
UNITED NATIONS ENVIRONMENT PROGRAMME
INTERNATIONAL LABOUR ORGANISATION
WORLD HEALTH ORGANIZATION
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
Environmental Health Criteria 218
FLAME RETARDANTS: TRIS(2-BUTOXYETHYL)
PHOSPHATE, TRIS(2-ETHYLHEXYL)
PHOSPHATE AND TETRAKIS(HYDROXYMETHYL)
PHOSPHONIUM SALTS
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization.
First draft prepared by Dr G.J. van Esch, Bilthoven, the Netherlands
Published under the joint sponsorship of the United Nations
Environment Programme, the International Labour Organisation, and the
World Health Organization, and produced within the framework of the
Inter-Organization Programme for the Sound Management of Chemicals.
World Health Organization
Geneva, 2000
The International Programme on Chemical Safety (IPCS),
established in 1980, is a joint venture of the United Nations
Environment Programme (UNEP), the International Labour Organisation
(ILO), and the World Health Organization (WHO). The overall
objectives of the IPCS are to establish the scientific basis for
assessment of the risk to human health and the environment from
exposure to chemicals, through international peer review processes, as
a prerequisite for the promotion of chemical safety, and to provide
technical assistance in strengthening national capacities for the
sound management of chemicals.
The Inter-Organization Programme for the Sound Management of
Chemicals (IOMC) was established in 1995 by UNEP, ILO, the Food and
Agriculture Organization of the United Nations, WHO, the United
Nations Industrial Development Organization, the United Nations
Institute for Training and Research, and the Organisation for Economic
Co-operation and Development (Participating Organizations), following
recommendations made by the 1992 UN Conference on Environment and
Development to strengthen cooperation and increase coordination in the
field of chemical safety. The purpose of the IOMC is to promote
coordination of the policies and activities pursued by the
Participating Organizations, jointly or separately, to achieve the
sound management of chemicals in relation to human health and the
environment.
WHO Library Cataloguing-in-Publication Data
Flame retardants : tris(2-butoxyethyl) phosphate, tris(2-ethylhexyl)
phosphate, tetrakis(hydroxymethyl) phosphonium salts.
(Environmental health criteria ; 218)
1.Organophosphorus compounds - toxicity 2.Phosphoric acid esters
- toxicity 3.Flame retardants - toxicity
4.No-observed-adverse-effect level 5.Environmental exposure
6.Occupational exposure I.Series
ISBN 92 4 157218 3 (NLM Classification: QU 131)
ISSN 0250-863X
The World Health Organization welcomes requests for permission to
reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made to
the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 2000
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved.
The designations employed and the presentation of the material in
this publication do not imply the expression of any opinion whatsoever
on the part of the Secretariat of the World Health Organization
concerning the legal status of any country, territory, city, or area
or of its authorities, or concerning the delimitation of its frontiers
or boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar nature
that are not mentioned. Errors and omissions excepted, the names of
proprietary products are distinguished by initial capital letters.
CONTENTS
ENVIRONMENTAL HEALTH CRITERIA FOR FLAME RETARDANTS:
TRIS(2-BUTOXYETHYL) PHOSPHATE, TRIS(2-ETHYLHEXYL) PHOSPHATE,
TETRAKIS-(HYDROXYMETHYL) PHOSPHONIUM SALTS
PREAMBLE
ABBREVIATIONS
PART A: TRIS(2-BUTOXYETHYL) PHOSPHATE (TBEP)
A1. SUMMARY, EVALUATION AND RECOMMENDATIONS
A1.1 Summary
A1.2 Evaluation
A1.3 Recommendations
A2. IDENTITY, PHYSICAL AND CHEMICAL
PROPERTIES, AND ANALYTICAL METHODS
A2.1 Identity
A2.2 Physical and chemicals properties
A2.3 Conversion factors
A2.4 Analytical methods
A2.4.1 Air
A2.4.2 Water
A2.4.3 Sediment
A2.4.4 Soils and foodstuffs
A2.4.5 Biological media
A3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
A3.1 Natural occurrence
A3.2 Anthropogenic sources
A3.2.1 Production levels and processes
A3.2.2 Uses
A4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION AND TRANSFORMATION
A4.1 Transport and distribution between media
A4.2 Biodegradation
A4.2.1 Migration
A5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
A5.1 Environmental levels
A5.1.1 Air
A5.1.2 Water (drinking-water and surface water)
A5.1.3 Soils and sediment
A5.1.4 Aquatic organisms
A5.2 Human tissue levels
A5.3 Food
A5.4 Occupational exposure
A6. KINETIC AND METABOLISM IN LABORATORY ANIMALS AND HUMANS
A7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
A7.1 Single exposure
A7.1.1 Oral and dermal
A7.1.2 Inhalation
A7.2 Short-term repeated exposure
A7.2.1 Oral
A7.2.2 Dermal
A7.3 Skin and eye irritation; sensitization
A7.4 Reproductive toxicity, embryotoxicity and
teratogenicity
A7.5 Mutagenicity and related end-points
A7.6 Carcinogenicity
A7.7 Special studies
A7.7.1 Neurotoxicity
A7.7.1.1 Acute administration
A7.7.1.2 Repeated oral administration
A7.7.1.3 Effects on esterase activity
A8. EFFECTS ON HUMANS
A9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
A9.1 Laboratory experiments
A9.1.1 Aquatics organisms
A9.1.1.1 Invertebrates
A9.1.1.2 Vertebrates
PART B: TRIS(2-ETHYLHEXYL) PHOSPHATE (TEHP)
B1. SUMMARY, EVALUATION AND RECOMMENDATIONS
B1.1 Summary
B1.2 Evaluation
B1.3 Recommendations
B2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, AND ANALYTICAL
METHODS
B2.1 Identity
B2.2 Physical and chemical properties
B2.3 Conversion factors
B2.4 Analytical methods
B2.4.1 Air
B2.4.2 Water
B2.4.3 Sediment
B3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
B3.1 Natural occurrence
B3.2 Anthropogenic sources
B3.2.1 Production levels and processes
B3.2.2 Uses
B4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION AND TRANSFORMATION
B4.1 Biodegradation
B4.2 Bioaccumulation
B5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
B5.1 Environmental levels
B5.1.1 Air
B5.1.2 Surface water
B5.1.3 Drinking-water
B5.1.4 Effluents
B5.1.5 Sediment
B5.1.6 Food
B6. KINETICS AND METABOLISM IN LABORATORY ANIMALS
B7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
B7.1 Single exposure
B7.2 Repeated exposure
B7.2.1 Oral
B7.2.2 Dermal
B7.2.3 Inhalation
B7.3 Skin and eye irritation; sensitization
B7.4 Reproductive toxicity, embryo toxicity and
teratogenicity
B7.5 Mutagenicity
B7.5.1 In vitro assays
B7.5.2 In vivo assays
B7.6 Carcinogenicity
B7.7 Special studies
B7.7.1 Neurotoxicity
B8. EFFECTS ON HUMANS
B9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
B9.1 Laboratory experiments
B9.1.1 Microorganisms
B9.1.2 Aquatic organisms
B9.1.2.1 Vertebrates
B9.1.3 Terrestrial organisms
PART C: TETRAKIS(HYDROXYMETHYL) PHOSPHONIUM SALTS
C1. SUMMARY AND EVALUATION
C1.1 Summary
C1.2 Evaluation
C2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, AND ANALYTICAL
METHODS
C2.1 Identity
C2.1.1 Tetrakis(hydroxymethyl) phosphonium
chloride (THPC)
C2.1.2 Tetrakis(hydroxymethyl) phosphonium
sulfate (THPS)
C2.1.3 Tetrakis(hydroxymethyl) phosphonium
chloride-urea condensate (THPC-urea)
C2.2 Physical and chemical properties
C2.2.1 Technical products
C2.3 Conversion factors
C2.4 Analytical methods
C3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
C3.1 Natural occurrence
C3.2 Anthropogenic sources
C3.2.1 Production levels and processes
C3.2.2 Uses
C4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION AND TRANSPORTATION
C4.1 Transport and distribution between media
C4.2 Transformation
C4.2.1 Biodegradation
C4.2.2 Abiotic degradation
C4.3 Migration from textiles
C5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
C6. KINETICS AND METABOLISM IN LABORATORY ANIMALS
C7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
C7.1 Single exposure
C7.1.1 Oral
C7.1.2 Dermal
C7.1.3 Inhalation
C7.2 Repeated exposure
C7.2.1 Oral
C7.2.1.1 THPC
C7.2.1.2 THPS
C7.2.2 Dermal
C7.3 Long-term exposure
C7.3.1 THPC
C7.3.2 THPS
C7.4 Skin and eye irritation; sensitization
C7.4.1 Skin irritation
C7.4.1.1 THPS
C7.4.1.2 THPC-urea
C7.4.2 Eye irritation
C7.4.3 Skin sensitization
C7.4.3.1 THPS
C7.4.3.2 THPC-urea
C7.5 Reproductive toxicity, embryotoxicity and
teratogenicity
C7.5.1 THPS
C7.5.2 THPC-urea
C7.6 Mutagenicity and related end-points
C7.6.1 THPC-urea
C7.6.1.1 In vitro studies
C7.6.1.2 In vivo studies
C7.6.2 THPC
C7.6.3 THPS
C7.6.4 THPO
C7.6.5 Treated fabrics
C7.7 Carcinogenicity
C7.7.1 Oral studies
C7.7.1.1 Mice
C7.7.1.2 Rats
C7.7.2 Dermal studies: initiation and promotion
C7.8 Special studies
C8. EFFECTS ON HUMANS
C9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
C9.1 Laboratory experiments
C9.1.1 Aquatic organisms
C9.1.2 Terrestrial organisms
C10. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
REFERENCES
APPENDIX
RÉSUMÉ, EVALUATION ET RECOMMANDATIONS
RESUMEN, EVALUACION Y RECOMENDACIONES
NOTE TO READERS OF THE CRITERIA MONOGRAPHS
Every effort has been made to present information in the criteria
monographs as accurately as possible without unduly delaying their
publication. In the interest of all users of the Environmental Health
Criteria monographs, readers are requested to communicate any errors
that may have occurred to the Director of the International Programme
on Chemical Safety, World Health Organization, Geneva, Switzerland, in
order that they may be included in corrigenda.
* * *
A detailed data profile and a legal file can be obtained from the
International Register of Potentially Toxic Chemicals, Case postale
356, 1219 Châtelaine, Geneva, Switzerland (telephone no. + 41 22
- 9799111, fax no. + 41 22 - 7973460, E-mail irptc@unep.ch).
* * *
This publication was made possible by grant number
5 U01 ES02617-15 from the National Institute of Environmental Health
Sciences, National Institutes of Health, USA, and by financial support
from the European Commission.
Environmental Health Criteria
PREAMBLE
Objectives
In 1973 the WHO Environmental Health Criteria Programme was
initiated with the following objectives:
(i) to assess information on the relationship between exposure
to environmental pollutants and human health, and to provide
guidelines for setting exposure limits;
(ii) to identify new or potential pollutants;
(iii) to identify gaps in knowledge concerning the health effects
of pollutants;
(iv) to promote the harmonization of toxicological and
epidemiological methods in order to have internationally
comparable results.
The first Environmental Health Criteria (EHC) monograph, on
mercury, was published in 1976 and since that time an ever-increasing
number of assessments of chemicals and of physical effects have been
produced. In addition, many EHC monographs have been devoted to
evaluating toxicological methodology, e.g., for genetic, neurotoxic,
teratogenic and nephrotoxic effects. Other publications have been
concerned with epidemiological guidelines, evaluation of short-term
tests for carcinogens, biomarkers, effects on the elderly and so
forth.
Since its inauguration the EHC Programme has widened its scope,
and the importance of environmental effects, in addition to health
effects, has been increasingly emphasized in the total evaluation of
chemicals.
The original impetus for the Programme came from World Health
Assembly resolutions and the recommendations of the 1972 UN Conference
on the Human Environment. Subsequently the work became an integral
part of the International Programme on Chemical Safety (IPCS), a
cooperative programme of UNEP, ILO and WHO. In this manner, with the
strong support of the new partners, the importance of occupational
health and environmental effects was fully recognized. The EHC
monographs have become widely established, used and recognized
throughout the world.
The recommendations of the 1992 UN Conference on Environment and
Development and the subsequent establishment of the Intergovernmental
Forum on Chemical Safety with the priorities for action in the six
programme areas of Chapter 19, Agenda 21, all lend further weight to
the need for EHC assessments of the risks of chemicals.
Scope
The criteria monographs are intended to provide critical reviews
on the effect on human health and the environment of chemicals and of
combinations of chemicals and physical and biological agents. As
such, they include and review studies that are of direct relevance for
the evaluation. However, they do not describe every study carried
out. Worldwide data are used and are quoted from original studies,
not from abstracts or reviews. Both published and unpublished reports
are considered and it is incumbent on the authors to assess all the
articles cited in the references. Preference is always given to
published data. Unpublished data are only used when relevant
published data are absent or when they are pivotal to the risk
assessment. A detailed policy statement is available that describes
the procedures used for unpublished proprietary data so that this
information can be used in the evaluation without compromising its
confidential nature (WHO (1990) Revised Guidelines for the Preparation
of Environmental Health Criteria Monographs. PCS/90.69, Geneva, World
Health Organization).
In the evaluation of human health risks, sound human data,
whenever available, are preferred to animal data. Animal and in
vitro studies provide support and are used mainly to supply evidence
missing from human studies. It is mandatory that research on human
subjects is conducted in full accord with ethical principles,
including the provisions of the Helsinki Declaration.
The EHC monographs are intended to assist national and
international authorities in making risk assessments and subsequent
risk management decisions. They represent a thorough evaluation of
risks and are not, in any sense, recommendations for regulation or
standard setting. These latter are the exclusive purview of national
and regional governments.
Content
The layout of EHC monographs for chemicals is outlined below.
* Summary -- a review of the salient facts and the risk evaluation
of the chemical
* Identity -- physical and chemical properties, analytical methods
* Sources of exposure
* Environmental transport, distribution and transformation
* Environmental levels and human exposure
* Kinetics and metabolism in laboratory animals and humans
* Effects on laboratory mammals and in vitro test systems
* Effects on humans
* Effects on other organisms in the laboratory and field
* Evaluation of human health risks and effects on the environment
* Conclusions and recommendations for protection of human health
and the environment
* Further research
* Previous evaluations by international bodies, e.g., IARC, JECFA,
JMPR
Selection of chemicals
Since the inception of the EHC Programme, the IPCS has organized
meetings of scientists to establish lists of priority chemicals for
subsequent evaluation. Such meetings have been held in: Ispra, Italy,
1980; Oxford, United Kingdom, 1984; Berlin, Germany, 1987; and North
Carolina, USA, 1995. The selection of chemicals has been based on the
following criteria: the existence of scientific evidence that the
substance presents a hazard to human health and/or the environment;
the possible use, persistence, accumulation or degradation of the
substance shows that there may be significant human or environmental
exposure; the size and nature of populations at risk (both human and
other species) and risks for environment; international concern, i.e.
the substance is of major interest to several countries; adequate data
on the hazards are available.
If an EHC monograph is proposed for a chemical not on the
priority list, the IPCS Secretariat consults with the Cooperating
Organizations and all the Participating Institutions before embarking
on the preparation of the monograph.
Procedures
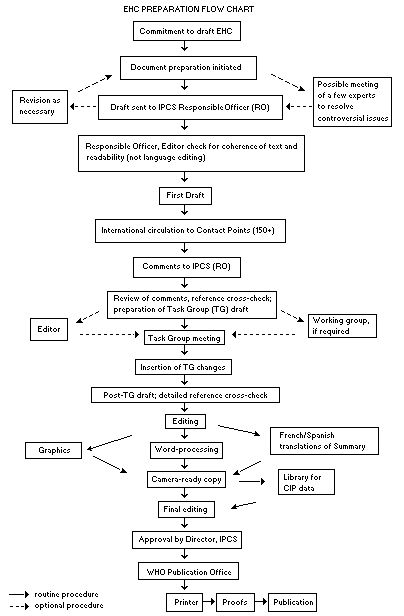
The order of procedures that result in the publication of an EHC
monograph is shown in the flow chart. A designated staff member of
IPCS, responsible for the scientific quality of the document, serves
as Responsible Officer (RO). The IPCS Editor is responsible for
layout and language. The first draft, prepared by consultants or,
more usually, staff from an IPCS Participating Institution, is based
initially on data provided from the International Register of
Potentially Toxic Chemicals, and reference data bases such as Medline
and Toxline.
The draft document, when received by the RO, may require an
initial review by a small panel of experts to determine its scientific
quality and objectivity. Once the RO finds the document acceptable as
a first draft, it is distributed, in its unedited form, to well over
150 EHC contact points throughout the world who are asked to comment
on its completeness and accuracy and, where necessary, provide
additional material. The contact points, usually designated by
governments, may be Participating Institutions, IPCS Focal Points, or
individual scientists known for their particular expertise. Generally
some four months are allowed before the comments are considered by the
RO and author(s). A second draft incorporating comments received and
approved by the Director, IPCS, is then distributed to Task Group
members, who carry out the peer review, at least six weeks before
their meeting.
The Task Group members serve as individual scientists, not as
representatives of any organization, government or industry. Their
function is to evaluate the accuracy, significance and relevance of
the information in the document and to assess the health and
environmental risks from exposure to the chemical. A summary and
recommendations for further research and improved safety aspects are
also required. The composition of the Task Group is dictated by the
range of expertise required for the subject of the meeting and by the
need for a balanced geographical distribution.
The three cooperating organizations of the IPCS recognize the
important role played by nongovernmental organizations.
Representatives from relevant national and international associations
may be invited to join the Task Group as observers. While observers
may provide a valuable contribution to the process, they can only
speak at the invitation of the Chairperson. Observers do not
participate in the final evaluation of the chemical; this is the sole
responsibility of the Task Group members. When the Task Group
considers it to be appropriate, it may meet in camera.
All individuals who as authors, consultants or advisers
participate in the preparation of the EHC monograph must, in addition
to serving in their personal capacity as scientists, inform the RO if
at any time a conflict of interest, whether actual or potential, could
be perceived in their work. They are required to sign a conflict of
interest statement. Such a procedure ensures the transparency and
probity of the process.
When the Task Group has completed its review and the RO is
satisfied as to the scientific correctness and completeness of the
document, it then goes for language editing, reference checking, and
preparation of camera-ready copy. After approval by the Director,
IPCS, the monograph is submitted to the WHO Office of Publications for
printing. At this time a copy of the final draft is sent to the
Chairperson and Rapporteur of the Task Group to check for any errors.
It is accepted that the following criteria should initiate the
updating of an EHC monograph: new data are available that would
substantially change the evaluation; there is public concern for
health or environmental effects of the agent because of greater
exposure; an appreciable time period has elapsed since the last
evaluation.
All Participating Institutions are informed, through the EHC
progress report, of the authors and institutions proposed for the
drafting of the documents. A comprehensive file of all comments
received on drafts of each EHC monograph is maintained and is
available on request. The Chairpersons of Task Groups are briefed
before each meeting on their role and responsibility in ensuring that
these rules are followed.
 WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR FLAME RETARDANTS:
TRIS(2-BUTOXYETHYL) PHOSPHATE, TRIS(2-ETHYLHEXYL) PHOSPHATE AND
TETRAKIS(HYDROXYMETHYL) PHOSPHONIUM SALTS
Members
Dr R. Benson, US Environmental Protection Agency, Denver, Colorado,
USA
Dr P. Brantom, British Industry Biological Research Association
(BIBRA) International, Carshalton, Surrey, United Kingdom
Dr S. Dobson, Institute of Terrestrial Ecology, Monks Wood,
Huntingdon, Cambridgeshire, United Kingdom (Chairman)
Professor J. Liesivuori, Department of Pharmacology and Toxicology,
University of Kuopio, Kuopio, Finland
Mr D. Renshaw, Department of Health, Elephant and Castle, London,
United Kingdom
Dr E. Söderlund, National Institute of Public Health, Department of
Environmental Medicine, Oslo, Norway (Rapporteur)
Observers
Dr L. Kotkoskic, FMC Corporation, Princetown, New Jersey, USA
Dr P. Martin, Albright and Wilson UK Limited, European Business
Services - Product Stewardship, Oldbury, West Midlands, United Kingdom
Secretariat
Dr M. Baril, International Programme on Chemical Safety, Montreal,
Quebec, Canada
ENVIRONMENTAL HEALTH CRITERIA FOR FLAME RETARDANTS:
TRIS(2-BUTOXYETHYL) PHOSPHATE, TRIS(2-ETHYLHEXYL) PHOSPHATE AND
TETRAKIS(HYDROXYMETHYL) PHOSPHONIUM SALTS
A WHO Task Group on Environmental Health Criteria for Flame
retardants: tris(2-butoxyethyl) phosphate, tris(2-ethylhexyl)
phosphate and tetrakis(hydroxymethyl) phosphonium salts met at the
British Industrial Biological Research Association, Carshalton, United
Kingdom from 18 to 22 January 1999. Dr P. Brantom opened the meeting
and welcome the participants on behalf of the host institute. Dr M.
Baril, IPCS, welcomed the participants on behalf of IPCS and the three
cooperating organizations (UNEP/ILO/WHO). The Task Group reviewed and
revised the draft criteria monograph and made an evaluation of the
risk to human health and the environment from exposure to these flame
retardants.
Financial support for this Task Group was provided by the United
Kingdom Department of Health as part of its contribution to the IPCS.
The first draft of this monograph was prepared by Dr G. J. van
Esch, Bilthoven, the Netherlands. The second draft prepared by Dr M.
Baril incorporated the comments received following circulation of the
first draft to the IPCS contact points for Environmental Health
Criteria.
Dr P.G. Jenkins (IPCS Central Unit, Geneva) and Dr M. Baril (IPCS
technical advisor, Montreal) were responsible for the overall
technical editing and scientific content,respectively.
The efforts of all who helped in the preparation and finalization
of the monograph are gratefully acknowledged.
* * *
ABBREVIATIONS
AChE acetylcholinesterase
ALAT alanine aminotransferase
ASAT aspartate aminotransferase
BCME bis(chloromethyl) ether
BEHP bis(2-ethylhexyl) phosphate
BMPA bishydroxymethyl phosphonic acid
BuCHE butyrylcholinesterase
CHO Chinese hamster ovary
DMSO dimethyl sulfoxide
EC50 median effective concentration
FDA Food and Drug Administration (USA)
GC gas chromatography
HPLC high performance liquid chromatography
IC50 median inhibitory concentration
LC50 median lethal concentration
LD50 median lethal dose
LOAEL lowest-observed-adverse-effect level
LOEL lowest-observed-effect level
MS mass spectrometry
nd not detected
NOAEL no-observed-adverse-effect level
NOEC no-observed-effect concentration
NOEL no-observed-effect level
NPD nitrogen-phosphorus sensitive detector
NTE neuropathy target esterase
NTP National Toxicology Program (USA)
OECD Organisation for Economic Co-operation and Development
PVC polyvinyl chloride
SCE sister-chromatid exchange
TBEP tris(2-butoxyethyl) phosphate
TEHP tris(2-ethylhexyl) phosphate
THP tetrakis(hydroxymethyl) phosphonium
THPC tetrakis(hydroxymethyl) phosphonium chloride
THPO trihydroxymethyl phosphine oxide
THPS tetrakis(hydroxymethyl) phosphonium sulfate
TOCP tri- ortho-cresyl phosphate
PART A
Tris(2-butoxyethyl) phosphate
(TBEP)
A. SUMMARY, EVALUATION AND RECOMMENDATIONS
A1. Tris(2-butoxyethyl) phosphate (TBEP)
A1.1 Summary
Tris(2-butoxyethyl) phosphate (TBEP) is used in floor polishes
and as a plasticizer in rubber and plastics. The worldwide production
volume is not available but is estimated to be in the range of
5000-6000 tonnes.
TBEP occurs in the environment only as a result of human
activity. Its distribution in the environment has been investigated in
certain industrialized countries. Concentrations in surface water were
found to be below 300 ng/litre, whereas concentrations in sediment
were between 100 and 1000 µg/kg. None of 167 analyses detected TBEP in
fish. It has been detected in outdoor air in a single study (<200
ng/m3). Measurement of TBEP in indoor air in offices showed
concentrations of 25 ng/m3 or less. TBEP is associated with
particulates and the source is considered to be the application of
floor polish. It has been detected at µg/kg levels in human adipose
tissue. The reported daily dietary intake from market basket studies,
for a range of age groups, was <0.02 µg/kg body weight per day.
Drinking-water concentrations of up to 270 µg/litre have been
reported, this is considered to arise from migration from rubber
gaskets in the plumbing.
TBEP is considered to be readily biodegradable. Sewage treatment
plant measurements and semi-continuous sludge laboratory tests have
indicated substantial elimination of TBEP (>80%). In river and
coastal water TBEP was completely degraded. The half-life in estuarine
water was reported to be about 50 days and there was little
degradation in unadapted seawater.
The acute systemic mammalian toxicity and irritation potential
are low.
Several subchronic studies in laboratory animals have shown that
the liver is the target organ for TBEP toxicity. One study in male
Sprague-Dawley rats suggested that TBEP might cause focal myocarditis.
Neurotoxic effects in rats after single doses of TBEP are
inconsistent. In rats repeatedly given high doses by gavage, TBEP
decreased nerve conduction velocity and increased the refractory
period. It did not cause delayed neurotoxicity in hens but did inhibit
brain and plasma cholinesterases.
Based on an 18-week repeated dose study in rats, the
no-observed-effect level (NOEL) for liver effects was reported to be
15 mg/kg body weight per day, while the lowest-observed-effect level
(LOEL) was 150 mg/kg body weight per day.
The long-term toxicity and carcinogenicity of TBEP have not been
studied.
Bacterial and mammalian cell tests for gene mutation gave
negative results, but no tests for chromosomal damage have been
reported.
Teratogenicity was not observed in one study in rats. Other
aspects of reproductive toxicity have not been reported.
A Repeat Human Insult Patch Test indicated no skin sensitization
and minimal skin irritation.
The toxicity of TBEP to aquatic organisms is moderate. The 48-h
LC50 in Daphnia magna is 75 mg/litre and the 96-h LC50 values in
fish range between 16 and 24 mg/litre.
A1.2 Evaluation
Occupational exposure to TBEP is likely to be by the dermal route
during manufacture (accidental exposure) and from the use of floor
polishes. The compound is absorbed dermally in experimental animals
but no information is available on its kinetics and metabolism. Dermal
exposure cannot, therefore, be quantified but is expected to be low.
Inhalation exposure in the office environment has been measured to be
25 ng/m3 or less.
Exposure of the general population is principally via food (from
use of TBEP as a plasticizer in packaging plastics) and drinking-water
(contaminated by leaching from synthetic rubbers used in plumbing
washers). Exposure from both sources is very low (estimated to be
<0.2 µg/kg body weight per day from the diet and concentrations in
drinking-water of <270 µg/litre).
Given the reported NOEL from animal studies of 15 mg/kg body
weight per day from a repeated dose oral study, the risk to the
general population is very low. The risk to the occupationally exposed
is also considered to be very low, though this cannot be quantified.
In the environment, TBEP is expected (from its low volatility,
high adsorption coefficient and moderate water solubility) to
partition to sediment. The few measured data confirm this. Degradation
in environmental media is expected to be rapid. No information is
available on breakdown products; phosphate released during breakdown
is not expected to contribute significantly to environmental nutrient
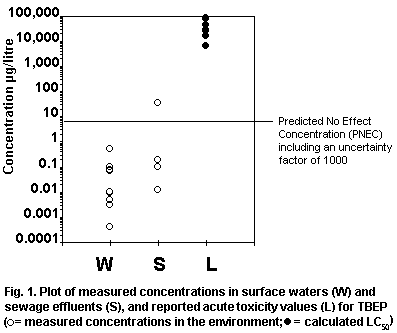
levels. Fig. 1 plots measured environmental concentrations in surface
water against reported acute toxicity values. The margin of safety
between highest reported concentrations and lowest reported toxicity
values is several orders of magnitude, indicating low risk to
organisms in the aquatic environment. No assessment of risk can be
made for the terrestrial compartment.
A1.3 Recommendations
For a full scientific evaluation of the compound, identification
and assessment of metabolites in mammals would be required, given the
toxicological profile of one of the suggested metabolites,
2-butoxyethanol.
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR FLAME RETARDANTS:
TRIS(2-BUTOXYETHYL) PHOSPHATE, TRIS(2-ETHYLHEXYL) PHOSPHATE AND
TETRAKIS(HYDROXYMETHYL) PHOSPHONIUM SALTS
Members
Dr R. Benson, US Environmental Protection Agency, Denver, Colorado,
USA
Dr P. Brantom, British Industry Biological Research Association
(BIBRA) International, Carshalton, Surrey, United Kingdom
Dr S. Dobson, Institute of Terrestrial Ecology, Monks Wood,
Huntingdon, Cambridgeshire, United Kingdom (Chairman)
Professor J. Liesivuori, Department of Pharmacology and Toxicology,
University of Kuopio, Kuopio, Finland
Mr D. Renshaw, Department of Health, Elephant and Castle, London,
United Kingdom
Dr E. Söderlund, National Institute of Public Health, Department of
Environmental Medicine, Oslo, Norway (Rapporteur)
Observers
Dr L. Kotkoskic, FMC Corporation, Princetown, New Jersey, USA
Dr P. Martin, Albright and Wilson UK Limited, European Business
Services - Product Stewardship, Oldbury, West Midlands, United Kingdom
Secretariat
Dr M. Baril, International Programme on Chemical Safety, Montreal,
Quebec, Canada
ENVIRONMENTAL HEALTH CRITERIA FOR FLAME RETARDANTS:
TRIS(2-BUTOXYETHYL) PHOSPHATE, TRIS(2-ETHYLHEXYL) PHOSPHATE AND
TETRAKIS(HYDROXYMETHYL) PHOSPHONIUM SALTS
A WHO Task Group on Environmental Health Criteria for Flame
retardants: tris(2-butoxyethyl) phosphate, tris(2-ethylhexyl)
phosphate and tetrakis(hydroxymethyl) phosphonium salts met at the
British Industrial Biological Research Association, Carshalton, United
Kingdom from 18 to 22 January 1999. Dr P. Brantom opened the meeting
and welcome the participants on behalf of the host institute. Dr M.
Baril, IPCS, welcomed the participants on behalf of IPCS and the three
cooperating organizations (UNEP/ILO/WHO). The Task Group reviewed and
revised the draft criteria monograph and made an evaluation of the
risk to human health and the environment from exposure to these flame
retardants.
Financial support for this Task Group was provided by the United
Kingdom Department of Health as part of its contribution to the IPCS.
The first draft of this monograph was prepared by Dr G. J. van
Esch, Bilthoven, the Netherlands. The second draft prepared by Dr M.
Baril incorporated the comments received following circulation of the
first draft to the IPCS contact points for Environmental Health
Criteria.
Dr P.G. Jenkins (IPCS Central Unit, Geneva) and Dr M. Baril (IPCS
technical advisor, Montreal) were responsible for the overall
technical editing and scientific content,respectively.
The efforts of all who helped in the preparation and finalization
of the monograph are gratefully acknowledged.
* * *
ABBREVIATIONS
AChE acetylcholinesterase
ALAT alanine aminotransferase
ASAT aspartate aminotransferase
BCME bis(chloromethyl) ether
BEHP bis(2-ethylhexyl) phosphate
BMPA bishydroxymethyl phosphonic acid
BuCHE butyrylcholinesterase
CHO Chinese hamster ovary
DMSO dimethyl sulfoxide
EC50 median effective concentration
FDA Food and Drug Administration (USA)
GC gas chromatography
HPLC high performance liquid chromatography
IC50 median inhibitory concentration
LC50 median lethal concentration
LD50 median lethal dose
LOAEL lowest-observed-adverse-effect level
LOEL lowest-observed-effect level
MS mass spectrometry
nd not detected
NOAEL no-observed-adverse-effect level
NOEC no-observed-effect concentration
NOEL no-observed-effect level
NPD nitrogen-phosphorus sensitive detector
NTE neuropathy target esterase
NTP National Toxicology Program (USA)
OECD Organisation for Economic Co-operation and Development
PVC polyvinyl chloride
SCE sister-chromatid exchange
TBEP tris(2-butoxyethyl) phosphate
TEHP tris(2-ethylhexyl) phosphate
THP tetrakis(hydroxymethyl) phosphonium
THPC tetrakis(hydroxymethyl) phosphonium chloride
THPO trihydroxymethyl phosphine oxide
THPS tetrakis(hydroxymethyl) phosphonium sulfate
TOCP tri- ortho-cresyl phosphate
PART A
Tris(2-butoxyethyl) phosphate
(TBEP)
A. SUMMARY, EVALUATION AND RECOMMENDATIONS
A1. Tris(2-butoxyethyl) phosphate (TBEP)
A1.1 Summary
Tris(2-butoxyethyl) phosphate (TBEP) is used in floor polishes
and as a plasticizer in rubber and plastics. The worldwide production
volume is not available but is estimated to be in the range of
5000-6000 tonnes.
TBEP occurs in the environment only as a result of human
activity. Its distribution in the environment has been investigated in
certain industrialized countries. Concentrations in surface water were
found to be below 300 ng/litre, whereas concentrations in sediment
were between 100 and 1000 µg/kg. None of 167 analyses detected TBEP in
fish. It has been detected in outdoor air in a single study (<200
ng/m3). Measurement of TBEP in indoor air in offices showed
concentrations of 25 ng/m3 or less. TBEP is associated with
particulates and the source is considered to be the application of
floor polish. It has been detected at µg/kg levels in human adipose
tissue. The reported daily dietary intake from market basket studies,
for a range of age groups, was <0.02 µg/kg body weight per day.
Drinking-water concentrations of up to 270 µg/litre have been
reported, this is considered to arise from migration from rubber
gaskets in the plumbing.
TBEP is considered to be readily biodegradable. Sewage treatment
plant measurements and semi-continuous sludge laboratory tests have
indicated substantial elimination of TBEP (>80%). In river and
coastal water TBEP was completely degraded. The half-life in estuarine
water was reported to be about 50 days and there was little
degradation in unadapted seawater.
The acute systemic mammalian toxicity and irritation potential
are low.
Several subchronic studies in laboratory animals have shown that
the liver is the target organ for TBEP toxicity. One study in male
Sprague-Dawley rats suggested that TBEP might cause focal myocarditis.
Neurotoxic effects in rats after single doses of TBEP are
inconsistent. In rats repeatedly given high doses by gavage, TBEP
decreased nerve conduction velocity and increased the refractory
period. It did not cause delayed neurotoxicity in hens but did inhibit
brain and plasma cholinesterases.
Based on an 18-week repeated dose study in rats, the
no-observed-effect level (NOEL) for liver effects was reported to be
15 mg/kg body weight per day, while the lowest-observed-effect level
(LOEL) was 150 mg/kg body weight per day.
The long-term toxicity and carcinogenicity of TBEP have not been
studied.
Bacterial and mammalian cell tests for gene mutation gave
negative results, but no tests for chromosomal damage have been
reported.
Teratogenicity was not observed in one study in rats. Other
aspects of reproductive toxicity have not been reported.
A Repeat Human Insult Patch Test indicated no skin sensitization
and minimal skin irritation.
The toxicity of TBEP to aquatic organisms is moderate. The 48-h
LC50 in Daphnia magna is 75 mg/litre and the 96-h LC50 values in
fish range between 16 and 24 mg/litre.
A1.2 Evaluation
Occupational exposure to TBEP is likely to be by the dermal route
during manufacture (accidental exposure) and from the use of floor
polishes. The compound is absorbed dermally in experimental animals
but no information is available on its kinetics and metabolism. Dermal
exposure cannot, therefore, be quantified but is expected to be low.
Inhalation exposure in the office environment has been measured to be
25 ng/m3 or less.
Exposure of the general population is principally via food (from
use of TBEP as a plasticizer in packaging plastics) and drinking-water
(contaminated by leaching from synthetic rubbers used in plumbing
washers). Exposure from both sources is very low (estimated to be
<0.2 µg/kg body weight per day from the diet and concentrations in
drinking-water of <270 µg/litre).
Given the reported NOEL from animal studies of 15 mg/kg body
weight per day from a repeated dose oral study, the risk to the
general population is very low. The risk to the occupationally exposed
is also considered to be very low, though this cannot be quantified.
In the environment, TBEP is expected (from its low volatility,
high adsorption coefficient and moderate water solubility) to
partition to sediment. The few measured data confirm this. Degradation
in environmental media is expected to be rapid. No information is
available on breakdown products; phosphate released during breakdown
is not expected to contribute significantly to environmental nutrient
levels. Fig. 1 plots measured environmental concentrations in surface
water against reported acute toxicity values. The margin of safety
between highest reported concentrations and lowest reported toxicity
values is several orders of magnitude, indicating low risk to
organisms in the aquatic environment. No assessment of risk can be
made for the terrestrial compartment.
A1.3 Recommendations
For a full scientific evaluation of the compound, identification
and assessment of metabolites in mammals would be required, given the
toxicological profile of one of the suggested metabolites,
2-butoxyethanol.
 A2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, AND ANALYTICAL
METHODS
A2.1 Identity
Molecular structure:
A2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, AND ANALYTICAL
METHODS
A2.1 Identity
Molecular structure:
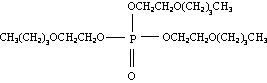 Empirical formula: C18H39O7P
Relative molecular mass: 398.54
Common name: tris(2-butoxyethyl) phosphate
Synonyms: phosphoric acid, tris(2-butoxyethyl) ester;
tri(2-butoxyethanol) phosphate;
tris(2- n-butoxyethyl) phosphate;
tributoxyethyl phosphate; TBOP; TBEP; TBXP
(only in Japanese literature);
2-butoxyethanol phosphate (RTCES, 1989);
tri(2-butylethylether) phosphate;
tris(butylglycol) phosphate; tributyl cello
solve phosphate
Trade names: Kronitex KP-140; KP-140; Phosflex T-BEP;
Phosflex 176C; Amgard TBEP
CAS registry number: 78-51-3
CAS name: Ethanol, 2-butoxy, phosphate (3:1)
EINECS number: 201-122-9
RTECS number: KJ9800000
A2.2 Physical and chemical properties
TBEP is a technical product that may contain as impurities
tributyl phosphate (about 3%) and traces of 2-butoxyethanol and
phosphoric acid (FMC, 1990; Albright & Wilson (1999) personal
communication to IPCS). There is no information on the concentration
of mono- or diesters or other impurities in the technical product.
TBEP is a light-coloured, high-boiling, non-flammable viscous
liquid with a butyl-like odour under normal conditions. It is more
soluble in non-polar than in polar solvents.
Boiling point: 200-230°C at 5.0-5.3 hPa
Melting point: -70°C
Density: 1.02 g/ml at 20°C
Viscosity: 11-15 mPa.s at 20°C
Vapour pressure:
at 25°C 2.8 × 10-7 hPa
at 150°C 0.33 hPa (0.03 mmHg)
Refractive index: 1.434 at 25°C
Solubility: 1.1-1.3 g/litre water at 20°C; miscible
in petroleum at 20°C
Acidity/alkalinity: neutral
(1 g/litre water at 20°C)
Flashpoint: 210°C (approximately);
159 ± 2°C
Ignition point: 251-52°C
Auto-ignition
temperature: 322 ± 5°C; 261°C
Log Koc: 4.38 (calculated)
n-Octanol/water
partition coefficient: 4.78 (calculated); 3.65
References: Eldefrawi et al. (1977); Keith & Walters (1985); Laham et
al. (1985b); Hoechst (1987); Watts & Moore (1988); Leo (1989); FMC
(1990); Hinckley et al. (1990); Lenga (1993); Tremain & Bartlett
(1994).
A2.3 Conversion factors
1 ppm = 16.53 mg/m3 at 20°C
1 mg/m3 = 0.0605 ppm at 20°C
A2.4 Analytical methods
TBEP is usually analysed by gas chromatography (GC) coupled with
mass spectrometry (MS), infrared spectroscopy or nuclear magnetic
resonance spectrometry. The detection limit is <1 ng/g (adipose
tissue) using any of these methods or a nitrogen/phosphorus-selective
detector (LeBel et al., 1981; Rivera et al., 1987).
A2.4.1 Air
TBEP has been found associated with particulate matter in the air
of offices. Of the methods that can be used to collect the particles,
Weschler (1980) used a four-stage impactor with a back-up filter and
extracted with a mixture of water and methanol. Later Weschler (1984)
and Weschler & Fong (1986) collected particles on Teflon(R) membranes,
separating the particles according to whether the aerodynamic diameter
was greater or less than 2.5 µm. The samples were analysed by GC/MS
after thermal desorption of the collector membranes. Sometimes samples
were desorbed or dissolved with toluene.
A2.4.2 Water
TBEP has been extracted either with dichloromethane after
acidification to pH 2 or by passage through a column filled with
Amberlite XAD-2 resin which is subsequently extracted with acetone and
hexane. After dehydration and concentration, extracts are analysed.
The concentrated extracts are determined by GC/MS, or with other
detection methods, as described above (LeBel et al., 1981; Watts &
Moore, 1988). LeBel et al. (1987) used large-volume resin sampling
cartridges to obtain sufficient organic extracts from water for
analysis. Recovery at 10 ng TBEP/litre fortification level was 103.4%.
Frimmel et al. (1987) described an analytical method to determine
TBEP in water by extracting TBEP with granulated activated carbon and
analysing the extract with GC/MS.
Rivera et al. (1987) analysed water samples with different
procedures, liquid-liquid extraction, adsorption on granular activated
carbon, extraction with dichloromethane, followed by GC/MS/DS
(Daughter spectral) detection. Ether-insoluble organic fractions were
analysed and fractionated by high-performance liquid chromatography
(HPLC) and ultraviolet absorbency detection was carried out with a
2140 diode-array detector, followed by fast atom bombardment (FAB) and
FAB-collision-induced dissociation - mass analysis kinetic energy
spectroscopy (CID-MIKES) mass spectrometry.
A2.4.3 Sediment
After decanting the supernatant water, the sediment samples are
mixed with an equal volume of pre-extracted anhydrous sodium sulfate
and transferred to a Soxhlet thimble. Soxhlet extraction is carried
out overnight using dichloromethane (300 ml) (Watts & Moore, 1988).
A2.4.4 Soils and foodstuffs
There are no reports of extraction or clean-up methods for soil
or food (ECETOC, 1992b).
A2.4.5 Biological media
LeBel & Williams (1983b, 1986) and LeBel et al. (1989) analysed
human adipose tissue for TBEP by extraction with a mixture of
acetone/hexane in the presence of anhydrous sodium sulfate. The
solution was centrifuged and the supernatant filtered and evaporated.
The resulting extract was dissolved in a mixture of 5% dichloromethane
in cyclohexane for gel permeation chromatography (GPC) to separate
residual lipids from phosphate esters. Using this method the recovery
of TBEP from adipose tissue was approximately 90%.
Anderson et al. (1984) measured peaks of TBEP determined by HPLC
in spiked samples of serum during the development of an analytical
refinement. There was a marked inter-individual variation in peak
height, which correlated with serum lipoprotein concentration.
A3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
A3.1 Natural occurrence
TBEP has not been found to occur naturally in the environment
(ECETOC, 1992b).
A3.2 Anthropogenic sources
A3.2.1 Production levels and processes
TBEP is produced by reacting phosphorus oxychloride and
butoxyethanol (butyl glycol) and stripping hydrochloric acid and
excess of butoxyethanol. Another production method uses the sodium
salt of the glycol. In this case, the by-product is sodium chloride
(ECETOC, 1992b).
The world global production has been estimated to be 5000-6000
tonnes, with less than 1000 tonnes in Europe.
A3.2.2 Uses
TBEP is used mainly as a component in floor polishes, a solvent
in some resins, a viscosity modifier in plastisols, an antifoam and
also as a plasticizer in synthetic rubber, plastics and lacquers. TBEP
is widely used as a plasticizer in rubber stoppers for vacutainer
tubes and plastic ware.
A4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION AND TRANSFORMATION
A4.1 Transport and distribution between media
All environmental TBEP derives from human activities but the
input rate to the environment cannot be estimated from the available
data. The input is expected to be mainly to soil, sediments and
surface waters from leachates from plastics on landfills, from
spillages and from effluents (ECETOC, 1992b).
The low vapour pressure, the high soil sorption coefficient
(Koc) and the water solubility of approximately 1 g/litre suggests
that TBEP in the environment will be found mainly in water and
sediment. TBEP has been detected in surface water and sediments
(ECETOC, 1992b).
A4.2 Biodegradation
No data are available on mechanisms of abiotic or biotic
transformation. Analogy with other phosphate esters suggests that
enzymatic hydrolysis would be expected to dominate (ECETOC, 1992b).
TBEP was readily biodegradable when tested in the OECD 301B
assay, achieving 87% degradation within 28 days (Mead & Handley 1998).
In a test of primary biodegradation using the semi-continuous
activated sludge procedure and an addition rate of 3 mg TBEP/litre per
test cycle, 88% of TBEP was eliminated. The ultimate biodegradability
(using the Monsanto shake-flask procedure) was 51% of the theoretical
CO2 generated after 28 days (Monsanto, 1976).
Hattori et al. (1981) studied the degradation of TBEP in
environmental water in 1979-1980. Using the molybdenum blue
colorimetric method, the increase of phosphate ions was analysed in Oh
and Neya river water and seawater from Osaka Bay to which
1 mg TBEP/litre had been added. The degradation depended on the source
of the water (Table 1).
Table 1. Biodegradation of TBEP in water in percentages
(from Hattori et al., 1981)
Test Oh River Neya River Osaka Bay
duration Tomagashima Senboku
(days) seawater seawater
7 29.1 0 1.9a 0
14 100b 100 17.6 100
a Test duration 8 days
b Test duration 15 days
A sterilized distilled water control did not show any degradation
after 15 days. TBEP was rapidly degraded in less than 14 days after an
acclimatization period of several days in water containing
micro-organisms. Where degradation was rapid, the phosphatase activity
increased during the test period.
TBEP was eliminated from estuarine water with a half-life of
approximately 50 days (Ernst, 1988).
A4.2.1 Migration
LeBel & Williams (1983a) investigated the difficulties of
obtaining representative water samples and the importance of designing
suitable sampling protocols. TBEP was detected in tap water at
concentrations from 11.0 to 5400 ng/litre. The authors suggested that
the TBEP originated from the O-ring and seal in the tap.
A5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
A5.1 Environmental levels
A5.1.1 Air
An indoor aerosol sample was collected in a large building in New
Jersey, USA. The abundance of TBEP was greatest both for particles
larger than 7.0 µm diameter and for those smaller than 1.1 µm; there
was considerably less material present in the intermediate size
ranges. This pattern is consistent with its use in floor polish.
Buffing operations generate relatively large particles which are
likely to contain TBEP. However, this compound may also migrate from
the floor polish and be attached to particles. In this case the
majority of the adsorbed TBEP would accumulate in the submicron size
range (Weschler, 1980). The mean concentrations measured in
representative samples of dust from air in 7 offices in the USA was
reported to be 15 ng/m3 (Weschler & Shields, 1986). The significance
of floor polish, which may contain 1% TBEP (Nakashima et al., 1993),
as a source of these particulates is suggested by the fact that the
highest concentration measured (25 ng/m3) was found immediately
following floor polishing work by a night crew.
Airborne concentrations of fine (2.5 µm) and coarse aerosol
(2.5-15 µm) particles were simultaneously measured outside and inside
two buildings, one in Wichita, Kansas, USA, during the fall and early
winter (1981-1982) and the second one in Lubbock, Texas, USA, during
late winter and spring 1982. The average indoor concentrations of TBEP
in Wichita and Lubbock were 4 and 25 ng/m3, mainly in fine aerosol
particles. TBEP was not found in outdoor aerosol particles (Weschler,
1984).
Yasuda (1980) reported the results of a study of 19 outdoor air
samples from 7 locations in 1976. Two samples from Kawauchi Town
contained 149.1 and 176.8 ng TBEP/m3 and one from Ehime University
9.6 ng TBEP/m3. TBEP was not detected in the other 16 samples.
A5.1.2 Water (drinking-water and surface water)
Levels of TBEP have been determined in rivers, sewage, tap water,
lakes and estuaries. The investigations have been carried out in the
Great Lakes area of Canada, USA, Japan, Germany and the United
Kingdom.
The lower part of the River Weser (over 33 km), Germany, was
examined for the presence of TBEP during the period May 1985 to April
1987. TBEP was found at a mean concentration of 125 ng/litre.
Systematic measurements of effluent samples from five sewage treatment
plants in the Bremen region showed concentrations of TBEP ranging from
800 to 34 900 ng/litre (Bohlen et al., 1989).
Ernst (1988) analysed water of the estuary of the Rivers Elbe and
Weser, Germany, for the presence of TBEP during the period 1983-1985.
The concentrations that were found ranged from 5 to 70 ng/litre.
One hundred samples of surface water were collected from various
locations throughout Japan in 1975 and analysed for the presence of
TBEP. TBEP was identified in none of the samples (the limit of
determination ranged from 0.02 to 0.5 µg/litre). In 1978, 114 samples
were analysed in Japan and TBEP was not identified (the limit of
determination ranged from 0.005 to 1.5 µg/litre) (Environmental Agency
Japan, 1978, 1983, 1987).
In a survey conducted between 1989 and 1990, Fukushima et al.
(1992) identified TBEP in Lake Biwa, Yodo River and also in the Yamato
Osaka Rivers and Osaka Bay at levels of about 0.2-2.5 µg/litre.
Drinking-water was collected in Japan over a 12-month period and
analysed. Concentrations ranging up to 0.0585 ng/litre were found
(Adachi et al., 1984).
Two samples of drinking-water collected from six Eastern Ontario
water treatment plants in the period June-October 1978 contained
0.9-75.4 ng/litre (LeBel et al., 1981). In another study two samples
of drinking-water were collected from five Great Lakes water treatment
plants of Eastern Ontario and analysed for TBEP. The concentration
found in surface water samples ranged from 9.8 to 54.4 ng/litre as
determined by GC/MS. When determined by GC/NPD, concentrations of 0.4
to 73.8 ng/litre were found (LeBel et al., 1987).
Williams et al. (1982) collected samples of drinking-water from
12 Ontario municipal water treatment plants which draw their water
from the Great Lakes system in January and August 1980. All samples
contained TBEP at concentrations ranging from 1.6 to 271.6 µg/litre.
The authors noted that TBEP is a common constituent of rubber gaskets
and washers and can be introduced into water from components of the
tap used for sampling.
In 1983, LeBel et al. (1983a) found up to 5400 ng/litre in a
sample of drinking-water taken after non-use of the tap for 65 h.
In the period August 1976 to March 1977, 16 grab samples of river
water were collected from the Delaware River, USA (between river mile
78 and 132). In addition to other compounds, TBEP was identified in
all samples. The concentrations ranged from 0.3 to 3.0 µg/litre in the
winter and from 0.4 to 2.0 µg/litre in the summer (Sheldon & Hites,
1978).
A5.1.3 Soils and sediment
TBEP was detected in 7 out of 80 samples of sediment collected at
different locations in Japan in 1975. The concentrations ranged from
0.22 to 0.54 mg/kg and the limit of determination was 0.002-0.1 mg/kg.
In 1978, none of the 114 sediment samples collected at different
places in Japan contained TBEP (limit of determination 0.0005-0.12
mg/kg) (Environmental Agency Japan, 1978, 1983).
Watts & Moore (1988) did not detect TBEP in suspended particles
or bottom sediments in a river in the United Kingdom, even though TBEP
was found in corresponding water columns.
A5.1.4 Aquatic organisms
No TBEP could be detected in 74 samples of fish from numerous
locations throughout Japan (limit of determination 0.005-0.1 mg/kg).
Another report from the same agency stated that TBEP was not found in
93 fish samples (limit of determination 0.0005-0.15 mg/kg)
(Environmental Agency Japan, 1978).
A5.2 Human tissue levels
LeBel & Williams (1983b) analysed 16 samples of human adipose
tissue for TBEP. Four of sixteen samples contained TBEP at
concentrations of 4.0-26.8 µg/kg. LeBel & Williams (1986) reported the
results of 115 human adipose tissue (omentum) samples for TBEP,
obtained at autopsy of humans from the Eastern Ontario cities,
Kingston and Ottawa, Canada. TBEP was detectable in 21 out of 68 male
adipose tissue samples and in 20 out of 47 female samples. Although
the frequency of detection was similar in the two cities, mean
concentrations in Ottawa were about 2.5 times those in Kingston. In
both cities the concentrations in women were 2-3 times greater than in
men. The arithmetic mean concentration of TBEP in the 41 detectable
samples was 11.3 µg/kg in extracted fat (in males 6.3 µg/kg and in
females 16.6 µg/kg). The mean concentration overall was 4.2 µg/kg in
extracted fat. In a different study, LeBel et al. (1989) showed the
presence of TBEP in human adipose tissue autopsy samples from 3 out of
6 Ontario (Canada) municipalities (based on a detection limit of 20
ng/g). No statistical difference between sexes was found, the mean
concentration being 396 ± 56 ng/g in Toronto and 173 ± 32 ng/g in
Cornwall.
A5.3 Food
In a series of articles Gunderson (1988, 1995a,b) reported data
on daily intake of TBEP for a range of age groups and for a period
between 1982 and 1991 from the USA FDA Total Diet Study (see Table 2).
Similar data were collected in a parallel study on ready-to-eat
food from 1982 to 1991. TBEP was found in 5 out of 230 food items
(baby food, ketchup, grapefruit juice, strawberries, tomatoes) and in
5 out of 17 050 chemical or pesticide samples, with an average
concentration per residue of 0.28 µg/g (Kan-Do Office and Pesticides
Team, 1995).
A5.4 Occupational exposure
The only data on occupational exposure to TBEP is from an office
environment. Weschler & Shields (1986) measured a mean concentration
of 15 ng/m3 in dust samples from some offices in the USA. NIOSH (USA)
has estimated that the number of workers exposed to TBEP is more than
200 000.
Table 2. Mean daily intake of TBEP per unit body weight (µg/kg body weight per day)
according to age and gender
6-11 2 years 14-16 years old 25-30 years old 60-65 years old
months old females males females males females males
old
1982-1984 0.0029 0.0144 0.0084 0.0077 0.0129 0.0107 0.0168 0.0137
1984-1986 0.0002 0.0015 0.0007 0.0011 0.0004 0.0008 0.0002 0.0002
1986-1991 0.0052 0.0037 0.0012 0.0011 0.0020 0.0009 0.0034 0.0028
A6. KINETICS AND METABOLISM IN LABORATORY ANIMALS AND HUMANS
No data are available on the kinetics or metabolism of TBEP
either in animals or humans.
The Task Group considered that 2-butoxyethanol is a metabolite.
Information on the toxicity of 2-butoxyethanol is given in IPCS
(1998).
A7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
A7.1 Single exposure
A7.1.1 Oral and dermal
The acute toxicity of TBEP following oral or dermal
administration is low (Table 3).
Table 3. Acute toxicity of TBEP
Species Route LD50 Reference
(mg/kg body weight)
Rat oral 3000 Eldefrawi et al. (1977)
Rat oral 4700 Monsanto (1984c)
Rabbit dermal >5000 Gabriel (1980c)
Rabbit dermal >10 000 Report ICD/T.76.019
by FMC Corporation,
Princeton, NJ, USA (1976)
An acute oral toxicity study was conducted according to the
"fixed dose" procedure. Two out of three male rats but no females died
at 5000 mg/kg body weight; no rats died at 500 mg/kg body weight.
Signs of toxicity included chromorhinorrhoea, dyspnoea and decreased
locomotion (Freeman, 1991a).
A7.1.2 Inhalation
The median lethal concentration in air has been investigated in a
4-h aerosol inhalation test (Hoechst, 1989). Groups of five male and
five female Wistar rats were exposed to measured TBEP concentrations
of 3.3, 3.4 or 6.4 mg/litre. No animal died but at all concentrations
the animals exhibited depressed and irregular respiration, increased
salivation, sneezing, unsteadiness and tremor, but these symptoms had
cleared in most animals 9 days later. There were no body weight
changes and gross necropsy revealed no abnormality. The 4-h LC50 was
thus >6.4 mg/litre.
The 4-h LC50 in rats was reported to be greater than 4.43
mg/litre determined gravimetrically (particle size 2.46 ± 2.52 µm)
(Mount 1991).
A7.2 Short-term repeated exposure
A7.2.1 Oral
In a 14-day oral dosing regime using male and female rats, where
the highest dose was 100 mg/kg body weight per day, a comprehensive
biochemical, haematological and histopathological evaluation showed no
changes (Komsta et al., 1989).
In a 4-week study, diets containing 0, 500, 2000, 7500 or
15 000 mg TBEP/kg were fed to male and female Sprague-Dawley rats.
No signs of toxicity were found in male rats of any group whereas
there was a slight decrease in body weight and food consumption in
females receiving diets containing 7500 or 15 000 mg/kg diet. No
compound-related changes were observed at necropsy (Monsanto, 1985a).
In a 14-week oral toxicity study with TBEP, Wistar rats (5 weeks
old, male and female, 15 rats/group) were given a diet containing 0,
0.3, 3 or 30 g TBEP/kg. Suppression of body weight gain was observed
in both sexes at 30 g/kg. Serum cholinesterase activity was
significantly decreased in both sexes at 3 and 30 g/kg, and serum
gammaglutamyl transferase activity was significantly increased in both
sexes at 30 g/kg. Examination of the liver in both sexes revealed
moderate periportal hepatocyte swelling in male rats at 30 g/kg after
14 weeks of exposure but this change was not found in male rats given
3 g/kg or less. The no-observed-effect level (NOEL) of TBEP in the
diet was 0.3 g/kg diet (for males 20 mg/kg body weight per day and for
females 22 mg/kg body weight per day. The Task Group considered the
NOAEL of this study to be 3 g/kg diet (Tsuda et al., 1993; Saitoh et
al., 1994).
In a gavage study, groups of 12 male and 12 female Sprague-Dawley
rats were administered 0, 0.25 or 0.5 ml/kg body weight undiluted TBEP
on 5 days/week for 18 weeks. During the first week, two high-dose
females showed muscular weakness and ataxia which had disappeared by
the end of the fourth week. After about 7 weeks, nearly all animals
exhibited some signs of toxicity, which seemed to be treatment
related. All treated animals appeared less active, and one female died
during week 13. Breathing difficulties and ataxia were present in
several males and females in both treatment groups, though the
low-dose group was affected to a lesser extent. Tremors, piloerection,
lacrimation and increased urination were observed in both males and
females of the high-dose group. After the last dose, the clinical
signs observed in the high-dose group decreased in intensity.
High-dose females had significantly elevated level of serum
gamma-glutamyltransferase. Red cell acetylcholinesterase (AchE)
activity was significantly reduced in males at both doses. There were
no haematological changes. Animals were necropsied one week after the
last dose. Liver weight was significantly increased (about 20%) in
both high- and low-dose groups. Kidney weight was increased by about
20% in both groups and the increase was statistically significant in
high-dose groups. Histopathological changes were confined to the heart
of male rats of both groups. Three of six high-dose and two of six
low-dose animals had multiple foci of mononuclear cell infiltration,
haemorrhages and/or myocardial fibre degeneration. Two of six
high-dose, three of six low-dose and one of six control rats
demonstrated multifocal interstitial fibrosis with or without
macrophage containing haemosiderin pigment. The authors concluded that
TBEP may have accelerated the development of focal myocarditis, which
is a normal feature of older male Sprague-Dawley rats. A NOAEL was not
ascertained in this study (Laham et al., 1984a, 1985a).
In an 18-week study, four groups of 20 male and 20 female
Sprague-Dawley rats were fed diets containing 0, 300, 3000 or 10 000
mg TBEP/kg. Body weight, food intake and clinical observations were
similar in treated and control rats. Haematological and clinical
chemistry parameters were normal except for increased platelet counts
in the 10 000 mg/kg group, and increased serum
gamma-glutamyl-transpeptidase and decreased plasma cholinesterase
activity in the 3000 and 10 000 mg/kg groups. Liver weight was
increased in the 10 000 mg/kg group. Microscopic examination showed
mild periportal hepatocellular hypertrophy and periportal
vacuolization in males receiving 3000 and 10 000 mg/kg in the diet.
The NOEL was 300 mg/kg diet, equivalent to 15 mg/kg body weight per
day (Monsanto, 1987a).
A7.2.2 Dermal
In a 21-day dermal toxicity study on New Zealand White rabbits,
groups of 6 male and 6 female animals were treated with TBEP
applications of 0, 10, 100 or 1000 mg/kg body weight per day,
5 days/week for 3 weeks. The unabraded dorsal clipped skin was used.
The tests sites were occluded for 6 h after each exposure. No animals
died and no adverse clinical signs of pharmacological/toxicological
effects were observed. There was no indication that dermal exposure to
1000 mg/kg body weight per day resulted in any adverse systemic
effect, but local irritation, oedema, atonia and desquamation occurred
at all dose levels (Monsanto, 1985b).
A7.3 Skin and eye irritation; sensitization
In three studies TBEP was non-irritating to intact and abraded
skin when applied topically to albino rabbits. (Gabriel 1980b;
Monsanto, 1984c; Freeman, 1991b).
In the 21-day dermal toxicity study on New Zealand White rabbits,
slight to moderate erythema was noted. The skin irritation was
dose-related and severity progressed over time. Microscopic
observations of the skin (of the 1000 mg/kg group) showed squamous
cell hyperplasia, hyperkeratosis, hair follicles distended with
keratin and surface accumulation of keratin and cellular debris,
erosions ulcers, acute/subacute inflammation and congestion and
haemorrhages in various combinations (Monsanto, 1985b) (see also
section A7.2.2).
In four studies TBEP was non-irritating to the eyes of albino
rabbits (Gabriel 1980a; Monsanto, 1984c; Freeman, 1991c; personal
communication from Hoechst AG, Frankfurt, Germany entitled: Eye
irritation test on New Zealand rabbit with TBEP, 1988).
No animal data are available on skin sensitization potential.
A7.4 Reproductive toxicity, embryotoxicity and teratogenicity
TBEP was administered by gavage in corn oil to three groups of 25
mated Charles River CD female rats at dose levels of 0 (corn oil),
250, 500 or 1500 mg/kg body weight per day on days 6 to 15 of
gestation. The treatment had no effect at any dose level on fetal
resorption, fetal viability, post-implantation loss, total
implantations or the incidence of fetal malformations. The NOEL was
the highest dose level tested, 1500 mg/kg body weight (Monsanto,
1985e). In an earlier range-finding study maternal weight loss was
observed in animals receiving 2000 mg/kg but not 1000 mg/kg body
weight per day (Monsanto, 1985d).
A7.5 Mutagenicity and related end-points
A mutagenicity test was carried out with Salmonella
typhimurium strains TA1535, TA1538, TA1537, TA98 and TA100, with and
without metabolic activation. Liver S9 fractions were used from male
Sprague-Dawley rats or from male Syrian hamsters induced by Aroclor
1254. TBEP was non-mutagenic (MacKeller, 1978)
TBEP was tested for mutagenic activity with Salmonella
typhimurium strains TA98, TA100, TA1535 and TA1537, in the presence
and absence of rat liver metabolic system, in comparison with positive
controls. The concentrations tested were 0, 50, 100, 500, 1000, 5000
and 10 000 µg/plate with and without S9. Toxicity to strain TA100 was
observed at 5000 and 10 000 µg/plate in the presence and absence of
metabolic activation. The same effect was seen at 10 000 µg/plate with
TA1535 and TA98 in the absence of S9 mix. TBEP did not cause any
mutagenic response either with or without metabolic activation
(Monsanto, 1984d).
A CHO/HGPRT mammalian cell forward gene mutation assay with TBEP
was carried out. The tests were conducted at 50, 100, 150, 225 and 300
µg/ml with S9 and at 5, 50, 75, 100 and 130 µg/ml without S9. TBEP was
not mutagenic (Monsanto, 1985c).
A7.6 Carcinogenicity
No data on the carcinogenicity of TBEP are available.
A7.7 Special studies
A7.7.1 Neurotoxicity
A7.7.1.1 Acute administration
An acute delayed neurotoxicity study was carried out using groups
of 20 hens. Dermal or oral (in gelatin capsules) TBEP doses of 5000
mg/kg body weight were administered at the start of the study and
again 21 days later. Positive control hens were given 750 mg/kg body
weight of tri- ortho-cresyl phosphate (TOCP) at the same time
intervals. Negative controls were either untreated (dermal study) or
given empty capsules (oral study). All hens were treated with 15 mg/kg
body weight of atropine sulfate three times a day for 5 days following
each dosing. Hens were killed 21 days after being given the final
dose, and histological preparations were made from brain, spinal cord
and peripheral nerves. No treatment-related lesions were detected in
the nerves of TBEP-treated hens. TBEP had no effect on neuropathy
target esterase (NTE). Brain and plasma cholinesterases were inhibited
in treated hens (Carrington et al., 1990).
In another study, groups of five hens were treated orally with
TBEP (5000 mg/kg), with TOCP (750 mg/kg) as positive control group, or
with the capsules alone. The animals were killed 24 h after treatment.
Brain AChE, brain neuropathy target esterase (NTE) and plasma
butyrylcholinesterase (BuChE) activity was measured. No differences
were seen between control and TBEP-treated brain NTE activity,
although plasma BuChE and brain AChE levels in TBEP-treated hens were
depressed to 5% and 13% of the control group, respectively (Monsanto,
1986).
