
AMITROLE
First draft prepared by
P.H. Arentzen and E.M. den Tonkelaar
National Institute of Public Health and Environmental Protection
Bilthoven, Netherlands
EXPLANATION
Amitrole was evaluated by the Joint Meeting in 1974 when a
conditional ADI of 0-0.00003 mg/kg bw was established (Annex I,
reference 22). The conditional ADI was extended in 1977 (Annex I,
reference 28). The compound was re-evaluated by the present Meeting
on the basis of the periodic review programme. The IPCS has
reviewed amitrole recently and will soon be publishing an
Environmental Health Criteria document on it (WHO, 1994). This
monograph summarizes the data received since the previous evaluation
and contains relevant data from the previous monograph on amitrole.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOLOGICAL DATA
Biochemical aspects
Absorption, distribution and excretion
Mice
The distribution of 14C-labelled amitrole was examined in non-
pregnant C57BL female mice. The mice received amitrole (doses not
specified) either intravenously or p.o. and the distribution of
radioactivity was studied by use of whole body autoradiography,
microautoradiography, impulse counting and cellular fractionation.
The distribution was characterized by an accumulation in tissues
with a rapid cell turnover, e.g., bone marrow, spleen, thymus and
gastrointestinal tract. Microautoradiographically, amitrole was
found mostly in the cytoplasm, suggesting possible involvement in
purine synthesis associated with cell division. Only a moderate
level of radioactivity was found in the thyroid (Tjälve, 1975).
The absorption and distribution of amitrole in fetal tissue of
mice were studied by whole body autoradiography upon administration
(i.v. or p.o.) of radiolabelled amitrole. Pregnant NMRI mice
received radioactive amitrole at day 18 of pregnancy and were
sacrificed 4 or 8 hours after administration. Amitrole passed
through the placenta into the fetus and the distribution pattern was
found to be similar to that in the mothers; the greatest
accumulation occurred in tissues with a rapid cell turnover. Most
of the radioactivity was present in the fetus in a non-metabolized
form (Tjälve, 1974).
Following intravenous administration of 14C-amitrole (3.4
mg/kg bw) to adult male ICR mice, radioactivity in the liver was
distributed homogeneously, but was gradually bound covalently to
hepatic macromolecules, located in the centrolobular region of the
liver (Fujii et al., 1984).
Rats
14C-Amitrole was administered to male and female Wistar rats
at a dose of 1 mg/rat and the expired air, urine, faeces, internal
organs and tissues were analyzed for radioactivity. Traces of 14C
were found in the expired air during the 3-day period following
dosing. High levels of radioactivity (70-95% of the administered
radioactivity) were found in the urine during the first 24 hours,
with only a small amount in the faeces, indicating rapid and almost
complete absorption from the gastrointestinal tract followed by
rapid excretion. Tissue levels were very low after 3 days with
significant amounts found only in the liver (Fang et al., 1964).
In a second experiment, 14C-amitrole was administered to male
and female Wistar rats at doses ranging from 1 mg/rat to 200 mg/rat.
There was no significant difference in the percentage recovery of
radioactivity in urine and faeces in relation to the dosage
administered. The formation of amitrole metabolites as percentage
of dose decreased with increasing dose. The rate of elimination of
amitrole from all tissues was slightly slower for rats fed 200 mg as
compared with those fed 1 mg amitrole. The fate of two unidentified
plant metabolites of amitrole, namely, metabolite 1 and metabolite 3
(isolated from bean plants) was also examined. Radioactivity from
metabolite 1 was excreted rapidly in the urine in the first 48 hours
and identified as unchanged metabolite 1. Elimination of metabolite
3 was mainly in the faeces (Fang et al., 1966).
14C-Amitrole was administered orally to male Wistar rats as a
single dose of 50 mg/kg bw. Excretion in urine and faeces was
followed during a period of 3 days. The majority of the
administered radioactivity was eliminated in the first 24 hours with
the urine (79%) and faeces (1%) (Grunow et al., 1975).
Inhalation exposure to 14C-amitrole aerosol in rats was
examined by MacDonald & Pullinger (1976) and Turner & Gilbert
(1976). Groups of 10 male and 10 female Sprague-Dawley rats were
exposed to 14C-amitrole aerosol for 1 hour either as `head-only' or
as `whole body' exposure at levels of 49.2 µg/l or 25.8 µg/l,
respectively. In both cases there was a rapid excretion of
radioactivity mainly via the urine. The calculated plasma
elimination half-life was approximately 21 hours for both studies.
The urine was the major route of excretion, 75% of the excreted
radioactivity appearing within the first 12 hours. From the whole
body exposure study it appeared that 33% of the radioactivity was
absorbed directly by inhalation and the other 67% by other routes
(probably dermal and oral). A significant proportion may have been
absorbed in the buccal cavity during normal respiration. In
addition, the ingestion of material deposited on the skin and fur by
grooming during and after exposure may have occurred.
Rabbits
In a comparative study of dermal penetration of pesticides,
14C-amitrole was applied on the skin of 3 New Zeeland white female
rabbits at a dose of 1 mg/kg bw. Blood samples were taken at early
time intervals up to 24 hours after application. Urine and faeces
were collected separately over a 24-hour period. Tissue
distribution was measured after 24 hours. After 24 hours, 70.5% of
the applied radioactivity was still at the site of application.
Penetration of the compound into the blood was already observed 1
minute after application, and within 15 minutes measurable amounts
of radioactivity appeared in the urine. The remaining 29.5% of
radioactivity was found 24 hours following topical application in
urine (26%), faeces (23%), gall bladder (23%), liver (10%), bladder
(6%), gastrointestinal tract (6%) and other organs (6%). Very small
amounts were found in fat (0.4%) (Shah & Guthrie, 1977).
Biotransformation
Little metabolic transformation of amitrole occurs in mammalian
species. In the mouse, tissue residues were identified by thin
layer chromatography as largely unchanged amitrole (Tjälve, 1975).
Similarly, in the rat, paper chromatographic analysis of liver
residues following oral administration revealed unchanged amitrole
plus one metabolite (Fang et al., 1964). The majority of the
radioactivity in the urine of rats was also unchanged amitrole; one
metabolite was isolated which represented approximately 20% of the
total radioactivity in urine (Fang et al., 1964).
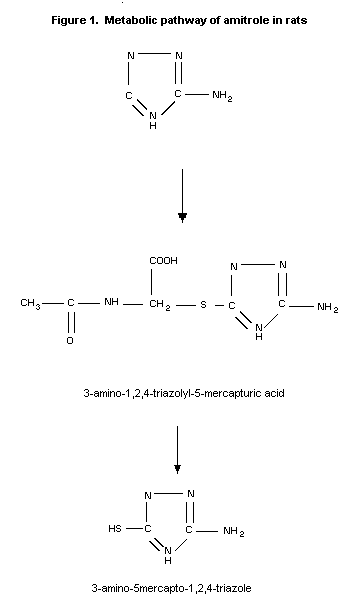
The metabolism of amitrole has been studied in rats. Unchanged
amitrole and 3 metabolites were present in the urine of the animals.
Comparison of these metabolites with the metabolites formed in beans
or E. coli revealed that the 2 major rat metabolites are also
found in these organisms and that the third metabolite is common to
both rats and beans (Franco & Municio, 1975).
In a more extensive analysis of the urinary metabolites in rats
by Grunow et al. (1975), the major part of the radioactivity
identified by paper chromatography corresponded to unchanged
amitrole. Two urinary metabolites were identified as 3-amino-1,2,4-
triazolyl-5-mercapturic acid and 3-amino-5-mercapto-1,2,4-triazole
which together amounted to approximately 6% of the administered
dose.
Following inhalation exposure to 14C-amitrole in the rat,
three urinary radioactive products were detected by paper
chromatography, the major one corresponding to unchanged amitrole
(60% of urinary radioactivity) (MacDonald & Pullinger, 1976; Turner
& Gilbert, 1976).
The metabolic pathway of amitrole in rats is given in Figure 1.
Effects on enzymes and other biochemical parameters
Amitrole inhibits the catalase activity in liver and kidneys of
rats (Alexander, 1959, Bagdon et al., 1956) and the peroxidase
activity in the thyroid of rats (Strum & Karnovsky, 1971; Tsuda et
al., 1973)
 Toxicological studies
Acute toxicity studies
The acute toxicity of amitrole to several animal species is
given in Table 1.
No signs of toxicity were observed in one beagle-type female
dog when given amitrole (96.1%) by gavage at a dosage of 2150 mg/kg
bw. Another dog receiving 4640 mg/kg bw vomited within 1 hour, and
no signs of toxicity were observed thereafter (Fogleman, 1954).
When a commercial product (water soluble powder with 50% active
ingredient) was administered to mice, the LD50 was reduced to 4.0
g/kg bw amitrole. By determining the mean lethal dose for amitrole
in different preparations, it was established that sodium carbonate,
sodium bicarbonate and wetting agents as admixtures in various
quantities considerably increase the toxicity of amitrole (Hapke,
1967). WHO has classified amitrole as unlikely to present acute
hazard in normal use (WHO, 1992).
Table 1. Acute toxicity of amitrole in animals
Species Sex Route LD50 LC50 Purity Reference
(mg/kg bw) (mg/m3)
Mouse M oral 14 700 7 9 96.1%* Fogleman, 1954
? oral 11 000 8 ? Hapke, 1967
? i.v. 5 000 10 ? Hecht, 1954
M i.v. > 1 600 4 6 94% Bagdon et al., 1956
M i.p. > 10 000 6 94% Bagdon et al., 1956
Rat ? oral > 4200 ? Seidenberg & Gee, 1953
? > 10 000 6 10 ? Hecht, 1954
M 25 000 1 9 94% Bagdon et al., 1956
M > 10 000 6 10 ? Hecht & Kimmerle, 1962
? > 2500 93.7% Kimmerle, 1968
M > 5000 ? Thyssen, 1974a
M > 5000 98% Thyssen, 1974b
M&F > 4080 6 99% Gaines et al., 1973
M > 5000 ? Heimann, 1982
M&F dermal > 2500 99% Gaines et al., 1973
M&F inhal (4 h) > 439 6 96.9% Thyssen, 1983
? i.v. 5000 10 ? Hecht, 1954
M i.p. > 4000 6 94% Bagdon et al., 1956
Rabbit ? dermal > 10 000 5 6 95% Elsea, 1954
Cat M&F oral > 5000 2 94% Bagdon et al., 1956
M&F i.v. > 1750 3 94% Bagdon et al., 1956
1 80% aqueous suspension
2 40% aqueous suspension; the 3 cats tested (limit test)
vomited within 2 hours after receiving the dose
3 10% solution in 0,9% saline; one cat per dose level
4 aqueous solution
5 moistened with 0.5% methyl cellulose solution
6 no signs of toxicity
7 mortality at 21 500 mg/kg; signs of toxicity at this and
higher dose levels: extreme depression, squinting eyes, slow,
laboured respiration, diarrhoea, ataxia, depressed or absent
placement and righting responses, mild clonic convulsions,
gasping, coma and death and irritation of gastrointestinal
tract. No signs of toxicity at the lower dose level
(10 000 mg/kg bw)
8 signs of toxicity seen from 5 mg/kg bw: severe hypokinesia,
slight tremor of the extremities, apathy ranging to catatonia,
prone position, individual deep inspirations.
9 only 5 animals/group
10 less than 4 animals/group
* no correction for purity was made.
Short-term toxicity studies
Dietary/gavage
Rats
In a two-week dietary study, administration of 60 or 120 ppm
amitrole resulted in enlargement of the thyroid gland of rats
(number and strain not specified) and a pronounced lowering of
iodine uptake. Over this two-week interval there were no
significant changes at levels of 15 or 30 ppm (Jukes & Shaffer,
1960; Annex I, reference 23).
Amitrole was administered by gavage to groups of rats (strain
unknown), 5 days a week, for 4 weeks at doses of 0, 100, 200 or 400
mg/kg bw. In all treated groups, growth rate was reduced, relative
thyroid weight increased and iodine content of the thyroid reduced
(Hapke, 1967; Annex 1, reference 23).
In a range-finding study, groups of albino Wistar rats (4/sex)
received 0, 125, 1250 or 12 500 ppm amitrole in their diet for 27
days. The low dose (125 ppm) was raised to 25 000 ppm on day 7 of
treatment. Body-weight was retarded and food intake reduced in all
treatment groups. Thyroid weight (the only organ investigated) was
increased in all dose groups (Ben-Dyke et al., 1973).
Reversibility of thyroid effects was studied in a two-week
dietary study in male albino rats (strain unknown). A dietary level
of 1000 ppm increased the (absolute) thyroid weights to about 3.5
times those in the untreated controls. Thyroid weights had nearly
completely returned to normal after two weeks recovery (Bagdon et
al., 1956; Annex I, reference 23).
Amitrole (purity 94.6%) was administered to groups of male
Sprague-Dawley rats (20/dose) at levels of 0, 30, 100, or 300 ppm in
their diet for 28 days, followed by a 28-day recovery period. Two
animals/group were killed weekly in order to monitor the thyroid
function by assaying the thyroid hormones T3 and T4. Body weight
was depressed and food consumption decreased at the 100 and 300 ppm
dose levels. Food consumption returned to normal during the
recovery period as did body weight, but only in the 100 ppm group.
T3 levels were significantly decreased at day 7 in the 300 ppm
group and at day 14 in the 100 ppm group. T3 levels returned to
normal by day 19 post-treatment. T4 levels followed the same
pattern although the extent of decrease was greater. The NOAEL in
this study was 30 ppm (equivalent to 3 mg/kg bw/day) (Babish, 1977).
In a thyroid function test amitrole (purity 96.9%) was
administered to groups of 30 female Wistar rats (12 week old,
weighing 200 g) at levels of 0, 0.5, 1, 2, or 4 ppm in their diet.
Ten animals per group were sacrificed 3, 9 or 29 days after study
initiation and the following parameters were examined: thyroid
weight, accumulation of radioiodine in the thyroid (24 hours after
administration of 131I), and serum levels of T3 and T4.
Significantly elevated thyroid weights were observed only in the 1
and 2 ppm groups, 3 days after study initiation. No significant
deviation was observed in any dose group at the later test dates.
The percentage of iodine accumulation in the thyroids of the 2 ppm
group exhibited significant elevation after a 9-day treatment
period. In the 4 ppm group a slight, but not significant increase
was found. No effect was observed at 29 days, and no clear effects
were observed on T3 or T4 levels. The effects on thyroid function
found in this study were considered marginal and not consistent.
Therefore the highest dose in this study (4 ppm, equivalent to 0.4
mg/kg bw/day) was considered to be the NOAEL (Weber, 1983).
Amitrole (96.1%) was administered to groups of Carworth Farm
male and female rats (5 animals/sex/group) at levels of 0, 100,
1000, or 10 000 ppm in the diet for 63 days. Both males and females
showed reduced body-weight gain at 1000 and 10 000 ppm, which was
accompanied by reduced food consumption. There were no deaths or
gross signs of toxicity. Histopathological examination of the
liver, kidneys, bladder, small intestine, spleen and testis (thyroid
was not examined) revealed only an increased vacuolization of the
liver cells around the central veins in the 1000 and 10 000 ppm
dosage groups. The vacuoles were identified as fat globules
indicative of fatty metamorphosis associated with liver cell damage.
No histological effects were noted at 100 ppm. Because the thyroid
was not examined in this study, it is of limited value in the
evaluation of amitrole (Fogleman, 1954).
Fregly (1968) investigated the dose-response relationship
between amitrole administered in the diet and a variety of clinical
parameters in order to establish the minimum effective dose on
thyroid activity. Amitrole was administered to groups of male
Spruce Farm strain rats (10/dose) at levels of 0, 2, 10, or 50 ppm
in the diet for 13 weeks. In a separate experiment, amitrole was
administered in the diet to similar groups at levels of 0, 0.25, or
0.50 ppm for 11 weeks. In the first experiment, there were no
treatment-related effects on body weight, food consumption,
haematocrit, haemoglobin, or the rate of oxygen consumption. Mean
rectal temperature (measured at week 12) was slightly, but
significantly increased at 50 ppm. Uptake of radioactive iodine,
measured in vivo during week 12 at various times 22-53 hours after
injection of Na131I, was significantly decreased at 50 ppm. At the
end of the first study, radioactivity in the thyroid gland, measured
24 hours after injection, and the level of PBI in serum were
significantly reduced in all treatment groups. The PBI-values were
5.1, 3.7, 3.8, and 3.3 µg/100 ml for the 0, 2, 10, and 50 ppm
groups, respectively. Thyroid weight was increased significantly
only in the 50 ppm group. Histological changes in the thyroid were
noted at 10 and 50 ppm in the follicular cells with regard to both
appearance and the presence of colloid. Capillary density, which is
characteristic of the TSH-stimulated thyroid, was increased
significantly at both 10 and 50 ppm. In the second experiment, at
lower dose levels, no significant differences were noted between the
treated and control groups. In this study PBI was not affected. In
fact the PBI values were 3.2, 3.9, and 4.5 µg/100 ml for the 0, 0.25
and 0.50 ppm groups, respectively. It should be noted that the PBI
control values, measured in the second experiment were much lower
than those measured in the first experiment. This means that there
is no biologically significant effect on PBI, because all values
fall in the same range. The NOAEL was therefore 2 ppm (equivalent
to 0.1 mg/kg bw/day) (Fregly, 1968).
Several short-term experiments were carried out in Wistar rats,
in order to establish a no-effect level on thyroid function. In all
experiments the uptake of 131I by the thyroid was measured in an in
vivo test 6, 24, or 48 hours after administration of 0.6 µCi 131I
per animal intraperitoneally. In addition, at the end of the study,
thyroid weight and PBI were measured and the thyroid was studied
histopathologically.
In the first experiment, four groups of 8 female rats received
0, 2, 20, or 200 ppm of amitrole in the diet for 6 weeks. The
uptake of 131I was measured after 5 days and 6 weeks. At both times
a significantly increased uptake was found in the 200 ppm group 6
hours after injection, which decreased rapidly after 24 and 48
hours. At that time the radioactivity was lower than that of the
controls. The thyroid weight was increased in the 200 ppm group and
histopathologically goitre was found in this group only. No
significant effects were found at lower dosages.
In the second experiment in which 8 female rats per group
received 0, 20, 50, or 200 ppm for 6 weeks, the same effect was
found in the 200 ppm group. In addition, a significantly decreased
PBI was observed at the end of the experiment compared with the
controls. At 50 ppm a statistically increased uptake was found 6
hours after injection of 131I. In this case the radioactivity in
the thyroid remained higher than the controls after 24 and 48 hours.
Histopathologically only a very slight activation was found, whereas
200 ppm showed strong activation and goitre.
In the third experiment, 10 female animals per group received
amitrole at dietary concentrations of 0, 20, 50, or 200 ppm for 13
weeks. The uptake of 131I by the thyroid was significantly
increased at 50 and 200 ppm after 6 and 12 weeks. At 50 ppm, the
radioactivity in the thyroid remained high after 24 and 48 hours,
whereas at 200 ppm a very high uptake was found 6 hours after
injection of 131I, followed by a rapid decrease with still lower
values than the controls after 48 hours. At 200 ppm, the PBI was
decreased and the thyroid/body-weight ratio increased by a factor of
6. At 50 ppm, only a slightly increased relative thyroid weight was
found. Histologically a strong activation and goitre were found at
200 ppm, and a slight activation at 50 ppm. In this experiment, a
tendency to a higher uptake of 131I was found in the 20 ppm group.
The three above mentioned experiments were carried out with an
iodine content of about 0.2-0.3 ppm in the diet. In the fourth
experiment an iodine content of about 2 ppm was used. In this
experiment, 8 female rats per group received 0, 20, 50, 200 or 500
ppm amitrole in the diet for 6 weeks, to determine whether iodine
could protect against the antithyroid action of amitrole. At 500
ppm, a small increase in iodine uptake was found 5 hours after
injection, but thereafter a very rapid decrease was found. At 200
ppm, the uptake was much higher and the same type of decrease was
found as in the other experiments, whereas with 50 ppm a
significantly increased thyroid radioactivity was found at all
times. PBI was decreased at 200 and 500 ppm only.
Histopathologically, goitre and strongly activated thyroids were
found at the two highest dose levels. Some activation was found in
the 20 ppm group. It can be concluded that measurement of 131I
uptake at different time points was a sensitive method for the
effects of amitrole on the thyroid. At 20 ppm, only slight effects
were found on iodine uptake and thyroid histopathology. The overall
NOAEL from these four experiments was 2 ppm, equivalent to 0.1 mg/kg
bw/day (Den Tonkelaar & Kroes, 1974).
Dogs
In a one-year study in male and female beagle dogs, amitrole
was given in capsules at daily dose levels of 0, 0.25, 1.25, 2.50 or
12.5 mg/kg bw, 6 days per week (4-6 animals/group). There were no
clinical signs of toxicity or pharmacological effects.
Haematological, biochemical and urinalysis parameters were
comparable to those of the control dogs, and within normal limits.
The dogs at the 12.5 mg/kg bw/day dose level had pale-coloured
pancreases as the only dose-related gross pathological effect.
Histopathological examination of all dogs did not reveal any
treatment-related effect. The thyroid, in particular, was normal at
all dose levels (Weir, 1958).
Drinking-water
Mice
Groups of male albino mice (strain unknown) were exposed to
amitrole in the drinking-water at concentrations of 0, 0.5, 1.0 or
2% for 30 days (water consumption was not reported). Light
microscopy revealed dose-related hypertrophy of hepatocytes with
nuclear deformations, increased pyknotic nucleoli, and increased
vacuoles in the cytoplasm. Electron microscopy revealed
proliferation of smooth endoplasmic reticulum (Reitze & Seitz,
1985).
Rats
Male Sprague-Dawley rats were provided with drinking-water
containing 400 ppm amitrole over periods of 12, 20, or 37 days.
Based on a body weight of about 200 g and a water intake of 30-35
ml/day, the daily intake was about 60-70 mg/kg bw. The catalase
activity of liver and kidney of the treated animals was inhibited by
50%, but the red cell catalase was unaltered. An increase in
thyroid weight was found, which correlated with the duration of
treatment. Microscopic examination showed hyperplasia of the
thyroid and a loss of colloid. It was postulated in this study that
the ability of the thyroid to concentrate plasma iodine was not
impaired by amitrole, but that the formation of organically-bound
iodine was inhibited (Alexander, 1959).
In a range-finding study, amitrole was administered to groups
of albino Wistar rats (4/sex) at levels of 0, 10, 104 or 1040 ppm in
drinking-water over a treatment period of 27 days. Body-weight gain
was retarded and food intake reduced at 104 ppm and above. Thyroid
weight (the only organ investigated) was increased in a dose-
dependent manner at 104 ppm and above. The NOAEL in this study was
10 ppm, equivalent to 1.5 mg/kg bw/day, based on a daily water
consumption of 30 ml and body weight of 200 g (Ben-Dyke et al.,
1973).
Amitrole (purity 94%) was administered in the drinking-water to
groups of male albino rats (10/dose; strain unknown) at
concentrations of 0, 50, 250, or 1250 ppm for 106 days. The
administration of amitrole resulted in a dose-dependent depression
of growth with a corresponding reduction of food and water intake.
Appearance, behaviour, and mortality were not affected by amitrole.
Haematology, except Ht, and clinical chemistry parameters were not
examined. At the end of the study, histopathology was performed on
the thyroid, hypophysis, liver, kidney, spleen, stomach, small and
large intestines, bladder, testis, adrenal and lung. Gross and
microscopic examination of tissues and organs showed a marked
increase in thyroid size at all dose levels. In rats where reduced
growth was noted, the kidneys, adrenals, liver and spleen were
proportionally smaller. Reproductive organs were not affected.
Microscopic examination showed general enlargement of the thyroid at
50 ppm, with moderate stimulation of the thyroid epithelium (no
evidence of hyperplasia). At 250 ppm, thyroid hyperplasia was
evident. At the two highest dose levels there was also absence of
follicles with stored colloid. Liver catalase activity was reduced
at 250 and 1250 ppm in a dose-dependent manner (Bagdon et al.,
1956; Annex I, reference 23).
In a study measuring the time-course of development of goitre,
Sprague-Dawley rats were given amitrole in the drinking-water (400
ppm). The thyroid of each animal was examined by light microscopy
at various periods from 3 days to 6 months. Each rat drank
approximately 30 ml per day. After 3 days, the thyroid size was
unchanged, although cellular changes were apparent. By one week,
the thyroid was twice its normal size with marked structural
changes. These changes continued to progress with prolonged
administration of the goitrogen. Goitre formation was accompanied
by increased vascularity and decreased colloid content in the
follicular cells. Electron microscopy revealed pronounced
dilatation of the endoplasmic reticulum. Peroxidase activity
measured in the thyroid progressively decreased with prolonged
administration of amitrole (Strum & Karnovsky, 1971).
The effects of amitrole on thyroid histology were examined in
seven groups of 5 female Wistar rats weighing about 200 g, which
were given amitrole in their drinking-water, 2500 ppm, and killed
after 1, 2, 3, 10, 30, 50, or 100 days. Water consumption was not
reported. After 1 and 2 days of exposure the only change noted was
a slight enlargement of some endoplasmic cisternae of follicular
cells. After 3 days the gland was slightly enlarged, follicular
colloid was slightly reduced and in some follicular cells the
cisternae were clearly dilated and stained more lightly for
peroxidase activity than in normal cells. By 10 days the glands had
doubled in size, the follicular epithelium consisted of low,
columnar cells, and colloid had been severely depleted. Nuclei had
become located basally and slightly elongated microvilli projected
into the lumen. Peroxidase activity was no longer seen in the
endoplasmic reticulum cisternae, whereas it was observed in portions
of perinuclear cisternae. These changes had progressed in the 30-
day exposure group, so that the glands were now several times their
normal size. In addition, fibrous thickening of both stroma and
capsule was prominent and cisternal peroxidase activity was absent.
Over 50-day exposure resulted in increased irregularity in
follicular size, more prominent papillary growth of the follicular
epithelium and greatly diminished peroxidase activity throughout the
cells (Tsuda et al., 1973).
Functional and morphological changes in the thyroid were
examined in male Wistar rats. Amitrole was administered via
drinking-water at a concentration of 1000 ppm for periods of up to
153 days. Within 2 weeks thyroid hormone synthesis (serum T3 and
T4) was inhibited. Serum TSH level increased rapidly during the
first 4 weeks, and remained essentially constant after that. The
accumulation of radioiodine in the thyroid (measured as the ratio
thyroid to serum inorganic iodide - T/S ratio) followed a similar
pattern. Thyroid weight increased rapidly during the first few
weeks and thereafter growth rate declined. After 4 months, total
thyroid weight was increased to a 12-fold plateau. During the first
week of amitrole administration there was also a rapid change in the
morphology of the gland; an increase in the proportional volume of
the epithelial cell compartment being accompanied by a corresponding
decrease in follicular lumen (colloid) and a marked increase in
vascularity (Wynford-Thomas et al., 1982a,b).
Dermal
Rabbits
Amitrole (97.6%) was applied to the shaved skin of the back and
flanks of groups of 6 male and 6 female New Zeeland white rabbits at
doses of 0, 25 or 100 mg/kg bw for 6 hours each day on 15
consecutive workdays. The skin was abraded in three animals per
group. The NOAEL for systemic and local effects was at the highest
dose level, i.e. 100 mg/kg bw/day (Mihail & Schilde, 1984).
Inhalation
Rats
Groups of Fischer 344 rats (15/sex/dose) were exposed to an
atmosphere containing amitrole (94.6% pure) at concentrations of 0,
0.1, 0.32, 0.99, or 4.05 mg/l (nominal concentrations adjusted for
non-nebulizing material), 5 hours/day 5 times per week, for 4 weeks
(particle size unknown). There were no adverse effects on behaviour
and no body-weight changes were noted. T3 and T4 levels were
depressed after 14 and 27 days at levels of 0.32 mg/l and above. At
these dose levels, thyroid hyperplasia and elevated thyroid weights
were also observed. At a concentration of 0.1 mg/l no effects on
the thyroid were observed (Cox & Re, 1978).
Long-term/carcinogenicity studies
Mice
Amitrole was used as a positive control in a study which
investigated the carcinogenicity of some 120 chemicals. Groups of
(C57BL/6 x C3H/Anf) F1 mice (18/sex) and (C57BL/6 x AKR) F1 mice
(18/sex) were given amitrole by gavage at a dose level of 1000 mg/kg
bw/day on days 7-28 of age, and in the diet at a level of 2200 ppm
from 28 days onwards until the end of the experiment. This was
planned to be an 80-week study but all of the mice in the amitrole
treatment groups had died by weeks 53-60. Thyroid tumours were
reported to have occurred in nearly all of the treated mice (64/72).
Liver tumours were observed in 34/36 (C57BL/6 x C3H/Anf) F1 treated
mice and in 33/36 (C57BL/6 x AKR) F1 treated mice. In pooled
control groups, 8/166 (C57BL/6 x C3H/Anf) F1 mice and 6/172
(C57BL/6 x AKR) F1 mice had liver tumours (Innes et al., 1969).
In a carcinogenicity study, amitrole (97.0% pure) was
administered to groups of NMRI mice (75/sex/dose level) at levels of
0, 1, 10, or 100 ppm in their diet for 18 months. There were no
treatment-related effects on appearance, behaviour, body weight,
food consumption or survival times (haematology not measured).
Histological examination of tissues of the major organs did not
reveal any treatment-related effects apart from a slight increase in
the number of hyperemias in the pituitary at 100 ppm (5 at 1 ppm, 2
at 10 ppm and 16 at 100 ppm). The incidence of treatment-related
tumours was not increased. Concurrent satellite groups of 5
animals/sex/dose were additionally used in this carcinogenicity
study for different thyroid function tests (thyroid weights,
incorporation of radioactive iodide in the thyroid gland, proportion
of PBI in total plasma iodine) performed at intervals of 3, 6, 9,
12, and 18 months. The thyroid weights (up to 3 times control
values), were elevated in the 100 ppm males at all test dates. The
percentage of iodine accumulation as well as the iodine level in the
thyroid were elevated in the male mice at 100 ppm. The fraction of
PBI in the male mice was elevated 9 months after study initiation,
but was depressed at later test dates. Comparable results were
observed in the high-dose females. However, the deviations relative
to the control group were generally smaller than in the males, and
were not significant in most cases. The NOAEL was 10 ppm equivalent
to 1.5 mg/kg bw/day (Steinhoff & Boehme, 1979b, Weber & Patzschke,
1978; Steinhoff et al., 1983).
In a study on C3H mice and their substrain without serum
catalase activity, ingestion of 10 000 ppm amitrole in the diet led
to a reduction in lifetime. In comparing the two substrains, it was
shown that the acatalasemic mice lived longer under treatment than
did the normal mice. The mean survival times were 35 and 25 weeks,
respectively. The C3H strain features a high rate of spontaneous
liver tumours (it was reported to be 9% in females and 46% in
males). Under amitrole treatment, liver tumours occurred earlier
and at a higher incidence in the acatalasemic mice than in the
normal mice (Feinstein et al., 1978).
Three groups of B6C3F1 mice were fed a diet containing 500
ppm amitrole (purity not specified): Group 1: the dams were treated
from day 12 of gestation to birth of the pups; group 2: dams
together with their pups were treated from birth to weaning; group
3: pups were treated for a period of 90 weeks after weaning.
Although not specifically stated, information on other chemicals
tested and described in this paper (benzidine, safrole etc.),
implies that pups of groups 1, 2, and 3 animals were examined only
for liver tumours after 90 weeks. It was not stated if treatment
with the other compounds were conducted in the same or different
rooms. The incidence of hepatocellular adenomas and carcinomas,
respectively, were: group 1 males, 4/74 and 2/74; group 2 males,
6/45 and 4/45; group 3 males, 15/55 and 11/55; group 1 females, 0/83
and 0/83; group 2 females, 0/55 and 0/55; group 3 females, 5/49 and
4/49. The incidence of these tumours in the untreated control
animals (which may or may not have been concurrent controls) at week
90 were: males, 1/98 and 0/98; females, 0/96 and 0/96. Other organs
were not examined. It was concluded that there was no effect of
amitrole treatment in group 1 or in females of group 2, but that
marginal increases occurred in males of group 2 and in males and
females of group 3 (Vesselinovitch, 1983).
The susceptibilities of three mouse strains to the development
of preneoplastic hepatic lesions were examined following amitrole
administration in their drinking-water at 10 000 ppm. The strains
were DS, ICR (Crj: CD-1) and NOD derived from ICR and found to
develop spontaneous insulitis followed by diabetes. There were
reported to be indications that NOD mice may carry immunological
abnormalities. In each of two experiments, 3 groups of female mice
were administered amitrole via drinking-water for 3 months
(experiment 1) or 6 months (experiment 2), after which they were
killed and their livers examined. The proportions of mice with
hyperplastic nodules were, in experiment 1: 15/19, NOD; 3/55, DS;
0/5, ICR, and in experiment 2: 19/19 NOD; 18/18 DS; 17/19 ICR. A
single hepatocellular carcinoma developed in a NOD mouse after 6
months (Mori et al., 1985).
Rats
In a limited chronic toxicity study (no haematology or clinical
chemistry) groups of Carworth Farm Wistar rats (35/sex/dose)
received amitrole in the diet at levels of 0, 10, 50, or 100 ppm for
two years. After 13 weeks, 5 animals/sex/dose and after 68 weeks 3
animals/sex/dose were killed for organ weight measurement and
histopathological examination. A separate group received 500 ppm
for 19 weeks. Due to marked reduction in body-weight gain and food
consumption, these animals were left on the control diet for 7 weeks
and were then killed (26 weeks). Animals in all groups, including
controls suffered from apparent respiratory infection and were in
poor condition. Death occurred in 67 rats but there was no
relationship with treatment. Body-weight gain was reduced at 100
ppm in male animals during the first 13 weeks of the study. After
68 and 104 weeks of treatment, relative thyroid weight was increased
at 100 ppm (not measured after 13 weeks). Histopathological
examination after 13 weeks showed hyperplasia and hypertrophy of the
thyroid at 500 ppm, but this was reversed after withdrawal of the
amitrole diet. Histopathological changes were also seen at 100 ppm
and in one animal at 50 ppm. At 68 weeks, 3 animals at 50 ppm
showed definitive evidence of hyperplasia while all animals at 100
ppm showed evidence of hyperplasia and hypofunctioning of the
thyroid. At 104 weeks tumours were found: thyroid tumours were
present in 1/10 animals of the 10 ppm group, 2/15 at 50 ppm and
15/27 at 100 ppm. No tumour was detected in 5 control group animals
but one rat exhibited early stages of an adenoma. One thyroid from
the 50 ppm group and 4 from the 100 ppm group exhibited lesions
interpreted as adenocarcinomas by several pathologists and benign
neoplasms by others. Based on thyroid hyperplasia the NOAEL was 10
ppm equivalent to 0.5 mg/kg bw/day (Keller, 1959; Jukes & Shaffer,
1960).
Groups of Fischer 344 rats (75/sex/dose) were treated with
amitrole (94.6% pure). Group A were the controls. Rats of group B
were fed 5 ppm of amitrole in their diet during 1-39 weeks and then
100 ppm during weeks 40-115 for males and 40-119 for females. Rats
in groups C, D, and E received amitrole in their diet at pulsed
intervals (alternate 4 week periods) at 1, 3, or 10 ppm,
respectively, during weeks 1-39 and 20, 60, or 200 ppm during the
last exposure period (week 40 onwards). On alternate 4-week
periods, groups C, D, and E received the basal diet containing no
amitrole. There were no treatment-related clinical signs of
toxicity or changes in body weight or food consumption. No
treatment-related effects on parameters of haematology, clinical
chemistry or urinalysis were observed (only 5 animals/sex/dose level
were examined). Thyroid weights were significantly increased in
both males and females in groups B and E after 60 weeks and at
termination. Increased thyroid weights, although not significant,
were also observed in group D. The T3 and T4 activities were
elevated in groups B and E from week 44 onwards. However, these
effects were not always consistent. These changes were not observed
up to week 36, when the groups were administered the lower dose
levels. Follicular epithelial hyperplasia in the thyroid was noted
in groups B, D, and E and to a much lesser extent in group C. An
increased incidence in thyroid tumours (mainly follicular adenomas)
was observed in male and female rats of groups B and E and in the
male animals of group D. There was no significant difference in
tumour incidence between groups B and E (in these dose groups the
incidence of follicular adenomas was 80-84% and of follicular
adenocarcinomas 5%, compared to 0-2% in the control group). Due to
the change in dosing regimen it was difficult to evaluate this
study. However, effects on the thyroid (hyperplasia) were seen in
all treated groups (Johnson, 1981).
In a study performed to characterize the oncogenes
participating in the genesis of thyroid tumours, male Wistar rats
(number not specified) were treated with a chemical carcinogen
(nitrosomethylurea, NMU) or by ionizing radiation (131I) to induce
thyroid tumours, and were then treated with amitrole (purity not
specified) as a goitrogen at a level of 0.1% in drinking-water
throughout their entire lifetimes. A control group was only
administered amitrole without prior tumour-initiating treatment.
Malignant thyroid tumours developed after even a relatively brief
period in rats treated with NMU or 131I. Animals exclusively
treated with amitrole exhibited only benign tumours which developed
at a markedly slower rate. A "H-ras" oncogene was determined to
be involved in treatment with NMU (13 out of 15 cases), whereas a
"K-ras" oncogene was involved in the animals treated with 131I
(8/15 cases) and amitrole (only 1/10 cases) (Lemoine et al.,
1988).
In a briefly worded description of a long-term study in rats,
thyroid and liver tumours were reported to occur in rats exposed to
20-25 mg amitrole per day via drinking-water or to 250 or 500 mg
amitrole per day in their diets for life. No control data were
reported (Napalkov, 1962). Due to the serious shortcomings in this
study, it was not considered relevant for the evaluation of amitrole
toxicity.
In a carcinogenicity study, groups of Wistar rats (75/sex/dose
level) were treated with amitrole (97.0% pure) in the diet at
concentrations of 0, 1, 10, or 100 ppm for two years. There were no
treatment-related effects on appearance, behaviour, body weight, or
food consumption (haematology not measured). A slight decrease in
survival time was found at 100 ppm. Average survival time at 100
ppm for males was 961 days and for females 919 days. The survival
times for control animals were 992 and 969 days for males and
females, respectively.
Concurrent satellite groups of 5 animals/sex/dose level were
additionally used in this carcinogenicity study for different
thyroid function tests (thyroid weights, incorporation of
radioactive iodide in the thyroid gland, proportion of protein-bound
iodine in total plasma iodine) performed at intervals of 3, 6, 9,
12, 18 or 24 months. The thyroid weights (up to 3.6 times control
values for males and 7 times for females) were increased at 100 ppm
as was the uptake of 131I by the thyroid, measured 24 hours after
oral administration of 131I. The fraction of PBI was not affected.
At 100 ppm, an elevated incidence of haemorrhages and hyperaemia of
the pituitary gland as well as a very high rate of cystically
dilated thyroid follicles were seen. Both benign and malignant
tumours of the thyroid were found at 100 ppm. There was also an
increase in the incidence of benign tumours in the pituitary gland
at the 100 ppm level (females). The NOAEL was 10 ppm (equivalent to
0.5 mg/kg bw/day) (Steinhoff & Boehme, 1979a; Weber & Patzschke,
1978; Steinhoff et al., 1983).
Hamsters
In a carcinogenicity study in Syrian golden hamsters
(76/sex/dose) amitrole (97%) was administered in the diet at levels
of 0, 1, 10, or 100 ppm for 18 months. There were no treatment-
related changes in appearance, behaviour or food intake. Body-
weight gain was decreased at 100 ppm from day 400 onward, and
mortality was significantly increased in the 100 ppm dose group.
Average survival time at 100 ppm for males was 557 days and for
females 438 days. The survival times for control animals were 631
and 508 days for males and females, respectively. The main cause of
death in all groups, including controls, was severe amyloidosis of
the kidneys. There was no evidence of amitrole-related
histopathological changes. There was no evidence of a treatment-
related carcinogenic effect. Concurrent satellite groups of 5
animals/sex/dose were additionally used in this carcinogenicity
study for different thyroid function tests (thyroid weights,
incorporation of radioactive iodide in the thyroid gland, proportion
of PBI in total plasma iodine) performed at intervals of 3, 6, 9,
12, or 18 months. There were no treatment-related effects observed.
The NOAEL was 10 ppm equivalent to 1 mg/kg bw/day (Steinhoff &
Boehme, 1978; Weber & Patzschke, 1978; Steinhoff et al., 1983).
Inhalation exposure
Rats
In an inhalation study involving intermittent treatment, groups
of 75 Fischer rats per dose and sex were exposed to aerosols at
nominal amitrole levels of 0, 50 or 500 µg/l air (one of the two
batches used had a purity of 94.6%). The actual amitrole
concentrations in the low-dose group varied between 15.8 and 32.2
µg/l air in the different exposure periods; the levels measured in
the high-dose group ranged between 97.9 and 376.4 µg/l air. The
animals were exposed for 5 hours each day five days per week.
Treatment phases during weeks 1-13, 40-52 and 78-90 were interrupted
by treatment-free intervals. Interim necropsies of 5 animals per
dose and sex were performed after 3, 9 and 18 months; the study was
concluded after 24 months. A total of 28 rats died in week 51 due
to a defect in the air conditioning system which led to an increase
in the room temperature. Treatment of the high-dose group was
thereupon concluded, and the surviving animals (6/75 males, and
18/75 females) were necropsied.
In the high-dose group, food intake and body-weight gain were
decreased and the rate of mortality was elevated. At week 13 of the
study, levels of cholesterol, ASAT and ALAT were higher and of
glucose lower in the high-dose group than in the control group.
Also T3 and T4 levels were lower. No differences in these
parameters were observed after the treatment-free period. Relative
thyroid weight was increased and epithelial hyperplasia of the
thyroid follicles was determined in both dose groups at the end of
the first treatment interval (week 13). This latter finding was no
longer observed after the first treatment-free interval of 24 weeks,
but the thyroid weights were still elevated in both dose groups.
Follicular epithelial hyperplasia was again present in most of the
animals of both treatment groups at the end of the second treatment
phase (week 51). This finding was still observed, in the remaining
treatment group (50 µg/l air), after a treatment-free interval of 26
weeks, which indicates that complete reversion no longer occurred at
this time. Neoplasms of the thyroid (adenomas and adenocarcinomas)
were found in addition to hyperplasia at terminal necropsy (Becci,
1983).
Reproduction studies
Rats
In a limited reproduction study, groups of 10 male and 10
female Sherman rats were fed amitrole in the diet. A preliminary
one-generation study was performed in which rats were fed levels of
0, 500, or 1000 ppm for 55 days before pair-mating. The offspring
were weaned at 21 days. Complete autopsies were performed on the
parents after total exposure of 107-110 days. Ten weanling rats
from each dose group were killed. Mean body-weight gain and food
consumption were reduced in the parents at all dose levels.
Relative kidney, spleen, and liver weights (only in males) were
reduced in parents fed 500 and 1000 ppm. The average number of pups
per litter was significantly reduced at the 500 and 1000 ppm dose
levels, as were the numbers surviving to weaning. Body weight of
pups at weaning was also reduced. Thirty-three out of 45 pups of
rats fed 500 ppm and 55/56 pups of rats fed 1000 ppm died within one
week after weaning. Relative weight of thymus and spleen of pups of
the 500 ppm group (1000 ppm not examined) were significantly
reduced. Thyroid hyperplasia was seen in all treated rats (Gaines
et al., 1973).
In a subsequent 2-generation study, rats were fed dose levels
of 0, 25, or 100 ppm for 61 and 173 days before pair-mating to
produce the F1a and F1b generations, respectively. Then 12
rats/sex of each dose level were pair-mated when about 100 days old
to produce the F2a generation. The offspring were weaned at 21
days. Food consumption was reduced in the F0 generation at
100 ppm. Thyroid hyperplasia was seen in all animals of the 100 ppm
group. In the 25 ppm group hyperplasia was seen in about half of
the F0 (4/10) and F1b (4/10) females and F1b (6/10) males, but
none of the F0 males (F1a litters not examined). Pups of the 100
ppm group showed reduced kidney and liver weights and also male pups
of the 25 ppm group showed reduced liver weights. There was a
decrease in the number of litters in the F2a generation at 100 ppm,
but there were no other changes. A NOAEL for toxic effects could
not be established. Due to the low number of animals used in this
study, a NOAEL for reproductive effects could not be established
(Gaines et al., 1973).
Groups of 5 rats/sex (strain unknown) were mated following a 3-
month pretreatment period during which only males, only females, or
both sexes were exposed to amitrole at a level of 100 ppm in their
drinking-water. The groups were compared with a common control
group. According to the author, the results did not point to a
reduction in reproductive ability for males or females. However,
due to limited data in the report this could not be confirmed. Pups
born in this study were reared and continued on the treatment.
Their growth was reduced (Hapke, 1967).
Special studies on embryotoxicity and/or teratogenicity
Mice
Pregnant NMRI mice (9-12/group) were administered amitrole
(purity not specified) at 0, 500, 1000, 2500 or 5000 ppm in
drinking-water (days 6-18 of pregnancy). There was a marked
decrease (22-28%) in body-weight gain in the dams and pronounced
retardation in the development (lower fetal weight and immature
skeletons) at concentrations of 1000 ppm and above. At the highest
concentration (5000 ppm), maternal toxicity was associated with an
increase in the rate of resorptions. No irreversible structural
changes were observed at any concentration (Tjälve, 1974).
Groups of 13 pregnant C57BL6 mice were treated subcutaneously
with 215 or 464 mg/kg bw/day from days 6 though 14 of gestation.
The highest dose tested resulted in an increased fetal mortality
rate. Subcutaneous treatment of 6 AKR mice from days 6-15 of
gestation at 464 mg/kg bw/day produced no effects in the fetuses.
Daily oral administration of 215 mg/kg bw to 7 C57BL6 mice from
days 6-14 of gestation produced increased fetal mortality and a
reduction of fetal weight. In nearly all groups maternal body
weight was reduced and liver weight increased. There were no
irreversible structural changes observed in any group (Bionetics
Research Laboratories, 1968).
Rats
In a limited teratogenicity study, groups of 8 pregnant female
Sherman rats were given amitrole (99%) by stomach tube at dose
levels of 0, 20 or 100 mg/kg bw/day during days 7 through 15 of
gestation. The animals were allowed to litter and to wean, and the
pups were observed for gross abnormalities. No abnormalities were
observed in the offspring at the time of birth or when weaned
(Gaines et al., 1973).
In a pilot study, 2 female rats (strain unknown) were orally
exposed to amitrole at a dosage of 1000 mg/kg bw/day and one rat to
500 mg/kg bw/day from days 8 through 13 of gestation. Weight gain
of the treated females was reduced. No effects were found on number
of corpora lutea, number of fetuses and no anomalies were observed
(Hapke, 1967).
In a teratogenicity study, groups of 20 presumed pregnant rats
(strain FB30, Long-Evans) were exposed to amitrole by gavage at dose
levels of 0, 100, 300, or 1000 mg/kg bw/day on days 6 to 15 of
gestation. Fetuses were examined on day 20 of gestation. There
were no deaths or signs of toxicity at any dose level. Body-weight
gain was not affected by treatment. There were no treatment-related
effects on the resorption rate, fetal weight, number of live
fetuses, placental weight, or sex ratio. There was no treatment-
related increase in gross, skeletal or visceral malformations. The
NOAEL for maternal and embryo/fetotoxicity was 1000 mg/kg bw/day
(Machemer, 1977b).
Groups of 24 presumed pregnant CD rats were exposed to amitrole
(91.83% pure) by gavage at dose levels of 0, 100, 500, or 1000 mg/kg
bw/day on days 6 through 15 of gestation. Apart from the normal
parameters measured in a teratogenicity study, the weights of liver
and thyroid of the dams were examined. Fetuses were examined on day
21 of gestation. Extra groups of 14 females per dose level were
allowed to litter and wean, and were maintained until postnatal day
21. In this postnatal period, body weight, food consumption and
thyroid weights were measured in the dams. Observations in the pups
included number, sex, weight and gross examination. Weight gain of
dams was reduced during gestation days 6-18 at 500 and 1000 mg/kg
bw/day. Food intake was reduced during gestation days 15-21 at the
same dose levels. Maternal thyroid weights (absolute and relative)
were increased at 500 and 1000 mg/kg bw/day, the increase still
persisting at termination of lactation. Fetotoxicity was observed
at 1000 mg/kg bw/day including reduced fetal body weight per litter,
increased incidence of fetuses with unossified or poorly ossified
bones and an increased number of fetuses with enlarged and/or dark
thyroid. These latter findings in the thyroid were also observed in
the 500 mg/kg bw/day dose group. There was no treatment-related
increased incidence of malformations at any dose employed.
Postnatal evaluations indicated no effects of treatment on survival
or growth of the pups, or on maternal body weights, weight gain or
food consumption in the lactation period. The NOAEL for
maternotoxicity and embryo/fetotoxicity was 100 mg/kg bw/day (Tyl,
1986a).
Rabbits
Groups of 22 presumed pregnant New Zeeland white rabbits were
exposed to amitrole (91.83% pure) by gavage at doses of 0, 4, 40, or
400 mg/kg bw/day on days 6 through 18 of gestation. Fetuses were
examined on day 29 of gestation. Maternotoxicity at 40 and 400
mg/kg bw/day included reduced body-weight gain and at 400 mg/kg
bw/day increased (relative) liver weight. A dose-related increase
in the incidence of abortions was observed (0, 1, 3 and 5 for the
control, 4, 40 and 400 mg/kg bw/day dose groups, respectively). The
number of non-viable implants/litter was increased and the
percentage of live fetuses/litter decreased at 40 and 400 mg/kg
bw/day (statistically significant at high dose). Decreased fetal
weight/litter was also seen at these dose levels, with statistical
significance at the high dose. At 40 and 400 mg/kg bw/day there
were significant increases in the incidence of numerous individual
malformations, especially of the head and limbs. The total
percentages of fetuses with malformations were 4.5, 7.2, 36.9 and
62.4% for the control, 4, 40 and 400 mg/kg bw/day dose groups,
respectively. The incidence of a number of individual visceral
(including craniofacial) and skeletal variants (mainly poorly or
absent ossification) was increased at 40 and 400 mg/kg bw/day.
Effects on the fetal thyroid were also observed. The percentages of
fetuses with enlarged thyroid were 1.3, 2.2, 3.6 and 8.2% and of
fetuses with dark/red thyroids were 2.6, 7.9, 8.1 and 12.9% for the
control, 4, 40 and 400 mg/kg bw/day dose groups, respectively.
Following oral administration amitrole was teratogenic,
embryo/fetotoxic and (slightly) maternotoxic at 40 and 400 mg/kg
bw/day. The NOAEL for all types of toxicity was 4 mg/kg bw/day
(Tyl, 1986b).
Four groups of 18 artificially inseminated Hra (NZW) SPF
rabbits were treated dermally from days 7 to 19 (inclusive) of
gestation with 0, 1000, 1500 or 2000 mg amitrole (93.9% purity)/kg
bw/day. The test material was applied as a mixture of 0.5 ml
deionized water/g amitrole for six hours/day. Rabbits were collared
during the exposure period, and remaining test material was removed
with warm water washing immediately following each exposure.
Clinical signs including dermal irritation, body weight and food
intake were measured in does. Dermal irritation, including erythema
and edema, was noted for treated animals. The number of animals
affected and the severity of these observations increased with
increasing dose. Numbers of pregnant females at term were 14, 15,
12 and 11. At 2000 mg/kg bw/day thin appearance and anorexia was
observed in does. Body weight was reduced on day 20, but was
virtually recovered by day 29; food intake was reduced on days 10-
14, with severe reductions on days 14-17 and 17-20 of gestation and
uterine weight was slightly reduced at term at 2000 mg/kg bw/day.
