
FENITROTHION JMPR 1977
Explanation
Fenitrothion was evaluated by the Joint Meeting in 1969, 1974 and 1976
(FAO/WHO, 1970, 1975, 1976,). An ADI of 0.005 mg/kg/day was
recommended in 1974. Because clinical signs were noted in humans at a
dose of 0.3 mg/kg further observation in humans was desired.
The Codex Committee on Pesticide Residues, at its 9th Session
considered the feasibility of applying the limit on citrus fruit
rather than simply on oranges. It was noted that the data available to
the Joint Meeting were limited to oranges only and that it would be
inappropriate to extend the limit to cover all citrus fruits.
Governments were invited to send data to the Joint Meeting. No
information was received on this topic, but other additional data on
both toxicological and residue aspects have become available. They are
summarized in the following monograph addendum.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Further information on absorption, distribution and excretion.
A metabolism study with special attention to the fate of the phenolic
moiety was carried out with fenitrothion (> 99.8%) 14C labelled at
the ring-methyl group.
Male rats were given a single oral dose of 15 mg/kg of labelled
fenitrothion and tissue levels were determined 1 and 24 hours p.a.
At 1 hour p.a. not more than 25% of the applied dose was present in
the gastro-intestinal tract and significant radio-activity was found
in the kidneys (11.7 mg/kg fenitrothion equivalents) stomach and
intestines (5.4), liver (2.6), blood (2.1) and lungs (1.1). In these
tissues > 80% of the radioactivity represented water soluble
metabolites, except in the stomach and intestines where 68% was
fenitrothion. In kidneys additionally a high content of
3-methyl-4-nitrophenol (MNP) (2.1 mg/kg) was detected; in other
tissues this amounted to less than 0.2 mg/kg. Fenitrooxon was scarcely
found with not more than 0.024 mg/kg in the stomach and intestine,
0.008 mg/kg in the kidneys and less than 0.005 mg/kg in other tissues.
Some fenitrothion was detected in the fat and pancreas (both 0.5
mg/kg).
Twenty-four hours p.a. all tissues contained less than 0.1 mg/kg of
total 14C, with highest levels in the liver, kidneys, fat and
stomach and intestine, partly as fenitrothion and partly as
metabolites. Other tissue levels were lower than 0.005 mg/kg for all
metabolites.
The same distribution was observed by autoradiograms of mice and rats,
shortly and 24 hours after application of 15 mg/kg labelled
fenitrothion.
The concentrations of fenitrothion in the blood of rats, rabbits, mice
and dogs were determined at 1, 3, 9, 24 and 48 hours after oral
application of 15 mg/kg. A maximum was observed at 1 or 3 hours and
levels were below 0.01 mg/kg at 24 hours.
Five male rabbits were fed 0.3 and 10 mg/kg/day fenitrothion for six
months. Fenitrothion (< 0.005 ppm) and fenitrooxon (< 0.001 ppm)
were not detectable (< 0.005 and < 0.001 mg/kg respectively) in
blood and skeletal muscle. In the fat 0.13 mg/kg of the oxygen
analogue was found.
After a single oral dose of 15 mg/kg labelled fenitrothion to mice,
rabbits, dogs and rats the major part (80.-90%) of the
14C-metabolites were excreted in the urine in 24 hours and 5%, with
the faeces; the excretion was nearly complete at 48 hours p.a. In rats
no 14C-metabolites were expired. In the urine 18 metabolites were
identified with 3-methyl-4-nitrophenol (as the sulphate, free or as
glucuronide) as the main metabolite in the rodents (50-70%, dogs only
35%) followed by 0-desmethyl-fenitrothion/-oxon (from 10% in rabbits
to 55% in dogs). In rabbits and rats small quantities of other
metabolites were determined with the nitro group reduced to amino
(total ca. 15%) or with an oxidized ring methyl group (ca. 1%).
Fenitrothion was not detectable and traces of fenitrooxon (ca. 0.4%)
were determined in rabbit urine only. About 5% remained unidentified.
In faeces of rats the same major metabolites as in urine,
3-methyl-4-nitrophenol (70%) and 0-desmethy-fenitrothion/oxon (20%),
occurred but also some fenitrothion (10%) was found (Miyamoto et al.,
1976).
Fenitrothion S-methyl isomer
Five female rats received a single oral dose of 3.1, 12.5, 50, 100 and
200 mg/kg S-methyl isomer of fenitrothion (SMF), an impurity. Urinary
excretion of the metabolite 3-methyl-4-nitrophenol was 65 to 75% of
the applied dose within 72 hours p.a. with 40 to 60%, within 5 hours
and less than 1.3% on the 3rd day.
Cholinesterase inhibition in plasma and erythrocytes was maximal at 6
hours (60-90%, dose-related) and 48 hours (25-90%) respectively.