Laham et al. (1985b) reported the results of the administration
by gavage to Sprague-Dawley rats of a single dose of TBEP (98.2%).
Groups of randomized female and male rats (10 rats of each sex per
dose level) were used. The doses were 1.0, 1.5, 1.75, 2.0 and 3.2 g/kg
for females and 1.0, 3.2, 6.8, 8.0 and 9.0 g/kg body weight for males.
Three weeks after the administration of TBEP, electrophysiological
parameters were determined in four or less surviving animals for each
group, selected from survivors showing overt clinical signs.
Reductions in caudal nerve conduction velocity and increases in
refractory period (in males) were observed. Sciatic nerve sections
showed degenerative changes in some myelinated and unmyelinated
fibres. It should be noticed that the doses were in the region of or
greater than the LD50. There was a high mortality. Survivors were ill
and had marked weight loss.
The Task Group considered this study of inadequate quality for
use in risk evaluation.
A study of similar design as the oral study of Monsanto (1986)
but with dermal application of 5000 mg/kg body weight both on day 0
and on day 21 showed no clinical signs of toxicity in chickens
(Monsanto, 1986).
A7.7.1.2 Repeated oral administration
In a 14-day repeated-dose study on Sprague-Dawley rats dosed at
0.8 to 2.24 ml/kg body weight (08-2.28 g/kg), electro-physiological
measurements were made on days 15 and 28. Apart from a significant
decrease in the body weight of low-dose females at 7 days, there were
no clinical signs or significant differences between dosed groups and
controls in the 14-day study. Minor and inconsistent changes in
electro-physiological parameters were reported. No morphological
changes were found using light or electron microscopy (Laham et al.,
1984b).
A second study (Lahman et al., 1984a) involved dosing on 5 days
per week for 18 weeks at dose levels of 0 (0.5 ml water), 0.25 and 0.5
ml/kg body weight (0.25-0.51 g/kg) with observations at 6, 12 and 18
weeks. There were no significant body weight differences between
exposed groups and their controls at any stage. A few females (2/12)
from the high-dose group showed, at the beginning of the experiment,
transient muscular weakness and ataxia which disappeared 4 weeks
later. In the second half of the study almost all treated animals
exhibited tremors, piloerection, lacrimation and increased urination.
Males were less affected than females.
Electro-physiological changes were observed at 18 weeks in all
test animals (Table 4) and included a statistically significant
reduction in nerve conduction velocity and a significant increase of
both relative and absolute refractory periods. The increased
refractory period and the decreased conduction velocity were
dose-related in females, but in males the maximum effect appears to
have been reached by the low dose, suggesting that the magnitude of
the maximum attainable neurophysiological changes is modest. Three
animals of each sex at each dose level were examined for
neurohistological abnormalities by light and electron microscopy of
the sciatic nerve. Most of the treated animals showed the presence of
some degenerative myelin sheaths accompanied by axonal swelling and an
advanced stage of degeneration, indicated by the presence of
lamellated electron-dense inclusions in unmyelinated nerve fibres
(Laham et al., 1984a).
In the 18-week studies of Monsanto (1987a,b), TBEP was
administered to four groups of 20 male and 20 female Sprague-Dawley
rats at concentrations of 0, 300, 3000 and 10 000 mg/kg diet for
approximately 18 weeks. No clinical signs of neurotoxicity were
observed. The only neurophysiological alteration observed was reduced
caudal nerve conduction velocity in high-dose females, and there were
no treatment-related changes in peripheral nerve or spinal cord
histopathology.
Table 4. Electro-physiological parameters at 18 weeks in rats treated with TBEP
(Laham et al., 1984a)a
Control (water) Low-dose TBEP High-dose TBEP
Males Females Males Females Males Females
Number of animals 12 12 12 12 12 12
Dose (ml/kg
per day) - - 0.25 0.25 0.5 0.5
Nerve conduction
velocity (m/s) 36.3 36.3 30.7b 32.0b 30.1b 30.8b
Absolute refractory
period in caudal nerve (ms) 1.02 0.95 1.24b 1.26b 1.24b 1.34b
Relative refractory
period in caudal nerve (ms) 2.06 1.93 2.39b 2.33b 2.32b 2.43b
a results at 6 and 12 weeks were quantitatively similar to those at 18 weeks
b P<0.001
A7.7.1.3 Effects on esterase activity
Laham et al. (1984b) reported a 5-7% reduction in red cell
cholinesterase activity at 18 weeks in male rats dosed by gavage with
0.25 or 0.5 ml TBEP/kg body weight per day but no reductions in female
rats.
A study was made of the effect of TBEP on NTE, brain AChE and
plasma BuChE in three groups of five hens. Each was administered a
single oral dose of 5000 mg TBEP/kg body weight. All animals were
killed 24 h after treatment. The NTE activity was unchanged but plasma
BuChE and brain AChE levels were depressed to 5% and 13%,
respectively, of control levels (Monsanto, 1986).
In an acute delayed neurotoxicity study in hens, two doses of
5000 mg TBEP/kg body weight were given 21 days apart, each followed by
antidote treatment with atropine. There was no effect on NTE activity,
whereas brain AChE and serum BuChE were inhibited (Carrington et al.,
1990).
A8. EFFECTS ON HUMANS
A repeat human insult patch test on a panel of 209 volunteers was
undertaken by Monsanto (1984e). In the 3-week induction period, four
applications per week of 0.2 ml of the test material were applied for
24 h to occluded skin. During the fourth week, four similar
applications were made to previously untreated sites. During
induction, minimal irritation was observed in 9 of the individuals.
The irritation was only seen once or twice during the 12 applications.
There was no dermal reaction to challenge applications. The results
indicate minimal skin irritation and do not indicate any sensitizing
potential.
A9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
A9.1 Laboratory experiments
A9.1.1 Aquatic organisms
A9.1.1.1 Invertebrates
The 24-h and 48-h LC50 values for TBEP in Daphnia magna were
84 mg/litre and 75 mg/litre, respectively. The no-observed-effect
concentration (NOEC) was 32 mg/litre (Monsanto, 1984a).
A9.1.1.2 Vertebrates
The 96-h LC50 in fathead minnow (Pimephales promelas) was
16 mg/litre (95% confidence interval 13-22 mg/litre) at 22°C
(Monsanto, 1984b). The 48-h LC50 values in killifish (Oryzias
latipes) at 10, 20 and 30°C were 44 mg, 27 mg and 6.8 mg/litre,
respectively (Tsuji et al., 1986).
In goldfish (Carassius auratus) Eldefrawi et al. (1977)
reported no death at 5 mg/litre after 168 h (temperature 20°C).
In rainbow trout (Oncorhynchus mykiss), a 96-h LC50 of
24 mg/litre and a NOEC of 10 mg/litre were reported in a test
conducted under OECD guideline 203 (Wetton & Handley, 1998).
PART B
TRIS(2-ETHYLHEXYL) PHOSPHATE
(TEHP)
B. SUMMARY, EVALUATION AND RECOMMENDATIONS
B1. Tris (2-ethylhexyl) phosphate (TEHP)
B1.1 Summary
Tris(2-ethylhexyl) phosphate (TEHP) is a non-flammable,
colourless liquid with low water solubility and very low vapour
pressure, which is used as a flame retardant and plasticizer for PVC
and cellulose acetate and as a solvent. It is produced from phosphorus
oxychloride and 2-ethylhexanol. Figures for current worldwide
production are not available. Approximately 1000 tonnes are currently
produced in Germany.
TEHP has not been detected in outdoor air; it has been detected
in indoor air at concentrations of less than 10 ng/m3, in river water
at concentrations of up to 7500 ng/litre and in sediments at
2-70 ng/g. TEHP was detected in a single sample of drinking-water at
0.3 ng/litre. Reported daily dietary intake from market basket
studies, from a range of age groups, was less than 0.05 µg/kg body
weight per day.
TEHP is rapidly biodegraded in natural waters, but in laboratory
tests with activated sludge the results were equivocal. There is no
significant abiotic degradation.
TEHP has a low acute toxicity for mammals, the oral LD50 being
>10 000 mg/kg body weight for rats.
TEHP is a skin irritant but not an eye irritant. Repeated
application of 0.1 ml (93 mg) TEHP to the skin of rabbits produced no
signs of systemic intoxication.
Thirteen-week gavage studies in rats and mice revealed no
significant toxic effects. The no-observed-adverse-effect level
(NOAEL) in rats was 2860 mg/kg body weight per day and in mice was
5710 mg/kg body weight per day, the highest dose tested in each
species.
In a 3-month inhalation study at concentrations up to 85.0 mg
TEHP/m3, the lungs of dogs showed mild chronic inflammatory changes,
and conditioned avoidance performance deteriorated in relation to the
concentration administered.
No studies on reproductive toxicity were available.
TEHP gave negative results in several in vivo and in vitro
tests for mutagenicity.
TEHP was tested for chronic toxicity and carcinogenicity in rats
and mice. The NOAEL for chronic toxicity in male rats was 2857 mg/kg
body weight per day and in female rats was 1428 mg/kg body weight per
day. In male and female mice, the lowest-observed-adverse-effect level
(LOAEL) for thyroid follicular cell hyperplasia was 357 mg/kg body
weight per day. A NOAEL in mice was not established. The authors
concluded there was some evidence of carcinogenicity based on an
increased incidence of hepatocellular carcinomas in female mice at the
high-dose level and equivocal evidence of carcinogenicity based on the
increased incidence of adrenal phaeochromocytomas in male rats in both
dose levels. Although there were increases in adrenal
phaeochromocytomas in both dose groups of male rats and in
hepatocellular carcinomas in female mice in the high-dose group, these
results are not considered to indicate that TEHP presents a
significant carcinogenic risk to humans. Phaeochromocytomas show a
variable background incidence in rats. The incidences of these tumours
in two previous National Toxicology Programme (NTP) bioassays were
equal to the incidence observed in the TEHP bioassay. The only other
significant neoplastic finding was hepatocellular carcinomas in the
high-dose group of female mice. Considering the low incidence of this
tumour, its occurrence in only one sex of one species, the lack of
evidence of genetic toxicity, and the low exposure of humans to TEHP,
it is unlikely that TEHP poses a significant carcinogenic risk to
humans.
Neurotoxicity studies have been conducted in several species.
TEHP causes no alteration in activity of plasma or red blood cell
cholinesterase. No studies on delayed neurotoxicity have been
reported.
In a study on human volunteers, no skin irritation was reported.
The few data available indicate a low acute aquatic toxicity of
TEHP. The IC50 for bacteria is greater than 100 mg/litre and the 96-h
LC50 for zebra fish (Brachydanio rerio) is greater than
100 mg/litre, which is the solubility limit of TEHP in water.
B1.2 Evaluation
Occupational exposure to TEHP is likely to be by the dermal route
during manufacture (accidental exposure) and from the use of some
products. The compound is absorbed dermally in experimental animals
but no information is available on its kinetics or metabolism via this
route. Dermal exposure cannot, therefore, be quantified but is
expected to be low. Inhalation exposure in the office environment has
been measured to be 10 ng/m3 or less.
Exposure of the general population is principally via food and
drinking-water. Exposure from both sources is very low (estimated to
be <0.05 µg/kg body weight per day from the diet; a single measured
concentration in drinking-water was 0.3 ng/litre).
Given the reported LOAEL for thyroid hyperplasia of 357 mg/kg
body weight per day in mice, the risk to the general population is
very low. The risk to those exposed occupationally is also considered
to be very low, although this cannot be quantified.
TEHP is not considered to be carcinogenic in humans.
In the environment, TEHP is expected (from its low volatility,
high adsorption coefficient and low water solubility) to partition to
sediment. Measured data are too few to confirm this. Degradation in
environmental media is expected, although laboratory data on
degradation in sewage sludges are equivocal. No information is
available on breakdown products; phosphate released during breakdown
is not expected to contribute significantly to environmental nutrient
levels. Fig. 2 plots measured environmental concentrations in
environmental media against reported acute toxicity values (the latter
indicating no toxic effects at the limit of water solubility). The
margin of safety between highest reported concentrations and lowest
reported toxicity values is several orders of magnitude, indicating
low risk to organisms in the aquatic environment. No assessment of
risk can be made for the terrestrial compartment.
B1.3 Recommendations
For full scientific evaluation of the compound, identification
and assessment of metabolites in mammals would be required, given
the toxicological profile of one of the suggested metabolites,
2-ethylhexanol.
Reproductive toxicity needs to be investigated, in particular the
potential for developmental effects.
Empirical formula: C18H39O7P
Relative molecular mass: 398.54
Common name: tris(2-butoxyethyl) phosphate
Synonyms: phosphoric acid, tris(2-butoxyethyl) ester;
tri(2-butoxyethanol) phosphate;
tris(2- n-butoxyethyl) phosphate;
tributoxyethyl phosphate; TBOP; TBEP; TBXP
(only in Japanese literature);
2-butoxyethanol phosphate (RTCES, 1989);
tri(2-butylethylether) phosphate;
tris(butylglycol) phosphate; tributyl cello
solve phosphate
Trade names: Kronitex KP-140; KP-140; Phosflex T-BEP;
Phosflex 176C; Amgard TBEP
CAS registry number: 78-51-3
CAS name: Ethanol, 2-butoxy, phosphate (3:1)
EINECS number: 201-122-9
RTECS number: KJ9800000
A2.2 Physical and chemical properties
TBEP is a technical product that may contain as impurities
tributyl phosphate (about 3%) and traces of 2-butoxyethanol and
phosphoric acid (FMC, 1990; Albright & Wilson (1999) personal
communication to IPCS). There is no information on the concentration
of mono- or diesters or other impurities in the technical product.
TBEP is a light-coloured, high-boiling, non-flammable viscous
liquid with a butyl-like odour under normal conditions. It is more
soluble in non-polar than in polar solvents.
Boiling point: 200-230°C at 5.0-5.3 hPa
Melting point: -70°C
Density: 1.02 g/ml at 20°C
Viscosity: 11-15 mPa.s at 20°C
Vapour pressure:
at 25°C 2.8 × 10-7 hPa
at 150°C 0.33 hPa (0.03 mmHg)
Refractive index: 1.434 at 25°C
Solubility: 1.1-1.3 g/litre water at 20°C; miscible
in petroleum at 20°C
Acidity/alkalinity: neutral
(1 g/litre water at 20°C)
Flashpoint: 210°C (approximately);
159 ± 2°C
Ignition point: 251-52°C
Auto-ignition
temperature: 322 ± 5°C; 261°C
Log Koc: 4.38 (calculated)
n-Octanol/water
partition coefficient: 4.78 (calculated); 3.65
References: Eldefrawi et al. (1977); Keith & Walters (1985); Laham et
al. (1985b); Hoechst (1987); Watts & Moore (1988); Leo (1989); FMC
(1990); Hinckley et al. (1990); Lenga (1993); Tremain & Bartlett
(1994).
A2.3 Conversion factors
1 ppm = 16.53 mg/m3 at 20°C
1 mg/m3 = 0.0605 ppm at 20°C
A2.4 Analytical methods
TBEP is usually analysed by gas chromatography (GC) coupled with
mass spectrometry (MS), infrared spectroscopy or nuclear magnetic
resonance spectrometry. The detection limit is <1 ng/g (adipose
tissue) using any of these methods or a nitrogen/phosphorus-selective
detector (LeBel et al., 1981; Rivera et al., 1987).
A2.4.1 Air
TBEP has been found associated with particulate matter in the air
of offices. Of the methods that can be used to collect the particles,
Weschler (1980) used a four-stage impactor with a back-up filter and
extracted with a mixture of water and methanol. Later Weschler (1984)
and Weschler & Fong (1986) collected particles on Teflon(R) membranes,
separating the particles according to whether the aerodynamic diameter
was greater or less than 2.5 µm. The samples were analysed by GC/MS
after thermal desorption of the collector membranes. Sometimes samples
were desorbed or dissolved with toluene.
A2.4.2 Water
TBEP has been extracted either with dichloromethane after
acidification to pH 2 or by passage through a column filled with
Amberlite XAD-2 resin which is subsequently extracted with acetone and
hexane. After dehydration and concentration, extracts are analysed.
The concentrated extracts are determined by GC/MS, or with other
detection methods, as described above (LeBel et al., 1981; Watts &
Moore, 1988). LeBel et al. (1987) used large-volume resin sampling
cartridges to obtain sufficient organic extracts from water for
analysis. Recovery at 10 ng TBEP/litre fortification level was 103.4%.
Frimmel et al. (1987) described an analytical method to determine
TBEP in water by extracting TBEP with granulated activated carbon and
analysing the extract with GC/MS.
Rivera et al. (1987) analysed water samples with different
procedures, liquid-liquid extraction, adsorption on granular activated
carbon, extraction with dichloromethane, followed by GC/MS/DS
(Daughter spectral) detection. Ether-insoluble organic fractions were
analysed and fractionated by high-performance liquid chromatography
(HPLC) and ultraviolet absorbency detection was carried out with a
2140 diode-array detector, followed by fast atom bombardment (FAB) and
FAB-collision-induced dissociation - mass analysis kinetic energy
spectroscopy (CID-MIKES) mass spectrometry.
A2.4.3 Sediment
After decanting the supernatant water, the sediment samples are
mixed with an equal volume of pre-extracted anhydrous sodium sulfate
and transferred to a Soxhlet thimble. Soxhlet extraction is carried
out overnight using dichloromethane (300 ml) (Watts & Moore, 1988).
A2.4.4 Soils and foodstuffs
There are no reports of extraction or clean-up methods for soil
or food (ECETOC, 1992b).
A2.4.5 Biological media
LeBel & Williams (1983b, 1986) and LeBel et al. (1989) analysed
human adipose tissue for TBEP by extraction with a mixture of
acetone/hexane in the presence of anhydrous sodium sulfate. The
solution was centrifuged and the supernatant filtered and evaporated.
The resulting extract was dissolved in a mixture of 5% dichloromethane
in cyclohexane for gel permeation chromatography (GPC) to separate
residual lipids from phosphate esters. Using this method the recovery
of TBEP from adipose tissue was approximately 90%.
Anderson et al. (1984) measured peaks of TBEP determined by HPLC
in spiked samples of serum during the development of an analytical
refinement. There was a marked inter-individual variation in peak
height, which correlated with serum lipoprotein concentration.
A3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
A3.1 Natural occurrence
TBEP has not been found to occur naturally in the environment
(ECETOC, 1992b).
A3.2 Anthropogenic sources
A3.2.1 Production levels and processes
TBEP is produced by reacting phosphorus oxychloride and
butoxyethanol (butyl glycol) and stripping hydrochloric acid and
excess of butoxyethanol. Another production method uses the sodium
salt of the glycol. In this case, the by-product is sodium chloride
(ECETOC, 1992b).
The world global production has been estimated to be 5000-6000
tonnes, with less than 1000 tonnes in Europe.
A3.2.2 Uses
TBEP is used mainly as a component in floor polishes, a solvent
in some resins, a viscosity modifier in plastisols, an antifoam and
also as a plasticizer in synthetic rubber, plastics and lacquers. TBEP
is widely used as a plasticizer in rubber stoppers for vacutainer
tubes and plastic ware.
A4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION AND TRANSFORMATION
A4.1 Transport and distribution between media
All environmental TBEP derives from human activities but the
input rate to the environment cannot be estimated from the available
data. The input is expected to be mainly to soil, sediments and
surface waters from leachates from plastics on landfills, from
spillages and from effluents (ECETOC, 1992b).
The low vapour pressure, the high soil sorption coefficient
(Koc) and the water solubility of approximately 1 g/litre suggests
that TBEP in the environment will be found mainly in water and
sediment. TBEP has been detected in surface water and sediments
(ECETOC, 1992b).
A4.2 Biodegradation
No data are available on mechanisms of abiotic or biotic
transformation. Analogy with other phosphate esters suggests that
enzymatic hydrolysis would be expected to dominate (ECETOC, 1992b).
TBEP was readily biodegradable when tested in the OECD 301B
assay, achieving 87% degradation within 28 days (Mead & Handley 1998).
In a test of primary biodegradation using the semi-continuous
activated sludge procedure and an addition rate of 3 mg TBEP/litre per
test cycle, 88% of TBEP was eliminated. The ultimate biodegradability
(using the Monsanto shake-flask procedure) was 51% of the theoretical
CO2 generated after 28 days (Monsanto, 1976).
Hattori et al. (1981) studied the degradation of TBEP in
environmental water in 1979-1980. Using the molybdenum blue
colorimetric method, the increase of phosphate ions was analysed in Oh
and Neya river water and seawater from Osaka Bay to which
1 mg TBEP/litre had been added. The degradation depended on the source
of the water (Table 1).
Table 1. Biodegradation of TBEP in water in percentages
(from Hattori et al., 1981)
Test Oh River Neya River Osaka Bay
duration Tomagashima Senboku
(days) seawater seawater
7 29.1 0 1.9a 0
14 100b 100 17.6 100
a Test duration 8 days
b Test duration 15 days
A sterilized distilled water control did not show any degradation
after 15 days. TBEP was rapidly degraded in less than 14 days after an
acclimatization period of several days in water containing
micro-organisms. Where degradation was rapid, the phosphatase activity
increased during the test period.
TBEP was eliminated from estuarine water with a half-life of
approximately 50 days (Ernst, 1988).
A4.2.1 Migration
LeBel & Williams (1983a) investigated the difficulties of
obtaining representative water samples and the importance of designing
suitable sampling protocols. TBEP was detected in tap water at
concentrations from 11.0 to 5400 ng/litre. The authors suggested that
the TBEP originated from the O-ring and seal in the tap.
A5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
A5.1 Environmental levels
A5.1.1 Air
An indoor aerosol sample was collected in a large building in New
Jersey, USA. The abundance of TBEP was greatest both for particles
larger than 7.0 µm diameter and for those smaller than 1.1 µm; there
was considerably less material present in the intermediate size
ranges. This pattern is consistent with its use in floor polish.
Buffing operations generate relatively large particles which are
likely to contain TBEP. However, this compound may also migrate from
the floor polish and be attached to particles. In this case the
majority of the adsorbed TBEP would accumulate in the submicron size
range (Weschler, 1980). The mean concentrations measured in
representative samples of dust from air in 7 offices in the USA was
reported to be 15 ng/m3 (Weschler & Shields, 1986). The significance
of floor polish, which may contain 1% TBEP (Nakashima et al., 1993),
as a source of these particulates is suggested by the fact that the
highest concentration measured (25 ng/m3) was found immediately
following floor polishing work by a night crew.
Airborne concentrations of fine (2.5 µm) and coarse aerosol
(2.5-15 µm) particles were simultaneously measured outside and inside
two buildings, one in Wichita, Kansas, USA, during the fall and early
winter (1981-1982) and the second one in Lubbock, Texas, USA, during
late winter and spring 1982. The average indoor concentrations of TBEP
in Wichita and Lubbock were 4 and 25 ng/m3, mainly in fine aerosol
particles. TBEP was not found in outdoor aerosol particles (Weschler,
1984).
Yasuda (1980) reported the results of a study of 19 outdoor air
samples from 7 locations in 1976. Two samples from Kawauchi Town
contained 149.1 and 176.8 ng TBEP/m3 and one from Ehime University
9.6 ng TBEP/m3. TBEP was not detected in the other 16 samples.
A5.1.2 Water (drinking-water and surface water)
Levels of TBEP have been determined in rivers, sewage, tap water,
lakes and estuaries. The investigations have been carried out in the
Great Lakes area of Canada, USA, Japan, Germany and the United
Kingdom.
The lower part of the River Weser (over 33 km), Germany, was
examined for the presence of TBEP during the period May 1985 to April
1987. TBEP was found at a mean concentration of 125 ng/litre.
Systematic measurements of effluent samples from five sewage treatment
plants in the Bremen region showed concentrations of TBEP ranging from
800 to 34 900 ng/litre (Bohlen et al., 1989).
Ernst (1988) analysed water of the estuary of the Rivers Elbe and
Weser, Germany, for the presence of TBEP during the period 1983-1985.
The concentrations that were found ranged from 5 to 70 ng/litre.
One hundred samples of surface water were collected from various
locations throughout Japan in 1975 and analysed for the presence of
TBEP. TBEP was identified in none of the samples (the limit of
determination ranged from 0.02 to 0.5 µg/litre). In 1978, 114 samples
were analysed in Japan and TBEP was not identified (the limit of
determination ranged from 0.005 to 1.5 µg/litre) (Environmental Agency
Japan, 1978, 1983, 1987).
In a survey conducted between 1989 and 1990, Fukushima et al.
(1992) identified TBEP in Lake Biwa, Yodo River and also in the Yamato
Osaka Rivers and Osaka Bay at levels of about 0.2-2.5 µg/litre.
Drinking-water was collected in Japan over a 12-month period and
analysed. Concentrations ranging up to 0.0585 ng/litre were found
(Adachi et al., 1984).
Two samples of drinking-water collected from six Eastern Ontario
water treatment plants in the period June-October 1978 contained
0.9-75.4 ng/litre (LeBel et al., 1981). In another study two samples
of drinking-water were collected from five Great Lakes water treatment
plants of Eastern Ontario and analysed for TBEP. The concentration
found in surface water samples ranged from 9.8 to 54.4 ng/litre as
determined by GC/MS. When determined by GC/NPD, concentrations of 0.4
to 73.8 ng/litre were found (LeBel et al., 1987).
Williams et al. (1982) collected samples of drinking-water from
12 Ontario municipal water treatment plants which draw their water
from the Great Lakes system in January and August 1980. All samples
contained TBEP at concentrations ranging from 1.6 to 271.6 µg/litre.
The authors noted that TBEP is a common constituent of rubber gaskets
and washers and can be introduced into water from components of the
tap used for sampling.
In 1983, LeBel et al. (1983a) found up to 5400 ng/litre in a
sample of drinking-water taken after non-use of the tap for 65 h.
In the period August 1976 to March 1977, 16 grab samples of river
water were collected from the Delaware River, USA (between river mile
78 and 132). In addition to other compounds, TBEP was identified in
all samples. The concentrations ranged from 0.3 to 3.0 µg/litre in the
winter and from 0.4 to 2.0 µg/litre in the summer (Sheldon & Hites,
1978).
A5.1.3 Soils and sediment
TBEP was detected in 7 out of 80 samples of sediment collected at
different locations in Japan in 1975. The concentrations ranged from
0.22 to 0.54 mg/kg and the limit of determination was 0.002-0.1 mg/kg.
In 1978, none of the 114 sediment samples collected at different
places in Japan contained TBEP (limit of determination 0.0005-0.12
mg/kg) (Environmental Agency Japan, 1978, 1983).
Watts & Moore (1988) did not detect TBEP in suspended particles
or bottom sediments in a river in the United Kingdom, even though TBEP
was found in corresponding water columns.
A5.1.4 Aquatic organisms
No TBEP could be detected in 74 samples of fish from numerous
locations throughout Japan (limit of determination 0.005-0.1 mg/kg).
Another report from the same agency stated that TBEP was not found in
93 fish samples (limit of determination 0.0005-0.15 mg/kg)
(Environmental Agency Japan, 1978).
A5.2 Human tissue levels
LeBel & Williams (1983b) analysed 16 samples of human adipose
tissue for TBEP. Four of sixteen samples contained TBEP at
concentrations of 4.0-26.8 µg/kg. LeBel & Williams (1986) reported the
results of 115 human adipose tissue (omentum) samples for TBEP,
obtained at autopsy of humans from the Eastern Ontario cities,
Kingston and Ottawa, Canada. TBEP was detectable in 21 out of 68 male
adipose tissue samples and in 20 out of 47 female samples. Although
the frequency of detection was similar in the two cities, mean
concentrations in Ottawa were about 2.5 times those in Kingston. In
both cities the concentrations in women were 2-3 times greater than in
men. The arithmetic mean concentration of TBEP in the 41 detectable
samples was 11.3 µg/kg in extracted fat (in males 6.3 µg/kg and in
females 16.6 µg/kg). The mean concentration overall was 4.2 µg/kg in
extracted fat. In a different study, LeBel et al. (1989) showed the
presence of TBEP in human adipose tissue autopsy samples from 3 out of
6 Ontario (Canada) municipalities (based on a detection limit of 20
ng/g). No statistical difference between sexes was found, the mean
concentration being 396 ± 56 ng/g in Toronto and 173 ± 32 ng/g in
Cornwall.
A5.3 Food
In a series of articles Gunderson (1988, 1995a,b) reported data
on daily intake of TBEP for a range of age groups and for a period
between 1982 and 1991 from the USA FDA Total Diet Study (see Table 2).
Similar data were collected in a parallel study on ready-to-eat
food from 1982 to 1991. TBEP was found in 5 out of 230 food items
(baby food, ketchup, grapefruit juice, strawberries, tomatoes) and in
5 out of 17 050 chemical or pesticide samples, with an average
concentration per residue of 0.28 µg/g (Kan-Do Office and Pesticides
Team, 1995).
A5.4 Occupational exposure
The only data on occupational exposure to TBEP is from an office
environment. Weschler & Shields (1986) measured a mean concentration
of 15 ng/m3 in dust samples from some offices in the USA. NIOSH (USA)
has estimated that the number of workers exposed to TBEP is more than
200 000.
Table 2. Mean daily intake of TBEP per unit body weight (µg/kg body weight per day)
according to age and gender
6-11 2 years 14-16 years old 25-30 years old 60-65 years old
months old females males females males females males
old
1982-1984 0.0029 0.0144 0.0084 0.0077 0.0129 0.0107 0.0168 0.0137
1984-1986 0.0002 0.0015 0.0007 0.0011 0.0004 0.0008 0.0002 0.0002
1986-1991 0.0052 0.0037 0.0012 0.0011 0.0020 0.0009 0.0034 0.0028
A6. KINETICS AND METABOLISM IN LABORATORY ANIMALS AND HUMANS
No data are available on the kinetics or metabolism of TBEP
either in animals or humans.
The Task Group considered that 2-butoxyethanol is a metabolite.
Information on the toxicity of 2-butoxyethanol is given in IPCS
(1998).
A7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
A7.1 Single exposure
A7.1.1 Oral and dermal
The acute toxicity of TBEP following oral or dermal
administration is low (Table 3).
Table 3. Acute toxicity of TBEP
Species Route LD50 Reference
(mg/kg body weight)
Rat oral 3000 Eldefrawi et al. (1977)
Rat oral 4700 Monsanto (1984c)
Rabbit dermal >5000 Gabriel (1980c)
Rabbit dermal >10 000 Report ICD/T.76.019
by FMC Corporation,
Princeton, NJ, USA (1976)
An acute oral toxicity study was conducted according to the
"fixed dose" procedure. Two out of three male rats but no females died
at 5000 mg/kg body weight; no rats died at 500 mg/kg body weight.
Signs of toxicity included chromorhinorrhoea, dyspnoea and decreased
locomotion (Freeman, 1991a).
A7.1.2 Inhalation
The median lethal concentration in air has been investigated in a
4-h aerosol inhalation test (Hoechst, 1989). Groups of five male and
five female Wistar rats were exposed to measured TBEP concentrations
of 3.3, 3.4 or 6.4 mg/litre. No animal died but at all concentrations
the animals exhibited depressed and irregular respiration, increased
salivation, sneezing, unsteadiness and tremor, but these symptoms had
cleared in most animals 9 days later. There were no body weight
changes and gross necropsy revealed no abnormality. The 4-h LC50 was
thus >6.4 mg/litre.
The 4-h LC50 in rats was reported to be greater than 4.43
mg/litre determined gravimetrically (particle size 2.46 ± 2.52 µm)
(Mount 1991).
A7.2 Short-term repeated exposure
A7.2.1 Oral
In a 14-day oral dosing regime using male and female rats, where
the highest dose was 100 mg/kg body weight per day, a comprehensive
biochemical, haematological and histopathological evaluation showed no
changes (Komsta et al., 1989).
In a 4-week study, diets containing 0, 500, 2000, 7500 or
15 000 mg TBEP/kg were fed to male and female Sprague-Dawley rats.
No signs of toxicity were found in male rats of any group whereas
there was a slight decrease in body weight and food consumption in
females receiving diets containing 7500 or 15 000 mg/kg diet. No
compound-related changes were observed at necropsy (Monsanto, 1985a).
In a 14-week oral toxicity study with TBEP, Wistar rats (5 weeks
old, male and female, 15 rats/group) were given a diet containing 0,
0.3, 3 or 30 g TBEP/kg. Suppression of body weight gain was observed
in both sexes at 30 g/kg. Serum cholinesterase activity was
significantly decreased in both sexes at 3 and 30 g/kg, and serum
gammaglutamyl transferase activity was significantly increased in both
sexes at 30 g/kg. Examination of the liver in both sexes revealed
moderate periportal hepatocyte swelling in male rats at 30 g/kg after
14 weeks of exposure but this change was not found in male rats given
3 g/kg or less. The no-observed-effect level (NOEL) of TBEP in the
diet was 0.3 g/kg diet (for males 20 mg/kg body weight per day and for
females 22 mg/kg body weight per day. The Task Group considered the
NOAEL of this study to be 3 g/kg diet (Tsuda et al., 1993; Saitoh et
al., 1994).
In a gavage study, groups of 12 male and 12 female Sprague-Dawley
rats were administered 0, 0.25 or 0.5 ml/kg body weight undiluted TBEP
on 5 days/week for 18 weeks. During the first week, two high-dose
females showed muscular weakness and ataxia which had disappeared by
the end of the fourth week. After about 7 weeks, nearly all animals
exhibited some signs of toxicity, which seemed to be treatment
related. All treated animals appeared less active, and one female died
during week 13. Breathing difficulties and ataxia were present in
several males and females in both treatment groups, though the
low-dose group was affected to a lesser extent. Tremors, piloerection,
lacrimation and increased urination were observed in both males and
females of the high-dose group. After the last dose, the clinical
signs observed in the high-dose group decreased in intensity.
High-dose females had significantly elevated level of serum
gamma-glutamyltransferase. Red cell acetylcholinesterase (AchE)
activity was significantly reduced in males at both doses. There were
no haematological changes. Animals were necropsied one week after the
last dose. Liver weight was significantly increased (about 20%) in
both high- and low-dose groups. Kidney weight was increased by about
20% in both groups and the increase was statistically significant in
high-dose groups. Histopathological changes were confined to the heart
of male rats of both groups. Three of six high-dose and two of six
low-dose animals had multiple foci of mononuclear cell infiltration,
haemorrhages and/or myocardial fibre degeneration. Two of six
high-dose, three of six low-dose and one of six control rats
demonstrated multifocal interstitial fibrosis with or without
macrophage containing haemosiderin pigment. The authors concluded that
TBEP may have accelerated the development of focal myocarditis, which
is a normal feature of older male Sprague-Dawley rats. A NOAEL was not
ascertained in this study (Laham et al., 1984a, 1985a).
In an 18-week study, four groups of 20 male and 20 female
Sprague-Dawley rats were fed diets containing 0, 300, 3000 or 10 000
mg TBEP/kg. Body weight, food intake and clinical observations were
similar in treated and control rats. Haematological and clinical
chemistry parameters were normal except for increased platelet counts
in the 10 000 mg/kg group, and increased serum
gamma-glutamyl-transpeptidase and decreased plasma cholinesterase
activity in the 3000 and 10 000 mg/kg groups. Liver weight was
increased in the 10 000 mg/kg group. Microscopic examination showed
mild periportal hepatocellular hypertrophy and periportal
vacuolization in males receiving 3000 and 10 000 mg/kg in the diet.
The NOEL was 300 mg/kg diet, equivalent to 15 mg/kg body weight per
day (Monsanto, 1987a).
A7.2.2 Dermal
In a 21-day dermal toxicity study on New Zealand White rabbits,
groups of 6 male and 6 female animals were treated with TBEP
applications of 0, 10, 100 or 1000 mg/kg body weight per day,
5 days/week for 3 weeks. The unabraded dorsal clipped skin was used.
The tests sites were occluded for 6 h after each exposure. No animals
died and no adverse clinical signs of pharmacological/toxicological
effects were observed. There was no indication that dermal exposure to
1000 mg/kg body weight per day resulted in any adverse systemic
effect, but local irritation, oedema, atonia and desquamation occurred
at all dose levels (Monsanto, 1985b).
A7.3 Skin and eye irritation; sensitization
In three studies TBEP was non-irritating to intact and abraded
skin when applied topically to albino rabbits. (Gabriel 1980b;
Monsanto, 1984c; Freeman, 1991b).
In the 21-day dermal toxicity study on New Zealand White rabbits,
slight to moderate erythema was noted. The skin irritation was
dose-related and severity progressed over time. Microscopic
observations of the skin (of the 1000 mg/kg group) showed squamous
cell hyperplasia, hyperkeratosis, hair follicles distended with
keratin and surface accumulation of keratin and cellular debris,
erosions ulcers, acute/subacute inflammation and congestion and
haemorrhages in various combinations (Monsanto, 1985b) (see also
section A7.2.2).
In four studies TBEP was non-irritating to the eyes of albino
rabbits (Gabriel 1980a; Monsanto, 1984c; Freeman, 1991c; personal
communication from Hoechst AG, Frankfurt, Germany entitled: Eye
irritation test on New Zealand rabbit with TBEP, 1988).
No animal data are available on skin sensitization potential.
A7.4 Reproductive toxicity, embryotoxicity and teratogenicity
TBEP was administered by gavage in corn oil to three groups of 25
mated Charles River CD female rats at dose levels of 0 (corn oil),
250, 500 or 1500 mg/kg body weight per day on days 6 to 15 of
gestation. The treatment had no effect at any dose level on fetal
resorption, fetal viability, post-implantation loss, total
implantations or the incidence of fetal malformations. The NOEL was
the highest dose level tested, 1500 mg/kg body weight (Monsanto,
1985e). In an earlier range-finding study maternal weight loss was
observed in animals receiving 2000 mg/kg but not 1000 mg/kg body
weight per day (Monsanto, 1985d).
A7.5 Mutagenicity and related end-points
A mutagenicity test was carried out with Salmonella
typhimurium strains TA1535, TA1538, TA1537, TA98 and TA100, with and
without metabolic activation. Liver S9 fractions were used from male
Sprague-Dawley rats or from male Syrian hamsters induced by Aroclor
1254. TBEP was non-mutagenic (MacKeller, 1978)
TBEP was tested for mutagenic activity with Salmonella
typhimurium strains TA98, TA100, TA1535 and TA1537, in the presence
and absence of rat liver metabolic system, in comparison with positive
controls. The concentrations tested were 0, 50, 100, 500, 1000, 5000
and 10 000 µg/plate with and without S9. Toxicity to strain TA100 was
observed at 5000 and 10 000 µg/plate in the presence and absence of
metabolic activation. The same effect was seen at 10 000 µg/plate with
TA1535 and TA98 in the absence of S9 mix. TBEP did not cause any
mutagenic response either with or without metabolic activation
(Monsanto, 1984d).
A CHO/HGPRT mammalian cell forward gene mutation assay with TBEP
was carried out. The tests were conducted at 50, 100, 150, 225 and 300
µg/ml with S9 and at 5, 50, 75, 100 and 130 µg/ml without S9. TBEP was
not mutagenic (Monsanto, 1985c).
A7.6 Carcinogenicity
No data on the carcinogenicity of TBEP are available.
A7.7 Special studies
A7.7.1 Neurotoxicity
A7.7.1.1 Acute administration
An acute delayed neurotoxicity study was carried out using groups
of 20 hens. Dermal or oral (in gelatin capsules) TBEP doses of 5000
mg/kg body weight were administered at the start of the study and
again 21 days later. Positive control hens were given 750 mg/kg body
weight of tri- ortho-cresyl phosphate (TOCP) at the same time
intervals. Negative controls were either untreated (dermal study) or
given empty capsules (oral study). All hens were treated with 15 mg/kg
body weight of atropine sulfate three times a day for 5 days following
each dosing. Hens were killed 21 days after being given the final
dose, and histological preparations were made from brain, spinal cord
and peripheral nerves. No treatment-related lesions were detected in
the nerves of TBEP-treated hens. TBEP had no effect on neuropathy
target esterase (NTE). Brain and plasma cholinesterases were inhibited
in treated hens (Carrington et al., 1990).
In another study, groups of five hens were treated orally with
TBEP (5000 mg/kg), with TOCP (750 mg/kg) as positive control group, or
with the capsules alone. The animals were killed 24 h after treatment.
Brain AChE, brain neuropathy target esterase (NTE) and plasma
butyrylcholinesterase (BuChE) activity was measured. No differences
were seen between control and TBEP-treated brain NTE activity,
although plasma BuChE and brain AChE levels in TBEP-treated hens were
depressed to 5% and 13% of the control group, respectively (Monsanto,
1986).
Laham et al. (1985b) reported the results of the administration
by gavage to Sprague-Dawley rats of a single dose of TBEP (98.2%).
Groups of randomized female and male rats (10 rats of each sex per
dose level) were used. The doses were 1.0, 1.5, 1.75, 2.0 and 3.2 g/kg
for females and 1.0, 3.2, 6.8, 8.0 and 9.0 g/kg body weight for males.
Three weeks after the administration of TBEP, electrophysiological
parameters were determined in four or less surviving animals for each
group, selected from survivors showing overt clinical signs.
Reductions in caudal nerve conduction velocity and increases in
refractory period (in males) were observed. Sciatic nerve sections
showed degenerative changes in some myelinated and unmyelinated
fibres. It should be noticed that the doses were in the region of or
greater than the LD50. There was a high mortality. Survivors were ill
and had marked weight loss.
The Task Group considered this study of inadequate quality for
use in risk evaluation.
A study of similar design as the oral study of Monsanto (1986)
but with dermal application of 5000 mg/kg body weight both on day 0
and on day 21 showed no clinical signs of toxicity in chickens
(Monsanto, 1986).
A7.7.1.2 Repeated oral administration
In a 14-day repeated-dose study on Sprague-Dawley rats dosed at
0.8 to 2.24 ml/kg body weight (08-2.28 g/kg), electro-physiological
measurements were made on days 15 and 28. Apart from a significant
decrease in the body weight of low-dose females at 7 days, there were
no clinical signs or significant differences between dosed groups and
controls in the 14-day study. Minor and inconsistent changes in
electro-physiological parameters were reported. No morphological
changes were found using light or electron microscopy (Laham et al.,
1984b).
A second study (Lahman et al., 1984a) involved dosing on 5 days
per week for 18 weeks at dose levels of 0 (0.5 ml water), 0.25 and 0.5
ml/kg body weight (0.25-0.51 g/kg) with observations at 6, 12 and 18
weeks. There were no significant body weight differences between
exposed groups and their controls at any stage. A few females (2/12)
from the high-dose group showed, at the beginning of the experiment,
transient muscular weakness and ataxia which disappeared 4 weeks
later. In the second half of the study almost all treated animals
exhibited tremors, piloerection, lacrimation and increased urination.
Males were less affected than females.
Electro-physiological changes were observed at 18 weeks in all
test animals (Table 4) and included a statistically significant
reduction in nerve conduction velocity and a significant increase of
both relative and absolute refractory periods. The increased
refractory period and the decreased conduction velocity were
dose-related in females, but in males the maximum effect appears to
have been reached by the low dose, suggesting that the magnitude of
the maximum attainable neurophysiological changes is modest. Three
animals of each sex at each dose level were examined for
neurohistological abnormalities by light and electron microscopy of
the sciatic nerve. Most of the treated animals showed the presence of
some degenerative myelin sheaths accompanied by axonal swelling and an
advanced stage of degeneration, indicated by the presence of
lamellated electron-dense inclusions in unmyelinated nerve fibres
(Laham et al., 1984a).
In the 18-week studies of Monsanto (1987a,b), TBEP was
administered to four groups of 20 male and 20 female Sprague-Dawley
rats at concentrations of 0, 300, 3000 and 10 000 mg/kg diet for
approximately 18 weeks. No clinical signs of neurotoxicity were
observed. The only neurophysiological alteration observed was reduced
caudal nerve conduction velocity in high-dose females, and there were
no treatment-related changes in peripheral nerve or spinal cord
histopathology.
Table 4. Electro-physiological parameters at 18 weeks in rats treated with TBEP
(Laham et al., 1984a)a
Control (water) Low-dose TBEP High-dose TBEP
Males Females Males Females Males Females
Number of animals 12 12 12 12 12 12
Dose (ml/kg
per day) - - 0.25 0.25 0.5 0.5
Nerve conduction
velocity (m/s) 36.3 36.3 30.7b 32.0b 30.1b 30.8b
Absolute refractory
period in caudal nerve (ms) 1.02 0.95 1.24b 1.26b 1.24b 1.34b
Relative refractory
period in caudal nerve (ms) 2.06 1.93 2.39b 2.33b 2.32b 2.43b
a results at 6 and 12 weeks were quantitatively similar to those at 18 weeks
b P<0.001
A7.7.1.3 Effects on esterase activity
Laham et al. (1984b) reported a 5-7% reduction in red cell
cholinesterase activity at 18 weeks in male rats dosed by gavage with
0.25 or 0.5 ml TBEP/kg body weight per day but no reductions in female
rats.
A study was made of the effect of TBEP on NTE, brain AChE and
plasma BuChE in three groups of five hens. Each was administered a
single oral dose of 5000 mg TBEP/kg body weight. All animals were
killed 24 h after treatment. The NTE activity was unchanged but plasma
BuChE and brain AChE levels were depressed to 5% and 13%,
respectively, of control levels (Monsanto, 1986).
In an acute delayed neurotoxicity study in hens, two doses of
5000 mg TBEP/kg body weight were given 21 days apart, each followed by
antidote treatment with atropine. There was no effect on NTE activity,
whereas brain AChE and serum BuChE were inhibited (Carrington et al.,
1990).
A8. EFFECTS ON HUMANS
A repeat human insult patch test on a panel of 209 volunteers was
undertaken by Monsanto (1984e). In the 3-week induction period, four
applications per week of 0.2 ml of the test material were applied for
24 h to occluded skin. During the fourth week, four similar
applications were made to previously untreated sites. During
induction, minimal irritation was observed in 9 of the individuals.
The irritation was only seen once or twice during the 12 applications.
There was no dermal reaction to challenge applications. The results
indicate minimal skin irritation and do not indicate any sensitizing
potential.
A9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
A9.1 Laboratory experiments
A9.1.1 Aquatic organisms
A9.1.1.1 Invertebrates
The 24-h and 48-h LC50 values for TBEP in Daphnia magna were
84 mg/litre and 75 mg/litre, respectively. The no-observed-effect
concentration (NOEC) was 32 mg/litre (Monsanto, 1984a).
A9.1.1.2 Vertebrates
The 96-h LC50 in fathead minnow (Pimephales promelas) was
16 mg/litre (95% confidence interval 13-22 mg/litre) at 22°C
(Monsanto, 1984b). The 48-h LC50 values in killifish (Oryzias
latipes) at 10, 20 and 30°C were 44 mg, 27 mg and 6.8 mg/litre,
respectively (Tsuji et al., 1986).
In goldfish (Carassius auratus) Eldefrawi et al. (1977)
reported no death at 5 mg/litre after 168 h (temperature 20°C).
In rainbow trout (Oncorhynchus mykiss), a 96-h LC50 of
24 mg/litre and a NOEC of 10 mg/litre were reported in a test
conducted under OECD guideline 203 (Wetton & Handley, 1998).
PART B
TRIS(2-ETHYLHEXYL) PHOSPHATE
(TEHP)
B. SUMMARY, EVALUATION AND RECOMMENDATIONS
B1. Tris (2-ethylhexyl) phosphate (TEHP)
B1.1 Summary
Tris(2-ethylhexyl) phosphate (TEHP) is a non-flammable,
colourless liquid with low water solubility and very low vapour
pressure, which is used as a flame retardant and plasticizer for PVC
and cellulose acetate and as a solvent. It is produced from phosphorus
oxychloride and 2-ethylhexanol. Figures for current worldwide
production are not available. Approximately 1000 tonnes are currently
produced in Germany.
TEHP has not been detected in outdoor air; it has been detected
in indoor air at concentrations of less than 10 ng/m3, in river water
at concentrations of up to 7500 ng/litre and in sediments at
2-70 ng/g. TEHP was detected in a single sample of drinking-water at
0.3 ng/litre. Reported daily dietary intake from market basket
studies, from a range of age groups, was less than 0.05 µg/kg body
weight per day.
TEHP is rapidly biodegraded in natural waters, but in laboratory
tests with activated sludge the results were equivocal. There is no
significant abiotic degradation.
TEHP has a low acute toxicity for mammals, the oral LD50 being
>10 000 mg/kg body weight for rats.
TEHP is a skin irritant but not an eye irritant. Repeated
application of 0.1 ml (93 mg) TEHP to the skin of rabbits produced no
signs of systemic intoxication.
Thirteen-week gavage studies in rats and mice revealed no
significant toxic effects. The no-observed-adverse-effect level
(NOAEL) in rats was 2860 mg/kg body weight per day and in mice was
5710 mg/kg body weight per day, the highest dose tested in each
species.
In a 3-month inhalation study at concentrations up to 85.0 mg
TEHP/m3, the lungs of dogs showed mild chronic inflammatory changes,
and conditioned avoidance performance deteriorated in relation to the
concentration administered.
No studies on reproductive toxicity were available.
TEHP gave negative results in several in vivo and in vitro
tests for mutagenicity.
TEHP was tested for chronic toxicity and carcinogenicity in rats
and mice. The NOAEL for chronic toxicity in male rats was 2857 mg/kg
body weight per day and in female rats was 1428 mg/kg body weight per
day. In male and female mice, the lowest-observed-adverse-effect level
(LOAEL) for thyroid follicular cell hyperplasia was 357 mg/kg body
weight per day. A NOAEL in mice was not established. The authors
concluded there was some evidence of carcinogenicity based on an
increased incidence of hepatocellular carcinomas in female mice at the
high-dose level and equivocal evidence of carcinogenicity based on the
increased incidence of adrenal phaeochromocytomas in male rats in both
dose levels. Although there were increases in adrenal
phaeochromocytomas in both dose groups of male rats and in
hepatocellular carcinomas in female mice in the high-dose group, these
results are not considered to indicate that TEHP presents a
significant carcinogenic risk to humans. Phaeochromocytomas show a
variable background incidence in rats. The incidences of these tumours
in two previous National Toxicology Programme (NTP) bioassays were
equal to the incidence observed in the TEHP bioassay. The only other
significant neoplastic finding was hepatocellular carcinomas in the
high-dose group of female mice. Considering the low incidence of this
tumour, its occurrence in only one sex of one species, the lack of
evidence of genetic toxicity, and the low exposure of humans to TEHP,
it is unlikely that TEHP poses a significant carcinogenic risk to
humans.
Neurotoxicity studies have been conducted in several species.
TEHP causes no alteration in activity of plasma or red blood cell
cholinesterase. No studies on delayed neurotoxicity have been
reported.
In a study on human volunteers, no skin irritation was reported.
The few data available indicate a low acute aquatic toxicity of
TEHP. The IC50 for bacteria is greater than 100 mg/litre and the 96-h
LC50 for zebra fish (Brachydanio rerio) is greater than
100 mg/litre, which is the solubility limit of TEHP in water.
B1.2 Evaluation
Occupational exposure to TEHP is likely to be by the dermal route
during manufacture (accidental exposure) and from the use of some
products. The compound is absorbed dermally in experimental animals
but no information is available on its kinetics or metabolism via this
route. Dermal exposure cannot, therefore, be quantified but is
expected to be low. Inhalation exposure in the office environment has
been measured to be 10 ng/m3 or less.
Exposure of the general population is principally via food and
drinking-water. Exposure from both sources is very low (estimated to
be <0.05 µg/kg body weight per day from the diet; a single measured
concentration in drinking-water was 0.3 ng/litre).
Given the reported LOAEL for thyroid hyperplasia of 357 mg/kg
body weight per day in mice, the risk to the general population is
very low. The risk to those exposed occupationally is also considered
to be very low, although this cannot be quantified.
TEHP is not considered to be carcinogenic in humans.
In the environment, TEHP is expected (from its low volatility,
high adsorption coefficient and low water solubility) to partition to
sediment. Measured data are too few to confirm this. Degradation in
environmental media is expected, although laboratory data on
degradation in sewage sludges are equivocal. No information is
available on breakdown products; phosphate released during breakdown
is not expected to contribute significantly to environmental nutrient
levels. Fig. 2 plots measured environmental concentrations in
environmental media against reported acute toxicity values (the latter
indicating no toxic effects at the limit of water solubility). The
margin of safety between highest reported concentrations and lowest
reported toxicity values is several orders of magnitude, indicating
low risk to organisms in the aquatic environment. No assessment of
risk can be made for the terrestrial compartment.
B1.3 Recommendations
For full scientific evaluation of the compound, identification
and assessment of metabolites in mammals would be required, given
the toxicological profile of one of the suggested metabolites,
2-ethylhexanol.
Reproductive toxicity needs to be investigated, in particular the
potential for developmental effects.
 B2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, AND ANALYTICAL
METHODS
B2.1 Identity
Chemical structure:
B2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, AND ANALYTICAL
METHODS
B2.1 Identity
Chemical structure:
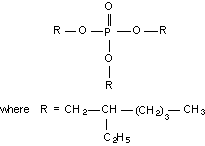 Chemical formula: C24H51O4P
Relative molecular mass: 434.64
CAS registry number: 78-42-2
EINECS number: 201-116-6
RTECS number: MP-0770000
CAS name: phosphoric acid, tris(2-ethylhexyl) ester
Synonyms:a 1-hexanol 2-ethyl-phosphate;
2-ethyl-1-hexanol phosphate; triethylhexyl
phosphate; tri(2-ethylhexyl) phosphate;
tris(isooctyl) phosphate
Trade names: Disflamoll TOF; Flexol TOF; Reomol TOP;
Amgard TOF; Antiblaze TOF
a The synonym trioctyl phosphate has also been
used. However, this chemical has a different
chemical abstracts (CAS) registry number
(1806-54-8).
B2.2 Physical and chemical properties
Tris(2-ethylhexyl) phosphate (TEHP) is a colourless to light
yellow liquid, non-flammable and nearly odourless (Arias, 1992).
Boiling point: 220°C at 6.67 hPa;
210°C at 5 hPa
Melting point: -74°C
Pour point: -70°C
Relative density: 0.926 at 20°C
Refractive index: 1.4426 at 20°C
Vapour pressure: <0.1 hPa at 20°C
Viscosity: 10.2 cP
Stability: stable under normal storage conditions; can
react with oxidizers
Flash point: 190-195°C
Solubility: soluble in acetone, ether and ethanol;
in DMSO 1.0 mg/litre at 18°C;
in water less than 0.1 g/litre at 20°C
n-Octanol/water
partition coefficient 4.22
From: MacFarland & Punte (1966); Saeger et al. (1979); Keith & Walters
(1987); Hinckley et al. (1990); FMC (1998).
B2.3 Conversion factors
1 ppm = 17.78 mg/m3
1 mg/m3 = 0.056 ppm
B2.4 Analytical methods
The analytical methods for TEHP are based on gas chromatography
combined with flame ionization detection (FID), flame photometric
detection (FPD), mass spectroscopy (MS) or nitrogen-phosphorus
sensitive detection (NPD). The detection limits are in the ng/m3
(air) and ng/litre (water) range.
Lerche & Morch (1973) determined TEHP using GC combined with FID
with a detection limit of 5-30 ng/litre. The separation of various
phosphoric acid esters by GC was achieved using columns filled with
various liquid silicone phases.
B2.4.1 Air
In a method described by Krzymien (1981), TEHP vapour and aerosol
were collected in glass absorber tubes packed with a plug of fine
platinum mesh coated with silicone packing material and subsequently
thermally desorbed into a GC for analysis. The capacity of the
absorber was found to be 2.1 ng pure TEHP when presented with 3 ng
TEHP/litre. Concentrations of 20 pg/litre were determined with a
precision of better than 10%. The aerosol concentration and its
drop-size distribution were determined at the picogram level with
around 5% precision using a cascade impactor. Armstrong & Yule (1978)
determined TEHP deposited on foliage and twigs by extraction with
toluene, drying with anhydrous sodium sulfate and using GC with FPD.
B2.4.2 Water
LeBel et al. (1981) used Amberlite(R) XAD-2 macroreticular resin
to collect TEHP from drinking-water. The resin was extracted with an
acetone/hexane mixture. TEHP was identified by GC and by GC/MS at
ng/litre levels. The recovery by direct fortification was 62%.
Determination of TEHP in extracts of activated carbon by means of
GC/MS was described by Frimmel et al. (1987). TEHP was extracted from
activated charcoal using a mixture of acetone, dichloromethane and
toluene.
Kawagoshi & Fukunaga (1994, 1995) showed, by extracting leachate
with dichloromethane and analysing the residue by GC-FPD, that it was
possible to detect organophosphoric acid triesters, including TEHP, at
a limit of detection of 2 ng/litre.
B2.4.3 Sediment
Sediment samples were extracted with acetone and dried,
concentrated and analysed by Ishikawa et al. (1985) in a similar way
to that used for water samples. The limit of determination was 10
ng/g.
B3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
B3.1 Natural occurrence
TEHP does not occur naturally in the environment.
B3.2 Anthropogenic sources
B3.2.1 Production levels and processes
In 1992, approximately 1000 tonnes of TEHP were manufactured in
Germany (BUA, 1997).
Figures for the world production of TEHP are not available.
ECETOC (1992a) estimated the world production to be between 1000 and
5000 tonnes/year.
TEHP is produced by reaction of phosphorus oxychloride and
2-ethylhexanol. The triester is separated by vacuum distillation.
Technical grade TEHP is usually 99% pure. The impurities are
2-ethylhexanol, bis(2-ethylhexyl) phosphate (BEHP) and traces of water
(ECETOC, 1992a).
B3.2.2 Uses
TEHP is used in PVC plastisols, as a flame retardant in cellulose
acetate and as a solvent for certain chemical reactions. It is also
used as a flame retardant plasticizer, particularly for PVC, in low
temperature application (ECETOC, 1992a).
B4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION AND TRANSFORMATION
B4.1 Biodegradation
Biodegradation of phosphoric acid esters involves stepwise
hydrolysis to ortho-phosphate and alcohol moieties. The alcohol
would then be expected to undergo further degradation (Saeger et al.,
1979).
In a ready biodegradability closed bottle test (OECD Guideline
301D), no biodegradation of TEHP was observed after 28 days (Bayer,
1982a).
An activated sludge method, based on a semi-continuous procedure,
was used to test primary degradation of TEHP. The addition rate of the
compound was 3 mg/litre per 24 h and the biodegradation was 20 (± 8)%
after 34 weeks (Saeger et al., 1979).
TEHP was rapidly biodegraded (50% in 48 h) by activated sludge
(Ishikawa et al., 1985). After a 48-h acclimation period, the
biodegradation increased to 60% during a further 48-h test period.
Hattori et al. (1981) studied the fate of TEHP in river water and
seawater from the Osaka Bay area, Japan. After addition of TEHP at a
level of 1 mg/litre, the biodegradation was followed by analysing the
increase in phosphate ion concentration using the molybdenum blue
colorimetric method. The percentages of biodegradation are given in
Table 5.
Table 5. Biodegradation of TEHP in river water and seawater
(in percentages)
Test duration Oh River Neya River Osaka Bay
(days) Tomogashima Senboku
seawater seawater
7 35.9 24.4 1.2b 9.9
14 65.2a 42.2 32.5 73.2
a Test period 15 days
b Test period 8 days
In sterilized water TEHP did not show any degradation after
15 days. The authors (Hattori et al., 1981) stated that the
degradation rate depended on the microbial content of the water, and
this view was supported by the increase of phosphatase activity
observed during the test period.
Similar results were reported by Kawai et al. (1985, 1986) for
river die-away tests with TEHP in water samples from rivers of the
Osaka City area, Japan. Depending on the bacterial content of the
water, up to 80% degradation was observed. Usually the TEHP
concentration decreased rapidly during the first 10 days. Fukushima &
Kawai (1986) found that the removal of TEHP in a wastewater treatment
plant (Osaka City) with aquatic bacteria was up to 99%.
B4.2 Bioaccumulation
Saeger et al. (1979) estimated the bioconcentration factor (BCF)
of TEHP to be 250, suggesting that some uptake by biota could occur.
B5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
B5.1 Environmental levels
B5.1.1 Air
In New Brunswick, Canada, forest ambient concentrations of TEHP
in air were below the limit of determination (20 ng/m3 with a
precision of ± 5%) (Krzymien, 1981).
When samples of particulates in air were collected in a large
building in New Jersey, USA, the relative abundance of TEHP in the
submicron particle size range was much higher than in any larger size
range, suggesting that its presence did not result from abrasion
(Weschler, 1980). Airborne concentrations of TEHP were measured in
fine (2.5 µm) and coarse (2.5 to 15 µm) particulate fractions
collected on filters simultaneously outdoors and indoors in Wichita,
Kansas, USA during the autumn and early winter of 1981-1982. The
average indoor concentration was 6 ng/m3, but TEHP was not detected
in outdoor air (Weschler, 1984). The mean concentration measured in
representative samples of dust of seven office buildings in the USA
was reported to be 5 ng/m3 (Weschler & Shields, 1986).
B5.1.2 Surface water
As a part of the German Monitoring Programme, water from the
River Weser was examined for concentrations and loads of various
chemicals including plasticizers. Several samples were taken at
various points over 35 km of the river. The average concentration of
TEHP over 10 sampling points did not exceed 10 ng/litre at any time.
On one day in 1987 peak values of 290 ng/litre were measured,
indicating direct emissions (Bohlen et al., 1989).
Water samples from estuaries of the German rivers Elbe, Weser and
Ems were analysed from 1977 to 1983. TEHP could be identified only in
water samples from the estuary of the River Elbe, where concentrations
were in the range of 1-5 ng/litre (Weber & Ernst, 1983). In a second
series of water samples from the estuaries of the Elbe and Weser
(taken in 1983-1985) concentrations of approximately 90 to 7500 ng
TEHP/litre were measured (Ernst, 1988).
The concentration of TEHP in Rhine water at Dusseldorf was
usually below 20 ng/litre. The maximum concentration found was
50 ng/litre (ARW, 1987).
Ishikawa et al. (1985) could not detect TEHP at 16 river sampling
sites or nine seawater sampling sites around Kitakyushu, Japan. The
limit of determination was 20 ng/litre.
TEHP was found in water of the Yodo River (Osaka area) at
concentrations of 80-2000 ng/litre, with a mean value of 100 ng/litre.
The detection limit was 80 ng/litre (Fukushima et al., 1986). In river
water of the Osaka City area, Kawai et al. (1985) detected 15-84
ng/litre (determination limit not reported).
No TEHP could be detected in 63 water samples from 21 locations
throughout Japan (with a limit of determination of 10 ng/litre)
collected during the period 1974-1981 (Environmental Agency Japan,
1983, 1987).
B5.1.3 Drinking-water
In drinking-water samples collected during October 1978 from two
Eastern Ontario (Canada) water treatment plants, TEHP was detected at
a concentration of 0.3 ng/litre in water from one plant (LeBel et al.,
1981).
B5.1.4 Effluents
In a study of organic pollutants from influent and effluent of
the Gothenburg regional sewage plant (Sweden) during the period 1989
to 1991, TEHP was not detected (limit of detection unknown) (Paxeus et
al., 1992). Effluent from water treatment plants into the River Weser,
Germany, contained up to 144 ng TEHP/litre (Bohlen et al., 1989).
In a study of leachate from Osaka North Port (Japan) sea-based
solid waste disposal site and surrounding seawater, no TEHP was
detected (Kawagoshi & Fukunaga, 1994).
B5.1.5 Sediment
An environmental monitoring programme was carried out during the
period 1974-1981. Bottom deposit samples were collected at 21 sites
all over Japan. In 43 out of 63 of the samples, levels of 2-7 µg/kg
were found. The limit of determination was 1-5 µg/kg (Environmental
Agency Japan, 1983).
In one river sediment and five sea sediment samples, Ishikawa et
al. (1985) could not detect TEHP. The limit of determination was
10 µg/kg.
B5.1.6 Food
Total-diet studies of the US Food and Drug Administration were
reported by Gartrell et al. (1986b). Baskets of 120 food items
representing a typical 14-day diet for infants, young children and
adults were collected from October 1980 to March 1982 from retail
markets throughout the USA. Foods were classified into various groups.
TEHP was found in the oil and fat food group of the diet used by young
children. The average concentration in this food group was 38.5 µg/kg,
and the average daily intake was calculated to be 0.385 µg/day.
In follow-up reports, Gunderson (1988, 1995a,b) presented data
for various groups of age (Table 6).
In adult total diet samples, collected during October 1980 to
March 1982, TEHP was found only in the meat, fish and poultry food
group at an average concentration of 6.7 µg/kg; the average daily
intake was calculated to be 1.73 µg/day. All the other food groups,
i.e., dairy products, grain and cereals, potatoes, leafy vegetables,
legume vegetables, root vegetables, fruits, oils and fats, sugar and
adjuncts and beverages (including water), were free of TEHP (Gartrell
et al., 1986a).
The daily intakes of TEHP with adult food for 1978, 1979, 1980
and 1981/1982 were nd, nd, nd and 0.025 µg/kg body weight per day,
respectively (Gartrell et al., 1986a).
In a similar study on ready-to-eat food sampled during 1982-1991,
TEHP was found in 22 out of 230 food items. Raw sweet cherries
contained the highest concentration (505 µg/kg) (Kan-Do Office and
Pesticide Team, 1995).
