
PESTICIDE RESIDUES IN FOOD - 1997
Sponsored jointly by FAO and WHO
with the support of the International Programme
on Chemical Safety (IPCS)
TOXICOLOGICAL AND ENVIRONMENTAL
EVALUATIONS 1994
Joint meeting of the
FAO Panel of Experts on Pesticide Residues
in Food and the Environment
and the
WHO Core Assessment Group
Lyon 22 September - 1 October 1997
The summaries and evaluations contained in this book are, in most
cases, based on unpublished proprietary data submitted for the purpose
of the JMPR assessment. A registration authority should not grant a
registration on the basis of an evaluation unless it has first
received authorization for such use from the owner who submitted the
data for JMPR review or has received the data on which the summaries
are based, either from the owner of the data or from a second party
that has obtained permission from the owner of the data for this
purpose.
AMITROLE (addendum)
First draft prepared by
S. Geertsen and T. Jones
Health Evaluation Division, Pest Management Regulatory Agency
Health Canada, Ottawa, Ontario, Canada
Explanation
Evaluation for acceptable daily intake
Biochemical aspects
Absorption, distribution, excretion
Biotransformation
Toxicological studies
Short-term toxicity
Reproductive toxicity
Multigeneration reproductive toxicity
Developmental toxicity
Special study: Maternal toxicity
Comments
Toxicological evaluation
References
Explanation
Amitrole was first considered by the Joint Meeting in 1974 (Annex
I, reference 22). A conditional ADI of 0-0.00003 mg/kg bw was
established at that time, which was extended by the 1977 Meeting
(Annex I, reference 28) after consideration of additional data, on the
basis of an NOAEL of 0.025 mg/kg bw per day in a three-month study in
rats and a 1000-fold safety factor. In 1993, amitrole was re-evaluated
within the CCPR periodic review programme (Annex I, reference 68),
when the Meeting established a temporary ADI of 0-0.0005 mg/kg bw on
the basis of an NOAEL of 0.5 mg/kg bw per day in a two-year study of
toxicity in rats and a 1000-fold safety factor because of the
inadequacy of the database. The 1993 Meeting requested submission of
the results of a two-generation study of reproductive toxicity in
rats, a one-year study in dogs, a study of developmental toxicity
after oral administration in rabbits, and a study of metabolism in
rats. The results of these studies and of a study of effects on the
thyroid gland of pregnant rabbits were reviewed at the present
Meeting.
WHO has published an Environmental Health Criteria monograph on
amitrole (WHO, 1994).
Evaluation for acceptable daily intake
1. Biochemical aspects
(a) Absorption, distribution, and excretion
Expiration of 14CO2 was measured in five male Wistar BOR:WISW
(SPF Cpb) rats given [5-14C]-amitrole (77.3 µCi/mg; radiochemical
purity, 97.5%) at a dose of 1 mg/kg bw over 72 h, and the presence of
radiolabel was determined by whole-body autoradiography in male rats
killed sequentially 1, 4, 8, 24, and 48 h after administration of 5
mg/kg bw labelled material. Groups of five male and five female rats
received single doses of labelled material at 1 mg/kg bw orally, 500
mg/kg bw orally, or 1 mg/kg bw intravenously and were killed after 48
h. A final group of five male and five female animals was given
unlabelled amitrole in daily doses of 1 mg/kg bw for 14 days, followed
on the 15th day by a single dose of 1 mg/kg bw 14C-labelled amitrole
and were killed 48 h after the final dose.
Amitrole was rapidly absorbed, attaining a maximal plasma level
40-60 min after oral administration and 5-20 min after intravenous
administration. The material was rapidly excreted, mainly in the
urine; more than 70% was eliminated within 8 h and 87-95% within 48 h.
Only a small amount (1.5-6.2%) was excreted in faeces and a negligible
amount in expired air. The total urinary excretion levels were the
same for the low dose (90%), high dose (94%), repeated doses (88%),
and intravenous dose (90% in males). The tissue levels of labelled
material were < 2.9% of the administered dose at sacrifice, the
majority being found in the liver. More than 94% of the administered
dose of radiolabel was recovered, except in females treated
intravenously with 1 mg/kg bw, from which only 77% was recovered,
indicating a problem in the dosing regime. The absorption,
distribution, and excretion of the labelled material were similar in
male and female animals.
Whole-body autoradiography showed wide distribution of radiolabel
in all organs and tissues at 1 and 4 h. The distribution shifted at 8
h to increasing density in the gastrointestinal tract, liver, and
kidneys. By 24 and 48 h after administration, most 14C-labelled
amitrole was found in the liver and minor amounts in the renal cortex
and nasal mucosa (Anderson & Brauner, 1995).
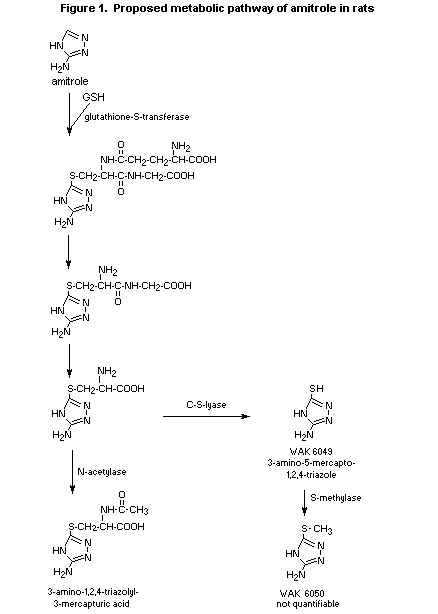
(b) Biotransformation
The metabolites in the urine were quantified for all groups in
the study described above. Most of the amitrole (60-90%) remained
unmetabolized. After administration of the low dose (1 mg/kg bw), the
primary metabolite was 3-amino-1,2,4-triazolyl-5-mercapturic acid,
which accounted for 2-7% of the administered dose.
3-Amino-5-mercapto-1,2,4-triazole (WAK 6049) was present in a very
small amount (< 2.6%). While the metabolic profile did not differ
significantly with route or frequency of dosing, mercapturic acid was
not found in urine collected after administration of the high dose of
500 mg/kg bw, and only samples from males contained trace amounts
(< 1%). WAK 6050 was identified in trace amounts only after repeated
dosing. Biotransformation of amitrole to volatile metabolites was
negligible (0.1%). Figure 1 summarizes the metabolic pathway of
amitrole in rats proposed in this study (Anderson & Brauner, 1995).
2. Toxicological studies
(a) Short-term toxicity
Groups of four beagle dogs of each sex were fed diets containing
amitrole (purity, 97%) at concentrations of 0, 10, 500, or 1500 ppm
(equal to 0, 0.29, 13, or 32 mg/kg bw per day in males and 0, 0.31,
13, or 37 mg/kg bw per day in females). The animals were obsrved for
deaths, clinical signs (including neurological reflexes and reactions,
cardiac waves, blood pressure, and heart rate), body weight, food
consumption, and ophthalmoscopic, haematological, urinary, and
clinical chemical parameters (including thyroxine, triiodothyronine,
and thyroid stimulating hormone); more than 45 tissues were examined
grossly and histologically.
The primary target for the toxicity of amitrole was the thyroid,
with evidence of hypothyroidism in males and females at the
intermediate and high doses characterized by statistically
significantly lower levels of thyroxine and triiodothyronine. Some
adaptation of these levels was seen after three months in males and
females at the intermediate dose, as the concentrations had returned
to normal by day 91; however, no such adaptation was seen in dogs at
1500 ppm. The levels were lower than those in controls throughout the
study. Although the authors stated that the level of thyroid
stimulating hormone was reduced in males and females at the high dose,
the results were too variable to conclude that there was a
treatment-related change. Clinical signs consistent with
hypothyroidism were observed in dogs at the intermediate and high
doses, including palpably enlarged thyroids and rough coats (in males
at 500 ppm and males and females at 1500 ppm); raised and/or
discoloured zones on the skin of the nose, outer ear margins, and
hindlimbs, described histologically as acanthosis, hyperkeratosis, and
inflammation, in animals at the high dose; and markedly increased
thyroid weights, by 5-8-fold in males and females at the intemediate
dose and by 16-33-fold at the high dose, coupled with pathological
changes in the thyroid and pituitary gland. A range of lesions in the
thyroids was noted, including follicular-cell hyperplasia (in males
and females at 500 and 1500 ppm) and capsular fibrosis, vasculitis,
and dilatation of the vasculature (in males at 500 ppm and males and
females at 1500 ppm). Thrombosis, pigmentation, and haemorrhage were
seen in the thyroids of males and females at the high dose; pituitary
hyperplasia (in males at 1500 ppm) and hypertrophy (in males at
500 ppm and males and females at 1500 ppm) were also seen. Males and
females at 500 and 1500 ppm also had ectopic thyroids in the adipose
tissue surrounding the aortas. Follicular hyperplasia with or without
capsular fibrosis was seen in these ectopic thyroids. Typical of
 hypothyroidism were the statistically significantly lower erythrocyte,
haemoglobin, and haematocrit counts and the slightly lower mean cell
volume and mean cell haemoglobin in males and females at 1500 ppm.
There was also a statistically significantly increased incidence of
hypochromasia. Although the authors concluded that there was mild,
non-regenerative, normochromic anaemia, the effect on mean cell volume
and mean cell haemoglobin would indicate mild, hypochromic, microcytic
anaemia. An increase in cholesterol levels was also seen in males at
the high dose.
Statistically significantly decreased absolute and relative heart
weights (by about 25%) were seen in males at 1500 ppm, which were
reflected in decreases in P-wave and R-wave amplitudes in this group.
The neurological examinations showed decreased jaw tone (related to
the trigeminal nerve) and/or temporal muscle atrophy in males at the
high dose. Limb muscle weakness and decreased postural hopping
reactions (hemiwalk and hemihop) accompanied by proprioceptive
deficits and reluctance to walk and/or dragging of the limbs were seen
in two males and one female at 1500 ppm. The authors stated that these
effects are consistent with the weakness, exercise intolerance, and
stiffness associated with canine hypothyroidism. While muscle atrophy
and inactivity are commonly observed in hypothyroidism, no
documentation was provided to specifically link proprioceptive
deficits and postural hopping reactions with hypothyroidism, and these
changes may be independent of the effects on the thyroid. Another
significant sign of systemic toxicity was a treatment-related
reduction in the body-weight gain of males at the high dose during the
first six months, which was coupled with 40% reductions in food
consumption throughout the study. Although the authors considered that
amitrole had no meaningful effects on body weight, the body weights of
these animals were consistently 10% lower and their weight gain 20-30%
lower than those of controls (not statistically significant). The food
consumption of females at the high-dose was also reduced, but to a
lesser extent (15-20%). Other indications of systemic toxicity
included a clear eye discharge in males at the intermediate dose and
males and females at the high dose, increased levels of lactic
dehydrogenase in males at the high dose, statistically significantly
increased platelet counts in males and females at the high dose,
statistically significantly lower brain weights (15%) and inflammation
with or without perivascular cuffing in the brains of males and
females at 1500 ppm, and pigmentation and epithelial-cell hyperplasia
in the gall-bladders of these animals. The authors also concluded that
amitrole induced changes in the blood levels of albumin, aspartate
aminotransferase, and sodium. Owing to the high variability, both over
time and among groups, the observed changes in these parameters could
not, however, be clearly attributed to treatment. There were no
effects on mortality, heart rate, blood pressure, or urinary
parameters. Males appeared to be more sensitive than females. The
NOAEL was 10 ppm, equal to 0.29 mg/kg bw per day, on the basis of
goitrogenic effects at doses of 500 ppm and above (Jones & Lake,
1994).
(b) Reproductive toxicity
(i) Multigeneration reproductive toxicity
In a one-generation, one-litter, dose-finding study of
reproductive toxicity, groups of 10 male and 10 female Sprague-Dawley
Crl:CD(SD)BR rats were fed amitrole (purity, 97.4%) in the diet at
levels of 2, 10, 40, or 160 ppm. F0 animals were treated for 29 days
before mating up to weaning of the F1 pups, providing doses of 0.12,
0.62, 2.5, and 8.3 mg/kg bw per day for F0 males; 0.19, 0.94, 3.7,
and 12 mg/kg bw per day for F0 females; 0.29, 1.4, 5.3, and 28 mg/kg
bw per day for F1 males; and 0.31, 1.6, 5.8, and 28 mg/kg bw per day
for F1 females. When possible, one F1 pup of each sex per litter was
killed for histopathological examination on day 14 post partum, and
two pups of each sex per litter were selected after weaning for
treatment for a further 36 days. Mortality, clinical signs, body
weight, and food consumption were monitored at all stages of the
study, and the following indices were calculated: male mating, female
mating, male and female fertility, gestation, viability on days 4, 14,
and 21 post partum, and numbers of live births. In addition, oestrus
cycles, litter size, number of implantations, gestation length, and
sex ratio were recorded. Gross and microscopic examinations were
performed.
Five F1 males and three females given 160 ppm died after
weaning, and three males and three females in this group showed one or
more of the following signs before premature sacrifice: piloerection,
cold to touch, pale extremities, hypersensitivity to touch, soiled
urogenital region, hypokinesia, dyspnoea, staggering gait, swollen
abdomen, sedation, and tremors. Statistically significantly decreased
body weights were seen at 160 ppm among F0 males, among F0 females
during gestation and lactation, among F1 pups on days 7-21 post
partum and after weaning, and in F1 males at 40 ppm. Significantly
decreased food consumption was seen throughout the study in F0 and
F1 animals given the high dose and in males of the F1 generation at
40 ppm. All of these effects were considered to be toxicologically
significant. Females at the high dose had a lower implantation rate
(12.1 implantation sites) than controls (16.6 sites), which was
reflected in significantly lower birth rates and day-1 litter sizes.
