been used by a number of workers to test the
potential of carbaryl to affect avian development. Olefir &
Vinogradova (1968) injected eggs with carbaryl at 0.01, 0.1, 1.0, or
10.0 mg/kg and examined embryos at different times after injection.
Death and anomalies were recorded at 5, 10, 15, and 20 days of
incubation at all doses above 0.01 mg/kg. Lillie (1973) administered
0, 250, or 500 mg/kg diet to pullets for 36 weeks beginning at 32
weeks of age. The adult birds showed reduced weights at both levels
of carbaryl in the adults and at 500 mg in the progeny. No
embryotoxicity or other effects were noted. Swartz (1981) examined
the effects of carbaryl on chicken embryo development. They examined
chick embryos at different days after injection, for 5 or 12 days
post-fertilization. They found vehicle-related increased mortality
(carbaryl was more toxic when administered in sesame oil than in
acetone) for both periods of exposure. Scattered anomalies were
recorded in surviving embryos. In another study (Swartz, 1985)
primordial germ cell migration was followed after injection of 10 mg
carbaryl per egg prior to incubation. Results did not indicate any
significant adverse effects on the primordial germ cells or
reproductive organs.
Bursian & Edens (1977) studied the effects of carbaryl on the
fertility and post hatching viability of Japanese quail ( Coturnix
coturnix japonica). Birds were exposed from hatching to 14 weeks
of age (breeding maturity). Fertility and the hatchability of eggs
were measured. The animals received 0, 50, 150, 300, 600, 900, or
1200 mg carbaryl/kg diet. Growth of the adult birds was reduced at
the 900 and 1200 mg/kg dose levels. There were no significant
effects on any reproductive parameter.
Fletcher & Leonard (1986a) investigated the effects of carbaryl
on reproduction in bobwhite quail ( Colinus virginianus) exposed to
0, 300, 1000, or 3000 mg/kg diet for 22 weeks. No adverse effects
were described in any factors related to hatchability, posthatching
viability, or gross pathology of newly hatched birds. These workers
used a similar protocol to study the effects of carbaryl on mallard
ducks ( Anas platyrhyncos) (Fletcher & Leonard, 1986b). The 3000
mg/kg diet level was toxic with some lethality, decreased numbers of
eggs, and thinner egg shells. No effects were seen at the 300 or
1000 mg/kg diet levels.
8.5.4 Appraisal
In summary, mammalian studies on the reproductive or
developmental toxicity of carbaryl clearly show that this compound
is capable of inducing adverse effects in utero and during the
reproductive process. These effects are always seen only at dose
levels at which there is concurrent maternal toxicity, with the
possible exception of a few studies on the rat which have not been
replicated by other workers. For a number of species, the dams
appear to be more sensitive than their litters. In general, the
adverse effects noted in developmental toxicology studies cannot be
simply attributed to maternal toxicity (Chernoff et al., 1990).
However, the pattern of maternal and fetal toxicity occurring at the
same dose levels indicates that the developing mammalian
embryo/fetus is not especially susceptible to carbaryl.
Carbaryl has been shown to be embryotoxic for fish, amphibians,
and birds, at some exposure concentrations.
8.6 Mutagenicity of carbaryl and N-nitrosocarbaryl
In this section on mutagenicity, and in the section on
carcinogenicity (8.7.1), the two compounds, carbaryl and
nitrosocarbaryl, are discussed (separately), since the formation of
N-nitrosocarbaryl was reported to occur in the stomach of rats and
guinea-pigs, in the presence of sodium nitrite and carbaryl, under
acid conditions (Elespuru & Lijinsky, 1973; Beraud et al., 1979;
Rickard & Dorough, 1984). See also section 8.9.3.
8.6.1 Genotoxicity assays in vitro
8.6.1.1 Primary DNA damage
(a) Carbaryl
Carbaryl did not cause DNA damage in different wild type
strains and DNA recombination lacking strains of Bacillus subtilis
(Table 49). Carbaryl was reported to be non-genotoxic for
B. subtilis Marburg 17A Rec+ and Marburg M45T Rec- strains,
highly sensitive to frameshift mutagens, even at the highest tested
concentration of 10 mg/plate (Uchiyama et al., 1975).
Carbaryl (10-4 mmol/litre) did not affect the sedimentation
profiles of DNA from human skin cells in culture (both normal and
xeroderma pigmentosum), either immediately or 20 h after treatment
(Regan et al., 1976).
There are two controversial reports on the genotoxicity of
carbaryl, evaluated by the induction of mitotic gene conversion in
the diploid strain of Saccharomyces cerevisiae, heteroallelic at
the two different loci ade2 and trp5. Siebert & Eisenbrand (1974)
used an assay system with a 16-h incubation time and a concentration
of carbaryl of 4.97 mmol/litre and did not observe any changes in
the control frequency of mitotic gene conversion. However, Jaszczuk
& Syrowatka (1979), reported a weak positive response with a lower
concentration of carbaryl (2.5 mmol/litre) and shorter (5 h)
incubation time. No converting activity of N-hydroxy carbaryl (2.5
mmol/litre) was found under the same assay conditions.
Table 49. Bacterial assays of genetic toxicity of carbaryl
Test Test organism Concentration Genetic Metabolic Result Reference
(µg/plate) end-point activation
DNA B. subtilis 700 mg/litre Rec assay - negative DeGiovanni-Donnelly et al. (1968)
damage
B. subtilis 20 Rec assay - negative Shirasu et al. (1976)
B. subtilis 0.4, 4, 40, 400 Rec assay - negative Eto et al. (1982)
Gene B. subtilis up to 10 000 Rec assay - negative Uchiyama et al. (1975)
mutation
E. Coli WP2 1000 Try- - negative Ashwood-Smith et al. (1972)
E. Coli WP2 1000-3000 Try- - negative Nagy et al. (1975)
E. Coli WP2 10 000 Try- - negative Uchiyama et al. (1975)
H. influenzae 10 µmol/litre Novobiocim - negative Elespuru et al. (1974)
S. typhimurium
TA 98 up to 2000 His- - negative McCann et al. (1975)
TA 98 up to 2000 His- Rat negative
TA 98 50 nmol/litre His- - negative Blevins et al. (1977)
TA 98 10-1500 His- - negative DeLorenzo et al. (1978)
Table 49 (continued)
Test Test organism Concentration Genetic Metabolic Result Reference
(µg/plate) end-point activation
TA 98 10-1500 His- Rat negative
TA 98 0.25, 2, 5, 50, 1000 His- - negative Jaszczuk et al. (1979)
TA 98 up to 5000 His- - negative Moriya et al. (1983)
TA 98 up to 5000 His- Rat negative
TA 98 0.2, 2, 20 His- - negative Eto et al. (1982)
TA 98 0.2, 2, 20 His- Rat negative
TA 98 5-2000 His- - negative Lawlor (1989)
TA 98 5-2000 His- Rat negative
TA 100 up to 2000 His- - negative McCann et al. (1975)
TA 100 up to 2000 His- Rat negative
TA 100 50 nmol/litre His- - negative Blevins et al. (1977)
TA 100 10-1500 His- - negative DeLorenzo et al. (1978)
TA 100 10-1500 His- Rat negative
TA 100 0.25, 2, 5, 50, 1000 His- - negative Jaszczuk et al. (1979)
TA 100 0.2, 2, 20 His- - negative Eto et al. (1982)
Table 49 (continued)
Test Test organism Concentration Genetic Metabolic Result Reference
(µg/plate) end-point activation
TA 100 0.2, 2, 20 His- Rat negative
TA 100 5-2000 His- - negative Lawlor (1989)
TA 100 5-2000 His- Rat negative
TA 1535 up to 2000 His- - negative McCann et al. (1975)
TA 1535 up to 2000 His- Rat negative
TA 1535 2500 His- - positive Marshall et al. (1976)
TA 1535 1000 His- Rat negative
TA 1535 50 nmol/litre His- - negative Blevins et al. (1977)
TA 1535 10-1500 His- - negative DeLorenzo et al. (1978)
TA 1535 10-1500 His- Rat negative
TA 1535 up to 5000 His- - negative Moriya et al. (1983)
TA 1535 up to 5000 His- Rat negative
TA 1535 5-2000 His- - negative Lawlor (1989)
TA 1535 5-2000 His- Rat negative
Table 49 (continued)
Test Test organism Concentration Genetic Metabolic Result Reference
(µg/plate) end-point activation
TA 1536 2500 His- - negative Marshall et al. (1976)
TA 1536 1000 His- Rat negative
TA 1537 up to 2000 His- - negative McCann et al. (1975)
TA 1537 up to 2000 His- Rat negative
TA 1537 50 nmol/litre His- - negative Blevins et al. (1977)
TA 1537 2500 His- - negative Marshall et al. (1976)
TA 1537 1000 His- Rat negative
TA 1537 10-1500 His- - negative DeLorenzo et al. (1978)
TA 1537 10-1500 His- Rat negative
TA 1537 0.25, 2, 5, 50, 1000 His- - negative Jaszczuk et al. (1979)
TA 1537 up to 5000 His- - negative Moriya et al. (1983)
TA 1537 up to 5000 His- Rat negative
TA 1537 5-2000 His- - negative Lawlor (1989)
TA 1537 5-2000 His- Rat negative
TA 1538 100 His- - positive Egert & Greim (1976)
Table 49 (continued)
Test Test organism Concentration Genetic Metabolic Result Reference
(µg/plate) end-point activation
TA 1538 100 His- Mouse positive
TA 1538 50 nmol/litre His- - negative Blevins et al. (1977)
TA 1538 2500 His- - negative Marshall et al. (1976)
TA 1538 1000 His- Rat negative
TA 1538 10-1500 His- - negative DeLorenzo et al. (1978)
TA 1538 10-1500 His- Rat negative
TA 1538 up to 5000 His- - negative Moriya et al. (1983)
TA 1538 up to 5000 His- Rat negative
TA 1538 5-2000 His- - negative Lawlor (1989)
TA 1538 5-2000 His- Rat negative
Carbaryl was inactive in the rat primary hepatocytes
unscheduled DNA synthesis assay (Cifone, 1989). It did not induce
significant changes in the nuclear labelling of rat primary
hepatocytes in two independent trials with applied concentrations
ranging from 5 to 25 µg/ml.
No DNA-damaging properties of carbaryl, assessed by its
capacity for inducing unscheduled DNA synthesis (UDS) in cultured
human lymphocytes, were reported by Rocchi et al. (1980). However,
the authors did not use a standardized test protocol meeting
established criteria for the UDS assay performance. They tested only
one concentration of carbaryl (50 µg/ml), did not use any metabolic
activation system, did not run negative and adequate positive
controls concomitantly (other than the ultraviolet irradiation), and
did not apply relevant criteria to assess data.
The lack of DNA-damaging properties of carbaryl, as measured by
the induction of UDS, was confirmed by Probst et al. (1981). They
used a standardized protocol and autoradiographic techniques in
metabolically competent, cultured rat hepatocytes with test
concentrations ranging from 0.5 to 1000 nmol/ml. In contrast, Ahmed
et al. (1977a) reported that carbaryl induced the UDS of the
ultraviolet type (long patch repair) in a cultured human fibroblast
VA-4 cell line with, and without, metabolic activation in
concentration ranges of 1, 10, 100, and 1000 µmol/litre. However,
this study had several experimental shortcomings. The protocol, the
control value, and the results of compounds tested were not in
accordance with comparable assays by other authors.
(b) N-nitrosocarbaryl
The DNA-damaging properties of N-nitrosocarbaryl in
B. subtilis have been reported (Table 50).
In one study (Uchiyama et al., 1975), more pronounced
genotoxicity of N-nitrosocarbaryl to B. subtilis was registered
compared with that of N-methyl- N'-nitro- N-nitrosoguanadine.
However, the genotoxicity of N-nitrosocarbaryl was the lowest when
compared with other concomitantly tested nitrocarbamates.
In contrast to carbaryl, N-nitrosocarbaryl showed pronounced
genotoxicity toward S. cerevisiae D4 (Siebert & Eisenbrand, 1974).
N-nitrosocarbaryl, but not carbaryl, reacted with human DNA
in cell culture to form alkaline-sensitive bonds (Regan et al.,
1976). The DNA of nitrosocarbaryl-treated (10-4 mmol/litre) cells
showed a substantial reduction in sedimentation rate immediately,
and up to 20 h, after treatment. Presumably, the effect observed was
related to the induction of numerous single-strand breaks in the DNA
and the formation of DNA adducts. On the basis of selective
labelling, the authors suggested that the methyl-containing moiety
of nitroso-carbaryl was separated from the naphthalene ring in a
human fibro-blast culture and bound irreversibly to DNA (Regan
et al., 1976).
8.6.1.2 Gene mutation assay
(a) Carbaryl
As summarized in Table 49, carbaryl did not exert a mutagenic
effect in studies with E. coli, H. influenzae, or Salmonella.
Among the many reports on Salmonella, only two indicated a
positive effect. Thus, Marshall et al. (1976) observed increased
mutagenicity at 1000 µg/plate with S9 mix for TA1535. An evaluation
of this data is difficult because no negative and positive control
data were presented. Egert & Greim (1976) reported a positive
response for TA1538 by 100 µg/plate. The mutagenicity of carbaryl in
this study greatly increased when a non-standard metabolic
activation system (mouse liver microsome) was used.
Ahmed et al. (1977b) reported a positive mutagenic response
to carbaryl in Chinese hamster V79 cells at a dose level of
0.01 mmol/litre, with no metabolic activation. There was a
concentration-related effect of carbaryl on cell survival; less than
50% of the cells survived at concentrations >0.01 mmol/litre.
Mutation studies carried out with a concentration of 0.01 mmol/litre
showed an approximately 8-fold increase in ouabain resistance
forward mutation in V79 cells as compared with the spontaneous
mutation rate.
However, Wojciechowski et al. (1982) found no
ouabain-resistance with carbaryl in a cell-mediated mutagenesis
assay when they used an exogenous activating system in which
irradiated fetal cells of Syrian hamsters were co-cultivated with
cells of Chinese hamsters V79. No mutagenic response was observed
for carbaryl at concentrations of 0.01, 0.05, 0.1 mmol/litre with,
or without metabolic activation. The toxicity of carbaryl at these
concentrations ranged from 7% at the lowest concentration to 23% at
the highest.
Carbaryl produced a negative result for inducing forward
mutations at the HPRT locus in Chinese hamster ovary cells both
with, and without, metabolic activation (Young, 1990).
Table 50. Bacterial assays of the genetic toxicity of nitrosocarbaryl
Test Test organism Concentration Genetic Metabolic Result Reference
(µg/plate) end-point activation
DNA B. subtilis 0.4, 4, 40 Rec assay - positive Eto et al. (1982)
damage
B. subtilis up to 100 Rec assay - positive Uchiyama et al. (1975)
Gene
mutation E. Coli 30 R 0.1 mmol/litre Try- - positive Elespuru et al. (1974)
E. Coli WP2 5, 10, 50, 100 Try- - positive Uchiyama et al. (1975)
E. Coli K12 100 Try- - positive Egert & Greim (1976)
E. Coli K12 100 Try- Mouse positive
H. influenzae 10 µmol/litre Novobiocin - positive Elespuru et al. (1974)
S. typhimurium
TA 98 0.001-11 His- - positive/ Blevins et al. (1977)
negative
TA 9 10-1500 His- - negative DeLorenzo et al. (1978)
TA 98 10-1500 His- Rat negative
TA 98 0.25-1000 His- - positive Jaszczuk et al. (1979)
Table 50 (continued)
Test Test organism Concentration Genetic Metabolic Result Reference
(µg/plate) end-point activation
TA 98 0.02, 0.2, 2 His- - negative Eto et al. (1982)
TA 98 0.02, 0.2, 2 His- Rat negative
TA 98 0.01-100 His- - negative Rickard et al. (1982)
TA 98 0.1-100 His- Rat negative
TA 100 0.001-11 His- - positive Blevins et al. (1977)
TA 100 0.02, 0.2, 2 His- - positive Eto et al. (1982)
TA 100 0.02, 0.2, 2 His- Rat negative
TA 100 0.1-100 His- - positive Rickard et al. (1982)
TA 100 0.1-100 His- Rat positive
TA 1535 0.5-100 His- - positive Marshall et al. (1976)
TA 1535 50-1000 His- Rat positive
TA 1535 0.001-11 His- - positive Blevins et al. (1977)
TA 1535 0.25-1000 His- - positive Jaszczuk et al. (1979)
TA 1535 0.1-100 His- - positive Rickard et al. (1982)
TA 1535 0.1-100 His- Rat positive
Table 50 (continued)
Test Test organism Concentration Genetic Metabolic Result Reference
(µg/plate) end-point activation
TA 1536 0.5-100 His- - negative Marshall et al. (1976)
TA 1536 50-1000 His- Rat negative
TA 1537 0.001-11 His- - negative Blevins et al. (1977)
TA 1537 0.5-100 His- - positive Marshall et al. (1976)
TA 1537 50-1000 His- Rat positive
TA 1537 0.25-1000 His- - positive Jaszczuk et al. (1979)
TA 1538 100 His- - positive Egert & Greim (1976)
TA 1538 100 His- Rat positive
TA 1538 0.001-11 His- - negative Blevins et al. (1977)
TA 1538 0.5-100 His- - positive Marshall et al. (1976)
TA 1538 50-1000 His- Rat negative
TA 1538 0.25-1000 His- - negative Jaszczuk et al. (1979)
(b) N-nitrosocarbaryl
N-nitrosocarbaryl has shown a positive response in a number
of bacterial assays (Table 50). In Salmonella, N-nitrosocarbaryl
was mutagenic to strains responding to both base substitution
(TA1535, TA100) and frame shift (TA1536, TA1537, TA1538, TA98). The
mutagenic potential of N-nitrosocarbaryl toward Salmonella TA1535
was greatly diminished by the added exogenous activation system
(Marshall et al., 1976). A decrease in mutagenic activity by the
addition of S9 mix on frame shift mutagenesis has also been reported
(Marshall et al., 1976; Jaszczuk et al., 1979).
8.6.1.3 Chromosomal aberration assays and sister chromatid exchange
(a) Carbaryl
Animals
Carbaryl induced a clastogenic response in the three in vitro
bioassays (Ishidate & Odashima, 1977; Kazarnovskaya & Vasilos, 1977;
Önfelt & Klasterska, 1983). All the positive responses were observed
at toxic dose levels (30-80 µg/ml; 50, 100 µmol/litre); exogenous
metabolic activation was not required for activity. Thus, Ishidate &
Odashima (1977) observed a strong positive response for carbaryl
(30 µg/ml) in a chromosomal aberration assay using a Chinese hamster
fibroblast cell line. Carbaryl produced predominantly chromatid type
gaps, breaks, translocations, rings, and fragmentation, 48 h after
treatment.
Carbaryl was negative for inducing chromosomal aberrations in
CHO cells without metabolic activation but was positive under
metabolic activation conditions (Murli, 1989).
Kazarnovskaya & Vasilos (1977) reported a positive response for
carbaryl in cultures of human embryonic fibroblast: levels of 40 and
80 µg/ml caused a 4-fold and 10-fold increase, respectively, in the
frequency of chromosomal aberrations, at 24 h exposure time,
compared with a control rate of 2% (without S9-mix). Again, the
aberrations produced were mainly of a chromatid type; the type most
frequently observed was single fragments. An increased number of
paired fragments was found only at 80 µg carbaryl/ml. Carbaryl
(80 µg/ml) increased the percentage of cells with chromosomal
coiling (9.8% in the control group; 17.9% in the test group) and
aneuploidy (3% in the control group; 29.2% in the test group).
Previously, Kazarnovskaya & Vasilos (1977) had shown that carbaryl
suppressed mitosis, changed the rate of the mitotic phase, and
significantly increased the number of pathological forms of mitosis
in a human embryonic fibroblast culture, with a dose-time response.
Onfelt & Klasterska (1983) observed induction of viable
aneuploid/polyploid cells and multiple chromatid exchanges after
treatment of V79 Chinese hamster cells with carbaryl. The compound
exerted a pronounced chromosome-breaking effect, at a concentration
of 100 µmol/litre, 26 and 50 h after treatment. There was an
increased frequency of multiple chromatid exchanges and fragments,
as well as pulverisation and more diffuse signs of chromosome
damage. The effects of carbaryl on chromosome structure and
distribution were almost abolished by the simultaneous addition of
Aroclor-induced 2 or 10% rat S9-mix and glutathion.
In two in vitro assays for chromosome damage using Chinese
hamster lung fibroblasts, carbaryl was found to be positive
(Ishidate et al., 1981; Onfelt & Klasterska, 1984). In the latter
study, the effect of carbaryl on the incidence of sister chromatid
exchange was decreased by the addition of rat liver microsomes.
Söderpalm & Önfelt (1988) related the mitotic aberrations in V79
Chinese hamster cells, in part, to a reduction in the intracellular
levels of glutathione, and increased lipid peroxidation. They also
hypothesized that the anticholinergic effects of carbaryl may play a
role in the cleavage process.
Plants
A negative clastogenic response for carbaryl in a concentration
range of 50-200 mg/litre was reported by Ma et al. (1984) who used
the Tradescantia micronucleus test. At present, this test is
considered the most established and standardized plant assay system
for the purposes of in situ monitoring of environmental mutagen
pollution (Ma et al., 1984).
Carbaryl induced chromosomal effects in different plant assays
(Wuu & Grant, 1966; Amer & Farah, 1968; Brankovan, 1972). Carbaryl
increased by approximately 10-fold the number of aberrant cells in
the root tips of barley seedings ( Hordeum vulgare) in C1 and C2
generations after the seeds were treated with concentrations of 500,
1000, or 1500 mg carbaryl/litre for 6 and 12 h. The cytogenetic
effects induced included mostly metaphase and anaphase fragments and
anaphase bridges and were time-dependent (Wuu & Grant, 1966).
The mitotic, cytogenetic effects of carbaryl were seen in
Vicia faba (Amer & Farah, 1968) and sugar corn (Brankovan, 1972).
Carbaryl caused chromosomal abnormalities (chromosome lagging;
stickiness, mainly in diakinesis, polyploidy, fragments, anaphase
bridges) in different meiotic states in the pollen mother cells of
Vicia faba after spraying flower buds of different ages (2 weeks,
1 month) with saturated aqueous solutions of a commercial carbaryl
preparation. The percentage of the chromosomal aberrations increased
with increase in the frequency of spraying (every week) or 2 weeks
for l month; daily for 8 days) and then decreased when the recovery
time was increased (Amer & Farah, 1968).
8.6.2 Genotoxicity in vivo
8.6.2.1 Host-mediated assay
(a) Carbaryl
Usha Rani et al. (1980) reported that carbaryl did not have a
gene-mutation potential in vivo, when given orally in a toxic dose
of 438 mg/kg, 3 times daily for 3 days, to 6 Swiss male mice, which
were subsequently injected with Salmonella strain G46. These data
are consistent with those that showed that carbaryl was not
mutagenic for Salmonella in vitro.
8.6.2.2 Drosophila melanogaster and other insects
(a) Carbaryl
There are several reports of bioassays for carbaryl
genotoxicity in which Drosophila melanogaster was used (Brzeskii,
1972; Brzeskii & Vaskov, 1972; Hoque, 1972; Woodruff et al., 1983;
Omer et al., 1986).
In studies by Hoque (1972), carbaryl at 1.5 and 10 mg/litre was
given to female Drosophila. It was reported that the treatment
caused a changed sex ratio, changes in eye colour, and various
chromosomal aberrations in the offspring. The small amounts of
material used and the lack of any detailed presentation of the
findings preclude any evaluation or conclusion.
At high doses (0.3 ml from a 1% suspension of 85% commercial
product in glucose), carbaryl caused a slight increase in mutation
frequency in the F1 generation males of a Drosophila line, which
were studied at different stages of spermatogenesis. There were no
deletions or disturbed fertility (Brzeskii, 1972; Brzeskii & Vaskov,
1972).
Woodruff et al. (1983), who used a sensitive experimental
protocol that incorporated the mating scheme with repair-deficient
females, reported that carbaryl was not mutagenic for Drosophila.
F1 male progeny of males that had ring X chromosomes and double
marked Y chromosomes, were treated with carbaryl at 200 mg/litre and
mated with mus-302, repair-deficient females of Drosophila. The
male progeny did not show any induced complete (ring chromosome) or
partial (Y chromosome markers) chromosome loss.
The mutagenic activity of carbaryl in Drosophila melanogaster
was studied by Omer et al. (1986). The results indicated that
carbaryl does not increase the rate of dominant and sex-linked
recessive lethal mutations.
Carbaryl did not cause chromosome abnormalities in the meiotic
cells of male grasshoppers, when they were given a single toxic dose
(not precisely indicated) of 0.25 ml of the supernatant of an
aqueous suspension/solution of carbaryl by ip injection; however,
there were morphological disturbances in the spermatocytes (Venkat
Reddy et al., 1974).
(b) N-nitrosocarbaryl
Results obtained by Omer et al. (1986) from the
nitrosocarbaryl-treated populations of Drosophila melanogaster
suggested that nitrosocarbaryl increased significantly the
percentage of dominant lethal mutations above the spontaneous
mutation frequency. The results obtained from the 0.05, 0.10, and
0.15% nitrosocarbaryl-treated populations also showed a significant
increase in the percentages of sex-linked recessive mutations.
8.6.2.3 Chromosomal aberrations and sister chromatid exchange
(a) Carbaryl
There are several negative chromosomal studies on carbaryl and
N-nitrosocarbaryl in somatic and germ cells in vivo in mammals
(Venkat Reddy et al., 1974; Degraeve et al., 1976; Seiler, 1977,
Usha Rani et al., 1980; Dzwonkowska & Hübner, 1986). In all these
studies, sufficiently high dose levels of carbaryl were used (up to
the LD50) in order to define the negative cytogenetic responses.
Using the micronucleus test and the cytogenetic analysis of
metaphase chromosomes, Degreave et al. (1976) found no clastogenic
effects in the bone marrow of mice that had been given a single oral
dose of carbaryl (0.2 ml), an intraperitoneal dose (0.5 ml) of
carbaryl, or 7 oral doses of carbaryl solution (1x10-3 mg/litre)
alone, or with sodium nitrite (2x10-3 mg/litre).
A negative response for a high dose of carbaryl (146 mg/kg,
orally, 2 times per 24 h; 30-h sampling time) was reported by Usha
Rani et al. (1980) with an adequate protocol for the mouse bone
marrow micronucleous test.
No increase in the rate of the micronucleated, polychromatic
erythrocytes in the bone marrow of mice was found during in vivo
nitrosation of carbaryl after its oral administration at the maximum
tolerated dose of 100 mg/kg, together with an excess of sodium
nitrite (100 mg/kg) (Seiler, 1977).
A dose of 64 mg carbaryl/kg produced a negative result in an
in vivo assay of chromosomal aberrations in the bone marrow cells
of the Syrian hamster (Dzwonkowska & Hubner, 1986).
No chromosome aberrations were observed in the bone marrow of
Syrian hamsters (6 per dose), given single intraperitoneal
injections of doses up to the LD50 (64, 128, 320, and 640 mg/kg)
of a commercial mixture of carbaryl/lindane (40:10) (Dzwonkowska &
Hubner, 1986).
Daily oral doses of technical carbaryl (10 mg/kg) suspended in
peanut oil were given to male, albino rats for a period of 5 days.
There were no significant chromosomal changes in the bone marrow
cells of the exposed animals (Dikshith, 1991).
8.6.2.4 Dominant lethal assays in rodents
(a) Carbaryl
Epstein et al. (1972) studied the mutagenicity of carbaryl
using the dominant lethal test. In this assay, male ICR/Ha Swiss
mice were treated orally with 1000 or 50 mg carbaryl/kg daily for 5
successive days and then caged with 3 untreated virgin females,
which were replaced weekly for 8 consecutive weeks. The frequency of
early fetal deaths and preimplantation losses in the test groups
were within the limits of the control values. Therefore, dominant
lethal mutations were not induced in mice given sufficiently high
oral doses of carbaryl.
8.6.3 Other end-points
8.6.3.1 Cell transformation
(a) Carbaryl
Transformation of the fibroblast clone A31 of the BALB/3T3
mouse was not induced by carbaryl when given in non-toxic (1.5 and
10 µg/ml) or moderately cytotoxic (20 and 40 µg/ml) doses, over 24 h
(Quarles & Tennant, 1975).
(b) N-nitrosocarbaryl
N-nitrosocarbaryl showed transforming activity, at
concentrations of 10-20 µg/ml, which was cytotoxic for the BALB/3T3,
A31 cells. That 3-10 cell passages are necessary for detecting the
transforming event suggests that the transformation frequency for
N-nitrosocarbaryl in this test system is low. Transformed cells
showed morphological alterations, loss of contact inhibition, and
growth in soft agar, as well as carcinogenic activity in normal
newborn, irradiated, suckling, or athymic BALB/C mice. The tumour
incidence (anaplastic sarcomas) was relatively low; in several
instances, tumours regressed in normal mice, but not in athymic
mice, after 2-3 weeks of growth. N-nitrosocarbaryl did not
activate detectable amounts of the endogenous murine leukaemia
viruses carried by the BALB/3T3 cells or viral protein. However,
viral antigen production in the transformed cells was induced by
iododeoxyuridin, which indicated the presence of the viral genome.
On the basis of this N-nitrosocarbaryl transforming activity in
mammalian cells in culture, Quarles & Tennant (1975) suggested that
the compound may be an active, though weak, carcinogen in vivo.
8.6.3.2 Aneuploidy induction
(a) Carbaryl
Tests of the chemical induction of spindle fibre inactivation
and c-mitosis (colchicine-mitosis) in Allium revealed the
existence of an unspecific physico/chemical mechanism, based on the
partitioning of compounds into hydrophobic compartments of the cell.
This means that chemical compounds, in general, cause c-mitosis
according to their lipophilic characteristics, as indicated by the
octanol/water partition coefficient. Thus, a close correlation
exists between the lipophilicity of compounds and the dose at which
spindle disturbance occurs. However, besides this unspecific effect
on the spindle fibres mechanism, there are some compounds that
exhibit specific effects causing inactivation of the spindle fibre
mechanism at lower doses than indicated by their lipophilicity. This
can occur via different mechanisms, such as specific binding to
actin (colchicine) or binding to sulfhydryl groups (organic
mercury). Onfelt (1987) analysed the c-mitotic effects of 22
compounds in V79 hamster cells. Five of these compounds fell outside
the regression line for lipophilicity/c-mitosis, among them
colchicine, methyl mercury, and carbaryl. Further analyses revealed
that carbaryl owes its pronounced c-mitotic action to its reactivity
with sulfhydryl groups. It is therefore not surprising that several
authors have reported mitotic disturbances caused by carbaryl in
various experimental systems. Onfelt & Klasterska (1983) reported
that a significant increase in the aneuploidy/polyploidy cells was
obtained with both 50 and 100 µmol carbaryl/litre, 26, 59, and 74 h
after treatment. Carbaryl caused mitotic disturbances in Allium
cepa, Vicia faba, Gossypium barbadense, Nigella damascena (Amer,
1965; Amer et al., 1971; Degraeve et al., 1976). Amer (1965)
reported c-mitotic effects of carbaryl in the roots of Allium cepa
that had been treated for 4 or 24 h with different concentrations of
pure (0.5, 0.25%) and commercial products (85% sprayable powder).
The increased rate of abnormal meta-telophases and ana-telophases
depended on the concentration and temperature of the test solutions.
The types of induced abnormal metaphases included star-metaphases
and a few prophase-metaphases. Two types of anaphases, the c-type
and the multipolar type, as well as multinuclear interphase cells,
were observed. Continuous treatment with carbaryl for 24 h nearly
arrested mitosis. There was a full recovery of mitosis in 48 h and
induced signs of polyploidy appeared.
Consistent with these findings are the c-mitotic effects of
carbaryl in Vicia faba and Gossypium barbadense (Amer et al.,
1971). The mitotic index of Vicia faba, after root treatment,
decreased with increased concentrations of carbaryl (25, 50, or
100 mg/litre for 4 h. There was no effect from carbaryl after
seed-soaking for different lengths of time. Mitotic anomalies
(mostly disturbed meta- and anaphases) in the roots of Vicia faba
and Gossypium barbadense showed a concentration-time response.
Carbaryl caused mitotic disturbances (c-mitotic effect,
multipolar anaphases) or cytotoxicity (pycnotic nuclei, tissue
degeneration) after root treatment of Nigella damascena with
concentrations of 2.5x10-4 and 1x10-3 mg/litre. When NaNO2 (2x10-3)
was added to carbaryl solutions of 1x10-2 and 1x10-3 mg/litre, the
effects increased, possibly because of the formation of
nitrosocarbaryl (Degreave et al., 1976). No effect was observed
after grain treatment.
Soaking sugar corn seeds for 48 h with a 0.12 or 0.25% aqueous
solution of 50% commercially prepared carbaryl, resulted in a
dose-response induction of aberrations of anaphase chromosomes of
types not seen in the controls (bridges with, or without, fragments,
dicentrics). Repeated treatment of young plants with a 0.25%
solution of carbaryl for 6 h during meiosis caused typical c-mitotic
effects in metaphase and anaphase through the arrest of cell
division and spindle inactivation. Some of the induced aberrations
persisted until the generative stage. They were fixed and
incorporated through the embryonal and generative development stages
and thus increased pollen sterility (Brankovan, 1972).
Carbaryl was reported to increase the number of aberrant forms
of mitosis (mostly c-mitotic effect) in the small intestine, cornea,
and spleen of rats given oral doses of 400 mg/kg. The authors also
reported that at lower doses of carbaryl (40 or 80 mg/kg), there was
an increased incidence of bridge and chromosome lagging in anaphase
and telophase, in addition to a high percentage of c-mitosis. The
authors reported that there were no effects at 20 mg/kg. Only the
result following the 400 mg/kg dose was fully reported (Vasilos
et al., 1975).
8.6.4 Appraisal
Carbaryl has been evaluated for its potential mutagenicity in a
number of tests in vitro as well as in vivo, in bacterial, yeast,
plant, insect, and mammalian systems, testing a variety of
end-points.
The available evidence indicates that carbaryl has no
DNA-damaging properties. No confirmed induction of mitotic
recombination, gene conversion, and UDS in prokaryotes
( H. influenzae, B. subtilis) and eukaryotes ( S. cerevisiae, A.
nidulans, cultured human lymphocytes, and rat hepatocytes)
in vitro has been reported.
Negative results were obtained in tests for gene mutations in a
number of bacterial assays, with the exception of two cases. In
several studies of gene mutations in mammalian cells in vitro,
carbaryl only produced one equivocal positive result in a cell
culture study. However, the study had several shortcomings and the
result has not been confirmed in any other comparable studies.
Chromosomal damage with high dosages of carbaryl, has been
reported in vitro in human, rat, and hamster cells and in plants.
No such effects have been observed in mammalian tests in vivo, even
at doses as high as 1000 mg/kg.
Carbaryl has been shown to induce disturbances in the spindle
fibre mechanism in plant and mammalian cells in vitro. The
relevance of plant assays for extrapolation to humans is unclear.
It can be concluded that the available data-base does not
support the presumption that carbaryl poses a risk of inducing
genetic changes in either the somatic or the germinal tissue of
humans.
The nitrosated product of carbaryl, N-nitrosocarbaryl, is
capable of inducing mitotic recombination and gene conversion in
prokaryotes (H. influenzae, B. subtilis) and eukaryotes (S.
cerevisiae) in vitro and gave positive results in E. coli spot
tests.
Furthermore, experimental results indicate that
N-nitrosocarbaryl binds to DNA, causing alkali-sensitive bonds and
single-strand breakage.
Nitrosocarbaryl has not been established as a clastogen
in vivo (bone marrow and germ cells), even at high toxic doses.
8.7 Carcinogenicity
8.7.1 Carcinogenicity studies of carbaryl in rodents
8.7.1.1 Mouse
Seven early carcinogenicity studies with carbaryl have been
reported (Table 51) involving different strains of mice,
subcutaneous injection, skin painting, ip and oral routes of
administration, different dose levels and dose schedules, and
various lengths of exposure and observation. None of these negative
studies (one marginal response) had sufficient data or data
reporting and it was not possible to evaluate them.
Table 51. Carcinogenicity studies on carbaryl in rodents
Chemical Species, strain Sex Route and Dose Duration Significant Evaluation Reference
(number) mode of (mg/kg) of study tumour/organ of individual
administration study
Carbaryl Mouse, A/Jax, C3H male SC 1/week 200 mg 20 weeks none inconclusive Carpenter et al.
30 in group (total) (1961)
Carbaryl Mouse, NS not skin painting not 24 months none inconclusive Weil & Carpenter
stated stated (1962)
Carbaryl Mouse, CD-1 male oral, diet 0.01% 80 weeks none inconclusive Mellon Institute
female 0.04% 2 years (1963)
Carbaryl Mouse, A,C3,HA not intraperitoneal 60 2 years none inconclusive Makovskaya et al.
stated 1/week (1965)
Carbaryl Mouse, (C57,B16x male oral gavage daily 4.64 18 months none inconclusive Innes et al.
C5H/Anf)F1 female 7 days-4 weeks 14 mg/kg (1969)
(C57B1/6xAKR)F1 72 of age; in diet body
4 weeks-18 weight
months of age
Carbaryl Mouse, A/He: 16 male intraperitoneal 6 mg 20 weeks marginal (±) inconclusive Shimkin et al.
3/week for (total) lung tumours (1969)
4 weeks response
Carbaryl Mouse, A/J: 124 female oral, diet 1000 10 weeks none inconclusive Triolo et al.
(1982)
Carbaryl Mouse CD-1 male diet 100,1000 53 weeks none no effect Hamada (1991b)
technical female 8000
Table 51 (continued)
Chemical Species, strain Sex Route and Dose Duration Significant Evaluation Reference
(number) mode of (mg/kg) of study tumour/organ of individual
administration study
Carbaryl Rat, CF-N 120 male oral, diet 0.005, 2 years none inconclusive Carpenter et al.
per level female 0.01, 0.02, (1961)
0.04% in
diet
Carbaryl Rat, Sprague-Dawley male diet 250, 1500 52 weeks none negative Hamada (1991b)
technical 80 or 90 per group and 7500
female
ß-Carbaryl Rat, mongrel 60 male oral gavage twice 30 22 months fibrosarcoma, positive but Andrianova &
48 control a week skin; polymorphic inconclusive Alexeev (1970)
cell sarcoma,
stomach;
osteosarcoma
with multiple
mestastases
48 single 20 mg 22 months fibrosarcoma, positive but
48 control subcutaneous skin inconclusive
implantation
Most of these studies involved the lung adenoma test in strain
A mice. This test is no longer considered to be a satisfactory
carcinogenicity bioassay because of the high rate of background
tumours that occur in untreated animals (Clayson, 1987).
Carbaryl did not increase the incidence of lung tumours in two
strains of male mice, A/Jax and C3H, which were given 20
consecutive, weekly, subcutaneous injections of 10 mg carbaryl
(Carpenter et al., 1961). As only one dose of carbaryl was tested
and the initial number of animals entered was insufficient, no
conclusion concerning the carcinogenic potential of carbaryl can be
drawn from this study.
There was no tumour development in mice (unspecified strain and
sex) after carbaryl application by skin painting for 24 months (dose
not specified, 48% water suspension) (Weil & Carpenter, 1962). This
study also lacked details about the experimental procedure and
pathology used and, so, was placed in the inconclusive category.
Carbaryl did not induce tumours in the lungs, liver, kidney,
heart, spleen, pancreas, and the thyroid and adrenal glands in two
strains of mice, A and C3HA, treated ip, once per week for about 2
years, with toxic doses of 60 mg/kg (Makovskaya et al., 1965). The
study involved a sufficient number of animals in the carbaryl group
(400), untreated control group (100), and urethan positive control
group (150), killed at 1, 3, 6, 9, 12, 15, 18, and 24 months after
the beginning of the study and studied histopathologically. However,
no numerical data on the background lung tumours in the untreated
control animals were presented. This lack of data, coupled with the
absence of tumours in the lungs of the carbaryl-treated mice strain
A, which are known for their high rate of naturally occurring lung
adenomas, makes the negative response of little significance.
Carbaryl yielded a marginal tumour response in the pulmonary
tumour induction test on mice (strain A) given a total dose of 6 mg
by ip injections, 3 times per week for 4 weeks (Shimkin et al.,
1969). Again, the lung adenoma test on strain A mice was not
reliable for evaluating possible carcinogenicity and, thus, the
results obtained from this study are not definitive.
No carcinogenic response was obtained with carbaryl
administered orally, by gavage, and/or in the diet to four different
strains of mice at doses ranging from 14 to 1000 mg/kg diet (Melon
Institute, 1963; Innes et al., 1969; Triolo et al., 1982).
There was no increase in the total tumour incidence and no
changes in tumour patterns in either sex of CD-1 mice fed diets
containing doses of 0.01 or 0.04% (100 or 400 mg/kg) carbaryl for 80
weeks and 2 years, respectively (Mellon Institute, 1969). The
survival rate in both test groups was too low for a response to be
observed; no information was given for one-third of the animals;
histopathology was performed only on animals that were suspected of
having tumours. Because of these deficiencies, no conclusion can be
drawn.
Carbaryl did not significantly increase the incidence of any
type of spontaneously occurring tumours (hepatomas, lung adenomas,
lymphoid sarcomas) in either sex of two hybrid strains of mice
(C57B1/6xC3HAnf) F1 and (C57B1/6xAKR) F1, treated orally with
4.64 mg/kg by gavage (mice from 7 days to 4 weeks of age), and in
the diet (mice from 4 weeks to 18 months of age) with 14 mg/kg diet
(Innes et al., 1969).
Another inadequate feeding study of carbaryl carcinogenicity
was conducted by Triolo et al. (1982), using the model of lung
tumour induction in strain A/J female mice. In two studies, the
feeding level of 1000 mg carbaryl/kg, incorporated in the diet of
mice for 20 weeks, did not cause a significant increase in the
incidence of background lung adenomas, nor did it induce tumours in
the glandular stomach or other tissues (spleen, intestinal tract).
However, there were a number of confounding factors in defining the
response in this study including: large variations in the incidence
of lung adenomas in the four separate control groups; in particular,
one such group had no tumours, despite high historical control
levels in strain A mice. In addition, only one dose level was
studied (1000 mg/kg), on the basis of which it is not possible to
establish a dose-response relationship.
In the second study, in which the same feeding level and
exposure period were used, carbaryl increased the lung
benzo( a)pyrene hydroxylase activity, which was associated with a
modest increase in the rate of lung tumours induced by the oral
administration of 3 mg BP twice, on days 7 and 21, respectively of
the study. However, there was great variability in the incidence of
BP-induced lung adenomas in the 3 control groups; the first
(7.2±0.8) was well above the values presented for the test group
carbaryl + BP (5.7±1.4); and the second fell within the limits of
the incidence of spontaneous tumours in the corn-oil control group,
(1.17±1.11), which make the results inconclusive.
