
FLUSILAZOLE
First draft prepared by
S. Ma,
Health Evaluation Division, Pest Management Regulatory Agency, Health
Canada, Ottawa, Canada
Explanation
Evaluation for acceptable daily intake
Biochemical aspects
Absorption, distribution, and excretion
Biotransformation
Toxicological studies
Acute toxicity
Short-term toxicity
Long-term toxicity and carcinogenicity
Reproductive toxicity
Developmental toxicity
Genotoxicity
Special studies
Dermal and ocular irritation and dermal sensitization
Mechanisms of Leydig-cell tumour induction
Comments
Toxicological evaluation
References
Explanation
Flusilazole was previously evaluated by the Joint Meeting in 1989
(Annex I, reference 56). An ADI of 0-0.001 mg/kg bw was allocated on
the basis of an NOAEL of 0.14 mg/kg bw per day (5 ppm) for liver
toxicity in a one-year feeding study in dogs. The compound was
reexamined at the present Meeting in response to a request from the
manufacturer. This monograph summarizes pertinent new (since 1989)
data as well as relevant data from the previous monograph.
Evaluation for acceptable daily intake
1. Biochemical aspects
The toxicokinetics of technical-grade flusilazole has been
studied after oral administration of radiolabelled test material in
rats. Summaries of the relevant data are presented below.
(a) Absorption, distribution, and excretion
14C-Flusilazole (uniformly phenyl-labelled) was administered
orally to groups of two male and two female Charles River CD rats by
one of three regimens: a single low dose of about 8 mg/kg bw; a single
low dose of about 8 mg/kg bw after pretreatment for 21 days with a
diet containing 100 ppm unlabelled flusilazole; or a single high dose
of about 200 mg/kg bw. By 96 h (first regimen) or 168 h (other two
regimens), about 90% of the administered radiolabel had been
eliminated in the urine and faeces, with an excretion half-life of
about 34 h. No significant amount of radiolabel was recovered in the
expired air as carbon dioxide or volatile metabolites. The faeces was
the main route of elimination, and there was an apparent sex
difference in the excretion pattern: in males, 87 and 8% of the dose
were eliminated in faeces and urine, respectively, while in females
the corresponding figures were 59 and 23%. Pretreatment with non-
radiolabelled flusilazole did not affect excretion. Tissue retention
of the radiolabel was very low, with total residues accounting for
< 2.5% of the administered dose. The largest amounts of radiolabel
were found in the carcass, gastrointestinal tract, and liver (means,
< 1% of dose). The tissue concentrations were proportional to the
amount of flusilazole administered. In the same study, an additional
group of one male and one female rat was given a single oral dose of
14C-flusilazole (triazole-3-labelled) of about 8 mg/kg bw. As with
the phenyl-labelled flusilazole, 88% of the administered radiolabel
was recovered in urine and faeces within 96 h of treatment; however,
urine was the predominant route of excretion. In males, urinary and
faecal radiolabel accounted for 78 and 11% of the dose, respectively,
while in females the corresponding figures were 59 and 26%. Tissue
retention of radiolabel was low, total residues accounting for about
3% of the administered dose. The highest concentrations of label were
found in the carcass (about 2% of dose), skin, gastrointestinal tract,
and liver (means, < 0.5% of dose) (Anderson et al., 1986).
14C-Flusilazole (triazole-3-labelled) was administered orally to
groups of five male and five female Charles River Crl:CD(SD)BR rats by
one of three regimens: a single dose of about 8 mg/kg bw; a single
dose of about 8 mg/kg bw after pretreatment with about 8 mg/kg bw per
day of non-radiolabelled flusilazole by gavage for 14 days; or a
single dose of about 224 mg/kg bw. At 96 h (first regimen) or 120 h
(other two regimens), the total recovery of radiolabel was 92.6-99.2%,
with about 90% eliminated within the first 48 h of treatment. Urine
was the primary route of excretion,, accounting for about 72% of the
administered dose, while faecal excretion accounted for about 17%. No
differences according to sex or regimen were observed. Tissue
retention of the radiolabel was low: the carcass accounted for < 3%
of dose and the remaining tissues for < 0.2.% (Cheng, 1986).
(b) Biotransformation
14C-Labelled flusilazole was extensively metabolized after oral
administration to Charles River CD rats. Recovered parent compound
accounted for only 2-11% of the dose in all animals, regardless of
dose or pretreatment with unlabelled compound, and was found
predominantly in the faeces; the urinary levels represented < 1% of
the dose. After absorption, flusilazole was cleaved at the triazole
ring. With phenyl-labelled material, the major faecal metabolites
identified were: [bis(4-fluorophenyl)methyl] silanol (about 30% of the
dose in males, about 19% in females); [bis(4-fluorophenyl)methylsilyl]
methanol (about 9% of the dose in animals of each sex); the fatty acid
conjugates of [bis(4-fluorophenyl)methylsilyl] methanol (19% of the
dose in males, 10% in females); and disiloxane (about 11% in males,
about 7% in females). Except for the fatty acid conjugates, the same
metabolites were found in urine. In males, all three urinary
metabolites represented < 1% of dose; in females, [bis(4-
fluorophenyl)methyl] silanol, [bis(4-fluorophenyl)methylsilyl]
methanol, and siloxane represented 7.5, 2.2, and 1.9% of the
administered dose, respectively. With triazole-labelled material, the
main metabolite identified was 1 H-1,2,4-triazole, which was found
predominantly in urine as 63.8% of the dose in males and 51.6% in
females. Faeces contained only a minor amount of the metabolite (4% of
the dose in males, 17% in females). A metabolic pathway for
flusilazole in rats was proposed on the basis of these results
(Figure 1) (Anderson et al., 1986).
2. Toxicological studies
(a) Acute toxicity
The results of studies of the acute toxicity of technical-grade
flusilazole are summarized in Table 1.
Flusilazole is moderately toxic to mice, rats, and rabbits when
given orally and minimally toxic to rats and rabbits when administered
dermally or by inhalation. Symptoms of toxicity after oral
administration included weight loss, weakness, lethargy, and, at
higher doses, prostration, salivation, laboured breathing,
convulsions, and loss of righting reflex. Dermal administration
resulted only in mild erythema at the site of application. The effects
of inhalation were mainly laboured breathing and lung sounds.
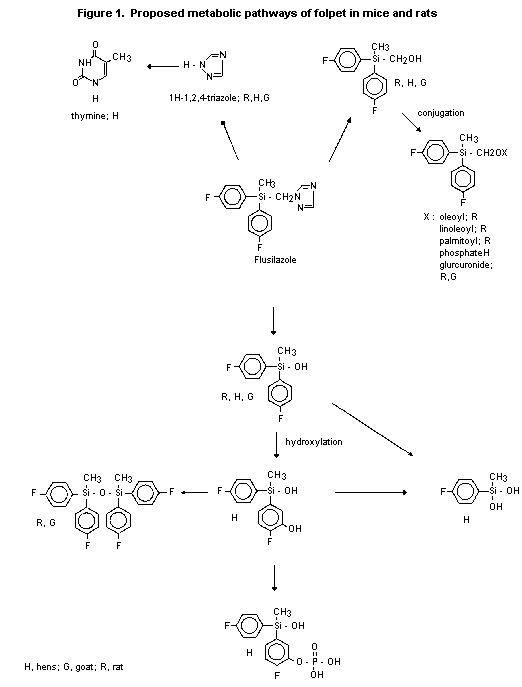 Table 1. Acute toxicity of flusilazole
Species Sex Route LD50 or LC50 Reference
(mg/kg bw or
mg/litre air)
Mouse Male Oral 680 Wylie et al. (1985)
female 1000
Rat Male Oral 1500 Wylie et al. (1983)
Male Oral 1110 Wylie et al. (1984a)
Female 674
Male Inhalation 2.7 Poindexter et al. (1984)
Female 3.7
Male, female Inhalation 6.8-7.7 Turner et al. (1985)
Rabbit Male, female Oral 450 Redgate et al. (1985)
Male, female Dermal > 2000 Gargus & Sutherland (1983)
(b) Short-term toxicity
Mice
Groups of 20 male and 20 female Crl:CD-1 mice were given
technical-grade flusilazole (purity, 96.7%) at dietary levels of 0,
25, 75, 225, 500, or 1000 ppm, equal to 0, 4, 12, 36, 82, or 164 mg/kg
bw per day for males and 0, 5, 15, 43, 92, or 222 mg/kg bw per day for
females, for up to 90 days. Ten mice of each sex per group were
sacrificed after four weeks of treatment; the remaining mice were
killed at the end of the study. No histopathological examination was
conducted on animals killed at four weeks. There were no clinical
symptoms of toxicity and no treatment-related effects on body weight
or food consumption in animals sacrificed at either interval. The
target organs of toxicity were liver and urinary bladder; females were
slightly more sensitive to flusilazole than males. No treatment-
related effects were observed at the lowest dose. At 75 ppm, increased
absolute and relative liver weights and an increased incidence (1/10)
of hepatocellular vacuolar cytoplasmic changes were observed in
females. At > 225 ppm, increased liver weight, dose-related
increases in the incidence of hepatocellular vacuolar cytoplasmic
changes, hepatocellular hypertrophy, and urinary bladder urothelial
cell hyperplasia were evident in animals of each sex. At the highest
dose, absolute and relative kidney weights were decreased in males,
but no treatment-related renal lesions were observed. In addition,
slight decreases in erythroid parameters (haemoglobin, haematocrit,
and erythrocyte counts) were noted in animals of each sex. The NOAEL
was 4 mg/kg bw per day (Pastoor et al., 1984).
Groups of 16 male and 16 female Crl:CD-1(ICR)BR mice were fed
diets containing technical-grade flusilazole (purity, 94%) at doses of
0, 1000, 2500, or 5000 ppm, equal to 0, 161, 436, and 1004 mg/kg bw
per day for males and 0, 239, 601, and 1414 mg/kg bw per day for
females, for at least 90 days. An additional six mice of each sex per
group were assessed for cellular proliferation in the liver and
urinary bladder on days 14 and 106. At the lowest dose, treatment-
related effects included reduced mean body weight and food efficiency
(males only), increased absolute and relative liver weights (animals
of each sex), decreased absolute and relative kidney weights (females
only), and hypertrophy or hyperplasia, cytoplasmic vacuolation, and
inflammation of the liver and urinary bladder (animals of each sex).
At the mid-dose, increased cellular proliferation of the urinary
bladder was also observed in animals of each sex. At the highest dose,
severe body-weight loss occurred in males and reduced body weight and
food efficiency were noted in females. The males at this dose were
sacrificed on day 44 owing to excess mortality and moribund condition.
There was no NOAEL (Keller, 1990).
Rats
Groups of six male Crl:CD rats were given technical-grade
flusilazole (purity, 95.5%) by gavage in corn oil at a dose of 0 or
300 mg/kg bw per day, five days per week for two weeks. Three rats per
group were killed at the end of the treatment period, and the
remaining rats were sacrificed after a two-week recovery period. All
animals were examined histopathologically. One treated animal died
after the fifth dose on day 7. Clinical signs of toxicity (lowered
body weight, alopecia, diarrhoea, stained or wet perineal area,
salivation, and hypersensitivity) were observed in four rats during
the treatment period. Treatment-related histopathological changes were
observed in liver, kidney, urinary bladder, and testis; the lesions
appeared to be less severe in rats killed after the recovery period.
Remarkable histopathological findings in treated rats included
hepatocellular vacuolation (six rats), hyperplasia and vacuolation of
the urinary bladder transitional epithelium (six rats) and renal
pelvis urothelium (two rats), and necrosis and cellular degeneration
of the germinal epithelium of the seminiferous tubules (two rats)
(Wylie et al., 1984b).
Four groups of 10 male and 10 female Charles River CD rats were
fed diets containing technical-grade flusilazole (purity, 96.7%) at
doses of 0, 25, 125, 375, or 750 ppm, equal to 0, 2, 9, 27, and
55 mg/kg bw per day for males and 0, 2, 11, 31, and 70 mg/kg bw per
day for females, for 90 days. No treatment-related effects were
oberved at the two lower doses. At 375 ppm, an increased serum
cholesterol level was seen in animals of each sex and an increased
incidence of mild bladder urothelial hyperplasia in one male and four
females. At the highest dose, bladder urothelial hyperplasia was seen
in five males and eight females. Additional treatment-related changes
included decreased body weight in females, increased absolute and
relative liver weights in animals of each sex, and remarkable
histopathological findings (hepatocellular hypertrophy, mild fatty
changes, and hepatocytolysis) in the livers of five males. The NOAEL
was 9 mg/kg bw per day (Pastoor et al., 1983).
Groups of 52 male and 52 female Crl:CD BR rats were fed diets
containing technical-grade flusilazole (purity, 95%) at doses of 0,
10, 125, 375, or 750 ppm, equal to 0, 0.58, 7.27, 22.1, and 44.7 mg/kg
bw per day for males and 0, 0.74, 9.40, 27.6, and 59.0 mg/kg bw per
day for females, for up to 91 days. Each group was divided into three
subgroups of 20 rats of each sex in order to study possible mechanisms
of action of toxicity in the liver and urinary bladder. Five rats of
each sex were sacrificed on days 7/8, 14, 46, and 91 for assessment of
cellular proliferation and histopathology. For evaluation of
cytochrome P450 and peroxisome proliferation, five rats of each sex
were sacrificed on days 14 and 90 (males) or 15 and 91 (females).
Serum levels of testosterone, estradiol, and luteinizing hormone were
determined in all males sacrificed on days 14 and 90. No adverse
treatment-related effects were observed at the two lower doses. At
125 ppm, absolute and relative liver weights were increased; in the
absence of histopathological changes or any other symptoms of
toxicity, the finding was not considered to be toxicologically
significant. At the two highest doses, liver hypertrophy was observed.
The response was sex-dependent: lamellar bodies and periportal
hypertrophy occurred in males and centrilobular hypertrophy without
lamellar bodies was seen in females. In the urinary bladder,
transitional-cell necrosis, exfoliation and hyperplasia were noted.
Hepatic cytochrome P450 levels were higher than control values, but
there was no evidence of peroxisome proliferation in the livers of
treated rats. No significant changes in the serum levels of
testosterone, estradiol, or luteinizing hormone were found in any of
the males examined. The NOAEL was 7.27 mg/kg bw per day (Keller,
1992a).
Rabbits
Groups of five male and five female New Zealand white rabbits
received dermal applications of technical-grade flusilazole (purity,
94.9%) at doses of 0, 1, 5, 25, or 200 mg/kg bw per day for 21 days.
The test material was applied daily as a paste in distilled water; the
exposure sites were occluded for 6 h and then washed with water. No
evidence of systemic toxicity was observed. The NOAEL for dermal
toxicity was 5 mg/kg bw per day, based on diffuse epidermal
hyperplasia or thickening (slight to mild) at > 25 mg/kg bw per
day. At the highest dose, mild erythema was seen on days 6-12. The
NOAEL for systemic toxicity was > 200 mg/kg bw per day (Sarver
et al., 1986).
Dogs
Groups of four male and four female beagle dogs were fed diets
containing technical-grade flusilazole (purity, 93%) at concentrations
of 0, 25, 125, or 750/500 ppm, equal to 0.9, 4.3, and 13.4 mg/kg bw
per day for males and 0, 0.9, 4.3, and 14.2 mg/kg bw per day for
females, for three months. The high-dose group received 750 ppm
flusilazole in the diet for the initial three weeks, then control diet
for one week, and 500 ppm flusilazole in the diet for the remaining
period. The dose was reduced because of marked body-weight loss and
decreased food intake at 750 ppm. At the lowest dose, there was a
treatment-related increase in the incidence of hyperplasia of the
lymphoid follicles in the pyloric glandular mucosa of the stomach in
males: 0/4, 3/4 3/4, and 4/4 at 0, 25, 125, and 750/500 ppm,
respectively. At 125 ppm, an increased incidence of pyloric granular
mucosa hyperplasia was also observed in females: 0/4, 0/4, 3/4, and
4/4 at 0, 25, 125, and 750/500 ppm, respectively. Higher levels (in
comparison with control values) of alanine aminotransferase activity
and an increased incidence of mild urinary bladder mucosal hyperplasia
were observed in males at 125 ppm and in animals of each sex at the
highest dose. Additional treatment-related effects seen at the highest
dose included clinical signs of toxicity (weakness or tremors) in
animals of each sex, reduced mean body-weight gain in males,
body-weight loss in females, decreased mean food consumption in
animals of each sex, a slight increase in leukocyte and monocyte
counts in males, decreased plasma levels of cholesterol, total
protein, and albumin in animals of each sex, and increased absolute
and relative liver weights in animals of each sex. There was no NOAEL
(Rickard et al., 1983).
Groups of five male and five female beagle dogs were fed
technical-grade flusilazole (purity, 95.8%) in the diet at doses of 0,
5, 20, or 75 ppm, equal to 0, 0.14, 0.7, and 2.4 mg/kg bw per day for
males and 0, 0.14, 0.7, and 2.6 mg/kg bw per day for females, for one
year. No treatment-related effects were observed at the lowest dose.
At the next dose, 20 ppm, the serum albumin level was decreased in
males and there was a dose-related increase in the incidence of
centrilobular hepatocellular hypertrophy in both males (0/5, 0/5, 4/5,
and 5/5) and females (0/5, 0/5, 2/5, and 5/5). More severe gastric
mucosal lymphoid hyperplasia, from minimal at 0 or 5 ppm to mild at
20 ppm, was also noted in males. At the highest dose, additional
treatment-related effects included higher leukocyte counts in animals
of each sex; elevated alkaline phosphatase and lowered cholesterol,
total protein, and albumin levels in males only; and increases in
relative liver (animals of each sex) and kidney weights (females). All
high-dose animals exhibited a greater degree of hepatic centrilobular
inflammatory infiltration, and distinct centrilobular hepatocellular
vacuolation was observed in three males. The lymphoid hyperplasia in
the gastric mucosa was of moderate severity in both males and females.
The NOAEL was 0.14 mg/kg bw per day (O'Neal et al., 1985).
(c) Long-term toxicity and carcinogenicity
Mice
Groups of 80 male and 80 female Crl:CD-1(ICR)BR mice received
technical-grade flusilazole (purity, 96.5%) in the diet at doses of 0,
5, 25, or 200 ppm, equal to 0, 0.66, 3.4, and 27 mg/kg bw per day for
males and 0, 0.92, 4.6, and 36 mg/kg bw per day for females, for 18
months. Each group included 10 mice of each sex that were killed at
six months. There were no treatment-related effects on mortality
(terminal survival rates were 76-86% in males and 57-80% in females),
body weight, food consumption, haematology, or serum chemistry at any
dose. At the highest dose, a toxicologically significant increase in
the incidence of hepatocellular fatty changes was observed, with
terminal incidences of 4/80, 3/80, 10/80, and 40/80 males and 2/80,
4/80, 3/80, and 24/80 females at 0, 5, 25, and 200 ppm. In addition,
there was an increase in absolute and relative liver weights in
animals of each sex, a decrease in absolute kidney weight in females,
and an increase in lymphocytic infiltration in the lung and urinary
bladder in males. There was no treatment-related increase in the
incidence of any tumour type at doses > 27 mg/kg bw per day, the
highest dose tested. The NOAEL for systemic toxicity was 3.4 mg/kg bw
per day (Brock et al., 1985).
Groups of 100 male and 100 female Crl:CD-1(ICR)BR mice received
diets containing technical-grade flusilazole (purity, 94%) at doses of
0, 100, 500, or 1000 ppm in males, equal to 0, 14.3, 73.1, and
144 mg/kg bw per day, and 0, 100, 1000, or 2000 ppm in females, equal
to 0, 19.4, 200, and 384 mg/kg bw per day, for 18 months. An
additional group of 100 mice of each sex was fed a diet containing
25 ppm technical-grade flusilazole (equal to 3.51 mg/kg bw per day for
males and 4.38 mg/kg bw per day for females) and was used to study
cell proliferation. The doses were selected on the basis of the
results of a 90-day study that indicated greater sensitivity of males
to the test compound. At the lowest dose tested, there was a slight
decrease in absolute kidney weight, an increase in focal necrosis of
the liver, and urinary bladder hyperplasia in males. No remarkable
changes were seen in females at this dose. At the two highest doses,
females had increased cellular hyperplasia in the urinary bladder and
urethra, but no focal necrosis of the liver. At these two doses,
additional treatment-related systemic effects included increased
absolute and relative liver weights, lower absolute and relative
kidney weights, increased numbers of foci of hepatocellular
alteration, and an increased incidence of hepatic vesicular or
vacuolar changes with cellular hypertrophy. Significantly higher
mortality occurred at 1000 ppm in males and 2000 ppm in females. In
addition, increased cellular proliferation in the urinary bladder (but
not in the liver) was observed in females at the highest dose. At
terminal sacrifice, an increased incidence of hepatocellular adenomas
and carcinomas was observed in males at all doses (13/80, 23/79,
20/80, and 18/78 at 0, 100, 500, and 1000 ppm, respectively;
historical control range, 6.3-13.8%) and in females at the two highest
doses (1/79, 3/80, 11/77, and 43/76 at 0, 100, 1000, and 2000 ppm,
respectively; historical control range, 0-2.6%). The increase in
tumour incidence was dose-related and statistically significant in
females but not in males. There was no NOAEL for systemic toxicity or
oncogenicity (Keller, 1992b).
Rats
Groups of 70 male and 70 female rats (Crl:CD(SD)BR strain)
received technical-grade flusilazole (purity, 95.6%) in the diet at
doses of 0, 10, 50, or 250 ppm, equal to 0, 0.4, 2.0, and 10 mg/kg bw
per day for males and 0, 0.5, 2.6, and 13 mg/kg bw per day for
females, for two years. Each group included two groups of 10 rats of
each sex that were killed at six months and one year. About 100 days
after the beginning of treatment, 20 rats of each sex in each group
were mated and were returned to the study after weaning of the second
litter. The rats killed at six months were examined for bladder
lesions only. No treatment-related effect on mortality was observed;
the survival rate was > 50% in all groups until week 98. No signs of
systemic toxicity was seen at the low dose. At 50 ppm, there was a
dose-related increase in the incidence of pyelonephritis in females at
two years (3/66, 3/62, 8/67, and 10/65 at 0, 10, 50, and 250 ppm,
respectively). Increases in relative liver weight in females and in
the incidence of hydronephrosis in males were observed at interim
sacrifice at one year. At the highest dose, additional treatment-
related changes included an increased incidence of hepatic lesions
(centrilobular hepatocellular hypertrophy and polyploidy) in females
and hydronephrosis in males at one year and an increased incidence of
acidophilic foci of hepatocellular alteration (13/65; 3/66 in
controls) and hepatic diffuse fatty changes (23/65; 9/66 in controls)
in females at two years. No hepatic lesions were observed in males
during the study, and no lesions of the urinary bladder were seen in
animals of either sex at either interim or terminal sacrifice. There
was no treatment-related increase in the incidence of any tumour type
at any dose. At 250 ppm, males had a slightly increased incidence of
squamous-cell carcinomas of the oral and nasal cavities (0/66, 1/63,
0/67, and 3/64 at 0, 10, 50, and 250 ppm, respectively). On the basis
of historical control data, from five two-year feeding studies in rats
with no tumours of this type and one study with an incidence of 2/60,
the incidence of nasal tumours in this study was judged to be
incidental. The NOAEL for systemic toxicity was 0.4 mg/kg bw per day;
however, the effects reported were relatively mild, and higher doses
might have been tolerated (Pastoor et al., 1986).
Groups of 65 male and 65 female Crl:CD(SD)BR rats received diets
containing technical-grade flusilazole (purity, 95%) at doses of 0,
125, 375, or 750 ppm, equal to 0, 5.03, 14.8, and 30.8 mg/kg bw per
day for males and 0, 6.83, 20.5, and 45.6 mg/kg bw per day for
females, for two years. Each group included 10 rats of each sex for
interim sacrifice at one year. Dose-related increases in the incidence
of hepatocellular hypertrophy were observed in animals of each sex at
both interim (males: 0/10, 4/10, 8/10, and 8/10; females: 0/10, 7/10,
10/10, and 10/10; at 0, 125, 375, and 750 ppm, respectively) and
terminal sacrifice (males: 2/53, 2/51, 10/53, and 19/53; females:
2/56, 4/55, 12/53, and 25/54). There was an apparent sex difference in
the hepatic lesions: periportal hepatocellular hypertrophy with
lamellar bodies was seen in males, while females showed centrilobular
hepatocellular hypertrophy with eosinophilic cytoplasm but no lamellar
bodies. At terminal sacrifice, there was also an increased incidence
of mixed foci of cellular alteration in males (6/53, 14/51, 17/53, and
19/53). At 375 and 750 ppm, there were also significant decreases in
terminal body weight in females, increases in absolute and/or relative
liver weights at interim and/or terminal sacrifice in males and/or
females, and an increase in the incidence of urinary bladder
transitional-cell hyperplasia at the end of the study in animals of
each sex (3/46, 6/45, 27/47, and 42/51 in males and 5/47, 3/49, 15/49,
and 33/53 in females). At 750 ppm, additional treatment-related
changes included an increased incidence of hepatic fatty changes in
males and increased cellular proliferation in the liver and urinary
bladder. At the highest dose, a treatment-related increase in the
incidence of urinary bladder transitional-cell papillomas and
carcinomas was observed in animals of each sex at the end of the study
(males: 0/46, 0/45, 1/47, and 5/51; and females: 0/47, 1/49, 0/49, and
13/53; at doses of 0, 125, 375, and 750 ppm, respectively). The
incidence of testicular interstitial-cell (Leydig-cell) tumours in
males at the highest dose was also increased: 2/53, 4/51, 2/53, and
9/53 at 0, 125, 375 and 750 ppm, respectively. There was no NOAEL for
systemic toxicity; the NOAEL for oncogenicity was 14.8 mg/kg bw per
day (Keller, 1992c).
(d) Reproductive toxicity
Rats
Groups of six male and six female Crl:CD(SD)BR rats from a 90-day
feeding study were used in a one-generation study of reproductive
toxicity. The rats were fed daily diets containing technical-grade
flusilazole (purity, 96.7%) at doses of 0, 25, 125, or 375 ppm, equal
to 0, 2, 9, and 27 mg/kg bw per day for males and 0, 2, 11, and
31 mg/kg bw per day for females, for 90 days before mating. Males and
females within the same dose group were mated for 15 days; females
were examined daily for evidence of a copulation plug. After the
mating period, females were housed individually. The fertility index
was low in all groups and especially in the control group (67.7%);
three to six females per group became pregnant. At the highest dose, a
lower gestation index, a lower percentage of liveborn pups, and lower
pup weight at day 4 were observed in comparison with controls (Pastoor
et al., 1983). The small group size and the absence of individual
data on some parameters limit the usefulness of this study for
evaluation of reproductive toxicity.
Groups of 20 male and 20 female Crl:CD(SD)BR rats from a two-year
study of toxicity and oncogenicity were used in a two-generation,
two-litter study. The rats were fed diets containing technical-grade
flusilazole (purity, 95.6%) at doses of 0, 10, 50, or 250 ppm, equal
to 0, 1, 3, and 18 mg/kg bw per day for males and 0, 1, 4, and
20 mg/kg bw per day for females (premating intake) for at least 100
days before mating. Males and females (F0) within the same dose group
were mated 1:1 for 15 days; the females were examined daily for
evidence of a copulation plug. After the mating period, F0 females
were housed individually and allowed to give birth to F1a litters.
About one week after the last F1a litter had been weaned, F0 females
were mated with different F0 males of the same dose group to produce
the F1b litters. After weaning of the F1b litters, 20 rats of each
sex per group were selected as the F1 parents for the F2 generation.
These rats were maintained on the same diets as their F0 parents for
90 days before mating to produce the F2a and F2b litters. Sibling
mating was avoided. The mean body weights of the F1b males at 250 ppm
were decreased during the premating period. No other treatment-related
systemic effect was observed in any F0 or F1 adult. There was no
evidence of a treatment-related effect on mating or fertility.
Treatment-related embryo-, feto-, and litter toxicity were observed at
the mid- (50 ppm) and high doses (250 ppm). An increased number of
stillborn pups and a decreased viability index (days 0-4) occurred in
the F2a litters at 50 ppm and in all litters at 250 ppm. Litter
survival after day 4 was similar in all groups in the F1 and F2
generations. At 250 ppm, the mean weights of F1b and F2b pups of each
sex at weaning were slightly reduced, and the absolute and relative
liver weights of F2b weanling male pups were increased. An increased
incidence of unilateral and/or bilateral hydronephrosis was noted in
F2b female weanlings: 1/10, 4/10, 3/10, and 5/10 at 0, 10, 50, and
250 ppm, respectively. Since neither the severity nor the incidence
of the lesions showed a dose-response relationship and since
hydronephrosis is a common lesion in weanling pups (historical control
range in 13 in-house studies, 0-30%), the finding was judged to be
toxicologically insignificant. The NOAEL was 1 mg/kg bw per day, based
on embryo- and fetotoxicity and decreased viability of pups in the
F2a litters at 50 ppm (Pastoor et al., 1986).
In a two-generation study with one or two litters per generation,
groups of 30 male and 30 female Crl:CD(SD)BR rats were fed diets
containing technical-grade flusilazole (purity, 94%) at doses of 0, 5,
50, or 250 ppm, equal to 0, 0.34, 3.46, and 17.3 mg/kg bw per day for
males and 0, 0.40, 4.04, and 19.6 mg/kg bw per day for females
(premating intake), for 73 days (F0 rats) or 91 days (F1 parents)
before mating. Males and females within the same dose group were
randomly paired 1:1 for 15 days; females were examined daily for
evidence of a copulation plug. One litter (F1a) was produced in the
F0 generation and two litters (F2a and F2b) in the F1 generation.
No treatment-related effects were seen in either the F0 or F1
generation at the low dose. At the middle dose, increased smooth
endoplasmic reticulum was seen in the hepatocytes of males and
centrilobular hepatocellular hypertrophy in females. At the highest
dose, F1 females had slightly but consistently lower body weights.
Reproductive toxicity was indicated by higher mortality during
parturition and increased gestational length of the F0 and F1 dams:
mean, 22.9-23.2 days in comparison with 22.4-22.6 days in controls. No
treatment-related effects on mating or fertility indices were
observed. Embryo-, feto-, and litter toxicity were seen as reduced
number of pups per litter and increased numbers of stillborn pups per
litter in the F1a, F2a, and F2b litters and decreased mean pup
weights on lactation days 14 and 21 (F2a litters only) at 250 ppm.
The NOAEL for systemic toxicity was 0.34 mg/kg bw per day, on the
basis of hepatic lesions at 50 ppm; the NOAEL for reproductive
toxicity was 4.04 mg/kg bw per day, on the basis of treatment-related
mortality during parturition, increased gestational length, and
embryo-, feto-, and litter toxicity at 250 ppm (Mullin, 1990).
(e) Developmental toxicity
Rats
In a range-finding study, groups of seven pregnant rats received
technical-grade flusilazole (purity, 99%) by gavage at doses of 0,
100, or 300 mg/kg bw per day on days 7-16 of gestation. Flusilazole
was maternally and embryotoxic at both doses, and the highest dose
induced cleft palates in about 51% of the fetuses in each litter. In
the main study of developmental toxicity, groups of 25 mated female
Crl:CD(SD)BR rats received technical-grade flusilazole (purity, 95.6%)
by gavage in corn oil at doses of 0, 10, 50, or 250 mg/kg bw per day
on days 7-16 of gestation; the day a copulation plug was observed was
designated as day 1 of gestation. On gestation day 21, all surviving
dams were sacrificed and necropsied; fetuses were delivered by
caesarean section and examined for external, visceral, and skeletal
abnormalities. No treatment-related signs of maternal toxicity were
seen at the lowest dose. At 50 mg/kg bw per day, slight decreases in
body-weight gain and food consumption and a slight increase in the
relative liver weight of dams were seen. At the highest dose,
additional maternal toxic effects included higher mortality and
clinical signs of toxicity (chromodacryorrhoea, chromorhinorrhoea, wet
or stained perineal areas, red vaginal discharges or stains, and focal
alopecia) in 23 animals. Fetuses had treatment-related increases in
the incidence of skeletal anomalies (misaligned sternebrae, extra
ossification centres in ribs, and delayed ossification in sternebrae)
at all doses. At 50 mg/kg bw per day, there was a reduced mean number
of liveborn fetuses per litter, a higher total number of stunted
fetuses (0, 1, 4, and 3 at 0, 10, 50, and 250 mg/kg bw per day,
respectively), and an increased incidence of rudimentary ribs. At the
highest dose, additional signs of embryo- and fetotoxicity were an
increased mean incidence of resorptions, reduced mean fetal weight per
litter, and all increased incidence of extra ribs in the fetuses. An
increased incidence of cleft palate (28/241; 0/331 in controls) and an
absence of renal papillae (21/155; 0/175 in controls) were observed in
fetuses of dams given 250 mg/kg bw per day but not at lower doses. An
unusually high incidence of hydrocephalus and/or dilated lateral
ventricles of the brain was also noted in all groups, including
controls; the effect was not dose-related and was not considered to be
related to treatment. The NOAEL for maternal toxicity was 10 mg/kg bw
per day; there was no NOAEL for embryo- or fetotoxicity; and the NOAEL
for teratogenicity was 50 mg/kg bw per day (Lamontia et al., 1984a).
Groups of 24 mated female Crl:CD(SD)BR rats received technical-
grade flusilazole (purity, 95.6%) by gavage in corn oil at doses of 0,
0.4, 2, 10, 50, or 250 (10 females only) mg/kg bw per day on days 7-16
of gestation; the day a copulation plug was observed was designated
day 1 of gestation. On day 21 of gestation, all surviving dams were
sacrificed and necropsied; fetuses were delivered by caesarean section
and examined for external, visceral, and skeletal abnormalities. No
treatment-related signs of maternal toxicity were observed at doses
> 10 mg/kg bw per day. Slightly reduced body-weight gain and food
consumption during treatment and increased relative liver weights of
dams were seen at 50 mg/kg bw per day. At the highest dose, clinical
signs of maternal toxicity (alopecia, brown stains on the face and
limbs, and stained perineal area) were also observed. No treatment-
related embryo- or fetotoxic effects were seen at the two lowest
doses. At > 10 mg/kg bw per day, an increased number of dams with
median or late resorptions, an increased total number of stunted
fetuses, a higher incidence of visceral (large renal pelvis and small
renal papilla) and skeletal (rib) anomalies, and an increased
incidence of delayed ossification (sternebrae and vertebral arches)
were observed. An increased incidence of cleft palate (21/116; 0/291
in controls) was observed in fetuses of dams receiving 250 mg/kg bw
per day but not at lower doses. Hydrocephalus was not observed. The
NOAEL for maternal toxicity was 10 mg/kg bw per day, that for embryo-
and fetotoxicity was 2 mg/kg bw per day, and that for teratogenicity
was 50 mg/kg bw per day (Lamontia et al., 1984b).
Groups of 24 mated female rats (Crl:CD (SD)BR strain) received
diets containing technical-grade flusilazole (purity, 96.5%) at doses
of 0, 50, 100, 300, or 900 ppm equal to 0, 4.6, 9.0, 26.6, and
79.2 mg/kg bw per day) on days 7-16 of gestation; the day a copulation
plug was observed was designated day 1 of gestation. On gestation day
21, all surviving dams were sacrificed and necropsied; fetuses were
delivered by caesarean section, weighed, sexed, and then examined for
external, visceral, and skeletal abnormalities. No treatment-related
signs of maternal toxicity were seen at doses > 100 ppm.
Significant, dose-related reductions in body-weight gain and food
consumption were observed during treatment at the two highest doses.
Treatment-related embryo- and fetotoxic effects were noted at
> 100 ppm, including an increased incidence of median or late
resorptions, small litters (< 10 fetuses per litter), and significant
dose-related increases in skeletal variations with extra ossification
of the sternebrae. Additional symptoms of fetal toxicity seen at the
two highest doses included an increased number of stunted fetuses, a
higher incidence of rudimentary ribs, extra ossification in cervical
ribs, and delayed ossification of the cervical vertebral arches. None
of the fetuses had cleft palate, and there were no treatment-related
malformations at any dose. The NOAEL for maternal toxicity was
9.0 mg/kg bw per day, that for embryo- and fetotoxicity was 4.6 mg/kg
bw per day, and that for teratogenicity was 79.2 mg/kg bw per day
(Alvarez et al., 1984).
In a study of prenatal and postnatal toxicity in rats, groups of
24 (phase I, prenatal study) or 22 (phase II, postnatal study) mated
female rats (Crl:CD (SD)BR strain) received technical-grade
flusilazole (purity, 96.5%) in 0.5% aqueous methylcellulose by gavage
at doses of 0, 0.2, 0.4, 2, 10, or 100 mg/kg bw per day on days 7-16
of gestation; the day a copulation plug was observed was designed day
1 of gestation. In phase I, dams were sacrificed on gestation day 21
for examination of the uterine contents. Additional control groups and
animals at 100 mg/kg bw per day were killed on gestation day 22 to
determine whether the absence of renal papillae was a compound-related
effect or an anomaly. As the concentrations of the first few solutions
used in phase I were found to be only 1-19% of the nominal
concentrations and the next analysis which showed 75-110% of the
nominal concentration, was done only on day 7, no definitive
conclusions could be drawn from the results of this part of the study.
Signs of maternal toxicity seen at the highest dose included clinical
signs (chin or perinasal staining and/or wet perineum), reduced
body-weight gain and food consumption during treatment, and increased
absolute and relative liver weights. Embryo- and fetotoxicity were
observed at > 10 mg/kg bw per day, manifested as increased numbers
of stunted fetuses and a higher incidence of visceral anomalies (small
renal papillae and distended ureter). Additional embryo- and
fetotoxicity seen at the highest dose included an increased incidence
of median or late resorptions and a decrease in the mean number of
live fetuses per litter. Treatment-related malformations (absence of
renal papillae) were observed in three fetuses from two litters of
dams at 100 mg/kg bw per day. In phase II of the experiment, dams were
permitted to deliver naturally and to raise their litters to weaning.