The viability indices on days 4, 14, and 21 were not decreased by
treatment. The number of females with a normal oestrus cycle was
decreased in a dose-related fashion among animals at 40 or 160 ppm,
due in the latter group to the smaller number of implantations.
The thyroid glands of F0 males and females showed a dose-related
increase in size and in the incidence of reddish colour, starting at
10 ppm in the males and at 40 ppm in the females. All animals at 160
ppm had reddened pituitary glands and greyish-white foci on the lungs,
and the adrenal glands and spleens were reduced in size. Male and
female F1 adults given 40 or 160 ppm and killed at the end of the
study had enlarged, reddened thyroid glands and reddened pituitary
glands. Histopathological examination showed dose-related effects in
the liver and pituitary gland in F0 and F1 animals at these doses,
decreased colloid content in females of both generations at 10, 40, or
160 ppm, vascular ectasia in all animals of both generations treated
with 40 or 160 ppm, and peri-adenitis in a small number of F0 males
at 160 ppm. No treatment-related histopathological lesions were seen
in pups killed on day 14 post partum.
Doses were selected for use in the main study on the basis of the
reproductive effects and toxicity seen at 160 ppm and the minimal
toxicity observed in males treated with 40 ppm amitrole (Savary,
1994).
In the main study, groups of 30 male and 30 female Sprague-Dawley
Crl:CD(SD)BR rats were fed diets containing amitrole (purity, 97-98%)
at levels of 0.5, 2, 15, or 110 ppm for two generations, with one
litter per generation. These doses were equivalent to 0.03, 0.12, 0.9,
or 5.9 mg/kg bw per day in F0 males; 0.04, 0.16, 1.2, or 12 mg/kg bw
per day in F1 males; 0.04, 0.16, 1.2, or 7.8 mg/kg bw per day in F0
females; and 0.05, 0.21, 1.6, or 16 mg/kg bw per day in F1 females.
Treatment was begun in the parental animals 72 days before mating and
was continued throughout gestation and lactation. Mortality, clinical
signs, body weight, and food consumption were monitored at all stages
of the study, and the following indices were calculated: male mating,
female mating, male and female fertility, gestation, viability on days
4 and 21 post partum, and numbers of live births. Additional
parameters measured included pup development, litter size, number of
implantations, and length of gestation. All animals were examined
grossly, and controls and animals at the high dose were examined
microscopically. The absolute and relative weights (as a percentage of
body weight) of 11 organs were determined.
Death or clinical signs were observed in 10/32 F1 males and
16/31 females treated with 110 ppm amitrole, and both F0 and F1
parental animals at this dose had statistically and toxicologically
significant decreases in body weight throughout the study. By the end
of pre-mating, the body weights of F0 males were 60% those of the
controls, and those of the females were 85% those of controls. The F1
animals at this dose were more severely affected, their mean body
weights being only about 35% of those of controls. All parental
animals at this dose had statistically and toxicologically
significantly decreased food consumption.
Animals at the two lower doses had no changes in organ weight or
histopathological appearance; however, those at 110 ppm had changes
indicative of widespread systemic toxicity, including significantly
increased relative thyroid gland weights and treatment-related
histopathological lesions such as follicular epithelial hyperplasia
and vascular ectasia, in most animals. The relative pituitary gland
weights were also significantly increased in F0 and F1 males and F1
females, with treatment-related histopathological changes, including
vacuolated cells and decreased numbers of acidophils, in F0 females
and F1 males and females. At this dose, significant increases were
also seen in the relative weights of the testis, epididymides, and
seminal vesicles (both generations), prostate (F1), and uterus (F1);
decreased relative ovarian weights were seen in the F0 generation.
Slightly increased incidences of histopathological effects were seen
in the testis and epididymides of F1 animals and in the ovaries,
uterus, and vagina in both generations. Many of the histopathological
effects on the reproductive tissues, including the prostate and
seminal vesicles, were related to immaturity, which is consistent with
the small size of the animals at this dose. Other signs of systemic
toxicity in animals at the high dose included significantly decreased
relative weights of the adrenal glands in F0 males and females and
F1 females but a significant increase in F1 males. Both generations
of males showed an increased frequency of ceroid-laden cortical cells,
and the F0 females had an increased incidence of cortical atrophy.
All animals at the high dose had significantly increased relative
spleen weights, and treatment-related histopathological effects were
seen in F0 males and females and F1 males. While only F1 males had
increased relative kidney weights, the remaining animals had
treatment-related increases in the incidence of numerous renal
lesions. Hepatocellular hypertrophy was seen in most animals at
110 ppm, but the decrease in relative liver weight was significant
only in F1 males. F1 pups at this dose had toxicologically
significantly increases in absolute and relative thyroid gland weights
and related gross pathological and histopathological lesions. Owing to
the low survival rate of F2 pups, treatment-related changes in organ
weight and pathological effects could not be identified in this group.
The following reproductive effects seen in animals at 110 ppm
were considered to be toxicologically significant: significantly
decreased mating indices among F1 males and females, decreased F1
male and female fertility indices, increased length of gestation in
F1 females (from 21-22 to 23 days), low implantation rate in F0 and
F1 females, low prenatal survival (59%) in the F2 generation,
significantly decreased mean litter size at day 1 (F1 and F2) and
day 21 (F2 only) post partum, significantly decreased mean litter
size on day 4 post partum (F2), very low viability indices for the
F2 generation on days 4 (33%) and 21 (37%), and decreased F1 and F2
pup body weights during lactation. In the F2 generation at 110 ppm,
the pups in four of five liveborn litters died during the first day,
and only three pups out of 24 liveborn pups of the remaining litter
survived to the end of lactation. There appeared to be no effects on
F1 pup development, sex ratio, or gross appearance at necropsy; there
were insufficient data to assess these parameters in the F2
generation. The author reported an NOAEL of 2 ppm for systemic
toxicity on the basis of minimally increased thyroid activity;
however, thyroid hormone levels were not measured and no other effects
on the thyroid were noted in rats at 15 ppm. The NOAEL for systemic
toxicity and reproductive toxicity was 15 ppm, equal to 0.9 mg/kg bw
per day (Richard, 1995).
(ii) Developmental toxicity
Groups of 16 mated female Mol:Russian rabbits were given amitrole
(purity, 97.5%) at doses of 0, 5, 20, or 80 mg/kg bw per day by gavage
on days 6-18 of gestation. The observations included daily monitoring
for clinical signs, food consumption during six periods, and maternal
body weight on day 0 and daily on days 6-18 and day 29. The animals
were killed on day 29, their fetuses were removed, and the does were
examined grossly for number of corpora lutea and implantation sites,
uterine weight, individual pup weights, appearance of fetuses or
embryos, sex of all live fetuses, individual weights of live fetuses,
and the occurrence of external malformations, visceral effects, and
effects on the skeletal system.
Statistically significantly lower maternal food consumption was
seen among does at 80 mg/kg bw per day during treatment on days 6-10
and 14-19 and throughout days 0-29, and their mean weight gain was 31%
lower than that of controls (not statistically significant) on days
6-18. Treatment at 80 mg/kg bw per day significantly reduced the body
weights of male fetuses and lowered the mean litter weight by 17%. The
NOAEL for maternal and fetal toxicity was 20 mg/kg bw per day. There
was no evidence of teratogenicity (Kolb, 1994a).
(iii) Special study: Maternal toxicity
Groups of five mated female Mol:Russian rabbits given amitrole
(purity, 97.5%) at doses of 0, 5, 20, or 80 mg/kg bw per day by gavage
on days 6-18 of gestation and monitored daily for clinical signs, food
consumption during four periods, maternal body weight on day 0 and
daily on days 6-18 and day 19. The animals were killed on day 19 and
examined grossly for the numbers of corpora lutea, implantations, and
resorptions. Clinical chemical and haematological parameters were
measured on days 6, 7, 13, and 19 before treatment, and the weights of
the liver, spleen, thyroid glands, and adrenal glands were recorded.
The liver, spleen, thyroid glands, adrenal glands, and pituitary
glands were examined histologically.
The absolute and relative weights of the liver and the levels of
albumin and protein were decreased on day 19 in rabbits treated with
20 mg/kg bw per day or more. Those at 80 mg/kg bw per day also had
lower food consumption during days 6-10 (but not days 10-18) of
treatment, increased creatine kinase activity by day 19, decreased
albumin and protein levels by day 13, decreased triiodothyronine and
thyroxine levels on days 7, 13, and 19, and slight hypertrophy of the
thyroid follicular cells, which was considered to be a minimal effect
related to the thyroid hormone levels. Changes observed in the liver
that were not considered to be adverse included a slight
treatment-related increase in the incidence and number of Kupffer-cell
foci in animals at 80 mg/kg bw per day and cytoplasmic changes
described as fine net-like markings on centrilobular heptocyte centres
and eosin-stained bands on the cell membrane in animals at 20 and 80
mg/kg bw per day. No deaths, adverse clinical signs, or body-weight
changes were reported. The numbers of corpora lutea, implantations,
and resorptions and the weights of the thyroid glands were not
affected by treatment.
The author concluded that treatment had increased cholesterol
levels in rabbits at 80 mg/kg bw per day, but that the increase was
due to one outlier and was thus spurious. The author considered the
NOAEL for maternal toxicity to be 20 mg/kg bw per day, as only
non-adverse cytoplasmic changes in the liver were seen at this dose
(Kolb, 1994b). The Meeting did not agree with this conclusion since
decreased absolute and relative liver weights and statistically
significantly decreased albumin and protein levels were seen at 20
mg/kg bw per day and above. The Meeting concluded that the NOAEL for
maternal toxicity was 5 mg/kg bw per day.
Comments
In rats, amitrole was rapidly absorbed, approximately 90% of the
administered dose being eliminated within 48 h. The primary route of
elimination was the urine, which accounted for over 87% of the dose;
faecal elimination accounted for less than 6% of the dose and volatile
metabolites represented only 0.1%. Forty-eight hours after dosing, the
tissue residues amounted to less than 3% of the dose, with the
majority found in the liver. Most of the radiolabel (62-90%) was
excreted as the parent compound. Several metabolites were present at
very low concentrations, a mercapturic acid derivative being
predominant (2.1-7.3% of the administered 14C).
In a one-year study of toxicity, dogs were fed diets containing
0, 10, 500, or 1500 ppm amitrole. At doses 500 ppm (equal to 13 mg/kg
bw per day) and above, the levels of triiodothyronine and thyroxine
were reduced and there were increases in the incidence of rough coats,
in thyroid weights, and in the frequency of lesions of the thyroid
(follicular-cell hyperplasia, capsular fibrosis, vasculitis, and
dilatation of the vasculature). In addition, ectopic thyroids with
follicular hyperplasia and capsular fibrosis were noted, and changes
in the pituitary (hyperplasia and hypertrophy) were evident in both
males and females. At 1500 ppm (equal to 32 mg/kg bw per day), both
males and females were anaemic and had increased platelet counts,
decreased food intake, decreased brain weights (with histopathological
lesions), and neurological effects. At this dose, males also had
decreased weight gain, increased lactate dehydrogenase activity and
cholesterol levels, decreased heart weights, and decreased P- and
R-wave amplitudes. The NOAEL was 10 ppm, equal to 0.29 mg/kg bw per
day.
In a two-generation study of reproductive toxicity, rats were fed
diets containing 0, 0.5, 2, 15, or 110 ppm amitrole. At 15 ppm (equal
to 0.9 mg/kg bw per day), the only effect observed was a slight
increase in the severity of some histopathological changes in the
thyroid, including small follicles, decreased colloid content, and
follicular epithelial hypertrophy. Parental toxicity at 110 ppm was
severe and was characterized by mortality, clinical signs,
considerably decreased weight gain, thyroid effects (increased
relative thyroid weights, follicular epithelial hyperplasia, and
vascular ectasia), changes in relative organ weights (increased
weights of pituitary, testes, epididymides, seminal vesicles,
prostate, uterus, spleen, and kidneys and decreased weights of
ovaries, adrenal glands, and liver), and histopathological lesions in
the liver, adrenal glands, kidneys, and numerous reproductive tissues.
In pups, the only observations were increased thyroid weights with
accompanying histopathological lesions. The effects on reproduction
included decreased mating and fertility indices, increased gestation
length, and decreased prenatal survival, litter size, pup body weight,
and viability. The NOAEL for systemic toxicity was 2 ppm, equal to
0.12 mg/kg bw per day. This value is supported by the findings of the
range-finding study, in which enlarged, reddened thyroids and
decreased colloid content were noted at 10 ppm (equal to 0.62 mg/kg bw
per day). The NOAEL for reproductive toxicity was 15 ppm, equal to 0.9
mg/kg bw per day.
In a study of developmental toxicity, rabbits were treated with
0, 5, 20, or 80 mg/kg bw per day amitrole on days 6-18 of gestation.
At 80 mg/kg bw per day, maternal food consumption and body-weight gain
were reduced during treatment. Male fetal body weights and mean litter
weights were reduced at 80 mg/kg bw per day. Clinical chemistry was
not evaluated in this study. The NOAEL for maternal and fetal toxicity
was 20 mg/kg bw per day.
