
PESTICIDE RESIDUES IN FOOD - 1983
Sponsored jointly by FAO and WHO
EVALUATIONS 1983
Data and recommendations of the joint meeting
of the FAO Panel of Experts on Pesticide Residues
in Food and the Environment and the
WHO Expert Group on Pesticide Residues
Geneva, 5 - 14 December 1983
Food and Agriculture Organization of the United Nations
Rome 1985
NITROFEN
TOXICOLOGY
IDENTITY
Chemical Name
2,4-dichlorophenyl 4-nitrophenyl ether (IUPAC)
2,4-dichloro-p-nitrophenyl ether
Synonyms
TOKR Herbicide, NIPR Herbicide, NIPDIAR Herbicide, NIPDINR
Herbicide, NIPQ-PR Herbicide, FW925
Common Names
nitrofen (ISO), niclofen (Canada), nitrofene (France).
Structural Formula
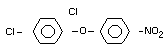 Molecular Formula
C12H9O3Cl2N
Other Information on Identify and Properties
Molecular mass 284. 11
Physical state dark brown to black semi-solid
(technical compound)
Melting point 70-71°C (pure compound)
Specific density 1.80 g/ml at 83°C
(technical product)
Vapour pressure 2 x 10-6 mm Hg at 250°C
Solubility (25°C) in
- water about 1 mg/l
- acetone about 25%
- ethyl acetate 50-60%
- methanol about 25%
- methylene chloride 58-68%
- xylene about 25%
Stability hydrolitically stable but rapidly
photodegraded in solution and on
surfaces
Purity of Technical Product
nitrofen technical contains > 95% pure compound
Formulations
Nitrofen is primarily formulated as a liquid emulsifiable concentrate
containing 25 percent active ingredient and a wettable powder
containing 50 percent active ingredient. It is also available in
combination with other herbicides and has been available as a granular
formulation.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
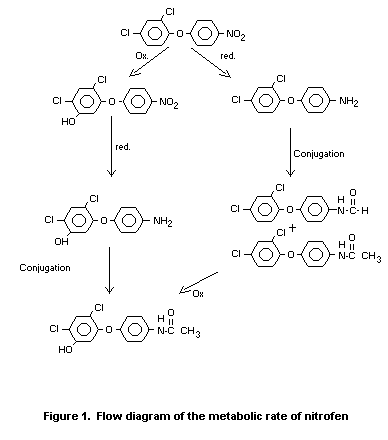
Absorption, Distribution, Excretion and Metabolism
Rat
Samples of urine and faeces from two experiments in which rats had
been fed nitrophenyl ring - and dichlorophenyl ring-14C-labelled
nitrofen, respectively, were used to determine the metabolic rate of
nitrofen. Faeces contained approximately 75 percent and urine
approximately 20 percent of the administered dose. Unmetabolized
nitrofen was excreted in the faeces but not in the urine and
represened ca. 36 percent of the total excretion. Nitrofen and
non-hydroxylated metabolites accounted for ca. 61 percent of the total
of the excreted dose, with relatively higher levels in urine (28
percent) than faecesœ
Hydroxylation occurred in the dichlorophenyl ring. Unidentified polar
metabolites (conjugates) were found in the urine. Cleavage of the
diphenyl ether moiety was not observed (Roser et al. 1971).
One male and one female albino rat were each given (by stomach tube)
nine daily doses of 125 mg/kg b.w. 14C-nitrofen uniformly labelled
in the nitrophenyl ring. After the dosing period, the rats were
maintained on a nitrofen-free diet for two days, and then sacrificed.
Recovery of total administered dose was 81.8 percent in the female
rate and 94.2 percent in the male rat, with 54.1 percent and 75.6
percent, respectively, appearing in the faeces and 18.0 percent and
13.7 percent in the urine. Metabolism to 14CO2 occurred to a minimal
extent (0.01 percent). A small portion (1.12 percent) of the
administered dose of nitrofen was found in all tissues and organs, the
highest concentrations of nitrofen and/or its metabolites were found
in the fat (211.9 ppm), spleen (54.3 ppm), stomach (42.5 ppm, blood
(40.7 ppm), kidneys and adrenals (58.1 ppm), liver (29.4 ppm) and skin
and hair (26.2 ppm). (Adler et al. 1970a)
Albino rats (one female and one male) were each given (by stomach
tube) daily doses of nitrofen (uniformly 14C-labelled in the
dichlorophenyl rins) at a level of 115 mg/kg b.w. for eight days.
After the dosing period, the rats were maintained on a nitrofen-free
diet for three days and were then sacrificed.
Throughout the experiment, urine, faeces and exhaled CO2 were
collected separately and were assayed for radioactivity, as were
tissues removed from the male rat at sacrificeœ
Recovery of total administered dose was 104 percent in the female rat
and 106 percent in the male rat, with 73.9 percent and 80.0 percent,
respectively, appearing in the faeces and 18.0 percent and 13.7
percent in the urine. Metabolism to 14CO2 occurs to a minimal extens
(0œ01 percent). Some radioactivity (0.89 percent) is found in all
tissues, with the highest concentrations being found in the blood
(21.9 ppm), fat (18.4 ppm), large intestine (13.5 ppm), liver (12.9
ppm), kidney and adrenals (11.0 ppm) and skin and hair (10œ6 ppm).
(Adler et al. 197Ob)
Groups of weanling albino Wistar rats (25 males and 25 females per
group) were fed diets containing technical grade (95 percent nitrofen
at levels of 0, 10, 100 and 1 000 ppm) for 97 weeks.
Tissues (fat, muscle, liver, kidney, blood) collected at week 97 and
excreta (urine, faeces) collected at week 65, were pooled and analysed
for nitrofen content by sas-chromatography.
The concentration of nitrofen was highest in fat (20.6, 166.0 and
814.0 ppm, respectively, at the three dietary levels tested), followed
by muscle (0.92, 8.2 and 18.0 ppm), kidney (0.15, 3.2 and 9.0), blood
(0.18, 0.92 and 3.4 ppm) and liver (0.04, 0.44 and <0.01). Excretion
of unchanged nitrofen occurs almost exclusively in the faeces (35.0
ppm at the highest level). (Ambrose et al. 1971) (Full experimental
data were not reported.)
In a comparative study of the fate of 14C-nitrofen, sets of two
female and two male Osborne-Mendel rats and two female Fischer-344
rats were fed diets containing technical nitrofen at levels of 60 or
1 300 ppm. After two weeks, the animals received a single oral pulse
dose of 3 and 65 mg/kg b.w. uniformly dichlorophenyl-rins-labelled
14C-nitrofen, respectively. The same treatment was repeated after
four weeks of dietary nitrofen administration. Urine and faeces were
collected daily after the first pulse dose until sacrifice. One animal
of each strain from each dose level was sacrificed at 24 and 96h after
the second 14C-nitrofen dose. Blood, liver, pancreas, fat and skin
samples were analysed for radioactivity and metabolites. By 92h after
the second pulse dose, the animals in all groups had eliminated
between 3 and 22 percent of the 14C in the urine and between 30 and
100 percent in the faeces. After the two-week exposure, the female
Osborne-Mendel rats excreted 14C in the urine more rapidly than the
males; both sexes of Osborne-Mendel rat excreted 14C more rapidly in
the faeces than did the female Fischer rats. There were no obvious
differences related to sex or strain in tissue concentrations, in
tissue binding or in the metabolite profiles in urine, faeces or
liver; some differences were noted in the pancreas. At 24h after
dosing, concentrations in the blood, fat, liver, pancreas and skin of
14C (calculated as nitrofen) were 0.15 to 0.25, 4.8 to 7.6, 0.5 to
0.8, 1.1 to 1.4 and 0.5 to 0.9 ppm, respectively, for the 3 mg/kg dose
groups and 2.7 to 4.1, 39 to 80, 6.2 to 14, 3.3 to 5.3 and 2.9 to 5.0
ppm, respectively, for the 65 mg/kg dose groups. By 92h after dosing,
the concentrations in the same tissues had declined to 0.06 to 0.09,
3.2 to 4.2, 0.21 to 0.22, 0.3 to 1.4 and 0.4 to 0.8 ppm, respectively,
for the 3 mg/kg groups and 1.6 to 2.2, 1.0 to 12.6, 1.3 to 2.5, 0.6 to
2.1 and 0.3 to 0.9 ppm, respectively, for the 64 mg/kg groups. About
14 to 29 percent and 22 to 42 percent of the 14C were bound in the
liver of low-dose and high-dose animals, respectively, while 1 to 6
percent and 16 to 36 percent were bound in the pancreas. The urinary
metabolites were generally very hydrophillic. Traces of
hydroxyaminonitrofen, acetamidonitrofen and aminonitrofen were
observed but urinary metabolites were not identified further. No
parent compound was detected; 33 percent of the total applied dose was
excreted in the faeces as the parent compound, 14.4 percent as a polar
unknown, 5 percent as acetamidonitrofen, 2.8 percent as 3'-hydroxy
acetamidonitrofen, 2.8 percent as 4-hydroxyaminonitrofen, 2.4 percent
as aminonitrofen and 0.2 percent as 5-hydroxynitrofen. With time, the
concentrations of nitrofen, aminonitrofen and hydroxyaminonitrofen
decreased, and the concentrations of the acetylated compounds
increased. While the metabolic profile in the liver paralleled that in
the urine, the pancreatic metabolites were more lipophilic and
consisted exclusively of the parent compound in the 3 mg/kg groups,
while the 65 ms/ks groups exhibited a variety of metabolites,
including aminonitrofen, hydroxyaminonitrofen, hydroxynitrofen and
2,4-dichloropheno) in addition to the 20 to 74 percent parent
compound. Nitrofen appeared to accumulate to a greater extent in the
pancreas of high-dose female Osborne-Mendel rats than in the pancreas
of the males of this strain or the Osborne-Mendel female pancreas
contained 2.23 ppm nitrofen while the pancreas of each of the other
two groups averaged 0.83 and 1.08 ppm. This strain and sex difference
was not seen in the 3 mg/kg animals which were also killed at 24h
(1.07, 1.19 and 1.36 ppm nitrofen) (Steiserwalt et al. 1980a).
Molecular Formula
C12H9O3Cl2N
Other Information on Identify and Properties
Molecular mass 284. 11
Physical state dark brown to black semi-solid
(technical compound)
Melting point 70-71°C (pure compound)
Specific density 1.80 g/ml at 83°C
(technical product)
Vapour pressure 2 x 10-6 mm Hg at 250°C
Solubility (25°C) in
- water about 1 mg/l
- acetone about 25%
- ethyl acetate 50-60%
- methanol about 25%
- methylene chloride 58-68%
- xylene about 25%
Stability hydrolitically stable but rapidly
photodegraded in solution and on
surfaces
Purity of Technical Product
nitrofen technical contains > 95% pure compound
Formulations
Nitrofen is primarily formulated as a liquid emulsifiable concentrate
containing 25 percent active ingredient and a wettable powder
containing 50 percent active ingredient. It is also available in
combination with other herbicides and has been available as a granular
formulation.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, Distribution, Excretion and Metabolism
Rat
Samples of urine and faeces from two experiments in which rats had
been fed nitrophenyl ring - and dichlorophenyl ring-14C-labelled
nitrofen, respectively, were used to determine the metabolic rate of
nitrofen. Faeces contained approximately 75 percent and urine
approximately 20 percent of the administered dose. Unmetabolized
nitrofen was excreted in the faeces but not in the urine and
represened ca. 36 percent of the total excretion. Nitrofen and
non-hydroxylated metabolites accounted for ca. 61 percent of the total
of the excreted dose, with relatively higher levels in urine (28
percent) than faecesœ
Hydroxylation occurred in the dichlorophenyl ring. Unidentified polar
metabolites (conjugates) were found in the urine. Cleavage of the
diphenyl ether moiety was not observed (Roser et al. 1971).
One male and one female albino rat were each given (by stomach tube)
nine daily doses of 125 mg/kg b.w. 14C-nitrofen uniformly labelled
in the nitrophenyl ring. After the dosing period, the rats were
maintained on a nitrofen-free diet for two days, and then sacrificed.
Recovery of total administered dose was 81.8 percent in the female
rate and 94.2 percent in the male rat, with 54.1 percent and 75.6
percent, respectively, appearing in the faeces and 18.0 percent and
13.7 percent in the urine. Metabolism to 14CO2 occurred to a minimal
extent (0.01 percent). A small portion (1.12 percent) of the
administered dose of nitrofen was found in all tissues and organs, the
highest concentrations of nitrofen and/or its metabolites were found
in the fat (211.9 ppm), spleen (54.3 ppm), stomach (42.5 ppm, blood
(40.7 ppm), kidneys and adrenals (58.1 ppm), liver (29.4 ppm) and skin
and hair (26.2 ppm). (Adler et al. 1970a)
Albino rats (one female and one male) were each given (by stomach
tube) daily doses of nitrofen (uniformly 14C-labelled in the
dichlorophenyl rins) at a level of 115 mg/kg b.w. for eight days.
After the dosing period, the rats were maintained on a nitrofen-free
diet for three days and were then sacrificed.
Throughout the experiment, urine, faeces and exhaled CO2 were
collected separately and were assayed for radioactivity, as were
tissues removed from the male rat at sacrificeœ
Recovery of total administered dose was 104 percent in the female rat
and 106 percent in the male rat, with 73.9 percent and 80.0 percent,
respectively, appearing in the faeces and 18.0 percent and 13.7
percent in the urine. Metabolism to 14CO2 occurs to a minimal extens
(0œ01 percent). Some radioactivity (0.89 percent) is found in all
tissues, with the highest concentrations being found in the blood
(21.9 ppm), fat (18.4 ppm), large intestine (13.5 ppm), liver (12.9
ppm), kidney and adrenals (11.0 ppm) and skin and hair (10œ6 ppm).
(Adler et al. 197Ob)
Groups of weanling albino Wistar rats (25 males and 25 females per
group) were fed diets containing technical grade (95 percent nitrofen
at levels of 0, 10, 100 and 1 000 ppm) for 97 weeks.
Tissues (fat, muscle, liver, kidney, blood) collected at week 97 and
excreta (urine, faeces) collected at week 65, were pooled and analysed
for nitrofen content by sas-chromatography.
The concentration of nitrofen was highest in fat (20.6, 166.0 and
814.0 ppm, respectively, at the three dietary levels tested), followed
by muscle (0.92, 8.2 and 18.0 ppm), kidney (0.15, 3.2 and 9.0), blood
(0.18, 0.92 and 3.4 ppm) and liver (0.04, 0.44 and <0.01). Excretion
of unchanged nitrofen occurs almost exclusively in the faeces (35.0
ppm at the highest level). (Ambrose et al. 1971) (Full experimental
data were not reported.)
In a comparative study of the fate of 14C-nitrofen, sets of two
female and two male Osborne-Mendel rats and two female Fischer-344
rats were fed diets containing technical nitrofen at levels of 60 or
1 300 ppm. After two weeks, the animals received a single oral pulse
dose of 3 and 65 mg/kg b.w. uniformly dichlorophenyl-rins-labelled
14C-nitrofen, respectively. The same treatment was repeated after
four weeks of dietary nitrofen administration. Urine and faeces were
collected daily after the first pulse dose until sacrifice. One animal
of each strain from each dose level was sacrificed at 24 and 96h after
the second 14C-nitrofen dose. Blood, liver, pancreas, fat and skin
samples were analysed for radioactivity and metabolites. By 92h after
the second pulse dose, the animals in all groups had eliminated
between 3 and 22 percent of the 14C in the urine and between 30 and
100 percent in the faeces. After the two-week exposure, the female
Osborne-Mendel rats excreted 14C in the urine more rapidly than the
males; both sexes of Osborne-Mendel rat excreted 14C more rapidly in
the faeces than did the female Fischer rats. There were no obvious
differences related to sex or strain in tissue concentrations, in
tissue binding or in the metabolite profiles in urine, faeces or
liver; some differences were noted in the pancreas. At 24h after
dosing, concentrations in the blood, fat, liver, pancreas and skin of
14C (calculated as nitrofen) were 0.15 to 0.25, 4.8 to 7.6, 0.5 to
0.8, 1.1 to 1.4 and 0.5 to 0.9 ppm, respectively, for the 3 mg/kg dose
groups and 2.7 to 4.1, 39 to 80, 6.2 to 14, 3.3 to 5.3 and 2.9 to 5.0
ppm, respectively, for the 65 mg/kg dose groups. By 92h after dosing,
the concentrations in the same tissues had declined to 0.06 to 0.09,
3.2 to 4.2, 0.21 to 0.22, 0.3 to 1.4 and 0.4 to 0.8 ppm, respectively,
for the 3 mg/kg groups and 1.6 to 2.2, 1.0 to 12.6, 1.3 to 2.5, 0.6 to
2.1 and 0.3 to 0.9 ppm, respectively, for the 64 mg/kg groups. About
14 to 29 percent and 22 to 42 percent of the 14C were bound in the
liver of low-dose and high-dose animals, respectively, while 1 to 6
percent and 16 to 36 percent were bound in the pancreas. The urinary
metabolites were generally very hydrophillic. Traces of
hydroxyaminonitrofen, acetamidonitrofen and aminonitrofen were
observed but urinary metabolites were not identified further. No
parent compound was detected; 33 percent of the total applied dose was
excreted in the faeces as the parent compound, 14.4 percent as a polar
unknown, 5 percent as acetamidonitrofen, 2.8 percent as 3'-hydroxy
acetamidonitrofen, 2.8 percent as 4-hydroxyaminonitrofen, 2.4 percent
as aminonitrofen and 0.2 percent as 5-hydroxynitrofen. With time, the
concentrations of nitrofen, aminonitrofen and hydroxyaminonitrofen
decreased, and the concentrations of the acetylated compounds
increased. While the metabolic profile in the liver paralleled that in
the urine, the pancreatic metabolites were more lipophilic and
consisted exclusively of the parent compound in the 3 mg/kg groups,
while the 65 ms/ks groups exhibited a variety of metabolites,
including aminonitrofen, hydroxyaminonitrofen, hydroxynitrofen and
2,4-dichloropheno) in addition to the 20 to 74 percent parent
compound. Nitrofen appeared to accumulate to a greater extent in the
pancreas of high-dose female Osborne-Mendel rats than in the pancreas
of the males of this strain or the Osborne-Mendel female pancreas
contained 2.23 ppm nitrofen while the pancreas of each of the other
two groups averaged 0.83 and 1.08 ppm. This strain and sex difference
was not seen in the 3 mg/kg animals which were also killed at 24h
(1.07, 1.19 and 1.36 ppm nitrofen) (Steiserwalt et al. 1980a).
 Groups of three female Sprague-Dawley rats received a single dermal
application of 14C-nitrofen (uniformly labelled in the dichlorophenyl
ring) as an undiluted emulsifiable concentrate at a dose level of 125
or 375 mg/kg or as an aqueous dilution (ca. 1.8 percent a.i.) at
levels of 9 or 27 mg/kg a.i.
Urine and faeces were collected twice daily. One rat per group was
sacrificed at 24, 48 and 96 h after dosing. Blood, liver and kidney
were assayed for radioactivity.
Less than 2 percent of the applied doses remained at the application
sites after 96 h while only 30-40 percent of the dose was excreted in
the urine (5-9 percent) and faeces (26-33 percent) during that time.
Blood, liver and kidneys also contained radioactivity at all sacrifice
times (Deckert & Steiserwalt 1979a).
14C-nitrofen (uniformly labelled in the dichlorophenyl ring) was
dermally applied as undiluted emulsifiable concentrate at a level of
120 mg/kg to a female Sprague-Dawley rat. Urine, faeces and volatile
materials were collected daily at 24 h intervals. Total 14C recovery
in this study was 87 percent. At 96 h post-dosing, the rat was
sacrificed and blood, organs and tissue specimens were removed for
14C analysis. After 96 h 8.7 percent of the dose was excreted in
urine and 36 percent in faeces. Only 0.02 percent of the dose was
found as 14CO2, while other volatile materials accounted for 0.001
percent. 14C was found in every tissue analysed. The highest
concentrations were found in skin (17.6 percent of the dose), fat
(484.5 ppm = 2.1 percent of the dose) and muscle at the application
site (158.3 ppm = 0.9 percent of the dose), whereas only 0.4 percent
of the dose remained unabsorbed. A total of 38 percent of the applied
dose was found in tissues. This figure, when compared with the lower
values found in oral studies, suggests that nitrofen may be better
absorbed dermally than by the oral route (Deckert & Steiserwalt
1979b).
Groups of 12 pregnant Sprague-Dawley rats were treated dermally with
14C nitrofen (uniformly labelled in the dichlorophenyl-ring) as
diluted emulsifiable formulation at levels of 0.3 or 27 mg/kg b.w.
a.i. for 6 h on day 11 of gestation. Another group received a single
dose intravenously at a level of 0.3 mg/kg b.w.
At the end of the six-hour application period, only 16 percent of the
dermally applied dose could be removed by washing. Low-dose dermal
application produced blood levels similar to those produced by i.v.
administration, except at the one hour sampling. Urinary 14C
excretion patterns were similar for both dermal application (low and
high dose) and i.v. administration. However, 11 days after dosing, 15
and 20 percent of the dermal (both levels) and i.v. doses,
respectively, were excreted in urine. Foetal 14C excretion patterns
were also similar for both routes of administration, with 45-48
percent and 75 percent of the dose being excreted by 11 days after
dermal and i.v. admin-istration, respectively. After 11 days, 61-65
percent and 97 percent of the dermal and i.v. doses, respectively, had
been excreted. For the higher dermal dose, most maternal tissue 14C
concentrations peaked one to two days after dosing (day 12-13 of
gestation) with the highest concentrations occurring in the fat and
gonads. At 0.3 mg/kg (both routes) heart, thryroid, adrenals,
Harderian glands and pancreas approached maximum levels after six
hours.
Peak 14C levels of most maternal tissues increased with increasing
dose increment and reached a higher level following i.v.
administration than after a dermal application of 0.3 mg/kg.
Placental and foetal 14-C concentrations also peaked within one to
two days after dosing. At 0.3 mg/kg, mean placental 14-C levels were
similar by either route, while the foetal 14-C level two days after
dermal application was five-fold higher than those after i.v.
administration, ten-fold higher than that found in maternal whole
blood at the same time, but only three-fold lower than after the high
dermal dose. At the low dermal dose, the peak or plateau placental
and foetal 14C levels were comparable with those in maternal tissues
(other than the fat and gonads). After i.v. administration and the
high dermal dose, tissue levels in the mother were much higher than in
the placenta or foetus.
In the amniotic fluid, both 0.3 mg/kg dose groups showed the highest
14C levels at 11 days after dosing (day 22 of gestation); the
high-dose dermal group had a steady high 14C level throughout this
period.
Eleven days after dermal application (27 mg/kg), 14C concentrations
in maternal organs were generally higher than in foetal organs, except
for the heart, where levels were nearly the same. Radioactivity was
detected in foetal organs in descending order from kidney to heart,
lung, liver and brain. Metabolic profiles in the placenta and the
embryo/foetus showed the presence of nitrofen, aminonitrofen and other
unidentified metabolites with peak or plateau concentrations occurring
within one to two days. Concentrations of nitrofen and minor
metabolites decreased with time, while aminonitrofen appeared to reach
a plateau and then decrease at a slower rate. More than 90 percent of
14 in the urine and faeces was readily extractable (Steiserwalt
et al. 1982).
Dog
Groups of six-month-old beagles (two males and two females/group) were
fed diets containing technical grade 95 percent pure) nitrofen at
levels of 0, 20, 200 or 2 000 ppm for two years. Tissues (fat, muscle,
bone, liver, kidney, spleen and blood) pooled at week 104 and excreta
(urine and faeces) at week 103 were analysed for unchanged nitrofen by
sas-chromatography. The nitrofen concentration was highest in body fat
(38, 175, 515 ppm, respectively, at the three dietary levels tested),
with lower levels found in bone (3.9, 19.2, 21.8 ppm), muscle (1.26,
4.76, 20.0 ppm), kidney (1.4, 4.6, 13.6 ppm), liver (1.0, 2.0, 3.95
ppm) and blood (0.1, 0.2, 0.4 ppm). Unchanged nitrofen was excreted
almost exclusively in the faeces (39.0 ppm at the highest level)
(Ambrose et al. 1971). (Full experimental data were not provided.)
In vitro dermal absorption
Skin sections from an adult female Sprague-Dawley rat and an adult
woman (postmortem) were mounted in Franz Diffusion Cells and 5 µl of
diluted 14C-nitrofen emulsifiable formulation (ca. 1.8 percent a.i.)
was applied to each. After six hours of exposure, the rat skin
absorbed from 24.7 to 61.7 percent of the 14C while the human skin
absorbed from 13.8 to 25.3 percent of the label. The cell-by-cell
average rat: human absorption ratio of 1.99+/-0.34 indicated that
nitrofen was absorbed by human skin at approximately half the rate
that it passed into rat skin (Steiserwalt et al. 1980b).
TOXICOLOGICAL STUDIES
Special Studies on Reproduction
Rat
In a three-generation, two-litter per generation, reproduction study
groups of 28-day-old Wistar rats (25 males and 25 females/group) were
fed diets containing technical grade (95 percent pure) nitrofen at
levels of 0, 10, 100 or 1 000 ppm for 11 weeks. Body weights for
parental rats before mating and at weaning of respective litters were
not adversely affected for Fo and F1b generations. Body weight of
F2b parent generation rats was slightly decreased for rats at 100
ppm, in breeding the F3a litter and in breeding the F3b litter fed 10
ppm. This decrease was due to lower initial body weights of rats used
for succeeding generations. Although there was considerable
inconsistency within and between matings, average data reported did
not support a dose-related effect on fertility and gestation indices.
Viability and lactation indices were not adversely affected at 100
ppm. However, the viability index for rats at 1 000 ppm was seriously
affected, as stillborn pups outnumbered live pups; indeed no pups were
available for continuance beyond the Fo generation. In all
generations, the number of stillborn pups from the 100 ppm group
numbered 64 compared with 30 in the control.
No structural or microscopic cellular abnormalities were detected in
any stillborn pups in F1b litters. Weaning weights indicated no
diet-related trends. Overall, only the 10 ppm dietary level of
nitrofen exhibited no adverse effects on reproduction. Examination of
a variety of tissues from 10 F3b generation rats of each sex from the
0, 10 and 100 ppm dietary levels showed no histopathological lesions
(Ambrose et al. 1971). (Full experimental data were not provided.)
Groups of Sherman rats (10 males and 20 females/group) were fed
dietary levels of 0, 20, 100 and 500 ppm technical nitrofen (89
percent) pure). Breeding and F1a and F1b litters commenced at 68 and
200 days, respectively. Offspring were observed through weaning. At
the time of weaning of the F1b litters, 20 female and ten male
weanlings were selected from the 0, 20 and 100 ppm groups, continued
on the parental diet and pair-mated when 112 days old to produce the
F2a generation. Neither the food consumption nor the body weight gain
were greatly affected in any of the nitrofen-treated groups. At the
500 ppm dietary level no offspring of the F1a and F1b generation
survived the neonatal period. The number of pups born alive and the
survivors-to-weaning were reduced at the 100 ppm dietary level but not
at 20 ppm (equal to 1.1 and 1.8 mg/kg b.w. in F1b and F1a,
respectively) (Kimbrough et al. 1974). (Full experimental data were
not provided.)
Groups of 25 male Sprague-Dawley rats were fed diets containing 0,
100, 500 or 2 500 ppm of nitrofen technical (95.7 percent pure) for 95
days. At that time, male and untreated female rats were cohabited
(1:1) for a maximum of 10 days. During co-habitation, males were fed
an untreated diet and daily doses of 0, 6, 30 or 155 mg/kg of nitrofen
were administered by gavage. The nitrofen dosage was based on the
dietary intake of the compound (mg/kg/day) during weeks 10-13 of the
study.
No significant effects were detected on the reproductive performance
of male as indicated by the average number of days for mating to
occur, percentage of females impregnated or number of mated females
that delivered. Furthermore, no differences were noted in the length
of gestation, litter size, percentage of live or dead births or sex
ratio of the offspring. No compound-related signs of toxicity were
evident in any of the progeny from birth to day 35. Necropsies of
offspring that died and of female parents which were killed after
lactation revealed no compound-related gross abnormalities. Neither
viability nor mean weights of offspring were affected at any dose
level. Male reproductive performance was not affected at dietary
concentrations up to and including 2 500 ppm, equal to 186 mg/kg/day
(O'Hara et al. 1983).
Special Studies on Teratogenicity and Postnatal Effects
Mouse
Two studies on postnatal dose response and standard teratology are
summarized together.
Purified nitrofen (99.6 percent pure) in maize oil was administered to
CD-1 mice by gavage. In the postnatal study, 24 animals were dosed
(50, 100, 150 and 200 mg/kg/day) on days 7-17 of gestation and allowed
to give birth. In the teratology study animals were dosed 50, 100 and
200 mg/kg/day) on days 7-17 and sacrificed on day 18. Foetuses were
removed and analysed for visceral and skeletal malformations.
Concomitant control groups were utilized in all the studies.
In the postnatal study there was a significant dose-related delay in
time of birth. Significant dose-related reductions in growth and
viability were noted at all dose levels. There was considerable litter
mortality in the 100 mg/kg/day groups between days 3 and 17
postpartum. Only 23 percent of the pups alive on day 3 were still
alive by day 17. Mortality in the 50 mg/kg/day dose group was not
different from control values during this period. Microphthalmia or
anophthalmia was seen in a high percentage of the animals in the 100
mg/kg/day dose group. A dose-related growth retardation was noted in
pups until 60 days of age at 50 mg/kg and above. There were delays in
the percent of female offspring showing vaginal opening at 30 days and
in the percentage of animals breeding by 60 days at 50 mg/kg and
above. Litter size was unaffected by treatment.
In the teratology study dose-related increases in maternal weight gain
and liver/ body weight ratios were recorded. Nitrofen significantly
increased the supraoccipital scores, indicating retarded skeletal
development of the skull. It was teratogenic at 100 and 200 mg/kg/day,
the most common defects noted being cleft palate and undescended
testes (Chernoff et al. 1980). (Full experimental data were not
available.)
After being dosed at levels of 100 mg/kg b.w. nitrofen on days 7 to 17
of gestation, pregnant CD-1 mice showed reduced mean litter size.
Surviving pups had delayed eye opening and absence of the Harderian
gland (Gray et al. 1982a).
Groups of pregnant CD-1 mice were dosed by gavage with nitrofen (99.6
percent pure) in maize oil at levels of 0, 6.25, 12.5, 25 and 50
mg/kg/day. There were 23, 19, 27, 21 and 17 litters, respectively, on
day 1. The animals were observed until necropsy at 110-130 days of
age.
The administration of nitrofen did not cause any adverse effects in
the dams, as measured by maternal viability or weight gain from days 7
to 19 of gestation. By day 3 the number of pups was reduced
significantly at 50 mg/kg (20 percent). Body weights were initially
unaffected after birth at 50 mg/kg but subsequent body weight was
reduced in the pups from the 12.5 mg/kg treatment group. There was a
dose-related increase in the frequency of lethal defects in the pups,
i.e. cleft palate, diaphragmatic hernia and extreme abdominal
distention. Lung weight was significantly reduced by nitrofen
treatment at all doses, including the lowest dose of 6.25 mg/kg.
Seminal vesicle weights were significantly reduced at 6.25, 12.5 and
50 mg/kg. Obvious intraorbital defects were present in some offspring
in the 25 mg/kg group and above. Prenatal nitrofen treatment
significantly reduced the weight of the Harderian glands at all doses,
including the lowest dose of 6.25 mg/kg. Prenatal nitrofen treatment
of 50 mg/kg significantly delayed puberty in the female mice, as
indicated by the percentage of females with patent vasina on day 30.
In addition, the 50 mg/kg females paired with treated males produced
significantly fewer pups in the second litter.
A cross-fostering experiment with 100 mg/kg demonstrated that the
defects noted in the present study were produced solely by prenatal
exposure; pups exposed to nitrofen in the milk alone as a consequence
of any accumulation of nitrofen in the dam during gestation were
unaffected (Gray et al. 1983).
Rat
Groups of 26 pregnant FDRL rats were administered nitrofen by gavage
on days 6-16 of gestation. The control animals received maize oil (1
ml/kg b.w.) whereas the test animals received 100 and 200 ppm of
nitrofen in the same volume of vehicle. Thus the dosage corresponds to
5 and 10 mg/kg b.w./day, respectively. On day 22, the animals were
sacrificed and Caesarean section performed. There were no differences
between control and treated groups with respect to mean dam weight,
implantation sites, foetal viability or mean live foetal weight.
Resorptions were increased in the high-dose group. No malformed
foetuses were seen at the time of Caesarean sections, nor was there
evidence of soft tissue anomalies. Numbers of incomplete cranial bone
ossification and scoliosis were slightly increased in both treated
groups. These variations were considered "consistent with changes
previously observed in the colony of rats" and, thus, not
treatment-related (Vosin 1971).
Groups of seven pregnant Wistar rats were administered by gavage daily
doses of nitrofen technical (96.2 percent pure) at levels of 68 mg/kg
b.w. on days 6 through 15 of gestation. Five pregnant control rats
received only peanut oil. Parameters evaluated were neonatal mortality
and histomorphology of the lungs of dead and moribund newborns in the
nitrofen-treated group and of four-day-old survivors from the control
and treated groups. All the offspring from the control group were
alive at birth. Most (85 percent) of the offspring from the treated
group were born dead or died within the first few hours after delivery
and exhibited generalized cyanosis. Histological examinations of the
lungs of moribund offspring from the treated group revealed a large
number of alveoli bordered by a cubical epithelium (Siou 1979b).
Groups of 9-12 pregnant Sherman rats were dosed by oral intubation on
days 7 through 15 of gestation. Dosage levels were 0 (peanut oil), 10,
20 or 50 mg/kg/day for technical nitrofen (89 percent pure); 0, 20, or
50 mg/kg/day for purified nitrofen (99 percent pure); and 0 or 0.04
mg/kg/day for 2,7-dichlorodibenzo-p-dioxins (DCDD) (this dose is
equivalent to a contamination level of 2 000 ppm (0.2 percent) in the
technical nitrofen for a 20 mg/kg dose). The pups were observed to
weaning. At the dosage levels of 20 and 50 mg/kg/day a dose-related
decrease of live-born rats was observed for both the technical and
purified nitrofen groups. None of the pups survived at the 50 mg/kg
dose level and survival-to-weaning was severely reduced (50 percent)
in both the technical and purified nitrofen groups. No effect of
technical nitrofen on the number of live pups born and their
survival-to-weaning was observed at 10 mg/kg dose level. Extensive
inflammation, fibrosis and epithelial proliferation of the lung were
seen in sucklings that died at 11 to 15 days. No effect on
survival-to-weaning was observed in DCDD-treated rats.
Additional groups of ten female and ten male rats were fed technical
nitrofen at dietary levels of 0 and 500 ppm. The rats were started on
the diet at weaning and pair-mated when they were 90 to 100 days old.
On day 20 of pregnancy, foetuses were removed by Caesarean section and
examined for malformations. The live foetuses were kept in an
incubator for six hours and closely checked for viability. Most of the
foetuses of the controls were pink and survived until they were killed
after six hours. The pups from nitrofen-treated dams were cyanotic and
died one to three hours following the Caesarean section.
Two more pups of pregnant rats were given by oral intubation technical
nitrofen at levels of 0 or 50 mg/kg/day on days 7 to 18 of gestation.
The foetuses from these rats were removed by Caesarean section on day
21 of pregnancy. Lungs from 18 exposed and 16 control foetuses from
four litters in each group were removed one to three hours following
the Caesarean section and studied with light- and electron-microscopy.
Pups from treated dams showed cyanosis at birth. The cyanosis
increased during the next 45 to 60 minutes and most of the pups from
treated dams died, while the control rats developed pink colour soon
after birth and survived. Lungs of treated foetuses were poorly
expanded and alveolar epithelium was cuboidal and resembled cells of
earlier gestational age (Kimbrough et al. 1974).
When purified nitrofen (containing only trace amounts (ppm) of
4-aminonitrofen) was administered to pregnant Long-Evans rats at
different days of gestation, gestation day 11 was demonstrated as
critical: a single dose of 150 mg/kg on day 11 killed 56 percent of
the newborn pups withing 48 h.
Groups of 4-7 Long-Evans dams were given a single dose of purified
nitrofen at levels of 0, 75, 115, 150, 200 or 250 mg/kg on day 11 of
gestation. Survival of neonates to five days was monitored. A
dose-related increase in neonatal mortality was observed. The LD50
for the neonates was estimated at 116 mg/kg of maternal body weight.
Initial experiments demonstrated that animals surviving the first five
days usually lived to maturity. Thus, the mortality is most accurately
described by the fraction dying within the first five postnatal days.
Surviving offspring were autopsied at 35 days of age and examined for
gross lesions. The predominant lesion observed in all treated groups
was hydronephrosis. The incidence of hydronephrosis in survivors
paralleled the neonatal mortality and the lesion was most frequent
among animals exposed on day 11. The incidence of hydronephrosis was
also dose-related among animals exposed only on day 11.
Nitrofen-induced hydronephrosis was not considered incompatible with
life.
In a further teratology study purified nitrofen was administered to
pregnant Long-Evans rats at dose levels of 0, 70, 115, 150, 250, 265
and 400 mg/kg b.w. on day 11 of gestation and foetuses collected on
day 22. Foetuses exposed to nitrofen and examined at term (day 22),
showed delayed skeletal ossification. There was a dose-related
depression in foetal body weight. Nitrofen exposure on day 11 induced
hydronephrosis, which was dose-related in incidence and severity.
Diaphragmatic hernias were also observed in the treated groups. The
overall frequency of soft tissue anomalies was also dose-dependent.
Mean litter frequencies of these anomalies ranged from 45 percent at
LD15 (70 mg/kg) to 91 percent at LD99+ (400 mg/kg). Nitrofen exposure
produced a dose-related increase in the frequency of cardiac
malformations, which included in order of decreasing frequency:
ventricular septal defects (VSD), double outlet right ventricules
(DORV), and transposition of great vessels. Incidence of lethal
cardiac malformations was 38 percent and 53 percent at 150 mg/kg and
250 mg/kg, respectively. No anomalies were seen in the control
litters. None of the cardiac or diaphragmatic defects in the term
foetuses were present in survivors, suggesting that the heart and
diaphragm are the target organs in nitrofen-induced neonatal deaths
(Costlow & Manson 1981).
Groups of five pregnant Sprague-Dawley rats were treated with nitrofen
(98 percent pure) at 0, 20, 31.2 or 50 mg/kg/day by gavage on days
8-18 of gestation. Significant differences in birth weights were
apparent in pups from the 31.2 and 50 mg/kg/day groups. A decrease in
the lung to body weight ratio of foetuses exposed to nitrofen was also
observed. Most, if not all, pups were born alive and lacked
externally-observable malformations. Within minutes after birth,
treated pups exhibited laboured breathing and became cyanotic. Death
usually ensued 30 min. to 4 h postpartum. Survival was significantly
depressed in all treatment groups and all time intervals (1 h, 24 h,
25 days) measured after birth. On examination, symptomatic
nitrofen-exposed pups suffered massive atelectasis at autopsy, with
few expanded air sacs in the 31.2 and 50 mg/kg groups. In contrast,
lungs from newborn controls exhibited normal alveolar expansion. The
level of glucocorticoids and capacity of the foetal lung to respond to
glucocorticoids by synthesizing and releasing surfactant were not
affected by prenatal exposure to nitrofen (Stone & Manson 1981).
Missing and/or malfunctioning Harderian glands were observed when
pregnant Sprague-Dawley CD rats were given daily doses of nitrofen at
a level of 12.5 mg/kg by gavage on days 8 to 16 of gestation (Gray
et al. 1982a).
A postnatal study, using rats (strain unknown) dosed on days 7-16 at
five doses from 0 to 25 mg/kg, demonstrated that nitrofen caused
diaphragmatic hernias at 1.39 mg/kg and above and affected the
haemoglobin level at 12.5 mg/kg (Gray et al. 1982b). (This
information was provided in abstract form and cannot be further
interpreted.)
Groups of five pregnant CD rats were given by gavage daily doses of
nitrofen at levels of 0, 12.5 or 25 mg/kg b.w. on days 8 to 16 of
gestation. Dams were sacrificed on day 21 of gestation and foetuses
removed for examinations. Dose-related increase in foetal mortality
and dose-related decrease of mean foetal weight were determined.
Diaphragmatic hernias occurred in both dose groups in a dose-related
fashion. Brain weight and brain DNA were statistically significantly
reduced at 25 mg/kg; lung weight and lung surfactants were reduced at
12.5 and 25.0 mg/kg in a dose-related fashion as were liver weight,
liver glycogen content, kidney weight and kidney alkaline phosphatase
levels. However, statistical analysis demonstrated that only effects
on lung and liver represented specific organ toxicity, while brain and
kidney effects were correlated to the foetal growth retardation
(Kavlock et al. 1982).
When pregnant Sprague-Dawley rats (35/group) were exposed dermally to
nitrofen emulsion at 0, 0.3, 3.0 or 30.0 mg/kg/day on days 6 to 15 of
gestation, foetal weight and foetal length were significantly lower in
the 30.0 mg/kg group compared to the control group. Soft tissue
anomalies (hydronephrosis, ectopic testes, liver and/or stomach and/or
intestine protruding into thorax, diaphragmatic hernia, right-sided
aorta) were observed almost exclusively in the foetuses of the 30.0
mg/kg group. The foetal viability (live at birth/implantations) in all
the treated groups was statistically significantly lower than the
control litters. Neonatal survival index (live at day 4/live at birth)
was significantly lower in the 30.0 mg/kg group than in the control
group (Weatherholtz 1979).
Groups of five to seven pregnant Charles River rats were treated
dermally with nitrofen (as a water-diluted emulsifiable formulation)
at levels of 0, 0.6, 4.8 or 19.2 mg/kg/day on days 6 to 15 of
gestation. A further group received dermally 4.8 mg/kg on day 11 only.
Dams were sacrificed on day 21 postpartum. Surviving offspring were
sacrificed on day 27. All maternal rats survived to sacrifice.
Treatment of pregnant rats with 19.2 mg/kg/day of nitrofen on days 6
to 15 of gestation resulted in slightly decreased weight gain during
the treatment interval. The no-observable effect level (NOEL) was 4.8
mg/kg/day. The treatment caused decreased viability of offspring,
decreased neonatal survival to day 4 and decreased live offspring of
pregnant rats treated dermally with 19.2 mg/kg/day of nitrofen on days
6 to 15 of gestation. Nitrofen did not affect neonatal viability,
neonatal survival, survival to weaning, growth of offspring or eye
opening in offspring of dams treated with 0.6 or 4.8 mg/kg/day on days
6 to 15 of gestation or 4.8 mg/kg/day on day 11 of gestation
(Hirsekorn & Kane 1981).
Groups of 25 pregnant Sprague-Dawley rats were treated dermally with
nitrofen in aqueous emulsion (0, 0.3, 0.6, 1.2 or 12.0 mg/kg b.w./day)
on days 6 to 15 of gestation. No maternal toxicity occurred. At 12
mg/kg neonatal survival was reduced. Necropsies of the dead neonates
showed a high incidence of diaphragmatic hernias. The number of pups
per litter and viability over days 4-41 of age were unaffected by
treatment. About half (randomly selected) of the pups from each litter
and group were necropsied on day 42. Chromo-dacryorrhea, reduced or
absent Harderian glands and diaphragmatic hernias occurred at 12.0
mg/kg. The frequency and the severity of dilation of the renal
pelvices increased with the dose; slight dilation was significantly
elevated at 0.3 mg/kg and moderate and severe dilation became
prominent at 0.6 and 12.0 mg/kg, respectively. In the 12.0 mg/kg group
nitrofen reduced the body weight of the survivors and also the
relative weights of the Harderian gland, the thyroid gland of both
sexes; lungs of females were significantly depressed with respect to
solvent and water control groups. The findings from necropsies
performed on the remaining offspring on days 146 to 149 postpartum
were similar to those at day 42. Body weight was unaffected by
nitrofen exposure but dilated kidneys and diaphragmatic hernias were
observed. Relative thyroid weight was significantly depressed only in
the 12 mg/kg dose group. A NOEL was not demonstrated (Costlow et al.
1983).
Rabbit
Groups of 15 pregnant New Zealand rabbits were given encapsulated
technical nitrofen (96.2 percent pure) at levels of 0, 7, 27 or 108
mg/kg on days 6 through 18 of gestation. Ten rabbits in each group
were sacrificed on day 28 of gestation to assess embryotoxic and
teratogenic effects. The average number of live foetuses and
implantation sites per pregnant female was slightly decreased in the
108 mg/kg group sacrificed on day 28 of gestation. Percentage of dead
foetuses with respect to implantation sites was increased in all
treated groups compared with the control group, but not in a
dose-related manner. The increased incidence of foetuses, with 13
pairs of ribs was dose-related. No other anomalies were observed. The
remaining five rabbits in each group were allowed to deliver, and they
and the newborns were sacrificed 48 h after delivery. The average
number of live offspring was slightly lower in the 108 mg/kg group.
Average weights of live foetuses after 48 h were slightly lower in the
treated groups, but not in a dose-related manner. No significant
differences were observed in survival rate to 48 h or in resorption
rates. An increased incidence of 13 ribs was observed in all treated
groups. Histological examinations of the lungs removed from between 5
and 17 of the 28-day foetuses and the 48-hour newborns in each group
did not indicate retardation of lung maturation (Siou 1979a).
Special Studies on Mutagenicity
Microbial systems
Nitrofen, when tested with Salmonella typhimurium strains TA1535,
TA1538, TA100 and TA98, both with and without S-9 activation, was
found only moderately mutagenic in TA100 with microsomal enzyme
activation (Byeon et al. 1976).
Purified nitrofen (99+ percent) and several samples of nitrofen
technical (90-98 percent pure) were tested at concentrations of
0.001-10.0 µg/plate with S. typhimurium strains TA1535, TA1537 TA98
and TA100. Tests were carried out both with and without microsomal
activation. Positive controls were used: 2-aminofluorene and
2-acetamidofluorene for TA98 and 2-anthramine for all the strains
tested. Only one sample of nitrofen technical (90-92 percent pure) was
completely negative in the test. The other samples of technical
nitrofen gave statistically significant increases of mutants/plate
compared with negative control in TA100 strain, both with and without
activation, but only one was positive in TA98 with activation.
However, a dose-response relationship was observed only with three
samples (97, 97 and 98 percent pure), but not with another three (93,
92.8 and 97.7 percent pure), generally at concentrations of 10-10 000
µg/plate. Purified nitrofen (99+ percent pure) gave a statistically
significant increase of revertants/plate in TA100, both with and
without activation; no dose-response relationship was observed.
Thus purified nitrofen and three technical samples gave an indication
of mutagenicity, but the other three technical samples were positive
(O'Neill & Scribner 1979).
Nitrofen was found mutagenic in a reversion test using
Saccharomyces cereviseae strain 632/4 (auxotrophic for methionine)
(Guerzoni et al. 1976).
Mammalian systems
The mutagenicity of nitrofen was assayed in the murine lymphoma cell
line L5178Y at concentrations of 0.1 x 10 (E-4), or 2 x 10 (E-4) M.
Doses were selected as those killing approximately 40 and 80 percent
of cells. Ethylmethanesulphonate was used as a positive control.
Induction of methotrexate-resistant mutants of L5178Y cells treated
in vitro with nitrofen was not significantly different from the
negative control (Paik & Lee 1977b).
Nitrofen was tested in the unscheduled DNA repair synthesis assay with
human lymphocytes (concentrations not clear). Nitromin was used as a
positive control. 3H-thymidine incorporation in the nitrofen-treated
cells was not significantly different from the negative control (Paik
& Lee 1977b).
Nitrofen was also tested in mouse cells in a BALB/3T3 in vitro
transformation assay. The compound did not induce a significant
increase in transformed foci over the applied concentration range of
0.1 to 60 micrograms/ml. This range corresponding to approximately 50
to 90 percent survival in the cytotoxicity tests. A negative control
consisting of assays performed on untreated cells was included. A
positive control with 3-methyl cholanthrene (MCA), used at level of 5
µg/ml for each assay, gave a marked increase of the average
number/flask of foci of transformed cells. Therefore, nitrofen was
considered to be inactive in the BALB/3T3 in vitro transformation
assay (Myhr & Brusick 1979).
Groups of three Swiss Webster albino mice were given intraperitoneally
two doses, separated by 24 h, of nitrofen (purity not specified) at
levels of 0, 500 or 1 000 mg/kg b.w. Six hours after the last dose,
three bone marrow smears from each mouse were prepared and about 2 000
polychromatic erythrocytes per mouse were analysed for the presence of
micronuclei. For both dose levels tested, the incidence of micronuclei
was not statistically significantly different from the control. A
positive control of cyclophosphamide gave the expected increase of the
incidence of micronuclei (Paik & Lee 1977a).
Groups of 5-10 male Swiss mice were given by gavage two doses of
nitrofen technical (96.2 percent pure) at levels of 0, 0.25, 1.00 and
1.25 ml/kg, spaced 24 h apart. The animals were sacrificed 6 h after
the second dose and bone marrow smears were prepared. The incidence of
polychromatic erythrocytes carrying "micronuclei" in the treated
groups was not significantly different from the control group (Siou
1978).
Groups of 24 male Charles-River CD-1 mice were given by gavage a
single oral dose of nitrofen technical (95.7 percent pure) at levels
of 0, 0.39, 0.79 and 1.58 g/kg, representing 1/8, 1/4 and 1/2 of the
acute oral LD50. Additional groups of 8 male mice were given once
daily five doses of nitrofen at the same levels. A positive control
group received triethylamine (TEM) 0.3 mg/kg i.p. Bone marrow slides
were prepared from 8 animals/group sacrificed at 6, 24 and 48 h after
the acute dose and 6 h after the last sub-acute dose and TEM dose. All
animals received colchicine at 2 mg/kg i.m. two hours prior to
sacrifice. Fifty metaphase cells per animal were scored when possible.
Owing to poor survival, the high dose subacute group was sacrificed on
day 4. No statistically significant increase in chromosomal
aberrations was observed in treated animals compared with controls,
both in the acute and subacute regimen (Reustle & Scribner 1980).
Nitrofen technical (95.7 percent pure) was administered in m-xylene
(99 percent) by dermal application to groups of eight male Charles
River CD rats at doses of 0, 0.05, 0.125 and 0.50 g/kg, to assess the
potential of nitrofen to induce chromosomal aberrations in mammalian
bone marrow cells. A positive control group received a single 0.3
mg/kg i.p. dose of triethylenemelamine. The doses were administered
according to both a single and repeated (daily × five days) regimen.
Animals were killed and bone marrow slides prepared at approximately
6, 24 and 48 h after the single dose, 24 h after the positive control
dose and 6 h after the last multiple dose. All animals received
colchicine (1 mg/kg, i.p.) three hours prior to being killed.
Metaphase cells (50 per animal) were examined for chromosomal
aberrations. Nitrofen at 0.5 g/kg and below did not induce a
significant increase in chromosomal aberrations in bone marrow cells
at either 6, 24 or 48 h after single or repeated exposure. Chromosomal
aberrations were consistently elevated at 24 h after treatment with
triethylmelamine (McLeod & McCarthy 1982).
Special Studies on Mutagenicity of Aminonitrofen
Aminonitrofen (99+ percent pure), a metabolite of nitrofen, was tested
in concentrations of 0.1-1 000 µg/plate with S. typhimurium strains
TA1535, TA1537, TA98 and TA100 with and without a microsomal enzyme
preparation. Statistically significant increases of revertants/plate
were observed with strains TA1535, TA98 and TA100 using a microsomal
enzyme preparation and a dose-response relationship was exhibited.
Positive controls were 2-anthramine, 2-aminofluorene and
2-acetamidofluorene (O'Neill & Scribner 1979).
Special Studies on Mutagenicity of 4,4'-Dichloroazobenzene
4,4'-Dichloroazobenzene (DCAB) (99+ percent technical), a low level
impurity in technical nitrofen, was tested with the S. typhimurium
strains TA1535, TA1537, TA98 and TA100 at concentrations of 1.0-1 000
µg/plate, with and without microsomal activation. Saline buffer
controls were used with each strain. Statistically significant
increases of revertants/plate were observed with strain TA100 at
10-1 000 µg/plate with metabolic activation. DCAB gave a greater
response with strain TA98 than with TA100. Positive metabolic
activation; and 2-anthramine (10 µg/plate), positive with TA1535,
TA1537 and TA100 with metabolic activation; and 2-acetamidofluorene
(50 µg/plate), positive with TA98 with metabolic activation (O'Neill &
Lohse 1980).
Special Studies on Carcinogenicity
Mouse
Groups of 50 male and 50 female young B6C3F1 mice (age not specified)
were fed diets containing technical nitrofen (stated to be >80
percent pure; impurities unspecified) dissolved in maize oil for 78
weeks at levels of 1 775-2 500 mg/kg (low-dose) or 3 550-5 000 mg/kg
(high-dose), to give time-weighted average concentrations of 2 348 and
4 696 mg/kg in the diet. A group of 20 male and 20 female controls
received the basal diet containing 2 percent maize oil. All mice were
observed for a further 12 weeks after treatment. Weight gain was
depressed in both groups of treated females and in the males exposed
to the high dose. Beginning at week 54, pronounced abdominal
distension was displayed by an increasing number of treated mice. By
the end of the study, only 10 percent of the control male mice, 34
percent of the high-dose group and 54 percent of the low-dose group
were still alive; and, of the female mice, 62 percent were still alive
in the high-dose group, 54 percent in the low-dose group and 85
percent of the controls. For neither sex could a positive association
be established between dosage of nitrofen and mortality. A high
incidence of hepatocellular carcinomas was found in exposed male and
female mice: in 4/20 control males, 36/49 low-dose males and 46/48
high-dose males, in none of the control female mice (both matched and
pooled controls), in 36/41 low-dose females and in 43/44 high-dose
females. A few of these tumours metastasized to other sites. The
difference in incidence between control and exposed male and female
mice was statistically significant at both dose levels.
Haemangiosarcomas of the spleen were found in 2/47 of high-dose males
and haemangiosarcomas of the liver in 1/49 low-dose males and 2/48
high-dose males. In female mice, haemangiosarcomas were seen in the
spleen in 1/18 controls, in the liver in 4/44 high-dose animals and in
the abdominal cavity in 1/43 of the high-dose group.
The incidence of haemangiosarcomas in the high-dose male mice, but not
females or low-dose males, was statistically higher than controls
(National Cancer Institute 1978).
Groups of 50 male and 50 female six-week-old B6C3F1 mice were fed
diets containing 3 000 or 6 000 mg/kg technical-grade nitrofen (purity
not specified) for 78 weeks. The 20 male and 20 female controls
received the basal diet. All animals received acidified (pH 2.5) water
ad libitum. After exposure to nitrofen, animals were observed for a
further 13 weeks. Consistent dose-related depressions in mean body
weight were noted in male and female mice, reaching about 25 percent
in high-dose males by the end of the study. At that time, 40/50 of the
high-dose males, 48/50 of the low-dose males and 19/20 of the control
males were still alive; of the females, 48/50 of the high-dose and
43/50 of the low-dose group and 12/20 off the controls survived until
the end of the study. Although a variety of tumours were noted, only
the incidence of liver tumours seemed to be related to nitrofen
exposure. Hepatocellular adenomas were seen in 1/20 control males,
18/49 low-dose males and 20/48 high-dose males, and hepatocellular
carcinomas in 0/20 controls, 13/49 low-dose males and 20/48 of the
high-dose group. In addition, none of the controls, but 3/49 of the
low-dose group and 4/48 of the high-dose group had hepatoblastoma, a
very rare tumour in this strain of mice. Of the females, 0/18
controls, 9/48 low-dose and 17/5 high-dose mice had hepatocellular
adenomas; hepatocellular carcinomas were observed in 0/18 control,
5/48 low-dose and 13/50 high-dose female mice. In addition, a
hepatoblastoma was found in 1/48 females in the low-dose group.