A new mouse oncogenicity study is in progress, under
proprietary sponsorship. The study design was as follows: carbaryl
was administered in the diet to male and female CDR-1 mice at
rates of 0, 100, 1000, or 8000 mg/kg diet, for up to 104 weeks.
Groups consisted of 80 mice/sex per group. Ten mice/sex per group
were sacrificed for clinical pathology evaluation after 52 weeks of
treatment. Results of this interim sacrifice are presented in
section 8.3. The remaining animals were designated for continued
exposure to the end of the 104-week treatment period. The study
design and evaluation parameters are consistent with guidelines set
out by the US EPA and OECD, with additional study parameters, for
studies of this nature (Rhône-Poulenc, 1992).a
8.7.1.2 Rats
Two reports by Carpenter et al. (1961) and Andrianova &
Alexeev (1970) are controversial. They studied the carcinogenicity
of carbaryl given to rats by oral gavage, in the diet, or by
subcutaneous implantation. Both studies had insufficiencies in the
protocols used.
Carpenter et al. (1961) noted no significant increase in the
total tumour incidence in either sex of CF-N rats fed a diet
containing 0.005, 0.01, 0.02, or 0.04% carbaryl, for 2 years. Female
mice had more pituitary tumours than male mice, but there was no
significant difference in the incidence of tumours between the
control and test groups. The small initial number of animals used,
their relatively old age (60 days), their low survival rate, and the
lack of detailed pathology are complicating factors in defining a
negative response.
However, Andrianova & Alexeev (1970) reported that carbaryl,
administered by both oral gavage and subcutaneous implantation,
produced positive carcinogenic responses in mongrel rats. Male rats
given oral doses of 30 mg carbaryl/kg, twice weekly for 22 months,
developed skin fibrosarcoma, polymorphic cell sarcoma in the
stomach, and osteosarcoma with multiple metastases. Carbaryl caused
high lethality (80%) during the exposure period. The authors did not
state whether the gross pathology was examined. One fibrosarcoma was
discovered among 46 control animals, at 11 months. No data were
presented on the number of control animals that lived for 22 months,
so a comparison of survival rates in control and test groups could
not be made.
In a parallel study, 20 mg of carbaryl in a purified paraffin
capsule was implanted subcutaneously in 48 male rats. At the end of
the 22-month exposure period, subcutaneous fibrosarcomas, in sites
far from the implantation area, and on the back and neck, were
diagnosed in 2 out of 10 surviving animals. There was no control
group (Andrianova & Alexeev, 1970). The carbaryl used was obtained
from a plant in the USSR and was of technical grade and 97.65%
purity. No information about the chemical composition and impurities
was given. Because of these deficiencies, this study is
inconclusive.
a Information to Task Group. These studies have not yet been
reviewed by the IPCS. The company performing these studies has
indicated that there is a significant increase in tumors at the
highest dose in both species.
A new combined long-term toxicity and oncogenicity study on
rats is in progress, under proprietary sponsorship. The study design
is as follows: carbaryl was administered in the diet to male and
female Sprague-Dawley rats at rates of 0, 250, 1500, or 7500 mg/kg
diet, for up to 104 weeks. Groups consisted of 80 rats/sex per group
for low- and middle-dose groups and 90 rats/sex per group for the
high-dose and control groups. Ten animals/sex per group were
sacrificed after 26 and 52 weeks exposure and evaluated for clinical
pathology and histopathology. In addition, 10 animals/sex from the
control and high-dose groups were used as a recovery group, kept on
a basal control group diet before sacrifice at 56 weeks. The results
of the interim sacrifices are presented in section 8.3. The
remaining animals were designated for continued exposure to the end
of the 104-week treatment period. The study design and evaluation
criteria are consistent with guidelines set out by the US EPA and
OECD, with additional study parameters, for studies of this nature
(Rhône-Poulenc, 1992).a
8.7.1.3 Overall appraisal of carbaryl carcinogenicity
Carbaryl has been studied for its carcinogenic potential in
numerous studies on rats and mice via various routes of
administration. Most of these studies are old and do not meet
contemporary standards.
Only one paper from the existing body of publications clearly
reports a tumorigenic action of carbaryl. Carbaryl induced malignant
tumours in an unidentified strain of rat by the oral and
subcutaneous routes of administration. This study does not meet
contemporary standards, because of insufficient reporting of control
data.
New studies, designed to meet with contemporary standards, are
in progress on rats and mice. Descriptions of the studies are given
in sections 8.7.1.1 and 8.7.1.2.
8.7.2 Carcinogenicity studies of N-nitrosocarbaryl
Carbaryl is a secondary amine and is, therefore, capable of
nitrosation in the presence of nitro donor groups, such as sodium
nitrate, to give a nitrosamide. This nitrosamide, nitrosocarbaryl,
has been proved to be mutagenic and carcinogenic, at high doses in
a Information to Task Group. These studies have not yet been
reviewed by the IPCS. The company performing these studies has
indicated that there is a significant increase in tumors at the
highest dose in both species.
animals (see Table 52). A condition of this nitrosation is an acidic
pH (less than 2), which is comparable to the one found in the human
stomach. However, nitrosocarbaryl is not stable at this pH. Its
maximum stability is between pH 3 and 5, at which pH no significant
amount of carbaryl can be nitrosated. Carbaryl was nitrosated in
several studies, in vitro as well as in vivo, in the guinea-pig,
which has a stomach acidity similar to that of humans. See also
section 8.9.3.
8.7.2.1 Rats
N-nitrosocarbaryl elicited a carcinogenic response in both
sexes of two strains of rats (Wistar and Sprague-Dawley) by two
routes of administration (oral gavage and subcutaneous injection)
(Eisenbrand et al., 1975, 1976; Lijinsky & Taylor, 1976;
Preussmann et al., 1976; Lijinsky & Schmähl, 1978).
A local carcinogenic effect of N-nitrosocarbaryl was reported
by Eisenbrand et al. (1975) in Wistar rats given a single
subcutaneous injection of 1000 mg/kg. Fifteen out of 16 treated
animals developed sarcomas at the injection site; there was no
histopathological evidence of any systemic carcinogenic effects. The
dose of N-nitrosocarbaryl was highly toxic and 14 out of 16
animals had died by day 450. No spontaneous tumours were observed in
control animals. When given to rats orally in single doses of
200-1500 mg/kg, N-nitrosocarbaryl did not induce tumours during 21
months of observation. No signs of toxicity were noted in any of the
test groups.
Further studies involving repeated oral administration of
N-nitrosocarbaryl to Sprague-Dawley rats gave unequivocal evidence
of local carcinogenic effects, produced by both oral and
subcutaneous routes of administration. (Eisenbrand et al., 1976;
Lijinsky & Taylor, 1976, 1977; Preussmann et al., 1976; Lijinsky &
Schmähl, 1978).
The nonglandular part of the stomach (forestomach) was the
target organ of the local carcinogenic action of N-nitrosocarbaryl,
when it was given orally to rats. All stages of malignant
transformation in the forestomach, from hyperplasia to squamous cell
carcinoma, were observed in male Sprague-Dawley rats treated with
oral doses of 130 mg N-nitrosocarbaryl/kg, twice weekly, until
spontaneous death. There was a substantial lowering of the survival
rate of treated animals because of the pronounced toxicity. By the
time of maximum increase of the tumour incidence (after 200 days),
most of the animals were dead. The average survival time of
tumour-bearing animals was 167 days after the onset of the study.
The low background tumour incidence (3 out of 29) in control animals
included lymphosarcomas and leukaemia.
Table 52. Carcinogenicity studies on N-nitrosocarbaryl in rodents
Chemica Species, strain Sex Route and Dose Duration Significant Evaluation Reference
(number) mode of (mg/kg) of study tumour/organ of individual
administration study
N-nitroso Rat, Wistar male subcutaneous, 1000 450 days polymorphic cell positive Eisenbrand et al.
carbaryl 8: male; female single injection sarcoma at (1975)
8: female injection site;
spindle cell
sarcoma
37 male, oral gavage, 200-1500 21 months none inconclusive
female single
N-nitroso Rat, Sprague-Dawley male oral gavage, 130 (5000 till squamous cell positive Eisenbrand et al.
carbaryl 31 twice per week total) spontaneous sarcoma, (1976)
death papilloma, Preussmann et al.
hyperkeratoses, (1976)
forestomach
N-nitroso Rat, Sprague-Dawley female oral gavage, 50 mg 110 weeks squamous cell positive Lijinsky & Taylor
carbaryl 12 once a week (total) carcinoma (1976)
for 10 weeks forestomach,
papilloma,
oesophagus
12 male oral gavage, 300 mg 90 weeks squamous cell
twice a week (total) carcinoma
for 20 weeks forestomach,
papilloma trachea
N-nitroso Rat, Sprague-Dawley male oral gavage, 600 80 weeks carcinoma positive Lijinsky &
carbaryl 16: male; female once a week (total) forestomach Schmahl (1978)
16: female for 10 weeks
In a comparative study of the carcinogenic potency of
N-nitroso- N-alkylcarbamate esters, Lijinsky & Taylor (1976)
showed that N-nitrosocarbaryl was a strong carcinogen, similar in
action to its highly potent carcinogenic analogue
N-nitrosomethylurethane. A high incidence (75-80%) of
squamous-cell carcinomas in the forestomach was detected in female
Sprague-Dawley rats given 10, weekly, equimolar oral doses
(0.22 mmol) of both compounds. Nitrosomethylurethane was a more
potent carcinogen, because the animals with tumours died earlier
than those in the nitrosocarbaryl group. The same rate of carcinomas
in the forestomach was induced in male rats treated orally with a
considerably higher dose of N-nitrosocarbaryl (1.3 mmol total),
over twice as long a period (20 weeks). The stronger carcinogenic
effect of this dose in male animals was expressed by the shorter
latent period of induced tumours and higher lethality compared with
female animals. The local carcinogenic effect of N-nitrosocarbaryl
in rats, the target organ being the forestomach, was seen in another
oral carcinogenicity study of a series of nitroso- N-methylcarbamate
insecticides carried out by Lijinsky & Schmähl, (1978). The oral
gavage of 10 weekly doses of 60 mg nitrosocarbaryl/kg to both sexes
of Sprague-Dawley rats led to a high incidence (over 70%) of
carcinomas in the forestomach, with no evidence of other systemic
carcinogenic effects.
No carcinogenic response for carbaryl was obtained with the
transplacental carcinogenicity test after in vivo nitrosation in
pregnant Sprague-Dawley rats (Lijinsky & Taylor, 1976). Pregnant
animals were given 30 mg carbaryl/rat, orally, for 10 days (4-18
days of gestation). Two other groups of animals received the same
dose of carbaryl, together with 0.6-1ml of 4% sodium nitrite on days
4-6 and 14-18 of pregnancy. The distribution of tumours in the rats
of various groups was that normally seen in Sprague-Dawley rats. It
is likely that an insufficient amount of nitrosocarbaryl was formed
under the conditions of in vivo nitrosation, or that no
significant amount of nitrosocarbaryl crossed the placenta.
A low rate of in vitro nitrosation of carbaryl under
conditions similar to those that exist in the human stomach was
reported by Eisenbrand et al. (1975). The reaction of
10-3 mol/litre carbaryl with a 5-fold molar excess of sodium nitrite
in 0.1N HC1 (pH, 1) led, after 15-60 min, to yields of only 1.2 and
1.7% of the maximum possible conversion to nitrosocarbaryl.
Reduction of the concentration of carbaryl and sodium nitrite by a
factor of 10 decreased the yield of nitrosocarbaryl by about
one-half. The yields of N-nitrosocarbaryl obtained by the
in vitro nitrosation of carbaryl, under the described conditions,
are low and the potential carcinogenic risk of in vitro nitrosation
and similar nitrosation reactions in vivo is difficult to evaluate
at present.
8.7.2.2 Mice
Nitrosocarbaryl administered for 104 weeks to the skin of
female CFLP mice (65 mice per group) in three doses (12.5, 50, and
200 µg/mouse) was found to be more potent regarding dermal
carcinogenic efficiency than nitrosomethylurea, applied under the
same conditions, though not as effective as benzo- a-pyrene
(Deutsch-Wenzel et al., 1985). Conclusions were drawn on the basis
of the number of animals bearing carcinomas, total cases with local
tumours, observed/expected ratios and dose-response relationships of
incidences of malignant tumours. These results were found to be in
contrast to the ones obtained by Lijinsky & Winter (1981), who used
a single total dose of 23 mg and found that nitrosocarbaryl was a
much less effective inducer of mouse skin tumours than
nitrosomethylurea.
8.7.2.3 Overall evaluation of the carcinogenicity of
N-nitrosocarbaryl
Sufficient evidence of carcinogenicity at the site of
application was seen in multiple studies on both sexes of
Sprague-Dawley rats, by different routes of administration
(subcutaneous and oral gavage), using different dose levels. The
non-glandular stomach was the target tumour site when
nitrosocarbaryl was administered by oral gavage; subcutaneous
injection of nitrosocarbaryl caused sarcomas at the injection site.
The local carcinogenic effect of N-nitrosocarbaryl in rats,
and the lack of any systemic carcinogenicity, characterizes it as a
direct-acting alkylating agent. N-nitrosocarbaryl was active as a
direct bacterial mutagen and interacted with human DNA in vitro.
These data agree with data that show that N-nitrosocarbaryl is an
effective in vivo genotoxin.
8.7.3 Carcinogenicity of ß-carbaryl
Beta-carbaryl (N-methyl ß-naphthyl carbamate) was a component
of technical carbaryl (alpha-carbaryl), suspected of having a
carcinogenic structure and a tumorigenic potential in mice and rats
(Zabezhinski, 1970). In life-time studies on mice, strain CC57W (24
months), and mongrel rats (33 months), using two routes of
administration, oral gavage (10 mg/kg in mice; 25 mg/kg in rats) and
subcutaneous injection (20 mg/kg in mice; 50 mg/kg in rats),
ß-carbaryl showed a weak carcinogenic response. High rates of
lethality (20-50%) were observed in both mice and rats, by the two
routes of administration. No data for survival rates and the
incidence of spontaneous tumours in control animals were presented.
ß-carbaryl caused a low rate of tumours in rats by both routes of
administration. The types of tumours (21%) observed after
subcutaneous injection of ß-carbaryl included: fibrosarcoma at the
injection site, subcutaneous rhabdomyosarcoma, intestinal sarcoma,
leukaemia, reticuloses, and reticulosarcoma. The pattern of
carcinogenic response after orally administered ß-carbaryl (25%)
involved sarcoma in the liver, fibroadenoma and adenocarcinoma in
the mammary glands, carcinoma in the thymus, and granulocellular
carcinoma in the ovaries. No local tumours at the injection site
were observed in control animals after subcutaneous administration
of corn oil. The other tumours (carcinoma in the mammary glands and
thymus, haematopoietic system malignancies) were unusual for control
animals, as well. ß-carbaryl caused a higher tumour incidence in
mice than in rats by both subcutaneous (60%) and oral (30%) routes.
It should be mentioned, however, that most of the tumours observed
(lung adenomas, leukaemia, and liver haemangiomas) occurred
spontaneously in untreated mice. Since no control data were
presented, the carcinogenic response to ß-carbaryl in mice is of
uncertain significance. Because of these deficiencies, this study is
inconclusive.
8.8 Special studies
8.8.1 Neurotoxicity
The effect of carbaryl on the nervous system is primarily
related to ChE inhibition.
Carpenter et al. (1961) studied the delayed neurotoxic
potential of carbaryl in chickens (Rhode Island hens) compared with
that of triorthocresyl-phosphate. Single doses of 250, 500, 1000, or
3000 mg/kg body weight, 25-40% in lard were administered
subcutaneously to chickens. At 2000 mg/kg, weakness was observed on
day 1 or 2 after dosing. In one case, the chicken was unable to walk
for 3 days. No evidence of demyelination was observed in any brain
sciatic nerve or spinal cord section examined microscopically.
According to the authors, there was a transient cholinergic effect
caused by the slow absorption of carbaryl.
The neurotoxic effect of carbaryl was studied in atropinized
chickens (Gaines, 1969) to protect against acute effects of the
subcutaneous injection of 800 or 1600 mg carbaryl/kg body weight.
The higher dose caused leg weakness within 24 h, which recovered by
day 24.
Carbaryl solution in corn oil at a daily dose of 100 mg/kg was
administered orally for 7 consecutive days to 35, 6-day-old, female
broilers, hybrids between Peterson strain roosters and Hubbard hens
(Farage-Elawar, 1989). Altered locomotion and abnormally shortened
gait were observed on the 7th day, and cases of delayed paralysis
20-40 days, after the last treatment. Locomotion changes were found
to have no association with the activities of brain
acetylcholinesterase and neuropathy target esterase, 24 h after the
first, second, third, and fifth doses as well as 1, 3, 6, 10, 20,
30, and 40 days after the last treatment, when no statistical
deviations from the control values of both enzymes were registered.
A similar, but lesser, effect on gait and stride length was
observed when 45 mg carbaryl/kg was injected into chick eggs on day
15 of incubation (Farage-Elawar, 1990). This treatment resulted in
significant inhibition of brain and plasma cholinesterase, and liver
carboxylesterase. Administration of the same dose on day 5 of
incubation resulted in 10% lethality. Brain NTE was not affected.
The cause of this delayed alteration is not known.
Effects of long-term carbaryl exposure on the neuromuscular
system of pigs were reported by Smalley et al. (1969). Six
Yorkshire pigs, 3 male and 3 female, received carbaryl in their diet
(150 mg/kg body weight); the male pigs for 72 days and the female
pigs for 83 days. Three pigs from the same litter were fed carbaryl
at 150 mg/kg body weight daily, for 4 weeks, and then 300 mg/kg body
weight, daily, for 46 days (2 male pigs), and for 85 days (the
female pig). The signs of intoxication started after about 1´
months, and were mostly typical of neuromuscular system damage (see
Table 44; section 8.3). Microscopic examination of the skeletal
muscle revealed myodegeneration. In the myelinated tracts of the
cerebellum, brain stem, and upper spinal cord, moderate to severe
edema was associated with vascular degenerative changes. No
demyelination of nerve tissue was observed. When carbaryl feeding
was stopped and hydrochlorothiazide applied as a diuretic, signs of
toxicity of carbaryl, such as ataxia, and partial paralysis,
disappeared.
Carbaryl disturbed the function of the myoneural synapses. It
produced a decrease in the spontaneous activity and an increase in
the permeability of the muscular fibre membrane for K+ and Na+
after multiple oral administrations of 8.5 mg/kg to rats(Kovtum &
Sokur, 1970).
Kovtun (1970) while analysing the effects of carbaryl on
myoneural formations, pointed out that, at a single intake
(425 mg/kg), multiple intakes during 2 months, or 1/100
LD50-8.5 mg/kg over 6 months, the frequency of tiny potentials of
an edge plate was suppressed by 62-65%. On the basis of the data
obtained it was concluded that carbaryl probably impairs the
function of presynaptic nerve endings. At the same time, carbaryl
does not affect the cholinereceptive membrane substance of muscular
fibres. Carbaryl increases the rest potential of muscular fibres by
11-35%, depending of the carbaryl dose. This increase can be
explained through high potassium ion accumulation inside the
muscular fibres.
Three different laboratories examined the effects of carbaryl
on motor activity in rats. All three reported decreased activity
after intraperitoneal administration, with ED50s that ranged from
13.3 to 17.6 mg/kg (Crofton et al., 1991).
Takahashi et al. (1991) compared the effects of carbaryl on
both young (3 months) and old (12 months) rats. A dose of 50 mg/kg
decreased activity in an open field test, prolonged the duration of
haloperidol-induced catalepsy, decreased body temperature, and
increased the nociception threshold, as measured by a hot-plate
test. A dose of 10 mg also prolonged haloperidol-induced catalepsy.
The effects on body temperature and nociception were significantly
greater in older rats.
Studies were carried out on the mechanical response
characteristics of the soleus muscle in situ. Female Holtzman rats
(6 treated-control pairs) were given 56 mg carbaryl/kg orally.
Carbaryl increased the tension developed during complete tetanus (by
electric stimulus) and decreased the time constant of tension
development (Santolucito & Whitcomb, 1971). The more forceful and
rapid contraction of the skeletal muscle is probably related to an
accelerated catecholamine release.
EEGs on 4 Rhesus monkeys given 0.01 mg carbaryl/kg and on 3
given 1 mg/kg per day, orally, for 18 months, showed only a
reduction in the amount of low-amplitude fast waves and an increased
bilateral synchrony between the right and left hemispheres
(Santolucito & Morrison, 1971). The authors did not relate these EEG
changes to the dose.
Belonozhko & Kuchak (1969) found some changes in the EEG in
rats, such as desynchronisation of rhythms after a single
application of carbaryl at 100 mg/kg. During repeated doses of
35 mg/kg for 90 days, no changes in the EEG were noted, due
(according to the authors) to adaptation of the nervous system. Oral
doses of 72 mg carbaryl/kg, administered to rats for 10 days, caused
a decrease in serotonin and an increase in dopamine levels in the
brain (Kuzminskaya et al., 1984).
Morphological changes in the nervous system caused by carbaryl
were dose-related. One to six months oral treatment of rabbits with
0.01 LD50 caused only haemodynamic disorders and cell
infiltrations, a dose of 0.02 LD50 caused cell dystrophic changes,
and a dose of 0.1 LD50 caused more progressive and serious
disorders (Azizova, 1976).
The behavioural effects of carbaryl were studied in rats and
monkeys. Disturbance of discrete (shock) avoidance behaviour by
carbaryl in rats was reported at ip doses > 2.5 mg/kg, the LD50
being 8 mg/kg (Goldberg et al., 1965). Carbaryl administered at
doses of 8, 16, or 28 mg/kg, ip, decreased maze activity in CD male
rats (10 in each group), whereas doses of 16 and 28 mg/kg reduced
open field activity. After acute exposure, behavioural changes
recovered within 60 min, whereas ChE of the blood and brain
recovered after 240 min (Ruppert et al., 1983).
Carbaryl at 10 mg/kg administered subcutaneously in 16, male,
Long Evans strain rats decreased the number of times they approached
the novel stimuli and explored the exploratory box, and increased
habituation. In familiar situations, carbaryl increased activity
(Albright & Simmel, 1977).
Anger & Setzer (1979) studied the effects of oral and im
administration of carbaryl on repeated chain acquisition in 5 male
monkeys ( Macaccus mascicularis). Oral doses of 50 mg/kg did not
alter the performance of repeated acquisition tasks. The im
injection resulted in consistent changes in performance at 5 and
10 mg/kg, but did not cause any changes at 1 mg/kg.
Carbaryl affected working memory (continuous delayed response
and continuous non-match) in 28 male Sprague-Dawley rats (Heise &
Hudson, 1985a,b). A dose of 10 mg carbaryl/kg, ip, affected
performance of working memory procedures; with increasing dose,
carbaryl nonselectively decreased response.
Administration of 2.24 mg carbaryl/kg, ip for 14 days, did not
affect the performance of rats in activity wheel cages, 24 h after
the last treatment. Acute ip administration of 0.54 or 2.24 mg/kg
significantly decreased the motor activity of rats in activity wheel
cages. This decrease was reversed by atropine sulfate (Singh, 1973).
Doses as low as 7.76 mg/kg ip caused mild tremors.
Oral administration of carbaryl (200 mg/kg, 3 days/week) for a
period of 90 days, though producing inhibition of ChE in blood (33%)
and brain (11%), did not result in any kind of overt signs of
toxicity in male albino rats (Dikshith et al., 1976).
Desi et al. (1974), in long-term studies, investigated the
effects of carbaryl on the learning process, on the performance of
previously learned tasks in mazes, and on the EEG in 40 male Wistar
strain rats. Carbaryl was given at doses of 10 or 20 mg/kg body
weight per day in the diet (100-200 mg/kg food), for 50 days. Mild,
but permanent and increased, functional deviations of the nervous
system were found. Because of increased irritability in the CNS, the
treated rats took less time than the untreated rats to find their
food. Later, the task was performed with difficulty when the
irritability of the CNS was reduced to below the normal level. The
performance of the learned task was impaired. EEG deviations
recorded at the end of the maze studies were slight, but permanent.
They consisted of increased electric activity of the brain,
increased number of moderately slow beta waves, and light flashes of
18 Hz accelerated electrical activity.
Viter (1978) found behavioural changes in rats treated via
inhalation for several months with 12 or 23 mg carbaryl/m3. The
latency period of the conditioned reflex on nutrition was prolonged.
Depression of investigative behaviour and spontaneous motor activity
were noted.
The behavioural effects of carbaryl on rats were examined by
Moser et al. (1988) using a functional observation battery.
Carbaryl was administered at doses of 3-30 mg/kg, ip, and tested
0.5, 3, 24, and 48 h afterwards. Carbaryl decreased spontaneous
activity, CNS excitability, motor and sensory function, and body
temperature and weight. Effects indicative of AChE inhibition were
also observed. All responses were dose dependent.
8.8.2 Effects on the immune system
8.8.2.1 Appraisal on immunotoxicology
The administration of carbaryl, or any other xenobiotic, at
doses resulting in overt toxicity can be expected to result also in
a variety of effects on the immune system. Carbaryl, when
administered in vivo, at a dose not causing overt clinical signs
has been reported to produce a variety of non-life-threatening
effects on the immune system. The effects included cellular as well
as humoral immunity, and several authors have suggested that they
were was due to subtle, treatment-related stress. Many of the
effects described were detected at doses close to the LD50.
Shortcomings of several of these studies were a lack of consistency
and, sometimes, overt contradiction between results, which prevents
the description of a defined immunotoxic mechanism.
Lifetime exposure to carbaryl did not result in increased
occurrence of disease in rats or mice. No enhancement of viral
infections was found with carbaryl, even at dosages close to the
LD50. Most studies on rabbits and mice, at doses permitting
survival, did not produce significant effects on the immune system.
In vitro, a number of researchers have demonstrated viral
enhancement by prior incubation with carbaryl. In vitro, carbaryl
can enhance herpes varicella-zoster, but not herpes simplex. In
goldfish cell culture, carbaryl has been shown to permit viral
enhancement by compromising interferon synthesis. Inhibition of
human serum complement activity as well as interleukine-2 driven
proliferation of large granular lymphocytes have been demonstrated
in vitro. These actions could be mediated through the inhibition
of serine esterases involved in the processes. No increased viral
infections have been seen in long-term in vivo feeding studies,
previously conducted or in progress at present. Carbaryl does not
enhance transformation of BALB/53T fibroblasts in culture or the
expression of endogenous murine leukaemia virus.
8.8.2.2 In vivo studies
Young mice weighing 10-12 g were infected with influenza by
applying 3-4 drops (0.05 g) 1% influenza virus in physiological
solution in the nose. The murine influenza virus strain A.P.R.8 was
used in the primary dilution 1:32. After 2-3 days, this group of 30
mice was treated orally with carbaryl in sunflower oil, at a dose of
500 mg/kg (a dose that killed 3 out of 10 mice). Two control groups
with the same number of mice, one treated with carbaryl alone, and
one infected, were compared with the experimental animals for
survival, blood biochemistry, and pathomorphological changes. The
greatest number of mice dying were in the experimental group. AChE
depression was more pronounced and recovery slower, and there were
more histological changes in the livers of mice infected and
intoxicated by carbaryl (Moreynis & Estrin, 1965). It is clear from
this study that a summation of the effects of both factors occurred,
but, because of the lack of statistical significance, it is
impossible to judge the eventual effect of the enhancement.
The effect of carbaryl on an experimental Erysipelothrix
rhusiopathiae infection in rats was studied by Shabanov et al.
(1983). Rats, treated with doses that increased gradually every 6
days from 2 to 5 mg/rat (mean weight of rats, 50-60 g) during 30
days, were infected iv with Erysipelothrix rhusiopathiae at
1.5-108 cells/rat. The mortality rate in carbaryl-treated rats was
36% versus 14% in control rats. Survival time was halved.
Bacteraemia persisted for 10-12 days in treated groups, and only 5-6
days in the control group. Both the gross and the histopathological
changes were more strongly manifested in the treated group.
Carbaryl, at 0, 2, 20, or 200 mg/kg was administered orally to
rabbits, daily, for 6 months. A decrease in the phagocytic activity
of leukocytes and antibody formation (4-5 times) after immunization
with a murine type of typhoid vaccine, was reported at the dose
level of 200 mg/kg. At the lower dose of 20 mg/kg, there were
different phases of reactivity; at the beginning of the first 2
months, there was an increase and later a decrease in immunological
reactivity (Perelygin et al., 1971).
The effects of oral ingestion of carbaryl on nonreaginic
antibody production in BALB/c mice were studied after pretreatment
with 150 mg carbaryl/kg diet for 10 weeks prior to commencement of
the study. Carbaryl produced significant effects on systemic
antibody production following oral immunization with sheep red blood
cells (SRBC). It significantly increased both IgG1 and IgG2b titres.
No reduction was seen in the synthesis of any antibody class. These
results suggest that, at the doses used, carbaryl increased systemic
antibody responses to orally ingested antigens (André et al.,
1983).
Changes in the immunological structures of lymphatic follicles
in the spleen during carbaryl administration were studied by Dinoeva
(1974, 1982), who used a micrometric method. Albino rats (30
treated-control pair) were given 1.5 mg carbaryl/kg, orally, daily
for 6 months. Plethora in the spleen and retardation in lymphatic
follicles were observed, which were similar to effects seen in the
positive control group treated with cyclophosphamide (2 mg/kg).
There were no changes in the structure and weight of the thymus in
carbaryl-treated rats, while in the cyclophosphamide positive
control group, the weight of the thymus was reduced 2.5 times. The
authors suggested that there is a different mechanism of the
immuno-suppressive effect of carbaryl that needs further research.
A single dose of 500 mg carbaryl/kg inhibited the production of
haemolysins and reduced the number of splenic germinal centres in
White Leghorn chickens (Roszkowski et al., 1976). Dose-dependent
immunosuppressive effects of continued dietary treatment of rabbits
with carbaryl were studied (Street & Sharma, 1975). Rabbits (male
White New Zealand, 7 in each group) were fed 0, 4, 20, 45, or 150 mg
carbaryl/kg diet (corresponding to 0, 0.23, 1.0, 2.3, or 8.38 mg/kg
body weight per day) for 57 days. The treatment reduced the germinal
centres in the spleen and caused atrophy of the thymus cortex. The
antigen-induced increase in serum gamma-globulin was decreased
significantly (no dose relationship was noted), at 10 days. No
changes were detected in the number of plasma cells in the lymph,
haemolysin and haemoglutinin titres, skin sensitivity to tuberculin,
leukocytes count, body weight, etc.
Rats pretreated, orally, with 0.05 LD50 carbaryl, daily, for
2.5 months, were immunized with typhoid antigen. Administration of
carbaryl continued for an additional 2 months. Signs of
insufficiency of the immune system, expressed as a reduction in
specific globulin production, were observed 3.5 months after
carbaryl application began. Carbaryl did not affect immunogenesis at
a dose of 0.001 LD50, daily, under the same exposure conditions.
No overall evaluation was given (Olefir, 1977).
A humoral immunity study in female BALB/C mice after oral
administration of carbaryl was performed by Wiltrout et al. (1978).
The effects of humoral immune competence were measured by the
immunoplaque in gel technique. The number of antibody plaque-forming
cells (PFC) per plate was counted and the PFC/spleen calculated.
Different groups of mice received carbaryl, orally, at about
1 LD50 (153 mg/kg body weight), 5 days before immunization (type
of antigen not specified), on the day of immunization, and 2 days
after. Only the last treatment resulted in significant suppression
of the humoral immune response. The ratio (experimental: control) of
antibody plaque-forming cells was 0.34. The oral administration of
0.1 LD50 for 8 and 28 days did not cause any significant effects
on humoral immune competence. Depression of the PFC/spleen level
corresponds to a decline in the total splenic lymphocyte population.
Akhundov et al. (1981) studied the effects of carbaryl on the
immunological reactivity of 40 rabbits and 40 guinea-pigs given oral
doses of 15 mg carbaryl/kg for 42 days. Heated vaccine Salmonella
typhimurium, at doses of 250 or 500 million microbial cells, was
applied 3 times at 7-day intervals, 21 days after the beginning of
carbaryl administration. The auto-sensitizing effect of the vaccine
was enhanced by carbaryl. Studies on guinea-pigs treated with 15 mg
carbaryl/kg per day for 3 months showed decreased macrophagial
migration activity.
Pipy et al. (1983) evaluated the cellular and humoral
mechanisms of carbaryl-induced reticuloendothelial phagocyte
depression. The function of the reticuloendothelial system (RES)
involved in specific and non-specific aspects of host resistance to
infection, neoplasma, etc., was quantitatively evaluated by the rate
of disappearance of colloids injected into the blood. Colloid
particles are extracted from the blood exclusively by the RES, in
particular liver and spleen macrophages. Plasma or serum factors
called opsonins have a strong stimulatory influence on inert
particle phagocytosis. Male Sprague-Dawley rats (200-240 g) were
given single, iv injections of labelled carbaryl at 0.5-32 mg/kg
body weight (7 different doses). The iv LD50 determined by the
authors was 50 mg/kg body weight. Colloidal carbon was administered
simultaneously. In another group, carbaryl was incubated with serum
from a normal rat for 20 min before the injection. The authors call
this carbaryl "opsonized". The results showed the ability of
carbaryl to induce a state of reticuloendothelial phagocytic
depression, mediated through depletion of opsonins. Carbaryl
inhibits the cell-bound aromatic amino acid esterase of the serine
esterase class. The authors concluded that, apart from a slight
deficiency in plasma opsonins, the inhibitory effect of carbaryl on
the phagocyte function was primarily due to a selective hepatic and
splenic macrophage impairment, which could be related to inhibition
of a cell-bound serine esterase. In another study by Pipy et al.
(1982), more details are given of this possible mechanism of
phagocytoxic blockade by carbaryl. Rats were treated with iv
carbaryl at 8 or 16 mg/kg body weight. The time-dependence of the
effects on phagocytic function and the activities of liver serine
esterases ( N-benzoyl-DL-phenylalanine-ß-naphthyl esterase and
acethylcholinesterase) was studied. Macrophages of the RES had
decreased phagocytic capacities after administration of carbaryl.
Depression of the phagocytosis of colloidal carbon persisted from 1
to 7 h and 1 to 24 h after administration of 8 and 16 mg/kg
carbaryl, respectively. Liver ( N-benzoyl-DL-phenylalanine-ß-
naphthyl esterase (cell-bound serine esterase) was susceptible to
reversible dose-related inhibition by carbaryl. A correlation
between the reactivation kinetics of liver serine esterases and
phagocytic activity was demonstrated.
8.8.2.3 In vitro studies
The replication of goldfish virus 2 (GFV-2) was enhanced in
vitro by pre-treatment of a piscine culture with subtoxic
concentrations (1 mg carbaryl/litre) (Shea, 1983). The mechanism of
enhancement was studied in vitro in goldfish-derived cell lines
and Air Bladder III infected with GFV-2 and pretreated with a
carbaryl solution of 1 µg/ml. Interferon synthesis was studied as a
possible mechanism of virus enhancement (Shea & Berry, 1984). The
authors demonstrated that interferon synthesis was induced in CAR
cells by GFV-2 cells. Antiviral protection provided by supernatants
from infected CAR cultures with carbaryl, against infection of
secondary CAR and Air Bladder III cultures was reduced. The authors
interpreted this phenomenon as a result of general mild suppression
of cellular metabolism, but other possible mechanisms, such as
metabolic interaction, were not excluded. In another study, Shea
(1985) demonstrated quantitative carbaryl inhibition of interferon
synthesis. Supernatants from infected cultures, not treated with
carbaryl, provided 10 times as much antiviral protection as compared
with non-infected cultures. Carbaryl-treated infected culture
provided only twice as much antiviral protection as uninfected
cultures. A similar relationship was observed when comparing the
amount of infectious viral progeny synthesized in the presence of
supernatant from infected cultures not pretreated with carbaryl
(10%), and from infected cultures pretreated with carbaryl (60% of
the amount of virus synthesized in control cultures). The author
speculated about the possibility of superimposition of a viral
infection in a population exposed to pesticides.
Characterization of varicella zoster virus enhancement by
carbaryl was carried out in vitro by Abrahamsen & Jerkofsky (1981)
and Jerkovsky & Abrahamsen (1983). In previous studies, the authors
demonstrated that the replication of varicella zoster virus (a human
herpes virus) is increased 12 to 15-fold by pretreatment of cultures
of human embryonic lung cells (HEL) with carbaryl, and, that
different strains of this virus show differences in sensitivity to
enhancement. Wild-type strains recently isolated from clinical
materials are more sensitive than laboratory adapted strains. In
this study, the authors demonstrated that the maximum enhancement
occurred 48-72 h post-inoculation and that the optimal time for the
pretreatment of monolayers of HEL is 20-24 h. 1-naphthol also
produced increased amounts of virus, but the treated cells cannot
pass on to the daughter cells the ability to enhance virus
replication.
Rodgers et al. (1986) determined that the EC50 for the
inhibition of a T-cell-mediated cytolytic (CTL) response by carbaryl
in vitro was 65.9 µg/ml. The addition of a supernatant containing
liver enzymes reduced the effects of several organophosphorous
pesticides on the CTL response, but had no significant effect on the
potency of carbaryl.
Casale et al. (1989) compared the ability of carbaryl to
interfere with the human serum complement mediated lysis of sheep
blood cells. At a concentration of 3 mmol/litre and with 2-h
preincubation, carbaryl inhibited lysis by 26-45%, depending on the
antibody concentration. Carbaryl was a more potent lysis inhibitor
than diisopropyl phosphorfluoridate or four other anticholinergic
insecticides; the potency was not correlated with anticholinergic
activity.
The effects of carbaryl at concentrations of
0.5-500 µmol/litre on lymphocyte proliferation were studied in
vitro (Bavari et al., 1991). Carbaryl inhibited (3H) thymidine
incorporation, in a concentration-dependent manner, by as much as
50%. Alpha-naphthol also had a slight effect at 50 µmol/litre. The
authors attributed the effect to inhibition of an esterase
responsible for interleukin activation.
8.8.3 Effects in blood
Carbaryl affects the coagulation process. Hyper- and
hypocoagulation were reported in different studies (Hassan & Cueto,
1970; Gapparov, 1974; Lox, 1984; Krug & Berndt, 1985; Krug et al.,
1988).
Gapparov (1974) studied the indices of blood coagulation in
dogs after oral treatment with a daily dose of 2 mg carbaryl/kg body
weight, over 5 months. A clearly manifested hypercoagulation was
established, which was connected with a rise in the general
coagulation activity of the blood, higher thromboplastic activity,
and prothrombin content, increased number of thrombocytes,
accelerated coagulation time, and increased activity of the
fibrinostabilizing factor. Also noted was a decrease in the
recalcification time of blood plasma, fibrinogen concentration, and
free heparin quantity, together with an inhibition of the fibrolytic
activity of the blood. The author interpreted all these changes as
being connected with the arousal of the parasympathetic system.
The blood coagulation time was considerably shortened in
rabbits that were given, orally, a mixture of carbaryl (5 mg/kg),
DDT (5 mg/kg), and parathion (0.5 mg/kg), for 222 days. This effect
corresponded to increased levels of 5-hydroxy-3-indolacetic acid
(5-HIAA) and 4-hydroxy-3-methoxymandelic acid (VMA) in the urine,
indicating an increased rate of metabolism of serotonin and
catecholamine (Hassan & Cueto, 1970). The authors suggested that
this effect was a manifestation of non-specific stress, since
adrenocortical hormones shorten the coagulation time.
Fifteen male Sprague-Dawley rats (250-300 g) were given
drinking-water containing 10 mg carbaryl/litre for 30 days. Platelet
count, prothrombin time, partial thromboplastin time, fibrinogen,
and clotting factor activity for coagulation factors II, V, VII,
VIII, IX, X, and XII were determined. A significant decrease in
platelet count and in the factor VII clotting activity was observed
compared with those in the same number of control rats. Microscopic
evaluation of the liver revealed hepatocyte degeneration, central
vein congestion, some leukocytic infiltration, and vacuolization of
the cytoplasm. The author suggested that carbaryl might have harmful
effects on haemostasis (Lox, 1984).
Carbaryl has an in vitro inhibitory effect on arachidonic
acid-induced platelet aggregation, which corresponds to its
inhibition of thromboxane B2-formation. The results suggest that
carbaryl affected platelet aggregation by inhibition of
cyclooxygenase (the key enzyme of prostaglandin synthesis) by
carbamoylation (Krug & Berndt, 1985). At a concentration of
10 µmol/litre, carbaryl completely blocked platelet aggregation and
cyclo-oxygenase activity (Krug et al., 1988). The carbamoylation
of several platelet proteins, including cyclo-oxygenase, may be
responsible for this effect.
Carbaryl produced, in vitro, a dose-dependent increase in
methaemoglobin (Met Hb) formation at 10 and 100 mg/litre, as well as
decreases in reduced glutathion levels in the erythrocytes of Dorset
sheep with low erythrocyte glucoso-6-phosphate dehydrogenase
(G-6-PD), which is similar to humans who have G-6-PD deficiency.
Carbaryl posed oxidative stress to G-6-PD-deficient red cells,
probably due to its major metabolite alpha-naphthol (Calabrese &
Geiger, 1986). Decreases in the K+ ion concentration in
erythrocytes (with more than 24%) and in haematocrits (from 43.5 to
38%) were found by Sokur (1971) in rats fed carbaryl 0.05 LD50/day
for 2 months.
In an in vitro study, Szczepaniak & Jeleniewicz (1981) found
that carbaryl binds free blood amino acids (plasma and
erythrocytes). These authors also performed a series of in vitro
studies to investigate the effect of carbaryl on amino acids. A
single application of 475 mg carbaryl/kg on 46 treated and 12
control rats produced significantly decreased amino acid values in
the brain, except for valine and phenylalanine. All amino acids
reached the control level after 72 and 120 h. Two hours after
administration, erythrocyte amino acids also decreased >50%
(Szczepaniak & Jeleniewicz, 1980; Jeleniewicz et al., 1984). A
slight decrease in erythrocyte amino acid concentrations was
observed after 30 days with 95 mg carbaryl/kg administered orally
(Jeleniewicz & Szczepaniak, 1980). Blood serum amino acids in 24
rats decreased 4 h after a single application of 189.6 mg/kg. There
was a larger decrease in valine, then in phenylalanine, alanine,
aspergic acid, serine, and glycine (Szczepaniak et al., 1980).
After 15-day dosing of 0.1 LD50(94.8 mg/kg) per day to 12 rats,
there were no changes in levels in serum and erythrocytes, but after
30-day dosing in 8 rats, there was a slight decrease in the alanine
concentration of the erythrocytes (Jeleniewicz & Szczepaniak, 1980).
The effects of carbaryl on the thermoresistance and fractional
content of blood serum proteins was studied by Subbotina &
Belonozhko (1968). A single dose of 150 mg carbaryl/kg administered
to rabbits and multiple applications of 100 mg carbaryl/kg for 2
months showed that, with a single application of carbaryl, there was
an increase in protein thermocoagulation from 28% on day 1 to 67% on
day 7, and with multiple applications of carbaryl there was a
lowering of albumin levels and a rise in globulins (mostly
alpha-globulins) in serum, on day 10. These changes were reversible.