In a special study to further characterize the toxicity in
pregnant rabbits, the animals were treated with amitrole at 0, 5, 20,
or 80 mg/kg bw per day on days 6-18 of gestation and were killed on
day 19 of gestation. At 20 and 80 mg/kg bw per day, albumin and
protein levels were decreased and absolute and relative liver weights
were decreased. At 80 mg/kg bw per day, there were reductions in food
consumption, increased creatine kinase levels, decreased
triiodothyronine and thyroxine levels, and thyroid follicular-cell
hypertrophy. The NOAEL for maternal toxicity was 5 mg/kg bw per day.
The results of these supplemental studies correlate well with the
results of the studies reviewed by the 1993 JMPR, showing that the
thyroid is the primary target organ of amitrole. Contrary to the
conclusions of the 1993 JMPR, the results demonstrate that amitrole is
also goitrogenic in the dog. In the one-year study in dogs reviewed
previously, no effects on the thyroid were noted at a dose of
12.5 mg/kg bw per day. In the current one-year study, however, there
were extensive effects at 13 mg/kg bw per day.
The rat was the most sensitive species, the NOAELs being 2 ppm in
both the two-generation study of reproductive toxicity (equal to 0.12
mg/kg bw per day) and the 90-day dietary study (equivalent to 0.1
mg/kg bw per day), on the basis of histopathological changes in the
thyroid. The Meeting noted that the rat is more sensitive to the
development of thyroid hyperplasia and subsequent neoplasia after
exposure to goitrogenic compounds than are humans. Therefore, a
smaller safety factor of 50 was used to take into account the reduced
uncertainty of interspecies extrapolation, giving an ADI of
0-0.002 mg/kg bw. This ADI provides a 150-fold margin of safety over
the NOAEL of 0.29 mg/kg bw per day in the one-year study in dogs.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 10 ppm, equivalent to 1.5 mg/kg bw per day (18-month
study of toxicity and carcinogenicity)
Rat: 10 ppm, equivalent to 0.5 mg/kg bw per day (two-year
study of toxicity and carcinogenicity)
2 ppm, equal to 0.12 mg/kg bw per day (parental
toxicity in a two-generation study of reproductive
toxicity)
100 mg/kg bw per day (maternal and fetal toxicity
in a study of developmental toxicity)
Rabbit: 5 mg/kg bw per day (maternal toxicity in a study of
developmental toxicity)
20 mg/kg bw per day (fetal toxicity in a study of
developmental toxicity)
Hamster: 10 ppm, equivalent to 1 mg/kg bw per day (18-month
study of toxicity)
Dog: 10 ppm, equal to 0.29 mg/kg bw per day (one-year study
of toxicity)
Estimate of acceptable daily intake for humans
0-0.002 mg/kg bw
Studies that would provide information useful for continued evaluation
of the compound
1. Clarification of the genotoxic potential of amitrole (e.g.
DNA adducts in vivo)
2. Further observations in humans.
Toxicological criteria for estimating guidance values for dietary and non-dietary exposure to amitrole
Human exposure Relevant route, study type, species Results, remarks
Short-term
(1-7 days) Oral, acute toxicity, rat LD50 > 2500 mg/kg bw
Dermal, acute toxicity, rat LD50 > 2500 mg/kg bw
Inhalation, acute toxicity, 4 h, rat LC50 > 439 mg/L
Dermal, irritation, rabbit Minimally irritating
Ocular, irritation, rabbit Mildly irritating
Dermal, sensitization, guinea-pig Moderately sensitizing (Magnusson-Kligman test)
Non-sensitizing (Klecak open epicutaneous test)
Medium-term Repeated oral, 2-4 weeks, rat NOAEL = 3 mg/kg bw per day: effects on the
(1-26 weeks) thyroid, food consumption and body weight
Repeated oral, drinking-water, 4 weeks, rat NOAEL = 10 mg/L water: decreased weight gain,
enlarged thyroids
Repeated oral, 6-13 weeks, rat NOAEL = 0.1 mg/kg bw per day: effects on the thyroid
Repeated dermal, 3 weeks, rabbit NOAEL = 100 mg/kg bw per day (highest dose
tested)
Repeated inhalation, 4 weeks, rat NOAEL = 0.1 mg/L: effects on the thyroid
Repeated oral, special maternal toxicity, rabbit NOAEL = 5 mg/kg bw per day: decreased liver
weights and protein/albumin levels
Repeated oral, developmental toxicity, rabbit NOAEL = 20 mg/kg bw per day: developmental
toxicity
Repeated dermal, developmental toxicity, rabbit NOAEL = 1500 mg/kg bw per day: maternal and
developmental toxicity
Repeated oral, reproductive toxicity, rat NOAEL = 0.12 mg/kg bw per day (systemic):
effects on the thyroid
NOAEL = 0.9 mg/kg bw per day: reproductive
toxicity
Long-term Repeated oral, 1 year, dog NOAEL = 0.29 mg/kg bw per day: effects on the
(> 1 year) thyroid
Repeated inhalation, intermittent dosing, 2 years, LOAEL = 50 µg/L (lowest dose tested): effects on
carcinogenicity, rat the thyroid
References
Anderson, C. & Brauner, A. (1995) Amitrole: Investigation of the
biokinetic behaviour and the metabolism in the rat. Unpublished
reports Nos MR-508/95, ANC82, and BNA81 (study No. M41819023) from
Bayer AG, Agrochemicals Division, Institute for Metabolism Research
and Residue Analysis, Leverkusen, Germany. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Jones, R.D. & Lake, S.G. (1994) Technical grade amitrole: A chronic
toxicity study in the beagle dog. Unpublished report No. 7413 (study
No. 92-276-RX) from Miles Inc., Agriculture Division, Toxicology,
Stilwell, KS, USA. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Kolb, J. (1994a) Amitrole: Developmental toxicity study in rabbits
after oral administration. Unpublished report No. 23486 (study No.
T5044250) from Bayer AG, Department of Toxicology, Wuppertal, Germany.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Kolb, J. (1994b) Amitrole: Supplementary study on maternal toxicity
study in pregnant rabbits after oral administration. Unpublished
report No. 23486 (study No. T5044284) from Bayer AG, Department of
Toxicology, Wuppertal, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Richard, J. (1995) Two generation study by oral route (dietary
admixture) in rats. Unpublished report No. 6432 (study No. T5041262)
from Centre International de Toxicologie, Evreux, France. Submitted to
WHO by Bayer AG, Leverkusen, Germany.
Savary, M.-H. (1994) Preliminary study to a two-generation study by
oral route (dietary admixture) in rats. Unpublished report No. 6306
(study No. T6041263) from Centre International de Toxicologie, Evreux,
France. Submitted to WHO by Bayer AG, Leverkusen, Germany.
WHO (1994) Amitrole (Environmental Health Criteria 158), Geneva
hypothyroidism were the statistically significantly lower erythrocyte,
haemoglobin, and haematocrit counts and the slightly lower mean cell
volume and mean cell haemoglobin in males and females at 1500 ppm.
There was also a statistically significantly increased incidence of
hypochromasia. Although the authors concluded that there was mild,
non-regenerative, normochromic anaemia, the effect on mean cell volume
and mean cell haemoglobin would indicate mild, hypochromic, microcytic
anaemia. An increase in cholesterol levels was also seen in males at
the high dose.
Statistically significantly decreased absolute and relative heart
weights (by about 25%) were seen in males at 1500 ppm, which were
reflected in decreases in P-wave and R-wave amplitudes in this group.
The neurological examinations showed decreased jaw tone (related to
the trigeminal nerve) and/or temporal muscle atrophy in males at the
high dose. Limb muscle weakness and decreased postural hopping
reactions (hemiwalk and hemihop) accompanied by proprioceptive
deficits and reluctance to walk and/or dragging of the limbs were seen
in two males and one female at 1500 ppm. The authors stated that these
effects are consistent with the weakness, exercise intolerance, and
stiffness associated with canine hypothyroidism. While muscle atrophy
and inactivity are commonly observed in hypothyroidism, no
documentation was provided to specifically link proprioceptive
deficits and postural hopping reactions with hypothyroidism, and these
changes may be independent of the effects on the thyroid. Another
significant sign of systemic toxicity was a treatment-related
reduction in the body-weight gain of males at the high dose during the
first six months, which was coupled with 40% reductions in food
consumption throughout the study. Although the authors considered that
amitrole had no meaningful effects on body weight, the body weights of
these animals were consistently 10% lower and their weight gain 20-30%
lower than those of controls (not statistically significant). The food
consumption of females at the high-dose was also reduced, but to a
lesser extent (15-20%). Other indications of systemic toxicity
included a clear eye discharge in males at the intermediate dose and
males and females at the high dose, increased levels of lactic
dehydrogenase in males at the high dose, statistically significantly
increased platelet counts in males and females at the high dose,
statistically significantly lower brain weights (15%) and inflammation
with or without perivascular cuffing in the brains of males and
females at 1500 ppm, and pigmentation and epithelial-cell hyperplasia
in the gall-bladders of these animals. The authors also concluded that
amitrole induced changes in the blood levels of albumin, aspartate
aminotransferase, and sodium. Owing to the high variability, both over
time and among groups, the observed changes in these parameters could
not, however, be clearly attributed to treatment. There were no
effects on mortality, heart rate, blood pressure, or urinary
parameters. Males appeared to be more sensitive than females. The
NOAEL was 10 ppm, equal to 0.29 mg/kg bw per day, on the basis of
goitrogenic effects at doses of 500 ppm and above (Jones & Lake,
1994).
(b) Reproductive toxicity
(i) Multigeneration reproductive toxicity
In a one-generation, one-litter, dose-finding study of
reproductive toxicity, groups of 10 male and 10 female Sprague-Dawley
Crl:CD(SD)BR rats were fed amitrole (purity, 97.4%) in the diet at
levels of 2, 10, 40, or 160 ppm. F0 animals were treated for 29 days
before mating up to weaning of the F1 pups, providing doses of 0.12,
0.62, 2.5, and 8.3 mg/kg bw per day for F0 males; 0.19, 0.94, 3.7,
and 12 mg/kg bw per day for F0 females; 0.29, 1.4, 5.3, and 28 mg/kg
bw per day for F1 males; and 0.31, 1.6, 5.8, and 28 mg/kg bw per day
for F1 females. When possible, one F1 pup of each sex per litter was
killed for histopathological examination on day 14 post partum, and
two pups of each sex per litter were selected after weaning for
treatment for a further 36 days. Mortality, clinical signs, body
weight, and food consumption were monitored at all stages of the
study, and the following indices were calculated: male mating, female
mating, male and female fertility, gestation, viability on days 4, 14,
and 21 post partum, and numbers of live births. In addition, oestrus
cycles, litter size, number of implantations, gestation length, and
sex ratio were recorded. Gross and microscopic examinations were
performed.
Five F1 males and three females given 160 ppm died after
weaning, and three males and three females in this group showed one or
more of the following signs before premature sacrifice: piloerection,
cold to touch, pale extremities, hypersensitivity to touch, soiled
urogenital region, hypokinesia, dyspnoea, staggering gait, swollen
abdomen, sedation, and tremors. Statistically significantly decreased
body weights were seen at 160 ppm among F0 males, among F0 females
during gestation and lactation, among F1 pups on days 7-21 post
partum and after weaning, and in F1 males at 40 ppm. Significantly
decreased food consumption was seen throughout the study in F0 and
F1 animals given the high dose and in males of the F1 generation at
40 ppm. All of these effects were considered to be toxicologically
significant. Females at the high dose had a lower implantation rate
(12.1 implantation sites) than controls (16.6 sites), which was
reflected in significantly lower birth rates and day-1 litter sizes.
The viability indices on days 4, 14, and 21 were not decreased by
treatment. The number of females with a normal oestrus cycle was
decreased in a dose-related fashion among animals at 40 or 160 ppm,
due in the latter group to the smaller number of implantations.
The thyroid glands of F0 males and females showed a dose-related
increase in size and in the incidence of reddish colour, starting at
10 ppm in the males and at 40 ppm in the females. All animals at 160
ppm had reddened pituitary glands and greyish-white foci on the lungs,
and the adrenal glands and spleens were reduced in size. Male and
female F1 adults given 40 or 160 ppm and killed at the end of the
study had enlarged, reddened thyroid glands and reddened pituitary
glands. Histopathological examination showed dose-related effects in
the liver and pituitary gland in F0 and F1 animals at these doses,
decreased colloid content in females of both generations at 10, 40, or
160 ppm, vascular ectasia in all animals of both generations treated
with 40 or 160 ppm, and peri-adenitis in a small number of F0 males
at 160 ppm. No treatment-related histopathological lesions were seen
in pups killed on day 14 post partum.
Doses were selected for use in the main study on the basis of the
reproductive effects and toxicity seen at 160 ppm and the minimal
toxicity observed in males treated with 40 ppm amitrole (Savary,
1994).
In the main study, groups of 30 male and 30 female Sprague-Dawley
Crl:CD(SD)BR rats were fed diets containing amitrole (purity, 97-98%)
at levels of 0.5, 2, 15, or 110 ppm for two generations, with one
litter per generation. These doses were equivalent to 0.03, 0.12, 0.9,
or 5.9 mg/kg bw per day in F0 males; 0.04, 0.16, 1.2, or 12 mg/kg bw
per day in F1 males; 0.04, 0.16, 1.2, or 7.8 mg/kg bw per day in F0
females; and 0.05, 0.21, 1.6, or 16 mg/kg bw per day in F1 females.
