
ENDOSULFAN JMPR 1998
First draft prepared by
D.B. McGregor
International Agency for Research on Cancer
Lyon, France
Explanation
Evaluation for acceptable daily intake
Biochemical aspects
Absorption, distribution, and excretion
Toxicological studies
Acute toxicity
Short term studies of toxicity
Long-term studies of toxicity and carcinogenicity
Genotoxicity
Reproductive toxicity
Multigeneration reproductive toxicity
Developmental toxicity
Special studies
Enzyme induction
Promotion
Immunotoxicity
Neurobehavioural effects and neurotoxicity
Effects on sperm
Endocrine effects
Observations in humans
Comments
Toxicological evaluation
References
Explanation
Endosulfan (6,7,8,9,10,10-hexachloro-1,5,5a,6,9,9a-hexahydro-6,9-
methano-2,4,3-benzo-dioxathiepin-3-oxide), an insecticide, has been
evaluated toxicologically on several occasions by previous Joint
Meetings (Annex 1, references 2, 4, 8, 10, 38, 44, and 56), the latest
being the 1989 JMPR (Annex 1, reference 56), when an ADI of 0-0.006
mg/kg bw was established. Endosulfan was reviewed by the present
Meeting within the Periodic Review Programme of the Codex Committee on
Pesticide Residues. In this evaluation, full use was made of the
review of endosulfan prepared by the Australian National Registration
Authority, the entire version of which may be obtained at
http://www.dpie.gov.au/nra/prsendo.html. This monograph summarizes the
new data and relevant data from the previous monographs and monograph
addenda on endoslufan (Annex 1, references 4, 9, 11, 39, and 58)
Evaluation for acceptable daily intake
1. Biochemical aspects
(a) Absorption, distribution, and excretion
When radiolabelled endosulfan was administered to mice as a
single dose of 4 mg/kg bw by gavage, a single dose of 4.7 mg/kg bw in
the diet, or a 21-day administration of 2.4 mg/kg bw per day in the
diet, most of the radiolabel was recovered from the faeces. Within
three weeks after cessation of treatment, a total of 87-100% of the
administered dose was recovered, with little difference with dosing
regime. While biliary excretion was not studied, the percent of
chemical absorbed after oral dosing would appear to have been moderate
to high. Three weeks after the final administration, the residues in
tissues were greater in animals fed the compound for 21 days, the
higher concentrations being found in the liver (about 2 ppm) and
spleen (about 1.4 ppm) at this time. Little residue was found in the
kidneys and fat, even after repeated administration, and there was no
accumulation of radiolabelled endosulfan residues (Christ & Kellner,
1968).
Single oral doses of 0.3 mg endosulfan and its two isomers
administered to male Balb/c mice were not completely absorbed from the
gastrointestinal tract but were excreted with the metabolites
endosulfan sulfate and diol in the faeces. Only the diol metabolite
was excreted via the urine; the sulfate metabolite was the only form
of endosulfan found in tissues, with relatively large amounts in
liver, small intestine, and visceral fat and trace amounts in muscle
and kidney. When endosulfan was fed to Balb/c mice in the diet at a
concentration of 10 ppm for up to 49 days, the sulfate metabolite was
detected in the liver and visceral fat of all animals. Both isomers
and the sulfate and diol metabolites of endosulfan were detected in
the faeces, while the only endosulfan product detected in the urine of
these animals in this early study was the diol metabolite. After a
single dose of up to 0.3 mg 14C-labelled endosulfan to Balb/c mice,
about 65% of the radiolabel was recovered; the faeces accounted for
the highest concentrations, followed (in rank order) by visceral fat
> urine > small intestine > kidney > brain > expired carbon
dioxide > blood (Deema et al., 1966).
At the end of a 24-month study in which NMRI mice were given
diets containing 0, 2, 6, or 18 ppm technical-grade endosulfan (see
Donaubauer, 1988), the concentrations of endosulfan and its main
metabolites endosulfan hydroxyether, sulfate, lactone, and diol were
measured in the liver and kidneys. No endosulfan was detected in
either the liver or the kidney. In mice given 18 ppm endosulfan, the
concentrations of the hydroxyether, lactone, and diol metabolites were
at or below the level of detection (0.02 ppm), while the endosulfan
sulfate concentrations were 0.1-0.2 ppm in kidney and 0.7-1.1 ppm in
liver. The tissue concentrations of endosulfan sulfate in mice at 2,
6, and 18 ppm, respectively, were: kidney, 0.2-0.4 ppm, 0.04 ppm, and
0.1-0.2 ppm; and liver, 0.06-0.07 ppm, 0.12-0.45 ppm, and 0.7-1.1 ppm
(Leist, 1989a).
After oral or intravenous administration of 14C-endosulfan to
male and female Wistar rats at a dose of 2 or 0.5 mg/kg bw,
respectively, > 80% (intravenous) or 90% (oral) of the dose was
eliminated in the urine and faeces within seven days; elimination was
essentially complete within the first 1-2 days. The half-lives for
urinary and faecal elimination for males and females were biphasic,
with an earlier half-life of 6-14 h and a later half-life of 33-67.5
h. Elimination in urine of the intravenous and oral doses,
respectively, accounted for 11 and 13% of the dose in males and 2 and
24% of the dose in females; the corresponding figures for elimination
in faeces were 65 and 82% in males and 60 and 72% in females. The
highest tissue concentrations were found in the kidneys (1.8 ppm),
liver (0.23 ppm in males; 0.48 ppm in females), and retroperitoneal
fat (0.16 ppm in females). The concentrations of residues were < 0.1
ppm in all other tissues examined. The absorption of endosulfan was
estimated to be 60-70% on the basis of a comparison of areas under the
curve after intravenous and oral administration and about 90% on the
basis of a comparison of elimination of radiolabel administered by the
two routes (Table 1; Kellner & Eckert, 1983; Stumf & Lehr, 1993).
14C-Endosulfan (alpha or ß isomer) was rapidly excreted by
female rats after a single oral dose of 2 mg/kg bw or administration
in the diet at a concentration of 5 ppm. After a single oral dose,
> 85% was excreted within 120 h (> 70% after 48 h), mainly in the
faeces and to a lesser extent in the urine. After dietary
administration for 14 days, followed by a 14-day recovery period,
> 72% of the administered dose was recovered. Biliary excretion of
radiolabel in male rats given 1.2 mg/kg bw as a single dose approached
50% for the alpha isomer and 30% for the ß isomer over 48 h. There
appeared to be little enterohepatic circulation. The tissue
concentrations of residues were generally highest in the kidneys and
liver and lower in other tissues, including fat. At the end of the
14-day recovery period, residues were confined to the kidneys and to a
lesser extent the liver, with half-lives of about seven days in
kidneys and three days in liver. By far the largest proportion of the
radiolabel administered was metabolized to highly polar products, most
of which could not be extracted from faeces (28%) or tissues (71%). Of
the extractable fraction, unidentifiable polar metabolites constituted
6.2% in faeces and 13% in urine. The apolar metabolites of endosulfan
identified in faeces and urine were the diol, the lactone, the
alpha-hydroxyether, and the sulfate. The metabolites occurred at
similar concentrations, ranging from 3.4 to 9.1% of the radiolabel in
the urine of rats given single doses and from 2.4 to 4.2% in the urine
of rats given endosulfan in the diet. The three apolar metabolites
(endosulfan diol, hydroxyether, and lactone) accounted for 7.5% of the
single dose and 3.2% of the dietary dose in the urine, 9% of the
administered dose in bile, and 21% in faeces. No accumulation in fatty
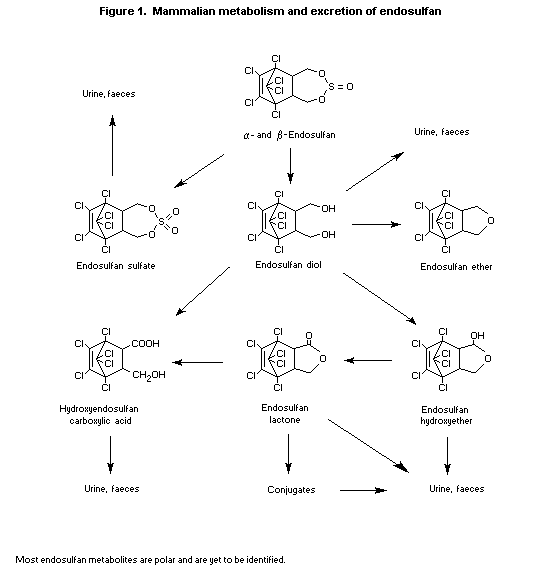
tissues was found (Dorough et al., 1978). A metabolic scheme is
presented in Figure 1.
Table 1. Pharmacokinetics of 14C-endosulfan in rats after oral and intravenous administration
Pharmacokinetic parameter Oral (2 mg/kg bw) Intravenous (0.5 mg/kg bw)
Tmax Males, 3-8 h; females, 18 h Males and females, 5 min
Cmax Males, 0.25 ± 0.06 µg/ml; Males and females, 0.18 ± 0.04 µg/ml
females, 0.18 ± 0.05 µg/ml
Elimination half-life Males (biphasic), 8 h , 110 h Males (triphasic), 0.77 h, 12.5 h, 157 h
Females (monophasic), 75 h Females (biphasic), 1.2 h, 47 h
Faecal excretion Males, 82%; females, 72% Males, 66%; females, 59%
Urinary excretion Males, 12%; females, 22% Males, 13%; females, 24%
Urinary excretion half-life Males (biphasic), 6.2 h, 67.5 h Males (biphasic), 7.5 h, 60 h
Females (biphasic), 5.6 h, 33 h Females (biphasic), 7.6 h, 42 h
Faecal excretion half-life Males (biphasic), 7.7 h, 34 h Males (biphasic), 8.6 h, 34.5 h
Females (biphasic), 11.4 h, 30 h Females (biphasic), 13.6 h, 40 h
 Groups of 24 male Sprague-Dawley rats received dermal
applications of [5a,9a14C]-endosulfan at a dose of 0.1, 0.76, or 10
mg/kg bw, without washing. Four animals from each group were killed at
0.5, 1, 2, 4, 10, and 24 h, and radiolabel was measured in the
collected excreta and various organs and tissues, including the
application site after washing with soapy water. No skin irritation
was seen at the application site. Adsorption onto the skin was
essentially complete within 0.5 h at all doses and accounted for
63-80% of the applied dose. Movement away from the application site
was slow, with 73, 73, and 89%, respectively, of the adsorbed doses
remaining at the application site after 24 h. By 10 h, each group had
excreted less than 1% of the applied dose. By 24 h, the excretion was
11, 10, and 4% of the applied dose in the groups given 0.1, 0.76, and
10 mg/kg bw, respectively (Craine, 1986).
Groups of 16 female Sprague-Dawley rats received dermal
applications of [5a,9a14C]-endosulfan at 0.09, 0.98, or 11 mg/kg bw,
and the site was washed with soapy water after 10 h. Four animals from
each group were killed at 24, 48, 72, and 168 h, and radiolabel was
measured in the collected excreta and various organs and tissues,
including the application site. No skin irritation was seen at the
application site, and there were no signs of systemic toxicity.
Recovery of radiolabel was 84-115%. The amount of the applied dose
that was removed by washing was 28, 47, and 69%, respectively, at the
three doses. After 168 h, 63, 87, and 65%, respectively, of the dose
that was not removed by washing was either adsorbed on to the skin or
had penetrated and been distributed and excreted; an average of
2.4-3.3% of the adhering dose remained at the application site.
Excretion was maximal between 24 and 48 h, faeces accounting for about
two-thirds of the label. The total residues at 168 h represented 2.5,
2.3, and 1.3% of the applied dose (about 3.5, 4.3, and 4.2% of the
adhering dose) at the doses of 0.09, 0.98, and 11 mg/kg bw,
respectively, and were present mainly in liver and kidney (Craine,
1988).
At the end of a 24-month study in which Sprague-Dawley rats were
given diets containing 0, 3, 7.5, 15, or 75 ppm technical-grade
endosulfan (see Ruckman et al., 1989), the concentrations of
endosulfan and its known metabolites, endosulfan hydroxyether,
sulfate, lactone, and diol, were measured in the liver and kidneys. As
no quantifiable residues were detected in organs of rats at 15 ppm,
the organs of animals at 7.5 and 3 ppm were not investigated further.
Neither alpha- nor ß-endosulfan, the substances administered in the
diet, or any of the metabolites was quantifiable in either organ, with
the exception of endosulfan sulfate in animals at 75 ppm, which was
found at concentrations of 0.2-0.4 mg/kg in liver. No residues were
observed in the kidneys of this group (Dorn & Werner, 1989; Leist,
1989a).
Twelve lactating goats were given endosulfan (purity unspecified)
in gelatine capsules at a dose of 1 mg/kg bw per day for 28 days. The
tissue concentrations of residues were generally low, the highest
being detected on the first day after cessation of treatment, with
0.29 ppm in kidney, 0.2 ppm in the gastrointestinal tract, and 0.12
ppm in liver. The concentrations in the kidney were increased one week
after treatment, reaching 0.49 ppm on day 8, but no residues were
detected 21 days after treatment ceased. Endosulfan residues did not
accumulate in the fat; the concentrations reached 0.06 ppm on day 1
after the end of treatment, but none were detected by day 8 after
treatment (Indraningsih, et al., 1993).
Groups of three lactating Holstein cows were given diets
containing 0, 0.3, 3, or 30 ppm 14C-endosulfan for 30 days. Between
days 7 and 29, the average concentrations in milk were 3.4, 40, and
462 ppb endosulfan equivalents in the three groups. After the dosing
period, the loss of radiolabel from milk (measured in one cow per
group) was 81% within seven days in the cow given 0.3 ppm and 96%
within 14 days in cows given 3 and 30 ppm (Bowman, 1959). The blood
concentrations rose during the first 21 days, to 0.15 and 1.97 ppm at
the doses of 3 and 30 ppm, respectively, but were always below the
detection level (0.06 ppm) in the groups at 0.3 ppm. The
concentrations of residues found at the three doses were: liver, 0.35,
2.45, and 25.3 ppm; kidney, 0.05, 0.35, and 6.29 ppm; and omental fat,
0.07, 0.71, and 7.08 ppm (Keller, 1959a).
Two lactating East Friesian sheep were given a single oral dose
of 0.3 mg/kg bw 14C-endosulfan and were killed after 40 days. The
radiolabel was maximal in blood after 24 h, when it was equivalent to
0.07 µg/ml. The total radiolabel eliminated in milk over 17 days was
0.37% and 1.82% of the dose in the two sheep, respectively. Radiolabel
was excreted mainly via the urine (41%) and faeces (50%). About half
of the 50% in faeces was unmetabolized endosulfan. Fat, kidney, and
liver of the sheep contained 0.02-0.03 µg/g endosulfan; all of the
remaining tissues had considerably lower concentrations. The total
radiolabel found in organs and tissues accounted for < 1% of the
administered dose (Gorbach et al., 1965).
In pigs fed endosulfan at 2 ppm in their diets for up to 81 days,
the compound was detected in fatty tissue at concentrations of 0.07,
0.09, and 0.04 ppm after 27, 54, and 81 days of treatment, much less
than the residues seen after administration of 7 ppm DDT: 8.3, 9.1,
and 9.7 ppm after 27, 54, and 81 days treatment, respectively. Liver
and muscle contained about 15-fold less DDT residues than fat. Thus,
while much less endosulfan was found in fatty tissues, it does not
appear to bioaccumulate as does DDT (Maier-Bode, 1966).
The systemic absorption of endosulfan over 96 h after dermal
administration to two rhesus monkeys of single doses of 2.2-3 mg/kg bw
of an aqueous suspension of 14C-endosulfan (purity, 94.6%) for 10 h
was 22% of the administered dose. An additional 11% remained in the
skin, 10.5% was found in the carcass, and 4.3 and 3.7% of the
administered dose was excreted in faeces and urine, respectively;
however, as only 50% of the administered dose was recovered, the
figures calculated for absorption may not be accurate indications of
the extent of dermal absorption of endosulfan. A plateau was reached
in blood and plasma concentrations at 36 h, and there may have been no
significant additional dermal absorption after that time.
Concentrations in the liver, kidneys, and fat were highest (0.48,
0.083, and 0.23 ppm, respectively), while there were negligible
concentrations in the brain (Lachmann, 1987).
In a study of the penetration of endosulfan through rat and human
skin in vitro, radiolabelled endosulfan formulated as an
emulsifiable concentrate containing 353 g/L endosulfan, which had been
diluted to concentrations of 0.4-4 mg/ml in water, was applied at
nominal doses of 0.01, 0.1, and 1 mg/cm2 to rat and human skin
mounted in dermal penetration cells. The rate of penetration was, on
average, 4.3 times greater through rat skin than that of humans. The
percentage of the applied dose varied with the concentration: 61% of
the lowest dose applied to human skin and 96% of that applied to rat
skin penetrated, and 20% of the highest dose applied to human skin and
40% of that applied to rat skin penetrated. When the skin was washed
10 h after application, the amount of endosulfan that penetrated
decreased to 4% in the human and 9% in the rat skin. Endosulfan that
passed through human skin was metabolized or degraded to a greater
extent than that which passed through rat skin (Noctor & John, 1995).
2. Toxicological studies
(a) Acute toxicity
The LD50 of endosulfan varies widely depending on the route of
administration, species, vehicle, and sex of the animal (see Table 2).
Certain other reports are available, but it has been argued that most
of them are not acceptable by current standards (Bremmer & Leist,
1998). Female rats are clearly more sensitive than male rats, and, on
the basis of a single study, this sex difference appears to apply to
mice also. Endosulfan is generally highly toxic after oral and
inhalation exposure. The lowest oral LD50 value is 9.6 mg/kg bw in
female Sprague-Dawley rats.
The isomers of endosulfan also show high acute toxicity after
oral administration. The clinical signs of poisoning include
piloerection, salivation, hyperactivity, respiratory distress,
diarrhoea, tremors, hunching, and convulsions. Like endosulfan, its
metabolites were more or less toxic according to the vehicle used and
the species exposed. In general, the toxicity of the lactone and
sulfate metabolites was similar to or less than that of the parent
compound, while the hydroether, ether, and, in particular, the diol
were far less toxic. The clinical signs of poisoning were similar to
those induced by the parent compound and included piloerection,
salivation, hyperactivity, respiratory distress, diarrhoea, tremors,
hunching, and convulsions.
Phenobarbital was an effective therapeutic measure against an
absolute lethal dose of endosulfan in rats, reducing the clinical
signs of poisoning and the mortality rate. Diazepam was not effective
(Ebert & Weigand, 1984).
Table 2. Acute toxicity of technical-grade endosulfan and its isomers and metabolites
Species Strain Sex Route Vehicle LD50 Reference
(mg/kg bw)
Technical-grade endosulfan
Rat Sprague-Dawley M Oral 25% in food 2 800 Bracha (1977)
Rat Sprague-Dawley F Oral 25% in food 45 Bracha (1977)
Rat CD M/F Oral Maize oil 43 Lightowler & Gardner (1978)
Rat Haffkine M Oral 110 Bhide & Naik (1984a)
Rat Haffkine F Oral 15 Bhide & Naik (1984a)
Rat Holtzman M Oral Corn oil 87 Elsea (1958)
Rat Wistar M Oral 2% starch 100-160 Diehl & Leist (1988a)
Rat Wistar F Oral 2% starch 23 Diehl & Leist (1988a)
Rat Sprague-Dawley M Oral 5% CMC 40 Reno (1975)
Rat Sprague-Dawley F Oral 5% CMC 9.6 Reno (1975)
Mouse Kasauli M Oral Tween 80 35 Bhide & Naik (1984b)
Mouse Kasauli F Oral Tween 80 14 Bhide & Naik (1984b)
Dog Mongrel M/F Oral Gelatin capsule 77 Nogami (1970)
Rat HoeWISKf M Dermala > 4 000 Diehl & Leist (1988b)
Rat HoeWISKf F Dermala 500 Diehl & Leist (1988b)
Rabbit NWS M Dermala 290 Bhide & Naik (1984c)
Rabbit New Zealand M Dermala 500-1 000 Bracha (1977)
Rabbit New Zealand F Dermala 1 000-2 000 Bracha (1977)
Rat Sprague-Dawley M/F Inhalation > 21 000 Bracha (1977)
mg/m3 for 1 h
Rat Wistar M Inhalation Ethanol + PEG 35 mg/m3 Hollander & Weigand (1983)
for 4 h
Rat Wistar F Inhalation Ethanol + PEG 13 mg/m3 Hollander & Weigand (1983)
for 4 h
Table 2. (continued)
Species Strain Sex Route Vehicle LD50 Reference
(mg/kg bw)
Endosulfan alpha isomer
Rat Oral 76 Goebel et al. (1982)
Mouse Albino F Oral Tween 80 11 Dorough et al. (1978)
Endosulfan ß isomer
Rat Oral 240 Goebel et al. (1982)
Mouse F Oral Tween 80 36 Dorough et al. (1978)
Endosulfan sulfate
Mouse Albino F Oral Tween 80 8 Dorough et al. (1978)
Rat Wistar F Oral Starch suspension 76 Hollander & Kramer (1975a)
Rat Wistar M Oral 570 Ehling & Leist (1991a)
Rat Wistar F Oral 40 Ehling & Leist (1991a)
Dog Beagle M Oral Starch suspension 15 Hollander & Kramer (1975b)
Rat Wistar M Dermal 2 700 Ehling & Leist (1991b)
Rat F Dermal 280 Ehling & Leist (1991b)
Endosulfan diol
Mouse Albino F Oral Tween 80 > 2 000 Dorough et al. (1978)
Rat F Oral Starch suspension > 1 500 Hollander & Kramer (1975c)
Rat Albino F Oral > 15 000 Weigand (1982a)
Rat Wistar M/F Oral > 5 000 Ehling & Leist (1991c)
Mouse Albino F Oral Tween 80 120 Dorough et al. (1978)
Rat F Oral Starch suspension 1 750 Hollander & Kramer (1975d)
Rat Wistar M/F Dermal > 2 000 Ehling & Leist (1991d)
Table 2. (continued)
Species Strain Sex Route Vehicle LD50 Reference
(mg/kg bw)
Endosulfan ether
Mouse Albino F Oral Tween 80 270 Dorough et al. (1978)
Rat F Oral Starch suspension > 15 000 Hollander & Kramer (1975c)
Rat Albino F Oral > 15 000 Weigand (1982b)
Endosulfan hydroxyether
Mouse Albino F Oral Tween 80 120 Dorough et al. (1978)
Rat F Oral Starch suspension 1 750 Hollander & Kramer (1975d)
Endosulfan lactone
Mouse Albino) F Oral Tween 80 120 Dorough et al. (1978)
Rat Wistar F Oral Starch suspension 290 Hollander & Kramer (1975e)
Rat Wistar M Oral Starch suspension 160 Hollander & Kramer (1975f)
Rat M/F Oral Sesame oil 100/120 Kramer & Weigand (1971)
M, male; F, female; CMC, carboxy methyl cellulose; PEG, polyethylene glycol
a 24 h on intact skin
The dermal irritancy of technical-grade endosulfan (purity,
98.6%) was tested in three New Zealand white rabbits by clipping the
hair from a dorsal area of about 25 cm2 and 24 h later applying 500
mg endosulfan moistened with deionized water on a 6.25-cm2 cellulose
patch, which was then covered with a semi-occlusive bandage. Exposure
was for 4 h, after which the test material was removed with warm
tap-water. The exposed area was examined 0.5-1, 24, 48, and 72 h after
removal of the patch. On the basis of the evaluation system defined by
EEC guideline B.4 ('Acute toxicity skin irritation' of Directive
92/69/EEC), the overall mean scores for dermal irritation were 0 for
both erythema and eschar formation and oedema formation. No signs of
systemic toxicity were observed (Bremmer, 1997a).
The ocular irritancy of technical-grade endosulfan (purity,
98.6%) was tested in three New Zealand white rabbits by applying 100
mg to the conjunctival sac of one eye of each rabbit; the other,
untreated eye served as the control. The eyes were exposed for 24 h,
after which the endosulfan was washed out, and the eyes were examined
for ocular lesions 1, 24, 48, and 72 h later. On the basis of the
evaluation system defined by EEC guideline B.5 ('Acute toxicity eye
irritation' of Directive 92/69/EEC), the overall mean scores for
irritation were 0.66 for redness of conjunctiva, 0 for chemosis of
conjunctiva, 0 for opacity of cornea, and 0.11 for irritation of the
iris. No signs of systemic toxicity were observed. Endosulfan was not
irritating to the eye (Bremmer, 1997b).
The cutaneous allergenic potential of endosulfan (purity, 98.6%)
was examined in 20 treated and 10 control male albino guinea-pigs. The
maximal tolerated concentration of endosulfan suitable for the
induction phase of the main study and a suitable non-irritating
concentration of topically applied endosulfan were identified for the
challenge application in a preliminary study. For intradermal
induction, injection of 0.1 ml of a 0.5% solution in corn oil
emulsified 1:1 (v:v) with Freund's complete adjuvant was selected. One
week after these injections, a 6-cm2 patch of filter paper saturated
with about 0.3 ml of a 50% solution of endosulfan in corn oil was
applied to the shaved skin of each guinea-pig, and the area was
occluded with aluminium foil secured by impermeable adhesive tape,
which was left in position for 48 h. Irritation was assessed 24 and 48
h later. On test day 22, the guinea-pigs were challenged with the
non-irritating 50% endosulfan in corn oil applied as during the
induction phase. The dressing was left in position for 24 h. The
application sites were assessed for erythema and oedema 24 and 48 h
later. On the basis of the evaluation system defined by EEC guideline
B.6 ('Acute toxicity -- Skin sensitization' of Directive 92/69/EEC),
none of the treated guinea-pigs developed skin reactions. Endosulfan
was therefore considered to be non-sensitizing for guinea-pig skin
(Arcelin, 1996).
(b) Short-term toxicity
Mice
Groups of 10 male and 10 female Hoe:NMRKf mice were fed diets
containing endosulfan at a concentration of 0 or 18 ppm for six weeks,
equal to 0 or 3.7 mg/kg bw per day for males and 0 or 4.6 mg/kg bw per
day for females, to determine whether 18 ppm was the NOAEL in this
strain, as it was found to be in CD-1 mice in a three-month study
(Barnard et al., 1984). There were no clinical signs attributable to
treatment. The mean food intake of mice receiving endosulfan was
slightly decreased in males and slightly increased in females. Mean
body-weight gain was reduced in treated males during the second half
of the experiment but was slightly increased in females throughout the
experiment. Two treated females died, on days 28 and 38; the causes
were not established, but no histological changes were found in the
mouse that was not autolysed. The absolute and relative weights of the
liver of males and females at 18 ppm were higher than those of
controls and statistically significantly so in females (17 and 12%,
respectively). A NOAEL was not identified, as an increase in liver
weights was seen in females at the only dose tested (Donaubauer et
al., 1985).
Groups of 20 CD-1 mice of each sex were fed diets containing
endosulfan (purity, 97.2%) at a concentration of 0, 2, 6, 18, or 54
ppm for three months, equal to 0, 0.24, 0.74, 2.1, and 7.3 mg/kg bw
per day for males and 0, 0.27, 0.8, 2.4, and 7.5 for females. Clinical
signs attributable to treatment, consisting of convulsions and
salivation, were seen in one male and one female at the high dose.
There was a marked treatment-related decrease in the survival rate
(about 50%) of male and female mice at the high dose; the mean food
intake of these animals was significantly reduced for the first two
weeks of the study, and the mean body-weight gain of males at the high
dose was reduced during the first week of treatment. A significant
reduction in neutrophil count was observed in males at 54 ppm at week
6 (72%, p < 0.01), and a lower count (62%), which was not
statistically significant, was also observed at week 13. The blood
glucose concentration was reduced by about 11% in females at all doses
(p < 0.01) at week 6, and the serum lipid concentration was
increased by 15% in females at 54 ppm at week 13. The NOAEL was
18 ppm, equivalent to 2.1 mg/kg bw per day, on the basis of
convulsions and salivation, decreased survival, and increased serum
lipid concentrations at 7.3 mg/kg bw per day (Barnard et al., 1984).
Groups of 10 four-week-old ddY mice of each sex were fed diets
containing technical-grade endosulfan (purity, 91.4%) at
concentrations of 0, 10, 30, 100, or 300 ppm, equal to 0, 1.2, 4.1,
15, and 42 mg/kg bw per day in males and 0, 1.4, 4.7, 14, and 42 mg/kg
bw per day in females, for 12 months. There were no apparent
treatment-related clinical signs or deaths. Males at the high dose
showed a small (1%) but significant decrease in mean corpuscular
volume, and transient, non-dose-related increases in haemoglobin
(11%), haematocrit (4%), and eosinophil counts (33%) were seen in
males at 30 ppm. A significant decrease in serum aspartate
aminotransferase activity was seen in males at 100 and 300 ppm and a
decrease in bilirubin concentration in males at the high dose. The
only change in organ weights was a dose-related increase in the
relative weight of the adrenals in females, which was about 30% and
statistically significant at 300 ppm. There were no treatment-related
changes on gross pathological examination; the histopathological
effects consisted of dose-related granulomatous changes in the liver
and lymph nodes. In the liver, granuloma, giant-cell infiltration,
and/or large histiocytic cells filled with brown pigment were found in
treated mice; these effects were significant at 100 and 300 ppm. In
the lymph nodes, giant-cell infiltration and/or reticuloendothelial
cell proliferation were found at the same doses. Testicular atrophy
was seen erratically, with incidences of 30, 70, 33, 50, and 80% in
the five groups, respectively, but was not considered to be related to
treatment. The NOAEL was 30 ppm, equal to 4.1 mg/kg bw per day, on the
basis of histological findings in the liver and lymphatic system (Arai
et al., 1981).
Rats
Groups of male albino rats were given endosulfan in peanut oil by
gavage at a dose of 0 or 11 mg/kg bw per day for 30 days. In addition,
the possible interaction between endosulfan and the chemosterilant,
metepa, was investigated in groups of rats receiving either metepa
alone at 30 mg/kg bw per day for 30 days or in combination with
endosulfan at 11 mg/kg bw per day. There were three deaths in the
endosulfan-treated group. Endosulfan alone had no significant effect
on body weights, organ weights, blood chemistry, or histopathological
appearance. No potentiation of the toxicity of metepa was seen (Nath
et al., 1978).
Groups of 100 male SPF Wistar rats were fed diets containing
technical-grade endosulfan (purity, 97.9%) at concentrations of 360 or
720 ppm, equal to 34 and 68 mg/kg bw per day for four weeks. Twenty
control rats received diet alone. Fifty treated and 10 control animals
were maintained for an additional four-week withdrawal period. The
objective of the experiment was to determine the toxicological
significance of yellow, granular deposits observed in the proximal
convoluted renal tubules in a 13-week and a two-generation feeding
study. One rat in each treated group died, with no signs of poisoning.
The behaviour and general condition of the rats and their food and
water consumption were unaffected by treatment, and body-weight gains
were comparable in treated and control groups. Enlargement of the
liver was observed in rats at 360 and 720 ppm at the end of the
treatment period, and the kidney and brain weights were significantly
elevated in those at 720 ppm; these treatment-related changes in organ
weights had, however, disappeared by the end of the withdrawal period.
The kidneys of treated rats were darkly discoloured, but their
appearance had returned to normal by the end of the withdrawal period.
Histopathological examination by light and electron microscopy showed
granular pigmentation and larger, more numerous lysosomes in renal
proximal tubule cells after treatment; these changes had decreased by
the end of the withdrawal period, and no lysosomal changes were found
in brain or liver. Analysis of residues showed that storage of
alpha-endosulfan was dose-dependent, temporary, and confined to the
kidney, where it was detected as endosulfan sulfate and endosulfan
lactone. The amount of ß-endosulfan was 230 times less than that of
alpha-endosulfan. The concentrations of the sulfate and lactone
metabolites were many times lower in liver, and only traces of
endosulfan remained in the kidney at the end of the withdrawal period
(Leist & Mayer, 1987).
Groups of 15 male and 15 female Wistar rats were exposed by
inhalation to technical-grade endosulfan (purity, 97.2%) at a
concentration of 0, 0.5, 1, or 2 mg/m3 air for 6 h/day, five days per
week for 29 days, for a total of 21 exposures. One male rat at the
high dose was emaciated, had a pale skin, and adopted a high-legged
position; no other clinical signs were seen in the treated animals. No
neurological disturbances, opacity of the refractive media, impairment
of dental growth, or changes in the oral mucosa were seen. The
body-weight gain of males at the high dose tended to be depressed from
day 20 of exposure until day 29, the end of the recovery period, but
no other changes in body-weight gain or food consumption were seen.
Non-dose-related increases in erythrocyte and haemoglobin
concentrations were seen at the end of exposure, but not at day 29;
these concentrations were reported to be within normal ranges for this
strain of rat. Apart from a transient, non-dose-related increase in
creatinine concentration and a decrease in serum aspartate
aminotransferase activity in females at the high dose, no
treatment-related change in blood chemistry was noted, and no
histological changes were seen in any of the rats (Hollander et al.,
1984)
Technical-grade endosulfan (purity, 97.2%) was applied in a
solution in sesame oil to the shaved skin of the nape of groups of six
Wistar rats of each sex 21 times over 30 days, for 6 h/day on five
days per week under an occlusive bandage. Males were given a dose of
0, 12, 48, 96, or 190 mg/kg bw per day, and females received 0, 3, 6,
12, or 48 mg/kg bw per day. In males, deaths and clinical signs of
poisoning consisting of tremors, tonoclonic convulsions, and/or
hypersalivation were observed only at the highest dose. In females,
clinical signs of poisoning were observed at > 2 mg/kg bw per day;
deaths occurred mainly in the group receiving 48 mg/kg bw per day,
although single animals died on day 18 after receiving 3, 6, or 12
mg/kg bw per day, none having shown signs of poisoning. Serum
cholinesterase activity was 33% lower in males at 192 mg/kg bw per
day, whereas the activities of the erythrocyte and brain enzymes were
reduced by 12 and 7%, respectively. In females at 48 mg/kg bw per day,
serum cholinesterase activity was 21% lower than in controls, and
there were no effects in the activities of erythrocyte and brain
enzymes. The NOAEL was 6 mg/kg bw per day (Ebert et al., 1985a,b).
Technical-grade endosulfan (purity, 97.2%) as a solution in
sesame oil was applied to the shaved skin of the nape of groups of six
male and six female Wistar rats 21 times over 30 days, for 6-h periods
under an occlusive bandage, at doses of 0, 1, 3, 9, 27, or 81 (males
only) mg/kg bw per day. No signs of toxicity were observed in males or
females at 1 and 3 mg/kg bw per day, but two males at 9 mg/kg bw per
day died, one on day 5 with no previous signs of poisoning and the
other on day 8 after piloerection, hypersalivation, blood-encrusted
nose, stagger, and dyspnoea. There were no deaths among males at 27
mg/kg bw per day, but three at 81 mg/kg bw per day died. None of the
females at 9 mg/kg bw per day died and no clinical signs of toxicity
were observed, but five females at 27 mg/kg bw per day died between
days 2 and 6 with no previous clinical signs of toxicity. Microscopic
changes were seen in the livers of animals at > 9 mg/kg bw per day,
consisting of enlargement of parenchymal cells in the periphery and
loss of cytoplasmic basoplilia. Serum cholinesterase activity was
statistically significantly reduced by treatment, by 70-80% in males
at doses of 9-81 mg/kg bw per day but in females by only about 40% at
9 mg/kg bw per day. Brain acetylcholinesterase activity was
statistically significantly reduced in males at 9 (21%), 27 (28%), and
81 (24%) mg/kg bw per day and in all treated females by 13-18%. The
responses were not dose-related, and the observations in females are
probably not biologically significant. Since a later study did not
demonstrate a direct inhibitory effect of endosulfan on rat brain
acetylcholinesterase activity in vitro, the reduced brain enzyme
activity found in this study was difficult to interpret. It is also
noted that serum enzyme activity was inhibited only at much higher
doses in the study of Ebert et al. (1985a,b). The two males at 9 mg/kg
bw per day that died had reduced or immature testes and/or sex organs,
and the livers of these animals has accentuated lobular markings. The
authors reasoned that these effects resulted from a
non-substance-related developmental disturbance already present before
treatment. No mechanism was proposed for these effects, which were not
seen at higher doses. The NOAEL was 3 mg/kg bw per day on the basis of
inhibition of serum and brain cholinesterase activity and microscopic
changes in the liver (Ebert et al., 1985b,c).
Endosulfan (purity not stated) as a solution in acetone was
applied daily to the shaved abdominal skin of groups of 24 albino rats
of each sex for 30 days at doses of 0, 19, 38, or
63 mg/kg bw per day for males and 0, 10, 20, or 32 mg/kg bw per day
for females. There were no deaths. All doses produced
hyperexcitability, tremor, dyspnoea, and salivation, which disappeared
after one week. No significant changes in organ:body weight ratios
occurred, and there were no treatment-associated effects on
histological, haematological, or blood chemical parameters. Liver
alanine and aspartate aminotransferase activities were reduced in
animals of each sex at the lowest dose, but there were no further
reductions with increasing dose. Liver alkaline phosphatase and
lactate dehydrogenase activities were increased in females but not in
males, again with no increase with dose. Cholinesterase activities
were not measured. A NOAEL was not identified (Dikshith et al., 1988).
Groups of 25 CD Sprague-Dawley rats of each sex were fed diets
containing technical-grade endosulfan (purity, 97.9%) at
concentrations of 0, 10, 30, 60, or 360 ppm, equal to 0, 0.64, 1.9,
3.8, and 23 mg/kg bw per day for males and 0, 0.75, 2.3, 4.6, and
27 mg/kg bw per day for females, for three months. Five animals of
each sex per group were maintained for an additional four-week
recovery period. Three females died, one each at 0, 60, and 360 ppm.
Slight but statistically significant, dose-related reductions in
erythrocyte counts and haemoglobin concentrations were seen in males
at > 30 ppm and in females at > 60 ppm, but were within the
reported normal range for this strain and age of rat (Leist & Bremmer,
1998); increased mean corpuscular volume was also seen at these doses.
Females at 360 ppm had statistically significant decreases in plasma
and erythrocyte cholinesterase activities (measured by the Ellman
method) at week 12 (by 41 and 12%, respectively), while increased
brain acetylcholinesterase activity was observed at 60 and 360 ppm (19
and 20%, respectively). In males at 360 ppm, urinalysis showed a
number of reversible changes, including increased urine volume and
urinary protein concentrations and decreased specific gravity. Gross
examination revealed enlargement of the liver in males at 360 ppm and
of the kidneys at 60 and 360 ppm; increases in the absolute weights of
the liver (18%), kidney (29%), and epididymides (8%) were seen in
males and of the liver (21%) and kidneys (10%) in females. The kidney
weights remained significantly elevated in male rats at 360 ppm (15%,
p < 0.01) at the end of the withdrawal period.
Histopathological examination revealed traces of brown pigment in
scattered hepatocytes in 25% of male rats and minimal centrilobular
enlargement of hepatocytes in 25% of females at 360 ppm. These changes
were not observed in rats at the end of the withdrawal period.
Yellowish discolouration of renal proximal tubular cells was seen in
males at all doses and in females at 30-360 ppm, the degree of
pigmentation increasing in a dose-related manner; however, no cell
death was associated with this finding. In addition, granular
pigmentation was seen in straight portions and occasionally in
proximal tubular cells in males at 60 and 360 ppm. The yellow
discolouration of the renal tubules in male rats had decreased by the
end of the withdrawal period, but trace or minimal pigmentation was
still evident. In females at > 60 ppm, the traces of pigmentation
persisted. Males at 360 ppm also had yellow protein aggregation in the
proximal convoluted with intracytoplasmic eosinophilic droplets in the
tubules. The increase in incidence and degree of the yellowish
discolouration of the proximal tubular cells appeared to be
treatment-related, since it did not develop in control rats; however,
no adverse effects were reported that might be associated with these
findings alone. All rats at 30 ppm showed either trace or minimal
discolouration and signs of granular or clumped pigment. Other
investigations suggest that the yellow pigmentation is no more than an
indication that endosulfan and some of its metabolites are being
temporarily stored before urinary excretion, and that it is therefore
an indicator of exposure rather than an expression of toxicity.
Consequently, this effect was not considered in the evaluation. At
doses of 60 and 360 ppm, other treatment-related effects were also
seen when the pigmentation was present, including enlarged kidneys and
centrilobular hepatocytes. No treatment-related increase in the
incidence of other renal effects was reported in animals at 10 ppm.
The NOAEL was thus 10 ppm, equal to 0.64 mg/kg bw per day, on the
basis of haematological changes (Barnard et al., 1985).
(c) Long-term studies of toxicity and carcinogenicity
Mice
Groups of 50 six- to seven-week-old B6C3F1 mice of each sex were
fed diets containing technical-grade endosulfan (purity, 98.8%) at
time-weighted average concentrations of 3.5 or 6.9 ppm for males and 2
or 3.9 ppm for females for 78 weeks. Groups of 20 controls received
untreated diet. There were no clear compound-related effects on
appearance or behaviour in the treated groups, and the body weights of
both males and females were unaffected by treatment. The mortality
rate of males at the high dose was increased early in treatment so
that, at the end of the experiment, the survival rates were 3/20
controls, 19/50 at the low dose, and 5/50 at the high dose. The
mortality rates of female mice were not affected by treatment. No
treatment-related clinical signs were recorded, and no
treatment-related neoplastic lesions were seen in the females. Owing
to the high early mortality rates, no conclusion could be drawn about
the carcinogenic potential of endosulfan in males. None of the
non-neoplastic changes seen in the kidneys and sex organs of male and
female mice could be attributed to treatment. The NOAEL for female
mice was 3.9 ppm, equal to 0.58 mg/kg bw per day (US National Cancer
Institute, 1978).
Groups of 60 NMRI mice of each sex were fed diets containing
technical-grade endosulfan (purity, 97.2%) at concentrations of 0, 2,
6, or 18 ppm, equal to 0.28, 0.84, and 2.5 mg/kg bw per day for males
and 0.32, 0.97, and 2.9 mg/kg bw per day for females, for up to 24
months. Ten mice of each sex per dose were killed at 12 and 18 months.
The behaviour and general health of the animals were not affected by
treatment. The mortality rate of females at 18 ppm was statistically
significant decreased at the end of the experiment: control, 45%; 18
ppm, 28% (p < 0.05); at week 78, the rates in these two groups of
female mice were 82 and 62%. Survival among treated male mice was not
statistically different from that of controls. The body weights of
males receiving 18 ppm were slightly but significantly lower than
those of controls during the first third of the study and remained
slightly but not significantly low throughout the remainder of the
study. In the other treated groups, there was a tendency to increased
body-weight gains, especially in the satellite groups killed at 12 and
18 months. No statistically significant changes were observed in
haematological or clinical chemical parameters, and macroscopic
examination did not reveal any findings that were related to
treatment. No statistically significant changes in organ weights were
seen in treated animals at the end of the experiment; however, slight
but statistically significant changes in organ weights were observed
in animals at 18 ppm at 12 and 18 months, consisting of decreased lung
and ovary weights in females at 12 months and decreased liver weights
in males and decreased ovary weights in females at 18 months.
Histopathological examination did not reveal any effects that were
related to treatment. No increase in the incidence of neoplastic or
non-neoplastic lesions was observed. The NOAEL was 6 ppm, equal to
0.84 mg/kg bw per day, on the basis of decreased body weights in males
at 24 months and decreased weights of the liver, ovaries, and lung in
males and females at 12 and/or 18 months (Donaubauer, 1988, 1989; Hack
et al., 1995).
Rats
Groups of 50 male and 50 female Osborne-Mendel rats were fed
diets containing technical-grade endosulfan (purity, 98.8%) at
time-weighted average doses of 220, 410, or 950 ppm for males and 220
or 400 ppm for females for 78 weeks, with a return to control diets
for a further four weeks. Groups of 20 rats of each sex received
untreated diet. A highly significant morbidity rate was seen in male
rats and, by week 54, 52% of those at the high dose had died. A
dose-related reduction in body weight was found in males at all doses.
Histopathological examination showed a high incidence of toxic
nephropathy (> 90%) in males at the low and high doses and in
females, but in none of the controls. Chronic renal inflammation was
observed in 40% of control males and 80% of treated males. The toxic
nephropathy observed was characterized as degenerative changes in the
proximal convoluted tubules at the junction of the cortex and medulla,
with associated cloudy swelling, fatty degeneration, and necrosis of
the tubular epithelium. Some tubules had hyaline casts, and enlarged,
dark-staining regenerative tubular epithelial cells were observed
infrequently. In treated males, parathyroid hyperplasia was observed,
as were calcium deposits in the stomach, kidney, testis, aorta, and
mesenteric artery. A dose-related increase in the incidence of
testicular atrophy was seen in treated males, characterized by
degeneration and necrosis of the germinal cells lining the
seminiferous tubules and multinucleated cells (fusion bodies),
resulting in aspermatogenesis. No treatment-related effects were noted
in the reproductive organs of female rats. No treatment-related
neoplastic lesions were seen in female rats; owing to the high
mortality rate in males, no valid conclusion can be drawn about
carcinogenicity. A NOAEL was not identified, as treatment-related
changes occurred in the kidneys and the testis at all doses (US
National Cancer Institute, 1978)
Groups of 50 five-to-six-week-old Sprague-Dawley rats of each sex
were fed diets containing endosulfan (purity, 97.1%) at concentrations
of 0, 3, 7.5, 15, or 75 ppm, equal to 0, 0.1, 0.3, 0.6, and 2.9 mg/kg
bw per day for males and 0, 0.1, 0.4, 0.7, and 3.8 mg/kg bw per day
for females, for 104 weeks. Satellite groups of 20 animals of each sex
were retained for blood sampling and examination at 104 weeks.
Reductions in body weights and body-weight gains were observed in
males (group mean, 17% at 104 weeks) and females (group mean, 18% at
104 weeks) at 75 ppm, but no clinical signs of poisoning were seen at
any dose. No increase in mortality rates was observed in treated
groups. Increased incidences of enlarged kidneys in females and of
aneurysms and enlarged lumbar lymph nodes in males were seen at 75
ppm. Histopathological examination showed that males at 75 ppm had an
increased incidence of aneurysm and marked progressive
glomerulonephrosis (controls, 20/70; 75 ppm, 30/70). The commonest
neoplasms were pituitary tumours in males and females and mammary
tumours in females, but the increased incidences did not appear to be
related to treatment. The NOAEL was 15 ppm, equal to 0.6 mg/kg bw per
day, on the basis of reduced body weights and pathological findings at
higher doses (Ruckman et al., 1989; Gopinath & Cannon, 1990; Hack et
al., 1995).
Groups of 25 Wistar rats of each sex were fed diets containing
technical-grade endosulfan (purity unspecified) at doses of 0, 10, 30,
or 100 ppm for up to 104 weeks; groups of five of each sex were killed
at 52 weeks. There were no treatment-related clinical signs, and the
body weights were unaffected, except for a nonsignificant decrease in
the body weights and food consumption of males at the high dose. The
survival rate of treated females was reduced, the deaths being
associated predominantly with respiratory infections. The weights of
the testes of males at 10 ppm were reduced by 7% with respect to
controls at 104 weeks (p < 0.05), and the kidney weights were
significantly (p < 0.001) increased (by 16%) in males at the high
dose at 104 weeks. Histopathological changes observed in males at the
high dose at 104 weeks consisted of enlarged kidneys, mild-to-severe
renal tubular dilatation (12/12), mild-to-moderate formation of
irregular albuminous casts (10/12), pronounced focal nephritis (7/12),
and mild-to-severe degeneration (11/12) of the renal tubular
epithelium. At 104 weeks, female rats at the high dose showed minimal
degeneration of renal tubules (2/3) and some focal nephritis (1/3).
The low survival rate precluded a clear conclusion about the renal
changes in female rats. Microscopic alterations in the liver were seen
in 50% of males at the high dose at week 104, consisting of focal
areas of hydropic cells, which were pale and swollen; the nuclei were
surrounded by a clear zone, and a few cells appeared to have
eosinophilic cytoplasmic inclusions. Few females at the high dose
showed changes in liver cells. A few tumours developed during the
experiment, but their occurrence was not dose-related. The NOAEL was
30 ppm, equal to 1.5 mg/kg bw per day, on the basis of effects on the
kidney (Keller, 1959c).
Dogs
Groups of six beagle dogs of each sex were fed diets containing
technical-grade endosulfan (purity, 96.5%) at concentrations of 0, 3,
10, or 30 ppm for one year, calculated by the authors to be equivalent
to 0, 0.23, 0.77, and 2.3 mg/kg bw per day. In addition, one group was
given a diet containing 30-60 ppm endosulfan, increasing in stages
from 30 ppm for 54 days, to 45 ppm for 52 days, and 60 ppm for 19-40
days; these dogs were killed in extremis before the scheduled
completion of the experiment and showed a number of signs of
poisoning, including tonic contraction and increased sensitivity to
noise and optical stimuli. Some animals given endosulfan at 30 ppm
throughout the 12-month study had violent contractions of the
abdominal muscles (without vomiting), and males at this dose had
reduced body-weight gains throughout the study and slightly reduced
body weights in the latter stages of the study, in comparison with
control animals. Cholinesterase activity was measured in serum,
erythrocytes, and brain, but difficulty appears to have been
experienced in measuring these activities, and there were large
variations within groups for the brain enzyme, the group mean of which
was increased in dogs at 30 ppm. No other effects related to treatment
were observed, and no increase in the incidence of neoplastic or non-
neoplastic lesions was observed in treated animals. The NOAEL was 10
ppm, calculated by the authors to be equivalent to 0.57 mg/kg bw per
day, on the basis of clinical signs and reductions in body weight
(Brunk 1989, 1990).
Groups of two male and two female mongrel dogs were given
technical-grade endosulfan in gelatin capsules at doses of 0, 3, 10,
or 30 ppm, equivalent to 0, 0.075, 0.25, or 0.75 mg/kg bw per day, on
six days per week for one year. The group receiving 3 ppm was given
100 ppm for the first three days of treatment, but clinical signs of
vomiting, tremors, convulsions, rapid respiration, mydriasis,
salivation, and tonic-clonic convulsions in one male and both females
led to a reduction in the dose for the remainder of the study. No
clinical signs or treatment-related effects on body-weight gain were
seen. Clinical chemical and haematological end-points were within
normal limits, and kidney function was unaffected by treatment. No
gross or histopathological changes associated with treatment were
noted. The NOAEL was 30 ppm, equivalent to 0.75 mg/kg bw per day, on
the basis of clinical signs at the initial high dose (Keller, 1959b).
(d) Genotoxicity
Endosulfan was tested for genotoxicity in a wide range of assays,
both in vitro (with and without metabolic activation) and in vivo
(Table 3). There was no evidence of genotoxicty in most of these
assays. In an assay for dominant lethal mutation in male Swiss mice
given endosulfan of a purity of 97.3%, there was a significant change
in the result of mating during the sixth mating week in the group at
16.6 mg/kg bw per day. The total numbers of implants per pregnancy
were 9 in controls and 4.5 at the high dose; the numbers of live
implants per pregnancy were 9 in controls and 2.2 at the high dose;
and the numbers of dead implants per pergnancy were none in controls
and 2.25 at the high dose (Pandey et al., 1990). While there is no
doubt about the statistical significance of these results, it is
unusual to find a true dominant lethal effect appearing so late in an
experimental mating schedule; however, it is not unknown, since a
reproducible effect of this type was demonstrated with some glycol
ethers (McGregor et al., 1983). In a later assay for dominant lethal
mutation (Dzwonkowska & Hübner, 1991), much lower doses were used, so
the results cannot be used as evidence for a non-reproducible effect.
Significant increases in the proportion of morphologically abnormal
sperm were also observed in the study of Pandey et al. (1990), as well
Table 3. Results of assays for the genotoxicity of endosulfan
End-point Test object Dose Result Reference
(LED or HID)a
In vitro
Differential toxicity B. subtilis rec strains H17 and M45 2000 µg/disc Negativea Shirasu et al. (1978)
Reverse mutation S. typhimurium TA100, TA1535, 5000 µg/plate Negativeb Shirasu et al. (1978)
TA1537, TA1538, TA98;
E. coli WP2 uvrA
Gene conversion S. cerevisiae, D4 5000 µg/ml Negativeb Mellano & Milone (1984b)
Forward mutation S. pombe 500 µg/ml Negativeb Mellano & Milone (1984a)
Unscheduled DNA Male F344 rat primary hepatocytes 51 µg/ml Negativea Cifone & Myhr (1984b)
synthesis
Gene mutation Mouse lymphoma L5178Y cells, 75 µg/ml [?] Negativeb Cifone & Myhr (1984a)
tk locus
Chromosomal Human lymphocytes 200 µg/ml Negativeb Asquith & Baillie (1989)
aberration
Chromosomal Human lymphocytes 200 µg/ml Negativeb Pirovano & Milone (1986)
aberration
In vivo
Micronucleus NMRI mouse bone-marrow cells 5 mg/kg bw, Negative Jung et al. (1983)
formation po × 1
Micronucleus NMRI mouse bone-marrow cells 10 mg/kg bw, Negative Müller (1988)
formation po × 1
Table 3. (continued)
End-point Test object Dose Result Reference
(LED or HID)a
Chromosomal Albino rat bone-marrow cells 55 mg/kg, po × 5 Negative Dikshith & Dotta (1978)
aberration
Dominant lethal Male Swiss mice 16.6 mg/kg bw, Equivocal Pandey et al. (1990)
mutation ip × 5
Dominant lethal Male Balb/c mice 0.64 mg/kg bw, Negative Dzwonkowska & Hübner
mutation ip × 1 and ip × 5 (1991)
Sperm morphology Mice 16.6 mg/kg bw, Positive Pandey et al. (1990)
ip × 5
Sperm morphology Mice in vivo 3 mg/kg bw, Positive Khan & Sinha (1996)
ip × 35
LED, lowest effective dose; HID, highest ineffective dose; po, oral; ip, intraperitoneal
a In the absence of exogenous metabolic activation; not tested in the presence of exogenous metabolic activation
b In the absence and presence of exogenous metabolic activation
as in a later study (Khan & Sinha, 1996) at lower daily doses of a 35%
emulsifiable concentrate.
(e) Reproductive toxicity
(i) Multigeneration reproductive toxicity
A preliminary investigation was conducted to determine the doses
of endosulfan to be used in a two-generation study of reproductive
toxicity. Four groups of 10 male and 10 female seven-week-old Crl:
COBS CD Sprague-Dawley rats were given diets containing
technical-grade endosulfan (purity, 97%) at concentrations of 0, 50,
75, or 100 ppm for two weeks and subsequently throughout mating and
the rearing of offspring to weaning. Food consumption and body weights
were decreased in adults at 75 and 100 ppm. At terminal autopsy, the
mean weights of the livers were significantly higher than the control
value in all treated groups. Mating performance, pregnancy rate, and
the duration of gestation were unaffected by treatment. The litter
weights of dams were significantly decreased at 75 ppm and to a
greater extent at 100 ppm from day 4 post partum. No
treatment-related abnormalities were found in the young (Edwards et
al., 1982).
In a two-generation study of reproductive toxicity with two
matings in each generation, four groups of six-week-old
Sprague-Dawley rats were fed diets containing technical-grade
endosulfan (purity, 97%) at concentrations of 0, 3, 15, or 75 ppm,
equal to 0.2-0.23, 1-1.2, and 5-5.7 mg/kg bw per day for males and
0.24-0.26, 1.2-1.3, and 6.2-6.9 mg/kg bw per day for females. The
group sizes were 32 of each sex for the F0 generation and 28 of each
sex for the F1b generation. No clinical signs or deaths related to
treatment were observed during the study. Single deaths occurred among
F0 females at 0, 3, and 15 ppm and among F1b control females. Mating
performance and pregnancy rates were not affected by treatment.
Statistically significant decreases in litter weight were occasionally
seen, but there was no effect on mean pup weights or on litter size.
No treatment-related effect on sex ratios was seen at any dose.
Statistically significantly increased relative kidney weights
were seen at 75 ppm in F0 and F1b males, and statistically
significantly increased relative liver weights were observed in F0
males and females at 75 ppm and in F1b females at 15 and 75 ppm. The
effect at 15 ppm in F1b dams was not seen at this dose in any other
matings. Yellowish discolouration of cells in the proximal convoluted
tubules were observed in male F1b rats at 3, 5, and 75 ppm and in
female F1b rats at 75 ppm. The incidence and extent of this effect
was dose-related; traces of discolouration were seen at all doses, and
minimal discolouration was seen in male rats at 15 and 75 ppm.
Granular or clumped pigment was seen in proximal convoluted tubular
cells in males at the high dose, but these findings were not
associated with histopathological evidence of renal damage. While the
increased incidence of cellular discolouration was related to
treatment, the finding is considered not to be toxicologically
significant as no adverse effects were seen on cells and the yellow
pigment was considered likely to be due to storage of endosulfan and
its metabolites in lysosomes before excretion (Annex 1, reference 58).
The presence of the pigment is thus an indication of exposure to
endosulfan rather than of toxicity. The NOAEL for maternal toxicity
was 15 ppm, equal to 1 mg/kg bw per day, on the basis of increased
relative liver and kidney weights at higher doses. The NOAEL for
reproductive effects was 75 ppm, equal to 6 mg/kg bw per day, the
highest dose tested (Edwards et al., 1984; Offer, 1985).
(ii) Developmental toxicity
Rats
Groups of mated female albino rats (strain and age unspecified)
were given oral doses of 0 (20 rats), 5 (26 rats), or 10 (32 rats)
mg/kg bw per day of endosulfan (purity unspecified) on days 6-14 of
gestation. No marked changes in behaviour or appearance were reported,
and the body weights of treated animals were similar to those of
controls. The numbers of pregnancies were 18, 20, and 21,
respectively. The dams were killed on day 21 of gestation. No
abortions occurred, but there was a significant increase in the
percent of litters with resorptions (5.5% in controls, 20% at 5 mg/kg
bw per day, and 23% at 10 mg/kg bw per day) and increased fetal
mortality, although this effect was slight and was not dose-related
(0, 2, and 1 in the three groups, respectively). Slight increases in
the incidences of cerebral hypoplasia and enlargement of the renal
pelvis were observed on visceral examination, but these effects were
not considered to be related to treatment as they were also seen in
control animals and the increases were small and were not
dose-dependent. No other increase in the incidence of visceral
abnormalities was reported. Skeletal examination showed statistically
significant increases in the incidences of absent fifth sternebrae and
of fetuses with incomplete ossification. A slight increase in the
incidence of absent fifth metacarpus, although not statistically
significant, was also seen in treated animals. These effects were not
considered to be related to treatment, as their magnitude was small
and they were not dose-dependent. No maternal toxicity was seen at any
dose. The level of reporting in this published paper was inadequate
for identifying a NOAEL for developmental toxicity (Gupta et al.,
1978).
Groups of 25 mated CD Sprague-Dawley rats were given
technical-grade endosulfan (purity, 97.3%) in corn oil by gavage on
days 6-19 of gestation at a dose of 0, 0.66, 2, or 6 mg/kg bw per day.
The clinical signs in dams at 6 mg/kg bw per day included flaccidity,
rough coat, alopecia, and hyperactivity. A dose-related decrease in
maternal body-weight gain was seen at 2 and 6 mg/kg bw per day. The
number of implantations and litter size were unaffected, but there was
a slight reduction in the weight and length of fetuses of dams at the
high dose. A non-dose-related reduction in the percent of live fetuses
and an increase in the number of resorbed fetuses were seen at 2 mg/kg
bw per day. No statistically significant treatment-related effect on
the sex ratio was observed. No external variations or malformations
were seen at 0.66 or 2 mg/kg bw per day; at the high dose, 5/405
fetuses exhibited lordosis (anteroposterior curvature of the spine)
and six had oedema. All five of the fetuses with lordosis and five of
those with oedema were from a single litter. In one fetus from the
same litter, the skin of the upper forelimb was webbed to the chest.
No significant treatment-related effects were seen on soft-tissue
development. Common minor skeletal variations were present in all
groups. The incidence of poorly ossified sixth sternebrae was
significantly greater in animals at the high dose than in the control
group, and two fetuses at this dose had clubbed left hindlimbs. The
five fetuses from the same litter that had oedema and lordosis also
had wide, thickened vertebral arches, ribs, and clavicles, and the
clavicles were shortened, curved, and twisted. Four of these fetuses
had shortened pubes, and two had an unossified hyoid bone. The
incidence of these effects was generally < 1%, and the effects were
largely related to delayed development and confined mainly to a single
litter from a single dam that showed numerous signs of poisoning
related to administration of endosulfan, including face rubbing,
alopecia, flaccidity, and hyperactivity. The developmental effects are
therefore probably related to the maternal toxicity of the high dose.
The NOAEL for maternal toxicity was 0.66 mg/kg bw per day on the basis
of decreased body-weight gain and clinical signs at higher doses. The
NOAEL for developmental toxicity was 2 mg/kg bw per day on the basis
of delayed development and a low incidence of isolated skeletal
variations (Mackenzie, 1980).