There was also a decrease in the coefficient of thermal dehydration.
The changes in proteins in both studies show lowering of their
lability.
Carbaryl inhibited the incorporation of 3H-uridine and
14C-labelled amino acids into RNA and proteins in cultures of HeLa
cells. The effect was dose-dependent. Incorporation was inhibited by
50% at a 150 µg/ml concentration after 30 min incubation. At a
concentration of 350 µg/ml, only 10% of amino acids and 32% of
uridine incorporation activity were retained (Myhr, 1973). Later,
Blevins & Dunn (1975) showed that carbaryl caused general metabolic
changes in Hela cells. A concentration of 1-2 mg carbaryl/litre
stimulated all divisions and, at 4-8 mg/litre, inhibited growth. A
decrease in cellular protein at 4 mg/litre was noted. Changes in the
phospholipid fraction at 8 mg/litre were probably related to the
alteration of the structure of cellular membranes. Disturbances in
the development of human cells in culture were also reported by
Shpirt (1973). An inhibitory effect of carbaryl on cell development
was demonstrated in Ehrlich ascites tumours in mice. A reduced rate
of incorporation of labelled uridine-5-3H-thymidine methyl-3H
and L-leucine 14C in the RNA, DNA, and proteins in Ehrlich cells
in vitro was also reported (Walker et al., 1975b). The authors
suggested that this effect could be a basis for investigating the
mechanism of the adverse effects that carbaryl has on reproduction.
Carbaryl and N-nitrosocarbaryl appeared to have different
action characteristics with regard to rat liver microsomal membrane
alterations (Beraud et al., 1989b); carbaryl was ineffective on
the lipoperoxidation indices while the nitroso compound had an
inhibitory action on the formation of malonaldehyde and conjugated
dienes as well as on the NADPH-dependent reductase activities.
8.8.4 Effects on the liver and other organs
Several authors reported data on disturbances in the
carbohydrate, protein-forming, and detoxicating functions of the
liver.
A single application of 300 mg carbaryl/kg in rats produced an
increase in albumin and alpha-globulins, and a decrease in ß-and
gamma-globulins (Zapko, 1970).
Kagan et al. (1970) studied the effects of carbaryl on the
liver of 180 rats and 18 rabbits. During an 11-month study, they
gave a daily, oral dose of 38 mg carbaryl/kg to rats. After 1 month,
they observed a rise in the alanine-aminotransferase and
alkaline-phosphatase activities in serum, and a decrease in succinic
dehydrogenase and glycogen in the liver. Doses of 0.76 mg/kg and
0.38 mg/kg, given in the diet to rabbits, caused retention of
bromsulfophthaleine in the blood. The authors also reported a
changed ratio in the protein fractions in serum and an increase in
liver weight. The pathomorphological changes in the liver were
destructive, necrobiotic, and proliferative. An effect on the liver
was also demonstrated in the study of Lox (1987) (see section
8.8.3).
Pavlova et al. (1968) found that, with acute and long-term
exposures, carbaryl affected the oxidative processes in tissues,
because of its direct action on the enzymes of cell respiration and
possible disturbance in the membrane processes. They performed
studies on rats treated with 0.2 LD50 for 3 days and on rats
treated with 0.01 LD50 for 20 weeks. At the higher dose, there
were decreases in the cytochromoxidase and succinedehydrogenase
activities in the liver and brain mitochondria. A histochemical
examination revealed a rather high, irregular activity of the
cytochromoxidase in the heart, as well as increased succinic
dehydrogenase activity. With the long-term dosing, the changes were
not significant, but there was a lowering of the cytochromoxidase
and succinedehydrogenase activities in the heart mitochondria.
Development of experimental cholesterol arteriosclerosis in rabbits
was facilitated by the application of 20 mg carbaryl/kg for 2.5
months (Lukaneva & Rodionov, 1973). Changes were found in the
following indices: general cholesterol, ß-lipoproteins, ECG changes,
and pathomorphological changes in the aorta and coronary vessels.
Carbaryl (100 mg/kg body weight) given to dogs in their diet
for 45 days caused disturbances in the secretion of the intestinal
enzymes. There was an increase in enterokinase secretion, as well as
in the excretion of alkaline phosphates and lipase into the
intestinal juice. The no-observed-effect level (NOEL) for these
effects was 700 µg/kg body weight, which corresponds to 7 mg
carbaryl/kg diet. Three dogs were used in each group (Georgiev,
1967).
8.8.5 Effects on serum glucose
Orzel & Weiss (1966) found that a rise in blood glucose
correlated with the onset and duration of tremors and the degree of
brain ChE inhibition in rats that were treated ip with 5 and 25 mg
carbaryl/kg. The hyperglycaemic response is blocked by
adrenal-ectomy and is unaltered by hypophysectomy. The authors
suggested that the hyperglycaemia was related to increased secretion
of epinephrine. Hyperglycaemia is thought to result from cholinergic
stimulations as it is also found in acute intoxications with
organophosphorous compounds (Kaloyanova, 1963). In their studies,
Orzel & Weiss did not find changes in liver glycogen. Muscle
glycogen was decreased only in non-fasted rats. Elevated levels of
blood glucose and slightly reduced immunoreactive insulin were found
with repeated oral exposure of rats at 3 mg/rat, weekly, for one
year (Wakakura et al., 1978). Glycogen disappeared in most of the
hepatocytes. The cells mainly contained related granular endoplasmic
reticulum with swollen mitochondria. A single application of
30 mg/rat produced transient hypoglycaemia at 20 h followed by
hyperglycaemia at 44 h. The effects of carbaryl on respiration,
glycolysis, and glycogenesis in isolated hepatocytes of male Wistar
rats were studied by Parafita et al. (1984) and Parafita &
Fernandez Otero (1984). The cells were treated with concentrations
of carbaryl of 0.01, 0.1, and 1.0 mmol/litre dissolved in 1%
dimethylsulfoxide (DMSO). DMSO slightly stimulates the respiratory
coefficients. Carbaryl decreased the metabolic output of CO2 at
all concentrations; oxygen consumption was reduced by 40% in
relation to the DMSO-treated groups only at the highest
concentration (1.0 mmol/litre). Glucose remained unchanged in the
presence of carbaryl. Endogenous production of lactic acid was not
affected, and net metabolic production was strongly inhibited by
both DMSO and carbaryl. At a maximum carbaryl concentration
(1 mmol/litre), the net lactic acid production was completely
blocked. Carbaryl inhibited lactate gluconeogenesis, and, to some
extent, gluconeogenesis from fructose pyruvate and alanin. Glycerol
glucogenesis was unaffected. Lactic dehydrogenase activity was
reduced by 38% and glucose 6-phosphate synthetic activity was
increased by 1.0 mmol carbaryl/litre. Aspartate aminotransferase
activities (cytoplasmic and mitochondrial fractions) were inhibited
by 0.1 and 1.0 mmol carbaryl/litre. The results indicated that
carbaryl causes a decrease in glucose production by hepatic cells
and suggested that carbaryl-induced hyperglycaemia in fasted animals
is caused by deficiencies in the peripheral utilization of the
glucose.
8.8.6 Interactions with the drug metabolizing enzyme system
Carbaryl is a weak inducer of hepatic microsomal
drug-metabolizing activity. Cress & Strother (1974) reported
depressed hexobarbital sleeping time in mice given 0.125 or 0.5
LD50 carbaryl, orally, for 10 days. These authors studied the
effects of a 2-week dietary administration of high levels of
carbaryl (10 times higher than the dose given in the earlier study).
Weanling male Swiss-Webster mice, weighing 17-20 g, were divided
into 5 groups of 40 animals each. The daily consumption of carbaryl
by each animal was approximately 119% of the acute LD50. This dose
was well tolerated with no deaths or overt symptoms of
cholinesterase inhibition, probably because carbaryl was
administered over a 24-h period, instead of in a single injection.
Feeding carbaryl resulted in a 44% increase in the rate of in vitro
p-hydroxylation of aniline, and increased in vitro demethylation
of benzphetamine. The hepatic levels of cytochrome P-450 and b5
were increased, but the microsomal protein concentration per gram of
liver was not affected. No changes were reported in
1-naphthol-treated mice. Increased metabolic activity was
demonstrated with phenobarbital and carbaryl. Phenobarbital sleeping
time was shortened to 45 min (control 74 min) in carbaryl-fed mice.
The rate of elimination for the carbaryl-treated animals was twice
as high as that for control animals. The oral LD50 of carbaryl in
carbaryl-pretreated animals (14 days feeding) was three times as
high as in the control animals. 1-Naphthol did not affect the levels
of cytochromes. Sleeping time and the LD50 for carbaryl were not
different in control and 1-naphthol pretreated animals.
Liver weight was low in carbaryl-fed mice, but higher as a
fraction of body weight. The authors concluded that the degree of
the enzyme induction was not high, because most indicators exceeded
the control values by 50% only, except for the lethal dose level.
The pretreatment of rats with 5 daily doses of 10 mg
carbaryl/kg, administered ip, resulted in a 4.2-fold increase in the
rate of benzo( a)pyrene metabolism (Lesca et al., 1984). Total
microsomal P-450 content and benzphetamine demethylase activity were
not significantly affected. The increased ability to hydroxylate
aniline in mice under carbaryl treatment was reported by Guthrie
et al. (1971).
Exposure of murine 3T3 fibroblasts to carbaryl at a
concentration of 10-6 mol/litre was found to increase aryl
hydrocarbon hydroxylase activity (Lahmy et al., 1988). The
magnitude of the effect increased with the duration of exposure.
Carbaryl induction of microsomal enzyme systems in White
Leghorn cockerels was demonstrated by Puyear & Paulson (1972). A
decrease in phenobarbital sleeping time, an increase in liver weight
and elevation of liver aniline hydroxylase activity were found after
oral treatment with carbaryl at 100 mg/kg per day (in gelatine
capsules) for 3 or 6 days. No effect was observed at a dose of
50 mg/kg. Thus, the sleeping time data indicated that the NOEL of
carbaryl for Leghorn cockerels was between 50 and 100 mg/kg per day.
Higher doses caused an increase in liver aminopyrine demethylase
activity (200 or more mg/kg per day) and in liver cytochrome P-450
content (300 or 400 mg/kg per day). Microsomal protein was not
changed. All study parameters returned to control values by day 11
after the treatment was terminated (Puyear & Paulson, 1972).
El-Toukhy et al. (1989) reported that the administration by
gavage of carbaryl to mice at a dose of 166 mg/kg resulted in small,
but statistically significant (15-20%), decreases in liver pyridoxal
phosphokinase and L-tryptophan 2,3-dioxygenase activity.
Administration over a 5-day period of 83 mg carbaryl/kg per day had
no significant effect. Hassan et al. (1990) administered 85 mg/kg,
orally, either once or five times daily. Both the single and
repeated regimens resulted in approximately 40% decreases in
L-tryptophan 2,3-dioxygenase. Carbaryl was also found to be a
competitive inhibitor of this enzyme in vitro.
Inhibition of monoamino-oxidase (MAO) was reported in the
presence of carbaryl in vitro in liver homogenate (Hassan et al.,
1966). The authors suggested that MAO and the catalase system are
involved in oxidative demethylation during carbaryl detoxification.
Exposure of rat liver microsomes to carbaryl in vitro at a
concentration of 0.5 mmol/litre resulted in decreases in the
activities of several monooxygenases (Knight et al., 1986). The
ability of carbaryl to inhibit the N-demethylation of
ethylmorphine appeared to be competitive with a Ki of
2.4x10-4 mol/litre. However, when administered to rats at doses of
up to 25 mg/kg, carbaryl did not have any significant inductive or
inhibitory effect.
Lechner & Abdel-Rahman (1986a) reported that the in vitro
incubation of 2 and 4 mmol carbaryl/litre for 60 min reduced
glutathione levels and increased glutathione S-alkyltransferase in a
rat liver homogenate in a dose- and time-dependent manner. Carbaryl
had no apparent effect on glutathione levels in whole blood with
120 min of incubation. Carbaryl inhibited N-demethylation and
O-demethylation by rat liver microsomes with apparent Ki of 0.1
and 0.7 mmol/litre, respectively (Beraud et al., 1989a).
Carbaryl had an antagonistic action on the release of
microsomal ß-glucuronidase by malathion in a microsomal suspension
in vitro (Lechner & Abdel-Rahman, 1985).
Jacob et al. (1985) studied the effects of carbaryl on the
metabolic activation of the environmental, carcinogenic, polycyclic
aromatic hydrocarbons. Carbaryl was a weak inducer of metabolism of
benz( a)anthracence in the rat liver, and altered its metabolite
profile by shifting from 10, 11-oxidation to 5, 6- and 8,
9-oxidation. Since carbaryl did not induce bay-region activation,
the authors concluded that tumour-promoting activity could not be
expected.
8.8.7 Effects on the endocrine system
The effect of carbaryl on the neuroendocrine system was studied
in rats. Carbaryl was given, orally, at 7, 14, or 70 mg/kg body
weight to male and female rats (each group contained 24 animals
weighing 90-130 g) for a period of 1 year. Growth retardation,
changes in the blood enzymes, and endocrine gland disturbances
varied with the dosage, being especially marked at the end of the
study in the group receiving 70 mg/kg body weight per day.
Acetylcholinesterase and butylcholinesterase activities were
inhibited significantly in these groups. Spermatozoon motility was
reduced progressively with the duration of the exposure. In the
prolonged estrus cycle, the diestrus period was particularly
affected. An increase in the number of corpora lutea and atrophic
follicles in the ovaries was correlated with this disturbance. There
was a dose-dependent increase in the gonadotropic function of the
hypophysis, which was determined by tests on immature mice.
Hypophyseal homogenate from a rat given 70 mg carbaryl/kg increased
the weight of the ovaries by 51.5%, and that of the uterus by 123%,
compared with the weights in control mice. At all doses,
histochemical studies of the hypophysis showed changes indicative of
an increase in the activity of the cells producing a luteinizing
gonadotrophy, i.e., an increase in the size of the cells, loss of
granules, and hyalinization of the cytoplasm. Histological
examination of the adrenal glands revealed an increase in the size
and mitotic activity of cells in the zone glomerulosa. Enlarged
cells with a large nucleus or two nuclei were present in the
fascicular zone. Impairment of thyroid gland functional activity in
the test group was indicated by the reduction in the rate of
absorption and excretion of 131I and its rather low recovery, as
well as by the corresponding histological findings, i.e.,
enlargement of follicles and more dense and basophilic colloid at
all doses. Histological findings were more pronounced in the
70 mg/kg per day groups. It is likely that the effects of carbaryl
on the reproductive organs is mediated by the endocrine glands.
However, the possibility of a direct effect was not excluded by the
authors (Rybakova, 1966; Shtenberg & Rybakova, 1968; Shtenberg
et al., 1970).
The effect of carbaryl on the thyroid gland in rats was studied
by Shtenberg & Hovaeva (1970). Rats were given carbaryl, orally, at
0.7, 2, 5, or 17 mg/kg per day for 6 months. They were fed a normal
iodine diet or an iodine-deficient diet. There was an initial
increase in function in the first 3.5 months of exposure and a
decrease after 6 months of exposure. Recovery was complete 2 months
after the end of the exposure. Decrease in iodine uptake and
reduction of function was 26.5% in rats given 5 mg/kg and 29.5% in
rats given 15 mg/kg. Rats fed an iodine-deficient diet were more
sensitive to carbaryl-induced changes in the thyroid.
The functional states of the thyroid gland and adrenal cortex
were affected by a daily administration of 36 mg carbaryl/kg to rats
for 4 months. The maximum accumulation of 131I occurred after 6 h
and, in the control animals, after 12 h. The 131I absorption rate
normalized after 45 days, but its excretion was delayed. The
concentration of sodium in the blood increased by 36.4% after day 15
and by 74% after 45 days, while the potassium concentrations were
reduced. The daily excretion of 17-corticosteroids in the urine
increased by 35.9%. The changes in the thyroid and adrenal glands
were transient (Dyadicheva, 1971).
Hassan (1971) studied the effects of carbaryl on the synthesis
and degradation of catecholamines in the rat. Rats were given
carbaryl in the diet at concentrations of 100 or 700 mg/kg for 7
months, or in peanut oil at single oral doses of 50, 80, or
250 mg/kg, or 3 single doses of 80 mg/kg body weight. On day 30, the
rats given 700 mg/kg diet eliminated amounts of urinary
4-hydroxy-3-methoxy mandelic acid (VMA) increased by more than 300%;
levels returned to normal after 195 days. A similar effect was
demonstrated after single oral doses with a dose-effect
relationship. The amount of 5-hydroxyindole acetic acid (5-HIAA) in
the urine increased in rats that were treated with a single dose and
3 successive daily doses of 80 mg/kg per day. Adrenalectomy and
hypophysectomy did not change the pattern of VMA excretion produced
by carbaryl. There was an increased turnover rate (68%) of heart
norepinephrine (NE-3H) in rats treated with 80 mg carbaryl/kg, 2 h
before application of NE-3H. The corticosterone level was increased
by about 125% at the same dose level. A carbaryl concentration of
16.2 µg/ml in the blood was enough to trigger maximal corticosterone
secretion. The authors suggested a probable activation of the enzyme
system involved in the catecholamine metabolism, because carbaryl
may also directly affect the adrenergic nerve endings. Activation of
the pituitary adrenal axis and an increase in sympaticoadrenergic
activity with concomitant increased VMA elimination was a
pharmacological effect due to carbaryl treatment.
The effect of a single oral dose of 60 mg carbaryl/kg body
weight on serotonin (5-HT) metabolism in the male Holtzman Albino
rat brain was studied by Hassan & Santolucito (1971); 2, 4, 6, and
24 h after carbaryl application, whole brain samples were analysed
for serotonin, 5-HIAA, and corticosterone. The concentrations of
5-HT and 5-HIAA in the brain increased by 20-30% (5-HT from 0.61 to
0.8; 5-HIAA from 0.25 to 0.31 µg/g) from 2 to 6 h and returned to
normal after 24 h. The plasma corticosterone level was also
increased by about 125%, 1 h after oral administration of carbaryl,
and returned to normal after 20 h. The inhibitory effect of
p-chlorophenylalanine on brain 5-HT formation was diminished by
carbaryl treatment. The serotonin level in the brain of
carbaryl-treated animals pretreated with p-chlorophenylalanine was
72% higher than in the control animals. Increased 5-HIAA levels were
probably a result of an increased rate of synthesis of serotonin.
The authors suggested the possible role of the increased synthesis
of serotonin and tryptophan-5-hydroxylase activity, which could be
related to stress conditions. Carbaryl activation of MAO is probably
related to the increased formation of cerebral 5-HIAA.
The uptake of noradrenaline in vitro by hypothalamic slices,
isolated from rats exposed in vivo to near lethal levels of
carbaryl, was increased in a dose-dependent manner (Jablonska &
Brzezinski, 1990).
Oral administration of 200 mg carbaryl/kg to rats was reported
to raise striatal levels of noradrenaline and homovanillic acid, a
metabolic product of dopamine, when measured 0.5-2 h later (Ray &
Poddar, 1985b).
The mechanism of the stimulation of corticosterone secretion is
not clear.
In vivo studies on male mice receiving 38 and 68 mg
carbaryl/kg showed that carbaryl can increase certain hepatic
androgen hydroxylase activities. In vitro incubation of carbaryl
with prostate tissue and testosterone 1,2-3H stimulated the
formation of dehydrotestosterone-3H, thus, suggesting a direct
action on steroidogenesis. Comparing these results with the effects
of other toxic substances, the authors concluded that carbaryl
cannot exert any major changes in steroid metabolism, nor can it
reduce hormonal disturbances (Dieringer & Thomas, 1974).
Attia et al. (1991) studied the effect of carbaryl on rat
melatonin production as alterations of the latter were found to have
marked consequences in reproduction, immunology, and tumour growth.
Male albino rats ( Rattus rattus), 8 animals in a group, were
exposed to light/dark cycles of 14/10 h. They were treated by
gastric gavage with carbaryl dissolved in corn oil for 6 successive
days. The total doses were 50, 125, and 250 mg/kg. The animals were
killed 2 and 4 h after the onset of darkness, which was 8 or 10 h
after the application of the insecticide. Carbaryl was found to
bring about an augmentation of the pineal melatonin content 4 h
after the onset of darkness, which coincided with the stimulated
N-acetyltransferase levels and hydroxyindole- O-methyltransferase
activity. However, at the same time, carbaryl treatment at all doses
significantly lowered the circulating melatonin titres; this was
supposed to be related to increased hepatic melatonin metabolism due
to the increased activity of the liver drug metabolizing enzyme
system (Gaillard et al., 1977). These results and the established
carbaryl-induced elevated pineal 5-hydroxytryptophan, serotonin, and
5-hydroxyindole acetic acid contents in the course of the night
cycle, support the concept that carbaryl has a significant effect on
pineal melatonin synthesis and secretion.
Kadir & Knowles (1981) reported that carbaryl inhibited rat
brain monamine oxidase activity in vitro.
8.8.8 Other studies
Human serum albumin reacted in vitro with the ester group of
carbaryl and catalysed the hydrolysis and liberation of 1-naphthol.
This reaction is similar to an "esterase type" action (Casida &
Augustinsson, 1959) called carbamoylation (Oonnithan & Casida,
1968).
8.9 Factors modifying toxicity, toxicity of metabolites
8.9.1 Factors modifying toxicity
The toxicity of carbaryl can be modified by altering liver
function by tranylcypromine treatment or 70% hepatectomy. A decrease
in LD50 and increase in ChE activity were more pronounced after
tranylcypromine treatment (Falzon et al., 1983).
The carbaryl LD50 in animals was increased three-fold by
pretreating animals with small doses of carbaryl (Cress & Strother,
1974).
Phenobarbital, administered 24 h before carbaryl treatment,
decreased the acute ip toxicity of carbaryl in mice, while
2-diethyl-amino-ethyl-2,2'-diphenyl-valerate-HC1 (SKF 525-A), given
1 h before carbaryl treatment, increased its acute intraperitoneal
toxicity (Neskovic et al., 1978). Enzyme-mediated binding of
carbaryl to rat hepatic microsomal protein occurred in vitro in
the presence of NADPH and oxygen. Incorporation of radioactivity of
14C-ring labelled carbaryl was studied. A 2- to 3-fold increase in
binding was produced by pretreatment of animals with MFO inducers,
e.g., phenobarbital or 3-methylcholanthrene. SKF-525-A inhibited
binding by approximately 77% of the radioactivity. It is likely that
binding of active carbaryl metabolites formed by MFO occurs. The
radiolabelled metabolic products were covalently bound to amino acid
residues of microsomal protein: 99.3-99.7% of the bound
radioactivity (Miller et al., 1979). A 50% decrease in microsomal
ß-glucuronidase activity was observed 1 h after a single oral
administration of 50 mg carbaryl/kg to female, Sprague-Dawley rats.
The whole liver homogenate ß-glucuronidase content was reduced by
40% after daily treatment with the same dose of carbaryl for 7 days.
This effect was attributed to the decreases in mitochondrial
lysosomal and microsomal ß-glucuronidase. The action of carbaryl in
depleting the endoplasmic reticulum of ß-glucuronidase led to an
increase in serum ß-glucuronidase activity. This was demonstrated in
an in vitro microsomal suspension study which showed that
incubation of 4 mmol carbaryl/litre with microsomal suspensions
effected a release of the ß-glucuronidase enzyme. The effect on the
endoplasmic reticulum was further exemplified by the induction in
microsomal UDP-glucuronyl transferase activity after 7 days of daily
treatment with carbaryl at a dose of 25 mg/kg. Thus, carbaryl
modifies the enzyme activities associated with the endoplasmic
reticulum by decreasing specific activity (90%) towards
ß-glucuronidase enzyme and activating the synthesis of endoplasmic
reticulum protein connected with drug metabolism, such as the
UDP-glucuronyl-transferase enzyme (Abdel-Rahman et al., 1985;
Lechner & Abdel-Rahman, 1985).
Osman & Brindley (1981) conducted bioassays to determine the
carbaryl susceptibility and synergism with piperonyl butoxide in
natural populations of three species of Lobops grassbugs, in order
to estimate monoxygenase detoxification. Pretreatment of the insects
with piperonyl butoxide inhibited the mixed-function oxidase system
and decreased the values of the LC50 for the insects (Osman &
Brindley, 1981). Mixed-function oxydase involvement in the
biochemistry of synergistic insecticides (including carbaryl) has
been reviewed by Casida (1970).
Diet may modify carbaryl toxicity. Boyd & Boulanger (1968)
reported an increased susceptibility to carbaryl toxicity in Albino
rats (272 Wistar strain rats were used) fed a protein-deficient
diet. Boyd & Krijnen (1969) also reported that the LD50 decreased
from 589 mg/kg in rats fed an 81% casein diet to 67 mg/kg in rats
fed a 0% casein diet; 285 male rats were used in this study.
The combined effects of different ambient temperature levels
and carbaryl were studied by Ahdaya et al. (1976). The LD50
values in mice injected ip were 263 mg/kg at 1 °C and 122 mg/kg at
38 °C (588 mg/kg is the LD50 at 27 °C). The thermoregulation
ability of mice treated with carbaryl was affected more than that of
mice treated with parathion which is a stronger inhibitor of ChE.
The authors suggested that this effect was due to an overall
reduction in the basal metabolic rate.
Atropine sulfate decreased signs of parasympathetic stimulation
in a group of pigs acutely poisoned with 1-2 g carbaryl/kg (Smally,
1970). During multiple administrations of carbaryl, drug-induced
(hydrochlorothiazide) diuresis helped in the detoxification
processes. ChE reactivations are contraindicated because they may
aggravate the signs of carbaryl intoxication (Sanderson, 1961;
Podolak & Warchocki, 1980).
Application of atropine or trepazin, 20 min before oral
intoxication with carbaryl in mice, decreased toxicity about 2
times. The results were similar when cholinolytics were applied
2 min after administration of carbaryl (Vyatchanikov & Alexachina,
1968). Atropine administered iv to rats at a dose of 8 mg/kg
increased the carbaryl ip LD50 by a factor of about 7 (70 to
460 mg/kg) (Harris et al., 1989). Co-administration of either of
two different oximes (2-PAM and HI-6) with atropine was found to
provide significantly less protection than that afforded by atropine
alone, indicating that oxime therapy is not a useful treatment for
carbaryl poisoning.
Co-administration of an oral dose of 10 mg malathion/kg in rats
significantly reduced the rates of both absorption and elimination
of an oral dose of 10 mg carbaryl/kg (Lechner & Abdel-Rahman,
1986a). Peak plasma levels of labelled carbaryl were observed about
1 h after the administration of carbaryl alone, and after 2 h when
malathion was administered simultaneously. The terminal rate of
elimination of carbaryl was decreased by a factor of about 4, with
the half-life increasing from 16.96 h to 64.41 h.
Concentrations of cimetidine in the range of 60-240 µg/ml were
observed to decrease the clearance rate of carbaryl by perfused rat
liver in a dose-dependent manner (Ward et al., 1988).
Ray & Poddar (1985a,b, 1990) reported that the intraperitoneal
administration of 1 mg haloperidol/kg, 12.5 to 100 mg
5-hydroxy-tryptamine (5-HTP)/kg, or 100 mg L-tryptophan/kg increased
the incidence of tremors in rats following an oral dose of 50-200 mg
carbaryl/kg. The potentiation of the tremorogenic effect of carbaryl
by 5-HTP was demonstrated to be dose dependent. The potentiating
effects of 5-HTP and haloperidol could be blocked by the
coadministration of the serotonergic antagonist methysergide
(20 mg/kg, ip) or the dopaminergic antagonist bromocriptine
(10 mg/kg, ip), respectively. These results suggest the possible
involvement of a central cholinergic, dopaminergic interaction in
the carbaryl-induced tremor.
The effects of humic acids in the detoxification of carbaryl
during oral application are very slight: only 9.9-18.6% decrease in
toxicity was observed (Golbs et al., 1984).
The combined effects of carbaryl and sodium nitrite have been
studied (Podolak-Majczak & Tyburczyk, 1984, 1986; Tyburczyk &
Podolak-Majczak, 1984a,b; Tyburczyk & Podolak-Majczak, 1986). Dosing
Wistar rats for 3 months with sodium nitrite at 20 mg/kg per day,
and carbaryl at 0.1 LD50/day (60 mg/kg), the brain
gamma-aminobutyryl acid level, methaemoglobin, blood serotonin, free
blood tryptophan, serum and liver alanine amino-transferase activity
levels increased and blood cholinesterase activity decreased.
Decreases were observed in the vitamin E and A levels,
glucose-6-phosphate dehydrogenase activity, and liver aminohexoses
and hydroxyproline levels with a simultaneous increase in the serum
aminohexoses level. This finding may indicate the increasing process
of connective tissue catabolism and lisosomal membranes
labilization.
8.9.2 Toxicity of metabolites
Hydrolysis of the carbamate ester bond of carbaryl results in
detoxification. The carbamate moiety is decomposed to carbon dioxide
and methylamine, and the phenolic part is conjugated and excreted.
Kuhr (1971) summarized the data on the toxicity of carbaryl
metabolites (Table 53).
Table 53. Toxicity of carbaryl and some of its metabolitesa
LD50 (mg/kg) 7-day Molar I50 bovine
NOELb anticholin-esterase
Mouse (mg/kg)
Rat oral Intraperitoneal Rat
Carbaryl 270 29-42 125-250 5 x 10-8
4-Hydroxycarbaryl 1190 74 > 1000 4 x 10-7
5-Hydroxycarbaryl 297 56 > 1000 4.6 x 10-8
7-Hydroxycarbaryl 4760 > 1000
Hydroxymethylcarbaryl > 5000 630-780 250-500 1.4 x 10-5
1-Naphthol 2570 500-1000 1 x 10-3
aFrom: Kuhr (1971).
bNOEL = No-observed-effect level.
Many of the known metabolites are much less toxic than
carbaryl. The pharmacological study of carbaryl in rats showed that
the metabolites with a methylcarbamate moiety are inhibitors of
plasma cholinesterase (Fernandez et al., 1982). None of the
carbaryl metabolites was appreciably more active as a cholinesterase
inhibitor than carbaryl itself (Oonnithan & Casida, 1968).
8.9.3 N-nitrosocarbaryl
Carbaryl is a secondary amine and is, therefore, capable of
nitrosation in the presence of nitro donor groups, such as sodium
nitrate, to give a nitrosamide. This nitrosamide, nitrosocarbaryl,
has been proved to be mutagenic and carcinogenic, at high doses in
animals. Conditions of this nitrosation include a strongly acidic pH
(less than 2), which is comparable with the pH in the human stomach.
However, nitrosocarbaryl is not stable at this pH. Its maximum
stability is between pH 3 and 5, pHs at which no significant amounts
of carbaryl can be nitrosated. Carbaryl has been nitrosated in
several studies, in vitro as well as in vivo, in the guinea-pig,
which has a stomach acidity close to that in man. If significant
yields of nitrosocarbaryl have been shown in these circumstances,
the significance of these findings is still not clear for human risk
evaluation. The pH in the human stomach is variable during food
intake and probably, more importantly, the stomach contains a lot of
food, which will minimize the contact between naturally occurring
nitrite and carbaryl residues, but which will also afford a lot of
nucleophilic sites for nitrosocarbaryl to react with. It is also
noteworthy that all studies that were conducted with jointly
administered carbaryl and nitrite yielded negative results for
oncogenicity. Cranmer (1986) estimated the potential intake of
nitrosocarbaryl at 6x10-9 mg/kg per day. Using different
mathematical models, Cranmer estimated a cancer risk for
nitrosocarbaryl between 10-6 (one-hit model) and 10-9 (probit
model). However, if such oncogenic nitroso-carbamates were to be
found in such quantities in the human stomach, an increased
incidence of stomach cancer would have been expected during the
period when drug and pesticide carbamates were widely used. However,
during this period, the incidence of gastric cancer in the USA
declined considerably.
The bacterial metabolite N-nitrosocarbaryl may act as a
noncompetitive inhibitor of the in vitro metabolism of
aminopyrine, p-nitroanisole, and aniline by rat liver microsomes
(Beraud et al., 1980, 1989a,b). N-nitrosocarbaryl was more
effective as an inhibitor of microsomal activity than the parent
compound.
8.10 Mechanism of toxicity - mode of action
The mechanism of toxicity and the mode of action of carbaryl
and carbamates, in general, have been described in EHC 64 (WHO,
1986).
8.10.1 Inhibition of cholinesterase activity
As a carbamate compound, carbaryl is an inhibitor of
cholinesterase (ChE) activity (for details see Reiner & Aldridge
(1967) and Aldridge (1971)).
A number of studies have been performed by Carpenter et al.
(1961) to assess the extent of ChE inhibition by carbaryl in
mammals. A single oral dose of carbaryl of 560 mg/kg produced a 43%
inhibition of the erythrocyte ChE in rats in 0.5 h, and a 30%
inhibition in brain AChE. However, they returned to normal in rats
that survived 24 h after administration of the dose. Carbaryl did
not depress plasma ChE significantly. Two groups of Beagle dogs were
injected once, iv, with 10 or 15 mg/kg as an 8% solution in 95%
alcohol. No significant effects were found on either erythrocyte or
plasma ChE. On the 5th day, several administrations of the same
doses depressed plasma ChE by 24% and erythrocyte AChE by 40%. It is
doubtful that a long incubation time for the samples (2 h for
plasma) played a role in the slight depression of ChE. Comparative
data on ChE inhibition in the brain, plasma, and erythrocytes of
rats that received single doses of carbaryl were reported by Mount
et al. (1981). As shown in Table 54, there was a dose-dependent
decrease in ChE in the brain, plasma, and erythrocytes.
Brain AChE was significantly different in dead rats and in
surviving rats given 800 mg/kg. AChE depression in the brain was
>70% in the lethally poisoned rats. The inhibition of red blood
cell AChE activity was the same as in plasma.
Table 54. Inhibition of ChE in % in comparison with the controlsa
Sprague-Dawley rats Post-dosing Brain Red blood Plasma
(h) cells
Controls 10 rats 0 0 0
450 mg/kg 0.5 56 44 51
(24 rats sacrificed) 1 74 29 53
2 74 42 68
4 66 48 61
8 55 25 31
24 27 60 38
48 22 23 46
96 6 19 -19
800 mg/kg 0.5 66 32 47
(34 rats sacrificed) 1 70 44 50
2 79 73 69
4 50 72 70
12 50 72 50
28 58 63 68
48 17 26 40
96 16 -6 16
800 mg/kg 3 80 - -
(24 rats died) 4 85 - -
18 88 - -
25 71 - -
29 88 - -
30 84 - -
1200 mg/kg 1-36 88-91 - -
(15 died)
aAdapted from: Mount et al. (1981).
The affinity of AChE of human brain caudate nucleus for
carbaryl was studied in vitro and some inhibition constants were
determined (Patocka & Bajgar, 1971). The value of the I50 affinity
constant was calculated from the dependence of AChE inhibition on
the molar concentration of the inhibitor in probit logarithm
transformation. The Hill coefficient was obtained from Hill plots.
The affinity constant for carbaryl pI50 was 5.59 and the Hill
coefficient was 1.50. According to the authors, some results of this
study suggest that AChE may be an allosteric enzyme.
Intravenous administration of colloidal carbon in the rat,
inhibiting the phagocytic activity of the RES, prolonged the
duration of the anticholinesterase effect of carbaryl (Pipy et al.,
1979). The authors speculated that the metabolism of carbaryl was
decreased by the RES inhibition and, as a result, the reactivation
speed of ChE was slower.
Pregnant rats were treated orally with 1 or 5 mg carbaryl/kg
from day 11 to the last day of gestation (Declume & Benard, 1977b;
Declume et al., 1979). No cholinesterase depression in blood,
brain, and liver were observed in either the dams or the newborn
offspring. However, after administration of 50 mg/kg from day 19 to
the last day of gestation, ChE inhibition was seen in both the
mother and newborn offspring.
Cambon et al. (1978) reported decreased cholinesterase
activity in the blood, brain, and liver in the mother and the fetus
after treatment of the dams at 6.25, 12.5, 25, and 50 mg/kg.
Variability of cholinesterase levels in the controls and
short-comings in the cholinesterase analysis protocol make these
results difficult to interpret.
9. EFFECTS ON HUMAN BEINGS
9.1 General population exposure
9.1.1 Acute toxicity, poisoning incidents
The clinical picture of carbaryl intoxication results from the
accumulation of ACh at nerve endings (WHO, 1986). The signs and
symptoms can be categorized into the following 3 groups:
(a) Muscarinic manifestations
- increased bronchial secretion, excessive sweating,
salivation, and lacrimation;
- pinpoint pupils, bronchoconstriction, abdominal cramps
(vomiting and diarrhoea); and
- bradycardia.
(b) Nicotinic manifestations
- fasciculation of fine muscles (in severe cases, diaphragm
and respiratory muscles also involved); and
- tachycardia.
(c) Central nervous system manifestations
- headache, dizziness, anxiety, mental confusion,
convulsions, and coma; and
- depression of respiratory centre.
All these signs and symptoms can occur in different
combinations and can vary in onset and sequence, depending on the
chemical, dose, and route of exposure. The duration of symptoms is
usually shorter than that observed in organophosphorus poisoning.
Mild poisoning might include muscarinic and nicotinic signs only.
Severe cases always show central nervous system involvement; the
clinical picture is dominated by respiratory failure sometimes
leading to pulmonary oedema, due to the combination of the
above-mentioned syndromes.
A review of carbaryl-related poisoning from 1966 to 1980 was
made by US EPA. During this period, 193 cases of over-exposure to
carbaryl as the sole active ingredient in the poisoning and 144
cases of over-exposure to a combination of carbaryl with other
active ingredients were recorded (Weston, 1982). Not all case
histories have been confirmed. There were 5 deaths. There is no
evidence that carbaryl was involved in these deaths, except for one,
which was acknowledged by the manufacturer.
Hayes (1963) reported two incidents of poisoning: one, a
19-month-old child who swallowed an unknown amount of carbaryl, and
the other, a man who swallowed 250 mg of carbaryl. Both developed
moderately severe ChE inhibition symptoms within 20 min: constricted
pupils, salivation, muscular incoordination in the child, and
epigastric pain, profuse sweating, lassitude, and vomiting in the
man. Both recovered after atropine treatment. Blood cholinesterase
was inhibited.
One death from carbaryl ingestion while drunk was reported by
Farago (1969) who concluded that 2-PAM application hastened the
fatal outcome (due to pulmonary oedema), 6 h after ingestion.
Carbaryl was found in various tissues.
Acute intoxication during the loading of an airplane was
reported by Long (1971).
In a case report of a suicide attempt involving about 25 g
(500 mg/kg) of carbaryl, dicumarin and boric acid, Dickoff et al.
(1987) described the occurrence of a peripheral neuropathy that
resembled the syndrome observed following exposure to some
organophosphorus compounds. Recovery continued for 9 months.
Electrophysiological findings were consistent with axonal peripheral
neuropathy. The cause-effect relationship was confounded because of
combined exposure.
9.1.2 Controlled human studies
Wills et al. (1968) carried out controlled studies in human
volunteers, aged 25-57 years. In a preliminary study, oral doses of
0.5, 1, and 2.0 mg carbaryl/kg in capsules (single application to 2
subjects) were tolerated without any subjective or objective
symptoms. In the main study, one group (5 subjects) took 0.06 mg
carbaryl/kg daily, and one group (6 subjects) took 0.13 mg
carbaryl/kg for 6 weeks. Physical examination, BSP removal from
blood, EEG examination, routine blood and urinalysis were performed,
and ChE of plasma and red blood cells was examined. No changes were
found in the low-dose group. An increase in the ratio of amino acid
nitrogen to creatinine in the urine, at the high dose, may represent
a decrease in the ability of the proximal convoluted tubule to
reabsorb amino acids. This change was reversible.
The urine of some subjects was analysed (Knaak et al., 1968).
The overall recovery (by the fluorometric method) of the carbaryl
equivalents in the urine was 26-28%, and, with the colorimetric
method, 37.8%, in subjects treated with 2 mg carbaryl/kg. The
following metabolites were found chromatographically in a 4-h urine
sample: alpha-naphthol glucuronide (10-15%), and sulfate (6-11%) and
4-(methylcarbamoyloxy)-alpha-naphthyl glucuronide (4%). Another
metabolite, alpha-naphthyl methylimido-carbonate O-glucuronide,
was identified by fluorometry.
A human ingestion study was conducted to determine the
relationship between a single oral dose of carbaryl and the rate of
its urinary excretion as metabolites. Elimination was apparently
first order over the dosage range of the studies (0.25-1 mg/kg). The
model predicts that, 24 h after ingestion, approximately 41% of the
dose can be accounted for as urinary metabolites (Hansen, 1978).
In a study involving one subject, Ward et al. (1988) observed
that pretreatment with a clinical regimen of cimetidine (3x200 mg
over a three-day period) reduced presystemic (first-pass) clearance
of a dose of 1 mg carbaryl/kg by about 46%.
A scientist studying the anthelminthic activity of carbaryl,
tested its human safety by ingesting 250 mg (about 2.8 mg/kg). After
20 min, he experienced epigastric pain and began to sweat profusely.
He was treated with a total dose of 3 mg atropine and recovered
completely 2 h after taking the carbaryl. Another scientist ingested
420 mg carbaryl (4.45 mg/kg), and after 85 min he had vision
troubles, weakness, profuse sweating, and felt lightheaded. He was
treated with a total dose of 4.8 mg atropine and recovered 4 h after
the onset of symptoms (Hayes & Laws, 1991).
9.1.3 Long-term exposure
Branch & Jacqz (1986a,b) reported the case of a 75-year-old man
who was exposed to carbaryl for 8 months, inside his home, after
repeated excessive applications of 10% dust formulation. He
experienced a series of signs and symptoms compatible with
cholinesterase depression in addition to a 40-lb (18-kg) weight
loss. After exposure ceased, the patient's condition improved
markedly. However, a few months later he started to experience
modification of his sleep pattern and peripheral neuropathy and
cerebral atrophy were demonstrated. Other pathologies, such as
recurrent gastric ulcer, cardiac fibrillations, a recent head injury
due to an automobile accident, and other less defined pathologies
were present in this patient which provide other, more likely,
causes for these later symptoms.
The uncertainties associated with long-term exposure to levels
sufficient to result in sustained suppression of plasma
pseudocholinesterase activity and possible brain damage are
discussed by Avashia (1987) and Branch (1987).
9.2 Occupational exposure
9.2.1 Epidemiological studies
The first report on workers exposed to carbaryl was published
by Best & Murray (1962). For 19 months, from the start of carbaryl
production, they studied men working on the production, handling,
and shipping of carbaryl. The most exposed group were bagging
workers occasionally exposed to carbaryl dust under abnormal
conditions (40 mg/m3). These showed a slight depression in blood
ChE activity, but this was below the rate at which clinical symptoms
might be expected. They found that 41% of 689 urine specimens
contained >1000 µg/100 ml total 1-naphthol (>400 µg/100 ml
indicates absorption), with no clinical or subjective symptoms. In
cases of acute intoxication, 3140 µg 1-naphthol/100 ml urine were
found. Knaak et al. (1965) using fluorometric analysis in
conjunction with chromatographic separation, found 5 times more
sulfate (25 mg/litre) than glucuronides (5 mg/litre) of 1-naphthol
in the urine of exposed workers.