All dams and pups were sacrificed on day 21 of lactation and subjected
to gross necropsy. The solutions were found to be adequately prepared
in this phase. No treatment-related maternal toxicity was evident at
doses > 10 mg/kg bw per day. At the next dose, 100 mg/kg bw per
day, increased mortality (5/22; 0/22 in controls), clinical signs of
difficult parturition (pallor, bunching, weakness, and/or dystocia
during parturition and lactation in four dams), reduced body-weight
gain and food consumption during the early part of treatment, and
increased liver weight were observed. No treatment-related embryo-
or fetotoxicity was noted at doses > 2 mg/kg bw per day. At
> 10 mg/kg bw per day, there were overt, dose-related increases in
mean gestational length (22.8, 23.1, 22.9, 23.1, 23.5, and 24.7 days
at 0, 0.2, 0.4, 2, 10, and 100 mg/kg bw per day, respectively),
decreases in mean litter size and the number of liveborn fetuses per
litter, an increased number of small litters (< 10 fetuses per
litter), and a higher incidence of dilated renal pelvis and/or ureter
in pups at weaning. Additional signs of treatment-related embryo- and
fetotoxicity at the highest dose included a reduced total number of
live litters, an increased mean number of dead fetuses per litter, and
reduced viability indexes (days 0-4; 82%; 98-99% in control and lower
dose groups). There were no treatment-related malformations in the
live pups examined. Of the 42 pups found dead, 29 were in the group at
100 mg/kg bw per day; two had no renal papillae and four had small
papillae. The NOAEL for maternal toxicity was 10 mg/kg bw per day,
that for embryo- and fetotoxicity was 2 mg/kg bw per day, and that for
teratogenicity was 100 mg/kg bw per day, the highest dose tested
(Alvarez et al., 1985a).
Rabbits
Four groups of 18 artificially inseminated female New Zealand
white rabbits received technical-grade flusilazole (purity, 96.5%) in
corn oil by gavage at nominal doses of 0, 2, 5, or 12 mg/kg bw per
day, equal to 0, 1.9, 4.8, and 10.1 mg/kg bw per day on the basis of
analysis of the solutions, on days 7-19 of gestation; the day of
insemination was designated gestation day 0. On gestation day 29, all
surviving does were sacrificed and necropsied; fetuses were delivered
by caesarean section and examined for external, visceral, and skeletal
abnormalities. There was no treatment-related mortality, no clinical
signs of maternal toxicity, and no disturbances of intrauterine
development of the conceptuses at any dose up to and including
12 mg/kg bw per day, the highest dose tested. All of the fetuses
delivered showed normal development. No evidence of fetotoxicity and
no treatment-related increases in malformations were observed in
fetuses at any dose. The higher incidence of hydrocephalus observed in
this study (one fetus in one litter, two in one litter, four in two
litters, and four in three litters at 0, 2, 5, and 12 mg/kg bw per
day, respectively), which was not confirmed in three subsequent
studies (in only one fetus at 35 mg/kg bw per day and in none at lower
doses or in controls), was not considered to be treatment-related. The
NOAEL for maternal toxicity, embryo- and fetotoxicity and
teratogenicity was 12 mg/kg bw per day (10.1 mg/kg bw per day by
analysis) (Solomon et al., 1984).
Three groups of 20 artificially inseminated female New Zealand
white rabbits received technical-grade flusilazole (purity, 96.5%) by
gavage at nominal doses of 0, 12, or 35 mg/kg bw per day, equal to 0,
11.2 and 31.5 mg/kg bw per day on the basis of analysis of the
solutions, on days 7-19 of gestation; the day of insemination was
designated gestation day 0. On gestation day 29, all surviving does
were killed and necropsied; fetuses were delivered by caesarean
section and examined by gross pathology. No evidence of maternal, or
embryo- or fetotoxicity was observed at the low dose, and the
incidence of malformations and fetal variations was not increased in
comparison with the controls. At the high dose, there was an increased
incidence of red vaginal discharge and stained tail and an increased
incidence of periodic anorexia. Two of 13 treated does and 0/16
controls aborted, and 10 of 13 had early total resorption in
comparison with 1/16 controls. Teratogenicity could not be assessed at
this dose because only one live litter was produced. The NOAEL for
maternal, embryo-, and fetotoxicity was 12 mg/kg bw per day
(11.2 mg/kg bw per day by analysis), and that for teratogenicity was
> 12 mg/kg bw per day (11.2 mg/kg bw per day by analysis) (Zellers
et al. 1985).
In a range-finding study, four groups of seven artificially
inseminated female New Zealand white rabbits were fed diets containing
technical-grade flusilazole (purity, 94.8%) at doses of 0, 500, 1000,
or 2000 ppm on days 7-19 of gestation. The pregnancy rate (3/7 per
group) was low at 500 and 1000 ppm, and the incidence of mortality
in utero was high at 2000 ppm (4/7 females with total resorptions).
In the main study, dietary levels of 0, 300, 600, or 1200 ppm
technical-grade flusilazole (purity, 94.8%), equal to 0, 8.9, 21.2,
and 37.8 mg/kg bw per day, were given to groups of 20 artificially
inseminated female New Zealand white rabbits on days 7-19 of
gestation; the day of insemination was designated day 0 of gestation.
All surviving does were sacrificed on gestation day 29 and necropsied;
fetuses were delivered by caesarean section and examined for external,
visceral, and skeletal abnormalities. During treatment with the
highest dose, maternal body weight and food consumption were
decreased. The pregnancy rate was reduced in all treated groups: 9/20,
10/20, and 7/20 at 300, 600, and 1200 ppm, respectively. The number of
does with total resorptions was increased at 600 and 1200 ppm. There
were no treatment-related effects on the mean number of live fetuses
per litter, the mean number of resorptions in dams with live fetuses,
or fetal weight. The small number of litters available from dams at
600 and 1200 ppm (three per group) precluded any definitive assessment
of fetotoxicity or teratogenic potential at these doses. There were no
apparent treatment-related effects at the low dose. In a supplementary
study, dietary levels of 0, 30, 100, or 300 ppm, equal to 0, 0.81,
2.84, and 8.32 mg/kg bw per day, of technical-grade flusilazole were
administered to groups of 18 or 25 (300 ppm) inseminated female New
Zealand white rabbits on days 7-19 of presumed gestation. The
pregnancy rate was again low in all groups, including the controls
(8/18). Total resorption occurred at 0 and 300 ppm in 25 and 29%,
respectively, of the pregnant does, but not at the low or mid-dose.
Because of the small number of live litters available for examination,
the results could not be adequately assessed for embryo- or
fetotoxicity or teratogenic potential. The NOAEL for maternal toxicity
was 21.2 mg/kg bw per day, based on decreased body weight and food
consumption at 1200 ppm. There was no NOAEL for embryo- or
fetotoxicity or teratogenicity (Alvarez et al., 1985b).
Four groups of 18 artificially inseminated female New Zealand
white rabbits received technical-grade flusilazole (purity, 93.8%) in
0.5% methylcellulose by gavage at doses of 0, 7, 15, or 30 mg/kg bw
per day on days 7-19 of gestation. The pregnancy rate was acceptable
in all groups: 12/18, 14/18, 16/18, and 16/18 at 0, 7, 15, and
30 mg/kg bw per day, respectively. No treatment-related maternal,
embryo- or fetotoxicity was seen at the low dose. At the two higher
doses, clinical signs of maternal toxicity (red discharge and
brown-yellow-stained tail) and increased incidences of abortion (one
dam per group) and total resorptions (4/16 and 12/16, respectively)
were observed. At the highest dose, food consumption was also
decreased. The mean numbers of liver fetuses per litter and of dead
fetuses, mean fetal weight and mean male:female ratio were comparable
in the control and treated groups. No treatment-related external,
visceral, or skeletal malformation or variation was observed in
fetuses of does at any dose; however, it should be noted that
assessment of fetotoxicity and teratogenic potential at the highest
dose was based on data for only three of 11-12 live litters in the
control and lower dose groups. These limited data reduce confidence in
the accuracy of any conclusions drawn on the basis of observations at
this dose. The NOAEL for maternal, embryo-, and fetal toxicity was
7 mg/kg bw per day, and that for teratogenicity was > 15 mg/kg bw
per day (Alvarez, 1900).
(f) Genotoxicity
A battery of studies on technical-grade flusilazole was conducted
to assess its potential to induce gene mutation, chromosomal
aberration, or unscheduled DNA synthesis. The results (summarized in
Table 2) were clearly negative.
(g) Special studies
(i) Dermal and ocular irritation and dermal sensitization
Two male New Zealand white rabbits received 0.01 ml of undiluted
flusilazole (purity, 90%) into the right conjunctival sac; the left
eye was not treated. The right eye of one rabbit was washed with
100 ml of water after 20 sec of exposure. Irritation was graded
according to the Draize scale 1, 4, 24, 48, and 72 h after
instillation. Both animals had mild conjunctival redness and chemosis
1-4 h after instillation. The washed eye also showed some discharge at
1 h, but by 24 h these effects were no longer observed. The unwashed
eye showed slight corneal opacity at 1-4 h, and biomicroscopic
examination revealed slight cloudiness at 24-48 h; the eye was normal
at 72 h. Flusilazole was thus minimally irritating to the eyes of male
New Zealand white rabbits, causing transient, mild irritation.
Flushing the eye with water after exposure eliminated the corneal but
not the conjunctival effects (Hall et al., 1984).
In a study of primary skin irritation, six young male New Zealand
white rabbits received a flusilazole formulation (Nustar 20DF,
containing 21% technical-grade flusilazole and 79% unspecified, inert
ingredients; undiluted, moistened with distilled water) topically on
shaved skin sites on the back at a concentration of about 0.5 g. The
test site was covered with a gauze patch and a sheet of rubber for
4 h; the coverings were then removed and the site washed gently with
warm water. Each site was evaluated for irritation potential according
to the Draize scale 4, 24, 28, and 72 h after treatment. At 4 h, grade
1 erythema was observed in four rabbits and grade 1 or 2 oedema in two
rabbits. By 24 h, only two rabbits had erythema and no oedema was
observed. No signs of dermal irritation were seen in any rabbit at
72 h. The primary irritation index was 0.167. Flusilazole as
formulated for this test was thus minimally irritating to the skin
(Brock, 1988).
Table 2. Results of tests for the genotoxicity of flusilazole
End-point Test system Concentration Purity Results Reference
or dose (%)
In vitro
Reverse mutation Salmonella typhimurium TA98, 1-250 µg/plate 90.0 Negativea Donovan & Irr (1982)
TA100, TA1535, TA1537
Reverse mutation Salmonella typhimurium TA97, 5-250 µg/plate 97.7 Negativea Arce et al. (1988)
TA98, TA100, TA1535
Reverse mutation Salmonella typhimurium TA97, 10-300 µg/plate 61.7 Negativea Reynolds (1991)
TA98, TA100, TA1535
hprt forward mutation Chinese hamster ovary cells 0.04-0.275 mmol/litre 95.5 Negative McCooey et al. (1983)
(KI/BH4 clone)
Chromosomal aberration Human lymphocytes 1.7-100 µg/ml 94.9 Negativea Vlachos et al. ( 1986),
Unscheduled DNA Rat primary hepatocytes 1 × 10-5 - 1.1 × 102 95.5 Negative Chromey et al. (1983)
synthesis mmol/litre
In vivo
Chromosomal aberration CrI:CD rat bone marrow 50-500 mg/kg bw NR Negative Farrow et al. (1983)
(single dose by gavage)
Micronucleus formation CD-1 mouse bone marrow 375 mg/kg bw 95.5 Negative Sorg et al. (1984)
(single dose by gavage)
NR, not reported
a With and without exogenous metabolic activation
A range-finding study in young adult Hartley guinea-pigs
indicated mild erythema 24 h after dermal application of undiluted
flusilazole (purity, 90%) in aliquots of 0.05 ml. No dermal irritation
was observed with concentrations > 50%. In a study of primary skin
irritation, flusilazole (purity, 90%) was applied topically to the
skin at concentrations of 5 or 50% (w/v, solution in dimethyl
phthalate), and two test sites per animal were scored for signs of
irritation at 24 and 48 h. No dermal irritation was seen in any of the
10 exposed animals. Flusilazole was thus not irritating to the skin of
guinea-pigs (Wylie el al., 1984c).
In a study of the sensitization of young adult Hartley
guinea-pigs, intradermal injections of a 1% solution (w/v) of
flusilazole (purity, 90%) in methyl phthalate weekly for four weeks
caused erythema and oedema with necrotic centres at the sites of
injection by 24 h. Challenge with topical applications of 5 or 50%
solutions to the skin did not induce sensitization (Wylie el al.,
1984c).
In another study of sensitization, 10 male and 10 female young
adult Duncan Hartley albino guinea-pigs were given three weekly dermal
applications of 0.4 ml (equivalent to 0.192 g) flusilazole (purity,
97.7%). The material, slightly moistened with dimethyl phthalate, was
applied to the intact shaved skin on the back and covered with plastic
wrap for 6 h, and dermal irritation was scored 24 and 48 h after
treatment. None was observed. Two weeks after the last induction, the
animals were challenged with a single dermal application of 0.4 ml of
flusilazole (0.192 g) on an untreated site, which was then covered for
6 h. The irritation response was again scored at 24 and 48 h. No signs
of dermal irritation were observed. A positive control group treated
with 1-chloro-2,4-dinitrobenzene showed severe erythema with necrosis
24 h after the second and third inductions and severe erythema after
the challenge dose two weeks later (Brock et al., 1988).
(ii) Mechanisms of Leydig-cell tumour induction
Groups of 10 Crl:CD BR male rats received technical-grade
flusilazole (purity, 94%) in corn oil by subcutaneous injection at
doses of 0, 20, 50, 150, or 250 mg/kg bw per day (given as two equal
half-doses, twice daily) for 14 days. The control group and that at
250 mg/kg bw per day each included an additional subgroup of 10 male
rats which were treated with human chorionic gonadotropin (hCG) 1 h
before sacrifice. Ketoconazole (a known inhibitor of 17ß-hydroxylase)
was used as the positive control: Groups of 10 male rats were given
subcutaneous injections of 0, 20, 50, 100, or 200 mg/kg bw per day
ketoconazole in saline (as two equal half-doses, twice daily) for 14
days. The control group and that at 200 mg/kg bw per day each included
an additional subgroup of 10 male rats which were treated with hCG 1 h
before sacrifice. All surviving animals were killed on day 15 of
treatment and necropsied. Testicular interstitial fluid and serum
samples were collected from rats that did not receive hCG; the
interstitial fluid was analysed for testosterone and the serum samples
for testosterone, estradiol, luteinizing hormone, and follicle-
stimulating hormone. The serum samples collected from the hCG-treated
rats were analysed for testosterone, androstenedione, 17ß-hydroxy-
progesterone, and progesterone. Increased absolute and relative liver
weights and a dose-related inhibition of serum testosterone levels
(statistically significant at doses > 150 mg/kg bw per day) and
estradiol (statistically significant at all doses) were observed at
doses > 20 mg/kg bw per day. At the two highest doses, clinical
symptoms of toxicity (sores, stained or wet fur, dehydration, and
diarrhoea), decreased body weight and body-weight gain, and reduced
food consumption were evident; eight of 10 rats at the highest dose
died. The rats at 250 mg/kg bw per day that were treated with hCG had
significantly lower serum testosterone levels than controls; no other
significant differences in hormone levels were noted. The positive
controls had significantly lower levels of testosterone,
androstenedione, and 17ß-hydroxyprogesterone and a higher level of
progesterone, indicating inhibition of 17ß-hydroxylase. There was no
NOAEL (Cook, 1993).
Leydig cells were collected from rat testes at termination of the
above study and cultured in microplate wells; the cells were then
incubated with either technical-grade flusilazole or ketoconazole at
doses of 0.05-100 µmol/litre in 70% ethanol for 2 h. The culture media
were sampled and analysed for testosterone, androstenedione,
17ß-hydroxyprogesterone, and progesterone. The results confirmed the
hormonal changes observed in vivo. Incubation of testicular Leydig
cells with technical-grade flusilazole caused a dose-dependent
lowering of testosterone and androstenedione levels, suggesting
inhibition of the enzymes involved in steroid biosynthesis. The IC50
for testosterone was 3.475 ± 1.455 µmol/litre without hCG and 2.774 ±
0.646 µmol/litre with hCG pretreatment. The positive control had an
IC50 for testosterone of 0.97 ± 0.83 µmol/litre without hCG and 0.154
± 0.065 µmol/litre with hCG (Cook, 1993).
Comments
In rats, orally administered 14C-labelled flusilazole was
readily absorbed and rapidly excreted in the urine and faeces, with
little radiolabel recovered in expired air. Over 90% of the
administered dose was eliminated within 96 h. 14C from phenyl-
labelled material was excreted predominantly in the faeces (87% in
males and 59% in females), while that from triazole-3-labelled
material was recovered primarily in the urine (72% in each sex).
Tissue retention of 14C was low (< 1%, excluding the carcass).
14C-Flusilazole was extensively metabolized in rats. After
absorption, it was cleaved at the triazole ring. Recovered parent
compound accounted for only 2-11% of the administered dose and was
found predominantly in faeces, the urinary level being < 1%.
Flusilazole is slightly toxic to mice, rats, and rabbits when
given as a single oral dose and is minimally toxic to rats and rabbits
when given dermally or by inhalation. The oral LD50 in rats was
> 500 mg/kg bw, the dermal LD50, in rabbits was > 2000 mg/kg bw,
and the inhalation LC50 in rats was 6.8-7.7 mg/litre. Flusilazole was
minimally irritating to the eyes and skin of rabbits; it was virtually
not irritating to the skin and was not a dermal sensitizer in guinea-
pigs. WHO has classified flusilazole as 'slightly hazardous'.
Repeated oral administration of flusilazole to mice (90 days),
rats (90 days), and dogs (90 days and one year) resulted primarily in
lesions of the liver (hepatocellular hypertrophy, hyperplasia and
vacuolation) and urinary bladder (urothelial hyperplasia and
vacuolation). On the basis of the hepatic and/or urinary bladder
changes, the NOAEL was 25 ppm (equal to 4 mg/kg bw per day) in mice,
125 ppm (equal to a mean of 8.1 mg/kg bw per day in two 90-day
studies) in rats, and 5 ppm (equal to 0.14 mg/kg bw per day) in dogs
(one-year study). Repeated dermal application of flusilazole to
rabbits for 21 days did not result in treatment-related systemic
toxicity at doses up to and including 200 mg/kg bw per day.
In two 18-month studies of carcinogenicity, flusilazole was
administered at dietary concentrations of 0, 5, 25, or 200 ppm to mice
of each sex in the first study and at 0, 100, 500, or 1000 ppm to
males and 0, 100, 1000, or 2000 ppm to females in the second study.
The overall NOAEL for the two studies was 25 ppm (equal to 3.4 mg/kg
bw per day) on the basis of hepatotoxicity and urinary bladder
hyperplasia in males at 100 ppm. In the second study, an increased
incidence of hepatocellular adenomas and carcinomas was observed in
males at > 100 ppm (equal to 14 mg/kg bw per day) and in females at
> 1000 ppm (equal to 200 mg/kg bw per day), doses at which lesions
of the liver (focal necrosis, hepatocellular hypertrophy and
hyperplasia, and vacuolation) were seen. Flusilazole was carcinogenic
in the second study.
In two two-year studies of toxicity and carcinogenicity, rats
received flusilazole at dietary concentrations of 0, 10, 50, or
250 ppm (first study) or 0, 125, 375, or 750 ppm (second study). In
the first study, the NOAEL was 10 ppm (equal to 0.4 mg/kg bw per day)
on the basis of mild nephrotoxicity (pyelonephritis in females and
hydronephrosis in males) at 50 ppm. In the second study, an increased
incidence of urinary bladder transitional-cell papillomas and
carcinomas in rats of each sex and testicular Leydig-cell tumours in
males were observed at 750 ppm (equal to 31 mg/kg bw per day), doses
at which lesions of the urinary bladder (urothelial necrosis,
exfoliation, and hyperplasia) were clearly demonstrated. Flusilazole
was carcinogenic in the second study.
A special two-week study to investigate the possible mechanism
by which testicular Leydig-cell tumours are induced was conducted
in rats. Flusilazole caused a dose-dependent lowering of serum
testosterone and estradiol levels at > 20 mg/kg bw per day in
vivo and a dose-related decrease in testosterone and androstenedione
production in cultured testicular Leydig cells by inhibiting enzymes
involved in steroid biosynthesis.
In a two-generation study of reproductive toxicity, rats were fed
diets containing flusilazole at concentrations of 0, 5, 50, or
250 ppm. The NOAEL for parental systemic toxicity was 5 ppm (equal to
0.34 mg/kg bw per day) on the basis of hepatic lesions at 50 ppm. The
NOAEL for reproductive toxicity was 50 ppm (equal to 4.0 mg/kg bw per
day) on the basis of treatment-related mortality during parturition,
increased length of gestation, reduced numbers of liveborn pups per
litter, and decreased pup growth at 250 ppm.
Three studies of developmental toxicity were performed in rats
with doses of 0, 10, 50, or 250 mg/kg bw per day in the first study,
0, 0.4, 2, 10, 50, or 250 mg/kg bw per day in the second study, and 0,
0.2, 0.4, 2, 10 or 100 mg/kg bw per day in the third study. A study of
developmental toxicity was also conducted in which rats were fed
flusilazole at dietary levels of 0, 50, 100, 300, or 900 ppm (equal to
0, 4.6, 9, 27, and 79 mg/kg bw per day) on days 7-16 of gestation. The
NOAEL for maternal toxicity was 10 mg/kg bw per day, on the basis of a
slight reduction in body-weight gain and food consumption during
treatment at > 27 mg/kg bw per day. The NOAEL for embryo- and
fetotoxicity was 4.6 mg/kg bw per day on the basis of increased
resorption, increased length of gestation, reduced litter size and a
higher incidence of skeletal variations or anomalies at > 9 mg/kg
bw per day. At 250 mg/kg bw per day, an increased incidence of cleft
palate was observed. The NOAEL for teratogenicity was 100 mg/kg bw per
day.
In three studies of developmental toxicity in rabbits, animals
were treated with flusilazole at 0, 2, 5, or 12 mg/kg bw per day
(first study), 0, 12, or 35 mg/kg bw per day (second study), or 0, 7,
15, or 30 mg/kg bw per day (third study) on days 7-19 of gestation.
The NOAEL for maternal and embryo- or fetotoxicity was 12 mg/kg bw per
day on the basis of clinical signs of toxicity and an increased
incidence of abortion and total resorptions at > 15 mg/kg bw per
day. There was no evidence of teratogenicity at doses up to and
including 15 mg/kg bw per day, the highest dose at which an adequate
number of live litters was available for an assessment of
teratogenicity.
Flusilazole has been adequately tested for genotoxicity in a
series of assays in vivo and in vitro. The Meeting concluded that
flusilazole is not genotoxic.
An ADI of 0-0.001 mg/kg bw was established on the basis of the
NOAEL of 5 ppm, equal to 0.14 mg/kg bw per day, in the one-year
dietary study in dogs, and a safety factor of 100.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 25 ppm, equal to 3.4 mg/kg bw per day (18-month study of
toxicity and carcinogenicity)
Rat: 10 ppm, equal to 0.4 mg/kg bw per day (two-year study of
toxicity and carcinogenicity)
5 ppm, equal to 0.34 mg/kg bw per day (maternal toxicity in
a two-generation study of reproductive toxicity)
50 ppm, equal to 4 mg/kg bw per day (two-generation study of
reproductive toxicity)
4.6 mg/kg bw per day (embryo- or fetotoxicity in a study of
developmental toxicity)
100 mg/kg bw per day (teratogenicity in a study of
developmental toxicity)
Rabbit: 12 mg/kg bw per day (maternal and embryo- or fetal toxicity
in a study of developmental toxicity)
15 mg/kg bw per day (teratogenicity in a study of
developmental toxicity)
Dog: 5 ppm, equal to 0.14 mg/kg bw per day (one-year study of
toxicity)
Estimate of acceptable daily in take for humans
0-0.001 mg/kg bw
Studies that would provide information useful for continued
evaluation of the compound
Observations in humans
Toxicological criteria for setting guidance values for dietary and non-dietary exposure to flusilazole
Exposure Relevant route, study type, species Result/remarks
Short-term (1-7 days) Dermal, irritation, rabbit Minimally irritating
Eye, irritation, rabbit Minimally irritating
Skin, sensitization, guinea-pig Not a skin sensitizer
Oral, toxicity, rat LD50 > 500 mg/kg bw
Dermal, toxicity, rabbit LD50 > 2000 mg/kg bw
Inhalation, 4 h, toxicity rat LC50 = 6.8-7.7 mg/litre
Medium-term (1-26 weeks) Repeated dermal, 21--day, toxicity, rabbit NOAEL = 200 mg/kg bw per day, highest
dose tested for systemic toxicity
Repeated dietary, reproductive toxicity, rat NOAEL = 0.34 mg/kg bw per day; hepatic
toxicity
Repeated gavage, developmental toxicity, NOAEL = 7 mg/kg bw per day for
rabbit maternal, embryo-, or fetotoxicity;
NOAEL > 15 mg/kg bw per day for
teratogenicity
Long-term (> one year) Repeated dietary, one year, toxicity, dog NOAEL = 0.14 mg/kg bw per day, primarily
for liver toxicity
References
Alvarez, L. (1990) Teratogenicity study of DPX-H6573-66 in rabbits.
Unpublished report No. HLR 216-90 from Haskell Laboratory for
Toxicology and Industrial Medicine, Wilmington, Delaware, USA.
Submitted to WHO by DuPont de Nemours and Co., Inc., Wilmington,
Delaware, USA.
Alvarez, L., Krauss, W.C. & Staples, R.E. (1984) Developmental
toxicity study in rats given INH-6573-66 in the diet on days 7-16
of gestation. Unpublished report No. HLR 431-84 from Haskell
Laboratory for Toxicology and Industrial Medicine, Wilmington,
Delaware, USA. Submitted to WHO by DuPont de Nemours and Co.,
Inc., Wilmington, Delaware, USA.
Alvarez, L., Staples, R.E. & Kaplan, A.M. (1985a) INH-6573: Prenatal
and postnatal toxicity study in rats dosed by gavage on days 7-16
of gestation. Unpublished report No. HLR 654-85 from Haskell
Laboratory for Toxicology and Industrial Medicine, Wilmington,
Delaware, USA. Submitted to WHO by DuPont de Nemours and Co..
Inc., Wilmington, Delaware, USA.
Alvarez, L., Staples, R.E., Driscoll, C.D. & Kaplan, A.M. (1985b)
INH-6573: Developmental toxicity study in rabbits treated by diet
on days 7-19 of gestation. Unpublished report No. HLR 337-85 from
Haskell Laboratory for Toxicology and Industrial Medicine,
Wilmington, Delaware, USA. Submitted to WHO by DuPont de Nemours
and Co., Inc., Wilmington, Delaware, USA.
Anderson, J.J., Stadalius, M.A. & Schlueter, D.D. (1986) Metabolism of
14C-DPX-H6573 in rats. Unpublished report No. AMR-196-84 from
DuPont de Nemours and Co., Inc., Wilmington, Delaware, USA.
Submitted to WHO by DuPont de Nemours and Co., Inc., Wilmington,
Delaware, USA.
Arce, G.T., Matarese, C.C. & Sarrif, A.M. (1988) Mutagenicity testing
of INH-6573-21 in Salmonella typhimurium plate incorporation
assay. Unpublished report No. HLR 59-88 from Haskell Laboratory
for Toxicology and Industrial Medicine, Wilmington, Delaware,
USA. Submitted to WHO by DuPont de Nemours and Co., Inc,
Wilmington, Delaware, USA.
Brock, W.J. (1988) Primary dermal irritation study with INH-6573-106
in rabbits. Unpublished report No. HLR 484-88 from Haskell
Laboratory for Toxicology and Industrial Medicine, Wilmington,
Delaware, USA. Submitted to WHO by DuPont de Nemours and Co.,
Inc., Wilmington, Delaware, USA.
Brock, W.J., Rickard, R.W., Kaplan, A.M. & Gibson, JR. (1985) Long-
term feeding study in mice with INH-6573. Unpublished report No.
HLR 278-85 from Haskell Laboratory for Toxicology and Industrial
Medicine, Wilmington, Delaware, USA. Submitted to WHO by DuPont
de Nemours and Co., Inc., Wilmington, Delaware, USA.
Brock, W.J., Vick, D.A. & Chromey, N.C. (1988) Closed-patch repeated
insult dermal sensitization study (Buehler method) with
INH-6573-21 in guinea pigs. Unpublished report No. HLR 34-88 from
Haskell Laboratory for Toxicology and Industrial Medicine,
Wilmington, Delaware, USA. Submitted to WHO by DuPont de Nemours
and Co., Inc., Wilmington, Delaware, USA.
Cheng, T. (1986) Rat metabolism, study of [triazole-3-14C] DPX-H6573.
Unpublished report No. HLR 337-85 from Haskell Laboratory for
Toxicology and Industrial Medicine, Wilmington, Delaware, USA.
Submitted to WHO by DuPont de Nemours and Co., Inc., Wilmington,
Delaware, USA.
Chromey, N.C., Horst, A.L., McCooey, K.T. & Sarrif. A.M. (1983)
Unscheduled DNA synthesis/rat hepatocytes in vitro. Unpublished
report No. HLR 209-83 from Haskell Laboratory for Toxicology and
Industrial Medicine, Wilmington, Delaware, USA. Submitted to WHO
by E.I. du Pont de Nemours & Co., Inc., Wilmington. Delaware,
USA.
Cook, J. (1993) Mechanisms of rat Leydig cell tumor induction by
DPX-H6573-193 (Flusilazole). Unpublished report No. HLR 410-93
from Haskell Laboratory for Toxicology and Industrial Medicine,
Wilmington, Delaware, USA. Submitted to WHO by E.I. du Pont de
Nemours & Co., Inc., Wilmington, Delaware, USA.
Donovan, S.M. & Irr, J.D. (1982) Mutagenicity evaluation in
Salmonella typhimurium. Unpublished report No. HLR 611-82 from
Haskell Laboratory for Toxicology and Industrial Medicine,
Wilmington, Delaware, USA. Submitted to WHO by E.I. du Pont de
Nemours & Co., Inc., Wilmington, Delaware, USA.
Farrow, M.G., Cortina, T. & Padilla-Nash, H. (1983) In vivo bone
marrow chromosome study in rats -- H # 14,728. Unpublished report
No. 201-636 from Hazleton Laboratories America, Inc., Virginia,
USA. Submitted to WHO by E.I. du Pont de Nemours & Co., Inc.,
Wilmington, Delaware, USA.
Gargus, J.L. & Sutherland, J.D. (1983) Acute skin absorption LD50
test on rabbits. Unpublished report No. 288-83 from Hazelton
Laboratories America Inc., Virginia, USA. Submitted to WHO by
E.I. du Pont de Nemours & Co., Inc., Wilmington, Delaware, USA.
Hall, J.A., Dashiell, O.L. & Kennedy, G.L. (1984) Eye irritation test
in rabbits. Unpublished report No. HLR 582-82 from Haskell
Laboratory for Toxicology and Industrial Medicine, Wilmington,
Delaware, USA. Submitted to WHO by E.L du Pont de Nemours & Co.,
Inc., Wilmington, Delaware. USA.
Keller, D.A. (1990) Subchronic oral toxicity: 90-day study with
DPX-H6573-193 feeding study in mice. Unpublished report No. HLR
60-90 from Haskell Laboratory for Toxicology and Industrial
Medicine, Wilmington, Delaware, USA. Submitted to WHO by E.I. du
Pont de Nemours & Co., Inc., Wilmington, Delaware, USA.
Keller, D.A. (1992a) Mechanism of toxicity: 90-day feeding study in
rats with DPX-H6573-194 (Flusilazole). Unpublished report No. HLR
628-92 from Haskell Laboratory for Toxicology and Industrial
Medicine, Wilmington, Delaware, USA. Submitted to WHO by E.I. du
Pont de Nemours & Co., Inc., Wilmington, Delaware, USA.
Keller, D.A. (1992b) Oncogenicity study with DPX-H6573-193
(Flusilazole): 18-month feeding study in mice. Unpublished report
No. HLR 35-92 from Haskell Laboratory for Toxicology and
Industrial Medicine, Wilmington, Delaware, USA. Submitted to WHO
by E.I. du Pont de Nemours & Co., Inc., Wilmington, Delaware,
USA.
Keller, D.A. (1992c) Oncogenicity study with DPX-H6573-194
(Flusilazole): 2-year feeding study in rats. Unpublished report
No. HLR 527-92 from Haskell Laboratory for Toxicology and
Industrial Medicine, Wilmington, Delaware, USA. Submitted to WHO
by E.I. du Pont de Nemours & Co., Inc., Wilmington, Delaware,
USA.
Lamontia, C.L., Staples, R.E. & Alvarez, L. (1984a) Embryo-fetal
toxicity and teratogenicity study of INH-6573-39 by gavage in the
rat Unpublished report No. HLR 444-83 from Haskell Laboratory for
Toxicology and Industrial Medicine, Wilmington, Delaware, USA.
Submitted to WHO by E.I. du Pont de Nemours & Co., Inc.,
Wilmington, Delaware, USA.
Lamontia, C.L., Staples, R.E. & Alvarez, L. (1984b) Embryo-fetal
toxicity and teratogenicity study of INH-6573-39 by gavage in the
rat. Unpublished report No. HLR 142-84 from Haskell Laboratory
for Toxicology and Industrial Medicine. Wilmington, Delaware,
USA. Submitted to WHO by E.I. du Pont de Nemours & Co., Inc.,
Wilmington, Delaware, USA.
McCooey, K.T., Chromey, N.C., Sarrif, A.M. & Hemingway, R.E. (1983)
CHO/HGPRT assay for gene mutation. Unpublished report No. HLR
449-83 from Haskell Laboratory for Toxicology and Industrial
Medicine, Wilmington, Delaware, USA. Submitted to WHO by E.I. du
Pont de Nemours & Co. Inc., Wilmington, Delaware, USA.
Mullin, L.S. (1990) Reproductive and fertility effects with
flusilazole: Multigeneration reproduction study in rats.
Unpublished report No. HLR 424-90 from Haskell Laboratory for
Toxicology and Industrial Medicine, Wilmington, Delaware, USA.
Submitted to WHO by E.I. du Pont de Nemours & Co., Inc.,
Wilmington, Delaware, USA.
O'Neal, F.O., Rickard, R.W., Kaplan, A.M. & Gibson, J.R. (1985)
One-year feeding study in dogs with INH-6573. Unpublished report
No. HLR 461-85 from Haskell Laboratory for Toxicology and
Industrial Medicine, Wilmington, Delaware, USA. Submitted to WHO
by E.I. du Pont de Nemours & Co. Inc., Wilmington, Delaware, USA.
Pastoor, T.P., Wood, C.K., Krahn, D.F. & Gibson, J.R. (1983)
Ninety-day feeding and one-generation reproduction study in rats
with silane [bis(4-fluorophenyl)](methyl) (1 H-1,2,4-triazol-
1-ylmethyl) (INH-6573). Unpublished report No. HLR 483-83 from
Haskell Laboratory for Toxicology and Industrial Medicine,
Wilmington, Delaware, USA. Submitted to WHO by E.I. du Pont de
Nemours & Co.. Inc., Wilmington, Delaware, USA.
Pastoor, T.P., Wood, C.K., Drahn, D.F. & Aftosmis, J.G. (1984)
Four-week range finding and ninety-day feeding study in mice with
silane [bis(4-fluorophenyl)](methyl) (1 H-1,2,4-triazol-
1-ylmethyl) (INH-6573). Revised unpublished report No. HLR
341-83 1-83 from Haskell Laboratory for Toxicology and Industrial
Medicine, Wilmington, Delaware, USA. Submitted to WHO by E.I. du
Pont de Nemours & Co., Inc., Wilmington, Delaware, USA.
Pastoor, T.P., Rickard, R.W., Sykes, G.P., Kaplan, A.M. & Gibson, J.R.
(1986) Long-term feeding (combined chronic toxicity/oncogenicity
study) and two-generation, four litter reproduction study in rats
with INH-6573. Unpublished report No. HLR 32-86 from Haskell
Laboratory for Toxicology and Industrial Medicine, Wilmington,
Delaware, USA. Submitted to WHO by E.I. du Pont de Nemours & Co.,
Inc., Wilmington, Delaware, USA.
Poindexter, G.L., Henry, J.E., Kenney, L.A., Burgess, B.A. & Kennedy,
G.L. (1984) Inhalation median lethal concentration (LC50) of
INH-6573-41 by EPA protocol. Unpublished report No. HLR 553-83
from Haskell Laboratory for Toxicology and Industrial Medicine,
Wilmington, Delaware, USA. Submitted to WHO by E.I. du Pont de
Nemours & Co., Inc., Wilmington, Delaware, USA.
Redgate, D., Sarver, J.W. & Chromey, N.C.(1985) Approximate lethal
dose (ALD) of INH-6573-66 in rabbits. Unpublished report No. HLR
54-85 from Haskell Laboratory for Toxicology and Industrial
Medicine, Wilmington, Delaware, USA. Submitted to WHO by E.I. du
Pont de Nemours & Co., Inc., Wilmington, Delaware, Wilmington,
USA.