Groups of 20-24 mated female Wistar rats were given
technical-grade endosulfan (purity, 97.3%) dissolved in sesame oil by
gavage on days 7-16 of gestation at doses of 0, 0.66, 2, or 6 mg/kg bw
per day. No clinical signs of toxicity were reported in females at
0.66 or 2 mg/kg bw per day; four dams at 6 mg/kg bw per day died after
6-10 doses of endosulfan, three of these rats having tonic-clonic
convulsions for several days before death. Thirteen of the surviving
animals had tonic-clonic convulsions for a number of days, generally
around day 10 of gestation. Some of these rats also showed
hypersalivation on a number of days during treatment. Statistically
significant decreases in body weight and body-weight gain were
observed at 6 mg/kg bw per day. No statistically significant changes
in reproductive or pup parameters were observed at any dose, and the
fetal sex ratio was relatively well balanced. No statistically
significant increase in the incidence of abnormalities was observed in
fetuses. A single oedematous, retarded fetus at 6 mg/kg bw per day
presented with superior brachygnathia and a relatively small alveolar
cavity in the upper jaw, combined with cleft palate, bending of both
hind feet in the tarsal joint, wavy clavicles, and bent and shortened
scapulae. These effects were considered to be spontaneous, as no other
limb or head defects were observed in any pup in any of the litters at
this dose. Skeletal examination revealed a statistically significant
increase in the incidence of fragmented thoracic vertebral centra at 6
mg/kg bw per day. This effect was considered to be treatment-related
and reflects the frank maternal toxicity of endosulfan at the high
dose. No treatment-related major malformations were observed. The
NOAEL for maternal toxicity was 2 mg/kg bw per day on the basis of
deaths, clinical signs, and decreased body weights at higher doses.
The NOAEL for developmental toxicity was 2 mg/kg bw per day on the
basis of the increased incidence of fragmented thoracic vertebral
centra (Albrecht & Baeder, 1993).
Rabbits
Groups of 20-26 mated New Zealand white rabbits were given
technical-grade endosulfan (purity, 97.3 %) in corn oil by gavage on
days 6-28 of gestation at doses of 0, 0.3, 0.7, or 1.8 mg/kg bw per
day. There were no changes in mean body weight. None of the does at
0.3 or 0.7 mg/kg bw per day aborted, and there were no signs of
toxicity and no deaths. The high dose was associated with signs of
maternal toxicity that included noisy, rapid breathing, hyperactivity,
and convulsions. The does were killed on day 29 of gestation. The
number of implantations, litter size, sex ratio, mean fetal weight and
length, and the numbers of live and resorbed fetuses were unaffected
by treatment. There were no dead fetuses in any group, and no gross
external alterations were reported. The only soft-tissue anomalies
occurred in 6/167 fetuses (2/20 litters examined) at the high dose and
consisted of the left carotid arising from the innominate; 1/141
control fetuses (1/18 litters examined) also showed this abnormality.
Common skeletal variations and minor anomalies occurred at similar
incidences in control and treated fetuses. Endosulfan did not have
teratogenic or developmental effects even at the maternally toxic dose
of 1.8 mg/kg bw per day. The NOAEL for maternal toxicity was 0.7 mg/kg
bw per day on the basis of clinical signs at higher doses (Dickie et
al., 1981).
(f) Special studies
(i) Enzyme induction
Endosulfan was one of 16 organochlorine pesticides tested for
their ability to induce hepatic microsomal enzyme activities. Wistar
rats were given diets containing endosulfan at concentrations of 0,
20, 50, or 200 ppm for two weeks. No difference from control enzyme
activity was observed at 20 or 50 ppm; at 200 ppm group, the
activities in comparison with the control were 123% for aniline
hydroxylase (statistically significant, one experiment), 191% for
aminopyrene demethylase (statistically significant, one experiment),
and 124% for hexobarbital oxidase (not significant, one experiment)
(den Tonkelaar & van Esch, 1974).
ICR mice given endosulfan at 5 mg/kg bw per day by oral gavage
for three days and killed on the fourth day showed no increase in
liver weight or total hepatic cytochrome P450 content. The dearylation
of O-ethyl O- para-nitrophenyl phenylphosphorothioate and
parathion and NAD(P)H-dependent reductase activity were
nonsignificantly increased (Robacker et al., 1981).
(ii) Promotion
Enhancement of gamma-glutamyl transpeptidase-positive foci in rat
liver was studied as an indicator of promotion. Young male
Sprague-Dawley rats were partially hepatectomized and injected
intraperitoneally 24 h later with N-nitrosodiethylamine at 30 mg/kg
bw. One week after the partial hepatectomy, the rats were randomized
to groups of 10 or 11 and dosed orally by gavage on five days per week
for 10 weeks with endosulfan in corn oil at 0, 1, or 5 mg/kg bw per
day. The numbers and volume of enzyme-altered foci were not increased
in the treated groups in comparison with controls.
In the same study, inhibition of intercellular communication was
studied in Chinese hamster lung V79 cells in a metabolic cooperation
assay and in rat liver WB epithelial cells in a scrape loading,
dye-transfer assay. At nontoxic concentrations, technical-grade
endosulfan, analytical-grade ensosulfan (alpha and ß isomers and a
mixture of the two), and endosulfan sulfate inhibited gap-junctional
intercellular communication in both systems. In addition, endosulfan
ether was effective in the rat liver WB cell system (Flodström et al.,
1988).
(iii) Immunotoxicity
In two published studies, endosulfan was administered to male
Wistar rats at dietary doses of up to 50 ppm for six weeks (Banarjee &
Hussain, 1987) or up to 20 ppm for 22 weeks (Banarjee & Hussain, 1986)
to evaluate humoral and cell-mediated immune responses. In the
six-week study, a significant decrease in total serum antibody titre
to tetanus toxoid was seen at 30 and 50 ppm, with a slight decrease
(not statistically significant) at 10 ppm. A decrease in both
immunoglobulin (Ig)M and IgG and in the total gamma-globulin content
of rat serum was observed at 50 ppm. Cellular immunity was assayed by
measuring inhibition of migration of activated leukocytes and
macrophages. Rats exposed to endosulfan and subsequently immunized
with tetanus toxoid showed a significant decrease in inhibition of
leukocyte and macrophage migration in a dose-dependent pattern, the
decrease becoming statistically significant at 30 and 50 ppm. These
results indicate that both humoural and cellular immunity was
depressed as a result of exposure to endosulfan at 30 and 50 ppm, with
no effect at 10 ppm, equivalent to 0.5 mg/kg bw per day. In the
22-week study, the specific response of serum antibody titre to
tetanus toxoid showed a marked decrease in rats exposed to 10 or 20
ppm endosulfan throughout the experiment, in a dose- and
time-dependent pattern. Treatment at 10 or 20 ppm diminished the
inhibition of migration of both leukocytes and macrophages throughout
the study.
The effect of endosulfan on the immune system did not appear to
be secondary to other toxic effects, since the body weights of the
animals were unaffected by treatment and endosulfan is not known to
affect the hormonal system. Immune responses were unaffected by
treatment at 5 ppm, equivalent to 0.25 mg/kg bw per day. The Committee
noted that the method used to assess cellular immunity in these
studies is far from ideal as it is flawed by large inherent errors,
lack of objectivity, and, except in very experienced hands, lack of
accuracy. Less subjective tests of cellular immunity, such as
cytotoxic T cell response to a virus, would have provided more
reliable results.
Technical-grade endosulfan (purity, 96%) was administered in
sesame oil on 10 occasions by gavage to groups of eight female Wistar
rats at doses of 0.5, 1.5, or 4.5 mg/kg bw per day from two days
before until seven days after infection by gavage with approximately
500 Trichonella spiralis larvae. As a positive control, prednisolone
was administered by subcutaneous injection at a dose of 25 mg/kg bw
per day two days before and three days after infection. Three rats
from each group were killed seven days after infection so that the
number of adult worms in the intestine could be counted; the remaining
rats were killed 54 days after infection in order to count the number
of larvae in the tongue. Thymus and spleen weights and the percentage
lymphocytes in the white cell count were measured at both times. Body
weights were measured weekly. There were no differences between
endosulfan-treated and untreated rats, whereas the
prednisolone-treated group had a sevenfold higher tongue larval count
and, at seven days, a 25% reduction in thymus weight, a 50% reduction
in spleen weight, and lymphocyte counts < 50% of the control value
(Hack & Leist, 1988).
Endosulfan was included in the first part of a study to screen
for immunotoxicity, but because no effect was observed it was not
examined in greater detail. Groups of six male, weanling Wistar rats
were given endosulfan in the diet at concentrations of 20, 100, or 250
ppm for three weeks. Body weights and food intake were recorded
weekly. At autopsy, the weights of the liver, kidneys, spleen, thymus,
pituitary, adrenals, thyroid, testis, and mesenteric and popliteal
lymph nodes were recorded, these organs were also examined
histologically. Haematological examination consisted of total and
differential leukocyte counts. Serum IgM and IgG were determined by
enzyme-linked immunosorbent assay. The only effects induced by
endosulfan were considered to be expressions of general toxicity, and
there was no evidence for any specifically immunotoxic effects. The
most sensitive parameter for the toxicity of endosulfan was a
reduction in body-weight gain, which was observed at 100 ppm (Vos et
al., 1982).
(iv) Neurobehavioural effects and neurotoxicity
In a number of studies conducted by the same group of
investigators, endosulfan (purity, 95%) was given by gavage to rats at
a dose of 2 mg/kg bw per day for 90 days (Paul et al., 1993, 1994) or
up to 6 mg/kg bw per day for 30 days (Paul et al., 1995), and
behavioural and biochemical changes were determined. Signs of frank
toxicity (reduced body weights, reduced food consumption, death,
increased intensity of tremors, and increased liver enzyme activity)
were observed in all studies, and some changes in behaviour were
noted, including increased motor activity and inhibition of
conditioned and unconditioned escape and avoidance responses.
Groups of adult domestic hens were given a single oral dose of 96
mg/kg bw endosulfan, the LD50, in corn oil, observed for 21 days,
re-dosed, and again observed for 21 days. As was to be expected, a
number of deaths occurred that were related to treatment. No signs of
ataxia and no treatment-related changes in nervous tissue were seen in
vehicle control or endosulfan-treated hens. Seven of 10 hens in a
concurrent positive control group given tri- ortho-cresyl phosphate
developed ataxia and signficant spinal cord and peripheral nerve
degeneration (Roberts et al., 1983).
No inhibition of rat brain acetylcholinesterase activity was
observed in a preparation incubated with 10 µmol/L alpha-endosulfan
for up to 75 min.. A similar concentration of aldicarb produced 15%
inhibition within 5 min and 80% inhibition within 75 min (Müllner,
1989).
Technical-grade endosulfan (purity, 98.6%) was administered to
groups of 10 rats as a single dose of 25, 50, or 100 mg/kg bw to males
and 3, 6, or 12 mg/kg bw to females. Deaths occurred at the highest
doses, and there was a dose-related increase in the frequency of
clinical signs, which were reversible and apparent only on the day of
dosing. These were assumed to be due to the known affinity of
endosulfan for the gamma-aminobutyric acid receptors in the brain. At
50 and 100 mg/kg bw in males and 6 and 12 mg/kg bw in females, various
serious neuropharmacological effects were seen, including coarse
tremor and tonic-clonic convulsions. At 25 mg/kg bw in males and
3 mg/kg bw in females, the clinical signs seen were typical of general
discomfort, such as stilted gait, squatting posture, and irregular
respiration. No compound-related effects on motor activity were
observed at non-lethal doses. No effects were seen on the rearing
frequency, fore- or hindlimb grip strength, or on landing foot-spread.
No histopathological effects were found in the central or peripheral
nervous system. The study was carried out in accordance with
prevailing OECD testing guidelines (April 1996) and the OECD
principles of good laboratory practice (12 May 1981 [C(81)30(Final)])
(Bury, 1997).
(v) Effects on sperm
In a study of biochemical changes induced by endosulfan in the
testis of Druckrey rats, the authors postulated that endosulfan
impairs testicular function by altering the enzyme activities
responsible for spermatogenesis, thus affecting the intratesticular
spermatid count and resulting in low sperm production and increased
sperm deformities. The data presented support the notion that
administration of endosulfan at relatively high doses (> 2.5 mg/kg
bw per day) for several months increases the activity of a number of
enzymes in the testes, including lactate dehydrogenase, sorbitol
dehydrogenase, gamma-glutamyl transpeptidase, and glucose-6-phosphate
dehydrogenase. At doses of 5 mg/kg bw per day and higher, there was a
marked reduction in sperm count (up to 47%) in comparison with
controls. In the absence of historical control data, it is unclear
whether the decrease in sperm count seen at 2.5 mg/kg bw per day (22%)
was within the normal biological range for the test animals. The sperm
abnormalities and the reductions in spermatid count and sperm
production, while statistically significantly different from those in
concurrent controls, were only slight. In the absence of consistent
dose-response relationships for these effects, they were considered
not biologically significant (Sinha et al., 1995).
The effects of endosulfan on gonadal hormones, measured as plasma
and testicular testosterone, plasma follicle-stimulating and
luteinizing hormones, and plasma and testicular 3ß- and
17ß-hydroxysteroid dehydrogenases, was investigated in Wistar rats
given endosulfan in peanut oil at doses up to 10 mg/kg bw per day for
up to 30 days. Significant inhibition of 3ß- and 17ß-hydroxysteroid
dehydrogenases occurred in the testes of treated animals after 30 days
of treatment, and the plasma follicle-stimulating hormone, luteinizing
hormone, and testosterone concentrations were significantly
(p < 0.05) reduced in rats treated for 15 or 30 days at either
dose. Plasma and testicular testosterone concentrations were not
significantly reduced after 15 days of treatment at 7.5 mg/kg. A
significant decrease in the content or activity of microsomal
cytochrome P450 and related mixed-function oxidases was observed in
the testes of treated animals, with marked inhibition of the activity
of glutathione- S-transferase at both doses. The latter changes were
reversed when endosulfan was withdrawn, but the testicular
testosterone concentrations remained significantly reduced (Singh &
Pandey, 1990).
(vi) Endocrine effects
An estrogen is a substance that can induce estrus or a biological
response associated with estrus; one such effect is proliferation of
cells in the female genital tract. In recent years, naturally
occurring and man-made substances in the environment that may be
estrogenic have come under increasing scrutiny. Suspicion that
endosulfan may have estrogenic properties was stimulated by
observation of the reduced sperm counts described above and of
testicular atrophy in rats given endosulfan in the diet in long-term
studies.
Perhaps the first study of estrogenic effects in vivo was
conducted by Raizada et al. (1991), who treated groups of eight
ovariectomized Wistar rats, weighing an average of 100 g, with
endosulfan at 1.5 mg/kg bw per day by gavage, estradiol dipropionate
intraperitoneally (dose unspecified), or a combination of these
treatments for 30 days. Endosulfan did not change the weights of the
uterus, cervix, or vagina, whereas estradiol propionate produced large
increases. The increased weights seen with the combined treatment were
similar to those with estradiol propionate alone.
Concern that endosulfan might be estrogenic persisted as a result
of the findings of an 'E-screen' assay, which was developed to assess
the estrogenic effects of environmental chemicals by observing their
proliferative effect on a target cell. The numbers of cells present
after similar inocula of the human breast cancer cell line, MCF-7,
were compared in the absence of estrogens (negative control), in the
presence of estradiol-17ß (positive control), and with a range of
concentrations of endosulfan. In this assay, endosulfan was estrogenic
at concentrations of 10-25 µmol/L, with a proliferative effect about
80% that of 17ß-estradiol at 10 µmol/L; the relative proliferative
potency (i.e. the ratio of the doses of endosulfan and estradiol-17ß
required to produce the maximum effect) was 0.0001%. In addition,
endosulfan competed with estradiol-17ß for binding to the estrogen
receptor and increased the concentrations of progesterone receptor and
pS2 in MCF-7 cells, as would be expected for a compound that mimics
estrogens (Soto et al., 1994, 1995).
As part of a study to optimize investigations of estrogenic
activity, endosulfan and nine other chemicals (17ß-estradiol,
diethylstilbestrol, tamoxifen, 4-hydroxytamoxifen, methoxychlor,
the methoxychlor metabolite
2,2-bis (para-hydroxyphenyl)-1,1,1-trichloroethane, nonylphenol,
ortho, para'-DDT, and kepone) with known or suspected estrogenic
activity were tested in three assays: competitive binding to the mouse
uterine estrogen receptor, transcriptional activation in HeLa cells
transfected with plasmids containing an estrogen receptor and a
response element, and the uterotropic assay in mice. The results of
the three assays were consistent with respect to the known estrogenic
activities of the chemicals tested and their requirements for
metabolic activation. There was no evidence from any of these tests
that endosulfan is estrogenic (Shelby et al., 1996).
A much publicized report indicated that even estrogens of low
potency, such as endosulfan, could have important effects because of
interaction with other chemicals. The estrogenic potencies of
combinations of chemicals were screened in a system in which the human
estrogen receptor sequence is incorporated into the yeast genome.
Combinations of two weak environmental estrogens, such as dieldrin,
endosulfan, and toxaphene, were 1000 times more potent in human
estrogen receptor-mediated transactivation than any chemical alone
(Arnold et al., 1996). This result was not reproduced in another
laboratory in which the same assay was used or in a uterotropic assay
in which sexually immature rats were treated with endosulfan or
dieldrin alone or in a combination on three successive days and the
uterine mass weighed on the following day. The highest doses used in
the human estrogen receptor assay were determined by the solubility of
the compounds, and the highest doses in the uterotropic assay were 100
mg/kg bw for endosulfan or dieldrin alone and 75 mg/kg bw of each in
combination. Both chemicals were inactive in both assays, and there
was no evidence of synergism (Ashby et al., 1997). In a further study
with the human estrogen receptor assay, however, 0.1 mmol/L endosulfan
increased the activity of ß-galactosidase (Ramamoorthy et al., 1997).
More doubt was cast upon the thesis of synergism by an
independent study in which endosulfan and dieldrin showed no additive
effect in displacing 3H-17ß-estradiol from rat uterine estrogen
receptors or in inducing the proliferation of MCF-7 breast cancer
cells. The weak proliferative potential described by Soto et al.
(1994, 1995) was, however, confirmed in this assay in vitro.
Endosulfan or dieldrin alone at 3 mg/kg bw per day or in combination,
injected intraperitoneally daily for three days, did not stimulate
uterotrophic activity and had no effect on pituitary prolactin or
other endocrine-related end-points in immature female rats, indicating
that these weakly estrogenic compounds do not interact in a
synergistic fashion in binding to estrogen receptors or in activating
estrogen receptor-dependent responses in mammalian tissues or cells
(Wade et al., 1997). The paper in which synergism was originally
proposed was later withdrawn, since the results could not be
reproduced, even in the same laboratory (McLachlan, 1997). Overall,
these results suggest that concomitant exposure to weakly estrogenic
compounds probably does not result in reproductive toxicity related to
estrogen action.
3. Observations in humans
In general, the doses of endosulfan involved in cases of
poisoning have been poorly characterized. In a summary of case reports
(Lehr, 1996), the lowest reported dose that resulted in death was 35
mg/kg bw; deaths have also been reported after ingestion of 295 and
467 mg/kg bw, within 1 h of ingestion in some cases. Intensive medical
treatment within 1 h was reported to be successful after ingestion of
doses of 100 and 1000 mg/kg bw. The clinical signs in these patient
were consistent with those seen in laboratory animals, dominated by
tonic-clonic spasms. In a case in which a dose of 1000 mg/kg bw was
ingested, neurological symptoms requiring anti-epileptic therapy were
still required one year after exposure.
Comments
More than 90% of an oral dose of endusulfan was absorbed in rats,
with maximum plasma concentrations occurring after 3-8 h in males and
about 18 h in females. Elimination occurs mainly in the faeces and to
a lesser extent in the urine, more than 85% being excreted within 120
h. The highest tissue concentrations were in the kidneys. The
metabolites of endosulfan include endosulfan sulfate, diol,
hydroxy-ether, ether, and lactone but most of its metabolites are
polar substances which have not yet been identified. Endosulfan would
not be expected to accumulate significantly in human tissues. No data
on plant metabolites were available to the Meeting.
A battery of tests for acute toxicity in several species with
technical-grade endosulfan showed that it is highly toxic after oral
or dermal administration, with respective LD50 values of 10-160 mg/kg
bw and 45-135 mg/kg bw. The LC50 value for rats in a single study was
13 mg/m3 in females and 35 mg/m3 in males. Endosulfan, administered
by any route, is more toxic to female than to male rats. Clinical
signs of acute intoxication include piloerection, salivation,
hyperactivity, respiratory distress, diarrhoea, tremors, hunching, and
convulsions.
WHO has classified endosulfan as moderately hazardous (WHO,
1996).
The kidney is the target organ for toxicity. The renal effects
include increased renal weights and granular pigment formation after
short-term administration and progressive, chronic glomerulo-nephrosis
or toxic nephropathy after long-term exposure, although the
observation of progressive glomerulonephrosis is complicated by the
fact that this is a common lesion in ageing laboratory rats and occurs
at high incidence in control rats.
In a 90-day feeding study in rats, the cytoplasm of isolated
cells in the renal proximal convoluted tubules had a yellowish colour,
particularly in males, at all dietary concentrations from 10 ppm. The
presence of this yellow pigmentation was largely reversible during a
four-week recovery period, and it did not appear to indicate
nephrotoxicity. A darker, more particulate, granular and/or clumped
pigment was also observed, predominantly in cells of the straight
portions and occasionally in the proximal convoluted tubules, at
dietary concentrations of 30 ppm and above. This darker pigment was
more persistent than the yellow one, and urinalysis revealed darker
urine and marginally more ketones at doses from 60 ppm, and marginally
more protein, particularly in males, indicating renal damage at doses
of 360 ppm and above. Similar findings emerged from a multigeneration
study but not from a two-year study of carcinogenicity in rats. The
changes in pigmentation were considered to be due to the presence of
endosulfan and/or its metabolites in the enlarged lysosomes. To test
this hypothesis, a four-week feeding study was conducted in which male
rats were given dietary concentrations of 360 or 720 ppm endosulfan.
Light and electron microscopy of the kidneys of these animals clearly
showed increases in the number of lysosomes and the size of cells in
the convoluted tubule, probably as a result of accumulation of the
test material and/or its metabolites. Lysosomal changes were not
observed in either brain or liver, and the renal changes receded
appreciably during a 30-day recovery period. Chemical analysis of the
kidneys indicated the presence of alpha-endosulfan and, to a lesser
extent ß-endosulfan sulfate, and endosulfan lactone. The
concentrations of the dominant alpha-endosulfan in the kidneys were
about 50 times those in the liver. The concentrations in blood were
usually below the level of detection. After the 30-day recovery
period, renal alpha-endosulfan was detected only in traces and ß-
endosulfan not at all. Similar analysis of tissues from rats in the
two-year study of toxicity and carcinogenicity did not reveal the
presence of these substances in the kidney, although measurable alpha-
endosulfan was found in the liver at 75 ppm. The yellow colour
therefore indicates the presence of endosulfan and/or its metabolites,
rather than either a stage in the pathogenesis of nephropathy or an
independent expression of toxicity. It was postulated that in longer
studies its removal from lysosomes is accelerated by enzyme induction,
which has not been investigated.
In a 78-week study, exposure of rats to endosulfan at a high dose
of 20 mg/kg bw per day resulted in testicular atrophy, characterized
by degeneration and necrosis of the germinal cells lining the
seminiferous tubules. In addition, decreased sperm counts accompanied
by an increased incidence of sperm abnormalities have been reported in
mice, again at high doses of endosulfan. Reductions in the activities
of some testicular xenobiotic-metabolizing enzymes and some hormones
that are necessary for normal testicular function were also seen in a
30-day study in rats at 10, but not at 7.5 mg/kg bw per day. The
functional significance of these findings was not clear, as studies of
reproductive and developmental toxicity in rats and rabbits showed
neither impaired fertility nor any increase in the incidence of
defects or abnormalities in offspring. Given the high doses at which
these testicular effects were observed, it would appear that they are
of little human significance.
No genotoxic activity was observed in an adequate battery of
tests for mutagenicity and clastogenicity in vitro and in vivo.
The Meeting concluded that endosulfan is not genotoxic.
No carcinogenic effect was observed in mice at 18 ppm for 24
months, in female rats at 445 ppm for 78 weeks in one study or in male
or female rats at 75 ppm or 100 ppm for two years in two other
studies. The Meeting noted the differences in the dietary
concentrations used in these studies, but non-neoplastic responses
were seen even at the lower doses.
Endosulfan at dietary concentrations of 0, 3, 15, or 75 ppm did
not affect reproductive performance or the growth or development of
the offspring of rats over the course of a two-generation study. The
NOAEL was 75 ppm, the highest dose tested, equal to 5 mg/kg bw per day
for males and 6.2 mg/kg bw per day for females. The NOAEL for parental
toxicity was 15 ppm, equal to 1 mg/kg bw per day for males and 1.2
mg/kg bw per day, on the basis of increased liver and kidney weights
at 75 ppm.
In two studies of developmental toxicity in rats given oral doses
of 0, 0.66, 2, or 6 mg/kg bw per day, the NOAEL for maternal toxicity
was 0.66 mg/kg bw per day in one study and 2 mg/kg bw per day in the
other. In the first case, the basis was decreased body-weight gain at
2 mg/kg bw per day and decreased body-weight gain and clinical signs
of toxicity at 6 mg/kg bw per day; in the second case, the basis was
mortality, clinical signs of toxicity, and decreased body-weight gain
at 6 mg/kg bw per day. In both studies, the NOAEL for developmental
toxicity was 2 mg/kg bw per day, in the first case on the basis of
delayed development and a low incidence of skeletal variations seen at
6 mg/kg per day and in the second on the basis of an increased
incidence of fragmented thoracic vertebral centra seen at 6 mg/kg bw
per day. In neither study was there any treatment-related major
malformation.
In a study of developmental toxicity in rabbits given oral doses
of 0, 0.3, 0.7, or 1.8 mg/kg bw per day, the NOAEL for maternal
tocicity was 0.7 mg/kg bw per day on the basis of clinical signs of
toxicity at 1.8 mg/kg bw per day. The NOAEL for developmental toxicity
was 1.8 mg/kg bw per day, the highest dose tested.
Several recent studies have shown that endosulfan, alone and in
combination with other pesticides, may bind to estrogen receptors and
may perturb the endocrine system. The available studies show only very
weak binding to hormone receptors in vitro, and the evidence for
their relevance to adverse physiological effects in vivo is
extremely limited. Long-term assays of toxicity and studies of
reproductive and developmental toxicity in experimental mammals did
not indicate that endosulfan induces functional aberrations that might
result from loss of endocrine homeostasis.
The absence of immunotoxic effects in a large number of bioassays
with endosulfan suggested that it does not have an adverse effect on
the immune function of laboratory animals. However, in two studies,
rats given endosulfan in the diet at 30 or 50 ppm for 6 weeks or 20
ppm for 22 weeks had reduced serum titres of tetanus toxoid antibody
and reduced immunoglobulins G and M, and inhibition of migration of
both leukocytes and macrophages. These findings have not been
confirmed.
In a summary of case reports of human poisoning incidents, the
lowest reported dose that caused death was 35 mg/kg bw. Higher doses
caused death within 1 h. The clinical signs in these patients were
dominated by tonic-clonic convulsions, consistent with the
observations in experimental animals.
An ADI of 0-0.006 mg/kg bw was established on the basis of the
NOAEL of 0.6 mg/kg bw per day in the two-year dietary study of
toxicity in rats and a safety factor of 100. The ADI is supported by
similar NOAEL values in the 78-week dietary study of toxicity in mice,
the one-year dietary study of toxicity in dogs, and the study of
developmental toxicity in rats.
An acute RfD of 0-0.02 mg/kg was established on the basis of the
NOAEL of 2 mg/kg bw per day in the study of neurotoxicity in rats and
a safety factor of 100.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 3.9 ppm, equal to 0.58 mg/kg bw per day (females in a
78-week study of toxicity)
Rat: 15 ppm, equal to 0.6 mg/kg bw per day (two-year dietary
study of toxicity)
75 ppm, equal to 6 mg/kg bw per day (reproductive
toxicity)
0.66 mg/kg bw per day (maternal toxicity in a study of
developmental toxicity)
2 mg/kg bw per day (fetotoxicity in a study of
developmental toxicity)
Rabbit: 0.7 mg/kg bw per day (maternal toxicity in a study of
developmental toxicity)
Dog: 10 ppm, equivalent to 0.57 mg/kg bw per day (one-year
study of toxicity)
Estimate of acceptable daily intake for humans
0-0.006 mg/kg bw
Estimate of acute reference dose for humans
0.02 mg/kg bw
Studies that would provide information useful for continued
evaluation of the compound
1. Studies of immunotoxicity with standard test protocols
2. Studies of the significant sex difference in acute toxicity,
particularly in rats
3. Further observations in humans
List of end-points relevant for setting guidance values for dietary and non-dietary exposure
Absorption, distribution, excretion, and metabolism in mammals
Rate and extent of absorption Rat: oral, > 90% absorption; max. concentration at
3-8 h (males) 18 h (females)
Distribution Mainly in kidney and liver
Potential for accumulation Low
Rate and extent of excretion Biphasic; urinary half-life was 6 h for 1st phase,
33-68 h for 2nd phase; faecal half-life was 10 h and
30 h; > 85% excretion within 120 h
Metabolism in animals Oxidation and hydrolysis; unidentified polar
metabolites
Toxicologically significant Parent; no data on plant metobolites
compounds (animals, plants and
environment)
Acute toxicity
Rat: LD50 oral 10 mg/kg bw (female)
Rat: LD50 dermal 500 mg/kg bw (female)
Rat: LC50 inhalation 13 mg/m3 4 h (female)
Skin irritation Not irritating
Eye irritation Not irritating
Skin sensitization Not sensitizing
Short-term toxicity
Target/critical effect Reduced survival, convulsions, salivation
Lowest relevant oral NOAEL Rat: 0.64 mg/kg bw per day, dietary
Lowest relevant dermal NOAEL Rat: 3 mg/kg bw per day
Lowest relevant inhalation NOAEL Rat: 2 mg/m3, no effect (highest concentration)
Genotoxicity Not genotoxic
Long-term toxicity and carcinogenicity
Target/critical effect Kidney
Lowest relevant NOAEL Rat: 0.6 mg/kg bw per day, 2-year study
Carcinogenicity Not carcinogenic
Reproductive toxicity
Reproduction target: critical effect None identified
Lowest relevant reproductive NOAEL Rat: 6 mg/kg bw per day
Developmental target /critical effect Fetoxicity at maternally toxic doses
Lowest relevant developmental NOAEL Rat: 2 mg/kg bw per day
Neurotoxicity/Delayed neurotoxicity Rat: 1.5 mg/kg bw (female); 12.5 mg/kg bw (male)
no effect
Other toxicological studies Immunotoxicity in certain special assays, not
confirmed in sensitization test or histologically
Some conflicting evidence of interaction with
estrogen receptors in vitro; none in vivo
Medical data Lowest lethal dose: 35 mg/kg bw, oral
Summary Value Study Safety factor
ADI 0-0.006 mg/kg bw Several different species 100
and end-points
Acute reference dose 0.02 mg/kg bw Study of neurotoxicity in 100
rats
References
Albrecht, M. & Baeder, C. (1993) Hoe 002671-substance technical (code:
Hoe 002671 00 ZD98 0005). Testing for embryotoxicity in the Wistar rat
after oral administration. Unpublished report No. 93.0716, document
A51695. Submitted to WHO by Hoechst Schering AgrEvo GmbH,
Frankfurt-am-Main, Germany.
Arai, M., Hiromori ,T., Ho, S., Okuno, Y. & Miyamoto, J. (1981) Life
span chronic toxicity study of endosulfan in mice -- 12 month report.
Unpublished report No. 0285, at-10 from Sumitomo Chemical Co Ltd,
Japan, 28 April 1981. Submitted to WHO by Hoechst Schering AgrEvo
GmbH, Frankfurt-am-Main, Germany.
Arcelin, G. (1996) Contact hypersensitivity to endosulfan (Code: Hoe
002671 00ZD99 0008) in albino guinea pigs maximumization test.
Unpublished report No. 630753 from RCC, Itingen, Switzerland. Hoechst
document A58132.A51695. Submitted to WHO by Hoechst Schering AgrEvo
GmbH, Frankfurt-am-Main, Germany.
Arnold, S.F., Klotz, D.M., Collins, B.M., Vonier, P.M., Guilette, L.J.
& McLachlan, J.A. (1996) Synergistic activation of oestrogen receptor
with combinations of environmental chemicals. Science, 272,
1489-1492.
Ashby, J., LeFevre, P.A., Odum, J., Harris, C.A., Routledge, E.J. &
Sumpter, J.P. (1997) Synergy between synthetic oestrogens? Nature,
385, 494.
Asquith, J.C. & Baillie, J.H. (1989) Endosulfan substance technical
(code Hoe 002671 0I ZD95 0005). Metaphase analysis of human
lymphocytes. Unpublished report No. m/hl/1307 from Toxicol
Laboratories, United Kingdom, 16 March 1989. Hoechst document A40411.
Submitted to WHO by Hoechst Schering AgrEvo GmbH, Frankfurt-am-Main,
Germany.
Banarjee, B.D. & Hussain, Q.Z. (1986) Effect of subchronic endosulfan
exposure on humoral and cell-mediated immune responses in albino rats.
Arch. Toxicol., 59, 279-284.
Banarjee, B.D. & Hussain, Q.Z. (1987) Effects of endosulfan on humoral
and cell-mediated immune responses in rats. Bull. Environ. Contam.
Toxicol., 38, 438-441.
Barnard, A.V., Atkinson, J.S., Heywood, R., Street, A.E, Gibson, W.A.,
Rao, R., Offer, J.M. & Gopinath, A.R.H. (1984) 13 week toxicity study
in mice. Unpublished report No. Hst 229/831052, 25 September 1984 from
Huntingdon Research Centre, Huntingdon, Cambridgeshire, United
Kingdom. Hoechst document A29663. Submitted to WHO by Hoechst Schering
AgrEvo GmbH, Frankfurt-am-Main, Germany.
Barnard, A.V., Jones, D.R., Powell, L.A.J., Heywood, R., Street, A.E.,
Gibson, W.A., Gopinath, C., Majeed, S.K. & Almond, R.H. (1985) 13-week
toxicity study in rats followed by a 4-week withdrawal period.
Unpublished report No. Hst 230/84176, 25 March 1985 from Huntingdon
Research Centre, Huntingdon, Cambridgeshire, United Kingdom. Hoechst
document A30700 Submitted to WHO by Hoechst Schering AgrEvo GmbH,
Frankfurt- am-Main, Germany.
Bhide, M.B. & Naik, P.Y. (1984a) The acute oral toxicity (LD50) of
Excel Industries Ltds endosulfan technical No. 2 to the albino rats.
Unpublished report from Indian Institute of Toxicology (report No. and
date not given). Submitted to WHO by the National Registration
Authority for Agricultural and Veterinary Chemicals, Australia.
Bhide, M.B. & Naik, P.Y. (1984b) The acute oral toxicity (LD50) of
Excel Industries Ltds endosulfan technical No. 1 to the albino mice.
Unpublished report from Indian Institute of Toxicology (report No. and
date not given). Submitted to WHO by the National Registration
Authority for Agricultural and Veterinary Chemicals, Australia.
Bhide, M.B. & Naik, P.Y. (1984c) The acute dermal toxicity (LD50) of
Excel Industries Ltds endosulfan technical No. 2 to the albino
rabbits. Unpublished report from Indian Institute of Toxicology
(report No. and date not given). Submitted to WHO by the National
Registration Authority for Agricultural and Veterinary Chemicals,
Australia.
Bowman, J.S. (1959) Preliminary report: Subacute feeding -- dairy
cows. Unpublished report from Hazleton Laboratories, USA. Hoechst
document No. A14205. Submitted to WHO by Hoechst Schering AgrEvo GmbH,
Frankfurt-am-Main, Germany.
Bracha, P. (1977) Thionex tech: Acute oral toxicity study in rats,
skin irritation study in rabbits, acute dermal toxicity study in
rabbits, eye irritation study in rabbits and acute inhalation study in
rats. Unpublished report No. 6111820 from Warf Institute Inc. Madison,
Wisconsin, USA. Submitted to WHO by Hoechst Schering AgrEvo GmbH,
Frankfurt-am-Main, Germany.
Bremmer, J.N. (1997a) Endosulfan: Substance technical (code: Hoe
002671 00 ZD99 0008) Testing for primary dermal irritation in the
rabbit. Unpublished Hoechst Marion Roussel preclinical development
study No. 96.0837. Hoechst document A58442. Submitted to WHO by
Hoechst Schering AgrEvo GmbH, Frankfurt-am-Main, Germany.
Bremmer, J.N. (1997b) Endosulfan: Substance technical (code: Hoe
002671 00 ZD99 0008) Testing for primary eye irritation in the rabbit.
Unpublished Hoechst Marion Roussel preclinical development study No.
96.0838. Hoechst document A58443. Submitted to WHO by Hoechst Schering
AgrEvo GmbH, Frankfurt-am-Main, Germany.
Bremmer, J.N. & Leist, K.-H. (1998) Endosulfan (AE F002671, substance
technical). Evaluation of the acute oral and dermal toxicity.
Unpublished report No. TOX98/003 from Hoechst Schering AgrEvo GmbH.
Hoechst document A59823. Submitted to WHO by Hoechst Schering AgrEvo
GmbH, Frankfurt-am-Main, Germany.
Brunk, R. (1989) Endosulfan-substance technical (code: Hoe 002671 00
ZD96 0002). Testing for toxicity by repeated oral administration
(1-year feeding study) to beagle dogs. Unpublished report No. 87.0643
from Pharma Research Toxicology and Pathology. Hoechst report No.
89.0188; document No. A40441 Submitted to WHO by Hoechst Schering
AgrEvo GmbH, Frankfurt-am-Main, Germany.
Brunk, R. (1990) Addendum to Hoechst report 89.0188; document No.
A44605. Submitted to WHO by Hoechst Schering AgrEvo GmbH,
Frankfurt-am-Main, Germany.
Bury, D. (1997) Endosulfan; substance, technical (code: Hoe 002671 00
ZD99 0008). Neurotoxicological screening in the male and female Wistar
rat. Acute toxicity. Unpublished report No. 96.0373 from Hoechst
Marion Roussel Preclinical Development, Germany. Hoechst report No.
A59088. Submitted to WHO by Hoechst Schering AgrEvo GmbH,
Frankfurt-am-Main, Germany.
Christ, O. & Kellner, H.M. (1968) Investigations with endosulfan-14C
in mice. Unpublished report No. ch/he-8412 from Hoechst Radiochemical,
Frankfurt, 31 December 1968. Hoechst document A53842, translation of
document A14217. Submitted to WHO by Hoechst Schering AgrEvo GmbH,
Frankfurt-am-Main, Germany.
Cifone, A.M. & Myhr, B.C. (1984a) Evaluation of Hoe 002671-substance
technical in the rat primary hepatocyte unscheduled DNA synthesis
assay. Unpublished LBI project No. 20991 from Litton Bionetics Inc.,
USA, November 1984. Hoechst document No. A29800. Submitted to WHO by
Hoechst Schering AgrEvo GmbH, Frankfurt-am-Main, Germany.
Cifone, A.M. & Myhr, B.C. (1984b) Mutagenicity evaluation of Hoe
002671-substance technical in the mouse lymphoma forward mutation
assay. Unpublished LBI project No. 20980 from Litton Bionetics Inc.,
USA, November 1984. Hoechst document No. A29801. Submitted to WHO by
Hoechst Schering AgrEvo GmbH, Frankfurt-am-Main, Germany.
Craine, E.M. (1986) A dermal absorption study in rats with
14C-endosulfan. Unpublished project No. Wil-39028, 11 December 1986
from Wil Research Lab, USA. Wil-39028. 11 Decemnber, 1986. Hoechst
document No. A35730. Submitted to WHO by Hoechst Schering AgrEvo GmbH,
Frankfurt-am-Main, Germany.
Craine, E.M. (1988) A dermal absorption study in rats with
14C-endosulfan with extended test duration. Unpublished project No.
Wil-39029 from Wil Research Lab, USA. Hoechst document No. A39677.
Submitted to WHO by Hoechst Schering AgrEvo GmbH, Frankfurt-am-Main,
Germany.
Deema, P. Thompson, E. & Ware, G. (1966) Metabolism, storage and
excretion of 14C-endosulfan in the mouse. J. Econom. Entomol., 59,
546-550.
Dickie, S.M., Mackenzie K.M. & Rao, G.N. (1981) Teratology study with
FMC 5462 in rabbits. Unpublished study No. 80070, 27 July 1981 from
Raltech Scientific Services. Hoechst document No. A23192. Submitted to
WHO by Hoechst Schering AgrEvo GmbH, Frankfurt-am-Main, Germany.
Diehl, K.H. & Leist, K.H. (1988a) Hoe 002671: Active ingredient
technical (code: Hoe 002671 0I ZD96 0002). Testing for acute oral
toxicity in the male and female Wistar rat. Unpublished study No.
88.0551, 27 July 1988, from Pharma Research Toxicology and Pathology,
Frankfurt, Germany. Hoechst document No. A39680. Submitted to WHO by
Hoechst Schering AgrEvo GmbH, Frankfurt-am-Main, Germany.
Diehl, K.H. & Leist, K.H. (1988b) Endosulfan-active ingredient
technical (code: Hoe 002671 0I ZD96 0002). Testing for acute dermal
toxicity in the male and female Wistar rat. Unpublished study No.
88.0552, 22 August 1988, from Pharma Research Toxicology and
Pathology, Germany. Hoechst document A39397, 1 September 1988.
Submitted to WHO by Hoechst Schering AgrEvo GmbH, Frankfurt-am-Main,
Germany.
Dikshith, T.S. & Datta, K.K. (1978) Endosulfan: Lack of cytogenetic
effects in male rats. Unpublished document No. A17140 from Industrial
Toxicology Research Centre. Submitted to WHO by Hoechst Schering
AgrEvo GmbH, Frankfurt-am-Main, Germany.
Dikshith, T.S.S., Raizada, R.B., Kumar, S.N., Srivastava, M.K.,
Kaushal, R.A., Singh, R.P. & Gupta, K.P. (1988) Effect of repeated
dermal application of endosulfan to rats. Vet. Hum. Toxicol., 30,
219-224.
Donaubauer, H.H. (1988) Endosulfan-substance technical (code: Hoe
002671 OI ZD97 0003). Carcinogenicity study in mice: 24 months feeding
study. Unpublished study No. 745 from Pharma Research Toxicology and
Pathology, Germany. Hoechst document No. A38008. Submitted to WHO by
Hoechst Schering AgrEvo GmbH, Frankfurt-am-Main, Germany.
Donaubauer, H.H. (1989) Amendment to the report No. 88.0278
endosulfan-substance technical (code: Hoe 002671 OI ZD97 0003).
Carcinogenicity study in mice: 24 months feeding study. Unpublished
study No. 745 from Pharma Research Toxicology and Pathology, Germany.
Hoechst document No. A41617. Submitted to WHO by Hoechst Schering
AgrEvo GmbH, Frankfurt-am-Main, Germany.
Donaubauer, H.H., Leist, K. & Kramer, M. (1985) Endosulfan-substance
technical (code: Hoe 002671 01 ZD97 0003). 42-Day feeding study in
mice. Unpublished study No. 744 from Pharma Research Toxicology,
Germany. Hoechst document No. A38104. Submitted to WHO by Hoechst
Schering AgrEvo GmbH, Frankfurt-am-Main, Germany.
Dorn, E. & Werner, H.-J. (1989) Endosulfan (Hoe 002671 OI ZD97 0003):
Determination of residues in the liver and kidneys of rats after
chronic (2-years) feeding (week 104). Unpublished Produktentwicklung,
GB-C Ökologie II Hoechst AG Study No. (Analytical part) CR065/88.
Hoechst document No. A41265. Submitted to WHO by Hoechst Schering
AgrEvo GmbH, Frankfurt-am-Main, Germany.
Dorough, M.W., Huhtanen, K., Marshall, T.C. & Bryant, H.E. (1978) Fate
of endosulfan in rats and toxicological considerations of apolar
metabolites. Pest. Biochem. Physiol., 8, 241-252.
Dzwonkowska, A. & Hübner, H. (1991) Studies on commercial insecticides
with the dominant lethal mutations test. Polish J. Occup. Med.
Environ. Health, 4, 43-53.
Ebert, E. & Weigand, W. (1984) Testing of the therapeutic effect of
diazapam (Valium R) and phenobarbital (Luminal R) in the event of
acute poisoning with endosulfan-active ingredient technical (code: Hoe
002671 oi ZD97 0003) in Wistar rats. Unpublished report No. 84.0062
from Pharma forschung Toxikologie. Hoechst document A29211. Submitted
to WHO by Hoechst Schering AgrEvo GmbH, Frankfurt-am-Main, Germany.
Ebert, E., Weigand, W. & Kramer, P. (1985a) Endosulfan-active
ingredient technical (code: Hoe 002671 01 ZD97 0003). Testing for
subchronic dermal toxicity (21 applications over 30 days) in SPF
Wistar rats. Unpublished study No. 83.0118 from Pharma forschung
Toxicologie. Hoechst document No. A30754, translation of document
A30751. Submitted to WHO by Hoechst Schering AgrEvo GmbH,
Frankfurt-am-Main, Germany.
Ebert, E., Leist, K.H. & Kramer, P. (1985b) Endosulfan-active
ingredient technical (code: Hoe 002671 01 ZD97 0003). Testing for
subchronic dermal toxicity (21 applications over 30 days) in Wistar
rats. Toxicological review of studies 721 and 729 (reports 84.0321 and
84.0223). Unpublished Hoechst document No. A30755, translation of
A30752. Submitted to WHO by Hoechst Schering AgrEvo GmbH,
Frankfurt-am-Main, Germany.
Ebert, E., Leist ,K.H. & Kramer, P. (1985c) Endosulfan-active
ingredient technical (code: Hoe 002671 01 ZD97 0003). Testing for
subchronic dermal toxicity (21 applications over 30 days) in Wistar
rats. Unpublished study No. 83.0508 from Pharma forschung Toxicologie.
Hoechst document No. A30753, translation of document A30750. Submitted
to WHO by Hoechst Schering AgrEvo GmbH, Frankfurt-am-Main, Germany.
Edwards, J.A., Hughes, E.W. & Almond, R.H. (1982) Preliminary
investigation of the effect of endosulfan (code, Hoe 02671 OI AT 209)
on reproduction of the rat. Unpublished report No. Hst 203/82252 from
Huntingdon Research Centre, Huntingdon, Cambridgeshire, United
Kingdom. Hoechst document No. A29563. Submitted to WHO by Hoechst
Schering AgrEvo GmbH, Frankfurt-am-Main, Germany.
Edwards, J.A., Reid, J.Y., Offer, M.J., Almond, H.R. & Gibson, A.W.
(1984) Effect of endosulfan-technical (code, Hoe 02671OI AT209) on
reproductive function of multiple generations in the rat. Unpublished
report No. Hst 204/83768 from Huntingdon Research Centre, Huntingdon,
Cambridgeshire, United Kingdom. Hoechst document No. A29428. Submitted
to WHO by Hoechst Schering AgrEvo GmbH, Frankfurt-am-Main, Germany.
Ehling, G. & Leist, K.-H. (1991a) Hoe 051327: substance technical
(code HOE 051327 00 ZB99 0002): Testing for acute oral toxicity in the
male and female Wistar rat. Pharma Research Toxicology, Germany.
Hoechst document No. A46286. Submitted to WHO by Hoechst Schering
AgrEvo GmbH, Frankfurt-am-Main, Germany.
Ehling, G. & Leist, K.-H. (1991b) Hoe 051327: substance technical
(code HOE 051327 00 ZB99 0002): Testing for acute dermal toxicity in
the male and female Wistar rat. Pharma Research Toxicology, Germany.
Unpublished Hoechst document No. A45783. Submitted to WHO by Hoechst
Schering AgrEvo GmbH, Frankfurt-am-Main, Germany.
Ehling, G. & Leist, K.-H. (1991c) Hoe 051329: substance technical
(code HOE 051329 00 ZD98 0001): Testing for acute oral toxicity in the
male and female Wistar rat. Pharma Research Toxicology, Germany.
Unpublished Hoechst document No. A45783. Submitted to WHO by Hoechst
Schering AgrEvo GmbH, Frankfurt-am-Main, Germany.
Ehling, G. & Leist, K.-H. (1991d) Hoe 051329: substance technical
(code HOE 051329 00 ZD98 0001): Testing for acute dermal toxicity in
the male and female Wistar rat. Pharma Research Toxicology, Germany.
Unpublished Hoechst document No. A45829. Submitted to WHO by Hoechst
Schering AgrEvo GmbH, Frankfurt-am-Main, Germany.
Elsea, J.R. (1958) Thiodan technical: Acute oral administration: Rats.
Hazleton Laboratories, USA, 28 February, 1958. Unpublished Hoechst
document No. A13686. Submitted to WHO by Hoechst Schering AgrEvo GmbH,
Frankfurt-am-Main, Germany.
Flodström, S., Wärngård, L., Hemming, H., Fransson, R. & Ahlborg, U.G.
(1988) Tumour promotion related effects by the cyclodiene insecticide
endosulfan studies in vitro and in vivo. Pharmacol Toxicol., 62,
230-235.
Goebel, H., Gorbach, S., Knauf, W., Rimpau, R.H. & Uttenbach, H.
(1982) Properties, effects, residues and analytics of the insecticide
endosulfan. Res. Rev., 83, 41. Submitted to WHO by the National
Registration Authority for Agricultural and Veterinary Chemicals,
Australia.
Gopinath, C. & Cannon, M.W.J. (1990) Photomicrographic addendum to
histopathology report No. Hst/289 Endosulfan, active ingredient
technical (code: Hoe 002671 OI ZD97 0003) combined chronic
toxicity/carcinogenicity study (104-week feeding in rats). Huntingdon
Research Centre Ltd, Huntingdon, Cambridgeshire, United Kingdom.
Unpublished Hoechst document No. A44604. Submitted to WHO by Hoechst
Schering AgrEvo GmbH, Frankfurt-am-Main, Germany.
Gorbach, S., Christ, O., Kellner, H.M., Kloss, G. & Börner, E. (1968)
The metabolism of endosulfan in milk sheep. J. Agric. Food Chem.,
16, 950-953.
Gupta, P.K., Chandra, S.V. & Saxena, D.K. (1978) Teratogenic and
embryotoxic effects of endosulfan in rats. Acta Pharmacol.
Toxicol., 42, 150-152.
Hack, R. & Leist, K.-H. (1988) Endosulfan-substance technical (code:
Hoe 002671 0I ZD96 0002) Testing of host resistance in the female
Wistar rat (immunological screening with Trichonella spiralis).
Unpublished study No. 87.1125 from Pharma Research Toxicology and
Pathology, Germany. Hoechst report A43829. Submitted to WHO by Agrevo
GmbH, Frankfurt-am-Main, Germany.
Hack, R., Ebert, E. & Leist, K.H. (1995) Chronic toxicity and
carcinogenicity studies with the insecticide endosulfan in rats and
mice. Food Chem. Toxicol., 33, 941-950.
Hollander, H. & Kramer, P. (1975a) Endosulfan sulfate = NIA 7985.
Acute oral toxicity in female SPF-Wistar rats (vehicle: starch
suspension). Unpublished Hoechst document No. A06966. Submitted to WHO
by Hoechst Schering AgrEvo GmbH, Frankfurt-am-Main, Germany.
Hollander, H. & Kramer, P. (1975b) Endosulfan sulfate = NIA 7985.
Acute oral toxicity in male beagle dogs. Unpublished Hoechst document
No. A06965. Submitted to WHO by Hoechst Schering AgrEvo GmbH,
Frankfurt-am-Main, Germany.
Hollander, H. & Kramer, P. (1975c) Comparative test on the acute
toxicity of endosulfan ether and endosulfan alcohol in female
SPF-Wistar rats (vehicle: starch suspension). Unpublished Hoechst
document No. A07170; translation of A05271. Submitted to WHO by
Hoechst Schering AgrEvo GmbH, Frankfurt-am-Main, Germany.
Hollander, H. & Kramer, P. (1975d) 1-Hydroxy endosulfan ether. Acute
oral toxicity in female SPF-Wistar rats (vehicle: starch suspension).
Unpublished Hoechst document No. A06967; translation of A05276
Submitted to WHO by Hoechst Schering AgrEvo GmbH, Frankfurt-am-Main,
Germany.
Hollander, H. & Kramer P. (1975e) Endosulfan lactone: Acute oral
toxicity in female SPF-Wistar rats (vehicle: starch suspension).
Unpublished Hoechst document No. A07171; translation of A05273.
Submitted to WHO by Hoechst Schering AgrEvo GmbH, Frankfurt-am-Main,
Germany.
Hollander, H. & Kramer, P. (1975f) Endosulfan lactone. Acute oral
toxicity in male SPF-Wistar rats. Unpublished Hoechst document No.
A06964.25. Submitted to WHO by Hoechst Schering AgrEvo GmbH,
Frankfurt-am-Main, Germany.
Hollander, H. & Weigand, W. (1983) Acute aerosol toxicity in male and
female SPF Wistar rats 4 hours-LC50. Unpublishd Hoechst report No.
83.0397; document No. A32087. Submitted to WHO by Hoechst Schering
AgrEvo GmbH, Frankfurt-am-Main, Germany.
Hollander, H., Weigand, W. & Kramer, P. (1984) Endosulfan-active
ingredient technical (code: Hoe 002671 0I ZD97 0003) Testing for
subchronic inhalation toxicity--21 exposures in 29 days -- in SPF
Wistar rats. Unpublished study No. 762; document No. A29823,
translation of A29766. Submitted to WHO by Hoechst Schering AgrEvo
GmbH, Frankfurt-am-Main, Germany.
Indraningsih, McSweeney, C.S. & Ladds, P.W. (1993) Residues of
endosulfan in the tissues of lactating goats. Aust. Vet. J., 70,
59-62.
Jung, Weigand, W. & Kramer, P. (1983) Mouse micronucleus test
following oral administration. Unpublished Hoechst AG report No.
83.0458; document No. A31628. Submitted to WHO by Hoechst Schering
AgrEvo GmbH, Frankfurt-am-Main, Germany.
Keller, J.G. (1959a) Subacute feeding--dairy cows. Hazleton
Laboratories, USA. Unpublished Hoechst document No. A14206. Submitted
to WHO by Hoechst Schering AgrEvo GmbH, Frankfurt-am-Main, Germany.
Keller, J.G. (1959b) One-year oral study in dog. Hazleton
Laboratories, 12 May 1959. Unpublished Hoechst document No. A13924.
Submitted to WHO by Hoechst Schering AgrEvo GmbH, Frankfurt-am-Main,
Germany.
Keller, J.G. (1959c) 2 year dietary study in rats. Hazleton
Laboratories, USA, 22 May 1959. Unpublished Hoechst document No.
A14037. Submitted to WHO by Hoechst Schering AgrEvo GmbH,
Frankfurt-am-Main, Germany.
Kellner, H.M. & Eckert (1983) Hoe 02671-14C. Pharmacokinetics and
residue determinations after oral and intravenous administration to
rats. Unpublished study No. tep 74/1; bereich c/analytisches project
oe 87/45; Hoechst report 01-l42-0382-83, 15 February 1983; document
No. A49475, translation of document A27971. Submitted to WHO by
Hoechst Schering AgrEvo GmbH, Frankfurt-am-Main, Germany.
Khan, P.K. & Sinha, S.P (1996) Ameliorating effect of vitamin C on
murine sperm toxicity induced by three pesticides (endosulfan,
phosphamidon and mancozeb). Mutagenesis, 11, 33-36
Kramer, P. & Weigand, W. (1971) Endosulfan lactone (vehicle: sesame
oil). Acute oral toxicity in male and female SPF-Wistar K-rat.
Unpublished Hoechst document No. A18276, 21 May 1971; translation of
A14326. Submitted to WHO by Hoechst Schering AgrEvo GmbH,
Frankfurt-am-Main, Germany.
Lachmann, G. & Siegemund, B. (1987) Hoe 002671-(5a, 9a-14C). Dermal
absorption of 14C- endosulfan in rhesus monkeys. Battelle-Institut,
Frankfurt. Unpublished Hoechst document No. A36685. Submitted to WHO
by Hoechst Schering AgrEvo GmbH, Frankfurt-am-Main, Germany.
Lehr, W (1996) Summary of intoxications with endosulfan. Clinical
cases and poisoning incidents. Unpublished Hoechst report No. psr
96/006, 12 March 1996; document A56361. Submitted to WHO by Hoechst
Schering AgrEvo GmbH, Frankfurt-am-Main, Germany.
Leist, K.H. (1989a) Endosulfan-substance technical (code: Hoe 002671
oi ZD97 0003). Carcinogenicity study in mice, 24 months feeding study
-- Residue determination. Pharma Research Toxicology and Pharmacology.
Unpublished Hoechst document No. A41284. Submitted to WHO by Hoechst
Schering AgrEvo GmbH, Frankfurt-am-Main, Germany.
Leist, K.-H. (1989b) Amendment to report No. HST 289/881067.
Endosulfan, active ingredient technical (code: Hoe 002671 OI ZD97
0003). Combined chronic toxicity/carcinogenicity study (104-week
feeding in rats). Residue determination. Unpublished Hoechst report
No. 89.1105; document No. A41265. Submitted to WHO by Hoechst Schering
AgrEvo GmbH, Frankfurt-am-Main, Germany.
Leist, K.-H. & Bremmer, J. (1998) Endosulfan (AE F002671, substance
technical). Re-evaluation of the NOAEL in the 90-day subchronic
toxicity study in rats. Unpublished AgrEvo document No. 59825.
Submitted to WHO by Hoechst Schering AgrEvo GmbH, Frankfurt-am-Main,
Germany.
Leist, K.-H. & Mayer, D. (1987) Endosulfan-active ingredient technical
(code: Hoe 002671 01 ZD97 0003). 30-Day feeding study in adult male
Wistar rats. Unpublished study No. 84 0585 from Pharma Research
Toxicology and Pharmacology. Hoechst report No. 87.0129, 27 March
1987; document No., A37112. Submitted to WHO by Hoechst Schering
AgrEvo GmbH, Frankfurt-am-Main, Germany.
Lightowler, J.E. & Gardner, J.R. (1978) Acute oral toxicity study in
rats. Unpublished report No. 78/mak1/428. 20 November 1978 from Life
Science Research. Submitted to WHO by Hoechst Schering AgrEvo GmbH,
Frankfurt-am-Main, Germany.
Mackenzie, K.M. (1980) Teratology study with FMC 5462 in rats. Raltech
Scientific Services, study No. 79041, 2 October 1980. Hoechst document
No. A21393. Submitted to WHO by Hoechst Schering AgrEvo GmbH,
Frankfurt-am-Main, Germany.
Maier-Bode, H. (1966) Investigations on the persistence of the
insecticide endosulfan in the vegetable and animal organism.
Pharmakologisches Institut der Rheinischen Friedrich Wilhelms
Universität. Unpublished Hoechst document No. A4047. Submitted to WHO
by Hoechst Schering AgrEvo GmbH, Frankfurt-am-Main, Germany.
McGregor, D.B., Willins, M.J., McDonald, P., Holström, M., McDonald,
D. & Niemeier, R.W. (1983) Genetic effects of 2-methoxyethanol and
bis(2-methoxyethyl)ether. Toxicol. Appl. Pharmacol., 70, 303-316.
McLachlan, J.A. (1997) Synergistic effect of environmental estrogens:
Report withdrawn. Science, 277, 462-463.
Mellano, D. & Milone, M.F. (1984a) Mutagenic activity of the compound
endosulfan-technical with Saccharomyces cerevisiae gene
conversion-DNA repair test. Instituto di Recherche Biomediche.
Unpublished Hoechst document No. A29313. Submitted to WHO by Hoechst
Schering AgrEvo GmbH, Frankfurt-am-Main, Germany.
Mellano, D. & Milone, M.F. (1984b) Mutagenic activity in vitro of the
compound endosulfan-technical with Schizosaccharomyces pombe.
Unpublished experiment No. M 708, 18 June 1984, from Instituto di
Recherche Biomediche. Hoechst document No. A29312. Submitted to WHO by
Hoechst Schering AgrEvo GmbH, Frankfurt-am-Main, Germany.
Müller, W. (1988) Endosulfan-substance technical (code: Hoe 002671 0I
ZD95 0005). Micronucleus test in male and female NMRI mice after oral
administration. Pharma Research Toxicology and Pathology. Unpublished
Hoechst document No. A38059. Submitted to WHO by Hoechst Schering
AgrEvo GmbH, Frankfurt-am-Main, Germany.
Müllner, H. (1989) Effects of endosulfan and aldicarb on rat brain
acetylcholinesterase. Unpublished Hoechst report 16 June 1989;
document No. A43395. Submitted to WHO by Hoechst Schering AgrEvo GmbH,
Frankfurt-am-Main, Germany.
Nath, G., Datta, K.K., Dikshith, T.S.S., Tandon, S.K. & Pandya, K.P.
(1978) 30 day oral administration in rats. Interaction of endosulfan
and metepa in rats. Industrial Toxicology Research Centre. Unpublished
Hoechst document No. A17906. Submitted to WHO by Hoechst Schering
AgrEvo GmbH, Frankfurt-am-Main, Germany.
Noctor, J.C. & John, S.A. (1995) (14C)-Endosulfan: Rates of
penetration through human and rat skin determined using an in vitro
system. Hazleton Europe, United Kingdom. Unpublished Hoechst document
No. A54103. Submitted to WHO by Hoechst Schering AgrEvo GmbH,
Frankfurt-am-Main, Germany.
Nogami, K. (translator, no other name available) (1970) Testing report
on the toxicity of endosulfan (Malix) to dogs through acute oral
administration (LD50). Unpublished Hoechst document No. A13834.
Submitted to WHO by Hoechst Schering AgrEvo GmbH, Frankfurt-am-Main,
Germany.
Offer, J.M. (1985) Addendum to HST 204. Effect of endosulfan-technical
(code: Hoe 02671 OI AT209) on the reproductive function of multiple
generations in the rat. Histopathological review of the kidneys in
adult rats of the F1b generation and in weanling rats of the F2b
generation. Huntingdon Research Centre, Huntingdon, Cambridgeshire,
United Kingdom. Unpublished Hoechst document No. A30757. Submitted to
WHO by Hoechst Schering AgrEvo GmbH, Frankfurt-am-Main, Germany.