Vandekar (1965) in a village-scale trial in Nigeria, assessed
the risk for the population of exposure to carbaryl. A slight
depression of plasma ChE was found in all spraymen, the day after
the spraying. Levels of 1-naphthol derivatives in their urine did
not increase on days 1 and 2 after spraying but increased slightly
on day 6.
In a study on 19 agricultural workers (Yakim, 1967), whole
blood ChE activity was measured before, and after, 3-4 days exposure
to airborne carbaryl. Men exposed to a mean airborne carbaryl
concentration of 2 mg/m3 showed a decrease of 20-24% in ChE
activity. Signalmen exposed to 4 mg/m3 (mean) showed a 13-30%
decrease in ChE activity. No objective signs of ill health were
observed. In the same study, a mean carbaryl concentration of
0.7 mg/m3 was reported in the cabin of an aeroplane used to apply
carbaryl, but no changes were reported in the biological parameters
of the pilots. During the agricultural application of carbaryl dust
at a maximum exposure level of 19 mg/m3 dust on cotton fields,
Adylov (1966) reported a decrease in catalase activity, and a 20%
decrease in ChE activity on day 14.
In cases of occupational overexposure to carbamates, mild
symptoms appear long before a dangerous dose is absorbed, which is
why severe occupational intoxications with carbaryl are rare. Tobin
(1970) gives reasons for the lack of severe intoxications: (1) there
is a very short time (´ h or less) between exposure to carbamates
and the onset of symptoms; (2) lack of symptoms progression, because
of a large margin between the median effective dose and the lethal
dose of carbamates and the early detection of intoxication.
Workers exposed to carbaryl used on pets for flea control,
experienced diarrhoea, increased salivation, cough, difficulty in
breathing, and phlegm (Ames et al., 1989).
Vandekar (1965) reported a skin rash in a sprayman who was
accidentally splashed with a carbaryl formulation. Although
carbamate compounds generally have not caused dermatitis or allergic
skin reactions, Vandekar suggested that they can appear in certain
individuals after unusually heavy exposure.
One out of a group of 30 farmers with contact dermatitis was
identified by a patch test as having a positive allergic reaction to
a 1% solution of carbaryl (Sharma & Kaur, 1990). No allergic
reaction were observed in a control population (No.=20).
To identify possible effects on reproduction, Whorton et al.
(1979) studied a cohort of 47 male workers who had worked for at
least 1 year in the production and packaging of carbaryl. A semen
sample was used for sperm count. Testosterone follicle-stimulating
hormone, and luteinizing hormone were determined by
radioimmunoassay. The range of airborne carbaryl concentrations in
the workplace was 0.03-14.21 mg/m3 with a mean of 4.9 mg/m3.
This cohort showed no seminal or blood abnormalities related to
carbaryl exposure. Although a small excess (not significant at
alpha=0.05) was observed in a small number of oligospermic men in
the exposed group, there was no evidence that testicular function or
fertility in the male workers was affected under these conditions of
exposure.
Wyrobek et al. (1981) used the same cohort of exposed workers
to study sperm abnormalities. Semen was collected from 50 men,
occupationally exposed to carbaryl for 1-18 years. Semen samples
were analysed for changes in sperm motility, sperm count,
morphology, and frequency of sperm-carrying double fluorescent
bodies (YFF). The YFF test represents sperm with two Y chromosomes
due to meiotic nondysjunction. The exposed workers showed changes in
sperm morphology with a higher proportion of sperm with abnormal
head shapes in comparison with the control group of newly hired
unexposed workers. There was no dose dependence as judged by job
classification. A negative correlation between number of years
working in the carbaryl area and the percentage of abnormal sperm
was observed. Workers who had once been exposed to carbaryl, but who
had not been exposed for an average of 6.3 years, showed a
marginally significant elevation in sperm abnormalities, possibly
not reversible. MacLeod (1982) reviewed the Wyrobek et al. (1981)
study and did not find any essential differences in the distribution
of the sperm types in the control and the carbaryl-exposed groups.
No significant changes in sperm count and fertility were reported in
100 workers (Thomas, 1981).
10. PREVIOUS EVALUATION BY INTERNATIONAL BODIES
The latest IARC evaluation of carbaryl was made during 1987
(IARC, 1987). It was concluded that there were no data on cancer in
humans and inadequate evidence of carcinogenicity in experimental
animals. Carbaryl could not be classified with regard to its
carcinogenicity to humans (Group 3).
The FAO/WHO Joint Meeting on Pesticide Residues (JMPR)
evaluated carbaryl at its meetings in 1963, 1965, 1966, 1967, 1968,
1969, 1970, 1971, 1973, 1975, 1976, 1977, 1979 and 1984 (FAO/WHO,
1964, 1965, 1967, 1968, 1969, 1970, 1971, 1972, 1974, 1976, 1977,
1978, 1980, 1985). Since 1973, an acceptable daily intake (ADI) of
0-0.01 mg/kg body weight has been established. This estimate is
based on the following experimental data: no-effect level for rats:
200 mg/kg in the diet = 10 mg/kg per day; for dogs: 100 mg/kg in the
diet = 1.8 mg/kg per day; and for human beings: 0.06 mg/kg per day
(FAO/WHO, 1965, 1967, 1974).
Maximum residue levels (MRLs) for carbaryl were recommended by
FAO/WHO (1986b) (see Table 55). The values recommended for tolerance
levels represent the sum of free carbaryl, combined carbaryl,
conjugated naphthol, and conjugated methylcarbaryl, expressed as
total toxic residues of carbaryl.
A WHO study group on occupational health recommended 5 mg
carbaryl/m3 as a tentative, health-based, maximum permissible
level in the working environment. A biological limit of 30%
inhibition of ChE activity in whole blood, plasma, or red cells with
respect to pre-exposure levels should not be exceeded (WHO, 1982).
In the WHO recommended classification of pesticides by hazard,
technical carbaryl is classified in Class II as moderately hazardous
in normal use (WHO, 1992). WHO/FAO (1975) issued a data sheet on
carbaryl (No. 3).
IRPTC in its series "Scientific reviews of Soviet literature on
toxicity and hazards of chemicals" has published a review on
carbaryl (IRPTC, 1982, 1989).
Table 55. Maximum Residue Limits (MRLs) established by the Codex
Alimentariusa
Commodity MRL
(mg/kg)
Animal feedstuffs (green alfalfa, clover, corn, forage, cow pea 100
foliage, grasses, peanut hay, sorghum forage, soybean vines,
sugarbeet tops, bean and pea vines)
Bran (wheat) 20
Apricots, blackberries, boysenberries, nectarines, peaches, 10
raspberries, asparagus, okra, leafy vegetables (except
brassica), nuts (whole), olives (fresh), sorghum grain, cherries,
plums, kiwi fruit
Blueberries, citrus fruit, cranberries, strawberries 7
Apples, bananas (pulp), grapes, beans, peas (including pod), 5
brassica, tomatoes, peppers, aubergines, pears, poultry skin,
barley, oats, rice (in husk and hulled), rye, wheat
Cucumbers, melons (Cantaloupe), pumpkins, squash 3
Root crop vegetables (beets, carrots, radishes, rutabagas, 2
parsnips), peanuts (ground-nuts, whole), wholemeal flour
Cottonseed (whole), sweet corn (kernels), nuts (shelled), olives 1
(processed), soybeans (dry mature seed), cow-peas
Poultry meat, eggs (without shells) 0.5
Potatoes, meat of cattle, sheep, and goats, sugarbeets, wheat 0.2
flour (white)
Milk and milk products 0.1
aFrom:FAO/WHO (1986b).
REFERENCES
Abdel-Rahman MS, Lechner DWH, & Klein KM (1985) Combination effect
of carbaryl and malathion in rats. Arch Environ Contam Toxicol, 14:
459-464.
Abrahamsen LH & Jerkofsky M (1981) Enhancement of varicella-zoster
virus replication in cultured human embryonic lung cells treated
with the pesticide carbaryl. Appl Environ Microbiol, 41(3): 652-656.
Addison JB, Silk PJ, & Unger I (1975) The photochemistry of
carbamates V. The photodecomposition of Sevin (1-naphthyl- N-methyl
carbamate) and of Phenmec (phenyl- N-methyl carbamate). Int J
Environ Anal Chem, 4: 135-140.
Adylov MA (1966) [Sanitary hygienic assessment of the working
conditions and health state of people working with Sevin in
Uzbekistan.] Gig Primen Toxicol Pestic Klin Otrav, 1866: 271-275 (in
Russian).
Ahdaya SM & Guthrie FE (1982) Stomach absorption of intubated
insecticides in fasted mice. Toxicology, 22(4): 311-317.
Ahdaya SM, Shah PV, & Guthrie FE (1976) Thermoregulation in mice
treated with parathion, carbaryl or DDT. Toxicol Appl Pharmacol, 35:
575.
Ahlrichs JL, Chandler L, Monke EJ, & Reuszler HW (1970) Effect of
pesticide residues and other organotoxicants on the quality of
surface and ground water resources. Lafayette, Indiana, Indiana
Water Research Center, 106 pp (Technical Report No. 10).
Ahmed FE, Hart RW, & Lewis NJ (1977a) Pesticide induced DNA damage
and its repair in cultured human cells. Mutat Res, 42: 161-174.
Ahmed FE, Lewis NJ, & Hart RW (1977b) Pesticide induced ouabain
resistant mutants in Chinese hamster V79 cells. Chem-Biol Interact,
19: 369-374.
Akhundov VY, Lurie LM, & Ismailova IM (1981) [Effect of Sevin on
immunological reactivity.] Gig i Sanit, 46(2): 25-28 (in Russian).
Albanis TA, Pomonis PJ, & Sdoukos AT (1986) Organophosphorous and
carbamates pesticide residues in the aquatic system of Ionnina basin
and Kalamas river (Greece). Chemosphere, 15(8): 1023-1034.
Albright LM & Simmel EC (1977) Behavioural effects of cholinesterase
inhibitor and insecticide carbaryl (Sevin). Paper presented at the
49th Annual Meeting of the Midwestern Physiological Association,
Chicago, 5-7 May 1977.
Aldridge WN (1971) The nature of the reaction of organophosphorous
compounds and carbamates with esterases. Bull World Health Organ,
44: 25.
Alvarez CC, Shimanuki H, & Argauer RJ (1970) Oral toxicity of
carbaryl to adult honey bees. J Econ Entomol, 63(6): 1834-1835.
Aly OM & El Dib MA (1971a) Studies on the persistence of some
carbamate insecticides in the aquatic environment. I. Hydrolysis of
Sevin, Baygon, Pyrolan and Dimetilan in waters. Water Res, 5:
1191-1205.
Aly OM & El Dib MA (1971b) Photodecomposition of some carbamate
insecticides in aquatic environments. In: Faust SJ & Hunter JV ed.
Proceedings of the Rudolfs Research Conference on Organic Compounds
in Aquatic Environments. New York, Basel, Marcel Dekker, Inc., pp
469-493.
Aly OM & El Dib MA (1972) Studies on the persistence of some
carbamate insecticides in the aquatic environment. In: Fate of
organic pesticides in the aquatic environment. Washington, DC,
American Chemical Society, pp 211-241 (Advances in Chemistry Series,
Volume 111).
Aly MI, Bakry N, Kishk F, & El-Sebae AH (1980) Carbaryl adsorption
on calcium-bentonite and soils. J Soil Sci Soc Am, 44(6): 1213-1215.
Amer S (1965) Cytological effects of pesticides. I. Mitotic effects
of N-methyl-1-naphthyl carbamate "Sevin". Cytologia, 30: 175-181.
Amer SM & Farah OR (1968) Cytological effects of pesticides. III.
Meiotic effects of N-methyl-1-naphthyl carbamate "Sevin".
Cytologia, 33: 337-344.
Amer SM, Hammouda MA, & Farah OR (1971) Cytological and
morphological effects of the insecticide N-methyl-1-naphthyl
carbamate "Sevin". Flora (Jena), 160: 433-439.
Ames RG, Brown SK, Rosenberg J, Jackson RJ, Stratton JW, & Quenon SG
(1989) Health symptoms and occupational exposure to flea control
product among California pet handlers. Am Ind Hyg Assoc J, 50(9):
466-472.
Andrawes NR & Bailey RH (1978a) Metabolism of carbaryl in the rat. A
reappraisal (File No. 25051). Research Triangle Park, North
Carolina, Union Carbide Corporation (Unpublished proprietary
information submitted to WHO by Rhône-Poulenc Agro, Lyon).
Andrawes NR & Bailey RH (1978b) Conjugated metabolites of carbaryl:
A comparison between rat and dog. South Charleston, West Virginia,
Union Carbide Research and Development (Project Report No. 811C20)
(Unpublished proprietary information submitted to WHO by
Rhône-Poulenc Agro, Lyon).
Andrawes NR & Bailey RH (1978c) Metabolism of carbaryl in Beagle
dogs. South Charleston, West Virginia, Union Carbide Research and
Development (Project Report No. 811C20) (Unpublished proprietary
information submitted to WHO by Rhône-Poulenc Agro, Lyon).
Andrawes NR & Bailey RH (1979) Carbaryl metabolites detected in the
urine of plant workers (File No. 25957). South Charleston, West
Virginia, Union Carbide Research and Development (Unpublished
proprietary information submitted to WHO by Rhône-Poulenc Agro,
Lyon).
Andrawes NR & Chancey EL (1970) The metabolism of 1-naphthyl-C14
carbaryl in wheat and bean plants. Clayton, North Carolina, Union
Carbide Corporation (Project Report No. 13749) (Unpublished
proprietary information submitted to WHO by Rhône-Poulenc Agro,
Lyon).
Andrawes NR & Myers WR (1976) Identification of carbaryl metabolites
in human urine (File No. 22983). South Charleston, West Virginia,
Union Carbide Corporation (Unpublished proprietary information
submitted to WHO by Rhône-Poulenc Agro, Lyon).
Andrawes NR, Chancey EL, Crabtree RJ, Herrett RA, & Weiden MHJ
(1972) Fate of naphthyl-1-14C carbaryl in laying chickens. J Agric
Food Chem, 20(3): 608-617.
André F, Gillon J, André C, Lafont S, & Jourdan G (1983)
Pesticide-containing diets augment anti-sheep red blood cell
nonreaginic antibody responses in mice but may prolong murine
infection with Giardia muris. Environ Res, 32: 145-150.
Andrianova MM & Alexeev IV (1970) [On the question of the
carcinogenic properties of the pesticides Sevin, Maneb, Ziram and
Zineb.] Vopr Pitan, 6: 71-74 (in Russian).
Anger WK & Setzer JV (1979) Effects of oral and intramuscular
carbaryl administrations on repeated chain acquisition in monkeys. J
Toxicol Environ Health, 5: 793-808.
Antonovich NA (1970) [On the residual quantities of Sevin in food
products.] Gig Primen Toxicol Pestic Klin Otrav, 1970: 176-179 (in
Russian).
Argauer RJ & Warthen JD (1975) Separation of 1- and 2-naphthols and
determination of trace amounts of 2-naphthyl methylcarbamate in
carbaryl formulations by high pressure liquid chromatography with
confirmation by spectrofluorometry. Anal Chem, 47(11): 2472-2474.
Armstrong DA & Millemann RE (1974a) Pathology of acute poisoning
with the insecticide Sevin in the bent-nosed clam, Macom nasuta. J
Invertebr Pathol, 24(2): 201-212.
Armstrong DA & Millemann RE (1974b) Effect of the pesticide carbaryl
on clams and some other intertidal mud flat animals. J Fish Res
Board Can, 31: 466-470.
Armstrong DA & Millemann RE (1974c) Effect of the insecticide Sevin
and its first hydrolytic product 1-naphthol on some developmental
stages of the bay mussel Mytilus edulis. Mar Biol, 28: 11-15.
Arunachalam S & Palanichamy S (1982) Sublethal effects of carbaryl
on surfacing behaviour and food utilization in the air breathing
fish, Macropodus cupanus. Physiol Behav, 29(1): 23-27.
Arunachalam S, Jeyalakshmi K, & Aboobucker S (1980) Toxic and
sublethal effects of carbaryl on a freshwater catfish, Mystus
vittatus (Bloch). Arch Environ Contam Toxicol, 9: 307-316.
Ashwood-Smith M, Trevino J, & Ring R (1972) Mutagenicity of
dichlorvos. Nature (Lond), 240: 418-420.
Atabaev ST (1972) [Carbamate compounds.] In: [Pesticides and hygiene
of the environment in the conditions of warm climate.] Tashkent,
Medizina, pp 114-121 (in Russian).
Attia AM, Reiter RJ, Nonaka KO, Mostafa MH, Soliman SA, & El-Sebae
AH (1991) Carbaryl-induced changes in indoleamine synthesis in the
Pineal gland and its effects on night-time serum melatonin
concentrations. Toxicology, 65: 305-314.
Avashia BH (1987) Is carbaryl safe as its reputation. Letter to the
Editor. Am J Med, 83: 1168-1169.
Azizova OM (1976) [Effect of Sevin and methyl-mercaptophos on
different levels of the nervous system.] In: Medved' LI ed. [Current
problems of the hygiene of application of pesticides in different
climatic and geographical zones. Proceedings of the Itinerary
Scientific Session of Vniigintoks, Erevan.] Kiev, Vniigintoks (in
Russian).
Bansal SK, Verma SR, Gupta AK, & Dalela RC (1980) Predicting
long-term toxicity by subacute screening of pesticides with larvae
and early juveniles of four species of freshwater major carp.
Ecotoxicol Environ Saf, 4: 224-231.
Baron RL (1968) Radioactive lactose in skim milk following
administration of carbonyl-14C-carbaryl to a lactating cow. J
Assoc Off Anal Chem, 51: 1046-1049.
Baron RL & Locke RK (1970) Utilization of cell culture techniques in
carbaryl metabolism studies. Bull Environ Contam Toxicol, 5:
287-291.
Barrett GW (1968) The effect of an acute insecticide stress on a
semi-enclosed grassland ecosystem. Ecology, 49(6): 1019-1035.
Bart J (1979) Effects of acephate and Sevin on forest birds. J Wildl
Manage, 43(2): 544-549.
Basha SM, Rao KSP, Rao KRSS, & Rao KVR (1983) Differential toxicity
of malathion, BHC, and carbaryl to the freshwater fish, Tilapia
mossambica (Peters). Bull Environ Contam Toxicol, 31(5): 543-546.
Bavari S, Casale GP, Gold RE, & Vitzthum EF (1991) Modulation of
interleukin-2 driven proliferation of human large granular
lymphocytes by carbaryl, an anticholinesterase insecticide. Fundam
Appl Toxicol, 17: 61-74.
Belonozhko GA & Kuchak JA (1969) [Electrocortical reactions to
treatment with Sevin in rats.] Gig i Sanit, 34(2): 111-113 (in
Russian).
Benard P, Cambon C, & Declume C (1979) Passage of radioactivity into
the milk of rats treated with [14C] carbaryl. Toxicol Lett, 4:
149-153.
Bend JR, Holder GM, Protos E, & Ryan AJ (1971) Water soluble
metabolites of carbaryl (1-naphthyl N-methylcarbamate) in mouse
liver preparations and in the rat. Aust J Biol Sci, 24: 535-546.
Benson BW, Scott WJ, & Beliles RP (1967) Sevin teratological study
in the mouse (Prepared by Woodard Research Corporation) (Union
Carbide proprietary report, submitted to WHO by Rhône-Poulenc Agro,
Lyon).
Beraud M, Pipy B, Derache R, & Gaillard D (1979) Formation d'un
cancérigène, le N-nitrosocarbaryl, par interaction entre un
insecticide de la série des carbamates, le carbaryl, et le nitrite
de sodium de suc gastrique de rat. Food Cosmet Toxicol, 17(6):
579-583.
Beraud M, Gaillard S, & Derache R (1980) Inhibition of two hepatic
microsomal drug-metabolizing enzymes by a carcinogenic
N-nitrosated pesticide: N-nitrosocarbaryl. Chem-Biol Interact,
31: 103-112.
Beraud M, Forgue M-F, Pipy B, & Derache P (1989a) Interaction of
carbaryl and N-nitrosocarbaryl with microsomal monooxygenase
activities. Toxicol Lett, 45(2-3): 251-260.
Beraud M, Forgue M-F, Pipy B, Didier A, & Carre P (1989b) Lipid
peroxidation in rat liver microsomes: Influence of carcinogenic
N-nitrosocarbaryl. Toxicology, 58: 299-311.
Best EM & Murray BL (1962) Observations on workers exposed to Sevin
insecticide: A preliminary report. J Occup Med, 4(10): 507-517.
Blase W & Loomis TA (1976) The uptake and metabolism of carbaryl by
isolated perfused rabbit lung. Toxicol Appl Pharmacol, 37: 481-490.
Blevins RD & Dunn WC (1975) Effects of carbaryl and dieldrin on the
growth, protein content and phospholipid content of Hela cells. J
Agric Food Chem, 23(3): 377-382.
Blevins RD, Lee M, & Regan JD (1977) Mutagenicity screening of five
methyl carbamate insecticides and their nitroso derivatives using
mutants of Salmonella typhimurium LT2. Mutat Res, 56(1): 1-6.
Bluzat R & Seuge J (1979) Effets de trois insecticides (lindane,
fenthion et carbaryl): toxicité aiguë sur quatre espèces
d'invertébrés limniques; toxicité chronique chez le mollusque
pulmoné lymnea. Environ Pollut, 18: 51-70.
Boethling RS & Alexander M (1979) Effect of concentration of organic
chemicals on their biodegradation by natural microbial communities.
Appl Environ Microbiol, 37(6): 1211-1216.
Bogacka T & Groba J (1980) [Toxicity and biodegradation of
chlorfenvinphos, carbaryl, and propoxur in water environment.]
Bromatol Chem Toksykol, 13(2): 151-158 (in Polish with English
summary).
Bogomolova ZN (1968) [Changes in the content of Sevin in fruitberry
cultures during the process of vegetation, storage and canning.] Gig
Primen Toxicol Pestic Klin Otrav, 1968: 363-367 (in Russian).
Bogomolova ZN (1970) [Content of Sevin in fruit cultures.] Gig
Primen Toxicol Pestic Klin Otrav, 1970: 197-180 (in Russian).
Bogus ER, Gallagher P, Cameron F, & Mumima R (1985) Analysis of
pesticide exposure pads using selective absorption and elution of
reversed-phase solid support. J Agric Food Chem, 33: 1018-1021.
Bollag JM (1979) Transformation of xenobiotics by microbial
activity. In: Proceedings of an EPA Workshop on Microbial
Degradation of Pollutants in Marine Environments. Gulf Breeze,
Florida, US Environmental Protection Agency, Environmental Research
Laboratory, pp 19-27 (EPA 600/9-79-012).
Bollag JM & Liu SY (1971) Degradation of Sevin by soil
microorganisms. Soil Biol Biochem, 3(4): 337-345.
Bollag JM & Liu SY (1972a) Fungal degradation of 1-naphthol. Can J
Microbiol, 18(7): 1113-1117.
Bollag JM & Liu SY (1972b) Hydroxylations of carbaryl by soil fungi.
Nature (Lond), 236: 177-178.
Bollag JM, Czaplicki EJ, & Minard RD (1975) Bacterial metabolism of
1-naphthol. J Agric Food Chem, 23(1): 85-90.
Bollag JM, Sjoblad RD, Czaplicki EJ, & Hoeppel RE (1976)
Transformation of 1-naphthol by the culture filtrate of Rhizoctonia
praticola. Soil Biol Biochem, 8: 7-11.
Bollag JM, Sjoblad RD, & Liu S-Y (1978) Characterization of an
enzyme from Rhizoctonia praticola which polymerizes phenolic
compounds. Can J Microbiol, 25: 229-233.
Borzelleca J & Skalsky HL (1980) The excretion of pesticides in
saliva and its value in assessing exposure. J Environ Sci Health,
B13(6): 843-866.
Bowman BT & Sans WW (1985) Effect of temperature on the water
solubility of insecticides. J Environ Sci Health, B20(6): 625-631.
Bowman MC, Oller W, Kendall D, Dosnell A, & Oliver K (1982) Stressed
bioassay systems for rapid screening of pesticide residues. Part II.
Determination of foliar residues for safe re-entry of agricultural
workers into the field. Arch Environ Contam Toxicol, 11: 447-455.
Boyd EM & Boulanger MA (1968) Insecticide toxicology. Augmented
susceptibility to carbaryl toxicity in albino rats fed purified
casein diets. J Agric Food Chem, 16: 834-838.
Boyd EM & Krijnen CJ (1969) The influence of protein intake on acute
oral toxicity of carbaryl. J Clin Pharmacol, 9(5): 292-297.
Brahmaprakash GP & Sethunathan N (1984) Mineralization of carbaryl
and carbofuran in soil planted to rice. Rice Newsl, 5(3-4): 2.
Brahmaprakash GP & Sethunathan N (1985) Metabolism of carbaryl and
carbofuran in soil planted to rice. Agric Ecosyst Environ, 13(1):
33-42.
Branch RA (1987) The reply. Letter to the Editor. Am J Med, 83:
1169.
Branch RA & Jacqz E (1986a) Is carbaryl as safe as its reputation?
Does it have the potential for causing chronic neurotoxicity in
humans? Am J Med, 80: 659-664.
Branch RA & Jacqz E (1986b) Subacute neurotoxicity following
long-term exposure to carbaryl. Am J Med, 80: 741-745.
Brankovan O (1972) Meiotic effects in corn in embryonic and
generative stages induced by Sevin 50. Arhiv Poljopr Nauke, 90:
125-132.
Briggs GG (1981) Adsorption of pesticides by some Australian soils.
Aust J Soil Res, 19: 61-68.
Brzeskii VV (1972) The study of the mutagenic properties of an
insecticide from the carbamate group - Sevin. Sov Genet, 8: 798-800.
Buchanan DV, Millemann RE, & Stewart NE (1970) Effects of the
insecticide Sevin on various stages of the dungeness crab, Cancer
magister. J Fish Res Board Can, 27: 93-104.
Bukin AL (1965) [Toxicity of Sevin for mammalia and birds.] Gig
Primen Toxicol Pestic Klin Otrav, 1965: 431-435 (in Russian).
Bukin AL & Filatov GV (1965) [Sevin toxicity for mammalia and
birds.] Veterinaria, 42: 93-95 (in Russian).
Burdick GE, Dean HJ, & Harris EJ (1960) Effect of Sevin upon the
aquatic environment. NY Fish Game J, 7(1): 14-25.
Bursian SJ & Edens FW (1977) The prolonged exposure of Japanese
quail to carbaryl and its effect on growth and reproductive
parameters. Bull Environ Contamin Toxicol, 17(3): 360-368.
Bursian SJ & Edens FW (1978) The effect of acute carbaryl
administration on various neurochemical and blood chemical
parameters in the Japanese quail. Toxicol Appl Pharmacol, 46:
463-473.
Bushy Run (1983a) Acute toxicity and irritancy study. Export,
Pennsylvania, Bushy Run Research Center (Project Report No. 46-96)
(Unpublished proprietary information submitted to WHO by
Rhône-Poulenc Agro, Lyon).
Bushy Run (1983b) Sevin 99% technical: Acute toxicity and irritancy
study. Export, Pennsylvania, Bushy Run Research Center (Project
Report No. 46-71) (Unpublished proprietary information submitted to
WHO by Rhône-Poulenc Agro, Lyon).
Bushy Run (1983c) Sevin 80 sprayable. Acute toxicity and irritancy
study. Export, Pennsylvania, Bushy Run Research Center (Project
Report No. 46-97) (Unpublished proprietary information submitted to
WHO by Rhône-Poulenc Agro, Lyon).
Butler PA (1962) Effects on commercial fisheries. In: Effects of
pesticides on fish and wildlife: A review of investigations during
1960. Washington, DC, US Department of the Interior, Fish and
Wildlife Service, pp 11-25 (Circular No. 143).
Butler PA (1963) Commercial fisheries investigations. In:
Pesticide-wildlife studies: A review of Fish and Wildlife Service
investigations during 1961 and 1962. Washington, DC, US Department
of the Interior, Fish and Wildlife Service, pp 11-25 (Circular No.
167).
Cairns T, Siegmund GA, & Doose MG (1983) Potential identification
problems with carbaryl. Bull Environ Contam Toxicol, 30: 93-98.
Calabrese EJ & Geiger CP (1986) Low erythrocyte glusose-6-phosphate
dehydrogenase (G-6-PD) activity and susceptibility to carbaryl
induced methaemoglobin formation and glutathion depletion. Bull
Environ Contam Toxicol, 36(4): 506-509.
Cambon C, Declume Ch, & Derache R (1978) Inhibitions of
acetylcholinesterase from foetal and maternal tissues after oral
intake of carbaryl (1-naphthyl- N-methyl carbamate) by pregnant
rats. Biochem Pharmacol, 27: 2647-2648.
Cambon C, Fernandez J, Falzon M, & Mitjavila S (1981) Variations of
digestive absorption kinetics of carbaryl with the nature of the
vehicle. Toxicology, 22(1): 45-51.
Cameron EA & Mastro VC (1976) Forest insect and disease management /
Evaluation Report: Effectiveness of disparlure in combination with
Sevin 4 oil for Gipsy moth suppression in Pennsylvania 1974. Upper
Darby, Pennsylvania, US Department of Agriculture, Northeastern Area
State and Private Forestry Service.
Cameron EA, Loerch CR, & Mumma RO (1985) Incidental and indirect
exposure to three chemical insecticides used for control of the
Gypsy moth, Lymantria Dispar (L.). Z Angew Entomol, 99: 241-248.
Carey AE & Kutz FW (1985) Trends in ambient concentrations of
agrochemicals in humans and the environment in USA. Environ Monit
Assess, 5(2): 155-164.
Carlson AR (1972) Effects of long-term exposure to carbaryl (Sevin)
on survival, growth and reproduction of the fathead minnow
(Pimephales promelas). J Fish Res Board Can, 29(5): 583-587.
Caro HJ, Freeman HP, & Turner BC (1974) Persistence in soil and
losses in run off of soil incorporated carbaryl in a small
watershed. J Agric Food Chem, 22(5): 860-863.
Carpenter M (1990) Hydrolysis of 14C-carbaryl in aqueous solutions
buffered at pH 5, 7, and 9. Columbia, Missouri, Analytical
Bio-Chemistry Laboratories, Inc. (ABC final report No. 38380)
(Unpublished proprietary information submitted to WHO by
Rhône-Poulenc Agro, Lyon).
Carpenter CP, Weil CW, Palm PH, Woodside MW, Nair JH III, & Smith HF
(1961) Mammalian toxicity of 1-naphthyl- N-methyl carbamate (Sevin
insecticide). J Agric Food Chem, 9: 30-39.
Casale GP, Bavari S, & Connolly JJ (1989) Inhibition of human serum
complement activity by diisopropylfluorophosphate and selected
anticholinesterase insecticides. Fundam Appl Toxicol, 12(3):
460-468.
Casida JE (1970) Mixed-function oxidase involvement in the
biochemistry of insecticide synergists. J Agric Food Chem, 18: 753.
Casida JE & Augustinsson KB (1959) Reaction of plasma albumin with
1-naphthyl- N-methylcarbamate and certain other esters. Biochim
Biophys Acta, 35: 411-426.
Casper HH, Pekas JC, & Dinusson WE (1973) Gastric absorption of a
pesticide (1-naphthyl N-methylcarbamate) in the fasted rat. Pestic
Biochem Physiol, 2: 391-396.
Chaiyarach S, Ratananun V, & Harrel RC (1975) Acute toxicity of the
insecticides toxaphene and carbaryl and the herbicides propanil and
molinate to four species of aquatic organisms. Bull Environ Contam
Toxicol, 14(3): 281-284.
Chancey EL (1974) Sevin carbaryl insecticide: metabolism of carbaryl
in apple fruit (File No. 19496). Clayton, North Carolina, Union
Carbide Corporation (Unpublished proprietary information submitted
to WHO by Rhône-Poulenc Agro, Lyon).
Chancey EL & Andrawes NR (1971a) The absorption and metabolism of
1-naphthyl-C14 carbaryl in corn and rice plants. Clayton, North
Carolina, Union Carbide Corporation (Project Report No. 15712)
(Unpublished proprietary information submitted to WHO by
Rhône-Poulenc Agro, Lyon).
Chancey EL & Andrawes NR (1971b) Metabolism and translocation of
1-naphthyl-C14 carbaryl in tomato fruit and foliage and potato
foliage. Clayton, North Carolina, Union Carbide Corporation (Project
Report No. 15715) (Unpublished proprietary information submitted to
WHO by Rhône-Poulenc Agro, Lyon).
Chapman RA & Cole CM (1982) Observations on the influence of water
and soil pH on the persistence of insecticides. J Environ Sci
Health, B17(5): 487-504.
Chaudhry GR & Wheeler WB (1988) Biodegradation of carbamates. Water
Sci Technol, 20(11/12): 89-94.
Chernoff N & Kavlock RJ (1982) An in vivo teratology screen
utilizing pregnant mice. J Toxicol Environ Health, 10: 541-550.
Chernoff N, Setzer RW, Miller DB, Rosen MB, & Rogers JM (1990)
Effects of chemically induced maternal toxicity on prenatal
development in the rat. Teratology, 42: 651-658.
Chib JS (1985a) The adsorption/desorption of 1-naphthyl
methylcarbamate on five soils (File No. 33820). Research Triangle
Park, North Carolina, Union Carbide Agricultural Products Co., Inc.
(Unpublished proprietary information submitted to WHO by
Rhône-Poulenc Agro, Lyon).
Chib JS (1985b) Sevin: Aerobic aquatic metabolism of carbaryl (File
No. 34234). Research Triangle Park, North Carolina, Union Carbide
Agricultural Products Co., Inc. (Unpublished proprietary information
submitted to WHO by Rhône-Poulenc Agro, Lyon).
Chib JS (1985c) Sevin (1-naphthyl methylcarbamate): Anaerobic
aquatic metabolism of carbaryl (File No. 34086). Research Triangle
Park, North Carolina, Union Carbide Agricultural Products Co., Inc.
(Unpublished proprietary information submitted to WHO by
Rhône-Poulenc Agro, Lyon).
Chib JS (1986a) Sevin brand carbaryl insecticide. Bioconcentration
and fate of carbaryl in bluegill sunfish (Lepomis macrochirus).
Research Triangle Park, North Carolina, Union Carbide Corporation
(Report No. 801R10) (Unpublished proprietary information submitted
to WHO by Rhône-Poulenc Agro, Lyon).
Chib JS (1986b) Sevin brand carbaryl insecticide: Photodegradation
of carbaryl on soil (File No. 34598). Research Triangle Park, North
Carolina, Union Carbide Agricultural Products Co., Inc. (Unpublished
proprietary information submitted to WHO by Rhône-Poulenc Agro,
Lyon).
Chib JS & Andrawes NR (1985) Carbaryl (1-naphthyl methylcarbamate) -
Soil mobility study (File No. 33770). Research Triangle Park, North
Carolina, Union Carbide Agricultural Products Co., Inc. (Unpublished
proprietary information submitted to WHO by Rhône-Poulenc Agro,
Lyon).
Chib JS, Noeth SP, & Smith BK (1986) Sevin brand carbaryl
insecticide. Bioconcentration and fate of carbaryl in Bluegill
Sunfish (Lepomis Macrochirus) (File No 34540). Research Triangle
Park, North Carolina, Union Carbide Agricultural Products Co., Inc.,
Research and Development Department (Unpublished proprietary
information submitted to WHO by Rhône-Poulenc Agro, Lyon).
Chin YN & Sudderuddin KI (1979) Effect of methamidophos on the
growth rate and esterase activity of the common carp Cyprinus
carpio L. Environ Pollut, 18(3): 213-220.
Chin BH, Eldridge JM, & Sullivan LJ (1974) Metabolism of carbaryl by
selected tissues using an organ-maintenance technique. Clin Toxicol,
7: 37-56.
Chin BH, Sullivan LJ, Eldridge JM, & Tallant J (1979a) Metabolism of
carbaryl by kidney, liver, and lung from human postembryonic fetal
autopsy tissue. Clin Toxicol, 14(5): 489-498.
Chin BH, Eldridge JM, Anderson JH, & Sullivan LJ (1979b) Carbaryl
metabolism in the rat. A comparison of in vivo, in vitro (tissue
explant), and liver perfusion techniques. J Agric Food Chem, 27(4):
716-720.
Christenson I (1964) Alkaline hydrolysis of some carbamic esters.
Acta Chem Scand, 18: 904-922.
Cifone MA (1989) Mutagenicity test on carbaryl technical in the in
vitro rat primary hepatocyte unscheduled DNA synthesis assay.
Final report (HLA Study No. 10862-0-435). Kensington, Maryland,
Hazelton Laboratories America, Inc. (Unpublished proprietary
information submitted to WHO by Rhône-Poulenc Agro, Lyon).
Clayson DB (1987) The need for biological risk assessment in
reaching decisions about carcinogens. Mutat Res, 185(3): 243-269.
Collins TFX, Hansen WH, & Keeler HV (1971) The effect of carbaryl
(Sevin) on reproduction of the rat and the gerbil. Toxicol Appl
Pharmacol, 19: 202-216.
Comer SW, Staiff DC, Armstrong JF, & Wolf HR (1975) Exposure of
workers to carbaryl. Bull Environ Contam Toxicol, 13(4): 385-391.
Coulston F, Rosenblum I, & Dougherty WJ (1974) Teratogenic
evaluation of carbaryl in the Rhesus monkey (Macacca mulatta).
Holloman Air Force Base, New Mexico, International Center of
Environmental Safety, Albany Medical College, 54 pp (Unpublished
proprietary information submitted to WHO by Rhône-Poulenc Agro,
Lyon).
Courtemanch DL & Gibbs KE (1978) The effects of Sevin-4-oil on
lenthic communities. In: Environmental monitoring of cooperative
spruce budworm control projects, Maine 1976 and 1977. Augusta,
Maine, Maine Department of Conservation, Forest Service, pp 141-150.
Courtemanch DL & Gibbs KE (1980) Short- and long-term effects of
forest spraying of carbaryl (Sevin-4-oil) on stream invertebrates.
Can Entomol, 112: 271-276.
Coutant CC (1964) Insecticide Sevin: Effect of aerial spraying on
drift of stream insects. Science, 146: 420-421.
Cranmer MF (1986) Carbaryl. A toxicological review and risk
analysis. Neurotoxicology, 7(1): 247-332.
Cress CR & Strother A (1974) Effects on drug metabolism of carbaryl
and 1-naphthol in the mouse. Life Sci, 14: 861-872.
Crofton KM, Howard JL, Moser VC, Gill MW, Reiter LW, Tilson HA, &
MacPhail RC (1991) Interlaboratory comparison of motor activity
experiments: Implications for neurotoxicological assessments.
Neurotoxicol Teratol, 13: 599-609.
Crosby DG, Leitis E, & Winterlin WL (1965) Photodecomposition of
carbamate insecticides. J Agric Food Chem, 13(3): 204-205.
Currier WW, MacCollom GB, & Baumann GI (1982) Drift residues of
air-applied carbaryl in an orchard environment. J Econ Entomol,
75(6): 1062-1068.
Czaplicki E (1979) Persistence in the soil of some more important
insecticides applied in Poland. I. Dynamics of insecticide
disappearance in the soil. Pr Nauk Inst Ochr Rosl, 21(2): 7-40.
Czaplicki EJ & Bollag JM (1975) Microbial degradation of 1-naphthol.
Biul Inst Ochr Rosl, 59: 241-248.
Das YT (1990a) Photodegradation of (1-naphthyl-14C) carbaryl in
aqueous solution buffered at pH 5 under artificial sunlight.
Piscataway, New Jersey, Innovative Scientific Services, Inc. (Report
No. 90060) (Unpublished proprietary information submitted to WHO by
Rhône-Poulenc Agro, Lyon).
Das YT (1990b) Photodegradation of (1-naphthyl-14C) carbaryl on
soil under artificial sunlight. Piscataway, New Jersey, Innovative
Scientific Services, Inc. (Report No. 90061) (Unpublished
proprietary information submitted to WHO by Rhône-Poulenc Agro,
Lyon).
Davies JI & Evans WC (1964) Oxidative metabolism of naphthalene by
soil Pseudomonads. The ring-fission mechanism. Biochem J, 91:
251-261.
Davis HC (1961) Effects of some pesticides on eggs and larvae of
oysters (Crassostrea virginica) and clams (Venus mercenaria).
Commer Fish Rev, 23(12): 8-23.
Davis CW (1986a) Sevin brand carbaryl insecticide: Dissipation of
soil residues under field use conditions (File No. 34620). Research
Triangle Park, North Carolina, Union Carbide Agricultural Products
Co., Inc. (Unpublished proprietary information submitted to WHO by
Rhône-Poulenc Agro, Lyon).
Davis CW (1986b) Sevin brand carbaryl insecticide: Magnitude of
carbaryl residues on rangeland and pasture grasses (File No. 34821).
Research Triangle Park, North Carolina, Union Carbide Agricultural
Products Co., Inc. (Unpublished proprietary information submitted to
WHO by Rhône-Poulenc Agro, Lyon).
Davis CW (1987) Sevin brand carbaryl insecticide: Comparison of the
magnitude of carbaryl residues resulting from applications of XLR
plus and 50 W formulations to tree fruit, leafy vegetables, and
cereals (File No. 35357). Research Triangle Park, North Carolina,
Union Carbide Agricultural Products Co., Inc. (Unpublished
proprietary information submitted to WHO by Rhône-Poulenc Agro,
Lyon).
Davis CW & Thomas SJ (1987) Carbaryl insecticide. Section D-Residues
barley (File No. 40092). Research Triangle Park, North Carolina,
Union Carbide Agricultural Products Co., Inc. (Unpublished
proprietary information submitted to WHO by Rhône-Poulenc Agro,
Lyon).
Declume C & Benard P (1977a) Foetal accumulation of [14C] carbaryl
in rats and mice autoradiographic study. Toxicology, 8: 95-105.
Declume C & Benard P (1977b) Etude autoradiographique de la
distribution d'un agent anticholinestérasique, le 1-naphthyl-
N-methyl [14C] carbamate chez la ratte gestante. Toxicol Appl
Pharmacol, 39: 451-460.
Declume C & Benard P (1978) Etude de la pharmacocinétique du
14C-carbaryl chez la souris gestante. Toxicol Eur Res, 1: 174-180.
Declume C & Derache M (1976) Biodisponibilité du 14C-carbaryl
après l'administration oral à la ratte gestante. C R Acad Sci Paris,
D283: 1799-1801.