Treatment was begun in the parental animals 72 days before mating and
was continued throughout gestation and lactation. Mortality, clinical
signs, body weight, and food consumption were monitored at all stages
of the study, and the following indices were calculated: male mating,
female mating, male and female fertility, gestation, viability on days
4 and 21 post partum, and numbers of live births. Additional
parameters measured included pup development, litter size, number of
implantations, and length of gestation. All animals were examined
grossly, and controls and animals at the high dose were examined
microscopically. The absolute and relative weights (as a percentage of
body weight) of 11 organs were determined.
Death or clinical signs were observed in 10/32 F1 males and
16/31 females treated with 110 ppm amitrole, and both F0 and F1
parental animals at this dose had statistically and toxicologically
significant decreases in body weight throughout the study. By the end
of pre-mating, the body weights of F0 males were 60% those of the
controls, and those of the females were 85% those of controls. The F1
animals at this dose were more severely affected, their mean body
weights being only about 35% of those of controls. All parental
animals at this dose had statistically and toxicologically
significantly decreased food consumption.
Animals at the two lower doses had no changes in organ weight or
histopathological appearance; however, those at 110 ppm had changes
indicative of widespread systemic toxicity, including significantly
increased relative thyroid gland weights and treatment-related
histopathological lesions such as follicular epithelial hyperplasia
and vascular ectasia, in most animals. The relative pituitary gland
weights were also significantly increased in F0 and F1 males and F1
females, with treatment-related histopathological changes, including
vacuolated cells and decreased numbers of acidophils, in F0 females
and F1 males and females. At this dose, significant increases were
also seen in the relative weights of the testis, epididymides, and
seminal vesicles (both generations), prostate (F1), and uterus (F1);
decreased relative ovarian weights were seen in the F0 generation.
Slightly increased incidences of histopathological effects were seen
in the testis and epididymides of F1 animals and in the ovaries,
uterus, and vagina in both generations. Many of the histopathological
effects on the reproductive tissues, including the prostate and
seminal vesicles, were related to immaturity, which is consistent with
the small size of the animals at this dose. Other signs of systemic
toxicity in animals at the high dose included significantly decreased
relative weights of the adrenal glands in F0 males and females and
F1 females but a significant increase in F1 males. Both generations
of males showed an increased frequency of ceroid-laden cortical cells,
and the F0 females had an increased incidence of cortical atrophy.
All animals at the high dose had significantly increased relative
spleen weights, and treatment-related histopathological effects were
seen in F0 males and females and F1 males. While only F1 males had
increased relative kidney weights, the remaining animals had
treatment-related increases in the incidence of numerous renal
lesions. Hepatocellular hypertrophy was seen in most animals at
110 ppm, but the decrease in relative liver weight was significant
only in F1 males. F1 pups at this dose had toxicologically
significantly increases in absolute and relative thyroid gland weights
and related gross pathological and histopathological lesions. Owing to
the low survival rate of F2 pups, treatment-related changes in organ
weight and pathological effects could not be identified in this group.
The following reproductive effects seen in animals at 110 ppm
were considered to be toxicologically significant: significantly
decreased mating indices among F1 males and females, decreased F1
male and female fertility indices, increased length of gestation in
F1 females (from 21-22 to 23 days), low implantation rate in F0 and
F1 females, low prenatal survival (59%) in the F2 generation,
significantly decreased mean litter size at day 1 (F1 and F2) and
day 21 (F2 only) post partum, significantly decreased mean litter
size on day 4 post partum (F2), very low viability indices for the
F2 generation on days 4 (33%) and 21 (37%), and decreased F1 and F2
pup body weights during lactation. In the F2 generation at 110 ppm,
the pups in four of five liveborn litters died during the first day,
and only three pups out of 24 liveborn pups of the remaining litter
survived to the end of lactation. There appeared to be no effects on
F1 pup development, sex ratio, or gross appearance at necropsy; there
were insufficient data to assess these parameters in the F2
generation. The author reported an NOAEL of 2 ppm for systemic
toxicity on the basis of minimally increased thyroid activity;
however, thyroid hormone levels were not measured and no other effects
on the thyroid were noted in rats at 15 ppm. The NOAEL for systemic
toxicity and reproductive toxicity was 15 ppm, equal to 0.9 mg/kg bw
per day (Richard, 1995).
(ii) Developmental toxicity
Groups of 16 mated female Mol:Russian rabbits were given amitrole
(purity, 97.5%) at doses of 0, 5, 20, or 80 mg/kg bw per day by gavage
on days 6-18 of gestation. The observations included daily monitoring
for clinical signs, food consumption during six periods, and maternal
body weight on day 0 and daily on days 6-18 and day 29. The animals
were killed on day 29, their fetuses were removed, and the does were
examined grossly for number of corpora lutea and implantation sites,
uterine weight, individual pup weights, appearance of fetuses or
embryos, sex of all live fetuses, individual weights of live fetuses,
and the occurrence of external malformations, visceral effects, and
effects on the skeletal system.
Statistically significantly lower maternal food consumption was
seen among does at 80 mg/kg bw per day during treatment on days 6-10
and 14-19 and throughout days 0-29, and their mean weight gain was 31%
lower than that of controls (not statistically significant) on days
6-18. Treatment at 80 mg/kg bw per day significantly reduced the body
weights of male fetuses and lowered the mean litter weight by 17%. The
NOAEL for maternal and fetal toxicity was 20 mg/kg bw per day. There
was no evidence of teratogenicity (Kolb, 1994a).
(iii) Special study: Maternal toxicity
Groups of five mated female Mol:Russian rabbits given amitrole
(purity, 97.5%) at doses of 0, 5, 20, or 80 mg/kg bw per day by gavage
on days 6-18 of gestation and monitored daily for clinical signs, food
consumption during four periods, maternal body weight on day 0 and
daily on days 6-18 and day 19. The animals were killed on day 19 and
examined grossly for the numbers of corpora lutea, implantations, and
resorptions. Clinical chemical and haematological parameters were
measured on days 6, 7, 13, and 19 before treatment, and the weights of
the liver, spleen, thyroid glands, and adrenal glands were recorded.
The liver, spleen, thyroid glands, adrenal glands, and pituitary
glands were examined histologically.
The absolute and relative weights of the liver and the levels of
albumin and protein were decreased on day 19 in rabbits treated with
20 mg/kg bw per day or more. Those at 80 mg/kg bw per day also had
lower food consumption during days 6-10 (but not days 10-18) of
treatment, increased creatine kinase activity by day 19, decreased
albumin and protein levels by day 13, decreased triiodothyronine and
thyroxine levels on days 7, 13, and 19, and slight hypertrophy of the
thyroid follicular cells, which was considered to be a minimal effect
related to the thyroid hormone levels. Changes observed in the liver
that were not considered to be adverse included a slight
treatment-related increase in the incidence and number of Kupffer-cell
foci in animals at 80 mg/kg bw per day and cytoplasmic changes
described as fine net-like markings on centrilobular heptocyte centres
and eosin-stained bands on the cell membrane in animals at 20 and 80
mg/kg bw per day. No deaths, adverse clinical signs, or body-weight
changes were reported. The numbers of corpora lutea, implantations,
and resorptions and the weights of the thyroid glands were not
affected by treatment.
The author concluded that treatment had increased cholesterol
levels in rabbits at 80 mg/kg bw per day, but that the increase was
due to one outlier and was thus spurious. The author considered the
NOAEL for maternal toxicity to be 20 mg/kg bw per day, as only
non-adverse cytoplasmic changes in the liver were seen at this dose
(Kolb, 1994b). The Meeting did not agree with this conclusion since
decreased absolute and relative liver weights and statistically
significantly decreased albumin and protein levels were seen at 20
mg/kg bw per day and above. The Meeting concluded that the NOAEL for
maternal toxicity was 5 mg/kg bw per day.
Comments
In rats, amitrole was rapidly absorbed, approximately 90% of the
administered dose being eliminated within 48 h. The primary route of
elimination was the urine, which accounted for over 87% of the dose;
faecal elimination accounted for less than 6% of the dose and volatile
metabolites represented only 0.1%. Forty-eight hours after dosing, the
tissue residues amounted to less than 3% of the dose, with the
majority found in the liver. Most of the radiolabel (62-90%) was
excreted as the parent compound. Several metabolites were present at
very low concentrations, a mercapturic acid derivative being
predominant (2.1-7.3% of the administered 14C).
In a one-year study of toxicity, dogs were fed diets containing
0, 10, 500, or 1500 ppm amitrole. At doses 500 ppm (equal to 13 mg/kg
bw per day) and above, the levels of triiodothyronine and thyroxine
were reduced and there were increases in the incidence of rough coats,
in thyroid weights, and in the frequency of lesions of the thyroid
(follicular-cell hyperplasia, capsular fibrosis, vasculitis, and
dilatation of the vasculature). In addition, ectopic thyroids with
follicular hyperplasia and capsular fibrosis were noted, and changes
in the pituitary (hyperplasia and hypertrophy) were evident in both
males and females. At 1500 ppm (equal to 32 mg/kg bw per day), both
males and females were anaemic and had increased platelet counts,
decreased food intake, decreased brain weights (with histopathological
lesions), and neurological effects. At this dose, males also had
decreased weight gain, increased lactate dehydrogenase activity and
cholesterol levels, decreased heart weights, and decreased P- and
R-wave amplitudes. The NOAEL was 10 ppm, equal to 0.29 mg/kg bw per
day.
In a two-generation study of reproductive toxicity, rats were fed
diets containing 0, 0.5, 2, 15, or 110 ppm amitrole. At 15 ppm (equal
to 0.9 mg/kg bw per day), the only effect observed was a slight
increase in the severity of some histopathological changes in the
thyroid, including small follicles, decreased colloid content, and
follicular epithelial hypertrophy. Parental toxicity at 110 ppm was
severe and was characterized by mortality, clinical signs,
considerably decreased weight gain, thyroid effects (increased
relative thyroid weights, follicular epithelial hyperplasia, and
vascular ectasia), changes in relative organ weights (increased
weights of pituitary, testes, epididymides, seminal vesicles,
prostate, uterus, spleen, and kidneys and decreased weights of
ovaries, adrenal glands, and liver), and histopathological lesions in
the liver, adrenal glands, kidneys, and numerous reproductive tissues.
In pups, the only observations were increased thyroid weights with
accompanying histopathological lesions. The effects on reproduction
included decreased mating and fertility indices, increased gestation
length, and decreased prenatal survival, litter size, pup body weight,
and viability. The NOAEL for systemic toxicity was 2 ppm, equal to
0.12 mg/kg bw per day. This value is supported by the findings of the
range-finding study, in which enlarged, reddened thyroids and
decreased colloid content were noted at 10 ppm (equal to 0.62 mg/kg bw
per day). The NOAEL for reproductive toxicity was 15 ppm, equal to 0.9
mg/kg bw per day.
In a study of developmental toxicity, rabbits were treated with
0, 5, 20, or 80 mg/kg bw per day amitrole on days 6-18 of gestation.
At 80 mg/kg bw per day, maternal food consumption and body-weight gain
were reduced during treatment. Male fetal body weights and mean litter
weights were reduced at 80 mg/kg bw per day. Clinical chemistry was
not evaluated in this study. The NOAEL for maternal and fetal toxicity
was 20 mg/kg bw per day.
In a special study to further characterize the toxicity in
pregnant rabbits, the animals were treated with amitrole at 0, 5, 20,
or 80 mg/kg bw per day on days 6-18 of gestation and were killed on
day 19 of gestation. At 20 and 80 mg/kg bw per day, albumin and
protein levels were decreased and absolute and relative liver weights
were decreased. At 80 mg/kg bw per day, there were reductions in food
consumption, increased creatine kinase levels, decreased
triiodothyronine and thyroxine levels, and thyroid follicular-cell
hypertrophy. The NOAEL for maternal toxicity was 5 mg/kg bw per day.
The results of these supplemental studies correlate well with the
results of the studies reviewed by the 1993 JMPR, showing that the
thyroid is the primary target organ of amitrole. Contrary to the
conclusions of the 1993 JMPR, the results demonstrate that amitrole is
also goitrogenic in the dog. In the one-year study in dogs reviewed
previously, no effects on the thyroid were noted at a dose of
12.5 mg/kg bw per day. In the current one-year study, however, there
were extensive effects at 13 mg/kg bw per day.