Recovery occurred slowly, depending on the dose, within 3 to 40 days
(HladkŠ and Krampl, 1975).
The anticholinesterase activity of SMF in vitro was found to be 2 to
3 times higher than that of fenitrothion (Rosival et al., 1976).
TOXICOLOGICAL STUDIES
Special studies on potentiation
After one treatment with CC14 the fenitrothion content of rat liver
was dose-dependant and significantly increased and the compound was
chemically bound to the oxygenated form of cytochrome P-450.
An in vitro study with liver homogenates, of rats (incubated in a
solution containing 0.3 mg/ml fenitrothion) showed a decreased
fenitrothion-degrading activity of the cytochrome P-450 in rats that
were pre-treated with 0.5 mg/ml CCI4 (oral).
The results may indicate that fenitrothion is metabolized by the liver
enzyme cytochrome P-450 to a toxically active component (Szutowski,
1975).
Special studies on teratogenecity
Fenitrothion was injected into the yolk of chicken eggs at doses of
0.1 ml of 0.1-30% fenitrothion. Dose levels of 1 and 0.1% produced
malformations (curled toe, leg weakness and abnormal gait). At the
high dose levels all the embryos died. There was also a dose-related
influence on the hatchibility (Paul and Vadlamudi, 1976),
Special studies on neurotoxicity
Sixteen hens received orally 500 mg/kg fenitrothion (97.2%) and were
protected against intoxication by atropine and PAM; this treatment was
repeated three weeks later. TOTP was used as a positive control. Five
hens died within two days after the first and none after the second
treatment; survivors showed symptoms of cholinesterase inhibition. No
paralysis was observed and histopathological findings in sciatic
nerves were normal.
In another test twelve hens received orally 35 mg/kg fenitrooxon (98%)
and were protected by atropine and PAM, the treatment was repeated
after three weeks. TOTP served as a positive control. Five hens died
and none showed paralysis in a three week observation period (Kadota
et al., 1975a).
Special studies on ocular toxicity
Dogs were dosed orally twice weekly with 0.5, 1 and 5 mg/kg
fenitrothion for 1 year. Myopization was observed 4 months after this
period.
The intraocular pressure was elevated at the fourth and ninth month on
test but recovered four months after the end. No significant changes
of corneal power and refractive components were observed (Tokoro et
al., 1976; summary only).
Rats that received 150 and 300 ppm fenitrothion in feed for 13 and 16
weeks respectively showed oedema and hyperaemia of the eyelids and
corneal infiltration or erosion. A decrease in body weight gain and in
serum cholinesterase was also observed (Fukami, 1976; summary only).
Acute toxicity
Fenitrothion
species route LD50(mg/kg) references
Hen oral. ca. 500 Kadota et al., 1975a
Jpn. quail M oral 115 (80-166) Kadato & Miyamota,
1975
Jpn. quail F oral 140 (105-186) Ibid.
Death occurred within two days; symptoms of cholinesterase, poisoning
disappeared in ca. eight days.
Fenitrothion S-methyl isomer
species route LD50(mg/kg) references
mouse oral ca. 320 Rosival et al, 1976
rat (M) oral 315 Ibid.
Fenitrooxon
species route LD50 (mg/kg) references
Hen oral 35 (21-57) Kadota et al., 1975a
Death occurred within 1 day; symptoms of cholinesterase poisoning, in
ca. eight days.
Short-term studies
Japanese quail, 60 males and 60 females per group (20 males and 20
females in the 1.5 ppm group) received 0, 1.5, 5, 15 and 50 ppm
fenitrothion (97.2%, dissolved in corn-oil in the diet 4 weeks. Every
two weeks 10 males and 10 females were sacrificed for ChE
determination in blood and brains respectively. Effects: no animals
died and no toxic symptoms were seen, nor abnormalities in body weight
or food consumption. A dose-related decrease of blood ChE activity was
observed in the females of the 5 ppm group (25%), males and females of
the 15 ppm (60%) and 50 ppm (85%) groups during the treatment, when on
the basal diet, recovery occurred within 2 weeks.
Brain ChE activity was reduced in the females of the 15 ppm group
(25%) and the males and females of the 50 ppm group (65%) and
recovered after 4 weeks. In the 50 ppm group the egg-laying rate was
decreased and recovered after 3 weeks (Kadota and Miyamoto, 1975).
Short-term studies (metabolites)
Groups of fifteen male and fifteen female Wistar rats received 0, 5,
15 and 50 ppm fenitrooxon (99%) respectively in the diet for six
months. Cholinesterase activity in plasma and brain was dose-related
and significantly decreased in males and females of the 15 and 50 ppm
groups; erythrocyte-ChE was significantly decreased in males and
females of the 50 ppm group. No clear dose dependent changes were
observed in body weight ratios, food consumption, and biochemical
parameters or histopathology (Kadota et al., 1975b).