Declume C & Derache M (1977) Passage placentaire d'un carbamate
anticholinestérasique à activité insecticide: Le carbaryl.
Chemosphere, 6: 141-146.
Declume C, Cambon C, & Derache R (1979) The effects on new-born rats
of repeated carbaryl administration during gestation. Toxicol Lett,
3: 191-196.
Degraeve N, Moutschen-Dahmen M, Houbrechts N, & Colizzi A (1976) A
propos des risques d'un insecticide: le carbaryl utilisé seul et en
combinaison avec les nitrites. Bull Soc R Sci Liège, 45(1-2): 46-57.
DeGiovanni-Donnelly R, Kolbye SM, & Greeves PD (1968) The effect of
IPC, CIPC, Sevin and Zectran on Bacillus subtilis. Experientia
(Basel), 24: 80-81.
Desi I, Gonczi L, Simon G, Farkas I, & Kneffel Z (1974)
Neurotoxicological studies of two carbamate pesticides in subacute
animal experiments. Toxicol Appl Pharmacol, 27: 456-476.
Deuel LE, Brown KW, Price JD, & Turner FT (1985) Dissipation of
carbaryl and the 1-naphthol metabolite in the flooded rice fields. J
Environ Qual, 14(3): 349-354.
Deutsch-Wenzel P, Brune H, Grimmer G, & Misfeld J (1985) Local
application to mouse skin as a carcinogen specific test system for
non-volatile nitroso compounds. Can Lett, 29: 85-92.
Dickoff DJ, Gerber O, & Turovsky Z (1987) Delayed neurotoxicity
after ingestion of carbamate pesticide. Neurology, 37(7): 1229-1231.
Dieringer CS & Thomas JA (1974) Effects of carbaryl on the
metabolism of androgens in the prostate and liver of the mouse.
Environ Res, 7: 381-386.
Dikshith TSS (1991) Mutagenicity and teratogenicity of pesticides.
In: Dikshith TSS ed. Toxicology of pesticides in animals. Boston,
Boca Raton, Ann Arbor, CRC Press, pp 189-198.
Dikshith TSS, Gupta PK, Gaur JS, Datta KK, & Mathur AK (1976)
Ninety-day toxicity of carbaryl in male rats. Environ Res, 12:
161-170.
Dikshith TSS, Kumar SN, Raizada RB, Srivastava MK, & Ray PK (1990)
Residues of 1-naphthol in soil and water samples in and around
Bhopal, India. Bull Environ Contam Toxicol, 44: 87-91.
Dinerman AA, Lavrent'eva NA, & Il'inskaia NA (1970) [The embryotoxic
action of some pesticides.] Gig i Sanit, 35: 39-42 (in Russian).
Dinoeva SK (1974) [Dynamics of the changes in the immunological
structures of lymphatic follicles in spleen during pesticide
poisoning.] Gig i Sanit, 39(3): 85-87 (in Russian).
Dinoeva S (1982) Humoral immune response in 1st, 2nd and 3rd
generations guinea-pigs in the period of reactivation after
intoxication. In: Immunology of reproduction. Proceedings of the
Fifth International Symposium, Varna, Bulgaria, 26-29 May 1982.
Sofia, Bulgarian Academy of Sciences Press, pp 393-395.
Dionne E, Kendall TB, & Suprenant D (1985) Acute toxicity of
carbaryl technical to embryos-larvae of Eastern oysters
(Crassostrea virginica). Wareham, Massachusetts, Springborn
Bionomics, Inc. (Report No. BW-85-7-1817) (Unpublished proprietary
information submitted to WHO by Rhône-Poulenc Agro, Lyon).
Dittert LW & Higuchi T (1963) Rates of hydrolysis of carbamate and
carbonate esters in alkaline solution. J Pharm Sci, 52: 852-857.
Dorough HW (1967) Carbaryl-C14 metabolism in a lactating cow. J
Agric Food Chem, 15(2): 261-266.
Dorough HW (1969) Carbaryl cow study No. 2. Lyon, Rhône-Poulenc Agro
(Unpublished proprietary information).
Dorough HW (1970a) Continuous feeding of carbaryl naphthyl-C14 to
lactating cows. Progress report. Lyon, Rhône-Poulenc Agro (Document
No. 25, Section C, Part III) (Unpublished proprietary information).
Dorough HW (1970b) Metabolism of insecticidal methylcarbamates in
animals. J Agric Food Chem, 18(6): 1015-1022.
Dorough HW (1971) Carbaryl residues in milk and meat of dairy
animals. Presented at the International Symposium on Pesticide
Terminal Residues, Tel-Aviv, Israel, 17-19 February 1971 (Document
No. 27, Section C, Part III).
Dorough HW (1973) Metabolism of carbamate insecticides. Washington,
DC, US Environmental Protection Agency, Office of Research and
Development, 246 pp (EPA-650/1-74-002).
Dorough HW (1982) Inhalation toxicology: Starting with the
environment. Environ Toxicol Chem, 1(3): 213-220.
Dorough HW & Casida JH ( 1964) Nature of certain carbamate
metabolites of the insecticide Sevin. J Agric Food Chem, 12(4):
294-304.
Dorough HW, Leeling NL, & Casida JE (1963) Nonhydrolytic pathway in
metabolism of N-methylcarbamate insecticides. Science, 140:
170-171.
Dougherty WJ, Goldberg L, & Coulston F (1971) The effect of carbaryl
on reproduction in the monkey (Macacca mulatta). Toxicol Appl
Pharmacol, 19: 365.
Duck BJ & Woolias M (1985) Reverse-phase high performance liquid
chromatographic determination of carbaryl in postmortem specimens. J
Anal Toxicol, 9: 177-179.
Duggan RE & Corneliussen PE (1972) Dietary intake of pesticide
chemicals in the United States (III). June 1968-April 1970. Pestic
Monit J, 5(4): 331-341.
Duggan RE, Lipscomb GQ, Cox EL, Heatwole RE, & Kling RC (1971)
Residues in food and feed. Pesticide residue levels in foods in the
United States from July 1, 1963, to June 30, 1969. Pestic Monit J,
5(2): 73-124.
Durham WF & Wolfe HR (1962) Measurements of the exposure of workers
to pesticides. Bull World Health Organ, 26: 75-91.
Dyadicheva TV (1971) [Experimental data on thyroid and adrenal
cortex function during prolonged effect of carbamine pesticides.]
Vrach Delo, 2: 120-123 (in Russian).
Dzwonkowska A & Hubner H (1986) Induction of chromosomal aberrations
in the Syrian hamster by insecticides tested in vivo. Arch
Toxicol, 58(3): 152-156.
Earl FL, Miller E, & Van Loon EJ (1973) Reproductive teratogenic and
neonatal effects of some pesticides and related compounds in beagle
dogs and miniature swine. In: Deichman W ed. Pesticides and the
environment: A continuing controversy. Proceedings of the 8th
Inter-American Conference on Toxicology and Occupational Medicine,
Miami, Florida, pp 253-266.
Edmiston CE Jr, Goheen M, Malaney GW, & Mills WL (1985) Evaluation
of carbamate toxicity: Acute toxicity in a culture of Paramecium
multimicronucleatum upon exposure to aldicarb, carbaryl, and
mexacarbate as measured by Warburg respirometry and acute plate
assay. Environ Res, 36(2): 338-350.
Egert G & Greim H (1976) Formation of mutagenic N-nitroso
compounds from the pesticides prometryne, dodine and carbaryl in the
presence of nitrite at ph 1. Mutat Res, 37: 179-186.
Eheart JF, Turner EC, & Dickinson J (1962) Residues of Sevin in
whole milk from sprayed and dusted cows. J Entomol, 55(4): 504-505.
Eichelberger J & Lichtenberg J (1971) Persistence of pesticides in
river water. Environ Sci Technol, 5: 541-544.
Eisenbrand G, Ungerer O, & Preussmann R (1975) The reaction of
nitrite with pesticides. II. Formation, chemical properties and
carcinogenic activity of the N-nitroso derivatives of N-methyl-
1-naphthyl carbamate (carbaryl). Food Cosmet Toxicol, 13: 365-367.
Eisenbrand G, Schmähl D, & Preussmann R (1976) Carcinogenicity in
rats of high oral doses of N-nitrosocarbaryl, a nitrosated
pesticide. Can Lett, 1: 281-284.
Elespuru RK & Lijinsky W (1973) The formation of carcinogenic
nitroso compounds from nitrite and some types of agricultural
chemicals. Food Cosmet Toxicol, 11: 807-817.
Elespuru R, Lijinsky W, & Setlow JK (1974) Nitrosocarbaryl as a
potent mutagene of environmental significance. Nature (Lond), 247:
386-387.
Elkins ER (1989) Effect of commercial processing on pesticide
residues in selected fruits and vegetables. J Assoc Off Anal Chem,
72(3): 533-535.
Elkins ER, Lamb FC, Farrow RP, Cook RW, Kawai M, & Kimball JR (1968)
Removal of DDT, malathion and carbaryl from green beans from
commercial and home preparative procedures. J Agric Food Chem,
16(6): 962-966.
Elliot-Feeley E & Armstrong JR (1982) Effects of fenitrothion and
carbaryl on Xenopus laevis development. Toxicology, 22(4):
319-335.
Epstein SS, Arnold E, Andrea J, Bass W, & Bishop Y (1972) Detection
of chemical mutagens by the dominant lethal assay in the mouse.
Toxicol Appl Pharmacol, 23: 288-325.
Estesen BJ, Buck NA, & Ware GW (1982) Dislodgeable insecticide
residues on cotton foliage: carbaryl, cypermethrin, and
methamidophos. Bull Environ Contam Toxicol, 28(4): 490-493.
Eto M, Seifert J, Engel J, & Casida J (1980) Organophosphorous and
methylcarbamate teratogens: Structural requirements for reducing
embryonic abnormalities in chickens and kynurenine formamidase
inhibition in mouse liver. Toxicol Appl Pharmacol, 54: 20-30.
Eto M, Kuwano E, Yoshikawa M, Suiko M, Kohno M, Kamihata M, &
Nakatsu S (1982) Mutagenicity and anticholinesterase activity of
possible metabolites of aryl N-methylcarbamates. J Fac Agric
Kyushu Univ, 26(4): 213-219.
Fait DW (1984) Sevin XLR acute inhalation toxicity test. Export,
Pennsylvania, Bushy Run Research Center (Report No. 47-84)
(Unpublished proprietary information submitted to WHO by
Rhône-Poulenc Agro, Lyon).
Falzon M, Fernandez Y, Cambon C, Gros C, & Mitjavila S (1983)
Influence of experimental hepatic impairment on the toxicokinetics
and the anticholinesterase activity of carbaryl in the rat. J Appl
Toxicol, 3(2): 87-89.
FAO (1982) Second Government Consultation on International
Harmonization of Pesticides Registration Requirements, Rome, 11-15
October 1982. Rome, Food and Agriculture Organization of the United
Nations.
FAO/WHO (1964) Carbaryl. In: Evaluation of the toxicity of
pesticides residues in food. Report of a Joint Meeting of the FAO
Committee on Pesticides in Agriculture and the WHO Expert Committee
on Pesticide Residues, Geneva, 30 September-7 October 1963. Geneva,
World Health Organization, pp 132-135 (FAO Meeting Report No.
PL:1963/13; WHO/Food Add./23).
FAO/WHO (1965) Carbaryl. In: Evaluation of the toxicity of pesticide
residues in food. Geneva, World Health Organization, pp 31-35 (FAO
Meeting Report No. PL:1965/10/1; WHO Food Add./27.65).
FAO/WHO (1967) Carbaryl. In: 1966 Evaluation of some pesticide
residues in food. Geneva, World Health Organization, pp 31-38 (FAO
PL/CP/15; WHO Food Add./67.32)
FAO/WHO (1968) Carbaryl. In: 1967 Evaluation of some pesticide
residues in food. Geneva, World Health Organization, pp 15-25 (FAO
Report No. PL: 1967/M/11/1; WHO Food Add./68.30).
FAO/WHO (1969) Carbaryl. In: 1968 Evaluation of some pesticide
residues in food. Geneva, World Health Organization, pp 35-38 (FAO
PL:1968/M/9/1; WHO Food Add./69.35).
FAO/WHO (1970) Carbaryl. In: 1969 Evaluations of some pesticide
residues in food. Geneva, World Health Organization, pp 45-57 (FAO
PL:1969/M/17/1; WHO Food Add./70.38).
FAO/WHO (1971) Carbaryl. In: 1970 Evaluations of some pesticide
residues in food. Geneva, World Health Organization, pp 3-6 (AGP
1979/M/12/1; WHO Food Add./71.42).
FAO/WHO (1972) 1971 Evaluations of some pesticide residues in food.
Geneva, World Health Organization (WHO Pesticide Residue Series No.
1).
FAO/WHO (1974) Carbaryl. In: 1973 Evaluations of some pesticide
residues in food. Geneva, World Health Organization, pp 141-176 (WHO
Pesticide Residue Series No. 3).
FAO/WHO (1976) Carbaryl. In: 1975 Evaluations of some pesticide
residues in food. Geneva, World Health Organization, pp 83-84 (WHO
Pesticide Residue Series No. 5).
FAO/WHO (1977) Carbaryl. In: 1976 Evaluations of some pesticide
residues in food. Rome, Food and Agriculture Organization of the
United Nations, pp 95-123 (AGP 1977/M/14).
FAO/WHO (1978) Carbaryl. In: Pesticide residues in food. Evaluations
1977. Rome, Food and Agriculture Organization of the United Nations,
pp 47-50 (FAO Plant Production and Protection Paper 10 Sup).
FAO/WHO (1980) Carbaryl. In: Pesticide residues in food. Evaluations
1979. Rome, Food and Agriculture Organization of the United Nations,
pp 101-102 (FAO Plant Production and Protection Paper 20 Sup).
FAO/WHO (1985) Carbaryl. In: Pesticide residues in food - 1984. The
monographs. Rome, Food and Agriculture Organization of the United
Nations, pp 79-83 (FAO Plant Production and Protection Paper 67).
FAO/WHO (1986a) Guide to Codex recommendations concerning pesticide
residues. Part 8. Recommendations for methods of analysis of
pesticide residues, 3rd ed. Rome, Food and Agriculture Organization
of the United Nations, Codex Alimentarius Commission.
FAO/WHO (1986b) Codex maximum limits for pesticide residues, 2nd ed.
Rome, Food and Agriculture Organization of the United Nations, Codex
Alimentarius Commission (CAC/Vol. XIII).
Farage-Elawar M (1989) Enzyme and behavioural changes in young
chicks as a result of carbaryl treatment. J Toxicol Environ Health,
26(1): 119-131.
Farage-Elawar M (1990) Effects of in ovo injection of carbamates
on chick embryo hatchability, esterase enzyme activity and
locomotion of chicks. J Appl Toxicol, 10(3): 197-201.
Farago A (1969) [Fatal suicidal case of Sevin (1-naphthol- N-methyl
carbamate) poisoning.] Arch Toxicol, 24: 309-315 (in German).
Farrow RP, Lamb FC, Cook RW, Kimball JR, & Elkins ER (1968) Removal
of DDT, malathion and carbaryl from tomatoes by commercial and home
preparative methods. J Agric Food Chem, 16(1): 65-71.
Farrow RP, Lamb FC, Elkins ER, Cook RW, Kawai M, & Cortes A (1969)
Effect of commercial and home preparative procedures on parathion
and carbaryl residues in broccoli. J Agric Food Chem, 17(1): 75-79.
Federle PF & Collins WJ (1976) Insecticide toxicity to three insects
from Ohio ponds. Ohio J Sci, 76(1): 19-24.
Feldmann RJ & Maibach HT (1974) Percutaneous penetration of some
pesticides and herbicides in man. Toxicol Appl Pharmacol, 28:
126-132.
Fernandez Y, Falzon M, Cambon C, Gros C, & Mitjavila S (1982)
Carbaryl tricompartmental toxicokinetics and anticholinesterase
activity. Toxicol Lett, 13(3-4): 253-258.
Ferreira GAL & Seiber JN (1981) Volatilization and exudation losses
of 3- N methylcarbamate insecticides applied systemically to rice.
J Agric Food Chem, 29(1): 93-99.
Field WE (1980a) Oral LD50 in rats of carbaryl, technical grade,
made in West Virginia. Clarks Summit, Pennsylvania, Chemicals, Drugs
and Cosmetics Research (Report No. CDC-UC-046-79) (Unpublished
proprietary information submitted to WHO by Rhône-Poulenc Agro,
Lyon).
Field WE (1980b) Oral LD50 in rats of carbaryl, technical grade,
made in India. Clarks Summit, Pennsylvania, Chemicals, Drugs and
Cosmetics Research (Report No. CDC-UC-047-79) (Unpublished
proprietary information submitted to WHO by Rhône-Poulenc Agro,
Lyon).
Filatov DV & Brytskov VE (1972) [Enzymic method of residue
determination of chlorophos and Sevin by means of indicator paper.]
Probl Vet Sanit, XLI: 233-241 (in Russian).
Fisher SW & Lohner TW (1986) Studies on the environmental fate of
carbaryl as a function of pH. Arch Environ Contam Toxicol, 15(6):
661-667.
Fisher HL, Most B, & Hall LL (1985) Dermal absorption of pesticides
calculated by deconvolution. J Appl Toxicol, 5(3): 163-177.
Fletcher DW & Leonard MI (1986a) Toxicity and reproduction study
with carbaryl technical in Bobwhite quail. Neillsville, Wisconsin,
Bio-Life Associate Ltd (Report No. 85-DR-15) (Unpublished
proprietary information submitted to WHO by Rhône-Poulenc Agro,
Lyon).
Fletcher DW & Leonard MI (1986b) 28-day dietary toxicity and
reproduction pilot study with carbaryl technical in Mallard ducks.
Neillsville, Wisconsin, Bio-Life Associate Ltd (Report No. 85 DR
P14) (Unpublished proprietary information submitted to WHO by
Rhône-Poulenc Agro, Lyon).
Flores-Ruegg E, De Andrea MM, Helene CG, & Hirata R (1980)
Persistence of pesticide residues in Brazilian soil samples related
to organic matter and microbiological activity. In: Meeting of the
Advisory Group on Agrochemical Residue Biota Interaction: Proceeding
report. Sao Paulo, Brazil, Biological Institute, pp 181-187.
Frank R, Braun HE, & Ripley BD (1989) Monitoring Ontario grown
apples for pest control chemicals used in their production, 1978-86.
Food Addit Contam, 6(2): 227-234.
Frank R, Braun HE, Ripley BD, & Pitblado R (1991) Residues of nine
insecticides and two fungicides in raw and processed tomatoes. J
Food Prot, 54(1): 41-46.
Fukuto TR (1973) Metabolism of carbamate insecticides. In: Di Carlo
FJ ed. Drug metabolism reviews. New York, Basel, Marcel Dekker,
Inc., vol 1, pp 117-151.
Gaillard D, Chamoiseau G, & Derache R (1977) Interaction du carbaryl
et du nitrite de sodium sur les activités enzymatiques de quelques
mono-oxygénases microsomiques du foie de rat. J Pharmacol, 8: 437.
Gaines TB (1960) The acute toxicity of pesticides to rat. Toxicol
Appl Pharmacol, 2: 88-99.
Gaines TB (1969) Acute toxicity of pesticides to rat. Toxicol Appl
Pharmacol, 14: 514-534.
Gangwar SK, Kavadia VS, Gupta HCL, & Srivastava BP (1978a)
Persistence of carbaryl in sandy loam soil and its uptake in bajra,
Pennistetum typhoides P. Ann Arid Zone, 17(4): 357-362.
Gangwar SK, Kavadia VS, Srivastava BP, & Gupta HCL (1978b)
Persistence of carbaryl residues in sandy loam soil and its uptake
by root crops. Indian J Plant Prot, 8(1): 11-18.
Gapparov Sh (1974) [Haemocoagulation indices in dogs chronically
intoxicated with low doses of Sevin.] [Health Health Care Organ
Uzbekistan], II: 146-149 (in Russian).
Gartrell MJ, Craun JC, Podrebarac DS, & Gunderson EL (1986)
Pesticides, selected elements, and other chemicals in infant and
toddler total diet samples, October 1980-March 1982. J Assoc Off
Anal Chem, 69(1): 123-145.
Georgiev IN (1967) [The effect of toxic and maximal permissible
amounts of Sevin on intestinal secretion.] Gig i Sanit, 32(8): 42-46
(in Russian with English summary).
Ghadiri M & Greenwood DA (1966) Toxicity and biologic effects of
malathion, phosdrin and Sevin in the chick embryo. Toxicol Appl
Pharmacol, 8: 342.
Ghosh P, Bhattacharya S, & Bhattacharya S (1990) Impairment of the
regulation of gonadal function in Channa punctatus by metacid 50
and carbaryl under laboratory and field conditions. Biomed Environ
Sci, 3(1): 106-112.
Gibbs E, Mingo TM, & Courtemanch DL (1981) The effect on pond
macroinvertebrates from forest spraying of carbaryl and its
persistence in water and sediment. Augusta, Maine, Maine Department
of Conservation, Forest Service.
Gibbs KE, Mingo TM, & Courtemanch DL (1984) Persistence of carbaryl
(Sevin-4-oil) in woodland ponds and its effects on ponds macro
invertebrates following forest spraying. Can Entomol, 116: 203-214.
Gill SS & Yeoh CL (1980) Degradation of carbaryl in three components
of the paddy-field ecosystem of Malaysia. In: Meeting of the
Advisory Group on Agrochemical Residue Biota Interaction: Proceeding
report. Sao Paulo, Brazil, Biological Institute, pp 229-243.
Gill TS, Pant JC, & Pant J (1988) Gill, liver, and kidney lesions
associated with experimental exposures to carbaryl and dimethoate in
the fish (Puntius conchonius Ham.). Bull Environ Contam Toxicol,
41(1): 71-78.
Gladenko IN & Malinin OA (1970) [Toxicity of Sevin for livestock.]
Gig Primen Toxicol Pestic Klin Otrav, 1970: 188-193 (in Russian).
Golbs S, Kühnert M, & Leue F (1975) [Prenatal toxicological study of
Sevin (carbaryl) for Wistar rats.] Arch Exp Veterinaermed, 29:
607-614 (in German with English summary).
Golbs S, Kühnert M, & Fuchs V (1984) [Influence on the acute
toxicity of selected pesticides by huminic acids.] Z Gesamte Hyg,
30(12): 720-723 (in German).
Gold RE, Leavitt JR, Holcslaw T, & Tupy D (1982) Exposure of urban
applicators to carbaryl. Arch Environ Contam Toxicol, 11: 63-67.
Goldberg HI, Johnson HE, & Knaak JB (1965) Inhibition of discrete
avoidance behaviour by three anticholinesterase agents.
Psychopharmacologia, 7(1): 72-76.
Guirguis GN & Brindley WA (1975) Carbaryl penetration into and
metabolism by alfalfa leafcutting bees, Megachile pacifica. Agric
Food Chem, 23(2): 274-279.
Guirguis GN & Brindley WA (1976) Effect of chlorcyclizin,
aminopyrine or phenobarbital on carbaryl metabolism in alfalfa
leafcutting bees. Environ Entomol, 5(3): 590-594.
Gunderson EL (1988) FDA total diet study, April 1982-April 1984.
Dietary intake of pesticides, selected elements, and other
chemicals. J Assoc Off Anal Chem, 71(6): 1200-1209.
Gupta SK & Sundararaman V (1991) Correlation between burrowing
capability and AChE activity in the earthworm, Pheretima posthuma,
on exposure to carbaryl. Bull Environ Contam Toxicol, 46: 859-865.
Guseinov MK (1970) [On the question of circulation of Sevin in
environment.] Gig Primen Toxicol Pestic Klin Otrav, 1970: 174-176
(in Russian).
Guthrie FE, Monro RJ, & Abernathy CO (1971) Response of laboratory
mouse to selection for resistance to insecticides. Toxicol Appl
Pharmacol, 18: 92-101.
Gyrisco GG, Lisk DJ, Fertig SN, Huddleston EW, Fox FH, Holland RF, &
Teimberger GW (1960) The effects of feeding high levels of Sevin on
residue, flavour, and odour of the milk of dairy cattle. Agric Food
Chem, 8(5): 409-410.
Haines TA (1981) Effect of an aerial application of carbaryl on
brook trout (Salvelinus fontinalis). Bull Environ Contam Toxicol,
27(4): 534-542.
Hamada NN (1987) One year oral toxicity study in Beagle dogs with
carbaryl technical. Vienna, Virginia, Hazleton Laboratories America,
Inc. (Report No. 400-715) (Unpublished proprietary information
submitted to WHO by Rhône-Poulenc Agro, Lyon).
Hamada NN (1990) Acute oral toxicity study in rats. Carbaryl
technical. Vienna, Virginia, Hazleton Laboratories America, Inc.
(Unpublished proprietary information submitted to WHO by
Rhône-Poulenc Agro, Lyon).
Hamada NN (1991a) Subchronic toxicity study in dogs with carbaryl
technical. Vienna, Virginia, Hazleton Laboratories America, Inc.
(Report No. N656-152) (Unpublished proprietary information submitted
to WHO by Rhône-Poulenc Agro, Lyon).
Hamada NN (1991b) Oncogenicity with carbaryl technical in CD-1(R)
mice. Vienna, Virginia, Hazleton Laboratories America, Inc. (Report
No. N656-138) (Unpublished proprietary information submitted to WHO
by Rhône-Poulenc Agro, Lyon).
Hamada NN (1991c) Combined chronic toxicity and oncogenicity study
with carbaryl technical in Sprague-Dawley rats - 52 week interim
report with 4-week recovery. Vienna, Virginia, Hazleton Laboratories
America, Inc. (Study No. 656-139) (Unpublished proprietary
information submitted to WHO by Rhône-Poulenc Agro, Lyon).
Hanazato T & Yasuno M (1987) Effects of a carbamate insecticide,
carbaryl, on the summer phyto- and zooplankton communities in ponds.
Environ Pollut, 48: 145-159.
Hanazato T & Yasuno M (1989) Influence of over-wintering Daphnia
on spring zooplankton communities: An experimental study. Ecol Res,
4: 323-338.
Hanazato T & Yasuno M (1990a) Influence of Chaoborus density on
the effects of an insecticide on zooplankton communities in ponds.
Hydrobiologia, 194: 183-197.
Hanazato T & Yasuno M (1990b) Influence of time in application of an
insecticide on recovery patterns of a zooplankton community in
experimental ponds. Arch Environ Contam Toxicol, 19: 77-83.
Hansen JL (1978) Carbaryl: Human ingestion study statistical
analysis (File No. 24971). Research Triangle Park, North Carolina,
Union Carbide Corporation (Unpublished proprietary information
submitted to WHO by Rhône-Poulenc Agro, Lyon).
Hansen CR & Kawatski JA (1976) Application of 24-hour post-exposure
observation to acute toxicity studies with invertebrates. J Fish Res
Board Can, 33: 1198-1201.
Harris LW, Talbot BG, Lennox WJ, & Anderson DR (1989) The
relationship between oxime induced reactivation of carbamylated
acetylcholinesterase and antidotal efficacy against carbamate
intoxication. Toxicol Appl Pharmacol, 98(1): 128-133.
Harry JB (1977) A review of human exposure to Sevin carbaryl
insecticide with particular reference to the USA. Research Triangle
Park, North Carolina, Union Carbide Agricultural Products Co., Inc.
(Unpublished proprietary information submitted to WHO by
Rhône-Poulenc Agro, Lyon).
Hart ER (1972) Teratology study. Sevin, vitamin A, aspirin and
malathion. South Charleston, West Virginia, Union Carbide
Corporation, 8 pp (Prepared by Bionetics Research Laboratories)
(Unpublished proprietary information submitted to WHO by
Rhône-Poulenc Agro, Lyon).
Hassan A (1971) Pharmacological effects of carbaryl. The effect of
carbaryl on the synthesis and degradation of catecholamines in the
rat. Biochem Pharmacol, 20: 2299-2308.
Hassan A & Cueto C Jr (1970) Biochemical effects in the rabbit of
repeated administration of a mixture of DDT, carbaryl and parathion.
Z Naturforsch, B25: 521-525.
Hassan A & Santolucito JA (1971) Pharmacological effects of
carbaryl. II. Modification of serotonin metabolism in the rat brain.
Experientia (Basel), 27: 287-288.
Hassan A, Zayed SMAD, & Abdel-Hamid FM (1966) Metabolism of
carbamate drugs. I. Metabolism of 1-naphthyl- N-methylcarbamate
(Sevin) in the rat. Biochem Pharmacol, 15: 2045-2055.
Hassan AA, El Sewedy SM, Minatogawa Y, & Kido R (1990) Effects of
some insecticides on several enzymes of tryptophan metabolism in
rats. J Environ Sci Health, B25(3): 333-346.
Haven D, Castagna M, Chanley P, Wass M, & Whitcomb J (1966) Effects
of the treatment of an oyster bed with polystream and Sevin.
Chesapeake Sci, 7(4): 179-188.
Haydock CI (1964) An experimental study to control oyster drills in
Tomales Bay, California. Calif Fish Game, 50: 11-28.
Hayes WJ (1963) Clinical handbook on economic poisons. Emergency
information for treating poisoning. Atlanta, Georgia, US Department
of Health, Education and Welfare, Communicable Disease Center, pp
44-46.
Hayes WJ Jr & Laws ER Jr ed. (1991) Carbaryl. In: Handbook of
pesticide toxicology. Volume 3 - Classes of pesticides. New York,
San Diego, Academic Press, Inc., pp 1144-1189.
Hazleton Laboratories America, Inc. (1982) Acute oral toxicity study
in rats - Sevin RF. Vienna, Virginia, Hazleton Laboratories America,
Inc. (Unpublished proprietary information submitted to WHO by
Rhône-Poulenc Agro, Lyon).
Heise GA & Hudson JD (1985a) Effects of pesticides and drugs on
working memory in rat: Continuous delayed response. Pharmacol
Biochem Behav, 23: 591-598.
Heise GA & Hudson JD (1985b) Effects of pesticides and drugs on
working memory in rats: Continuous non-match. Pharmacol Biochem
Behav, 23: 599-605.
Hernandez DA, Lombardo RL, Ferrari L, & Tortorelli MC (1990)
Toxicity of ethyl-parathion and carbaryl on early development of sea
urchin. Bull Environ Contam Toxicol, 45: 734-741.
Heywood DL (1975) Degradation of carbamate insecticides in soil.
Environ Qual Saf, 4: 128-133.
Hill EF & Camardese MB (1986) Lethal dietary toxicities of
environmental contaminants and pesticides to Coturnix. Washington,
DC, US Department of the Interior, Fish and Wildlife Service (Fish
and Wildlife Technical Report No. 2).
Hill EF, Heath RG, Spann JW, & Williams JD (1975) Lethal dietary
toxicities of environmental pollutants to birds. Washington, DC, US
Department of the Interior, Fish and Wildlife Service (Special
Scientific Report, Wildlife No. 191).
Hodgson E & Casida JE (1961) Metabolism of N:N-dialkyl carbamate and
related compounds by rat liver. Biochem Pharmacol, 8: 179-191.
Hoffman DJ & Albers PH (1984) Evaluation of potential embryotoxicity
and teratogenicity of 42 herbicides, insecticides, and petroleum
contaminants to mallard eggs. Arch Environ Contam Toxicol, 13:
15-27.
Hoque H (1972) Radio tracer studies on chemical residues in food and
agriculture. Proc. Comb. Panel Res. Coord. Meet. 1971.
Hoque MZ (1972) Carbaryl - A new chemical mutagen. Curr Sci, 41(23):
855-856.
Houston JB, Upshall DG, & Bridges JW (1974) Pharmacokinetics and
metabolism of two carbamates, carbaryl and landrin, in the rat.
Xenobiotica, 5(10): 637-648.
Hudson RH, Tucker RK, & Haegele MA (1984) Handbook of toxicity of
pesticides to wildlife, 2nd ed. Washington, DC, US Department of the
Interior, Fish and Wildlife Service (Resource Publication 153).
Hughes L Jr (1971) A study of the fate of carbaryl insecticide in
surface waters. Lafayette, Indiana, Purdue University (Ph.D
Dissertation) (University microfilms, High Wycombe, England).
Hughes LB & Reuszer HW (1970) A study of bacterial decomposition of
carbaryl in farm pond waters. Lafayette, Indiana, Purdue University,
Water Resources Research Center (Technical Report No. 10).
Hundley HK, Cairns T, Luke AM, & Masumoto HT (1988) Pesticide
residue findings by the Luke method in domestic and imported foods
and animal feeds for fiscal years 1982-1986. J Assoc Off Anal Chem,
71(5): 875-892.
Huque H (1972) Preliminary report on the residues of carbaryl
granules in rice plants. Radiotracer Stud. Chem. Residues Food Agr.,
Proc. Comb. Panel Res. Coord. Meet. 1971. Vienna, International
Atomic Energy Agency, pp 107-109.
Hurlburt E, McMillan R, & Vialle M (1989) Supplemental environmental
impact statement: use of the insecticide carbaryl to control ghosts
and mud shrimp in oyster beds of Willapa Bay and Grays Harbour.
Olympia, Washington, Washington State Department of Fisheries and
Washington State Department of Ecology (Unpublished report submitted
to WHO by Rhône-Poulenc Agro, Lyon).
Hurwood IS (1967) Studies on pesticide residues. 2. Carbaryl
residues in the body tissues and milk of cattle following dermal
application. Qld J Agric Anim Sci, 24: 69-74.
Hwang SW & Schanker S (1974) Absorption of carbaryl from lung and
small intestine of the rat. Environ Res, 7(2): 206-211.
IARC (1987) Overall evaluations of carcinogenicity: An updating of
IARC monographs, volumes 1 to 42. Lyon, International Agency for
Research on Cancer (IARC Monographs on the Evaluation of the
Carcinogenic Risk of Chemicals to Humans, Supplement 7).
Imming RJ, Shaffer BC, & Woodard G (1969) Sevin, safety evaluation
by feeding to female Beagles from day one of gestation through
weaning of the offspring. (Prepared by Woodard Research Corporation
for Union Carbide Agricultural Products Co., Inc., Research Triangle
Park) (Unpublished proprietary information submitted to WHO by
Rhône-Poulenc Agro, Lyon).
Innes JRM, Ulland MB, Valerio MG, Petrucel L, Fishbein L, Hart ER,
Pallotta AJ, Bates RR, Falk HL, Gart JJ, Klein M, Mitchell I, &
Peters H (1969) Bioassay of pesticides and industrial chemicals for
tumorigenicity in mice: a preliminary note. J Natl Cancer Inst, 42:
1101-1114.
IRPTC (1982) In: Izmerov NF ed. IRPTC scientific reviews of Soviet
literature on toxicity and hazard of chemicals No. 7: Carbaryl.
Moscow, Centre of International Projects, GKNT, 13 pp.
IRPTC (1989) In: Izmerov NF ed. [Scientific reviews of Soviet
literature on toxicity and hazard of chemicals No. 7: Carbaryl], 2nd
ed. Moscow, Centre of International Projects, GKNT, 82 pp (in
Russian).
Ishidate M Jr & Odashima S (1977) Chromosome tests with 134
compounds on Chinese hamster cells in vitro: a screening for
chemical carcinogens. Mutat Res,48: 337-354.
Ishidate M Jr, Sofuni T, & Yoshikawa K (1981) Chromosomal aberration
tests in vitro as a primary screening tool for environmental
mutagens and/or carcinogens. Gann Monogr Cancer Res, 27: 95-108.
Ivanova LN & Molozhanova YG (1973) [Static and dynamic distribution
of carbaryl in the environment.] Gig i Sanit, 38(2): 24-28 (in
Russian).
Iwata Y, Dusch ME, Garman GE, & Gunther FA (1979) Worker environment
research: Residues for carbaryl, chlorobenzilate, dimethoate and
trichlorfon applied to citrus trees. J Agric Food Chem, 27(6):
1141-1145.
Jablonska J & Brzezinski J (1990) The influence of carbaryl on the
uptake of (3H) noradrenaline (3H) NA by rat hypothalamic slices.
Arch Toxciol, 64(5): 417-419.
Jacob J, Schmoldt A, Raab J, Hamman M, & Grimmer G (1985)
Monoxygenase induction by various xenobiotics and its influence on
the rat liver microsomal metabolite profile of benza(a)anthracene.
Cancer Lett, 27: 105-113.
Jaszczuk E & Syrowatka T (1979) [Induction of mitotic conversion in
Saccharomyces cerevisiae D4 with propoxur, carbaryl,
dimethylnitrosamine and products of their metabolic activation.]
Rocz Panstw Zakl Hig, 30(4): 323-330 (in Polish).
Jaszczuk E, Syrowatka T, & Cybulski J (1979) [Mutagenic activity of
propoxur, carbaryl and their nitroso derivatives. Induction of
reversion in Salmonella typhimurium.] Rocz Pantsw Zakl Hig, 30(1):
81-88 (in Polish).
Jegier Z (1964) Health hazards in insecticide spraying of crops.
Arch Environ Health, 8: 670-674.
Jeleniewicz KK & Szczepaniak St (1980) [Free aminoacids and amino
nitrogen levels in serum and blood erythrocytes of rat exposed to
subacute intoxication with carbaryl.] Bromatol Chem Toksykol, 13(3):
237-240 (in Polish).
Jeleniewicz KK, Szczepaniak St, & Slenkiewicz E (1984) [Effect of
acute intoxication with carbaryl on brain free aminoacids level in
rat.] Bromatol Chem Toksykol, 17(3): 225-227 (in Polish).
Jerkofsky M & Abrahamsen LH (1983) Variation on human herpes virus
susceptibility to enhancement by the pesticide carbaryl. Appl
Environ Microbiol, 45(5): 1555-1559.
Johnson WW & Finley MT (1980) Handbook of acute toxicity of
chemicals to fish and aquatic invertebrates. Washington, DC, US
Department of the Interior, Fish and Wildlife Service (Resource
Publication 137).
Johnson RD & Manske DD (1976) Pesticide residues in total diet
samples (IX). Pestic Monit J, 9(4): 157-169.
Johnson DP & Stansbury HA (1965) Adaptation of Sevin insecticide
(carbaryl) residue method to various crops. J Agric Food Chem, 13:
235-238.
Junk GA, Richard JJ, & Dahm PA (1984) Degradation of pesticides in
controlled water-soil systems (treatment and disposal of pesticides
wastes). Washington, DC, American Chemical Society, pp 37-67 (ACS
Symposium Series No. 259).
Kadir HA & Knowles CO (1981) Inhibition of rat brain monoamine
oxidase by insecticides, acaricides and related compounds. Gen
Pharmacol, 12(4): 239-247.
Kagan J, Rodionov G, Voronina L, Velitsco L, Kulagin O, & Peremitina
A (1970) [Effect of Sevin on liver function and structure.]
Pharmacol Toxicol, 33: 219-224 (in Russian with English summary).
Kaloyanova F (1963) [Changes in the blood sugar in cases of
intoxications with certain phosphorus organic compounds.] In: [Works
of the Scientific Research Institute on Work Safety and Occupational
Diseases - Volume X.] Sofia, Institute of Hygiene and Occupational
Health, pp 63-67 (in Bulgarian).
Kanazawa J (1975) Uptake and excretion of organophosphorus and
carbamate insecticides by freshwater fish, Motsugo, Pseudorasbora
parva. Bull Environ Contam Toxicol, 14(3): 346-352.
Kanazawa J (1981) Measurement of the bioconcentration factors of
pesticides by freshwater fish and their correlation with
physicochemical properties or acute toxicities. Pestic Sci, 12(4):
417-424.
Kanazawa J, Isensee AR, & Kearney PC (1975) Distribution of carbaryl
and 3,5-xylylmethyl carbamate in an aquatic model ecosystem. J Agric
Food Chem, 23(4): 760-763.
Karinen FJ, Lamberton JG, Stewart NE, & Terrier LC (1967)
Persistence of carbaryl in the marine estuarine environment. J Agric
Food Chem, 15(1): 148-156.
Karnak RE & Collins WJ (1974) The susceptibility to selected
insecticides and acetylcholinesterase activity in a laboratory
colony of midge larvae, Chironomus tentans (Diptera:
chironomidae). Bull Environ Contam Toxicol, 12: 62-69.
Kassin VJ (1968) [Structural changes in endocrine organs under
repeated action of Sevin.] Gig Primen Toxicol Pestic Klin Otrav,
1968: 631-635 (in Russian).
Kaur K & Toor HS (1977) Toxicity of pesticides to embryonic stages
of Cyprinus carpio communis Linn. Indian J Exp Biol, 15: 193-196.
Kavadia VS, Gangwar SK, Srivastava BP, Kathpal TS, & Gupta HCL
(1978) Persistence of carbaryl in clay loam soil and its uptake in
maize and root crops. Indian J Entomol, 40(3): 265-272.
Kavlock RJ, Short RD, & Chernoff N (1987) Further evaluation of an
in vivo teratology screen. Teratog Carcinog Mutagen, 7: 7-16.
Kazano H, Kearney PC, & Kaufman DD (1972) Metabolism of
methylcarbamate insecticides in soil. J Agric Food Chem, 20(5):
975-979.
Kazarnovskaya ML & Vasilos AF (1977) [The effect of Sevin on
chromosomal apparatus of cells in vitro.] Zdravookhranenije,
20(4): 14-16 (in Russian).
Kendrick PN, Trim AJ, Atwal JK, & Brown PM (1991) Direct gas
chromatographic determination of carbaryl residues in honeybees
( Apis mellifera L.) using a nitrogen-phosphorus detector with
confirmation by formation of a chemical derivative. Bull Environ
Contam Toxicol, 46: 654-661.
Khasawinah AM (1977) Hydrolysis of carbaryl in aqueous buffer
solutions (Project No. 111A12, File No. 23092). South Charleston,
West Virginia, Union Carbide Corporation (Prepared in cooperation
with the International Research and Development Corporation)
(Unpublished proprietary information submitted to WHO by
Rhône-Poulenc Agro, Lyon).
Khasawinah AM (1978) Fate of carbaryl in soils (Project No. 285).
Monument, Colorado, Analytical Development Corporation (Unpublished
proprietary information submitted to WHO by Rhône-Poulenc Agro,
Lyon).
Khera K (1966) Toxic and teratogenic effects of insecticides in duck
and chick embryos. Toxicol Appl Pharmacol, 8: 345.
Klisenko MA (1965) [Determination of Sevin in air and biological
material.] J Anal Chim, XX(5): 634-636 (in Russian).
Klisenko MA & Yakim VS (1966) [Accumulation and excretion of Sevin
from the body of warm-blooded animals.] Gig Primen Toxicol Pestic
Klin Otrav, 1966: 146-149 (in Russian).
Klisenko MA, Lebedeva TA, & Yurkova ZF (1972) [Chemical analysis of
micro amounts of pesticides.] Moscow, Medizina, pp 197-201 (in
Russian).
Knaak JB (1971) Biological and nonbiological modifications of
carbamates. Bull World Health Organ, 44: 121-131.
Knaak JB & Sullivan LJ (1967) Metabolism of carbaryl in dog. J Agric
Food Chem, 15(6): 1125-1126.