Fetal weights were reduced at 2000 mg/kg bw/day and the incidence of
total resorptions was increased significantly (mainly early
resorptions). Malformations, including microphthalmia (3 fetuses in
2 litters), dilated brain lateral ventricles (2 pups in 1 litter),
anencephaly (2 fetuses in 2 litters), unossified pubis (4 pups in 1
litter, but several other pups in the same litter with thyroid,
skeleton and rib anomalies) were observed at 2000 mg/kg bw/day.
Following dermal administration, amitrole was teratogenic,
embryo/fetotoxic and maternotoxic at 2000 mg/kg bw/day. The NOAEL
for all types of toxicity was 1500 mg/kg bw/day (Henwood, 1988).
Special studies on genotoxicity
A number of genotoxicity tests has been carried out with
amitrole. The results are summarized in Table 2. The important
features of these data are described below.
Gene mutations
Numerous assays for in vitro gene-mutations were performed
and were predominantly negative. One study with E. coli and S.
typhimurium strains gave positive responses (Venitt & Crofton-
Sleigh, 1981). Of two other bacterial mutation assays, one assay
gave a positive result in absence of metabolic activation and the
other gave an equivocal response. Many other in vitro assays gave
negative results. A positive response was obtained in one out of
two mouse peritoneal host mediated assays with S. typhimurium
(Simmon et al., 1979). No mutation induction was observed in
fungi and yeast. No mutation induction was observed in several sex-
linked recessive assays with Drosophila melangolaster. Mutations
(TK +/-) were not induced in mouse lymphoma cells, whereas HPRT
locus and Na+/K+ATPase locus mutations were induced in Syrian
hamster embryo cells (Tsutsui et al., 1984).
Chromosomal damage
Weakly positive results were obtained in tests with fungi. A
cytogenetic study in human lymphocytes was negative, but sister-
chromatid exchanges were increased in a single study. No effects of
amitrole were observed in mice subjected to bone marrow micronucleus
tests or male dominant lethal tests.
Table 2. Results of genotoxicity assays on amitrole
Tests for gene mutations
Test system Test object Concentration/ Purity Results References
dose (%)
reverse mut. S.typhimurium 100 µg/plate 99.4 negative Brusick, 1975
(*) TA1535, TA1537
TA1538
S.cerevisiae, D4 100 µg/plate 99.4 negative
reverse mut. S.typhimurium 0.4% 93.2 negative Bamford et al.
(-) LT 2 trp 1976
(A8, B4, C3)
reverse mut. S.typhimurium 1000 µg/plate ? negative Prince, 1977
(*) TA98, TA100
TA1538, TA1535
point mut. Aspergillus up to 2000 µg ? negative Bignami et al.
(-) nidulans 1977
strain 35
reverse mut. S.typhimurium 20-12 500 µg/ 97.6 negative Herbold, 1980
(*) TA98, TA100 plate
TA1535, TA1537
reverse mut. S.typhmurium 0.2-2000 µg/ ? negative Brooks &
(*) TA1535, TA1537 plate Dean, 1981
TA1538, TA98
TA100, TA92
Tests for gene mutations (contd)
Test system Test object Concentration/ Purity Results References
dose (%)
reverse mut. S.typhimurium up to 5000 µg/ ? negative McDonald,
(*) TA98, TA100 plate 1981
TA1537
reverse mut. S.typhimurium 10-10 000 µg/ ? negative Richold &
(*) TA1535, TA1537 plate Jones, 1981
TA1538, TA98
TA100
reverse mut. S.typhimurium 0.1-2000 µg/ ? negative Rowland &
(*) TA1535, TA1537 plate Severn, 1981
TA1538, TA98
TA100
reverse mut. S.typhimurium 4-2500 µg/ ? negative Trueman,
(+) TA1535, TA1537 plate 1981
TA1538, TA98
TA100
reverse mut. E.coli 0.5-500 µg/ml ? positive Venitt &
(+) WP2uvrA(p) Crofton-
S. typhimurium positive Sleigh, 1981
TA98, TA100
reverse mut. S.typhimurium up to 500 µg/ ? equivocal Hubbard et al.
(*) TA98, TA100 ml 1981
(fluctuation test)
Tests for gene mutations (contd)
Test system Test object Concentration/ Purity Results References
dose (%)
reverse mut. E. coli WP2uvrA 10-500 µg/ml ? negative Gatehouse,
1981
(*) S. typhimurium negative
(microtiter TA98, TA1535
fluctuation test) TA1537
reverse mut. S. cerevisiae 88.9 & 889 µg/ ? negative Mehta & Von
(*) XV185-14C ml Borstel, 1981
reverse mut. S. typhimurium up to 5000 µg/ ? negative Moriya et al.
(*) TA98, TA100 plate 1983
TA1535, TA1537
TA1538
E. coli WP2 hcr negative
forward mut. E. coli CHY832 ? Hayes et al.
+ S9-mix: 10 000 µg/ml negative 1984
- S9-mix 2500 µg/ml positive
reverse mut. S. typhimurium 0.3-333.3 µg/ 98 negative Dunkel et al.
(*) TA1535, TA1537 plate 1984
(comparative TA1538, TA98
study in 4 TA100
laboratories) E. coli 0.3-333.3 µg/ negative
WP-2 uvrA plate
HPRT mutation Syrian hamster 0.3-10 µg/ml ? positive Tsutsui et al.
assay (-) embryo cells 1984
Tests for gene mutations (contd)
Test system Test object Concentration/ Purity Results References
dose (%)
mutation Na+/K+ Syrian hamster 0.3-10 µg/ml ? positive Tsutsui et al.
ATPase locus (-) embryo cells 1984
TK +/- forward mouse lymphoma up to 5000 µg/ ? negative McGregor et
mutation assay(*) cells L5178Y ml al. 1987
host mediated S. typhimurium 1450-2900 µmol/ ? negative1 Braun et al.
assay host: NMRI-mice kg 1977
host mediated S. typhimurium 12-1585 mg/kg ? positive Simmon et al.
TA1530, TA1535 ip 1979
TA1538
host:
Swiss Webster mice
1: weakly positive when given simultaneously with sodium nitrite
Tests for chromosome effects
chromosome aberr. human 0.00001-1% 93.2- negative Meretoja et
assay (-) lymphocytes 96.3 al. 1976
crossing over Aspergillus up to 2000 µg ? positive1 Bignami et al.
test (-) nidulans 1977
non-disjunct. strain P positive1
test (-)
non-disjunct. Aspergillus up to 0.4 mg/ml ? positive Morpurgo et
test (-) nidulans al. 1979
strain P
Tests for chromosome effects (contd)
Test system Test object Concentration/ Purity Results References
dose (%)
sister Chinese 0.01-100 µg/ ? positive1 Perry &
chromatid hamster ovary ml (+S9) Thomson 1981
exchange(*) cells (CHO)
1: weakly positive
Tests for DNA damage/repair
rec assay (-) B. subtilis 20 µg/plate ? negative Shirasu et al.
H17 rec+, 1976
M45 rec-
rec assay (*) B. subtilis up to 1 mg/plate ? positive1 Kada, 1981
H17 rec+,
M45 rec-
rec assay (*) E. coli 500 µg/ml ? negative Ichinotsubo et
JC 2921, 9238 al. 1981
8471, 5519, 7623
7689
rec assay (+) E. coli WP2, 4000 µg/ml ? negative Mamber et al.
WP100 1983
pol A test(-) E. coli 5 mg/plate 93.2 negative Bamford et al.
pol A1, pol A+ 1976
pol A test(*) E. coli 333 µg/plate ? negative Rosenkranz et
WP3110, P3478 al. 1981
Tests for DNA damage/repair (contd)
Test system Test object Concentration/ Purity Results References
dose (%)
differential E. coli WP2, 250-1000 µg/ ? negative Tweats, 1981
killing assay(*) WP67, CM871 ml
mitotic S. cerevisiae 100 & 1000 µg/ ? negative Kassinova et
recombination(*) T1, T2 ml al. 1981
DNA repair S. cerevisiae 100-750 µg/ ? positive Sharp &
test(*) 197/2d, rad3, ml (-S9) Parry, 1981b
rad18, rad52,
trp2
UDS test(*) HeLa cells 0.1-100 µg/ml ? positive Martin &
(+S9) McDermid, 1981
prophage induct. E. coli 58-161 1-10 mg/ml ? negative Thomson,
assay(+) Prophage lambda 1981
prophage induct. E. coli 2000 µg/plate ? negative Mamber et al.
assay(+) GY5027, GY4015 1984
mitotic crossing S. cerevisiae 100 & 1000 µg/ ? negative Kassinova et
over(*) T1, T2 ml al. 1981
mitotic gene S. cerevisiae 12.5 mg/ml ? negative Zimmermann
conversion(*) D7 & Scheel, 1981
mitotic gene S. cerevisiae 300 µg/ml ? positive Sharp & Parry
conversion(*) JD1 (-S9) 1981a
1: positive results were obtained only after metabolic activation with liver homogenates from
Japanese clam and Yellowtail fish
Tests for in vitro transformation
Test system Test object Concentration/ Purity Results References
dose (%)
Tests for in vitro transformation
cell transf. BALB/3T3 cells 0.01-2.5 mg/ml ? equivocal1 Brusick, 1976
assay(-)
cell transf. BHK-21 cells 0.025-25 µg/ml ? positive Styles, 1979
assay(+)
cell transf. BHK-21 cells 0.025-250 µg/ml ? positive Styles, 1981
assay(+)
cell transf. BHK-21 cells 4000 µg/ml ? negative Daniel &
assay(*) Dehnel, 1981
cell transf. hamster embryo 1-100 µg/ml ? positive Inoue et al.
assay(+) cells 1981
cell transf. hamster embryo 0.1-100 µg/ml ? positive Dunkel et al.
assay(-) cells 1981
rat embryo 10-1200 µg/ml ? positive
cells
cell transf. Syrian hamster 0.3-10 µg/ml ? positive Tsutsui et al.
assay(-) embryo cells 1984
Tests for in vitro transformation
Test system Test object Concentration/ Purity Results References
dose (%)
cell transf. mouse embryo ? Dunkel et al.
assay(-) cells 1984
C3H/10T1/2
laboratory A: 2-250 µg/ml negative
laboratory B: 125-1000 positive
µg/ml
1: positive only in 1 out of 3 tests
In vivo tests
non-disjunct. Drosophila 10 ppm in diet 93.2% negative Laamanen et
test females al. 1976
recessive Drosophila 10 ppm in diet 93.2% negative Laamanen et
lethal test males al. 1976
recessive Drosophila 2000 ppm in diet ? negative Vogel et al.
lethal test males 1981
dominant lethal NMRI-mice 1 x 1000 mg/kg ? negative Machemer,
test males 1977a
dominant lethal Ha(ICR)-mice 1 or 10 ppm in 94.59 negative Knickerbocker,
test males the diet for 1978
49 days
micronucleus CD-1 mice 2 x 500 mg/kg ? negative Tsuchimoto &
test males + females Matter, 1981
In vivo tests (contd)
Test system Test object Concentration/ Purity Results References
dose (%)
micronucleus NMRI-mice 1 x 10 000 mg/kg 96.9 negative Herbold, 1982
test males + females
sperm morphol. (CBA x BALB/c)F1 50-500 mg/kg/day ? negative Topham, 1980
assay 5 ip-injections
Tests were done: (*) = with and without metabolic activation (S9-mix)
(+) = with S9-mix activation
(-) = without S9-mix
DNA damage and repair
The possibility of inducing DNA damage by amitrole has been
investigated frequently and in a number of different ways. In
bacteria the results have been negative except for one recombinant
assay, which was positive when an exogenous metabolic activation was
provided by "liver" preparations from a mollusc and a fish. A DNA
repair assay in yeast gave a positive result as did a repair assay
in mammalian cells.
Cell transformation
Assays for cell transformation in several systems gave
predominantly positive results.
Other endpoints
Amitrole did not increase the frequency of morphologically
abnormal sperm in mice.
Amitrole has therefore been tested adequately in series of in
vitro and in vivo genotoxicity assays. Positive responses were
obtained in a number of mutation assays in bacteria,
recombinogenicity assays in yeast and some mammalian cell assays for
mutation, sister-chromatid exchange and cell transformation. No
genotoxicity was demonstrated in vivo. The Meeting concluded that
the genotoxic potential of amitrole was equivocal.
Special studies on skin and eye irritation and skin sensitization
In a limited study, the potential for dermal irritation by
amitrole (95%) was examined in 4 albino rabbits (strain and sex not
specified) over a 24-hour period following a single application of
dosages from 1000 up to 10 000 mg/kg bw. Minimal dermal irritation
was observed with mild erythema at the high-dose level only. By 48
hours, the skin appeared normal (Elsea, 1954).
In a limited study, the potential for eye irritation by
amitrole (95%) was examined in 3 albino rabbits (strain and sex not
specified) following application of 3 mg into the conjunctival sac
of the left eye. Observations were made at 1, 4 and 24 hours and at
daily intervals for 6 days. Mild irritation was observed at 4
hours, which subsided within 24 hours (Elsea, 1954).
In a Magnusson-Kligman maximization test in Pirbright White
guinea-pigs, amitrole (97.6%) was found to be a moderate skin
sensitizer. Concentrations employed were 2.5% for intracutaneous
induction, 25% for topical induction, and 12% for the first and
second challenges (Mihail, 1984).
In a Klecak open epicutaneous test in BOR:DHPW/SPF guinea-pigs,
amitrole (97.3%) did not have a sensitizing effect. Concentrations
employed were 0, 3, 10, or 30% for induction, and 1, 3, 10, or 30%
for the first and second challenges (Mihail, 1985).
Special studies on farm animals
Cattle tolerated administration of both a single oral dose of
amitrole at 1000 mg/kg bw as well as repeated 100 mg/kg bw doses on
10 consecutive days without symptoms (Stendel, 1965).
Female sheep (1/dose level) were orally treated with amitrole
at doses of 0, 750, or 1000 mg/kg bw at intervals of 5-7 days over a
period of 8 weeks. The only symptom observed was lethargy
immediately following treatment. Three days after the last dose the
animals were slaughtered. Residues were found in muscular tissues
at levels of 80 ± 30 ppm and 120 ± 40 ppm in the low- and high-dose
respectively (semi-quantitative determination). The thyroids were
grey-red discoloured. At histopathology complete cessation of
colloid formation, with adenomatoid proliferation of the follicle
epithelia was observed (Hapke et al., 1965).
In sheep, a single oral dose of 750 mg/kg bw led to clinical
symptoms (lethargic behaviour, frequent lying down). At higher
doses, the animals became apathetic and did not eat. At a dose
level of 4000 mg/kg bw the animals died. A single oral dose of 200
mg/kg bw was tolerated by a horse without any symptoms. Adult geese
tolerated oral doses up to 1000 mg/kg bw. At higher doses, these
birds became somnolent. Mortality occurred at 10 000 mg/kg bw
(Hapke, 1967).
Young cattle and sheep were orally exposed to amitrole at
dosages of 10, 25 or 50 mg/kg bw for up to 10 consecutive days. The
10 mg/kg bw dose was tolerated without symptoms by the cattle.
Doses of 25 or 50 mg/kg bw led to toxic symptoms after 3 and 2 days,
respectively. One out of three sheep exhibited retarded body-weight
development at 10 mg/kg bw, whereas at the higher doses all sheep
showed toxic symptoms. Hens were treated orally at dosages of 50,
100 or 250 mg/kg bw on 10 consecutive days. Retarded body-weight
development was observed at 100 and 250 mg/kg bw. Hens dosed at 375
or 500 mg/kg bw died within 3-7 days after the start of the study
(Palmer, 1972).
Observations in humans
In a patch test conducted with a human volunteer, amitrole
exerted no primary dermal irritant effect after exposure periods of
4 or 8 hours. A slight irritant effect was observed in 3 out of 6
subjects after 24 hours of exposure (no further details) (Hecht,
1954).
A case study of a 41-year old weed control operator with a 6-
month history of dermatitis involving his face, hands, back, thighs,
and feet was reported. Patch testing with 1% amitrole showed a
strong positive vesicular reaction at 2 and 4 days, indicative of
allergic contact dermatitis (English et al., 1986).
The intentional ingestion of a commercial mixture of amitrole
and diuron, at a dose equivalent to 20 mg/kg bw amitrole (together
with about 38 mg/kg bw diuron), was reported to have caused no
symptoms of poisoning in a female subject. Within a few hours, the
compound appeared in the urine at a concentration of 100 mg/100 ml.
Metabolites could not be detected in urine (Geldmacher-von
Mallinckrodt & Schmidt, 1970).
Astwood (1960) reported in a brief communication that a single
oral dose of 100 mg amitrole inhibited radioiodine uptake by the
thyroid of both normal and thyrotoxic subjects for 24 hours.
However, a dose of 10 mg was said to have had a slight effect on
iodine uptake. Astwood suggested that amitrole could be used
therapeutically in the treatment of hyperthyroidism.
An epidemiological study was conducted on Swedish railway
workers exposed to various herbicides. A small increase in tumours
was observed, particularly of the lung. Amitrole was only one of
the pesticides (other pesticides were for example phenoxy acids) to
which these workers were exposed (Axelson, 1980).
Mild cases of dermatitis on the face due to contact with
amitrole occurred yearly in one or two production workers of the
American Cyanamid Company. The dermatitis was of a primary irritant
type rather than the sensitive type. No other medical findings, not
occurring in the general public, and, in particular, no thyroid or
liver tumours were observed during the years of observation
(1955-1970) (Clyne, 1970).
Skin irritation was also occasionally observed in employees of
Bayer engaged in production of amitrole. No other adverse effects
occurred (Miksche, 1982).
The thyroid function in 5 employees who had been engaged in
production and packaging of amitrole for periods between 3 and 16
years was checked as part of the occupational medical programme.
Scintigraphic thyroid imaging findings and determinations of the T3
and T4 levels afforded no evidence for the presence of an effect on
thyroid function (Miksche, 1983).
In a study for a possible phototoxic potential of amitrole, 20
test subjects were exposed to patches containing amitrole at a level
of 1% in an ointment base (hydrous eucerin) over a period of 48
hours. The treatment areas were then irradiated with ultraviolet
light (UVA and UVB), and the dermal reactions assessed (no further
details). No evidence was found for phototoxic skin reactions
(Tronnier, 1983).
General mode of action
Amitrole is considered a goitrogenic compound. The mechanisms
by which goitrogens produce their pharmacologic and potential
neoplastic effects is through the induction of hormonal imbalance.
Most commonly this is the result of interference with the thyroid
iodide transport system or interference with peroxidases essential
to the synthesis and secretion of competent thyroid hormone. The
sequence of events triggered by this interference is generally
understood. Because of the ability of goitrogens to inhibit thyroid
hormone synthesis, these compounds have the potential to reduce
circulating levels of T3 and T4 and, consequently, to induce the
secretion of TSH by the pituitary. As a result, prolonged exposure
to such compounds can be expected to induce thyroid gland
hypertrophy and hyperplasia, nodular hyperplasia and, ultimately,
may lead to neoplasia. Evidence indicates that if the pharmacologic
effects, thyroid hypertrophy and hyperplasia, are prevented or
controlled within the feedback system, follicular cell neoplasia
does not develop. The data also suggest that some degree of
hypertrophy or hyperplasia of the thyroid gland is tolerated within
the oscillation of the feedback system without induction of
neoplasia. The rat, and to a lesser extent the mouse, appear to be
very sensitive to goitrogens, since after short exposure periods
their thyroid glands exhibit hypertrophic and hyperplastic changes.
Following continuous, long-term exposure of rats and mice to these
agents, both the thyroid and the pituitary glands frequently exhibit
neoplastic changes. In contrast, humans appear to be less sensitive
(Paynter et al., 1988; Hill et al., 1989).
COMMENTS
Amitrole is rapidly and almost completely absorbed from the
gastrointestinal tract following oral administration to rats and
mice. It is rapidly distributed throughout most body tissues, but
with a slight accumulation in tissues with a rapid cell turnover
(bone marrow, spleen, thymus, gastrointestinal tract). In a study
with pregnant mice it was observed that amitrole passes through the
placenta into the fetuses with the same distribution pattern as in
the mothers. Excretion is rapid after oral exposure. Within 24
hours, 70-95% of the administered radioactivity is excreted via the
urine, mainly as the parent compound.
The metabolic transformation in mammals produces two minor
metabolites detectable in the urine. Metabolism of amitrole occurs
mainly in the liver and involves substitution of the hydrogen atom
in the 5-position. The metabolites identified were 3-amino-5-
mercapto-1,2,4-triazole and 3-amino-1,2,4-triazolyl-5-mercapturic
acid.
Amitrole has a low acute toxicity when tested in several
species by various routes of administration. In old studies,
amitrole was reported to have slight irritating effects on the skin
and eyes. Evidence of a moderate sensitizing potential was observed
in a Magnusson-Kligman test but not in a Klecak open epicutaneous
test. WHO has classified amitrole as unlikely to present acute
hazard in normal use.
Oral exposures of up to four weeks in rats revealed that
effects on the thyroid occurred at levels > 60 ppm in the diet or
104 ppm in drinking-water. No effects were observed at 30 ppm in
the diet (equivalent to 3 mg/kg bw/day) or 10 ppm in drinking-water
(equivalent to 1.5 mg/kg bw/day). Furthermore, it was shown that
after a recovery period the effects on the thyroid were reversible.
In a 30-day study in mice at concentrations in drinking-water
of 0, 5000, 10 000 or 20 000 mg/l, liver effects were observed at
all dose levels.
Several short-term oral studies were performed with rats.
These were mainly focused on the effects on the thyroid, as this is
the target organ in rats.
Only two oral studies were suitable for assessment. In one
study, male rats were exposed to dietary concentrations of 0, 2, 10
or 50 ppm for 13 weeks or to 0, 0.25 or 0.50 ppm for 11 weeks. The
NOAEL was 2 ppm (equivalent to 0.1 mg/kg bw/day) based on
histological changes in the thyroid (appearance of follicular cells,
contents of colloid and capillary density). Decreases in protein-
bound iodine were not considered to be biologically significant.
The other study consisted of four short-term experiments in
female rats. Dietary concentrations in one experiment were 0, 2, 20
or 200 ppm with exposure for six weeks, in two subsequent
experiments 0, 20, 50 or 200 ppm with exposure for 6 or 13 weeks and
in the fourth experiment 0, 20, 50, 200 or 500 ppm with exposure for
six weeks. From these four experiments, the overall NOAEL was 2 ppm
(equivalent to 0.1 mg/kg bw/day) based on increased iodine uptake
(shortly after injection), increased thyroid weight and
histopathological changes of the thyroid (goitre and clearly
activated thyroids).
From several short-term studies in rats with administration in
drinking-water, slight effects on the thyroid (moderate stimulation
of the thyroid epithelium) were seen at the lowest concentration
tested, 50 ppm.
In a one-year study in dogs, no effects on the thyroid were
observed at the highest dose tested (12.5 mg/kg bw/day). The only
effect observed at this level was pale-coloured pancreas. The test,
however, was performed with a low number of animals.
Long-term toxicity and/or carcinogenicity studies have been
performed in mice, rats, and golden hamsters. Studies in mice were
focused on induction of liver and thyroid tumours. In a
carcinogenicity study in mice with only one but very high dose level
(1000 mg/kg bw/day by gavage), survival time was significantly
reduced and liver and thyroid tumours were observed in all treated
mice. A slight increase in the incidence of liver tumours was
observed in a special carcinogenicity study in which pups were
treated for a period of 90 weeks at a level of 500 ppm in the diet.
In an 18-month carcinogenicity study in mice at levels of 0, 1,
10 or 100 ppm in the diet, an increased incidence of tumours was not
observed. In this study, a thyroid function test was also performed
with a small number of animals. At 100 ppm an increase in thyroid
weight and in iodine accumulation in the thyroid was observed. The
NOAEL was 10 ppm (equivalent to 1.5 mg/kg bw/day).
In a carcinogenicity study in rats with levels of 0, 1, 10 or
100 ppm in the diet, a slight decrease in survival time, an increase
in the incidence of thyroid tumours and an increase in the incidence
of (mainly benign) pituitary tumours were observed at 100 ppm. In
this study, a thyroid function test was also performed with a small
number of animals. At 100 ppm, thyroid weight was increased during
the whole study period as was the percentage accumulation of
radioiodine in the thyroid. The NOAEL was 10 ppm (equivalent to 0.5
mg/kg bw/day).
In another limited long-term toxicity study in rats, the NOAEL
was 10 ppm (equivalent to 0.5 mg/kg bw/day) based on thyroid
hyperplasia. A clearly enhanced thyroid tumour incidence was found
at 50 and 100 ppm. In this study, animals suffered from apparent
respiratory infection.
In a third study in rats, thyroid hyperplasia and thyroid
tumours were observed in animals fed 100 ppm (during the first 40
weeks of the 115-120 week study, the dose level was 5 ppm). In rats
treated at pulsed intervals (alternate four week periods) at levels
of 60 ppm (first 3 ppm) and 200 ppm (first 10 ppm) thyroid tumours
were also observed. Slight thyroid hyperplasia was also observed at
the lowest dose level of 20 ppm (first 1 ppm; intermittent dosing
regimen). A NOAEL could not be established.
In an 18-month carcinogenicity study in Syrian hamsters at
dietary concentrations of 0, 1, 10 or 100 ppm, the NOAEL was 10 ppm,
equivalent to 1 mg/kg bw/day, based on decreased body-weight gain
and increased mortality. No effects on the thyroid were observed at
100 ppm. There was no evidence of carcinogenic potential.
A well-performed reproduction study was not available. From a
limited study in rats at dietary concentrations ranging from 25 to
1000 ppm, effects on reproductive capability were observed at 500
ppm and above. Reduction of liver weight and thyroid hyperplasia
were the most sensitive effects observed at the lowest dose level
(25 ppm, equivalent to 1.3 mg/kg bw/day).
In a teratogenicity study, rats were exposed by gavage at doses
of 0, 100, 300 or 1000 mg/kg bw/day on days 6 to 15 of gestation.
No effects were observed in this study. The NOAEL for
maternotoxicity and embryo/ fetotoxicity was 1000 mg/kg bw/day.
In another teratogenicity study, rats were exposed by gavage at
doses of 0, 100, 500 or 1000 mg/kg bw/day. Slight maternal toxicity
(reduced weight gain and food consumption and increased thyroid
weights) was observed at doses of 500 and 1000 mg/kg bw/day.
Reduced fetal body weight/litter and reduced skeletal ossifications
were observed in the high-dose group. Increased incidences of
enlarged and/or dark thyroids were seen in fetuses at 500 and 1000
mg/kg bw/day. The NOAEL for maternotoxicity and embryo/fetotoxicity
was 100 mg/kg bw/day. Amitrole was considered not to be teratogenic
in rats at dose levels up to 1000 mg/kg bw/day.
In a teratogenicity study in rabbits the animals were exposed
by gavage to dose levels of 0, 4, 40 or 400 mg/kg bw/day. Decreased
weight gain during the gestation period was observed at 40 and 400
mg/kg bw/day and increased liver weight at 400 mg/kg bw/day. A
dose-related increased incidence of abortions was observed in all
treated groups. Embryo/fetotoxicity were observed at 40 and 400
mg/kg bw/day. Increased incidences of irreversible structural
changes were also found at these dose levels, which involved mainly
the head and limbs. The NOAEL for maternotoxicity,
embryo/fetotoxicity and teratogenicity was 4 mg/kg bw/day.
In a dermal teratogenicity study in rabbits at dose levels of
0, 1000, 1500 or 2000 mg/kg bw/day, maternotoxicity (decreased body
weight and food consumption, thin appearance and anorexia) was
observed at 2000 mg/kg bw/day. At this level, irreversible
structural changes (anencephaly and microphthalmia) were observed.
The NOAEL for maternotoxicity, embryo/fetotoxicity and
teratogenicity after dermal exposure was 1500 mg/kg bw/day.
Amitrole has been tested adequately in series of in vitro and
in vivo genotoxicity assays. Positive responses were obtained in
a number of mutation assays in bacteria, recombinogenicity assays in
yeast and some mammalian cell assays for mutation, sister-chromatid
exchange and cell transformation. No genotoxicity was demonstrated
in vivo. The Meeting concluded that the genotoxic potential of
amitrole was equivocal.
Amitrole is a goitrogen in mice, rats and sheep but not in
Syrian hamsters, dogs, or cattle at the doses that have been tested.
The mechanism of thyroid toxicity involves inhibition of thyroid
peroxidase. This inhibition results in decreases in circulating
levels of T4 and T3 which stimulate the pituitary to increase
secretion of TSH which in turn may cause thyroid hypertrophy,
hyperplasia and neoplasia. Threshold doses have been identified in
the sensitive species. Amitrole is not genotoxic in in vivo
assays.
The Meeting withdrew the conditional ADI and established a
temporary ADI, based on the NOAEL of 0.5 mg/kg bw/day in the 24-
month dietary study in rats, using a safety factor of 1000 because
of inadequacy of the data.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Mouse: 10 ppm, equivalent to 1.5 mg/kg bw/day
(18-month study)
Rat: 10 ppm, equivalent to 0.5 mg/kg bw/day
(24-month study)
100 mg/kg bw/day (teratogenicity study)
Hamster: 10 ppm, equivalent to 1.0 mg/kg bw/day
(18-month study)
Dog 12.5 mg/kg bw/day (12 month study)
Estimate of temporary acceptable daily intake for humans
0-0.0005 mg/kg bw
Studies without which the determination of a full ADI is
impracticable
Results be submitted to WHO by 1996
(all known to have been initiated):
Two-generation reproduction study in rats
One-year study in dogs
Oral teratogenicity study in rabbits
Metabolism study in rats
Studies which will provide information valuable in the continued
evaluation of the compound
Further observations in humans.
Comparative biotransformation (including humans).
Clarification of genotoxic potential of amitrole.
REFERENCES
Alexander, N.M. (1959). Antithyroid action of 3-amino-1,2,4-
triazole. J. Biol. Chem. 234: 148-150.
Astwood, E.B. (1960). Cranberries, turnips and goiter. J. American
Med. Assoc. 172: 1319-1320.
Axelson, O., Sundell, L., Andersson, K., Edling, C., Hogstedt, C.,
Kling, H. (1980). Herbicide exposure and tumor mortality. An updated
epidemiologic investigation on Swedish railroad workers. Scan. J.
Work Environ. Health, 6: 73-79.
Babish, J.G. (1977). Triiodothyronine (T3) and Thyroxine (T4)
levels in male rats consuming Amitrole (3-amino-1,2,4-triazole) in
their diets for four weeks followed by a four week recovery period.
Unpublished report No 5539 (dated: 29-11-1977) from Food and Drug
Research Laboratories, Inc.. Submitted to WHO by Bayer AG.
Bagdon, R.E., Shaffer, C.B., Vidone, L.B., Golz, H.H. (1956).
Aminotriazole - acute and subacute toxicity. Unpublished report No
56-29 (dated: 14-12-1956) from American Cyanamid Company. Submitted
to WHO by Bayer AG.
Bamford, D., Sorsa, M., Gripenberg, U., Laamanen, I., Meretoja, T.
(1976). Mutagenicity and toxicity of amitrole. III. Microbial tests.
Mutation Research, 40: 197-202.
Becci, P. (1983). Evaluation of the chronic inhalation toxicity and
carcinogenicity of amitrole in rats. Unpublished report No 5821
(dated: 13-01-1983) from Food and Drug Research Laboratories, Inc..
Submitted to WHO by Bayer AG.
Ben-Dyke, R., Perry, M.C., Wall, I. (1973). The short-term
cumulative toxicity of aminotriazole in the rat. Unpublished report
Tox/73/39-6 (dated: September 1973) from Fisons Limited Agrochemical
division, Toxicology department. Submitted to WHO by Bayer AG.
Bignami, M., Aulicino, F., Velcich, A., Carere, A., Morpurgo, G.
(1977). Mutagenic and recombinogenic action of pesticides in
Aspergillus nidulans. Mutation Research, 46: 395-402.
Bionetics Research Laboratories (1968). Evaluation of carcinogenic,
teratogenic, and mutagenic activities of selected pesticides and
industrial chemicals. Volume II: Teratogenic study in mice and rats.
Report PB-223 160 (dated: August 1968) from Bionetic Research Labs,
Inc., prepared for National Cancer Institute.
Braun, R., Schöneich, J., Ziebarth, D. (1977). In vivo formation
of N-nitroso compounds and detection of their mutagenic activity in
the host-mediated assay. Cancer Research, 37: 4572-4579.
Brooks, T.M. & Dean, B.J. (1981). Mutagenic activity of 42 coded
compounds in the salmonella/microsome assay with preincubation.
Progress in Mutation Research, 1: 261-270.
Brusick, D.J. (1975). Mutagenic evaluation of compound 3-amino-
1,2,4-triazole. Unpublished report No 2547 (dated: 14-08-1975) from
Litton Bionetics, Inc. Submitted to WHO by Bayer AG.
Brusick, D.J. (1976). Mutagenicity evaluation of 3-amino-1,2,4-
triazole; in vitro cellular transformation in BALB/3T3 cells.
Unpublished report No 2549 (dated: 26-10-1976) from Litton
Bionetics, Inc. Submitted to WHO by Bayer AG.
Clyne, R.M. (1970). Statement before the amitrole advisory
committee. Unpublished document from the American Cyanamid Company
dd 16 December 1970. Submitted to WHO by Bayer AG.
Cox, G.E. & Re, T.A. (1978). Inhalation toxicity study with 3-amino-
1,2,4-triazole (Amitrole) in adult Fisher 344 rats. Unpublished
report No 5492 (dated: 08-09-1978) from Food and Drug Research
Laboratories, Inc., Submitted to WHO by Bayer AG.
Daniel, M.R. & Dehnel, J.M. (1981). Cell transformation test with
baby hamster kidney cells. Progress in Mutation Research, 1: 626-
637.
Den Tonkelaar, E.M. & Kroes, R. (1974). Schildklierfunctieonderzoek
na subacute en semichronische toediening van aminotriazol.
Unpublished report No. 64/74 Tox. from the National Institute of
Public Health, Bilthoven, the Netherlands.
Dunkel, V.C., Pienta, R.J., Sivak, A., Traul, K.A. (1981).
Comparative neoplastic transformation responses of Balb/3T3 cells,
Syrian hamster embryo cells, and Rauscher murine leukemia virus-
infected Fischer 344 rat embryo cells to chemical compounds. J.
Nat. Cancer Inst. 67: 1303-1315.
Dunkel, V.C., Zeiger, E., Brusick, D., McCoy, E., McGregor, D.,
Mortelmans, K., Rosenkranz, H.S., Simmon, V.F. (1984).
Reproducibility of microbial mutagenicity assays: I. Tests with
Salmonella typhimurium and Escherichia coli using a standardized
protocol. Environmental Mutagenesis, 6: 1-25.
Dunkel, V.C., Schechtman L.M., Tu, A.S., Sivak, A., Lubet, R.A.,
Cameron, T.P. (1988). Interlaboratory evaluation of the C3H/10T1/2
cell transformation assay. Env. Mol. Mutagen. 12: 21-31.
Elsea, J.R. (1954). Amino Triazole. Acute dermal application - acute
eye application. Unpublished report from Hazleton Laboratories,
Virginia (dated: 23-12-1954). Submitted to WHO by Bayer AG.
English, J.S., Rycroft, R.J., Calnan, C.D. (1986). Allergic contact
dermatitis from aminotriazole. Contact Dermatitis, 14: 255-256.
Fang, S.C., George, M., Yu, T.C. (1964). Metabolism of 3-amino-
1,2,4-triazole-5-C14 by rats. J. Agric. Food Chem. 12: 219-223.
Fang, S.C., Khanna, S., Rao, A.V. (1966). Further study on the
metabolism of labelled 3-amino-1,2,4-triazole (ATA) and its plant
metabolites in rats. J. Agric. Food Chem. 14: 262-265.
Feinstein, R.N., Fry, R.J.M, Staffeldt, E.F. (1978). Carcinogenic
and Antitumor effects of aminotriazole on acatalasemic and normal
catalase mice. J. Natl. Cancer Inst. 60: 1113-1116.
Fogleman, R.W. (1954). Amizole: Acute administration -
pharmacodynamics - subacute feeding. Unpublished report from
Hazleton Laboratories, Virginia (dated: 02-12-1954). Submitted to
WHO by Bayer AG.
Franco, L., Municio, A.M. (1975). Comparative metabolism of 3-amino-
1,2,4-triazole. Gen. Pharmac. 6: 163-169.
Fregly, M.J. (1968). Effect of aminotriazole and thyroid function in
the rat. Toxicol. Appl. Pharmacol. 13: 271-286.
Fujii, T., Miyazaki, H., Hashimoto, M. (1984). Autoradiographic and
biochemical studies of drug distribution in the liver. III. (14C)
Aminotriazole. Eur. J. Drug Metab. Pharmacokinet. 9: 257-265.
Gaines, T.B., Kimbrough, R.D., Linder, R.E. (1973). The toxicity of
amitrole in the rat. Toxicol. Appl. Pharmacol. 26: 118-129.
Gatehouse, D. (1981). Mutagenic activity of 42 coded compounds in
the microtiter fluctuation assay. Progress in Mutation Research,
1: 376-386.
Geldmacher-von Mallinckrodt, M. & Schmidt, H.P. (1970). Toxicity and
metabolism of aminotriazole in man. Arch. Toxikol. 27: 13-18.
Grunow, W., Altmann, H.-J., Böhme, Chr. (1975). Metabolism of 3-
amino-1,2,4-triazole in rats. Arch. Toxicol. 34: 315-324.
Hapke, H.-J., Rüssel, H., Ueberschär, S. (1965). Dangers in the use
of aminotriazol. Dtsch. Tierärztl. Wochenschrift, 72: 204-206.
Hapke, H.-J. (1967). The toxicity of aminotriazole for domestic
animals. Zbl. Vet. Med. 14: 469-486.
Hayes, S., Gordon, A., Sadowski, I., Hayes, C. (1984). RK bacterial
test for independently measuring chemical toxicity and mutagenicity:
Short-term forward selection assay. Mutation Research, 130: 97-
106.
Hecht (1954). Aminotriazol - Letter report of May 11, 1954 from
Bayer AG Institute of Toxicology. Submitted to WHO by Bayer AG.
Hecht & Kimmerle (1962). Aminotriazol - Letter report of August 23,
1962 from Bayer AG Institute of Toxicology. Submitted to WHO by
Bayer AG.
Heimann, K.G. (1982). Aminotriazol techn. - Letter report of April
7, 1982 from Bayer AG Institute of Toxicology. Submitted to WHO by
Bayer AG.
Henwood, S.M. (1988). Percutaneous teratology study with amitrole
technical in rabbits. Unpublished report on number HLA 6224-107
from Hazelton Laboratories America, Inc., submitted to WHO by Rhône-
Poulenc Ag. Company
Herbold, B. (1980). Amitrole; Salmonella/microsome test for
detection of point-mutagenic effects. Unpublished report No 9339
(dated: 01-08-1980) from Bayer AG Institute of Toxicology. Submitted
to WHO by Bayer AG.
Herbold, B. (1982). Amitrole; Micronucleus test on the mouse to
evaluate for mutagenic effect. Unpublished report No 11139 (dated:
13-09-1982) from Bayer AG Institute of Toxicology. Submitted to WHO
by Bayer AG.
Hill, R.N., Erdreich, L.S., Paynter, O.E., Roberts, P.A., Rosenthal,
S.L., Wilkinson, C.F. (1989). Thyroid follicular cell
carcinogenesis. Fund. Appl. Toxicol. 12: 629-697.
Hubbard, S.A., Green, M.H.L., Bridges, B.A., Wain, A.J., Bridges,
J.W. (1981). Fluctuation test with S9 and hepatocyte activation.
Progress in Mutation Research, 1: 361-370.
Ichinotsubo, D., Mower, H., Mandel, M. (1981). Testing of a series
of paired compounds (carcinogen and non-carcinogenic structural
analog) by DNA repair-deficient E. coli strains. Progress in
Mutation Research, 1: 195-198.
Innes, J.R., Ulland, B.M., Valerio, M.G., Petrucelli, L., Fishbein,
L., Hart, E.R., Palotta, A.J. (1969). Bioassay of pesticides and
industrial chemicals for tumorigenicity in mice: A preliminary note.
J. Nat. Cancer Inst. 42: 1101-1114.
Inoue, K., Katoh, I., Takayama, S. (1981). In vitro transformation
of hamster embryo cells by 3-(N-salicycloyl)amino-1,2,4-triazole.
Toxicol. Letters, 7: 211-215.
Johnson, W.D. (1981). Lifetime feeding study of amitrole in Fischer
344 rats. Unpublished report No 5651 (dated: 07-08-1981) from Food
and Drug Research Laboratories, Inc. Submitted to WHO by Bayer AG.
Jukes, T.H & Shafer, C.B. (1960). Antithyroid effects of
aminotriazole. Science, 132: 296-297.
Kada, T. (1981). The DNA-damaging activity of 42 coded compounds in
the rec-assay. Progress in Mutation Research, 1: 171-182.
Kassinova, G.V., Kovaltzova, S.V., Marfin, S.V., Zakharov, I.A.
(1981). Activity of 40 coded compounds in differential inhibition
and mitotic crossing-over assays in yeast. Progress in Mutation
Research, 1: 434-455.
Keller, J. (1959). Aminotriazole: Two-year chronic feeding - rats.
Unpublished report from Hazleton Laboratories Virginia (dated: 02-
01-1959). Submitted to WHO by Bayer AG.
Kimmerle (1968). Aminotriazol - Letter report of June 12, 1968 from
Bayer AG Institute of Toxicology. Submitted to WHO by Bayer AG.
Knickerbocker, M. (1978). A study to determine the potential of
amitrole to induce dominant lethal mutations in Ha (ICR) mice.
Unpublished report no 5502 (dated: 28-02-1978) from Food and Drug
Research Laboratories, Inc. Submitted to WHO by Bayer AG.
Laamanen, I., Sorsa, M., Bamford, D., Gripenberg, U. Meretoja, T.
(1976). Mutagenicity and toxicity of amitrole. I. Drosophila
tests. Mutation Research, 40: 185-190.
Lemoine, N.R., Mayall, E.S., Williams, E.D., Thurston, V., Wynford-
Thomas, D. (1988). Agent-specific ras oncogene activation in rat
thyroid tumours. Oncogene, 3: 541-544.
MacDonald, C.M., Pullinger, D.H. (1976). A pharmacokinetic study to
compare "head only" and "whole body" inhalation methods of 14C-
amitrole exposure. Unpublished report No 291/1 (dated: March 1976)
from Hazleton Laboratories Europe Ltd. Submitted to WHO by Bayer AG.
Machemer, L. (1977a). Amitrole/Dominant lethal test on the male
mouse to evaluate for mutagenic effects. Unpublished report no 6679
(dated: 23-03-1977) from Bayer AG, Institute of Toxicology.
Submitted to WHO by Bayer AG.
Machemer, L. (1977b). Amitrole; Study for embryotoxic and
teratogenic effects after oral administration to the rat.
Unpublished report No 6634 (dated: 24-02-77) from Bayer AG Institute
of Toxicology. Submitted to WHO by Bayer AG.
Mamber, S.W., Bryson, V., Katz, S.E. (1983). The Escherichia coli
WP2/WP100 rec assay for detection of potential chemical carcinogens.
Mutation Research, 119: 135-144.
Mamber, S.W., Bryson, V., Katz, S.E. (1984). Evaluation of the
Escherichia coli K12 inductest for detection of potential chemical
carcinogens. Mutation Research, 130: 141-151.
Martin, C.N. & McDermid, A.C. (1981). Testing of 42 coded compounds
for their ability to induce unscheduled DNA repair synthesis in HeLa
cells. Progress in Mutation Research, 1: 533-537.
McDonald, D.J. (1981). Salmonella/microsome tests on 42 coded
chemicals. Progress in Mutation Research, 1: 285-297.
McGregor, D.B., Martin, R., Cattanach, P., Edwards, I., McBride, D.,
Caspary, W.J. (1987). Response of the L5178Y Tk+/Tk-mouse lymphoma
cell forward mutation assay to coded chemicals. I. Results for nine
compounds. Env. Mutagen. 9: 143-160.
Mehta, R.D., von Borstel, R.C. (1981). Mutagenic activity of 42
coded compounds in the haploid yeast reversion assay, strain XV185-
14C. Progress in Mutation Research, 1: 414-423.
Meretoja, T., Gripenberg, U., Bamford, D., Laamanen, I., Sorsa, S.
(1976). Mutagenicity and toxicity of amitrole, II. Human lymphocyte
culture tests. Mutation Research, 40: 191-196.
Mihail, F. (1984). Aminotriazole - Studies for skin sensitization
effect on guinea pigs. Unpublished report No 12607 (dated 12-04-
1984) from Bayer AG Institute of Toxicology. Submitted to WHO by
Bayer AG.
Mihail, F. (1985). Aminotriazole - Study for skin-sensitising effect
on guinea pigs in the open epicutaneous test. Unpublished report No
13775 (dated: 27-08-1985) from Bayer AG Institute of Toxicology.
Submitted to WHO by Bayer AG.
Mihail, F. & Schilde, B. (1984). Aminotriazole - Study for subacute
dermal toxicity to rabbits Unpublished report No 12369 (dated: 09-
01-1984) from Bayer AG Institute of Toxicology. Submitted to WHO by
Bayer AG.
Miksche, L.W. (1982). Letter report of 22-06-82 - BBA request-
effects on man. Unpublished document from the Bayer AG, LE Medical
Department Leverkusen. Submitted to WHO by Bayer AG.
Miksche, L.W. (1983). Letter report of 22-04-83 - Production of
amitrole-medical monitoring. Unpublished document from the Bayer AG,
LE Medical Department Leverkusen. Submitted to WHO by Bayer AG.
Mori, S., Takeuchi, Y., Toyama, M., Makino, S., Ohhara, T., Tochino,
Y., Hayashi, Y. (1985). Amitrole: Strain differences in
morphological response of the liver following subchronic
administration to mice. Toxicology Letters, 29: 145-152.
Moriya, M., Ohta, T., Watanabe, K., Miyazawa, T., Kato, K., Shirasu,
Y. (1983). Further mutagenicity studies on pesticides in bacterial
reversion assay systems. Mutation Research, 116: 185-216.
Morpurgo, G., Bellincampi, D., Gualandi, G., Baldinelli, L., Serlupi
Crescenzi, O. (1979). Analysis of mitotic nondisjunction with
Aspergillus nidulans. Env. Health Perspect. 31: 81-95.
Napalkov, N.P. (1962). Blastomogenic action of 3-amino-1,2,4-
triazole. (Russ). Gig. Tr. prof. Zabol. 6: 48-51. In: Amitrole; IARC
Monographs on the evaluation of carcinogenic risk of chemicals to
humans (1986). World Health Organization, Vol. 41: 293-317, Lyon.
Palmer, J.S. (1972). Toxicity of 45 organic herbicides to cattle
sheep, and chickens. Production Research report no 137 (dated: March
1972) from US Department of Agriculture. Submitted to WHO by Bayer
AG.
Paynter O.E., Burin, G.J., Jaeger, R.B., Gregorio C.A. (1988).
Goitrogens and thyroid follicular cell neoplasia: evidence for a
threshold process. Regul. Toxicol. Pharmacol. 8: 102-119.
Perry, P.E. & Thomson, E.J. (1981). Evaluation of sister chromatid
exchange method in mammalian cells as a screening system for
carcinogens. Progress in Mutation Research, 1: 560-569.
Prince, H.N. (1977). Microbial mutagen assays with amitrole (3-
amino-1,2,4-triazole). Unpublished report no 5634 (dated: 12-09-
1977) from Gibraltar Biological Laboratories (submitted via Food and
Drug Research Laboratories, Inc.). Submitted to WHO by Bayer AG.
Reitze, H.K. & Seitz, K.A. (1985). Light and electron microscopical
changes in the liver of mice following treatment with aminotriazole.
Exp. Pathol. 27: 17-31.
Richold, M. & Jones, E. (1981). Mutagenic activity of 42 coded
compounds in the Salmonella/microsome assay. Progress in Mutation
Research, 1: 314-322.
Rosenkranz, H.S., Hyman, J., Leifer, Z. (1981). DNA polymerase
deficient assay. Progress in Mutation Research, 1: 210-218.
Rowland, I. & Severn, B. (1981). Mutagenic of carcinogens and
noncarcinogens in the salmonella/micrososme test. Progress in
Mutation Research, 1: 323-332.
Seidenberg, I.L. & Gee, A.H. (1953). Report on acute toxicity of
aminotriazole as established by oral administration to rats.
Unpublished report from Foster D. Snell Inc. (dated: 08-06-1953).
Submitted to WHO by Bayer AG.
Shah, P.V. & Guthrie, F.E. (1977). Dermal absorption, distribution,
and the fate of six pesticides in the rabbit. In: Pesticide
Management and Insecticide Resistance. pp 547-554. Academic Press.
Sharp, D.C. & Parry, J.M. (1981a). Induction of mitotic gene
conversion by 41 coded compounds using the yeast culture JD1.
Progress in Mutation Research, 1: 491-501.
Sharp, D.C. & Parry, J.M. (1981b). Use of repair deficient strains
of yeast to assay the activity of 40 coded compounds. Progress in
Mutation Research, 1: 502-516.
Shirasu, Y., Moriya, M., kato, K., Furuhashi, A., Kada, T. (1976).
Mutagenicity screening of pesticides in the microbial system.
Mutation Research, 40: 19-30.
Simmon, V.H., Rosenkranz, H.S., Zeiger, E., Poirer, L.A. (1979).
Mutagenic activity of chemical carcinogens and related compounds in
the intraperitoneal host-mediated assay. J. Natl. Cancer Inst. 62:
911-918.
Steinhoff, D. & Boehme, K. (1978). Aminotriazole; carcinogenesis
study with oral administration to Syrian golden hamsters.
Unpublished report No 7521 (dated: 16-05-1978) from Bayer AG,
Institute of Toxicology. Submitted to WHO by Bayer AG.
Steinhoff, D. & Boehme, K. (1979a). Aminotriazole; carcinogenesis
study with oral administration to rats. Unpublished report No 8450
(dated: 11-06-1979) from Bayer AG, Institute of Toxicology.
Submitted to WHO by Bayer AG.
Steinhoff, D. & Boehme, K. (1979b). Aminotriazole; carcinogenesis
study with oral administration to mice. Unpublished report No 8490
(dated: 17-07-1979) from Bayer AG, Institute of Toxicology.
Submitted to WHO by Bayer AG.
Steinhoff, D., Weber, H., Mohr, U., Boehme, K. (1983). Evaluation of
Amitrole (Aminotriazole) for potential carcinogenicity in orally
dosed rats, mice, and golden hamsters. Toxicol. Appl. Pharmacol.
69: 161-169.
Stendel (1965). Aminotriazole/oral toxicity to cattle. Unpublished
letter report of March 15, 1965 from Bayer AG, Institute of
Toxicology. Submitted to WHO by Bayer AG.
Strum, J.M. & Karnovsky, M.B. (1971). Aminotriazole goiter. Fine
structure and localization of thyroid peroxidase activity.
Laboratory Investigation, 24: 1-12.