For both the low- and high-dose groups there was a statistically
significant increase from the control group in the incidences of
hepatocellular adenomas and hepatocellular carcinomas (National Cancer
Institute 1979).
Rat
Groups of 50 male and 50 female Osborne-Mendel rats (age not
specified) were fed diets containing technical grade nitrofen (stated
to be >80 percent pure; impurities unspecified) in 2 percent maize
oil for 78 weeks. The dietary concentration for males was 2 300 mg/kg
(low-dose) or 2 300-4 600 (high-dose), to give a time-weighted average
of 3 627 mg/kg. The dietary concentrations for female rate were 1 300
mg/kg (low-dose) or 2 600 mg/kg (high-dose). The low-dose males, the
control animals and the high- and low-dose females were observed for
an additional 32 weeks, during which they were maintained on a basal
laboratory diet plus maize oil; the high-dose males were observed for
an additional five weeks after dosing was stopped. Groups of 20 male
and 20 female controls received the basal diet plus 2 percent maize
oil. A dose-related decrease in body weight was noted; by 75 weeks
after onset of dosing, the high-dose females weighed roughly one-third
less than the controls. Fifty percent of the high-dose males were dead
by week 45 and only 15 survived to week 83; 60 percent of the low-dose
and 45 percent of the control group survived until the end of the
study. Of the females, 58 percent at the high-dose, 74 percent at the
low-dose and 80 percent of the controls survivied until the end of the
study. Adenocarcinomas of the exocrine pancreas were seen in 2/50
females at the low dose and in 7/50 at the high dose. All tumours
showed local invasion and had metastasized to the lungs. No such
tumour was seen in the 20 matched controls or in the 110 pooled
controls. The difference was statistically significant for high-dose
animals. No other tumour showed a statistically significant difference
in incidence between control and exposed rats. Poor survival precluded
the evaluation of carcinogenicity in male rats (National Cancer
Institute 1978).
Groups of 50 male and 50 female six-week-old Fischer 344 rate were fed
diets containing 3 000 or 6 000 mg/kg technical-grade nitrofen (purity
not specified) for 78 weeks. A group of 20 male and 20 female rats
were given the control diet. All animals received acidified water (pH
2.5). The animals were followed for an additional 26 weeks, when all
surviving rats were killed. A dose-related depression in mean body
weight was noted in animals of both sexes, reaching 15 percent for
those given the high dose for 60 to 75 weeks. Of the male rats, 45/50
given the high dose, 42/50 given the low dose and 17/20 of the
controls survived until termination of the study; of the females,
38/50, 42/50 and 17/20, respectively, survived. No statistically
significant difference in the incidence of tumours was observed
between the exposed and control groups (National Cancer Institute
1979). However, subsequent detailed histological and morphological
studies distinguished the nitrofen-induced neoplasm from those
occurring naturally in the controls (Hoover et al. 1980; Stinson
et al. 1981).
Special Studies on Enzyme Induction
Groups of B6C3F1 mice (six animals per sex) were fed diets containing
nitrofen (technical, 92 percent pure) at levels of 0, 10, 100 or 1000
ppm or two weeks prior to determination of individual liver to body
weight (L:BW) rations and in vitro hepatic p-nitroanisole (pNA)
O-demethylase activity. The dose levels were chosen after acute
studies (three-day gavage dosing), using CD-1 mice, showed a
sex-dependent minimum effect level for hepatic pNA-O-demethylase
activity between 10 and 50 mg/kg/day. The 100 and 1000 ppm dietary
nitrofen levels resulted in elevated liver mixed function oxidase
(MFO) activity in both sexes, with females showing a greater response
(170 percent and 236 percent of control, respectively, for the two
diet levels). L:BW ratios were increased 20 percent and 30 percent in
males and females, respectively, but only at the 1000 ppm level. No
significant changes were observed in either sex fed the 10 ppm
nitrofen diet. The no-effect level found for MFO induction was much
lower than that for oncogenicity (2 348 ppm in the National Cancer
Institute 1978 oncogenicity study). (Deckert & Steigerwalt 1979c)
Special Studies on Dermal Irritation
No evidence of irritation developed when both technical grade (95
percent pure) and pure nitrofen were applied either as a 10 percent
solution in maize oil or as moistened powder to the skin of albino
rabbits (Ambrose et al. 1971).
Special Studies on Cutaneous Sensitization
There was no visible evidence of erythema, swelling, or discolouration
of the test sites after each sensitizing injection or after the
challenging dose of aqueous suspension of technical grade (95 percent)
nitrofen. Under the conditions of the test, these finding indicate
that nitrofen is not a sensitizing agent (Ambrose et al. 1971).
Special Studies on Cataractogenic Effect
Groups of 30 two-week-old Peking ducklings were fed diets containing
nitrofen at levels of 0, 0.05 and 0.1 percent for 13 weeks. Distinct
growth depression occurred at the 0.1 percent nitrofen dose level and
slight retardation at 0.05 percent. The mortality rate in the highest
dose group was distinctly increased. No clinical or histologic signs
of cataract were observed in any of the treated or control animals,
nor were other eye abnormalities found (Ensel & Seinen 1970).
Special Studies on Methaemoglobin Formation by Aminonitrofen
Methaemoglobin formation in vitro by nitroso and amino derivatives
of nitrofen and other chlorinated biphenyl ethers was investigated in
suspensions of human erythrocytes. Ability to induce methaemoglobin
formation among the nitroso and amino derivatives decreased with
increasing chlorine substitution of the biphenyl ether. However, amino
derivatives from no methaemoglobin in suspensions of erythrocytes
alone, but do so after activation by liver homogenate. Methaemoglobin
formation after intraperitoneal and oral administration to male Wistar
rats of chlorinated p-aminobiphenyl ether at levels of 0.77 mg/kg b.w.
gave almost the same result (ca. 40 percent methaemoglobin within
60-120 min.) after either route was used (Miyauchi et al. 1981).
Special Studies on the Stability of the Metabolite Azoxynitrofen
Azoxynitrofen (a possible vegetable metabolite of nitrofen) was added
to simulated gastric fluid and simulated intestinal fluid at level of
7 ppm. There is a slight degradation of azoxynitrofen in gastric fluid
during 24 h. The intestinal fluid completely decomposes azoxynitrofen
within 24 h. After 14 days, there is evidence of azoxynitrofen in
either digestive fluid, and it appears that aminonitrofen is a major
product of the decomposition (Warso 1972).
Acute Toxicity
The acute toxicity of nitrofen in animals is summarized in Table 1.
Most deaths occurred two to eight days after dosing and were preceded
by progressive depression. There was no evidence of diarrhoea.
Postmortem findings were negative in rats that died and in survivors
autopsied at 14 days (Ambrose et al. 1971).
Short-Term Studies
Rat
Groups of albino Wistar rats (10 males and 10 females per group) were
fed diets containing technical grade nitrofen (95 percent pure) at
levels of 0, 100, 500, 2 500, 12 500 and 50 000 ppm for 13 weeks.
Rats on the 50 000 ppm diet failed to survive beyond the first week
and survival of rats on the 12 500 ppm diet was adversely affected.
Growth was depressed in both sexes at 12 500 ppm and in males at 2
500. In rats on the lower dietary concentrations of nitrofen, no
adverse effects were noted in growth, food consumption and mortality.
Haematologic values appeared to be within normal range and urinary
tests for reducing substances and protein showed no marked differences
from the controls. Significantly higher liver-to-body weight ratios
were found in rats at all dietary levels of nitrofen, except for male
rats at 100 ppm, and appeared to be dose-related. Kidney body weight
ratios were significantly elevated for females at 12 500 ppm and for
males at 2 500 and 12 500 ppm. Testes body weight ratios were
significantly elevated for rats at 2 500 and 12 500 ppm. Lesser
effects were noted for heart and spleen. Histopathologic findings
related to treatment appeared to be confined to rats receiving diets
containing 12 500 and 50 00 ppm nitrofen. The principal findings
consisted of a hepatitis characterized by oedema, peripheral
localization of glycogen granules, swelling of the cytoplasm and liver
nuclei with prominent nucleoli (Ambrose et al. 1971). (Full
experimental data were not available.)
Rabbit
Groups of five male and five female rabbits received dermal
applications of nitrofen (as a water-diluted emulsifiable formulation)
at levels of 0, 250 or 1 250 mg/kg b.w., 7 h/day, 5 days/week for
three weeks. Additional groups received applications on abraded skin.
There was no mortality and no evidence of any skin irritation. No
histological changes were noted in the skin of exposed animals. There
was no evidence of subacute systemic damage to liver, kidney, lung or
spleen on histological examination (Brown 1964).
Table 1 Acute Toxicity of Nitrofen in Animals
Animal Sex Route Vehicle LD50 a.i. Reference
g/kg b.w. purity
Rat M oral maize oil 2.63 95% Ambrose et al. 1971
M oral maize oil 3.58 100% Ambrose et al. 1971
M oral peanut oil 2.84 99% Kimbrough et al. 1974
F oral peanat oil 2.40 99% Kimbrough et al. 1974
oral undiluted 5.00 Swann 1973
formulation (as 25%
formulation)
M+F dermal xylene 5.00 99% Kimbrough et al. 1974
inhalation undiluted 205 mg/l Swann 1973
(1h exper.) formulation (as 25%
formulation)
M+F inhalation undiluted >271 mg/cu.m. Brown 1965
(1h exper.) formation (as a.i.)
Rabbit dermal undiluted >2.00 Swann 1973
(24 h) fomulation (as 25%
formulation)
Dog
Groups of six-month-old beagles (two males and two females/group) were
fed diets containing technical grade (95 percent pure) nitrofen at
levels of 0, 20, 200 and 2 000 ppm for two years. The dogs on the
various dietary levels of nitrofen survived the two-year feeding
period. No toxic signs attributable to nitrofen were seen at any time.
The general health, appearance, behaviourial pattern, body weight gain
and cumulative food consumption were not different than those of the
respective controls. Haematologic values and urinary tests for
reducing substances and protein showed no effect of treatment at any
of the test periods. BSP, SGOT, SAP and BUN tests made during the
month 24 showed no adverse trends. Significantly higher liver-to-body
weight ratios appeared only in dogs on 2 000 ppm nitrofen.
Histopathologic examination of major organs revealed no abnormalities
or lesions resulting from the ingestion of nitrofen for two years, in
kind or incidence, that were not found in control dogs. In general,
only the 20 and 200 ppm diet levels of nitrofen exhibited no adverse
effects (Ambrose et al. 1971).
Long-Term Studies
Rat
Groups of weanling Wistar rats (25 males and 25 females/group)
received diets containing technical grade (95 percents pure) nitrofen
at levels of 0, 1, 10, 100 and 1 000 ppm for 97 weeks. The study was
terminated at 97 weeks, owing to poor survival (50 percent) in all
groups, including the controls, beyond week 65. Statistically
significant decreases in mean body weight were observed in the males
at 100 and 1000 ppm after the week 52 and in the females at the same
dose levels until week 26.
Food consumption, haematological and urinalysis parameters were not
affected. Significantly higher kidney/body weight and liver/body
weight ratios in male rats on 1 000 ppm were notable and appeared to
be attributable to nitrofen. Histopathologic findings revealed no
lesions in kind or incidence that were not found in the respective
controls. Overall, the 10 ppm and below diet level of nitrofen was
reported to cause no adverse effects (Ambrose et al. 1971). (Lack of
individual animal data precludes further interpretation of this
study.)
COMMENTS
Nitrofen has low acute oral toxicity. Following oral administration,
nitrofen is partially absorbed and rapidly excreted. A small
proportion of the administered dose is distributed to all tissues and
organs. Nitrofen and its metabolites are found primarily in faeces
(accounting for about 75 percent of the administered dose), while
urine contains metabolites accounting for about 15 percent of the
dose. The metabolism of nitrofen involves reduction, hydroxylation and
conjugation.
Two reproduction studies were presented. Because of inadequacies in
the reports the studies were not considered.
Oral exposures to nitrofen during the period of organogenesis produced
neonatal mortality, decreases in the time of survival to weaning, and
a series of abnormalities in rodent offspring. In a mouse
teratology/post-natal study, the lowest dose tested (6.25 mg/kg b.w.)
produced increased incidence of lethal defects at birth and resulted
in decreased lung and seminal vesicle weights at maturity. In a rat
teratology study, diaphragmatic hernias were observed at and above
1.39 mg/kg b.w., but full details were not provided.
Nitrofen in some cases was found mutagenic in the
Salmonella/microsome assay but it did not display any mutagenic
potential in several mammalian systems either in vitro or in vivo.
Two carcinogenicity studies on B6C3F1 mice indicated that nitrofen is
a liver carcinogen, causing hepatocellular carcinomas and
hepatocellular adenomas in both sexes, and haemangiosarcomas in male
mice. In addition, the compound is carcinogenic to female
Osborne-Mendel rats, causing adenocarcinomas of the pancreas. Nitrofen
was not found to be carcinogenic in Fischer 344 rats. Detailed
histological examinations suggest that naturally occurring and
nitrofen-induced liver tumours in mice are morphologically different.
Both studies were of short duration. In the study on Osborne-Mendel
rats, poor survival precluded the evaluation of carcinogenicity in
males.
In a long-term rat-feeding study, a NOEL of 10 ppm was observed.
However, the duration of the study (97 weeks), the poor survival in
all groups (50 percent beyond week 65), and the fact that the study
was not available in extenso, precluded its full evaluation.
An acceptable daily intake could not be estimated, owing to the
evidence of carcinogenicity, the lack of a NOEL for the teratology and
post-natal effects and the inadequacies of several studies, including
reproduction and long-term studies.
REFERENCES - TOXICOLOGY
Adler, I.L. et al. A material balance study in rats using
1970a 14C- TOK labelled in the nitrophenyl
ring. Rohm and Haas Report No. 23-24.
(Unpublished)
Adler, I.L. et al. A material balance study in rats using
1970b 14C - TOK labelled in the dichlorophenyl
ring. Rohm and Haas Report No. 23-25.
(Unpublished)
Ambrose, A.M., Larson, Toxicologic studies on
P.S., Borzelleca, J.F., 2,4-dichlorophenyl-p-nitrophenyl
Blackwell Smith, R. & ether (TOK). Toxicol. Appl. Pharmacol.,
Hennigar, G.R. 19:263-275.
Brown, J.R. A study on the acute inhalation toxicity
1965 of TOK EC-25. Report from the University
of Toronto, Canada submitted to WHO by
Rohm and Haas. (Unpublished)
Brown, J.R. & Mastromateo, E. Subacute percutaneous toxicity of TOK
1964 E-25 in the rabbit. Report from the
University of Toronto, Canada,
submitted to WHO by Rohm and Haas.
(Unpublished)
Byeon, W.H., Hyun, H.H. & Mutagenicity of pesticides in the
Lee, S.Y. Salmonella/microsome system. Korean J.
1976 Microbiol., 14:128-134.
Chernoff, N., Kavlock, R.J., Preliminary report on the perinatal
Gray, L.E. toxicity of nitrofen administered to
1980 mice. Rohm and Haas Report.
(Unpublished)
Costlow, R.D. & Manson, J.M. The heart and diaphragm: target organs
1981 in the neonatal death induced by
nitrofen (2,4-dichlorophenyl-p-
nitrophenyl ether). Toxicology,
20:209-227.
Costlow, R.D., The effect on rat pups when nitrofen
Hirsekorn, J.M., (4-(2,4-dichlorophenoxy) nitrobenzene)
Stiratelli, R.G., was applied dermally to the dam
O'Hara G.P., Black, D.L., during organogenesis. Toxicology
Kane, W.K., Burke, S.S., (in press). Submitted to WHO by
Smith, J.M. & Hayes, A.W. Rohm and Haas.
Decker, F.W. & Percutaneous absorption of 14C-TOK in
Steigerwalt, R.B. rats. Rohm and Haas Report TD77P-59.
1979a (Unpublished)
Deckert, F.W. & Disposition of 14C-TOK after
Steigerwalt, R.B. percutaneous application to a rat.
1979b Rohm and Haas Report TD78P-53.
(Unpublished)
Deckert, F.W. & Fourteen day dietary TOK enzyme
Steigerwalt, R.B. induction study in mice. Rohm
1979c and Haas Report TD78P-58.
(Unpublished)
Ensel, A.B. & Seinen, W. Investigation of TOK on a possible
1970 cataractogenic effect in ducklings.
Central Institut Voor Voedingsonderzock.
Report no. R-3306 submitted to WHO by
Rohm and Haas. (Unpublished)
Gray, L.E., Kavlock, R.J., Prenatal exposure to the herbicide
Chernoff, N., Ferrell, J., 2,4-dichlorophenyl-p-nitrophenyl
McLamb, J. & Ostby, J. ether destroys the rodent Harderian
1982a gland. Science, 215:293-294.
Gray, L.E., Kavlock, R.J., The effects of the prenatal exposure
Chernoff, N. & Ferrell, J. to the herbicide TOK on the postnatal
1982b development of the Harderian gland of
the mouse, rat and hamster. Presented
at Soc. Teratology Meeting 1982.
Submitted to WHO by Rohm and Haas.
Gray, L.E., Kavlock, R.J., Postnatal development alterations
Chernoff,N., Ostby, J. & following prenatal exposure to the
Ferrell, J. herbicide 2,4-dichlorophenyl-p-
1983 nitrophenyl ether. A dose response
evaluation in the mouse. Toxicol.
Appl. Pharmacol. 67:1-14.
Guerzoni, M.E. & Mutagenic activity of pesticides.
Del Cupolo, L. Riv. Sci. Technol. Alimenti Nutr.
1976 Um., 6:161-168.
Hirsekorn, J.M. & Kane, W.W. TOK E-25 percutaneous range-findings
1981 teratology study with postpartum
evaluation. Rohm and Haas Report No.
81R-63. (Unpublished)
Hoover, K.L., Ward, J.M. & Histopathological differences between
Stinson, S.F. nitrofen-induced and naturally occurring
1980 hepatocellular carcinomas in the B6C3F1
mouse. J. Nat. Cancer Inst., 65:937-948.
Kavlock, R.J., Chernoff, N., An analysis of fetotoxicity using
Rogers, E., Whitehouse, D., biochemical end-points of organ
Carver, B., Gray, J. & differentiation. Teratology,
Robinson, K. 26:183-194.
1982
Kimbrough, R.D., 2,4-dichlorophenyl-p-nitrophenyl ether
Gaines, T.B. & Linder, R.B. (TOK). Effect on the lung maturation of
1974 rat fetus. Arch. Environ. Health,
28:316-320
McLeod, P.L. & McCarthy, K.L. TOK dermal cytogenetic study in rats.
1982 Rohm and Haas Report No. 81R-268.
(Unpublished)
Miyauchi, M., Koizumi, M. & Studies on the toxicity of chlorinated
Uematsu, T. p-nitro-biphenyl ether.
1981 I - Methemoglobin formation in vitro
and in vivo induced by nitroso and
amino derivatives of chlorinated
biphenyl ether. Biochem. Pharmacol.,
30:3341-3346.
Myhr, B.C. & Brusik, G. Evaluation of 78-232 in the in vitro
1979 transformation of BALB/3T3 cells assay,
Litton Bionetics Report No. 20992
submitted to WHO by Rohm and Haas.
(Unpublished)
National Cancer Institute. Bioassay of nitrofen for possible
1978 carcinogenicity. Carcinogenesis
Technical Report Series No. 26,
DHEW Publ. No. (NIH) 78-826.
National Cancer Institute. Bioassay of nitrofen for possible
1979 carcinogenicity. Carcinogenesis
Technical Report Series No. 184,
DHEW Publ. No. (NIH) 79-1740,1.
O'Hara, G.P., Can, P.K., The effect of nitrofen
Harris, J.C., Burke, S.S., 4-(2,4-dichlorophenoxy) nitrobenzene
Smith, J.M. & Hayes, A.W. on the reproductive performance
1983 of male rats.Rohm and Haas Report
(Unpublished)
O'Neill, P.J. & Lohse, E. 4,4-DCAB microbial mutagen test. Rohm
1980 and Haas Report No. 80R-4. (Unpublished)
O'Neill, P.J. & TOK: Microbial mutagen tests. Rohm and
Scribner, H.E. Haas Report No. TD79M-307. (Unpublished)
Paik, S.G. & Lee, S.Y. Genetic effects of pesticides in the
1977a mammalian cells. I. Induction of
micronucleous. Kor. J. Zool.,
20:19-28.
Paik, S.G. & Lee, S.Y. Genetic effects of pesticides in the
1977b mammalian cells. II. Mutagenesis in
L5178Y cells and DNA repair induction.
Kor. J. Zool., 20:159-168.
Reustle, J.A. & TOK cytogenetic study in mice. Rohm and
Scribner, H.E. Haas Report No. 79R-173. (Unpublished)
1980
Roser, R.L., Adler, I.L. & A study of the metabolism of 14-C TOK in
Allen, S.S. rats. Rohm and Haas Report No. 23-33.
1971 (Unpublished)
Siou, G. Study of the potential mutagenic
1978 activity of TOK by the Howell-Jolly
body technique. Cabinet d'Etudes et
de Recherches en Toxicologie
Industrielle, Report No. MBL-2199
submitted to WHO by Rohm and Haas.
(Unpublished)
Siou, G. Study of the effect of TOK on the
1979a prenatal and postnatal development
of the rabbit. Cabinet d'Etudes et
de Recherches en Toxicologie
Industrielle, Report No. MBL-2212
and Addendum submitted to WHO by
Rohm and Haas. (Unpublished)
Siou, G. Study of the effect of TOK on the
1979b postnatal development of the rat.
Cabinet d'Etudes et de Recherches
en Toxicologie Industrielle Report
No. MBL-2248 submitted to WHO by
Rohm and Haas. (Unpublished)
Steigerwalt, R.B., TOK comparative metabolism study.
Godfrey, W.J. & Deckert, Rohm and Haas Report No. 79R-169.
F.W. 1980a (Unpublished)
Steigerwalt, R.B., Lisk, D.C. Range-finding study on 14C-TOK. Dermal
& Deckert, F.W. absorption by rat and human skin in
vitro. Rohm and Haas Report No.
80R-110. (Unpublished)
Steigerwalt, R.B., TOK: disposition after percutaneous
Udinsky, J.R. & Deckert, applications to pregnant rats. Rohm
F.W. 1982 and Haas Report No. 81R-65.
(Unpublished)
Stinson, S.F., Hoover, K.L. Quantitation of differences between
& Ward, J.M. spontaneous and induced liver tumors in
1981 mice with an automated image analyzer.
Cancer Letters, 14:143-150.
Stone, L.C. & Manson, J.M. Effects of the herbicide
1981 2,4-dichlorophenyl-p-nitrophenyl
ether (nitrofen) on fetal lung
development in rats. Toxicology,
20:195-207.
Swann, H.E. Acute toxicity report on Rohm and Haas
1973 TOK E-25, dark liquid. Food Drug
Res. Labs. Report No. 1-3784-851
submitted to WHO by Rohm and Haas.
(Unpublished)
Vogin, E.E. Effects of FW925 on the fetal
1971 development in rats. Food and Drug
Research Laboratories Report,
August 10 submitted to WHO by
Rohm and Haas. (Unpublished)
Warso, J.P. Degradation of azoxynitrofen in
1972 simulated digestion fluids. Rohm
and Haas Report No. 23-72-27.
(Unpublished)
Weatherholtz, W.M., Teratogenicity study in rats, TOK
Kapp, R.W. & E-25, TOK E-25 solvent control.
Greenspun, K.S. Report from Hazleton Laboratories,
1979 America Inc. submitted to WHO by
Rohm and Haas. (Unpublished)
RESIDUES
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Nitrofen is a selective pre- and early post-emergence herbicide for
the control of annual grasses and various broad-leaved weeds in cereal
grains and vegetables.
Nitrofen is used in France and some other countries under labels that
prohibit women from handling the product. It is primarily used to
control backgrass (Alopecurus agrestis), Veronica hederaefolia,
Galium aparine, Viola tricolor and other weeds that in winter
wheat and other cereal grains are not adequately controlled by other
commercially available herbicides.
The effective use rate is generally between 1.5 and 2 kg/ha applied to
the soil surface; activity is lost on soil incorporation. Adequate
soil moisture is necessary for efficacy, but excessive moisture may
result in crop injury.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Residue data have been obtained in several countries where nitrofen
was applied to various crops at or about the recommended rates. Two to
four samples were taken at random from the fields and were transported
to the laboratory within two days. The preparation of the sample and
the portion of sample to be analysed were in agreement with the
recommended Codex procedure with two exceptions: only the cloves of
garlic were sent to the laboratory after removing the outer leaves and
all the samples were washed before chopping and deep-freezing.
The analytical results from the United States were obtained by a
method (Adler and Wargo 1975) that measures the parent compound and
the majority of metabolites in the form of the derivative of amine
moiety. The data from other countries indicate the residue level of
the parent compound only. The results of supervised trials are
summarized in Table 1.
FATE OF RESIDUES
In general, nitrofen undergoes metabolic degradation in all systems
(animals, plants, soil and Water) studied. Metabolic pathways include
reduction of the nitro group, conjugation of the resulting amino
group, hydroxylation of the dichlorophenyl ring and formation of
naturally occurring materials, as well as total conversion to CO2.
The structural formula and the occurrence of the important identified
metabolites are shown in Table 2.
In Animals
Cattle milk, urine and faeces collected during the dosing period from
a cow fed a daily ration of 26.2 kg containing 5 mg/kg purified
nitrofen for four days did not contain detectable levels of nitrofen
or aminonitrofen (sensitivity of the method was approximately 0.3
mg/kg). In vitro studies demonstrated that nitrofen is rapidly
reduced to aminonitrofen in fresh rumen fluid (T1/2 is less than or
equal to 10 min). Aminonitrofen was stable in the rumen fluid for 24 h
(Gutemann & List 1967).
Similarly, only trace levels of radioactivity (0.02 to 0.07 mg/kg,
calculated as nitrofen) were found in samples of urine, faeces and
milk from a cow dosed at 5 mg/kg with radiolabelled nitrofen. The
levels of total radioactivity were 1.4 and 1.7 mg/kg in the faeces and
urine, respectively, and 0.14 and 0.16 mg/kg in two samples of milk. A
fat sample contained 1.06 mg/kg 14C, of which 0.25 mg/kg was parent
compound (Roser & Adler 1971).
Table 1 Residues of Nitrofen Resulting from Supervised Trials
Crop and variety Country Application Proharvest Residue (mg/kg)
rate interval Mean
(kg/a.i./ha) No. (days)
Broccoli (head) United States1 3.7 1 74 <0.01 <0.015
Futura 4.5 2 63 <0.01 <0.01
Gem 4.5 1 85 <0.01 <0.01
Green Duke 4.75 2 63 <0.01 <0.01
4.75 2 69 <0.01 <0.01
5.0 1 70 <0.01 <0.01
5.3 2 70 <0.01 <0.01
5.4 1 77 <0.01 <0,01
Cabbage (head) United States1
Head Start 4.5 1 69 <0.01 <0.01
4.5 1 84 <0.01 <0.01
Green 4.5 1 89 <0.01 <0.01
6.7 1 76 <0.01 <0.01
6.7 1 90 <0.01 <0.016
Cauliflower (head) United States1
Snoball 4.5 1 90 <0.01 <0.01
4.5 1 93 <0.01 <0.01
4.6 1 88 <0.01 <0.016
5.6 1 83 <0.01 <0.01
6.7 1 59 <0.01 <0.01
6.7 1 107 <0.01 <0.016
Celery (stalk) United States1
5270 RIMP 4.5 1 109 <0.01 <0.01
5.4 1 127 <0.01 <0.01
5270 5.6 1 52 <0.01 <0.01
5270R 5.6 1 165 <0.01 <0.01
Table 1 (continued)
Crop and variety Country Application Proharvest Residue (mg/kg)
rate interval Mean
(kg/a.i./ha) No. (days)
Garlic (bulb) United States1
Cal Late 5.6 1 234 <0.01 <0.01
5.6 1 250 <0.01 <0.01
6.7 1 229 <0.01 <0.01
7.8 1 241 <0.01 <0.01
Rape (grain) Denmark2 1.2 1 100 - <0.01
1.2 1 101 - <0.01
1.2 1 105 - <0.01
1.2 1 100 - <0.01
1.2 1 101 - <0.01
1.2 1 105 - <0.01
Rioe (grain) United States1
Early Rose 2.2 1 145 <0.01 <0.01
3.4 1 145 <0.01 <0.01
3.4 1 160 <0.01 <0.01
4.5 1 160 <0.01 <0.01
6.7 1 160 <0.01 <0.01
9.0 1 166 <0.01 <0.01
Rice (straw)
Early Rose 2.2 1 145 <0.01 <0.01
3.4 1 145 <0.01 <0.01
4.5 1 166 <0.01 <0.01
9.0 1 166 <0.01 <0.01
Table 1 (continued)
Crop and variety Country Application Proharvest Residue (mg/kg)
rate interval Mean
(kg/a.i./ha) No. (days)
Wheat (grain) France3
Chamlein
4.0 1 239 <0.01 <0.01
4.0 1 264 <0.01 <0.01
Joss 4.5 1 266 <0.01 <0.01
Hardy 8.0 1 236 <0.01 <0.01
Chamlein 8.0 1 264 <0.01 <0.01
Hardy 8.0 1 265 <0.01 <0,01
Wheat (grain) Federal
Republic
Joss of 1.6 1 248 - <0.002
Carstacht Germany4 1.6 1 280 - <0.002
Kranich 1.6 1 284 - <0.002
Caribo 1.6 1 286 - <0.002
2.0 1 270 - <0.002
Benno 2.0 1 279 - <0.002
Carito 2.0 1 286 - <0.002
Hyslop United States1 1.7 1 294 <0.01 <0.01
Druchamp 2.2 1 163 0.00-0.02 0.01
Yamhill 2.2 1 250 <0.01 <0.01
2.2 1 267 <0.01 <0.01
2.2 1 269 <0.01 <0.01
2.2 1 272 0.01-0.09 0.04
Nugaines 2.2 1 272 0.02-0.03 0.02
Hyslop 2.2 1 281 <0.01 <0.01
Yamhill 2.2 1 284 <0.01 <0.01
2.2 1 289 <0.01 <0.01
Table 1 (continued)
Crop and variety Country Application Proharvest Residue (mg/kg)
rate interval Mean
(kg/a.i./ha) No. (days)
Cajame 3.4 1 146 <0.01-0.01 <0.01
Tehame 3.4 1 151 <0.01 <0.01
Hyslop 3.4 1 294 <0.01 <0.01
Cajame 4.5 1 146 <0.01 <0.01
Tehame 4.5 1 151 <0.01-0.01 <0.01
Druchamp United States1 4.5 1 163 <0.01-0.06 0.03
Yamhill 4.5 1 250 <0.01-0.01 <0.01
4.5 1 267 <0.01 <0.01
4.5 1 269 <0.01 <0.01
4.5 1 272 0.01-0.12 0.06
Nugaines 4.5 1 272 0.02-0.06 0.04
Hylop 4.5 1 281 <0.01 <0.01
Yamhill 4.5 1 284 <0.01 <0.01
4.5 1 289 <0.01 <0.01
Cajame 5.6 1 146 <0.01-0.02 0.01
6.7 1 146 <0.01-0.01 <0.01
Tehame 6.7 1 151 <0.01-0.01 <0.01
Yamhill 6.7 1 267 <0.01 <0.01
Wheat (straw) Federal Republic
of Germany4
Joss 1.6 1 248 - <0.002
Carstacht 1.6 1 280 - 0.020
Kranich 1.6 1 284 - 0.007
Caribo 1.6 1 286 - <0.004
2.0 1 270 - 0.004
Benno 2.0 1 279 - 0.010
Carito 2.0 1 286 - 0.005
Table 1 (continued)
Crop and variety Country Application Proharvest Residue (mg/kg)
rate interval Mean
(kg/a.i./ha) No. (days)
Hyslop United States1 1.7 1 294 <0.01 <0.01
Yamhill 2.2 1 252 <0.01 <0.01
2.2 1 267 <0.01 <0.01
2.2 1 269 <0.01 <0.01
Nugaines 2.2 1 272 0.02-0.11 0.06
Yamhill 2.2 1 273 <0.01-0.02 0.01
Druchamp 2.2 1 276 0.01-0.04 0.02
Hyslop 2.2 1 281 <0.01 <0.01
Yamhill 2.2 1 284 <0.01 <0.01
2.2 1 291 <0.01 <0.01
Hyslop 3.4 1 294 <0.01 <0.01
Yamhill 4.5 1 252 <0.01 <0.01
4.5 1 267 <0.01-0.01 <0.01
4.5 1 269 <0.01 <0.01
Nagaines 4.5 1 272 0.02-0.05 0.03
Yamhill 4.5 1 273 <0.01-0.03 0.01
Druchamp 4.5 1 276 0.01-0.02 0.01
Hyslop 4.5 1 281 <0.01 <0.01
Yamhill 4.5 1 284 <0.01 <0.01
4.5 1 291 <0.01-0.02 0.01
6.7 1 267 <0.01 <0.01
Sources:
1 Rohm and Haas 1969-1980;
2 Institute National de Recherche Agronomique 1975b;
3 Institute National de Recherche Agronomique 1975a;
4 Institute National de Recherche Agroncmique 1978;
5 Samples taken from four experimental fields;
6 Samples taken from three experimental fields.
Table 2 Nitrofen Metabolites
Metabolite Occurrence
Aminals Plants Soil
Groups of three female Sprague-Dawley rats received a single dermal
application of 14C-nitrofen (uniformly labelled in the dichlorophenyl
ring) as an undiluted emulsifiable concentrate at a dose level of 125
or 375 mg/kg or as an aqueous dilution (ca. 1.8 percent a.i.) at
levels of 9 or 27 mg/kg a.i.
Urine and faeces were collected twice daily. One rat per group was
sacrificed at 24, 48 and 96 h after dosing. Blood, liver and kidney
were assayed for radioactivity.
Less than 2 percent of the applied doses remained at the application
sites after 96 h while only 30-40 percent of the dose was excreted in
the urine (5-9 percent) and faeces (26-33 percent) during that time.
Blood, liver and kidneys also contained radioactivity at all sacrifice
times (Deckert & Steiserwalt 1979a).
14C-nitrofen (uniformly labelled in the dichlorophenyl ring) was
dermally applied as undiluted emulsifiable concentrate at a level of
120 mg/kg to a female Sprague-Dawley rat. Urine, faeces and volatile
materials were collected daily at 24 h intervals. Total 14C recovery
in this study was 87 percent. At 96 h post-dosing, the rat was
sacrificed and blood, organs and tissue specimens were removed for
14C analysis. After 96 h 8.7 percent of the dose was excreted in
urine and 36 percent in faeces. Only 0.02 percent of the dose was
found as 14CO2, while other volatile materials accounted for 0.001
percent. 14C was found in every tissue analysed. The highest
concentrations were found in skin (17.6 percent of the dose), fat
(484.5 ppm = 2.1 percent of the dose) and muscle at the application
site (158.3 ppm = 0.9 percent of the dose), whereas only 0.4 percent
of the dose remained unabsorbed. A total of 38 percent of the applied
dose was found in tissues. This figure, when compared with the lower
values found in oral studies, suggests that nitrofen may be better
absorbed dermally than by the oral route (Deckert & Steiserwalt
1979b).
Groups of 12 pregnant Sprague-Dawley rats were treated dermally with
14C nitrofen (uniformly labelled in the dichlorophenyl-ring) as
diluted emulsifiable formulation at levels of 0.3 or 27 mg/kg b.w.
a.i. for 6 h on day 11 of gestation. Another group received a single
dose intravenously at a level of 0.3 mg/kg b.w.
At the end of the six-hour application period, only 16 percent of the
dermally applied dose could be removed by washing. Low-dose dermal
application produced blood levels similar to those produced by i.v.
administration, except at the one hour sampling. Urinary 14C
excretion patterns were similar for both dermal application (low and
high dose) and i.v. administration. However, 11 days after dosing, 15
and 20 percent of the dermal (both levels) and i.v. doses,
respectively, were excreted in urine. Foetal 14C excretion patterns
were also similar for both routes of administration, with 45-48
percent and 75 percent of the dose being excreted by 11 days after
dermal and i.v. admin-istration, respectively. After 11 days, 61-65
percent and 97 percent of the dermal and i.v. doses, respectively, had
been excreted. For the higher dermal dose, most maternal tissue 14C
concentrations peaked one to two days after dosing (day 12-13 of
gestation) with the highest concentrations occurring in the fat and
gonads. At 0.3 mg/kg (both routes) heart, thryroid, adrenals,
Harderian glands and pancreas approached maximum levels after six
hours.
Peak 14C levels of most maternal tissues increased with increasing
dose increment and reached a higher level following i.v.
administration than after a dermal application of 0.3 mg/kg.
Placental and foetal 14-C concentrations also peaked within one to
two days after dosing. At 0.3 mg/kg, mean placental 14-C levels were
similar by either route, while the foetal 14-C level two days after
dermal application was five-fold higher than those after i.v.
administration, ten-fold higher than that found in maternal whole
blood at the same time, but only three-fold lower than after the high
dermal dose. At the low dermal dose, the peak or plateau placental
and foetal 14C levels were comparable with those in maternal tissues
(other than the fat and gonads). After i.v. administration and the
high dermal dose, tissue levels in the mother were much higher than in
the placenta or foetus.
In the amniotic fluid, both 0.3 mg/kg dose groups showed the highest
14C levels at 11 days after dosing (day 22 of gestation); the
high-dose dermal group had a steady high 14C level throughout this
period.
Eleven days after dermal application (27 mg/kg), 14C concentrations
in maternal organs were generally higher than in foetal organs, except
for the heart, where levels were nearly the same. Radioactivity was
detected in foetal organs in descending order from kidney to heart,
lung, liver and brain. Metabolic profiles in the placenta and the
embryo/foetus showed the presence of nitrofen, aminonitrofen and other
unidentified metabolites with peak or plateau concentrations occurring
within one to two days. Concentrations of nitrofen and minor
metabolites decreased with time, while aminonitrofen appeared to reach
a plateau and then decrease at a slower rate. More than 90 percent of
14 in the urine and faeces was readily extractable (Steiserwalt
et al. 1982).
Dog
Groups of six-month-old beagles (two males and two females/group) were
fed diets containing technical grade 95 percent pure) nitrofen at
levels of 0, 20, 200 or 2 000 ppm for two years. Tissues (fat, muscle,
bone, liver, kidney, spleen and blood) pooled at week 104 and excreta
(urine and faeces) at week 103 were analysed for unchanged nitrofen by
sas-chromatography. The nitrofen concentration was highest in body fat
(38, 175, 515 ppm, respectively, at the three dietary levels tested),
with lower levels found in bone (3.9, 19.2, 21.8 ppm), muscle (1.26,
4.76, 20.0 ppm), kidney (1.4, 4.6, 13.6 ppm), liver (1.0, 2.0, 3.95
ppm) and blood (0.1, 0.2, 0.4 ppm). Unchanged nitrofen was excreted
almost exclusively in the faeces (39.0 ppm at the highest level)
(Ambrose et al. 1971). (Full experimental data were not provided.)
In vitro dermal absorption
Skin sections from an adult female Sprague-Dawley rat and an adult
woman (postmortem) were mounted in Franz Diffusion Cells and 5 µl of
diluted 14C-nitrofen emulsifiable formulation (ca. 1.8 percent a.i.)
was applied to each. After six hours of exposure, the rat skin
absorbed from 24.7 to 61.7 percent of the 14C while the human skin
absorbed from 13.8 to 25.3 percent of the label. The cell-by-cell
average rat: human absorption ratio of 1.99+/-0.34 indicated that
nitrofen was absorbed by human skin at approximately half the rate
that it passed into rat skin (Steiserwalt et al. 1980b).
TOXICOLOGICAL STUDIES
Special Studies on Reproduction
Rat
In a three-generation, two-litter per generation, reproduction study
groups of 28-day-old Wistar rats (25 males and 25 females/group) were
fed diets containing technical grade (95 percent pure) nitrofen at
levels of 0, 10, 100 or 1 000 ppm for 11 weeks. Body weights for
parental rats before mating and at weaning of respective litters were
not adversely affected for Fo and F1b generations. Body weight of
F2b parent generation rats was slightly decreased for rats at 100
ppm, in breeding the F3a litter and in breeding the F3b litter fed 10
ppm. This decrease was due to lower initial body weights of rats used
for succeeding generations. Although there was considerable
inconsistency within and between matings, average data reported did
not support a dose-related effect on fertility and gestation indices.
Viability and lactation indices were not adversely affected at 100
ppm. However, the viability index for rats at 1 000 ppm was seriously
affected, as stillborn pups outnumbered live pups; indeed no pups were
available for continuance beyond the Fo generation. In all
generations, the number of stillborn pups from the 100 ppm group
numbered 64 compared with 30 in the control.
No structural or microscopic cellular abnormalities were detected in
any stillborn pups in F1b litters. Weaning weights indicated no
diet-related trends. Overall, only the 10 ppm dietary level of
nitrofen exhibited no adverse effects on reproduction. Examination of
a variety of tissues from 10 F3b generation rats of each sex from the
0, 10 and 100 ppm dietary levels showed no histopathological lesions
(Ambrose et al. 1971). (Full experimental data were not provided.)
Groups of Sherman rats (10 males and 20 females/group) were fed
dietary levels of 0, 20, 100 and 500 ppm technical nitrofen (89
percent) pure). Breeding and F1a and F1b litters commenced at 68 and
200 days, respectively. Offspring were observed through weaning. At
the time of weaning of the F1b litters, 20 female and ten male
weanlings were selected from the 0, 20 and 100 ppm groups, continued
on the parental diet and pair-mated when 112 days old to produce the
F2a generation. Neither the food consumption nor the body weight gain
were greatly affected in any of the nitrofen-treated groups. At the
500 ppm dietary level no offspring of the F1a and F1b generation
survived the neonatal period. The number of pups born alive and the
survivors-to-weaning were reduced at the 100 ppm dietary level but not
at 20 ppm (equal to 1.1 and 1.8 mg/kg b.w. in F1b and F1a,
respectively) (Kimbrough et al. 1974). (Full experimental data were
not provided.)
Groups of 25 male Sprague-Dawley rats were fed diets containing 0,
100, 500 or 2 500 ppm of nitrofen technical (95.7 percent pure) for 95
days. At that time, male and untreated female rats were cohabited
(1:1) for a maximum of 10 days. During co-habitation, males were fed
an untreated diet and daily doses of 0, 6, 30 or 155 mg/kg of nitrofen
were administered by gavage. The nitrofen dosage was based on the
dietary intake of the compound (mg/kg/day) during weeks 10-13 of the
study.
No significant effects were detected on the reproductive performance
of male as indicated by the average number of days for mating to
occur, percentage of females impregnated or number of mated females
that delivered. Furthermore, no differences were noted in the length
of gestation, litter size, percentage of live or dead births or sex
ratio of the offspring. No compound-related signs of toxicity were
evident in any of the progeny from birth to day 35. Necropsies of
offspring that died and of female parents which were killed after
lactation revealed no compound-related gross abnormalities. Neither
viability nor mean weights of offspring were affected at any dose
level. Male reproductive performance was not affected at dietary
concentrations up to and including 2 500 ppm, equal to 186 mg/kg/day
(O'Hara et al. 1983).
Special Studies on Teratogenicity and Postnatal Effects
Mouse
Two studies on postnatal dose response and standard teratology are
summarized together.
Purified nitrofen (99.6 percent pure) in maize oil was administered to
CD-1 mice by gavage. In the postnatal study, 24 animals were dosed
(50, 100, 150 and 200 mg/kg/day) on days 7-17 of gestation and allowed
to give birth. In the teratology study animals were dosed 50, 100 and
200 mg/kg/day) on days 7-17 and sacrificed on day 18. Foetuses were
removed and analysed for visceral and skeletal malformations.
Concomitant control groups were utilized in all the studies.
In the postnatal study there was a significant dose-related delay in
time of birth. Significant dose-related reductions in growth and
viability were noted at all dose levels. There was considerable litter
mortality in the 100 mg/kg/day groups between days 3 and 17
postpartum. Only 23 percent of the pups alive on day 3 were still
alive by day 17. Mortality in the 50 mg/kg/day dose group was not
different from control values during this period. Microphthalmia or
anophthalmia was seen in a high percentage of the animals in the 100
mg/kg/day dose group. A dose-related growth retardation was noted in
pups until 60 days of age at 50 mg/kg and above. There were delays in
the percent of female offspring showing vaginal opening at 30 days and
in the percentage of animals breeding by 60 days at 50 mg/kg and
above. Litter size was unaffected by treatment.
In the teratology study dose-related increases in maternal weight gain
and liver/ body weight ratios were recorded. Nitrofen significantly
increased the supraoccipital scores, indicating retarded skeletal
development of the skull. It was teratogenic at 100 and 200 mg/kg/day,
the most common defects noted being cleft palate and undescended
testes (Chernoff et al. 1980). (Full experimental data were not
available.)
After being dosed at levels of 100 mg/kg b.w. nitrofen on days 7 to 17
of gestation, pregnant CD-1 mice showed reduced mean litter size.
Surviving pups had delayed eye opening and absence of the Harderian
gland (Gray et al. 1982a).
Groups of pregnant CD-1 mice were dosed by gavage with nitrofen (99.6
percent pure) in maize oil at levels of 0, 6.25, 12.5, 25 and 50
mg/kg/day. There were 23, 19, 27, 21 and 17 litters, respectively, on
day 1. The animals were observed until necropsy at 110-130 days of
age.
The administration of nitrofen did not cause any adverse effects in
the dams, as measured by maternal viability or weight gain from days 7
to 19 of gestation. By day 3 the number of pups was reduced
significantly at 50 mg/kg (20 percent). Body weights were initially
unaffected after birth at 50 mg/kg but subsequent body weight was
reduced in the pups from the 12.5 mg/kg treatment group. There was a
dose-related increase in the frequency of lethal defects in the pups,
i.e. cleft palate, diaphragmatic hernia and extreme abdominal
distention. Lung weight was significantly reduced by nitrofen
treatment at all doses, including the lowest dose of 6.25 mg/kg.
Seminal vesicle weights were significantly reduced at 6.25, 12.5 and
50 mg/kg. Obvious intraorbital defects were present in some offspring
in the 25 mg/kg group and above. Prenatal nitrofen treatment
significantly reduced the weight of the Harderian glands at all doses,
including the lowest dose of 6.25 mg/kg. Prenatal nitrofen treatment
of 50 mg/kg significantly delayed puberty in the female mice, as
indicated by the percentage of females with patent vasina on day 30.
In addition, the 50 mg/kg females paired with treated males produced
significantly fewer pups in the second litter.
A cross-fostering experiment with 100 mg/kg demonstrated that the
defects noted in the present study were produced solely by prenatal
exposure; pups exposed to nitrofen in the milk alone as a consequence
of any accumulation of nitrofen in the dam during gestation were
unaffected (Gray et al. 1983).
Rat
Groups of 26 pregnant FDRL rats were administered nitrofen by gavage
on days 6-16 of gestation. The control animals received maize oil (1
ml/kg b.w.) whereas the test animals received 100 and 200 ppm of
nitrofen in the same volume of vehicle. Thus the dosage corresponds to
5 and 10 mg/kg b.w./day, respectively. On day 22, the animals were
sacrificed and Caesarean section performed. There were no differences
between control and treated groups with respect to mean dam weight,
implantation sites, foetal viability or mean live foetal weight.
Resorptions were increased in the high-dose group. No malformed
foetuses were seen at the time of Caesarean sections, nor was there
evidence of soft tissue anomalies. Numbers of incomplete cranial bone
ossification and scoliosis were slightly increased in both treated
groups. These variations were considered "consistent with changes
previously observed in the colony of rats" and, thus, not
treatment-related (Vosin 1971).
Groups of seven pregnant Wistar rats were administered by gavage daily
doses of nitrofen technical (96.2 percent pure) at levels of 68 mg/kg
b.w. on days 6 through 15 of gestation. Five pregnant control rats
received only peanut oil. Parameters evaluated were neonatal mortality
and histomorphology of the lungs of dead and moribund newborns in the
nitrofen-treated group and of four-day-old survivors from the control
and treated groups. All the offspring from the control group were
alive at birth. Most (85 percent) of the offspring from the treated
group were born dead or died within the first few hours after delivery
and exhibited generalized cyanosis. Histological examinations of the
lungs of moribund offspring from the treated group revealed a large
number of alveoli bordered by a cubical epithelium (Siou 1979b).
Groups of 9-12 pregnant Sherman rats were dosed by oral intubation on
days 7 through 15 of gestation. Dosage levels were 0 (peanut oil), 10,
20 or 50 mg/kg/day for technical nitrofen (89 percent pure); 0, 20, or
50 mg/kg/day for purified nitrofen (99 percent pure); and 0 or 0.04
mg/kg/day for 2,7-dichlorodibenzo-p-dioxins (DCDD) (this dose is
equivalent to a contamination level of 2 000 ppm (0.2 percent) in the
technical nitrofen for a 20 mg/kg dose). The pups were observed to
weaning. At the dosage levels of 20 and 50 mg/kg/day a dose-related
decrease of live-born rats was observed for both the technical and
purified nitrofen groups. None of the pups survived at the 50 mg/kg
dose level and survival-to-weaning was severely reduced (50 percent)
in both the technical and purified nitrofen groups. No effect of
technical nitrofen on the number of live pups born and their
survival-to-weaning was observed at 10 mg/kg dose level. Extensive
inflammation, fibrosis and epithelial proliferation of the lung were
seen in sucklings that died at 11 to 15 days. No effect on
survival-to-weaning was observed in DCDD-treated rats.
Additional groups of ten female and ten male rats were fed technical
nitrofen at dietary levels of 0 and 500 ppm. The rats were started on
the diet at weaning and pair-mated when they were 90 to 100 days old.
On day 20 of pregnancy, foetuses were removed by Caesarean section and
examined for malformations. The live foetuses were kept in an
incubator for six hours and closely checked for viability. Most of the
foetuses of the controls were pink and survived until they were killed
after six hours. The pups from nitrofen-treated dams were cyanotic and
died one to three hours following the Caesarean section.
Two more pups of pregnant rats were given by oral intubation technical
nitrofen at levels of 0 or 50 mg/kg/day on days 7 to 18 of gestation.
The foetuses from these rats were removed by Caesarean section on day
21 of pregnancy. Lungs from 18 exposed and 16 control foetuses from
four litters in each group were removed one to three hours following
the Caesarean section and studied with light- and electron-microscopy.
Pups from treated dams showed cyanosis at birth. The cyanosis
increased during the next 45 to 60 minutes and most of the pups from
treated dams died, while the control rats developed pink colour soon
after birth and survived. Lungs of treated foetuses were poorly
expanded and alveolar epithelium was cuboidal and resembled cells of
earlier gestational age (Kimbrough et al. 1974).
When purified nitrofen (containing only trace amounts (ppm) of
4-aminonitrofen) was administered to pregnant Long-Evans rats at
different days of gestation, gestation day 11 was demonstrated as
critical: a single dose of 150 mg/kg on day 11 killed 56 percent of
the newborn pups withing 48 h.
Groups of 4-7 Long-Evans dams were given a single dose of purified
nitrofen at levels of 0, 75, 115, 150, 200 or 250 mg/kg on day 11 of
gestation. Survival of neonates to five days was monitored. A
dose-related increase in neonatal mortality was observed. The LD50
for the neonates was estimated at 116 mg/kg of maternal body weight.
Initial experiments demonstrated that animals surviving the first five
days usually lived to maturity. Thus, the mortality is most accurately
described by the fraction dying within the first five postnatal days.
Surviving offspring were autopsied at 35 days of age and examined for
gross lesions. The predominant lesion observed in all treated groups
was hydronephrosis. The incidence of hydronephrosis in survivors
paralleled the neonatal mortality and the lesion was most frequent
among animals exposed on day 11. The incidence of hydronephrosis was
also dose-related among animals exposed only on day 11.
Nitrofen-induced hydronephrosis was not considered incompatible with
life.
In a further teratology study purified nitrofen was administered to
pregnant Long-Evans rats at dose levels of 0, 70, 115, 150, 250, 265
and 400 mg/kg b.w. on day 11 of gestation and foetuses collected on
day 22. Foetuses exposed to nitrofen and examined at term (day 22),
showed delayed skeletal ossification. There was a dose-related
depression in foetal body weight. Nitrofen exposure on day 11 induced
hydronephrosis, which was dose-related in incidence and severity.
Diaphragmatic hernias were also observed in the treated groups. The
overall frequency of soft tissue anomalies was also dose-dependent.
Mean litter frequencies of these anomalies ranged from 45 percent at
LD15 (70 mg/kg) to 91 percent at LD99+ (400 mg/kg). Nitrofen exposure
produced a dose-related increase in the frequency of cardiac
malformations, which included in order of decreasing frequency:
ventricular septal defects (VSD), double outlet right ventricules
(DORV), and transposition of great vessels. Incidence of lethal
cardiac malformations was 38 percent and 53 percent at 150 mg/kg and
250 mg/kg, respectively. No anomalies were seen in the control
litters. None of the cardiac or diaphragmatic defects in the term
foetuses were present in survivors, suggesting that the heart and
diaphragm are the target organs in nitrofen-induced neonatal deaths
(Costlow & Manson 1981).
Groups of five pregnant Sprague-Dawley rats were treated with nitrofen
(98 percent pure) at 0, 20, 31.2 or 50 mg/kg/day by gavage on days
8-18 of gestation. Significant differences in birth weights were
apparent in pups from the 31.2 and 50 mg/kg/day groups. A decrease in
the lung to body weight ratio of foetuses exposed to nitrofen was also
observed. Most, if not all, pups were born alive and lacked
externally-observable malformations. Within minutes after birth,
treated pups exhibited laboured breathing and became cyanotic. Death
usually ensued 30 min. to 4 h postpartum. Survival was significantly
depressed in all treatment groups and all time intervals (1 h, 24 h,
25 days) measured after birth. On examination, symptomatic
nitrofen-exposed pups suffered massive atelectasis at autopsy, with
few expanded air sacs in the 31.2 and 50 mg/kg groups. In contrast,
lungs from newborn controls exhibited normal alveolar expansion. The
level of glucocorticoids and capacity of the foetal lung to respond to
glucocorticoids by synthesizing and releasing surfactant were not
affected by prenatal exposure to nitrofen (Stone & Manson 1981).
Missing and/or malfunctioning Harderian glands were observed when
pregnant Sprague-Dawley CD rats were given daily doses of nitrofen at
a level of 12.5 mg/kg by gavage on days 8 to 16 of gestation (Gray
et al. 1982a).
A postnatal study, using rats (strain unknown) dosed on days 7-16 at
five doses from 0 to 25 mg/kg, demonstrated that nitrofen caused
diaphragmatic hernias at 1.39 mg/kg and above and affected the
haemoglobin level at 12.5 mg/kg (Gray et al. 1982b). (This
information was provided in abstract form and cannot be further
interpreted.)
Groups of five pregnant CD rats were given by gavage daily doses of
nitrofen at levels of 0, 12.5 or 25 mg/kg b.w. on days 8 to 16 of
gestation. Dams were sacrificed on day 21 of gestation and foetuses
removed for examinations. Dose-related increase in foetal mortality
and dose-related decrease of mean foetal weight were determined.
