
WHO FOOD ADDITIVES SERIES: 52
First draft prepared by
Mrs I.M.E.J. Pronk
Centre for Substances and Integrated Risk Assessment, National Institute for Public Health and the Environment, Bilthoven, Netherlands
and
Dr C. Leclercq
National Research Institute for Food and Nutrition, Rome, Italy
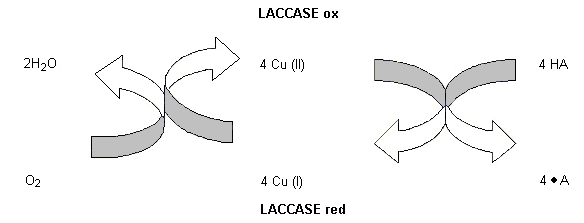
The enzyme preparation under evaluation contains the active enzyme laccase, which has not been evaluated previously by the Committee. Laccase is an enzyme that catalyses the oxidation of phenolic compounds such as ortho- and para-diphenols to their corresponding quinones, with the concomitant reduction of oxygen to water. This enzyme is marketed for use in the brewing of beer to prevent the formation of off-flavour compounds, such as trans-2-nonenal. Laccase scavenges oxygen which otherwise would react with fatty acids, amino acids, proteins, and alcohols to form off-flavour precursors.
The enzyme preparation (trade name, Flavourstar) is produced by submerged fermentation of Aspergillus oryzae carrying a gene encoding a laccase from Myceliophthora thermophila. Many fungi and plants produce laccases, and enzymes related to laccase are also produced by bacteria. They form a group of multi-copper proteins of low specifity and catalyse the oxidation of a wide range of both o- and p-quinols, and also aminophenols and phenylenediamine, with the concomittant reduction of oxygen to water. Although the specificity for the electron donor is low, the specificity for the acceptor (oxygen) is essential. Peroxides are not produced in the reaction. The reaction can be described by the chemical equation given in Figure 1.

Figure 1. Mechanism of oxidation catalysed by laccase
The activity of the laccase enzyme preparation is measured by a colorimetric method based on oxidation of the substrate chromophore syringaldazine under formation of tetramethoxy-azo-bismethylene-quinone, and is expressed as laccase Myceliophthora units, or LAMU. Flavourstar has a typical activity of 1000 LAMU/g, and has the following composition: total organic solids (TOS), approximately 5.3%; sorbitol, approximately 30%; sodium lactate, approximately 5%; glucose, approximately 3%; glycine, approximately 2%; water, approximately 54.3%; potassium sorbate, 0.2%; and sodium benzoate, 0.2%.
The evaluated enzyme preparation Flavourstar is used in the food industry as a processing aid in the brewing of beer to prevent the formation of off-flavour components, such as trans-2-nonenal. During mashing, laccase scavenges oxygen which otherwise would react with sensitive substances to form off-flavour components. Flavourstar is to be used at a maximum dosage of 350 g/ton of malt and barley, corresponding to 350 000 LAMU/ton of malt and barley. One ton of malt and barley renders approximately 6000 l of beer.
Toxicological studies have been performed with a liquid enzyme concentrate (batch PPX 5720). This batch was obtained by mixing of three sub-batches, omitting formulation, stabilization and standardization. The composition of test batch PPX 5720 is given in Table 1.
Table 1. Composition of laccase enzyme preparation, batch PPX 5720
|
PPX 5720 |
|
|
Enzyme activity, LAMU/g |
3130 |
|
TOS (% w/w) |
16.6 |
|
Ash (% w/w) |
0.3 |
|
Water (% w/w) |
83.1 |
|
Density (g/ml) |
1.039 |
From Brinch & Pedersen, 2002; Jensen, 2002 TOS, total organic solids
The A. oryzae production strain, designated as Mt, was developed by transfection of the A. oryzae host strain How B711 (derived from the A 1560 strain) with two plasmids, pRaMB17.WT and pToC90. The pRaMB17.WT plasmid contains the laccase gene from the thermophilic fungus, M. thermophila, which is found in decaying organic matter. The laccase gene is linked to DNA regulatory sequences, a promoter and a terminator. The pRAMB17.WT plasmid also contains the bla gene, which confers resistance to ampicillin, and other well-characterized DNA sequences. The pToC90 plasmid contains the amdS gene, which encodes acetamidase and enables A. oryzae to metabolize acetamide in the absence of other sources of carbon or nitrogen; it is also used as a selection marker. The pToC90 plasmid also contains the bla gene.