COMMENTS
Additional information on absorption, distribution, excretion,
potentiation, terato-genicity and neurotoxicity was available.
Fenitrothion is easily absorbed from the gastro-intestinal tract and
distributed into various tissues. The main metabolites are
3-methyl-4-nitrophenol and 0-desmethyl-fenitrothion and/or its oxygen
analogue, which are eliminated rapidly, predominantly by the kidneys.
Tissue residues are very low. There are some indications that
fenitrothion induces increased ocular pressure after oral application
in both dogs and rats. However, this can be expected since it
depresses cholinesterases. No-effect levels in both rats and dogs were
demonstrated at 5 ppm in the diet. There are no reasons to alter the
previously established ADI for humans.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 5 mg/kg in the diet, equivalent to 0.25 mg/kg bw
Dog: 5 mg/kg in the diet, equivalent to 0.125 mg/kg bw
ESTIMATE OF ACCEPTABLE DAILY INTAKE FOR HUMANS
0.005 mg/kg bw
RESIDUES IN FOOD AND THEIR EVALUATION
RESIDUES RESULTING FROM SUPERVISED TRIALS
Pre-harvest
The Japanese Science and Technology Agency advised the Meeting that
extensive trials were carried out between 1973 and 1975 in many
prefecture of Japan where a variety of fenitrothion formulations were
applied to rice crops. From 1 to 7 applications were made with
pre-harvest intervals ranging from 14 to 120 days. Most of the samples
of hulled rice contained no detectable residues at harvest (limit of
determination 0.001 mg/kg) but a few contained small residues ranging
up to a maximum of 0.025 mg/kg.
Post-harvest
Having been established for post-harvest use on wheat, studies were
made into the usefulness and fate of fenitrothion on barley, oats and
rice and milling products derived therefrom (FAO-WHO 1977). These,
studies have been extended to include sorghum and further experience
has confirmed that it is possible and convenient to predict the
residues of fenitrothion on any raw cereal variety when the
temperature and relative humidity of the intergrain air is known.
Following extensive pilot and commercial-scale trials, the Australian
Wheat Board has treated tons of wheat with fenitrothion at rates
ranging from 6 to 12 mg/kg depending, on grain temperature, moisture
content, and anticipated storage period. Following systematic
monitoring of the fenitrothion content of samples drawn from over 20
different storage cites each month for over eight months,
Desmarchelier (1977a) reported that the rate of loss of fenitrothion
was consistent with predicted values.
Bengston et al. (1977a) carried out field experiments with grain
protectant insecticides for the control of malathion-resistant insects
in stored sorghum in which the level of residues determined by
analysis and the insecticidal activity was measured by bio-assay using
10 species of malathion-resistant insects. These workers reported that
fenitrothion applied at the nominal rate of 12 mg/kg was effective in
all species except Rhizopertha dominica for more than 12 weeks (test
still in progress). During the first 24 weeks of storage the residue
levels were as indicated in Table 1.
TABLE 1. Fenitrothion residues on stored sorghum
Residue (mg/kg) at intervals (weeks) after storage.
Site 1 4 8 12 18 24
1 13.4 10.5 10.0 7.9 9.0 8.3
2 7.7 7.8 8.6 7.7 5.9 5.6
3 16.4 11.5 11.1 9.3 8.8 9.1
4 8.2 7.4 10.1 8.5 5.2 4.0
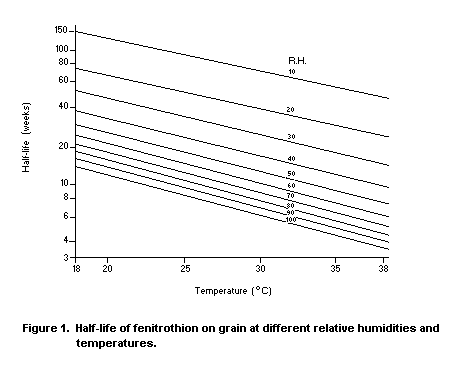
Desmarchelier (1977b) found that the loss of fenitrothion from
post-harvest application to wheat, oats, paddy rice and sorghum was a
second-order process, with rate of loss being proportional, at a fixed
temperature, to the amount of fenitrothion and the activity of water,
which was obtained from the equilibrium partial pressure of water
vapour in the inter-grain space. Figure 1 relates the half-life of the
deposit of temperature and relative humidity. Tables are published
elsewhere showing the relative humidity of the inter-grain air
equilibrium with different varieties of grain of varying moisture
content.
It is obvious from these and other studies by the same author (FAO/WHO
1977) in respect of fenitrothion, pirimiphos-methyl, carbaryl,
bioresmethrin and other insecticides that the fate of residues of
grain protectant insectides can be predicted from calculations based
on several well-defined parameters (Desmarchelier, 1977c). The
recommended maximum residue limits can therefore be extended to cover
all raw grains in the confident expectation that the level of the
initial deposit will decline at a predicted rate dependent only on
temperature and relative humidity.