Styles, J.A. (1979). Studies on the detection of carcinogens using a
mammalian cell transformation assay with liver homogenate
activation. In: Norpoth, K. & Garner, R.C. (eds), Short-term
mutagenicity test systems for detecting carcinogens. Proceedings of
the International Symposium on short-term mutagenicity test systems.
Dortmund, Nov. 15-17, 1978 pp 226-238. Springer Verlag, New York.
Styles, J.A. (1981). Activity of 42 coded compounds in the BHK-21
cell transformation test. Progress in Mutation Research, 1: 638-
646.
Thomson, J.A. (1981). Mutagenic activity of 42 coded compounds in
the lambda induction assay. Progress in Mutation Research, 1: 224-
235.
Thyssen, J. (1974a). Aminotriazol Batch no 143 - Determination of
acute toxicity. Letter report of June 10, 1974 from Bayer AG,
Institute of Toxicology. Submitted to WHO by Bayer AG.
Thyssen, J. (1974b). Aminotriazol - Determination of acute toxicity.
Letter report of September 26, 1974 from Bayer AG, Institute of
Toxicology. Submitted to WHO by Bayer AG.
Thyssen, J. (1983). Aminotriazole - Acute inhalation toxicity.
Unpublished report No 11707 (dated: 11-04-1983) from Bayer AG,
Institute of Toxicology. Submitted to WHO by Bayer AG.
Tjälve, H. (1974). Fetal uptake and embryogenetic effects of
aminotriazole in mice. Arch. Toxicol. 33: 41-48.
Tjälve, H. (1975). The distribution of labelled aminotriazole in
mice. Toxicology, 3: 49-67.
Topham, J.C. (1980). Do induced sperm-head abnormalities in mice
specifically identify mammalian mutagens rather than carcinogens?
Mutation Research, 74: 379-387.
Tronnier, H. (1983). Aminotriazole - Expertise by consultant
dermatologist on testing for phototoxicity in test subjects.
Unpublished report No R 2544 (dated: 05-08-1983) from City Clinics
Dortmund. Submitted to WHO by Bayer AG
Trueman, R.W. (1981). Activity of 42 coded compounds in the
Salmonella reverse mutation test. Progress in Mutation Research,
1: 343-350.
Tsuchimoto, T. & Matter, B.E. (1981). Activity of coded compounds in
the micronucleus test. Progress in Mutation Research, 1: 705-711.
Tsuda, H., Takahashi, M., Fukushima, S., Endo, Y. (1973). Fine
structure and localization of peroxidase activity in aminotriazole
goiter. Nagoya Med. J., 18: 183-197.
Tsutsui, T., Maizumi, H., Barrett, J.C. (1984). Amitrole-induced
cell transformation and gene mutations in Syrian hamster embryo
cells in culture. Mutation Research, 140: 205-207.
Turner, D.M., Gilbert, C.M. (1976). Evidence of metabolismus of
(14C) Amitrole in the rat after inhalation administration.
Unpublished report No 291/1a (dated May 1976) from Hazleton
Laboratories Europe Ltd. Submitted to WHO by Bayer AG.
Tweats, D.J. (1981). Activity of 42 coded compounds in a
differential killing test using Escherichia coli strains Wp2, WP67
(uvrA polA), and CM871 (uvrA lexA recA). Progress in Mutation
Research, 1: 199-209.
Tyl, R.W. (1986a). Teratogenicity evaluation of aminotriazole
technical administered by gavage to CD rats. Unpublished report No
49-45 (dated: 29-05-1986) from Union Carbide Bushy Run Research
Center. Submitted to WHO by Bayer AG and Rhône-Poulenc Ag. Company.
Tyl, R.W. (1986b). Teratogenicity evaluation of aminotriazole
technical administered by gavage to New Zealand White rabbits.
Unpublished report No 49-66 (dated: 30-05-1986) from Union Carbide
Bushy Run Research Center. Submitted to WHO by Rhône-Poulenc Ag.
Company.
Venitt, S. & Crofton-Sleigh, S. (1981). Mutagenicity of 42 coded
compounds in a bacterial assay using Escherichia coli and
Salmonella typhimurium. Progress in Mutation Research, 1: 351-360.
Vesselinovitch, S.D. (1983). Perinatal hepatocarcinogenesis.
Biological Research in Pregnancy and Perinatology, 4: 22-25.
Vogel, E., Blijleven, W.G.H., Kortselius, M.J.H., Zijlstra, J.A.
(1981). Mutagenic activity of 17 coded compounds in the sex-linked
recessive lethal test in Drosophila melangolaster. Progress in
Mutation Research, 1: 660-665.
Weber, H. (1983). Aminotriazole feeding study: Determination of the
threshold dose level affecting the thyroid function of female rats
by radioiodine assay and by T3 and T4 radioimmunoassay. Unpublished
report No. 11739 (F) (dated: 13-04-1983) from Bayer Health Care
Division. Submitted to WHO by Bayer AG.
Weber, H. & Patzschke, K. (1978). Effect of long-term administration
of aminotriazole on thyroid function of male and female rats, mice
and hamsters. Unpublished report No 7690 (dated: 14-07-1978) from
Bayer AG, Institute of Pharmacokinetics. Submitted to WHO by Bayer
AG.
Weir, R. (1958). 3-Amino-1,2,4-triazole Chronic oral administration
- beagle dogs. Unpublished report from Hazleton Laboratories
Virginia dd December 1, 1958. Submitted to WHO by Bayer AG.
WHO (1992). The WHO recommended classification of pesticides by
hazard and guidelines to classification 1992-1993 (WHO/PCD/92.14).
Available from the International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland.
WHO (1994). International Programme on Chemical Safety Environmental
Health Criteria 158: Amitrole. Geneva, World Health Organization.
Wynford-Thomas, D., Stringer, B.M., Williams, E.D. (1982a).
Dissociation of growth and function in the rat thyroid during
prolonged goitrogen administration. Acta Endocrinologica, 101:
210-216.
Wynford-Thomas, D., Stringer, B.M., Williams, E.D. (1982b).
Goitrogen-induced thyroid growth in the rat: a quantitative
morphometric study. J. Endocr. 94: 131-140.
Zimmermann, F.K. & Scheel, I. (1981). Induction of mitotic gene
conversion in strain D7 of Saccharomyces cerevisiae by 42 coded
chemicals. Progress in Mutation Research, 1: 481-490.
Toxicological studies
Acute toxicity studies
The acute toxicity of amitrole to several animal species is
given in Table 1.
No signs of toxicity were observed in one beagle-type female
dog when given amitrole (96.1%) by gavage at a dosage of 2150 mg/kg
bw. Another dog receiving 4640 mg/kg bw vomited within 1 hour, and
no signs of toxicity were observed thereafter (Fogleman, 1954).
When a commercial product (water soluble powder with 50% active
ingredient) was administered to mice, the LD50 was reduced to 4.0
g/kg bw amitrole. By determining the mean lethal dose for amitrole
in different preparations, it was established that sodium carbonate,
sodium bicarbonate and wetting agents as admixtures in various
quantities considerably increase the toxicity of amitrole (Hapke,
1967). WHO has classified amitrole as unlikely to present acute
hazard in normal use (WHO, 1992).
Table 1. Acute toxicity of amitrole in animals
Species Sex Route LD50 LC50 Purity Reference
(mg/kg bw) (mg/m3)
Mouse M oral 14 700 7 9 96.1%* Fogleman, 1954
? oral 11 000 8 ? Hapke, 1967
? i.v. 5 000 10 ? Hecht, 1954
M i.v. > 1 600 4 6 94% Bagdon et al., 1956
M i.p. > 10 000 6 94% Bagdon et al., 1956
Rat ? oral > 4200 ? Seidenberg & Gee, 1953
? > 10 000 6 10 ? Hecht, 1954
M 25 000 1 9 94% Bagdon et al., 1956
M > 10 000 6 10 ? Hecht & Kimmerle, 1962
? > 2500 93.7% Kimmerle, 1968
M > 5000 ? Thyssen, 1974a
M > 5000 98% Thyssen, 1974b
M&F > 4080 6 99% Gaines et al., 1973
M > 5000 ? Heimann, 1982
M&F dermal > 2500 99% Gaines et al., 1973
M&F inhal (4 h) > 439 6 96.9% Thyssen, 1983
? i.v. 5000 10 ? Hecht, 1954
M i.p. > 4000 6 94% Bagdon et al., 1956
Rabbit ? dermal > 10 000 5 6 95% Elsea, 1954
Cat M&F oral > 5000 2 94% Bagdon et al., 1956
M&F i.v. > 1750 3 94% Bagdon et al., 1956
1 80% aqueous suspension
2 40% aqueous suspension; the 3 cats tested (limit test)
vomited within 2 hours after receiving the dose
3 10% solution in 0,9% saline; one cat per dose level
4 aqueous solution
5 moistened with 0.5% methyl cellulose solution
6 no signs of toxicity
7 mortality at 21 500 mg/kg; signs of toxicity at this and
higher dose levels: extreme depression, squinting eyes, slow,
laboured respiration, diarrhoea, ataxia, depressed or absent
placement and righting responses, mild clonic convulsions,
gasping, coma and death and irritation of gastrointestinal
tract. No signs of toxicity at the lower dose level
(10 000 mg/kg bw)
8 signs of toxicity seen from 5 mg/kg bw: severe hypokinesia,
slight tremor of the extremities, apathy ranging to catatonia,
prone position, individual deep inspirations.
9 only 5 animals/group
10 less than 4 animals/group
* no correction for purity was made.
Short-term toxicity studies
Dietary/gavage
Rats
In a two-week dietary study, administration of 60 or 120 ppm
amitrole resulted in enlargement of the thyroid gland of rats
(number and strain not specified) and a pronounced lowering of
iodine uptake. Over this two-week interval there were no
significant changes at levels of 15 or 30 ppm (Jukes & Shaffer,
1960; Annex I, reference 23).
Amitrole was administered by gavage to groups of rats (strain
unknown), 5 days a week, for 4 weeks at doses of 0, 100, 200 or 400
mg/kg bw. In all treated groups, growth rate was reduced, relative
thyroid weight increased and iodine content of the thyroid reduced
(Hapke, 1967; Annex 1, reference 23).
In a range-finding study, groups of albino Wistar rats (4/sex)
received 0, 125, 1250 or 12 500 ppm amitrole in their diet for 27
days. The low dose (125 ppm) was raised to 25 000 ppm on day 7 of
treatment. Body-weight was retarded and food intake reduced in all
treatment groups. Thyroid weight (the only organ investigated) was
increased in all dose groups (Ben-Dyke et al., 1973).
Reversibility of thyroid effects was studied in a two-week
dietary study in male albino rats (strain unknown). A dietary level
of 1000 ppm increased the (absolute) thyroid weights to about 3.5
times those in the untreated controls. Thyroid weights had nearly
completely returned to normal after two weeks recovery (Bagdon et
al., 1956; Annex I, reference 23).
Amitrole (purity 94.6%) was administered to groups of male
Sprague-Dawley rats (20/dose) at levels of 0, 30, 100, or 300 ppm in
their diet for 28 days, followed by a 28-day recovery period. Two
animals/group were killed weekly in order to monitor the thyroid
function by assaying the thyroid hormones T3 and T4. Body weight
was depressed and food consumption decreased at the 100 and 300 ppm
dose levels. Food consumption returned to normal during the
recovery period as did body weight, but only in the 100 ppm group.
T3 levels were significantly decreased at day 7 in the 300 ppm
group and at day 14 in the 100 ppm group. T3 levels returned to
normal by day 19 post-treatment. T4 levels followed the same
pattern although the extent of decrease was greater. The NOAEL in
this study was 30 ppm (equivalent to 3 mg/kg bw/day) (Babish, 1977).
In a thyroid function test amitrole (purity 96.9%) was
administered to groups of 30 female Wistar rats (12 week old,
weighing 200 g) at levels of 0, 0.5, 1, 2, or 4 ppm in their diet.
Ten animals per group were sacrificed 3, 9 or 29 days after study
initiation and the following parameters were examined: thyroid
weight, accumulation of radioiodine in the thyroid (24 hours after
administration of 131I), and serum levels of T3 and T4.
Significantly elevated thyroid weights were observed only in the 1
and 2 ppm groups, 3 days after study initiation. No significant
deviation was observed in any dose group at the later test dates.
The percentage of iodine accumulation in the thyroids of the 2 ppm
group exhibited significant elevation after a 9-day treatment
period. In the 4 ppm group a slight, but not significant increase
was found. No effect was observed at 29 days, and no clear effects
were observed on T3 or T4 levels. The effects on thyroid function
found in this study were considered marginal and not consistent.
Therefore the highest dose in this study (4 ppm, equivalent to 0.4
mg/kg bw/day) was considered to be the NOAEL (Weber, 1983).
Amitrole (96.1%) was administered to groups of Carworth Farm
male and female rats (5 animals/sex/group) at levels of 0, 100,
1000, or 10 000 ppm in the diet for 63 days. Both males and females
showed reduced body-weight gain at 1000 and 10 000 ppm, which was
accompanied by reduced food consumption. There were no deaths or
gross signs of toxicity. Histopathological examination of the
liver, kidneys, bladder, small intestine, spleen and testis (thyroid
was not examined) revealed only an increased vacuolization of the
liver cells around the central veins in the 1000 and 10 000 ppm
dosage groups. The vacuoles were identified as fat globules
indicative of fatty metamorphosis associated with liver cell damage.
No histological effects were noted at 100 ppm. Because the thyroid
was not examined in this study, it is of limited value in the
evaluation of amitrole (Fogleman, 1954).
Fregly (1968) investigated the dose-response relationship
between amitrole administered in the diet and a variety of clinical
parameters in order to establish the minimum effective dose on
thyroid activity. Amitrole was administered to groups of male
Spruce Farm strain rats (10/dose) at levels of 0, 2, 10, or 50 ppm
in the diet for 13 weeks. In a separate experiment, amitrole was
administered in the diet to similar groups at levels of 0, 0.25, or
0.50 ppm for 11 weeks. In the first experiment, there were no
treatment-related effects on body weight, food consumption,
haematocrit, haemoglobin, or the rate of oxygen consumption. Mean
rectal temperature (measured at week 12) was slightly, but
significantly increased at 50 ppm. Uptake of radioactive iodine,
measured in vivo during week 12 at various times 22-53 hours after
injection of Na131I, was significantly decreased at 50 ppm. At the
end of the first study, radioactivity in the thyroid gland, measured
24 hours after injection, and the level of PBI in serum were
significantly reduced in all treatment groups. The PBI-values were
5.1, 3.7, 3.8, and 3.3 µg/100 ml for the 0, 2, 10, and 50 ppm
groups, respectively. Thyroid weight was increased significantly
only in the 50 ppm group. Histological changes in the thyroid were
noted at 10 and 50 ppm in the follicular cells with regard to both
appearance and the presence of colloid. Capillary density, which is
characteristic of the TSH-stimulated thyroid, was increased
significantly at both 10 and 50 ppm. In the second experiment, at
lower dose levels, no significant differences were noted between the
treated and control groups. In this study PBI was not affected. In
fact the PBI values were 3.2, 3.9, and 4.5 µg/100 ml for the 0, 0.25
and 0.50 ppm groups, respectively. It should be noted that the PBI
control values, measured in the second experiment were much lower
than those measured in the first experiment. This means that there
is no biologically significant effect on PBI, because all values
fall in the same range. The NOAEL was therefore 2 ppm (equivalent
to 0.1 mg/kg bw/day) (Fregly, 1968).
Several short-term experiments were carried out in Wistar rats,
in order to establish a no-effect level on thyroid function. In all
experiments the uptake of 131I by the thyroid was measured in an in
vivo test 6, 24, or 48 hours after administration of 0.6 µCi 131I
per animal intraperitoneally. In addition, at the end of the study,
thyroid weight and PBI were measured and the thyroid was studied
histopathologically.
In the first experiment, four groups of 8 female rats received
0, 2, 20, or 200 ppm of amitrole in the diet for 6 weeks. The
uptake of 131I was measured after 5 days and 6 weeks. At both times
a significantly increased uptake was found in the 200 ppm group 6
hours after injection, which decreased rapidly after 24 and 48
hours. At that time the radioactivity was lower than that of the
controls. The thyroid weight was increased in the 200 ppm group and
histopathologically goitre was found in this group only. No
significant effects were found at lower dosages.
In the second experiment in which 8 female rats per group
received 0, 20, 50, or 200 ppm for 6 weeks, the same effect was
found in the 200 ppm group. In addition, a significantly decreased
PBI was observed at the end of the experiment compared with the
controls. At 50 ppm a statistically increased uptake was found 6
hours after injection of 131I. In this case the radioactivity in
the thyroid remained higher than the controls after 24 and 48 hours.
Histopathologically only a very slight activation was found, whereas
200 ppm showed strong activation and goitre.
In the third experiment, 10 female animals per group received
amitrole at dietary concentrations of 0, 20, 50, or 200 ppm for 13
weeks. The uptake of 131I by the thyroid was significantly
increased at 50 and 200 ppm after 6 and 12 weeks. At 50 ppm, the
radioactivity in the thyroid remained high after 24 and 48 hours,
whereas at 200 ppm a very high uptake was found 6 hours after
injection of 131I, followed by a rapid decrease with still lower
values than the controls after 48 hours. At 200 ppm, the PBI was
decreased and the thyroid/body-weight ratio increased by a factor of
6. At 50 ppm, only a slightly increased relative thyroid weight was
found. Histologically a strong activation and goitre were found at
200 ppm, and a slight activation at 50 ppm. In this experiment, a
tendency to a higher uptake of 131I was found in the 20 ppm group.
The three above mentioned experiments were carried out with an
iodine content of about 0.2-0.3 ppm in the diet. In the fourth
experiment an iodine content of about 2 ppm was used. In this
experiment, 8 female rats per group received 0, 20, 50, 200 or 500
ppm amitrole in the diet for 6 weeks, to determine whether iodine
could protect against the antithyroid action of amitrole. At 500
ppm, a small increase in iodine uptake was found 5 hours after
injection, but thereafter a very rapid decrease was found. At 200
ppm, the uptake was much higher and the same type of decrease was
found as in the other experiments, whereas with 50 ppm a
significantly increased thyroid radioactivity was found at all
times. PBI was decreased at 200 and 500 ppm only.
Histopathologically, goitre and strongly activated thyroids were
found at the two highest dose levels. Some activation was found in
the 20 ppm group. It can be concluded that measurement of 131I
uptake at different time points was a sensitive method for the
effects of amitrole on the thyroid. At 20 ppm, only slight effects
were found on iodine uptake and thyroid histopathology. The overall
NOAEL from these four experiments was 2 ppm, equivalent to 0.1 mg/kg
bw/day (Den Tonkelaar & Kroes, 1974).
Dogs
In a one-year study in male and female beagle dogs, amitrole
was given in capsules at daily dose levels of 0, 0.25, 1.25, 2.50 or
12.5 mg/kg bw, 6 days per week (4-6 animals/group). There were no
clinical signs of toxicity or pharmacological effects.
Haematological, biochemical and urinalysis parameters were
comparable to those of the control dogs, and within normal limits.
The dogs at the 12.5 mg/kg bw/day dose level had pale-coloured
pancreases as the only dose-related gross pathological effect.
Histopathological examination of all dogs did not reveal any
treatment-related effect. The thyroid, in particular, was normal at
all dose levels (Weir, 1958).
Drinking-water
Mice
Groups of male albino mice (strain unknown) were exposed to
amitrole in the drinking-water at concentrations of 0, 0.5, 1.0 or
2% for 30 days (water consumption was not reported). Light
microscopy revealed dose-related hypertrophy of hepatocytes with
nuclear deformations, increased pyknotic nucleoli, and increased
vacuoles in the cytoplasm. Electron microscopy revealed
proliferation of smooth endoplasmic reticulum (Reitze & Seitz,
1985).
Rats
Male Sprague-Dawley rats were provided with drinking-water
containing 400 ppm amitrole over periods of 12, 20, or 37 days.
Based on a body weight of about 200 g and a water intake of 30-35
ml/day, the daily intake was about 60-70 mg/kg bw. The catalase
activity of liver and kidney of the treated animals was inhibited by
50%, but the red cell catalase was unaltered. An increase in
thyroid weight was found, which correlated with the duration of
treatment. Microscopic examination showed hyperplasia of the
thyroid and a loss of colloid. It was postulated in this study that
the ability of the thyroid to concentrate plasma iodine was not
impaired by amitrole, but that the formation of organically-bound
iodine was inhibited (Alexander, 1959).
In a range-finding study, amitrole was administered to groups
of albino Wistar rats (4/sex) at levels of 0, 10, 104 or 1040 ppm in
drinking-water over a treatment period of 27 days. Body-weight gain
was retarded and food intake reduced at 104 ppm and above. Thyroid
weight (the only organ investigated) was increased in a dose-
dependent manner at 104 ppm and above. The NOAEL in this study was
10 ppm, equivalent to 1.5 mg/kg bw/day, based on a daily water
consumption of 30 ml and body weight of 200 g (Ben-Dyke et al.,
1973).
Amitrole (purity 94%) was administered in the drinking-water to
groups of male albino rats (10/dose; strain unknown) at
concentrations of 0, 50, 250, or 1250 ppm for 106 days. The
administration of amitrole resulted in a dose-dependent depression
of growth with a corresponding reduction of food and water intake.
Appearance, behaviour, and mortality were not affected by amitrole.
Haematology, except Ht, and clinical chemistry parameters were not
examined. At the end of the study, histopathology was performed on
the thyroid, hypophysis, liver, kidney, spleen, stomach, small and
large intestines, bladder, testis, adrenal and lung. Gross and
microscopic examination of tissues and organs showed a marked
increase in thyroid size at all dose levels. In rats where reduced
growth was noted, the kidneys, adrenals, liver and spleen were
proportionally smaller. Reproductive organs were not affected.
Microscopic examination showed general enlargement of the thyroid at
50 ppm, with moderate stimulation of the thyroid epithelium (no
evidence of hyperplasia). At 250 ppm, thyroid hyperplasia was
evident. At the two highest dose levels there was also absence of
follicles with stored colloid. Liver catalase activity was reduced
at 250 and 1250 ppm in a dose-dependent manner (Bagdon et al.,
1956; Annex I, reference 23).
In a study measuring the time-course of development of goitre,
Sprague-Dawley rats were given amitrole in the drinking-water (400
ppm). The thyroid of each animal was examined by light microscopy
at various periods from 3 days to 6 months. Each rat drank
approximately 30 ml per day. After 3 days, the thyroid size was
unchanged, although cellular changes were apparent. By one week,
the thyroid was twice its normal size with marked structural
changes. These changes continued to progress with prolonged
administration of the goitrogen. Goitre formation was accompanied
by increased vascularity and decreased colloid content in the
follicular cells. Electron microscopy revealed pronounced
dilatation of the endoplasmic reticulum. Peroxidase activity
measured in the thyroid progressively decreased with prolonged
administration of amitrole (Strum & Karnovsky, 1971).
The effects of amitrole on thyroid histology were examined in
seven groups of 5 female Wistar rats weighing about 200 g, which
were given amitrole in their drinking-water, 2500 ppm, and killed
after 1, 2, 3, 10, 30, 50, or 100 days. Water consumption was not
reported. After 1 and 2 days of exposure the only change noted was
a slight enlargement of some endoplasmic cisternae of follicular
cells. After 3 days the gland was slightly enlarged, follicular
colloid was slightly reduced and in some follicular cells the
cisternae were clearly dilated and stained more lightly for
peroxidase activity than in normal cells. By 10 days the glands had
doubled in size, the follicular epithelium consisted of low,
columnar cells, and colloid had been severely depleted. Nuclei had
become located basally and slightly elongated microvilli projected
into the lumen. Peroxidase activity was no longer seen in the
endoplasmic reticulum cisternae, whereas it was observed in portions
of perinuclear cisternae. These changes had progressed in the 30-
day exposure group, so that the glands were now several times their
normal size. In addition, fibrous thickening of both stroma and
capsule was prominent and cisternal peroxidase activity was absent.
Over 50-day exposure resulted in increased irregularity in
follicular size, more prominent papillary growth of the follicular
epithelium and greatly diminished peroxidase activity throughout the
cells (Tsuda et al., 1973).
Functional and morphological changes in the thyroid were
examined in male Wistar rats. Amitrole was administered via
drinking-water at a concentration of 1000 ppm for periods of up to
153 days. Within 2 weeks thyroid hormone synthesis (serum T3 and
T4) was inhibited. Serum TSH level increased rapidly during the
first 4 weeks, and remained essentially constant after that. The
accumulation of radioiodine in the thyroid (measured as the ratio
thyroid to serum inorganic iodide - T/S ratio) followed a similar
pattern. Thyroid weight increased rapidly during the first few
weeks and thereafter growth rate declined. After 4 months, total
thyroid weight was increased to a 12-fold plateau. During the first
week of amitrole administration there was also a rapid change in the
morphology of the gland; an increase in the proportional volume of
the epithelial cell compartment being accompanied by a corresponding
decrease in follicular lumen (colloid) and a marked increase in
vascularity (Wynford-Thomas et al., 1982a,b).
Dermal
Rabbits
Amitrole (97.6%) was applied to the shaved skin of the back and
flanks of groups of 6 male and 6 female New Zeeland white rabbits at
doses of 0, 25 or 100 mg/kg bw for 6 hours each day on 15
consecutive workdays. The skin was abraded in three animals per
group. The NOAEL for systemic and local effects was at the highest
dose level, i.e. 100 mg/kg bw/day (Mihail & Schilde, 1984).
Inhalation
Rats
Groups of Fischer 344 rats (15/sex/dose) were exposed to an
atmosphere containing amitrole (94.6% pure) at concentrations of 0,
0.1, 0.32, 0.99, or 4.05 mg/l (nominal concentrations adjusted for
non-nebulizing material), 5 hours/day 5 times per week, for 4 weeks
(particle size unknown). There were no adverse effects on behaviour
and no body-weight changes were noted. T3 and T4 levels were
depressed after 14 and 27 days at levels of 0.32 mg/l and above. At
these dose levels, thyroid hyperplasia and elevated thyroid weights
were also observed. At a concentration of 0.1 mg/l no effects on
the thyroid were observed (Cox & Re, 1978).
Long-term/carcinogenicity studies
Mice
Amitrole was used as a positive control in a study which
investigated the carcinogenicity of some 120 chemicals. Groups of
(C57BL/6 x C3H/Anf) F1 mice (18/sex) and (C57BL/6 x AKR) F1 mice
(18/sex) were given amitrole by gavage at a dose level of 1000 mg/kg
bw/day on days 7-28 of age, and in the diet at a level of 2200 ppm
from 28 days onwards until the end of the experiment. This was
planned to be an 80-week study but all of the mice in the amitrole
treatment groups had died by weeks 53-60. Thyroid tumours were
reported to have occurred in nearly all of the treated mice (64/72).
Liver tumours were observed in 34/36 (C57BL/6 x C3H/Anf) F1 treated
mice and in 33/36 (C57BL/6 x AKR) F1 treated mice. In pooled
control groups, 8/166 (C57BL/6 x C3H/Anf) F1 mice and 6/172
(C57BL/6 x AKR) F1 mice had liver tumours (Innes et al., 1969).
In a carcinogenicity study, amitrole (97.0% pure) was
administered to groups of NMRI mice (75/sex/dose level) at levels of
0, 1, 10, or 100 ppm in their diet for 18 months. There were no
treatment-related effects on appearance, behaviour, body weight,
food consumption or survival times (haematology not measured).
Histological examination of tissues of the major organs did not
reveal any treatment-related effects apart from a slight increase in
the number of hyperemias in the pituitary at 100 ppm (5 at 1 ppm, 2
at 10 ppm and 16 at 100 ppm). The incidence of treatment-related
tumours was not increased. Concurrent satellite groups of 5
animals/sex/dose were additionally used in this carcinogenicity
study for different thyroid function tests (thyroid weights,
incorporation of radioactive iodide in the thyroid gland, proportion
of PBI in total plasma iodine) performed at intervals of 3, 6, 9,
12, and 18 months. The thyroid weights (up to 3 times control
values), were elevated in the 100 ppm males at all test dates. The
percentage of iodine accumulation as well as the iodine level in the
thyroid were elevated in the male mice at 100 ppm. The fraction of
PBI in the male mice was elevated 9 months after study initiation,
but was depressed at later test dates. Comparable results were
observed in the high-dose females. However, the deviations relative
to the control group were generally smaller than in the males, and
were not significant in most cases. The NOAEL was 10 ppm equivalent
to 1.5 mg/kg bw/day (Steinhoff & Boehme, 1979b, Weber & Patzschke,
1978; Steinhoff et al., 1983).
In a study on C3H mice and their substrain without serum
catalase activity, ingestion of 10 000 ppm amitrole in the diet led
to a reduction in lifetime. In comparing the two substrains, it was
shown that the acatalasemic mice lived longer under treatment than
did the normal mice. The mean survival times were 35 and 25 weeks,
respectively. The C3H strain features a high rate of spontaneous
liver tumours (it was reported to be 9% in females and 46% in
males). Under amitrole treatment, liver tumours occurred earlier
and at a higher incidence in the acatalasemic mice than in the
normal mice (Feinstein et al., 1978).
Three groups of B6C3F1 mice were fed a diet containing 500
ppm amitrole (purity not specified): Group 1: the dams were treated
from day 12 of gestation to birth of the pups; group 2: dams
together with their pups were treated from birth to weaning; group
3: pups were treated for a period of 90 weeks after weaning.
Although not specifically stated, information on other chemicals
tested and described in this paper (benzidine, safrole etc.),
implies that pups of groups 1, 2, and 3 animals were examined only
for liver tumours after 90 weeks. It was not stated if treatment
with the other compounds were conducted in the same or different
rooms. The incidence of hepatocellular adenomas and carcinomas,
respectively, were: group 1 males, 4/74 and 2/74; group 2 males,
6/45 and 4/45; group 3 males, 15/55 and 11/55; group 1 females, 0/83
and 0/83; group 2 females, 0/55 and 0/55; group 3 females, 5/49 and
4/49. The incidence of these tumours in the untreated control
animals (which may or may not have been concurrent controls) at week
90 were: males, 1/98 and 0/98; females, 0/96 and 0/96. Other organs
were not examined. It was concluded that there was no effect of
amitrole treatment in group 1 or in females of group 2, but that
marginal increases occurred in males of group 2 and in males and
females of group 3 (Vesselinovitch, 1983).
The susceptibilities of three mouse strains to the development
of preneoplastic hepatic lesions were examined following amitrole
administration in their drinking-water at 10 000 ppm. The strains
were DS, ICR (Crj: CD-1) and NOD derived from ICR and found to
develop spontaneous insulitis followed by diabetes. There were
reported to be indications that NOD mice may carry immunological
abnormalities. In each of two experiments, 3 groups of female mice
were administered amitrole via drinking-water for 3 months
(experiment 1) or 6 months (experiment 2), after which they were
killed and their livers examined. The proportions of mice with
hyperplastic nodules were, in experiment 1: 15/19, NOD; 3/55, DS;
0/5, ICR, and in experiment 2: 19/19 NOD; 18/18 DS; 17/19 ICR. A
single hepatocellular carcinoma developed in a NOD mouse after 6
months (Mori et al., 1985).
Rats
In a limited chronic toxicity study (no haematology or clinical
chemistry) groups of Carworth Farm Wistar rats (35/sex/dose)
received amitrole in the diet at levels of 0, 10, 50, or 100 ppm for
two years. After 13 weeks, 5 animals/sex/dose and after 68 weeks 3
animals/sex/dose were killed for organ weight measurement and
histopathological examination. A separate group received 500 ppm
for 19 weeks. Due to marked reduction in body-weight gain and food
consumption, these animals were left on the control diet for 7 weeks
and were then killed (26 weeks). Animals in all groups, including
controls suffered from apparent respiratory infection and were in
poor condition. Death occurred in 67 rats but there was no
relationship with treatment. Body-weight gain was reduced at 100
ppm in male animals during the first 13 weeks of the study. After
68 and 104 weeks of treatment, relative thyroid weight was increased
at 100 ppm (not measured after 13 weeks). Histopathological
examination after 13 weeks showed hyperplasia and hypertrophy of the
thyroid at 500 ppm, but this was reversed after withdrawal of the
amitrole diet. Histopathological changes were also seen at 100 ppm
and in one animal at 50 ppm. At 68 weeks, 3 animals at 50 ppm
showed definitive evidence of hyperplasia while all animals at 100
ppm showed evidence of hyperplasia and hypofunctioning of the
thyroid. At 104 weeks tumours were found: thyroid tumours were
present in 1/10 animals of the 10 ppm group, 2/15 at 50 ppm and
15/27 at 100 ppm. No tumour was detected in 5 control group animals
but one rat exhibited early stages of an adenoma. One thyroid from
the 50 ppm group and 4 from the 100 ppm group exhibited lesions
interpreted as adenocarcinomas by several pathologists and benign
neoplasms by others. Based on thyroid hyperplasia the NOAEL was 10
ppm equivalent to 0.5 mg/kg bw/day (Keller, 1959; Jukes & Shaffer,
1960).
Groups of Fischer 344 rats (75/sex/dose) were treated with
amitrole (94.6% pure). Group A were the controls. Rats of group B
were fed 5 ppm of amitrole in their diet during 1-39 weeks and then
100 ppm during weeks 40-115 for males and 40-119 for females. Rats
in groups C, D, and E received amitrole in their diet at pulsed
intervals (alternate 4 week periods) at 1, 3, or 10 ppm,
respectively, during weeks 1-39 and 20, 60, or 200 ppm during the
last exposure period (week 40 onwards). On alternate 4-week
periods, groups C, D, and E received the basal diet containing no
amitrole. There were no treatment-related clinical signs of
toxicity or changes in body weight or food consumption. No
treatment-related effects on parameters of haematology, clinical
chemistry or urinalysis were observed (only 5 animals/sex/dose level
were examined). Thyroid weights were significantly increased in
both males and females in groups B and E after 60 weeks and at
termination. Increased thyroid weights, although not significant,
were also observed in group D. The T3 and T4 activities were
elevated in groups B and E from week 44 onwards. However, these
effects were not always consistent. These changes were not observed
up to week 36, when the groups were administered the lower dose
levels. Follicular epithelial hyperplasia in the thyroid was noted
in groups B, D, and E and to a much lesser extent in group C. An
increased incidence in thyroid tumours (mainly follicular adenomas)
was observed in male and female rats of groups B and E and in the
male animals of group D. There was no significant difference in
tumour incidence between groups B and E (in these dose groups the
incidence of follicular adenomas was 80-84% and of follicular
adenocarcinomas 5%, compared to 0-2% in the control group). Due to
the change in dosing regimen it was difficult to evaluate this
study. However, effects on the thyroid (hyperplasia) were seen in
all treated groups (Johnson, 1981).
In a study performed to characterize the oncogenes
participating in the genesis of thyroid tumours, male Wistar rats
(number not specified) were treated with a chemical carcinogen
(nitrosomethylurea, NMU) or by ionizing radiation (131I) to induce
thyroid tumours, and were then treated with amitrole (purity not
specified) as a goitrogen at a level of 0.1% in drinking-water
throughout their entire lifetimes. A control group was only
administered amitrole without prior tumour-initiating treatment.
Malignant thyroid tumours developed after even a relatively brief
period in rats treated with NMU or 131I. Animals exclusively
treated with amitrole exhibited only benign tumours which developed
at a markedly slower rate. A "H-ras" oncogene was determined to
be involved in treatment with NMU (13 out of 15 cases), whereas a
"K-ras" oncogene was involved in the animals treated with 131I
(8/15 cases) and amitrole (only 1/10 cases) (Lemoine et al.,
1988).
In a briefly worded description of a long-term study in rats,
thyroid and liver tumours were reported to occur in rats exposed to
20-25 mg amitrole per day via drinking-water or to 250 or 500 mg
amitrole per day in their diets for life. No control data were
reported (Napalkov, 1962). Due to the serious shortcomings in this
study, it was not considered relevant for the evaluation of amitrole
toxicity.
In a carcinogenicity study, groups of Wistar rats (75/sex/dose
level) were treated with amitrole (97.0% pure) in the diet at
concentrations of 0, 1, 10, or 100 ppm for two years. There were no
treatment-related effects on appearance, behaviour, body weight, or
food consumption (haematology not measured). A slight decrease in
survival time was found at 100 ppm. Average survival time at 100
ppm for males was 961 days and for females 919 days. The survival
times for control animals were 992 and 969 days for males and
females, respectively.
Concurrent satellite groups of 5 animals/sex/dose level were
additionally used in this carcinogenicity study for different
thyroid function tests (thyroid weights, incorporation of
radioactive iodide in the thyroid gland, proportion of protein-bound
iodine in total plasma iodine) performed at intervals of 3, 6, 9,
12, 18 or 24 months. The thyroid weights (up to 3.6 times control
values for males and 7 times for females) were increased at 100 ppm
as was the uptake of 131I by the thyroid, measured 24 hours after
oral administration of 131I. The fraction of PBI was not affected.
At 100 ppm, an elevated incidence of haemorrhages and hyperaemia of
the pituitary gland as well as a very high rate of cystically
dilated thyroid follicles were seen. Both benign and malignant
tumours of the thyroid were found at 100 ppm. There was also an
increase in the incidence of benign tumours in the pituitary gland
at the 100 ppm level (females). The NOAEL was 10 ppm (equivalent to
0.5 mg/kg bw/day) (Steinhoff & Boehme, 1979a; Weber & Patzschke,
1978; Steinhoff et al., 1983).
Hamsters
In a carcinogenicity study in Syrian golden hamsters
(76/sex/dose) amitrole (97%) was administered in the diet at levels
of 0, 1, 10, or 100 ppm for 18 months. There were no treatment-
related changes in appearance, behaviour or food intake. Body-
weight gain was decreased at 100 ppm from day 400 onward, and
mortality was significantly increased in the 100 ppm dose group.
Average survival time at 100 ppm for males was 557 days and for
females 438 days. The survival times for control animals were 631
and 508 days for males and females, respectively. The main cause of
death in all groups, including controls, was severe amyloidosis of
the kidneys. There was no evidence of amitrole-related
histopathological changes. There was no evidence of a treatment-
related carcinogenic effect. Concurrent satellite groups of 5
animals/sex/dose were additionally used in this carcinogenicity
study for different thyroid function tests (thyroid weights,
incorporation of radioactive iodide in the thyroid gland, proportion
of PBI in total plasma iodine) performed at intervals of 3, 6, 9,
12, or 18 months. There were no treatment-related effects observed.
The NOAEL was 10 ppm equivalent to 1 mg/kg bw/day (Steinhoff &
Boehme, 1978; Weber & Patzschke, 1978; Steinhoff et al., 1983).
Inhalation exposure
Rats
In an inhalation study involving intermittent treatment, groups
of 75 Fischer rats per dose and sex were exposed to aerosols at
nominal amitrole levels of 0, 50 or 500 µg/l air (one of the two
batches used had a purity of 94.6%). The actual amitrole
concentrations in the low-dose group varied between 15.8 and 32.2
µg/l air in the different exposure periods; the levels measured in
the high-dose group ranged between 97.9 and 376.4 µg/l air. The
animals were exposed for 5 hours each day five days per week.
Treatment phases during weeks 1-13, 40-52 and 78-90 were interrupted
by treatment-free intervals. Interim necropsies of 5 animals per
dose and sex were performed after 3, 9 and 18 months; the study was
concluded after 24 months. A total of 28 rats died in week 51 due
to a defect in the air conditioning system which led to an increase
in the room temperature. Treatment of the high-dose group was
thereupon concluded, and the surviving animals (6/75 males, and
18/75 females) were necropsied.
In the high-dose group, food intake and body-weight gain were
decreased and the rate of mortality was elevated. At week 13 of the
study, levels of cholesterol, ASAT and ALAT were higher and of
glucose lower in the high-dose group than in the control group.
Also T3 and T4 levels were lower. No differences in these
parameters were observed after the treatment-free period. Relative
thyroid weight was increased and epithelial hyperplasia of the
thyroid follicles was determined in both dose groups at the end of
the first treatment interval (week 13). This latter finding was no
longer observed after the first treatment-free interval of 24 weeks,
but the thyroid weights were still elevated in both dose groups.
Follicular epithelial hyperplasia was again present in most of the
animals of both treatment groups at the end of the second treatment
phase (week 51). This finding was still observed, in the remaining
treatment group (50 µg/l air), after a treatment-free interval of 26
weeks, which indicates that complete reversion no longer occurred at
this time. Neoplasms of the thyroid (adenomas and adenocarcinomas)
were found in addition to hyperplasia at terminal necropsy (Becci,
1983).
Reproduction studies
Rats
In a limited reproduction study, groups of 10 male and 10
female Sherman rats were fed amitrole in the diet. A preliminary
one-generation study was performed in which rats were fed levels of
0, 500, or 1000 ppm for 55 days before pair-mating. The offspring
were weaned at 21 days. Complete autopsies were performed on the
parents after total exposure of 107-110 days. Ten weanling rats
from each dose group were killed. Mean body-weight gain and food
consumption were reduced in the parents at all dose levels.
Relative kidney, spleen, and liver weights (only in males) were
reduced in parents fed 500 and 1000 ppm. The average number of pups
per litter was significantly reduced at the 500 and 1000 ppm dose
levels, as were the numbers surviving to weaning. Body weight of
pups at weaning was also reduced. Thirty-three out of 45 pups of
rats fed 500 ppm and 55/56 pups of rats fed 1000 ppm died within one
week after weaning. Relative weight of thymus and spleen of pups of
the 500 ppm group (1000 ppm not examined) were significantly
reduced. Thyroid hyperplasia was seen in all treated rats (Gaines
et al., 1973).
In a subsequent 2-generation study, rats were fed dose levels
of 0, 25, or 100 ppm for 61 and 173 days before pair-mating to
produce the F1a and F1b generations, respectively. Then 12
rats/sex of each dose level were pair-mated when about 100 days old
to produce the F2a generation. The offspring were weaned at 21
days. Food consumption was reduced in the F0 generation at
100 ppm. Thyroid hyperplasia was seen in all animals of the 100 ppm
group. In the 25 ppm group hyperplasia was seen in about half of
the F0 (4/10) and F1b (4/10) females and F1b (6/10) males, but
none of the F0 males (F1a litters not examined). Pups of the 100
ppm group showed reduced kidney and liver weights and also male pups
of the 25 ppm group showed reduced liver weights. There was a
decrease in the number of litters in the F2a generation at 100 ppm,
but there were no other changes. A NOAEL for toxic effects could
not be established. Due to the low number of animals used in this
study, a NOAEL for reproductive effects could not be established
(Gaines et al., 1973).
Groups of 5 rats/sex (strain unknown) were mated following a 3-
month pretreatment period during which only males, only females, or
both sexes were exposed to amitrole at a level of 100 ppm in their
drinking-water. The groups were compared with a common control
group. According to the author, the results did not point to a
reduction in reproductive ability for males or females. However,
due to limited data in the report this could not be confirmed. Pups
born in this study were reared and continued on the treatment.
Their growth was reduced (Hapke, 1967).
Special studies on embryotoxicity and/or teratogenicity
Mice
Pregnant NMRI mice (9-12/group) were administered amitrole
(purity not specified) at 0, 500, 1000, 2500 or 5000 ppm in
drinking-water (days 6-18 of pregnancy). There was a marked
decrease (22-28%) in body-weight gain in the dams and pronounced
retardation in the development (lower fetal weight and immature
skeletons) at concentrations of 1000 ppm and above. At the highest
concentration (5000 ppm), maternal toxicity was associated with an
increase in the rate of resorptions. No irreversible structural
changes were observed at any concentration (Tjälve, 1974).
Groups of 13 pregnant C57BL6 mice were treated subcutaneously
with 215 or 464 mg/kg bw/day from days 6 though 14 of gestation.
The highest dose tested resulted in an increased fetal mortality
rate. Subcutaneous treatment of 6 AKR mice from days 6-15 of
gestation at 464 mg/kg bw/day produced no effects in the fetuses.
Daily oral administration of 215 mg/kg bw to 7 C57BL6 mice from
days 6-14 of gestation produced increased fetal mortality and a
reduction of fetal weight. In nearly all groups maternal body
weight was reduced and liver weight increased. There were no
irreversible structural changes observed in any group (Bionetics
Research Laboratories, 1968).
Rats
In a limited teratogenicity study, groups of 8 pregnant female
Sherman rats were given amitrole (99%) by stomach tube at dose
levels of 0, 20 or 100 mg/kg bw/day during days 7 through 15 of
gestation. The animals were allowed to litter and to wean, and the
pups were observed for gross abnormalities. No abnormalities were
observed in the offspring at the time of birth or when weaned
(Gaines et al., 1973).
In a pilot study, 2 female rats (strain unknown) were orally
exposed to amitrole at a dosage of 1000 mg/kg bw/day and one rat to
500 mg/kg bw/day from days 8 through 13 of gestation. Weight gain
of the treated females was reduced. No effects were found on number
of corpora lutea, number of fetuses and no anomalies were observed
(Hapke, 1967).
In a teratogenicity study, groups of 20 presumed pregnant rats
(strain FB30, Long-Evans) were exposed to amitrole by gavage at dose
levels of 0, 100, 300, or 1000 mg/kg bw/day on days 6 to 15 of
gestation. Fetuses were examined on day 20 of gestation. There
were no deaths or signs of toxicity at any dose level. Body-weight
gain was not affected by treatment. There were no treatment-related
effects on the resorption rate, fetal weight, number of live
fetuses, placental weight, or sex ratio. There was no treatment-
related increase in gross, skeletal or visceral malformations. The
NOAEL for maternal and embryo/fetotoxicity was 1000 mg/kg bw/day
(Machemer, 1977b).
Groups of 24 presumed pregnant CD rats were exposed to amitrole
(91.83% pure) by gavage at dose levels of 0, 100, 500, or 1000 mg/kg
bw/day on days 6 through 15 of gestation. Apart from the normal
parameters measured in a teratogenicity study, the weights of liver
and thyroid of the dams were examined. Fetuses were examined on day
21 of gestation. Extra groups of 14 females per dose level were
allowed to litter and wean, and were maintained until postnatal day
21. In this postnatal period, body weight, food consumption and
thyroid weights were measured in the dams. Observations in the pups
included number, sex, weight and gross examination. Weight gain of
dams was reduced during gestation days 6-18 at 500 and 1000 mg/kg
bw/day. Food intake was reduced during gestation days 15-21 at the
same dose levels. Maternal thyroid weights (absolute and relative)
were increased at 500 and 1000 mg/kg bw/day, the increase still
persisting at termination of lactation. Fetotoxicity was observed
at 1000 mg/kg bw/day including reduced fetal body weight per litter,
increased incidence of fetuses with unossified or poorly ossified
bones and an increased number of fetuses with enlarged and/or dark
thyroid. These latter findings in the thyroid were also observed in
the 500 mg/kg bw/day dose group. There was no treatment-related
increased incidence of malformations at any dose employed.
Postnatal evaluations indicated no effects of treatment on survival
or growth of the pups, or on maternal body weights, weight gain or
food consumption in the lactation period. The NOAEL for
maternotoxicity and embryo/fetotoxicity was 100 mg/kg bw/day (Tyl,
1986a).
Rabbits
Groups of 22 presumed pregnant New Zeeland white rabbits were
exposed to amitrole (91.83% pure) by gavage at doses of 0, 4, 40, or
400 mg/kg bw/day on days 6 through 18 of gestation. Fetuses were
examined on day 29 of gestation. Maternotoxicity at 40 and 400
mg/kg bw/day included reduced body-weight gain and at 400 mg/kg
bw/day increased (relative) liver weight. A dose-related increase
in the incidence of abortions was observed (0, 1, 3 and 5 for the
control, 4, 40 and 400 mg/kg bw/day dose groups, respectively). The
number of non-viable implants/litter was increased and the
percentage of live fetuses/litter decreased at 40 and 400 mg/kg
bw/day (statistically significant at high dose). Decreased fetal
weight/litter was also seen at these dose levels, with statistical
significance at the high dose. At 40 and 400 mg/kg bw/day there
were significant increases in the incidence of numerous individual
malformations, especially of the head and limbs. The total
percentages of fetuses with malformations were 4.5, 7.2, 36.9 and
62.4% for the control, 4, 40 and 400 mg/kg bw/day dose groups,
respectively. The incidence of a number of individual visceral
(including craniofacial) and skeletal variants (mainly poorly or
absent ossification) was increased at 40 and 400 mg/kg bw/day.
Effects on the fetal thyroid were also observed. The percentages of
fetuses with enlarged thyroid were 1.3, 2.2, 3.6 and 8.2% and of
fetuses with dark/red thyroids were 2.6, 7.9, 8.1 and 12.9% for the
control, 4, 40 and 400 mg/kg bw/day dose groups, respectively.
Following oral administration amitrole was teratogenic,
embryo/fetotoxic and (slightly) maternotoxic at 40 and 400 mg/kg
bw/day. The NOAEL for all types of toxicity was 4 mg/kg bw/day
(Tyl, 1986b).
Four groups of 18 artificially inseminated Hra (NZW) SPF
rabbits were treated dermally from days 7 to 19 (inclusive) of
gestation with 0, 1000, 1500 or 2000 mg amitrole (93.9% purity)/kg
bw/day. The test material was applied as a mixture of 0.5 ml
deionized water/g amitrole for six hours/day. Rabbits were collared
during the exposure period, and remaining test material was removed
with warm water washing immediately following each exposure.
Clinical signs including dermal irritation, body weight and food
intake were measured in does. Dermal irritation, including erythema
and edema, was noted for treated animals. The number of animals
affected and the severity of these observations increased with
increasing dose. Numbers of pregnant females at term were 14, 15,
12 and 11. At 2000 mg/kg bw/day thin appearance and anorexia was
observed in does. Body weight was reduced on day 20, but was
virtually recovered by day 29; food intake was reduced on days 10-
14, with severe reductions on days 14-17 and 17-20 of gestation and
uterine weight was slightly reduced at term at 2000 mg/kg bw/day.
Fetal weights were reduced at 2000 mg/kg bw/day and the incidence of
total resorptions was increased significantly (mainly early
resorptions). Malformations, including microphthalmia (3 fetuses in
2 litters), dilated brain lateral ventricles (2 pups in 1 litter),
anencephaly (2 fetuses in 2 litters), unossified pubis (4 pups in 1
litter, but several other pups in the same litter with thyroid,
skeleton and rib anomalies) were observed at 2000 mg/kg bw/day.
Following dermal administration, amitrole was teratogenic,
embryo/fetotoxic and maternotoxic at 2000 mg/kg bw/day. The NOAEL
for all types of toxicity was 1500 mg/kg bw/day (Henwood, 1988).
Special studies on genotoxicity
A number of genotoxicity tests has been carried out with
amitrole. The results are summarized in Table 2. The important
features of these data are described below.
Gene mutations
Numerous assays for in vitro gene-mutations were performed
and were predominantly negative. One study with E. coli and S.
typhimurium strains gave positive responses (Venitt & Crofton-
Sleigh, 1981). Of two other bacterial mutation assays, one assay
gave a positive result in absence of metabolic activation and the
other gave an equivocal response. Many other in vitro assays gave
negative results. A positive response was obtained in one out of
two mouse peritoneal host mediated assays with S. typhimurium
(Simmon et al., 1979). No mutation induction was observed in
fungi and yeast. No mutation induction was observed in several sex-
linked recessive assays with Drosophila melangolaster. Mutations
(TK +/-) were not induced in mouse lymphoma cells, whereas HPRT
locus and Na+/K+ATPase locus mutations were induced in Syrian
hamster embryo cells (Tsutsui et al., 1984).