The rat was the most sensitive species, the NOAELs being 2 ppm in
both the two-generation study of reproductive toxicity (equal to 0.12
mg/kg bw per day) and the 90-day dietary study (equivalent to 0.1
mg/kg bw per day), on the basis of histopathological changes in the
thyroid. The Meeting noted that the rat is more sensitive to the
development of thyroid hyperplasia and subsequent neoplasia after
exposure to goitrogenic compounds than are humans. Therefore, a
smaller safety factor of 50 was used to take into account the reduced
uncertainty of interspecies extrapolation, giving an ADI of
0-0.002 mg/kg bw. This ADI provides a 150-fold margin of safety over
the NOAEL of 0.29 mg/kg bw per day in the one-year study in dogs.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 10 ppm, equivalent to 1.5 mg/kg bw per day (18-month
study of toxicity and carcinogenicity)
Rat: 10 ppm, equivalent to 0.5 mg/kg bw per day (two-year
study of toxicity and carcinogenicity)
2 ppm, equal to 0.12 mg/kg bw per day (parental
toxicity in a two-generation study of reproductive
toxicity)
100 mg/kg bw per day (maternal and fetal toxicity
in a study of developmental toxicity)
Rabbit: 5 mg/kg bw per day (maternal toxicity in a study of
developmental toxicity)
20 mg/kg bw per day (fetal toxicity in a study of
developmental toxicity)
Hamster: 10 ppm, equivalent to 1 mg/kg bw per day (18-month
study of toxicity)
Dog: 10 ppm, equal to 0.29 mg/kg bw per day (one-year study
of toxicity)
Estimate of acceptable daily intake for humans
0-0.002 mg/kg bw
Studies that would provide information useful for continued evaluation
of the compound
1. Clarification of the genotoxic potential of amitrole (e.g.
DNA adducts in vivo)
2. Further observations in humans.
Toxicological criteria for estimating guidance values for dietary and non-dietary exposure to amitrole
Human exposure Relevant route, study type, species Results, remarks
Short-term
(1-7 days) Oral, acute toxicity, rat LD50 > 2500 mg/kg bw
Dermal, acute toxicity, rat LD50 > 2500 mg/kg bw
Inhalation, acute toxicity, 4 h, rat LC50 > 439 mg/L
Dermal, irritation, rabbit Minimally irritating
Ocular, irritation, rabbit Mildly irritating
Dermal, sensitization, guinea-pig Moderately sensitizing (Magnusson-Kligman test)
Non-sensitizing (Klecak open epicutaneous test)
Medium-term Repeated oral, 2-4 weeks, rat NOAEL = 3 mg/kg bw per day: effects on the
(1-26 weeks) thyroid, food consumption and body weight
Repeated oral, drinking-water, 4 weeks, rat NOAEL = 10 mg/L water: decreased weight gain,
enlarged thyroids
Repeated oral, 6-13 weeks, rat NOAEL = 0.1 mg/kg bw per day: effects on the thyroid
Repeated dermal, 3 weeks, rabbit NOAEL = 100 mg/kg bw per day (highest dose
tested)
Repeated inhalation, 4 weeks, rat NOAEL = 0.1 mg/L: effects on the thyroid
Repeated oral, special maternal toxicity, rabbit NOAEL = 5 mg/kg bw per day: decreased liver
weights and protein/albumin levels
Repeated oral, developmental toxicity, rabbit NOAEL = 20 mg/kg bw per day: developmental
toxicity
Repeated dermal, developmental toxicity, rabbit NOAEL = 1500 mg/kg bw per day: maternal and
developmental toxicity
Repeated oral, reproductive toxicity, rat NOAEL = 0.12 mg/kg bw per day (systemic):
effects on the thyroid
NOAEL = 0.9 mg/kg bw per day: reproductive
toxicity
Long-term Repeated oral, 1 year, dog NOAEL = 0.29 mg/kg bw per day: effects on the
(> 1 year) thyroid
Repeated inhalation, intermittent dosing, 2 years, LOAEL = 50 µg/L (lowest dose tested): effects on
carcinogenicity, rat the thyroid
References
Anderson, C. & Brauner, A. (1995) Amitrole: Investigation of the
biokinetic behaviour and the metabolism in the rat. Unpublished
reports Nos MR-508/95, ANC82, and BNA81 (study No. M41819023) from
Bayer AG, Agrochemicals Division, Institute for Metabolism Research
and Residue Analysis, Leverkusen, Germany. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Jones, R.D. & Lake, S.G. (1994) Technical grade amitrole: A chronic
toxicity study in the beagle dog. Unpublished report No. 7413 (study
No. 92-276-RX) from Miles Inc., Agriculture Division, Toxicology,
Stilwell, KS, USA. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Kolb, J. (1994a) Amitrole: Developmental toxicity study in rabbits
after oral administration. Unpublished report No. 23486 (study No.
T5044250) from Bayer AG, Department of Toxicology, Wuppertal, Germany.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Kolb, J. (1994b) Amitrole: Supplementary study on maternal toxicity
study in pregnant rabbits after oral administration. Unpublished
report No. 23486 (study No. T5044284) from Bayer AG, Department of
Toxicology, Wuppertal, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Richard, J. (1995) Two generation study by oral route (dietary
admixture) in rats. Unpublished report No. 6432 (study No. T5041262)
from Centre International de Toxicologie, Evreux, France. Submitted to
WHO by Bayer AG, Leverkusen, Germany.
Savary, M.-H. (1994) Preliminary study to a two-generation study by
oral route (dietary admixture) in rats. Unpublished report No. 6306
(study No. T6041263) from Centre International de Toxicologie, Evreux,
France. Submitted to WHO by Bayer AG, Leverkusen, Germany.
WHO (1994) Amitrole (Environmental Health Criteria 158), Geneva
 hypothyroidism were the statistically significantly lower erythrocyte,
haemoglobin, and haematocrit counts and the slightly lower mean cell
volume and mean cell haemoglobin in males and females at 1500 ppm.
There was also a statistically significantly increased incidence of
hypochromasia. Although the authors concluded that there was mild,
non-regenerative, normochromic anaemia, the effect on mean cell volume
and mean cell haemoglobin would indicate mild, hypochromic, microcytic
anaemia. An increase in cholesterol levels was also seen in males at
the high dose.
Statistically significantly decreased absolute and relative heart
weights (by about 25%) were seen in males at 1500 ppm, which were
reflected in decreases in P-wave and R-wave amplitudes in this group.
The neurological examinations showed decreased jaw tone (related to
the trigeminal nerve) and/or temporal muscle atrophy in males at the
high dose. Limb muscle weakness and decreased postural hopping
reactions (hemiwalk and hemihop) accompanied by proprioceptive
deficits and reluctance to walk and/or dragging of the limbs were seen
in two males and one female at 1500 ppm. The authors stated that these
effects are consistent with the weakness, exercise intolerance, and
stiffness associated with canine hypothyroidism. While muscle atrophy
and inactivity are commonly observed in hypothyroidism, no
documentation was provided to specifically link proprioceptive
deficits and postural hopping reactions with hypothyroidism, and these
changes may be independent of the effects on the thyroid. Another
significant sign of systemic toxicity was a treatment-related
reduction in the body-weight gain of males at the high dose during the
first six months, which was coupled with 40% reductions in food
consumption throughout the study. Although the authors considered that
amitrole had no meaningful effects on body weight, the body weights of
these animals were consistently 10% lower and their weight gain 20-30%
lower than those of controls (not statistically significant). The food
consumption of females at the high-dose was also reduced, but to a
lesser extent (15-20%). Other indications of systemic toxicity
included a clear eye discharge in males at the intermediate dose and
males and females at the high dose, increased levels of lactic
dehydrogenase in males at the high dose, statistically significantly
increased platelet counts in males and females at the high dose,
statistically significantly lower brain weights (15%) and inflammation
with or without perivascular cuffing in the brains of males and
females at 1500 ppm, and pigmentation and epithelial-cell hyperplasia
in the gall-bladders of these animals. The authors also concluded that
amitrole induced changes in the blood levels of albumin, aspartate
aminotransferase, and sodium. Owing to the high variability, both over
time and among groups, the observed changes in these parameters could
not, however, be clearly attributed to treatment. There were no
effects on mortality, heart rate, blood pressure, or urinary
parameters. Males appeared to be more sensitive than females. The
NOAEL was 10 ppm, equal to 0.29 mg/kg bw per day, on the basis of
goitrogenic effects at doses of 500 ppm and above (Jones & Lake,
1994).
(b) Reproductive toxicity
(i) Multigeneration reproductive toxicity
In a one-generation, one-litter, dose-finding study of
reproductive toxicity, groups of 10 male and 10 female Sprague-Dawley
Crl:CD(SD)BR rats were fed amitrole (purity, 97.4%) in the diet at
levels of 2, 10, 40, or 160 ppm. F0 animals were treated for 29 days
before mating up to weaning of the F1 pups, providing doses of 0.12,
0.62, 2.5, and 8.3 mg/kg bw per day for F0 males; 0.19, 0.94, 3.7,
and 12 mg/kg bw per day for F0 females; 0.29, 1.4, 5.3, and 28 mg/kg
bw per day for F1 males; and 0.31, 1.6, 5.8, and 28 mg/kg bw per day
for F1 females. When possible, one F1 pup of each sex per litter was
killed for histopathological examination on day 14 post partum, and
two pups of each sex per litter were selected after weaning for
treatment for a further 36 days. Mortality, clinical signs, body
weight, and food consumption were monitored at all stages of the
study, and the following indices were calculated: male mating, female
mating, male and female fertility, gestation, viability on days 4, 14,
and 21 post partum, and numbers of live births. In addition, oestrus
cycles, litter size, number of implantations, gestation length, and
sex ratio were recorded. Gross and microscopic examinations were
performed.
Five F1 males and three females given 160 ppm died after
weaning, and three males and three females in this group showed one or
more of the following signs before premature sacrifice: piloerection,
cold to touch, pale extremities, hypersensitivity to touch, soiled
urogenital region, hypokinesia, dyspnoea, staggering gait, swollen
abdomen, sedation, and tremors. Statistically significantly decreased
body weights were seen at 160 ppm among F0 males, among F0 females
during gestation and lactation, among F1 pups on days 7-21 post
partum and after weaning, and in F1 males at 40 ppm. Significantly
decreased food consumption was seen throughout the study in F0 and
F1 animals given the high dose and in males of the F1 generation at
40 ppm. All of these effects were considered to be toxicologically
significant. Females at the high dose had a lower implantation rate
(12.1 implantation sites) than controls (16.6 sites), which was
reflected in significantly lower birth rates and day-1 litter sizes.
The viability indices on days 4, 14, and 21 were not decreased by
treatment. The number of females with a normal oestrus cycle was
decreased in a dose-related fashion among animals at 40 or 160 ppm,
due in the latter group to the smaller number of implantations.
The thyroid glands of F0 males and females showed a dose-related
increase in size and in the incidence of reddish colour, starting at
10 ppm in the males and at 40 ppm in the females. All animals at 160
ppm had reddened pituitary glands and greyish-white foci on the lungs,
and the adrenal glands and spleens were reduced in size. Male and
female F1 adults given 40 or 160 ppm and killed at the end of the
study had enlarged, reddened thyroid glands and reddened pituitary
glands. Histopathological examination showed dose-related effects in
the liver and pituitary gland in F0 and F1 animals at these doses,
decreased colloid content in females of both generations at 10, 40, or
160 ppm, vascular ectasia in all animals of both generations treated
with 40 or 160 ppm, and peri-adenitis in a small number of F0 males
at 160 ppm. No treatment-related histopathological lesions were seen
in pups killed on day 14 post partum.
Doses were selected for use in the main study on the basis of the
reproductive effects and toxicity seen at 160 ppm and the minimal
toxicity observed in males treated with 40 ppm amitrole (Savary,
1994).
In the main study, groups of 30 male and 30 female Sprague-Dawley
Crl:CD(SD)BR rats were fed diets containing amitrole (purity, 97-98%)
at levels of 0.5, 2, 15, or 110 ppm for two generations, with one
litter per generation. These doses were equivalent to 0.03, 0.12, 0.9,
or 5.9 mg/kg bw per day in F0 males; 0.04, 0.16, 1.2, or 12 mg/kg bw
per day in F1 males; 0.04, 0.16, 1.2, or 7.8 mg/kg bw per day in F0
females; and 0.05, 0.21, 1.6, or 16 mg/kg bw per day in F1 females.
Treatment was begun in the parental animals 72 days before mating and
was continued throughout gestation and lactation. Mortality, clinical
signs, body weight, and food consumption were monitored at all stages
of the study, and the following indices were calculated: male mating,
female mating, male and female fertility, gestation, viability on days
4 and 21 post partum, and numbers of live births. Additional
parameters measured included pup development, litter size, number of
implantations, and length of gestation. All animals were examined
grossly, and controls and animals at the high dose were examined
microscopically. The absolute and relative weights (as a percentage of
body weight) of 11 organs were determined.
Death or clinical signs were observed in 10/32 F1 males and
16/31 females treated with 110 ppm amitrole, and both F0 and F1
parental animals at this dose had statistically and toxicologically
significant decreases in body weight throughout the study. By the end
of pre-mating, the body weights of F0 males were 60% those of the
controls, and those of the females were 85% those of controls. The F1
animals at this dose were more severely affected, their mean body
weights being only about 35% of those of controls. All parental
animals at this dose had statistically and toxicologically
significantly decreased food consumption.
Animals at the two lower doses had no changes in organ weight or
histopathological appearance; however, those at 110 ppm had changes
indicative of widespread systemic toxicity, including significantly
increased relative thyroid gland weights and treatment-related
histopathological lesions such as follicular epithelial hyperplasia
and vascular ectasia, in most animals. The relative pituitary gland
weights were also significantly increased in F0 and F1 males and F1
females, with treatment-related histopathological changes, including
vacuolated cells and decreased numbers of acidophils, in F0 females
and F1 males and females. At this dose, significant increases were
also seen in the relative weights of the testis, epididymides, and
seminal vesicles (both generations), prostate (F1), and uterus (F1);
decreased relative ovarian weights were seen in the F0 generation.
Slightly increased incidences of histopathological effects were seen
in the testis and epididymides of F1 animals and in the ovaries,
uterus, and vagina in both generations. Many of the histopathological
effects on the reproductive tissues, including the prostate and
seminal vesicles, were related to immaturity, which is consistent with
the small size of the animals at this dose. Other signs of systemic
toxicity in animals at the high dose included significantly decreased
relative weights of the adrenal glands in F0 males and females and
F1 females but a significant increase in F1 males. Both generations
of males showed an increased frequency of ceroid-laden cortical cells,
and the F0 females had an increased incidence of cortical atrophy.