Results of three studies with fenitrothion on barley (Green and Tyler,
1966; Rengston et al., 1977b and Desmarchelier et al., 1977) were used
to test the predictions of Desmarchelier (19770) and a high level of
agreement was found.
 APPRAISAL
The usefulness of fenitrothion as a grain protectant insecticide and
the fate of fenitrothion applied to various grains have been evaluated
in 1974 and 1976. The results of further studies on a variety of
grains including barley, oats, rice, sorghum and wheat have revealed
that the rate of decline in residue levels in stored raw cereals can
be predicted accurately from a knowledge of the temperature, relative
humidity and residue concentration.
These data, taken in conjunction with information contained in
previous monographs, have provided adequate assurance that the maximum
residue limits already recommended for rice and wheat can be extended
to embrace all raw cereals, including sorghum.
Results from extensive commercial-scale trials on sorghum confirm that
these assumptions are correct. Additional studies following the
application of fenitrothion to malting barley have confirmed that
there is no adverse effect from the treatment on the quality of the
malt produced. Residues in malt and beer are in keeping with
anticipated values.
RECOMMENDATIONS
The previously recommended maximum residue limit for fenitrothion on
rice and wheat is replaced by a limit at the same level for raw
cereals. The limit is for the sum of fenitrothion and its oxygen
analogue, expressed as fenitrothion.
Commodity Limit, mg/kg
raw cereal 10
FURTHER WORK OR INFORMATION
DESIRABLE
1. Further observations in humans.
2. Information on the level and fate of fenitrothion residues on
citrus varieties other than oranges.
REFERENCES
Bengston, M., Cooper, L.M., Davies, R.A.H., Desmarchelier, J.M., Hart,
R.J. and Phillips, M. (1977a) Grain Protectants for the Control of
Malathion-resistant insects in stored sorghum. J. Stored Prod. Res.
(submitted for publication).
Bengston, M., Davies, R.A.H., Desmarchelier, J.H., Hart, R.J., Moore,
B., Murray, W., Phillips, M., Ridley, E., Ripp, E., Scewakowski, C.,
Snelson J.T., Sticka, R., Wallbank, B. and Wilson, A. (1977b),
Extensive pilot usage of the grain protectants chlopyrifos-methyl plus
bioresmethrin, fenitrothion plus d-fenothrin methacrifos and
primiphos-methyl plus carbaryl (in preparation).
Desmarchelier, J.M. (CSIRO Division of Entomology, Canberra) (1977a)
Analysis of fenitrothion 1976-77 Pilot trials. Report to the
Australian Wheat Board Pest Control Conference (July 1977) (To be
published)
Desmarchelier, J.M. (1977b) Loss of fenitrothion on grains in storage.
J. Stored Prod. Res. (in-press).
Desmarchelier, J.M. (1977c) Loss of bioresmethrin, carbaryl,
d-fenothrin etc. on grain in (manuscripts of papers being prepared for
publication).
Desmarchelier, J.M., Bengston, M., Connell, M., Phillips, M., Ridley,
E., Snelson, J.T., Sticka, R. and Wilson, A. (1977) Extensive pilot
usage of the grain protectant combinations fenitrothion plus
bioresmethrin and primiphos-methyl plus bioresmethrin (in
preparation).
Fukami Y. (1976) The ocular injurious level of organic phosphates in
rats. Jpn. J, Clin. Ophthalmol., 30, 841-848.
Green, A.A. and Tyler, P.S. (1966) A field comparison of malathion,
dichlorvos and fenitrothion for the control of Oryzaepholus
surenamensis infesting stored barley. J. Stored Prod. Research, I,
197.
HladkŠ, A. and Krampl, V. (1975) Effect of S-methylfenitrothion on the
activity of cholinesterase and on the excretion of its metabolites in
rats. Int. Arch. Occup. Environ. Hlth., 36, 67-73.
Kadota, T., Okuno, Y, and Miyamoto, J. (1975a) Acute oral toxicity and
delayed neurotoxicity of 5 organophosphorus compounds, salithion,
cyanox, surecide, sumithion and sumiaxon in adult hens. Botyu-Kagaki
(Sci. Pest. Contr.), 40, 49-53.
Kadota T. and Miyamoto, J. (1975) Acute and sub-acute toxicity of
sumithion in japanese quails, Botyu-Kagaku (Sci. Pest. Contr.), 40,
54-58.
Yiyamoto, J., Mihara, K. and Hosokawa, S. (1976) Comparative
metabolism of m-methyl-14C-sumithion in several species of mammals
in vivo. J. Pesticide Sci., 1, 9-21.