Pandey, N., Gundevia, F., Prem, A.S. & Ray, P.K. (1990) Studies on the
genotoxicity of endosulfan, and organochlorine insecticide, in
mammalian germ cells. Mutat. Res., 242, 1-7.
Paul, V., Sheela, S., Balasubramaniam, E. & Kazi ,M. (1993)
Behavioural and biochemical changes produced by repeated oral
administration of the insecticide endosulfan in immature rats.
Indian J. Physiol. Pharmacol., 37, 204-208.
Paul, V., Easwaramoorthy, B. & Kazi, M. (1994) The neurobehavioural
toxicity of endosulfan: A serotonergic involvement in learning
impairment. Eur.J. Pharmacol. Environ. Toxicol. Pharmacol., 270,
1-7.
Paul, V., Easwaramoorthy, B., Arumugam, R.J. & Kazi, M. (1995) A
sex-related difference in the neurobehavioural and hepatic effects
following chronic endosulfan treatment in rats. Eur. J. Pharmacol.
Environ. Toxicol. Pharmacol., 293, 355-360.
Pirovano, R. & Milone, M.F. (1986) Study of the capacity of the test
article endosulfan, substance technical to induce chromosome
aberrations in human lymphocytes cultured in vitro. Unpublished RBM
experiment No. m 822, 20 March 1986. Hoechst document A33127.
Submitted to WHO by Hoechst Schering AgrEvo GmbH, Frankfurt-am-Main,
Germany.
Raizada, R.B., Srivastava, M.K. & Dikshith, T.S.S. (1991) Lack of
estrogenic effects of endosulfan: An organochlorine insecticide in
rat. Natl Acad. Sci. Lett., 14, 103-107.
Ramamoorthy, K., Wang, F., Chen, I.-C., Norris, J.D., McDonnel, D.P.,
Leonard, L.S., Gaido, K.W., Bocchinfuso, W.P., Korach, K.S. & Safe, S.
(1997) Estrogenic activity of a dieldrin/toxaphene mixture in the
mouse uterus, MCF-7 human breast cancer cells, and yeast-based
estrogen receptor assays: No apparent synergism. Endocrinology, 138,
1520-1527.
Robacker, K.M., Kulkarni, A.P. & Hodgson, E. (1981) Pesticide induced
changes in the mouse hepatic microsomal cytochrome P-450-dependent
monooxygenase system and other enzymes. J. Environ. Sci. Health,
B16, 529-545.
Reno, F.E. (1975) Acute oral toxicity study in rats. Endosulfan
technical. Unpublished final report, 18 December 1975, from Hazleton
Laboratories America. Hoechst document No. A33732. Submitted to WHO by
Hoechst Schering AgrEvo GmbH, Frankfurt-am-Main, Germany.
Roberts, N.L., Phillips, N.K. & Gopinath, C. (1983) Acute delayed
neurotoxicity study with endosulfan-technical (code: Hoe 002671 OI
ZD97 0003) in the domestic hen. Huntingdon Research Centre,
Huntingdon, Cambridgeshire, United Kingdom. Unpublished Hoechst
document No. A32153. Submitted to WHO by Hoechst Schering AgrEvo GmbH,
Frankfurt-am-Main, Germany.
Ruckman, S.A., Waterson, L.A., Crook, D., Gopinath, C., Majeed, S.K.,
Anderson, A. & Chanter, D.O. (1989) Endosulfan, active ingrediant
technical (code: Hoe 002671 OI ZD97 0003). Combined chronic
toxicity/carcinogenicity study (104-week feeding study in rats).
Huntingdon Research Centre, Huntingdon, Cambridgeshire, United
Kingdom. Unpublished Hoechst document No.A40440. Submitted to WHO by
Hoechst Schering AgrEvo GmbH, Frankfurt-am-Main, Germany.
Shelby, M.D., Newbold, R.R., Tully, D.B., Chae, K. & Davis, V.L.
(1996) Assessing environmental chemicals for estrogenicity using a
combination of in vitro and in vivo assays. Environ. Health
Perspectives, 104, 1296-1300.
Shirasu, Y., Moriya, M. & Ohta, T. (1978) Microbial mutagenicity
testing on endosulfan. Institute of Environmental Toxicology, Japan.
Unpublished Hoechst document No. A21215, 1978. Submitted to WHO by
Hoechst Schering AgrEvo GmbH, Frankfurt-am-Main, Germany.
Singh, S.K. & Pandey, R.S. (1990) Effect of sub-chronic endosulfan
exposures on plasma gonadotrophins, testosterone, testicular
testosterone and enzymes of androgen biosynthesis in rat. Indian J.
Exp. Biol., 28, 953-956.
Sinha, N., Narayan, R., Shanker, R. & Saxena, D.X. (1995)
Endosulfan-induced biochemical changes in the testis of rats. Vet.
Hum. Toxicol., 37, 547-549.
Soto, A.M., Chung, K.L. & Sonnenschein, C. (1994) The pesticides
endosulfan, toxaphene and dieldrin have oestrogenic effects on human
oestrogen-sensitive cells. Environ. Health Perspectives, 102,
380-383.
Soto, A.M., Sonnenschein, C., Chung, K.L., Fernandez, M.F., Olea, N. &
Serrano, F.O. (1995) The E-screen assay as a tool to identify
estrogens: An update on estrogenic environmental pollutants.
Environ. Health Perspectives, 103 (Suppl. 7), 113-122.
Stumf, K. & Lehr, W. (1993) Amendment to document A49584. Unpublished
Hoechst document A49584, 2 February 1993. Submitted to WHO by Hoechst
Schering AgrEvo GmbH, Frankfurt-am-Main, Germany.
den Tonkelaar, E.M. & van Esch, G.J. (1974) No-effect levels of
organochlorine pesticides based on induction of microsomal liver
enzymes in short-term toxicity experiments. Toxicology, 2, 371-380.
US National Cancer Institute (1978) 78-week dietary study in
Osborne-Mendel rats and B6C3F1 mice. NCI study No. NCI-CG-TR62,
Technical Report Series No. 62, Bethesda, Maryland, USA.
Vos, J.G., Krajnc, E.I., Beekhof, P.K. & van Logten, M.J. (1982)
Methods for testing immune effects of toxic chemicals: Evaluation of
the immunotoxicity of various pesticides in the rat. In: Matsunaka,
S., Hutson, D.H. & Murphy, S.D., eds, Pesticide Chemistry: Human
Welfare and the Environment, Oxford, Pergamon Press, pp. 497-504.
Wade, M.G., Desaulniers, D., Leingartner, K. & Foster, W.G. (1997)
Interactions between endosulfan and dieldrin on estrogen-mediated
processes in vitro and in vivo. Reprod. Toxicol., 11, 791-798.
Weigand, W. (1982a) Acute oral toxicity of Hoe 51329 in albino rats.
Pharma Research Toxicology, Germany. Unpublished Hoechst document No.
A23296. Submitted to WHO by Hoechst Schering AgrEvo GmbH,
Frankfurt-am-Main, Germany (in German).
Weigand, W. (1982b) Acute oral toxicity of Hoe 51330 in albino rats.
Pharma Research Toxicology, Germany. Unpublished Hoechst document No.
A23297. Submitted to WHO by Hoechst Schering AgrEvo GmbH,
Frankfurt-am-Main, Germany (in German).
WHO (1996) The WHO Recommended Classification of Pesticides by
Hazard and Guidelines to Classification 1996-1997 (WHO/PCS/96.3),
International Programme on Chemical Safety, Geneva.
Groups of 24 male Sprague-Dawley rats received dermal
applications of [5a,9a14C]-endosulfan at a dose of 0.1, 0.76, or 10
mg/kg bw, without washing. Four animals from each group were killed at
0.5, 1, 2, 4, 10, and 24 h, and radiolabel was measured in the
collected excreta and various organs and tissues, including the
application site after washing with soapy water. No skin irritation
was seen at the application site. Adsorption onto the skin was
essentially complete within 0.5 h at all doses and accounted for
63-80% of the applied dose. Movement away from the application site
was slow, with 73, 73, and 89%, respectively, of the adsorbed doses
remaining at the application site after 24 h. By 10 h, each group had
excreted less than 1% of the applied dose. By 24 h, the excretion was
11, 10, and 4% of the applied dose in the groups given 0.1, 0.76, and
10 mg/kg bw, respectively (Craine, 1986).
Groups of 16 female Sprague-Dawley rats received dermal
applications of [5a,9a14C]-endosulfan at 0.09, 0.98, or 11 mg/kg bw,
and the site was washed with soapy water after 10 h. Four animals from
each group were killed at 24, 48, 72, and 168 h, and radiolabel was
measured in the collected excreta and various organs and tissues,
including the application site. No skin irritation was seen at the
application site, and there were no signs of systemic toxicity.
Recovery of radiolabel was 84-115%. The amount of the applied dose
that was removed by washing was 28, 47, and 69%, respectively, at the
three doses. After 168 h, 63, 87, and 65%, respectively, of the dose
that was not removed by washing was either adsorbed on to the skin or
had penetrated and been distributed and excreted; an average of
2.4-3.3% of the adhering dose remained at the application site.
Excretion was maximal between 24 and 48 h, faeces accounting for about
two-thirds of the label. The total residues at 168 h represented 2.5,
2.3, and 1.3% of the applied dose (about 3.5, 4.3, and 4.2% of the
adhering dose) at the doses of 0.09, 0.98, and 11 mg/kg bw,
respectively, and were present mainly in liver and kidney (Craine,
1988).
At the end of a 24-month study in which Sprague-Dawley rats were
given diets containing 0, 3, 7.5, 15, or 75 ppm technical-grade
endosulfan (see Ruckman et al., 1989), the concentrations of
endosulfan and its known metabolites, endosulfan hydroxyether,
sulfate, lactone, and diol, were measured in the liver and kidneys. As
no quantifiable residues were detected in organs of rats at 15 ppm,
the organs of animals at 7.5 and 3 ppm were not investigated further.
Neither alpha- nor ß-endosulfan, the substances administered in the
diet, or any of the metabolites was quantifiable in either organ, with
the exception of endosulfan sulfate in animals at 75 ppm, which was
found at concentrations of 0.2-0.4 mg/kg in liver. No residues were
observed in the kidneys of this group (Dorn & Werner, 1989; Leist,
1989a).
Twelve lactating goats were given endosulfan (purity unspecified)
in gelatine capsules at a dose of 1 mg/kg bw per day for 28 days. The
tissue concentrations of residues were generally low, the highest
being detected on the first day after cessation of treatment, with
0.29 ppm in kidney, 0.2 ppm in the gastrointestinal tract, and 0.12
ppm in liver. The concentrations in the kidney were increased one week
after treatment, reaching 0.49 ppm on day 8, but no residues were
detected 21 days after treatment ceased. Endosulfan residues did not
accumulate in the fat; the concentrations reached 0.06 ppm on day 1
after the end of treatment, but none were detected by day 8 after
treatment (Indraningsih, et al., 1993).
Groups of three lactating Holstein cows were given diets
containing 0, 0.3, 3, or 30 ppm 14C-endosulfan for 30 days. Between
days 7 and 29, the average concentrations in milk were 3.4, 40, and
462 ppb endosulfan equivalents in the three groups. After the dosing
period, the loss of radiolabel from milk (measured in one cow per
group) was 81% within seven days in the cow given 0.3 ppm and 96%
within 14 days in cows given 3 and 30 ppm (Bowman, 1959). The blood
concentrations rose during the first 21 days, to 0.15 and 1.97 ppm at
the doses of 3 and 30 ppm, respectively, but were always below the
detection level (0.06 ppm) in the groups at 0.3 ppm. The
concentrations of residues found at the three doses were: liver, 0.35,
2.45, and 25.3 ppm; kidney, 0.05, 0.35, and 6.29 ppm; and omental fat,
0.07, 0.71, and 7.08 ppm (Keller, 1959a).
Two lactating East Friesian sheep were given a single oral dose
of 0.3 mg/kg bw 14C-endosulfan and were killed after 40 days. The
radiolabel was maximal in blood after 24 h, when it was equivalent to
0.07 µg/ml. The total radiolabel eliminated in milk over 17 days was
0.37% and 1.82% of the dose in the two sheep, respectively. Radiolabel
was excreted mainly via the urine (41%) and faeces (50%). About half
of the 50% in faeces was unmetabolized endosulfan. Fat, kidney, and
liver of the sheep contained 0.02-0.03 µg/g endosulfan; all of the
remaining tissues had considerably lower concentrations. The total
radiolabel found in organs and tissues accounted for < 1% of the
administered dose (Gorbach et al., 1965).
In pigs fed endosulfan at 2 ppm in their diets for up to 81 days,
the compound was detected in fatty tissue at concentrations of 0.07,
0.09, and 0.04 ppm after 27, 54, and 81 days of treatment, much less
than the residues seen after administration of 7 ppm DDT: 8.3, 9.1,
and 9.7 ppm after 27, 54, and 81 days treatment, respectively. Liver
and muscle contained about 15-fold less DDT residues than fat. Thus,
while much less endosulfan was found in fatty tissues, it does not
appear to bioaccumulate as does DDT (Maier-Bode, 1966).
The systemic absorption of endosulfan over 96 h after dermal
administration to two rhesus monkeys of single doses of 2.2-3 mg/kg bw
of an aqueous suspension of 14C-endosulfan (purity, 94.6%) for 10 h
was 22% of the administered dose. An additional 11% remained in the
skin, 10.5% was found in the carcass, and 4.3 and 3.7% of the
administered dose was excreted in faeces and urine, respectively;
however, as only 50% of the administered dose was recovered, the
figures calculated for absorption may not be accurate indications of
the extent of dermal absorption of endosulfan. A plateau was reached
in blood and plasma concentrations at 36 h, and there may have been no
significant additional dermal absorption after that time.
Concentrations in the liver, kidneys, and fat were highest (0.48,
0.083, and 0.23 ppm, respectively), while there were negligible
concentrations in the brain (Lachmann, 1987).
In a study of the penetration of endosulfan through rat and human
skin in vitro, radiolabelled endosulfan formulated as an
emulsifiable concentrate containing 353 g/L endosulfan, which had been
diluted to concentrations of 0.4-4 mg/ml in water, was applied at
nominal doses of 0.01, 0.1, and 1 mg/cm2 to rat and human skin
mounted in dermal penetration cells. The rate of penetration was, on
average, 4.3 times greater through rat skin than that of humans. The
percentage of the applied dose varied with the concentration: 61% of
the lowest dose applied to human skin and 96% of that applied to rat
skin penetrated, and 20% of the highest dose applied to human skin and
40% of that applied to rat skin penetrated. When the skin was washed
10 h after application, the amount of endosulfan that penetrated
decreased to 4% in the human and 9% in the rat skin. Endosulfan that
passed through human skin was metabolized or degraded to a greater
extent than that which passed through rat skin (Noctor & John, 1995).
2. Toxicological studies
(a) Acute toxicity
The LD50 of endosulfan varies widely depending on the route of
administration, species, vehicle, and sex of the animal (see Table 2).
Certain other reports are available, but it has been argued that most
of them are not acceptable by current standards (Bremmer & Leist,
1998). Female rats are clearly more sensitive than male rats, and, on
the basis of a single study, this sex difference appears to apply to
mice also. Endosulfan is generally highly toxic after oral and
inhalation exposure. The lowest oral LD50 value is 9.6 mg/kg bw in
female Sprague-Dawley rats.
The isomers of endosulfan also show high acute toxicity after
oral administration. The clinical signs of poisoning include
piloerection, salivation, hyperactivity, respiratory distress,
diarrhoea, tremors, hunching, and convulsions. Like endosulfan, its
metabolites were more or less toxic according to the vehicle used and
the species exposed. In general, the toxicity of the lactone and
sulfate metabolites was similar to or less than that of the parent
compound, while the hydroether, ether, and, in particular, the diol
were far less toxic. The clinical signs of poisoning were similar to
those induced by the parent compound and included piloerection,
salivation, hyperactivity, respiratory distress, diarrhoea, tremors,
hunching, and convulsions.
Phenobarbital was an effective therapeutic measure against an
absolute lethal dose of endosulfan in rats, reducing the clinical
signs of poisoning and the mortality rate. Diazepam was not effective
(Ebert & Weigand, 1984).
Table 2. Acute toxicity of technical-grade endosulfan and its isomers and metabolites
Species Strain Sex Route Vehicle LD50 Reference
(mg/kg bw)
Technical-grade endosulfan
Rat Sprague-Dawley M Oral 25% in food 2 800 Bracha (1977)
Rat Sprague-Dawley F Oral 25% in food 45 Bracha (1977)
Rat CD M/F Oral Maize oil 43 Lightowler & Gardner (1978)
Rat Haffkine M Oral 110 Bhide & Naik (1984a)
Rat Haffkine F Oral 15 Bhide & Naik (1984a)
Rat Holtzman M Oral Corn oil 87 Elsea (1958)
Rat Wistar M Oral 2% starch 100-160 Diehl & Leist (1988a)
Rat Wistar F Oral 2% starch 23 Diehl & Leist (1988a)
Rat Sprague-Dawley M Oral 5% CMC 40 Reno (1975)
Rat Sprague-Dawley F Oral 5% CMC 9.6 Reno (1975)
Mouse Kasauli M Oral Tween 80 35 Bhide & Naik (1984b)
Mouse Kasauli F Oral Tween 80 14 Bhide & Naik (1984b)
Dog Mongrel M/F Oral Gelatin capsule 77 Nogami (1970)
Rat HoeWISKf M Dermala > 4 000 Diehl & Leist (1988b)
Rat HoeWISKf F Dermala 500 Diehl & Leist (1988b)
Rabbit NWS M Dermala 290 Bhide & Naik (1984c)
Rabbit New Zealand M Dermala 500-1 000 Bracha (1977)
Rabbit New Zealand F Dermala 1 000-2 000 Bracha (1977)
Rat Sprague-Dawley M/F Inhalation > 21 000 Bracha (1977)
mg/m3 for 1 h
Rat Wistar M Inhalation Ethanol + PEG 35 mg/m3 Hollander & Weigand (1983)
for 4 h
Rat Wistar F Inhalation Ethanol + PEG 13 mg/m3 Hollander & Weigand (1983)
for 4 h
Table 2. (continued)
Species Strain Sex Route Vehicle LD50 Reference
(mg/kg bw)
Endosulfan alpha isomer
Rat Oral 76 Goebel et al. (1982)
Mouse Albino F Oral Tween 80 11 Dorough et al. (1978)
Endosulfan ß isomer
Rat Oral 240 Goebel et al. (1982)
Mouse F Oral Tween 80 36 Dorough et al. (1978)
Endosulfan sulfate
Mouse Albino F Oral Tween 80 8 Dorough et al. (1978)
Rat Wistar F Oral Starch suspension 76 Hollander & Kramer (1975a)
Rat Wistar M Oral 570 Ehling & Leist (1991a)
Rat Wistar F Oral 40 Ehling & Leist (1991a)
Dog Beagle M Oral Starch suspension 15 Hollander & Kramer (1975b)
Rat Wistar M Dermal 2 700 Ehling & Leist (1991b)
Rat F Dermal 280 Ehling & Leist (1991b)
Endosulfan diol
Mouse Albino F Oral Tween 80 > 2 000 Dorough et al. (1978)
Rat F Oral Starch suspension > 1 500 Hollander & Kramer (1975c)
Rat Albino F Oral > 15 000 Weigand (1982a)
Rat Wistar M/F Oral > 5 000 Ehling & Leist (1991c)
Mouse Albino F Oral Tween 80 120 Dorough et al. (1978)
Rat F Oral Starch suspension 1 750 Hollander & Kramer (1975d)
Rat Wistar M/F Dermal > 2 000 Ehling & Leist (1991d)
Table 2. (continued)
Species Strain Sex Route Vehicle LD50 Reference
(mg/kg bw)
Endosulfan ether
Mouse Albino F Oral Tween 80 270 Dorough et al. (1978)
Rat F Oral Starch suspension > 15 000 Hollander & Kramer (1975c)
Rat Albino F Oral > 15 000 Weigand (1982b)
Endosulfan hydroxyether
Mouse Albino F Oral Tween 80 120 Dorough et al. (1978)
Rat F Oral Starch suspension 1 750 Hollander & Kramer (1975d)
Endosulfan lactone
Mouse Albino) F Oral Tween 80 120 Dorough et al. (1978)
Rat Wistar F Oral Starch suspension 290 Hollander & Kramer (1975e)
Rat Wistar M Oral Starch suspension 160 Hollander & Kramer (1975f)
Rat M/F Oral Sesame oil 100/120 Kramer & Weigand (1971)
M, male; F, female; CMC, carboxy methyl cellulose; PEG, polyethylene glycol
a 24 h on intact skin
The dermal irritancy of technical-grade endosulfan (purity,
98.6%) was tested in three New Zealand white rabbits by clipping the
hair from a dorsal area of about 25 cm2 and 24 h later applying 500
mg endosulfan moistened with deionized water on a 6.25-cm2 cellulose
patch, which was then covered with a semi-occlusive bandage. Exposure
was for 4 h, after which the test material was removed with warm
tap-water. The exposed area was examined 0.5-1, 24, 48, and 72 h after
removal of the patch. On the basis of the evaluation system defined by
EEC guideline B.4 ('Acute toxicity skin irritation' of Directive
92/69/EEC), the overall mean scores for dermal irritation were 0 for
both erythema and eschar formation and oedema formation. No signs of
systemic toxicity were observed (Bremmer, 1997a).
The ocular irritancy of technical-grade endosulfan (purity,
98.6%) was tested in three New Zealand white rabbits by applying 100
mg to the conjunctival sac of one eye of each rabbit; the other,
untreated eye served as the control. The eyes were exposed for 24 h,
after which the endosulfan was washed out, and the eyes were examined
for ocular lesions 1, 24, 48, and 72 h later. On the basis of the
evaluation system defined by EEC guideline B.5 ('Acute toxicity eye
irritation' of Directive 92/69/EEC), the overall mean scores for
irritation were 0.66 for redness of conjunctiva, 0 for chemosis of
conjunctiva, 0 for opacity of cornea, and 0.11 for irritation of the
iris. No signs of systemic toxicity were observed. Endosulfan was not
irritating to the eye (Bremmer, 1997b).
The cutaneous allergenic potential of endosulfan (purity, 98.6%)
was examined in 20 treated and 10 control male albino guinea-pigs. The
maximal tolerated concentration of endosulfan suitable for the
induction phase of the main study and a suitable non-irritating
concentration of topically applied endosulfan were identified for the
challenge application in a preliminary study. For intradermal
induction, injection of 0.1 ml of a 0.5% solution in corn oil
emulsified 1:1 (v:v) with Freund's complete adjuvant was selected. One
week after these injections, a 6-cm2 patch of filter paper saturated
with about 0.3 ml of a 50% solution of endosulfan in corn oil was
applied to the shaved skin of each guinea-pig, and the area was
occluded with aluminium foil secured by impermeable adhesive tape,
which was left in position for 48 h. Irritation was assessed 24 and 48
h later. On test day 22, the guinea-pigs were challenged with the
non-irritating 50% endosulfan in corn oil applied as during the
induction phase. The dressing was left in position for 24 h. The
application sites were assessed for erythema and oedema 24 and 48 h
later. On the basis of the evaluation system defined by EEC guideline
B.6 ('Acute toxicity -- Skin sensitization' of Directive 92/69/EEC),
none of the treated guinea-pigs developed skin reactions. Endosulfan
was therefore considered to be non-sensitizing for guinea-pig skin
(Arcelin, 1996).
(b) Short-term toxicity
Mice
Groups of 10 male and 10 female Hoe:NMRKf mice were fed diets
containing endosulfan at a concentration of 0 or 18 ppm for six weeks,
equal to 0 or 3.7 mg/kg bw per day for males and 0 or 4.6 mg/kg bw per
day for females, to determine whether 18 ppm was the NOAEL in this
strain, as it was found to be in CD-1 mice in a three-month study
(Barnard et al., 1984). There were no clinical signs attributable to
treatment. The mean food intake of mice receiving endosulfan was
slightly decreased in males and slightly increased in females. Mean
body-weight gain was reduced in treated males during the second half
of the experiment but was slightly increased in females throughout the
experiment. Two treated females died, on days 28 and 38; the causes
were not established, but no histological changes were found in the
mouse that was not autolysed. The absolute and relative weights of the
liver of males and females at 18 ppm were higher than those of
controls and statistically significantly so in females (17 and 12%,
respectively). A NOAEL was not identified, as an increase in liver
weights was seen in females at the only dose tested (Donaubauer et
al., 1985).
Groups of 20 CD-1 mice of each sex were fed diets containing
endosulfan (purity, 97.2%) at a concentration of 0, 2, 6, 18, or 54
ppm for three months, equal to 0, 0.24, 0.74, 2.1, and 7.3 mg/kg bw
per day for males and 0, 0.27, 0.8, 2.4, and 7.5 for females. Clinical
signs attributable to treatment, consisting of convulsions and
salivation, were seen in one male and one female at the high dose.
There was a marked treatment-related decrease in the survival rate
(about 50%) of male and female mice at the high dose; the mean food
intake of these animals was significantly reduced for the first two
weeks of the study, and the mean body-weight gain of males at the high
dose was reduced during the first week of treatment. A significant
reduction in neutrophil count was observed in males at 54 ppm at week
6 (72%, p < 0.01), and a lower count (62%), which was not
statistically significant, was also observed at week 13. The blood
glucose concentration was reduced by about 11% in females at all doses
(p < 0.01) at week 6, and the serum lipid concentration was
increased by 15% in females at 54 ppm at week 13. The NOAEL was
18 ppm, equivalent to 2.1 mg/kg bw per day, on the basis of
convulsions and salivation, decreased survival, and increased serum
lipid concentrations at 7.3 mg/kg bw per day (Barnard et al., 1984).
Groups of 10 four-week-old ddY mice of each sex were fed diets
containing technical-grade endosulfan (purity, 91.4%) at
concentrations of 0, 10, 30, 100, or 300 ppm, equal to 0, 1.2, 4.1,
15, and 42 mg/kg bw per day in males and 0, 1.4, 4.7, 14, and 42 mg/kg
bw per day in females, for 12 months. There were no apparent
treatment-related clinical signs or deaths. Males at the high dose
showed a small (1%) but significant decrease in mean corpuscular
volume, and transient, non-dose-related increases in haemoglobin
(11%), haematocrit (4%), and eosinophil counts (33%) were seen in
males at 30 ppm. A significant decrease in serum aspartate
aminotransferase activity was seen in males at 100 and 300 ppm and a
decrease in bilirubin concentration in males at the high dose. The
only change in organ weights was a dose-related increase in the
relative weight of the adrenals in females, which was about 30% and
statistically significant at 300 ppm. There were no treatment-related
changes on gross pathological examination; the histopathological
effects consisted of dose-related granulomatous changes in the liver
and lymph nodes. In the liver, granuloma, giant-cell infiltration,
and/or large histiocytic cells filled with brown pigment were found in
treated mice; these effects were significant at 100 and 300 ppm. In
the lymph nodes, giant-cell infiltration and/or reticuloendothelial
cell proliferation were found at the same doses. Testicular atrophy
was seen erratically, with incidences of 30, 70, 33, 50, and 80% in
the five groups, respectively, but was not considered to be related to
treatment. The NOAEL was 30 ppm, equal to 4.1 mg/kg bw per day, on the
basis of histological findings in the liver and lymphatic system (Arai
et al., 1981).
Rats
Groups of male albino rats were given endosulfan in peanut oil by
gavage at a dose of 0 or 11 mg/kg bw per day for 30 days. In addition,
the possible interaction between endosulfan and the chemosterilant,
metepa, was investigated in groups of rats receiving either metepa
alone at 30 mg/kg bw per day for 30 days or in combination with
endosulfan at 11 mg/kg bw per day. There were three deaths in the
endosulfan-treated group. Endosulfan alone had no significant effect
on body weights, organ weights, blood chemistry, or histopathological
appearance. No potentiation of the toxicity of metepa was seen (Nath
et al., 1978).
Groups of 100 male SPF Wistar rats were fed diets containing
technical-grade endosulfan (purity, 97.9%) at concentrations of 360 or
720 ppm, equal to 34 and 68 mg/kg bw per day for four weeks. Twenty
control rats received diet alone. Fifty treated and 10 control animals
were maintained for an additional four-week withdrawal period. The
objective of the experiment was to determine the toxicological
significance of yellow, granular deposits observed in the proximal
convoluted renal tubules in a 13-week and a two-generation feeding
study. One rat in each treated group died, with no signs of poisoning.
The behaviour and general condition of the rats and their food and
water consumption were unaffected by treatment, and body-weight gains
were comparable in treated and control groups. Enlargement of the
liver was observed in rats at 360 and 720 ppm at the end of the
treatment period, and the kidney and brain weights were significantly
elevated in those at 720 ppm; these treatment-related changes in organ
weights had, however, disappeared by the end of the withdrawal period.
The kidneys of treated rats were darkly discoloured, but their
appearance had returned to normal by the end of the withdrawal period.
Histopathological examination by light and electron microscopy showed
granular pigmentation and larger, more numerous lysosomes in renal
proximal tubule cells after treatment; these changes had decreased by
the end of the withdrawal period, and no lysosomal changes were found
in brain or liver. Analysis of residues showed that storage of
alpha-endosulfan was dose-dependent, temporary, and confined to the
kidney, where it was detected as endosulfan sulfate and endosulfan
lactone. The amount of ß-endosulfan was 230 times less than that of
alpha-endosulfan. The concentrations of the sulfate and lactone
metabolites were many times lower in liver, and only traces of
endosulfan remained in the kidney at the end of the withdrawal period
(Leist & Mayer, 1987).
Groups of 15 male and 15 female Wistar rats were exposed by
inhalation to technical-grade endosulfan (purity, 97.2%) at a
concentration of 0, 0.5, 1, or 2 mg/m3 air for 6 h/day, five days per
week for 29 days, for a total of 21 exposures. One male rat at the
high dose was emaciated, had a pale skin, and adopted a high-legged
position; no other clinical signs were seen in the treated animals. No
neurological disturbances, opacity of the refractive media, impairment
of dental growth, or changes in the oral mucosa were seen. The
body-weight gain of males at the high dose tended to be depressed from
day 20 of exposure until day 29, the end of the recovery period, but
no other changes in body-weight gain or food consumption were seen.
Non-dose-related increases in erythrocyte and haemoglobin
concentrations were seen at the end of exposure, but not at day 29;
these concentrations were reported to be within normal ranges for this
strain of rat. Apart from a transient, non-dose-related increase in
creatinine concentration and a decrease in serum aspartate
aminotransferase activity in females at the high dose, no
treatment-related change in blood chemistry was noted, and no
histological changes were seen in any of the rats (Hollander et al.,
1984)
Technical-grade endosulfan (purity, 97.2%) was applied in a
solution in sesame oil to the shaved skin of the nape of groups of six
Wistar rats of each sex 21 times over 30 days, for 6 h/day on five
days per week under an occlusive bandage. Males were given a dose of
0, 12, 48, 96, or 190 mg/kg bw per day, and females received 0, 3, 6,
12, or 48 mg/kg bw per day. In males, deaths and clinical signs of
poisoning consisting of tremors, tonoclonic convulsions, and/or
hypersalivation were observed only at the highest dose. In females,
clinical signs of poisoning were observed at > 2 mg/kg bw per day;
deaths occurred mainly in the group receiving 48 mg/kg bw per day,
although single animals died on day 18 after receiving 3, 6, or 12
mg/kg bw per day, none having shown signs of poisoning. Serum
cholinesterase activity was 33% lower in males at 192 mg/kg bw per
day, whereas the activities of the erythrocyte and brain enzymes were
reduced by 12 and 7%, respectively. In females at 48 mg/kg bw per day,
serum cholinesterase activity was 21% lower than in controls, and
there were no effects in the activities of erythrocyte and brain
enzymes. The NOAEL was 6 mg/kg bw per day (Ebert et al., 1985a,b).
Technical-grade endosulfan (purity, 97.2%) as a solution in
sesame oil was applied to the shaved skin of the nape of groups of six
male and six female Wistar rats 21 times over 30 days, for 6-h periods
under an occlusive bandage, at doses of 0, 1, 3, 9, 27, or 81 (males
only) mg/kg bw per day. No signs of toxicity were observed in males or
females at 1 and 3 mg/kg bw per day, but two males at 9 mg/kg bw per
day died, one on day 5 with no previous signs of poisoning and the
other on day 8 after piloerection, hypersalivation, blood-encrusted
nose, stagger, and dyspnoea. There were no deaths among males at 27
mg/kg bw per day, but three at 81 mg/kg bw per day died. None of the
females at 9 mg/kg bw per day died and no clinical signs of toxicity
were observed, but five females at 27 mg/kg bw per day died between
days 2 and 6 with no previous clinical signs of toxicity. Microscopic
changes were seen in the livers of animals at > 9 mg/kg bw per day,
consisting of enlargement of parenchymal cells in the periphery and
loss of cytoplasmic basoplilia. Serum cholinesterase activity was
statistically significantly reduced by treatment, by 70-80% in males
at doses of 9-81 mg/kg bw per day but in females by only about 40% at
9 mg/kg bw per day. Brain acetylcholinesterase activity was
statistically significantly reduced in males at 9 (21%), 27 (28%), and
81 (24%) mg/kg bw per day and in all treated females by 13-18%. The
responses were not dose-related, and the observations in females are
probably not biologically significant. Since a later study did not
demonstrate a direct inhibitory effect of endosulfan on rat brain
acetylcholinesterase activity in vitro, the reduced brain enzyme
activity found in this study was difficult to interpret. It is also
noted that serum enzyme activity was inhibited only at much higher
doses in the study of Ebert et al. (1985a,b). The two males at 9 mg/kg
bw per day that died had reduced or immature testes and/or sex organs,
and the livers of these animals has accentuated lobular markings. The
authors reasoned that these effects resulted from a
non-substance-related developmental disturbance already present before
treatment. No mechanism was proposed for these effects, which were not
seen at higher doses. The NOAEL was 3 mg/kg bw per day on the basis of
inhibition of serum and brain cholinesterase activity and microscopic
changes in the liver (Ebert et al., 1985b,c).
Endosulfan (purity not stated) as a solution in acetone was
applied daily to the shaved abdominal skin of groups of 24 albino rats
of each sex for 30 days at doses of 0, 19, 38, or
63 mg/kg bw per day for males and 0, 10, 20, or 32 mg/kg bw per day
for females. There were no deaths. All doses produced
hyperexcitability, tremor, dyspnoea, and salivation, which disappeared
after one week. No significant changes in organ:body weight ratios
occurred, and there were no treatment-associated effects on
histological, haematological, or blood chemical parameters. Liver
alanine and aspartate aminotransferase activities were reduced in
animals of each sex at the lowest dose, but there were no further
reductions with increasing dose. Liver alkaline phosphatase and
lactate dehydrogenase activities were increased in females but not in
males, again with no increase with dose. Cholinesterase activities
were not measured. A NOAEL was not identified (Dikshith et al., 1988).
Groups of 25 CD Sprague-Dawley rats of each sex were fed diets
containing technical-grade endosulfan (purity, 97.9%) at
concentrations of 0, 10, 30, 60, or 360 ppm, equal to 0, 0.64, 1.9,
3.8, and 23 mg/kg bw per day for males and 0, 0.75, 2.3, 4.6, and
27 mg/kg bw per day for females, for three months. Five animals of
each sex per group were maintained for an additional four-week
recovery period. Three females died, one each at 0, 60, and 360 ppm.
Slight but statistically significant, dose-related reductions in
erythrocyte counts and haemoglobin concentrations were seen in males
at > 30 ppm and in females at > 60 ppm, but were within the
reported normal range for this strain and age of rat (Leist & Bremmer,
1998); increased mean corpuscular volume was also seen at these doses.
Females at 360 ppm had statistically significant decreases in plasma
and erythrocyte cholinesterase activities (measured by the Ellman
method) at week 12 (by 41 and 12%, respectively), while increased
brain acetylcholinesterase activity was observed at 60 and 360 ppm (19
and 20%, respectively). In males at 360 ppm, urinalysis showed a
number of reversible changes, including increased urine volume and
urinary protein concentrations and decreased specific gravity. Gross
examination revealed enlargement of the liver in males at 360 ppm and
of the kidneys at 60 and 360 ppm; increases in the absolute weights of
the liver (18%), kidney (29%), and epididymides (8%) were seen in
males and of the liver (21%) and kidneys (10%) in females. The kidney
weights remained significantly elevated in male rats at 360 ppm (15%,
p < 0.01) at the end of the withdrawal period.
Histopathological examination revealed traces of brown pigment in
scattered hepatocytes in 25% of male rats and minimal centrilobular
enlargement of hepatocytes in 25% of females at 360 ppm. These changes
were not observed in rats at the end of the withdrawal period.
Yellowish discolouration of renal proximal tubular cells was seen in
males at all doses and in females at 30-360 ppm, the degree of
pigmentation increasing in a dose-related manner; however, no cell
death was associated with this finding. In addition, granular
pigmentation was seen in straight portions and occasionally in
proximal tubular cells in males at 60 and 360 ppm. The yellow
discolouration of the renal tubules in male rats had decreased by the
end of the withdrawal period, but trace or minimal pigmentation was
still evident. In females at > 60 ppm, the traces of pigmentation
persisted. Males at 360 ppm also had yellow protein aggregation in the
proximal convoluted with intracytoplasmic eosinophilic droplets in the
tubules. The increase in incidence and degree of the yellowish
discolouration of the proximal tubular cells appeared to be
treatment-related, since it did not develop in control rats; however,
no adverse effects were reported that might be associated with these
findings alone. All rats at 30 ppm showed either trace or minimal
discolouration and signs of granular or clumped pigment. Other
investigations suggest that the yellow pigmentation is no more than an
indication that endosulfan and some of its metabolites are being
temporarily stored before urinary excretion, and that it is therefore
an indicator of exposure rather than an expression of toxicity.
Consequently, this effect was not considered in the evaluation. At
doses of 60 and 360 ppm, other treatment-related effects were also
seen when the pigmentation was present, including enlarged kidneys and
centrilobular hepatocytes. No treatment-related increase in the
incidence of other renal effects was reported in animals at 10 ppm.
The NOAEL was thus 10 ppm, equal to 0.64 mg/kg bw per day, on the
basis of haematological changes (Barnard et al., 1985).
(c) Long-term studies of toxicity and carcinogenicity
Mice
Groups of 50 six- to seven-week-old B6C3F1 mice of each sex were
fed diets containing technical-grade endosulfan (purity, 98.8%) at
time-weighted average concentrations of 3.5 or 6.9 ppm for males and 2
or 3.9 ppm for females for 78 weeks. Groups of 20 controls received
untreated diet. There were no clear compound-related effects on
appearance or behaviour in the treated groups, and the body weights of
both males and females were unaffected by treatment. The mortality
rate of males at the high dose was increased early in treatment so
that, at the end of the experiment, the survival rates were 3/20
controls, 19/50 at the low dose, and 5/50 at the high dose. The
mortality rates of female mice were not affected by treatment. No
treatment-related clinical signs were recorded, and no
treatment-related neoplastic lesions were seen in the females. Owing
to the high early mortality rates, no conclusion could be drawn about
the carcinogenic potential of endosulfan in males. None of the
non-neoplastic changes seen in the kidneys and sex organs of male and
female mice could be attributed to treatment. The NOAEL for female
mice was 3.9 ppm, equal to 0.58 mg/kg bw per day (US National Cancer
Institute, 1978).
Groups of 60 NMRI mice of each sex were fed diets containing
technical-grade endosulfan (purity, 97.2%) at concentrations of 0, 2,
6, or 18 ppm, equal to 0.28, 0.84, and 2.5 mg/kg bw per day for males
and 0.32, 0.97, and 2.9 mg/kg bw per day for females, for up to 24
months. Ten mice of each sex per dose were killed at 12 and 18 months.
The behaviour and general health of the animals were not affected by
treatment. The mortality rate of females at 18 ppm was statistically
significant decreased at the end of the experiment: control, 45%; 18
ppm, 28% (p < 0.05); at week 78, the rates in these two groups of
female mice were 82 and 62%. Survival among treated male mice was not
statistically different from that of controls. The body weights of
males receiving 18 ppm were slightly but significantly lower than
those of controls during the first third of the study and remained
slightly but not significantly low throughout the remainder of the
study. In the other treated groups, there was a tendency to increased
body-weight gains, especially in the satellite groups killed at 12 and
18 months. No statistically significant changes were observed in
haematological or clinical chemical parameters, and macroscopic
examination did not reveal any findings that were related to
treatment. No statistically significant changes in organ weights were
seen in treated animals at the end of the experiment; however, slight
but statistically significant changes in organ weights were observed
in animals at 18 ppm at 12 and 18 months, consisting of decreased lung
and ovary weights in females at 12 months and decreased liver weights
in males and decreased ovary weights in females at 18 months.
Histopathological examination did not reveal any effects that were
related to treatment. No increase in the incidence of neoplastic or
non-neoplastic lesions was observed. The NOAEL was 6 ppm, equal to
0.84 mg/kg bw per day, on the basis of decreased body weights in males
at 24 months and decreased weights of the liver, ovaries, and lung in
males and females at 12 and/or 18 months (Donaubauer, 1988, 1989; Hack
et al., 1995).
Rats
Groups of 50 male and 50 female Osborne-Mendel rats were fed
diets containing technical-grade endosulfan (purity, 98.8%) at
time-weighted average doses of 220, 410, or 950 ppm for males and 220
or 400 ppm for females for 78 weeks, with a return to control diets
for a further four weeks. Groups of 20 rats of each sex received
untreated diet. A highly significant morbidity rate was seen in male
rats and, by week 54, 52% of those at the high dose had died. A
dose-related reduction in body weight was found in males at all doses.
Histopathological examination showed a high incidence of toxic
nephropathy (> 90%) in males at the low and high doses and in
females, but in none of the controls. Chronic renal inflammation was
observed in 40% of control males and 80% of treated males. The toxic
nephropathy observed was characterized as degenerative changes in the
proximal convoluted tubules at the junction of the cortex and medulla,
with associated cloudy swelling, fatty degeneration, and necrosis of
the tubular epithelium. Some tubules had hyaline casts, and enlarged,
dark-staining regenerative tubular epithelial cells were observed
infrequently. In treated males, parathyroid hyperplasia was observed,
as were calcium deposits in the stomach, kidney, testis, aorta, and
mesenteric artery. A dose-related increase in the incidence of
testicular atrophy was seen in treated males, characterized by
degeneration and necrosis of the germinal cells lining the
seminiferous tubules and multinucleated cells (fusion bodies),
resulting in aspermatogenesis. No treatment-related effects were noted
in the reproductive organs of female rats. No treatment-related
neoplastic lesions were seen in female rats; owing to the high
mortality rate in males, no valid conclusion can be drawn about
carcinogenicity. A NOAEL was not identified, as treatment-related
changes occurred in the kidneys and the testis at all doses (US
National Cancer Institute, 1978)
Groups of 50 five-to-six-week-old Sprague-Dawley rats of each sex
were fed diets containing endosulfan (purity, 97.1%) at concentrations
of 0, 3, 7.5, 15, or 75 ppm, equal to 0, 0.1, 0.3, 0.6, and 2.9 mg/kg
bw per day for males and 0, 0.1, 0.4, 0.7, and 3.8 mg/kg bw per day
for females, for 104 weeks. Satellite groups of 20 animals of each sex
were retained for blood sampling and examination at 104 weeks.
Reductions in body weights and body-weight gains were observed in
males (group mean, 17% at 104 weeks) and females (group mean, 18% at
104 weeks) at 75 ppm, but no clinical signs of poisoning were seen at
any dose. No increase in mortality rates was observed in treated
groups. Increased incidences of enlarged kidneys in females and of
aneurysms and enlarged lumbar lymph nodes in males were seen at 75
ppm. Histopathological examination showed that males at 75 ppm had an
increased incidence of aneurysm and marked progressive
glomerulonephrosis (controls, 20/70; 75 ppm, 30/70). The commonest
neoplasms were pituitary tumours in males and females and mammary
tumours in females, but the increased incidences did not appear to be
related to treatment. The NOAEL was 15 ppm, equal to 0.6 mg/kg bw per
day, on the basis of reduced body weights and pathological findings at
higher doses (Ruckman et al., 1989; Gopinath & Cannon, 1990; Hack et
al., 1995).
Groups of 25 Wistar rats of each sex were fed diets containing
technical-grade endosulfan (purity unspecified) at doses of 0, 10, 30,
or 100 ppm for up to 104 weeks; groups of five of each sex were killed
at 52 weeks. There were no treatment-related clinical signs, and the
body weights were unaffected, except for a nonsignificant decrease in
the body weights and food consumption of males at the high dose. The
survival rate of treated females was reduced, the deaths being
associated predominantly with respiratory infections. The weights of
the testes of males at 10 ppm were reduced by 7% with respect to
controls at 104 weeks (p < 0.05), and the kidney weights were
significantly (p < 0.001) increased (by 16%) in males at the high
dose at 104 weeks. Histopathological changes observed in males at the
high dose at 104 weeks consisted of enlarged kidneys, mild-to-severe
renal tubular dilatation (12/12), mild-to-moderate formation of
irregular albuminous casts (10/12), pronounced focal nephritis (7/12),
and mild-to-severe degeneration (11/12) of the renal tubular
epithelium. At 104 weeks, female rats at the high dose showed minimal
degeneration of renal tubules (2/3) and some focal nephritis (1/3).
The low survival rate precluded a clear conclusion about the renal
changes in female rats. Microscopic alterations in the liver were seen
in 50% of males at the high dose at week 104, consisting of focal
areas of hydropic cells, which were pale and swollen; the nuclei were
surrounded by a clear zone, and a few cells appeared to have
eosinophilic cytoplasmic inclusions. Few females at the high dose
showed changes in liver cells. A few tumours developed during the
experiment, but their occurrence was not dose-related. The NOAEL was
30 ppm, equal to 1.5 mg/kg bw per day, on the basis of effects on the
kidney (Keller, 1959c).
Dogs
Groups of six beagle dogs of each sex were fed diets containing
technical-grade endosulfan (purity, 96.5%) at concentrations of 0, 3,
10, or 30 ppm for one year, calculated by the authors to be equivalent
to 0, 0.23, 0.77, and 2.3 mg/kg bw per day. In addition, one group was
given a diet containing 30-60 ppm endosulfan, increasing in stages
from 30 ppm for 54 days, to 45 ppm for 52 days, and 60 ppm for 19-40
days; these dogs were killed in extremis before the scheduled
completion of the experiment and showed a number of signs of
poisoning, including tonic contraction and increased sensitivity to
noise and optical stimuli. Some animals given endosulfan at 30 ppm
throughout the 12-month study had violent contractions of the
abdominal muscles (without vomiting), and males at this dose had
reduced body-weight gains throughout the study and slightly reduced
body weights in the latter stages of the study, in comparison with
control animals. Cholinesterase activity was measured in serum,
erythrocytes, and brain, but difficulty appears to have been
experienced in measuring these activities, and there were large
variations within groups for the brain enzyme, the group mean of which
was increased in dogs at 30 ppm. No other effects related to treatment
were observed, and no increase in the incidence of neoplastic or non-
neoplastic lesions was observed in treated animals. The NOAEL was 10
ppm, calculated by the authors to be equivalent to 0.57 mg/kg bw per
day, on the basis of clinical signs and reductions in body weight
(Brunk 1989, 1990).
Groups of two male and two female mongrel dogs were given
technical-grade endosulfan in gelatin capsules at doses of 0, 3, 10,
or 30 ppm, equivalent to 0, 0.075, 0.25, or 0.75 mg/kg bw per day, on
six days per week for one year. The group receiving 3 ppm was given
100 ppm for the first three days of treatment, but clinical signs of
vomiting, tremors, convulsions, rapid respiration, mydriasis,
salivation, and tonic-clonic convulsions in one male and both females
led to a reduction in the dose for the remainder of the study. No
clinical signs or treatment-related effects on body-weight gain were
seen. Clinical chemical and haematological end-points were within
normal limits, and kidney function was unaffected by treatment. No
gross or histopathological changes associated with treatment were
noted. The NOAEL was 30 ppm, equivalent to 0.75 mg/kg bw per day, on
the basis of clinical signs at the initial high dose (Keller, 1959b).
(d) Genotoxicity
Endosulfan was tested for genotoxicity in a wide range of assays,
both in vitro (with and without metabolic activation) and in vivo
(Table 3). There was no evidence of genotoxicty in most of these
assays. In an assay for dominant lethal mutation in male Swiss mice
given endosulfan of a purity of 97.3%, there was a significant change
in the result of mating during the sixth mating week in the group at
16.6 mg/kg bw per day. The total numbers of implants per pregnancy
were 9 in controls and 4.5 at the high dose; the numbers of live
implants per pregnancy were 9 in controls and 2.2 at the high dose;
and the numbers of dead implants per pergnancy were none in controls
and 2.25 at the high dose (Pandey et al., 1990). While there is no
doubt about the statistical significance of these results, it is
unusual to find a true dominant lethal effect appearing so late in an
experimental mating schedule; however, it is not unknown, since a
reproducible effect of this type was demonstrated with some glycol
ethers (McGregor et al., 1983). In a later assay for dominant lethal
mutation (Dzwonkowska & Hübner, 1991), much lower doses were used, so
the results cannot be used as evidence for a non-reproducible effect.
Significant increases in the proportion of morphologically abnormal
sperm were also observed in the study of Pandey et al. (1990), as well
Table 3. Results of assays for the genotoxicity of endosulfan
End-point Test object Dose Result Reference
(LED or HID)a
In vitro
Differential toxicity B. subtilis rec strains H17 and M45 2000 µg/disc Negativea Shirasu et al. (1978)
Reverse mutation S. typhimurium TA100, TA1535, 5000 µg/plate Negativeb Shirasu et al. (1978)
TA1537, TA1538, TA98;
E. coli WP2 uvrA
Gene conversion S. cerevisiae, D4 5000 µg/ml Negativeb Mellano & Milone (1984b)
Forward mutation S. pombe 500 µg/ml Negativeb Mellano & Milone (1984a)
Unscheduled DNA Male F344 rat primary hepatocytes 51 µg/ml Negativea Cifone & Myhr (1984b)
synthesis
Gene mutation Mouse lymphoma L5178Y cells, 75 µg/ml [?] Negativeb Cifone & Myhr (1984a)
tk locus
Chromosomal Human lymphocytes 200 µg/ml Negativeb Asquith & Baillie (1989)
aberration
Chromosomal Human lymphocytes 200 µg/ml Negativeb Pirovano & Milone (1986)
aberration
In vivo
Micronucleus NMRI mouse bone-marrow cells 5 mg/kg bw, Negative Jung et al. (1983)
formation po × 1
Micronucleus NMRI mouse bone-marrow cells 10 mg/kg bw, Negative Müller (1988)
formation po × 1
Table 3. (continued)
End-point Test object Dose Result Reference
(LED or HID)a
Chromosomal Albino rat bone-marrow cells 55 mg/kg, po × 5 Negative Dikshith & Dotta (1978)
aberration
Dominant lethal Male Swiss mice 16.6 mg/kg bw, Equivocal Pandey et al. (1990)
mutation ip × 5
Dominant lethal Male Balb/c mice 0.64 mg/kg bw, Negative Dzwonkowska & Hübner
mutation ip × 1 and ip × 5 (1991)
Sperm morphology Mice 16.6 mg/kg bw, Positive Pandey et al. (1990)
ip × 5
Sperm morphology Mice in vivo 3 mg/kg bw, Positive Khan & Sinha (1996)
ip × 35
LED, lowest effective dose; HID, highest ineffective dose; po, oral; ip, intraperitoneal
a In the absence of exogenous metabolic activation; not tested in the presence of exogenous metabolic activation
b In the absence and presence of exogenous metabolic activation
as in a later study (Khan & Sinha, 1996) at lower daily doses of a 35%
emulsifiable concentrate.
(e) Reproductive toxicity
(i) Multigeneration reproductive toxicity
A preliminary investigation was conducted to determine the doses
of endosulfan to be used in a two-generation study of reproductive
toxicity. Four groups of 10 male and 10 female seven-week-old Crl:
COBS CD Sprague-Dawley rats were given diets containing
technical-grade endosulfan (purity, 97%) at concentrations of 0, 50,
75, or 100 ppm for two weeks and subsequently throughout mating and
the rearing of offspring to weaning. Food consumption and body weights
were decreased in adults at 75 and 100 ppm. At terminal autopsy, the
mean weights of the livers were significantly higher than the control
value in all treated groups. Mating performance, pregnancy rate, and
the duration of gestation were unaffected by treatment. The litter
weights of dams were significantly decreased at 75 ppm and to a
greater extent at 100 ppm from day 4 post partum. No
treatment-related abnormalities were found in the young (Edwards et
al., 1982).
In a two-generation study of reproductive toxicity with two
matings in each generation, four groups of six-week-old
Sprague-Dawley rats were fed diets containing technical-grade
endosulfan (purity, 97%) at concentrations of 0, 3, 15, or 75 ppm,
equal to 0.2-0.23, 1-1.2, and 5-5.7 mg/kg bw per day for males and
0.24-0.26, 1.2-1.3, and 6.2-6.9 mg/kg bw per day for females. The
group sizes were 32 of each sex for the F0 generation and 28 of each
sex for the F1b generation. No clinical signs or deaths related to
treatment were observed during the study. Single deaths occurred among
F0 females at 0, 3, and 15 ppm and among F1b control females. Mating
performance and pregnancy rates were not affected by treatment.
Statistically significant decreases in litter weight were occasionally
seen, but there was no effect on mean pup weights or on litter size.
No treatment-related effect on sex ratios was seen at any dose.
Statistically significantly increased relative kidney weights
were seen at 75 ppm in F0 and F1b males, and statistically
significantly increased relative liver weights were observed in F0
males and females at 75 ppm and in F1b females at 15 and 75 ppm. The
effect at 15 ppm in F1b dams was not seen at this dose in any other
matings. Yellowish discolouration of cells in the proximal convoluted
tubules were observed in male F1b rats at 3, 5, and 75 ppm and in
female F1b rats at 75 ppm. The incidence and extent of this effect
was dose-related; traces of discolouration were seen at all doses, and
minimal discolouration was seen in male rats at 15 and 75 ppm.
Granular or clumped pigment was seen in proximal convoluted tubular
cells in males at the high dose, but these findings were not
associated with histopathological evidence of renal damage. While the
increased incidence of cellular discolouration was related to
treatment, the finding is considered not to be toxicologically
significant as no adverse effects were seen on cells and the yellow
pigment was considered likely to be due to storage of endosulfan and
its metabolites in lysosomes before excretion (Annex 1, reference 58).
The presence of the pigment is thus an indication of exposure to
endosulfan rather than of toxicity. The NOAEL for maternal toxicity
was 15 ppm, equal to 1 mg/kg bw per day, on the basis of increased
relative liver and kidney weights at higher doses. The NOAEL for
reproductive effects was 75 ppm, equal to 6 mg/kg bw per day, the
highest dose tested (Edwards et al., 1984; Offer, 1985).
(ii) Developmental toxicity
Rats
Groups of mated female albino rats (strain and age unspecified)
were given oral doses of 0 (20 rats), 5 (26 rats), or 10 (32 rats)
mg/kg bw per day of endosulfan (purity unspecified) on days 6-14 of
gestation. No marked changes in behaviour or appearance were reported,
and the body weights of treated animals were similar to those of
controls. The numbers of pregnancies were 18, 20, and 21,
respectively. The dams were killed on day 21 of gestation. No
abortions occurred, but there was a significant increase in the
percent of litters with resorptions (5.5% in controls, 20% at 5 mg/kg
bw per day, and 23% at 10 mg/kg bw per day) and increased fetal
mortality, although this effect was slight and was not dose-related
(0, 2, and 1 in the three groups, respectively). Slight increases in
the incidences of cerebral hypoplasia and enlargement of the renal
pelvis were observed on visceral examination, but these effects were
not considered to be related to treatment as they were also seen in
control animals and the increases were small and were not
dose-dependent. No other increase in the incidence of visceral
abnormalities was reported. Skeletal examination showed statistically
significant increases in the incidences of absent fifth sternebrae and
of fetuses with incomplete ossification. A slight increase in the
incidence of absent fifth metacarpus, although not statistically
significant, was also seen in treated animals. These effects were not
considered to be related to treatment, as their magnitude was small
and they were not dose-dependent. No maternal toxicity was seen at any
dose. The level of reporting in this published paper was inadequate
for identifying a NOAEL for developmental toxicity (Gupta et al.,
1978).
Groups of 25 mated CD Sprague-Dawley rats were given
technical-grade endosulfan (purity, 97.3%) in corn oil by gavage on
days 6-19 of gestation at a dose of 0, 0.66, 2, or 6 mg/kg bw per day.
The clinical signs in dams at 6 mg/kg bw per day included flaccidity,
rough coat, alopecia, and hyperactivity. A dose-related decrease in
maternal body-weight gain was seen at 2 and 6 mg/kg bw per day. The
number of implantations and litter size were unaffected, but there was
a slight reduction in the weight and length of fetuses of dams at the
high dose. A non-dose-related reduction in the percent of live fetuses
and an increase in the number of resorbed fetuses were seen at 2 mg/kg
bw per day. No statistically significant treatment-related effect on
the sex ratio was observed. No external variations or malformations
were seen at 0.66 or 2 mg/kg bw per day; at the high dose, 5/405
fetuses exhibited lordosis (anteroposterior curvature of the spine)
and six had oedema. All five of the fetuses with lordosis and five of
those with oedema were from a single litter. In one fetus from the
same litter, the skin of the upper forelimb was webbed to the chest.
No significant treatment-related effects were seen on soft-tissue
development. Common minor skeletal variations were present in all
groups. The incidence of poorly ossified sixth sternebrae was
significantly greater in animals at the high dose than in the control
group, and two fetuses at this dose had clubbed left hindlimbs. The
five fetuses from the same litter that had oedema and lordosis also
had wide, thickened vertebral arches, ribs, and clavicles, and the
clavicles were shortened, curved, and twisted. Four of these fetuses
had shortened pubes, and two had an unossified hyoid bone. The
incidence of these effects was generally < 1%, and the effects were
largely related to delayed development and confined mainly to a single
litter from a single dam that showed numerous signs of poisoning
related to administration of endosulfan, including face rubbing,
alopecia, flaccidity, and hyperactivity. The developmental effects are
therefore probably related to the maternal toxicity of the high dose.
The NOAEL for maternal toxicity was 0.66 mg/kg bw per day on the basis
of decreased body-weight gain and clinical signs at higher doses. The
NOAEL for developmental toxicity was 2 mg/kg bw per day on the basis
of delayed development and a low incidence of isolated skeletal
variations (Mackenzie, 1980).
Groups of 20-24 mated female Wistar rats were given
technical-grade endosulfan (purity, 97.3%) dissolved in sesame oil by
gavage on days 7-16 of gestation at doses of 0, 0.66, 2, or 6 mg/kg bw
per day. No clinical signs of toxicity were reported in females at
0.66 or 2 mg/kg bw per day; four dams at 6 mg/kg bw per day died after
6-10 doses of endosulfan, three of these rats having tonic-clonic
convulsions for several days before death. Thirteen of the surviving
animals had tonic-clonic convulsions for a number of days, generally
around day 10 of gestation. Some of these rats also showed
hypersalivation on a number of days during treatment. Statistically
significant decreases in body weight and body-weight gain were
observed at 6 mg/kg bw per day. No statistically significant changes
in reproductive or pup parameters were observed at any dose, and the
fetal sex ratio was relatively well balanced. No statistically
significant increase in the incidence of abnormalities was observed in
fetuses. A single oedematous, retarded fetus at 6 mg/kg bw per day
presented with superior brachygnathia and a relatively small alveolar
cavity in the upper jaw, combined with cleft palate, bending of both
hind feet in the tarsal joint, wavy clavicles, and bent and shortened
scapulae. These effects were considered to be spontaneous, as no other
limb or head defects were observed in any pup in any of the litters at
this dose. Skeletal examination revealed a statistically significant
increase in the incidence of fragmented thoracic vertebral centra at 6
mg/kg bw per day. This effect was considered to be treatment-related
and reflects the frank maternal toxicity of endosulfan at the high
dose. No treatment-related major malformations were observed. The
NOAEL for maternal toxicity was 2 mg/kg bw per day on the basis of
deaths, clinical signs, and decreased body weights at higher doses.
The NOAEL for developmental toxicity was 2 mg/kg bw per day on the
basis of the increased incidence of fragmented thoracic vertebral
centra (Albrecht & Baeder, 1993).
Rabbits
Groups of 20-26 mated New Zealand white rabbits were given
technical-grade endosulfan (purity, 97.3 %) in corn oil by gavage on
days 6-28 of gestation at doses of 0, 0.3, 0.7, or 1.8 mg/kg bw per
day. There were no changes in mean body weight. None of the does at
0.3 or 0.7 mg/kg bw per day aborted, and there were no signs of
toxicity and no deaths. The high dose was associated with signs of
maternal toxicity that included noisy, rapid breathing, hyperactivity,
and convulsions. The does were killed on day 29 of gestation. The
number of implantations, litter size, sex ratio, mean fetal weight and
length, and the numbers of live and resorbed fetuses were unaffected
by treatment. There were no dead fetuses in any group, and no gross
external alterations were reported. The only soft-tissue anomalies
occurred in 6/167 fetuses (2/20 litters examined) at the high dose and
consisted of the left carotid arising from the innominate; 1/141
control fetuses (1/18 litters examined) also showed this abnormality.
Common skeletal variations and minor anomalies occurred at similar
incidences in control and treated fetuses. Endosulfan did not have
teratogenic or developmental effects even at the maternally toxic dose
of 1.8 mg/kg bw per day. The NOAEL for maternal toxicity was 0.7 mg/kg
bw per day on the basis of clinical signs at higher doses (Dickie et
al., 1981).