Table 6. Mean daily intake (µg/kg body weight per day) of TEHP according to age and gender
6-11 months 2 years 14-16 years old 25-30 years old 60-65 years old
old old females males females males females males
1982-84 0.0272 0.071 0.0249 0.0317 0.024 0.0282 0.0232 0.0264
1984-86 0.0018 0.015 0.011 0.0101 0.0392 0.0385 0.0444 0.0471
1986-91 0.0015 0.0051 0.0029 0.0033 0.0039 0.0055 0.0033 0.0037
B6. KINETICS AND METABOLISM IN LABORATORY ANIMALS
In an inhalation study, nine male rats received a single,
head-only exposure of 20 min to an aerosol of [32P]-TEHP. The
animals were killed after the following post-exposure intervals: 5
min, 30 min; 1, 4, 17, 18, 24, 48 and 70 h. Exposure concentrations
were 0.72 and 0.91 mg/litre. TEHP and/or its metabolites were
distributed into the lungs (13% of total radioactivity after 5 min),
stomach contents (64% after first hour), brain and liver (9 and 16%,
respectively, after 30 min). Spleen, kidney, bone, muscle and fat
retained less than 2% of the radioactivity at any time. Faecal
excretion was high but urinary excretion was relatively low.
Chromatographic analysis of urine and faeces showed TEHP was partly
biotransformed but the nature of the metabolites was not mentioned
(MacFarland & Punte, 1966).
Kluwe et al. (1985) assumed, although no confirmatory data are
available, that TEHP is hydrolysed to 2-ethylhexanol and
di(2-ethylhexyl) phosphate. This may be analogous to other compounds
containing 2-ethylhexyl ester groups, which could be readily
hydrolysed to the corresponding mono- or di-ester and 2-ethylhexanol.
B7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
B7.1 Single exposure
The LD50 values for TEHP are summarized in Table 7.
Table 7. Acute toxicity of TEHP
Route Species LD50 Reference
Oral rat 37.08 g/kg body weight Smyth & Carpenter (1948)
Oral rat >10.0 g/kg body weight Bayer (1958)
Oral rat >36.8 g/kg body weight MacFarland & Punte (1966)
Oral rabbit 46.0 g/kg body weight MacFarland & Punte (1966)
From Table 7, it can be concluded that TEHP has low acute
toxicity by the oral and dermal routes.
Acute inhalation toxicity of TEHP has been investigated in Wistar
rats and Hartley guinea-pigs. Groups of 10 animals of each species
(sex not stated) were used. Rats were exposed to air concentrations of
287 to 460 mg/m3 for 30 to 210 min without any mortality in any
group. Guinea-pigs were exposed to air concentration of 287 to 460
mg/m3 for 30 to 180 min, with some mortality in each group, varying
from 30% (at 450 mg/m3 for 30 min, 298 mg/m3 for 60 min, and 460
mg/m3 for 60 min) to 80% (at 287 mg/m3 for 120 min). The mass median
diameter for the TEHP aerosol was 1.5 µm (MacFarland & Punte, 1966).
B7.2 Repeated exposure
B7.2.1 Oral
Feeding doses of 110-1550 mg TEHP/kg body weight per day to rats
in their diet for 30 days revealed a NOEL of 430 mg/kg body weight. At
1550 mg/kg body weight, weight loss was observed (Smyth & Carpenter,
1948).
In a 2-week dose range-finding study, groups of five male and
five female F 344/N rats and five male and five female B6C3F1 mice
were administered 0, 375, 750, 1500, 3000 or 6000 mg TEHP/kg body
weight in corn oil by gavage for 14 consecutive days. No animals died.
There was no effect on body weight gain in mice. The final mean body
weights of male rats that received 1500-6000 mg/kg body weight and
female rats that received 3000-6000 mg/kg body weight were lower than
those of the vehicle controls (US NTP, 1984).
Groups of 10 Fisher-344 rats of each sex received 0, 250, 500,
1000, 2000 or 4000 mg TEHP/kg body weight by gavage in corn oil on 5
days/week for 13 weeks and groups of 10 mice of each sex were
administered 0, 500, 1000, 2000, 4000 or 8000 mg/kg body weight. The
animals were examined twice daily and body weights were recorded
weekly. Postmortem and histopathological examinations were performed
on all animals except those excessively autolysed or cannibalized. No
deaths, toxic effects or induced histological alteration were
attributed to TEHP administration at any of these treatment dosage
levels other than a slight to moderate suppression in body weight gain
(US NTP, 1984; Kluwe et al., 1985). The suppression of body weight
gain at the highest dose in male and female rats was 5%; in male and
female mice the suppression of weight gain at the highest dose was 7%
and 5%, respectively. The Task Group did not consider these minimal
changes biologically significant. Hence, the NOAEL in rats was 2860
mg/kg body weight per day and in mice 5710 mg/kg body weight per day.
Results of a 2-year study on rats and mice are given in
section B7.6.
Two cats were administered 1.0 ml (926 mg) TEHP/kg body weight
per day by gavage 5 days/week for 4 weeks. During the treatment and
the recovery periods there were no signs of intoxication. Measurements
of erythrocyte cholinesterase activity during the test period revealed
no inhibitory effects (Bayer, 1958).
B7.2.2 Dermal
A daily dose of 0.1 ml (93 mg) undiluted TEHP was applied 5 days
a week to the clipped intact skin of six male rabbits (2-3 kg). Four
animals received ten applications and the remaining two animals 20
applications. No evidence of systemic intoxication was seen based on
gross necropsies and on the fact that animals, with one exception,
gained weight throughout the study (MacFarland & Punte, 1966).
B7.2.3 Inhalation
In a 3-month inhalation study, three test groups and a control
group, each consisting of equal numbers of males and females and
comprising 20 guinea-pigs, two dogs and two rhesus monkeys, were
exposed whole body for 6 h/day, 5 days/week for a total of 60
exposures (MacFarland & Punte, 1966). Three concentrations of TEHP
aerosol were tested; controls received the same air-flow but without
TEHP. The mean concentrations and standard deviations received by the
three test groups over the 12-week period were: low-dose, 10.8 ± 6.0
mg/m3; mid-dose, 24.4 ± 16.8 mg/m3; high-dose, 85.0 ± 33.3 mg/m3.
The median particle size was 4.4 µm with a geometric standard
deviation of 3.0.
No mortality and a normal increase in body weight was observed in
dogs and monkeys. There were no treatment-related alterations in a
limited range of biochemical and haematological parameters and organs
function test. Activities of plasma and erythrocyte cholinesterases
were unaffected. While the lungs of monkeys were normal, the lungs of
dogs showed mild, chronic parenchymal inflammatory changes. In an
evaluation of effects on trained behaviour, no effects were detected
in the performance of monkeys in a visual discrimination test; the
performance of dogs trained in conditional avoidance deteriorated as
the exposure concentration increased. The guinea-pig portion of the
study was invalidated due to the high mortality from intercurrent
respiratory infections.
The 3-month inhalation study was repeated with guinea-pigs. Two
groups of 20 male guinea-pigs were exposed to two concentrations of
TEHP. A third group acted as control and inhaled uncontaminated air in
the exposure chamber. Tetracycline was administered prophylactically
in the drinking-water throughout the study. The mean concentrations
and standard deviations for the two test groups were: low dose, 1.6 ±
0.8 mg/m3; high-dose, 9.6 ± 1/5 mg/m3. Exposures were for 6 h/day, 5
days/week for a total of 60 exposures. The mean particle size was 3.8
µm with a geometric standard deviation of 1.7.
The high-dose guinea-pigs showed a significantly increased body
weight in comparison with the controls. Plasma and erythrocyte
cholinesterase activities were unaffected in terminal blood samples.
Both test groups exhibited a lower kidney-to-body weight ratio than
the controls. Histopathological alterations in the lung, liver and
kidneys were not related to the treatment. Sections of the spinal cord
and sciatic nerve, stained to demonstrate the myelin sheaths, showed
no pathological changes (MacFarland & Punte, 1966).
B7.3 Skin and eye irritation; sensitization
No irritation was seen after exposure to TEHP by a saturated
cotton swab placed on the inside of the ears of rabbits for 24 h
(Kimmerle, 1958).
TEHP was tested in three albino rabbits according to OECD 404
test guideline. Well-defined erythema, slight to moderate oedema,
crust formation and desquamation were observed. TEHP produced a
primary irritation index of 4.2/8.0 and was classified as a moderate
irritant to rabbit skin. No corrosive effects were observed (Guest,
1993b).
A single dose of 250 mg undiluted TEHP applied to the clipped
skin of rabbits produced moderate erythema, which persisted for a week
(MacFarland & Punte, 1966). Repeated applications of 0.1 ml on 5
days/week (10 or 20 applications) produced moderate erythema after the
first application. With further applications a spreading zone of
erythema developed with desquamation, leatheriness and some fissuring
with haemorrhages. At the end of the observation period, thickening
and severe hyperkeratosis of the skin was apparent.
TEHP was non-irritating when tested in the eyes of three albino
rabbits according to OECD 405 Test Guideline (Guest, 1993a).
TEHP was instilled into the conjunctival sac of one eye of each
of two rabbits at dose levels of 0.01 to 0.5 ml. Doses up to 0.05 ml
produced slight conjunctivitis, while doses of 0.1 and 0.5 ml produced
moderate conjunctivitis which cleared up in 24 h (MacFarland & Punte,
1966).
B7.4 Reproductive toxicity, embryo toxicity and teratogenicity
No data on the reproductive toxicity of TEHP are available.
B7.5 Mutagenicity
TEHP was shown to be non-genotoxic in a range of mutagenicity
assays.
B7.5.1 In vitro assays
TEHP was tested for mutagenicity in a Salmonella/microsome
assay using strains TA1535, TA1537, TA98 and TA100 in the presence and
absence of S9 derived from livers of Aroclor 1254-treated
Sprague-Dawley rats. Results were negative (Zeiger et al., 1985).
In a mouse lymphoma assay, concentrations of TEHP of up to and
exceeding the apparent solubility limit of 62.5 µl/litre produced no
gene mutations. The assay was carried out in the presence and absence
of S9 from livers of Aroclor 1254-treated male F-344 rats (Myhr &
Caspary, 1991).
An in vitro cytogenetic assay in Chinese hamster ovary (CHO)
cells was carried out using concentrations of TEHP up to 251 µg/ml in
the presence and absence of S9 derived from livers of Aroclor
1254-treated Sprague-Dawley rats. There was no evidence of induction
of chromosome damage (Ivett et al., 1989).
An in vitro sister-chromatid exchange (SCE) assay was carried
out in CHO cells in the presence and absence of S9 derived from livers
of Aroclor 1254-treated Sprague-Dawley rats. Concentrations of up to
251 µg/ml were used, but at 16.7 µg/ml and above there was severe cell
cycle delay, which limited the number of cells available for analysis.
TEHP did not increase the number of SCEs (Ivett et al., 1989).
B7.5.2 In vivo assays
A mouse bone marrow micronucleus assay was carried out in male
B6C3F1 mice, which were given intraperitoneal TEHP injections of 500,
1000 or 2000 mg/kg body weight on three consecutive days. Bone marrow
was harvested at 24 h after the last dose, and was examined for
micronucleus-containing polychromatic erythrocytes (MN-PCEs). A
statistically significant (P < 0.001) dose-related increase in the
number of MN-PCEs was detected in the bone marrow from treated mice.
The assay was repeated in two further experiments using doses of 1500
and 2000 mg/kg body weight in one and 2000 and 3000 mg/kg body weight
in the other. No increase in the number of bone marrow MN-PCEs was
detected in either of these experiments. It is concluded that the
initial result was an artefact and that TEHP is not mutagenic in this
assay (Shelby et al., 1993).
An in vivo cytogenetic assay was carried out in male B6C3F1
mice, which were given a single intraperitoneal injection of TEHP
(dose not stated). Bone marrow was harvested for analysis at 17 and 36
h post-dosing and metaphase cells were examined for chromosomal
aberrations. The number of chromosomal aberrations was not elevated in
TEHP-treated mice (Shelby & Witt, 1995).
In an in vivo liver unscheduled DNA synthesis (UDS) assay, male
B6C3F1 mice were given TEHP doses of 1000 or 2000 mg/kg body weight
by gavage. Mice were killed at 24, 39 and 48 h post-dosing and liver
preparations were made. There was no evidence of increased UDS in
hepatocytes from TEHP-treated mice (Miyagawa et al., 1995).
B7.6 Carcinogenicity
In a US NTP (1984) study, TEHP was administered in corn oil (10
ml/kg body weight) by gavage 5 days/week for 103 weeks to groups of 50
male and 50 female F-344/N rats and B6C3F1 mice. The doses
administered were:
Fischer-344 rats B6C3F1 mice
Dose Male Female Male Female
Control Vehicle Vehicle Vehicle Vehicle
Low dose 2000 mg/kg 1000 mg/kg 500 mg/kg 500 mg/kg
High dose 4000 mg/kg 2000 mg/kg 1000 mg/kg 1000 mg/kg
The animals were observed twice daily and body weight was
measured weekly for the first 13 weeks and once every 4 weeks
thereafter. Clinical examinations were performed once every 4 weeks.
Necropsies and histopathological examinations were performed on all
animals, but organ weight changes were not reported.
No compound-related clinical toxicity was observed in either sex
of either species. Decrease in body weight, compared with controls,
was limited to male rats at the low dose (11.5%) and the high dose
(15.8%). The decreased body weight did not affect survival.
In male rats the incidence of phaeochromocytomas of the adrenal
gland increased with dose and two (4%) were malignant in the high-dose
group. The incidence of adrenal phaeochromocytomas in male rats was:
control 2/50 (4%), low-dose 9/50 (18%) and high-dose 12/50 (24%). In
two previous gavage studies in the same laboratory, the incidence of
phaeochromocytomas in control male rats was 24 and 26%.
In female mice the incidence of hepatocellular carcinomas was:
control 0/48 (0%), low dose 4/50 (8%) and high dose 7/50 (14%). The
incidence of hepatocellular carcinomas showed a dose-related increase
and the incidence at the high-dose level was statistically
significant.
The results of these 2-year gavage studies in rats and mice were
interpreted by NTP as showing some evidence of carcinogenicity in
female mice based on the increase in hepatocellular carcinomas and
equivocal evidence of carcinogenicity in male rats based on the
increased incidence of phaeochromocytomas (US NTP, 1984; Kluwe et al.,
1985)
In this same study (US NTP, 1984), TEHP caused a dose-related
increase in the incidence of follicular cell hyperplasia of the
thyroid in male and female B6C3F1 mice. The incidence of hyperplasia
was: in males, control 0/49 (0%), low dose 12/48 (25%) and high dose
24/47 (51%); in females, control 1/44 (2%), low dose 13/47 (28%) and
high dose 12/46 (26%). There was no dose-related increase in thyroid
tumours. The LOAEL for thyroid hyperplasia was 357 mg/kg body weight
per day; a NOAEL was not established.
B7.7 Special studies
B7.7.1 Neurotoxicity
MacFarland & Punte (1966) tested TEHP for its neurotoxic
potential. Four groups of female chickens, each weighing 1.5-2.0 kg,
received a single dose of test material into the crops as follows:
Group Number of chickens Test material Dose
1 4 Saline 1.5 ml/kg
2 8 TOCP 500 mg/kg
3 8 TEHP 500 mg/kg
4 8 TEHP 2500 mg/kg
After receiving a single dose of the test material the animals
were kept under observation for 4 weeks and then killed. Body weights
were recorded weekly and changes in appearance and behaviour noted
daily. Gross necropsy was performed on all chickens. Sections of
brain, three levels of the spinal cord and the sciatic nerve were
examined microscopically.
In the tri- ortho-cresyl phosphate (TOCP) group used as positive
control, weight loss become apparent by the end of the first week and
signs of ataxia and muscular weakness were evident by the 12th day.
These signs increased in intensity, so that the chickens were
prostate by the end of the study. The microscopic examination of the
nerve tissue sections confirmed that TOCP was producing
demyelinization. The chickens in the saline and TEHP groups appeared
normal and maintained or gained weight throughout the study. There
were no macroscopic signs of neurotoxicity and microscopically no
demyelinization was observed.
No evidence of systemic intoxication or, in particular,
neurotoxicity was seen in chickens dosed with a single dose of up to
2.500 mg TEHP/kg body weight (MacFarland & Punte, 1966).
Single hens were administered a single dose of 0.25, 0.5 or
1.0 g/kg body weight by gavage. The animals were kept under
observation for 2 months and examined for neurotoxicity twice weekly.
No abnormalities in behaviour were detected. A single intramuscular
injection of 0.25, 0.5 or 1.0 g TEHP/kg body weight to single chicken
again induced no signs of intoxication (Kimmerle, 1958).
In 3-month inhalation studies (see section B7.2.3) with
guinea-pigs, dogs and rhesus monkeys, determination of plasma and
erythrocyte cholinesterase activity and histological examination of
sections of tissue including spinal cord and sciatic nerve did not
reveal any abnormalities. In dogs and rhesus monkeys, the
cholinesterase were measured after 4, 8 and 12 weeks of exposure to
10.8, 26.4 or 85 mg TEHP/m3, but in guinea-pigs measurements were
made after 12 weeks of exposure to 1.6 or 9.6 mg/m3
(MacFarland & Punte, 1966).
In two cats receiving 28 doses of 1.0 ml TEHP/kg body weight by
gavage (see section 7.2.1) no signs of neurotoxicity and no inhibition
in the erythrocyte cholinesterase activity was found (Bayer, 1958).
B8. EFFECTS ON HUMANS
No irritant effects were seen after 24 h of exposure to a
TEHP-saturated cotton swab placed on the skin of the forearm of six
volunteers. A piece (2 cm2) of PVC plastic containing 40% TEHP was
placed on the arm of 8 volunteers for 72 h. Slight redness but no
irritation was observed (Kimmerle, 1958).
No other data are available concerning the effect of TEHP on
humans.
B9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
B9.1 Laboratory experiments
B9.1.1 Microorganisms
A bacterial growth inhibition test, carried out according to ISO
8192 indicated an IC50 for TEHP greater than 100 mg/litre (Bayer,
1982b).
B9.1.2 Aquatic organisms
B9.1.2.1 Vertebrates
A 96-h exposure of zebra fish (Brachydanio rerio) under static
conditions to TEHP (100 mg/litre) produced no deaths (Bayer, 1989).
B9.1.3 Terrestrial organisms
No data on the toxicity of TEHP to terrestrial organisms are
available.
PART C
TETRAKIS(HYDROXYMETHYL) PHOSPHONIUM SALTS
C. SUMMARY AND EVALUATION
C1. Tetrakis(hydroxymethyl) phosphonium salts
C1.1 Summary
Tetrakis(hydroxymethyl) phosphonium salts represent the major
class of chemicals used as a flame retardant for cotton, cellulose and
cellulose-blend fabrics. There is low migration from fabrics treated
with tetrakis(hydroxymethyl) phosphonium chloride (THPC)-urea. The
sulfate salt (THPS) is mainly used as a biocide. Combined world
production is estimated to be >3000 tonnes for THP salts and around
3000 tonnes for the THPC-urea condensate.
Photodegradation and hydrolysis of THP salts are significant
abiotic degradation pathways in the environment. THPS binds poorly to
environmental particulates and is, therefore, mobile. THPS degrades
rapidly under both aerobic and anaerobic conditions. Trihydroxymethyl
phosphine oxide (THPO) and bishydroxymethyl phosphonic acid (BMPA)
have been identified as breakdown products.
Since no monitoring has been reported, no estimates can be made
of exposure to humans or organisms in the environment.
The acute oral toxicity of THPC and THPS is moderate; dermal
toxicity is low.
In short-term (up to 28 days) studies in rats and mice, the main
toxic effect for both THPC and THPS is decreased body weight. The
NOAEL for both chemicals in both species is approximately 8 mg/kg body
weight per day. In longer-term studies (13 weeks), the main target
organ for toxicity is the liver. The NOAEL for this effect ranged from
3 to 7 mg/kg body weight per day for both salts in both species.
Carcinogenicity bioassays on THPC also showed effects on the liver,
but a NOAEL was not established. The LOAEL was approximately 3 mg/kg
body weight per day for both species. In a carcinogenicity bioassay on
THPS in mice, the NOAEL for focal hyperplasia in the adrenal medulla
was 3.6 mg/kg body weight per day; in rats the LOAEL for mortality was
3.6 mg/kg body weight per day.
THPS did not cause skin irritation when administered as a single
dose to rabbits. However repeated dermal exposure of rats resulted in
severe skin reaction. THPC-urea was corrosive. THPS was identified as
severe eye irritant in rabbits.
THPS and THPC-urea cause skin sensitization guinea-pigs
(Magnusson & Kilman Maximization test).
THPS and THPC-urea did not cause developmental toxicity in orally
dosed experimental animals.
THPC and THPS have mutagenic potential in vitro, but THPS is
not mutagenic in vivo (no in vivo mutagenicity data are available
for THPC). Limited mutagenicity data for THPC-urea suggest that it is
not mutagenic in vivo. THPO is non-genotoxic. There is no convincing
evidence to suggest that fabrics treated with THP salts are mutagenic.
Available information indicates that there is no genotoxic hazard to
humans.
THPC and THPS were not carcinogenic in rats and mice in 2-year
bioassays. Dermal studies have shown that THP salts are promoters of
skin cancer but not initiators.
THPS and THPO did not inhibit acetylchlolinesterase activity in
vitro, suggesting a lack of neurotoxic hazard for humans.
THPC-urea-treated fabric did not cause skin irritation in humans.
For THPS, reported acute toxicity values for algae are less than
1 mg/litre, with one no-observed-effect concentration (NOEC) of
0.06 mg/litre. The acute NOEC for the water flea is 10 mg/litre.
Reported acute toxicity values for marine invertebrates range from 1.6
to 340 mg/litre.
Fish 96-h LC50 values range from 72 to 119 mg/litre, with NOEC
values in the range of 18 to 41 mg/litre. An acute avian LD50 of 311
mg/kg body weight and dietary LC50 values of 1300 and 2400 mg/kg diet
have been reported.
C1.2 Evaluation
No exposure information is available for either humans or
organisms in the environment. Therefore no quantitative risk
assessment could be made.
C2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, AND ANALYTICAL
METHODS
C2.1 Identity
Tetrakis(hydroxymethyl) phosphonium salts (THP salts) have the
following general chemical structure :
Chemical formula: C24H51O4P
Relative molecular mass: 434.64
CAS registry number: 78-42-2
EINECS number: 201-116-6
RTECS number: MP-0770000
CAS name: phosphoric acid, tris(2-ethylhexyl) ester
Synonyms:a 1-hexanol 2-ethyl-phosphate;
2-ethyl-1-hexanol phosphate; triethylhexyl
phosphate; tri(2-ethylhexyl) phosphate;
tris(isooctyl) phosphate
Trade names: Disflamoll TOF; Flexol TOF; Reomol TOP;
Amgard TOF; Antiblaze TOF
a The synonym trioctyl phosphate has also been
used. However, this chemical has a different
chemical abstracts (CAS) registry number
(1806-54-8).
B2.2 Physical and chemical properties
Tris(2-ethylhexyl) phosphate (TEHP) is a colourless to light
yellow liquid, non-flammable and nearly odourless (Arias, 1992).
Boiling point: 220°C at 6.67 hPa;
210°C at 5 hPa
Melting point: -74°C
Pour point: -70°C
Relative density: 0.926 at 20°C
Refractive index: 1.4426 at 20°C
Vapour pressure: <0.1 hPa at 20°C
Viscosity: 10.2 cP
Stability: stable under normal storage conditions; can
react with oxidizers
Flash point: 190-195°C
Solubility: soluble in acetone, ether and ethanol;
in DMSO 1.0 mg/litre at 18°C;
in water less than 0.1 g/litre at 20°C
n-Octanol/water
partition coefficient 4.22
From: MacFarland & Punte (1966); Saeger et al. (1979); Keith & Walters
(1987); Hinckley et al. (1990); FMC (1998).
B2.3 Conversion factors
1 ppm = 17.78 mg/m3
1 mg/m3 = 0.056 ppm
B2.4 Analytical methods
The analytical methods for TEHP are based on gas chromatography
combined with flame ionization detection (FID), flame photometric
detection (FPD), mass spectroscopy (MS) or nitrogen-phosphorus
sensitive detection (NPD). The detection limits are in the ng/m3
(air) and ng/litre (water) range.
Lerche & Morch (1973) determined TEHP using GC combined with FID
with a detection limit of 5-30 ng/litre. The separation of various
phosphoric acid esters by GC was achieved using columns filled with
various liquid silicone phases.
B2.4.1 Air
In a method described by Krzymien (1981), TEHP vapour and aerosol
were collected in glass absorber tubes packed with a plug of fine
platinum mesh coated with silicone packing material and subsequently
thermally desorbed into a GC for analysis. The capacity of the
absorber was found to be 2.1 ng pure TEHP when presented with 3 ng
TEHP/litre. Concentrations of 20 pg/litre were determined with a
precision of better than 10%. The aerosol concentration and its
drop-size distribution were determined at the picogram level with
around 5% precision using a cascade impactor. Armstrong & Yule (1978)
determined TEHP deposited on foliage and twigs by extraction with
toluene, drying with anhydrous sodium sulfate and using GC with FPD.
B2.4.2 Water
LeBel et al. (1981) used Amberlite(R) XAD-2 macroreticular resin
to collect TEHP from drinking-water. The resin was extracted with an
acetone/hexane mixture. TEHP was identified by GC and by GC/MS at
ng/litre levels. The recovery by direct fortification was 62%.
Determination of TEHP in extracts of activated carbon by means of
GC/MS was described by Frimmel et al. (1987). TEHP was extracted from
activated charcoal using a mixture of acetone, dichloromethane and
toluene.
Kawagoshi & Fukunaga (1994, 1995) showed, by extracting leachate
with dichloromethane and analysing the residue by GC-FPD, that it was
possible to detect organophosphoric acid triesters, including TEHP, at
a limit of detection of 2 ng/litre.
B2.4.3 Sediment
Sediment samples were extracted with acetone and dried,
concentrated and analysed by Ishikawa et al. (1985) in a similar way
to that used for water samples. The limit of determination was 10
ng/g.
B3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
B3.1 Natural occurrence
TEHP does not occur naturally in the environment.
B3.2 Anthropogenic sources
B3.2.1 Production levels and processes
In 1992, approximately 1000 tonnes of TEHP were manufactured in
Germany (BUA, 1997).
Figures for the world production of TEHP are not available.
ECETOC (1992a) estimated the world production to be between 1000 and
5000 tonnes/year.
TEHP is produced by reaction of phosphorus oxychloride and
2-ethylhexanol. The triester is separated by vacuum distillation.
Technical grade TEHP is usually 99% pure. The impurities are
2-ethylhexanol, bis(2-ethylhexyl) phosphate (BEHP) and traces of water
(ECETOC, 1992a).
B3.2.2 Uses
TEHP is used in PVC plastisols, as a flame retardant in cellulose
acetate and as a solvent for certain chemical reactions. It is also
used as a flame retardant plasticizer, particularly for PVC, in low
temperature application (ECETOC, 1992a).
B4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION AND TRANSFORMATION
B4.1 Biodegradation
Biodegradation of phosphoric acid esters involves stepwise
hydrolysis to ortho-phosphate and alcohol moieties. The alcohol
would then be expected to undergo further degradation (Saeger et al.,
1979).
In a ready biodegradability closed bottle test (OECD Guideline
301D), no biodegradation of TEHP was observed after 28 days (Bayer,
1982a).
An activated sludge method, based on a semi-continuous procedure,
was used to test primary degradation of TEHP. The addition rate of the
compound was 3 mg/litre per 24 h and the biodegradation was 20 (± 8)%
after 34 weeks (Saeger et al., 1979).
TEHP was rapidly biodegraded (50% in 48 h) by activated sludge
(Ishikawa et al., 1985). After a 48-h acclimation period, the
biodegradation increased to 60% during a further 48-h test period.
Hattori et al. (1981) studied the fate of TEHP in river water and
seawater from the Osaka Bay area, Japan. After addition of TEHP at a
level of 1 mg/litre, the biodegradation was followed by analysing the
increase in phosphate ion concentration using the molybdenum blue
colorimetric method. The percentages of biodegradation are given in
Table 5.
Table 5. Biodegradation of TEHP in river water and seawater
(in percentages)
Test duration Oh River Neya River Osaka Bay
(days) Tomogashima Senboku
seawater seawater
7 35.9 24.4 1.2b 9.9
14 65.2a 42.2 32.5 73.2
a Test period 15 days
b Test period 8 days
In sterilized water TEHP did not show any degradation after
15 days. The authors (Hattori et al., 1981) stated that the
degradation rate depended on the microbial content of the water, and
this view was supported by the increase of phosphatase activity
observed during the test period.
Similar results were reported by Kawai et al. (1985, 1986) for
river die-away tests with TEHP in water samples from rivers of the
Osaka City area, Japan. Depending on the bacterial content of the
water, up to 80% degradation was observed. Usually the TEHP
concentration decreased rapidly during the first 10 days. Fukushima &
Kawai (1986) found that the removal of TEHP in a wastewater treatment
plant (Osaka City) with aquatic bacteria was up to 99%.
B4.2 Bioaccumulation
Saeger et al. (1979) estimated the bioconcentration factor (BCF)
of TEHP to be 250, suggesting that some uptake by biota could occur.
B5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
B5.1 Environmental levels
B5.1.1 Air
In New Brunswick, Canada, forest ambient concentrations of TEHP
in air were below the limit of determination (20 ng/m3 with a
precision of ± 5%) (Krzymien, 1981).
When samples of particulates in air were collected in a large
building in New Jersey, USA, the relative abundance of TEHP in the
submicron particle size range was much higher than in any larger size
range, suggesting that its presence did not result from abrasion
(Weschler, 1980). Airborne concentrations of TEHP were measured in
fine (2.5 µm) and coarse (2.5 to 15 µm) particulate fractions
collected on filters simultaneously outdoors and indoors in Wichita,
Kansas, USA during the autumn and early winter of 1981-1982. The
average indoor concentration was 6 ng/m3, but TEHP was not detected
in outdoor air (Weschler, 1984). The mean concentration measured in
representative samples of dust of seven office buildings in the USA
was reported to be 5 ng/m3 (Weschler & Shields, 1986).
B5.1.2 Surface water
As a part of the German Monitoring Programme, water from the
River Weser was examined for concentrations and loads of various
chemicals including plasticizers. Several samples were taken at
various points over 35 km of the river. The average concentration of
TEHP over 10 sampling points did not exceed 10 ng/litre at any time.
On one day in 1987 peak values of 290 ng/litre were measured,
indicating direct emissions (Bohlen et al., 1989).
Water samples from estuaries of the German rivers Elbe, Weser and
Ems were analysed from 1977 to 1983. TEHP could be identified only in
water samples from the estuary of the River Elbe, where concentrations
were in the range of 1-5 ng/litre (Weber & Ernst, 1983). In a second
series of water samples from the estuaries of the Elbe and Weser
(taken in 1983-1985) concentrations of approximately 90 to 7500 ng
TEHP/litre were measured (Ernst, 1988).
The concentration of TEHP in Rhine water at Dusseldorf was
usually below 20 ng/litre. The maximum concentration found was
50 ng/litre (ARW, 1987).
Ishikawa et al. (1985) could not detect TEHP at 16 river sampling
sites or nine seawater sampling sites around Kitakyushu, Japan. The
limit of determination was 20 ng/litre.
TEHP was found in water of the Yodo River (Osaka area) at
concentrations of 80-2000 ng/litre, with a mean value of 100 ng/litre.
The detection limit was 80 ng/litre (Fukushima et al., 1986). In river
water of the Osaka City area, Kawai et al. (1985) detected 15-84
ng/litre (determination limit not reported).
No TEHP could be detected in 63 water samples from 21 locations
throughout Japan (with a limit of determination of 10 ng/litre)
collected during the period 1974-1981 (Environmental Agency Japan,
1983, 1987).
B5.1.3 Drinking-water
In drinking-water samples collected during October 1978 from two
Eastern Ontario (Canada) water treatment plants, TEHP was detected at
a concentration of 0.3 ng/litre in water from one plant (LeBel et al.,
1981).
B5.1.4 Effluents
In a study of organic pollutants from influent and effluent of
the Gothenburg regional sewage plant (Sweden) during the period 1989
to 1991, TEHP was not detected (limit of detection unknown) (Paxeus et
al., 1992). Effluent from water treatment plants into the River Weser,
Germany, contained up to 144 ng TEHP/litre (Bohlen et al., 1989).
In a study of leachate from Osaka North Port (Japan) sea-based
solid waste disposal site and surrounding seawater, no TEHP was
detected (Kawagoshi & Fukunaga, 1994).
B5.1.5 Sediment
An environmental monitoring programme was carried out during the
period 1974-1981. Bottom deposit samples were collected at 21 sites
all over Japan. In 43 out of 63 of the samples, levels of 2-7 µg/kg
were found. The limit of determination was 1-5 µg/kg (Environmental
Agency Japan, 1983).
In one river sediment and five sea sediment samples, Ishikawa et
al. (1985) could not detect TEHP. The limit of determination was
10 µg/kg.
B5.1.6 Food
Total-diet studies of the US Food and Drug Administration were
reported by Gartrell et al. (1986b). Baskets of 120 food items
representing a typical 14-day diet for infants, young children and
adults were collected from October 1980 to March 1982 from retail
markets throughout the USA. Foods were classified into various groups.
TEHP was found in the oil and fat food group of the diet used by young
children. The average concentration in this food group was 38.5 µg/kg,
and the average daily intake was calculated to be 0.385 µg/day.
In follow-up reports, Gunderson (1988, 1995a,b) presented data
for various groups of age (Table 6).
In adult total diet samples, collected during October 1980 to
March 1982, TEHP was found only in the meat, fish and poultry food
group at an average concentration of 6.7 µg/kg; the average daily
intake was calculated to be 1.73 µg/day. All the other food groups,
i.e., dairy products, grain and cereals, potatoes, leafy vegetables,
legume vegetables, root vegetables, fruits, oils and fats, sugar and
adjuncts and beverages (including water), were free of TEHP (Gartrell
et al., 1986a).
The daily intakes of TEHP with adult food for 1978, 1979, 1980
and 1981/1982 were nd, nd, nd and 0.025 µg/kg body weight per day,
respectively (Gartrell et al., 1986a).
In a similar study on ready-to-eat food sampled during 1982-1991,
TEHP was found in 22 out of 230 food items. Raw sweet cherries
contained the highest concentration (505 µg/kg) (Kan-Do Office and
Pesticide Team, 1995).
Table 6. Mean daily intake (µg/kg body weight per day) of TEHP according to age and gender
6-11 months 2 years 14-16 years old 25-30 years old 60-65 years old
old old females males females males females males
1982-84 0.0272 0.071 0.0249 0.0317 0.024 0.0282 0.0232 0.0264
1984-86 0.0018 0.015 0.011 0.0101 0.0392 0.0385 0.0444 0.0471
1986-91 0.0015 0.0051 0.0029 0.0033 0.0039 0.0055 0.0033 0.0037
B6. KINETICS AND METABOLISM IN LABORATORY ANIMALS
In an inhalation study, nine male rats received a single,
head-only exposure of 20 min to an aerosol of [32P]-TEHP. The
animals were killed after the following post-exposure intervals: 5
min, 30 min; 1, 4, 17, 18, 24, 48 and 70 h. Exposure concentrations
were 0.72 and 0.91 mg/litre. TEHP and/or its metabolites were
distributed into the lungs (13% of total radioactivity after 5 min),
stomach contents (64% after first hour), brain and liver (9 and 16%,
respectively, after 30 min). Spleen, kidney, bone, muscle and fat
retained less than 2% of the radioactivity at any time. Faecal
excretion was high but urinary excretion was relatively low.
Chromatographic analysis of urine and faeces showed TEHP was partly
biotransformed but the nature of the metabolites was not mentioned
(MacFarland & Punte, 1966).
Kluwe et al. (1985) assumed, although no confirmatory data are
available, that TEHP is hydrolysed to 2-ethylhexanol and
di(2-ethylhexyl) phosphate. This may be analogous to other compounds
containing 2-ethylhexyl ester groups, which could be readily
hydrolysed to the corresponding mono- or di-ester and 2-ethylhexanol.
B7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
B7.1 Single exposure
The LD50 values for TEHP are summarized in Table 7.
Table 7. Acute toxicity of TEHP
Route Species LD50 Reference
Oral rat 37.08 g/kg body weight Smyth & Carpenter (1948)
Oral rat >10.0 g/kg body weight Bayer (1958)
Oral rat >36.8 g/kg body weight MacFarland & Punte (1966)
Oral rabbit 46.0 g/kg body weight MacFarland & Punte (1966)
From Table 7, it can be concluded that TEHP has low acute
toxicity by the oral and dermal routes.
Acute inhalation toxicity of TEHP has been investigated in Wistar
rats and Hartley guinea-pigs. Groups of 10 animals of each species
(sex not stated) were used. Rats were exposed to air concentrations of
287 to 460 mg/m3 for 30 to 210 min without any mortality in any
group. Guinea-pigs were exposed to air concentration of 287 to 460
mg/m3 for 30 to 180 min, with some mortality in each group, varying
from 30% (at 450 mg/m3 for 30 min, 298 mg/m3 for 60 min, and 460
mg/m3 for 60 min) to 80% (at 287 mg/m3 for 120 min). The mass median
diameter for the TEHP aerosol was 1.5 µm (MacFarland & Punte, 1966).
B7.2 Repeated exposure
B7.2.1 Oral
Feeding doses of 110-1550 mg TEHP/kg body weight per day to rats
in their diet for 30 days revealed a NOEL of 430 mg/kg body weight. At
1550 mg/kg body weight, weight loss was observed (Smyth & Carpenter,
1948).
In a 2-week dose range-finding study, groups of five male and
five female F 344/N rats and five male and five female B6C3F1 mice
were administered 0, 375, 750, 1500, 3000 or 6000 mg TEHP/kg body
weight in corn oil by gavage for 14 consecutive days. No animals died.
There was no effect on body weight gain in mice. The final mean body
weights of male rats that received 1500-6000 mg/kg body weight and
female rats that received 3000-6000 mg/kg body weight were lower than
those of the vehicle controls (US NTP, 1984).
Groups of 10 Fisher-344 rats of each sex received 0, 250, 500,
1000, 2000 or 4000 mg TEHP/kg body weight by gavage in corn oil on 5
days/week for 13 weeks and groups of 10 mice of each sex were
administered 0, 500, 1000, 2000, 4000 or 8000 mg/kg body weight. The
animals were examined twice daily and body weights were recorded
weekly. Postmortem and histopathological examinations were performed
on all animals except those excessively autolysed or cannibalized. No
deaths, toxic effects or induced histological alteration were
attributed to TEHP administration at any of these treatment dosage
levels other than a slight to moderate suppression in body weight gain
(US NTP, 1984; Kluwe et al., 1985). The suppression of body weight
gain at the highest dose in male and female rats was 5%; in male and
female mice the suppression of weight gain at the highest dose was 7%
and 5%, respectively. The Task Group did not consider these minimal
changes biologically significant. Hence, the NOAEL in rats was 2860
mg/kg body weight per day and in mice 5710 mg/kg body weight per day.
Results of a 2-year study on rats and mice are given in
section B7.6.
Two cats were administered 1.0 ml (926 mg) TEHP/kg body weight
per day by gavage 5 days/week for 4 weeks. During the treatment and
the recovery periods there were no signs of intoxication. Measurements
of erythrocyte cholinesterase activity during the test period revealed
no inhibitory effects (Bayer, 1958).
B7.2.2 Dermal
A daily dose of 0.1 ml (93 mg) undiluted TEHP was applied 5 days
a week to the clipped intact skin of six male rabbits (2-3 kg). Four
animals received ten applications and the remaining two animals 20
applications. No evidence of systemic intoxication was seen based on
gross necropsies and on the fact that animals, with one exception,
gained weight throughout the study (MacFarland & Punte, 1966).
B7.2.3 Inhalation
In a 3-month inhalation study, three test groups and a control
group, each consisting of equal numbers of males and females and
comprising 20 guinea-pigs, two dogs and two rhesus monkeys, were
exposed whole body for 6 h/day, 5 days/week for a total of 60
exposures (MacFarland & Punte, 1966). Three concentrations of TEHP
aerosol were tested; controls received the same air-flow but without
TEHP. The mean concentrations and standard deviations received by the
three test groups over the 12-week period were: low-dose, 10.8 ± 6.0
mg/m3; mid-dose, 24.4 ± 16.8 mg/m3; high-dose, 85.0 ± 33.3 mg/m3.
The median particle size was 4.4 µm with a geometric standard
deviation of 3.0.
No mortality and a normal increase in body weight was observed in
dogs and monkeys. There were no treatment-related alterations in a
limited range of biochemical and haematological parameters and organs
function test. Activities of plasma and erythrocyte cholinesterases
were unaffected. While the lungs of monkeys were normal, the lungs of
dogs showed mild, chronic parenchymal inflammatory changes. In an
evaluation of effects on trained behaviour, no effects were detected
in the performance of monkeys in a visual discrimination test; the
performance of dogs trained in conditional avoidance deteriorated as
the exposure concentration increased. The guinea-pig portion of the
study was invalidated due to the high mortality from intercurrent
respiratory infections.
The 3-month inhalation study was repeated with guinea-pigs. Two
groups of 20 male guinea-pigs were exposed to two concentrations of
TEHP. A third group acted as control and inhaled uncontaminated air in
the exposure chamber. Tetracycline was administered prophylactically
in the drinking-water throughout the study. The mean concentrations
and standard deviations for the two test groups were: low dose, 1.6 ±
0.8 mg/m3; high-dose, 9.6 ± 1/5 mg/m3. Exposures were for 6 h/day, 5
days/week for a total of 60 exposures. The mean particle size was 3.8
µm with a geometric standard deviation of 1.7.
The high-dose guinea-pigs showed a significantly increased body
weight in comparison with the controls. Plasma and erythrocyte
cholinesterase activities were unaffected in terminal blood samples.
Both test groups exhibited a lower kidney-to-body weight ratio than
the controls. Histopathological alterations in the lung, liver and
kidneys were not related to the treatment. Sections of the spinal cord
and sciatic nerve, stained to demonstrate the myelin sheaths, showed
no pathological changes (MacFarland & Punte, 1966).
B7.3 Skin and eye irritation; sensitization
No irritation was seen after exposure to TEHP by a saturated
cotton swab placed on the inside of the ears of rabbits for 24 h
(Kimmerle, 1958).
TEHP was tested in three albino rabbits according to OECD 404
test guideline. Well-defined erythema, slight to moderate oedema,
crust formation and desquamation were observed. TEHP produced a
primary irritation index of 4.2/8.0 and was classified as a moderate
irritant to rabbit skin. No corrosive effects were observed (Guest,
1993b).
A single dose of 250 mg undiluted TEHP applied to the clipped
skin of rabbits produced moderate erythema, which persisted for a week
(MacFarland & Punte, 1966). Repeated applications of 0.1 ml on 5
days/week (10 or 20 applications) produced moderate erythema after the
first application. With further applications a spreading zone of
erythema developed with desquamation, leatheriness and some fissuring
with haemorrhages. At the end of the observation period, thickening
and severe hyperkeratosis of the skin was apparent.
TEHP was non-irritating when tested in the eyes of three albino
rabbits according to OECD 405 Test Guideline (Guest, 1993a).
TEHP was instilled into the conjunctival sac of one eye of each
of two rabbits at dose levels of 0.01 to 0.5 ml. Doses up to 0.05 ml
produced slight conjunctivitis, while doses of 0.1 and 0.5 ml produced
moderate conjunctivitis which cleared up in 24 h (MacFarland & Punte,
1966).
B7.4 Reproductive toxicity, embryo toxicity and teratogenicity
No data on the reproductive toxicity of TEHP are available.
B7.5 Mutagenicity
TEHP was shown to be non-genotoxic in a range of mutagenicity
assays.
B7.5.1 In vitro assays
TEHP was tested for mutagenicity in a Salmonella/microsome
assay using strains TA1535, TA1537, TA98 and TA100 in the presence and
absence of S9 derived from livers of Aroclor 1254-treated
Sprague-Dawley rats. Results were negative (Zeiger et al., 1985).
In a mouse lymphoma assay, concentrations of TEHP of up to and
exceeding the apparent solubility limit of 62.5 µl/litre produced no
gene mutations. The assay was carried out in the presence and absence
of S9 from livers of Aroclor 1254-treated male F-344 rats (Myhr &
Caspary, 1991).
An in vitro cytogenetic assay in Chinese hamster ovary (CHO)
cells was carried out using concentrations of TEHP up to 251 µg/ml in
the presence and absence of S9 derived from livers of Aroclor
1254-treated Sprague-Dawley rats. There was no evidence of induction
of chromosome damage (Ivett et al., 1989).
An in vitro sister-chromatid exchange (SCE) assay was carried
out in CHO cells in the presence and absence of S9 derived from livers
of Aroclor 1254-treated Sprague-Dawley rats. Concentrations of up to
251 µg/ml were used, but at 16.7 µg/ml and above there was severe cell
cycle delay, which limited the number of cells available for analysis.
TEHP did not increase the number of SCEs (Ivett et al., 1989).
B7.5.2 In vivo assays
A mouse bone marrow micronucleus assay was carried out in male
B6C3F1 mice, which were given intraperitoneal TEHP injections of 500,
1000 or 2000 mg/kg body weight on three consecutive days. Bone marrow
was harvested at 24 h after the last dose, and was examined for
micronucleus-containing polychromatic erythrocytes (MN-PCEs). A
statistically significant (P < 0.001) dose-related increase in the
number of MN-PCEs was detected in the bone marrow from treated mice.
The assay was repeated in two further experiments using doses of 1500
and 2000 mg/kg body weight in one and 2000 and 3000 mg/kg body weight
in the other. No increase in the number of bone marrow MN-PCEs was
detected in either of these experiments. It is concluded that the
initial result was an artefact and that TEHP is not mutagenic in this
assay (Shelby et al., 1993).
An in vivo cytogenetic assay was carried out in male B6C3F1
mice, which were given a single intraperitoneal injection of TEHP
(dose not stated). Bone marrow was harvested for analysis at 17 and 36
h post-dosing and metaphase cells were examined for chromosomal
aberrations. The number of chromosomal aberrations was not elevated in
TEHP-treated mice (Shelby & Witt, 1995).
In an in vivo liver unscheduled DNA synthesis (UDS) assay, male
B6C3F1 mice were given TEHP doses of 1000 or 2000 mg/kg body weight
by gavage. Mice were killed at 24, 39 and 48 h post-dosing and liver
preparations were made. There was no evidence of increased UDS in
hepatocytes from TEHP-treated mice (Miyagawa et al., 1995).
B7.6 Carcinogenicity
In a US NTP (1984) study, TEHP was administered in corn oil (10
ml/kg body weight) by gavage 5 days/week for 103 weeks to groups of 50
male and 50 female F-344/N rats and B6C3F1 mice. The doses
administered were:
Fischer-344 rats B6C3F1 mice
Dose Male Female Male Female
Control Vehicle Vehicle Vehicle Vehicle
Low dose 2000 mg/kg 1000 mg/kg 500 mg/kg 500 mg/kg
High dose 4000 mg/kg 2000 mg/kg 1000 mg/kg 1000 mg/kg
The animals were observed twice daily and body weight was
measured weekly for the first 13 weeks and once every 4 weeks
thereafter. Clinical examinations were performed once every 4 weeks.
Necropsies and histopathological examinations were performed on all
animals, but organ weight changes were not reported.
No compound-related clinical toxicity was observed in either sex
of either species. Decrease in body weight, compared with controls,
was limited to male rats at the low dose (11.5%) and the high dose
(15.8%). The decreased body weight did not affect survival.
In male rats the incidence of phaeochromocytomas of the adrenal
gland increased with dose and two (4%) were malignant in the high-dose
group. The incidence of adrenal phaeochromocytomas in male rats was:
control 2/50 (4%), low-dose 9/50 (18%) and high-dose 12/50 (24%). In
two previous gavage studies in the same laboratory, the incidence of
phaeochromocytomas in control male rats was 24 and 26%.
In female mice the incidence of hepatocellular carcinomas was:
control 0/48 (0%), low dose 4/50 (8%) and high dose 7/50 (14%). The
incidence of hepatocellular carcinomas showed a dose-related increase
and the incidence at the high-dose level was statistically
significant.
The results of these 2-year gavage studies in rats and mice were
interpreted by NTP as showing some evidence of carcinogenicity in
female mice based on the increase in hepatocellular carcinomas and
equivocal evidence of carcinogenicity in male rats based on the
increased incidence of phaeochromocytomas (US NTP, 1984; Kluwe et al.,
1985)
In this same study (US NTP, 1984), TEHP caused a dose-related
increase in the incidence of follicular cell hyperplasia of the
thyroid in male and female B6C3F1 mice. The incidence of hyperplasia
was: in males, control 0/49 (0%), low dose 12/48 (25%) and high dose
24/47 (51%); in females, control 1/44 (2%), low dose 13/47 (28%) and
high dose 12/46 (26%). There was no dose-related increase in thyroid
tumours. The LOAEL for thyroid hyperplasia was 357 mg/kg body weight
per day; a NOAEL was not established.
B7.7 Special studies
B7.7.1 Neurotoxicity
MacFarland & Punte (1966) tested TEHP for its neurotoxic
potential. Four groups of female chickens, each weighing 1.5-2.0 kg,
received a single dose of test material into the crops as follows:
Group Number of chickens Test material Dose
1 4 Saline 1.5 ml/kg
2 8 TOCP 500 mg/kg
3 8 TEHP 500 mg/kg
4 8 TEHP 2500 mg/kg
After receiving a single dose of the test material the animals
were kept under observation for 4 weeks and then killed. Body weights
were recorded weekly and changes in appearance and behaviour noted
daily. Gross necropsy was performed on all chickens. Sections of
brain, three levels of the spinal cord and the sciatic nerve were
examined microscopically.
In the tri- ortho-cresyl phosphate (TOCP) group used as positive
control, weight loss become apparent by the end of the first week and
signs of ataxia and muscular weakness were evident by the 12th day.
These signs increased in intensity, so that the chickens were
prostate by the end of the study. The microscopic examination of the
nerve tissue sections confirmed that TOCP was producing
demyelinization. The chickens in the saline and TEHP groups appeared
normal and maintained or gained weight throughout the study. There
were no macroscopic signs of neurotoxicity and microscopically no
demyelinization was observed.
No evidence of systemic intoxication or, in particular,
neurotoxicity was seen in chickens dosed with a single dose of up to
2.500 mg TEHP/kg body weight (MacFarland & Punte, 1966).
Single hens were administered a single dose of 0.25, 0.5 or
1.0 g/kg body weight by gavage. The animals were kept under
observation for 2 months and examined for neurotoxicity twice weekly.
No abnormalities in behaviour were detected. A single intramuscular
injection of 0.25, 0.5 or 1.0 g TEHP/kg body weight to single chicken
again induced no signs of intoxication (Kimmerle, 1958).
In 3-month inhalation studies (see section B7.2.3) with
guinea-pigs, dogs and rhesus monkeys, determination of plasma and
erythrocyte cholinesterase activity and histological examination of
sections of tissue including spinal cord and sciatic nerve did not
reveal any abnormalities. In dogs and rhesus monkeys, the
cholinesterase were measured after 4, 8 and 12 weeks of exposure to
10.8, 26.4 or 85 mg TEHP/m3, but in guinea-pigs measurements were
made after 12 weeks of exposure to 1.6 or 9.6 mg/m3
(MacFarland & Punte, 1966).
In two cats receiving 28 doses of 1.0 ml TEHP/kg body weight by
gavage (see section 7.2.1) no signs of neurotoxicity and no inhibition
in the erythrocyte cholinesterase activity was found (Bayer, 1958).
B8. EFFECTS ON HUMANS
No irritant effects were seen after 24 h of exposure to a
TEHP-saturated cotton swab placed on the skin of the forearm of six
volunteers. A piece (2 cm2) of PVC plastic containing 40% TEHP was
placed on the arm of 8 volunteers for 72 h. Slight redness but no
irritation was observed (Kimmerle, 1958).
No other data are available concerning the effect of TEHP on
humans.
B9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
B9.1 Laboratory experiments
B9.1.1 Microorganisms
A bacterial growth inhibition test, carried out according to ISO
8192 indicated an IC50 for TEHP greater than 100 mg/litre (Bayer,
1982b).
B9.1.2 Aquatic organisms
B9.1.2.1 Vertebrates
A 96-h exposure of zebra fish (Brachydanio rerio) under static
conditions to TEHP (100 mg/litre) produced no deaths (Bayer, 1989).
B9.1.3 Terrestrial organisms
No data on the toxicity of TEHP to terrestrial organisms are
available.
PART C
TETRAKIS(HYDROXYMETHYL) PHOSPHONIUM SALTS
C. SUMMARY AND EVALUATION
C1. Tetrakis(hydroxymethyl) phosphonium salts
C1.1 Summary
Tetrakis(hydroxymethyl) phosphonium salts represent the major
class of chemicals used as a flame retardant for cotton, cellulose and
cellulose-blend fabrics. There is low migration from fabrics treated
with tetrakis(hydroxymethyl) phosphonium chloride (THPC)-urea. The
sulfate salt (THPS) is mainly used as a biocide. Combined world
production is estimated to be >3000 tonnes for THP salts and around
3000 tonnes for the THPC-urea condensate.
Photodegradation and hydrolysis of THP salts are significant
abiotic degradation pathways in the environment. THPS binds poorly to
environmental particulates and is, therefore, mobile. THPS degrades
rapidly under both aerobic and anaerobic conditions. Trihydroxymethyl
phosphine oxide (THPO) and bishydroxymethyl phosphonic acid (BMPA)
have been identified as breakdown products.
Since no monitoring has been reported, no estimates can be made
of exposure to humans or organisms in the environment.
The acute oral toxicity of THPC and THPS is moderate; dermal
toxicity is low.
In short-term (up to 28 days) studies in rats and mice, the main
toxic effect for both THPC and THPS is decreased body weight. The
NOAEL for both chemicals in both species is approximately 8 mg/kg body
weight per day. In longer-term studies (13 weeks), the main target
organ for toxicity is the liver. The NOAEL for this effect ranged from
3 to 7 mg/kg body weight per day for both salts in both species.
Carcinogenicity bioassays on THPC also showed effects on the liver,
but a NOAEL was not established. The LOAEL was approximately 3 mg/kg
body weight per day for both species. In a carcinogenicity bioassay on
THPS in mice, the NOAEL for focal hyperplasia in the adrenal medulla
was 3.6 mg/kg body weight per day; in rats the LOAEL for mortality was
3.6 mg/kg body weight per day.
THPS did not cause skin irritation when administered as a single
dose to rabbits. However repeated dermal exposure of rats resulted in
severe skin reaction. THPC-urea was corrosive. THPS was identified as
severe eye irritant in rabbits.
THPS and THPC-urea cause skin sensitization guinea-pigs
(Magnusson & Kilman Maximization test).
THPS and THPC-urea did not cause developmental toxicity in orally
dosed experimental animals.
THPC and THPS have mutagenic potential in vitro, but THPS is
not mutagenic in vivo (no in vivo mutagenicity data are available
for THPC). Limited mutagenicity data for THPC-urea suggest that it is
not mutagenic in vivo. THPO is non-genotoxic. There is no convincing
evidence to suggest that fabrics treated with THP salts are mutagenic.
Available information indicates that there is no genotoxic hazard to
humans.
THPC and THPS were not carcinogenic in rats and mice in 2-year
bioassays. Dermal studies have shown that THP salts are promoters of
skin cancer but not initiators.
THPS and THPO did not inhibit acetylchlolinesterase activity in
vitro, suggesting a lack of neurotoxic hazard for humans.
THPC-urea-treated fabric did not cause skin irritation in humans.
For THPS, reported acute toxicity values for algae are less than
1 mg/litre, with one no-observed-effect concentration (NOEC) of
0.06 mg/litre. The acute NOEC for the water flea is 10 mg/litre.
Reported acute toxicity values for marine invertebrates range from 1.6
to 340 mg/litre.
Fish 96-h LC50 values range from 72 to 119 mg/litre, with NOEC
values in the range of 18 to 41 mg/litre. An acute avian LD50 of 311
mg/kg body weight and dietary LC50 values of 1300 and 2400 mg/kg diet
have been reported.
C1.2 Evaluation
No exposure information is available for either humans or
organisms in the environment. Therefore no quantitative risk
assessment could be made.
C2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, AND ANALYTICAL
METHODS
C2.1 Identity
Tetrakis(hydroxymethyl) phosphonium salts (THP salts) have the
following general chemical structure :
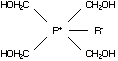 The commercially relevant salts of THP are the sulfate (THPS) and
the chloride (THPC). In addition, tetrakis(hydroxymethyl) phosphonium
chloride-urea condensate is the major commercially available flame
retardant product.
In the past other salts and salt-urea condensates have been used;
their names and CAS numbers are listed in IARC (1990).
C2.1.1 Tetrakis(hydroxymethyl) phosphonium chloride (THPC)
Chemical formula: C4H12O4PCl
Chemical structure:
The commercially relevant salts of THP are the sulfate (THPS) and
the chloride (THPC). In addition, tetrakis(hydroxymethyl) phosphonium
chloride-urea condensate is the major commercially available flame
retardant product.
In the past other salts and salt-urea condensates have been used;
their names and CAS numbers are listed in IARC (1990).
C2.1.1 Tetrakis(hydroxymethyl) phosphonium chloride (THPC)
Chemical formula: C4H12O4PCl
Chemical structure:
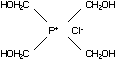 Chemical name: Phosphonium, tetrakis(hydroxymethyl)
chloride
Relative molecular mass: 190.56
CAS registry number: 124-64-1
CAS name: Phosphonium tetrakis(hydroxymethyl)
chloride
IUPAC name: Tetrakis(hydroxymethyl) phosphonium
chloride
Trade names: Tolcide PC800; Tolcide THPC; Retardol C
Synonyms: Tetrahydroxymethyl phosphonium chloride
Tetramethylol phosphonium chloride
C2.1.2 Tetrakis(hydroxymethyl) phosphonium sulfate (THPS)
Chemical formula: C8H24O8P204S
Chemical structure:
Chemical name: Phosphonium, tetrakis(hydroxymethyl)
chloride
Relative molecular mass: 190.56
CAS registry number: 124-64-1
CAS name: Phosphonium tetrakis(hydroxymethyl)
chloride
IUPAC name: Tetrakis(hydroxymethyl) phosphonium
chloride
Trade names: Tolcide PC800; Tolcide THPC; Retardol C
Synonyms: Tetrahydroxymethyl phosphonium chloride
Tetramethylol phosphonium chloride
C2.1.2 Tetrakis(hydroxymethyl) phosphonium sulfate (THPS)
Chemical formula: C8H24O8P204S
Chemical structure:
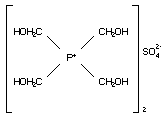 Chemical name: Phosphonium, tetrakis(hydroxymethyl)
sulfate
Relative molecular mass: 406.28
CAS registry number: 55566-30-8
CAS name: Phosphonium, tetrakis(hydroxymethyl)
sulfate (2:1)
IUPAC name: bis[tetrakis(hydroxymethyl) phosphonium]
sulfate (salt)
Trade names: Tolcide PS75; Tolcide THPS, Retardol S
Synonyms: Octakis(hydroxymethyl) phosphonium sulfate
C2.1.3 Tetrakis(hydroxymethyl) phosphonium chloride-urea condensate
(THPC-urea)
Chemical formula: [C4H12O4P.CH4N2O.Cl]x
Chemical name: Tetrakis(hydroxymethyl) phosphonium
chloride-urea copolymer
Chemical structure:
Chemical name: Phosphonium, tetrakis(hydroxymethyl)
sulfate
Relative molecular mass: 406.28
CAS registry number: 55566-30-8
CAS name: Phosphonium, tetrakis(hydroxymethyl)
sulfate (2:1)
IUPAC name: bis[tetrakis(hydroxymethyl) phosphonium]
sulfate (salt)
Trade names: Tolcide PS75; Tolcide THPS, Retardol S
Synonyms: Octakis(hydroxymethyl) phosphonium sulfate
C2.1.3 Tetrakis(hydroxymethyl) phosphonium chloride-urea condensate
(THPC-urea)
Chemical formula: [C4H12O4P.CH4N2O.Cl]x
Chemical name: Tetrakis(hydroxymethyl) phosphonium
chloride-urea copolymer
Chemical structure:
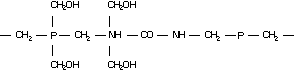 Relative molecular mass: 300 for the repeat unit shown above
CAS registry number: 27104-30-9
CAS name: Phosphonium, tetrakis(hydroxymethyl)-
chloride, polymer with urea
Trade names: Proban CC; Retardol AC
Proban 210 is no longer produced.
C2.2 Physical and chemical properties
Physical and chemical properties are given in Table 8.
C2.2.1 Technical products
THPC and THPS are marketed in concentrated aqueous solutions at
approximately 80 and 75% (by weight), respectively (Albright & Wilson,
personal communication to IPCS). Typically THPS is marketed with less
then 1% of formaldehyde content (Albright & Wilson, personal
communication to IPCS). In the past values ranging from 3.79% at pH
0.4 to 14.1% at pH > 5.0 have been reported (Loewengart & Van Duuren,
1977; Ulsamer et al., 1980). Tetrakis(hydroxymethyl) phosphonium
acetate/phosphate (THPA/P) was previously available in the USA as a
clear, nearly colourless solution with a pH of approximately 5,
containing 10% active phosphorus (Hooper et al., 1976).
C2.3 Conversion factors
THPC 1 ppm = 7.76 mg/m3
1 mg/m3 = 0.128 ppm
THPS 1 ppm = 16.61 mg/m3
1 mg/m3 = 0.0602 ppm
C2.4 Analytical methods
A standard method for THPS and THPC determination is by iodine
titration. However, this not substance specific and is therefore
subject to interference by many other chemicals that may be present in
the sample to be analysed. The method involves dilution in water
containing an aliquot of a saturated solution of disodium hydrogen
orthophosphate. A solution of polystyrene sulfonic acid is then added
followed by a few drops of a starch indicator. Titration against a
previously standardized iodine solution is then carried out (Albright
& Wilson, personal communication to IPCS).
The most accurate analytical technique for the quantitative
substance-specific determination of THP salts is currently ion
chromatography. In this method the sample is chromatographed using an
Ionpac CS5 column with a CG5 guard column. The mobile phase is
hydrochloric acid (0.1 mol/litre) at 1 ml/min. The ion chromatography
separates the THP salt from any free formaldehyde, which is largely
retained. The THP salt is then detected using a visible wavelength
detector at 425 nm following a post-column reaction with an
acetylacetone reagent containing acetic acid, ammonium acetate and
acetyl acetone. The reagent breaks down the THP salt to form free
formaldehyde, which forms a cyclic coloured complex. Free formaldehyde
also reacts but two separate and distinct peaks are seen on the
chromatogram.
Table 8. Physical and chemical properties of THP salts
Parameter THPS THPC THPC-urea copolymer
100% 75% 80-85%
Appearance Soft waxy solid Colourless liquid Clear straw-coloured Straw-coloured liquid
liquid
Odour Resembles Resembles aldehyde Pungent Pungent
aldehyde
Boiling point (°C) 108.5 115
Melting point (°C) 54.2-81.5 -21
Flash point >100
Vapour pressure <2.6 × 10-4 Pascal 26.7 mmHg at 25°C
at 20°C
Viscosity 38 cStp at 25°C 0.27 Pa.s at 29°C
pH 3.19 (0.01 M solution) < 2 5
Stability 21°C -- stable for 21°C -- stable for 14 days Stable under normal Stable under normal
14 days conditions conditions
54°C -- stable for 54°C -- stable for 14 days
14 days
Table 8. (continued)
Parameter THPS THPC THPC-urea copolymer
100% 75% 80-85%
Decomposition Oxides of sulfur, phosphorus Oxides of phosphorus; Oxides of phosphorus;
products and carbon; phosphine chlorine, ammonia chlorine, ammonia
Relative density 1.53 1.39 1.34 1.31
Solubility Infinitely soluble in Completely soluble Completely soluble Miscible with water
water
Log n-octanol/water -9.8 (calculated)
partition coefficient
From: Cowlyn (1991a,b); Antony (1993); Barth (1994); Willis (1995)
C3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURES
C3.1 Natural occurrence
These compounds are not known to occur as natural products.
C3.2 Anthropogenic sources
C3.2.1 Production levels and processes
THP salts have been produced for commercial use since the 1950s.
The first of these, THPC, was introduced in 1953.
THP salts are synthesized in high yields through the reaction of
formaldehyde with phosphine and the corresponding acid in an enclosed
process (Weil, 1980; Hawley, 1981).
PH3 + HCl + 4CH2O --> [(HOCH2)4P]Cl
The resulting products exist in an equilibrium with THP+, which
is highly pH dependent. Increasing the pH shifts this equilibrium to
the right with the resultant production of formaldehyde, i.e., one of
the methylol groups from the THP salt becomes hydrolysed.