Paul, B.S. and Vadlamudi, V.P. (1976) Teratogenic studies of
fenitrothion on white leghorn chick embryos. Bull, of Environ.
Contamination and Toxic., 15, 223-229.
Rosival, L., Vargova, M., SzokolayovŠ J., Cerey, K., HladkŠ, A.,
BŠtora, V., KovacicovŠ, J. and Truchlik, S. (1976) Contribution to the
toxic action of S-Methyl fenitrothion. Pest. Biochem. and Physiol.,
6, 280.286.
Szutowski, M.M. (1975) Effect of carbontetrachloride on activation and
detoxification of organophosphorus insecticides in the rat. Toxical.
Appl. Pharmacol., 33, 350-355.
Tokoro T., Suzuki, K., Hayashi, K. and Otsuka, J. (1976) Development
of myopia induced by organic phosphorous pesticide (sumithion) in
beagle dogs. J. Jpn. Ophthalmol. Soc., 80, 51-53.
FAO/WHO (1970) 1969 evaluations of some pesticide residues in food.
FAO/PL:1969/M/17/1; WHO/Food Add./70.38.
FAO/WHO (1975) 1974 evaluations of some pesticide residues in food.
AGP:1974/M/11; WHO Pesticide Residues Series No. 4.
FAO/WHO (1976) 1975 evaluations of some pesticide residues in food.
AGP:1975/m/13; WHO Pesticide Residues Series No. 5.
FAO/WHO (1977) 1976 evaluations of some pesticide residues in food.
AGP:1976/M/14.
APPRAISAL
The usefulness of fenitrothion as a grain protectant insecticide and
the fate of fenitrothion applied to various grains have been evaluated
in 1974 and 1976. The results of further studies on a variety of
grains including barley, oats, rice, sorghum and wheat have revealed
that the rate of decline in residue levels in stored raw cereals can
be predicted accurately from a knowledge of the temperature, relative
humidity and residue concentration.
These data, taken in conjunction with information contained in
previous monographs, have provided adequate assurance that the maximum
residue limits already recommended for rice and wheat can be extended
to embrace all raw cereals, including sorghum.
Results from extensive commercial-scale trials on sorghum confirm that
these assumptions are correct. Additional studies following the
application of fenitrothion to malting barley have confirmed that
there is no adverse effect from the treatment on the quality of the
malt produced. Residues in malt and beer are in keeping with
anticipated values.
RECOMMENDATIONS
The previously recommended maximum residue limit for fenitrothion on
rice and wheat is replaced by a limit at the same level for raw
cereals. The limit is for the sum of fenitrothion and its oxygen
analogue, expressed as fenitrothion.
Commodity Limit, mg/kg
raw cereal 10
FURTHER WORK OR INFORMATION
DESIRABLE
1. Further observations in humans.
2. Information on the level and fate of fenitrothion residues on
citrus varieties other than oranges.
REFERENCES
Bengston, M., Cooper, L.M., Davies, R.A.H., Desmarchelier, J.M., Hart,
R.J. and Phillips, M. (1977a) Grain Protectants for the Control of
Malathion-resistant insects in stored sorghum. J. Stored Prod. Res.
(submitted for publication).
Bengston, M., Davies, R.A.H., Desmarchelier, J.H., Hart, R.J., Moore,
B., Murray, W., Phillips, M., Ridley, E., Ripp, E., Scewakowski, C.,
Snelson J.T., Sticka, R., Wallbank, B. and Wilson, A. (1977b),
Extensive pilot usage of the grain protectants chlopyrifos-methyl plus
bioresmethrin, fenitrothion plus d-fenothrin methacrifos and
primiphos-methyl plus carbaryl (in preparation).
Desmarchelier, J.M. (CSIRO Division of Entomology, Canberra) (1977a)
Analysis of fenitrothion 1976-77 Pilot trials. Report to the
Australian Wheat Board Pest Control Conference (July 1977) (To be
published)
Desmarchelier, J.M. (1977b) Loss of fenitrothion on grains in storage.
J. Stored Prod. Res. (in-press).
Desmarchelier, J.M. (1977c) Loss of bioresmethrin, carbaryl,
d-fenothrin etc. on grain in (manuscripts of papers being prepared for
publication).
Desmarchelier, J.M., Bengston, M., Connell, M., Phillips, M., Ridley,
E., Snelson, J.T., Sticka, R. and Wilson, A. (1977) Extensive pilot
usage of the grain protectant combinations fenitrothion plus
bioresmethrin and primiphos-methyl plus bioresmethrin (in
preparation).
Fukami Y. (1976) The ocular injurious level of organic phosphates in
rats. Jpn. J, Clin. Ophthalmol., 30, 841-848.
Green, A.A. and Tyler, P.S. (1966) A field comparison of malathion,
dichlorvos and fenitrothion for the control of Oryzaepholus
surenamensis infesting stored barley. J. Stored Prod. Research, I,
197.
