
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
ENVIRONMENTAL HEALTH CRITERIA 159
GLYPHOSATE
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization.
First draft prepared by Dr H. Mensink and
Dr. P. Janssen, National Institute of Public
Health and Environmental Hygiene,
Bilthoven, The Netherlands
Published under the joint sponsorship of
the United Nations Environment Programme,
the International Labour Organisation,
and the World Health Organization
World Health Orgnization
Geneva, 1994
The International Programme on Chemical Safety (IPCS) is a
joint venture of the United Nations Environment Programme, the
International Labour Organisation, and the World Health
Organization. The main objective of the IPCS is to carry out and
disseminate evaluations of the effects of chemicals on human health
and the quality of the environment. Supporting activities include
the development of epidemiological, experimental laboratory, and
risk-assessment methods that could produce internationally
comparable results, and the development of manpower in the field of
toxicology. Other activities carried out by the IPCS include the
development of know-how for coping with chemical accidents,
coordination of laboratory testing and epidemiological studies, and
promotion of research on the mechanisms of the biological action of
chemicals.
WHO Library Cataloguing in Publication Data
Glyphosate.
(Environmental health criteria ; 159)
1.Glycine - analogs and derivatives 2.Herbicides
3.Environmental exposure I.Series
ISBN 92 4 157159 4 (NLM Classification: WA 240)
ISSN 0250-863X
The World Health Organization welcomes requests for permission
to reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made
to the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 1994
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved.
The designations employed and the presentation of the material
in this publication do not imply the expression of any opinion
whatsoever on the part of the Secretariat of the World Health
Organization concerning the legal status of any country, territory,
city or area or of its authorities, or concerning the delimitation
of its frontiers or boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar
nature that are not mentioned. Errors and omissions excepted, the
names of proprietary products are distinguished by initial capital
letters.
CONTENTS
ENVIRONMENTAL HEALTH CRITERIA FOR GLYPHOSATE
1. SUMMARY
1.1. Identity, physical and chemical properties,
and analytical methods
1.2. Sources of human and environmental exposure
1.3. Environmental transport, distribution and
transformation
1.4. Environmental levels and human exposure
1.5. Kinetics and metabolism in laboratory animals
and humans
1.6. Effects on laboratory mammals, and in vitro
test systems
1.7. Effects on humans
1.8. Effects on other organisms in the laboratory
and field
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, AND ANALYTICAL
METHODS
2.1. Identity
2.2. Physical and chemical properties
2.3. Formulations
2.4. Conversion factors
2.5. Analytical methods
2.5.1. Sample handling and preparation
2.5.2. Analytical methods
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1. Anthropogenic sources
3.1.1. Production levels and processes
3.1.2. Uses
3.1.3. Drinking-water
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION AND TRANSFORMATION
4.1. Transport and distribution between media
4.1.1. Water
4.1.2. Soil sorption
4.1.3. Mobility in soils
4.1.4. Dissipation from the soil in the field
4.1.5. Uptake and dissipation from plants
4.1.6. Ingestion by animals
4.2. Abiotic degradation
4.2.1. Hydrolytic cleavage
4.2.2. Photodegradation
4.3. Biodegradation
4.4. Bioaccumulation
4.5. Waste disposal
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1. Environmental levels
5.2. General population exposure
5.3. Occupational exposure during manufacture,
formulation, or use
6. KINETICS AND METABOLISM IN LABORATORY ANIMALS AND HUMANS
6.1. Absorption
6.2. Distribution
6.3. Metabolic transformation
6.4. Elimination and excretion
6.5. Retention and turnover
7. EFFECTS ON LABORATORY ANIMALS AND IN VITRO TEST SYSTEMS
7.1. Single exposure
7.2. Short-term exposure
7.2.1. Oral studies
7.2.2. Dermal studies
7.2.3. Inhalational studies
7.3. Long-term toxicity and carcinogenicity
7.4. Skin and eye irritation; sensitization
7.5. Reproductive toxicity, embryotoxicity and
teratogenicity
7.5.1. Teratogenicity studies
7.5.2. Reproduction studies
7.6. Mutagenicity and related end-points
8. EFFECTS ON HUMANS
8.1. Cases of intentional and accidental exposure
8.2. Occupational exposure
8.3. Subpopulations at special risk
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
9.1. Laboratory experiments
9.1.1. Microorganisms
9.1.1.1 Water
9.1.1.2 Soil
9.1.2. Aquatic organisms
9.1.2.1 Plants
9.1.2.2 Invertebrates
9.1.2.3 Vertebrates
9.1.3. Terrestrial organisms
9.1.3.1 Plants
9.1.3.2 Invertebrates
9.1.3.3 Vertebrates
9.2. Field observations
9.2.1. Microorganisms
9.2.1.1 Water
9.2.1.2 Soil
9.2.2. Aquatic organisms
9.2.2.1 Plants
9.2.2.2 Invertebrates
9.2.2.3 Vertebrates
9.2.3. Terrestrial organisms
9.2.3.1 Plants
9.2.3.2 Invertebrates
9.2.3.3 Vertebrates
10. EVALUATION OF HUMAN HEALTH HAZARDS AND EFFECTS ON THE
ENVIRONMENT
10.1. Human health hazards
10.2. Evaluation of effects on the environment
10.2.1. Exposure levels and toxic effects
10.2.2. Hazard evaluation for aquatic organisms
10.2.3. Hazard evaluation for terrestrial organisms
11. RECOMMENDATIONS FOR PROTECTION OF HUMAN HEALTH
12. FURTHER RESEARCH
REFERENCES
RESUME
RESUMEN
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR GLYPHOSATE
Members
Dr S. Dobson, Institute of Terrestrial Ecology, Monks Wood
Experimental Station, Huntingdon, United Kingdom
(Chairman)
Dr A.H. El-Sebae, College of Agriculture, Alexandria University,
El Shatby, Alexandria, Egypt
Dr P. Janssen, National Institute of Public Health and
Environmental Hygiene, Bilthoven, The Netherlands
Dr H. Mensink, National Institute of Public Health and
Environmental Hygiene, Bilthoven, The Netherlands
Dr M.S. Morrow, Health Effects Division, Office of Pesticide
Programs, US Environmental Protection Agency, Washington, DC,
USA
Professor R. Nilsson, Department of Scientific Documentation and
Research, National Chemicals Inspectorate, Solna, Swedena
Dr R. Ye, National Environmental Protection Agency, Beijing,
People's Republic of China
Observers
Dr C. Hastings, Agricultural Group, Monsanto, Missouri, St.
Louis, USA
Secretariat
Dr M. Gilbert, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland (Secretary)
___________
a Invited but unable to attend.
NOTE TO READERS OF THE CRITERIA MONOGRAPHS
Every effort has been made to present information in the
criteria monographs as accurately as possible without unduly
delaying their publication. In the interest of all users of the
Environmental Health Criteria monographs, readers are kindly
requested to communicate any errors that may have occurred to the
Director of the International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland, in order that they may be
included in corrigenda.
* * *
A detailed data profile and a legal file can be obtained from
the International Register of Potentially Toxic Chemicals, Case
postale 356, 1219 Châtelaine, Geneva, Switzerland (Telephone No.
9799111).
* * *
This publication was made possible by grant number 5 U01
ES02617-14 from the National Institute of Environmental Health
Sciences, National Institutes of Health, USA.
ENVIRONMENTAL HEALTH CRITERIA FOR GLYPHOSATE
A Task Group on Environmental Health Criteria for Glyphosate
met at the Institute of Terrestrial Ecology, Monks Wood, United
Kingdom, from 23 to 27 August 1993. Dr S. Dobson welcomed the
participants on behalf of the host institution, and Dr M. Gilbert
opened the Meeting on behalf of the three cooperating organizations
of the IPCS (UNEP/ILO/WHO). The Task Group reviewed and revised the
draft monograph and made an evaluation of the risks for human health
and the environment from exposure to glyphosate.
The first draft of this monograph was prepared by Dr H. Mensink
and Dr P. Janssen, National Institute of Public Health and
Environmental Hygiene, Bilthoven, The Netherlands.
Dr M. Gilbert was responsible for the overall scientific
content of the monograph and for the organization of the meeting,
and Dr P.G. Jenkins, IPCS, for the technical editing of the
monograph.
The efforts of all who helped in the preparation and
finalization of the monograph are gratefully acknowledged.
ABBREVIATIONS
a.i. active ingredient
ALAT alanine aminotransferase
AMPA aminomethylphosphonic acid
AP alkaline phosphatase
CHO Chinese hamster ovary
CNS central nervous system
HPLC high-performance liquid chromatography
i.p. intraperitoneal
IPA isopropylamine
MATC maximum acceptable toxicant concentration
NOAEL no-observed-adverse-effect level
NOEC no-observed-effect concentration
1. SUMMARY
1.1 Identity, physical and chemical properties, and analytical
methods
Glyphosate is a weak organic acid consisting of a glycine and a
phosphonomethyl moiety. The empirical formula is C3H8NO5P.
Glyphosate is usually formulated as a salt of the deprotonated acid
of glyphosate and a cation, e.g., isopropylamine or
trimethylsulfonium. The purity of technical grade glyphosate is
generally above 90%. Technical grade glyphosate is an odourless
white crystalline powder with a specific gravity of 1.704, a very
low vapour pressure, and a high solubility in water. The
octanol-water partition coefficient (log Kow) is -2.8. Glyphosate
is amphoteric and may exist as different ionic species, dependent on
the actual pH.
Determination of glyphosate is in general laborious, complex,
and costly. Derivatization with fluorogenic substances is the most
common method and may be applied pre- or post-column. Determination
is usually carried out with high performance liquid chromatography
or gas liquid chromatography. Limits of determination for glyphosate
in water, plants, soil and human urine, are 0.02-3.2 µg/litre,
0.01-0.3 mg/kg, 0.05-1 mg/kg and 0.1 mg/litre, respectively.
1.2 Sources of human and environmental exposure
Glyphosate is a post-emergent, systemic and non-selective
herbicide that is used in both agricultural and non-agricultural
areas all over the world. Glyphosate is applied to many crops and in
various commercial formulations. The major formulation is Roundup in
which glyphosate is formulated as the isopropylamine salt.
Recommended application rates do not exceed 5.8 kg a.i./ha and are
dependent on the type of use. Environmental exposure may occur
because of deposition due to drift and accidental releases.
1.3 Environmental transport, distribution and transformation
The most important processes of dissipation that may be
involved after application of glyphosate are complexation in water
with ions, e.g., Ca2+ and Mg2+, sorption to sediment, suspended
particles in water, and soil, photodegradation in water, uptake by
plants, and biodegradation.
Glyphosate dissipates from the water with DT50 values
(dissipation) ranging from a few days to more than 91 days. Sediment
or suspended particles are shown to be the major sink.
The adsorption coefficients (Ks/l) of glyphosate in
laboratory experiments vary between 8 and 377 dm3/kg for various
soils and clay minerals. No data on the sorption of
aminomethylphosphonic acid (AMPA), the major metabolite, under
laboratory conditions are available.
Rf values of glyphosate do not exceed 0.2 in soil thin-layer
chromatography experiments. Between less than 0.1% and 11% of the
applied activity is recovered in the eluate of soil columns under
leaching conditions simulating an extremely high rainfall. From
field experiments it appears that AMPA is not likely to leach.
Glyphosate dissipates in field experiments from the soil with
DT50 values between 3 and 174 days, mainly depending on edaphic
and climatic conditions. Up to 1.8% of the applied dose dissipated
from the soil due to run-off in some field experiments.
Under laboratory conditions, up to 45% of the applied activity
may be absorbed by treated leaves, and this is followed by a
substantial translocation.
Hydrolysis of glyphosate in sterile buffers is very slow with
DT50 values >> 35 days. Photodegradation in water under natural
conditions occurs with DT50 values < 28 days. No substantial
photodegradation in soil was recorded in a study lasting 31 days.
The time needed for 50% biodegradation of glyphosate in the
whole system of a test with water and sediment is > 14 days under
aerobic conditions and 14-22 days under anaerobic conditions in the
laboratory. The time needed for 50% biodegradation of glyphosate in
the soil is 2-3 days under aerobic conditions.
The major metabolite in soil and water is AMPA. Maximum amounts
of AMPA in soils are approximately 20% of the applied activity under
aerobic conditions and 0.5% under anaerobic conditions. Maximum
amounts of AMPA in sediments are 25% under both aerobic and
anaerobic conditions.
Bioconcentration factors are low in laboratory tests with
invertebrates and fish. Bluegill sunfish in a flow-through test
showed a depuration half-life of 35 days, after being exposed for 35
days. AMPA is recovered in bluegill sunfish up to 21 days after
continuous exposure to glyphosate. Glyphosate has not been detected
in fish living in directly sprayed water in field experiments. In
one experiment, AMPA was detectable in carp up to 90 days after
application. No biomagnification of glyphosate in litter by
herbivorous and omnivorous small mammals in a forest brush ecosystem
was indicated in a field experiment. Concentrations of up to 5 mg
a.i./kg were measured in deermice immediately after spraying in this
experiment.
A range of bacterial strains can degrade glyphosate. Bacteria
capable of using the compound as sole phosphorus, sole carbon or
sole nitrogen source have been identified. Growth is slow compared
to growth on inorganic sources of P, C and N. There is evidence from
the field that bacterial populations adapted to metabolise
glyphosate. The presence of inorganic phosphate inhibits degradation
of glyphosate with some, but not all, bacteria. Biodegradation of
glyphosate may involve co-metabolism with other energy sources.
1.4 Environmental levels and human exposure
Data on the occurrence of glyphosate in environmental biota and
abiota as part of regular monitoring programmes are very scarce.
Data from field experiments in which common agricultural practice is
simulated are used to indicate maximum environmental concentrations:
< 1-1700 µg/litre surface water, 0.07-40 mg/kg dry weight soil,
< 0.05-19 mg/kg dry weight sediment, 261-1300 mg/kg foliage, 5 mg/kg
the viscera of deermice, 1.6-19 mg/kg wild berries, and 45 mg/kg
lichens. The corresponding maximum concentrations of AMPA are:
< 1-35 µg/litre (surface water), 0.1-9 mg/kg dry weight (soil),
< 0.05-1.8 mg/kg dry weight (sediment), 1.7-< 9 mg/kg (foliage),
0.02-0.1 mg/kg (wild berries), and 2.1 mg/kg (lichens). The
above-mentioned concentrations of glyphosate are generally found
immediately after application. The concentration in lichens was
found 270 days after application.
Measurements of daily human intake of glyphosate via food and
drinking-water (total diet studies) are not available. The few data
on occupational exposure indicate that exposure levels for workers
applying glyphosate as the herbicide formulation Roundup are low.
1.5 Kinetics and metabolism in laboratory animals and humans
Technical glyphosate is only partially absorbed from the
gastrointestinal tract. In studies with 14C-labelled glyphosate,
absorption percentages of 30-36% were found in several species.
Dermal absorption is low. From the herbicide formulation Roundup,
< 5.5% of the glyphosate present is absorbed through the skin
(contact time about 24 h). In body tissues, the highest
concentrations, approximately 1% of the oral dose, are found in
bone. Following a single oral dose, 62-69% is eliminated in the
faeces without absorption. Of the absorbed glyphosate, 14-29% is
excreted in urine and 0.2% or less in expired air. Biliary excretion
following intravenous application was only 5-8%. In lactating goats,
excretion in milk was shown to occur to a minor extent only
(concentration < 0.1 mg/kg whole milk at a dose level of
120 mg/kg diet). Biotransformation of glyphosate occurs to a very
low degree only. The only metabolite, AMPA, accounts for 0.3% of the
dose or less; the rest is unchanged glyphosate. Whole body clearance
(99% of an oral dose) occurs in approximately 168 h.
1.6 Effects on laboratory mammals, and in vitro test systems
In experimental animals, technical glyphosate has very low
acute toxicity by the oral and dermal administration routes; it is
markedly more toxic by the intraperitoneal route than by other
routes. Short-term feeding studies have been conducted in several
species, but few effects were seen in most of these tests. In one
13-week study in mice with technical glyphosate, increased weights
of several organs and growth retardation were observed at
50 000 mg/kg diet. In a 13-week study in rats no effect occurred
(technical glyphosate dose levels up to 20 000 mg/kg diet). In
another 13-week study, lesions of the salivary glands were found in
rats and mice. In mice, the NOAEL was 3125 mg/kg diet; in rats, it
was < 3125 mg/kg diet. These findings were not present in any other
short-term or long-term studies conducted in different strains and
species. The salivary lesions suggest that glyphosate may be acting
as a weak adrenergic agonist.
Long-term toxicity was studied in mice and rats. Few effects
were observed and, in almost all cases, at relatively high dose
levels only. In mice, technical glyphosate produced growth
retardation, hepatocyte hypertrophy or necrosis and urinary bladder
epithelial hyperplasia at 30 000 mg/kg. In rats, the same test
compound produced decreased growth, increased liver weights,
degenerative lens changes and gastric inflammation at 20 000 mg/kg
diet.
The available studies do not indicate that technical glyphosate
is mutagenic, carcinogenic or teratogenic. Two multigeneration
studies were carried out in rats. The main effects of technical
glyphosate were decreased body weights of parent animals and pups
and decreased litter size at 30 000 mg/kg diet. In one reproduction
study, an increase in the incidence of unilateral renal tubular
dilation in F3b male pups at 30 mg/kg body weight was reported.
The absence of a renal effect in pups at a higher dose level in the
other reproduction study indicates that the reproducibility of this
lesion is uncertain.
1.7 Effects on humans
The available controlled studies are limited to three
irritation/sensitization studies in human volunteers, the results of
which indicated no effect. Several cases of (mostly intentional)
intoxications with technical glyphosate herbicide formulation
Roundup have been reported. In a study on health effects in workers
applying Roundup herbicide formulation, no adverse effects were
found. Available data on occupational exposure for workers applying
Roundup indicate exposure levels far below the NOAELs from the
relevant animal experiments.
1.8 Effects on other organisms in the laboratory and field
Technical grade glyphosate is moderately to slightly toxic to
aquatic microorganisms, with EC50 (3-4 days) values of
1.2-7.8 mg/litre, and 7-day NOEC values of 0.3-34 mg/litre.
Formulations of glyphosate are slightly to highly toxic to aquatic
microorganisms with 3-day EC50 values of 1.0 to > 55 mg product
per litre. Cyanophyta (blue-green algae) are more sensitive to
Roundup than true algae. Physiological processes that are affected
include the greening process, respiration, photosynthesis, and the
synthesis of aromatic amino acids.
Soil bacteria in culture have shown effects of glyphosate on
nitrogen fixation, denitrification and nitrification. However, field
studies after application of formulations have not shown significant
effects. Closely related species of bacteria have been shown capable
of degrading glyphosate.
Mycelial growth of ectomycorrhizal fungi in pure cultures is
inhibited at concentrations of > 29 µg Roundup/litre. Sensitive
genera are Cenococcum, Hebeloma and Laccaria.
Glyphosate is slightly toxic to aquatic macrophytes with a
14-day NOEC value of 9 mg/litre, when dissolved in water. Roundup is
also slightly toxic with 14-day NOEC values of 2.4-56 mg
Roundup/litre, when dissolved in water. No data on acute toxicity
are available. Phytotoxicity is much higher when sprayed deposits
are not washed off.
Technical grade glyphosate is slightly to very slightly toxic
to aquatic invertebrates with 2- to 4-day LC50 or EC50 values of
> 55 mg/litre, and a 21-day NOEC value of 100 mg/litre.
Formulations of glyphosate are moderately to very slightly toxic to
aquatic invertebrates with 2-day EC50 values of 5.3-5600 mg
product/litre and 21-day MATC values of 1.4-4.9 mg product per
litre. The higher toxicity of Roundup is mainly due to the presence
of surfactants.
Technical grade glyphosate is moderately to very slightly toxic
to fish, with 4-day LC50 values of 10 to > 1000 mg/litre, a
21-day NOEC value of 52 mg/litre, and an MATC value of >
26 mg/litre. Formulations of glyphosate are also moderately to very
slightly toxic to fish with 4-day LC50 values of 2.4 to > 1000 mg
product per litre, and 21-day NOEC values of 0.8-2.4 mg
product/litre. The most sensitive species is the carp, when exposed
to the formulation Sting. No treatment-related effects of Roundup on
fish have been found under field conditions, with the exception of
stress immediately after application of a recommended rate and
avoidance of concentrations of > 40 mg Roundup/litre.
Nodulation of sub-clover inoculated with Rhizobium is inhibited
in a dose-related way in soil-free systems with nutrient solutions
at concentrations of > 2 mg a.i./litre. Seed germination of
various forest species is not affected by glyphosate at the
recommended application rates. The root length of red pine seedlings
is decreased under laboratory conditions in a dose-related way at
application rates of > 0.54 kg a.i./ha. This decrease was not
confirmed in a comparable field experiment.
Technical grade glyphosate and Roundup are slightly toxic to
bees when applied either orally or topically. The 2-day LD50
values are > 100 µg (a.i. or product) per bee. The oral 2-day
LD50 of Sting to bees is > 100 µg/bee. Roundup and Roundup D-pak
are slightly toxic to earthworms with 14-day NOEC values of 500 and
158 mg product per kg dry weight, respectively. No adverse effects
of Roundup were found on the fecundity and fertility of green
lacewings, and there were no effects of Sting on the food uptake and
mortality of the beetle Poecilus.
Technical grade glyphosate is slightly toxic to birds, with an
LD50 of >3851 mg/kg body weight, an 8-day LC50 of >4640 mg/kg
feed, and 112- to 119-day NOEC values of > 1000 mg/kg feed.
Roundup and an unknown formulation are also slightly toxic to birds,
with an LD50 of > 2686 mg product/kg body weight and an 8-day
LC50 of > 5620 mg product/kg feed. Generally no treatment-related
effects of technical grade glyphosate or Roundup on mammals are
found under laboratory conditions, except at very high application
rates. Treatment-related effects on birds and mammals under field
conditions appear to be primarily due to habitat changes after
treatment with Roundup.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, AND ANALYTICAL
METHODS
2.1 Identity
Glyphosate is the primary name of a weak organic acid that
consists of a glycine moiety and a phosphonomethyl moiety. The
chemical name is N-(phosphonomethyl)glycine according to IUPAC
nomenclature. The CAS name is glycine, N-(phosphonomethyl)-, and
its CAS registry number is 1071-83-6. The empirical formula is
C3H8NO5P, and the structural formula is as follows:
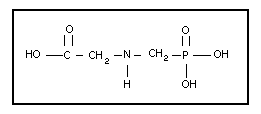 The relative molecular mass of glyphosate is 169.07. Technical
grade glyphosate has a purity of > 80%, but the purity generally
exceeds 90%. Glyphosate usually is formulated as a salt of the
deprotonated acid of glyphosate and a cation, e.g., isopropylamine.
The CAS registry number of the salt of glyphosate and
isopropyl-amine is 38641-94-0.
Surfactants and inerts may be added to formulations of
glyphosate. The type of surfactant and its concentration may differ
per formulation. A common surfactant in the major formulation
Roundup is polyoxyethylene amine. Other known surfactants are ortho
X-77 (Mitchell et al., 1987), LI-700, R-11 and Widespread (Monsanto,
1990a). Other additives in formulations may be sulfuric and
phosphoric acids.
2.2 Physical and chemical properties
The physical and chemical properties of glyphosate are
tabulated in Table 1. Glyphosate is an amphoteric compound of which
the ionic species and their pKa values are presented in Fig. 1. Due
to its high polarity glyphosate is practically insoluble in, for
instance, ethanol, acetone and benzene.
Table 1. Physical and chemical properties of glyphosatea
Remarks
Physical state crystalline powder
Colour white
Odour none
Melting pointb 184.5 °C decomposition at 187 °C
Boiling point n.a.