Knaak JB, Marilyn J, Tallant MJ, Bartley WJ, & Sullivan LF (1965)
The metabolism of carbaryl in rat, guinea-pig, and man. J Agric Food
Chem, 13(6): 537-543.
Knaak JB, Tallant MJ, Kozbelt SJ, & Sullivan LJ (1968) The
metabolism of carbaryl in man, monkey, pig, and sheep. J Agric Food
Chem, 16(3): 465-470.
Knaak JB, Yee K, Ackerman CR, Zweig G, Fry DM, & Wilson BW (1984)
Percutaneous absorption and dermal dose - ChE response studies with
parathion and carbaryl in the rat. Toxicol Appl Pharmacol, 76:
252-263.
Knight EV, Chin BH, Ueng TH, & Alvares AP (1986) Differences in the
in vivo and in vitro effects of the carbamate insecticide,
carbaryl on rat hepatic monooxygenases. Drug Chem Toxicol, 9(3-4):
253-273.
Knight EV, Alvares AP, & Chin BH (1987) Effects of phenobarbital
pre-treatment on the in vivo metabolism of carbaryl in rats. Bull
Environ Contam Toxicol, 39(5): 815-821.
Korn S (1973) The uptake and persistence of carbaryl in channel
catfish. Trans Am Fish Soc, 1: 137-139.
Koundinya PR & Ramamurthi R (1980) Toxicity of Sunithion and Sevin
to the freshwater fish Sarotherodon mossambicus (Peters). Curr
Sci, 49: 875-876.
Kovaleva ES & Talanov GA (1978a) [Methods for determination of Sevin
in soil and plants by means of thin-layer chromatography.] Gig i
Sanit, 7: 69-70 (in Russian).
Kovaleva ES & Talanov GA (1978b) [Residual amounts of Sevin in
plants and soil.] Khim Sel'sk Khoz, 16(8): 77-79 (in Russian).
Kovaleva ES & Talanov GA (1980) [Plant and soil dynamics of Sevin
residues and dithiocarbamic acid derivatives.] In: Bobovnikova TI &
Malakhov SG ed. [Migration of contaminants in soil and contiguous
media. Proceedings of the All-Union Congress, 2nd Meeting, 1978.]
Leningrad, Gidrometeoizdat, pp 155-159 (in Russian).
Kovtun SD (1970) Analysis of the action exerted by Sevine on the
myoneural formations. Pharmacol Toxicol, 35(5): 543-545.
Kovtun SD & Sokur AI (1970) [The effect of Sevin on the spontaneous
electrical activity of myoneural synaps and permeability of the
membrane of skeletal muscle in white rats.] Gig Primen Toxicol
Pestic Klin Otrav, 1970: 211-214 (in Russian).
Krause RT (1985a) Pesticide and industrial chemical residues. Liquid
chromatographic determination of N-methylcarbamate insecticides
and metabolites in crops. I. Collaborative study. J Assoc Off Anal
Chem, 68(4): 726-733.
Krause RT (1985b) Liquid chromatographic determination of
N-methylcarbamate insecticides and metabolites in crops. II.
Analytical characteristics and residue findings. J Assoc Off Anal
Chem, 68(4): 734-740.
Krechniak V & Foss W (1981) Gas chromatography determination of
carbaryl. Pr Cent Inst Ochr Pr, XXXI(110): 183-188.
Krishna JG & Casida JE (1966) Fate in rats of the radiocarbon from
ten variously labelled methyl- and dimethylcarbamate-C14
insecticide chemicals and their hydrolysis products. J Agric Food
Chem, 14(2): 98-105.
Krishna JG, Dorough HW, & Casida JE (1962) Synthesis of
N-methylcarbamates via methyl isocyanate-C14 and chromatographic
purification. J Agric Food Chem, 10(6): 462-466.
Krishnaih K, Prasad VG, & Jagan Mohan N (1978) Carbaryl residues on
brinjal and peas. In: Edwards CA, Veeresh GK, & Krueger HR ed.
Proceedings of the Symposium on Pesticide Residues in the
Environment in India, 7-10 November 1978. Hebbal, Bangalore,
University of Agricultural Sciences, pp 229-231 (UAS Technical
Series No. 32).
Krug HG & Berndt J (1985) Inhibition by pesticides of prostglandin
formation in blood platelets. Blut, 51: 19-23.
Krug HF, Hamm U, & Berndt J (1988) Mechanism of inhibition of
cyclo-oxygenase in human blood platelets by carbamate insecticides.
Biochem. J, 250(1): 103-110.
Krylov DG (1970) [The effect of Sevin on red vole population.] Gig
Primen Toxicol Pestic Klin Otrav, 1970: 218-110 (in Russian).
Kuhn JO (1991a) Acute oral toxicity study in rats with Sevin XLR
plus. Sugar Land, Texas, Stillmeadow, Inc. (Laboratory Study No. IV
8150-91) (Unpublished proprietary information submitted to WHO by
Rhône-Poulenc Agro, Lyon).
Kuhn JO (1991b) Sevin-4-oil 47.0% (w/w). Acute oral toxicity study
in rats . Sugar Land, Texas, Stillmeadow, Inc. (Laboratory Study No.
N 7712-90) (Unpublished proprietary information submitted to WHO by
Rhône-Poulenc Agro, Lyon).
Kuhr RJ (1970) Metabolism of carbamate insecticide chemicals in
plants and insects. J Agric Food Chem, 18(6): 1023-1030.
Kuhr RJ (1971) The formation and importance of carbamate insecticide
metabolites as terminal residues. Pure Appl Chem, Suppl: 199-220.
Kuhr RJ & Dorough HW (1976) Carbamate insecticides: Chemistry,
biochemistry and toxicology. Cleveland, Ohio, CRC Press, Inc., pp
125-200.
Kuhr RJ, Davis AC, & Bourke JB (1974) Dissipation of Guthion, Sevin,
Polyram, Phygon and Systox from apple orchard soil. Bull Environ
Contam Toxicol, 11(3): 224-230.
Kulshrestha SK & Arora N (1984) Impairments induced by sublethal
doses of two pesticides in the ovaries of a freshwater teleost
Channa striatus Bloch. Toxicol Lett, 20(1): 93-98.
Kuribayashi S (1981) Studies on the effect of pesticides on the
reproduction of the silkworm, Bombyx Mori L. I. Effects of
chemicals administered during the larval stage on egg-laying and
hatching. J Toxicol Sci, 6: 169-176.
Kurtz DA & Studholme GA (1974) Recovery of trichlorfon (Dylox) and
carbaryl (Sevin) in songbirds following spraying of forest for Gipsy
moth. Bull Environ Contam Toxicol, 11(1): 78-84.
Kuzminskaya UA, Ivanitskij VA, & Shilina VA (1984) [The influence of
pesticides having anticholinesterase effect upon the content of
biogenic amines in brain.] Gig i Sanit, 5: 80-81 (in Russian).
LaFleur KS (1976a) Carbaryl desorption and movement in soil columns.
Soil Sci, 121(4): 212-216.
LaFleur KS (1976b) Movement of carbaryl through Congaree soil into
ground water. J Environ Qual, 5(1): 91-92.
Lahmy S, Salmon JM, & Viallet P (1988) Influence of long-term
treatment with 3 methylcholanthrene or carbaryl on mixed function
oxidase activity in 3T3 fibroblasts: studies by
microspectro-fluorimetry on single cells. Cell Biochem Funct, 6(4):
275-282.
Lamb FC, Farrow RP, Elkins ER, Kimball JR, & Cook RW (1968) Removal
of DDT, parathion and carbaryl from spinach by commercial and home
preparative methods. J Agric Food Chem, 16: 967-973.
Lamberton JG & Claeys RR (1970) Degradation of 1-naphthol in sea
water. J Agric Food Chem, 18(1): 92-96.
Larkin MJ & Day MJ (1985) The effect of pH on the selection of
carbaryl-degrading bacteria from garden soil. J Appl Bacteriol,
58(2): 175-186.
Larkin MJ & Day MJ (1986) The metabolism of carbaryl by three
bacterial isolates, Pseudomonas spp. (NCIB 12042 & 12043) and
Rodococcus sp. (NCIB 12038) from garden soil. J Appl Bacteriol,
60(3): 233-242.
Lawlor TE (1989) Mutagenicity test on carbaryl (technical) in the
Ames Salmonella/Microsome reverse mutation assay. Final report.
Kensington, Maryland, Hazleton Laboratories America, Inc. (Report
No. 10862-0-401) (Unpublished proprietary information submitted to
WHO by Rhône-Poulenc Agro, Lyon).
Leavitt JRC, Gold RE, Holcslaw T, & Tupy D (1982) Exposure of
professional pesticide applicators to carbaryl. Arch Environ Contam
Toxicol, 11(1): 57-62.
Lechner DMW & Abdel-Rahman MS (1984) A teratology study of carbaryl
and malathion mixtures in rat. J Toxicol Environ Health, 14:
267-278.
Lechner DMW & Abdel-Rahman MS (1985) Alterations in rat liver
microsomal enzymes following exposure to carbaryl and malathion in
combination. Arch Environ Contam Toxicol, 1: 451-457.
Lechner DMW & Abdel-Rahman MS (1986a) The effect of carbaryl and
malathion in combination on gamma glutamyl transpeptidase and
glutathione- S-alkyltransferase activity in vitro. Arch Environ
Contam Toxicol, 15(6): 647-651.
Lechner DMW & Abdel-Rahman MS (1986b) Kinetics of carbaryl and
malathion in combination in the rat. J Toxicol Environ Health,
18(2): 241-256.
Lee RM & Brindley WA (1974) Synergist ratios, EPN detoxication,
lipid and drug-induced changes in carbaryl toxicity in Megachile
pacifica. Environ Entomol, 3: 899-907.
Lee DY, Chen CT, & Houng KH (1990) Behaviour assessment of eight
major pesticides used in Taiwan in soils by mechanistic models. In:
Soils and fertilizers in Taiwan, pp 33-50.
Leeling NC & Casida JE (1966) Metabolites of carbaryl (1-naphthyl
methyl carbamate) in mammals and enzymatic systems for their
formation. J Agric Food Chem, 14(3): 281-290.
Leenheer JA & Ahlrichs (1971) A kinetic and equilibrium study of the
adsorption of carbaryl and parathion upon soil organic matter
surfaces. Soil Sci Soc Am Proc, 35: 700-705.
Lejczak B (1977) Effect of insecticides: chlorfenvinphos, carbaryl
and propoxur on aquatic organisms. Pol Arch Hydrobiol, 24(4):
583-591.
Leonard JA & Yearly RA (1990) Exposure of workers using hand-held
equipment during urban application of pesticides to trees and
ornamental shrubs. Am Ind Hyg Assoc J, 51: 605-609.
Lesca P, Fournier A, Lecointe P, & Cresteil T (1984) A dual assay
for the specific screening of 3 methylcholanthrene and phenobarbital
like chemical inducers of cytochrome P450 monooxygenases. Mutat Res,
129: 229-310.
Lijinsky W & Schmähl D (1978) Carcinogenicity of N-nitroso
derivatives of N-methylcarbamate insecticides in rats. Ecotoxicol
Environ Saf, 2: 413-419.
Lijinsky W & Taylor HW (1976) Carcinogenesis in Sprague-Dawley rats
on N-nitroso- N-alkylcarbamate esters. Cancer Lett, 1: 275-279.
Lijinsky W & Taylor HW (1977) Transplacental chronic toxicity test
of carbaryl with nitrite in rats. Food Cosmet Toxicol, 15: 229-232.
Lijinsky W & Winter C (1981) Skin tumours induced by painting
nitrosoalkylureas on mouse skin. J Cancer Res Clin Oncol, 102:
13-20.
Lillie RJ (1973) Studies on the reproductive performance and progeny
performance of caged white leghorns fed malathion and carbaryl.
Poult Sci, 52: 266-272.
Lin TH, North HH, & Menzer RE (1975) Metabolism of carbaryl
(1-naphthyl N-methylcarbamate) in human embryonic lung cell
cultures. J Agric Food Chem, 23(2): 253-256.
Lindsay CE (1961) Pesticide tests in the marine environment in the
State of Washington. Proc Natl Shellfish Assoc, 52: 87-97.
Lingaraja T & Venugopalan UK (1978) Pesticide induced physiological
and behavioural changes in an estuarine teleost Therapon jarbua
(Forsk). Fish Technol, 15: 115-119.
Little EE, Archeski RD, Flerov BA, & Kozlovskaya VI (1990)
Behavioral indicators of sublethal toxicity in rainbow trout. Arch
Environ Contam Toxicol, 19(3): 380-385.
Liu SY & Bollag JM (1971a) Metabolism of carbaryl by a soil fungus.
J Agric Food Chem, 19(3): 487-490.
Liu SY & Bollag JM (1971b) Carbaryl decomposition to 1-naphthyl
carbamate by Aspergillus terreus. Pestic Biochem Physiol, 1(3-4):
366-372.
Liu D, Thomson K, & Strachan WMJ (1981) Biodegradation of carbaryl
in simulated aquatic environment. Bull Environ Contam Toxicol,
27(3): 412-417.
Long KR (1971) Pesticides and occupational hazards on farms. Am J
Nurs, 71(4): 740-743.
Looney NE & Knight JN (1985) Effects of initial set and carbaryl
treatment in final fruit set on "Greensleeves" apple. Hortic Sci,
20(3): 400-401.
Looney NE & McKellar JE (1984) Thinning Spartan apples with carbaryl
and 1-napthaleneacetic acid: influence of spray volume and
combinations of chemicals. Can J Plant Sci, 64: 161-166.
Loosanoff VL (1961) Recent advances in the control of shellfish
predators and competitors. Proc Gulf Caribb Fish Inst, 13: 113-128.
Loosanoff VL, MacKenzie CL Jr, & Shearer LW (1960a) Use of chemical
barriers to protect shellfish beds from predators. In: Fisheries:
Fish farming. Volume 3 - Fisheries management. Olympia, Washington,
Washington State Department of Fisheries, pp 86-90.
Loosanoff VL, MacKenzie CL Jr, & Shearer LW (1960b) Use of chemical
to control shellfish predators. Science, 131: 1522-1523.
Lowe JI (1967) Effect of prolonged exposure to Sevin on an estuarine
fish, Leiostomus xanthurus Lacépède. Bull Environ Contam Toxicol,
2(3): 147-155.
Lox CD (1984) The effects of acute carbaryl exposure on clotting
factor activity in rat. Ecotoxicol Environ Saf, 8: 280-283.
Luca D & Balan M (1987) Sperm abnormality assay in the evaluation of
the genotoxic potential of carbaryl in rats. Morphol Embryol
(Bucur), 33(1): 19-22.
Lucier GW, McDaniel OS, Williams C, & Klein R (1972) Effects of
chlordane and methylmercury on the metabolism of carbaryl and
carbofuran in rats. Pestic Biochem Physiol, 2: 244-255.
Lukaneva AM & Rodionov GA (1973) [Some experimental data on the
effect of DDT and Sevin upon the development of cholesterin
atherosclerosis.] Gig i Sanit, 9: 41-45 (in Russian).
Luke MA, Masumoto HT, Cairns T, & Hundley HK (1988) Levels and
incidences of pesticide residues in various foods and animal feeds
analyzed by the Luke multiresidue methodology for fiscal years
1982-1986. J Assoc Off Anal Chem, 71(2): 415-420.
Lykken L & Casida JE (1969) Metabolism of organic insecticide
chemicals. Can Med Assoc J, 100: 145-154.
Ma TH, Harris MM, Anderson VA, Ahmed I, Mohammed K, Bare JL, & Lin G
(1984) Tradescantia-Micronucleus (Trad-MCN) tests on 140
health-related agents. Mutat Res, 138: 157-167.
McCann J, Choi E, Yamasaki E, & Ames BM (1975) Detection of
carcinogen as mutagens in the Salmonella/microsome test: Assay of
300 chemicals. Proc Natl Acad Sci (USA), 72(12): 5135-5139.
MacCollom GB, Currier WW, & Baumann GL (1985) Pesticide drift and
quantification from air and ground applications to a single orchard
site. In: Honeycutt RC, Zweg D, & Ragsdale N ed. Dermal exposure
related to pesticide use. Washington, DC, American Chemical Society,
pp 190-198 (ACS Symposium Series No. 273).
MacCollom GB, Currier WW, & Baumann GL (1986) Drift comparisons
between aerial and ground orchard application. J Econ Entomol,
79(2): 459-464.
Macek KJ & McAllister WA (1970) Insecticide susceptibility of some
common fish family representatives. Trans Am Fish Soc, 99(1): 20-27.
MacLeod J (1982) Report on UCC sperm morphology readings submitted
to Union Carbide Agricultural Products Co., Inc. (Unpublished
proprietary information submitted to WHO by Rhône-Poulenc Agro,
Lyon).
MacPherson SE, Scott RC, & Williams FM (1991) Fate of carbaryl in
rat skin. Arch Toxicol, 65: 594-598.
Maibach HI, Feldmann RJ, Milby TH, & Serat WF (1971) Regional
variations in percutaneous penetration in man - Pesticides. Arch
Environ Health, 23: 208-211.
Makovskaya EL, Rapoport MB, & Pintshuk VG (1965) [On the possibility
of carcinogenic effect of some insecticides belonging to the
carbamate group.] Vopr Exp Onkol, 1: 67-74 (in Russian).
Maliwal BP & Guthrie FE (1981) Interaction of insecticides with
human plasma lipoproteins. Chem-Biol Interact, 35(2): 177-188.
Mann GS & Chopra SL (1969) Residues of carbaryl on crops. Pestic
Monit J, 2(4): 163-165.
Manske DD & Corneliussen PE (1974) Pesticides in food and feed.
Pesticide residues in total diet samples (VII). Pestic Monit J,
8(2): 110-124.
Manske DD & Johnson RD (1975) Residues in food and feed. Pesticide
residues in total diet samples (VIII). Pestic Monit J, 9(2): 94-105.
Manske DD & Johnson RD (1977) Residues in food and feed. Pesticide
and other chemical residues in total diet samples (X). Pestic Monit
J, 10: 134-148.
Marian MP, Arul V, & Pandian TJ (1983) Acute and chronic effect of
carbaryl on survival, growth, and metamorphosis in the bullfrog
(Rana tigrina). Arch Environ Contam Toxicol, 12(3): 271-275.
Marshall TC, Dorough HW, & Swin HE (1976) Screening of pesticides
for mutagenic potential using Salmonella typhimurium mutants. J
Agric Food Chem, 24(3): 500-563.
Marshall TC, Dorough HW, & Swin HE (1979) Biliary excretion of
carbamate insecticides in the rat. Pestic Biochem Physiol, 11:
56-63.
Mason Y, Choshen E, & Rav-Acha C (1990) Carbamate insecticides:
Removal from water by chlorination and ozonation. Water Res, 24(1):
11-22.
Mastro VC & Cameron EA (1976) Control of Archips semiferanus on
Quercus, spp with two chemical insecticides. J Econ Entomol,
69(4): 554-556.
Mathur A & Bhatnagar P (1991) A teratogenic study of carbaryl in
Swiss albino mice. Food Chem Toxicol, 29(9): 629-632.
Matsumura F & Sakai K (1968) Degradation of insecticides by
esterases of the American cockroach. J Econ Entomol, 61(3): 598-605.
Matsumura F & Ward CT (1966) Degradation of insecticides by the
human and the rat liver. Arch Environ Health, 13: 257-261.
Mayer FL Jr (1987) Acute toxicity handbook of chemicals to estuarine
organisms. Gulf Breeze, Florida, US Environmental Protection Agency,
Gulf Breeze Laboratory (EPA 600/8-87/017).
Mayer FL & Ellersieck MR (1986) Manual of acute toxicity:
Interpretation and data base for 410 chemicals and 66 species of
freshwater animals. Washington, DC, US Department of the Interior,
Fish and Wildlife Service (Resource Publication 160).
Mehendale HM & Dorough HW (1971) Glucuronidation mechanisms in the
rat and their significance in the metabolism of insecticides. Pestic
Biochem Physiol, 1: 307-318.
Mellon Institute (1958) Acute toxicity of carbaryl. Pittsburgh,
Pennsylvania, Mellon Institute (Unpublished proprietary information
submitted to WHO by Rhône-Poulenc Agro, Lyon).
Mellon Institute (1963) Results of the eighty weeks of inclusion of
Sevin in the diet of mice. Pittsburgh, Pennsylvania, Mellon
Institute, pp 1-12 (Special report No. 26-53) (Unpublished
proprietary information submitted to WHO by Rhône-Poulenc Agro,
Lyon).
Menzie CM (1969) Metabolism of pesticides. Washington, DC, US
Department of the Interior, Bureau of Sport Fisheries and Wildlife,
pp 72-76 (Special Scientific Report, Wildlife No. 127).
Miles CJ, Trehy ML, & Yost RA (1988a) Degradation of
N-methylcarbamate and carbamoyl oximes pesticides in chlorinated
water. Bull Environ Contam Toxicol, 41(6): 838-843.
Miles CJ, Trehy ML, & Yost RA (1988b) Fate of pesticides in
chlorinated water. Paper presented at the 195th Meeting of the
American Chemical Society. Washington, DC, American Chemical Society
(Abstract ENVR 139).
Miller NE (1990) Metabolism of C14-carbaryl under aerobic soil
conditions. Research Triangle Park, North Carolina, Rhône-Poulenc Ag
Company (Unpublished proprietary information submitted to WHO by
Rhône-Poulenc Agro, Lyon).
Miller A III, Henderson MC, & Buhler DR (1979) Covalent binding of
carbaryl (1-naphthyl- N-methylcarbamate) to rat liver microsome in
vitro. Chem-Biol Interact, 24: 1-17.
Molozhanova EG (1968) [Circulation of Sevin in compartments of the
environment.] Gig Primen Toxicol Pestic Klin Otrav, 1968: 355-362
(in Russian).
Molozhanova EG (1970) [Factual content of Sevin in compartments of
the environment and quantities of the formulation apt at getting
into human organism.] Gig Primen Toxicol Pestic Klin Otrav, 1970:
170-174 (in Russian).
Molozhanova EG & Kanevskij AA (1971) [Application of mathematical
analysis to modelling the behaviour of Sevin in soil.] Gig Primen
Toxicol Pestic Klin Otrav, 1971: 77-83 (in Russian).
Moreynis JA & Estrin IM (1965) [Sevin action on animals infected
with the virus of influenza.] Gig Primen Toxicol Pestic Klin Otrav,
1965: 435-442 (in Russian).
Moriya M, Ohta T, Watanabe K, Miyazawa T, Kato K, & Shirasu Y (1983)
Further mutagenicity studies on pesticides in bacterial reversion
assay systems. Mutat Res, 116: 185-216.
Moser VC, McCormick JP, Creason JP, & MacPhail RC (1988) Comparison
of chlordimeform and carbaryl using a functional observational
battery. Fundam Appl Toxicol, 11(2): 189-206.
Mount ME & Oehme FW (1981a) Carbaryl. A literature review. Residue
Rev, 80: 1-64.
Mount ME & Oehme FW (1981b) Diagnostic criteria for carbaryl
poisoning in sheep. Arch Environ Contam Toxicol, 10(4): 483-495.
Mount ME, Dayton AD & Oehme FW (1981) Carbaryl residues in tissues
in cholinesterase activities in brain and blood of rats receiving
carbaryl. Toxicol Appl Pharmacol, 58(2): 282-296.
Mumma RO, Khalifa S, & Hamilton RH (1971) Spectroscopic
identification of metabolites of carbaryl in plants. J Agric Food
Chem, 19(3): 445-451.
Murli H (1989) Mutagenicity test on carbaryl technical in an in
vitro cytogenetic assay measuring chromosomal aberration
frequencies in Chinese hamster ovary (CHO) cells. Kensington,
Maryland, Hazleton Laboratories America, Inc. (Report No.
10862-0-437) (Unpublished proprietary information submitted to WHO
by Rhône-Poulenc Agro, Lyon).
Murray HE & Guthrie RK (1980) Effects of carbaryl, diazinon and
malathion on native aquatic populations of microorganisms. Bull
Environ Contam Toxicol, 24: 535-542.
Murray FJ, Staples RE, & Schwets BA (1979) Teratogenic potential of
carbaryl given to rabbits and mice by gavage or by dietary
inclusion. Toxicol Appl Pharmacol, 51: 81-89.
Murthy NBK & Raghu K (1988) Soil bound residues of carbaryl and
1-naphthol: release and mineralization in soil, and uptake by
plants. J Environ Sci Health, B23(6): 575-585.
Murthy NBK & Raghu K (1989) Metabolism of 14C-carbaryl and
14C-naphthol in moist and flooded soils. J Environ Sci Health,
B24(5): 479-491.
Murthy NBK & Raghu K (1991) Fate of 14C-carbaryl in soils as a
function of pH. Bull Environ Contam Toxicol, 46(3): 374-379.
Myers WF (1977) Carbaryl insecticide. Estimation of carbaryl
exposure to humans. South Charleston, West Virginia, Union Carbide
Corporation (Project Report No. 111A12) (Unpublished proprietary
information submitted to WHO by Rhône-Poulenc Agro, Lyon).
Myers RC & Christopher SM (1987) Sevin technical dermal
sensitization study in the guinea-pig (Project No. ID-49-146).
Export, Pennsylvania, Bushy Run Research Center (Proprietary report
submitted to WHO by Rhône-Poulenc Agro, Lyon).
Myers RC, Weil CS, & Carpenter CP (1975) Sevin liquid, 44% carbaryl,
range-finding toxicity studies. Pittsburgh, Pennsylvania,
Carnegie-Mellon Research Institute (Unpublished proprietary
information submitted to WHO by Rhône-Poulenc Agro, Lyon).
Myhr S (1973) A screen for pesticide toxicity to protein and RNA
synthesis in HeLa cells. J Agric Food Chem, 21(3): 362-367.
Nagy Z, Mile I, & Antony F (1975) Mutagenic effect of pesticides on
Escherichia coli WP2 try. Acta Microbiol Acad Sci Hung, 22:
309-314.
Neskovic N, Terzic M, & Vitorovic S (1978) [Acute toxicity of
carbaryl and propoxur in mice previously treated with phenobarbital
and SKF-525-A.] Arh Hig Rada, 29: 251-256 (in Croatian).
Neuhauser EF, Loehr RC, Malecki MR, Milligan DL, & Durkin PR (1985)
The toxicity of selected organic chemicals to the earthworm Eisenia
fetida. J Environ Qual, 14(3): 383-388.
Nimmo DR, Hamaker TL, Matthews E, & Moore JC (1981) An overview of
the acute and chronic effects of first and second generation
pesticides on an estuarine Mysid. In: Vernberg FJ, Calabrese A,
Thurberg FP, & Vernberg WB ed. Biological monitoring of marine
pollutants. New York, Academic Press, Inc., pp 3-19.
NIOSH (1976) Criteria for a recommended standard ... Occupational
exposure to carbaryl. Cincinnati, Ohio, National Institute for
Occupational Safety and Health, 191 pp (DHEW (NIOSH) Publication No.
77-107).
Norris FA 1991) A terrestrial field soil dissipation study with
carbaryl (Study No. EC 89-080, File No. 40862). Research Triangle
Park, North Carolina, Rhône-Poulenc Ag Company (Unpublished
proprietary information submitted to WHO by Rhône-Poulenc Agro,
Lyon).
Nye DE & Dorough HW (1976) Fate of insecticides administered
endotracheally to rats. Bull Environ Contam Toxicol, 15(3): 291-296.
Odeyemi O (1982) Relative stability of carbaryl in tropical
ecosystems. Environ Pollut, A29(3): 207-213.
Okumura D, Melnicoe R, Jackson T, Drefs C, Maddy K, & Wells J (1991)
Pesticide residues in food crops analyzed by the California
Department of Food and Agriculture in 1989. Rev Environ Contam
Toxicol, 118: 87-150.
Olefir AI (1977) [Effects of pesticides on immunogenesis.] Vrach
Delo, 9: 121-123 (in Russian).
Olefir AI & Vinogradova VKh (1968) [A study of the embryotropic
effect of the pesticides Sevin and Zyram.] Vrach Delo, 11: 103-106
(in Russian).
Omer AA, Youssef MK, Abou-Youssef AY, & Khalil AM (1986)
Mutagenicity of insecticide Sevin and its derivative nitrosocarbaryl
on D. melanogaster. Alex J Agric Res, 31(3): 239-248.
Omkar & Shukla GS (1985) Toxicity of insecticides to Macrobrachium
lamarrei (H. Milne Edwards) (Decapoda, Palaemonidae). Crustaceana,
48: 1-5.
Önfelt A (1987) Spindle disturbances in mammalian cells. III.
Toxicity, c-mitosis and aneuploidy with 22 different compounds.
Specific and unspecific mechanisms. Mutat Res, 182: 135-154.
Önfelt A & Klasterska I (1983) Spindle disturbances in mammalian
cells. II. Induction of viable aneuploid/polyploid cells and
multiple chromatid exchanges after treatment of V-70 Chinese hamster
cells with carbaryl. Modifying effects of glutathion and S9. Mutat
Res, 119: 319-330.
Önfelt A & Klasterska I (1984) Sister chromatid exchanges and
thioguanine resistance in V79 Chinese hamster cells after treatment
with the aneuploidy inducing agent carbaryl +/ S9 mix. Mutat Res,
125: 269-274.
Oonnithan ES & Casida JE (1968) Oxidation of methyl- and
dimethylcarbamate insecticide chemicals by microsomal enzymes and
anticholinesterase activity of the metabolites. J Agric Food Chem,
18(1): 28-44.
Orlova NV & Zhalbe EP (1968) [Experimental material contributive to
the problem of maximal permissible amounts of Sevin in food
products.] Vopr Pitan, 27(6): 49-55 (in Russian).
Orzel RA & Weiss LR (1966) The effect of carbaryl
(1-naphthyl- N-methylcarbamate) on blood glucose, and liver and
muscle glycogen in fasted and nonfasted rats. Biochem Pharmacol, 15:
995-998.
Osborne BG, Barrett GM, Laal-Khoshab A, & Willis KH (1989) The
occurrence of pesticide residues in UK home-grown and imported
wheat. Pestic Sci, 27: 103-109.
Osman MA & Belal MH (1980) Persistence of carbaryl in canal water. J
Environ Sci Health, B15(3): 307-311.
Osman DH & Brindley WA (1981) Estimating monoxygenase detoxification
in field populations: toxicity and distribution of carbaryl in three
species of Labops grassbugs. Environ Entomol, 10(5): 676-680.
Osterloh J, Letz G, Pond S, & Becker Ch (1983) An assessment of the
potential testicular toxicity of 10 pesticides using the mouse sperm
morphology assay. Mutat Res, 116(3-4): 407-415.
Ott FT, McCullogh R, & Stanley JG (1981) Effects of forest spraying
on the ecology of brook trout. Report to the Maine Department of
Conservation, Forest Service, Augusta.
Owen BB Jr (1965) Aquatic insect populations reduced by aerial
spraying of insecticide Sevin. Philadelphia, Pennsylvania,
Pennsylvania Academy of Science, pp 63-69.
Page M, Haverty MJ, & Richmond ChE (1985) Residual activity of
carbaryl protected Lodgepole Pine against Mountain Pine Beetle,
Dillon, Colorado, 1982 and 1983. Berkeley, California, US Department
of Agriculture, Forest Service, Pacific Southwest Forest and Range
Experiment Station (Research Note PSW-375).
Panciera RJ (1967) Determination of teratogenic properties of orally
administrated 1-naphthyl- N-methylcarbamate (Sevin) in sheep.
Research Triangle Park, North Carolina, Union Carbide Agricultural
Products Co., Inc. (Unpublished proprietary information submitted to
WHO by Rhône-Poulenc Agro, Lyon).
Parafita MA & Fernandez-Otero P (1984) The interaction of carbaryl
with the metabolism of isolated hepatocytes. II. Effect on
glycogenesis. Gen Pharmacol, 15(4): 333-337.
Parafita MA, Fernandez-Otero P, & Aldegunde MA (1984) The
interaction of carbaryl with the metabolism of isolated hepatocytes.
I. Effect on respiration and glycolysis. Gen Pharmacol, 15(4):
327-331.
Paris DF & Lewis DL (1973) Chemical and microbiological degradation
of ten selected pesticides in aquatic system. Residue Rev, 45:
95-124.
Paris D, Lewis D, Barnett J, & Baugham G (1975) Microbial
degradation and accumulation of pesticides in aquatic systems.
Corvallis, Oregon, US Environmental Protection Agency, Office of
Research and Development, National Environmental Research Center, pp
1-45 (EPA-660/3-75-007).
Patocka JJ & Bajgar J (1971) Affinity of human brain
acetylcholinesterase to some organophosphates and carbamates in
vitro. J Neurochem, 18: 2545-2546.
Paulson GD & Feil VJ (1969) The fate of a single oral dose of
carbaryl (1-naphthyl- N-methyl-carbamate) in the chicken. Poult
Sci, 48: 1593-1597.
Paulson GD, Zaylskie RG, Zehr MV, Portnoy CE, & Feil VJ (1970)
Metabolites of carbaryl (1-naphthyl N-methyl-carbamate) in chicken
urine. J Agric Food Chem, 18: 110-115.
Pavlova II, Petrovskaya OG, & Ivanova SI (1968) [Biochemical and
histochemical studies on the oxidative-reductive enzymes after an
exposure to Sevin.] Gig Primen Toxicol Pestic Klin Otrav, 1968:
625-630 (in Russian).
Payne JA, Dutcher JD, & Ellis HC (1985) Low pressure soil
application of carbaryl for control of the pecan weevil, Curculio
carvae. J Agric Entomol, 2(1): 1-5.
Pekas JC (1972) Intestinal hydrolysis, metabolism and transport of a
pesticidal carbamate in pH G.5 medium. Toxicol Appl Pharmacol, 23:
62-70.
Pekas JC & Paulson GD (1970) Intestinal hydrolysis and conjugation
of a pesticidal carbamate in vitro. Science, 170: 77-78.
Perelygin VM, Shpirt MB, Aripov OA, & Ershova VI (1971) [Effect of
some pesticides on immunological response reactivity.] Gig i Sanit,
36(12): 29-33 (in Russian).
Petrovski VV (1970) [The level of content and duration of excretion
of Sevin in the milk of cows treated against ticks.] Gig Primen
Toxicol Pestic Klin Otrav, 1970: 193-196 (in Russian).
Pieper GR (1979) Residues analysis of carbaryl on forest foliage and
in stream water using HPLC. Bull Environ Contam Toxicol, 22(1-2):
167-171.
Pieper GR & Roberts RB (1978) Residue analysis of carbaryl on forest
foliage and in creek water. US Department of Agriculture, Forest
Service, Northern Region, pp 1-9 (Report No. 78-5).
Pipy B, Gaillard D, & Derache R (1979) Effect of blockage of the
reticulo-endothelial system on the carbaryl toxicity in the rat.
Toxicology, 12: 135-142.
Pipy B, Gaillard D, Beraud M, & Derache R (1980) Phagocytic activity
of the rat reticuloendothelial system and the pharmakokinetics of an
anticholinesterasic insecticide: carbaryl. Xenobiotica, 10(10):
785-793.
Pipy B, Gaillard D, & Derache R (1981) Phagocytic activity of the
reticuloendothelial system in the rat and rates of in vivo
excretion of metabolites of carbaryl and in vitro microsomal
metabolism. Biochem Pharmacol, 30(6): 669-672.
Pipy B, Gaillard D, Beraud M, & Derache R (1982) Enzymatic
activities of liver serine esterases during the reticuloendothelial
system phagocytosis blockade by carbaryl, an anticholinesterasic
insecticide. Toxicol Appl Pharmacol, 62: 11-18.
Pipy B, de Maroussem D, Beraud M, & Derache R (1983) Evaluation of
cellular and humoral mechanisms of carbaryl-induced
reticuloendothelial phagocytic depression. J Reticuloendothel Soc,
34(5): 395-412.
Podolak M & Warchocki B (1980) [Effect of toxobidin on the kinetics
of reaction of ChE inhibition by chlorfenvinfos and carbaryl.] Rocz
Panstw Zakl Hyg, 31(2): 125-131 (in Polish).
Podolak-Majczak M & Tyburczyk W (1984) Effect of joint action of
sodium nitrite and carbaryl on rats organism. Bromatol Chem
Toksykol, XVII(3): 211-214 (in Polish with English summary).
Podolak-Majczak M & Tyburczyk W (1986) [Effect of joint action of
sodium nitrite and carbaryl on rats organism. Part IV. Hepatotoxic
action.] Bromatol Chem Toksykol, XIX(3): 164-166 (in Polish with
English summary).
Post G & Schroeder TR (1971) The toxicity of four insecticides to
four salmonid species. Bull Environ Contam Toxicol, 6(2): 144-155.
Preussmann RG, Eisenbrand G, & Schmahl D (1976) Carcinogenicity
testing of low doses of nitrosopyrrolidine and of
nitrosobenzo-thiazuron and nitrosocarbaryl in rats. In: Walker EA,
Bogovski P, & Griciute L ed. Environmental N-nitroso compounds
analysis and formation. Proceedings of a Working Conference held at
the Polytechnical Institute, Tallinn, Estonian SSR, 1-3 October
1975. Lyon, International Agency for Research on Cancer, pp 429-433
(IARC Scientific Publications No. 14).
Prima NG, Prima AP, Sem'yan AI, Solov'eva VG, & Bondarenko VF (1976)
[Model studies of the rate of self-purification of river water from
Sevin and 1-naphthol residues.] Probl Okhr Vod, 7: 8-13 (in
Russian).
Probst GS, McMahon RE, Hill LE, Thompson CZ, Epp JK, & Neal SB
(1981) Chemically induced unscheduled DNA synthesis in primary rat
hepatocyte cultures: A comparison with bacterial mutagenicity using
218 compounds. Environ Mutagen, 3(1): 11-32.
Puech AA (undated) A study of the potential for human dermal and
inhalation exposure of Sevin, carbaryl insecticide during normal
garden use. Research Triangle Park, North Carolina, Union Carbide
Corporation (Unpublished proprietary information submitted to WHO by
Rhône-Poulenc Agro, Lyon).
Puyear RL & Paulson GD (1972) Effect of carbaryl (1-naphthyl-
N-methylcarbamate) on phenobarbital-induced sleeping time and some
liver microsomal enzymes in white leghorn cockerels. Toxicol Appl
Pharmacol, 22: 621-627.
Quarles JM & Tennant RW (1975) Effects of nitrosocarbaryl on
Balb/3T3 cells. Cancer Res, 35: 2637-2645.
Rajagopal BS & Sethunathan N (1984) Influence of nitrogen
fertilizers on the persistence of carbaryl and carbofuran in flooded
soils. Pestic Sci, 15: 591-599.
Rajagopal BS, Chendrayan K, Reddy B, Rajasekhar-Reddy B, &
Sethunathan N (1983) Persistence of carbaryl in flooded soils and
its degradation by soil enrichment cultures. Plant Soil, 73(1):
35-45.
Rajagopal BS, Rao VR, Nagendrappa G, & Sethunathan N (1984)
Metabolism of carbaryl and carbofuran by soil-enrichment and
bacterial cultures. Can J Microbiol, 30(12): 1457-1465.
Rajagopal BS, Panda S, & Sethunathan N (1986) Accelerated
degradation of carbaryl and carbofuran in a flooded soil pretreated
with hydrolysis products, 1-naphthol and carbofuran phenol. Bull
Environ Contam Toxicol, 36(6): 827-832.
Rajukkannu K, Basha AA, Habeebullah B, Duraisamy P, &
Balasubramanian M (1985) Degradation and persistence of DDT, BHC,
carbaryl and malathion in soils. Indian J Environ Health, 27(3):
237-243.
Rao KRSS, Rao KSP, Sahib IKA, & Rao KVR (1985a) Combined action of
carbaryl and phenthoate on a freshwater fish ( Carina punctatus
bloch). Ecotoxicol Environ Saf, 10(2): 209-217.
Rao KS, Rao KR, Sahib IK, & Rao KV (1985b) Combined action of
carbaryl and phenthoate on a tissue lipid derivatives of murrel,
Channa punctatus (Bloch). Ecotoxicol Environ Saf, 9(1): 107-111.
Ray SK & Poddar MK (1985a) Central cholinergic dopaminergic
interaction in carbaryl induced tremor. Eur J Pharmacol, 119(3):
251-253.
Ray SK & Poddar MK (1985b) Effect of pentylenetetrazol on carbaryl
induced changes in striatal catecholamines. Biochem Pharmacol,
34(4): 553-557.
Ray SK & Poddar MK (1990) Interaction of central serotonin and
dopamine in the regulation of carbaryl induced tremor. Eur J
Pharmacol, 181(3): 159-166.
Rawash IA, Gaaboub IA, El-Gayar FM, & El-Shazli AY (1975) Standard
curves for Nuvacron, Malathion, Sevin, DDT, and Kelthane tested
against the mosquito Culex pipiens L. and the microcrustacean
Daphnia magna Straus. Toxicology, 4: 133-144.
Regan JD, Seltow RB, Francis AA, & Lijinsky W (1976)
Nitrosocarbaryl: Its effect on human DNA. Mutat Res, 38: 239-302.
Reiner E & Aldridge WN (1967) Effect of pH on inhibition and
spontaneous reactivation of acethylcholinesterase treated with
esters of phosphorus acids and of carbamic acids. Biochem J, 105:
171-179.
Rickard RW & Dorough HW (1984) in vivo formation of
nitrosocarbamates in the stomach of rats and guinea-pigs. J Toxicol
Environ Health, 14(2-3): 279-290.
Rickard RW, Walter-Echols G, Dorough HW, & Lawrence LJ (1982)
Importance of pH in assessing the potential for nitrosocarbamate
formation in the stomach. Pestic Biochem Physiol, 18(3): 325-333.
Robel J, Felthausen RW, & Dayton D (1982) Effects of carbamates on
bobwhite food intake, body weight and locomotor activity. Arch
Environ Contam Toxicol, 11(5): 611-615.
Robens JF (1969) Teratologic studies of carbaryl, diazinon, norea,
disulfiram and thiram in small laboratory animals. Toxicol Appl
Pharmacol, 15: 152-163.
Roberts D (1975) The effect of pesticides on byssus formation in the
common mussel, Mytilus edulis. Environ Pollut, 8(4): 241-254.
Roberts BL & Dorough HW (1984) Relative toxicity of chemicals to the
earthworm Eisenia foetida. Environ Toxicol Chem, 3: 67-78.
Rocchi P, Perocco P, Alberhin W, Fini A, & Prodi G (1980) Effects of
pesticides on scheduled and unscheduled DNA synthesis of rat
thymocytes and human lymphocytes. Arch Toxicol, 45(2): 101-108.
Rodgers KE, Leung N, Imamura T, & Devens BH (1986) Rapid in vitro
screening assay for immunotoxic effects of organophosphorus and
carbamate insecticides in the generation of cytotoxic T-lymphocyte
responses. Pestic Biochem Physiol, 26(3): 292-301.