HladkŠ, A. and Krampl, V. (1975) Effect of S-methylfenitrothion on the
activity of cholinesterase and on the excretion of its metabolites in
rats. Int. Arch. Occup. Environ. Hlth., 36, 67-73.
Kadota, T., Okuno, Y, and Miyamoto, J. (1975a) Acute oral toxicity and
delayed neurotoxicity of 5 organophosphorus compounds, salithion,
cyanox, surecide, sumithion and sumiaxon in adult hens. Botyu-Kagaki
(Sci. Pest. Contr.), 40, 49-53.
Kadota T. and Miyamoto, J. (1975) Acute and sub-acute toxicity of
sumithion in japanese quails, Botyu-Kagaku (Sci. Pest. Contr.), 40,
54-58.
Yiyamoto, J., Mihara, K. and Hosokawa, S. (1976) Comparative
metabolism of m-methyl-14C-sumithion in several species of mammals
in vivo. J. Pesticide Sci., 1, 9-21.
Paul, B.S. and Vadlamudi, V.P. (1976) Teratogenic studies of
fenitrothion on white leghorn chick embryos. Bull, of Environ.
Contamination and Toxic., 15, 223-229.
Rosival, L., Vargova, M., SzokolayovŠ J., Cerey, K., HladkŠ, A.,
BŠtora, V., KovacicovŠ, J. and Truchlik, S. (1976) Contribution to the
toxic action of S-Methyl fenitrothion. Pest. Biochem. and Physiol.,
6, 280.286.
Szutowski, M.M. (1975) Effect of carbontetrachloride on activation and
detoxification of organophosphorus insecticides in the rat. Toxical.
Appl. Pharmacol., 33, 350-355.
Tokoro T., Suzuki, K., Hayashi, K. and Otsuka, J. (1976) Development
of myopia induced by organic phosphorous pesticide (sumithion) in
beagle dogs. J. Jpn. Ophthalmol. Soc., 80, 51-53.
FAO/WHO (1970) 1969 evaluations of some pesticide residues in food.
FAO/PL:1969/M/17/1; WHO/Food Add./70.38.
FAO/WHO (1975) 1974 evaluations of some pesticide residues in food.
AGP:1974/M/11; WHO Pesticide Residues Series No. 4.
FAO/WHO (1976) 1975 evaluations of some pesticide residues in food.
AGP:1975/m/13; WHO Pesticide Residues Series No. 5.
FAO/WHO (1977) 1976 evaluations of some pesticide residues in food.
AGP:1976/M/14.
 APPRAISAL
The usefulness of fenitrothion as a grain protectant insecticide and
the fate of fenitrothion applied to various grains have been evaluated
in 1974 and 1976. The results of further studies on a variety of
grains including barley, oats, rice, sorghum and wheat have revealed
that the rate of decline in residue levels in stored raw cereals can
be predicted accurately from a knowledge of the temperature, relative
humidity and residue concentration.
These data, taken in conjunction with information contained in
previous monographs, have provided adequate assurance that the maximum
residue limits already recommended for rice and wheat can be extended
to embrace all raw cereals, including sorghum.
Results from extensive commercial-scale trials on sorghum confirm that
these assumptions are correct. Additional studies following the
application of fenitrothion to malting barley have confirmed that
there is no adverse effect from the treatment on the quality of the
malt produced. Residues in malt and beer are in keeping with
anticipated values.
RECOMMENDATIONS
The previously recommended maximum residue limit for fenitrothion on
rice and wheat is replaced by a limit at the same level for raw
cereals. The limit is for the sum of fenitrothion and its oxygen
analogue, expressed as fenitrothion.
Commodity Limit, mg/kg
raw cereal 10
FURTHER WORK OR INFORMATION
DESIRABLE
1. Further observations in humans.
2. Information on the level and fate of fenitrothion residues on
citrus varieties other than oranges.
REFERENCES
Bengston, M., Cooper, L.M., Davies, R.A.H., Desmarchelier, J.M., Hart,
R.J. and Phillips, M. (1977a) Grain Protectants for the Control of
Malathion-resistant insects in stored sorghum. J. Stored Prod. Res.
(submitted for publication).
Bengston, M., Davies, R.A.H., Desmarchelier, J.H., Hart, R.J., Moore,
B., Murray, W., Phillips, M., Ridley, E., Ripp, E., Scewakowski, C.,
Snelson J.T., Sticka, R., Wallbank, B. and Wilson, A. (1977b),
Extensive pilot usage of the grain protectants chlopyrifos-methyl plus
bioresmethrin, fenitrothion plus d-fenothrin methacrifos and
primiphos-methyl plus carbaryl (in preparation).