Diaphragmatic hernias occurred in both dose groups in a dose-related
fashion. Brain weight and brain DNA were statistically significantly
reduced at 25 mg/kg; lung weight and lung surfactants were reduced at
12.5 and 25.0 mg/kg in a dose-related fashion as were liver weight,
liver glycogen content, kidney weight and kidney alkaline phosphatase
levels. However, statistical analysis demonstrated that only effects
on lung and liver represented specific organ toxicity, while brain and
kidney effects were correlated to the foetal growth retardation
(Kavlock et al. 1982).
When pregnant Sprague-Dawley rats (35/group) were exposed dermally to
nitrofen emulsion at 0, 0.3, 3.0 or 30.0 mg/kg/day on days 6 to 15 of
gestation, foetal weight and foetal length were significantly lower in
the 30.0 mg/kg group compared to the control group. Soft tissue
anomalies (hydronephrosis, ectopic testes, liver and/or stomach and/or
intestine protruding into thorax, diaphragmatic hernia, right-sided
aorta) were observed almost exclusively in the foetuses of the 30.0
mg/kg group. The foetal viability (live at birth/implantations) in all
the treated groups was statistically significantly lower than the
control litters. Neonatal survival index (live at day 4/live at birth)
was significantly lower in the 30.0 mg/kg group than in the control
group (Weatherholtz 1979).
Groups of five to seven pregnant Charles River rats were treated
dermally with nitrofen (as a water-diluted emulsifiable formulation)
at levels of 0, 0.6, 4.8 or 19.2 mg/kg/day on days 6 to 15 of
gestation. A further group received dermally 4.8 mg/kg on day 11 only.
Dams were sacrificed on day 21 postpartum. Surviving offspring were
sacrificed on day 27. All maternal rats survived to sacrifice.
Treatment of pregnant rats with 19.2 mg/kg/day of nitrofen on days 6
to 15 of gestation resulted in slightly decreased weight gain during
the treatment interval. The no-observable effect level (NOEL) was 4.8
mg/kg/day. The treatment caused decreased viability of offspring,
decreased neonatal survival to day 4 and decreased live offspring of
pregnant rats treated dermally with 19.2 mg/kg/day of nitrofen on days
6 to 15 of gestation. Nitrofen did not affect neonatal viability,
neonatal survival, survival to weaning, growth of offspring or eye
opening in offspring of dams treated with 0.6 or 4.8 mg/kg/day on days
6 to 15 of gestation or 4.8 mg/kg/day on day 11 of gestation
(Hirsekorn & Kane 1981).
Groups of 25 pregnant Sprague-Dawley rats were treated dermally with
nitrofen in aqueous emulsion (0, 0.3, 0.6, 1.2 or 12.0 mg/kg b.w./day)
on days 6 to 15 of gestation. No maternal toxicity occurred. At 12
mg/kg neonatal survival was reduced. Necropsies of the dead neonates
showed a high incidence of diaphragmatic hernias. The number of pups
per litter and viability over days 4-41 of age were unaffected by
treatment. About half (randomly selected) of the pups from each litter
and group were necropsied on day 42. Chromo-dacryorrhea, reduced or
absent Harderian glands and diaphragmatic hernias occurred at 12.0
mg/kg. The frequency and the severity of dilation of the renal
pelvices increased with the dose; slight dilation was significantly
elevated at 0.3 mg/kg and moderate and severe dilation became
prominent at 0.6 and 12.0 mg/kg, respectively. In the 12.0 mg/kg group
nitrofen reduced the body weight of the survivors and also the
relative weights of the Harderian gland, the thyroid gland of both
sexes; lungs of females were significantly depressed with respect to
solvent and water control groups. The findings from necropsies
performed on the remaining offspring on days 146 to 149 postpartum
were similar to those at day 42. Body weight was unaffected by
nitrofen exposure but dilated kidneys and diaphragmatic hernias were
observed. Relative thyroid weight was significantly depressed only in
the 12 mg/kg dose group. A NOEL was not demonstrated (Costlow et al.
1983).
Rabbit
Groups of 15 pregnant New Zealand rabbits were given encapsulated
technical nitrofen (96.2 percent pure) at levels of 0, 7, 27 or 108
mg/kg on days 6 through 18 of gestation. Ten rabbits in each group
were sacrificed on day 28 of gestation to assess embryotoxic and
teratogenic effects. The average number of live foetuses and
implantation sites per pregnant female was slightly decreased in the
108 mg/kg group sacrificed on day 28 of gestation. Percentage of dead
foetuses with respect to implantation sites was increased in all
treated groups compared with the control group, but not in a
dose-related manner. The increased incidence of foetuses, with 13
pairs of ribs was dose-related. No other anomalies were observed. The
remaining five rabbits in each group were allowed to deliver, and they
and the newborns were sacrificed 48 h after delivery. The average
number of live offspring was slightly lower in the 108 mg/kg group.
Average weights of live foetuses after 48 h were slightly lower in the
treated groups, but not in a dose-related manner. No significant
differences were observed in survival rate to 48 h or in resorption
rates. An increased incidence of 13 ribs was observed in all treated
groups. Histological examinations of the lungs removed from between 5
and 17 of the 28-day foetuses and the 48-hour newborns in each group
did not indicate retardation of lung maturation (Siou 1979a).
Special Studies on Mutagenicity
Microbial systems
Nitrofen, when tested with Salmonella typhimurium strains TA1535,
TA1538, TA100 and TA98, both with and without S-9 activation, was
found only moderately mutagenic in TA100 with microsomal enzyme
activation (Byeon et al. 1976).
Purified nitrofen (99+ percent) and several samples of nitrofen
technical (90-98 percent pure) were tested at concentrations of
0.001-10.0 µg/plate with S. typhimurium strains TA1535, TA1537 TA98
and TA100. Tests were carried out both with and without microsomal
activation. Positive controls were used: 2-aminofluorene and
2-acetamidofluorene for TA98 and 2-anthramine for all the strains
tested. Only one sample of nitrofen technical (90-92 percent pure) was
completely negative in the test. The other samples of technical
nitrofen gave statistically significant increases of mutants/plate
compared with negative control in TA100 strain, both with and without
activation, but only one was positive in TA98 with activation.
However, a dose-response relationship was observed only with three
samples (97, 97 and 98 percent pure), but not with another three (93,
92.8 and 97.7 percent pure), generally at concentrations of 10-10 000
µg/plate. Purified nitrofen (99+ percent pure) gave a statistically
significant increase of revertants/plate in TA100, both with and
without activation; no dose-response relationship was observed.
Thus purified nitrofen and three technical samples gave an indication
of mutagenicity, but the other three technical samples were positive
(O'Neill & Scribner 1979).
Nitrofen was found mutagenic in a reversion test using
Saccharomyces cereviseae strain 632/4 (auxotrophic for methionine)
(Guerzoni et al. 1976).
Mammalian systems
The mutagenicity of nitrofen was assayed in the murine lymphoma cell
line L5178Y at concentrations of 0.1 x 10 (E-4), or 2 x 10 (E-4) M.
Doses were selected as those killing approximately 40 and 80 percent
of cells. Ethylmethanesulphonate was used as a positive control.
Induction of methotrexate-resistant mutants of L5178Y cells treated
in vitro with nitrofen was not significantly different from the
negative control (Paik & Lee 1977b).
Nitrofen was tested in the unscheduled DNA repair synthesis assay with
human lymphocytes (concentrations not clear). Nitromin was used as a
positive control. 3H-thymidine incorporation in the nitrofen-treated
cells was not significantly different from the negative control (Paik
& Lee 1977b).
Nitrofen was also tested in mouse cells in a BALB/3T3 in vitro
transformation assay. The compound did not induce a significant
increase in transformed foci over the applied concentration range of
0.1 to 60 micrograms/ml. This range corresponding to approximately 50
to 90 percent survival in the cytotoxicity tests. A negative control
consisting of assays performed on untreated cells was included. A
positive control with 3-methyl cholanthrene (MCA), used at level of 5
µg/ml for each assay, gave a marked increase of the average
number/flask of foci of transformed cells. Therefore, nitrofen was
considered to be inactive in the BALB/3T3 in vitro transformation
assay (Myhr & Brusick 1979).
Groups of three Swiss Webster albino mice were given intraperitoneally
two doses, separated by 24 h, of nitrofen (purity not specified) at
levels of 0, 500 or 1 000 mg/kg b.w. Six hours after the last dose,
three bone marrow smears from each mouse were prepared and about 2 000
polychromatic erythrocytes per mouse were analysed for the presence of
micronuclei. For both dose levels tested, the incidence of micronuclei
was not statistically significantly different from the control. A
positive control of cyclophosphamide gave the expected increase of the
incidence of micronuclei (Paik & Lee 1977a).
Groups of 5-10 male Swiss mice were given by gavage two doses of
nitrofen technical (96.2 percent pure) at levels of 0, 0.25, 1.00 and
1.25 ml/kg, spaced 24 h apart. The animals were sacrificed 6 h after
the second dose and bone marrow smears were prepared. The incidence of
polychromatic erythrocytes carrying "micronuclei" in the treated
groups was not significantly different from the control group (Siou
1978).
Groups of 24 male Charles-River CD-1 mice were given by gavage a
single oral dose of nitrofen technical (95.7 percent pure) at levels
of 0, 0.39, 0.79 and 1.58 g/kg, representing 1/8, 1/4 and 1/2 of the
acute oral LD50. Additional groups of 8 male mice were given once
daily five doses of nitrofen at the same levels. A positive control
group received triethylamine (TEM) 0.3 mg/kg i.p. Bone marrow slides
were prepared from 8 animals/group sacrificed at 6, 24 and 48 h after
the acute dose and 6 h after the last sub-acute dose and TEM dose. All
animals received colchicine at 2 mg/kg i.m. two hours prior to
sacrifice. Fifty metaphase cells per animal were scored when possible.
Owing to poor survival, the high dose subacute group was sacrificed on
day 4. No statistically significant increase in chromosomal
aberrations was observed in treated animals compared with controls,
both in the acute and subacute regimen (Reustle & Scribner 1980).
Nitrofen technical (95.7 percent pure) was administered in m-xylene
(99 percent) by dermal application to groups of eight male Charles
River CD rats at doses of 0, 0.05, 0.125 and 0.50 g/kg, to assess the
potential of nitrofen to induce chromosomal aberrations in mammalian
bone marrow cells. A positive control group received a single 0.3
mg/kg i.p. dose of triethylenemelamine. The doses were administered
according to both a single and repeated (daily × five days) regimen.
Animals were killed and bone marrow slides prepared at approximately
6, 24 and 48 h after the single dose, 24 h after the positive control
dose and 6 h after the last multiple dose. All animals received
colchicine (1 mg/kg, i.p.) three hours prior to being killed.
Metaphase cells (50 per animal) were examined for chromosomal
aberrations. Nitrofen at 0.5 g/kg and below did not induce a
significant increase in chromosomal aberrations in bone marrow cells
at either 6, 24 or 48 h after single or repeated exposure. Chromosomal
aberrations were consistently elevated at 24 h after treatment with
triethylmelamine (McLeod & McCarthy 1982).
Special Studies on Mutagenicity of Aminonitrofen
Aminonitrofen (99+ percent pure), a metabolite of nitrofen, was tested
in concentrations of 0.1-1 000 µg/plate with S. typhimurium strains
TA1535, TA1537, TA98 and TA100 with and without a microsomal enzyme
preparation. Statistically significant increases of revertants/plate
were observed with strains TA1535, TA98 and TA100 using a microsomal
enzyme preparation and a dose-response relationship was exhibited.
Positive controls were 2-anthramine, 2-aminofluorene and
2-acetamidofluorene (O'Neill & Scribner 1979).
Special Studies on Mutagenicity of 4,4'-Dichloroazobenzene
4,4'-Dichloroazobenzene (DCAB) (99+ percent technical), a low level
impurity in technical nitrofen, was tested with the S. typhimurium
strains TA1535, TA1537, TA98 and TA100 at concentrations of 1.0-1 000
µg/plate, with and without microsomal activation. Saline buffer
controls were used with each strain. Statistically significant
increases of revertants/plate were observed with strain TA100 at
10-1 000 µg/plate with metabolic activation. DCAB gave a greater
response with strain TA98 than with TA100. Positive metabolic
activation; and 2-anthramine (10 µg/plate), positive with TA1535,
TA1537 and TA100 with metabolic activation; and 2-acetamidofluorene
(50 µg/plate), positive with TA98 with metabolic activation (O'Neill &
Lohse 1980).
Special Studies on Carcinogenicity
Mouse
Groups of 50 male and 50 female young B6C3F1 mice (age not specified)
were fed diets containing technical nitrofen (stated to be >80
percent pure; impurities unspecified) dissolved in maize oil for 78
weeks at levels of 1 775-2 500 mg/kg (low-dose) or 3 550-5 000 mg/kg
(high-dose), to give time-weighted average concentrations of 2 348 and
4 696 mg/kg in the diet. A group of 20 male and 20 female controls
received the basal diet containing 2 percent maize oil. All mice were
observed for a further 12 weeks after treatment. Weight gain was
depressed in both groups of treated females and in the males exposed
to the high dose. Beginning at week 54, pronounced abdominal
distension was displayed by an increasing number of treated mice. By
the end of the study, only 10 percent of the control male mice, 34
percent of the high-dose group and 54 percent of the low-dose group
were still alive; and, of the female mice, 62 percent were still alive
in the high-dose group, 54 percent in the low-dose group and 85
percent of the controls. For neither sex could a positive association
be established between dosage of nitrofen and mortality. A high
incidence of hepatocellular carcinomas was found in exposed male and
female mice: in 4/20 control males, 36/49 low-dose males and 46/48
high-dose males, in none of the control female mice (both matched and
pooled controls), in 36/41 low-dose females and in 43/44 high-dose
females. A few of these tumours metastasized to other sites. The
difference in incidence between control and exposed male and female
mice was statistically significant at both dose levels.
Haemangiosarcomas of the spleen were found in 2/47 of high-dose males
and haemangiosarcomas of the liver in 1/49 low-dose males and 2/48
high-dose males. In female mice, haemangiosarcomas were seen in the
spleen in 1/18 controls, in the liver in 4/44 high-dose animals and in
the abdominal cavity in 1/43 of the high-dose group.
The incidence of haemangiosarcomas in the high-dose male mice, but not
females or low-dose males, was statistically higher than controls
(National Cancer Institute 1978).
Groups of 50 male and 50 female six-week-old B6C3F1 mice were fed
diets containing 3 000 or 6 000 mg/kg technical-grade nitrofen (purity
not specified) for 78 weeks. The 20 male and 20 female controls
received the basal diet. All animals received acidified (pH 2.5) water
ad libitum. After exposure to nitrofen, animals were observed for a
further 13 weeks. Consistent dose-related depressions in mean body
weight were noted in male and female mice, reaching about 25 percent
in high-dose males by the end of the study. At that time, 40/50 of the
high-dose males, 48/50 of the low-dose males and 19/20 of the control
males were still alive; of the females, 48/50 of the high-dose and
43/50 of the low-dose group and 12/20 off the controls survived until
the end of the study. Although a variety of tumours were noted, only
the incidence of liver tumours seemed to be related to nitrofen
exposure. Hepatocellular adenomas were seen in 1/20 control males,
18/49 low-dose males and 20/48 high-dose males, and hepatocellular
carcinomas in 0/20 controls, 13/49 low-dose males and 20/48 of the
high-dose group. In addition, none of the controls, but 3/49 of the
low-dose group and 4/48 of the high-dose group had hepatoblastoma, a
very rare tumour in this strain of mice. Of the females, 0/18
controls, 9/48 low-dose and 17/5 high-dose mice had hepatocellular
adenomas; hepatocellular carcinomas were observed in 0/18 control,
5/48 low-dose and 13/50 high-dose female mice. In addition, a
hepatoblastoma was found in 1/48 females in the low-dose group.
For both the low- and high-dose groups there was a statistically
significant increase from the control group in the incidences of
hepatocellular adenomas and hepatocellular carcinomas (National Cancer
Institute 1979).
Rat
Groups of 50 male and 50 female Osborne-Mendel rats (age not
specified) were fed diets containing technical grade nitrofen (stated
to be >80 percent pure; impurities unspecified) in 2 percent maize
oil for 78 weeks. The dietary concentration for males was 2 300 mg/kg
(low-dose) or 2 300-4 600 (high-dose), to give a time-weighted average
of 3 627 mg/kg. The dietary concentrations for female rate were 1 300
mg/kg (low-dose) or 2 600 mg/kg (high-dose). The low-dose males, the
control animals and the high- and low-dose females were observed for
an additional 32 weeks, during which they were maintained on a basal
laboratory diet plus maize oil; the high-dose males were observed for
an additional five weeks after dosing was stopped. Groups of 20 male
and 20 female controls received the basal diet plus 2 percent maize
oil. A dose-related decrease in body weight was noted; by 75 weeks
after onset of dosing, the high-dose females weighed roughly one-third
less than the controls. Fifty percent of the high-dose males were dead
by week 45 and only 15 survived to week 83; 60 percent of the low-dose
and 45 percent of the control group survived until the end of the
study. Of the females, 58 percent at the high-dose, 74 percent at the
low-dose and 80 percent of the controls survivied until the end of the
study. Adenocarcinomas of the exocrine pancreas were seen in 2/50
females at the low dose and in 7/50 at the high dose. All tumours
showed local invasion and had metastasized to the lungs. No such
tumour was seen in the 20 matched controls or in the 110 pooled
controls. The difference was statistically significant for high-dose
animals. No other tumour showed a statistically significant difference
in incidence between control and exposed rats. Poor survival precluded
the evaluation of carcinogenicity in male rats (National Cancer
Institute 1978).
Groups of 50 male and 50 female six-week-old Fischer 344 rate were fed
diets containing 3 000 or 6 000 mg/kg technical-grade nitrofen (purity
not specified) for 78 weeks. A group of 20 male and 20 female rats
were given the control diet. All animals received acidified water (pH
2.5). The animals were followed for an additional 26 weeks, when all
surviving rats were killed. A dose-related depression in mean body
weight was noted in animals of both sexes, reaching 15 percent for
those given the high dose for 60 to 75 weeks. Of the male rats, 45/50
given the high dose, 42/50 given the low dose and 17/20 of the
controls survived until termination of the study; of the females,
38/50, 42/50 and 17/20, respectively, survived. No statistically
significant difference in the incidence of tumours was observed
between the exposed and control groups (National Cancer Institute
1979). However, subsequent detailed histological and morphological
studies distinguished the nitrofen-induced neoplasm from those
occurring naturally in the controls (Hoover et al. 1980; Stinson
et al. 1981).
Special Studies on Enzyme Induction
Groups of B6C3F1 mice (six animals per sex) were fed diets containing
nitrofen (technical, 92 percent pure) at levels of 0, 10, 100 or 1000
ppm or two weeks prior to determination of individual liver to body
weight (L:BW) rations and in vitro hepatic p-nitroanisole (pNA)
O-demethylase activity. The dose levels were chosen after acute
studies (three-day gavage dosing), using CD-1 mice, showed a
sex-dependent minimum effect level for hepatic pNA-O-demethylase
activity between 10 and 50 mg/kg/day. The 100 and 1000 ppm dietary
nitrofen levels resulted in elevated liver mixed function oxidase
(MFO) activity in both sexes, with females showing a greater response
(170 percent and 236 percent of control, respectively, for the two
diet levels). L:BW ratios were increased 20 percent and 30 percent in
males and females, respectively, but only at the 1000 ppm level. No
significant changes were observed in either sex fed the 10 ppm
nitrofen diet. The no-effect level found for MFO induction was much
lower than that for oncogenicity (2 348 ppm in the National Cancer
Institute 1978 oncogenicity study). (Deckert & Steigerwalt 1979c)
Special Studies on Dermal Irritation
No evidence of irritation developed when both technical grade (95
percent pure) and pure nitrofen were applied either as a 10 percent
solution in maize oil or as moistened powder to the skin of albino
rabbits (Ambrose et al. 1971).
Special Studies on Cutaneous Sensitization
There was no visible evidence of erythema, swelling, or discolouration
of the test sites after each sensitizing injection or after the
challenging dose of aqueous suspension of technical grade (95 percent)
nitrofen. Under the conditions of the test, these finding indicate
that nitrofen is not a sensitizing agent (Ambrose et al. 1971).
Special Studies on Cataractogenic Effect
Groups of 30 two-week-old Peking ducklings were fed diets containing
nitrofen at levels of 0, 0.05 and 0.1 percent for 13 weeks. Distinct
growth depression occurred at the 0.1 percent nitrofen dose level and
slight retardation at 0.05 percent. The mortality rate in the highest
dose group was distinctly increased. No clinical or histologic signs
of cataract were observed in any of the treated or control animals,
nor were other eye abnormalities found (Ensel & Seinen 1970).
Special Studies on Methaemoglobin Formation by Aminonitrofen
Methaemoglobin formation in vitro by nitroso and amino derivatives
of nitrofen and other chlorinated biphenyl ethers was investigated in
suspensions of human erythrocytes. Ability to induce methaemoglobin
formation among the nitroso and amino derivatives decreased with
increasing chlorine substitution of the biphenyl ether. However, amino
derivatives from no methaemoglobin in suspensions of erythrocytes
alone, but do so after activation by liver homogenate. Methaemoglobin
formation after intraperitoneal and oral administration to male Wistar
rats of chlorinated p-aminobiphenyl ether at levels of 0.77 mg/kg b.w.
gave almost the same result (ca. 40 percent methaemoglobin within
60-120 min.) after either route was used (Miyauchi et al. 1981).
Special Studies on the Stability of the Metabolite Azoxynitrofen
Azoxynitrofen (a possible vegetable metabolite of nitrofen) was added
to simulated gastric fluid and simulated intestinal fluid at level of
7 ppm. There is a slight degradation of azoxynitrofen in gastric fluid
during 24 h. The intestinal fluid completely decomposes azoxynitrofen
within 24 h. After 14 days, there is evidence of azoxynitrofen in
either digestive fluid, and it appears that aminonitrofen is a major
product of the decomposition (Warso 1972).
Acute Toxicity
The acute toxicity of nitrofen in animals is summarized in Table 1.
Most deaths occurred two to eight days after dosing and were preceded
by progressive depression. There was no evidence of diarrhoea.
Postmortem findings were negative in rats that died and in survivors
autopsied at 14 days (Ambrose et al. 1971).
Short-Term Studies
Rat
Groups of albino Wistar rats (10 males and 10 females per group) were
fed diets containing technical grade nitrofen (95 percent pure) at
levels of 0, 100, 500, 2 500, 12 500 and 50 000 ppm for 13 weeks.
Rats on the 50 000 ppm diet failed to survive beyond the first week
and survival of rats on the 12 500 ppm diet was adversely affected.
Growth was depressed in both sexes at 12 500 ppm and in males at 2
500. In rats on the lower dietary concentrations of nitrofen, no
adverse effects were noted in growth, food consumption and mortality.
Haematologic values appeared to be within normal range and urinary
tests for reducing substances and protein showed no marked differences
from the controls. Significantly higher liver-to-body weight ratios
were found in rats at all dietary levels of nitrofen, except for male
rats at 100 ppm, and appeared to be dose-related. Kidney body weight
ratios were significantly elevated for females at 12 500 ppm and for
males at 2 500 and 12 500 ppm. Testes body weight ratios were
significantly elevated for rats at 2 500 and 12 500 ppm. Lesser
effects were noted for heart and spleen. Histopathologic findings
related to treatment appeared to be confined to rats receiving diets
containing 12 500 and 50 00 ppm nitrofen. The principal findings
consisted of a hepatitis characterized by oedema, peripheral
localization of glycogen granules, swelling of the cytoplasm and liver
nuclei with prominent nucleoli (Ambrose et al. 1971). (Full
experimental data were not available.)
Rabbit
Groups of five male and five female rabbits received dermal
applications of nitrofen (as a water-diluted emulsifiable formulation)
at levels of 0, 250 or 1 250 mg/kg b.w., 7 h/day, 5 days/week for
three weeks. Additional groups received applications on abraded skin.
There was no mortality and no evidence of any skin irritation. No
histological changes were noted in the skin of exposed animals. There
was no evidence of subacute systemic damage to liver, kidney, lung or
spleen on histological examination (Brown 1964).
Table 1 Acute Toxicity of Nitrofen in Animals
Animal Sex Route Vehicle LD50 a.i. Reference
g/kg b.w. purity
Rat M oral maize oil 2.63 95% Ambrose et al. 1971
M oral maize oil 3.58 100% Ambrose et al. 1971
M oral peanut oil 2.84 99% Kimbrough et al. 1974
F oral peanat oil 2.40 99% Kimbrough et al. 1974
oral undiluted 5.00 Swann 1973
formulation (as 25%
formulation)
M+F dermal xylene 5.00 99% Kimbrough et al. 1974
inhalation undiluted 205 mg/l Swann 1973
(1h exper.) formulation (as 25%
formulation)
M+F inhalation undiluted >271 mg/cu.m. Brown 1965
(1h exper.) formation (as a.i.)
Rabbit dermal undiluted >2.00 Swann 1973
(24 h) fomulation (as 25%
formulation)
Dog
Groups of six-month-old beagles (two males and two females/group) were
fed diets containing technical grade (95 percent pure) nitrofen at
levels of 0, 20, 200 and 2 000 ppm for two years. The dogs on the
various dietary levels of nitrofen survived the two-year feeding
period. No toxic signs attributable to nitrofen were seen at any time.
The general health, appearance, behaviourial pattern, body weight gain
and cumulative food consumption were not different than those of the
respective controls. Haematologic values and urinary tests for
reducing substances and protein showed no effect of treatment at any
of the test periods. BSP, SGOT, SAP and BUN tests made during the
month 24 showed no adverse trends. Significantly higher liver-to-body
weight ratios appeared only in dogs on 2 000 ppm nitrofen.
Histopathologic examination of major organs revealed no abnormalities
or lesions resulting from the ingestion of nitrofen for two years, in
kind or incidence, that were not found in control dogs. In general,
only the 20 and 200 ppm diet levels of nitrofen exhibited no adverse
effects (Ambrose et al. 1971).
Long-Term Studies
Rat
Groups of weanling Wistar rats (25 males and 25 females/group)
received diets containing technical grade (95 percents pure) nitrofen
at levels of 0, 1, 10, 100 and 1 000 ppm for 97 weeks. The study was
terminated at 97 weeks, owing to poor survival (50 percent) in all
groups, including the controls, beyond week 65. Statistically
significant decreases in mean body weight were observed in the males
at 100 and 1000 ppm after the week 52 and in the females at the same
dose levels until week 26.
Food consumption, haematological and urinalysis parameters were not
affected. Significantly higher kidney/body weight and liver/body
weight ratios in male rats on 1 000 ppm were notable and appeared to
be attributable to nitrofen. Histopathologic findings revealed no
lesions in kind or incidence that were not found in the respective
controls. Overall, the 10 ppm and below diet level of nitrofen was
reported to cause no adverse effects (Ambrose et al. 1971). (Lack of
individual animal data precludes further interpretation of this
study.)
COMMENTS
Nitrofen has low acute oral toxicity. Following oral administration,
nitrofen is partially absorbed and rapidly excreted. A small
proportion of the administered dose is distributed to all tissues and
organs. Nitrofen and its metabolites are found primarily in faeces
(accounting for about 75 percent of the administered dose), while
urine contains metabolites accounting for about 15 percent of the
dose. The metabolism of nitrofen involves reduction, hydroxylation and
conjugation.
Two reproduction studies were presented. Because of inadequacies in
the reports the studies were not considered.
Oral exposures to nitrofen during the period of organogenesis produced
neonatal mortality, decreases in the time of survival to weaning, and
a series of abnormalities in rodent offspring. In a mouse
teratology/post-natal study, the lowest dose tested (6.25 mg/kg b.w.)
produced increased incidence of lethal defects at birth and resulted
in decreased lung and seminal vesicle weights at maturity. In a rat
teratology study, diaphragmatic hernias were observed at and above
1.39 mg/kg b.w., but full details were not provided.
Nitrofen in some cases was found mutagenic in the
Salmonella/microsome assay but it did not display any mutagenic
potential in several mammalian systems either in vitro or in vivo.
Two carcinogenicity studies on B6C3F1 mice indicated that nitrofen is
a liver carcinogen, causing hepatocellular carcinomas and
hepatocellular adenomas in both sexes, and haemangiosarcomas in male
mice. In addition, the compound is carcinogenic to female
Osborne-Mendel rats, causing adenocarcinomas of the pancreas. Nitrofen
was not found to be carcinogenic in Fischer 344 rats. Detailed
histological examinations suggest that naturally occurring and
nitrofen-induced liver tumours in mice are morphologically different.
Both studies were of short duration. In the study on Osborne-Mendel
rats, poor survival precluded the evaluation of carcinogenicity in
males.
In a long-term rat-feeding study, a NOEL of 10 ppm was observed.
However, the duration of the study (97 weeks), the poor survival in
all groups (50 percent beyond week 65), and the fact that the study
was not available in extenso, precluded its full evaluation.
An acceptable daily intake could not be estimated, owing to the
evidence of carcinogenicity, the lack of a NOEL for the teratology and
post-natal effects and the inadequacies of several studies, including
reproduction and long-term studies.
REFERENCES - TOXICOLOGY
Adler, I.L. et al. A material balance study in rats using
1970a 14C- TOK labelled in the nitrophenyl
ring. Rohm and Haas Report No. 23-24.
(Unpublished)
Adler, I.L. et al. A material balance study in rats using
1970b 14C - TOK labelled in the dichlorophenyl
ring. Rohm and Haas Report No. 23-25.
(Unpublished)
Ambrose, A.M., Larson, Toxicologic studies on
P.S., Borzelleca, J.F., 2,4-dichlorophenyl-p-nitrophenyl
Blackwell Smith, R. & ether (TOK). Toxicol. Appl. Pharmacol.,
Hennigar, G.R. 19:263-275.
Brown, J.R. A study on the acute inhalation toxicity
1965 of TOK EC-25. Report from the University
of Toronto, Canada submitted to WHO by
Rohm and Haas. (Unpublished)
Brown, J.R. & Mastromateo, E. Subacute percutaneous toxicity of TOK
1964 E-25 in the rabbit. Report from the
University of Toronto, Canada,
submitted to WHO by Rohm and Haas.
(Unpublished)
Byeon, W.H., Hyun, H.H. & Mutagenicity of pesticides in the
Lee, S.Y. Salmonella/microsome system. Korean J.
1976 Microbiol., 14:128-134.
Chernoff, N., Kavlock, R.J., Preliminary report on the perinatal
Gray, L.E. toxicity of nitrofen administered to
1980 mice. Rohm and Haas Report.
(Unpublished)
Costlow, R.D. & Manson, J.M. The heart and diaphragm: target organs
1981 in the neonatal death induced by
nitrofen (2,4-dichlorophenyl-p-
nitrophenyl ether). Toxicology,
20:209-227.
Costlow, R.D., The effect on rat pups when nitrofen
Hirsekorn, J.M., (4-(2,4-dichlorophenoxy) nitrobenzene)
Stiratelli, R.G., was applied dermally to the dam
O'Hara G.P., Black, D.L., during organogenesis. Toxicology
Kane, W.K., Burke, S.S., (in press). Submitted to WHO by
Smith, J.M. & Hayes, A.W. Rohm and Haas.
Decker, F.W. & Percutaneous absorption of 14C-TOK in
Steigerwalt, R.B. rats. Rohm and Haas Report TD77P-59.
1979a (Unpublished)
Deckert, F.W. & Disposition of 14C-TOK after
Steigerwalt, R.B. percutaneous application to a rat.
1979b Rohm and Haas Report TD78P-53.
(Unpublished)
Deckert, F.W. & Fourteen day dietary TOK enzyme
Steigerwalt, R.B. induction study in mice. Rohm
1979c and Haas Report TD78P-58.
(Unpublished)
Ensel, A.B. & Seinen, W. Investigation of TOK on a possible
1970 cataractogenic effect in ducklings.
Central Institut Voor Voedingsonderzock.
Report no. R-3306 submitted to WHO by
Rohm and Haas. (Unpublished)
Gray, L.E., Kavlock, R.J., Prenatal exposure to the herbicide
Chernoff, N., Ferrell, J., 2,4-dichlorophenyl-p-nitrophenyl
McLamb, J. & Ostby, J. ether destroys the rodent Harderian
1982a gland. Science, 215:293-294.
Gray, L.E., Kavlock, R.J., The effects of the prenatal exposure
Chernoff, N. & Ferrell, J. to the herbicide TOK on the postnatal
1982b development of the Harderian gland of
the mouse, rat and hamster. Presented
at Soc. Teratology Meeting 1982.
Submitted to WHO by Rohm and Haas.
Gray, L.E., Kavlock, R.J., Postnatal development alterations
Chernoff,N., Ostby, J. & following prenatal exposure to the
Ferrell, J. herbicide 2,4-dichlorophenyl-p-
1983 nitrophenyl ether. A dose response
evaluation in the mouse. Toxicol.
Appl. Pharmacol. 67:1-14.
Guerzoni, M.E. & Mutagenic activity of pesticides.
Del Cupolo, L. Riv. Sci. Technol. Alimenti Nutr.
1976 Um., 6:161-168.
Hirsekorn, J.M. & Kane, W.W. TOK E-25 percutaneous range-findings
1981 teratology study with postpartum
evaluation. Rohm and Haas Report No.
81R-63. (Unpublished)
Hoover, K.L., Ward, J.M. & Histopathological differences between
Stinson, S.F. nitrofen-induced and naturally occurring
1980 hepatocellular carcinomas in the B6C3F1
mouse. J. Nat. Cancer Inst., 65:937-948.
Kavlock, R.J., Chernoff, N., An analysis of fetotoxicity using
Rogers, E., Whitehouse, D., biochemical end-points of organ
Carver, B., Gray, J. & differentiation. Teratology,
Robinson, K. 26:183-194.
1982
Kimbrough, R.D., 2,4-dichlorophenyl-p-nitrophenyl ether
Gaines, T.B. & Linder, R.B. (TOK). Effect on the lung maturation of
1974 rat fetus. Arch. Environ. Health,
28:316-320
McLeod, P.L. & McCarthy, K.L. TOK dermal cytogenetic study in rats.
1982 Rohm and Haas Report No. 81R-268.
(Unpublished)
Miyauchi, M., Koizumi, M. & Studies on the toxicity of chlorinated
Uematsu, T. p-nitro-biphenyl ether.
1981 I - Methemoglobin formation in vitro
and in vivo induced by nitroso and
amino derivatives of chlorinated
biphenyl ether. Biochem. Pharmacol.,
30:3341-3346.
Myhr, B.C. & Brusik, G. Evaluation of 78-232 in the in vitro
1979 transformation of BALB/3T3 cells assay,
Litton Bionetics Report No. 20992
submitted to WHO by Rohm and Haas.
(Unpublished)
National Cancer Institute. Bioassay of nitrofen for possible
1978 carcinogenicity. Carcinogenesis
Technical Report Series No. 26,
DHEW Publ. No. (NIH) 78-826.
National Cancer Institute. Bioassay of nitrofen for possible
1979 carcinogenicity. Carcinogenesis
Technical Report Series No. 184,
DHEW Publ. No. (NIH) 79-1740,1.
O'Hara, G.P., Can, P.K., The effect of nitrofen
Harris, J.C., Burke, S.S., 4-(2,4-dichlorophenoxy) nitrobenzene
Smith, J.M. & Hayes, A.W. on the reproductive performance
1983 of male rats.Rohm and Haas Report
(Unpublished)
O'Neill, P.J. & Lohse, E. 4,4-DCAB microbial mutagen test. Rohm
1980 and Haas Report No. 80R-4. (Unpublished)
O'Neill, P.J. & TOK: Microbial mutagen tests. Rohm and
Scribner, H.E. Haas Report No. TD79M-307. (Unpublished)
Paik, S.G. & Lee, S.Y. Genetic effects of pesticides in the
1977a mammalian cells. I. Induction of
micronucleous. Kor. J. Zool.,
20:19-28.
Paik, S.G. & Lee, S.Y. Genetic effects of pesticides in the
1977b mammalian cells. II. Mutagenesis in
L5178Y cells and DNA repair induction.
Kor. J. Zool., 20:159-168.
Reustle, J.A. & TOK cytogenetic study in mice. Rohm and
Scribner, H.E. Haas Report No. 79R-173. (Unpublished)
1980
Roser, R.L., Adler, I.L. & A study of the metabolism of 14-C TOK in
Allen, S.S. rats. Rohm and Haas Report No. 23-33.
1971 (Unpublished)
Siou, G. Study of the potential mutagenic
1978 activity of TOK by the Howell-Jolly
body technique. Cabinet d'Etudes et
de Recherches en Toxicologie
Industrielle, Report No. MBL-2199
submitted to WHO by Rohm and Haas.
(Unpublished)
Siou, G. Study of the effect of TOK on the
1979a prenatal and postnatal development
of the rabbit. Cabinet d'Etudes et
de Recherches en Toxicologie
Industrielle, Report No. MBL-2212
and Addendum submitted to WHO by
Rohm and Haas. (Unpublished)
Siou, G. Study of the effect of TOK on the
1979b postnatal development of the rat.
Cabinet d'Etudes et de Recherches
en Toxicologie Industrielle Report
No. MBL-2248 submitted to WHO by
Rohm and Haas. (Unpublished)
Steigerwalt, R.B., TOK comparative metabolism study.
Godfrey, W.J. & Deckert, Rohm and Haas Report No. 79R-169.
F.W. 1980a (Unpublished)
Steigerwalt, R.B., Lisk, D.C. Range-finding study on 14C-TOK. Dermal
& Deckert, F.W. absorption by rat and human skin in
vitro. Rohm and Haas Report No.
80R-110. (Unpublished)
Steigerwalt, R.B., TOK: disposition after percutaneous
Udinsky, J.R. & Deckert, applications to pregnant rats. Rohm
F.W. 1982 and Haas Report No. 81R-65.
(Unpublished)
Stinson, S.F., Hoover, K.L. Quantitation of differences between
& Ward, J.M. spontaneous and induced liver tumors in
1981 mice with an automated image analyzer.
Cancer Letters, 14:143-150.
Stone, L.C. & Manson, J.M. Effects of the herbicide
1981 2,4-dichlorophenyl-p-nitrophenyl
ether (nitrofen) on fetal lung
development in rats. Toxicology,
20:195-207.
Swann, H.E. Acute toxicity report on Rohm and Haas
1973 TOK E-25, dark liquid. Food Drug
Res. Labs. Report No. 1-3784-851
submitted to WHO by Rohm and Haas.
(Unpublished)
Vogin, E.E. Effects of FW925 on the fetal
1971 development in rats. Food and Drug
Research Laboratories Report,
August 10 submitted to WHO by
Rohm and Haas. (Unpublished)
Warso, J.P. Degradation of azoxynitrofen in
1972 simulated digestion fluids. Rohm
and Haas Report No. 23-72-27.
(Unpublished)
Weatherholtz, W.M., Teratogenicity study in rats, TOK
Kapp, R.W. & E-25, TOK E-25 solvent control.
Greenspun, K.S. Report from Hazleton Laboratories,
1979 America Inc. submitted to WHO by
Rohm and Haas. (Unpublished)
RESIDUES
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Nitrofen is a selective pre- and early post-emergence herbicide for
the control of annual grasses and various broad-leaved weeds in cereal
grains and vegetables.
Nitrofen is used in France and some other countries under labels that
prohibit women from handling the product. It is primarily used to
control backgrass (Alopecurus agrestis), Veronica hederaefolia,
Galium aparine, Viola tricolor and other weeds that in winter
wheat and other cereal grains are not adequately controlled by other
commercially available herbicides.
The effective use rate is generally between 1.5 and 2 kg/ha applied to
the soil surface; activity is lost on soil incorporation. Adequate
soil moisture is necessary for efficacy, but excessive moisture may
result in crop injury.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Residue data have been obtained in several countries where nitrofen
was applied to various crops at or about the recommended rates. Two to
four samples were taken at random from the fields and were transported
to the laboratory within two days. The preparation of the sample and
the portion of sample to be analysed were in agreement with the
recommended Codex procedure with two exceptions: only the cloves of
garlic were sent to the laboratory after removing the outer leaves and
all the samples were washed before chopping and deep-freezing.
The analytical results from the United States were obtained by a
method (Adler and Wargo 1975) that measures the parent compound and
the majority of metabolites in the form of the derivative of amine
moiety. The data from other countries indicate the residue level of
the parent compound only. The results of supervised trials are
summarized in Table 1.
FATE OF RESIDUES
In general, nitrofen undergoes metabolic degradation in all systems
(animals, plants, soil and Water) studied. Metabolic pathways include
reduction of the nitro group, conjugation of the resulting amino
group, hydroxylation of the dichlorophenyl ring and formation of
naturally occurring materials, as well as total conversion to CO2.
The structural formula and the occurrence of the important identified
metabolites are shown in Table 2.
In Animals
Cattle milk, urine and faeces collected during the dosing period from
a cow fed a daily ration of 26.2 kg containing 5 mg/kg purified
nitrofen for four days did not contain detectable levels of nitrofen
or aminonitrofen (sensitivity of the method was approximately 0.3
mg/kg). In vitro studies demonstrated that nitrofen is rapidly
reduced to aminonitrofen in fresh rumen fluid (T1/2 is less than or
equal to 10 min). Aminonitrofen was stable in the rumen fluid for 24 h
(Gutemann & List 1967).
Similarly, only trace levels of radioactivity (0.02 to 0.07 mg/kg,
calculated as nitrofen) were found in samples of urine, faeces and
milk from a cow dosed at 5 mg/kg with radiolabelled nitrofen. The
levels of total radioactivity were 1.4 and 1.7 mg/kg in the faeces and
urine, respectively, and 0.14 and 0.16 mg/kg in two samples of milk. A
fat sample contained 1.06 mg/kg 14C, of which 0.25 mg/kg was parent
compound (Roser & Adler 1971).
Table 1 Residues of Nitrofen Resulting from Supervised Trials
Crop and variety Country Application Proharvest Residue (mg/kg)
rate interval Mean
(kg/a.i./ha) No. (days)
Broccoli (head) United States1 3.7 1 74 <0.01 <0.015
Futura 4.5 2 63 <0.01 <0.01
Gem 4.5 1 85 <0.01 <0.01
Green Duke 4.75 2 63 <0.01 <0.01
4.75 2 69 <0.01 <0.01
5.0 1 70 <0.01 <0.01
5.3 2 70 <0.01 <0.01
5.4 1 77 <0.01 <0,01
Cabbage (head) United States1
Head Start 4.5 1 69 <0.01 <0.01
4.5 1 84 <0.01 <0.01
Green 4.5 1 89 <0.01 <0.01
6.7 1 76 <0.01 <0.01
6.7 1 90 <0.01 <0.016
Cauliflower (head) United States1
Snoball 4.5 1 90 <0.01 <0.01
4.5 1 93 <0.01 <0.01
4.6 1 88 <0.01 <0.016
5.6 1 83 <0.01 <0.01
6.7 1 59 <0.01 <0.01
6.7 1 107 <0.01 <0.016
Celery (stalk) United States1
5270 RIMP 4.5 1 109 <0.01 <0.01
5.4 1 127 <0.01 <0.01
5270 5.6 1 52 <0.01 <0.01
5270R 5.6 1 165 <0.01 <0.01
Table 1 (continued)
Crop and variety Country Application Proharvest Residue (mg/kg)
rate interval Mean
(kg/a.i./ha) No. (days)
Garlic (bulb) United States1
Cal Late 5.6 1 234 <0.01 <0.01
5.6 1 250 <0.01 <0.01
6.7 1 229 <0.01 <0.01
7.8 1 241 <0.01 <0.01
Rape (grain) Denmark2 1.2 1 100 - <0.01
1.2 1 101 - <0.01
1.2 1 105 - <0.01
1.2 1 100 - <0.01
1.2 1 101 - <0.01
1.2 1 105 - <0.01
Rioe (grain) United States1
Early Rose 2.2 1 145 <0.01 <0.01
3.4 1 145 <0.01 <0.01
3.4 1 160 <0.01 <0.01
4.5 1 160 <0.01 <0.01
6.7 1 160 <0.01 <0.01
9.0 1 166 <0.01 <0.01
Rice (straw)
Early Rose 2.2 1 145 <0.01 <0.01
3.4 1 145 <0.01 <0.01
4.5 1 166 <0.01 <0.01
9.0 1 166 <0.01 <0.01
Table 1 (continued)
Crop and variety Country Application Proharvest Residue (mg/kg)
rate interval Mean
(kg/a.i./ha) No. (days)
Wheat (grain) France3
Chamlein
4.0 1 239 <0.01 <0.01
4.0 1 264 <0.01 <0.01
Joss 4.5 1 266 <0.01 <0.01
Hardy 8.0 1 236 <0.01 <0.01
Chamlein 8.0 1 264 <0.01 <0.01
Hardy 8.0 1 265 <0.01 <0,01
Wheat (grain) Federal
Republic
Joss of 1.6 1 248 - <0.002
Carstacht Germany4 1.6 1 280 - <0.002
Kranich 1.6 1 284 - <0.002
Caribo 1.6 1 286 - <0.002
2.0 1 270 - <0.002
Benno 2.0 1 279 - <0.002
Carito 2.0 1 286 - <0.002
Hyslop United States1 1.7 1 294 <0.01 <0.01
Druchamp 2.2 1 163 0.00-0.02 0.01
Yamhill 2.2 1 250 <0.01 <0.01
2.2 1 267 <0.01 <0.01
2.2 1 269 <0.01 <0.01
2.2 1 272 0.01-0.09 0.04
Nugaines 2.2 1 272 0.02-0.03 0.02
Hyslop 2.2 1 281 <0.01 <0.01
Yamhill 2.2 1 284 <0.01 <0.01
2.2 1 289 <0.01 <0.01
Table 1 (continued)
Crop and variety Country Application Proharvest Residue (mg/kg)
rate interval Mean
(kg/a.i./ha) No. (days)
Cajame 3.4 1 146 <0.01-0.01 <0.01
Tehame 3.4 1 151 <0.01 <0.01
Hyslop 3.4 1 294 <0.01 <0.01
Cajame 4.5 1 146 <0.01 <0.01
Tehame 4.5 1 151 <0.01-0.01 <0.01
Druchamp United States1 4.5 1 163 <0.01-0.06 0.03
Yamhill 4.5 1 250 <0.01-0.01 <0.01
4.5 1 267 <0.01 <0.01
4.5 1 269 <0.01 <0.01
4.5 1 272 0.01-0.12 0.06
Nugaines 4.5 1 272 0.02-0.06 0.04
Hylop 4.5 1 281 <0.01 <0.01
Yamhill 4.5 1 284 <0.01 <0.01
4.5 1 289 <0.01 <0.01
Cajame 5.6 1 146 <0.01-0.02 0.01
6.7 1 146 <0.01-0.01 <0.01
Tehame 6.7 1 151 <0.01-0.01 <0.01
Yamhill 6.7 1 267 <0.01 <0.01
Wheat (straw) Federal Republic
of Germany4
Joss 1.6 1 248 - <0.002
Carstacht 1.6 1 280 - 0.020
Kranich 1.6 1 284 - 0.007
Caribo 1.6 1 286 - <0.004
2.0 1 270 - 0.004
Benno 2.0 1 279 - 0.010
Carito 2.0 1 286 - 0.005
Table 1 (continued)
Crop and variety Country Application Proharvest Residue (mg/kg)
rate interval Mean
(kg/a.i./ha) No. (days)
Hyslop United States1 1.7 1 294 <0.01 <0.01
Yamhill 2.2 1 252 <0.01 <0.01
2.2 1 267 <0.01 <0.01
2.2 1 269 <0.01 <0.01
Nugaines 2.2 1 272 0.02-0.11 0.06
Yamhill 2.2 1 273 <0.01-0.02 0.01
Druchamp 2.2 1 276 0.01-0.04 0.02
Hyslop 2.2 1 281 <0.01 <0.01
Yamhill 2.2 1 284 <0.01 <0.01
2.2 1 291 <0.01 <0.01
Hyslop 3.4 1 294 <0.01 <0.01
Yamhill 4.5 1 252 <0.01 <0.01
4.5 1 267 <0.01-0.01 <0.01
4.5 1 269 <0.01 <0.01
Nagaines 4.5 1 272 0.02-0.05 0.03
Yamhill 4.5 1 273 <0.01-0.03 0.01
Druchamp 4.5 1 276 0.01-0.02 0.01
Hyslop 4.5 1 281 <0.01 <0.01
Yamhill 4.5 1 284 <0.01 <0.01
4.5 1 291 <0.01-0.02 0.01
6.7 1 267 <0.01 <0.01
Sources:
1 Rohm and Haas 1969-1980;
2 Institute National de Recherche Agronomique 1975b;
3 Institute National de Recherche Agronomique 1975a;
4 Institute National de Recherche Agroncmique 1978;
5 Samples taken from four experimental fields;
6 Samples taken from three experimental fields.
Table 2 Nitrofen Metabolites
Metabolite Occurrence
Aminals Plants Soil
 azoxy-nitrofen 4,4'-bis
(2,4-dichlorophenoxy) azoxy benzene x x
azoxy-nitrofen 4,4'-bis
(2,4-dichlorophenoxy) azoxy benzene x x
 hydroxy nitrofen chloro,
hydroxy-4 '- nitrodiphenylether1 x
hydroxy nitrofen chloro,
hydroxy-4 '- nitrodiphenylether1 x
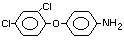 aminonitrofen
2,4-dichloro-4'-aminodiphenylether x x x
aminonitrofen
2,4-dichloro-4'-aminodiphenylether x x x
 formamidonitrofen
2,4-dichloro-4'-formamido-diphenylether x x x
Table 2 (Continued)
Metabolite Occurrence
Aminals Plants Soil
formamidonitrofen
2,4-dichloro-4'-formamido-diphenylether x x x
Table 2 (Continued)
Metabolite Occurrence
Aminals Plants Soil
 acetamidonitrofen
2,4-dichloro-4'-acetamidodiphenyletherx x x x
acetamidonitrofen
2,4-dichloro-4'-acetamidodiphenyletherx x x x
 5-hydroxyaminonitrofen
2,4-dichloro, 5-hydroxy
4'-aminodiphenyl-ether x x
5-hydroxyaminonitrofen
2,4-dichloro, 5-hydroxy
4'-aminodiphenyl-ether x x
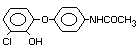 hydroxy-acetamido-nitrofen x x x
chloro,hydroxy 4'acetamido
diphenylether1 x
hydroxy-acetamido-nitrofen x x x
chloro,hydroxy 4'acetamido
diphenylether1 x
 monochloronitrofen 2 chloro-4'
nitrodiphenyl ether x
Table 2 (Continued)
Metabolite Occurrence
Aminals Plants Soil
monochloronitrofen 2 chloro-4'
nitrodiphenyl ether x
Table 2 (Continued)
Metabolite Occurrence
Aminals Plants Soil
 hydroxy-propionamido-nitrofen1 x
hydroxy-propionamido-nitrofen1 x
 2,5-dichlorophenol x
1 The substituted carbon atoms have not been identified.
Cattle feeding studies were conducted twice at feeding levels of 0.05,
0.5 and 5 mg/kg administered in gelatin capsules. Combined residues in
meat and milk were determined once in four cows and a second time with
14C-labelled nitrofen. In the multiple cow feeding study, no residues
of nitrofen were detected in milk or tissues of animals fed 0.05 or
0.5 mg/kg, based on the total daily diet. Barely detectable residues,
ranging from 0.002 to 0.004 mg/kg, were found in the milk of a cow fed
5 mg/kg. At this feeding level, no residue was found in any of the
tissues except fat (0.01 mg/kg). The sensitivity of the method was
0.01 mg/kg for tissue, and 0.002 mg/kg for milk (Rohm & Haas 1967b).
In the radioactive feeding study a cow was dosed orally twice a day
with 14C-labelled nitrofen. Nitrofen residues were not found in the
milk from the lowest feeding level (0.05 mg/kg) and only 0.003 and
0.08 mg/kg were found in milk from the two higher feeding levels, i.e.
0.5 and 5 mg/kg. The residue in milk was 0.3 percent and 0.8 percent
of the applied dosage, respectively. Residues were found in edible
tissues taken from the cow dosed at the 5 mg/kg feeding level.
Calculated as nitrofen, these residues ranged from 0.05 mg/kg (trips)
and 0.24 mg/kg (muscle) to 1.15 mg/kg (fat) (Rohm & Haas 1971). The
parent compound constituted approximately 47, 32 and 23 percent of the
total residue in milk, kidney and fat, respectively.
The lowest feeding level (0.05 mg/kg) of radioactive nitrofen is
almost ten times greater than the calculated maximum level of residue
that might be eaten by livestock kept on feed containing rice bran,
rice milling fractions or any other plant by-products that had been
treated with nitrofen. Consequently, no detectable residues are likely
to be found in milk and edible tissues.
Poultry
Three groups of 15 laying hens each were fed dichlorophenyl
ring-labelled 14C nitrofen incorporated in their feed for ten weeks
at levels of 0.04, 0.12 and 0.47 mg/kg, respectively. Residues in
tissues and eggs were studied and analysed by liquid scintillation
counting. Residues in eggs reached plateaux at levels of 0.02, 0.05
and 0.17 mg/kg, respectively, by week 6 of feeding for the X, 3X and
10X test group. The residue was located almost exclusively in the egg
yolk. The excreta collected for one 24 h period in week 3 contained
98.9 and 80 percent of the total radioactivity of feeding levels of
0.04 and 0.47 mg/kg, respectively. Hens from each group were
sacrificed after weeks 3, 5 and 10 of test feeding. Selected tissues
were removed and analysed by combustion and liquid scintillation
counting. Average residue levels in tissues from the 1X test group
ranged from non-detectable (white meat) to 0.18 mg/kg (fat).
Prolonged feeding of nitrofen to laying hens at a level of 0.04 mg/kg
daily resulted in total residue of approximately 0.01-0.02 mg/kg in
eggs. The residue was found almost exclusively in the egg yolk (Adler
1972).
Sheep
A female sheep was given a single oral, 40 mg/kg dose of 14C-labelled
nitrofen. After 99 h, 76.2 percent of the 14C had been recovered in
the excreta, with 39 percent appearing in the urine and 37.2 percent
in the faeces. The residue in the blood peaked at 19 h with a plateau
from 11 to 31 h; the concentrations were between 3 and 4 mg/kg. When
killed at 100 h, the highest tissue concentrations were found in the
fat (23 to 24 mg/kg total radioactivity expressed as nitrofen); the
gut, liver, thyroid and mammary glands contained from 2 to 4 mg/kg and
14 other organs had residues of 0.5 to 2 mg/kg (expressed as
nitrofen). The highest urinary excretion of nitrofen occurred during
the 11 to 19 h post-treatment collection period; the peak in the
faeces was during the 39 to 47 h post-treatment collection. Only 2 to
5.5 percent of the radioactivity in the urine was extractable with
chloroform. Aminoitrofen and 5-hydroxynitrofen were the principal
metabolites, with the parent compound accounting for less than 0.3
percent of the total radioactivity. Small amounts of phenol,
acetamidonitrofen and the 2-chloro ether, as well as several unknown
compounds, were found. The bulk of the radioactivity consisted of
sulphates, glucuronides and glycine conjugates. In contrast, 47 to 86
percent of the total radioactivity in the faeces could be extracted
with chloroform. Approximately 30 percent of the total radiocarbon
excreted in the faeces was the parent nitrofen (11 percent of the
total dose administered). Aminonitrofen accounted for an average of 21
percent of the 14C in the faeces, with the concentration increasing
over time from 14 percent at 23 h to 25 percent at 33 h. In blood
samples, the parent nitrofen averaged 33.9 percent of the total
radioactivity, aminonitrofen 20 percent and an unknown labelled
compound 13.4 percent. The amount of parent compound peaked at 43
percent at 19 h post-treatment and declined to 28 percent of the total
by 31 h (Hunt et al. 1977).