(f) Special studies
(i) Enzyme induction
Endosulfan was one of 16 organochlorine pesticides tested for
their ability to induce hepatic microsomal enzyme activities. Wistar
rats were given diets containing endosulfan at concentrations of 0,
20, 50, or 200 ppm for two weeks. No difference from control enzyme
activity was observed at 20 or 50 ppm; at 200 ppm group, the
activities in comparison with the control were 123% for aniline
hydroxylase (statistically significant, one experiment), 191% for
aminopyrene demethylase (statistically significant, one experiment),
and 124% for hexobarbital oxidase (not significant, one experiment)
(den Tonkelaar & van Esch, 1974).
ICR mice given endosulfan at 5 mg/kg bw per day by oral gavage
for three days and killed on the fourth day showed no increase in
liver weight or total hepatic cytochrome P450 content. The dearylation
of O-ethyl O- para-nitrophenyl phenylphosphorothioate and
parathion and NAD(P)H-dependent reductase activity were
nonsignificantly increased (Robacker et al., 1981).
(ii) Promotion
Enhancement of gamma-glutamyl transpeptidase-positive foci in rat
liver was studied as an indicator of promotion. Young male
Sprague-Dawley rats were partially hepatectomized and injected
intraperitoneally 24 h later with N-nitrosodiethylamine at 30 mg/kg
bw. One week after the partial hepatectomy, the rats were randomized
to groups of 10 or 11 and dosed orally by gavage on five days per week
for 10 weeks with endosulfan in corn oil at 0, 1, or 5 mg/kg bw per
day. The numbers and volume of enzyme-altered foci were not increased
in the treated groups in comparison with controls.
In the same study, inhibition of intercellular communication was
studied in Chinese hamster lung V79 cells in a metabolic cooperation
assay and in rat liver WB epithelial cells in a scrape loading,
dye-transfer assay. At nontoxic concentrations, technical-grade
endosulfan, analytical-grade ensosulfan (alpha and ß isomers and a
mixture of the two), and endosulfan sulfate inhibited gap-junctional
intercellular communication in both systems. In addition, endosulfan
ether was effective in the rat liver WB cell system (Flodström et al.,
1988).
(iii) Immunotoxicity
In two published studies, endosulfan was administered to male
Wistar rats at dietary doses of up to 50 ppm for six weeks (Banarjee &
Hussain, 1987) or up to 20 ppm for 22 weeks (Banarjee & Hussain, 1986)
to evaluate humoral and cell-mediated immune responses. In the
six-week study, a significant decrease in total serum antibody titre
to tetanus toxoid was seen at 30 and 50 ppm, with a slight decrease
(not statistically significant) at 10 ppm. A decrease in both
immunoglobulin (Ig)M and IgG and in the total gamma-globulin content
of rat serum was observed at 50 ppm. Cellular immunity was assayed by
measuring inhibition of migration of activated leukocytes and
macrophages. Rats exposed to endosulfan and subsequently immunized
with tetanus toxoid showed a significant decrease in inhibition of
leukocyte and macrophage migration in a dose-dependent pattern, the
decrease becoming statistically significant at 30 and 50 ppm. These
results indicate that both humoural and cellular immunity was
depressed as a result of exposure to endosulfan at 30 and 50 ppm, with
no effect at 10 ppm, equivalent to 0.5 mg/kg bw per day. In the
22-week study, the specific response of serum antibody titre to
tetanus toxoid showed a marked decrease in rats exposed to 10 or 20
ppm endosulfan throughout the experiment, in a dose- and
time-dependent pattern. Treatment at 10 or 20 ppm diminished the
inhibition of migration of both leukocytes and macrophages throughout
the study.
The effect of endosulfan on the immune system did not appear to
be secondary to other toxic effects, since the body weights of the
animals were unaffected by treatment and endosulfan is not known to
affect the hormonal system. Immune responses were unaffected by
treatment at 5 ppm, equivalent to 0.25 mg/kg bw per day. The Committee
noted that the method used to assess cellular immunity in these
studies is far from ideal as it is flawed by large inherent errors,
lack of objectivity, and, except in very experienced hands, lack of
accuracy. Less subjective tests of cellular immunity, such as
cytotoxic T cell response to a virus, would have provided more
reliable results.
Technical-grade endosulfan (purity, 96%) was administered in
sesame oil on 10 occasions by gavage to groups of eight female Wistar
rats at doses of 0.5, 1.5, or 4.5 mg/kg bw per day from two days
before until seven days after infection by gavage with approximately
500 Trichonella spiralis larvae. As a positive control, prednisolone
was administered by subcutaneous injection at a dose of 25 mg/kg bw
per day two days before and three days after infection. Three rats
from each group were killed seven days after infection so that the
number of adult worms in the intestine could be counted; the remaining
rats were killed 54 days after infection in order to count the number
of larvae in the tongue. Thymus and spleen weights and the percentage
lymphocytes in the white cell count were measured at both times. Body
weights were measured weekly. There were no differences between
endosulfan-treated and untreated rats, whereas the
prednisolone-treated group had a sevenfold higher tongue larval count
and, at seven days, a 25% reduction in thymus weight, a 50% reduction
in spleen weight, and lymphocyte counts < 50% of the control value
(Hack & Leist, 1988).
Endosulfan was included in the first part of a study to screen
for immunotoxicity, but because no effect was observed it was not
examined in greater detail. Groups of six male, weanling Wistar rats
were given endosulfan in the diet at concentrations of 20, 100, or 250
ppm for three weeks. Body weights and food intake were recorded
weekly. At autopsy, the weights of the liver, kidneys, spleen, thymus,
pituitary, adrenals, thyroid, testis, and mesenteric and popliteal
lymph nodes were recorded, these organs were also examined
histologically. Haematological examination consisted of total and
differential leukocyte counts. Serum IgM and IgG were determined by
enzyme-linked immunosorbent assay. The only effects induced by
endosulfan were considered to be expressions of general toxicity, and
there was no evidence for any specifically immunotoxic effects. The
most sensitive parameter for the toxicity of endosulfan was a
reduction in body-weight gain, which was observed at 100 ppm (Vos et
al., 1982).
(iv) Neurobehavioural effects and neurotoxicity
In a number of studies conducted by the same group of
investigators, endosulfan (purity, 95%) was given by gavage to rats at
a dose of 2 mg/kg bw per day for 90 days (Paul et al., 1993, 1994) or
up to 6 mg/kg bw per day for 30 days (Paul et al., 1995), and
behavioural and biochemical changes were determined. Signs of frank
toxicity (reduced body weights, reduced food consumption, death,
increased intensity of tremors, and increased liver enzyme activity)
were observed in all studies, and some changes in behaviour were
noted, including increased motor activity and inhibition of
conditioned and unconditioned escape and avoidance responses.
Groups of adult domestic hens were given a single oral dose of 96
mg/kg bw endosulfan, the LD50, in corn oil, observed for 21 days,
re-dosed, and again observed for 21 days. As was to be expected, a
number of deaths occurred that were related to treatment. No signs of
ataxia and no treatment-related changes in nervous tissue were seen in
vehicle control or endosulfan-treated hens. Seven of 10 hens in a
concurrent positive control group given tri- ortho-cresyl phosphate
developed ataxia and signficant spinal cord and peripheral nerve
degeneration (Roberts et al., 1983).
No inhibition of rat brain acetylcholinesterase activity was
observed in a preparation incubated with 10 µmol/L alpha-endosulfan
for up to 75 min.. A similar concentration of aldicarb produced 15%
inhibition within 5 min and 80% inhibition within 75 min (Müllner,
1989).
Technical-grade endosulfan (purity, 98.6%) was administered to
groups of 10 rats as a single dose of 25, 50, or 100 mg/kg bw to males
and 3, 6, or 12 mg/kg bw to females. Deaths occurred at the highest
doses, and there was a dose-related increase in the frequency of
clinical signs, which were reversible and apparent only on the day of
dosing. These were assumed to be due to the known affinity of
endosulfan for the gamma-aminobutyric acid receptors in the brain. At
50 and 100 mg/kg bw in males and 6 and 12 mg/kg bw in females, various
serious neuropharmacological effects were seen, including coarse
tremor and tonic-clonic convulsions. At 25 mg/kg bw in males and
3 mg/kg bw in females, the clinical signs seen were typical of general
discomfort, such as stilted gait, squatting posture, and irregular
respiration. No compound-related effects on motor activity were
observed at non-lethal doses. No effects were seen on the rearing
frequency, fore- or hindlimb grip strength, or on landing foot-spread.
No histopathological effects were found in the central or peripheral
nervous system. The study was carried out in accordance with
prevailing OECD testing guidelines (April 1996) and the OECD
principles of good laboratory practice (12 May 1981 [C(81)30(Final)])
(Bury, 1997).
(v) Effects on sperm
In a study of biochemical changes induced by endosulfan in the
testis of Druckrey rats, the authors postulated that endosulfan
impairs testicular function by altering the enzyme activities
responsible for spermatogenesis, thus affecting the intratesticular
spermatid count and resulting in low sperm production and increased
sperm deformities. The data presented support the notion that
administration of endosulfan at relatively high doses (> 2.5 mg/kg
bw per day) for several months increases the activity of a number of
enzymes in the testes, including lactate dehydrogenase, sorbitol
dehydrogenase, gamma-glutamyl transpeptidase, and glucose-6-phosphate
dehydrogenase. At doses of 5 mg/kg bw per day and higher, there was a
marked reduction in sperm count (up to 47%) in comparison with
controls. In the absence of historical control data, it is unclear
whether the decrease in sperm count seen at 2.5 mg/kg bw per day (22%)
was within the normal biological range for the test animals. The sperm
abnormalities and the reductions in spermatid count and sperm
production, while statistically significantly different from those in
concurrent controls, were only slight. In the absence of consistent
dose-response relationships for these effects, they were considered
not biologically significant (Sinha et al., 1995).
The effects of endosulfan on gonadal hormones, measured as plasma
and testicular testosterone, plasma follicle-stimulating and
luteinizing hormones, and plasma and testicular 3ß- and
17ß-hydroxysteroid dehydrogenases, was investigated in Wistar rats
given endosulfan in peanut oil at doses up to 10 mg/kg bw per day for
up to 30 days. Significant inhibition of 3ß- and 17ß-hydroxysteroid
dehydrogenases occurred in the testes of treated animals after 30 days
of treatment, and the plasma follicle-stimulating hormone, luteinizing
hormone, and testosterone concentrations were significantly
(p < 0.05) reduced in rats treated for 15 or 30 days at either
dose. Plasma and testicular testosterone concentrations were not
significantly reduced after 15 days of treatment at 7.5 mg/kg. A
significant decrease in the content or activity of microsomal
cytochrome P450 and related mixed-function oxidases was observed in
the testes of treated animals, with marked inhibition of the activity
of glutathione- S-transferase at both doses. The latter changes were
reversed when endosulfan was withdrawn, but the testicular
testosterone concentrations remained significantly reduced (Singh &
Pandey, 1990).
(vi) Endocrine effects
An estrogen is a substance that can induce estrus or a biological
response associated with estrus; one such effect is proliferation of
cells in the female genital tract. In recent years, naturally
occurring and man-made substances in the environment that may be
estrogenic have come under increasing scrutiny. Suspicion that
endosulfan may have estrogenic properties was stimulated by
observation of the reduced sperm counts described above and of
testicular atrophy in rats given endosulfan in the diet in long-term
studies.
Perhaps the first study of estrogenic effects in vivo was
conducted by Raizada et al. (1991), who treated groups of eight
ovariectomized Wistar rats, weighing an average of 100 g, with
endosulfan at 1.5 mg/kg bw per day by gavage, estradiol dipropionate
intraperitoneally (dose unspecified), or a combination of these
treatments for 30 days. Endosulfan did not change the weights of the
uterus, cervix, or vagina, whereas estradiol propionate produced large
increases. The increased weights seen with the combined treatment were
similar to those with estradiol propionate alone.
Concern that endosulfan might be estrogenic persisted as a result
of the findings of an 'E-screen' assay, which was developed to assess
the estrogenic effects of environmental chemicals by observing their
proliferative effect on a target cell. The numbers of cells present
after similar inocula of the human breast cancer cell line, MCF-7,
were compared in the absence of estrogens (negative control), in the
presence of estradiol-17ß (positive control), and with a range of
concentrations of endosulfan. In this assay, endosulfan was estrogenic
at concentrations of 10-25 µmol/L, with a proliferative effect about
80% that of 17ß-estradiol at 10 µmol/L; the relative proliferative
potency (i.e. the ratio of the doses of endosulfan and estradiol-17ß
required to produce the maximum effect) was 0.0001%. In addition,
endosulfan competed with estradiol-17ß for binding to the estrogen
receptor and increased the concentrations of progesterone receptor and
pS2 in MCF-7 cells, as would be expected for a compound that mimics
estrogens (Soto et al., 1994, 1995).
As part of a study to optimize investigations of estrogenic
activity, endosulfan and nine other chemicals (17ß-estradiol,
diethylstilbestrol, tamoxifen, 4-hydroxytamoxifen, methoxychlor,
the methoxychlor metabolite
2,2-bis (para-hydroxyphenyl)-1,1,1-trichloroethane, nonylphenol,
ortho, para'-DDT, and kepone) with known or suspected estrogenic
activity were tested in three assays: competitive binding to the mouse
uterine estrogen receptor, transcriptional activation in HeLa cells
transfected with plasmids containing an estrogen receptor and a
response element, and the uterotropic assay in mice. The results of
the three assays were consistent with respect to the known estrogenic
activities of the chemicals tested and their requirements for
metabolic activation. There was no evidence from any of these tests
that endosulfan is estrogenic (Shelby et al., 1996).
A much publicized report indicated that even estrogens of low
potency, such as endosulfan, could have important effects because of
interaction with other chemicals. The estrogenic potencies of
combinations of chemicals were screened in a system in which the human
estrogen receptor sequence is incorporated into the yeast genome.
Combinations of two weak environmental estrogens, such as dieldrin,
endosulfan, and toxaphene, were 1000 times more potent in human
estrogen receptor-mediated transactivation than any chemical alone
(Arnold et al., 1996). This result was not reproduced in another
laboratory in which the same assay was used or in a uterotropic assay
in which sexually immature rats were treated with endosulfan or
dieldrin alone or in a combination on three successive days and the
uterine mass weighed on the following day. The highest doses used in
the human estrogen receptor assay were determined by the solubility of
the compounds, and the highest doses in the uterotropic assay were 100
mg/kg bw for endosulfan or dieldrin alone and 75 mg/kg bw of each in
combination. Both chemicals were inactive in both assays, and there
was no evidence of synergism (Ashby et al., 1997). In a further study
with the human estrogen receptor assay, however, 0.1 mmol/L endosulfan
increased the activity of ß-galactosidase (Ramamoorthy et al., 1997).
More doubt was cast upon the thesis of synergism by an
independent study in which endosulfan and dieldrin showed no additive
effect in displacing 3H-17ß-estradiol from rat uterine estrogen
receptors or in inducing the proliferation of MCF-7 breast cancer
cells. The weak proliferative potential described by Soto et al.
(1994, 1995) was, however, confirmed in this assay in vitro.
Endosulfan or dieldrin alone at 3 mg/kg bw per day or in combination,
injected intraperitoneally daily for three days, did not stimulate
uterotrophic activity and had no effect on pituitary prolactin or
other endocrine-related end-points in immature female rats, indicating
that these weakly estrogenic compounds do not interact in a
synergistic fashion in binding to estrogen receptors or in activating
estrogen receptor-dependent responses in mammalian tissues or cells
(Wade et al., 1997). The paper in which synergism was originally
proposed was later withdrawn, since the results could not be
reproduced, even in the same laboratory (McLachlan, 1997). Overall,
these results suggest that concomitant exposure to weakly estrogenic
compounds probably does not result in reproductive toxicity related to
estrogen action.
3. Observations in humans
In general, the doses of endosulfan involved in cases of
poisoning have been poorly characterized. In a summary of case reports
(Lehr, 1996), the lowest reported dose that resulted in death was 35
mg/kg bw; deaths have also been reported after ingestion of 295 and
467 mg/kg bw, within 1 h of ingestion in some cases. Intensive medical
treatment within 1 h was reported to be successful after ingestion of
doses of 100 and 1000 mg/kg bw. The clinical signs in these patient
were consistent with those seen in laboratory animals, dominated by
tonic-clonic spasms. In a case in which a dose of 1000 mg/kg bw was
ingested, neurological symptoms requiring anti-epileptic therapy were
still required one year after exposure.
Comments
More than 90% of an oral dose of endusulfan was absorbed in rats,
with maximum plasma concentrations occurring after 3-8 h in males and
about 18 h in females. Elimination occurs mainly in the faeces and to
a lesser extent in the urine, more than 85% being excreted within 120
h. The highest tissue concentrations were in the kidneys. The
metabolites of endosulfan include endosulfan sulfate, diol,
hydroxy-ether, ether, and lactone but most of its metabolites are
polar substances which have not yet been identified. Endosulfan would
not be expected to accumulate significantly in human tissues. No data
on plant metabolites were available to the Meeting.
A battery of tests for acute toxicity in several species with
technical-grade endosulfan showed that it is highly toxic after oral
or dermal administration, with respective LD50 values of 10-160 mg/kg
bw and 45-135 mg/kg bw. The LC50 value for rats in a single study was
13 mg/m3 in females and 35 mg/m3 in males. Endosulfan, administered
by any route, is more toxic to female than to male rats. Clinical
signs of acute intoxication include piloerection, salivation,
hyperactivity, respiratory distress, diarrhoea, tremors, hunching, and
convulsions.
WHO has classified endosulfan as moderately hazardous (WHO,
1996).
The kidney is the target organ for toxicity. The renal effects
include increased renal weights and granular pigment formation after
short-term administration and progressive, chronic glomerulo-nephrosis
or toxic nephropathy after long-term exposure, although the
observation of progressive glomerulonephrosis is complicated by the
fact that this is a common lesion in ageing laboratory rats and occurs
at high incidence in control rats.
In a 90-day feeding study in rats, the cytoplasm of isolated
cells in the renal proximal convoluted tubules had a yellowish colour,
particularly in males, at all dietary concentrations from 10 ppm. The
presence of this yellow pigmentation was largely reversible during a
four-week recovery period, and it did not appear to indicate
nephrotoxicity. A darker, more particulate, granular and/or clumped
pigment was also observed, predominantly in cells of the straight
portions and occasionally in the proximal convoluted tubules, at
dietary concentrations of 30 ppm and above. This darker pigment was
more persistent than the yellow one, and urinalysis revealed darker
urine and marginally more ketones at doses from 60 ppm, and marginally
more protein, particularly in males, indicating renal damage at doses
of 360 ppm and above. Similar findings emerged from a multigeneration
study but not from a two-year study of carcinogenicity in rats. The
changes in pigmentation were considered to be due to the presence of
endosulfan and/or its metabolites in the enlarged lysosomes. To test
this hypothesis, a four-week feeding study was conducted in which male
rats were given dietary concentrations of 360 or 720 ppm endosulfan.
Light and electron microscopy of the kidneys of these animals clearly
showed increases in the number of lysosomes and the size of cells in
the convoluted tubule, probably as a result of accumulation of the
test material and/or its metabolites. Lysosomal changes were not
observed in either brain or liver, and the renal changes receded
appreciably during a 30-day recovery period. Chemical analysis of the
kidneys indicated the presence of alpha-endosulfan and, to a lesser
extent ß-endosulfan sulfate, and endosulfan lactone. The
concentrations of the dominant alpha-endosulfan in the kidneys were
about 50 times those in the liver. The concentrations in blood were
usually below the level of detection. After the 30-day recovery
period, renal alpha-endosulfan was detected only in traces and ß-
endosulfan not at all. Similar analysis of tissues from rats in the
two-year study of toxicity and carcinogenicity did not reveal the
presence of these substances in the kidney, although measurable alpha-
endosulfan was found in the liver at 75 ppm. The yellow colour
therefore indicates the presence of endosulfan and/or its metabolites,
rather than either a stage in the pathogenesis of nephropathy or an
independent expression of toxicity. It was postulated that in longer
studies its removal from lysosomes is accelerated by enzyme induction,
which has not been investigated.
In a 78-week study, exposure of rats to endosulfan at a high dose
of 20 mg/kg bw per day resulted in testicular atrophy, characterized
by degeneration and necrosis of the germinal cells lining the
seminiferous tubules. In addition, decreased sperm counts accompanied
by an increased incidence of sperm abnormalities have been reported in
mice, again at high doses of endosulfan. Reductions in the activities
of some testicular xenobiotic-metabolizing enzymes and some hormones
that are necessary for normal testicular function were also seen in a
30-day study in rats at 10, but not at 7.5 mg/kg bw per day. The
functional significance of these findings was not clear, as studies of
reproductive and developmental toxicity in rats and rabbits showed
neither impaired fertility nor any increase in the incidence of
defects or abnormalities in offspring. Given the high doses at which
these testicular effects were observed, it would appear that they are
of little human significance.
No genotoxic activity was observed in an adequate battery of
tests for mutagenicity and clastogenicity in vitro and in vivo.
The Meeting concluded that endosulfan is not genotoxic.
No carcinogenic effect was observed in mice at 18 ppm for 24
months, in female rats at 445 ppm for 78 weeks in one study or in male
or female rats at 75 ppm or 100 ppm for two years in two other
studies. The Meeting noted the differences in the dietary
concentrations used in these studies, but non-neoplastic responses
were seen even at the lower doses.
Endosulfan at dietary concentrations of 0, 3, 15, or 75 ppm did
not affect reproductive performance or the growth or development of
the offspring of rats over the course of a two-generation study. The
NOAEL was 75 ppm, the highest dose tested, equal to 5 mg/kg bw per day
for males and 6.2 mg/kg bw per day for females. The NOAEL for parental
toxicity was 15 ppm, equal to 1 mg/kg bw per day for males and 1.2
mg/kg bw per day, on the basis of increased liver and kidney weights
at 75 ppm.
In two studies of developmental toxicity in rats given oral doses
of 0, 0.66, 2, or 6 mg/kg bw per day, the NOAEL for maternal toxicity
was 0.66 mg/kg bw per day in one study and 2 mg/kg bw per day in the
other. In the first case, the basis was decreased body-weight gain at
2 mg/kg bw per day and decreased body-weight gain and clinical signs
of toxicity at 6 mg/kg bw per day; in the second case, the basis was
mortality, clinical signs of toxicity, and decreased body-weight gain
at 6 mg/kg bw per day. In both studies, the NOAEL for developmental
toxicity was 2 mg/kg bw per day, in the first case on the basis of
delayed development and a low incidence of skeletal variations seen at
6 mg/kg per day and in the second on the basis of an increased
incidence of fragmented thoracic vertebral centra seen at 6 mg/kg bw
per day. In neither study was there any treatment-related major
malformation.
In a study of developmental toxicity in rabbits given oral doses
of 0, 0.3, 0.7, or 1.8 mg/kg bw per day, the NOAEL for maternal
tocicity was 0.7 mg/kg bw per day on the basis of clinical signs of
toxicity at 1.8 mg/kg bw per day. The NOAEL for developmental toxicity
was 1.8 mg/kg bw per day, the highest dose tested.
Several recent studies have shown that endosulfan, alone and in
combination with other pesticides, may bind to estrogen receptors and
may perturb the endocrine system. The available studies show only very
weak binding to hormone receptors in vitro, and the evidence for
their relevance to adverse physiological effects in vivo is
extremely limited. Long-term assays of toxicity and studies of
reproductive and developmental toxicity in experimental mammals did
not indicate that endosulfan induces functional aberrations that might
result from loss of endocrine homeostasis.
The absence of immunotoxic effects in a large number of bioassays
with endosulfan suggested that it does not have an adverse effect on
the immune function of laboratory animals. However, in two studies,
rats given endosulfan in the diet at 30 or 50 ppm for 6 weeks or 20
ppm for 22 weeks had reduced serum titres of tetanus toxoid antibody
and reduced immunoglobulins G and M, and inhibition of migration of
both leukocytes and macrophages. These findings have not been
confirmed.
In a summary of case reports of human poisoning incidents, the
lowest reported dose that caused death was 35 mg/kg bw. Higher doses
caused death within 1 h. The clinical signs in these patients were
dominated by tonic-clonic convulsions, consistent with the
observations in experimental animals.
An ADI of 0-0.006 mg/kg bw was established on the basis of the
NOAEL of 0.6 mg/kg bw per day in the two-year dietary study of
toxicity in rats and a safety factor of 100. The ADI is supported by
similar NOAEL values in the 78-week dietary study of toxicity in mice,
the one-year dietary study of toxicity in dogs, and the study of
developmental toxicity in rats.
An acute RfD of 0-0.02 mg/kg was established on the basis of the
NOAEL of 2 mg/kg bw per day in the study of neurotoxicity in rats and
a safety factor of 100.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 3.9 ppm, equal to 0.58 mg/kg bw per day (females in a
78-week study of toxicity)
Rat: 15 ppm, equal to 0.6 mg/kg bw per day (two-year dietary
study of toxicity)
75 ppm, equal to 6 mg/kg bw per day (reproductive
toxicity)
0.66 mg/kg bw per day (maternal toxicity in a study of
developmental toxicity)
2 mg/kg bw per day (fetotoxicity in a study of
developmental toxicity)
Rabbit: 0.7 mg/kg bw per day (maternal toxicity in a study of
developmental toxicity)
Dog: 10 ppm, equivalent to 0.57 mg/kg bw per day (one-year
study of toxicity)
Estimate of acceptable daily intake for humans
0-0.006 mg/kg bw
Estimate of acute reference dose for humans
0.02 mg/kg bw
Studies that would provide information useful for continued
evaluation of the compound
1. Studies of immunotoxicity with standard test protocols
2. Studies of the significant sex difference in acute toxicity,
particularly in rats
3. Further observations in humans
List of end-points relevant for setting guidance values for dietary and non-dietary exposure
Absorption, distribution, excretion, and metabolism in mammals
Rate and extent of absorption Rat: oral, > 90% absorption; max. concentration at
3-8 h (males) 18 h (females)
Distribution Mainly in kidney and liver
Potential for accumulation Low
Rate and extent of excretion Biphasic; urinary half-life was 6 h for 1st phase,
33-68 h for 2nd phase; faecal half-life was 10 h and
30 h; > 85% excretion within 120 h
Metabolism in animals Oxidation and hydrolysis; unidentified polar
metabolites
Toxicologically significant Parent; no data on plant metobolites
compounds (animals, plants and
environment)
Acute toxicity
Rat: LD50 oral 10 mg/kg bw (female)
Rat: LD50 dermal 500 mg/kg bw (female)
Rat: LC50 inhalation 13 mg/m3 4 h (female)
Skin irritation Not irritating
Eye irritation Not irritating
Skin sensitization Not sensitizing
Short-term toxicity
Target/critical effect Reduced survival, convulsions, salivation
Lowest relevant oral NOAEL Rat: 0.64 mg/kg bw per day, dietary
Lowest relevant dermal NOAEL Rat: 3 mg/kg bw per day
Lowest relevant inhalation NOAEL Rat: 2 mg/m3, no effect (highest concentration)
Genotoxicity Not genotoxic
Long-term toxicity and carcinogenicity
Target/critical effect Kidney
Lowest relevant NOAEL Rat: 0.6 mg/kg bw per day, 2-year study
Carcinogenicity Not carcinogenic
Reproductive toxicity
Reproduction target: critical effect None identified
Lowest relevant reproductive NOAEL Rat: 6 mg/kg bw per day
Developmental target /critical effect Fetoxicity at maternally toxic doses
Lowest relevant developmental NOAEL Rat: 2 mg/kg bw per day
Neurotoxicity/Delayed neurotoxicity Rat: 1.5 mg/kg bw (female); 12.5 mg/kg bw (male)
no effect
Other toxicological studies Immunotoxicity in certain special assays, not
confirmed in sensitization test or histologically
Some conflicting evidence of interaction with
estrogen receptors in vitro; none in vivo
Medical data Lowest lethal dose: 35 mg/kg bw, oral
Summary Value Study Safety factor
ADI 0-0.006 mg/kg bw Several different species 100
and end-points
Acute reference dose 0.02 mg/kg bw Study of neurotoxicity in 100
rats
References
Albrecht, M. & Baeder, C. (1993) Hoe 002671-substance technical (code:
Hoe 002671 00 ZD98 0005). Testing for embryotoxicity in the Wistar rat
after oral administration. Unpublished report No. 93.0716, document
A51695. Submitted to WHO by Hoechst Schering AgrEvo GmbH,
Frankfurt-am-Main, Germany.
Arai, M., Hiromori ,T., Ho, S., Okuno, Y. & Miyamoto, J. (1981) Life
span chronic toxicity study of endosulfan in mice -- 12 month report.
Unpublished report No. 0285, at-10 from Sumitomo Chemical Co Ltd,
Japan, 28 April 1981. Submitted to WHO by Hoechst Schering AgrEvo
GmbH, Frankfurt-am-Main, Germany.
Arcelin, G. (1996) Contact hypersensitivity to endosulfan (Code: Hoe
002671 00ZD99 0008) in albino guinea pigs maximumization test.
Unpublished report No. 630753 from RCC, Itingen, Switzerland. Hoechst
document A58132.A51695. Submitted to WHO by Hoechst Schering AgrEvo
GmbH, Frankfurt-am-Main, Germany.
Arnold, S.F., Klotz, D.M., Collins, B.M., Vonier, P.M., Guilette, L.J.
& McLachlan, J.A. (1996) Synergistic activation of oestrogen receptor
with combinations of environmental chemicals. Science, 272,
1489-1492.
Ashby, J., LeFevre, P.A., Odum, J., Harris, C.A., Routledge, E.J. &
Sumpter, J.P. (1997) Synergy between synthetic oestrogens? Nature,
385, 494.
Asquith, J.C. & Baillie, J.H. (1989) Endosulfan substance technical
(code Hoe 002671 0I ZD95 0005). Metaphase analysis of human
lymphocytes. Unpublished report No. m/hl/1307 from Toxicol
Laboratories, United Kingdom, 16 March 1989. Hoechst document A40411.
Submitted to WHO by Hoechst Schering AgrEvo GmbH, Frankfurt-am-Main,
Germany.
Banarjee, B.D. & Hussain, Q.Z. (1986) Effect of subchronic endosulfan
exposure on humoral and cell-mediated immune responses in albino rats.
Arch. Toxicol., 59, 279-284.
Banarjee, B.D. & Hussain, Q.Z. (1987) Effects of endosulfan on humoral
and cell-mediated immune responses in rats. Bull. Environ. Contam.
Toxicol., 38, 438-441.
Barnard, A.V., Atkinson, J.S., Heywood, R., Street, A.E, Gibson, W.A.,
Rao, R., Offer, J.M. & Gopinath, A.R.H. (1984) 13 week toxicity study
in mice. Unpublished report No. Hst 229/831052, 25 September 1984 from
Huntingdon Research Centre, Huntingdon, Cambridgeshire, United
Kingdom. Hoechst document A29663. Submitted to WHO by Hoechst Schering
AgrEvo GmbH, Frankfurt-am-Main, Germany.
Barnard, A.V., Jones, D.R., Powell, L.A.J., Heywood, R., Street, A.E.,
Gibson, W.A., Gopinath, C., Majeed, S.K. & Almond, R.H. (1985) 13-week
toxicity study in rats followed by a 4-week withdrawal period.
Unpublished report No. Hst 230/84176, 25 March 1985 from Huntingdon
Research Centre, Huntingdon, Cambridgeshire, United Kingdom. Hoechst
document A30700 Submitted to WHO by Hoechst Schering AgrEvo GmbH,
Frankfurt- am-Main, Germany.
Bhide, M.B. & Naik, P.Y. (1984a) The acute oral toxicity (LD50) of
Excel Industries Ltds endosulfan technical No. 2 to the albino rats.
Unpublished report from Indian Institute of Toxicology (report No. and
date not given). Submitted to WHO by the National Registration
Authority for Agricultural and Veterinary Chemicals, Australia.
Bhide, M.B. & Naik, P.Y. (1984b) The acute oral toxicity (LD50) of
Excel Industries Ltds endosulfan technical No. 1 to the albino mice.
Unpublished report from Indian Institute of Toxicology (report No. and
date not given). Submitted to WHO by the National Registration
Authority for Agricultural and Veterinary Chemicals, Australia.
Bhide, M.B. & Naik, P.Y. (1984c) The acute dermal toxicity (LD50) of
Excel Industries Ltds endosulfan technical No. 2 to the albino
rabbits. Unpublished report from Indian Institute of Toxicology
(report No. and date not given). Submitted to WHO by the National
Registration Authority for Agricultural and Veterinary Chemicals,
Australia.
Bowman, J.S. (1959) Preliminary report: Subacute feeding -- dairy
cows. Unpublished report from Hazleton Laboratories, USA. Hoechst
document No. A14205. Submitted to WHO by Hoechst Schering AgrEvo GmbH,
Frankfurt-am-Main, Germany.
Bracha, P. (1977) Thionex tech: Acute oral toxicity study in rats,
skin irritation study in rabbits, acute dermal toxicity study in
rabbits, eye irritation study in rabbits and acute inhalation study in
rats. Unpublished report No. 6111820 from Warf Institute Inc. Madison,
Wisconsin, USA. Submitted to WHO by Hoechst Schering AgrEvo GmbH,
Frankfurt-am-Main, Germany.
Bremmer, J.N. (1997a) Endosulfan: Substance technical (code: Hoe
002671 00 ZD99 0008) Testing for primary dermal irritation in the
rabbit. Unpublished Hoechst Marion Roussel preclinical development
study No. 96.0837. Hoechst document A58442. Submitted to WHO by
Hoechst Schering AgrEvo GmbH, Frankfurt-am-Main, Germany.
Bremmer, J.N. (1997b) Endosulfan: Substance technical (code: Hoe
002671 00 ZD99 0008) Testing for primary eye irritation in the rabbit.
Unpublished Hoechst Marion Roussel preclinical development study No.
96.0838. Hoechst document A58443. Submitted to WHO by Hoechst Schering
AgrEvo GmbH, Frankfurt-am-Main, Germany.
Bremmer, J.N. & Leist, K.-H. (1998) Endosulfan (AE F002671, substance
technical). Evaluation of the acute oral and dermal toxicity.
Unpublished report No. TOX98/003 from Hoechst Schering AgrEvo GmbH.
Hoechst document A59823. Submitted to WHO by Hoechst Schering AgrEvo
GmbH, Frankfurt-am-Main, Germany.
Brunk, R. (1989) Endosulfan-substance technical (code: Hoe 002671 00
ZD96 0002). Testing for toxicity by repeated oral administration
(1-year feeding study) to beagle dogs. Unpublished report No. 87.0643
from Pharma Research Toxicology and Pathology. Hoechst report No.
89.0188; document No. A40441 Submitted to WHO by Hoechst Schering
AgrEvo GmbH, Frankfurt-am-Main, Germany.
Brunk, R. (1990) Addendum to Hoechst report 89.0188; document No.
A44605. Submitted to WHO by Hoechst Schering AgrEvo GmbH,
Frankfurt-am-Main, Germany.
Bury, D. (1997) Endosulfan; substance, technical (code: Hoe 002671 00
ZD99 0008). Neurotoxicological screening in the male and female Wistar
rat. Acute toxicity. Unpublished report No. 96.0373 from Hoechst
Marion Roussel Preclinical Development, Germany. Hoechst report No.
A59088. Submitted to WHO by Hoechst Schering AgrEvo GmbH,
Frankfurt-am-Main, Germany.
Christ, O. & Kellner, H.M. (1968) Investigations with endosulfan-14C
in mice. Unpublished report No. ch/he-8412 from Hoechst Radiochemical,
Frankfurt, 31 December 1968. Hoechst document A53842, translation of
document A14217. Submitted to WHO by Hoechst Schering AgrEvo GmbH,
Frankfurt-am-Main, Germany.
Cifone, A.M. & Myhr, B.C. (1984a) Evaluation of Hoe 002671-substance
technical in the rat primary hepatocyte unscheduled DNA synthesis
assay. Unpublished LBI project No. 20991 from Litton Bionetics Inc.,
USA, November 1984. Hoechst document No. A29800. Submitted to WHO by
Hoechst Schering AgrEvo GmbH, Frankfurt-am-Main, Germany.
Cifone, A.M. & Myhr, B.C. (1984b) Mutagenicity evaluation of Hoe
002671-substance technical in the mouse lymphoma forward mutation
assay. Unpublished LBI project No. 20980 from Litton Bionetics Inc.,
USA, November 1984. Hoechst document No. A29801. Submitted to WHO by
Hoechst Schering AgrEvo GmbH, Frankfurt-am-Main, Germany.
Craine, E.M. (1986) A dermal absorption study in rats with
14C-endosulfan. Unpublished project No. Wil-39028, 11 December 1986
from Wil Research Lab, USA. Wil-39028. 11 Decemnber, 1986. Hoechst
document No. A35730. Submitted to WHO by Hoechst Schering AgrEvo GmbH,
Frankfurt-am-Main, Germany.
Craine, E.M. (1988) A dermal absorption study in rats with
14C-endosulfan with extended test duration. Unpublished project No.
Wil-39029 from Wil Research Lab, USA. Hoechst document No. A39677.
Submitted to WHO by Hoechst Schering AgrEvo GmbH, Frankfurt-am-Main,
Germany.
Deema, P. Thompson, E. & Ware, G. (1966) Metabolism, storage and
excretion of 14C-endosulfan in the mouse. J. Econom. Entomol., 59,
546-550.
Dickie, S.M., Mackenzie K.M. & Rao, G.N. (1981) Teratology study with
FMC 5462 in rabbits. Unpublished study No. 80070, 27 July 1981 from
Raltech Scientific Services. Hoechst document No. A23192. Submitted to
WHO by Hoechst Schering AgrEvo GmbH, Frankfurt-am-Main, Germany.
Diehl, K.H. & Leist, K.H. (1988a) Hoe 002671: Active ingredient
technical (code: Hoe 002671 0I ZD96 0002). Testing for acute oral
toxicity in the male and female Wistar rat. Unpublished study No.
88.0551, 27 July 1988, from Pharma Research Toxicology and Pathology,
Frankfurt, Germany. Hoechst document No. A39680. Submitted to WHO by
Hoechst Schering AgrEvo GmbH, Frankfurt-am-Main, Germany.
Diehl, K.H. & Leist, K.H. (1988b) Endosulfan-active ingredient
technical (code: Hoe 002671 0I ZD96 0002). Testing for acute dermal
toxicity in the male and female Wistar rat. Unpublished study No.
88.0552, 22 August 1988, from Pharma Research Toxicology and
Pathology, Germany. Hoechst document A39397, 1 September 1988.
Submitted to WHO by Hoechst Schering AgrEvo GmbH, Frankfurt-am-Main,
Germany.
Dikshith, T.S. & Datta, K.K. (1978) Endosulfan: Lack of cytogenetic
effects in male rats. Unpublished document No. A17140 from Industrial
Toxicology Research Centre. Submitted to WHO by Hoechst Schering
AgrEvo GmbH, Frankfurt-am-Main, Germany.
Dikshith, T.S.S., Raizada, R.B., Kumar, S.N., Srivastava, M.K.,
Kaushal, R.A., Singh, R.P. & Gupta, K.P. (1988) Effect of repeated
dermal application of endosulfan to rats. Vet. Hum. Toxicol., 30,
219-224.
Donaubauer, H.H. (1988) Endosulfan-substance technical (code: Hoe
002671 OI ZD97 0003). Carcinogenicity study in mice: 24 months feeding
study. Unpublished study No. 745 from Pharma Research Toxicology and
Pathology, Germany. Hoechst document No. A38008. Submitted to WHO by
Hoechst Schering AgrEvo GmbH, Frankfurt-am-Main, Germany.
Donaubauer, H.H. (1989) Amendment to the report No. 88.0278
endosulfan-substance technical (code: Hoe 002671 OI ZD97 0003).
Carcinogenicity study in mice: 24 months feeding study. Unpublished
study No. 745 from Pharma Research Toxicology and Pathology, Germany.
Hoechst document No. A41617. Submitted to WHO by Hoechst Schering
AgrEvo GmbH, Frankfurt-am-Main, Germany.
Donaubauer, H.H., Leist, K. & Kramer, M. (1985) Endosulfan-substance
technical (code: Hoe 002671 01 ZD97 0003). 42-Day feeding study in
mice. Unpublished study No. 744 from Pharma Research Toxicology,
Germany. Hoechst document No. A38104. Submitted to WHO by Hoechst
Schering AgrEvo GmbH, Frankfurt-am-Main, Germany.
Dorn, E. & Werner, H.-J. (1989) Endosulfan (Hoe 002671 OI ZD97 0003):
Determination of residues in the liver and kidneys of rats after
chronic (2-years) feeding (week 104). Unpublished Produktentwicklung,
GB-C Ökologie II Hoechst AG Study No. (Analytical part) CR065/88.
Hoechst document No. A41265. Submitted to WHO by Hoechst Schering
AgrEvo GmbH, Frankfurt-am-Main, Germany.
Dorough, M.W., Huhtanen, K., Marshall, T.C. & Bryant, H.E. (1978) Fate
of endosulfan in rats and toxicological considerations of apolar
metabolites. Pest. Biochem. Physiol., 8, 241-252.
Dzwonkowska, A. & Hübner, H. (1991) Studies on commercial insecticides
with the dominant lethal mutations test. Polish J. Occup. Med.
Environ. Health, 4, 43-53.
Ebert, E. & Weigand, W. (1984) Testing of the therapeutic effect of
diazapam (Valium R) and phenobarbital (Luminal R) in the event of
acute poisoning with endosulfan-active ingredient technical (code: Hoe
002671 oi ZD97 0003) in Wistar rats. Unpublished report No. 84.0062
from Pharma forschung Toxikologie. Hoechst document A29211. Submitted
to WHO by Hoechst Schering AgrEvo GmbH, Frankfurt-am-Main, Germany.
Ebert, E., Weigand, W. & Kramer, P. (1985a) Endosulfan-active
ingredient technical (code: Hoe 002671 01 ZD97 0003). Testing for
subchronic dermal toxicity (21 applications over 30 days) in SPF
Wistar rats. Unpublished study No. 83.0118 from Pharma forschung
Toxicologie. Hoechst document No. A30754, translation of document
A30751. Submitted to WHO by Hoechst Schering AgrEvo GmbH,
Frankfurt-am-Main, Germany.
Ebert, E., Leist, K.H. & Kramer, P. (1985b) Endosulfan-active
ingredient technical (code: Hoe 002671 01 ZD97 0003). Testing for
subchronic dermal toxicity (21 applications over 30 days) in Wistar
rats. Toxicological review of studies 721 and 729 (reports 84.0321 and
84.0223). Unpublished Hoechst document No. A30755, translation of
A30752. Submitted to WHO by Hoechst Schering AgrEvo GmbH,
Frankfurt-am-Main, Germany.
Ebert, E., Leist ,K.H. & Kramer, P. (1985c) Endosulfan-active
ingredient technical (code: Hoe 002671 01 ZD97 0003). Testing for
subchronic dermal toxicity (21 applications over 30 days) in Wistar
rats. Unpublished study No. 83.0508 from Pharma forschung Toxicologie.
Hoechst document No. A30753, translation of document A30750. Submitted
to WHO by Hoechst Schering AgrEvo GmbH, Frankfurt-am-Main, Germany.
Edwards, J.A., Hughes, E.W. & Almond, R.H. (1982) Preliminary
investigation of the effect of endosulfan (code, Hoe 02671 OI AT 209)
on reproduction of the rat. Unpublished report No. Hst 203/82252 from
Huntingdon Research Centre, Huntingdon, Cambridgeshire, United
Kingdom. Hoechst document No. A29563. Submitted to WHO by Hoechst
Schering AgrEvo GmbH, Frankfurt-am-Main, Germany.
Edwards, J.A., Reid, J.Y., Offer, M.J., Almond, H.R. & Gibson, A.W.
(1984) Effect of endosulfan-technical (code, Hoe 02671OI AT209) on
reproductive function of multiple generations in the rat. Unpublished
report No. Hst 204/83768 from Huntingdon Research Centre, Huntingdon,
Cambridgeshire, United Kingdom. Hoechst document No. A29428. Submitted
to WHO by Hoechst Schering AgrEvo GmbH, Frankfurt-am-Main, Germany.
Ehling, G. & Leist, K.-H. (1991a) Hoe 051327: substance technical
(code HOE 051327 00 ZB99 0002): Testing for acute oral toxicity in the
male and female Wistar rat. Pharma Research Toxicology, Germany.
Hoechst document No. A46286. Submitted to WHO by Hoechst Schering
AgrEvo GmbH, Frankfurt-am-Main, Germany.
Ehling, G. & Leist, K.-H. (1991b) Hoe 051327: substance technical
(code HOE 051327 00 ZB99 0002): Testing for acute dermal toxicity in
the male and female Wistar rat. Pharma Research Toxicology, Germany.
Unpublished Hoechst document No. A45783. Submitted to WHO by Hoechst
Schering AgrEvo GmbH, Frankfurt-am-Main, Germany.
Ehling, G. & Leist, K.-H. (1991c) Hoe 051329: substance technical
(code HOE 051329 00 ZD98 0001): Testing for acute oral toxicity in the
male and female Wistar rat. Pharma Research Toxicology, Germany.
Unpublished Hoechst document No. A45783. Submitted to WHO by Hoechst
Schering AgrEvo GmbH, Frankfurt-am-Main, Germany.
Ehling, G. & Leist, K.-H. (1991d) Hoe 051329: substance technical
(code HOE 051329 00 ZD98 0001): Testing for acute dermal toxicity in
the male and female Wistar rat. Pharma Research Toxicology, Germany.
Unpublished Hoechst document No. A45829. Submitted to WHO by Hoechst
Schering AgrEvo GmbH, Frankfurt-am-Main, Germany.
Elsea, J.R. (1958) Thiodan technical: Acute oral administration: Rats.
Hazleton Laboratories, USA, 28 February, 1958. Unpublished Hoechst
document No. A13686. Submitted to WHO by Hoechst Schering AgrEvo GmbH,
Frankfurt-am-Main, Germany.
Flodström, S., Wärngård, L., Hemming, H., Fransson, R. & Ahlborg, U.G.
(1988) Tumour promotion related effects by the cyclodiene insecticide
endosulfan studies in vitro and in vivo. Pharmacol Toxicol., 62,
230-235.
Goebel, H., Gorbach, S., Knauf, W., Rimpau, R.H. & Uttenbach, H.
(1982) Properties, effects, residues and analytics of the insecticide
endosulfan. Res. Rev., 83, 41. Submitted to WHO by the National
Registration Authority for Agricultural and Veterinary Chemicals,
Australia.
Gopinath, C. & Cannon, M.W.J. (1990) Photomicrographic addendum to
histopathology report No. Hst/289 Endosulfan, active ingredient
technical (code: Hoe 002671 OI ZD97 0003) combined chronic
toxicity/carcinogenicity study (104-week feeding in rats). Huntingdon
Research Centre Ltd, Huntingdon, Cambridgeshire, United Kingdom.
Unpublished Hoechst document No. A44604. Submitted to WHO by Hoechst
Schering AgrEvo GmbH, Frankfurt-am-Main, Germany.
Gorbach, S., Christ, O., Kellner, H.M., Kloss, G. & Börner, E. (1968)
The metabolism of endosulfan in milk sheep. J. Agric. Food Chem.,
16, 950-953.
Gupta, P.K., Chandra, S.V. & Saxena, D.K. (1978) Teratogenic and
embryotoxic effects of endosulfan in rats. Acta Pharmacol.
Toxicol., 42, 150-152.
Hack, R. & Leist, K.-H. (1988) Endosulfan-substance technical (code:
Hoe 002671 0I ZD96 0002) Testing of host resistance in the female
Wistar rat (immunological screening with Trichonella spiralis).
Unpublished study No. 87.1125 from Pharma Research Toxicology and
Pathology, Germany. Hoechst report A43829. Submitted to WHO by Agrevo
GmbH, Frankfurt-am-Main, Germany.
Hack, R., Ebert, E. & Leist, K.H. (1995) Chronic toxicity and
carcinogenicity studies with the insecticide endosulfan in rats and
mice. Food Chem. Toxicol., 33, 941-950.
Hollander, H. & Kramer, P. (1975a) Endosulfan sulfate = NIA 7985.
Acute oral toxicity in female SPF-Wistar rats (vehicle: starch
suspension). Unpublished Hoechst document No. A06966. Submitted to WHO
by Hoechst Schering AgrEvo GmbH, Frankfurt-am-Main, Germany.
Hollander, H. & Kramer, P. (1975b) Endosulfan sulfate = NIA 7985.
Acute oral toxicity in male beagle dogs. Unpublished Hoechst document
No. A06965. Submitted to WHO by Hoechst Schering AgrEvo GmbH,
Frankfurt-am-Main, Germany.
Hollander, H. & Kramer, P. (1975c) Comparative test on the acute
toxicity of endosulfan ether and endosulfan alcohol in female
SPF-Wistar rats (vehicle: starch suspension). Unpublished Hoechst
document No. A07170; translation of A05271. Submitted to WHO by
Hoechst Schering AgrEvo GmbH, Frankfurt-am-Main, Germany.
Hollander, H. & Kramer, P. (1975d) 1-Hydroxy endosulfan ether. Acute
oral toxicity in female SPF-Wistar rats (vehicle: starch suspension).
Unpublished Hoechst document No. A06967; translation of A05276
Submitted to WHO by Hoechst Schering AgrEvo GmbH, Frankfurt-am-Main,
Germany.
Hollander, H. & Kramer P. (1975e) Endosulfan lactone: Acute oral
toxicity in female SPF-Wistar rats (vehicle: starch suspension).
Unpublished Hoechst document No. A07171; translation of A05273.
Submitted to WHO by Hoechst Schering AgrEvo GmbH, Frankfurt-am-Main,
Germany.
Hollander, H. & Kramer, P. (1975f) Endosulfan lactone. Acute oral
toxicity in male SPF-Wistar rats. Unpublished Hoechst document No.
A06964.25. Submitted to WHO by Hoechst Schering AgrEvo GmbH,
Frankfurt-am-Main, Germany.
Hollander, H. & Weigand, W. (1983) Acute aerosol toxicity in male and
female SPF Wistar rats 4 hours-LC50. Unpublishd Hoechst report No.
83.0397; document No. A32087. Submitted to WHO by Hoechst Schering
AgrEvo GmbH, Frankfurt-am-Main, Germany.
Hollander, H., Weigand, W. & Kramer, P. (1984) Endosulfan-active
ingredient technical (code: Hoe 002671 0I ZD97 0003) Testing for
subchronic inhalation toxicity--21 exposures in 29 days -- in SPF
Wistar rats. Unpublished study No. 762; document No. A29823,
translation of A29766. Submitted to WHO by Hoechst Schering AgrEvo
GmbH, Frankfurt-am-Main, Germany.
Indraningsih, McSweeney, C.S. & Ladds, P.W. (1993) Residues of
endosulfan in the tissues of lactating goats. Aust. Vet. J., 70,
59-62.
Jung, Weigand, W. & Kramer, P. (1983) Mouse micronucleus test
following oral administration. Unpublished Hoechst AG report No.
83.0458; document No. A31628. Submitted to WHO by Hoechst Schering
AgrEvo GmbH, Frankfurt-am-Main, Germany.
Keller, J.G. (1959a) Subacute feeding--dairy cows. Hazleton
Laboratories, USA. Unpublished Hoechst document No. A14206. Submitted
to WHO by Hoechst Schering AgrEvo GmbH, Frankfurt-am-Main, Germany.
Keller, J.G. (1959b) One-year oral study in dog. Hazleton
Laboratories, 12 May 1959. Unpublished Hoechst document No. A13924.
Submitted to WHO by Hoechst Schering AgrEvo GmbH, Frankfurt-am-Main,
Germany.
Keller, J.G. (1959c) 2 year dietary study in rats. Hazleton
Laboratories, USA, 22 May 1959. Unpublished Hoechst document No.
A14037. Submitted to WHO by Hoechst Schering AgrEvo GmbH,
Frankfurt-am-Main, Germany.
Kellner, H.M. & Eckert (1983) Hoe 02671-14C. Pharmacokinetics and
residue determinations after oral and intravenous administration to
rats. Unpublished study No. tep 74/1; bereich c/analytisches project
oe 87/45; Hoechst report 01-l42-0382-83, 15 February 1983; document
No. A49475, translation of document A27971. Submitted to WHO by
Hoechst Schering AgrEvo GmbH, Frankfurt-am-Main, Germany.
Khan, P.K. & Sinha, S.P (1996) Ameliorating effect of vitamin C on
murine sperm toxicity induced by three pesticides (endosulfan,
phosphamidon and mancozeb). Mutagenesis, 11, 33-36
Kramer, P. & Weigand, W. (1971) Endosulfan lactone (vehicle: sesame
oil). Acute oral toxicity in male and female SPF-Wistar K-rat.
Unpublished Hoechst document No. A18276, 21 May 1971; translation of
A14326. Submitted to WHO by Hoechst Schering AgrEvo GmbH,
Frankfurt-am-Main, Germany.
Lachmann, G. & Siegemund, B. (1987) Hoe 002671-(5a, 9a-14C). Dermal
absorption of 14C- endosulfan in rhesus monkeys. Battelle-Institut,
Frankfurt. Unpublished Hoechst document No. A36685. Submitted to WHO
by Hoechst Schering AgrEvo GmbH, Frankfurt-am-Main, Germany.
Lehr, W (1996) Summary of intoxications with endosulfan. Clinical
cases and poisoning incidents. Unpublished Hoechst report No. psr
96/006, 12 March 1996; document A56361. Submitted to WHO by Hoechst
Schering AgrEvo GmbH, Frankfurt-am-Main, Germany.
Leist, K.H. (1989a) Endosulfan-substance technical (code: Hoe 002671
oi ZD97 0003). Carcinogenicity study in mice, 24 months feeding study
-- Residue determination. Pharma Research Toxicology and Pharmacology.
Unpublished Hoechst document No. A41284. Submitted to WHO by Hoechst
Schering AgrEvo GmbH, Frankfurt-am-Main, Germany.
Leist, K.-H. (1989b) Amendment to report No. HST 289/881067.
Endosulfan, active ingredient technical (code: Hoe 002671 OI ZD97
0003). Combined chronic toxicity/carcinogenicity study (104-week
feeding in rats). Residue determination. Unpublished Hoechst report
No. 89.1105; document No. A41265. Submitted to WHO by Hoechst Schering
AgrEvo GmbH, Frankfurt-am-Main, Germany.
Leist, K.-H. & Bremmer, J. (1998) Endosulfan (AE F002671, substance
technical). Re-evaluation of the NOAEL in the 90-day subchronic
toxicity study in rats. Unpublished AgrEvo document No. 59825.
Submitted to WHO by Hoechst Schering AgrEvo GmbH, Frankfurt-am-Main,
Germany.
Leist, K.-H. & Mayer, D. (1987) Endosulfan-active ingredient technical
(code: Hoe 002671 01 ZD97 0003). 30-Day feeding study in adult male
Wistar rats. Unpublished study No. 84 0585 from Pharma Research
Toxicology and Pharmacology. Hoechst report No. 87.0129, 27 March
1987; document No., A37112. Submitted to WHO by Hoechst Schering
AgrEvo GmbH, Frankfurt-am-Main, Germany.
Lightowler, J.E. & Gardner, J.R. (1978) Acute oral toxicity study in
rats. Unpublished report No. 78/mak1/428. 20 November 1978 from Life
Science Research. Submitted to WHO by Hoechst Schering AgrEvo GmbH,
Frankfurt-am-Main, Germany.
Mackenzie, K.M. (1980) Teratology study with FMC 5462 in rats. Raltech
Scientific Services, study No. 79041, 2 October 1980. Hoechst document
No. A21393. Submitted to WHO by Hoechst Schering AgrEvo GmbH,
Frankfurt-am-Main, Germany.
Maier-Bode, H. (1966) Investigations on the persistence of the
insecticide endosulfan in the vegetable and animal organism.
Pharmakologisches Institut der Rheinischen Friedrich Wilhelms
Universität. Unpublished Hoechst document No. A4047. Submitted to WHO
by Hoechst Schering AgrEvo GmbH, Frankfurt-am-Main, Germany.
McGregor, D.B., Willins, M.J., McDonald, P., Holström, M., McDonald,
D. & Niemeier, R.W. (1983) Genetic effects of 2-methoxyethanol and
bis(2-methoxyethyl)ether. Toxicol. Appl. Pharmacol., 70, 303-316.
McLachlan, J.A. (1997) Synergistic effect of environmental estrogens:
Report withdrawn. Science, 277, 462-463.
Mellano, D. & Milone, M.F. (1984a) Mutagenic activity of the compound
endosulfan-technical with Saccharomyces cerevisiae gene
conversion-DNA repair test. Instituto di Recherche Biomediche.
Unpublished Hoechst document No. A29313. Submitted to WHO by Hoechst
Schering AgrEvo GmbH, Frankfurt-am-Main, Germany.
Mellano, D. & Milone, M.F. (1984b) Mutagenic activity in vitro of the
compound endosulfan-technical with Schizosaccharomyces pombe.
Unpublished experiment No. M 708, 18 June 1984, from Instituto di
Recherche Biomediche. Hoechst document No. A29312. Submitted to WHO by
Hoechst Schering AgrEvo GmbH, Frankfurt-am-Main, Germany.
Müller, W. (1988) Endosulfan-substance technical (code: Hoe 002671 0I
ZD95 0005). Micronucleus test in male and female NMRI mice after oral
administration. Pharma Research Toxicology and Pathology. Unpublished
Hoechst document No. A38059. Submitted to WHO by Hoechst Schering
AgrEvo GmbH, Frankfurt-am-Main, Germany.
Müllner, H. (1989) Effects of endosulfan and aldicarb on rat brain
acetylcholinesterase. Unpublished Hoechst report 16 June 1989;
document No. A43395. Submitted to WHO by Hoechst Schering AgrEvo GmbH,
Frankfurt-am-Main, Germany.
Nath, G., Datta, K.K., Dikshith, T.S.S., Tandon, S.K. & Pandya, K.P.
(1978) 30 day oral administration in rats. Interaction of endosulfan
and metepa in rats. Industrial Toxicology Research Centre. Unpublished
Hoechst document No. A17906. Submitted to WHO by Hoechst Schering
AgrEvo GmbH, Frankfurt-am-Main, Germany.
Noctor, J.C. & John, S.A. (1995) (14C)-Endosulfan: Rates of
penetration through human and rat skin determined using an in vitro
system. Hazleton Europe, United Kingdom. Unpublished Hoechst document
No. A54103. Submitted to WHO by Hoechst Schering AgrEvo GmbH,
Frankfurt-am-Main, Germany.
Nogami, K. (translator, no other name available) (1970) Testing report
on the toxicity of endosulfan (Malix) to dogs through acute oral
administration (LD50). Unpublished Hoechst document No. A13834.
Submitted to WHO by Hoechst Schering AgrEvo GmbH, Frankfurt-am-Main,
Germany.
Offer, J.M. (1985) Addendum to HST 204. Effect of endosulfan-technical
(code: Hoe 02671 OI AT209) on the reproductive function of multiple
generations in the rat. Histopathological review of the kidneys in
adult rats of the F1b generation and in weanling rats of the F2b
generation. Huntingdon Research Centre, Huntingdon, Cambridgeshire,
United Kingdom. Unpublished Hoechst document No. A30757. Submitted to
WHO by Hoechst Schering AgrEvo GmbH, Frankfurt-am-Main, Germany.
Pandey, N., Gundevia, F., Prem, A.S. & Ray, P.K. (1990) Studies on the
genotoxicity of endosulfan, and organochlorine insecticide, in
mammalian germ cells. Mutat. Res., 242, 1-7.
Paul, V., Sheela, S., Balasubramaniam, E. & Kazi ,M. (1993)
Behavioural and biochemical changes produced by repeated oral
administration of the insecticide endosulfan in immature rats.
Indian J. Physiol. Pharmacol., 37, 204-208.
Paul, V., Easwaramoorthy, B. & Kazi, M. (1994) The neurobehavioural
toxicity of endosulfan: A serotonergic involvement in learning
impairment. Eur.J. Pharmacol. Environ. Toxicol. Pharmacol., 270,
1-7.
Paul, V., Easwaramoorthy, B., Arumugam, R.J. & Kazi, M. (1995) A
sex-related difference in the neurobehavioural and hepatic effects
following chronic endosulfan treatment in rats. Eur. J. Pharmacol.
Environ. Toxicol. Pharmacol., 293, 355-360.
Pirovano, R. & Milone, M.F. (1986) Study of the capacity of the test
article endosulfan, substance technical to induce chromosome
aberrations in human lymphocytes cultured in vitro. Unpublished RBM
experiment No. m 822, 20 March 1986. Hoechst document A33127.
Submitted to WHO by Hoechst Schering AgrEvo GmbH, Frankfurt-am-Main,
Germany.
Raizada, R.B., Srivastava, M.K. & Dikshith, T.S.S. (1991) Lack of
estrogenic effects of endosulfan: An organochlorine insecticide in
rat. Natl Acad. Sci. Lett., 14, 103-107.
Ramamoorthy, K., Wang, F., Chen, I.-C., Norris, J.D., McDonnel, D.P.,
Leonard, L.S., Gaido, K.W., Bocchinfuso, W.P., Korach, K.S. & Safe, S.
(1997) Estrogenic activity of a dieldrin/toxaphene mixture in the
mouse uterus, MCF-7 human breast cancer cells, and yeast-based
estrogen receptor assays: No apparent synergism. Endocrinology, 138,
1520-1527.
Robacker, K.M., Kulkarni, A.P. & Hodgson, E. (1981) Pesticide induced
changes in the mouse hepatic microsomal cytochrome P-450-dependent
monooxygenase system and other enzymes. J. Environ. Sci. Health,
B16, 529-545.