Reynolds, V. (1991) Mutagenicity testing of DPX-H6573-194 in the
Salmonella typhimurium plate incorporation assay. Unpublished
report No. HLR 33-91 by Haskell Laboratory for Toxicology and
Industrial Medicine, Wilmington, Delaware, USA. Submitted to WHO
by E.I. du Pont de Nemours & Co., Inc., Wilmington, Delaware,
USA.
Rickard, R.W., Wood, C.K., Krahn, D.F. & Aftosmis, J.G. (1983)
Three-month feeding study in dogs with silane [bis(4-
fluorophenyl)](methyl) (1 H-1,2,4-triazol- 1-ylmethyl)
(INH-6573). Unpublished report No. HLR 461-83 from Haskell
Laboratory for Toxicology and Industrial Medicine, Wilmington,
Delaware, USA. Submitted to WHO by E.I. du Pont de Nemours & Co.,
Inc., Wilmington, Delaware, USA.
Sarver, J.W., Vick, D.A., Valentine, R., Chromey, N.C. & Kaplan, A.M.
(1986) Twenty-one dose dermal toxicity study with INH-6573-82 in
rabbits. Unpublished report No. HLR 744-86 from Haskell
Laboratory for Toxicology and Industrial Medicine, Wilmington,
Delaware, USA. Submitted to WHO by E.I. du Pont de Nemours & Co.,
Inc., Wilmington, Delaware, USA.
Solomon, H.M., Alvarez, L., Staples, R.E. & Hamill, J.C. (1984)
Developmental toxicity study in rabbits given INH-6573 by gavage
on days 7-19 of gestation. Unpublished report No. HLR 333-84 from
Haskell Laboratory for Toxicology and Industrial Medicine,
Wilmington, Delaware, USA. Submitted to WHO by E.I. du Pont de
Nemours & Co., Inc., Wilmington, Delaware, USA.
Sorg, R.M., Naismith, R.W. & Mathews, R.J. (1984) Micronucleus test
(MNT)-OECD H # 15,314. Unpublished Haskell report No. HLR 437-84
from Pharmakon Research International. Submitted to WHO by E.I.
du Pont de Nemours & Co., Inc., Wilmington, Delaware, USA.
Turner, R.J., Kinney, L.A. & Chromey, N.C. (1985) Inhalation median
lethal concentration (LC50) of INH-6573 by EPA guidelines.
Unpublished report No. HLR 1-85 by Haskell Laboratory for
Toxicology and Industrial Medicine, Wilmington, Delaware, USA.
Submitted to WHO by E.I. du Pont de Nemours & Co., Inc.,
Wilmington, Delaware, USA.
Vlachos, D.A., Covell, D.L. & Sarrif, A.M. (1986) Evaluation of
INH-6573-82 in the in vitro assay for chromosome aberrations in
human lymphocytes. Unpublished report No. HLR 745-86 from Haskell
Laboratory for Toxicology and Industrial Medicine, Wilmington,
Delaware, USA. Submitted to WHO by E.I. du Pont de Nemours & Co.,
Inc., Wilmington, Delaware, USA.
Wylie, C.N., Burgess, B.A. & Kennedy, G.L. (1983) Acute oral test in
rats. Unpublished report No. HLR 78-83 from Haskell Laboratory
for Toxicology and Industrial Medicine, Wilmington, Delaware,
USA. Submitted to WHO by E.I. du Pont de Nemours & Co., Inc.,
Wilmington, Delaware, USA.
Wylie, C.N., Henry, J.E., Ferenz, R.L., Burgess, B.A. & Kennedy, G.L.
(1984a) Median lethal dose (LD50) in rats -- EPA proposed
guidelines, Newark, Delaware, USA. Unpublished report No. HLR
433-83 by Haskell Laboratory for Toxicology and Industrial
Medicine, Wilmington, Delaware, USA. Submitted to WHO by E.I. du
Pont de Nemours & Co., Inc., Wilmington, Delaware, USA.
Wylie, C.N., Henry, J.E., Dashiell, O.L. & Kennedy, G.L. (1984b)
Primary skin irritation and sensitization test on guinea pigs.
Unpublished report No. HLR 626-82 from Haskell Laboratory for
Toxicology and Industrial Medicine, Wilmington, Delaware, USA.
Submitted to WHO by E.I. du Pont de Nemours & Co., Inc.,
Wilmington, Delaware, USA.
Wylie, C.N., Henry, J.E., Burgess, B.A. & Kennedy, G.L. (1984c)
Ten-dose oral subacute test in rats. Unpublished report No. HLR
78-83 from Haskell Laboratory for Toxicology and Industrial
Medicine, Wilmington, Delaware, USA. Submitted to WHO by E.I. du
Pont de Nemours & Co, Inc., Wilmington, Delaware, USA.
Wylie, C.N., Redgate, D., Warheit, D.B. & Chromey, N.C. (1985)
Approximate lethal dose (ALD) of INH-6573-66 in male and female
mice. Unpublished report No. HLR 531-84 from Haskell Laboratory
for Toxicology and Industrial Medicine, Wilmington, Delaware,
USA. Submitted to WHO by E.I. du Pont de Nemours & Co., Inc.,
Wilmington, Delaware, USA.
Zellers, J.E., Staples, R.E., Alvarez, L. & Kaplan, A.M. (1985)
Developmental toxicity study (supplemental) in rabbits dosed by
gavage on days 7-19 of gestation. Unpublished report No. HLR
669-85, from Haskell Laboratory for Toxicology and Industrial
Medicine, Wilmington, Delaware, USA. Submitted to WHO by E.I. du
Pont de Nemours & Co., Inc., Wilmington, Delaware, USA.
Table 1. Acute toxicity of flusilazole
Species Sex Route LD50 or LC50 Reference
(mg/kg bw or
mg/litre air)
Mouse Male Oral 680 Wylie et al. (1985)
female 1000
Rat Male Oral 1500 Wylie et al. (1983)
Male Oral 1110 Wylie et al. (1984a)
Female 674
Male Inhalation 2.7 Poindexter et al. (1984)
Female 3.7
Male, female Inhalation 6.8-7.7 Turner et al. (1985)
Rabbit Male, female Oral 450 Redgate et al. (1985)
Male, female Dermal > 2000 Gargus & Sutherland (1983)
(b) Short-term toxicity
Mice
Groups of 20 male and 20 female Crl:CD-1 mice were given
technical-grade flusilazole (purity, 96.7%) at dietary levels of 0,
25, 75, 225, 500, or 1000 ppm, equal to 0, 4, 12, 36, 82, or 164 mg/kg
bw per day for males and 0, 5, 15, 43, 92, or 222 mg/kg bw per day for
females, for up to 90 days. Ten mice of each sex per group were
sacrificed after four weeks of treatment; the remaining mice were
killed at the end of the study. No histopathological examination was
conducted on animals killed at four weeks. There were no clinical
symptoms of toxicity and no treatment-related effects on body weight
or food consumption in animals sacrificed at either interval. The
target organs of toxicity were liver and urinary bladder; females were
slightly more sensitive to flusilazole than males. No treatment-
related effects were observed at the lowest dose. At 75 ppm, increased
absolute and relative liver weights and an increased incidence (1/10)
of hepatocellular vacuolar cytoplasmic changes were observed in
females. At > 225 ppm, increased liver weight, dose-related
increases in the incidence of hepatocellular vacuolar cytoplasmic
changes, hepatocellular hypertrophy, and urinary bladder urothelial
cell hyperplasia were evident in animals of each sex. At the highest
dose, absolute and relative kidney weights were decreased in males,
but no treatment-related renal lesions were observed. In addition,
slight decreases in erythroid parameters (haemoglobin, haematocrit,
and erythrocyte counts) were noted in animals of each sex. The NOAEL
was 4 mg/kg bw per day (Pastoor et al., 1984).
Groups of 16 male and 16 female Crl:CD-1(ICR)BR mice were fed
diets containing technical-grade flusilazole (purity, 94%) at doses of
0, 1000, 2500, or 5000 ppm, equal to 0, 161, 436, and 1004 mg/kg bw
per day for males and 0, 239, 601, and 1414 mg/kg bw per day for
females, for at least 90 days. An additional six mice of each sex per
group were assessed for cellular proliferation in the liver and
urinary bladder on days 14 and 106. At the lowest dose, treatment-
related effects included reduced mean body weight and food efficiency
(males only), increased absolute and relative liver weights (animals
of each sex), decreased absolute and relative kidney weights (females
only), and hypertrophy or hyperplasia, cytoplasmic vacuolation, and
inflammation of the liver and urinary bladder (animals of each sex).
At the mid-dose, increased cellular proliferation of the urinary
bladder was also observed in animals of each sex. At the highest dose,
severe body-weight loss occurred in males and reduced body weight and
food efficiency were noted in females. The males at this dose were
sacrificed on day 44 owing to excess mortality and moribund condition.
There was no NOAEL (Keller, 1990).
Rats
Groups of six male Crl:CD rats were given technical-grade
flusilazole (purity, 95.5%) by gavage in corn oil at a dose of 0 or
300 mg/kg bw per day, five days per week for two weeks. Three rats per
group were killed at the end of the treatment period, and the
remaining rats were sacrificed after a two-week recovery period. All
animals were examined histopathologically. One treated animal died
after the fifth dose on day 7. Clinical signs of toxicity (lowered
body weight, alopecia, diarrhoea, stained or wet perineal area,
salivation, and hypersensitivity) were observed in four rats during
the treatment period. Treatment-related histopathological changes were
observed in liver, kidney, urinary bladder, and testis; the lesions
appeared to be less severe in rats killed after the recovery period.
Remarkable histopathological findings in treated rats included
hepatocellular vacuolation (six rats), hyperplasia and vacuolation of
the urinary bladder transitional epithelium (six rats) and renal
pelvis urothelium (two rats), and necrosis and cellular degeneration
of the germinal epithelium of the seminiferous tubules (two rats)
(Wylie et al., 1984b).
Four groups of 10 male and 10 female Charles River CD rats were
fed diets containing technical-grade flusilazole (purity, 96.7%) at
doses of 0, 25, 125, 375, or 750 ppm, equal to 0, 2, 9, 27, and
55 mg/kg bw per day for males and 0, 2, 11, 31, and 70 mg/kg bw per
day for females, for 90 days. No treatment-related effects were
oberved at the two lower doses. At 375 ppm, an increased serum
cholesterol level was seen in animals of each sex and an increased
incidence of mild bladder urothelial hyperplasia in one male and four
females. At the highest dose, bladder urothelial hyperplasia was seen
in five males and eight females. Additional treatment-related changes
included decreased body weight in females, increased absolute and
relative liver weights in animals of each sex, and remarkable
histopathological findings (hepatocellular hypertrophy, mild fatty
changes, and hepatocytolysis) in the livers of five males. The NOAEL
was 9 mg/kg bw per day (Pastoor et al., 1983).
Groups of 52 male and 52 female Crl:CD BR rats were fed diets
containing technical-grade flusilazole (purity, 95%) at doses of 0,
10, 125, 375, or 750 ppm, equal to 0, 0.58, 7.27, 22.1, and 44.7 mg/kg
bw per day for males and 0, 0.74, 9.40, 27.6, and 59.0 mg/kg bw per
day for females, for up to 91 days. Each group was divided into three
subgroups of 20 rats of each sex in order to study possible mechanisms
of action of toxicity in the liver and urinary bladder. Five rats of
each sex were sacrificed on days 7/8, 14, 46, and 91 for assessment of
cellular proliferation and histopathology. For evaluation of
cytochrome P450 and peroxisome proliferation, five rats of each sex
were sacrificed on days 14 and 90 (males) or 15 and 91 (females).
Serum levels of testosterone, estradiol, and luteinizing hormone were
determined in all males sacrificed on days 14 and 90. No adverse
treatment-related effects were observed at the two lower doses. At
125 ppm, absolute and relative liver weights were increased; in the
absence of histopathological changes or any other symptoms of
toxicity, the finding was not considered to be toxicologically
significant. At the two highest doses, liver hypertrophy was observed.
The response was sex-dependent: lamellar bodies and periportal
hypertrophy occurred in males and centrilobular hypertrophy without
lamellar bodies was seen in females. In the urinary bladder,
transitional-cell necrosis, exfoliation and hyperplasia were noted.
Hepatic cytochrome P450 levels were higher than control values, but
there was no evidence of peroxisome proliferation in the livers of
treated rats. No significant changes in the serum levels of
testosterone, estradiol, or luteinizing hormone were found in any of
the males examined. The NOAEL was 7.27 mg/kg bw per day (Keller,
1992a).
Rabbits
Groups of five male and five female New Zealand white rabbits
received dermal applications of technical-grade flusilazole (purity,
94.9%) at doses of 0, 1, 5, 25, or 200 mg/kg bw per day for 21 days.
The test material was applied daily as a paste in distilled water; the
exposure sites were occluded for 6 h and then washed with water. No
evidence of systemic toxicity was observed. The NOAEL for dermal
toxicity was 5 mg/kg bw per day, based on diffuse epidermal
hyperplasia or thickening (slight to mild) at > 25 mg/kg bw per
day. At the highest dose, mild erythema was seen on days 6-12. The
NOAEL for systemic toxicity was > 200 mg/kg bw per day (Sarver
et al., 1986).
Dogs
Groups of four male and four female beagle dogs were fed diets
containing technical-grade flusilazole (purity, 93%) at concentrations
of 0, 25, 125, or 750/500 ppm, equal to 0.9, 4.3, and 13.4 mg/kg bw
per day for males and 0, 0.9, 4.3, and 14.2 mg/kg bw per day for
females, for three months. The high-dose group received 750 ppm
flusilazole in the diet for the initial three weeks, then control diet
for one week, and 500 ppm flusilazole in the diet for the remaining
period. The dose was reduced because of marked body-weight loss and
decreased food intake at 750 ppm. At the lowest dose, there was a
treatment-related increase in the incidence of hyperplasia of the
lymphoid follicles in the pyloric glandular mucosa of the stomach in
males: 0/4, 3/4 3/4, and 4/4 at 0, 25, 125, and 750/500 ppm,
respectively. At 125 ppm, an increased incidence of pyloric granular
mucosa hyperplasia was also observed in females: 0/4, 0/4, 3/4, and
4/4 at 0, 25, 125, and 750/500 ppm, respectively. Higher levels (in
comparison with control values) of alanine aminotransferase activity
and an increased incidence of mild urinary bladder mucosal hyperplasia
were observed in males at 125 ppm and in animals of each sex at the
highest dose. Additional treatment-related effects seen at the highest
dose included clinical signs of toxicity (weakness or tremors) in
animals of each sex, reduced mean body-weight gain in males,
body-weight loss in females, decreased mean food consumption in
animals of each sex, a slight increase in leukocyte and monocyte
counts in males, decreased plasma levels of cholesterol, total
protein, and albumin in animals of each sex, and increased absolute
and relative liver weights in animals of each sex. There was no NOAEL
(Rickard et al., 1983).
Groups of five male and five female beagle dogs were fed
technical-grade flusilazole (purity, 95.8%) in the diet at doses of 0,
5, 20, or 75 ppm, equal to 0, 0.14, 0.7, and 2.4 mg/kg bw per day for
males and 0, 0.14, 0.7, and 2.6 mg/kg bw per day for females, for one
year. No treatment-related effects were observed at the lowest dose.
At the next dose, 20 ppm, the serum albumin level was decreased in
males and there was a dose-related increase in the incidence of
centrilobular hepatocellular hypertrophy in both males (0/5, 0/5, 4/5,
and 5/5) and females (0/5, 0/5, 2/5, and 5/5). More severe gastric
mucosal lymphoid hyperplasia, from minimal at 0 or 5 ppm to mild at
20 ppm, was also noted in males. At the highest dose, additional
treatment-related effects included higher leukocyte counts in animals
of each sex; elevated alkaline phosphatase and lowered cholesterol,
total protein, and albumin levels in males only; and increases in
relative liver (animals of each sex) and kidney weights (females). All
high-dose animals exhibited a greater degree of hepatic centrilobular
inflammatory infiltration, and distinct centrilobular hepatocellular
vacuolation was observed in three males. The lymphoid hyperplasia in
the gastric mucosa was of moderate severity in both males and females.
The NOAEL was 0.14 mg/kg bw per day (O'Neal et al., 1985).
(c) Long-term toxicity and carcinogenicity
Mice
Groups of 80 male and 80 female Crl:CD-1(ICR)BR mice received
technical-grade flusilazole (purity, 96.5%) in the diet at doses of 0,
5, 25, or 200 ppm, equal to 0, 0.66, 3.4, and 27 mg/kg bw per day for
males and 0, 0.92, 4.6, and 36 mg/kg bw per day for females, for 18
months. Each group included 10 mice of each sex that were killed at
six months. There were no treatment-related effects on mortality
(terminal survival rates were 76-86% in males and 57-80% in females),
body weight, food consumption, haematology, or serum chemistry at any
dose. At the highest dose, a toxicologically significant increase in
the incidence of hepatocellular fatty changes was observed, with
terminal incidences of 4/80, 3/80, 10/80, and 40/80 males and 2/80,
4/80, 3/80, and 24/80 females at 0, 5, 25, and 200 ppm. In addition,
there was an increase in absolute and relative liver weights in
animals of each sex, a decrease in absolute kidney weight in females,
and an increase in lymphocytic infiltration in the lung and urinary
bladder in males. There was no treatment-related increase in the
incidence of any tumour type at doses > 27 mg/kg bw per day, the
highest dose tested. The NOAEL for systemic toxicity was 3.4 mg/kg bw
per day (Brock et al., 1985).
Groups of 100 male and 100 female Crl:CD-1(ICR)BR mice received
diets containing technical-grade flusilazole (purity, 94%) at doses of
0, 100, 500, or 1000 ppm in males, equal to 0, 14.3, 73.1, and
144 mg/kg bw per day, and 0, 100, 1000, or 2000 ppm in females, equal
to 0, 19.4, 200, and 384 mg/kg bw per day, for 18 months. An
additional group of 100 mice of each sex was fed a diet containing
25 ppm technical-grade flusilazole (equal to 3.51 mg/kg bw per day for
males and 4.38 mg/kg bw per day for females) and was used to study
cell proliferation. The doses were selected on the basis of the
results of a 90-day study that indicated greater sensitivity of males
to the test compound. At the lowest dose tested, there was a slight
decrease in absolute kidney weight, an increase in focal necrosis of
the liver, and urinary bladder hyperplasia in males. No remarkable
changes were seen in females at this dose. At the two highest doses,
females had increased cellular hyperplasia in the urinary bladder and
urethra, but no focal necrosis of the liver. At these two doses,
additional treatment-related systemic effects included increased
absolute and relative liver weights, lower absolute and relative
kidney weights, increased numbers of foci of hepatocellular
alteration, and an increased incidence of hepatic vesicular or
vacuolar changes with cellular hypertrophy. Significantly higher
mortality occurred at 1000 ppm in males and 2000 ppm in females. In
addition, increased cellular proliferation in the urinary bladder (but
not in the liver) was observed in females at the highest dose. At
terminal sacrifice, an increased incidence of hepatocellular adenomas
and carcinomas was observed in males at all doses (13/80, 23/79,
20/80, and 18/78 at 0, 100, 500, and 1000 ppm, respectively;
historical control range, 6.3-13.8%) and in females at the two highest
doses (1/79, 3/80, 11/77, and 43/76 at 0, 100, 1000, and 2000 ppm,
respectively; historical control range, 0-2.6%). The increase in
tumour incidence was dose-related and statistically significant in
females but not in males. There was no NOAEL for systemic toxicity or
oncogenicity (Keller, 1992b).
Rats
Groups of 70 male and 70 female rats (Crl:CD(SD)BR strain)
received technical-grade flusilazole (purity, 95.6%) in the diet at
doses of 0, 10, 50, or 250 ppm, equal to 0, 0.4, 2.0, and 10 mg/kg bw
per day for males and 0, 0.5, 2.6, and 13 mg/kg bw per day for
females, for two years. Each group included two groups of 10 rats of
each sex that were killed at six months and one year. About 100 days
after the beginning of treatment, 20 rats of each sex in each group
were mated and were returned to the study after weaning of the second
litter. The rats killed at six months were examined for bladder
lesions only. No treatment-related effect on mortality was observed;
the survival rate was > 50% in all groups until week 98. No signs of
systemic toxicity was seen at the low dose. At 50 ppm, there was a
dose-related increase in the incidence of pyelonephritis in females at
two years (3/66, 3/62, 8/67, and 10/65 at 0, 10, 50, and 250 ppm,
respectively). Increases in relative liver weight in females and in
the incidence of hydronephrosis in males were observed at interim
sacrifice at one year. At the highest dose, additional treatment-
related changes included an increased incidence of hepatic lesions
(centrilobular hepatocellular hypertrophy and polyploidy) in females
and hydronephrosis in males at one year and an increased incidence of
acidophilic foci of hepatocellular alteration (13/65; 3/66 in
controls) and hepatic diffuse fatty changes (23/65; 9/66 in controls)
in females at two years. No hepatic lesions were observed in males
during the study, and no lesions of the urinary bladder were seen in
animals of either sex at either interim or terminal sacrifice. There
was no treatment-related increase in the incidence of any tumour type
at any dose. At 250 ppm, males had a slightly increased incidence of
squamous-cell carcinomas of the oral and nasal cavities (0/66, 1/63,
0/67, and 3/64 at 0, 10, 50, and 250 ppm, respectively). On the basis
of historical control data, from five two-year feeding studies in rats
with no tumours of this type and one study with an incidence of 2/60,
the incidence of nasal tumours in this study was judged to be
incidental. The NOAEL for systemic toxicity was 0.4 mg/kg bw per day;
however, the effects reported were relatively mild, and higher doses
might have been tolerated (Pastoor et al., 1986).
Groups of 65 male and 65 female Crl:CD(SD)BR rats received diets
containing technical-grade flusilazole (purity, 95%) at doses of 0,
125, 375, or 750 ppm, equal to 0, 5.03, 14.8, and 30.8 mg/kg bw per
day for males and 0, 6.83, 20.5, and 45.6 mg/kg bw per day for
females, for two years. Each group included 10 rats of each sex for
interim sacrifice at one year. Dose-related increases in the incidence
of hepatocellular hypertrophy were observed in animals of each sex at
both interim (males: 0/10, 4/10, 8/10, and 8/10; females: 0/10, 7/10,
10/10, and 10/10; at 0, 125, 375, and 750 ppm, respectively) and
terminal sacrifice (males: 2/53, 2/51, 10/53, and 19/53; females:
2/56, 4/55, 12/53, and 25/54). There was an apparent sex difference in
the hepatic lesions: periportal hepatocellular hypertrophy with
lamellar bodies was seen in males, while females showed centrilobular
hepatocellular hypertrophy with eosinophilic cytoplasm but no lamellar
bodies. At terminal sacrifice, there was also an increased incidence
of mixed foci of cellular alteration in males (6/53, 14/51, 17/53, and
19/53). At 375 and 750 ppm, there were also significant decreases in
terminal body weight in females, increases in absolute and/or relative
liver weights at interim and/or terminal sacrifice in males and/or
females, and an increase in the incidence of urinary bladder
transitional-cell hyperplasia at the end of the study in animals of
each sex (3/46, 6/45, 27/47, and 42/51 in males and 5/47, 3/49, 15/49,
and 33/53 in females). At 750 ppm, additional treatment-related
changes included an increased incidence of hepatic fatty changes in
males and increased cellular proliferation in the liver and urinary
bladder. At the highest dose, a treatment-related increase in the
incidence of urinary bladder transitional-cell papillomas and
carcinomas was observed in animals of each sex at the end of the study
(males: 0/46, 0/45, 1/47, and 5/51; and females: 0/47, 1/49, 0/49, and
13/53; at doses of 0, 125, 375, and 750 ppm, respectively). The
incidence of testicular interstitial-cell (Leydig-cell) tumours in
males at the highest dose was also increased: 2/53, 4/51, 2/53, and
9/53 at 0, 125, 375 and 750 ppm, respectively. There was no NOAEL for
systemic toxicity; the NOAEL for oncogenicity was 14.8 mg/kg bw per
day (Keller, 1992c).
(d) Reproductive toxicity
Rats
Groups of six male and six female Crl:CD(SD)BR rats from a 90-day
feeding study were used in a one-generation study of reproductive
toxicity. The rats were fed daily diets containing technical-grade
flusilazole (purity, 96.7%) at doses of 0, 25, 125, or 375 ppm, equal
to 0, 2, 9, and 27 mg/kg bw per day for males and 0, 2, 11, and
31 mg/kg bw per day for females, for 90 days before mating. Males and
females within the same dose group were mated for 15 days; females
were examined daily for evidence of a copulation plug. After the
mating period, females were housed individually. The fertility index
was low in all groups and especially in the control group (67.7%);
three to six females per group became pregnant. At the highest dose, a
lower gestation index, a lower percentage of liveborn pups, and lower
pup weight at day 4 were observed in comparison with controls (Pastoor
et al., 1983). The small group size and the absence of individual
data on some parameters limit the usefulness of this study for
evaluation of reproductive toxicity.
Groups of 20 male and 20 female Crl:CD(SD)BR rats from a two-year
study of toxicity and oncogenicity were used in a two-generation,
two-litter study. The rats were fed diets containing technical-grade
flusilazole (purity, 95.6%) at doses of 0, 10, 50, or 250 ppm, equal
to 0, 1, 3, and 18 mg/kg bw per day for males and 0, 1, 4, and
20 mg/kg bw per day for females (premating intake) for at least 100
days before mating. Males and females (F0) within the same dose group
were mated 1:1 for 15 days; the females were examined daily for
evidence of a copulation plug. After the mating period, F0 females
were housed individually and allowed to give birth to F1a litters.
About one week after the last F1a litter had been weaned, F0 females
were mated with different F0 males of the same dose group to produce
the F1b litters. After weaning of the F1b litters, 20 rats of each
sex per group were selected as the F1 parents for the F2 generation.
These rats were maintained on the same diets as their F0 parents for
90 days before mating to produce the F2a and F2b litters. Sibling
mating was avoided. The mean body weights of the F1b males at 250 ppm
were decreased during the premating period. No other treatment-related
systemic effect was observed in any F0 or F1 adult. There was no
evidence of a treatment-related effect on mating or fertility.
Treatment-related embryo-, feto-, and litter toxicity were observed at
the mid- (50 ppm) and high doses (250 ppm). An increased number of
stillborn pups and a decreased viability index (days 0-4) occurred in
the F2a litters at 50 ppm and in all litters at 250 ppm. Litter
survival after day 4 was similar in all groups in the F1 and F2
generations. At 250 ppm, the mean weights of F1b and F2b pups of each
sex at weaning were slightly reduced, and the absolute and relative
liver weights of F2b weanling male pups were increased. An increased
incidence of unilateral and/or bilateral hydronephrosis was noted in
F2b female weanlings: 1/10, 4/10, 3/10, and 5/10 at 0, 10, 50, and
250 ppm, respectively. Since neither the severity nor the incidence
of the lesions showed a dose-response relationship and since
hydronephrosis is a common lesion in weanling pups (historical control
range in 13 in-house studies, 0-30%), the finding was judged to be
toxicologically insignificant. The NOAEL was 1 mg/kg bw per day, based
on embryo- and fetotoxicity and decreased viability of pups in the
F2a litters at 50 ppm (Pastoor et al., 1986).
In a two-generation study with one or two litters per generation,
groups of 30 male and 30 female Crl:CD(SD)BR rats were fed diets
containing technical-grade flusilazole (purity, 94%) at doses of 0, 5,
50, or 250 ppm, equal to 0, 0.34, 3.46, and 17.3 mg/kg bw per day for
males and 0, 0.40, 4.04, and 19.6 mg/kg bw per day for females
(premating intake), for 73 days (F0 rats) or 91 days (F1 parents)
before mating. Males and females within the same dose group were
randomly paired 1:1 for 15 days; females were examined daily for
evidence of a copulation plug. One litter (F1a) was produced in the
F0 generation and two litters (F2a and F2b) in the F1 generation.
No treatment-related effects were seen in either the F0 or F1
generation at the low dose. At the middle dose, increased smooth
endoplasmic reticulum was seen in the hepatocytes of males and
centrilobular hepatocellular hypertrophy in females. At the highest
dose, F1 females had slightly but consistently lower body weights.
Reproductive toxicity was indicated by higher mortality during
parturition and increased gestational length of the F0 and F1 dams:
mean, 22.9-23.2 days in comparison with 22.4-22.6 days in controls. No
treatment-related effects on mating or fertility indices were
observed. Embryo-, feto-, and litter toxicity were seen as reduced
number of pups per litter and increased numbers of stillborn pups per
litter in the F1a, F2a, and F2b litters and decreased mean pup
weights on lactation days 14 and 21 (F2a litters only) at 250 ppm.
The NOAEL for systemic toxicity was 0.34 mg/kg bw per day, on the
basis of hepatic lesions at 50 ppm; the NOAEL for reproductive
toxicity was 4.04 mg/kg bw per day, on the basis of treatment-related
mortality during parturition, increased gestational length, and
embryo-, feto-, and litter toxicity at 250 ppm (Mullin, 1990).
(e) Developmental toxicity
Rats
In a range-finding study, groups of seven pregnant rats received
technical-grade flusilazole (purity, 99%) by gavage at doses of 0,
100, or 300 mg/kg bw per day on days 7-16 of gestation. Flusilazole
was maternally and embryotoxic at both doses, and the highest dose
induced cleft palates in about 51% of the fetuses in each litter. In
the main study of developmental toxicity, groups of 25 mated female
Crl:CD(SD)BR rats received technical-grade flusilazole (purity, 95.6%)
by gavage in corn oil at doses of 0, 10, 50, or 250 mg/kg bw per day
on days 7-16 of gestation; the day a copulation plug was observed was
designated as day 1 of gestation. On gestation day 21, all surviving
dams were sacrificed and necropsied; fetuses were delivered by
caesarean section and examined for external, visceral, and skeletal
abnormalities. No treatment-related signs of maternal toxicity were
seen at the lowest dose. At 50 mg/kg bw per day, slight decreases in
body-weight gain and food consumption and a slight increase in the
relative liver weight of dams were seen. At the highest dose,
additional maternal toxic effects included higher mortality and
clinical signs of toxicity (chromodacryorrhoea, chromorhinorrhoea, wet
or stained perineal areas, red vaginal discharges or stains, and focal
alopecia) in 23 animals. Fetuses had treatment-related increases in
the incidence of skeletal anomalies (misaligned sternebrae, extra
ossification centres in ribs, and delayed ossification in sternebrae)
at all doses. At 50 mg/kg bw per day, there was a reduced mean number
of liveborn fetuses per litter, a higher total number of stunted
fetuses (0, 1, 4, and 3 at 0, 10, 50, and 250 mg/kg bw per day,
respectively), and an increased incidence of rudimentary ribs. At the
highest dose, additional signs of embryo- and fetotoxicity were an
increased mean incidence of resorptions, reduced mean fetal weight per
litter, and all increased incidence of extra ribs in the fetuses. An
increased incidence of cleft palate (28/241; 0/331 in controls) and an
absence of renal papillae (21/155; 0/175 in controls) were observed in
fetuses of dams given 250 mg/kg bw per day but not at lower doses. An
unusually high incidence of hydrocephalus and/or dilated lateral
ventricles of the brain was also noted in all groups, including
controls; the effect was not dose-related and was not considered to be
related to treatment. The NOAEL for maternal toxicity was 10 mg/kg bw
per day; there was no NOAEL for embryo- or fetotoxicity; and the NOAEL
for teratogenicity was 50 mg/kg bw per day (Lamontia et al., 1984a).
Groups of 24 mated female Crl:CD(SD)BR rats received technical-
grade flusilazole (purity, 95.6%) by gavage in corn oil at doses of 0,
0.4, 2, 10, 50, or 250 (10 females only) mg/kg bw per day on days 7-16
of gestation; the day a copulation plug was observed was designated
day 1 of gestation. On day 21 of gestation, all surviving dams were
sacrificed and necropsied; fetuses were delivered by caesarean section
and examined for external, visceral, and skeletal abnormalities. No
treatment-related signs of maternal toxicity were observed at doses
> 10 mg/kg bw per day. Slightly reduced body-weight gain and food
consumption during treatment and increased relative liver weights of
dams were seen at 50 mg/kg bw per day. At the highest dose, clinical
signs of maternal toxicity (alopecia, brown stains on the face and
limbs, and stained perineal area) were also observed. No treatment-
related embryo- or fetotoxic effects were seen at the two lowest
doses. At > 10 mg/kg bw per day, an increased number of dams with
median or late resorptions, an increased total number of stunted
fetuses, a higher incidence of visceral (large renal pelvis and small
renal papilla) and skeletal (rib) anomalies, and an increased
incidence of delayed ossification (sternebrae and vertebral arches)
were observed. An increased incidence of cleft palate (21/116; 0/291
in controls) was observed in fetuses of dams receiving 250 mg/kg bw
per day but not at lower doses. Hydrocephalus was not observed. The
NOAEL for maternal toxicity was 10 mg/kg bw per day, that for embryo-
and fetotoxicity was 2 mg/kg bw per day, and that for teratogenicity
was 50 mg/kg bw per day (Lamontia et al., 1984b).
Groups of 24 mated female rats (Crl:CD (SD)BR strain) received
diets containing technical-grade flusilazole (purity, 96.5%) at doses
of 0, 50, 100, 300, or 900 ppm equal to 0, 4.6, 9.0, 26.6, and
79.2 mg/kg bw per day) on days 7-16 of gestation; the day a copulation
plug was observed was designated day 1 of gestation. On gestation day
21, all surviving dams were sacrificed and necropsied; fetuses were
delivered by caesarean section, weighed, sexed, and then examined for
external, visceral, and skeletal abnormalities. No treatment-related
signs of maternal toxicity were seen at doses > 100 ppm.
Significant, dose-related reductions in body-weight gain and food
consumption were observed during treatment at the two highest doses.
Treatment-related embryo- and fetotoxic effects were noted at
> 100 ppm, including an increased incidence of median or late
resorptions, small litters (< 10 fetuses per litter), and significant
dose-related increases in skeletal variations with extra ossification
of the sternebrae. Additional symptoms of fetal toxicity seen at the
two highest doses included an increased number of stunted fetuses, a
higher incidence of rudimentary ribs, extra ossification in cervical
ribs, and delayed ossification of the cervical vertebral arches. None
of the fetuses had cleft palate, and there were no treatment-related
malformations at any dose. The NOAEL for maternal toxicity was
9.0 mg/kg bw per day, that for embryo- and fetotoxicity was 4.6 mg/kg
bw per day, and that for teratogenicity was 79.2 mg/kg bw per day
(Alvarez et al., 1984).
In a study of prenatal and postnatal toxicity in rats, groups of
24 (phase I, prenatal study) or 22 (phase II, postnatal study) mated
female rats (Crl:CD (SD)BR strain) received technical-grade
flusilazole (purity, 96.5%) in 0.5% aqueous methylcellulose by gavage
at doses of 0, 0.2, 0.4, 2, 10, or 100 mg/kg bw per day on days 7-16
of gestation; the day a copulation plug was observed was designed day
1 of gestation. In phase I, dams were sacrificed on gestation day 21
for examination of the uterine contents. Additional control groups and
animals at 100 mg/kg bw per day were killed on gestation day 22 to
determine whether the absence of renal papillae was a compound-related
effect or an anomaly. As the concentrations of the first few solutions
used in phase I were found to be only 1-19% of the nominal
concentrations and the next analysis which showed 75-110% of the
nominal concentration, was done only on day 7, no definitive
conclusions could be drawn from the results of this part of the study.
Signs of maternal toxicity seen at the highest dose included clinical
signs (chin or perinasal staining and/or wet perineum), reduced
body-weight gain and food consumption during treatment, and increased
absolute and relative liver weights. Embryo- and fetotoxicity were
observed at > 10 mg/kg bw per day, manifested as increased numbers
of stunted fetuses and a higher incidence of visceral anomalies (small
renal papillae and distended ureter). Additional embryo- and
fetotoxicity seen at the highest dose included an increased incidence
of median or late resorptions and a decrease in the mean number of
live fetuses per litter. Treatment-related malformations (absence of
renal papillae) were observed in three fetuses from two litters of
dams at 100 mg/kg bw per day. In phase II of the experiment, dams were
permitted to deliver naturally and to raise their litters to weaning.
All dams and pups were sacrificed on day 21 of lactation and subjected
to gross necropsy. The solutions were found to be adequately prepared
in this phase. No treatment-related maternal toxicity was evident at
doses > 10 mg/kg bw per day. At the next dose, 100 mg/kg bw per
day, increased mortality (5/22; 0/22 in controls), clinical signs of
difficult parturition (pallor, bunching, weakness, and/or dystocia
during parturition and lactation in four dams), reduced body-weight
gain and food consumption during the early part of treatment, and
increased liver weight were observed. No treatment-related embryo-
or fetotoxicity was noted at doses > 2 mg/kg bw per day. At
> 10 mg/kg bw per day, there were overt, dose-related increases in
mean gestational length (22.8, 23.1, 22.9, 23.1, 23.5, and 24.7 days
at 0, 0.2, 0.4, 2, 10, and 100 mg/kg bw per day, respectively),
decreases in mean litter size and the number of liveborn fetuses per
litter, an increased number of small litters (< 10 fetuses per
litter), and a higher incidence of dilated renal pelvis and/or ureter
in pups at weaning. Additional signs of treatment-related embryo- and
fetotoxicity at the highest dose included a reduced total number of
live litters, an increased mean number of dead fetuses per litter, and
reduced viability indexes (days 0-4; 82%; 98-99% in control and lower
dose groups). There were no treatment-related malformations in the
live pups examined. Of the 42 pups found dead, 29 were in the group at
100 mg/kg bw per day; two had no renal papillae and four had small
papillae. The NOAEL for maternal toxicity was 10 mg/kg bw per day,
that for embryo- and fetotoxicity was 2 mg/kg bw per day, and that for
teratogenicity was 100 mg/kg bw per day, the highest dose tested
(Alvarez et al., 1985a).