Desmarchelier, J.M. (CSIRO Division of Entomology, Canberra) (1977a)
Analysis of fenitrothion 1976-77 Pilot trials. Report to the
Australian Wheat Board Pest Control Conference (July 1977) (To be
published)
Desmarchelier, J.M. (1977b) Loss of fenitrothion on grains in storage.
J. Stored Prod. Res. (in-press).
Desmarchelier, J.M. (1977c) Loss of bioresmethrin, carbaryl,
d-fenothrin etc. on grain in (manuscripts of papers being prepared for
publication).
Desmarchelier, J.M., Bengston, M., Connell, M., Phillips, M., Ridley,
E., Snelson, J.T., Sticka, R. and Wilson, A. (1977) Extensive pilot
usage of the grain protectant combinations fenitrothion plus
bioresmethrin and primiphos-methyl plus bioresmethrin (in
preparation).
Fukami Y. (1976) The ocular injurious level of organic phosphates in
rats. Jpn. J, Clin. Ophthalmol., 30, 841-848.
Green, A.A. and Tyler, P.S. (1966) A field comparison of malathion,
dichlorvos and fenitrothion for the control of Oryzaepholus
surenamensis infesting stored barley. J. Stored Prod. Research, I,
197.
HladkŠ, A. and Krampl, V. (1975) Effect of S-methylfenitrothion on the
activity of cholinesterase and on the excretion of its metabolites in
rats. Int. Arch. Occup. Environ. Hlth., 36, 67-73.
Kadota, T., Okuno, Y, and Miyamoto, J. (1975a) Acute oral toxicity and
delayed neurotoxicity of 5 organophosphorus compounds, salithion,
cyanox, surecide, sumithion and sumiaxon in adult hens. Botyu-Kagaki
(Sci. Pest. Contr.), 40, 49-53.
Kadota T. and Miyamoto, J. (1975) Acute and sub-acute toxicity of
sumithion in japanese quails, Botyu-Kagaku (Sci. Pest. Contr.), 40,
54-58.
Yiyamoto, J., Mihara, K. and Hosokawa, S. (1976) Comparative
metabolism of m-methyl-14C-sumithion in several species of mammals
in vivo. J. Pesticide Sci., 1, 9-21.
Paul, B.S. and Vadlamudi, V.P. (1976) Teratogenic studies of
fenitrothion on white leghorn chick embryos. Bull, of Environ.
Contamination and Toxic., 15, 223-229.
Rosival, L., Vargova, M., SzokolayovŠ J., Cerey, K., HladkŠ, A.,
BŠtora, V., KovacicovŠ, J. and Truchlik, S. (1976) Contribution to the
toxic action of S-Methyl fenitrothion. Pest. Biochem. and Physiol.,
6, 280.286.
Szutowski, M.M. (1975) Effect of carbontetrachloride on activation and
detoxification of organophosphorus insecticides in the rat. Toxical.
Appl. Pharmacol., 33, 350-355.
Tokoro T., Suzuki, K., Hayashi, K. and Otsuka, J. (1976) Development
of myopia induced by organic phosphorous pesticide (sumithion) in
beagle dogs. J. Jpn. Ophthalmol. Soc., 80, 51-53.
FAO/WHO (1970) 1969 evaluations of some pesticide residues in food.
FAO/PL:1969/M/17/1; WHO/Food Add./70.38.
FAO/WHO (1975) 1974 evaluations of some pesticide residues in food.
AGP:1974/M/11; WHO Pesticide Residues Series No. 4.
FAO/WHO (1976) 1975 evaluations of some pesticide residues in food.
AGP:1975/m/13; WHO Pesticide Residues Series No. 5.
FAO/WHO (1977) 1976 evaluations of some pesticide residues in food.
AGP:1976/M/14.
APPRAISAL
The usefulness of fenitrothion as a grain protectant insecticide and
the fate of fenitrothion applied to various grains have been evaluated
in 1974 and 1976. The results of further studies on a variety of
grains including barley, oats, rice, sorghum and wheat have revealed
that the rate of decline in residue levels in stored raw cereals can
be predicted accurately from a knowledge of the temperature, relative
humidity and residue concentration.
These data, taken in conjunction with information contained in
previous monographs, have provided adequate assurance that the maximum
residue limits already recommended for rice and wheat can be extended
to embrace all raw cereals, including sorghum.
Results from extensive commercial-scale trials on sorghum confirm that
these assumptions are correct. Additional studies following the
application of fenitrothion to malting barley have confirmed that
there is no adverse effect from the treatment on the quality of the
malt produced. Residues in malt and beer are in keeping with
anticipated values.
RECOMMENDATIONS
The previously recommended maximum residue limit for fenitrothion on
rice and wheat is replaced by a limit at the same level for raw
cereals. The limit is for the sum of fenitrothion and its oxygen
analogue, expressed as fenitrothion.