Specific gravity (density)c 1.704 20 °C
Vapour pressured < 1 x 10-5 Pa 25 °C
Solubility in waterb,e 10 100 mg/litre 20 °C
Henry's law constant < 7 x 10-11
Octanol-water partition
coefficient (log Kow)d -2.8
Surface tensiond 0.072 N/m 0.5% (w/v) at approx. 25 °C
pKa valuesd,f < 2, 2.6, 5.6, 10.6 Sprankle et al. (1975)
Molar absorptivityc 0.086 litre/mol per cm at 295 nm
Flammabilityd not flammable
Explosivenessd not explosive
pHd 2.5 1% solution
a data provided by Monsanto Ltd
b purity 96%
c purity 100%
d purity not reported
e pure glyphosate had been reported to have a water solubility of
11 600 mg/litre at 25 °C
f free acid
n.a. = not applicable
2.3 Formulations
Glyphosate can be applied in various formulations. A synopsis
of these formulations, their concentrations of active ingredient,
and the countries in which the use is permitted is presented in
Table 2. This synopsis is not complete. Formulations may contain
specific surfactants. The major formulation of glyphosate is Roundup
containing 480 g/litre of the isopropylamine salt, which is
equivalent to 360 g/litre of the free acid. Some other Roundup
formulations that are characterized by other a.i. concentrations or
other surfactants have been developed for specific applications.
Other formulations that have been developed for special equipment
are Roundup Ultrabax for CDA equipment, Glyphosate Nomix for Nomix
equipment, and EZ-JECT for tree injections. In Canada, Roundup was
re-labelled as Vision in 1987 for use in forestry.
The relative molecular mass of glyphosate is 169.07. Technical
grade glyphosate has a purity of > 80%, but the purity generally
exceeds 90%. Glyphosate usually is formulated as a salt of the
deprotonated acid of glyphosate and a cation, e.g., isopropylamine.
The CAS registry number of the salt of glyphosate and
isopropyl-amine is 38641-94-0.
Surfactants and inerts may be added to formulations of
glyphosate. The type of surfactant and its concentration may differ
per formulation. A common surfactant in the major formulation
Roundup is polyoxyethylene amine. Other known surfactants are ortho
X-77 (Mitchell et al., 1987), LI-700, R-11 and Widespread (Monsanto,
1990a). Other additives in formulations may be sulfuric and
phosphoric acids.
2.2 Physical and chemical properties
The physical and chemical properties of glyphosate are
tabulated in Table 1. Glyphosate is an amphoteric compound of which
the ionic species and their pKa values are presented in Fig. 1. Due
to its high polarity glyphosate is practically insoluble in, for
instance, ethanol, acetone and benzene.
Table 1. Physical and chemical properties of glyphosatea
Remarks
Physical state crystalline powder
Colour white
Odour none
Melting pointb 184.5 °C decomposition at 187 °C
Boiling point n.a.
Specific gravity (density)c 1.704 20 °C
Vapour pressured < 1 x 10-5 Pa 25 °C
Solubility in waterb,e 10 100 mg/litre 20 °C
Henry's law constant < 7 x 10-11
Octanol-water partition
coefficient (log Kow)d -2.8
Surface tensiond 0.072 N/m 0.5% (w/v) at approx. 25 °C
pKa valuesd,f < 2, 2.6, 5.6, 10.6 Sprankle et al. (1975)
Molar absorptivityc 0.086 litre/mol per cm at 295 nm
Flammabilityd not flammable
Explosivenessd not explosive
pHd 2.5 1% solution
a data provided by Monsanto Ltd
b purity 96%
c purity 100%
d purity not reported
e pure glyphosate had been reported to have a water solubility of
11 600 mg/litre at 25 °C
f free acid
n.a. = not applicable
2.3 Formulations
Glyphosate can be applied in various formulations. A synopsis
of these formulations, their concentrations of active ingredient,
and the countries in which the use is permitted is presented in
Table 2. This synopsis is not complete. Formulations may contain
specific surfactants. The major formulation of glyphosate is Roundup
containing 480 g/litre of the isopropylamine salt, which is
equivalent to 360 g/litre of the free acid. Some other Roundup
formulations that are characterized by other a.i. concentrations or
other surfactants have been developed for specific applications.
Other formulations that have been developed for special equipment
are Roundup Ultrabax for CDA equipment, Glyphosate Nomix for Nomix
equipment, and EZ-JECT for tree injections. In Canada, Roundup was
re-labelled as Vision in 1987 for use in forestry.
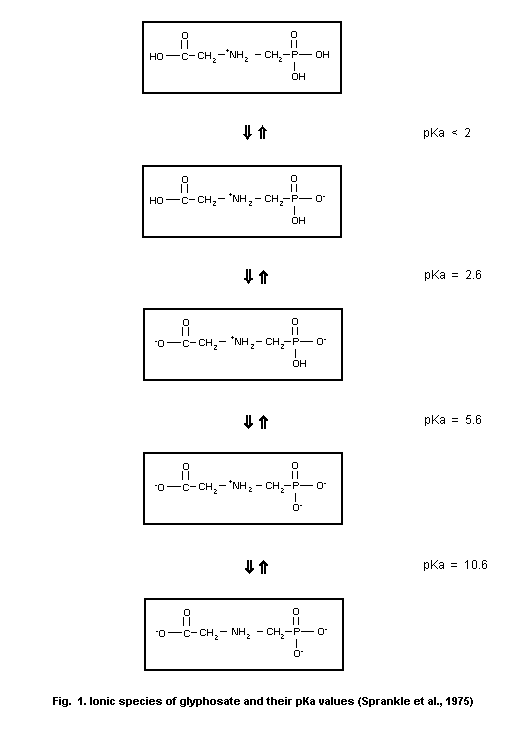 Table 2. Composition of various commercial formulations with glyphosatea
Name Synonyms Concentration Country
a.i. (%)
Roundup Spasor, 48.0 (w/v); Most countries
Sting Vision, 41.0 (w/w)b
Swing, 21.7 (w/w) Belgium, Cameroon, France,
Arcade, Holland, Kenya, Malawi,
Tomahawk Portugal, South Africa,
United Kingdom
Armada Frontier 16.6 (w/w) Belgium, Cameroon, Ivory
Coast, Gabon, Greece, Zaire
Dardo Ricochetg, 12.2 (w/w) Cameroon, Egypt, France,
Rival, Greece, Israel, Italy,
Ultrasonic Portugal, Spain, United
Kingdom
Squadron 20.2 (w/w) Argentina, Australia, Columbia
Stirrup Nomix, Expedite 18.3 (w/w) France, United Kingdom
Wallop 20.8 (w/w) Malaysia
Deploy Dryc 94.0 (w/w) USA
Quotamakerd 75.0 (w/w) USA
Landmaster IIe 13.3 (w/w) USA
Landmaster BW,
Campaignf 12.9 (w/w) USA
Roundup D-Pak 62.0 (w/w) USA
Rodeo 53.8 (w/w) USA
Ranger 28.6 (w/w) USA
Roundup Lawn and
Garden Conc. 18.0 (w/w) USA
Roundup-Ready-
To-Use 0.96 (w/w) USA
Fusta 22.5 (w/w) Spain
Table 2 (continued)
a all formulations produced by Monsanto Ltd; data provided by Monsanto Ltd
b based on the isopropylamine salt; equivalent to 36.0% (w/v) and 30.5% (w/w)
of the free acid
c dry formulation of the monoammonium salt
d dry formulation of the sodium sesqui salt
e also contains 11.1% 2,4-D (isopropylamine salt)
f also contains 20.6% 2,4-D (isopropylamine salt)
g also contains simazine
Formulations may contain other active ingredients, e.g.,
simazine in Ricochet, 2,4-D in Landmaster, and MCPA in Fusta.
2.4 Conversion factors
1 ppm = 6.91 mg/m3 at 25 °C and 101.3 kPa
1 mg/m3 = 0.145 ppm
2.5 Analytical methods
2.5.1 Sample handling and preparation
The first preparative step before detection and measurement of
glyphosate is generally extraction. As both glyphosate and its main
metabolite aminomethylphosphonic acid (AMPA, see Fig. 2) show high
polarity, and are therefore highly water soluble, they are difficult
to extract with organic solvents. However, various methods have been
developed. Some recently developed extraction methods for different
media are summarized in Table 3.
Table 2. Composition of various commercial formulations with glyphosatea
Name Synonyms Concentration Country
a.i. (%)
Roundup Spasor, 48.0 (w/v); Most countries
Sting Vision, 41.0 (w/w)b
Swing, 21.7 (w/w) Belgium, Cameroon, France,
Arcade, Holland, Kenya, Malawi,
Tomahawk Portugal, South Africa,
United Kingdom
Armada Frontier 16.6 (w/w) Belgium, Cameroon, Ivory
Coast, Gabon, Greece, Zaire
Dardo Ricochetg, 12.2 (w/w) Cameroon, Egypt, France,
Rival, Greece, Israel, Italy,
Ultrasonic Portugal, Spain, United
Kingdom
Squadron 20.2 (w/w) Argentina, Australia, Columbia
Stirrup Nomix, Expedite 18.3 (w/w) France, United Kingdom
Wallop 20.8 (w/w) Malaysia
Deploy Dryc 94.0 (w/w) USA
Quotamakerd 75.0 (w/w) USA
Landmaster IIe 13.3 (w/w) USA
Landmaster BW,
Campaignf 12.9 (w/w) USA
Roundup D-Pak 62.0 (w/w) USA
Rodeo 53.8 (w/w) USA
Ranger 28.6 (w/w) USA
Roundup Lawn and
Garden Conc. 18.0 (w/w) USA
Roundup-Ready-
To-Use 0.96 (w/w) USA
Fusta 22.5 (w/w) Spain
Table 2 (continued)
a all formulations produced by Monsanto Ltd; data provided by Monsanto Ltd
b based on the isopropylamine salt; equivalent to 36.0% (w/v) and 30.5% (w/w)
of the free acid
c dry formulation of the monoammonium salt
d dry formulation of the sodium sesqui salt
e also contains 11.1% 2,4-D (isopropylamine salt)
f also contains 20.6% 2,4-D (isopropylamine salt)
g also contains simazine
Formulations may contain other active ingredients, e.g.,
simazine in Ricochet, 2,4-D in Landmaster, and MCPA in Fusta.
2.4 Conversion factors
1 ppm = 6.91 mg/m3 at 25 °C and 101.3 kPa
1 mg/m3 = 0.145 ppm
2.5 Analytical methods
2.5.1 Sample handling and preparation
The first preparative step before detection and measurement of
glyphosate is generally extraction. As both glyphosate and its main
metabolite aminomethylphosphonic acid (AMPA, see Fig. 2) show high
polarity, and are therefore highly water soluble, they are difficult
to extract with organic solvents. However, various methods have been
developed. Some recently developed extraction methods for different
media are summarized in Table 3.
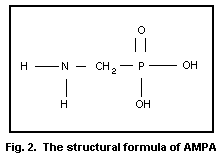 Table 3. Sampling, preparation, and analysis of glyphosate
Medium Sampling Preparations Derivatization Analytical Limit of Recovery Reference
volume or reagent method determinationa
weight
Air n.r. collected onto an trifluoroacetic GC-MS and approx. 0.3 94% Jauhiainen
absorption liquid; anhydride and GC-EC µg/m3 et al. (1991)
evaporation to trifluoroethanol
dryness
Cyano-bacteria 100 ml dry, resuspend in PITC HPLC with a n.r.b 78% Powell et al.
methanol/sodium - radically (1990)
acetate/ column compressed
triethylamine
Plants 5 g extraction with trifluoroacetic GC-NPD 0.03 mg/kg 72-92% Konar & Roy,
water/chloroform; anhydride and (1990)
preconcentration trifluoroethanol
and clean-up on
cation-exchange
resin
Plants 25-50 g extraction with TLC with 0.01 mg/kg n.r. Bunyathyan &
water/chloroform; ninhydrin Gevorgyan
preconcentration detection (1984)
and clean-up on
anion-exchange
and cation-exchange
resin; evaporation
to dryness
Table 3. cont'd (2)
Medium Sampling Preparations Derivatization Analytical Limit of Recovery Reference
volume or reagent method determinationa
weight
Water 250 ml extraction with o-phthalaldehyde LC 3.2 µg/litre 89% Wigfield &
dichloromethane; Lanouette
adsorption on (1990)
anion-exchange
resin
Water 25 ml extraction with FMOCCl HPLC and TLC 0.02 µg/litre 80% Gauch et al.
dichloromethane/ (1989)
2-propanol;
acidification
with H2SO4;
evaporation
to dryness
Water 1-1.5 litre no extraction; TLC with - 0.05 mg/litre n.r. Bunyathyan &
preconcentration ninhydrin Gevorgyan
and clean-up detectionc (1984)
with anion-exchange
and cation-exchange
resin
Soil 5 g extraction with trifluoroacetic GC-NPD 0.05 mg/kg 75% Roy & Konar
deionized water/ anhydride/ (1989)
H3PO4; addition trifluoroethanol
of Darco
charcoal
Table 3. cont'd (3)
Medium Sampling Preparations Derivatization Analytical Limit of Recovery Reference
volume or reagent method determinationa
weight
Soil 2 g (sandy extraction with FMOCCl HPLC 1 mg/kg 80-119% Miles & Moye,
soil); 25 g KH2PO4 (sandy soil), (1988b)
(clayish KOH (clayish soil);
soil) no clean-up
Soil, 5 g (soil); extraction with NH4OH; ninhydrin LC 0.05-0.1 73-79% Thompson
sediment, 20 g (sed); adsorption on mg/kg (soil); (soil) et al. (1989)
foliage 5 g (fol) anion-exchange resin; 0.1 mg/kg 65-84%
further clean-up with (sed); 0.3 (sed)
Dowex cation-exchange mg/kg (fol)d 81-84%
resin (fol)
Urine and 5-6 g extraction with H2O HPLC (ion n.r. 81-99% Monsanto
faeces of (only faeces); protein pair, strong (1988a)
the rat precipitation and anion and
lyophilization cation-exchange),
(only urine); LSC, 1H NMR, 31P
clean-up with C18 NMR, GC/MS
column
Urine n.r. adsorption on trifluoroacetic GC-MS and 0.1 mg/litre n.r. Jauhiainen
(human anion-exchange resin anhydride/ GC-EC et al. (1991)
male) (SAX); elution of the trifluoroethanol
resin with HCl;
evaporation to
dryness
Table 3. cont'd (4)
Medium Sampling Preparations Derivatization Analytical Limit of Recovery Reference
volume or reagent method determinationa
weight
Serum 0.5 ml extraction with p-toluene HPLC with UV n.r.e n.r Tomita et
(human) trichloroacetic sulfonyl chloride detection al. (1991)
acid; adsorption
on anion-exchange
resin; elution
with HCl; evaporation
to dryness
a In no study with a non-liquid medium was it reported whether the limit of determination was based on dry or fresh weight,
except in the study of Thompson et al. (1989).
b The order of magnitude was reported to be picomol.
c The use of TLC with ninhydrin, copper nitrate and rhodamine B detection is reported for glyphosate in distilled water in
Ragab (1978).
d The limits of determination in soil, sediment, and foliage are expressed per kg dry weight.
e Only the limit of detection was reported: 0.3 mg/litre (approximately 75% recovery).
PITC = phenylisothiocyanate; FMOCCl = 9-fluorenyl-methyl chloroformate; GC =gas chromatography;
(HP)LC = (high-performance) liquid chromatography; TLC = thin layer chromatography; MS = mass spectroscopy;
EC = electron capture detector; NPD = nitrogen-phosphorus detector; n.r. = not reported;
LSC = liquid scintillation counting; NMR = nuclear magnetic resonance; sed = sediment; fol = foliage
The second preparative step is the clean-up, which may include
extraction, preconcentration by evaporation, ion-exchange
chromatography or gel chromatography. Clean-up procedures may
involve different combinations of chromatographic techniques. In a
validation study in which plant tissues and water were analysed, a
Chelex column was combined with anion-exchange clean-up (Cowell
et al., 1986). No chromatography was included in the clean-up
procedures for analysing glyphosate and AMPA in natural waters
(Miles et al., 1986). In this procedure samples were successively
filtrated, supplied with phosphate buffer, concentrated by
evaporation, and filtrated, prior to derivatization.
Samples with urine and faeces of the rat were subjected to clean-up
with a C18 column (Monsanto, 1988a). Prior to this extraction,
proteins were precipitated and the samples were lyophilized; samples
of faeces were, however, only extracted with water.
The third preparative step is derivatization. Derivatization
with a fluorogenic reagent is common. Burns (1983), however,
developed a preparation technique without derivatization.
Derivatization prior to detection and measurement with HPLC can be
pre-column (Miles et al., 1986; Lundgren, 1986; Miles & Moye,
1988a) or post-column (Moye et al., 1983; Tuinstra & Kienhuis,
1987). 9-Fluorenylmethyl chloroformate, phenylisothiocyanate and
1-fluoro-2,4-dinitrobenzene may be used as pre-column reagents,
whereas ortho-phthalaldehyde-mercaptoethanol and ninhydrin may be
used as post-column fluorogenic reagents. With post-column
techniques, derivatives can be formed on-line, but it requires more
equipment and experience. On the other hand, pre-column techniques
are often more rapid and require less equipment and experience. In
general the facilities required for derivatization with fluorogenic
substances are very specific, and therefore not available in many
laboratories (Konar & Roy, 1990). These authors proposed
derivatization with a mixture of trifluoroacetic anhydride and
trifluoroethanol prior to analysis with gas chromatography as a
simpler, less laborious and more economical method. This proposal
referred to the determination of glyphosate and AMPA in plant
tissues. This and other recently developed techniques of clean-up
and derivatization are summarized in Table 2. These techniques are
intended to simplify and improve preparative techniques, which in
general used to be complex and costly (Marcotte et al., 1977;
Guinivan et al., 1982; Roseboom & Berkhoff, 1982; Moye et al.,
1983; Moye & Deyrup, 1984; Deyrup et al., 1985; Miles et al.,
1986; Lundgren, 1986; Miles & Moye, 1988b).
Sample preparation and derivatization, as developed by Powell
et al. (1990) for cyanobacteria without deproteinization (see
Table 3), should also be usable for plant and animal tissue. In this
case, a simple maceration step prior to ethanol extraction should be
included. Bunyathyan & Gevorgyan (1984) developed preparative
techniques for different media prior to analysis with TLC. Only
their procedures for plants and water are summarized in Table 3. The
preparative technique for soil samples was comparable with that of
Thompson et al. (1989), although samples of 25-50 g were required.
Bunyathyan & Gevorgyan (1984) also developed a method for preparing
20-litre air samples prior to TLC. They extracted the residues
collected on a filter with water before clean-up on a
cation-exchange resin.
2.5.2 Analytical methods
Various analytical methods for the determination of glyphosate
have been described, including thin-layer chromatography (Young
et al., 1977; Ragab, 1978; Bunyathyan & Gevorgyan, 1984),
colorimetry (Glass, 1981), differential pulse polarography (Friestad
& Bronstad, 1985), gas chromatography (Guinivan et al., 1982; Moye
& Deyrup, 1984; Deyrup et al., 1985), high-performance liquid
chromatography (Miles & Moye, 1988a; Powell et al., 1990), and
31P NMR (Dickson et al., 1988). Some of these techniques, their
analytical recoveries and limits of determination are listed in
Table 3. The corresponding determination limits for AMPA, i.e.
analysed with the same techniques, are listed in Table 4. Recoveries
in the different media appear to be higher for glyphosate than for
AMPA. This is probably due to optimization of the systems for
glyphosate, as was done by Thompson et al.(1989).
Table 4. Limits of determination of AMPA
Medium Limit of Recovery Reference
determination
Plants 0.01 mg/kg 61-73% Konar & Roy (1990)
Water 1.2 µg/litre 86% Wigfield & Lanouette (1990)
Soil 0.01 mg/kg 66% Roy & Konar (1989)
Soil 0.03-0.05 mg/kg 58-68% Thompson et al. (1989)
Sediment 0.03 mg/kg 54-67% Thompson et al. (1989)
Foliage 0.008 mg/kg 55-70% Thompson et al. (1989)
Urine (human) 0.05 mg/litre n.r. Jauhiainen et al. (1991)
Serum (human) n.r.a n.r. Tomita et al. (1991)
a n.r. = not reported; only the limit of detection was reported: 0.2 mg/litre
(approximately 88% recovery)
TLC techniques are generally based on silica gel or cellulose
plates; cellulose plates give a better separation (Dubelman, 1988).
Ninhydrin and phosphate sensitive reagents may be used for
detection, although interference from co-extractives may occur.
According to Dubelman (1988), fluorogenic reagents may be preferable
in case of interference.
Fluorogenic derivatives can be determined in HPLC analysis with
fluorescence detectors (Wigfield & Lanouette, 1990) and also with a
spectrophotometer (Powell et al., 1990). In a GC analysis a
nitrogen-phosphorus, electron capture or a flame photometric
detector can be used.
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1 Anthropogenic sources
3.1.1 Production levels and processes
No data on the world production of glyphosate and its
formulations are available. In addition, no data on losses to the
environment during normal production and formulation or accidental
losses have been reported.
The first phase of the production of glyphosate consists of
refluxing a mixture of glycine (50 parts), chloromethylphosphonic
acid (92 parts), an aqueous solution with 50% sodium hydroxide (150
parts), and water (100 parts) in a suitable reaction vessel. Another
50 parts of an aqueous solution with 50% sodium hydroxide are added
to maintain the pH between 10 and 12, whereafter the reaction
mixture is refluxed for another 20 h. The mixture is then cooled to
room temperature and filtered. After adding 160 parts of
concentrated hydrochloric acid, this mixture is again filtered.
Glyphosate will slowly precipitate in the filtrate (IRPTC, 1991).
3.1.2 Uses
Glyphosate is a post-emergent, systemic and non-selective
herbicide intended for use against deep-rooted perennial species,
and also biennial and annual broad-leaved, grass and sedge species
(WSSA, 1983; Monsanto, personal communication to the IPCS, 1991).
Glyphosate is used in both agriculture and forestry. Fields of
agricultural use include grassland renovation, horticulture,
fructiculture, arable cultivation, and rice cultivation. Use in
forestry includes the killing of fast growing competitors in conifer
plantations or conservation areas, and the treatment of tree stumps.
Glyphosate may also be used for weed killing in non-agricultural
areas such as water systems, including irrigation and temporarily
drained waters, parks, road verges and gardens.
The uses of glyphosate indicate that it can be applied in
various crops for specific purposes. The major formulation Roundup
may, for instance, be used in pre-plant treatments for seed bed
preparations, and also against bracken infestations in forestry,
against couchgrass (Elytrigia repens) infestations on pastures, in
direct treatments between rows of crops, or by direct wiping of the
leaves of the weed, assuming the weeds are taller than the existing
crop.
Glyphosate is used worldwide. In 1987, 35 160 ha of the area in
British Columbia where vegetation management activities took place
had been treated with Roundup. This was 94% of the total area where
there were such activities (Ackurst, 1989).
The application rates of glyphosate are dependent on the
formulation and type of use. In the Netherlands, recommended rates
for the application of Roundup are 0.3-2.9 kg a.i./ha. In Canada the
recommended application rates of Roundup are 1.1-1.7 kg a.i./ha for
annual weeds and 1.2-5.8 kg a.i./ha for perennial weeds. The
recommended application rates for Vision in Canadian forestry are
1.1-2.1 kg a.i./ha (Task Force on Water Quality Guidelines, 1991).
Glyphosate is generally applied as a 0.5-5% solution in water by
spraying, and as a 10-50% solution in water by wiping with, for
instance, a rope-wick (Monsanto, personal communication to the IPCS,
1991).
The timing of application is dependent on the use. Application
in late summer or autumn is recommended for use in forestry in
Canada (Hildebrand et al., 1982). Application in agriculture may
be pre- or post-harvest. In the Netherlands, for instance,
glyphosate may be applied to cereals, potatoes and asparagus
immediately (up to 7 days) before harvest, but only when the
ripening is complete. Treatment of immature crops would result in
higher residue levels, early crop desiccation and reduced yields.
Glyphosate may be applied in different ways. For large-scale
treatments aerial application can be appropriate, small-scale
treatments can be done with spraying equipment on the back or behind
vehicles, or by wiping equipment.
Aerial applications will lead to losses due to wind-drift.
Exposure of flora and fauna due to off-target deposits may take
place. These downwind deposits depend on the meteorological
conditions, the plant canopy structure and the application method,
including the release height (Payne et al., 1989; Feng et al.,
1990; Payne, 1992; Payne & Thompson, 1992). The non-volatile
tank-mix fraction and the speed of the aircraft may influence the
drop-size spectrum, and it can be expected that dispersal systems
causing relatively small droplets and having a relatively low
non-volatile fraction will cause the highest off-target deposits.