Currently there is one major producer of these salts and
THPC-urea condensates in the United Kingdom and some production
potential in the USA. Combined worldwide production of THP salts is
greater than 3000 tonnes; the urea-condensate production is around
3000 tonnes annually of which 40% is consumed in the USA (Albright &
Wilson, personal communication to IPCS).
C3.2.2 Uses
THPC-based products represent the major class of chemicals used
as flame retardants for cotton, cellulose and cellulose-blend fabrics.
Until 1976, THPC was the major THP salt used as a flame retardant. In
addition, THPS and some mixed salts were commercially available.
THPC-based flame retardants have been found to be more reactive
and efficient as flame retardants when compared with similar
THPS-based products (Albright & Wilson, personal communication to
IPCS). Nowadays, the THPC-urea condensates dominant the market for
flame retardant treatment of cellulose and cellulose-blend fabrics
where durability to laundering and dry cleaning is required.
It has been suggested that bis(chloromethyl) ether (BCME) may be
formed during the manufacture of THPC and thus may present an
occupational hazard (Loewengart & Van Duuren, 1977). However, an
extensive airborne monitoring survey coupled with chemical analysis
(using mass spectroscopy) conducted at the United Kingdom
manufacturing site during 1975 did not detect the presence of BCME at
the minimum level of analytical detection of 2.35 µg/m3 (0.5 ppb).
These results were later independently confirmed in a separate survey
carried out by the United Kingdom Factory Inspectorate (personal
communication by R. Williams, HM Inspector of Factories, United
Kingdom Health and Safety Executive to Albright & Wilson, 1975).
The results of the two occupational hygiene surveys are supported
by a study in 1974 in which kinetic measurements in aqueous media
(manufacture is via an aqueous route) showed that BCME undergoes rapid
hydrolysis with a half-life of approximately 10-40 seconds at ambient
temperature (Tou et al.,1974). BCME, if formed, would therefore exist
as a transient species in aqueous media containing formaldehyde and
hydrochloric acid, or other acid chlorides.
The major application of THPS now is as a biocide in a variety of
preservative applications which include leather, textile, paper and
photographic films, as well as industrial water treatment and offshore
oil production processes.
C4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION AND TRANSFORMATION
C4.1 Transport and distribution between media
A field study was conducted on the use of THPS as a biocide in
the water of an industrial cooling tower. THPS was added to produce an
initial concentration of approximately 100 mg/litre and lithium
chloride was added simultaneously as a marker for determination of
dilution volumes in the tower and associated drainage systems.
Analysis of THPS was by HPLC. The half-life of lithium (and therefore
of THPS by dilution) was calculated to be 3 days. THPS levels were
found to decrease more rapidly than simple dilution would have
suggested, indicating hydrolysis; actual concentrations in the Rhyne,
the drainage channel of the cooling tower, were <0.5 mg/litre
(Heaton, 1991).
Adsorption of radiolabelled THPS was studied using agricultural
sand, silt loam, sandy loam, pond sediment and marine sediment; the
percentage of organic carbon in the tested soil/sediments ranged from
0.17 to 1.1%. Estimated Koc values ranged from 72 to 266 (mean
153 ± 69.2), indicating medium-to-high mobility for the compound
(Heim, 1998).
C4.2 Transformation
C4.2.1 Biodegradation
Inherent biodegradability of THPS was assessed using the OECD
302B guideline; >20% degradation occurred within a 28-day period
(Douglas & Pell, 1985).
Aquatic aerobic degradation of THPS was assessed in a soil/water
system under US EPA Guidelines. The soil was dosed with THPS at
1 µg/g. The compound was metabolized with 60% of applied radioactivity
appearing as CO2 within 7 days. Major metabolites were
trihydroxymethyl phosphine oxide (THPO) and bishydroxymethyl
phosphonic acid (BMPA), which were both found in the water; neither
degradate reached a concentration of 10% of the applied dose (Gorman,
1996). A comparable study at the same initial concentration of THPS
but under anaerobic conditions also showed 60% degradation within 30
days, and the same breakdown products were identified (Gorman, 1997).
Natural seawater was dosed with THPS to an initial concentration
of 4.16 mg/litre. Biodegradation (measured as oxygen demand) reached
17.7% after 28 days. A parallel toxicity test showed that the
substance was inhibitory to bacteria at the concentration used in the
test (McWilliam, 1994).
C4.2.2 Abiotic degradation
Laboratory studies using UV light showed that THPS photodegrades
to THPO when at low concentrations in aqueous solution. Conversion to
THPO was almost complete at concentrations up to about 20 mg/litre
within 2 h. Conversion also took place in synthetic seawater.
Photodegradation was pH-dependent, with greater conversion at
environmentally relevant pH. Exposure to natural sunlight showed high
levels (not stated) of conversion over a 3-month period (Lloyd, 1994).
Hydrolysis of THPS is pH-dependent; half-lives for the compound
at 25°C were 131, 72 and 7 days at pH 5, 7 and 9, respectively
(O'Connor, 1992).
C4.3 Migration from textiles
A method is available for the determination of the fixation
efficiency of the THPC-urea copolymer in terms of the percentage
phosphorus applied and fixed during the flame retardant treatment of
various textiles. A fixation efficiency of greater than 90% is common
for the PROBANR process (Albright & Wilson, personal communication
to IPCS). The THPC-urea copolymer is chemically converted to a
water-insoluble polymer of high relative molecular mass during the
textile processing, first by exposure to ammonia in an enclosed
chamber and then by an oxidation process involving hydrogen peroxide.
This latter process converts any phosphorus to the inert pentavalent
form. At the same time the hydrogen peroxide will convert any unfixed
THPC-urea copolymer to tetrakis(hydroxymethyl) phosphine hydroxide
(THPOH), which is subsequently removed from the fabric during the
final washing-off steps.
Wetting the treated fabric with water, beverages, liquid foods or
urine does not release the flame retardant polymer. The lack of water
solubility and the physical entrapment of the polymer inside the
fibres (which make up the treated fabric) make the polymer resistant
to removal by cleaning materials that a consumer might normally employ
to clean these flame-retardant-treated articles.
Industrial work clothing treated with THPC-urea condensate flame
retardants have been shown to be flame resistant after 50-100 washings
or dry cleanings, demonstrating the durability of the polymer to
leaching and washing (Albright & Wilson, personal communication to
IPCS).
Appendix A lists flammability standards met regularly by products
treated with THPC-urea condensates.
C5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
Approximately 20 workers are currently potentially exposed during
production of THP salts and THPC-urea condensate in the United Kingdom
(Albright & Wilson, personal communication to IPCS). No data on levels
of exposure are available.
C6. KINETICS AND METABOLISM IN LABORATORY ANIMALS
A metabolism study on rats has been conducted using
14C-radiolabelled THPS. THPS was not found in rat urine. However,
three metabolites were present, identified as trihydroxymethyl
phosphine oxide, bishydroxymethylphosphonic acid and possibly a
formaldehyde adduct of the trihydroxy compound (Dr P. Martin, Albright
& Wilson, personal communication to IPCS).
C7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
C7.1 Single exposure
C7.1.1 Oral
Acute oral toxicity results are given in Table 9.
Table 9. Oral LD50 values for THPC, THPS and THPC-urea condensate in
mice and rats (mg/kg body weight) a
Species Sex THPCb THPSc THPC-urea
Mice female 280 200 (none died) 400 (all died)
Rats male 282
Rats both sexes 575 962
Rats male 185 333
Rats female 161 248
a From Ulsamer et al. (1980); US NTP (1987); Tuffnell (1991);
Guest (1994a)
b 75% solution in water
c 72% solution in water
In THPC-treated rats that survived, reddish fluids around the
nostrils and laboured breathing were observed. All mice were lethargic
and had a rough coat. With THPS no signs of intoxication were noted
(Ulsamer et al., 1980; US NTP, 1987).
C7.1.2 Dermal
The dermal LD50 value in albino rabbits was greater than 4084 mg
THPC/kg body weight after a 24-h exposure. Erythema and oedema of the
integumentary system were observed (US NTP, 1987).
No deaths occurred when rats were treated dermally with THPS or
THPC-urea at 2000 mg/kg body weight (Liggett & Allen, 1989; Snell,
1994).
C7.1.3 Inhalation
The LC50 4-h value for THPS was 5.5 mg/litre in rats exposed to
respirable aerosol (nose only) (McDonald & Anderson, 1989).
C7.2 Repeated exposure
C7.2.1 Oral
C7.2.1.1 THPC
Groups of five 6-week-old, F-344/N rats of each sex were
administered 0, 9.4, 18.8, 37.5, 75 or 150 mg THPC (as a 75% aqueous
solution) per kg body weight in deionized water by gavage for 14 days.
At the two highest dose levels an increased mortality was observed. At
75 mg/kg the mortality for males was 20%, and at 150 mg/kg all males
and females died. The body weight gain of the male animals
administered 18.8 and 37.5 mg/kg was decreased, respectively, by 7 and
11%. This effect was also found in the females administered 75 mg/kg
(27%). Rats with 150 mg/kg had yellow to tan or mottled red livers. No
histopathology was carried out (US NTP, 1987).
Groups of ten 4-week-old F-344/N rats of each sex were gavaged 5
days/week for 13 weeks with 0, 3.75, 7.5, 15, 30 or 60 mg THPC (as a
75% aqueous solution) per kg. All males and 5/10 females that received
60 mg/kg died. The final mean body weight of males that received 30
mg/kg was 89% of vehicle controls. The final mean body weight of
females that received 60 mg/kg was 80% of vehicle controls.
At the highest dose level, clinical signs of toxicity included
rough coat, hunched back, diarrhoea, lethargy, paresis and
hyperextension of back legs. Periportal hepatocellular necrosis was
observed in 9/10 males and 7/10 females that received 15 mg/kg, all
males and females that received 30 mg/kg, and 7/10 males and 8/10
females that received 60 mg/kg.
Periportal cytoplasmic vacuolization was observed in 8/10 males
that received 7.5 mg/kg, 9/10 males and 8/10 females that received
15 mg/kg, and all rats that received 30 or 60 mg/kg. Degeneration of
the axons was found in 2/10 females that received 60 mg/kg. The NOAEL
for this study was 2.7 mg/kg body weight per day; the LOAEL was 5.4
mg/kg body weight per day (US NTP, 1987).
Groups of five 5-week-old B6C3F1 mice of each sex were
administered 0, 18.8, 37.5, 75, 150 or 300 mg THPC (as a 75% aqueous
solution) per kg body weight by gavage for 14 consecutive days. At the
highest dose level all mice died. At 150 mg/kg, body weight was
depressed by 18% in males and 20% in females compared to control mice.
No clinical sign of toxicity were observed in animals surviving to the
end of the study. No compound-related effects were observed at
necropsy (US NTP, 1987).
Groups of ten 4-week-old B6C3F1 mice of each sex were
administered by gavage 0, 1.5, 4.5, 15, 45 or 135 mg/kg body weight
5 days/week for 13 weeks. Seven of 10 males and 6/20 females that
received 135 mg/kg died. The final mean body weight for mice that
received 135 mg/kg was 8% lower than that of the controls for males
and 19% lower for females. Paresis of the hind legs and loss of
coordination were observed in all males and 9/10 females that received
135 mg/kg. Mice in this group also had marked axonal degeneration,
characterized by swollen axon sheaths, missing fragmented axons and
some proliferation of neurolemmal cells in the sciatic nerve, dorsal
roots of the caudal spinal nerves and tracts of the spinal cord
particularly in the dorsal column of the lumbar cord. Intracytoplasmic
vacuoles were seen in periportal hepatocytes of all mice in the 15, 45
and 135 mg/kg groups. The NOAEL for this study was 3.2 mg/kg body
weight per day; the LOAEL was 10.7 mg/kg body weight per day (US NTP,
1987).
C7.2.1.2 THPS
Group of 12 male ICR Swiss mice were administered daily by gavage
THPS in saline (2, 10 or 50 mg/kg body weight) for 14 days. In the
high-dose group 75% of the animals died (Connor et al., 1980).
Groups of five, 5-week-old, B6C3F1 mice of each sex were
administered 0, 12.5, 25, 50, 100 or 200 mg THPS (obtained as a 72%
aqueous solution) per kg body weight by gavage for 14 consecutive
days. Mice of the two highest dose groups had increased mortality at
100 mg/kg (20% male, 40% female) and at 200 mg/kg (80% male, 100%
female). At 25 mg/kg or more there was a decrease in body weight gain.
The animals given 100 or 200 mg/kg had laboured breathing and rough
coat and female mice of these groups also showed loss of movement in
their hind legs (US NTP, 1987).
Groups of 10, 5- to 6-week-old B6C3F1 mice of each sex were
administered 0, 5, 10, 20, 40 or 60 mg THPS (obtained from a 72%
aqueous solution) per kg body weight by gavage on 5 days/week for 13
weeks. One of 10 females that received 60 mg/kg and 2/10 males and
1/10 females that received 40 mg/kg died. The final mean body weights
of mice that received 20, 40 or 60 mg/kg were 4%, 7% or 11%,
respectively, lower than those of the controls for males and 3%, 5%
and 11% lower for females. Periportal vacuolar degeneration occurred
in all male and female mice that received 60 mg/kg, all males and 9/10
females that received 40 mg/kg, and 8/10 male mice that received 20
mg/kg. The NOAEL for this study was 7.1 mg/kg body weight per day, and
the LOAEL was 14.3 mg/kg body weight per day (US NTP, 1987).
Groups of five, 4-week-old, F-344/N rats of each sex were
administered 0, 12.5, 25, 50, 100 or 200 mg THPS (obtained as a 72%
aqueous solution) per kg body weight by gavage for 14 consecutive
days. All rats given 100 or 200 mg/kg died. Animals that received 25
or 50 mg/kg gained less weight than controls by 11% and 21%,
respectively, in males and 1% and 7% in females. The animals given the
two highest dose levels showed tremors, and one animal had partial
loss of movement of the hind legs. At necropsy no abnormalities were
seen (US NTP, 1987).
In an oral 28-day study, Charles River derived CD rats (five
females and five males/group) were given by gavage 6, 30 or 60 mg THPS
(75% obtain as aqueous solution) per kg body weight daily. At the
highest dose level, one male rat died on day 20, one female rat on day
21 and the remaining rats on day 22. Post-dose salivation, emaciation,
hypoactivity, hunched posture, noisy breathing, urogenital staining
and ptosis were seen only in the 60 mg/kg group. There was severe
weight loss during week 3 (body weight 52% for male and 74% for female
relative to controls at the end of week 3). The NOEL was considered to
be 6 mg/kg body weight per day (Hill, 1989).
Groups of ten, 5- to 6-week-old F-344/N rats of each sex were
administered 0, 5, 10, 20, 40 or 60 mg THPS (obtained from a 72%
aqueous solution) per kg body weight by gavage 5 days/week for
13 weeks. Three of the male rats that received 60 mg/kg died. Final
mean body weights were 5%, 15% and 22% lower than those of the
controls for males that received 20, 40 or 60 mg/kg and 9%, 12% and
19% lower for females that received 20, 40 or 60 mg/kg. Vacuolar
degeneration of hepatocytes occurred in all males receiving 10 mg/kg
or more, in all females receiving 40 or 60 mg/kg, and in 5/10 females
receiving 20 mg/kg. Lymphoid depletion in the spleen was seen in three
males in the 60 mg/kg group. Bone marrow hypoplasia was diagnosed in
3/10 male and 4/10 female rats in the 60 mg/kg groups. The NOAEL in
this study was 3.6 mg/kg body weight per day; the LOAEL was 7.1 mg/kg
body weight per day (US NTP, 1987).
Groups of 10 Charles River derived CD rats of each sex received
0, 1, 5 or 10 mg THPS (75% aqueous solution) per kg body weight per
day by gavage daily for 13 weeks. One female rat from the 5 mg/kg
group died on day 14, one female from the 1 mg/kg group on day 53 and
another on day 81. No clinical signs or changes in body weight were
attributed to THPS administration at any dose level other than mean
plasma ALAT and ASAT levels being twice control values for males of
the highest dose group. Histopathology showed moderate to marked
cytoplasmic vacuolation of periportal hepatocytes in all male rats and
moderate vacuolation in one female rat of the highest dose group. The
NOEL for this study was 1 mg/kg body weight per day (Hill & Newman,
1990).
C7.2.2 Dermal
Application of 125, 350, 700 or 1000 mg THPS/kg body weight on
chemically depilated back skin of groups of 12 male ICR mice daily for
up to 14 days caused reduced body weight, paralysed back muscles at
700 mg/kg body weight or more, and some superficial necrosis at all
doses (Connor et al., 1980). Similar effects were reported in mice by
Afansa'eva & Evseenko (1971).
Both THPC and THPS were toxic when applied dermally for long
periods on the skin. Rats and rabbits were dosed daily for 20 days
with 15%, 20% or 30% aqueous solutions of THPC. Severe skin lesions
occurred and all rats in the highest dose group died after 9 days of
application (Aoyama, 1975).
In a study by Wragg et al. (1996), trihydroxymethyl phosphine
oxide was administered by dermal application to three groups, each of
five male and five female Sprague-Dawley CD rats, for up to 28
consecutive days at dose levels of 0, 300, 650 and 1000 mg/kg body
weight per day. There were no deaths or clinical abnormalities
attributable to the test material. Body weight gain and food
consumption were similar in treated groups and controls. Females
treated with 1000 mg/kg showed an increase in plasma total protein and
a reduction in albumin/globulin ratio compared to controls. Males
treated with 1000 mg/kg and both males and females in the remaining
treatment groups showed no toxicologically significant changes in
these parameters. Both males and females dosed at 1000 mg/kg showed
cortical hypertrophy of the adrenal glands. Adverse dermal reactions
(scabs sometimes accompanied by scar tissue) were seen at all dose
levels. The incidence of these adverse dermal reactions gradually
increased during the second half of the treatment period amongst males
(but not females) dosed at 1000 mg/kg, and by day 28 all five males
showed dermal abnormalities. A NOAEL for dermal reactions was not
established. The NOAEL for systemic toxicity was determined to be 650
mg/kg body weight per day (Wragg et al., 1996).
C7.3 Long-term exposure
C7.3.1 THPC
Groups of 50 F-344/N rats of each sex (7 weeks of age) were
administered 0, 3.75 or 7.5 mg THPC (obtained from a 75% aqueous
solution) per kg body weight by gavage on 5 days/week for 103 weeks.
The mean body weights of different groups were comparable. Clinical
signs noted were rough hair coat and diarrhoea. The survival of
high-dose female rats was significantly lower than that of controls
after week 70 (P = 0.013). A dose-related increase in the
incidence of hepatocellular lesions, primarily cytoplasmic
vacuolization, was found (males: controls 0%, low-dose 18% and
high-dose 47%; females: controls 6%, low-dose 22% and high-dose 50%).
The LOAEL for this study was 2.7 mg/kg body weight per day; a NOAEL
was not established (US NTP, 1987).
Groups of 50 male B6C3F1 mice (8 weeks of age) were administered
0, 7.5 or 15 mg THPC (obtained from a 75% aqueous solution) per kg
body weight, and groups of 50 female B6C3F1 mice received 0, 15 or 30
mg/kg body weight by gavage 5 days/week for 103 weeks. No clear
difference between control and treated groups concerning body weight
gain and mortality was observed. Compound-related clinical signs
consisted of rough hair coat and diarrhoea. No signs of neurotoxicity
were observed. There was a dose-related increase in the incidence of
hepatocellular cytoplasmic vacuolization (males: controls 0%, low-dose
80%, and high-dose 88%; females: controls 0%, low-dose 84%, and
high-dose 96%). Follicular cell hyperplasia of the thyroid gland was
observed in the high-dose females (22% versus 6% in controls). The
LOAEL for this study was 5.4 mg/kg body weight per day; a NOAEL was
not established (US NTP, 1987).
C7.3.2 THPS
Groups of 49 or 50 F-344/N rats of each sex were administered 0,
5 or 10 mg THPS (obtained from a 72% aqueous solution) per kg body
weight by gavage 5 days/week for 104 weeks. Mean body weights of the
different groups were comparable. Clinical signs were rough hair coat
and diarrhoea. The survival of both the male low-dose (after week 102)
and high-dose (after week 67) animals was significantly lower
(P = 0.036 and 0.006, respectively). The effect level for mortality
in this study was 3.6 mg/kg body weight per day, the lowest dose
tested (US NTP, 1987).
Groups of 50 B6C3F1 mice of each sex (7 weeks of age) were
administered 0, 5 or 10 mg THPS (obtained from a 72% aqueous solution)
per kg body weight by gavage 5 days/week for 104 weeks. Mean body
weight and survival of control and treated groups were comparable.
Clinical signs were limited to rough hair coat and diarrhoea.
Non-neoplastic lesions seen were focal hyperplasia of the adrenal
medulla, but the numbers were statistically unrelated to dose
(controls 3/49, low-dose 5/48, high-dose 10/49). The NOAEL for this
study was 3.6 mg/kg body weight per day; the LOAEL was 7.1 mg/kg body
weight per day (US NTP, 1987).
C7.4 Skin and eye irritation; sensitization
C7.4.1 Skin irritation
C7.4.1.1 THPS
When 0.5 ml of THPS (75%) was applied to the skin of six New
Zealand white rabbits for a period of 4 h (OECD 404), no dermal
irritation was observed (Liggett, 1989a).
In a dermal study, daily doses of 25, 250 or 500 mg THPS (75%
aqueous solution) were applied to the shaved neck skin of ten (five
females, five males) Charles River derived CD rats. The treatment had
to be terminated and animals killed after 6 days due to the nature and
severity of the skin reaction observed at the application site (Hill,
1989).
C7.4.1.2 THPC-urea
A single 4-h semi-occluded application of THPC-urea to the intact
skin of six rabbits produced corrosive effects at two skin sites and
slight-to-well-defined erythema and very slight to slight oedema at
the other four treated skin sites (Snell, 1994).
C7.4.2 Eye irritation
In a test for eye irritation (OECD 405), an aliquot of 0.1 ml
THPS (75%) was introduced to one eye of a New Zealand rabbit. Opacity
was observed 24 h after application and lasted at least for 24 h. Red
coloration of the conjunctiva accompanied by considerable swelling was
observed. On the basis of these effects, THPS (75%) is considered to
be a severe eye irritant (Liggett, 1989b).
C7.4.3 Skin sensitization
C7.4.3.1 THPS
THPS (75%) was assessed for skin sensitization using the
Magnusson & Kligman Maximisation test (OECD 406). Fourteen out of 20
animals challenged with the test substance were sensitized (Guest,
1994b). These data clearly demonstrate a sensitization potential for
THPS (75%).
C7.4.3.2 THPC-urea
When THPC-urea was tested for skin sensitization potential using
the guinea-pig Magnusson & Kligman Maximisation test, 53% of the
animals were sensitized (Tufnell, 1992).
C7.5 Reproductive toxicity, embryotoxicity and teratogenicity
C7.5.1 THPS
Three groups of 16 New Zealand white rabbits were administered
(OECD 414) by gavage THPS (75%) at 6, 18 and 60 mg/kg body weight per
day from day 7 to 19 of gestation. All animals were kill on day 21. At
60 mg/kg mean body weight gain was significantly lower than that of
controls, showing maternal toxicity. Treatment at 60 mg/kg resulted in
increase incidence (42/120) of fetuses with eye malformation and some
with additional hydrocephaly or limb/phalangeal reduction defects. An
increase incidence of specific skeleton variation was also observed.
No adverse effects were noticed in the two lower-dose group (Barker,
1991a).
Charles River CD rats (24 animals per group) were administered by
gavage THPS (75%) at 15, 30 and 60 mg/kg body weight per day from day
6 to 15. All animals were killed on day 21. Treatment at 60 mg/kg
reduced significantly body weight gain from day 12 of gestation
onwards, indicating maternal toxicity. No treatment-related effects
were observed in dams at the low-dose level. At the high-dose level,
the incidence of fetuses showing extra thoraco-lumbar ribs was
significantly higher than for controls. At 30 mg/kg only minor signs
of maternal toxicity were observed (Barker, 1991b).
From these studies a NOAEL of 18 mg/kg body weight per day based
on maternal toxicity could be derived. No developmental effects were
observed in the absence of maternal toxicity.
C7.5.2 THPC-urea
Dose levels of 10, 30 and 100 mg THPC-urea/kg body weight per day
were administered to groups of 16 rabbits from day 7 to 19 of
gestation. All animals were killed on day 29. At the high-dose level,
an initial mean weight loss was followed by significantly reduced
weight gain over the treatment period. Treatment did not affect the
incidence of fetuses showing external, visceral or skeletal
malformations or variations (Barker, 1992).
C7.6 Mutagenicity and related end-points
C7.6.1 THPC-urea
C7.6.1.1 In vitro studies
An in vitro cytogenetic assay was performed with THPC-urea
using human lymphocytes. Harvest times of 16 and 40 h were used for
cells incubated in the presence of S9 from induced rat liver, and
times of 20 and 44 h were used when S9 was absent. In one experiment,
THPC-urea had no effect on the number of aberrant cells at
concentrations up to 40 mg/ml, but, in a repeat experiment, increased
numbers of aberrant cells were seen at 20 and 40 mg/ml. The reason for
the difference in results between the two experiments is not clear
(Durward, 1995; Bailey, 1995).
C7.6.1.2 In vivo studies
No mutagenicity of THPC-urea was evident in a mouse micronucleus
test in which oral doses of 212.5, 425 and 850 mg/kg body weight were
administered (Durward, 1996).
C7.6.2 THPC
Salmonella microsomal assays have shown uniformly negative
results for THPC (Table 10).
THPC produced chromosomal aberrations in Chinese hamster ovary
(CHO) cells in the absence of metabolic activation, but the results
were equivocal when it was tested in the presence of S9 from livers of
Sprague-Dawley rats treated with Aroclor 1254 (US NTP, 1987; Loveday
et al., 1989). THPC induced chromosomal aberrations in Chinese hamster
DON-6 cells in the absence of metabolic activation (Sasaki et al.,
1980).
THPC induced sister-chromatid exchange (SCE) in CHO cells when
incubated in the absence of metabolic activation (US NTP, 1987;
Loveday et al., 1989).
Table 10. Mutagenicity tests with Salmonella typhimurium
Strain Dose Metabolic activation Reference
(µg/plate)
TA100, TA1535, TA1537, TA98 0.33-33 none, S9 rat liver (male), US NTP (1987)a
S9 Syrian hamster liver (male)
TA98, TA100, TA1535, TA1537 10-1000 none, S9 liver (Aroclor 1254, rat) MacGregor et al. (1980)
TA98, TA100, TA1535, TA1537 10-300 none, S9 liver (Aroclor 1254, rat) MacGregor et al. (1980)
TA98, TA100, TA1535, TA1537 Up to 10 000 none, S9 liver (Aroclor 1254, rat) Zeiger et al. (1987)
TA98, TA100 not know none, S9 Kawachi et al. (1980b)b
a THPC gave positive results in a mouse lymphoma assay in the absence of metabolic activation (US NTP, 1987).
b Kawachi et al. (1980b) found positive results with THPC in the Bacillus subtilis rec assay with and without
metabolic activation.
C7.6.3 THPS
THPS produced no mutagenicity when tested in the Salmonella
microsome assay using strains TA98, TA100, TA1535, TA1537 and
TA1538 in the presence and absence of S9 from the livers of
Aroclor-1254-treated rats (Dillon & Riach, 1990). Leachate from
THPS-treated paper also showed no mutagenicity when tested with
strains TA98, TA100, TA102, TA1535 and TA1537 in the presence and
absence of S9 from Aroclor-1254-treated rats (Ballentyne, 1996b).
When tested in the mouse lymphoma assay, THPS caused mutations
both in the presence and absence of S9 from Aroclor-treated rats
(Riach, 1996). US NTP (1987) also reported positive results for THPS
in the mouse lymphoma assay in the absence of S9.
High levels of structural chromosomal aberrations were detected
at metaphase in CHO cells treated with THPS in the presence or absence
of S9 from liver of Aroclor-treated rats (Leddy, 1990). Anaphase
analysis of THPS-treated CHO cells also showed that chromosomal
aberrations were produced along with abnormal spindles (Coutino,
1979).
The results of an in vitro assay for unscheduled DNA synthesis
in a primary culture of rat hepatocytes were negative (Downey et al.,
1990; Riach, 1994).
Bone marrow from THPS-treated mice was analysed for
micronucleated polychromatic erythrocytes (MN-PCEs) and for metaphase
cells showing chromosomal aberrations. There was no effect on the
number of MN-PCEs nor on the number of chromosomal aberrations (Connor
et al., 1980).
The in vivo mutagenicity of dermal doses of THPS was
investigated in Swiss (ICR) mice using a similar protocol to that of
the previous study. Dermal doses of 125, 350, 700 and 1000 mg/kg body
weight per day were used. Urine from treated mice was not mutagenic in
the Salmonella microsome assay. Analysis of bone marrow from treated
mice showed a slight increase in the number of polyploid cells at the
highest dose level, but otherwise there was no indication of
mutagenicity (Connor et al., 1980).
In a dominant lethal assay in Swiss (ICR) mice, males were dosed
with up to 1000 mg/kg body weight. There was no evidence to suggest
that THPS produced dominant lethal mutations (personal communication
by M. Legator to Hooker Chemicals and Plastics Corp., 1977).
In a dominant lethal study in rats, gavage doses of 5, 10 or 15
mg/kg body weight per day were given to males for 10 weeks. After
mating, investigation of the pregnant females indicated no dominant
lethal mutations (Clode, 1996).
C7.6.4 THPO
THPO is a metabolite and breakdown product of THP salts. It was
not mutagenic in Salmonella typhimurium strains TA98, TA100, TA1535
and TA1537 or in Escherichia coli strain WP2 uvra when tested in
the presence and absence of S9 from livers of Aroclor-1254-treated
rats (Ballentyne, 1996a).
THPO was not mutagenic in the mouse lymphoma assay in either the
presence or absence of liver S9 from Aroclor-1254-treated rats
(Fellows, 1996). It did not produce chromosomal aberrations in CHO
cells cultured in either the presence or absence of S9 (Marshall,
1996).
C7.6.5 Treated fabrics
Groups of 12 male ICR mice received 0 (no fabric), 2500 mg
untreated fabric per kg diet or 250, 1250 or 2500 mg THPS-treated
fabric per kg diet for 5 successive days. Femurs were collected for
analyse of bone marrow for MN-PCEs and chromosomal aberrations. All
tests were negative (Connor et al., 1980).
C7.7 Carcinogenicity
C7.7.1 Oral studies
C7.7.1.1 Mice
In a 103-week study of THPC (75%) in mice (see section C7.3.1),
there was no evidence of carcinogenicity (US NTP, 1987).
In a 104 week study of THPS (72%) in mice(see section C7.3.2),
there was no evidence of carcinogenicity (US NTP, 1987).
C7.7.1.2 Rats
In a 103 week study for THPC (75%) in rat (see section C7.3.1),
there was no evidence of carcinogenicity (US NTP, 1987).
In a 104 week study for THPS (72%) in rat (see section C7.3.2),
there was no evidence of carcinogenicity (US NTP, 1987).
C7.7.2 Dermal studies: Initiation and promotion
A group of 60 female ICR/Ha Swiss mice, 6-8 weeks of age,
received skin applications of THPC (2 mg/mouse) in acetone three times
a week for 71 weeks. The control group received acetone only. There
was no significant increase in tumours (papillary tumours of the lung
and papillomas of the forestomach) in the animals treated with THPC
(Van Duuren et al., 1978).
Groups of 20 female ICR/Ha Swiss mice, 6-8 weeks of age, received
skin applications of THPC (2 mg/mouse) or Pyroset TKP
(acetate/phosphate mixture of THP) (7 mg/mouse) in dimethyl sulfoxide
three times a week for 57 weeks to examine the initiating, promoting
and complete carcinogenic potential in skin carcinogenesis bioassays.
Both chemicals were active as tumour promoters using a single
application of 7,12-dimethylbenz[ a]anthracene (DMBA) (20 µg in 0.1
ml acetone) as initiator. Neither chemical was active as a tumour
initiator or complete carcinogen (Loewengart & van Duuren, 1977).
C7.8 Special studies
THPS and THPO did not inhibit acetyl cholinesterase when tested
in vitro using malathion (a known inhibitor of cholinesterase) as a
positive control (Thompson, 1997a,b).
C8. EFFECTS ON HUMANS
Fabric treated with THPC-urea condensate was tested on human
volunteers in a 48-h skin patch test. The treated fabric was not
irritant to exposed human skin (Jackson, 1982).
C9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
C9.1 Laboratory experiments
C9.1.1 Aquatic organisms
THPS showed an EC50 for growth inhibition of the marine
microalga Skeletonema costatum of 0.16 mg/litre over 72 h (Hushagen
& McWilliam, 1994). For the freshwater green alga Selenastrum
capricornutum, EC50 values of 0.652 mg/litre (based on growth rate)
and 0.204 mg/litre (based on biomass) were determined. The NOEC for
both end-points was 0.063 mg/litre. The test was conducted under OECD
Guideline 201 (Jenkins, 1991c).
The 48-h LC50, based on immobilization and using nominal
concentrations, for THPS in the water flea (Daphnia magna) was
determined to be 19.4 mg/litre, with a NOEC of 10.4 mg/litre. The test
was conducted under US EPA/OECD Guideline 202 (Jenkins, 1989).
Concerning estuarine/marine invertebrates, the 96-h LC50 for the
mysid shrimp (Mysidopsis bahia) is 7.3 mg/litre (NOEC 3.5 mg/litre)
(Boeri et al., 1995a), the 96-h LC50 for the brown shrimp (Crangon
crangon) is 340 mg/litre (Douglas & Pell, 1986), and the 48-h LC50
for the brine shrimp (Acartia tonsa) is 0.6 mg/litre (Torp &
McWilliam, 1994). The 96-h EC50 for shell deposition in juvenile
Eastern oysters (Crassostrea virginica) was reported to be
1.6 mg/litre, with a NOEC of 0.67 mg/litre for THPS in a test
following US EPA guidelines (Boeri et al., 1995c). The 10-day LC50
for THPS was determined for the sediment-dwelling amphipod Corophium
volutator to be 2174 mg/kg dry sediment weight (Roddie, 1994).
The 96-h LC50, based on nominal concentrations of THPS, for the
rainbow trout (Oncorhynchus mykiss) was determined to be 119
mg/litre with a NOEC of 18.1 mg/litre in a test conducted according to
OECD Guideline 203 (Jenkins, 1991a). A test conducted under US
EPA/OECD Guideline 203 determined the 96-h LC50 for THPS in bluegill
sunfish (Lepomis macrochirus) to be 93 mg/litre, with a NOEC of 22.7
mg/litre (Jenkins, 1991b). The 96-h LC50 for the marine sheepshead
minnow (Cyprinodon variegatus) was determined to be 72 mg/litre,
with a NOEC of 41 mg/litre using US EPA guidelines (Boeri et al.,
1995b). For juvenile plaice (Pleuronecta platessa), the 96-h LC50
was 86 mg/litre (Douglas & Handley, 1989).
C9.1.2 Terrestrial organisms
The acute oral LD50 for young adult mallard ducks (Anas
platyrynchus) was 311 mg/kg body weight (Roberts & Phillips, 1988a).
Dietary LC50 values for the mallard duck and bobwhite quail
(Colinus virginianus) were 1313 and 2414 mg/kg diet, respectively
(Roberts & Fairley, 1988; Roberts & Phillips, 1988b).
C10. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
The International Agency for Research on Cancer evaluated the
carcinogenicity of tetrakis(hydroxymethyl) phosphonium salts in 1989
(IARC, 1990) and concluded:
a) There is inadequate evidence for the carcinogenicity of
tetrakis(hydroxymethyl) phosphonium salts in experimental
animals.
b) No data were available from studies in humans on the
carcinogenicity of tetrakis(hydroxymethyl) phosphonium salts.
c) Tetrakis(hydroxymethyl) phosphonium salts are not classifiable
as to their carcinogenicity to humans (Group 3).
REFERENCES
Adachi K, Mitsuhashi M, & Ohkuni N (1984) Pesticides and trialkyl
phosphates in tap water (Jpn.) Hyogo-ken Eisei Kenkyusho Kenkyu
Hokoku, 19: 1-6 (Abstract).
Afansa'eva LV & Evseenko NS (1971) Hygiene evaluation of fire-proof
textiles processed with an organophosphorus impregnant based on
tetrahydroxymethyl phosphonium chloride. Hyg Sanit , 36(3): 450-453.
Anderson GD, Elvin AT, & Lalka D (1984) Estimation of
tris(2-butoxyethyl) phosphate in biological fluids: Novel intersubject
variability in recovery from human serum. J Pharm Sci, 73(12):
1791-1793.
Antony C (1993) THPS 75% viscosity (Study sponsored by Albright &
Wilson). Whippany, New Jersey, Case Consulting Laboratories Inc.
Aoyama M (1975) Effect of anti-flame treating agents on the skin.
Nagoya Med J, 20: 11-19.
Arias P (1992) Brominated diphenyloxides as flame retardants: Bromine
based chemicals (Unpublished report to the OECD).
Armstrong JA & Yule WN (1978) The distribution of aerially-applied
spray deposits in spruce trees. Can Entomol , 110: 1259-1267.
ARW (1987) [Annual report 1987.] Düsseldorf, Working Group
Rhine-Waterworks, p 86 (in German).
Ashby J & Tennant RW (1988) Chemical structure, Salmonella
mutagenicity and extent of carcinogenicity as indicators of genotoxic
carcinogens among 222 chemicals tested in rodents by the US NCI/NTP.
Mutat Res, 204: 17-115.
Bailey IA (1995) Proban CC: in vitro cytogenetic assay review (Study
sponsored by Albright & Wilson). Cheshire, United Kingdom, Zeneca Ltd,
Central Toxicology Laboratory (Report No. CTL/B/31).
Ballentyne M (1996a) Trishydroxymethyl phosphine oxide: Reverse
mutation in five histidine-requiring strains of Salmonella
typhimurium. Harrogate, United Kingdom, Corning Hazleton (Europe)
(Unpublished report No. 254/47-1052).
Ballentyne M (1996b) THPS paper leachate PLO1: Reverse mutation in
four histidine-requiring strains of Salmonella typhimurium and one
tryptophan-requiring strain of Escherichia coli. Harrogate, United
Kingdom, Corning Hazleton (Europe) (Unpublished report No.
254/45-1052).
Barker L (1991a) THPS: Oral (gavage) teratology study in the rabbit
(Study sponsored by Albright & Wilson). Harrogate, United Kingdom,
Corning Hazleton (Europe) (Unpublished report No. 6380-254/19).
Barker L (1991b) THPS: Oral (gavage) teratology study in the rat
(Study sponsored by Albright & Wilson). Harrogate, United Kingdom,
Corning Hazleton (Europe) (Unpublished report No. 6358-254/23).
Barker L (1992) Proban CC: Oral (gavage) teratology study in the
rabbit (Study sponsored by Albright & Wilson). Harrogate, United
Kingdom, Corning Hazleton (Europe) (Unpublished report No.
6742-254/26).
Barth T (1994) Bioaccumulation OECD 117 partition coefficient
n-octanol/water (HPLC)- tolcide PS75 (Study sponsored by Albright &
Wilson). Bergen, Norway, Terra Miljö-Laboratories A/S.
Bayer (1958) [Toxicological testing of the softener
tri-2-ethylhexylphosphate (TOF).] Leverkusen, Germany, Bayer AG,
Institute of Toxicology (Unpublished report) (in German).
Bayer (1989) [Phosphoric acid, bis(2-ethylhexyl)ester ecotoxicological
data -- Fish toxicity: Basic data set for existing chemicals above
1000 t/year.] Leverkusen, Germany, Bayer AG (in German).
Bayer (1982a) [Biological degradation of tris(2-ethylhexyl)phosphate.]
Leverkusen, Germany, Bayer AG, Institute for Environmental Analysis
and Assessment (Unpublished report) (in German).
Bayer (1982b) [Bacterial test on chemicals, ETAD 3002/ISO8192.]
Leverkusen, Germany, Bayer AG, Institute for Environmental Analysis
and Assessment (Unpublished report) (in German).
Boeri RL, Magazu JP, & Ward TJ (1995a) Acute toxicity of tetrakis
(hydroxymethyl) phosphonium sulphate (THPS) to the Mysid (Mysidopsis
bahia), under flow-through conditions (Study No. 41238). Columbia,
Missouri, ABC Laboratories Inc. (Unpublished report).
Boeri RL, Magazu JP, & Ward TJ (1995b) Acute toxicity of
tetrakis(hydroxymethyl) phosphonium sulphate (THPS) to the sheepshead
minnow, (Cyprinodon variegatus), under flow-through conditions
(Study No. 41239). Columbia, Missouri, ABC Laboratories Inc.
(Unpublished report).
Boeri RL, Magazu JP, & Ward TJ (1995c) Acute flow-through mollusc
shell deposition test with tetrakis(hydroxymethyl) phosphonium
sulphate (THPS) (Study No. 41240). Columbia, Missouri, ABC
Laboratories Inc. (Unpublished report).
Bohlen H, Hicke K, Stobel A-O, Zierott M, & Thiemann W (1989) [The
contamination of the lower part of the Weser River by organochlorine
and organophosphorus compounds I.] Vom Wasser, 72: 185-197 (in
German).
BUA (1996) [German Chemical Society-Advisory Committee on Existing
Chemicals of Environmental Relevance (BUA): Di(2-ethylhexyl)
phosphate, tri(2-ethylhexyl) phosphate.] Stuttgart, Germany, S. Hirzel
(BUA Report 172) (in German).
Carrington CD, Lapadula DM, Othman M, Farr C, Nair RS, Johanssen F, &
Abou-Donia M (1990) Assessment of the delayed neurotoxicity of
tributyl phosphate, tributoxyethyl phosphate and dibutylphenyl
phosphate. Toxicol Ind Health, 6(3/4): 415-423.
Clode SA (1996) THPS: Oral (gavage) dominant lethal study in the rat.
Harrogate, United Kingdom, Corning Hazleton (Europe) (Unpublished
report No. 254/43-1050l).
Connor T, Meyne J, & Legator M (1980) The mutagenic evaluation of
tetrakis(hydroxymethyl) phosphonium sulfate using a combined testing
protocol approach. J Environ Pathol Toxicol, 1: 145-158.
Coutino R (1979) Analysis of anaphase in cell culture: An adequate
test system for the distinction between compounds which selectively
alter the chromosome structure or the mitotic apparatus. Environ
Health Perspect, 31: 131-136.
Cowlyn TC (1991a) THPS: Determination of physical-chemical properties
(Study sponsored by Albright & Wilson). Eye, United Kingdom, Life
Science Research Limited
Cowlyn TC (1991b) THPS-75: Determination of physical-chemical
properties (Study sponsored by Albright & Wilson). Eye, United
Kingdom, Life Science Research Limited.
Dillon DM & Riach CG (1990) THPS-75, batch No 88/22: Testing for
mutagenic activity with Salmonella typhimurium TA1535, TA1537,
TA1538, TA98 and TA100 (Project No.740423). Falls Church, Virginia,
Inveresk Research International.
Douglas MT & Handley JW (1989) The acute toxicity of DP435 to juvenile
plaice (Pleuronectes platessa) (Study sponsored by Albright &
Wilson). Huntingdon, United Kingdom, Huntingdon Research Centre
Limited (Unpublished report No. A&W 491/881590).
Douglas MT & Pell IB (1985) Assessment of inherent biodegradability of
DP 435 (Study sponsored by Albright & Wilson). Huntingdon, United
Kingdom, Huntingdon Research Centre Limited (Report No.
A&W457(C)/85679).
Douglas MT & Pell IB (1986) The acute toxicity of DP435 to Brown
shrimp (Crangon crangon) (Study sponsored by Albright & Wilson).
Huntingdon, United Kingdom, Huntingdon Research Centre Limited
(Unpublished report No. A&W 470(b)/8677).
Downey E, Riach CG, & Mohammed R (1990)
Tetrakishydroxymethylphosphonium sulphate: assessment of genotoxicity
in an unscheduled DNA synthesis assay using adult rat hepatocyte
primary culture (Project No. 741343). Falls Church, Virginia, Inveresk
Research International (Unpublished Report No. 6230).
Durward R (1995) Proban CC: Chromosomal aberration test in human
lymphocytes in vitro (SPL project No. 071/346, sponsored by Albright
& Wilson). Derby, United Kingdom, Safepharm Laboratories Ltd.
Durward R (1996) Proban CC: Micronucleus test in the mouse (SPL
project No. 071/372, sponsored by Albright & Wilson). Derby, United
Kingdom, Safepharm Laboratories Ltd.
ECETOC (1992a) Tris(2-ethylhexyl) phosphate (CAS No. 78-42-2);
Bis(2-ethylhexyl) phosphate (CAS No. 298-07-7); and Mono(2-ethylhexyl)
phosphate (CAS No. 12645-31-7). Brussels, European Chemical Industry
Ecology and Toxicology Centre (Joint Assessment of Commodity Chemicals
No. 20).
ECETOC (1992b) Tris(2-butoxylethyl) phosphate. Brussels, European
Chemical Industry Ecology and Toxicology Centre ( Joint Assessment of
Commodity Chemicals No. 21).
Eldefrawi AT, Mansour NA, Brattsten LB, Ahrens VD, & Lisk DJ (1977)
Further toxicologic studies with commercial and candidate flame
retardant chemicals: Part II. Bull Environ Contam Toxicol, 17(6):
720-726.
Environmental Agency Japan (1983) Environmental monitoring of
chemicals: Environmental survey report of FYs 1980 and 1981. Tokyo,
Environmental Agency, Department of Environmental Health, Office of
Health Studies..
Environmental Agency Japan (1987) Chemicals in the environment: Report
on environmental survey and wildlife monitoring of chemicals in FYs
1984 and 1985. Tokyo, Environmental Agency, Department of
Environmental Health, Office of Health Studies.
Environmental Agency Japan (1978) Environmental monitoring of
chemicals: Environmental survey report of 1977 FY. Tokyo,
Environmental Agency, Department of Environmental Health, Office of
Health Studies.
Ernst W (1988) [Evaluation of contaminants of low degradability in
estuaries.] Biotechnology, 129(3): 60-63 (in German).
Fellow SM (1996) Tris hydroxymethyl phosphine oxide (THPO); mutation
at the thymidine kinase (tk) locus of mouse lymphoma L517BY cells
using the microtitre fluctuation technique (Study sponsored by
Albright & Wilson). Harrogate, United Kingdom, Corning Hazleton
(Europe).
FMC (1990) Material safety data sheet on KP-140: Tributoxyethyl
phosphate. Princeton, New Jersey, FMC Corporation.
FMC (1998) Material safety data sheet: Reomol TOP. Princeton, New
Jersey, FMC Corporation (Rev. 1).
Freeman C (1991a) KP-140: Non-definitive primary skin irritation study
in rabbits (Study No. I91-1206). Princeton, New Jersey, FMC
Corporation, Toxicology Laboratory
Freeman C (1991b) KP-140: Non-definitive acute oral toxicity study in
rats (Study No. I91-1207). Princeton, New Jersey, FMC Corporation,
Toxicology Laboratory.
Freeman C (1991c) KP-140: Non-definitive primary eye irritation study
in rabbits (Study No. 191-1205). Princeton, New Jersey, FMC
Corporation, Toxicology Laboratory.
Frimmel FH, Millington DS, & Christman RF (1987) [Quantification of
organic compounds in charcoal extracts using gas chromatography/mass
spectrometry.] Fresenius Z Anal Chem, 327: 149-153 (in German).
Fukushima M & Kawai S (1986) Present status and fate of selected
organophosphoric acid triesters in the water area of Osaka City.
Seitai Kagaku, 8(4): 13-24.
Fukushima M, Kawai S, Chimaki S, & Morioka T (1986) Urban runoff as a
source of selected chemicals to river waters: An observation at
Isojima sampling station in the Yodo River Basin. Health Environ Sci,
49: 11-20.
Fukushima M, Kawai S, & Yamaguchi Y (1992) Behavior of
organophosphoric acid triesters in Japanese riverine and coastal
environment. Water Sci Technol, 25(11): 271-278.
Gabriel KL (1980a) Primary eye irritation study -- rabbits (Project
No. 80-1907A). Philadelphia, Pennsylvania, Bioresearch Inc.
Gabriel KL (1980b) Primary skin irritation study -- rabbits (Project
No. 80-1907A). Philadelphia, Pennsylvania, Bioresearch Inc.
Gabriel KL (1980c) Acute dermal toxicity -- rabbits (Project No.
80-1907A). Philadelphia, Pennsylvania, Bioresearch Inc.
Gartrell MJ, Craun JC, Podrebarac DS, & Gunderson EL (1986a)
Pesticides, selected elements and other chemicals in infant and adult
total diet samples: October 1980-March 1982. J Assoc Off Anal Chem,
69: 146-161.
Gartrell MJ, Craun JC, Podrebarac DS, & Gunderson EL (1986b) Chemical
contaminants monitoring: Pesticides, selected elements and other
chemicals in infant and toddler total diet samples, October 1980-March
1982. J Assoc Off Anal Chem, 69: 123-145.
Gorman M (1996) Aerobic aquatic metabolism of
14C-tetrakishydroxylmethyl phosphonium sulphate (THPS): Final report
(Study Sponsored by Albright & Wilson). Columbia, Missouri, ABC
Laboratories Inc. (Unpublished report No. 40054).
Gorman M (1997) Anaerobic aquatic metabolism of
14C-tetrakishydroxylmethyl phosphonium sulphate (THPS): Final report
(Study sponsored by Albright & Wilson). Columbia, Missouri, ABC
Laboratories Inc. (Unpublished report No. 40055).
Guest RL (1993a) Amgard TOF: Acute eye irritation test in the rabbit
(Project No. 71/200, sponsored by Albright & Wilson). Derby, United
Kingdom, Safepharm Laboratories Ltd.
Guest RL (1993b) Amgard TOF: Acute dermal irritation test in the
rabbit (Project No. 71/199, sponsored by Albright & Wilson). Derby,
United Kingdom, Safepharm Laboratories Ltd.
Guest RL (1994a) Tolcide THPS 75 %: Acute oral toxicity test in the
rat (Study sponsored by Albright & Wilson). Derby, United Kingdom,
Safepharm Laboratories Ltd (Unpublished report).
Guest RL (1994b) Tolcide THPS 75 %: Magnusson & Kligman maximisation
study in the guinea pig (Project No. 71/288). Derby, United Kingdom,
Safepharm Laboratories Ltd (Unpublished report).
Gunderson EL (1988) Dietary intakes of pesticides, selected elements
and other chemicals: FDA total diet study, April 1982-April 1984. J
Assoc Off Anal Chem, 71(6): 1200-1209.
Gunderson EL (1995a) Dietary intakes of pesticides, selected elements
and other chemicals: FDA total diet study, June 1984-April 1986. J
Assoc Off Anal Chem, 78(4): 910-921.
Gunderson EL (1995b) Dietary intakes of pesticides, selected elements
and other chemicals: FDA total diet study, July 1986-April 1991. J
Assoc Off Anal Chem, 78(6): 1353-1363.
Haseman JK, Crawford DD, Huff JE, Boorman GA, & McConnell EE (1984)
Results from 86 two-year carcinogenicity studies conducted by the
National Toxicology Program. J Toxicol Environ Health, 14: 621-639.
Hattori Y, Ishitani H, Kuge Y, & Nakamoto M (1981) Environmental fate
of organic phosphate esters. Suishitsu Odaku Kenkyu, 4(3): 137-141.
Hawley GG (1981) Condensed chemical dictionary, 12th ed. New York, Van
Nostrand Reinhold, p 1133.
Heaton PE (1991) Determination of the environment fate of THPS in
water treatment applications. Oldbury, United Kingdom, Albright &
Wilson UK Ltd, Biocides Group (Unpublished report No. BTSP-4A).
Heim LC (1998) Soil/sediment adsorption-desorption of
14C-tetrakishydroxymethyl phosphonium sulphate (THPS) (Study
sponsored by Albright & Wilson). Columbia, Missouri, Analytical
Bio-Chemistry (ABC) Laboratories, Inc. (Final report No. 40053).
Hill RE (1989) DP435: 28 day dose range finding study in the rat
-- Sub-acute dermal study in the rat. Toxicol Laboratories Limited
(Unpublished report No. ALW/7/89).
Hill RE & Newman AJ (1990) DP435: 13 week oral (gavage) toxicity study
in the rat -- Oral 90-day studies. Toxicol Laboratories Limited
(Unpublished report No. ALW/4/90).
Hinckley DA, Bidleman TF, Foreman WT, & Tuschall JR (1990)
Determination of vapor pressures for nonpolar and semipolar organic
compounds from gas chromatographic retention data. J Chem Eng Data,
35: 232-237.
Hoechst (1987) [Safety data sheet on tributoxyethyl phosphate, TBEP.]
Frankfurt, Germany, Hoechst AG (in German).
Hoechst (1989) [TBEP: Assessment of acute aerosol inhalation toxicity
in male and female SPF-Wistar rats, 4 hours LC50.] Frankfurt,
Germany, Hoechst AG, Pharma-Research Toxicology and Pathology (in
German).
Hooper G, Nakajima WN, & Herbes WF (1976) The use of various
phosphonium salts for flame retardancy by the ammonium cure technique.
In: Bhatnagar VM ed. Fire retardants: Proceedings of the International
Symposium on Flammability and Fire Retardants, Montreal, Canada, 22-23
May 1975. Westport, Connecticut, Technomic Publishing Co., pp 98-114.
Hushagen H & McWilliam P (1994) Tolcide PS75 (THPS): Marine algal
growth inhibition test with Skeletonema costatum. Bergen, Norway,
Terra Miljö-Laboratories A/S (Unpublished report No.
60090.aw\tm.001\skelet
IARC (1990) Some flame retardants and textile chemicals, and exposures
in the textile manufacturing industry. Lyon, International Agency for
Research on Cancer, pp 95-107 (IARC Monographs on the Evaluation of
Carcinogenic Risks to Humans, Volume 48).
IPCS (1998) Concise international chemical assessment document No. 10:
2-Butoxyethanol. Geneva, World Health Organization, International
Programme on Chemical Safety, 29 pp.
Ishikawa S, Taketomi M, & Shinohara R (1985) Determination of trialkyl
and triaryl phosphates in environmental samples. Water Res, 19(1):
119-125.
Ivett JL, Brown BM, Rodgers C, Anderson BE, Resnick MA, & Zeiger E
(1989) Chromosomal aberrations and sister chromatid exchange test in
Chinese hamster ovary cells in vitro: IV. Results with 15 chemicals.
Environ Mol. Mutagen, 14: 165-187.
Jackson JR (1982) Study to determine the primary irritancy of
untreated fabric. Proban 210 treated fabric, Proban NX treated fabric,
laundered Proban NX treated fabric in human volunteers. Oldbury,
United Kingdom, Albright & Wilson UK Ltd.
Jenkins WR (1989) THPS: Acute toxicity to Daphnia magna (Study
sponsored by Albright & Wilson). Eye, United Kingdom, Life Science
Research Limited (Unpublished report No. 90/AWL007/0471).
Jenkins WR (1991a) Acute toxicity to Rainbow trout (Study sponsored by
Albright & Wilson). Eye, United Kingdom, Life Science Research Limited
(Unpublished report No. 90/AWL006/0470).
Jenkins WR (1991b) THPS -- Acute toxicity to Bluegill Sunfish (Study
sponsored by Albright & Wilson). Eye, United Kingdom, Life Science
Research Limited (Unpublished report No. 90/AWL005/0469).
Jenkins WR (1991c) THPS -- Determination of its EC50 to Selenastrum
capricornutum under non-axenic conditions (Study sponsored by
Albright & Wilson). Eye, United Kingdom, Life Science Research Limited
(Unpublished report No. 90/AWL009/0473).
KAN-DO Office and Pesticides Team (1995) Accumulated pesticide and
industrial chemical finding from a ten-year study of ready-to-eat
foods. J Assoc Off Anal Chem, 78(3): 614-631.
Kawachi T, Komatsu T, Kada T, Ishidate M, Sasaki M, Sugiyama T, &
Tazima Y (1980a) Results of recent studies on the relevance of various
short-term screening tests in Japan: The predictive value of
short-term screening tests in carcinogenicity evaluation. Appl Methods
Oncol, 3: 253-267.
Kawachi T, Yahagi T, Kada T, Tazima T, Ishidate M, Sasaki M, &
Sugiyama T (1980b) Cooperative programme on short-term assays for
carcinogenicity in Japan. In: Montesano R, Bartsch H, & Tomatis L ed.
Molecular and cellular aspects of carcinogen screening tests, pp
323-330 (IARC Scientific Publications No. 27).
Kawagoshi Y & Fukunaga I (1994) Levels and features of
organophosphoric acid triesters at Osaka North Port Sea-Based solid
waste disposal site. J Environ Chem, 4(4): 797-804.
Kawagoshi Y & Fukunaga I (1995) Organophosphoric acid triesters in
leachate from solid waste disposal site. Kankyo Kagaku, 5: 390-391.
Kawai S, Fukushima M, Kitano M, & Morishita H (1985) Degradation of
organophosphoric acid triesters by the bacteria in the river water of
Osaka City. Annu Rep Osaka City Inst Public Health Environ Sci, 48:
175-183.
Kawai S, Fukushima M, Kitano M, Takayuki N, & Morishita H (1986)
Degradation of organophosphoric acid triesters by the bacteria in the
river water -- (ii) Properties of TBP (tributyl phosphate) degrading
bacteria and their enzymes. Annu Rep Osaka City Inst Public Health
Environ Sci , 49: 160-166.
Keith LH & Walters DB (1985) Compendium of safety data sheet for
research and industrial chemicals. New York, VCH Publishers Inc., part
III, pp 1630-1631.
Keith LH & Walters DB (1987) Compendium of safety data sheets for
research and industrial chemicals. New York, VCH Publishers Inc., part
IV, p 856.
Kimmerle G (1958) [Softener tri-2-ethylhexylphosphate (TOF).]
Leverkusen, Germany, Bayer AG, Laboratory of Toxicology and Pathology
(Unpublished report No. 1764) (in German).
Kluwe WM, Huff JE, Matthews HB, Irwin R, & Haseman JK (1985)
Comparative chronic toxicities and carcinogenic potentials of
2-ethylhexyl containing compounds in rats and mice. Carcinogenesis,
6(11): 1577-1583.
Komsta E, Secours VE, Chu I, Valli VE, Morris R, Harrison J,
Baranowski E, & Villeneuve DC (1989) Short-term toxicity of nine
industrials chemicals. Bull Environ Contamin Toxicol, 43: 87-94.
Krzymien M (1981) Sample collection and field analysis of
tris(2-ethylhexyl) phosphate used in a study of pesticide spray drift.
J Chromatogr, 204: 453-459.
Laham S, Long G, Schrader K, & Szabo J (1984a) Induction of electro
physiological and morphological changes in Sprague-Dawley rats fed
tributoxyethyl phosphate. J Appl Toxicol, 4(1): 42-48.
Laham S, Szabo J, & Long G (1984b) Short-term neurotoxicity studies on
tributoxyethyl phosphate orally administered to Sprague-Dawley rats.
Chemosphere, 13(7): 801-812.
Laham S, Long GW, & Broxup BR (1985a) Subchronic oral toxicity of
tributoxyethyl phosphate in the Sprague-Dawley rat. Arch Environ
Health, 40(1): 12-17.
Laham S, Szabo J, Long G, & Schrader K (1985b) Dose-response toxicity
studies on tributoxyethyl phosphate orally administered to
Sprague-Dawley rats. Am Ind Hyg Assoc J, 46(8): 442-448.
Lebel GL & Williams DT (1983a) Problems in collection of
representative samples for determination of tributoxyethyl phosphate
in potable water. J Assoc Off Anal Chem, 66(1): 202-203.
Lebel GL & Williams DT (1983b) Determination of organic phosphate
triesters in human adipose tissue. J Assoc Off Anal Chem, 66(1):
691-699.
Lebel GL & Williams DT (1986) Levels of triaryl/alkyl phosphates in
human adipose tissue from eastern Ontario. Bull Environ Contam
Toxicol, 37: 41-46.
Lebel GL, Williams DT, & Benoit FM (1981) Gas chromatographic
determination of trialkyl/aryl phosphates in drinking water, following
isolation using macroreticular resin. J Assoc Off Anal Chem, 64(4):
991-998.
Lebel GL, Williams DT, & Benoit FM (1987) Use of large volume resin
cartridges for the determination of organic contaminants in drinking
water derived from the Great Lakes. Adv Chem Ser, 214: 309-325.
Lebel GL ,Williams DT, & Berard D (1989) Triaryl/alkyl phosphate
residues in human adipose autopsy samples from six Ontario
municipalities. Bull Environ Contam Toxicol, 43: 225-230.
Leddy IA (1990) THPS-75: Chromosal aberrations assay with Chinese
hamster ovary cells in vitro (Project No.740444). Falls Church,
Virginia, Inveresk Research International (Unpublished report No.
6144).
Lenga RE (1993) The Sigma-Aldrich library of chemical safety data.
Buchs, Switzerland, Sigma-Aldrich, pp 1117-1118.
Leo A (1989) CLOGP-3.54 MedChem software 1989. Claremont, California,
Daylight Chemical Information Systems.
Lerche J & Morch J (1973) Qualitative and quantitative gas
chromatographic determination of plasticizers in polyvinyl chloride.
Arch Pharm OG Chem, 1: 25-30.
Liggett MP (1989a) Irritant effects on rabbit skin of THPS (Study
sponsored by Albright & Wilson). Peterborough, United Kingdom,
Huntingdon Research Center Limited (Unpublished report No.89361D/A&W
498/SE).
Liggett MP (1989b) Irritant effects on rabbit eye of THPS (Study
sponsored by Albright & Wilson). Peterborough, United Kingdom,
Huntingdon Research Center Limited (Unpublished report No. 89447D/A&W
499/SE).
Liggett MP & Allen SA (1989) Acute dermal toxicity to rats of THPS
(Study sponsored by Albright & Wilson). Peterborough, United Kingdom,
Huntingdon Research Center Limited.
Lloyd GR (1994) UV degradation of THPS. Oldbury, United Kingdom,
Albright & Wilson UK Ltd, Specialities Technical Laboratory.
Loewengart G & Van Duuren BL (1977) Evaluation of chemical flame
retardants for carcinogenic potential. J Toxicol Environ Health, 2:
539-546.
Loveday KS, Lugo MH, Resnick MA, Anderson BE, & Zeiger E (1989)
Chromosome aberration and sister chromatid exchange tests in Chinese
hamster ovary cells in vitro: II. Results with 20 chemicals. Environ
Mol Mutagen, 13: 60-94.
McDonald P & Anderson BT (1989) THPS acute inhalation toxicity study
in rats (Study sponsored by Albright & Wilson). Tranent, Edinburgh,
United Kingdom, Inveresk Research International.
MacFarland HN & Punte CL (1966) Toxicological studies on
tri-(2-ethylhexyl)-phosphate. Arch Environ Health, 13: 13-20.
MacGregor JT, Diamond MJ, Mazzeno LW Jr, & Friedman M (1980)
Mutagenicity tests of fabric-finishing agents in Salmonella
typhimurium : Fiber-reactive wool dyes and cotton flame retardants.
Environ Mutagen, 2: 405-418.
MacKeller DG (1978) Kp-140 tris(butoxyethyl) phosphate mutagenicity
screening test salmonella microsomal assay (Ames test). Princeton, New
Jersey, FMC Corporation (Report No. ICG/T-78-006).
McWilliam P (1994) Tolcide PS355A: Biodegradability in seawater
-- Closed bottle test (Study sponsored by Albright & Wilson). Bergen,
Norway, Terra Miljö-Laboratories A/S (Unpublished report No.
60090.aw\tm.002\biodeg).
Marshall R (1996) Trishydroxymethyl phosphine oxide: Induction of
chromosome aberrations in cultured chinese hamster ovary (CHO) cells.
Harrogate, United Kingdom, Corning Hazelton (Europe) (Unpublished
report No. 254/44-1052).
Mead C & Handley JW (1998) Assessment of ready biodegradability CO2
evolution test (modified sturm test) of Amgard TBEP (Project No.
071/607). Derby, United Kingdom, Safepharm Laboratories Ltd.
Miyagawa M, Takasawa H, Sugiyama A, Inoue Y, Murata T, Uno Y, &
Yoshikawa K (1995) The in vivo-in vitro replicative DNA synthesis
(RDS) test with hepatocytes prepared from male B6C3F1 mice as an early
prediction assay for putative nongenotoxic (Ames-negative) mouse
hepatocarcinogens. Mutat Res, 343: 157-183.
Monsanto (1976) Biodegradability of phosphate esters: Analytical
chemistry -- Special study AC-75-55-9. St Louis, Missouri, Monsanto
Industrial Chemicals, Applied Sciences.
Monsanto (1984a) Summary sheet: t-Butoxyethyl phosphate (Daphnia
magna). St Louis, Missouri, Monsanto Industrial Chemicals,
Environmental Sciences Toxicity.
Monsanto (1984b) Acute toxicity of TBEP to fathead minnow
(Pimephales promelas). Wareham, Massachusetts, Springborn Bionomics
Inc. (Unpublished report No. SB 89-9160, prepared for Monsanto
Industrial Chemicals, Environmental Sciences Toxicity, St Louis,
Missouri).
Monsanto (1984c) Tributoxyethyl phosphate: Acute toxicity/irritation
studies. St Louis, Missouri, Monsanto, Department of Medicine and
Environmental Health (Unpublished report No. BD-84-038).