Reno, F.E. (1975) Acute oral toxicity study in rats. Endosulfan
technical. Unpublished final report, 18 December 1975, from Hazleton
Laboratories America. Hoechst document No. A33732. Submitted to WHO by
Hoechst Schering AgrEvo GmbH, Frankfurt-am-Main, Germany.
Roberts, N.L., Phillips, N.K. & Gopinath, C. (1983) Acute delayed
neurotoxicity study with endosulfan-technical (code: Hoe 002671 OI
ZD97 0003) in the domestic hen. Huntingdon Research Centre,
Huntingdon, Cambridgeshire, United Kingdom. Unpublished Hoechst
document No. A32153. Submitted to WHO by Hoechst Schering AgrEvo GmbH,
Frankfurt-am-Main, Germany.
Ruckman, S.A., Waterson, L.A., Crook, D., Gopinath, C., Majeed, S.K.,
Anderson, A. & Chanter, D.O. (1989) Endosulfan, active ingrediant
technical (code: Hoe 002671 OI ZD97 0003). Combined chronic
toxicity/carcinogenicity study (104-week feeding study in rats).
Huntingdon Research Centre, Huntingdon, Cambridgeshire, United
Kingdom. Unpublished Hoechst document No.A40440. Submitted to WHO by
Hoechst Schering AgrEvo GmbH, Frankfurt-am-Main, Germany.
Shelby, M.D., Newbold, R.R., Tully, D.B., Chae, K. & Davis, V.L.
(1996) Assessing environmental chemicals for estrogenicity using a
combination of in vitro and in vivo assays. Environ. Health
Perspectives, 104, 1296-1300.
Shirasu, Y., Moriya, M. & Ohta, T. (1978) Microbial mutagenicity
testing on endosulfan. Institute of Environmental Toxicology, Japan.
Unpublished Hoechst document No. A21215, 1978. Submitted to WHO by
Hoechst Schering AgrEvo GmbH, Frankfurt-am-Main, Germany.
Singh, S.K. & Pandey, R.S. (1990) Effect of sub-chronic endosulfan
exposures on plasma gonadotrophins, testosterone, testicular
testosterone and enzymes of androgen biosynthesis in rat. Indian J.
Exp. Biol., 28, 953-956.
Sinha, N., Narayan, R., Shanker, R. & Saxena, D.X. (1995)
Endosulfan-induced biochemical changes in the testis of rats. Vet.
Hum. Toxicol., 37, 547-549.
Soto, A.M., Chung, K.L. & Sonnenschein, C. (1994) The pesticides
endosulfan, toxaphene and dieldrin have oestrogenic effects on human
oestrogen-sensitive cells. Environ. Health Perspectives, 102,
380-383.
Soto, A.M., Sonnenschein, C., Chung, K.L., Fernandez, M.F., Olea, N. &
Serrano, F.O. (1995) The E-screen assay as a tool to identify
estrogens: An update on estrogenic environmental pollutants.
Environ. Health Perspectives, 103 (Suppl. 7), 113-122.
Stumf, K. & Lehr, W. (1993) Amendment to document A49584. Unpublished
Hoechst document A49584, 2 February 1993. Submitted to WHO by Hoechst
Schering AgrEvo GmbH, Frankfurt-am-Main, Germany.
den Tonkelaar, E.M. & van Esch, G.J. (1974) No-effect levels of
organochlorine pesticides based on induction of microsomal liver
enzymes in short-term toxicity experiments. Toxicology, 2, 371-380.
US National Cancer Institute (1978) 78-week dietary study in
Osborne-Mendel rats and B6C3F1 mice. NCI study No. NCI-CG-TR62,
Technical Report Series No. 62, Bethesda, Maryland, USA.
Vos, J.G., Krajnc, E.I., Beekhof, P.K. & van Logten, M.J. (1982)
Methods for testing immune effects of toxic chemicals: Evaluation of
the immunotoxicity of various pesticides in the rat. In: Matsunaka,
S., Hutson, D.H. & Murphy, S.D., eds, Pesticide Chemistry: Human
Welfare and the Environment, Oxford, Pergamon Press, pp. 497-504.
Wade, M.G., Desaulniers, D., Leingartner, K. & Foster, W.G. (1997)
Interactions between endosulfan and dieldrin on estrogen-mediated
processes in vitro and in vivo. Reprod. Toxicol., 11, 791-798.
Weigand, W. (1982a) Acute oral toxicity of Hoe 51329 in albino rats.
Pharma Research Toxicology, Germany. Unpublished Hoechst document No.
A23296. Submitted to WHO by Hoechst Schering AgrEvo GmbH,
Frankfurt-am-Main, Germany (in German).
Weigand, W. (1982b) Acute oral toxicity of Hoe 51330 in albino rats.
Pharma Research Toxicology, Germany. Unpublished Hoechst document No.
A23297. Submitted to WHO by Hoechst Schering AgrEvo GmbH,
Frankfurt-am-Main, Germany (in German).
WHO (1996) The WHO Recommended Classification of Pesticides by
Hazard and Guidelines to Classification 1996-1997 (WHO/PCS/96.3),
International Programme on Chemical Safety, Geneva.
 Groups of 24 male Sprague-Dawley rats received dermal
applications of [5a,9a14C]-endosulfan at a dose of 0.1, 0.76, or 10
mg/kg bw, without washing. Four animals from each group were killed at
0.5, 1, 2, 4, 10, and 24 h, and radiolabel was measured in the
collected excreta and various organs and tissues, including the
application site after washing with soapy water. No skin irritation
was seen at the application site. Adsorption onto the skin was
essentially complete within 0.5 h at all doses and accounted for
63-80% of the applied dose. Movement away from the application site
was slow, with 73, 73, and 89%, respectively, of the adsorbed doses
remaining at the application site after 24 h. By 10 h, each group had
excreted less than 1% of the applied dose. By 24 h, the excretion was
11, 10, and 4% of the applied dose in the groups given 0.1, 0.76, and
10 mg/kg bw, respectively (Craine, 1986).
Groups of 16 female Sprague-Dawley rats received dermal
applications of [5a,9a14C]-endosulfan at 0.09, 0.98, or 11 mg/kg bw,
and the site was washed with soapy water after 10 h. Four animals from
each group were killed at 24, 48, 72, and 168 h, and radiolabel was
measured in the collected excreta and various organs and tissues,
including the application site. No skin irritation was seen at the
application site, and there were no signs of systemic toxicity.
Recovery of radiolabel was 84-115%. The amount of the applied dose
that was removed by washing was 28, 47, and 69%, respectively, at the
three doses. After 168 h, 63, 87, and 65%, respectively, of the dose
that was not removed by washing was either adsorbed on to the skin or
had penetrated and been distributed and excreted; an average of
2.4-3.3% of the adhering dose remained at the application site.
Excretion was maximal between 24 and 48 h, faeces accounting for about
two-thirds of the label. The total residues at 168 h represented 2.5,
2.3, and 1.3% of the applied dose (about 3.5, 4.3, and 4.2% of the
adhering dose) at the doses of 0.09, 0.98, and 11 mg/kg bw,
respectively, and were present mainly in liver and kidney (Craine,
1988).
At the end of a 24-month study in which Sprague-Dawley rats were
given diets containing 0, 3, 7.5, 15, or 75 ppm technical-grade
endosulfan (see Ruckman et al., 1989), the concentrations of
endosulfan and its known metabolites, endosulfan hydroxyether,
sulfate, lactone, and diol, were measured in the liver and kidneys. As
no quantifiable residues were detected in organs of rats at 15 ppm,
the organs of animals at 7.5 and 3 ppm were not investigated further.
Neither alpha- nor ß-endosulfan, the substances administered in the
diet, or any of the metabolites was quantifiable in either organ, with
the exception of endosulfan sulfate in animals at 75 ppm, which was
found at concentrations of 0.2-0.4 mg/kg in liver. No residues were
observed in the kidneys of this group (Dorn & Werner, 1989; Leist,
1989a).
Twelve lactating goats were given endosulfan (purity unspecified)
in gelatine capsules at a dose of 1 mg/kg bw per day for 28 days. The
tissue concentrations of residues were generally low, the highest
being detected on the first day after cessation of treatment, with
0.29 ppm in kidney, 0.2 ppm in the gastrointestinal tract, and 0.12
ppm in liver. The concentrations in the kidney were increased one week
after treatment, reaching 0.49 ppm on day 8, but no residues were
detected 21 days after treatment ceased. Endosulfan residues did not
accumulate in the fat; the concentrations reached 0.06 ppm on day 1
after the end of treatment, but none were detected by day 8 after
treatment (Indraningsih, et al., 1993).
Groups of three lactating Holstein cows were given diets
containing 0, 0.3, 3, or 30 ppm 14C-endosulfan for 30 days. Between
days 7 and 29, the average concentrations in milk were 3.4, 40, and
462 ppb endosulfan equivalents in the three groups. After the dosing
period, the loss of radiolabel from milk (measured in one cow per
group) was 81% within seven days in the cow given 0.3 ppm and 96%
within 14 days in cows given 3 and 30 ppm (Bowman, 1959). The blood
concentrations rose during the first 21 days, to 0.15 and 1.97 ppm at
the doses of 3 and 30 ppm, respectively, but were always below the
detection level (0.06 ppm) in the groups at 0.3 ppm. The
concentrations of residues found at the three doses were: liver, 0.35,
2.45, and 25.3 ppm; kidney, 0.05, 0.35, and 6.29 ppm; and omental fat,
0.07, 0.71, and 7.08 ppm (Keller, 1959a).
Two lactating East Friesian sheep were given a single oral dose
of 0.3 mg/kg bw 14C-endosulfan and were killed after 40 days. The
radiolabel was maximal in blood after 24 h, when it was equivalent to
0.07 µg/ml. The total radiolabel eliminated in milk over 17 days was
0.37% and 1.82% of the dose in the two sheep, respectively. Radiolabel
was excreted mainly via the urine (41%) and faeces (50%). About half
of the 50% in faeces was unmetabolized endosulfan. Fat, kidney, and
liver of the sheep contained 0.02-0.03 µg/g endosulfan; all of the
remaining tissues had considerably lower concentrations. The total
radiolabel found in organs and tissues accounted for < 1% of the
administered dose (Gorbach et al., 1965).
In pigs fed endosulfan at 2 ppm in their diets for up to 81 days,
the compound was detected in fatty tissue at concentrations of 0.07,
0.09, and 0.04 ppm after 27, 54, and 81 days of treatment, much less
than the residues seen after administration of 7 ppm DDT: 8.3, 9.1,
and 9.7 ppm after 27, 54, and 81 days treatment, respectively. Liver
and muscle contained about 15-fold less DDT residues than fat. Thus,
while much less endosulfan was found in fatty tissues, it does not
appear to bioaccumulate as does DDT (Maier-Bode, 1966).
The systemic absorption of endosulfan over 96 h after dermal
administration to two rhesus monkeys of single doses of 2.2-3 mg/kg bw
of an aqueous suspension of 14C-endosulfan (purity, 94.6%) for 10 h
was 22% of the administered dose. An additional 11% remained in the
skin, 10.5% was found in the carcass, and 4.3 and 3.7% of the
administered dose was excreted in faeces and urine, respectively;
however, as only 50% of the administered dose was recovered, the
figures calculated for absorption may not be accurate indications of
the extent of dermal absorption of endosulfan. A plateau was reached
in blood and plasma concentrations at 36 h, and there may have been no
significant additional dermal absorption after that time.
Concentrations in the liver, kidneys, and fat were highest (0.48,
0.083, and 0.23 ppm, respectively), while there were negligible
concentrations in the brain (Lachmann, 1987).
In a study of the penetration of endosulfan through rat and human
skin in vitro, radiolabelled endosulfan formulated as an
emulsifiable concentrate containing 353 g/L endosulfan, which had been
diluted to concentrations of 0.4-4 mg/ml in water, was applied at
nominal doses of 0.01, 0.1, and 1 mg/cm2 to rat and human skin
mounted in dermal penetration cells. The rate of penetration was, on
average, 4.3 times greater through rat skin than that of humans. The
percentage of the applied dose varied with the concentration: 61% of
the lowest dose applied to human skin and 96% of that applied to rat
skin penetrated, and 20% of the highest dose applied to human skin and
40% of that applied to rat skin penetrated. When the skin was washed
10 h after application, the amount of endosulfan that penetrated
decreased to 4% in the human and 9% in the rat skin. Endosulfan that
passed through human skin was metabolized or degraded to a greater
extent than that which passed through rat skin (Noctor & John, 1995).
2. Toxicological studies
(a) Acute toxicity
The LD50 of endosulfan varies widely depending on the route of
administration, species, vehicle, and sex of the animal (see Table 2).
Certain other reports are available, but it has been argued that most
of them are not acceptable by current standards (Bremmer & Leist,
1998). Female rats are clearly more sensitive than male rats, and, on
the basis of a single study, this sex difference appears to apply to
mice also. Endosulfan is generally highly toxic after oral and
inhalation exposure. The lowest oral LD50 value is 9.6 mg/kg bw in
female Sprague-Dawley rats.
The isomers of endosulfan also show high acute toxicity after
oral administration. The clinical signs of poisoning include
piloerection, salivation, hyperactivity, respiratory distress,
diarrhoea, tremors, hunching, and convulsions. Like endosulfan, its
metabolites were more or less toxic according to the vehicle used and
the species exposed. In general, the toxicity of the lactone and
sulfate metabolites was similar to or less than that of the parent
compound, while the hydroether, ether, and, in particular, the diol
were far less toxic. The clinical signs of poisoning were similar to
those induced by the parent compound and included piloerection,
salivation, hyperactivity, respiratory distress, diarrhoea, tremors,
hunching, and convulsions.
Phenobarbital was an effective therapeutic measure against an
absolute lethal dose of endosulfan in rats, reducing the clinical
signs of poisoning and the mortality rate. Diazepam was not effective
(Ebert & Weigand, 1984).
Table 2. Acute toxicity of technical-grade endosulfan and its isomers and metabolites
Species Strain Sex Route Vehicle LD50 Reference
(mg/kg bw)
Technical-grade endosulfan
Rat Sprague-Dawley M Oral 25% in food 2 800 Bracha (1977)
Rat Sprague-Dawley F Oral 25% in food 45 Bracha (1977)
Rat CD M/F Oral Maize oil 43 Lightowler & Gardner (1978)
Rat Haffkine M Oral 110 Bhide & Naik (1984a)
Rat Haffkine F Oral 15 Bhide & Naik (1984a)
Rat Holtzman M Oral Corn oil 87 Elsea (1958)
Rat Wistar M Oral 2% starch 100-160 Diehl & Leist (1988a)
Rat Wistar F Oral 2% starch 23 Diehl & Leist (1988a)
Rat Sprague-Dawley M Oral 5% CMC 40 Reno (1975)
Rat Sprague-Dawley F Oral 5% CMC 9.6 Reno (1975)
Mouse Kasauli M Oral Tween 80 35 Bhide & Naik (1984b)
Mouse Kasauli F Oral Tween 80 14 Bhide & Naik (1984b)
Dog Mongrel M/F Oral Gelatin capsule 77 Nogami (1970)
Rat HoeWISKf M Dermala > 4 000 Diehl & Leist (1988b)
Rat HoeWISKf F Dermala 500 Diehl & Leist (1988b)
Rabbit NWS M Dermala 290 Bhide & Naik (1984c)
Rabbit New Zealand M Dermala 500-1 000 Bracha (1977)
Rabbit New Zealand F Dermala 1 000-2 000 Bracha (1977)
Rat Sprague-Dawley M/F Inhalation > 21 000 Bracha (1977)
mg/m3 for 1 h
Rat Wistar M Inhalation Ethanol + PEG 35 mg/m3 Hollander & Weigand (1983)
for 4 h
Rat Wistar F Inhalation Ethanol + PEG 13 mg/m3 Hollander & Weigand (1983)
for 4 h
Table 2. (continued)
Species Strain Sex Route Vehicle LD50 Reference
(mg/kg bw)
Endosulfan alpha isomer
Rat Oral 76 Goebel et al. (1982)
Mouse Albino F Oral Tween 80 11 Dorough et al. (1978)
Endosulfan ß isomer
Rat Oral 240 Goebel et al. (1982)
Mouse F Oral Tween 80 36 Dorough et al. (1978)
Endosulfan sulfate
Mouse Albino F Oral Tween 80 8 Dorough et al. (1978)
Rat Wistar F Oral Starch suspension 76 Hollander & Kramer (1975a)
Rat Wistar M Oral 570 Ehling & Leist (1991a)
Rat Wistar F Oral 40 Ehling & Leist (1991a)
Dog Beagle M Oral Starch suspension 15 Hollander & Kramer (1975b)
Rat Wistar M Dermal 2 700 Ehling & Leist (1991b)
Rat F Dermal 280 Ehling & Leist (1991b)
Endosulfan diol
Mouse Albino F Oral Tween 80 > 2 000 Dorough et al. (1978)
Rat F Oral Starch suspension > 1 500 Hollander & Kramer (1975c)
Rat Albino F Oral > 15 000 Weigand (1982a)
Rat Wistar M/F Oral > 5 000 Ehling & Leist (1991c)
Mouse Albino F Oral Tween 80 120 Dorough et al. (1978)
Rat F Oral Starch suspension 1 750 Hollander & Kramer (1975d)
Rat Wistar M/F Dermal > 2 000 Ehling & Leist (1991d)
Table 2. (continued)
Species Strain Sex Route Vehicle LD50 Reference
(mg/kg bw)
Endosulfan ether
Mouse Albino F Oral Tween 80 270 Dorough et al. (1978)
Rat F Oral Starch suspension > 15 000 Hollander & Kramer (1975c)
Rat Albino F Oral > 15 000 Weigand (1982b)
Endosulfan hydroxyether
Mouse Albino F Oral Tween 80 120 Dorough et al. (1978)
Rat F Oral Starch suspension 1 750 Hollander & Kramer (1975d)
Endosulfan lactone
Mouse Albino) F Oral Tween 80 120 Dorough et al. (1978)
Rat Wistar F Oral Starch suspension 290 Hollander & Kramer (1975e)
Rat Wistar M Oral Starch suspension 160 Hollander & Kramer (1975f)
Rat M/F Oral Sesame oil 100/120 Kramer & Weigand (1971)
M, male; F, female; CMC, carboxy methyl cellulose; PEG, polyethylene glycol
a 24 h on intact skin
The dermal irritancy of technical-grade endosulfan (purity,
98.6%) was tested in three New Zealand white rabbits by clipping the
hair from a dorsal area of about 25 cm2 and 24 h later applying 500
mg endosulfan moistened with deionized water on a 6.25-cm2 cellulose
patch, which was then covered with a semi-occlusive bandage. Exposure
was for 4 h, after which the test material was removed with warm
tap-water. The exposed area was examined 0.5-1, 24, 48, and 72 h after
removal of the patch. On the basis of the evaluation system defined by
EEC guideline B.4 ('Acute toxicity skin irritation' of Directive
92/69/EEC), the overall mean scores for dermal irritation were 0 for
both erythema and eschar formation and oedema formation. No signs of
systemic toxicity were observed (Bremmer, 1997a).
The ocular irritancy of technical-grade endosulfan (purity,
98.6%) was tested in three New Zealand white rabbits by applying 100
mg to the conjunctival sac of one eye of each rabbit; the other,
untreated eye served as the control. The eyes were exposed for 24 h,
after which the endosulfan was washed out, and the eyes were examined
for ocular lesions 1, 24, 48, and 72 h later. On the basis of the
evaluation system defined by EEC guideline B.5 ('Acute toxicity eye
irritation' of Directive 92/69/EEC), the overall mean scores for
irritation were 0.66 for redness of conjunctiva, 0 for chemosis of
conjunctiva, 0 for opacity of cornea, and 0.11 for irritation of the
iris. No signs of systemic toxicity were observed. Endosulfan was not
irritating to the eye (Bremmer, 1997b).
The cutaneous allergenic potential of endosulfan (purity, 98.6%)
was examined in 20 treated and 10 control male albino guinea-pigs. The
maximal tolerated concentration of endosulfan suitable for the
induction phase of the main study and a suitable non-irritating
concentration of topically applied endosulfan were identified for the
challenge application in a preliminary study. For intradermal
induction, injection of 0.1 ml of a 0.5% solution in corn oil
emulsified 1:1 (v:v) with Freund's complete adjuvant was selected. One
week after these injections, a 6-cm2 patch of filter paper saturated
with about 0.3 ml of a 50% solution of endosulfan in corn oil was
applied to the shaved skin of each guinea-pig, and the area was
occluded with aluminium foil secured by impermeable adhesive tape,
which was left in position for 48 h. Irritation was assessed 24 and 48
h later. On test day 22, the guinea-pigs were challenged with the
non-irritating 50% endosulfan in corn oil applied as during the
induction phase. The dressing was left in position for 24 h. The
application sites were assessed for erythema and oedema 24 and 48 h
later. On the basis of the evaluation system defined by EEC guideline
B.6 ('Acute toxicity -- Skin sensitization' of Directive 92/69/EEC),
none of the treated guinea-pigs developed skin reactions. Endosulfan
was therefore considered to be non-sensitizing for guinea-pig skin
(Arcelin, 1996).
(b) Short-term toxicity
Mice
Groups of 10 male and 10 female Hoe:NMRKf mice were fed diets
containing endosulfan at a concentration of 0 or 18 ppm for six weeks,
equal to 0 or 3.7 mg/kg bw per day for males and 0 or 4.6 mg/kg bw per
day for females, to determine whether 18 ppm was the NOAEL in this
strain, as it was found to be in CD-1 mice in a three-month study
(Barnard et al., 1984). There were no clinical signs attributable to
treatment. The mean food intake of mice receiving endosulfan was
slightly decreased in males and slightly increased in females. Mean
body-weight gain was reduced in treated males during the second half
of the experiment but was slightly increased in females throughout the
experiment. Two treated females died, on days 28 and 38; the causes
were not established, but no histological changes were found in the
mouse that was not autolysed. The absolute and relative weights of the
liver of males and females at 18 ppm were higher than those of
controls and statistically significantly so in females (17 and 12%,
respectively). A NOAEL was not identified, as an increase in liver
weights was seen in females at the only dose tested (Donaubauer et
al., 1985).
Groups of 20 CD-1 mice of each sex were fed diets containing
endosulfan (purity, 97.2%) at a concentration of 0, 2, 6, 18, or 54
ppm for three months, equal to 0, 0.24, 0.74, 2.1, and 7.3 mg/kg bw
per day for males and 0, 0.27, 0.8, 2.4, and 7.5 for females. Clinical
signs attributable to treatment, consisting of convulsions and
salivation, were seen in one male and one female at the high dose.
There was a marked treatment-related decrease in the survival rate
(about 50%) of male and female mice at the high dose; the mean food
intake of these animals was significantly reduced for the first two
weeks of the study, and the mean body-weight gain of males at the high
dose was reduced during the first week of treatment. A significant
reduction in neutrophil count was observed in males at 54 ppm at week
6 (72%, p < 0.01), and a lower count (62%), which was not
statistically significant, was also observed at week 13. The blood
glucose concentration was reduced by about 11% in females at all doses
(p < 0.01) at week 6, and the serum lipid concentration was
increased by 15% in females at 54 ppm at week 13. The NOAEL was
18 ppm, equivalent to 2.1 mg/kg bw per day, on the basis of
convulsions and salivation, decreased survival, and increased serum
lipid concentrations at 7.3 mg/kg bw per day (Barnard et al., 1984).
Groups of 10 four-week-old ddY mice of each sex were fed diets
containing technical-grade endosulfan (purity, 91.4%) at
concentrations of 0, 10, 30, 100, or 300 ppm, equal to 0, 1.2, 4.1,
15, and 42 mg/kg bw per day in males and 0, 1.4, 4.7, 14, and 42 mg/kg
bw per day in females, for 12 months. There were no apparent
treatment-related clinical signs or deaths. Males at the high dose
showed a small (1%) but significant decrease in mean corpuscular
volume, and transient, non-dose-related increases in haemoglobin
(11%), haematocrit (4%), and eosinophil counts (33%) were seen in
males at 30 ppm. A significant decrease in serum aspartate
aminotransferase activity was seen in males at 100 and 300 ppm and a
decrease in bilirubin concentration in males at the high dose. The
only change in organ weights was a dose-related increase in the
relative weight of the adrenals in females, which was about 30% and
statistically significant at 300 ppm. There were no treatment-related
changes on gross pathological examination; the histopathological
effects consisted of dose-related granulomatous changes in the liver
and lymph nodes. In the liver, granuloma, giant-cell infiltration,
and/or large histiocytic cells filled with brown pigment were found in
treated mice; these effects were significant at 100 and 300 ppm. In
the lymph nodes, giant-cell infiltration and/or reticuloendothelial
cell proliferation were found at the same doses. Testicular atrophy
was seen erratically, with incidences of 30, 70, 33, 50, and 80% in
the five groups, respectively, but was not considered to be related to
treatment. The NOAEL was 30 ppm, equal to 4.1 mg/kg bw per day, on the
basis of histological findings in the liver and lymphatic system (Arai
et al., 1981).
Rats
Groups of male albino rats were given endosulfan in peanut oil by
gavage at a dose of 0 or 11 mg/kg bw per day for 30 days. In addition,
the possible interaction between endosulfan and the chemosterilant,
metepa, was investigated in groups of rats receiving either metepa
alone at 30 mg/kg bw per day for 30 days or in combination with
endosulfan at 11 mg/kg bw per day. There were three deaths in the
endosulfan-treated group. Endosulfan alone had no significant effect
on body weights, organ weights, blood chemistry, or histopathological
appearance. No potentiation of the toxicity of metepa was seen (Nath
et al., 1978).
Groups of 100 male SPF Wistar rats were fed diets containing
technical-grade endosulfan (purity, 97.9%) at concentrations of 360 or
720 ppm, equal to 34 and 68 mg/kg bw per day for four weeks. Twenty
control rats received diet alone. Fifty treated and 10 control animals
were maintained for an additional four-week withdrawal period. The
objective of the experiment was to determine the toxicological
significance of yellow, granular deposits observed in the proximal
convoluted renal tubules in a 13-week and a two-generation feeding
study. One rat in each treated group died, with no signs of poisoning.
The behaviour and general condition of the rats and their food and
water consumption were unaffected by treatment, and body-weight gains
were comparable in treated and control groups. Enlargement of the
liver was observed in rats at 360 and 720 ppm at the end of the
treatment period, and the kidney and brain weights were significantly
elevated in those at 720 ppm; these treatment-related changes in organ
weights had, however, disappeared by the end of the withdrawal period.
The kidneys of treated rats were darkly discoloured, but their
appearance had returned to normal by the end of the withdrawal period.
Histopathological examination by light and electron microscopy showed
granular pigmentation and larger, more numerous lysosomes in renal
proximal tubule cells after treatment; these changes had decreased by
the end of the withdrawal period, and no lysosomal changes were found
in brain or liver. Analysis of residues showed that storage of
alpha-endosulfan was dose-dependent, temporary, and confined to the
kidney, where it was detected as endosulfan sulfate and endosulfan
lactone. The amount of ß-endosulfan was 230 times less than that of
alpha-endosulfan. The concentrations of the sulfate and lactone
metabolites were many times lower in liver, and only traces of
endosulfan remained in the kidney at the end of the withdrawal period
(Leist & Mayer, 1987).
Groups of 15 male and 15 female Wistar rats were exposed by
inhalation to technical-grade endosulfan (purity, 97.2%) at a
concentration of 0, 0.5, 1, or 2 mg/m3 air for 6 h/day, five days per
week for 29 days, for a total of 21 exposures. One male rat at the
high dose was emaciated, had a pale skin, and adopted a high-legged
position; no other clinical signs were seen in the treated animals. No
neurological disturbances, opacity of the refractive media, impairment
of dental growth, or changes in the oral mucosa were seen. The
body-weight gain of males at the high dose tended to be depressed from
day 20 of exposure until day 29, the end of the recovery period, but
no other changes in body-weight gain or food consumption were seen.
Non-dose-related increases in erythrocyte and haemoglobin
concentrations were seen at the end of exposure, but not at day 29;
these concentrations were reported to be within normal ranges for this
strain of rat. Apart from a transient, non-dose-related increase in
creatinine concentration and a decrease in serum aspartate
aminotransferase activity in females at the high dose, no
treatment-related change in blood chemistry was noted, and no
histological changes were seen in any of the rats (Hollander et al.,
1984)
Technical-grade endosulfan (purity, 97.2%) was applied in a
solution in sesame oil to the shaved skin of the nape of groups of six
Wistar rats of each sex 21 times over 30 days, for 6 h/day on five
days per week under an occlusive bandage. Males were given a dose of
0, 12, 48, 96, or 190 mg/kg bw per day, and females received 0, 3, 6,
12, or 48 mg/kg bw per day. In males, deaths and clinical signs of
poisoning consisting of tremors, tonoclonic convulsions, and/or
hypersalivation were observed only at the highest dose. In females,
clinical signs of poisoning were observed at > 2 mg/kg bw per day;
deaths occurred mainly in the group receiving 48 mg/kg bw per day,
although single animals died on day 18 after receiving 3, 6, or 12
mg/kg bw per day, none having shown signs of poisoning. Serum
cholinesterase activity was 33% lower in males at 192 mg/kg bw per
day, whereas the activities of the erythrocyte and brain enzymes were
reduced by 12 and 7%, respectively. In females at 48 mg/kg bw per day,
serum cholinesterase activity was 21% lower than in controls, and
there were no effects in the activities of erythrocyte and brain
enzymes. The NOAEL was 6 mg/kg bw per day (Ebert et al., 1985a,b).
Technical-grade endosulfan (purity, 97.2%) as a solution in
sesame oil was applied to the shaved skin of the nape of groups of six
male and six female Wistar rats 21 times over 30 days, for 6-h periods
under an occlusive bandage, at doses of 0, 1, 3, 9, 27, or 81 (males
only) mg/kg bw per day. No signs of toxicity were observed in males or
females at 1 and 3 mg/kg bw per day, but two males at 9 mg/kg bw per
day died, one on day 5 with no previous signs of poisoning and the
other on day 8 after piloerection, hypersalivation, blood-encrusted
nose, stagger, and dyspnoea. There were no deaths among males at 27
mg/kg bw per day, but three at 81 mg/kg bw per day died. None of the
females at 9 mg/kg bw per day died and no clinical signs of toxicity
were observed, but five females at 27 mg/kg bw per day died between
days 2 and 6 with no previous clinical signs of toxicity. Microscopic
changes were seen in the livers of animals at > 9 mg/kg bw per day,
consisting of enlargement of parenchymal cells in the periphery and
loss of cytoplasmic basoplilia. Serum cholinesterase activity was
statistically significantly reduced by treatment, by 70-80% in males
at doses of 9-81 mg/kg bw per day but in females by only about 40% at
9 mg/kg bw per day. Brain acetylcholinesterase activity was
statistically significantly reduced in males at 9 (21%), 27 (28%), and
81 (24%) mg/kg bw per day and in all treated females by 13-18%. The
responses were not dose-related, and the observations in females are
probably not biologically significant. Since a later study did not
demonstrate a direct inhibitory effect of endosulfan on rat brain
acetylcholinesterase activity in vitro, the reduced brain enzyme
activity found in this study was difficult to interpret. It is also
noted that serum enzyme activity was inhibited only at much higher
doses in the study of Ebert et al. (1985a,b). The two males at 9 mg/kg
bw per day that died had reduced or immature testes and/or sex organs,
and the livers of these animals has accentuated lobular markings. The
authors reasoned that these effects resulted from a
non-substance-related developmental disturbance already present before
treatment. No mechanism was proposed for these effects, which were not
seen at higher doses. The NOAEL was 3 mg/kg bw per day on the basis of
inhibition of serum and brain cholinesterase activity and microscopic
changes in the liver (Ebert et al., 1985b,c).
Endosulfan (purity not stated) as a solution in acetone was
applied daily to the shaved abdominal skin of groups of 24 albino rats
of each sex for 30 days at doses of 0, 19, 38, or
63 mg/kg bw per day for males and 0, 10, 20, or 32 mg/kg bw per day
for females. There were no deaths. All doses produced
hyperexcitability, tremor, dyspnoea, and salivation, which disappeared
after one week. No significant changes in organ:body weight ratios
occurred, and there were no treatment-associated effects on
histological, haematological, or blood chemical parameters. Liver
alanine and aspartate aminotransferase activities were reduced in
animals of each sex at the lowest dose, but there were no further
reductions with increasing dose. Liver alkaline phosphatase and
lactate dehydrogenase activities were increased in females but not in
males, again with no increase with dose. Cholinesterase activities
were not measured. A NOAEL was not identified (Dikshith et al., 1988).
Groups of 25 CD Sprague-Dawley rats of each sex were fed diets
containing technical-grade endosulfan (purity, 97.9%) at
concentrations of 0, 10, 30, 60, or 360 ppm, equal to 0, 0.64, 1.9,
3.8, and 23 mg/kg bw per day for males and 0, 0.75, 2.3, 4.6, and
27 mg/kg bw per day for females, for three months. Five animals of
each sex per group were maintained for an additional four-week
recovery period. Three females died, one each at 0, 60, and 360 ppm.
Slight but statistically significant, dose-related reductions in
erythrocyte counts and haemoglobin concentrations were seen in males
at > 30 ppm and in females at > 60 ppm, but were within the
reported normal range for this strain and age of rat (Leist & Bremmer,
1998); increased mean corpuscular volume was also seen at these doses.
Females at 360 ppm had statistically significant decreases in plasma
and erythrocyte cholinesterase activities (measured by the Ellman
method) at week 12 (by 41 and 12%, respectively), while increased
brain acetylcholinesterase activity was observed at 60 and 360 ppm (19
and 20%, respectively). In males at 360 ppm, urinalysis showed a
number of reversible changes, including increased urine volume and
urinary protein concentrations and decreased specific gravity. Gross
examination revealed enlargement of the liver in males at 360 ppm and
of the kidneys at 60 and 360 ppm; increases in the absolute weights of
the liver (18%), kidney (29%), and epididymides (8%) were seen in
males and of the liver (21%) and kidneys (10%) in females. The kidney
weights remained significantly elevated in male rats at 360 ppm (15%,
p < 0.01) at the end of the withdrawal period.
Histopathological examination revealed traces of brown pigment in
scattered hepatocytes in 25% of male rats and minimal centrilobular
enlargement of hepatocytes in 25% of females at 360 ppm. These changes
were not observed in rats at the end of the withdrawal period.
Yellowish discolouration of renal proximal tubular cells was seen in
males at all doses and in females at 30-360 ppm, the degree of
pigmentation increasing in a dose-related manner; however, no cell
death was associated with this finding. In addition, granular
pigmentation was seen in straight portions and occasionally in
proximal tubular cells in males at 60 and 360 ppm. The yellow
discolouration of the renal tubules in male rats had decreased by the
end of the withdrawal period, but trace or minimal pigmentation was
still evident. In females at > 60 ppm, the traces of pigmentation
persisted. Males at 360 ppm also had yellow protein aggregation in the
proximal convoluted with intracytoplasmic eosinophilic droplets in the
tubules. The increase in incidence and degree of the yellowish
discolouration of the proximal tubular cells appeared to be
treatment-related, since it did not develop in control rats; however,
no adverse effects were reported that might be associated with these
findings alone. All rats at 30 ppm showed either trace or minimal
discolouration and signs of granular or clumped pigment. Other
investigations suggest that the yellow pigmentation is no more than an
indication that endosulfan and some of its metabolites are being
temporarily stored before urinary excretion, and that it is therefore
an indicator of exposure rather than an expression of toxicity.
Consequently, this effect was not considered in the evaluation. At
doses of 60 and 360 ppm, other treatment-related effects were also
seen when the pigmentation was present, including enlarged kidneys and
centrilobular hepatocytes. No treatment-related increase in the
incidence of other renal effects was reported in animals at 10 ppm.
The NOAEL was thus 10 ppm, equal to 0.64 mg/kg bw per day, on the
basis of haematological changes (Barnard et al., 1985).
(c) Long-term studies of toxicity and carcinogenicity
Mice
Groups of 50 six- to seven-week-old B6C3F1 mice of each sex were
fed diets containing technical-grade endosulfan (purity, 98.8%) at
time-weighted average concentrations of 3.5 or 6.9 ppm for males and 2
or 3.9 ppm for females for 78 weeks. Groups of 20 controls received
untreated diet. There were no clear compound-related effects on
appearance or behaviour in the treated groups, and the body weights of
both males and females were unaffected by treatment. The mortality
rate of males at the high dose was increased early in treatment so
that, at the end of the experiment, the survival rates were 3/20
controls, 19/50 at the low dose, and 5/50 at the high dose. The
mortality rates of female mice were not affected by treatment. No
treatment-related clinical signs were recorded, and no
treatment-related neoplastic lesions were seen in the females. Owing
to the high early mortality rates, no conclusion could be drawn about
the carcinogenic potential of endosulfan in males. None of the
non-neoplastic changes seen in the kidneys and sex organs of male and
female mice could be attributed to treatment. The NOAEL for female
mice was 3.9 ppm, equal to 0.58 mg/kg bw per day (US National Cancer
Institute, 1978).
Groups of 60 NMRI mice of each sex were fed diets containing
technical-grade endosulfan (purity, 97.2%) at concentrations of 0, 2,
6, or 18 ppm, equal to 0.28, 0.84, and 2.5 mg/kg bw per day for males
and 0.32, 0.97, and 2.9 mg/kg bw per day for females, for up to 24
months. Ten mice of each sex per dose were killed at 12 and 18 months.
The behaviour and general health of the animals were not affected by
treatment. The mortality rate of females at 18 ppm was statistically
significant decreased at the end of the experiment: control, 45%; 18
ppm, 28% (p < 0.05); at week 78, the rates in these two groups of
female mice were 82 and 62%. Survival among treated male mice was not
statistically different from that of controls. The body weights of
males receiving 18 ppm were slightly but significantly lower than
those of controls during the first third of the study and remained
slightly but not significantly low throughout the remainder of the
study. In the other treated groups, there was a tendency to increased
body-weight gains, especially in the satellite groups killed at 12 and
18 months. No statistically significant changes were observed in
haematological or clinical chemical parameters, and macroscopic
examination did not reveal any findings that were related to
treatment. No statistically significant changes in organ weights were
seen in treated animals at the end of the experiment; however, slight
but statistically significant changes in organ weights were observed
in animals at 18 ppm at 12 and 18 months, consisting of decreased lung
and ovary weights in females at 12 months and decreased liver weights
in males and decreased ovary weights in females at 18 months.
Histopathological examination did not reveal any effects that were
related to treatment. No increase in the incidence of neoplastic or
non-neoplastic lesions was observed. The NOAEL was 6 ppm, equal to
0.84 mg/kg bw per day, on the basis of decreased body weights in males
at 24 months and decreased weights of the liver, ovaries, and lung in
males and females at 12 and/or 18 months (Donaubauer, 1988, 1989; Hack
et al., 1995).
Rats
Groups of 50 male and 50 female Osborne-Mendel rats were fed
diets containing technical-grade endosulfan (purity, 98.8%) at
time-weighted average doses of 220, 410, or 950 ppm for males and 220
or 400 ppm for females for 78 weeks, with a return to control diets
for a further four weeks. Groups of 20 rats of each sex received
untreated diet. A highly significant morbidity rate was seen in male
rats and, by week 54, 52% of those at the high dose had died. A
dose-related reduction in body weight was found in males at all doses.
Histopathological examination showed a high incidence of toxic
nephropathy (> 90%) in males at the low and high doses and in
females, but in none of the controls. Chronic renal inflammation was
observed in 40% of control males and 80% of treated males. The toxic
nephropathy observed was characterized as degenerative changes in the
proximal convoluted tubules at the junction of the cortex and medulla,
with associated cloudy swelling, fatty degeneration, and necrosis of
the tubular epithelium. Some tubules had hyaline casts, and enlarged,
dark-staining regenerative tubular epithelial cells were observed
infrequently. In treated males, parathyroid hyperplasia was observed,
as were calcium deposits in the stomach, kidney, testis, aorta, and
mesenteric artery. A dose-related increase in the incidence of
testicular atrophy was seen in treated males, characterized by
degeneration and necrosis of the germinal cells lining the
seminiferous tubules and multinucleated cells (fusion bodies),
resulting in aspermatogenesis. No treatment-related effects were noted
in the reproductive organs of female rats. No treatment-related
neoplastic lesions were seen in female rats; owing to the high
mortality rate in males, no valid conclusion can be drawn about
carcinogenicity. A NOAEL was not identified, as treatment-related
changes occurred in the kidneys and the testis at all doses (US
National Cancer Institute, 1978)
Groups of 50 five-to-six-week-old Sprague-Dawley rats of each sex
were fed diets containing endosulfan (purity, 97.1%) at concentrations
of 0, 3, 7.5, 15, or 75 ppm, equal to 0, 0.1, 0.3, 0.6, and 2.9 mg/kg
bw per day for males and 0, 0.1, 0.4, 0.7, and 3.8 mg/kg bw per day
for females, for 104 weeks. Satellite groups of 20 animals of each sex
were retained for blood sampling and examination at 104 weeks.
Reductions in body weights and body-weight gains were observed in
males (group mean, 17% at 104 weeks) and females (group mean, 18% at
104 weeks) at 75 ppm, but no clinical signs of poisoning were seen at
any dose. No increase in mortality rates was observed in treated
groups. Increased incidences of enlarged kidneys in females and of
aneurysms and enlarged lumbar lymph nodes in males were seen at 75
ppm. Histopathological examination showed that males at 75 ppm had an
increased incidence of aneurysm and marked progressive
glomerulonephrosis (controls, 20/70; 75 ppm, 30/70). The commonest
neoplasms were pituitary tumours in males and females and mammary
tumours in females, but the increased incidences did not appear to be
related to treatment. The NOAEL was 15 ppm, equal to 0.6 mg/kg bw per
day, on the basis of reduced body weights and pathological findings at
higher doses (Ruckman et al., 1989; Gopinath & Cannon, 1990; Hack et
al., 1995).
Groups of 25 Wistar rats of each sex were fed diets containing
technical-grade endosulfan (purity unspecified) at doses of 0, 10, 30,
or 100 ppm for up to 104 weeks; groups of five of each sex were killed
at 52 weeks. There were no treatment-related clinical signs, and the
body weights were unaffected, except for a nonsignificant decrease in
the body weights and food consumption of males at the high dose. The
survival rate of treated females was reduced, the deaths being
associated predominantly with respiratory infections. The weights of
the testes of males at 10 ppm were reduced by 7% with respect to
controls at 104 weeks (p < 0.05), and the kidney weights were
significantly (p < 0.001) increased (by 16%) in males at the high
dose at 104 weeks. Histopathological changes observed in males at the
high dose at 104 weeks consisted of enlarged kidneys, mild-to-severe
renal tubular dilatation (12/12), mild-to-moderate formation of
irregular albuminous casts (10/12), pronounced focal nephritis (7/12),
and mild-to-severe degeneration (11/12) of the renal tubular
epithelium. At 104 weeks, female rats at the high dose showed minimal
degeneration of renal tubules (2/3) and some focal nephritis (1/3).
The low survival rate precluded a clear conclusion about the renal
changes in female rats. Microscopic alterations in the liver were seen
in 50% of males at the high dose at week 104, consisting of focal
areas of hydropic cells, which were pale and swollen; the nuclei were
surrounded by a clear zone, and a few cells appeared to have
eosinophilic cytoplasmic inclusions. Few females at the high dose
showed changes in liver cells. A few tumours developed during the
experiment, but their occurrence was not dose-related. The NOAEL was
30 ppm, equal to 1.5 mg/kg bw per day, on the basis of effects on the
kidney (Keller, 1959c).
Dogs
Groups of six beagle dogs of each sex were fed diets containing
technical-grade endosulfan (purity, 96.5%) at concentrations of 0, 3,
10, or 30 ppm for one year, calculated by the authors to be equivalent
to 0, 0.23, 0.77, and 2.3 mg/kg bw per day. In addition, one group was
given a diet containing 30-60 ppm endosulfan, increasing in stages
from 30 ppm for 54 days, to 45 ppm for 52 days, and 60 ppm for 19-40
days; these dogs were killed in extremis before the scheduled
completion of the experiment and showed a number of signs of
poisoning, including tonic contraction and increased sensitivity to
noise and optical stimuli. Some animals given endosulfan at 30 ppm
throughout the 12-month study had violent contractions of the
abdominal muscles (without vomiting), and males at this dose had
reduced body-weight gains throughout the study and slightly reduced
body weights in the latter stages of the study, in comparison with
control animals. Cholinesterase activity was measured in serum,
erythrocytes, and brain, but difficulty appears to have been
experienced in measuring these activities, and there were large
variations within groups for the brain enzyme, the group mean of which
was increased in dogs at 30 ppm. No other effects related to treatment
were observed, and no increase in the incidence of neoplastic or non-
neoplastic lesions was observed in treated animals. The NOAEL was 10
ppm, calculated by the authors to be equivalent to 0.57 mg/kg bw per
day, on the basis of clinical signs and reductions in body weight
(Brunk 1989, 1990).
Groups of two male and two female mongrel dogs were given
technical-grade endosulfan in gelatin capsules at doses of 0, 3, 10,
or 30 ppm, equivalent to 0, 0.075, 0.25, or 0.75 mg/kg bw per day, on
six days per week for one year. The group receiving 3 ppm was given
100 ppm for the first three days of treatment, but clinical signs of
vomiting, tremors, convulsions, rapid respiration, mydriasis,
salivation, and tonic-clonic convulsions in one male and both females
led to a reduction in the dose for the remainder of the study. No
clinical signs or treatment-related effects on body-weight gain were
seen. Clinical chemical and haematological end-points were within
normal limits, and kidney function was unaffected by treatment. No
gross or histopathological changes associated with treatment were
noted. The NOAEL was 30 ppm, equivalent to 0.75 mg/kg bw per day, on
the basis of clinical signs at the initial high dose (Keller, 1959b).
(d) Genotoxicity
Endosulfan was tested for genotoxicity in a wide range of assays,
both in vitro (with and without metabolic activation) and in vivo
(Table 3). There was no evidence of genotoxicty in most of these
assays. In an assay for dominant lethal mutation in male Swiss mice
given endosulfan of a purity of 97.3%, there was a significant change
in the result of mating during the sixth mating week in the group at
16.6 mg/kg bw per day. The total numbers of implants per pregnancy
were 9 in controls and 4.5 at the high dose; the numbers of live
implants per pregnancy were 9 in controls and 2.2 at the high dose;
and the numbers of dead implants per pergnancy were none in controls
and 2.25 at the high dose (Pandey et al., 1990). While there is no
doubt about the statistical significance of these results, it is
unusual to find a true dominant lethal effect appearing so late in an
experimental mating schedule; however, it is not unknown, since a
reproducible effect of this type was demonstrated with some glycol
ethers (McGregor et al., 1983). In a later assay for dominant lethal
mutation (Dzwonkowska & Hübner, 1991), much lower doses were used, so
the results cannot be used as evidence for a non-reproducible effect.
Significant increases in the proportion of morphologically abnormal
sperm were also observed in the study of Pandey et al. (1990), as well
Table 3. Results of assays for the genotoxicity of endosulfan
End-point Test object Dose Result Reference
(LED or HID)a
In vitro
Differential toxicity B. subtilis rec strains H17 and M45 2000 µg/disc Negativea Shirasu et al. (1978)
Reverse mutation S. typhimurium TA100, TA1535, 5000 µg/plate Negativeb Shirasu et al. (1978)
TA1537, TA1538, TA98;
E. coli WP2 uvrA
Gene conversion S. cerevisiae, D4 5000 µg/ml Negativeb Mellano & Milone (1984b)
Forward mutation S. pombe 500 µg/ml Negativeb Mellano & Milone (1984a)
Unscheduled DNA Male F344 rat primary hepatocytes 51 µg/ml Negativea Cifone & Myhr (1984b)
synthesis
Gene mutation Mouse lymphoma L5178Y cells, 75 µg/ml [?] Negativeb Cifone & Myhr (1984a)
tk locus
Chromosomal Human lymphocytes 200 µg/ml Negativeb Asquith & Baillie (1989)
aberration
Chromosomal Human lymphocytes 200 µg/ml Negativeb Pirovano & Milone (1986)
aberration
In vivo
Micronucleus NMRI mouse bone-marrow cells 5 mg/kg bw, Negative Jung et al. (1983)
formation po × 1
Micronucleus NMRI mouse bone-marrow cells 10 mg/kg bw, Negative Müller (1988)
formation po × 1
Table 3. (continued)
End-point Test object Dose Result Reference
(LED or HID)a
Chromosomal Albino rat bone-marrow cells 55 mg/kg, po × 5 Negative Dikshith & Dotta (1978)
aberration
Dominant lethal Male Swiss mice 16.6 mg/kg bw, Equivocal Pandey et al. (1990)
mutation ip × 5
Dominant lethal Male Balb/c mice 0.64 mg/kg bw, Negative Dzwonkowska & Hübner
mutation ip × 1 and ip × 5 (1991)
Sperm morphology Mice 16.6 mg/kg bw, Positive Pandey et al. (1990)
ip × 5
Sperm morphology Mice in vivo 3 mg/kg bw, Positive Khan & Sinha (1996)
ip × 35
LED, lowest effective dose; HID, highest ineffective dose; po, oral; ip, intraperitoneal
a In the absence of exogenous metabolic activation; not tested in the presence of exogenous metabolic activation
b In the absence and presence of exogenous metabolic activation
as in a later study (Khan & Sinha, 1996) at lower daily doses of a 35%
emulsifiable concentrate.
(e) Reproductive toxicity
(i) Multigeneration reproductive toxicity
A preliminary investigation was conducted to determine the doses
of endosulfan to be used in a two-generation study of reproductive
toxicity. Four groups of 10 male and 10 female seven-week-old Crl:
COBS CD Sprague-Dawley rats were given diets containing
technical-grade endosulfan (purity, 97%) at concentrations of 0, 50,
75, or 100 ppm for two weeks and subsequently throughout mating and
the rearing of offspring to weaning. Food consumption and body weights
were decreased in adults at 75 and 100 ppm. At terminal autopsy, the
mean weights of the livers were significantly higher than the control
value in all treated groups. Mating performance, pregnancy rate, and
the duration of gestation were unaffected by treatment. The litter
weights of dams were significantly decreased at 75 ppm and to a
greater extent at 100 ppm from day 4 post partum. No
treatment-related abnormalities were found in the young (Edwards et
al., 1982).
In a two-generation study of reproductive toxicity with two
matings in each generation, four groups of six-week-old
Sprague-Dawley rats were fed diets containing technical-grade
endosulfan (purity, 97%) at concentrations of 0, 3, 15, or 75 ppm,
equal to 0.2-0.23, 1-1.2, and 5-5.7 mg/kg bw per day for males and
0.24-0.26, 1.2-1.3, and 6.2-6.9 mg/kg bw per day for females. The
group sizes were 32 of each sex for the F0 generation and 28 of each
sex for the F1b generation. No clinical signs or deaths related to
treatment were observed during the study. Single deaths occurred among
F0 females at 0, 3, and 15 ppm and among F1b control females. Mating
performance and pregnancy rates were not affected by treatment.
Statistically significant decreases in litter weight were occasionally
seen, but there was no effect on mean pup weights or on litter size.
No treatment-related effect on sex ratios was seen at any dose.
Statistically significantly increased relative kidney weights
were seen at 75 ppm in F0 and F1b males, and statistically
significantly increased relative liver weights were observed in F0
males and females at 75 ppm and in F1b females at 15 and 75 ppm. The
effect at 15 ppm in F1b dams was not seen at this dose in any other
matings. Yellowish discolouration of cells in the proximal convoluted
tubules were observed in male F1b rats at 3, 5, and 75 ppm and in
female F1b rats at 75 ppm. The incidence and extent of this effect
was dose-related; traces of discolouration were seen at all doses, and
minimal discolouration was seen in male rats at 15 and 75 ppm.
Granular or clumped pigment was seen in proximal convoluted tubular
cells in males at the high dose, but these findings were not
associated with histopathological evidence of renal damage. While the
increased incidence of cellular discolouration was related to
treatment, the finding is considered not to be toxicologically
significant as no adverse effects were seen on cells and the yellow
pigment was considered likely to be due to storage of endosulfan and
its metabolites in lysosomes before excretion (Annex 1, reference 58).
The presence of the pigment is thus an indication of exposure to
endosulfan rather than of toxicity. The NOAEL for maternal toxicity
was 15 ppm, equal to 1 mg/kg bw per day, on the basis of increased
relative liver and kidney weights at higher doses. The NOAEL for
reproductive effects was 75 ppm, equal to 6 mg/kg bw per day, the
highest dose tested (Edwards et al., 1984; Offer, 1985).
(ii) Developmental toxicity
Rats
Groups of mated female albino rats (strain and age unspecified)
were given oral doses of 0 (20 rats), 5 (26 rats), or 10 (32 rats)
mg/kg bw per day of endosulfan (purity unspecified) on days 6-14 of
gestation. No marked changes in behaviour or appearance were reported,
and the body weights of treated animals were similar to those of
controls. The numbers of pregnancies were 18, 20, and 21,
respectively. The dams were killed on day 21 of gestation. No
abortions occurred, but there was a significant increase in the
percent of litters with resorptions (5.5% in controls, 20% at 5 mg/kg
bw per day, and 23% at 10 mg/kg bw per day) and increased fetal
mortality, although this effect was slight and was not dose-related
(0, 2, and 1 in the three groups, respectively). Slight increases in
the incidences of cerebral hypoplasia and enlargement of the renal
pelvis were observed on visceral examination, but these effects were
not considered to be related to treatment as they were also seen in
control animals and the increases were small and were not
dose-dependent. No other increase in the incidence of visceral
abnormalities was reported. Skeletal examination showed statistically
significant increases in the incidences of absent fifth sternebrae and
of fetuses with incomplete ossification. A slight increase in the
incidence of absent fifth metacarpus, although not statistically
significant, was also seen in treated animals. These effects were not
considered to be related to treatment, as their magnitude was small
and they were not dose-dependent. No maternal toxicity was seen at any
dose. The level of reporting in this published paper was inadequate
for identifying a NOAEL for developmental toxicity (Gupta et al.,
1978).
Groups of 25 mated CD Sprague-Dawley rats were given
technical-grade endosulfan (purity, 97.3%) in corn oil by gavage on
days 6-19 of gestation at a dose of 0, 0.66, 2, or 6 mg/kg bw per day.
The clinical signs in dams at 6 mg/kg bw per day included flaccidity,
rough coat, alopecia, and hyperactivity. A dose-related decrease in
maternal body-weight gain was seen at 2 and 6 mg/kg bw per day. The
number of implantations and litter size were unaffected, but there was
a slight reduction in the weight and length of fetuses of dams at the
high dose. A non-dose-related reduction in the percent of live fetuses
and an increase in the number of resorbed fetuses were seen at 2 mg/kg
bw per day. No statistically significant treatment-related effect on
the sex ratio was observed. No external variations or malformations
were seen at 0.66 or 2 mg/kg bw per day; at the high dose, 5/405
fetuses exhibited lordosis (anteroposterior curvature of the spine)
and six had oedema. All five of the fetuses with lordosis and five of
those with oedema were from a single litter. In one fetus from the
same litter, the skin of the upper forelimb was webbed to the chest.
No significant treatment-related effects were seen on soft-tissue
development. Common minor skeletal variations were present in all
groups. The incidence of poorly ossified sixth sternebrae was
significantly greater in animals at the high dose than in the control
group, and two fetuses at this dose had clubbed left hindlimbs. The
five fetuses from the same litter that had oedema and lordosis also
had wide, thickened vertebral arches, ribs, and clavicles, and the
clavicles were shortened, curved, and twisted. Four of these fetuses
had shortened pubes, and two had an unossified hyoid bone. The
incidence of these effects was generally < 1%, and the effects were
largely related to delayed development and confined mainly to a single
litter from a single dam that showed numerous signs of poisoning
related to administration of endosulfan, including face rubbing,
alopecia, flaccidity, and hyperactivity. The developmental effects are
therefore probably related to the maternal toxicity of the high dose.
The NOAEL for maternal toxicity was 0.66 mg/kg bw per day on the basis
of decreased body-weight gain and clinical signs at higher doses. The
NOAEL for developmental toxicity was 2 mg/kg bw per day on the basis
of delayed development and a low incidence of isolated skeletal
variations (Mackenzie, 1980).
Groups of 20-24 mated female Wistar rats were given
technical-grade endosulfan (purity, 97.3%) dissolved in sesame oil by
gavage on days 7-16 of gestation at doses of 0, 0.66, 2, or 6 mg/kg bw
per day. No clinical signs of toxicity were reported in females at
0.66 or 2 mg/kg bw per day; four dams at 6 mg/kg bw per day died after
6-10 doses of endosulfan, three of these rats having tonic-clonic
convulsions for several days before death. Thirteen of the surviving
animals had tonic-clonic convulsions for a number of days, generally
around day 10 of gestation. Some of these rats also showed
hypersalivation on a number of days during treatment. Statistically
significant decreases in body weight and body-weight gain were
observed at 6 mg/kg bw per day. No statistically significant changes
in reproductive or pup parameters were observed at any dose, and the
fetal sex ratio was relatively well balanced. No statistically
significant increase in the incidence of abnormalities was observed in
fetuses. A single oedematous, retarded fetus at 6 mg/kg bw per day
presented with superior brachygnathia and a relatively small alveolar
cavity in the upper jaw, combined with cleft palate, bending of both
hind feet in the tarsal joint, wavy clavicles, and bent and shortened
scapulae. These effects were considered to be spontaneous, as no other
limb or head defects were observed in any pup in any of the litters at
this dose. Skeletal examination revealed a statistically significant
increase in the incidence of fragmented thoracic vertebral centra at 6
mg/kg bw per day. This effect was considered to be treatment-related
and reflects the frank maternal toxicity of endosulfan at the high
dose. No treatment-related major malformations were observed. The
NOAEL for maternal toxicity was 2 mg/kg bw per day on the basis of
deaths, clinical signs, and decreased body weights at higher doses.
The NOAEL for developmental toxicity was 2 mg/kg bw per day on the
basis of the increased incidence of fragmented thoracic vertebral
centra (Albrecht & Baeder, 1993).
Rabbits
Groups of 20-26 mated New Zealand white rabbits were given
technical-grade endosulfan (purity, 97.3 %) in corn oil by gavage on
days 6-28 of gestation at doses of 0, 0.3, 0.7, or 1.8 mg/kg bw per
day. There were no changes in mean body weight. None of the does at
0.3 or 0.7 mg/kg bw per day aborted, and there were no signs of
toxicity and no deaths. The high dose was associated with signs of
maternal toxicity that included noisy, rapid breathing, hyperactivity,
and convulsions. The does were killed on day 29 of gestation. The
number of implantations, litter size, sex ratio, mean fetal weight and
length, and the numbers of live and resorbed fetuses were unaffected
by treatment. There were no dead fetuses in any group, and no gross
external alterations were reported. The only soft-tissue anomalies
occurred in 6/167 fetuses (2/20 litters examined) at the high dose and
consisted of the left carotid arising from the innominate; 1/141
control fetuses (1/18 litters examined) also showed this abnormality.
Common skeletal variations and minor anomalies occurred at similar
incidences in control and treated fetuses. Endosulfan did not have
teratogenic or developmental effects even at the maternally toxic dose
of 1.8 mg/kg bw per day. The NOAEL for maternal toxicity was 0.7 mg/kg
bw per day on the basis of clinical signs at higher doses (Dickie et
al., 1981).
(f) Special studies
(i) Enzyme induction
Endosulfan was one of 16 organochlorine pesticides tested for
their ability to induce hepatic microsomal enzyme activities. Wistar
rats were given diets containing endosulfan at concentrations of 0,
20, 50, or 200 ppm for two weeks. No difference from control enzyme
activity was observed at 20 or 50 ppm; at 200 ppm group, the
activities in comparison with the control were 123% for aniline
hydroxylase (statistically significant, one experiment), 191% for
aminopyrene demethylase (statistically significant, one experiment),
and 124% for hexobarbital oxidase (not significant, one experiment)
(den Tonkelaar & van Esch, 1974).
ICR mice given endosulfan at 5 mg/kg bw per day by oral gavage
for three days and killed on the fourth day showed no increase in
liver weight or total hepatic cytochrome P450 content. The dearylation
of O-ethyl O- para-nitrophenyl phenylphosphorothioate and
parathion and NAD(P)H-dependent reductase activity were
nonsignificantly increased (Robacker et al., 1981).
(ii) Promotion
Enhancement of gamma-glutamyl transpeptidase-positive foci in rat
liver was studied as an indicator of promotion. Young male
Sprague-Dawley rats were partially hepatectomized and injected
intraperitoneally 24 h later with N-nitrosodiethylamine at 30 mg/kg
bw. One week after the partial hepatectomy, the rats were randomized
to groups of 10 or 11 and dosed orally by gavage on five days per week
for 10 weeks with endosulfan in corn oil at 0, 1, or 5 mg/kg bw per
day. The numbers and volume of enzyme-altered foci were not increased
in the treated groups in comparison with controls.
In the same study, inhibition of intercellular communication was
studied in Chinese hamster lung V79 cells in a metabolic cooperation
assay and in rat liver WB epithelial cells in a scrape loading,
dye-transfer assay. At nontoxic concentrations, technical-grade
endosulfan, analytical-grade ensosulfan (alpha and ß isomers and a
mixture of the two), and endosulfan sulfate inhibited gap-junctional
intercellular communication in both systems. In addition, endosulfan
ether was effective in the rat liver WB cell system (Flodström et al.,
1988).