Payne (1992) assumed that the large differences in deposits in two
comparable experiments were due more to different aircraft airspeeds
than to different wind speeds. In these experiments the maximum
deposits at a downwind distance of 50 m were 19 and 3 mg a.i./m2
at aircraft airspeeds of 45 and 11-20 m/second, respectively. The
application rate in both experiments was 2.1 kg a.i./ha. In other
experiments with the same application rate, Payne & Thompson (1992)
found that the meteorological conditions had a considerable impact
on the off-target deposition up to 400 m downwind when spraying at
different wind speeds (2.2-5.7 m/second) and turbulences. The
deposits at a downwind distance of 400 m varied between 0.001 and
0.06 mg a.i./m2, whereas they varied between 0.6 and 4 mg
a.i./m2 at a downwind distance of 50 m. Remarkably, the deposition
was highest with an intermediate wind speed and intensity of
turbulence. Payne et al.(1989) investigated the deposits for
aerial applications of Roundup with different dispersal systems.
When 2.1 kg a.i./ha was applied with a helicopter in a single
crosswind swath over 100 ha, up to 13.4 mg a.i./m2 was deposited
on a downwind distance of 50 m. This maximum deposition was caused
by a D8-46 hydraulic nozzle, whereas the highest depositions with a
Thru Valve Boom and a Microfoil Boom were 2 and 0.4 mg a.i./m2,
respectively. These depositions were also found at a downwind
distance of 50 m. At the time of application the windspeed 13 m
above ground level was 0.4-0.5 m/second. Riley et al.(1991)
modelled spray deposition of glyphosate using results from
helicopter applications under semi-operational conditions. The study
was designed to test the appropriateness of a New Brunswick "buffer
zone" of 65 m to minimize the effects of spray drift. At a distance
of 65 m, it was estimated that between 3.7% and 5.6% of the nominal
spray rate was deposited.
3.1.3 Drinking-water
Appraisal
The low mobility of glyphosate in soil would indicate a
minimal potential for the contamination of drinking-water from
groundwater aquifers. The only possible source of drinking-water
contamination is, therefore, surface waters. There have been no
reported incidences of drinking-water contamination with
glyphosate.
Conventional plants for processing of drinking-water would not
remove glyphosate, but this could be achieved by coprecipitation
after adding iron salts (AMA van der Linden, personal communication
to the IPCS, 1991). Ozone, increasingly used as an alternative to
chlorine in drinking-water treatment, does effectively remove
glyphosate through the hydroxyl radical (HO. ) chain processes that
occur in most ozonated waters (Yao & Haag, 1991; Haag & Yao, 1992).
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION AND TRANSFORMATION
4.1 Transport and distribution between media
Appraisal
Following application, glyphosate selectively partitions to
particulate matter suspended in surface water or to the soil
substrate. This partitioning is usually rapid and occurred within 14
days in reported studies. The mechanism of sorption to soil is only
partially understood. Glyphosate can adsorb to soils through
phosphate binding sites. Competition with inorganic phosphate has
been demonstrated in the laboratory but not measured in the field.
Specific ions (Fe2+, Fe3+ and Al3+ ) complex glyphosate; metal
complexes with humic acids in soil may be a main binding mechanism
for glyphosate in soil. There is little reported information on
desorption from soil; the data available suggest "strong" binding.
This is supported by mobility studies which show little leaching of
glyphosate below the upper few centimetres of the soil profile. The
major metabolite, AMPA, is also retained in the upper soil layers.
There is very little information on the bioavailability of
sediment-bound glyphosate to either aquatic or terrestrial
organisms. Bioaccumulation and ecotoxicity studies have not,
generally, been performed with added sediment.
Applied glyphosate can be translocated in plants. Glyphosate
in plant foliage or leaf litter does not seem to represent a source
of contamination of aquatic systems. Animals can ingest the
herbicide residues in or on plants.
Dissipation of glyphosate from soil has been widely studied
with very variable results (DT50 between 3 and 174 days).
Biodegradation appears to be the major source of dissipation.
Run-off was minimal in experimental studies, but field results
suggest that aquatic systems may be receiving glyphosate bound to
soil particles following rainfall.
In this chapter the terms biodegradation and dissipation are
used to distinguish between the decrease of the concentration in,
for instance, the soil that is due to microbes transforming the
molecule to a smaller size (biodegradation) and the decrease of the
concentration that might be due to microbial activity but also to
other processes, e.g., sorption, leaching and run-off (dissipation).
4.1.1 Water
Glyphosate dissipates from the water with 50% dissipation times
ranging from a few days to 2 weeks (Newton et al., 1984; Monsanto
1990a; see also Table 5). These DT50 values were deduced from both
laboratory and field experiments in which sediment or suspended
particles were shown to be the major sink.
In water with a near-neutral pH, the formation of an insoluble
complex of Ca2+ with glyphosate was demonstrated in a laboratory
experiment (Subramaniam & Hoggard, 1988). It was confirmed with
X-ray powder diffraction and infrared spectra that this complex was
not an ionic salt. At a near-neutral pH, the dianionic species of
glyphosate is dominant. Insoluble complexes have also been found
with Mg2+, Fe3+ and Cu2+.
In a field experiment in a temperate coastal rainforest in
British Columbia, Canada, the highest concentration of glyphosate in
water was 162 µg/litre (Feng et al., 1990). This maximum was found
in a directly sprayed tributary 2 h after an aerial application of
Roundup at a rate of 2 kg a.i./ha. Concentrations in oversprayed
tributaries without a high cover of overhanging riparian vegetation
increased after the first rainfall. In oversprayed tributaries with
a high cover of riparian vegetation almost no residues were found.
Within 96 h after application the residues in all waters had
declined below detection limits, indicating rapid dissipation. After
rainstorms, peak concentrations of glyphosate were found in the
sediments and on suspended particles of the oversprayed tributaries,
with maximum concentrations of 7 mg a.i./kg dry weight and 0.06 µg
a.i./litre unfiltered water, respectively. The amounts in the
sediments of these waters were variable but declined over time. As
0.1-2 mg residue/kg dry weight sediment was found between 196 and
364 days after application, the residues appear to be persistent in
sediments of oversprayed waters. Feng et al.(1990) concluded,
therefore, that after rainstorms sediments appear to be the major
sink.
In another field experiment in the same forest ecosystem,
glyphosate dissipated rapidly from a small perennial, very slow
flowing stream, in a site of 8 ha aerially sprayed with Roundup at a
rate of 3.3 kg a.i./ha (Newton et al., 1984). In water, 50% of the
initial concentration had dissipated in 2 days. In sediment, maximum
concentrations of approximately 0.6 mg a.i./kg were found 14 days
after application. These were reduced to approximately 0.3 mg
a.i./kg in 28 days, and to < 0.2 mg a.i./kg in 55 days after
application. A comparable rapid dissipation from the water column
was found for small forest ponds in a boreal forest in Manitoba,
Canada, after applying Roundup at a rate of 0.9 kg a.i./ha
(Goldsborough & Beck, 1989). The highest concentration in filtered
water was 141 µg a.i./litre, within 6 h after application. The main
mechanism of dissipation was probably sorption to the sediment. This
was confirmed by additional experiments with polyethylene basins
filled with unfiltered water and sediment that were placed in the
spray zone. Without sediment, only a very small amount of the dose
actually applied had dissipated after 30 days, whereas with sediment
the initial concentrations in the water had decreased by 50%,
approximately 6 days after application.
Comparable dissipation patterns were found in a field
experiment (Monsanto, 1990a) in which Accord (30.5% a.i. w/w) was
applied at a rate of 4.2 kg Accord/ha on three forestry sites with
non-flowing pond water and flowing water. Concentrations of up to
1700 µg/litre filtered water were found in the pond water
immediately after spraying. The initial concentrations in both pond
and flowing water were reduced by 50% within 7 days. Concomitantly
initial AMPA concentrations (maximally 35 µg/litre) were reduced by
50% within the same period. In flowing water the dissipation of both
glyphosate and AMPA was even more rapid. Concentrations of
glyphosate increased up to 19 mg/kg dry weight in the sediment of
one pond 28 days after application. Concentrations of up to 1 mg/kg
of both compounds were measured in the sediments of non-flowing
ponds up to 400 days after application.
In field experiments in turbid Australian irrigation water,
glyphosate adsorbed to suspended particles at different rates,
apparently mainly depending on the application rate (Bowmer et al.,
1986). At an initial concentration of 5 mg a.i./litre, 10-16% of the
load adsorbed to suspended matter, whereas at an initial
concentration of 0.05 mg a.i./litre, 53-71% adsorbed. In more saline
water the degree of sorption was less, probably due to rapid
flocculation. Maximum adsorbed amounts were approximately 7000 mg
a.i./kg in less saline supply water, and approximately 2500 mg
a.i./kg in more saline drainage water. When a supply channel was
emptied before spraying with 3.6 kg a.i./ha for control of aquatic
weeds, and filled again with water 4 days after the treatment, the
amount in the unfiltered water used for irrigation was 7% of the
applied dose.
4.1.2 Soil sorption
Glyphosate is readily bound to many soils and clay minerals
(Sprankle et al., 1975; Hance, 1976; Glass, 1987; Miles & Moye,
1988b). In laboratory experiments in which glyphosate was added to
aqueous soil suspensions, the adsorption coefficient Ks/l was
18-377 dm3/kg in nine soils ranging from sandy loam to peat
(Hance, 1976), and 33-76 dm3/kg in three soils ranging from sandy
loam to clay loam (Glass, 1987). These Ks/l values indicate strong
sorption. In both experiments the sorption could be described by the
Freundlich equation. Glass (1987) found Ks/l values for the clay
minerals montmorillonite, illite and kaolinite of 138, 115 and 8
dm3/kg, respectively.
Table 5. Biodegradationa of technical grade glyphosate in water and sediments in the laboratory
Water type Sediment Test Sediment Organic Temperature pH of Experimental Parameter Time Reference
type type (%) matter in (°C) water time (days)
sediment (days)
(%)
Pond water silty A 17 0.9 23-25 5.9-7.0 30 DT50 14b PTRL East
clay Inc. (1990a)
loam
Pond water silty An 16 0.9 20-27 5.7-6.5 365 DT50 14c,e PTRL East
clay Inc. (1990b)
loam
Surface waterd n.r. A 9 n.r. 30 8.2-8.6 14 DT50 < 14 Monsanto
(1972a)
Lake water sandy An 33 1.4 30 6.6 42 DT50 22e Monsanto
clay (1978a)
loam
a Biodegradation in the whole system
b The biodegradation stopped after approximately 15 days
c The biodegradation stopped after approximately 150 days
d Three rivers and one lake in the USA
e Approximate value derived from data of the author(s)
A = aerobic; An = anaerobic; n.r. = not reported.
The mechanism of sorption of glyphosate to soil is only
partially understood. Several factors may be involved. The
phosphonic moiety adsorbs weakly to unoccupied phosphate binding
sites and can be displaced by phosphate (Hance, 1976). In laboratory
experiments with nine soils the author showed that sorption was
positively correlated with the unoccupied phosphate sorption
capacity, and not correlated with the total phosphate sorption
capacity, organic matter, clay, iron or aluminium content. No data
are available that confirm competition of glyphosate and phosphate
under field conditions, e.g., after application of artificial
fertiliser. Miles & Moye (1988b) suggested that the main mechanism
was probably by H-bonding and ion-exchange, as the degree of
sorption in their experiments was not correlated with cation
exchange capacity (CEC) values or surface areas. Contrary to the
results of Miles & Moye (1988b) and of Hance (1976), sorption
appeared to be correlated with CEC values and clay content in a
sorption study with clay loam, silt loam and sandy loam (Glass,
1987).
The binding is also influenced by the presence of specific
cations. Hensley et al.(1978) demonstrated that Fe2+, Fe3+
and Al3+ inactivated glyphosate much more than Ca2+, K+ and
Na+. This was confirmed by Glass (1987) and Sprankle et al.
(1975). Glass (1987) suggested that glyphosate was complexed by
cations, released from cation-saturated clays via a cation-exchange
with solution protons.
According to Heinonen-Tanski (1989), most of the soil-bound
residues of glyphosate were recovered in the fulvic acid fraction
(21-33%). Sorption of glyphosate to fulvic acids was also reported
by Madhun et al.(1986), who added 14C-glyphosate to an aqueous
soil extract (ASE) of peat. In this study sorption was mainly on ASE
fractions with a relative molecular mass ¾ 1000. Piccolo et al.
(1992) studied the interaction of glyphosate with a pure iron-humic
acid complex. Maximum adsorption values indicated that adsorption to
the complex occurred to as great an extent as to whole soils. This
suggested that humic acid complexes with polyvalent cations might
represent a main binding substrate for glyphosate in soils. There
was no desorption of bound residues of glyphosate following shaking
with 0.01 mol CaCl2/litre solution for 48 h, the maximum shaking
time for the adsorption studies.
Desorption of glyphosate with ionized water from
montmorillonite and illite needed three days before reaching an
equilibrium in a study of Miles & Moye (1988b).
It can be concluded that sorption of glyphosate can be expected
in the presence of available phosphate binding sites, the presence
of iron and aluminium (oxides or hydroxides), and appropriate
combinations of clay and organic matter.
4.1.3 Mobility in soils
In view of its Ks/l, glyphosate can be expected to be
immobile or slightly mobile in many soils. This was confirmed by
several experiments, both in the laboratory and in the field. In
thin-layer chromatography studies with sandy loam, clay loam and
sandy clay loam, the Rf values of 14C-glyphosate were 0.14-0.20
(Sprankle et al., 1975). In comparable studies with silt loam,
silty clay loam, and sandy loam Rf values were < 0.2 (Monsanto,
1972c). In a leaching study with columns of 30 cm and a high water
flux of 51 cm over less than 2 days, < 0.1-6.6% of the applied
activity was leached (Monsanto, 1978b). This experiment was
performed with eight soils, ranging from sandy loam (organic matter
content 0.7%) to volcanic ash (organic matter content 9.5%). More
than 90% of the applied activity was recovered in the upper 0-14 cm
layer.
Only one leaching study under laboratory conditions with
respect to the mobility of AMPA has been reported. In this
experiment with 30-day-old residues, < 0.1-1.6% of the applied
activity was leached over 45 days (Monsanto, 1978b). The columns
were 30 cm and the water flux over 45 days was low (17 cm). The
amount of AMPA that was recovered after 45 days in the upper 0-2 cm
layer was low (0.5-12% of the applied activity), due to a high rate
of mineralisation.
4.1.4 Dissipation from the soil in the field
Many field experiments on the dissipation of glyphosate from
the soil have been performed. Some relevant studies are summarized
in Table 6. They indicate DT50 values based on dissipation that
range from 3 to 174 days depending on edaphic and climatic
conditions. In a forest brush ecosystem in Oregon, USA, the DT50
value in loam was 29 days with and 40 days without litter (Newton
et al., 1984). In field experiments in Sweden, Roundup was sprayed
over reforestated sites (Torstensson et al., 1989). In the soils
of these sites the DT50 values were < 50 days, apparently
depending on the soil respiration rate. The dissipation consisted of
a fast first, and a much slower second phase, especially in sites in
northern Sweden, which was possibly due to a longer frost period. In
these sites 1-2% of the actually applied dose was recovered 1080
days after application. A comparable dissipation pattern was found
in a field experiment on Finnish agricultural soils (Heinonen-Tanski
et al., 1985). In this experiment 25% of the concentration in a
sandy loam 2 days after the treatment was recovered one year after
application. The application rate was 1.4 kg a.i./ha.
A study in a temperate coastal rain forest in British Columbia,
Canada, showed that, 360 days after application, 6-18% of the
initial levels was recovered (Feng et al., 1990). In this
experiment Roundup was applied at a rate of 2 kg a.i./ha. The soils
were alluvial sandy loam or sandy clay loam with highly organic
surface horizons. Some of these soils were well drained, others were
seasonally flooded. At each sampling time more than 90% of the
recovered residues was in the upper 0-15 cm layer. Under all
conditions the amount of glyphosate declined over time, whereas
there was a transient increase of AMPA.
In other field experiments on boreal forest soils, comparable
dissipation patterns were found. Stark (1983) reported DT90 values
of 30-720 days, and Roy et al.(1989b) found a DT50 value of
approximately 20 days on a sandy soil planted with jackpines (Pinus
banksiana). In the field experiments of Roy et al.(1989b),
glyphosate was detectable up to 335 days after application; almost
all residues in the sandy soil were recovered in the organic top
layer. In field experiments of Monsanto (1990a) in three forest
locations in the USA, the concentration course of glyphosate
appeared to be rather irregular, especially during the first four
months. However, 50% of the initial concentrations in the soil had
mostly dissipated within 120 days. One clear exception was a site in
Corvallis in which glyphosate increased up to 0.15 mg/kg dry weight,
180 days after application. On the same site AMPA increased up to
0.32 mg/kg, 346 days after application. The application rate in
these experiments was 4.2 kg Accord/ha.
On a clay soil of a clear-cut boreal forest area, Roy et al.
(1989b) found no dissipation of glyphosate due to run-off on a 8°
slope. In a field experiment on agricultural soils without
conventional tillage, the dissipation of glyphosate due to run-off
on 6-16° slopes was < 1% of the applied dose when 1.1-3.4 kg
a.i./ha was applied (Edwards et al., 1980). However, when 9.0 kg
a.i./ha was applied, 1.8% of the applied dose dissipated due to
run-off, mainly because of a rainstorm shortly after application.
4.1.5 Uptake and dissipation from plants
Uptake of 14C-glyphosate by leaves of trembling aspen
seedlings (Populus tremuloides) was initially rapid, after which
it slowed down (Sundaram, 1990). The seedlings were exposed to
Roundup that was dripped with a micro-applicator on some central
leaves. The application rate was 0.35 kg a.i./ha leaf surface area.
Most activity was washable from the leaves (61-77%), and 22-28% was
recovered in the treated leaves within 48 h. As only 1-10% was
recovered in the other parts of the seedlings, this indicated a
rather low translocation after absorption. A rapid uptake of
14C-glyphosate within a few hours was indicated for sugar beets
(Beta vulgaris), when applied to a mature leaf (Gougler & Geiger,
1981). 14C-glyphosate probably entered the phloem in a
non-facilitated way. The subsequent transport through the phloem
Table 6. Biodegradation and dissipation of glyphosate in soils
Soil type Compound Test Moisture Temperature pH Organic Experimental DT50 Reference
type content (°C) matter duration (days)
(%) (%) (days)
Biodegradation
Sandy loam Tgg L,A 14-16 25 7.3 2.8 360 2b PTRL East Inc. (1991)
Silt loam Tgg L,A 12-14 25 7.5 1.0 360 2b PTRL East Inc. (1991)
Dissipation
Sandy loam Tgg G 11 32 5.7 1.0 112 130b Monsanto (1972b); Rueppel
et al. (1977)
Silt loam Tgg G 11 32 6.5 1.0 112 3b Monsanto (1972b); Rueppel
et al. (1977)
Silty clay loam Tgg G 11 32 7.0 6.0 112 25-27b Monsanto (1972b); Rueppel
et al. (1977)
Sand Ru F n.r. n.r. 3.5-3.7 40 762 approx Roy et al. (1989b)
(humoferric 20a
podsol)
Sandy loam, Ru F n.r. n.r. 4.2-4.9 15-31 360 45-60b Feng & Thompson (1990)
sandy clay loam
Loam Ru F n.r. n.r. 4.0-4.7 3.8-5.2 55 29-40b Newton et al. (1984)
Loamy sand Ru F n.r. n.r. n.r. 0.8 370 3-4b Monsanto (1983a)
Sandy clay loam Ru F n.r. n.r. n.r. 7.0 370 122-174b Monsanto (1983a)
a Based on data of the author(s) b Data reported by the author(s)
L = laboratory study; F = field study; G = greenhouse study; A = aerobic; An = anaerobic;
Tgg = technical grade glyphosate; Ru = Roundup; n.r. = not reported
appeared to be according to an "intermediate permeability
mechanism". When exposed for a longer time, plants may show
substantial translocation of absorbed 14C-glyphosate, as was shown
for potatoes (Solanum tuberosum) by Smid & Hiller (1981). In the
treated leaves of the potatoes 45% of the absorbed activity was
recovered, whereas the rest was mainly translocated to the apical
meristem and the roots. Up to 5% was recovered in the mother tuber.
The degree of translocation was age-dependent, as older plants
showed less translocation than younger plants.
The uptake of glyphosate by red raspberries (Rubus strigosus)
was 9% of the amount that was deposited on the leaves after spraying
Roundup at a rate of 2 kg a.i./ha (Roy et al., 1989a). In the same
field experiment the uptake was 14% by wild blueberries (Vaccinium
myrilloides). Most glyphosate was recovered in the washings, which
was also found under laboratory conditions. The initial absorbed
amounts were 0.92-2.0 mg a.i./kg dry weight. The absorbed and
washable amounts together were reduced by 50% within 13 days in the
raspberries and within 20 days in the blueberries. AMPA was
detectable up to 33 days after application. Metabolism occurred to
only a minor extent as AMPA concentrations were less than 1.5% of
the concurrent concentrations of glyphosate (similar results were
reported by FAO/WHO, 1986b). In a field experiment by Feng &
Thompson (1990) in a temperate coastal rainforest in British
Columbia, Canada, the main target species for treatment with Roundup
were red alder (Alnus rubra) and salmonberry (Rubus spectabilis).
Immediately after spraying, the concentrations in leaf tissue were
up to 448 mg a.i./kg dry weight. Glyphosate dissipated rapidly from
the leaf litter with a DT50 value of 8-9 days. The leaf litter
included leaves directly exposed on the trees and existing leaf
litter from natural defoliation before treatment with Roundup. The
authors assumed that leaf litter of these major brush species is an
insignificant source of glyphosate input into streams or onto forest
floor, because of the fast dissipation. A rapid dissipation of
glyphosate from fresh foliage was also found in a field study
(Monsanto, 1990a) in which initial concentrations of up to 1300 mg
a.i./kg and 2.6 mg AMPA/kg decreased rapidly. A transient
accumulation of glyphosate and AMPA was found in the leaf litter on
some sites, but these amounts were reduced by approximately 90%
within 100 days.
Glyphosate dissipated completely from wild berries (Vaccinium
vitis-idaea, Vaccinium myrtilus) within one year in a field
experiment in Finland in which Roundup was applied at a rate of
0.25-2.2 kg a.i./ha with a knapsack sprayer (Siltanen et al.,
1981). Contrary to this dissipation pattern was that of glyphosate
in reindeer lichens (Cladonia rangiferina) that were sampled in
the same experimental plots. Around 270 days after application,
dose-related concentrations of glyphosate and AMPA were recovered in
lichens with maxima of 45 and 2.1 mg/kg for glyphosate and AMPA,
respectively. Approximately 390 days after application of 0.8 kg
a.i./ha, 6.4 and 0.3 mg/kg of glyphosate and AMPA were still
detectable.
4.1.6 Ingestion by animals
As the concentration in the foliage may increase up to high
amounts immediately after application, this implies the possibility
of entry into the food chain through ingestion by herbivorous or
omnivorous animals. This was confirmed by Sullivan & Sullivan (1979)
who investigated the effects of glyphosate on the feed preference
and daily chow consumption of black-tailed deer (Odocoileus
hemionus columbianus). These herbivores did not avoid eating
browse of alder (Alnus rubra) and alfalfa (Medicago sativa) that
was treated with glyphosate at a rate of 2.2 kg/ha. Sometimes the
treated alder browse was even preferred. Reindeer may be exposed to
glyphosate, since reindeer lichens, which are an important food
source, can take up a substantial amount of glyphosate (see above).
4.2 Abiotic degradation
Appraisal
Hydrolysis of glyphosate is very slow. Photodegradation in the
field may occur.
4.2.1 Hydrolytic cleavage
Hydrolysis of glyphosate in sterile buffers is very slow. After
32 days < 6.3% of the applied activity was recovered as AMPA,
after applying 14C-glyphosate at rates of 25 and 250 mg/litre to
aqueous buffer solutions of pH 3, 6 and 9 (Monsanto, 1978b). These
tests were performed at both 5 and 35 °C.