The transformants were selected after growth on a medium containing acetamide as the sole nitrogen source and screening for ability to produce laccase. One colony was selected and subjected to chemical mutagenesis and screening for high yield of laccase. A transformant producing an adequately high quantity of laccase was selected for use as the laccase production strain A. oryzae Mt.
The genetic material introduced into the production strain has been well characterized by known molecular biology methods and does not contain any sequences that would encode proteins that are toxic or that produce toxic or undesirable substances. The laccase gene is stably integrated into the A. oryzae genome. Although the introduced DNA contains the bla gene, this gene is not expressed because it is under the control of a bacterial promoter that is not functional in the eukaryotic fungus A. oryzae. Furthermore, the bla gene is stably integrated into the host genome. Thus, the laccase preparation does not contain the bla gene product, i.e. the enzyme beta-lactamase that hydrolyses and inactivates ampicillin. No bla DNA was detected in the laccase preparation.
The M. thermophila laccase enzyme was assessed for potential allergenicity by amino acid sequence comparison with allergens listed in publicly available protein databases. No immunologically significant sequence homology was detected.
The results of studies of acute toxicity with (undiluted) laccase (batch PPX 5720) are summarized in Table 1. The original test reports were not available, but it was stated that the studies were performed in compliance with good laboratory practice (GLP) and OECD test guidelines.
Rats
In a 2-week range-finding study, groups of five male and five female rats (strain, age and body weight not stated) received laccase (batch PPX 5720) at a dose of 0, 1, 3.3, or 10 ml/kg bw (equivalent to 0, 3252, 10 732, or 32 521 LAMU/kg bw per day, or 0, 0.17, 0.56, or 1.7 g/kg bw per day expressed as TOS) by oral gavage for 2 weeks. No adverse effects on survival, clinical signs, body weights, food and water consumption, macroscopy or organ weights were observed at up to the highest dose of 10 ml/kg bw per day (Brinch & Pedersen, 2002). The original test report was not available.
Groups of 10 male and 10 female CD rats (aged 30–37 days) received water containing laccase (batch PPX 5720) by oral gavage at a dose of 0, 0.1, 1, or 10 ml/kg bw per day (equivalent to 0, 325, 3252, or 32 521 LAMU/kg bw per day, or 0, 0.017, 0.17, or 1.7 g/kg bw per day expressed as TOS) for 13 weeks. The study followed OECD test guideline 408 (1981), and was certified for compliance with GLP and quality assurance. Animals and cage-trays were inspected at least twice a day for reactions to treatment or ill health. All animals were observed individually before and after dosing daily during week 1 of treatment, twice weekly during weeks 2–4 and once a week during weeks 5–13. In addition, all animals underwent weekly a detailed physical examination, including palpation. Body weight and food consumption were recorded weekly. Food conversion efficiency was calculated. Food and water were freely available. Ophthalmoscopy was carried out on all animals before treatment and during week 12 on animals in the control group and the group receiving the highest dose. During week 13, haematological and clinical chemistry parameters were analysed for all animals. The absolute weights of 12 organs were determined and adjusted for body weight. All animals were examined macroscopically. Microscopy was carried out on about 30 organs and tissues of all animals in the control group and the group receiving the highest dose, and on all macrospically abnormal tissues.
No effects on survival, behaviour, ophthalmoscopy, body weight, food consumption or feed efficiency were observed. Haematological analysis only showed slightly higher platelet counts in treated females (statistically significant only at the highest dose, p <0.01). As this parameter was not affected in males, and prothrombin time (another parameter relating to blood clotting) was not affected in either males or females, this effect is not considered to be toxicologically relevant. Clinical chemistry revealed slightly higher (but significantly, p <0.05) activities of serum alkaline phosphatase and slightly lower (but significantly, p <0.01) creatinine values in females at the highest dose. As this effect was not observed in females at the lower doses, nor in males, these changes were not considered to be toxicologically relevant. Other minor but significant changes in clinical chemical parameters did not show any dose–response relationship and were thus considered to represent normal biological variation. Organ weights, macroscopy and microscopy did not show any changes related to treatment. The Committee concluded that in this 13-week study in rats treated orally, the NOEL for laccase (batch PPX 5720) was the highest dose, 10 ml/kg bw per day (equivalent to 32 521 LAMU/kg bw per day, or 1.7 g/kg bw per day expressed as TOS) (Bolton, 1997; Brinch & Pedersen, 2002).