Rabbits
Four groups of 18 artificially inseminated female New Zealand
white rabbits received technical-grade flusilazole (purity, 96.5%) in
corn oil by gavage at nominal doses of 0, 2, 5, or 12 mg/kg bw per
day, equal to 0, 1.9, 4.8, and 10.1 mg/kg bw per day on the basis of
analysis of the solutions, on days 7-19 of gestation; the day of
insemination was designated gestation day 0. On gestation day 29, all
surviving does were sacrificed and necropsied; fetuses were delivered
by caesarean section and examined for external, visceral, and skeletal
abnormalities. There was no treatment-related mortality, no clinical
signs of maternal toxicity, and no disturbances of intrauterine
development of the conceptuses at any dose up to and including
12 mg/kg bw per day, the highest dose tested. All of the fetuses
delivered showed normal development. No evidence of fetotoxicity and
no treatment-related increases in malformations were observed in
fetuses at any dose. The higher incidence of hydrocephalus observed in
this study (one fetus in one litter, two in one litter, four in two
litters, and four in three litters at 0, 2, 5, and 12 mg/kg bw per
day, respectively), which was not confirmed in three subsequent
studies (in only one fetus at 35 mg/kg bw per day and in none at lower
doses or in controls), was not considered to be treatment-related. The
NOAEL for maternal toxicity, embryo- and fetotoxicity and
teratogenicity was 12 mg/kg bw per day (10.1 mg/kg bw per day by
analysis) (Solomon et al., 1984).
Three groups of 20 artificially inseminated female New Zealand
white rabbits received technical-grade flusilazole (purity, 96.5%) by
gavage at nominal doses of 0, 12, or 35 mg/kg bw per day, equal to 0,
11.2 and 31.5 mg/kg bw per day on the basis of analysis of the
solutions, on days 7-19 of gestation; the day of insemination was
designated gestation day 0. On gestation day 29, all surviving does
were killed and necropsied; fetuses were delivered by caesarean
section and examined by gross pathology. No evidence of maternal, or
embryo- or fetotoxicity was observed at the low dose, and the
incidence of malformations and fetal variations was not increased in
comparison with the controls. At the high dose, there was an increased
incidence of red vaginal discharge and stained tail and an increased
incidence of periodic anorexia. Two of 13 treated does and 0/16
controls aborted, and 10 of 13 had early total resorption in
comparison with 1/16 controls. Teratogenicity could not be assessed at
this dose because only one live litter was produced. The NOAEL for
maternal, embryo-, and fetotoxicity was 12 mg/kg bw per day
(11.2 mg/kg bw per day by analysis), and that for teratogenicity was
> 12 mg/kg bw per day (11.2 mg/kg bw per day by analysis) (Zellers
et al. 1985).
In a range-finding study, four groups of seven artificially
inseminated female New Zealand white rabbits were fed diets containing
technical-grade flusilazole (purity, 94.8%) at doses of 0, 500, 1000,
or 2000 ppm on days 7-19 of gestation. The pregnancy rate (3/7 per
group) was low at 500 and 1000 ppm, and the incidence of mortality
in utero was high at 2000 ppm (4/7 females with total resorptions).
In the main study, dietary levels of 0, 300, 600, or 1200 ppm
technical-grade flusilazole (purity, 94.8%), equal to 0, 8.9, 21.2,
and 37.8 mg/kg bw per day, were given to groups of 20 artificially
inseminated female New Zealand white rabbits on days 7-19 of
gestation; the day of insemination was designated day 0 of gestation.
All surviving does were sacrificed on gestation day 29 and necropsied;
fetuses were delivered by caesarean section and examined for external,
visceral, and skeletal abnormalities. During treatment with the
highest dose, maternal body weight and food consumption were
decreased. The pregnancy rate was reduced in all treated groups: 9/20,
10/20, and 7/20 at 300, 600, and 1200 ppm, respectively. The number of
does with total resorptions was increased at 600 and 1200 ppm. There
were no treatment-related effects on the mean number of live fetuses
per litter, the mean number of resorptions in dams with live fetuses,
or fetal weight. The small number of litters available from dams at
600 and 1200 ppm (three per group) precluded any definitive assessment
of fetotoxicity or teratogenic potential at these doses. There were no
apparent treatment-related effects at the low dose. In a supplementary
study, dietary levels of 0, 30, 100, or 300 ppm, equal to 0, 0.81,
2.84, and 8.32 mg/kg bw per day, of technical-grade flusilazole were
administered to groups of 18 or 25 (300 ppm) inseminated female New
Zealand white rabbits on days 7-19 of presumed gestation. The
pregnancy rate was again low in all groups, including the controls
(8/18). Total resorption occurred at 0 and 300 ppm in 25 and 29%,
respectively, of the pregnant does, but not at the low or mid-dose.
Because of the small number of live litters available for examination,
the results could not be adequately assessed for embryo- or
fetotoxicity or teratogenic potential. The NOAEL for maternal toxicity
was 21.2 mg/kg bw per day, based on decreased body weight and food
consumption at 1200 ppm. There was no NOAEL for embryo- or
fetotoxicity or teratogenicity (Alvarez et al., 1985b).
Four groups of 18 artificially inseminated female New Zealand
white rabbits received technical-grade flusilazole (purity, 93.8%) in
0.5% methylcellulose by gavage at doses of 0, 7, 15, or 30 mg/kg bw
per day on days 7-19 of gestation. The pregnancy rate was acceptable
in all groups: 12/18, 14/18, 16/18, and 16/18 at 0, 7, 15, and
30 mg/kg bw per day, respectively. No treatment-related maternal,
embryo- or fetotoxicity was seen at the low dose. At the two higher
doses, clinical signs of maternal toxicity (red discharge and
brown-yellow-stained tail) and increased incidences of abortion (one
dam per group) and total resorptions (4/16 and 12/16, respectively)
were observed. At the highest dose, food consumption was also
decreased. The mean numbers of liver fetuses per litter and of dead
fetuses, mean fetal weight and mean male:female ratio were comparable
in the control and treated groups. No treatment-related external,
visceral, or skeletal malformation or variation was observed in
fetuses of does at any dose; however, it should be noted that
assessment of fetotoxicity and teratogenic potential at the highest
dose was based on data for only three of 11-12 live litters in the
control and lower dose groups. These limited data reduce confidence in
the accuracy of any conclusions drawn on the basis of observations at
this dose. The NOAEL for maternal, embryo-, and fetal toxicity was
7 mg/kg bw per day, and that for teratogenicity was > 15 mg/kg bw
per day (Alvarez, 1900).
(f) Genotoxicity
A battery of studies on technical-grade flusilazole was conducted
to assess its potential to induce gene mutation, chromosomal
aberration, or unscheduled DNA synthesis. The results (summarized in
Table 2) were clearly negative.
(g) Special studies
(i) Dermal and ocular irritation and dermal sensitization
Two male New Zealand white rabbits received 0.01 ml of undiluted
flusilazole (purity, 90%) into the right conjunctival sac; the left
eye was not treated. The right eye of one rabbit was washed with
100 ml of water after 20 sec of exposure. Irritation was graded
according to the Draize scale 1, 4, 24, 48, and 72 h after
instillation. Both animals had mild conjunctival redness and chemosis
1-4 h after instillation. The washed eye also showed some discharge at
1 h, but by 24 h these effects were no longer observed. The unwashed
eye showed slight corneal opacity at 1-4 h, and biomicroscopic
examination revealed slight cloudiness at 24-48 h; the eye was normal
at 72 h. Flusilazole was thus minimally irritating to the eyes of male
New Zealand white rabbits, causing transient, mild irritation.
Flushing the eye with water after exposure eliminated the corneal but
not the conjunctival effects (Hall et al., 1984).
In a study of primary skin irritation, six young male New Zealand
white rabbits received a flusilazole formulation (Nustar 20DF,
containing 21% technical-grade flusilazole and 79% unspecified, inert
ingredients; undiluted, moistened with distilled water) topically on
shaved skin sites on the back at a concentration of about 0.5 g. The
test site was covered with a gauze patch and a sheet of rubber for
4 h; the coverings were then removed and the site washed gently with
warm water. Each site was evaluated for irritation potential according
to the Draize scale 4, 24, 28, and 72 h after treatment. At 4 h, grade
1 erythema was observed in four rabbits and grade 1 or 2 oedema in two
rabbits. By 24 h, only two rabbits had erythema and no oedema was
observed. No signs of dermal irritation were seen in any rabbit at
72 h. The primary irritation index was 0.167. Flusilazole as
formulated for this test was thus minimally irritating to the skin
(Brock, 1988).
Table 2. Results of tests for the genotoxicity of flusilazole
End-point Test system Concentration Purity Results Reference
or dose (%)
In vitro
Reverse mutation Salmonella typhimurium TA98, 1-250 µg/plate 90.0 Negativea Donovan & Irr (1982)
TA100, TA1535, TA1537
Reverse mutation Salmonella typhimurium TA97, 5-250 µg/plate 97.7 Negativea Arce et al. (1988)
TA98, TA100, TA1535
Reverse mutation Salmonella typhimurium TA97, 10-300 µg/plate 61.7 Negativea Reynolds (1991)
TA98, TA100, TA1535
hprt forward mutation Chinese hamster ovary cells 0.04-0.275 mmol/litre 95.5 Negative McCooey et al. (1983)
(KI/BH4 clone)
Chromosomal aberration Human lymphocytes 1.7-100 µg/ml 94.9 Negativea Vlachos et al. ( 1986),
Unscheduled DNA Rat primary hepatocytes 1 × 10-5 - 1.1 × 102 95.5 Negative Chromey et al. (1983)
synthesis mmol/litre
In vivo
Chromosomal aberration CrI:CD rat bone marrow 50-500 mg/kg bw NR Negative Farrow et al. (1983)
(single dose by gavage)
Micronucleus formation CD-1 mouse bone marrow 375 mg/kg bw 95.5 Negative Sorg et al. (1984)
(single dose by gavage)
NR, not reported
a With and without exogenous metabolic activation
A range-finding study in young adult Hartley guinea-pigs
indicated mild erythema 24 h after dermal application of undiluted
flusilazole (purity, 90%) in aliquots of 0.05 ml. No dermal irritation
was observed with concentrations > 50%. In a study of primary skin
irritation, flusilazole (purity, 90%) was applied topically to the
skin at concentrations of 5 or 50% (w/v, solution in dimethyl
phthalate), and two test sites per animal were scored for signs of
irritation at 24 and 48 h. No dermal irritation was seen in any of the
10 exposed animals. Flusilazole was thus not irritating to the skin of
guinea-pigs (Wylie el al., 1984c).
In a study of the sensitization of young adult Hartley
guinea-pigs, intradermal injections of a 1% solution (w/v) of
flusilazole (purity, 90%) in methyl phthalate weekly for four weeks
caused erythema and oedema with necrotic centres at the sites of
injection by 24 h. Challenge with topical applications of 5 or 50%
solutions to the skin did not induce sensitization (Wylie el al.,
1984c).
In another study of sensitization, 10 male and 10 female young
adult Duncan Hartley albino guinea-pigs were given three weekly dermal
applications of 0.4 ml (equivalent to 0.192 g) flusilazole (purity,
97.7%). The material, slightly moistened with dimethyl phthalate, was
applied to the intact shaved skin on the back and covered with plastic
wrap for 6 h, and dermal irritation was scored 24 and 48 h after
treatment. None was observed. Two weeks after the last induction, the
animals were challenged with a single dermal application of 0.4 ml of
flusilazole (0.192 g) on an untreated site, which was then covered for
6 h. The irritation response was again scored at 24 and 48 h. No signs
of dermal irritation were observed. A positive control group treated
with 1-chloro-2,4-dinitrobenzene showed severe erythema with necrosis
24 h after the second and third inductions and severe erythema after
the challenge dose two weeks later (Brock et al., 1988).
(ii) Mechanisms of Leydig-cell tumour induction
Groups of 10 Crl:CD BR male rats received technical-grade
flusilazole (purity, 94%) in corn oil by subcutaneous injection at
doses of 0, 20, 50, 150, or 250 mg/kg bw per day (given as two equal
half-doses, twice daily) for 14 days. The control group and that at
250 mg/kg bw per day each included an additional subgroup of 10 male
rats which were treated with human chorionic gonadotropin (hCG) 1 h
before sacrifice. Ketoconazole (a known inhibitor of 17ß-hydroxylase)
was used as the positive control: Groups of 10 male rats were given
subcutaneous injections of 0, 20, 50, 100, or 200 mg/kg bw per day
ketoconazole in saline (as two equal half-doses, twice daily) for 14
days. The control group and that at 200 mg/kg bw per day each included
an additional subgroup of 10 male rats which were treated with hCG 1 h
before sacrifice. All surviving animals were killed on day 15 of
treatment and necropsied. Testicular interstitial fluid and serum
samples were collected from rats that did not receive hCG; the
interstitial fluid was analysed for testosterone and the serum samples
for testosterone, estradiol, luteinizing hormone, and follicle-
stimulating hormone. The serum samples collected from the hCG-treated
rats were analysed for testosterone, androstenedione, 17ß-hydroxy-
progesterone, and progesterone. Increased absolute and relative liver
weights and a dose-related inhibition of serum testosterone levels
(statistically significant at doses > 150 mg/kg bw per day) and
estradiol (statistically significant at all doses) were observed at
doses > 20 mg/kg bw per day. At the two highest doses, clinical
symptoms of toxicity (sores, stained or wet fur, dehydration, and
diarrhoea), decreased body weight and body-weight gain, and reduced
food consumption were evident; eight of 10 rats at the highest dose
died. The rats at 250 mg/kg bw per day that were treated with hCG had
significantly lower serum testosterone levels than controls; no other
significant differences in hormone levels were noted. The positive
controls had significantly lower levels of testosterone,
androstenedione, and 17ß-hydroxyprogesterone and a higher level of
progesterone, indicating inhibition of 17ß-hydroxylase. There was no
NOAEL (Cook, 1993).
Leydig cells were collected from rat testes at termination of the
above study and cultured in microplate wells; the cells were then
incubated with either technical-grade flusilazole or ketoconazole at
doses of 0.05-100 µmol/litre in 70% ethanol for 2 h. The culture media
were sampled and analysed for testosterone, androstenedione,
17ß-hydroxyprogesterone, and progesterone. The results confirmed the
hormonal changes observed in vivo. Incubation of testicular Leydig
cells with technical-grade flusilazole caused a dose-dependent
lowering of testosterone and androstenedione levels, suggesting
inhibition of the enzymes involved in steroid biosynthesis. The IC50
for testosterone was 3.475 ± 1.455 µmol/litre without hCG and 2.774 ±
0.646 µmol/litre with hCG pretreatment. The positive control had an
IC50 for testosterone of 0.97 ± 0.83 µmol/litre without hCG and 0.154
± 0.065 µmol/litre with hCG (Cook, 1993).
Comments
In rats, orally administered 14C-labelled flusilazole was
readily absorbed and rapidly excreted in the urine and faeces, with
little radiolabel recovered in expired air. Over 90% of the
administered dose was eliminated within 96 h. 14C from phenyl-
labelled material was excreted predominantly in the faeces (87% in
males and 59% in females), while that from triazole-3-labelled
material was recovered primarily in the urine (72% in each sex).
Tissue retention of 14C was low (< 1%, excluding the carcass).
14C-Flusilazole was extensively metabolized in rats. After
absorption, it was cleaved at the triazole ring. Recovered parent
compound accounted for only 2-11% of the administered dose and was
found predominantly in faeces, the urinary level being < 1%.
Flusilazole is slightly toxic to mice, rats, and rabbits when
given as a single oral dose and is minimally toxic to rats and rabbits
when given dermally or by inhalation. The oral LD50 in rats was
> 500 mg/kg bw, the dermal LD50, in rabbits was > 2000 mg/kg bw,
and the inhalation LC50 in rats was 6.8-7.7 mg/litre. Flusilazole was
minimally irritating to the eyes and skin of rabbits; it was virtually
not irritating to the skin and was not a dermal sensitizer in guinea-
pigs. WHO has classified flusilazole as 'slightly hazardous'.
Repeated oral administration of flusilazole to mice (90 days),
rats (90 days), and dogs (90 days and one year) resulted primarily in
lesions of the liver (hepatocellular hypertrophy, hyperplasia and
vacuolation) and urinary bladder (urothelial hyperplasia and
vacuolation). On the basis of the hepatic and/or urinary bladder
changes, the NOAEL was 25 ppm (equal to 4 mg/kg bw per day) in mice,
125 ppm (equal to a mean of 8.1 mg/kg bw per day in two 90-day
studies) in rats, and 5 ppm (equal to 0.14 mg/kg bw per day) in dogs
(one-year study). Repeated dermal application of flusilazole to
rabbits for 21 days did not result in treatment-related systemic
toxicity at doses up to and including 200 mg/kg bw per day.
In two 18-month studies of carcinogenicity, flusilazole was
administered at dietary concentrations of 0, 5, 25, or 200 ppm to mice
of each sex in the first study and at 0, 100, 500, or 1000 ppm to
males and 0, 100, 1000, or 2000 ppm to females in the second study.
The overall NOAEL for the two studies was 25 ppm (equal to 3.4 mg/kg
bw per day) on the basis of hepatotoxicity and urinary bladder
hyperplasia in males at 100 ppm. In the second study, an increased
incidence of hepatocellular adenomas and carcinomas was observed in
males at > 100 ppm (equal to 14 mg/kg bw per day) and in females at
> 1000 ppm (equal to 200 mg/kg bw per day), doses at which lesions
of the liver (focal necrosis, hepatocellular hypertrophy and
hyperplasia, and vacuolation) were seen. Flusilazole was carcinogenic
in the second study.
In two two-year studies of toxicity and carcinogenicity, rats
received flusilazole at dietary concentrations of 0, 10, 50, or
250 ppm (first study) or 0, 125, 375, or 750 ppm (second study). In
the first study, the NOAEL was 10 ppm (equal to 0.4 mg/kg bw per day)
on the basis of mild nephrotoxicity (pyelonephritis in females and
hydronephrosis in males) at 50 ppm. In the second study, an increased
incidence of urinary bladder transitional-cell papillomas and
carcinomas in rats of each sex and testicular Leydig-cell tumours in
males were observed at 750 ppm (equal to 31 mg/kg bw per day), doses
at which lesions of the urinary bladder (urothelial necrosis,
exfoliation, and hyperplasia) were clearly demonstrated. Flusilazole
was carcinogenic in the second study.
A special two-week study to investigate the possible mechanism
by which testicular Leydig-cell tumours are induced was conducted
in rats. Flusilazole caused a dose-dependent lowering of serum
testosterone and estradiol levels at > 20 mg/kg bw per day in
vivo and a dose-related decrease in testosterone and androstenedione
production in cultured testicular Leydig cells by inhibiting enzymes
involved in steroid biosynthesis.
In a two-generation study of reproductive toxicity, rats were fed
diets containing flusilazole at concentrations of 0, 5, 50, or
250 ppm. The NOAEL for parental systemic toxicity was 5 ppm (equal to
0.34 mg/kg bw per day) on the basis of hepatic lesions at 50 ppm. The
NOAEL for reproductive toxicity was 50 ppm (equal to 4.0 mg/kg bw per
day) on the basis of treatment-related mortality during parturition,
increased length of gestation, reduced numbers of liveborn pups per
litter, and decreased pup growth at 250 ppm.
Three studies of developmental toxicity were performed in rats
with doses of 0, 10, 50, or 250 mg/kg bw per day in the first study,
0, 0.4, 2, 10, 50, or 250 mg/kg bw per day in the second study, and 0,
0.2, 0.4, 2, 10 or 100 mg/kg bw per day in the third study. A study of
developmental toxicity was also conducted in which rats were fed
flusilazole at dietary levels of 0, 50, 100, 300, or 900 ppm (equal to
0, 4.6, 9, 27, and 79 mg/kg bw per day) on days 7-16 of gestation. The
NOAEL for maternal toxicity was 10 mg/kg bw per day, on the basis of a
slight reduction in body-weight gain and food consumption during
treatment at > 27 mg/kg bw per day. The NOAEL for embryo- and
fetotoxicity was 4.6 mg/kg bw per day on the basis of increased
resorption, increased length of gestation, reduced litter size and a
higher incidence of skeletal variations or anomalies at > 9 mg/kg
bw per day. At 250 mg/kg bw per day, an increased incidence of cleft
palate was observed. The NOAEL for teratogenicity was 100 mg/kg bw per
day.
In three studies of developmental toxicity in rabbits, animals
were treated with flusilazole at 0, 2, 5, or 12 mg/kg bw per day
(first study), 0, 12, or 35 mg/kg bw per day (second study), or 0, 7,
15, or 30 mg/kg bw per day (third study) on days 7-19 of gestation.
The NOAEL for maternal and embryo- or fetotoxicity was 12 mg/kg bw per
day on the basis of clinical signs of toxicity and an increased
incidence of abortion and total resorptions at > 15 mg/kg bw per
day. There was no evidence of teratogenicity at doses up to and
including 15 mg/kg bw per day, the highest dose at which an adequate
number of live litters was available for an assessment of
teratogenicity.
Flusilazole has been adequately tested for genotoxicity in a
series of assays in vivo and in vitro. The Meeting concluded that
flusilazole is not genotoxic.
An ADI of 0-0.001 mg/kg bw was established on the basis of the
NOAEL of 5 ppm, equal to 0.14 mg/kg bw per day, in the one-year
dietary study in dogs, and a safety factor of 100.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 25 ppm, equal to 3.4 mg/kg bw per day (18-month study of
toxicity and carcinogenicity)
Rat: 10 ppm, equal to 0.4 mg/kg bw per day (two-year study of
toxicity and carcinogenicity)
5 ppm, equal to 0.34 mg/kg bw per day (maternal toxicity in
a two-generation study of reproductive toxicity)
50 ppm, equal to 4 mg/kg bw per day (two-generation study of
reproductive toxicity)
4.6 mg/kg bw per day (embryo- or fetotoxicity in a study of
developmental toxicity)
100 mg/kg bw per day (teratogenicity in a study of
developmental toxicity)
Rabbit: 12 mg/kg bw per day (maternal and embryo- or fetal toxicity
in a study of developmental toxicity)
15 mg/kg bw per day (teratogenicity in a study of
developmental toxicity)
Dog: 5 ppm, equal to 0.14 mg/kg bw per day (one-year study of
toxicity)
Estimate of acceptable daily in take for humans
0-0.001 mg/kg bw
Studies that would provide information useful for continued
evaluation of the compound
Observations in humans
Toxicological criteria for setting guidance values for dietary and non-dietary exposure to flusilazole
Exposure Relevant route, study type, species Result/remarks
Short-term (1-7 days) Dermal, irritation, rabbit Minimally irritating
Eye, irritation, rabbit Minimally irritating
Skin, sensitization, guinea-pig Not a skin sensitizer
Oral, toxicity, rat LD50 > 500 mg/kg bw
Dermal, toxicity, rabbit LD50 > 2000 mg/kg bw
Inhalation, 4 h, toxicity rat LC50 = 6.8-7.7 mg/litre
Medium-term (1-26 weeks) Repeated dermal, 21--day, toxicity, rabbit NOAEL = 200 mg/kg bw per day, highest
dose tested for systemic toxicity
Repeated dietary, reproductive toxicity, rat NOAEL = 0.34 mg/kg bw per day; hepatic
toxicity
Repeated gavage, developmental toxicity, NOAEL = 7 mg/kg bw per day for
rabbit maternal, embryo-, or fetotoxicity;
NOAEL > 15 mg/kg bw per day for
teratogenicity
Long-term (> one year) Repeated dietary, one year, toxicity, dog NOAEL = 0.14 mg/kg bw per day, primarily
for liver toxicity
References
Alvarez, L. (1990) Teratogenicity study of DPX-H6573-66 in rabbits.
Unpublished report No. HLR 216-90 from Haskell Laboratory for
Toxicology and Industrial Medicine, Wilmington, Delaware, USA.
Submitted to WHO by DuPont de Nemours and Co., Inc., Wilmington,
Delaware, USA.
Alvarez, L., Krauss, W.C. & Staples, R.E. (1984) Developmental
toxicity study in rats given INH-6573-66 in the diet on days 7-16
of gestation. Unpublished report No. HLR 431-84 from Haskell
Laboratory for Toxicology and Industrial Medicine, Wilmington,
Delaware, USA. Submitted to WHO by DuPont de Nemours and Co.,
Inc., Wilmington, Delaware, USA.
Alvarez, L., Staples, R.E. & Kaplan, A.M. (1985a) INH-6573: Prenatal
and postnatal toxicity study in rats dosed by gavage on days 7-16
of gestation. Unpublished report No. HLR 654-85 from Haskell
Laboratory for Toxicology and Industrial Medicine, Wilmington,
Delaware, USA. Submitted to WHO by DuPont de Nemours and Co..
Inc., Wilmington, Delaware, USA.
Alvarez, L., Staples, R.E., Driscoll, C.D. & Kaplan, A.M. (1985b)
INH-6573: Developmental toxicity study in rabbits treated by diet
on days 7-19 of gestation. Unpublished report No. HLR 337-85 from
Haskell Laboratory for Toxicology and Industrial Medicine,
Wilmington, Delaware, USA. Submitted to WHO by DuPont de Nemours
and Co., Inc., Wilmington, Delaware, USA.
Anderson, J.J., Stadalius, M.A. & Schlueter, D.D. (1986) Metabolism of
14C-DPX-H6573 in rats. Unpublished report No. AMR-196-84 from
DuPont de Nemours and Co., Inc., Wilmington, Delaware, USA.
Submitted to WHO by DuPont de Nemours and Co., Inc., Wilmington,
Delaware, USA.
Arce, G.T., Matarese, C.C. & Sarrif, A.M. (1988) Mutagenicity testing
of INH-6573-21 in Salmonella typhimurium plate incorporation
assay. Unpublished report No. HLR 59-88 from Haskell Laboratory
for Toxicology and Industrial Medicine, Wilmington, Delaware,
USA. Submitted to WHO by DuPont de Nemours and Co., Inc,
Wilmington, Delaware, USA.
Brock, W.J. (1988) Primary dermal irritation study with INH-6573-106
in rabbits. Unpublished report No. HLR 484-88 from Haskell
Laboratory for Toxicology and Industrial Medicine, Wilmington,
Delaware, USA. Submitted to WHO by DuPont de Nemours and Co.,
Inc., Wilmington, Delaware, USA.
Brock, W.J., Rickard, R.W., Kaplan, A.M. & Gibson, JR. (1985) Long-
term feeding study in mice with INH-6573. Unpublished report No.
HLR 278-85 from Haskell Laboratory for Toxicology and Industrial
Medicine, Wilmington, Delaware, USA. Submitted to WHO by DuPont
de Nemours and Co., Inc., Wilmington, Delaware, USA.
Brock, W.J., Vick, D.A. & Chromey, N.C. (1988) Closed-patch repeated
insult dermal sensitization study (Buehler method) with
INH-6573-21 in guinea pigs. Unpublished report No. HLR 34-88 from
Haskell Laboratory for Toxicology and Industrial Medicine,
Wilmington, Delaware, USA. Submitted to WHO by DuPont de Nemours
and Co., Inc., Wilmington, Delaware, USA.
Cheng, T. (1986) Rat metabolism, study of [triazole-3-14C] DPX-H6573.
Unpublished report No. HLR 337-85 from Haskell Laboratory for
Toxicology and Industrial Medicine, Wilmington, Delaware, USA.
Submitted to WHO by DuPont de Nemours and Co., Inc., Wilmington,
Delaware, USA.
Chromey, N.C., Horst, A.L., McCooey, K.T. & Sarrif. A.M. (1983)
Unscheduled DNA synthesis/rat hepatocytes in vitro. Unpublished
report No. HLR 209-83 from Haskell Laboratory for Toxicology and
Industrial Medicine, Wilmington, Delaware, USA. Submitted to WHO
by E.I. du Pont de Nemours & Co., Inc., Wilmington. Delaware,
USA.
Cook, J. (1993) Mechanisms of rat Leydig cell tumor induction by
DPX-H6573-193 (Flusilazole). Unpublished report No. HLR 410-93
from Haskell Laboratory for Toxicology and Industrial Medicine,
Wilmington, Delaware, USA. Submitted to WHO by E.I. du Pont de
Nemours & Co., Inc., Wilmington, Delaware, USA.
Donovan, S.M. & Irr, J.D. (1982) Mutagenicity evaluation in
Salmonella typhimurium. Unpublished report No. HLR 611-82 from
Haskell Laboratory for Toxicology and Industrial Medicine,
Wilmington, Delaware, USA. Submitted to WHO by E.I. du Pont de
Nemours & Co., Inc., Wilmington, Delaware, USA.
Farrow, M.G., Cortina, T. & Padilla-Nash, H. (1983) In vivo bone
marrow chromosome study in rats -- H # 14,728. Unpublished report
No. 201-636 from Hazleton Laboratories America, Inc., Virginia,
USA. Submitted to WHO by E.I. du Pont de Nemours & Co., Inc.,
Wilmington, Delaware, USA.
Gargus, J.L. & Sutherland, J.D. (1983) Acute skin absorption LD50
test on rabbits. Unpublished report No. 288-83 from Hazelton
Laboratories America Inc., Virginia, USA. Submitted to WHO by
E.I. du Pont de Nemours & Co., Inc., Wilmington, Delaware, USA.
Hall, J.A., Dashiell, O.L. & Kennedy, G.L. (1984) Eye irritation test
in rabbits. Unpublished report No. HLR 582-82 from Haskell
Laboratory for Toxicology and Industrial Medicine, Wilmington,
Delaware, USA. Submitted to WHO by E.L du Pont de Nemours & Co.,
Inc., Wilmington, Delaware. USA.
Keller, D.A. (1990) Subchronic oral toxicity: 90-day study with
DPX-H6573-193 feeding study in mice. Unpublished report No. HLR
60-90 from Haskell Laboratory for Toxicology and Industrial
Medicine, Wilmington, Delaware, USA. Submitted to WHO by E.I. du
Pont de Nemours & Co., Inc., Wilmington, Delaware, USA.
Keller, D.A. (1992a) Mechanism of toxicity: 90-day feeding study in
rats with DPX-H6573-194 (Flusilazole). Unpublished report No. HLR
628-92 from Haskell Laboratory for Toxicology and Industrial
Medicine, Wilmington, Delaware, USA. Submitted to WHO by E.I. du
Pont de Nemours & Co., Inc., Wilmington, Delaware, USA.
Keller, D.A. (1992b) Oncogenicity study with DPX-H6573-193
(Flusilazole): 18-month feeding study in mice. Unpublished report
No. HLR 35-92 from Haskell Laboratory for Toxicology and
Industrial Medicine, Wilmington, Delaware, USA. Submitted to WHO
by E.I. du Pont de Nemours & Co., Inc., Wilmington, Delaware,
USA.
Keller, D.A. (1992c) Oncogenicity study with DPX-H6573-194
(Flusilazole): 2-year feeding study in rats. Unpublished report
No. HLR 527-92 from Haskell Laboratory for Toxicology and
Industrial Medicine, Wilmington, Delaware, USA. Submitted to WHO
by E.I. du Pont de Nemours & Co., Inc., Wilmington, Delaware,
USA.
Lamontia, C.L., Staples, R.E. & Alvarez, L. (1984a) Embryo-fetal
toxicity and teratogenicity study of INH-6573-39 by gavage in the
rat Unpublished report No. HLR 444-83 from Haskell Laboratory for
Toxicology and Industrial Medicine, Wilmington, Delaware, USA.
Submitted to WHO by E.I. du Pont de Nemours & Co., Inc.,
Wilmington, Delaware, USA.
Lamontia, C.L., Staples, R.E. & Alvarez, L. (1984b) Embryo-fetal
toxicity and teratogenicity study of INH-6573-39 by gavage in the
rat. Unpublished report No. HLR 142-84 from Haskell Laboratory
for Toxicology and Industrial Medicine. Wilmington, Delaware,
USA. Submitted to WHO by E.I. du Pont de Nemours & Co., Inc.,
Wilmington, Delaware, USA.
McCooey, K.T., Chromey, N.C., Sarrif, A.M. & Hemingway, R.E. (1983)
CHO/HGPRT assay for gene mutation. Unpublished report No. HLR
449-83 from Haskell Laboratory for Toxicology and Industrial
Medicine, Wilmington, Delaware, USA. Submitted to WHO by E.I. du
Pont de Nemours & Co. Inc., Wilmington, Delaware, USA.
Mullin, L.S. (1990) Reproductive and fertility effects with
flusilazole: Multigeneration reproduction study in rats.
Unpublished report No. HLR 424-90 from Haskell Laboratory for
Toxicology and Industrial Medicine, Wilmington, Delaware, USA.
Submitted to WHO by E.I. du Pont de Nemours & Co., Inc.,
Wilmington, Delaware, USA.
O'Neal, F.O., Rickard, R.W., Kaplan, A.M. & Gibson, J.R. (1985)
One-year feeding study in dogs with INH-6573. Unpublished report
No. HLR 461-85 from Haskell Laboratory for Toxicology and
Industrial Medicine, Wilmington, Delaware, USA. Submitted to WHO
by E.I. du Pont de Nemours & Co. Inc., Wilmington, Delaware, USA.
Pastoor, T.P., Wood, C.K., Krahn, D.F. & Gibson, J.R. (1983)
Ninety-day feeding and one-generation reproduction study in rats
with silane [bis(4-fluorophenyl)](methyl) (1 H-1,2,4-triazol-
1-ylmethyl) (INH-6573). Unpublished report No. HLR 483-83 from
Haskell Laboratory for Toxicology and Industrial Medicine,
Wilmington, Delaware, USA. Submitted to WHO by E.I. du Pont de
Nemours & Co.. Inc., Wilmington, Delaware, USA.
Pastoor, T.P., Wood, C.K., Drahn, D.F. & Aftosmis, J.G. (1984)
Four-week range finding and ninety-day feeding study in mice with
silane [bis(4-fluorophenyl)](methyl) (1 H-1,2,4-triazol-
1-ylmethyl) (INH-6573). Revised unpublished report No. HLR
341-83 1-83 from Haskell Laboratory for Toxicology and Industrial
Medicine, Wilmington, Delaware, USA. Submitted to WHO by E.I. du
Pont de Nemours & Co., Inc., Wilmington, Delaware, USA.
Pastoor, T.P., Rickard, R.W., Sykes, G.P., Kaplan, A.M. & Gibson, J.R.
(1986) Long-term feeding (combined chronic toxicity/oncogenicity
study) and two-generation, four litter reproduction study in rats
with INH-6573. Unpublished report No. HLR 32-86 from Haskell
Laboratory for Toxicology and Industrial Medicine, Wilmington,
Delaware, USA. Submitted to WHO by E.I. du Pont de Nemours & Co.,
Inc., Wilmington, Delaware, USA.
Poindexter, G.L., Henry, J.E., Kenney, L.A., Burgess, B.A. & Kennedy,
G.L. (1984) Inhalation median lethal concentration (LC50) of
INH-6573-41 by EPA protocol. Unpublished report No. HLR 553-83
from Haskell Laboratory for Toxicology and Industrial Medicine,
Wilmington, Delaware, USA. Submitted to WHO by E.I. du Pont de
Nemours & Co., Inc., Wilmington, Delaware, USA.
Redgate, D., Sarver, J.W. & Chromey, N.C.(1985) Approximate lethal
dose (ALD) of INH-6573-66 in rabbits. Unpublished report No. HLR
54-85 from Haskell Laboratory for Toxicology and Industrial
Medicine, Wilmington, Delaware, USA. Submitted to WHO by E.I. du
Pont de Nemours & Co., Inc., Wilmington, Delaware, Wilmington,
USA.
Reynolds, V. (1991) Mutagenicity testing of DPX-H6573-194 in the
Salmonella typhimurium plate incorporation assay. Unpublished
report No. HLR 33-91 by Haskell Laboratory for Toxicology and
Industrial Medicine, Wilmington, Delaware, USA. Submitted to WHO
by E.I. du Pont de Nemours & Co., Inc., Wilmington, Delaware,
USA.
Rickard, R.W., Wood, C.K., Krahn, D.F. & Aftosmis, J.G. (1983)
Three-month feeding study in dogs with silane [bis(4-
fluorophenyl)](methyl) (1 H-1,2,4-triazol- 1-ylmethyl)
(INH-6573). Unpublished report No. HLR 461-83 from Haskell
Laboratory for Toxicology and Industrial Medicine, Wilmington,
Delaware, USA. Submitted to WHO by E.I. du Pont de Nemours & Co.,
Inc., Wilmington, Delaware, USA.
Sarver, J.W., Vick, D.A., Valentine, R., Chromey, N.C. & Kaplan, A.M.
(1986) Twenty-one dose dermal toxicity study with INH-6573-82 in
rabbits. Unpublished report No. HLR 744-86 from Haskell
Laboratory for Toxicology and Industrial Medicine, Wilmington,
Delaware, USA. Submitted to WHO by E.I. du Pont de Nemours & Co.,
Inc., Wilmington, Delaware, USA.
Solomon, H.M., Alvarez, L., Staples, R.E. & Hamill, J.C. (1984)
Developmental toxicity study in rabbits given INH-6573 by gavage
on days 7-19 of gestation. Unpublished report No. HLR 333-84 from
Haskell Laboratory for Toxicology and Industrial Medicine,
Wilmington, Delaware, USA. Submitted to WHO by E.I. du Pont de
Nemours & Co., Inc., Wilmington, Delaware, USA.