4.2.2 Photodegradation
Photochemical degradation in water may occur under both
laboratory and field conditions, mainly depending on the type of
light source. In sterile aqueous buffers of pH 5, 7, and 9, less
than 1% of the applied dose was degraded (photodecomposition of
14C-phosphonomethyl-labelled glyphosate) during 29-31 days, when
exposed to sunlight (PTRL Inc., 1990).
Lund-Hoie & Friestad (1986) exposed Roundup to several light
sources under different conditions. When exposed to UV light (lambda
= 254 nm) under laboratory conditions, concentrations of 1 and
2000 mg a.i./litre in deionized water showed DT50 values of 4 and 14
days, respectively. When exposed to sunlight under field conditions
1 mg a.i./litre in polluted water without sediment showed a much
slower decomposition (DT50 > 63 days), possibly due to pollution
preventing adequate UV penetration in the water. Polluted water with
sediments showed a rapid dissipation from water, probably due to
adsorption onto the sediments. In another field experiment 2 and
100 mg a.i./litre in deionized or polluted water without sediment
showed DT50 values of < 28 days, when exposed to sunlight. At
the low concentration the dissipation in polluted water was more
rapid than in deionized water. In the dark no dissipation occurred.
In laboratory experiments 1 mg/litre of glyphosate in
sterilized natural and deionized water showed DT50 values of 4 to >
14 days when exposed to artificial light (350-450 nm) in
photoreactors without sediment (Monsanto, 1978a). In these
experiments Ca2+ acted as a photosensitizing agent.
Photodegradation by sunlight of glyphosate applied to a soil
appeared to be an insignificant route of dissipation (PTRL Inc.,
1989). In this study, 14C-glyphosate mixed with unlabelled
glyphosate was exposed for 31 days to natural sunlight, after
application to a sandy loam at a rate of 4.5 kg a.i./ha.
Extrapolated DT50 values that were based on first-order kinetics
were 90 days in the sunlight and 96 days in the dark, indicating no
substantial degradation due to photolysis. The temperature of the
soil surface was 22-23 °C. Under unnatural light conditions
glyphosate appeared not to be photodegraded substantially (Monsanto,
1972c; Rueppel et al., 1977; Monsanto, 1978a).
4.3 Biodegradation
Appraisal
Selected studies of the biodegradation of glyphosate have been
considered; selection was on the basis of test conditions and modern
methodologies. There is considerable variation in rate of breakdown
in water, aquatic sediment and soil. Degradation occurs more rapidly
in aerobic than anaerobic conditions. Half-times for biodegradation
in the three media under laboratory conditions range between a few
days and approximately 20 days. No data on biodegradation under
anaerobic conditions are available.
The main route of biodegradation of glyphosate appears to be
by splitting the C-N bond to produce AMPA. However, a second route
with splitting of the C-P bond can also occur.
A range of bacterial strains can degrade glyphosate. Bacteria
capable of using the compound as sole phosphorus, sole carbon or
sole nitrogen source have been identified. Growth is slow compared
to growth on inorganic sources of P, C or N. There is evidence from
the field that bacterial populations adapt to the metabolism of
glyphosate. Presence of inorganic phosphate inhibits degradation of
glyphosate with some, but not all, bacteria. Biodegradation of
glyphosate may involve co-metabolism.
The most relevant laboratory experiments in which the
biodegradation in systems with water and sediment have been studied
are summarized in Table 5. These studies indicate that the rate of
biodegradation may vary substantially, depending on experimental
conditions, e.g., the availability of oxygen, temperature and type
of sediment. The time needed for 50% biodegradation of glyphosate in
the whole system of a test with water and sediment is < 14 days
under aerobic and 14-22 days under anaerobic conditions in the
laboratory.
In the experiments of PTRL East Inc. (1990a,b), less then 10%
of the applied activity was recovered in the pond water over a
period of 30 days under aerobic condition and 365 days under
anaerobic conditions. During all experiments more than 50% of the
applied activity was recovered in the sediment.
In experiments with water and their associated sediments the
amount of a.i. declines over time with a generally transient
increase of 14C-AMPA, an increase of 14CO2, and an increase of
sediment-bound residues. An exception to this pattern of
biodegradation can be observed in some aerobic and anaerobic
experiments that were performed with pond water and a silty clay
loam sediment (PTRL East Inc, 1990a,b). In this water/sediment
system the biodegradation stopped after approximately 15 days under
aerobic conditions and after approximately 150 days under anaerobic
conditions. The glyphosate residues (a.i. plus AMPA) at both time
points remained approximately 40% of the applied dose, which
indicated substantial persistence in spite of the rapid initial
degradation.
AMPA is the main metabolite of glyphosate found in both the
water column and the sediment. Maximum amounts of AMPA under both
aerobic and anaerobic conditions in the sediment were 25% of the
applied activity (PTRL East Inc., 1990a,b). These maxima were found
at 7-20 days after application. In the same experiments maximum
amounts of sediment-bound residue were 9% of the applied activity
under aerobic conditions and 4% under anaerobic conditions. These
maxima were found at the end of the experiments. The amounts of
evolved 14CO2 in these studies gradually increased in most cases
up to 24 and 35% of the applied activity after 30 days (aerobic),
and 365 days (anaerobic), respectively. This indicates substantial
differences in the mineralization rate. These differences are partly
due to the availability of oxygen, since under anaerobic conditions
the mineralization rate was slower than under aerobic conditions.
This was also found by Monsanto (1972a, 1978a). In the aerobic
experiments of Monsanto (1972a), four sediments that differed by up
to two orders of magnitude in the total number of micro-organisms
did not show substantial differences in mineralization rate.
Biodegradation studies with glyphosate in the soil under
conditions where unequivocal interpretation is justified are scarce.
Table 6 summarizes some relevant studies, indicating that the
biodegradation rate may differ substantially, depending on the
experimental conditions. The laboratory and greenhouse experiments
in Table 6 were performed with moisture contents (> 75% of the
field capacity) that were adequate for optimal biodegradation.
In most laboratory experiments the biodegradation rate of
glyphosate in soils appears to be rapid (see Table 6). Mostly
biodegradation can be described with linear first-order kinetics.
Sometimes a non-linear first-order model taking into account
spatial variability better describes the results observed (PTRL East
Inc., 1991):
C = C0 (1 + ßt)-alpha
C in this equation is the concentration in the soil at time t,
C0 the initial concentration, and alpha and ß are rate constants
reflecting spatial variability.
The main metabolite under aerobic conditions of glyphosate in
soil is AMPA. In aerobic laboratory experiments the maximum amounts
in sandy loam and silt loam were 27 and 29%, respectively, of the
applied activity. These maxima were reached 14 days after
application (PTRL East Inc., 1991). From the data of PTRL East Inc.
(1991), DT50 values for AMPA of approximately 50 days in sandy and
silty loam can be derived. That AMPA is more persistent than
glyphosate was also shown in a laboratory experiment with sandy loam
(Monsanto, 1972b). The amounts of AMPA after 111 days were 10-17% of
the applied activity. In this study, the temperature (32 °C) was
higher than in the other studies discussed above.
Some minor unidentified metabolites were quantified in an
aerobic laboratory experiment lasting 364 days with sandy loam and
silt loam (PTRL East Inc., 1991). Two unknown metabolites did not
exceed 3.5% of the applied activity, whereas some other unknown
metabolites did not exceed 1.5% each. Rueppel et al.(1977)
quantified some minor metabolites that did not exceed 1% of the
applied activity. These metabolites were
N-methylamino-methylphosphonic acid, glycine,
N,N-dimethylaminomethylphos-phonic acid, hydroxymethylphosphonic
acid, and two unknown metabolites.
In aerobic laboratory experiments, the amounts of soil-bound
residues immediately after application were 9-35% of the applied
dose, after which they showed an irregular time-course during these
experiments of approximately 112 days (Monsanto, 1972b). In general,
the initial amounts were also the maximum amounts. In other
laboratory experiments however, maximum amounts of soil-bound
residues appeared to be reached after 14 days, whereafter they
remained more or less constant or even decreased (PTRL East Inc.,
1991). These maximum amounts were 7-9% of the applied activity, and
were probably lower compared with other studies due to better
extraction procedures.
Mineralization in the soil occurs under both aerobic and
anaerobic conditions in the laboratory, although the rates may
differ greatly, apparently mainly depending on the soil respiration
rate and the temperature. When 14C-phosphonomethyl-labelled
glyphosate was applied to sandy loam and silt loam, 70-78% 14CO2
evolved during an aerobic laboratory experiment of 360 days (PTRL
East Inc., 1991). In this study the application rate was 4 mg
a.i./kg dry weight. In an aerobic laboratory study with 15 Swedish
forest soils, DT50 values based on 14CO2 evolution varied
between 6 and 200 days. Mineralization was highly correlated with
the soil respiration rate, but not with pH or organic matter content
(Torstensson & Stark, 1981). This was confirmed by Torstensson &
Stenström (1986) and Heinonen-Tanski (1989). Torstensson & Stenström
(1986) reported that glyphosate was co-metabolized. In this case,
co-metabolizing microorganisms are not supplied with energy by
biodegrading glyphosate.
Establishing the correlation between soil respiration and
mineralization requires both a standardized measurement of the
respiration rate and an accurate measurement of the actual dose that
reaches the soil (Torstensson & Stenström, 1986). In a laboratory
experiment simulating temperatures under arctic conditions in forest
soils, 51-71% of the applied activity was recovered as 14CO2 217
days after application of 14C-glyphosate. In this study the
mineralization rate was reduced 10-15 times during a temperature
decrease of 10 °C over the first part of the study. The rate
increased only 3.7-4 times with a temperature increase of 10 °C
during the second part (Heinonen-Tanski, 1989).
Glyphosate in the soil appears to be degradable by
micro-organisms in two ways (Jacob et al., 1988), as shown in
Fig. 3. One route is via the formation of AMPA and a C2 fragment
which might be glyoxylate. This scheme for degradation was proposed
by many researchers (Monsanto, 1972b; PTRL East Inc., 1991). In this
route the splitting of the C-N bond is the first step. There is,
however, another route of biodegradation via sarcosine
(N-methyl-glycine) and orthophosphate, after which sarcosine is
degraded to glycine and a one-carbon unit that eventually might form
CO2 via formaldehyde (Kishore & Jacob, 1987; Jacob et al., 1988).
In this route the splitting of the C-P bond is the first step. In
experiments with 14C-glyphosate, isolated cultures of Pseudomonas
sp. strain LBr were able to degrade glyphosate according to both
routes (Jacob et al., 1988). Approximately 5% of the applied
14C-glyphosate was not degraded via AMPA, but via sarcosine.
The growth rate of bacteria isolated from a sandy loam garden
soil that was sprayed with Tumbleweed (a garden product) was less
inhibited by technical grade glyphosate than the growth rate of
bacteria from an unsprayed control (Quin et al., 1988). This
indicated adaptation of the bacterial populations of the sprayed
site. As addition of aromatic amino acids prevented growth
inhibition in the population of the unsprayed site to a greater
extent than in the population of the sprayed site, different
mechanisms of biochemical interference were indicated. The
composition of the bacterial population on the unsprayed site was
also different from the sprayed one. Pseudomonas sp. and
lactose-fermenting bacteria could be identified in an inoculum from
the sprayed soil able to use glyphosate as a sole source of
phosphorus (Quinn et al., 1988). A different regulatory mechanism
for biodegradation in unsprayed and sprayed sites was assumed: in
the latter the aromatic amino acid pathway might be regulated by
direct control of 3-deoxy-D-arabino-heptulosonate-7-phosphate (DAHP)
by the end-products, whereas in the unsprayed site DAHP synthase
might be indirectly regulated by prephenate. Also in other
experiments bacteria were shown to use glyphosate as a sole P source
(Kishore & Jacob, 1987; Pipke & Amrhein, 1988; Weidhase et al.,
1990), thereby primarily degrading glyphosate to orthophosphate and
sarcosine, by splitting the C-P bond. In the study of Weidhase et
al. (1990), 18.2% of the applied activity was recovered as sarcosine
8 h after application of 14C-1-methyl-labelled glyphosate to a
pure culture of Pseudomonas sp. GS. This biodegradation route of
glyphosate via sarcosine was also demonstrated by Kishore & Jacob
(1987). In their experiments with glyphosate as sole P source for
Pseudomonas sp. PG2982, one hour after application of
14C-labelled glyphosate, glycine, phosphate, and a one-carbon
unit, possibly formaldehyde, were identified as metabolites. After
one hour, the 14CO2 evolution when the phosphonomethyl moiety
was labelled was substantially higher, as compared with the 1- or
2-glycine-labelled moieties. The authors suggested that the
so-called phosphate-starvation-inducible proteins, as identified by
others, might be responsible for cleaving the C-P bond. In an
experiment with pure cultures of a mutant of Arthrobacter sp.
GLP-1 able to use glyphosate as a sole P source, 90% of the applied
activity was released as orthophosphate at 240 h after application
of 14C-1-methyl-labelled glyphosate (Pipke & Amrhein, 1988).
Orthophosphate inhibited further biodegradation of glyphosate.
Flavobacterium sp. was found by Balthazor & Hallas (1986) to be
able to degrade glyphosate in spite of the presence of
orthophosphate. Liu et al. (1991) showed that 12 strains of bacteria
from the family Rhizobiaceae could degrade glyphosate present in the
medium as the sole phosphorus source; although growth of the
bacteria was slower than with inorganic phosphate. Sarcosine was the
intermediate breakdown product, indicating initial cleavage of the
C-P bond, in Rhizobium meliloti, the strain used for detailed
metabolic studies.
Table 3. Sampling, preparation, and analysis of glyphosate
Medium Sampling Preparations Derivatization Analytical Limit of Recovery Reference
volume or reagent method determinationa
weight
Air n.r. collected onto an trifluoroacetic GC-MS and approx. 0.3 94% Jauhiainen
absorption liquid; anhydride and GC-EC µg/m3 et al. (1991)
evaporation to trifluoroethanol
dryness
Cyano-bacteria 100 ml dry, resuspend in PITC HPLC with a n.r.b 78% Powell et al.
methanol/sodium - radically (1990)
acetate/ column compressed
triethylamine
Plants 5 g extraction with trifluoroacetic GC-NPD 0.03 mg/kg 72-92% Konar & Roy,
water/chloroform; anhydride and (1990)
preconcentration trifluoroethanol
and clean-up on
cation-exchange
resin
Plants 25-50 g extraction with TLC with 0.01 mg/kg n.r. Bunyathyan &
water/chloroform; ninhydrin Gevorgyan
preconcentration detection (1984)
and clean-up on
anion-exchange
and cation-exchange
resin; evaporation
to dryness
Table 3. cont'd (2)
Medium Sampling Preparations Derivatization Analytical Limit of Recovery Reference
volume or reagent method determinationa
weight
Water 250 ml extraction with o-phthalaldehyde LC 3.2 µg/litre 89% Wigfield &
dichloromethane; Lanouette
adsorption on (1990)
anion-exchange
resin
Water 25 ml extraction with FMOCCl HPLC and TLC 0.02 µg/litre 80% Gauch et al.
dichloromethane/ (1989)
2-propanol;
acidification
with H2SO4;
evaporation
to dryness
Water 1-1.5 litre no extraction; TLC with - 0.05 mg/litre n.r. Bunyathyan &
preconcentration ninhydrin Gevorgyan
and clean-up detectionc (1984)
with anion-exchange
and cation-exchange
resin
Soil 5 g extraction with trifluoroacetic GC-NPD 0.05 mg/kg 75% Roy & Konar
deionized water/ anhydride/ (1989)
H3PO4; addition trifluoroethanol
of Darco
charcoal
Table 3. cont'd (3)
Medium Sampling Preparations Derivatization Analytical Limit of Recovery Reference
volume or reagent method determinationa
weight
Soil 2 g (sandy extraction with FMOCCl HPLC 1 mg/kg 80-119% Miles & Moye,
soil); 25 g KH2PO4 (sandy soil), (1988b)
(clayish KOH (clayish soil);
soil) no clean-up
Soil, 5 g (soil); extraction with NH4OH; ninhydrin LC 0.05-0.1 73-79% Thompson
sediment, 20 g (sed); adsorption on mg/kg (soil); (soil) et al. (1989)
foliage 5 g (fol) anion-exchange resin; 0.1 mg/kg 65-84%
further clean-up with (sed); 0.3 (sed)
Dowex cation-exchange mg/kg (fol)d 81-84%
resin (fol)
Urine and 5-6 g extraction with H2O HPLC (ion n.r. 81-99% Monsanto
faeces of (only faeces); protein pair, strong (1988a)
the rat precipitation and anion and
lyophilization cation-exchange),
(only urine); LSC, 1H NMR, 31P
clean-up with C18 NMR, GC/MS
column
Urine n.r. adsorption on trifluoroacetic GC-MS and 0.1 mg/litre n.r. Jauhiainen
(human anion-exchange resin anhydride/ GC-EC et al. (1991)
male) (SAX); elution of the trifluoroethanol
resin with HCl;
evaporation to
dryness
Table 3. cont'd (4)
Medium Sampling Preparations Derivatization Analytical Limit of Recovery Reference
volume or reagent method determinationa
weight
Serum 0.5 ml extraction with p-toluene HPLC with UV n.r.e n.r Tomita et
(human) trichloroacetic sulfonyl chloride detection al. (1991)
acid; adsorption
on anion-exchange
resin; elution
with HCl; evaporation
to dryness
a In no study with a non-liquid medium was it reported whether the limit of determination was based on dry or fresh weight,
except in the study of Thompson et al. (1989).
b The order of magnitude was reported to be picomol.
c The use of TLC with ninhydrin, copper nitrate and rhodamine B detection is reported for glyphosate in distilled water in
Ragab (1978).
d The limits of determination in soil, sediment, and foliage are expressed per kg dry weight.
e Only the limit of detection was reported: 0.3 mg/litre (approximately 75% recovery).
PITC = phenylisothiocyanate; FMOCCl = 9-fluorenyl-methyl chloroformate; GC =gas chromatography;
(HP)LC = (high-performance) liquid chromatography; TLC = thin layer chromatography; MS = mass spectroscopy;
EC = electron capture detector; NPD = nitrogen-phosphorus detector; n.r. = not reported;
LSC = liquid scintillation counting; NMR = nuclear magnetic resonance; sed = sediment; fol = foliage
The second preparative step is the clean-up, which may include
extraction, preconcentration by evaporation, ion-exchange
chromatography or gel chromatography. Clean-up procedures may
involve different combinations of chromatographic techniques. In a
validation study in which plant tissues and water were analysed, a
Chelex column was combined with anion-exchange clean-up (Cowell
et al., 1986). No chromatography was included in the clean-up
procedures for analysing glyphosate and AMPA in natural waters
(Miles et al., 1986). In this procedure samples were successively
filtrated, supplied with phosphate buffer, concentrated by
evaporation, and filtrated, prior to derivatization.
Samples with urine and faeces of the rat were subjected to clean-up
with a C18 column (Monsanto, 1988a). Prior to this extraction,
proteins were precipitated and the samples were lyophilized; samples
of faeces were, however, only extracted with water.
The third preparative step is derivatization. Derivatization
with a fluorogenic reagent is common. Burns (1983), however,
developed a preparation technique without derivatization.
Derivatization prior to detection and measurement with HPLC can be
pre-column (Miles et al., 1986; Lundgren, 1986; Miles & Moye,
1988a) or post-column (Moye et al., 1983; Tuinstra & Kienhuis,
1987). 9-Fluorenylmethyl chloroformate, phenylisothiocyanate and
1-fluoro-2,4-dinitrobenzene may be used as pre-column reagents,
whereas ortho-phthalaldehyde-mercaptoethanol and ninhydrin may be
used as post-column fluorogenic reagents. With post-column
techniques, derivatives can be formed on-line, but it requires more
equipment and experience. On the other hand, pre-column techniques
are often more rapid and require less equipment and experience. In
general the facilities required for derivatization with fluorogenic
substances are very specific, and therefore not available in many
laboratories (Konar & Roy, 1990). These authors proposed
derivatization with a mixture of trifluoroacetic anhydride and
trifluoroethanol prior to analysis with gas chromatography as a
simpler, less laborious and more economical method. This proposal
referred to the determination of glyphosate and AMPA in plant
tissues. This and other recently developed techniques of clean-up
and derivatization are summarized in Table 2. These techniques are
intended to simplify and improve preparative techniques, which in
general used to be complex and costly (Marcotte et al., 1977;
Guinivan et al., 1982; Roseboom & Berkhoff, 1982; Moye et al.,
1983; Moye & Deyrup, 1984; Deyrup et al., 1985; Miles et al.,
1986; Lundgren, 1986; Miles & Moye, 1988b).
Sample preparation and derivatization, as developed by Powell
et al. (1990) for cyanobacteria without deproteinization (see
Table 3), should also be usable for plant and animal tissue. In this
case, a simple maceration step prior to ethanol extraction should be
included. Bunyathyan & Gevorgyan (1984) developed preparative
techniques for different media prior to analysis with TLC. Only
their procedures for plants and water are summarized in Table 3. The
preparative technique for soil samples was comparable with that of
Thompson et al. (1989), although samples of 25-50 g were required.
Bunyathyan & Gevorgyan (1984) also developed a method for preparing
20-litre air samples prior to TLC. They extracted the residues
collected on a filter with water before clean-up on a
cation-exchange resin.
2.5.2 Analytical methods
Various analytical methods for the determination of glyphosate
have been described, including thin-layer chromatography (Young
et al., 1977; Ragab, 1978; Bunyathyan & Gevorgyan, 1984),
colorimetry (Glass, 1981), differential pulse polarography (Friestad
& Bronstad, 1985), gas chromatography (Guinivan et al., 1982; Moye
& Deyrup, 1984; Deyrup et al., 1985), high-performance liquid
chromatography (Miles & Moye, 1988a; Powell et al., 1990), and
31P NMR (Dickson et al., 1988). Some of these techniques, their
analytical recoveries and limits of determination are listed in
Table 3. The corresponding determination limits for AMPA, i.e.
analysed with the same techniques, are listed in Table 4. Recoveries
in the different media appear to be higher for glyphosate than for
AMPA. This is probably due to optimization of the systems for
glyphosate, as was done by Thompson et al.(1989).
Table 4. Limits of determination of AMPA
Medium Limit of Recovery Reference
determination
Plants 0.01 mg/kg 61-73% Konar & Roy (1990)
Water 1.2 µg/litre 86% Wigfield & Lanouette (1990)
Soil 0.01 mg/kg 66% Roy & Konar (1989)
Soil 0.03-0.05 mg/kg 58-68% Thompson et al. (1989)
Sediment 0.03 mg/kg 54-67% Thompson et al. (1989)
Foliage 0.008 mg/kg 55-70% Thompson et al. (1989)
Urine (human) 0.05 mg/litre n.r. Jauhiainen et al. (1991)
Serum (human) n.r.a n.r. Tomita et al. (1991)
a n.r. = not reported; only the limit of detection was reported: 0.2 mg/litre
(approximately 88% recovery)
TLC techniques are generally based on silica gel or cellulose
plates; cellulose plates give a better separation (Dubelman, 1988).
Ninhydrin and phosphate sensitive reagents may be used for
detection, although interference from co-extractives may occur.
According to Dubelman (1988), fluorogenic reagents may be preferable
in case of interference.
Fluorogenic derivatives can be determined in HPLC analysis with
fluorescence detectors (Wigfield & Lanouette, 1990) and also with a
spectrophotometer (Powell et al., 1990). In a GC analysis a
nitrogen-phosphorus, electron capture or a flame photometric
detector can be used.
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1 Anthropogenic sources
3.1.1 Production levels and processes
No data on the world production of glyphosate and its
formulations are available. In addition, no data on losses to the
environment during normal production and formulation or accidental
losses have been reported.
The first phase of the production of glyphosate consists of
refluxing a mixture of glycine (50 parts), chloromethylphosphonic
acid (92 parts), an aqueous solution with 50% sodium hydroxide (150
parts), and water (100 parts) in a suitable reaction vessel. Another
50 parts of an aqueous solution with 50% sodium hydroxide are added
to maintain the pH between 10 and 12, whereafter the reaction
mixture is refluxed for another 20 h. The mixture is then cooled to
room temperature and filtered. After adding 160 parts of
concentrated hydrochloric acid, this mixture is again filtered.