No information was available.
The results of two studies of genotoxicity in vitro with laccase (batch PPX 5720) are summarized in Table 2. The two studies followed OECD test guidelines, 471 (1997) and 473 (1983), respectively, and were certified for compliance with GLP and quality assurance.
Table 2. Results of studies of acute toxicity with laccase (batch PPX 5720) in male and female rats
|
Species |
Route of administration |
LD(C)50 |
|
Rat |
Oral |
>12 ml/kg bwa |
|
Dermal |
>12 ml/kg bwa |
|
|
Inhalation |
>5.16 mg/lb |
|
From Brinch & Pedersen (2002) |
|
|
a |
Equivalent to 39 025 LAMU or 2.07 g of TOS/kg bw |
|
b |
Equivalent to 16.12 LAMU or 0.85 mg of TOS/l. Exposure was to aerosolized laccase by snout only |
No information was available.
A semioccluded application of 0.5 ml laccase (PPX 5720) was applied to the closely-clipped dorsa of three New Zealand white rabbits for 4 h. Dermal reactions were assessed at 0.5–1, 24, 48 and 72 h after removal of the test substance. After the period of observation, the animals were killed and examined macroscopically. No dermal reactions were observed (Brinch & Pedersen, 2002). The original test report was not available. It was stated that the study complied with GLP and OECD test guidelines.
Table 3. Results of studies of genotoxicity with laccase (batch PPX 5720)
|
Endpoint |
Test object |
Concentration |
Results |
References |
|
In vitro |
|
|
|
|
|
Reverse mutation |
S. typhimurium TA98, TA100, TA1535, TA1537 and E. coli WP2uvrA |
156–5000 µg/ml for S. typhimurium strains and 156– 5000 µg/plate for E. coli. Solvent sterile water |
Negativea |
Pedersen (1998); Brinch & Pedersen (2002) |
|
Chromosomal aberration |
Human lymphocytes |
25–100 µg/ml -S9; 1000–5000 µg/ml +S9 in culture medium |
Negativeb |
Edwards (1997); Brinch & Pedersen (2002) |
|
S9, 9000 × g supernatant of rat liver homogenate |
|
|
a |
In the presence and absence of metabolic activation from S9; no cytotoxicity was seen. Owing to the presence of free amino acids (e.g. histidine and tryptophan) in the laccase preparation, the growth of Salmonella strains requiring histidine was significantly increased after direct-plate incorporation. Therefore, the Salmonella strains were exposed to the alpha-amylase preparation in a phosphate-buffered nutrient broth in liquid culture ("treat-and-plate assay") at six concentrations (highest dose, 5 mg/ml) for 3 h. After incubation, the test substance was removed by centrifugation before plating. Stimulation of growth of E. coli strains requiring tryptophan was only weak and insignificant |
|
b |
In the presence and absence of metabolic activation from S9. Cell cultures in the absence of S9 were treated for 19 or 43 h continuously and then harvested. Cell cultures in the presence of S9 were treated for 3 h and harvested 16 or 40 h later. Concentrations tested in the absence of S9 were 25, 50, and 100 µg/ml at 19 h and 100 µg/ml at 43 h. Concentrations tested in the presence of S9 were 1000, 3000, and 5000 µg/ml at 19 h and 5000 µg/ml at 43 h. The test was duplicated. Mitotic inhibition in the absence of S9 was 62–69% at 100 µg/ml at both sampling times. In the presence of S9, mitotic inhibition at 5000 µg/ml was 42–61% at 19 h and 10–18% at 43 h. Some small but significant increases in the percentage of cells with chromosomal aberrations were seen both in the absence and presence of S9. However, these increases did not show any dose–response relationship, were poorly reproducible between the duplicate assays and were within the range of historical control values (except in one case). Therefore the increases were not considered to be biologically relevant |
Three New Zealand white rabbits received a single ocular instillation of 0.1 ml of laccase (batch PPX 5720). Ocular reactions were assessed at 1, 24, 48 and 72 h after instillation. No ocular reactions were observed (Brinch & Pedersen, 2002). The original test report was not available. It was stated that the study complied with GLP and OECD test guidelines.