Fish
The accumulation of residue in fish was studied in a system consisting
of two tanks. The first tank contained soil treated with 14C-labelled
nitrofen, while the second one contained water only. The tanks were
connected and the water circulated. During the first six days, the
residue in the soil and water declined from 6.29 mg/kg and 0.016 mg/kg
to 4.31 mg/kg and 0.004 mg/kg, respectively. From day 10, the water
did not contain detectable residue, while the total residue in soil
was 3.62 mg/kg at day 28. The residue in the tail (edible portion) of
crayfish placed in the tank containing soil was 0.48 mg/kg on the
first day and varied in the range of 0.13-1.04 mg/kg thereafter. In
the tissue of catfish, kept in the second tank, the highest residue
(7.42 mg/kg) was measured at day 2. The residue level declined to 0.17
mg/kg at day 28. Approximately 55 percent of total 14C residue was
nitrofen (Adler 1971).
Cultures of intestinal micro-organisms from carp were capable of
reducing nitrofen to aminonitrofen under both aerobic and anaerobic
conditions (Miyauchi et al. 1980).
In Plants
Soil in which paddy rice was grown was treated pre-emergence with
dichlorophenyl ring-labelled nitrofen. The rice plants were sprayed
with nitrophenyl ring-labelled nitrofen in one experiment and with
dichlorophenyl ring-labelled nitrofen in another. The post-emergence
spray application was carried out to provide samples with sufficiently
large residues for metabolic identification and to study metabolic
routes that might correlate with post-emergence applications of the
compound to other commodities. Metabolites identified and their
percentage ratio in the extractable residue are shown in Table 3
(Roser & Adler 1971).
The parent compound amounted to 72 percent of the organic extractable
residue (73.9 percent of total residue) in rice plants treated 10 days
before sampling. The parent compound (13.7 percent) hydroxy
acetamidonitrofen (15 percent) and the amino derivative (11.8 percent)
were the major components of the organic extractable residue (29.1
percent of total residue) in rice straw 90 days after treatment. The
bound residues in rice and wheat grain and straw from a 14C
pre-emergence application were characterized by Honeycutt and Adler
(1975).
64 to 96 percent of the radioactive residues in wheat and rice grain
were in the starch 110 and 147 days, respectively, after planting and
treatment. The authors conclude that nitrofen must undergo
diphenylether bond cleavage followed by ring opening and incorporation
of the ring fragments into glucose and starch. Similar
characterization of the wheat and rice straw reaveled approximately 30
percent of total 14C residues identifiable in the lignin fraction.
Negligible (5 percent or less) 14C residues were associated with the
cellulose fraction, except for rice treated pre-emergence (25
percent).
Two experimental plots were treated with nitrofen labelled either on
the dichlorophenyl ring or on the nitrophenyl ring. Each treated plot
and the control were planted with two varieties, Anza and Era, of
wheat. Samples were taken at various intervals and analysed with
methods measuring the parent nitrofen only and all of the residues
containing an amino group or capable of being converted to amino
nitrofen. The results of analyses are shown in Table 4 (Wargo 1974).
Only about half of the total radioactivity could be extracted from the
earlier samples and only about 20 percent from straw at harvest. The
method for measuring aminonitrofen, which includes several
metabolites, resulted in substantially higher residues in the earlier
samples than the method for the parent compound only. No residues were
detected by either method in grain and straw at harvest. Most of the
radioactivity present in grain and straw at harvest was in the form of
non-toxic natural substances.
Table 3 Metabolites of Nitrofen Identified in Rice Grain and Straw
Compound1 Extracted 14C(%)
10 days 90 days 90 days
plant straw grain
Nitrofen 72.3 13.7 2
Azoxynitrofen 4.6 2.5
Aminonitrofen 3.3 11.8 4
Formamidonitrofen 4.8 )
Acetamidonitrofen 0.5 ) 19
5-Hydroxy aminonitrofen 1.9 )
Hydroxy acetamidonitrofen 2.8 15
Unknowns and polar conjugates 9.8 38 94
1 Structural formulas of compounds are shown in Table 2.
Table 4 Residues of 14C Nitrofen in Wheat
Residues (mg/kg calculated as nitrofen)
Label Variety Radioassay Nitrofen Aminonitrofen
(parent compound) Method
Sample Extract Method
Immature Plants (at 26 days)
Control Era 0.105 - 0.04 0.04
Control Anza 0.134 - 0.04 0.02
NO2 Era 4.04 2.26 0.99 1.57
NO2 Anza 2.42 1.08 0.63 0.77
Cl2 Era 2.80 - 0.47 1.02
Cl2 Anza 1.12 0.61 0.25 0.32
Immatare Plants (at 44 days)
Control Era 0.035 - NDR1 NDR
Control Anza 0.053 - NDR NDR
NO2 Era 2.19 1.17 0.55 0.87
NO2 Anza 1.18 0.68 0.44 0.51
Cl2 Era 2.55 1.34 0.41 1.12
Cl2 Anza 1.44 1.00 0.48 0.54
Table 4 (continued)
Residues (mg/kg calculated as nitrofen)
Label Variety Radioassay Nitrofen Aminonitrofen
(parent compound) Method
Sample Extract Method
Final Harvest (at 110 days)
Grain Straw Straw2 Grain Straw Grain Straw
Control Era 0.025 0.087 - NDR NDR NDR NDR
Control Anza 0.034 0.028 - NDR NDR NDR NDR
NO2 Era 0.173 0.992 0.19 NDR NDR NDR NDR
NO2 Anza 0.148 0.460 0.10 NDR NDR NDR NDR
Cl2 Era 0.192 1.123 0.22 NDR NDR NDR NDR
Cl2 Anza 0.122 0.624 0.14 NDR NDR NDR NDR
1 NDR = no detectable residue; sensitivity = <0.01 mg/kg
2 No detectable 14C activity was present in the extract from any sample of grain
In Soil and Water
Roser and Adler (1971) identified the soil metabolites resulting from
a preplant/prefood application of dichlorophenyl ring 14C-labelled
nitrofen to simulated field rice paddies. Characterization of residues
in soil samples taken 147 days after treatment revealed that the
parent compound represented 23 percent of the extractable residue,
which amounted to 36 percent of total 14C activity. The major
metabolites were the aminonitrofen (35.4 percent) and
hydroxyacetamidonitrofen (10.7 percent), while the levels of
azoxynitrofen, formamidonitrofen and acetamidonitrofen were below 10
percent of extractable activity. Following a laboratory leaching
study, Adler and Roser (1971) characterized the 14C products in the
top 2.5 cm from soil columns. The extracts (6396 percent of total
activity) contained primarily parent compound (>90 percent), but a
qualitatively similar spectrum of products was observed as those
identified in the previous experiment. Niki and Kuwatsuka (1976)
studied several diphenyl ether herbicides in two rice paddy soils and
found rapid metabolism of nitrofen occurring in both soils. Under
flooded conditions, the half-life was less than 10 days while the
dissipation rate was slower under upland conditions. The primary
degradation product was the corresponding 4'-amino derivative.
Watanabe (1973) reported similar rapid dissipation under field
conditions.
In sealed metabolism flasks (biometer flasks), the metabolism of 14C
nitrofen under aerobic conditions were followed through approximately
100 days (Fischer 1971). Evolution of 14CO2 was detectable from both
dichlorophenyl and nitrophenyl ring-labelled preparations (ca. 3 and
1.5 percent of applied 14C, respectively). In order to characterize
accurately the residue decline patterns of nitrofen from field soil, a
plot was surface-treated with dichlorophenyl - 14C compound and left
fallow through the growing season (Fischer 1976). Radioassay of soil
samples collected through 316 days demonstrated a very rapid initial
decline of 0-15 cm residues (50 percent dissipation within 18 days )
followed by a slower decline. In contrast to field conditions nitrofen
did not undergo dissipation and metabolism under either aerobic or
anaerobic conditions in the greenhouse (Fischer 1975).
Fadayomi and Warren (1977) reported essentially no downward movement
of 14C nitrofen (nitrophenyl ring) that was spiked onto columns of a
silt loam and a fine sand soil and subjected to 5.4 cm of simulated
rainfall. Likewise, Abernathy (1972) found essentially no movement of
nitrofen on thin-layer plates coated with 12 different soil types. The
leaching tendencies for aged nitrofen residues were also determined
(Fischer 1974). After aging for 30 days under greenhouse conditions, a
14C nitrofen (both ring labelled) fortified sandy loam soil was
placed in 30 cm columns and subjected to daily simulated rainfalls of
12 mm/day for 45 days. Radioassay of the leachates as well as the
dissected columns revealed 83 to 102 percent of the applied 14C in
the top 5 cm. No significant 14C was detectable in the leachates.
Using nitrophenyl 14C-labelled nitrofen, a total of five soils of
different types plus river silt were compared as to the extent of
nitrofen adsorption from an aqueous solution (Adler & Allen 1971). The
average amount of 14C adsorbed was greater than 90 percent in every
case from 0.1 ms/l solutions and in all but one (high kaolinite clay
soil) from 1.0 ms/l solutions. These findings are consistent with
those of Fadayomi & Warren (1977), in which most or complete
adsorption of 14C nitrofen was observed by muck soil and bentonite
clays but not by kaolinite clays. Nitrofen was desorbed slowly when
the adsorbents were resuspended in distilled water four successive
times.
The observed immobility of nitrofen is consistent with its low water
solubility and relatively high adsorptive properties. The findings of
laboratory experiments are supported by the results of field
experiments in which water from wells surrounded by or near to treated
fields were sampled. No residue of nitrofen (<0.001 mg/l) was
detectable in 28 samples taken from depths of 3 to 27 m (Zagorski &
Rogerson 1980).
Nitrofen has not demonstrated any tendency to hydrolyse in aqueous
media either in the environment or in the laboratory (Adler 1973).
Photodecomposition
Nakagawa & Crosby (1974a) reported that nitrofen exposed in aqueous
suspensions to sunlight or simulated sunlight underwent rapid ether
cleavage to form 2,4-dichlorophenol and p-nitrophenol, along with
other numerous degradation products. In sunlight, more than 50 percent
of the initial nitrofen degraded after one week of outside exposure.
In a related publication, Nakagawa & Crosby (1974b) reported that the
sunlight photolysis of nitrofen in aqueous methanol represented a
photonucleophilic displacement of nitrophenol by the hydroxide ion of
water.
Both nitrophenyl ring-labelled nitrofen and dichlorophenyl
ring-labelled nitrofen were placed on glass plates as a thin film and
irradiated with simulated sunlight. Samples were takes after 48, 165,
239, 359 and 498 h of illumination. Some of the resulting
photoproducts were identified by thin-layer chromatography and mass
spectrometry. Nitrofen containing both ring labels gave rise to at
least 13 14C-photoproducts. Eleven of the radioactive components of
the nitrophenyl ring-labelled photoproduct mixture were identical (by
TLC Rf values) to corresponding 14C-components of the dichlorophenyl
ring-labelled photoproduct mixture. One unique component of the
nitrophenyl ring-labelled 14C nitrofen photolysis mixture was
identified as 14C-p-nitrophenol. 14C-2,4-dichlorophenol was also
detected among the photoproducts of the dichlorophenyl ring-labelled
mixtures. This indicates that diphenyl ether cleavage had occurred to
some extent during photolysis.
Most photoproducts of nitrofen were identified as diphenyl ethers,
such as amino-nitrofen, hydroxyacetamido nitrofen, isocyano nitrofen,
4-hydroxy-2,5-dichloro-1 (4-nitrophenoxy) benzene, 5-hydroxy-nitrofen,
acetamidonitrofen, formamido nitrofen, nitroso-nitrofen and
5-hydroxy-amino-nitrofen. Total 14C from both labelled compounds
declined to less than 50 percent within 100 h and it was below 20
percent after 498 h (Honeycutt 1975).
METHODS OF RESIDUE ANALYSIS
Methods are available for determining either the parent compound only,
or all of the residues containing amino group or those that can be
converted to aminonitrofen. The parent compound is extracted with
dichloromethane from crops and with isopropanol-benzene 1:2 (v/v) from
soil. The extract is cleaned on a Florisil column and the residue is
determined by gas-liquid chromatography (GLC) using an electron
capture detector (ECD). The limit of determination is 0.01 mg/kg (Rohm
& Haas 1967a). Nitrofen residue can also be analysed with
multi-residue procedures. The limit of determination was reported to
be 0.01-0.02 mg/kg depending on the type of sample (Ambrus et al.
1981).
Either method is suitable for regulatory purposes. The majority of
metabolites can be determined with the method of Adler & Wargo (1975),
which is based on the reduction of the residues to a common amine
intermediate. The latter compound is further derivatized and
quantitatively measured by ECD.
NATIONAL MAXIMUM RESIDUE LIMITS
National maximum residue limits (MRLs) reported to the Meeting are
summarized in Table 6.
APPRAISAL
Nitrofen is a selective pre- and early post-emergence herbicide for
the control of animal grasses and various broad-leaved weeds in cereal
grains and vegetables. Its recommendations for use either prohibit
women from handling the product or demand stringent handling
precaution that prevent exposure during application. It has a limited
use, which is concentrated mainly on wheat. The technical product,
containing a minimum of 95 percent nitrofen, is formulated as a 25
percent EC, 50 percent WP or in combination with other compounds. It
is applied at rates of 2 to 6 kg a.i./ha to the soil surface or to
weeds at the two to four leaf growth stage.
Table 6 National Maximum Residue Limits for Nitrofen
Crop Country MRL
(mg/kg)
Barley Greece 0.5
Broccoli United States 0.75
Brussels sprouts 0.75
Cabbage 0.75
Cauliflower 0.75
Carrots 0.75
Celery 0.75
Eggs 0.75
Fat (poultry) 0.2
Fruits Japan 0.1
Horseradish United States 0.05
Kohlrabi 0.75
Legumes Japan 0.1
Meat, meat by-products and
fat (except poultry) in
cattle, goats, pigs, horses,
sheep United States 0.05
Milk (fat) 0.5
Milk (whole) 0.02
Onion (dry bulb) Austria 0.02
United States 0.75
Parsley United States 0.75
Rice (grain) Japan 0.1
Rice (grain and straw) United States 0.1
Roots Japan 0.1
Rye The Netherlands 0.01
Sugarbeet United States 0.05
Taro 0.02
Vegetables Japan 0.1
Wheat Greece 0.5
The Netherlands 0.01
Yugoslavia 0.01
Supervised trials were carried out in several countries on various
crops at or about the recommended rates and at twice these rates.
Broccoli, cabbage, cauliflower, celery, garlic, oilseed rape and rice
contained no measurable residues (<0.01 mg/kg) regardless of dosage,
pre-harvest interval or analytical method. No parent compound was
detectable in many of the wheat samples; in others treated at the
recommended rate the total residue, measured as the amino derivative,
ranged, up to 0.09 mg/kg. In contrast to the grain, wheat straw
contained the parent compound at levels of <0.002-0.02 mg/kg while
the sum of metabolites and parent compound ranged from 0.01 mg/kg to
0.11 mg/kg.
The metabolic pathways of nitrofen include reduction of the nitro
group, conjugation of the resulting amino group, hydroxylation of the
dichlorophenyl ring and incorporation into naturally occurring
materials. The major metabolites identified in plants were largely
similar to those in animals. In rice plants, the organo-extractable
residue amounted to 73.9 percent and 29.1 percent of the total at 10
and 90 days after application. The parent compound accounted for 72
percent of the extractable residue at day 10, while
hydroxyacetamidonitrofen (15 percent), the parent compound (13.7
percent) and the amino derivative (11.8 percent) were the major
components after 90 days. A similar pattern of metabolite formation
was observed in wheat. Neither the grain nor the straw contained
extractable residues at harvest. In studies with the radio-labelled
compound, 64 to 96 percent of the radioactive carbon was found in the
starch of rice and wheat grains. Approximately 50 percent of the
unextractable activity was found in the lignin fraction of the rice
straw.
No residue was detectable in milk or tissues of cattle given nitrofen
by capsule at rates equivalent to 0.05 and 0.5 mg/kg of total daily
diet. In cattle dosed at 5 mg/kg, the total residue ranged from 0.002
to 0.004 mg/kg in milk, while the only tissue containing a measurable
residue was fat (0.01 mg/kg). Further studies with the labelled
compound administered by capsule resulted in similar levels of
residues. In milk, the total radioactivity expressed as the parent
compound was 0.003 mg/kg and 0.08 mg/kg at feeding levels of 0.5 mg/kg
and 5 mg/kg in the diet, respectively. At the highest dose, the muscle
and fat contained 0.24 mg/kg and 1.15 mg/kg of radioactive residue,
respectively. However, no residue was detectable in the milk, urine or
faeces of cattle when nitrofen was mixed with the ration at a level of
5 mg/kg, owing to the rapid degradation of the parent compound and
aminonitrofen in rumen fluid.
Metabolites were identified in the urine, faeces and blood of sheep
dosed at the extreme rate of 40 mg/kg. In urine, the bulk of the
radioactivity consisted of sulphate, glucuronide and glycine
conjugates. The organo-soluble fraction (2 to 5.5 percent of the total
residue) consisted mainly of aminonitrofen and hydroxynitrofen, while
the parent compound amounted to only 0.3 percent. In blood, the parent
compound averaged 33.9 percent of the total activity, while
aminonitrofen and unknown compounds amounted to 20 percent and 13.4
percent, respectively.
Eggs and tissues of laying hens kept on a diet containing 0.04 mg/kg,
0.12 mg/kg and 0.48 mg/kg radio-labelled nitrofen were analysed. After
a 6-week feeding period the residue in eggs reached plateaus at levels
of 0.02, 0.05 and 0.17 mg/kg, respectively. Almost all of the residue
in eggs was found in the yolk. Residues ranged from non-detectable in
white meat to 0.18 mg/kg in fat at the 0.04 mg/kg feeding level.
As the lowest feeding level is approximately 10 times the calculated
maximum residue resulting from treated feed components, no detectable
residue can be expected in the milk and tissues of cattle or in eggs
and meat of poultry.
Fish take up nitrofen residues from water. The residue in fish tissues
is of about the same magnitude as that in the water shortly after the
beginning of exposure but declines more slowly.
Under field conditions, the nitrofen concentration in or on soil
declined rapidly at first (50 percent dissipation within 18 days) but
much more slowly thereafter. The major metabolites identified in the
soil were aminonitrofen and hydroxyacetamidonitrofen. Both nitrofen
and its aged derivates were immobile in soil, which is in keeping with
their high adsorption coefficient on different soils and the low water
solubility of parent compound.
Analytical methods are available for the determination of the parent
compound alone or the majority of the organo-extractable residue after
derivatization of aminonitrofen. The former method is recommended for
regulatory purposes.
RECOMMENDATIONS
The Meeting examined the results of supervised trials and metabolite
studies and concluded that the data available were sufficient for
estimating MRLs. As no acceptable daily intake was allocated, the MRLs
were recorded as guideline levels. They refer to the parent compound
only.
Commodity Guideline levels Preharvest interval
(mg/kg) on which levels are
(based months)
Wheat 0.01* 5
Wheat straw 0.05 5
*Residue at or about the limit of determination
FURTHER WORK OR INFORMATION
Desirable
Determination of nitrofen residue in wheat bran.
REFERENCES - RESIDUES
Abernathy, J.R. Linuron, chlorbromuron, nitrofen and
1972 fluorodifen absorption and movement in
12 selected Illinois soils. PhD. Thesis,
University of Illinois, Champaign-
Urbana. (Unpublished)
Adler, I.L. A fish residues study using 14C-labelled
1971 TOK. Rohm and Haas Research Report No.
23-41. (Unpublished)
Adler, I.L. A study to determine radioactive residue
1972 levels in eggs, tissues and excreta of
laying hens fed 14C TOK. Rohm & Haas
Report No. 23-47. (Unpublished)
Adler, I.L. Study of the hydrolysis of the herbicide
1973 TOK in water. Rohm & Haas. Report No.
3923-73-3. (Unpublished)
Adler, I.L, & Allen, Soil absorption studies with 14C TOK.
S.S. Jr. 1971 Rohm and Haas Report No. 23-77-11.
(Unpublished)
Adler, I.L. & Roser, R.L. A study of the leaching and metabolism
1971 of 14C TOK in soils. Rohm and Haas
Report No. 23-22. (Unpublished)
Adler, I.L. & Wargo, J.P. Determination of residues from the
1975 herbicide 2,4-dichloro-1-(4-
nitrophenoxy)-benzene in rice and wheat
by electron capture gas-liquid
chromatography. J. Assoc Off. Anal.
Chem., 58:551.
Ambrus, A., Csatlos, I., General method for determination of
Hargitai, E., Lautos, J., pesticide residues in samples of
Szabó, L., Visi, E. & plant origin, soil and water. Part
Zakar, F. I-III, H, Assoc. Off. Anal. Chem.,
64:733.
Fadayomi, O. & Warren, G.F. Absorption, desorption and leaching of
1977 nitrofen and oxyfluorten. Weed Sci.,
25:97.
Fischer, J.D. Dissipation study of TOK in soil and its
1971 effects on microbial activity. Rohm and
Haas Report. (Unpublished)
Fischer, J.D. Laboratory leaching study with aged TOK
soil. Rohm & Haas Report No. 3923-74-78.
(Unpublished)
Fischer, J.D. TOK greenhouse soil metabolism study.
1975 Rohm & Haas Report No. 3923-75-13.
(Unpublished)
Fischer, J.D. TOK fallow field study. Rohm & Haas
1976 Report No. 34H-76-16. (Unpublished)
Gutemann, W.H. & List, D.J. Metabolism of TOK herbicide in the dairy
1967 cow. J. Dairy Sci. 50:1516.
Honeycutt, R.C. Photolysis of 14C nitrofen by simulated
1975 sunlight. Rohm & Haas Report No. 3923-
75-6. (Unpublished)
Honeycutt, R.C. & Adler, Characterization of bound residues of
I.L. 1975 nitrofen in rice and wheat straw.
J. Agric. Food Chem. 23:1097-1101.
Hunt, L.M., Absorption, excretion, and metabolism
Chamberlain, W.F., of nitrofen by a sheep. J. Agric. Food
Gilbert, B.N., Chem., 25:1062-1065.
Hopkins, D.E. &
Gingrich, A.R.
1977
Institut National de la Report on residues of nitrofen on
Recherche Agronomique. wheat. Laboratorie de Phytopharmacie,
1975a Versailles. (Unpublished)
Institut National de la Reports on residues of nitrofen in rape.
Recherche Agronomique. (Unpublished)
1975b
Institut National de la Reports on residues of nitrofen in
Recherche Agronomique. wheat. (Unpublished)
1978
Miyauchi, M., Takagi, M. & Studies on the toxicity of chlorinated
Takayoshi, M. nitrobiphenyl ethers on fish-III. Bull.
1980 Japanese Soc. Sci. Fish., 46(7):837-844.
Nakagawa, M. & Crosby, D.G. Photodecomposition of nitrofen. J.
1974a Agric. Food. Chem. 22:849-853.
Nakagawa, M. & Crosby, D.G. Photonucleophilic reactions of nitrofen.
1974b J. Agric. Food Chem., 22:930-933.
Niki, Y. & Kuwatsuka, S. Degradation of diphenyl ether herbicides
1976 in soils. Soil Sci. Plant Nutr. (Tokyo),
22:223.
Rohm & Haas. Determination of TOK residues in crops
1967a and soil. RAR Memorandum No. 514.
(Unpublished)
Rohm & Haas. A study to determine residues levels in
1967b milk and tissues from a cow fed TOK.
Rohm and Haas Report No. 23-6.
(Unpublished)
Rohm & Haas. A study to determine residue levels in
1971 milk, tissues and excreta of a cow fed
14 C-labelled TOK. Rohm and Haas Report
No. 23-30. (Unpublished)
Rohm & Haas. Reports on residues of nitrofen: In
1969-1980 broccoli, Report Nos. 78-0172,
78-0173, 78-0174, 78-0175, 78-0176,
78-0177, 80-0249, 80-0250, 80-0279,
80-0280, 80-0290, 80-0291; in cabbage,
Report Nos. 78-0336, 78-0337, 80-0245,
80-0246, 80-0286, 0-0287; in
cauliflower, report nos. 78-0166,
78-0167, 78-0168, 78-0169, 78-0170,
78-0171, 80-0247, 80-0248, 80-288,
80-0289; in celery, Report Nos. 80-0238,
80-0251, 80-0292, 80-0293; garlic,
Report Nos. 80-0252, 80-0253, 80-0254,
80-0255; in rice, Report Nos. 2-69-227,
2-70-173, 2-70-190; in wheat, Report
Nos. 72-121-02, 72-122-02, 72-123-02,
72-292-02, 72-293-02, 72-294-02,
73-266-02, 73-267-02, 73-268-02,
73-269-02, 73-270-02, 73-271-02,
73-272-02, 73-309-02, 73-301-02,
73-311-02, 73-312-02, 73-313-02,
73-314-02, 73-315-02, 74-075-02,
74-076-02. (Unpublished)
Roser, R.L. & Adler, I.L. The nature of aged residues of TOK.
1971 Rohm & Haas Report No. 23-34.
(Unpublished)
Wargo, J.P. TOK and amino-TOK analysis of 14C-TOK
1974 treated wheat. Rohm and Haas Report No.
3923-74-8. (Unpublished)
Watanabe, I. Decomposition of pesticides, by soil
1973 micro-organisms - Special emphasis on
the flooded soil condition. Jap. Agric.
Res. Quar., 7:15.
Zorgorski, W.J. & Analysis of ground water samples for
Rogerson, T.D. residues of KELTHANE and TOK. Rohm
and Haas Report No. 34H-80-1.
(Unpublished)
2,5-dichlorophenol x
1 The substituted carbon atoms have not been identified.
Cattle feeding studies were conducted twice at feeding levels of 0.05,
0.5 and 5 mg/kg administered in gelatin capsules. Combined residues in
meat and milk were determined once in four cows and a second time with
14C-labelled nitrofen. In the multiple cow feeding study, no residues
of nitrofen were detected in milk or tissues of animals fed 0.05 or
0.5 mg/kg, based on the total daily diet. Barely detectable residues,
ranging from 0.002 to 0.004 mg/kg, were found in the milk of a cow fed
5 mg/kg. At this feeding level, no residue was found in any of the
tissues except fat (0.01 mg/kg). The sensitivity of the method was
0.01 mg/kg for tissue, and 0.002 mg/kg for milk (Rohm & Haas 1967b).
In the radioactive feeding study a cow was dosed orally twice a day
with 14C-labelled nitrofen. Nitrofen residues were not found in the
milk from the lowest feeding level (0.05 mg/kg) and only 0.003 and
0.08 mg/kg were found in milk from the two higher feeding levels, i.e.
0.5 and 5 mg/kg. The residue in milk was 0.3 percent and 0.8 percent
of the applied dosage, respectively. Residues were found in edible
tissues taken from the cow dosed at the 5 mg/kg feeding level.
Calculated as nitrofen, these residues ranged from 0.05 mg/kg (trips)
and 0.24 mg/kg (muscle) to 1.15 mg/kg (fat) (Rohm & Haas 1971). The
parent compound constituted approximately 47, 32 and 23 percent of the
total residue in milk, kidney and fat, respectively.
The lowest feeding level (0.05 mg/kg) of radioactive nitrofen is
almost ten times greater than the calculated maximum level of residue
that might be eaten by livestock kept on feed containing rice bran,
rice milling fractions or any other plant by-products that had been
treated with nitrofen. Consequently, no detectable residues are likely
to be found in milk and edible tissues.
Poultry
Three groups of 15 laying hens each were fed dichlorophenyl
ring-labelled 14C nitrofen incorporated in their feed for ten weeks
at levels of 0.04, 0.12 and 0.47 mg/kg, respectively. Residues in
tissues and eggs were studied and analysed by liquid scintillation
counting. Residues in eggs reached plateaux at levels of 0.02, 0.05
and 0.17 mg/kg, respectively, by week 6 of feeding for the X, 3X and
10X test group. The residue was located almost exclusively in the egg
yolk. The excreta collected for one 24 h period in week 3 contained
98.9 and 80 percent of the total radioactivity of feeding levels of
0.04 and 0.47 mg/kg, respectively. Hens from each group were
sacrificed after weeks 3, 5 and 10 of test feeding. Selected tissues
were removed and analysed by combustion and liquid scintillation
counting. Average residue levels in tissues from the 1X test group
ranged from non-detectable (white meat) to 0.18 mg/kg (fat).
Prolonged feeding of nitrofen to laying hens at a level of 0.04 mg/kg
daily resulted in total residue of approximately 0.01-0.02 mg/kg in
eggs. The residue was found almost exclusively in the egg yolk (Adler
1972).
Sheep
A female sheep was given a single oral, 40 mg/kg dose of 14C-labelled
nitrofen. After 99 h, 76.2 percent of the 14C had been recovered in
the excreta, with 39 percent appearing in the urine and 37.2 percent
in the faeces. The residue in the blood peaked at 19 h with a plateau
from 11 to 31 h; the concentrations were between 3 and 4 mg/kg. When
killed at 100 h, the highest tissue concentrations were found in the
fat (23 to 24 mg/kg total radioactivity expressed as nitrofen); the
gut, liver, thyroid and mammary glands contained from 2 to 4 mg/kg and
14 other organs had residues of 0.5 to 2 mg/kg (expressed as
nitrofen). The highest urinary excretion of nitrofen occurred during
the 11 to 19 h post-treatment collection period; the peak in the
faeces was during the 39 to 47 h post-treatment collection. Only 2 to
5.5 percent of the radioactivity in the urine was extractable with
chloroform. Aminoitrofen and 5-hydroxynitrofen were the principal
metabolites, with the parent compound accounting for less than 0.3
percent of the total radioactivity. Small amounts of phenol,
acetamidonitrofen and the 2-chloro ether, as well as several unknown
compounds, were found. The bulk of the radioactivity consisted of
sulphates, glucuronides and glycine conjugates. In contrast, 47 to 86
percent of the total radioactivity in the faeces could be extracted
with chloroform. Approximately 30 percent of the total radiocarbon
excreted in the faeces was the parent nitrofen (11 percent of the
total dose administered). Aminonitrofen accounted for an average of 21
percent of the 14C in the faeces, with the concentration increasing
over time from 14 percent at 23 h to 25 percent at 33 h. In blood
samples, the parent nitrofen averaged 33.9 percent of the total
radioactivity, aminonitrofen 20 percent and an unknown labelled
compound 13.4 percent. The amount of parent compound peaked at 43
percent at 19 h post-treatment and declined to 28 percent of the total
by 31 h (Hunt et al. 1977).
Fish
The accumulation of residue in fish was studied in a system consisting
of two tanks. The first tank contained soil treated with 14C-labelled
nitrofen, while the second one contained water only. The tanks were
connected and the water circulated. During the first six days, the
residue in the soil and water declined from 6.29 mg/kg and 0.016 mg/kg
to 4.31 mg/kg and 0.004 mg/kg, respectively. From day 10, the water
did not contain detectable residue, while the total residue in soil
was 3.62 mg/kg at day 28. The residue in the tail (edible portion) of
crayfish placed in the tank containing soil was 0.48 mg/kg on the
first day and varied in the range of 0.13-1.04 mg/kg thereafter. In
the tissue of catfish, kept in the second tank, the highest residue
(7.42 mg/kg) was measured at day 2. The residue level declined to 0.17
mg/kg at day 28. Approximately 55 percent of total 14C residue was
nitrofen (Adler 1971).
Cultures of intestinal micro-organisms from carp were capable of
reducing nitrofen to aminonitrofen under both aerobic and anaerobic
conditions (Miyauchi et al. 1980).
In Plants
Soil in which paddy rice was grown was treated pre-emergence with
dichlorophenyl ring-labelled nitrofen. The rice plants were sprayed
with nitrophenyl ring-labelled nitrofen in one experiment and with
dichlorophenyl ring-labelled nitrofen in another. The post-emergence
spray application was carried out to provide samples with sufficiently
large residues for metabolic identification and to study metabolic
routes that might correlate with post-emergence applications of the
compound to other commodities. Metabolites identified and their
percentage ratio in the extractable residue are shown in Table 3
(Roser & Adler 1971).
The parent compound amounted to 72 percent of the organic extractable
residue (73.9 percent of total residue) in rice plants treated 10 days
before sampling. The parent compound (13.7 percent) hydroxy
acetamidonitrofen (15 percent) and the amino derivative (11.8 percent)
were the major components of the organic extractable residue (29.1
percent of total residue) in rice straw 90 days after treatment. The
bound residues in rice and wheat grain and straw from a 14C
pre-emergence application were characterized by Honeycutt and Adler
(1975).
64 to 96 percent of the radioactive residues in wheat and rice grain
were in the starch 110 and 147 days, respectively, after planting and
treatment. The authors conclude that nitrofen must undergo
diphenylether bond cleavage followed by ring opening and incorporation
of the ring fragments into glucose and starch. Similar
characterization of the wheat and rice straw reaveled approximately 30
percent of total 14C residues identifiable in the lignin fraction.
Negligible (5 percent or less) 14C residues were associated with the
cellulose fraction, except for rice treated pre-emergence (25
percent).
Two experimental plots were treated with nitrofen labelled either on
the dichlorophenyl ring or on the nitrophenyl ring. Each treated plot
and the control were planted with two varieties, Anza and Era, of
wheat. Samples were taken at various intervals and analysed with
methods measuring the parent nitrofen only and all of the residues
containing an amino group or capable of being converted to amino
nitrofen. The results of analyses are shown in Table 4 (Wargo 1974).
Only about half of the total radioactivity could be extracted from the
earlier samples and only about 20 percent from straw at harvest. The
method for measuring aminonitrofen, which includes several
metabolites, resulted in substantially higher residues in the earlier
samples than the method for the parent compound only. No residues were
detected by either method in grain and straw at harvest. Most of the
radioactivity present in grain and straw at harvest was in the form of
non-toxic natural substances.
Table 3 Metabolites of Nitrofen Identified in Rice Grain and Straw
Compound1 Extracted 14C(%)
10 days 90 days 90 days
plant straw grain
Nitrofen 72.3 13.7 2
Azoxynitrofen 4.6 2.5
Aminonitrofen 3.3 11.8 4
Formamidonitrofen 4.8 )
Acetamidonitrofen 0.5 ) 19
5-Hydroxy aminonitrofen 1.9 )
Hydroxy acetamidonitrofen 2.8 15
Unknowns and polar conjugates 9.8 38 94
1 Structural formulas of compounds are shown in Table 2.
Table 4 Residues of 14C Nitrofen in Wheat
Residues (mg/kg calculated as nitrofen)
Label Variety Radioassay Nitrofen Aminonitrofen
(parent compound) Method
Sample Extract Method
Immature Plants (at 26 days)
Control Era 0.105 - 0.04 0.04
Control Anza 0.134 - 0.04 0.02
NO2 Era 4.04 2.26 0.99 1.57
NO2 Anza 2.42 1.08 0.63 0.77
Cl2 Era 2.80 - 0.47 1.02
Cl2 Anza 1.12 0.61 0.25 0.32
Immatare Plants (at 44 days)
Control Era 0.035 - NDR1 NDR
Control Anza 0.053 - NDR NDR
NO2 Era 2.19 1.17 0.55 0.87
NO2 Anza 1.18 0.68 0.44 0.51
Cl2 Era 2.55 1.34 0.41 1.12
Cl2 Anza 1.44 1.00 0.48 0.54
Table 4 (continued)
Residues (mg/kg calculated as nitrofen)
Label Variety Radioassay Nitrofen Aminonitrofen
(parent compound) Method
Sample Extract Method
Final Harvest (at 110 days)
Grain Straw Straw2 Grain Straw Grain Straw
Control Era 0.025 0.087 - NDR NDR NDR NDR
Control Anza 0.034 0.028 - NDR NDR NDR NDR
NO2 Era 0.173 0.992 0.19 NDR NDR NDR NDR
NO2 Anza 0.148 0.460 0.10 NDR NDR NDR NDR
Cl2 Era 0.192 1.123 0.22 NDR NDR NDR NDR
Cl2 Anza 0.122 0.624 0.14 NDR NDR NDR NDR
1 NDR = no detectable residue; sensitivity = <0.01 mg/kg
2 No detectable 14C activity was present in the extract from any sample of grain
In Soil and Water
Roser and Adler (1971) identified the soil metabolites resulting from
a preplant/prefood application of dichlorophenyl ring 14C-labelled
nitrofen to simulated field rice paddies. Characterization of residues
in soil samples taken 147 days after treatment revealed that the
parent compound represented 23 percent of the extractable residue,
which amounted to 36 percent of total 14C activity. The major
metabolites were the aminonitrofen (35.4 percent) and
hydroxyacetamidonitrofen (10.7 percent), while the levels of
azoxynitrofen, formamidonitrofen and acetamidonitrofen were below 10
percent of extractable activity. Following a laboratory leaching
study, Adler and Roser (1971) characterized the 14C products in the
top 2.5 cm from soil columns. The extracts (6396 percent of total
activity) contained primarily parent compound (>90 percent), but a
qualitatively similar spectrum of products was observed as those
identified in the previous experiment. Niki and Kuwatsuka (1976)
studied several diphenyl ether herbicides in two rice paddy soils and
found rapid metabolism of nitrofen occurring in both soils. Under
flooded conditions, the half-life was less than 10 days while the
dissipation rate was slower under upland conditions. The primary
degradation product was the corresponding 4'-amino derivative.
Watanabe (1973) reported similar rapid dissipation under field
conditions.
In sealed metabolism flasks (biometer flasks), the metabolism of 14C
nitrofen under aerobic conditions were followed through approximately
100 days (Fischer 1971). Evolution of 14CO2 was detectable from both
dichlorophenyl and nitrophenyl ring-labelled preparations (ca. 3 and
1.5 percent of applied 14C, respectively). In order to characterize
accurately the residue decline patterns of nitrofen from field soil, a
plot was surface-treated with dichlorophenyl - 14C compound and left
fallow through the growing season (Fischer 1976). Radioassay of soil
samples collected through 316 days demonstrated a very rapid initial
decline of 0-15 cm residues (50 percent dissipation within 18 days )
followed by a slower decline. In contrast to field conditions nitrofen
did not undergo dissipation and metabolism under either aerobic or
anaerobic conditions in the greenhouse (Fischer 1975).
Fadayomi and Warren (1977) reported essentially no downward movement
of 14C nitrofen (nitrophenyl ring) that was spiked onto columns of a
silt loam and a fine sand soil and subjected to 5.4 cm of simulated
rainfall. Likewise, Abernathy (1972) found essentially no movement of
nitrofen on thin-layer plates coated with 12 different soil types. The
leaching tendencies for aged nitrofen residues were also determined
(Fischer 1974). After aging for 30 days under greenhouse conditions, a
14C nitrofen (both ring labelled) fortified sandy loam soil was
placed in 30 cm columns and subjected to daily simulated rainfalls of
12 mm/day for 45 days. Radioassay of the leachates as well as the
dissected columns revealed 83 to 102 percent of the applied 14C in
the top 5 cm. No significant 14C was detectable in the leachates.
Using nitrophenyl 14C-labelled nitrofen, a total of five soils of
different types plus river silt were compared as to the extent of
nitrofen adsorption from an aqueous solution (Adler & Allen 1971). The
average amount of 14C adsorbed was greater than 90 percent in every
case from 0.1 ms/l solutions and in all but one (high kaolinite clay
soil) from 1.0 ms/l solutions. These findings are consistent with
those of Fadayomi & Warren (1977), in which most or complete
adsorption of 14C nitrofen was observed by muck soil and bentonite
clays but not by kaolinite clays. Nitrofen was desorbed slowly when
the adsorbents were resuspended in distilled water four successive
times.
The observed immobility of nitrofen is consistent with its low water
solubility and relatively high adsorptive properties. The findings of
laboratory experiments are supported by the results of field
experiments in which water from wells surrounded by or near to treated
fields were sampled. No residue of nitrofen (<0.001 mg/l) was
detectable in 28 samples taken from depths of 3 to 27 m (Zagorski &
Rogerson 1980).
Nitrofen has not demonstrated any tendency to hydrolyse in aqueous
media either in the environment or in the laboratory (Adler 1973).
Photodecomposition
Nakagawa & Crosby (1974a) reported that nitrofen exposed in aqueous
suspensions to sunlight or simulated sunlight underwent rapid ether
cleavage to form 2,4-dichlorophenol and p-nitrophenol, along with
other numerous degradation products. In sunlight, more than 50 percent
of the initial nitrofen degraded after one week of outside exposure.
In a related publication, Nakagawa & Crosby (1974b) reported that the
sunlight photolysis of nitrofen in aqueous methanol represented a
photonucleophilic displacement of nitrophenol by the hydroxide ion of
water.
Both nitrophenyl ring-labelled nitrofen and dichlorophenyl
ring-labelled nitrofen were placed on glass plates as a thin film and
irradiated with simulated sunlight. Samples were takes after 48, 165,
239, 359 and 498 h of illumination. Some of the resulting
photoproducts were identified by thin-layer chromatography and mass
spectrometry. Nitrofen containing both ring labels gave rise to at
least 13 14C-photoproducts. Eleven of the radioactive components of
the nitrophenyl ring-labelled photoproduct mixture were identical (by
TLC Rf values) to corresponding 14C-components of the dichlorophenyl
ring-labelled photoproduct mixture. One unique component of the
nitrophenyl ring-labelled 14C nitrofen photolysis mixture was
identified as 14C-p-nitrophenol. 14C-2,4-dichlorophenol was also
detected among the photoproducts of the dichlorophenyl ring-labelled
mixtures. This indicates that diphenyl ether cleavage had occurred to
some extent during photolysis.
Most photoproducts of nitrofen were identified as diphenyl ethers,
such as amino-nitrofen, hydroxyacetamido nitrofen, isocyano nitrofen,
4-hydroxy-2,5-dichloro-1 (4-nitrophenoxy) benzene, 5-hydroxy-nitrofen,
acetamidonitrofen, formamido nitrofen, nitroso-nitrofen and
5-hydroxy-amino-nitrofen. Total 14C from both labelled compounds
declined to less than 50 percent within 100 h and it was below 20
percent after 498 h (Honeycutt 1975).
METHODS OF RESIDUE ANALYSIS
Methods are available for determining either the parent compound only,
or all of the residues containing amino group or those that can be
converted to aminonitrofen. The parent compound is extracted with
dichloromethane from crops and with isopropanol-benzene 1:2 (v/v) from
soil. The extract is cleaned on a Florisil column and the residue is
determined by gas-liquid chromatography (GLC) using an electron
capture detector (ECD). The limit of determination is 0.01 mg/kg (Rohm
& Haas 1967a). Nitrofen residue can also be analysed with
multi-residue procedures. The limit of determination was reported to
be 0.01-0.02 mg/kg depending on the type of sample (Ambrus et al.
1981).
Either method is suitable for regulatory purposes. The majority of
metabolites can be determined with the method of Adler & Wargo (1975),
which is based on the reduction of the residues to a common amine
intermediate. The latter compound is further derivatized and
quantitatively measured by ECD.
NATIONAL MAXIMUM RESIDUE LIMITS
National maximum residue limits (MRLs) reported to the Meeting are
summarized in Table 6.
APPRAISAL
Nitrofen is a selective pre- and early post-emergence herbicide for
the control of animal grasses and various broad-leaved weeds in cereal
grains and vegetables. Its recommendations for use either prohibit
women from handling the product or demand stringent handling
precaution that prevent exposure during application. It has a limited
use, which is concentrated mainly on wheat. The technical product,
containing a minimum of 95 percent nitrofen, is formulated as a 25
percent EC, 50 percent WP or in combination with other compounds. It
is applied at rates of 2 to 6 kg a.i./ha to the soil surface or to
weeds at the two to four leaf growth stage.
Table 6 National Maximum Residue Limits for Nitrofen
Crop Country MRL
(mg/kg)
Barley Greece 0.5
Broccoli United States 0.75
Brussels sprouts 0.75
Cabbage 0.75
Cauliflower 0.75
Carrots 0.75
Celery 0.75
Eggs 0.75
Fat (poultry) 0.2
Fruits Japan 0.1
Horseradish United States 0.05
Kohlrabi 0.75
Legumes Japan 0.1
Meat, meat by-products and
fat (except poultry) in
cattle, goats, pigs, horses,
sheep United States 0.05
Milk (fat) 0.5
Milk (whole) 0.02
Onion (dry bulb) Austria 0.02
United States 0.75
Parsley United States 0.75
Rice (grain) Japan 0.1
Rice (grain and straw) United States 0.1
Roots Japan 0.1
Rye The Netherlands 0.01
Sugarbeet United States 0.05
Taro 0.02
Vegetables Japan 0.1
Wheat Greece 0.5
The Netherlands 0.01
Yugoslavia 0.01
Supervised trials were carried out in several countries on various
crops at or about the recommended rates and at twice these rates.
Broccoli, cabbage, cauliflower, celery, garlic, oilseed rape and rice
contained no measurable residues (<0.01 mg/kg) regardless of dosage,
pre-harvest interval or analytical method. No parent compound was
detectable in many of the wheat samples; in others treated at the
recommended rate the total residue, measured as the amino derivative,
ranged, up to 0.09 mg/kg. In contrast to the grain, wheat straw
contained the parent compound at levels of <0.002-0.02 mg/kg while
the sum of metabolites and parent compound ranged from 0.01 mg/kg to
0.11 mg/kg.
The metabolic pathways of nitrofen include reduction of the nitro
group, conjugation of the resulting amino group, hydroxylation of the
dichlorophenyl ring and incorporation into naturally occurring
materials. The major metabolites identified in plants were largely
similar to those in animals. In rice plants, the organo-extractable
residue amounted to 73.9 percent and 29.1 percent of the total at 10
and 90 days after application. The parent compound accounted for 72
percent of the extractable residue at day 10, while
hydroxyacetamidonitrofen (15 percent), the parent compound (13.7
percent) and the amino derivative (11.8 percent) were the major
components after 90 days. A similar pattern of metabolite formation
was observed in wheat. Neither the grain nor the straw contained
extractable residues at harvest. In studies with the radio-labelled
compound, 64 to 96 percent of the radioactive carbon was found in the
starch of rice and wheat grains. Approximately 50 percent of the
unextractable activity was found in the lignin fraction of the rice
straw.
No residue was detectable in milk or tissues of cattle given nitrofen
by capsule at rates equivalent to 0.05 and 0.5 mg/kg of total daily
diet. In cattle dosed at 5 mg/kg, the total residue ranged from 0.002
to 0.004 mg/kg in milk, while the only tissue containing a measurable
residue was fat (0.01 mg/kg). Further studies with the labelled
compound administered by capsule resulted in similar levels of
residues. In milk, the total radioactivity expressed as the parent
compound was 0.003 mg/kg and 0.08 mg/kg at feeding levels of 0.5 mg/kg
and 5 mg/kg in the diet, respectively. At the highest dose, the muscle
and fat contained 0.24 mg/kg and 1.15 mg/kg of radioactive residue,
respectively. However, no residue was detectable in the milk, urine or
faeces of cattle when nitrofen was mixed with the ration at a level of
5 mg/kg, owing to the rapid degradation of the parent compound and
aminonitrofen in rumen fluid.
Metabolites were identified in the urine, faeces and blood of sheep
dosed at the extreme rate of 40 mg/kg. In urine, the bulk of the
radioactivity consisted of sulphate, glucuronide and glycine
conjugates. The organo-soluble fraction (2 to 5.5 percent of the total
residue) consisted mainly of aminonitrofen and hydroxynitrofen, while
the parent compound amounted to only 0.3 percent. In blood, the parent
compound averaged 33.9 percent of the total activity, while
aminonitrofen and unknown compounds amounted to 20 percent and 13.4
percent, respectively.
Eggs and tissues of laying hens kept on a diet containing 0.04 mg/kg,
0.12 mg/kg and 0.48 mg/kg radio-labelled nitrofen were analysed. After
a 6-week feeding period the residue in eggs reached plateaus at levels
of 0.02, 0.05 and 0.17 mg/kg, respectively. Almost all of the residue
in eggs was found in the yolk. Residues ranged from non-detectable in
white meat to 0.18 mg/kg in fat at the 0.04 mg/kg feeding level.
As the lowest feeding level is approximately 10 times the calculated
maximum residue resulting from treated feed components, no detectable
residue can be expected in the milk and tissues of cattle or in eggs
and meat of poultry.
Fish take up nitrofen residues from water. The residue in fish tissues
is of about the same magnitude as that in the water shortly after the
beginning of exposure but declines more slowly.
Under field conditions, the nitrofen concentration in or on soil
declined rapidly at first (50 percent dissipation within 18 days) but
much more slowly thereafter. The major metabolites identified in the
soil were aminonitrofen and hydroxyacetamidonitrofen. Both nitrofen
and its aged derivates were immobile in soil, which is in keeping with
their high adsorption coefficient on different soils and the low water
solubility of parent compound.
Analytical methods are available for the determination of the parent
compound alone or the majority of the organo-extractable residue after
derivatization of aminonitrofen. The former method is recommended for
regulatory purposes.
RECOMMENDATIONS
The Meeting examined the results of supervised trials and metabolite
studies and concluded that the data available were sufficient for
estimating MRLs. As no acceptable daily intake was allocated, the MRLs
were recorded as guideline levels. They refer to the parent compound
only.
Commodity Guideline levels Preharvest interval
(mg/kg) on which levels are
(based months)
Wheat 0.01* 5
Wheat straw 0.05 5
*Residue at or about the limit of determination
FURTHER WORK OR INFORMATION
Desirable
Determination of nitrofen residue in wheat bran.
REFERENCES - RESIDUES
Abernathy, J.R. Linuron, chlorbromuron, nitrofen and
1972 fluorodifen absorption and movement in
12 selected Illinois soils. PhD. Thesis,
University of Illinois, Champaign-
Urbana. (Unpublished)
Adler, I.L. A fish residues study using 14C-labelled
1971 TOK. Rohm and Haas Research Report No.
23-41. (Unpublished)
Adler, I.L. A study to determine radioactive residue
1972 levels in eggs, tissues and excreta of
laying hens fed 14C TOK. Rohm & Haas
Report No. 23-47. (Unpublished)
Adler, I.L. Study of the hydrolysis of the herbicide
1973 TOK in water. Rohm & Haas. Report No.
3923-73-3. (Unpublished)
Adler, I.L, & Allen, Soil absorption studies with 14C TOK.
S.S. Jr. 1971 Rohm and Haas Report No. 23-77-11.
(Unpublished)
Adler, I.L. & Roser, R.L. A study of the leaching and metabolism
1971 of 14C TOK in soils. Rohm and Haas
Report No. 23-22. (Unpublished)
Adler, I.L. & Wargo, J.P. Determination of residues from the
1975 herbicide 2,4-dichloro-1-(4-
nitrophenoxy)-benzene in rice and wheat
by electron capture gas-liquid
chromatography. J. Assoc Off. Anal.
Chem., 58:551.
Ambrus, A., Csatlos, I., General method for determination of
Hargitai, E., Lautos, J., pesticide residues in samples of
Szabó, L., Visi, E. & plant origin, soil and water. Part
Zakar, F. I-III, H, Assoc. Off. Anal. Chem.,
64:733.
Fadayomi, O. & Warren, G.F. Absorption, desorption and leaching of
1977 nitrofen and oxyfluorten. Weed Sci.,
25:97.
Fischer, J.D. Dissipation study of TOK in soil and its
1971 effects on microbial activity. Rohm and
Haas Report. (Unpublished)
Fischer, J.D. Laboratory leaching study with aged TOK
soil. Rohm & Haas Report No. 3923-74-78.
(Unpublished)
Fischer, J.D. TOK greenhouse soil metabolism study.
1975 Rohm & Haas Report No. 3923-75-13.
(Unpublished)
Fischer, J.D. TOK fallow field study. Rohm & Haas
1976 Report No. 34H-76-16. (Unpublished)
Gutemann, W.H. & List, D.J. Metabolism of TOK herbicide in the dairy
1967 cow. J. Dairy Sci. 50:1516.
Honeycutt, R.C. Photolysis of 14C nitrofen by simulated
1975 sunlight. Rohm & Haas Report No. 3923-
75-6. (Unpublished)
Honeycutt, R.C. & Adler, Characterization of bound residues of
I.L. 1975 nitrofen in rice and wheat straw.
J. Agric. Food Chem. 23:1097-1101.
Hunt, L.M., Absorption, excretion, and metabolism
Chamberlain, W.F., of nitrofen by a sheep. J. Agric. Food
Gilbert, B.N., Chem., 25:1062-1065.
Hopkins, D.E. &
Gingrich, A.R.
1977
Institut National de la Report on residues of nitrofen on
Recherche Agronomique. wheat. Laboratorie de Phytopharmacie,
1975a Versailles. (Unpublished)
Institut National de la Reports on residues of nitrofen in rape.
Recherche Agronomique. (Unpublished)
1975b
Institut National de la Reports on residues of nitrofen in
Recherche Agronomique. wheat. (Unpublished)
1978
Miyauchi, M., Takagi, M. & Studies on the toxicity of chlorinated
Takayoshi, M. nitrobiphenyl ethers on fish-III. Bull.
1980 Japanese Soc. Sci. Fish., 46(7):837-844.
Nakagawa, M. & Crosby, D.G. Photodecomposition of nitrofen. J.
1974a Agric. Food. Chem. 22:849-853.
Nakagawa, M. & Crosby, D.G. Photonucleophilic reactions of nitrofen.
1974b J. Agric. Food Chem., 22:930-933.
Niki, Y. & Kuwatsuka, S. Degradation of diphenyl ether herbicides
1976 in soils. Soil Sci. Plant Nutr. (Tokyo),
22:223.
Rohm & Haas. Determination of TOK residues in crops
1967a and soil. RAR Memorandum No. 514.
(Unpublished)
Rohm & Haas. A study to determine residues levels in
1967b milk and tissues from a cow fed TOK.
Rohm and Haas Report No. 23-6.
(Unpublished)
Rohm & Haas. A study to determine residue levels in
1971 milk, tissues and excreta of a cow fed
14 C-labelled TOK. Rohm and Haas Report
No. 23-30. (Unpublished)
Rohm & Haas. Reports on residues of nitrofen: In
1969-1980 broccoli, Report Nos. 78-0172,
78-0173, 78-0174, 78-0175, 78-0176,
78-0177, 80-0249, 80-0250, 80-0279,
80-0280, 80-0290, 80-0291; in cabbage,
Report Nos. 78-0336, 78-0337, 80-0245,
80-0246, 80-0286, 0-0287; in
cauliflower, report nos. 78-0166,
78-0167, 78-0168, 78-0169, 78-0170,
78-0171, 80-0247, 80-0248, 80-288,
80-0289; in celery, Report Nos. 80-0238,
80-0251, 80-0292, 80-0293; garlic,
Report Nos. 80-0252, 80-0253, 80-0254,
80-0255; in rice, Report Nos. 2-69-227,
2-70-173, 2-70-190; in wheat, Report
Nos. 72-121-02, 72-122-02, 72-123-02,
72-292-02, 72-293-02, 72-294-02,
73-266-02, 73-267-02, 73-268-02,
73-269-02, 73-270-02, 73-271-02,
73-272-02, 73-309-02, 73-301-02,
73-311-02, 73-312-02, 73-313-02,
73-314-02, 73-315-02, 74-075-02,
74-076-02. (Unpublished)
Roser, R.L. & Adler, I.L. The nature of aged residues of TOK.