(iii) Immunotoxicity
In two published studies, endosulfan was administered to male
Wistar rats at dietary doses of up to 50 ppm for six weeks (Banarjee &
Hussain, 1987) or up to 20 ppm for 22 weeks (Banarjee & Hussain, 1986)
to evaluate humoral and cell-mediated immune responses. In the
six-week study, a significant decrease in total serum antibody titre
to tetanus toxoid was seen at 30 and 50 ppm, with a slight decrease
(not statistically significant) at 10 ppm. A decrease in both
immunoglobulin (Ig)M and IgG and in the total gamma-globulin content
of rat serum was observed at 50 ppm. Cellular immunity was assayed by
measuring inhibition of migration of activated leukocytes and
macrophages. Rats exposed to endosulfan and subsequently immunized
with tetanus toxoid showed a significant decrease in inhibition of
leukocyte and macrophage migration in a dose-dependent pattern, the
decrease becoming statistically significant at 30 and 50 ppm. These
results indicate that both humoural and cellular immunity was
depressed as a result of exposure to endosulfan at 30 and 50 ppm, with
no effect at 10 ppm, equivalent to 0.5 mg/kg bw per day. In the
22-week study, the specific response of serum antibody titre to
tetanus toxoid showed a marked decrease in rats exposed to 10 or 20
ppm endosulfan throughout the experiment, in a dose- and
time-dependent pattern. Treatment at 10 or 20 ppm diminished the
inhibition of migration of both leukocytes and macrophages throughout
the study.
The effect of endosulfan on the immune system did not appear to
be secondary to other toxic effects, since the body weights of the
animals were unaffected by treatment and endosulfan is not known to
affect the hormonal system. Immune responses were unaffected by
treatment at 5 ppm, equivalent to 0.25 mg/kg bw per day. The Committee
noted that the method used to assess cellular immunity in these
studies is far from ideal as it is flawed by large inherent errors,
lack of objectivity, and, except in very experienced hands, lack of
accuracy. Less subjective tests of cellular immunity, such as
cytotoxic T cell response to a virus, would have provided more
reliable results.
Technical-grade endosulfan (purity, 96%) was administered in
sesame oil on 10 occasions by gavage to groups of eight female Wistar
rats at doses of 0.5, 1.5, or 4.5 mg/kg bw per day from two days
before until seven days after infection by gavage with approximately
500 Trichonella spiralis larvae. As a positive control, prednisolone
was administered by subcutaneous injection at a dose of 25 mg/kg bw
per day two days before and three days after infection. Three rats
from each group were killed seven days after infection so that the
number of adult worms in the intestine could be counted; the remaining
rats were killed 54 days after infection in order to count the number
of larvae in the tongue. Thymus and spleen weights and the percentage
lymphocytes in the white cell count were measured at both times. Body
weights were measured weekly. There were no differences between
endosulfan-treated and untreated rats, whereas the
prednisolone-treated group had a sevenfold higher tongue larval count
and, at seven days, a 25% reduction in thymus weight, a 50% reduction
in spleen weight, and lymphocyte counts < 50% of the control value
(Hack & Leist, 1988).
Endosulfan was included in the first part of a study to screen
for immunotoxicity, but because no effect was observed it was not
examined in greater detail. Groups of six male, weanling Wistar rats
were given endosulfan in the diet at concentrations of 20, 100, or 250
ppm for three weeks. Body weights and food intake were recorded
weekly. At autopsy, the weights of the liver, kidneys, spleen, thymus,
pituitary, adrenals, thyroid, testis, and mesenteric and popliteal
lymph nodes were recorded, these organs were also examined
histologically. Haematological examination consisted of total and
differential leukocyte counts. Serum IgM and IgG were determined by
enzyme-linked immunosorbent assay. The only effects induced by
endosulfan were considered to be expressions of general toxicity, and
there was no evidence for any specifically immunotoxic effects. The
most sensitive parameter for the toxicity of endosulfan was a
reduction in body-weight gain, which was observed at 100 ppm (Vos et
al., 1982).
(iv) Neurobehavioural effects and neurotoxicity
In a number of studies conducted by the same group of
investigators, endosulfan (purity, 95%) was given by gavage to rats at
a dose of 2 mg/kg bw per day for 90 days (Paul et al., 1993, 1994) or
up to 6 mg/kg bw per day for 30 days (Paul et al., 1995), and
behavioural and biochemical changes were determined. Signs of frank
toxicity (reduced body weights, reduced food consumption, death,
increased intensity of tremors, and increased liver enzyme activity)
were observed in all studies, and some changes in behaviour were
noted, including increased motor activity and inhibition of
conditioned and unconditioned escape and avoidance responses.
Groups of adult domestic hens were given a single oral dose of 96
mg/kg bw endosulfan, the LD50, in corn oil, observed for 21 days,
re-dosed, and again observed for 21 days. As was to be expected, a
number of deaths occurred that were related to treatment. No signs of
ataxia and no treatment-related changes in nervous tissue were seen in
vehicle control or endosulfan-treated hens. Seven of 10 hens in a
concurrent positive control group given tri- ortho-cresyl phosphate
developed ataxia and signficant spinal cord and peripheral nerve
degeneration (Roberts et al., 1983).
No inhibition of rat brain acetylcholinesterase activity was
observed in a preparation incubated with 10 µmol/L alpha-endosulfan
for up to 75 min.. A similar concentration of aldicarb produced 15%
inhibition within 5 min and 80% inhibition within 75 min (Müllner,
1989).
Technical-grade endosulfan (purity, 98.6%) was administered to
groups of 10 rats as a single dose of 25, 50, or 100 mg/kg bw to males
and 3, 6, or 12 mg/kg bw to females. Deaths occurred at the highest
doses, and there was a dose-related increase in the frequency of
clinical signs, which were reversible and apparent only on the day of
dosing. These were assumed to be due to the known affinity of
endosulfan for the gamma-aminobutyric acid receptors in the brain. At
50 and 100 mg/kg bw in males and 6 and 12 mg/kg bw in females, various
serious neuropharmacological effects were seen, including coarse
tremor and tonic-clonic convulsions. At 25 mg/kg bw in males and
3 mg/kg bw in females, the clinical signs seen were typical of general
discomfort, such as stilted gait, squatting posture, and irregular
respiration. No compound-related effects on motor activity were
observed at non-lethal doses. No effects were seen on the rearing
frequency, fore- or hindlimb grip strength, or on landing foot-spread.
No histopathological effects were found in the central or peripheral
nervous system. The study was carried out in accordance with
prevailing OECD testing guidelines (April 1996) and the OECD
principles of good laboratory practice (12 May 1981 [C(81)30(Final)])
(Bury, 1997).
(v) Effects on sperm
In a study of biochemical changes induced by endosulfan in the
testis of Druckrey rats, the authors postulated that endosulfan
impairs testicular function by altering the enzyme activities
responsible for spermatogenesis, thus affecting the intratesticular
spermatid count and resulting in low sperm production and increased
sperm deformities. The data presented support the notion that
administration of endosulfan at relatively high doses (> 2.5 mg/kg
bw per day) for several months increases the activity of a number of
enzymes in the testes, including lactate dehydrogenase, sorbitol
dehydrogenase, gamma-glutamyl transpeptidase, and glucose-6-phosphate
dehydrogenase. At doses of 5 mg/kg bw per day and higher, there was a
marked reduction in sperm count (up to 47%) in comparison with
controls. In the absence of historical control data, it is unclear
whether the decrease in sperm count seen at 2.5 mg/kg bw per day (22%)
was within the normal biological range for the test animals. The sperm
abnormalities and the reductions in spermatid count and sperm
production, while statistically significantly different from those in
concurrent controls, were only slight. In the absence of consistent
dose-response relationships for these effects, they were considered
not biologically significant (Sinha et al., 1995).
The effects of endosulfan on gonadal hormones, measured as plasma
and testicular testosterone, plasma follicle-stimulating and
luteinizing hormones, and plasma and testicular 3ß- and
17ß-hydroxysteroid dehydrogenases, was investigated in Wistar rats
given endosulfan in peanut oil at doses up to 10 mg/kg bw per day for
up to 30 days. Significant inhibition of 3ß- and 17ß-hydroxysteroid
dehydrogenases occurred in the testes of treated animals after 30 days
of treatment, and the plasma follicle-stimulating hormone, luteinizing
hormone, and testosterone concentrations were significantly
(p < 0.05) reduced in rats treated for 15 or 30 days at either
dose. Plasma and testicular testosterone concentrations were not
significantly reduced after 15 days of treatment at 7.5 mg/kg. A
significant decrease in the content or activity of microsomal
cytochrome P450 and related mixed-function oxidases was observed in
the testes of treated animals, with marked inhibition of the activity
of glutathione- S-transferase at both doses. The latter changes were
reversed when endosulfan was withdrawn, but the testicular
testosterone concentrations remained significantly reduced (Singh &
Pandey, 1990).
(vi) Endocrine effects
An estrogen is a substance that can induce estrus or a biological
response associated with estrus; one such effect is proliferation of
cells in the female genital tract. In recent years, naturally
occurring and man-made substances in the environment that may be
estrogenic have come under increasing scrutiny. Suspicion that
endosulfan may have estrogenic properties was stimulated by
observation of the reduced sperm counts described above and of
testicular atrophy in rats given endosulfan in the diet in long-term
studies.
Perhaps the first study of estrogenic effects in vivo was
conducted by Raizada et al. (1991), who treated groups of eight
ovariectomized Wistar rats, weighing an average of 100 g, with
endosulfan at 1.5 mg/kg bw per day by gavage, estradiol dipropionate
intraperitoneally (dose unspecified), or a combination of these
treatments for 30 days. Endosulfan did not change the weights of the
uterus, cervix, or vagina, whereas estradiol propionate produced large
increases. The increased weights seen with the combined treatment were
similar to those with estradiol propionate alone.
Concern that endosulfan might be estrogenic persisted as a result
of the findings of an 'E-screen' assay, which was developed to assess
the estrogenic effects of environmental chemicals by observing their
proliferative effect on a target cell. The numbers of cells present
after similar inocula of the human breast cancer cell line, MCF-7,
were compared in the absence of estrogens (negative control), in the
presence of estradiol-17ß (positive control), and with a range of
concentrations of endosulfan. In this assay, endosulfan was estrogenic
at concentrations of 10-25 µmol/L, with a proliferative effect about
80% that of 17ß-estradiol at 10 µmol/L; the relative proliferative
potency (i.e. the ratio of the doses of endosulfan and estradiol-17ß
required to produce the maximum effect) was 0.0001%. In addition,
endosulfan competed with estradiol-17ß for binding to the estrogen
receptor and increased the concentrations of progesterone receptor and
pS2 in MCF-7 cells, as would be expected for a compound that mimics
estrogens (Soto et al., 1994, 1995).
As part of a study to optimize investigations of estrogenic
activity, endosulfan and nine other chemicals (17ß-estradiol,
diethylstilbestrol, tamoxifen, 4-hydroxytamoxifen, methoxychlor,
the methoxychlor metabolite
2,2-bis (para-hydroxyphenyl)-1,1,1-trichloroethane, nonylphenol,
ortho, para'-DDT, and kepone) with known or suspected estrogenic
activity were tested in three assays: competitive binding to the mouse
uterine estrogen receptor, transcriptional activation in HeLa cells
transfected with plasmids containing an estrogen receptor and a
response element, and the uterotropic assay in mice. The results of
the three assays were consistent with respect to the known estrogenic
activities of the chemicals tested and their requirements for
metabolic activation. There was no evidence from any of these tests
that endosulfan is estrogenic (Shelby et al., 1996).
A much publicized report indicated that even estrogens of low
potency, such as endosulfan, could have important effects because of
interaction with other chemicals. The estrogenic potencies of
combinations of chemicals were screened in a system in which the human
estrogen receptor sequence is incorporated into the yeast genome.
Combinations of two weak environmental estrogens, such as dieldrin,
endosulfan, and toxaphene, were 1000 times more potent in human
estrogen receptor-mediated transactivation than any chemical alone
(Arnold et al., 1996). This result was not reproduced in another
laboratory in which the same assay was used or in a uterotropic assay
in which sexually immature rats were treated with endosulfan or
dieldrin alone or in a combination on three successive days and the
uterine mass weighed on the following day. The highest doses used in
the human estrogen receptor assay were determined by the solubility of
the compounds, and the highest doses in the uterotropic assay were 100
mg/kg bw for endosulfan or dieldrin alone and 75 mg/kg bw of each in
combination. Both chemicals were inactive in both assays, and there
was no evidence of synergism (Ashby et al., 1997). In a further study
with the human estrogen receptor assay, however, 0.1 mmol/L endosulfan
increased the activity of ß-galactosidase (Ramamoorthy et al., 1997).
More doubt was cast upon the thesis of synergism by an
independent study in which endosulfan and dieldrin showed no additive
effect in displacing 3H-17ß-estradiol from rat uterine estrogen
receptors or in inducing the proliferation of MCF-7 breast cancer
cells. The weak proliferative potential described by Soto et al.
(1994, 1995) was, however, confirmed in this assay in vitro.
Endosulfan or dieldrin alone at 3 mg/kg bw per day or in combination,
injected intraperitoneally daily for three days, did not stimulate
uterotrophic activity and had no effect on pituitary prolactin or
other endocrine-related end-points in immature female rats, indicating
that these weakly estrogenic compounds do not interact in a
synergistic fashion in binding to estrogen receptors or in activating
estrogen receptor-dependent responses in mammalian tissues or cells
(Wade et al., 1997). The paper in which synergism was originally
proposed was later withdrawn, since the results could not be
reproduced, even in the same laboratory (McLachlan, 1997). Overall,
these results suggest that concomitant exposure to weakly estrogenic
compounds probably does not result in reproductive toxicity related to
estrogen action.
3. Observations in humans
In general, the doses of endosulfan involved in cases of
poisoning have been poorly characterized. In a summary of case reports
(Lehr, 1996), the lowest reported dose that resulted in death was 35
mg/kg bw; deaths have also been reported after ingestion of 295 and
467 mg/kg bw, within 1 h of ingestion in some cases. Intensive medical
treatment within 1 h was reported to be successful after ingestion of
doses of 100 and 1000 mg/kg bw. The clinical signs in these patient
were consistent with those seen in laboratory animals, dominated by
tonic-clonic spasms. In a case in which a dose of 1000 mg/kg bw was
ingested, neurological symptoms requiring anti-epileptic therapy were
still required one year after exposure.
Comments
More than 90% of an oral dose of endusulfan was absorbed in rats,
with maximum plasma concentrations occurring after 3-8 h in males and
about 18 h in females. Elimination occurs mainly in the faeces and to
a lesser extent in the urine, more than 85% being excreted within 120
h. The highest tissue concentrations were in the kidneys. The
metabolites of endosulfan include endosulfan sulfate, diol,
hydroxy-ether, ether, and lactone but most of its metabolites are
polar substances which have not yet been identified. Endosulfan would
not be expected to accumulate significantly in human tissues. No data
on plant metabolites were available to the Meeting.
A battery of tests for acute toxicity in several species with
technical-grade endosulfan showed that it is highly toxic after oral
or dermal administration, with respective LD50 values of 10-160 mg/kg
bw and 45-135 mg/kg bw. The LC50 value for rats in a single study was
13 mg/m3 in females and 35 mg/m3 in males. Endosulfan, administered
by any route, is more toxic to female than to male rats. Clinical
signs of acute intoxication include piloerection, salivation,
hyperactivity, respiratory distress, diarrhoea, tremors, hunching, and
convulsions.
WHO has classified endosulfan as moderately hazardous (WHO,
1996).
The kidney is the target organ for toxicity. The renal effects
include increased renal weights and granular pigment formation after
short-term administration and progressive, chronic glomerulo-nephrosis
or toxic nephropathy after long-term exposure, although the
observation of progressive glomerulonephrosis is complicated by the
fact that this is a common lesion in ageing laboratory rats and occurs
at high incidence in control rats.
In a 90-day feeding study in rats, the cytoplasm of isolated
cells in the renal proximal convoluted tubules had a yellowish colour,
particularly in males, at all dietary concentrations from 10 ppm. The
presence of this yellow pigmentation was largely reversible during a
four-week recovery period, and it did not appear to indicate
nephrotoxicity. A darker, more particulate, granular and/or clumped
pigment was also observed, predominantly in cells of the straight
portions and occasionally in the proximal convoluted tubules, at
dietary concentrations of 30 ppm and above. This darker pigment was
more persistent than the yellow one, and urinalysis revealed darker
urine and marginally more ketones at doses from 60 ppm, and marginally
more protein, particularly in males, indicating renal damage at doses
of 360 ppm and above. Similar findings emerged from a multigeneration
study but not from a two-year study of carcinogenicity in rats. The
changes in pigmentation were considered to be due to the presence of
endosulfan and/or its metabolites in the enlarged lysosomes. To test
this hypothesis, a four-week feeding study was conducted in which male
rats were given dietary concentrations of 360 or 720 ppm endosulfan.
Light and electron microscopy of the kidneys of these animals clearly
showed increases in the number of lysosomes and the size of cells in
the convoluted tubule, probably as a result of accumulation of the
test material and/or its metabolites. Lysosomal changes were not
observed in either brain or liver, and the renal changes receded
appreciably during a 30-day recovery period. Chemical analysis of the
kidneys indicated the presence of alpha-endosulfan and, to a lesser
extent ß-endosulfan sulfate, and endosulfan lactone. The
concentrations of the dominant alpha-endosulfan in the kidneys were
about 50 times those in the liver. The concentrations in blood were
usually below the level of detection. After the 30-day recovery
period, renal alpha-endosulfan was detected only in traces and ß-
endosulfan not at all. Similar analysis of tissues from rats in the
two-year study of toxicity and carcinogenicity did not reveal the
presence of these substances in the kidney, although measurable alpha-
endosulfan was found in the liver at 75 ppm. The yellow colour
therefore indicates the presence of endosulfan and/or its metabolites,
rather than either a stage in the pathogenesis of nephropathy or an
independent expression of toxicity. It was postulated that in longer
studies its removal from lysosomes is accelerated by enzyme induction,
which has not been investigated.
In a 78-week study, exposure of rats to endosulfan at a high dose
of 20 mg/kg bw per day resulted in testicular atrophy, characterized
by degeneration and necrosis of the germinal cells lining the
seminiferous tubules. In addition, decreased sperm counts accompanied
by an increased incidence of sperm abnormalities have been reported in
mice, again at high doses of endosulfan. Reductions in the activities
of some testicular xenobiotic-metabolizing enzymes and some hormones
that are necessary for normal testicular function were also seen in a
30-day study in rats at 10, but not at 7.5 mg/kg bw per day. The
functional significance of these findings was not clear, as studies of
reproductive and developmental toxicity in rats and rabbits showed
neither impaired fertility nor any increase in the incidence of
defects or abnormalities in offspring. Given the high doses at which
these testicular effects were observed, it would appear that they are
of little human significance.
No genotoxic activity was observed in an adequate battery of
tests for mutagenicity and clastogenicity in vitro and in vivo.
The Meeting concluded that endosulfan is not genotoxic.
No carcinogenic effect was observed in mice at 18 ppm for 24
months, in female rats at 445 ppm for 78 weeks in one study or in male
or female rats at 75 ppm or 100 ppm for two years in two other
studies. The Meeting noted the differences in the dietary
concentrations used in these studies, but non-neoplastic responses
were seen even at the lower doses.
Endosulfan at dietary concentrations of 0, 3, 15, or 75 ppm did
not affect reproductive performance or the growth or development of
the offspring of rats over the course of a two-generation study. The
NOAEL was 75 ppm, the highest dose tested, equal to 5 mg/kg bw per day
for males and 6.2 mg/kg bw per day for females. The NOAEL for parental
toxicity was 15 ppm, equal to 1 mg/kg bw per day for males and 1.2
mg/kg bw per day, on the basis of increased liver and kidney weights
at 75 ppm.
In two studies of developmental toxicity in rats given oral doses
of 0, 0.66, 2, or 6 mg/kg bw per day, the NOAEL for maternal toxicity
was 0.66 mg/kg bw per day in one study and 2 mg/kg bw per day in the
other. In the first case, the basis was decreased body-weight gain at
2 mg/kg bw per day and decreased body-weight gain and clinical signs
of toxicity at 6 mg/kg bw per day; in the second case, the basis was
mortality, clinical signs of toxicity, and decreased body-weight gain
at 6 mg/kg bw per day. In both studies, the NOAEL for developmental
toxicity was 2 mg/kg bw per day, in the first case on the basis of
delayed development and a low incidence of skeletal variations seen at
6 mg/kg per day and in the second on the basis of an increased
incidence of fragmented thoracic vertebral centra seen at 6 mg/kg bw
per day. In neither study was there any treatment-related major
malformation.
In a study of developmental toxicity in rabbits given oral doses
of 0, 0.3, 0.7, or 1.8 mg/kg bw per day, the NOAEL for maternal
tocicity was 0.7 mg/kg bw per day on the basis of clinical signs of
toxicity at 1.8 mg/kg bw per day. The NOAEL for developmental toxicity
was 1.8 mg/kg bw per day, the highest dose tested.
Several recent studies have shown that endosulfan, alone and in
combination with other pesticides, may bind to estrogen receptors and
may perturb the endocrine system. The available studies show only very
weak binding to hormone receptors in vitro, and the evidence for
their relevance to adverse physiological effects in vivo is
extremely limited. Long-term assays of toxicity and studies of
reproductive and developmental toxicity in experimental mammals did
not indicate that endosulfan induces functional aberrations that might
result from loss of endocrine homeostasis.
The absence of immunotoxic effects in a large number of bioassays
with endosulfan suggested that it does not have an adverse effect on
the immune function of laboratory animals. However, in two studies,
rats given endosulfan in the diet at 30 or 50 ppm for 6 weeks or 20
ppm for 22 weeks had reduced serum titres of tetanus toxoid antibody
and reduced immunoglobulins G and M, and inhibition of migration of
both leukocytes and macrophages. These findings have not been
confirmed.
In a summary of case reports of human poisoning incidents, the
lowest reported dose that caused death was 35 mg/kg bw. Higher doses
caused death within 1 h. The clinical signs in these patients were
dominated by tonic-clonic convulsions, consistent with the
observations in experimental animals.
An ADI of 0-0.006 mg/kg bw was established on the basis of the
NOAEL of 0.6 mg/kg bw per day in the two-year dietary study of
toxicity in rats and a safety factor of 100. The ADI is supported by
similar NOAEL values in the 78-week dietary study of toxicity in mice,
the one-year dietary study of toxicity in dogs, and the study of
developmental toxicity in rats.
An acute RfD of 0-0.02 mg/kg was established on the basis of the
NOAEL of 2 mg/kg bw per day in the study of neurotoxicity in rats and
a safety factor of 100.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 3.9 ppm, equal to 0.58 mg/kg bw per day (females in a
78-week study of toxicity)
Rat: 15 ppm, equal to 0.6 mg/kg bw per day (two-year dietary
study of toxicity)
75 ppm, equal to 6 mg/kg bw per day (reproductive
toxicity)
0.66 mg/kg bw per day (maternal toxicity in a study of
developmental toxicity)
2 mg/kg bw per day (fetotoxicity in a study of
developmental toxicity)
Rabbit: 0.7 mg/kg bw per day (maternal toxicity in a study of
developmental toxicity)
Dog: 10 ppm, equivalent to 0.57 mg/kg bw per day (one-year
study of toxicity)
Estimate of acceptable daily intake for humans
0-0.006 mg/kg bw
Estimate of acute reference dose for humans
0.02 mg/kg bw
Studies that would provide information useful for continued
evaluation of the compound
1. Studies of immunotoxicity with standard test protocols
2. Studies of the significant sex difference in acute toxicity,
particularly in rats
3. Further observations in humans
List of end-points relevant for setting guidance values for dietary and non-dietary exposure
Absorption, distribution, excretion, and metabolism in mammals
Rate and extent of absorption Rat: oral, > 90% absorption; max. concentration at
3-8 h (males) 18 h (females)
Distribution Mainly in kidney and liver
Potential for accumulation Low
Rate and extent of excretion Biphasic; urinary half-life was 6 h for 1st phase,
33-68 h for 2nd phase; faecal half-life was 10 h and
30 h; > 85% excretion within 120 h
Metabolism in animals Oxidation and hydrolysis; unidentified polar
metabolites
Toxicologically significant Parent; no data on plant metobolites
compounds (animals, plants and
environment)
Acute toxicity
Rat: LD50 oral 10 mg/kg bw (female)
Rat: LD50 dermal 500 mg/kg bw (female)
Rat: LC50 inhalation 13 mg/m3 4 h (female)
Skin irritation Not irritating
Eye irritation Not irritating
Skin sensitization Not sensitizing
Short-term toxicity
Target/critical effect Reduced survival, convulsions, salivation
Lowest relevant oral NOAEL Rat: 0.64 mg/kg bw per day, dietary
Lowest relevant dermal NOAEL Rat: 3 mg/kg bw per day
Lowest relevant inhalation NOAEL Rat: 2 mg/m3, no effect (highest concentration)
Genotoxicity Not genotoxic
Long-term toxicity and carcinogenicity
Target/critical effect Kidney
Lowest relevant NOAEL Rat: 0.6 mg/kg bw per day, 2-year study
Carcinogenicity Not carcinogenic
Reproductive toxicity
Reproduction target: critical effect None identified
Lowest relevant reproductive NOAEL Rat: 6 mg/kg bw per day
Developmental target /critical effect Fetoxicity at maternally toxic doses
Lowest relevant developmental NOAEL Rat: 2 mg/kg bw per day
Neurotoxicity/Delayed neurotoxicity Rat: 1.5 mg/kg bw (female); 12.5 mg/kg bw (male)
no effect
Other toxicological studies Immunotoxicity in certain special assays, not
confirmed in sensitization test or histologically
Some conflicting evidence of interaction with
estrogen receptors in vitro; none in vivo
Medical data Lowest lethal dose: 35 mg/kg bw, oral
Summary Value Study Safety factor
ADI 0-0.006 mg/kg bw Several different species 100
and end-points
Acute reference dose 0.02 mg/kg bw Study of neurotoxicity in 100
rats
References
Albrecht, M. & Baeder, C. (1993) Hoe 002671-substance technical (code:
Hoe 002671 00 ZD98 0005). Testing for embryotoxicity in the Wistar rat
after oral administration. Unpublished report No. 93.0716, document
A51695. Submitted to WHO by Hoechst Schering AgrEvo GmbH,
Frankfurt-am-Main, Germany.
Arai, M., Hiromori ,T., Ho, S., Okuno, Y. & Miyamoto, J. (1981) Life
span chronic toxicity study of endosulfan in mice -- 12 month report.
Unpublished report No. 0285, at-10 from Sumitomo Chemical Co Ltd,
Japan, 28 April 1981. Submitted to WHO by Hoechst Schering AgrEvo
GmbH, Frankfurt-am-Main, Germany.
Arcelin, G. (1996) Contact hypersensitivity to endosulfan (Code: Hoe
002671 00ZD99 0008) in albino guinea pigs maximumization test.
Unpublished report No. 630753 from RCC, Itingen, Switzerland. Hoechst
document A58132.A51695. Submitted to WHO by Hoechst Schering AgrEvo
GmbH, Frankfurt-am-Main, Germany.
Arnold, S.F., Klotz, D.M., Collins, B.M., Vonier, P.M., Guilette, L.J.
& McLachlan, J.A. (1996) Synergistic activation of oestrogen receptor
with combinations of environmental chemicals. Science, 272,
1489-1492.
Ashby, J., LeFevre, P.A., Odum, J., Harris, C.A., Routledge, E.J. &
Sumpter, J.P. (1997) Synergy between synthetic oestrogens? Nature,
385, 494.
Asquith, J.C. & Baillie, J.H. (1989) Endosulfan substance technical
(code Hoe 002671 0I ZD95 0005). Metaphase analysis of human
lymphocytes. Unpublished report No. m/hl/1307 from Toxicol
Laboratories, United Kingdom, 16 March 1989. Hoechst document A40411.
Submitted to WHO by Hoechst Schering AgrEvo GmbH, Frankfurt-am-Main,
Germany.
Banarjee, B.D. & Hussain, Q.Z. (1986) Effect of subchronic endosulfan
exposure on humoral and cell-mediated immune responses in albino rats.
Arch. Toxicol., 59, 279-284.
Banarjee, B.D. & Hussain, Q.Z. (1987) Effects of endosulfan on humoral
and cell-mediated immune responses in rats. Bull. Environ. Contam.
Toxicol., 38, 438-441.
Barnard, A.V., Atkinson, J.S., Heywood, R., Street, A.E, Gibson, W.A.,
Rao, R., Offer, J.M. & Gopinath, A.R.H. (1984) 13 week toxicity study
in mice. Unpublished report No. Hst 229/831052, 25 September 1984 from
Huntingdon Research Centre, Huntingdon, Cambridgeshire, United
Kingdom. Hoechst document A29663. Submitted to WHO by Hoechst Schering
AgrEvo GmbH, Frankfurt-am-Main, Germany.
Barnard, A.V., Jones, D.R., Powell, L.A.J., Heywood, R., Street, A.E.,
Gibson, W.A., Gopinath, C., Majeed, S.K. & Almond, R.H. (1985) 13-week
toxicity study in rats followed by a 4-week withdrawal period.
Unpublished report No. Hst 230/84176, 25 March 1985 from Huntingdon
Research Centre, Huntingdon, Cambridgeshire, United Kingdom. Hoechst
document A30700 Submitted to WHO by Hoechst Schering AgrEvo GmbH,
Frankfurt- am-Main, Germany.
Bhide, M.B. & Naik, P.Y. (1984a) The acute oral toxicity (LD50) of
Excel Industries Ltds endosulfan technical No. 2 to the albino rats.
Unpublished report from Indian Institute of Toxicology (report No. and
date not given). Submitted to WHO by the National Registration
Authority for Agricultural and Veterinary Chemicals, Australia.
Bhide, M.B. & Naik, P.Y. (1984b) The acute oral toxicity (LD50) of
Excel Industries Ltds endosulfan technical No. 1 to the albino mice.
Unpublished report from Indian Institute of Toxicology (report No. and
date not given). Submitted to WHO by the National Registration
Authority for Agricultural and Veterinary Chemicals, Australia.
Bhide, M.B. & Naik, P.Y. (1984c) The acute dermal toxicity (LD50) of
Excel Industries Ltds endosulfan technical No. 2 to the albino
rabbits. Unpublished report from Indian Institute of Toxicology
(report No. and date not given). Submitted to WHO by the National
Registration Authority for Agricultural and Veterinary Chemicals,
Australia.
Bowman, J.S. (1959) Preliminary report: Subacute feeding -- dairy
cows. Unpublished report from Hazleton Laboratories, USA. Hoechst
document No. A14205. Submitted to WHO by Hoechst Schering AgrEvo GmbH,
Frankfurt-am-Main, Germany.
Bracha, P. (1977) Thionex tech: Acute oral toxicity study in rats,
skin irritation study in rabbits, acute dermal toxicity study in
rabbits, eye irritation study in rabbits and acute inhalation study in
rats. Unpublished report No. 6111820 from Warf Institute Inc. Madison,
Wisconsin, USA. Submitted to WHO by Hoechst Schering AgrEvo GmbH,
Frankfurt-am-Main, Germany.
Bremmer, J.N. (1997a) Endosulfan: Substance technical (code: Hoe
002671 00 ZD99 0008) Testing for primary dermal irritation in the
rabbit. Unpublished Hoechst Marion Roussel preclinical development
study No. 96.0837. Hoechst document A58442. Submitted to WHO by
Hoechst Schering AgrEvo GmbH, Frankfurt-am-Main, Germany.
Bremmer, J.N. (1997b) Endosulfan: Substance technical (code: Hoe
002671 00 ZD99 0008) Testing for primary eye irritation in the rabbit.
Unpublished Hoechst Marion Roussel preclinical development study No.
96.0838. Hoechst document A58443. Submitted to WHO by Hoechst Schering
AgrEvo GmbH, Frankfurt-am-Main, Germany.
Bremmer, J.N. & Leist, K.-H. (1998) Endosulfan (AE F002671, substance
technical). Evaluation of the acute oral and dermal toxicity.
Unpublished report No. TOX98/003 from Hoechst Schering AgrEvo GmbH.
Hoechst document A59823. Submitted to WHO by Hoechst Schering AgrEvo
GmbH, Frankfurt-am-Main, Germany.
Brunk, R. (1989) Endosulfan-substance technical (code: Hoe 002671 00
ZD96 0002). Testing for toxicity by repeated oral administration
(1-year feeding study) to beagle dogs. Unpublished report No. 87.0643
from Pharma Research Toxicology and Pathology. Hoechst report No.
89.0188; document No. A40441 Submitted to WHO by Hoechst Schering
AgrEvo GmbH, Frankfurt-am-Main, Germany.
Brunk, R. (1990) Addendum to Hoechst report 89.0188; document No.
A44605. Submitted to WHO by Hoechst Schering AgrEvo GmbH,
Frankfurt-am-Main, Germany.
Bury, D. (1997) Endosulfan; substance, technical (code: Hoe 002671 00
ZD99 0008). Neurotoxicological screening in the male and female Wistar
rat. Acute toxicity. Unpublished report No. 96.0373 from Hoechst
Marion Roussel Preclinical Development, Germany. Hoechst report No.
A59088. Submitted to WHO by Hoechst Schering AgrEvo GmbH,
Frankfurt-am-Main, Germany.
Christ, O. & Kellner, H.M. (1968) Investigations with endosulfan-14C
in mice. Unpublished report No. ch/he-8412 from Hoechst Radiochemical,
Frankfurt, 31 December 1968. Hoechst document A53842, translation of
document A14217. Submitted to WHO by Hoechst Schering AgrEvo GmbH,
Frankfurt-am-Main, Germany.
Cifone, A.M. & Myhr, B.C. (1984a) Evaluation of Hoe 002671-substance
technical in the rat primary hepatocyte unscheduled DNA synthesis
assay. Unpublished LBI project No. 20991 from Litton Bionetics Inc.,
USA, November 1984. Hoechst document No. A29800. Submitted to WHO by
Hoechst Schering AgrEvo GmbH, Frankfurt-am-Main, Germany.
Cifone, A.M. & Myhr, B.C. (1984b) Mutagenicity evaluation of Hoe
002671-substance technical in the mouse lymphoma forward mutation
assay. Unpublished LBI project No. 20980 from Litton Bionetics Inc.,
USA, November 1984. Hoechst document No. A29801. Submitted to WHO by
Hoechst Schering AgrEvo GmbH, Frankfurt-am-Main, Germany.
Craine, E.M. (1986) A dermal absorption study in rats with
14C-endosulfan. Unpublished project No. Wil-39028, 11 December 1986
from Wil Research Lab, USA. Wil-39028. 11 Decemnber, 1986. Hoechst
document No. A35730. Submitted to WHO by Hoechst Schering AgrEvo GmbH,
Frankfurt-am-Main, Germany.
Craine, E.M. (1988) A dermal absorption study in rats with
14C-endosulfan with extended test duration. Unpublished project No.
Wil-39029 from Wil Research Lab, USA. Hoechst document No. A39677.
Submitted to WHO by Hoechst Schering AgrEvo GmbH, Frankfurt-am-Main,
Germany.
Deema, P. Thompson, E. & Ware, G. (1966) Metabolism, storage and
excretion of 14C-endosulfan in the mouse. J. Econom. Entomol., 59,
546-550.
Dickie, S.M., Mackenzie K.M. & Rao, G.N. (1981) Teratology study with
FMC 5462 in rabbits. Unpublished study No. 80070, 27 July 1981 from
Raltech Scientific Services. Hoechst document No. A23192. Submitted to
WHO by Hoechst Schering AgrEvo GmbH, Frankfurt-am-Main, Germany.
Diehl, K.H. & Leist, K.H. (1988a) Hoe 002671: Active ingredient
technical (code: Hoe 002671 0I ZD96 0002). Testing for acute oral
toxicity in the male and female Wistar rat. Unpublished study No.
88.0551, 27 July 1988, from Pharma Research Toxicology and Pathology,
Frankfurt, Germany. Hoechst document No. A39680. Submitted to WHO by
Hoechst Schering AgrEvo GmbH, Frankfurt-am-Main, Germany.
Diehl, K.H. & Leist, K.H. (1988b) Endosulfan-active ingredient
technical (code: Hoe 002671 0I ZD96 0002). Testing for acute dermal
toxicity in the male and female Wistar rat. Unpublished study No.
88.0552, 22 August 1988, from Pharma Research Toxicology and
Pathology, Germany. Hoechst document A39397, 1 September 1988.
Submitted to WHO by Hoechst Schering AgrEvo GmbH, Frankfurt-am-Main,
Germany.
Dikshith, T.S. & Datta, K.K. (1978) Endosulfan: Lack of cytogenetic
effects in male rats. Unpublished document No. A17140 from Industrial
Toxicology Research Centre. Submitted to WHO by Hoechst Schering
AgrEvo GmbH, Frankfurt-am-Main, Germany.
Dikshith, T.S.S., Raizada, R.B., Kumar, S.N., Srivastava, M.K.,
Kaushal, R.A., Singh, R.P. & Gupta, K.P. (1988) Effect of repeated
dermal application of endosulfan to rats. Vet. Hum. Toxicol., 30,
219-224.
Donaubauer, H.H. (1988) Endosulfan-substance technical (code: Hoe
002671 OI ZD97 0003). Carcinogenicity study in mice: 24 months feeding
study. Unpublished study No. 745 from Pharma Research Toxicology and
Pathology, Germany. Hoechst document No. A38008. Submitted to WHO by
Hoechst Schering AgrEvo GmbH, Frankfurt-am-Main, Germany.
Donaubauer, H.H. (1989) Amendment to the report No. 88.0278
endosulfan-substance technical (code: Hoe 002671 OI ZD97 0003).
Carcinogenicity study in mice: 24 months feeding study. Unpublished
study No. 745 from Pharma Research Toxicology and Pathology, Germany.
Hoechst document No. A41617. Submitted to WHO by Hoechst Schering
AgrEvo GmbH, Frankfurt-am-Main, Germany.
Donaubauer, H.H., Leist, K. & Kramer, M. (1985) Endosulfan-substance
technical (code: Hoe 002671 01 ZD97 0003). 42-Day feeding study in
mice. Unpublished study No. 744 from Pharma Research Toxicology,
Germany. Hoechst document No. A38104. Submitted to WHO by Hoechst
Schering AgrEvo GmbH, Frankfurt-am-Main, Germany.
Dorn, E. & Werner, H.-J. (1989) Endosulfan (Hoe 002671 OI ZD97 0003):
Determination of residues in the liver and kidneys of rats after
chronic (2-years) feeding (week 104). Unpublished Produktentwicklung,
GB-C Ökologie II Hoechst AG Study No. (Analytical part) CR065/88.
Hoechst document No. A41265. Submitted to WHO by Hoechst Schering
AgrEvo GmbH, Frankfurt-am-Main, Germany.
Dorough, M.W., Huhtanen, K., Marshall, T.C. & Bryant, H.E. (1978) Fate
of endosulfan in rats and toxicological considerations of apolar
metabolites. Pest. Biochem. Physiol., 8, 241-252.
Dzwonkowska, A. & Hübner, H. (1991) Studies on commercial insecticides
with the dominant lethal mutations test. Polish J. Occup. Med.
Environ. Health, 4, 43-53.
Ebert, E. & Weigand, W. (1984) Testing of the therapeutic effect of
diazapam (Valium R) and phenobarbital (Luminal R) in the event of
acute poisoning with endosulfan-active ingredient technical (code: Hoe
002671 oi ZD97 0003) in Wistar rats. Unpublished report No. 84.0062
from Pharma forschung Toxikologie. Hoechst document A29211. Submitted
to WHO by Hoechst Schering AgrEvo GmbH, Frankfurt-am-Main, Germany.
Ebert, E., Weigand, W. & Kramer, P. (1985a) Endosulfan-active
ingredient technical (code: Hoe 002671 01 ZD97 0003). Testing for
subchronic dermal toxicity (21 applications over 30 days) in SPF
Wistar rats. Unpublished study No. 83.0118 from Pharma forschung
Toxicologie. Hoechst document No. A30754, translation of document
A30751. Submitted to WHO by Hoechst Schering AgrEvo GmbH,
Frankfurt-am-Main, Germany.
Ebert, E., Leist, K.H. & Kramer, P. (1985b) Endosulfan-active
ingredient technical (code: Hoe 002671 01 ZD97 0003). Testing for
subchronic dermal toxicity (21 applications over 30 days) in Wistar
rats. Toxicological review of studies 721 and 729 (reports 84.0321 and
84.0223). Unpublished Hoechst document No. A30755, translation of
A30752. Submitted to WHO by Hoechst Schering AgrEvo GmbH,
Frankfurt-am-Main, Germany.
Ebert, E., Leist ,K.H. & Kramer, P. (1985c) Endosulfan-active
ingredient technical (code: Hoe 002671 01 ZD97 0003). Testing for
subchronic dermal toxicity (21 applications over 30 days) in Wistar
rats. Unpublished study No. 83.0508 from Pharma forschung Toxicologie.
Hoechst document No. A30753, translation of document A30750. Submitted
to WHO by Hoechst Schering AgrEvo GmbH, Frankfurt-am-Main, Germany.
Edwards, J.A., Hughes, E.W. & Almond, R.H. (1982) Preliminary
investigation of the effect of endosulfan (code, Hoe 02671 OI AT 209)
on reproduction of the rat. Unpublished report No. Hst 203/82252 from
Huntingdon Research Centre, Huntingdon, Cambridgeshire, United
Kingdom. Hoechst document No. A29563. Submitted to WHO by Hoechst
Schering AgrEvo GmbH, Frankfurt-am-Main, Germany.
Edwards, J.A., Reid, J.Y., Offer, M.J., Almond, H.R. & Gibson, A.W.
(1984) Effect of endosulfan-technical (code, Hoe 02671OI AT209) on
reproductive function of multiple generations in the rat. Unpublished
report No. Hst 204/83768 from Huntingdon Research Centre, Huntingdon,
Cambridgeshire, United Kingdom. Hoechst document No. A29428. Submitted
to WHO by Hoechst Schering AgrEvo GmbH, Frankfurt-am-Main, Germany.
Ehling, G. & Leist, K.-H. (1991a) Hoe 051327: substance technical
(code HOE 051327 00 ZB99 0002): Testing for acute oral toxicity in the
male and female Wistar rat. Pharma Research Toxicology, Germany.
Hoechst document No. A46286. Submitted to WHO by Hoechst Schering
AgrEvo GmbH, Frankfurt-am-Main, Germany.
Ehling, G. & Leist, K.-H. (1991b) Hoe 051327: substance technical
(code HOE 051327 00 ZB99 0002): Testing for acute dermal toxicity in
the male and female Wistar rat. Pharma Research Toxicology, Germany.
Unpublished Hoechst document No. A45783. Submitted to WHO by Hoechst
Schering AgrEvo GmbH, Frankfurt-am-Main, Germany.
Ehling, G. & Leist, K.-H. (1991c) Hoe 051329: substance technical
(code HOE 051329 00 ZD98 0001): Testing for acute oral toxicity in the
male and female Wistar rat. Pharma Research Toxicology, Germany.
Unpublished Hoechst document No. A45783. Submitted to WHO by Hoechst
Schering AgrEvo GmbH, Frankfurt-am-Main, Germany.
Ehling, G. & Leist, K.-H. (1991d) Hoe 051329: substance technical
(code HOE 051329 00 ZD98 0001): Testing for acute dermal toxicity in
the male and female Wistar rat. Pharma Research Toxicology, Germany.
Unpublished Hoechst document No. A45829. Submitted to WHO by Hoechst
Schering AgrEvo GmbH, Frankfurt-am-Main, Germany.
Elsea, J.R. (1958) Thiodan technical: Acute oral administration: Rats.
Hazleton Laboratories, USA, 28 February, 1958. Unpublished Hoechst
document No. A13686. Submitted to WHO by Hoechst Schering AgrEvo GmbH,
Frankfurt-am-Main, Germany.
Flodström, S., Wärngård, L., Hemming, H., Fransson, R. & Ahlborg, U.G.
(1988) Tumour promotion related effects by the cyclodiene insecticide
endosulfan studies in vitro and in vivo. Pharmacol Toxicol., 62,
230-235.
Goebel, H., Gorbach, S., Knauf, W., Rimpau, R.H. & Uttenbach, H.
(1982) Properties, effects, residues and analytics of the insecticide
endosulfan. Res. Rev., 83, 41. Submitted to WHO by the National
Registration Authority for Agricultural and Veterinary Chemicals,
Australia.
Gopinath, C. & Cannon, M.W.J. (1990) Photomicrographic addendum to
histopathology report No. Hst/289 Endosulfan, active ingredient
technical (code: Hoe 002671 OI ZD97 0003) combined chronic
toxicity/carcinogenicity study (104-week feeding in rats). Huntingdon
Research Centre Ltd, Huntingdon, Cambridgeshire, United Kingdom.
Unpublished Hoechst document No. A44604. Submitted to WHO by Hoechst
Schering AgrEvo GmbH, Frankfurt-am-Main, Germany.
Gorbach, S., Christ, O., Kellner, H.M., Kloss, G. & Börner, E. (1968)
The metabolism of endosulfan in milk sheep. J. Agric. Food Chem.,
16, 950-953.
Gupta, P.K., Chandra, S.V. & Saxena, D.K. (1978) Teratogenic and
embryotoxic effects of endosulfan in rats. Acta Pharmacol.
Toxicol., 42, 150-152.
Hack, R. & Leist, K.-H. (1988) Endosulfan-substance technical (code:
Hoe 002671 0I ZD96 0002) Testing of host resistance in the female
Wistar rat (immunological screening with Trichonella spiralis).
Unpublished study No. 87.1125 from Pharma Research Toxicology and
Pathology, Germany. Hoechst report A43829. Submitted to WHO by Agrevo
GmbH, Frankfurt-am-Main, Germany.
Hack, R., Ebert, E. & Leist, K.H. (1995) Chronic toxicity and
carcinogenicity studies with the insecticide endosulfan in rats and
mice. Food Chem. Toxicol., 33, 941-950.
Hollander, H. & Kramer, P. (1975a) Endosulfan sulfate = NIA 7985.
Acute oral toxicity in female SPF-Wistar rats (vehicle: starch
suspension). Unpublished Hoechst document No. A06966. Submitted to WHO
by Hoechst Schering AgrEvo GmbH, Frankfurt-am-Main, Germany.
Hollander, H. & Kramer, P. (1975b) Endosulfan sulfate = NIA 7985.
Acute oral toxicity in male beagle dogs. Unpublished Hoechst document
No. A06965. Submitted to WHO by Hoechst Schering AgrEvo GmbH,
Frankfurt-am-Main, Germany.
Hollander, H. & Kramer, P. (1975c) Comparative test on the acute
toxicity of endosulfan ether and endosulfan alcohol in female
SPF-Wistar rats (vehicle: starch suspension). Unpublished Hoechst
document No. A07170; translation of A05271. Submitted to WHO by
Hoechst Schering AgrEvo GmbH, Frankfurt-am-Main, Germany.
Hollander, H. & Kramer, P. (1975d) 1-Hydroxy endosulfan ether. Acute
oral toxicity in female SPF-Wistar rats (vehicle: starch suspension).
Unpublished Hoechst document No. A06967; translation of A05276
Submitted to WHO by Hoechst Schering AgrEvo GmbH, Frankfurt-am-Main,
Germany.
Hollander, H. & Kramer P. (1975e) Endosulfan lactone: Acute oral
toxicity in female SPF-Wistar rats (vehicle: starch suspension).
Unpublished Hoechst document No. A07171; translation of A05273.
Submitted to WHO by Hoechst Schering AgrEvo GmbH, Frankfurt-am-Main,
Germany.
Hollander, H. & Kramer, P. (1975f) Endosulfan lactone. Acute oral
toxicity in male SPF-Wistar rats. Unpublished Hoechst document No.
A06964.25. Submitted to WHO by Hoechst Schering AgrEvo GmbH,
Frankfurt-am-Main, Germany.
Hollander, H. & Weigand, W. (1983) Acute aerosol toxicity in male and
female SPF Wistar rats 4 hours-LC50. Unpublishd Hoechst report No.
83.0397; document No. A32087. Submitted to WHO by Hoechst Schering
AgrEvo GmbH, Frankfurt-am-Main, Germany.
Hollander, H., Weigand, W. & Kramer, P. (1984) Endosulfan-active
ingredient technical (code: Hoe 002671 0I ZD97 0003) Testing for
subchronic inhalation toxicity--21 exposures in 29 days -- in SPF
Wistar rats. Unpublished study No. 762; document No. A29823,
translation of A29766. Submitted to WHO by Hoechst Schering AgrEvo
GmbH, Frankfurt-am-Main, Germany.
Indraningsih, McSweeney, C.S. & Ladds, P.W. (1993) Residues of
endosulfan in the tissues of lactating goats. Aust. Vet. J., 70,
59-62.
Jung, Weigand, W. & Kramer, P. (1983) Mouse micronucleus test
following oral administration. Unpublished Hoechst AG report No.
83.0458; document No. A31628. Submitted to WHO by Hoechst Schering
AgrEvo GmbH, Frankfurt-am-Main, Germany.
Keller, J.G. (1959a) Subacute feeding--dairy cows. Hazleton
Laboratories, USA. Unpublished Hoechst document No. A14206. Submitted
to WHO by Hoechst Schering AgrEvo GmbH, Frankfurt-am-Main, Germany.
Keller, J.G. (1959b) One-year oral study in dog. Hazleton
Laboratories, 12 May 1959. Unpublished Hoechst document No. A13924.
Submitted to WHO by Hoechst Schering AgrEvo GmbH, Frankfurt-am-Main,
Germany.
Keller, J.G. (1959c) 2 year dietary study in rats. Hazleton
Laboratories, USA, 22 May 1959. Unpublished Hoechst document No.
A14037. Submitted to WHO by Hoechst Schering AgrEvo GmbH,
Frankfurt-am-Main, Germany.
Kellner, H.M. & Eckert (1983) Hoe 02671-14C. Pharmacokinetics and
residue determinations after oral and intravenous administration to
rats. Unpublished study No. tep 74/1; bereich c/analytisches project
oe 87/45; Hoechst report 01-l42-0382-83, 15 February 1983; document
No. A49475, translation of document A27971. Submitted to WHO by
Hoechst Schering AgrEvo GmbH, Frankfurt-am-Main, Germany.
Khan, P.K. & Sinha, S.P (1996) Ameliorating effect of vitamin C on
murine sperm toxicity induced by three pesticides (endosulfan,
phosphamidon and mancozeb). Mutagenesis, 11, 33-36
Kramer, P. & Weigand, W. (1971) Endosulfan lactone (vehicle: sesame
oil). Acute oral toxicity in male and female SPF-Wistar K-rat.
Unpublished Hoechst document No. A18276, 21 May 1971; translation of
A14326. Submitted to WHO by Hoechst Schering AgrEvo GmbH,
Frankfurt-am-Main, Germany.
Lachmann, G. & Siegemund, B. (1987) Hoe 002671-(5a, 9a-14C). Dermal
absorption of 14C- endosulfan in rhesus monkeys. Battelle-Institut,
Frankfurt. Unpublished Hoechst document No. A36685. Submitted to WHO
by Hoechst Schering AgrEvo GmbH, Frankfurt-am-Main, Germany.
Lehr, W (1996) Summary of intoxications with endosulfan. Clinical
cases and poisoning incidents. Unpublished Hoechst report No. psr
96/006, 12 March 1996; document A56361. Submitted to WHO by Hoechst
Schering AgrEvo GmbH, Frankfurt-am-Main, Germany.
Leist, K.H. (1989a) Endosulfan-substance technical (code: Hoe 002671
oi ZD97 0003). Carcinogenicity study in mice, 24 months feeding study
-- Residue determination. Pharma Research Toxicology and Pharmacology.
Unpublished Hoechst document No. A41284. Submitted to WHO by Hoechst
Schering AgrEvo GmbH, Frankfurt-am-Main, Germany.
Leist, K.-H. (1989b) Amendment to report No. HST 289/881067.
Endosulfan, active ingredient technical (code: Hoe 002671 OI ZD97
0003). Combined chronic toxicity/carcinogenicity study (104-week
feeding in rats). Residue determination. Unpublished Hoechst report
No. 89.1105; document No. A41265. Submitted to WHO by Hoechst Schering
AgrEvo GmbH, Frankfurt-am-Main, Germany.
Leist, K.-H. & Bremmer, J. (1998) Endosulfan (AE F002671, substance
technical). Re-evaluation of the NOAEL in the 90-day subchronic
toxicity study in rats. Unpublished AgrEvo document No. 59825.
Submitted to WHO by Hoechst Schering AgrEvo GmbH, Frankfurt-am-Main,
Germany.
Leist, K.-H. & Mayer, D. (1987) Endosulfan-active ingredient technical
(code: Hoe 002671 01 ZD97 0003). 30-Day feeding study in adult male
Wistar rats. Unpublished study No. 84 0585 from Pharma Research
Toxicology and Pharmacology. Hoechst report No. 87.0129, 27 March
1987; document No., A37112. Submitted to WHO by Hoechst Schering
AgrEvo GmbH, Frankfurt-am-Main, Germany.
Lightowler, J.E. & Gardner, J.R. (1978) Acute oral toxicity study in
rats. Unpublished report No. 78/mak1/428. 20 November 1978 from Life
Science Research. Submitted to WHO by Hoechst Schering AgrEvo GmbH,
Frankfurt-am-Main, Germany.
Mackenzie, K.M. (1980) Teratology study with FMC 5462 in rats. Raltech
Scientific Services, study No. 79041, 2 October 1980. Hoechst document
No. A21393. Submitted to WHO by Hoechst Schering AgrEvo GmbH,
Frankfurt-am-Main, Germany.
Maier-Bode, H. (1966) Investigations on the persistence of the
insecticide endosulfan in the vegetable and animal organism.
Pharmakologisches Institut der Rheinischen Friedrich Wilhelms
Universität. Unpublished Hoechst document No. A4047. Submitted to WHO
by Hoechst Schering AgrEvo GmbH, Frankfurt-am-Main, Germany.
McGregor, D.B., Willins, M.J., McDonald, P., Holström, M., McDonald,
D. & Niemeier, R.W. (1983) Genetic effects of 2-methoxyethanol and
bis(2-methoxyethyl)ether. Toxicol. Appl. Pharmacol., 70, 303-316.
McLachlan, J.A. (1997) Synergistic effect of environmental estrogens:
Report withdrawn. Science, 277, 462-463.
Mellano, D. & Milone, M.F. (1984a) Mutagenic activity of the compound
endosulfan-technical with Saccharomyces cerevisiae gene
conversion-DNA repair test. Instituto di Recherche Biomediche.
Unpublished Hoechst document No. A29313. Submitted to WHO by Hoechst
Schering AgrEvo GmbH, Frankfurt-am-Main, Germany.
Mellano, D. & Milone, M.F. (1984b) Mutagenic activity in vitro of the
compound endosulfan-technical with Schizosaccharomyces pombe.
Unpublished experiment No. M 708, 18 June 1984, from Instituto di
Recherche Biomediche. Hoechst document No. A29312. Submitted to WHO by
Hoechst Schering AgrEvo GmbH, Frankfurt-am-Main, Germany.
Müller, W. (1988) Endosulfan-substance technical (code: Hoe 002671 0I
ZD95 0005). Micronucleus test in male and female NMRI mice after oral
administration. Pharma Research Toxicology and Pathology. Unpublished
Hoechst document No. A38059. Submitted to WHO by Hoechst Schering
AgrEvo GmbH, Frankfurt-am-Main, Germany.
Müllner, H. (1989) Effects of endosulfan and aldicarb on rat brain
acetylcholinesterase. Unpublished Hoechst report 16 June 1989;
document No. A43395. Submitted to WHO by Hoechst Schering AgrEvo GmbH,
Frankfurt-am-Main, Germany.
Nath, G., Datta, K.K., Dikshith, T.S.S., Tandon, S.K. & Pandya, K.P.
(1978) 30 day oral administration in rats. Interaction of endosulfan
and metepa in rats. Industrial Toxicology Research Centre. Unpublished
Hoechst document No. A17906. Submitted to WHO by Hoechst Schering
AgrEvo GmbH, Frankfurt-am-Main, Germany.
Noctor, J.C. & John, S.A. (1995) (14C)-Endosulfan: Rates of
penetration through human and rat skin determined using an in vitro
system. Hazleton Europe, United Kingdom. Unpublished Hoechst document
No. A54103. Submitted to WHO by Hoechst Schering AgrEvo GmbH,
Frankfurt-am-Main, Germany.
Nogami, K. (translator, no other name available) (1970) Testing report
on the toxicity of endosulfan (Malix) to dogs through acute oral
administration (LD50). Unpublished Hoechst document No. A13834.
Submitted to WHO by Hoechst Schering AgrEvo GmbH, Frankfurt-am-Main,
Germany.
Offer, J.M. (1985) Addendum to HST 204. Effect of endosulfan-technical
(code: Hoe 02671 OI AT209) on the reproductive function of multiple
generations in the rat. Histopathological review of the kidneys in
adult rats of the F1b generation and in weanling rats of the F2b
generation. Huntingdon Research Centre, Huntingdon, Cambridgeshire,
United Kingdom. Unpublished Hoechst document No. A30757. Submitted to
WHO by Hoechst Schering AgrEvo GmbH, Frankfurt-am-Main, Germany.
Pandey, N., Gundevia, F., Prem, A.S. & Ray, P.K. (1990) Studies on the
genotoxicity of endosulfan, and organochlorine insecticide, in
mammalian germ cells. Mutat. Res., 242, 1-7.
Paul, V., Sheela, S., Balasubramaniam, E. & Kazi ,M. (1993)
Behavioural and biochemical changes produced by repeated oral
administration of the insecticide endosulfan in immature rats.
Indian J. Physiol. Pharmacol., 37, 204-208.
Paul, V., Easwaramoorthy, B. & Kazi, M. (1994) The neurobehavioural
toxicity of endosulfan: A serotonergic involvement in learning
impairment. Eur.J. Pharmacol. Environ. Toxicol. Pharmacol., 270,
1-7.
Paul, V., Easwaramoorthy, B., Arumugam, R.J. & Kazi, M. (1995) A
sex-related difference in the neurobehavioural and hepatic effects
following chronic endosulfan treatment in rats. Eur. J. Pharmacol.
Environ. Toxicol. Pharmacol., 293, 355-360.
Pirovano, R. & Milone, M.F. (1986) Study of the capacity of the test
article endosulfan, substance technical to induce chromosome
aberrations in human lymphocytes cultured in vitro. Unpublished RBM
experiment No. m 822, 20 March 1986. Hoechst document A33127.
Submitted to WHO by Hoechst Schering AgrEvo GmbH, Frankfurt-am-Main,
Germany.
Raizada, R.B., Srivastava, M.K. & Dikshith, T.S.S. (1991) Lack of
estrogenic effects of endosulfan: An organochlorine insecticide in
rat. Natl Acad. Sci. Lett., 14, 103-107.
Ramamoorthy, K., Wang, F., Chen, I.-C., Norris, J.D., McDonnel, D.P.,
Leonard, L.S., Gaido, K.W., Bocchinfuso, W.P., Korach, K.S. & Safe, S.
(1997) Estrogenic activity of a dieldrin/toxaphene mixture in the
mouse uterus, MCF-7 human breast cancer cells, and yeast-based
estrogen receptor assays: No apparent synergism. Endocrinology, 138,
1520-1527.
Robacker, K.M., Kulkarni, A.P. & Hodgson, E. (1981) Pesticide induced
changes in the mouse hepatic microsomal cytochrome P-450-dependent
monooxygenase system and other enzymes. J. Environ. Sci. Health,
B16, 529-545.
Reno, F.E. (1975) Acute oral toxicity study in rats. Endosulfan
technical. Unpublished final report, 18 December 1975, from Hazleton
Laboratories America. Hoechst document No. A33732. Submitted to WHO by
Hoechst Schering AgrEvo GmbH, Frankfurt-am-Main, Germany.
Roberts, N.L., Phillips, N.K. & Gopinath, C. (1983) Acute delayed
neurotoxicity study with endosulfan-technical (code: Hoe 002671 OI
ZD97 0003) in the domestic hen. Huntingdon Research Centre,
Huntingdon, Cambridgeshire, United Kingdom. Unpublished Hoechst
document No. A32153. Submitted to WHO by Hoechst Schering AgrEvo GmbH,
Frankfurt-am-Main, Germany.
Ruckman, S.A., Waterson, L.A., Crook, D., Gopinath, C., Majeed, S.K.,
Anderson, A. & Chanter, D.O. (1989) Endosulfan, active ingrediant
technical (code: Hoe 002671 OI ZD97 0003). Combined chronic
toxicity/carcinogenicity study (104-week feeding study in rats).
Huntingdon Research Centre, Huntingdon, Cambridgeshire, United
Kingdom. Unpublished Hoechst document No.A40440. Submitted to WHO by
Hoechst Schering AgrEvo GmbH, Frankfurt-am-Main, Germany.
Shelby, M.D., Newbold, R.R., Tully, D.B., Chae, K. & Davis, V.L.
(1996) Assessing environmental chemicals for estrogenicity using a
combination of in vitro and in vivo assays. Environ. Health
Perspectives, 104, 1296-1300.
Shirasu, Y., Moriya, M. & Ohta, T. (1978) Microbial mutagenicity
testing on endosulfan. Institute of Environmental Toxicology, Japan.
Unpublished Hoechst document No. A21215, 1978. Submitted to WHO by
Hoechst Schering AgrEvo GmbH, Frankfurt-am-Main, Germany.
Singh, S.K. & Pandey, R.S. (1990) Effect of sub-chronic endosulfan
exposures on plasma gonadotrophins, testosterone, testicular
testosterone and enzymes of androgen biosynthesis in rat. Indian J.
Exp. Biol., 28, 953-956.
Sinha, N., Narayan, R., Shanker, R. & Saxena, D.X. (1995)
Endosulfan-induced biochemical changes in the testis of rats. Vet.
Hum. Toxicol., 37, 547-549.
Soto, A.M., Chung, K.L. & Sonnenschein, C. (1994) The pesticides
endosulfan, toxaphene and dieldrin have oestrogenic effects on human
oestrogen-sensitive cells. Environ. Health Perspectives, 102,
380-383.