Glyphosate will slowly precipitate in the filtrate (IRPTC, 1991).
3.1.2 Uses
Glyphosate is a post-emergent, systemic and non-selective
herbicide intended for use against deep-rooted perennial species,
and also biennial and annual broad-leaved, grass and sedge species
(WSSA, 1983; Monsanto, personal communication to the IPCS, 1991).
Glyphosate is used in both agriculture and forestry. Fields of
agricultural use include grassland renovation, horticulture,
fructiculture, arable cultivation, and rice cultivation. Use in
forestry includes the killing of fast growing competitors in conifer
plantations or conservation areas, and the treatment of tree stumps.
Glyphosate may also be used for weed killing in non-agricultural
areas such as water systems, including irrigation and temporarily
drained waters, parks, road verges and gardens.
The uses of glyphosate indicate that it can be applied in
various crops for specific purposes. The major formulation Roundup
may, for instance, be used in pre-plant treatments for seed bed
preparations, and also against bracken infestations in forestry,
against couchgrass (Elytrigia repens) infestations on pastures, in
direct treatments between rows of crops, or by direct wiping of the
leaves of the weed, assuming the weeds are taller than the existing
crop.
Glyphosate is used worldwide. In 1987, 35 160 ha of the area in
British Columbia where vegetation management activities took place
had been treated with Roundup. This was 94% of the total area where
there were such activities (Ackurst, 1989).
The application rates of glyphosate are dependent on the
formulation and type of use. In the Netherlands, recommended rates
for the application of Roundup are 0.3-2.9 kg a.i./ha. In Canada the
recommended application rates of Roundup are 1.1-1.7 kg a.i./ha for
annual weeds and 1.2-5.8 kg a.i./ha for perennial weeds. The
recommended application rates for Vision in Canadian forestry are
1.1-2.1 kg a.i./ha (Task Force on Water Quality Guidelines, 1991).
Glyphosate is generally applied as a 0.5-5% solution in water by
spraying, and as a 10-50% solution in water by wiping with, for
instance, a rope-wick (Monsanto, personal communication to the IPCS,
1991).
The timing of application is dependent on the use. Application
in late summer or autumn is recommended for use in forestry in
Canada (Hildebrand et al., 1982). Application in agriculture may
be pre- or post-harvest. In the Netherlands, for instance,
glyphosate may be applied to cereals, potatoes and asparagus
immediately (up to 7 days) before harvest, but only when the
ripening is complete. Treatment of immature crops would result in
higher residue levels, early crop desiccation and reduced yields.
Glyphosate may be applied in different ways. For large-scale
treatments aerial application can be appropriate, small-scale
treatments can be done with spraying equipment on the back or behind
vehicles, or by wiping equipment.
Aerial applications will lead to losses due to wind-drift.
Exposure of flora and fauna due to off-target deposits may take
place. These downwind deposits depend on the meteorological
conditions, the plant canopy structure and the application method,
including the release height (Payne et al., 1989; Feng et al.,
1990; Payne, 1992; Payne & Thompson, 1992). The non-volatile
tank-mix fraction and the speed of the aircraft may influence the
drop-size spectrum, and it can be expected that dispersal systems
causing relatively small droplets and having a relatively low
non-volatile fraction will cause the highest off-target deposits.
Payne (1992) assumed that the large differences in deposits in two
comparable experiments were due more to different aircraft airspeeds
than to different wind speeds. In these experiments the maximum
deposits at a downwind distance of 50 m were 19 and 3 mg a.i./m2
at aircraft airspeeds of 45 and 11-20 m/second, respectively. The
application rate in both experiments was 2.1 kg a.i./ha. In other
experiments with the same application rate, Payne & Thompson (1992)
found that the meteorological conditions had a considerable impact
on the off-target deposition up to 400 m downwind when spraying at
different wind speeds (2.2-5.7 m/second) and turbulences. The
deposits at a downwind distance of 400 m varied between 0.001 and
0.06 mg a.i./m2, whereas they varied between 0.6 and 4 mg
a.i./m2 at a downwind distance of 50 m. Remarkably, the deposition
was highest with an intermediate wind speed and intensity of
turbulence. Payne et al.(1989) investigated the deposits for
aerial applications of Roundup with different dispersal systems.
When 2.1 kg a.i./ha was applied with a helicopter in a single
crosswind swath over 100 ha, up to 13.4 mg a.i./m2 was deposited
on a downwind distance of 50 m. This maximum deposition was caused
by a D8-46 hydraulic nozzle, whereas the highest depositions with a
Thru Valve Boom and a Microfoil Boom were 2 and 0.4 mg a.i./m2,
respectively. These depositions were also found at a downwind
distance of 50 m. At the time of application the windspeed 13 m
above ground level was 0.4-0.5 m/second. Riley et al.(1991)
modelled spray deposition of glyphosate using results from
helicopter applications under semi-operational conditions. The study
was designed to test the appropriateness of a New Brunswick "buffer
zone" of 65 m to minimize the effects of spray drift. At a distance
of 65 m, it was estimated that between 3.7% and 5.6% of the nominal
spray rate was deposited.
3.1.3 Drinking-water
Appraisal
The low mobility of glyphosate in soil would indicate a
minimal potential for the contamination of drinking-water from
groundwater aquifers. The only possible source of drinking-water
contamination is, therefore, surface waters. There have been no
reported incidences of drinking-water contamination with
glyphosate.
Conventional plants for processing of drinking-water would not
remove glyphosate, but this could be achieved by coprecipitation
after adding iron salts (AMA van der Linden, personal communication
to the IPCS, 1991). Ozone, increasingly used as an alternative to
chlorine in drinking-water treatment, does effectively remove
glyphosate through the hydroxyl radical (HO. ) chain processes that
occur in most ozonated waters (Yao & Haag, 1991; Haag & Yao, 1992).
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION AND TRANSFORMATION
4.1 Transport and distribution between media
Appraisal
Following application, glyphosate selectively partitions to
particulate matter suspended in surface water or to the soil
substrate. This partitioning is usually rapid and occurred within 14
days in reported studies. The mechanism of sorption to soil is only
partially understood. Glyphosate can adsorb to soils through
phosphate binding sites. Competition with inorganic phosphate has
been demonstrated in the laboratory but not measured in the field.
Specific ions (Fe2+, Fe3+ and Al3+ ) complex glyphosate; metal
complexes with humic acids in soil may be a main binding mechanism
for glyphosate in soil. There is little reported information on
desorption from soil; the data available suggest "strong" binding.
This is supported by mobility studies which show little leaching of
glyphosate below the upper few centimetres of the soil profile. The
major metabolite, AMPA, is also retained in the upper soil layers.
There is very little information on the bioavailability of
sediment-bound glyphosate to either aquatic or terrestrial
organisms. Bioaccumulation and ecotoxicity studies have not,
generally, been performed with added sediment.
Applied glyphosate can be translocated in plants. Glyphosate
in plant foliage or leaf litter does not seem to represent a source
of contamination of aquatic systems. Animals can ingest the
herbicide residues in or on plants.
Dissipation of glyphosate from soil has been widely studied
with very variable results (DT50 between 3 and 174 days).
Biodegradation appears to be the major source of dissipation.
Run-off was minimal in experimental studies, but field results
suggest that aquatic systems may be receiving glyphosate bound to
soil particles following rainfall.
In this chapter the terms biodegradation and dissipation are
used to distinguish between the decrease of the concentration in,
for instance, the soil that is due to microbes transforming the
molecule to a smaller size (biodegradation) and the decrease of the
concentration that might be due to microbial activity but also to
other processes, e.g., sorption, leaching and run-off (dissipation).
4.1.1 Water
Glyphosate dissipates from the water with 50% dissipation times
ranging from a few days to 2 weeks (Newton et al., 1984; Monsanto
1990a; see also Table 5). These DT50 values were deduced from both
laboratory and field experiments in which sediment or suspended
particles were shown to be the major sink.
In water with a near-neutral pH, the formation of an insoluble
complex of Ca2+ with glyphosate was demonstrated in a laboratory
experiment (Subramaniam & Hoggard, 1988). It was confirmed with
X-ray powder diffraction and infrared spectra that this complex was
not an ionic salt. At a near-neutral pH, the dianionic species of
glyphosate is dominant. Insoluble complexes have also been found
with Mg2+, Fe3+ and Cu2+.
In a field experiment in a temperate coastal rainforest in
British Columbia, Canada, the highest concentration of glyphosate in
water was 162 µg/litre (Feng et al., 1990). This maximum was found
in a directly sprayed tributary 2 h after an aerial application of
Roundup at a rate of 2 kg a.i./ha. Concentrations in oversprayed
tributaries without a high cover of overhanging riparian vegetation
increased after the first rainfall. In oversprayed tributaries with
a high cover of riparian vegetation almost no residues were found.
Within 96 h after application the residues in all waters had
declined below detection limits, indicating rapid dissipation. After
rainstorms, peak concentrations of glyphosate were found in the
sediments and on suspended particles of the oversprayed tributaries,
with maximum concentrations of 7 mg a.i./kg dry weight and 0.06 µg
a.i./litre unfiltered water, respectively. The amounts in the
sediments of these waters were variable but declined over time. As
0.1-2 mg residue/kg dry weight sediment was found between 196 and
364 days after application, the residues appear to be persistent in
sediments of oversprayed waters. Feng et al.(1990) concluded,
therefore, that after rainstorms sediments appear to be the major
sink.
In another field experiment in the same forest ecosystem,
glyphosate dissipated rapidly from a small perennial, very slow
flowing stream, in a site of 8 ha aerially sprayed with Roundup at a
rate of 3.3 kg a.i./ha (Newton et al., 1984). In water, 50% of the
initial concentration had dissipated in 2 days. In sediment, maximum
concentrations of approximately 0.6 mg a.i./kg were found 14 days
after application. These were reduced to approximately 0.3 mg
a.i./kg in 28 days, and to < 0.2 mg a.i./kg in 55 days after
application. A comparable rapid dissipation from the water column
was found for small forest ponds in a boreal forest in Manitoba,
Canada, after applying Roundup at a rate of 0.9 kg a.i./ha
(Goldsborough & Beck, 1989). The highest concentration in filtered
water was 141 µg a.i./litre, within 6 h after application. The main
mechanism of dissipation was probably sorption to the sediment. This
was confirmed by additional experiments with polyethylene basins
filled with unfiltered water and sediment that were placed in the
spray zone. Without sediment, only a very small amount of the dose
actually applied had dissipated after 30 days, whereas with sediment
the initial concentrations in the water had decreased by 50%,
approximately 6 days after application.
Comparable dissipation patterns were found in a field
experiment (Monsanto, 1990a) in which Accord (30.5% a.i. w/w) was
applied at a rate of 4.2 kg Accord/ha on three forestry sites with
non-flowing pond water and flowing water. Concentrations of up to
1700 µg/litre filtered water were found in the pond water
immediately after spraying. The initial concentrations in both pond
and flowing water were reduced by 50% within 7 days. Concomitantly
initial AMPA concentrations (maximally 35 µg/litre) were reduced by
50% within the same period. In flowing water the dissipation of both
glyphosate and AMPA was even more rapid. Concentrations of
glyphosate increased up to 19 mg/kg dry weight in the sediment of
one pond 28 days after application. Concentrations of up to 1 mg/kg
of both compounds were measured in the sediments of non-flowing
ponds up to 400 days after application.
In field experiments in turbid Australian irrigation water,
glyphosate adsorbed to suspended particles at different rates,
apparently mainly depending on the application rate (Bowmer et al.,
1986). At an initial concentration of 5 mg a.i./litre, 10-16% of the
load adsorbed to suspended matter, whereas at an initial
concentration of 0.05 mg a.i./litre, 53-71% adsorbed. In more saline
water the degree of sorption was less, probably due to rapid
flocculation. Maximum adsorbed amounts were approximately 7000 mg
a.i./kg in less saline supply water, and approximately 2500 mg
a.i./kg in more saline drainage water. When a supply channel was
emptied before spraying with 3.6 kg a.i./ha for control of aquatic
weeds, and filled again with water 4 days after the treatment, the
amount in the unfiltered water used for irrigation was 7% of the
applied dose.
4.1.2 Soil sorption
Glyphosate is readily bound to many soils and clay minerals
(Sprankle et al., 1975; Hance, 1976; Glass, 1987; Miles & Moye,
1988b). In laboratory experiments in which glyphosate was added to
aqueous soil suspensions, the adsorption coefficient Ks/l was
18-377 dm3/kg in nine soils ranging from sandy loam to peat
(Hance, 1976), and 33-76 dm3/kg in three soils ranging from sandy
loam to clay loam (Glass, 1987). These Ks/l values indicate strong
sorption. In both experiments the sorption could be described by the
Freundlich equation. Glass (1987) found Ks/l values for the clay
minerals montmorillonite, illite and kaolinite of 138, 115 and 8
dm3/kg, respectively.
Table 5. Biodegradationa of technical grade glyphosate in water and sediments in the laboratory
Water type Sediment Test Sediment Organic Temperature pH of Experimental Parameter Time Reference
type type (%) matter in (°C) water time (days)
sediment (days)
(%)
Pond water silty A 17 0.9 23-25 5.9-7.0 30 DT50 14b PTRL East
clay Inc. (1990a)
loam
Pond water silty An 16 0.9 20-27 5.7-6.5 365 DT50 14c,e PTRL East
clay Inc. (1990b)
loam
Surface waterd n.r. A 9 n.r. 30 8.2-8.6 14 DT50 < 14 Monsanto
(1972a)
Lake water sandy An 33 1.4 30 6.6 42 DT50 22e Monsanto
clay (1978a)
loam
a Biodegradation in the whole system
b The biodegradation stopped after approximately 15 days
c The biodegradation stopped after approximately 150 days
d Three rivers and one lake in the USA
e Approximate value derived from data of the author(s)
A = aerobic; An = anaerobic; n.r. = not reported.
The mechanism of sorption of glyphosate to soil is only
partially understood. Several factors may be involved. The
phosphonic moiety adsorbs weakly to unoccupied phosphate binding
sites and can be displaced by phosphate (Hance, 1976). In laboratory
experiments with nine soils the author showed that sorption was
positively correlated with the unoccupied phosphate sorption
capacity, and not correlated with the total phosphate sorption
capacity, organic matter, clay, iron or aluminium content. No data
are available that confirm competition of glyphosate and phosphate
under field conditions, e.g., after application of artificial
fertiliser. Miles & Moye (1988b) suggested that the main mechanism
was probably by H-bonding and ion-exchange, as the degree of
sorption in their experiments was not correlated with cation
exchange capacity (CEC) values or surface areas. Contrary to the
results of Miles & Moye (1988b) and of Hance (1976), sorption
appeared to be correlated with CEC values and clay content in a
sorption study with clay loam, silt loam and sandy loam (Glass,
1987).
The binding is also influenced by the presence of specific
cations. Hensley et al.(1978) demonstrated that Fe2+, Fe3+
and Al3+ inactivated glyphosate much more than Ca2+, K+ and
Na+. This was confirmed by Glass (1987) and Sprankle et al.
(1975). Glass (1987) suggested that glyphosate was complexed by
cations, released from cation-saturated clays via a cation-exchange
with solution protons.
According to Heinonen-Tanski (1989), most of the soil-bound
residues of glyphosate were recovered in the fulvic acid fraction
(21-33%). Sorption of glyphosate to fulvic acids was also reported
by Madhun et al.(1986), who added 14C-glyphosate to an aqueous
soil extract (ASE) of peat. In this study sorption was mainly on ASE
fractions with a relative molecular mass ¾ 1000. Piccolo et al.
(1992) studied the interaction of glyphosate with a pure iron-humic
acid complex. Maximum adsorption values indicated that adsorption to
the complex occurred to as great an extent as to whole soils. This
suggested that humic acid complexes with polyvalent cations might
represent a main binding substrate for glyphosate in soils. There
was no desorption of bound residues of glyphosate following shaking
with 0.01 mol CaCl2/litre solution for 48 h, the maximum shaking
time for the adsorption studies.
Desorption of glyphosate with ionized water from
montmorillonite and illite needed three days before reaching an
equilibrium in a study of Miles & Moye (1988b).
It can be concluded that sorption of glyphosate can be expected
in the presence of available phosphate binding sites, the presence
of iron and aluminium (oxides or hydroxides), and appropriate
combinations of clay and organic matter.
4.1.3 Mobility in soils
In view of its Ks/l, glyphosate can be expected to be
immobile or slightly mobile in many soils. This was confirmed by
several experiments, both in the laboratory and in the field. In
thin-layer chromatography studies with sandy loam, clay loam and
sandy clay loam, the Rf values of 14C-glyphosate were 0.14-0.20
(Sprankle et al., 1975). In comparable studies with silt loam,
silty clay loam, and sandy loam Rf values were < 0.2 (Monsanto,
1972c). In a leaching study with columns of 30 cm and a high water
flux of 51 cm over less than 2 days, < 0.1-6.6% of the applied
activity was leached (Monsanto, 1978b). This experiment was
performed with eight soils, ranging from sandy loam (organic matter
content 0.7%) to volcanic ash (organic matter content 9.5%). More
than 90% of the applied activity was recovered in the upper 0-14 cm
layer.
Only one leaching study under laboratory conditions with
respect to the mobility of AMPA has been reported. In this
experiment with 30-day-old residues, < 0.1-1.6% of the applied
activity was leached over 45 days (Monsanto, 1978b). The columns
were 30 cm and the water flux over 45 days was low (17 cm). The
amount of AMPA that was recovered after 45 days in the upper 0-2 cm
layer was low (0.5-12% of the applied activity), due to a high rate
of mineralisation.
4.1.4 Dissipation from the soil in the field
Many field experiments on the dissipation of glyphosate from
the soil have been performed. Some relevant studies are summarized
in Table 6. They indicate DT50 values based on dissipation that
range from 3 to 174 days depending on edaphic and climatic
conditions. In a forest brush ecosystem in Oregon, USA, the DT50
value in loam was 29 days with and 40 days without litter (Newton
et al., 1984). In field experiments in Sweden, Roundup was sprayed
over reforestated sites (Torstensson et al., 1989). In the soils
of these sites the DT50 values were < 50 days, apparently
depending on the soil respiration rate. The dissipation consisted of
a fast first, and a much slower second phase, especially in sites in
northern Sweden, which was possibly due to a longer frost period. In
these sites 1-2% of the actually applied dose was recovered 1080
days after application. A comparable dissipation pattern was found
in a field experiment on Finnish agricultural soils (Heinonen-Tanski
et al., 1985). In this experiment 25% of the concentration in a
sandy loam 2 days after the treatment was recovered one year after
application. The application rate was 1.4 kg a.i./ha.
A study in a temperate coastal rain forest in British Columbia,
Canada, showed that, 360 days after application, 6-18% of the
initial levels was recovered (Feng et al., 1990). In this
experiment Roundup was applied at a rate of 2 kg a.i./ha. The soils
were alluvial sandy loam or sandy clay loam with highly organic
surface horizons. Some of these soils were well drained, others were
seasonally flooded. At each sampling time more than 90% of the
recovered residues was in the upper 0-15 cm layer. Under all
conditions the amount of glyphosate declined over time, whereas
there was a transient increase of AMPA.
In other field experiments on boreal forest soils, comparable
dissipation patterns were found. Stark (1983) reported DT90 values
of 30-720 days, and Roy et al.(1989b) found a DT50 value of
approximately 20 days on a sandy soil planted with jackpines (Pinus
banksiana). In the field experiments of Roy et al.(1989b),
glyphosate was detectable up to 335 days after application; almost
all residues in the sandy soil were recovered in the organic top
layer. In field experiments of Monsanto (1990a) in three forest
locations in the USA, the concentration course of glyphosate
appeared to be rather irregular, especially during the first four
months. However, 50% of the initial concentrations in the soil had
mostly dissipated within 120 days. One clear exception was a site in
Corvallis in which glyphosate increased up to 0.15 mg/kg dry weight,
180 days after application. On the same site AMPA increased up to
0.32 mg/kg, 346 days after application. The application rate in
these experiments was 4.2 kg Accord/ha.
On a clay soil of a clear-cut boreal forest area, Roy et al.
(1989b) found no dissipation of glyphosate due to run-off on a 8°
slope. In a field experiment on agricultural soils without
conventional tillage, the dissipation of glyphosate due to run-off
on 6-16° slopes was < 1% of the applied dose when 1.1-3.4 kg
a.i./ha was applied (Edwards et al., 1980). However, when 9.0 kg
a.i./ha was applied, 1.8% of the applied dose dissipated due to
run-off, mainly because of a rainstorm shortly after application.
4.1.5 Uptake and dissipation from plants
Uptake of 14C-glyphosate by leaves of trembling aspen
seedlings (Populus tremuloides) was initially rapid, after which
it slowed down (Sundaram, 1990). The seedlings were exposed to
Roundup that was dripped with a micro-applicator on some central
leaves. The application rate was 0.35 kg a.i./ha leaf surface area.
Most activity was washable from the leaves (61-77%), and 22-28% was
recovered in the treated leaves within 48 h. As only 1-10% was
recovered in the other parts of the seedlings, this indicated a
rather low translocation after absorption. A rapid uptake of
14C-glyphosate within a few hours was indicated for sugar beets
(Beta vulgaris), when applied to a mature leaf (Gougler & Geiger,
1981). 14C-glyphosate probably entered the phloem in a
non-facilitated way. The subsequent transport through the phloem
Table 6. Biodegradation and dissipation of glyphosate in soils
Soil type Compound Test Moisture Temperature pH Organic Experimental DT50 Reference
type content (°C) matter duration (days)
(%) (%) (days)
Biodegradation
Sandy loam Tgg L,A 14-16 25 7.3 2.8 360 2b PTRL East Inc. (1991)
Silt loam Tgg L,A 12-14 25 7.5 1.0 360 2b PTRL East Inc. (1991)
Dissipation
Sandy loam Tgg G 11 32 5.7 1.0 112 130b Monsanto (1972b); Rueppel
et al. (1977)
Silt loam Tgg G 11 32 6.5 1.0 112 3b Monsanto (1972b); Rueppel
et al. (1977)
Silty clay loam Tgg G 11 32 7.0 6.0 112 25-27b Monsanto (1972b); Rueppel
et al. (1977)
Sand Ru F n.r. n.r. 3.5-3.7 40 762 approx Roy et al. (1989b)
(humoferric 20a
podsol)
Sandy loam, Ru F n.r. n.r. 4.2-4.9 15-31 360 45-60b Feng & Thompson (1990)
sandy clay loam
Loam Ru F n.r. n.r. 4.0-4.7 3.8-5.2 55 29-40b Newton et al. (1984)
Loamy sand Ru F n.r. n.r. n.r. 0.8 370 3-4b Monsanto (1983a)
Sandy clay loam Ru F n.r. n.r. n.r. 7.0 370 122-174b Monsanto (1983a)
a Based on data of the author(s) b Data reported by the author(s)
L = laboratory study; F = field study; G = greenhouse study; A = aerobic; An = anaerobic;
Tgg = technical grade glyphosate; Ru = Roundup; n.r. = not reported
appeared to be according to an "intermediate permeability
mechanism". When exposed for a longer time, plants may show
substantial translocation of absorbed 14C-glyphosate, as was shown
for potatoes (Solanum tuberosum) by Smid & Hiller (1981). In the
treated leaves of the potatoes 45% of the absorbed activity was
recovered, whereas the rest was mainly translocated to the apical
meristem and the roots. Up to 5% was recovered in the mother tuber.
The degree of translocation was age-dependent, as older plants
showed less translocation than younger plants.
The uptake of glyphosate by red raspberries (Rubus strigosus)
was 9% of the amount that was deposited on the leaves after spraying
Roundup at a rate of 2 kg a.i./ha (Roy et al., 1989a). In the same
field experiment the uptake was 14% by wild blueberries (Vaccinium
myrilloides). Most glyphosate was recovered in the washings, which
was also found under laboratory conditions. The initial absorbed
amounts were 0.92-2.0 mg a.i./kg dry weight. The absorbed and
washable amounts together were reduced by 50% within 13 days in the
raspberries and within 20 days in the blueberries. AMPA was
detectable up to 33 days after application. Metabolism occurred to
only a minor extent as AMPA concentrations were less than 1.5% of
the concurrent concentrations of glyphosate (similar results were
reported by FAO/WHO, 1986b). In a field experiment by Feng &
Thompson (1990) in a temperate coastal rainforest in British
Columbia, Canada, the main target species for treatment with Roundup
were red alder (Alnus rubra) and salmonberry (Rubus spectabilis).