In a repeated patch test, 100 volunteers received nine applications of 0.5 ml of a 10% (w/v) solution of laccase (batch PPX 5720) in distilled water on an occlusive patch (area, 2 × 2 cm), each for 24 h, on a Monday–Wednesday–Friday schedule lasting 3 weeks. Two weeks after the final application, each person received on each arm a patch soaked with a 10% solution of laccase (batch PPX 5720), which was removed after 24 h. Reactions were scored after 48 and 96 h. Three out of 100 persons had evidence of possible skin sensitization and showed similar responses to one or both of two other enzymes that were tested at the same time in this study. When rechallenged with laccase at two concentrations (10% and 1% w/v) after approximately 1 month, none of these three persons had any evidence of skin sensitization to laccase only when tested at both 10 and 1% (w/v) (Brinch & Pedersen, 2002). The original test report was not available, but it was stated that the study was performed in compliance with GLP and OECD test guidelines.
When the laccase preparation is used in the brewing of beer during mashing of malt, barley, or other starch sources, it is inactivated during wort boiling and removed during subsequent stages of the production process. Therefore, an estimate of intake would not normally be necessary for this application. Nevertheless, a very conservative estimate of daily intake resulting from consumption of beer was performed on the basis of the following assumptions:
According to the budget method, the upper physiological intake of liquid is 100 ml/kg bw per day, or 6 l for a 60-kg person (Hansen, 1979). A "worst-case" scenario is that of ingestion of beer of 50 ml/kg bw per day of beer, leading to an intake of 0.15 mg of TOS/kg bw per day (350 × 5.3% × 0.05 / 6), i.e. 9 mg of TOS per day for a 60-kg person.
Since laccase is the subject of increasing interest for various applications other than brewing, conservative estimates were performed to account for its use in other products. The Food & Drug Administration of the United States had received "generally recognized as safe" (GRAS) notices for the specific laccase under evaluation by the Committee for use in chewing gum, mouthwash, breath mints and toothpaste (Food & Drug Administration, 2003). Laccase enzyme preparations were not reported by any Member State of the European Union in the inventory of enzymes used as processing aids that was compiled for scientific cooperation (SCOOP) Task 7.4 (European Commission, 2000), but this inventory is not exhaustive.
Conservative estimates were therefore performed for the use of laccase in chewing gum, mouthwash, breath mints and toothpaste on the basis of recommended dosages and TOS content provided by the producer (Jensen, personal communication).
A "worst-case" value for human intake via chewing gum was estimated on the basis of the following assumptions:
On the basis of these assumptions, the "worst-case" daily intake of laccase enzyme preparation in chewing gum was 0.35 mg of TOS/kg bw per day (0.16 × 1000 × 1.33% × 10 sticks / 60), i.e. 21 mg of TOS per day for a 60-kg subject.
A "worst-case" estimate of intake of laccase from mouthwash was made on the basis of the following assumptions:
• All mouthwash products contain laccase at the highest recommended dosage (0.4 g per application);
• All the mouthwash is swallowed (12 g per application) and all TOS (1.33%) are ingested;
• The maximum frequency of use of mouthwash is three times per day.
On the basis of these assumptions, the "worst-case" daily intake of laccase enzyme preparation in mouthwash was 0.26 mg of TOS/kg bw per day ([0.4 × 1000 × 1.33% × 3] / 60), i.e. 16 mg of TOS per day for a 60-kg person.
A "worst-case" intake of laccase enzyme preparation from breath mints was estimated on the basis of the following assumptions:
On the basis of these assumptions, the "worst-case" daily intake of laccase enzyme preparation on breath-mint tablets was 0.21 mg of TOS/kg bw per day (0.08 × 1000 × 1.33% × 12 / 60), i.e. 12.8 mg of TOS per day for a 60-kg subject.
An exaggerated intake from toothpaste was estimated on the basis of the following assumptions:
On the basis of these assumptions, the "worst-case" daily intake of laccase enzyme preparation in toothpaste was 0.05 mg of TOS/kg bw per day (0.08 × 1000 × 1.33% × 3 / 60), i.e. 3.2 mg of TOS per day for a 60-kg person.
The ratio between the NOEL for TOS of 1.7 g/kg bw per day in the 13-week study of oral toxicity and the cumulative intake deriving from all the conservative estimates is nearly 2000.
Toxicological studies were conducted on the liquid enzyme concentrate (LEC). The materials added to the LEC for stabilization, formulation and standardization have either been evaluated previously by the Committee or are common food constituents and do not raise safety concerns.