Soto, A.M., Sonnenschein, C., Chung, K.L., Fernandez, M.F., Olea, N. &
Serrano, F.O. (1995) The E-screen assay as a tool to identify
estrogens: An update on estrogenic environmental pollutants.
Environ. Health Perspectives, 103 (Suppl. 7), 113-122.
Stumf, K. & Lehr, W. (1993) Amendment to document A49584. Unpublished
Hoechst document A49584, 2 February 1993. Submitted to WHO by Hoechst
Schering AgrEvo GmbH, Frankfurt-am-Main, Germany.
den Tonkelaar, E.M. & van Esch, G.J. (1974) No-effect levels of
organochlorine pesticides based on induction of microsomal liver
enzymes in short-term toxicity experiments. Toxicology, 2, 371-380.
US National Cancer Institute (1978) 78-week dietary study in
Osborne-Mendel rats and B6C3F1 mice. NCI study No. NCI-CG-TR62,
Technical Report Series No. 62, Bethesda, Maryland, USA.
Vos, J.G., Krajnc, E.I., Beekhof, P.K. & van Logten, M.J. (1982)
Methods for testing immune effects of toxic chemicals: Evaluation of
the immunotoxicity of various pesticides in the rat. In: Matsunaka,
S., Hutson, D.H. & Murphy, S.D., eds, Pesticide Chemistry: Human
Welfare and the Environment, Oxford, Pergamon Press, pp. 497-504.
Wade, M.G., Desaulniers, D., Leingartner, K. & Foster, W.G. (1997)
Interactions between endosulfan and dieldrin on estrogen-mediated
processes in vitro and in vivo. Reprod. Toxicol., 11, 791-798.
Weigand, W. (1982a) Acute oral toxicity of Hoe 51329 in albino rats.
Pharma Research Toxicology, Germany. Unpublished Hoechst document No.
A23296. Submitted to WHO by Hoechst Schering AgrEvo GmbH,
Frankfurt-am-Main, Germany (in German).
Weigand, W. (1982b) Acute oral toxicity of Hoe 51330 in albino rats.
Pharma Research Toxicology, Germany. Unpublished Hoechst document No.
A23297. Submitted to WHO by Hoechst Schering AgrEvo GmbH,
Frankfurt-am-Main, Germany (in German).
WHO (1996) The WHO Recommended Classification of Pesticides by
Hazard and Guidelines to Classification 1996-1997 (WHO/PCS/96.3),
International Programme on Chemical Safety, Geneva.
Groups of 24 male Sprague-Dawley rats received dermal
applications of [5a,9a14C]-endosulfan at a dose of 0.1, 0.76, or 10
mg/kg bw, without washing. Four animals from each group were killed at
0.5, 1, 2, 4, 10, and 24 h, and radiolabel was measured in the
collected excreta and various organs and tissues, including the
application site after washing with soapy water. No skin irritation
was seen at the application site. Adsorption onto the skin was
essentially complete within 0.5 h at all doses and accounted for
63-80% of the applied dose. Movement away from the application site
was slow, with 73, 73, and 89%, respectively, of the adsorbed doses
remaining at the application site after 24 h. By 10 h, each group had
excreted less than 1% of the applied dose. By 24 h, the excretion was
11, 10, and 4% of the applied dose in the groups given 0.1, 0.76, and
10 mg/kg bw, respectively (Craine, 1986).
Groups of 16 female Sprague-Dawley rats received dermal
applications of [5a,9a14C]-endosulfan at 0.09, 0.98, or 11 mg/kg bw,
and the site was washed with soapy water after 10 h. Four animals from
each group were killed at 24, 48, 72, and 168 h, and radiolabel was
measured in the collected excreta and various organs and tissues,
including the application site. No skin irritation was seen at the
application site, and there were no signs of systemic toxicity.
Recovery of radiolabel was 84-115%. The amount of the applied dose
that was removed by washing was 28, 47, and 69%, respectively, at the
three doses. After 168 h, 63, 87, and 65%, respectively, of the dose
that was not removed by washing was either adsorbed on to the skin or
had penetrated and been distributed and excreted; an average of
2.4-3.3% of the adhering dose remained at the application site.
Excretion was maximal between 24 and 48 h, faeces accounting for about
two-thirds of the label. The total residues at 168 h represented 2.5,
2.3, and 1.3% of the applied dose (about 3.5, 4.3, and 4.2% of the
adhering dose) at the doses of 0.09, 0.98, and 11 mg/kg bw,
respectively, and were present mainly in liver and kidney (Craine,
1988).
At the end of a 24-month study in which Sprague-Dawley rats were
given diets containing 0, 3, 7.5, 15, or 75 ppm technical-grade
endosulfan (see Ruckman et al., 1989), the concentrations of
endosulfan and its known metabolites, endosulfan hydroxyether,
sulfate, lactone, and diol, were measured in the liver and kidneys. As
no quantifiable residues were detected in organs of rats at 15 ppm,
the organs of animals at 7.5 and 3 ppm were not investigated further.
Neither alpha- nor ß-endosulfan, the substances administered in the
diet, or any of the metabolites was quantifiable in either organ, with
the exception of endosulfan sulfate in animals at 75 ppm, which was
found at concentrations of 0.2-0.4 mg/kg in liver. No residues were
observed in the kidneys of this group (Dorn & Werner, 1989; Leist,
1989a).
Twelve lactating goats were given endosulfan (purity unspecified)
in gelatine capsules at a dose of 1 mg/kg bw per day for 28 days. The
tissue concentrations of residues were generally low, the highest
being detected on the first day after cessation of treatment, with
0.29 ppm in kidney, 0.2 ppm in the gastrointestinal tract, and 0.12
ppm in liver. The concentrations in the kidney were increased one week
after treatment, reaching 0.49 ppm on day 8, but no residues were
detected 21 days after treatment ceased. Endosulfan residues did not
accumulate in the fat; the concentrations reached 0.06 ppm on day 1
after the end of treatment, but none were detected by day 8 after
treatment (Indraningsih, et al., 1993).
Groups of three lactating Holstein cows were given diets
containing 0, 0.3, 3, or 30 ppm 14C-endosulfan for 30 days. Between
days 7 and 29, the average concentrations in milk were 3.4, 40, and
462 ppb endosulfan equivalents in the three groups. After the dosing
period, the loss of radiolabel from milk (measured in one cow per
group) was 81% within seven days in the cow given 0.3 ppm and 96%
within 14 days in cows given 3 and 30 ppm (Bowman, 1959). The blood
concentrations rose during the first 21 days, to 0.15 and 1.97 ppm at
the doses of 3 and 30 ppm, respectively, but were always below the
detection level (0.06 ppm) in the groups at 0.3 ppm. The
concentrations of residues found at the three doses were: liver, 0.35,
2.45, and 25.3 ppm; kidney, 0.05, 0.35, and 6.29 ppm; and omental fat,
0.07, 0.71, and 7.08 ppm (Keller, 1959a).
Two lactating East Friesian sheep were given a single oral dose
of 0.3 mg/kg bw 14C-endosulfan and were killed after 40 days. The
radiolabel was maximal in blood after 24 h, when it was equivalent to
0.07 µg/ml. The total radiolabel eliminated in milk over 17 days was
0.37% and 1.82% of the dose in the two sheep, respectively. Radiolabel
was excreted mainly via the urine (41%) and faeces (50%). About half
of the 50% in faeces was unmetabolized endosulfan. Fat, kidney, and
liver of the sheep contained 0.02-0.03 µg/g endosulfan; all of the
remaining tissues had considerably lower concentrations. The total
radiolabel found in organs and tissues accounted for < 1% of the
administered dose (Gorbach et al., 1965).
In pigs fed endosulfan at 2 ppm in their diets for up to 81 days,
the compound was detected in fatty tissue at concentrations of 0.07,
0.09, and 0.04 ppm after 27, 54, and 81 days of treatment, much less
than the residues seen after administration of 7 ppm DDT: 8.3, 9.1,
and 9.7 ppm after 27, 54, and 81 days treatment, respectively. Liver
and muscle contained about 15-fold less DDT residues than fat. Thus,
while much less endosulfan was found in fatty tissues, it does not
appear to bioaccumulate as does DDT (Maier-Bode, 1966).
The systemic absorption of endosulfan over 96 h after dermal
administration to two rhesus monkeys of single doses of 2.2-3 mg/kg bw
of an aqueous suspension of 14C-endosulfan (purity, 94.6%) for 10 h
was 22% of the administered dose. An additional 11% remained in the
skin, 10.5% was found in the carcass, and 4.3 and 3.7% of the
administered dose was excreted in faeces and urine, respectively;
however, as only 50% of the administered dose was recovered, the
figures calculated for absorption may not be accurate indications of
the extent of dermal absorption of endosulfan. A plateau was reached
in blood and plasma concentrations at 36 h, and there may have been no
significant additional dermal absorption after that time.
Concentrations in the liver, kidneys, and fat were highest (0.48,
0.083, and 0.23 ppm, respectively), while there were negligible
concentrations in the brain (Lachmann, 1987).
In a study of the penetration of endosulfan through rat and human
skin in vitro, radiolabelled endosulfan formulated as an
emulsifiable concentrate containing 353 g/L endosulfan, which had been
diluted to concentrations of 0.4-4 mg/ml in water, was applied at
nominal doses of 0.01, 0.1, and 1 mg/cm2 to rat and human skin
mounted in dermal penetration cells. The rate of penetration was, on
average, 4.3 times greater through rat skin than that of humans. The
percentage of the applied dose varied with the concentration: 61% of
the lowest dose applied to human skin and 96% of that applied to rat
skin penetrated, and 20% of the highest dose applied to human skin and
40% of that applied to rat skin penetrated. When the skin was washed
10 h after application, the amount of endosulfan that penetrated
decreased to 4% in the human and 9% in the rat skin. Endosulfan that
passed through human skin was metabolized or degraded to a greater
extent than that which passed through rat skin (Noctor & John, 1995).
2. Toxicological studies
(a) Acute toxicity
The LD50 of endosulfan varies widely depending on the route of
administration, species, vehicle, and sex of the animal (see Table 2).
Certain other reports are available, but it has been argued that most
of them are not acceptable by current standards (Bremmer & Leist,
1998). Female rats are clearly more sensitive than male rats, and, on
the basis of a single study, this sex difference appears to apply to
mice also. Endosulfan is generally highly toxic after oral and
inhalation exposure. The lowest oral LD50 value is 9.6 mg/kg bw in
female Sprague-Dawley rats.
The isomers of endosulfan also show high acute toxicity after
oral administration. The clinical signs of poisoning include
piloerection, salivation, hyperactivity, respiratory distress,
diarrhoea, tremors, hunching, and convulsions. Like endosulfan, its
metabolites were more or less toxic according to the vehicle used and
the species exposed. In general, the toxicity of the lactone and
sulfate metabolites was similar to or less than that of the parent
compound, while the hydroether, ether, and, in particular, the diol
were far less toxic. The clinical signs of poisoning were similar to
those induced by the parent compound and included piloerection,
salivation, hyperactivity, respiratory distress, diarrhoea, tremors,
hunching, and convulsions.
Phenobarbital was an effective therapeutic measure against an
absolute lethal dose of endosulfan in rats, reducing the clinical
signs of poisoning and the mortality rate. Diazepam was not effective
(Ebert & Weigand, 1984).
Table 2. Acute toxicity of technical-grade endosulfan and its isomers and metabolites
Species Strain Sex Route Vehicle LD50 Reference
(mg/kg bw)
Technical-grade endosulfan
Rat Sprague-Dawley M Oral 25% in food 2 800 Bracha (1977)
Rat Sprague-Dawley F Oral 25% in food 45 Bracha (1977)
Rat CD M/F Oral Maize oil 43 Lightowler & Gardner (1978)
Rat Haffkine M Oral 110 Bhide & Naik (1984a)
Rat Haffkine F Oral 15 Bhide & Naik (1984a)
Rat Holtzman M Oral Corn oil 87 Elsea (1958)
Rat Wistar M Oral 2% starch 100-160 Diehl & Leist (1988a)
Rat Wistar F Oral 2% starch 23 Diehl & Leist (1988a)
Rat Sprague-Dawley M Oral 5% CMC 40 Reno (1975)
Rat Sprague-Dawley F Oral 5% CMC 9.6 Reno (1975)
Mouse Kasauli M Oral Tween 80 35 Bhide & Naik (1984b)
Mouse Kasauli F Oral Tween 80 14 Bhide & Naik (1984b)
Dog Mongrel M/F Oral Gelatin capsule 77 Nogami (1970)
Rat HoeWISKf M Dermala > 4 000 Diehl & Leist (1988b)
Rat HoeWISKf F Dermala 500 Diehl & Leist (1988b)
Rabbit NWS M Dermala 290 Bhide & Naik (1984c)
Rabbit New Zealand M Dermala 500-1 000 Bracha (1977)
Rabbit New Zealand F Dermala 1 000-2 000 Bracha (1977)
Rat Sprague-Dawley M/F Inhalation > 21 000 Bracha (1977)
mg/m3 for 1 h
Rat Wistar M Inhalation Ethanol + PEG 35 mg/m3 Hollander & Weigand (1983)
for 4 h
Rat Wistar F Inhalation Ethanol + PEG 13 mg/m3 Hollander & Weigand (1983)
for 4 h
Table 2. (continued)
Species Strain Sex Route Vehicle LD50 Reference
(mg/kg bw)
Endosulfan alpha isomer
Rat Oral 76 Goebel et al. (1982)
Mouse Albino F Oral Tween 80 11 Dorough et al. (1978)
Endosulfan ß isomer
Rat Oral 240 Goebel et al. (1982)
Mouse F Oral Tween 80 36 Dorough et al. (1978)
Endosulfan sulfate
Mouse Albino F Oral Tween 80 8 Dorough et al. (1978)
Rat Wistar F Oral Starch suspension 76 Hollander & Kramer (1975a)
Rat Wistar M Oral 570 Ehling & Leist (1991a)
Rat Wistar F Oral 40 Ehling & Leist (1991a)
Dog Beagle M Oral Starch suspension 15 Hollander & Kramer (1975b)
Rat Wistar M Dermal 2 700 Ehling & Leist (1991b)
Rat F Dermal 280 Ehling & Leist (1991b)
Endosulfan diol
Mouse Albino F Oral Tween 80 > 2 000 Dorough et al. (1978)
Rat F Oral Starch suspension > 1 500 Hollander & Kramer (1975c)
Rat Albino F Oral > 15 000 Weigand (1982a)
Rat Wistar M/F Oral > 5 000 Ehling & Leist (1991c)
Mouse Albino F Oral Tween 80 120 Dorough et al. (1978)
Rat F Oral Starch suspension 1 750 Hollander & Kramer (1975d)
Rat Wistar M/F Dermal > 2 000 Ehling & Leist (1991d)
Table 2. (continued)
Species Strain Sex Route Vehicle LD50 Reference
(mg/kg bw)
Endosulfan ether
Mouse Albino F Oral Tween 80 270 Dorough et al. (1978)
Rat F Oral Starch suspension > 15 000 Hollander & Kramer (1975c)
Rat Albino F Oral > 15 000 Weigand (1982b)
Endosulfan hydroxyether
Mouse Albino F Oral Tween 80 120 Dorough et al. (1978)
Rat F Oral Starch suspension 1 750 Hollander & Kramer (1975d)
Endosulfan lactone
Mouse Albino) F Oral Tween 80 120 Dorough et al. (1978)
Rat Wistar F Oral Starch suspension 290 Hollander & Kramer (1975e)
Rat Wistar M Oral Starch suspension 160 Hollander & Kramer (1975f)
Rat M/F Oral Sesame oil 100/120 Kramer & Weigand (1971)
M, male; F, female; CMC, carboxy methyl cellulose; PEG, polyethylene glycol
a 24 h on intact skin
The dermal irritancy of technical-grade endosulfan (purity,
98.6%) was tested in three New Zealand white rabbits by clipping the
hair from a dorsal area of about 25 cm2 and 24 h later applying 500
mg endosulfan moistened with deionized water on a 6.25-cm2 cellulose
patch, which was then covered with a semi-occlusive bandage. Exposure
was for 4 h, after which the test material was removed with warm
tap-water. The exposed area was examined 0.5-1, 24, 48, and 72 h after
removal of the patch. On the basis of the evaluation system defined by
EEC guideline B.4 ('Acute toxicity skin irritation' of Directive
92/69/EEC), the overall mean scores for dermal irritation were 0 for
both erythema and eschar formation and oedema formation. No signs of
systemic toxicity were observed (Bremmer, 1997a).
The ocular irritancy of technical-grade endosulfan (purity,
98.6%) was tested in three New Zealand white rabbits by applying 100
mg to the conjunctival sac of one eye of each rabbit; the other,
untreated eye served as the control. The eyes were exposed for 24 h,
after which the endosulfan was washed out, and the eyes were examined
for ocular lesions 1, 24, 48, and 72 h later. On the basis of the
evaluation system defined by EEC guideline B.5 ('Acute toxicity eye
irritation' of Directive 92/69/EEC), the overall mean scores for
irritation were 0.66 for redness of conjunctiva, 0 for chemosis of
conjunctiva, 0 for opacity of cornea, and 0.11 for irritation of the
iris. No signs of systemic toxicity were observed. Endosulfan was not
irritating to the eye (Bremmer, 1997b).
The cutaneous allergenic potential of endosulfan (purity, 98.6%)
was examined in 20 treated and 10 control male albino guinea-pigs. The
maximal tolerated concentration of endosulfan suitable for the
induction phase of the main study and a suitable non-irritating
concentration of topically applied endosulfan were identified for the
challenge application in a preliminary study. For intradermal
induction, injection of 0.1 ml of a 0.5% solution in corn oil
emulsified 1:1 (v:v) with Freund's complete adjuvant was selected. One
week after these injections, a 6-cm2 patch of filter paper saturated
with about 0.3 ml of a 50% solution of endosulfan in corn oil was
applied to the shaved skin of each guinea-pig, and the area was
occluded with aluminium foil secured by impermeable adhesive tape,
which was left in position for 48 h. Irritation was assessed 24 and 48
h later. On test day 22, the guinea-pigs were challenged with the
non-irritating 50% endosulfan in corn oil applied as during the
induction phase. The dressing was left in position for 24 h. The
application sites were assessed for erythema and oedema 24 and 48 h
later. On the basis of the evaluation system defined by EEC guideline
B.6 ('Acute toxicity -- Skin sensitization' of Directive 92/69/EEC),
none of the treated guinea-pigs developed skin reactions. Endosulfan
was therefore considered to be non-sensitizing for guinea-pig skin
(Arcelin, 1996).
(b) Short-term toxicity
Mice
Groups of 10 male and 10 female Hoe:NMRKf mice were fed diets
containing endosulfan at a concentration of 0 or 18 ppm for six weeks,
equal to 0 or 3.7 mg/kg bw per day for males and 0 or 4.6 mg/kg bw per
day for females, to determine whether 18 ppm was the NOAEL in this
strain, as it was found to be in CD-1 mice in a three-month study
(Barnard et al., 1984). There were no clinical signs attributable to
treatment. The mean food intake of mice receiving endosulfan was
slightly decreased in males and slightly increased in females. Mean
body-weight gain was reduced in treated males during the second half
of the experiment but was slightly increased in females throughout the
experiment. Two treated females died, on days 28 and 38; the causes
were not established, but no histological changes were found in the
mouse that was not autolysed. The absolute and relative weights of the
liver of males and females at 18 ppm were higher than those of
controls and statistically significantly so in females (17 and 12%,
respectively). A NOAEL was not identified, as an increase in liver
weights was seen in females at the only dose tested (Donaubauer et
al., 1985).
Groups of 20 CD-1 mice of each sex were fed diets containing
endosulfan (purity, 97.2%) at a concentration of 0, 2, 6, 18, or 54
ppm for three months, equal to 0, 0.24, 0.74, 2.1, and 7.3 mg/kg bw
per day for males and 0, 0.27, 0.8, 2.4, and 7.5 for females. Clinical
signs attributable to treatment, consisting of convulsions and
salivation, were seen in one male and one female at the high dose.
There was a marked treatment-related decrease in the survival rate
(about 50%) of male and female mice at the high dose; the mean food
intake of these animals was significantly reduced for the first two
weeks of the study, and the mean body-weight gain of males at the high
dose was reduced during the first week of treatment. A significant
reduction in neutrophil count was observed in males at 54 ppm at week
6 (72%, p < 0.01), and a lower count (62%), which was not
statistically significant, was also observed at week 13. The blood
glucose concentration was reduced by about 11% in females at all doses
(p < 0.01) at week 6, and the serum lipid concentration was
increased by 15% in females at 54 ppm at week 13. The NOAEL was
18 ppm, equivalent to 2.1 mg/kg bw per day, on the basis of
convulsions and salivation, decreased survival, and increased serum
lipid concentrations at 7.3 mg/kg bw per day (Barnard et al., 1984).
Groups of 10 four-week-old ddY mice of each sex were fed diets
containing technical-grade endosulfan (purity, 91.4%) at
concentrations of 0, 10, 30, 100, or 300 ppm, equal to 0, 1.2, 4.1,
15, and 42 mg/kg bw per day in males and 0, 1.4, 4.7, 14, and 42 mg/kg
bw per day in females, for 12 months. There were no apparent
treatment-related clinical signs or deaths. Males at the high dose
showed a small (1%) but significant decrease in mean corpuscular
volume, and transient, non-dose-related increases in haemoglobin
(11%), haematocrit (4%), and eosinophil counts (33%) were seen in
males at 30 ppm. A significant decrease in serum aspartate
aminotransferase activity was seen in males at 100 and 300 ppm and a
decrease in bilirubin concentration in males at the high dose. The
only change in organ weights was a dose-related increase in the
relative weight of the adrenals in females, which was about 30% and
statistically significant at 300 ppm. There were no treatment-related
changes on gross pathological examination; the histopathological
effects consisted of dose-related granulomatous changes in the liver
and lymph nodes. In the liver, granuloma, giant-cell infiltration,
and/or large histiocytic cells filled with brown pigment were found in
treated mice; these effects were significant at 100 and 300 ppm. In
the lymph nodes, giant-cell infiltration and/or reticuloendothelial
cell proliferation were found at the same doses. Testicular atrophy
was seen erratically, with incidences of 30, 70, 33, 50, and 80% in
the five groups, respectively, but was not considered to be related to
treatment. The NOAEL was 30 ppm, equal to 4.1 mg/kg bw per day, on the
basis of histological findings in the liver and lymphatic system (Arai
et al., 1981).
Rats
Groups of male albino rats were given endosulfan in peanut oil by
gavage at a dose of 0 or 11 mg/kg bw per day for 30 days. In addition,
the possible interaction between endosulfan and the chemosterilant,
metepa, was investigated in groups of rats receiving either metepa
alone at 30 mg/kg bw per day for 30 days or in combination with
endosulfan at 11 mg/kg bw per day. There were three deaths in the
endosulfan-treated group. Endosulfan alone had no significant effect
on body weights, organ weights, blood chemistry, or histopathological
appearance. No potentiation of the toxicity of metepa was seen (Nath
et al., 1978).
Groups of 100 male SPF Wistar rats were fed diets containing
technical-grade endosulfan (purity, 97.9%) at concentrations of 360 or
720 ppm, equal to 34 and 68 mg/kg bw per day for four weeks. Twenty
control rats received diet alone. Fifty treated and 10 control animals
were maintained for an additional four-week withdrawal period. The
objective of the experiment was to determine the toxicological
significance of yellow, granular deposits observed in the proximal
convoluted renal tubules in a 13-week and a two-generation feeding
study. One rat in each treated group died, with no signs of poisoning.
The behaviour and general condition of the rats and their food and
water consumption were unaffected by treatment, and body-weight gains
were comparable in treated and control groups. Enlargement of the
liver was observed in rats at 360 and 720 ppm at the end of the
treatment period, and the kidney and brain weights were significantly
elevated in those at 720 ppm; these treatment-related changes in organ
weights had, however, disappeared by the end of the withdrawal period.
The kidneys of treated rats were darkly discoloured, but their
appearance had returned to normal by the end of the withdrawal period.
Histopathological examination by light and electron microscopy showed
granular pigmentation and larger, more numerous lysosomes in renal
proximal tubule cells after treatment; these changes had decreased by
the end of the withdrawal period, and no lysosomal changes were found
in brain or liver. Analysis of residues showed that storage of
alpha-endosulfan was dose-dependent, temporary, and confined to the
kidney, where it was detected as endosulfan sulfate and endosulfan
lactone. The amount of ß-endosulfan was 230 times less than that of
alpha-endosulfan. The concentrations of the sulfate and lactone
metabolites were many times lower in liver, and only traces of
endosulfan remained in the kidney at the end of the withdrawal period
(Leist & Mayer, 1987).
Groups of 15 male and 15 female Wistar rats were exposed by
inhalation to technical-grade endosulfan (purity, 97.2%) at a
concentration of 0, 0.5, 1, or 2 mg/m3 air for 6 h/day, five days per
week for 29 days, for a total of 21 exposures. One male rat at the
high dose was emaciated, had a pale skin, and adopted a high-legged
position; no other clinical signs were seen in the treated animals. No
neurological disturbances, opacity of the refractive media, impairment
of dental growth, or changes in the oral mucosa were seen. The
body-weight gain of males at the high dose tended to be depressed from
day 20 of exposure until day 29, the end of the recovery period, but
no other changes in body-weight gain or food consumption were seen.
Non-dose-related increases in erythrocyte and haemoglobin
concentrations were seen at the end of exposure, but not at day 29;
these concentrations were reported to be within normal ranges for this
strain of rat. Apart from a transient, non-dose-related increase in
creatinine concentration and a decrease in serum aspartate
aminotransferase activity in females at the high dose, no
treatment-related change in blood chemistry was noted, and no
histological changes were seen in any of the rats (Hollander et al.,
1984)
Technical-grade endosulfan (purity, 97.2%) was applied in a
solution in sesame oil to the shaved skin of the nape of groups of six
Wistar rats of each sex 21 times over 30 days, for 6 h/day on five
days per week under an occlusive bandage. Males were given a dose of
0, 12, 48, 96, or 190 mg/kg bw per day, and females received 0, 3, 6,
12, or 48 mg/kg bw per day. In males, deaths and clinical signs of
poisoning consisting of tremors, tonoclonic convulsions, and/or
hypersalivation were observed only at the highest dose. In females,
clinical signs of poisoning were observed at > 2 mg/kg bw per day;
deaths occurred mainly in the group receiving 48 mg/kg bw per day,
although single animals died on day 18 after receiving 3, 6, or 12
mg/kg bw per day, none having shown signs of poisoning. Serum
cholinesterase activity was 33% lower in males at 192 mg/kg bw per
day, whereas the activities of the erythrocyte and brain enzymes were
reduced by 12 and 7%, respectively. In females at 48 mg/kg bw per day,
serum cholinesterase activity was 21% lower than in controls, and
there were no effects in the activities of erythrocyte and brain
enzymes. The NOAEL was 6 mg/kg bw per day (Ebert et al., 1985a,b).
Technical-grade endosulfan (purity, 97.2%) as a solution in
sesame oil was applied to the shaved skin of the nape of groups of six
male and six female Wistar rats 21 times over 30 days, for 6-h periods
under an occlusive bandage, at doses of 0, 1, 3, 9, 27, or 81 (males
only) mg/kg bw per day. No signs of toxicity were observed in males or
females at 1 and 3 mg/kg bw per day, but two males at 9 mg/kg bw per
day died, one on day 5 with no previous signs of poisoning and the
other on day 8 after piloerection, hypersalivation, blood-encrusted
nose, stagger, and dyspnoea. There were no deaths among males at 27
mg/kg bw per day, but three at 81 mg/kg bw per day died. None of the
females at 9 mg/kg bw per day died and no clinical signs of toxicity
were observed, but five females at 27 mg/kg bw per day died between
days 2 and 6 with no previous clinical signs of toxicity. Microscopic
changes were seen in the livers of animals at > 9 mg/kg bw per day,
consisting of enlargement of parenchymal cells in the periphery and
loss of cytoplasmic basoplilia. Serum cholinesterase activity was
statistically significantly reduced by treatment, by 70-80% in males
at doses of 9-81 mg/kg bw per day but in females by only about 40% at
9 mg/kg bw per day. Brain acetylcholinesterase activity was
statistically significantly reduced in males at 9 (21%), 27 (28%), and
81 (24%) mg/kg bw per day and in all treated females by 13-18%. The
responses were not dose-related, and the observations in females are
probably not biologically significant. Since a later study did not
demonstrate a direct inhibitory effect of endosulfan on rat brain
acetylcholinesterase activity in vitro, the reduced brain enzyme
activity found in this study was difficult to interpret. It is also
noted that serum enzyme activity was inhibited only at much higher
doses in the study of Ebert et al. (1985a,b). The two males at 9 mg/kg
bw per day that died had reduced or immature testes and/or sex organs,
and the livers of these animals has accentuated lobular markings. The
authors reasoned that these effects resulted from a
non-substance-related developmental disturbance already present before
treatment. No mechanism was proposed for these effects, which were not
seen at higher doses. The NOAEL was 3 mg/kg bw per day on the basis of
inhibition of serum and brain cholinesterase activity and microscopic
changes in the liver (Ebert et al., 1985b,c).
Endosulfan (purity not stated) as a solution in acetone was
applied daily to the shaved abdominal skin of groups of 24 albino rats
of each sex for 30 days at doses of 0, 19, 38, or
63 mg/kg bw per day for males and 0, 10, 20, or 32 mg/kg bw per day
for females. There were no deaths. All doses produced
hyperexcitability, tremor, dyspnoea, and salivation, which disappeared
after one week. No significant changes in organ:body weight ratios
occurred, and there were no treatment-associated effects on
histological, haematological, or blood chemical parameters. Liver
alanine and aspartate aminotransferase activities were reduced in
animals of each sex at the lowest dose, but there were no further
reductions with increasing dose. Liver alkaline phosphatase and
lactate dehydrogenase activities were increased in females but not in
males, again with no increase with dose. Cholinesterase activities
were not measured. A NOAEL was not identified (Dikshith et al., 1988).
Groups of 25 CD Sprague-Dawley rats of each sex were fed diets
containing technical-grade endosulfan (purity, 97.9%) at
concentrations of 0, 10, 30, 60, or 360 ppm, equal to 0, 0.64, 1.9,
3.8, and 23 mg/kg bw per day for males and 0, 0.75, 2.3, 4.6, and
27 mg/kg bw per day for females, for three months. Five animals of
each sex per group were maintained for an additional four-week
recovery period. Three females died, one each at 0, 60, and 360 ppm.
Slight but statistically significant, dose-related reductions in
erythrocyte counts and haemoglobin concentrations were seen in males
at > 30 ppm and in females at > 60 ppm, but were within the
reported normal range for this strain and age of rat (Leist & Bremmer,
1998); increased mean corpuscular volume was also seen at these doses.
Females at 360 ppm had statistically significant decreases in plasma
and erythrocyte cholinesterase activities (measured by the Ellman
method) at week 12 (by 41 and 12%, respectively), while increased
brain acetylcholinesterase activity was observed at 60 and 360 ppm (19
and 20%, respectively). In males at 360 ppm, urinalysis showed a
number of reversible changes, including increased urine volume and
urinary protein concentrations and decreased specific gravity. Gross
examination revealed enlargement of the liver in males at 360 ppm and
of the kidneys at 60 and 360 ppm; increases in the absolute weights of
the liver (18%), kidney (29%), and epididymides (8%) were seen in
males and of the liver (21%) and kidneys (10%) in females. The kidney
weights remained significantly elevated in male rats at 360 ppm (15%,
p < 0.01) at the end of the withdrawal period.
Histopathological examination revealed traces of brown pigment in
scattered hepatocytes in 25% of male rats and minimal centrilobular
enlargement of hepatocytes in 25% of females at 360 ppm. These changes
were not observed in rats at the end of the withdrawal period.
Yellowish discolouration of renal proximal tubular cells was seen in
males at all doses and in females at 30-360 ppm, the degree of
pigmentation increasing in a dose-related manner; however, no cell
death was associated with this finding. In addition, granular
pigmentation was seen in straight portions and occasionally in
proximal tubular cells in males at 60 and 360 ppm. The yellow
discolouration of the renal tubules in male rats had decreased by the
end of the withdrawal period, but trace or minimal pigmentation was
still evident. In females at > 60 ppm, the traces of pigmentation
persisted. Males at 360 ppm also had yellow protein aggregation in the
proximal convoluted with intracytoplasmic eosinophilic droplets in the
tubules. The increase in incidence and degree of the yellowish
discolouration of the proximal tubular cells appeared to be
treatment-related, since it did not develop in control rats; however,
no adverse effects were reported that might be associated with these
findings alone. All rats at 30 ppm showed either trace or minimal
discolouration and signs of granular or clumped pigment. Other
investigations suggest that the yellow pigmentation is no more than an
indication that endosulfan and some of its metabolites are being
temporarily stored before urinary excretion, and that it is therefore
an indicator of exposure rather than an expression of toxicity.
Consequently, this effect was not considered in the evaluation. At
doses of 60 and 360 ppm, other treatment-related effects were also
seen when the pigmentation was present, including enlarged kidneys and
centrilobular hepatocytes. No treatment-related increase in the
incidence of other renal effects was reported in animals at 10 ppm.
The NOAEL was thus 10 ppm, equal to 0.64 mg/kg bw per day, on the
basis of haematological changes (Barnard et al., 1985).
(c) Long-term studies of toxicity and carcinogenicity
Mice
Groups of 50 six- to seven-week-old B6C3F1 mice of each sex were
fed diets containing technical-grade endosulfan (purity, 98.8%) at
time-weighted average concentrations of 3.5 or 6.9 ppm for males and 2
or 3.9 ppm for females for 78 weeks. Groups of 20 controls received
untreated diet. There were no clear compound-related effects on
appearance or behaviour in the treated groups, and the body weights of
both males and females were unaffected by treatment. The mortality
rate of males at the high dose was increased early in treatment so
that, at the end of the experiment, the survival rates were 3/20
controls, 19/50 at the low dose, and 5/50 at the high dose. The
mortality rates of female mice were not affected by treatment. No
treatment-related clinical signs were recorded, and no
treatment-related neoplastic lesions were seen in the females. Owing
to the high early mortality rates, no conclusion could be drawn about
the carcinogenic potential of endosulfan in males. None of the
non-neoplastic changes seen in the kidneys and sex organs of male and
female mice could be attributed to treatment. The NOAEL for female
mice was 3.9 ppm, equal to 0.58 mg/kg bw per day (US National Cancer
Institute, 1978).
Groups of 60 NMRI mice of each sex were fed diets containing
technical-grade endosulfan (purity, 97.2%) at concentrations of 0, 2,
6, or 18 ppm, equal to 0.28, 0.84, and 2.5 mg/kg bw per day for males
and 0.32, 0.97, and 2.9 mg/kg bw per day for females, for up to 24
months. Ten mice of each sex per dose were killed at 12 and 18 months.
The behaviour and general health of the animals were not affected by
treatment. The mortality rate of females at 18 ppm was statistically
significant decreased at the end of the experiment: control, 45%; 18
ppm, 28% (p < 0.05); at week 78, the rates in these two groups of
female mice were 82 and 62%. Survival among treated male mice was not
statistically different from that of controls. The body weights of
males receiving 18 ppm were slightly but significantly lower than
those of controls during the first third of the study and remained
slightly but not significantly low throughout the remainder of the
study. In the other treated groups, there was a tendency to increased
body-weight gains, especially in the satellite groups killed at 12 and
18 months. No statistically significant changes were observed in
haematological or clinical chemical parameters, and macroscopic
examination did not reveal any findings that were related to
treatment. No statistically significant changes in organ weights were
seen in treated animals at the end of the experiment; however, slight
but statistically significant changes in organ weights were observed
in animals at 18 ppm at 12 and 18 months, consisting of decreased lung
and ovary weights in females at 12 months and decreased liver weights
in males and decreased ovary weights in females at 18 months.
Histopathological examination did not reveal any effects that were
related to treatment. No increase in the incidence of neoplastic or
non-neoplastic lesions was observed. The NOAEL was 6 ppm, equal to
0.84 mg/kg bw per day, on the basis of decreased body weights in males
at 24 months and decreased weights of the liver, ovaries, and lung in
males and females at 12 and/or 18 months (Donaubauer, 1988, 1989; Hack
et al., 1995).
Rats
Groups of 50 male and 50 female Osborne-Mendel rats were fed
diets containing technical-grade endosulfan (purity, 98.8%) at
time-weighted average doses of 220, 410, or 950 ppm for males and 220
or 400 ppm for females for 78 weeks, with a return to control diets
for a further four weeks. Groups of 20 rats of each sex received
untreated diet. A highly significant morbidity rate was seen in male
rats and, by week 54, 52% of those at the high dose had died. A
dose-related reduction in body weight was found in males at all doses.
Histopathological examination showed a high incidence of toxic
nephropathy (> 90%) in males at the low and high doses and in
females, but in none of the controls. Chronic renal inflammation was
observed in 40% of control males and 80% of treated males. The toxic
nephropathy observed was characterized as degenerative changes in the
proximal convoluted tubules at the junction of the cortex and medulla,
with associated cloudy swelling, fatty degeneration, and necrosis of
the tubular epithelium. Some tubules had hyaline casts, and enlarged,
dark-staining regenerative tubular epithelial cells were observed
infrequently. In treated males, parathyroid hyperplasia was observed,
as were calcium deposits in the stomach, kidney, testis, aorta, and
mesenteric artery. A dose-related increase in the incidence of
testicular atrophy was seen in treated males, characterized by
degeneration and necrosis of the germinal cells lining the
seminiferous tubules and multinucleated cells (fusion bodies),
resulting in aspermatogenesis. No treatment-related effects were noted
in the reproductive organs of female rats. No treatment-related
neoplastic lesions were seen in female rats; owing to the high
mortality rate in males, no valid conclusion can be drawn about
carcinogenicity. A NOAEL was not identified, as treatment-related
changes occurred in the kidneys and the testis at all doses (US
National Cancer Institute, 1978)
Groups of 50 five-to-six-week-old Sprague-Dawley rats of each sex
were fed diets containing endosulfan (purity, 97.1%) at concentrations
of 0, 3, 7.5, 15, or 75 ppm, equal to 0, 0.1, 0.3, 0.6, and 2.9 mg/kg
bw per day for males and 0, 0.1, 0.4, 0.7, and 3.8 mg/kg bw per day
for females, for 104 weeks. Satellite groups of 20 animals of each sex
were retained for blood sampling and examination at 104 weeks.
Reductions in body weights and body-weight gains were observed in
males (group mean, 17% at 104 weeks) and females (group mean, 18% at
104 weeks) at 75 ppm, but no clinical signs of poisoning were seen at
any dose. No increase in mortality rates was observed in treated
groups. Increased incidences of enlarged kidneys in females and of
aneurysms and enlarged lumbar lymph nodes in males were seen at 75
ppm. Histopathological examination showed that males at 75 ppm had an
increased incidence of aneurysm and marked progressive
glomerulonephrosis (controls, 20/70; 75 ppm, 30/70). The commonest
neoplasms were pituitary tumours in males and females and mammary
tumours in females, but the increased incidences did not appear to be
related to treatment. The NOAEL was 15 ppm, equal to 0.6 mg/kg bw per
day, on the basis of reduced body weights and pathological findings at
higher doses (Ruckman et al., 1989; Gopinath & Cannon, 1990; Hack et
al., 1995).
Groups of 25 Wistar rats of each sex were fed diets containing
technical-grade endosulfan (purity unspecified) at doses of 0, 10, 30,
or 100 ppm for up to 104 weeks; groups of five of each sex were killed
at 52 weeks. There were no treatment-related clinical signs, and the
body weights were unaffected, except for a nonsignificant decrease in
the body weights and food consumption of males at the high dose. The
survival rate of treated females was reduced, the deaths being
associated predominantly with respiratory infections. The weights of
the testes of males at 10 ppm were reduced by 7% with respect to
controls at 104 weeks (p < 0.05), and the kidney weights were
significantly (p < 0.001) increased (by 16%) in males at the high
dose at 104 weeks. Histopathological changes observed in males at the
high dose at 104 weeks consisted of enlarged kidneys, mild-to-severe
renal tubular dilatation (12/12), mild-to-moderate formation of
irregular albuminous casts (10/12), pronounced focal nephritis (7/12),
and mild-to-severe degeneration (11/12) of the renal tubular
epithelium. At 104 weeks, female rats at the high dose showed minimal
degeneration of renal tubules (2/3) and some focal nephritis (1/3).
The low survival rate precluded a clear conclusion about the renal
changes in female rats. Microscopic alterations in the liver were seen
in 50% of males at the high dose at week 104, consisting of focal
areas of hydropic cells, which were pale and swollen; the nuclei were
surrounded by a clear zone, and a few cells appeared to have
eosinophilic cytoplasmic inclusions. Few females at the high dose
showed changes in liver cells. A few tumours developed during the
experiment, but their occurrence was not dose-related. The NOAEL was
30 ppm, equal to 1.5 mg/kg bw per day, on the basis of effects on the
kidney (Keller, 1959c).
Dogs
Groups of six beagle dogs of each sex were fed diets containing
technical-grade endosulfan (purity, 96.5%) at concentrations of 0, 3,
10, or 30 ppm for one year, calculated by the authors to be equivalent
to 0, 0.23, 0.77, and 2.3 mg/kg bw per day. In addition, one group was
given a diet containing 30-60 ppm endosulfan, increasing in stages
from 30 ppm for 54 days, to 45 ppm for 52 days, and 60 ppm for 19-40
days; these dogs were killed in extremis before the scheduled
completion of the experiment and showed a number of signs of
poisoning, including tonic contraction and increased sensitivity to
noise and optical stimuli. Some animals given endosulfan at 30 ppm
throughout the 12-month study had violent contractions of the
abdominal muscles (without vomiting), and males at this dose had
reduced body-weight gains throughout the study and slightly reduced
body weights in the latter stages of the study, in comparison with
control animals. Cholinesterase activity was measured in serum,
erythrocytes, and brain, but difficulty appears to have been
experienced in measuring these activities, and there were large
variations within groups for the brain enzyme, the group mean of which
was increased in dogs at 30 ppm. No other effects related to treatment
were observed, and no increase in the incidence of neoplastic or non-
neoplastic lesions was observed in treated animals. The NOAEL was 10
ppm, calculated by the authors to be equivalent to 0.57 mg/kg bw per
day, on the basis of clinical signs and reductions in body weight
(Brunk 1989, 1990).
Groups of two male and two female mongrel dogs were given
technical-grade endosulfan in gelatin capsules at doses of 0, 3, 10,
or 30 ppm, equivalent to 0, 0.075, 0.25, or 0.75 mg/kg bw per day, on
six days per week for one year. The group receiving 3 ppm was given
100 ppm for the first three days of treatment, but clinical signs of
vomiting, tremors, convulsions, rapid respiration, mydriasis,
salivation, and tonic-clonic convulsions in one male and both females
led to a reduction in the dose for the remainder of the study. No
clinical signs or treatment-related effects on body-weight gain were
seen. Clinical chemical and haematological end-points were within
normal limits, and kidney function was unaffected by treatment. No
gross or histopathological changes associated with treatment were
noted. The NOAEL was 30 ppm, equivalent to 0.75 mg/kg bw per day, on
the basis of clinical signs at the initial high dose (Keller, 1959b).
(d) Genotoxicity
Endosulfan was tested for genotoxicity in a wide range of assays,
both in vitro (with and without metabolic activation) and in vivo
(Table 3). There was no evidence of genotoxicty in most of these
assays. In an assay for dominant lethal mutation in male Swiss mice
given endosulfan of a purity of 97.3%, there was a significant change
in the result of mating during the sixth mating week in the group at
16.6 mg/kg bw per day. The total numbers of implants per pregnancy
were 9 in controls and 4.5 at the high dose; the numbers of live
implants per pregnancy were 9 in controls and 2.2 at the high dose;
and the numbers of dead implants per pergnancy were none in controls
and 2.25 at the high dose (Pandey et al., 1990). While there is no
doubt about the statistical significance of these results, it is
unusual to find a true dominant lethal effect appearing so late in an
experimental mating schedule; however, it is not unknown, since a
reproducible effect of this type was demonstrated with some glycol
ethers (McGregor et al., 1983). In a later assay for dominant lethal
mutation (Dzwonkowska & Hübner, 1991), much lower doses were used, so
the results cannot be used as evidence for a non-reproducible effect.
Significant increases in the proportion of morphologically abnormal
sperm were also observed in the study of Pandey et al. (1990), as well
Table 3. Results of assays for the genotoxicity of endosulfan
End-point Test object Dose Result Reference
(LED or HID)a
In vitro
Differential toxicity B. subtilis rec strains H17 and M45 2000 µg/disc Negativea Shirasu et al. (1978)
Reverse mutation S. typhimurium TA100, TA1535, 5000 µg/plate Negativeb Shirasu et al. (1978)
TA1537, TA1538, TA98;
E. coli WP2 uvrA
Gene conversion S. cerevisiae, D4 5000 µg/ml Negativeb Mellano & Milone (1984b)
Forward mutation S. pombe 500 µg/ml Negativeb Mellano & Milone (1984a)
Unscheduled DNA Male F344 rat primary hepatocytes 51 µg/ml Negativea Cifone & Myhr (1984b)
synthesis
Gene mutation Mouse lymphoma L5178Y cells, 75 µg/ml [?] Negativeb Cifone & Myhr (1984a)
tk locus
Chromosomal Human lymphocytes 200 µg/ml Negativeb Asquith & Baillie (1989)
aberration
Chromosomal Human lymphocytes 200 µg/ml Negativeb Pirovano & Milone (1986)
aberration
In vivo
Micronucleus NMRI mouse bone-marrow cells 5 mg/kg bw, Negative Jung et al. (1983)
formation po × 1
Micronucleus NMRI mouse bone-marrow cells 10 mg/kg bw, Negative Müller (1988)
formation po × 1
Table 3. (continued)
End-point Test object Dose Result Reference
(LED or HID)a
Chromosomal Albino rat bone-marrow cells 55 mg/kg, po × 5 Negative Dikshith & Dotta (1978)
aberration
Dominant lethal Male Swiss mice 16.6 mg/kg bw, Equivocal Pandey et al. (1990)
mutation ip × 5
Dominant lethal Male Balb/c mice 0.64 mg/kg bw, Negative Dzwonkowska & Hübner
mutation ip × 1 and ip × 5 (1991)
Sperm morphology Mice 16.6 mg/kg bw, Positive Pandey et al. (1990)
ip × 5
Sperm morphology Mice in vivo 3 mg/kg bw, Positive Khan & Sinha (1996)
ip × 35
LED, lowest effective dose; HID, highest ineffective dose; po, oral; ip, intraperitoneal
a In the absence of exogenous metabolic activation; not tested in the presence of exogenous metabolic activation
b In the absence and presence of exogenous metabolic activation
as in a later study (Khan & Sinha, 1996) at lower daily doses of a 35%
emulsifiable concentrate.
(e) Reproductive toxicity
(i) Multigeneration reproductive toxicity
A preliminary investigation was conducted to determine the doses
of endosulfan to be used in a two-generation study of reproductive
toxicity. Four groups of 10 male and 10 female seven-week-old Crl:
COBS CD Sprague-Dawley rats were given diets containing
technical-grade endosulfan (purity, 97%) at concentrations of 0, 50,
75, or 100 ppm for two weeks and subsequently throughout mating and
the rearing of offspring to weaning. Food consumption and body weights
were decreased in adults at 75 and 100 ppm. At terminal autopsy, the
mean weights of the livers were significantly higher than the control
value in all treated groups. Mating performance, pregnancy rate, and
the duration of gestation were unaffected by treatment. The litter
weights of dams were significantly decreased at 75 ppm and to a
greater extent at 100 ppm from day 4 post partum. No
treatment-related abnormalities were found in the young (Edwards et
al., 1982).
In a two-generation study of reproductive toxicity with two
matings in each generation, four groups of six-week-old
Sprague-Dawley rats were fed diets containing technical-grade
endosulfan (purity, 97%) at concentrations of 0, 3, 15, or 75 ppm,
equal to 0.2-0.23, 1-1.2, and 5-5.7 mg/kg bw per day for males and
0.24-0.26, 1.2-1.3, and 6.2-6.9 mg/kg bw per day for females. The
group sizes were 32 of each sex for the F0 generation and 28 of each
sex for the F1b generation. No clinical signs or deaths related to
treatment were observed during the study. Single deaths occurred among
F0 females at 0, 3, and 15 ppm and among F1b control females. Mating
performance and pregnancy rates were not affected by treatment.
Statistically significant decreases in litter weight were occasionally
seen, but there was no effect on mean pup weights or on litter size.
No treatment-related effect on sex ratios was seen at any dose.
Statistically significantly increased relative kidney weights
were seen at 75 ppm in F0 and F1b males, and statistically
significantly increased relative liver weights were observed in F0
males and females at 75 ppm and in F1b females at 15 and 75 ppm. The
effect at 15 ppm in F1b dams was not seen at this dose in any other
matings. Yellowish discolouration of cells in the proximal convoluted
tubules were observed in male F1b rats at 3, 5, and 75 ppm and in
female F1b rats at 75 ppm. The incidence and extent of this effect
was dose-related; traces of discolouration were seen at all doses, and
minimal discolouration was seen in male rats at 15 and 75 ppm.
Granular or clumped pigment was seen in proximal convoluted tubular
cells in males at the high dose, but these findings were not
associated with histopathological evidence of renal damage. While the
increased incidence of cellular discolouration was related to
treatment, the finding is considered not to be toxicologically
significant as no adverse effects were seen on cells and the yellow
pigment was considered likely to be due to storage of endosulfan and
its metabolites in lysosomes before excretion (Annex 1, reference 58).
The presence of the pigment is thus an indication of exposure to
endosulfan rather than of toxicity. The NOAEL for maternal toxicity
was 15 ppm, equal to 1 mg/kg bw per day, on the basis of increased
relative liver and kidney weights at higher doses. The NOAEL for
reproductive effects was 75 ppm, equal to 6 mg/kg bw per day, the
highest dose tested (Edwards et al., 1984; Offer, 1985).
(ii) Developmental toxicity
Rats
Groups of mated female albino rats (strain and age unspecified)
were given oral doses of 0 (20 rats), 5 (26 rats), or 10 (32 rats)
mg/kg bw per day of endosulfan (purity unspecified) on days 6-14 of
gestation. No marked changes in behaviour or appearance were reported,
and the body weights of treated animals were similar to those of
controls. The numbers of pregnancies were 18, 20, and 21,
respectively. The dams were killed on day 21 of gestation. No
abortions occurred, but there was a significant increase in the
percent of litters with resorptions (5.5% in controls, 20% at 5 mg/kg
bw per day, and 23% at 10 mg/kg bw per day) and increased fetal
mortality, although this effect was slight and was not dose-related
(0, 2, and 1 in the three groups, respectively). Slight increases in
the incidences of cerebral hypoplasia and enlargement of the renal
pelvis were observed on visceral examination, but these effects were
not considered to be related to treatment as they were also seen in
control animals and the increases were small and were not
dose-dependent. No other increase in the incidence of visceral
abnormalities was reported. Skeletal examination showed statistically
significant increases in the incidences of absent fifth sternebrae and
of fetuses with incomplete ossification. A slight increase in the
incidence of absent fifth metacarpus, although not statistically
significant, was also seen in treated animals. These effects were not
considered to be related to treatment, as their magnitude was small
and they were not dose-dependent. No maternal toxicity was seen at any
dose. The level of reporting in this published paper was inadequate
for identifying a NOAEL for developmental toxicity (Gupta et al.,
1978).
Groups of 25 mated CD Sprague-Dawley rats were given
technical-grade endosulfan (purity, 97.3%) in corn oil by gavage on
days 6-19 of gestation at a dose of 0, 0.66, 2, or 6 mg/kg bw per day.
The clinical signs in dams at 6 mg/kg bw per day included flaccidity,
rough coat, alopecia, and hyperactivity. A dose-related decrease in
maternal body-weight gain was seen at 2 and 6 mg/kg bw per day. The
number of implantations and litter size were unaffected, but there was
a slight reduction in the weight and length of fetuses of dams at the
high dose. A non-dose-related reduction in the percent of live fetuses
and an increase in the number of resorbed fetuses were seen at 2 mg/kg
bw per day. No statistically significant treatment-related effect on
the sex ratio was observed. No external variations or malformations
were seen at 0.66 or 2 mg/kg bw per day; at the high dose, 5/405
fetuses exhibited lordosis (anteroposterior curvature of the spine)
and six had oedema. All five of the fetuses with lordosis and five of
those with oedema were from a single litter. In one fetus from the
same litter, the skin of the upper forelimb was webbed to the chest.
No significant treatment-related effects were seen on soft-tissue
development. Common minor skeletal variations were present in all
groups. The incidence of poorly ossified sixth sternebrae was
significantly greater in animals at the high dose than in the control
group, and two fetuses at this dose had clubbed left hindlimbs. The
five fetuses from the same litter that had oedema and lordosis also
had wide, thickened vertebral arches, ribs, and clavicles, and the
clavicles were shortened, curved, and twisted. Four of these fetuses
had shortened pubes, and two had an unossified hyoid bone. The
incidence of these effects was generally < 1%, and the effects were
largely related to delayed development and confined mainly to a single
litter from a single dam that showed numerous signs of poisoning
related to administration of endosulfan, including face rubbing,
alopecia, flaccidity, and hyperactivity. The developmental effects are
therefore probably related to the maternal toxicity of the high dose.
The NOAEL for maternal toxicity was 0.66 mg/kg bw per day on the basis
of decreased body-weight gain and clinical signs at higher doses. The
NOAEL for developmental toxicity was 2 mg/kg bw per day on the basis
of delayed development and a low incidence of isolated skeletal
variations (Mackenzie, 1980).
Groups of 20-24 mated female Wistar rats were given
technical-grade endosulfan (purity, 97.3%) dissolved in sesame oil by
gavage on days 7-16 of gestation at doses of 0, 0.66, 2, or 6 mg/kg bw
per day. No clinical signs of toxicity were reported in females at
0.66 or 2 mg/kg bw per day; four dams at 6 mg/kg bw per day died after
6-10 doses of endosulfan, three of these rats having tonic-clonic
convulsions for several days before death. Thirteen of the surviving
animals had tonic-clonic convulsions for a number of days, generally
around day 10 of gestation. Some of these rats also showed
hypersalivation on a number of days during treatment. Statistically
significant decreases in body weight and body-weight gain were
observed at 6 mg/kg bw per day. No statistically significant changes
in reproductive or pup parameters were observed at any dose, and the
fetal sex ratio was relatively well balanced. No statistically
significant increase in the incidence of abnormalities was observed in
fetuses. A single oedematous, retarded fetus at 6 mg/kg bw per day
presented with superior brachygnathia and a relatively small alveolar
cavity in the upper jaw, combined with cleft palate, bending of both
hind feet in the tarsal joint, wavy clavicles, and bent and shortened
scapulae. These effects were considered to be spontaneous, as no other
limb or head defects were observed in any pup in any of the litters at
this dose. Skeletal examination revealed a statistically significant
increase in the incidence of fragmented thoracic vertebral centra at 6
mg/kg bw per day. This effect was considered to be treatment-related
and reflects the frank maternal toxicity of endosulfan at the high
dose. No treatment-related major malformations were observed. The
NOAEL for maternal toxicity was 2 mg/kg bw per day on the basis of
deaths, clinical signs, and decreased body weights at higher doses.
The NOAEL for developmental toxicity was 2 mg/kg bw per day on the
basis of the increased incidence of fragmented thoracic vertebral
centra (Albrecht & Baeder, 1993).
Rabbits
Groups of 20-26 mated New Zealand white rabbits were given
technical-grade endosulfan (purity, 97.3 %) in corn oil by gavage on
days 6-28 of gestation at doses of 0, 0.3, 0.7, or 1.8 mg/kg bw per
day. There were no changes in mean body weight. None of the does at
0.3 or 0.7 mg/kg bw per day aborted, and there were no signs of
toxicity and no deaths. The high dose was associated with signs of
maternal toxicity that included noisy, rapid breathing, hyperactivity,
and convulsions. The does were killed on day 29 of gestation. The
number of implantations, litter size, sex ratio, mean fetal weight and
length, and the numbers of live and resorbed fetuses were unaffected
by treatment. There were no dead fetuses in any group, and no gross
external alterations were reported. The only soft-tissue anomalies
occurred in 6/167 fetuses (2/20 litters examined) at the high dose and
consisted of the left carotid arising from the innominate; 1/141
control fetuses (1/18 litters examined) also showed this abnormality.
Common skeletal variations and minor anomalies occurred at similar
incidences in control and treated fetuses. Endosulfan did not have
teratogenic or developmental effects even at the maternally toxic dose
of 1.8 mg/kg bw per day. The NOAEL for maternal toxicity was 0.7 mg/kg
bw per day on the basis of clinical signs at higher doses (Dickie et
al., 1981).
(f) Special studies
(i) Enzyme induction
Endosulfan was one of 16 organochlorine pesticides tested for
their ability to induce hepatic microsomal enzyme activities. Wistar
rats were given diets containing endosulfan at concentrations of 0,
20, 50, or 200 ppm for two weeks. No difference from control enzyme
activity was observed at 20 or 50 ppm; at 200 ppm group, the
activities in comparison with the control were 123% for aniline
hydroxylase (statistically significant, one experiment), 191% for
aminopyrene demethylase (statistically significant, one experiment),
and 124% for hexobarbital oxidase (not significant, one experiment)
(den Tonkelaar & van Esch, 1974).
ICR mice given endosulfan at 5 mg/kg bw per day by oral gavage
for three days and killed on the fourth day showed no increase in
liver weight or total hepatic cytochrome P450 content. The dearylation
of O-ethyl O- para-nitrophenyl phenylphosphorothioate and
parathion and NAD(P)H-dependent reductase activity were
nonsignificantly increased (Robacker et al., 1981).
(ii) Promotion
Enhancement of gamma-glutamyl transpeptidase-positive foci in rat
liver was studied as an indicator of promotion. Young male
Sprague-Dawley rats were partially hepatectomized and injected
intraperitoneally 24 h later with N-nitrosodiethylamine at 30 mg/kg
bw. One week after the partial hepatectomy, the rats were randomized
to groups of 10 or 11 and dosed orally by gavage on five days per week
for 10 weeks with endosulfan in corn oil at 0, 1, or 5 mg/kg bw per
day. The numbers and volume of enzyme-altered foci were not increased
in the treated groups in comparison with controls.
In the same study, inhibition of intercellular communication was
studied in Chinese hamster lung V79 cells in a metabolic cooperation
assay and in rat liver WB epithelial cells in a scrape loading,
dye-transfer assay. At nontoxic concentrations, technical-grade
endosulfan, analytical-grade ensosulfan (alpha and ß isomers and a
mixture of the two), and endosulfan sulfate inhibited gap-junctional
intercellular communication in both systems. In addition, endosulfan
ether was effective in the rat liver WB cell system (Flodström et al.,
1988).
(iii) Immunotoxicity
In two published studies, endosulfan was administered to male
Wistar rats at dietary doses of up to 50 ppm for six weeks (Banarjee &
Hussain, 1987) or up to 20 ppm for 22 weeks (Banarjee & Hussain, 1986)
to evaluate humoral and cell-mediated immune responses. In the
six-week study, a significant decrease in total serum antibody titre
to tetanus toxoid was seen at 30 and 50 ppm, with a slight decrease
(not statistically significant) at 10 ppm. A decrease in both
immunoglobulin (Ig)M and IgG and in the total gamma-globulin content
of rat serum was observed at 50 ppm. Cellular immunity was assayed by
measuring inhibition of migration of activated leukocytes and
macrophages. Rats exposed to endosulfan and subsequently immunized
with tetanus toxoid showed a significant decrease in inhibition of
leukocyte and macrophage migration in a dose-dependent pattern, the
decrease becoming statistically significant at 30 and 50 ppm. These
results indicate that both humoural and cellular immunity was
depressed as a result of exposure to endosulfan at 30 and 50 ppm, with
no effect at 10 ppm, equivalent to 0.5 mg/kg bw per day. In the
22-week study, the specific response of serum antibody titre to
tetanus toxoid showed a marked decrease in rats exposed to 10 or 20
ppm endosulfan throughout the experiment, in a dose- and
time-dependent pattern. Treatment at 10 or 20 ppm diminished the
inhibition of migration of both leukocytes and macrophages throughout
the study.
The effect of endosulfan on the immune system did not appear to
be secondary to other toxic effects, since the body weights of the
animals were unaffected by treatment and endosulfan is not known to
affect the hormonal system. Immune responses were unaffected by
treatment at 5 ppm, equivalent to 0.25 mg/kg bw per day. The Committee
noted that the method used to assess cellular immunity in these
studies is far from ideal as it is flawed by large inherent errors,
lack of objectivity, and, except in very experienced hands, lack of
accuracy. Less subjective tests of cellular immunity, such as
cytotoxic T cell response to a virus, would have provided more
reliable results.
Technical-grade endosulfan (purity, 96%) was administered in
sesame oil on 10 occasions by gavage to groups of eight female Wistar
rats at doses of 0.5, 1.5, or 4.5 mg/kg bw per day from two days
before until seven days after infection by gavage with approximately
500 Trichonella spiralis larvae. As a positive control, prednisolone
was administered by subcutaneous injection at a dose of 25 mg/kg bw
per day two days before and three days after infection. Three rats
from each group were killed seven days after infection so that the
number of adult worms in the intestine could be counted; the remaining
rats were killed 54 days after infection in order to count the number
of larvae in the tongue. Thymus and spleen weights and the percentage
lymphocytes in the white cell count were measured at both times. Body
weights were measured weekly. There were no differences between
endosulfan-treated and untreated rats, whereas the
prednisolone-treated group had a sevenfold higher tongue larval count
and, at seven days, a 25% reduction in thymus weight, a 50% reduction
in spleen weight, and lymphocyte counts < 50% of the control value
(Hack & Leist, 1988).