Immediately after spraying, the concentrations in leaf tissue were
up to 448 mg a.i./kg dry weight. Glyphosate dissipated rapidly from
the leaf litter with a DT50 value of 8-9 days. The leaf litter
included leaves directly exposed on the trees and existing leaf
litter from natural defoliation before treatment with Roundup. The
authors assumed that leaf litter of these major brush species is an
insignificant source of glyphosate input into streams or onto forest
floor, because of the fast dissipation. A rapid dissipation of
glyphosate from fresh foliage was also found in a field study
(Monsanto, 1990a) in which initial concentrations of up to 1300 mg
a.i./kg and 2.6 mg AMPA/kg decreased rapidly. A transient
accumulation of glyphosate and AMPA was found in the leaf litter on
some sites, but these amounts were reduced by approximately 90%
within 100 days.
Glyphosate dissipated completely from wild berries (Vaccinium
vitis-idaea, Vaccinium myrtilus) within one year in a field
experiment in Finland in which Roundup was applied at a rate of
0.25-2.2 kg a.i./ha with a knapsack sprayer (Siltanen et al.,
1981). Contrary to this dissipation pattern was that of glyphosate
in reindeer lichens (Cladonia rangiferina) that were sampled in
the same experimental plots. Around 270 days after application,
dose-related concentrations of glyphosate and AMPA were recovered in
lichens with maxima of 45 and 2.1 mg/kg for glyphosate and AMPA,
respectively. Approximately 390 days after application of 0.8 kg
a.i./ha, 6.4 and 0.3 mg/kg of glyphosate and AMPA were still
detectable.
4.1.6 Ingestion by animals
As the concentration in the foliage may increase up to high
amounts immediately after application, this implies the possibility
of entry into the food chain through ingestion by herbivorous or
omnivorous animals. This was confirmed by Sullivan & Sullivan (1979)
who investigated the effects of glyphosate on the feed preference
and daily chow consumption of black-tailed deer (Odocoileus
hemionus columbianus). These herbivores did not avoid eating
browse of alder (Alnus rubra) and alfalfa (Medicago sativa) that
was treated with glyphosate at a rate of 2.2 kg/ha. Sometimes the
treated alder browse was even preferred. Reindeer may be exposed to
glyphosate, since reindeer lichens, which are an important food
source, can take up a substantial amount of glyphosate (see above).
4.2 Abiotic degradation
Appraisal
Hydrolysis of glyphosate is very slow. Photodegradation in the
field may occur.
4.2.1 Hydrolytic cleavage
Hydrolysis of glyphosate in sterile buffers is very slow. After
32 days < 6.3% of the applied activity was recovered as AMPA,
after applying 14C-glyphosate at rates of 25 and 250 mg/litre to
aqueous buffer solutions of pH 3, 6 and 9 (Monsanto, 1978b). These
tests were performed at both 5 and 35 °C.
4.2.2 Photodegradation
Photochemical degradation in water may occur under both
laboratory and field conditions, mainly depending on the type of
light source. In sterile aqueous buffers of pH 5, 7, and 9, less
than 1% of the applied dose was degraded (photodecomposition of
14C-phosphonomethyl-labelled glyphosate) during 29-31 days, when
exposed to sunlight (PTRL Inc., 1990).
Lund-Hoie & Friestad (1986) exposed Roundup to several light
sources under different conditions. When exposed to UV light (lambda
= 254 nm) under laboratory conditions, concentrations of 1 and
2000 mg a.i./litre in deionized water showed DT50 values of 4 and 14
days, respectively. When exposed to sunlight under field conditions
1 mg a.i./litre in polluted water without sediment showed a much
slower decomposition (DT50 > 63 days), possibly due to pollution
preventing adequate UV penetration in the water. Polluted water with
sediments showed a rapid dissipation from water, probably due to
adsorption onto the sediments. In another field experiment 2 and
100 mg a.i./litre in deionized or polluted water without sediment
showed DT50 values of < 28 days, when exposed to sunlight. At
the low concentration the dissipation in polluted water was more
rapid than in deionized water. In the dark no dissipation occurred.
In laboratory experiments 1 mg/litre of glyphosate in
sterilized natural and deionized water showed DT50 values of 4 to >
14 days when exposed to artificial light (350-450 nm) in
photoreactors without sediment (Monsanto, 1978a). In these
experiments Ca2+ acted as a photosensitizing agent.
Photodegradation by sunlight of glyphosate applied to a soil
appeared to be an insignificant route of dissipation (PTRL Inc.,
1989). In this study, 14C-glyphosate mixed with unlabelled
glyphosate was exposed for 31 days to natural sunlight, after
application to a sandy loam at a rate of 4.5 kg a.i./ha.
Extrapolated DT50 values that were based on first-order kinetics
were 90 days in the sunlight and 96 days in the dark, indicating no
substantial degradation due to photolysis. The temperature of the
soil surface was 22-23 °C. Under unnatural light conditions
glyphosate appeared not to be photodegraded substantially (Monsanto,
1972c; Rueppel et al., 1977; Monsanto, 1978a).
4.3 Biodegradation
Appraisal
Selected studies of the biodegradation of glyphosate have been
considered; selection was on the basis of test conditions and modern
methodologies. There is considerable variation in rate of breakdown
in water, aquatic sediment and soil. Degradation occurs more rapidly
in aerobic than anaerobic conditions. Half-times for biodegradation
in the three media under laboratory conditions range between a few
days and approximately 20 days. No data on biodegradation under
anaerobic conditions are available.
The main route of biodegradation of glyphosate appears to be
by splitting the C-N bond to produce AMPA. However, a second route
with splitting of the C-P bond can also occur.
A range of bacterial strains can degrade glyphosate. Bacteria
capable of using the compound as sole phosphorus, sole carbon or
sole nitrogen source have been identified. Growth is slow compared
to growth on inorganic sources of P, C or N. There is evidence from
the field that bacterial populations adapt to the metabolism of
glyphosate. Presence of inorganic phosphate inhibits degradation of
glyphosate with some, but not all, bacteria. Biodegradation of
glyphosate may involve co-metabolism.
The most relevant laboratory experiments in which the
biodegradation in systems with water and sediment have been studied
are summarized in Table 5. These studies indicate that the rate of
biodegradation may vary substantially, depending on experimental
conditions, e.g., the availability of oxygen, temperature and type
of sediment. The time needed for 50% biodegradation of glyphosate in
the whole system of a test with water and sediment is < 14 days
under aerobic and 14-22 days under anaerobic conditions in the
laboratory.
In the experiments of PTRL East Inc. (1990a,b), less then 10%
of the applied activity was recovered in the pond water over a
period of 30 days under aerobic condition and 365 days under
anaerobic conditions. During all experiments more than 50% of the
applied activity was recovered in the sediment.
In experiments with water and their associated sediments the
amount of a.i. declines over time with a generally transient
increase of 14C-AMPA, an increase of 14CO2, and an increase of
sediment-bound residues. An exception to this pattern of
biodegradation can be observed in some aerobic and anaerobic
experiments that were performed with pond water and a silty clay
loam sediment (PTRL East Inc, 1990a,b). In this water/sediment
system the biodegradation stopped after approximately 15 days under
aerobic conditions and after approximately 150 days under anaerobic
conditions. The glyphosate residues (a.i. plus AMPA) at both time
points remained approximately 40% of the applied dose, which
indicated substantial persistence in spite of the rapid initial
degradation.
AMPA is the main metabolite of glyphosate found in both the
water column and the sediment. Maximum amounts of AMPA under both
aerobic and anaerobic conditions in the sediment were 25% of the
applied activity (PTRL East Inc., 1990a,b). These maxima were found
at 7-20 days after application. In the same experiments maximum
amounts of sediment-bound residue were 9% of the applied activity
under aerobic conditions and 4% under anaerobic conditions. These
maxima were found at the end of the experiments. The amounts of
evolved 14CO2 in these studies gradually increased in most cases
up to 24 and 35% of the applied activity after 30 days (aerobic),
and 365 days (anaerobic), respectively. This indicates substantial
differences in the mineralization rate. These differences are partly
due to the availability of oxygen, since under anaerobic conditions
the mineralization rate was slower than under aerobic conditions.
This was also found by Monsanto (1972a, 1978a). In the aerobic
experiments of Monsanto (1972a), four sediments that differed by up
to two orders of magnitude in the total number of micro-organisms
did not show substantial differences in mineralization rate.
Biodegradation studies with glyphosate in the soil under
conditions where unequivocal interpretation is justified are scarce.
Table 6 summarizes some relevant studies, indicating that the
biodegradation rate may differ substantially, depending on the
experimental conditions. The laboratory and greenhouse experiments
in Table 6 were performed with moisture contents (> 75% of the
field capacity) that were adequate for optimal biodegradation.
In most laboratory experiments the biodegradation rate of
glyphosate in soils appears to be rapid (see Table 6). Mostly
biodegradation can be described with linear first-order kinetics.
Sometimes a non-linear first-order model taking into account
spatial variability better describes the results observed (PTRL East
Inc., 1991):
C = C0 (1 + ßt)-alpha
C in this equation is the concentration in the soil at time t,
C0 the initial concentration, and alpha and ß are rate constants
reflecting spatial variability.
The main metabolite under aerobic conditions of glyphosate in
soil is AMPA. In aerobic laboratory experiments the maximum amounts
in sandy loam and silt loam were 27 and 29%, respectively, of the
applied activity. These maxima were reached 14 days after
application (PTRL East Inc., 1991). From the data of PTRL East Inc.
(1991), DT50 values for AMPA of approximately 50 days in sandy and
silty loam can be derived. That AMPA is more persistent than
glyphosate was also shown in a laboratory experiment with sandy loam
(Monsanto, 1972b). The amounts of AMPA after 111 days were 10-17% of
the applied activity. In this study, the temperature (32 °C) was
higher than in the other studies discussed above.
Some minor unidentified metabolites were quantified in an
aerobic laboratory experiment lasting 364 days with sandy loam and
silt loam (PTRL East Inc., 1991). Two unknown metabolites did not
exceed 3.5% of the applied activity, whereas some other unknown
metabolites did not exceed 1.5% each. Rueppel et al.(1977)
quantified some minor metabolites that did not exceed 1% of the
applied activity. These metabolites were
N-methylamino-methylphosphonic acid, glycine,
N,N-dimethylaminomethylphos-phonic acid, hydroxymethylphosphonic
acid, and two unknown metabolites.
In aerobic laboratory experiments, the amounts of soil-bound
residues immediately after application were 9-35% of the applied
dose, after which they showed an irregular time-course during these
experiments of approximately 112 days (Monsanto, 1972b). In general,
the initial amounts were also the maximum amounts. In other
laboratory experiments however, maximum amounts of soil-bound
residues appeared to be reached after 14 days, whereafter they
remained more or less constant or even decreased (PTRL East Inc.,
1991). These maximum amounts were 7-9% of the applied activity, and
were probably lower compared with other studies due to better
extraction procedures.
Mineralization in the soil occurs under both aerobic and
anaerobic conditions in the laboratory, although the rates may
differ greatly, apparently mainly depending on the soil respiration
rate and the temperature. When 14C-phosphonomethyl-labelled
glyphosate was applied to sandy loam and silt loam, 70-78% 14CO2
evolved during an aerobic laboratory experiment of 360 days (PTRL
East Inc., 1991). In this study the application rate was 4 mg
a.i./kg dry weight. In an aerobic laboratory study with 15 Swedish
forest soils, DT50 values based on 14CO2 evolution varied
between 6 and 200 days. Mineralization was highly correlated with
the soil respiration rate, but not with pH or organic matter content
(Torstensson & Stark, 1981). This was confirmed by Torstensson &
Stenström (1986) and Heinonen-Tanski (1989). Torstensson & Stenström
(1986) reported that glyphosate was co-metabolized. In this case,
co-metabolizing microorganisms are not supplied with energy by
biodegrading glyphosate.
Establishing the correlation between soil respiration and
mineralization requires both a standardized measurement of the
respiration rate and an accurate measurement of the actual dose that
reaches the soil (Torstensson & Stenström, 1986). In a laboratory
experiment simulating temperatures under arctic conditions in forest
soils, 51-71% of the applied activity was recovered as 14CO2 217
days after application of 14C-glyphosate. In this study the
mineralization rate was reduced 10-15 times during a temperature
decrease of 10 °C over the first part of the study. The rate
increased only 3.7-4 times with a temperature increase of 10 °C
during the second part (Heinonen-Tanski, 1989).
Glyphosate in the soil appears to be degradable by
micro-organisms in two ways (Jacob et al., 1988), as shown in
Fig. 3. One route is via the formation of AMPA and a C2 fragment
which might be glyoxylate. This scheme for degradation was proposed
by many researchers (Monsanto, 1972b; PTRL East Inc., 1991). In this
route the splitting of the C-N bond is the first step. There is,
however, another route of biodegradation via sarcosine
(N-methyl-glycine) and orthophosphate, after which sarcosine is
degraded to glycine and a one-carbon unit that eventually might form
CO2 via formaldehyde (Kishore & Jacob, 1987; Jacob et al., 1988).
In this route the splitting of the C-P bond is the first step. In
experiments with 14C-glyphosate, isolated cultures of Pseudomonas
sp. strain LBr were able to degrade glyphosate according to both
routes (Jacob et al., 1988). Approximately 5% of the applied
14C-glyphosate was not degraded via AMPA, but via sarcosine.
The growth rate of bacteria isolated from a sandy loam garden
soil that was sprayed with Tumbleweed (a garden product) was less
inhibited by technical grade glyphosate than the growth rate of
bacteria from an unsprayed control (Quin et al., 1988). This
indicated adaptation of the bacterial populations of the sprayed
site. As addition of aromatic amino acids prevented growth
inhibition in the population of the unsprayed site to a greater
extent than in the population of the sprayed site, different
mechanisms of biochemical interference were indicated. The
composition of the bacterial population on the unsprayed site was
also different from the sprayed one. Pseudomonas sp. and
lactose-fermenting bacteria could be identified in an inoculum from
the sprayed soil able to use glyphosate as a sole source of
phosphorus (Quinn et al., 1988). A different regulatory mechanism
for biodegradation in unsprayed and sprayed sites was assumed: in
the latter the aromatic amino acid pathway might be regulated by
direct control of 3-deoxy-D-arabino-heptulosonate-7-phosphate (DAHP)
by the end-products, whereas in the unsprayed site DAHP synthase
might be indirectly regulated by prephenate. Also in other
experiments bacteria were shown to use glyphosate as a sole P source
(Kishore & Jacob, 1987; Pipke & Amrhein, 1988; Weidhase et al.,
1990), thereby primarily degrading glyphosate to orthophosphate and
sarcosine, by splitting the C-P bond. In the study of Weidhase et
al. (1990), 18.2% of the applied activity was recovered as sarcosine
8 h after application of 14C-1-methyl-labelled glyphosate to a
pure culture of Pseudomonas sp. GS. This biodegradation route of
glyphosate via sarcosine was also demonstrated by Kishore & Jacob
(1987). In their experiments with glyphosate as sole P source for
Pseudomonas sp. PG2982, one hour after application of
14C-labelled glyphosate, glycine, phosphate, and a one-carbon
unit, possibly formaldehyde, were identified as metabolites. After
one hour, the 14CO2 evolution when the phosphonomethyl moiety
was labelled was substantially higher, as compared with the 1- or
2-glycine-labelled moieties. The authors suggested that the
so-called phosphate-starvation-inducible proteins, as identified by
others, might be responsible for cleaving the C-P bond. In an
experiment with pure cultures of a mutant of Arthrobacter sp.
GLP-1 able to use glyphosate as a sole P source, 90% of the applied
activity was released as orthophosphate at 240 h after application
of 14C-1-methyl-labelled glyphosate (Pipke & Amrhein, 1988).
Orthophosphate inhibited further biodegradation of glyphosate.
Flavobacterium sp. was found by Balthazor & Hallas (1986) to be
able to degrade glyphosate in spite of the presence of
orthophosphate. Liu et al. (1991) showed that 12 strains of bacteria
from the family Rhizobiaceae could degrade glyphosate present in the
medium as the sole phosphorus source; although growth of the
bacteria was slower than with inorganic phosphate. Sarcosine was the
intermediate breakdown product, indicating initial cleavage of the
C-P bond, in Rhizobium meliloti, the strain used for detailed
metabolic studies.
 Carlisle & Trevors (1986a) deduced from their experiments that
nitrate-reducing bacteria are involved in metabolizing glyphosate.
Involvement of nitrifying bacteria in the biodegradation of
glyphosate was also demonstrated by Murthy et al. (1989), when they
investigated the treatment of waste water from a Roundup formulating
factory.
Pseudomonas sp. may use glyphosate as a sole P or C source,
as demonstrated by Weidhase et al. (1990). Only slight growth of the
wild-type strain of the bacterium Pseudomonas fluorescens was
observed with glyphosate as sole carbon or nitrogen source. The
herbicide was metabolized to aminomethylphosphonate (Zboinska
et al., 1992). Murthy et al. (1989) isolated a denitrifying
bacterial species that was also able to use glyphosate as a C
source. This species was isolated from activated sludge in a
waste-water treatment plant. A mutant of Arthrobacter sp. strain
GLP-1 was able to utilize glyphosate as a sole N source, whereas
this was not possible for the normal strain (Pipke & Amrhrein,
1988), probably due to the uptake of inorganic P released during
biodegradation.
As the Biological Oxygen Demand and the Chemical Oxygen Demand
of glyphosate are < 2 mg/g and 0.53 g/g, respectively, glyphosate
cannot be considered as readily biodegradable (LISEC, 1990a,b). In
suitable systems, however, glyphosate is biodegradable, as shown by
Murthy et al. (1989), who investigated the biodegradation of
glyphosate in waste-water treatment plants under different
conditions in sequencing batch reactors on a laboratory scale. These
reactors were fed with waste water from a Roundup manufacturing
facility. Glyphosate was degraded completely within one cycle of
24 h, independent of whether there was an initial aerated or anoxic
phase of 4 h. However, more glyphosate could be processed with an
anoxic initial phase, probably due to better conditions for
denitrification. Not only denitrifiers but also ammonifiers and
nitrifiers appeared to be involved in the biodegradation of
glyphosate. Only at the very high concentration of approximately
5000 mg a.i./litre was biodegradation repressed by non-glyphosate
COD and inhibited by excess ammonia production.
Pseudomonas sp. strain LBr, Flavobacterium sp. and a
denitrifying bacterial species were isolated from activated sludge
as species with the ability to use glyphosate as a P source
(Balthazor & Hallas, 1986; Jacob et al., 1988; Murthy et al., 1989).
The denitrifier was also able to use glyphosate as a sole C source.
Flavobacterium sp. degraded glyphosate to AMPA in both the
presence and absence of PO43- (Balthazor & Hallas, 1986). In
this experiment the further degradation of AMPA appeared to be
hampered in the presence of PO43-.
Pseudomonas sp. strain LBr was capable of completely
eliminating amounts of glyphosate up to 3212 mg/litre from a growth
medium (Jacob et al., 1988).
Continuous exposure of an activated sludge treatment system in
a pilot plant increased the ability of the sludge to metabolize
glyphosate to AMPA (Hallas et al., 1992). In this trial an influent
concentration of 50 mg a.i./litre was reduced to less than 5 mg
a.i./litre under continuous-flow conditions with an average
residence time of 10 min. The sludge was inoculated with immobilized
bacteria capable of degrading glyphosate. The effectiveness of the
treatment was dependent on the presence of a nitrogen source and a
non-glyphosate carbon source, and required a pH range of 6.0 to 8.0.
No data are available on the amounts of glyphosate that can be
eliminated in conventional waste-water treatment plants under
practical conditions. In waste water from glyphosate-producing
plants, 28-45% is reported to be eliminated through biological
treatment (Task Force on Water Quality Guidelines, 1991).
No data are available on the biodegradability of the
surfactants in formulations. It is, however, probable that
polyoxyethylene amine is biodegraded fairly rapidly in view of the
biodegradability of structurally related compounds (Swisher, 1987).
4.4 Bioaccumulation
Appraisal
Glyphosate is not expected to bioaccumulate in view of its
high water solubility and its ionic character. This was confirmed by
several laboratory experiments with fish, crustaceans and molluscs
and by field experiments.
In a static test, channel catfish (Ictalurus punctatus) were
exposed to 0.94-0.99 mg 14C-labelled a.i./litre (actual
concentrations) for 10 days (ABC Inc, 1981d; Monsanto, 1981a). Of
the absorbed amount, 76% was recovered in the viscera. More than 90%
of the extractable residues in the viscera and the fillet was
identified as glyphosate, whereas less than 2% was identified as
AMPA. After 10 days of depuration 80% of the absorbed activity was
eliminated. For exposed channel catfish the calculated
bioconcentration factor based on the activity absorbed by the whole
fish was 0.27. For depurated channel catfish the calculated
bioconcentration factor was 0.052.
The marsh clam (Rangia cuneata) and crayfish (Procambarus
simulans) were exposed in static tests lasting 28 days to
synthetic uncontaminated sea water and a sandy loam sediment that
was incorporated with 3 mg 14C-labelled a.i./kg (ABC Inc.,
1982d,e). These experiments were set up to assess the degree of
bioconcentration of glyphosate when used in flooded rice levees and
tidal water. The calculated bioconcentration factor for the edible
parts of the clam increased during exposure up to 4.8, whereas for
the whole crayfish it increased up to 12. The highest concentrations
in the edible parts of the clam and the whole crayfish were 0.3 mg
14C-labelled residues/kg for both. After 28 days of depuration 48%
of the accumulated residues were eliminated from the edible parts of
the clam. The concentration in these parts was then 0.16 mg
14C-residues/kg. The crayfish finally had eliminated 91% after 14
days of depuration. The concentration in the whole crayfish was then
0.02 mg 14C-residues/kg. It must be stated that this test refers
to the accumulation of 14C and not glyphosate.
In a static test without sediment, in which rainbow trout
(Salmo gairdnerii) were exposed to 2 mg a.i./litre (nominal
concentration) for 12 h, the fillets of the fish contained 80 µg
a.i./kg (in the original article the erroneous figure of 80 mg/kg
was reported), and the eggs 60 µg a.i./kg (Folmar et al., 1979).
This indicates a bioconcentration factor of 0.04 for the edible
parts. Roundup was applied in this test.
In a flow-through test in which bluegill sunfish (Lepomis
macrochirus) were exposed to 11-13 mg 14C-labelled a.i./litre
(actual concentrations) for 35 days, calculated daily
bioconcentration factors based on the whole fish increased from <
0.1, 0.2 days after the start of the test, to 0.4-0.5 at the end
(ABC Inc., 1989f). Maximum concentrations in the whole fish, viscera
and fillet were 13, 7.6 and 4.8 mg 14C-residues/kg, respectively.
The time required to reach 90% of the steady state and the uptake
rate constant were calculated to be 120 days and 0.02 mg/kg fish x
(mg/litre water)-1 x day-1, respectively. During 21 days of
depuration, the half-life of depuration was calculated to be 35. A
slow decrease in tissue concentration during depuration was
indicated. After the period of depuration 2.2 mg 14C-residues/kg
whole fish was still present. In an additional study to characterize
the 14C-residues, 95-97% of the residues in the water was
glyphosate, whereas in the whole fish and tissues 28-91% of the
recovered activity was glyphosate (ABC Inc., 1989g). In a whole fish
sample 21 days after starting the test, 49% of the recovered
activity was found to be AMPA. By treating homogenates with
proteinase K it was indicated that a substantial amount of the
absorbed residues was tightly associated with, or incorporated into,
protein.
In a field experiment in a forest ecosystem in Oregon, USA,
neither glyphosate nor AMPA were recovered in salmon fingerlings
(Oncorhynchus kisutch) after aerial application of Roundup at a
rate of 3.3 kg a.i./ha (Newton et al., 1984). The fingerlings were
released at the downstream edge of the sprayed site and analysed up
to 55 days after treatment. Glyphosate was not recovered in carp
(Cyprinus carpio) in a field experiment in which ponds were
sprayed with Roundup at rates of 1.3-1.4 kg a.i./ha (Monsanto,
1980). In this experiment of approximately 90 days, AMPA was not
recovered until 30 days after application. It then increased up to
0.21 mg/kg whole fish, remained constant for another 30 days, and
then decreased to around the limit of determination (0.1 mg/kg) at
the end of the experiment.