Chromosomal damage
Weakly positive results were obtained in tests with fungi. A
cytogenetic study in human lymphocytes was negative, but sister-
chromatid exchanges were increased in a single study. No effects of
amitrole were observed in mice subjected to bone marrow micronucleus
tests or male dominant lethal tests.
Table 2. Results of genotoxicity assays on amitrole
Tests for gene mutations
Test system Test object Concentration/ Purity Results References
dose (%)
reverse mut. S.typhimurium 100 µg/plate 99.4 negative Brusick, 1975
(*) TA1535, TA1537
TA1538
S.cerevisiae, D4 100 µg/plate 99.4 negative
reverse mut. S.typhimurium 0.4% 93.2 negative Bamford et al.
(-) LT 2 trp 1976
(A8, B4, C3)
reverse mut. S.typhimurium 1000 µg/plate ? negative Prince, 1977
(*) TA98, TA100
TA1538, TA1535
point mut. Aspergillus up to 2000 µg ? negative Bignami et al.
(-) nidulans 1977
strain 35
reverse mut. S.typhimurium 20-12 500 µg/ 97.6 negative Herbold, 1980
(*) TA98, TA100 plate
TA1535, TA1537
reverse mut. S.typhmurium 0.2-2000 µg/ ? negative Brooks &
(*) TA1535, TA1537 plate Dean, 1981
TA1538, TA98
TA100, TA92
Tests for gene mutations (contd)
Test system Test object Concentration/ Purity Results References
dose (%)
reverse mut. S.typhimurium up to 5000 µg/ ? negative McDonald,
(*) TA98, TA100 plate 1981
TA1537
reverse mut. S.typhimurium 10-10 000 µg/ ? negative Richold &
(*) TA1535, TA1537 plate Jones, 1981
TA1538, TA98
TA100
reverse mut. S.typhimurium 0.1-2000 µg/ ? negative Rowland &
(*) TA1535, TA1537 plate Severn, 1981
TA1538, TA98
TA100
reverse mut. S.typhimurium 4-2500 µg/ ? negative Trueman,
(+) TA1535, TA1537 plate 1981
TA1538, TA98
TA100
reverse mut. E.coli 0.5-500 µg/ml ? positive Venitt &
(+) WP2uvrA(p) Crofton-
S. typhimurium positive Sleigh, 1981
TA98, TA100
reverse mut. S.typhimurium up to 500 µg/ ? equivocal Hubbard et al.
(*) TA98, TA100 ml 1981
(fluctuation test)
Tests for gene mutations (contd)
Test system Test object Concentration/ Purity Results References
dose (%)
reverse mut. E. coli WP2uvrA 10-500 µg/ml ? negative Gatehouse,
1981
(*) S. typhimurium negative
(microtiter TA98, TA1535
fluctuation test) TA1537
reverse mut. S. cerevisiae 88.9 & 889 µg/ ? negative Mehta & Von
(*) XV185-14C ml Borstel, 1981
reverse mut. S. typhimurium up to 5000 µg/ ? negative Moriya et al.
(*) TA98, TA100 plate 1983
TA1535, TA1537
TA1538
E. coli WP2 hcr negative
forward mut. E. coli CHY832 ? Hayes et al.
+ S9-mix: 10 000 µg/ml negative 1984
- S9-mix 2500 µg/ml positive
reverse mut. S. typhimurium 0.3-333.3 µg/ 98 negative Dunkel et al.
(*) TA1535, TA1537 plate 1984
(comparative TA1538, TA98
study in 4 TA100
laboratories) E. coli 0.3-333.3 µg/ negative
WP-2 uvrA plate
HPRT mutation Syrian hamster 0.3-10 µg/ml ? positive Tsutsui et al.
assay (-) embryo cells 1984
Tests for gene mutations (contd)
Test system Test object Concentration/ Purity Results References
dose (%)
mutation Na+/K+ Syrian hamster 0.3-10 µg/ml ? positive Tsutsui et al.
ATPase locus (-) embryo cells 1984
TK +/- forward mouse lymphoma up to 5000 µg/ ? negative McGregor et
mutation assay(*) cells L5178Y ml al. 1987
host mediated S. typhimurium 1450-2900 µmol/ ? negative1 Braun et al.
assay host: NMRI-mice kg 1977
host mediated S. typhimurium 12-1585 mg/kg ? positive Simmon et al.
TA1530, TA1535 ip 1979
TA1538
host:
Swiss Webster mice
1: weakly positive when given simultaneously with sodium nitrite
Tests for chromosome effects
chromosome aberr. human 0.00001-1% 93.2- negative Meretoja et
assay (-) lymphocytes 96.3 al. 1976
crossing over Aspergillus up to 2000 µg ? positive1 Bignami et al.
test (-) nidulans 1977
non-disjunct. strain P positive1
test (-)
non-disjunct. Aspergillus up to 0.4 mg/ml ? positive Morpurgo et
test (-) nidulans al. 1979
strain P
Tests for chromosome effects (contd)
Test system Test object Concentration/ Purity Results References
dose (%)
sister Chinese 0.01-100 µg/ ? positive1 Perry &
chromatid hamster ovary ml (+S9) Thomson 1981
exchange(*) cells (CHO)
1: weakly positive
Tests for DNA damage/repair
rec assay (-) B. subtilis 20 µg/plate ? negative Shirasu et al.
H17 rec+, 1976
M45 rec-
rec assay (*) B. subtilis up to 1 mg/plate ? positive1 Kada, 1981
H17 rec+,
M45 rec-
rec assay (*) E. coli 500 µg/ml ? negative Ichinotsubo et
JC 2921, 9238 al. 1981
8471, 5519, 7623
7689
rec assay (+) E. coli WP2, 4000 µg/ml ? negative Mamber et al.
WP100 1983
pol A test(-) E. coli 5 mg/plate 93.2 negative Bamford et al.
pol A1, pol A+ 1976
pol A test(*) E. coli 333 µg/plate ? negative Rosenkranz et
WP3110, P3478 al. 1981
Tests for DNA damage/repair (contd)
Test system Test object Concentration/ Purity Results References
dose (%)
differential E. coli WP2, 250-1000 µg/ ? negative Tweats, 1981
killing assay(*) WP67, CM871 ml
mitotic S. cerevisiae 100 & 1000 µg/ ? negative Kassinova et
recombination(*) T1, T2 ml al. 1981
DNA repair S. cerevisiae 100-750 µg/ ? positive Sharp &
test(*) 197/2d, rad3, ml (-S9) Parry, 1981b
rad18, rad52,
trp2
UDS test(*) HeLa cells 0.1-100 µg/ml ? positive Martin &
(+S9) McDermid, 1981
prophage induct. E. coli 58-161 1-10 mg/ml ? negative Thomson,
assay(+) Prophage lambda 1981
prophage induct. E. coli 2000 µg/plate ? negative Mamber et al.
assay(+) GY5027, GY4015 1984
mitotic crossing S. cerevisiae 100 & 1000 µg/ ? negative Kassinova et
over(*) T1, T2 ml al. 1981
mitotic gene S. cerevisiae 12.5 mg/ml ? negative Zimmermann
conversion(*) D7 & Scheel, 1981
mitotic gene S. cerevisiae 300 µg/ml ? positive Sharp & Parry
conversion(*) JD1 (-S9) 1981a
1: positive results were obtained only after metabolic activation with liver homogenates from
Japanese clam and Yellowtail fish
Tests for in vitro transformation
Test system Test object Concentration/ Purity Results References
dose (%)
Tests for in vitro transformation
cell transf. BALB/3T3 cells 0.01-2.5 mg/ml ? equivocal1 Brusick, 1976
assay(-)
cell transf. BHK-21 cells 0.025-25 µg/ml ? positive Styles, 1979
assay(+)
cell transf. BHK-21 cells 0.025-250 µg/ml ? positive Styles, 1981
assay(+)
cell transf. BHK-21 cells 4000 µg/ml ? negative Daniel &
assay(*) Dehnel, 1981
cell transf. hamster embryo 1-100 µg/ml ? positive Inoue et al.
assay(+) cells 1981
cell transf. hamster embryo 0.1-100 µg/ml ? positive Dunkel et al.
assay(-) cells 1981
rat embryo 10-1200 µg/ml ? positive
cells
cell transf. Syrian hamster 0.3-10 µg/ml ? positive Tsutsui et al.
assay(-) embryo cells 1984
Tests for in vitro transformation
Test system Test object Concentration/ Purity Results References
dose (%)
cell transf. mouse embryo ? Dunkel et al.
assay(-) cells 1984
C3H/10T1/2
laboratory A: 2-250 µg/ml negative
laboratory B: 125-1000 positive
µg/ml
1: positive only in 1 out of 3 tests
In vivo tests
non-disjunct. Drosophila 10 ppm in diet 93.2% negative Laamanen et
test females al. 1976
recessive Drosophila 10 ppm in diet 93.2% negative Laamanen et
lethal test males al. 1976
recessive Drosophila 2000 ppm in diet ? negative Vogel et al.
lethal test males 1981
dominant lethal NMRI-mice 1 x 1000 mg/kg ? negative Machemer,
test males 1977a
dominant lethal Ha(ICR)-mice 1 or 10 ppm in 94.59 negative Knickerbocker,
test males the diet for 1978
49 days
micronucleus CD-1 mice 2 x 500 mg/kg ? negative Tsuchimoto &
test males + females Matter, 1981
In vivo tests (contd)
Test system Test object Concentration/ Purity Results References
dose (%)
micronucleus NMRI-mice 1 x 10 000 mg/kg 96.9 negative Herbold, 1982
test males + females
sperm morphol. (CBA x BALB/c)F1 50-500 mg/kg/day ? negative Topham, 1980
assay 5 ip-injections
Tests were done: (*) = with and without metabolic activation (S9-mix)
(+) = with S9-mix activation
(-) = without S9-mix
DNA damage and repair
The possibility of inducing DNA damage by amitrole has been
investigated frequently and in a number of different ways. In
bacteria the results have been negative except for one recombinant
assay, which was positive when an exogenous metabolic activation was
provided by "liver" preparations from a mollusc and a fish. A DNA
repair assay in yeast gave a positive result as did a repair assay
in mammalian cells.
Cell transformation
Assays for cell transformation in several systems gave
predominantly positive results.
Other endpoints
Amitrole did not increase the frequency of morphologically
abnormal sperm in mice.
Amitrole has therefore been tested adequately in series of in
vitro and in vivo genotoxicity assays. Positive responses were
obtained in a number of mutation assays in bacteria,
recombinogenicity assays in yeast and some mammalian cell assays for
mutation, sister-chromatid exchange and cell transformation. No
genotoxicity was demonstrated in vivo. The Meeting concluded that
the genotoxic potential of amitrole was equivocal.
Special studies on skin and eye irritation and skin sensitization
In a limited study, the potential for dermal irritation by
amitrole (95%) was examined in 4 albino rabbits (strain and sex not
specified) over a 24-hour period following a single application of
dosages from 1000 up to 10 000 mg/kg bw. Minimal dermal irritation
was observed with mild erythema at the high-dose level only. By 48
hours, the skin appeared normal (Elsea, 1954).
In a limited study, the potential for eye irritation by
amitrole (95%) was examined in 3 albino rabbits (strain and sex not
specified) following application of 3 mg into the conjunctival sac
of the left eye. Observations were made at 1, 4 and 24 hours and at
daily intervals for 6 days. Mild irritation was observed at 4
hours, which subsided within 24 hours (Elsea, 1954).
In a Magnusson-Kligman maximization test in Pirbright White
guinea-pigs, amitrole (97.6%) was found to be a moderate skin
sensitizer. Concentrations employed were 2.5% for intracutaneous
induction, 25% for topical induction, and 12% for the first and
second challenges (Mihail, 1984).
In a Klecak open epicutaneous test in BOR:DHPW/SPF guinea-pigs,
amitrole (97.3%) did not have a sensitizing effect. Concentrations
employed were 0, 3, 10, or 30% for induction, and 1, 3, 10, or 30%
for the first and second challenges (Mihail, 1985).
Special studies on farm animals
Cattle tolerated administration of both a single oral dose of
amitrole at 1000 mg/kg bw as well as repeated 100 mg/kg bw doses on
10 consecutive days without symptoms (Stendel, 1965).
Female sheep (1/dose level) were orally treated with amitrole
at doses of 0, 750, or 1000 mg/kg bw at intervals of 5-7 days over a
period of 8 weeks. The only symptom observed was lethargy
immediately following treatment. Three days after the last dose the
animals were slaughtered. Residues were found in muscular tissues
at levels of 80 ± 30 ppm and 120 ± 40 ppm in the low- and high-dose
respectively (semi-quantitative determination). The thyroids were
grey-red discoloured. At histopathology complete cessation of
colloid formation, with adenomatoid proliferation of the follicle
epithelia was observed (Hapke et al., 1965).
In sheep, a single oral dose of 750 mg/kg bw led to clinical
symptoms (lethargic behaviour, frequent lying down). At higher
doses, the animals became apathetic and did not eat. At a dose
level of 4000 mg/kg bw the animals died. A single oral dose of 200
mg/kg bw was tolerated by a horse without any symptoms. Adult geese
tolerated oral doses up to 1000 mg/kg bw. At higher doses, these
birds became somnolent. Mortality occurred at 10 000 mg/kg bw
(Hapke, 1967).
Young cattle and sheep were orally exposed to amitrole at
dosages of 10, 25 or 50 mg/kg bw for up to 10 consecutive days. The
10 mg/kg bw dose was tolerated without symptoms by the cattle.
Doses of 25 or 50 mg/kg bw led to toxic symptoms after 3 and 2 days,
respectively. One out of three sheep exhibited retarded body-weight
development at 10 mg/kg bw, whereas at the higher doses all sheep
showed toxic symptoms. Hens were treated orally at dosages of 50,
100 or 250 mg/kg bw on 10 consecutive days. Retarded body-weight
development was observed at 100 and 250 mg/kg bw. Hens dosed at 375
or 500 mg/kg bw died within 3-7 days after the start of the study
(Palmer, 1972).
Observations in humans
In a patch test conducted with a human volunteer, amitrole
exerted no primary dermal irritant effect after exposure periods of
4 or 8 hours. A slight irritant effect was observed in 3 out of 6
subjects after 24 hours of exposure (no further details) (Hecht,
1954).
A case study of a 41-year old weed control operator with a 6-
month history of dermatitis involving his face, hands, back, thighs,
and feet was reported. Patch testing with 1% amitrole showed a
strong positive vesicular reaction at 2 and 4 days, indicative of
allergic contact dermatitis (English et al., 1986).
The intentional ingestion of a commercial mixture of amitrole
and diuron, at a dose equivalent to 20 mg/kg bw amitrole (together
with about 38 mg/kg bw diuron), was reported to have caused no
symptoms of poisoning in a female subject. Within a few hours, the
compound appeared in the urine at a concentration of 100 mg/100 ml.
Metabolites could not be detected in urine (Geldmacher-von
Mallinckrodt & Schmidt, 1970).
Astwood (1960) reported in a brief communication that a single
oral dose of 100 mg amitrole inhibited radioiodine uptake by the
thyroid of both normal and thyrotoxic subjects for 24 hours.
However, a dose of 10 mg was said to have had a slight effect on
iodine uptake. Astwood suggested that amitrole could be used
therapeutically in the treatment of hyperthyroidism.
An epidemiological study was conducted on Swedish railway
workers exposed to various herbicides. A small increase in tumours
was observed, particularly of the lung. Amitrole was only one of
the pesticides (other pesticides were for example phenoxy acids) to
which these workers were exposed (Axelson, 1980).
Mild cases of dermatitis on the face due to contact with
amitrole occurred yearly in one or two production workers of the
American Cyanamid Company. The dermatitis was of a primary irritant
type rather than the sensitive type. No other medical findings, not
occurring in the general public, and, in particular, no thyroid or
liver tumours were observed during the years of observation
(1955-1970) (Clyne, 1970).
Skin irritation was also occasionally observed in employees of
Bayer engaged in production of amitrole. No other adverse effects
occurred (Miksche, 1982).
The thyroid function in 5 employees who had been engaged in
production and packaging of amitrole for periods between 3 and 16
years was checked as part of the occupational medical programme.
Scintigraphic thyroid imaging findings and determinations of the T3
and T4 levels afforded no evidence for the presence of an effect on
thyroid function (Miksche, 1983).
In a study for a possible phototoxic potential of amitrole, 20
test subjects were exposed to patches containing amitrole at a level
of 1% in an ointment base (hydrous eucerin) over a period of 48
hours. The treatment areas were then irradiated with ultraviolet
light (UVA and UVB), and the dermal reactions assessed (no further
details). No evidence was found for phototoxic skin reactions
(Tronnier, 1983).
General mode of action
Amitrole is considered a goitrogenic compound. The mechanisms
by which goitrogens produce their pharmacologic and potential
neoplastic effects is through the induction of hormonal imbalance.
Most commonly this is the result of interference with the thyroid
iodide transport system or interference with peroxidases essential
to the synthesis and secretion of competent thyroid hormone. The
sequence of events triggered by this interference is generally
understood. Because of the ability of goitrogens to inhibit thyroid
hormone synthesis, these compounds have the potential to reduce
circulating levels of T3 and T4 and, consequently, to induce the
secretion of TSH by the pituitary. As a result, prolonged exposure
to such compounds can be expected to induce thyroid gland
hypertrophy and hyperplasia, nodular hyperplasia and, ultimately,
may lead to neoplasia. Evidence indicates that if the pharmacologic
effects, thyroid hypertrophy and hyperplasia, are prevented or
controlled within the feedback system, follicular cell neoplasia
does not develop. The data also suggest that some degree of
hypertrophy or hyperplasia of the thyroid gland is tolerated within
the oscillation of the feedback system without induction of
neoplasia. The rat, and to a lesser extent the mouse, appear to be
very sensitive to goitrogens, since after short exposure periods
their thyroid glands exhibit hypertrophic and hyperplastic changes.
Following continuous, long-term exposure of rats and mice to these
agents, both the thyroid and the pituitary glands frequently exhibit
neoplastic changes. In contrast, humans appear to be less sensitive
(Paynter et al., 1988; Hill et al., 1989).
COMMENTS
Amitrole is rapidly and almost completely absorbed from the
gastrointestinal tract following oral administration to rats and
mice. It is rapidly distributed throughout most body tissues, but
with a slight accumulation in tissues with a rapid cell turnover
(bone marrow, spleen, thymus, gastrointestinal tract). In a study
with pregnant mice it was observed that amitrole passes through the
placenta into the fetuses with the same distribution pattern as in
the mothers. Excretion is rapid after oral exposure. Within 24
hours, 70-95% of the administered radioactivity is excreted via the
urine, mainly as the parent compound.
The metabolic transformation in mammals produces two minor
metabolites detectable in the urine. Metabolism of amitrole occurs
mainly in the liver and involves substitution of the hydrogen atom
in the 5-position. The metabolites identified were 3-amino-5-
mercapto-1,2,4-triazole and 3-amino-1,2,4-triazolyl-5-mercapturic
acid.
Amitrole has a low acute toxicity when tested in several
species by various routes of administration. In old studies,
amitrole was reported to have slight irritating effects on the skin
and eyes. Evidence of a moderate sensitizing potential was observed
in a Magnusson-Kligman test but not in a Klecak open epicutaneous
test. WHO has classified amitrole as unlikely to present acute
hazard in normal use.
Oral exposures of up to four weeks in rats revealed that
effects on the thyroid occurred at levels > 60 ppm in the diet or
104 ppm in drinking-water. No effects were observed at 30 ppm in
the diet (equivalent to 3 mg/kg bw/day) or 10 ppm in drinking-water
(equivalent to 1.5 mg/kg bw/day). Furthermore, it was shown that
after a recovery period the effects on the thyroid were reversible.
In a 30-day study in mice at concentrations in drinking-water
of 0, 5000, 10 000 or 20 000 mg/l, liver effects were observed at
all dose levels.
Several short-term oral studies were performed with rats.
These were mainly focused on the effects on the thyroid, as this is
the target organ in rats.
Only two oral studies were suitable for assessment. In one
study, male rats were exposed to dietary concentrations of 0, 2, 10
or 50 ppm for 13 weeks or to 0, 0.25 or 0.50 ppm for 11 weeks. The
NOAEL was 2 ppm (equivalent to 0.1 mg/kg bw/day) based on
histological changes in the thyroid (appearance of follicular cells,
contents of colloid and capillary density). Decreases in protein-
bound iodine were not considered to be biologically significant.
The other study consisted of four short-term experiments in
female rats. Dietary concentrations in one experiment were 0, 2, 20
or 200 ppm with exposure for six weeks, in two subsequent
experiments 0, 20, 50 or 200 ppm with exposure for 6 or 13 weeks and
in the fourth experiment 0, 20, 50, 200 or 500 ppm with exposure for
six weeks. From these four experiments, the overall NOAEL was 2 ppm
(equivalent to 0.1 mg/kg bw/day) based on increased iodine uptake
(shortly after injection), increased thyroid weight and
histopathological changes of the thyroid (goitre and clearly
activated thyroids).
From several short-term studies in rats with administration in
drinking-water, slight effects on the thyroid (moderate stimulation
of the thyroid epithelium) were seen at the lowest concentration
tested, 50 ppm.
In a one-year study in dogs, no effects on the thyroid were
observed at the highest dose tested (12.5 mg/kg bw/day). The only
effect observed at this level was pale-coloured pancreas. The test,
however, was performed with a low number of animals.
Long-term toxicity and/or carcinogenicity studies have been
performed in mice, rats, and golden hamsters. Studies in mice were
focused on induction of liver and thyroid tumours. In a
carcinogenicity study in mice with only one but very high dose level
(1000 mg/kg bw/day by gavage), survival time was significantly
reduced and liver and thyroid tumours were observed in all treated
mice. A slight increase in the incidence of liver tumours was
observed in a special carcinogenicity study in which pups were
treated for a period of 90 weeks at a level of 500 ppm in the diet.
In an 18-month carcinogenicity study in mice at levels of 0, 1,
10 or 100 ppm in the diet, an increased incidence of tumours was not
observed. In this study, a thyroid function test was also performed
with a small number of animals. At 100 ppm an increase in thyroid
weight and in iodine accumulation in the thyroid was observed. The
NOAEL was 10 ppm (equivalent to 1.5 mg/kg bw/day).
In a carcinogenicity study in rats with levels of 0, 1, 10 or
100 ppm in the diet, a slight decrease in survival time, an increase
in the incidence of thyroid tumours and an increase in the incidence
of (mainly benign) pituitary tumours were observed at 100 ppm. In
this study, a thyroid function test was also performed with a small
number of animals. At 100 ppm, thyroid weight was increased during
the whole study period as was the percentage accumulation of
radioiodine in the thyroid. The NOAEL was 10 ppm (equivalent to 0.5
mg/kg bw/day).
In another limited long-term toxicity study in rats, the NOAEL
was 10 ppm (equivalent to 0.5 mg/kg bw/day) based on thyroid
hyperplasia. A clearly enhanced thyroid tumour incidence was found
at 50 and 100 ppm. In this study, animals suffered from apparent
respiratory infection.
In a third study in rats, thyroid hyperplasia and thyroid
tumours were observed in animals fed 100 ppm (during the first 40
weeks of the 115-120 week study, the dose level was 5 ppm). In rats
treated at pulsed intervals (alternate four week periods) at levels
of 60 ppm (first 3 ppm) and 200 ppm (first 10 ppm) thyroid tumours
were also observed. Slight thyroid hyperplasia was also observed at
the lowest dose level of 20 ppm (first 1 ppm; intermittent dosing
regimen). A NOAEL could not be established.
In an 18-month carcinogenicity study in Syrian hamsters at
dietary concentrations of 0, 1, 10 or 100 ppm, the NOAEL was 10 ppm,
equivalent to 1 mg/kg bw/day, based on decreased body-weight gain
and increased mortality. No effects on the thyroid were observed at
100 ppm. There was no evidence of carcinogenic potential.
A well-performed reproduction study was not available. From a
limited study in rats at dietary concentrations ranging from 25 to
1000 ppm, effects on reproductive capability were observed at 500
ppm and above. Reduction of liver weight and thyroid hyperplasia
were the most sensitive effects observed at the lowest dose level
(25 ppm, equivalent to 1.3 mg/kg bw/day).
In a teratogenicity study, rats were exposed by gavage at doses
of 0, 100, 300 or 1000 mg/kg bw/day on days 6 to 15 of gestation.
No effects were observed in this study. The NOAEL for
maternotoxicity and embryo/ fetotoxicity was 1000 mg/kg bw/day.
In another teratogenicity study, rats were exposed by gavage at
doses of 0, 100, 500 or 1000 mg/kg bw/day. Slight maternal toxicity
(reduced weight gain and food consumption and increased thyroid
weights) was observed at doses of 500 and 1000 mg/kg bw/day.
Reduced fetal body weight/litter and reduced skeletal ossifications
were observed in the high-dose group. Increased incidences of
enlarged and/or dark thyroids were seen in fetuses at 500 and 1000
mg/kg bw/day. The NOAEL for maternotoxicity and embryo/fetotoxicity
was 100 mg/kg bw/day. Amitrole was considered not to be teratogenic
in rats at dose levels up to 1000 mg/kg bw/day.
In a teratogenicity study in rabbits the animals were exposed
by gavage to dose levels of 0, 4, 40 or 400 mg/kg bw/day. Decreased
weight gain during the gestation period was observed at 40 and 400
mg/kg bw/day and increased liver weight at 400 mg/kg bw/day. A
dose-related increased incidence of abortions was observed in all
treated groups. Embryo/fetotoxicity were observed at 40 and 400
mg/kg bw/day. Increased incidences of irreversible structural
changes were also found at these dose levels, which involved mainly
the head and limbs. The NOAEL for maternotoxicity,
embryo/fetotoxicity and teratogenicity was 4 mg/kg bw/day.
In a dermal teratogenicity study in rabbits at dose levels of
0, 1000, 1500 or 2000 mg/kg bw/day, maternotoxicity (decreased body
weight and food consumption, thin appearance and anorexia) was
observed at 2000 mg/kg bw/day. At this level, irreversible
structural changes (anencephaly and microphthalmia) were observed.
The NOAEL for maternotoxicity, embryo/fetotoxicity and
teratogenicity after dermal exposure was 1500 mg/kg bw/day.
Amitrole has been tested adequately in series of in vitro and
in vivo genotoxicity assays. Positive responses were obtained in
a number of mutation assays in bacteria, recombinogenicity assays in
yeast and some mammalian cell assays for mutation, sister-chromatid
exchange and cell transformation. No genotoxicity was demonstrated
in vivo. The Meeting concluded that the genotoxic potential of
amitrole was equivocal.
Amitrole is a goitrogen in mice, rats and sheep but not in
Syrian hamsters, dogs, or cattle at the doses that have been tested.
The mechanism of thyroid toxicity involves inhibition of thyroid
peroxidase. This inhibition results in decreases in circulating
levels of T4 and T3 which stimulate the pituitary to increase
secretion of TSH which in turn may cause thyroid hypertrophy,
hyperplasia and neoplasia. Threshold doses have been identified in
the sensitive species. Amitrole is not genotoxic in in vivo
assays.
The Meeting withdrew the conditional ADI and established a
temporary ADI, based on the NOAEL of 0.5 mg/kg bw/day in the 24-
month dietary study in rats, using a safety factor of 1000 because
of inadequacy of the data.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Mouse: 10 ppm, equivalent to 1.5 mg/kg bw/day
(18-month study)
Rat: 10 ppm, equivalent to 0.5 mg/kg bw/day
(24-month study)
100 mg/kg bw/day (teratogenicity study)
Hamster: 10 ppm, equivalent to 1.0 mg/kg bw/day
(18-month study)
Dog 12.5 mg/kg bw/day (12 month study)
Estimate of temporary acceptable daily intake for humans
0-0.0005 mg/kg bw
Studies without which the determination of a full ADI is
impracticable
Results be submitted to WHO by 1996
(all known to have been initiated):
Two-generation reproduction study in rats
One-year study in dogs
Oral teratogenicity study in rabbits
Metabolism study in rats
Studies which will provide information valuable in the continued
evaluation of the compound
Further observations in humans.
Comparative biotransformation (including humans).
Clarification of genotoxic potential of amitrole.
REFERENCES
Alexander, N.M. (1959). Antithyroid action of 3-amino-1,2,4-
triazole. J. Biol. Chem. 234: 148-150.
Astwood, E.B. (1960). Cranberries, turnips and goiter. J. American
Med. Assoc. 172: 1319-1320.
Axelson, O., Sundell, L., Andersson, K., Edling, C., Hogstedt, C.,
Kling, H. (1980). Herbicide exposure and tumor mortality. An updated
epidemiologic investigation on Swedish railroad workers. Scan. J.
Work Environ. Health, 6: 73-79.
Babish, J.G. (1977). Triiodothyronine (T3) and Thyroxine (T4)
levels in male rats consuming Amitrole (3-amino-1,2,4-triazole) in
their diets for four weeks followed by a four week recovery period.
Unpublished report No 5539 (dated: 29-11-1977) from Food and Drug
Research Laboratories, Inc.. Submitted to WHO by Bayer AG.
Bagdon, R.E., Shaffer, C.B., Vidone, L.B., Golz, H.H. (1956).
Aminotriazole - acute and subacute toxicity. Unpublished report No
56-29 (dated: 14-12-1956) from American Cyanamid Company. Submitted
to WHO by Bayer AG.
Bamford, D., Sorsa, M., Gripenberg, U., Laamanen, I., Meretoja, T.
(1976). Mutagenicity and toxicity of amitrole. III. Microbial tests.
Mutation Research, 40: 197-202.
Becci, P. (1983). Evaluation of the chronic inhalation toxicity and
carcinogenicity of amitrole in rats. Unpublished report No 5821
(dated: 13-01-1983) from Food and Drug Research Laboratories, Inc..
Submitted to WHO by Bayer AG.
Ben-Dyke, R., Perry, M.C., Wall, I. (1973). The short-term
cumulative toxicity of aminotriazole in the rat. Unpublished report
Tox/73/39-6 (dated: September 1973) from Fisons Limited Agrochemical
division, Toxicology department. Submitted to WHO by Bayer AG.
Bignami, M., Aulicino, F., Velcich, A., Carere, A., Morpurgo, G.
(1977). Mutagenic and recombinogenic action of pesticides in
Aspergillus nidulans. Mutation Research, 46: 395-402.
Bionetics Research Laboratories (1968). Evaluation of carcinogenic,
teratogenic, and mutagenic activities of selected pesticides and
industrial chemicals. Volume II: Teratogenic study in mice and rats.
Report PB-223 160 (dated: August 1968) from Bionetic Research Labs,
Inc., prepared for National Cancer Institute.
Braun, R., Schöneich, J., Ziebarth, D. (1977). In vivo formation
of N-nitroso compounds and detection of their mutagenic activity in
the host-mediated assay. Cancer Research, 37: 4572-4579.
Brooks, T.M. & Dean, B.J. (1981). Mutagenic activity of 42 coded
compounds in the salmonella/microsome assay with preincubation.
Progress in Mutation Research, 1: 261-270.
Brusick, D.J. (1975). Mutagenic evaluation of compound 3-amino-
1,2,4-triazole. Unpublished report No 2547 (dated: 14-08-1975) from
Litton Bionetics, Inc. Submitted to WHO by Bayer AG.
Brusick, D.J. (1976). Mutagenicity evaluation of 3-amino-1,2,4-
triazole; in vitro cellular transformation in BALB/3T3 cells.
Unpublished report No 2549 (dated: 26-10-1976) from Litton
Bionetics, Inc. Submitted to WHO by Bayer AG.
Clyne, R.M. (1970). Statement before the amitrole advisory
committee. Unpublished document from the American Cyanamid Company
dd 16 December 1970. Submitted to WHO by Bayer AG.
Cox, G.E. & Re, T.A. (1978). Inhalation toxicity study with 3-amino-
1,2,4-triazole (Amitrole) in adult Fisher 344 rats. Unpublished
report No 5492 (dated: 08-09-1978) from Food and Drug Research
Laboratories, Inc., Submitted to WHO by Bayer AG.
Daniel, M.R. & Dehnel, J.M. (1981). Cell transformation test with
baby hamster kidney cells. Progress in Mutation Research, 1: 626-
637.
Den Tonkelaar, E.M. & Kroes, R. (1974). Schildklierfunctieonderzoek
na subacute en semichronische toediening van aminotriazol.
Unpublished report No. 64/74 Tox. from the National Institute of
Public Health, Bilthoven, the Netherlands.
Dunkel, V.C., Pienta, R.J., Sivak, A., Traul, K.A. (1981).
Comparative neoplastic transformation responses of Balb/3T3 cells,
Syrian hamster embryo cells, and Rauscher murine leukemia virus-
infected Fischer 344 rat embryo cells to chemical compounds. J.
Nat. Cancer Inst. 67: 1303-1315.
Dunkel, V.C., Zeiger, E., Brusick, D., McCoy, E., McGregor, D.,
Mortelmans, K., Rosenkranz, H.S., Simmon, V.F. (1984).
Reproducibility of microbial mutagenicity assays: I. Tests with
Salmonella typhimurium and Escherichia coli using a standardized
protocol. Environmental Mutagenesis, 6: 1-25.
Dunkel, V.C., Schechtman L.M., Tu, A.S., Sivak, A., Lubet, R.A.,
Cameron, T.P. (1988). Interlaboratory evaluation of the C3H/10T1/2
cell transformation assay. Env. Mol. Mutagen. 12: 21-31.
Elsea, J.R. (1954). Amino Triazole. Acute dermal application - acute
eye application. Unpublished report from Hazleton Laboratories,
Virginia (dated: 23-12-1954). Submitted to WHO by Bayer AG.
English, J.S., Rycroft, R.J., Calnan, C.D. (1986). Allergic contact
dermatitis from aminotriazole. Contact Dermatitis, 14: 255-256.
Fang, S.C., George, M., Yu, T.C. (1964). Metabolism of 3-amino-
1,2,4-triazole-5-C14 by rats. J. Agric. Food Chem. 12: 219-223.
Fang, S.C., Khanna, S., Rao, A.V. (1966). Further study on the
metabolism of labelled 3-amino-1,2,4-triazole (ATA) and its plant
metabolites in rats. J. Agric. Food Chem. 14: 262-265.
Feinstein, R.N., Fry, R.J.M, Staffeldt, E.F. (1978). Carcinogenic
and Antitumor effects of aminotriazole on acatalasemic and normal
catalase mice. J. Natl. Cancer Inst. 60: 1113-1116.
Fogleman, R.W. (1954). Amizole: Acute administration -
pharmacodynamics - subacute feeding. Unpublished report from
Hazleton Laboratories, Virginia (dated: 02-12-1954). Submitted to
WHO by Bayer AG.
Franco, L., Municio, A.M. (1975). Comparative metabolism of 3-amino-
1,2,4-triazole. Gen. Pharmac. 6: 163-169.
Fregly, M.J. (1968). Effect of aminotriazole and thyroid function in
the rat. Toxicol. Appl. Pharmacol. 13: 271-286.
Fujii, T., Miyazaki, H., Hashimoto, M. (1984). Autoradiographic and
biochemical studies of drug distribution in the liver. III. (14C)
Aminotriazole. Eur. J. Drug Metab. Pharmacokinet. 9: 257-265.
Gaines, T.B., Kimbrough, R.D., Linder, R.E. (1973). The toxicity of
amitrole in the rat. Toxicol. Appl. Pharmacol. 26: 118-129.
Gatehouse, D. (1981). Mutagenic activity of 42 coded compounds in
the microtiter fluctuation assay. Progress in Mutation Research,
1: 376-386.
Geldmacher-von Mallinckrodt, M. & Schmidt, H.P. (1970). Toxicity and
metabolism of aminotriazole in man. Arch. Toxikol. 27: 13-18.
Grunow, W., Altmann, H.-J., Böhme, Chr. (1975). Metabolism of 3-
amino-1,2,4-triazole in rats. Arch. Toxicol. 34: 315-324.
Hapke, H.-J., Rüssel, H., Ueberschär, S. (1965). Dangers in the use
of aminotriazol. Dtsch. Tierärztl. Wochenschrift, 72: 204-206.
Hapke, H.-J. (1967). The toxicity of aminotriazole for domestic
animals. Zbl. Vet. Med. 14: 469-486.
Hayes, S., Gordon, A., Sadowski, I., Hayes, C. (1984). RK bacterial
test for independently measuring chemical toxicity and mutagenicity:
Short-term forward selection assay. Mutation Research, 130: 97-
106.
Hecht (1954). Aminotriazol - Letter report of May 11, 1954 from
Bayer AG Institute of Toxicology. Submitted to WHO by Bayer AG.
Hecht & Kimmerle (1962). Aminotriazol - Letter report of August 23,
1962 from Bayer AG Institute of Toxicology. Submitted to WHO by
Bayer AG.
Heimann, K.G. (1982). Aminotriazol techn. - Letter report of April
7, 1982 from Bayer AG Institute of Toxicology. Submitted to WHO by
Bayer AG.
Henwood, S.M. (1988). Percutaneous teratology study with amitrole
technical in rabbits. Unpublished report on number HLA 6224-107
from Hazelton Laboratories America, Inc., submitted to WHO by Rhône-
Poulenc Ag. Company
Herbold, B. (1980). Amitrole; Salmonella/microsome test for
detection of point-mutagenic effects. Unpublished report No 9339
(dated: 01-08-1980) from Bayer AG Institute of Toxicology. Submitted
to WHO by Bayer AG.
Herbold, B. (1982). Amitrole; Micronucleus test on the mouse to
evaluate for mutagenic effect. Unpublished report No 11139 (dated:
13-09-1982) from Bayer AG Institute of Toxicology. Submitted to WHO
by Bayer AG.
Hill, R.N., Erdreich, L.S., Paynter, O.E., Roberts, P.A., Rosenthal,
S.L., Wilkinson, C.F. (1989). Thyroid follicular cell
carcinogenesis. Fund. Appl. Toxicol. 12: 629-697.
Hubbard, S.A., Green, M.H.L., Bridges, B.A., Wain, A.J., Bridges,
J.W. (1981). Fluctuation test with S9 and hepatocyte activation.
Progress in Mutation Research, 1: 361-370.
Ichinotsubo, D., Mower, H., Mandel, M. (1981). Testing of a series
of paired compounds (carcinogen and non-carcinogenic structural
analog) by DNA repair-deficient E. coli strains. Progress in
Mutation Research, 1: 195-198.
Innes, J.R., Ulland, B.M., Valerio, M.G., Petrucelli, L., Fishbein,
L., Hart, E.R., Palotta, A.J. (1969). Bioassay of pesticides and
industrial chemicals for tumorigenicity in mice: A preliminary note.
J. Nat. Cancer Inst. 42: 1101-1114.
Inoue, K., Katoh, I., Takayama, S. (1981). In vitro transformation
of hamster embryo cells by 3-(N-salicycloyl)amino-1,2,4-triazole.
Toxicol. Letters, 7: 211-215.
Johnson, W.D. (1981). Lifetime feeding study of amitrole in Fischer
344 rats. Unpublished report No 5651 (dated: 07-08-1981) from Food
and Drug Research Laboratories, Inc. Submitted to WHO by Bayer AG.
Jukes, T.H & Shafer, C.B. (1960). Antithyroid effects of
aminotriazole. Science, 132: 296-297.
Kada, T. (1981). The DNA-damaging activity of 42 coded compounds in
the rec-assay. Progress in Mutation Research, 1: 171-182.
Kassinova, G.V., Kovaltzova, S.V., Marfin, S.V., Zakharov, I.A.
(1981). Activity of 40 coded compounds in differential inhibition
and mitotic crossing-over assays in yeast. Progress in Mutation
Research, 1: 434-455.
Keller, J. (1959). Aminotriazole: Two-year chronic feeding - rats.
Unpublished report from Hazleton Laboratories Virginia (dated: 02-
01-1959). Submitted to WHO by Bayer AG.
Kimmerle (1968). Aminotriazol - Letter report of June 12, 1968 from
Bayer AG Institute of Toxicology. Submitted to WHO by Bayer AG.
Knickerbocker, M. (1978). A study to determine the potential of
amitrole to induce dominant lethal mutations in Ha (ICR) mice.
Unpublished report no 5502 (dated: 28-02-1978) from Food and Drug
Research Laboratories, Inc. Submitted to WHO by Bayer AG.
Laamanen, I., Sorsa, M., Bamford, D., Gripenberg, U. Meretoja, T.
(1976). Mutagenicity and toxicity of amitrole. I. Drosophila
tests. Mutation Research, 40: 185-190.
Lemoine, N.R., Mayall, E.S., Williams, E.D., Thurston, V., Wynford-
Thomas, D. (1988). Agent-specific ras oncogene activation in rat
thyroid tumours. Oncogene, 3: 541-544.
MacDonald, C.M., Pullinger, D.H. (1976). A pharmacokinetic study to
compare "head only" and "whole body" inhalation methods of 14C-
amitrole exposure. Unpublished report No 291/1 (dated: March 1976)
from Hazleton Laboratories Europe Ltd. Submitted to WHO by Bayer AG.
Machemer, L. (1977a). Amitrole/Dominant lethal test on the male
mouse to evaluate for mutagenic effects. Unpublished report no 6679
(dated: 23-03-1977) from Bayer AG, Institute of Toxicology.
Submitted to WHO by Bayer AG.
Machemer, L. (1977b). Amitrole; Study for embryotoxic and
teratogenic effects after oral administration to the rat.
Unpublished report No 6634 (dated: 24-02-77) from Bayer AG Institute
of Toxicology. Submitted to WHO by Bayer AG.
Mamber, S.W., Bryson, V., Katz, S.E. (1983). The Escherichia coli
WP2/WP100 rec assay for detection of potential chemical carcinogens.
Mutation Research, 119: 135-144.
Mamber, S.W., Bryson, V., Katz, S.E. (1984). Evaluation of the
Escherichia coli K12 inductest for detection of potential chemical
carcinogens. Mutation Research, 130: 141-151.
Martin, C.N. & McDermid, A.C. (1981). Testing of 42 coded compounds
for their ability to induce unscheduled DNA repair synthesis in HeLa
cells. Progress in Mutation Research, 1: 533-537.
McDonald, D.J. (1981). Salmonella/microsome tests on 42 coded
chemicals. Progress in Mutation Research, 1: 285-297.
McGregor, D.B., Martin, R., Cattanach, P., Edwards, I., McBride, D.,
Caspary, W.J. (1987). Response of the L5178Y Tk+/Tk-mouse lymphoma
cell forward mutation assay to coded chemicals. I. Results for nine
compounds. Env. Mutagen. 9: 143-160.
Mehta, R.D., von Borstel, R.C. (1981). Mutagenic activity of 42
coded compounds in the haploid yeast reversion assay, strain XV185-
14C. Progress in Mutation Research, 1: 414-423.
Meretoja, T., Gripenberg, U., Bamford, D., Laamanen, I., Sorsa, S.
(1976). Mutagenicity and toxicity of amitrole, II. Human lymphocyte
culture tests. Mutation Research, 40: 191-196.
Mihail, F. (1984). Aminotriazole - Studies for skin sensitization
effect on guinea pigs. Unpublished report No 12607 (dated 12-04-
1984) from Bayer AG Institute of Toxicology. Submitted to WHO by
Bayer AG.
Mihail, F. (1985). Aminotriazole - Study for skin-sensitising effect
on guinea pigs in the open epicutaneous test. Unpublished report No
13775 (dated: 27-08-1985) from Bayer AG Institute of Toxicology.
Submitted to WHO by Bayer AG.
Mihail, F. & Schilde, B. (1984). Aminotriazole - Study for subacute
dermal toxicity to rabbits Unpublished report No 12369 (dated: 09-
01-1984) from Bayer AG Institute of Toxicology. Submitted to WHO by
Bayer AG.
Miksche, L.W. (1982). Letter report of 22-06-82 - BBA request-
effects on man. Unpublished document from the Bayer AG, LE Medical
Department Leverkusen. Submitted to WHO by Bayer AG.
Miksche, L.W. (1983). Letter report of 22-04-83 - Production of
amitrole-medical monitoring. Unpublished document from the Bayer AG,
LE Medical Department Leverkusen. Submitted to WHO by Bayer AG.
Mori, S., Takeuchi, Y., Toyama, M., Makino, S., Ohhara, T., Tochino,
Y., Hayashi, Y. (1985). Amitrole: Strain differences in
morphological response of the liver following subchronic
administration to mice. Toxicology Letters, 29: 145-152.
Moriya, M., Ohta, T., Watanabe, K., Miyazawa, T., Kato, K., Shirasu,
Y. (1983). Further mutagenicity studies on pesticides in bacterial
reversion assay systems. Mutation Research, 116: 185-216.
Morpurgo, G., Bellincampi, D., Gualandi, G., Baldinelli, L., Serlupi
Crescenzi, O. (1979). Analysis of mitotic nondisjunction with
Aspergillus nidulans. Env. Health Perspect. 31: 81-95.
Napalkov, N.P. (1962). Blastomogenic action of 3-amino-1,2,4-
triazole. (Russ). Gig. Tr. prof. Zabol. 6: 48-51. In: Amitrole; IARC
Monographs on the evaluation of carcinogenic risk of chemicals to
humans (1986). World Health Organization, Vol. 41: 293-317, Lyon.
Palmer, J.S. (1972). Toxicity of 45 organic herbicides to cattle
sheep, and chickens. Production Research report no 137 (dated: March
1972) from US Department of Agriculture. Submitted to WHO by Bayer
AG.
Paynter O.E., Burin, G.J., Jaeger, R.B., Gregorio C.A. (1988).
Goitrogens and thyroid follicular cell neoplasia: evidence for a
threshold process. Regul. Toxicol. Pharmacol. 8: 102-119.
Perry, P.E. & Thomson, E.J. (1981). Evaluation of sister chromatid
exchange method in mammalian cells as a screening system for
carcinogens. Progress in Mutation Research, 1: 560-569.
Prince, H.N. (1977). Microbial mutagen assays with amitrole (3-
amino-1,2,4-triazole). Unpublished report no 5634 (dated: 12-09-
1977) from Gibraltar Biological Laboratories (submitted via Food and
Drug Research Laboratories, Inc.). Submitted to WHO by Bayer AG.
Reitze, H.K. & Seitz, K.A. (1985). Light and electron microscopical
changes in the liver of mice following treatment with aminotriazole.
Exp. Pathol. 27: 17-31.
Richold, M. & Jones, E. (1981). Mutagenic activity of 42 coded
compounds in the Salmonella/microsome assay. Progress in Mutation
Research, 1: 314-322.
Rosenkranz, H.S., Hyman, J., Leifer, Z. (1981). DNA polymerase
deficient assay. Progress in Mutation Research, 1: 210-218.
Rowland, I. & Severn, B. (1981). Mutagenic of carcinogens and
noncarcinogens in the salmonella/micrososme test. Progress in
Mutation Research, 1: 323-332.
Seidenberg, I.L. & Gee, A.H. (1953). Report on acute toxicity of
aminotriazole as established by oral administration to rats.
Unpublished report from Foster D. Snell Inc. (dated: 08-06-1953).
Submitted to WHO by Bayer AG.
Shah, P.V. & Guthrie, F.E. (1977). Dermal absorption, distribution,
and the fate of six pesticides in the rabbit. In: Pesticide
Management and Insecticide Resistance. pp 547-554. Academic Press.
Sharp, D.C. & Parry, J.M. (1981a). Induction of mitotic gene
conversion by 41 coded compounds using the yeast culture JD1.
Progress in Mutation Research, 1: 491-501.
Sharp, D.C. & Parry, J.M. (1981b). Use of repair deficient strains
of yeast to assay the activity of 40 coded compounds. Progress in
Mutation Research, 1: 502-516.
Shirasu, Y., Moriya, M., kato, K., Furuhashi, A., Kada, T. (1976).
Mutagenicity screening of pesticides in the microbial system.
Mutation Research, 40: 19-30.
Simmon, V.H., Rosenkranz, H.S., Zeiger, E., Poirer, L.A. (1979).
Mutagenic activity of chemical carcinogens and related compounds in
the intraperitoneal host-mediated assay. J. Natl. Cancer Inst. 62:
911-918.
Steinhoff, D. & Boehme, K. (1978). Aminotriazole; carcinogenesis
study with oral administration to Syrian golden hamsters.
Unpublished report No 7521 (dated: 16-05-1978) from Bayer AG,
Institute of Toxicology. Submitted to WHO by Bayer AG.
Steinhoff, D. & Boehme, K. (1979a). Aminotriazole; carcinogenesis
study with oral administration to rats. Unpublished report No 8450
(dated: 11-06-1979) from Bayer AG, Institute of Toxicology.
Submitted to WHO by Bayer AG.
Steinhoff, D. & Boehme, K. (1979b). Aminotriazole; carcinogenesis
study with oral administration to mice. Unpublished report No 8490
(dated: 17-07-1979) from Bayer AG, Institute of Toxicology.
Submitted to WHO by Bayer AG.
Steinhoff, D., Weber, H., Mohr, U., Boehme, K. (1983). Evaluation of
Amitrole (Aminotriazole) for potential carcinogenicity in orally
dosed rats, mice, and golden hamsters. Toxicol. Appl. Pharmacol.
69: 161-169.
Stendel (1965). Aminotriazole/oral toxicity to cattle. Unpublished
letter report of March 15, 1965 from Bayer AG, Institute of
Toxicology. Submitted to WHO by Bayer AG.
Strum, J.M. & Karnovsky, M.B. (1971). Aminotriazole goiter. Fine
structure and localization of thyroid peroxidase activity.
Laboratory Investigation, 24: 1-12.
Styles, J.A. (1979). Studies on the detection of carcinogens using a
mammalian cell transformation assay with liver homogenate
activation. In: Norpoth, K. & Garner, R.C. (eds), Short-term
mutagenicity test systems for detecting carcinogens. Proceedings of
the International Symposium on short-term mutagenicity test systems.
Dortmund, Nov. 15-17, 1978 pp 226-238. Springer Verlag, New York.
Styles, J.A. (1981). Activity of 42 coded compounds in the BHK-21
cell transformation test. Progress in Mutation Research, 1: 638-
646.
Thomson, J.A. (1981). Mutagenic activity of 42 coded compounds in
the lambda induction assay. Progress in Mutation Research, 1: 224-
235.
Thyssen, J. (1974a). Aminotriazol Batch no 143 - Determination of
acute toxicity. Letter report of June 10, 1974 from Bayer AG,
Institute of Toxicology. Submitted to WHO by Bayer AG.
Thyssen, J. (1974b). Aminotriazol - Determination of acute toxicity.
Letter report of September 26, 1974 from Bayer AG, Institute of
Toxicology. Submitted to WHO by Bayer AG.
Thyssen, J. (1983). Aminotriazole - Acute inhalation toxicity.
Unpublished report No 11707 (dated: 11-04-1983) from Bayer AG,
Institute of Toxicology. Submitted to WHO by Bayer AG.
Tjälve, H. (1974). Fetal uptake and embryogenetic effects of
aminotriazole in mice. Arch. Toxicol. 33: 41-48.
Tjälve, H. (1975). The distribution of labelled aminotriazole in
mice. Toxicology, 3: 49-67.
Topham, J.C. (1980). Do induced sperm-head abnormalities in mice
specifically identify mammalian mutagens rather than carcinogens?
Mutation Research, 74: 379-387.
Tronnier, H. (1983). Aminotriazole - Expertise by consultant
dermatologist on testing for phototoxicity in test subjects.
Unpublished report No R 2544 (dated: 05-08-1983) from City Clinics
Dortmund. Submitted to WHO by Bayer AG
Trueman, R.W. (1981). Activity of 42 coded compounds in the
Salmonella reverse mutation test. Progress in Mutation Research,
1: 343-350.