1971 Rohm & Haas Report No. 23-34.
(Unpublished)
Wargo, J.P. TOK and amino-TOK analysis of 14C-TOK
1974 treated wheat. Rohm and Haas Report No.
3923-74-8. (Unpublished)
Watanabe, I. Decomposition of pesticides, by soil
1973 micro-organisms - Special emphasis on
the flooded soil condition. Jap. Agric.
Res. Quar., 7:15.
Zorgorski, W.J. & Analysis of ground water samples for
Rogerson, T.D. residues of KELTHANE and TOK. Rohm
and Haas Report No. 34H-80-1.
(Unpublished)
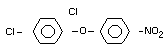 Molecular Formula
C12H9O3Cl2N
Other Information on Identify and Properties
Molecular mass 284. 11
Physical state dark brown to black semi-solid
(technical compound)
Melting point 70-71°C (pure compound)
Specific density 1.80 g/ml at 83°C
(technical product)
Vapour pressure 2 x 10-6 mm Hg at 250°C
Solubility (25°C) in
- water about 1 mg/l
- acetone about 25%
- ethyl acetate 50-60%
- methanol about 25%
- methylene chloride 58-68%
- xylene about 25%
Stability hydrolitically stable but rapidly
photodegraded in solution and on
surfaces
Purity of Technical Product
nitrofen technical contains > 95% pure compound
Formulations
Nitrofen is primarily formulated as a liquid emulsifiable concentrate
containing 25 percent active ingredient and a wettable powder
containing 50 percent active ingredient. It is also available in
combination with other herbicides and has been available as a granular
formulation.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, Distribution, Excretion and Metabolism
Rat
Samples of urine and faeces from two experiments in which rats had
been fed nitrophenyl ring - and dichlorophenyl ring-14C-labelled
nitrofen, respectively, were used to determine the metabolic rate of
nitrofen. Faeces contained approximately 75 percent and urine
approximately 20 percent of the administered dose. Unmetabolized
nitrofen was excreted in the faeces but not in the urine and
represened ca. 36 percent of the total excretion. Nitrofen and
non-hydroxylated metabolites accounted for ca. 61 percent of the total
of the excreted dose, with relatively higher levels in urine (28
percent) than faecesœ
Hydroxylation occurred in the dichlorophenyl ring. Unidentified polar
metabolites (conjugates) were found in the urine. Cleavage of the
diphenyl ether moiety was not observed (Roser et al. 1971).
One male and one female albino rat were each given (by stomach tube)
nine daily doses of 125 mg/kg b.w. 14C-nitrofen uniformly labelled
in the nitrophenyl ring. After the dosing period, the rats were
maintained on a nitrofen-free diet for two days, and then sacrificed.
Recovery of total administered dose was 81.8 percent in the female
rate and 94.2 percent in the male rat, with 54.1 percent and 75.6
percent, respectively, appearing in the faeces and 18.0 percent and
13.7 percent in the urine. Metabolism to 14CO2 occurred to a minimal
extent (0.01 percent). A small portion (1.12 percent) of the
administered dose of nitrofen was found in all tissues and organs, the
highest concentrations of nitrofen and/or its metabolites were found
in the fat (211.9 ppm), spleen (54.3 ppm), stomach (42.5 ppm, blood
(40.7 ppm), kidneys and adrenals (58.1 ppm), liver (29.4 ppm) and skin
and hair (26.2 ppm). (Adler et al. 1970a)
Albino rats (one female and one male) were each given (by stomach
tube) daily doses of nitrofen (uniformly 14C-labelled in the
dichlorophenyl rins) at a level of 115 mg/kg b.w. for eight days.
After the dosing period, the rats were maintained on a nitrofen-free
diet for three days and were then sacrificed.
Throughout the experiment, urine, faeces and exhaled CO2 were
collected separately and were assayed for radioactivity, as were
tissues removed from the male rat at sacrificeœ
Recovery of total administered dose was 104 percent in the female rat
and 106 percent in the male rat, with 73.9 percent and 80.0 percent,
respectively, appearing in the faeces and 18.0 percent and 13.7
percent in the urine. Metabolism to 14CO2 occurs to a minimal extens
(0œ01 percent). Some radioactivity (0.89 percent) is found in all
tissues, with the highest concentrations being found in the blood
(21.9 ppm), fat (18.4 ppm), large intestine (13.5 ppm), liver (12.9
ppm), kidney and adrenals (11.0 ppm) and skin and hair (10œ6 ppm).
(Adler et al. 197Ob)
Groups of weanling albino Wistar rats (25 males and 25 females per
group) were fed diets containing technical grade (95 percent nitrofen
at levels of 0, 10, 100 and 1 000 ppm) for 97 weeks.
Tissues (fat, muscle, liver, kidney, blood) collected at week 97 and
excreta (urine, faeces) collected at week 65, were pooled and analysed
for nitrofen content by sas-chromatography.
The concentration of nitrofen was highest in fat (20.6, 166.0 and
814.0 ppm, respectively, at the three dietary levels tested), followed
by muscle (0.92, 8.2 and 18.0 ppm), kidney (0.15, 3.2 and 9.0), blood
(0.18, 0.92 and 3.4 ppm) and liver (0.04, 0.44 and <0.01). Excretion
of unchanged nitrofen occurs almost exclusively in the faeces (35.0
ppm at the highest level). (Ambrose et al. 1971) (Full experimental
data were not reported.)
In a comparative study of the fate of 14C-nitrofen, sets of two
female and two male Osborne-Mendel rats and two female Fischer-344
rats were fed diets containing technical nitrofen at levels of 60 or
1 300 ppm. After two weeks, the animals received a single oral pulse
dose of 3 and 65 mg/kg b.w. uniformly dichlorophenyl-rins-labelled
14C-nitrofen, respectively. The same treatment was repeated after
four weeks of dietary nitrofen administration. Urine and faeces were
collected daily after the first pulse dose until sacrifice. One animal
of each strain from each dose level was sacrificed at 24 and 96h after
the second 14C-nitrofen dose. Blood, liver, pancreas, fat and skin
samples were analysed for radioactivity and metabolites. By 92h after
the second pulse dose, the animals in all groups had eliminated
between 3 and 22 percent of the 14C in the urine and between 30 and
100 percent in the faeces. After the two-week exposure, the female
Osborne-Mendel rats excreted 14C in the urine more rapidly than the
males; both sexes of Osborne-Mendel rat excreted 14C more rapidly in
the faeces than did the female Fischer rats. There were no obvious
differences related to sex or strain in tissue concentrations, in
tissue binding or in the metabolite profiles in urine, faeces or
liver; some differences were noted in the pancreas. At 24h after
dosing, concentrations in the blood, fat, liver, pancreas and skin of
14C (calculated as nitrofen) were 0.15 to 0.25, 4.8 to 7.6, 0.5 to
0.8, 1.1 to 1.4 and 0.5 to 0.9 ppm, respectively, for the 3 mg/kg dose
groups and 2.7 to 4.1, 39 to 80, 6.2 to 14, 3.3 to 5.3 and 2.9 to 5.0
ppm, respectively, for the 65 mg/kg dose groups. By 92h after dosing,
the concentrations in the same tissues had declined to 0.06 to 0.09,
3.2 to 4.2, 0.21 to 0.22, 0.3 to 1.4 and 0.4 to 0.8 ppm, respectively,
for the 3 mg/kg groups and 1.6 to 2.2, 1.0 to 12.6, 1.3 to 2.5, 0.6 to
2.1 and 0.3 to 0.9 ppm, respectively, for the 64 mg/kg groups. About
14 to 29 percent and 22 to 42 percent of the 14C were bound in the
liver of low-dose and high-dose animals, respectively, while 1 to 6
percent and 16 to 36 percent were bound in the pancreas. The urinary
metabolites were generally very hydrophillic. Traces of
hydroxyaminonitrofen, acetamidonitrofen and aminonitrofen were
observed but urinary metabolites were not identified further. No
parent compound was detected; 33 percent of the total applied dose was
excreted in the faeces as the parent compound, 14.4 percent as a polar
unknown, 5 percent as acetamidonitrofen, 2.8 percent as 3'-hydroxy
acetamidonitrofen, 2.8 percent as 4-hydroxyaminonitrofen, 2.4 percent
as aminonitrofen and 0.2 percent as 5-hydroxynitrofen. With time, the
concentrations of nitrofen, aminonitrofen and hydroxyaminonitrofen
decreased, and the concentrations of the acetylated compounds
increased. While the metabolic profile in the liver paralleled that in
the urine, the pancreatic metabolites were more lipophilic and
consisted exclusively of the parent compound in the 3 mg/kg groups,
while the 65 ms/ks groups exhibited a variety of metabolites,
including aminonitrofen, hydroxyaminonitrofen, hydroxynitrofen and
2,4-dichloropheno) in addition to the 20 to 74 percent parent
compound. Nitrofen appeared to accumulate to a greater extent in the
pancreas of high-dose female Osborne-Mendel rats than in the pancreas
of the males of this strain or the Osborne-Mendel female pancreas
contained 2.23 ppm nitrofen while the pancreas of each of the other
two groups averaged 0.83 and 1.08 ppm. This strain and sex difference
was not seen in the 3 mg/kg animals which were also killed at 24h
(1.07, 1.19 and 1.36 ppm nitrofen) (Steiserwalt et al. 1980a).
Molecular Formula
C12H9O3Cl2N
Other Information on Identify and Properties
Molecular mass 284. 11
Physical state dark brown to black semi-solid
(technical compound)
Melting point 70-71°C (pure compound)
Specific density 1.80 g/ml at 83°C
(technical product)
Vapour pressure 2 x 10-6 mm Hg at 250°C
Solubility (25°C) in
- water about 1 mg/l
- acetone about 25%
- ethyl acetate 50-60%
- methanol about 25%
- methylene chloride 58-68%
- xylene about 25%
Stability hydrolitically stable but rapidly
photodegraded in solution and on
surfaces
Purity of Technical Product
nitrofen technical contains > 95% pure compound
Formulations
Nitrofen is primarily formulated as a liquid emulsifiable concentrate
containing 25 percent active ingredient and a wettable powder
containing 50 percent active ingredient. It is also available in
combination with other herbicides and has been available as a granular
formulation.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, Distribution, Excretion and Metabolism
Rat
Samples of urine and faeces from two experiments in which rats had
been fed nitrophenyl ring - and dichlorophenyl ring-14C-labelled
nitrofen, respectively, were used to determine the metabolic rate of
nitrofen. Faeces contained approximately 75 percent and urine
approximately 20 percent of the administered dose. Unmetabolized
nitrofen was excreted in the faeces but not in the urine and
represened ca. 36 percent of the total excretion. Nitrofen and
non-hydroxylated metabolites accounted for ca. 61 percent of the total
of the excreted dose, with relatively higher levels in urine (28
percent) than faecesœ
Hydroxylation occurred in the dichlorophenyl ring. Unidentified polar
metabolites (conjugates) were found in the urine. Cleavage of the
diphenyl ether moiety was not observed (Roser et al. 1971).
One male and one female albino rat were each given (by stomach tube)
nine daily doses of 125 mg/kg b.w. 14C-nitrofen uniformly labelled
in the nitrophenyl ring. After the dosing period, the rats were
maintained on a nitrofen-free diet for two days, and then sacrificed.
Recovery of total administered dose was 81.8 percent in the female
rate and 94.2 percent in the male rat, with 54.1 percent and 75.6
percent, respectively, appearing in the faeces and 18.0 percent and
13.7 percent in the urine. Metabolism to 14CO2 occurred to a minimal
extent (0.01 percent). A small portion (1.12 percent) of the
administered dose of nitrofen was found in all tissues and organs, the
highest concentrations of nitrofen and/or its metabolites were found
in the fat (211.9 ppm), spleen (54.3 ppm), stomach (42.5 ppm, blood
(40.7 ppm), kidneys and adrenals (58.1 ppm), liver (29.4 ppm) and skin
and hair (26.2 ppm). (Adler et al. 1970a)
Albino rats (one female and one male) were each given (by stomach
tube) daily doses of nitrofen (uniformly 14C-labelled in the
dichlorophenyl rins) at a level of 115 mg/kg b.w. for eight days.
After the dosing period, the rats were maintained on a nitrofen-free
diet for three days and were then sacrificed.
Throughout the experiment, urine, faeces and exhaled CO2 were
collected separately and were assayed for radioactivity, as were
tissues removed from the male rat at sacrificeœ
Recovery of total administered dose was 104 percent in the female rat
and 106 percent in the male rat, with 73.9 percent and 80.0 percent,
respectively, appearing in the faeces and 18.0 percent and 13.7
percent in the urine. Metabolism to 14CO2 occurs to a minimal extens
(0œ01 percent). Some radioactivity (0.89 percent) is found in all
tissues, with the highest concentrations being found in the blood
(21.9 ppm), fat (18.4 ppm), large intestine (13.5 ppm), liver (12.9
ppm), kidney and adrenals (11.0 ppm) and skin and hair (10œ6 ppm).
(Adler et al. 197Ob)
Groups of weanling albino Wistar rats (25 males and 25 females per
group) were fed diets containing technical grade (95 percent nitrofen
at levels of 0, 10, 100 and 1 000 ppm) for 97 weeks.
Tissues (fat, muscle, liver, kidney, blood) collected at week 97 and
excreta (urine, faeces) collected at week 65, were pooled and analysed
for nitrofen content by sas-chromatography.
The concentration of nitrofen was highest in fat (20.6, 166.0 and
814.0 ppm, respectively, at the three dietary levels tested), followed
by muscle (0.92, 8.2 and 18.0 ppm), kidney (0.15, 3.2 and 9.0), blood
(0.18, 0.92 and 3.4 ppm) and liver (0.04, 0.44 and <0.01). Excretion
of unchanged nitrofen occurs almost exclusively in the faeces (35.0
ppm at the highest level). (Ambrose et al. 1971) (Full experimental
data were not reported.)
In a comparative study of the fate of 14C-nitrofen, sets of two
female and two male Osborne-Mendel rats and two female Fischer-344
rats were fed diets containing technical nitrofen at levels of 60 or
1 300 ppm. After two weeks, the animals received a single oral pulse
dose of 3 and 65 mg/kg b.w. uniformly dichlorophenyl-rins-labelled
14C-nitrofen, respectively. The same treatment was repeated after
four weeks of dietary nitrofen administration. Urine and faeces were
collected daily after the first pulse dose until sacrifice. One animal
of each strain from each dose level was sacrificed at 24 and 96h after
the second 14C-nitrofen dose. Blood, liver, pancreas, fat and skin
samples were analysed for radioactivity and metabolites. By 92h after
the second pulse dose, the animals in all groups had eliminated
between 3 and 22 percent of the 14C in the urine and between 30 and
100 percent in the faeces. After the two-week exposure, the female
Osborne-Mendel rats excreted 14C in the urine more rapidly than the
males; both sexes of Osborne-Mendel rat excreted 14C more rapidly in
the faeces than did the female Fischer rats. There were no obvious
differences related to sex or strain in tissue concentrations, in
tissue binding or in the metabolite profiles in urine, faeces or
liver; some differences were noted in the pancreas. At 24h after
dosing, concentrations in the blood, fat, liver, pancreas and skin of
14C (calculated as nitrofen) were 0.15 to 0.25, 4.8 to 7.6, 0.5 to
0.8, 1.1 to 1.4 and 0.5 to 0.9 ppm, respectively, for the 3 mg/kg dose
groups and 2.7 to 4.1, 39 to 80, 6.2 to 14, 3.3 to 5.3 and 2.9 to 5.0
ppm, respectively, for the 65 mg/kg dose groups. By 92h after dosing,
the concentrations in the same tissues had declined to 0.06 to 0.09,
3.2 to 4.2, 0.21 to 0.22, 0.3 to 1.4 and 0.4 to 0.8 ppm, respectively,
for the 3 mg/kg groups and 1.6 to 2.2, 1.0 to 12.6, 1.3 to 2.5, 0.6 to
2.1 and 0.3 to 0.9 ppm, respectively, for the 64 mg/kg groups. About
14 to 29 percent and 22 to 42 percent of the 14C were bound in the
liver of low-dose and high-dose animals, respectively, while 1 to 6
percent and 16 to 36 percent were bound in the pancreas. The urinary
metabolites were generally very hydrophillic. Traces of
hydroxyaminonitrofen, acetamidonitrofen and aminonitrofen were
observed but urinary metabolites were not identified further. No
parent compound was detected; 33 percent of the total applied dose was
excreted in the faeces as the parent compound, 14.4 percent as a polar
unknown, 5 percent as acetamidonitrofen, 2.8 percent as 3'-hydroxy
acetamidonitrofen, 2.8 percent as 4-hydroxyaminonitrofen, 2.4 percent
as aminonitrofen and 0.2 percent as 5-hydroxynitrofen. With time, the
concentrations of nitrofen, aminonitrofen and hydroxyaminonitrofen
decreased, and the concentrations of the acetylated compounds
increased. While the metabolic profile in the liver paralleled that in
the urine, the pancreatic metabolites were more lipophilic and
consisted exclusively of the parent compound in the 3 mg/kg groups,
while the 65 ms/ks groups exhibited a variety of metabolites,
including aminonitrofen, hydroxyaminonitrofen, hydroxynitrofen and
2,4-dichloropheno) in addition to the 20 to 74 percent parent
compound. Nitrofen appeared to accumulate to a greater extent in the
pancreas of high-dose female Osborne-Mendel rats than in the pancreas
of the males of this strain or the Osborne-Mendel female pancreas
contained 2.23 ppm nitrofen while the pancreas of each of the other
two groups averaged 0.83 and 1.08 ppm. This strain and sex difference
was not seen in the 3 mg/kg animals which were also killed at 24h
(1.07, 1.19 and 1.36 ppm nitrofen) (Steiserwalt et al. 1980a).
 Groups of three female Sprague-Dawley rats received a single dermal
application of 14C-nitrofen (uniformly labelled in the dichlorophenyl
ring) as an undiluted emulsifiable concentrate at a dose level of 125
or 375 mg/kg or as an aqueous dilution (ca. 1.8 percent a.i.) at
levels of 9 or 27 mg/kg a.i.
Urine and faeces were collected twice daily. One rat per group was
sacrificed at 24, 48 and 96 h after dosing. Blood, liver and kidney
were assayed for radioactivity.
Less than 2 percent of the applied doses remained at the application
sites after 96 h while only 30-40 percent of the dose was excreted in
the urine (5-9 percent) and faeces (26-33 percent) during that time.
Blood, liver and kidneys also contained radioactivity at all sacrifice
times (Deckert & Steiserwalt 1979a).
14C-nitrofen (uniformly labelled in the dichlorophenyl ring) was
dermally applied as undiluted emulsifiable concentrate at a level of
120 mg/kg to a female Sprague-Dawley rat. Urine, faeces and volatile
materials were collected daily at 24 h intervals. Total 14C recovery
in this study was 87 percent. At 96 h post-dosing, the rat was
sacrificed and blood, organs and tissue specimens were removed for
14C analysis. After 96 h 8.7 percent of the dose was excreted in
urine and 36 percent in faeces. Only 0.02 percent of the dose was
found as 14CO2, while other volatile materials accounted for 0.001
percent. 14C was found in every tissue analysed. The highest
concentrations were found in skin (17.6 percent of the dose), fat
(484.5 ppm = 2.1 percent of the dose) and muscle at the application
site (158.3 ppm = 0.9 percent of the dose), whereas only 0.4 percent
of the dose remained unabsorbed. A total of 38 percent of the applied
dose was found in tissues. This figure, when compared with the lower
values found in oral studies, suggests that nitrofen may be better
absorbed dermally than by the oral route (Deckert & Steiserwalt
1979b).
Groups of 12 pregnant Sprague-Dawley rats were treated dermally with
14C nitrofen (uniformly labelled in the dichlorophenyl-ring) as
diluted emulsifiable formulation at levels of 0.3 or 27 mg/kg b.w.
a.i. for 6 h on day 11 of gestation. Another group received a single
dose intravenously at a level of 0.3 mg/kg b.w.
At the end of the six-hour application period, only 16 percent of the
dermally applied dose could be removed by washing. Low-dose dermal
application produced blood levels similar to those produced by i.v.
administration, except at the one hour sampling. Urinary 14C
excretion patterns were similar for both dermal application (low and
high dose) and i.v. administration. However, 11 days after dosing, 15
and 20 percent of the dermal (both levels) and i.v. doses,
respectively, were excreted in urine. Foetal 14C excretion patterns
were also similar for both routes of administration, with 45-48
percent and 75 percent of the dose being excreted by 11 days after
dermal and i.v. admin-istration, respectively. After 11 days, 61-65
percent and 97 percent of the dermal and i.v. doses, respectively, had
been excreted. For the higher dermal dose, most maternal tissue 14C
concentrations peaked one to two days after dosing (day 12-13 of
gestation) with the highest concentrations occurring in the fat and
gonads. At 0.3 mg/kg (both routes) heart, thryroid, adrenals,
Harderian glands and pancreas approached maximum levels after six
hours.
Peak 14C levels of most maternal tissues increased with increasing
dose increment and reached a higher level following i.v.
administration than after a dermal application of 0.3 mg/kg.
Placental and foetal 14-C concentrations also peaked within one to
two days after dosing. At 0.3 mg/kg, mean placental 14-C levels were
similar by either route, while the foetal 14-C level two days after
dermal application was five-fold higher than those after i.v.
administration, ten-fold higher than that found in maternal whole
blood at the same time, but only three-fold lower than after the high
dermal dose. At the low dermal dose, the peak or plateau placental
and foetal 14C levels were comparable with those in maternal tissues
(other than the fat and gonads). After i.v. administration and the
high dermal dose, tissue levels in the mother were much higher than in
the placenta or foetus.
In the amniotic fluid, both 0.3 mg/kg dose groups showed the highest
14C levels at 11 days after dosing (day 22 of gestation); the
high-dose dermal group had a steady high 14C level throughout this
period.
Eleven days after dermal application (27 mg/kg), 14C concentrations
in maternal organs were generally higher than in foetal organs, except
for the heart, where levels were nearly the same. Radioactivity was
detected in foetal organs in descending order from kidney to heart,
lung, liver and brain. Metabolic profiles in the placenta and the
embryo/foetus showed the presence of nitrofen, aminonitrofen and other
unidentified metabolites with peak or plateau concentrations occurring
within one to two days. Concentrations of nitrofen and minor
metabolites decreased with time, while aminonitrofen appeared to reach
a plateau and then decrease at a slower rate. More than 90 percent of
14 in the urine and faeces was readily extractable (Steiserwalt
et al. 1982).
Dog
Groups of six-month-old beagles (two males and two females/group) were
fed diets containing technical grade 95 percent pure) nitrofen at
levels of 0, 20, 200 or 2 000 ppm for two years. Tissues (fat, muscle,
bone, liver, kidney, spleen and blood) pooled at week 104 and excreta
(urine and faeces) at week 103 were analysed for unchanged nitrofen by
sas-chromatography. The nitrofen concentration was highest in body fat
(38, 175, 515 ppm, respectively, at the three dietary levels tested),
with lower levels found in bone (3.9, 19.2, 21.8 ppm), muscle (1.26,
4.76, 20.0 ppm), kidney (1.4, 4.6, 13.6 ppm), liver (1.0, 2.0, 3.95
ppm) and blood (0.1, 0.2, 0.4 ppm). Unchanged nitrofen was excreted
almost exclusively in the faeces (39.0 ppm at the highest level)
(Ambrose et al. 1971). (Full experimental data were not provided.)
In vitro dermal absorption
Skin sections from an adult female Sprague-Dawley rat and an adult
woman (postmortem) were mounted in Franz Diffusion Cells and 5 µl of
diluted 14C-nitrofen emulsifiable formulation (ca. 1.8 percent a.i.)
was applied to each. After six hours of exposure, the rat skin
absorbed from 24.7 to 61.7 percent of the 14C while the human skin
absorbed from 13.8 to 25.3 percent of the label. The cell-by-cell
average rat: human absorption ratio of 1.99+/-0.34 indicated that
nitrofen was absorbed by human skin at approximately half the rate
that it passed into rat skin (Steiserwalt et al. 1980b).
TOXICOLOGICAL STUDIES
Special Studies on Reproduction
Rat
In a three-generation, two-litter per generation, reproduction study
groups of 28-day-old Wistar rats (25 males and 25 females/group) were
fed diets containing technical grade (95 percent pure) nitrofen at
levels of 0, 10, 100 or 1 000 ppm for 11 weeks. Body weights for
parental rats before mating and at weaning of respective litters were
not adversely affected for Fo and F1b generations. Body weight of
F2b parent generation rats was slightly decreased for rats at 100
ppm, in breeding the F3a litter and in breeding the F3b litter fed 10
ppm. This decrease was due to lower initial body weights of rats used
for succeeding generations. Although there was considerable
inconsistency within and between matings, average data reported did
not support a dose-related effect on fertility and gestation indices.
Viability and lactation indices were not adversely affected at 100
ppm. However, the viability index for rats at 1 000 ppm was seriously
affected, as stillborn pups outnumbered live pups; indeed no pups were
available for continuance beyond the Fo generation. In all
generations, the number of stillborn pups from the 100 ppm group
numbered 64 compared with 30 in the control.
No structural or microscopic cellular abnormalities were detected in
any stillborn pups in F1b litters. Weaning weights indicated no
diet-related trends. Overall, only the 10 ppm dietary level of
nitrofen exhibited no adverse effects on reproduction. Examination of
a variety of tissues from 10 F3b generation rats of each sex from the
0, 10 and 100 ppm dietary levels showed no histopathological lesions
(Ambrose et al. 1971). (Full experimental data were not provided.)
Groups of Sherman rats (10 males and 20 females/group) were fed
dietary levels of 0, 20, 100 and 500 ppm technical nitrofen (89
percent) pure). Breeding and F1a and F1b litters commenced at 68 and
200 days, respectively. Offspring were observed through weaning. At
the time of weaning of the F1b litters, 20 female and ten male
weanlings were selected from the 0, 20 and 100 ppm groups, continued
on the parental diet and pair-mated when 112 days old to produce the
F2a generation. Neither the food consumption nor the body weight gain
were greatly affected in any of the nitrofen-treated groups. At the
500 ppm dietary level no offspring of the F1a and F1b generation
survived the neonatal period. The number of pups born alive and the
survivors-to-weaning were reduced at the 100 ppm dietary level but not
at 20 ppm (equal to 1.1 and 1.8 mg/kg b.w. in F1b and F1a,
respectively) (Kimbrough et al. 1974). (Full experimental data were
not provided.)
Groups of 25 male Sprague-Dawley rats were fed diets containing 0,
100, 500 or 2 500 ppm of nitrofen technical (95.7 percent pure) for 95
days. At that time, male and untreated female rats were cohabited
(1:1) for a maximum of 10 days. During co-habitation, males were fed
an untreated diet and daily doses of 0, 6, 30 or 155 mg/kg of nitrofen
were administered by gavage. The nitrofen dosage was based on the
dietary intake of the compound (mg/kg/day) during weeks 10-13 of the
study.
No significant effects were detected on the reproductive performance
of male as indicated by the average number of days for mating to
occur, percentage of females impregnated or number of mated females
that delivered. Furthermore, no differences were noted in the length
of gestation, litter size, percentage of live or dead births or sex
ratio of the offspring. No compound-related signs of toxicity were
evident in any of the progeny from birth to day 35. Necropsies of
offspring that died and of female parents which were killed after
lactation revealed no compound-related gross abnormalities. Neither
viability nor mean weights of offspring were affected at any dose
level. Male reproductive performance was not affected at dietary
concentrations up to and including 2 500 ppm, equal to 186 mg/kg/day
(O'Hara et al. 1983).
Special Studies on Teratogenicity and Postnatal Effects
Mouse
Two studies on postnatal dose response and standard teratology are
summarized together.
Purified nitrofen (99.6 percent pure) in maize oil was administered to
CD-1 mice by gavage. In the postnatal study, 24 animals were dosed
(50, 100, 150 and 200 mg/kg/day) on days 7-17 of gestation and allowed
to give birth. In the teratology study animals were dosed 50, 100 and
200 mg/kg/day) on days 7-17 and sacrificed on day 18. Foetuses were
removed and analysed for visceral and skeletal malformations.
Concomitant control groups were utilized in all the studies.
In the postnatal study there was a significant dose-related delay in
time of birth. Significant dose-related reductions in growth and
viability were noted at all dose levels. There was considerable litter
mortality in the 100 mg/kg/day groups between days 3 and 17
postpartum. Only 23 percent of the pups alive on day 3 were still
alive by day 17. Mortality in the 50 mg/kg/day dose group was not
different from control values during this period. Microphthalmia or
anophthalmia was seen in a high percentage of the animals in the 100
mg/kg/day dose group. A dose-related growth retardation was noted in
pups until 60 days of age at 50 mg/kg and above. There were delays in
the percent of female offspring showing vaginal opening at 30 days and
in the percentage of animals breeding by 60 days at 50 mg/kg and
above. Litter size was unaffected by treatment.
In the teratology study dose-related increases in maternal weight gain
and liver/ body weight ratios were recorded. Nitrofen significantly
increased the supraoccipital scores, indicating retarded skeletal
development of the skull. It was teratogenic at 100 and 200 mg/kg/day,
the most common defects noted being cleft palate and undescended
testes (Chernoff et al. 1980). (Full experimental data were not
available.)
After being dosed at levels of 100 mg/kg b.w. nitrofen on days 7 to 17
of gestation, pregnant CD-1 mice showed reduced mean litter size.
Surviving pups had delayed eye opening and absence of the Harderian
gland (Gray et al. 1982a).
Groups of pregnant CD-1 mice were dosed by gavage with nitrofen (99.6
percent pure) in maize oil at levels of 0, 6.25, 12.5, 25 and 50
mg/kg/day. There were 23, 19, 27, 21 and 17 litters, respectively, on
day 1. The animals were observed until necropsy at 110-130 days of
age.
The administration of nitrofen did not cause any adverse effects in
the dams, as measured by maternal viability or weight gain from days 7
to 19 of gestation. By day 3 the number of pups was reduced
significantly at 50 mg/kg (20 percent). Body weights were initially
unaffected after birth at 50 mg/kg but subsequent body weight was
reduced in the pups from the 12.5 mg/kg treatment group. There was a
dose-related increase in the frequency of lethal defects in the pups,
i.e. cleft palate, diaphragmatic hernia and extreme abdominal
distention. Lung weight was significantly reduced by nitrofen
treatment at all doses, including the lowest dose of 6.25 mg/kg.
Seminal vesicle weights were significantly reduced at 6.25, 12.5 and
50 mg/kg. Obvious intraorbital defects were present in some offspring
in the 25 mg/kg group and above. Prenatal nitrofen treatment
significantly reduced the weight of the Harderian glands at all doses,
including the lowest dose of 6.25 mg/kg. Prenatal nitrofen treatment
of 50 mg/kg significantly delayed puberty in the female mice, as
indicated by the percentage of females with patent vasina on day 30.
In addition, the 50 mg/kg females paired with treated males produced
significantly fewer pups in the second litter.
A cross-fostering experiment with 100 mg/kg demonstrated that the
defects noted in the present study were produced solely by prenatal
exposure; pups exposed to nitrofen in the milk alone as a consequence
of any accumulation of nitrofen in the dam during gestation were
unaffected (Gray et al. 1983).
Rat
Groups of 26 pregnant FDRL rats were administered nitrofen by gavage
on days 6-16 of gestation. The control animals received maize oil (1
ml/kg b.w.) whereas the test animals received 100 and 200 ppm of
nitrofen in the same volume of vehicle. Thus the dosage corresponds to
5 and 10 mg/kg b.w./day, respectively. On day 22, the animals were
sacrificed and Caesarean section performed. There were no differences
between control and treated groups with respect to mean dam weight,
implantation sites, foetal viability or mean live foetal weight.
Resorptions were increased in the high-dose group. No malformed
foetuses were seen at the time of Caesarean sections, nor was there
evidence of soft tissue anomalies. Numbers of incomplete cranial bone
ossification and scoliosis were slightly increased in both treated
groups. These variations were considered "consistent with changes
previously observed in the colony of rats" and, thus, not
treatment-related (Vosin 1971).
Groups of seven pregnant Wistar rats were administered by gavage daily
doses of nitrofen technical (96.2 percent pure) at levels of 68 mg/kg
b.w. on days 6 through 15 of gestation. Five pregnant control rats
received only peanut oil. Parameters evaluated were neonatal mortality
and histomorphology of the lungs of dead and moribund newborns in the
nitrofen-treated group and of four-day-old survivors from the control
and treated groups. All the offspring from the control group were
alive at birth. Most (85 percent) of the offspring from the treated
group were born dead or died within the first few hours after delivery
and exhibited generalized cyanosis. Histological examinations of the
lungs of moribund offspring from the treated group revealed a large
number of alveoli bordered by a cubical epithelium (Siou 1979b).
Groups of 9-12 pregnant Sherman rats were dosed by oral intubation on
days 7 through 15 of gestation. Dosage levels were 0 (peanut oil), 10,
20 or 50 mg/kg/day for technical nitrofen (89 percent pure); 0, 20, or
50 mg/kg/day for purified nitrofen (99 percent pure); and 0 or 0.04
mg/kg/day for 2,7-dichlorodibenzo-p-dioxins (DCDD) (this dose is
equivalent to a contamination level of 2 000 ppm (0.2 percent) in the
technical nitrofen for a 20 mg/kg dose). The pups were observed to
weaning. At the dosage levels of 20 and 50 mg/kg/day a dose-related
decrease of live-born rats was observed for both the technical and
purified nitrofen groups. None of the pups survived at the 50 mg/kg
dose level and survival-to-weaning was severely reduced (50 percent)
in both the technical and purified nitrofen groups. No effect of
technical nitrofen on the number of live pups born and their
survival-to-weaning was observed at 10 mg/kg dose level. Extensive
inflammation, fibrosis and epithelial proliferation of the lung were
seen in sucklings that died at 11 to 15 days. No effect on
survival-to-weaning was observed in DCDD-treated rats.
Additional groups of ten female and ten male rats were fed technical
nitrofen at dietary levels of 0 and 500 ppm. The rats were started on
the diet at weaning and pair-mated when they were 90 to 100 days old.
On day 20 of pregnancy, foetuses were removed by Caesarean section and
examined for malformations. The live foetuses were kept in an
incubator for six hours and closely checked for viability. Most of the
foetuses of the controls were pink and survived until they were killed
after six hours. The pups from nitrofen-treated dams were cyanotic and
died one to three hours following the Caesarean section.
Two more pups of pregnant rats were given by oral intubation technical
nitrofen at levels of 0 or 50 mg/kg/day on days 7 to 18 of gestation.
The foetuses from these rats were removed by Caesarean section on day
21 of pregnancy. Lungs from 18 exposed and 16 control foetuses from
four litters in each group were removed one to three hours following
the Caesarean section and studied with light- and electron-microscopy.
Pups from treated dams showed cyanosis at birth. The cyanosis
increased during the next 45 to 60 minutes and most of the pups from
treated dams died, while the control rats developed pink colour soon
after birth and survived. Lungs of treated foetuses were poorly
expanded and alveolar epithelium was cuboidal and resembled cells of
earlier gestational age (Kimbrough et al. 1974).
When purified nitrofen (containing only trace amounts (ppm) of
4-aminonitrofen) was administered to pregnant Long-Evans rats at
different days of gestation, gestation day 11 was demonstrated as
critical: a single dose of 150 mg/kg on day 11 killed 56 percent of
the newborn pups withing 48 h.
Groups of 4-7 Long-Evans dams were given a single dose of purified
nitrofen at levels of 0, 75, 115, 150, 200 or 250 mg/kg on day 11 of
gestation. Survival of neonates to five days was monitored. A
dose-related increase in neonatal mortality was observed. The LD50
for the neonates was estimated at 116 mg/kg of maternal body weight.
Initial experiments demonstrated that animals surviving the first five
days usually lived to maturity. Thus, the mortality is most accurately
described by the fraction dying within the first five postnatal days.
Surviving offspring were autopsied at 35 days of age and examined for
gross lesions. The predominant lesion observed in all treated groups
was hydronephrosis. The incidence of hydronephrosis in survivors
paralleled the neonatal mortality and the lesion was most frequent
among animals exposed on day 11. The incidence of hydronephrosis was
also dose-related among animals exposed only on day 11.
Nitrofen-induced hydronephrosis was not considered incompatible with
life.
In a further teratology study purified nitrofen was administered to
pregnant Long-Evans rats at dose levels of 0, 70, 115, 150, 250, 265
and 400 mg/kg b.w. on day 11 of gestation and foetuses collected on
day 22. Foetuses exposed to nitrofen and examined at term (day 22),
showed delayed skeletal ossification. There was a dose-related
depression in foetal body weight. Nitrofen exposure on day 11 induced
hydronephrosis, which was dose-related in incidence and severity.
Diaphragmatic hernias were also observed in the treated groups. The
overall frequency of soft tissue anomalies was also dose-dependent.
Mean litter frequencies of these anomalies ranged from 45 percent at
LD15 (70 mg/kg) to 91 percent at LD99+ (400 mg/kg). Nitrofen exposure
produced a dose-related increase in the frequency of cardiac
malformations, which included in order of decreasing frequency:
ventricular septal defects (VSD), double outlet right ventricules
(DORV), and transposition of great vessels. Incidence of lethal
cardiac malformations was 38 percent and 53 percent at 150 mg/kg and
250 mg/kg, respectively. No anomalies were seen in the control
litters. None of the cardiac or diaphragmatic defects in the term
foetuses were present in survivors, suggesting that the heart and
diaphragm are the target organs in nitrofen-induced neonatal deaths
(Costlow & Manson 1981).
Groups of five pregnant Sprague-Dawley rats were treated with nitrofen
(98 percent pure) at 0, 20, 31.2 or 50 mg/kg/day by gavage on days
8-18 of gestation. Significant differences in birth weights were
apparent in pups from the 31.2 and 50 mg/kg/day groups. A decrease in
the lung to body weight ratio of foetuses exposed to nitrofen was also
observed. Most, if not all, pups were born alive and lacked
externally-observable malformations. Within minutes after birth,
treated pups exhibited laboured breathing and became cyanotic. Death
usually ensued 30 min. to 4 h postpartum. Survival was significantly
depressed in all treatment groups and all time intervals (1 h, 24 h,
25 days) measured after birth. On examination, symptomatic
nitrofen-exposed pups suffered massive atelectasis at autopsy, with
few expanded air sacs in the 31.2 and 50 mg/kg groups. In contrast,
lungs from newborn controls exhibited normal alveolar expansion. The
level of glucocorticoids and capacity of the foetal lung to respond to
glucocorticoids by synthesizing and releasing surfactant were not
affected by prenatal exposure to nitrofen (Stone & Manson 1981).
Missing and/or malfunctioning Harderian glands were observed when
pregnant Sprague-Dawley CD rats were given daily doses of nitrofen at
a level of 12.5 mg/kg by gavage on days 8 to 16 of gestation (Gray
et al. 1982a).
A postnatal study, using rats (strain unknown) dosed on days 7-16 at
five doses from 0 to 25 mg/kg, demonstrated that nitrofen caused
diaphragmatic hernias at 1.39 mg/kg and above and affected the
haemoglobin level at 12.5 mg/kg (Gray et al. 1982b). (This
information was provided in abstract form and cannot be further
interpreted.)
Groups of five pregnant CD rats were given by gavage daily doses of
nitrofen at levels of 0, 12.5 or 25 mg/kg b.w. on days 8 to 16 of
gestation. Dams were sacrificed on day 21 of gestation and foetuses
removed for examinations. Dose-related increase in foetal mortality
and dose-related decrease of mean foetal weight were determined.
Diaphragmatic hernias occurred in both dose groups in a dose-related
fashion. Brain weight and brain DNA were statistically significantly
reduced at 25 mg/kg; lung weight and lung surfactants were reduced at
12.5 and 25.0 mg/kg in a dose-related fashion as were liver weight,
liver glycogen content, kidney weight and kidney alkaline phosphatase
levels. However, statistical analysis demonstrated that only effects
on lung and liver represented specific organ toxicity, while brain and
kidney effects were correlated to the foetal growth retardation
(Kavlock et al. 1982).
When pregnant Sprague-Dawley rats (35/group) were exposed dermally to
nitrofen emulsion at 0, 0.3, 3.0 or 30.0 mg/kg/day on days 6 to 15 of
gestation, foetal weight and foetal length were significantly lower in
the 30.0 mg/kg group compared to the control group. Soft tissue
anomalies (hydronephrosis, ectopic testes, liver and/or stomach and/or
intestine protruding into thorax, diaphragmatic hernia, right-sided
aorta) were observed almost exclusively in the foetuses of the 30.0
mg/kg group. The foetal viability (live at birth/implantations) in all
the treated groups was statistically significantly lower than the
control litters. Neonatal survival index (live at day 4/live at birth)
was significantly lower in the 30.0 mg/kg group than in the control
group (Weatherholtz 1979).
Groups of five to seven pregnant Charles River rats were treated
dermally with nitrofen (as a water-diluted emulsifiable formulation)
at levels of 0, 0.6, 4.8 or 19.2 mg/kg/day on days 6 to 15 of
gestation. A further group received dermally 4.8 mg/kg on day 11 only.
Dams were sacrificed on day 21 postpartum. Surviving offspring were
sacrificed on day 27. All maternal rats survived to sacrifice.
Treatment of pregnant rats with 19.2 mg/kg/day of nitrofen on days 6
to 15 of gestation resulted in slightly decreased weight gain during
the treatment interval. The no-observable effect level (NOEL) was 4.8
mg/kg/day. The treatment caused decreased viability of offspring,
decreased neonatal survival to day 4 and decreased live offspring of
pregnant rats treated dermally with 19.2 mg/kg/day of nitrofen on days
6 to 15 of gestation. Nitrofen did not affect neonatal viability,
neonatal survival, survival to weaning, growth of offspring or eye
opening in offspring of dams treated with 0.6 or 4.8 mg/kg/day on days
6 to 15 of gestation or 4.8 mg/kg/day on day 11 of gestation
(Hirsekorn & Kane 1981).
Groups of 25 pregnant Sprague-Dawley rats were treated dermally with
nitrofen in aqueous emulsion (0, 0.3, 0.6, 1.2 or 12.0 mg/kg b.w./day)
on days 6 to 15 of gestation. No maternal toxicity occurred. At 12
mg/kg neonatal survival was reduced. Necropsies of the dead neonates
showed a high incidence of diaphragmatic hernias. The number of pups
per litter and viability over days 4-41 of age were unaffected by
treatment. About half (randomly selected) of the pups from each litter
and group were necropsied on day 42. Chromo-dacryorrhea, reduced or
absent Harderian glands and diaphragmatic hernias occurred at 12.0
mg/kg. The frequency and the severity of dilation of the renal
pelvices increased with the dose; slight dilation was significantly
elevated at 0.3 mg/kg and moderate and severe dilation became
prominent at 0.6 and 12.0 mg/kg, respectively. In the 12.0 mg/kg group
nitrofen reduced the body weight of the survivors and also the
relative weights of the Harderian gland, the thyroid gland of both
sexes; lungs of females were significantly depressed with respect to
solvent and water control groups. The findings from necropsies
performed on the remaining offspring on days 146 to 149 postpartum
were similar to those at day 42. Body weight was unaffected by
nitrofen exposure but dilated kidneys and diaphragmatic hernias were
observed. Relative thyroid weight was significantly depressed only in
the 12 mg/kg dose group. A NOEL was not demonstrated (Costlow et al.
1983).
Rabbit
Groups of 15 pregnant New Zealand rabbits were given encapsulated
technical nitrofen (96.2 percent pure) at levels of 0, 7, 27 or 108
mg/kg on days 6 through 18 of gestation. Ten rabbits in each group
were sacrificed on day 28 of gestation to assess embryotoxic and
teratogenic effects. The average number of live foetuses and
implantation sites per pregnant female was slightly decreased in the
108 mg/kg group sacrificed on day 28 of gestation. Percentage of dead
foetuses with respect to implantation sites was increased in all
treated groups compared with the control group, but not in a
dose-related manner. The increased incidence of foetuses, with 13
pairs of ribs was dose-related. No other anomalies were observed. The
remaining five rabbits in each group were allowed to deliver, and they
and the newborns were sacrificed 48 h after delivery. The average
number of live offspring was slightly lower in the 108 mg/kg group.
Average weights of live foetuses after 48 h were slightly lower in the
treated groups, but not in a dose-related manner. No significant
differences were observed in survival rate to 48 h or in resorption
rates. An increased incidence of 13 ribs was observed in all treated
groups. Histological examinations of the lungs removed from between 5
and 17 of the 28-day foetuses and the 48-hour newborns in each group
did not indicate retardation of lung maturation (Siou 1979a).
Special Studies on Mutagenicity
Microbial systems
Nitrofen, when tested with Salmonella typhimurium strains TA1535,
TA1538, TA100 and TA98, both with and without S-9 activation, was
found only moderately mutagenic in TA100 with microsomal enzyme
activation (Byeon et al. 1976).
Purified nitrofen (99+ percent) and several samples of nitrofen
technical (90-98 percent pure) were tested at concentrations of
0.001-10.0 µg/plate with S. typhimurium strains TA1535, TA1537 TA98
and TA100. Tests were carried out both with and without microsomal
activation. Positive controls were used: 2-aminofluorene and
2-acetamidofluorene for TA98 and 2-anthramine for all the strains
tested. Only one sample of nitrofen technical (90-92 percent pure) was
completely negative in the test. The other samples of technical
nitrofen gave statistically significant increases of mutants/plate
compared with negative control in TA100 strain, both with and without
activation, but only one was positive in TA98 with activation.
However, a dose-response relationship was observed only with three
samples (97, 97 and 98 percent pure), but not with another three (93,
92.8 and 97.7 percent pure), generally at concentrations of 10-10 000
µg/plate. Purified nitrofen (99+ percent pure) gave a statistically
significant increase of revertants/plate in TA100, both with and
without activation; no dose-response relationship was observed.
Thus purified nitrofen and three technical samples gave an indication
of mutagenicity, but the other three technical samples were positive
(O'Neill & Scribner 1979).
Nitrofen was found mutagenic in a reversion test using
Saccharomyces cereviseae strain 632/4 (auxotrophic for methionine)
(Guerzoni et al. 1976).
Mammalian systems
The mutagenicity of nitrofen was assayed in the murine lymphoma cell
line L5178Y at concentrations of 0.1 x 10 (E-4), or 2 x 10 (E-4) M.
Doses were selected as those killing approximately 40 and 80 percent
of cells. Ethylmethanesulphonate was used as a positive control.
Induction of methotrexate-resistant mutants of L5178Y cells treated
in vitro with nitrofen was not significantly different from the
negative control (Paik & Lee 1977b).
Nitrofen was tested in the unscheduled DNA repair synthesis assay with
human lymphocytes (concentrations not clear). Nitromin was used as a
positive control. 3H-thymidine incorporation in the nitrofen-treated
cells was not significantly different from the negative control (Paik
& Lee 1977b).
Nitrofen was also tested in mouse cells in a BALB/3T3 in vitro
transformation assay. The compound did not induce a significant
increase in transformed foci over the applied concentration range of
0.1 to 60 micrograms/ml. This range corresponding to approximately 50
to 90 percent survival in the cytotoxicity tests. A negative control
consisting of assays performed on untreated cells was included. A
positive control with 3-methyl cholanthrene (MCA), used at level of 5
µg/ml for each assay, gave a marked increase of the average
number/flask of foci of transformed cells. Therefore, nitrofen was
considered to be inactive in the BALB/3T3 in vitro transformation
assay (Myhr & Brusick 1979).
Groups of three Swiss Webster albino mice were given intraperitoneally
two doses, separated by 24 h, of nitrofen (purity not specified) at
levels of 0, 500 or 1 000 mg/kg b.w. Six hours after the last dose,
three bone marrow smears from each mouse were prepared and about 2 000
polychromatic erythrocytes per mouse were analysed for the presence of
micronuclei. For both dose levels tested, the incidence of micronuclei
was not statistically significantly different from the control. A
positive control of cyclophosphamide gave the expected increase of the
incidence of micronuclei (Paik & Lee 1977a).
Groups of 5-10 male Swiss mice were given by gavage two doses of
nitrofen technical (96.2 percent pure) at levels of 0, 0.25, 1.00 and
1.25 ml/kg, spaced 24 h apart. The animals were sacrificed 6 h after
the second dose and bone marrow smears were prepared. The incidence of
polychromatic erythrocytes carrying "micronuclei" in the treated
groups was not significantly different from the control group (Siou
1978).
Groups of 24 male Charles-River CD-1 mice were given by gavage a
single oral dose of nitrofen technical (95.7 percent pure) at levels
of 0, 0.39, 0.79 and 1.58 g/kg, representing 1/8, 1/4 and 1/2 of the
acute oral LD50. Additional groups of 8 male mice were given once
daily five doses of nitrofen at the same levels. A positive control
group received triethylamine (TEM) 0.3 mg/kg i.p. Bone marrow slides
were prepared from 8 animals/group sacrificed at 6, 24 and 48 h after
the acute dose and 6 h after the last sub-acute dose and TEM dose. All
animals received colchicine at 2 mg/kg i.m. two hours prior to
sacrifice. Fifty metaphase cells per animal were scored when possible.
Owing to poor survival, the high dose subacute group was sacrificed on
day 4. No statistically significant increase in chromosomal
aberrations was observed in treated animals compared with controls,
both in the acute and subacute regimen (Reustle & Scribner 1980).
Nitrofen technical (95.7 percent pure) was administered in m-xylene
(99 percent) by dermal application to groups of eight male Charles
River CD rats at doses of 0, 0.05, 0.125 and 0.50 g/kg, to assess the
potential of nitrofen to induce chromosomal aberrations in mammalian
bone marrow cells. A positive control group received a single 0.3
mg/kg i.p. dose of triethylenemelamine. The doses were administered
according to both a single and repeated (daily × five days) regimen.
Animals were killed and bone marrow slides prepared at approximately
6, 24 and 48 h after the single dose, 24 h after the positive control
dose and 6 h after the last multiple dose. All animals received
colchicine (1 mg/kg, i.p.) three hours prior to being killed.
Metaphase cells (50 per animal) were examined for chromosomal
aberrations. Nitrofen at 0.5 g/kg and below did not induce a
significant increase in chromosomal aberrations in bone marrow cells
at either 6, 24 or 48 h after single or repeated exposure. Chromosomal
aberrations were consistently elevated at 24 h after treatment with
triethylmelamine (McLeod & McCarthy 1982).
Special Studies on Mutagenicity of Aminonitrofen
Aminonitrofen (99+ percent pure), a metabolite of nitrofen, was tested
in concentrations of 0.1-1 000 µg/plate with S. typhimurium strains
TA1535, TA1537, TA98 and TA100 with and without a microsomal enzyme
preparation. Statistically significant increases of revertants/plate
were observed with strains TA1535, TA98 and TA100 using a microsomal
enzyme preparation and a dose-response relationship was exhibited.
Positive controls were 2-anthramine, 2-aminofluorene and
2-acetamidofluorene (O'Neill & Scribner 1979).
Special Studies on Mutagenicity of 4,4'-Dichloroazobenzene
4,4'-Dichloroazobenzene (DCAB) (99+ percent technical), a low level
impurity in technical nitrofen, was tested with the S. typhimurium
strains TA1535, TA1537, TA98 and TA100 at concentrations of 1.0-1 000
µg/plate, with and without microsomal activation. Saline buffer
controls were used with each strain. Statistically significant
increases of revertants/plate were observed with strain TA100 at
10-1 000 µg/plate with metabolic activation. DCAB gave a greater
response with strain TA98 than with TA100. Positive metabolic
activation; and 2-anthramine (10 µg/plate), positive with TA1535,
TA1537 and TA100 with metabolic activation; and 2-acetamidofluorene
(50 µg/plate), positive with TA98 with metabolic activation (O'Neill &
Lohse 1980).
Special Studies on Carcinogenicity
Mouse
Groups of 50 male and 50 female young B6C3F1 mice (age not specified)
were fed diets containing technical nitrofen (stated to be >80
percent pure; impurities unspecified) dissolved in maize oil for 78
weeks at levels of 1 775-2 500 mg/kg (low-dose) or 3 550-5 000 mg/kg
(high-dose), to give time-weighted average concentrations of 2 348 and
4 696 mg/kg in the diet. A group of 20 male and 20 female controls
received the basal diet containing 2 percent maize oil. All mice were
observed for a further 12 weeks after treatment. Weight gain was
depressed in both groups of treated females and in the males exposed
to the high dose. Beginning at week 54, pronounced abdominal
distension was displayed by an increasing number of treated mice. By
the end of the study, only 10 percent of the control male mice, 34
percent of the high-dose group and 54 percent of the low-dose group
were still alive; and, of the female mice, 62 percent were still alive
in the high-dose group, 54 percent in the low-dose group and 85
percent of the controls. For neither sex could a positive association
be established between dosage of nitrofen and mortality. A high
incidence of hepatocellular carcinomas was found in exposed male and
female mice: in 4/20 control males, 36/49 low-dose males and 46/48
high-dose males, in none of the control female mice (both matched and
pooled controls), in 36/41 low-dose females and in 43/44 high-dose
females. A few of these tumours metastasized to other sites. The
difference in incidence between control and exposed male and female
mice was statistically significant at both dose levels.
Haemangiosarcomas of the spleen were found in 2/47 of high-dose males
and haemangiosarcomas of the liver in 1/49 low-dose males and 2/48
high-dose males. In female mice, haemangiosarcomas were seen in the
spleen in 1/18 controls, in the liver in 4/44 high-dose animals and in
the abdominal cavity in 1/43 of the high-dose group.
The incidence of haemangiosarcomas in the high-dose male mice, but not
females or low-dose males, was statistically higher than controls
(National Cancer Institute 1978).
Groups of 50 male and 50 female six-week-old B6C3F1 mice were fed
diets containing 3 000 or 6 000 mg/kg technical-grade nitrofen (purity
not specified) for 78 weeks. The 20 male and 20 female controls
received the basal diet. All animals received acidified (pH 2.5) water
ad libitum. After exposure to nitrofen, animals were observed for a
further 13 weeks. Consistent dose-related depressions in mean body
weight were noted in male and female mice, reaching about 25 percent
in high-dose males by the end of the study. At that time, 40/50 of the
high-dose males, 48/50 of the low-dose males and 19/20 of the control
males were still alive; of the females, 48/50 of the high-dose and
43/50 of the low-dose group and 12/20 off the controls survived until
the end of the study. Although a variety of tumours were noted, only
the incidence of liver tumours seemed to be related to nitrofen
exposure. Hepatocellular adenomas were seen in 1/20 control males,
18/49 low-dose males and 20/48 high-dose males, and hepatocellular
carcinomas in 0/20 controls, 13/49 low-dose males and 20/48 of the
high-dose group. In addition, none of the controls, but 3/49 of the
low-dose group and 4/48 of the high-dose group had hepatoblastoma, a
very rare tumour in this strain of mice. Of the females, 0/18
controls, 9/48 low-dose and 17/5 high-dose mice had hepatocellular
adenomas; hepatocellular carcinomas were observed in 0/18 control,
5/48 low-dose and 13/50 high-dose female mice. In addition, a
hepatoblastoma was found in 1/48 females in the low-dose group.
For both the low- and high-dose groups there was a statistically
significant increase from the control group in the incidences of
hepatocellular adenomas and hepatocellular carcinomas (National Cancer
Institute 1979).