Sorg, R.M., Naismith, R.W. & Mathews, R.J. (1984) Micronucleus test
(MNT)-OECD H # 15,314. Unpublished Haskell report No. HLR 437-84
from Pharmakon Research International. Submitted to WHO by E.I.
du Pont de Nemours & Co., Inc., Wilmington, Delaware, USA.
Turner, R.J., Kinney, L.A. & Chromey, N.C. (1985) Inhalation median
lethal concentration (LC50) of INH-6573 by EPA guidelines.
Unpublished report No. HLR 1-85 by Haskell Laboratory for
Toxicology and Industrial Medicine, Wilmington, Delaware, USA.
Submitted to WHO by E.I. du Pont de Nemours & Co., Inc.,
Wilmington, Delaware, USA.
Vlachos, D.A., Covell, D.L. & Sarrif, A.M. (1986) Evaluation of
INH-6573-82 in the in vitro assay for chromosome aberrations in
human lymphocytes. Unpublished report No. HLR 745-86 from Haskell
Laboratory for Toxicology and Industrial Medicine, Wilmington,
Delaware, USA. Submitted to WHO by E.I. du Pont de Nemours & Co.,
Inc., Wilmington, Delaware, USA.
Wylie, C.N., Burgess, B.A. & Kennedy, G.L. (1983) Acute oral test in
rats. Unpublished report No. HLR 78-83 from Haskell Laboratory
for Toxicology and Industrial Medicine, Wilmington, Delaware,
USA. Submitted to WHO by E.I. du Pont de Nemours & Co., Inc.,
Wilmington, Delaware, USA.
Wylie, C.N., Henry, J.E., Ferenz, R.L., Burgess, B.A. & Kennedy, G.L.
(1984a) Median lethal dose (LD50) in rats -- EPA proposed
guidelines, Newark, Delaware, USA. Unpublished report No. HLR
433-83 by Haskell Laboratory for Toxicology and Industrial
Medicine, Wilmington, Delaware, USA. Submitted to WHO by E.I. du
Pont de Nemours & Co., Inc., Wilmington, Delaware, USA.
Wylie, C.N., Henry, J.E., Dashiell, O.L. & Kennedy, G.L. (1984b)
Primary skin irritation and sensitization test on guinea pigs.
Unpublished report No. HLR 626-82 from Haskell Laboratory for
Toxicology and Industrial Medicine, Wilmington, Delaware, USA.
Submitted to WHO by E.I. du Pont de Nemours & Co., Inc.,
Wilmington, Delaware, USA.
Wylie, C.N., Henry, J.E., Burgess, B.A. & Kennedy, G.L. (1984c)
Ten-dose oral subacute test in rats. Unpublished report No. HLR
78-83 from Haskell Laboratory for Toxicology and Industrial
Medicine, Wilmington, Delaware, USA. Submitted to WHO by E.I. du
Pont de Nemours & Co, Inc., Wilmington, Delaware, USA.
Wylie, C.N., Redgate, D., Warheit, D.B. & Chromey, N.C. (1985)
Approximate lethal dose (ALD) of INH-6573-66 in male and female
mice. Unpublished report No. HLR 531-84 from Haskell Laboratory
for Toxicology and Industrial Medicine, Wilmington, Delaware,
USA. Submitted to WHO by E.I. du Pont de Nemours & Co., Inc.,
Wilmington, Delaware, USA.
Zellers, J.E., Staples, R.E., Alvarez, L. & Kaplan, A.M. (1985)
Developmental toxicity study (supplemental) in rabbits dosed by
gavage on days 7-19 of gestation. Unpublished report No. HLR
669-85, from Haskell Laboratory for Toxicology and Industrial
Medicine, Wilmington, Delaware, USA. Submitted to WHO by E.I. du
Pont de Nemours & Co., Inc., Wilmington, Delaware, USA.
 Table 1. Acute toxicity of flusilazole
Species Sex Route LD50 or LC50 Reference
(mg/kg bw or
mg/litre air)
Mouse Male Oral 680 Wylie et al. (1985)
female 1000
Rat Male Oral 1500 Wylie et al. (1983)
Male Oral 1110 Wylie et al. (1984a)
Female 674
Male Inhalation 2.7 Poindexter et al. (1984)
Female 3.7
Male, female Inhalation 6.8-7.7 Turner et al. (1985)
Rabbit Male, female Oral 450 Redgate et al. (1985)
Male, female Dermal > 2000 Gargus & Sutherland (1983)
(b) Short-term toxicity
Mice
Groups of 20 male and 20 female Crl:CD-1 mice were given
technical-grade flusilazole (purity, 96.7%) at dietary levels of 0,
25, 75, 225, 500, or 1000 ppm, equal to 0, 4, 12, 36, 82, or 164 mg/kg
bw per day for males and 0, 5, 15, 43, 92, or 222 mg/kg bw per day for
females, for up to 90 days. Ten mice of each sex per group were
sacrificed after four weeks of treatment; the remaining mice were
killed at the end of the study. No histopathological examination was
conducted on animals killed at four weeks. There were no clinical
symptoms of toxicity and no treatment-related effects on body weight
or food consumption in animals sacrificed at either interval. The
target organs of toxicity were liver and urinary bladder; females were
slightly more sensitive to flusilazole than males. No treatment-
related effects were observed at the lowest dose. At 75 ppm, increased
absolute and relative liver weights and an increased incidence (1/10)
of hepatocellular vacuolar cytoplasmic changes were observed in
females. At > 225 ppm, increased liver weight, dose-related
increases in the incidence of hepatocellular vacuolar cytoplasmic
changes, hepatocellular hypertrophy, and urinary bladder urothelial
cell hyperplasia were evident in animals of each sex. At the highest
dose, absolute and relative kidney weights were decreased in males,
but no treatment-related renal lesions were observed. In addition,
slight decreases in erythroid parameters (haemoglobin, haematocrit,
and erythrocyte counts) were noted in animals of each sex. The NOAEL
was 4 mg/kg bw per day (Pastoor et al., 1984).
Groups of 16 male and 16 female Crl:CD-1(ICR)BR mice were fed
diets containing technical-grade flusilazole (purity, 94%) at doses of
0, 1000, 2500, or 5000 ppm, equal to 0, 161, 436, and 1004 mg/kg bw
per day for males and 0, 239, 601, and 1414 mg/kg bw per day for
females, for at least 90 days. An additional six mice of each sex per
group were assessed for cellular proliferation in the liver and
urinary bladder on days 14 and 106. At the lowest dose, treatment-
related effects included reduced mean body weight and food efficiency
(males only), increased absolute and relative liver weights (animals
of each sex), decreased absolute and relative kidney weights (females
only), and hypertrophy or hyperplasia, cytoplasmic vacuolation, and
inflammation of the liver and urinary bladder (animals of each sex).
At the mid-dose, increased cellular proliferation of the urinary
bladder was also observed in animals of each sex. At the highest dose,
severe body-weight loss occurred in males and reduced body weight and
food efficiency were noted in females. The males at this dose were
sacrificed on day 44 owing to excess mortality and moribund condition.
There was no NOAEL (Keller, 1990).
Rats
Groups of six male Crl:CD rats were given technical-grade
flusilazole (purity, 95.5%) by gavage in corn oil at a dose of 0 or
300 mg/kg bw per day, five days per week for two weeks. Three rats per
group were killed at the end of the treatment period, and the
remaining rats were sacrificed after a two-week recovery period. All
animals were examined histopathologically. One treated animal died
after the fifth dose on day 7. Clinical signs of toxicity (lowered
body weight, alopecia, diarrhoea, stained or wet perineal area,
salivation, and hypersensitivity) were observed in four rats during
the treatment period. Treatment-related histopathological changes were
observed in liver, kidney, urinary bladder, and testis; the lesions
appeared to be less severe in rats killed after the recovery period.
Remarkable histopathological findings in treated rats included
hepatocellular vacuolation (six rats), hyperplasia and vacuolation of
the urinary bladder transitional epithelium (six rats) and renal
pelvis urothelium (two rats), and necrosis and cellular degeneration
of the germinal epithelium of the seminiferous tubules (two rats)
(Wylie et al., 1984b).
Four groups of 10 male and 10 female Charles River CD rats were
fed diets containing technical-grade flusilazole (purity, 96.7%) at
doses of 0, 25, 125, 375, or 750 ppm, equal to 0, 2, 9, 27, and
55 mg/kg bw per day for males and 0, 2, 11, 31, and 70 mg/kg bw per
day for females, for 90 days. No treatment-related effects were
oberved at the two lower doses. At 375 ppm, an increased serum
cholesterol level was seen in animals of each sex and an increased
incidence of mild bladder urothelial hyperplasia in one male and four
females. At the highest dose, bladder urothelial hyperplasia was seen
in five males and eight females. Additional treatment-related changes
included decreased body weight in females, increased absolute and
relative liver weights in animals of each sex, and remarkable
histopathological findings (hepatocellular hypertrophy, mild fatty
changes, and hepatocytolysis) in the livers of five males. The NOAEL
was 9 mg/kg bw per day (Pastoor et al., 1983).
Groups of 52 male and 52 female Crl:CD BR rats were fed diets
containing technical-grade flusilazole (purity, 95%) at doses of 0,
10, 125, 375, or 750 ppm, equal to 0, 0.58, 7.27, 22.1, and 44.7 mg/kg
bw per day for males and 0, 0.74, 9.40, 27.6, and 59.0 mg/kg bw per
day for females, for up to 91 days. Each group was divided into three
subgroups of 20 rats of each sex in order to study possible mechanisms
of action of toxicity in the liver and urinary bladder. Five rats of
each sex were sacrificed on days 7/8, 14, 46, and 91 for assessment of
cellular proliferation and histopathology. For evaluation of
cytochrome P450 and peroxisome proliferation, five rats of each sex
were sacrificed on days 14 and 90 (males) or 15 and 91 (females).
Serum levels of testosterone, estradiol, and luteinizing hormone were
determined in all males sacrificed on days 14 and 90. No adverse
treatment-related effects were observed at the two lower doses. At
125 ppm, absolute and relative liver weights were increased; in the
absence of histopathological changes or any other symptoms of
toxicity, the finding was not considered to be toxicologically
significant. At the two highest doses, liver hypertrophy was observed.
The response was sex-dependent: lamellar bodies and periportal
hypertrophy occurred in males and centrilobular hypertrophy without
lamellar bodies was seen in females. In the urinary bladder,
transitional-cell necrosis, exfoliation and hyperplasia were noted.
Hepatic cytochrome P450 levels were higher than control values, but
there was no evidence of peroxisome proliferation in the livers of
treated rats. No significant changes in the serum levels of
testosterone, estradiol, or luteinizing hormone were found in any of
the males examined. The NOAEL was 7.27 mg/kg bw per day (Keller,
1992a).
Rabbits
Groups of five male and five female New Zealand white rabbits
received dermal applications of technical-grade flusilazole (purity,
94.9%) at doses of 0, 1, 5, 25, or 200 mg/kg bw per day for 21 days.
The test material was applied daily as a paste in distilled water; the
exposure sites were occluded for 6 h and then washed with water. No
evidence of systemic toxicity was observed. The NOAEL for dermal
toxicity was 5 mg/kg bw per day, based on diffuse epidermal
hyperplasia or thickening (slight to mild) at > 25 mg/kg bw per
day. At the highest dose, mild erythema was seen on days 6-12. The
NOAEL for systemic toxicity was > 200 mg/kg bw per day (Sarver
et al., 1986).
Dogs
Groups of four male and four female beagle dogs were fed diets
containing technical-grade flusilazole (purity, 93%) at concentrations
of 0, 25, 125, or 750/500 ppm, equal to 0.9, 4.3, and 13.4 mg/kg bw
per day for males and 0, 0.9, 4.3, and 14.2 mg/kg bw per day for
females, for three months. The high-dose group received 750 ppm
flusilazole in the diet for the initial three weeks, then control diet
for one week, and 500 ppm flusilazole in the diet for the remaining
period. The dose was reduced because of marked body-weight loss and
decreased food intake at 750 ppm. At the lowest dose, there was a
treatment-related increase in the incidence of hyperplasia of the
lymphoid follicles in the pyloric glandular mucosa of the stomach in
males: 0/4, 3/4 3/4, and 4/4 at 0, 25, 125, and 750/500 ppm,
respectively. At 125 ppm, an increased incidence of pyloric granular
mucosa hyperplasia was also observed in females: 0/4, 0/4, 3/4, and
4/4 at 0, 25, 125, and 750/500 ppm, respectively. Higher levels (in
comparison with control values) of alanine aminotransferase activity
and an increased incidence of mild urinary bladder mucosal hyperplasia
were observed in males at 125 ppm and in animals of each sex at the
highest dose. Additional treatment-related effects seen at the highest
dose included clinical signs of toxicity (weakness or tremors) in
animals of each sex, reduced mean body-weight gain in males,
body-weight loss in females, decreased mean food consumption in
animals of each sex, a slight increase in leukocyte and monocyte
counts in males, decreased plasma levels of cholesterol, total
protein, and albumin in animals of each sex, and increased absolute
and relative liver weights in animals of each sex. There was no NOAEL
(Rickard et al., 1983).
Groups of five male and five female beagle dogs were fed
technical-grade flusilazole (purity, 95.8%) in the diet at doses of 0,
5, 20, or 75 ppm, equal to 0, 0.14, 0.7, and 2.4 mg/kg bw per day for
males and 0, 0.14, 0.7, and 2.6 mg/kg bw per day for females, for one
year. No treatment-related effects were observed at the lowest dose.
At the next dose, 20 ppm, the serum albumin level was decreased in
males and there was a dose-related increase in the incidence of
centrilobular hepatocellular hypertrophy in both males (0/5, 0/5, 4/5,
and 5/5) and females (0/5, 0/5, 2/5, and 5/5). More severe gastric
mucosal lymphoid hyperplasia, from minimal at 0 or 5 ppm to mild at
20 ppm, was also noted in males. At the highest dose, additional
treatment-related effects included higher leukocyte counts in animals
of each sex; elevated alkaline phosphatase and lowered cholesterol,
total protein, and albumin levels in males only; and increases in
relative liver (animals of each sex) and kidney weights (females). All
high-dose animals exhibited a greater degree of hepatic centrilobular
inflammatory infiltration, and distinct centrilobular hepatocellular
vacuolation was observed in three males. The lymphoid hyperplasia in
the gastric mucosa was of moderate severity in both males and females.
The NOAEL was 0.14 mg/kg bw per day (O'Neal et al., 1985).
(c) Long-term toxicity and carcinogenicity
Mice
Groups of 80 male and 80 female Crl:CD-1(ICR)BR mice received
technical-grade flusilazole (purity, 96.5%) in the diet at doses of 0,
5, 25, or 200 ppm, equal to 0, 0.66, 3.4, and 27 mg/kg bw per day for
males and 0, 0.92, 4.6, and 36 mg/kg bw per day for females, for 18
months. Each group included 10 mice of each sex that were killed at
six months. There were no treatment-related effects on mortality
(terminal survival rates were 76-86% in males and 57-80% in females),
body weight, food consumption, haematology, or serum chemistry at any
dose. At the highest dose, a toxicologically significant increase in
the incidence of hepatocellular fatty changes was observed, with
terminal incidences of 4/80, 3/80, 10/80, and 40/80 males and 2/80,
4/80, 3/80, and 24/80 females at 0, 5, 25, and 200 ppm. In addition,
there was an increase in absolute and relative liver weights in
animals of each sex, a decrease in absolute kidney weight in females,
and an increase in lymphocytic infiltration in the lung and urinary
bladder in males. There was no treatment-related increase in the
incidence of any tumour type at doses > 27 mg/kg bw per day, the
highest dose tested. The NOAEL for systemic toxicity was 3.4 mg/kg bw
per day (Brock et al., 1985).
Groups of 100 male and 100 female Crl:CD-1(ICR)BR mice received
diets containing technical-grade flusilazole (purity, 94%) at doses of
0, 100, 500, or 1000 ppm in males, equal to 0, 14.3, 73.1, and
144 mg/kg bw per day, and 0, 100, 1000, or 2000 ppm in females, equal
to 0, 19.4, 200, and 384 mg/kg bw per day, for 18 months. An
additional group of 100 mice of each sex was fed a diet containing
25 ppm technical-grade flusilazole (equal to 3.51 mg/kg bw per day for
males and 4.38 mg/kg bw per day for females) and was used to study
cell proliferation. The doses were selected on the basis of the
results of a 90-day study that indicated greater sensitivity of males
to the test compound. At the lowest dose tested, there was a slight
decrease in absolute kidney weight, an increase in focal necrosis of
the liver, and urinary bladder hyperplasia in males. No remarkable
changes were seen in females at this dose. At the two highest doses,
females had increased cellular hyperplasia in the urinary bladder and
urethra, but no focal necrosis of the liver. At these two doses,
additional treatment-related systemic effects included increased
absolute and relative liver weights, lower absolute and relative
kidney weights, increased numbers of foci of hepatocellular
alteration, and an increased incidence of hepatic vesicular or
vacuolar changes with cellular hypertrophy. Significantly higher
mortality occurred at 1000 ppm in males and 2000 ppm in females. In
addition, increased cellular proliferation in the urinary bladder (but
not in the liver) was observed in females at the highest dose. At
terminal sacrifice, an increased incidence of hepatocellular adenomas
and carcinomas was observed in males at all doses (13/80, 23/79,
20/80, and 18/78 at 0, 100, 500, and 1000 ppm, respectively;
historical control range, 6.3-13.8%) and in females at the two highest
doses (1/79, 3/80, 11/77, and 43/76 at 0, 100, 1000, and 2000 ppm,
respectively; historical control range, 0-2.6%). The increase in
tumour incidence was dose-related and statistically significant in
females but not in males. There was no NOAEL for systemic toxicity or
oncogenicity (Keller, 1992b).
Rats
Groups of 70 male and 70 female rats (Crl:CD(SD)BR strain)
received technical-grade flusilazole (purity, 95.6%) in the diet at
doses of 0, 10, 50, or 250 ppm, equal to 0, 0.4, 2.0, and 10 mg/kg bw
per day for males and 0, 0.5, 2.6, and 13 mg/kg bw per day for
females, for two years. Each group included two groups of 10 rats of
each sex that were killed at six months and one year. About 100 days
after the beginning of treatment, 20 rats of each sex in each group
were mated and were returned to the study after weaning of the second
litter. The rats killed at six months were examined for bladder
lesions only. No treatment-related effect on mortality was observed;
the survival rate was > 50% in all groups until week 98. No signs of
systemic toxicity was seen at the low dose. At 50 ppm, there was a
dose-related increase in the incidence of pyelonephritis in females at
two years (3/66, 3/62, 8/67, and 10/65 at 0, 10, 50, and 250 ppm,
respectively). Increases in relative liver weight in females and in
the incidence of hydronephrosis in males were observed at interim
sacrifice at one year. At the highest dose, additional treatment-
related changes included an increased incidence of hepatic lesions
(centrilobular hepatocellular hypertrophy and polyploidy) in females
and hydronephrosis in males at one year and an increased incidence of
acidophilic foci of hepatocellular alteration (13/65; 3/66 in
controls) and hepatic diffuse fatty changes (23/65; 9/66 in controls)
in females at two years. No hepatic lesions were observed in males
during the study, and no lesions of the urinary bladder were seen in
animals of either sex at either interim or terminal sacrifice. There
was no treatment-related increase in the incidence of any tumour type
at any dose. At 250 ppm, males had a slightly increased incidence of
squamous-cell carcinomas of the oral and nasal cavities (0/66, 1/63,
0/67, and 3/64 at 0, 10, 50, and 250 ppm, respectively). On the basis
of historical control data, from five two-year feeding studies in rats
with no tumours of this type and one study with an incidence of 2/60,
the incidence of nasal tumours in this study was judged to be
incidental. The NOAEL for systemic toxicity was 0.4 mg/kg bw per day;
however, the effects reported were relatively mild, and higher doses
might have been tolerated (Pastoor et al., 1986).
Groups of 65 male and 65 female Crl:CD(SD)BR rats received diets
containing technical-grade flusilazole (purity, 95%) at doses of 0,
125, 375, or 750 ppm, equal to 0, 5.03, 14.8, and 30.8 mg/kg bw per
day for males and 0, 6.83, 20.5, and 45.6 mg/kg bw per day for
females, for two years. Each group included 10 rats of each sex for
interim sacrifice at one year. Dose-related increases in the incidence
of hepatocellular hypertrophy were observed in animals of each sex at
both interim (males: 0/10, 4/10, 8/10, and 8/10; females: 0/10, 7/10,
10/10, and 10/10; at 0, 125, 375, and 750 ppm, respectively) and
terminal sacrifice (males: 2/53, 2/51, 10/53, and 19/53; females:
2/56, 4/55, 12/53, and 25/54). There was an apparent sex difference in
the hepatic lesions: periportal hepatocellular hypertrophy with
lamellar bodies was seen in males, while females showed centrilobular
hepatocellular hypertrophy with eosinophilic cytoplasm but no lamellar
bodies. At terminal sacrifice, there was also an increased incidence
of mixed foci of cellular alteration in males (6/53, 14/51, 17/53, and
19/53). At 375 and 750 ppm, there were also significant decreases in
terminal body weight in females, increases in absolute and/or relative
liver weights at interim and/or terminal sacrifice in males and/or
females, and an increase in the incidence of urinary bladder
transitional-cell hyperplasia at the end of the study in animals of
each sex (3/46, 6/45, 27/47, and 42/51 in males and 5/47, 3/49, 15/49,
and 33/53 in females). At 750 ppm, additional treatment-related
changes included an increased incidence of hepatic fatty changes in
males and increased cellular proliferation in the liver and urinary
bladder. At the highest dose, a treatment-related increase in the
incidence of urinary bladder transitional-cell papillomas and
carcinomas was observed in animals of each sex at the end of the study
(males: 0/46, 0/45, 1/47, and 5/51; and females: 0/47, 1/49, 0/49, and
13/53; at doses of 0, 125, 375, and 750 ppm, respectively). The
incidence of testicular interstitial-cell (Leydig-cell) tumours in
males at the highest dose was also increased: 2/53, 4/51, 2/53, and
9/53 at 0, 125, 375 and 750 ppm, respectively. There was no NOAEL for
systemic toxicity; the NOAEL for oncogenicity was 14.8 mg/kg bw per
day (Keller, 1992c).
(d) Reproductive toxicity
Rats
Groups of six male and six female Crl:CD(SD)BR rats from a 90-day
feeding study were used in a one-generation study of reproductive
toxicity. The rats were fed daily diets containing technical-grade
flusilazole (purity, 96.7%) at doses of 0, 25, 125, or 375 ppm, equal
to 0, 2, 9, and 27 mg/kg bw per day for males and 0, 2, 11, and
31 mg/kg bw per day for females, for 90 days before mating. Males and
females within the same dose group were mated for 15 days; females
were examined daily for evidence of a copulation plug. After the
mating period, females were housed individually. The fertility index
was low in all groups and especially in the control group (67.7%);
three to six females per group became pregnant. At the highest dose, a
lower gestation index, a lower percentage of liveborn pups, and lower
pup weight at day 4 were observed in comparison with controls (Pastoor
et al., 1983). The small group size and the absence of individual
data on some parameters limit the usefulness of this study for
evaluation of reproductive toxicity.
Groups of 20 male and 20 female Crl:CD(SD)BR rats from a two-year
study of toxicity and oncogenicity were used in a two-generation,
two-litter study. The rats were fed diets containing technical-grade
flusilazole (purity, 95.6%) at doses of 0, 10, 50, or 250 ppm, equal
to 0, 1, 3, and 18 mg/kg bw per day for males and 0, 1, 4, and
20 mg/kg bw per day for females (premating intake) for at least 100
days before mating. Males and females (F0) within the same dose group
were mated 1:1 for 15 days; the females were examined daily for
evidence of a copulation plug. After the mating period, F0 females
were housed individually and allowed to give birth to F1a litters.
About one week after the last F1a litter had been weaned, F0 females
were mated with different F0 males of the same dose group to produce
the F1b litters. After weaning of the F1b litters, 20 rats of each
sex per group were selected as the F1 parents for the F2 generation.
These rats were maintained on the same diets as their F0 parents for
90 days before mating to produce the F2a and F2b litters. Sibling
mating was avoided. The mean body weights of the F1b males at 250 ppm
were decreased during the premating period. No other treatment-related
systemic effect was observed in any F0 or F1 adult. There was no
evidence of a treatment-related effect on mating or fertility.
Treatment-related embryo-, feto-, and litter toxicity were observed at
the mid- (50 ppm) and high doses (250 ppm). An increased number of
stillborn pups and a decreased viability index (days 0-4) occurred in
the F2a litters at 50 ppm and in all litters at 250 ppm. Litter
survival after day 4 was similar in all groups in the F1 and F2
generations. At 250 ppm, the mean weights of F1b and F2b pups of each
sex at weaning were slightly reduced, and the absolute and relative
liver weights of F2b weanling male pups were increased. An increased
incidence of unilateral and/or bilateral hydronephrosis was noted in
F2b female weanlings: 1/10, 4/10, 3/10, and 5/10 at 0, 10, 50, and
250 ppm, respectively. Since neither the severity nor the incidence
of the lesions showed a dose-response relationship and since
hydronephrosis is a common lesion in weanling pups (historical control
range in 13 in-house studies, 0-30%), the finding was judged to be
toxicologically insignificant. The NOAEL was 1 mg/kg bw per day, based
on embryo- and fetotoxicity and decreased viability of pups in the
F2a litters at 50 ppm (Pastoor et al., 1986).
In a two-generation study with one or two litters per generation,
groups of 30 male and 30 female Crl:CD(SD)BR rats were fed diets
containing technical-grade flusilazole (purity, 94%) at doses of 0, 5,
50, or 250 ppm, equal to 0, 0.34, 3.46, and 17.3 mg/kg bw per day for
males and 0, 0.40, 4.04, and 19.6 mg/kg bw per day for females
(premating intake), for 73 days (F0 rats) or 91 days (F1 parents)
before mating. Males and females within the same dose group were
randomly paired 1:1 for 15 days; females were examined daily for
evidence of a copulation plug. One litter (F1a) was produced in the
F0 generation and two litters (F2a and F2b) in the F1 generation.
No treatment-related effects were seen in either the F0 or F1
generation at the low dose. At the middle dose, increased smooth
endoplasmic reticulum was seen in the hepatocytes of males and
centrilobular hepatocellular hypertrophy in females. At the highest
dose, F1 females had slightly but consistently lower body weights.
Reproductive toxicity was indicated by higher mortality during
parturition and increased gestational length of the F0 and F1 dams:
mean, 22.9-23.2 days in comparison with 22.4-22.6 days in controls. No
treatment-related effects on mating or fertility indices were
observed. Embryo-, feto-, and litter toxicity were seen as reduced
number of pups per litter and increased numbers of stillborn pups per
litter in the F1a, F2a, and F2b litters and decreased mean pup
weights on lactation days 14 and 21 (F2a litters only) at 250 ppm.
The NOAEL for systemic toxicity was 0.34 mg/kg bw per day, on the
basis of hepatic lesions at 50 ppm; the NOAEL for reproductive
toxicity was 4.04 mg/kg bw per day, on the basis of treatment-related
mortality during parturition, increased gestational length, and
embryo-, feto-, and litter toxicity at 250 ppm (Mullin, 1990).
(e) Developmental toxicity
Rats
In a range-finding study, groups of seven pregnant rats received
technical-grade flusilazole (purity, 99%) by gavage at doses of 0,
100, or 300 mg/kg bw per day on days 7-16 of gestation. Flusilazole
was maternally and embryotoxic at both doses, and the highest dose
induced cleft palates in about 51% of the fetuses in each litter. In
the main study of developmental toxicity, groups of 25 mated female
Crl:CD(SD)BR rats received technical-grade flusilazole (purity, 95.6%)
by gavage in corn oil at doses of 0, 10, 50, or 250 mg/kg bw per day
on days 7-16 of gestation; the day a copulation plug was observed was
designated as day 1 of gestation. On gestation day 21, all surviving
dams were sacrificed and necropsied; fetuses were delivered by
caesarean section and examined for external, visceral, and skeletal
abnormalities. No treatment-related signs of maternal toxicity were
seen at the lowest dose. At 50 mg/kg bw per day, slight decreases in
body-weight gain and food consumption and a slight increase in the
relative liver weight of dams were seen. At the highest dose,
additional maternal toxic effects included higher mortality and
clinical signs of toxicity (chromodacryorrhoea, chromorhinorrhoea, wet
or stained perineal areas, red vaginal discharges or stains, and focal
alopecia) in 23 animals. Fetuses had treatment-related increases in
the incidence of skeletal anomalies (misaligned sternebrae, extra
ossification centres in ribs, and delayed ossification in sternebrae)
at all doses. At 50 mg/kg bw per day, there was a reduced mean number
of liveborn fetuses per litter, a higher total number of stunted
fetuses (0, 1, 4, and 3 at 0, 10, 50, and 250 mg/kg bw per day,
respectively), and an increased incidence of rudimentary ribs. At the
highest dose, additional signs of embryo- and fetotoxicity were an
increased mean incidence of resorptions, reduced mean fetal weight per
litter, and all increased incidence of extra ribs in the fetuses. An
increased incidence of cleft palate (28/241; 0/331 in controls) and an
absence of renal papillae (21/155; 0/175 in controls) were observed in
fetuses of dams given 250 mg/kg bw per day but not at lower doses. An
unusually high incidence of hydrocephalus and/or dilated lateral
ventricles of the brain was also noted in all groups, including
controls; the effect was not dose-related and was not considered to be
related to treatment. The NOAEL for maternal toxicity was 10 mg/kg bw
per day; there was no NOAEL for embryo- or fetotoxicity; and the NOAEL
for teratogenicity was 50 mg/kg bw per day (Lamontia et al., 1984a).
Groups of 24 mated female Crl:CD(SD)BR rats received technical-
grade flusilazole (purity, 95.6%) by gavage in corn oil at doses of 0,
0.4, 2, 10, 50, or 250 (10 females only) mg/kg bw per day on days 7-16
of gestation; the day a copulation plug was observed was designated
day 1 of gestation. On day 21 of gestation, all surviving dams were
sacrificed and necropsied; fetuses were delivered by caesarean section
and examined for external, visceral, and skeletal abnormalities. No
treatment-related signs of maternal toxicity were observed at doses
> 10 mg/kg bw per day. Slightly reduced body-weight gain and food
consumption during treatment and increased relative liver weights of
dams were seen at 50 mg/kg bw per day. At the highest dose, clinical
signs of maternal toxicity (alopecia, brown stains on the face and
limbs, and stained perineal area) were also observed. No treatment-
related embryo- or fetotoxic effects were seen at the two lowest
doses. At > 10 mg/kg bw per day, an increased number of dams with
median or late resorptions, an increased total number of stunted
fetuses, a higher incidence of visceral (large renal pelvis and small
renal papilla) and skeletal (rib) anomalies, and an increased
incidence of delayed ossification (sternebrae and vertebral arches)
were observed. An increased incidence of cleft palate (21/116; 0/291
in controls) was observed in fetuses of dams receiving 250 mg/kg bw
per day but not at lower doses. Hydrocephalus was not observed. The
NOAEL for maternal toxicity was 10 mg/kg bw per day, that for embryo-
and fetotoxicity was 2 mg/kg bw per day, and that for teratogenicity
was 50 mg/kg bw per day (Lamontia et al., 1984b).
Groups of 24 mated female rats (Crl:CD (SD)BR strain) received
diets containing technical-grade flusilazole (purity, 96.5%) at doses
of 0, 50, 100, 300, or 900 ppm equal to 0, 4.6, 9.0, 26.6, and
79.2 mg/kg bw per day) on days 7-16 of gestation; the day a copulation
plug was observed was designated day 1 of gestation. On gestation day
21, all surviving dams were sacrificed and necropsied; fetuses were
delivered by caesarean section, weighed, sexed, and then examined for
external, visceral, and skeletal abnormalities. No treatment-related
signs of maternal toxicity were seen at doses > 100 ppm.
Significant, dose-related reductions in body-weight gain and food
consumption were observed during treatment at the two highest doses.
Treatment-related embryo- and fetotoxic effects were noted at
> 100 ppm, including an increased incidence of median or late
resorptions, small litters (< 10 fetuses per litter), and significant
dose-related increases in skeletal variations with extra ossification
of the sternebrae. Additional symptoms of fetal toxicity seen at the
two highest doses included an increased number of stunted fetuses, a
higher incidence of rudimentary ribs, extra ossification in cervical
ribs, and delayed ossification of the cervical vertebral arches. None
of the fetuses had cleft palate, and there were no treatment-related
malformations at any dose. The NOAEL for maternal toxicity was
9.0 mg/kg bw per day, that for embryo- and fetotoxicity was 4.6 mg/kg
bw per day, and that for teratogenicity was 79.2 mg/kg bw per day
(Alvarez et al., 1984).
In a study of prenatal and postnatal toxicity in rats, groups of
24 (phase I, prenatal study) or 22 (phase II, postnatal study) mated
female rats (Crl:CD (SD)BR strain) received technical-grade
flusilazole (purity, 96.5%) in 0.5% aqueous methylcellulose by gavage
at doses of 0, 0.2, 0.4, 2, 10, or 100 mg/kg bw per day on days 7-16
of gestation; the day a copulation plug was observed was designed day
1 of gestation. In phase I, dams were sacrificed on gestation day 21
for examination of the uterine contents. Additional control groups and
animals at 100 mg/kg bw per day were killed on gestation day 22 to
determine whether the absence of renal papillae was a compound-related
effect or an anomaly. As the concentrations of the first few solutions
used in phase I were found to be only 1-19% of the nominal
concentrations and the next analysis which showed 75-110% of the
nominal concentration, was done only on day 7, no definitive
conclusions could be drawn from the results of this part of the study.
Signs of maternal toxicity seen at the highest dose included clinical
signs (chin or perinasal staining and/or wet perineum), reduced
body-weight gain and food consumption during treatment, and increased
absolute and relative liver weights. Embryo- and fetotoxicity were
observed at > 10 mg/kg bw per day, manifested as increased numbers
of stunted fetuses and a higher incidence of visceral anomalies (small
renal papillae and distended ureter). Additional embryo- and
fetotoxicity seen at the highest dose included an increased incidence
of median or late resorptions and a decrease in the mean number of
live fetuses per litter. Treatment-related malformations (absence of
renal papillae) were observed in three fetuses from two litters of
dams at 100 mg/kg bw per day. In phase II of the experiment, dams were
permitted to deliver naturally and to raise their litters to weaning.
All dams and pups were sacrificed on day 21 of lactation and subjected
to gross necropsy. The solutions were found to be adequately prepared
in this phase. No treatment-related maternal toxicity was evident at
doses > 10 mg/kg bw per day. At the next dose, 100 mg/kg bw per
day, increased mortality (5/22; 0/22 in controls), clinical signs of
difficult parturition (pallor, bunching, weakness, and/or dystocia
during parturition and lactation in four dams), reduced body-weight
gain and food consumption during the early part of treatment, and
increased liver weight were observed. No treatment-related embryo-
or fetotoxicity was noted at doses > 2 mg/kg bw per day. At
> 10 mg/kg bw per day, there were overt, dose-related increases in
mean gestational length (22.8, 23.1, 22.9, 23.1, 23.5, and 24.7 days
at 0, 0.2, 0.4, 2, 10, and 100 mg/kg bw per day, respectively),
decreases in mean litter size and the number of liveborn fetuses per
litter, an increased number of small litters (< 10 fetuses per
litter), and a higher incidence of dilated renal pelvis and/or ureter
in pups at weaning. Additional signs of treatment-related embryo- and
fetotoxicity at the highest dose included a reduced total number of
live litters, an increased mean number of dead fetuses per litter, and
reduced viability indexes (days 0-4; 82%; 98-99% in control and lower
dose groups). There were no treatment-related malformations in the
live pups examined. Of the 42 pups found dead, 29 were in the group at
100 mg/kg bw per day; two had no renal papillae and four had small
papillae. The NOAEL for maternal toxicity was 10 mg/kg bw per day,
that for embryo- and fetotoxicity was 2 mg/kg bw per day, and that for
teratogenicity was 100 mg/kg bw per day, the highest dose tested
(Alvarez et al., 1985a).
Rabbits
Four groups of 18 artificially inseminated female New Zealand
white rabbits received technical-grade flusilazole (purity, 96.5%) in
corn oil by gavage at nominal doses of 0, 2, 5, or 12 mg/kg bw per
day, equal to 0, 1.9, 4.8, and 10.1 mg/kg bw per day on the basis of
analysis of the solutions, on days 7-19 of gestation; the day of
insemination was designated gestation day 0. On gestation day 29, all
surviving does were sacrificed and necropsied; fetuses were delivered
by caesarean section and examined for external, visceral, and skeletal
abnormalities. There was no treatment-related mortality, no clinical
signs of maternal toxicity, and no disturbances of intrauterine
development of the conceptuses at any dose up to and including
12 mg/kg bw per day, the highest dose tested. All of the fetuses
delivered showed normal development. No evidence of fetotoxicity and
no treatment-related increases in malformations were observed in
fetuses at any dose. The higher incidence of hydrocephalus observed in
this study (one fetus in one litter, two in one litter, four in two
litters, and four in three litters at 0, 2, 5, and 12 mg/kg bw per
day, respectively), which was not confirmed in three subsequent
studies (in only one fetus at 35 mg/kg bw per day and in none at lower
doses or in controls), was not considered to be treatment-related. The
NOAEL for maternal toxicity, embryo- and fetotoxicity and
teratogenicity was 12 mg/kg bw per day (10.1 mg/kg bw per day by
analysis) (Solomon et al., 1984).