In a 13-week study in rats, no significant treatment-related effects were seen when the LEC was administered by oral gavage at doses of up to and including 10 ml/kg bw per day. This highest dose tested (equivalent to an intake of 1700 mg of TOS/kg bw per day) was the NOEL. The LEC was not active in an assay for mutagenicity in bacteria in vitro or in an assay for chromosomal aberrations in mammalian cells in vitro. Studies of dermal and ocular irritation in rabbits revealed no treatment-related effects. The LEC did not show skin sensitizing potential in a repeated patch test in humans.
In the brewing of beer, the laccase preparation is added during the mashing process and is denatured and inactivated during the subsequent wort-boiling stage. The beer filtration process is likely to remove the denatured enzymes along with other insoluble materials. Thus no residual LE399 alpha-amylase is expected to be present in food processed with this enzyme preparation.
A very conservative estimate of daily intake from beer was performed on the basis of the assumption that all the TOS would persist in the final product. This gave an estimated daily intake of 9 mg of TOS per day (equivalent to 0.15 mg/kg bw per day). As the Committee was aware that laccases are receiving increasing interest for various applications other than brewing, e.g. in chewing gum, mouthwash, breath mints and toothpaste, conservative estimates of daily intakes resulting from these uses were made, resulting in the following values: chewing gum, 2 mg of TOS per day (equivalent to 0.35 mg/kg bw per day for a 60-kg person); mouthwash, 16 mg of TOS per day (equivalent to 0.26 mg/kg bw per day for a 60-kg person); breath mints, 12.8 mg of TOS per day (equivalent to 0.21 mg/kg bw per day for a 60-kg person); and toothpaste, 3.2 mg of TOS per day (equivalent to 0.05 mg/kg bw per day for a 60-kg person). The ratio between the NOEL for TOS of 1700 mg/kg bw per day from the 13-week study of oral toxicity and the cumulative intake deriving from all these conservative estimates is nearly 2000.
The Committee allocated an ADI "not specified" to laccase from this recombinant strain of A. oryzae, used in the applications specified and in accordance with good manufacturing practice.
Bolton, N. (1997) Laccase, PPX 5720: Toxicity study by oral (gavage) administration to CD rats for 13 weeks. Unpublished report No. NLE186/9703426 from Huntingdon Life Sciences Ltd., Suffolk, England. Submitted to WHO by Novozymes A/S, Bagsvaerd, Denmark.
Brinch, D.S. & Pedersen, P.B. (2002) Toxicological studies on laccase from Myceliophthora thermophila expressed in Aspergillus oryzae. Regul. Toxicol. Pharmacol., 35, 296–307.
Edwards, C.N. (1997) Laccase, PPX 5720: Lymphocyte cytogenetic study. Unpublished report No. NLE188/970101 from Huntingdon Life Sciences Ltd., Suffolk, England. Submitted to WHO by Novozymes A/S, Bagsvaerd, Denmark.
Jensen, B.R. (2002) Laccase enzyme preparation produced by a strain of Aspergillus oryzae containing the gene coding for laccase in Myceliophthora thermophila inserted by recombinant DNA techniques. Laccase. FlavourstarTM. Unpublished report No. 20024691401 from Novozymes A/S, Bagsvaerd, Denmark. Submitted to WHO by Novozymes A/S, Bagsvaerd, Denmark.
Pedersen, P.B. (1998) Laccase (batch number: PPX 5720): Test for mutagenic activity with strains of Salmonella typhimurium and Escherichia coli. Unpublished report No. 988041 from Novo Nordisk A/S, Bagsvaerd, Denmark. Submitted to WHO by Novozymes A/S, Bagsvaerd, Denmark
European Commission (2000) Report on task for scientific cooperation (SCOOP). Report of experts participating in Task 7.4. Study of the enzymes used in foodstuffs and collation of data on their safety (http://www.europa.eu.int/comm/food/fs/scoop/index_en.html).
Food & Drug Administration (2003) List of the substances that are the subject of each GRAS Notice (http://www.cfsan.fda.gov/~rdb/opagras.html).
Jensen, B.R. (2002) Laccase enzyme preparation produced by a strain of Aspergillus oryzae containing the gene coding for laccase in Myceliophthora thermophila inserted by recombinant DNA techniques. Laccase. FlavourstarTM. Unpublished report No. 20024691401 from Novozymes A/S, Bagsvaerd, Denmark. Submitted to WHO by Novozymes A/S, Bagsvaerd, Denmark.
See Also:
Toxicological Abbreviations
LACCASE FROM MYCELIOPHTHORA THERMOPHILA EXPRESSED IN ASPERGILLUS ORYZAE (JECFA Evaluation)