Rat
Groups of 50 male and 50 female Osborne-Mendel rats (age not
specified) were fed diets containing technical grade nitrofen (stated
to be >80 percent pure; impurities unspecified) in 2 percent maize
oil for 78 weeks. The dietary concentration for males was 2 300 mg/kg
(low-dose) or 2 300-4 600 (high-dose), to give a time-weighted average
of 3 627 mg/kg. The dietary concentrations for female rate were 1 300
mg/kg (low-dose) or 2 600 mg/kg (high-dose). The low-dose males, the
control animals and the high- and low-dose females were observed for
an additional 32 weeks, during which they were maintained on a basal
laboratory diet plus maize oil; the high-dose males were observed for
an additional five weeks after dosing was stopped. Groups of 20 male
and 20 female controls received the basal diet plus 2 percent maize
oil. A dose-related decrease in body weight was noted; by 75 weeks
after onset of dosing, the high-dose females weighed roughly one-third
less than the controls. Fifty percent of the high-dose males were dead
by week 45 and only 15 survived to week 83; 60 percent of the low-dose
and 45 percent of the control group survived until the end of the
study. Of the females, 58 percent at the high-dose, 74 percent at the
low-dose and 80 percent of the controls survivied until the end of the
study. Adenocarcinomas of the exocrine pancreas were seen in 2/50
females at the low dose and in 7/50 at the high dose. All tumours
showed local invasion and had metastasized to the lungs. No such
tumour was seen in the 20 matched controls or in the 110 pooled
controls. The difference was statistically significant for high-dose
animals. No other tumour showed a statistically significant difference
in incidence between control and exposed rats. Poor survival precluded
the evaluation of carcinogenicity in male rats (National Cancer
Institute 1978).
Groups of 50 male and 50 female six-week-old Fischer 344 rate were fed
diets containing 3 000 or 6 000 mg/kg technical-grade nitrofen (purity
not specified) for 78 weeks. A group of 20 male and 20 female rats
were given the control diet. All animals received acidified water (pH
2.5). The animals were followed for an additional 26 weeks, when all
surviving rats were killed. A dose-related depression in mean body
weight was noted in animals of both sexes, reaching 15 percent for
those given the high dose for 60 to 75 weeks. Of the male rats, 45/50
given the high dose, 42/50 given the low dose and 17/20 of the
controls survived until termination of the study; of the females,
38/50, 42/50 and 17/20, respectively, survived. No statistically
significant difference in the incidence of tumours was observed
between the exposed and control groups (National Cancer Institute
1979). However, subsequent detailed histological and morphological
studies distinguished the nitrofen-induced neoplasm from those
occurring naturally in the controls (Hoover et al. 1980; Stinson
et al. 1981).
Special Studies on Enzyme Induction
Groups of B6C3F1 mice (six animals per sex) were fed diets containing
nitrofen (technical, 92 percent pure) at levels of 0, 10, 100 or 1000
ppm or two weeks prior to determination of individual liver to body
weight (L:BW) rations and in vitro hepatic p-nitroanisole (pNA)
O-demethylase activity. The dose levels were chosen after acute
studies (three-day gavage dosing), using CD-1 mice, showed a
sex-dependent minimum effect level for hepatic pNA-O-demethylase
activity between 10 and 50 mg/kg/day. The 100 and 1000 ppm dietary
nitrofen levels resulted in elevated liver mixed function oxidase
(MFO) activity in both sexes, with females showing a greater response
(170 percent and 236 percent of control, respectively, for the two
diet levels). L:BW ratios were increased 20 percent and 30 percent in
males and females, respectively, but only at the 1000 ppm level. No
significant changes were observed in either sex fed the 10 ppm
nitrofen diet. The no-effect level found for MFO induction was much
lower than that for oncogenicity (2 348 ppm in the National Cancer
Institute 1978 oncogenicity study). (Deckert & Steigerwalt 1979c)
Special Studies on Dermal Irritation
No evidence of irritation developed when both technical grade (95
percent pure) and pure nitrofen were applied either as a 10 percent
solution in maize oil or as moistened powder to the skin of albino
rabbits (Ambrose et al. 1971).
Special Studies on Cutaneous Sensitization
There was no visible evidence of erythema, swelling, or discolouration
of the test sites after each sensitizing injection or after the
challenging dose of aqueous suspension of technical grade (95 percent)
nitrofen. Under the conditions of the test, these finding indicate
that nitrofen is not a sensitizing agent (Ambrose et al. 1971).
Special Studies on Cataractogenic Effect
Groups of 30 two-week-old Peking ducklings were fed diets containing
nitrofen at levels of 0, 0.05 and 0.1 percent for 13 weeks. Distinct
growth depression occurred at the 0.1 percent nitrofen dose level and
slight retardation at 0.05 percent. The mortality rate in the highest
dose group was distinctly increased. No clinical or histologic signs
of cataract were observed in any of the treated or control animals,
nor were other eye abnormalities found (Ensel & Seinen 1970).
Special Studies on Methaemoglobin Formation by Aminonitrofen
Methaemoglobin formation in vitro by nitroso and amino derivatives
of nitrofen and other chlorinated biphenyl ethers was investigated in
suspensions of human erythrocytes. Ability to induce methaemoglobin
formation among the nitroso and amino derivatives decreased with
increasing chlorine substitution of the biphenyl ether. However, amino
derivatives from no methaemoglobin in suspensions of erythrocytes
alone, but do so after activation by liver homogenate. Methaemoglobin
formation after intraperitoneal and oral administration to male Wistar
rats of chlorinated p-aminobiphenyl ether at levels of 0.77 mg/kg b.w.
gave almost the same result (ca. 40 percent methaemoglobin within
60-120 min.) after either route was used (Miyauchi et al. 1981).
Special Studies on the Stability of the Metabolite Azoxynitrofen
Azoxynitrofen (a possible vegetable metabolite of nitrofen) was added
to simulated gastric fluid and simulated intestinal fluid at level of
7 ppm. There is a slight degradation of azoxynitrofen in gastric fluid
during 24 h. The intestinal fluid completely decomposes azoxynitrofen
within 24 h. After 14 days, there is evidence of azoxynitrofen in
either digestive fluid, and it appears that aminonitrofen is a major
product of the decomposition (Warso 1972).
Acute Toxicity
The acute toxicity of nitrofen in animals is summarized in Table 1.
Most deaths occurred two to eight days after dosing and were preceded
by progressive depression. There was no evidence of diarrhoea.
Postmortem findings were negative in rats that died and in survivors
autopsied at 14 days (Ambrose et al. 1971).
Short-Term Studies
Rat
Groups of albino Wistar rats (10 males and 10 females per group) were
fed diets containing technical grade nitrofen (95 percent pure) at
levels of 0, 100, 500, 2 500, 12 500 and 50 000 ppm for 13 weeks.
Rats on the 50 000 ppm diet failed to survive beyond the first week
and survival of rats on the 12 500 ppm diet was adversely affected.
Growth was depressed in both sexes at 12 500 ppm and in males at 2
500. In rats on the lower dietary concentrations of nitrofen, no
adverse effects were noted in growth, food consumption and mortality.
Haematologic values appeared to be within normal range and urinary
tests for reducing substances and protein showed no marked differences
from the controls. Significantly higher liver-to-body weight ratios
were found in rats at all dietary levels of nitrofen, except for male
rats at 100 ppm, and appeared to be dose-related. Kidney body weight
ratios were significantly elevated for females at 12 500 ppm and for
males at 2 500 and 12 500 ppm. Testes body weight ratios were
significantly elevated for rats at 2 500 and 12 500 ppm. Lesser
effects were noted for heart and spleen. Histopathologic findings
related to treatment appeared to be confined to rats receiving diets
containing 12 500 and 50 00 ppm nitrofen. The principal findings
consisted of a hepatitis characterized by oedema, peripheral
localization of glycogen granules, swelling of the cytoplasm and liver
nuclei with prominent nucleoli (Ambrose et al. 1971). (Full
experimental data were not available.)
Rabbit
Groups of five male and five female rabbits received dermal
applications of nitrofen (as a water-diluted emulsifiable formulation)
at levels of 0, 250 or 1 250 mg/kg b.w., 7 h/day, 5 days/week for
three weeks. Additional groups received applications on abraded skin.
There was no mortality and no evidence of any skin irritation. No
histological changes were noted in the skin of exposed animals. There
was no evidence of subacute systemic damage to liver, kidney, lung or
spleen on histological examination (Brown 1964).
Table 1 Acute Toxicity of Nitrofen in Animals
Animal Sex Route Vehicle LD50 a.i. Reference
g/kg b.w. purity
Rat M oral maize oil 2.63 95% Ambrose et al. 1971
M oral maize oil 3.58 100% Ambrose et al. 1971
M oral peanut oil 2.84 99% Kimbrough et al. 1974
F oral peanat oil 2.40 99% Kimbrough et al. 1974
oral undiluted 5.00 Swann 1973
formulation (as 25%
formulation)
M+F dermal xylene 5.00 99% Kimbrough et al. 1974
inhalation undiluted 205 mg/l Swann 1973
(1h exper.) formulation (as 25%
formulation)
M+F inhalation undiluted >271 mg/cu.m. Brown 1965
(1h exper.) formation (as a.i.)
Rabbit dermal undiluted >2.00 Swann 1973
(24 h) fomulation (as 25%
formulation)
Dog
Groups of six-month-old beagles (two males and two females/group) were
fed diets containing technical grade (95 percent pure) nitrofen at
levels of 0, 20, 200 and 2 000 ppm for two years. The dogs on the
various dietary levels of nitrofen survived the two-year feeding
period. No toxic signs attributable to nitrofen were seen at any time.
The general health, appearance, behaviourial pattern, body weight gain
and cumulative food consumption were not different than those of the
respective controls. Haematologic values and urinary tests for
reducing substances and protein showed no effect of treatment at any
of the test periods. BSP, SGOT, SAP and BUN tests made during the
month 24 showed no adverse trends. Significantly higher liver-to-body
weight ratios appeared only in dogs on 2 000 ppm nitrofen.
Histopathologic examination of major organs revealed no abnormalities
or lesions resulting from the ingestion of nitrofen for two years, in
kind or incidence, that were not found in control dogs. In general,
only the 20 and 200 ppm diet levels of nitrofen exhibited no adverse
effects (Ambrose et al. 1971).
Long-Term Studies
Rat
Groups of weanling Wistar rats (25 males and 25 females/group)
received diets containing technical grade (95 percents pure) nitrofen
at levels of 0, 1, 10, 100 and 1 000 ppm for 97 weeks. The study was
terminated at 97 weeks, owing to poor survival (50 percent) in all
groups, including the controls, beyond week 65. Statistically
significant decreases in mean body weight were observed in the males
at 100 and 1000 ppm after the week 52 and in the females at the same
dose levels until week 26.
Food consumption, haematological and urinalysis parameters were not
affected. Significantly higher kidney/body weight and liver/body
weight ratios in male rats on 1 000 ppm were notable and appeared to
be attributable to nitrofen. Histopathologic findings revealed no
lesions in kind or incidence that were not found in the respective
controls. Overall, the 10 ppm and below diet level of nitrofen was
reported to cause no adverse effects (Ambrose et al. 1971). (Lack of
individual animal data precludes further interpretation of this
study.)
COMMENTS
Nitrofen has low acute oral toxicity. Following oral administration,
nitrofen is partially absorbed and rapidly excreted. A small
proportion of the administered dose is distributed to all tissues and
organs. Nitrofen and its metabolites are found primarily in faeces
(accounting for about 75 percent of the administered dose), while
urine contains metabolites accounting for about 15 percent of the
dose. The metabolism of nitrofen involves reduction, hydroxylation and
conjugation.
Two reproduction studies were presented. Because of inadequacies in
the reports the studies were not considered.
Oral exposures to nitrofen during the period of organogenesis produced
neonatal mortality, decreases in the time of survival to weaning, and
a series of abnormalities in rodent offspring. In a mouse
teratology/post-natal study, the lowest dose tested (6.25 mg/kg b.w.)
produced increased incidence of lethal defects at birth and resulted
in decreased lung and seminal vesicle weights at maturity. In a rat
teratology study, diaphragmatic hernias were observed at and above
1.39 mg/kg b.w., but full details were not provided.
Nitrofen in some cases was found mutagenic in the
Salmonella/microsome assay but it did not display any mutagenic
potential in several mammalian systems either in vitro or in vivo.
Two carcinogenicity studies on B6C3F1 mice indicated that nitrofen is
a liver carcinogen, causing hepatocellular carcinomas and
hepatocellular adenomas in both sexes, and haemangiosarcomas in male
mice. In addition, the compound is carcinogenic to female
Osborne-Mendel rats, causing adenocarcinomas of the pancreas. Nitrofen
was not found to be carcinogenic in Fischer 344 rats. Detailed
histological examinations suggest that naturally occurring and
nitrofen-induced liver tumours in mice are morphologically different.
Both studies were of short duration. In the study on Osborne-Mendel
rats, poor survival precluded the evaluation of carcinogenicity in
males.
In a long-term rat-feeding study, a NOEL of 10 ppm was observed.
However, the duration of the study (97 weeks), the poor survival in
all groups (50 percent beyond week 65), and the fact that the study
was not available in extenso, precluded its full evaluation.
An acceptable daily intake could not be estimated, owing to the
evidence of carcinogenicity, the lack of a NOEL for the teratology and
post-natal effects and the inadequacies of several studies, including
reproduction and long-term studies.
REFERENCES - TOXICOLOGY
Adler, I.L. et al. A material balance study in rats using
1970a 14C- TOK labelled in the nitrophenyl
ring. Rohm and Haas Report No. 23-24.
(Unpublished)
Adler, I.L. et al. A material balance study in rats using
1970b 14C - TOK labelled in the dichlorophenyl
ring. Rohm and Haas Report No. 23-25.
(Unpublished)
Ambrose, A.M., Larson, Toxicologic studies on
P.S., Borzelleca, J.F., 2,4-dichlorophenyl-p-nitrophenyl
Blackwell Smith, R. & ether (TOK). Toxicol. Appl. Pharmacol.,
Hennigar, G.R. 19:263-275.
Brown, J.R. A study on the acute inhalation toxicity
1965 of TOK EC-25. Report from the University
of Toronto, Canada submitted to WHO by
Rohm and Haas. (Unpublished)
Brown, J.R. & Mastromateo, E. Subacute percutaneous toxicity of TOK
1964 E-25 in the rabbit. Report from the
University of Toronto, Canada,
submitted to WHO by Rohm and Haas.
(Unpublished)
Byeon, W.H., Hyun, H.H. & Mutagenicity of pesticides in the
Lee, S.Y. Salmonella/microsome system. Korean J.
1976 Microbiol., 14:128-134.
Chernoff, N., Kavlock, R.J., Preliminary report on the perinatal
Gray, L.E. toxicity of nitrofen administered to
1980 mice. Rohm and Haas Report.
(Unpublished)
Costlow, R.D. & Manson, J.M. The heart and diaphragm: target organs
1981 in the neonatal death induced by
nitrofen (2,4-dichlorophenyl-p-
nitrophenyl ether). Toxicology,
20:209-227.
Costlow, R.D., The effect on rat pups when nitrofen
Hirsekorn, J.M., (4-(2,4-dichlorophenoxy) nitrobenzene)
Stiratelli, R.G., was applied dermally to the dam
O'Hara G.P., Black, D.L., during organogenesis. Toxicology
Kane, W.K., Burke, S.S., (in press). Submitted to WHO by
Smith, J.M. & Hayes, A.W. Rohm and Haas.
Decker, F.W. & Percutaneous absorption of 14C-TOK in
Steigerwalt, R.B. rats. Rohm and Haas Report TD77P-59.
1979a (Unpublished)
Deckert, F.W. & Disposition of 14C-TOK after
Steigerwalt, R.B. percutaneous application to a rat.
1979b Rohm and Haas Report TD78P-53.
(Unpublished)
Deckert, F.W. & Fourteen day dietary TOK enzyme
Steigerwalt, R.B. induction study in mice. Rohm
1979c and Haas Report TD78P-58.
(Unpublished)
Ensel, A.B. & Seinen, W. Investigation of TOK on a possible
1970 cataractogenic effect in ducklings.
Central Institut Voor Voedingsonderzock.
Report no. R-3306 submitted to WHO by
Rohm and Haas. (Unpublished)
Gray, L.E., Kavlock, R.J., Prenatal exposure to the herbicide
Chernoff, N., Ferrell, J., 2,4-dichlorophenyl-p-nitrophenyl
McLamb, J. & Ostby, J. ether destroys the rodent Harderian
1982a gland. Science, 215:293-294.
Gray, L.E., Kavlock, R.J., The effects of the prenatal exposure
Chernoff, N. & Ferrell, J. to the herbicide TOK on the postnatal
1982b development of the Harderian gland of
the mouse, rat and hamster. Presented
at Soc. Teratology Meeting 1982.
Submitted to WHO by Rohm and Haas.
Gray, L.E., Kavlock, R.J., Postnatal development alterations
Chernoff,N., Ostby, J. & following prenatal exposure to the
Ferrell, J. herbicide 2,4-dichlorophenyl-p-
1983 nitrophenyl ether. A dose response
evaluation in the mouse. Toxicol.
Appl. Pharmacol. 67:1-14.
Guerzoni, M.E. & Mutagenic activity of pesticides.
Del Cupolo, L. Riv. Sci. Technol. Alimenti Nutr.
1976 Um., 6:161-168.
Hirsekorn, J.M. & Kane, W.W. TOK E-25 percutaneous range-findings
1981 teratology study with postpartum
evaluation. Rohm and Haas Report No.
81R-63. (Unpublished)
Hoover, K.L., Ward, J.M. & Histopathological differences between
Stinson, S.F. nitrofen-induced and naturally occurring
1980 hepatocellular carcinomas in the B6C3F1
mouse. J. Nat. Cancer Inst., 65:937-948.
Kavlock, R.J., Chernoff, N., An analysis of fetotoxicity using
Rogers, E., Whitehouse, D., biochemical end-points of organ
Carver, B., Gray, J. & differentiation. Teratology,
Robinson, K. 26:183-194.
1982
Kimbrough, R.D., 2,4-dichlorophenyl-p-nitrophenyl ether
Gaines, T.B. & Linder, R.B. (TOK). Effect on the lung maturation of
1974 rat fetus. Arch. Environ. Health,
28:316-320
McLeod, P.L. & McCarthy, K.L. TOK dermal cytogenetic study in rats.
1982 Rohm and Haas Report No. 81R-268.
(Unpublished)
Miyauchi, M., Koizumi, M. & Studies on the toxicity of chlorinated
Uematsu, T. p-nitro-biphenyl ether.
1981 I - Methemoglobin formation in vitro
and in vivo induced by nitroso and
amino derivatives of chlorinated
biphenyl ether. Biochem. Pharmacol.,
30:3341-3346.
Myhr, B.C. & Brusik, G. Evaluation of 78-232 in the in vitro
1979 transformation of BALB/3T3 cells assay,
Litton Bionetics Report No. 20992
submitted to WHO by Rohm and Haas.
(Unpublished)
National Cancer Institute. Bioassay of nitrofen for possible
1978 carcinogenicity. Carcinogenesis
Technical Report Series No. 26,
DHEW Publ. No. (NIH) 78-826.
National Cancer Institute. Bioassay of nitrofen for possible
1979 carcinogenicity. Carcinogenesis
Technical Report Series No. 184,
DHEW Publ. No. (NIH) 79-1740,1.
O'Hara, G.P., Can, P.K., The effect of nitrofen
Harris, J.C., Burke, S.S., 4-(2,4-dichlorophenoxy) nitrobenzene
Smith, J.M. & Hayes, A.W. on the reproductive performance
1983 of male rats.Rohm and Haas Report
(Unpublished)
O'Neill, P.J. & Lohse, E. 4,4-DCAB microbial mutagen test. Rohm
1980 and Haas Report No. 80R-4. (Unpublished)
O'Neill, P.J. & TOK: Microbial mutagen tests. Rohm and
Scribner, H.E. Haas Report No. TD79M-307. (Unpublished)
Paik, S.G. & Lee, S.Y. Genetic effects of pesticides in the
1977a mammalian cells. I. Induction of
micronucleous. Kor. J. Zool.,
20:19-28.
Paik, S.G. & Lee, S.Y. Genetic effects of pesticides in the
1977b mammalian cells. II. Mutagenesis in
L5178Y cells and DNA repair induction.
Kor. J. Zool., 20:159-168.
Reustle, J.A. & TOK cytogenetic study in mice. Rohm and
Scribner, H.E. Haas Report No. 79R-173. (Unpublished)
1980
Roser, R.L., Adler, I.L. & A study of the metabolism of 14-C TOK in
Allen, S.S. rats. Rohm and Haas Report No. 23-33.
1971 (Unpublished)
Siou, G. Study of the potential mutagenic
1978 activity of TOK by the Howell-Jolly
body technique. Cabinet d'Etudes et
de Recherches en Toxicologie
Industrielle, Report No. MBL-2199
submitted to WHO by Rohm and Haas.
(Unpublished)
Siou, G. Study of the effect of TOK on the
1979a prenatal and postnatal development
of the rabbit. Cabinet d'Etudes et
de Recherches en Toxicologie
Industrielle, Report No. MBL-2212
and Addendum submitted to WHO by
Rohm and Haas. (Unpublished)
Siou, G. Study of the effect of TOK on the
1979b postnatal development of the rat.
Cabinet d'Etudes et de Recherches
en Toxicologie Industrielle Report
No. MBL-2248 submitted to WHO by
Rohm and Haas. (Unpublished)
Steigerwalt, R.B., TOK comparative metabolism study.
Godfrey, W.J. & Deckert, Rohm and Haas Report No. 79R-169.
F.W. 1980a (Unpublished)
Steigerwalt, R.B., Lisk, D.C. Range-finding study on 14C-TOK. Dermal
& Deckert, F.W. absorption by rat and human skin in
vitro. Rohm and Haas Report No.
80R-110. (Unpublished)
Steigerwalt, R.B., TOK: disposition after percutaneous
Udinsky, J.R. & Deckert, applications to pregnant rats. Rohm
F.W. 1982 and Haas Report No. 81R-65.
(Unpublished)
Stinson, S.F., Hoover, K.L. Quantitation of differences between
& Ward, J.M. spontaneous and induced liver tumors in
1981 mice with an automated image analyzer.
Cancer Letters, 14:143-150.
Stone, L.C. & Manson, J.M. Effects of the herbicide
1981 2,4-dichlorophenyl-p-nitrophenyl
ether (nitrofen) on fetal lung
development in rats. Toxicology,
20:195-207.
Swann, H.E. Acute toxicity report on Rohm and Haas
1973 TOK E-25, dark liquid. Food Drug
Res. Labs. Report No. 1-3784-851
submitted to WHO by Rohm and Haas.
(Unpublished)
Vogin, E.E. Effects of FW925 on the fetal
1971 development in rats. Food and Drug
Research Laboratories Report,
August 10 submitted to WHO by
Rohm and Haas. (Unpublished)
Warso, J.P. Degradation of azoxynitrofen in
1972 simulated digestion fluids. Rohm
and Haas Report No. 23-72-27.
(Unpublished)
Weatherholtz, W.M., Teratogenicity study in rats, TOK
Kapp, R.W. & E-25, TOK E-25 solvent control.
Greenspun, K.S. Report from Hazleton Laboratories,
1979 America Inc. submitted to WHO by
Rohm and Haas. (Unpublished)
RESIDUES
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Nitrofen is a selective pre- and early post-emergence herbicide for
the control of annual grasses and various broad-leaved weeds in cereal
grains and vegetables.
Nitrofen is used in France and some other countries under labels that
prohibit women from handling the product. It is primarily used to
control backgrass (Alopecurus agrestis), Veronica hederaefolia,
Galium aparine, Viola tricolor and other weeds that in winter
wheat and other cereal grains are not adequately controlled by other
commercially available herbicides.
The effective use rate is generally between 1.5 and 2 kg/ha applied to
the soil surface; activity is lost on soil incorporation. Adequate
soil moisture is necessary for efficacy, but excessive moisture may
result in crop injury.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Residue data have been obtained in several countries where nitrofen
was applied to various crops at or about the recommended rates. Two to
four samples were taken at random from the fields and were transported
to the laboratory within two days. The preparation of the sample and
the portion of sample to be analysed were in agreement with the
recommended Codex procedure with two exceptions: only the cloves of
garlic were sent to the laboratory after removing the outer leaves and
all the samples were washed before chopping and deep-freezing.
The analytical results from the United States were obtained by a
method (Adler and Wargo 1975) that measures the parent compound and
the majority of metabolites in the form of the derivative of amine
moiety. The data from other countries indicate the residue level of
the parent compound only. The results of supervised trials are
summarized in Table 1.
FATE OF RESIDUES
In general, nitrofen undergoes metabolic degradation in all systems
(animals, plants, soil and Water) studied. Metabolic pathways include
reduction of the nitro group, conjugation of the resulting amino
group, hydroxylation of the dichlorophenyl ring and formation of
naturally occurring materials, as well as total conversion to CO2.
The structural formula and the occurrence of the important identified
metabolites are shown in Table 2.
In Animals
Cattle milk, urine and faeces collected during the dosing period from
a cow fed a daily ration of 26.2 kg containing 5 mg/kg purified
nitrofen for four days did not contain detectable levels of nitrofen
or aminonitrofen (sensitivity of the method was approximately 0.3
mg/kg). In vitro studies demonstrated that nitrofen is rapidly
reduced to aminonitrofen in fresh rumen fluid (T1/2 is less than or
equal to 10 min). Aminonitrofen was stable in the rumen fluid for 24 h
(Gutemann & List 1967).
Similarly, only trace levels of radioactivity (0.02 to 0.07 mg/kg,
calculated as nitrofen) were found in samples of urine, faeces and
milk from a cow dosed at 5 mg/kg with radiolabelled nitrofen. The
levels of total radioactivity were 1.4 and 1.7 mg/kg in the faeces and
urine, respectively, and 0.14 and 0.16 mg/kg in two samples of milk. A
fat sample contained 1.06 mg/kg 14C, of which 0.25 mg/kg was parent
compound (Roser & Adler 1971).
Table 1 Residues of Nitrofen Resulting from Supervised Trials
Crop and variety Country Application Proharvest Residue (mg/kg)
rate interval Mean
(kg/a.i./ha) No. (days)
Broccoli (head) United States1 3.7 1 74 <0.01 <0.015
Futura 4.5 2 63 <0.01 <0.01
Gem 4.5 1 85 <0.01 <0.01
Green Duke 4.75 2 63 <0.01 <0.01
4.75 2 69 <0.01 <0.01
5.0 1 70 <0.01 <0.01
5.3 2 70 <0.01 <0.01
5.4 1 77 <0.01 <0,01
Cabbage (head) United States1
Head Start 4.5 1 69 <0.01 <0.01
4.5 1 84 <0.01 <0.01
Green 4.5 1 89 <0.01 <0.01
6.7 1 76 <0.01 <0.01
6.7 1 90 <0.01 <0.016
Cauliflower (head) United States1
Snoball 4.5 1 90 <0.01 <0.01
4.5 1 93 <0.01 <0.01
4.6 1 88 <0.01 <0.016
5.6 1 83 <0.01 <0.01
6.7 1 59 <0.01 <0.01
6.7 1 107 <0.01 <0.016
Celery (stalk) United States1
5270 RIMP 4.5 1 109 <0.01 <0.01
5.4 1 127 <0.01 <0.01
5270 5.6 1 52 <0.01 <0.01
5270R 5.6 1 165 <0.01 <0.01
Table 1 (continued)
Crop and variety Country Application Proharvest Residue (mg/kg)
rate interval Mean
(kg/a.i./ha) No. (days)
Garlic (bulb) United States1
Cal Late 5.6 1 234 <0.01 <0.01
5.6 1 250 <0.01 <0.01
6.7 1 229 <0.01 <0.01
7.8 1 241 <0.01 <0.01
Rape (grain) Denmark2 1.2 1 100 - <0.01
1.2 1 101 - <0.01
1.2 1 105 - <0.01
1.2 1 100 - <0.01
1.2 1 101 - <0.01
1.2 1 105 - <0.01
Rioe (grain) United States1
Early Rose 2.2 1 145 <0.01 <0.01
3.4 1 145 <0.01 <0.01
3.4 1 160 <0.01 <0.01
4.5 1 160 <0.01 <0.01
6.7 1 160 <0.01 <0.01
9.0 1 166 <0.01 <0.01
Rice (straw)
Early Rose 2.2 1 145 <0.01 <0.01
3.4 1 145 <0.01 <0.01
4.5 1 166 <0.01 <0.01
9.0 1 166 <0.01 <0.01
Table 1 (continued)
Crop and variety Country Application Proharvest Residue (mg/kg)
rate interval Mean
(kg/a.i./ha) No. (days)
Wheat (grain) France3
Chamlein
4.0 1 239 <0.01 <0.01
4.0 1 264 <0.01 <0.01
Joss 4.5 1 266 <0.01 <0.01
Hardy 8.0 1 236 <0.01 <0.01
Chamlein 8.0 1 264 <0.01 <0.01
Hardy 8.0 1 265 <0.01 <0,01
Wheat (grain) Federal
Republic
Joss of 1.6 1 248 - <0.002
Carstacht Germany4 1.6 1 280 - <0.002
Kranich 1.6 1 284 - <0.002
Caribo 1.6 1 286 - <0.002
2.0 1 270 - <0.002
Benno 2.0 1 279 - <0.002
Carito 2.0 1 286 - <0.002
Hyslop United States1 1.7 1 294 <0.01 <0.01
Druchamp 2.2 1 163 0.00-0.02 0.01
Yamhill 2.2 1 250 <0.01 <0.01
2.2 1 267 <0.01 <0.01
2.2 1 269 <0.01 <0.01
2.2 1 272 0.01-0.09 0.04
Nugaines 2.2 1 272 0.02-0.03 0.02
Hyslop 2.2 1 281 <0.01 <0.01
Yamhill 2.2 1 284 <0.01 <0.01
2.2 1 289 <0.01 <0.01
Table 1 (continued)
Crop and variety Country Application Proharvest Residue (mg/kg)
rate interval Mean
(kg/a.i./ha) No. (days)
Cajame 3.4 1 146 <0.01-0.01 <0.01
Tehame 3.4 1 151 <0.01 <0.01
Hyslop 3.4 1 294 <0.01 <0.01
Cajame 4.5 1 146 <0.01 <0.01
Tehame 4.5 1 151 <0.01-0.01 <0.01
Druchamp United States1 4.5 1 163 <0.01-0.06 0.03
Yamhill 4.5 1 250 <0.01-0.01 <0.01
4.5 1 267 <0.01 <0.01
4.5 1 269 <0.01 <0.01
4.5 1 272 0.01-0.12 0.06
Nugaines 4.5 1 272 0.02-0.06 0.04
Hylop 4.5 1 281 <0.01 <0.01
Yamhill 4.5 1 284 <0.01 <0.01
4.5 1 289 <0.01 <0.01
Cajame 5.6 1 146 <0.01-0.02 0.01
6.7 1 146 <0.01-0.01 <0.01
Tehame 6.7 1 151 <0.01-0.01 <0.01
Yamhill 6.7 1 267 <0.01 <0.01
Wheat (straw) Federal Republic
of Germany4
Joss 1.6 1 248 - <0.002
Carstacht 1.6 1 280 - 0.020
Kranich 1.6 1 284 - 0.007
Caribo 1.6 1 286 - <0.004
2.0 1 270 - 0.004
Benno 2.0 1 279 - 0.010
Carito 2.0 1 286 - 0.005
Table 1 (continued)
Crop and variety Country Application Proharvest Residue (mg/kg)
rate interval Mean
(kg/a.i./ha) No. (days)
Hyslop United States1 1.7 1 294 <0.01 <0.01
Yamhill 2.2 1 252 <0.01 <0.01
2.2 1 267 <0.01 <0.01
2.2 1 269 <0.01 <0.01
Nugaines 2.2 1 272 0.02-0.11 0.06
Yamhill 2.2 1 273 <0.01-0.02 0.01
Druchamp 2.2 1 276 0.01-0.04 0.02
Hyslop 2.2 1 281 <0.01 <0.01
Yamhill 2.2 1 284 <0.01 <0.01
2.2 1 291 <0.01 <0.01
Hyslop 3.4 1 294 <0.01 <0.01
Yamhill 4.5 1 252 <0.01 <0.01
4.5 1 267 <0.01-0.01 <0.01
4.5 1 269 <0.01 <0.01
Nagaines 4.5 1 272 0.02-0.05 0.03
Yamhill 4.5 1 273 <0.01-0.03 0.01
Druchamp 4.5 1 276 0.01-0.02 0.01
Hyslop 4.5 1 281 <0.01 <0.01
Yamhill 4.5 1 284 <0.01 <0.01
4.5 1 291 <0.01-0.02 0.01
6.7 1 267 <0.01 <0.01
Sources:
1 Rohm and Haas 1969-1980;
2 Institute National de Recherche Agronomique 1975b;
3 Institute National de Recherche Agronomique 1975a;
4 Institute National de Recherche Agroncmique 1978;
5 Samples taken from four experimental fields;
6 Samples taken from three experimental fields.
Table 2 Nitrofen Metabolites
Metabolite Occurrence
Aminals Plants Soil
Groups of three female Sprague-Dawley rats received a single dermal
application of 14C-nitrofen (uniformly labelled in the dichlorophenyl
ring) as an undiluted emulsifiable concentrate at a dose level of 125
or 375 mg/kg or as an aqueous dilution (ca. 1.8 percent a.i.) at
levels of 9 or 27 mg/kg a.i.
Urine and faeces were collected twice daily. One rat per group was
sacrificed at 24, 48 and 96 h after dosing. Blood, liver and kidney
were assayed for radioactivity.
Less than 2 percent of the applied doses remained at the application
sites after 96 h while only 30-40 percent of the dose was excreted in
the urine (5-9 percent) and faeces (26-33 percent) during that time.
Blood, liver and kidneys also contained radioactivity at all sacrifice
times (Deckert & Steiserwalt 1979a).
14C-nitrofen (uniformly labelled in the dichlorophenyl ring) was
dermally applied as undiluted emulsifiable concentrate at a level of
120 mg/kg to a female Sprague-Dawley rat. Urine, faeces and volatile
materials were collected daily at 24 h intervals. Total 14C recovery
in this study was 87 percent. At 96 h post-dosing, the rat was
sacrificed and blood, organs and tissue specimens were removed for
14C analysis. After 96 h 8.7 percent of the dose was excreted in
urine and 36 percent in faeces. Only 0.02 percent of the dose was
found as 14CO2, while other volatile materials accounted for 0.001
percent. 14C was found in every tissue analysed. The highest
concentrations were found in skin (17.6 percent of the dose), fat
(484.5 ppm = 2.1 percent of the dose) and muscle at the application
site (158.3 ppm = 0.9 percent of the dose), whereas only 0.4 percent
of the dose remained unabsorbed. A total of 38 percent of the applied
dose was found in tissues. This figure, when compared with the lower
values found in oral studies, suggests that nitrofen may be better
absorbed dermally than by the oral route (Deckert & Steiserwalt
1979b).
Groups of 12 pregnant Sprague-Dawley rats were treated dermally with
14C nitrofen (uniformly labelled in the dichlorophenyl-ring) as
diluted emulsifiable formulation at levels of 0.3 or 27 mg/kg b.w.
a.i. for 6 h on day 11 of gestation. Another group received a single
dose intravenously at a level of 0.3 mg/kg b.w.
At the end of the six-hour application period, only 16 percent of the
dermally applied dose could be removed by washing. Low-dose dermal
application produced blood levels similar to those produced by i.v.
administration, except at the one hour sampling. Urinary 14C
excretion patterns were similar for both dermal application (low and
high dose) and i.v. administration. However, 11 days after dosing, 15
and 20 percent of the dermal (both levels) and i.v. doses,
respectively, were excreted in urine. Foetal 14C excretion patterns
were also similar for both routes of administration, with 45-48
percent and 75 percent of the dose being excreted by 11 days after
dermal and i.v. admin-istration, respectively. After 11 days, 61-65
percent and 97 percent of the dermal and i.v. doses, respectively, had
been excreted. For the higher dermal dose, most maternal tissue 14C
concentrations peaked one to two days after dosing (day 12-13 of
gestation) with the highest concentrations occurring in the fat and
gonads. At 0.3 mg/kg (both routes) heart, thryroid, adrenals,
Harderian glands and pancreas approached maximum levels after six
hours.
Peak 14C levels of most maternal tissues increased with increasing
dose increment and reached a higher level following i.v.
administration than after a dermal application of 0.3 mg/kg.
Placental and foetal 14-C concentrations also peaked within one to
two days after dosing. At 0.3 mg/kg, mean placental 14-C levels were
similar by either route, while the foetal 14-C level two days after
dermal application was five-fold higher than those after i.v.
administration, ten-fold higher than that found in maternal whole
blood at the same time, but only three-fold lower than after the high
dermal dose. At the low dermal dose, the peak or plateau placental
and foetal 14C levels were comparable with those in maternal tissues
(other than the fat and gonads). After i.v. administration and the
high dermal dose, tissue levels in the mother were much higher than in
the placenta or foetus.
In the amniotic fluid, both 0.3 mg/kg dose groups showed the highest
14C levels at 11 days after dosing (day 22 of gestation); the
high-dose dermal group had a steady high 14C level throughout this
period.
Eleven days after dermal application (27 mg/kg), 14C concentrations
in maternal organs were generally higher than in foetal organs, except
for the heart, where levels were nearly the same. Radioactivity was
detected in foetal organs in descending order from kidney to heart,
lung, liver and brain. Metabolic profiles in the placenta and the
embryo/foetus showed the presence of nitrofen, aminonitrofen and other
unidentified metabolites with peak or plateau concentrations occurring
within one to two days. Concentrations of nitrofen and minor
metabolites decreased with time, while aminonitrofen appeared to reach
a plateau and then decrease at a slower rate. More than 90 percent of
14 in the urine and faeces was readily extractable (Steiserwalt
et al. 1982).
Dog
Groups of six-month-old beagles (two males and two females/group) were
fed diets containing technical grade 95 percent pure) nitrofen at
levels of 0, 20, 200 or 2 000 ppm for two years. Tissues (fat, muscle,
bone, liver, kidney, spleen and blood) pooled at week 104 and excreta
(urine and faeces) at week 103 were analysed for unchanged nitrofen by
sas-chromatography. The nitrofen concentration was highest in body fat
(38, 175, 515 ppm, respectively, at the three dietary levels tested),
with lower levels found in bone (3.9, 19.2, 21.8 ppm), muscle (1.26,
4.76, 20.0 ppm), kidney (1.4, 4.6, 13.6 ppm), liver (1.0, 2.0, 3.95
ppm) and blood (0.1, 0.2, 0.4 ppm). Unchanged nitrofen was excreted
almost exclusively in the faeces (39.0 ppm at the highest level)
(Ambrose et al. 1971). (Full experimental data were not provided.)
In vitro dermal absorption
Skin sections from an adult female Sprague-Dawley rat and an adult
woman (postmortem) were mounted in Franz Diffusion Cells and 5 µl of
diluted 14C-nitrofen emulsifiable formulation (ca. 1.8 percent a.i.)
was applied to each. After six hours of exposure, the rat skin
absorbed from 24.7 to 61.7 percent of the 14C while the human skin
absorbed from 13.8 to 25.3 percent of the label. The cell-by-cell
average rat: human absorption ratio of 1.99+/-0.34 indicated that
nitrofen was absorbed by human skin at approximately half the rate
that it passed into rat skin (Steiserwalt et al. 1980b).
TOXICOLOGICAL STUDIES
Special Studies on Reproduction
Rat
In a three-generation, two-litter per generation, reproduction study
groups of 28-day-old Wistar rats (25 males and 25 females/group) were
fed diets containing technical grade (95 percent pure) nitrofen at
levels of 0, 10, 100 or 1 000 ppm for 11 weeks. Body weights for
parental rats before mating and at weaning of respective litters were
not adversely affected for Fo and F1b generations. Body weight of
F2b parent generation rats was slightly decreased for rats at 100
ppm, in breeding the F3a litter and in breeding the F3b litter fed 10
ppm. This decrease was due to lower initial body weights of rats used
for succeeding generations. Although there was considerable
inconsistency within and between matings, average data reported did
not support a dose-related effect on fertility and gestation indices.
Viability and lactation indices were not adversely affected at 100
ppm. However, the viability index for rats at 1 000 ppm was seriously
affected, as stillborn pups outnumbered live pups; indeed no pups were
available for continuance beyond the Fo generation. In all
generations, the number of stillborn pups from the 100 ppm group
numbered 64 compared with 30 in the control.
No structural or microscopic cellular abnormalities were detected in
any stillborn pups in F1b litters. Weaning weights indicated no
diet-related trends. Overall, only the 10 ppm dietary level of
nitrofen exhibited no adverse effects on reproduction. Examination of
a variety of tissues from 10 F3b generation rats of each sex from the
0, 10 and 100 ppm dietary levels showed no histopathological lesions
(Ambrose et al. 1971). (Full experimental data were not provided.)
Groups of Sherman rats (10 males and 20 females/group) were fed
dietary levels of 0, 20, 100 and 500 ppm technical nitrofen (89
percent) pure). Breeding and F1a and F1b litters commenced at 68 and
200 days, respectively. Offspring were observed through weaning. At
the time of weaning of the F1b litters, 20 female and ten male
weanlings were selected from the 0, 20 and 100 ppm groups, continued
on the parental diet and pair-mated when 112 days old to produce the
F2a generation. Neither the food consumption nor the body weight gain
were greatly affected in any of the nitrofen-treated groups. At the
500 ppm dietary level no offspring of the F1a and F1b generation
survived the neonatal period. The number of pups born alive and the
survivors-to-weaning were reduced at the 100 ppm dietary level but not
at 20 ppm (equal to 1.1 and 1.8 mg/kg b.w. in F1b and F1a,
respectively) (Kimbrough et al. 1974). (Full experimental data were
not provided.)
Groups of 25 male Sprague-Dawley rats were fed diets containing 0,
100, 500 or 2 500 ppm of nitrofen technical (95.7 percent pure) for 95
days. At that time, male and untreated female rats were cohabited
(1:1) for a maximum of 10 days. During co-habitation, males were fed
an untreated diet and daily doses of 0, 6, 30 or 155 mg/kg of nitrofen
were administered by gavage. The nitrofen dosage was based on the
dietary intake of the compound (mg/kg/day) during weeks 10-13 of the
study.
No significant effects were detected on the reproductive performance
of male as indicated by the average number of days for mating to
occur, percentage of females impregnated or number of mated females
that delivered. Furthermore, no differences were noted in the length
of gestation, litter size, percentage of live or dead births or sex
ratio of the offspring. No compound-related signs of toxicity were
evident in any of the progeny from birth to day 35. Necropsies of
offspring that died and of female parents which were killed after
lactation revealed no compound-related gross abnormalities. Neither
viability nor mean weights of offspring were affected at any dose
level. Male reproductive performance was not affected at dietary
concentrations up to and including 2 500 ppm, equal to 186 mg/kg/day
(O'Hara et al. 1983).
Special Studies on Teratogenicity and Postnatal Effects
Mouse
Two studies on postnatal dose response and standard teratology are
summarized together.
Purified nitrofen (99.6 percent pure) in maize oil was administered to
CD-1 mice by gavage. In the postnatal study, 24 animals were dosed
(50, 100, 150 and 200 mg/kg/day) on days 7-17 of gestation and allowed
to give birth. In the teratology study animals were dosed 50, 100 and
200 mg/kg/day) on days 7-17 and sacrificed on day 18. Foetuses were
removed and analysed for visceral and skeletal malformations.
Concomitant control groups were utilized in all the studies.
In the postnatal study there was a significant dose-related delay in
time of birth. Significant dose-related reductions in growth and
viability were noted at all dose levels. There was considerable litter
mortality in the 100 mg/kg/day groups between days 3 and 17
postpartum. Only 23 percent of the pups alive on day 3 were still
alive by day 17. Mortality in the 50 mg/kg/day dose group was not
different from control values during this period. Microphthalmia or
anophthalmia was seen in a high percentage of the animals in the 100
mg/kg/day dose group. A dose-related growth retardation was noted in
pups until 60 days of age at 50 mg/kg and above. There were delays in
the percent of female offspring showing vaginal opening at 30 days and
in the percentage of animals breeding by 60 days at 50 mg/kg and
above. Litter size was unaffected by treatment.
In the teratology study dose-related increases in maternal weight gain
and liver/ body weight ratios were recorded. Nitrofen significantly
increased the supraoccipital scores, indicating retarded skeletal
development of the skull. It was teratogenic at 100 and 200 mg/kg/day,
the most common defects noted being cleft palate and undescended
testes (Chernoff et al. 1980). (Full experimental data were not
available.)
After being dosed at levels of 100 mg/kg b.w. nitrofen on days 7 to 17
of gestation, pregnant CD-1 mice showed reduced mean litter size.
Surviving pups had delayed eye opening and absence of the Harderian
gland (Gray et al. 1982a).
Groups of pregnant CD-1 mice were dosed by gavage with nitrofen (99.6
percent pure) in maize oil at levels of 0, 6.25, 12.5, 25 and 50
mg/kg/day. There were 23, 19, 27, 21 and 17 litters, respectively, on
day 1. The animals were observed until necropsy at 110-130 days of
age.
The administration of nitrofen did not cause any adverse effects in
the dams, as measured by maternal viability or weight gain from days 7
to 19 of gestation. By day 3 the number of pups was reduced
significantly at 50 mg/kg (20 percent). Body weights were initially
unaffected after birth at 50 mg/kg but subsequent body weight was
reduced in the pups from the 12.5 mg/kg treatment group. There was a
dose-related increase in the frequency of lethal defects in the pups,
i.e. cleft palate, diaphragmatic hernia and extreme abdominal
distention. Lung weight was significantly reduced by nitrofen
treatment at all doses, including the lowest dose of 6.25 mg/kg.
Seminal vesicle weights were significantly reduced at 6.25, 12.5 and
50 mg/kg. Obvious intraorbital defects were present in some offspring
in the 25 mg/kg group and above. Prenatal nitrofen treatment
significantly reduced the weight of the Harderian glands at all doses,
including the lowest dose of 6.25 mg/kg. Prenatal nitrofen treatment
of 50 mg/kg significantly delayed puberty in the female mice, as
indicated by the percentage of females with patent vasina on day 30.
In addition, the 50 mg/kg females paired with treated males produced
significantly fewer pups in the second litter.
A cross-fostering experiment with 100 mg/kg demonstrated that the
defects noted in the present study were produced solely by prenatal
exposure; pups exposed to nitrofen in the milk alone as a consequence
of any accumulation of nitrofen in the dam during gestation were
unaffected (Gray et al. 1983).
Rat
Groups of 26 pregnant FDRL rats were administered nitrofen by gavage
on days 6-16 of gestation. The control animals received maize oil (1
ml/kg b.w.) whereas the test animals received 100 and 200 ppm of
nitrofen in the same volume of vehicle. Thus the dosage corresponds to
5 and 10 mg/kg b.w./day, respectively. On day 22, the animals were
sacrificed and Caesarean section performed. There were no differences
between control and treated groups with respect to mean dam weight,
implantation sites, foetal viability or mean live foetal weight.
Resorptions were increased in the high-dose group. No malformed
foetuses were seen at the time of Caesarean sections, nor was there
evidence of soft tissue anomalies. Numbers of incomplete cranial bone
ossification and scoliosis were slightly increased in both treated
groups. These variations were considered "consistent with changes
previously observed in the colony of rats" and, thus, not
treatment-related (Vosin 1971).
Groups of seven pregnant Wistar rats were administered by gavage daily
doses of nitrofen technical (96.2 percent pure) at levels of 68 mg/kg
b.w. on days 6 through 15 of gestation. Five pregnant control rats
received only peanut oil. Parameters evaluated were neonatal mortality
and histomorphology of the lungs of dead and moribund newborns in the
nitrofen-treated group and of four-day-old survivors from the control
and treated groups. All the offspring from the control group were
alive at birth. Most (85 percent) of the offspring from the treated
group were born dead or died within the first few hours after delivery
and exhibited generalized cyanosis. Histological examinations of the
lungs of moribund offspring from the treated group revealed a large
number of alveoli bordered by a cubical epithelium (Siou 1979b).
Groups of 9-12 pregnant Sherman rats were dosed by oral intubation on
days 7 through 15 of gestation. Dosage levels were 0 (peanut oil), 10,
20 or 50 mg/kg/day for technical nitrofen (89 percent pure); 0, 20, or
50 mg/kg/day for purified nitrofen (99 percent pure); and 0 or 0.04
mg/kg/day for 2,7-dichlorodibenzo-p-dioxins (DCDD) (this dose is
equivalent to a contamination level of 2 000 ppm (0.2 percent) in the
technical nitrofen for a 20 mg/kg dose). The pups were observed to
weaning. At the dosage levels of 20 and 50 mg/kg/day a dose-related
decrease of live-born rats was observed for both the technical and
purified nitrofen groups. None of the pups survived at the 50 mg/kg
dose level and survival-to-weaning was severely reduced (50 percent)
in both the technical and purified nitrofen groups. No effect of
technical nitrofen on the number of live pups born and their
survival-to-weaning was observed at 10 mg/kg dose level. Extensive
inflammation, fibrosis and epithelial proliferation of the lung were
seen in sucklings that died at 11 to 15 days. No effect on
survival-to-weaning was observed in DCDD-treated rats.
Additional groups of ten female and ten male rats were fed technical
nitrofen at dietary levels of 0 and 500 ppm. The rats were started on
the diet at weaning and pair-mated when they were 90 to 100 days old.
On day 20 of pregnancy, foetuses were removed by Caesarean section and
examined for malformations. The live foetuses were kept in an
incubator for six hours and closely checked for viability. Most of the
foetuses of the controls were pink and survived until they were killed
after six hours. The pups from nitrofen-treated dams were cyanotic and
died one to three hours following the Caesarean section.
Two more pups of pregnant rats were given by oral intubation technical
nitrofen at levels of 0 or 50 mg/kg/day on days 7 to 18 of gestation.
The foetuses from these rats were removed by Caesarean section on day
21 of pregnancy. Lungs from 18 exposed and 16 control foetuses from
four litters in each group were removed one to three hours following
the Caesarean section and studied with light- and electron-microscopy.
Pups from treated dams showed cyanosis at birth. The cyanosis
increased during the next 45 to 60 minutes and most of the pups from
treated dams died, while the control rats developed pink colour soon
after birth and survived. Lungs of treated foetuses were poorly
expanded and alveolar epithelium was cuboidal and resembled cells of
earlier gestational age (Kimbrough et al. 1974).
When purified nitrofen (containing only trace amounts (ppm) of
4-aminonitrofen) was administered to pregnant Long-Evans rats at
different days of gestation, gestation day 11 was demonstrated as
critical: a single dose of 150 mg/kg on day 11 killed 56 percent of
the newborn pups withing 48 h.
Groups of 4-7 Long-Evans dams were given a single dose of purified
nitrofen at levels of 0, 75, 115, 150, 200 or 250 mg/kg on day 11 of
gestation. Survival of neonates to five days was monitored. A
dose-related increase in neonatal mortality was observed. The LD50
for the neonates was estimated at 116 mg/kg of maternal body weight.
Initial experiments demonstrated that animals surviving the first five
days usually lived to maturity. Thus, the mortality is most accurately
described by the fraction dying within the first five postnatal days.
Surviving offspring were autopsied at 35 days of age and examined for
gross lesions. The predominant lesion observed in all treated groups
was hydronephrosis. The incidence of hydronephrosis in survivors
paralleled the neonatal mortality and the lesion was most frequent
among animals exposed on day 11. The incidence of hydronephrosis was
also dose-related among animals exposed only on day 11.
Nitrofen-induced hydronephrosis was not considered incompatible with
life.
In a further teratology study purified nitrofen was administered to
pregnant Long-Evans rats at dose levels of 0, 70, 115, 150, 250, 265
and 400 mg/kg b.w. on day 11 of gestation and foetuses collected on
day 22. Foetuses exposed to nitrofen and examined at term (day 22),
showed delayed skeletal ossification. There was a dose-related
depression in foetal body weight. Nitrofen exposure on day 11 induced
hydronephrosis, which was dose-related in incidence and severity.
Diaphragmatic hernias were also observed in the treated groups. The
overall frequency of soft tissue anomalies was also dose-dependent.
Mean litter frequencies of these anomalies ranged from 45 percent at
LD15 (70 mg/kg) to 91 percent at LD99+ (400 mg/kg). Nitrofen exposure
produced a dose-related increase in the frequency of cardiac
malformations, which included in order of decreasing frequency:
ventricular septal defects (VSD), double outlet right ventricules
(DORV), and transposition of great vessels. Incidence of lethal
cardiac malformations was 38 percent and 53 percent at 150 mg/kg and
250 mg/kg, respectively. No anomalies were seen in the control
litters. None of the cardiac or diaphragmatic defects in the term
foetuses were present in survivors, suggesting that the heart and
diaphragm are the target organs in nitrofen-induced neonatal deaths
(Costlow & Manson 1981).
Groups of five pregnant Sprague-Dawley rats were treated with nitrofen
(98 percent pure) at 0, 20, 31.2 or 50 mg/kg/day by gavage on days
8-18 of gestation. Significant differences in birth weights were
apparent in pups from the 31.2 and 50 mg/kg/day groups. A decrease in
the lung to body weight ratio of foetuses exposed to nitrofen was also
observed. Most, if not all, pups were born alive and lacked
externally-observable malformations. Within minutes after birth,
treated pups exhibited laboured breathing and became cyanotic. Death
usually ensued 30 min. to 4 h postpartum. Survival was significantly
depressed in all treatment groups and all time intervals (1 h, 24 h,
25 days) measured after birth. On examination, symptomatic
nitrofen-exposed pups suffered massive atelectasis at autopsy, with
few expanded air sacs in the 31.2 and 50 mg/kg groups. In contrast,
lungs from newborn controls exhibited normal alveolar expansion. The
level of glucocorticoids and capacity of the foetal lung to respond to
glucocorticoids by synthesizing and releasing surfactant were not
affected by prenatal exposure to nitrofen (Stone & Manson 1981).
Missing and/or malfunctioning Harderian glands were observed when
pregnant Sprague-Dawley CD rats were given daily doses of nitrofen at
a level of 12.5 mg/kg by gavage on days 8 to 16 of gestation (Gray
et al. 1982a).
A postnatal study, using rats (strain unknown) dosed on days 7-16 at
five doses from 0 to 25 mg/kg, demonstrated that nitrofen caused
diaphragmatic hernias at 1.39 mg/kg and above and affected the
haemoglobin level at 12.5 mg/kg (Gray et al. 1982b). (This
information was provided in abstract form and cannot be further
interpreted.)
Groups of five pregnant CD rats were given by gavage daily doses of
nitrofen at levels of 0, 12.5 or 25 mg/kg b.w. on days 8 to 16 of
gestation. Dams were sacrificed on day 21 of gestation and foetuses
removed for examinations. Dose-related increase in foetal mortality
and dose-related decrease of mean foetal weight were determined.
Diaphragmatic hernias occurred in both dose groups in a dose-related
fashion. Brain weight and brain DNA were statistically significantly
reduced at 25 mg/kg; lung weight and lung surfactants were reduced at
12.5 and 25.0 mg/kg in a dose-related fashion as were liver weight,
liver glycogen content, kidney weight and kidney alkaline phosphatase
levels. However, statistical analysis demonstrated that only effects
on lung and liver represented specific organ toxicity, while brain and
kidney effects were correlated to the foetal growth retardation
(Kavlock et al. 1982).