Three groups of 20 artificially inseminated female New Zealand
white rabbits received technical-grade flusilazole (purity, 96.5%) by
gavage at nominal doses of 0, 12, or 35 mg/kg bw per day, equal to 0,
11.2 and 31.5 mg/kg bw per day on the basis of analysis of the
solutions, on days 7-19 of gestation; the day of insemination was
designated gestation day 0. On gestation day 29, all surviving does
were killed and necropsied; fetuses were delivered by caesarean
section and examined by gross pathology. No evidence of maternal, or
embryo- or fetotoxicity was observed at the low dose, and the
incidence of malformations and fetal variations was not increased in
comparison with the controls. At the high dose, there was an increased
incidence of red vaginal discharge and stained tail and an increased
incidence of periodic anorexia. Two of 13 treated does and 0/16
controls aborted, and 10 of 13 had early total resorption in
comparison with 1/16 controls. Teratogenicity could not be assessed at
this dose because only one live litter was produced. The NOAEL for
maternal, embryo-, and fetotoxicity was 12 mg/kg bw per day
(11.2 mg/kg bw per day by analysis), and that for teratogenicity was
> 12 mg/kg bw per day (11.2 mg/kg bw per day by analysis) (Zellers
et al. 1985).
In a range-finding study, four groups of seven artificially
inseminated female New Zealand white rabbits were fed diets containing
technical-grade flusilazole (purity, 94.8%) at doses of 0, 500, 1000,
or 2000 ppm on days 7-19 of gestation. The pregnancy rate (3/7 per
group) was low at 500 and 1000 ppm, and the incidence of mortality
in utero was high at 2000 ppm (4/7 females with total resorptions).
In the main study, dietary levels of 0, 300, 600, or 1200 ppm
technical-grade flusilazole (purity, 94.8%), equal to 0, 8.9, 21.2,
and 37.8 mg/kg bw per day, were given to groups of 20 artificially
inseminated female New Zealand white rabbits on days 7-19 of
gestation; the day of insemination was designated day 0 of gestation.
All surviving does were sacrificed on gestation day 29 and necropsied;
fetuses were delivered by caesarean section and examined for external,
visceral, and skeletal abnormalities. During treatment with the
highest dose, maternal body weight and food consumption were
decreased. The pregnancy rate was reduced in all treated groups: 9/20,
10/20, and 7/20 at 300, 600, and 1200 ppm, respectively. The number of
does with total resorptions was increased at 600 and 1200 ppm. There
were no treatment-related effects on the mean number of live fetuses
per litter, the mean number of resorptions in dams with live fetuses,
or fetal weight. The small number of litters available from dams at
600 and 1200 ppm (three per group) precluded any definitive assessment
of fetotoxicity or teratogenic potential at these doses. There were no
apparent treatment-related effects at the low dose. In a supplementary
study, dietary levels of 0, 30, 100, or 300 ppm, equal to 0, 0.81,
2.84, and 8.32 mg/kg bw per day, of technical-grade flusilazole were
administered to groups of 18 or 25 (300 ppm) inseminated female New
Zealand white rabbits on days 7-19 of presumed gestation. The
pregnancy rate was again low in all groups, including the controls
(8/18). Total resorption occurred at 0 and 300 ppm in 25 and 29%,
respectively, of the pregnant does, but not at the low or mid-dose.
Because of the small number of live litters available for examination,
the results could not be adequately assessed for embryo- or
fetotoxicity or teratogenic potential. The NOAEL for maternal toxicity
was 21.2 mg/kg bw per day, based on decreased body weight and food
consumption at 1200 ppm. There was no NOAEL for embryo- or
fetotoxicity or teratogenicity (Alvarez et al., 1985b).
Four groups of 18 artificially inseminated female New Zealand
white rabbits received technical-grade flusilazole (purity, 93.8%) in
0.5% methylcellulose by gavage at doses of 0, 7, 15, or 30 mg/kg bw
per day on days 7-19 of gestation. The pregnancy rate was acceptable
in all groups: 12/18, 14/18, 16/18, and 16/18 at 0, 7, 15, and
30 mg/kg bw per day, respectively. No treatment-related maternal,
embryo- or fetotoxicity was seen at the low dose. At the two higher
doses, clinical signs of maternal toxicity (red discharge and
brown-yellow-stained tail) and increased incidences of abortion (one
dam per group) and total resorptions (4/16 and 12/16, respectively)
were observed. At the highest dose, food consumption was also
decreased. The mean numbers of liver fetuses per litter and of dead
fetuses, mean fetal weight and mean male:female ratio were comparable
in the control and treated groups. No treatment-related external,
visceral, or skeletal malformation or variation was observed in
fetuses of does at any dose; however, it should be noted that
assessment of fetotoxicity and teratogenic potential at the highest
dose was based on data for only three of 11-12 live litters in the
control and lower dose groups. These limited data reduce confidence in
the accuracy of any conclusions drawn on the basis of observations at
this dose. The NOAEL for maternal, embryo-, and fetal toxicity was
7 mg/kg bw per day, and that for teratogenicity was > 15 mg/kg bw
per day (Alvarez, 1900).
(f) Genotoxicity
A battery of studies on technical-grade flusilazole was conducted
to assess its potential to induce gene mutation, chromosomal
aberration, or unscheduled DNA synthesis. The results (summarized in
Table 2) were clearly negative.
(g) Special studies
(i) Dermal and ocular irritation and dermal sensitization
Two male New Zealand white rabbits received 0.01 ml of undiluted
flusilazole (purity, 90%) into the right conjunctival sac; the left
eye was not treated. The right eye of one rabbit was washed with
100 ml of water after 20 sec of exposure. Irritation was graded
according to the Draize scale 1, 4, 24, 48, and 72 h after
instillation. Both animals had mild conjunctival redness and chemosis
1-4 h after instillation. The washed eye also showed some discharge at
1 h, but by 24 h these effects were no longer observed. The unwashed
eye showed slight corneal opacity at 1-4 h, and biomicroscopic
examination revealed slight cloudiness at 24-48 h; the eye was normal
at 72 h. Flusilazole was thus minimally irritating to the eyes of male
New Zealand white rabbits, causing transient, mild irritation.
Flushing the eye with water after exposure eliminated the corneal but
not the conjunctival effects (Hall et al., 1984).
In a study of primary skin irritation, six young male New Zealand
white rabbits received a flusilazole formulation (Nustar 20DF,
containing 21% technical-grade flusilazole and 79% unspecified, inert
ingredients; undiluted, moistened with distilled water) topically on
shaved skin sites on the back at a concentration of about 0.5 g. The
test site was covered with a gauze patch and a sheet of rubber for
4 h; the coverings were then removed and the site washed gently with
warm water. Each site was evaluated for irritation potential according
to the Draize scale 4, 24, 28, and 72 h after treatment. At 4 h, grade
1 erythema was observed in four rabbits and grade 1 or 2 oedema in two
rabbits. By 24 h, only two rabbits had erythema and no oedema was
observed. No signs of dermal irritation were seen in any rabbit at
72 h. The primary irritation index was 0.167. Flusilazole as
formulated for this test was thus minimally irritating to the skin
(Brock, 1988).
Table 2. Results of tests for the genotoxicity of flusilazole
End-point Test system Concentration Purity Results Reference
or dose (%)
In vitro
Reverse mutation Salmonella typhimurium TA98, 1-250 µg/plate 90.0 Negativea Donovan & Irr (1982)
TA100, TA1535, TA1537
Reverse mutation Salmonella typhimurium TA97, 5-250 µg/plate 97.7 Negativea Arce et al. (1988)
TA98, TA100, TA1535
Reverse mutation Salmonella typhimurium TA97, 10-300 µg/plate 61.7 Negativea Reynolds (1991)
TA98, TA100, TA1535
hprt forward mutation Chinese hamster ovary cells 0.04-0.275 mmol/litre 95.5 Negative McCooey et al. (1983)
(KI/BH4 clone)
Chromosomal aberration Human lymphocytes 1.7-100 µg/ml 94.9 Negativea Vlachos et al. ( 1986),
Unscheduled DNA Rat primary hepatocytes 1 × 10-5 - 1.1 × 102 95.5 Negative Chromey et al. (1983)
synthesis mmol/litre
In vivo
Chromosomal aberration CrI:CD rat bone marrow 50-500 mg/kg bw NR Negative Farrow et al. (1983)
(single dose by gavage)
Micronucleus formation CD-1 mouse bone marrow 375 mg/kg bw 95.5 Negative Sorg et al. (1984)
(single dose by gavage)
NR, not reported
a With and without exogenous metabolic activation
A range-finding study in young adult Hartley guinea-pigs
indicated mild erythema 24 h after dermal application of undiluted
flusilazole (purity, 90%) in aliquots of 0.05 ml. No dermal irritation
was observed with concentrations > 50%. In a study of primary skin
irritation, flusilazole (purity, 90%) was applied topically to the
skin at concentrations of 5 or 50% (w/v, solution in dimethyl
phthalate), and two test sites per animal were scored for signs of
irritation at 24 and 48 h. No dermal irritation was seen in any of the
10 exposed animals. Flusilazole was thus not irritating to the skin of
guinea-pigs (Wylie el al., 1984c).
In a study of the sensitization of young adult Hartley
guinea-pigs, intradermal injections of a 1% solution (w/v) of
flusilazole (purity, 90%) in methyl phthalate weekly for four weeks
caused erythema and oedema with necrotic centres at the sites of
injection by 24 h. Challenge with topical applications of 5 or 50%
solutions to the skin did not induce sensitization (Wylie el al.,
1984c).
In another study of sensitization, 10 male and 10 female young
adult Duncan Hartley albino guinea-pigs were given three weekly dermal
applications of 0.4 ml (equivalent to 0.192 g) flusilazole (purity,
97.7%). The material, slightly moistened with dimethyl phthalate, was
applied to the intact shaved skin on the back and covered with plastic
wrap for 6 h, and dermal irritation was scored 24 and 48 h after
treatment. None was observed. Two weeks after the last induction, the
animals were challenged with a single dermal application of 0.4 ml of
flusilazole (0.192 g) on an untreated site, which was then covered for
6 h. The irritation response was again scored at 24 and 48 h. No signs
of dermal irritation were observed. A positive control group treated
with 1-chloro-2,4-dinitrobenzene showed severe erythema with necrosis
24 h after the second and third inductions and severe erythema after
the challenge dose two weeks later (Brock et al., 1988).
(ii) Mechanisms of Leydig-cell tumour induction
Groups of 10 Crl:CD BR male rats received technical-grade
flusilazole (purity, 94%) in corn oil by subcutaneous injection at
doses of 0, 20, 50, 150, or 250 mg/kg bw per day (given as two equal
half-doses, twice daily) for 14 days. The control group and that at
250 mg/kg bw per day each included an additional subgroup of 10 male
rats which were treated with human chorionic gonadotropin (hCG) 1 h
before sacrifice. Ketoconazole (a known inhibitor of 17ß-hydroxylase)
was used as the positive control: Groups of 10 male rats were given
subcutaneous injections of 0, 20, 50, 100, or 200 mg/kg bw per day
ketoconazole in saline (as two equal half-doses, twice daily) for 14
days. The control group and that at 200 mg/kg bw per day each included
an additional subgroup of 10 male rats which were treated with hCG 1 h
before sacrifice. All surviving animals were killed on day 15 of
treatment and necropsied. Testicular interstitial fluid and serum
samples were collected from rats that did not receive hCG; the
interstitial fluid was analysed for testosterone and the serum samples
for testosterone, estradiol, luteinizing hormone, and follicle-
stimulating hormone. The serum samples collected from the hCG-treated
rats were analysed for testosterone, androstenedione, 17ß-hydroxy-
progesterone, and progesterone. Increased absolute and relative liver
weights and a dose-related inhibition of serum testosterone levels
(statistically significant at doses > 150 mg/kg bw per day) and
estradiol (statistically significant at all doses) were observed at
doses > 20 mg/kg bw per day. At the two highest doses, clinical
symptoms of toxicity (sores, stained or wet fur, dehydration, and
diarrhoea), decreased body weight and body-weight gain, and reduced
food consumption were evident; eight of 10 rats at the highest dose
died. The rats at 250 mg/kg bw per day that were treated with hCG had
significantly lower serum testosterone levels than controls; no other
significant differences in hormone levels were noted. The positive
controls had significantly lower levels of testosterone,
androstenedione, and 17ß-hydroxyprogesterone and a higher level of
progesterone, indicating inhibition of 17ß-hydroxylase. There was no
NOAEL (Cook, 1993).
Leydig cells were collected from rat testes at termination of the
above study and cultured in microplate wells; the cells were then
incubated with either technical-grade flusilazole or ketoconazole at
doses of 0.05-100 µmol/litre in 70% ethanol for 2 h. The culture media
were sampled and analysed for testosterone, androstenedione,
17ß-hydroxyprogesterone, and progesterone. The results confirmed the
hormonal changes observed in vivo. Incubation of testicular Leydig
cells with technical-grade flusilazole caused a dose-dependent
lowering of testosterone and androstenedione levels, suggesting
inhibition of the enzymes involved in steroid biosynthesis. The IC50
for testosterone was 3.475 ± 1.455 µmol/litre without hCG and 2.774 ±
0.646 µmol/litre with hCG pretreatment. The positive control had an
IC50 for testosterone of 0.97 ± 0.83 µmol/litre without hCG and 0.154
± 0.065 µmol/litre with hCG (Cook, 1993).
Comments
In rats, orally administered 14C-labelled flusilazole was
readily absorbed and rapidly excreted in the urine and faeces, with
little radiolabel recovered in expired air. Over 90% of the
administered dose was eliminated within 96 h. 14C from phenyl-
labelled material was excreted predominantly in the faeces (87% in
males and 59% in females), while that from triazole-3-labelled
material was recovered primarily in the urine (72% in each sex).
Tissue retention of 14C was low (< 1%, excluding the carcass).
14C-Flusilazole was extensively metabolized in rats. After
absorption, it was cleaved at the triazole ring. Recovered parent
compound accounted for only 2-11% of the administered dose and was
found predominantly in faeces, the urinary level being < 1%.
Flusilazole is slightly toxic to mice, rats, and rabbits when
given as a single oral dose and is minimally toxic to rats and rabbits
when given dermally or by inhalation. The oral LD50 in rats was
> 500 mg/kg bw, the dermal LD50, in rabbits was > 2000 mg/kg bw,
and the inhalation LC50 in rats was 6.8-7.7 mg/litre. Flusilazole was
minimally irritating to the eyes and skin of rabbits; it was virtually
not irritating to the skin and was not a dermal sensitizer in guinea-
pigs. WHO has classified flusilazole as 'slightly hazardous'.
Repeated oral administration of flusilazole to mice (90 days),
rats (90 days), and dogs (90 days and one year) resulted primarily in
lesions of the liver (hepatocellular hypertrophy, hyperplasia and
vacuolation) and urinary bladder (urothelial hyperplasia and
vacuolation). On the basis of the hepatic and/or urinary bladder
changes, the NOAEL was 25 ppm (equal to 4 mg/kg bw per day) in mice,
125 ppm (equal to a mean of 8.1 mg/kg bw per day in two 90-day
studies) in rats, and 5 ppm (equal to 0.14 mg/kg bw per day) in dogs
(one-year study). Repeated dermal application of flusilazole to
rabbits for 21 days did not result in treatment-related systemic
toxicity at doses up to and including 200 mg/kg bw per day.
In two 18-month studies of carcinogenicity, flusilazole was
administered at dietary concentrations of 0, 5, 25, or 200 ppm to mice
of each sex in the first study and at 0, 100, 500, or 1000 ppm to
males and 0, 100, 1000, or 2000 ppm to females in the second study.
The overall NOAEL for the two studies was 25 ppm (equal to 3.4 mg/kg
bw per day) on the basis of hepatotoxicity and urinary bladder
hyperplasia in males at 100 ppm. In the second study, an increased
incidence of hepatocellular adenomas and carcinomas was observed in
males at > 100 ppm (equal to 14 mg/kg bw per day) and in females at
> 1000 ppm (equal to 200 mg/kg bw per day), doses at which lesions
of the liver (focal necrosis, hepatocellular hypertrophy and
hyperplasia, and vacuolation) were seen. Flusilazole was carcinogenic
in the second study.
In two two-year studies of toxicity and carcinogenicity, rats
received flusilazole at dietary concentrations of 0, 10, 50, or
250 ppm (first study) or 0, 125, 375, or 750 ppm (second study). In
the first study, the NOAEL was 10 ppm (equal to 0.4 mg/kg bw per day)
on the basis of mild nephrotoxicity (pyelonephritis in females and
hydronephrosis in males) at 50 ppm. In the second study, an increased
incidence of urinary bladder transitional-cell papillomas and
carcinomas in rats of each sex and testicular Leydig-cell tumours in
males were observed at 750 ppm (equal to 31 mg/kg bw per day), doses
at which lesions of the urinary bladder (urothelial necrosis,
exfoliation, and hyperplasia) were clearly demonstrated. Flusilazole
was carcinogenic in the second study.
A special two-week study to investigate the possible mechanism
by which testicular Leydig-cell tumours are induced was conducted
in rats. Flusilazole caused a dose-dependent lowering of serum
testosterone and estradiol levels at > 20 mg/kg bw per day in
vivo and a dose-related decrease in testosterone and androstenedione
production in cultured testicular Leydig cells by inhibiting enzymes
involved in steroid biosynthesis.
In a two-generation study of reproductive toxicity, rats were fed
diets containing flusilazole at concentrations of 0, 5, 50, or
250 ppm. The NOAEL for parental systemic toxicity was 5 ppm (equal to
0.34 mg/kg bw per day) on the basis of hepatic lesions at 50 ppm. The
NOAEL for reproductive toxicity was 50 ppm (equal to 4.0 mg/kg bw per
day) on the basis of treatment-related mortality during parturition,
increased length of gestation, reduced numbers of liveborn pups per
litter, and decreased pup growth at 250 ppm.
Three studies of developmental toxicity were performed in rats
with doses of 0, 10, 50, or 250 mg/kg bw per day in the first study,
0, 0.4, 2, 10, 50, or 250 mg/kg bw per day in the second study, and 0,
0.2, 0.4, 2, 10 or 100 mg/kg bw per day in the third study. A study of
developmental toxicity was also conducted in which rats were fed
flusilazole at dietary levels of 0, 50, 100, 300, or 900 ppm (equal to
0, 4.6, 9, 27, and 79 mg/kg bw per day) on days 7-16 of gestation. The
NOAEL for maternal toxicity was 10 mg/kg bw per day, on the basis of a
slight reduction in body-weight gain and food consumption during
treatment at > 27 mg/kg bw per day. The NOAEL for embryo- and
fetotoxicity was 4.6 mg/kg bw per day on the basis of increased
resorption, increased length of gestation, reduced litter size and a
higher incidence of skeletal variations or anomalies at > 9 mg/kg
bw per day. At 250 mg/kg bw per day, an increased incidence of cleft
palate was observed. The NOAEL for teratogenicity was 100 mg/kg bw per
day.
In three studies of developmental toxicity in rabbits, animals
were treated with flusilazole at 0, 2, 5, or 12 mg/kg bw per day
(first study), 0, 12, or 35 mg/kg bw per day (second study), or 0, 7,
15, or 30 mg/kg bw per day (third study) on days 7-19 of gestation.
The NOAEL for maternal and embryo- or fetotoxicity was 12 mg/kg bw per
day on the basis of clinical signs of toxicity and an increased
incidence of abortion and total resorptions at > 15 mg/kg bw per
day. There was no evidence of teratogenicity at doses up to and
including 15 mg/kg bw per day, the highest dose at which an adequate
number of live litters was available for an assessment of
teratogenicity.
Flusilazole has been adequately tested for genotoxicity in a
series of assays in vivo and in vitro. The Meeting concluded that
flusilazole is not genotoxic.
An ADI of 0-0.001 mg/kg bw was established on the basis of the
NOAEL of 5 ppm, equal to 0.14 mg/kg bw per day, in the one-year
dietary study in dogs, and a safety factor of 100.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 25 ppm, equal to 3.4 mg/kg bw per day (18-month study of
toxicity and carcinogenicity)
Rat: 10 ppm, equal to 0.4 mg/kg bw per day (two-year study of
toxicity and carcinogenicity)
5 ppm, equal to 0.34 mg/kg bw per day (maternal toxicity in
a two-generation study of reproductive toxicity)
50 ppm, equal to 4 mg/kg bw per day (two-generation study of
reproductive toxicity)
4.6 mg/kg bw per day (embryo- or fetotoxicity in a study of
developmental toxicity)
100 mg/kg bw per day (teratogenicity in a study of
developmental toxicity)
Rabbit: 12 mg/kg bw per day (maternal and embryo- or fetal toxicity
in a study of developmental toxicity)
15 mg/kg bw per day (teratogenicity in a study of
developmental toxicity)
Dog: 5 ppm, equal to 0.14 mg/kg bw per day (one-year study of
toxicity)
Estimate of acceptable daily in take for humans
0-0.001 mg/kg bw
Studies that would provide information useful for continued
evaluation of the compound
Observations in humans
Toxicological criteria for setting guidance values for dietary and non-dietary exposure to flusilazole
Exposure Relevant route, study type, species Result/remarks
Short-term (1-7 days) Dermal, irritation, rabbit Minimally irritating
Eye, irritation, rabbit Minimally irritating
Skin, sensitization, guinea-pig Not a skin sensitizer
Oral, toxicity, rat LD50 > 500 mg/kg bw
Dermal, toxicity, rabbit LD50 > 2000 mg/kg bw
Inhalation, 4 h, toxicity rat LC50 = 6.8-7.7 mg/litre
Medium-term (1-26 weeks) Repeated dermal, 21--day, toxicity, rabbit NOAEL = 200 mg/kg bw per day, highest
dose tested for systemic toxicity
Repeated dietary, reproductive toxicity, rat NOAEL = 0.34 mg/kg bw per day; hepatic
toxicity
Repeated gavage, developmental toxicity, NOAEL = 7 mg/kg bw per day for
rabbit maternal, embryo-, or fetotoxicity;
NOAEL > 15 mg/kg bw per day for
teratogenicity
Long-term (> one year) Repeated dietary, one year, toxicity, dog NOAEL = 0.14 mg/kg bw per day, primarily
for liver toxicity
References
Alvarez, L. (1990) Teratogenicity study of DPX-H6573-66 in rabbits.
Unpublished report No. HLR 216-90 from Haskell Laboratory for
Toxicology and Industrial Medicine, Wilmington, Delaware, USA.
Submitted to WHO by DuPont de Nemours and Co., Inc., Wilmington,
Delaware, USA.
Alvarez, L., Krauss, W.C. & Staples, R.E. (1984) Developmental
toxicity study in rats given INH-6573-66 in the diet on days 7-16
of gestation. Unpublished report No. HLR 431-84 from Haskell
Laboratory for Toxicology and Industrial Medicine, Wilmington,
Delaware, USA. Submitted to WHO by DuPont de Nemours and Co.,
Inc., Wilmington, Delaware, USA.
Alvarez, L., Staples, R.E. & Kaplan, A.M. (1985a) INH-6573: Prenatal
and postnatal toxicity study in rats dosed by gavage on days 7-16
of gestation. Unpublished report No. HLR 654-85 from Haskell
Laboratory for Toxicology and Industrial Medicine, Wilmington,
Delaware, USA. Submitted to WHO by DuPont de Nemours and Co..
Inc., Wilmington, Delaware, USA.
Alvarez, L., Staples, R.E., Driscoll, C.D. & Kaplan, A.M. (1985b)
INH-6573: Developmental toxicity study in rabbits treated by diet
on days 7-19 of gestation. Unpublished report No. HLR 337-85 from
Haskell Laboratory for Toxicology and Industrial Medicine,
Wilmington, Delaware, USA. Submitted to WHO by DuPont de Nemours
and Co., Inc., Wilmington, Delaware, USA.
Anderson, J.J., Stadalius, M.A. & Schlueter, D.D. (1986) Metabolism of
14C-DPX-H6573 in rats. Unpublished report No. AMR-196-84 from
DuPont de Nemours and Co., Inc., Wilmington, Delaware, USA.
Submitted to WHO by DuPont de Nemours and Co., Inc., Wilmington,
Delaware, USA.
Arce, G.T., Matarese, C.C. & Sarrif, A.M. (1988) Mutagenicity testing
of INH-6573-21 in Salmonella typhimurium plate incorporation
assay. Unpublished report No. HLR 59-88 from Haskell Laboratory
for Toxicology and Industrial Medicine, Wilmington, Delaware,
USA. Submitted to WHO by DuPont de Nemours and Co., Inc,
Wilmington, Delaware, USA.
Brock, W.J. (1988) Primary dermal irritation study with INH-6573-106
in rabbits. Unpublished report No. HLR 484-88 from Haskell
Laboratory for Toxicology and Industrial Medicine, Wilmington,
Delaware, USA. Submitted to WHO by DuPont de Nemours and Co.,
Inc., Wilmington, Delaware, USA.
Brock, W.J., Rickard, R.W., Kaplan, A.M. & Gibson, JR. (1985) Long-
term feeding study in mice with INH-6573. Unpublished report No.
HLR 278-85 from Haskell Laboratory for Toxicology and Industrial
Medicine, Wilmington, Delaware, USA. Submitted to WHO by DuPont
de Nemours and Co., Inc., Wilmington, Delaware, USA.
Brock, W.J., Vick, D.A. & Chromey, N.C. (1988) Closed-patch repeated
insult dermal sensitization study (Buehler method) with
INH-6573-21 in guinea pigs. Unpublished report No. HLR 34-88 from
Haskell Laboratory for Toxicology and Industrial Medicine,
Wilmington, Delaware, USA. Submitted to WHO by DuPont de Nemours
and Co., Inc., Wilmington, Delaware, USA.
Cheng, T. (1986) Rat metabolism, study of [triazole-3-14C] DPX-H6573.
Unpublished report No. HLR 337-85 from Haskell Laboratory for
Toxicology and Industrial Medicine, Wilmington, Delaware, USA.
Submitted to WHO by DuPont de Nemours and Co., Inc., Wilmington,
Delaware, USA.
Chromey, N.C., Horst, A.L., McCooey, K.T. & Sarrif. A.M. (1983)
Unscheduled DNA synthesis/rat hepatocytes in vitro. Unpublished
report No. HLR 209-83 from Haskell Laboratory for Toxicology and
Industrial Medicine, Wilmington, Delaware, USA. Submitted to WHO
by E.I. du Pont de Nemours & Co., Inc., Wilmington. Delaware,
USA.
Cook, J. (1993) Mechanisms of rat Leydig cell tumor induction by
DPX-H6573-193 (Flusilazole). Unpublished report No. HLR 410-93
from Haskell Laboratory for Toxicology and Industrial Medicine,
Wilmington, Delaware, USA. Submitted to WHO by E.I. du Pont de
Nemours & Co., Inc., Wilmington, Delaware, USA.
Donovan, S.M. & Irr, J.D. (1982) Mutagenicity evaluation in
Salmonella typhimurium. Unpublished report No. HLR 611-82 from
Haskell Laboratory for Toxicology and Industrial Medicine,
Wilmington, Delaware, USA. Submitted to WHO by E.I. du Pont de
Nemours & Co., Inc., Wilmington, Delaware, USA.
Farrow, M.G., Cortina, T. & Padilla-Nash, H. (1983) In vivo bone
marrow chromosome study in rats -- H # 14,728. Unpublished report
No. 201-636 from Hazleton Laboratories America, Inc., Virginia,
USA. Submitted to WHO by E.I. du Pont de Nemours & Co., Inc.,
Wilmington, Delaware, USA.
Gargus, J.L. & Sutherland, J.D. (1983) Acute skin absorption LD50
test on rabbits. Unpublished report No. 288-83 from Hazelton
Laboratories America Inc., Virginia, USA. Submitted to WHO by
E.I. du Pont de Nemours & Co., Inc., Wilmington, Delaware, USA.
Hall, J.A., Dashiell, O.L. & Kennedy, G.L. (1984) Eye irritation test
in rabbits. Unpublished report No. HLR 582-82 from Haskell
Laboratory for Toxicology and Industrial Medicine, Wilmington,
Delaware, USA. Submitted to WHO by E.L du Pont de Nemours & Co.,
Inc., Wilmington, Delaware. USA.
Keller, D.A. (1990) Subchronic oral toxicity: 90-day study with
DPX-H6573-193 feeding study in mice. Unpublished report No. HLR
60-90 from Haskell Laboratory for Toxicology and Industrial
Medicine, Wilmington, Delaware, USA. Submitted to WHO by E.I. du
Pont de Nemours & Co., Inc., Wilmington, Delaware, USA.
Keller, D.A. (1992a) Mechanism of toxicity: 90-day feeding study in
rats with DPX-H6573-194 (Flusilazole). Unpublished report No. HLR
628-92 from Haskell Laboratory for Toxicology and Industrial
Medicine, Wilmington, Delaware, USA. Submitted to WHO by E.I. du
Pont de Nemours & Co., Inc., Wilmington, Delaware, USA.
Keller, D.A. (1992b) Oncogenicity study with DPX-H6573-193
(Flusilazole): 18-month feeding study in mice. Unpublished report
No. HLR 35-92 from Haskell Laboratory for Toxicology and
Industrial Medicine, Wilmington, Delaware, USA. Submitted to WHO
by E.I. du Pont de Nemours & Co., Inc., Wilmington, Delaware,
USA.
Keller, D.A. (1992c) Oncogenicity study with DPX-H6573-194
(Flusilazole): 2-year feeding study in rats. Unpublished report
No. HLR 527-92 from Haskell Laboratory for Toxicology and
Industrial Medicine, Wilmington, Delaware, USA. Submitted to WHO
by E.I. du Pont de Nemours & Co., Inc., Wilmington, Delaware,
USA.
Lamontia, C.L., Staples, R.E. & Alvarez, L. (1984a) Embryo-fetal
toxicity and teratogenicity study of INH-6573-39 by gavage in the
rat Unpublished report No. HLR 444-83 from Haskell Laboratory for
Toxicology and Industrial Medicine, Wilmington, Delaware, USA.
Submitted to WHO by E.I. du Pont de Nemours & Co., Inc.,
Wilmington, Delaware, USA.
Lamontia, C.L., Staples, R.E. & Alvarez, L. (1984b) Embryo-fetal
toxicity and teratogenicity study of INH-6573-39 by gavage in the
rat. Unpublished report No. HLR 142-84 from Haskell Laboratory
for Toxicology and Industrial Medicine. Wilmington, Delaware,
USA. Submitted to WHO by E.I. du Pont de Nemours & Co., Inc.,
Wilmington, Delaware, USA.
McCooey, K.T., Chromey, N.C., Sarrif, A.M. & Hemingway, R.E. (1983)
CHO/HGPRT assay for gene mutation. Unpublished report No. HLR
449-83 from Haskell Laboratory for Toxicology and Industrial
Medicine, Wilmington, Delaware, USA. Submitted to WHO by E.I. du
Pont de Nemours & Co. Inc., Wilmington, Delaware, USA.
Mullin, L.S. (1990) Reproductive and fertility effects with
flusilazole: Multigeneration reproduction study in rats.
Unpublished report No. HLR 424-90 from Haskell Laboratory for
Toxicology and Industrial Medicine, Wilmington, Delaware, USA.
Submitted to WHO by E.I. du Pont de Nemours & Co., Inc.,
Wilmington, Delaware, USA.
O'Neal, F.O., Rickard, R.W., Kaplan, A.M. & Gibson, J.R. (1985)
One-year feeding study in dogs with INH-6573. Unpublished report
No. HLR 461-85 from Haskell Laboratory for Toxicology and
Industrial Medicine, Wilmington, Delaware, USA. Submitted to WHO
by E.I. du Pont de Nemours & Co. Inc., Wilmington, Delaware, USA.
Pastoor, T.P., Wood, C.K., Krahn, D.F. & Gibson, J.R. (1983)
Ninety-day feeding and one-generation reproduction study in rats
with silane [bis(4-fluorophenyl)](methyl) (1 H-1,2,4-triazol-
1-ylmethyl) (INH-6573). Unpublished report No. HLR 483-83 from
Haskell Laboratory for Toxicology and Industrial Medicine,
Wilmington, Delaware, USA. Submitted to WHO by E.I. du Pont de
Nemours & Co.. Inc., Wilmington, Delaware, USA.
Pastoor, T.P., Wood, C.K., Drahn, D.F. & Aftosmis, J.G. (1984)
Four-week range finding and ninety-day feeding study in mice with
silane [bis(4-fluorophenyl)](methyl) (1 H-1,2,4-triazol-
1-ylmethyl) (INH-6573). Revised unpublished report No. HLR
341-83 1-83 from Haskell Laboratory for Toxicology and Industrial
Medicine, Wilmington, Delaware, USA. Submitted to WHO by E.I. du
Pont de Nemours & Co., Inc., Wilmington, Delaware, USA.
Pastoor, T.P., Rickard, R.W., Sykes, G.P., Kaplan, A.M. & Gibson, J.R.
(1986) Long-term feeding (combined chronic toxicity/oncogenicity
study) and two-generation, four litter reproduction study in rats
with INH-6573. Unpublished report No. HLR 32-86 from Haskell
Laboratory for Toxicology and Industrial Medicine, Wilmington,
Delaware, USA. Submitted to WHO by E.I. du Pont de Nemours & Co.,
Inc., Wilmington, Delaware, USA.
Poindexter, G.L., Henry, J.E., Kenney, L.A., Burgess, B.A. & Kennedy,
G.L. (1984) Inhalation median lethal concentration (LC50) of
INH-6573-41 by EPA protocol. Unpublished report No. HLR 553-83
from Haskell Laboratory for Toxicology and Industrial Medicine,
Wilmington, Delaware, USA. Submitted to WHO by E.I. du Pont de
Nemours & Co., Inc., Wilmington, Delaware, USA.
Redgate, D., Sarver, J.W. & Chromey, N.C.(1985) Approximate lethal
dose (ALD) of INH-6573-66 in rabbits. Unpublished report No. HLR
54-85 from Haskell Laboratory for Toxicology and Industrial
Medicine, Wilmington, Delaware, USA. Submitted to WHO by E.I. du
Pont de Nemours & Co., Inc., Wilmington, Delaware, Wilmington,
USA.
Reynolds, V. (1991) Mutagenicity testing of DPX-H6573-194 in the
Salmonella typhimurium plate incorporation assay. Unpublished
report No. HLR 33-91 by Haskell Laboratory for Toxicology and
Industrial Medicine, Wilmington, Delaware, USA. Submitted to WHO
by E.I. du Pont de Nemours & Co., Inc., Wilmington, Delaware,
USA.
Rickard, R.W., Wood, C.K., Krahn, D.F. & Aftosmis, J.G. (1983)
Three-month feeding study in dogs with silane [bis(4-
fluorophenyl)](methyl) (1 H-1,2,4-triazol- 1-ylmethyl)
(INH-6573). Unpublished report No. HLR 461-83 from Haskell
Laboratory for Toxicology and Industrial Medicine, Wilmington,
Delaware, USA. Submitted to WHO by E.I. du Pont de Nemours & Co.,
Inc., Wilmington, Delaware, USA.
Sarver, J.W., Vick, D.A., Valentine, R., Chromey, N.C. & Kaplan, A.M.
(1986) Twenty-one dose dermal toxicity study with INH-6573-82 in
rabbits. Unpublished report No. HLR 744-86 from Haskell
Laboratory for Toxicology and Industrial Medicine, Wilmington,
Delaware, USA. Submitted to WHO by E.I. du Pont de Nemours & Co.,
Inc., Wilmington, Delaware, USA.
Solomon, H.M., Alvarez, L., Staples, R.E. & Hamill, J.C. (1984)
Developmental toxicity study in rabbits given INH-6573 by gavage
on days 7-19 of gestation. Unpublished report No. HLR 333-84 from
Haskell Laboratory for Toxicology and Industrial Medicine,
Wilmington, Delaware, USA. Submitted to WHO by E.I. du Pont de
Nemours & Co., Inc., Wilmington, Delaware, USA.
Sorg, R.M., Naismith, R.W. & Mathews, R.J. (1984) Micronucleus test
(MNT)-OECD H # 15,314. Unpublished Haskell report No. HLR 437-84
from Pharmakon Research International. Submitted to WHO by E.I.
du Pont de Nemours & Co., Inc., Wilmington, Delaware, USA.
Turner, R.J., Kinney, L.A. & Chromey, N.C. (1985) Inhalation median
lethal concentration (LC50) of INH-6573 by EPA guidelines.
Unpublished report No. HLR 1-85 by Haskell Laboratory for
Toxicology and Industrial Medicine, Wilmington, Delaware, USA.
Submitted to WHO by E.I. du Pont de Nemours & Co., Inc.,
Wilmington, Delaware, USA.
Vlachos, D.A., Covell, D.L. & Sarrif, A.M. (1986) Evaluation of
INH-6573-82 in the in vitro assay for chromosome aberrations in
human lymphocytes. Unpublished report No. HLR 745-86 from Haskell
Laboratory for Toxicology and Industrial Medicine, Wilmington,
Delaware, USA. Submitted to WHO by E.I. du Pont de Nemours & Co.,
Inc., Wilmington, Delaware, USA.
Wylie, C.N., Burgess, B.A. & Kennedy, G.L. (1983) Acute oral test in
rats. Unpublished report No. HLR 78-83 from Haskell Laboratory
for Toxicology and Industrial Medicine, Wilmington, Delaware,
USA. Submitted to WHO by E.I. du Pont de Nemours & Co., Inc.,
Wilmington, Delaware, USA.
Wylie, C.N., Henry, J.E., Ferenz, R.L., Burgess, B.A. & Kennedy, G.L.
(1984a) Median lethal dose (LD50) in rats -- EPA proposed
guidelines, Newark, Delaware, USA. Unpublished report No. HLR
433-83 by Haskell Laboratory for Toxicology and Industrial
Medicine, Wilmington, Delaware, USA. Submitted to WHO by E.I. du
Pont de Nemours & Co., Inc., Wilmington, Delaware, USA.