Endosulfan was included in the first part of a study to screen
for immunotoxicity, but because no effect was observed it was not
examined in greater detail. Groups of six male, weanling Wistar rats
were given endosulfan in the diet at concentrations of 20, 100, or 250
ppm for three weeks. Body weights and food intake were recorded
weekly. At autopsy, the weights of the liver, kidneys, spleen, thymus,
pituitary, adrenals, thyroid, testis, and mesenteric and popliteal
lymph nodes were recorded, these organs were also examined
histologically. Haematological examination consisted of total and
differential leukocyte counts. Serum IgM and IgG were determined by
enzyme-linked immunosorbent assay. The only effects induced by
endosulfan were considered to be expressions of general toxicity, and
there was no evidence for any specifically immunotoxic effects. The
most sensitive parameter for the toxicity of endosulfan was a
reduction in body-weight gain, which was observed at 100 ppm (Vos et
al., 1982).
(iv) Neurobehavioural effects and neurotoxicity
In a number of studies conducted by the same group of
investigators, endosulfan (purity, 95%) was given by gavage to rats at
a dose of 2 mg/kg bw per day for 90 days (Paul et al., 1993, 1994) or
up to 6 mg/kg bw per day for 30 days (Paul et al., 1995), and
behavioural and biochemical changes were determined. Signs of frank
toxicity (reduced body weights, reduced food consumption, death,
increased intensity of tremors, and increased liver enzyme activity)
were observed in all studies, and some changes in behaviour were
noted, including increased motor activity and inhibition of
conditioned and unconditioned escape and avoidance responses.
Groups of adult domestic hens were given a single oral dose of 96
mg/kg bw endosulfan, the LD50, in corn oil, observed for 21 days,
re-dosed, and again observed for 21 days. As was to be expected, a
number of deaths occurred that were related to treatment. No signs of
ataxia and no treatment-related changes in nervous tissue were seen in
vehicle control or endosulfan-treated hens. Seven of 10 hens in a
concurrent positive control group given tri- ortho-cresyl phosphate
developed ataxia and signficant spinal cord and peripheral nerve
degeneration (Roberts et al., 1983).
No inhibition of rat brain acetylcholinesterase activity was
observed in a preparation incubated with 10 µmol/L alpha-endosulfan
for up to 75 min.. A similar concentration of aldicarb produced 15%
inhibition within 5 min and 80% inhibition within 75 min (Müllner,
1989).
Technical-grade endosulfan (purity, 98.6%) was administered to
groups of 10 rats as a single dose of 25, 50, or 100 mg/kg bw to males
and 3, 6, or 12 mg/kg bw to females. Deaths occurred at the highest
doses, and there was a dose-related increase in the frequency of
clinical signs, which were reversible and apparent only on the day of
dosing. These were assumed to be due to the known affinity of
endosulfan for the gamma-aminobutyric acid receptors in the brain. At
50 and 100 mg/kg bw in males and 6 and 12 mg/kg bw in females, various
serious neuropharmacological effects were seen, including coarse
tremor and tonic-clonic convulsions. At 25 mg/kg bw in males and
3 mg/kg bw in females, the clinical signs seen were typical of general
discomfort, such as stilted gait, squatting posture, and irregular
respiration. No compound-related effects on motor activity were
observed at non-lethal doses. No effects were seen on the rearing
frequency, fore- or hindlimb grip strength, or on landing foot-spread.
No histopathological effects were found in the central or peripheral
nervous system. The study was carried out in accordance with
prevailing OECD testing guidelines (April 1996) and the OECD
principles of good laboratory practice (12 May 1981 [C(81)30(Final)])
(Bury, 1997).
(v) Effects on sperm
In a study of biochemical changes induced by endosulfan in the
testis of Druckrey rats, the authors postulated that endosulfan
impairs testicular function by altering the enzyme activities
responsible for spermatogenesis, thus affecting the intratesticular
spermatid count and resulting in low sperm production and increased
sperm deformities. The data presented support the notion that
administration of endosulfan at relatively high doses (> 2.5 mg/kg
bw per day) for several months increases the activity of a number of
enzymes in the testes, including lactate dehydrogenase, sorbitol
dehydrogenase, gamma-glutamyl transpeptidase, and glucose-6-phosphate
dehydrogenase. At doses of 5 mg/kg bw per day and higher, there was a
marked reduction in sperm count (up to 47%) in comparison with
controls. In the absence of historical control data, it is unclear
whether the decrease in sperm count seen at 2.5 mg/kg bw per day (22%)
was within the normal biological range for the test animals. The sperm
abnormalities and the reductions in spermatid count and sperm
production, while statistically significantly different from those in
concurrent controls, were only slight. In the absence of consistent
dose-response relationships for these effects, they were considered
not biologically significant (Sinha et al., 1995).
The effects of endosulfan on gonadal hormones, measured as plasma
and testicular testosterone, plasma follicle-stimulating and
luteinizing hormones, and plasma and testicular 3ß- and
17ß-hydroxysteroid dehydrogenases, was investigated in Wistar rats
given endosulfan in peanut oil at doses up to 10 mg/kg bw per day for
up to 30 days. Significant inhibition of 3ß- and 17ß-hydroxysteroid
dehydrogenases occurred in the testes of treated animals after 30 days
of treatment, and the plasma follicle-stimulating hormone, luteinizing
hormone, and testosterone concentrations were significantly
(p < 0.05) reduced in rats treated for 15 or 30 days at either
dose. Plasma and testicular testosterone concentrations were not
significantly reduced after 15 days of treatment at 7.5 mg/kg. A
significant decrease in the content or activity of microsomal
cytochrome P450 and related mixed-function oxidases was observed in
the testes of treated animals, with marked inhibition of the activity
of glutathione- S-transferase at both doses. The latter changes were
reversed when endosulfan was withdrawn, but the testicular
testosterone concentrations remained significantly reduced (Singh &
Pandey, 1990).
(vi) Endocrine effects
An estrogen is a substance that can induce estrus or a biological
response associated with estrus; one such effect is proliferation of
cells in the female genital tract. In recent years, naturally
occurring and man-made substances in the environment that may be
estrogenic have come under increasing scrutiny. Suspicion that
endosulfan may have estrogenic properties was stimulated by
observation of the reduced sperm counts described above and of
testicular atrophy in rats given endosulfan in the diet in long-term
studies.
Perhaps the first study of estrogenic effects in vivo was
conducted by Raizada et al. (1991), who treated groups of eight
ovariectomized Wistar rats, weighing an average of 100 g, with
endosulfan at 1.5 mg/kg bw per day by gavage, estradiol dipropionate
intraperitoneally (dose unspecified), or a combination of these
treatments for 30 days. Endosulfan did not change the weights of the
uterus, cervix, or vagina, whereas estradiol propionate produced large
increases. The increased weights seen with the combined treatment were
similar to those with estradiol propionate alone.
Concern that endosulfan might be estrogenic persisted as a result
of the findings of an 'E-screen' assay, which was developed to assess
the estrogenic effects of environmental chemicals by observing their
proliferative effect on a target cell. The numbers of cells present
after similar inocula of the human breast cancer cell line, MCF-7,
were compared in the absence of estrogens (negative control), in the
presence of estradiol-17ß (positive control), and with a range of
concentrations of endosulfan. In this assay, endosulfan was estrogenic
at concentrations of 10-25 µmol/L, with a proliferative effect about
80% that of 17ß-estradiol at 10 µmol/L; the relative proliferative
potency (i.e. the ratio of the doses of endosulfan and estradiol-17ß
required to produce the maximum effect) was 0.0001%. In addition,
endosulfan competed with estradiol-17ß for binding to the estrogen
receptor and increased the concentrations of progesterone receptor and
pS2 in MCF-7 cells, as would be expected for a compound that mimics
estrogens (Soto et al., 1994, 1995).
As part of a study to optimize investigations of estrogenic
activity, endosulfan and nine other chemicals (17ß-estradiol,
diethylstilbestrol, tamoxifen, 4-hydroxytamoxifen, methoxychlor,
the methoxychlor metabolite
2,2-bis (para-hydroxyphenyl)-1,1,1-trichloroethane, nonylphenol,
ortho, para'-DDT, and kepone) with known or suspected estrogenic
activity were tested in three assays: competitive binding to the mouse
uterine estrogen receptor, transcriptional activation in HeLa cells
transfected with plasmids containing an estrogen receptor and a
response element, and the uterotropic assay in mice. The results of
the three assays were consistent with respect to the known estrogenic
activities of the chemicals tested and their requirements for
metabolic activation. There was no evidence from any of these tests
that endosulfan is estrogenic (Shelby et al., 1996).
A much publicized report indicated that even estrogens of low
potency, such as endosulfan, could have important effects because of
interaction with other chemicals. The estrogenic potencies of
combinations of chemicals were screened in a system in which the human
estrogen receptor sequence is incorporated into the yeast genome.
Combinations of two weak environmental estrogens, such as dieldrin,
endosulfan, and toxaphene, were 1000 times more potent in human
estrogen receptor-mediated transactivation than any chemical alone
(Arnold et al., 1996). This result was not reproduced in another
laboratory in which the same assay was used or in a uterotropic assay
in which sexually immature rats were treated with endosulfan or
dieldrin alone or in a combination on three successive days and the
uterine mass weighed on the following day. The highest doses used in
the human estrogen receptor assay were determined by the solubility of
the compounds, and the highest doses in the uterotropic assay were 100
mg/kg bw for endosulfan or dieldrin alone and 75 mg/kg bw of each in
combination. Both chemicals were inactive in both assays, and there
was no evidence of synergism (Ashby et al., 1997). In a further study
with the human estrogen receptor assay, however, 0.1 mmol/L endosulfan
increased the activity of ß-galactosidase (Ramamoorthy et al., 1997).
More doubt was cast upon the thesis of synergism by an
independent study in which endosulfan and dieldrin showed no additive
effect in displacing 3H-17ß-estradiol from rat uterine estrogen
receptors or in inducing the proliferation of MCF-7 breast cancer
cells. The weak proliferative potential described by Soto et al.
(1994, 1995) was, however, confirmed in this assay in vitro.
Endosulfan or dieldrin alone at 3 mg/kg bw per day or in combination,
injected intraperitoneally daily for three days, did not stimulate
uterotrophic activity and had no effect on pituitary prolactin or
other endocrine-related end-points in immature female rats, indicating
that these weakly estrogenic compounds do not interact in a
synergistic fashion in binding to estrogen receptors or in activating
estrogen receptor-dependent responses in mammalian tissues or cells
(Wade et al., 1997). The paper in which synergism was originally
proposed was later withdrawn, since the results could not be
reproduced, even in the same laboratory (McLachlan, 1997). Overall,
these results suggest that concomitant exposure to weakly estrogenic
compounds probably does not result in reproductive toxicity related to
estrogen action.
3. Observations in humans
In general, the doses of endosulfan involved in cases of
poisoning have been poorly characterized. In a summary of case reports
(Lehr, 1996), the lowest reported dose that resulted in death was 35
mg/kg bw; deaths have also been reported after ingestion of 295 and
467 mg/kg bw, within 1 h of ingestion in some cases. Intensive medical
treatment within 1 h was reported to be successful after ingestion of
doses of 100 and 1000 mg/kg bw. The clinical signs in these patient
were consistent with those seen in laboratory animals, dominated by
tonic-clonic spasms. In a case in which a dose of 1000 mg/kg bw was
ingested, neurological symptoms requiring anti-epileptic therapy were
still required one year after exposure.
Comments
More than 90% of an oral dose of endusulfan was absorbed in rats,
with maximum plasma concentrations occurring after 3-8 h in males and
about 18 h in females. Elimination occurs mainly in the faeces and to
a lesser extent in the urine, more than 85% being excreted within 120
h. The highest tissue concentrations were in the kidneys. The
metabolites of endosulfan include endosulfan sulfate, diol,
hydroxy-ether, ether, and lactone but most of its metabolites are
polar substances which have not yet been identified. Endosulfan would
not be expected to accumulate significantly in human tissues. No data
on plant metabolites were available to the Meeting.
A battery of tests for acute toxicity in several species with
technical-grade endosulfan showed that it is highly toxic after oral
or dermal administration, with respective LD50 values of 10-160 mg/kg
bw and 45-135 mg/kg bw. The LC50 value for rats in a single study was
13 mg/m3 in females and 35 mg/m3 in males. Endosulfan, administered
by any route, is more toxic to female than to male rats. Clinical
signs of acute intoxication include piloerection, salivation,
hyperactivity, respiratory distress, diarrhoea, tremors, hunching, and
convulsions.
WHO has classified endosulfan as moderately hazardous (WHO,
1996).
The kidney is the target organ for toxicity. The renal effects
include increased renal weights and granular pigment formation after
short-term administration and progressive, chronic glomerulo-nephrosis
or toxic nephropathy after long-term exposure, although the
observation of progressive glomerulonephrosis is complicated by the
fact that this is a common lesion in ageing laboratory rats and occurs
at high incidence in control rats.
In a 90-day feeding study in rats, the cytoplasm of isolated
cells in the renal proximal convoluted tubules had a yellowish colour,
particularly in males, at all dietary concentrations from 10 ppm. The
presence of this yellow pigmentation was largely reversible during a
four-week recovery period, and it did not appear to indicate
nephrotoxicity. A darker, more particulate, granular and/or clumped
pigment was also observed, predominantly in cells of the straight
portions and occasionally in the proximal convoluted tubules, at
dietary concentrations of 30 ppm and above. This darker pigment was
more persistent than the yellow one, and urinalysis revealed darker
urine and marginally more ketones at doses from 60 ppm, and marginally
more protein, particularly in males, indicating renal damage at doses
of 360 ppm and above. Similar findings emerged from a multigeneration
study but not from a two-year study of carcinogenicity in rats. The
changes in pigmentation were considered to be due to the presence of
endosulfan and/or its metabolites in the enlarged lysosomes. To test
this hypothesis, a four-week feeding study was conducted in which male
rats were given dietary concentrations of 360 or 720 ppm endosulfan.
Light and electron microscopy of the kidneys of these animals clearly
showed increases in the number of lysosomes and the size of cells in
the convoluted tubule, probably as a result of accumulation of the
test material and/or its metabolites. Lysosomal changes were not
observed in either brain or liver, and the renal changes receded
appreciably during a 30-day recovery period. Chemical analysis of the
kidneys indicated the presence of alpha-endosulfan and, to a lesser
extent ß-endosulfan sulfate, and endosulfan lactone. The
concentrations of the dominant alpha-endosulfan in the kidneys were
about 50 times those in the liver. The concentrations in blood were
usually below the level of detection. After the 30-day recovery
period, renal alpha-endosulfan was detected only in traces and ß-
endosulfan not at all. Similar analysis of tissues from rats in the
two-year study of toxicity and carcinogenicity did not reveal the
presence of these substances in the kidney, although measurable alpha-
endosulfan was found in the liver at 75 ppm. The yellow colour
therefore indicates the presence of endosulfan and/or its metabolites,
rather than either a stage in the pathogenesis of nephropathy or an
independent expression of toxicity. It was postulated that in longer
studies its removal from lysosomes is accelerated by enzyme induction,
which has not been investigated.
In a 78-week study, exposure of rats to endosulfan at a high dose
of 20 mg/kg bw per day resulted in testicular atrophy, characterized
by degeneration and necrosis of the germinal cells lining the
seminiferous tubules. In addition, decreased sperm counts accompanied
by an increased incidence of sperm abnormalities have been reported in
mice, again at high doses of endosulfan. Reductions in the activities
of some testicular xenobiotic-metabolizing enzymes and some hormones
that are necessary for normal testicular function were also seen in a
30-day study in rats at 10, but not at 7.5 mg/kg bw per day. The
functional significance of these findings was not clear, as studies of
reproductive and developmental toxicity in rats and rabbits showed
neither impaired fertility nor any increase in the incidence of
defects or abnormalities in offspring. Given the high doses at which
these testicular effects were observed, it would appear that they are
of little human significance.
No genotoxic activity was observed in an adequate battery of
tests for mutagenicity and clastogenicity in vitro and in vivo.
The Meeting concluded that endosulfan is not genotoxic.
No carcinogenic effect was observed in mice at 18 ppm for 24
months, in female rats at 445 ppm for 78 weeks in one study or in male
or female rats at 75 ppm or 100 ppm for two years in two other
studies. The Meeting noted the differences in the dietary
concentrations used in these studies, but non-neoplastic responses
were seen even at the lower doses.
Endosulfan at dietary concentrations of 0, 3, 15, or 75 ppm did
not affect reproductive performance or the growth or development of
the offspring of rats over the course of a two-generation study. The
NOAEL was 75 ppm, the highest dose tested, equal to 5 mg/kg bw per day
for males and 6.2 mg/kg bw per day for females. The NOAEL for parental
toxicity was 15 ppm, equal to 1 mg/kg bw per day for males and 1.2
mg/kg bw per day, on the basis of increased liver and kidney weights
at 75 ppm.
In two studies of developmental toxicity in rats given oral doses
of 0, 0.66, 2, or 6 mg/kg bw per day, the NOAEL for maternal toxicity
was 0.66 mg/kg bw per day in one study and 2 mg/kg bw per day in the
other. In the first case, the basis was decreased body-weight gain at
2 mg/kg bw per day and decreased body-weight gain and clinical signs
of toxicity at 6 mg/kg bw per day; in the second case, the basis was
mortality, clinical signs of toxicity, and decreased body-weight gain
at 6 mg/kg bw per day. In both studies, the NOAEL for developmental
toxicity was 2 mg/kg bw per day, in the first case on the basis of
delayed development and a low incidence of skeletal variations seen at
6 mg/kg per day and in the second on the basis of an increased
incidence of fragmented thoracic vertebral centra seen at 6 mg/kg bw
per day. In neither study was there any treatment-related major
malformation.
In a study of developmental toxicity in rabbits given oral doses
of 0, 0.3, 0.7, or 1.8 mg/kg bw per day, the NOAEL for maternal
tocicity was 0.7 mg/kg bw per day on the basis of clinical signs of
toxicity at 1.8 mg/kg bw per day. The NOAEL for developmental toxicity
was 1.8 mg/kg bw per day, the highest dose tested.
Several recent studies have shown that endosulfan, alone and in
combination with other pesticides, may bind to estrogen receptors and
may perturb the endocrine system. The available studies show only very
weak binding to hormone receptors in vitro, and the evidence for
their relevance to adverse physiological effects in vivo is
extremely limited. Long-term assays of toxicity and studies of
reproductive and developmental toxicity in experimental mammals did
not indicate that endosulfan induces functional aberrations that might
result from loss of endocrine homeostasis.
The absence of immunotoxic effects in a large number of bioassays
with endosulfan suggested that it does not have an adverse effect on
the immune function of laboratory animals. However, in two studies,
rats given endosulfan in the diet at 30 or 50 ppm for 6 weeks or 20
ppm for 22 weeks had reduced serum titres of tetanus toxoid antibody
and reduced immunoglobulins G and M, and inhibition of migration of
both leukocytes and macrophages. These findings have not been
confirmed.
In a summary of case reports of human poisoning incidents, the
lowest reported dose that caused death was 35 mg/kg bw. Higher doses
caused death within 1 h. The clinical signs in these patients were
dominated by tonic-clonic convulsions, consistent with the
observations in experimental animals.
An ADI of 0-0.006 mg/kg bw was established on the basis of the
NOAEL of 0.6 mg/kg bw per day in the two-year dietary study of
toxicity in rats and a safety factor of 100. The ADI is supported by
similar NOAEL values in the 78-week dietary study of toxicity in mice,
the one-year dietary study of toxicity in dogs, and the study of
developmental toxicity in rats.
An acute RfD of 0-0.02 mg/kg was established on the basis of the
NOAEL of 2 mg/kg bw per day in the study of neurotoxicity in rats and
a safety factor of 100.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 3.9 ppm, equal to 0.58 mg/kg bw per day (females in a
78-week study of toxicity)
Rat: 15 ppm, equal to 0.6 mg/kg bw per day (two-year dietary
study of toxicity)
75 ppm, equal to 6 mg/kg bw per day (reproductive
toxicity)
0.66 mg/kg bw per day (maternal toxicity in a study of
developmental toxicity)
2 mg/kg bw per day (fetotoxicity in a study of
developmental toxicity)
Rabbit: 0.7 mg/kg bw per day (maternal toxicity in a study of
developmental toxicity)
Dog: 10 ppm, equivalent to 0.57 mg/kg bw per day (one-year
study of toxicity)
Estimate of acceptable daily intake for humans
0-0.006 mg/kg bw
Estimate of acute reference dose for humans
0.02 mg/kg bw
Studies that would provide information useful for continued
evaluation of the compound
1. Studies of immunotoxicity with standard test protocols
2. Studies of the significant sex difference in acute toxicity,
particularly in rats
3. Further observations in humans
List of end-points relevant for setting guidance values for dietary and non-dietary exposure
Absorption, distribution, excretion, and metabolism in mammals
Rate and extent of absorption Rat: oral, > 90% absorption; max. concentration at
3-8 h (males) 18 h (females)
Distribution Mainly in kidney and liver
Potential for accumulation Low
Rate and extent of excretion Biphasic; urinary half-life was 6 h for 1st phase,
33-68 h for 2nd phase; faecal half-life was 10 h and
30 h; > 85% excretion within 120 h
Metabolism in animals Oxidation and hydrolysis; unidentified polar
metabolites
Toxicologically significant Parent; no data on plant metobolites
compounds (animals, plants and
environment)
Acute toxicity
Rat: LD50 oral 10 mg/kg bw (female)
Rat: LD50 dermal 500 mg/kg bw (female)
Rat: LC50 inhalation 13 mg/m3 4 h (female)
Skin irritation Not irritating
Eye irritation Not irritating
Skin sensitization Not sensitizing
Short-term toxicity
Target/critical effect Reduced survival, convulsions, salivation
Lowest relevant oral NOAEL Rat: 0.64 mg/kg bw per day, dietary
Lowest relevant dermal NOAEL Rat: 3 mg/kg bw per day
Lowest relevant inhalation NOAEL Rat: 2 mg/m3, no effect (highest concentration)
Genotoxicity Not genotoxic
Long-term toxicity and carcinogenicity
Target/critical effect Kidney
Lowest relevant NOAEL Rat: 0.6 mg/kg bw per day, 2-year study
Carcinogenicity Not carcinogenic
Reproductive toxicity
Reproduction target: critical effect None identified
Lowest relevant reproductive NOAEL Rat: 6 mg/kg bw per day
Developmental target /critical effect Fetoxicity at maternally toxic doses
Lowest relevant developmental NOAEL Rat: 2 mg/kg bw per day
Neurotoxicity/Delayed neurotoxicity Rat: 1.5 mg/kg bw (female); 12.5 mg/kg bw (male)
no effect
Other toxicological studies Immunotoxicity in certain special assays, not
confirmed in sensitization test or histologically
Some conflicting evidence of interaction with
estrogen receptors in vitro; none in vivo
Medical data Lowest lethal dose: 35 mg/kg bw, oral
Summary Value Study Safety factor
ADI 0-0.006 mg/kg bw Several different species 100
and end-points
Acute reference dose 0.02 mg/kg bw Study of neurotoxicity in 100
rats
References
Albrecht, M. & Baeder, C. (1993) Hoe 002671-substance technical (code:
Hoe 002671 00 ZD98 0005). Testing for embryotoxicity in the Wistar rat
after oral administration. Unpublished report No. 93.0716, document
A51695. Submitted to WHO by Hoechst Schering AgrEvo GmbH,
Frankfurt-am-Main, Germany.
Arai, M., Hiromori ,T., Ho, S., Okuno, Y. & Miyamoto, J. (1981) Life
span chronic toxicity study of endosulfan in mice -- 12 month report.
Unpublished report No. 0285, at-10 from Sumitomo Chemical Co Ltd,
Japan, 28 April 1981. Submitted to WHO by Hoechst Schering AgrEvo
GmbH, Frankfurt-am-Main, Germany.
Arcelin, G. (1996) Contact hypersensitivity to endosulfan (Code: Hoe
002671 00ZD99 0008) in albino guinea pigs maximumization test.
Unpublished report No. 630753 from RCC, Itingen, Switzerland. Hoechst
document A58132.A51695. Submitted to WHO by Hoechst Schering AgrEvo
GmbH, Frankfurt-am-Main, Germany.
Arnold, S.F., Klotz, D.M., Collins, B.M., Vonier, P.M., Guilette, L.J.
& McLachlan, J.A. (1996) Synergistic activation of oestrogen receptor
with combinations of environmental chemicals. Science, 272,
1489-1492.
Ashby, J., LeFevre, P.A., Odum, J., Harris, C.A., Routledge, E.J. &
Sumpter, J.P. (1997) Synergy between synthetic oestrogens? Nature,
385, 494.
Asquith, J.C. & Baillie, J.H. (1989) Endosulfan substance technical
(code Hoe 002671 0I ZD95 0005). Metaphase analysis of human
lymphocytes. Unpublished report No. m/hl/1307 from Toxicol
Laboratories, United Kingdom, 16 March 1989. Hoechst document A40411.
Submitted to WHO by Hoechst Schering AgrEvo GmbH, Frankfurt-am-Main,
Germany.
Banarjee, B.D. & Hussain, Q.Z. (1986) Effect of subchronic endosulfan
exposure on humoral and cell-mediated immune responses in albino rats.
Arch. Toxicol., 59, 279-284.
Banarjee, B.D. & Hussain, Q.Z. (1987) Effects of endosulfan on humoral
and cell-mediated immune responses in rats. Bull. Environ. Contam.
Toxicol., 38, 438-441.
Barnard, A.V., Atkinson, J.S., Heywood, R., Street, A.E, Gibson, W.A.,
Rao, R., Offer, J.M. & Gopinath, A.R.H. (1984) 13 week toxicity study
in mice. Unpublished report No. Hst 229/831052, 25 September 1984 from
Huntingdon Research Centre, Huntingdon, Cambridgeshire, United
Kingdom. Hoechst document A29663. Submitted to WHO by Hoechst Schering
AgrEvo GmbH, Frankfurt-am-Main, Germany.
Barnard, A.V., Jones, D.R., Powell, L.A.J., Heywood, R., Street, A.E.,
Gibson, W.A., Gopinath, C., Majeed, S.K. & Almond, R.H. (1985) 13-week
toxicity study in rats followed by a 4-week withdrawal period.
Unpublished report No. Hst 230/84176, 25 March 1985 from Huntingdon
Research Centre, Huntingdon, Cambridgeshire, United Kingdom. Hoechst
document A30700 Submitted to WHO by Hoechst Schering AgrEvo GmbH,
Frankfurt- am-Main, Germany.
Bhide, M.B. & Naik, P.Y. (1984a) The acute oral toxicity (LD50) of
Excel Industries Ltds endosulfan technical No. 2 to the albino rats.
Unpublished report from Indian Institute of Toxicology (report No. and
date not given). Submitted to WHO by the National Registration
Authority for Agricultural and Veterinary Chemicals, Australia.
Bhide, M.B. & Naik, P.Y. (1984b) The acute oral toxicity (LD50) of
Excel Industries Ltds endosulfan technical No. 1 to the albino mice.
Unpublished report from Indian Institute of Toxicology (report No. and
date not given). Submitted to WHO by the National Registration
Authority for Agricultural and Veterinary Chemicals, Australia.
Bhide, M.B. & Naik, P.Y. (1984c) The acute dermal toxicity (LD50) of
Excel Industries Ltds endosulfan technical No. 2 to the albino
rabbits. Unpublished report from Indian Institute of Toxicology
(report No. and date not given). Submitted to WHO by the National
Registration Authority for Agricultural and Veterinary Chemicals,
Australia.
Bowman, J.S. (1959) Preliminary report: Subacute feeding -- dairy
cows. Unpublished report from Hazleton Laboratories, USA. Hoechst
document No. A14205. Submitted to WHO by Hoechst Schering AgrEvo GmbH,
Frankfurt-am-Main, Germany.
Bracha, P. (1977) Thionex tech: Acute oral toxicity study in rats,
skin irritation study in rabbits, acute dermal toxicity study in
rabbits, eye irritation study in rabbits and acute inhalation study in
rats. Unpublished report No. 6111820 from Warf Institute Inc. Madison,
Wisconsin, USA. Submitted to WHO by Hoechst Schering AgrEvo GmbH,
Frankfurt-am-Main, Germany.
Bremmer, J.N. (1997a) Endosulfan: Substance technical (code: Hoe
002671 00 ZD99 0008) Testing for primary dermal irritation in the
rabbit. Unpublished Hoechst Marion Roussel preclinical development
study No. 96.0837. Hoechst document A58442. Submitted to WHO by
Hoechst Schering AgrEvo GmbH, Frankfurt-am-Main, Germany.
Bremmer, J.N. (1997b) Endosulfan: Substance technical (code: Hoe
002671 00 ZD99 0008) Testing for primary eye irritation in the rabbit.
Unpublished Hoechst Marion Roussel preclinical development study No.
96.0838. Hoechst document A58443. Submitted to WHO by Hoechst Schering
AgrEvo GmbH, Frankfurt-am-Main, Germany.
Bremmer, J.N. & Leist, K.-H. (1998) Endosulfan (AE F002671, substance
technical). Evaluation of the acute oral and dermal toxicity.
Unpublished report No. TOX98/003 from Hoechst Schering AgrEvo GmbH.
Hoechst document A59823. Submitted to WHO by Hoechst Schering AgrEvo
GmbH, Frankfurt-am-Main, Germany.
Brunk, R. (1989) Endosulfan-substance technical (code: Hoe 002671 00
ZD96 0002). Testing for toxicity by repeated oral administration
(1-year feeding study) to beagle dogs. Unpublished report No. 87.0643
from Pharma Research Toxicology and Pathology. Hoechst report No.
89.0188; document No. A40441 Submitted to WHO by Hoechst Schering
AgrEvo GmbH, Frankfurt-am-Main, Germany.
Brunk, R. (1990) Addendum to Hoechst report 89.0188; document No.
A44605. Submitted to WHO by Hoechst Schering AgrEvo GmbH,
Frankfurt-am-Main, Germany.
Bury, D. (1997) Endosulfan; substance, technical (code: Hoe 002671 00
ZD99 0008). Neurotoxicological screening in the male and female Wistar
rat. Acute toxicity. Unpublished report No. 96.0373 from Hoechst
Marion Roussel Preclinical Development, Germany. Hoechst report No.
A59088. Submitted to WHO by Hoechst Schering AgrEvo GmbH,
Frankfurt-am-Main, Germany.
Christ, O. & Kellner, H.M. (1968) Investigations with endosulfan-14C
in mice. Unpublished report No. ch/he-8412 from Hoechst Radiochemical,
Frankfurt, 31 December 1968. Hoechst document A53842, translation of
document A14217. Submitted to WHO by Hoechst Schering AgrEvo GmbH,
Frankfurt-am-Main, Germany.
Cifone, A.M. & Myhr, B.C. (1984a) Evaluation of Hoe 002671-substance
technical in the rat primary hepatocyte unscheduled DNA synthesis
assay. Unpublished LBI project No. 20991 from Litton Bionetics Inc.,
USA, November 1984. Hoechst document No. A29800. Submitted to WHO by
Hoechst Schering AgrEvo GmbH, Frankfurt-am-Main, Germany.
Cifone, A.M. & Myhr, B.C. (1984b) Mutagenicity evaluation of Hoe
002671-substance technical in the mouse lymphoma forward mutation
assay. Unpublished LBI project No. 20980 from Litton Bionetics Inc.,
USA, November 1984. Hoechst document No. A29801. Submitted to WHO by
Hoechst Schering AgrEvo GmbH, Frankfurt-am-Main, Germany.
Craine, E.M. (1986) A dermal absorption study in rats with
14C-endosulfan. Unpublished project No. Wil-39028, 11 December 1986
from Wil Research Lab, USA. Wil-39028. 11 Decemnber, 1986. Hoechst
document No. A35730. Submitted to WHO by Hoechst Schering AgrEvo GmbH,
Frankfurt-am-Main, Germany.
Craine, E.M. (1988) A dermal absorption study in rats with
14C-endosulfan with extended test duration. Unpublished project No.
Wil-39029 from Wil Research Lab, USA. Hoechst document No. A39677.
Submitted to WHO by Hoechst Schering AgrEvo GmbH, Frankfurt-am-Main,
Germany.
Deema, P. Thompson, E. & Ware, G. (1966) Metabolism, storage and
excretion of 14C-endosulfan in the mouse. J. Econom. Entomol., 59,
546-550.
Dickie, S.M., Mackenzie K.M. & Rao, G.N. (1981) Teratology study with
FMC 5462 in rabbits. Unpublished study No. 80070, 27 July 1981 from
Raltech Scientific Services. Hoechst document No. A23192. Submitted to
WHO by Hoechst Schering AgrEvo GmbH, Frankfurt-am-Main, Germany.
Diehl, K.H. & Leist, K.H. (1988a) Hoe 002671: Active ingredient
technical (code: Hoe 002671 0I ZD96 0002). Testing for acute oral
toxicity in the male and female Wistar rat. Unpublished study No.
88.0551, 27 July 1988, from Pharma Research Toxicology and Pathology,
Frankfurt, Germany. Hoechst document No. A39680. Submitted to WHO by
Hoechst Schering AgrEvo GmbH, Frankfurt-am-Main, Germany.
Diehl, K.H. & Leist, K.H. (1988b) Endosulfan-active ingredient
technical (code: Hoe 002671 0I ZD96 0002). Testing for acute dermal
toxicity in the male and female Wistar rat. Unpublished study No.
88.0552, 22 August 1988, from Pharma Research Toxicology and
Pathology, Germany. Hoechst document A39397, 1 September 1988.
Submitted to WHO by Hoechst Schering AgrEvo GmbH, Frankfurt-am-Main,
Germany.
Dikshith, T.S. & Datta, K.K. (1978) Endosulfan: Lack of cytogenetic
effects in male rats. Unpublished document No. A17140 from Industrial
Toxicology Research Centre. Submitted to WHO by Hoechst Schering
AgrEvo GmbH, Frankfurt-am-Main, Germany.
Dikshith, T.S.S., Raizada, R.B., Kumar, S.N., Srivastava, M.K.,
Kaushal, R.A., Singh, R.P. & Gupta, K.P. (1988) Effect of repeated
dermal application of endosulfan to rats. Vet. Hum. Toxicol., 30,
219-224.
Donaubauer, H.H. (1988) Endosulfan-substance technical (code: Hoe
002671 OI ZD97 0003). Carcinogenicity study in mice: 24 months feeding
study. Unpublished study No. 745 from Pharma Research Toxicology and
Pathology, Germany. Hoechst document No. A38008. Submitted to WHO by
Hoechst Schering AgrEvo GmbH, Frankfurt-am-Main, Germany.
Donaubauer, H.H. (1989) Amendment to the report No. 88.0278
endosulfan-substance technical (code: Hoe 002671 OI ZD97 0003).
Carcinogenicity study in mice: 24 months feeding study. Unpublished
study No. 745 from Pharma Research Toxicology and Pathology, Germany.
Hoechst document No. A41617. Submitted to WHO by Hoechst Schering
AgrEvo GmbH, Frankfurt-am-Main, Germany.
Donaubauer, H.H., Leist, K. & Kramer, M. (1985) Endosulfan-substance
technical (code: Hoe 002671 01 ZD97 0003). 42-Day feeding study in
mice. Unpublished study No. 744 from Pharma Research Toxicology,
Germany. Hoechst document No. A38104. Submitted to WHO by Hoechst
Schering AgrEvo GmbH, Frankfurt-am-Main, Germany.
Dorn, E. & Werner, H.-J. (1989) Endosulfan (Hoe 002671 OI ZD97 0003):
Determination of residues in the liver and kidneys of rats after
chronic (2-years) feeding (week 104). Unpublished Produktentwicklung,
GB-C Ökologie II Hoechst AG Study No. (Analytical part) CR065/88.
Hoechst document No. A41265. Submitted to WHO by Hoechst Schering
AgrEvo GmbH, Frankfurt-am-Main, Germany.
Dorough, M.W., Huhtanen, K., Marshall, T.C. & Bryant, H.E. (1978) Fate
of endosulfan in rats and toxicological considerations of apolar
metabolites. Pest. Biochem. Physiol., 8, 241-252.
Dzwonkowska, A. & Hübner, H. (1991) Studies on commercial insecticides
with the dominant lethal mutations test. Polish J. Occup. Med.
Environ. Health, 4, 43-53.
Ebert, E. & Weigand, W. (1984) Testing of the therapeutic effect of
diazapam (Valium R) and phenobarbital (Luminal R) in the event of
acute poisoning with endosulfan-active ingredient technical (code: Hoe
002671 oi ZD97 0003) in Wistar rats. Unpublished report No. 84.0062
from Pharma forschung Toxikologie. Hoechst document A29211. Submitted
to WHO by Hoechst Schering AgrEvo GmbH, Frankfurt-am-Main, Germany.
Ebert, E., Weigand, W. & Kramer, P. (1985a) Endosulfan-active
ingredient technical (code: Hoe 002671 01 ZD97 0003). Testing for
subchronic dermal toxicity (21 applications over 30 days) in SPF
Wistar rats. Unpublished study No. 83.0118 from Pharma forschung
Toxicologie. Hoechst document No. A30754, translation of document
A30751. Submitted to WHO by Hoechst Schering AgrEvo GmbH,
Frankfurt-am-Main, Germany.
Ebert, E., Leist, K.H. & Kramer, P. (1985b) Endosulfan-active
ingredient technical (code: Hoe 002671 01 ZD97 0003). Testing for
subchronic dermal toxicity (21 applications over 30 days) in Wistar
rats. Toxicological review of studies 721 and 729 (reports 84.0321 and
84.0223). Unpublished Hoechst document No. A30755, translation of
A30752. Submitted to WHO by Hoechst Schering AgrEvo GmbH,
Frankfurt-am-Main, Germany.
Ebert, E., Leist ,K.H. & Kramer, P. (1985c) Endosulfan-active
ingredient technical (code: Hoe 002671 01 ZD97 0003). Testing for
subchronic dermal toxicity (21 applications over 30 days) in Wistar
rats. Unpublished study No. 83.0508 from Pharma forschung Toxicologie.
Hoechst document No. A30753, translation of document A30750. Submitted
to WHO by Hoechst Schering AgrEvo GmbH, Frankfurt-am-Main, Germany.
Edwards, J.A., Hughes, E.W. & Almond, R.H. (1982) Preliminary
investigation of the effect of endosulfan (code, Hoe 02671 OI AT 209)
on reproduction of the rat. Unpublished report No. Hst 203/82252 from
Huntingdon Research Centre, Huntingdon, Cambridgeshire, United
Kingdom. Hoechst document No. A29563. Submitted to WHO by Hoechst
Schering AgrEvo GmbH, Frankfurt-am-Main, Germany.
Edwards, J.A., Reid, J.Y., Offer, M.J., Almond, H.R. & Gibson, A.W.
(1984) Effect of endosulfan-technical (code, Hoe 02671OI AT209) on
reproductive function of multiple generations in the rat. Unpublished
report No. Hst 204/83768 from Huntingdon Research Centre, Huntingdon,
Cambridgeshire, United Kingdom. Hoechst document No. A29428. Submitted
to WHO by Hoechst Schering AgrEvo GmbH, Frankfurt-am-Main, Germany.
Ehling, G. & Leist, K.-H. (1991a) Hoe 051327: substance technical
(code HOE 051327 00 ZB99 0002): Testing for acute oral toxicity in the
male and female Wistar rat. Pharma Research Toxicology, Germany.
Hoechst document No. A46286. Submitted to WHO by Hoechst Schering
AgrEvo GmbH, Frankfurt-am-Main, Germany.
Ehling, G. & Leist, K.-H. (1991b) Hoe 051327: substance technical
(code HOE 051327 00 ZB99 0002): Testing for acute dermal toxicity in
the male and female Wistar rat. Pharma Research Toxicology, Germany.
Unpublished Hoechst document No. A45783. Submitted to WHO by Hoechst
Schering AgrEvo GmbH, Frankfurt-am-Main, Germany.
Ehling, G. & Leist, K.-H. (1991c) Hoe 051329: substance technical
(code HOE 051329 00 ZD98 0001): Testing for acute oral toxicity in the
male and female Wistar rat. Pharma Research Toxicology, Germany.
Unpublished Hoechst document No. A45783. Submitted to WHO by Hoechst
Schering AgrEvo GmbH, Frankfurt-am-Main, Germany.
Ehling, G. & Leist, K.-H. (1991d) Hoe 051329: substance technical
(code HOE 051329 00 ZD98 0001): Testing for acute dermal toxicity in
the male and female Wistar rat. Pharma Research Toxicology, Germany.
Unpublished Hoechst document No. A45829. Submitted to WHO by Hoechst
Schering AgrEvo GmbH, Frankfurt-am-Main, Germany.
Elsea, J.R. (1958) Thiodan technical: Acute oral administration: Rats.
Hazleton Laboratories, USA, 28 February, 1958. Unpublished Hoechst
document No. A13686. Submitted to WHO by Hoechst Schering AgrEvo GmbH,
Frankfurt-am-Main, Germany.
Flodström, S., Wärngård, L., Hemming, H., Fransson, R. & Ahlborg, U.G.
(1988) Tumour promotion related effects by the cyclodiene insecticide
endosulfan studies in vitro and in vivo. Pharmacol Toxicol., 62,
230-235.
Goebel, H., Gorbach, S., Knauf, W., Rimpau, R.H. & Uttenbach, H.
(1982) Properties, effects, residues and analytics of the insecticide
endosulfan. Res. Rev., 83, 41. Submitted to WHO by the National
Registration Authority for Agricultural and Veterinary Chemicals,
Australia.
Gopinath, C. & Cannon, M.W.J. (1990) Photomicrographic addendum to
histopathology report No. Hst/289 Endosulfan, active ingredient
technical (code: Hoe 002671 OI ZD97 0003) combined chronic
toxicity/carcinogenicity study (104-week feeding in rats). Huntingdon
Research Centre Ltd, Huntingdon, Cambridgeshire, United Kingdom.
Unpublished Hoechst document No. A44604. Submitted to WHO by Hoechst
Schering AgrEvo GmbH, Frankfurt-am-Main, Germany.
Gorbach, S., Christ, O., Kellner, H.M., Kloss, G. & Börner, E. (1968)
The metabolism of endosulfan in milk sheep. J. Agric. Food Chem.,
16, 950-953.
Gupta, P.K., Chandra, S.V. & Saxena, D.K. (1978) Teratogenic and
embryotoxic effects of endosulfan in rats. Acta Pharmacol.
Toxicol., 42, 150-152.
Hack, R. & Leist, K.-H. (1988) Endosulfan-substance technical (code:
Hoe 002671 0I ZD96 0002) Testing of host resistance in the female
Wistar rat (immunological screening with Trichonella spiralis).
Unpublished study No. 87.1125 from Pharma Research Toxicology and
Pathology, Germany. Hoechst report A43829. Submitted to WHO by Agrevo
GmbH, Frankfurt-am-Main, Germany.
Hack, R., Ebert, E. & Leist, K.H. (1995) Chronic toxicity and
carcinogenicity studies with the insecticide endosulfan in rats and
mice. Food Chem. Toxicol., 33, 941-950.
Hollander, H. & Kramer, P. (1975a) Endosulfan sulfate = NIA 7985.
Acute oral toxicity in female SPF-Wistar rats (vehicle: starch
suspension). Unpublished Hoechst document No. A06966. Submitted to WHO
by Hoechst Schering AgrEvo GmbH, Frankfurt-am-Main, Germany.
Hollander, H. & Kramer, P. (1975b) Endosulfan sulfate = NIA 7985.
Acute oral toxicity in male beagle dogs. Unpublished Hoechst document
No. A06965. Submitted to WHO by Hoechst Schering AgrEvo GmbH,
Frankfurt-am-Main, Germany.
Hollander, H. & Kramer, P. (1975c) Comparative test on the acute
toxicity of endosulfan ether and endosulfan alcohol in female
SPF-Wistar rats (vehicle: starch suspension). Unpublished Hoechst
document No. A07170; translation of A05271. Submitted to WHO by
Hoechst Schering AgrEvo GmbH, Frankfurt-am-Main, Germany.
Hollander, H. & Kramer, P. (1975d) 1-Hydroxy endosulfan ether. Acute
oral toxicity in female SPF-Wistar rats (vehicle: starch suspension).
Unpublished Hoechst document No. A06967; translation of A05276
Submitted to WHO by Hoechst Schering AgrEvo GmbH, Frankfurt-am-Main,
Germany.
Hollander, H. & Kramer P. (1975e) Endosulfan lactone: Acute oral
toxicity in female SPF-Wistar rats (vehicle: starch suspension).
Unpublished Hoechst document No. A07171; translation of A05273.
Submitted to WHO by Hoechst Schering AgrEvo GmbH, Frankfurt-am-Main,
Germany.
Hollander, H. & Kramer, P. (1975f) Endosulfan lactone. Acute oral
toxicity in male SPF-Wistar rats. Unpublished Hoechst document No.
A06964.25. Submitted to WHO by Hoechst Schering AgrEvo GmbH,
Frankfurt-am-Main, Germany.
Hollander, H. & Weigand, W. (1983) Acute aerosol toxicity in male and
female SPF Wistar rats 4 hours-LC50. Unpublishd Hoechst report No.
83.0397; document No. A32087. Submitted to WHO by Hoechst Schering
AgrEvo GmbH, Frankfurt-am-Main, Germany.
Hollander, H., Weigand, W. & Kramer, P. (1984) Endosulfan-active
ingredient technical (code: Hoe 002671 0I ZD97 0003) Testing for
subchronic inhalation toxicity--21 exposures in 29 days -- in SPF
Wistar rats. Unpublished study No. 762; document No. A29823,
translation of A29766. Submitted to WHO by Hoechst Schering AgrEvo
GmbH, Frankfurt-am-Main, Germany.
Indraningsih, McSweeney, C.S. & Ladds, P.W. (1993) Residues of
endosulfan in the tissues of lactating goats. Aust. Vet. J., 70,
59-62.
Jung, Weigand, W. & Kramer, P. (1983) Mouse micronucleus test
following oral administration. Unpublished Hoechst AG report No.
83.0458; document No. A31628. Submitted to WHO by Hoechst Schering
AgrEvo GmbH, Frankfurt-am-Main, Germany.
Keller, J.G. (1959a) Subacute feeding--dairy cows. Hazleton
Laboratories, USA. Unpublished Hoechst document No. A14206. Submitted
to WHO by Hoechst Schering AgrEvo GmbH, Frankfurt-am-Main, Germany.
Keller, J.G. (1959b) One-year oral study in dog. Hazleton
Laboratories, 12 May 1959. Unpublished Hoechst document No. A13924.
Submitted to WHO by Hoechst Schering AgrEvo GmbH, Frankfurt-am-Main,
Germany.
Keller, J.G. (1959c) 2 year dietary study in rats. Hazleton
Laboratories, USA, 22 May 1959. Unpublished Hoechst document No.
A14037. Submitted to WHO by Hoechst Schering AgrEvo GmbH,
Frankfurt-am-Main, Germany.
Kellner, H.M. & Eckert (1983) Hoe 02671-14C. Pharmacokinetics and
residue determinations after oral and intravenous administration to
rats. Unpublished study No. tep 74/1; bereich c/analytisches project
oe 87/45; Hoechst report 01-l42-0382-83, 15 February 1983; document
No. A49475, translation of document A27971. Submitted to WHO by
Hoechst Schering AgrEvo GmbH, Frankfurt-am-Main, Germany.
Khan, P.K. & Sinha, S.P (1996) Ameliorating effect of vitamin C on
murine sperm toxicity induced by three pesticides (endosulfan,
phosphamidon and mancozeb). Mutagenesis, 11, 33-36
Kramer, P. & Weigand, W. (1971) Endosulfan lactone (vehicle: sesame
oil). Acute oral toxicity in male and female SPF-Wistar K-rat.
Unpublished Hoechst document No. A18276, 21 May 1971; translation of
A14326. Submitted to WHO by Hoechst Schering AgrEvo GmbH,
Frankfurt-am-Main, Germany.
Lachmann, G. & Siegemund, B. (1987) Hoe 002671-(5a, 9a-14C). Dermal
absorption of 14C- endosulfan in rhesus monkeys. Battelle-Institut,
Frankfurt. Unpublished Hoechst document No. A36685. Submitted to WHO
by Hoechst Schering AgrEvo GmbH, Frankfurt-am-Main, Germany.
Lehr, W (1996) Summary of intoxications with endosulfan. Clinical
cases and poisoning incidents. Unpublished Hoechst report No. psr
96/006, 12 March 1996; document A56361. Submitted to WHO by Hoechst
Schering AgrEvo GmbH, Frankfurt-am-Main, Germany.
Leist, K.H. (1989a) Endosulfan-substance technical (code: Hoe 002671
oi ZD97 0003). Carcinogenicity study in mice, 24 months feeding study
-- Residue determination. Pharma Research Toxicology and Pharmacology.
Unpublished Hoechst document No. A41284. Submitted to WHO by Hoechst
Schering AgrEvo GmbH, Frankfurt-am-Main, Germany.
Leist, K.-H. (1989b) Amendment to report No. HST 289/881067.
Endosulfan, active ingredient technical (code: Hoe 002671 OI ZD97
0003). Combined chronic toxicity/carcinogenicity study (104-week
feeding in rats). Residue determination. Unpublished Hoechst report
No. 89.1105; document No. A41265. Submitted to WHO by Hoechst Schering
AgrEvo GmbH, Frankfurt-am-Main, Germany.
Leist, K.-H. & Bremmer, J. (1998) Endosulfan (AE F002671, substance
technical). Re-evaluation of the NOAEL in the 90-day subchronic
toxicity study in rats. Unpublished AgrEvo document No. 59825.
Submitted to WHO by Hoechst Schering AgrEvo GmbH, Frankfurt-am-Main,
Germany.
Leist, K.-H. & Mayer, D. (1987) Endosulfan-active ingredient technical
(code: Hoe 002671 01 ZD97 0003). 30-Day feeding study in adult male
Wistar rats. Unpublished study No. 84 0585 from Pharma Research
Toxicology and Pharmacology. Hoechst report No. 87.0129, 27 March
1987; document No., A37112. Submitted to WHO by Hoechst Schering
AgrEvo GmbH, Frankfurt-am-Main, Germany.
Lightowler, J.E. & Gardner, J.R. (1978) Acute oral toxicity study in
rats. Unpublished report No. 78/mak1/428. 20 November 1978 from Life
Science Research. Submitted to WHO by Hoechst Schering AgrEvo GmbH,
Frankfurt-am-Main, Germany.
Mackenzie, K.M. (1980) Teratology study with FMC 5462 in rats. Raltech
Scientific Services, study No. 79041, 2 October 1980. Hoechst document
No. A21393. Submitted to WHO by Hoechst Schering AgrEvo GmbH,
Frankfurt-am-Main, Germany.
Maier-Bode, H. (1966) Investigations on the persistence of the
insecticide endosulfan in the vegetable and animal organism.
Pharmakologisches Institut der Rheinischen Friedrich Wilhelms
Universität. Unpublished Hoechst document No. A4047. Submitted to WHO
by Hoechst Schering AgrEvo GmbH, Frankfurt-am-Main, Germany.
McGregor, D.B., Willins, M.J., McDonald, P., Holström, M., McDonald,
D. & Niemeier, R.W. (1983) Genetic effects of 2-methoxyethanol and
bis(2-methoxyethyl)ether. Toxicol. Appl. Pharmacol., 70, 303-316.
McLachlan, J.A. (1997) Synergistic effect of environmental estrogens:
Report withdrawn. Science, 277, 462-463.
Mellano, D. & Milone, M.F. (1984a) Mutagenic activity of the compound
endosulfan-technical with Saccharomyces cerevisiae gene
conversion-DNA repair test. Instituto di Recherche Biomediche.
Unpublished Hoechst document No. A29313. Submitted to WHO by Hoechst
Schering AgrEvo GmbH, Frankfurt-am-Main, Germany.
Mellano, D. & Milone, M.F. (1984b) Mutagenic activity in vitro of the
compound endosulfan-technical with Schizosaccharomyces pombe.
Unpublished experiment No. M 708, 18 June 1984, from Instituto di
Recherche Biomediche. Hoechst document No. A29312. Submitted to WHO by
Hoechst Schering AgrEvo GmbH, Frankfurt-am-Main, Germany.
Müller, W. (1988) Endosulfan-substance technical (code: Hoe 002671 0I
ZD95 0005). Micronucleus test in male and female NMRI mice after oral
administration. Pharma Research Toxicology and Pathology. Unpublished
Hoechst document No. A38059. Submitted to WHO by Hoechst Schering
AgrEvo GmbH, Frankfurt-am-Main, Germany.
Müllner, H. (1989) Effects of endosulfan and aldicarb on rat brain
acetylcholinesterase. Unpublished Hoechst report 16 June 1989;
document No. A43395. Submitted to WHO by Hoechst Schering AgrEvo GmbH,
Frankfurt-am-Main, Germany.
Nath, G., Datta, K.K., Dikshith, T.S.S., Tandon, S.K. & Pandya, K.P.
(1978) 30 day oral administration in rats. Interaction of endosulfan
and metepa in rats. Industrial Toxicology Research Centre. Unpublished
Hoechst document No. A17906. Submitted to WHO by Hoechst Schering
AgrEvo GmbH, Frankfurt-am-Main, Germany.
Noctor, J.C. & John, S.A. (1995) (14C)-Endosulfan: Rates of
penetration through human and rat skin determined using an in vitro
system. Hazleton Europe, United Kingdom. Unpublished Hoechst document
No. A54103. Submitted to WHO by Hoechst Schering AgrEvo GmbH,
Frankfurt-am-Main, Germany.
Nogami, K. (translator, no other name available) (1970) Testing report
on the toxicity of endosulfan (Malix) to dogs through acute oral
administration (LD50). Unpublished Hoechst document No. A13834.
Submitted to WHO by Hoechst Schering AgrEvo GmbH, Frankfurt-am-Main,
Germany.
Offer, J.M. (1985) Addendum to HST 204. Effect of endosulfan-technical
(code: Hoe 02671 OI AT209) on the reproductive function of multiple
generations in the rat. Histopathological review of the kidneys in
adult rats of the F1b generation and in weanling rats of the F2b
generation. Huntingdon Research Centre, Huntingdon, Cambridgeshire,
United Kingdom. Unpublished Hoechst document No. A30757. Submitted to
WHO by Hoechst Schering AgrEvo GmbH, Frankfurt-am-Main, Germany.
Pandey, N., Gundevia, F., Prem, A.S. & Ray, P.K. (1990) Studies on the
genotoxicity of endosulfan, and organochlorine insecticide, in
mammalian germ cells. Mutat. Res., 242, 1-7.
Paul, V., Sheela, S., Balasubramaniam, E. & Kazi ,M. (1993)
Behavioural and biochemical changes produced by repeated oral
administration of the insecticide endosulfan in immature rats.
Indian J. Physiol. Pharmacol., 37, 204-208.
Paul, V., Easwaramoorthy, B. & Kazi, M. (1994) The neurobehavioural
toxicity of endosulfan: A serotonergic involvement in learning
impairment. Eur.J. Pharmacol. Environ. Toxicol. Pharmacol., 270,
1-7.
Paul, V., Easwaramoorthy, B., Arumugam, R.J. & Kazi, M. (1995) A
sex-related difference in the neurobehavioural and hepatic effects
following chronic endosulfan treatment in rats. Eur. J. Pharmacol.
Environ. Toxicol. Pharmacol., 293, 355-360.
Pirovano, R. & Milone, M.F. (1986) Study of the capacity of the test
article endosulfan, substance technical to induce chromosome
aberrations in human lymphocytes cultured in vitro. Unpublished RBM
experiment No. m 822, 20 March 1986. Hoechst document A33127.
Submitted to WHO by Hoechst Schering AgrEvo GmbH, Frankfurt-am-Main,
Germany.
Raizada, R.B., Srivastava, M.K. & Dikshith, T.S.S. (1991) Lack of
estrogenic effects of endosulfan: An organochlorine insecticide in
rat. Natl Acad. Sci. Lett., 14, 103-107.
Ramamoorthy, K., Wang, F., Chen, I.-C., Norris, J.D., McDonnel, D.P.,
Leonard, L.S., Gaido, K.W., Bocchinfuso, W.P., Korach, K.S. & Safe, S.
(1997) Estrogenic activity of a dieldrin/toxaphene mixture in the
mouse uterus, MCF-7 human breast cancer cells, and yeast-based
estrogen receptor assays: No apparent synergism. Endocrinology, 138,
1520-1527.
Robacker, K.M., Kulkarni, A.P. & Hodgson, E. (1981) Pesticide induced
changes in the mouse hepatic microsomal cytochrome P-450-dependent
monooxygenase system and other enzymes. J. Environ. Sci. Health,
B16, 529-545.
Reno, F.E. (1975) Acute oral toxicity study in rats. Endosulfan
technical. Unpublished final report, 18 December 1975, from Hazleton
Laboratories America. Hoechst document No. A33732. Submitted to WHO by
Hoechst Schering AgrEvo GmbH, Frankfurt-am-Main, Germany.
Roberts, N.L., Phillips, N.K. & Gopinath, C. (1983) Acute delayed
neurotoxicity study with endosulfan-technical (code: Hoe 002671 OI
ZD97 0003) in the domestic hen. Huntingdon Research Centre,
Huntingdon, Cambridgeshire, United Kingdom. Unpublished Hoechst
document No. A32153. Submitted to WHO by Hoechst Schering AgrEvo GmbH,
Frankfurt-am-Main, Germany.
Ruckman, S.A., Waterson, L.A., Crook, D., Gopinath, C., Majeed, S.K.,
Anderson, A. & Chanter, D.O. (1989) Endosulfan, active ingrediant
technical (code: Hoe 002671 OI ZD97 0003). Combined chronic
toxicity/carcinogenicity study (104-week feeding study in rats).
Huntingdon Research Centre, Huntingdon, Cambridgeshire, United
Kingdom. Unpublished Hoechst document No.A40440. Submitted to WHO by
Hoechst Schering AgrEvo GmbH, Frankfurt-am-Main, Germany.
Shelby, M.D., Newbold, R.R., Tully, D.B., Chae, K. & Davis, V.L.
(1996) Assessing environmental chemicals for estrogenicity using a
combination of in vitro and in vivo assays. Environ. Health
Perspectives, 104, 1296-1300.
Shirasu, Y., Moriya, M. & Ohta, T. (1978) Microbial mutagenicity
testing on endosulfan. Institute of Environmental Toxicology, Japan.
Unpublished Hoechst document No. A21215, 1978. Submitted to WHO by
Hoechst Schering AgrEvo GmbH, Frankfurt-am-Main, Germany.
Singh, S.K. & Pandey, R.S. (1990) Effect of sub-chronic endosulfan
exposures on plasma gonadotrophins, testosterone, testicular
testosterone and enzymes of androgen biosynthesis in rat. Indian J.
Exp. Biol., 28, 953-956.
Sinha, N., Narayan, R., Shanker, R. & Saxena, D.X. (1995)
Endosulfan-induced biochemical changes in the testis of rats. Vet.
Hum. Toxicol., 37, 547-549.
Soto, A.M., Chung, K.L. & Sonnenschein, C. (1994) The pesticides
endosulfan, toxaphene and dieldrin have oestrogenic effects on human
oestrogen-sensitive cells. Environ. Health Perspectives, 102,
380-383.
Soto, A.M., Sonnenschein, C., Chung, K.L., Fernandez, M.F., Olea, N. &
Serrano, F.O. (1995) The E-screen assay as a tool to identify
estrogens: An update on estrogenic environmental pollutants.
Environ. Health Perspectives, 103 (Suppl. 7), 113-122.
Stumf, K. & Lehr, W. (1993) Amendment to document A49584. Unpublished
Hoechst document A49584, 2 February 1993. Submitted to WHO by Hoechst
Schering AgrEvo GmbH, Frankfurt-am-Main, Germany.
den Tonkelaar, E.M. & van Esch, G.J. (1974) No-effect levels of
organochlorine pesticides based on induction of microsomal liver
enzymes in short-term toxicity experiments. Toxicology, 2, 371-380.
US National Cancer Institute (1978) 78-week dietary study in
Osborne-Mendel rats and B6C3F1 mice. NCI study No. NCI-CG-TR62,
Technical Report Series No. 62, Bethesda, Maryland, USA.
Vos, J.G., Krajnc, E.I., Beekhof, P.K. & van Logten, M.J. (1982)
Methods for testing immune effects of toxic chemicals: Evaluation of
the immunotoxicity of various pesticides in the rat. In: Matsunaka,
S., Hutson, D.H. & Murphy, S.D., eds, Pesticide Chemistry: Human
Welfare and the Environment, Oxford, Pergamon Press, pp. 497-504.
Wade, M.G., Desaulniers, D., Leingartner, K. & Foster, W.G. (1997)
Interactions between endosulfan and dieldrin on estrogen-mediated
processes in vitro and in vivo. Reprod. Toxicol., 11, 791-798.
Weigand, W. (1982a) Acute oral toxicity of Hoe 51329 in albino rats.
Pharma Research Toxicology, Germany. Unpublished Hoechst document No.
A23296. Submitted to WHO by Hoechst Schering AgrEvo GmbH,
Frankfurt-am-Main, Germany (in German).
Weigand, W. (1982b) Acute oral toxicity of Hoe 51330 in albino rats.
Pharma Research Toxicology, Germany. Unpublished Hoechst document No.
A23297. Submitted to WHO by Hoechst Schering AgrEvo GmbH,
Frankfurt-am-Main, Germany (in German).
WHO (1996) The WHO Recommended Classification of Pesticides by
Hazard and Guidelines to Classification 1996-1997 (WHO/PCS/96.3),
International Programme on Chemical Safety, Geneva.