Monsanto (1984d) Tributoxyethyl phosphate microbial (AMES)
mutagenicity. St Louis, Missouri, Monsanto, Department of Medicine and
Environmental Health (Unpublished report No. SR-84-143).
Monsanto (1984e) Repeat human insult patch test of tributoxyethyl
phosphate. St Louis, Missouri, Monsanto, Department of Medicine and
Environmental Health (Unpublished report No. SH-84-002).
Monsanto (1985a) Four-week feeding study of tributoxyethyl phosphate
in male and female Sprague-Dawley rats. St Louis, Missouri, Monsanto,
Department of Medicine and Environmental Health (Unpublished report
No. ML-84-093).
Monsanto (1985b) Twenty-one-day dermal toxicity study in rabbits with
tributoxyethyl phosphate. St Louis, Missouri, Monsanto, Department of
Medicine and Environmental Health (Unpublished report No. BD-84-130).
Monsanto (1985c) CHO/HGPRT mammalian cell forward gene mutation assay
with tributoxyethyl phosphate. St Louis, Missouri, Monsanto,
Department of Medicine and Environmental Health (Unpublished report
No. PK-84-408).
Monsanto (1985d) Range-finding teratology study in rats with
tributoxyethyl phosphate. St Louis, Missouri, Monsanto, Department of
Medicine and Environmental Health (Unpublished report No. IR-84-224).
Monsanto (1985e) Teratology study in rats with tributoxyethyl
phosphate. St Louis, Missouri, Monsanto, Department of Medicine and
Environmental Health (Unpublished report No. IR-84-225).
Monsanto (1986) Neurotoxicity studies of tributoxyethyl phosphate in
chickens. St Louis, Missouri, Monsanto, Department of Medicine and
Environmental Health (Unpublished report No. DU-84-358).
Monsanto (1987a) Eighteen-week feeding study of tributoxyethyl
phosphate with Sprague-Dawley rats. St Louis, Missouri, Monsanto,
Department of Medicine and Health Sciences (Unpublished report No.
ML-84-437 [EHL No. 84108]).
Monsanto (1987b) Peripheral nerve conduction after an eighteen-week
feeding exposure to tributoxyethyl phosphate in rats. St Louis,
Missouri, Monsanto, Department of Medicine and Health Sciences
(Unpublished report No. ML-84-435 [EHL No. 84110]).
Mount E (1991) KP-140 non-definitive acute inhalation toxicity study
in rats (Study No. 191-1208). Princeton, New Jersey, FMC Corporation,
Toxicology Laboratory.
Myhr BC & Caspary WJ (1991) Chemical mutagenesis at the thymidine
kinase locus in L5178Y mouse lymphoma cells: Results for 31 coded
compounds in the National Toxicology Program. Environ Mol Mutagen, 18:
51-83.
Nakashima H, Matsunaga I, & Miyano N (1993) Determination of tris
(2-butoxyethyl) phosphate in textiles and household wax products by
capillary gas chromatography. Jpn J Toxicol Environ Health, 39(6):
549-553.
O'Conner J (1992) THPS: Determination of hydrolysis as a function of
pH. Eye, United Kingdom, Life Science Research Limited (LSR report No.
91/AWL/015/0737, prepared for Albright & Wilson, International
Technical Center, Oldbury, United Kingdom).
Paxéus N, Robinson P, & Balmér P (1992) Study of organic pollutants in
municipal wastewater in Göteborg, Sweden. Water Sci Technol, 25(11):
249-256.
Piegorsch WW & Hoel DG (1988) Exploring relationships between
mutagenic and carcinogenic potencies. Mutat Res, 196: 161-175.
Riach CG (1994) THPS-75%: Assessment of genotoxicity in an unscheduled
DNA synthesis assay using adult rat hepatocyte primary cultures
-- Supplemental information to report MRID No. 4222363-17. Falls
Church, Virginia, Inveresk Research International (Unpublished
report).
Riach CG (1996) THPS mouse lymphoma mutation assay (Project No.
756936). Falls Church, Virginia, Inveresk Research International
(Unpublished report No. 12110).
Rivera J, Ventura F, Caixach J, de Torres M, & Figueras A (1987)
GC/MS, HPLC and FAB mass spectrometric analysis of organic pollutants
in Barcelona's water supply. J Environ Chem, 29: 15.
Roberts NL & Fairley C (1988) The dietary toxicity (LC50) of DP 435
to the mallard duck. Huntingdon, United Kingdom, Huntingdon Research
Center Limited (Unpublished report No. A&W 473/87982).
Roberts NL & Phillips C (1988a) The dietary toxicity (LC50) of DP 435
to the Bobwhite quail. Huntingdon, United Kingdom, Huntingdon Research
Center Limited (Unpublished report No. A&W 474/871332).
Roberts NL & Phillips C (1988b) The acute oral toxicity (LD50) of DP
435 to the mallard duck. Huntingdon, United Kingdom, Huntingdon
Research Center Limited (Unpublished report No. A&W 475/87999).
Roddie B (1994) Assessment of the sediment-phase toxicity of Tolcide
PS 75 to the sediment-dwelling amphipod Coronium volutator.
Edinburgh, United Kingdom, Environment & Resource Technology Limited
(Unpublished report No. ERT 94/005/V1).
Saeger VW, Hicks O, Kaley RG, Michael PR, Mieure JP, & Tucker ES
(1979) Environmental fate of selected phosphate esters. Environ Sci
Technol, 13(7): 840-844.
Saitoh M, Umemura T, Kawasaki Y, Momma J, Matsushima Y, Matsumoto M,
Eshita N, Isama K, & Kaniwa M (1994) Subchronic toxicity study of
tributoxyethyl phosphate in Wistar rats. Eiser Shikensko Hokoku, 112:
27-39.
Sasaki M, Sugimura K, Yoshida MA, & Abe S (1980) Cytogenic effects of
60 chemicals on cultured human and Chinese hamster cells. Senshokutai,
20: 574-584.
Shelby MD & Witt KL (1995) Comparison of results from mouse bone
marrow chromosome aberration and micro nucleus tests. Environ Mol
Mutagen, 25: 302-313.
Shelby MD, Erexson GL, Hook GJ, & Tice RR (1993) Evaluation of a three
exposure mouse bone marrow micronucleus protocol: Results with 49
chemicals. Environ Mol Mutagen, 21: 160-179.
Sheldon LS & Hites RA (1978) Organic compounds in the Delaware River.
Environ Sci Technol, 12(10): 1188-1194.
Smyth HF & Carpenter CP (1948) Further experience with the range
finding test in the industrial toxicology laboratory. J Ind Hyg
Toxicol, 30(1): 63-68.
Snell K (1994) Proban CC: Acute dermal irritation test in the rabbit
(Project No. 71/344, sponsored by Albright & Wilson). Derby, United
Kingdom, Safepharm Laboratories Ltd.
Thompson PW (1997a) THPS cholinesterase inhibition test (Project No.
071/506, sponsored by Albright & Wilson). Derby, United Kingdom,
Safepharm Laboratories Ltd.
Thompson PW (1997b) THPO cholinesterase inhibition test (Project No.
071/507, sponsored by Albright & Wilson). Derby, United Kingdom,
Safepharm Laboratories Ltd.
Torp UM & McWilliam P (1994) Tolcide PS75: Determination of acute
lethal toxicity to Acartia tonsa. Bergen, Norway, Terra
Miljö-Laboratories A/S (Unpublished report No.
60090.aw\tm.001\acartia).
Tou JC, Westover LB, & Sonnabend LF (1974) Kinetic studies of
bis(chloromethyl)ether hydrolysis by mass spectrometry. J Phys Chem,
78(11): 1096-1098.
Tremain SP & Bartlett AJ (1994) Amgard TBEP determination of hazardous
physico-chemical properties (Project No. 583/014, sponsored by
Albright & Wilson). Derby, United Kingdom, Safepharm Laboratories Ltd.
Tsuda M, Saito M, Umemura T, Kawasaki Y, Momma J, Matsushima Y,
Matsumoto M, Isama K, Kawiwa M, & Kurokawa Y (1993) A 14-week oral
toxicity study of tributoxyethyl phosphate (TBEP) in rats. J Toxicol
Sci, 18(4): 421, 429.
Tsuji S, Tonogai Y, Ito Y, & Kanoh S (1986) The influence of rearing
temperatures on the toxicity of various environmental pollutants for
killifish (Oryzias latipes). Eisei Kagaku, 32(1): 46-53.
Tuffnell PP (1991) Proban CC: Acute oral toxicity test in the rat
(Study sponsored by Albright & Wilson). Derby, United Kingdom,
Safepharm Laboratories Ltd.
Tuffnell PP (1992) Proban CC: Magnusson & Kligman maximisation study
in the guinea pig (Project No. 71/127, sponsored by Albright &
Wilson). Derby, United Kingdom, Safepharm Laboratories Ltd.
Ulsamer AG, Osterberg RE, & McLaughlin J Jr (1980) Flame-retardant
chemicals in textiles. Clin Toxicol, 17(1): 101-131.
US NTP (1984) Toxicology and carcinogenesis studies of
tris(2-ethylhexyl)phosphate (CAS No. 78-42-2) in F344/N rats and
B6C3F1 mice (gavage studies). Research Triangle Park, North Carolina,
US Department of Health and Human Services, National Toxicology
Program (Technical Report Series No. 274; NIH Publication No.
84-2530).
US NTP (1987) Toxicology and carcinogenesis studies of
tetrakis(hydroxymethyl) phosphonium sulfate (THPS) (CAS No.
55566-30-8) an tetrakis(hydroxymethyl) phosphonium chloride (THPC)
(CAS No. 124-64-1) in F344/N rats and B6C3F1 mice (gavage studies).
Research Triangle Park, North Carolina, US Department of Health and
Human Services, National Toxicology Program (Technical Report Series
No. 29; NIH Publication No. 87-2552).
Van Duuren B, Loewengart G, Seidman I, Smith A, & Melchionne S (1978)
Mouse skin carcinogenicity tests of the flame retardants
tris(2,3-dibromopropyl) phosphate, tetrakis(hydroxymethyl) phosphonium
chloride, and polyvinylbromide. Cancer Res, 38: 3236-3240.
Watts CD & Moore K (1988) Fate and transport of organic compounds in
rivers. In: Angeletti G & Bjorseth A ed. Organic micropollutants in
the aquatic environment: Proceedings of the 5th European Symposium,
Rome, 20-22 October 1987. Boston, Massachusetts, Kluwer Academic
Publishers, pp 154-169 (Water Pollution Research Reports Series, No.
4; EUR 11350).
Weber K & Ernst W (1983) [Presence and fluctuation of organic
environmental chemicals in German estuaries.] Vom Wasser, 61: 111-123
(in German).
Weil ED (1980) Kirk-Othmer encyclopedia of chemical technology. New
York, John Wiley and Sons, vol 10, pp 488-489.
Weschler CJ (1984) Indoor-outdoor relationships for nonpolar
constituents of aerosol particles. Environ Sci Technol, 18(9):
648-652.
Weschler CJ (1980) Characterization of selected organic compounds in
size-fractionated indoor aerosols. Environ Sci Technol, 14(4):
428-431.
Weschler CJ & Fong KL (1986) Characterization of organics species
associated with indoor aerosol particles. Environ Int, 12: 93-97.
Weschler CJ & Shields HC (1986) The accumulation of "Additives" in
office air -- Proceedings of the 79th Annual Meeting of the Air
Pollution Control Association, Minneapolis, 22-27 June 1986.
Pittsburgh, Pennsylvania, Air Pollution Control Association.
Wetton PM & Handley JW (1998) Acute toxicity to Rainbow trout (Project
No. 071/608R, sponsored by Albright & Wilson). Derby, United Kingdom,
Safepharm Laboratories Ltd.
Williams DT, Nestmann ER, Lebel GL, Benoit FM, Otson R, & Lee EGH
(1982) Determination of mutagenic potential and organic contaminants
of Great Lakes drinking water. Chemosphere, 11(3): 263-276.
Willis CV (1995) Tolcide PS75 final report. Physical and chemical
characteristics of Tolcide PS75: vapor pressure (Study sponsored by
Albright & Wilson). Whippany, New Jersey, Case Consulting Laboratories
Inc.
Wragg MS, Thomas ON, & Brooks PN (1996) Twenty-eight day subacute
dermal toxicity study in the rat (Sponsored by Albright & Wilson).
Derby, United Kingdom, SafePharm Laboratories Ltd. Derby UK.
Yasuda H (1980) Concentration of organic phosphorus pesticides in the
atmosphere above the Dogo and Ozun Basin. J Chem Soc Jpn, 4: 645-653.
Zeiger E, Anderson B, Haworth S, Lawlor T, Mortelmans K, & Speck W
(1987) Salmonella mutagenicity test: III. Results from the testing of
255 chemicals. Environ Mutagen, 9(suppl 9): 1-110.
Zeiger E, Haworth S, Mortelmans K, & Speck W (1985) Mutagenicity
testing of di(2-ethylhexyl)phthalate and related chemicals in
Salmonella. Environ Mutagen, 7: 213-232.
Appendix A
Flammability standards met by products treated by THPC-urea
condensates a
Items Flammability standards
Protective clothing ASTM F-1506
ASTM F-955
NFPA 1975
NFPA 1977
EN 533 : 1977 Index 3 after 50 washes at 75°C
EN 531 : 1995 para. 6.2.2 after 50 washes at 75°C
EN 470-1 : 1995 para. 6.1 after 50 washes at 75°C
EN 470-1 : para. 6.2
EN 531 : 1995 para. 6.3
EN 531 : 1995 para. 6.4
EN 531 : 1995 para. 6.6
Sheeting, blankets and Ingnition sources 0, 1 and 5.
BS 7175 counterpanes When tested on top of and below the test
fabric, after 200 washes at 74°C (BS 5651 HLPN).
BS 5815 : Part 3 : 1991
Curtains and drapes NFPA 701
BS 5867 : 1980 Part 2 Type B after 200 washes
at 74°C (BS 5651 HLPN).
Mattress ticking and Ignition sources 0, 1 and 5 when tested
BS 7175 mattresses on top of and below the test fabric, after 3
washes at 74°C
BS 597-1 : 1995 (fabric tested in combination
with a non-fire-retardant polyurethane foam block).
BS 597-2 : 1995 (fabric tested in combination with a
non-fire-retardant polyurethane foam block).
Sleepwear DOC FF 3-71
BS 5722 : 1984 when testing in accordance with
BS 5438 : 1976 Test 2.
BS 5722 : 1991 Level 1.
Upholstery BS 5852 part 1 Ignition sources 0 (smouldering
cigarette) and 1 (simulated match) after a 30-min
water soak, BS 5651 amended.
EN 1021-1 : 1994 (fabric tested in combination with
a non-fire-retardant polyurethane foam block).
EN 1021-2 : 1994 (fabric tested in combination with
a non-fire-retardant polyurethane foam block).
a From: Dr P. Martin, Albright & Wilson, personal communication to IPCS
RÉSUMÉ, EVALUATION ET RECOMMANDATIONS
1. Phosphate de tris(2-butoxyéthyle) (TBEP)
1.1 Résumé
Le phosphate de tris(butoxyéthyle) ou TBEP est utilisé pour la
confection d'encaustiques destinés à l'entretien des sols ou encore
comme plastifiant dans les caoutchoucs et les matières plastiques. On
ne connaît pas le volume de la production annuelle mondiale, mais on
pense qu'il est de l'ordre de 5000 à 6000 tonnes.
La présence du TBEP dans l'environnement résulte exclusivement de
l'activité humaine. Sa répartition dans l'environnement a été étudiée
dans certains pays industrialisés. On a trouvé une concentration
inférieure à 300 ng/litre dans les eaux de surface et comprise entre
100 et 1000 µg/kg dans les matières particulaires. Aucune des 167
analyses effectuées n'a permis d'en mettre en évidence dans les
poissons. Une unique étude en a décelé sa présence dans l'air
extérieur (< 200 ng/m3). Le dosage du TBEP dans l'air de bureaux a
donné une concentration de 25 ng/m3 tout au plus. Le TBEP est associé
aux matières particulaires et la source en est dans ce cas les
encaustiques que l'on applique sur le sol. On l'a décelé à des
concentrations de l'ordre du µg/kg dans les tissus adipeux humains.
D'après des études basées sur le panier de la ménagère, la dose
journalière ingérée serait de moins de 0,02 µg/kg de poids corporel
pour diverses tranches d'âge. On en a également signalé la présence
dans l'eau de boisson à des concentrations pouvant atteindre 270
µg/litre. Elle est due, semble-t-il, à la migration du produit contenu
dans les joints de caoutchouc de l'installation sanitaire.
On estime que le TBEP est facilement biodégradable. Des mesures
effectuées dans des stations d'épuration des eaux d'égout et des
dosages pratiqués en semi-continu au laboratoire sur des boues de même
provenance montrent que le TBEP s'élimine en grande partie (> 80 %).
Dans les cours d'eau et les eaux littorales, le TBEP est totalement
décomposé. On a fait état d'une demi-vie d'environ 50 jours dans les
eaux estuarielles, la décomposition étant minime dans l'eau de mer non
adaptée.
Le composé présente une faible toxicité aiguë pour les mammifères
et son pouvoir irritant est également faible.
Plusieurs études subchroniques sur des animaux de laboratoire
montrent que la toxicité du TBEP s'exerce au niveau du foie qui en est
l'organe cible. Une étude sur des rats Sprague-Dawley incite à penser
que le TBEP pourrait provoquer une myocardite focale. Des effets
neurologiques ont été observés chez le rat après ingestion d'une seule
dose, mais ils n'apparaissent pas systématiquement. Lorsqu'on
l'administre répétitivement à forte dose à des rats par gavage, le
TBEP réduit la vitesse de conduction nerveuse et augmente la durée de
la période réfractaire. Après administration à des poules, on n'a pas
constaté de neurotoxicité retardée, mais il y avait par contre
inhibition de la cholinestérase cérébrale et plasmatique.
Une étude de 18 semaines au cours desquelles des rats ont reçu
des doses répétées de TBEP a permis de fixer à 15 mg.kg-1j-1 pc la
dose sans effet observable (NOEL) sur le foie, la dose la plus faible
produisant un effet observable (LOEL) étant de 150 mg.kg-1.j-1 pc.
Ni la toxicité à long terme ni le pouvoir cancérogène de ce
composé n'ont été étudiés.
Les tests de mutation génique effectués sur des cellules
mammaliennes et sur des bactéries n'ont donné que des résultats
négatifs mais il n'existe aucun compte rendu de recherche de lésions
chromosomiques.
Une étude effectuée sur des rats n'a révélé aucun effet
tératogène. Il n'existe pas de publication faisant état d'autres
effets toxiques sur la reproduction.
Les tests de sensibilisation effectués sur des sujets humains par
apposition d'un timbre cutané ne font ressortir aucune sensibilisation
mais seulement une légère irritation.
Le TBEP est modérément toxique pour les organismes aquatiques. La
CL50 à 48 h pour Daphnia magna est de 75 mg/litre et la CL50 à 96 h
se situe entre 16 et 24 mg/litre pour les poissons.
1.2 Evaluation
L'exposition sur les lieux de travail se produit
vraisemblablement au niveau de la peau pendant la fabrication ou
l'utilisation d'encaustiques pour sols (exposition accidentelle). Le
composé est absorbé par voie percutanée chez l'animal de laboratoire,
mais on ne possède aucune information sur sa cinétique ou son
métabolisme. On ne peut donc pas évaluer quantitativement l'exposition
par cette voie, mais on peut penser qu'elle doit être faible. Les
mesures montrent que l'exposition par la voie respiratoire dans un
bureau est au plus égale à 25 ng/m3.
L'exposition de la population générale s'opère principalement par
la voie alimentaire, (du fait que le TBEP est présent comme
plastifiant dans les plastiques utilisés pour l'emballage des produits
alimentaires) et par la consommation d'eau de boisson contaminée par
le TBEP contenu dans les joints de caoutchouc synthétique de la
plomberie. Elle est cependant très faible dans les deux cas (estimée à
moins de 0,2 µg par kg pc et par jour avec une concentration dans
l'eau de boisson inférieure à 270 µg/litre).
Compte tenu de la valeur de la NOEL tirée des études sur l'animal
(15 mg.kg-1.j-1, valeur obtenue après administration répétée du
composé par voie orale), on peut considérer que le risque est très
faible pour la population générale. On estime qu'il est également très
faible sur les lieux de travail, encore qu'il ne soit pas possible
d'en donner une évaluation chiffrée.
Dans l'environnement, on peut déduire de la faible volatilité, du
fort coefficient d'adsorption et de sa solubilité modérée dans l'eau,
que le TBEP va se répartir entre les différents types de matières
particulaires. Les quelques mesures dont on dispose confirment cette
hypothèse. Sa décomposition dans les différents compartiments de
l'environnement devrait être rapide. On ne dispose d'aucune donnée sur
ces produits de décomposition ; le reste phosphate libéré au cours de
ce processus ne devrait pas sensiblement augmenter la concentration
globale des nutriments présents dans l'environnement. La Fig. 1 donne
la valeur de la concentration relevée dans les eaux de surface en
fonction de la toxicité aiguë observée. Il y a plusieurs ordres de
grandeur entre la concentration la plus élevée mesurée et la plus
faible de la toxicité observée, d'où une marge de sécurité élevée et
donc un faible risque pour les organismes aquatiques. On n'est pas en
mesure d'évaluer l'importance du risque pour les organismes
terrestres.
Relative molecular mass: 300 for the repeat unit shown above
CAS registry number: 27104-30-9
CAS name: Phosphonium, tetrakis(hydroxymethyl)-
chloride, polymer with urea
Trade names: Proban CC; Retardol AC
Proban 210 is no longer produced.
C2.2 Physical and chemical properties
Physical and chemical properties are given in Table 8.
C2.2.1 Technical products
THPC and THPS are marketed in concentrated aqueous solutions at
approximately 80 and 75% (by weight), respectively (Albright & Wilson,
personal communication to IPCS). Typically THPS is marketed with less
then 1% of formaldehyde content (Albright & Wilson, personal
communication to IPCS). In the past values ranging from 3.79% at pH
0.4 to 14.1% at pH > 5.0 have been reported (Loewengart & Van Duuren,
1977; Ulsamer et al., 1980). Tetrakis(hydroxymethyl) phosphonium
acetate/phosphate (THPA/P) was previously available in the USA as a
clear, nearly colourless solution with a pH of approximately 5,
containing 10% active phosphorus (Hooper et al., 1976).
C2.3 Conversion factors
THPC 1 ppm = 7.76 mg/m3
1 mg/m3 = 0.128 ppm
THPS 1 ppm = 16.61 mg/m3
1 mg/m3 = 0.0602 ppm
C2.4 Analytical methods
A standard method for THPS and THPC determination is by iodine
titration. However, this not substance specific and is therefore
subject to interference by many other chemicals that may be present in
the sample to be analysed. The method involves dilution in water
containing an aliquot of a saturated solution of disodium hydrogen
orthophosphate. A solution of polystyrene sulfonic acid is then added
followed by a few drops of a starch indicator. Titration against a
previously standardized iodine solution is then carried out (Albright
& Wilson, personal communication to IPCS).
The most accurate analytical technique for the quantitative
substance-specific determination of THP salts is currently ion
chromatography. In this method the sample is chromatographed using an
Ionpac CS5 column with a CG5 guard column. The mobile phase is
hydrochloric acid (0.1 mol/litre) at 1 ml/min. The ion chromatography
separates the THP salt from any free formaldehyde, which is largely
retained. The THP salt is then detected using a visible wavelength
detector at 425 nm following a post-column reaction with an
acetylacetone reagent containing acetic acid, ammonium acetate and
acetyl acetone. The reagent breaks down the THP salt to form free
formaldehyde, which forms a cyclic coloured complex. Free formaldehyde
also reacts but two separate and distinct peaks are seen on the
chromatogram.
Table 8. Physical and chemical properties of THP salts
Parameter THPS THPC THPC-urea copolymer
100% 75% 80-85%
Appearance Soft waxy solid Colourless liquid Clear straw-coloured Straw-coloured liquid
liquid
Odour Resembles Resembles aldehyde Pungent Pungent
aldehyde
Boiling point (°C) 108.5 115
Melting point (°C) 54.2-81.5 -21
Flash point >100
Vapour pressure <2.6 × 10-4 Pascal 26.7 mmHg at 25°C
at 20°C
Viscosity 38 cStp at 25°C 0.27 Pa.s at 29°C
pH 3.19 (0.01 M solution) < 2 5
Stability 21°C -- stable for 21°C -- stable for 14 days Stable under normal Stable under normal
14 days conditions conditions
54°C -- stable for 54°C -- stable for 14 days
14 days
Table 8. (continued)
Parameter THPS THPC THPC-urea copolymer
100% 75% 80-85%
Decomposition Oxides of sulfur, phosphorus Oxides of phosphorus; Oxides of phosphorus;
products and carbon; phosphine chlorine, ammonia chlorine, ammonia
Relative density 1.53 1.39 1.34 1.31
Solubility Infinitely soluble in Completely soluble Completely soluble Miscible with water
water
Log n-octanol/water -9.8 (calculated)
partition coefficient
From: Cowlyn (1991a,b); Antony (1993); Barth (1994); Willis (1995)
C3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURES
C3.1 Natural occurrence
These compounds are not known to occur as natural products.
C3.2 Anthropogenic sources
C3.2.1 Production levels and processes
THP salts have been produced for commercial use since the 1950s.
The first of these, THPC, was introduced in 1953.
THP salts are synthesized in high yields through the reaction of
formaldehyde with phosphine and the corresponding acid in an enclosed
process (Weil, 1980; Hawley, 1981).
PH3 + HCl + 4CH2O --> [(HOCH2)4P]Cl
The resulting products exist in an equilibrium with THP+, which
is highly pH dependent. Increasing the pH shifts this equilibrium to
the right with the resultant production of formaldehyde, i.e., one of
the methylol groups from the THP salt becomes hydrolysed.
Currently there is one major producer of these salts and
THPC-urea condensates in the United Kingdom and some production
potential in the USA. Combined worldwide production of THP salts is
greater than 3000 tonnes; the urea-condensate production is around
3000 tonnes annually of which 40% is consumed in the USA (Albright &
Wilson, personal communication to IPCS).
C3.2.2 Uses
THPC-based products represent the major class of chemicals used
as flame retardants for cotton, cellulose and cellulose-blend fabrics.
Until 1976, THPC was the major THP salt used as a flame retardant. In
addition, THPS and some mixed salts were commercially available.
THPC-based flame retardants have been found to be more reactive
and efficient as flame retardants when compared with similar
THPS-based products (Albright & Wilson, personal communication to
IPCS). Nowadays, the THPC-urea condensates dominant the market for
flame retardant treatment of cellulose and cellulose-blend fabrics
where durability to laundering and dry cleaning is required.
It has been suggested that bis(chloromethyl) ether (BCME) may be
formed during the manufacture of THPC and thus may present an
occupational hazard (Loewengart & Van Duuren, 1977). However, an
extensive airborne monitoring survey coupled with chemical analysis
(using mass spectroscopy) conducted at the United Kingdom
manufacturing site during 1975 did not detect the presence of BCME at
the minimum level of analytical detection of 2.35 µg/m3 (0.5 ppb).
These results were later independently confirmed in a separate survey
carried out by the United Kingdom Factory Inspectorate (personal
communication by R. Williams, HM Inspector of Factories, United
Kingdom Health and Safety Executive to Albright & Wilson, 1975).
The results of the two occupational hygiene surveys are supported
by a study in 1974 in which kinetic measurements in aqueous media
(manufacture is via an aqueous route) showed that BCME undergoes rapid
hydrolysis with a half-life of approximately 10-40 seconds at ambient
temperature (Tou et al.,1974). BCME, if formed, would therefore exist
as a transient species in aqueous media containing formaldehyde and
hydrochloric acid, or other acid chlorides.
The major application of THPS now is as a biocide in a variety of
preservative applications which include leather, textile, paper and
photographic films, as well as industrial water treatment and offshore
oil production processes.
C4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION AND TRANSFORMATION
C4.1 Transport and distribution between media
A field study was conducted on the use of THPS as a biocide in
the water of an industrial cooling tower. THPS was added to produce an
initial concentration of approximately 100 mg/litre and lithium
chloride was added simultaneously as a marker for determination of
dilution volumes in the tower and associated drainage systems.
Analysis of THPS was by HPLC. The half-life of lithium (and therefore
of THPS by dilution) was calculated to be 3 days. THPS levels were
found to decrease more rapidly than simple dilution would have
suggested, indicating hydrolysis; actual concentrations in the Rhyne,
the drainage channel of the cooling tower, were <0.5 mg/litre
(Heaton, 1991).
Adsorption of radiolabelled THPS was studied using agricultural
sand, silt loam, sandy loam, pond sediment and marine sediment; the
percentage of organic carbon in the tested soil/sediments ranged from
0.17 to 1.1%. Estimated Koc values ranged from 72 to 266 (mean
153 ± 69.2), indicating medium-to-high mobility for the compound
(Heim, 1998).
C4.2 Transformation
C4.2.1 Biodegradation
Inherent biodegradability of THPS was assessed using the OECD
302B guideline; >20% degradation occurred within a 28-day period
(Douglas & Pell, 1985).
Aquatic aerobic degradation of THPS was assessed in a soil/water
system under US EPA Guidelines. The soil was dosed with THPS at
1 µg/g. The compound was metabolized with 60% of applied radioactivity
appearing as CO2 within 7 days. Major metabolites were
trihydroxymethyl phosphine oxide (THPO) and bishydroxymethyl
phosphonic acid (BMPA), which were both found in the water; neither
degradate reached a concentration of 10% of the applied dose (Gorman,
1996). A comparable study at the same initial concentration of THPS
but under anaerobic conditions also showed 60% degradation within 30
days, and the same breakdown products were identified (Gorman, 1997).
Natural seawater was dosed with THPS to an initial concentration
of 4.16 mg/litre. Biodegradation (measured as oxygen demand) reached
17.7% after 28 days. A parallel toxicity test showed that the
substance was inhibitory to bacteria at the concentration used in the
test (McWilliam, 1994).
C4.2.2 Abiotic degradation
Laboratory studies using UV light showed that THPS photodegrades
to THPO when at low concentrations in aqueous solution. Conversion to
THPO was almost complete at concentrations up to about 20 mg/litre
within 2 h. Conversion also took place in synthetic seawater.
Photodegradation was pH-dependent, with greater conversion at
environmentally relevant pH. Exposure to natural sunlight showed high
levels (not stated) of conversion over a 3-month period (Lloyd, 1994).
Hydrolysis of THPS is pH-dependent; half-lives for the compound
at 25°C were 131, 72 and 7 days at pH 5, 7 and 9, respectively
(O'Connor, 1992).
C4.3 Migration from textiles
A method is available for the determination of the fixation
efficiency of the THPC-urea copolymer in terms of the percentage
phosphorus applied and fixed during the flame retardant treatment of
various textiles. A fixation efficiency of greater than 90% is common
for the PROBANR process (Albright & Wilson, personal communication
to IPCS). The THPC-urea copolymer is chemically converted to a
water-insoluble polymer of high relative molecular mass during the
textile processing, first by exposure to ammonia in an enclosed
chamber and then by an oxidation process involving hydrogen peroxide.
This latter process converts any phosphorus to the inert pentavalent
form. At the same time the hydrogen peroxide will convert any unfixed
THPC-urea copolymer to tetrakis(hydroxymethyl) phosphine hydroxide
(THPOH), which is subsequently removed from the fabric during the
final washing-off steps.
Wetting the treated fabric with water, beverages, liquid foods or
urine does not release the flame retardant polymer. The lack of water
solubility and the physical entrapment of the polymer inside the
fibres (which make up the treated fabric) make the polymer resistant
to removal by cleaning materials that a consumer might normally employ
to clean these flame-retardant-treated articles.
Industrial work clothing treated with THPC-urea condensate flame
retardants have been shown to be flame resistant after 50-100 washings
or dry cleanings, demonstrating the durability of the polymer to
leaching and washing (Albright & Wilson, personal communication to
IPCS).
Appendix A lists flammability standards met regularly by products
treated with THPC-urea condensates.
C5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
Approximately 20 workers are currently potentially exposed during
production of THP salts and THPC-urea condensate in the United Kingdom
(Albright & Wilson, personal communication to IPCS). No data on levels
of exposure are available.
C6. KINETICS AND METABOLISM IN LABORATORY ANIMALS
A metabolism study on rats has been conducted using
14C-radiolabelled THPS. THPS was not found in rat urine. However,
three metabolites were present, identified as trihydroxymethyl
phosphine oxide, bishydroxymethylphosphonic acid and possibly a
formaldehyde adduct of the trihydroxy compound (Dr P. Martin, Albright
& Wilson, personal communication to IPCS).
C7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
C7.1 Single exposure
C7.1.1 Oral
Acute oral toxicity results are given in Table 9.
Table 9. Oral LD50 values for THPC, THPS and THPC-urea condensate in
mice and rats (mg/kg body weight) a
Species Sex THPCb THPSc THPC-urea
Mice female 280 200 (none died) 400 (all died)
Rats male 282
Rats both sexes 575 962
Rats male 185 333
Rats female 161 248
a From Ulsamer et al. (1980); US NTP (1987); Tuffnell (1991);
Guest (1994a)
b 75% solution in water
c 72% solution in water
In THPC-treated rats that survived, reddish fluids around the
nostrils and laboured breathing were observed. All mice were lethargic
and had a rough coat. With THPS no signs of intoxication were noted
(Ulsamer et al., 1980; US NTP, 1987).
C7.1.2 Dermal
The dermal LD50 value in albino rabbits was greater than 4084 mg
THPC/kg body weight after a 24-h exposure. Erythema and oedema of the
integumentary system were observed (US NTP, 1987).
No deaths occurred when rats were treated dermally with THPS or
THPC-urea at 2000 mg/kg body weight (Liggett & Allen, 1989; Snell,
1994).
C7.1.3 Inhalation
The LC50 4-h value for THPS was 5.5 mg/litre in rats exposed to
respirable aerosol (nose only) (McDonald & Anderson, 1989).
C7.2 Repeated exposure
C7.2.1 Oral
C7.2.1.1 THPC
Groups of five 6-week-old, F-344/N rats of each sex were
administered 0, 9.4, 18.8, 37.5, 75 or 150 mg THPC (as a 75% aqueous
solution) per kg body weight in deionized water by gavage for 14 days.
At the two highest dose levels an increased mortality was observed. At
75 mg/kg the mortality for males was 20%, and at 150 mg/kg all males
and females died. The body weight gain of the male animals
administered 18.8 and 37.5 mg/kg was decreased, respectively, by 7 and
11%. This effect was also found in the females administered 75 mg/kg
(27%). Rats with 150 mg/kg had yellow to tan or mottled red livers. No
histopathology was carried out (US NTP, 1987).
Groups of ten 4-week-old F-344/N rats of each sex were gavaged 5
days/week for 13 weeks with 0, 3.75, 7.5, 15, 30 or 60 mg THPC (as a
75% aqueous solution) per kg. All males and 5/10 females that received
60 mg/kg died. The final mean body weight of males that received 30
mg/kg was 89% of vehicle controls. The final mean body weight of
females that received 60 mg/kg was 80% of vehicle controls.
At the highest dose level, clinical signs of toxicity included
rough coat, hunched back, diarrhoea, lethargy, paresis and
hyperextension of back legs. Periportal hepatocellular necrosis was
observed in 9/10 males and 7/10 females that received 15 mg/kg, all
males and females that received 30 mg/kg, and 7/10 males and 8/10
females that received 60 mg/kg.
Periportal cytoplasmic vacuolization was observed in 8/10 males
that received 7.5 mg/kg, 9/10 males and 8/10 females that received
15 mg/kg, and all rats that received 30 or 60 mg/kg. Degeneration of
the axons was found in 2/10 females that received 60 mg/kg. The NOAEL
for this study was 2.7 mg/kg body weight per day; the LOAEL was 5.4
mg/kg body weight per day (US NTP, 1987).
Groups of five 5-week-old B6C3F1 mice of each sex were
administered 0, 18.8, 37.5, 75, 150 or 300 mg THPC (as a 75% aqueous
solution) per kg body weight by gavage for 14 consecutive days. At the
highest dose level all mice died. At 150 mg/kg, body weight was
depressed by 18% in males and 20% in females compared to control mice.
No clinical sign of toxicity were observed in animals surviving to the
end of the study. No compound-related effects were observed at
necropsy (US NTP, 1987).
Groups of ten 4-week-old B6C3F1 mice of each sex were
administered by gavage 0, 1.5, 4.5, 15, 45 or 135 mg/kg body weight
5 days/week for 13 weeks. Seven of 10 males and 6/20 females that
received 135 mg/kg died. The final mean body weight for mice that
received 135 mg/kg was 8% lower than that of the controls for males
and 19% lower for females. Paresis of the hind legs and loss of
coordination were observed in all males and 9/10 females that received
135 mg/kg. Mice in this group also had marked axonal degeneration,
characterized by swollen axon sheaths, missing fragmented axons and
some proliferation of neurolemmal cells in the sciatic nerve, dorsal
roots of the caudal spinal nerves and tracts of the spinal cord
particularly in the dorsal column of the lumbar cord. Intracytoplasmic
vacuoles were seen in periportal hepatocytes of all mice in the 15, 45
and 135 mg/kg groups. The NOAEL for this study was 3.2 mg/kg body
weight per day; the LOAEL was 10.7 mg/kg body weight per day (US NTP,
1987).
C7.2.1.2 THPS
Group of 12 male ICR Swiss mice were administered daily by gavage
THPS in saline (2, 10 or 50 mg/kg body weight) for 14 days. In the
high-dose group 75% of the animals died (Connor et al., 1980).
Groups of five, 5-week-old, B6C3F1 mice of each sex were
administered 0, 12.5, 25, 50, 100 or 200 mg THPS (obtained as a 72%
aqueous solution) per kg body weight by gavage for 14 consecutive
days. Mice of the two highest dose groups had increased mortality at
100 mg/kg (20% male, 40% female) and at 200 mg/kg (80% male, 100%
female). At 25 mg/kg or more there was a decrease in body weight gain.
The animals given 100 or 200 mg/kg had laboured breathing and rough
coat and female mice of these groups also showed loss of movement in
their hind legs (US NTP, 1987).
Groups of 10, 5- to 6-week-old B6C3F1 mice of each sex were
administered 0, 5, 10, 20, 40 or 60 mg THPS (obtained from a 72%
aqueous solution) per kg body weight by gavage on 5 days/week for 13
weeks. One of 10 females that received 60 mg/kg and 2/10 males and
1/10 females that received 40 mg/kg died. The final mean body weights
of mice that received 20, 40 or 60 mg/kg were 4%, 7% or 11%,
respectively, lower than those of the controls for males and 3%, 5%
and 11% lower for females. Periportal vacuolar degeneration occurred
in all male and female mice that received 60 mg/kg, all males and 9/10
females that received 40 mg/kg, and 8/10 male mice that received 20
mg/kg. The NOAEL for this study was 7.1 mg/kg body weight per day, and
the LOAEL was 14.3 mg/kg body weight per day (US NTP, 1987).
Groups of five, 4-week-old, F-344/N rats of each sex were
administered 0, 12.5, 25, 50, 100 or 200 mg THPS (obtained as a 72%
aqueous solution) per kg body weight by gavage for 14 consecutive
days. All rats given 100 or 200 mg/kg died. Animals that received 25
or 50 mg/kg gained less weight than controls by 11% and 21%,
respectively, in males and 1% and 7% in females. The animals given the
two highest dose levels showed tremors, and one animal had partial
loss of movement of the hind legs. At necropsy no abnormalities were
seen (US NTP, 1987).
In an oral 28-day study, Charles River derived CD rats (five
females and five males/group) were given by gavage 6, 30 or 60 mg THPS
(75% obtain as aqueous solution) per kg body weight daily. At the
highest dose level, one male rat died on day 20, one female rat on day
21 and the remaining rats on day 22. Post-dose salivation, emaciation,
hypoactivity, hunched posture, noisy breathing, urogenital staining
and ptosis were seen only in the 60 mg/kg group. There was severe
weight loss during week 3 (body weight 52% for male and 74% for female
relative to controls at the end of week 3). The NOEL was considered to
be 6 mg/kg body weight per day (Hill, 1989).
Groups of ten, 5- to 6-week-old F-344/N rats of each sex were
administered 0, 5, 10, 20, 40 or 60 mg THPS (obtained from a 72%
aqueous solution) per kg body weight by gavage 5 days/week for
13 weeks. Three of the male rats that received 60 mg/kg died. Final
mean body weights were 5%, 15% and 22% lower than those of the
controls for males that received 20, 40 or 60 mg/kg and 9%, 12% and
19% lower for females that received 20, 40 or 60 mg/kg. Vacuolar
degeneration of hepatocytes occurred in all males receiving 10 mg/kg
or more, in all females receiving 40 or 60 mg/kg, and in 5/10 females
receiving 20 mg/kg. Lymphoid depletion in the spleen was seen in three
males in the 60 mg/kg group. Bone marrow hypoplasia was diagnosed in
3/10 male and 4/10 female rats in the 60 mg/kg groups. The NOAEL in
this study was 3.6 mg/kg body weight per day; the LOAEL was 7.1 mg/kg
body weight per day (US NTP, 1987).
Groups of 10 Charles River derived CD rats of each sex received
0, 1, 5 or 10 mg THPS (75% aqueous solution) per kg body weight per
day by gavage daily for 13 weeks. One female rat from the 5 mg/kg
group died on day 14, one female from the 1 mg/kg group on day 53 and
another on day 81. No clinical signs or changes in body weight were
attributed to THPS administration at any dose level other than mean
plasma ALAT and ASAT levels being twice control values for males of
the highest dose group. Histopathology showed moderate to marked
cytoplasmic vacuolation of periportal hepatocytes in all male rats and
moderate vacuolation in one female rat of the highest dose group. The
NOEL for this study was 1 mg/kg body weight per day (Hill & Newman,
1990).
C7.2.2 Dermal
Application of 125, 350, 700 or 1000 mg THPS/kg body weight on
chemically depilated back skin of groups of 12 male ICR mice daily for
up to 14 days caused reduced body weight, paralysed back muscles at
700 mg/kg body weight or more, and some superficial necrosis at all
doses (Connor et al., 1980). Similar effects were reported in mice by
Afansa'eva & Evseenko (1971).
Both THPC and THPS were toxic when applied dermally for long
periods on the skin. Rats and rabbits were dosed daily for 20 days
with 15%, 20% or 30% aqueous solutions of THPC. Severe skin lesions
occurred and all rats in the highest dose group died after 9 days of
application (Aoyama, 1975).
In a study by Wragg et al. (1996), trihydroxymethyl phosphine
oxide was administered by dermal application to three groups, each of
five male and five female Sprague-Dawley CD rats, for up to 28
consecutive days at dose levels of 0, 300, 650 and 1000 mg/kg body
weight per day. There were no deaths or clinical abnormalities
attributable to the test material. Body weight gain and food
consumption were similar in treated groups and controls. Females
treated with 1000 mg/kg showed an increase in plasma total protein and
a reduction in albumin/globulin ratio compared to controls. Males
treated with 1000 mg/kg and both males and females in the remaining
treatment groups showed no toxicologically significant changes in
these parameters. Both males and females dosed at 1000 mg/kg showed
cortical hypertrophy of the adrenal glands. Adverse dermal reactions
(scabs sometimes accompanied by scar tissue) were seen at all dose
levels. The incidence of these adverse dermal reactions gradually
increased during the second half of the treatment period amongst males
(but not females) dosed at 1000 mg/kg, and by day 28 all five males
showed dermal abnormalities. A NOAEL for dermal reactions was not
established. The NOAEL for systemic toxicity was determined to be 650
mg/kg body weight per day (Wragg et al., 1996).
C7.3 Long-term exposure
C7.3.1 THPC
Groups of 50 F-344/N rats of each sex (7 weeks of age) were
administered 0, 3.75 or 7.5 mg THPC (obtained from a 75% aqueous
solution) per kg body weight by gavage on 5 days/week for 103 weeks.
The mean body weights of different groups were comparable. Clinical
signs noted were rough hair coat and diarrhoea. The survival of
high-dose female rats was significantly lower than that of controls
after week 70 (P = 0.013). A dose-related increase in the
incidence of hepatocellular lesions, primarily cytoplasmic
vacuolization, was found (males: controls 0%, low-dose 18% and
high-dose 47%; females: controls 6%, low-dose 22% and high-dose 50%).
The LOAEL for this study was 2.7 mg/kg body weight per day; a NOAEL
was not established (US NTP, 1987).
Groups of 50 male B6C3F1 mice (8 weeks of age) were administered
0, 7.5 or 15 mg THPC (obtained from a 75% aqueous solution) per kg
body weight, and groups of 50 female B6C3F1 mice received 0, 15 or 30
mg/kg body weight by gavage 5 days/week for 103 weeks. No clear
difference between control and treated groups concerning body weight
gain and mortality was observed. Compound-related clinical signs
consisted of rough hair coat and diarrhoea. No signs of neurotoxicity
were observed. There was a dose-related increase in the incidence of
hepatocellular cytoplasmic vacuolization (males: controls 0%, low-dose
80%, and high-dose 88%; females: controls 0%, low-dose 84%, and
high-dose 96%). Follicular cell hyperplasia of the thyroid gland was
observed in the high-dose females (22% versus 6% in controls). The
LOAEL for this study was 5.4 mg/kg body weight per day; a NOAEL was
not established (US NTP, 1987).
C7.3.2 THPS
Groups of 49 or 50 F-344/N rats of each sex were administered 0,
5 or 10 mg THPS (obtained from a 72% aqueous solution) per kg body
weight by gavage 5 days/week for 104 weeks. Mean body weights of the
different groups were comparable. Clinical signs were rough hair coat
and diarrhoea. The survival of both the male low-dose (after week 102)
and high-dose (after week 67) animals was significantly lower
(P = 0.036 and 0.006, respectively). The effect level for mortality
in this study was 3.6 mg/kg body weight per day, the lowest dose
tested (US NTP, 1987).
Groups of 50 B6C3F1 mice of each sex (7 weeks of age) were
administered 0, 5 or 10 mg THPS (obtained from a 72% aqueous solution)
per kg body weight by gavage 5 days/week for 104 weeks. Mean body
weight and survival of control and treated groups were comparable.
Clinical signs were limited to rough hair coat and diarrhoea.
Non-neoplastic lesions seen were focal hyperplasia of the adrenal
medulla, but the numbers were statistically unrelated to dose
(controls 3/49, low-dose 5/48, high-dose 10/49). The NOAEL for this
study was 3.6 mg/kg body weight per day; the LOAEL was 7.1 mg/kg body
weight per day (US NTP, 1987).
C7.4 Skin and eye irritation; sensitization
C7.4.1 Skin irritation
C7.4.1.1 THPS
When 0.5 ml of THPS (75%) was applied to the skin of six New
Zealand white rabbits for a period of 4 h (OECD 404), no dermal
irritation was observed (Liggett, 1989a).
In a dermal study, daily doses of 25, 250 or 500 mg THPS (75%
aqueous solution) were applied to the shaved neck skin of ten (five
females, five males) Charles River derived CD rats. The treatment had
to be terminated and animals killed after 6 days due to the nature and
severity of the skin reaction observed at the application site (Hill,
1989).
C7.4.1.2 THPC-urea
A single 4-h semi-occluded application of THPC-urea to the intact
skin of six rabbits produced corrosive effects at two skin sites and
slight-to-well-defined erythema and very slight to slight oedema at
the other four treated skin sites (Snell, 1994).
C7.4.2 Eye irritation
In a test for eye irritation (OECD 405), an aliquot of 0.1 ml
THPS (75%) was introduced to one eye of a New Zealand rabbit. Opacity
was observed 24 h after application and lasted at least for 24 h. Red
coloration of the conjunctiva accompanied by considerable swelling was
observed. On the basis of these effects, THPS (75%) is considered to
be a severe eye irritant (Liggett, 1989b).
C7.4.3 Skin sensitization
C7.4.3.1 THPS
THPS (75%) was assessed for skin sensitization using the
Magnusson & Kligman Maximisation test (OECD 406). Fourteen out of 20
animals challenged with the test substance were sensitized (Guest,
1994b). These data clearly demonstrate a sensitization potential for
THPS (75%).
C7.4.3.2 THPC-urea
When THPC-urea was tested for skin sensitization potential using
the guinea-pig Magnusson & Kligman Maximisation test, 53% of the
animals were sensitized (Tufnell, 1992).
C7.5 Reproductive toxicity, embryotoxicity and teratogenicity
C7.5.1 THPS
Three groups of 16 New Zealand white rabbits were administered
(OECD 414) by gavage THPS (75%) at 6, 18 and 60 mg/kg body weight per
day from day 7 to 19 of gestation. All animals were kill on day 21. At
60 mg/kg mean body weight gain was significantly lower than that of
controls, showing maternal toxicity. Treatment at 60 mg/kg resulted in
increase incidence (42/120) of fetuses with eye malformation and some
with additional hydrocephaly or limb/phalangeal reduction defects. An
increase incidence of specific skeleton variation was also observed.
No adverse effects were noticed in the two lower-dose group (Barker,
1991a).
Charles River CD rats (24 animals per group) were administered by
gavage THPS (75%) at 15, 30 and 60 mg/kg body weight per day from day
6 to 15. All animals were killed on day 21. Treatment at 60 mg/kg
reduced significantly body weight gain from day 12 of gestation
onwards, indicating maternal toxicity. No treatment-related effects
were observed in dams at the low-dose level. At the high-dose level,
the incidence of fetuses showing extra thoraco-lumbar ribs was
significantly higher than for controls. At 30 mg/kg only minor signs
of maternal toxicity were observed (Barker, 1991b).
From these studies a NOAEL of 18 mg/kg body weight per day based
on maternal toxicity could be derived. No developmental effects were
observed in the absence of maternal toxicity.
C7.5.2 THPC-urea
Dose levels of 10, 30 and 100 mg THPC-urea/kg body weight per day
were administered to groups of 16 rabbits from day 7 to 19 of
gestation. All animals were killed on day 29. At the high-dose level,
an initial mean weight loss was followed by significantly reduced
weight gain over the treatment period. Treatment did not affect the
incidence of fetuses showing external, visceral or skeletal
malformations or variations (Barker, 1992).
C7.6 Mutagenicity and related end-points
C7.6.1 THPC-urea
C7.6.1.1 In vitro studies
An in vitro cytogenetic assay was performed with THPC-urea
using human lymphocytes. Harvest times of 16 and 40 h were used for
cells incubated in the presence of S9 from induced rat liver, and
times of 20 and 44 h were used when S9 was absent. In one experiment,
THPC-urea had no effect on the number of aberrant cells at
concentrations up to 40 mg/ml, but, in a repeat experiment, increased
numbers of aberrant cells were seen at 20 and 40 mg/ml. The reason for
the difference in results between the two experiments is not clear
(Durward, 1995; Bailey, 1995).
C7.6.1.2 In vivo studies
No mutagenicity of THPC-urea was evident in a mouse micronucleus
test in which oral doses of 212.5, 425 and 850 mg/kg body weight were
administered (Durward, 1996).
C7.6.2 THPC
Salmonella microsomal assays have shown uniformly negative
results for THPC (Table 10).
THPC produced chromosomal aberrations in Chinese hamster ovary
(CHO) cells in the absence of metabolic activation, but the results
were equivocal when it was tested in the presence of S9 from livers of
Sprague-Dawley rats treated with Aroclor 1254 (US NTP, 1987; Loveday
et al., 1989). THPC induced chromosomal aberrations in Chinese hamster
DON-6 cells in the absence of metabolic activation (Sasaki et al.,
1980).
THPC induced sister-chromatid exchange (SCE) in CHO cells when
incubated in the absence of metabolic activation (US NTP, 1987;
Loveday et al., 1989).
Table 10. Mutagenicity tests with Salmonella typhimurium
Strain Dose Metabolic activation Reference
(µg/plate)
TA100, TA1535, TA1537, TA98 0.33-33 none, S9 rat liver (male), US NTP (1987)a
S9 Syrian hamster liver (male)
TA98, TA100, TA1535, TA1537 10-1000 none, S9 liver (Aroclor 1254, rat) MacGregor et al. (1980)
TA98, TA100, TA1535, TA1537 10-300 none, S9 liver (Aroclor 1254, rat) MacGregor et al. (1980)
TA98, TA100, TA1535, TA1537 Up to 10 000 none, S9 liver (Aroclor 1254, rat) Zeiger et al. (1987)
TA98, TA100 not know none, S9 Kawachi et al. (1980b)b
a THPC gave positive results in a mouse lymphoma assay in the absence of metabolic activation (US NTP, 1987).
b Kawachi et al. (1980b) found positive results with THPC in the Bacillus subtilis rec assay with and without
metabolic activation.
C7.6.3 THPS
THPS produced no mutagenicity when tested in the Salmonella
microsome assay using strains TA98, TA100, TA1535, TA1537 and
TA1538 in the presence and absence of S9 from the livers of
Aroclor-1254-treated rats (Dillon & Riach, 1990). Leachate from
THPS-treated paper also showed no mutagenicity when tested with
strains TA98, TA100, TA102, TA1535 and TA1537 in the presence and
absence of S9 from Aroclor-1254-treated rats (Ballentyne, 1996b).
When tested in the mouse lymphoma assay, THPS caused mutations
both in the presence and absence of S9 from Aroclor-treated rats
(Riach, 1996). US NTP (1987) also reported positive results for THPS
in the mouse lymphoma assay in the absence of S9.
High levels of structural chromosomal aberrations were detected
at metaphase in CHO cells treated with THPS in the presence or absence
of S9 from liver of Aroclor-treated rats (Leddy, 1990). Anaphase
analysis of THPS-treated CHO cells also showed that chromosomal
aberrations were produced along with abnormal spindles (Coutino,
1979).
The results of an in vitro assay for unscheduled DNA synthesis
in a primary culture of rat hepatocytes were negative (Downey et al.,
1990; Riach, 1994).
Bone marrow from THPS-treated mice was analysed for
micronucleated polychromatic erythrocytes (MN-PCEs) and for metaphase
cells showing chromosomal aberrations. There was no effect on the
number of MN-PCEs nor on the number of chromosomal aberrations (Connor
et al., 1980).
The in vivo mutagenicity of dermal doses of THPS was
investigated in Swiss (ICR) mice using a similar protocol to that of
the previous study. Dermal doses of 125, 350, 700 and 1000 mg/kg body
weight per day were used. Urine from treated mice was not mutagenic in
the Salmonella microsome assay. Analysis of bone marrow from treated
mice showed a slight increase in the number of polyploid cells at the
highest dose level, but otherwise there was no indication of
mutagenicity (Connor et al., 1980).
In a dominant lethal assay in Swiss (ICR) mice, males were dosed
with up to 1000 mg/kg body weight. There was no evidence to suggest
that THPS produced dominant lethal mutations (personal communication
by M. Legator to Hooker Chemicals and Plastics Corp., 1977).
In a dominant lethal study in rats, gavage doses of 5, 10 or 15
mg/kg body weight per day were given to males for 10 weeks. After
mating, investigation of the pregnant females indicated no dominant
lethal mutations (Clode, 1996).
C7.6.4 THPO
THPO is a metabolite and breakdown product of THP salts. It was
not mutagenic in Salmonella typhimurium strains TA98, TA100, TA1535
and TA1537 or in Escherichia coli strain WP2 uvra when tested in
the presence and absence of S9 from livers of Aroclor-1254-treated
rats (Ballentyne, 1996a).
THPO was not mutagenic in the mouse lymphoma assay in either the
presence or absence of liver S9 from Aroclor-1254-treated rats
(Fellows, 1996). It did not produce chromosomal aberrations in CHO
cells cultured in either the presence or absence of S9 (Marshall,
1996).
C7.6.5 Treated fabrics
Groups of 12 male ICR mice received 0 (no fabric), 2500 mg
untreated fabric per kg diet or 250, 1250 or 2500 mg THPS-treated
fabric per kg diet for 5 successive days. Femurs were collected for
analyse of bone marrow for MN-PCEs and chromosomal aberrations. All
tests were negative (Connor et al., 1980).
C7.7 Carcinogenicity
C7.7.1 Oral studies
C7.7.1.1 Mice
In a 103-week study of THPC (75%) in mice (see section C7.3.1),
there was no evidence of carcinogenicity (US NTP, 1987).
In a 104 week study of THPS (72%) in mice(see section C7.3.2),
there was no evidence of carcinogenicity (US NTP, 1987).
C7.7.1.2 Rats
In a 103 week study for THPC (75%) in rat (see section C7.3.1),
there was no evidence of carcinogenicity (US NTP, 1987).
In a 104 week study for THPS (72%) in rat (see section C7.3.2),
there was no evidence of carcinogenicity (US NTP, 1987).
C7.7.2 Dermal studies: Initiation and promotion
A group of 60 female ICR/Ha Swiss mice, 6-8 weeks of age,
received skin applications of THPC (2 mg/mouse) in acetone three times
a week for 71 weeks. The control group received acetone only. There
was no significant increase in tumours (papillary tumours of the lung
and papillomas of the forestomach) in the animals treated with THPC
(Van Duuren et al., 1978).
Groups of 20 female ICR/Ha Swiss mice, 6-8 weeks of age, received
skin applications of THPC (2 mg/mouse) or Pyroset TKP
(acetate/phosphate mixture of THP) (7 mg/mouse) in dimethyl sulfoxide
three times a week for 57 weeks to examine the initiating, promoting
and complete carcinogenic potential in skin carcinogenesis bioassays.
Both chemicals were active as tumour promoters using a single
application of 7,12-dimethylbenz[ a]anthracene (DMBA) (20 µg in 0.1
ml acetone) as initiator. Neither chemical was active as a tumour
initiator or complete carcinogen (Loewengart & van Duuren, 1977).
C7.8 Special studies
THPS and THPO did not inhibit acetyl cholinesterase when tested
in vitro using malathion (a known inhibitor of cholinesterase) as a
positive control (Thompson, 1997a,b).
C8. EFFECTS ON HUMANS
Fabric treated with THPC-urea condensate was tested on human
volunteers in a 48-h skin patch test. The treated fabric was not
irritant to exposed human skin (Jackson, 1982).
C9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
C9.1 Laboratory experiments
C9.1.1 Aquatic organisms
THPS showed an EC50 for growth inhibition of the marine
microalga Skeletonema costatum of 0.16 mg/litre over 72 h (Hushagen
& McWilliam, 1994). For the freshwater green alga Selenastrum
capricornutum, EC50 values of 0.652 mg/litre (based on growth rate)
and 0.204 mg/litre (based on biomass) were determined. The NOEC for
both end-points was 0.063 mg/litre. The test was conducted under OECD
Guideline 201 (Jenkins, 1991c).
The 48-h LC50, based on immobilization and using nominal
concentrations, for THPS in the water flea (Daphnia magna) was
determined to be 19.4 mg/litre, with a NOEC of 10.4 mg/litre. The test
was conducted under US EPA/OECD Guideline 202 (Jenkins, 1989).
Concerning estuarine/marine invertebrates, the 96-h LC50 for the
mysid shrimp (Mysidopsis bahia) is 7.3 mg/litre (NOEC 3.5 mg/litre)
(Boeri et al., 1995a), the 96-h LC50 for the brown shrimp (Crangon
crangon) is 340 mg/litre (Douglas & Pell, 1986), and the 48-h LC50
for the brine shrimp (Acartia tonsa) is 0.6 mg/litre (Torp &
McWilliam, 1994). The 96-h EC50 for shell deposition in juvenile
Eastern oysters (Crassostrea virginica) was reported to be
1.6 mg/litre, with a NOEC of 0.67 mg/litre for THPS in a test
following US EPA guidelines (Boeri et al., 1995c). The 10-day LC50
for THPS was determined for the sediment-dwelling amphipod Corophium
volutator to be 2174 mg/kg dry sediment weight (Roddie, 1994).
The 96-h LC50, based on nominal concentrations of THPS, for the
rainbow trout (Oncorhynchus mykiss) was determined to be 119
mg/litre with a NOEC of 18.1 mg/litre in a test conducted according to
OECD Guideline 203 (Jenkins, 1991a). A test conducted under US
EPA/OECD Guideline 203 determined the 96-h LC50 for THPS in bluegill
sunfish (Lepomis macrochirus) to be 93 mg/litre, with a NOEC of 22.7
mg/litre (Jenkins, 1991b). The 96-h LC50 for the marine sheepshead
minnow (Cyprinodon variegatus) was determined to be 72 mg/litre,
with a NOEC of 41 mg/litre using US EPA guidelines (Boeri et al.,
1995b). For juvenile plaice (Pleuronecta platessa), the 96-h LC50
was 86 mg/litre (Douglas & Handley, 1989).
C9.1.2 Terrestrial organisms
The acute oral LD50 for young adult mallard ducks (Anas
platyrynchus) was 311 mg/kg body weight (Roberts & Phillips, 1988a).
Dietary LC50 values for the mallard duck and bobwhite quail
(Colinus virginianus) were 1313 and 2414 mg/kg diet, respectively
(Roberts & Fairley, 1988; Roberts & Phillips, 1988b).
C10. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
The International Agency for Research on Cancer evaluated the
carcinogenicity of tetrakis(hydroxymethyl) phosphonium salts in 1989
(IARC, 1990) and concluded:
a) There is inadequate evidence for the carcinogenicity of
tetrakis(hydroxymethyl) phosphonium salts in experimental
animals.
b) No data were available from studies in humans on the
carcinogenicity of tetrakis(hydroxymethyl) phosphonium salts.
c) Tetrakis(hydroxymethyl) phosphonium salts are not classifiable
as to their carcinogenicity to humans (Group 3).
REFERENCES
Adachi K, Mitsuhashi M, & Ohkuni N (1984) Pesticides and trialkyl
phosphates in tap water (Jpn.) Hyogo-ken Eisei Kenkyusho Kenkyu
Hokoku, 19: 1-6 (Abstract).
Afansa'eva LV & Evseenko NS (1971) Hygiene evaluation of fire-proof
textiles processed with an organophosphorus impregnant based on
tetrahydroxymethyl phosphonium chloride. Hyg Sanit , 36(3): 450-453.
Anderson GD, Elvin AT, & Lalka D (1984) Estimation of
tris(2-butoxyethyl) phosphate in biological fluids: Novel intersubject
variability in recovery from human serum. J Pharm Sci, 73(12):
1791-1793.
Antony C (1993) THPS 75% viscosity (Study sponsored by Albright &
Wilson). Whippany, New Jersey, Case Consulting Laboratories Inc.
Aoyama M (1975) Effect of anti-flame treating agents on the skin.
Nagoya Med J, 20: 11-19.
Arias P (1992) Brominated diphenyloxides as flame retardants: Bromine
based chemicals (Unpublished report to the OECD).
Armstrong JA & Yule WN (1978) The distribution of aerially-applied
spray deposits in spruce trees. Can Entomol , 110: 1259-1267.
ARW (1987) [Annual report 1987.] Düsseldorf, Working Group
Rhine-Waterworks, p 86 (in German).
Ashby J & Tennant RW (1988) Chemical structure, Salmonella
mutagenicity and extent of carcinogenicity as indicators of genotoxic
carcinogens among 222 chemicals tested in rodents by the US NCI/NTP.
Mutat Res, 204: 17-115.
Bailey IA (1995) Proban CC: in vitro cytogenetic assay review (Study
sponsored by Albright & Wilson). Cheshire, United Kingdom, Zeneca Ltd,
Central Toxicology Laboratory (Report No. CTL/B/31).
Ballentyne M (1996a) Trishydroxymethyl phosphine oxide: Reverse
mutation in five histidine-requiring strains of Salmonella
typhimurium. Harrogate, United Kingdom, Corning Hazleton (Europe)
(Unpublished report No. 254/47-1052).
Ballentyne M (1996b) THPS paper leachate PLO1: Reverse mutation in
four histidine-requiring strains of Salmonella typhimurium and one
tryptophan-requiring strain of Escherichia coli. Harrogate, United
Kingdom, Corning Hazleton (Europe) (Unpublished report No.
254/45-1052).
Barker L (1991a) THPS: Oral (gavage) teratology study in the rabbit
(Study sponsored by Albright & Wilson). Harrogate, United Kingdom,
Corning Hazleton (Europe) (Unpublished report No. 6380-254/19).
Barker L (1991b) THPS: Oral (gavage) teratology study in the rat
(Study sponsored by Albright & Wilson). Harrogate, United Kingdom,
Corning Hazleton (Europe) (Unpublished report No. 6358-254/23).
Barker L (1992) Proban CC: Oral (gavage) teratology study in the
rabbit (Study sponsored by Albright & Wilson). Harrogate, United
Kingdom, Corning Hazleton (Europe) (Unpublished report No.
6742-254/26).
Barth T (1994) Bioaccumulation OECD 117 partition coefficient
n-octanol/water (HPLC)- tolcide PS75 (Study sponsored by Albright &
Wilson). Bergen, Norway, Terra Miljö-Laboratories A/S.
Bayer (1958) [Toxicological testing of the softener
tri-2-ethylhexylphosphate (TOF).] Leverkusen, Germany, Bayer AG,
Institute of Toxicology (Unpublished report) (in German).