Wylie, C.N., Henry, J.E., Dashiell, O.L. & Kennedy, G.L. (1984b)
Primary skin irritation and sensitization test on guinea pigs.
Unpublished report No. HLR 626-82 from Haskell Laboratory for
Toxicology and Industrial Medicine, Wilmington, Delaware, USA.
Submitted to WHO by E.I. du Pont de Nemours & Co., Inc.,
Wilmington, Delaware, USA.
Wylie, C.N., Henry, J.E., Burgess, B.A. & Kennedy, G.L. (1984c)
Ten-dose oral subacute test in rats. Unpublished report No. HLR
78-83 from Haskell Laboratory for Toxicology and Industrial
Medicine, Wilmington, Delaware, USA. Submitted to WHO by E.I. du
Pont de Nemours & Co, Inc., Wilmington, Delaware, USA.
Wylie, C.N., Redgate, D., Warheit, D.B. & Chromey, N.C. (1985)
Approximate lethal dose (ALD) of INH-6573-66 in male and female
mice. Unpublished report No. HLR 531-84 from Haskell Laboratory
for Toxicology and Industrial Medicine, Wilmington, Delaware,
USA. Submitted to WHO by E.I. du Pont de Nemours & Co., Inc.,
Wilmington, Delaware, USA.
Zellers, J.E., Staples, R.E., Alvarez, L. & Kaplan, A.M. (1985)
Developmental toxicity study (supplemental) in rabbits dosed by
gavage on days 7-19 of gestation. Unpublished report No. HLR
669-85, from Haskell Laboratory for Toxicology and Industrial
Medicine, Wilmington, Delaware, USA. Submitted to WHO by E.I. du
Pont de Nemours & Co., Inc., Wilmington, Delaware, USA.
Table 1. Acute toxicity of flusilazole
Species Sex Route LD50 or LC50 Reference
(mg/kg bw or
mg/litre air)
Mouse Male Oral 680 Wylie et al. (1985)
female 1000
Rat Male Oral 1500 Wylie et al. (1983)
Male Oral 1110 Wylie et al. (1984a)
Female 674
Male Inhalation 2.7 Poindexter et al. (1984)
Female 3.7
Male, female Inhalation 6.8-7.7 Turner et al. (1985)
Rabbit Male, female Oral 450 Redgate et al. (1985)
Male, female Dermal > 2000 Gargus & Sutherland (1983)
(b) Short-term toxicity
Mice
Groups of 20 male and 20 female Crl:CD-1 mice were given
technical-grade flusilazole (purity, 96.7%) at dietary levels of 0,
25, 75, 225, 500, or 1000 ppm, equal to 0, 4, 12, 36, 82, or 164 mg/kg
bw per day for males and 0, 5, 15, 43, 92, or 222 mg/kg bw per day for
females, for up to 90 days. Ten mice of each sex per group were
sacrificed after four weeks of treatment; the remaining mice were
killed at the end of the study. No histopathological examination was
conducted on animals killed at four weeks. There were no clinical
symptoms of toxicity and no treatment-related effects on body weight
or food consumption in animals sacrificed at either interval. The
target organs of toxicity were liver and urinary bladder; females were
slightly more sensitive to flusilazole than males. No treatment-
related effects were observed at the lowest dose. At 75 ppm, increased
absolute and relative liver weights and an increased incidence (1/10)
of hepatocellular vacuolar cytoplasmic changes were observed in
females. At > 225 ppm, increased liver weight, dose-related
increases in the incidence of hepatocellular vacuolar cytoplasmic
changes, hepatocellular hypertrophy, and urinary bladder urothelial
cell hyperplasia were evident in animals of each sex. At the highest
dose, absolute and relative kidney weights were decreased in males,
but no treatment-related renal lesions were observed. In addition,
slight decreases in erythroid parameters (haemoglobin, haematocrit,
and erythrocyte counts) were noted in animals of each sex. The NOAEL
was 4 mg/kg bw per day (Pastoor et al., 1984).
Groups of 16 male and 16 female Crl:CD-1(ICR)BR mice were fed
diets containing technical-grade flusilazole (purity, 94%) at doses of
0, 1000, 2500, or 5000 ppm, equal to 0, 161, 436, and 1004 mg/kg bw
per day for males and 0, 239, 601, and 1414 mg/kg bw per day for
females, for at least 90 days. An additional six mice of each sex per
group were assessed for cellular proliferation in the liver and
urinary bladder on days 14 and 106. At the lowest dose, treatment-
related effects included reduced mean body weight and food efficiency
(males only), increased absolute and relative liver weights (animals
of each sex), decreased absolute and relative kidney weights (females
only), and hypertrophy or hyperplasia, cytoplasmic vacuolation, and
inflammation of the liver and urinary bladder (animals of each sex).
At the mid-dose, increased cellular proliferation of the urinary
bladder was also observed in animals of each sex. At the highest dose,
severe body-weight loss occurred in males and reduced body weight and
food efficiency were noted in females. The males at this dose were
sacrificed on day 44 owing to excess mortality and moribund condition.
There was no NOAEL (Keller, 1990).
Rats
Groups of six male Crl:CD rats were given technical-grade
flusilazole (purity, 95.5%) by gavage in corn oil at a dose of 0 or
300 mg/kg bw per day, five days per week for two weeks. Three rats per
group were killed at the end of the treatment period, and the
remaining rats were sacrificed after a two-week recovery period. All
animals were examined histopathologically. One treated animal died
after the fifth dose on day 7. Clinical signs of toxicity (lowered
body weight, alopecia, diarrhoea, stained or wet perineal area,
salivation, and hypersensitivity) were observed in four rats during
the treatment period. Treatment-related histopathological changes were
observed in liver, kidney, urinary bladder, and testis; the lesions
appeared to be less severe in rats killed after the recovery period.
Remarkable histopathological findings in treated rats included
hepatocellular vacuolation (six rats), hyperplasia and vacuolation of
the urinary bladder transitional epithelium (six rats) and renal
pelvis urothelium (two rats), and necrosis and cellular degeneration
of the germinal epithelium of the seminiferous tubules (two rats)
(Wylie et al., 1984b).
Four groups of 10 male and 10 female Charles River CD rats were
fed diets containing technical-grade flusilazole (purity, 96.7%) at
doses of 0, 25, 125, 375, or 750 ppm, equal to 0, 2, 9, 27, and
55 mg/kg bw per day for males and 0, 2, 11, 31, and 70 mg/kg bw per
day for females, for 90 days. No treatment-related effects were
oberved at the two lower doses. At 375 ppm, an increased serum
cholesterol level was seen in animals of each sex and an increased
incidence of mild bladder urothelial hyperplasia in one male and four
females. At the highest dose, bladder urothelial hyperplasia was seen
in five males and eight females. Additional treatment-related changes
included decreased body weight in females, increased absolute and
relative liver weights in animals of each sex, and remarkable
histopathological findings (hepatocellular hypertrophy, mild fatty
changes, and hepatocytolysis) in the livers of five males. The NOAEL
was 9 mg/kg bw per day (Pastoor et al., 1983).
Groups of 52 male and 52 female Crl:CD BR rats were fed diets
containing technical-grade flusilazole (purity, 95%) at doses of 0,
10, 125, 375, or 750 ppm, equal to 0, 0.58, 7.27, 22.1, and 44.7 mg/kg
bw per day for males and 0, 0.74, 9.40, 27.6, and 59.0 mg/kg bw per
day for females, for up to 91 days. Each group was divided into three
subgroups of 20 rats of each sex in order to study possible mechanisms
of action of toxicity in the liver and urinary bladder. Five rats of
each sex were sacrificed on days 7/8, 14, 46, and 91 for assessment of
cellular proliferation and histopathology. For evaluation of
cytochrome P450 and peroxisome proliferation, five rats of each sex
were sacrificed on days 14 and 90 (males) or 15 and 91 (females).
Serum levels of testosterone, estradiol, and luteinizing hormone were
determined in all males sacrificed on days 14 and 90. No adverse
treatment-related effects were observed at the two lower doses. At
125 ppm, absolute and relative liver weights were increased; in the
absence of histopathological changes or any other symptoms of
toxicity, the finding was not considered to be toxicologically
significant. At the two highest doses, liver hypertrophy was observed.
The response was sex-dependent: lamellar bodies and periportal
hypertrophy occurred in males and centrilobular hypertrophy without
lamellar bodies was seen in females. In the urinary bladder,
transitional-cell necrosis, exfoliation and hyperplasia were noted.
Hepatic cytochrome P450 levels were higher than control values, but
there was no evidence of peroxisome proliferation in the livers of
treated rats. No significant changes in the serum levels of
testosterone, estradiol, or luteinizing hormone were found in any of
the males examined. The NOAEL was 7.27 mg/kg bw per day (Keller,
1992a).
Rabbits
Groups of five male and five female New Zealand white rabbits
received dermal applications of technical-grade flusilazole (purity,
94.9%) at doses of 0, 1, 5, 25, or 200 mg/kg bw per day for 21 days.
The test material was applied daily as a paste in distilled water; the
exposure sites were occluded for 6 h and then washed with water. No
evidence of systemic toxicity was observed. The NOAEL for dermal
toxicity was 5 mg/kg bw per day, based on diffuse epidermal
hyperplasia or thickening (slight to mild) at > 25 mg/kg bw per
day. At the highest dose, mild erythema was seen on days 6-12. The
NOAEL for systemic toxicity was > 200 mg/kg bw per day (Sarver
et al., 1986).
Dogs
Groups of four male and four female beagle dogs were fed diets
containing technical-grade flusilazole (purity, 93%) at concentrations
of 0, 25, 125, or 750/500 ppm, equal to 0.9, 4.3, and 13.4 mg/kg bw
per day for males and 0, 0.9, 4.3, and 14.2 mg/kg bw per day for
females, for three months. The high-dose group received 750 ppm
flusilazole in the diet for the initial three weeks, then control diet
for one week, and 500 ppm flusilazole in the diet for the remaining
period. The dose was reduced because of marked body-weight loss and
decreased food intake at 750 ppm. At the lowest dose, there was a
treatment-related increase in the incidence of hyperplasia of the
lymphoid follicles in the pyloric glandular mucosa of the stomach in
males: 0/4, 3/4 3/4, and 4/4 at 0, 25, 125, and 750/500 ppm,
respectively. At 125 ppm, an increased incidence of pyloric granular
mucosa hyperplasia was also observed in females: 0/4, 0/4, 3/4, and
4/4 at 0, 25, 125, and 750/500 ppm, respectively. Higher levels (in
comparison with control values) of alanine aminotransferase activity
and an increased incidence of mild urinary bladder mucosal hyperplasia
were observed in males at 125 ppm and in animals of each sex at the
highest dose. Additional treatment-related effects seen at the highest
dose included clinical signs of toxicity (weakness or tremors) in
animals of each sex, reduced mean body-weight gain in males,
body-weight loss in females, decreased mean food consumption in
animals of each sex, a slight increase in leukocyte and monocyte
counts in males, decreased plasma levels of cholesterol, total
protein, and albumin in animals of each sex, and increased absolute
and relative liver weights in animals of each sex. There was no NOAEL
(Rickard et al., 1983).
Groups of five male and five female beagle dogs were fed
technical-grade flusilazole (purity, 95.8%) in the diet at doses of 0,
5, 20, or 75 ppm, equal to 0, 0.14, 0.7, and 2.4 mg/kg bw per day for
males and 0, 0.14, 0.7, and 2.6 mg/kg bw per day for females, for one
year. No treatment-related effects were observed at the lowest dose.
At the next dose, 20 ppm, the serum albumin level was decreased in
males and there was a dose-related increase in the incidence of
centrilobular hepatocellular hypertrophy in both males (0/5, 0/5, 4/5,
and 5/5) and females (0/5, 0/5, 2/5, and 5/5). More severe gastric
mucosal lymphoid hyperplasia, from minimal at 0 or 5 ppm to mild at
20 ppm, was also noted in males. At the highest dose, additional
treatment-related effects included higher leukocyte counts in animals
of each sex; elevated alkaline phosphatase and lowered cholesterol,
total protein, and albumin levels in males only; and increases in
relative liver (animals of each sex) and kidney weights (females). All
high-dose animals exhibited a greater degree of hepatic centrilobular
inflammatory infiltration, and distinct centrilobular hepatocellular
vacuolation was observed in three males. The lymphoid hyperplasia in
the gastric mucosa was of moderate severity in both males and females.
The NOAEL was 0.14 mg/kg bw per day (O'Neal et al., 1985).
(c) Long-term toxicity and carcinogenicity
Mice
Groups of 80 male and 80 female Crl:CD-1(ICR)BR mice received
technical-grade flusilazole (purity, 96.5%) in the diet at doses of 0,
5, 25, or 200 ppm, equal to 0, 0.66, 3.4, and 27 mg/kg bw per day for
males and 0, 0.92, 4.6, and 36 mg/kg bw per day for females, for 18
months. Each group included 10 mice of each sex that were killed at
six months. There were no treatment-related effects on mortality
(terminal survival rates were 76-86% in males and 57-80% in females),
body weight, food consumption, haematology, or serum chemistry at any
dose. At the highest dose, a toxicologically significant increase in
the incidence of hepatocellular fatty changes was observed, with
terminal incidences of 4/80, 3/80, 10/80, and 40/80 males and 2/80,
4/80, 3/80, and 24/80 females at 0, 5, 25, and 200 ppm. In addition,
there was an increase in absolute and relative liver weights in
animals of each sex, a decrease in absolute kidney weight in females,
and an increase in lymphocytic infiltration in the lung and urinary
bladder in males. There was no treatment-related increase in the
incidence of any tumour type at doses > 27 mg/kg bw per day, the
highest dose tested. The NOAEL for systemic toxicity was 3.4 mg/kg bw
per day (Brock et al., 1985).
Groups of 100 male and 100 female Crl:CD-1(ICR)BR mice received
diets containing technical-grade flusilazole (purity, 94%) at doses of
0, 100, 500, or 1000 ppm in males, equal to 0, 14.3, 73.1, and
144 mg/kg bw per day, and 0, 100, 1000, or 2000 ppm in females, equal
to 0, 19.4, 200, and 384 mg/kg bw per day, for 18 months. An
additional group of 100 mice of each sex was fed a diet containing
25 ppm technical-grade flusilazole (equal to 3.51 mg/kg bw per day for
males and 4.38 mg/kg bw per day for females) and was used to study
cell proliferation. The doses were selected on the basis of the
results of a 90-day study that indicated greater sensitivity of males
to the test compound. At the lowest dose tested, there was a slight
decrease in absolute kidney weight, an increase in focal necrosis of
the liver, and urinary bladder hyperplasia in males. No remarkable
changes were seen in females at this dose. At the two highest doses,
females had increased cellular hyperplasia in the urinary bladder and
urethra, but no focal necrosis of the liver. At these two doses,
additional treatment-related systemic effects included increased
absolute and relative liver weights, lower absolute and relative
kidney weights, increased numbers of foci of hepatocellular
alteration, and an increased incidence of hepatic vesicular or
vacuolar changes with cellular hypertrophy. Significantly higher
mortality occurred at 1000 ppm in males and 2000 ppm in females. In
addition, increased cellular proliferation in the urinary bladder (but
not in the liver) was observed in females at the highest dose. At
terminal sacrifice, an increased incidence of hepatocellular adenomas
and carcinomas was observed in males at all doses (13/80, 23/79,
20/80, and 18/78 at 0, 100, 500, and 1000 ppm, respectively;
historical control range, 6.3-13.8%) and in females at the two highest
doses (1/79, 3/80, 11/77, and 43/76 at 0, 100, 1000, and 2000 ppm,
respectively; historical control range, 0-2.6%). The increase in
tumour incidence was dose-related and statistically significant in
females but not in males. There was no NOAEL for systemic toxicity or
oncogenicity (Keller, 1992b).
Rats
Groups of 70 male and 70 female rats (Crl:CD(SD)BR strain)
received technical-grade flusilazole (purity, 95.6%) in the diet at
doses of 0, 10, 50, or 250 ppm, equal to 0, 0.4, 2.0, and 10 mg/kg bw
per day for males and 0, 0.5, 2.6, and 13 mg/kg bw per day for
females, for two years. Each group included two groups of 10 rats of
each sex that were killed at six months and one year. About 100 days
after the beginning of treatment, 20 rats of each sex in each group
were mated and were returned to the study after weaning of the second
litter. The rats killed at six months were examined for bladder
lesions only. No treatment-related effect on mortality was observed;
the survival rate was > 50% in all groups until week 98. No signs of
systemic toxicity was seen at the low dose. At 50 ppm, there was a
dose-related increase in the incidence of pyelonephritis in females at
two years (3/66, 3/62, 8/67, and 10/65 at 0, 10, 50, and 250 ppm,
respectively). Increases in relative liver weight in females and in
the incidence of hydronephrosis in males were observed at interim
sacrifice at one year. At the highest dose, additional treatment-
related changes included an increased incidence of hepatic lesions
(centrilobular hepatocellular hypertrophy and polyploidy) in females
and hydronephrosis in males at one year and an increased incidence of
acidophilic foci of hepatocellular alteration (13/65; 3/66 in
controls) and hepatic diffuse fatty changes (23/65; 9/66 in controls)
in females at two years. No hepatic lesions were observed in males
during the study, and no lesions of the urinary bladder were seen in
animals of either sex at either interim or terminal sacrifice. There
was no treatment-related increase in the incidence of any tumour type
at any dose. At 250 ppm, males had a slightly increased incidence of
squamous-cell carcinomas of the oral and nasal cavities (0/66, 1/63,
0/67, and 3/64 at 0, 10, 50, and 250 ppm, respectively). On the basis
of historical control data, from five two-year feeding studies in rats
with no tumours of this type and one study with an incidence of 2/60,
the incidence of nasal tumours in this study was judged to be
incidental. The NOAEL for systemic toxicity was 0.4 mg/kg bw per day;
however, the effects reported were relatively mild, and higher doses
might have been tolerated (Pastoor et al., 1986).
Groups of 65 male and 65 female Crl:CD(SD)BR rats received diets
containing technical-grade flusilazole (purity, 95%) at doses of 0,
125, 375, or 750 ppm, equal to 0, 5.03, 14.8, and 30.8 mg/kg bw per
day for males and 0, 6.83, 20.5, and 45.6 mg/kg bw per day for
females, for two years. Each group included 10 rats of each sex for
interim sacrifice at one year. Dose-related increases in the incidence
of hepatocellular hypertrophy were observed in animals of each sex at
both interim (males: 0/10, 4/10, 8/10, and 8/10; females: 0/10, 7/10,
10/10, and 10/10; at 0, 125, 375, and 750 ppm, respectively) and
terminal sacrifice (males: 2/53, 2/51, 10/53, and 19/53; females:
2/56, 4/55, 12/53, and 25/54). There was an apparent sex difference in
the hepatic lesions: periportal hepatocellular hypertrophy with
lamellar bodies was seen in males, while females showed centrilobular
hepatocellular hypertrophy with eosinophilic cytoplasm but no lamellar
bodies. At terminal sacrifice, there was also an increased incidence
of mixed foci of cellular alteration in males (6/53, 14/51, 17/53, and
19/53). At 375 and 750 ppm, there were also significant decreases in
terminal body weight in females, increases in absolute and/or relative
liver weights at interim and/or terminal sacrifice in males and/or
females, and an increase in the incidence of urinary bladder
transitional-cell hyperplasia at the end of the study in animals of
each sex (3/46, 6/45, 27/47, and 42/51 in males and 5/47, 3/49, 15/49,
and 33/53 in females). At 750 ppm, additional treatment-related
changes included an increased incidence of hepatic fatty changes in
males and increased cellular proliferation in the liver and urinary
bladder. At the highest dose, a treatment-related increase in the
incidence of urinary bladder transitional-cell papillomas and
carcinomas was observed in animals of each sex at the end of the study
(males: 0/46, 0/45, 1/47, and 5/51; and females: 0/47, 1/49, 0/49, and
13/53; at doses of 0, 125, 375, and 750 ppm, respectively). The
incidence of testicular interstitial-cell (Leydig-cell) tumours in
males at the highest dose was also increased: 2/53, 4/51, 2/53, and
9/53 at 0, 125, 375 and 750 ppm, respectively. There was no NOAEL for
systemic toxicity; the NOAEL for oncogenicity was 14.8 mg/kg bw per
day (Keller, 1992c).
(d) Reproductive toxicity
Rats
Groups of six male and six female Crl:CD(SD)BR rats from a 90-day
feeding study were used in a one-generation study of reproductive
toxicity. The rats were fed daily diets containing technical-grade
flusilazole (purity, 96.7%) at doses of 0, 25, 125, or 375 ppm, equal
to 0, 2, 9, and 27 mg/kg bw per day for males and 0, 2, 11, and
31 mg/kg bw per day for females, for 90 days before mating. Males and
females within the same dose group were mated for 15 days; females
were examined daily for evidence of a copulation plug. After the
mating period, females were housed individually. The fertility index
was low in all groups and especially in the control group (67.7%);
three to six females per group became pregnant. At the highest dose, a
lower gestation index, a lower percentage of liveborn pups, and lower
pup weight at day 4 were observed in comparison with controls (Pastoor
et al., 1983). The small group size and the absence of individual
data on some parameters limit the usefulness of this study for
evaluation of reproductive toxicity.
Groups of 20 male and 20 female Crl:CD(SD)BR rats from a two-year
study of toxicity and oncogenicity were used in a two-generation,
two-litter study. The rats were fed diets containing technical-grade
flusilazole (purity, 95.6%) at doses of 0, 10, 50, or 250 ppm, equal
to 0, 1, 3, and 18 mg/kg bw per day for males and 0, 1, 4, and
20 mg/kg bw per day for females (premating intake) for at least 100
days before mating. Males and females (F0) within the same dose group
were mated 1:1 for 15 days; the females were examined daily for
evidence of a copulation plug. After the mating period, F0 females
were housed individually and allowed to give birth to F1a litters.
About one week after the last F1a litter had been weaned, F0 females
were mated with different F0 males of the same dose group to produce
the F1b litters. After weaning of the F1b litters, 20 rats of each
sex per group were selected as the F1 parents for the F2 generation.
These rats were maintained on the same diets as their F0 parents for
90 days before mating to produce the F2a and F2b litters. Sibling
mating was avoided. The mean body weights of the F1b males at 250 ppm
were decreased during the premating period. No other treatment-related
systemic effect was observed in any F0 or F1 adult. There was no
evidence of a treatment-related effect on mating or fertility.
Treatment-related embryo-, feto-, and litter toxicity were observed at
the mid- (50 ppm) and high doses (250 ppm). An increased number of
stillborn pups and a decreased viability index (days 0-4) occurred in
the F2a litters at 50 ppm and in all litters at 250 ppm. Litter
survival after day 4 was similar in all groups in the F1 and F2
generations. At 250 ppm, the mean weights of F1b and F2b pups of each
sex at weaning were slightly reduced, and the absolute and relative
liver weights of F2b weanling male pups were increased. An increased
incidence of unilateral and/or bilateral hydronephrosis was noted in
F2b female weanlings: 1/10, 4/10, 3/10, and 5/10 at 0, 10, 50, and
250 ppm, respectively. Since neither the severity nor the incidence
of the lesions showed a dose-response relationship and since
hydronephrosis is a common lesion in weanling pups (historical control
range in 13 in-house studies, 0-30%), the finding was judged to be
toxicologically insignificant. The NOAEL was 1 mg/kg bw per day, based
on embryo- and fetotoxicity and decreased viability of pups in the
F2a litters at 50 ppm (Pastoor et al., 1986).
In a two-generation study with one or two litters per generation,
groups of 30 male and 30 female Crl:CD(SD)BR rats were fed diets
containing technical-grade flusilazole (purity, 94%) at doses of 0, 5,
50, or 250 ppm, equal to 0, 0.34, 3.46, and 17.3 mg/kg bw per day for
males and 0, 0.40, 4.04, and 19.6 mg/kg bw per day for females
(premating intake), for 73 days (F0 rats) or 91 days (F1 parents)
before mating. Males and females within the same dose group were
randomly paired 1:1 for 15 days; females were examined daily for
evidence of a copulation plug. One litter (F1a) was produced in the
F0 generation and two litters (F2a and F2b) in the F1 generation.
No treatment-related effects were seen in either the F0 or F1
generation at the low dose. At the middle dose, increased smooth
endoplasmic reticulum was seen in the hepatocytes of males and
centrilobular hepatocellular hypertrophy in females. At the highest
dose, F1 females had slightly but consistently lower body weights.
Reproductive toxicity was indicated by higher mortality during
parturition and increased gestational length of the F0 and F1 dams:
mean, 22.9-23.2 days in comparison with 22.4-22.6 days in controls. No
treatment-related effects on mating or fertility indices were
observed. Embryo-, feto-, and litter toxicity were seen as reduced
number of pups per litter and increased numbers of stillborn pups per
litter in the F1a, F2a, and F2b litters and decreased mean pup
weights on lactation days 14 and 21 (F2a litters only) at 250 ppm.
The NOAEL for systemic toxicity was 0.34 mg/kg bw per day, on the
basis of hepatic lesions at 50 ppm; the NOAEL for reproductive
toxicity was 4.04 mg/kg bw per day, on the basis of treatment-related
mortality during parturition, increased gestational length, and
embryo-, feto-, and litter toxicity at 250 ppm (Mullin, 1990).
(e) Developmental toxicity
Rats
In a range-finding study, groups of seven pregnant rats received
technical-grade flusilazole (purity, 99%) by gavage at doses of 0,
100, or 300 mg/kg bw per day on days 7-16 of gestation. Flusilazole
was maternally and embryotoxic at both doses, and the highest dose
induced cleft palates in about 51% of the fetuses in each litter. In
the main study of developmental toxicity, groups of 25 mated female
Crl:CD(SD)BR rats received technical-grade flusilazole (purity, 95.6%)
by gavage in corn oil at doses of 0, 10, 50, or 250 mg/kg bw per day
on days 7-16 of gestation; the day a copulation plug was observed was
designated as day 1 of gestation. On gestation day 21, all surviving
dams were sacrificed and necropsied; fetuses were delivered by
caesarean section and examined for external, visceral, and skeletal
abnormalities. No treatment-related signs of maternal toxicity were
seen at the lowest dose. At 50 mg/kg bw per day, slight decreases in
body-weight gain and food consumption and a slight increase in the
relative liver weight of dams were seen. At the highest dose,
additional maternal toxic effects included higher mortality and
clinical signs of toxicity (chromodacryorrhoea, chromorhinorrhoea, wet
or stained perineal areas, red vaginal discharges or stains, and focal
alopecia) in 23 animals. Fetuses had treatment-related increases in
the incidence of skeletal anomalies (misaligned sternebrae, extra
ossification centres in ribs, and delayed ossification in sternebrae)
at all doses. At 50 mg/kg bw per day, there was a reduced mean number
of liveborn fetuses per litter, a higher total number of stunted
fetuses (0, 1, 4, and 3 at 0, 10, 50, and 250 mg/kg bw per day,
respectively), and an increased incidence of rudimentary ribs. At the
highest dose, additional signs of embryo- and fetotoxicity were an
increased mean incidence of resorptions, reduced mean fetal weight per
litter, and all increased incidence of extra ribs in the fetuses. An
increased incidence of cleft palate (28/241; 0/331 in controls) and an
absence of renal papillae (21/155; 0/175 in controls) were observed in
fetuses of dams given 250 mg/kg bw per day but not at lower doses. An
unusually high incidence of hydrocephalus and/or dilated lateral
ventricles of the brain was also noted in all groups, including
controls; the effect was not dose-related and was not considered to be
related to treatment. The NOAEL for maternal toxicity was 10 mg/kg bw
per day; there was no NOAEL for embryo- or fetotoxicity; and the NOAEL
for teratogenicity was 50 mg/kg bw per day (Lamontia et al., 1984a).
Groups of 24 mated female Crl:CD(SD)BR rats received technical-
grade flusilazole (purity, 95.6%) by gavage in corn oil at doses of 0,
0.4, 2, 10, 50, or 250 (10 females only) mg/kg bw per day on days 7-16
of gestation; the day a copulation plug was observed was designated
day 1 of gestation. On day 21 of gestation, all surviving dams were
sacrificed and necropsied; fetuses were delivered by caesarean section
and examined for external, visceral, and skeletal abnormalities. No
treatment-related signs of maternal toxicity were observed at doses
> 10 mg/kg bw per day. Slightly reduced body-weight gain and food
consumption during treatment and increased relative liver weights of
dams were seen at 50 mg/kg bw per day. At the highest dose, clinical
signs of maternal toxicity (alopecia, brown stains on the face and
limbs, and stained perineal area) were also observed. No treatment-
related embryo- or fetotoxic effects were seen at the two lowest
doses. At > 10 mg/kg bw per day, an increased number of dams with
median or late resorptions, an increased total number of stunted
fetuses, a higher incidence of visceral (large renal pelvis and small
renal papilla) and skeletal (rib) anomalies, and an increased
incidence of delayed ossification (sternebrae and vertebral arches)
were observed. An increased incidence of cleft palate (21/116; 0/291
in controls) was observed in fetuses of dams receiving 250 mg/kg bw
per day but not at lower doses. Hydrocephalus was not observed. The
NOAEL for maternal toxicity was 10 mg/kg bw per day, that for embryo-
and fetotoxicity was 2 mg/kg bw per day, and that for teratogenicity
was 50 mg/kg bw per day (Lamontia et al., 1984b).
Groups of 24 mated female rats (Crl:CD (SD)BR strain) received
diets containing technical-grade flusilazole (purity, 96.5%) at doses
of 0, 50, 100, 300, or 900 ppm equal to 0, 4.6, 9.0, 26.6, and
79.2 mg/kg bw per day) on days 7-16 of gestation; the day a copulation
plug was observed was designated day 1 of gestation. On gestation day
21, all surviving dams were sacrificed and necropsied; fetuses were
delivered by caesarean section, weighed, sexed, and then examined for
external, visceral, and skeletal abnormalities. No treatment-related
signs of maternal toxicity were seen at doses > 100 ppm.
Significant, dose-related reductions in body-weight gain and food
consumption were observed during treatment at the two highest doses.
Treatment-related embryo- and fetotoxic effects were noted at
> 100 ppm, including an increased incidence of median or late
resorptions, small litters (< 10 fetuses per litter), and significant
dose-related increases in skeletal variations with extra ossification
of the sternebrae. Additional symptoms of fetal toxicity seen at the
two highest doses included an increased number of stunted fetuses, a
higher incidence of rudimentary ribs, extra ossification in cervical
ribs, and delayed ossification of the cervical vertebral arches. None
of the fetuses had cleft palate, and there were no treatment-related
malformations at any dose. The NOAEL for maternal toxicity was
9.0 mg/kg bw per day, that for embryo- and fetotoxicity was 4.6 mg/kg
bw per day, and that for teratogenicity was 79.2 mg/kg bw per day
(Alvarez et al., 1984).
In a study of prenatal and postnatal toxicity in rats, groups of
24 (phase I, prenatal study) or 22 (phase II, postnatal study) mated
female rats (Crl:CD (SD)BR strain) received technical-grade
flusilazole (purity, 96.5%) in 0.5% aqueous methylcellulose by gavage
at doses of 0, 0.2, 0.4, 2, 10, or 100 mg/kg bw per day on days 7-16
of gestation; the day a copulation plug was observed was designed day
1 of gestation. In phase I, dams were sacrificed on gestation day 21
for examination of the uterine contents. Additional control groups and
animals at 100 mg/kg bw per day were killed on gestation day 22 to
determine whether the absence of renal papillae was a compound-related
effect or an anomaly. As the concentrations of the first few solutions
used in phase I were found to be only 1-19% of the nominal
concentrations and the next analysis which showed 75-110% of the
nominal concentration, was done only on day 7, no definitive
conclusions could be drawn from the results of this part of the study.
Signs of maternal toxicity seen at the highest dose included clinical
signs (chin or perinasal staining and/or wet perineum), reduced
body-weight gain and food consumption during treatment, and increased
absolute and relative liver weights. Embryo- and fetotoxicity were
observed at > 10 mg/kg bw per day, manifested as increased numbers
of stunted fetuses and a higher incidence of visceral anomalies (small
renal papillae and distended ureter). Additional embryo- and
fetotoxicity seen at the highest dose included an increased incidence
of median or late resorptions and a decrease in the mean number of
live fetuses per litter. Treatment-related malformations (absence of
renal papillae) were observed in three fetuses from two litters of
dams at 100 mg/kg bw per day. In phase II of the experiment, dams were
permitted to deliver naturally and to raise their litters to weaning.
All dams and pups were sacrificed on day 21 of lactation and subjected
to gross necropsy. The solutions were found to be adequately prepared
in this phase. No treatment-related maternal toxicity was evident at
doses > 10 mg/kg bw per day. At the next dose, 100 mg/kg bw per
day, increased mortality (5/22; 0/22 in controls), clinical signs of
difficult parturition (pallor, bunching, weakness, and/or dystocia
during parturition and lactation in four dams), reduced body-weight
gain and food consumption during the early part of treatment, and
increased liver weight were observed. No treatment-related embryo-
or fetotoxicity was noted at doses > 2 mg/kg bw per day. At
> 10 mg/kg bw per day, there were overt, dose-related increases in
mean gestational length (22.8, 23.1, 22.9, 23.1, 23.5, and 24.7 days
at 0, 0.2, 0.4, 2, 10, and 100 mg/kg bw per day, respectively),
decreases in mean litter size and the number of liveborn fetuses per
litter, an increased number of small litters (< 10 fetuses per
litter), and a higher incidence of dilated renal pelvis and/or ureter
in pups at weaning. Additional signs of treatment-related embryo- and
fetotoxicity at the highest dose included a reduced total number of
live litters, an increased mean number of dead fetuses per litter, and
reduced viability indexes (days 0-4; 82%; 98-99% in control and lower
dose groups). There were no treatment-related malformations in the
live pups examined. Of the 42 pups found dead, 29 were in the group at
100 mg/kg bw per day; two had no renal papillae and four had small
papillae. The NOAEL for maternal toxicity was 10 mg/kg bw per day,
that for embryo- and fetotoxicity was 2 mg/kg bw per day, and that for
teratogenicity was 100 mg/kg bw per day, the highest dose tested
(Alvarez et al., 1985a).
Rabbits
Four groups of 18 artificially inseminated female New Zealand
white rabbits received technical-grade flusilazole (purity, 96.5%) in
corn oil by gavage at nominal doses of 0, 2, 5, or 12 mg/kg bw per
day, equal to 0, 1.9, 4.8, and 10.1 mg/kg bw per day on the basis of
analysis of the solutions, on days 7-19 of gestation; the day of
insemination was designated gestation day 0. On gestation day 29, all
surviving does were sacrificed and necropsied; fetuses were delivered
by caesarean section and examined for external, visceral, and skeletal
abnormalities. There was no treatment-related mortality, no clinical
signs of maternal toxicity, and no disturbances of intrauterine
development of the conceptuses at any dose up to and including
12 mg/kg bw per day, the highest dose tested. All of the fetuses
delivered showed normal development. No evidence of fetotoxicity and
no treatment-related increases in malformations were observed in
fetuses at any dose. The higher incidence of hydrocephalus observed in
this study (one fetus in one litter, two in one litter, four in two
litters, and four in three litters at 0, 2, 5, and 12 mg/kg bw per
day, respectively), which was not confirmed in three subsequent
studies (in only one fetus at 35 mg/kg bw per day and in none at lower
doses or in controls), was not considered to be treatment-related. The
NOAEL for maternal toxicity, embryo- and fetotoxicity and
teratogenicity was 12 mg/kg bw per day (10.1 mg/kg bw per day by
analysis) (Solomon et al., 1984).
Three groups of 20 artificially inseminated female New Zealand
white rabbits received technical-grade flusilazole (purity, 96.5%) by
gavage at nominal doses of 0, 12, or 35 mg/kg bw per day, equal to 0,
11.2 and 31.5 mg/kg bw per day on the basis of analysis of the
solutions, on days 7-19 of gestation; the day of insemination was
designated gestation day 0. On gestation day 29, all surviving does
were killed and necropsied; fetuses were delivered by caesarean
section and examined by gross pathology. No evidence of maternal, or
embryo- or fetotoxicity was observed at the low dose, and the
incidence of malformations and fetal variations was not increased in
comparison with the controls. At the high dose, there was an increased
incidence of red vaginal discharge and stained tail and an increased
incidence of periodic anorexia. Two of 13 treated does and 0/16
controls aborted, and 10 of 13 had early total resorption in
comparison with 1/16 controls. Teratogenicity could not be assessed at
this dose because only one live litter was produced. The NOAEL for
maternal, embryo-, and fetotoxicity was 12 mg/kg bw per day
(11.2 mg/kg bw per day by analysis), and that for teratogenicity was
> 12 mg/kg bw per day (11.2 mg/kg bw per day by analysis) (Zellers
et al. 1985).
In a range-finding study, four groups of seven artificially
inseminated female New Zealand white rabbits were fed diets containing
technical-grade flusilazole (purity, 94.8%) at doses of 0, 500, 1000,
or 2000 ppm on days 7-19 of gestation. The pregnancy rate (3/7 per
group) was low at 500 and 1000 ppm, and the incidence of mortality
in utero was high at 2000 ppm (4/7 females with total resorptions).
In the main study, dietary levels of 0, 300, 600, or 1200 ppm
technical-grade flusilazole (purity, 94.8%), equal to 0, 8.9, 21.2,
and 37.8 mg/kg bw per day, were given to groups of 20 artificially
inseminated female New Zealand white rabbits on days 7-19 of
gestation; the day of insemination was designated day 0 of gestation.