Commodity Limit, mg/kg
raw cereal 10
FURTHER WORK OR INFORMATION
DESIRABLE
1. Further observations in humans.
2. Information on the level and fate of fenitrothion residues on
citrus varieties other than oranges.
REFERENCES
Bengston, M., Cooper, L.M., Davies, R.A.H., Desmarchelier, J.M., Hart,
R.J. and Phillips, M. (1977a) Grain Protectants for the Control of
Malathion-resistant insects in stored sorghum. J. Stored Prod. Res.
(submitted for publication).
Bengston, M., Davies, R.A.H., Desmarchelier, J.H., Hart, R.J., Moore,
B., Murray, W., Phillips, M., Ridley, E., Ripp, E., Scewakowski, C.,
Snelson J.T., Sticka, R., Wallbank, B. and Wilson, A. (1977b),
Extensive pilot usage of the grain protectants chlopyrifos-methyl plus
bioresmethrin, fenitrothion plus d-fenothrin methacrifos and
primiphos-methyl plus carbaryl (in preparation).
Desmarchelier, J.M. (CSIRO Division of Entomology, Canberra) (1977a)
Analysis of fenitrothion 1976-77 Pilot trials. Report to the
Australian Wheat Board Pest Control Conference (July 1977) (To be
published)
Desmarchelier, J.M. (1977b) Loss of fenitrothion on grains in storage.
J. Stored Prod. Res. (in-press).
Desmarchelier, J.M. (1977c) Loss of bioresmethrin, carbaryl,
d-fenothrin etc. on grain in (manuscripts of papers being prepared for
publication).
Desmarchelier, J.M., Bengston, M., Connell, M., Phillips, M., Ridley,
E., Snelson, J.T., Sticka, R. and Wilson, A. (1977) Extensive pilot
usage of the grain protectant combinations fenitrothion plus
bioresmethrin and primiphos-methyl plus bioresmethrin (in
preparation).
Fukami Y. (1976) The ocular injurious level of organic phosphates in
rats. Jpn. J, Clin. Ophthalmol., 30, 841-848.
Green, A.A. and Tyler, P.S. (1966) A field comparison of malathion,
dichlorvos and fenitrothion for the control of Oryzaepholus
surenamensis infesting stored barley. J. Stored Prod. Research, I,
197.
HladkŠ, A. and Krampl, V. (1975) Effect of S-methylfenitrothion on the
activity of cholinesterase and on the excretion of its metabolites in
rats. Int. Arch. Occup. Environ. Hlth., 36, 67-73.
Kadota, T., Okuno, Y, and Miyamoto, J. (1975a) Acute oral toxicity and
delayed neurotoxicity of 5 organophosphorus compounds, salithion,
cyanox, surecide, sumithion and sumiaxon in adult hens. Botyu-Kagaki
(Sci. Pest. Contr.), 40, 49-53.
Kadota T. and Miyamoto, J. (1975) Acute and sub-acute toxicity of
sumithion in japanese quails, Botyu-Kagaku (Sci. Pest. Contr.), 40,
54-58.
Yiyamoto, J., Mihara, K. and Hosokawa, S. (1976) Comparative
metabolism of m-methyl-14C-sumithion in several species of mammals
in vivo. J. Pesticide Sci., 1, 9-21.
Paul, B.S. and Vadlamudi, V.P. (1976) Teratogenic studies of
fenitrothion on white leghorn chick embryos. Bull, of Environ.
Contamination and Toxic., 15, 223-229.
Rosival, L., Vargova, M., SzokolayovŠ J., Cerey, K., HladkŠ, A.,
BŠtora, V., KovacicovŠ, J. and Truchlik, S. (1976) Contribution to the
toxic action of S-Methyl fenitrothion. Pest. Biochem. and Physiol.,
6, 280.286.
Szutowski, M.M. (1975) Effect of carbontetrachloride on activation and
detoxification of organophosphorus insecticides in the rat. Toxical.
Appl. Pharmacol., 33, 350-355.
Tokoro T., Suzuki, K., Hayashi, K. and Otsuka, J. (1976) Development
of myopia induced by organic phosphorous pesticide (sumithion) in
beagle dogs. J. Jpn. Ophthalmol. Soc., 80, 51-53.
FAO/WHO (1970) 1969 evaluations of some pesticide residues in food.
FAO/PL:1969/M/17/1; WHO/Food Add./70.38.
FAO/WHO (1975) 1974 evaluations of some pesticide residues in food.
AGP:1974/M/11; WHO Pesticide Residues Series No. 4.
FAO/WHO (1976) 1975 evaluations of some pesticide residues in food.
AGP:1975/m/13; WHO Pesticide Residues Series No. 5.
FAO/WHO (1977) 1976 evaluations of some pesticide residues in food.
AGP:1976/M/14.