In a forest ecosystem in Oregon, USA, Roundup was aerially
applied at a rate of 3.3 kg a.i./ha (Newton et al., 1984).
Concentrations in mammals were of the same order of magnitude as the
concentrations in litter and ground cover. The concentrations of
glyphosate in the viscera of herbivorous small mammals decreased
more slowly than in omnivorous and carnivorous small mammals, which
was probably due to a higher ingestion of contaminated litter. The
highest concentration was found in the viscera of omnivorous
deermice (Peromysces maniculatus) immediately after spraying: 5 mg
a.i./kg. Only small traces of AMPA were found in mammalian viscera.
4.5 Waste disposal
Small amounts of glyphosate can be disposed of by mixing with
alkali and soil prior to burial in a pit or trench, whereas large
amounts should be incinerated in units equipped with effluent gas
scrubbing (IRPTC, 1991).
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
Appraisal
The low toxicity, low volatility and low body absorption of
glyphosate makes its application by backpack sprayer safe under
field condition provided that the worker wears full protective
clothing.
5.1 Environmental levels
A synopsis of concentrations of glyphosate is tabulated in
Table 7. Measurements as part of regular monitoring programmes are
very scarce; measurements in field experiments with recommended
application rates simulating common agricultural practice are
therefore included in Table 7. Only maximum amounts are tabulated as
indicative values, since the rate at which they dissipate is not
included here (see sections 4.1 and 4.3). Data on the occurrence of
glyphosate and AMPA in sewage sludge are not available.
In biota the highest concentrations of glyphosate and AMPA were
found in fresh foliage and reindeer lichen (Cladonia rangiferina).
In abiota the highest concentrations of both compounds were found in
the soil (see Table 7). The occurrence of glyphosate in the
groundwater of Texas, USA, was reported by Hallberg (1989), but the
measured concentration and the year of measurement were not
specified.
Use of glyphosate as a herbicide may result in the presence of
residues in crops and animal tissues destined for human consumption.
Application as a herbicide may also be responsible for the presence
of glyphosate in drinking-water. Direct measurements of glyphosate
in foodstuffs (as part of food surveillance), drinking-water or
total diets have not been carried out. The only information
available comes from controlled residue studies. With technical
glyphosate formulated as the isopropylamine salt in aqueous
solution, numerous residue studies have been carried out in
vegetables, grasses, oil seeds, mammalian products, poultry products
and primary feed commodities. The results are summarized in the
various reports of the FAO/WHO Joint Meeting on Pesticide Residues
(FAO/WHO, 1986a, 1987, 1988). For detailed information on these
studies the reader is referred to these reports. The appraisals made
by the JMPR included the following more general statements.
Pre-harvest (5-14 days) application of glyphosate (isopropylamine
salt) in the cultivation of cereals results in significant residues
in the grain and plant materials. Studies are available to show the
fate of glyphosate in milling, baking and brewing. Residue levels in
white flour were approximately 10-20% of the levels in wheat, while
the bran residue levels were 2 to 4 times as high as those in the
wheat. Glyphosate residues were not lost during baking, but residue
levels decreased when bread was made from flour because of dilution.
Glyphosate residue levels in malt and beer derived from
field-treated barley were, respectively, about 25% and 4% of the
original level in the barley. Some glyphosate is lost during
washing, but most of the decrease can be attributed to dilution. The
levels in groats (processed oats) were about 50% of the levels in
the pre-harvest-treated oats. In all these cases, AMPA contributed
only a small proportion (average < 2.5%) of the total residues
(FAO/WHO, 1986a, 1987).
When administered to animals glyphosate is rapidly excreted
without degradation. Residues in cattle, pig and poultry meat, eggs
and milk were negligible after the animals were fed with a diet
containing 100 mg/kg glyphosate and aminoglyphosate acid. The
highest residues were in pig liver and kidney (up to 0.16 and
0.91 mg/kg, respectively) and cattle kidney (up to 1.4 mg/kg).
Residues in animal feeds arising from pre-harvest glyphosate
applications to cereals will result in only low residues in meat,
milk and eggs (FAO/WHO, 1986a).
On the basis of these residue studies the JMPR has estimated
the maximum residue levels that are likely to occur when glyphosate
(as isopropylamine salt) is used in practice, and recommended these
levels as Maximum Residue Limits (MRLs). These MRLs are presented in
FAO/WHO (1986a, 1987, 1988).
5.2 General population exposure
Apart from the controlled residue studies mentioned above, no
data are available.
Table 7. Maximum concentrations of glyphosate in environmental air, water, soil, sediment and biota
Sample Compound Location Yeara Concentration Reference
Netherlands
Surface water glyphosate Drentsche Aa 1988-1989 0.5-1 µg/litreb (Soppe, personal
Surface water AMPA Drentsche Aa 1988-1989 6 µg/litreb communication to the IPCS, 1991)
Finland
Loam soil (agric.) glyphosate Kettula 1978 17 mg/kg d.w. Müller et al. (1981)
Loam soil (agric.) AMPA Kettula 1978 3.2 mg/kg d.w. Müller et al. (1981)
Silt soil (agric.) glyphosate Kettula 1978 3.8 mg/kg d.w. Müller et al. (1981)
Silt soil (agric.) AMPA Kettula 1978 0.4 mg/kg d.w. Müller et al. (1981)
Wild berries glyphosate Laukaa, Konnevesi 1977 1.6-2.1 mg/kgc Siltanen et al. (1981)
Wild berries AMPA Laukaa, Konnevesi 1977 0.02-0.07 mg/kgc Siltanen et al. (1981)
Reindeer lichen glyphosate Laukaa, Konnevesi 1976 45 mg/kgc Siltanen et al. (1981)
Reindeer lichen AMPA Laukaa, Konnevesi 1976 2.1 mg/kgc Siltanen et al. (1981)
Canada
Wild berries glyphosate Harker, Lamplugh 1985 8-19 mg/kg f.w. Roy et al. (1989a)
Wild berries AMPA Harker, Lamplugh 1985 0.06-0.1 mg/kg f.w. Roy et al. (1989a)
Surface waterd glyphosate Carnation Creek 1984 162 µg/litre Feng et al. (1990)
Surface watere glyphosate Carnation Creek 1984 < 1 µg/litre Feng et al. (1990)
Surface waterd AMPA Carnation Creek 1984 approx 3 µg/litre Feng et al. (1990)
Pond water glyphosate Manitoba 1986 141 µg/litre Goldsborough & Beck (1989)
Pond water AMPA Manitoba 1986 2.2 µg/litre Goldsborough & Beck (1989)
Sedimentd glyphosate Carnation Creek 1984 6.8 mg/kg d.w. Feng et al. (1990)
Suspended sediment glyphosate Carnation Creek 1984 0.06 µg/litre Feng et al. (1990)
Soil glyphosate Carnation Creek 1984 40 mg/kg d.w. Feng & Thompson (1990)
Soil AMPA Carnation Creek 1984 9 mg/kg d.w. Feng & Thompson (1990)
Table 7. (cont'd)
Sample Compound Location Yeara Concentration Reference
Canada (cont'd)
Foliage (fresh) glyphosate Carnation Creek 1984 261-448 mg/kg d.w. Feng & Thompson (1990)
Foliage (fresh) AMPA Carnation Creek 1984 < 9 mg/kg d.w. Feng & Thompson (1990)
Topcrown foliage glyphosate Oregon Coast Range 1978 489 mg/kgc Newton et al. (1984)
Topcrown foliage AMPA Oregon Coast Range 1978 2.1 mg/kgc Newton et al. (1984)
Deermice (viscera) glyphosate Oregon Coast Range 1978 5.1 mg/kgc Newton et al. (1984)
USA
Pond water glyphosate Chassell, Corvallis, 1987 90-1700 µg/litre Monsanto (1990a)
Cuthbert
Pond water AMPA Chassell, Corvallis, 1987 2-35 µg/litre Monsanto (1990a)
Cuthbert
Stream water glyphosate Chassell, Corvallis, 1987 35-1237 µg/litre Monsanto (1990a)
Cuthbert
Stream water AMPA Chassell, Corvallis, 1987 < 1.0-10 µg/litre Monsanto (1990a)
Cuthbert
Pond sediment glyphosate Chassell, Corvallis, 1987 0.26-19 mg/kg d.w. Monsanto (1990a)
Cuthbert
Pond sediment AMPA Chassell, Corvallis, 1987 0.13-1.8 mg/kg d.w. Monsanto (1990a)
Cuthbert
Stream sediment glyphosate Chassell, Corvallis, 1987 0.11-0.69 mg/kg d.w. Monsanto (1990a)
Cuthbert
Stream sediment AMPA Chassell, Corvallis, 1987 < 0.05-0.38 mg/kg d.w. Monsanto (1990a)
Cuthbert
Soil (no litter on it) glyphosate Chassell, Corvallis, 1987 0.15-4.7 mg/kg d.w. Monsanto (1990a)
Cuthbert
Table 7. (cont'd)
Sample Compound Location Yeara Concentration Reference
USA (cont'd)
Soil (no litter on it) AMPA Chassell, Corvallis, 1987 0.18-0.51 mg/kg d.w. Monsanto (1990a)
Cuthbert
Soil (litter on it) glyphosate Chassell, Corvallis, 1987 0.07-1.4 mg/kg d.w. Monsanto (1990a)
Cuthbert
Soil (litter on it) AMPA Chassell, Corvallis, 1987 0.14-0.68 mg/kg d.w. Monsanto (1990a)
Cuthbert
Foliage (fresh) glyphosate Chassell, Corvallis, 1987 650-1300 mg/kgb Monsanto (1990a)
Cuthbert
Foliage (fresh) AMPA Chassell, Corvallis, 1987 1.7-2.6 mg/kgb Monsanto (1990a)
Cuthbert
a Sampling invariably took place during the autumn period.
b Analytical procedure was unvalidated; the water was sampled at an inlet point of
a drinking-water processing facility
c It was not reported whether values were based on dry or fresh weight
d Water unbuffered by vegetation
e Water buffered by vegetation
5.3 Occupational exposure during manufacture, formulation or use
In the study of Monsanto (1977), worker exposure to Roundup was
measured during herbicide mixing and application operations and upon
re-entry of treated fields. The formulation was applied on separate
plots using three application devices, i.e. a broadcast boom
sprayer, a handgun broadcast sprayer and a backpack/hand-gun
sprayer. Exposure time during mixing was < 5 min; during
application this was about 45 to 60 min. Inhalational exposure
during mixing and spraying was determined using a sampling device
placed close to the applicator's face; the total air volume sampled
was 15-20 times the daily ventilation volume. Dermal exposure during
mixing and spraying was monitored by determination of deposition on
gauze pads placed outside or inside the operator's clothing on
different parts of the body. Operator exposure (inhalation and skin,
determination method as above) upon re-entry was determined at 1, 3
and 7 days after application. In one treated plot, this was done by
having operators walk through the plot for 28 to 44 min; in two
other plots a dummy sampling device was used to determine exposure
upon re-entry. Using the measured glyphosate residues total worker
exposure was estimated. The results are presented in Table 8.
Table 8. Applicator/worker exposure to glyphosatea
Operation Average dermal Average inhalation Total exposure
exposure exposure (µg/h)
(µg/h) (µg/h)b
Tank filling 805.1 17.9 823.0
Boom spraying 271.4 0.19 271.59
Handgun spraying 7957.0 2.47 7959.47
Backpack spraying 3619.0 0.92 3619.92
Field re-entry:
1 day after treatment 2046.6 4.58 2051.18
3 days after treatment 2919.7 0.12 2919.82
7 days after treatment 15.9 0.12 16.02
a From: Monsanto (1977)
b Calculation based on an estimated breathing rate of 1.8 m3/h
Monsanto (1990) conducted another collaborative study at three
sites maintained by the USDA Forestry Service near Clayton, Georgia;
the Savanah River Plant, South Carolina; and Edgefield, South
Carolina. Glyphosate was being used to control vegetative growth
around pine seedlings planted in clear-cut forest areas. The workers
were biologically monitored by analysis of collected composite urine
specimens. Additionally, dermal/clothing deposition and simulated
inhalation exposure were monitored by passive dosimetry with cotton
cloth patches, hand rinses and air filters. At the three sites,
exposure of workers to glyphosate using backpack sprayers while
performing their duties under normal use conditions was monitored.
The analytical results indicated that the majority of urine
composite samples had unmeasurable residues of glyphosate.
Deposition on air filters, patches and hands from measurement of
washes, however, indicated a small amount of body exposure. It was
concluded that penetration of clothing did not exceed 3.84% and thus
clothing produced 96.2% protection. Body burden, as shown in urine
samples, was extremely low and in most cases below the detection
limit.
A new Monsanto study was conducted for the assessment of worker
exposure to glyphosate during mist blower application of Roundup
herbicide. Exposure was determined by a passive dosimetry technique
while workers sprayed weeds around palm trees in a plantation in
Malaysia. The workers were fitted with gauze patches at different
locations on their clothing. Air sampling was performed in the
breathing zone and the workers hands were washed at the end of the
day. The passive dosimetry body dose estimates were calculated for a
fully clothed worker with a long-sleeved shirt, long pants and
rubber boots. The hand exposure would account for bare hands during
the loading and spraying operations. Passive dosimetry estimates for
the four replicates, corrected for clothing and dermal penetration,
transport/storage/analytical recovery and normalized for body weight
and amount of chemical handled, averaged 1.88 µg/kg body weight per
kg a.i. This is little higher than the passive dosimetry estimates
of forestry workers who applied Roundup with knapsack sprayers,
which was 1.75 µg/kg body weight per kg a.i. Thus, it can be
concluded that workers applying Roundup herbicide with mist blowers,
experience some dermal exposure. In addition, inhalation exposure to
glyphosate may be higher during mist blower application. However,
Roundup demonstrated essentially no volatility. The only possible
route would be via air-borne particles. The actual amount of
glyphosate absorbed through inhalation would be much lower than the
estimated values because the measurement includes particles too
large to be inhaled (Monsanto, 1991).
Jauhiainen et al. (1991) examined the exposure of workers to
glyphosate during silvicultural clearing with brush saws equipped
with herbicide sprayers. Measurements of air concentrations during
spraying and urinary glyphosate levels both during and following
spraying were carried out. Most of the air samples had glyphosate
concentrations below 1.25 µg/m3; the highest value observed was
15.7 µg/m3. All urine samples taken had glyphosate concentrations
below the limit of detection of 0.1 mg/litre (Jauhiainen et al.,
1991).
Lavy et al. (1992) used two methods to monitor glyphosate
exposure of workers planting conifer seedlings. Firstly, they
estimated dermal exposure based on the rate of deposition on cotton
gauze patches, surface area exposed, and a dermal penetration rate
of 1.8%. This yielded dose estimates in the range of 0-875 or
0-1.7 µg/kg body weight per h. Secondly, they attempted to measure
urinary concentration levels but found no samples above the
detection limit of 0.01 mg/litre. Based on the negative results with
the urine samples, the authors concluded that the estimates based on
patch deposition overestimated exposure by at least a factor of 11
for the most highly exposed workers (Lavy et al., 1992).
It should be noted that a dermal penetration rate of 1.8% was
used in this calculation. In view of the discussion of the dermal
penetration studies (section 6.1), a value of 5.5% is to be
preferred. However, since the estimate based on 1.8% is already an
overestimation of exposure, it is not considered necessary to adjust
the estimate of Lavy et al. (1992) to 5.5%. In their final data
assessment, the authors estimated exposure from the biological
monitoring using postulated data, i.e. assuming a concentration in
urine of half the lower limit of method validation. This yielded
mean exposure values of 0.039 to 0.080 µg/kg body weight per h (Lavy
et al., 1992).
6. KINETICS AND METABOLISM IN LABORATORY
ANIMALS AND HUMANS
Appraisal
Absorption from the gastrointestinal tract after oral intake
is limited to 30-36% of the dose or less in various species, i.e.
rats, rabbits, laying hens and lactating goats. Percutaneous
absorption in Rhesus monkeys amounts to 5.5% only, and glyphosate is
very poorly absorbed through excised human abdominal skin.
Radiolabelled glyphosate distributes widely in the body, but
is primarily found in the bones where approximately 1% can be
detected after oral administration.
Glyphosate is essentially not metabolized. This validates
kinetic studies performed with radiolabelled compound.
After absorption, excretion of glyphosate occurs mainly in the
urine. Biliary excretion is limited and elimination through exhaled
air is very low. Total body clearance is 99% after 168 h.
6.1 Absorption
The absorption percentage in rats was reported to be 30-36%
after single oral dosage at 10 and 1000 mg/kg body weight;
calculations were based on excretion percentages in urine and
faeces, and on the fact that biliary elimination is probably a minor
route (Monsanto, 1988b; Brewster et al., 1991). From a similar study
carried out in 1973, total absorption percentages of approximately
20% (male rats) and 45% (female rats) can be derived (Monsanto,
1973a). Comparable results were obtained in a recent single dose
(5.6 or 56 mg/kg body weight) disposition study in F344/N rats (NTP,
1992), which indicated that 30% of the dose was absorbed.
In a 14-day oral study in rats with application of
14C-glyphosate via the diet (dose levels 1, 10 and 100 mg/kg
feed), the observed total excretion in urine was < 10% and in
faeces approximately 80-90%. Given the minor importance of the
biliary elimination route, these data indicate absorption levels of
about 15% or less (Monsanto, 1973c). The results of oral studies
with 14C-glyphosate in rabbits (Monsanto, 1973d), laying hens
(Hazleton Lab. Inc., 1988a) and lactating goats (Hazleton Lab. Inc.,
1988b) indicate gastrointestinal absorption percentages of
approximately 30% or less.
Percutaneous absorption has been studied in Rhesus monkeys and
in human tissue in vitro. After a single application of
14C-glyphosate (isoproylamine salt) as the undiluted Roundup
formulation to the shaven intact abdominal skin (contact time 24 h)
of Rhesus monkeys, absorption amounted to only 1.8% of the dose. In
this study, however, only 16% of the dose could be accounted for at
the end of the study (Maibach, 1983). This low recovery strongly
reduces the value of the study result (possibly skin absorption is
seriously underestimated in this study). From an identical study
with diluted Roundup formulation (1:29 with water), conducted by
Wester et al. (1991), also in Rhesus monkeys (contact time 12 h),
total absorption percentages of 3.7% (at low dose) or 5.5% (high
dose) can be derived. Using both undiluted and diluted Roundup
formulation, Wester et al. (1991) observed that percutaneous
absorption of 14C-glyphosate through human skin in vitro into
human plasma as receptor fluid was < 2% (contact-time up to
16 h). In another in vitro study with human skin, absorption of
14C-glyphosate from three undiluted formulations (i.e. MON 0139,
Roundup and Roundup spray mix) was studied (contact time 24 h); very
low absorption percentages of 0.028 to 0.152% were found (Franz,
1983). With regard to these in vitro results it should be pointed
out that this technique has not yet been fully validated and
therefore direct extrapolation to in vivo human skin absorption
should be undertaken cautiously.
The absorption after inhalational intake has not been
determined.
6.2 Distribution
Concentrations of 14C label in tissues were determined on day
7 after administration of a single oral dose (10 or 1000 mg/kg body
weight) of 14C-glyphosate to rats (Monsanto, 1988b). Although only
a small proportion was absorbed, the isotope was widely distributed
throughout the body, but was primarily found in bone. The principal
results are presented in Table 9.
In rats tissue concentrations of 14C label were determined on
several occasions throughout a treatment period of 14 days and a
post-dosing withdrawal period of 10 days (dietary administration of
14C-glyphosate at 1, 10 and 100 mg/kg diet). Maximum tissue levels
were reached after 10 days or less, with highest concentrations
(maximum 0.85 mg/kg at the 100 mg/kg dose level) in kidneys
(Monsanto, 1973c). It should be noted, however, that in this study
concentrations in bone or bone marrow were not measured. An increase
of 14C in excreta was observed during the withdrawal period after
an initial rapid decrease; this indicated mobilization from storage
in depot tissue (Monsanto, 1973c).
Table 9. Concentrations of 14C label (as mg glyphosate-equivalents/kg
fresh weight) in selected tissues of rats on day 7 after a single
oral dose (rounded values) (Monsanto, 1988b)
Dose: 10 mg/kg body weight Dose: 1000 mg/kg body weight
male female male female
Blood 0.0045 0.0027 0.33 0.17
Liver 0.030 0.014 1.9 1.3
Kidney 0.022 0.013 1.9 1.4
Spleen 0.012 0.0073 2.6 3.0
Lung 0.015 0.012 1.5 1.1
Thyroid 0.00080 0.00036 1.5 1.2
Nasal mucosa 0.0050 0.023 1.7 1.8
Stomach 0.0080 0.0037 2.4 2.4
Small intestines 0.022 0.018 1.9 1.6
Colon 0.034 0.016 11.0 9.2
Bone 0.55 0.31 30.6 19.7
Bone marrow 0.029 0.0064 4.1 12.5
In lactating goats concentrations of 14C label in milk were
measured after giving capsules containing a 9:1 mixture of
14C-glyphosate and 14C-aminomethylphosphonic acid (AMPA) to a
dose level equivalent to 120 mg/kg diet (expressed as free acid) for
5 days. Concentrations in milk (as mg equivalents glyphosate/kg
whole milk) ranged from 0.019 to 0.086 mg/kg during the test period;
at day 5 after the last dose the concentration was 0.006 mg/kg
(Hazleton Lab. Inc., 1988b; Monsanto, 1988d).
In a study on laying hens, carried out using a 9:1 mixture of
14C-glyphosate and 14C-AMPA, concentrations of radiolabel were
measured in eggs collected during a 7-day period of dietary
administration at 120 or 400 mg/kg diet. At 400 mg/kg, residues in
egg white were 0.010-0.032 mg/kg (expressed as
glyphosate-equivalents) and in egg yolk 0.096-0.753 mg/kg; at
120 mg/kg the corresponding concentration ranges were 0.003-0.017
and 0.002-0.24 mg/kg, respectively. At 120 mg/kg diet, no 14C was
detectable in egg white after 10 withdrawal days; in yolk
0.019 mg/kg was present at that time (at 400 mg/kg no withdrawal
test was conducted) (Hazleton Lab. Inc., 1988a).
6.3 Metabolic transformation
Biotransformation of glyphosate occurs to a very low degree
only. In rats it was shown that all of the 14C in urine and
faeces, after a single oral application of 14C-glyphosate, was
present as unchanged parent compound (Monsanto, 1973b). Also in
rats, > 97% of the 14C in excreta, after a single oral dose,
was shown to be unchanged compound. AMPA was the only metabolite,
covering only 0.2-0.3% of the applied 14C (Monsanto, 1988a). In
laying hens also, AMPA was the only metabolite, accounting for only
a minor part of the applied amount (Monsanto, 1988c).
6.4 Elimination and excretion
In the period of 0-5 days after a single oral application of
14C-glyphosate (6.7 mg/kg body weight) to rats the total excretion
in urine was 15% (males) and 35-43% (females) of the administered
dose; total excretion in faeces was 85% (males) or 50-55% (females).
Less than 1% of the radiolabel was expired as 14CO2 (Monsanto,
1973a). In a more recent study (Monsanto, 1988b), the very low level
of expiration as 14CO2 was confirmed but no significant
inter-sex difference in the level of 14C in excreta was observed.
The result of the latter study was that at both oral dose levels (10
and 1000 mg/kg body weight) elimination in faeces was 62-70% and
excretion in urine was 14-18% (1000 mg/kg body weight) or 22-29%
(10 mg/kg body weight); less than 0.2% of the dose was expired as
14CO2. After single intravenous application (dose 10 mg/kg body
weight) 75-79% appeared in urine and only 5-8% in faeces, a finding
that shows that biliary elimination occurs to a limited degree only
(Monsanto, 1988b).