Tsuchimoto, T. & Matter, B.E. (1981). Activity of coded compounds in
the micronucleus test. Progress in Mutation Research, 1: 705-711.
Tsuda, H., Takahashi, M., Fukushima, S., Endo, Y. (1973). Fine
structure and localization of peroxidase activity in aminotriazole
goiter. Nagoya Med. J., 18: 183-197.
Tsutsui, T., Maizumi, H., Barrett, J.C. (1984). Amitrole-induced
cell transformation and gene mutations in Syrian hamster embryo
cells in culture. Mutation Research, 140: 205-207.
Turner, D.M., Gilbert, C.M. (1976). Evidence of metabolismus of
(14C) Amitrole in the rat after inhalation administration.
Unpublished report No 291/1a (dated May 1976) from Hazleton
Laboratories Europe Ltd. Submitted to WHO by Bayer AG.
Tweats, D.J. (1981). Activity of 42 coded compounds in a
differential killing test using Escherichia coli strains Wp2, WP67
(uvrA polA), and CM871 (uvrA lexA recA). Progress in Mutation
Research, 1: 199-209.
Tyl, R.W. (1986a). Teratogenicity evaluation of aminotriazole
technical administered by gavage to CD rats. Unpublished report No
49-45 (dated: 29-05-1986) from Union Carbide Bushy Run Research
Center. Submitted to WHO by Bayer AG and Rhône-Poulenc Ag. Company.
Tyl, R.W. (1986b). Teratogenicity evaluation of aminotriazole
technical administered by gavage to New Zealand White rabbits.
Unpublished report No 49-66 (dated: 30-05-1986) from Union Carbide
Bushy Run Research Center. Submitted to WHO by Rhône-Poulenc Ag.
Company.
Venitt, S. & Crofton-Sleigh, S. (1981). Mutagenicity of 42 coded
compounds in a bacterial assay using Escherichia coli and
Salmonella typhimurium. Progress in Mutation Research, 1: 351-360.
Vesselinovitch, S.D. (1983). Perinatal hepatocarcinogenesis.
Biological Research in Pregnancy and Perinatology, 4: 22-25.
Vogel, E., Blijleven, W.G.H., Kortselius, M.J.H., Zijlstra, J.A.
(1981). Mutagenic activity of 17 coded compounds in the sex-linked
recessive lethal test in Drosophila melangolaster. Progress in
Mutation Research, 1: 660-665.
Weber, H. (1983). Aminotriazole feeding study: Determination of the
threshold dose level affecting the thyroid function of female rats
by radioiodine assay and by T3 and T4 radioimmunoassay. Unpublished
report No. 11739 (F) (dated: 13-04-1983) from Bayer Health Care
Division. Submitted to WHO by Bayer AG.
Weber, H. & Patzschke, K. (1978). Effect of long-term administration
of aminotriazole on thyroid function of male and female rats, mice
and hamsters. Unpublished report No 7690 (dated: 14-07-1978) from
Bayer AG, Institute of Pharmacokinetics. Submitted to WHO by Bayer
AG.
Weir, R. (1958). 3-Amino-1,2,4-triazole Chronic oral administration
- beagle dogs. Unpublished report from Hazleton Laboratories
Virginia dd December 1, 1958. Submitted to WHO by Bayer AG.
WHO (1992). The WHO recommended classification of pesticides by
hazard and guidelines to classification 1992-1993 (WHO/PCD/92.14).
Available from the International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland.
WHO (1994). International Programme on Chemical Safety Environmental
Health Criteria 158: Amitrole. Geneva, World Health Organization.
Wynford-Thomas, D., Stringer, B.M., Williams, E.D. (1982a).
Dissociation of growth and function in the rat thyroid during
prolonged goitrogen administration. Acta Endocrinologica, 101:
210-216.
Wynford-Thomas, D., Stringer, B.M., Williams, E.D. (1982b).
Goitrogen-induced thyroid growth in the rat: a quantitative
morphometric study. J. Endocr. 94: 131-140.
Zimmermann, F.K. & Scheel, I. (1981). Induction of mitotic gene
conversion in strain D7 of Saccharomyces cerevisiae by 42 coded
chemicals. Progress in Mutation Research, 1: 481-490.
 Toxicological studies
Acute toxicity studies
The acute toxicity of amitrole to several animal species is
given in Table 1.
No signs of toxicity were observed in one beagle-type female
dog when given amitrole (96.1%) by gavage at a dosage of 2150 mg/kg
bw. Another dog receiving 4640 mg/kg bw vomited within 1 hour, and
no signs of toxicity were observed thereafter (Fogleman, 1954).
When a commercial product (water soluble powder with 50% active
ingredient) was administered to mice, the LD50 was reduced to 4.0
g/kg bw amitrole. By determining the mean lethal dose for amitrole
in different preparations, it was established that sodium carbonate,
sodium bicarbonate and wetting agents as admixtures in various
quantities considerably increase the toxicity of amitrole (Hapke,
1967). WHO has classified amitrole as unlikely to present acute
hazard in normal use (WHO, 1992).
Table 1. Acute toxicity of amitrole in animals
Species Sex Route LD50 LC50 Purity Reference
(mg/kg bw) (mg/m3)
Mouse M oral 14 700 7 9 96.1%* Fogleman, 1954
? oral 11 000 8 ? Hapke, 1967
? i.v. 5 000 10 ? Hecht, 1954
M i.v. > 1 600 4 6 94% Bagdon et al., 1956
M i.p. > 10 000 6 94% Bagdon et al., 1956
Rat ? oral > 4200 ? Seidenberg & Gee, 1953
? > 10 000 6 10 ? Hecht, 1954
M 25 000 1 9 94% Bagdon et al., 1956
M > 10 000 6 10 ? Hecht & Kimmerle, 1962
? > 2500 93.7% Kimmerle, 1968
M > 5000 ? Thyssen, 1974a
M > 5000 98% Thyssen, 1974b
M&F > 4080 6 99% Gaines et al., 1973
M > 5000 ? Heimann, 1982
M&F dermal > 2500 99% Gaines et al., 1973
M&F inhal (4 h) > 439 6 96.9% Thyssen, 1983
? i.v. 5000 10 ? Hecht, 1954
M i.p. > 4000 6 94% Bagdon et al., 1956
Rabbit ? dermal > 10 000 5 6 95% Elsea, 1954
Cat M&F oral > 5000 2 94% Bagdon et al., 1956
M&F i.v. > 1750 3 94% Bagdon et al., 1956
1 80% aqueous suspension
2 40% aqueous suspension; the 3 cats tested (limit test)
vomited within 2 hours after receiving the dose
3 10% solution in 0,9% saline; one cat per dose level
4 aqueous solution
5 moistened with 0.5% methyl cellulose solution
6 no signs of toxicity
7 mortality at 21 500 mg/kg; signs of toxicity at this and
higher dose levels: extreme depression, squinting eyes, slow,
laboured respiration, diarrhoea, ataxia, depressed or absent
placement and righting responses, mild clonic convulsions,
gasping, coma and death and irritation of gastrointestinal
tract. No signs of toxicity at the lower dose level
(10 000 mg/kg bw)
8 signs of toxicity seen from 5 mg/kg bw: severe hypokinesia,
slight tremor of the extremities, apathy ranging to catatonia,
prone position, individual deep inspirations.
9 only 5 animals/group
10 less than 4 animals/group
* no correction for purity was made.
Short-term toxicity studies
Dietary/gavage
Rats
In a two-week dietary study, administration of 60 or 120 ppm
amitrole resulted in enlargement of the thyroid gland of rats
(number and strain not specified) and a pronounced lowering of
iodine uptake. Over this two-week interval there were no
significant changes at levels of 15 or 30 ppm (Jukes & Shaffer,
1960; Annex I, reference 23).
Amitrole was administered by gavage to groups of rats (strain
unknown), 5 days a week, for 4 weeks at doses of 0, 100, 200 or 400
mg/kg bw. In all treated groups, growth rate was reduced, relative
thyroid weight increased and iodine content of the thyroid reduced
(Hapke, 1967; Annex 1, reference 23).
In a range-finding study, groups of albino Wistar rats (4/sex)
received 0, 125, 1250 or 12 500 ppm amitrole in their diet for 27
days. The low dose (125 ppm) was raised to 25 000 ppm on day 7 of
treatment. Body-weight was retarded and food intake reduced in all
treatment groups. Thyroid weight (the only organ investigated) was
increased in all dose groups (Ben-Dyke et al., 1973).
Reversibility of thyroid effects was studied in a two-week
dietary study in male albino rats (strain unknown). A dietary level
of 1000 ppm increased the (absolute) thyroid weights to about 3.5
times those in the untreated controls. Thyroid weights had nearly
completely returned to normal after two weeks recovery (Bagdon et
al., 1956; Annex I, reference 23).
Amitrole (purity 94.6%) was administered to groups of male
Sprague-Dawley rats (20/dose) at levels of 0, 30, 100, or 300 ppm in
their diet for 28 days, followed by a 28-day recovery period. Two
animals/group were killed weekly in order to monitor the thyroid
function by assaying the thyroid hormones T3 and T4. Body weight
was depressed and food consumption decreased at the 100 and 300 ppm
dose levels. Food consumption returned to normal during the
recovery period as did body weight, but only in the 100 ppm group.
T3 levels were significantly decreased at day 7 in the 300 ppm
group and at day 14 in the 100 ppm group. T3 levels returned to
normal by day 19 post-treatment. T4 levels followed the same
pattern although the extent of decrease was greater. The NOAEL in
this study was 30 ppm (equivalent to 3 mg/kg bw/day) (Babish, 1977).
In a thyroid function test amitrole (purity 96.9%) was
administered to groups of 30 female Wistar rats (12 week old,
weighing 200 g) at levels of 0, 0.5, 1, 2, or 4 ppm in their diet.
Ten animals per group were sacrificed 3, 9 or 29 days after study
initiation and the following parameters were examined: thyroid
weight, accumulation of radioiodine in the thyroid (24 hours after
administration of 131I), and serum levels of T3 and T4.
Significantly elevated thyroid weights were observed only in the 1
and 2 ppm groups, 3 days after study initiation. No significant
deviation was observed in any dose group at the later test dates.
The percentage of iodine accumulation in the thyroids of the 2 ppm
group exhibited significant elevation after a 9-day treatment
period. In the 4 ppm group a slight, but not significant increase
was found. No effect was observed at 29 days, and no clear effects
were observed on T3 or T4 levels. The effects on thyroid function
found in this study were considered marginal and not consistent.
Therefore the highest dose in this study (4 ppm, equivalent to 0.4
mg/kg bw/day) was considered to be the NOAEL (Weber, 1983).
Amitrole (96.1%) was administered to groups of Carworth Farm
male and female rats (5 animals/sex/group) at levels of 0, 100,
1000, or 10 000 ppm in the diet for 63 days. Both males and females
showed reduced body-weight gain at 1000 and 10 000 ppm, which was
accompanied by reduced food consumption. There were no deaths or
gross signs of toxicity. Histopathological examination of the
liver, kidneys, bladder, small intestine, spleen and testis (thyroid
was not examined) revealed only an increased vacuolization of the
liver cells around the central veins in the 1000 and 10 000 ppm
dosage groups. The vacuoles were identified as fat globules
indicative of fatty metamorphosis associated with liver cell damage.
No histological effects were noted at 100 ppm. Because the thyroid
was not examined in this study, it is of limited value in the
evaluation of amitrole (Fogleman, 1954).
Fregly (1968) investigated the dose-response relationship
between amitrole administered in the diet and a variety of clinical
parameters in order to establish the minimum effective dose on
thyroid activity. Amitrole was administered to groups of male
Spruce Farm strain rats (10/dose) at levels of 0, 2, 10, or 50 ppm
in the diet for 13 weeks. In a separate experiment, amitrole was
administered in the diet to similar groups at levels of 0, 0.25, or
0.50 ppm for 11 weeks. In the first experiment, there were no
treatment-related effects on body weight, food consumption,
haematocrit, haemoglobin, or the rate of oxygen consumption. Mean
rectal temperature (measured at week 12) was slightly, but
significantly increased at 50 ppm. Uptake of radioactive iodine,
measured in vivo during week 12 at various times 22-53 hours after
injection of Na131I, was significantly decreased at 50 ppm. At the
end of the first study, radioactivity in the thyroid gland, measured
24 hours after injection, and the level of PBI in serum were
significantly reduced in all treatment groups. The PBI-values were
5.1, 3.7, 3.8, and 3.3 µg/100 ml for the 0, 2, 10, and 50 ppm
groups, respectively. Thyroid weight was increased significantly
only in the 50 ppm group. Histological changes in the thyroid were
noted at 10 and 50 ppm in the follicular cells with regard to both
appearance and the presence of colloid. Capillary density, which is
characteristic of the TSH-stimulated thyroid, was increased
significantly at both 10 and 50 ppm. In the second experiment, at
lower dose levels, no significant differences were noted between the
treated and control groups. In this study PBI was not affected. In
fact the PBI values were 3.2, 3.9, and 4.5 µg/100 ml for the 0, 0.25
and 0.50 ppm groups, respectively. It should be noted that the PBI
control values, measured in the second experiment were much lower
than those measured in the first experiment. This means that there
is no biologically significant effect on PBI, because all values
fall in the same range. The NOAEL was therefore 2 ppm (equivalent
to 0.1 mg/kg bw/day) (Fregly, 1968).
Several short-term experiments were carried out in Wistar rats,
in order to establish a no-effect level on thyroid function. In all
experiments the uptake of 131I by the thyroid was measured in an in
vivo test 6, 24, or 48 hours after administration of 0.6 µCi 131I
per animal intraperitoneally. In addition, at the end of the study,
thyroid weight and PBI were measured and the thyroid was studied
histopathologically.
In the first experiment, four groups of 8 female rats received
0, 2, 20, or 200 ppm of amitrole in the diet for 6 weeks. The
uptake of 131I was measured after 5 days and 6 weeks. At both times
a significantly increased uptake was found in the 200 ppm group 6
hours after injection, which decreased rapidly after 24 and 48
hours. At that time the radioactivity was lower than that of the
controls. The thyroid weight was increased in the 200 ppm group and
histopathologically goitre was found in this group only. No
significant effects were found at lower dosages.
In the second experiment in which 8 female rats per group
received 0, 20, 50, or 200 ppm for 6 weeks, the same effect was
found in the 200 ppm group. In addition, a significantly decreased
PBI was observed at the end of the experiment compared with the
controls. At 50 ppm a statistically increased uptake was found 6
hours after injection of 131I. In this case the radioactivity in
the thyroid remained higher than the controls after 24 and 48 hours.
Histopathologically only a very slight activation was found, whereas
200 ppm showed strong activation and goitre.
In the third experiment, 10 female animals per group received
amitrole at dietary concentrations of 0, 20, 50, or 200 ppm for 13
weeks. The uptake of 131I by the thyroid was significantly
increased at 50 and 200 ppm after 6 and 12 weeks. At 50 ppm, the
radioactivity in the thyroid remained high after 24 and 48 hours,
whereas at 200 ppm a very high uptake was found 6 hours after
injection of 131I, followed by a rapid decrease with still lower
values than the controls after 48 hours. At 200 ppm, the PBI was
decreased and the thyroid/body-weight ratio increased by a factor of
6. At 50 ppm, only a slightly increased relative thyroid weight was
found. Histologically a strong activation and goitre were found at
200 ppm, and a slight activation at 50 ppm. In this experiment, a
tendency to a higher uptake of 131I was found in the 20 ppm group.
The three above mentioned experiments were carried out with an
iodine content of about 0.2-0.3 ppm in the diet. In the fourth
experiment an iodine content of about 2 ppm was used. In this
experiment, 8 female rats per group received 0, 20, 50, 200 or 500
ppm amitrole in the diet for 6 weeks, to determine whether iodine
could protect against the antithyroid action of amitrole. At 500
ppm, a small increase in iodine uptake was found 5 hours after
injection, but thereafter a very rapid decrease was found. At 200
ppm, the uptake was much higher and the same type of decrease was
found as in the other experiments, whereas with 50 ppm a
significantly increased thyroid radioactivity was found at all
times. PBI was decreased at 200 and 500 ppm only.
Histopathologically, goitre and strongly activated thyroids were
found at the two highest dose levels. Some activation was found in
the 20 ppm group. It can be concluded that measurement of 131I
uptake at different time points was a sensitive method for the
effects of amitrole on the thyroid. At 20 ppm, only slight effects
were found on iodine uptake and thyroid histopathology. The overall
NOAEL from these four experiments was 2 ppm, equivalent to 0.1 mg/kg
bw/day (Den Tonkelaar & Kroes, 1974).
Dogs
In a one-year study in male and female beagle dogs, amitrole
was given in capsules at daily dose levels of 0, 0.25, 1.25, 2.50 or
12.5 mg/kg bw, 6 days per week (4-6 animals/group). There were no
clinical signs of toxicity or pharmacological effects.
Haematological, biochemical and urinalysis parameters were
comparable to those of the control dogs, and within normal limits.
The dogs at the 12.5 mg/kg bw/day dose level had pale-coloured
pancreases as the only dose-related gross pathological effect.
Histopathological examination of all dogs did not reveal any
treatment-related effect. The thyroid, in particular, was normal at
all dose levels (Weir, 1958).
Drinking-water
Mice
Groups of male albino mice (strain unknown) were exposed to
amitrole in the drinking-water at concentrations of 0, 0.5, 1.0 or
2% for 30 days (water consumption was not reported). Light
microscopy revealed dose-related hypertrophy of hepatocytes with
nuclear deformations, increased pyknotic nucleoli, and increased
vacuoles in the cytoplasm. Electron microscopy revealed
proliferation of smooth endoplasmic reticulum (Reitze & Seitz,
1985).
Rats
Male Sprague-Dawley rats were provided with drinking-water
containing 400 ppm amitrole over periods of 12, 20, or 37 days.
Based on a body weight of about 200 g and a water intake of 30-35
ml/day, the daily intake was about 60-70 mg/kg bw. The catalase
activity of liver and kidney of the treated animals was inhibited by
50%, but the red cell catalase was unaltered. An increase in
thyroid weight was found, which correlated with the duration of
treatment. Microscopic examination showed hyperplasia of the
thyroid and a loss of colloid. It was postulated in this study that
the ability of the thyroid to concentrate plasma iodine was not
impaired by amitrole, but that the formation of organically-bound
iodine was inhibited (Alexander, 1959).
In a range-finding study, amitrole was administered to groups
of albino Wistar rats (4/sex) at levels of 0, 10, 104 or 1040 ppm in
drinking-water over a treatment period of 27 days. Body-weight gain
was retarded and food intake reduced at 104 ppm and above. Thyroid
weight (the only organ investigated) was increased in a dose-
dependent manner at 104 ppm and above. The NOAEL in this study was
10 ppm, equivalent to 1.5 mg/kg bw/day, based on a daily water
consumption of 30 ml and body weight of 200 g (Ben-Dyke et al.,
1973).
Amitrole (purity 94%) was administered in the drinking-water to
groups of male albino rats (10/dose; strain unknown) at
concentrations of 0, 50, 250, or 1250 ppm for 106 days. The
administration of amitrole resulted in a dose-dependent depression
of growth with a corresponding reduction of food and water intake.
Appearance, behaviour, and mortality were not affected by amitrole.
Haematology, except Ht, and clinical chemistry parameters were not
examined. At the end of the study, histopathology was performed on
the thyroid, hypophysis, liver, kidney, spleen, stomach, small and
large intestines, bladder, testis, adrenal and lung. Gross and
microscopic examination of tissues and organs showed a marked
increase in thyroid size at all dose levels. In rats where reduced
growth was noted, the kidneys, adrenals, liver and spleen were
proportionally smaller. Reproductive organs were not affected.
Microscopic examination showed general enlargement of the thyroid at
50 ppm, with moderate stimulation of the thyroid epithelium (no
evidence of hyperplasia). At 250 ppm, thyroid hyperplasia was
evident. At the two highest dose levels there was also absence of
follicles with stored colloid. Liver catalase activity was reduced
at 250 and 1250 ppm in a dose-dependent manner (Bagdon et al.,
1956; Annex I, reference 23).
In a study measuring the time-course of development of goitre,
Sprague-Dawley rats were given amitrole in the drinking-water (400
ppm). The thyroid of each animal was examined by light microscopy
at various periods from 3 days to 6 months. Each rat drank
approximately 30 ml per day. After 3 days, the thyroid size was
unchanged, although cellular changes were apparent. By one week,
the thyroid was twice its normal size with marked structural
changes. These changes continued to progress with prolonged
administration of the goitrogen. Goitre formation was accompanied
by increased vascularity and decreased colloid content in the
follicular cells. Electron microscopy revealed pronounced
dilatation of the endoplasmic reticulum. Peroxidase activity
measured in the thyroid progressively decreased with prolonged
administration of amitrole (Strum & Karnovsky, 1971).
The effects of amitrole on thyroid histology were examined in
seven groups of 5 female Wistar rats weighing about 200 g, which
were given amitrole in their drinking-water, 2500 ppm, and killed
after 1, 2, 3, 10, 30, 50, or 100 days. Water consumption was not
reported. After 1 and 2 days of exposure the only change noted was
a slight enlargement of some endoplasmic cisternae of follicular
cells. After 3 days the gland was slightly enlarged, follicular
colloid was slightly reduced and in some follicular cells the
cisternae were clearly dilated and stained more lightly for
peroxidase activity than in normal cells. By 10 days the glands had
doubled in size, the follicular epithelium consisted of low,
columnar cells, and colloid had been severely depleted. Nuclei had
become located basally and slightly elongated microvilli projected
into the lumen. Peroxidase activity was no longer seen in the
endoplasmic reticulum cisternae, whereas it was observed in portions
of perinuclear cisternae. These changes had progressed in the 30-
day exposure group, so that the glands were now several times their
normal size. In addition, fibrous thickening of both stroma and
capsule was prominent and cisternal peroxidase activity was absent.
Over 50-day exposure resulted in increased irregularity in
follicular size, more prominent papillary growth of the follicular
epithelium and greatly diminished peroxidase activity throughout the
cells (Tsuda et al., 1973).
Functional and morphological changes in the thyroid were
examined in male Wistar rats. Amitrole was administered via
drinking-water at a concentration of 1000 ppm for periods of up to
153 days. Within 2 weeks thyroid hormone synthesis (serum T3 and
T4) was inhibited. Serum TSH level increased rapidly during the
first 4 weeks, and remained essentially constant after that. The
accumulation of radioiodine in the thyroid (measured as the ratio
thyroid to serum inorganic iodide - T/S ratio) followed a similar
pattern. Thyroid weight increased rapidly during the first few
weeks and thereafter growth rate declined. After 4 months, total
thyroid weight was increased to a 12-fold plateau. During the first
week of amitrole administration there was also a rapid change in the
morphology of the gland; an increase in the proportional volume of
the epithelial cell compartment being accompanied by a corresponding
decrease in follicular lumen (colloid) and a marked increase in
vascularity (Wynford-Thomas et al., 1982a,b).
Dermal
Rabbits
Amitrole (97.6%) was applied to the shaved skin of the back and
flanks of groups of 6 male and 6 female New Zeeland white rabbits at
doses of 0, 25 or 100 mg/kg bw for 6 hours each day on 15
consecutive workdays. The skin was abraded in three animals per
group. The NOAEL for systemic and local effects was at the highest
dose level, i.e. 100 mg/kg bw/day (Mihail & Schilde, 1984).
Inhalation
Rats
Groups of Fischer 344 rats (15/sex/dose) were exposed to an
atmosphere containing amitrole (94.6% pure) at concentrations of 0,
0.1, 0.32, 0.99, or 4.05 mg/l (nominal concentrations adjusted for
non-nebulizing material), 5 hours/day 5 times per week, for 4 weeks
(particle size unknown). There were no adverse effects on behaviour
and no body-weight changes were noted. T3 and T4 levels were
depressed after 14 and 27 days at levels of 0.32 mg/l and above. At
these dose levels, thyroid hyperplasia and elevated thyroid weights
were also observed. At a concentration of 0.1 mg/l no effects on
the thyroid were observed (Cox & Re, 1978).
Long-term/carcinogenicity studies
Mice
Amitrole was used as a positive control in a study which
investigated the carcinogenicity of some 120 chemicals. Groups of
(C57BL/6 x C3H/Anf) F1 mice (18/sex) and (C57BL/6 x AKR) F1 mice
(18/sex) were given amitrole by gavage at a dose level of 1000 mg/kg
bw/day on days 7-28 of age, and in the diet at a level of 2200 ppm
from 28 days onwards until the end of the experiment. This was
planned to be an 80-week study but all of the mice in the amitrole
treatment groups had died by weeks 53-60. Thyroid tumours were
reported to have occurred in nearly all of the treated mice (64/72).
Liver tumours were observed in 34/36 (C57BL/6 x C3H/Anf) F1 treated
mice and in 33/36 (C57BL/6 x AKR) F1 treated mice. In pooled
control groups, 8/166 (C57BL/6 x C3H/Anf) F1 mice and 6/172
(C57BL/6 x AKR) F1 mice had liver tumours (Innes et al., 1969).
In a carcinogenicity study, amitrole (97.0% pure) was
administered to groups of NMRI mice (75/sex/dose level) at levels of
0, 1, 10, or 100 ppm in their diet for 18 months. There were no
treatment-related effects on appearance, behaviour, body weight,
food consumption or survival times (haematology not measured).
Histological examination of tissues of the major organs did not
reveal any treatment-related effects apart from a slight increase in
the number of hyperemias in the pituitary at 100 ppm (5 at 1 ppm, 2
at 10 ppm and 16 at 100 ppm). The incidence of treatment-related
tumours was not increased. Concurrent satellite groups of 5
animals/sex/dose were additionally used in this carcinogenicity
study for different thyroid function tests (thyroid weights,
incorporation of radioactive iodide in the thyroid gland, proportion
of PBI in total plasma iodine) performed at intervals of 3, 6, 9,
12, and 18 months. The thyroid weights (up to 3 times control
values), were elevated in the 100 ppm males at all test dates. The
percentage of iodine accumulation as well as the iodine level in the
thyroid were elevated in the male mice at 100 ppm. The fraction of
PBI in the male mice was elevated 9 months after study initiation,
but was depressed at later test dates. Comparable results were
observed in the high-dose females. However, the deviations relative
to the control group were generally smaller than in the males, and
were not significant in most cases. The NOAEL was 10 ppm equivalent
to 1.5 mg/kg bw/day (Steinhoff & Boehme, 1979b, Weber & Patzschke,
1978; Steinhoff et al., 1983).
In a study on C3H mice and their substrain without serum
catalase activity, ingestion of 10 000 ppm amitrole in the diet led
to a reduction in lifetime. In comparing the two substrains, it was
shown that the acatalasemic mice lived longer under treatment than
did the normal mice. The mean survival times were 35 and 25 weeks,
respectively. The C3H strain features a high rate of spontaneous
liver tumours (it was reported to be 9% in females and 46% in
males). Under amitrole treatment, liver tumours occurred earlier
and at a higher incidence in the acatalasemic mice than in the
normal mice (Feinstein et al., 1978).
Three groups of B6C3F1 mice were fed a diet containing 500
ppm amitrole (purity not specified): Group 1: the dams were treated
from day 12 of gestation to birth of the pups; group 2: dams
together with their pups were treated from birth to weaning; group
3: pups were treated for a period of 90 weeks after weaning.
Although not specifically stated, information on other chemicals
tested and described in this paper (benzidine, safrole etc.),
implies that pups of groups 1, 2, and 3 animals were examined only
for liver tumours after 90 weeks. It was not stated if treatment
with the other compounds were conducted in the same or different
rooms. The incidence of hepatocellular adenomas and carcinomas,
respectively, were: group 1 males, 4/74 and 2/74; group 2 males,
6/45 and 4/45; group 3 males, 15/55 and 11/55; group 1 females, 0/83
and 0/83; group 2 females, 0/55 and 0/55; group 3 females, 5/49 and
4/49. The incidence of these tumours in the untreated control
animals (which may or may not have been concurrent controls) at week
90 were: males, 1/98 and 0/98; females, 0/96 and 0/96. Other organs
were not examined. It was concluded that there was no effect of
amitrole treatment in group 1 or in females of group 2, but that
marginal increases occurred in males of group 2 and in males and
females of group 3 (Vesselinovitch, 1983).
The susceptibilities of three mouse strains to the development
of preneoplastic hepatic lesions were examined following amitrole
administration in their drinking-water at 10 000 ppm. The strains
were DS, ICR (Crj: CD-1) and NOD derived from ICR and found to
develop spontaneous insulitis followed by diabetes. There were
reported to be indications that NOD mice may carry immunological
abnormalities. In each of two experiments, 3 groups of female mice
were administered amitrole via drinking-water for 3 months
(experiment 1) or 6 months (experiment 2), after which they were
killed and their livers examined. The proportions of mice with
hyperplastic nodules were, in experiment 1: 15/19, NOD; 3/55, DS;
0/5, ICR, and in experiment 2: 19/19 NOD; 18/18 DS; 17/19 ICR. A
single hepatocellular carcinoma developed in a NOD mouse after 6
months (Mori et al., 1985).
Rats
In a limited chronic toxicity study (no haematology or clinical
chemistry) groups of Carworth Farm Wistar rats (35/sex/dose)
received amitrole in the diet at levels of 0, 10, 50, or 100 ppm for
two years. After 13 weeks, 5 animals/sex/dose and after 68 weeks 3
animals/sex/dose were killed for organ weight measurement and
histopathological examination. A separate group received 500 ppm
for 19 weeks. Due to marked reduction in body-weight gain and food
consumption, these animals were left on the control diet for 7 weeks
and were then killed (26 weeks). Animals in all groups, including
controls suffered from apparent respiratory infection and were in
poor condition. Death occurred in 67 rats but there was no
relationship with treatment. Body-weight gain was reduced at 100
ppm in male animals during the first 13 weeks of the study. After
68 and 104 weeks of treatment, relative thyroid weight was increased
at 100 ppm (not measured after 13 weeks). Histopathological
examination after 13 weeks showed hyperplasia and hypertrophy of the
thyroid at 500 ppm, but this was reversed after withdrawal of the
amitrole diet. Histopathological changes were also seen at 100 ppm
and in one animal at 50 ppm. At 68 weeks, 3 animals at 50 ppm
showed definitive evidence of hyperplasia while all animals at 100
ppm showed evidence of hyperplasia and hypofunctioning of the
thyroid. At 104 weeks tumours were found: thyroid tumours were
present in 1/10 animals of the 10 ppm group, 2/15 at 50 ppm and
15/27 at 100 ppm. No tumour was detected in 5 control group animals
but one rat exhibited early stages of an adenoma. One thyroid from
the 50 ppm group and 4 from the 100 ppm group exhibited lesions
interpreted as adenocarcinomas by several pathologists and benign
neoplasms by others. Based on thyroid hyperplasia the NOAEL was 10
ppm equivalent to 0.5 mg/kg bw/day (Keller, 1959; Jukes & Shaffer,
1960).
Groups of Fischer 344 rats (75/sex/dose) were treated with
amitrole (94.6% pure). Group A were the controls. Rats of group B
were fed 5 ppm of amitrole in their diet during 1-39 weeks and then
100 ppm during weeks 40-115 for males and 40-119 for females. Rats
in groups C, D, and E received amitrole in their diet at pulsed
intervals (alternate 4 week periods) at 1, 3, or 10 ppm,
respectively, during weeks 1-39 and 20, 60, or 200 ppm during the
last exposure period (week 40 onwards). On alternate 4-week
periods, groups C, D, and E received the basal diet containing no
amitrole. There were no treatment-related clinical signs of
toxicity or changes in body weight or food consumption. No
treatment-related effects on parameters of haematology, clinical
chemistry or urinalysis were observed (only 5 animals/sex/dose level
were examined). Thyroid weights were significantly increased in
both males and females in groups B and E after 60 weeks and at
termination. Increased thyroid weights, although not significant,
were also observed in group D. The T3 and T4 activities were
elevated in groups B and E from week 44 onwards. However, these
effects were not always consistent. These changes were not observed
up to week 36, when the groups were administered the lower dose
levels. Follicular epithelial hyperplasia in the thyroid was noted
in groups B, D, and E and to a much lesser extent in group C. An
increased incidence in thyroid tumours (mainly follicular adenomas)
was observed in male and female rats of groups B and E and in the
male animals of group D. There was no significant difference in
tumour incidence between groups B and E (in these dose groups the
incidence of follicular adenomas was 80-84% and of follicular
adenocarcinomas 5%, compared to 0-2% in the control group). Due to
the change in dosing regimen it was difficult to evaluate this
study. However, effects on the thyroid (hyperplasia) were seen in
all treated groups (Johnson, 1981).
In a study performed to characterize the oncogenes
participating in the genesis of thyroid tumours, male Wistar rats
(number not specified) were treated with a chemical carcinogen
(nitrosomethylurea, NMU) or by ionizing radiation (131I) to induce
thyroid tumours, and were then treated with amitrole (purity not
specified) as a goitrogen at a level of 0.1% in drinking-water
throughout their entire lifetimes. A control group was only
administered amitrole without prior tumour-initiating treatment.
Malignant thyroid tumours developed after even a relatively brief
period in rats treated with NMU or 131I. Animals exclusively
treated with amitrole exhibited only benign tumours which developed
at a markedly slower rate. A "H-ras" oncogene was determined to
be involved in treatment with NMU (13 out of 15 cases), whereas a
"K-ras" oncogene was involved in the animals treated with 131I
(8/15 cases) and amitrole (only 1/10 cases) (Lemoine et al.,
1988).
In a briefly worded description of a long-term study in rats,
thyroid and liver tumours were reported to occur in rats exposed to
20-25 mg amitrole per day via drinking-water or to 250 or 500 mg
amitrole per day in their diets for life. No control data were
reported (Napalkov, 1962). Due to the serious shortcomings in this
study, it was not considered relevant for the evaluation of amitrole
toxicity.
In a carcinogenicity study, groups of Wistar rats (75/sex/dose
level) were treated with amitrole (97.0% pure) in the diet at
concentrations of 0, 1, 10, or 100 ppm for two years. There were no
treatment-related effects on appearance, behaviour, body weight, or
food consumption (haematology not measured). A slight decrease in
survival time was found at 100 ppm. Average survival time at 100
ppm for males was 961 days and for females 919 days. The survival
times for control animals were 992 and 969 days for males and
females, respectively.
Concurrent satellite groups of 5 animals/sex/dose level were
additionally used in this carcinogenicity study for different
thyroid function tests (thyroid weights, incorporation of
radioactive iodide in the thyroid gland, proportion of protein-bound
iodine in total plasma iodine) performed at intervals of 3, 6, 9,
12, 18 or 24 months. The thyroid weights (up to 3.6 times control
values for males and 7 times for females) were increased at 100 ppm
as was the uptake of 131I by the thyroid, measured 24 hours after
oral administration of 131I. The fraction of PBI was not affected.
At 100 ppm, an elevated incidence of haemorrhages and hyperaemia of
the pituitary gland as well as a very high rate of cystically
dilated thyroid follicles were seen. Both benign and malignant
tumours of the thyroid were found at 100 ppm. There was also an
increase in the incidence of benign tumours in the pituitary gland
at the 100 ppm level (females). The NOAEL was 10 ppm (equivalent to
0.5 mg/kg bw/day) (Steinhoff & Boehme, 1979a; Weber & Patzschke,
1978; Steinhoff et al., 1983).
Hamsters
In a carcinogenicity study in Syrian golden hamsters
(76/sex/dose) amitrole (97%) was administered in the diet at levels
of 0, 1, 10, or 100 ppm for 18 months. There were no treatment-
related changes in appearance, behaviour or food intake. Body-
weight gain was decreased at 100 ppm from day 400 onward, and
mortality was significantly increased in the 100 ppm dose group.
Average survival time at 100 ppm for males was 557 days and for
females 438 days. The survival times for control animals were 631
and 508 days for males and females, respectively. The main cause of
death in all groups, including controls, was severe amyloidosis of
the kidneys. There was no evidence of amitrole-related
histopathological changes. There was no evidence of a treatment-
related carcinogenic effect. Concurrent satellite groups of 5
animals/sex/dose were additionally used in this carcinogenicity
study for different thyroid function tests (thyroid weights,
incorporation of radioactive iodide in the thyroid gland, proportion
of PBI in total plasma iodine) performed at intervals of 3, 6, 9,
12, or 18 months. There were no treatment-related effects observed.
The NOAEL was 10 ppm equivalent to 1 mg/kg bw/day (Steinhoff &
Boehme, 1978; Weber & Patzschke, 1978; Steinhoff et al., 1983).
Inhalation exposure
Rats
In an inhalation study involving intermittent treatment, groups
of 75 Fischer rats per dose and sex were exposed to aerosols at
nominal amitrole levels of 0, 50 or 500 µg/l air (one of the two
batches used had a purity of 94.6%). The actual amitrole
concentrations in the low-dose group varied between 15.8 and 32.2
µg/l air in the different exposure periods; the levels measured in
the high-dose group ranged between 97.9 and 376.4 µg/l air. The
animals were exposed for 5 hours each day five days per week.
Treatment phases during weeks 1-13, 40-52 and 78-90 were interrupted
by treatment-free intervals. Interim necropsies of 5 animals per
dose and sex were performed after 3, 9 and 18 months; the study was
concluded after 24 months. A total of 28 rats died in week 51 due
to a defect in the air conditioning system which led to an increase
in the room temperature. Treatment of the high-dose group was
thereupon concluded, and the surviving animals (6/75 males, and
18/75 females) were necropsied.
In the high-dose group, food intake and body-weight gain were
decreased and the rate of mortality was elevated. At week 13 of the
study, levels of cholesterol, ASAT and ALAT were higher and of
glucose lower in the high-dose group than in the control group.
Also T3 and T4 levels were lower. No differences in these
parameters were observed after the treatment-free period. Relative
thyroid weight was increased and epithelial hyperplasia of the
thyroid follicles was determined in both dose groups at the end of
the first treatment interval (week 13). This latter finding was no
longer observed after the first treatment-free interval of 24 weeks,
but the thyroid weights were still elevated in both dose groups.
Follicular epithelial hyperplasia was again present in most of the
animals of both treatment groups at the end of the second treatment
phase (week 51). This finding was still observed, in the remaining
treatment group (50 µg/l air), after a treatment-free interval of 26
weeks, which indicates that complete reversion no longer occurred at
this time. Neoplasms of the thyroid (adenomas and adenocarcinomas)
were found in addition to hyperplasia at terminal necropsy (Becci,
1983).
Reproduction studies
Rats
In a limited reproduction study, groups of 10 male and 10
female Sherman rats were fed amitrole in the diet. A preliminary
one-generation study was performed in which rats were fed levels of
0, 500, or 1000 ppm for 55 days before pair-mating. The offspring
were weaned at 21 days. Complete autopsies were performed on the
parents after total exposure of 107-110 days. Ten weanling rats
from each dose group were killed. Mean body-weight gain and food
consumption were reduced in the parents at all dose levels.
Relative kidney, spleen, and liver weights (only in males) were
reduced in parents fed 500 and 1000 ppm. The average number of pups
per litter was significantly reduced at the 500 and 1000 ppm dose
levels, as were the numbers surviving to weaning. Body weight of
pups at weaning was also reduced. Thirty-three out of 45 pups of
rats fed 500 ppm and 55/56 pups of rats fed 1000 ppm died within one
week after weaning. Relative weight of thymus and spleen of pups of
the 500 ppm group (1000 ppm not examined) were significantly
reduced. Thyroid hyperplasia was seen in all treated rats (Gaines
et al., 1973).
In a subsequent 2-generation study, rats were fed dose levels
of 0, 25, or 100 ppm for 61 and 173 days before pair-mating to
produce the F1a and F1b generations, respectively. Then 12
rats/sex of each dose level were pair-mated when about 100 days old
to produce the F2a generation. The offspring were weaned at 21
days. Food consumption was reduced in the F0 generation at
100 ppm. Thyroid hyperplasia was seen in all animals of the 100 ppm
group. In the 25 ppm group hyperplasia was seen in about half of
the F0 (4/10) and F1b (4/10) females and F1b (6/10) males, but
none of the F0 males (F1a litters not examined). Pups of the 100
ppm group showed reduced kidney and liver weights and also male pups
of the 25 ppm group showed reduced liver weights. There was a
decrease in the number of litters in the F2a generation at 100 ppm,
but there were no other changes. A NOAEL for toxic effects could
not be established. Due to the low number of animals used in this
study, a NOAEL for reproductive effects could not be established
(Gaines et al., 1973).
Groups of 5 rats/sex (strain unknown) were mated following a 3-
month pretreatment period during which only males, only females, or
both sexes were exposed to amitrole at a level of 100 ppm in their
drinking-water. The groups were compared with a common control
group. According to the author, the results did not point to a
reduction in reproductive ability for males or females. However,
due to limited data in the report this could not be confirmed. Pups
born in this study were reared and continued on the treatment.
Their growth was reduced (Hapke, 1967).
Special studies on embryotoxicity and/or teratogenicity
Mice
Pregnant NMRI mice (9-12/group) were administered amitrole
(purity not specified) at 0, 500, 1000, 2500 or 5000 ppm in
drinking-water (days 6-18 of pregnancy). There was a marked
decrease (22-28%) in body-weight gain in the dams and pronounced
retardation in the development (lower fetal weight and immature
skeletons) at concentrations of 1000 ppm and above. At the highest
concentration (5000 ppm), maternal toxicity was associated with an
increase in the rate of resorptions. No irreversible structural
changes were observed at any concentration (Tjälve, 1974).
Groups of 13 pregnant C57BL6 mice were treated subcutaneously
with 215 or 464 mg/kg bw/day from days 6 though 14 of gestation.
The highest dose tested resulted in an increased fetal mortality
rate. Subcutaneous treatment of 6 AKR mice from days 6-15 of
gestation at 464 mg/kg bw/day produced no effects in the fetuses.
Daily oral administration of 215 mg/kg bw to 7 C57BL6 mice from
days 6-14 of gestation produced increased fetal mortality and a
reduction of fetal weight. In nearly all groups maternal body
weight was reduced and liver weight increased. There were no
irreversible structural changes observed in any group (Bionetics
Research Laboratories, 1968).
Rats
In a limited teratogenicity study, groups of 8 pregnant female
Sherman rats were given amitrole (99%) by stomach tube at dose
levels of 0, 20 or 100 mg/kg bw/day during days 7 through 15 of
gestation. The animals were allowed to litter and to wean, and the
pups were observed for gross abnormalities. No abnormalities were
observed in the offspring at the time of birth or when weaned
(Gaines et al., 1973).
In a pilot study, 2 female rats (strain unknown) were orally
exposed to amitrole at a dosage of 1000 mg/kg bw/day and one rat to
500 mg/kg bw/day from days 8 through 13 of gestation. Weight gain
of the treated females was reduced. No effects were found on number
of corpora lutea, number of fetuses and no anomalies were observed
(Hapke, 1967).
In a teratogenicity study, groups of 20 presumed pregnant rats
(strain FB30, Long-Evans) were exposed to amitrole by gavage at dose
levels of 0, 100, 300, or 1000 mg/kg bw/day on days 6 to 15 of
gestation. Fetuses were examined on day 20 of gestation. There
were no deaths or signs of toxicity at any dose level. Body-weight
gain was not affected by treatment. There were no treatment-related
effects on the resorption rate, fetal weight, number of live
fetuses, placental weight, or sex ratio. There was no treatment-
related increase in gross, skeletal or visceral malformations. The
NOAEL for maternal and embryo/fetotoxicity was 1000 mg/kg bw/day
(Machemer, 1977b).
Groups of 24 presumed pregnant CD rats were exposed to amitrole
(91.83% pure) by gavage at dose levels of 0, 100, 500, or 1000 mg/kg
bw/day on days 6 through 15 of gestation. Apart from the normal
parameters measured in a teratogenicity study, the weights of liver
and thyroid of the dams were examined. Fetuses were examined on day
21 of gestation. Extra groups of 14 females per dose level were
allowed to litter and wean, and were maintained until postnatal day
21. In this postnatal period, body weight, food consumption and
thyroid weights were measured in the dams. Observations in the pups
included number, sex, weight and gross examination. Weight gain of
dams was reduced during gestation days 6-18 at 500 and 1000 mg/kg
bw/day. Food intake was reduced during gestation days 15-21 at the
same dose levels. Maternal thyroid weights (absolute and relative)
were increased at 500 and 1000 mg/kg bw/day, the increase still
persisting at termination of lactation. Fetotoxicity was observed
at 1000 mg/kg bw/day including reduced fetal body weight per litter,
increased incidence of fetuses with unossified or poorly ossified
bones and an increased number of fetuses with enlarged and/or dark
thyroid. These latter findings in the thyroid were also observed in
the 500 mg/kg bw/day dose group. There was no treatment-related
increased incidence of malformations at any dose employed.
Postnatal evaluations indicated no effects of treatment on survival
or growth of the pups, or on maternal body weights, weight gain or
food consumption in the lactation period. The NOAEL for
maternotoxicity and embryo/fetotoxicity was 100 mg/kg bw/day (Tyl,
1986a).
Rabbits
Groups of 22 presumed pregnant New Zeeland white rabbits were
exposed to amitrole (91.83% pure) by gavage at doses of 0, 4, 40, or
400 mg/kg bw/day on days 6 through 18 of gestation. Fetuses were
examined on day 29 of gestation. Maternotoxicity at 40 and 400
mg/kg bw/day included reduced body-weight gain and at 400 mg/kg
bw/day increased (relative) liver weight. A dose-related increase
in the incidence of abortions was observed (0, 1, 3 and 5 for the
control, 4, 40 and 400 mg/kg bw/day dose groups, respectively). The
number of non-viable implants/litter was increased and the
percentage of live fetuses/litter decreased at 40 and 400 mg/kg
bw/day (statistically significant at high dose). Decreased fetal
weight/litter was also seen at these dose levels, with statistical
significance at the high dose. At 40 and 400 mg/kg bw/day there
were significant increases in the incidence of numerous individual
malformations, especially of the head and limbs. The total
percentages of fetuses with malformations were 4.5, 7.2, 36.9 and
62.4% for the control, 4, 40 and 400 mg/kg bw/day dose groups,
respectively. The incidence of a number of individual visceral
(including craniofacial) and skeletal variants (mainly poorly or
absent ossification) was increased at 40 and 400 mg/kg bw/day.
Effects on the fetal thyroid were also observed. The percentages of
fetuses with enlarged thyroid were 1.3, 2.2, 3.6 and 8.2% and of
fetuses with dark/red thyroids were 2.6, 7.9, 8.1 and 12.9% for the
control, 4, 40 and 400 mg/kg bw/day dose groups, respectively.
Following oral administration amitrole was teratogenic,
embryo/fetotoxic and (slightly) maternotoxic at 40 and 400 mg/kg
bw/day. The NOAEL for all types of toxicity was 4 mg/kg bw/day
(Tyl, 1986b).
Four groups of 18 artificially inseminated Hra (NZW) SPF
rabbits were treated dermally from days 7 to 19 (inclusive) of
gestation with 0, 1000, 1500 or 2000 mg amitrole (93.9% purity)/kg
bw/day. The test material was applied as a mixture of 0.5 ml
deionized water/g amitrole for six hours/day. Rabbits were collared
during the exposure period, and remaining test material was removed
with warm water washing immediately following each exposure.
Clinical signs including dermal irritation, body weight and food
intake were measured in does. Dermal irritation, including erythema
and edema, was noted for treated animals. The number of animals
affected and the severity of these observations increased with
increasing dose. Numbers of pregnant females at term were 14, 15,
12 and 11. At 2000 mg/kg bw/day thin appearance and anorexia was
observed in does. Body weight was reduced on day 20, but was
virtually recovered by day 29; food intake was reduced on days 10-
14, with severe reductions on days 14-17 and 17-20 of gestation and
uterine weight was slightly reduced at term at 2000 mg/kg bw/day.
Fetal weights were reduced at 2000 mg/kg bw/day and the incidence of
total resorptions was increased significantly (mainly early
resorptions). Malformations, including microphthalmia (3 fetuses in
2 litters), dilated brain lateral ventricles (2 pups in 1 litter),
anencephaly (2 fetuses in 2 litters), unossified pubis (4 pups in 1
litter, but several other pups in the same litter with thyroid,
skeleton and rib anomalies) were observed at 2000 mg/kg bw/day.
Following dermal administration, amitrole was teratogenic,
embryo/fetotoxic and maternotoxic at 2000 mg/kg bw/day. The NOAEL
for all types of toxicity was 1500 mg/kg bw/day (Henwood, 1988).
Special studies on genotoxicity
A number of genotoxicity tests has been carried out with
amitrole. The results are summarized in Table 2. The important
features of these data are described below.
Gene mutations
Numerous assays for in vitro gene-mutations were performed
and were predominantly negative. One study with E. coli and S.
typhimurium strains gave positive responses (Venitt & Crofton-
Sleigh, 1981). Of two other bacterial mutation assays, one assay
gave a positive result in absence of metabolic activation and the
other gave an equivocal response. Many other in vitro assays gave
negative results. A positive response was obtained in one out of
two mouse peritoneal host mediated assays with S. typhimurium
(Simmon et al., 1979). No mutation induction was observed in
fungi and yeast. No mutation induction was observed in several sex-
linked recessive assays with Drosophila melangolaster. Mutations
(TK +/-) were not induced in mouse lymphoma cells, whereas HPRT
locus and Na+/K+ATPase locus mutations were induced in Syrian
hamster embryo cells (Tsutsui et al., 1984).
Chromosomal damage
Weakly positive results were obtained in tests with fungi. A
cytogenetic study in human lymphocytes was negative, but sister-
chromatid exchanges were increased in a single study. No effects of
amitrole were observed in mice subjected to bone marrow micronucleus
tests or male dominant lethal tests.
Table 2. Results of genotoxicity assays on amitrole
Tests for gene mutations
Test system Test object Concentration/ Purity Results References
dose (%)
reverse mut. S.typhimurium 100 µg/plate 99.4 negative Brusick, 1975
(*) TA1535, TA1537
TA1538
S.cerevisiae, D4 100 µg/plate 99.4 negative
reverse mut. S.typhimurium 0.4% 93.2 negative Bamford et al.
(-) LT 2 trp 1976
(A8, B4, C3)
reverse mut. S.typhimurium 1000 µg/plate ? negative Prince, 1977
(*) TA98, TA100
TA1538, TA1535
point mut. Aspergillus up to 2000 µg ? negative Bignami et al.
(-) nidulans 1977
strain 35
reverse mut. S.typhimurium 20-12 500 µg/ 97.6 negative Herbold, 1980
(*) TA98, TA100 plate
TA1535, TA1537
reverse mut. S.typhmurium 0.2-2000 µg/ ? negative Brooks &
(*) TA1535, TA1537 plate Dean, 1981
TA1538, TA98
TA100, TA92
Tests for gene mutations (contd)
Test system Test object Concentration/ Purity Results References
dose (%)
reverse mut. S.typhimurium up to 5000 µg/ ? negative McDonald,
(*) TA98, TA100 plate 1981
TA1537
reverse mut. S.typhimurium 10-10 000 µg/ ? negative Richold &
(*) TA1535, TA1537 plate Jones, 1981
TA1538, TA98
TA100
reverse mut. S.typhimurium 0.1-2000 µg/ ? negative Rowland &
(*) TA1535, TA1537 plate Severn, 1981
TA1538, TA98
TA100
reverse mut. S.typhimurium 4-2500 µg/ ? negative Trueman,
(+) TA1535, TA1537 plate 1981
TA1538, TA98
TA100
reverse mut. E.coli 0.5-500 µg/ml ? positive Venitt &
(+) WP2uvrA(p) Crofton-
S. typhimurium positive Sleigh, 1981
TA98, TA100
reverse mut. S.typhimurium up to 500 µg/ ? equivocal Hubbard et al.