All animals at the high dose had significantly increased relative
spleen weights, and treatment-related histopathological effects were
seen in F0 males and females and F1 males. While only F1 males had
increased relative kidney weights, the remaining animals had
treatment-related increases in the incidence of numerous renal
lesions. Hepatocellular hypertrophy was seen in most animals at
110 ppm, but the decrease in relative liver weight was significant
only in F1 males. F1 pups at this dose had toxicologically
significantly increases in absolute and relative thyroid gland weights
and related gross pathological and histopathological lesions. Owing to
the low survival rate of F2 pups, treatment-related changes in organ
weight and pathological effects could not be identified in this group.
The following reproductive effects seen in animals at 110 ppm
were considered to be toxicologically significant: significantly
decreased mating indices among F1 males and females, decreased F1
male and female fertility indices, increased length of gestation in
F1 females (from 21-22 to 23 days), low implantation rate in F0 and
F1 females, low prenatal survival (59%) in the F2 generation,
significantly decreased mean litter size at day 1 (F1 and F2) and
day 21 (F2 only) post partum, significantly decreased mean litter
size on day 4 post partum (F2), very low viability indices for the
F2 generation on days 4 (33%) and 21 (37%), and decreased F1 and F2
pup body weights during lactation. In the F2 generation at 110 ppm,
the pups in four of five liveborn litters died during the first day,
and only three pups out of 24 liveborn pups of the remaining litter
survived to the end of lactation. There appeared to be no effects on
F1 pup development, sex ratio, or gross appearance at necropsy; there
were insufficient data to assess these parameters in the F2
generation. The author reported an NOAEL of 2 ppm for systemic
toxicity on the basis of minimally increased thyroid activity;
however, thyroid hormone levels were not measured and no other effects
on the thyroid were noted in rats at 15 ppm. The NOAEL for systemic
toxicity and reproductive toxicity was 15 ppm, equal to 0.9 mg/kg bw
per day (Richard, 1995).
(ii) Developmental toxicity
Groups of 16 mated female Mol:Russian rabbits were given amitrole
(purity, 97.5%) at doses of 0, 5, 20, or 80 mg/kg bw per day by gavage
on days 6-18 of gestation. The observations included daily monitoring
for clinical signs, food consumption during six periods, and maternal
body weight on day 0 and daily on days 6-18 and day 29. The animals
were killed on day 29, their fetuses were removed, and the does were
examined grossly for number of corpora lutea and implantation sites,
uterine weight, individual pup weights, appearance of fetuses or
embryos, sex of all live fetuses, individual weights of live fetuses,
and the occurrence of external malformations, visceral effects, and
effects on the skeletal system.
Statistically significantly lower maternal food consumption was
seen among does at 80 mg/kg bw per day during treatment on days 6-10
and 14-19 and throughout days 0-29, and their mean weight gain was 31%
lower than that of controls (not statistically significant) on days
6-18. Treatment at 80 mg/kg bw per day significantly reduced the body
weights of male fetuses and lowered the mean litter weight by 17%. The
NOAEL for maternal and fetal toxicity was 20 mg/kg bw per day. There
was no evidence of teratogenicity (Kolb, 1994a).
(iii) Special study: Maternal toxicity
Groups of five mated female Mol:Russian rabbits given amitrole
(purity, 97.5%) at doses of 0, 5, 20, or 80 mg/kg bw per day by gavage
on days 6-18 of gestation and monitored daily for clinical signs, food
consumption during four periods, maternal body weight on day 0 and
daily on days 6-18 and day 19. The animals were killed on day 19 and
examined grossly for the numbers of corpora lutea, implantations, and
resorptions. Clinical chemical and haematological parameters were
measured on days 6, 7, 13, and 19 before treatment, and the weights of
the liver, spleen, thyroid glands, and adrenal glands were recorded.
The liver, spleen, thyroid glands, adrenal glands, and pituitary
glands were examined histologically.
The absolute and relative weights of the liver and the levels of
albumin and protein were decreased on day 19 in rabbits treated with
20 mg/kg bw per day or more. Those at 80 mg/kg bw per day also had
lower food consumption during days 6-10 (but not days 10-18) of
treatment, increased creatine kinase activity by day 19, decreased
albumin and protein levels by day 13, decreased triiodothyronine and
thyroxine levels on days 7, 13, and 19, and slight hypertrophy of the
thyroid follicular cells, which was considered to be a minimal effect
related to the thyroid hormone levels. Changes observed in the liver
that were not considered to be adverse included a slight
treatment-related increase in the incidence and number of Kupffer-cell
foci in animals at 80 mg/kg bw per day and cytoplasmic changes
described as fine net-like markings on centrilobular heptocyte centres
and eosin-stained bands on the cell membrane in animals at 20 and 80
mg/kg bw per day. No deaths, adverse clinical signs, or body-weight
changes were reported. The numbers of corpora lutea, implantations,
and resorptions and the weights of the thyroid glands were not
affected by treatment.
The author concluded that treatment had increased cholesterol
levels in rabbits at 80 mg/kg bw per day, but that the increase was
due to one outlier and was thus spurious. The author considered the
NOAEL for maternal toxicity to be 20 mg/kg bw per day, as only
non-adverse cytoplasmic changes in the liver were seen at this dose
(Kolb, 1994b). The Meeting did not agree with this conclusion since
decreased absolute and relative liver weights and statistically
significantly decreased albumin and protein levels were seen at 20
mg/kg bw per day and above. The Meeting concluded that the NOAEL for
maternal toxicity was 5 mg/kg bw per day.
Comments
In rats, amitrole was rapidly absorbed, approximately 90% of the
administered dose being eliminated within 48 h. The primary route of
elimination was the urine, which accounted for over 87% of the dose;
faecal elimination accounted for less than 6% of the dose and volatile
metabolites represented only 0.1%. Forty-eight hours after dosing, the
tissue residues amounted to less than 3% of the dose, with the
majority found in the liver. Most of the radiolabel (62-90%) was
excreted as the parent compound. Several metabolites were present at
very low concentrations, a mercapturic acid derivative being
predominant (2.1-7.3% of the administered 14C).
In a one-year study of toxicity, dogs were fed diets containing
0, 10, 500, or 1500 ppm amitrole. At doses 500 ppm (equal to 13 mg/kg
bw per day) and above, the levels of triiodothyronine and thyroxine
were reduced and there were increases in the incidence of rough coats,
in thyroid weights, and in the frequency of lesions of the thyroid
(follicular-cell hyperplasia, capsular fibrosis, vasculitis, and
dilatation of the vasculature). In addition, ectopic thyroids with
follicular hyperplasia and capsular fibrosis were noted, and changes
in the pituitary (hyperplasia and hypertrophy) were evident in both
males and females. At 1500 ppm (equal to 32 mg/kg bw per day), both
males and females were anaemic and had increased platelet counts,
decreased food intake, decreased brain weights (with histopathological
lesions), and neurological effects. At this dose, males also had
decreased weight gain, increased lactate dehydrogenase activity and
cholesterol levels, decreased heart weights, and decreased P- and
R-wave amplitudes. The NOAEL was 10 ppm, equal to 0.29 mg/kg bw per
day.
In a two-generation study of reproductive toxicity, rats were fed
diets containing 0, 0.5, 2, 15, or 110 ppm amitrole. At 15 ppm (equal
to 0.9 mg/kg bw per day), the only effect observed was a slight
increase in the severity of some histopathological changes in the
thyroid, including small follicles, decreased colloid content, and
follicular epithelial hypertrophy. Parental toxicity at 110 ppm was
severe and was characterized by mortality, clinical signs,
considerably decreased weight gain, thyroid effects (increased
relative thyroid weights, follicular epithelial hyperplasia, and
vascular ectasia), changes in relative organ weights (increased
weights of pituitary, testes, epididymides, seminal vesicles,
prostate, uterus, spleen, and kidneys and decreased weights of
ovaries, adrenal glands, and liver), and histopathological lesions in
the liver, adrenal glands, kidneys, and numerous reproductive tissues.
In pups, the only observations were increased thyroid weights with
accompanying histopathological lesions. The effects on reproduction
included decreased mating and fertility indices, increased gestation
length, and decreased prenatal survival, litter size, pup body weight,
and viability. The NOAEL for systemic toxicity was 2 ppm, equal to
0.12 mg/kg bw per day. This value is supported by the findings of the
range-finding study, in which enlarged, reddened thyroids and
decreased colloid content were noted at 10 ppm (equal to 0.62 mg/kg bw
per day). The NOAEL for reproductive toxicity was 15 ppm, equal to 0.9
mg/kg bw per day.
In a study of developmental toxicity, rabbits were treated with
0, 5, 20, or 80 mg/kg bw per day amitrole on days 6-18 of gestation.
At 80 mg/kg bw per day, maternal food consumption and body-weight gain
were reduced during treatment. Male fetal body weights and mean litter
weights were reduced at 80 mg/kg bw per day. Clinical chemistry was
not evaluated in this study. The NOAEL for maternal and fetal toxicity
was 20 mg/kg bw per day.
In a special study to further characterize the toxicity in
pregnant rabbits, the animals were treated with amitrole at 0, 5, 20,
or 80 mg/kg bw per day on days 6-18 of gestation and were killed on
day 19 of gestation. At 20 and 80 mg/kg bw per day, albumin and
protein levels were decreased and absolute and relative liver weights
were decreased. At 80 mg/kg bw per day, there were reductions in food
consumption, increased creatine kinase levels, decreased
triiodothyronine and thyroxine levels, and thyroid follicular-cell
hypertrophy. The NOAEL for maternal toxicity was 5 mg/kg bw per day.
The results of these supplemental studies correlate well with the
results of the studies reviewed by the 1993 JMPR, showing that the
thyroid is the primary target organ of amitrole. Contrary to the
conclusions of the 1993 JMPR, the results demonstrate that amitrole is
also goitrogenic in the dog. In the one-year study in dogs reviewed
previously, no effects on the thyroid were noted at a dose of
12.5 mg/kg bw per day. In the current one-year study, however, there
were extensive effects at 13 mg/kg bw per day.
The rat was the most sensitive species, the NOAELs being 2 ppm in
both the two-generation study of reproductive toxicity (equal to 0.12
mg/kg bw per day) and the 90-day dietary study (equivalent to 0.1
mg/kg bw per day), on the basis of histopathological changes in the
thyroid. The Meeting noted that the rat is more sensitive to the
development of thyroid hyperplasia and subsequent neoplasia after
exposure to goitrogenic compounds than are humans. Therefore, a
smaller safety factor of 50 was used to take into account the reduced
uncertainty of interspecies extrapolation, giving an ADI of
0-0.002 mg/kg bw. This ADI provides a 150-fold margin of safety over
the NOAEL of 0.29 mg/kg bw per day in the one-year study in dogs.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 10 ppm, equivalent to 1.5 mg/kg bw per day (18-month
study of toxicity and carcinogenicity)
Rat: 10 ppm, equivalent to 0.5 mg/kg bw per day (two-year
study of toxicity and carcinogenicity)
2 ppm, equal to 0.12 mg/kg bw per day (parental
toxicity in a two-generation study of reproductive
toxicity)
100 mg/kg bw per day (maternal and fetal toxicity
in a study of developmental toxicity)
Rabbit: 5 mg/kg bw per day (maternal toxicity in a study of
developmental toxicity)
20 mg/kg bw per day (fetal toxicity in a study of
developmental toxicity)
Hamster: 10 ppm, equivalent to 1 mg/kg bw per day (18-month
study of toxicity)
Dog: 10 ppm, equal to 0.29 mg/kg bw per day (one-year study
of toxicity)
Estimate of acceptable daily intake for humans
0-0.002 mg/kg bw
Studies that would provide information useful for continued evaluation
of the compound
1. Clarification of the genotoxic potential of amitrole (e.g.
DNA adducts in vivo)
2. Further observations in humans.
Toxicological criteria for estimating guidance values for dietary and non-dietary exposure to amitrole
Human exposure Relevant route, study type, species Results, remarks
Short-term
(1-7 days) Oral, acute toxicity, rat LD50 > 2500 mg/kg bw
Dermal, acute toxicity, rat LD50 > 2500 mg/kg bw
Inhalation, acute toxicity, 4 h, rat LC50 > 439 mg/L
Dermal, irritation, rabbit Minimally irritating
Ocular, irritation, rabbit Mildly irritating
Dermal, sensitization, guinea-pig Moderately sensitizing (Magnusson-Kligman test)
Non-sensitizing (Klecak open epicutaneous test)
Medium-term Repeated oral, 2-4 weeks, rat NOAEL = 3 mg/kg bw per day: effects on the
(1-26 weeks) thyroid, food consumption and body weight
Repeated oral, drinking-water, 4 weeks, rat NOAEL = 10 mg/L water: decreased weight gain,
enlarged thyroids
Repeated oral, 6-13 weeks, rat NOAEL = 0.1 mg/kg bw per day: effects on the thyroid
Repeated dermal, 3 weeks, rabbit NOAEL = 100 mg/kg bw per day (highest dose
tested)
Repeated inhalation, 4 weeks, rat NOAEL = 0.1 mg/L: effects on the thyroid
Repeated oral, special maternal toxicity, rabbit NOAEL = 5 mg/kg bw per day: decreased liver
weights and protein/albumin levels
Repeated oral, developmental toxicity, rabbit NOAEL = 20 mg/kg bw per day: developmental
toxicity
Repeated dermal, developmental toxicity, rabbit NOAEL = 1500 mg/kg bw per day: maternal and
developmental toxicity
Repeated oral, reproductive toxicity, rat NOAEL = 0.12 mg/kg bw per day (systemic):
effects on the thyroid
NOAEL = 0.9 mg/kg bw per day: reproductive
toxicity
Long-term Repeated oral, 1 year, dog NOAEL = 0.29 mg/kg bw per day: effects on the
(> 1 year) thyroid
Repeated inhalation, intermittent dosing, 2 years, LOAEL = 50 µg/L (lowest dose tested): effects on
carcinogenicity, rat the thyroid
References
Anderson, C. & Brauner, A. (1995) Amitrole: Investigation of the
biokinetic behaviour and the metabolism in the rat. Unpublished
reports Nos MR-508/95, ANC82, and BNA81 (study No. M41819023) from
Bayer AG, Agrochemicals Division, Institute for Metabolism Research
and Residue Analysis, Leverkusen, Germany. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Jones, R.D. & Lake, S.G. (1994) Technical grade amitrole: A chronic
toxicity study in the beagle dog. Unpublished report No. 7413 (study
No. 92-276-RX) from Miles Inc., Agriculture Division, Toxicology,
Stilwell, KS, USA. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Kolb, J. (1994a) Amitrole: Developmental toxicity study in rabbits
after oral administration. Unpublished report No. 23486 (study No.