Bayer (1989) [Phosphoric acid, bis(2-ethylhexyl)ester ecotoxicological
data -- Fish toxicity: Basic data set for existing chemicals above
1000 t/year.] Leverkusen, Germany, Bayer AG (in German).
Bayer (1982a) [Biological degradation of tris(2-ethylhexyl)phosphate.]
Leverkusen, Germany, Bayer AG, Institute for Environmental Analysis
and Assessment (Unpublished report) (in German).
Bayer (1982b) [Bacterial test on chemicals, ETAD 3002/ISO8192.]
Leverkusen, Germany, Bayer AG, Institute for Environmental Analysis
and Assessment (Unpublished report) (in German).
Boeri RL, Magazu JP, & Ward TJ (1995a) Acute toxicity of tetrakis
(hydroxymethyl) phosphonium sulphate (THPS) to the Mysid (Mysidopsis
bahia), under flow-through conditions (Study No. 41238). Columbia,
Missouri, ABC Laboratories Inc. (Unpublished report).
Boeri RL, Magazu JP, & Ward TJ (1995b) Acute toxicity of
tetrakis(hydroxymethyl) phosphonium sulphate (THPS) to the sheepshead
minnow, (Cyprinodon variegatus), under flow-through conditions
(Study No. 41239). Columbia, Missouri, ABC Laboratories Inc.
(Unpublished report).
Boeri RL, Magazu JP, & Ward TJ (1995c) Acute flow-through mollusc
shell deposition test with tetrakis(hydroxymethyl) phosphonium
sulphate (THPS) (Study No. 41240). Columbia, Missouri, ABC
Laboratories Inc. (Unpublished report).
Bohlen H, Hicke K, Stobel A-O, Zierott M, & Thiemann W (1989) [The
contamination of the lower part of the Weser River by organochlorine
and organophosphorus compounds I.] Vom Wasser, 72: 185-197 (in
German).
BUA (1996) [German Chemical Society-Advisory Committee on Existing
Chemicals of Environmental Relevance (BUA): Di(2-ethylhexyl)
phosphate, tri(2-ethylhexyl) phosphate.] Stuttgart, Germany, S. Hirzel
(BUA Report 172) (in German).
Carrington CD, Lapadula DM, Othman M, Farr C, Nair RS, Johanssen F, &
Abou-Donia M (1990) Assessment of the delayed neurotoxicity of
tributyl phosphate, tributoxyethyl phosphate and dibutylphenyl
phosphate. Toxicol Ind Health, 6(3/4): 415-423.
Clode SA (1996) THPS: Oral (gavage) dominant lethal study in the rat.
Harrogate, United Kingdom, Corning Hazleton (Europe) (Unpublished
report No. 254/43-1050l).
Connor T, Meyne J, & Legator M (1980) The mutagenic evaluation of
tetrakis(hydroxymethyl) phosphonium sulfate using a combined testing
protocol approach. J Environ Pathol Toxicol, 1: 145-158.
Coutino R (1979) Analysis of anaphase in cell culture: An adequate
test system for the distinction between compounds which selectively
alter the chromosome structure or the mitotic apparatus. Environ
Health Perspect, 31: 131-136.
Cowlyn TC (1991a) THPS: Determination of physical-chemical properties
(Study sponsored by Albright & Wilson). Eye, United Kingdom, Life
Science Research Limited
Cowlyn TC (1991b) THPS-75: Determination of physical-chemical
properties (Study sponsored by Albright & Wilson). Eye, United
Kingdom, Life Science Research Limited.
Dillon DM & Riach CG (1990) THPS-75, batch No 88/22: Testing for
mutagenic activity with Salmonella typhimurium TA1535, TA1537,
TA1538, TA98 and TA100 (Project No.740423). Falls Church, Virginia,
Inveresk Research International.
Douglas MT & Handley JW (1989) The acute toxicity of DP435 to juvenile
plaice (Pleuronectes platessa) (Study sponsored by Albright &
Wilson). Huntingdon, United Kingdom, Huntingdon Research Centre
Limited (Unpublished report No. A&W 491/881590).
Douglas MT & Pell IB (1985) Assessment of inherent biodegradability of
DP 435 (Study sponsored by Albright & Wilson). Huntingdon, United
Kingdom, Huntingdon Research Centre Limited (Report No.
A&W457(C)/85679).
Douglas MT & Pell IB (1986) The acute toxicity of DP435 to Brown
shrimp (Crangon crangon) (Study sponsored by Albright & Wilson).
Huntingdon, United Kingdom, Huntingdon Research Centre Limited
(Unpublished report No. A&W 470(b)/8677).
Downey E, Riach CG, & Mohammed R (1990)
Tetrakishydroxymethylphosphonium sulphate: assessment of genotoxicity
in an unscheduled DNA synthesis assay using adult rat hepatocyte
primary culture (Project No. 741343). Falls Church, Virginia, Inveresk
Research International (Unpublished Report No. 6230).
Durward R (1995) Proban CC: Chromosomal aberration test in human
lymphocytes in vitro (SPL project No. 071/346, sponsored by Albright
& Wilson). Derby, United Kingdom, Safepharm Laboratories Ltd.
Durward R (1996) Proban CC: Micronucleus test in the mouse (SPL
project No. 071/372, sponsored by Albright & Wilson). Derby, United
Kingdom, Safepharm Laboratories Ltd.
ECETOC (1992a) Tris(2-ethylhexyl) phosphate (CAS No. 78-42-2);
Bis(2-ethylhexyl) phosphate (CAS No. 298-07-7); and Mono(2-ethylhexyl)
phosphate (CAS No. 12645-31-7). Brussels, European Chemical Industry
Ecology and Toxicology Centre (Joint Assessment of Commodity Chemicals
No. 20).
ECETOC (1992b) Tris(2-butoxylethyl) phosphate. Brussels, European
Chemical Industry Ecology and Toxicology Centre ( Joint Assessment of
Commodity Chemicals No. 21).
Eldefrawi AT, Mansour NA, Brattsten LB, Ahrens VD, & Lisk DJ (1977)
Further toxicologic studies with commercial and candidate flame
retardant chemicals: Part II. Bull Environ Contam Toxicol, 17(6):
720-726.
Environmental Agency Japan (1983) Environmental monitoring of
chemicals: Environmental survey report of FYs 1980 and 1981. Tokyo,
Environmental Agency, Department of Environmental Health, Office of
Health Studies..
Environmental Agency Japan (1987) Chemicals in the environment: Report
on environmental survey and wildlife monitoring of chemicals in FYs
1984 and 1985. Tokyo, Environmental Agency, Department of
Environmental Health, Office of Health Studies.
Environmental Agency Japan (1978) Environmental monitoring of
chemicals: Environmental survey report of 1977 FY. Tokyo,
Environmental Agency, Department of Environmental Health, Office of
Health Studies.
Ernst W (1988) [Evaluation of contaminants of low degradability in
estuaries.] Biotechnology, 129(3): 60-63 (in German).
Fellow SM (1996) Tris hydroxymethyl phosphine oxide (THPO); mutation
at the thymidine kinase (tk) locus of mouse lymphoma L517BY cells
using the microtitre fluctuation technique (Study sponsored by
Albright & Wilson). Harrogate, United Kingdom, Corning Hazleton
(Europe).
FMC (1990) Material safety data sheet on KP-140: Tributoxyethyl
phosphate. Princeton, New Jersey, FMC Corporation.
FMC (1998) Material safety data sheet: Reomol TOP. Princeton, New
Jersey, FMC Corporation (Rev. 1).
Freeman C (1991a) KP-140: Non-definitive primary skin irritation study
in rabbits (Study No. I91-1206). Princeton, New Jersey, FMC
Corporation, Toxicology Laboratory
Freeman C (1991b) KP-140: Non-definitive acute oral toxicity study in
rats (Study No. I91-1207). Princeton, New Jersey, FMC Corporation,
Toxicology Laboratory.
Freeman C (1991c) KP-140: Non-definitive primary eye irritation study
in rabbits (Study No. 191-1205). Princeton, New Jersey, FMC
Corporation, Toxicology Laboratory.
Frimmel FH, Millington DS, & Christman RF (1987) [Quantification of
organic compounds in charcoal extracts using gas chromatography/mass
spectrometry.] Fresenius Z Anal Chem, 327: 149-153 (in German).
Fukushima M & Kawai S (1986) Present status and fate of selected
organophosphoric acid triesters in the water area of Osaka City.
Seitai Kagaku, 8(4): 13-24.
Fukushima M, Kawai S, Chimaki S, & Morioka T (1986) Urban runoff as a
source of selected chemicals to river waters: An observation at
Isojima sampling station in the Yodo River Basin. Health Environ Sci,
49: 11-20.
Fukushima M, Kawai S, & Yamaguchi Y (1992) Behavior of
organophosphoric acid triesters in Japanese riverine and coastal
environment. Water Sci Technol, 25(11): 271-278.
Gabriel KL (1980a) Primary eye irritation study -- rabbits (Project
No. 80-1907A). Philadelphia, Pennsylvania, Bioresearch Inc.
Gabriel KL (1980b) Primary skin irritation study -- rabbits (Project
No. 80-1907A). Philadelphia, Pennsylvania, Bioresearch Inc.
Gabriel KL (1980c) Acute dermal toxicity -- rabbits (Project No.
80-1907A). Philadelphia, Pennsylvania, Bioresearch Inc.
Gartrell MJ, Craun JC, Podrebarac DS, & Gunderson EL (1986a)
Pesticides, selected elements and other chemicals in infant and adult
total diet samples: October 1980-March 1982. J Assoc Off Anal Chem,
69: 146-161.
Gartrell MJ, Craun JC, Podrebarac DS, & Gunderson EL (1986b) Chemical
contaminants monitoring: Pesticides, selected elements and other
chemicals in infant and toddler total diet samples, October 1980-March
1982. J Assoc Off Anal Chem, 69: 123-145.
Gorman M (1996) Aerobic aquatic metabolism of
14C-tetrakishydroxylmethyl phosphonium sulphate (THPS): Final report
(Study Sponsored by Albright & Wilson). Columbia, Missouri, ABC
Laboratories Inc. (Unpublished report No. 40054).
Gorman M (1997) Anaerobic aquatic metabolism of
14C-tetrakishydroxylmethyl phosphonium sulphate (THPS): Final report
(Study sponsored by Albright & Wilson). Columbia, Missouri, ABC
Laboratories Inc. (Unpublished report No. 40055).
Guest RL (1993a) Amgard TOF: Acute eye irritation test in the rabbit
(Project No. 71/200, sponsored by Albright & Wilson). Derby, United
Kingdom, Safepharm Laboratories Ltd.
Guest RL (1993b) Amgard TOF: Acute dermal irritation test in the
rabbit (Project No. 71/199, sponsored by Albright & Wilson). Derby,
United Kingdom, Safepharm Laboratories Ltd.
Guest RL (1994a) Tolcide THPS 75 %: Acute oral toxicity test in the
rat (Study sponsored by Albright & Wilson). Derby, United Kingdom,
Safepharm Laboratories Ltd (Unpublished report).
Guest RL (1994b) Tolcide THPS 75 %: Magnusson & Kligman maximisation
study in the guinea pig (Project No. 71/288). Derby, United Kingdom,
Safepharm Laboratories Ltd (Unpublished report).
Gunderson EL (1988) Dietary intakes of pesticides, selected elements
and other chemicals: FDA total diet study, April 1982-April 1984. J
Assoc Off Anal Chem, 71(6): 1200-1209.
Gunderson EL (1995a) Dietary intakes of pesticides, selected elements
and other chemicals: FDA total diet study, June 1984-April 1986. J
Assoc Off Anal Chem, 78(4): 910-921.
Gunderson EL (1995b) Dietary intakes of pesticides, selected elements
and other chemicals: FDA total diet study, July 1986-April 1991. J
Assoc Off Anal Chem, 78(6): 1353-1363.
Haseman JK, Crawford DD, Huff JE, Boorman GA, & McConnell EE (1984)
Results from 86 two-year carcinogenicity studies conducted by the
National Toxicology Program. J Toxicol Environ Health, 14: 621-639.
Hattori Y, Ishitani H, Kuge Y, & Nakamoto M (1981) Environmental fate
of organic phosphate esters. Suishitsu Odaku Kenkyu, 4(3): 137-141.
Hawley GG (1981) Condensed chemical dictionary, 12th ed. New York, Van
Nostrand Reinhold, p 1133.
Heaton PE (1991) Determination of the environment fate of THPS in
water treatment applications. Oldbury, United Kingdom, Albright &
Wilson UK Ltd, Biocides Group (Unpublished report No. BTSP-4A).
Heim LC (1998) Soil/sediment adsorption-desorption of
14C-tetrakishydroxymethyl phosphonium sulphate (THPS) (Study
sponsored by Albright & Wilson). Columbia, Missouri, Analytical
Bio-Chemistry (ABC) Laboratories, Inc. (Final report No. 40053).
Hill RE (1989) DP435: 28 day dose range finding study in the rat
-- Sub-acute dermal study in the rat. Toxicol Laboratories Limited
(Unpublished report No. ALW/7/89).
Hill RE & Newman AJ (1990) DP435: 13 week oral (gavage) toxicity study
in the rat -- Oral 90-day studies. Toxicol Laboratories Limited
(Unpublished report No. ALW/4/90).
Hinckley DA, Bidleman TF, Foreman WT, & Tuschall JR (1990)
Determination of vapor pressures for nonpolar and semipolar organic
compounds from gas chromatographic retention data. J Chem Eng Data,
35: 232-237.
Hoechst (1987) [Safety data sheet on tributoxyethyl phosphate, TBEP.]
Frankfurt, Germany, Hoechst AG (in German).
Hoechst (1989) [TBEP: Assessment of acute aerosol inhalation toxicity
in male and female SPF-Wistar rats, 4 hours LC50.] Frankfurt,
Germany, Hoechst AG, Pharma-Research Toxicology and Pathology (in
German).
Hooper G, Nakajima WN, & Herbes WF (1976) The use of various
phosphonium salts for flame retardancy by the ammonium cure technique.
In: Bhatnagar VM ed. Fire retardants: Proceedings of the International
Symposium on Flammability and Fire Retardants, Montreal, Canada, 22-23
May 1975. Westport, Connecticut, Technomic Publishing Co., pp 98-114.
Hushagen H & McWilliam P (1994) Tolcide PS75 (THPS): Marine algal
growth inhibition test with Skeletonema costatum. Bergen, Norway,
Terra Miljö-Laboratories A/S (Unpublished report No.
60090.aw\tm.001\skelet
IARC (1990) Some flame retardants and textile chemicals, and exposures
in the textile manufacturing industry. Lyon, International Agency for
Research on Cancer, pp 95-107 (IARC Monographs on the Evaluation of
Carcinogenic Risks to Humans, Volume 48).
IPCS (1998) Concise international chemical assessment document No. 10:
2-Butoxyethanol. Geneva, World Health Organization, International
Programme on Chemical Safety, 29 pp.
Ishikawa S, Taketomi M, & Shinohara R (1985) Determination of trialkyl
and triaryl phosphates in environmental samples. Water Res, 19(1):
119-125.
Ivett JL, Brown BM, Rodgers C, Anderson BE, Resnick MA, & Zeiger E
(1989) Chromosomal aberrations and sister chromatid exchange test in
Chinese hamster ovary cells in vitro: IV. Results with 15 chemicals.
Environ Mol. Mutagen, 14: 165-187.
Jackson JR (1982) Study to determine the primary irritancy of
untreated fabric. Proban 210 treated fabric, Proban NX treated fabric,
laundered Proban NX treated fabric in human volunteers. Oldbury,
United Kingdom, Albright & Wilson UK Ltd.
Jenkins WR (1989) THPS: Acute toxicity to Daphnia magna (Study
sponsored by Albright & Wilson). Eye, United Kingdom, Life Science
Research Limited (Unpublished report No. 90/AWL007/0471).
Jenkins WR (1991a) Acute toxicity to Rainbow trout (Study sponsored by
Albright & Wilson). Eye, United Kingdom, Life Science Research Limited
(Unpublished report No. 90/AWL006/0470).
Jenkins WR (1991b) THPS -- Acute toxicity to Bluegill Sunfish (Study
sponsored by Albright & Wilson). Eye, United Kingdom, Life Science
Research Limited (Unpublished report No. 90/AWL005/0469).
Jenkins WR (1991c) THPS -- Determination of its EC50 to Selenastrum
capricornutum under non-axenic conditions (Study sponsored by
Albright & Wilson). Eye, United Kingdom, Life Science Research Limited
(Unpublished report No. 90/AWL009/0473).
KAN-DO Office and Pesticides Team (1995) Accumulated pesticide and
industrial chemical finding from a ten-year study of ready-to-eat
foods. J Assoc Off Anal Chem, 78(3): 614-631.
Kawachi T, Komatsu T, Kada T, Ishidate M, Sasaki M, Sugiyama T, &
Tazima Y (1980a) Results of recent studies on the relevance of various
short-term screening tests in Japan: The predictive value of
short-term screening tests in carcinogenicity evaluation. Appl Methods
Oncol, 3: 253-267.
Kawachi T, Yahagi T, Kada T, Tazima T, Ishidate M, Sasaki M, &
Sugiyama T (1980b) Cooperative programme on short-term assays for
carcinogenicity in Japan. In: Montesano R, Bartsch H, & Tomatis L ed.
Molecular and cellular aspects of carcinogen screening tests, pp
323-330 (IARC Scientific Publications No. 27).
Kawagoshi Y & Fukunaga I (1994) Levels and features of
organophosphoric acid triesters at Osaka North Port Sea-Based solid
waste disposal site. J Environ Chem, 4(4): 797-804.
Kawagoshi Y & Fukunaga I (1995) Organophosphoric acid triesters in
leachate from solid waste disposal site. Kankyo Kagaku, 5: 390-391.
Kawai S, Fukushima M, Kitano M, & Morishita H (1985) Degradation of
organophosphoric acid triesters by the bacteria in the river water of
Osaka City. Annu Rep Osaka City Inst Public Health Environ Sci, 48:
175-183.
Kawai S, Fukushima M, Kitano M, Takayuki N, & Morishita H (1986)
Degradation of organophosphoric acid triesters by the bacteria in the
river water -- (ii) Properties of TBP (tributyl phosphate) degrading
bacteria and their enzymes. Annu Rep Osaka City Inst Public Health
Environ Sci , 49: 160-166.
Keith LH & Walters DB (1985) Compendium of safety data sheet for
research and industrial chemicals. New York, VCH Publishers Inc., part
III, pp 1630-1631.
Keith LH & Walters DB (1987) Compendium of safety data sheets for
research and industrial chemicals. New York, VCH Publishers Inc., part
IV, p 856.
Kimmerle G (1958) [Softener tri-2-ethylhexylphosphate (TOF).]
Leverkusen, Germany, Bayer AG, Laboratory of Toxicology and Pathology
(Unpublished report No. 1764) (in German).
Kluwe WM, Huff JE, Matthews HB, Irwin R, & Haseman JK (1985)
Comparative chronic toxicities and carcinogenic potentials of
2-ethylhexyl containing compounds in rats and mice. Carcinogenesis,
6(11): 1577-1583.
Komsta E, Secours VE, Chu I, Valli VE, Morris R, Harrison J,
Baranowski E, & Villeneuve DC (1989) Short-term toxicity of nine
industrials chemicals. Bull Environ Contamin Toxicol, 43: 87-94.
Krzymien M (1981) Sample collection and field analysis of
tris(2-ethylhexyl) phosphate used in a study of pesticide spray drift.
J Chromatogr, 204: 453-459.
Laham S, Long G, Schrader K, & Szabo J (1984a) Induction of electro
physiological and morphological changes in Sprague-Dawley rats fed
tributoxyethyl phosphate. J Appl Toxicol, 4(1): 42-48.
Laham S, Szabo J, & Long G (1984b) Short-term neurotoxicity studies on
tributoxyethyl phosphate orally administered to Sprague-Dawley rats.
Chemosphere, 13(7): 801-812.
Laham S, Long GW, & Broxup BR (1985a) Subchronic oral toxicity of
tributoxyethyl phosphate in the Sprague-Dawley rat. Arch Environ
Health, 40(1): 12-17.
Laham S, Szabo J, Long G, & Schrader K (1985b) Dose-response toxicity
studies on tributoxyethyl phosphate orally administered to
Sprague-Dawley rats. Am Ind Hyg Assoc J, 46(8): 442-448.
Lebel GL & Williams DT (1983a) Problems in collection of
representative samples for determination of tributoxyethyl phosphate
in potable water. J Assoc Off Anal Chem, 66(1): 202-203.
Lebel GL & Williams DT (1983b) Determination of organic phosphate
triesters in human adipose tissue. J Assoc Off Anal Chem, 66(1):
691-699.
Lebel GL & Williams DT (1986) Levels of triaryl/alkyl phosphates in
human adipose tissue from eastern Ontario. Bull Environ Contam
Toxicol, 37: 41-46.
Lebel GL, Williams DT, & Benoit FM (1981) Gas chromatographic
determination of trialkyl/aryl phosphates in drinking water, following
isolation using macroreticular resin. J Assoc Off Anal Chem, 64(4):
991-998.
Lebel GL, Williams DT, & Benoit FM (1987) Use of large volume resin
cartridges for the determination of organic contaminants in drinking
water derived from the Great Lakes. Adv Chem Ser, 214: 309-325.
Lebel GL ,Williams DT, & Berard D (1989) Triaryl/alkyl phosphate
residues in human adipose autopsy samples from six Ontario
municipalities. Bull Environ Contam Toxicol, 43: 225-230.
Leddy IA (1990) THPS-75: Chromosal aberrations assay with Chinese
hamster ovary cells in vitro (Project No.740444). Falls Church,
Virginia, Inveresk Research International (Unpublished report No.
6144).
Lenga RE (1993) The Sigma-Aldrich library of chemical safety data.
Buchs, Switzerland, Sigma-Aldrich, pp 1117-1118.
Leo A (1989) CLOGP-3.54 MedChem software 1989. Claremont, California,
Daylight Chemical Information Systems.
Lerche J & Morch J (1973) Qualitative and quantitative gas
chromatographic determination of plasticizers in polyvinyl chloride.
Arch Pharm OG Chem, 1: 25-30.
Liggett MP (1989a) Irritant effects on rabbit skin of THPS (Study
sponsored by Albright & Wilson). Peterborough, United Kingdom,
Huntingdon Research Center Limited (Unpublished report No.89361D/A&W
498/SE).
Liggett MP (1989b) Irritant effects on rabbit eye of THPS (Study
sponsored by Albright & Wilson). Peterborough, United Kingdom,
Huntingdon Research Center Limited (Unpublished report No. 89447D/A&W
499/SE).
Liggett MP & Allen SA (1989) Acute dermal toxicity to rats of THPS
(Study sponsored by Albright & Wilson). Peterborough, United Kingdom,
Huntingdon Research Center Limited.
Lloyd GR (1994) UV degradation of THPS. Oldbury, United Kingdom,
Albright & Wilson UK Ltd, Specialities Technical Laboratory.
Loewengart G & Van Duuren BL (1977) Evaluation of chemical flame
retardants for carcinogenic potential. J Toxicol Environ Health, 2:
539-546.
Loveday KS, Lugo MH, Resnick MA, Anderson BE, & Zeiger E (1989)
Chromosome aberration and sister chromatid exchange tests in Chinese
hamster ovary cells in vitro: II. Results with 20 chemicals. Environ
Mol Mutagen, 13: 60-94.
McDonald P & Anderson BT (1989) THPS acute inhalation toxicity study
in rats (Study sponsored by Albright & Wilson). Tranent, Edinburgh,
United Kingdom, Inveresk Research International.
MacFarland HN & Punte CL (1966) Toxicological studies on
tri-(2-ethylhexyl)-phosphate. Arch Environ Health, 13: 13-20.
MacGregor JT, Diamond MJ, Mazzeno LW Jr, & Friedman M (1980)
Mutagenicity tests of fabric-finishing agents in Salmonella
typhimurium : Fiber-reactive wool dyes and cotton flame retardants.
Environ Mutagen, 2: 405-418.
MacKeller DG (1978) Kp-140 tris(butoxyethyl) phosphate mutagenicity
screening test salmonella microsomal assay (Ames test). Princeton, New
Jersey, FMC Corporation (Report No. ICG/T-78-006).
McWilliam P (1994) Tolcide PS355A: Biodegradability in seawater
-- Closed bottle test (Study sponsored by Albright & Wilson). Bergen,
Norway, Terra Miljö-Laboratories A/S (Unpublished report No.
60090.aw\tm.002\biodeg).
Marshall R (1996) Trishydroxymethyl phosphine oxide: Induction of
chromosome aberrations in cultured chinese hamster ovary (CHO) cells.
Harrogate, United Kingdom, Corning Hazelton (Europe) (Unpublished
report No. 254/44-1052).
Mead C & Handley JW (1998) Assessment of ready biodegradability CO2
evolution test (modified sturm test) of Amgard TBEP (Project No.
071/607). Derby, United Kingdom, Safepharm Laboratories Ltd.
Miyagawa M, Takasawa H, Sugiyama A, Inoue Y, Murata T, Uno Y, &
Yoshikawa K (1995) The in vivo-in vitro replicative DNA synthesis
(RDS) test with hepatocytes prepared from male B6C3F1 mice as an early
prediction assay for putative nongenotoxic (Ames-negative) mouse
hepatocarcinogens. Mutat Res, 343: 157-183.
Monsanto (1976) Biodegradability of phosphate esters: Analytical
chemistry -- Special study AC-75-55-9. St Louis, Missouri, Monsanto
Industrial Chemicals, Applied Sciences.
Monsanto (1984a) Summary sheet: t-Butoxyethyl phosphate (Daphnia
magna). St Louis, Missouri, Monsanto Industrial Chemicals,
Environmental Sciences Toxicity.
Monsanto (1984b) Acute toxicity of TBEP to fathead minnow
(Pimephales promelas). Wareham, Massachusetts, Springborn Bionomics
Inc. (Unpublished report No. SB 89-9160, prepared for Monsanto
Industrial Chemicals, Environmental Sciences Toxicity, St Louis,
Missouri).
Monsanto (1984c) Tributoxyethyl phosphate: Acute toxicity/irritation
studies. St Louis, Missouri, Monsanto, Department of Medicine and
Environmental Health (Unpublished report No. BD-84-038).
Monsanto (1984d) Tributoxyethyl phosphate microbial (AMES)
mutagenicity. St Louis, Missouri, Monsanto, Department of Medicine and
Environmental Health (Unpublished report No. SR-84-143).
Monsanto (1984e) Repeat human insult patch test of tributoxyethyl
phosphate. St Louis, Missouri, Monsanto, Department of Medicine and
Environmental Health (Unpublished report No. SH-84-002).
Monsanto (1985a) Four-week feeding study of tributoxyethyl phosphate
in male and female Sprague-Dawley rats. St Louis, Missouri, Monsanto,
Department of Medicine and Environmental Health (Unpublished report
No. ML-84-093).
Monsanto (1985b) Twenty-one-day dermal toxicity study in rabbits with
tributoxyethyl phosphate. St Louis, Missouri, Monsanto, Department of
Medicine and Environmental Health (Unpublished report No. BD-84-130).
Monsanto (1985c) CHO/HGPRT mammalian cell forward gene mutation assay
with tributoxyethyl phosphate. St Louis, Missouri, Monsanto,
Department of Medicine and Environmental Health (Unpublished report
No. PK-84-408).
Monsanto (1985d) Range-finding teratology study in rats with
tributoxyethyl phosphate. St Louis, Missouri, Monsanto, Department of
Medicine and Environmental Health (Unpublished report No. IR-84-224).
Monsanto (1985e) Teratology study in rats with tributoxyethyl
phosphate. St Louis, Missouri, Monsanto, Department of Medicine and
Environmental Health (Unpublished report No. IR-84-225).
Monsanto (1986) Neurotoxicity studies of tributoxyethyl phosphate in
chickens. St Louis, Missouri, Monsanto, Department of Medicine and
Environmental Health (Unpublished report No. DU-84-358).
Monsanto (1987a) Eighteen-week feeding study of tributoxyethyl
phosphate with Sprague-Dawley rats. St Louis, Missouri, Monsanto,
Department of Medicine and Health Sciences (Unpublished report No.
ML-84-437 [EHL No. 84108]).
Monsanto (1987b) Peripheral nerve conduction after an eighteen-week
feeding exposure to tributoxyethyl phosphate in rats. St Louis,
Missouri, Monsanto, Department of Medicine and Health Sciences
(Unpublished report No. ML-84-435 [EHL No. 84110]).
Mount E (1991) KP-140 non-definitive acute inhalation toxicity study
in rats (Study No. 191-1208). Princeton, New Jersey, FMC Corporation,
Toxicology Laboratory.
Myhr BC & Caspary WJ (1991) Chemical mutagenesis at the thymidine
kinase locus in L5178Y mouse lymphoma cells: Results for 31 coded
compounds in the National Toxicology Program. Environ Mol Mutagen, 18:
51-83.
Nakashima H, Matsunaga I, & Miyano N (1993) Determination of tris
(2-butoxyethyl) phosphate in textiles and household wax products by
capillary gas chromatography. Jpn J Toxicol Environ Health, 39(6):
549-553.
O'Conner J (1992) THPS: Determination of hydrolysis as a function of
pH. Eye, United Kingdom, Life Science Research Limited (LSR report No.
91/AWL/015/0737, prepared for Albright & Wilson, International
Technical Center, Oldbury, United Kingdom).
Paxéus N, Robinson P, & Balmér P (1992) Study of organic pollutants in
municipal wastewater in Göteborg, Sweden. Water Sci Technol, 25(11):
249-256.
Piegorsch WW & Hoel DG (1988) Exploring relationships between
mutagenic and carcinogenic potencies. Mutat Res, 196: 161-175.
Riach CG (1994) THPS-75%: Assessment of genotoxicity in an unscheduled
DNA synthesis assay using adult rat hepatocyte primary cultures
-- Supplemental information to report MRID No. 4222363-17. Falls
Church, Virginia, Inveresk Research International (Unpublished
report).
Riach CG (1996) THPS mouse lymphoma mutation assay (Project No.
756936). Falls Church, Virginia, Inveresk Research International
(Unpublished report No. 12110).
Rivera J, Ventura F, Caixach J, de Torres M, & Figueras A (1987)
GC/MS, HPLC and FAB mass spectrometric analysis of organic pollutants
in Barcelona's water supply. J Environ Chem, 29: 15.
Roberts NL & Fairley C (1988) The dietary toxicity (LC50) of DP 435
to the mallard duck. Huntingdon, United Kingdom, Huntingdon Research
Center Limited (Unpublished report No. A&W 473/87982).
Roberts NL & Phillips C (1988a) The dietary toxicity (LC50) of DP 435
to the Bobwhite quail. Huntingdon, United Kingdom, Huntingdon Research
Center Limited (Unpublished report No. A&W 474/871332).
Roberts NL & Phillips C (1988b) The acute oral toxicity (LD50) of DP
435 to the mallard duck. Huntingdon, United Kingdom, Huntingdon
Research Center Limited (Unpublished report No. A&W 475/87999).
Roddie B (1994) Assessment of the sediment-phase toxicity of Tolcide
PS 75 to the sediment-dwelling amphipod Coronium volutator.
Edinburgh, United Kingdom, Environment & Resource Technology Limited
(Unpublished report No. ERT 94/005/V1).
Saeger VW, Hicks O, Kaley RG, Michael PR, Mieure JP, & Tucker ES
(1979) Environmental fate of selected phosphate esters. Environ Sci
Technol, 13(7): 840-844.
Saitoh M, Umemura T, Kawasaki Y, Momma J, Matsushima Y, Matsumoto M,
Eshita N, Isama K, & Kaniwa M (1994) Subchronic toxicity study of
tributoxyethyl phosphate in Wistar rats. Eiser Shikensko Hokoku, 112:
27-39.
Sasaki M, Sugimura K, Yoshida MA, & Abe S (1980) Cytogenic effects of
60 chemicals on cultured human and Chinese hamster cells. Senshokutai,
20: 574-584.
Shelby MD & Witt KL (1995) Comparison of results from mouse bone
marrow chromosome aberration and micro nucleus tests. Environ Mol
Mutagen, 25: 302-313.
Shelby MD, Erexson GL, Hook GJ, & Tice RR (1993) Evaluation of a three
exposure mouse bone marrow micronucleus protocol: Results with 49
chemicals. Environ Mol Mutagen, 21: 160-179.
Sheldon LS & Hites RA (1978) Organic compounds in the Delaware River.
Environ Sci Technol, 12(10): 1188-1194.
Smyth HF & Carpenter CP (1948) Further experience with the range
finding test in the industrial toxicology laboratory. J Ind Hyg
Toxicol, 30(1): 63-68.
Snell K (1994) Proban CC: Acute dermal irritation test in the rabbit
(Project No. 71/344, sponsored by Albright & Wilson). Derby, United
Kingdom, Safepharm Laboratories Ltd.
Thompson PW (1997a) THPS cholinesterase inhibition test (Project No.
071/506, sponsored by Albright & Wilson). Derby, United Kingdom,
Safepharm Laboratories Ltd.
Thompson PW (1997b) THPO cholinesterase inhibition test (Project No.
071/507, sponsored by Albright & Wilson). Derby, United Kingdom,
Safepharm Laboratories Ltd.
Torp UM & McWilliam P (1994) Tolcide PS75: Determination of acute
lethal toxicity to Acartia tonsa. Bergen, Norway, Terra
Miljö-Laboratories A/S (Unpublished report No.
60090.aw\tm.001\acartia).
Tou JC, Westover LB, & Sonnabend LF (1974) Kinetic studies of
bis(chloromethyl)ether hydrolysis by mass spectrometry. J Phys Chem,
78(11): 1096-1098.
Tremain SP & Bartlett AJ (1994) Amgard TBEP determination of hazardous
physico-chemical properties (Project No. 583/014, sponsored by
Albright & Wilson). Derby, United Kingdom, Safepharm Laboratories Ltd.
Tsuda M, Saito M, Umemura T, Kawasaki Y, Momma J, Matsushima Y,
Matsumoto M, Isama K, Kawiwa M, & Kurokawa Y (1993) A 14-week oral
toxicity study of tributoxyethyl phosphate (TBEP) in rats. J Toxicol
Sci, 18(4): 421, 429.
Tsuji S, Tonogai Y, Ito Y, & Kanoh S (1986) The influence of rearing
temperatures on the toxicity of various environmental pollutants for
killifish (Oryzias latipes). Eisei Kagaku, 32(1): 46-53.
Tuffnell PP (1991) Proban CC: Acute oral toxicity test in the rat
(Study sponsored by Albright & Wilson). Derby, United Kingdom,
Safepharm Laboratories Ltd.
Tuffnell PP (1992) Proban CC: Magnusson & Kligman maximisation study
in the guinea pig (Project No. 71/127, sponsored by Albright &
Wilson). Derby, United Kingdom, Safepharm Laboratories Ltd.
Ulsamer AG, Osterberg RE, & McLaughlin J Jr (1980) Flame-retardant
chemicals in textiles. Clin Toxicol, 17(1): 101-131.
US NTP (1984) Toxicology and carcinogenesis studies of
tris(2-ethylhexyl)phosphate (CAS No. 78-42-2) in F344/N rats and
B6C3F1 mice (gavage studies). Research Triangle Park, North Carolina,
US Department of Health and Human Services, National Toxicology
Program (Technical Report Series No. 274; NIH Publication No.
84-2530).
US NTP (1987) Toxicology and carcinogenesis studies of
tetrakis(hydroxymethyl) phosphonium sulfate (THPS) (CAS No.
55566-30-8) an tetrakis(hydroxymethyl) phosphonium chloride (THPC)
(CAS No. 124-64-1) in F344/N rats and B6C3F1 mice (gavage studies).
Research Triangle Park, North Carolina, US Department of Health and
Human Services, National Toxicology Program (Technical Report Series
No. 29; NIH Publication No. 87-2552).
Van Duuren B, Loewengart G, Seidman I, Smith A, & Melchionne S (1978)
Mouse skin carcinogenicity tests of the flame retardants
tris(2,3-dibromopropyl) phosphate, tetrakis(hydroxymethyl) phosphonium
chloride, and polyvinylbromide. Cancer Res, 38: 3236-3240.
Watts CD & Moore K (1988) Fate and transport of organic compounds in
rivers. In: Angeletti G & Bjorseth A ed. Organic micropollutants in
the aquatic environment: Proceedings of the 5th European Symposium,
Rome, 20-22 October 1987. Boston, Massachusetts, Kluwer Academic
Publishers, pp 154-169 (Water Pollution Research Reports Series, No.
4; EUR 11350).
Weber K & Ernst W (1983) [Presence and fluctuation of organic
environmental chemicals in German estuaries.] Vom Wasser, 61: 111-123
(in German).
Weil ED (1980) Kirk-Othmer encyclopedia of chemical technology. New
York, John Wiley and Sons, vol 10, pp 488-489.
Weschler CJ (1984) Indoor-outdoor relationships for nonpolar
constituents of aerosol particles. Environ Sci Technol, 18(9):
648-652.
Weschler CJ (1980) Characterization of selected organic compounds in
size-fractionated indoor aerosols. Environ Sci Technol, 14(4):
428-431.
Weschler CJ & Fong KL (1986) Characterization of organics species
associated with indoor aerosol particles. Environ Int, 12: 93-97.
Weschler CJ & Shields HC (1986) The accumulation of "Additives" in
office air -- Proceedings of the 79th Annual Meeting of the Air
Pollution Control Association, Minneapolis, 22-27 June 1986.
Pittsburgh, Pennsylvania, Air Pollution Control Association.
Wetton PM & Handley JW (1998) Acute toxicity to Rainbow trout (Project
No. 071/608R, sponsored by Albright & Wilson). Derby, United Kingdom,
Safepharm Laboratories Ltd.
Williams DT, Nestmann ER, Lebel GL, Benoit FM, Otson R, & Lee EGH
(1982) Determination of mutagenic potential and organic contaminants
of Great Lakes drinking water. Chemosphere, 11(3): 263-276.
Willis CV (1995) Tolcide PS75 final report. Physical and chemical
characteristics of Tolcide PS75: vapor pressure (Study sponsored by
Albright & Wilson). Whippany, New Jersey, Case Consulting Laboratories
Inc.
Wragg MS, Thomas ON, & Brooks PN (1996) Twenty-eight day subacute
dermal toxicity study in the rat (Sponsored by Albright & Wilson).
Derby, United Kingdom, SafePharm Laboratories Ltd. Derby UK.
Yasuda H (1980) Concentration of organic phosphorus pesticides in the
atmosphere above the Dogo and Ozun Basin. J Chem Soc Jpn, 4: 645-653.
Zeiger E, Anderson B, Haworth S, Lawlor T, Mortelmans K, & Speck W
(1987) Salmonella mutagenicity test: III. Results from the testing of
255 chemicals. Environ Mutagen, 9(suppl 9): 1-110.
Zeiger E, Haworth S, Mortelmans K, & Speck W (1985) Mutagenicity
testing of di(2-ethylhexyl)phthalate and related chemicals in
Salmonella. Environ Mutagen, 7: 213-232.
Appendix A
Flammability standards met by products treated by THPC-urea
condensates a
Items Flammability standards
Protective clothing ASTM F-1506
ASTM F-955
NFPA 1975
NFPA 1977
EN 533 : 1977 Index 3 after 50 washes at 75°C
EN 531 : 1995 para. 6.2.2 after 50 washes at 75°C
EN 470-1 : 1995 para. 6.1 after 50 washes at 75°C
EN 470-1 : para. 6.2
EN 531 : 1995 para. 6.3
EN 531 : 1995 para. 6.4
EN 531 : 1995 para. 6.6
Sheeting, blankets and Ingnition sources 0, 1 and 5.
BS 7175 counterpanes When tested on top of and below the test
fabric, after 200 washes at 74°C (BS 5651 HLPN).
BS 5815 : Part 3 : 1991
Curtains and drapes NFPA 701
BS 5867 : 1980 Part 2 Type B after 200 washes
at 74°C (BS 5651 HLPN).
Mattress ticking and Ignition sources 0, 1 and 5 when tested
BS 7175 mattresses on top of and below the test fabric, after 3
washes at 74°C
BS 597-1 : 1995 (fabric tested in combination
with a non-fire-retardant polyurethane foam block).
BS 597-2 : 1995 (fabric tested in combination with a
non-fire-retardant polyurethane foam block).
Sleepwear DOC FF 3-71
BS 5722 : 1984 when testing in accordance with
BS 5438 : 1976 Test 2.
BS 5722 : 1991 Level 1.
Upholstery BS 5852 part 1 Ignition sources 0 (smouldering
cigarette) and 1 (simulated match) after a 30-min
water soak, BS 5651 amended.
EN 1021-1 : 1994 (fabric tested in combination with
a non-fire-retardant polyurethane foam block).
EN 1021-2 : 1994 (fabric tested in combination with
a non-fire-retardant polyurethane foam block).
a From: Dr P. Martin, Albright & Wilson, personal communication to IPCS
RÉSUMÉ, EVALUATION ET RECOMMANDATIONS
1. Phosphate de tris(2-butoxyéthyle) (TBEP)
1.1 Résumé
Le phosphate de tris(butoxyéthyle) ou TBEP est utilisé pour la
confection d'encaustiques destinés à l'entretien des sols ou encore
comme plastifiant dans les caoutchoucs et les matières plastiques. On
ne connaît pas le volume de la production annuelle mondiale, mais on
pense qu'il est de l'ordre de 5000 à 6000 tonnes.
La présence du TBEP dans l'environnement résulte exclusivement de
l'activité humaine. Sa répartition dans l'environnement a été étudiée
dans certains pays industrialisés. On a trouvé une concentration
inférieure à 300 ng/litre dans les eaux de surface et comprise entre
100 et 1000 µg/kg dans les matières particulaires. Aucune des 167
analyses effectuées n'a permis d'en mettre en évidence dans les
poissons. Une unique étude en a décelé sa présence dans l'air
extérieur (< 200 ng/m3). Le dosage du TBEP dans l'air de bureaux a
donné une concentration de 25 ng/m3 tout au plus. Le TBEP est associé
aux matières particulaires et la source en est dans ce cas les
encaustiques que l'on applique sur le sol. On l'a décelé à des
concentrations de l'ordre du µg/kg dans les tissus adipeux humains.
D'après des études basées sur le panier de la ménagère, la dose
journalière ingérée serait de moins de 0,02 µg/kg de poids corporel
pour diverses tranches d'âge. On en a également signalé la présence
dans l'eau de boisson à des concentrations pouvant atteindre 270
µg/litre. Elle est due, semble-t-il, à la migration du produit contenu
dans les joints de caoutchouc de l'installation sanitaire.
On estime que le TBEP est facilement biodégradable. Des mesures
effectuées dans des stations d'épuration des eaux d'égout et des
dosages pratiqués en semi-continu au laboratoire sur des boues de même
provenance montrent que le TBEP s'élimine en grande partie (> 80 %).
Dans les cours d'eau et les eaux littorales, le TBEP est totalement
décomposé. On a fait état d'une demi-vie d'environ 50 jours dans les
eaux estuarielles, la décomposition étant minime dans l'eau de mer non
adaptée.
Le composé présente une faible toxicité aiguë pour les mammifères
et son pouvoir irritant est également faible.
Plusieurs études subchroniques sur des animaux de laboratoire
montrent que la toxicité du TBEP s'exerce au niveau du foie qui en est
l'organe cible. Une étude sur des rats Sprague-Dawley incite à penser
que le TBEP pourrait provoquer une myocardite focale. Des effets
neurologiques ont été observés chez le rat après ingestion d'une seule
dose, mais ils n'apparaissent pas systématiquement. Lorsqu'on
l'administre répétitivement à forte dose à des rats par gavage, le
TBEP réduit la vitesse de conduction nerveuse et augmente la durée de
la période réfractaire. Après administration à des poules, on n'a pas
constaté de neurotoxicité retardée, mais il y avait par contre
inhibition de la cholinestérase cérébrale et plasmatique.
Une étude de 18 semaines au cours desquelles des rats ont reçu
des doses répétées de TBEP a permis de fixer à 15 mg.kg-1j-1 pc la
dose sans effet observable (NOEL) sur le foie, la dose la plus faible
produisant un effet observable (LOEL) étant de 150 mg.kg-1.j-1 pc.
Ni la toxicité à long terme ni le pouvoir cancérogène de ce
composé n'ont été étudiés.
Les tests de mutation génique effectués sur des cellules
mammaliennes et sur des bactéries n'ont donné que des résultats
négatifs mais il n'existe aucun compte rendu de recherche de lésions
chromosomiques.
Une étude effectuée sur des rats n'a révélé aucun effet
tératogène. Il n'existe pas de publication faisant état d'autres
effets toxiques sur la reproduction.
Les tests de sensibilisation effectués sur des sujets humains par
apposition d'un timbre cutané ne font ressortir aucune sensibilisation
mais seulement une légère irritation.
Le TBEP est modérément toxique pour les organismes aquatiques. La
CL50 à 48 h pour Daphnia magna est de 75 mg/litre et la CL50 à 96 h
se situe entre 16 et 24 mg/litre pour les poissons.
1.2 Evaluation
L'exposition sur les lieux de travail se produit
vraisemblablement au niveau de la peau pendant la fabrication ou
l'utilisation d'encaustiques pour sols (exposition accidentelle). Le
composé est absorbé par voie percutanée chez l'animal de laboratoire,
mais on ne possède aucune information sur sa cinétique ou son
métabolisme. On ne peut donc pas évaluer quantitativement l'exposition
par cette voie, mais on peut penser qu'elle doit être faible. Les
mesures montrent que l'exposition par la voie respiratoire dans un
bureau est au plus égale à 25 ng/m3.
L'exposition de la population générale s'opère principalement par
la voie alimentaire, (du fait que le TBEP est présent comme
plastifiant dans les plastiques utilisés pour l'emballage des produits
alimentaires) et par la consommation d'eau de boisson contaminée par
le TBEP contenu dans les joints de caoutchouc synthétique de la
plomberie. Elle est cependant très faible dans les deux cas (estimée à
moins de 0,2 µg par kg pc et par jour avec une concentration dans
l'eau de boisson inférieure à 270 µg/litre).
Compte tenu de la valeur de la NOEL tirée des études sur l'animal
(15 mg.kg-1.j-1, valeur obtenue après administration répétée du
composé par voie orale), on peut considérer que le risque est très
faible pour la population générale. On estime qu'il est également très
faible sur les lieux de travail, encore qu'il ne soit pas possible
d'en donner une évaluation chiffrée.
Dans l'environnement, on peut déduire de la faible volatilité, du
fort coefficient d'adsorption et de sa solubilité modérée dans l'eau,
que le TBEP va se répartir entre les différents types de matières
particulaires. Les quelques mesures dont on dispose confirment cette
hypothèse. Sa décomposition dans les différents compartiments de
l'environnement devrait être rapide. On ne dispose d'aucune donnée sur
ces produits de décomposition ; le reste phosphate libéré au cours de
ce processus ne devrait pas sensiblement augmenter la concentration
globale des nutriments présents dans l'environnement. La Fig. 1 donne
la valeur de la concentration relevée dans les eaux de surface en
fonction de la toxicité aiguë observée. Il y a plusieurs ordres de
grandeur entre la concentration la plus élevée mesurée et la plus
faible de la toxicité observée, d'où une marge de sécurité élevée et
donc un faible risque pour les organismes aquatiques. On n'est pas en
mesure d'évaluer l'importance du risque pour les organismes
terrestres.
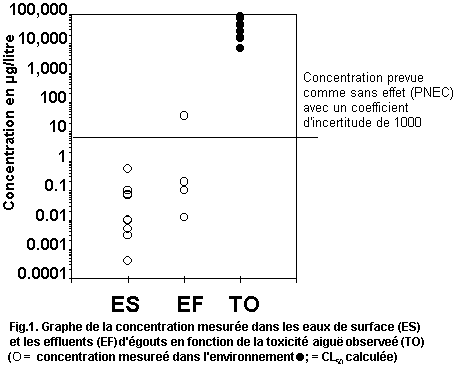 1.3 Recommandations
Pour procéder à une évaluation scientifique complète de ce
composé, il faudrait identifier et étudier chacun de ses métabolites
chez des mammifères, étant donné le profil toxicologique d'un des
métabolites possibles, le 2-butoxyéthanol.
2. Phosphate de tris (2-éthylhexyle) (TEHP)
2.1 Résumé
Le phosphate de tris (2-éthylhexyle) ou THEP, se présente sous la
forme d'un liquide incolore et ininflammable, dont la solubilité dans
l'eau et la tension de vapeur sont faibles et qui est utilisé comme
retardateur de flammes et comme plastifiant dans le PVC et l'acétate
de cellulose ou encore comme solvant. On le prépare à partir de
l'oxychlorure de phosphore et du 2-éthyléthanol. On ne connaît pas les
chiffres actuels de production dans le monde. En Allemagne, la
production annuelle est actuellement d'environ 1000 tonnes.
On n'a pas décelé la présence de TEHP dans l'air extérieur ; à
l'intérieur des bâtiments, sa concentration dans l'air est inférieure
à 10 ng/m3. Dans les cours d'eau, la concentration peut atteindre
7500 ng/litre et dans les matières particulaires, 2 à 70 ng/g. On en a
trouvé 0,3 ng/litre dans un seul et unique échantillon d'eau de
boisson. D'après des études sur le panier de la ménagère, la dose
ingérée journalière pour diverses tranches d'âge serait inférieure à
0,05 µg/kg pc.
Le TEHP est rapidement décomposé dans les eaux naturelles, mais
des essais en laboratoire portant sur des boues activées ont donné des
résultats équivoques. Il ne subit pas de décomposition abiotique
importante.
Le TEHP présente une faible toxicité aiguë pour les mammifères,
la DL50 par voie orale étant > 10 000 mg/kg pc pour le rat.
Le TEHP irrite la peau mais il n'est pas irritant pour l'oeil.
Des applications répétées de TEHP sur la peau de lapins à raison de
0,1 ml (93 mg) n'ont produit aucun signe d'intoxication générale.
Des études au cours desquelles des rats et des souris ont reçu
pendant 13 semaines du TEHP par gavage n'ont pas permis d'observer
d'effets toxiques importants. Pour les rats, la dose sans effet nocif
observable (NOAEL) était égale à 2860 mg/kg pc et pour les souris, à
5710 mg/kg pc, soit les deux plus fortes doses utilisées chez chaque
espèce.
Lors d'une étude de 3 mois au cours de laquelle on a fait inhaler
du TEHP à des chiens à des doses allant jusqu'à 85,0 mg/m3, on a
observé de légères altérations inflammatoires chroniques au niveau des
poumons et constaté que le réflexe conditionné d'évitement
s'affaiblissait parallèlement à l'augmentation des doses.
Il n'existe pas d'étude consacrée à la toxicité génésique du
TEHP.
Le composé a donné des résultats négatifs dans plusieurs tests de
mutagénicité in vitro et in vivo.
On a étudié la toxicité chronique et le pouvoir cancérogène du
TEHP sur des rats et des souris. La NOAEL relative à la toxicité
chronique était de 2857 mg.kg-1.j-1 pour les rats mâles et de 1428
mg.kg-1.j-1 pour les femelles. Chez les souris mâles et femelles, la
dose la plus faible produisant un effet nocif observable (LOAEL) était
de 357 mg.kg-1.j-1, le critère retenu étant une hyperplasie des
cellules folliculaires de la thyroïde. On n'a pas établi de NOAEL pour
les souris. Les auteurs de ces études concluent que le composé
présente un certain pouvoir cancérogène, compte tenu de l'augmentation
des carcinomes hépatocellulaires qui a été observée chez les souris
femelles, cette cancérogénicité étant plus équivoque pour les rats
mâles chez qui a été relevée une augmentation de l'incidence des
phéochromocytomes surrénaliens aux deux doses administrées. Malgré
l'augmentation de l'incidence des phéochromocytomes aux deux doses
chez les rats mâles et de celle des carcinomes hépatocellulaires chez
les souris soumises à la plus forte dose, on estime qu'il n'y a pas
lieu de considérer que le TEHP comporte un risque cancérogène
important pour l'Homme. En effet, chez le rat l'incidence
« naturelle » des phéochromocytomes est variable. Ainsi, dans deux
études toxicologiques effectuées antérieurement par le National
Toxicology Programme (NTP ) on a observé des phéochromocytomes dont
l'incidence était égale à celle constatée dans l'étude précédente.
Pour ce qui est de l'autre type de tumeur observé, à savoir le
carcinome hépatocellulaire chez les souris femelles soumises à la dose
la plus forte, le fait que son incidence soit faible, qu'elle ne se
soit manifestée que dans un seul sexe et chez une seule espèce, qu'on
n'ait pas de preuve d'une quelconque activité génotoxique et que
l'Homme ne soit guère exposé au TEHP, rend improbable la possibilité
d'un risque cancérogène notable pour l'Homme.
Des études sur la neurotoxicité du TEHP ont été effectuées sur
plusieurs espèces. Le composé ne provoque aucune modification dans
l'activité de la cholinestérase plasmatique ou érythrocytaire. On n'a
pas connaissance d'études sur la neurotoxicité retardée du TEHP.
Une étude sur des volontaires humains n'a pas révélé d'irritation
cutanée.
Les quelques données disponibles montrent que le composé présente
une faible toxicité aiguë pour les organismes aquatiques. Pour les
bactéries, la CI50 à 96 h est supérieure à 100 mg/litre et pour un
poisson comme le danio (Brachydanio rerio), elle dépasse également
cette valeur, qui correspond d'ailleurs à la limite de solubilité du
TEHP dans l'eau.
2.2 Evaluation
L'exposition au TEHP se produit vraisemblablement par voie
cutanée au cours de la préparation du composé (exposition
accidentelle) ou par suite de l'utilisation de produits qui en
contiennent. Le TEHP est absorbé par voie percutanée chez l'animal de
laboratoire mais on dispose d'aucune donnée sur sa cinétique ou son
métabolisme après absorption par cette voie. On ne peut donc pas
évaluer quantitativement ce type d'exposition, mais on peut penser
qu'elle est faible. La mesure de l'exposition par inhalation de l'air
des bureaux a donné une valeur au plus égale à 10 ng/m3.
L'exposition de la population générale se produit principalement
par la consommation de nourriture et d'eau de boisson. Quelle que soit
la source, cette exposition est très faible (l'exposition par voie
alimentaire est estimée à moins de 0,05 µg.kg-1.j-1 ; une seule et
unique mesure effectuée sur de l'eau de boisson a donné 0,3 ng/litre).
Si l'on se base sur la LOAEL de 357 mg.kg-1.j-1 obtenue chez la
souris avec comme critère une hyperplasie de la thyroïde, le risque
est très faible à l'échelon de la population générale. On estime que
le risque est également très faible sur les lieux de travail, encore
qu'il ne soit pas possible d'en donner une évaluation quantitative.
On estime que le TEHP n'est pas cancérogène pour l'Homme.
Dans l'environnement, on peut déduire de la faible volatilité, du
fort coefficient d'adsorption et de sa solubilité modérée dans l'eau,
que le TEHP va se répartir entre les différents types de matières
particulaires. Les mesures dont on dispose sont cependant trop peu
nombreuses pour le confirmer. On peut s'attendre à une décomposition
dans l'environnement, mais les données de laboratoire relatives à la
décomposition du TEHP dans les boues d'égout sont ambiguës. On ne
dispose d'aucune donnée sur ses produits de décomposition ; le reste
phosphate libéré au cours de ce processus ne devrait pas sensiblement
augmenter la concentration globale des nutriments. La Fig. 2 donne la
valeur de la concentration relevée dans divers compartiments du milieu
en fonction de la toxicité aiguë relevée (aucun effet toxique observé
à la limite de solubilité dans l'eau). Il y a plusieurs ordres de
grandeur entre la concentration la plus élevée mesurée et la plus
faible valeur de la toxicité observée, d'où une marge de sécurité
élevée et donc un faible risque pour les organismes aquatiques. On
n'est pas en mesure d'évaluer l'importance du risque pour les
organismes terrestres.
2.3 Recommandations
Pour procéder à une évaluation scientifique complète de ce
composé, il faudrait identifier et étudier chacun de ses métabolites
chez des mammifères, étant donné le profil toxicologique d'un des
métabolites possibles, le 2-éthylhexanol.
La toxicité génésique doit être étudiée, notamment en ce qui
concerne d'éventuels effets sur le développement.
3. Sels de tétrakis(hydroxyméthyl) phosphonium
3.1 Résumé
Les sels de tétrakis(hydroxyméthyl)phosphonium (THP) constituent
un groupe important de composés utilisés comme retardateurs de flammes
pour le coton, la cellulose et les toiles constituées de mélanges de
cellulose. On constate que la migration du chlorure de
tétrakis(hydroxyméthyl)phosphonium (THPC) à partir des tissus traités
par le condensat de ce composé avec l'urée reste faible. Le sulfate de
THP (THPS) est surtout utilisé comme produit biocide. On estime que la
production mondiale est de moins de 3000 tonnes par an pour les sels
de THP et d'environ 3000 tonnes pour le condensat chlorure de tétrakis
(hydroxyméthyl)phosphonium-urée.
La photodécomposition et l'hydrolyse des sels de THP constituent
des voies de dégradation abiotiques importantes dans l'environnement.
Le sulfate de THP ne se fixe guère sur les matières particulaires et
il est donc mobile. Le THPS se décompose rapidement en aérobiose comme
en anaérobiose. On a constaté la présence d'oxyde de
trihydroxyméthylphosphine (THPO) et d'acide
bishydroxyméthylphosphonique ou BMPA dans les produits de
décomposition.
Comme il ne semble pas y avoir de surveillance de ces composés,
on ne peut pas évaluer l'exposition de l'Homme ni celle des autres
êtres vivants dans leur milieu naturel.
Le THPC et le THPS présentent une toxicité aiguë modérée par voie
orale ; au niveau cutané, leur toxicité est faible.
Des études à court terme (jusqu'à 28 jours) effectuées sur des
rats et des souris ont montré que le principal effet toxique du THPC
et du THPS était une réduction du poids corporel. Chez les deux
espèces, la NOAEL est d'environ 8 mg.kg-1.j-1. Des études plus
longues (13 semaines) montrent que le principal organe cible est le
foie. La NOAEL relative à cet effet varie de 3 à 7 mg.kg-1.j-1 pour
les deux sels chez les deux espèces. Les tests biologiques de
cancérogénicité effectués sur le THPC ont également montré que ces
effets se produisaient au niveau du foie, mais on n'a pas établi de
NOAEL. Chez les deux espèces, la LOAEL était d'environ 3 mg.kg-1.j-1.
Lors d'une étude de cancérogénicité portant sur le THPS et effectuée
sur des souris, on a évalué à 3,6 mg.kg-1.j-1 la NOAEL pour une
hyperplasie médullo-surrénalienne focale; chez les rats, la LOAEL pour
la mortalité avait la même valeur.
Administré en dose unique à des lapins, le THPS n'a pas provoqué
d'irritation cutanée. Cependant, l'exposition répétée de rats à ce
composé par la voie cutanée a entraîné une sérieuse réaction à ce
niveau. Le condensat THPC-urée s'est révélé corrosif. Chez le lapin,
le THPS irrite fortement la muqueuse oculaire.
1.3 Recommandations
Pour procéder à une évaluation scientifique complète de ce
composé, il faudrait identifier et étudier chacun de ses métabolites
chez des mammifères, étant donné le profil toxicologique d'un des
métabolites possibles, le 2-butoxyéthanol.
2. Phosphate de tris (2-éthylhexyle) (TEHP)
2.1 Résumé
Le phosphate de tris (2-éthylhexyle) ou THEP, se présente sous la
forme d'un liquide incolore et ininflammable, dont la solubilité dans
l'eau et la tension de vapeur sont faibles et qui est utilisé comme
retardateur de flammes et comme plastifiant dans le PVC et l'acétate
de cellulose ou encore comme solvant. On le prépare à partir de
l'oxychlorure de phosphore et du 2-éthyléthanol. On ne connaît pas les
chiffres actuels de production dans le monde. En Allemagne, la
production annuelle est actuellement d'environ 1000 tonnes.
On n'a pas décelé la présence de TEHP dans l'air extérieur ; à
l'intérieur des bâtiments, sa concentration dans l'air est inférieure
à 10 ng/m3. Dans les cours d'eau, la concentration peut atteindre
7500 ng/litre et dans les matières particulaires, 2 à 70 ng/g. On en a
trouvé 0,3 ng/litre dans un seul et unique échantillon d'eau de
boisson. D'après des études sur le panier de la ménagère, la dose
ingérée journalière pour diverses tranches d'âge serait inférieure à
0,05 µg/kg pc.
Le TEHP est rapidement décomposé dans les eaux naturelles, mais
des essais en laboratoire portant sur des boues activées ont donné des
résultats équivoques. Il ne subit pas de décomposition abiotique
importante.
Le TEHP présente une faible toxicité aiguë pour les mammifères,
la DL50 par voie orale étant > 10 000 mg/kg pc pour le rat.
Le TEHP irrite la peau mais il n'est pas irritant pour l'oeil.
Des applications répétées de TEHP sur la peau de lapins à raison de
0,1 ml (93 mg) n'ont produit aucun signe d'intoxication générale.
Des études au cours desquelles des rats et des souris ont reçu
pendant 13 semaines du TEHP par gavage n'ont pas permis d'observer
d'effets toxiques importants. Pour les rats, la dose sans effet nocif
observable (NOAEL) était égale à 2860 mg/kg pc et pour les souris, à
5710 mg/kg pc, soit les deux plus fortes doses utilisées chez chaque
espèce.
Lors d'une étude de 3 mois au cours de laquelle on a fait inhaler
du TEHP à des chiens à des doses allant jusqu'à 85,0 mg/m3, on a
observé de légères altérations inflammatoires chroniques au niveau des
poumons et constaté que le réflexe conditionné d'évitement
s'affaiblissait parallèlement à l'augmentation des doses.
Il n'existe pas d'étude consacrée à la toxicité génésique du
TEHP.
Le composé a donné des résultats négatifs dans plusieurs tests de
mutagénicité in vitro et in vivo.
On a étudié la toxicité chronique et le pouvoir cancérogène du
TEHP sur des rats et des souris. La NOAEL relative à la toxicité
chronique était de 2857 mg.kg-1.j-1 pour les rats mâles et de 1428
mg.kg-1.j-1 pour les femelles. Chez les souris mâles et femelles, la
dose la plus faible produisant un effet nocif observable (LOAEL) était
de 357 mg.kg-1.j-1, le critère retenu étant une hyperplasie des
cellules folliculaires de la thyroïde. On n'a pas établi de NOAEL pour
les souris. Les auteurs de ces études concluent que le composé
présente un certain pouvoir cancérogène, compte tenu de l'augmentation
des carcinomes hépatocellulaires qui a été observée chez les souris
femelles, cette cancérogénicité étant plus équivoque pour les rats
mâles chez qui a été relevée une augmentation de l'incidence des
phéochromocytomes surrénaliens aux deux doses administrées. Malgré
l'augmentation de l'incidence des phéochromocytomes aux deux doses
chez les rats mâles et de celle des carcinomes hépatocellulaires chez
les souris soumises à la plus forte dose, on estime qu'il n'y a pas
lieu de considérer que le TEHP comporte un risque cancérogène
important pour l'Homme. En effet, chez le rat l'incidence
« naturelle » des phéochromocytomes est variable. Ainsi, dans deux
études toxicologiques effectuées antérieurement par le National
Toxicology Programme (NTP ) on a observé des phéochromocytomes dont
l'incidence était égale à celle constatée dans l'étude précédente.
Pour ce qui est de l'autre type de tumeur observé, à savoir le
carcinome hépatocellulaire chez les souris femelles soumises à la dose
la plus forte, le fait que son incidence soit faible, qu'elle ne se
soit manifestée que dans un seul sexe et chez une seule espèce, qu'on
n'ait pas de preuve d'une quelconque activité génotoxique et que
l'Homme ne soit guère exposé au TEHP, rend improbable la possibilité
d'un risque cancérogène notable pour l'Homme.
Des études sur la neurotoxicité du TEHP ont été effectuées sur
plusieurs espèces. Le composé ne provoque aucune modification dans
l'activité de la cholinestérase plasmatique ou érythrocytaire. On n'a
pas connaissance d'études sur la neurotoxicité retardée du TEHP.
Une étude sur des volontaires humains n'a pas révélé d'irritation
cutanée.
Les quelques données disponibles montrent que le composé présente
une faible toxicité aiguë pour les organismes aquatiques. Pour les
bactéries, la CI50 à 96 h est supérieure à 100 mg/litre et pour un
poisson comme le danio (Brachydanio rerio), elle dépasse également
cette valeur, qui correspond d'ailleurs à la limite de solubilité du
TEHP dans l'eau.
2.2 Evaluation
L'exposition au TEHP se produit vraisemblablement par voie
cutanée au cours de la préparation du composé (exposition
accidentelle) ou par suite de l'utilisation de produits qui en
contiennent. Le TEHP est absorbé par voie percutanée chez l'animal de
laboratoire mais on dispose d'aucune donnée sur sa cinétique ou son
métabolisme après absorption par cette voie. On ne peut donc pas
évaluer quantitativement ce type d'exposition, mais on peut penser
qu'elle est faible. La mesure de l'exposition par inhalation de l'air
des bureaux a donné une valeur au plus égale à 10 ng/m3.