(*) TA98, TA100 ml 1981
(fluctuation test)
Tests for gene mutations (contd)
Test system Test object Concentration/ Purity Results References
dose (%)
reverse mut. E. coli WP2uvrA 10-500 µg/ml ? negative Gatehouse,
1981
(*) S. typhimurium negative
(microtiter TA98, TA1535
fluctuation test) TA1537
reverse mut. S. cerevisiae 88.9 & 889 µg/ ? negative Mehta & Von
(*) XV185-14C ml Borstel, 1981
reverse mut. S. typhimurium up to 5000 µg/ ? negative Moriya et al.
(*) TA98, TA100 plate 1983
TA1535, TA1537
TA1538
E. coli WP2 hcr negative
forward mut. E. coli CHY832 ? Hayes et al.
+ S9-mix: 10 000 µg/ml negative 1984
- S9-mix 2500 µg/ml positive
reverse mut. S. typhimurium 0.3-333.3 µg/ 98 negative Dunkel et al.
(*) TA1535, TA1537 plate 1984
(comparative TA1538, TA98
study in 4 TA100
laboratories) E. coli 0.3-333.3 µg/ negative
WP-2 uvrA plate
HPRT mutation Syrian hamster 0.3-10 µg/ml ? positive Tsutsui et al.
assay (-) embryo cells 1984
Tests for gene mutations (contd)
Test system Test object Concentration/ Purity Results References
dose (%)
mutation Na+/K+ Syrian hamster 0.3-10 µg/ml ? positive Tsutsui et al.
ATPase locus (-) embryo cells 1984
TK +/- forward mouse lymphoma up to 5000 µg/ ? negative McGregor et
mutation assay(*) cells L5178Y ml al. 1987
host mediated S. typhimurium 1450-2900 µmol/ ? negative1 Braun et al.
assay host: NMRI-mice kg 1977
host mediated S. typhimurium 12-1585 mg/kg ? positive Simmon et al.
TA1530, TA1535 ip 1979
TA1538
host:
Swiss Webster mice
1: weakly positive when given simultaneously with sodium nitrite
Tests for chromosome effects
chromosome aberr. human 0.00001-1% 93.2- negative Meretoja et
assay (-) lymphocytes 96.3 al. 1976
crossing over Aspergillus up to 2000 µg ? positive1 Bignami et al.
test (-) nidulans 1977
non-disjunct. strain P positive1
test (-)
non-disjunct. Aspergillus up to 0.4 mg/ml ? positive Morpurgo et
test (-) nidulans al. 1979
strain P
Tests for chromosome effects (contd)
Test system Test object Concentration/ Purity Results References
dose (%)
sister Chinese 0.01-100 µg/ ? positive1 Perry &
chromatid hamster ovary ml (+S9) Thomson 1981
exchange(*) cells (CHO)
1: weakly positive
Tests for DNA damage/repair
rec assay (-) B. subtilis 20 µg/plate ? negative Shirasu et al.
H17 rec+, 1976
M45 rec-
rec assay (*) B. subtilis up to 1 mg/plate ? positive1 Kada, 1981
H17 rec+,
M45 rec-
rec assay (*) E. coli 500 µg/ml ? negative Ichinotsubo et
JC 2921, 9238 al. 1981
8471, 5519, 7623
7689
rec assay (+) E. coli WP2, 4000 µg/ml ? negative Mamber et al.
WP100 1983
pol A test(-) E. coli 5 mg/plate 93.2 negative Bamford et al.
pol A1, pol A+ 1976
pol A test(*) E. coli 333 µg/plate ? negative Rosenkranz et
WP3110, P3478 al. 1981
Tests for DNA damage/repair (contd)
Test system Test object Concentration/ Purity Results References
dose (%)
differential E. coli WP2, 250-1000 µg/ ? negative Tweats, 1981
killing assay(*) WP67, CM871 ml
mitotic S. cerevisiae 100 & 1000 µg/ ? negative Kassinova et
recombination(*) T1, T2 ml al. 1981
DNA repair S. cerevisiae 100-750 µg/ ? positive Sharp &
test(*) 197/2d, rad3, ml (-S9) Parry, 1981b
rad18, rad52,
trp2
UDS test(*) HeLa cells 0.1-100 µg/ml ? positive Martin &
(+S9) McDermid, 1981
prophage induct. E. coli 58-161 1-10 mg/ml ? negative Thomson,
assay(+) Prophage lambda 1981
prophage induct. E. coli 2000 µg/plate ? negative Mamber et al.
assay(+) GY5027, GY4015 1984
mitotic crossing S. cerevisiae 100 & 1000 µg/ ? negative Kassinova et
over(*) T1, T2 ml al. 1981
mitotic gene S. cerevisiae 12.5 mg/ml ? negative Zimmermann
conversion(*) D7 & Scheel, 1981
mitotic gene S. cerevisiae 300 µg/ml ? positive Sharp & Parry
conversion(*) JD1 (-S9) 1981a
1: positive results were obtained only after metabolic activation with liver homogenates from
Japanese clam and Yellowtail fish
Tests for in vitro transformation
Test system Test object Concentration/ Purity Results References
dose (%)
Tests for in vitro transformation
cell transf. BALB/3T3 cells 0.01-2.5 mg/ml ? equivocal1 Brusick, 1976
assay(-)
cell transf. BHK-21 cells 0.025-25 µg/ml ? positive Styles, 1979
assay(+)
cell transf. BHK-21 cells 0.025-250 µg/ml ? positive Styles, 1981
assay(+)
cell transf. BHK-21 cells 4000 µg/ml ? negative Daniel &
assay(*) Dehnel, 1981
cell transf. hamster embryo 1-100 µg/ml ? positive Inoue et al.
assay(+) cells 1981
cell transf. hamster embryo 0.1-100 µg/ml ? positive Dunkel et al.
assay(-) cells 1981
rat embryo 10-1200 µg/ml ? positive
cells
cell transf. Syrian hamster 0.3-10 µg/ml ? positive Tsutsui et al.
assay(-) embryo cells 1984
Tests for in vitro transformation
Test system Test object Concentration/ Purity Results References
dose (%)
cell transf. mouse embryo ? Dunkel et al.
assay(-) cells 1984
C3H/10T1/2
laboratory A: 2-250 µg/ml negative
laboratory B: 125-1000 positive
µg/ml
1: positive only in 1 out of 3 tests
In vivo tests
non-disjunct. Drosophila 10 ppm in diet 93.2% negative Laamanen et
test females al. 1976
recessive Drosophila 10 ppm in diet 93.2% negative Laamanen et
lethal test males al. 1976
recessive Drosophila 2000 ppm in diet ? negative Vogel et al.
lethal test males 1981
dominant lethal NMRI-mice 1 x 1000 mg/kg ? negative Machemer,
test males 1977a
dominant lethal Ha(ICR)-mice 1 or 10 ppm in 94.59 negative Knickerbocker,
test males the diet for 1978
49 days
micronucleus CD-1 mice 2 x 500 mg/kg ? negative Tsuchimoto &
test males + females Matter, 1981
In vivo tests (contd)
Test system Test object Concentration/ Purity Results References
dose (%)
micronucleus NMRI-mice 1 x 10 000 mg/kg 96.9 negative Herbold, 1982
test males + females
sperm morphol. (CBA x BALB/c)F1 50-500 mg/kg/day ? negative Topham, 1980
assay 5 ip-injections
Tests were done: (*) = with and without metabolic activation (S9-mix)
(+) = with S9-mix activation
(-) = without S9-mix
DNA damage and repair
The possibility of inducing DNA damage by amitrole has been
investigated frequently and in a number of different ways. In
bacteria the results have been negative except for one recombinant
assay, which was positive when an exogenous metabolic activation was
provided by "liver" preparations from a mollusc and a fish. A DNA
repair assay in yeast gave a positive result as did a repair assay
in mammalian cells.
Cell transformation
Assays for cell transformation in several systems gave
predominantly positive results.
Other endpoints
Amitrole did not increase the frequency of morphologically
abnormal sperm in mice.
Amitrole has therefore been tested adequately in series of in
vitro and in vivo genotoxicity assays. Positive responses were
obtained in a number of mutation assays in bacteria,
recombinogenicity assays in yeast and some mammalian cell assays for
mutation, sister-chromatid exchange and cell transformation. No
genotoxicity was demonstrated in vivo. The Meeting concluded that
the genotoxic potential of amitrole was equivocal.
Special studies on skin and eye irritation and skin sensitization
In a limited study, the potential for dermal irritation by
amitrole (95%) was examined in 4 albino rabbits (strain and sex not
specified) over a 24-hour period following a single application of
dosages from 1000 up to 10 000 mg/kg bw. Minimal dermal irritation
was observed with mild erythema at the high-dose level only. By 48
hours, the skin appeared normal (Elsea, 1954).
In a limited study, the potential for eye irritation by
amitrole (95%) was examined in 3 albino rabbits (strain and sex not
specified) following application of 3 mg into the conjunctival sac
of the left eye. Observations were made at 1, 4 and 24 hours and at
daily intervals for 6 days. Mild irritation was observed at 4
hours, which subsided within 24 hours (Elsea, 1954).
In a Magnusson-Kligman maximization test in Pirbright White
guinea-pigs, amitrole (97.6%) was found to be a moderate skin
sensitizer. Concentrations employed were 2.5% for intracutaneous
induction, 25% for topical induction, and 12% for the first and
second challenges (Mihail, 1984).
In a Klecak open epicutaneous test in BOR:DHPW/SPF guinea-pigs,
amitrole (97.3%) did not have a sensitizing effect. Concentrations
employed were 0, 3, 10, or 30% for induction, and 1, 3, 10, or 30%
for the first and second challenges (Mihail, 1985).
Special studies on farm animals
Cattle tolerated administration of both a single oral dose of
amitrole at 1000 mg/kg bw as well as repeated 100 mg/kg bw doses on
10 consecutive days without symptoms (Stendel, 1965).
Female sheep (1/dose level) were orally treated with amitrole
at doses of 0, 750, or 1000 mg/kg bw at intervals of 5-7 days over a
period of 8 weeks. The only symptom observed was lethargy
immediately following treatment. Three days after the last dose the
animals were slaughtered. Residues were found in muscular tissues
at levels of 80 ± 30 ppm and 120 ± 40 ppm in the low- and high-dose
respectively (semi-quantitative determination). The thyroids were
grey-red discoloured. At histopathology complete cessation of
colloid formation, with adenomatoid proliferation of the follicle
epithelia was observed (Hapke et al., 1965).
In sheep, a single oral dose of 750 mg/kg bw led to clinical
symptoms (lethargic behaviour, frequent lying down). At higher
doses, the animals became apathetic and did not eat. At a dose
level of 4000 mg/kg bw the animals died. A single oral dose of 200
mg/kg bw was tolerated by a horse without any symptoms. Adult geese
tolerated oral doses up to 1000 mg/kg bw. At higher doses, these
birds became somnolent. Mortality occurred at 10 000 mg/kg bw
(Hapke, 1967).
Young cattle and sheep were orally exposed to amitrole at
dosages of 10, 25 or 50 mg/kg bw for up to 10 consecutive days. The
10 mg/kg bw dose was tolerated without symptoms by the cattle.
Doses of 25 or 50 mg/kg bw led to toxic symptoms after 3 and 2 days,
respectively. One out of three sheep exhibited retarded body-weight
development at 10 mg/kg bw, whereas at the higher doses all sheep
showed toxic symptoms. Hens were treated orally at dosages of 50,
100 or 250 mg/kg bw on 10 consecutive days. Retarded body-weight
development was observed at 100 and 250 mg/kg bw. Hens dosed at 375
or 500 mg/kg bw died within 3-7 days after the start of the study
(Palmer, 1972).
Observations in humans
In a patch test conducted with a human volunteer, amitrole
exerted no primary dermal irritant effect after exposure periods of
4 or 8 hours. A slight irritant effect was observed in 3 out of 6
subjects after 24 hours of exposure (no further details) (Hecht,
1954).
A case study of a 41-year old weed control operator with a 6-
month history of dermatitis involving his face, hands, back, thighs,
and feet was reported. Patch testing with 1% amitrole showed a
strong positive vesicular reaction at 2 and 4 days, indicative of
allergic contact dermatitis (English et al., 1986).
The intentional ingestion of a commercial mixture of amitrole
and diuron, at a dose equivalent to 20 mg/kg bw amitrole (together
with about 38 mg/kg bw diuron), was reported to have caused no
symptoms of poisoning in a female subject. Within a few hours, the
compound appeared in the urine at a concentration of 100 mg/100 ml.
Metabolites could not be detected in urine (Geldmacher-von
Mallinckrodt & Schmidt, 1970).
Astwood (1960) reported in a brief communication that a single
oral dose of 100 mg amitrole inhibited radioiodine uptake by the
thyroid of both normal and thyrotoxic subjects for 24 hours.
However, a dose of 10 mg was said to have had a slight effect on
iodine uptake. Astwood suggested that amitrole could be used
therapeutically in the treatment of hyperthyroidism.
An epidemiological study was conducted on Swedish railway
workers exposed to various herbicides. A small increase in tumours
was observed, particularly of the lung. Amitrole was only one of
the pesticides (other pesticides were for example phenoxy acids) to
which these workers were exposed (Axelson, 1980).
Mild cases of dermatitis on the face due to contact with
amitrole occurred yearly in one or two production workers of the
American Cyanamid Company. The dermatitis was of a primary irritant
type rather than the sensitive type. No other medical findings, not
occurring in the general public, and, in particular, no thyroid or
liver tumours were observed during the years of observation
(1955-1970) (Clyne, 1970).
Skin irritation was also occasionally observed in employees of
Bayer engaged in production of amitrole. No other adverse effects
occurred (Miksche, 1982).
The thyroid function in 5 employees who had been engaged in
production and packaging of amitrole for periods between 3 and 16
years was checked as part of the occupational medical programme.
Scintigraphic thyroid imaging findings and determinations of the T3
and T4 levels afforded no evidence for the presence of an effect on
thyroid function (Miksche, 1983).
In a study for a possible phototoxic potential of amitrole, 20
test subjects were exposed to patches containing amitrole at a level
of 1% in an ointment base (hydrous eucerin) over a period of 48
hours. The treatment areas were then irradiated with ultraviolet
light (UVA and UVB), and the dermal reactions assessed (no further
details). No evidence was found for phototoxic skin reactions
(Tronnier, 1983).
General mode of action
Amitrole is considered a goitrogenic compound. The mechanisms
by which goitrogens produce their pharmacologic and potential
neoplastic effects is through the induction of hormonal imbalance.
Most commonly this is the result of interference with the thyroid
iodide transport system or interference with peroxidases essential
to the synthesis and secretion of competent thyroid hormone. The
sequence of events triggered by this interference is generally
understood. Because of the ability of goitrogens to inhibit thyroid
hormone synthesis, these compounds have the potential to reduce
circulating levels of T3 and T4 and, consequently, to induce the
secretion of TSH by the pituitary. As a result, prolonged exposure
to such compounds can be expected to induce thyroid gland
hypertrophy and hyperplasia, nodular hyperplasia and, ultimately,
may lead to neoplasia. Evidence indicates that if the pharmacologic
effects, thyroid hypertrophy and hyperplasia, are prevented or
controlled within the feedback system, follicular cell neoplasia
does not develop. The data also suggest that some degree of
hypertrophy or hyperplasia of the thyroid gland is tolerated within
the oscillation of the feedback system without induction of
neoplasia. The rat, and to a lesser extent the mouse, appear to be
very sensitive to goitrogens, since after short exposure periods
their thyroid glands exhibit hypertrophic and hyperplastic changes.
Following continuous, long-term exposure of rats and mice to these
agents, both the thyroid and the pituitary glands frequently exhibit
neoplastic changes. In contrast, humans appear to be less sensitive
(Paynter et al., 1988; Hill et al., 1989).
COMMENTS
Amitrole is rapidly and almost completely absorbed from the
gastrointestinal tract following oral administration to rats and
mice. It is rapidly distributed throughout most body tissues, but
with a slight accumulation in tissues with a rapid cell turnover
(bone marrow, spleen, thymus, gastrointestinal tract). In a study
with pregnant mice it was observed that amitrole passes through the
placenta into the fetuses with the same distribution pattern as in
the mothers. Excretion is rapid after oral exposure. Within 24
hours, 70-95% of the administered radioactivity is excreted via the
urine, mainly as the parent compound.
The metabolic transformation in mammals produces two minor
metabolites detectable in the urine. Metabolism of amitrole occurs
mainly in the liver and involves substitution of the hydrogen atom
in the 5-position. The metabolites identified were 3-amino-5-
mercapto-1,2,4-triazole and 3-amino-1,2,4-triazolyl-5-mercapturic
acid.
Amitrole has a low acute toxicity when tested in several
species by various routes of administration. In old studies,
amitrole was reported to have slight irritating effects on the skin
and eyes. Evidence of a moderate sensitizing potential was observed
in a Magnusson-Kligman test but not in a Klecak open epicutaneous
test. WHO has classified amitrole as unlikely to present acute
hazard in normal use.
Oral exposures of up to four weeks in rats revealed that
effects on the thyroid occurred at levels > 60 ppm in the diet or
104 ppm in drinking-water. No effects were observed at 30 ppm in
the diet (equivalent to 3 mg/kg bw/day) or 10 ppm in drinking-water
(equivalent to 1.5 mg/kg bw/day). Furthermore, it was shown that
after a recovery period the effects on the thyroid were reversible.
In a 30-day study in mice at concentrations in drinking-water
of 0, 5000, 10 000 or 20 000 mg/l, liver effects were observed at
all dose levels.
Several short-term oral studies were performed with rats.
These were mainly focused on the effects on the thyroid, as this is
the target organ in rats.
Only two oral studies were suitable for assessment. In one
study, male rats were exposed to dietary concentrations of 0, 2, 10
or 50 ppm for 13 weeks or to 0, 0.25 or 0.50 ppm for 11 weeks. The
NOAEL was 2 ppm (equivalent to 0.1 mg/kg bw/day) based on
histological changes in the thyroid (appearance of follicular cells,
contents of colloid and capillary density). Decreases in protein-
bound iodine were not considered to be biologically significant.
The other study consisted of four short-term experiments in
female rats. Dietary concentrations in one experiment were 0, 2, 20
or 200 ppm with exposure for six weeks, in two subsequent
experiments 0, 20, 50 or 200 ppm with exposure for 6 or 13 weeks and
in the fourth experiment 0, 20, 50, 200 or 500 ppm with exposure for
six weeks. From these four experiments, the overall NOAEL was 2 ppm
(equivalent to 0.1 mg/kg bw/day) based on increased iodine uptake
(shortly after injection), increased thyroid weight and
histopathological changes of the thyroid (goitre and clearly
activated thyroids).
From several short-term studies in rats with administration in
drinking-water, slight effects on the thyroid (moderate stimulation
of the thyroid epithelium) were seen at the lowest concentration
tested, 50 ppm.
In a one-year study in dogs, no effects on the thyroid were
observed at the highest dose tested (12.5 mg/kg bw/day). The only
effect observed at this level was pale-coloured pancreas. The test,
however, was performed with a low number of animals.
Long-term toxicity and/or carcinogenicity studies have been
performed in mice, rats, and golden hamsters. Studies in mice were
focused on induction of liver and thyroid tumours. In a
carcinogenicity study in mice with only one but very high dose level
(1000 mg/kg bw/day by gavage), survival time was significantly
reduced and liver and thyroid tumours were observed in all treated
mice. A slight increase in the incidence of liver tumours was
observed in a special carcinogenicity study in which pups were
treated for a period of 90 weeks at a level of 500 ppm in the diet.
In an 18-month carcinogenicity study in mice at levels of 0, 1,
10 or 100 ppm in the diet, an increased incidence of tumours was not
observed. In this study, a thyroid function test was also performed
with a small number of animals. At 100 ppm an increase in thyroid
weight and in iodine accumulation in the thyroid was observed. The
NOAEL was 10 ppm (equivalent to 1.5 mg/kg bw/day).
In a carcinogenicity study in rats with levels of 0, 1, 10 or
100 ppm in the diet, a slight decrease in survival time, an increase
in the incidence of thyroid tumours and an increase in the incidence
of (mainly benign) pituitary tumours were observed at 100 ppm. In
this study, a thyroid function test was also performed with a small
number of animals. At 100 ppm, thyroid weight was increased during
the whole study period as was the percentage accumulation of
radioiodine in the thyroid. The NOAEL was 10 ppm (equivalent to 0.5
mg/kg bw/day).
In another limited long-term toxicity study in rats, the NOAEL
was 10 ppm (equivalent to 0.5 mg/kg bw/day) based on thyroid
hyperplasia. A clearly enhanced thyroid tumour incidence was found
at 50 and 100 ppm. In this study, animals suffered from apparent
respiratory infection.
In a third study in rats, thyroid hyperplasia and thyroid
tumours were observed in animals fed 100 ppm (during the first 40
weeks of the 115-120 week study, the dose level was 5 ppm). In rats
treated at pulsed intervals (alternate four week periods) at levels
of 60 ppm (first 3 ppm) and 200 ppm (first 10 ppm) thyroid tumours
were also observed. Slight thyroid hyperplasia was also observed at
the lowest dose level of 20 ppm (first 1 ppm; intermittent dosing
regimen). A NOAEL could not be established.
In an 18-month carcinogenicity study in Syrian hamsters at
dietary concentrations of 0, 1, 10 or 100 ppm, the NOAEL was 10 ppm,
equivalent to 1 mg/kg bw/day, based on decreased body-weight gain
and increased mortality. No effects on the thyroid were observed at
100 ppm. There was no evidence of carcinogenic potential.
A well-performed reproduction study was not available. From a
limited study in rats at dietary concentrations ranging from 25 to
1000 ppm, effects on reproductive capability were observed at 500
ppm and above. Reduction of liver weight and thyroid hyperplasia
were the most sensitive effects observed at the lowest dose level
(25 ppm, equivalent to 1.3 mg/kg bw/day).
In a teratogenicity study, rats were exposed by gavage at doses
of 0, 100, 300 or 1000 mg/kg bw/day on days 6 to 15 of gestation.
No effects were observed in this study. The NOAEL for
maternotoxicity and embryo/ fetotoxicity was 1000 mg/kg bw/day.
In another teratogenicity study, rats were exposed by gavage at
doses of 0, 100, 500 or 1000 mg/kg bw/day. Slight maternal toxicity
(reduced weight gain and food consumption and increased thyroid
weights) was observed at doses of 500 and 1000 mg/kg bw/day.
Reduced fetal body weight/litter and reduced skeletal ossifications
were observed in the high-dose group. Increased incidences of
enlarged and/or dark thyroids were seen in fetuses at 500 and 1000
mg/kg bw/day. The NOAEL for maternotoxicity and embryo/fetotoxicity
was 100 mg/kg bw/day. Amitrole was considered not to be teratogenic
in rats at dose levels up to 1000 mg/kg bw/day.
In a teratogenicity study in rabbits the animals were exposed
by gavage to dose levels of 0, 4, 40 or 400 mg/kg bw/day. Decreased
weight gain during the gestation period was observed at 40 and 400
mg/kg bw/day and increased liver weight at 400 mg/kg bw/day. A
dose-related increased incidence of abortions was observed in all
treated groups. Embryo/fetotoxicity were observed at 40 and 400
mg/kg bw/day. Increased incidences of irreversible structural
changes were also found at these dose levels, which involved mainly
the head and limbs. The NOAEL for maternotoxicity,
embryo/fetotoxicity and teratogenicity was 4 mg/kg bw/day.
In a dermal teratogenicity study in rabbits at dose levels of
0, 1000, 1500 or 2000 mg/kg bw/day, maternotoxicity (decreased body
weight and food consumption, thin appearance and anorexia) was
observed at 2000 mg/kg bw/day. At this level, irreversible
structural changes (anencephaly and microphthalmia) were observed.
The NOAEL for maternotoxicity, embryo/fetotoxicity and
teratogenicity after dermal exposure was 1500 mg/kg bw/day.
Amitrole has been tested adequately in series of in vitro and
in vivo genotoxicity assays. Positive responses were obtained in
a number of mutation assays in bacteria, recombinogenicity assays in
yeast and some mammalian cell assays for mutation, sister-chromatid
exchange and cell transformation. No genotoxicity was demonstrated
in vivo. The Meeting concluded that the genotoxic potential of
amitrole was equivocal.
Amitrole is a goitrogen in mice, rats and sheep but not in
Syrian hamsters, dogs, or cattle at the doses that have been tested.
The mechanism of thyroid toxicity involves inhibition of thyroid
peroxidase. This inhibition results in decreases in circulating
levels of T4 and T3 which stimulate the pituitary to increase
secretion of TSH which in turn may cause thyroid hypertrophy,
hyperplasia and neoplasia. Threshold doses have been identified in
the sensitive species. Amitrole is not genotoxic in in vivo
assays.
The Meeting withdrew the conditional ADI and established a
temporary ADI, based on the NOAEL of 0.5 mg/kg bw/day in the 24-
month dietary study in rats, using a safety factor of 1000 because
of inadequacy of the data.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Mouse: 10 ppm, equivalent to 1.5 mg/kg bw/day
(18-month study)
Rat: 10 ppm, equivalent to 0.5 mg/kg bw/day
(24-month study)
100 mg/kg bw/day (teratogenicity study)
Hamster: 10 ppm, equivalent to 1.0 mg/kg bw/day
(18-month study)
Dog 12.5 mg/kg bw/day (12 month study)
Estimate of temporary acceptable daily intake for humans
0-0.0005 mg/kg bw
Studies without which the determination of a full ADI is
impracticable
Results be submitted to WHO by 1996
(all known to have been initiated):
Two-generation reproduction study in rats
One-year study in dogs
Oral teratogenicity study in rabbits
Metabolism study in rats
Studies which will provide information valuable in the continued
evaluation of the compound
Further observations in humans.
Comparative biotransformation (including humans).
Clarification of genotoxic potential of amitrole.
REFERENCES
Alexander, N.M. (1959). Antithyroid action of 3-amino-1,2,4-
triazole. J. Biol. Chem. 234: 148-150.
Astwood, E.B. (1960). Cranberries, turnips and goiter. J. American
Med. Assoc. 172: 1319-1320.
Axelson, O., Sundell, L., Andersson, K., Edling, C., Hogstedt, C.,
Kling, H. (1980). Herbicide exposure and tumor mortality. An updated
epidemiologic investigation on Swedish railroad workers. Scan. J.
Work Environ. Health, 6: 73-79.
Babish, J.G. (1977). Triiodothyronine (T3) and Thyroxine (T4)
levels in male rats consuming Amitrole (3-amino-1,2,4-triazole) in
their diets for four weeks followed by a four week recovery period.
Unpublished report No 5539 (dated: 29-11-1977) from Food and Drug
Research Laboratories, Inc.. Submitted to WHO by Bayer AG.
Bagdon, R.E., Shaffer, C.B., Vidone, L.B., Golz, H.H. (1956).
Aminotriazole - acute and subacute toxicity. Unpublished report No
56-29 (dated: 14-12-1956) from American Cyanamid Company. Submitted
to WHO by Bayer AG.
Bamford, D., Sorsa, M., Gripenberg, U., Laamanen, I., Meretoja, T.
(1976). Mutagenicity and toxicity of amitrole. III. Microbial tests.
Mutation Research, 40: 197-202.
Becci, P. (1983). Evaluation of the chronic inhalation toxicity and
carcinogenicity of amitrole in rats. Unpublished report No 5821
(dated: 13-01-1983) from Food and Drug Research Laboratories, Inc..
Submitted to WHO by Bayer AG.
Ben-Dyke, R., Perry, M.C., Wall, I. (1973). The short-term
cumulative toxicity of aminotriazole in the rat. Unpublished report
Tox/73/39-6 (dated: September 1973) from Fisons Limited Agrochemical
division, Toxicology department. Submitted to WHO by Bayer AG.
Bignami, M., Aulicino, F., Velcich, A., Carere, A., Morpurgo, G.
(1977). Mutagenic and recombinogenic action of pesticides in
Aspergillus nidulans. Mutation Research, 46: 395-402.
Bionetics Research Laboratories (1968). Evaluation of carcinogenic,
teratogenic, and mutagenic activities of selected pesticides and
industrial chemicals. Volume II: Teratogenic study in mice and rats.
Report PB-223 160 (dated: August 1968) from Bionetic Research Labs,
Inc., prepared for National Cancer Institute.
Braun, R., Schöneich, J., Ziebarth, D. (1977). In vivo formation
of N-nitroso compounds and detection of their mutagenic activity in
the host-mediated assay. Cancer Research, 37: 4572-4579.
Brooks, T.M. & Dean, B.J. (1981). Mutagenic activity of 42 coded
compounds in the salmonella/microsome assay with preincubation.
Progress in Mutation Research, 1: 261-270.
Brusick, D.J. (1975). Mutagenic evaluation of compound 3-amino-
1,2,4-triazole. Unpublished report No 2547 (dated: 14-08-1975) from
Litton Bionetics, Inc. Submitted to WHO by Bayer AG.
Brusick, D.J. (1976). Mutagenicity evaluation of 3-amino-1,2,4-
triazole; in vitro cellular transformation in BALB/3T3 cells.
Unpublished report No 2549 (dated: 26-10-1976) from Litton
Bionetics, Inc. Submitted to WHO by Bayer AG.
Clyne, R.M. (1970). Statement before the amitrole advisory
committee. Unpublished document from the American Cyanamid Company
dd 16 December 1970. Submitted to WHO by Bayer AG.
Cox, G.E. & Re, T.A. (1978). Inhalation toxicity study with 3-amino-
1,2,4-triazole (Amitrole) in adult Fisher 344 rats. Unpublished
report No 5492 (dated: 08-09-1978) from Food and Drug Research
Laboratories, Inc., Submitted to WHO by Bayer AG.
Daniel, M.R. & Dehnel, J.M. (1981). Cell transformation test with
baby hamster kidney cells. Progress in Mutation Research, 1: 626-
637.
Den Tonkelaar, E.M. & Kroes, R. (1974). Schildklierfunctieonderzoek
na subacute en semichronische toediening van aminotriazol.
Unpublished report No. 64/74 Tox. from the National Institute of
Public Health, Bilthoven, the Netherlands.
Dunkel, V.C., Pienta, R.J., Sivak, A., Traul, K.A. (1981).
Comparative neoplastic transformation responses of Balb/3T3 cells,
Syrian hamster embryo cells, and Rauscher murine leukemia virus-
infected Fischer 344 rat embryo cells to chemical compounds. J.
Nat. Cancer Inst. 67: 1303-1315.
Dunkel, V.C., Zeiger, E., Brusick, D., McCoy, E., McGregor, D.,
Mortelmans, K., Rosenkranz, H.S., Simmon, V.F. (1984).
Reproducibility of microbial mutagenicity assays: I. Tests with
Salmonella typhimurium and Escherichia coli using a standardized
protocol. Environmental Mutagenesis, 6: 1-25.
Dunkel, V.C., Schechtman L.M., Tu, A.S., Sivak, A., Lubet, R.A.,
Cameron, T.P. (1988). Interlaboratory evaluation of the C3H/10T1/2
cell transformation assay. Env. Mol. Mutagen. 12: 21-31.
Elsea, J.R. (1954). Amino Triazole. Acute dermal application - acute
eye application. Unpublished report from Hazleton Laboratories,
Virginia (dated: 23-12-1954). Submitted to WHO by Bayer AG.
English, J.S., Rycroft, R.J., Calnan, C.D. (1986). Allergic contact
dermatitis from aminotriazole. Contact Dermatitis, 14: 255-256.
Fang, S.C., George, M., Yu, T.C. (1964). Metabolism of 3-amino-
1,2,4-triazole-5-C14 by rats. J. Agric. Food Chem. 12: 219-223.
Fang, S.C., Khanna, S., Rao, A.V. (1966). Further study on the
metabolism of labelled 3-amino-1,2,4-triazole (ATA) and its plant
metabolites in rats. J. Agric. Food Chem. 14: 262-265.
Feinstein, R.N., Fry, R.J.M, Staffeldt, E.F. (1978). Carcinogenic
and Antitumor effects of aminotriazole on acatalasemic and normal
catalase mice. J. Natl. Cancer Inst. 60: 1113-1116.
Fogleman, R.W. (1954). Amizole: Acute administration -
pharmacodynamics - subacute feeding. Unpublished report from
Hazleton Laboratories, Virginia (dated: 02-12-1954). Submitted to
WHO by Bayer AG.
Franco, L., Municio, A.M. (1975). Comparative metabolism of 3-amino-
1,2,4-triazole. Gen. Pharmac. 6: 163-169.
Fregly, M.J. (1968). Effect of aminotriazole and thyroid function in
the rat. Toxicol. Appl. Pharmacol. 13: 271-286.
Fujii, T., Miyazaki, H., Hashimoto, M. (1984). Autoradiographic and
biochemical studies of drug distribution in the liver. III. (14C)
Aminotriazole. Eur. J. Drug Metab. Pharmacokinet. 9: 257-265.
Gaines, T.B., Kimbrough, R.D., Linder, R.E. (1973). The toxicity of
amitrole in the rat. Toxicol. Appl. Pharmacol. 26: 118-129.
Gatehouse, D. (1981). Mutagenic activity of 42 coded compounds in
the microtiter fluctuation assay. Progress in Mutation Research,
1: 376-386.
Geldmacher-von Mallinckrodt, M. & Schmidt, H.P. (1970). Toxicity and
metabolism of aminotriazole in man. Arch. Toxikol. 27: 13-18.
Grunow, W., Altmann, H.-J., Böhme, Chr. (1975). Metabolism of 3-
amino-1,2,4-triazole in rats. Arch. Toxicol. 34: 315-324.
Hapke, H.-J., Rüssel, H., Ueberschär, S. (1965). Dangers in the use
of aminotriazol. Dtsch. Tierärztl. Wochenschrift, 72: 204-206.
Hapke, H.-J. (1967). The toxicity of aminotriazole for domestic
animals. Zbl. Vet. Med. 14: 469-486.
Hayes, S., Gordon, A., Sadowski, I., Hayes, C. (1984). RK bacterial
test for independently measuring chemical toxicity and mutagenicity:
Short-term forward selection assay. Mutation Research, 130: 97-
106.
Hecht (1954). Aminotriazol - Letter report of May 11, 1954 from
Bayer AG Institute of Toxicology. Submitted to WHO by Bayer AG.
Hecht & Kimmerle (1962). Aminotriazol - Letter report of August 23,
1962 from Bayer AG Institute of Toxicology. Submitted to WHO by
Bayer AG.
Heimann, K.G. (1982). Aminotriazol techn. - Letter report of April
7, 1982 from Bayer AG Institute of Toxicology. Submitted to WHO by
Bayer AG.
Henwood, S.M. (1988). Percutaneous teratology study with amitrole
technical in rabbits. Unpublished report on number HLA 6224-107
from Hazelton Laboratories America, Inc., submitted to WHO by Rhône-
Poulenc Ag. Company
Herbold, B. (1980). Amitrole; Salmonella/microsome test for
detection of point-mutagenic effects. Unpublished report No 9339
(dated: 01-08-1980) from Bayer AG Institute of Toxicology. Submitted
to WHO by Bayer AG.
Herbold, B. (1982). Amitrole; Micronucleus test on the mouse to
evaluate for mutagenic effect. Unpublished report No 11139 (dated:
13-09-1982) from Bayer AG Institute of Toxicology. Submitted to WHO
by Bayer AG.
Hill, R.N., Erdreich, L.S., Paynter, O.E., Roberts, P.A., Rosenthal,
S.L., Wilkinson, C.F. (1989). Thyroid follicular cell
carcinogenesis. Fund. Appl. Toxicol. 12: 629-697.
Hubbard, S.A., Green, M.H.L., Bridges, B.A., Wain, A.J., Bridges,
J.W. (1981). Fluctuation test with S9 and hepatocyte activation.
Progress in Mutation Research, 1: 361-370.
Ichinotsubo, D., Mower, H., Mandel, M. (1981). Testing of a series
of paired compounds (carcinogen and non-carcinogenic structural
analog) by DNA repair-deficient E. coli strains. Progress in
Mutation Research, 1: 195-198.
Innes, J.R., Ulland, B.M., Valerio, M.G., Petrucelli, L., Fishbein,
L., Hart, E.R., Palotta, A.J. (1969). Bioassay of pesticides and
industrial chemicals for tumorigenicity in mice: A preliminary note.
J. Nat. Cancer Inst. 42: 1101-1114.
Inoue, K., Katoh, I., Takayama, S. (1981). In vitro transformation
of hamster embryo cells by 3-(N-salicycloyl)amino-1,2,4-triazole.
Toxicol. Letters, 7: 211-215.
Johnson, W.D. (1981). Lifetime feeding study of amitrole in Fischer
344 rats. Unpublished report No 5651 (dated: 07-08-1981) from Food
and Drug Research Laboratories, Inc. Submitted to WHO by Bayer AG.
Jukes, T.H & Shafer, C.B. (1960). Antithyroid effects of
aminotriazole. Science, 132: 296-297.
Kada, T. (1981). The DNA-damaging activity of 42 coded compounds in
the rec-assay. Progress in Mutation Research, 1: 171-182.
Kassinova, G.V., Kovaltzova, S.V., Marfin, S.V., Zakharov, I.A.
(1981). Activity of 40 coded compounds in differential inhibition
and mitotic crossing-over assays in yeast. Progress in Mutation
Research, 1: 434-455.
Keller, J. (1959). Aminotriazole: Two-year chronic feeding - rats.
Unpublished report from Hazleton Laboratories Virginia (dated: 02-
01-1959). Submitted to WHO by Bayer AG.
Kimmerle (1968). Aminotriazol - Letter report of June 12, 1968 from
Bayer AG Institute of Toxicology. Submitted to WHO by Bayer AG.
Knickerbocker, M. (1978). A study to determine the potential of
amitrole to induce dominant lethal mutations in Ha (ICR) mice.
Unpublished report no 5502 (dated: 28-02-1978) from Food and Drug
Research Laboratories, Inc. Submitted to WHO by Bayer AG.
Laamanen, I., Sorsa, M., Bamford, D., Gripenberg, U. Meretoja, T.
(1976). Mutagenicity and toxicity of amitrole. I. Drosophila
tests. Mutation Research, 40: 185-190.
Lemoine, N.R., Mayall, E.S., Williams, E.D., Thurston, V., Wynford-
Thomas, D. (1988). Agent-specific ras oncogene activation in rat
thyroid tumours. Oncogene, 3: 541-544.
MacDonald, C.M., Pullinger, D.H. (1976). A pharmacokinetic study to
compare "head only" and "whole body" inhalation methods of 14C-
amitrole exposure. Unpublished report No 291/1 (dated: March 1976)
from Hazleton Laboratories Europe Ltd. Submitted to WHO by Bayer AG.
Machemer, L. (1977a). Amitrole/Dominant lethal test on the male
mouse to evaluate for mutagenic effects. Unpublished report no 6679
(dated: 23-03-1977) from Bayer AG, Institute of Toxicology.
Submitted to WHO by Bayer AG.
Machemer, L. (1977b). Amitrole; Study for embryotoxic and
teratogenic effects after oral administration to the rat.
Unpublished report No 6634 (dated: 24-02-77) from Bayer AG Institute
of Toxicology. Submitted to WHO by Bayer AG.
Mamber, S.W., Bryson, V., Katz, S.E. (1983). The Escherichia coli
WP2/WP100 rec assay for detection of potential chemical carcinogens.
Mutation Research, 119: 135-144.
Mamber, S.W., Bryson, V., Katz, S.E. (1984). Evaluation of the
Escherichia coli K12 inductest for detection of potential chemical
carcinogens. Mutation Research, 130: 141-151.
Martin, C.N. & McDermid, A.C. (1981). Testing of 42 coded compounds
for their ability to induce unscheduled DNA repair synthesis in HeLa
cells. Progress in Mutation Research, 1: 533-537.
McDonald, D.J. (1981). Salmonella/microsome tests on 42 coded
chemicals. Progress in Mutation Research, 1: 285-297.
McGregor, D.B., Martin, R., Cattanach, P., Edwards, I., McBride, D.,
Caspary, W.J. (1987). Response of the L5178Y Tk+/Tk-mouse lymphoma
cell forward mutation assay to coded chemicals. I. Results for nine
compounds. Env. Mutagen. 9: 143-160.
Mehta, R.D., von Borstel, R.C. (1981). Mutagenic activity of 42
coded compounds in the haploid yeast reversion assay, strain XV185-
14C. Progress in Mutation Research, 1: 414-423.
Meretoja, T., Gripenberg, U., Bamford, D., Laamanen, I., Sorsa, S.
(1976). Mutagenicity and toxicity of amitrole, II. Human lymphocyte
culture tests. Mutation Research, 40: 191-196.
Mihail, F. (1984). Aminotriazole - Studies for skin sensitization
effect on guinea pigs. Unpublished report No 12607 (dated 12-04-
1984) from Bayer AG Institute of Toxicology. Submitted to WHO by
Bayer AG.
Mihail, F. (1985). Aminotriazole - Study for skin-sensitising effect
on guinea pigs in the open epicutaneous test. Unpublished report No
13775 (dated: 27-08-1985) from Bayer AG Institute of Toxicology.
Submitted to WHO by Bayer AG.
Mihail, F. & Schilde, B. (1984). Aminotriazole - Study for subacute
dermal toxicity to rabbits Unpublished report No 12369 (dated: 09-
01-1984) from Bayer AG Institute of Toxicology. Submitted to WHO by
Bayer AG.
Miksche, L.W. (1982). Letter report of 22-06-82 - BBA request-
effects on man. Unpublished document from the Bayer AG, LE Medical
Department Leverkusen. Submitted to WHO by Bayer AG.
Miksche, L.W. (1983). Letter report of 22-04-83 - Production of
amitrole-medical monitoring. Unpublished document from the Bayer AG,
LE Medical Department Leverkusen. Submitted to WHO by Bayer AG.
Mori, S., Takeuchi, Y., Toyama, M., Makino, S., Ohhara, T., Tochino,
Y., Hayashi, Y. (1985). Amitrole: Strain differences in
morphological response of the liver following subchronic
administration to mice. Toxicology Letters, 29: 145-152.
Moriya, M., Ohta, T., Watanabe, K., Miyazawa, T., Kato, K., Shirasu,
Y. (1983). Further mutagenicity studies on pesticides in bacterial
reversion assay systems. Mutation Research, 116: 185-216.
Morpurgo, G., Bellincampi, D., Gualandi, G., Baldinelli, L., Serlupi
Crescenzi, O. (1979). Analysis of mitotic nondisjunction with
Aspergillus nidulans. Env. Health Perspect. 31: 81-95.
Napalkov, N.P. (1962). Blastomogenic action of 3-amino-1,2,4-
triazole. (Russ). Gig. Tr. prof. Zabol. 6: 48-51. In: Amitrole; IARC
Monographs on the evaluation of carcinogenic risk of chemicals to
humans (1986). World Health Organization, Vol. 41: 293-317, Lyon.
Palmer, J.S. (1972). Toxicity of 45 organic herbicides to cattle
sheep, and chickens. Production Research report no 137 (dated: March
1972) from US Department of Agriculture. Submitted to WHO by Bayer
AG.
Paynter O.E., Burin, G.J., Jaeger, R.B., Gregorio C.A. (1988).
Goitrogens and thyroid follicular cell neoplasia: evidence for a
threshold process. Regul. Toxicol. Pharmacol. 8: 102-119.
Perry, P.E. & Thomson, E.J. (1981). Evaluation of sister chromatid
exchange method in mammalian cells as a screening system for
carcinogens. Progress in Mutation Research, 1: 560-569.
Prince, H.N. (1977). Microbial mutagen assays with amitrole (3-
amino-1,2,4-triazole). Unpublished report no 5634 (dated: 12-09-
1977) from Gibraltar Biological Laboratories (submitted via Food and
Drug Research Laboratories, Inc.). Submitted to WHO by Bayer AG.
Reitze, H.K. & Seitz, K.A. (1985). Light and electron microscopical
changes in the liver of mice following treatment with aminotriazole.
Exp. Pathol. 27: 17-31.
Richold, M. & Jones, E. (1981). Mutagenic activity of 42 coded
compounds in the Salmonella/microsome assay. Progress in Mutation
Research, 1: 314-322.
Rosenkranz, H.S., Hyman, J., Leifer, Z. (1981). DNA polymerase
deficient assay. Progress in Mutation Research, 1: 210-218.
Rowland, I. & Severn, B. (1981). Mutagenic of carcinogens and
noncarcinogens in the salmonella/micrososme test. Progress in
Mutation Research, 1: 323-332.
Seidenberg, I.L. & Gee, A.H. (1953). Report on acute toxicity of
aminotriazole as established by oral administration to rats.
Unpublished report from Foster D. Snell Inc. (dated: 08-06-1953).
Submitted to WHO by Bayer AG.
Shah, P.V. & Guthrie, F.E. (1977). Dermal absorption, distribution,
and the fate of six pesticides in the rabbit. In: Pesticide
Management and Insecticide Resistance. pp 547-554. Academic Press.
Sharp, D.C. & Parry, J.M. (1981a). Induction of mitotic gene
conversion by 41 coded compounds using the yeast culture JD1.
Progress in Mutation Research, 1: 491-501.
Sharp, D.C. & Parry, J.M. (1981b). Use of repair deficient strains
of yeast to assay the activity of 40 coded compounds. Progress in
Mutation Research, 1: 502-516.
Shirasu, Y., Moriya, M., kato, K., Furuhashi, A., Kada, T. (1976).
Mutagenicity screening of pesticides in the microbial system.
Mutation Research, 40: 19-30.
Simmon, V.H., Rosenkranz, H.S., Zeiger, E., Poirer, L.A. (1979).
Mutagenic activity of chemical carcinogens and related compounds in
the intraperitoneal host-mediated assay. J. Natl. Cancer Inst. 62:
911-918.
Steinhoff, D. & Boehme, K. (1978). Aminotriazole; carcinogenesis
study with oral administration to Syrian golden hamsters.
Unpublished report No 7521 (dated: 16-05-1978) from Bayer AG,
Institute of Toxicology. Submitted to WHO by Bayer AG.
Steinhoff, D. & Boehme, K. (1979a). Aminotriazole; carcinogenesis
study with oral administration to rats. Unpublished report No 8450
(dated: 11-06-1979) from Bayer AG, Institute of Toxicology.
Submitted to WHO by Bayer AG.
Steinhoff, D. & Boehme, K. (1979b). Aminotriazole; carcinogenesis
study with oral administration to mice. Unpublished report No 8490
(dated: 17-07-1979) from Bayer AG, Institute of Toxicology.
Submitted to WHO by Bayer AG.
Steinhoff, D., Weber, H., Mohr, U., Boehme, K. (1983). Evaluation of
Amitrole (Aminotriazole) for potential carcinogenicity in orally
dosed rats, mice, and golden hamsters. Toxicol. Appl. Pharmacol.
69: 161-169.
Stendel (1965). Aminotriazole/oral toxicity to cattle. Unpublished
letter report of March 15, 1965 from Bayer AG, Institute of
Toxicology. Submitted to WHO by Bayer AG.
Strum, J.M. & Karnovsky, M.B. (1971). Aminotriazole goiter. Fine
structure and localization of thyroid peroxidase activity.
Laboratory Investigation, 24: 1-12.
Styles, J.A. (1979). Studies on the detection of carcinogens using a
mammalian cell transformation assay with liver homogenate
activation. In: Norpoth, K. & Garner, R.C. (eds), Short-term
mutagenicity test systems for detecting carcinogens. Proceedings of
the International Symposium on short-term mutagenicity test systems.
Dortmund, Nov. 15-17, 1978 pp 226-238. Springer Verlag, New York.
Styles, J.A. (1981). Activity of 42 coded compounds in the BHK-21
cell transformation test. Progress in Mutation Research, 1: 638-
646.
Thomson, J.A. (1981). Mutagenic activity of 42 coded compounds in
the lambda induction assay. Progress in Mutation Research, 1: 224-
235.
Thyssen, J. (1974a). Aminotriazol Batch no 143 - Determination of
acute toxicity. Letter report of June 10, 1974 from Bayer AG,
Institute of Toxicology. Submitted to WHO by Bayer AG.
Thyssen, J. (1974b). Aminotriazol - Determination of acute toxicity.
Letter report of September 26, 1974 from Bayer AG, Institute of
Toxicology. Submitted to WHO by Bayer AG.
Thyssen, J. (1983). Aminotriazole - Acute inhalation toxicity.
Unpublished report No 11707 (dated: 11-04-1983) from Bayer AG,
Institute of Toxicology. Submitted to WHO by Bayer AG.
Tjälve, H. (1974). Fetal uptake and embryogenetic effects of
aminotriazole in mice. Arch. Toxicol. 33: 41-48.
Tjälve, H. (1975). The distribution of labelled aminotriazole in
mice. Toxicology, 3: 49-67.
Topham, J.C. (1980). Do induced sperm-head abnormalities in mice
specifically identify mammalian mutagens rather than carcinogens?
Mutation Research, 74: 379-387.
Tronnier, H. (1983). Aminotriazole - Expertise by consultant
dermatologist on testing for phototoxicity in test subjects.
Unpublished report No R 2544 (dated: 05-08-1983) from City Clinics
Dortmund. Submitted to WHO by Bayer AG
Trueman, R.W. (1981). Activity of 42 coded compounds in the
Salmonella reverse mutation test. Progress in Mutation Research,
1: 343-350.
Tsuchimoto, T. & Matter, B.E. (1981). Activity of coded compounds in
the micronucleus test. Progress in Mutation Research, 1: 705-711.
Tsuda, H., Takahashi, M., Fukushima, S., Endo, Y. (1973). Fine
structure and localization of peroxidase activity in aminotriazole
goiter. Nagoya Med. J., 18: 183-197.
Tsutsui, T., Maizumi, H., Barrett, J.C. (1984). Amitrole-induced
cell transformation and gene mutations in Syrian hamster embryo
cells in culture. Mutation Research, 140: 205-207.
Turner, D.M., Gilbert, C.M. (1976). Evidence of metabolismus of
(14C) Amitrole in the rat after inhalation administration.
Unpublished report No 291/1a (dated May 1976) from Hazleton
Laboratories Europe Ltd. Submitted to WHO by Bayer AG.
Tweats, D.J. (1981). Activity of 42 coded compounds in a
differential killing test using Escherichia coli strains Wp2, WP67
(uvrA polA), and CM871 (uvrA lexA recA). Progress in Mutation
Research, 1: 199-209.
Tyl, R.W. (1986a). Teratogenicity evaluation of aminotriazole
technical administered by gavage to CD rats. Unpublished report No
49-45 (dated: 29-05-1986) from Union Carbide Bushy Run Research
Center. Submitted to WHO by Bayer AG and Rhône-Poulenc Ag. Company.
Tyl, R.W. (1986b). Teratogenicity evaluation of aminotriazole
technical administered by gavage to New Zealand White rabbits.
Unpublished report No 49-66 (dated: 30-05-1986) from Union Carbide
Bushy Run Research Center. Submitted to WHO by Rhône-Poulenc Ag.
Company.
Venitt, S. & Crofton-Sleigh, S. (1981). Mutagenicity of 42 coded
compounds in a bacterial assay using Escherichia coli and
Salmonella typhimurium. Progress in Mutation Research, 1: 351-360.
Vesselinovitch, S.D. (1983). Perinatal hepatocarcinogenesis.
Biological Research in Pregnancy and Perinatology, 4: 22-25.