Rodriguez LD & Dorough HW (1977) Degradation of carbaryl by soil
microorganisms. Arch Environ Contam Toxicol, 6(1): 47-56.
Romine RR & Bussian RA (1971) Sevin Insecticide: The degradation of
carbaryl after surface application to a farm pond (Project No
111A13, File No. 15265). South Charleston, West Virginia, Union
Carbide Corporation (Unpublished proprietary information submitted
to WHO by Rhône-Poulenc Agro, Lyon).
Roszkowski J, Zadura J, & Skwarek P (1976) The effect of carbaryl on
immune response in chickens. Bull Vet Inst Pulawy, 20(3-4): 85-88.
Ruppert P, Cook L, Dean F, & Reiter W (1983) Acute behavioural
toxicity of carbaryl and propoxur in adult rats. Pharmacol Biochem
Behav, 18(4): 579-584.
Ryan AJ (1971) The metabolism of pesticidal carbamates. CRC Crit Rev
Toxicol, 1: 35-54.
Rybakova MN (1966) [Toxic effect of Sevin on animals.] Gig i Sanit,
31(9): 42-47 (in Russian with English summary).
Rybakova MN (1967) [Comparative toxic effect of Sevin and DDT used
in the treatment of food crops.] Vopr Pitan, 26: 9-15 (in Russian
with English summary).
Saini ML & Dorough HW (1978) Metabolism of carbaryl-naphthyl-C14
in a lactating cow, when administered orally. In: Edwards CA,
Veeresh GK, & Krueger HR ed. Proceedings of the Symposium on
Pesticide Residues in the Environment in India, 7-10 November 1978.
Hebbal, Bangalore, University of Agricultural Sciences, pp 74-87
(UAS Technical Series No. 32).
Sakai K & Matsumura F (1971) Degradation of certain organophosphate
and carbamate insecticides by human brain esterases. Toxicol Appl
Pharmacol, 19: 660-666.
Samanidou V, Fytianos K, Pfister G, & Bahadir M (1988) Photochemical
decomposition of carbamate pesticides in natural waters of Northern
Greece. Sci Total Environ, 76(1): 85-92.
Sanders HO & Cope DB (1966) Toxicity of several pesticides in two
species of Cladocerans. Trans Am Fish Soc, 95(2): 165-169.
Sanders HO, Finley MT, & Hunn JB (1983) Acute toxicity of six forest
insecticides to three aquatic invertebrates and four fishes.
Washington, DC, US Department of the Interior, Fish and Wildlife
Service, 5 pp (Technical Paper 110).
Sanderson DM (1961) Treatment of poisoning by anticholinesterase
insecticides in the rat. J Pharm Pharmacol, 13: 435-442.
Santolucito JA & Morrison G (1971) EEC of Rhesus monkeys following
prolonged low level feeding of pesticides. Toxicol Appl Pharmacol,
19: 147-154.
Santolucito JA & Whitcomb E (1971) Mechanical response of skeletal
muscle following oral administration of pesticides. Toxicol Appl
Pharmacol, 20: 66-72.
Schafer EA (1972) The acute oral toxicity of 369 pesticidal,
pharmaceutical and other chemicals to wild birds. Toxicol Appl
Pharmacol, 21: 315-330.
Schomburg CJ, Glottfeldy DE, & Seiber JN (1991) Pesticide occurrence
and distribution in fog collected near Monterey, California. Environ
Sci Technol, 25: 155-160.
Schulze TL, Hauge PN, Mitchell WJ, Remley WJ, Shahied SI, & Marshall
FJ (1979) New Jersey Epidemiologic Studies Program-Pesticides -
Assessment of human exposure to carbaryl during aerial application
for Gypsy moth control in New Jersey. New Jersey State Department of
Health.
Seiler JP (1977) Nitrosation in vivo and in vitro by sodium
nitrate and mutagenicity of nitrogenous pesticides. Mutat Res, 48:
225-236.
Shabanov M, Toshkov A, Georgiev D, & Ibrishimov N (1983) [Effect of
carbaryl on experimental Erysipelothrix rhusiopathiae infection in
rats.] Vet Med Nauk, 20(5-6): 9-15 (in Russian).
Shafik MT, Sullivan HC, & Enos NF (1971) A method for the
determination of 1-naphthol in urine. Bull Environ Contam Toxicol,
6(1): 34-39.
Shah PV & Guthrie FE (1983) Percutaneous penetration of three
insecticides in rats: a comparison of two methods for in vivo
determination. J Invest Dermatol, 80(4): 291-293.
Shah PV, Monroe RV, & Guthrie FE (1983) Comparative penetration of
insecticides in target and nontarget species. Drug Chem Toxicol,
6(2): 155-179.
Shah PV, Fisher HL, Sumler MR, Monroe RJ, Chernoff N, & Hall LL
(1987) Comparison of the penetration of 14 pesticides through the
skin of young and adult rats. J Toxicol Environ Health, 21(3):
353-366.
Sharma VK & Kaur S (1990) Contact sensitization by pesticides in
farmers. Contact Dermatitis, 23(2): 77-80.
Sharom MS, Miles JRW, Harris CR, & McEwen FL (1980) Behaviour of 12
insecticides in soil and aqueous suspensions of soil and sediment.
Water Res, 14(8): 1095-1100.
Shea TB (1985) Carbaryl inhibition of interferon synthesis in
cultured goldfish cells. Bull Environ Contam Toxicol, 34: 109-113.
Shea TB & Berry ES (1983) Toxicity of carbaryl and 1-naphthol to
goldfish (Carassius auratus) and Killifish (Fundulus
heteroclitus). Bull Environ Contam Toxicol, 31: 526-529.
Shea TB & Berry ES (1984) Suppression of interferon synthesis by the
pesticide carbaryl as a mechanism for enhancement of goldfish
virus-2 replication. Appl Environ Microbiol, 47(2): 250-252.
Shehata T, Richardson E, & Cotton E (1984) Assessment of human
population exposure to carbaryl from the 1982 Main Spruce budworm
spray project. J Environ Health, 46(6): 293-297.
Shilova SA, Denisova AV, Sedykh EL, & Efron KM (1973) [After effects
of insecticide treatment in the subartics.] Zool Zh, 52(7):
1008-1012 (in Russian).
Shimkin MB, Wieder R, McDonough M, Fishbein L, & Swern D (1969) Lung
tumour response in strain A mice as a quantitative bioassay of
carcinogenic activity of some carbamates and aziridines. Cancer Res,
29: 2184-2190.
Shpirt MB (1973) [Toxicological assessment of DDT, HCHC, TMTD, Sevin
and Zineb acting on human cell cultures.] Gig Tr Prof Zabol, 14:
32-35 (in Russian).
Shtenberg AI & Hovaeva LA (1970) [The effect of Sevin on the state
of thyroid gland of rats having received artificial full-value
ration with different content of iodine.] Gig i Sanit, 8: 119-122
(in Russian).
Shtenberg AI & Ozhovan MV (1971) [The effect of small doses of Sevin
upon the reproduction function of animals in several generations.]
Vopr Pitan, 1: 42-47 (in Russian).
Shtenberg AI & Rybakova MN (1968) Effect of carbaryl on the
neuroendocrine system in rats. Food Cosmet Toxicol, 6: 461-467.
Shtenberg AI, Orlova NV, Pozdnyakov AL, Tropova GP, Zhalbe JP,
Khovayeva LA, & Akincheva MJ (1970) [Functional and morpho-logical
parallels associated with the state of the sex glands of animals
under the influence of Sevin.] In: [Problems of the hygiene and
toxicology of pesticides.] Moscow, Meditsina, pp 124-129 (in
Russian).
Shtenberg AI, Orlova NV, & Torchinskij AM (1973) [On the effect of
pesticides of different chemical structure on gonads and
embryogenesis in experimental animals.] Gig i Sanit, 8: 16-20 (in
Russian).
Shukla GS, Omkar & Upadhyay VB (1982) Acute toxicity of few
pesticides to an aquatic insect, Ranatra elongata (Fabr.) J Adv
Zool, 3(2): 148-150.
Siboulet R, Grinfeld S, Deparis P, & Jaylet A (1984) Micronuclei in
red blood cells of the newt Pleurodeles waltl Michah: Induction
with X rays and chemicals. Mutat Res, 125(2): 275-281.
Siebert D & Eisenbrand G (1974) Induction of mitotic gene
conversions in Saccharomyces cerevisiae by N-nitrosated
pesticides. Mutat Res, 22: 121-126.
Sikka HC, Miyazaki S, & Rice CP (1973) Metabolism of selected
pesticides by marine microorganisms. Springfield, Virginia, National
Technical Information Service (Syracuse University Annual Report No.
2 - NTIS AS AD-763 410).
Sikka HC, Miyazaki S, & Lynch RS (1975) Degradation of carbaryl and
1-naphthol by marine microorganisms. Bull Environ Contam Toxicol,
13(6): 666-672.
Simpson JR (1965) Exposure to orchard pesticides, dermal and
inhalation exposures. Arch Environ Health, 10: 884-885.
Singh JM (1973) Decreased performance behaviour with carbaryl - an
indication of clinical toxicity. Clin Toxicol, 6(1): 97-108.
Singh KP, Pandey SY, Divekar VV, Surana KC, & Paralikar AB (1978)
Dissipation of carbaryl residues in and on cauliflower. In: Edwards
CA, Veeresh GK, & Krueger HR ed. Proceedings of the Symposium on
Pesticide Residues in the Environment in India, 7-10 November 1978.
Hebbal, Bangalore, University of Agricultural Sciences, pp 210-214
(UAS Technical Series No. 32).
Singh VP, Gupta S, & Saxena PK (1984) Evaluation of acute toxicity
of carbaryl and malathion to freshwater teleosts, Chauna punctatis
(bloch) and Heteropneustes fossilis (bloch). Toxicol Lett, 20:
271-276.
Sjoblad RD, Minard RD, & Bollag JM (1976) Polymerisation of
1-naphthol and related phenolic compounds by an extracellular fungal
enzyme. Pestic Biochem Physiol, 6: 457-463.
Skraba WJ & Young FG (1959) Radioactive Sevin (1-naphthyl-
1-carbon-14 N-methylcarbamate): a convenient synthesis. Agric Food
Chem, 7(9): 612-613.
Smalley HE (1970) Diagnosis and treatment of carbaryl poisoning in
swine. J Am Vet Med Assoc, 156(3): 339-344.
Smalley HE, Curtis JM, & Earl FL (1968) Teratogenic action of
carbaryl in beagle dogs. Toxicol Appl Pharmacol, 13: 392-403.
Smalley HE, O'Hara PJ, Bridges CH, & Radeleef RD (1969) The effect
of chronic carbaryl administration on the neuromuscular system of
swine. Toxicol Appl Pharmacol, 14: 409-419.
Snow CD & Stewart NE (1963) Treatment of Tikamook Bay oyster beds
with MGS-90 (Sevin) (Unpublished report from the Oregon Fish
Commission, submitted to WHO by Rhône-Poulenc Agro, Lyon).
Söderpalm BC & Önfelt A (1988) The action of carbaryl and its
metabolite alpha naphthol on mitosis in V79 Chinese hamster
fibroblasts. Indications of the involvement of some cholinester in
cell division. Mutat Res, 201(2): 349-363.
Sokur AJ (1971) [Hypokaliaemia in erythrocytes after intoxication of
white rats with some pesticides (DDT, Sevin, chlorophos).] Gig
Primen Toxicol Pestic Klin Otrav, 1971: 213-216 (in Russian).
Solomon H & Weis J (1979) Abnormal circulatory development in medaka
caused by the insecticides carbaryl, malathion and parathion.
Toxicology, 19: 51-62.
Spain AV (1974) The effects of carbaryl and DDT on the litter fauna
of a Corsican pine forest (Pinus nigra var. maritima): A
multivariate comparison. J Appl Ecol, 11: 467-481.
Springborn Bionomics, Inc. (1988a) Environmental fate of spray
applications of the rice insecticide Sevin XLR (a.i. carbaryl):
California (Study No. 565-6118/6112). Wareham, Massachusetts,
Springborn Bionomics, Inc. (Unpublished proprietary information
submitted to WHO by Rhône-Poulenc Agro, Lyon).
Springborn Bionomics, Inc. (1988b) Environmental fate of spray
applications of the forest insecticide Sevin-4-oil (a.i. carbaryl):
Oregon (Study No. 565-6120/6114). Wareham, Massachusetts, Springborn
Bionomics, Inc. (Unpublished proprietary information submitted to
WHO by Rhône-Poulenc Agro, Lyon).
Springborn Bionomics, Inc. (1988c) Environmental fate of spray
applications of the forest insecticide Sevin-4-oil (a.i. carbaryl):
Pennsylvania (Study No. 565-6121/6115). Wareham, Massachusetts,
Springborn Bionomics, Inc. (Unpublished proprietary information
submitted to WHO by Rhône-Poulenc Agro, Lyon).
Stanley JG & Trial JG (1980) Disappearance constants of carbaryl
from streams contaminated by forest spraying. Bull Environ Contam
Toxicol, 25(5): 771-776.
Statham CN & Lech JJ (1975) Potentiation of the acute toxicity of
several pesticides and herbicides in trouts by carbaryl. Toxicol
Appl Pharmacol, 34: 83-87.
Stegeman LC (1964) The effects of the carbamate insecticide carbaryl
upon forest soil mites and Collembola. J Econ Entomol, 57(6):
803-808.
Stevenson JH (1978) The acute toxicity of unformulated pesticides to
worker honey bees ( Apis Mellifera L.). Plant Pathol, 27: 38-40.
Stevenson JH, Needham PH, & Walker J (1977) Poisoning of honey bees
by pesticides: Investigations of the changing pattern in Britain
over 20 years. Rothamsted Rep, 2: 55-66.
Stewart NE, Millemann RE, & Breese WP (1967) Acute toxicity of the
insecticide Sevin and its hydrolytic product 1-naphthol to some
marine organisms. Trans Am Fish Soc, 96: 25-30.
Strait JR, Thornwall GC, & Ehrich M (1991) Sensitive
high-performance liquid chromatographic analysis for toxicological
studies with carbaryl. Washington, DC, American Chemical Society.
Street JC & Sharma RP (1975) Alteration of induced cellular and
humoral immune responses by pesticides and chemicals of
environmental concern: Quantitative studies on immunosuppression by
DDT, Aroclor 1254, carbaryl, carbofuran and methylparathion. Toxicol
Appl Pharmacol, 32(5): 587-602.
Strother A (1970) Comparative metabolism of selected
1-methylcarbamates by human and rat liver fractions. Biochem.
Pharmacol, 19: 2525-2529.
Strother A & Wheeler L (1976) Disposition of 14C-carbaryl in
pregnant, nonpregant and fetal tissues of rat. Fed Proc, 35: 327.
Strother A & Wheeler L (1980) Excretion and disposition of
[14C]carbaryl in pregnant, non-pregant and foetal tissues of the
rat after acute administration. Xenobiotica, 10(2): 113-124.
Struble CB, Feil VJ, Pecas JC, & Gerst JW (1983a) Biliary secretion
of 14C and identification of 5,6-dihydro-5,6-dihydroxycarbaryl
glucoronide as a biliary metabolite of (14C) carbaryl in the rat.
Pestic Biochem Physiol, 19: 85-94.
Struble CB, Pecas JC, & Gerst JW (1983b) Enterohepatic circulation
and biliary secretion of 5,6-dihydro-5,6-dihydroxy(14C)carbaryl
glucoronide in the rat. Pestic Biochem Physiol, 19(1): 95-103.
Subbotina SG & Belonozhko GA (1968) [The effect of Sevin on the
thermoresistance and fractionary protein content of blood serum.]
Gig Primen Toxicol Pestic Klin Otrav, 1968: 442-444 (in Russian).
Sud RK, Sud AK, & Gupta KG (1972) Degradation of Sevin (1-naphthyl
N-methylcarbamate) by Achromobacter sp. Arch Microbiol, 87:
353-358.
Sullivan LJ, Chin BH, & Carpenter CP (1972) in vitro vs. in vivo
chromatographic profiles of carbaryl anionic metabolites in man and
lower animals. Toxicol Appl Pharmacol, 22: 161-174.
Sundaram KMS & Szeto SY (1987) Distribution and persistence of
carbaryl in some terrestrial and aquatic components of a forest
environment. J Environ Sci Health, B22(5): 579-599.
Surprenant DC (1985a) Acute toxicity of carbaryl technical to
embryo-larvae of eastern oysters (Crassostrea virginica). Wareham,
Massachusetts, Springborn Bionomics, Inc. (Report No. BW-85-7-1817)
(Unpublished proprietary information submitted to WHO by
Rhône-Poulenc Agro, Lyon).
Surprenant DC (1985b) Acute toxicity of carbaryl technical to mysid
shrimp (Mysidopsis bahia). Wareham, Massachusetts, Springborn
Bionomics, Inc. (Report No. BW-85-6-1790) (Unpublished proprietary
information submitted to WHO by Rhône-Poulenc Agro, Lyon).
Surprenant DC (1985c) The chronic toxicity of carbaryl technical to
Daphnia magna under flow-through conditions. Wareham,
Massachusetts, Springborn Bionomics, Inc. (Report No. BW-85-7-1813)
(Unpublished proprietary information submitted to WHO by
Rhône-Poulenc Agro, Lyon).
Sussman HF, Cooke SB, & Bloch V (1969) Human louse infestation;
treatment with carbacide. Arch Dermatol, 100(1): 82-83.
Swartz WJ (1981) Long- and short-term effects of carbaryl exposure
in chick embryos. Environ Res, 26(2): 463-471.
Swartz WJ (1985) Effects of carbaryl on gonadal development in the
chick embryo. Bull Environ Contam Toxicol, 34: 481-485.
Szczepaniak S & Jeleniewicz K (1980) [Alterations of free amino
acids concentrations in rat blood erythrocytes following the acute
intoxication with carbaryl.] Bromatol Chem Toksykol, XIII(2):
159-163 (in Polish with English summary).
Szczepaniak S & Jeleniewicz K (1981) [ In vitro binding of carbaryl
and alpha naphthol with plasma and erythrocytes amino acids.]
Bromatol Chem Toksykol, XIV(2): 165-168 (in Polish with English
summary).
Szczepaniak S & Jeleniewicz K (1986) [Body amino acids balance in
acute poisoning with carbaryl.] Farm Pol, 9: 475-479 (in Polish).
Szczepaniak S, Siemkievicz E, Jeleniewicz K, & Nowinski H (1980)
[Effects of carbaryl and chlorfenvinfos on the level of free amino
acids in rat blood serum. Part II. Intoxication with a single dose
corresponding to 1/5 LD50.] Bromatol Chem Toksykol, XIII(4):
351-353 (in Polish with English summary).
Szeto SY, MacCarty HR, Oloffs PC, & Shephard RF (1979) The fate of
acephate and carbaryl in water. J Environ Sci Health, B14(6):
635-654.
Tagatz ME, Ivey JM, & Lehman HK (1979) Effects of Sevin on
development of experimental estuarine communities. J Toxicol Environ
Health, 5: 643-651.
Takahashi RN, Poli A, Morato GS, Lima TCM, & Zanin M (1991) Effects
of age on behavioral and physiological responses to carbaryl in
rats. Neurotoxicol Teratol, 13: 21-26.
Tewfik MS & Hamdi YA (1970) Decomposition of Sevin by a soil
bacterium. Acta Microbiol Pol Ser B, 19(2): 133-135.
Thomas A (1981) Introductory remarks: The testes. Environ Health
Perspect, 38: 3-4.
Thomas SJ (1986) Sevin brand carbaryl insecticide: Magnitude of
carbaryl residues in sugar beet roots (File No. 34934). Research
Triangle Park, North Carolina, Union Carbide Agricultural Products
Co., Inc. (Prepared in collaboration with Hazleton Laboratories
America, Inc., Chemical and Biomedical Sciences Division, Madison)
(Unpublished proprietary information submitted to WHO by
Rhône-Poulenc Agro, Lyon.
Thomas JH, Dieringer CS, & Schein L (1974) Effects of carbaryl on
mouse organs of reproduction. Toxicol Appl Pharmacol, 28: 142-145.
Thomas J, Visalakshi A, & Mohandas N (1982) Contamination of water
in rice fields sprayed with carbaryl for insect control. Entomon,
7(3): 379-380.
Thompson AR (1971) Effects of nine insecticides on the numbers and
biomass of earthworms in pasture. Bull Environ Contam Toxicol, 5(6):
577-586.
Tilak KS, Rao DMR, Devi AP, & Murty AS (1981) Toxicity of carbaryl
and 1-naphthol to four species of freshwater fish. J Biosci, 3(4):
457-462.
Tilden RL & Van Middelem CH (1970) Determination of carbaryl as an
amide derivative by electroncapture gas chromatography. J Agric Food
Chem, 18(1): 154-158.
Ting KC, Kho PK, Musselman AS, Root JA, & Tichelaar R (1984) High
performance liquid chromatographic method for determination of six
N-methylcarbamates in vegetables and fruits. Bull Environ Contam
Toxicol, 33: 538-547.
Tobin JS (1970) Carbofuran. A new carbamate insecticide. J Occup
Med, 12(1): 16-19.
Toor HS & Kaur K (1974) Toxicity of pesticides to the fish Cyprinus
carpio communis Linn. Indian J Exp Biol, 12: 334-336.
Tos-Luty S, Puchala-Matysek W, & Latuszynska J (1973) [Carbaryl
toxicity in chick embryo.] Bromatol Chem Toksykol, 6: 409-411 (in
Polish with English summary).
Trial JG (1978) The effects of Sevin-4-oil on aquatic insect
communities of streams: a continuation of 1976 studies. In:
Environmental monitoring of cooperative spruce budworm control
projects, Maine 1976 and 1977. Augusta, Maine, Maine Department of
Conservation, Forest Service, pp 124-140.
Trial JG (1979) The effects of Sevin-4-oil on aquatic insect
communities of stream (1976-1978). In: Environmental moitoring of
cooperative spruce budworm control projects, Maine 1978. Augusta,
Maine, Maine Department of Conservation, Forest Service, pp 6-22.
Triolo AJ, Lang WR, Coon JM, Lindstrom D, & Herr DL (1982) Effect of
the insecticide toxaphene and carbaryl on induction of lung tumors
by benzo(a)pyrene in the mouse. J Toxicol Environ Health, 9(4):
637-649.
Tripathi G & Shukla SP (1988) Toxicity bioassay of technical and
commercial formulations of carbaryl to the freshwater catfish,
Clarias batrachus. Ecotoxicol Environ Saf, 15(3): 277-281.
Tyburczyk W & Podolak-Majczak M (1984a) [Effect of joint action of
sodium nitrite and carbaryl on rats organism.] Bromatol Chem
Toksykol, XVII(2): 125-129 (in Polish with English summary).
Tyburczyk W & Podolak-Majczak M (1984b) [Effect of joint action of
sodium nitrite and carbaryl on rats organism.] Bromatol Chem
Toksykol, XVII(3): 215-219 (in Polish with English summary).
Tyburczyk W & Podolak-Majczak M (1986) [Effect of joint action of
sodium nitrite and carbaryl on rats organism. Part V. The role of
glucose-6-phosphate dehydrogenase and haematologic studies.]
Bromatol Chem Toksykol, XIX(3): 167-173 (in Polish with English
summary).
Uchiyama M, Takeda M, Suzuki T, & Yoshikawa K (1975) Mutagenicity of
nitroso derivatives of N-methylcarbamate insecticides in
microbiological method. Bull Environ Contam Toxicol, 14: 389-394.
US Department of Labour (1978) Occupational health guideline for
carbaryl (Sevin/R). Washington, DC, US Department of Labour, pp 1-5.
US EPA (1977) Aspects of pesticidal uses of carbaryl on man and the
environment. Washington, DC, US Environmental Protection Agency,
Office of Pesticide Programs, 286 pp.
US EPA (1980a) Carbaryl. Decision document. Washington, DC, US
Environmental Protection Agency, Office of Pesticides and Toxic
Substances, 22 pp.
US EPA (1980b) FIFRA Scientific Advisory Panel. Subcommittee
meeting, Arlington, Virginia, 23 July 1980. Washington, DC, US
Environmental Protection Agency, 202 pp.
US EPA (1981) Environmental fates and impacts of major forest use of
pesticides. Washington, DC, US Environmental Protection Agency,
Office of Pesticides and Toxic Substances.
US EPA (1984) Health and environmental profile for carbaryl.
Cincinnati. Ohio, US Environmental Protection Agency.
Usha Rani MV, Reddi OS, & Reddy PP (1980) Mutagenicity studies
involving aldrin, endosulfan, demethoate, phosphamidon, carbaryl and
ceresan. Bull Environ Contam Toxicol, 25(2): 277-282.
Vandekar M (1965) Observations of the toxicity of carbaryl,
folithion and 3-isopropylphenyl N-methylcarbamate in a village
scale trail in Southern Nigeria. Bull World Health Organ, 33:
107-115.
Vandekar M, Plestina R, & Wilhelm M (1971) Toxicity of carbamates
for mammals. Bull World Health Organ, 44: 241-249.
Varshney TN & Gaur AC (1972) Effect of DDT and Sevin on soil fungi.
Acta Microbiol Acad Sci Hung, 19: 97-102.
Vashakidze VI (1966) [On the question of the embryotropic,
gonadotropic and mutagenic effect of some pesticides.] Gig Primen
Toxicol Pestic Klin Otrav, 1966: 80-83 (in Russian).
Vashakidze VI (1975) [Effect of small doses of Sevin on gonad
function following its repeated effect on white rats SbTr NII.] Gig
Tr Prof Zabol, 14: 235-266 (in Russian).
Vasilos AF, Dmitrienko VD, & Shroyt IG (1975) [Disruption of the
mitotic system following acute Sevin poisoning.] Izv Akad Nauk
Moldavskoj SSR Ser Khim Nauk, 3: 64-67 (in Russian).
Venkateswarlu K, Chendrayan K, & Sethunathan N (1980) Persistence
and biodegradation of carbaryl in soils. J Environ Sci Health,
B15(4): 421-429.
Venkat Reddy P, Prem Veer Reddy G, & Subramanyams S (1974)
Cytological effects of the insecticide Sevin on the mitotic cells of
Poecilocerus pictus. Curr Sci, 43(6): 187-189.
Verma SR, Tonk IF, Gupta AK, & Saxena M (1984) Evaluation of an
application factor for determining the safe concentration of
agricultural and industrial chemicals. Water Res, 18(1): 111-115.
Viter VF (1978) [Monotonous and intermittent inhalatory effect of
Sevin upon some behavioural reactions of experimental animals.] Gig
i Sanit, 11: 33-34 (in Russian).
Vyatchanikov KA & Alexachina ZA (1968) [Experimental investigations
for procuring therapeutic and protective means at intoxications by
Sevin.] Gig Primen Toxicol Pestic Klin Otrav, 1968: 442-444 (in
Russian).
Wagner R (1973) [Identification and estimation of organophosphate
insecticides and carbaryl on ready made thin-layer plates.] Z
Gesamte Hyg Grenzgeb, 19(7): 504-510 (in German).
Wakakura M, Ishikawa S, & Uga S (1978) Ultrastructural hepatic
changes by carbamate pesticide (Sevin) in rats. Environ Res, 16:
191-204.
Walker N, Janes NF, Spokes JR, & Van Berkum P (1975a) Degradation of
1-naphthol by a soil pseudomonad. J Appl Bacteriol, 39: 281-286.
Walker M, Gale GR, Atkins LM, & Gadse-Den RH (1975b) Some effects of
carbaryl on Ehrlich ascites tumor cells in vitro and in vivo.
Bull Environ Contam Toxicol, 14(4): 441-448.
Waller GD (1969) Susceptibility of an alfalfa leaf-cutting bee to
residues of insecticides on foliage. J Econ Entomol, 62(1): 189-192.
Ward SA, May DG, Heath AJ, & Branch RA (1988) Carbaryl metabolism is
inhibited by Cimetidine in the isolated perfused rat liver and in
man. J Toxicol Clin Toxicol, 26(5/6): 269-281.
Wauchope RD & Haque R (1973) Effects of pH, light and temperature on
carbaryl in aqueous media. Bull Environ Contam Toxicol, 9(5):
257-260.
Weber FJ, Shea TB, & Berry SE (1982) Toxicity of certain
insecticides to protozoa. Bull Environ Contam Toxicol, 28: 618-631.
Weil CS & Carpenter CP (1962) Studies on carcinogenesis completed in
1962. Pittsburgh, Pennsylvania, Carnegie-Mellon Research Institute
(Special Report 25-122) (Unpublished proprietary information
submitted to WHO by Rhône-Poulenc Agro, Lyon).
Weil CS & Carpenter CP (1965) Results of a three generations
reproductive study on rats fed Sevin in their diet. Pittsburgh,
Pennsylvania, Carnegie-Mellon Research Institute, 18 pp (Report No.
28-53) (Unpublished proprietary information submitted to WHO by
Rhône-Poulenc Agro, Lyon).
Weil CS & Carpenter CP (1974) Sevin: range finding toxicity studies.
Pittsburgh, Pennsylvania, Carnegie Mellon (Report No. 37-53)
(Unpublished proprietary information submitted to WHO by
Rhône-Poulenc Agro, Lyon).
Weil CS, Woodside MD, Carpenter CP, & Smyth HF Jr (1972) Current
studies of tests of carbaryl for reproductive and teratogenic
effect. Toxicol Appl Pharmacol, 21: 390-404.
Weil CS, Woodside MD, Bernard JB, Condra NI, King JM, & Carpenter CP
(1973) Comparative effect of carbaryl on rat reproduction and
guinea-pig teratology when fed either in the diet or by stomach
intubation. Toxicol Appl Pharmacol, 26: 621-638.
Weston RF, Inc. (1982) Carbaryl: A profile on its characteristics as
pesticide. Rockville, Maryland, Roy F. Weston, Inc., Environmental
Health Sciences Centre.
Whitehurst WE, Bishop ET, Critchfield FE, Gyrisco GG, Huddleston EW,
Arnold H, & Lisk OJ (1963) The metabolism of Sevin in dairy cows. J
Agric Food Chem, 11: 167-169.
WHO (1982) Recommended health-based limits in occupational exposure
to pesticides. Report of a WHO study group. Geneva, World Health
Organization, 110 pp (WHO Technical Report Series 677).
WHO (1986) IPCS Environmental Health Criteria 64: Carbamate
pesticides - A general introduction. Geneva, World Health
Organization, 136 pp.
WHO (1992) The WHO recommended classification of pesticides by
hazard and guidelines to classification 1992-1993. Geneva, World
Health Organization, 66 pp (Unpublished document WHO/PCS/92.14).
WHO/FAO (1975) Data sheets on pesticides No. 3: Carbaryl. Geneva,
World Health Organization, 8 pp (Unpublished document VBC/DS/75.3,
Rev. 1).
Whorton MD, Milby TH, Stubbs HA, Avasha BH, & Hull EG (1979)
Testicular function among carbaryl exposed employees. J Toxicol
Environ Health, 5: 925-941.
Wiggins OG & Weiden MHJ (1969) Fate of Sevin applied to the surface
of bean fruit and bean plants under field conditions. Clayton, North
Carolina, Union Carbide Corporation (Report No. 11377) (Unpublished
proprietary information submitted to WHO by Rhône-Poulenc Agro,
Lyon).
Wilkes L, Mulkey N, & Wargo J (1977) Research report laboratory soil
metabolism study of carbaryl (Project No. 285). Monument, Colorado,
Analytical Development Corp. (Unpublished proprietary information
submitted to WHO by Rhône-Poulenc Agro, Lyon).
Wills JH, Jameson E, & Coulston F (1968) Effects of oral doses of
carbaryl on man. Clin Toxicol, 1:(3) 265-271.
Wiltrout RW, Ercegovich CD, & Ceglowski WS (1978) Humoral immunity
in mice following oral administration of selected pesticides. Bull
Environ Contam Toxicol, 20(3): 423-431.
Winterlin W & Walker G (1973) Carbaryl residues in bees, honey, and
bee bread following exposure to carbaryl via the food supply. Arch
Environ Contam Toxicol, 1(4): 362-374.
Wojciechowski JP, Kaur P, & Sabharual PS (1982) Induction of ouabain
resistance in V-79 cells by four carbamate pesticides. Environ Res,
29: 48-53.
Wolfe HP, Durham W, & Armstrong J (1967) Exposure of workers to
pesticides. Arch Environ Health, 14: 622-633.
Wolfe NL, Zepp RG, & Paris DF (1978) Carbaryl, propham and
chlorpropham: A comparison of the rates of hydrolysis and photolysis
with the rate of biolysis. Water Res, 12(8): 565-571.
Woodruff RC, Phillips JP, & Irwin D (1983) Pesticide-induced
complete and partial chromosome loss in screens with
repair-defective females of Drosophila melanogaster. Environ
Mutagen, 5: 835-846.
Woodward DF & Mauck WL (1980) Toxicity of five forest insecticides
to cutthroat trout and two species of aquatic invertebrates. Bull
Environ Contam Toxicol, 25: 846-853.
Wright CG, Leidy RB, & Dupress HE (1981) Insecticides in the ambient
air of rooms following their application for control of pests. Bull
Environ Contam Toxicol, 26(4): 548-553.
Wuu K & Grant W (1966) Morphological and somatic chromosomal
aberrations induced by pesticides in barley (Hordeum vulgare). Can
J Genet Cytol, 8: 481-501.
Wyrobek AJ, Watchmaker G, Gordon L, Wong K, Moore D, & Whorton D
(1981) Sperm shape abnormalities in carbaryl-exposed employees.
Environ Health Perspect, 40: 225-265.
Yadav PR & Jaglan RS (1982) Persistence of carbaryl residues in and
on unprocessed and processed cauliflower curds. Beitr Trop
Landwirtsch Veterinärmed, 20(1): 89-95.
Yadav GS, Kathpal TS, & Singh H (1985) Efficacy of monocrotophos,
carbaryl, and endosulfan against Antigastra catalaunalis Dup. and
their persistence in soil and sesamum seeds. Indian J Agric Sci,
55(6): 438-442.
Yakim VS (1965) [Toxicological hygienic characteristics of Sevin.]
Gig Primen Toxicol Pestic Klin Otrav, 1965: 423-431 (in Russian).
Yakim VS (1967) [Data for substantiating the MAC values for Sevin in
the air of the working zone.] Gig i Sanit, 4: 23-33 (in Russian).
Yakim VS (1968) [The toxicity of Sevin at inhalatory intake.] Gig
Primen Toxicol Pestic Klin Otrav, 1968: 115-117 (in Russian).
Yakim VS (1970) [Content and distribution of Sevin in warm-blooded
animals organism and its effect on ChE activity.] Gig Primen Toxicol
Pestic Klin Otrav, 1970: 214-218 (in Russian).
Yess NJ, Houston MG, & Gunderson EL (1991a) Food and Drug
Administration pesticide residue monitoring of foods: 1978-1982. J
Assoc Off Anal Chem, 75(2): 265-272.
Yess NJ, Houston MG, & Gunderson EL (1991b) Food and Drug
Administration pesticide residue monitoring of foods: 1983-1986. J
Assoc Off Anal Chem, 74(2): 273-280.
Young RR (1990) Mutagenicity test on carbaryl (technical) in the
CHO/HGPRT forward mutation assay. Kensington, Maryland, Hazleton
Laboratories America, Inc. (Study No. 10862-0-435) (Unpublished
proprietary information submitted to WHO by Rhône-Poulenc Agro,
Lyon).
Zabezhinski MA (1970) [Possible carcinogenic effect of ß-Sevin.]
Vopr Onkol, 16: 106-107 (in Russian).
Zapko WG (1970) [Effect on some liver functions of acute
intoxication of experimental animals with cuprosin, Sevin, Eptam and
TMTD.] Gig Primen Toxicol Pestic Klin Otrav, 1970: 237-240 (in
Russian).
Zeakes SJ, Hansen MF, & Robel RJ (1981) Increased susceptibility of
Bobwhites (Colinus virginianus) to Histomonas meleagridis after
exposure to Sevin insecticide. Avian Dis, 25(4): 981-987.
Zepp RG & Cline DM (1977) Rates of direct photolysis in the aquatic
environment. Environ Sci Technol, 11: 359-366.
Zepp RG, Wolfe N, Gordon L, & Fincher RC (1976) Light-induced
transformations of methoxychlor in aquatic systems. J Agric Food
Chem, 24: 727-733.
Zinkl JG, Hennys ChJ, & Dewelse LR (1977) Brain cholinesterase
activity in birds from forest sprayed trichlorfon (Dylox) and
carbaryl (Sevin-4-oil). Bull Environ Contam Toxicol, 17(4):
379-386.
Zuberli R & Zubairi MY (1971) Carbaryl degradation by Pseudomonas
phaseolicola and Aspergillus niger. Pak J Sci Ind Res, 14(4-5):
383-384.
Zweig G, Gao R-Y, Witt JM, Propendorf W, & Bogen K (1984) Dermal
exposure to carbaryl by strawberry harvesters. J Agric Food Chem,
32(6): 1232-1236.
Zweig G, Leffingwell JT, & Propendorf W (1985) The relationship
between dermal pesticide exposure to fruit harvesters and
dislodgeable foliar residues. J Environ Sci Health, B20(1): 27-59.
ANNEX I. TREATMENT OF CARBAMATE PESTICIDE POISONING IN MAN
(From EHC 64: Carbamate Pesticides - A General Introduction)
All cases of carbamate poisoning should be dealt with as an
emergency and the patient should be hospitalized as quickly as
possible.
Extensive descriptions of the treatment of poisoning by
anticholinesterase agents are given in several major references
(Kagan, 1977;Taylor, 1980; Plestina, 1984).
The treatment is based on:
(a) minimizing the absorption;
(b) general supportive treatment; and
(c) specific pharmacological treatment.
I.1 Minimizing the absorption
When dermal exposure occurs, decontamination procedures include
removal of contaminated clothes and washing of the skin with
alkaline soap or with a sodium bicarbonate solution. Particular care
should be taken in the cleaning of the skin area where venupuncture
is performed. Blood might be contaminated with carbamates and
therefore inaccurate measures of ChE inhibition might result.
Extensive eye irrigation with water or saline should also be
performed. In the case of ingestion, vomiting can be induced, if the
patient is conscious, by the administration of ipecacuanha syrup
(10-30 ml) followed by 200 ml of water. However, this treatment is
contraindicated in the case of pesticides dissolved in hydrocarbon
solvents. Gastric lavage (with the addition of bicarbonate solution
or activated charcoal) can also be performed, particularly in
unconscious patients, taking care to prevent aspiration of fluids
into the lungs (i.e., only after a tracheal tube has been put in
place).
The volumes of the fluids introduced in the stomach should be
recorded and samples of gastric lavage frozen and stored for
subsequent chemical analysis. If the formulation of the pesticide
involved is available, it should also be stored for further analysis
(i.e., detection of toxicologically relevant impurities). A purge to
remove the ingested compound can be administered.
I.2 General supportive treatment
Artificial respiration (via a tracheal tube) should be started
at the first sign of respiratory failure and maintained for as long
as necessary.
Cautious administration of fluids is advised as well as general
supportive and symptomatic pharmacological treatment and absolute
rest.
I.3 Specific pharmacological treatment
I.3.1 Atropine
Atropine should be given, beginning with 2 mg iv repeated at 15
to 30-min intervals. The dose and the frequency of atropine
treatment varies from case to case, but should maintain the patient
fully atropinized (dilated pupils, dry mouth, skin flushing, etc.).
I.3.2 Oxime reactivations
Although it might be suspected that oxime cholinesterase
reactivators would be as helpful in carbamate poisoning as they are
in organophosphorous poisoning, this is not the case. There is
experimental evidence that the pyridinium oxime 2-PAM is not
effective in carbamate poisoning and there is some evidence that it
makes poisoning by certain carbamates, including carbaryl, worse.
I.3.3 Diazepam
Diazepam should be included in the therapy of all but the
mildest cases. Besides relieving anxiety it appears to counteract
some aspects of CNS-derived symptoms that are not affected by
atropine. Doses of 10 mg sc or iv are appropriate and may be
repeated as required.
Other centrally acting drugs and drugs that may depress
respiration are not usually recommended in the absence of artificial
respiration procedures.
References to Annex I
KAGAN, J.S. (1977) [The toxicity of organophosphorus pesticides.]
Moscow (in Russian).
PLESTINA, R. (1984) Prevention, diagnosis, and treatment of
insecticide poisoning. Geneva, World Health Organization.
(Unpublished WHO document VBC/84.889).
TAYLOR, P. (1980) Anticholinesterase agents. In: Goodman, L.S. &
Gilman, A., ed. The pharmacological basis of therapeutics. 6th ed.,
New York, Macmillan Publishing Co., pp. 100-119.
RESUME ET EVALUATION, CONCLUSIONS ET RECOMMANDATIONS
1. Résumé et évaluation
1.1 Identité, propriétés et méthodes d'analyse
Carbaryl est la dénomination commune d'un dérivé de l'acide
carbamique, le N-méthylcarbamate de 1-naphtyle. Le produit
technique consiste en un solide cristallin blanc, peut volatil et
peu soluble dans l'eau, qui est stable à la lumière et à la chaleur
mais qui s'hydrolyse facilement en milieu alcalin. Il existe une
norme FAO pour le carbaryl qui stipule un degré de pureté de 98%,
avec une limite pour les impuretés ( N-méthylcarmate de ß-naphtyle)
de 0,05%.
Pour les analyses portant sur le carbaryl et ses métabolites,
il existe de nombreuses méthodes: chromatographie sur couche mince,
spectrophotométrie, chromatographie en phase gazeuse,
chromatographie en phase liquide à haute pression et spectrométrie
de masse à ionisation chimique. La limite de détection peut
descendre en-dessous du nanogramme et le taux de récupération
dépasse généralement 80%.
1.2 Production et usages
On utilise le carbaryl depuis une trentaine d'années comme
insecticide agissant par contact et par ingestion avec certaines
propriétés endothérapiques; il permet de lutter contre de nombreux
vecteurs et ravageurs. La principale unité de production se trouve
aux Etats-Unis d'Amérique. Plus de 290 fabricants proposent une
gamme de formulations de carbaryl qui dépasse les 1500 produits.
1.3 Transport, distribution et transformation dans l'environnement
Dans la plupart des conditions, le carbaryl ne persiste pas
dans l'environnement. Dans l'eau, son temps de demi-hydrolyse dépend
de la température, du pH et de la concentration initiale; il varie
de quelques minutes à plusieurs semaines. Le principal produit de
décomposition est le 1-naphtol.
L'accumulation du carbaryl, exprimée sous la forme d'un facteur
de bioconcentration dans l'environnement aquatique, et plus
précisément chez les poissons d'eau douce, varie de 14 à 75. Le
carbaryl s'adsorbe plus facilement sur les sols à forte teneur
organique que sur les sols sableux. Aux doses d'emploi usuelles, et
lorsqu'il est appliqué conformément "aux bonnes pratiques
agricoles", il se dissipe rapidement, avec une demi-vie de 8 jours à
un mois dans les conditions normales. Il arrive que le carbaryl soit
entraîné par les pluies ou par les travaux agricoles de la surface
vers les couches sous-jacentes (à un mètre de profondeur).