L'exposition de la population générale se produit principalement
par la consommation de nourriture et d'eau de boisson. Quelle que soit
la source, cette exposition est très faible (l'exposition par voie
alimentaire est estimée à moins de 0,05 µg.kg-1.j-1 ; une seule et
unique mesure effectuée sur de l'eau de boisson a donné 0,3 ng/litre).
Si l'on se base sur la LOAEL de 357 mg.kg-1.j-1 obtenue chez la
souris avec comme critère une hyperplasie de la thyroïde, le risque
est très faible à l'échelon de la population générale. On estime que
le risque est également très faible sur les lieux de travail, encore
qu'il ne soit pas possible d'en donner une évaluation quantitative.
On estime que le TEHP n'est pas cancérogène pour l'Homme.
Dans l'environnement, on peut déduire de la faible volatilité, du
fort coefficient d'adsorption et de sa solubilité modérée dans l'eau,
que le TEHP va se répartir entre les différents types de matières
particulaires. Les mesures dont on dispose sont cependant trop peu
nombreuses pour le confirmer. On peut s'attendre à une décomposition
dans l'environnement, mais les données de laboratoire relatives à la
décomposition du TEHP dans les boues d'égout sont ambiguës. On ne
dispose d'aucune donnée sur ses produits de décomposition ; le reste
phosphate libéré au cours de ce processus ne devrait pas sensiblement
augmenter la concentration globale des nutriments. La Fig. 2 donne la
valeur de la concentration relevée dans divers compartiments du milieu
en fonction de la toxicité aiguë relevée (aucun effet toxique observé
à la limite de solubilité dans l'eau). Il y a plusieurs ordres de
grandeur entre la concentration la plus élevée mesurée et la plus
faible valeur de la toxicité observée, d'où une marge de sécurité
élevée et donc un faible risque pour les organismes aquatiques. On
n'est pas en mesure d'évaluer l'importance du risque pour les
organismes terrestres.
2.3 Recommandations
Pour procéder à une évaluation scientifique complète de ce
composé, il faudrait identifier et étudier chacun de ses métabolites
chez des mammifères, étant donné le profil toxicologique d'un des
métabolites possibles, le 2-éthylhexanol.
La toxicité génésique doit être étudiée, notamment en ce qui
concerne d'éventuels effets sur le développement.
3. Sels de tétrakis(hydroxyméthyl) phosphonium
3.1 Résumé
Les sels de tétrakis(hydroxyméthyl)phosphonium (THP) constituent
un groupe important de composés utilisés comme retardateurs de flammes
pour le coton, la cellulose et les toiles constituées de mélanges de
cellulose. On constate que la migration du chlorure de
tétrakis(hydroxyméthyl)phosphonium (THPC) à partir des tissus traités
par le condensat de ce composé avec l'urée reste faible. Le sulfate de
THP (THPS) est surtout utilisé comme produit biocide. On estime que la
production mondiale est de moins de 3000 tonnes par an pour les sels
de THP et d'environ 3000 tonnes pour le condensat chlorure de tétrakis
(hydroxyméthyl)phosphonium-urée.
La photodécomposition et l'hydrolyse des sels de THP constituent
des voies de dégradation abiotiques importantes dans l'environnement.
Le sulfate de THP ne se fixe guère sur les matières particulaires et
il est donc mobile. Le THPS se décompose rapidement en aérobiose comme
en anaérobiose. On a constaté la présence d'oxyde de
trihydroxyméthylphosphine (THPO) et d'acide
bishydroxyméthylphosphonique ou BMPA dans les produits de
décomposition.
Comme il ne semble pas y avoir de surveillance de ces composés,
on ne peut pas évaluer l'exposition de l'Homme ni celle des autres
êtres vivants dans leur milieu naturel.
Le THPC et le THPS présentent une toxicité aiguë modérée par voie
orale ; au niveau cutané, leur toxicité est faible.
Des études à court terme (jusqu'à 28 jours) effectuées sur des
rats et des souris ont montré que le principal effet toxique du THPC
et du THPS était une réduction du poids corporel. Chez les deux
espèces, la NOAEL est d'environ 8 mg.kg-1.j-1. Des études plus
longues (13 semaines) montrent que le principal organe cible est le
foie. La NOAEL relative à cet effet varie de 3 à 7 mg.kg-1.j-1 pour
les deux sels chez les deux espèces. Les tests biologiques de
cancérogénicité effectués sur le THPC ont également montré que ces
effets se produisaient au niveau du foie, mais on n'a pas établi de
NOAEL. Chez les deux espèces, la LOAEL était d'environ 3 mg.kg-1.j-1.
Lors d'une étude de cancérogénicité portant sur le THPS et effectuée
sur des souris, on a évalué à 3,6 mg.kg-1.j-1 la NOAEL pour une
hyperplasie médullo-surrénalienne focale; chez les rats, la LOAEL pour
la mortalité avait la même valeur.
Administré en dose unique à des lapins, le THPS n'a pas provoqué
d'irritation cutanée. Cependant, l'exposition répétée de rats à ce
composé par la voie cutanée a entraîné une sérieuse réaction à ce
niveau. Le condensat THPC-urée s'est révélé corrosif. Chez le lapin,
le THPS irrite fortement la muqueuse oculaire.
 Le THPS et le condensat THPC-urée provoquent une sensibilisation
cutanée chez le cobaye (test de sensibilisation maximale de Magnusson
& Kilman).
Ni le THPS ni le condensat THPC-urée n'ont eu d'effets toxiques
sur le développement lorsqu'ils étaient administrés par voie orale à
des animaux de laboratoire.
Le THPC et le THPS manifestent une activité mutagène in vitro,
mais celle-ci disparaît in vivo dans le cas du THPS (on ne dispose
pas de données sur l'activité mutagène du THPC in vivo). Les
résultats limités dont on dispose au sujet du condensat THPC-urée
incitent à penser qu'il n'est pas mutagène in vivo. Le THPO n'est
pas génotoxique. Rien n'indique de façon probante que les tissus
traités par des sels de THP puissent avoir des effets mutagènes. Les
données disponibles montrent qu'il n'y a pas de risque de génotoxicité
pour l'Homme.
Le THPS et le THPC ne sont pas révélés cancérogènes chez le rat
ou la souris lors d'études biologiques d'une durée de deux ans. Les
tests cutanés montrent que les sels de THP agissent comme promoteurs
dans le processus de cancérisation, mais pas comme initiateurs.
Le THPS et le THPO n'inhibent pas l'activité cholinesterasique in
vitro, ce qui indique qu'ils ne sont pas neurotoxiques pour l'Homme.
Les tissus traités par le condensat THPC-urée ne provoquent pas
d'irritation cutanée chez l'Homme.
Dans le cas du THPS, la valeur de la concentration entraînant des
effets toxiques aigus pour les algues est de l'ordre de 1 mg/litre,
avec une concentration sans effet observable (NOEC) de 0,06 mg/litre.
En ce qui concerne la daphnie, la valeur de la NOEC relative aux
effets aigus est de 10 mg/litre. Chez les invertébrés marins, les
valeurs correspondant à des effets toxiques aigus oscillent entre 1,6
et 340 mg/litre.
Chez les poissons, la valeur de la CL50 à 96 h va de 72 à 119
mg/litre, avec une NOEC comprise entre 18 et 41 mg/litre. Pour les
oiseaux, on donne pour la DL50 une valeur de 311 mg/litre (effets
aigus). Dans le cas de la toxicité par ingestion, on a retenu, pour la
CL50, une valeur comprise entre 1300 et 2400 mg/kg de nourriture.
3.2 Evaluation
On ne dispose d'aucune information sur l'exposition de l'Homme ou
des autres êtres vivants dans leur milieu naturel. Dans ces
conditions, il n'est pas possible de donner une estimation
quantitative du risque.
RESUMEN, EVALUACION Y RECOMENDACIONES
1. Tris(2-butoxietil)fosfato (TBEP)
1.1 Resumen
El tris(2-butoxietil)fosfato (TBEP) se utiliza en ceras para el
suelo y como plastificante del caucho y del plástico. No se dispone de
datos sobre el volumen de producción mundial, pero se calcula que es
del orden de 5000 a 6000 toneladas.
El TBEP se encuentra en el medio ambiente sólo como consecuencia
de la actividad humana. Se ha investigado en determinados países
industrializados su distribución en la naturaleza. Se comprobó que la
concentración en las aguas superficiales era inferior a 300 ng/litro,
mientras que en los sedimentos oscilaba entre 100 y 1000 ìg/kg. No se
detectó TBEP en ninguno de los 167 análisis realizados en peces. Se ha
detectado en un estudio único en el aire exterior (<200 ng/m3). La
medición del TBEP en el aire de espacios cerrados de oficina puso de
manifiesto concentraciones de 25 ng/m3 o inferiores. El TBEP se
asocia a partículas y se considera que la fuente es la aplicación de
cera al suelo. Se ha detectado a niveles del orden de ìg/kg en el
tejido adiposo humano. La ingesta diaria con los alimentos notificada
a partir de estudios de la cesta de la compra, para una gama de grupos
de edad, fue <0,02 ìg/kg de peso corporal al día. Se han notificado
concentraciones en el agua de bebida de hasta 270 ìg/litro,
estimándose que procede de la migración desde las juntas de caucho de
las tuberías.
Se considera que el TBEP es fácilmente biodegradable. Las
mediciones en depuradoras de aguas residuales y las pruebas
semicontinuas de laboratorio de los lodos cloacales han indicado una
eliminación sustancial de TBEP (>80%). En aguas fluviales y costeras,
el TBEP se degradó completamente. Se notificó que la semivida en el
agua de los estuarios era de unos 50 días y que había poca degradación
en el agua marina no adaptada.
La toxicidad aguda sistémica en mamíferos y el potencial de
irritación son bajos.
En varios estudios subcrónicos en animales de laboratorio se ha
comprobado que el hígado es el órgano destinatario de la toxicidad del
TBEP. Los resultados de un estudio en ratas Sprague-Dawley macho
parecen indicar que el TBEP podría causar miocarditis focal. Los
efectos neurotóxicos en ratas tras dosis únicas de TBEP no son
uniformes. La administración repetida de dosis elevadas de TBEP a
ratas mediante sonda produjo una disminución de la velocidad de
conducción nerviosa y un aumento del período de refracción. No produjo
neurotoxicidad retardada en gallinas, pero inhibió las colinesterasas
del cerebro y del plasma.
Tomando como base un estudio de dosis repetidas de 18 semanas en
ratas, se notificó una concentración sin efectos observados (NOEL) en
el hígado de 15 mg/kg de peso corporal al día, mientras que la
concentración más baja con efectos observados (LOEL), fue de 150 mg/kg
de peso corporal al día.
No se han estudiado la toxicidad y la carcinogenicidad del TBEP a
largo plazo.
Las pruebas de mutación genética en bacterias y células de
mamíferos dieron resultados negativos, pero no se han notificado
pruebas sobre los daños cromosómicos.
En un estudio realizado en ratas no se observó teratogenicidad.
No se han notificado otros aspectos de toxicidad reproductiva.
En una prueba epicutánea (con parche) repetida para estudiar los
efectos del TBEP en la piel humana se vio que no se producía
sensibilización y que la irritación era mínima.
La toxicidad del TBEP para los organismos acuáticos es moderada.
La CL50 a las 48 horas en Daphnia magna es de 75 mg/litro y los
valores de la CL50 a las 96 horas en peces oscilan entre 16 y
24 mg/litro.
1.2 Evaluación
Es probable que se produzca exposición ocupacional al TBEP por
vía cutánea durante la fabricación (exposición occidental) y a partir
de las ceras del suelo. El compuesto se absorbe por vía cutánea en
animales de experimentación, pero no se dispone de información sobre
su cinética y metabolismo. Por consiguiente, no se puede cuantificar
la exposición cutánea, pero es previsible que sea baja. La exposición
por inhalación medida en el entorno de oficina ha sido de 25 ng/m3 o
inferior.
La exposición de la población general se produce fundamentalmente
a través de los alimentos (debido al uso de TBEP como plastificante en
los plásticos de envasado) y del agua de bebida (contaminada por
lixiviación del caucho sintético utilizado en las arandelas de las
cañerías). La exposición a partir de ambas fuentes es muy baja
(estimada en <0,2 ìg/kg de peso corporal al día a partir de los
alimentos y concentraciones en el agua de bebida de <270 ìg/litro).
Teniendo en cuenta la NOEL notificada a partir de estudios en
animales de 15 mg/kg de peso corporal al día obtenida en un estudio de
administración oral con dosis repetidas, el riesgo para la población
general es muy bajo. El riesgo para las personas expuestas en el
trabajo se considera también muy bajo, aunque no se puede cuantificar.
En el medio ambiente, se supone que el TBEP (dada su baja
volatilidad, su elevado coeficiente de adsorción y su solubilidad
moderada en agua) se reparte en los sedimentos. Los escasos datos
medidos así lo confirman. La degradación en los compartimentos del
medio ambiente se supone que es rápida. No se dispone de información
sobre los productos de su degradación; no parece que el fosfato
liberado durante la degradación contribuya de manera significativa a
la concentración de nutrientes del medio ambiente. La Fig. 1 es una
representación gráfica de las concentraciones en el medio ambiente
medidas en aguas superficiales frente a los valores notificados de
toxicidad aguda. El margen de inocuidad entre las concentraciones más
altas y los valores de toxicidad más bajos notificados es de varios
órdenes de magnitud, lo que indica un riesgo bajo para los organismos
del medio ambiente acuático. No se puede hacer una evaluación del
riesgo para el compartimento terrestre.
1.3 Recomendaciones
Para una evaluación científica completa del compuesto, sería
necesaria la identificación y evaluación de los metabolitos en los
mamíferos, dado el perfil toxicológico de uno de los metabolitos
propuestos, el 2-butoxietanol.
2. Tris(2-etilhexil)fosfato (TEHP)
El tris(2-etilhexil)fosfato (TEHP) es un líquido incoloro no
inflamable, poco soluble en agua y de presión de vapor muy baja, que
se utiliza como pirorretardante y plastificante para el PVC y el
acetato de celulosa, y como disolvente. Se produce a partir del
oxicloruro de fósforo y el 2-etilhexanol. No se dispone de cifras para
la producción mundial actual. En Alemania se producen actualmente unas
1000 toneladas.
Le THPS et le condensat THPC-urée provoquent une sensibilisation
cutanée chez le cobaye (test de sensibilisation maximale de Magnusson
& Kilman).
Ni le THPS ni le condensat THPC-urée n'ont eu d'effets toxiques
sur le développement lorsqu'ils étaient administrés par voie orale à
des animaux de laboratoire.
Le THPC et le THPS manifestent une activité mutagène in vitro,
mais celle-ci disparaît in vivo dans le cas du THPS (on ne dispose
pas de données sur l'activité mutagène du THPC in vivo). Les
résultats limités dont on dispose au sujet du condensat THPC-urée
incitent à penser qu'il n'est pas mutagène in vivo. Le THPO n'est
pas génotoxique. Rien n'indique de façon probante que les tissus
traités par des sels de THP puissent avoir des effets mutagènes. Les
données disponibles montrent qu'il n'y a pas de risque de génotoxicité
pour l'Homme.
Le THPS et le THPC ne sont pas révélés cancérogènes chez le rat
ou la souris lors d'études biologiques d'une durée de deux ans. Les
tests cutanés montrent que les sels de THP agissent comme promoteurs
dans le processus de cancérisation, mais pas comme initiateurs.
Le THPS et le THPO n'inhibent pas l'activité cholinesterasique in
vitro, ce qui indique qu'ils ne sont pas neurotoxiques pour l'Homme.
Les tissus traités par le condensat THPC-urée ne provoquent pas
d'irritation cutanée chez l'Homme.
Dans le cas du THPS, la valeur de la concentration entraînant des
effets toxiques aigus pour les algues est de l'ordre de 1 mg/litre,
avec une concentration sans effet observable (NOEC) de 0,06 mg/litre.
En ce qui concerne la daphnie, la valeur de la NOEC relative aux
effets aigus est de 10 mg/litre. Chez les invertébrés marins, les
valeurs correspondant à des effets toxiques aigus oscillent entre 1,6
et 340 mg/litre.
Chez les poissons, la valeur de la CL50 à 96 h va de 72 à 119
mg/litre, avec une NOEC comprise entre 18 et 41 mg/litre. Pour les
oiseaux, on donne pour la DL50 une valeur de 311 mg/litre (effets
aigus). Dans le cas de la toxicité par ingestion, on a retenu, pour la
CL50, une valeur comprise entre 1300 et 2400 mg/kg de nourriture.
3.2 Evaluation
On ne dispose d'aucune information sur l'exposition de l'Homme ou
des autres êtres vivants dans leur milieu naturel. Dans ces
conditions, il n'est pas possible de donner une estimation
quantitative du risque.
RESUMEN, EVALUACION Y RECOMENDACIONES
1. Tris(2-butoxietil)fosfato (TBEP)
1.1 Resumen
El tris(2-butoxietil)fosfato (TBEP) se utiliza en ceras para el
suelo y como plastificante del caucho y del plástico. No se dispone de
datos sobre el volumen de producción mundial, pero se calcula que es
del orden de 5000 a 6000 toneladas.
El TBEP se encuentra en el medio ambiente sólo como consecuencia
de la actividad humana. Se ha investigado en determinados países
industrializados su distribución en la naturaleza. Se comprobó que la
concentración en las aguas superficiales era inferior a 300 ng/litro,
mientras que en los sedimentos oscilaba entre 100 y 1000 ìg/kg. No se
detectó TBEP en ninguno de los 167 análisis realizados en peces. Se ha
detectado en un estudio único en el aire exterior (<200 ng/m3). La
medición del TBEP en el aire de espacios cerrados de oficina puso de
manifiesto concentraciones de 25 ng/m3 o inferiores. El TBEP se
asocia a partículas y se considera que la fuente es la aplicación de
cera al suelo. Se ha detectado a niveles del orden de ìg/kg en el
tejido adiposo humano. La ingesta diaria con los alimentos notificada
a partir de estudios de la cesta de la compra, para una gama de grupos
de edad, fue <0,02 ìg/kg de peso corporal al día. Se han notificado
concentraciones en el agua de bebida de hasta 270 ìg/litro,
estimándose que procede de la migración desde las juntas de caucho de
las tuberías.
Se considera que el TBEP es fácilmente biodegradable. Las
mediciones en depuradoras de aguas residuales y las pruebas
semicontinuas de laboratorio de los lodos cloacales han indicado una
eliminación sustancial de TBEP (>80%). En aguas fluviales y costeras,
el TBEP se degradó completamente. Se notificó que la semivida en el
agua de los estuarios era de unos 50 días y que había poca degradación
en el agua marina no adaptada.
La toxicidad aguda sistémica en mamíferos y el potencial de
irritación son bajos.
En varios estudios subcrónicos en animales de laboratorio se ha
comprobado que el hígado es el órgano destinatario de la toxicidad del
TBEP. Los resultados de un estudio en ratas Sprague-Dawley macho
parecen indicar que el TBEP podría causar miocarditis focal. Los
efectos neurotóxicos en ratas tras dosis únicas de TBEP no son
uniformes. La administración repetida de dosis elevadas de TBEP a
ratas mediante sonda produjo una disminución de la velocidad de
conducción nerviosa y un aumento del período de refracción. No produjo
neurotoxicidad retardada en gallinas, pero inhibió las colinesterasas
del cerebro y del plasma.
Tomando como base un estudio de dosis repetidas de 18 semanas en
ratas, se notificó una concentración sin efectos observados (NOEL) en
el hígado de 15 mg/kg de peso corporal al día, mientras que la
concentración más baja con efectos observados (LOEL), fue de 150 mg/kg
de peso corporal al día.
No se han estudiado la toxicidad y la carcinogenicidad del TBEP a
largo plazo.
Las pruebas de mutación genética en bacterias y células de
mamíferos dieron resultados negativos, pero no se han notificado
pruebas sobre los daños cromosómicos.
En un estudio realizado en ratas no se observó teratogenicidad.
No se han notificado otros aspectos de toxicidad reproductiva.
En una prueba epicutánea (con parche) repetida para estudiar los
efectos del TBEP en la piel humana se vio que no se producía
sensibilización y que la irritación era mínima.
La toxicidad del TBEP para los organismos acuáticos es moderada.
La CL50 a las 48 horas en Daphnia magna es de 75 mg/litro y los
valores de la CL50 a las 96 horas en peces oscilan entre 16 y
24 mg/litro.
1.2 Evaluación
Es probable que se produzca exposición ocupacional al TBEP por
vía cutánea durante la fabricación (exposición occidental) y a partir
de las ceras del suelo. El compuesto se absorbe por vía cutánea en
animales de experimentación, pero no se dispone de información sobre
su cinética y metabolismo. Por consiguiente, no se puede cuantificar
la exposición cutánea, pero es previsible que sea baja. La exposición
por inhalación medida en el entorno de oficina ha sido de 25 ng/m3 o
inferior.
La exposición de la población general se produce fundamentalmente
a través de los alimentos (debido al uso de TBEP como plastificante en
los plásticos de envasado) y del agua de bebida (contaminada por
lixiviación del caucho sintético utilizado en las arandelas de las
cañerías). La exposición a partir de ambas fuentes es muy baja
(estimada en <0,2 ìg/kg de peso corporal al día a partir de los
alimentos y concentraciones en el agua de bebida de <270 ìg/litro).
Teniendo en cuenta la NOEL notificada a partir de estudios en
animales de 15 mg/kg de peso corporal al día obtenida en un estudio de
administración oral con dosis repetidas, el riesgo para la población
general es muy bajo. El riesgo para las personas expuestas en el
trabajo se considera también muy bajo, aunque no se puede cuantificar.
En el medio ambiente, se supone que el TBEP (dada su baja
volatilidad, su elevado coeficiente de adsorción y su solubilidad
moderada en agua) se reparte en los sedimentos. Los escasos datos
medidos así lo confirman. La degradación en los compartimentos del
medio ambiente se supone que es rápida. No se dispone de información
sobre los productos de su degradación; no parece que el fosfato
liberado durante la degradación contribuya de manera significativa a
la concentración de nutrientes del medio ambiente. La Fig. 1 es una
representación gráfica de las concentraciones en el medio ambiente
medidas en aguas superficiales frente a los valores notificados de
toxicidad aguda. El margen de inocuidad entre las concentraciones más
altas y los valores de toxicidad más bajos notificados es de varios
órdenes de magnitud, lo que indica un riesgo bajo para los organismos
del medio ambiente acuático. No se puede hacer una evaluación del
riesgo para el compartimento terrestre.
1.3 Recomendaciones
Para una evaluación científica completa del compuesto, sería
necesaria la identificación y evaluación de los metabolitos en los
mamíferos, dado el perfil toxicológico de uno de los metabolitos
propuestos, el 2-butoxietanol.
2. Tris(2-etilhexil)fosfato (TEHP)
El tris(2-etilhexil)fosfato (TEHP) es un líquido incoloro no
inflamable, poco soluble en agua y de presión de vapor muy baja, que
se utiliza como pirorretardante y plastificante para el PVC y el
acetato de celulosa, y como disolvente. Se produce a partir del
oxicloruro de fósforo y el 2-etilhexanol. No se dispone de cifras para
la producción mundial actual. En Alemania se producen actualmente unas
1000 toneladas.
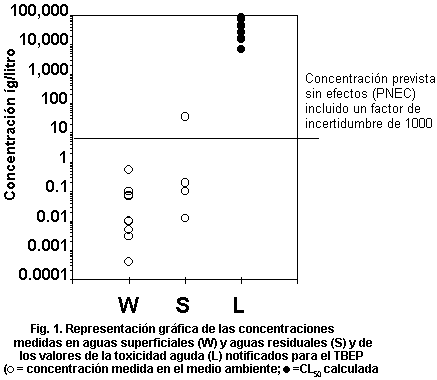 No se ha detectado TEHP en el aire exterior; se ha encontrado en
el aire de espacios cerrados en concentraciones de menos de 10 ng/m3,
en aguas fluviales en concentraciones de hasta 7500 ng/litro y en
sedimentos de 2-70 ng/g. Se detectó TEHP en una sola muestra de agua
de bebida en una concentración de 0,3 ng/litro. La ingesta diaria
notificada en los alimentos a partir de estudios de la cesta de la
compra, para una gama de grupos de edad, fue inferior a 0,05 ìg/kg de
peso corporal al día.
El TEHP se degrada rápidamente en las aguas naturales, pero las
pruebas de laboratorio con lodos activados dieron resultados
equívocos. No hay una degradación abiótica significativa.
El TEHP tiene una toxicidad aguda baja para los mamíferos, siendo
la DL50 por vía oral >10 000 mg/kg de peso corporal en ratas.
El TEHP es irritante cutáneo, pero no ocular. La aplicación
repetida de 0,1 ml (93 mg) de TEHP a la piel de conejos no produjo
signos de intoxicación sistémica.
En estudios de administración con sonda durante 13 semanas a
ratas y ratones no aparecieron efectos tóxicos significativos. La
concentración sin efectos adversos observados (NOAEL) en ratas fue de
2860 mg/kg de peso corporal al día y en ratones de 5710 mg/kg de peso
corporal al día, la dosis más elevada probada en ambas especies.
En un estudio de inhalación de tres meses con concentraciones de
hasta 85 mg de TEHP/m3 se observaron cambios inflamatorios crónicos
leves en los pulmones de perros y los resultados de rechazo
condicionado empeoraron en relación con la concentración administrada.
No se dispuso de estudios sobre toxicidad reproductiva.
El TEHP dio resultados negativos en varias pruebas de
mutagenicidad in vivo e in vitro.
Se realizaron pruebas de toxicidad crónica y carcinogenicidad del
TEHP en ratas y ratones. La NOAEL para la toxicidad crónica en ratas
macho fue de 2857 mg/kg de peso corporal al día y en ratas hembras de
1428 mg/kg de peso corporal al día. La concentración más baja con
efectos adversos observados (LOAEL) en ratones machos y hembras para
la hiperplasia de las células foliculares del tiroides fue de 357
mg/kg de peso corporal al día. No se estableció una NOAEL en ratones.
Los autores llegaron a la conclusión de que había algunas pruebas de
carcinogenicidad basadas en una mayor incidencia de carcinomas
hepatocelulares en ratones hembra con un nivel de dosificación alto y
pruebas equívocas de carcinogenicidad basadas en la mayor incidencia
de feocromocitomas suprarrenales en ratas macho en ambos grupos de
dosis. Aunque se produjo un aumento de feocromocitomas suprarrenales
en ambos grupos de dosis de ratas macho y de carcinomas
hepatocelulares en ratones hembra del grupo de dosificación alta, no
se considera que estos resultados indiquen que el TEHP presenta un
riesgo carcinogénico significativo para el ser humano. Los
feocromocitomas muestran una incidencia de base variable en ratas. La
incidencia de estos tumores en dos biovaloraciones anteriores del
Programa Nacional de Toxicología fue igual a la observada en la
biovaloración del TEHP. Solamente hubo otro resultado neoplásico
significativo, consistente en carcinomas hepatocelulares, en el grupo
de ratones hembra de dosificación alta. Teniendo en cuenta la baja
incidencia de este tumor, su presencia en un solo sexo de una especie,
la falta de pruebas de toxicidad genética y la baja exposición del ser
humano al TEHP, no es probable que este producto cree un riesgo
carcinogénico significativo para el ser humano.
Se han realizado estudios de neurotoxicidad en varias especies.
El TEHP no produce ninguna alteración de la actividad de la
colinesterasa del plasma o los glóbulos rojos. No se han notificado
estudios sobre neurotoxicidad retardada.
En un estudio realizado en voluntarios humanos, no se notificó
irritación cutánea.
Los escasos datos disponibles indican una toxicidad aguda baja
del TEHP en el medio acuático. La CL50 para bacterias es superior a
100 mg/litro y la CL50 a las 96 horas para el pez Brachydanio rerio
es superior a 100 mg/litro, que es el límite de la solubilidad del
TEHP en agua.
2.2 Evaluación
Es probable que se produzca exposición ocupacional al TEHP por
vía cutánea durante la fabricación (exposición occidental) y por el
uso de algunos productos. El compuesto se absorbe por vía cutánea en
los animales de experimentación, pero no se dispone de información
sobre su cinética o metabolismo en esta vía. Por consiguiente, no se
puede cuantificar la exposición cutánea, pero es previsible que sea
baja. La exposición por inhalación medida en el entorno de oficina es
de 10 ng/m3 o menor.
La exposición de la población general se produce fundamentalmente
a través de los alimentos y el agua de bebida. La exposición a partir
de ambas fuentes es muy baja (estimada en <0,05 ìg/kg de peso
corporal día a partir de los alimentos; una concentración única medida
en el agua de bebida fue de 0,3 ng/litro).
Teniendo cuenta la LOAEL notificada para la hiperplasia tiroidea
de 357 mg/kg de peso corporal día en ratones, el riesgo para la
población general es muy bajo. El riesgo para las personas expuestas
en el trabajo se considera también muy bajo, aunque no se puede
cuantificar.
El TEHP no parece ser carcinogénico para el ser humano.
En el medio ambiente, se supone que el TEHP (dada su baja
volatilidad, su elevado coeficiente de adsorción y su solubilidad baja
en agua) se repartirá en los sedimentos. Los datos medidos son
demasiado escasos para confirmarlo. Se prevé su degradación en los
compartimentos del medio ambiente, aunque los datos de laboratorio
sobre la degradación en lodos cloacales son equívocos. No se dispone
de información sobre los productos de la degradación; no parece que el
fosfato liberado durante su degradación contribuya de manera
significativa a la concentración de nutrientes del medio ambiente. La
Fig. 2 es una representación gráfica de las concentraciones en el
medio ambiente medidas en sus compartimentos frente a los valores
notificados de toxicidad aguda (éstos indican que no tiene efectos
tóxicos en el límite de solubilidad en el agua). El margen de
inocuidad entre las concentraciones más altas y los valores de
toxicidad más bajos notificados es de varios órdenes de magnitud, lo
que indica un riesgo bajo para los organismos del medio ambiente
acuático. No se puede hacer una evaluación del riesgo para el
compartimento terrestre.
2.3 Recomendaciones
Para una evaluación científica completa del compuesto, sería
necesaria la identificación y evaluación de los metabolitos en los
mamíferos, dado el perfil toxicológico de uno de los metabolitos
propuestos, el 2-etilhexanol.
Es necesario investigar la toxicidad reproductiva, en particular
los posibles efectos en el desarrollo.
No se ha detectado TEHP en el aire exterior; se ha encontrado en
el aire de espacios cerrados en concentraciones de menos de 10 ng/m3,
en aguas fluviales en concentraciones de hasta 7500 ng/litro y en
sedimentos de 2-70 ng/g. Se detectó TEHP en una sola muestra de agua
de bebida en una concentración de 0,3 ng/litro. La ingesta diaria
notificada en los alimentos a partir de estudios de la cesta de la
compra, para una gama de grupos de edad, fue inferior a 0,05 ìg/kg de
peso corporal al día.
El TEHP se degrada rápidamente en las aguas naturales, pero las
pruebas de laboratorio con lodos activados dieron resultados
equívocos. No hay una degradación abiótica significativa.
El TEHP tiene una toxicidad aguda baja para los mamíferos, siendo
la DL50 por vía oral >10 000 mg/kg de peso corporal en ratas.
El TEHP es irritante cutáneo, pero no ocular. La aplicación
repetida de 0,1 ml (93 mg) de TEHP a la piel de conejos no produjo
signos de intoxicación sistémica.
En estudios de administración con sonda durante 13 semanas a
ratas y ratones no aparecieron efectos tóxicos significativos. La
concentración sin efectos adversos observados (NOAEL) en ratas fue de
2860 mg/kg de peso corporal al día y en ratones de 5710 mg/kg de peso
corporal al día, la dosis más elevada probada en ambas especies.
En un estudio de inhalación de tres meses con concentraciones de
hasta 85 mg de TEHP/m3 se observaron cambios inflamatorios crónicos
leves en los pulmones de perros y los resultados de rechazo
condicionado empeoraron en relación con la concentración administrada.
No se dispuso de estudios sobre toxicidad reproductiva.
El TEHP dio resultados negativos en varias pruebas de
mutagenicidad in vivo e in vitro.
Se realizaron pruebas de toxicidad crónica y carcinogenicidad del
TEHP en ratas y ratones. La NOAEL para la toxicidad crónica en ratas
macho fue de 2857 mg/kg de peso corporal al día y en ratas hembras de
1428 mg/kg de peso corporal al día. La concentración más baja con
efectos adversos observados (LOAEL) en ratones machos y hembras para
la hiperplasia de las células foliculares del tiroides fue de 357
mg/kg de peso corporal al día. No se estableció una NOAEL en ratones.
Los autores llegaron a la conclusión de que había algunas pruebas de
carcinogenicidad basadas en una mayor incidencia de carcinomas
hepatocelulares en ratones hembra con un nivel de dosificación alto y
pruebas equívocas de carcinogenicidad basadas en la mayor incidencia
de feocromocitomas suprarrenales en ratas macho en ambos grupos de
dosis. Aunque se produjo un aumento de feocromocitomas suprarrenales
en ambos grupos de dosis de ratas macho y de carcinomas
hepatocelulares en ratones hembra del grupo de dosificación alta, no
se considera que estos resultados indiquen que el TEHP presenta un
riesgo carcinogénico significativo para el ser humano. Los
feocromocitomas muestran una incidencia de base variable en ratas. La
incidencia de estos tumores en dos biovaloraciones anteriores del
Programa Nacional de Toxicología fue igual a la observada en la
biovaloración del TEHP. Solamente hubo otro resultado neoplásico
significativo, consistente en carcinomas hepatocelulares, en el grupo
de ratones hembra de dosificación alta. Teniendo en cuenta la baja
incidencia de este tumor, su presencia en un solo sexo de una especie,
la falta de pruebas de toxicidad genética y la baja exposición del ser
humano al TEHP, no es probable que este producto cree un riesgo
carcinogénico significativo para el ser humano.
Se han realizado estudios de neurotoxicidad en varias especies.
El TEHP no produce ninguna alteración de la actividad de la
colinesterasa del plasma o los glóbulos rojos. No se han notificado
estudios sobre neurotoxicidad retardada.
En un estudio realizado en voluntarios humanos, no se notificó
irritación cutánea.
Los escasos datos disponibles indican una toxicidad aguda baja
del TEHP en el medio acuático. La CL50 para bacterias es superior a
100 mg/litro y la CL50 a las 96 horas para el pez Brachydanio rerio
es superior a 100 mg/litro, que es el límite de la solubilidad del
TEHP en agua.
2.2 Evaluación
Es probable que se produzca exposición ocupacional al TEHP por
vía cutánea durante la fabricación (exposición occidental) y por el
uso de algunos productos. El compuesto se absorbe por vía cutánea en
los animales de experimentación, pero no se dispone de información
sobre su cinética o metabolismo en esta vía. Por consiguiente, no se
puede cuantificar la exposición cutánea, pero es previsible que sea
baja. La exposición por inhalación medida en el entorno de oficina es
de 10 ng/m3 o menor.
La exposición de la población general se produce fundamentalmente
a través de los alimentos y el agua de bebida. La exposición a partir
de ambas fuentes es muy baja (estimada en <0,05 ìg/kg de peso
corporal día a partir de los alimentos; una concentración única medida
en el agua de bebida fue de 0,3 ng/litro).
Teniendo cuenta la LOAEL notificada para la hiperplasia tiroidea
de 357 mg/kg de peso corporal día en ratones, el riesgo para la
población general es muy bajo. El riesgo para las personas expuestas
en el trabajo se considera también muy bajo, aunque no se puede
cuantificar.
El TEHP no parece ser carcinogénico para el ser humano.
En el medio ambiente, se supone que el TEHP (dada su baja
volatilidad, su elevado coeficiente de adsorción y su solubilidad baja
en agua) se repartirá en los sedimentos. Los datos medidos son
demasiado escasos para confirmarlo. Se prevé su degradación en los
compartimentos del medio ambiente, aunque los datos de laboratorio
sobre la degradación en lodos cloacales son equívocos. No se dispone
de información sobre los productos de la degradación; no parece que el
fosfato liberado durante su degradación contribuya de manera
significativa a la concentración de nutrientes del medio ambiente. La
Fig. 2 es una representación gráfica de las concentraciones en el
medio ambiente medidas en sus compartimentos frente a los valores
notificados de toxicidad aguda (éstos indican que no tiene efectos
tóxicos en el límite de solubilidad en el agua). El margen de
inocuidad entre las concentraciones más altas y los valores de
toxicidad más bajos notificados es de varios órdenes de magnitud, lo
que indica un riesgo bajo para los organismos del medio ambiente
acuático. No se puede hacer una evaluación del riesgo para el
compartimento terrestre.
2.3 Recomendaciones
Para una evaluación científica completa del compuesto, sería
necesaria la identificación y evaluación de los metabolitos en los
mamíferos, dado el perfil toxicológico de uno de los metabolitos
propuestos, el 2-etilhexanol.
Es necesario investigar la toxicidad reproductiva, en particular
los posibles efectos en el desarrollo.
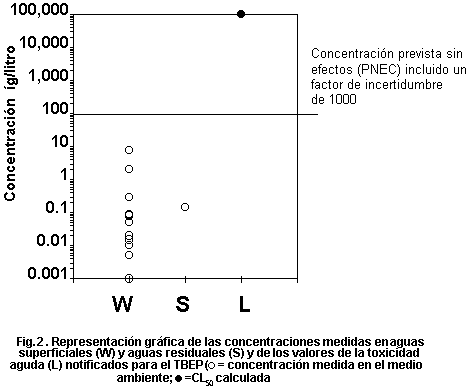 3. Sales de tetrakis(hidroximetil)fosfonio
3.1 Resumen
Las sales de tetrakis(hidroximetil)fosfonio representan la clase
principal de productos químicos utilizados como pirorretardantes en el
algodón, la celulosa y los tejidos con celulosa. Hay una migración
baja a partir de los tejidos tratados con cloruro de
tetrakis(hidroximetil)fosfonio (THPC)-urea. La sal sulfatada (THPS) se
utiliza fundamentalmente como biocida. La producción mundial combinada
se estima que es >3000 toneladas para las sales de THP y de unas 3000
toneladas para el condensado de THPC-urea.
La fotodegradación y la hidrólisis de las sales de THP son vías
importantes de degradación abiótica en el medio ambiente. El THPS se
une débilmente a las partículas del medio ambiente, por lo que es
móvil. Se degrada rápidamente tanto en condiciones aerobias como
anaerobias. Se han identificado como productos de su degradación el
óxido de trihidroximetilfosfuro (THPO) y el ácido
bihidroximetilfosfónico (BMPA).
Puesto que no se ha notificado ninguna vigilancia, no se pueden
hacer estimaciones de la exposición del ser humano o de los organismos
en el medio ambiente.
La toxicidad aguda por vía oral del THPC y el THPS es moderada;
la toxicidad cutánea es baja.
En estudios breves (hasta 28 días) en ratas y ratones, el
principal efecto tóxico tanto del THPC como del THPS es la disminución
del peso corporal. La NOAEL para ambos productos químicos en las dos
especies es de unos 8 mg/kg de peso corporal al día. En estudios más
prolongados (13 semanas), el principal órgano destinatario de la
toxicidad es el hígado. La NOAEL para este efecto osciló entre 3 y 7
mg/kg de peso corporal al día para ambas sales en las dos especies.
Las biovaloraciones de la carcinogenicidad del THPC también pusieron
de manifiesto efectos en el hígado, pero no se estableció una NOAEL.
La LOAEL fue de unos 3 mg/kg de peso corporal al día para ambas
especies. En una biovaloración de la carcinogenicidad del THPS en
ratones, la NOAEL para la hiperplasia focal en la médula suprarrenal
fue de 3,6 mg/kg de peso corporal al día; en ratas, la LOAEL para la
mortalidad fue de 3,6 mg/kg de peso corporal al día.
El THPS administrado en dosis única a conejos no provocó
irritación cutánea. Sin embargo, la exposición cutánea repetida en
ratas produjo una reacción grave en la piel. El THPC-urea fue
corrosivo. Se comprobó que el THPS ocasionaba una irritación grave de
los ojos en conejos.
El THPS y el THPC-urea producen sensibilización cutánea en
cobayas (prueba de maximización de Magnusson y Kilman).
En animales de experimentación tratados por vía oral, el THPS y
el THPC-urea no produjeron toxicidad en el desarrollo.
El THPC y el THPS tienen potencial mutagénico in vitro, pero el
THPS no es mutagénico in vivo (no hay datos de mutagenicidad in
vivo para el THPC). Los limitados datos de mutagenicidad para el
THPC-urea parecen indicar que no es mutagénico in vivo. El THPO no
es genotóxico. No hay pruebas convincentes que indiquen que las telas
tratadas con sales de THP sean mutagénicas. La información disponible
pone de manifiesto que no hay peligro genotóxico para el ser humano.
El THPC y el THPS no fueron carcinogénicos en ratas y ratones en
biovaloraciones de dos años. En estudios cutáneos se ha observado que
las sales son promotoras de cáncer cutáneo, pero no iniciadoras.
El THPS y el THPO no inhibieron la actividad de la
acetilcolinesterasa in vivo, lo que parece indicar una ausencia de
peligro neurotóxico para el ser humano.
Las telas tratadas con THPC-urea no produjeron irritación cutánea
en el ser humano.
Para el THPS, los valores de toxicidad aguda notificados en algas
son inferiores a 1 mg/litro, con una concentración sin efectos
observados (NOEC) de 0,06 mg/litro. La NOEC para la pulga de agua es
de 10 mg/litro. Los valores de toxicidad aguda notificados para los
invertebrados marinos oscilan entre 1,6 y 340 mg/litro.
Los valores de la DL50 a las 96 horas para los peces van de 72 a
119 mg/litro, con valores de la NOEC entre 18 y 41 mg/litro. Se ha
notificado una DL50 aguda para las aves de 311 mg/kg de peso corporal
y valores de la CL50 de 1 300 y 2 400 mg/kg de alimentos.
3.2 Evaluación
No se dispone de información sobre la exposición para el ser
humano ni para los organismos del medio ambiente. Por consiguiente, no
se pudo realizar una evaluación cuantitativa del riesgo.
3. Sales de tetrakis(hidroximetil)fosfonio
3.1 Resumen
Las sales de tetrakis(hidroximetil)fosfonio representan la clase
principal de productos químicos utilizados como pirorretardantes en el
algodón, la celulosa y los tejidos con celulosa. Hay una migración
baja a partir de los tejidos tratados con cloruro de
tetrakis(hidroximetil)fosfonio (THPC)-urea. La sal sulfatada (THPS) se
utiliza fundamentalmente como biocida. La producción mundial combinada
se estima que es >3000 toneladas para las sales de THP y de unas 3000
toneladas para el condensado de THPC-urea.
La fotodegradación y la hidrólisis de las sales de THP son vías
importantes de degradación abiótica en el medio ambiente. El THPS se
une débilmente a las partículas del medio ambiente, por lo que es
móvil. Se degrada rápidamente tanto en condiciones aerobias como
anaerobias. Se han identificado como productos de su degradación el
óxido de trihidroximetilfosfuro (THPO) y el ácido
bihidroximetilfosfónico (BMPA).
Puesto que no se ha notificado ninguna vigilancia, no se pueden
hacer estimaciones de la exposición del ser humano o de los organismos
en el medio ambiente.
La toxicidad aguda por vía oral del THPC y el THPS es moderada;
la toxicidad cutánea es baja.
En estudios breves (hasta 28 días) en ratas y ratones, el
principal efecto tóxico tanto del THPC como del THPS es la disminución
del peso corporal. La NOAEL para ambos productos químicos en las dos
especies es de unos 8 mg/kg de peso corporal al día. En estudios más
prolongados (13 semanas), el principal órgano destinatario de la
toxicidad es el hígado. La NOAEL para este efecto osciló entre 3 y 7
mg/kg de peso corporal al día para ambas sales en las dos especies.
Las biovaloraciones de la carcinogenicidad del THPC también pusieron
de manifiesto efectos en el hígado, pero no se estableció una NOAEL.
La LOAEL fue de unos 3 mg/kg de peso corporal al día para ambas
especies. En una biovaloración de la carcinogenicidad del THPS en
ratones, la NOAEL para la hiperplasia focal en la médula suprarrenal
fue de 3,6 mg/kg de peso corporal al día; en ratas, la LOAEL para la
mortalidad fue de 3,6 mg/kg de peso corporal al día.
El THPS administrado en dosis única a conejos no provocó
irritación cutánea. Sin embargo, la exposición cutánea repetida en
ratas produjo una reacción grave en la piel. El THPC-urea fue
corrosivo. Se comprobó que el THPS ocasionaba una irritación grave de
los ojos en conejos.
El THPS y el THPC-urea producen sensibilización cutánea en
cobayas (prueba de maximización de Magnusson y Kilman).
En animales de experimentación tratados por vía oral, el THPS y
el THPC-urea no produjeron toxicidad en el desarrollo.
El THPC y el THPS tienen potencial mutagénico in vitro, pero el
THPS no es mutagénico in vivo (no hay datos de mutagenicidad in
vivo para el THPC). Los limitados datos de mutagenicidad para el
THPC-urea parecen indicar que no es mutagénico in vivo. El THPO no
es genotóxico. No hay pruebas convincentes que indiquen que las telas
tratadas con sales de THP sean mutagénicas. La información disponible
pone de manifiesto que no hay peligro genotóxico para el ser humano.
El THPC y el THPS no fueron carcinogénicos en ratas y ratones en
biovaloraciones de dos años. En estudios cutáneos se ha observado que
las sales son promotoras de cáncer cutáneo, pero no iniciadoras.
El THPS y el THPO no inhibieron la actividad de la
acetilcolinesterasa in vivo, lo que parece indicar una ausencia de
peligro neurotóxico para el ser humano.
Las telas tratadas con THPC-urea no produjeron irritación cutánea
en el ser humano.
Para el THPS, los valores de toxicidad aguda notificados en algas
son inferiores a 1 mg/litro, con una concentración sin efectos
observados (NOEC) de 0,06 mg/litro. La NOEC para la pulga de agua es
de 10 mg/litro. Los valores de toxicidad aguda notificados para los
invertebrados marinos oscilan entre 1,6 y 340 mg/litro.
Los valores de la DL50 a las 96 horas para los peces van de 72 a
119 mg/litro, con valores de la NOEC entre 18 y 41 mg/litro. Se ha
notificado una DL50 aguda para las aves de 311 mg/kg de peso corporal
y valores de la CL50 de 1 300 y 2 400 mg/kg de alimentos.
3.2 Evaluación
No se dispone de información sobre la exposición para el ser
humano ni para los organismos del medio ambiente. Por consiguiente, no
se pudo realizar una evaluación cuantitativa del riesgo.
 WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR FLAME RETARDANTS:
TRIS(2-BUTOXYETHYL) PHOSPHATE, TRIS(2-ETHYLHEXYL) PHOSPHATE AND
TETRAKIS(HYDROXYMETHYL) PHOSPHONIUM SALTS
Members
Dr R. Benson, US Environmental Protection Agency, Denver, Colorado,
USA
Dr P. Brantom, British Industry Biological Research Association
(BIBRA) International, Carshalton, Surrey, United Kingdom
Dr S. Dobson, Institute of Terrestrial Ecology, Monks Wood,
Huntingdon, Cambridgeshire, United Kingdom (Chairman)
Professor J. Liesivuori, Department of Pharmacology and Toxicology,
University of Kuopio, Kuopio, Finland
Mr D. Renshaw, Department of Health, Elephant and Castle, London,
United Kingdom
Dr E. Söderlund, National Institute of Public Health, Department of
Environmental Medicine, Oslo, Norway (Rapporteur)
Observers
Dr L. Kotkoskic, FMC Corporation, Princetown, New Jersey, USA
Dr P. Martin, Albright and Wilson UK Limited, European Business
Services - Product Stewardship, Oldbury, West Midlands, United Kingdom
Secretariat
Dr M. Baril, International Programme on Chemical Safety, Montreal,
Quebec, Canada
ENVIRONMENTAL HEALTH CRITERIA FOR FLAME RETARDANTS:
TRIS(2-BUTOXYETHYL) PHOSPHATE, TRIS(2-ETHYLHEXYL) PHOSPHATE AND
TETRAKIS(HYDROXYMETHYL) PHOSPHONIUM SALTS
A WHO Task Group on Environmental Health Criteria for Flame
retardants: tris(2-butoxyethyl) phosphate, tris(2-ethylhexyl)
phosphate and tetrakis(hydroxymethyl) phosphonium salts met at the
British Industrial Biological Research Association, Carshalton, United
Kingdom from 18 to 22 January 1999. Dr P. Brantom opened the meeting
and welcome the participants on behalf of the host institute. Dr M.
Baril, IPCS, welcomed the participants on behalf of IPCS and the three
cooperating organizations (UNEP/ILO/WHO). The Task Group reviewed and
revised the draft criteria monograph and made an evaluation of the
risk to human health and the environment from exposure to these flame
retardants.
Financial support for this Task Group was provided by the United
Kingdom Department of Health as part of its contribution to the IPCS.
The first draft of this monograph was prepared by Dr G. J. van
Esch, Bilthoven, the Netherlands. The second draft prepared by Dr M.
Baril incorporated the comments received following circulation of the
first draft to the IPCS contact points for Environmental Health
Criteria.
Dr P.G. Jenkins (IPCS Central Unit, Geneva) and Dr M. Baril (IPCS
technical advisor, Montreal) were responsible for the overall
technical editing and scientific content,respectively.
The efforts of all who helped in the preparation and finalization
of the monograph are gratefully acknowledged.
* * *
ABBREVIATIONS
AChE acetylcholinesterase
ALAT alanine aminotransferase
ASAT aspartate aminotransferase
BCME bis(chloromethyl) ether
BEHP bis(2-ethylhexyl) phosphate
BMPA bishydroxymethyl phosphonic acid
BuCHE butyrylcholinesterase
CHO Chinese hamster ovary
DMSO dimethyl sulfoxide
EC50 median effective concentration
FDA Food and Drug Administration (USA)
GC gas chromatography
HPLC high performance liquid chromatography
IC50 median inhibitory concentration
LC50 median lethal concentration
LD50 median lethal dose
LOAEL lowest-observed-adverse-effect level
LOEL lowest-observed-effect level
MS mass spectrometry
nd not detected
NOAEL no-observed-adverse-effect level
NOEC no-observed-effect concentration
NOEL no-observed-effect level
NPD nitrogen-phosphorus sensitive detector
NTE neuropathy target esterase
NTP National Toxicology Program (USA)
OECD Organisation for Economic Co-operation and Development
PVC polyvinyl chloride
SCE sister-chromatid exchange
TBEP tris(2-butoxyethyl) phosphate
TEHP tris(2-ethylhexyl) phosphate
THP tetrakis(hydroxymethyl) phosphonium
THPC tetrakis(hydroxymethyl) phosphonium chloride
THPO trihydroxymethyl phosphine oxide
THPS tetrakis(hydroxymethyl) phosphonium sulfate
TOCP tri- ortho-cresyl phosphate
PART A
Tris(2-butoxyethyl) phosphate
(TBEP)
A. SUMMARY, EVALUATION AND RECOMMENDATIONS
A1. Tris(2-butoxyethyl) phosphate (TBEP)
A1.1 Summary
Tris(2-butoxyethyl) phosphate (TBEP) is used in floor polishes
and as a plasticizer in rubber and plastics. The worldwide production
volume is not available but is estimated to be in the range of
5000-6000 tonnes.
TBEP occurs in the environment only as a result of human
activity. Its distribution in the environment has been investigated in
certain industrialized countries. Concentrations in surface water were
found to be below 300 ng/litre, whereas concentrations in sediment
were between 100 and 1000 µg/kg. None of 167 analyses detected TBEP in
fish. It has been detected in outdoor air in a single study (<200
ng/m3). Measurement of TBEP in indoor air in offices showed
concentrations of 25 ng/m3 or less. TBEP is associated with
particulates and the source is considered to be the application of
floor polish. It has been detected at µg/kg levels in human adipose
tissue. The reported daily dietary intake from market basket studies,
for a range of age groups, was <0.02 µg/kg body weight per day.
Drinking-water concentrations of up to 270 µg/litre have been
reported, this is considered to arise from migration from rubber
gaskets in the plumbing.
TBEP is considered to be readily biodegradable. Sewage treatment
plant measurements and semi-continuous sludge laboratory tests have
indicated substantial elimination of TBEP (>80%). In river and
coastal water TBEP was completely degraded. The half-life in estuarine
water was reported to be about 50 days and there was little
degradation in unadapted seawater.
The acute systemic mammalian toxicity and irritation potential
are low.
Several subchronic studies in laboratory animals have shown that
the liver is the target organ for TBEP toxicity. One study in male
Sprague-Dawley rats suggested that TBEP might cause focal myocarditis.
Neurotoxic effects in rats after single doses of TBEP are
inconsistent. In rats repeatedly given high doses by gavage, TBEP
decreased nerve conduction velocity and increased the refractory
period. It did not cause delayed neurotoxicity in hens but did inhibit
brain and plasma cholinesterases.
Based on an 18-week repeated dose study in rats, the
no-observed-effect level (NOEL) for liver effects was reported to be
15 mg/kg body weight per day, while the lowest-observed-effect level
(LOEL) was 150 mg/kg body weight per day.
The long-term toxicity and carcinogenicity of TBEP have not been
studied.
Bacterial and mammalian cell tests for gene mutation gave
negative results, but no tests for chromosomal damage have been
reported.
Teratogenicity was not observed in one study in rats. Other
aspects of reproductive toxicity have not been reported.
A Repeat Human Insult Patch Test indicated no skin sensitization
and minimal skin irritation.
The toxicity of TBEP to aquatic organisms is moderate. The 48-h
LC50 in Daphnia magna is 75 mg/litre and the 96-h LC50 values in
fish range between 16 and 24 mg/litre.
A1.2 Evaluation
Occupational exposure to TBEP is likely to be by the dermal route
during manufacture (accidental exposure) and from the use of floor
polishes. The compound is absorbed dermally in experimental animals
but no information is available on its kinetics and metabolism. Dermal
exposure cannot, therefore, be quantified but is expected to be low.
Inhalation exposure in the office environment has been measured to be
25 ng/m3 or less.
Exposure of the general population is principally via food (from
use of TBEP as a plasticizer in packaging plastics) and drinking-water
(contaminated by leaching from synthetic rubbers used in plumbing
washers). Exposure from both sources is very low (estimated to be
<0.2 µg/kg body weight per day from the diet and concentrations in
drinking-water of <270 µg/litre).
Given the reported NOEL from animal studies of 15 mg/kg body
weight per day from a repeated dose oral study, the risk to the
general population is very low. The risk to the occupationally exposed
is also considered to be very low, though this cannot be quantified.
In the environment, TBEP is expected (from its low volatility,
high adsorption coefficient and moderate water solubility) to
partition to sediment. The few measured data confirm this. Degradation
in environmental media is expected to be rapid. No information is
available on breakdown products; phosphate released during breakdown
is not expected to contribute significantly to environmental nutrient
levels. Fig. 1 plots measured environmental concentrations in surface
water against reported acute toxicity values. The margin of safety
between highest reported concentrations and lowest reported toxicity
values is several orders of magnitude, indicating low risk to
organisms in the aquatic environment. No assessment of risk can be
made for the terrestrial compartment.
A1.3 Recommendations
For a full scientific evaluation of the compound, identification
and assessment of metabolites in mammals would be required, given the
toxicological profile of one of the suggested metabolites,
2-butoxyethanol.
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR FLAME RETARDANTS:
TRIS(2-BUTOXYETHYL) PHOSPHATE, TRIS(2-ETHYLHEXYL) PHOSPHATE AND
TETRAKIS(HYDROXYMETHYL) PHOSPHONIUM SALTS
Members
Dr R. Benson, US Environmental Protection Agency, Denver, Colorado,
USA
Dr P. Brantom, British Industry Biological Research Association
(BIBRA) International, Carshalton, Surrey, United Kingdom
Dr S. Dobson, Institute of Terrestrial Ecology, Monks Wood,
Huntingdon, Cambridgeshire, United Kingdom (Chairman)
Professor J. Liesivuori, Department of Pharmacology and Toxicology,
University of Kuopio, Kuopio, Finland
Mr D. Renshaw, Department of Health, Elephant and Castle, London,
United Kingdom
Dr E. Söderlund, National Institute of Public Health, Department of
Environmental Medicine, Oslo, Norway (Rapporteur)
Observers
Dr L. Kotkoskic, FMC Corporation, Princetown, New Jersey, USA
Dr P. Martin, Albright and Wilson UK Limited, European Business
Services - Product Stewardship, Oldbury, West Midlands, United Kingdom
Secretariat
Dr M. Baril, International Programme on Chemical Safety, Montreal,
Quebec, Canada
ENVIRONMENTAL HEALTH CRITERIA FOR FLAME RETARDANTS:
TRIS(2-BUTOXYETHYL) PHOSPHATE, TRIS(2-ETHYLHEXYL) PHOSPHATE AND
TETRAKIS(HYDROXYMETHYL) PHOSPHONIUM SALTS
A WHO Task Group on Environmental Health Criteria for Flame
retardants: tris(2-butoxyethyl) phosphate, tris(2-ethylhexyl)
phosphate and tetrakis(hydroxymethyl) phosphonium salts met at the
British Industrial Biological Research Association, Carshalton, United
Kingdom from 18 to 22 January 1999. Dr P. Brantom opened the meeting
and welcome the participants on behalf of the host institute. Dr M.
Baril, IPCS, welcomed the participants on behalf of IPCS and the three
cooperating organizations (UNEP/ILO/WHO). The Task Group reviewed and
revised the draft criteria monograph and made an evaluation of the
risk to human health and the environment from exposure to these flame
retardants.
Financial support for this Task Group was provided by the United
Kingdom Department of Health as part of its contribution to the IPCS.
The first draft of this monograph was prepared by Dr G. J. van
Esch, Bilthoven, the Netherlands. The second draft prepared by Dr M.
Baril incorporated the comments received following circulation of the
first draft to the IPCS contact points for Environmental Health
Criteria.
Dr P.G. Jenkins (IPCS Central Unit, Geneva) and Dr M. Baril (IPCS
technical advisor, Montreal) were responsible for the overall
technical editing and scientific content,respectively.
The efforts of all who helped in the preparation and finalization
of the monograph are gratefully acknowledged.
* * *
ABBREVIATIONS
AChE acetylcholinesterase
ALAT alanine aminotransferase
ASAT aspartate aminotransferase
BCME bis(chloromethyl) ether
BEHP bis(2-ethylhexyl) phosphate
BMPA bishydroxymethyl phosphonic acid
BuCHE butyrylcholinesterase
CHO Chinese hamster ovary
DMSO dimethyl sulfoxide
EC50 median effective concentration
FDA Food and Drug Administration (USA)
GC gas chromatography
HPLC high performance liquid chromatography
IC50 median inhibitory concentration
LC50 median lethal concentration
LD50 median lethal dose
LOAEL lowest-observed-adverse-effect level
LOEL lowest-observed-effect level
MS mass spectrometry
nd not detected
NOAEL no-observed-adverse-effect level
NOEC no-observed-effect concentration
NOEL no-observed-effect level
NPD nitrogen-phosphorus sensitive detector
NTE neuropathy target esterase
NTP National Toxicology Program (USA)
OECD Organisation for Economic Co-operation and Development
PVC polyvinyl chloride
SCE sister-chromatid exchange
TBEP tris(2-butoxyethyl) phosphate
TEHP tris(2-ethylhexyl) phosphate
THP tetrakis(hydroxymethyl) phosphonium
THPC tetrakis(hydroxymethyl) phosphonium chloride
THPO trihydroxymethyl phosphine oxide
THPS tetrakis(hydroxymethyl) phosphonium sulfate
TOCP tri- ortho-cresyl phosphate
PART A
Tris(2-butoxyethyl) phosphate
(TBEP)
A. SUMMARY, EVALUATION AND RECOMMENDATIONS
A1. Tris(2-butoxyethyl) phosphate (TBEP)
A1.1 Summary
Tris(2-butoxyethyl) phosphate (TBEP) is used in floor polishes
and as a plasticizer in rubber and plastics. The worldwide production
volume is not available but is estimated to be in the range of
5000-6000 tonnes.
TBEP occurs in the environment only as a result of human
activity. Its distribution in the environment has been investigated in
certain industrialized countries. Concentrations in surface water were
found to be below 300 ng/litre, whereas concentrations in sediment
were between 100 and 1000 µg/kg. None of 167 analyses detected TBEP in
fish. It has been detected in outdoor air in a single study (<200
ng/m3). Measurement of TBEP in indoor air in offices showed
concentrations of 25 ng/m3 or less. TBEP is associated with
particulates and the source is considered to be the application of
floor polish. It has been detected at µg/kg levels in human adipose
tissue. The reported daily dietary intake from market basket studies,
for a range of age groups, was <0.02 µg/kg body weight per day.
Drinking-water concentrations of up to 270 µg/litre have been
reported, this is considered to arise from migration from rubber
gaskets in the plumbing.
TBEP is considered to be readily biodegradable. Sewage treatment
plant measurements and semi-continuous sludge laboratory tests have
indicated substantial elimination of TBEP (>80%). In river and
coastal water TBEP was completely degraded. The half-life in estuarine
water was reported to be about 50 days and there was little
degradation in unadapted seawater.
The acute systemic mammalian toxicity and irritation potential
are low.
Several subchronic studies in laboratory animals have shown that
the liver is the target organ for TBEP toxicity. One study in male
Sprague-Dawley rats suggested that TBEP might cause focal myocarditis.
Neurotoxic effects in rats after single doses of TBEP are
inconsistent. In rats repeatedly given high doses by gavage, TBEP
decreased nerve conduction velocity and increased the refractory
period. It did not cause delayed neurotoxicity in hens but did inhibit
brain and plasma cholinesterases.
Based on an 18-week repeated dose study in rats, the
no-observed-effect level (NOEL) for liver effects was reported to be
15 mg/kg body weight per day, while the lowest-observed-effect level
(LOEL) was 150 mg/kg body weight per day.
The long-term toxicity and carcinogenicity of TBEP have not been
studied.
Bacterial and mammalian cell tests for gene mutation gave
negative results, but no tests for chromosomal damage have been
reported.
Teratogenicity was not observed in one study in rats. Other
aspects of reproductive toxicity have not been reported.
A Repeat Human Insult Patch Test indicated no skin sensitization
and minimal skin irritation.
The toxicity of TBEP to aquatic organisms is moderate. The 48-h
LC50 in Daphnia magna is 75 mg/litre and the 96-h LC50 values in
fish range between 16 and 24 mg/litre.
A1.2 Evaluation
Occupational exposure to TBEP is likely to be by the dermal route
during manufacture (accidental exposure) and from the use of floor
polishes. The compound is absorbed dermally in experimental animals
but no information is available on its kinetics and metabolism. Dermal
exposure cannot, therefore, be quantified but is expected to be low.
Inhalation exposure in the office environment has been measured to be
25 ng/m3 or less.
Exposure of the general population is principally via food (from
use of TBEP as a plasticizer in packaging plastics) and drinking-water
(contaminated by leaching from synthetic rubbers used in plumbing
washers). Exposure from both sources is very low (estimated to be
<0.2 µg/kg body weight per day from the diet and concentrations in
drinking-water of <270 µg/litre).
Given the reported NOEL from animal studies of 15 mg/kg body
weight per day from a repeated dose oral study, the risk to the
general population is very low. The risk to the occupationally exposed
is also considered to be very low, though this cannot be quantified.
In the environment, TBEP is expected (from its low volatility,
high adsorption coefficient and moderate water solubility) to
partition to sediment. The few measured data confirm this. Degradation
in environmental media is expected to be rapid. No information is
available on breakdown products; phosphate released during breakdown
is not expected to contribute significantly to environmental nutrient
levels. Fig. 1 plots measured environmental concentrations in surface
water against reported acute toxicity values. The margin of safety
between highest reported concentrations and lowest reported toxicity
values is several orders of magnitude, indicating low risk to
organisms in the aquatic environment. No assessment of risk can be
made for the terrestrial compartment.
A1.3 Recommendations
For a full scientific evaluation of the compound, identification
and assessment of metabolites in mammals would be required, given the
toxicological profile of one of the suggested metabolites,
2-butoxyethanol.
 A2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, AND ANALYTICAL
METHODS
A2.1 Identity
Molecular structure:
A2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, AND ANALYTICAL
METHODS
A2.1 Identity
Molecular structure:
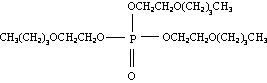 Empirical formula: C18H39O7P
Relative molecular mass: 398.54
Common name: tris(2-butoxyethyl) phosphate
Synonyms: phosphoric acid, tris(2-butoxyethyl) ester;
tri(2-butoxyethanol) phosphate;
tris(2- n-butoxyethyl) phosphate;
tributoxyethyl phosphate; TBOP; TBEP; TBXP
(only in Japanese literature);
2-butoxyethanol phosphate (RTCES, 1989);
tri(2-butylethylether) phosphate;
tris(butylglycol) phosphate; tributyl cello
solve phosphate
Trade names: Kronitex KP-140; KP-140; Phosflex T-BEP;
Phosflex 176C; Amgard TBEP
CAS registry number:
Empirical formula: C18H39O7P
Relative molecular mass: 398.54
Common name: tris(2-butoxyethyl) phosphate
Synonyms: phosphoric acid, tris(2-butoxyethyl) ester;
tri(2-butoxyethanol) phosphate;
tris(2- n-butoxyethyl) phosphate;
tributoxyethyl phosphate; TBOP; TBEP; TBXP
(only in Japanese literature);
2-butoxyethanol phosphate (RTCES, 1989);
tri(2-butylethylether) phosphate;
tris(butylglycol) phosphate; tributyl cello
solve phosphate
Trade names: Kronitex KP-140; KP-140; Phosflex T-BEP;
Phosflex 176C; Amgard TBEP
CAS registry number:  B2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, AND ANALYTICAL
METHODS
B2.1 Identity
Chemical structure:
B2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, AND ANALYTICAL
METHODS
B2.1 Identity
Chemical structure:
 Chemical formula: C24H51O4P
Relative molecular mass: 434.64
CAS registry number:
Chemical formula: C24H51O4P
Relative molecular mass: 434.64
CAS registry number:  The commercially relevant salts of THP are the sulfate (THPS) and
the chloride (THPC). In addition, tetrakis(hydroxymethyl) phosphonium
chloride-urea condensate is the major commercially available flame
retardant product.