Vogel, E., Blijleven, W.G.H., Kortselius, M.J.H., Zijlstra, J.A.
(1981). Mutagenic activity of 17 coded compounds in the sex-linked
recessive lethal test in Drosophila melangolaster. Progress in
Mutation Research, 1: 660-665.
Weber, H. (1983). Aminotriazole feeding study: Determination of the
threshold dose level affecting the thyroid function of female rats
by radioiodine assay and by T3 and T4 radioimmunoassay. Unpublished
report No. 11739 (F) (dated: 13-04-1983) from Bayer Health Care
Division. Submitted to WHO by Bayer AG.
Weber, H. & Patzschke, K. (1978). Effect of long-term administration
of aminotriazole on thyroid function of male and female rats, mice
and hamsters. Unpublished report No 7690 (dated: 14-07-1978) from
Bayer AG, Institute of Pharmacokinetics. Submitted to WHO by Bayer
AG.
Weir, R. (1958). 3-Amino-1,2,4-triazole Chronic oral administration
- beagle dogs. Unpublished report from Hazleton Laboratories
Virginia dd December 1, 1958. Submitted to WHO by Bayer AG.
WHO (1992). The WHO recommended classification of pesticides by
hazard and guidelines to classification 1992-1993 (WHO/PCD/92.14).
Available from the International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland.
WHO (1994). International Programme on Chemical Safety Environmental
Health Criteria 158: Amitrole. Geneva, World Health Organization.
Wynford-Thomas, D., Stringer, B.M., Williams, E.D. (1982a).
Dissociation of growth and function in the rat thyroid during
prolonged goitrogen administration. Acta Endocrinologica, 101:
210-216.
Wynford-Thomas, D., Stringer, B.M., Williams, E.D. (1982b).
Goitrogen-induced thyroid growth in the rat: a quantitative
morphometric study. J. Endocr. 94: 131-140.
Zimmermann, F.K. & Scheel, I. (1981). Induction of mitotic gene
conversion in strain D7 of Saccharomyces cerevisiae by 42 coded
chemicals. Progress in Mutation Research, 1: 481-490.
Toxicological studies
Acute toxicity studies
The acute toxicity of amitrole to several animal species is
given in Table 1.
No signs of toxicity were observed in one beagle-type female
dog when given amitrole (96.1%) by gavage at a dosage of 2150 mg/kg
bw. Another dog receiving 4640 mg/kg bw vomited within 1 hour, and
no signs of toxicity were observed thereafter (Fogleman, 1954).
When a commercial product (water soluble powder with 50% active
ingredient) was administered to mice, the LD50 was reduced to 4.0
g/kg bw amitrole. By determining the mean lethal dose for amitrole
in different preparations, it was established that sodium carbonate,
sodium bicarbonate and wetting agents as admixtures in various
quantities considerably increase the toxicity of amitrole (Hapke,
1967). WHO has classified amitrole as unlikely to present acute
hazard in normal use (WHO, 1992).
Table 1. Acute toxicity of amitrole in animals
Species Sex Route LD50 LC50 Purity Reference
(mg/kg bw) (mg/m3)
Mouse M oral 14 700 7 9 96.1%* Fogleman, 1954
? oral 11 000 8 ? Hapke, 1967
? i.v. 5 000 10 ? Hecht, 1954
M i.v. > 1 600 4 6 94% Bagdon et al., 1956
M i.p. > 10 000 6 94% Bagdon et al., 1956
Rat ? oral > 4200 ? Seidenberg & Gee, 1953
? > 10 000 6 10 ? Hecht, 1954
M 25 000 1 9 94% Bagdon et al., 1956
M > 10 000 6 10 ? Hecht & Kimmerle, 1962
? > 2500 93.7% Kimmerle, 1968
M > 5000 ? Thyssen, 1974a
M > 5000 98% Thyssen, 1974b
M&F > 4080 6 99% Gaines et al., 1973
M > 5000 ? Heimann, 1982
M&F dermal > 2500 99% Gaines et al., 1973
M&F inhal (4 h) > 439 6 96.9% Thyssen, 1983
? i.v. 5000 10 ? Hecht, 1954
M i.p. > 4000 6 94% Bagdon et al., 1956
Rabbit ? dermal > 10 000 5 6 95% Elsea, 1954
Cat M&F oral > 5000 2 94% Bagdon et al., 1956
M&F i.v. > 1750 3 94% Bagdon et al., 1956
1 80% aqueous suspension
2 40% aqueous suspension; the 3 cats tested (limit test)
vomited within 2 hours after receiving the dose
3 10% solution in 0,9% saline; one cat per dose level
4 aqueous solution
5 moistened with 0.5% methyl cellulose solution
6 no signs of toxicity
7 mortality at 21 500 mg/kg; signs of toxicity at this and
higher dose levels: extreme depression, squinting eyes, slow,
laboured respiration, diarrhoea, ataxia, depressed or absent
placement and righting responses, mild clonic convulsions,
gasping, coma and death and irritation of gastrointestinal
tract. No signs of toxicity at the lower dose level
(10 000 mg/kg bw)
8 signs of toxicity seen from 5 mg/kg bw: severe hypokinesia,
slight tremor of the extremities, apathy ranging to catatonia,
prone position, individual deep inspirations.
9 only 5 animals/group
10 less than 4 animals/group
* no correction for purity was made.
Short-term toxicity studies
Dietary/gavage
Rats
In a two-week dietary study, administration of 60 or 120 ppm
amitrole resulted in enlargement of the thyroid gland of rats
(number and strain not specified) and a pronounced lowering of
iodine uptake. Over this two-week interval there were no
significant changes at levels of 15 or 30 ppm (Jukes & Shaffer,
1960; Annex I, reference 23).
Amitrole was administered by gavage to groups of rats (strain
unknown), 5 days a week, for 4 weeks at doses of 0, 100, 200 or 400
mg/kg bw. In all treated groups, growth rate was reduced, relative
thyroid weight increased and iodine content of the thyroid reduced
(Hapke, 1967; Annex 1, reference 23).
In a range-finding study, groups of albino Wistar rats (4/sex)
received 0, 125, 1250 or 12 500 ppm amitrole in their diet for 27
days. The low dose (125 ppm) was raised to 25 000 ppm on day 7 of
treatment. Body-weight was retarded and food intake reduced in all
treatment groups. Thyroid weight (the only organ investigated) was
increased in all dose groups (Ben-Dyke et al., 1973).
Reversibility of thyroid effects was studied in a two-week
dietary study in male albino rats (strain unknown). A dietary level
of 1000 ppm increased the (absolute) thyroid weights to about 3.5
times those in the untreated controls. Thyroid weights had nearly
completely returned to normal after two weeks recovery (Bagdon et
al., 1956; Annex I, reference 23).
Amitrole (purity 94.6%) was administered to groups of male
Sprague-Dawley rats (20/dose) at levels of 0, 30, 100, or 300 ppm in
their diet for 28 days, followed by a 28-day recovery period. Two
animals/group were killed weekly in order to monitor the thyroid
function by assaying the thyroid hormones T3 and T4. Body weight
was depressed and food consumption decreased at the 100 and 300 ppm
dose levels. Food consumption returned to normal during the
recovery period as did body weight, but only in the 100 ppm group.
T3 levels were significantly decreased at day 7 in the 300 ppm
group and at day 14 in the 100 ppm group. T3 levels returned to
normal by day 19 post-treatment. T4 levels followed the same
pattern although the extent of decrease was greater. The NOAEL in
this study was 30 ppm (equivalent to 3 mg/kg bw/day) (Babish, 1977).
In a thyroid function test amitrole (purity 96.9%) was
administered to groups of 30 female Wistar rats (12 week old,
weighing 200 g) at levels of 0, 0.5, 1, 2, or 4 ppm in their diet.
Ten animals per group were sacrificed 3, 9 or 29 days after study
initiation and the following parameters were examined: thyroid
weight, accumulation of radioiodine in the thyroid (24 hours after
administration of 131I), and serum levels of T3 and T4.
Significantly elevated thyroid weights were observed only in the 1
and 2 ppm groups, 3 days after study initiation. No significant
deviation was observed in any dose group at the later test dates.
The percentage of iodine accumulation in the thyroids of the 2 ppm
group exhibited significant elevation after a 9-day treatment
period. In the 4 ppm group a slight, but not significant increase
was found. No effect was observed at 29 days, and no clear effects
were observed on T3 or T4 levels. The effects on thyroid function
found in this study were considered marginal and not consistent.
Therefore the highest dose in this study (4 ppm, equivalent to 0.4
mg/kg bw/day) was considered to be the NOAEL (Weber, 1983).
Amitrole (96.1%) was administered to groups of Carworth Farm
male and female rats (5 animals/sex/group) at levels of 0, 100,
1000, or 10 000 ppm in the diet for 63 days. Both males and females
showed reduced body-weight gain at 1000 and 10 000 ppm, which was
accompanied by reduced food consumption. There were no deaths or
gross signs of toxicity. Histopathological examination of the
liver, kidneys, bladder, small intestine, spleen and testis (thyroid
was not examined) revealed only an increased vacuolization of the
liver cells around the central veins in the 1000 and 10 000 ppm
dosage groups. The vacuoles were identified as fat globules
indicative of fatty metamorphosis associated with liver cell damage.
No histological effects were noted at 100 ppm. Because the thyroid
was not examined in this study, it is of limited value in the
evaluation of amitrole (Fogleman, 1954).
Fregly (1968) investigated the dose-response relationship
between amitrole administered in the diet and a variety of clinical
parameters in order to establish the minimum effective dose on
thyroid activity. Amitrole was administered to groups of male
Spruce Farm strain rats (10/dose) at levels of 0, 2, 10, or 50 ppm
in the diet for 13 weeks. In a separate experiment, amitrole was
administered in the diet to similar groups at levels of 0, 0.25, or
0.50 ppm for 11 weeks. In the first experiment, there were no
treatment-related effects on body weight, food consumption,
haematocrit, haemoglobin, or the rate of oxygen consumption. Mean
rectal temperature (measured at week 12) was slightly, but
significantly increased at 50 ppm. Uptake of radioactive iodine,
measured in vivo during week 12 at various times 22-53 hours after
injection of Na131I, was significantly decreased at 50 ppm. At the
end of the first study, radioactivity in the thyroid gland, measured
24 hours after injection, and the level of PBI in serum were
significantly reduced in all treatment groups. The PBI-values were
5.1, 3.7, 3.8, and 3.3 µg/100 ml for the 0, 2, 10, and 50 ppm
groups, respectively. Thyroid weight was increased significantly
only in the 50 ppm group. Histological changes in the thyroid were
noted at 10 and 50 ppm in the follicular cells with regard to both
appearance and the presence of colloid. Capillary density, which is
characteristic of the TSH-stimulated thyroid, was increased
significantly at both 10 and 50 ppm. In the second experiment, at
lower dose levels, no significant differences were noted between the
treated and control groups. In this study PBI was not affected. In
fact the PBI values were 3.2, 3.9, and 4.5 µg/100 ml for the 0, 0.25
and 0.50 ppm groups, respectively. It should be noted that the PBI
control values, measured in the second experiment were much lower
than those measured in the first experiment. This means that there
is no biologically significant effect on PBI, because all values
fall in the same range. The NOAEL was therefore 2 ppm (equivalent
to 0.1 mg/kg bw/day) (Fregly, 1968).
Several short-term experiments were carried out in Wistar rats,
in order to establish a no-effect level on thyroid function. In all
experiments the uptake of 131I by the thyroid was measured in an in
vivo test 6, 24, or 48 hours after administration of 0.6 µCi 131I
per animal intraperitoneally. In addition, at the end of the study,
thyroid weight and PBI were measured and the thyroid was studied
histopathologically.
In the first experiment, four groups of 8 female rats received
0, 2, 20, or 200 ppm of amitrole in the diet for 6 weeks. The
uptake of 131I was measured after 5 days and 6 weeks. At both times
a significantly increased uptake was found in the 200 ppm group 6
hours after injection, which decreased rapidly after 24 and 48
hours. At that time the radioactivity was lower than that of the
controls. The thyroid weight was increased in the 200 ppm group and
histopathologically goitre was found in this group only. No
significant effects were found at lower dosages.
In the second experiment in which 8 female rats per group
received 0, 20, 50, or 200 ppm for 6 weeks, the same effect was
found in the 200 ppm group. In addition, a significantly decreased
PBI was observed at the end of the experiment compared with the
controls. At 50 ppm a statistically increased uptake was found 6
hours after injection of 131I. In this case the radioactivity in
the thyroid remained higher than the controls after 24 and 48 hours.
Histopathologically only a very slight activation was found, whereas
200 ppm showed strong activation and goitre.
In the third experiment, 10 female animals per group received
amitrole at dietary concentrations of 0, 20, 50, or 200 ppm for 13
weeks. The uptake of 131I by the thyroid was significantly
increased at 50 and 200 ppm after 6 and 12 weeks. At 50 ppm, the
radioactivity in the thyroid remained high after 24 and 48 hours,
whereas at 200 ppm a very high uptake was found 6 hours after
injection of 131I, followed by a rapid decrease with still lower
values than the controls after 48 hours. At 200 ppm, the PBI was
decreased and the thyroid/body-weight ratio increased by a factor of
6. At 50 ppm, only a slightly increased relative thyroid weight was
found. Histologically a strong activation and goitre were found at
200 ppm, and a slight activation at 50 ppm. In this experiment, a
tendency to a higher uptake of 131I was found in the 20 ppm group.
The three above mentioned experiments were carried out with an
iodine content of about 0.2-0.3 ppm in the diet. In the fourth
experiment an iodine content of about 2 ppm was used. In this
experiment, 8 female rats per group received 0, 20, 50, 200 or 500
ppm amitrole in the diet for 6 weeks, to determine whether iodine
could protect against the antithyroid action of amitrole. At 500
ppm, a small increase in iodine uptake was found 5 hours after
injection, but thereafter a very rapid decrease was found. At 200
ppm, the uptake was much higher and the same type of decrease was
found as in the other experiments, whereas with 50 ppm a
significantly increased thyroid radioactivity was found at all
times. PBI was decreased at 200 and 500 ppm only.
Histopathologically, goitre and strongly activated thyroids were
found at the two highest dose levels. Some activation was found in
the 20 ppm group. It can be concluded that measurement of 131I
uptake at different time points was a sensitive method for the
effects of amitrole on the thyroid. At 20 ppm, only slight effects
were found on iodine uptake and thyroid histopathology. The overall
NOAEL from these four experiments was 2 ppm, equivalent to 0.1 mg/kg
bw/day (Den Tonkelaar & Kroes, 1974).
Dogs
In a one-year study in male and female beagle dogs, amitrole
was given in capsules at daily dose levels of 0, 0.25, 1.25, 2.50 or
12.5 mg/kg bw, 6 days per week (4-6 animals/group). There were no
clinical signs of toxicity or pharmacological effects.
Haematological, biochemical and urinalysis parameters were
comparable to those of the control dogs, and within normal limits.
The dogs at the 12.5 mg/kg bw/day dose level had pale-coloured
pancreases as the only dose-related gross pathological effect.
Histopathological examination of all dogs did not reveal any
treatment-related effect. The thyroid, in particular, was normal at
all dose levels (Weir, 1958).
Drinking-water
Mice
Groups of male albino mice (strain unknown) were exposed to
amitrole in the drinking-water at concentrations of 0, 0.5, 1.0 or
2% for 30 days (water consumption was not reported). Light
microscopy revealed dose-related hypertrophy of hepatocytes with
nuclear deformations, increased pyknotic nucleoli, and increased
vacuoles in the cytoplasm. Electron microscopy revealed
proliferation of smooth endoplasmic reticulum (Reitze & Seitz,
1985).
Rats
Male Sprague-Dawley rats were provided with drinking-water
containing 400 ppm amitrole over periods of 12, 20, or 37 days.
Based on a body weight of about 200 g and a water intake of 30-35
ml/day, the daily intake was about 60-70 mg/kg bw. The catalase
activity of liver and kidney of the treated animals was inhibited by
50%, but the red cell catalase was unaltered. An increase in
thyroid weight was found, which correlated with the duration of
treatment. Microscopic examination showed hyperplasia of the
thyroid and a loss of colloid. It was postulated in this study that
the ability of the thyroid to concentrate plasma iodine was not
impaired by amitrole, but that the formation of organically-bound
iodine was inhibited (Alexander, 1959).
In a range-finding study, amitrole was administered to groups
of albino Wistar rats (4/sex) at levels of 0, 10, 104 or 1040 ppm in
drinking-water over a treatment period of 27 days. Body-weight gain
was retarded and food intake reduced at 104 ppm and above. Thyroid
weight (the only organ investigated) was increased in a dose-
dependent manner at 104 ppm and above. The NOAEL in this study was
10 ppm, equivalent to 1.5 mg/kg bw/day, based on a daily water
consumption of 30 ml and body weight of 200 g (Ben-Dyke et al.,
1973).
Amitrole (purity 94%) was administered in the drinking-water to
groups of male albino rats (10/dose; strain unknown) at
concentrations of 0, 50, 250, or 1250 ppm for 106 days. The
administration of amitrole resulted in a dose-dependent depression
of growth with a corresponding reduction of food and water intake.
Appearance, behaviour, and mortality were not affected by amitrole.
Haematology, except Ht, and clinical chemistry parameters were not
examined. At the end of the study, histopathology was performed on
the thyroid, hypophysis, liver, kidney, spleen, stomach, small and
large intestines, bladder, testis, adrenal and lung. Gross and
microscopic examination of tissues and organs showed a marked
increase in thyroid size at all dose levels. In rats where reduced
growth was noted, the kidneys, adrenals, liver and spleen were
proportionally smaller. Reproductive organs were not affected.
Microscopic examination showed general enlargement of the thyroid at
50 ppm, with moderate stimulation of the thyroid epithelium (no
evidence of hyperplasia). At 250 ppm, thyroid hyperplasia was
evident. At the two highest dose levels there was also absence of
follicles with stored colloid. Liver catalase activity was reduced
at 250 and 1250 ppm in a dose-dependent manner (Bagdon et al.,
1956; Annex I, reference 23).
In a study measuring the time-course of development of goitre,
Sprague-Dawley rats were given amitrole in the drinking-water (400
ppm). The thyroid of each animal was examined by light microscopy
at various periods from 3 days to 6 months. Each rat drank
approximately 30 ml per day. After 3 days, the thyroid size was
unchanged, although cellular changes were apparent. By one week,
the thyroid was twice its normal size with marked structural
changes. These changes continued to progress with prolonged
administration of the goitrogen. Goitre formation was accompanied
by increased vascularity and decreased colloid content in the
follicular cells. Electron microscopy revealed pronounced
dilatation of the endoplasmic reticulum. Peroxidase activity
measured in the thyroid progressively decreased with prolonged
administration of amitrole (Strum & Karnovsky, 1971).
The effects of amitrole on thyroid histology were examined in
seven groups of 5 female Wistar rats weighing about 200 g, which
were given amitrole in their drinking-water, 2500 ppm, and killed
after 1, 2, 3, 10, 30, 50, or 100 days. Water consumption was not
reported. After 1 and 2 days of exposure the only change noted was
a slight enlargement of some endoplasmic cisternae of follicular
cells. After 3 days the gland was slightly enlarged, follicular
colloid was slightly reduced and in some follicular cells the
cisternae were clearly dilated and stained more lightly for
peroxidase activity than in normal cells. By 10 days the glands had
doubled in size, the follicular epithelium consisted of low,
columnar cells, and colloid had been severely depleted. Nuclei had
become located basally and slightly elongated microvilli projected
into the lumen. Peroxidase activity was no longer seen in the
endoplasmic reticulum cisternae, whereas it was observed in portions
of perinuclear cisternae. These changes had progressed in the 30-
day exposure group, so that the glands were now several times their
normal size. In addition, fibrous thickening of both stroma and
capsule was prominent and cisternal peroxidase activity was absent.
Over 50-day exposure resulted in increased irregularity in
follicular size, more prominent papillary growth of the follicular
epithelium and greatly diminished peroxidase activity throughout the
cells (Tsuda et al., 1973).
Functional and morphological changes in the thyroid were
examined in male Wistar rats. Amitrole was administered via
drinking-water at a concentration of 1000 ppm for periods of up to
153 days. Within 2 weeks thyroid hormone synthesis (serum T3 and
T4) was inhibited. Serum TSH level increased rapidly during the
first 4 weeks, and remained essentially constant after that. The
accumulation of radioiodine in the thyroid (measured as the ratio
thyroid to serum inorganic iodide - T/S ratio) followed a similar
pattern. Thyroid weight increased rapidly during the first few
weeks and thereafter growth rate declined. After 4 months, total
thyroid weight was increased to a 12-fold plateau. During the first
week of amitrole administration there was also a rapid change in the
morphology of the gland; an increase in the proportional volume of
the epithelial cell compartment being accompanied by a corresponding
decrease in follicular lumen (colloid) and a marked increase in
vascularity (Wynford-Thomas et al., 1982a,b).
Dermal
Rabbits
Amitrole (97.6%) was applied to the shaved skin of the back and
flanks of groups of 6 male and 6 female New Zeeland white rabbits at
doses of 0, 25 or 100 mg/kg bw for 6 hours each day on 15
consecutive workdays. The skin was abraded in three animals per
group. The NOAEL for systemic and local effects was at the highest
dose level, i.e. 100 mg/kg bw/day (Mihail & Schilde, 1984).
Inhalation
Rats
Groups of Fischer 344 rats (15/sex/dose) were exposed to an
atmosphere containing amitrole (94.6% pure) at concentrations of 0,
0.1, 0.32, 0.99, or 4.05 mg/l (nominal concentrations adjusted for
non-nebulizing material), 5 hours/day 5 times per week, for 4 weeks
(particle size unknown). There were no adverse effects on behaviour
and no body-weight changes were noted. T3 and T4 levels were
depressed after 14 and 27 days at levels of 0.32 mg/l and above. At
these dose levels, thyroid hyperplasia and elevated thyroid weights
were also observed. At a concentration of 0.1 mg/l no effects on
the thyroid were observed (Cox & Re, 1978).
Long-term/carcinogenicity studies
Mice
Amitrole was used as a positive control in a study which
investigated the carcinogenicity of some 120 chemicals. Groups of
(C57BL/6 x C3H/Anf) F1 mice (18/sex) and (C57BL/6 x AKR) F1 mice
(18/sex) were given amitrole by gavage at a dose level of 1000 mg/kg
bw/day on days 7-28 of age, and in the diet at a level of 2200 ppm
from 28 days onwards until the end of the experiment. This was
planned to be an 80-week study but all of the mice in the amitrole
treatment groups had died by weeks 53-60. Thyroid tumours were
reported to have occurred in nearly all of the treated mice (64/72).
Liver tumours were observed in 34/36 (C57BL/6 x C3H/Anf) F1 treated
mice and in 33/36 (C57BL/6 x AKR) F1 treated mice. In pooled
control groups, 8/166 (C57BL/6 x C3H/Anf) F1 mice and 6/172
(C57BL/6 x AKR) F1 mice had liver tumours (Innes et al., 1969).
In a carcinogenicity study, amitrole (97.0% pure) was
administered to groups of NMRI mice (75/sex/dose level) at levels of
0, 1, 10, or 100 ppm in their diet for 18 months. There were no
treatment-related effects on appearance, behaviour, body weight,
food consumption or survival times (haematology not measured).
Histological examination of tissues of the major organs did not
reveal any treatment-related effects apart from a slight increase in
the number of hyperemias in the pituitary at 100 ppm (5 at 1 ppm, 2
at 10 ppm and 16 at 100 ppm). The incidence of treatment-related
tumours was not increased. Concurrent satellite groups of 5
animals/sex/dose were additionally used in this carcinogenicity
study for different thyroid function tests (thyroid weights,
incorporation of radioactive iodide in the thyroid gland, proportion
of PBI in total plasma iodine) performed at intervals of 3, 6, 9,
12, and 18 months. The thyroid weights (up to 3 times control
values), were elevated in the 100 ppm males at all test dates. The
percentage of iodine accumulation as well as the iodine level in the
thyroid were elevated in the male mice at 100 ppm. The fraction of
PBI in the male mice was elevated 9 months after study initiation,
but was depressed at later test dates. Comparable results were
observed in the high-dose females. However, the deviations relative
to the control group were generally smaller than in the males, and
were not significant in most cases. The NOAEL was 10 ppm equivalent
to 1.5 mg/kg bw/day (Steinhoff & Boehme, 1979b, Weber & Patzschke,
1978; Steinhoff et al., 1983).
In a study on C3H mice and their substrain without serum
catalase activity, ingestion of 10 000 ppm amitrole in the diet led
to a reduction in lifetime. In comparing the two substrains, it was
shown that the acatalasemic mice lived longer under treatment than
did the normal mice. The mean survival times were 35 and 25 weeks,
respectively. The C3H strain features a high rate of spontaneous
liver tumours (it was reported to be 9% in females and 46% in
males). Under amitrole treatment, liver tumours occurred earlier
and at a higher incidence in the acatalasemic mice than in the
normal mice (Feinstein et al., 1978).
Three groups of B6C3F1 mice were fed a diet containing 500
ppm amitrole (purity not specified): Group 1: the dams were treated
from day 12 of gestation to birth of the pups; group 2: dams
together with their pups were treated from birth to weaning; group
3: pups were treated for a period of 90 weeks after weaning.
Although not specifically stated, information on other chemicals
tested and described in this paper (benzidine, safrole etc.),
implies that pups of groups 1, 2, and 3 animals were examined only
for liver tumours after 90 weeks. It was not stated if treatment
with the other compounds were conducted in the same or different
rooms. The incidence of hepatocellular adenomas and carcinomas,
respectively, were: group 1 males, 4/74 and 2/74; group 2 males,
6/45 and 4/45; group 3 males, 15/55 and 11/55; group 1 females, 0/83
and 0/83; group 2 females, 0/55 and 0/55; group 3 females, 5/49 and
4/49. The incidence of these tumours in the untreated control
animals (which may or may not have been concurrent controls) at week
90 were: males, 1/98 and 0/98; females, 0/96 and 0/96. Other organs
were not examined. It was concluded that there was no effect of
amitrole treatment in group 1 or in females of group 2, but that
marginal increases occurred in males of group 2 and in males and
females of group 3 (Vesselinovitch, 1983).
The susceptibilities of three mouse strains to the development
of preneoplastic hepatic lesions were examined following amitrole
administration in their drinking-water at 10 000 ppm. The strains
were DS, ICR (Crj: CD-1) and NOD derived from ICR and found to
develop spontaneous insulitis followed by diabetes. There were
reported to be indications that NOD mice may carry immunological
abnormalities. In each of two experiments, 3 groups of female mice
were administered amitrole via drinking-water for 3 months
(experiment 1) or 6 months (experiment 2), after which they were
killed and their livers examined. The proportions of mice with
hyperplastic nodules were, in experiment 1: 15/19, NOD; 3/55, DS;
0/5, ICR, and in experiment 2: 19/19 NOD; 18/18 DS; 17/19 ICR. A
single hepatocellular carcinoma developed in a NOD mouse after 6
months (Mori et al., 1985).
Rats
In a limited chronic toxicity study (no haematology or clinical
chemistry) groups of Carworth Farm Wistar rats (35/sex/dose)
received amitrole in the diet at levels of 0, 10, 50, or 100 ppm for
two years. After 13 weeks, 5 animals/sex/dose and after 68 weeks 3
animals/sex/dose were killed for organ weight measurement and
histopathological examination. A separate group received 500 ppm
for 19 weeks. Due to marked reduction in body-weight gain and food
consumption, these animals were left on the control diet for 7 weeks
and were then killed (26 weeks). Animals in all groups, including
controls suffered from apparent respiratory infection and were in
poor condition. Death occurred in 67 rats but there was no
relationship with treatment. Body-weight gain was reduced at 100
ppm in male animals during the first 13 weeks of the study. After
68 and 104 weeks of treatment, relative thyroid weight was increased
at 100 ppm (not measured after 13 weeks). Histopathological
examination after 13 weeks showed hyperplasia and hypertrophy of the
thyroid at 500 ppm, but this was reversed after withdrawal of the
amitrole diet. Histopathological changes were also seen at 100 ppm
and in one animal at 50 ppm. At 68 weeks, 3 animals at 50 ppm
showed definitive evidence of hyperplasia while all animals at 100
ppm showed evidence of hyperplasia and hypofunctioning of the
thyroid. At 104 weeks tumours were found: thyroid tumours were
present in 1/10 animals of the 10 ppm group, 2/15 at 50 ppm and
15/27 at 100 ppm. No tumour was detected in 5 control group animals
but one rat exhibited early stages of an adenoma. One thyroid from
the 50 ppm group and 4 from the 100 ppm group exhibited lesions
interpreted as adenocarcinomas by several pathologists and benign
neoplasms by others. Based on thyroid hyperplasia the NOAEL was 10
ppm equivalent to 0.5 mg/kg bw/day (Keller, 1959; Jukes & Shaffer,
1960).
Groups of Fischer 344 rats (75/sex/dose) were treated with
amitrole (94.6% pure). Group A were the controls. Rats of group B
were fed 5 ppm of amitrole in their diet during 1-39 weeks and then
100 ppm during weeks 40-115 for males and 40-119 for females. Rats
in groups C, D, and E received amitrole in their diet at pulsed
intervals (alternate 4 week periods) at 1, 3, or 10 ppm,
respectively, during weeks 1-39 and 20, 60, or 200 ppm during the
last exposure period (week 40 onwards). On alternate 4-week
periods, groups C, D, and E received the basal diet containing no
amitrole. There were no treatment-related clinical signs of
toxicity or changes in body weight or food consumption. No
treatment-related effects on parameters of haematology, clinical
chemistry or urinalysis were observed (only 5 animals/sex/dose level
were examined). Thyroid weights were significantly increased in
both males and females in groups B and E after 60 weeks and at
termination. Increased thyroid weights, although not significant,
were also observed in group D. The T3 and T4 activities were
elevated in groups B and E from week 44 onwards. However, these
effects were not always consistent. These changes were not observed
up to week 36, when the groups were administered the lower dose
levels. Follicular epithelial hyperplasia in the thyroid was noted
in groups B, D, and E and to a much lesser extent in group C. An
increased incidence in thyroid tumours (mainly follicular adenomas)
was observed in male and female rats of groups B and E and in the
male animals of group D. There was no significant difference in
tumour incidence between groups B and E (in these dose groups the
incidence of follicular adenomas was 80-84% and of follicular
adenocarcinomas 5%, compared to 0-2% in the control group). Due to
the change in dosing regimen it was difficult to evaluate this
study. However, effects on the thyroid (hyperplasia) were seen in
all treated groups (Johnson, 1981).
In a study performed to characterize the oncogenes
participating in the genesis of thyroid tumours, male Wistar rats
(number not specified) were treated with a chemical carcinogen
(nitrosomethylurea, NMU) or by ionizing radiation (131I) to induce
thyroid tumours, and were then treated with amitrole (purity not
specified) as a goitrogen at a level of 0.1% in drinking-water
throughout their entire lifetimes. A control group was only
administered amitrole without prior tumour-initiating treatment.
Malignant thyroid tumours developed after even a relatively brief
period in rats treated with NMU or 131I. Animals exclusively
treated with amitrole exhibited only benign tumours which developed
at a markedly slower rate. A "H-ras" oncogene was determined to
be involved in treatment with NMU (13 out of 15 cases), whereas a
"K-ras" oncogene was involved in the animals treated with 131I
(8/15 cases) and amitrole (only 1/10 cases) (Lemoine et al.,
1988).
In a briefly worded description of a long-term study in rats,
thyroid and liver tumours were reported to occur in rats exposed to
20-25 mg amitrole per day via drinking-water or to 250 or 500 mg
amitrole per day in their diets for life. No control data were
reported (Napalkov, 1962). Due to the serious shortcomings in this
study, it was not considered relevant for the evaluation of amitrole
toxicity.
In a carcinogenicity study, groups of Wistar rats (75/sex/dose
level) were treated with amitrole (97.0% pure) in the diet at
concentrations of 0, 1, 10, or 100 ppm for two years. There were no
treatment-related effects on appearance, behaviour, body weight, or
food consumption (haematology not measured). A slight decrease in
survival time was found at 100 ppm. Average survival time at 100
ppm for males was 961 days and for females 919 days. The survival
times for control animals were 992 and 969 days for males and
females, respectively.
Concurrent satellite groups of 5 animals/sex/dose level were
additionally used in this carcinogenicity study for different
thyroid function tests (thyroid weights, incorporation of
radioactive iodide in the thyroid gland, proportion of protein-bound
iodine in total plasma iodine) performed at intervals of 3, 6, 9,
12, 18 or 24 months. The thyroid weights (up to 3.6 times control
values for males and 7 times for females) were increased at 100 ppm
as was the uptake of 131I by the thyroid, measured 24 hours after
oral administration of 131I. The fraction of PBI was not affected.
At 100 ppm, an elevated incidence of haemorrhages and hyperaemia of
the pituitary gland as well as a very high rate of cystically
dilated thyroid follicles were seen. Both benign and malignant
tumours of the thyroid were found at 100 ppm. There was also an
increase in the incidence of benign tumours in the pituitary gland
at the 100 ppm level (females). The NOAEL was 10 ppm (equivalent to
0.5 mg/kg bw/day) (Steinhoff & Boehme, 1979a; Weber & Patzschke,
1978; Steinhoff et al., 1983).
Hamsters
In a carcinogenicity study in Syrian golden hamsters
(76/sex/dose) amitrole (97%) was administered in the diet at levels
of 0, 1, 10, or 100 ppm for 18 months. There were no treatment-
related changes in appearance, behaviour or food intake. Body-
weight gain was decreased at 100 ppm from day 400 onward, and
mortality was significantly increased in the 100 ppm dose group.
Average survival time at 100 ppm for males was 557 days and for
females 438 days. The survival times for control animals were 631
and 508 days for males and females, respectively. The main cause of
death in all groups, including controls, was severe amyloidosis of
the kidneys. There was no evidence of amitrole-related
histopathological changes. There was no evidence of a treatment-
related carcinogenic effect. Concurrent satellite groups of 5
animals/sex/dose were additionally used in this carcinogenicity
study for different thyroid function tests (thyroid weights,
incorporation of radioactive iodide in the thyroid gland, proportion
of PBI in total plasma iodine) performed at intervals of 3, 6, 9,
12, or 18 months. There were no treatment-related effects observed.
The NOAEL was 10 ppm equivalent to 1 mg/kg bw/day (Steinhoff &
Boehme, 1978; Weber & Patzschke, 1978; Steinhoff et al., 1983).
Inhalation exposure
Rats
In an inhalation study involving intermittent treatment, groups
of 75 Fischer rats per dose and sex were exposed to aerosols at
nominal amitrole levels of 0, 50 or 500 µg/l air (one of the two
batches used had a purity of 94.6%). The actual amitrole
concentrations in the low-dose group varied between 15.8 and 32.2
µg/l air in the different exposure periods; the levels measured in
the high-dose group ranged between 97.9 and 376.4 µg/l air. The
animals were exposed for 5 hours each day five days per week.
Treatment phases during weeks 1-13, 40-52 and 78-90 were interrupted
by treatment-free intervals. Interim necropsies of 5 animals per
dose and sex were performed after 3, 9 and 18 months; the study was
concluded after 24 months. A total of 28 rats died in week 51 due
to a defect in the air conditioning system which led to an increase
in the room temperature. Treatment of the high-dose group was
thereupon concluded, and the surviving animals (6/75 males, and
18/75 females) were necropsied.
In the high-dose group, food intake and body-weight gain were
decreased and the rate of mortality was elevated. At week 13 of the
study, levels of cholesterol, ASAT and ALAT were higher and of
glucose lower in the high-dose group than in the control group.
Also T3 and T4 levels were lower. No differences in these
parameters were observed after the treatment-free period. Relative
thyroid weight was increased and epithelial hyperplasia of the
thyroid follicles was determined in both dose groups at the end of
the first treatment interval (week 13). This latter finding was no
longer observed after the first treatment-free interval of 24 weeks,
but the thyroid weights were still elevated in both dose groups.
Follicular epithelial hyperplasia was again present in most of the
animals of both treatment groups at the end of the second treatment
phase (week 51). This finding was still observed, in the remaining
treatment group (50 µg/l air), after a treatment-free interval of 26
weeks, which indicates that complete reversion no longer occurred at
this time. Neoplasms of the thyroid (adenomas and adenocarcinomas)
were found in addition to hyperplasia at terminal necropsy (Becci,
1983).
Reproduction studies
Rats
In a limited reproduction study, groups of 10 male and 10
female Sherman rats were fed amitrole in the diet. A preliminary
one-generation study was performed in which rats were fed levels of
0, 500, or 1000 ppm for 55 days before pair-mating. The offspring
were weaned at 21 days. Complete autopsies were performed on the
parents after total exposure of 107-110 days. Ten weanling rats
from each dose group were killed. Mean body-weight gain and food
consumption were reduced in the parents at all dose levels.
Relative kidney, spleen, and liver weights (only in males) were
reduced in parents fed 500 and 1000 ppm. The average number of pups
per litter was significantly reduced at the 500 and 1000 ppm dose
levels, as were the numbers surviving to weaning. Body weight of
pups at weaning was also reduced. Thirty-three out of 45 pups of
rats fed 500 ppm and 55/56 pups of rats fed 1000 ppm died within one
week after weaning. Relative weight of thymus and spleen of pups of
the 500 ppm group (1000 ppm not examined) were significantly
reduced. Thyroid hyperplasia was seen in all treated rats (Gaines
et al., 1973).
In a subsequent 2-generation study, rats were fed dose levels
of 0, 25, or 100 ppm for 61 and 173 days before pair-mating to
produce the F1a and F1b generations, respectively. Then 12
rats/sex of each dose level were pair-mated when about 100 days old
to produce the F2a generation. The offspring were weaned at 21
days. Food consumption was reduced in the F0 generation at
100 ppm. Thyroid hyperplasia was seen in all animals of the 100 ppm
group. In the 25 ppm group hyperplasia was seen in about half of
the F0 (4/10) and F1b (4/10) females and F1b (6/10) males, but
none of the F0 males (F1a litters not examined). Pups of the 100
ppm group showed reduced kidney and liver weights and also male pups
of the 25 ppm group showed reduced liver weights. There was a
decrease in the number of litters in the F2a generation at 100 ppm,
but there were no other changes. A NOAEL for toxic effects could
not be established. Due to the low number of animals used in this
study, a NOAEL for reproductive effects could not be established
(Gaines et al., 1973).
Groups of 5 rats/sex (strain unknown) were mated following a 3-
month pretreatment period during which only males, only females, or
both sexes were exposed to amitrole at a level of 100 ppm in their
drinking-water. The groups were compared with a common control
group. According to the author, the results did not point to a
reduction in reproductive ability for males or females. However,
due to limited data in the report this could not be confirmed. Pups
born in this study were reared and continued on the treatment.
Their growth was reduced (Hapke, 1967).
Special studies on embryotoxicity and/or teratogenicity
Mice
Pregnant NMRI mice (9-12/group) were administered amitrole
(purity not specified) at 0, 500, 1000, 2500 or 5000 ppm in
drinking-water (days 6-18 of pregnancy). There was a marked
decrease (22-28%) in body-weight gain in the dams and pronounced
retardation in the development (lower fetal weight and immature
skeletons) at concentrations of 1000 ppm and above. At the highest
concentration (5000 ppm), maternal toxicity was associated with an
increase in the rate of resorptions. No irreversible structural
changes were observed at any concentration (Tjälve, 1974).
Groups of 13 pregnant C57BL6 mice were treated subcutaneously
with 215 or 464 mg/kg bw/day from days 6 though 14 of gestation.
The highest dose tested resulted in an increased fetal mortality
rate. Subcutaneous treatment of 6 AKR mice from days 6-15 of
gestation at 464 mg/kg bw/day produced no effects in the fetuses.
Daily oral administration of 215 mg/kg bw to 7 C57BL6 mice from
days 6-14 of gestation produced increased fetal mortality and a
reduction of fetal weight. In nearly all groups maternal body
weight was reduced and liver weight increased. There were no
irreversible structural changes observed in any group (Bionetics
Research Laboratories, 1968).
Rats
In a limited teratogenicity study, groups of 8 pregnant female
Sherman rats were given amitrole (99%) by stomach tube at dose
levels of 0, 20 or 100 mg/kg bw/day during days 7 through 15 of
gestation. The animals were allowed to litter and to wean, and the
pups were observed for gross abnormalities. No abnormalities were
observed in the offspring at the time of birth or when weaned
(Gaines et al., 1973).
In a pilot study, 2 female rats (strain unknown) were orally
exposed to amitrole at a dosage of 1000 mg/kg bw/day and one rat to
500 mg/kg bw/day from days 8 through 13 of gestation. Weight gain
of the treated females was reduced. No effects were found on number
of corpora lutea, number of fetuses and no anomalies were observed
(Hapke, 1967).
In a teratogenicity study, groups of 20 presumed pregnant rats
(strain FB30, Long-Evans) were exposed to amitrole by gavage at dose
levels of 0, 100, 300, or 1000 mg/kg bw/day on days 6 to 15 of
gestation. Fetuses were examined on day 20 of gestation. There
were no deaths or signs of toxicity at any dose level. Body-weight
gain was not affected by treatment. There were no treatment-related
effects on the resorption rate, fetal weight, number of live
fetuses, placental weight, or sex ratio. There was no treatment-
related increase in gross, skeletal or visceral malformations. The
NOAEL for maternal and embryo/fetotoxicity was 1000 mg/kg bw/day
(Machemer, 1977b).
Groups of 24 presumed pregnant CD rats were exposed to amitrole
(91.83% pure) by gavage at dose levels of 0, 100, 500, or 1000 mg/kg
bw/day on days 6 through 15 of gestation. Apart from the normal
parameters measured in a teratogenicity study, the weights of liver
and thyroid of the dams were examined. Fetuses were examined on day
21 of gestation. Extra groups of 14 females per dose level were
allowed to litter and wean, and were maintained until postnatal day
21. In this postnatal period, body weight, food consumption and
thyroid weights were measured in the dams. Observations in the pups
included number, sex, weight and gross examination. Weight gain of
dams was reduced during gestation days 6-18 at 500 and 1000 mg/kg
bw/day. Food intake was reduced during gestation days 15-21 at the
same dose levels. Maternal thyroid weights (absolute and relative)
were increased at 500 and 1000 mg/kg bw/day, the increase still
persisting at termination of lactation. Fetotoxicity was observed
at 1000 mg/kg bw/day including reduced fetal body weight per litter,
increased incidence of fetuses with unossified or poorly ossified
bones and an increased number of fetuses with enlarged and/or dark
thyroid. These latter findings in the thyroid were also observed in
the 500 mg/kg bw/day dose group. There was no treatment-related
increased incidence of malformations at any dose employed.
Postnatal evaluations indicated no effects of treatment on survival
or growth of the pups, or on maternal body weights, weight gain or
food consumption in the lactation period. The NOAEL for
maternotoxicity and embryo/fetotoxicity was 100 mg/kg bw/day (Tyl,
1986a).
Rabbits
Groups of 22 presumed pregnant New Zeeland white rabbits were
exposed to amitrole (91.83% pure) by gavage at doses of 0, 4, 40, or
400 mg/kg bw/day on days 6 through 18 of gestation. Fetuses were
examined on day 29 of gestation. Maternotoxicity at 40 and 400
mg/kg bw/day included reduced body-weight gain and at 400 mg/kg
bw/day increased (relative) liver weight. A dose-related increase
in the incidence of abortions was observed (0, 1, 3 and 5 for the
control, 4, 40 and 400 mg/kg bw/day dose groups, respectively). The
number of non-viable implants/litter was increased and the
percentage of live fetuses/litter decreased at 40 and 400 mg/kg
bw/day (statistically significant at high dose). Decreased fetal
weight/litter was also seen at these dose levels, with statistical
significance at the high dose. At 40 and 400 mg/kg bw/day there
were significant increases in the incidence of numerous individual
malformations, especially of the head and limbs. The total
percentages of fetuses with malformations were 4.5, 7.2, 36.9 and
62.4% for the control, 4, 40 and 400 mg/kg bw/day dose groups,
respectively. The incidence of a number of individual visceral
(including craniofacial) and skeletal variants (mainly poorly or
absent ossification) was increased at 40 and 400 mg/kg bw/day.
Effects on the fetal thyroid were also observed. The percentages of
fetuses with enlarged thyroid were 1.3, 2.2, 3.6 and 8.2% and of
fetuses with dark/red thyroids were 2.6, 7.9, 8.1 and 12.9% for the
control, 4, 40 and 400 mg/kg bw/day dose groups, respectively.
Following oral administration amitrole was teratogenic,
embryo/fetotoxic and (slightly) maternotoxic at 40 and 400 mg/kg
bw/day. The NOAEL for all types of toxicity was 4 mg/kg bw/day
(Tyl, 1986b).
Four groups of 18 artificially inseminated Hra (NZW) SPF
rabbits were treated dermally from days 7 to 19 (inclusive) of
gestation with 0, 1000, 1500 or 2000 mg amitrole (93.9% purity)/kg
bw/day. The test material was applied as a mixture of 0.5 ml
deionized water/g amitrole for six hours/day. Rabbits were collared
during the exposure period, and remaining test material was removed
with warm water washing immediately following each exposure.
Clinical signs including dermal irritation, body weight and food
intake were measured in does. Dermal irritation, including erythema
and edema, was noted for treated animals. The number of animals
affected and the severity of these observations increased with
increasing dose. Numbers of pregnant females at term were 14, 15,
12 and 11. At 2000 mg/kg bw/day thin appearance and anorexia was
observed in does. Body weight was reduced on day 20, but was
virtually recovered by day 29; food intake was reduced on days 10-
14, with severe reductions on days 14-17 and 17-20 of gestation and
uterine weight was slightly reduced at term at 2000 mg/kg bw/day.
Fetal weights were reduced at 2000 mg/kg bw/day and the incidence of
total resorptions was increased significantly (mainly early
resorptions). Malformations, including microphthalmia (3 fetuses in
2 litters), dilated brain lateral ventricles (2 pups in 1 litter),
anencephaly (2 fetuses in 2 litters), unossified pubis (4 pups in 1
litter, but several other pups in the same litter with thyroid,
skeleton and rib anomalies) were observed at 2000 mg/kg bw/day.
Following dermal administration, amitrole was teratogenic,
embryo/fetotoxic and maternotoxic at 2000 mg/kg bw/day. The NOAEL
for all types of toxicity was 1500 mg/kg bw/day (Henwood, 1988).
Special studies on genotoxicity
A number of genotoxicity tests has been carried out with
amitrole. The results are summarized in Table 2. The important
features of these data are described below.
Gene mutations
Numerous assays for in vitro gene-mutations were performed
and were predominantly negative. One study with E. coli and S.
typhimurium strains gave positive responses (Venitt & Crofton-
Sleigh, 1981). Of two other bacterial mutation assays, one assay
gave a positive result in absence of metabolic activation and the
other gave an equivocal response. Many other in vitro assays gave
negative results. A positive response was obtained in one out of
two mouse peritoneal host mediated assays with S. typhimurium
(Simmon et al., 1979). No mutation induction was observed in
fungi and yeast. No mutation induction was observed in several sex-
linked recessive assays with Drosophila melangolaster. Mutations
(TK +/-) were not induced in mouse lymphoma cells, whereas HPRT
locus and Na+/K+ATPase locus mutations were induced in Syrian
hamster embryo cells (Tsutsui et al., 1984).
Chromosomal damage
Weakly positive results were obtained in tests with fungi. A
cytogenetic study in human lymphocytes was negative, but sister-
chromatid exchanges were increased in a single study. No effects of
amitrole were observed in mice subjected to bone marrow micronucleus
tests or male dominant lethal tests.
Table 2. Results of genotoxicity assays on amitrole
Tests for gene mutations
Test system Test object Concentration/ Purity Results References
dose (%)
reverse mut. S.typhimurium 100 µg/plate 99.4 negative Brusick, 1975
(*) TA1535, TA1537
TA1538
S.cerevisiae, D4 100 µg/plate 99.4 negative
reverse mut. S.typhimurium 0.4% 93.2 negative Bamford et al.
(-) LT 2 trp 1976
(A8, B4, C3)
reverse mut. S.typhimurium 1000 µg/plate ? negative Prince, 1977
(*) TA98, TA100
TA1538, TA1535
point mut. Aspergillus up to 2000 µg ? negative Bignami et al.
(-) nidulans 1977
strain 35
reverse mut. S.typhimurium 20-12 500 µg/ 97.6 negative Herbold, 1980
(*) TA98, TA100 plate
TA1535, TA1537
reverse mut. S.typhmurium 0.2-2000 µg/ ? negative Brooks &
(*) TA1535, TA1537 plate Dean, 1981
TA1538, TA98
TA100, TA92
Tests for gene mutations (contd)
Test system Test object Concentration/ Purity Results References
dose (%)
reverse mut. S.typhimurium up to 5000 µg/ ? negative McDonald,
(*) TA98, TA100 plate 1981
TA1537
reverse mut. S.typhimurium 10-10 000 µg/ ? negative Richold &
(*) TA1535, TA1537 plate Jones, 1981
TA1538, TA98
TA100
reverse mut. S.typhimurium 0.1-2000 µg/ ? negative Rowland &
(*) TA1535, TA1537 plate Severn, 1981
TA1538, TA98
TA100
reverse mut. S.typhimurium 4-2500 µg/ ? negative Trueman,
(+) TA1535, TA1537 plate 1981
TA1538, TA98
TA100
reverse mut. E.coli 0.5-500 µg/ml ? positive Venitt &
(+) WP2uvrA(p) Crofton-
S. typhimurium positive Sleigh, 1981
TA98, TA100
reverse mut. S.typhimurium up to 500 µg/ ? equivocal Hubbard et al.
(*) TA98, TA100 ml 1981
(fluctuation test)
Tests for gene mutations (contd)
Test system Test object Concentration/ Purity Results References
dose (%)
reverse mut. E. coli WP2uvrA 10-500 µg/ml ? negative Gatehouse,
1981
(*) S. typhimurium negative
(microtiter TA98, TA1535
fluctuation test) TA1537
reverse mut. S. cerevisiae 88.9 & 889 µg/ ? negative Mehta & Von
(*) XV185-14C ml Borstel, 1981
reverse mut. S. typhimurium up to 5000 µg/ ? negative Moriya et al.
(*) TA98, TA100 plate 1983
TA1535, TA1537
TA1538
E. coli WP2 hcr negative
forward mut. E. coli CHY832 ? Hayes et al.
+ S9-mix: 10 000 µg/ml negative 1984
- S9-mix 2500 µg/ml positive
reverse mut. S. typhimurium 0.3-333.3 µg/ 98 negative Dunkel et al.
(*) TA1535, TA1537 plate 1984
(comparative TA1538, TA98
study in 4 TA100
laboratories) E. coli 0.3-333.3 µg/ negative
WP-2 uvrA plate
HPRT mutation Syrian hamster 0.3-10 µg/ml ? positive Tsutsui et al.
assay (-) embryo cells 1984
Tests for gene mutations (contd)
Test system Test object Concentration/ Purity Results References
dose (%)
mutation Na+/K+ Syrian hamster 0.3-10 µg/ml ? positive Tsutsui et al.
ATPase locus (-) embryo cells 1984
TK +/- forward mouse lymphoma up to 5000 µg/ ? negative McGregor et
mutation assay(*) cells L5178Y ml al. 1987
host mediated S. typhimurium 1450-2900 µmol/ ? negative1 Braun et al.
assay host: NMRI-mice kg 1977
host mediated S. typhimurium 12-1585 mg/kg ? positive Simmon et al.