All surviving does were sacrificed on gestation day 29 and necropsied;
fetuses were delivered by caesarean section and examined for external,
visceral, and skeletal abnormalities. During treatment with the
highest dose, maternal body weight and food consumption were
decreased. The pregnancy rate was reduced in all treated groups: 9/20,
10/20, and 7/20 at 300, 600, and 1200 ppm, respectively. The number of
does with total resorptions was increased at 600 and 1200 ppm. There
were no treatment-related effects on the mean number of live fetuses
per litter, the mean number of resorptions in dams with live fetuses,
or fetal weight. The small number of litters available from dams at
600 and 1200 ppm (three per group) precluded any definitive assessment
of fetotoxicity or teratogenic potential at these doses. There were no
apparent treatment-related effects at the low dose. In a supplementary
study, dietary levels of 0, 30, 100, or 300 ppm, equal to 0, 0.81,
2.84, and 8.32 mg/kg bw per day, of technical-grade flusilazole were
administered to groups of 18 or 25 (300 ppm) inseminated female New
Zealand white rabbits on days 7-19 of presumed gestation. The
pregnancy rate was again low in all groups, including the controls
(8/18). Total resorption occurred at 0 and 300 ppm in 25 and 29%,
respectively, of the pregnant does, but not at the low or mid-dose.
Because of the small number of live litters available for examination,
the results could not be adequately assessed for embryo- or
fetotoxicity or teratogenic potential. The NOAEL for maternal toxicity
was 21.2 mg/kg bw per day, based on decreased body weight and food
consumption at 1200 ppm. There was no NOAEL for embryo- or
fetotoxicity or teratogenicity (Alvarez et al., 1985b).
Four groups of 18 artificially inseminated female New Zealand
white rabbits received technical-grade flusilazole (purity, 93.8%) in
0.5% methylcellulose by gavage at doses of 0, 7, 15, or 30 mg/kg bw
per day on days 7-19 of gestation. The pregnancy rate was acceptable
in all groups: 12/18, 14/18, 16/18, and 16/18 at 0, 7, 15, and
30 mg/kg bw per day, respectively. No treatment-related maternal,
embryo- or fetotoxicity was seen at the low dose. At the two higher
doses, clinical signs of maternal toxicity (red discharge and
brown-yellow-stained tail) and increased incidences of abortion (one
dam per group) and total resorptions (4/16 and 12/16, respectively)
were observed. At the highest dose, food consumption was also
decreased. The mean numbers of liver fetuses per litter and of dead
fetuses, mean fetal weight and mean male:female ratio were comparable
in the control and treated groups. No treatment-related external,
visceral, or skeletal malformation or variation was observed in
fetuses of does at any dose; however, it should be noted that
assessment of fetotoxicity and teratogenic potential at the highest
dose was based on data for only three of 11-12 live litters in the
control and lower dose groups. These limited data reduce confidence in
the accuracy of any conclusions drawn on the basis of observations at
this dose. The NOAEL for maternal, embryo-, and fetal toxicity was
7 mg/kg bw per day, and that for teratogenicity was > 15 mg/kg bw
per day (Alvarez, 1900).
(f) Genotoxicity
A battery of studies on technical-grade flusilazole was conducted
to assess its potential to induce gene mutation, chromosomal
aberration, or unscheduled DNA synthesis. The results (summarized in
Table 2) were clearly negative.
(g) Special studies
(i) Dermal and ocular irritation and dermal sensitization
Two male New Zealand white rabbits received 0.01 ml of undiluted
flusilazole (purity, 90%) into the right conjunctival sac; the left
eye was not treated. The right eye of one rabbit was washed with
100 ml of water after 20 sec of exposure. Irritation was graded
according to the Draize scale 1, 4, 24, 48, and 72 h after
instillation. Both animals had mild conjunctival redness and chemosis
1-4 h after instillation. The washed eye also showed some discharge at
1 h, but by 24 h these effects were no longer observed. The unwashed
eye showed slight corneal opacity at 1-4 h, and biomicroscopic
examination revealed slight cloudiness at 24-48 h; the eye was normal
at 72 h. Flusilazole was thus minimally irritating to the eyes of male
New Zealand white rabbits, causing transient, mild irritation.
Flushing the eye with water after exposure eliminated the corneal but
not the conjunctival effects (Hall et al., 1984).
In a study of primary skin irritation, six young male New Zealand
white rabbits received a flusilazole formulation (Nustar 20DF,
containing 21% technical-grade flusilazole and 79% unspecified, inert
ingredients; undiluted, moistened with distilled water) topically on
shaved skin sites on the back at a concentration of about 0.5 g. The
test site was covered with a gauze patch and a sheet of rubber for
4 h; the coverings were then removed and the site washed gently with
warm water. Each site was evaluated for irritation potential according
to the Draize scale 4, 24, 28, and 72 h after treatment. At 4 h, grade
1 erythema was observed in four rabbits and grade 1 or 2 oedema in two
rabbits. By 24 h, only two rabbits had erythema and no oedema was
observed. No signs of dermal irritation were seen in any rabbit at
72 h. The primary irritation index was 0.167. Flusilazole as
formulated for this test was thus minimally irritating to the skin
(Brock, 1988).
Table 2. Results of tests for the genotoxicity of flusilazole
End-point Test system Concentration Purity Results Reference
or dose (%)
In vitro
Reverse mutation Salmonella typhimurium TA98, 1-250 µg/plate 90.0 Negativea Donovan & Irr (1982)
TA100, TA1535, TA1537
Reverse mutation Salmonella typhimurium TA97, 5-250 µg/plate 97.7 Negativea Arce et al. (1988)
TA98, TA100, TA1535
Reverse mutation Salmonella typhimurium TA97, 10-300 µg/plate 61.7 Negativea Reynolds (1991)
TA98, TA100, TA1535
hprt forward mutation Chinese hamster ovary cells 0.04-0.275 mmol/litre 95.5 Negative McCooey et al. (1983)
(KI/BH4 clone)
Chromosomal aberration Human lymphocytes 1.7-100 µg/ml 94.9 Negativea Vlachos et al. ( 1986),
Unscheduled DNA Rat primary hepatocytes 1 × 10-5 - 1.1 × 102 95.5 Negative Chromey et al. (1983)
synthesis mmol/litre
In vivo
Chromosomal aberration CrI:CD rat bone marrow 50-500 mg/kg bw NR Negative Farrow et al. (1983)
(single dose by gavage)
Micronucleus formation CD-1 mouse bone marrow 375 mg/kg bw 95.5 Negative Sorg et al. (1984)
(single dose by gavage)
NR, not reported
a With and without exogenous metabolic activation
A range-finding study in young adult Hartley guinea-pigs
indicated mild erythema 24 h after dermal application of undiluted
flusilazole (purity, 90%) in aliquots of 0.05 ml. No dermal irritation
was observed with concentrations > 50%. In a study of primary skin
irritation, flusilazole (purity, 90%) was applied topically to the
skin at concentrations of 5 or 50% (w/v, solution in dimethyl
phthalate), and two test sites per animal were scored for signs of
irritation at 24 and 48 h. No dermal irritation was seen in any of the
10 exposed animals. Flusilazole was thus not irritating to the skin of
guinea-pigs (Wylie el al., 1984c).
In a study of the sensitization of young adult Hartley
guinea-pigs, intradermal injections of a 1% solution (w/v) of
flusilazole (purity, 90%) in methyl phthalate weekly for four weeks
caused erythema and oedema with necrotic centres at the sites of
injection by 24 h. Challenge with topical applications of 5 or 50%
solutions to the skin did not induce sensitization (Wylie el al.,
1984c).
In another study of sensitization, 10 male and 10 female young
adult Duncan Hartley albino guinea-pigs were given three weekly dermal
applications of 0.4 ml (equivalent to 0.192 g) flusilazole (purity,
97.7%). The material, slightly moistened with dimethyl phthalate, was
applied to the intact shaved skin on the back and covered with plastic
wrap for 6 h, and dermal irritation was scored 24 and 48 h after
treatment. None was observed. Two weeks after the last induction, the
animals were challenged with a single dermal application of 0.4 ml of
flusilazole (0.192 g) on an untreated site, which was then covered for
6 h. The irritation response was again scored at 24 and 48 h. No signs
of dermal irritation were observed. A positive control group treated
with 1-chloro-2,4-dinitrobenzene showed severe erythema with necrosis
24 h after the second and third inductions and severe erythema after
the challenge dose two weeks later (Brock et al., 1988).
(ii) Mechanisms of Leydig-cell tumour induction
Groups of 10 Crl:CD BR male rats received technical-grade
flusilazole (purity, 94%) in corn oil by subcutaneous injection at
doses of 0, 20, 50, 150, or 250 mg/kg bw per day (given as two equal
half-doses, twice daily) for 14 days. The control group and that at
250 mg/kg bw per day each included an additional subgroup of 10 male
rats which were treated with human chorionic gonadotropin (hCG) 1 h
before sacrifice. Ketoconazole (a known inhibitor of 17ß-hydroxylase)
was used as the positive control: Groups of 10 male rats were given
subcutaneous injections of 0, 20, 50, 100, or 200 mg/kg bw per day
ketoconazole in saline (as two equal half-doses, twice daily) for 14
days. The control group and that at 200 mg/kg bw per day each included
an additional subgroup of 10 male rats which were treated with hCG 1 h
before sacrifice. All surviving animals were killed on day 15 of
treatment and necropsied. Testicular interstitial fluid and serum
samples were collected from rats that did not receive hCG; the
interstitial fluid was analysed for testosterone and the serum samples
for testosterone, estradiol, luteinizing hormone, and follicle-
stimulating hormone. The serum samples collected from the hCG-treated
rats were analysed for testosterone, androstenedione, 17ß-hydroxy-
progesterone, and progesterone. Increased absolute and relative liver
weights and a dose-related inhibition of serum testosterone levels
(statistically significant at doses > 150 mg/kg bw per day) and
estradiol (statistically significant at all doses) were observed at
doses > 20 mg/kg bw per day. At the two highest doses, clinical
symptoms of toxicity (sores, stained or wet fur, dehydration, and
diarrhoea), decreased body weight and body-weight gain, and reduced
food consumption were evident; eight of 10 rats at the highest dose
died. The rats at 250 mg/kg bw per day that were treated with hCG had
significantly lower serum testosterone levels than controls; no other
significant differences in hormone levels were noted. The positive
controls had significantly lower levels of testosterone,
androstenedione, and 17ß-hydroxyprogesterone and a higher level of
progesterone, indicating inhibition of 17ß-hydroxylase. There was no
NOAEL (Cook, 1993).
Leydig cells were collected from rat testes at termination of the
above study and cultured in microplate wells; the cells were then
incubated with either technical-grade flusilazole or ketoconazole at
doses of 0.05-100 µmol/litre in 70% ethanol for 2 h. The culture media
were sampled and analysed for testosterone, androstenedione,
17ß-hydroxyprogesterone, and progesterone. The results confirmed the
hormonal changes observed in vivo. Incubation of testicular Leydig
cells with technical-grade flusilazole caused a dose-dependent
lowering of testosterone and androstenedione levels, suggesting
inhibition of the enzymes involved in steroid biosynthesis. The IC50
for testosterone was 3.475 ± 1.455 µmol/litre without hCG and 2.774 ±
0.646 µmol/litre with hCG pretreatment. The positive control had an
IC50 for testosterone of 0.97 ± 0.83 µmol/litre without hCG and 0.154
± 0.065 µmol/litre with hCG (Cook, 1993).
Comments
In rats, orally administered 14C-labelled flusilazole was
readily absorbed and rapidly excreted in the urine and faeces, with
little radiolabel recovered in expired air. Over 90% of the
administered dose was eliminated within 96 h. 14C from phenyl-
labelled material was excreted predominantly in the faeces (87% in
males and 59% in females), while that from triazole-3-labelled
material was recovered primarily in the urine (72% in each sex).
Tissue retention of 14C was low (< 1%, excluding the carcass).
14C-Flusilazole was extensively metabolized in rats. After
absorption, it was cleaved at the triazole ring. Recovered parent
compound accounted for only 2-11% of the administered dose and was
found predominantly in faeces, the urinary level being < 1%.
Flusilazole is slightly toxic to mice, rats, and rabbits when
given as a single oral dose and is minimally toxic to rats and rabbits
when given dermally or by inhalation. The oral LD50 in rats was
> 500 mg/kg bw, the dermal LD50, in rabbits was > 2000 mg/kg bw,
and the inhalation LC50 in rats was 6.8-7.7 mg/litre. Flusilazole was
minimally irritating to the eyes and skin of rabbits; it was virtually
not irritating to the skin and was not a dermal sensitizer in guinea-
pigs. WHO has classified flusilazole as 'slightly hazardous'.
Repeated oral administration of flusilazole to mice (90 days),
rats (90 days), and dogs (90 days and one year) resulted primarily in
lesions of the liver (hepatocellular hypertrophy, hyperplasia and
vacuolation) and urinary bladder (urothelial hyperplasia and
vacuolation). On the basis of the hepatic and/or urinary bladder
changes, the NOAEL was 25 ppm (equal to 4 mg/kg bw per day) in mice,
125 ppm (equal to a mean of 8.1 mg/kg bw per day in two 90-day
studies) in rats, and 5 ppm (equal to 0.14 mg/kg bw per day) in dogs
(one-year study). Repeated dermal application of flusilazole to
rabbits for 21 days did not result in treatment-related systemic
toxicity at doses up to and including 200 mg/kg bw per day.
In two 18-month studies of carcinogenicity, flusilazole was
administered at dietary concentrations of 0, 5, 25, or 200 ppm to mice
of each sex in the first study and at 0, 100, 500, or 1000 ppm to
males and 0, 100, 1000, or 2000 ppm to females in the second study.
The overall NOAEL for the two studies was 25 ppm (equal to 3.4 mg/kg
bw per day) on the basis of hepatotoxicity and urinary bladder
hyperplasia in males at 100 ppm. In the second study, an increased
incidence of hepatocellular adenomas and carcinomas was observed in
males at > 100 ppm (equal to 14 mg/kg bw per day) and in females at
> 1000 ppm (equal to 200 mg/kg bw per day), doses at which lesions
of the liver (focal necrosis, hepatocellular hypertrophy and
hyperplasia, and vacuolation) were seen. Flusilazole was carcinogenic
in the second study.
In two two-year studies of toxicity and carcinogenicity, rats
received flusilazole at dietary concentrations of 0, 10, 50, or
250 ppm (first study) or 0, 125, 375, or 750 ppm (second study). In
the first study, the NOAEL was 10 ppm (equal to 0.4 mg/kg bw per day)
on the basis of mild nephrotoxicity (pyelonephritis in females and
hydronephrosis in males) at 50 ppm. In the second study, an increased
incidence of urinary bladder transitional-cell papillomas and
carcinomas in rats of each sex and testicular Leydig-cell tumours in
males were observed at 750 ppm (equal to 31 mg/kg bw per day), doses
at which lesions of the urinary bladder (urothelial necrosis,
exfoliation, and hyperplasia) were clearly demonstrated. Flusilazole
was carcinogenic in the second study.
A special two-week study to investigate the possible mechanism
by which testicular Leydig-cell tumours are induced was conducted
in rats. Flusilazole caused a dose-dependent lowering of serum
testosterone and estradiol levels at > 20 mg/kg bw per day in
vivo and a dose-related decrease in testosterone and androstenedione
production in cultured testicular Leydig cells by inhibiting enzymes
involved in steroid biosynthesis.
In a two-generation study of reproductive toxicity, rats were fed
diets containing flusilazole at concentrations of 0, 5, 50, or
250 ppm. The NOAEL for parental systemic toxicity was 5 ppm (equal to
0.34 mg/kg bw per day) on the basis of hepatic lesions at 50 ppm. The
NOAEL for reproductive toxicity was 50 ppm (equal to 4.0 mg/kg bw per
day) on the basis of treatment-related mortality during parturition,
increased length of gestation, reduced numbers of liveborn pups per
litter, and decreased pup growth at 250 ppm.
Three studies of developmental toxicity were performed in rats
with doses of 0, 10, 50, or 250 mg/kg bw per day in the first study,
0, 0.4, 2, 10, 50, or 250 mg/kg bw per day in the second study, and 0,
0.2, 0.4, 2, 10 or 100 mg/kg bw per day in the third study. A study of
developmental toxicity was also conducted in which rats were fed
flusilazole at dietary levels of 0, 50, 100, 300, or 900 ppm (equal to
0, 4.6, 9, 27, and 79 mg/kg bw per day) on days 7-16 of gestation. The
NOAEL for maternal toxicity was 10 mg/kg bw per day, on the basis of a
slight reduction in body-weight gain and food consumption during
treatment at > 27 mg/kg bw per day. The NOAEL for embryo- and
fetotoxicity was 4.6 mg/kg bw per day on the basis of increased
resorption, increased length of gestation, reduced litter size and a
higher incidence of skeletal variations or anomalies at > 9 mg/kg
bw per day. At 250 mg/kg bw per day, an increased incidence of cleft
palate was observed. The NOAEL for teratogenicity was 100 mg/kg bw per
day.
In three studies of developmental toxicity in rabbits, animals
were treated with flusilazole at 0, 2, 5, or 12 mg/kg bw per day
(first study), 0, 12, or 35 mg/kg bw per day (second study), or 0, 7,
15, or 30 mg/kg bw per day (third study) on days 7-19 of gestation.
The NOAEL for maternal and embryo- or fetotoxicity was 12 mg/kg bw per
day on the basis of clinical signs of toxicity and an increased
incidence of abortion and total resorptions at > 15 mg/kg bw per
day. There was no evidence of teratogenicity at doses up to and
including 15 mg/kg bw per day, the highest dose at which an adequate
number of live litters was available for an assessment of
teratogenicity.
Flusilazole has been adequately tested for genotoxicity in a
series of assays in vivo and in vitro. The Meeting concluded that
flusilazole is not genotoxic.
An ADI of 0-0.001 mg/kg bw was established on the basis of the
NOAEL of 5 ppm, equal to 0.14 mg/kg bw per day, in the one-year
dietary study in dogs, and a safety factor of 100.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 25 ppm, equal to 3.4 mg/kg bw per day (18-month study of
toxicity and carcinogenicity)
Rat: 10 ppm, equal to 0.4 mg/kg bw per day (two-year study of
toxicity and carcinogenicity)
5 ppm, equal to 0.34 mg/kg bw per day (maternal toxicity in
a two-generation study of reproductive toxicity)
50 ppm, equal to 4 mg/kg bw per day (two-generation study of
reproductive toxicity)
4.6 mg/kg bw per day (embryo- or fetotoxicity in a study of
developmental toxicity)
100 mg/kg bw per day (teratogenicity in a study of
developmental toxicity)
Rabbit: 12 mg/kg bw per day (maternal and embryo- or fetal toxicity
in a study of developmental toxicity)
15 mg/kg bw per day (teratogenicity in a study of
developmental toxicity)
Dog: 5 ppm, equal to 0.14 mg/kg bw per day (one-year study of
toxicity)
Estimate of acceptable daily in take for humans
0-0.001 mg/kg bw
Studies that would provide information useful for continued
evaluation of the compound
Observations in humans
Toxicological criteria for setting guidance values for dietary and non-dietary exposure to flusilazole
Exposure Relevant route, study type, species Result/remarks
Short-term (1-7 days) Dermal, irritation, rabbit Minimally irritating
Eye, irritation, rabbit Minimally irritating
Skin, sensitization, guinea-pig Not a skin sensitizer
Oral, toxicity, rat LD50 > 500 mg/kg bw
Dermal, toxicity, rabbit LD50 > 2000 mg/kg bw
Inhalation, 4 h, toxicity rat LC50 = 6.8-7.7 mg/litre
Medium-term (1-26 weeks) Repeated dermal, 21--day, toxicity, rabbit NOAEL = 200 mg/kg bw per day, highest
dose tested for systemic toxicity
Repeated dietary, reproductive toxicity, rat NOAEL = 0.34 mg/kg bw per day; hepatic
toxicity
Repeated gavage, developmental toxicity, NOAEL = 7 mg/kg bw per day for
rabbit maternal, embryo-, or fetotoxicity;
NOAEL > 15 mg/kg bw per day for
teratogenicity
Long-term (> one year) Repeated dietary, one year, toxicity, dog NOAEL = 0.14 mg/kg bw per day, primarily
for liver toxicity
References
Alvarez, L. (1990) Teratogenicity study of DPX-H6573-66 in rabbits.
Unpublished report No. HLR 216-90 from Haskell Laboratory for
Toxicology and Industrial Medicine, Wilmington, Delaware, USA.
Submitted to WHO by DuPont de Nemours and Co., Inc., Wilmington,
Delaware, USA.
Alvarez, L., Krauss, W.C. & Staples, R.E. (1984) Developmental
toxicity study in rats given INH-6573-66 in the diet on days 7-16
of gestation. Unpublished report No. HLR 431-84 from Haskell
Laboratory for Toxicology and Industrial Medicine, Wilmington,
Delaware, USA. Submitted to WHO by DuPont de Nemours and Co.,
Inc., Wilmington, Delaware, USA.
Alvarez, L., Staples, R.E. & Kaplan, A.M. (1985a) INH-6573: Prenatal
and postnatal toxicity study in rats dosed by gavage on days 7-16
of gestation. Unpublished report No. HLR 654-85 from Haskell
Laboratory for Toxicology and Industrial Medicine, Wilmington,
Delaware, USA. Submitted to WHO by DuPont de Nemours and Co..
Inc., Wilmington, Delaware, USA.
Alvarez, L., Staples, R.E., Driscoll, C.D. & Kaplan, A.M. (1985b)
INH-6573: Developmental toxicity study in rabbits treated by diet
on days 7-19 of gestation. Unpublished report No. HLR 337-85 from
Haskell Laboratory for Toxicology and Industrial Medicine,
Wilmington, Delaware, USA. Submitted to WHO by DuPont de Nemours
and Co., Inc., Wilmington, Delaware, USA.
Anderson, J.J., Stadalius, M.A. & Schlueter, D.D. (1986) Metabolism of
14C-DPX-H6573 in rats. Unpublished report No. AMR-196-84 from
DuPont de Nemours and Co., Inc., Wilmington, Delaware, USA.
Submitted to WHO by DuPont de Nemours and Co., Inc., Wilmington,
Delaware, USA.
Arce, G.T., Matarese, C.C. & Sarrif, A.M. (1988) Mutagenicity testing
of INH-6573-21 in Salmonella typhimurium plate incorporation
assay. Unpublished report No. HLR 59-88 from Haskell Laboratory
for Toxicology and Industrial Medicine, Wilmington, Delaware,
USA. Submitted to WHO by DuPont de Nemours and Co., Inc,
Wilmington, Delaware, USA.
Brock, W.J. (1988) Primary dermal irritation study with INH-6573-106
in rabbits. Unpublished report No. HLR 484-88 from Haskell
Laboratory for Toxicology and Industrial Medicine, Wilmington,
Delaware, USA. Submitted to WHO by DuPont de Nemours and Co.,
Inc., Wilmington, Delaware, USA.
Brock, W.J., Rickard, R.W., Kaplan, A.M. & Gibson, JR. (1985) Long-
term feeding study in mice with INH-6573. Unpublished report No.
HLR 278-85 from Haskell Laboratory for Toxicology and Industrial
Medicine, Wilmington, Delaware, USA. Submitted to WHO by DuPont
de Nemours and Co., Inc., Wilmington, Delaware, USA.
Brock, W.J., Vick, D.A. & Chromey, N.C. (1988) Closed-patch repeated
insult dermal sensitization study (Buehler method) with
INH-6573-21 in guinea pigs. Unpublished report No. HLR 34-88 from
Haskell Laboratory for Toxicology and Industrial Medicine,
Wilmington, Delaware, USA. Submitted to WHO by DuPont de Nemours
and Co., Inc., Wilmington, Delaware, USA.
Cheng, T. (1986) Rat metabolism, study of [triazole-3-14C] DPX-H6573.
Unpublished report No. HLR 337-85 from Haskell Laboratory for
Toxicology and Industrial Medicine, Wilmington, Delaware, USA.
Submitted to WHO by DuPont de Nemours and Co., Inc., Wilmington,
Delaware, USA.
Chromey, N.C., Horst, A.L., McCooey, K.T. & Sarrif. A.M. (1983)
Unscheduled DNA synthesis/rat hepatocytes in vitro. Unpublished
report No. HLR 209-83 from Haskell Laboratory for Toxicology and
Industrial Medicine, Wilmington, Delaware, USA. Submitted to WHO
by E.I. du Pont de Nemours & Co., Inc., Wilmington. Delaware,
USA.
Cook, J. (1993) Mechanisms of rat Leydig cell tumor induction by
DPX-H6573-193 (Flusilazole). Unpublished report No. HLR 410-93
from Haskell Laboratory for Toxicology and Industrial Medicine,
Wilmington, Delaware, USA. Submitted to WHO by E.I. du Pont de
Nemours & Co., Inc., Wilmington, Delaware, USA.
Donovan, S.M. & Irr, J.D. (1982) Mutagenicity evaluation in
Salmonella typhimurium. Unpublished report No. HLR 611-82 from
Haskell Laboratory for Toxicology and Industrial Medicine,
Wilmington, Delaware, USA. Submitted to WHO by E.I. du Pont de
Nemours & Co., Inc., Wilmington, Delaware, USA.
Farrow, M.G., Cortina, T. & Padilla-Nash, H. (1983) In vivo bone
marrow chromosome study in rats -- H # 14,728. Unpublished report
No. 201-636 from Hazleton Laboratories America, Inc., Virginia,
USA. Submitted to WHO by E.I. du Pont de Nemours & Co., Inc.,
Wilmington, Delaware, USA.
Gargus, J.L. & Sutherland, J.D. (1983) Acute skin absorption LD50
test on rabbits. Unpublished report No. 288-83 from Hazelton
Laboratories America Inc., Virginia, USA. Submitted to WHO by
E.I. du Pont de Nemours & Co., Inc., Wilmington, Delaware, USA.
Hall, J.A., Dashiell, O.L. & Kennedy, G.L. (1984) Eye irritation test
in rabbits. Unpublished report No. HLR 582-82 from Haskell
Laboratory for Toxicology and Industrial Medicine, Wilmington,
Delaware, USA. Submitted to WHO by E.L du Pont de Nemours & Co.,
Inc., Wilmington, Delaware. USA.
Keller, D.A. (1990) Subchronic oral toxicity: 90-day study with
DPX-H6573-193 feeding study in mice. Unpublished report No. HLR
60-90 from Haskell Laboratory for Toxicology and Industrial
Medicine, Wilmington, Delaware, USA. Submitted to WHO by E.I. du
Pont de Nemours & Co., Inc., Wilmington, Delaware, USA.
Keller, D.A. (1992a) Mechanism of toxicity: 90-day feeding study in
rats with DPX-H6573-194 (Flusilazole). Unpublished report No. HLR
628-92 from Haskell Laboratory for Toxicology and Industrial
Medicine, Wilmington, Delaware, USA. Submitted to WHO by E.I. du
Pont de Nemours & Co., Inc., Wilmington, Delaware, USA.
Keller, D.A. (1992b) Oncogenicity study with DPX-H6573-193
(Flusilazole): 18-month feeding study in mice. Unpublished report
No. HLR 35-92 from Haskell Laboratory for Toxicology and
Industrial Medicine, Wilmington, Delaware, USA. Submitted to WHO
by E.I. du Pont de Nemours & Co., Inc., Wilmington, Delaware,
USA.
Keller, D.A. (1992c) Oncogenicity study with DPX-H6573-194
(Flusilazole): 2-year feeding study in rats. Unpublished report
No. HLR 527-92 from Haskell Laboratory for Toxicology and
Industrial Medicine, Wilmington, Delaware, USA. Submitted to WHO
by E.I. du Pont de Nemours & Co., Inc., Wilmington, Delaware,
USA.
Lamontia, C.L., Staples, R.E. & Alvarez, L. (1984a) Embryo-fetal
toxicity and teratogenicity study of INH-6573-39 by gavage in the
rat Unpublished report No. HLR 444-83 from Haskell Laboratory for
Toxicology and Industrial Medicine, Wilmington, Delaware, USA.
Submitted to WHO by E.I. du Pont de Nemours & Co., Inc.,
Wilmington, Delaware, USA.
Lamontia, C.L., Staples, R.E. & Alvarez, L. (1984b) Embryo-fetal
toxicity and teratogenicity study of INH-6573-39 by gavage in the
rat. Unpublished report No. HLR 142-84 from Haskell Laboratory
for Toxicology and Industrial Medicine. Wilmington, Delaware,
USA. Submitted to WHO by E.I. du Pont de Nemours & Co., Inc.,
Wilmington, Delaware, USA.
McCooey, K.T., Chromey, N.C., Sarrif, A.M. & Hemingway, R.E. (1983)
CHO/HGPRT assay for gene mutation. Unpublished report No. HLR
449-83 from Haskell Laboratory for Toxicology and Industrial
Medicine, Wilmington, Delaware, USA. Submitted to WHO by E.I. du
Pont de Nemours & Co. Inc., Wilmington, Delaware, USA.
Mullin, L.S. (1990) Reproductive and fertility effects with
flusilazole: Multigeneration reproduction study in rats.
Unpublished report No. HLR 424-90 from Haskell Laboratory for
Toxicology and Industrial Medicine, Wilmington, Delaware, USA.
Submitted to WHO by E.I. du Pont de Nemours & Co., Inc.,
Wilmington, Delaware, USA.
O'Neal, F.O., Rickard, R.W., Kaplan, A.M. & Gibson, J.R. (1985)
One-year feeding study in dogs with INH-6573. Unpublished report
No. HLR 461-85 from Haskell Laboratory for Toxicology and
Industrial Medicine, Wilmington, Delaware, USA. Submitted to WHO
by E.I. du Pont de Nemours & Co. Inc., Wilmington, Delaware, USA.
Pastoor, T.P., Wood, C.K., Krahn, D.F. & Gibson, J.R. (1983)
Ninety-day feeding and one-generation reproduction study in rats
with silane [bis(4-fluorophenyl)](methyl) (1 H-1,2,4-triazol-
1-ylmethyl) (INH-6573). Unpublished report No. HLR 483-83 from
Haskell Laboratory for Toxicology and Industrial Medicine,
Wilmington, Delaware, USA. Submitted to WHO by E.I. du Pont de
Nemours & Co.. Inc., Wilmington, Delaware, USA.
Pastoor, T.P., Wood, C.K., Drahn, D.F. & Aftosmis, J.G. (1984)
Four-week range finding and ninety-day feeding study in mice with
silane [bis(4-fluorophenyl)](methyl) (1 H-1,2,4-triazol-
1-ylmethyl) (INH-6573). Revised unpublished report No. HLR
341-83 1-83 from Haskell Laboratory for Toxicology and Industrial
Medicine, Wilmington, Delaware, USA. Submitted to WHO by E.I. du
Pont de Nemours & Co., Inc., Wilmington, Delaware, USA.
Pastoor, T.P., Rickard, R.W., Sykes, G.P., Kaplan, A.M. & Gibson, J.R.
(1986) Long-term feeding (combined chronic toxicity/oncogenicity
study) and two-generation, four litter reproduction study in rats
with INH-6573. Unpublished report No. HLR 32-86 from Haskell
Laboratory for Toxicology and Industrial Medicine, Wilmington,
Delaware, USA. Submitted to WHO by E.I. du Pont de Nemours & Co.,
Inc., Wilmington, Delaware, USA.
Poindexter, G.L., Henry, J.E., Kenney, L.A., Burgess, B.A. & Kennedy,
G.L. (1984) Inhalation median lethal concentration (LC50) of
INH-6573-41 by EPA protocol. Unpublished report No. HLR 553-83
from Haskell Laboratory for Toxicology and Industrial Medicine,
Wilmington, Delaware, USA. Submitted to WHO by E.I. du Pont de
Nemours & Co., Inc., Wilmington, Delaware, USA.
Redgate, D., Sarver, J.W. & Chromey, N.C.(1985) Approximate lethal
dose (ALD) of INH-6573-66 in rabbits. Unpublished report No. HLR
54-85 from Haskell Laboratory for Toxicology and Industrial
Medicine, Wilmington, Delaware, USA. Submitted to WHO by E.I. du
Pont de Nemours & Co., Inc., Wilmington, Delaware, Wilmington,
USA.
Reynolds, V. (1991) Mutagenicity testing of DPX-H6573-194 in the
Salmonella typhimurium plate incorporation assay. Unpublished
report No. HLR 33-91 by Haskell Laboratory for Toxicology and
Industrial Medicine, Wilmington, Delaware, USA. Submitted to WHO
by E.I. du Pont de Nemours & Co., Inc., Wilmington, Delaware,
USA.
Rickard, R.W., Wood, C.K., Krahn, D.F. & Aftosmis, J.G. (1983)
Three-month feeding study in dogs with silane [bis(4-
fluorophenyl)](methyl) (1 H-1,2,4-triazol- 1-ylmethyl)
(INH-6573). Unpublished report No. HLR 461-83 from Haskell
Laboratory for Toxicology and Industrial Medicine, Wilmington,
Delaware, USA. Submitted to WHO by E.I. du Pont de Nemours & Co.,
Inc., Wilmington, Delaware, USA.
Sarver, J.W., Vick, D.A., Valentine, R., Chromey, N.C. & Kaplan, A.M.
(1986) Twenty-one dose dermal toxicity study with INH-6573-82 in
rabbits. Unpublished report No. HLR 744-86 from Haskell
Laboratory for Toxicology and Industrial Medicine, Wilmington,
Delaware, USA. Submitted to WHO by E.I. du Pont de Nemours & Co.,
Inc., Wilmington, Delaware, USA.
Solomon, H.M., Alvarez, L., Staples, R.E. & Hamill, J.C. (1984)
Developmental toxicity study in rabbits given INH-6573 by gavage
on days 7-19 of gestation. Unpublished report No. HLR 333-84 from
Haskell Laboratory for Toxicology and Industrial Medicine,
Wilmington, Delaware, USA. Submitted to WHO by E.I. du Pont de
Nemours & Co., Inc., Wilmington, Delaware, USA.
Sorg, R.M., Naismith, R.W. & Mathews, R.J. (1984) Micronucleus test
(MNT)-OECD H # 15,314. Unpublished Haskell report No. HLR 437-84
from Pharmakon Research International. Submitted to WHO by E.I.
du Pont de Nemours & Co., Inc., Wilmington, Delaware, USA.
Turner, R.J., Kinney, L.A. & Chromey, N.C. (1985) Inhalation median
lethal concentration (LC50) of INH-6573 by EPA guidelines.
Unpublished report No. HLR 1-85 by Haskell Laboratory for
Toxicology and Industrial Medicine, Wilmington, Delaware, USA.
Submitted to WHO by E.I. du Pont de Nemours & Co., Inc.,
Wilmington, Delaware, USA.
Vlachos, D.A., Covell, D.L. & Sarrif, A.M. (1986) Evaluation of
INH-6573-82 in the in vitro assay for chromosome aberrations in
human lymphocytes. Unpublished report No. HLR 745-86 from Haskell
Laboratory for Toxicology and Industrial Medicine, Wilmington,
Delaware, USA. Submitted to WHO by E.I. du Pont de Nemours & Co.,
Inc., Wilmington, Delaware, USA.
Wylie, C.N., Burgess, B.A. & Kennedy, G.L. (1983) Acute oral test in
rats. Unpublished report No. HLR 78-83 from Haskell Laboratory
for Toxicology and Industrial Medicine, Wilmington, Delaware,
USA. Submitted to WHO by E.I. du Pont de Nemours & Co., Inc.,
Wilmington, Delaware, USA.
Wylie, C.N., Henry, J.E., Ferenz, R.L., Burgess, B.A. & Kennedy, G.L.
(1984a) Median lethal dose (LD50) in rats -- EPA proposed
guidelines, Newark, Delaware, USA. Unpublished report No. HLR
433-83 by Haskell Laboratory for Toxicology and Industrial
Medicine, Wilmington, Delaware, USA. Submitted to WHO by E.I. du
Pont de Nemours & Co., Inc., Wilmington, Delaware, USA.
Wylie, C.N., Henry, J.E., Dashiell, O.L. & Kennedy, G.L. (1984b)
Primary skin irritation and sensitization test on guinea pigs.
Unpublished report No. HLR 626-82 from Haskell Laboratory for
Toxicology and Industrial Medicine, Wilmington, Delaware, USA.
Submitted to WHO by E.I. du Pont de Nemours & Co., Inc.,
Wilmington, Delaware, USA.
Wylie, C.N., Henry, J.E., Burgess, B.A. & Kennedy, G.L. (1984c)
Ten-dose oral subacute test in rats. Unpublished report No. HLR
78-83 from Haskell Laboratory for Toxicology and Industrial
Medicine, Wilmington, Delaware, USA. Submitted to WHO by E.I. du
Pont de Nemours & Co, Inc., Wilmington, Delaware, USA.
Wylie, C.N., Redgate, D., Warheit, D.B. & Chromey, N.C. (1985)
Approximate lethal dose (ALD) of INH-6573-66 in male and female
mice. Unpublished report No. HLR 531-84 from Haskell Laboratory
for Toxicology and Industrial Medicine, Wilmington, Delaware,
USA. Submitted to WHO by E.I. du Pont de Nemours & Co., Inc.,
Wilmington, Delaware, USA.
Zellers, J.E., Staples, R.E., Alvarez, L. & Kaplan, A.M. (1985)
Developmental toxicity study (supplemental) in rabbits dosed by
gavage on days 7-19 of gestation. Unpublished report No. HLR
669-85, from Haskell Laboratory for Toxicology and Industrial
Medicine, Wilmington, Delaware, USA. Submitted to WHO by E.I. du
Pont de Nemours & Co., Inc., Wilmington, Delaware, USA.