When pregnant Sprague-Dawley rats (35/group) were exposed dermally to
nitrofen emulsion at 0, 0.3, 3.0 or 30.0 mg/kg/day on days 6 to 15 of
gestation, foetal weight and foetal length were significantly lower in
the 30.0 mg/kg group compared to the control group. Soft tissue
anomalies (hydronephrosis, ectopic testes, liver and/or stomach and/or
intestine protruding into thorax, diaphragmatic hernia, right-sided
aorta) were observed almost exclusively in the foetuses of the 30.0
mg/kg group. The foetal viability (live at birth/implantations) in all
the treated groups was statistically significantly lower than the
control litters. Neonatal survival index (live at day 4/live at birth)
was significantly lower in the 30.0 mg/kg group than in the control
group (Weatherholtz 1979).
Groups of five to seven pregnant Charles River rats were treated
dermally with nitrofen (as a water-diluted emulsifiable formulation)
at levels of 0, 0.6, 4.8 or 19.2 mg/kg/day on days 6 to 15 of
gestation. A further group received dermally 4.8 mg/kg on day 11 only.
Dams were sacrificed on day 21 postpartum. Surviving offspring were
sacrificed on day 27. All maternal rats survived to sacrifice.
Treatment of pregnant rats with 19.2 mg/kg/day of nitrofen on days 6
to 15 of gestation resulted in slightly decreased weight gain during
the treatment interval. The no-observable effect level (NOEL) was 4.8
mg/kg/day. The treatment caused decreased viability of offspring,
decreased neonatal survival to day 4 and decreased live offspring of
pregnant rats treated dermally with 19.2 mg/kg/day of nitrofen on days
6 to 15 of gestation. Nitrofen did not affect neonatal viability,
neonatal survival, survival to weaning, growth of offspring or eye
opening in offspring of dams treated with 0.6 or 4.8 mg/kg/day on days
6 to 15 of gestation or 4.8 mg/kg/day on day 11 of gestation
(Hirsekorn & Kane 1981).
Groups of 25 pregnant Sprague-Dawley rats were treated dermally with
nitrofen in aqueous emulsion (0, 0.3, 0.6, 1.2 or 12.0 mg/kg b.w./day)
on days 6 to 15 of gestation. No maternal toxicity occurred. At 12
mg/kg neonatal survival was reduced. Necropsies of the dead neonates
showed a high incidence of diaphragmatic hernias. The number of pups
per litter and viability over days 4-41 of age were unaffected by
treatment. About half (randomly selected) of the pups from each litter
and group were necropsied on day 42. Chromo-dacryorrhea, reduced or
absent Harderian glands and diaphragmatic hernias occurred at 12.0
mg/kg. The frequency and the severity of dilation of the renal
pelvices increased with the dose; slight dilation was significantly
elevated at 0.3 mg/kg and moderate and severe dilation became
prominent at 0.6 and 12.0 mg/kg, respectively. In the 12.0 mg/kg group
nitrofen reduced the body weight of the survivors and also the
relative weights of the Harderian gland, the thyroid gland of both
sexes; lungs of females were significantly depressed with respect to
solvent and water control groups. The findings from necropsies
performed on the remaining offspring on days 146 to 149 postpartum
were similar to those at day 42. Body weight was unaffected by
nitrofen exposure but dilated kidneys and diaphragmatic hernias were
observed. Relative thyroid weight was significantly depressed only in
the 12 mg/kg dose group. A NOEL was not demonstrated (Costlow et al.
1983).
Rabbit
Groups of 15 pregnant New Zealand rabbits were given encapsulated
technical nitrofen (96.2 percent pure) at levels of 0, 7, 27 or 108
mg/kg on days 6 through 18 of gestation. Ten rabbits in each group
were sacrificed on day 28 of gestation to assess embryotoxic and
teratogenic effects. The average number of live foetuses and
implantation sites per pregnant female was slightly decreased in the
108 mg/kg group sacrificed on day 28 of gestation. Percentage of dead
foetuses with respect to implantation sites was increased in all
treated groups compared with the control group, but not in a
dose-related manner. The increased incidence of foetuses, with 13
pairs of ribs was dose-related. No other anomalies were observed. The
remaining five rabbits in each group were allowed to deliver, and they
and the newborns were sacrificed 48 h after delivery. The average
number of live offspring was slightly lower in the 108 mg/kg group.
Average weights of live foetuses after 48 h were slightly lower in the
treated groups, but not in a dose-related manner. No significant
differences were observed in survival rate to 48 h or in resorption
rates. An increased incidence of 13 ribs was observed in all treated
groups. Histological examinations of the lungs removed from between 5
and 17 of the 28-day foetuses and the 48-hour newborns in each group
did not indicate retardation of lung maturation (Siou 1979a).
Special Studies on Mutagenicity
Microbial systems
Nitrofen, when tested with Salmonella typhimurium strains TA1535,
TA1538, TA100 and TA98, both with and without S-9 activation, was
found only moderately mutagenic in TA100 with microsomal enzyme
activation (Byeon et al. 1976).
Purified nitrofen (99+ percent) and several samples of nitrofen
technical (90-98 percent pure) were tested at concentrations of
0.001-10.0 µg/plate with S. typhimurium strains TA1535, TA1537 TA98
and TA100. Tests were carried out both with and without microsomal
activation. Positive controls were used: 2-aminofluorene and
2-acetamidofluorene for TA98 and 2-anthramine for all the strains
tested. Only one sample of nitrofen technical (90-92 percent pure) was
completely negative in the test. The other samples of technical
nitrofen gave statistically significant increases of mutants/plate
compared with negative control in TA100 strain, both with and without
activation, but only one was positive in TA98 with activation.
However, a dose-response relationship was observed only with three
samples (97, 97 and 98 percent pure), but not with another three (93,
92.8 and 97.7 percent pure), generally at concentrations of 10-10 000
µg/plate. Purified nitrofen (99+ percent pure) gave a statistically
significant increase of revertants/plate in TA100, both with and
without activation; no dose-response relationship was observed.
Thus purified nitrofen and three technical samples gave an indication
of mutagenicity, but the other three technical samples were positive
(O'Neill & Scribner 1979).
Nitrofen was found mutagenic in a reversion test using
Saccharomyces cereviseae strain 632/4 (auxotrophic for methionine)
(Guerzoni et al. 1976).
Mammalian systems
The mutagenicity of nitrofen was assayed in the murine lymphoma cell
line L5178Y at concentrations of 0.1 x 10 (E-4), or 2 x 10 (E-4) M.
Doses were selected as those killing approximately 40 and 80 percent
of cells. Ethylmethanesulphonate was used as a positive control.
Induction of methotrexate-resistant mutants of L5178Y cells treated
in vitro with nitrofen was not significantly different from the
negative control (Paik & Lee 1977b).
Nitrofen was tested in the unscheduled DNA repair synthesis assay with
human lymphocytes (concentrations not clear). Nitromin was used as a
positive control. 3H-thymidine incorporation in the nitrofen-treated
cells was not significantly different from the negative control (Paik
& Lee 1977b).
Nitrofen was also tested in mouse cells in a BALB/3T3 in vitro
transformation assay. The compound did not induce a significant
increase in transformed foci over the applied concentration range of
0.1 to 60 micrograms/ml. This range corresponding to approximately 50
to 90 percent survival in the cytotoxicity tests. A negative control
consisting of assays performed on untreated cells was included. A
positive control with 3-methyl cholanthrene (MCA), used at level of 5
µg/ml for each assay, gave a marked increase of the average
number/flask of foci of transformed cells. Therefore, nitrofen was
considered to be inactive in the BALB/3T3 in vitro transformation
assay (Myhr & Brusick 1979).
Groups of three Swiss Webster albino mice were given intraperitoneally
two doses, separated by 24 h, of nitrofen (purity not specified) at
levels of 0, 500 or 1 000 mg/kg b.w. Six hours after the last dose,
three bone marrow smears from each mouse were prepared and about 2 000
polychromatic erythrocytes per mouse were analysed for the presence of
micronuclei. For both dose levels tested, the incidence of micronuclei
was not statistically significantly different from the control. A
positive control of cyclophosphamide gave the expected increase of the
incidence of micronuclei (Paik & Lee 1977a).
Groups of 5-10 male Swiss mice were given by gavage two doses of
nitrofen technical (96.2 percent pure) at levels of 0, 0.25, 1.00 and
1.25 ml/kg, spaced 24 h apart. The animals were sacrificed 6 h after
the second dose and bone marrow smears were prepared. The incidence of
polychromatic erythrocytes carrying "micronuclei" in the treated
groups was not significantly different from the control group (Siou
1978).
Groups of 24 male Charles-River CD-1 mice were given by gavage a
single oral dose of nitrofen technical (95.7 percent pure) at levels
of 0, 0.39, 0.79 and 1.58 g/kg, representing 1/8, 1/4 and 1/2 of the
acute oral LD50. Additional groups of 8 male mice were given once
daily five doses of nitrofen at the same levels. A positive control
group received triethylamine (TEM) 0.3 mg/kg i.p. Bone marrow slides
were prepared from 8 animals/group sacrificed at 6, 24 and 48 h after
the acute dose and 6 h after the last sub-acute dose and TEM dose. All
animals received colchicine at 2 mg/kg i.m. two hours prior to
sacrifice. Fifty metaphase cells per animal were scored when possible.
Owing to poor survival, the high dose subacute group was sacrificed on
day 4. No statistically significant increase in chromosomal
aberrations was observed in treated animals compared with controls,
both in the acute and subacute regimen (Reustle & Scribner 1980).
Nitrofen technical (95.7 percent pure) was administered in m-xylene
(99 percent) by dermal application to groups of eight male Charles
River CD rats at doses of 0, 0.05, 0.125 and 0.50 g/kg, to assess the
potential of nitrofen to induce chromosomal aberrations in mammalian
bone marrow cells. A positive control group received a single 0.3
mg/kg i.p. dose of triethylenemelamine. The doses were administered
according to both a single and repeated (daily × five days) regimen.
Animals were killed and bone marrow slides prepared at approximately
6, 24 and 48 h after the single dose, 24 h after the positive control
dose and 6 h after the last multiple dose. All animals received
colchicine (1 mg/kg, i.p.) three hours prior to being killed.
Metaphase cells (50 per animal) were examined for chromosomal
aberrations. Nitrofen at 0.5 g/kg and below did not induce a
significant increase in chromosomal aberrations in bone marrow cells
at either 6, 24 or 48 h after single or repeated exposure. Chromosomal
aberrations were consistently elevated at 24 h after treatment with
triethylmelamine (McLeod & McCarthy 1982).
Special Studies on Mutagenicity of Aminonitrofen
Aminonitrofen (99+ percent pure), a metabolite of nitrofen, was tested
in concentrations of 0.1-1 000 µg/plate with S. typhimurium strains
TA1535, TA1537, TA98 and TA100 with and without a microsomal enzyme
preparation. Statistically significant increases of revertants/plate
were observed with strains TA1535, TA98 and TA100 using a microsomal
enzyme preparation and a dose-response relationship was exhibited.
Positive controls were 2-anthramine, 2-aminofluorene and
2-acetamidofluorene (O'Neill & Scribner 1979).
Special Studies on Mutagenicity of 4,4'-Dichloroazobenzene
4,4'-Dichloroazobenzene (DCAB) (99+ percent technical), a low level
impurity in technical nitrofen, was tested with the S. typhimurium
strains TA1535, TA1537, TA98 and TA100 at concentrations of 1.0-1 000
µg/plate, with and without microsomal activation. Saline buffer
controls were used with each strain. Statistically significant
increases of revertants/plate were observed with strain TA100 at
10-1 000 µg/plate with metabolic activation. DCAB gave a greater
response with strain TA98 than with TA100. Positive metabolic
activation; and 2-anthramine (10 µg/plate), positive with TA1535,
TA1537 and TA100 with metabolic activation; and 2-acetamidofluorene
(50 µg/plate), positive with TA98 with metabolic activation (O'Neill &
Lohse 1980).
Special Studies on Carcinogenicity
Mouse
Groups of 50 male and 50 female young B6C3F1 mice (age not specified)
were fed diets containing technical nitrofen (stated to be >80
percent pure; impurities unspecified) dissolved in maize oil for 78
weeks at levels of 1 775-2 500 mg/kg (low-dose) or 3 550-5 000 mg/kg
(high-dose), to give time-weighted average concentrations of 2 348 and
4 696 mg/kg in the diet. A group of 20 male and 20 female controls
received the basal diet containing 2 percent maize oil. All mice were
observed for a further 12 weeks after treatment. Weight gain was
depressed in both groups of treated females and in the males exposed
to the high dose. Beginning at week 54, pronounced abdominal
distension was displayed by an increasing number of treated mice. By
the end of the study, only 10 percent of the control male mice, 34
percent of the high-dose group and 54 percent of the low-dose group
were still alive; and, of the female mice, 62 percent were still alive
in the high-dose group, 54 percent in the low-dose group and 85
percent of the controls. For neither sex could a positive association
be established between dosage of nitrofen and mortality. A high
incidence of hepatocellular carcinomas was found in exposed male and
female mice: in 4/20 control males, 36/49 low-dose males and 46/48
high-dose males, in none of the control female mice (both matched and
pooled controls), in 36/41 low-dose females and in 43/44 high-dose
females. A few of these tumours metastasized to other sites. The
difference in incidence between control and exposed male and female
mice was statistically significant at both dose levels.
Haemangiosarcomas of the spleen were found in 2/47 of high-dose males
and haemangiosarcomas of the liver in 1/49 low-dose males and 2/48
high-dose males. In female mice, haemangiosarcomas were seen in the
spleen in 1/18 controls, in the liver in 4/44 high-dose animals and in
the abdominal cavity in 1/43 of the high-dose group.
The incidence of haemangiosarcomas in the high-dose male mice, but not
females or low-dose males, was statistically higher than controls
(National Cancer Institute 1978).
Groups of 50 male and 50 female six-week-old B6C3F1 mice were fed
diets containing 3 000 or 6 000 mg/kg technical-grade nitrofen (purity
not specified) for 78 weeks. The 20 male and 20 female controls
received the basal diet. All animals received acidified (pH 2.5) water
ad libitum. After exposure to nitrofen, animals were observed for a
further 13 weeks. Consistent dose-related depressions in mean body
weight were noted in male and female mice, reaching about 25 percent
in high-dose males by the end of the study. At that time, 40/50 of the
high-dose males, 48/50 of the low-dose males and 19/20 of the control
males were still alive; of the females, 48/50 of the high-dose and
43/50 of the low-dose group and 12/20 off the controls survived until
the end of the study. Although a variety of tumours were noted, only
the incidence of liver tumours seemed to be related to nitrofen
exposure. Hepatocellular adenomas were seen in 1/20 control males,
18/49 low-dose males and 20/48 high-dose males, and hepatocellular
carcinomas in 0/20 controls, 13/49 low-dose males and 20/48 of the
high-dose group. In addition, none of the controls, but 3/49 of the
low-dose group and 4/48 of the high-dose group had hepatoblastoma, a
very rare tumour in this strain of mice. Of the females, 0/18
controls, 9/48 low-dose and 17/5 high-dose mice had hepatocellular
adenomas; hepatocellular carcinomas were observed in 0/18 control,
5/48 low-dose and 13/50 high-dose female mice. In addition, a
hepatoblastoma was found in 1/48 females in the low-dose group.
For both the low- and high-dose groups there was a statistically
significant increase from the control group in the incidences of
hepatocellular adenomas and hepatocellular carcinomas (National Cancer
Institute 1979).
Rat
Groups of 50 male and 50 female Osborne-Mendel rats (age not
specified) were fed diets containing technical grade nitrofen (stated
to be >80 percent pure; impurities unspecified) in 2 percent maize
oil for 78 weeks. The dietary concentration for males was 2 300 mg/kg
(low-dose) or 2 300-4 600 (high-dose), to give a time-weighted average
of 3 627 mg/kg. The dietary concentrations for female rate were 1 300
mg/kg (low-dose) or 2 600 mg/kg (high-dose). The low-dose males, the
control animals and the high- and low-dose females were observed for
an additional 32 weeks, during which they were maintained on a basal
laboratory diet plus maize oil; the high-dose males were observed for
an additional five weeks after dosing was stopped. Groups of 20 male
and 20 female controls received the basal diet plus 2 percent maize
oil. A dose-related decrease in body weight was noted; by 75 weeks
after onset of dosing, the high-dose females weighed roughly one-third
less than the controls. Fifty percent of the high-dose males were dead
by week 45 and only 15 survived to week 83; 60 percent of the low-dose
and 45 percent of the control group survived until the end of the
study. Of the females, 58 percent at the high-dose, 74 percent at the
low-dose and 80 percent of the controls survivied until the end of the
study. Adenocarcinomas of the exocrine pancreas were seen in 2/50
females at the low dose and in 7/50 at the high dose. All tumours
showed local invasion and had metastasized to the lungs. No such
tumour was seen in the 20 matched controls or in the 110 pooled
controls. The difference was statistically significant for high-dose
animals. No other tumour showed a statistically significant difference
in incidence between control and exposed rats. Poor survival precluded
the evaluation of carcinogenicity in male rats (National Cancer
Institute 1978).
Groups of 50 male and 50 female six-week-old Fischer 344 rate were fed
diets containing 3 000 or 6 000 mg/kg technical-grade nitrofen (purity
not specified) for 78 weeks. A group of 20 male and 20 female rats
were given the control diet. All animals received acidified water (pH
2.5). The animals were followed for an additional 26 weeks, when all
surviving rats were killed. A dose-related depression in mean body
weight was noted in animals of both sexes, reaching 15 percent for
those given the high dose for 60 to 75 weeks. Of the male rats, 45/50
given the high dose, 42/50 given the low dose and 17/20 of the
controls survived until termination of the study; of the females,
38/50, 42/50 and 17/20, respectively, survived. No statistically
significant difference in the incidence of tumours was observed
between the exposed and control groups (National Cancer Institute
1979). However, subsequent detailed histological and morphological
studies distinguished the nitrofen-induced neoplasm from those
occurring naturally in the controls (Hoover et al. 1980; Stinson
et al. 1981).
Special Studies on Enzyme Induction
Groups of B6C3F1 mice (six animals per sex) were fed diets containing
nitrofen (technical, 92 percent pure) at levels of 0, 10, 100 or 1000
ppm or two weeks prior to determination of individual liver to body
weight (L:BW) rations and in vitro hepatic p-nitroanisole (pNA)
O-demethylase activity. The dose levels were chosen after acute
studies (three-day gavage dosing), using CD-1 mice, showed a
sex-dependent minimum effect level for hepatic pNA-O-demethylase
activity between 10 and 50 mg/kg/day. The 100 and 1000 ppm dietary
nitrofen levels resulted in elevated liver mixed function oxidase
(MFO) activity in both sexes, with females showing a greater response
(170 percent and 236 percent of control, respectively, for the two
diet levels). L:BW ratios were increased 20 percent and 30 percent in
males and females, respectively, but only at the 1000 ppm level. No
significant changes were observed in either sex fed the 10 ppm
nitrofen diet. The no-effect level found for MFO induction was much
lower than that for oncogenicity (2 348 ppm in the National Cancer
Institute 1978 oncogenicity study). (Deckert & Steigerwalt 1979c)
Special Studies on Dermal Irritation
No evidence of irritation developed when both technical grade (95
percent pure) and pure nitrofen were applied either as a 10 percent
solution in maize oil or as moistened powder to the skin of albino
rabbits (Ambrose et al. 1971).
Special Studies on Cutaneous Sensitization
There was no visible evidence of erythema, swelling, or discolouration
of the test sites after each sensitizing injection or after the
challenging dose of aqueous suspension of technical grade (95 percent)
nitrofen. Under the conditions of the test, these finding indicate
that nitrofen is not a sensitizing agent (Ambrose et al. 1971).
Special Studies on Cataractogenic Effect
Groups of 30 two-week-old Peking ducklings were fed diets containing
nitrofen at levels of 0, 0.05 and 0.1 percent for 13 weeks. Distinct
growth depression occurred at the 0.1 percent nitrofen dose level and
slight retardation at 0.05 percent. The mortality rate in the highest
dose group was distinctly increased. No clinical or histologic signs
of cataract were observed in any of the treated or control animals,
nor were other eye abnormalities found (Ensel & Seinen 1970).
Special Studies on Methaemoglobin Formation by Aminonitrofen
Methaemoglobin formation in vitro by nitroso and amino derivatives
of nitrofen and other chlorinated biphenyl ethers was investigated in
suspensions of human erythrocytes. Ability to induce methaemoglobin
formation among the nitroso and amino derivatives decreased with
increasing chlorine substitution of the biphenyl ether. However, amino
derivatives from no methaemoglobin in suspensions of erythrocytes
alone, but do so after activation by liver homogenate. Methaemoglobin
formation after intraperitoneal and oral administration to male Wistar
rats of chlorinated p-aminobiphenyl ether at levels of 0.77 mg/kg b.w.
gave almost the same result (ca. 40 percent methaemoglobin within
60-120 min.) after either route was used (Miyauchi et al. 1981).
Special Studies on the Stability of the Metabolite Azoxynitrofen
Azoxynitrofen (a possible vegetable metabolite of nitrofen) was added
to simulated gastric fluid and simulated intestinal fluid at level of
7 ppm. There is a slight degradation of azoxynitrofen in gastric fluid
during 24 h. The intestinal fluid completely decomposes azoxynitrofen
within 24 h. After 14 days, there is evidence of azoxynitrofen in
either digestive fluid, and it appears that aminonitrofen is a major
product of the decomposition (Warso 1972).
Acute Toxicity
The acute toxicity of nitrofen in animals is summarized in Table 1.
Most deaths occurred two to eight days after dosing and were preceded
by progressive depression. There was no evidence of diarrhoea.
Postmortem findings were negative in rats that died and in survivors
autopsied at 14 days (Ambrose et al. 1971).
Short-Term Studies
Rat
Groups of albino Wistar rats (10 males and 10 females per group) were
fed diets containing technical grade nitrofen (95 percent pure) at
levels of 0, 100, 500, 2 500, 12 500 and 50 000 ppm for 13 weeks.
Rats on the 50 000 ppm diet failed to survive beyond the first week
and survival of rats on the 12 500 ppm diet was adversely affected.
Growth was depressed in both sexes at 12 500 ppm and in males at 2
500. In rats on the lower dietary concentrations of nitrofen, no
adverse effects were noted in growth, food consumption and mortality.
Haematologic values appeared to be within normal range and urinary
tests for reducing substances and protein showed no marked differences
from the controls. Significantly higher liver-to-body weight ratios
were found in rats at all dietary levels of nitrofen, except for male
rats at 100 ppm, and appeared to be dose-related. Kidney body weight
ratios were significantly elevated for females at 12 500 ppm and for
males at 2 500 and 12 500 ppm. Testes body weight ratios were
significantly elevated for rats at 2 500 and 12 500 ppm. Lesser
effects were noted for heart and spleen. Histopathologic findings
related to treatment appeared to be confined to rats receiving diets
containing 12 500 and 50 00 ppm nitrofen. The principal findings
consisted of a hepatitis characterized by oedema, peripheral
localization of glycogen granules, swelling of the cytoplasm and liver
nuclei with prominent nucleoli (Ambrose et al. 1971). (Full
experimental data were not available.)
Rabbit
Groups of five male and five female rabbits received dermal
applications of nitrofen (as a water-diluted emulsifiable formulation)
at levels of 0, 250 or 1 250 mg/kg b.w., 7 h/day, 5 days/week for
three weeks. Additional groups received applications on abraded skin.
There was no mortality and no evidence of any skin irritation. No
histological changes were noted in the skin of exposed animals. There
was no evidence of subacute systemic damage to liver, kidney, lung or
spleen on histological examination (Brown 1964).
Table 1 Acute Toxicity of Nitrofen in Animals
Animal Sex Route Vehicle LD50 a.i. Reference
g/kg b.w. purity
Rat M oral maize oil 2.63 95% Ambrose et al. 1971
M oral maize oil 3.58 100% Ambrose et al. 1971
M oral peanut oil 2.84 99% Kimbrough et al. 1974
F oral peanat oil 2.40 99% Kimbrough et al. 1974
oral undiluted 5.00 Swann 1973
formulation (as 25%
formulation)
M+F dermal xylene 5.00 99% Kimbrough et al. 1974
inhalation undiluted 205 mg/l Swann 1973
(1h exper.) formulation (as 25%
formulation)
M+F inhalation undiluted >271 mg/cu.m. Brown 1965
(1h exper.) formation (as a.i.)
Rabbit dermal undiluted >2.00 Swann 1973
(24 h) fomulation (as 25%
formulation)
Dog
Groups of six-month-old beagles (two males and two females/group) were
fed diets containing technical grade (95 percent pure) nitrofen at
levels of 0, 20, 200 and 2 000 ppm for two years. The dogs on the
various dietary levels of nitrofen survived the two-year feeding
period. No toxic signs attributable to nitrofen were seen at any time.
The general health, appearance, behaviourial pattern, body weight gain
and cumulative food consumption were not different than those of the
respective controls. Haematologic values and urinary tests for
reducing substances and protein showed no effect of treatment at any
of the test periods. BSP, SGOT, SAP and BUN tests made during the
month 24 showed no adverse trends. Significantly higher liver-to-body
weight ratios appeared only in dogs on 2 000 ppm nitrofen.
Histopathologic examination of major organs revealed no abnormalities
or lesions resulting from the ingestion of nitrofen for two years, in
kind or incidence, that were not found in control dogs. In general,
only the 20 and 200 ppm diet levels of nitrofen exhibited no adverse
effects (Ambrose et al. 1971).
Long-Term Studies
Rat
Groups of weanling Wistar rats (25 males and 25 females/group)
received diets containing technical grade (95 percents pure) nitrofen
at levels of 0, 1, 10, 100 and 1 000 ppm for 97 weeks. The study was
terminated at 97 weeks, owing to poor survival (50 percent) in all
groups, including the controls, beyond week 65. Statistically
significant decreases in mean body weight were observed in the males
at 100 and 1000 ppm after the week 52 and in the females at the same
dose levels until week 26.
Food consumption, haematological and urinalysis parameters were not
affected. Significantly higher kidney/body weight and liver/body
weight ratios in male rats on 1 000 ppm were notable and appeared to
be attributable to nitrofen. Histopathologic findings revealed no
lesions in kind or incidence that were not found in the respective
controls. Overall, the 10 ppm and below diet level of nitrofen was
reported to cause no adverse effects (Ambrose et al. 1971). (Lack of
individual animal data precludes further interpretation of this
study.)
COMMENTS
Nitrofen has low acute oral toxicity. Following oral administration,
nitrofen is partially absorbed and rapidly excreted. A small
proportion of the administered dose is distributed to all tissues and
organs. Nitrofen and its metabolites are found primarily in faeces
(accounting for about 75 percent of the administered dose), while
urine contains metabolites accounting for about 15 percent of the
dose. The metabolism of nitrofen involves reduction, hydroxylation and
conjugation.
Two reproduction studies were presented. Because of inadequacies in
the reports the studies were not considered.
Oral exposures to nitrofen during the period of organogenesis produced
neonatal mortality, decreases in the time of survival to weaning, and
a series of abnormalities in rodent offspring. In a mouse
teratology/post-natal study, the lowest dose tested (6.25 mg/kg b.w.)
produced increased incidence of lethal defects at birth and resulted
in decreased lung and seminal vesicle weights at maturity. In a rat
teratology study, diaphragmatic hernias were observed at and above
1.39 mg/kg b.w., but full details were not provided.
Nitrofen in some cases was found mutagenic in the
Salmonella/microsome assay but it did not display any mutagenic
potential in several mammalian systems either in vitro or in vivo.
Two carcinogenicity studies on B6C3F1 mice indicated that nitrofen is
a liver carcinogen, causing hepatocellular carcinomas and
hepatocellular adenomas in both sexes, and haemangiosarcomas in male
mice. In addition, the compound is carcinogenic to female
Osborne-Mendel rats, causing adenocarcinomas of the pancreas. Nitrofen
was not found to be carcinogenic in Fischer 344 rats. Detailed
histological examinations suggest that naturally occurring and
nitrofen-induced liver tumours in mice are morphologically different.
Both studies were of short duration. In the study on Osborne-Mendel
rats, poor survival precluded the evaluation of carcinogenicity in
males.
In a long-term rat-feeding study, a NOEL of 10 ppm was observed.
However, the duration of the study (97 weeks), the poor survival in
all groups (50 percent beyond week 65), and the fact that the study
was not available in extenso, precluded its full evaluation.
An acceptable daily intake could not be estimated, owing to the
evidence of carcinogenicity, the lack of a NOEL for the teratology and
post-natal effects and the inadequacies of several studies, including
reproduction and long-term studies.
REFERENCES - TOXICOLOGY
Adler, I.L. et al. A material balance study in rats using
1970a 14C- TOK labelled in the nitrophenyl
ring. Rohm and Haas Report No. 23-24.
(Unpublished)
Adler, I.L. et al. A material balance study in rats using
1970b 14C - TOK labelled in the dichlorophenyl
ring. Rohm and Haas Report No. 23-25.
(Unpublished)
Ambrose, A.M., Larson, Toxicologic studies on
P.S., Borzelleca, J.F., 2,4-dichlorophenyl-p-nitrophenyl
Blackwell Smith, R. & ether (TOK). Toxicol. Appl. Pharmacol.,
Hennigar, G.R. 19:263-275.
Brown, J.R. A study on the acute inhalation toxicity
1965 of TOK EC-25. Report from the University
of Toronto, Canada submitted to WHO by
Rohm and Haas. (Unpublished)
Brown, J.R. & Mastromateo, E. Subacute percutaneous toxicity of TOK
1964 E-25 in the rabbit. Report from the
University of Toronto, Canada,
submitted to WHO by Rohm and Haas.
(Unpublished)
Byeon, W.H., Hyun, H.H. & Mutagenicity of pesticides in the
Lee, S.Y. Salmonella/microsome system. Korean J.
1976 Microbiol., 14:128-134.
Chernoff, N., Kavlock, R.J., Preliminary report on the perinatal
Gray, L.E. toxicity of nitrofen administered to
1980 mice. Rohm and Haas Report.
(Unpublished)
Costlow, R.D. & Manson, J.M. The heart and diaphragm: target organs
1981 in the neonatal death induced by
nitrofen (2,4-dichlorophenyl-p-
nitrophenyl ether). Toxicology,
20:209-227.
Costlow, R.D., The effect on rat pups when nitrofen
Hirsekorn, J.M., (4-(2,4-dichlorophenoxy) nitrobenzene)
Stiratelli, R.G., was applied dermally to the dam
O'Hara G.P., Black, D.L., during organogenesis. Toxicology
Kane, W.K., Burke, S.S., (in press). Submitted to WHO by
Smith, J.M. & Hayes, A.W. Rohm and Haas.
Decker, F.W. & Percutaneous absorption of 14C-TOK in
Steigerwalt, R.B. rats. Rohm and Haas Report TD77P-59.
1979a (Unpublished)
Deckert, F.W. & Disposition of 14C-TOK after
Steigerwalt, R.B. percutaneous application to a rat.
1979b Rohm and Haas Report TD78P-53.
(Unpublished)
Deckert, F.W. & Fourteen day dietary TOK enzyme
Steigerwalt, R.B. induction study in mice. Rohm
1979c and Haas Report TD78P-58.
(Unpublished)
Ensel, A.B. & Seinen, W. Investigation of TOK on a possible
1970 cataractogenic effect in ducklings.
Central Institut Voor Voedingsonderzock.
Report no. R-3306 submitted to WHO by
Rohm and Haas. (Unpublished)
Gray, L.E., Kavlock, R.J., Prenatal exposure to the herbicide
Chernoff, N., Ferrell, J., 2,4-dichlorophenyl-p-nitrophenyl
McLamb, J. & Ostby, J. ether destroys the rodent Harderian
1982a gland. Science, 215:293-294.
Gray, L.E., Kavlock, R.J., The effects of the prenatal exposure
Chernoff, N. & Ferrell, J. to the herbicide TOK on the postnatal
1982b development of the Harderian gland of
the mouse, rat and hamster. Presented
at Soc. Teratology Meeting 1982.
Submitted to WHO by Rohm and Haas.
Gray, L.E., Kavlock, R.J., Postnatal development alterations
Chernoff,N., Ostby, J. & following prenatal exposure to the
Ferrell, J. herbicide 2,4-dichlorophenyl-p-
1983 nitrophenyl ether. A dose response
evaluation in the mouse. Toxicol.
Appl. Pharmacol. 67:1-14.
Guerzoni, M.E. & Mutagenic activity of pesticides.
Del Cupolo, L. Riv. Sci. Technol. Alimenti Nutr.
1976 Um., 6:161-168.
Hirsekorn, J.M. & Kane, W.W. TOK E-25 percutaneous range-findings
1981 teratology study with postpartum
evaluation. Rohm and Haas Report No.
81R-63. (Unpublished)
Hoover, K.L., Ward, J.M. & Histopathological differences between
Stinson, S.F. nitrofen-induced and naturally occurring
1980 hepatocellular carcinomas in the B6C3F1
mouse. J. Nat. Cancer Inst., 65:937-948.
Kavlock, R.J., Chernoff, N., An analysis of fetotoxicity using
Rogers, E., Whitehouse, D., biochemical end-points of organ
Carver, B., Gray, J. & differentiation. Teratology,
Robinson, K. 26:183-194.
1982
Kimbrough, R.D., 2,4-dichlorophenyl-p-nitrophenyl ether
Gaines, T.B. & Linder, R.B. (TOK). Effect on the lung maturation of
1974 rat fetus. Arch. Environ. Health,
28:316-320
McLeod, P.L. & McCarthy, K.L. TOK dermal cytogenetic study in rats.
1982 Rohm and Haas Report No. 81R-268.
(Unpublished)
Miyauchi, M., Koizumi, M. & Studies on the toxicity of chlorinated
Uematsu, T. p-nitro-biphenyl ether.
1981 I - Methemoglobin formation in vitro
and in vivo induced by nitroso and
amino derivatives of chlorinated
biphenyl ether. Biochem. Pharmacol.,
30:3341-3346.
Myhr, B.C. & Brusik, G. Evaluation of 78-232 in the in vitro
1979 transformation of BALB/3T3 cells assay,
Litton Bionetics Report No. 20992
submitted to WHO by Rohm and Haas.
(Unpublished)
National Cancer Institute. Bioassay of nitrofen for possible
1978 carcinogenicity. Carcinogenesis
Technical Report Series No. 26,
DHEW Publ. No. (NIH) 78-826.
National Cancer Institute. Bioassay of nitrofen for possible
1979 carcinogenicity. Carcinogenesis
Technical Report Series No. 184,
DHEW Publ. No. (NIH) 79-1740,1.
O'Hara, G.P., Can, P.K., The effect of nitrofen
Harris, J.C., Burke, S.S., 4-(2,4-dichlorophenoxy) nitrobenzene
Smith, J.M. & Hayes, A.W. on the reproductive performance
1983 of male rats.Rohm and Haas Report
(Unpublished)
O'Neill, P.J. & Lohse, E. 4,4-DCAB microbial mutagen test. Rohm
1980 and Haas Report No. 80R-4. (Unpublished)
O'Neill, P.J. & TOK: Microbial mutagen tests. Rohm and
Scribner, H.E. Haas Report No. TD79M-307. (Unpublished)
Paik, S.G. & Lee, S.Y. Genetic effects of pesticides in the
1977a mammalian cells. I. Induction of
micronucleous. Kor. J. Zool.,
20:19-28.
Paik, S.G. & Lee, S.Y. Genetic effects of pesticides in the
1977b mammalian cells. II. Mutagenesis in
L5178Y cells and DNA repair induction.
Kor. J. Zool., 20:159-168.
Reustle, J.A. & TOK cytogenetic study in mice. Rohm and
Scribner, H.E. Haas Report No. 79R-173. (Unpublished)
1980
Roser, R.L., Adler, I.L. & A study of the metabolism of 14-C TOK in
Allen, S.S. rats. Rohm and Haas Report No. 23-33.
1971 (Unpublished)
Siou, G. Study of the potential mutagenic
1978 activity of TOK by the Howell-Jolly
body technique. Cabinet d'Etudes et
de Recherches en Toxicologie
Industrielle, Report No. MBL-2199
submitted to WHO by Rohm and Haas.
(Unpublished)
Siou, G. Study of the effect of TOK on the
1979a prenatal and postnatal development
of the rabbit. Cabinet d'Etudes et
de Recherches en Toxicologie
Industrielle, Report No. MBL-2212
and Addendum submitted to WHO by
Rohm and Haas. (Unpublished)
Siou, G. Study of the effect of TOK on the
1979b postnatal development of the rat.
Cabinet d'Etudes et de Recherches
en Toxicologie Industrielle Report
No. MBL-2248 submitted to WHO by
Rohm and Haas. (Unpublished)
Steigerwalt, R.B., TOK comparative metabolism study.
Godfrey, W.J. & Deckert, Rohm and Haas Report No. 79R-169.
F.W. 1980a (Unpublished)
Steigerwalt, R.B., Lisk, D.C. Range-finding study on 14C-TOK. Dermal
& Deckert, F.W. absorption by rat and human skin in
vitro. Rohm and Haas Report No.
80R-110. (Unpublished)
Steigerwalt, R.B., TOK: disposition after percutaneous
Udinsky, J.R. & Deckert, applications to pregnant rats. Rohm
F.W. 1982 and Haas Report No. 81R-65.
(Unpublished)
Stinson, S.F., Hoover, K.L. Quantitation of differences between
& Ward, J.M. spontaneous and induced liver tumors in
1981 mice with an automated image analyzer.
Cancer Letters, 14:143-150.
Stone, L.C. & Manson, J.M. Effects of the herbicide
1981 2,4-dichlorophenyl-p-nitrophenyl
ether (nitrofen) on fetal lung
development in rats. Toxicology,
20:195-207.
Swann, H.E. Acute toxicity report on Rohm and Haas
1973 TOK E-25, dark liquid. Food Drug
Res. Labs. Report No. 1-3784-851
submitted to WHO by Rohm and Haas.
(Unpublished)
Vogin, E.E. Effects of FW925 on the fetal
1971 development in rats. Food and Drug
Research Laboratories Report,
August 10 submitted to WHO by
Rohm and Haas. (Unpublished)
Warso, J.P. Degradation of azoxynitrofen in
1972 simulated digestion fluids. Rohm
and Haas Report No. 23-72-27.
(Unpublished)
Weatherholtz, W.M., Teratogenicity study in rats, TOK
Kapp, R.W. & E-25, TOK E-25 solvent control.
Greenspun, K.S. Report from Hazleton Laboratories,
1979 America Inc. submitted to WHO by
Rohm and Haas. (Unpublished)
RESIDUES
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Nitrofen is a selective pre- and early post-emergence herbicide for
the control of annual grasses and various broad-leaved weeds in cereal
grains and vegetables.
Nitrofen is used in France and some other countries under labels that
prohibit women from handling the product. It is primarily used to
control backgrass (Alopecurus agrestis), Veronica hederaefolia,
Galium aparine, Viola tricolor and other weeds that in winter
wheat and other cereal grains are not adequately controlled by other
commercially available herbicides.
The effective use rate is generally between 1.5 and 2 kg/ha applied to
the soil surface; activity is lost on soil incorporation. Adequate
soil moisture is necessary for efficacy, but excessive moisture may
result in crop injury.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Residue data have been obtained in several countries where nitrofen
was applied to various crops at or about the recommended rates. Two to
four samples were taken at random from the fields and were transported
to the laboratory within two days. The preparation of the sample and
the portion of sample to be analysed were in agreement with the
recommended Codex procedure with two exceptions: only the cloves of
garlic were sent to the laboratory after removing the outer leaves and
all the samples were washed before chopping and deep-freezing.
The analytical results from the United States were obtained by a
method (Adler and Wargo 1975) that measures the parent compound and
the majority of metabolites in the form of the derivative of amine
moiety. The data from other countries indicate the residue level of
the parent compound only. The results of supervised trials are
summarized in Table 1.
FATE OF RESIDUES
In general, nitrofen undergoes metabolic degradation in all systems
(animals, plants, soil and Water) studied. Metabolic pathways include
reduction of the nitro group, conjugation of the resulting amino
group, hydroxylation of the dichlorophenyl ring and formation of
naturally occurring materials, as well as total conversion to CO2.
The structural formula and the occurrence of the important identified
metabolites are shown in Table 2.
In Animals
Cattle milk, urine and faeces collected during the dosing period from
a cow fed a daily ration of 26.2 kg containing 5 mg/kg purified
nitrofen for four days did not contain detectable levels of nitrofen
or aminonitrofen (sensitivity of the method was approximately 0.3
mg/kg). In vitro studies demonstrated that nitrofen is rapidly
reduced to aminonitrofen in fresh rumen fluid (T1/2 is less than or
equal to 10 min). Aminonitrofen was stable in the rumen fluid for 24 h
(Gutemann & List 1967).
Similarly, only trace levels of radioactivity (0.02 to 0.07 mg/kg,
calculated as nitrofen) were found in samples of urine, faeces and
milk from a cow dosed at 5 mg/kg with radiolabelled nitrofen. The
levels of total radioactivity were 1.4 and 1.7 mg/kg in the faeces and
urine, respectively, and 0.14 and 0.16 mg/kg in two samples of milk. A
fat sample contained 1.06 mg/kg 14C, of which 0.25 mg/kg was parent
compound (Roser & Adler 1971).
Table 1 Residues of Nitrofen Resulting from Supervised Trials
Crop and variety Country Application Proharvest Residue (mg/kg)
rate interval Mean
(kg/a.i./ha) No. (days)
Broccoli (head) United States1 3.7 1 74 <0.01 <0.015
Futura 4.5 2 63 <0.01 <0.01
Gem 4.5 1 85 <0.01 <0.01
Green Duke 4.75 2 63 <0.01 <0.01
4.75 2 69 <0.01 <0.01
5.0 1 70 <0.01 <0.01
5.3 2 70 <0.01 <0.01
5.4 1 77 <0.01 <0,01
Cabbage (head) United States1
Head Start 4.5 1 69 <0.01 <0.01
4.5 1 84 <0.01 <0.01
Green 4.5 1 89 <0.01 <0.01
6.7 1 76 <0.01 <0.01
6.7 1 90 <0.01 <0.016
Cauliflower (head) United States1
Snoball 4.5 1 90 <0.01 <0.01
4.5 1 93 <0.01 <0.01
4.6 1 88 <0.01 <0.016
5.6 1 83 <0.01 <0.01
6.7 1 59 <0.01 <0.01
6.7 1 107 <0.01 <0.016
Celery (stalk) United States1
5270 RIMP 4.5 1 109 <0.01 <0.01
5.4 1 127 <0.01 <0.01
5270 5.6 1 52 <0.01 <0.01
5270R 5.6 1 165 <0.01 <0.01
Table 1 (continued)
Crop and variety Country Application Proharvest Residue (mg/kg)
rate interval Mean
(kg/a.i./ha) No. (days)
Garlic (bulb) United States1
Cal Late 5.6 1 234 <0.01 <0.01
5.6 1 250 <0.01 <0.01
6.7 1 229 <0.01 <0.01
7.8 1 241 <0.01 <0.01
Rape (grain) Denmark2 1.2 1 100 - <0.01
1.2 1 101 - <0.01
1.2 1 105 - <0.01
1.2 1 100 - <0.01
1.2 1 101 - <0.01
1.2 1 105 - <0.01
Rioe (grain) United States1
Early Rose 2.2 1 145 <0.01 <0.01
3.4 1 145 <0.01 <0.01
3.4 1 160 <0.01 <0.01
4.5 1 160 <0.01 <0.01
6.7 1 160 <0.01 <0.01
9.0 1 166 <0.01 <0.01
Rice (straw)
Early Rose 2.2 1 145 <0.01 <0.01
3.4 1 145 <0.01 <0.01
4.5 1 166 <0.01 <0.01
9.0 1 166 <0.01 <0.01
Table 1 (continued)
Crop and variety Country Application Proharvest Residue (mg/kg)
rate interval Mean
(kg/a.i./ha) No. (days)
Wheat (grain) France3
Chamlein
4.0 1 239 <0.01 <0.01
4.0 1 264 <0.01 <0.01
Joss 4.5 1 266 <0.01 <0.01
Hardy 8.0 1 236 <0.01 <0.01
Chamlein 8.0 1 264 <0.01 <0.01
Hardy 8.0 1 265 <0.01 <0,01
Wheat (grain) Federal
Republic
Joss of 1.6 1 248 - <0.002
Carstacht Germany4 1.6 1 280 - <0.002
Kranich 1.6 1 284 - <0.002
Caribo 1.6 1 286 - <0.002
2.0 1 270 - <0.002
Benno 2.0 1 279 - <0.002
Carito 2.0 1 286 - <0.002
Hyslop United States1 1.7 1 294 <0.01 <0.01
Druchamp 2.2 1 163 0.00-0.02 0.01
Yamhill 2.2 1 250 <0.01 <0.01
2.2 1 267 <0.01 <0.01
2.2 1 269 <0.01 <0.01
2.2 1 272 0.01-0.09 0.04
Nugaines 2.2 1 272 0.02-0.03 0.02
Hyslop 2.2 1 281 <0.01 <0.01
Yamhill 2.2 1 284 <0.01 <0.01
2.2 1 289 <0.01 <0.01
Table 1 (continued)
Crop and variety Country Application Proharvest Residue (mg/kg)
rate interval Mean
(kg/a.i./ha) No. (days)
Cajame 3.4 1 146 <0.01-0.01 <0.01
Tehame 3.4 1 151 <0.01 <0.01
Hyslop 3.4 1 294 <0.01 <0.01
Cajame 4.5 1 146 <0.01 <0.01
Tehame 4.5 1 151 <0.01-0.01 <0.01
Druchamp United States1 4.5 1 163 <0.01-0.06 0.03
Yamhill 4.5 1 250 <0.01-0.01 <0.01
4.5 1 267 <0.01 <0.01
4.5 1 269 <0.01 <0.01
4.5 1 272 0.01-0.12 0.06
Nugaines 4.5 1 272 0.02-0.06 0.04
Hylop 4.5 1 281 <0.01 <0.01
Yamhill 4.5 1 284 <0.01 <0.01
4.5 1 289 <0.01 <0.01
Cajame 5.6 1 146 <0.01-0.02 0.01
6.7 1 146 <0.01-0.01 <0.01
Tehame 6.7 1 151 <0.01-0.01 <0.01
Yamhill 6.7 1 267 <0.01 <0.01
Wheat (straw) Federal Republic
of Germany4
Joss 1.6 1 248 - <0.002
Carstacht 1.6 1 280 - 0.020
Kranich 1.6 1 284 - 0.007
Caribo 1.6 1 286 - <0.004
2.0 1 270 - 0.004
Benno 2.0 1 279 - 0.010
Carito 2.0 1 286 - 0.005
Table 1 (continued)
Crop and variety Country Application Proharvest Residue (mg/kg)
rate interval Mean
(kg/a.i./ha) No. (days)
Hyslop United States1 1.7 1 294 <0.01 <0.01
Yamhill 2.2 1 252 <0.01 <0.01
2.2 1 267 <0.01 <0.01
2.2 1 269 <0.01 <0.01
Nugaines 2.2 1 272 0.02-0.11 0.06
Yamhill 2.2 1 273 <0.01-0.02 0.01
Druchamp 2.2 1 276 0.01-0.04 0.02
Hyslop 2.2 1 281 <0.01 <0.01
Yamhill 2.2 1 284 <0.01 <0.01
2.2 1 291 <0.01 <0.01
Hyslop 3.4 1 294 <0.01 <0.01
Yamhill 4.5 1 252 <0.01 <0.01
4.5 1 267 <0.01-0.01 <0.01
4.5 1 269 <0.01 <0.01
Nagaines 4.5 1 272 0.02-0.05 0.03
Yamhill 4.5 1 273 <0.01-0.03 0.01
Druchamp 4.5 1 276 0.01-0.02 0.01
Hyslop 4.5 1 281 <0.01 <0.01
Yamhill 4.5 1 284 <0.01 <0.01
4.5 1 291 <0.01-0.02 0.01
6.7 1 267 <0.01 <0.01
Sources:
1 Rohm and Haas 1969-1980;
2 Institute National de Recherche Agronomique 1975b;
3 Institute National de Recherche Agronomique 1975a;
4 Institute National de Recherche Agroncmique 1978;
5 Samples taken from four experimental fields;
6 Samples taken from three experimental fields.
Table 2 Nitrofen Metabolites
Metabolite Occurrence
Aminals Plants Soil
 azoxy-nitrofen 4,4'-bis
(2,4-dichlorophenoxy) azoxy benzene x x
azoxy-nitrofen 4,4'-bis
(2,4-dichlorophenoxy) azoxy benzene x x
 hydroxy nitrofen chloro,
hydroxy-4 '- nitrodiphenylether1 x
hydroxy nitrofen chloro,
hydroxy-4 '- nitrodiphenylether1 x
 aminonitrofen
2,4-dichloro-4'-aminodiphenylether x x x
aminonitrofen
2,4-dichloro-4'-aminodiphenylether x x x
 formamidonitrofen
2,4-dichloro-4'-formamido-diphenylether x x x
Table 2 (Continued)
Metabolite Occurrence
Aminals Plants Soil
formamidonitrofen
2,4-dichloro-4'-formamido-diphenylether x x x
Table 2 (Continued)
Metabolite Occurrence
Aminals Plants Soil
 acetamidonitrofen
2,4-dichloro-4'-acetamidodiphenyletherx x x x
acetamidonitrofen
2,4-dichloro-4'-acetamidodiphenyletherx x x x
 5-hydroxyaminonitrofen
2,4-dichloro, 5-hydroxy
4'-aminodiphenyl-ether x x
5-hydroxyaminonitrofen
2,4-dichloro, 5-hydroxy
4'-aminodiphenyl-ether x x
 hydroxy-acetamido-nitrofen x x x
chloro,hydroxy 4'acetamido
diphenylether1 x
hydroxy-acetamido-nitrofen x x x
chloro,hydroxy 4'acetamido
diphenylether1 x
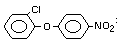 monochloronitrofen 2 chloro-4'
nitrodiphenyl ether x
Table 2 (Continued)
Metabolite Occurrence
Aminals Plants Soil
monochloronitrofen 2 chloro-4'
nitrodiphenyl ether x
Table 2 (Continued)
Metabolite Occurrence
Aminals Plants Soil
 hydroxy-propionamido-nitrofen1 x
hydroxy-propionamido-nitrofen1 x
 2,5-dichlorophenol x
1 The substituted carbon atoms have not been identified.
Cattle feeding studies were conducted twice at feeding levels of 0.05,
0.5 and 5 mg/kg administered in gelatin capsules. Combined residues in
meat and milk were determined once in four cows and a second time with
14C-labelled nitrofen. In the multiple cow feeding study, no residues
of nitrofen were detected in milk or tissues of animals fed 0.05 or
0.5 mg/kg, based on the total daily diet. Barely detectable residues,
ranging from 0.002 to 0.004 mg/kg, were found in the milk of a cow fed
5 mg/kg. At this feeding level, no residue was found in any of the
tissues except fat (0.01 mg/kg). The sensitivity of the method was
0.01 mg/kg for tissue, and 0.002 mg/kg for milk (Rohm & Haas 1967b).
In the radioactive feeding study a cow was dosed orally twice a day
with 14C-labelled nitrofen. Nitrofen residues were not found in the
milk from the lowest feeding level (0.05 mg/kg) and only 0.003 and
0.08 mg/kg were found in milk from the two higher feeding levels, i.e.
0.5 and 5 mg/kg. The residue in milk was 0.3 percent and 0.8 percent
of the applied dosage, respectively. Residues were found in edible
tissues taken from the cow dosed at the 5 mg/kg feeding level.
Calculated as nitrofen, these residues ranged from 0.05 mg/kg (trips)
and 0.24 mg/kg (muscle) to 1.15 mg/kg (fat) (Rohm & Haas 1971). The
parent compound constituted approximately 47, 32 and 23 percent of the
total residue in milk, kidney and fat, respectively.
The lowest feeding level (0.05 mg/kg) of radioactive nitrofen is
almost ten times greater than the calculated maximum level of residue
that might be eaten by livestock kept on feed containing rice bran,
rice milling fractions or any other plant by-products that had been
treated with nitrofen. Consequently, no detectable residues are likely
to be found in milk and edible tissues.
Poultry
Three groups of 15 laying hens each were fed dichlorophenyl
ring-labelled 14C nitrofen incorporated in their feed for ten weeks
at levels of 0.04, 0.12 and 0.47 mg/kg, respectively. Residues in
tissues and eggs were studied and analysed by liquid scintillation
counting. Residues in eggs reached plateaux at levels of 0.02, 0.05
and 0.17 mg/kg, respectively, by week 6 of feeding for the X, 3X and
10X test group. The residue was located almost exclusively in the egg
yolk. The excreta collected for one 24 h period in week 3 contained
98.9 and 80 percent of the total radioactivity of feeding levels of
0.04 and 0.47 mg/kg, respectively. Hens from each group were
sacrificed after weeks 3, 5 and 10 of test feeding. Selected tissues
were removed and analysed by combustion and liquid scintillation
counting. Average residue levels in tissues from the 1X test group
ranged from non-detectable (white meat) to 0.18 mg/kg (fat).
Prolonged feeding of nitrofen to laying hens at a level of 0.04 mg/kg
daily resulted in total residue of approximately 0.01-0.02 mg/kg in
eggs. The residue was found almost exclusively in the egg yolk (Adler
1972).
Sheep
A female sheep was given a single oral, 40 mg/kg dose of 14C-labelled
nitrofen. After 99 h, 76.2 percent of the 14C had been recovered in
the excreta, with 39 percent appearing in the urine and 37.2 percent
in the faeces. The residue in the blood peaked at 19 h with a plateau
from 11 to 31 h; the concentrations were between 3 and 4 mg/kg. When
killed at 100 h, the highest tissue concentrations were found in the
fat (23 to 24 mg/kg total radioactivity expressed as nitrofen); the
gut, liver, thyroid and mammary glands contained from 2 to 4 mg/kg and
14 other organs had residues of 0.5 to 2 mg/kg (expressed as
nitrofen). The highest urinary excretion of nitrofen occurred during
the 11 to 19 h post-treatment collection period; the peak in the
faeces was during the 39 to 47 h post-treatment collection. Only 2 to
5.5 percent of the radioactivity in the urine was extractable with
chloroform. Aminoitrofen and 5-hydroxynitrofen were the principal
metabolites, with the parent compound accounting for less than 0.3
percent of the total radioactivity. Small amounts of phenol,
acetamidonitrofen and the 2-chloro ether, as well as several unknown
compounds, were found. The bulk of the radioactivity consisted of
sulphates, glucuronides and glycine conjugates. In contrast, 47 to 86
percent of the total radioactivity in the faeces could be extracted
with chloroform. Approximately 30 percent of the total radiocarbon
excreted in the faeces was the parent nitrofen (11 percent of the
total dose administered). Aminonitrofen accounted for an average of 21
percent of the 14C in the faeces, with the concentration increasing
over time from 14 percent at 23 h to 25 percent at 33 h. In blood
samples, the parent nitrofen averaged 33.9 percent of the total
radioactivity, aminonitrofen 20 percent and an unknown labelled
compound 13.4 percent. The amount of parent compound peaked at 43
percent at 19 h post-treatment and declined to 28 percent of the total
by 31 h (Hunt et al. 1977).