TA1530, TA1535 ip 1979
TA1538
host:
Swiss Webster mice
1: weakly positive when given simultaneously with sodium nitrite
Tests for chromosome effects
chromosome aberr. human 0.00001-1% 93.2- negative Meretoja et
assay (-) lymphocytes 96.3 al. 1976
crossing over Aspergillus up to 2000 µg ? positive1 Bignami et al.
test (-) nidulans 1977
non-disjunct. strain P positive1
test (-)
non-disjunct. Aspergillus up to 0.4 mg/ml ? positive Morpurgo et
test (-) nidulans al. 1979
strain P
Tests for chromosome effects (contd)
Test system Test object Concentration/ Purity Results References
dose (%)
sister Chinese 0.01-100 µg/ ? positive1 Perry &
chromatid hamster ovary ml (+S9) Thomson 1981
exchange(*) cells (CHO)
1: weakly positive
Tests for DNA damage/repair
rec assay (-) B. subtilis 20 µg/plate ? negative Shirasu et al.
H17 rec+, 1976
M45 rec-
rec assay (*) B. subtilis up to 1 mg/plate ? positive1 Kada, 1981
H17 rec+,
M45 rec-
rec assay (*) E. coli 500 µg/ml ? negative Ichinotsubo et
JC 2921, 9238 al. 1981
8471, 5519, 7623
7689
rec assay (+) E. coli WP2, 4000 µg/ml ? negative Mamber et al.
WP100 1983
pol A test(-) E. coli 5 mg/plate 93.2 negative Bamford et al.
pol A1, pol A+ 1976
pol A test(*) E. coli 333 µg/plate ? negative Rosenkranz et
WP3110, P3478 al. 1981
Tests for DNA damage/repair (contd)
Test system Test object Concentration/ Purity Results References
dose (%)
differential E. coli WP2, 250-1000 µg/ ? negative Tweats, 1981
killing assay(*) WP67, CM871 ml
mitotic S. cerevisiae 100 & 1000 µg/ ? negative Kassinova et
recombination(*) T1, T2 ml al. 1981
DNA repair S. cerevisiae 100-750 µg/ ? positive Sharp &
test(*) 197/2d, rad3, ml (-S9) Parry, 1981b
rad18, rad52,
trp2
UDS test(*) HeLa cells 0.1-100 µg/ml ? positive Martin &
(+S9) McDermid, 1981
prophage induct. E. coli 58-161 1-10 mg/ml ? negative Thomson,
assay(+) Prophage lambda 1981
prophage induct. E. coli 2000 µg/plate ? negative Mamber et al.
assay(+) GY5027, GY4015 1984
mitotic crossing S. cerevisiae 100 & 1000 µg/ ? negative Kassinova et
over(*) T1, T2 ml al. 1981
mitotic gene S. cerevisiae 12.5 mg/ml ? negative Zimmermann
conversion(*) D7 & Scheel, 1981
mitotic gene S. cerevisiae 300 µg/ml ? positive Sharp & Parry
conversion(*) JD1 (-S9) 1981a
1: positive results were obtained only after metabolic activation with liver homogenates from
Japanese clam and Yellowtail fish
Tests for in vitro transformation
Test system Test object Concentration/ Purity Results References
dose (%)
Tests for in vitro transformation
cell transf. BALB/3T3 cells 0.01-2.5 mg/ml ? equivocal1 Brusick, 1976
assay(-)
cell transf. BHK-21 cells 0.025-25 µg/ml ? positive Styles, 1979
assay(+)
cell transf. BHK-21 cells 0.025-250 µg/ml ? positive Styles, 1981
assay(+)
cell transf. BHK-21 cells 4000 µg/ml ? negative Daniel &
assay(*) Dehnel, 1981
cell transf. hamster embryo 1-100 µg/ml ? positive Inoue et al.
assay(+) cells 1981
cell transf. hamster embryo 0.1-100 µg/ml ? positive Dunkel et al.
assay(-) cells 1981
rat embryo 10-1200 µg/ml ? positive
cells
cell transf. Syrian hamster 0.3-10 µg/ml ? positive Tsutsui et al.
assay(-) embryo cells 1984
Tests for in vitro transformation
Test system Test object Concentration/ Purity Results References
dose (%)
cell transf. mouse embryo ? Dunkel et al.
assay(-) cells 1984
C3H/10T1/2
laboratory A: 2-250 µg/ml negative
laboratory B: 125-1000 positive
µg/ml
1: positive only in 1 out of 3 tests
In vivo tests
non-disjunct. Drosophila 10 ppm in diet 93.2% negative Laamanen et
test females al. 1976
recessive Drosophila 10 ppm in diet 93.2% negative Laamanen et
lethal test males al. 1976
recessive Drosophila 2000 ppm in diet ? negative Vogel et al.
lethal test males 1981
dominant lethal NMRI-mice 1 x 1000 mg/kg ? negative Machemer,
test males 1977a
dominant lethal Ha(ICR)-mice 1 or 10 ppm in 94.59 negative Knickerbocker,
test males the diet for 1978
49 days
micronucleus CD-1 mice 2 x 500 mg/kg ? negative Tsuchimoto &
test males + females Matter, 1981
In vivo tests (contd)
Test system Test object Concentration/ Purity Results References
dose (%)
micronucleus NMRI-mice 1 x 10 000 mg/kg 96.9 negative Herbold, 1982
test males + females
sperm morphol. (CBA x BALB/c)F1 50-500 mg/kg/day ? negative Topham, 1980
assay 5 ip-injections
Tests were done: (*) = with and without metabolic activation (S9-mix)
(+) = with S9-mix activation
(-) = without S9-mix
DNA damage and repair
The possibility of inducing DNA damage by amitrole has been
investigated frequently and in a number of different ways. In
bacteria the results have been negative except for one recombinant
assay, which was positive when an exogenous metabolic activation was
provided by "liver" preparations from a mollusc and a fish. A DNA
repair assay in yeast gave a positive result as did a repair assay
in mammalian cells.
Cell transformation
Assays for cell transformation in several systems gave
predominantly positive results.
Other endpoints
Amitrole did not increase the frequency of morphologically
abnormal sperm in mice.
Amitrole has therefore been tested adequately in series of in
vitro and in vivo genotoxicity assays. Positive responses were
obtained in a number of mutation assays in bacteria,
recombinogenicity assays in yeast and some mammalian cell assays for
mutation, sister-chromatid exchange and cell transformation. No
genotoxicity was demonstrated in vivo. The Meeting concluded that
the genotoxic potential of amitrole was equivocal.
Special studies on skin and eye irritation and skin sensitization
In a limited study, the potential for dermal irritation by
amitrole (95%) was examined in 4 albino rabbits (strain and sex not
specified) over a 24-hour period following a single application of
dosages from 1000 up to 10 000 mg/kg bw. Minimal dermal irritation
was observed with mild erythema at the high-dose level only. By 48
hours, the skin appeared normal (Elsea, 1954).
In a limited study, the potential for eye irritation by
amitrole (95%) was examined in 3 albino rabbits (strain and sex not
specified) following application of 3 mg into the conjunctival sac
of the left eye. Observations were made at 1, 4 and 24 hours and at
daily intervals for 6 days. Mild irritation was observed at 4
hours, which subsided within 24 hours (Elsea, 1954).
In a Magnusson-Kligman maximization test in Pirbright White
guinea-pigs, amitrole (97.6%) was found to be a moderate skin
sensitizer. Concentrations employed were 2.5% for intracutaneous
induction, 25% for topical induction, and 12% for the first and
second challenges (Mihail, 1984).
In a Klecak open epicutaneous test in BOR:DHPW/SPF guinea-pigs,
amitrole (97.3%) did not have a sensitizing effect. Concentrations
employed were 0, 3, 10, or 30% for induction, and 1, 3, 10, or 30%
for the first and second challenges (Mihail, 1985).
Special studies on farm animals
Cattle tolerated administration of both a single oral dose of
amitrole at 1000 mg/kg bw as well as repeated 100 mg/kg bw doses on
10 consecutive days without symptoms (Stendel, 1965).
Female sheep (1/dose level) were orally treated with amitrole
at doses of 0, 750, or 1000 mg/kg bw at intervals of 5-7 days over a
period of 8 weeks. The only symptom observed was lethargy
immediately following treatment. Three days after the last dose the
animals were slaughtered. Residues were found in muscular tissues
at levels of 80 ± 30 ppm and 120 ± 40 ppm in the low- and high-dose
respectively (semi-quantitative determination). The thyroids were
grey-red discoloured. At histopathology complete cessation of
colloid formation, with adenomatoid proliferation of the follicle
epithelia was observed (Hapke et al., 1965).
In sheep, a single oral dose of 750 mg/kg bw led to clinical
symptoms (lethargic behaviour, frequent lying down). At higher
doses, the animals became apathetic and did not eat. At a dose
level of 4000 mg/kg bw the animals died. A single oral dose of 200
mg/kg bw was tolerated by a horse without any symptoms. Adult geese
tolerated oral doses up to 1000 mg/kg bw. At higher doses, these
birds became somnolent. Mortality occurred at 10 000 mg/kg bw
(Hapke, 1967).
Young cattle and sheep were orally exposed to amitrole at
dosages of 10, 25 or 50 mg/kg bw for up to 10 consecutive days. The
10 mg/kg bw dose was tolerated without symptoms by the cattle.
Doses of 25 or 50 mg/kg bw led to toxic symptoms after 3 and 2 days,
respectively. One out of three sheep exhibited retarded body-weight
development at 10 mg/kg bw, whereas at the higher doses all sheep
showed toxic symptoms. Hens were treated orally at dosages of 50,
100 or 250 mg/kg bw on 10 consecutive days. Retarded body-weight
development was observed at 100 and 250 mg/kg bw. Hens dosed at 375
or 500 mg/kg bw died within 3-7 days after the start of the study
(Palmer, 1972).
Observations in humans
In a patch test conducted with a human volunteer, amitrole
exerted no primary dermal irritant effect after exposure periods of
4 or 8 hours. A slight irritant effect was observed in 3 out of 6
subjects after 24 hours of exposure (no further details) (Hecht,
1954).
A case study of a 41-year old weed control operator with a 6-
month history of dermatitis involving his face, hands, back, thighs,
and feet was reported. Patch testing with 1% amitrole showed a
strong positive vesicular reaction at 2 and 4 days, indicative of
allergic contact dermatitis (English et al., 1986).
The intentional ingestion of a commercial mixture of amitrole
and diuron, at a dose equivalent to 20 mg/kg bw amitrole (together
with about 38 mg/kg bw diuron), was reported to have caused no
symptoms of poisoning in a female subject. Within a few hours, the
compound appeared in the urine at a concentration of 100 mg/100 ml.
Metabolites could not be detected in urine (Geldmacher-von
Mallinckrodt & Schmidt, 1970).
Astwood (1960) reported in a brief communication that a single
oral dose of 100 mg amitrole inhibited radioiodine uptake by the
thyroid of both normal and thyrotoxic subjects for 24 hours.
However, a dose of 10 mg was said to have had a slight effect on
iodine uptake. Astwood suggested that amitrole could be used
therapeutically in the treatment of hyperthyroidism.
An epidemiological study was conducted on Swedish railway
workers exposed to various herbicides. A small increase in tumours
was observed, particularly of the lung. Amitrole was only one of
the pesticides (other pesticides were for example phenoxy acids) to
which these workers were exposed (Axelson, 1980).
Mild cases of dermatitis on the face due to contact with
amitrole occurred yearly in one or two production workers of the
American Cyanamid Company. The dermatitis was of a primary irritant
type rather than the sensitive type. No other medical findings, not
occurring in the general public, and, in particular, no thyroid or
liver tumours were observed during the years of observation
(1955-1970) (Clyne, 1970).
Skin irritation was also occasionally observed in employees of
Bayer engaged in production of amitrole. No other adverse effects
occurred (Miksche, 1982).
The thyroid function in 5 employees who had been engaged in
production and packaging of amitrole for periods between 3 and 16
years was checked as part of the occupational medical programme.
Scintigraphic thyroid imaging findings and determinations of the T3
and T4 levels afforded no evidence for the presence of an effect on
thyroid function (Miksche, 1983).
In a study for a possible phototoxic potential of amitrole, 20
test subjects were exposed to patches containing amitrole at a level
of 1% in an ointment base (hydrous eucerin) over a period of 48
hours. The treatment areas were then irradiated with ultraviolet
light (UVA and UVB), and the dermal reactions assessed (no further
details). No evidence was found for phototoxic skin reactions
(Tronnier, 1983).
General mode of action
Amitrole is considered a goitrogenic compound. The mechanisms
by which goitrogens produce their pharmacologic and potential
neoplastic effects is through the induction of hormonal imbalance.
Most commonly this is the result of interference with the thyroid
iodide transport system or interference with peroxidases essential
to the synthesis and secretion of competent thyroid hormone. The
sequence of events triggered by this interference is generally
understood. Because of the ability of goitrogens to inhibit thyroid
hormone synthesis, these compounds have the potential to reduce
circulating levels of T3 and T4 and, consequently, to induce the
secretion of TSH by the pituitary. As a result, prolonged exposure
to such compounds can be expected to induce thyroid gland
hypertrophy and hyperplasia, nodular hyperplasia and, ultimately,
may lead to neoplasia. Evidence indicates that if the pharmacologic
effects, thyroid hypertrophy and hyperplasia, are prevented or
controlled within the feedback system, follicular cell neoplasia
does not develop. The data also suggest that some degree of
hypertrophy or hyperplasia of the thyroid gland is tolerated within
the oscillation of the feedback system without induction of
neoplasia. The rat, and to a lesser extent the mouse, appear to be
very sensitive to goitrogens, since after short exposure periods
their thyroid glands exhibit hypertrophic and hyperplastic changes.
Following continuous, long-term exposure of rats and mice to these
agents, both the thyroid and the pituitary glands frequently exhibit
neoplastic changes. In contrast, humans appear to be less sensitive
(Paynter et al., 1988; Hill et al., 1989).
COMMENTS
Amitrole is rapidly and almost completely absorbed from the
gastrointestinal tract following oral administration to rats and
mice. It is rapidly distributed throughout most body tissues, but
with a slight accumulation in tissues with a rapid cell turnover
(bone marrow, spleen, thymus, gastrointestinal tract). In a study
with pregnant mice it was observed that amitrole passes through the
placenta into the fetuses with the same distribution pattern as in
the mothers. Excretion is rapid after oral exposure. Within 24
hours, 70-95% of the administered radioactivity is excreted via the
urine, mainly as the parent compound.
The metabolic transformation in mammals produces two minor
metabolites detectable in the urine. Metabolism of amitrole occurs
mainly in the liver and involves substitution of the hydrogen atom
in the 5-position. The metabolites identified were 3-amino-5-
mercapto-1,2,4-triazole and 3-amino-1,2,4-triazolyl-5-mercapturic
acid.
Amitrole has a low acute toxicity when tested in several
species by various routes of administration. In old studies,
amitrole was reported to have slight irritating effects on the skin
and eyes. Evidence of a moderate sensitizing potential was observed
in a Magnusson-Kligman test but not in a Klecak open epicutaneous
test. WHO has classified amitrole as unlikely to present acute
hazard in normal use.
Oral exposures of up to four weeks in rats revealed that
effects on the thyroid occurred at levels > 60 ppm in the diet or
104 ppm in drinking-water. No effects were observed at 30 ppm in
the diet (equivalent to 3 mg/kg bw/day) or 10 ppm in drinking-water
(equivalent to 1.5 mg/kg bw/day). Furthermore, it was shown that
after a recovery period the effects on the thyroid were reversible.
In a 30-day study in mice at concentrations in drinking-water
of 0, 5000, 10 000 or 20 000 mg/l, liver effects were observed at
all dose levels.
Several short-term oral studies were performed with rats.
These were mainly focused on the effects on the thyroid, as this is
the target organ in rats.
Only two oral studies were suitable for assessment. In one
study, male rats were exposed to dietary concentrations of 0, 2, 10
or 50 ppm for 13 weeks or to 0, 0.25 or 0.50 ppm for 11 weeks. The
NOAEL was 2 ppm (equivalent to 0.1 mg/kg bw/day) based on
histological changes in the thyroid (appearance of follicular cells,
contents of colloid and capillary density). Decreases in protein-
bound iodine were not considered to be biologically significant.
The other study consisted of four short-term experiments in
female rats. Dietary concentrations in one experiment were 0, 2, 20
or 200 ppm with exposure for six weeks, in two subsequent
experiments 0, 20, 50 or 200 ppm with exposure for 6 or 13 weeks and
in the fourth experiment 0, 20, 50, 200 or 500 ppm with exposure for
six weeks. From these four experiments, the overall NOAEL was 2 ppm
(equivalent to 0.1 mg/kg bw/day) based on increased iodine uptake
(shortly after injection), increased thyroid weight and
histopathological changes of the thyroid (goitre and clearly
activated thyroids).
From several short-term studies in rats with administration in
drinking-water, slight effects on the thyroid (moderate stimulation
of the thyroid epithelium) were seen at the lowest concentration
tested, 50 ppm.
In a one-year study in dogs, no effects on the thyroid were
observed at the highest dose tested (12.5 mg/kg bw/day). The only
effect observed at this level was pale-coloured pancreas. The test,
however, was performed with a low number of animals.
Long-term toxicity and/or carcinogenicity studies have been
performed in mice, rats, and golden hamsters. Studies in mice were
focused on induction of liver and thyroid tumours. In a
carcinogenicity study in mice with only one but very high dose level
(1000 mg/kg bw/day by gavage), survival time was significantly
reduced and liver and thyroid tumours were observed in all treated
mice. A slight increase in the incidence of liver tumours was
observed in a special carcinogenicity study in which pups were
treated for a period of 90 weeks at a level of 500 ppm in the diet.
In an 18-month carcinogenicity study in mice at levels of 0, 1,
10 or 100 ppm in the diet, an increased incidence of tumours was not
observed. In this study, a thyroid function test was also performed
with a small number of animals. At 100 ppm an increase in thyroid
weight and in iodine accumulation in the thyroid was observed. The
NOAEL was 10 ppm (equivalent to 1.5 mg/kg bw/day).
In a carcinogenicity study in rats with levels of 0, 1, 10 or
100 ppm in the diet, a slight decrease in survival time, an increase
in the incidence of thyroid tumours and an increase in the incidence
of (mainly benign) pituitary tumours were observed at 100 ppm. In
this study, a thyroid function test was also performed with a small
number of animals. At 100 ppm, thyroid weight was increased during
the whole study period as was the percentage accumulation of
radioiodine in the thyroid. The NOAEL was 10 ppm (equivalent to 0.5
mg/kg bw/day).
In another limited long-term toxicity study in rats, the NOAEL
was 10 ppm (equivalent to 0.5 mg/kg bw/day) based on thyroid
hyperplasia. A clearly enhanced thyroid tumour incidence was found
at 50 and 100 ppm. In this study, animals suffered from apparent
respiratory infection.
In a third study in rats, thyroid hyperplasia and thyroid
tumours were observed in animals fed 100 ppm (during the first 40
weeks of the 115-120 week study, the dose level was 5 ppm). In rats
treated at pulsed intervals (alternate four week periods) at levels
of 60 ppm (first 3 ppm) and 200 ppm (first 10 ppm) thyroid tumours
were also observed. Slight thyroid hyperplasia was also observed at
the lowest dose level of 20 ppm (first 1 ppm; intermittent dosing
regimen). A NOAEL could not be established.
In an 18-month carcinogenicity study in Syrian hamsters at
dietary concentrations of 0, 1, 10 or 100 ppm, the NOAEL was 10 ppm,
equivalent to 1 mg/kg bw/day, based on decreased body-weight gain
and increased mortality. No effects on the thyroid were observed at
100 ppm. There was no evidence of carcinogenic potential.
A well-performed reproduction study was not available. From a
limited study in rats at dietary concentrations ranging from 25 to
1000 ppm, effects on reproductive capability were observed at 500
ppm and above. Reduction of liver weight and thyroid hyperplasia
were the most sensitive effects observed at the lowest dose level
(25 ppm, equivalent to 1.3 mg/kg bw/day).
In a teratogenicity study, rats were exposed by gavage at doses
of 0, 100, 300 or 1000 mg/kg bw/day on days 6 to 15 of gestation.
No effects were observed in this study. The NOAEL for
maternotoxicity and embryo/ fetotoxicity was 1000 mg/kg bw/day.
In another teratogenicity study, rats were exposed by gavage at
doses of 0, 100, 500 or 1000 mg/kg bw/day. Slight maternal toxicity
(reduced weight gain and food consumption and increased thyroid
weights) was observed at doses of 500 and 1000 mg/kg bw/day.
Reduced fetal body weight/litter and reduced skeletal ossifications
were observed in the high-dose group. Increased incidences of
enlarged and/or dark thyroids were seen in fetuses at 500 and 1000
mg/kg bw/day. The NOAEL for maternotoxicity and embryo/fetotoxicity
was 100 mg/kg bw/day. Amitrole was considered not to be teratogenic
in rats at dose levels up to 1000 mg/kg bw/day.
In a teratogenicity study in rabbits the animals were exposed
by gavage to dose levels of 0, 4, 40 or 400 mg/kg bw/day. Decreased
weight gain during the gestation period was observed at 40 and 400
mg/kg bw/day and increased liver weight at 400 mg/kg bw/day. A
dose-related increased incidence of abortions was observed in all
treated groups. Embryo/fetotoxicity were observed at 40 and 400
mg/kg bw/day. Increased incidences of irreversible structural
changes were also found at these dose levels, which involved mainly
the head and limbs. The NOAEL for maternotoxicity,
embryo/fetotoxicity and teratogenicity was 4 mg/kg bw/day.
In a dermal teratogenicity study in rabbits at dose levels of
0, 1000, 1500 or 2000 mg/kg bw/day, maternotoxicity (decreased body
weight and food consumption, thin appearance and anorexia) was
observed at 2000 mg/kg bw/day. At this level, irreversible
structural changes (anencephaly and microphthalmia) were observed.
The NOAEL for maternotoxicity, embryo/fetotoxicity and
teratogenicity after dermal exposure was 1500 mg/kg bw/day.
Amitrole has been tested adequately in series of in vitro and
in vivo genotoxicity assays. Positive responses were obtained in
a number of mutation assays in bacteria, recombinogenicity assays in
yeast and some mammalian cell assays for mutation, sister-chromatid
exchange and cell transformation. No genotoxicity was demonstrated
in vivo. The Meeting concluded that the genotoxic potential of
amitrole was equivocal.
Amitrole is a goitrogen in mice, rats and sheep but not in
Syrian hamsters, dogs, or cattle at the doses that have been tested.
The mechanism of thyroid toxicity involves inhibition of thyroid
peroxidase. This inhibition results in decreases in circulating
levels of T4 and T3 which stimulate the pituitary to increase
secretion of TSH which in turn may cause thyroid hypertrophy,
hyperplasia and neoplasia. Threshold doses have been identified in
the sensitive species. Amitrole is not genotoxic in in vivo
assays.
The Meeting withdrew the conditional ADI and established a
temporary ADI, based on the NOAEL of 0.5 mg/kg bw/day in the 24-
month dietary study in rats, using a safety factor of 1000 because
of inadequacy of the data.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Mouse: 10 ppm, equivalent to 1.5 mg/kg bw/day
(18-month study)
Rat: 10 ppm, equivalent to 0.5 mg/kg bw/day
(24-month study)
100 mg/kg bw/day (teratogenicity study)
Hamster: 10 ppm, equivalent to 1.0 mg/kg bw/day
(18-month study)
Dog 12.5 mg/kg bw/day (12 month study)
Estimate of temporary acceptable daily intake for humans
0-0.0005 mg/kg bw
Studies without which the determination of a full ADI is
impracticable
Results be submitted to WHO by 1996
(all known to have been initiated):
Two-generation reproduction study in rats
One-year study in dogs
Oral teratogenicity study in rabbits
Metabolism study in rats
Studies which will provide information valuable in the continued
evaluation of the compound
Further observations in humans.
Comparative biotransformation (including humans).
Clarification of genotoxic potential of amitrole.
REFERENCES
Alexander, N.M. (1959). Antithyroid action of 3-amino-1,2,4-
triazole. J. Biol. Chem. 234: 148-150.
Astwood, E.B. (1960). Cranberries, turnips and goiter. J. American
Med. Assoc. 172: 1319-1320.
Axelson, O., Sundell, L., Andersson, K., Edling, C., Hogstedt, C.,
Kling, H. (1980). Herbicide exposure and tumor mortality. An updated
epidemiologic investigation on Swedish railroad workers. Scan. J.
Work Environ. Health, 6: 73-79.
Babish, J.G. (1977). Triiodothyronine (T3) and Thyroxine (T4)
levels in male rats consuming Amitrole (3-amino-1,2,4-triazole) in
their diets for four weeks followed by a four week recovery period.
Unpublished report No 5539 (dated: 29-11-1977) from Food and Drug
Research Laboratories, Inc.. Submitted to WHO by Bayer AG.
Bagdon, R.E., Shaffer, C.B., Vidone, L.B., Golz, H.H. (1956).
Aminotriazole - acute and subacute toxicity. Unpublished report No
56-29 (dated: 14-12-1956) from American Cyanamid Company. Submitted
to WHO by Bayer AG.
Bamford, D., Sorsa, M., Gripenberg, U., Laamanen, I., Meretoja, T.
(1976). Mutagenicity and toxicity of amitrole. III. Microbial tests.
Mutation Research, 40: 197-202.
Becci, P. (1983). Evaluation of the chronic inhalation toxicity and
carcinogenicity of amitrole in rats. Unpublished report No 5821
(dated: 13-01-1983) from Food and Drug Research Laboratories, Inc..
Submitted to WHO by Bayer AG.
Ben-Dyke, R., Perry, M.C., Wall, I. (1973). The short-term
cumulative toxicity of aminotriazole in the rat. Unpublished report
Tox/73/39-6 (dated: September 1973) from Fisons Limited Agrochemical
division, Toxicology department. Submitted to WHO by Bayer AG.
Bignami, M., Aulicino, F., Velcich, A., Carere, A., Morpurgo, G.
(1977). Mutagenic and recombinogenic action of pesticides in
Aspergillus nidulans. Mutation Research, 46: 395-402.
Bionetics Research Laboratories (1968). Evaluation of carcinogenic,
teratogenic, and mutagenic activities of selected pesticides and
industrial chemicals. Volume II: Teratogenic study in mice and rats.
Report PB-223 160 (dated: August 1968) from Bionetic Research Labs,
Inc., prepared for National Cancer Institute.
Braun, R., Schöneich, J., Ziebarth, D. (1977). In vivo formation
of N-nitroso compounds and detection of their mutagenic activity in
the host-mediated assay. Cancer Research, 37: 4572-4579.
Brooks, T.M. & Dean, B.J. (1981). Mutagenic activity of 42 coded
compounds in the salmonella/microsome assay with preincubation.
Progress in Mutation Research, 1: 261-270.
Brusick, D.J. (1975). Mutagenic evaluation of compound 3-amino-
1,2,4-triazole. Unpublished report No 2547 (dated: 14-08-1975) from
Litton Bionetics, Inc. Submitted to WHO by Bayer AG.
Brusick, D.J. (1976). Mutagenicity evaluation of 3-amino-1,2,4-
triazole; in vitro cellular transformation in BALB/3T3 cells.
Unpublished report No 2549 (dated: 26-10-1976) from Litton
Bionetics, Inc. Submitted to WHO by Bayer AG.
Clyne, R.M. (1970). Statement before the amitrole advisory
committee. Unpublished document from the American Cyanamid Company
dd 16 December 1970. Submitted to WHO by Bayer AG.
Cox, G.E. & Re, T.A. (1978). Inhalation toxicity study with 3-amino-
1,2,4-triazole (Amitrole) in adult Fisher 344 rats. Unpublished
report No 5492 (dated: 08-09-1978) from Food and Drug Research
Laboratories, Inc., Submitted to WHO by Bayer AG.
Daniel, M.R. & Dehnel, J.M. (1981). Cell transformation test with
baby hamster kidney cells. Progress in Mutation Research, 1: 626-
637.
Den Tonkelaar, E.M. & Kroes, R. (1974). Schildklierfunctieonderzoek
na subacute en semichronische toediening van aminotriazol.
Unpublished report No. 64/74 Tox. from the National Institute of
Public Health, Bilthoven, the Netherlands.
Dunkel, V.C., Pienta, R.J., Sivak, A., Traul, K.A. (1981).
Comparative neoplastic transformation responses of Balb/3T3 cells,
Syrian hamster embryo cells, and Rauscher murine leukemia virus-
infected Fischer 344 rat embryo cells to chemical compounds. J.
Nat. Cancer Inst. 67: 1303-1315.
Dunkel, V.C., Zeiger, E., Brusick, D., McCoy, E., McGregor, D.,
Mortelmans, K., Rosenkranz, H.S., Simmon, V.F. (1984).
Reproducibility of microbial mutagenicity assays: I. Tests with
Salmonella typhimurium and Escherichia coli using a standardized
protocol. Environmental Mutagenesis, 6: 1-25.
Dunkel, V.C., Schechtman L.M., Tu, A.S., Sivak, A., Lubet, R.A.,
Cameron, T.P. (1988). Interlaboratory evaluation of the C3H/10T1/2
cell transformation assay. Env. Mol. Mutagen. 12: 21-31.
Elsea, J.R. (1954). Amino Triazole. Acute dermal application - acute
eye application. Unpublished report from Hazleton Laboratories,
Virginia (dated: 23-12-1954). Submitted to WHO by Bayer AG.
English, J.S., Rycroft, R.J., Calnan, C.D. (1986). Allergic contact
dermatitis from aminotriazole. Contact Dermatitis, 14: 255-256.
Fang, S.C., George, M., Yu, T.C. (1964). Metabolism of 3-amino-
1,2,4-triazole-5-C14 by rats. J. Agric. Food Chem. 12: 219-223.
Fang, S.C., Khanna, S., Rao, A.V. (1966). Further study on the
metabolism of labelled 3-amino-1,2,4-triazole (ATA) and its plant
metabolites in rats. J. Agric. Food Chem. 14: 262-265.
Feinstein, R.N., Fry, R.J.M, Staffeldt, E.F. (1978). Carcinogenic
and Antitumor effects of aminotriazole on acatalasemic and normal
catalase mice. J. Natl. Cancer Inst. 60: 1113-1116.
Fogleman, R.W. (1954). Amizole: Acute administration -
pharmacodynamics - subacute feeding. Unpublished report from
Hazleton Laboratories, Virginia (dated: 02-12-1954). Submitted to
WHO by Bayer AG.
Franco, L., Municio, A.M. (1975). Comparative metabolism of 3-amino-
1,2,4-triazole. Gen. Pharmac. 6: 163-169.
Fregly, M.J. (1968). Effect of aminotriazole and thyroid function in
the rat. Toxicol. Appl. Pharmacol. 13: 271-286.
Fujii, T., Miyazaki, H., Hashimoto, M. (1984). Autoradiographic and
biochemical studies of drug distribution in the liver. III. (14C)
Aminotriazole. Eur. J. Drug Metab. Pharmacokinet. 9: 257-265.
Gaines, T.B., Kimbrough, R.D., Linder, R.E. (1973). The toxicity of
amitrole in the rat. Toxicol. Appl. Pharmacol. 26: 118-129.
Gatehouse, D. (1981). Mutagenic activity of 42 coded compounds in
the microtiter fluctuation assay. Progress in Mutation Research,
1: 376-386.
Geldmacher-von Mallinckrodt, M. & Schmidt, H.P. (1970). Toxicity and
metabolism of aminotriazole in man. Arch. Toxikol. 27: 13-18.
Grunow, W., Altmann, H.-J., Böhme, Chr. (1975). Metabolism of 3-
amino-1,2,4-triazole in rats. Arch. Toxicol. 34: 315-324.
Hapke, H.-J., Rüssel, H., Ueberschär, S. (1965). Dangers in the use
of aminotriazol. Dtsch. Tierärztl. Wochenschrift, 72: 204-206.
Hapke, H.-J. (1967). The toxicity of aminotriazole for domestic
animals. Zbl. Vet. Med. 14: 469-486.
Hayes, S., Gordon, A., Sadowski, I., Hayes, C. (1984). RK bacterial
test for independently measuring chemical toxicity and mutagenicity:
Short-term forward selection assay. Mutation Research, 130: 97-
106.
Hecht (1954). Aminotriazol - Letter report of May 11, 1954 from
Bayer AG Institute of Toxicology. Submitted to WHO by Bayer AG.
Hecht & Kimmerle (1962). Aminotriazol - Letter report of August 23,
1962 from Bayer AG Institute of Toxicology. Submitted to WHO by
Bayer AG.
Heimann, K.G. (1982). Aminotriazol techn. - Letter report of April
7, 1982 from Bayer AG Institute of Toxicology. Submitted to WHO by
Bayer AG.
Henwood, S.M. (1988). Percutaneous teratology study with amitrole
technical in rabbits. Unpublished report on number HLA 6224-107
from Hazelton Laboratories America, Inc., submitted to WHO by Rhône-
Poulenc Ag. Company
Herbold, B. (1980). Amitrole; Salmonella/microsome test for
detection of point-mutagenic effects. Unpublished report No 9339
(dated: 01-08-1980) from Bayer AG Institute of Toxicology. Submitted
to WHO by Bayer AG.
Herbold, B. (1982). Amitrole; Micronucleus test on the mouse to
evaluate for mutagenic effect. Unpublished report No 11139 (dated:
13-09-1982) from Bayer AG Institute of Toxicology. Submitted to WHO
by Bayer AG.
Hill, R.N., Erdreich, L.S., Paynter, O.E., Roberts, P.A., Rosenthal,
S.L., Wilkinson, C.F. (1989). Thyroid follicular cell
carcinogenesis. Fund. Appl. Toxicol. 12: 629-697.
Hubbard, S.A., Green, M.H.L., Bridges, B.A., Wain, A.J., Bridges,
J.W. (1981). Fluctuation test with S9 and hepatocyte activation.
Progress in Mutation Research, 1: 361-370.
Ichinotsubo, D., Mower, H., Mandel, M. (1981). Testing of a series
of paired compounds (carcinogen and non-carcinogenic structural
analog) by DNA repair-deficient E. coli strains. Progress in
Mutation Research, 1: 195-198.
Innes, J.R., Ulland, B.M., Valerio, M.G., Petrucelli, L., Fishbein,
L., Hart, E.R., Palotta, A.J. (1969). Bioassay of pesticides and
industrial chemicals for tumorigenicity in mice: A preliminary note.
J. Nat. Cancer Inst. 42: 1101-1114.
Inoue, K., Katoh, I., Takayama, S. (1981). In vitro transformation
of hamster embryo cells by 3-(N-salicycloyl)amino-1,2,4-triazole.
Toxicol. Letters, 7: 211-215.
Johnson, W.D. (1981). Lifetime feeding study of amitrole in Fischer
344 rats. Unpublished report No 5651 (dated: 07-08-1981) from Food
and Drug Research Laboratories, Inc. Submitted to WHO by Bayer AG.
Jukes, T.H & Shafer, C.B. (1960). Antithyroid effects of
aminotriazole. Science, 132: 296-297.
Kada, T. (1981). The DNA-damaging activity of 42 coded compounds in
the rec-assay. Progress in Mutation Research, 1: 171-182.
Kassinova, G.V., Kovaltzova, S.V., Marfin, S.V., Zakharov, I.A.
(1981). Activity of 40 coded compounds in differential inhibition
and mitotic crossing-over assays in yeast. Progress in Mutation
Research, 1: 434-455.
Keller, J. (1959). Aminotriazole: Two-year chronic feeding - rats.
Unpublished report from Hazleton Laboratories Virginia (dated: 02-
01-1959). Submitted to WHO by Bayer AG.
Kimmerle (1968). Aminotriazol - Letter report of June 12, 1968 from
Bayer AG Institute of Toxicology. Submitted to WHO by Bayer AG.
Knickerbocker, M. (1978). A study to determine the potential of
amitrole to induce dominant lethal mutations in Ha (ICR) mice.
Unpublished report no 5502 (dated: 28-02-1978) from Food and Drug
Research Laboratories, Inc. Submitted to WHO by Bayer AG.
Laamanen, I., Sorsa, M., Bamford, D., Gripenberg, U. Meretoja, T.
(1976). Mutagenicity and toxicity of amitrole. I. Drosophila
tests. Mutation Research, 40: 185-190.
Lemoine, N.R., Mayall, E.S., Williams, E.D., Thurston, V., Wynford-
Thomas, D. (1988). Agent-specific ras oncogene activation in rat
thyroid tumours. Oncogene, 3: 541-544.
MacDonald, C.M., Pullinger, D.H. (1976). A pharmacokinetic study to
compare "head only" and "whole body" inhalation methods of 14C-
amitrole exposure. Unpublished report No 291/1 (dated: March 1976)
from Hazleton Laboratories Europe Ltd. Submitted to WHO by Bayer AG.
Machemer, L. (1977a). Amitrole/Dominant lethal test on the male
mouse to evaluate for mutagenic effects. Unpublished report no 6679
(dated: 23-03-1977) from Bayer AG, Institute of Toxicology.
Submitted to WHO by Bayer AG.
Machemer, L. (1977b). Amitrole; Study for embryotoxic and
teratogenic effects after oral administration to the rat.
Unpublished report No 6634 (dated: 24-02-77) from Bayer AG Institute
of Toxicology. Submitted to WHO by Bayer AG.
Mamber, S.W., Bryson, V., Katz, S.E. (1983). The Escherichia coli
WP2/WP100 rec assay for detection of potential chemical carcinogens.
Mutation Research, 119: 135-144.
Mamber, S.W., Bryson, V., Katz, S.E. (1984). Evaluation of the
Escherichia coli K12 inductest for detection of potential chemical
carcinogens. Mutation Research, 130: 141-151.
Martin, C.N. & McDermid, A.C. (1981). Testing of 42 coded compounds
for their ability to induce unscheduled DNA repair synthesis in HeLa
cells. Progress in Mutation Research, 1: 533-537.
McDonald, D.J. (1981). Salmonella/microsome tests on 42 coded
chemicals. Progress in Mutation Research, 1: 285-297.
McGregor, D.B., Martin, R., Cattanach, P., Edwards, I., McBride, D.,
Caspary, W.J. (1987). Response of the L5178Y Tk+/Tk-mouse lymphoma
cell forward mutation assay to coded chemicals. I. Results for nine
compounds. Env. Mutagen. 9: 143-160.
Mehta, R.D., von Borstel, R.C. (1981). Mutagenic activity of 42
coded compounds in the haploid yeast reversion assay, strain XV185-
14C. Progress in Mutation Research, 1: 414-423.
Meretoja, T., Gripenberg, U., Bamford, D., Laamanen, I., Sorsa, S.
(1976). Mutagenicity and toxicity of amitrole, II. Human lymphocyte
culture tests. Mutation Research, 40: 191-196.
Mihail, F. (1984). Aminotriazole - Studies for skin sensitization
effect on guinea pigs. Unpublished report No 12607 (dated 12-04-
1984) from Bayer AG Institute of Toxicology. Submitted to WHO by
Bayer AG.
Mihail, F. (1985). Aminotriazole - Study for skin-sensitising effect
on guinea pigs in the open epicutaneous test. Unpublished report No
13775 (dated: 27-08-1985) from Bayer AG Institute of Toxicology.
Submitted to WHO by Bayer AG.
Mihail, F. & Schilde, B. (1984). Aminotriazole - Study for subacute
dermal toxicity to rabbits Unpublished report No 12369 (dated: 09-
01-1984) from Bayer AG Institute of Toxicology. Submitted to WHO by
Bayer AG.
Miksche, L.W. (1982). Letter report of 22-06-82 - BBA request-
effects on man. Unpublished document from the Bayer AG, LE Medical
Department Leverkusen. Submitted to WHO by Bayer AG.
Miksche, L.W. (1983). Letter report of 22-04-83 - Production of
amitrole-medical monitoring. Unpublished document from the Bayer AG,
LE Medical Department Leverkusen. Submitted to WHO by Bayer AG.
Mori, S., Takeuchi, Y., Toyama, M., Makino, S., Ohhara, T., Tochino,
Y., Hayashi, Y. (1985). Amitrole: Strain differences in
morphological response of the liver following subchronic
administration to mice. Toxicology Letters, 29: 145-152.
Moriya, M., Ohta, T., Watanabe, K., Miyazawa, T., Kato, K., Shirasu,
Y. (1983). Further mutagenicity studies on pesticides in bacterial
reversion assay systems. Mutation Research, 116: 185-216.
Morpurgo, G., Bellincampi, D., Gualandi, G., Baldinelli, L., Serlupi
Crescenzi, O. (1979). Analysis of mitotic nondisjunction with
Aspergillus nidulans. Env. Health Perspect. 31: 81-95.
Napalkov, N.P. (1962). Blastomogenic action of 3-amino-1,2,4-
triazole. (Russ). Gig. Tr. prof. Zabol. 6: 48-51. In: Amitrole; IARC
Monographs on the evaluation of carcinogenic risk of chemicals to
humans (1986). World Health Organization, Vol. 41: 293-317, Lyon.
Palmer, J.S. (1972). Toxicity of 45 organic herbicides to cattle
sheep, and chickens. Production Research report no 137 (dated: March
1972) from US Department of Agriculture. Submitted to WHO by Bayer
AG.
Paynter O.E., Burin, G.J., Jaeger, R.B., Gregorio C.A. (1988).
Goitrogens and thyroid follicular cell neoplasia: evidence for a
threshold process. Regul. Toxicol. Pharmacol. 8: 102-119.
Perry, P.E. & Thomson, E.J. (1981). Evaluation of sister chromatid
exchange method in mammalian cells as a screening system for
carcinogens. Progress in Mutation Research, 1: 560-569.
Prince, H.N. (1977). Microbial mutagen assays with amitrole (3-
amino-1,2,4-triazole). Unpublished report no 5634 (dated: 12-09-
1977) from Gibraltar Biological Laboratories (submitted via Food and
Drug Research Laboratories, Inc.). Submitted to WHO by Bayer AG.
Reitze, H.K. & Seitz, K.A. (1985). Light and electron microscopical
changes in the liver of mice following treatment with aminotriazole.
Exp. Pathol. 27: 17-31.
Richold, M. & Jones, E. (1981). Mutagenic activity of 42 coded
compounds in the Salmonella/microsome assay. Progress in Mutation
Research, 1: 314-322.
Rosenkranz, H.S., Hyman, J., Leifer, Z. (1981). DNA polymerase
deficient assay. Progress in Mutation Research, 1: 210-218.
Rowland, I. & Severn, B. (1981). Mutagenic of carcinogens and
noncarcinogens in the salmonella/micrososme test. Progress in
Mutation Research, 1: 323-332.
Seidenberg, I.L. & Gee, A.H. (1953). Report on acute toxicity of
aminotriazole as established by oral administration to rats.
Unpublished report from Foster D. Snell Inc. (dated: 08-06-1953).
Submitted to WHO by Bayer AG.
Shah, P.V. & Guthrie, F.E. (1977). Dermal absorption, distribution,
and the fate of six pesticides in the rabbit. In: Pesticide
Management and Insecticide Resistance. pp 547-554. Academic Press.
Sharp, D.C. & Parry, J.M. (1981a). Induction of mitotic gene
conversion by 41 coded compounds using the yeast culture JD1.
Progress in Mutation Research, 1: 491-501.
Sharp, D.C. & Parry, J.M. (1981b). Use of repair deficient strains
of yeast to assay the activity of 40 coded compounds. Progress in
Mutation Research, 1: 502-516.
Shirasu, Y., Moriya, M., kato, K., Furuhashi, A., Kada, T. (1976).
Mutagenicity screening of pesticides in the microbial system.
Mutation Research, 40: 19-30.
Simmon, V.H., Rosenkranz, H.S., Zeiger, E., Poirer, L.A. (1979).
Mutagenic activity of chemical carcinogens and related compounds in
the intraperitoneal host-mediated assay. J. Natl. Cancer Inst. 62:
911-918.
Steinhoff, D. & Boehme, K. (1978). Aminotriazole; carcinogenesis
study with oral administration to Syrian golden hamsters.
Unpublished report No 7521 (dated: 16-05-1978) from Bayer AG,
Institute of Toxicology. Submitted to WHO by Bayer AG.
Steinhoff, D. & Boehme, K. (1979a). Aminotriazole; carcinogenesis
study with oral administration to rats. Unpublished report No 8450
(dated: 11-06-1979) from Bayer AG, Institute of Toxicology.
Submitted to WHO by Bayer AG.
Steinhoff, D. & Boehme, K. (1979b). Aminotriazole; carcinogenesis
study with oral administration to mice. Unpublished report No 8490
(dated: 17-07-1979) from Bayer AG, Institute of Toxicology.
Submitted to WHO by Bayer AG.
Steinhoff, D., Weber, H., Mohr, U., Boehme, K. (1983). Evaluation of
Amitrole (Aminotriazole) for potential carcinogenicity in orally
dosed rats, mice, and golden hamsters. Toxicol. Appl. Pharmacol.
69: 161-169.
Stendel (1965). Aminotriazole/oral toxicity to cattle. Unpublished
letter report of March 15, 1965 from Bayer AG, Institute of
Toxicology. Submitted to WHO by Bayer AG.
Strum, J.M. & Karnovsky, M.B. (1971). Aminotriazole goiter. Fine
structure and localization of thyroid peroxidase activity.
Laboratory Investigation, 24: 1-12.
Styles, J.A. (1979). Studies on the detection of carcinogens using a
mammalian cell transformation assay with liver homogenate
activation. In: Norpoth, K. & Garner, R.C. (eds), Short-term
mutagenicity test systems for detecting carcinogens. Proceedings of
the International Symposium on short-term mutagenicity test systems.
Dortmund, Nov. 15-17, 1978 pp 226-238. Springer Verlag, New York.
Styles, J.A. (1981). Activity of 42 coded compounds in the BHK-21
cell transformation test. Progress in Mutation Research, 1: 638-
646.
Thomson, J.A. (1981). Mutagenic activity of 42 coded compounds in
the lambda induction assay. Progress in Mutation Research, 1: 224-
235.
Thyssen, J. (1974a). Aminotriazol Batch no 143 - Determination of
acute toxicity. Letter report of June 10, 1974 from Bayer AG,
Institute of Toxicology. Submitted to WHO by Bayer AG.
Thyssen, J. (1974b). Aminotriazol - Determination of acute toxicity.
Letter report of September 26, 1974 from Bayer AG, Institute of
Toxicology. Submitted to WHO by Bayer AG.
Thyssen, J. (1983). Aminotriazole - Acute inhalation toxicity.
Unpublished report No 11707 (dated: 11-04-1983) from Bayer AG,
Institute of Toxicology. Submitted to WHO by Bayer AG.
Tjälve, H. (1974). Fetal uptake and embryogenetic effects of
aminotriazole in mice. Arch. Toxicol. 33: 41-48.
Tjälve, H. (1975). The distribution of labelled aminotriazole in
mice. Toxicology, 3: 49-67.
Topham, J.C. (1980). Do induced sperm-head abnormalities in mice
specifically identify mammalian mutagens rather than carcinogens?
Mutation Research, 74: 379-387.
Tronnier, H. (1983). Aminotriazole - Expertise by consultant
dermatologist on testing for phototoxicity in test subjects.
Unpublished report No R 2544 (dated: 05-08-1983) from City Clinics
Dortmund. Submitted to WHO by Bayer AG
Trueman, R.W. (1981). Activity of 42 coded compounds in the
Salmonella reverse mutation test. Progress in Mutation Research,
1: 343-350.
Tsuchimoto, T. & Matter, B.E. (1981). Activity of coded compounds in
the micronucleus test. Progress in Mutation Research, 1: 705-711.
Tsuda, H., Takahashi, M., Fukushima, S., Endo, Y. (1973). Fine
structure and localization of peroxidase activity in aminotriazole
goiter. Nagoya Med. J., 18: 183-197.
Tsutsui, T., Maizumi, H., Barrett, J.C. (1984). Amitrole-induced
cell transformation and gene mutations in Syrian hamster embryo
cells in culture. Mutation Research, 140: 205-207.
Turner, D.M., Gilbert, C.M. (1976). Evidence of metabolismus of
(14C) Amitrole in the rat after inhalation administration.
Unpublished report No 291/1a (dated May 1976) from Hazleton
Laboratories Europe Ltd. Submitted to WHO by Bayer AG.
Tweats, D.J. (1981). Activity of 42 coded compounds in a
differential killing test using Escherichia coli strains Wp2, WP67
(uvrA polA), and CM871 (uvrA lexA recA). Progress in Mutation
Research, 1: 199-209.
Tyl, R.W. (1986a). Teratogenicity evaluation of aminotriazole
technical administered by gavage to CD rats. Unpublished report No
49-45 (dated: 29-05-1986) from Union Carbide Bushy Run Research
Center. Submitted to WHO by Bayer AG and Rhône-Poulenc Ag. Company.
Tyl, R.W. (1986b). Teratogenicity evaluation of aminotriazole
technical administered by gavage to New Zealand White rabbits.
Unpublished report No 49-66 (dated: 30-05-1986) from Union Carbide
Bushy Run Research Center. Submitted to WHO by Rhône-Poulenc Ag.
Company.
Venitt, S. & Crofton-Sleigh, S. (1981). Mutagenicity of 42 coded
compounds in a bacterial assay using Escherichia coli and
Salmonella typhimurium. Progress in Mutation Research, 1: 351-360.
Vesselinovitch, S.D. (1983). Perinatal hepatocarcinogenesis.
Biological Research in Pregnancy and Perinatology, 4: 22-25.
Vogel, E., Blijleven, W.G.H., Kortselius, M.J.H., Zijlstra, J.A.
(1981). Mutagenic activity of 17 coded compounds in the sex-linked
recessive lethal test in Drosophila melangolaster. Progress in
Mutation Research, 1: 660-665.
Weber, H. (1983). Aminotriazole feeding study: Determination of the
threshold dose level affecting the thyroid function of female rats
by radioiodine assay and by T3 and T4 radioimmunoassay. Unpublished
report No. 11739 (F) (dated: 13-04-1983) from Bayer Health Care
Division. Submitted to WHO by Bayer AG.
Weber, H. & Patzschke, K. (1978). Effect of long-term administration
of aminotriazole on thyroid function of male and female rats, mice
and hamsters. Unpublished report No 7690 (dated: 14-07-1978) from
Bayer AG, Institute of Pharmacokinetics. Submitted to WHO by Bayer
AG.
Weir, R. (1958). 3-Amino-1,2,4-triazole Chronic oral administration
- beagle dogs. Unpublished report from Hazleton Laboratories
Virginia dd December 1, 1958. Submitted to WHO by Bayer AG.
WHO (1992). The WHO recommended classification of pesticides by
hazard and guidelines to classification 1992-1993 (WHO/PCD/92.14).
Available from the International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland.
WHO (1994). International Programme on Chemical Safety Environmental
Health Criteria 158: Amitrole. Geneva, World Health Organization.
Wynford-Thomas, D., Stringer, B.M., Williams, E.D. (1982a).
Dissociation of growth and function in the rat thyroid during
prolonged goitrogen administration. Acta Endocrinologica, 101:
210-216.
Wynford-Thomas, D., Stringer, B.M., Williams, E.D. (1982b).
Goitrogen-induced thyroid growth in the rat: a quantitative
morphometric study. J. Endocr. 94: 131-140.
Zimmermann, F.K. & Scheel, I. (1981). Induction of mitotic gene
conversion in strain D7 of Saccharomyces cerevisiae by 42 coded
chemicals. Progress in Mutation Research, 1: 481-490.