In the past other salts and salt-urea condensates have been used;
their names and CAS numbers are listed in IARC (1990).
C2.1.1 Tetrakis(hydroxymethyl) phosphonium chloride (THPC)
Chemical formula: C4H12O4PCl
Chemical structure:
The commercially relevant salts of THP are the sulfate (THPS) and
the chloride (THPC). In addition, tetrakis(hydroxymethyl) phosphonium
chloride-urea condensate is the major commercially available flame
retardant product.
In the past other salts and salt-urea condensates have been used;
their names and CAS numbers are listed in IARC (1990).
C2.1.1 Tetrakis(hydroxymethyl) phosphonium chloride (THPC)
Chemical formula: C4H12O4PCl
Chemical structure:
 Chemical name: Phosphonium, tetrakis(hydroxymethyl)
chloride
Relative molecular mass: 190.56
CAS registry number:
Chemical name: Phosphonium, tetrakis(hydroxymethyl)
chloride
Relative molecular mass: 190.56
CAS registry number:  Chemical name: Phosphonium, tetrakis(hydroxymethyl)
sulfate
Relative molecular mass: 406.28
CAS registry number:
Chemical name: Phosphonium, tetrakis(hydroxymethyl)
sulfate
Relative molecular mass: 406.28
CAS registry number:  Relative molecular mass: 300 for the repeat unit shown above
CAS registry number:
Relative molecular mass: 300 for the repeat unit shown above
CAS registry number:  1.3 Recommandations
Pour procéder à une évaluation scientifique complète de ce
composé, il faudrait identifier et étudier chacun de ses métabolites
chez des mammifères, étant donné le profil toxicologique d'un des
métabolites possibles, le 2-butoxyéthanol.
2. Phosphate de tris (2-éthylhexyle) (TEHP)
2.1 Résumé
Le phosphate de tris (2-éthylhexyle) ou THEP, se présente sous la
forme d'un liquide incolore et ininflammable, dont la solubilité dans
l'eau et la tension de vapeur sont faibles et qui est utilisé comme
retardateur de flammes et comme plastifiant dans le PVC et l'acétate
de cellulose ou encore comme solvant. On le prépare à partir de
l'oxychlorure de phosphore et du 2-éthyléthanol. On ne connaît pas les
chiffres actuels de production dans le monde. En Allemagne, la
production annuelle est actuellement d'environ 1000 tonnes.
On n'a pas décelé la présence de TEHP dans l'air extérieur ; à
l'intérieur des bâtiments, sa concentration dans l'air est inférieure
à 10 ng/m3. Dans les cours d'eau, la concentration peut atteindre
7500 ng/litre et dans les matières particulaires, 2 à 70 ng/g. On en a
trouvé 0,3 ng/litre dans un seul et unique échantillon d'eau de
boisson. D'après des études sur le panier de la ménagère, la dose
ingérée journalière pour diverses tranches d'âge serait inférieure à
0,05 µg/kg pc.
Le TEHP est rapidement décomposé dans les eaux naturelles, mais
des essais en laboratoire portant sur des boues activées ont donné des
résultats équivoques. Il ne subit pas de décomposition abiotique
importante.
Le TEHP présente une faible toxicité aiguë pour les mammifères,
la DL50 par voie orale étant > 10 000 mg/kg pc pour le rat.
Le TEHP irrite la peau mais il n'est pas irritant pour l'oeil.
Des applications répétées de TEHP sur la peau de lapins à raison de
0,1 ml (93 mg) n'ont produit aucun signe d'intoxication générale.
Des études au cours desquelles des rats et des souris ont reçu
pendant 13 semaines du TEHP par gavage n'ont pas permis d'observer
d'effets toxiques importants. Pour les rats, la dose sans effet nocif
observable (NOAEL) était égale à 2860 mg/kg pc et pour les souris, à
5710 mg/kg pc, soit les deux plus fortes doses utilisées chez chaque
espèce.
Lors d'une étude de 3 mois au cours de laquelle on a fait inhaler
du TEHP à des chiens à des doses allant jusqu'à 85,0 mg/m3, on a
observé de légères altérations inflammatoires chroniques au niveau des
poumons et constaté que le réflexe conditionné d'évitement
s'affaiblissait parallèlement à l'augmentation des doses.
Il n'existe pas d'étude consacrée à la toxicité génésique du
TEHP.
Le composé a donné des résultats négatifs dans plusieurs tests de
mutagénicité in vitro et in vivo.
On a étudié la toxicité chronique et le pouvoir cancérogène du
TEHP sur des rats et des souris. La NOAEL relative à la toxicité
chronique était de 2857 mg.kg-1.j-1 pour les rats mâles et de 1428
mg.kg-1.j-1 pour les femelles. Chez les souris mâles et femelles, la
dose la plus faible produisant un effet nocif observable (LOAEL) était
de 357 mg.kg-1.j-1, le critère retenu étant une hyperplasie des
cellules folliculaires de la thyroïde. On n'a pas établi de NOAEL pour
les souris. Les auteurs de ces études concluent que le composé
présente un certain pouvoir cancérogène, compte tenu de l'augmentation
des carcinomes hépatocellulaires qui a été observée chez les souris
femelles, cette cancérogénicité étant plus équivoque pour les rats
mâles chez qui a été relevée une augmentation de l'incidence des
phéochromocytomes surrénaliens aux deux doses administrées. Malgré
l'augmentation de l'incidence des phéochromocytomes aux deux doses
chez les rats mâles et de celle des carcinomes hépatocellulaires chez
les souris soumises à la plus forte dose, on estime qu'il n'y a pas
lieu de considérer que le TEHP comporte un risque cancérogène
important pour l'Homme. En effet, chez le rat l'incidence
« naturelle » des phéochromocytomes est variable. Ainsi, dans deux
études toxicologiques effectuées antérieurement par le National
Toxicology Programme (NTP ) on a observé des phéochromocytomes dont
l'incidence était égale à celle constatée dans l'étude précédente.
Pour ce qui est de l'autre type de tumeur observé, à savoir le
carcinome hépatocellulaire chez les souris femelles soumises à la dose
la plus forte, le fait que son incidence soit faible, qu'elle ne se
soit manifestée que dans un seul sexe et chez une seule espèce, qu'on
n'ait pas de preuve d'une quelconque activité génotoxique et que
l'Homme ne soit guère exposé au TEHP, rend improbable la possibilité
d'un risque cancérogène notable pour l'Homme.
Des études sur la neurotoxicité du TEHP ont été effectuées sur
plusieurs espèces. Le composé ne provoque aucune modification dans
l'activité de la cholinestérase plasmatique ou érythrocytaire. On n'a
pas connaissance d'études sur la neurotoxicité retardée du TEHP.
Une étude sur des volontaires humains n'a pas révélé d'irritation
cutanée.
Les quelques données disponibles montrent que le composé présente
une faible toxicité aiguë pour les organismes aquatiques. Pour les
bactéries, la CI50 à 96 h est supérieure à 100 mg/litre et pour un
poisson comme le danio (Brachydanio rerio), elle dépasse également
cette valeur, qui correspond d'ailleurs à la limite de solubilité du
TEHP dans l'eau.
2.2 Evaluation
L'exposition au TEHP se produit vraisemblablement par voie
cutanée au cours de la préparation du composé (exposition
accidentelle) ou par suite de l'utilisation de produits qui en
contiennent. Le TEHP est absorbé par voie percutanée chez l'animal de
laboratoire mais on dispose d'aucune donnée sur sa cinétique ou son
métabolisme après absorption par cette voie. On ne peut donc pas
évaluer quantitativement ce type d'exposition, mais on peut penser
qu'elle est faible. La mesure de l'exposition par inhalation de l'air
des bureaux a donné une valeur au plus égale à 10 ng/m3.
L'exposition de la population générale se produit principalement
par la consommation de nourriture et d'eau de boisson. Quelle que soit
la source, cette exposition est très faible (l'exposition par voie
alimentaire est estimée à moins de 0,05 µg.kg-1.j-1 ; une seule et
unique mesure effectuée sur de l'eau de boisson a donné 0,3 ng/litre).
Si l'on se base sur la LOAEL de 357 mg.kg-1.j-1 obtenue chez la
souris avec comme critère une hyperplasie de la thyroïde, le risque
est très faible à l'échelon de la population générale. On estime que
le risque est également très faible sur les lieux de travail, encore
qu'il ne soit pas possible d'en donner une évaluation quantitative.
On estime que le TEHP n'est pas cancérogène pour l'Homme.
Dans l'environnement, on peut déduire de la faible volatilité, du
fort coefficient d'adsorption et de sa solubilité modérée dans l'eau,
que le TEHP va se répartir entre les différents types de matières
particulaires. Les mesures dont on dispose sont cependant trop peu
nombreuses pour le confirmer. On peut s'attendre à une décomposition
dans l'environnement, mais les données de laboratoire relatives à la
décomposition du TEHP dans les boues d'égout sont ambiguës. On ne
dispose d'aucune donnée sur ses produits de décomposition ; le reste
phosphate libéré au cours de ce processus ne devrait pas sensiblement
augmenter la concentration globale des nutriments. La Fig. 2 donne la
valeur de la concentration relevée dans divers compartiments du milieu
en fonction de la toxicité aiguë relevée (aucun effet toxique observé
à la limite de solubilité dans l'eau). Il y a plusieurs ordres de
grandeur entre la concentration la plus élevée mesurée et la plus
faible valeur de la toxicité observée, d'où une marge de sécurité
élevée et donc un faible risque pour les organismes aquatiques. On
n'est pas en mesure d'évaluer l'importance du risque pour les
organismes terrestres.
2.3 Recommandations
Pour procéder à une évaluation scientifique complète de ce
composé, il faudrait identifier et étudier chacun de ses métabolites
chez des mammifères, étant donné le profil toxicologique d'un des
métabolites possibles, le 2-éthylhexanol.
La toxicité génésique doit être étudiée, notamment en ce qui
concerne d'éventuels effets sur le développement.
3. Sels de tétrakis(hydroxyméthyl) phosphonium
3.1 Résumé
Les sels de tétrakis(hydroxyméthyl)phosphonium (THP) constituent
un groupe important de composés utilisés comme retardateurs de flammes
pour le coton, la cellulose et les toiles constituées de mélanges de
cellulose. On constate que la migration du chlorure de
tétrakis(hydroxyméthyl)phosphonium (THPC) à partir des tissus traités
par le condensat de ce composé avec l'urée reste faible. Le sulfate de
THP (THPS) est surtout utilisé comme produit biocide. On estime que la
production mondiale est de moins de 3000 tonnes par an pour les sels
de THP et d'environ 3000 tonnes pour le condensat chlorure de tétrakis
(hydroxyméthyl)phosphonium-urée.
La photodécomposition et l'hydrolyse des sels de THP constituent
des voies de dégradation abiotiques importantes dans l'environnement.
Le sulfate de THP ne se fixe guère sur les matières particulaires et
il est donc mobile. Le THPS se décompose rapidement en aérobiose comme
en anaérobiose. On a constaté la présence d'oxyde de
trihydroxyméthylphosphine (THPO) et d'acide
bishydroxyméthylphosphonique ou BMPA dans les produits de
décomposition.
Comme il ne semble pas y avoir de surveillance de ces composés,
on ne peut pas évaluer l'exposition de l'Homme ni celle des autres
êtres vivants dans leur milieu naturel.
Le THPC et le THPS présentent une toxicité aiguë modérée par voie
orale ; au niveau cutané, leur toxicité est faible.
Des études à court terme (jusqu'à 28 jours) effectuées sur des
rats et des souris ont montré que le principal effet toxique du THPC
et du THPS était une réduction du poids corporel. Chez les deux
espèces, la NOAEL est d'environ 8 mg.kg-1.j-1. Des études plus
longues (13 semaines) montrent que le principal organe cible est le
foie. La NOAEL relative à cet effet varie de 3 à 7 mg.kg-1.j-1 pour
les deux sels chez les deux espèces. Les tests biologiques de
cancérogénicité effectués sur le THPC ont également montré que ces
effets se produisaient au niveau du foie, mais on n'a pas établi de
NOAEL. Chez les deux espèces, la LOAEL était d'environ 3 mg.kg-1.j-1.
Lors d'une étude de cancérogénicité portant sur le THPS et effectuée
sur des souris, on a évalué à 3,6 mg.kg-1.j-1 la NOAEL pour une
hyperplasie médullo-surrénalienne focale; chez les rats, la LOAEL pour
la mortalité avait la même valeur.
Administré en dose unique à des lapins, le THPS n'a pas provoqué
d'irritation cutanée. Cependant, l'exposition répétée de rats à ce
composé par la voie cutanée a entraîné une sérieuse réaction à ce
niveau. Le condensat THPC-urée s'est révélé corrosif. Chez le lapin,
le THPS irrite fortement la muqueuse oculaire.
1.3 Recommandations
Pour procéder à une évaluation scientifique complète de ce
composé, il faudrait identifier et étudier chacun de ses métabolites
chez des mammifères, étant donné le profil toxicologique d'un des
métabolites possibles, le 2-butoxyéthanol.
2. Phosphate de tris (2-éthylhexyle) (TEHP)
2.1 Résumé
Le phosphate de tris (2-éthylhexyle) ou THEP, se présente sous la
forme d'un liquide incolore et ininflammable, dont la solubilité dans
l'eau et la tension de vapeur sont faibles et qui est utilisé comme
retardateur de flammes et comme plastifiant dans le PVC et l'acétate
de cellulose ou encore comme solvant. On le prépare à partir de
l'oxychlorure de phosphore et du 2-éthyléthanol. On ne connaît pas les
chiffres actuels de production dans le monde. En Allemagne, la
production annuelle est actuellement d'environ 1000 tonnes.
On n'a pas décelé la présence de TEHP dans l'air extérieur ; à
l'intérieur des bâtiments, sa concentration dans l'air est inférieure
à 10 ng/m3. Dans les cours d'eau, la concentration peut atteindre
7500 ng/litre et dans les matières particulaires, 2 à 70 ng/g. On en a
trouvé 0,3 ng/litre dans un seul et unique échantillon d'eau de
boisson. D'après des études sur le panier de la ménagère, la dose
ingérée journalière pour diverses tranches d'âge serait inférieure à
0,05 µg/kg pc.
Le TEHP est rapidement décomposé dans les eaux naturelles, mais
des essais en laboratoire portant sur des boues activées ont donné des
résultats équivoques. Il ne subit pas de décomposition abiotique
importante.
Le TEHP présente une faible toxicité aiguë pour les mammifères,
la DL50 par voie orale étant > 10 000 mg/kg pc pour le rat.
Le TEHP irrite la peau mais il n'est pas irritant pour l'oeil.
Des applications répétées de TEHP sur la peau de lapins à raison de
0,1 ml (93 mg) n'ont produit aucun signe d'intoxication générale.
Des études au cours desquelles des rats et des souris ont reçu
pendant 13 semaines du TEHP par gavage n'ont pas permis d'observer
d'effets toxiques importants. Pour les rats, la dose sans effet nocif
observable (NOAEL) était égale à 2860 mg/kg pc et pour les souris, à
5710 mg/kg pc, soit les deux plus fortes doses utilisées chez chaque
espèce.
Lors d'une étude de 3 mois au cours de laquelle on a fait inhaler
du TEHP à des chiens à des doses allant jusqu'à 85,0 mg/m3, on a
observé de légères altérations inflammatoires chroniques au niveau des
poumons et constaté que le réflexe conditionné d'évitement
s'affaiblissait parallèlement à l'augmentation des doses.
Il n'existe pas d'étude consacrée à la toxicité génésique du
TEHP.
Le composé a donné des résultats négatifs dans plusieurs tests de
mutagénicité in vitro et in vivo.
On a étudié la toxicité chronique et le pouvoir cancérogène du
TEHP sur des rats et des souris. La NOAEL relative à la toxicité
chronique était de 2857 mg.kg-1.j-1 pour les rats mâles et de 1428
mg.kg-1.j-1 pour les femelles. Chez les souris mâles et femelles, la
dose la plus faible produisant un effet nocif observable (LOAEL) était
de 357 mg.kg-1.j-1, le critère retenu étant une hyperplasie des
cellules folliculaires de la thyroïde. On n'a pas établi de NOAEL pour
les souris. Les auteurs de ces études concluent que le composé
présente un certain pouvoir cancérogène, compte tenu de l'augmentation
des carcinomes hépatocellulaires qui a été observée chez les souris
femelles, cette cancérogénicité étant plus équivoque pour les rats
mâles chez qui a été relevée une augmentation de l'incidence des
phéochromocytomes surrénaliens aux deux doses administrées. Malgré
l'augmentation de l'incidence des phéochromocytomes aux deux doses
chez les rats mâles et de celle des carcinomes hépatocellulaires chez
les souris soumises à la plus forte dose, on estime qu'il n'y a pas
lieu de considérer que le TEHP comporte un risque cancérogène
important pour l'Homme. En effet, chez le rat l'incidence
« naturelle » des phéochromocytomes est variable. Ainsi, dans deux
études toxicologiques effectuées antérieurement par le National
Toxicology Programme (NTP ) on a observé des phéochromocytomes dont
l'incidence était égale à celle constatée dans l'étude précédente.
Pour ce qui est de l'autre type de tumeur observé, à savoir le
carcinome hépatocellulaire chez les souris femelles soumises à la dose
la plus forte, le fait que son incidence soit faible, qu'elle ne se
soit manifestée que dans un seul sexe et chez une seule espèce, qu'on
n'ait pas de preuve d'une quelconque activité génotoxique et que
l'Homme ne soit guère exposé au TEHP, rend improbable la possibilité
d'un risque cancérogène notable pour l'Homme.
Des études sur la neurotoxicité du TEHP ont été effectuées sur
plusieurs espèces. Le composé ne provoque aucune modification dans
l'activité de la cholinestérase plasmatique ou érythrocytaire. On n'a
pas connaissance d'études sur la neurotoxicité retardée du TEHP.
Une étude sur des volontaires humains n'a pas révélé d'irritation
cutanée.
Les quelques données disponibles montrent que le composé présente
une faible toxicité aiguë pour les organismes aquatiques. Pour les
bactéries, la CI50 à 96 h est supérieure à 100 mg/litre et pour un
poisson comme le danio (Brachydanio rerio), elle dépasse également
cette valeur, qui correspond d'ailleurs à la limite de solubilité du
TEHP dans l'eau.
2.2 Evaluation
L'exposition au TEHP se produit vraisemblablement par voie
cutanée au cours de la préparation du composé (exposition
accidentelle) ou par suite de l'utilisation de produits qui en
contiennent. Le TEHP est absorbé par voie percutanée chez l'animal de
laboratoire mais on dispose d'aucune donnée sur sa cinétique ou son
métabolisme après absorption par cette voie. On ne peut donc pas
évaluer quantitativement ce type d'exposition, mais on peut penser
qu'elle est faible. La mesure de l'exposition par inhalation de l'air
des bureaux a donné une valeur au plus égale à 10 ng/m3.
L'exposition de la population générale se produit principalement
par la consommation de nourriture et d'eau de boisson. Quelle que soit
la source, cette exposition est très faible (l'exposition par voie
alimentaire est estimée à moins de 0,05 µg.kg-1.j-1 ; une seule et
unique mesure effectuée sur de l'eau de boisson a donné 0,3 ng/litre).
Si l'on se base sur la LOAEL de 357 mg.kg-1.j-1 obtenue chez la
souris avec comme critère une hyperplasie de la thyroïde, le risque
est très faible à l'échelon de la population générale. On estime que
le risque est également très faible sur les lieux de travail, encore
qu'il ne soit pas possible d'en donner une évaluation quantitative.
On estime que le TEHP n'est pas cancérogène pour l'Homme.
Dans l'environnement, on peut déduire de la faible volatilité, du
fort coefficient d'adsorption et de sa solubilité modérée dans l'eau,
que le TEHP va se répartir entre les différents types de matières
particulaires. Les mesures dont on dispose sont cependant trop peu
nombreuses pour le confirmer. On peut s'attendre à une décomposition
dans l'environnement, mais les données de laboratoire relatives à la
décomposition du TEHP dans les boues d'égout sont ambiguës. On ne
dispose d'aucune donnée sur ses produits de décomposition ; le reste
phosphate libéré au cours de ce processus ne devrait pas sensiblement
augmenter la concentration globale des nutriments. La Fig. 2 donne la
valeur de la concentration relevée dans divers compartiments du milieu
en fonction de la toxicité aiguë relevée (aucun effet toxique observé
à la limite de solubilité dans l'eau). Il y a plusieurs ordres de
grandeur entre la concentration la plus élevée mesurée et la plus
faible valeur de la toxicité observée, d'où une marge de sécurité
élevée et donc un faible risque pour les organismes aquatiques. On
n'est pas en mesure d'évaluer l'importance du risque pour les
organismes terrestres.
2.3 Recommandations
Pour procéder à une évaluation scientifique complète de ce
composé, il faudrait identifier et étudier chacun de ses métabolites
chez des mammifères, étant donné le profil toxicologique d'un des
métabolites possibles, le 2-éthylhexanol.
La toxicité génésique doit être étudiée, notamment en ce qui
concerne d'éventuels effets sur le développement.
3. Sels de tétrakis(hydroxyméthyl) phosphonium
3.1 Résumé
Les sels de tétrakis(hydroxyméthyl)phosphonium (THP) constituent
un groupe important de composés utilisés comme retardateurs de flammes
pour le coton, la cellulose et les toiles constituées de mélanges de
cellulose. On constate que la migration du chlorure de
tétrakis(hydroxyméthyl)phosphonium (THPC) à partir des tissus traités
par le condensat de ce composé avec l'urée reste faible. Le sulfate de
THP (THPS) est surtout utilisé comme produit biocide. On estime que la
production mondiale est de moins de 3000 tonnes par an pour les sels
de THP et d'environ 3000 tonnes pour le condensat chlorure de tétrakis
(hydroxyméthyl)phosphonium-urée.
La photodécomposition et l'hydrolyse des sels de THP constituent
des voies de dégradation abiotiques importantes dans l'environnement.
Le sulfate de THP ne se fixe guère sur les matières particulaires et
il est donc mobile. Le THPS se décompose rapidement en aérobiose comme
en anaérobiose. On a constaté la présence d'oxyde de
trihydroxyméthylphosphine (THPO) et d'acide
bishydroxyméthylphosphonique ou BMPA dans les produits de
décomposition.
Comme il ne semble pas y avoir de surveillance de ces composés,
on ne peut pas évaluer l'exposition de l'Homme ni celle des autres
êtres vivants dans leur milieu naturel.
Le THPC et le THPS présentent une toxicité aiguë modérée par voie
orale ; au niveau cutané, leur toxicité est faible.
Des études à court terme (jusqu'à 28 jours) effectuées sur des
rats et des souris ont montré que le principal effet toxique du THPC
et du THPS était une réduction du poids corporel. Chez les deux
espèces, la NOAEL est d'environ 8 mg.kg-1.j-1. Des études plus
longues (13 semaines) montrent que le principal organe cible est le
foie. La NOAEL relative à cet effet varie de 3 à 7 mg.kg-1.j-1 pour
les deux sels chez les deux espèces. Les tests biologiques de
cancérogénicité effectués sur le THPC ont également montré que ces
effets se produisaient au niveau du foie, mais on n'a pas établi de
NOAEL. Chez les deux espèces, la LOAEL était d'environ 3 mg.kg-1.j-1.
Lors d'une étude de cancérogénicité portant sur le THPS et effectuée
sur des souris, on a évalué à 3,6 mg.kg-1.j-1 la NOAEL pour une
hyperplasie médullo-surrénalienne focale; chez les rats, la LOAEL pour
la mortalité avait la même valeur.
Administré en dose unique à des lapins, le THPS n'a pas provoqué
d'irritation cutanée. Cependant, l'exposition répétée de rats à ce
composé par la voie cutanée a entraîné une sérieuse réaction à ce
niveau. Le condensat THPC-urée s'est révélé corrosif. Chez le lapin,
le THPS irrite fortement la muqueuse oculaire.
 Le THPS et le condensat THPC-urée provoquent une sensibilisation
cutanée chez le cobaye (test de sensibilisation maximale de Magnusson
& Kilman).
Ni le THPS ni le condensat THPC-urée n'ont eu d'effets toxiques
sur le développement lorsqu'ils étaient administrés par voie orale à
des animaux de laboratoire.
Le THPC et le THPS manifestent une activité mutagène in vitro,
mais celle-ci disparaît in vivo dans le cas du THPS (on ne dispose
pas de données sur l'activité mutagène du THPC in vivo). Les
résultats limités dont on dispose au sujet du condensat THPC-urée
incitent à penser qu'il n'est pas mutagène in vivo. Le THPO n'est
pas génotoxique. Rien n'indique de façon probante que les tissus
traités par des sels de THP puissent avoir des effets mutagènes. Les
données disponibles montrent qu'il n'y a pas de risque de génotoxicité
pour l'Homme.
Le THPS et le THPC ne sont pas révélés cancérogènes chez le rat
ou la souris lors d'études biologiques d'une durée de deux ans. Les
tests cutanés montrent que les sels de THP agissent comme promoteurs
dans le processus de cancérisation, mais pas comme initiateurs.
Le THPS et le THPO n'inhibent pas l'activité cholinesterasique in
vitro, ce qui indique qu'ils ne sont pas neurotoxiques pour l'Homme.
Les tissus traités par le condensat THPC-urée ne provoquent pas
d'irritation cutanée chez l'Homme.
Dans le cas du THPS, la valeur de la concentration entraînant des
effets toxiques aigus pour les algues est de l'ordre de 1 mg/litre,
avec une concentration sans effet observable (NOEC) de 0,06 mg/litre.
En ce qui concerne la daphnie, la valeur de la NOEC relative aux
effets aigus est de 10 mg/litre. Chez les invertébrés marins, les
valeurs correspondant à des effets toxiques aigus oscillent entre 1,6
et 340 mg/litre.
Chez les poissons, la valeur de la CL50 à 96 h va de 72 à 119
mg/litre, avec une NOEC comprise entre 18 et 41 mg/litre. Pour les
oiseaux, on donne pour la DL50 une valeur de 311 mg/litre (effets
aigus). Dans le cas de la toxicité par ingestion, on a retenu, pour la
CL50, une valeur comprise entre 1300 et 2400 mg/kg de nourriture.
3.2 Evaluation
On ne dispose d'aucune information sur l'exposition de l'Homme ou
des autres êtres vivants dans leur milieu naturel. Dans ces
conditions, il n'est pas possible de donner une estimation
quantitative du risque.
RESUMEN, EVALUACION Y RECOMENDACIONES
1. Tris(2-butoxietil)fosfato (TBEP)
1.1 Resumen
El tris(2-butoxietil)fosfato (TBEP) se utiliza en ceras para el
suelo y como plastificante del caucho y del plástico. No se dispone de
datos sobre el volumen de producción mundial, pero se calcula que es
del orden de 5000 a 6000 toneladas.
El TBEP se encuentra en el medio ambiente sólo como consecuencia
de la actividad humana. Se ha investigado en determinados países
industrializados su distribución en la naturaleza. Se comprobó que la
concentración en las aguas superficiales era inferior a 300 ng/litro,
mientras que en los sedimentos oscilaba entre 100 y 1000 ìg/kg. No se
detectó TBEP en ninguno de los 167 análisis realizados en peces. Se ha
detectado en un estudio único en el aire exterior (<200 ng/m3). La
medición del TBEP en el aire de espacios cerrados de oficina puso de
manifiesto concentraciones de 25 ng/m3 o inferiores. El TBEP se
asocia a partículas y se considera que la fuente es la aplicación de
cera al suelo. Se ha detectado a niveles del orden de ìg/kg en el
tejido adiposo humano. La ingesta diaria con los alimentos notificada
a partir de estudios de la cesta de la compra, para una gama de grupos
de edad, fue <0,02 ìg/kg de peso corporal al día. Se han notificado
concentraciones en el agua de bebida de hasta 270 ìg/litro,
estimándose que procede de la migración desde las juntas de caucho de
las tuberías.
Se considera que el TBEP es fácilmente biodegradable. Las
mediciones en depuradoras de aguas residuales y las pruebas
semicontinuas de laboratorio de los lodos cloacales han indicado una
eliminación sustancial de TBEP (>80%). En aguas fluviales y costeras,
el TBEP se degradó completamente. Se notificó que la semivida en el
agua de los estuarios era de unos 50 días y que había poca degradación
en el agua marina no adaptada.
La toxicidad aguda sistémica en mamíferos y el potencial de
irritación son bajos.
En varios estudios subcrónicos en animales de laboratorio se ha
comprobado que el hígado es el órgano destinatario de la toxicidad del
TBEP. Los resultados de un estudio en ratas Sprague-Dawley macho
parecen indicar que el TBEP podría causar miocarditis focal. Los
efectos neurotóxicos en ratas tras dosis únicas de TBEP no son
uniformes. La administración repetida de dosis elevadas de TBEP a
ratas mediante sonda produjo una disminución de la velocidad de
conducción nerviosa y un aumento del período de refracción. No produjo
neurotoxicidad retardada en gallinas, pero inhibió las colinesterasas
del cerebro y del plasma.
Tomando como base un estudio de dosis repetidas de 18 semanas en
ratas, se notificó una concentración sin efectos observados (NOEL) en
el hígado de 15 mg/kg de peso corporal al día, mientras que la
concentración más baja con efectos observados (LOEL), fue de 150 mg/kg
de peso corporal al día.
No se han estudiado la toxicidad y la carcinogenicidad del TBEP a
largo plazo.
Las pruebas de mutación genética en bacterias y células de
mamíferos dieron resultados negativos, pero no se han notificado
pruebas sobre los daños cromosómicos.
En un estudio realizado en ratas no se observó teratogenicidad.
No se han notificado otros aspectos de toxicidad reproductiva.
En una prueba epicutánea (con parche) repetida para estudiar los
efectos del TBEP en la piel humana se vio que no se producía
sensibilización y que la irritación era mínima.
La toxicidad del TBEP para los organismos acuáticos es moderada.
La CL50 a las 48 horas en Daphnia magna es de 75 mg/litro y los
valores de la CL50 a las 96 horas en peces oscilan entre 16 y
24 mg/litro.
1.2 Evaluación
Es probable que se produzca exposición ocupacional al TBEP por
vía cutánea durante la fabricación (exposición occidental) y a partir
de las ceras del suelo. El compuesto se absorbe por vía cutánea en
animales de experimentación, pero no se dispone de información sobre
su cinética y metabolismo. Por consiguiente, no se puede cuantificar
la exposición cutánea, pero es previsible que sea baja. La exposición
por inhalación medida en el entorno de oficina ha sido de 25 ng/m3 o
inferior.
La exposición de la población general se produce fundamentalmente
a través de los alimentos (debido al uso de TBEP como plastificante en
los plásticos de envasado) y del agua de bebida (contaminada por
lixiviación del caucho sintético utilizado en las arandelas de las
cañerías). La exposición a partir de ambas fuentes es muy baja
(estimada en <0,2 ìg/kg de peso corporal al día a partir de los
alimentos y concentraciones en el agua de bebida de <270 ìg/litro).
Teniendo en cuenta la NOEL notificada a partir de estudios en
animales de 15 mg/kg de peso corporal al día obtenida en un estudio de
administración oral con dosis repetidas, el riesgo para la población
general es muy bajo. El riesgo para las personas expuestas en el
trabajo se considera también muy bajo, aunque no se puede cuantificar.
En el medio ambiente, se supone que el TBEP (dada su baja
volatilidad, su elevado coeficiente de adsorción y su solubilidad
moderada en agua) se reparte en los sedimentos. Los escasos datos
medidos así lo confirman. La degradación en los compartimentos del
medio ambiente se supone que es rápida. No se dispone de información
sobre los productos de su degradación; no parece que el fosfato
liberado durante la degradación contribuya de manera significativa a
la concentración de nutrientes del medio ambiente. La Fig. 1 es una
representación gráfica de las concentraciones en el medio ambiente
medidas en aguas superficiales frente a los valores notificados de
toxicidad aguda. El margen de inocuidad entre las concentraciones más
altas y los valores de toxicidad más bajos notificados es de varios
órdenes de magnitud, lo que indica un riesgo bajo para los organismos
del medio ambiente acuático. No se puede hacer una evaluación del
riesgo para el compartimento terrestre.
1.3 Recomendaciones
Para una evaluación científica completa del compuesto, sería
necesaria la identificación y evaluación de los metabolitos en los
mamíferos, dado el perfil toxicológico de uno de los metabolitos
propuestos, el 2-butoxietanol.
2. Tris(2-etilhexil)fosfato (TEHP)
El tris(2-etilhexil)fosfato (TEHP) es un líquido incoloro no
inflamable, poco soluble en agua y de presión de vapor muy baja, que
se utiliza como pirorretardante y plastificante para el PVC y el
acetato de celulosa, y como disolvente. Se produce a partir del
oxicloruro de fósforo y el 2-etilhexanol. No se dispone de cifras para
la producción mundial actual. En Alemania se producen actualmente unas
1000 toneladas.
Le THPS et le condensat THPC-urée provoquent une sensibilisation
cutanée chez le cobaye (test de sensibilisation maximale de Magnusson
& Kilman).
Ni le THPS ni le condensat THPC-urée n'ont eu d'effets toxiques
sur le développement lorsqu'ils étaient administrés par voie orale à
des animaux de laboratoire.
Le THPC et le THPS manifestent une activité mutagène in vitro,
mais celle-ci disparaît in vivo dans le cas du THPS (on ne dispose
pas de données sur l'activité mutagène du THPC in vivo). Les
résultats limités dont on dispose au sujet du condensat THPC-urée
incitent à penser qu'il n'est pas mutagène in vivo. Le THPO n'est
pas génotoxique. Rien n'indique de façon probante que les tissus
traités par des sels de THP puissent avoir des effets mutagènes. Les
données disponibles montrent qu'il n'y a pas de risque de génotoxicité
pour l'Homme.
Le THPS et le THPC ne sont pas révélés cancérogènes chez le rat
ou la souris lors d'études biologiques d'une durée de deux ans. Les
tests cutanés montrent que les sels de THP agissent comme promoteurs
dans le processus de cancérisation, mais pas comme initiateurs.
Le THPS et le THPO n'inhibent pas l'activité cholinesterasique in
vitro, ce qui indique qu'ils ne sont pas neurotoxiques pour l'Homme.
Les tissus traités par le condensat THPC-urée ne provoquent pas
d'irritation cutanée chez l'Homme.
Dans le cas du THPS, la valeur de la concentration entraînant des
effets toxiques aigus pour les algues est de l'ordre de 1 mg/litre,
avec une concentration sans effet observable (NOEC) de 0,06 mg/litre.
En ce qui concerne la daphnie, la valeur de la NOEC relative aux
effets aigus est de 10 mg/litre. Chez les invertébrés marins, les
valeurs correspondant à des effets toxiques aigus oscillent entre 1,6
et 340 mg/litre.
Chez les poissons, la valeur de la CL50 à 96 h va de 72 à 119
mg/litre, avec une NOEC comprise entre 18 et 41 mg/litre. Pour les
oiseaux, on donne pour la DL50 une valeur de 311 mg/litre (effets
aigus). Dans le cas de la toxicité par ingestion, on a retenu, pour la
CL50, une valeur comprise entre 1300 et 2400 mg/kg de nourriture.
3.2 Evaluation
On ne dispose d'aucune information sur l'exposition de l'Homme ou
des autres êtres vivants dans leur milieu naturel. Dans ces
conditions, il n'est pas possible de donner une estimation
quantitative du risque.
RESUMEN, EVALUACION Y RECOMENDACIONES
1. Tris(2-butoxietil)fosfato (TBEP)
1.1 Resumen
El tris(2-butoxietil)fosfato (TBEP) se utiliza en ceras para el
suelo y como plastificante del caucho y del plástico. No se dispone de
datos sobre el volumen de producción mundial, pero se calcula que es
del orden de 5000 a 6000 toneladas.
El TBEP se encuentra en el medio ambiente sólo como consecuencia
de la actividad humana. Se ha investigado en determinados países
industrializados su distribución en la naturaleza. Se comprobó que la
concentración en las aguas superficiales era inferior a 300 ng/litro,
mientras que en los sedimentos oscilaba entre 100 y 1000 ìg/kg. No se
detectó TBEP en ninguno de los 167 análisis realizados en peces. Se ha
detectado en un estudio único en el aire exterior (<200 ng/m3). La
medición del TBEP en el aire de espacios cerrados de oficina puso de
manifiesto concentraciones de 25 ng/m3 o inferiores. El TBEP se
asocia a partículas y se considera que la fuente es la aplicación de
cera al suelo. Se ha detectado a niveles del orden de ìg/kg en el
tejido adiposo humano. La ingesta diaria con los alimentos notificada
a partir de estudios de la cesta de la compra, para una gama de grupos
de edad, fue <0,02 ìg/kg de peso corporal al día. Se han notificado
concentraciones en el agua de bebida de hasta 270 ìg/litro,
estimándose que procede de la migración desde las juntas de caucho de
las tuberías.
Se considera que el TBEP es fácilmente biodegradable. Las
mediciones en depuradoras de aguas residuales y las pruebas
semicontinuas de laboratorio de los lodos cloacales han indicado una
eliminación sustancial de TBEP (>80%). En aguas fluviales y costeras,
el TBEP se degradó completamente. Se notificó que la semivida en el
agua de los estuarios era de unos 50 días y que había poca degradación
en el agua marina no adaptada.
La toxicidad aguda sistémica en mamíferos y el potencial de
irritación son bajos.
En varios estudios subcrónicos en animales de laboratorio se ha
comprobado que el hígado es el órgano destinatario de la toxicidad del
TBEP. Los resultados de un estudio en ratas Sprague-Dawley macho
parecen indicar que el TBEP podría causar miocarditis focal. Los
efectos neurotóxicos en ratas tras dosis únicas de TBEP no son
uniformes. La administración repetida de dosis elevadas de TBEP a
ratas mediante sonda produjo una disminución de la velocidad de
conducción nerviosa y un aumento del período de refracción. No produjo
neurotoxicidad retardada en gallinas, pero inhibió las colinesterasas
del cerebro y del plasma.
Tomando como base un estudio de dosis repetidas de 18 semanas en
ratas, se notificó una concentración sin efectos observados (NOEL) en
el hígado de 15 mg/kg de peso corporal al día, mientras que la
concentración más baja con efectos observados (LOEL), fue de 150 mg/kg
de peso corporal al día.
No se han estudiado la toxicidad y la carcinogenicidad del TBEP a
largo plazo.
Las pruebas de mutación genética en bacterias y células de
mamíferos dieron resultados negativos, pero no se han notificado
pruebas sobre los daños cromosómicos.
En un estudio realizado en ratas no se observó teratogenicidad.
No se han notificado otros aspectos de toxicidad reproductiva.
En una prueba epicutánea (con parche) repetida para estudiar los
efectos del TBEP en la piel humana se vio que no se producía
sensibilización y que la irritación era mínima.
La toxicidad del TBEP para los organismos acuáticos es moderada.
La CL50 a las 48 horas en Daphnia magna es de 75 mg/litro y los
valores de la CL50 a las 96 horas en peces oscilan entre 16 y
24 mg/litro.
1.2 Evaluación
Es probable que se produzca exposición ocupacional al TBEP por
vía cutánea durante la fabricación (exposición occidental) y a partir
de las ceras del suelo. El compuesto se absorbe por vía cutánea en
animales de experimentación, pero no se dispone de información sobre
su cinética y metabolismo. Por consiguiente, no se puede cuantificar
la exposición cutánea, pero es previsible que sea baja. La exposición
por inhalación medida en el entorno de oficina ha sido de 25 ng/m3 o
inferior.
La exposición de la población general se produce fundamentalmente
a través de los alimentos (debido al uso de TBEP como plastificante en
los plásticos de envasado) y del agua de bebida (contaminada por
lixiviación del caucho sintético utilizado en las arandelas de las
cañerías). La exposición a partir de ambas fuentes es muy baja
(estimada en <0,2 ìg/kg de peso corporal al día a partir de los
alimentos y concentraciones en el agua de bebida de <270 ìg/litro).
Teniendo en cuenta la NOEL notificada a partir de estudios en
animales de 15 mg/kg de peso corporal al día obtenida en un estudio de
administración oral con dosis repetidas, el riesgo para la población
general es muy bajo. El riesgo para las personas expuestas en el
trabajo se considera también muy bajo, aunque no se puede cuantificar.
En el medio ambiente, se supone que el TBEP (dada su baja
volatilidad, su elevado coeficiente de adsorción y su solubilidad
moderada en agua) se reparte en los sedimentos. Los escasos datos
medidos así lo confirman. La degradación en los compartimentos del
medio ambiente se supone que es rápida. No se dispone de información
sobre los productos de su degradación; no parece que el fosfato
liberado durante la degradación contribuya de manera significativa a
la concentración de nutrientes del medio ambiente. La Fig. 1 es una
representación gráfica de las concentraciones en el medio ambiente
medidas en aguas superficiales frente a los valores notificados de
toxicidad aguda. El margen de inocuidad entre las concentraciones más
altas y los valores de toxicidad más bajos notificados es de varios
órdenes de magnitud, lo que indica un riesgo bajo para los organismos
del medio ambiente acuático. No se puede hacer una evaluación del
riesgo para el compartimento terrestre.
1.3 Recomendaciones
Para una evaluación científica completa del compuesto, sería
necesaria la identificación y evaluación de los metabolitos en los
mamíferos, dado el perfil toxicológico de uno de los metabolitos
propuestos, el 2-butoxietanol.
2. Tris(2-etilhexil)fosfato (TEHP)
El tris(2-etilhexil)fosfato (TEHP) es un líquido incoloro no
inflamable, poco soluble en agua y de presión de vapor muy baja, que
se utiliza como pirorretardante y plastificante para el PVC y el
acetato de celulosa, y como disolvente. Se produce a partir del
oxicloruro de fósforo y el 2-etilhexanol. No se dispone de cifras para
la producción mundial actual. En Alemania se producen actualmente unas
1000 toneladas.
 No se ha detectado TEHP en el aire exterior; se ha encontrado en
el aire de espacios cerrados en concentraciones de menos de 10 ng/m3,
en aguas fluviales en concentraciones de hasta 7500 ng/litro y en
sedimentos de 2-70 ng/g. Se detectó TEHP en una sola muestra de agua
de bebida en una concentración de 0,3 ng/litro. La ingesta diaria
notificada en los alimentos a partir de estudios de la cesta de la
compra, para una gama de grupos de edad, fue inferior a 0,05 ìg/kg de
peso corporal al día.
El TEHP se degrada rápidamente en las aguas naturales, pero las
pruebas de laboratorio con lodos activados dieron resultados
equívocos. No hay una degradación abiótica significativa.
El TEHP tiene una toxicidad aguda baja para los mamíferos, siendo
la DL50 por vía oral >10 000 mg/kg de peso corporal en ratas.
El TEHP es irritante cutáneo, pero no ocular. La aplicación
repetida de 0,1 ml (93 mg) de TEHP a la piel de conejos no produjo
signos de intoxicación sistémica.
En estudios de administración con sonda durante 13 semanas a
ratas y ratones no aparecieron efectos tóxicos significativos. La
concentración sin efectos adversos observados (NOAEL) en ratas fue de
2860 mg/kg de peso corporal al día y en ratones de 5710 mg/kg de peso
corporal al día, la dosis más elevada probada en ambas especies.
En un estudio de inhalación de tres meses con concentraciones de
hasta 85 mg de TEHP/m3 se observaron cambios inflamatorios crónicos
leves en los pulmones de perros y los resultados de rechazo
condicionado empeoraron en relación con la concentración administrada.
No se dispuso de estudios sobre toxicidad reproductiva.
El TEHP dio resultados negativos en varias pruebas de
mutagenicidad in vivo e in vitro.
Se realizaron pruebas de toxicidad crónica y carcinogenicidad del
TEHP en ratas y ratones. La NOAEL para la toxicidad crónica en ratas
macho fue de 2857 mg/kg de peso corporal al día y en ratas hembras de
1428 mg/kg de peso corporal al día. La concentración más baja con
efectos adversos observados (LOAEL) en ratones machos y hembras para
la hiperplasia de las células foliculares del tiroides fue de 357
mg/kg de peso corporal al día. No se estableció una NOAEL en ratones.
Los autores llegaron a la conclusión de que había algunas pruebas de
carcinogenicidad basadas en una mayor incidencia de carcinomas
hepatocelulares en ratones hembra con un nivel de dosificación alto y
pruebas equívocas de carcinogenicidad basadas en la mayor incidencia
de feocromocitomas suprarrenales en ratas macho en ambos grupos de
dosis. Aunque se produjo un aumento de feocromocitomas suprarrenales
en ambos grupos de dosis de ratas macho y de carcinomas
hepatocelulares en ratones hembra del grupo de dosificación alta, no
se considera que estos resultados indiquen que el TEHP presenta un
riesgo carcinogénico significativo para el ser humano. Los
feocromocitomas muestran una incidencia de base variable en ratas. La
incidencia de estos tumores en dos biovaloraciones anteriores del
Programa Nacional de Toxicología fue igual a la observada en la
biovaloración del TEHP. Solamente hubo otro resultado neoplásico
significativo, consistente en carcinomas hepatocelulares, en el grupo
de ratones hembra de dosificación alta. Teniendo en cuenta la baja
incidencia de este tumor, su presencia en un solo sexo de una especie,
la falta de pruebas de toxicidad genética y la baja exposición del ser
humano al TEHP, no es probable que este producto cree un riesgo
carcinogénico significativo para el ser humano.
Se han realizado estudios de neurotoxicidad en varias especies.
El TEHP no produce ninguna alteración de la actividad de la
colinesterasa del plasma o los glóbulos rojos. No se han notificado
estudios sobre neurotoxicidad retardada.
En un estudio realizado en voluntarios humanos, no se notificó
irritación cutánea.
Los escasos datos disponibles indican una toxicidad aguda baja
del TEHP en el medio acuático. La CL50 para bacterias es superior a
100 mg/litro y la CL50 a las 96 horas para el pez Brachydanio rerio
es superior a 100 mg/litro, que es el límite de la solubilidad del
TEHP en agua.
2.2 Evaluación
Es probable que se produzca exposición ocupacional al TEHP por
vía cutánea durante la fabricación (exposición occidental) y por el
uso de algunos productos. El compuesto se absorbe por vía cutánea en
los animales de experimentación, pero no se dispone de información
sobre su cinética o metabolismo en esta vía. Por consiguiente, no se
puede cuantificar la exposición cutánea, pero es previsible que sea
baja. La exposición por inhalación medida en el entorno de oficina es
de 10 ng/m3 o menor.
La exposición de la población general se produce fundamentalmente
a través de los alimentos y el agua de bebida. La exposición a partir
de ambas fuentes es muy baja (estimada en <0,05 ìg/kg de peso
corporal día a partir de los alimentos; una concentración única medida
en el agua de bebida fue de 0,3 ng/litro).
Teniendo cuenta la LOAEL notificada para la hiperplasia tiroidea
de 357 mg/kg de peso corporal día en ratones, el riesgo para la
población general es muy bajo. El riesgo para las personas expuestas
en el trabajo se considera también muy bajo, aunque no se puede
cuantificar.
El TEHP no parece ser carcinogénico para el ser humano.
En el medio ambiente, se supone que el TEHP (dada su baja
volatilidad, su elevado coeficiente de adsorción y su solubilidad baja
en agua) se repartirá en los sedimentos. Los datos medidos son
demasiado escasos para confirmarlo. Se prevé su degradación en los
compartimentos del medio ambiente, aunque los datos de laboratorio
sobre la degradación en lodos cloacales son equívocos. No se dispone
de información sobre los productos de la degradación; no parece que el
fosfato liberado durante su degradación contribuya de manera
significativa a la concentración de nutrientes del medio ambiente. La
Fig. 2 es una representación gráfica de las concentraciones en el
medio ambiente medidas en sus compartimentos frente a los valores
notificados de toxicidad aguda (éstos indican que no tiene efectos
tóxicos en el límite de solubilidad en el agua). El margen de
inocuidad entre las concentraciones más altas y los valores de
toxicidad más bajos notificados es de varios órdenes de magnitud, lo
que indica un riesgo bajo para los organismos del medio ambiente
acuático. No se puede hacer una evaluación del riesgo para el
compartimento terrestre.
2.3 Recomendaciones
Para una evaluación científica completa del compuesto, sería
necesaria la identificación y evaluación de los metabolitos en los
mamíferos, dado el perfil toxicológico de uno de los metabolitos
propuestos, el 2-etilhexanol.
Es necesario investigar la toxicidad reproductiva, en particular
los posibles efectos en el desarrollo.
No se ha detectado TEHP en el aire exterior; se ha encontrado en
el aire de espacios cerrados en concentraciones de menos de 10 ng/m3,
en aguas fluviales en concentraciones de hasta 7500 ng/litro y en
sedimentos de 2-70 ng/g. Se detectó TEHP en una sola muestra de agua
de bebida en una concentración de 0,3 ng/litro. La ingesta diaria
notificada en los alimentos a partir de estudios de la cesta de la
compra, para una gama de grupos de edad, fue inferior a 0,05 ìg/kg de
peso corporal al día.
El TEHP se degrada rápidamente en las aguas naturales, pero las
pruebas de laboratorio con lodos activados dieron resultados
equívocos. No hay una degradación abiótica significativa.
El TEHP tiene una toxicidad aguda baja para los mamíferos, siendo
la DL50 por vía oral >10 000 mg/kg de peso corporal en ratas.
El TEHP es irritante cutáneo, pero no ocular. La aplicación
repetida de 0,1 ml (93 mg) de TEHP a la piel de conejos no produjo
signos de intoxicación sistémica.
En estudios de administración con sonda durante 13 semanas a
ratas y ratones no aparecieron efectos tóxicos significativos. La
concentración sin efectos adversos observados (NOAEL) en ratas fue de
2860 mg/kg de peso corporal al día y en ratones de 5710 mg/kg de peso
corporal al día, la dosis más elevada probada en ambas especies.
En un estudio de inhalación de tres meses con concentraciones de
hasta 85 mg de TEHP/m3 se observaron cambios inflamatorios crónicos
leves en los pulmones de perros y los resultados de rechazo
condicionado empeoraron en relación con la concentración administrada.
No se dispuso de estudios sobre toxicidad reproductiva.
El TEHP dio resultados negativos en varias pruebas de
mutagenicidad in vivo e in vitro.
Se realizaron pruebas de toxicidad crónica y carcinogenicidad del
TEHP en ratas y ratones. La NOAEL para la toxicidad crónica en ratas
macho fue de 2857 mg/kg de peso corporal al día y en ratas hembras de
1428 mg/kg de peso corporal al día. La concentración más baja con
efectos adversos observados (LOAEL) en ratones machos y hembras para
la hiperplasia de las células foliculares del tiroides fue de 357
mg/kg de peso corporal al día. No se estableció una NOAEL en ratones.
Los autores llegaron a la conclusión de que había algunas pruebas de
carcinogenicidad basadas en una mayor incidencia de carcinomas
hepatocelulares en ratones hembra con un nivel de dosificación alto y
pruebas equívocas de carcinogenicidad basadas en la mayor incidencia
de feocromocitomas suprarrenales en ratas macho en ambos grupos de
dosis. Aunque se produjo un aumento de feocromocitomas suprarrenales
en ambos grupos de dosis de ratas macho y de carcinomas
hepatocelulares en ratones hembra del grupo de dosificación alta, no
se considera que estos resultados indiquen que el TEHP presenta un
riesgo carcinogénico significativo para el ser humano. Los
feocromocitomas muestran una incidencia de base variable en ratas. La
incidencia de estos tumores en dos biovaloraciones anteriores del
Programa Nacional de Toxicología fue igual a la observada en la
biovaloración del TEHP. Solamente hubo otro resultado neoplásico
significativo, consistente en carcinomas hepatocelulares, en el grupo
de ratones hembra de dosificación alta. Teniendo en cuenta la baja
incidencia de este tumor, su presencia en un solo sexo de una especie,
la falta de pruebas de toxicidad genética y la baja exposición del ser
humano al TEHP, no es probable que este producto cree un riesgo
carcinogénico significativo para el ser humano.
Se han realizado estudios de neurotoxicidad en varias especies.
El TEHP no produce ninguna alteración de la actividad de la
colinesterasa del plasma o los glóbulos rojos. No se han notificado
estudios sobre neurotoxicidad retardada.
En un estudio realizado en voluntarios humanos, no se notificó
irritación cutánea.
Los escasos datos disponibles indican una toxicidad aguda baja
del TEHP en el medio acuático. La CL50 para bacterias es superior a
100 mg/litro y la CL50 a las 96 horas para el pez Brachydanio rerio
es superior a 100 mg/litro, que es el límite de la solubilidad del
TEHP en agua.
2.2 Evaluación
Es probable que se produzca exposición ocupacional al TEHP por
vía cutánea durante la fabricación (exposición occidental) y por el
uso de algunos productos. El compuesto se absorbe por vía cutánea en
los animales de experimentación, pero no se dispone de información
sobre su cinética o metabolismo en esta vía. Por consiguiente, no se
puede cuantificar la exposición cutánea, pero es previsible que sea
baja. La exposición por inhalación medida en el entorno de oficina es
de 10 ng/m3 o menor.
La exposición de la población general se produce fundamentalmente
a través de los alimentos y el agua de bebida. La exposición a partir
de ambas fuentes es muy baja (estimada en <0,05 ìg/kg de peso
corporal día a partir de los alimentos; una concentración única medida
en el agua de bebida fue de 0,3 ng/litro).
Teniendo cuenta la LOAEL notificada para la hiperplasia tiroidea
de 357 mg/kg de peso corporal día en ratones, el riesgo para la
población general es muy bajo. El riesgo para las personas expuestas
en el trabajo se considera también muy bajo, aunque no se puede
cuantificar.
El TEHP no parece ser carcinogénico para el ser humano.
En el medio ambiente, se supone que el TEHP (dada su baja
volatilidad, su elevado coeficiente de adsorción y su solubilidad baja
en agua) se repartirá en los sedimentos. Los datos medidos son
demasiado escasos para confirmarlo. Se prevé su degradación en los
compartimentos del medio ambiente, aunque los datos de laboratorio
sobre la degradación en lodos cloacales son equívocos. No se dispone
de información sobre los productos de la degradación; no parece que el
fosfato liberado durante su degradación contribuya de manera
significativa a la concentración de nutrientes del medio ambiente. La
Fig. 2 es una representación gráfica de las concentraciones en el
medio ambiente medidas en sus compartimentos frente a los valores
notificados de toxicidad aguda (éstos indican que no tiene efectos
tóxicos en el límite de solubilidad en el agua). El margen de
inocuidad entre las concentraciones más altas y los valores de
toxicidad más bajos notificados es de varios órdenes de magnitud, lo
que indica un riesgo bajo para los organismos del medio ambiente
acuático. No se puede hacer una evaluación del riesgo para el
compartimento terrestre.
2.3 Recomendaciones
Para una evaluación científica completa del compuesto, sería
necesaria la identificación y evaluación de los metabolitos en los
mamíferos, dado el perfil toxicológico de uno de los metabolitos
propuestos, el 2-etilhexanol.
Es necesario investigar la toxicidad reproductiva, en particular
los posibles efectos en el desarrollo.
 3. Sales de tetrakis(hidroximetil)fosfonio
3.1 Resumen
Las sales de tetrakis(hidroximetil)fosfonio representan la clase
principal de productos químicos utilizados como pirorretardantes en el
algodón, la celulosa y los tejidos con celulosa. Hay una migración
baja a partir de los tejidos tratados con cloruro de
tetrakis(hidroximetil)fosfonio (THPC)-urea. La sal sulfatada (THPS) se
utiliza fundamentalmente como biocida. La producción mundial combinada
se estima que es >3000 toneladas para las sales de THP y de unas 3000
toneladas para el condensado de THPC-urea.
La fotodegradación y la hidrólisis de las sales de THP son vías
importantes de degradación abiótica en el medio ambiente. El THPS se
une débilmente a las partículas del medio ambiente, por lo que es
móvil. Se degrada rápidamente tanto en condiciones aerobias como
anaerobias. Se han identificado como productos de su degradación el
óxido de trihidroximetilfosfuro (THPO) y el ácido
bihidroximetilfosfónico (BMPA).
Puesto que no se ha notificado ninguna vigilancia, no se pueden
hacer estimaciones de la exposición del ser humano o de los organismos
en el medio ambiente.
La toxicidad aguda por vía oral del THPC y el THPS es moderada;
la toxicidad cutánea es baja.
En estudios breves (hasta 28 días) en ratas y ratones, el
principal efecto tóxico tanto del THPC como del THPS es la disminución
del peso corporal. La NOAEL para ambos productos químicos en las dos
especies es de unos 8 mg/kg de peso corporal al día. En estudios más
prolongados (13 semanas), el principal órgano destinatario de la
toxicidad es el hígado. La NOAEL para este efecto osciló entre 3 y 7
mg/kg de peso corporal al día para ambas sales en las dos especies.
Las biovaloraciones de la carcinogenicidad del THPC también pusieron
de manifiesto efectos en el hígado, pero no se estableció una NOAEL.
La LOAEL fue de unos 3 mg/kg de peso corporal al día para ambas
especies. En una biovaloración de la carcinogenicidad del THPS en
ratones, la NOAEL para la hiperplasia focal en la médula suprarrenal
fue de 3,6 mg/kg de peso corporal al día; en ratas, la LOAEL para la
mortalidad fue de 3,6 mg/kg de peso corporal al día.
El THPS administrado en dosis única a conejos no provocó
irritación cutánea. Sin embargo, la exposición cutánea repetida en
ratas produjo una reacción grave en la piel. El THPC-urea fue
corrosivo. Se comprobó que el THPS ocasionaba una irritación grave de
los ojos en conejos.
El THPS y el THPC-urea producen sensibilización cutánea en
cobayas (prueba de maximización de Magnusson y Kilman).
En animales de experimentación tratados por vía oral, el THPS y
el THPC-urea no produjeron toxicidad en el desarrollo.
El THPC y el THPS tienen potencial mutagénico in vitro, pero el
THPS no es mutagénico in vivo (no hay datos de mutagenicidad in
vivo para el THPC). Los limitados datos de mutagenicidad para el
THPC-urea parecen indicar que no es mutagénico in vivo. El THPO no
es genotóxico. No hay pruebas convincentes que indiquen que las telas
tratadas con sales de THP sean mutagénicas. La información disponible
pone de manifiesto que no hay peligro genotóxico para el ser humano.
El THPC y el THPS no fueron carcinogénicos en ratas y ratones en
biovaloraciones de dos años. En estudios cutáneos se ha observado que
las sales son promotoras de cáncer cutáneo, pero no iniciadoras.
El THPS y el THPO no inhibieron la actividad de la
acetilcolinesterasa in vivo, lo que parece indicar una ausencia de
peligro neurotóxico para el ser humano.
Las telas tratadas con THPC-urea no produjeron irritación cutánea
en el ser humano.
Para el THPS, los valores de toxicidad aguda notificados en algas
son inferiores a 1 mg/litro, con una concentración sin efectos
observados (NOEC) de 0,06 mg/litro. La NOEC para la pulga de agua es
de 10 mg/litro. Los valores de toxicidad aguda notificados para los
invertebrados marinos oscilan entre 1,6 y 340 mg/litro.
Los valores de la DL50 a las 96 horas para los peces van de 72 a
119 mg/litro, con valores de la NOEC entre 18 y 41 mg/litro. Se ha
notificado una DL50 aguda para las aves de 311 mg/kg de peso corporal
y valores de la CL50 de 1 300 y 2 400 mg/kg de alimentos.
3.2 Evaluación
No se dispone de información sobre la exposición para el ser
humano ni para los organismos del medio ambiente. Por consiguiente, no
se pudo realizar una evaluación cuantitativa del riesgo.
3. Sales de tetrakis(hidroximetil)fosfonio
3.1 Resumen
Las sales de tetrakis(hidroximetil)fosfonio representan la clase
principal de productos químicos utilizados como pirorretardantes en el
algodón, la celulosa y los tejidos con celulosa. Hay una migración
baja a partir de los tejidos tratados con cloruro de
tetrakis(hidroximetil)fosfonio (THPC)-urea. La sal sulfatada (THPS) se
utiliza fundamentalmente como biocida. La producción mundial combinada
se estima que es >3000 toneladas para las sales de THP y de unas 3000
toneladas para el condensado de THPC-urea.
La fotodegradación y la hidrólisis de las sales de THP son vías
importantes de degradación abiótica en el medio ambiente. El THPS se
une débilmente a las partículas del medio ambiente, por lo que es
móvil. Se degrada rápidamente tanto en condiciones aerobias como
anaerobias. Se han identificado como productos de su degradación el
óxido de trihidroximetilfosfuro (THPO) y el ácido
bihidroximetilfosfónico (BMPA).
Puesto que no se ha notificado ninguna vigilancia, no se pueden
hacer estimaciones de la exposición del ser humano o de los organismos
en el medio ambiente.
La toxicidad aguda por vía oral del THPC y el THPS es moderada;
la toxicidad cutánea es baja.
En estudios breves (hasta 28 días) en ratas y ratones, el
principal efecto tóxico tanto del THPC como del THPS es la disminución
del peso corporal. La NOAEL para ambos productos químicos en las dos
especies es de unos 8 mg/kg de peso corporal al día. En estudios más
prolongados (13 semanas), el principal órgano destinatario de la
toxicidad es el hígado. La NOAEL para este efecto osciló entre 3 y 7
mg/kg de peso corporal al día para ambas sales en las dos especies.
Las biovaloraciones de la carcinogenicidad del THPC también pusieron
de manifiesto efectos en el hígado, pero no se estableció una NOAEL.
La LOAEL fue de unos 3 mg/kg de peso corporal al día para ambas
especies. En una biovaloración de la carcinogenicidad del THPS en
ratones, la NOAEL para la hiperplasia focal en la médula suprarrenal
fue de 3,6 mg/kg de peso corporal al día; en ratas, la LOAEL para la
mortalidad fue de 3,6 mg/kg de peso corporal al día.
El THPS administrado en dosis única a conejos no provocó
irritación cutánea. Sin embargo, la exposición cutánea repetida en
ratas produjo una reacción grave en la piel. El THPC-urea fue
corrosivo. Se comprobó que el THPS ocasionaba una irritación grave de
los ojos en conejos.
El THPS y el THPC-urea producen sensibilización cutánea en
cobayas (prueba de maximización de Magnusson y Kilman).
En animales de experimentación tratados por vía oral, el THPS y
el THPC-urea no produjeron toxicidad en el desarrollo.
El THPC y el THPS tienen potencial mutagénico in vitro, pero el
THPS no es mutagénico in vivo (no hay datos de mutagenicidad in
vivo para el THPC). Los limitados datos de mutagenicidad para el
THPC-urea parecen indicar que no es mutagénico in vivo. El THPO no
es genotóxico. No hay pruebas convincentes que indiquen que las telas
tratadas con sales de THP sean mutagénicas. La información disponible
pone de manifiesto que no hay peligro genotóxico para el ser humano.
El THPC y el THPS no fueron carcinogénicos en ratas y ratones en
biovaloraciones de dos años. En estudios cutáneos se ha observado que
las sales son promotoras de cáncer cutáneo, pero no iniciadoras.
El THPS y el THPO no inhibieron la actividad de la
acetilcolinesterasa in vivo, lo que parece indicar una ausencia de
peligro neurotóxico para el ser humano.
Las telas tratadas con THPC-urea no produjeron irritación cutánea
en el ser humano.
Para el THPS, los valores de toxicidad aguda notificados en algas
son inferiores a 1 mg/litro, con una concentración sin efectos
observados (NOEC) de 0,06 mg/litro. La NOEC para la pulga de agua es
de 10 mg/litro. Los valores de toxicidad aguda notificados para los
invertebrados marinos oscilan entre 1,6 y 340 mg/litro.
Los valores de la DL50 a las 96 horas para los peces van de 72 a
119 mg/litro, con valores de la NOEC entre 18 y 41 mg/litro. Se ha
notificado una DL50 aguda para las aves de 311 mg/kg de peso corporal
y valores de la CL50 de 1 300 y 2 400 mg/kg de alimentos.
3.2 Evaluación
No se dispone de información sobre la exposición para el ser
humano ni para los organismos del medio ambiente. Por consiguiente, no
se pudo realizar una evaluación cuantitativa del riesgo.