T5044250) from Bayer AG, Department of Toxicology, Wuppertal, Germany.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Kolb, J. (1994b) Amitrole: Supplementary study on maternal toxicity
study in pregnant rabbits after oral administration. Unpublished
report No. 23486 (study No. T5044284) from Bayer AG, Department of
Toxicology, Wuppertal, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Richard, J. (1995) Two generation study by oral route (dietary
admixture) in rats. Unpublished report No. 6432 (study No. T5041262)
from Centre International de Toxicologie, Evreux, France. Submitted to
WHO by Bayer AG, Leverkusen, Germany.
Savary, M.-H. (1994) Preliminary study to a two-generation study by
oral route (dietary admixture) in rats. Unpublished report No. 6306
(study No. T6041263) from Centre International de Toxicologie, Evreux,
France. Submitted to WHO by Bayer AG, Leverkusen, Germany.
WHO (1994) Amitrole (Environmental Health Criteria 158), Geneva
hypothyroidism were the statistically significantly lower erythrocyte,
haemoglobin, and haematocrit counts and the slightly lower mean cell
volume and mean cell haemoglobin in males and females at 1500 ppm.
There was also a statistically significantly increased incidence of
hypochromasia. Although the authors concluded that there was mild,
non-regenerative, normochromic anaemia, the effect on mean cell volume
and mean cell haemoglobin would indicate mild, hypochromic, microcytic
anaemia. An increase in cholesterol levels was also seen in males at
the high dose.
Statistically significantly decreased absolute and relative heart
weights (by about 25%) were seen in males at 1500 ppm, which were
reflected in decreases in P-wave and R-wave amplitudes in this group.
The neurological examinations showed decreased jaw tone (related to
the trigeminal nerve) and/or temporal muscle atrophy in males at the
high dose. Limb muscle weakness and decreased postural hopping
reactions (hemiwalk and hemihop) accompanied by proprioceptive
deficits and reluctance to walk and/or dragging of the limbs were seen
in two males and one female at 1500 ppm. The authors stated that these
effects are consistent with the weakness, exercise intolerance, and
stiffness associated with canine hypothyroidism. While muscle atrophy
and inactivity are commonly observed in hypothyroidism, no
documentation was provided to specifically link proprioceptive
deficits and postural hopping reactions with hypothyroidism, and these
changes may be independent of the effects on the thyroid. Another
significant sign of systemic toxicity was a treatment-related
reduction in the body-weight gain of males at the high dose during the
first six months, which was coupled with 40% reductions in food
consumption throughout the study. Although the authors considered that
amitrole had no meaningful effects on body weight, the body weights of
these animals were consistently 10% lower and their weight gain 20-30%
lower than those of controls (not statistically significant). The food
consumption of females at the high-dose was also reduced, but to a
lesser extent (15-20%). Other indications of systemic toxicity
included a clear eye discharge in males at the intermediate dose and
males and females at the high dose, increased levels of lactic
dehydrogenase in males at the high dose, statistically significantly
increased platelet counts in males and females at the high dose,
statistically significantly lower brain weights (15%) and inflammation
with or without perivascular cuffing in the brains of males and
females at 1500 ppm, and pigmentation and epithelial-cell hyperplasia
in the gall-bladders of these animals. The authors also concluded that
amitrole induced changes in the blood levels of albumin, aspartate
aminotransferase, and sodium. Owing to the high variability, both over
time and among groups, the observed changes in these parameters could
not, however, be clearly attributed to treatment. There were no
effects on mortality, heart rate, blood pressure, or urinary
parameters. Males appeared to be more sensitive than females. The
NOAEL was 10 ppm, equal to 0.29 mg/kg bw per day, on the basis of
goitrogenic effects at doses of 500 ppm and above (Jones & Lake,
1994).
(b) Reproductive toxicity
(i) Multigeneration reproductive toxicity
In a one-generation, one-litter, dose-finding study of
reproductive toxicity, groups of 10 male and 10 female Sprague-Dawley
Crl:CD(SD)BR rats were fed amitrole (purity, 97.4%) in the diet at
levels of 2, 10, 40, or 160 ppm. F0 animals were treated for 29 days
before mating up to weaning of the F1 pups, providing doses of 0.12,
0.62, 2.5, and 8.3 mg/kg bw per day for F0 males; 0.19, 0.94, 3.7,
and 12 mg/kg bw per day for F0 females; 0.29, 1.4, 5.3, and 28 mg/kg
bw per day for F1 males; and 0.31, 1.6, 5.8, and 28 mg/kg bw per day
for F1 females. When possible, one F1 pup of each sex per litter was
killed for histopathological examination on day 14 post partum, and
two pups of each sex per litter were selected after weaning for
treatment for a further 36 days. Mortality, clinical signs, body
weight, and food consumption were monitored at all stages of the
study, and the following indices were calculated: male mating, female
mating, male and female fertility, gestation, viability on days 4, 14,
and 21 post partum, and numbers of live births. In addition, oestrus
cycles, litter size, number of implantations, gestation length, and
sex ratio were recorded. Gross and microscopic examinations were
performed.
Five F1 males and three females given 160 ppm died after
weaning, and three males and three females in this group showed one or
more of the following signs before premature sacrifice: piloerection,
cold to touch, pale extremities, hypersensitivity to touch, soiled
urogenital region, hypokinesia, dyspnoea, staggering gait, swollen
abdomen, sedation, and tremors. Statistically significantly decreased
body weights were seen at 160 ppm among F0 males, among F0 females
during gestation and lactation, among F1 pups on days 7-21 post
partum and after weaning, and in F1 males at 40 ppm. Significantly
decreased food consumption was seen throughout the study in F0 and
F1 animals given the high dose and in males of the F1 generation at
40 ppm. All of these effects were considered to be toxicologically
significant. Females at the high dose had a lower implantation rate
(12.1 implantation sites) than controls (16.6 sites), which was
reflected in significantly lower birth rates and day-1 litter sizes.
The viability indices on days 4, 14, and 21 were not decreased by
treatment. The number of females with a normal oestrus cycle was
decreased in a dose-related fashion among animals at 40 or 160 ppm,
due in the latter group to the smaller number of implantations.
The thyroid glands of F0 males and females showed a dose-related
increase in size and in the incidence of reddish colour, starting at
10 ppm in the males and at 40 ppm in the females. All animals at 160
ppm had reddened pituitary glands and greyish-white foci on the lungs,
and the adrenal glands and spleens were reduced in size. Male and
female F1 adults given 40 or 160 ppm and killed at the end of the
study had enlarged, reddened thyroid glands and reddened pituitary
glands. Histopathological examination showed dose-related effects in
the liver and pituitary gland in F0 and F1 animals at these doses,
decreased colloid content in females of both generations at 10, 40, or
160 ppm, vascular ectasia in all animals of both generations treated
with 40 or 160 ppm, and peri-adenitis in a small number of F0 males
at 160 ppm. No treatment-related histopathological lesions were seen
in pups killed on day 14 post partum.
Doses were selected for use in the main study on the basis of the
reproductive effects and toxicity seen at 160 ppm and the minimal
toxicity observed in males treated with 40 ppm amitrole (Savary,
1994).
In the main study, groups of 30 male and 30 female Sprague-Dawley
Crl:CD(SD)BR rats were fed diets containing amitrole (purity, 97-98%)
at levels of 0.5, 2, 15, or 110 ppm for two generations, with one
litter per generation. These doses were equivalent to 0.03, 0.12, 0.9,
or 5.9 mg/kg bw per day in F0 males; 0.04, 0.16, 1.2, or 12 mg/kg bw
per day in F1 males; 0.04, 0.16, 1.2, or 7.8 mg/kg bw per day in F0
females; and 0.05, 0.21, 1.6, or 16 mg/kg bw per day in F1 females.
Treatment was begun in the parental animals 72 days before mating and
was continued throughout gestation and lactation. Mortality, clinical
signs, body weight, and food consumption were monitored at all stages
of the study, and the following indices were calculated: male mating,
female mating, male and female fertility, gestation, viability on days
4 and 21 post partum, and numbers of live births. Additional
parameters measured included pup development, litter size, number of
implantations, and length of gestation. All animals were examined
grossly, and controls and animals at the high dose were examined
microscopically. The absolute and relative weights (as a percentage of
body weight) of 11 organs were determined.
Death or clinical signs were observed in 10/32 F1 males and
16/31 females treated with 110 ppm amitrole, and both F0 and F1
parental animals at this dose had statistically and toxicologically
significant decreases in body weight throughout the study. By the end
of pre-mating, the body weights of F0 males were 60% those of the
controls, and those of the females were 85% those of controls. The F1
animals at this dose were more severely affected, their mean body
weights being only about 35% of those of controls. All parental
animals at this dose had statistically and toxicologically
significantly decreased food consumption.
Animals at the two lower doses had no changes in organ weight or
histopathological appearance; however, those at 110 ppm had changes
indicative of widespread systemic toxicity, including significantly
increased relative thyroid gland weights and treatment-related
histopathological lesions such as follicular epithelial hyperplasia
and vascular ectasia, in most animals. The relative pituitary gland
weights were also significantly increased in F0 and F1 males and F1
females, with treatment-related histopathological changes, including
vacuolated cells and decreased numbers of acidophils, in F0 females
and F1 males and females. At this dose, significant increases were
also seen in the relative weights of the testis, epididymides, and
seminal vesicles (both generations), prostate (F1), and uterus (F1);
decreased relative ovarian weights were seen in the F0 generation.
Slightly increased incidences of histopathological effects were seen
in the testis and epididymides of F1 animals and in the ovaries,
uterus, and vagina in both generations. Many of the histopathological
effects on the reproductive tissues, including the prostate and
seminal vesicles, were related to immaturity, which is consistent with
the small size of the animals at this dose. Other signs of systemic
toxicity in animals at the high dose included significantly decreased
relative weights of the adrenal glands in F0 males and females and
F1 females but a significant increase in F1 males. Both generations
of males showed an increased frequency of ceroid-laden cortical cells,
and the F0 females had an increased incidence of cortical atrophy.
All animals at the high dose had significantly increased relative
spleen weights, and treatment-related histopathological effects were
seen in F0 males and females and F1 males. While only F1 males had
increased relative kidney weights, the remaining animals had
treatment-related increases in the incidence of numerous renal
lesions. Hepatocellular hypertrophy was seen in most animals at
110 ppm, but the decrease in relative liver weight was significant
only in F1 males. F1 pups at this dose had toxicologically
significantly increases in absolute and relative thyroid gland weights
and related gross pathological and histopathological lesions. Owing to
the low survival rate of F2 pups, treatment-related changes in organ
weight and pathological effects could not be identified in this group.
The following reproductive effects seen in animals at 110 ppm
were considered to be toxicologically significant: significantly
decreased mating indices among F1 males and females, decreased F1
male and female fertility indices, increased length of gestation in
F1 females (from 21-22 to 23 days), low implantation rate in F0 and
F1 females, low prenatal survival (59%) in the F2 generation,
significantly decreased mean litter size at day 1 (F1 and F2) and
day 21 (F2 only) post partum, significantly decreased mean litter
size on day 4 post partum (F2), very low viability indices for the
F2 generation on days 4 (33%) and 21 (37%), and decreased F1 and F2
pup body weights during lactation. In the F2 generation at 110 ppm,
the pups in four of five liveborn litters died during the first day,
and only three pups out of 24 liveborn pups of the remaining litter
survived to the end of lactation. There appeared to be no effects on
F1 pup development, sex ratio, or gross appearance at necropsy; there
were insufficient data to assess these parameters in the F2
generation. The author reported an NOAEL of 2 ppm for systemic
toxicity on the basis of minimally increased thyroid activity;
however, thyroid hormone levels were not measured and no other effects
on the thyroid were noted in rats at 15 ppm. The NOAEL for systemic
toxicity and reproductive toxicity was 15 ppm, equal to 0.9 mg/kg bw
per day (Richard, 1995).
(ii) Developmental toxicity
Groups of 16 mated female Mol:Russian rabbits were given amitrole
(purity, 97.5%) at doses of 0, 5, 20, or 80 mg/kg bw per day by gavage
on days 6-18 of gestation. The observations included daily monitoring
for clinical signs, food consumption during six periods, and maternal
body weight on day 0 and daily on days 6-18 and day 29. The animals
were killed on day 29, their fetuses were removed, and the does were
examined grossly for number of corpora lutea and implantation sites,
uterine weight, individual pup weights, appearance of fetuses or
embryos, sex of all live fetuses, individual weights of live fetuses,
and the occurrence of external malformations, visceral effects, and
effects on the skeletal system.