Le carbaryl peut contaminer la végétation, soit au cours de
l'épandage soit par migration à partir d'un sol contaminé.
La décomposition du carbaryl dans l'environnement dépend de son
degré de volatilisation, de photodécomposition et de dégradation
chimique ou microbienne dans le sol, l'eau et les végétaux. la
vitesse de décomposition est plus rapide en climat chaud.
1.4 Concentrations dans l'environnement et exposition humaine
Dans la population générale, le carbaryl est principalement
absorbé par la voie alimentaire.
Les résidus présents dans des échantillons de rations totales
sont relativement faibles, et ils vont de traces à 0,05 mg/kg. Aux
Etats-Unis d'Amérique, on estime que pendant les premières années
d'utilisation du carbaryl, l'ingestion journalière de carbaryl était
de 0,15 mg/personne/jour (dans 7,4% des composites); cette dose est
tombée à 0,003 mg/personne/jour en 1969 (dans seulement 0,8% des
composites). Pendant la période d'épandage, on peut retrouver
parfois du carbaryl dans les eaux de surface et les retenues d'eau.
La population peut être exposée au carbaryl lors d'opérations
de lutte de contre les ravageurs ou les vecteurs tant au domicile
que sur les aires de loisir.
Les travailleurs peuvent être exposés au carbaryl lors de la
fabrication, de la formulation, de l'emballage, du transport et du
stockage du produit ainsi que pendant son épandage. Les
concentrations dans l'atmosphère des lieux de travail au cours de la
production varient de < 1 mg/m3 à 30 mg/m3. Il peut y avoir une
importante exposition cutanée chez les travailleurs de l'industrie
et les ouvriers agricoles en cas de mesures de protection
insuffisantes.
1.5 Cinétique et métabolisme
Le carbaryl est rapidement absorbé au niveau des poumons et des
voies digestives. Une dose de carbaryl dans l'acétone appliquée sur
la peau de volontaires humains a été absorbée par voie percutanée à
raison de 45% en 8 heures. Toutefois, les données obtenues in vitro
concernant la pénétration cutanée ainsi que les données
toxicologiques indiquent que l'absorption percutanée s'effectue
généralement à un rythme beaucoup plus lent.
Les principales voies métaboliques du carbaryl consistent en
une hydroxylation du cycle et une hydrolyse. Il en résulte la
formation de nombreux métabolites qui subissent ensuite une
conjugaison avec formation de sulfates, de glucuronides et de
mercapturates hydrosolubles qui sont excrétés dans l'urine.
L'hydrolyse conduit à la formation de 1-naphtol, de dioxyde de
carbone et de méthylamine. L'hydroxylation produit du
4-hydroxycarbaryl, du 5-hydroxycarbaryl, du
N-hydroxyméthylcarbaryl, du 5,6-dihydro-5,6-dihydroxycarbaryl et
du 1,4-naphtalènediol. Le principal métabolite chez l'homme est le
1-naphtol.
Dans les conditions normales d'exposition, il est improbable
que le carbaryl s'accumule chez les animaux. Il est excrété
principalement par la voie urinaire étant donné que la
détoxification de son produit d'hydrolyse, le 1-naphtol, s'effectue
essentiellement par la formation de conjugués hydrosolubles. Dans le
cas des métabolites du carbaryl, le cycle entérohépatique joue
également un rôle considérable, en particulier après administration
par voie orale.
Le produit d'hydrolyse, l'acide N-naphtolcarbamique, se
décompose spontanément en méthylamine et dioxyde de carbone. La
méthylamine subit ensuite une déméthylation en dioxyde de carbone et
formiate, ce dernier étant ultérieurement excrété, en majeure partie
dans l'urine.
Des métabolites du carbaryl sont également présents en faible
proportion de la dose absorbée dans la salive et le lait.
1.6 Effets sur les êtres vivants dans leur milieu naturel
Les valeurs de la CL50 pour les crustacés varient de 5 à 9
µg/litre (puces d'eau et mysidés), 8 à 25 µg/litre (orchesties) et
500 à 2500 µg/litre (écrevisses). Chez les insectes aquatiques, les
limites de sensibilité sont du même ordre. Les plécoptères et
éphémèroptères (perles et éphémères) en constituent les groupes les
plus sensibles. Les mollusques sont moins sensibles avec des valeurs
de la CE50 de l'ordre de quelques mg/litre. Dans le cas des
poissons, la plupart des valeurs de la CL50 se situent entre 1 et
30 mg/litre. Les salmonidés constituent le groupe le plus sensible.
La toxicité aiguë est faible pour les oiseaux. La DL50 pour
la sauvagine et le gibier à plumes en général est > 1000 mg/kg.
D'après les tests, l'oiseau le plus sensible est un francolin
(Francolinus levaillanti) (DL50 = 56 mg/kg). Rien n'indique que
les oiseaux aient eu à souffrir de l'effet des épandages effectués
sur les zones forestières à la dose de 1,1 kg de carbaryl/ha.
Le carbaryl est très toxique pour les abeilles et les lombrics.
Dans le cas des abeilles, la DL50 par voie orale est de 0,16
µg/insecte (soit 1-2 mg/kg).
On est fondé à penser que le carbaryl puisse avoir une
influence temporaire sur la composition en espèces des écosystèmes
terrestres et aquatiques. Par exemple, une étude a montré que les
effets exercés sur certaines communautés d'invertébrés terrestres
pourraient persister au moins 10 mois après un seul épandage.
1.7 Effets sur les animaux d'expérience et les systèmes d'épreuve
in vitro
La toxicité aiguë, exprimée sous la forme de la DL50, varie
considérablement selon l'espèce, la formulation et le véhicule du
produit. La DL50 estimative par voie orale pour le rat varie de
200 à 850 mg(kg. Les chats sont plus sensibles, avec une DL50 de
150 mg/kg. Les porcs et les singes le sont moins, avec une DL50 >
1000 mg/kg.
Une concentration de 792 mg de matière active par m3, qui
constitue la valeur maximale qu'on puisse obtenir pour un aérosol, a
produit, au cours d'une exposition de 4 heures, une mortalité de 20%
(1/5) chez des rattes. A des concentrations de 20 mg/m3, des
aérosols de carbaryl ont provoqué une réduction de l'activité
cholinestérasique chez des chats lors d'une exposition de 4 heures,
mais cette concentration n'a eu aucun effet observable chez des
rats.
Le carbaryl est légèrement irritant pour l'oeil et n'a que peu
ou pas de pouvoir sensibilisateur. Lors d'études à long terme, la
concentration sans effet nocif observable a été évaluée à 10 mg/kg
de poids corporel (200 mg/kg de nourriture) pour le rat et à 1,8
mg/kg de poids corporel (100 mg/kg de nourriture) pour le chien.
Chez le chat, soumis à une exposition de longue durée par
inhalation, la concentration sans effet nocif observable est de 0,16
mg/m3. Le carbaryl a un faible potentiel d'accumulation.
1.7.1 Reproduction
On a montré que le carbaryl avait des effets indésirables sur
la reproduction des mammifères et le développement périnatal chez un
certain nombre d'espèces. Ces effets sur la reproduction consistent
en une réduction de la fécondité, une diminution de l'effectif des
portées et une réduction de la viabilité postnatale. Les effets
toxiques du carbaryl sur le développement se traduisent pas un
certain nombre de morts foetales, une réduction du poids foetal et
la présence de malformations. A l'exception d'un petit nombre
d'études, les effets nocifs sur la reproduction et le développement
n'ont été constatés en totalité qu'à des doses manifestement
toxiques pour la mère, et dans un certain nombre de cas, la mère
était plus sensible au carbaryl que l'embryon ou le foetus. Ces
effets toxiques sur la femelle gestante consistaient en une
mortalité accrue, une réduction de la croissance et des dystocies.
Les données indiquent que, par rapport à l'organisme adulte, la
fonction de reproduction et le processus de développement des
mammifères n'est pas particulièrement sensible au carbaryl.
1.7.2 Mutagénicité
Un certain nombre de tests in vitro et in vivo ont été
effectués sur la carbaryl afin d'en évaluer le pouvoir mutagène;
divers points d'aboutissement de ces effets ont été étudiés sur
divers systèmes (bactéries, levures, végétaux, insectes et
mammifères).
Les données disponibles montrent que le carbaryl n'a aucune
tendance à endommager l'ADN. On ne dispose d'aucun rapport
confirmant les caractères mutagènes suivants: induction de
recombinaisons mitotiques, conversion génique, et synthèse non
programmée de l'ADN chez les procaryotes (H. influenzae, B.
subtilis) ni chez les eucaryotes ( S. cerevisiae, A. nidulans,
lymphocytes humains en culture et hépatocytes de rat) in vitro.
La recherche de mutations géniques, qui a donné lieu à un grand
nombre d'épreuves sur systèmes bactériens, a toujours donné des
résultats négatifs, sauf dans deux cas. Lors de plusieurs études
portant sur les mutations géniques et effectuées sur des cellules
mammaliennes in vitro, le carbaryl n'a donné qu'un seul résultat
positif équivoque dans une de ces études. Toutefois, cette étude
présentait un certain nombre de défauts et ce résultat n'a pas été
confirmé par ceux d'autres études comparables.
Des lésions chromosomiques ont été observées in vitro sur des
cellules humaines, des cellules de rat et de hamster ainsi que des
cellules végétales exposées à des fortes doses de carbaryl. Aucun
effet de ce genre n'a été observé lors d'épreuves in vivo sur des
systèmes mammaliens, même à des doses atteignant 1000 mg/kg.
On a montré que le carbaryl perturbe le mécanisme d'élongation
des fibres du fuseau chez les cellules végétales et mammaliennes in
vitro. On ne sait pas encore très bien si les résultats des
épreuves effectuées sur des végétaux sont extrapolables à l'homme.
Compte tenu de la base de données sont on dispose actuellement,
on peut conclure que rien n'autorise à soupçonner le carbaryl de
présenter un risque d'effets mutagènes pour les cellules somatiques
ou germinales de l'homme.
Un dérivé nitrosé du carbaryl, le N-nitrosocarbaryl, peut
provoquer des recombinaisons mitotiques et des conversions géniques
chez les procaryotes (H. influenzae, B. subtilis) et les
eucaryotes (S. cerevisiae) in vitro, et il donne des résultats
positifs lors de "spot tests" effectués sur E. coli.
En outre, les résultats expérimentaux indiquent que le
N-nitrosocarbaryl se lie à l'ADN provoquant la formation de
liaisons alcalino-sensibles et des cassures monocaténaires.
Il n'est pas établi que le nitrosocarbaryl soit un agent
clastogène in vivo (cellules de la moelle osseuse et cellules
germinales) même à doses toxiques élevées.
1.7.3 Cancérogénicité
De nombreuses études ont été consacrées au pouvoir cancérogène
du carbaryl chez le rat et la souris. La plupart de ces études ont
donné des résultats négatifs mais il s'agit de travaux anciens qui
ne satisfont pas aux normes actuelles. Quoiqu'il en soit, de
nouvelles études portant sur ces mêmes animaux et qui, elles,
satisfont aux exigences modernes, sont actuellement en coursa. La
dernière évaluation du CIRC (CIRC, 1987) a conclu que l'existence de
cancers imputables au carbaryl n'était pas documentée chez l'homme
et qu'on ne possédait pas de preuves suffisantes d'un pouvoir
cancérogène de cette substance chez les animaux de laboratoire. Il
n'a donc pas été possible de classer le carbaryl en fonction de son
pouvoir cancérogène pour l'homme (Groupe 3).
On a montré que le N-nitrosocarbaryl induisait des tumeurs
locales chez les rats (sarcomes au point d'injection ou carcinomes
spinocellulaires au niveau de la portion cardiaque de l'estomac,
lorsque la substance est administrée par voie orale). Etant donné la
biochimie du carbaryl chez l'homme, on peut considérer comme
négligeable le risque de cancérisation par le N-nitrosocarbaryl
résultant d'une exposition humaine au carbaryl.
1.7.4 Effets sur les différents organes et systèmes
a) Système nerveux
Les effets du carbaryl sur le système nerveux tiennent
essentiellement à l'inhibition de la cholinestérase qui est
généralement passagère. Ces effets ont été étudiés sur des rats et
des singes. Des doses de 10 à 20 mg de carbaryl par kg, administrées
pendant 50 jours par voie orale, ont provoqué des perturbations dans
l'apprentissage et l'exécution de certaines épreuves par les rats
traités.
______________
a Ces études n'ont pas encore fait l'objet d'un examen par le
PISC. La société qui effectue ces travaux indique qu'aux doses
les plus fortes étudiées, on note une augmentation
significative de la fréquence des tumeurs chez les deux
espèces.
Lors d'une petite étude portant sur des porcs, du carbaryl
administré pendant 72 à 82 jours en mélange avec la nourriture à
raison de 150 mg/kg de poids corporel, a produit un certain nombre
d'effets neuromusculaires. On a observé en outre chez des poulets
ayant reçu de fortes doses de carbaryl, une faiblesse réversible des
pattes. Aucun signe de démyélinisation n'a été observé dans le
cerveau, le nerf sciatique ou les coupes de moelle épinière
examinées au microscope. On n'a pas observé d'effets de ce genre à
l'issue d'études à long terme sur des rongeurs.
b) Système immunitaire
Administré in vivo à des doses provoquant des signes
cliniques manifestes, le carbaryl exerce divers effets sur le
système immunitaire. Nombre de ces effets ont été observés à des
doses voisines de la DL50. Dans la plupart des études où les doses
utilisées permettaient la survie des lapins et des souris examinés,
on n'a pas observé d'effets significatifs sur le système
immunitaire. Plusieurs de ces travaux présentaient des défauts, par
exemple un manque de cohérence et parfois des contradictions
manifestes dans les résultats, défauts qui ne permettent pas de
dégager des résultats obtenus un mécanisme immunotoxique bien
défini.
c) Sang
Le carbaryl affecterait l'hémostase mais les résultats
concernant le sens de cet effet sont contradictoires. On a observé
que le carbaryl produisait une augmentation de la formation de
méthémoglobine liée à la dose dans des érythrocytes de moutons
présentant un déficit en glucose-6-phosphate-déshydrogénase. La
sérum-albumine humaine réagit in vitro sur le groupement ester du
carbaryl. Le carbaryl se lie aux acides aminés libres.
d) Foie
On a fait état de troubles affectant le métabolisme des
glucides et la synthèse des protéines au niveau du foie ainsi que
d'une perturbation de la fonction détoxifiante de cet organe chez
les mammifères. Le carbaryl induit faiblement l'activité
pharmacométabolisante des microsomes hépatiques. Il y a
raccourcissement de la durée du sommeil induit par le phénobarbital.
Les taux hépatiques de cytochrome P-450 et b5 sont augmentés. Ces
modifi-cations du métabolisme hépatique pourraient expliquer en
partie le triplement de la DL50 pour le carbaryl observé chez des
rats préalablement traités par cette substance.
e) Fonction gonadotrope
Il a été signalé que le carbaryl augmentait la fonction
gonadotrope de l'hypophyse chez le rat.
1.7.5 Mécanisme fondamental de la toxicité
Le carbaryl est un inhibiteur de l'activité cholinestérasique.
Cet effet est lié à la dose et rapidement réversible. On n'a pas
observé de vieillissement de la cholinestérase carbamylée. Tous les
métabolites reconnus du carbaryl ont une activité
anticholinestérasique sensiblement plus faible que le carbaryl
lui-même.
1.8 Effets sur l'homme
Le carbaryl est facilement absorbé par inhalation et après
administration par voie orale mais moins facilement pas la voie
percutanée. Etant donné que l'inhibition de la cholinestérase est le
principal mécanisme de l'action du carbaryl, le tableau clinique
d'une intoxication par cette substance est dominé par les symptômes
correspondants, à savoir: augmentation de la sécrétion bronchique,
sueurs profuses, salivation et larmoiement; myosis,
bronchoconstriction, crampes abdominales (vomissements et diarrhée);
bradycardie; fasciculation des petits muscles (dans les cas graves
le diaphragme et les muscles respiratoires sont également atteints);
tachycardie, céphalées, vertiges, angoisses, confusion mentale, coma
et dépression des centres respiratoires. Ces signes d'intoxication
se manifestent rapidement après l'absorption et disparaissent aussi
vite une fois que l'exposition a cessé.
Des études contrôlées sur des volontaires humains ont montré
que des doses uniques inférieures à 2 mg/kg étaient bien tolérées.
Une dose unique de 250 mg (2,8 mg/kg) a suscité des symptômes
modérés d'inhibition cholinestérasique (douleurs épigastriques et
sueurs) en l'espace de 20 minutes. Les sujets ont complètement
récupéré dans les 2 heures suivant un traitement par le sulfate
d'atropine.
En cas d'exposition excessive d'origine professionnelle au
carbaryl, on observe des symptômes légers bien avant qu'une dose
dangereuse ne soit absorbée, ce qui explique que les cas graves
d'intoxication professionnelle par le carbaryl soient rares. Lors
des épandages agricoles, l'exposition percutanée peut être
importante. Cependant on n'observe en général aucun effet irritant
local, encore que l'on ait décrit des éruptions cutanées à la suite
l'éclaboussures accidentelles de carbaryl en formulation liquide.
Les données concernant les effets du carbaryl sur le nombre de
spermatozoïdes et la modification de leur morphologie chez les
travailleurs de l'industrie sont contradictoires. Aucun effet
indésirable sur la reproduction n'a été signalé.
L'indicateur biologique le plus sensible de l'exposition au
carbaryl est l'apparition de 1-naphtol dans les urines et la
diminution de l'activité cholinestérasique du sang. On peut donc
utiliser la concentration urinaire du 1-naphtol comme indicateur
biologique à condition qu'il n'y ait pas de 1-naphtol sur le lieu de
travail. Lors de certains cas d'exposition professionnelle, on a
constaté que 40% des échantillons d'urine contenaient plus de 10 mg
de 1-naphtol total par litre. Dans un cas d'intoxication aiguë, on
en a trouvé 31 mg/litre d'urine. On considère qu'il y a danger à
partir de 10 mg/litre et que les symptômes apparaissent à partir de
30 mg de 1-naphtol par litre d'urine (Fiche d'information sur le
carbaryl, OMS, 1973, VBC/DS/75.3).
La mesure de l'activité cholinestérasique peut constituer un
test très sensible pour la surveillance médical des travailleurs, à
condition que le dosage soit effectué peu après l'exposition.
2. Conclusions
On estime que le carbaryl est peu dangereux pour l'homme en
raison de sa faible tension de vapeur, de sa décomposition rapide,
de la désinhibition spontanée également rapide de la cholinestérase
et en raison du fait que les symptômes d'intoxication apparaissent
bien avant qu'une dose dangereuse ne se soit accumulée dans
l'organisme. On ne dispose pas encore de bonnes études de
cancérogénicité qui satisfassent aux normes actuelles en la matière.
2.1 Exposition de la population générale
Les quantités résiduelles de carbaryl qui demeurent dans les
denrées alimentaires et l'eau de boisson après l'utilisation normale
de cet insecticide en agriculture, sont très inférieures à la dose
journalière acceptable (DJA) (0,01 mg/kg de poids corporel/jour) et
il est improbable qu'elles puissent constituer un risque pour la
santé de la population dans son ensemble.
2.2 Sous-groupes de population exposés à un risque élevé
Lorsqu'on utilise du carbaryl à des fins de santé publique,
soit au domicile soit sur des aires de loisir, il y a un risque
d'exposition excessive si l'on ne suit pas les règles concernant son
emploi.
2.3 Exposition professionnelle
En faisant respecter des méthodes de travail raisonnables et
notamment un certain nombre de précautions de sécurité et des
mesures de protection individuelle, avec une surveillance
convenable, il n'existe aucun risque qui puisse résulter d'une
exposition professionnelle au cours de la fabrication, de la
formulation et de l'épandage du carbaryl. Les produits non dilués
doivent être manipulés avec de grandes précautions car toute faute
de manipulation peut entraîner une contamination cutanée. Sur le
lieu de travail, la concentration atmosphérique ne doit pas dépasser
5 mg/m3.
2.4 Effets sur l'environnement
Le carbaryl est toxique pour les abeilles et les lombrics. On
ne doit pas procéder à son épandage pendant la floraison.
En utilisation normale, le carbaryl ne devrait pas poser des
problème écologique. Il est adsorbé en grande partie sur les
particules de sol et il ne passe pas facilement par lessivage dans
les eaux souterraines. Il subit une décomposition rapide dans
l'environnement et n'est donc pas persistant. L'emploi de carbaryl
ne devrait pas entraîner d'effets nocifs à court terme sur
l'écosystème.
3. Recommandations
* La manipulation et l'épandage du carbaryl doivent s'effectuer
en observant les précautions qui s'imposent pour tous les
pesticides. On suivra rigoureusement les instructions
d'utilisation qui figurent sur l'emballage.
* La fabrication, la formulation, l'emploi et l'élimination du
carbaryl doivent se faire avec toutes les précautions voulues
pour réduire au minimum la contamination de l'environnement.
* Les travailleurs qui sont soumis à une exposition régulière
doivent faire l'objet d'un contrôle médical périodique.
* La chronologie des épandages de carbaryl doit être réglée de
manière à éviter tout effet sur les espèces non visées.
* Il importe d'effectuer des études de cancérogénicité
satisfaisant aux normes modernes.
RESUMEN Y EVALUACION, CONCLUSIONES Y RECOMENDACIONES
1. Resumen y evaluación
1.1 Identidad, propiedades y métodos analíticos
El 1-naftil N-metil carbamato, derivado del ácido carbámico,
se conoce por el nombre común de carbarilo. El producto de calidad
técnica es un sólido cristalino blanco, de baja volatilidad y escasa
hidrosolubilidad, y estable a la luz y al calor, pero fácilmente
hidrolizable en medio alcalino. La FAO ha establecido una
especificación mínima de pureza del 98%, con un límite de impureza
del 0,05% para el ß-naftil N-metil carbamato.
Para analizar el carbarilo y sus metabolitos, se pueden
utilizar numerosas técnicas, como la cromatografía de capa fina, la
espectrofotometría, la cromatografía de gases, la cromatografía
líquida de alta presión y la espectrometría de masas con ionización
química. Pueden alcanzarse límites de detección inferiores a un
nanogramo, y la recuperación supera por lo general el 80%.
1.2 Producción y usos
El carbarilo se viene utilizando desde hace unos 30 años como
insecticida de contacto y de ingestión, posee algunas propiedades
sistémicas y permite combatir una amplia serie de plagas. La planta
de fabricación más importante está en los Estados Unidos. Más de 290
fabricantes transforman el carbarilo para integrarlo en más de 1500
productos diferentes.
1.3 Transporte, distribución y transformación en el medio ambiente
En la mayoría de las situaciones, el carbarilo no persiste en
el entorno. En el agua, su semivida por hidrólisis depende de la
temperatura, del pH y de la concentración inicial, y varía entre
varios minutos y varias semanas. El principal producto de
degradación es el 1-naftol.
Se ha estudiado la acumulación de carbarilo en peces de agua
dulce, y expresada como factor de bioconcentración en el medio
acuático se ha cifrado en valores comprendidos entre 14 y 75. El
carbarilo es adsorbido más fácilmente en los suelos que poseen un
alto contenido orgánico que en los suelos arenosos. A un ritmo
habitual de aplicación conforme con unas "prácticas agrícolas
adecuadas", la disipación es rápida, con una semivida de entre 8
días y un mes en condiciones normales. Ocasionalmente, por efecto de
la lluvia y del cultivo agrícola, el carbarilo es transportado de la
superficie al subsuelo (a un metro de la superficie).
El carbarilo contamina la vegetación durante el rociamiento o
por desplazamiento hasta las plantas a través del suelo contaminado.
La degradación del carbarilo en el entorno depende del grado de
volatilización, fotodescomposición y degradación química y
microbiana que se produzca en el suelo, el agua y las plantas. La
descomposición es más rápida cuando el clima es cálido.
1.4 Niveles ambientales y exposición humana
La principal fuente de ingestión de carbarilo entre la
población general son los alimentos.
Los residuos hallados en muestras de la ingesta alimentaria
total son relativamente escasos, pues oscilan entre cantidades
ínfimas y 0,05 mg/kg. En los Estados Unidos, la ingesta diaria
durante los primeros años de aplicación de carbarilo fue de 0,15
mg/día por persona (lo contenían el 7,4% de los compuestos); la
cifra se redujo a 0,003 mg/día por persona en 1969 (sólo lo
contenían un 0,8% de los compuestos). Durante el periodo de
aplicación se encuentra carbarilo ocasionalmente en las aguas
superficiales y los embalses.
La población general puede estar expuesta al carbarilo durante
las operaciones de lucha contra las plagas, en su vivienda o en
zonas recreativas.
Los trabajadores pueden estar expuestos al carbarilo durante su
fabricación, formulación, envasado, transporte y almacenamiento, así
como durante y después de su aplicación. Las concentraciones
halladas en la atmósfera del lugar de trabajo durante su producción
oscilaron entre < 1 mg/m3 y 30 mg/m3. Si las medidas de
protección son inadecuadas, los trabajadores industriales y
agrícolas pueden sufrir exposiciones cutáneas importantes.
1.5 Cinética y metabolismo
El carbarilo es absorbido rápidamente por los pulmones y el
tracto digestivo. En voluntarios se observó una absorción cutánea
del 45% a las 8 horas de aplicar una dosis del producto diluida en
acetona. Sin embargo, los datos referentes a la penetración cutánea
in vitro y a la toxicidad indican que la absorción cutánea se
produce por lo general a una velocidad mucho menor.
Las principales vías metabólicas del carbarilo son la
hidroxilación del anillo y la hidrólisis. El resultado son numerosos
metabolitos que experimentan conjugación, con formación de sulfatos,
glucurónidos y mercapturatos hidrosolubles, que se excretan por la
orina. Como resultado de la hidrólisis se forman 1-naftol, dióxido
de carbono y metilamina. La hidroxilación da lugar a
4-hidroxicarbarilo, 5-hidroxicarbarilo, N-hidroximetilcarbarilo,
5-6-dihidro-5-6-dihidroxicarbarilo y 1,4-naftalendiol. El metabolito
principal en el hombre es el 1-naftol.
En condiciones normales de exposición, el carbarilo rara vez se
acumula en los animales. El producto se excreta principalmente por
la orina, debido a que la detoxificación del producto de su
hidrólisis, el 1-naftol, se produce principalmente por
transformación en conjugados hidrosolubles. La circulación
enterohepática de los metabolitos del carbarilo es también
considerable, sobre todo tras su administración oral.
El producto de la hidrólisis, el ácido carbámico N-naftol, se
descompone espontáneamente en metilamina y dióxido de carbono. La
posterior desmetilación de la metilamina da lugar a dióxido de
carbono y formato, siendo este último excretado principalmente por
la orina.
Un pequeño porcentaje de las dosis de carbarilo absorbidas
aparecen como metabolitos en la saliva y la leche.
1.6 Efectos en otros organismos en el medio ambiente
En los crustáceos, las CL50 oscilan entre 5 y 9 µg/litro
(pulgas de agua, mísidos), 8 y 25 µg/litro (anfípodos), y 500 y 2500
µg/litro (cangrejos de río). El margen de sensibilidad es parecido
en los insectos acuáticos; Plecoptera y Ephemeroptera (gusarapa y
cachipollas) son los grupos más sensibles. Los moluscos son menos
sensibles, situándose su CE50 en niveles de unos pocos mg/litro.
En cuanto a los peces, la mayoría de las CL50 están comprendidas
entre 1 y 30 mg/litro; el grupo más sensible son los salmónidos.
En el caso de las aves la toxicidad aguda es baja. La DL50
para las aves de caza, sean acuáticas o terrestres, es > 1000
mg/kg. El ave más sensible analizada es el mirlo de alas rojas
(DL50 = 56 mg/kg). En zonas forestales rociadas con 1,1 kg de
carbarilo por hectárea no se observó ningún efecto en las aves
locales.
El carbarilo es muy tóxico para las abejas y las lombrices de
tierra. La DL50 oral para las primeras es de 0,18 µg/abeja
(aproximadamente 1-2 mg/kg).
Hay indicios de que el carbarilo puede alterar temporalmente la
composición de especies en los ecosistemas tanto terrestres como
acuáticos. Por ejemplo, un estudio reveló que en determinadas
colonias de invertebrados terrestres sus efectos pueden persistir
durante por lo menos 10 meses tras una sola aplicación.
1.7 Efectos en animales de experimentación y en sistemas de prueba
in vitro
La toxicidad aguda, expresada como DL50, varía
considerablemente según las especies, fórmulas y vehículos. Las
estimaciones de la DL50 oral en la rata oscilan entre 200 y 850
mg/kg. Los gatos son más sensibles, pues presentan una DL50 de 150
mg/kg. Los cerdos y los monos son menos sensibles, pues su DL50 es
> 1000 mg/kg.
La exposición a 792 mg de ingrediente activo de carbarilo
nebulizado, que es la máxima concentración a la que se llegó durante
4 horas, provocó la muerte de una de cinco ratas hembra. Aerosoles
de carbarilo a concentraciones de 20 mg/m3 dieron lugar a una
disminución de la actividad colinesterasa (ChEA) en gatos durante
exposiciones únicas de 4 horas, pero esa misma concentración no tuvo
efectos observables en ratas.
El carbarilo produce leves irritaciones oculares y tiene un
potencial de sensibilización escaso o nulo. Estudios prolongados
revelaron un NOEL de 10 mg/kg de peso corporal (200 mg/kg ingesta
alimentaria) en las ratas, y de 1,8 mg/kg de peso corporal (100
mg/kg dieta) en los perros. El NOEL por inhalación prolongada es de
0,16 mg/m3 en los gatos. El potencial de acumulación de carbarilo
es bajo.
1.7.1 Reproducción
Se ha demostrado que el carbarilo tiene efectos adversos sobre
la reproducción y el desarrollo perinatal en diversas especies de
mamíferos. Los efectos sobre la reproducción comprenden problemas de
infertilidad, una disminución del tamaño de las camadas y una
reducción de la viabilidad postnatal. Los efectos tóxicos sobre el
desarrollo observados son un aumento de la mortalidad in utero,
una disminución del peso del feto y la aparición de malformaciones.
Salvo en un reducido número de estudios, todos los efectos adversos
sobre la reproducción y el desarrollo se observaron sólo a dosis
manifiestamente tóxicas para la madre, y en varios casos ésta
resultó ser más sensible al carbarilo que su prole. Entre los
efectos tóxicos para la madre cabe citar la letalidad, una
disminución del crecimiento y la distocia. Los datos disponibles
indican que los procesos de reproducción y desarrollo de los
mamíferos no son especialmente sensibles al carbarilo en comparación
con la susceptibilidad del organismo adulto.
1.7.2 Mutagenicidad
Se ha evaluado la posible mutagenicidad del carbarilo mediante
diversas pruebas in vitro e in vivo, empleando para ello
bacterias, levadura, plantas, insectos y mamíferos, y analizando
diversos puntos finales.
Según los datos disponibles, el carbarilo no es lesivo para el
ADN. No se ha notificado ningún dato que confirme que ha habido
inducción de la recombinación mitótica, conversión génica o síntesis
imprevista de ADN en procariotas (H. influenzae, B. subtilis) y
eucariotas ( S. cerevisiae, A. nidulans, linfocitos humanos en
cultivo, y hepatocitos de rata) in vitro.
Se obtuvieron resultados negativos en las pruebas de detección
de mutaciones génicas en un gran número de ensayos realizados con
bacterias, salvo en dos casos. En varios estudios de mutagenicidad
del carbarilo llevados a cabo con células de mamífero in vitro, se
obtuvo sólo un resultado positivo equívoco en un estudio de células
en cultivo. Ese estudio, sin embargo, presentaba varias deficiencias
y sus resultados no han sido confirmados en estudios comparables.
Se han notificado lesiones cromosómicas a altas dosis de
carbarilo en células humanas y de rata y hámster in vitro, así
como en plantas. No se han observado efectos de ese tipo en pruebas
in vivo con mamíferos, ni siquiera a dosis de hasta 1000 mg/kg.
Se ha mostrado que el carbarilo altera el mecanismo de las
fibras del huso en células de plantas y mamíferos in vitro. Es
dudoso el interés de realizar ensayos con plantas para extrapolar
sus resultados al hombre.
Cabe concluir que los datos disponibles no corroboran la
suposición de que el carbarilo plantea un riesgo de inducción de
cambios génicos en las células somáticas o germinales del hombre.
El producto nitrosado del carbarilo, el N-nitrosocarbarilo,
puede inducir fenómenos de recombinación mitótica y conversión
génica en los procariotas (H. influenzae, B. subtilis) y
eucariotas (S. cerevisiae) in vitro, y arroja resultados positivos
en las pruebas in situ con E. coli.
Además, resultados experimentales indican que el
N-nitrosocarbarilo se une al ADN, causando la ruptura de los
enlaces alcalisensibles y de las cadenas simples.
No se ha demostrado que el nitrosocarbarilo sea clastógeno in
vivo (médula ósea y células germinales), ni siquiera a dosis
tóxicas elevadas.
1.7.3 Carcinogenicidad
Se han realizado numerosos estudios en la rata y el ratón para
determinar los posibles efectos carcinógenos del carbarilo. Los
resultados de la mayoría de esos estudios fueron negativos, pero se
trata de trabajos realizados hace muchos años y que no satisfacían
los criterios actuales. Se están realizando nuevos estudios
conformes con los actuales criterios en ratones y ratas.a En la
más reciente evaluación del CIIC (CIIC, 1987) se llegaba a la
conclusión de que no había información sobre el cáncer en el hombre
y de que los indicios de carcinogenicidad en animales de
experimentación eran insuficientes. No se podía clasificar el
carbarilo en lo que se refiere a su carcinogenicidad para la especie
humana (Grupo 3).
Se ha mostrado que el N-nitrosocarbarilo induce la aparición
de tumores localmente en la rata (sarcoma en el lugar de la
inyección o carcinoma de células escamosas del antro cardiaco al
administrarlo por vía oral). Teniendo en cuenta las transformaciones
químicas que sufre el carbarilo en el hombre, el riesgo de
carcinogenicidad por N-nitrosocarbarilo como consecuencia de la
exposición a esta sustancia puede considerarse insignificante.
1.7.4 Efectos en distintos órganos y sistemas
a) Sistema nervioso
Los efectos del carbarilo sobre el sistema nervioso se deben
principalmente a la inhibición de la colinesterasa y son por lo
general temporales. En unos estudios sobre los efectos en el sistema
nervioso central de ratas y monos se observó que la administración
oral de 10-20 mg/kg durante 50 días provocaba trastornos del
aprendizaje y del comportamiento en las ratas.
En un pequeño estudio realizado con cerdos, el carbarilo (150
mg/kg de peso corporal en la alimentación durante 72-82 días) tuvo
diversos efectos neuromusculares. Se observó una debilidad
reversible de las patas en pollos sometidos a altas dosis de
carbarilo. No se observaron signos de desmielinización en los cortes
de cerebro, nervio ciático o médula espinal examinados al
microscopio. Tampoco se notaron efectos de esa índole en estudios
prolongados realizados con roedores.
b) Sistema inmunitario
Se ha notificado que el carbarilo administrado in vivo a
dosis que producen claros signos clínicos tiene diversos efectos en
el sistema inmunitario. Muchos de los efectos descritos se
detectaron a dosis cercanas a la DL50. La mayoría de los estudios
llevados a cabo con conejos y ratones a dosis compatibles con la
supervivencia no han revelado efectos de importancia en el sistema
______________
a El IPCS aún no ha examinado esos estudios. La sociedad
encargada de realizarlos ha indicado que se observa un aumento
significativo de tumores a la dosis màs elevada en las dos
especies.
inmunitario. Sin embargo, varios de esos estudios adolecían de
incoherencia y a veces de contradicción patente entre los
resultados, lo cual no permite describir un mecanismo inmunotóxico
bien definido.
c) Sangre
Se ha señalado que el carbarilo afecta a la coagulación, pero
existe cierta controversia en cuanto al tipo de efecto. En
eritrocitos de oveja con déficit de glucosa-6-fosfato
deshidrogenasa, el carbarilo provocó un aumento dosis-dependiente de
la formación de metahemoglobina (Met-Hb). La albúmina sérica humana
reaccionó in vitro con el grupo éster del carbarilo. El carbarilo
se une a los aminoácidos libres de la sangre.
d) Hígado
Se han señalado trastornos del metabolismo de los carbohidratos
y de la síntesis de proteínas, así como de la función de
detoxificación en el hígado de ciertos mamíferos. El carbarilo es un
inductor ligero de la actividad de metabolización de medicamentos
que reside en los microsomas hepáticos. Se observa un acortamiento
del periodo de sueño inducido por el fenobarbital. Los niveles
hepáticos de citocromo P-450 y b5 aumentan. Los cambios del
metabolismo hepático son tal vez parcialmente responsables de la
triplicación de la DL50 por carbarilo en ratas tratadas
previamente con dicho compuesto.
e) Función gonadotrópica
Se ha señalado que el carbarilo estimula la función
gonadotrópica de la hipófisis de la rata.
1.7.5 Mecanismo principal de toxicidad
El carbarilo inhibe la acción de la colinesterasa, efecto que
depende de la dosis y es fácilmente reversible. No se observó ningún
fenómeno de "maduración" de la colinesterasa carbamilada. Todos los
metabolitos identificados del carbarilo son
considerablemente menos activos que éste como inhibidores de la
colinesterasa.
1.8 Efectos en el hombre
El carbarilo es fácilmente absorbido por inhalación y por vía
oral, y menos fácilmente por vía cutánea. Como su principal
mecanismo de acción es la inhibición de la colinesterasa (ChE), en
el cuadro clínico de la intoxicación predominan los síntomas de esa
inhibición, tales como: hipersecreción bronquial, aumento de la
sudación, de la salivación y del lagrimeo; pupilas puntiformes,
broncoconstricción, espasmos abdominales (vómitos y diarrea);
bradicardia; fasciculación de los músculos finos (que también afecta
en los casos graves al diafragma y a los músculos respiratorios);
taquicardia; cefalea, vértigo, ansiedad, confusión mental,
convulsiones y coma; y depresión del centro respiratorio. Los signos
de intoxicación aparecen rápidamente tras la absorción y desaparecen
pronto al cesar la exposición.
En estudios controlados realizados con voluntarios se observó
una buena tolerancia a dosis únicas inferiores a 2 mg/kg. Una dosis
única de 250 mg (2,8 mg/kg) provocó síntomas moderados de inhibición
de la ChE (dolor epigástrico y sudación) al cabo de 20 minutos. La
recuperación completa se produjo tras dos horas de tratamiento con
sulfato de atropina.
En los casos de sobreexposición ocupacional al carbarilo, se
observan síntomas leves mucho antes de que llegue a absorberse una
dosis peligrosa; de ahí que raras veces se produzcan casos graves de
intoxicación ocupacional por este compuesto. Durante las
aplicaciones agrícolas, la exposición cutánea puede desempeñar un
papel importante. No suelen observarse efectos irritantes locales,
pero se ha descrito la aparición de exantema cutáneo tras la
salpicadura accidental con formulaciones de carbarilo.
Los datos acerca de los efectos del carbarilo sobre el número y
la morfología de los espermatozoides en trabajadores industriales
son discordantes. No se han notificado efectos adversos sobre la
reproducción.
El indicador biológico más sensible de la exposición al
carbarilo es la aparición de 1-naftol en la orina y una disminución
de la actividad ChE de la sangre. Si no hay 1-naftol en el entorno
de trabajo, los niveles urinarios de este producto se pueden emplear
como indicador biológico. Durante la exposición ocupacional, el 40%
de las muestras de orina contenían más de 10 mg de 1-naftol/litro.
En un caso de intoxicación aguda se hallaron 31 mg/litro en la
orina. El nivel de riesgo es > 10 mg/litro, y el nivel de aparición
de síntomas, 30 mg 1-naftol/litro de orina (hoja de datos sobre el
carbarilo, OMS, 1973, VBC/DS/75.3).
El análisis de la actividad ChE puede ser una prueba de gran
sensibilidad a efectos de vigilancia, siempre que se realice poco
después de la exposición.
2. Conclusiones
Se considera que los riesgos que plantea el carbarilo para el
ser humano son escasos, debido a su baja presión de vapor, a su
rápida degradación y a la recuperación espontánea y rápida de la
colinesterasa inhibida, así como al hecho de que los síntomas
aparecen normalmente mucho antes de que pueda haberse acumulado en
el organismo una dosis peligrosa. Aún no se dispone de estudios
satisfactorios sobre la carcinogenicidad conformes con los criterios
actuales.
2.1 Exposición de la población general
Los niveles de residuos de carbarilo presentes en los alimentos
y el agua de bebida, resultantes de su uso normal en la agricultura,
son muy inferiores a la ingesta diaria admisible (IDA) (0,01 mg/kg
de peso corporal al día), y difícilmente pueden representar un
riesgo para la salud de la población general.
2.2 Subpoblaciones de alto riesgo
El uso del carbarilo con fines de salud pública en la vivienda
o en zonas recreativas puede ser causa de sobreexposición si se
descuidan las normas aconsejadas para su aplicación.
2.3 Exposición ocupacional
Si se vela por el cumplimiento de unas prácticas laborales
razonables, en particular las medidas de seguridad, la protección
del personal y una supervisión adecuada, la exposición ocupacional
durante la fabricación, formulación y aplicación de carbarilo no
planteará riesgos. Las concentraciones no diluidas se deben manejar
con sumo cuidado, dado que unas prácticas laborales incorrectas
pueden ser causa de contaminación cutánea. Las concentraciones en el
aire del lugar de trabajo no deberían superar los 5 mg/m3.
2.4 Efectos en el medio ambiente
El carbarilo es tóxico para las abejas y las lombrices. No
debería aplicarse a los cultivos durante la floración.
Si se emplea normalmente, el carbarilo no debería suscitar
preocupación desde el punto de vista del medio ambiente. El
carbarilo se adsorbe en buena parte en el suelo y no se lixivia
fácilmente hacia las aguas subterráneas. Se degrada rápidamente en
el entorno, por lo que no tiende a persistir. El uso de carbarilo no
debería tener efectos nocivos a corto plazo en el ecosistema.
3. Recomendaciones
* La manipulación y la aplicación del carbarilo se deberían
realizar adoptando las precauciones previstas para todo
plaguicida y siguiendo minuciosamente las instrucciones
suministradas en el envase para emplear correctamente el
producto.
* Deberían controlarse cuidadosamente la fabricación, la
formulación, el uso y la eliminación del carbarilo, con objeto
de reducir al mínimo la contaminación del medio ambiente.
* Los trabajadores expuestos regularmente al producto deberían
someterse a chequeos periódicos.
* Debería determinarse la época de aplicación del carbarilo de
manera que no afecte a las especies que no se desee combatir.
* Deberían realizarse estudios de carcinogenicidad que satisfagan
los criterios actuales.