Fish
The accumulation of residue in fish was studied in a system consisting
of two tanks. The first tank contained soil treated with 14C-labelled
nitrofen, while the second one contained water only. The tanks were
connected and the water circulated. During the first six days, the
residue in the soil and water declined from 6.29 mg/kg and 0.016 mg/kg
to 4.31 mg/kg and 0.004 mg/kg, respectively. From day 10, the water
did not contain detectable residue, while the total residue in soil
was 3.62 mg/kg at day 28. The residue in the tail (edible portion) of
crayfish placed in the tank containing soil was 0.48 mg/kg on the
first day and varied in the range of 0.13-1.04 mg/kg thereafter. In
the tissue of catfish, kept in the second tank, the highest residue
(7.42 mg/kg) was measured at day 2. The residue level declined to 0.17
mg/kg at day 28. Approximately 55 percent of total 14C residue was
nitrofen (Adler 1971).
Cultures of intestinal micro-organisms from carp were capable of
reducing nitrofen to aminonitrofen under both aerobic and anaerobic
conditions (Miyauchi et al. 1980).
In Plants
Soil in which paddy rice was grown was treated pre-emergence with
dichlorophenyl ring-labelled nitrofen. The rice plants were sprayed
with nitrophenyl ring-labelled nitrofen in one experiment and with
dichlorophenyl ring-labelled nitrofen in another. The post-emergence
spray application was carried out to provide samples with sufficiently
large residues for metabolic identification and to study metabolic
routes that might correlate with post-emergence applications of the
compound to other commodities. Metabolites identified and their
percentage ratio in the extractable residue are shown in Table 3
(Roser & Adler 1971).
The parent compound amounted to 72 percent of the organic extractable
residue (73.9 percent of total residue) in rice plants treated 10 days
before sampling. The parent compound (13.7 percent) hydroxy
acetamidonitrofen (15 percent) and the amino derivative (11.8 percent)
were the major components of the organic extractable residue (29.1
percent of total residue) in rice straw 90 days after treatment. The
bound residues in rice and wheat grain and straw from a 14C
pre-emergence application were characterized by Honeycutt and Adler
(1975).
64 to 96 percent of the radioactive residues in wheat and rice grain
were in the starch 110 and 147 days, respectively, after planting and
treatment. The authors conclude that nitrofen must undergo
diphenylether bond cleavage followed by ring opening and incorporation
of the ring fragments into glucose and starch. Similar
characterization of the wheat and rice straw reaveled approximately 30
percent of total 14C residues identifiable in the lignin fraction.
Negligible (5 percent or less) 14C residues were associated with the
cellulose fraction, except for rice treated pre-emergence (25
percent).
Two experimental plots were treated with nitrofen labelled either on
the dichlorophenyl ring or on the nitrophenyl ring. Each treated plot
and the control were planted with two varieties, Anza and Era, of
wheat. Samples were taken at various intervals and analysed with
methods measuring the parent nitrofen only and all of the residues
containing an amino group or capable of being converted to amino
nitrofen. The results of analyses are shown in Table 4 (Wargo 1974).
Only about half of the total radioactivity could be extracted from the
earlier samples and only about 20 percent from straw at harvest. The
method for measuring aminonitrofen, which includes several
metabolites, resulted in substantially higher residues in the earlier
samples than the method for the parent compound only. No residues were
detected by either method in grain and straw at harvest. Most of the
radioactivity present in grain and straw at harvest was in the form of
non-toxic natural substances.
Table 3 Metabolites of Nitrofen Identified in Rice Grain and Straw
Compound1 Extracted 14C(%)
10 days 90 days 90 days
plant straw grain
Nitrofen 72.3 13.7 2
Azoxynitrofen 4.6 2.5
Aminonitrofen 3.3 11.8 4
Formamidonitrofen 4.8 )
Acetamidonitrofen 0.5 ) 19
5-Hydroxy aminonitrofen 1.9 )
Hydroxy acetamidonitrofen 2.8 15
Unknowns and polar conjugates 9.8 38 94
1 Structural formulas of compounds are shown in Table 2.
Table 4 Residues of 14C Nitrofen in Wheat
Residues (mg/kg calculated as nitrofen)
Label Variety Radioassay Nitrofen Aminonitrofen
(parent compound) Method
Sample Extract Method
Immature Plants (at 26 days)
Control Era 0.105 - 0.04 0.04
Control Anza 0.134 - 0.04 0.02
NO2 Era 4.04 2.26 0.99 1.57
NO2 Anza 2.42 1.08 0.63 0.77
Cl2 Era 2.80 - 0.47 1.02
Cl2 Anza 1.12 0.61 0.25 0.32
Immatare Plants (at 44 days)
Control Era 0.035 - NDR1 NDR
Control Anza 0.053 - NDR NDR
NO2 Era 2.19 1.17 0.55 0.87
NO2 Anza 1.18 0.68 0.44 0.51
Cl2 Era 2.55 1.34 0.41 1.12
Cl2 Anza 1.44 1.00 0.48 0.54
Table 4 (continued)
Residues (mg/kg calculated as nitrofen)
Label Variety Radioassay Nitrofen Aminonitrofen
(parent compound) Method
Sample Extract Method
Final Harvest (at 110 days)
Grain Straw Straw2 Grain Straw Grain Straw
Control Era 0.025 0.087 - NDR NDR NDR NDR
Control Anza 0.034 0.028 - NDR NDR NDR NDR
NO2 Era 0.173 0.992 0.19 NDR NDR NDR NDR
NO2 Anza 0.148 0.460 0.10 NDR NDR NDR NDR
Cl2 Era 0.192 1.123 0.22 NDR NDR NDR NDR
Cl2 Anza 0.122 0.624 0.14 NDR NDR NDR NDR
1 NDR = no detectable residue; sensitivity = <0.01 mg/kg
2 No detectable 14C activity was present in the extract from any sample of grain
In Soil and Water
Roser and Adler (1971) identified the soil metabolites resulting from
a preplant/prefood application of dichlorophenyl ring 14C-labelled
nitrofen to simulated field rice paddies. Characterization of residues
in soil samples taken 147 days after treatment revealed that the
parent compound represented 23 percent of the extractable residue,
which amounted to 36 percent of total 14C activity. The major
metabolites were the aminonitrofen (35.4 percent) and
hydroxyacetamidonitrofen (10.7 percent), while the levels of
azoxynitrofen, formamidonitrofen and acetamidonitrofen were below 10
percent of extractable activity. Following a laboratory leaching
study, Adler and Roser (1971) characterized the 14C products in the
top 2.5 cm from soil columns. The extracts (6396 percent of total
activity) contained primarily parent compound (>90 percent), but a
qualitatively similar spectrum of products was observed as those
identified in the previous experiment. Niki and Kuwatsuka (1976)
studied several diphenyl ether herbicides in two rice paddy soils and
found rapid metabolism of nitrofen occurring in both soils. Under
flooded conditions, the half-life was less than 10 days while the
dissipation rate was slower under upland conditions. The primary
degradation product was the corresponding 4'-amino derivative.
Watanabe (1973) reported similar rapid dissipation under field
conditions.
In sealed metabolism flasks (biometer flasks), the metabolism of 14C
nitrofen under aerobic conditions were followed through approximately
100 days (Fischer 1971). Evolution of 14CO2 was detectable from both
dichlorophenyl and nitrophenyl ring-labelled preparations (ca. 3 and
1.5 percent of applied 14C, respectively). In order to characterize
accurately the residue decline patterns of nitrofen from field soil, a
plot was surface-treated with dichlorophenyl - 14C compound and left
fallow through the growing season (Fischer 1976). Radioassay of soil
samples collected through 316 days demonstrated a very rapid initial
decline of 0-15 cm residues (50 percent dissipation within 18 days )
followed by a slower decline. In contrast to field conditions nitrofen
did not undergo dissipation and metabolism under either aerobic or
anaerobic conditions in the greenhouse (Fischer 1975).
Fadayomi and Warren (1977) reported essentially no downward movement
of 14C nitrofen (nitrophenyl ring) that was spiked onto columns of a
silt loam and a fine sand soil and subjected to 5.4 cm of simulated
rainfall. Likewise, Abernathy (1972) found essentially no movement of
nitrofen on thin-layer plates coated with 12 different soil types. The
leaching tendencies for aged nitrofen residues were also determined
(Fischer 1974). After aging for 30 days under greenhouse conditions, a
14C nitrofen (both ring labelled) fortified sandy loam soil was
placed in 30 cm columns and subjected to daily simulated rainfalls of
12 mm/day for 45 days. Radioassay of the leachates as well as the
dissected columns revealed 83 to 102 percent of the applied 14C in
the top 5 cm. No significant 14C was detectable in the leachates.
Using nitrophenyl 14C-labelled nitrofen, a total of five soils of
different types plus river silt were compared as to the extent of
nitrofen adsorption from an aqueous solution (Adler & Allen 1971). The
average amount of 14C adsorbed was greater than 90 percent in every
case from 0.1 ms/l solutions and in all but one (high kaolinite clay
soil) from 1.0 ms/l solutions. These findings are consistent with
those of Fadayomi & Warren (1977), in which most or complete
adsorption of 14C nitrofen was observed by muck soil and bentonite
clays but not by kaolinite clays. Nitrofen was desorbed slowly when
the adsorbents were resuspended in distilled water four successive
times.
The observed immobility of nitrofen is consistent with its low water
solubility and relatively high adsorptive properties. The findings of
laboratory experiments are supported by the results of field
experiments in which water from wells surrounded by or near to treated
fields were sampled. No residue of nitrofen (<0.001 mg/l) was
detectable in 28 samples taken from depths of 3 to 27 m (Zagorski &
Rogerson 1980).
Nitrofen has not demonstrated any tendency to hydrolyse in aqueous
media either in the environment or in the laboratory (Adler 1973).
Photodecomposition
Nakagawa & Crosby (1974a) reported that nitrofen exposed in aqueous
suspensions to sunlight or simulated sunlight underwent rapid ether
cleavage to form 2,4-dichlorophenol and p-nitrophenol, along with
other numerous degradation products. In sunlight, more than 50 percent
of the initial nitrofen degraded after one week of outside exposure.
In a related publication, Nakagawa & Crosby (1974b) reported that the
sunlight photolysis of nitrofen in aqueous methanol represented a
photonucleophilic displacement of nitrophenol by the hydroxide ion of
water.
Both nitrophenyl ring-labelled nitrofen and dichlorophenyl
ring-labelled nitrofen were placed on glass plates as a thin film and
irradiated with simulated sunlight. Samples were takes after 48, 165,
239, 359 and 498 h of illumination. Some of the resulting
photoproducts were identified by thin-layer chromatography and mass
spectrometry. Nitrofen containing both ring labels gave rise to at
least 13 14C-photoproducts. Eleven of the radioactive components of
the nitrophenyl ring-labelled photoproduct mixture were identical (by
TLC Rf values) to corresponding 14C-components of the dichlorophenyl
ring-labelled photoproduct mixture. One unique component of the
nitrophenyl ring-labelled 14C nitrofen photolysis mixture was
identified as 14C-p-nitrophenol. 14C-2,4-dichlorophenol was also
detected among the photoproducts of the dichlorophenyl ring-labelled
mixtures. This indicates that diphenyl ether cleavage had occurred to
some extent during photolysis.
Most photoproducts of nitrofen were identified as diphenyl ethers,
such as amino-nitrofen, hydroxyacetamido nitrofen, isocyano nitrofen,
4-hydroxy-2,5-dichloro-1 (4-nitrophenoxy) benzene, 5-hydroxy-nitrofen,
acetamidonitrofen, formamido nitrofen, nitroso-nitrofen and
5-hydroxy-amino-nitrofen. Total 14C from both labelled compounds
declined to less than 50 percent within 100 h and it was below 20
percent after 498 h (Honeycutt 1975).
METHODS OF RESIDUE ANALYSIS
Methods are available for determining either the parent compound only,
or all of the residues containing amino group or those that can be
converted to aminonitrofen. The parent compound is extracted with
dichloromethane from crops and with isopropanol-benzene 1:2 (v/v) from
soil. The extract is cleaned on a Florisil column and the residue is
determined by gas-liquid chromatography (GLC) using an electron
capture detector (ECD). The limit of determination is 0.01 mg/kg (Rohm
& Haas 1967a). Nitrofen residue can also be analysed with
multi-residue procedures. The limit of determination was reported to
be 0.01-0.02 mg/kg depending on the type of sample (Ambrus et al.
1981).
Either method is suitable for regulatory purposes. The majority of
metabolites can be determined with the method of Adler & Wargo (1975),
which is based on the reduction of the residues to a common amine
intermediate. The latter compound is further derivatized and
quantitatively measured by ECD.
NATIONAL MAXIMUM RESIDUE LIMITS
National maximum residue limits (MRLs) reported to the Meeting are
summarized in Table 6.
APPRAISAL
Nitrofen is a selective pre- and early post-emergence herbicide for
the control of animal grasses and various broad-leaved weeds in cereal
grains and vegetables. Its recommendations for use either prohibit
women from handling the product or demand stringent handling
precaution that prevent exposure during application. It has a limited
use, which is concentrated mainly on wheat. The technical product,
containing a minimum of 95 percent nitrofen, is formulated as a 25
percent EC, 50 percent WP or in combination with other compounds. It
is applied at rates of 2 to 6 kg a.i./ha to the soil surface or to
weeds at the two to four leaf growth stage.
Table 6 National Maximum Residue Limits for Nitrofen
Crop Country MRL
(mg/kg)
Barley Greece 0.5
Broccoli United States 0.75
Brussels sprouts 0.75
Cabbage 0.75
Cauliflower 0.75
Carrots 0.75
Celery 0.75
Eggs 0.75
Fat (poultry) 0.2
Fruits Japan 0.1
Horseradish United States 0.05
Kohlrabi 0.75
Legumes Japan 0.1
Meat, meat by-products and
fat (except poultry) in
cattle, goats, pigs, horses,
sheep United States 0.05
Milk (fat) 0.5
Milk (whole) 0.02
Onion (dry bulb) Austria 0.02
United States 0.75
Parsley United States 0.75
Rice (grain) Japan 0.1
Rice (grain and straw) United States 0.1
Roots Japan 0.1
Rye The Netherlands 0.01
Sugarbeet United States 0.05
Taro 0.02
Vegetables Japan 0.1
Wheat Greece 0.5
The Netherlands 0.01
Yugoslavia 0.01
Supervised trials were carried out in several countries on various
crops at or about the recommended rates and at twice these rates.
Broccoli, cabbage, cauliflower, celery, garlic, oilseed rape and rice
contained no measurable residues (<0.01 mg/kg) regardless of dosage,
pre-harvest interval or analytical method. No parent compound was
detectable in many of the wheat samples; in others treated at the
recommended rate the total residue, measured as the amino derivative,
ranged, up to 0.09 mg/kg. In contrast to the grain, wheat straw
contained the parent compound at levels of <0.002-0.02 mg/kg while
the sum of metabolites and parent compound ranged from 0.01 mg/kg to
0.11 mg/kg.
The metabolic pathways of nitrofen include reduction of the nitro
group, conjugation of the resulting amino group, hydroxylation of the
dichlorophenyl ring and incorporation into naturally occurring
materials. The major metabolites identified in plants were largely
similar to those in animals. In rice plants, the organo-extractable
residue amounted to 73.9 percent and 29.1 percent of the total at 10
and 90 days after application. The parent compound accounted for 72
percent of the extractable residue at day 10, while
hydroxyacetamidonitrofen (15 percent), the parent compound (13.7
percent) and the amino derivative (11.8 percent) were the major
components after 90 days. A similar pattern of metabolite formation
was observed in wheat. Neither the grain nor the straw contained
extractable residues at harvest. In studies with the radio-labelled
compound, 64 to 96 percent of the radioactive carbon was found in the
starch of rice and wheat grains. Approximately 50 percent of the
unextractable activity was found in the lignin fraction of the rice
straw.
No residue was detectable in milk or tissues of cattle given nitrofen
by capsule at rates equivalent to 0.05 and 0.5 mg/kg of total daily
diet. In cattle dosed at 5 mg/kg, the total residue ranged from 0.002
to 0.004 mg/kg in milk, while the only tissue containing a measurable
residue was fat (0.01 mg/kg). Further studies with the labelled
compound administered by capsule resulted in similar levels of
residues. In milk, the total radioactivity expressed as the parent
compound was 0.003 mg/kg and 0.08 mg/kg at feeding levels of 0.5 mg/kg
and 5 mg/kg in the diet, respectively. At the highest dose, the muscle
and fat contained 0.24 mg/kg and 1.15 mg/kg of radioactive residue,
respectively. However, no residue was detectable in the milk, urine or
faeces of cattle when nitrofen was mixed with the ration at a level of
5 mg/kg, owing to the rapid degradation of the parent compound and
aminonitrofen in rumen fluid.
Metabolites were identified in the urine, faeces and blood of sheep
dosed at the extreme rate of 40 mg/kg. In urine, the bulk of the
radioactivity consisted of sulphate, glucuronide and glycine
conjugates. The organo-soluble fraction (2 to 5.5 percent of the total
residue) consisted mainly of aminonitrofen and hydroxynitrofen, while
the parent compound amounted to only 0.3 percent. In blood, the parent
compound averaged 33.9 percent of the total activity, while
aminonitrofen and unknown compounds amounted to 20 percent and 13.4
percent, respectively.
Eggs and tissues of laying hens kept on a diet containing 0.04 mg/kg,
0.12 mg/kg and 0.48 mg/kg radio-labelled nitrofen were analysed. After
a 6-week feeding period the residue in eggs reached plateaus at levels
of 0.02, 0.05 and 0.17 mg/kg, respectively. Almost all of the residue
in eggs was found in the yolk. Residues ranged from non-detectable in
white meat to 0.18 mg/kg in fat at the 0.04 mg/kg feeding level.
As the lowest feeding level is approximately 10 times the calculated
maximum residue resulting from treated feed components, no detectable
residue can be expected in the milk and tissues of cattle or in eggs
and meat of poultry.
Fish take up nitrofen residues from water. The residue in fish tissues
is of about the same magnitude as that in the water shortly after the
beginning of exposure but declines more slowly.
Under field conditions, the nitrofen concentration in or on soil
declined rapidly at first (50 percent dissipation within 18 days) but
much more slowly thereafter. The major metabolites identified in the
soil were aminonitrofen and hydroxyacetamidonitrofen. Both nitrofen
and its aged derivates were immobile in soil, which is in keeping with
their high adsorption coefficient on different soils and the low water
solubility of parent compound.
Analytical methods are available for the determination of the parent
compound alone or the majority of the organo-extractable residue after
derivatization of aminonitrofen. The former method is recommended for
regulatory purposes.
RECOMMENDATIONS
The Meeting examined the results of supervised trials and metabolite
studies and concluded that the data available were sufficient for
estimating MRLs. As no acceptable daily intake was allocated, the MRLs
were recorded as guideline levels. They refer to the parent compound
only.
Commodity Guideline levels Preharvest interval
(mg/kg) on which levels are
(based months)
Wheat 0.01* 5
Wheat straw 0.05 5
*Residue at or about the limit of determination
FURTHER WORK OR INFORMATION
Desirable
Determination of nitrofen residue in wheat bran.
REFERENCES - RESIDUES
Abernathy, J.R. Linuron, chlorbromuron, nitrofen and
1972 fluorodifen absorption and movement in
12 selected Illinois soils. PhD. Thesis,
University of Illinois, Champaign-
Urbana. (Unpublished)
Adler, I.L. A fish residues study using 14C-labelled
1971 TOK. Rohm and Haas Research Report No.
23-41. (Unpublished)
Adler, I.L. A study to determine radioactive residue
1972 levels in eggs, tissues and excreta of
laying hens fed 14C TOK. Rohm & Haas
Report No. 23-47. (Unpublished)
Adler, I.L. Study of the hydrolysis of the herbicide
1973 TOK in water. Rohm & Haas. Report No.
3923-73-3. (Unpublished)
Adler, I.L, & Allen, Soil absorption studies with 14C TOK.
S.S. Jr. 1971 Rohm and Haas Report No. 23-77-11.
(Unpublished)
Adler, I.L. & Roser, R.L. A study of the leaching and metabolism
1971 of 14C TOK in soils. Rohm and Haas
Report No. 23-22. (Unpublished)
Adler, I.L. & Wargo, J.P. Determination of residues from the
1975 herbicide 2,4-dichloro-1-(4-
nitrophenoxy)-benzene in rice and wheat
by electron capture gas-liquid
chromatography. J. Assoc Off. Anal.
Chem., 58:551.
Ambrus, A., Csatlos, I., General method for determination of
Hargitai, E., Lautos, J., pesticide residues in samples of
Szabó, L., Visi, E. & plant origin, soil and water. Part
Zakar, F. I-III, H, Assoc. Off. Anal. Chem.,
64:733.
Fadayomi, O. & Warren, G.F. Absorption, desorption and leaching of
1977 nitrofen and oxyfluorten. Weed Sci.,
25:97.
Fischer, J.D. Dissipation study of TOK in soil and its
1971 effects on microbial activity. Rohm and
Haas Report. (Unpublished)
Fischer, J.D. Laboratory leaching study with aged TOK
soil. Rohm & Haas Report No. 3923-74-78.
(Unpublished)
Fischer, J.D. TOK greenhouse soil metabolism study.
1975 Rohm & Haas Report No. 3923-75-13.
(Unpublished)
Fischer, J.D. TOK fallow field study. Rohm & Haas
1976 Report No. 34H-76-16. (Unpublished)
Gutemann, W.H. & List, D.J. Metabolism of TOK herbicide in the dairy
1967 cow. J. Dairy Sci. 50:1516.
Honeycutt, R.C. Photolysis of 14C nitrofen by simulated
1975 sunlight. Rohm & Haas Report No. 3923-
75-6. (Unpublished)
Honeycutt, R.C. & Adler, Characterization of bound residues of
I.L. 1975 nitrofen in rice and wheat straw.
J. Agric. Food Chem. 23:1097-1101.
Hunt, L.M., Absorption, excretion, and metabolism
Chamberlain, W.F., of nitrofen by a sheep. J. Agric. Food
Gilbert, B.N., Chem., 25:1062-1065.
Hopkins, D.E. &
Gingrich, A.R.
1977
Institut National de la Report on residues of nitrofen on
Recherche Agronomique. wheat. Laboratorie de Phytopharmacie,
1975a Versailles. (Unpublished)
Institut National de la Reports on residues of nitrofen in rape.
Recherche Agronomique. (Unpublished)
1975b
Institut National de la Reports on residues of nitrofen in
Recherche Agronomique. wheat. (Unpublished)
1978
Miyauchi, M., Takagi, M. & Studies on the toxicity of chlorinated
Takayoshi, M. nitrobiphenyl ethers on fish-III. Bull.
1980 Japanese Soc. Sci. Fish., 46(7):837-844.
Nakagawa, M. & Crosby, D.G. Photodecomposition of nitrofen. J.
1974a Agric. Food. Chem. 22:849-853.
Nakagawa, M. & Crosby, D.G. Photonucleophilic reactions of nitrofen.
1974b J. Agric. Food Chem., 22:930-933.
Niki, Y. & Kuwatsuka, S. Degradation of diphenyl ether herbicides
1976 in soils. Soil Sci. Plant Nutr. (Tokyo),
22:223.
Rohm & Haas. Determination of TOK residues in crops
1967a and soil. RAR Memorandum No. 514.
(Unpublished)
Rohm & Haas. A study to determine residues levels in
1967b milk and tissues from a cow fed TOK.
Rohm and Haas Report No. 23-6.
(Unpublished)
Rohm & Haas. A study to determine residue levels in
1971 milk, tissues and excreta of a cow fed
14 C-labelled TOK. Rohm and Haas Report
No. 23-30. (Unpublished)
Rohm & Haas. Reports on residues of nitrofen: In
1969-1980 broccoli, Report Nos. 78-0172,
78-0173, 78-0174, 78-0175, 78-0176,
78-0177, 80-0249, 80-0250, 80-0279,
80-0280, 80-0290, 80-0291; in cabbage,
Report Nos. 78-0336, 78-0337, 80-0245,
80-0246, 80-0286, 0-0287; in
cauliflower, report nos. 78-0166,
78-0167, 78-0168, 78-0169, 78-0170,
78-0171, 80-0247, 80-0248, 80-288,
80-0289; in celery, Report Nos. 80-0238,
80-0251, 80-0292, 80-0293; garlic,
Report Nos. 80-0252, 80-0253, 80-0254,
80-0255; in rice, Report Nos. 2-69-227,
2-70-173, 2-70-190; in wheat, Report
Nos. 72-121-02, 72-122-02, 72-123-02,
72-292-02, 72-293-02, 72-294-02,
73-266-02, 73-267-02, 73-268-02,
73-269-02, 73-270-02, 73-271-02,
73-272-02, 73-309-02, 73-301-02,
73-311-02, 73-312-02, 73-313-02,
73-314-02, 73-315-02, 74-075-02,
74-076-02. (Unpublished)
Roser, R.L. & Adler, I.L. The nature of aged residues of TOK.
1971 Rohm & Haas Report No. 23-34.
(Unpublished)
Wargo, J.P. TOK and amino-TOK analysis of 14C-TOK
1974 treated wheat. Rohm and Haas Report No.
3923-74-8. (Unpublished)
Watanabe, I. Decomposition of pesticides, by soil
1973 micro-organisms - Special emphasis on
the flooded soil condition. Jap. Agric.
Res. Quar., 7:15.
Zorgorski, W.J. & Analysis of ground water samples for
Rogerson, T.D. residues of KELTHANE and TOK. Rohm
and Haas Report No. 34H-80-1.
(Unpublished)
2,5-dichlorophenol x
1 The substituted carbon atoms have not been identified.
Cattle feeding studies were conducted twice at feeding levels of 0.05,
0.5 and 5 mg/kg administered in gelatin capsules. Combined residues in
meat and milk were determined once in four cows and a second time with
14C-labelled nitrofen. In the multiple cow feeding study, no residues
of nitrofen were detected in milk or tissues of animals fed 0.05 or
0.5 mg/kg, based on the total daily diet. Barely detectable residues,
ranging from 0.002 to 0.004 mg/kg, were found in the milk of a cow fed
5 mg/kg. At this feeding level, no residue was found in any of the
tissues except fat (0.01 mg/kg). The sensitivity of the method was
0.01 mg/kg for tissue, and 0.002 mg/kg for milk (Rohm & Haas 1967b).
In the radioactive feeding study a cow was dosed orally twice a day
with 14C-labelled nitrofen. Nitrofen residues were not found in the
milk from the lowest feeding level (0.05 mg/kg) and only 0.003 and
0.08 mg/kg were found in milk from the two higher feeding levels, i.e.
0.5 and 5 mg/kg. The residue in milk was 0.3 percent and 0.8 percent
of the applied dosage, respectively. Residues were found in edible
tissues taken from the cow dosed at the 5 mg/kg feeding level.
Calculated as nitrofen, these residues ranged from 0.05 mg/kg (trips)
and 0.24 mg/kg (muscle) to 1.15 mg/kg (fat) (Rohm & Haas 1971). The
parent compound constituted approximately 47, 32 and 23 percent of the
total residue in milk, kidney and fat, respectively.
The lowest feeding level (0.05 mg/kg) of radioactive nitrofen is
almost ten times greater than the calculated maximum level of residue
that might be eaten by livestock kept on feed containing rice bran,
rice milling fractions or any other plant by-products that had been
treated with nitrofen. Consequently, no detectable residues are likely
to be found in milk and edible tissues.
Poultry
Three groups of 15 laying hens each were fed dichlorophenyl
ring-labelled 14C nitrofen incorporated in their feed for ten weeks
at levels of 0.04, 0.12 and 0.47 mg/kg, respectively. Residues in
tissues and eggs were studied and analysed by liquid scintillation
counting. Residues in eggs reached plateaux at levels of 0.02, 0.05
and 0.17 mg/kg, respectively, by week 6 of feeding for the X, 3X and
10X test group. The residue was located almost exclusively in the egg
yolk. The excreta collected for one 24 h period in week 3 contained
98.9 and 80 percent of the total radioactivity of feeding levels of
0.04 and 0.47 mg/kg, respectively. Hens from each group were
sacrificed after weeks 3, 5 and 10 of test feeding. Selected tissues
were removed and analysed by combustion and liquid scintillation
counting. Average residue levels in tissues from the 1X test group
ranged from non-detectable (white meat) to 0.18 mg/kg (fat).
Prolonged feeding of nitrofen to laying hens at a level of 0.04 mg/kg
daily resulted in total residue of approximately 0.01-0.02 mg/kg in
eggs. The residue was found almost exclusively in the egg yolk (Adler
1972).
Sheep
A female sheep was given a single oral, 40 mg/kg dose of 14C-labelled
nitrofen. After 99 h, 76.2 percent of the 14C had been recovered in
the excreta, with 39 percent appearing in the urine and 37.2 percent
in the faeces. The residue in the blood peaked at 19 h with a plateau
from 11 to 31 h; the concentrations were between 3 and 4 mg/kg. When
killed at 100 h, the highest tissue concentrations were found in the
fat (23 to 24 mg/kg total radioactivity expressed as nitrofen); the
gut, liver, thyroid and mammary glands contained from 2 to 4 mg/kg and
14 other organs had residues of 0.5 to 2 mg/kg (expressed as
nitrofen). The highest urinary excretion of nitrofen occurred during
the 11 to 19 h post-treatment collection period; the peak in the
faeces was during the 39 to 47 h post-treatment collection. Only 2 to
5.5 percent of the radioactivity in the urine was extractable with
chloroform. Aminoitrofen and 5-hydroxynitrofen were the principal
metabolites, with the parent compound accounting for less than 0.3
percent of the total radioactivity. Small amounts of phenol,
acetamidonitrofen and the 2-chloro ether, as well as several unknown
compounds, were found. The bulk of the radioactivity consisted of
sulphates, glucuronides and glycine conjugates. In contrast, 47 to 86
percent of the total radioactivity in the faeces could be extracted
with chloroform. Approximately 30 percent of the total radiocarbon
excreted in the faeces was the parent nitrofen (11 percent of the
total dose administered). Aminonitrofen accounted for an average of 21
percent of the 14C in the faeces, with the concentration increasing
over time from 14 percent at 23 h to 25 percent at 33 h. In blood
samples, the parent nitrofen averaged 33.9 percent of the total
radioactivity, aminonitrofen 20 percent and an unknown labelled
compound 13.4 percent. The amount of parent compound peaked at 43
percent at 19 h post-treatment and declined to 28 percent of the total
by 31 h (Hunt et al. 1977).
Fish
The accumulation of residue in fish was studied in a system consisting
of two tanks. The first tank contained soil treated with 14C-labelled
nitrofen, while the second one contained water only. The tanks were
connected and the water circulated. During the first six days, the
residue in the soil and water declined from 6.29 mg/kg and 0.016 mg/kg
to 4.31 mg/kg and 0.004 mg/kg, respectively. From day 10, the water
did not contain detectable residue, while the total residue in soil
was 3.62 mg/kg at day 28. The residue in the tail (edible portion) of
crayfish placed in the tank containing soil was 0.48 mg/kg on the
first day and varied in the range of 0.13-1.04 mg/kg thereafter. In
the tissue of catfish, kept in the second tank, the highest residue
(7.42 mg/kg) was measured at day 2. The residue level declined to 0.17
mg/kg at day 28. Approximately 55 percent of total 14C residue was
nitrofen (Adler 1971).
Cultures of intestinal micro-organisms from carp were capable of
reducing nitrofen to aminonitrofen under both aerobic and anaerobic
conditions (Miyauchi et al. 1980).
In Plants
Soil in which paddy rice was grown was treated pre-emergence with
dichlorophenyl ring-labelled nitrofen. The rice plants were sprayed
with nitrophenyl ring-labelled nitrofen in one experiment and with
dichlorophenyl ring-labelled nitrofen in another. The post-emergence
spray application was carried out to provide samples with sufficiently
large residues for metabolic identification and to study metabolic
routes that might correlate with post-emergence applications of the
compound to other commodities. Metabolites identified and their
percentage ratio in the extractable residue are shown in Table 3
(Roser & Adler 1971).
The parent compound amounted to 72 percent of the organic extractable
residue (73.9 percent of total residue) in rice plants treated 10 days
before sampling. The parent compound (13.7 percent) hydroxy
acetamidonitrofen (15 percent) and the amino derivative (11.8 percent)
were the major components of the organic extractable residue (29.1
percent of total residue) in rice straw 90 days after treatment. The
bound residues in rice and wheat grain and straw from a 14C
pre-emergence application were characterized by Honeycutt and Adler
(1975).
64 to 96 percent of the radioactive residues in wheat and rice grain
were in the starch 110 and 147 days, respectively, after planting and
treatment. The authors conclude that nitrofen must undergo
diphenylether bond cleavage followed by ring opening and incorporation
of the ring fragments into glucose and starch. Similar
characterization of the wheat and rice straw reaveled approximately 30
percent of total 14C residues identifiable in the lignin fraction.
Negligible (5 percent or less) 14C residues were associated with the
cellulose fraction, except for rice treated pre-emergence (25
percent).
Two experimental plots were treated with nitrofen labelled either on
the dichlorophenyl ring or on the nitrophenyl ring. Each treated plot
and the control were planted with two varieties, Anza and Era, of
wheat. Samples were taken at various intervals and analysed with
methods measuring the parent nitrofen only and all of the residues
containing an amino group or capable of being converted to amino
nitrofen. The results of analyses are shown in Table 4 (Wargo 1974).
Only about half of the total radioactivity could be extracted from the
earlier samples and only about 20 percent from straw at harvest. The
method for measuring aminonitrofen, which includes several
metabolites, resulted in substantially higher residues in the earlier
samples than the method for the parent compound only. No residues were
detected by either method in grain and straw at harvest. Most of the
radioactivity present in grain and straw at harvest was in the form of
non-toxic natural substances.
Table 3 Metabolites of Nitrofen Identified in Rice Grain and Straw
Compound1 Extracted 14C(%)
10 days 90 days 90 days
plant straw grain
Nitrofen 72.3 13.7 2
Azoxynitrofen 4.6 2.5
Aminonitrofen 3.3 11.8 4
Formamidonitrofen 4.8 )
Acetamidonitrofen 0.5 ) 19
5-Hydroxy aminonitrofen 1.9 )
Hydroxy acetamidonitrofen 2.8 15
Unknowns and polar conjugates 9.8 38 94
1 Structural formulas of compounds are shown in Table 2.
Table 4 Residues of 14C Nitrofen in Wheat
Residues (mg/kg calculated as nitrofen)
Label Variety Radioassay Nitrofen Aminonitrofen
(parent compound) Method
Sample Extract Method
Immature Plants (at 26 days)
Control Era 0.105 - 0.04 0.04
Control Anza 0.134 - 0.04 0.02
NO2 Era 4.04 2.26 0.99 1.57
NO2 Anza 2.42 1.08 0.63 0.77
Cl2 Era 2.80 - 0.47 1.02
Cl2 Anza 1.12 0.61 0.25 0.32
Immatare Plants (at 44 days)
Control Era 0.035 - NDR1 NDR
Control Anza 0.053 - NDR NDR
NO2 Era 2.19 1.17 0.55 0.87
NO2 Anza 1.18 0.68 0.44 0.51
Cl2 Era 2.55 1.34 0.41 1.12
Cl2 Anza 1.44 1.00 0.48 0.54
Table 4 (continued)
Residues (mg/kg calculated as nitrofen)
Label Variety Radioassay Nitrofen Aminonitrofen
(parent compound) Method
Sample Extract Method
Final Harvest (at 110 days)
Grain Straw Straw2 Grain Straw Grain Straw
Control Era 0.025 0.087 - NDR NDR NDR NDR
Control Anza 0.034 0.028 - NDR NDR NDR NDR
NO2 Era 0.173 0.992 0.19 NDR NDR NDR NDR
NO2 Anza 0.148 0.460 0.10 NDR NDR NDR NDR
Cl2 Era 0.192 1.123 0.22 NDR NDR NDR NDR
Cl2 Anza 0.122 0.624 0.14 NDR NDR NDR NDR
1 NDR = no detectable residue; sensitivity = <0.01 mg/kg
2 No detectable 14C activity was present in the extract from any sample of grain
In Soil and Water
Roser and Adler (1971) identified the soil metabolites resulting from
a preplant/prefood application of dichlorophenyl ring 14C-labelled
nitrofen to simulated field rice paddies. Characterization of residues
in soil samples taken 147 days after treatment revealed that the
parent compound represented 23 percent of the extractable residue,
which amounted to 36 percent of total 14C activity. The major
metabolites were the aminonitrofen (35.4 percent) and
hydroxyacetamidonitrofen (10.7 percent), while the levels of
azoxynitrofen, formamidonitrofen and acetamidonitrofen were below 10
percent of extractable activity. Following a laboratory leaching
study, Adler and Roser (1971) characterized the 14C products in the
top 2.5 cm from soil columns. The extracts (6396 percent of total
activity) contained primarily parent compound (>90 percent), but a
qualitatively similar spectrum of products was observed as those
identified in the previous experiment. Niki and Kuwatsuka (1976)
studied several diphenyl ether herbicides in two rice paddy soils and
found rapid metabolism of nitrofen occurring in both soils. Under
flooded conditions, the half-life was less than 10 days while the
dissipation rate was slower under upland conditions. The primary
degradation product was the corresponding 4'-amino derivative.
Watanabe (1973) reported similar rapid dissipation under field
conditions.
In sealed metabolism flasks (biometer flasks), the metabolism of 14C
nitrofen under aerobic conditions were followed through approximately
100 days (Fischer 1971). Evolution of 14CO2 was detectable from both
dichlorophenyl and nitrophenyl ring-labelled preparations (ca. 3 and
1.5 percent of applied 14C, respectively). In order to characterize
accurately the residue decline patterns of nitrofen from field soil, a
plot was surface-treated with dichlorophenyl - 14C compound and left
fallow through the growing season (Fischer 1976). Radioassay of soil
samples collected through 316 days demonstrated a very rapid initial
decline of 0-15 cm residues (50 percent dissipation within 18 days )
followed by a slower decline. In contrast to field conditions nitrofen
did not undergo dissipation and metabolism under either aerobic or
anaerobic conditions in the greenhouse (Fischer 1975).
Fadayomi and Warren (1977) reported essentially no downward movement
of 14C nitrofen (nitrophenyl ring) that was spiked onto columns of a
silt loam and a fine sand soil and subjected to 5.4 cm of simulated
rainfall. Likewise, Abernathy (1972) found essentially no movement of
nitrofen on thin-layer plates coated with 12 different soil types. The
leaching tendencies for aged nitrofen residues were also determined
(Fischer 1974). After aging for 30 days under greenhouse conditions, a
14C nitrofen (both ring labelled) fortified sandy loam soil was
placed in 30 cm columns and subjected to daily simulated rainfalls of
12 mm/day for 45 days. Radioassay of the leachates as well as the
dissected columns revealed 83 to 102 percent of the applied 14C in
the top 5 cm. No significant 14C was detectable in the leachates.
Using nitrophenyl 14C-labelled nitrofen, a total of five soils of
different types plus river silt were compared as to the extent of
nitrofen adsorption from an aqueous solution (Adler & Allen 1971). The
average amount of 14C adsorbed was greater than 90 percent in every
case from 0.1 ms/l solutions and in all but one (high kaolinite clay
soil) from 1.0 ms/l solutions. These findings are consistent with
those of Fadayomi & Warren (1977), in which most or complete
adsorption of 14C nitrofen was observed by muck soil and bentonite
clays but not by kaolinite clays. Nitrofen was desorbed slowly when
the adsorbents were resuspended in distilled water four successive
times.
The observed immobility of nitrofen is consistent with its low water
solubility and relatively high adsorptive properties. The findings of
laboratory experiments are supported by the results of field
experiments in which water from wells surrounded by or near to treated
fields were sampled. No residue of nitrofen (<0.001 mg/l) was
detectable in 28 samples taken from depths of 3 to 27 m (Zagorski &
Rogerson 1980).
Nitrofen has not demonstrated any tendency to hydrolyse in aqueous
media either in the environment or in the laboratory (Adler 1973).
Photodecomposition
Nakagawa & Crosby (1974a) reported that nitrofen exposed in aqueous
suspensions to sunlight or simulated sunlight underwent rapid ether
cleavage to form 2,4-dichlorophenol and p-nitrophenol, along with
other numerous degradation products. In sunlight, more than 50 percent
of the initial nitrofen degraded after one week of outside exposure.
In a related publication, Nakagawa & Crosby (1974b) reported that the
sunlight photolysis of nitrofen in aqueous methanol represented a
photonucleophilic displacement of nitrophenol by the hydroxide ion of
water.
Both nitrophenyl ring-labelled nitrofen and dichlorophenyl
ring-labelled nitrofen were placed on glass plates as a thin film and
irradiated with simulated sunlight. Samples were takes after 48, 165,
239, 359 and 498 h of illumination. Some of the resulting
photoproducts were identified by thin-layer chromatography and mass
spectrometry. Nitrofen containing both ring labels gave rise to at
least 13 14C-photoproducts. Eleven of the radioactive components of
the nitrophenyl ring-labelled photoproduct mixture were identical (by
TLC Rf values) to corresponding 14C-components of the dichlorophenyl
ring-labelled photoproduct mixture. One unique component of the
nitrophenyl ring-labelled 14C nitrofen photolysis mixture was
identified as 14C-p-nitrophenol. 14C-2,4-dichlorophenol was also
detected among the photoproducts of the dichlorophenyl ring-labelled
mixtures. This indicates that diphenyl ether cleavage had occurred to
some extent during photolysis.
Most photoproducts of nitrofen were identified as diphenyl ethers,
such as amino-nitrofen, hydroxyacetamido nitrofen, isocyano nitrofen,
4-hydroxy-2,5-dichloro-1 (4-nitrophenoxy) benzene, 5-hydroxy-nitrofen,
acetamidonitrofen, formamido nitrofen, nitroso-nitrofen and
5-hydroxy-amino-nitrofen. Total 14C from both labelled compounds
declined to less than 50 percent within 100 h and it was below 20
percent after 498 h (Honeycutt 1975).
METHODS OF RESIDUE ANALYSIS
Methods are available for determining either the parent compound only,
or all of the residues containing amino group or those that can be
converted to aminonitrofen. The parent compound is extracted with
dichloromethane from crops and with isopropanol-benzene 1:2 (v/v) from
soil. The extract is cleaned on a Florisil column and the residue is
determined by gas-liquid chromatography (GLC) using an electron
capture detector (ECD). The limit of determination is 0.01 mg/kg (Rohm
& Haas 1967a). Nitrofen residue can also be analysed with
multi-residue procedures. The limit of determination was reported to
be 0.01-0.02 mg/kg depending on the type of sample (Ambrus et al.
1981).
Either method is suitable for regulatory purposes. The majority of
metabolites can be determined with the method of Adler & Wargo (1975),
which is based on the reduction of the residues to a common amine
intermediate. The latter compound is further derivatized and
quantitatively measured by ECD.
NATIONAL MAXIMUM RESIDUE LIMITS
National maximum residue limits (MRLs) reported to the Meeting are
summarized in Table 6.
APPRAISAL
Nitrofen is a selective pre- and early post-emergence herbicide for
the control of animal grasses and various broad-leaved weeds in cereal
grains and vegetables. Its recommendations for use either prohibit
women from handling the product or demand stringent handling
precaution that prevent exposure during application. It has a limited
use, which is concentrated mainly on wheat. The technical product,
containing a minimum of 95 percent nitrofen, is formulated as a 25
percent EC, 50 percent WP or in combination with other compounds. It
is applied at rates of 2 to 6 kg a.i./ha to the soil surface or to
weeds at the two to four leaf growth stage.
Table 6 National Maximum Residue Limits for Nitrofen
Crop Country MRL
(mg/kg)
Barley Greece 0.5
Broccoli United States 0.75
Brussels sprouts 0.75
Cabbage 0.75
Cauliflower 0.75
Carrots 0.75
Celery 0.75
Eggs 0.75
Fat (poultry) 0.2
Fruits Japan 0.1
Horseradish United States 0.05
Kohlrabi 0.75
Legumes Japan 0.1
Meat, meat by-products and
fat (except poultry) in
cattle, goats, pigs, horses,
sheep United States 0.05
Milk (fat) 0.5
Milk (whole) 0.02
Onion (dry bulb) Austria 0.02
United States 0.75
Parsley United States 0.75
Rice (grain) Japan 0.1
Rice (grain and straw) United States 0.1
Roots Japan 0.1
Rye The Netherlands 0.01
Sugarbeet United States 0.05
Taro 0.02
Vegetables Japan 0.1
Wheat Greece 0.5
The Netherlands 0.01
Yugoslavia 0.01
Supervised trials were carried out in several countries on various
crops at or about the recommended rates and at twice these rates.
Broccoli, cabbage, cauliflower, celery, garlic, oilseed rape and rice
contained no measurable residues (<0.01 mg/kg) regardless of dosage,
pre-harvest interval or analytical method. No parent compound was
detectable in many of the wheat samples; in others treated at the
recommended rate the total residue, measured as the amino derivative,
ranged, up to 0.09 mg/kg. In contrast to the grain, wheat straw
contained the parent compound at levels of <0.002-0.02 mg/kg while
the sum of metabolites and parent compound ranged from 0.01 mg/kg to
0.11 mg/kg.
The metabolic pathways of nitrofen include reduction of the nitro
group, conjugation of the resulting amino group, hydroxylation of the
dichlorophenyl ring and incorporation into naturally occurring
materials. The major metabolites identified in plants were largely
similar to those in animals. In rice plants, the organo-extractable
residue amounted to 73.9 percent and 29.1 percent of the total at 10
and 90 days after application. The parent compound accounted for 72
percent of the extractable residue at day 10, while
hydroxyacetamidonitrofen (15 percent), the parent compound (13.7
percent) and the amino derivative (11.8 percent) were the major
components after 90 days. A similar pattern of metabolite formation
was observed in wheat. Neither the grain nor the straw contained
extractable residues at harvest. In studies with the radio-labelled
compound, 64 to 96 percent of the radioactive carbon was found in the
starch of rice and wheat grains. Approximately 50 percent of the
unextractable activity was found in the lignin fraction of the rice
straw.
No residue was detectable in milk or tissues of cattle given nitrofen
by capsule at rates equivalent to 0.05 and 0.5 mg/kg of total daily
diet. In cattle dosed at 5 mg/kg, the total residue ranged from 0.002
to 0.004 mg/kg in milk, while the only tissue containing a measurable
residue was fat (0.01 mg/kg). Further studies with the labelled
compound administered by capsule resulted in similar levels of
residues. In milk, the total radioactivity expressed as the parent
compound was 0.003 mg/kg and 0.08 mg/kg at feeding levels of 0.5 mg/kg
and 5 mg/kg in the diet, respectively. At the highest dose, the muscle
and fat contained 0.24 mg/kg and 1.15 mg/kg of radioactive residue,
respectively. However, no residue was detectable in the milk, urine or
faeces of cattle when nitrofen was mixed with the ration at a level of
5 mg/kg, owing to the rapid degradation of the parent compound and
aminonitrofen in rumen fluid.
Metabolites were identified in the urine, faeces and blood of sheep
dosed at the extreme rate of 40 mg/kg. In urine, the bulk of the
radioactivity consisted of sulphate, glucuronide and glycine
conjugates. The organo-soluble fraction (2 to 5.5 percent of the total
residue) consisted mainly of aminonitrofen and hydroxynitrofen, while
the parent compound amounted to only 0.3 percent. In blood, the parent
compound averaged 33.9 percent of the total activity, while
aminonitrofen and unknown compounds amounted to 20 percent and 13.4
percent, respectively.
Eggs and tissues of laying hens kept on a diet containing 0.04 mg/kg,
0.12 mg/kg and 0.48 mg/kg radio-labelled nitrofen were analysed. After
a 6-week feeding period the residue in eggs reached plateaus at levels
of 0.02, 0.05 and 0.17 mg/kg, respectively. Almost all of the residue
in eggs was found in the yolk. Residues ranged from non-detectable in
white meat to 0.18 mg/kg in fat at the 0.04 mg/kg feeding level.
As the lowest feeding level is approximately 10 times the calculated
maximum residue resulting from treated feed components, no detectable
residue can be expected in the milk and tissues of cattle or in eggs
and meat of poultry.
Fish take up nitrofen residues from water. The residue in fish tissues
is of about the same magnitude as that in the water shortly after the
beginning of exposure but declines more slowly.
Under field conditions, the nitrofen concentration in or on soil
declined rapidly at first (50 percent dissipation within 18 days) but
much more slowly thereafter. The major metabolites identified in the
soil were aminonitrofen and hydroxyacetamidonitrofen. Both nitrofen
and its aged derivates were immobile in soil, which is in keeping with
their high adsorption coefficient on different soils and the low water
solubility of parent compound.
Analytical methods are available for the determination of the parent
compound alone or the majority of the organo-extractable residue after
derivatization of aminonitrofen. The former method is recommended for
regulatory purposes.
RECOMMENDATIONS
The Meeting examined the results of supervised trials and metabolite
studies and concluded that the data available were sufficient for
estimating MRLs. As no acceptable daily intake was allocated, the MRLs
were recorded as guideline levels. They refer to the parent compound
only.
Commodity Guideline levels Preharvest interval
(mg/kg) on which levels are
(based months)
Wheat 0.01* 5
Wheat straw 0.05 5
*Residue at or about the limit of determination
FURTHER WORK OR INFORMATION
Desirable
Determination of nitrofen residue in wheat bran.
REFERENCES - RESIDUES
Abernathy, J.R. Linuron, chlorbromuron, nitrofen and
1972 fluorodifen absorption and movement in
12 selected Illinois soils. PhD. Thesis,
University of Illinois, Champaign-
Urbana. (Unpublished)
Adler, I.L. A fish residues study using 14C-labelled
1971 TOK. Rohm and Haas Research Report No.
23-41. (Unpublished)
Adler, I.L. A study to determine radioactive residue
1972 levels in eggs, tissues and excreta of
laying hens fed 14C TOK. Rohm & Haas
Report No. 23-47. (Unpublished)
Adler, I.L. Study of the hydrolysis of the herbicide
1973 TOK in water. Rohm & Haas. Report No.
3923-73-3. (Unpublished)
Adler, I.L, & Allen, Soil absorption studies with 14C TOK.
S.S. Jr. 1971 Rohm and Haas Report No. 23-77-11.
(Unpublished)
Adler, I.L. & Roser, R.L. A study of the leaching and metabolism
1971 of 14C TOK in soils. Rohm and Haas
Report No. 23-22. (Unpublished)
Adler, I.L. & Wargo, J.P. Determination of residues from the
1975 herbicide 2,4-dichloro-1-(4-
nitrophenoxy)-benzene in rice and wheat
by electron capture gas-liquid
chromatography. J. Assoc Off. Anal.
Chem., 58:551.
Ambrus, A., Csatlos, I., General method for determination of
Hargitai, E., Lautos, J., pesticide residues in samples of
Szabó, L., Visi, E. & plant origin, soil and water. Part
Zakar, F. I-III, H, Assoc. Off. Anal. Chem.,
64:733.
Fadayomi, O. & Warren, G.F. Absorption, desorption and leaching of
1977 nitrofen and oxyfluorten. Weed Sci.,
25:97.
Fischer, J.D. Dissipation study of TOK in soil and its
1971 effects on microbial activity. Rohm and
Haas Report. (Unpublished)
Fischer, J.D. Laboratory leaching study with aged TOK
soil. Rohm & Haas Report No. 3923-74-78.
(Unpublished)
Fischer, J.D. TOK greenhouse soil metabolism study.
1975 Rohm & Haas Report No. 3923-75-13.
(Unpublished)
Fischer, J.D. TOK fallow field study. Rohm & Haas
1976 Report No. 34H-76-16. (Unpublished)
Gutemann, W.H. & List, D.J. Metabolism of TOK herbicide in the dairy
1967 cow. J. Dairy Sci. 50:1516.
Honeycutt, R.C. Photolysis of 14C nitrofen by simulated
1975 sunlight. Rohm & Haas Report No. 3923-
75-6. (Unpublished)
Honeycutt, R.C. & Adler, Characterization of bound residues of
I.L. 1975 nitrofen in rice and wheat straw.
J. Agric. Food Chem. 23:1097-1101.
Hunt, L.M., Absorption, excretion, and metabolism
Chamberlain, W.F., of nitrofen by a sheep. J. Agric. Food
Gilbert, B.N., Chem., 25:1062-1065.
Hopkins, D.E. &
Gingrich, A.R.
1977
Institut National de la Report on residues of nitrofen on
Recherche Agronomique. wheat. Laboratorie de Phytopharmacie,
1975a Versailles. (Unpublished)
Institut National de la Reports on residues of nitrofen in rape.
Recherche Agronomique. (Unpublished)
1975b
Institut National de la Reports on residues of nitrofen in
Recherche Agronomique. wheat. (Unpublished)
1978
Miyauchi, M., Takagi, M. & Studies on the toxicity of chlorinated
Takayoshi, M. nitrobiphenyl ethers on fish-III. Bull.
1980 Japanese Soc. Sci. Fish., 46(7):837-844.
Nakagawa, M. & Crosby, D.G. Photodecomposition of nitrofen. J.
1974a Agric. Food. Chem. 22:849-853.
Nakagawa, M. & Crosby, D.G. Photonucleophilic reactions of nitrofen.
1974b J. Agric. Food Chem., 22:930-933.
Niki, Y. & Kuwatsuka, S. Degradation of diphenyl ether herbicides
1976 in soils. Soil Sci. Plant Nutr. (Tokyo),
22:223.
Rohm & Haas. Determination of TOK residues in crops
1967a and soil. RAR Memorandum No. 514.
(Unpublished)
Rohm & Haas. A study to determine residues levels in
1967b milk and tissues from a cow fed TOK.
Rohm and Haas Report No. 23-6.
(Unpublished)
Rohm & Haas. A study to determine residue levels in
1971 milk, tissues and excreta of a cow fed
14 C-labelled TOK. Rohm and Haas Report
No. 23-30. (Unpublished)
Rohm & Haas. Reports on residues of nitrofen: In
1969-1980 broccoli, Report Nos. 78-0172,
78-0173, 78-0174, 78-0175, 78-0176,
78-0177, 80-0249, 80-0250, 80-0279,
80-0280, 80-0290, 80-0291; in cabbage,
Report Nos. 78-0336, 78-0337, 80-0245,
80-0246, 80-0286, 0-0287; in
cauliflower, report nos. 78-0166,
78-0167, 78-0168, 78-0169, 78-0170,
78-0171, 80-0247, 80-0248, 80-288,
80-0289; in celery, Report Nos. 80-0238,
80-0251, 80-0292, 80-0293; garlic,
Report Nos. 80-0252, 80-0253, 80-0254,
80-0255; in rice, Report Nos. 2-69-227,
2-70-173, 2-70-190; in wheat, Report
Nos. 72-121-02, 72-122-02, 72-123-02,
72-292-02, 72-293-02, 72-294-02,
73-266-02, 73-267-02, 73-268-02,
73-269-02, 73-270-02, 73-271-02,
73-272-02, 73-309-02, 73-301-02,
73-311-02, 73-312-02, 73-313-02,
73-314-02, 73-315-02, 74-075-02,
74-076-02. (Unpublished)
Roser, R.L. & Adler, I.L. The nature of aged residues of TOK.
1971 Rohm & Haas Report No. 23-34.
(Unpublished)
Wargo, J.P. TOK and amino-TOK analysis of 14C-TOK
1974 treated wheat. Rohm and Haas Report No.
3923-74-8. (Unpublished)
Watanabe, I. Decomposition of pesticides, by soil
1973 micro-organisms - Special emphasis on
the flooded soil condition. Jap. Agric.
Res. Quar., 7:15.
Zorgorski, W.J. & Analysis of ground water samples for
Rogerson, T.D. residues of KELTHANE and TOK. Rohm
and Haas Report No. 34H-80-1.
(Unpublished)