Statistically significantly lower maternal food consumption was
seen among does at 80 mg/kg bw per day during treatment on days 6-10
and 14-19 and throughout days 0-29, and their mean weight gain was 31%
lower than that of controls (not statistically significant) on days
6-18. Treatment at 80 mg/kg bw per day significantly reduced the body
weights of male fetuses and lowered the mean litter weight by 17%. The
NOAEL for maternal and fetal toxicity was 20 mg/kg bw per day. There
was no evidence of teratogenicity (Kolb, 1994a).
(iii) Special study: Maternal toxicity
Groups of five mated female Mol:Russian rabbits given amitrole
(purity, 97.5%) at doses of 0, 5, 20, or 80 mg/kg bw per day by gavage
on days 6-18 of gestation and monitored daily for clinical signs, food
consumption during four periods, maternal body weight on day 0 and
daily on days 6-18 and day 19. The animals were killed on day 19 and
examined grossly for the numbers of corpora lutea, implantations, and
resorptions. Clinical chemical and haematological parameters were
measured on days 6, 7, 13, and 19 before treatment, and the weights of
the liver, spleen, thyroid glands, and adrenal glands were recorded.
The liver, spleen, thyroid glands, adrenal glands, and pituitary
glands were examined histologically.
The absolute and relative weights of the liver and the levels of
albumin and protein were decreased on day 19 in rabbits treated with
20 mg/kg bw per day or more. Those at 80 mg/kg bw per day also had
lower food consumption during days 6-10 (but not days 10-18) of
treatment, increased creatine kinase activity by day 19, decreased
albumin and protein levels by day 13, decreased triiodothyronine and
thyroxine levels on days 7, 13, and 19, and slight hypertrophy of the
thyroid follicular cells, which was considered to be a minimal effect
related to the thyroid hormone levels. Changes observed in the liver
that were not considered to be adverse included a slight
treatment-related increase in the incidence and number of Kupffer-cell
foci in animals at 80 mg/kg bw per day and cytoplasmic changes
described as fine net-like markings on centrilobular heptocyte centres
and eosin-stained bands on the cell membrane in animals at 20 and 80
mg/kg bw per day. No deaths, adverse clinical signs, or body-weight
changes were reported. The numbers of corpora lutea, implantations,
and resorptions and the weights of the thyroid glands were not
affected by treatment.
The author concluded that treatment had increased cholesterol
levels in rabbits at 80 mg/kg bw per day, but that the increase was
due to one outlier and was thus spurious. The author considered the
NOAEL for maternal toxicity to be 20 mg/kg bw per day, as only
non-adverse cytoplasmic changes in the liver were seen at this dose
(Kolb, 1994b). The Meeting did not agree with this conclusion since
decreased absolute and relative liver weights and statistically
significantly decreased albumin and protein levels were seen at 20
mg/kg bw per day and above. The Meeting concluded that the NOAEL for
maternal toxicity was 5 mg/kg bw per day.
Comments
In rats, amitrole was rapidly absorbed, approximately 90% of the
administered dose being eliminated within 48 h. The primary route of
elimination was the urine, which accounted for over 87% of the dose;
faecal elimination accounted for less than 6% of the dose and volatile
metabolites represented only 0.1%. Forty-eight hours after dosing, the
tissue residues amounted to less than 3% of the dose, with the
majority found in the liver. Most of the radiolabel (62-90%) was
excreted as the parent compound. Several metabolites were present at
very low concentrations, a mercapturic acid derivative being
predominant (2.1-7.3% of the administered 14C).
In a one-year study of toxicity, dogs were fed diets containing
0, 10, 500, or 1500 ppm amitrole. At doses 500 ppm (equal to 13 mg/kg
bw per day) and above, the levels of triiodothyronine and thyroxine
were reduced and there were increases in the incidence of rough coats,
in thyroid weights, and in the frequency of lesions of the thyroid
(follicular-cell hyperplasia, capsular fibrosis, vasculitis, and
dilatation of the vasculature). In addition, ectopic thyroids with
follicular hyperplasia and capsular fibrosis were noted, and changes
in the pituitary (hyperplasia and hypertrophy) were evident in both
males and females. At 1500 ppm (equal to 32 mg/kg bw per day), both
males and females were anaemic and had increased platelet counts,
decreased food intake, decreased brain weights (with histopathological
lesions), and neurological effects. At this dose, males also had
decreased weight gain, increased lactate dehydrogenase activity and
cholesterol levels, decreased heart weights, and decreased P- and
R-wave amplitudes. The NOAEL was 10 ppm, equal to 0.29 mg/kg bw per
day.
In a two-generation study of reproductive toxicity, rats were fed
diets containing 0, 0.5, 2, 15, or 110 ppm amitrole. At 15 ppm (equal
to 0.9 mg/kg bw per day), the only effect observed was a slight
increase in the severity of some histopathological changes in the
thyroid, including small follicles, decreased colloid content, and
follicular epithelial hypertrophy. Parental toxicity at 110 ppm was
severe and was characterized by mortality, clinical signs,
considerably decreased weight gain, thyroid effects (increased
relative thyroid weights, follicular epithelial hyperplasia, and
vascular ectasia), changes in relative organ weights (increased
weights of pituitary, testes, epididymides, seminal vesicles,
prostate, uterus, spleen, and kidneys and decreased weights of
ovaries, adrenal glands, and liver), and histopathological lesions in
the liver, adrenal glands, kidneys, and numerous reproductive tissues.
In pups, the only observations were increased thyroid weights with
accompanying histopathological lesions. The effects on reproduction
included decreased mating and fertility indices, increased gestation
length, and decreased prenatal survival, litter size, pup body weight,
and viability. The NOAEL for systemic toxicity was 2 ppm, equal to
0.12 mg/kg bw per day. This value is supported by the findings of the
range-finding study, in which enlarged, reddened thyroids and
decreased colloid content were noted at 10 ppm (equal to 0.62 mg/kg bw
per day). The NOAEL for reproductive toxicity was 15 ppm, equal to 0.9
mg/kg bw per day.
In a study of developmental toxicity, rabbits were treated with
0, 5, 20, or 80 mg/kg bw per day amitrole on days 6-18 of gestation.
At 80 mg/kg bw per day, maternal food consumption and body-weight gain
were reduced during treatment. Male fetal body weights and mean litter
weights were reduced at 80 mg/kg bw per day. Clinical chemistry was
not evaluated in this study. The NOAEL for maternal and fetal toxicity
was 20 mg/kg bw per day.
In a special study to further characterize the toxicity in
pregnant rabbits, the animals were treated with amitrole at 0, 5, 20,
or 80 mg/kg bw per day on days 6-18 of gestation and were killed on
day 19 of gestation. At 20 and 80 mg/kg bw per day, albumin and
protein levels were decreased and absolute and relative liver weights
were decreased. At 80 mg/kg bw per day, there were reductions in food
consumption, increased creatine kinase levels, decreased
triiodothyronine and thyroxine levels, and thyroid follicular-cell
hypertrophy. The NOAEL for maternal toxicity was 5 mg/kg bw per day.
The results of these supplemental studies correlate well with the
results of the studies reviewed by the 1993 JMPR, showing that the
thyroid is the primary target organ of amitrole. Contrary to the
conclusions of the 1993 JMPR, the results demonstrate that amitrole is
also goitrogenic in the dog. In the one-year study in dogs reviewed
previously, no effects on the thyroid were noted at a dose of
12.5 mg/kg bw per day. In the current one-year study, however, there
were extensive effects at 13 mg/kg bw per day.
The rat was the most sensitive species, the NOAELs being 2 ppm in
both the two-generation study of reproductive toxicity (equal to 0.12
mg/kg bw per day) and the 90-day dietary study (equivalent to 0.1
mg/kg bw per day), on the basis of histopathological changes in the
thyroid. The Meeting noted that the rat is more sensitive to the
development of thyroid hyperplasia and subsequent neoplasia after
exposure to goitrogenic compounds than are humans. Therefore, a
smaller safety factor of 50 was used to take into account the reduced
uncertainty of interspecies extrapolation, giving an ADI of
0-0.002 mg/kg bw. This ADI provides a 150-fold margin of safety over
the NOAEL of 0.29 mg/kg bw per day in the one-year study in dogs.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 10 ppm, equivalent to 1.5 mg/kg bw per day (18-month
study of toxicity and carcinogenicity)
Rat: 10 ppm, equivalent to 0.5 mg/kg bw per day (two-year
study of toxicity and carcinogenicity)
2 ppm, equal to 0.12 mg/kg bw per day (parental
toxicity in a two-generation study of reproductive
toxicity)
100 mg/kg bw per day (maternal and fetal toxicity
in a study of developmental toxicity)
Rabbit: 5 mg/kg bw per day (maternal toxicity in a study of
developmental toxicity)
20 mg/kg bw per day (fetal toxicity in a study of
developmental toxicity)
Hamster: 10 ppm, equivalent to 1 mg/kg bw per day (18-month
study of toxicity)
Dog: 10 ppm, equal to 0.29 mg/kg bw per day (one-year study
of toxicity)
Estimate of acceptable daily intake for humans
0-0.002 mg/kg bw
Studies that would provide information useful for continued evaluation
of the compound
1. Clarification of the genotoxic potential of amitrole (e.g.
DNA adducts in vivo)
2. Further observations in humans.
Toxicological criteria for estimating guidance values for dietary and non-dietary exposure to amitrole
Human exposure Relevant route, study type, species Results, remarks
Short-term
(1-7 days) Oral, acute toxicity, rat LD50 > 2500 mg/kg bw
Dermal, acute toxicity, rat LD50 > 2500 mg/kg bw
Inhalation, acute toxicity, 4 h, rat LC50 > 439 mg/L
Dermal, irritation, rabbit Minimally irritating
Ocular, irritation, rabbit Mildly irritating
Dermal, sensitization, guinea-pig Moderately sensitizing (Magnusson-Kligman test)
Non-sensitizing (Klecak open epicutaneous test)
Medium-term Repeated oral, 2-4 weeks, rat NOAEL = 3 mg/kg bw per day: effects on the
(1-26 weeks) thyroid, food consumption and body weight
Repeated oral, drinking-water, 4 weeks, rat NOAEL = 10 mg/L water: decreased weight gain,
enlarged thyroids
Repeated oral, 6-13 weeks, rat NOAEL = 0.1 mg/kg bw per day: effects on the thyroid
Repeated dermal, 3 weeks, rabbit NOAEL = 100 mg/kg bw per day (highest dose
tested)
Repeated inhalation, 4 weeks, rat NOAEL = 0.1 mg/L: effects on the thyroid
Repeated oral, special maternal toxicity, rabbit NOAEL = 5 mg/kg bw per day: decreased liver
weights and protein/albumin levels
Repeated oral, developmental toxicity, rabbit NOAEL = 20 mg/kg bw per day: developmental
toxicity
Repeated dermal, developmental toxicity, rabbit NOAEL = 1500 mg/kg bw per day: maternal and
developmental toxicity
Repeated oral, reproductive toxicity, rat NOAEL = 0.12 mg/kg bw per day (systemic):
effects on the thyroid
NOAEL = 0.9 mg/kg bw per day: reproductive
toxicity
Long-term Repeated oral, 1 year, dog NOAEL = 0.29 mg/kg bw per day: effects on the
(> 1 year) thyroid
Repeated inhalation, intermittent dosing, 2 years, LOAEL = 50 µg/L (lowest dose tested): effects on
carcinogenicity, rat the thyroid
References
Anderson, C. & Brauner, A. (1995) Amitrole: Investigation of the
biokinetic behaviour and the metabolism in the rat. Unpublished
reports Nos MR-508/95, ANC82, and BNA81 (study No. M41819023) from
Bayer AG, Agrochemicals Division, Institute for Metabolism Research
and Residue Analysis, Leverkusen, Germany. Submitted to WHO by Bayer
AG, Leverkusen, Germany.
Jones, R.D. & Lake, S.G. (1994) Technical grade amitrole: A chronic
toxicity study in the beagle dog. Unpublished report No. 7413 (study
No. 92-276-RX) from Miles Inc., Agriculture Division, Toxicology,
Stilwell, KS, USA. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Kolb, J. (1994a) Amitrole: Developmental toxicity study in rabbits
after oral administration. Unpublished report No. 23486 (study No.
T5044250) from Bayer AG, Department of Toxicology, Wuppertal, Germany.
Submitted to WHO by Bayer AG, Leverkusen, Germany.
Kolb, J. (1994b) Amitrole: Supplementary study on maternal toxicity
study in pregnant rabbits after oral administration. Unpublished
report No. 23486 (study No. T5044284) from Bayer AG, Department of
Toxicology, Wuppertal, Germany. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Richard, J. (1995) Two generation study by oral route (dietary
admixture) in rats. Unpublished report No. 6432 (study No. T5041262)
from Centre International de Toxicologie, Evreux, France. Submitted to
WHO by Bayer AG, Leverkusen, Germany.
Savary, M.-H. (1994) Preliminary study to a two-generation study by
oral route (dietary admixture) in rats. Unpublished report No. 6306
(study No. T6041263) from Centre International de Toxicologie, Evreux,
France. Submitted to WHO by Bayer AG, Leverkusen, Germany.
WHO (1994) Amitrole (Environmental Health Criteria 158), Geneva