Delayed excretion in rats during a 10-day post-dosing
withdrawal period was observed after daily oral administration via
the diet for 14 days (Monsanto, 1973c); this suggests that some
storage in tissue(s) occurs when uptake is prolonged. Tissue
equilibrium was attained by day 10 of the dosing period and
excretion equalled intake by day 6 of the dosing period.
In rabbits > 80% was eliminated in faeces (with additional
14C present in the gut) and 7-11% in urine within 5 days after
administration of a single oral dose (6.7 mg/kg body weight) of
14C-glyphosate. Less than 1% of the dose was expired as 14CO2
(Monsanto, 1973d). In one oral study in lactating goats lasting 5
days, total excretion in urine varied from 20 to 24% and in faeces
from 60 to 66% (Hazleton Lab. Inc., 1988b).
6.5 Retention and turnover
Total body clearance in the study of Monsanto (1973a) was
94-98% (males) or 82-84% (females) over a 48-h period after giving a
single oral dose of 14C-glyphosate; at 120 h post-dosing this was
99% (both sexes). In Monsanto (1988b), the kinetics of whole body
elimination were estimated using the radioactivity (14C) measured
in urine and faeces after a single oral dose of 14C-glyphosate (10
or 1000 mg/kg body weight). Because of the lack of biotransformation
of glyphosate it is valid to base kinetics on total radioactivity.
The elimination appeared to be biphasic. The half-life of the alpha
elimination phase at 10 mg/kg body weight was 5.87 h (males) or
6.22 h (females); at 1000 mg/kg body weight this was 5.26 h (males)
or 6.44 h (females). The half-life of the beta phase at 10 mg/kg
body weight was 79 h (males) or 106 h (females); at 1000 mg/kg body
weight this was 181 h (males) or 337 h (females). Pretreatment with
unlabelled compound for 14 days (carried out at the low dose level)
did not have an effect on whole body elimination. Seven days after
dosing, < 0.05% of the dose was present in organs and < 0.5% in
the remaining carcass. Highest concentrations were present in bone.
It was estimated that 0.2-0.6% of the oral dose was associated with
this site; after intravenous dosing this was approximately 1%
(Monsanto, 1988b). Brewster et al. (1991) reported that in rats
nearly all of the absorbed material had been eliminated from the
body 168 h after oral administration of 10 mg/kg body weight;
approximately 1% of the dose was still associated with the bone.
In a recent study on the disposition of glyphosate in F-344/N
rats, 1% of a single oral dose (5.6 or 56 mg/kg) was found in the
tissues 72 h after dosing; 20-30% of the administered radioactivity
was eliminated via urine and 70-80% via the faeces (NTP, 1992).
7. EFFECTS ON LABORATORY ANIMALS AND IN VITRO TEST SYSTEMS
Appraisal
Glyphosate, administered by oral and dermal routes, has a very
low acute toxicity. Both glyphosate and its concentrated
formulations produce moderate to severe eye irritation, but only
slight dermal irritation. Neither glyphosate nor tested formulations
induce sensitization.
Short-term feeding studies have been conducted in several
species. In CD-1 mice, increased liver, brain, heart and kidney
weights, and growth retardation were reported at 50 000 mg/kg diet.
At 10 000 mg/kg diet, an increase in relative liver weight was
reported; however, there were no differences in absolute liver
weights when this group was compared to controls. The relative
increase represented only a 9% increase over the liver weight
reported for controls and was not considered toxicologically
significant. Additionally, there were no gross or histopathological
changes in the liver at doses of 10 000 mg/kg or more. The Task
Group considered the NOAEL to be 50 000 mg/kg diet.
In a 13-week study conducted in Charles River CD
(Sprague-Dawley) BR rats, no treatment-related effects were observed
at doses up to 20 000 mg/kg diet. The NOAEL was greater than the
highest dose tested.
Two additional 13-week studies (one in rats and the other in
mice) were conducted by NTP in which lesions of the salivary glands
were observed in both species. The NOAEL in the rat study was
< 3125 mg/kg diet and that in the mouse study was 3125 mg/kg diet.
Other short-term and long-term studies conducted in different
strains and species did not reveal similar lesions. The lesions
indicate that glyphosate may be acting as a weak adrenergic agonist.
The toxicological significance of the salivary gland lesions
observed in the NTP studies is unknown.
In a 52-week study conducted in beagles, no compound-related
effects were reported. The NOAEL was 500 mg/kg body weight per day.
In a 7-day study with the Roundup formulation in female cattle, a
NOAEL of 400 mg Roundup/kg body weight was reported. At higher dose
levels, decreased feed intake and diarrhoea occurred.
In long-term feeding studies in both rats and mice, few toxic
effects were observed. These effects were present at relatively high
dose levels only. In mice, technical glyphosate produced growth
retardation, hepatocyte hypertrophy or necrosis at 30 000 mg/kg diet
only. At 5000 and 30 000 mg/kg diet an increase in epithelial
hyperplasia of the urinary bladder was reported. The increased
incidence of this lesion did not follow a dose-related trend and in
the highest dose tested the incidence was actually lower than that
reported at the medium dose level, in spite of a 6-fold increase in
glyphosate. The observation at the medium dose (5000 mg/kg) is not
considered a compound-related effect and the NOAEL is, therefore,
considered to be 5000 mg/kg diet (814 mg/kg body weight).
Long-term feeding studies in rats resulted in decreased
growth, increased liver weight and degenerative liver changes at
20 000 mg/kg diet only. At 8000 and 20 000 mg/kg diet, there was an
apparent increase in the incidence of inflammation of the gastric
mucosa in both sexes. The only statistically significant increase
was observed in the medium-dose females (15%). This value was also
outside the historical control range of 0-13%. This finding was not
considered to be a treatment-related effect. There was no
dose-related trend across all groups of treated females and there
was no statistically significant difference in any treated male
groups. The NOAEL was therefore 8000 mg/kg diet (410 mg/kg body
weight).
Studies in rats and rabbits indicated that technical
glyphosate is not teratogenic. Two multigeneration studies were
conducted with technical glyphosate. In the first study, the only
effect noted was an increased incidence of unilateral renal tubular
dilation in F3b male pups at 30 mg/kg body weight. In the second
study, decreased body weights were reported for parents and pups and
decreased litter size was associated with dose levels of
30 000 mg/kg diet. Decreased body weights reported for parents and
pups at 10 000 mg/kg diet were not toxicologically significant. In
parents, the decrease was only 2 to 4% below controls and for pups
the decrease was 5.6 to 6.6% lower than controls. The findings in
pups were also transient and did not occur consistently in all
litters. The NOAEL was 10 000 mg/kg diet. The absence of a renal
effect in pups at a higher dose level (1500 mg/kg body weight),
though not invalidating earlier findings of unilateral renal tubular
dilation in male F3b pups, indicates that the reproducibility of
this lesion and its toxicological significance are uncertain. It
should be noted that in no other toxicological study was an effect
on kidneys found.
Bioassays in mice and rats did not indicate that technical
glyphosate was carcinogenic.
Glyphosate has been shown to have no genotoxic potential in a
range of in vitro and in vivo studies.
7.1 Single exposure
Numerous acute toxicity studies have been performed to
determine LD50 values of glyphosate and of herbicide formulations
containing glyphosate as active ingredient. The results of these
studies are summarized in Tables 10 (results for glyphosate) and 11
(results for formulations). These data show that glyphosate and its
formulations have very low toxicity by the oral and dermal
administration routes. By the intraperitoneal route glyphosate is
markedly more toxic than by the other routes. General intoxication
symptoms include breathing difficulties, ataxia and convulsions.
The mechanism of the toxic action of glyphosate has been
studied in rats. Olorunsaga et al. (1979) observed dose-related
reduced respiratory control ratios and increased phosphatase
activity in mitochondria isolated from rat livers 5 h after single
intraperitoneal doses ranging from 15 to 120 mg/kg body weight. This
effect was also seen in rat liver mitochondria in vitro (Bababunmi
et al., 1979; Olorunsaga, 1982a,b). The authors suggest that acute
toxicity at lethal doses may occur as a result of the uncoupling of
oxidative phosphorylation.
The acute toxicity in rats of the surfactant
polyoxyethyleneamine, with which glyphosate is commonly formulated
in Roundup, was compared to that of glyphosate in a study by
Martinez et al. (1990). Both compounds exhibited pulmonary toxicity
following either oral or intratracheal administration. The toxicity
of the herbicide formulation was greater than can be accounted for
on the basis of the dose response data from either compound alone
(Martinez et al., 1990; Martinez & Brown, 1991).
A study was undertaken by Tai et al. (1990) to investigate the
effects of glyphosate, surfactant, and their combination in Roundup
on cardiovascular function in female beagles. They found that
glyphosate alone at plasma levels ranging from 923 to 3450 mg/litre,
which simulates the human ingestion situation, were shown to
increase the myocardial contractility. The surfactant alone
considerably reduced the cardiac output, the left ventricular stroke
work index and the mean arterial pressure. The joint effect of both
glyphosate and the surfactant in Roundup formulation resulted in
cardiac depression, which was mostly due to the surfactant since
glyphosate itself increased myocardial contractility. The authors
indicated that the probable cause of the observed increases in
pulmonary vascular resistance index and pulmonary artery pressure
was a direct vasoactive effect of glyphosate on the pulmonary
artery.
Table 10. Acute toxicity of glyphosate to experimental animals
Species (sex) Product tested LD50/LC50a Reference
Oral studies
Rat (m,f) glyphosate techn, > 5000 mg/kg bw FDRL (1988d)
purity 97.8%
Rat (m,f) glyphosate techn, > 5000 mg/kg bw Inveresk Research Int.
purity 96-99% (1989a)
Rat (m,f) glyphosate techn, > 5000 mg/kg bw NOTOX (1988)
purity 96-99%
Rat (m,f) 85.5% techn. > 5000 mg/kg bw Bio/Dynamics Inc.
glyphosate in (1988c)
water
Rat (m,f) glyphosate, IPA salt, > 5000 mg/kg bw Monsanto (1981b)
65% in water
Rat (m.f) glyphosate, EO saltb 2047 mg/kg bw Knapek et al. (1986)
Goat (f) glyphosate techn, 3500 mg/kg bw USDA (1987c)
purity 98.7%
Goat (f) glyphosate, IPA salt, 5700 mg/kg bw USDA (1987b)
65% in water
Dermal studies
Rat (m,f) glyphosate techn, > 2000 mg/kg bw Inveresk Research Int.
purity 96-99% (1989c)
Rabbit (m,f) glyphosate techn, > 5000 mg/kg bw FDRL (1988b)
purity 97.8%
Rabbit (m,f) 85.5% techn. > 5000 mg/kg bw Bio/Dynamics Inc.
glyphosate in water (1988a)
Rabbit (m,f) glyphosate, IPA salt, > 5000 mg/kg bw Monsanto (1981c)
65% in water
Intraperitoneal studies
Mouse (m) glyphosate (not 545 mg/kg bw
further specified)
Mouse (f) glyphosate (not 740 mg/kg bw FAO/WHO (1986b)
further specified)
Mouse (m,f) glyphosate (not 134 mg/kg bw FAO/WHO (1986b)
further specified)
Rat (m) glyphosate (not 281 mg/kg bw
further specified
Table 10. (cont'd)
Species (sex) Product tested LD50/LC50a Reference
Rat (f) glyphosate (not 467 mg/kg bw FAO/WHO (1986b)
further specified)
Rat (m,f) glyphosate (not 238 mg/kg bw FAO/WHO (1986b)
further specified)
a All values expressed as mg of product tested (as presented in "product tested"
column)
b EO is an abbreviation of 5-ethoxy-oleinamine salt.
Table 11. Acute toxicity of glyphosate formulations to experimental animals
Species (sex) Product testeda LD50/LC50b Reference
Oral studies
Mouse (m,f) Roundup >5000 mg/kg bw Mitsukaido Labs (1986)
Rat (m,f) Roundup 5000 mg/kg bw Bio/Dynamics Inc.
(1988e)
Rat (m,f) "Compound No. 3607" >5000 mg/kg bw Inveresk Research Int.
(1988a)
Rat (m,f) Roundup TX >5000 mg/kg bw NOTOX (1987a,b)
Rat (m,f) Alphee >5000 mg/kg bw Bio/Dynamics Inc.
(1987a)
Rat (m,f) Sting TX >5000 mg/kg bw NOTOX (1987f,g)
Rat (m,f) Sting 2510 mg/kg bw Younger Labs Inc.
(1984)
Rat (m,f) Sting 1950 mg/kg bw Bio/Dynamics Inc.
(1984b)
Rat (m,f) MON 8780 >5000 mg/kg bw Bio/Dynamics Inc.
(1985a)
Rat (m,f) Agrichem Glyfosaat B >5000 mg/kg bw NOTOX (1990e)
Rat (m,f) "Glyfosaat 360 g/litre" >2000 mg/kg bw NOTOX (1989c)
Rat (m,f) Legend >2000 mg/kg bw CIT (1991a)
Goat (f) Roundup 4860 mg/kg bw USDA (1983)
Table 11. (cont'd)
Species (sex) Product testeda LD50/LC50b Reference
Dermal studies
Rat (m,f) "Compound No. 3607" >2000 mg/kg bw Inveresk Research Int.
(1988b)
Rat (m,f) Roundup TX >4000 mg/kg bw NOTOX (1987c)
Rat (m,f) Sting TX >4000 mg/kg bw NOTOX (1987h)
Rat (m,f) MON 8780 >5000 mg/kg bw Bio/Dynamics Inc.
(1985b)
Rat (m,f) Agrichem Glyfosaat >4000 mg/kg bw NOTOX (1990f)
B or 2
Rat (m,f) "Glyfosaat 360 g/litre" >2000 mg/kg bw NOTOX (1989b)
Rat (m,f) Legend >2000 mg/kg bw CIT (1991b)
Rabbit (m,f) Roundup >5000 mg/kg bw Bio/Dynamics Inc.
(1988f)
Rabbit (m,f) Alphee >5000 mg/kg bw Bio/Dynamics Inc.
(1987b)
Rabbit (m,f) Sting >5000 mg/kg bw Bio/Dynamics Inc.
(1984a)
Inhalation studies
Rat (m,f) Roundup (aerosol) 3180 mg/m3 Monsanto (1983d)
Rat (m,f) "Compound No. 3607" >4860 mg/m3 Inveresk Research Int.
(1989d)
Rat (m,f) Alphee >8900 mg/m3 Monsanto (1987b)
a Composition of the formulations is given in Table 2, with the exceptions of
Agrichem Glyphosaat B (or 2), "Glyfosaat 360 g/litre" and "Compound No. 3607",
which all contain approximately 480 g/litre of the isopropylamine salt, and of MON
8780 (32.8% isopropylamine salt), and Legend (40% isopropylamine salt).
b All values given as mg formulation.
7.2 Short-term exposure
7.2.1 Oral studies
In CD-1 mice, a 13-week feeding study was conducted with
technical glyphosate (purity 98.7%) using dose levels of 5000,
10 000 and 50 000 mg/kg diet (equal to 940, 1890 and 9710 mg/kg body
weight per day in males and 1530, 2730 and 14 860 mg/kg body weight
per day in females). No effect on appearance or survival was
observed. Growth retardation and increased weights of brain, heart
and kidneys were observed at 50 000 mg/kg. Liver weights were
increased at 50 000 and 10 000 mg/kg. Limited histopathology showed
no adverse effects. The authors of the study concluded that the
NOAEL was 10 000 mg/kg diet (1890 mg/kg body weight per day)
(Bio/Dynamics Inc., 1979).
In a 13-week feeding study with technical glyphosate,
Sprague-Dawley rats received 1000, 5000 or 20 000 mg/kg diet (equal
to 63, 317 and 1267 mg/kg body weight per day in males and 84, 404
and 1623 mg/kg body weight per day in females). No effect on
appearance, survival or growth occurred. Haematology, blood
biochemistry and urinalysis, carried out at test end only, were also
unaffected. Organ weights (determined for liver, kidneys and testes
only) were not affected. Limited histopathology showed no adverse
effect in any tissue. The NOAEL in this study was 20 000 mg/kg diet
(1267 mg/kg body weight per day) (Monsanto, 1987a). Absence of
toxicity was also found in another 13-week feeding study on rats
using technical glyphosate and dose levels of 200 to 12 500 mg/kg
diet (Tauchi, 1979 as cited by FAO/WHO, 1986b).
Two further 13-week studies in rodents were conducted on behalf
of the US National Toxicology Program (1992). Both rats (F-344/N)
and mice (B6C3F1) were administered glyphosate (purity
approximately 99%) in feed at levels of 3125, 6250, 12 500, 25 000
and 50 000 mg/kg diet.
In rats, reduced weight gains were observed at 25 000 mg/kg
diet (males only) and at 50 000 mg/kg diet (males and females). No
changes in feed consumption were found. Minor increases in the
relative organ weight of liver, kidney and testes, and decreased
thymus weight were observed in males only at several dose levels;
these changes did not show a clear dose relation and therefore are
not considered to be compound-related effects. Small increases in
haematocrit and red cell blood counts were observed in male rats at
> 12 500 mg/kg diet. Clinical chemistry showed increased alkaline
phosphatase (AP) and alanine aminotransferase (ALAT) at >
6250 mg/kg diet in males and at > 12 500 mg/kg diet in females.
Bile acid levels in blood were increased at 25 000 mg/kg diet (males
only) and at 50 000 mg/kg diet (males and females). Decreases in
sperm count were observed in males at > 25 000 mg/kg diet. The
only histopathological lesions found were cytoplasmic alterations of
the parotid and submandibular salivary glands, consisting of
basophilic changes and hypertrophy of acinar cells. The parotid
gland was more severely affected. The magnitude of the effect was
dose-dependent, with focal lesions in less severe cases to diffuse
involvement at higher doses. Lesions of a similar nature and
magnitude were observed in both sexes. The sublingual gland was not
detectably altered. Effects on the salivary glands were observed
already at the lowest dose level tested of 3125 mg/kg diet (equal to
205 mg/kg body weight per day for males and 213 mg/kg body weight
per day for females). Thus, this study did not yield a NOAEL (NOAEL
< 3125 mg/kg diet) (NTP, 1992).
In mice, reduced weight gains were observed at 50 000 mg/kg
diet in both sexes. Increased organ weights were noted in heart,
kidney, liver, thymus, lung and testis; these changes did not show a
clear dose relation and therefore are not considered to be
compound-related effects. Feed consumption levels were not changed
significantly. Lesions in the parotid gland were observed, similar
to those observed in rats. The sublingual gland and the
submandibular salivary glands were not detectably altered. The
effects on the parotid gland were observed in mice at 6250 mg/kg
diet (equal to 1065 mg/kg body weight per day for males and
1411 mg/kg body weight per day for females), but were not seen at
the lowest dose level tested of 3125 mg/kg diet (equal to 507 mg/kg
body weight per day for males and 753 mg/kg body weight per day for
females). The NOAEL in this study was 3125 mg/kg diet (507 mg/kg
body weight per day) (NTP, 1992).
The salivary gland lesions could also be induced in rats by
14-day exposure at feed levels of 50 000 mg/kg diet. The salivary
glands lesions induced by glyphosate were similar to those which
could be induced by exposure to high subcutaneous doses of the
ß-adrenergic agonist isoproterenol and could be partially
ameliorated with the ß-adrenergic antagonist propanolol. This
indicates that glyphosate may induce the salivary gland lesions by
acting as a weak adrenergic agonist (NTP, 1992).
The short-term toxicity of technical glyphosate was also
studied in dogs. Beagle dogs received technical glyphosate in
gelatin capsules at dose levels of 0, 20, 100 or 500 mg/kg body
weight per day for 52 weeks. No effect occurred with respect to
clinical signs, body weight, feed consumption, ophthalmoscopy,
haematology, blood biochemistry, urinalysis, gross pathology and
histopathology. The only changes in treated groups relative to
controls were increased pituitary weights (absolute and relative) in
the medium- and high-dose males. Because there were no concomitant
histological changes present in pituitaries and given the absence of
an effect on this organ (and related organs) in all other studies,
the toxicological significance of the increased pituitary weights is
questionable. The NOAEL in this study was 500 mg/kg body weight per
day (Monsanto, 1985).
A 7-day oral study was carried out with Roundup in female
cattle weighing 160 to 272 kg. Brahman-cross heifers received 400,
500, 630 or 790 mg Roundup/kg body weight per day by nasogastric
tube. At 790 mg/kg, 1/3 animals died before test end, showing
laboured breathing and pneumonia caused by aspiration of rumen
contents. Decreased feed intake was seen at 630 and 790 mg/kg;
diarrhoea occurred at 500, 630 and 790 mg/kg body weight. Slight
increases in a number of blood parameters, occurring at 790 mg/kg
only, were probably due to extracellular fluid shifts and
haemoconcentration secondary to diarrhoea. The NOAEL in this study
was 400 mg Roundup/kg body weight per day (USDA, 1987a).
7.2.2 Dermal studies
Short-term dermal toxicity studies were carried out in rabbits
with technical glyphosate and the formulation Roundup.
Technical glyphosate, moistened with saline, was applied under
occlusion at dose levels of 100, 1000 and 5000 mg/kg body weight per
day to the shaven intact or abraded skin of rabbits for 6 h/day, 5
days/week for 3 weeks. No effect on survival and growth occurred.
Slight dermal irritation (barely perceptible erythema and oedema)
was observed at 5000 mg/kg body weight only. No evidence of systemic
toxicity was found (parameters: haematology and blood biochemistry
in five animals of each sex per group, organ weights, gross
pathology, limited histopathology) (IRDC, 1982). Absence of systemic
effects was also found in the somewhat more limited 21-day study in
rabbits (no haematology and blood biochemistry) with Roundup. Skin
effects were more severe in this study: at both dose levels (76 and
114 mg/kg body weight per day, undiluted formulation applied)
erythema and oedema were seen and, in addition, exudate and
fissuring occurred at the abraded skin sites. After a 4-week
recovery period the skin effects were no longer present
(Bio/Dynamics Inc., 1975).
7.2.3 Inhalational studies
A 4-week inhalation study was carried out on rats with a 1:3
dilution of Roundup formulation. Test concentrations of 50, 160 and
360 mg/m3 of the diluted formulation (equivalent to 17, 53 and
120 mg Roundup/m3) were administered as an aerosol spray for
6 h/day, 5 days/week. The mass median aerodynamic diameter of the
test material ranged from 1.8 to 2.7 µm with geometric standard
deviations between 1.7 and 2.0. An increased incidence of irritation
of the nasal turbinates (subacute inflammation), trachea
(mononuclear cell infiltration) and lungs (perivascular lymphoid
infiltrates/aggregates) was observed among the high-dose females
only. No signs of systemic toxicity were found (parameters:
survival, growth, limited haematology and blood biochemistry, organ
weights, limited histopathology) (Monsanto, 1983e).
7.3 Long-term toxicity and carcinogenicity
Only oral studies are available. Dietary studies using
technical glyphosate were performed in mice and rats.
In Charles River CD-1 mice technical glyphosate was fed in the
diet at concentrations of 0, 1000, 5000 or 30 000 mg/kg diet for 24
months (dose levels equal to 0, 157, 814 and 4841 mg/kg body weight
per day for males and 0, 190, 955 and 5874 mg/kg body weight per day
for females). No effect on survival or appearance was noted. Body
weights were decreased in the males of the high-dose group.
Haematology and organ weights showed no effects. Histopathology in
liver revealed an increased incidence of central lobular hepatocyte
hypertrophy among high-dose males (incidences: 9/49, 5/50, 3/50 and
17/50 in control, low-dose, medium-dose and high-dose males,
respectively) and an increased incidence of central lobular
hepatocyte necrosis also among high-dose males (incidences: 0/49,
2/50, 2/50 and 10/50). Increased incidences of epithelial
hyperplasia in the urinary bladder were present in the medium-dose
and high-dose males (incidences: 3/49, 3/50, 10/50 and 8/50). There
were no statistically significant increases in the frequency of
neoplastic lesions. The NOAEL in this study was 5000 mg/kg diet
(814 mg/kg body weight per day) (Bio/Dynamics Inc., 1983a).
Two chronic feeding studies on rats were conducted with
technical glyphosate, one in 1979-1981 and the other in 1988-1990.
The first study, carried out using Charles River CD (Sprague-Dawley)
BR rats, had dose levels of 0, 60, 200 and 600 mg technical
glyphosate/kg diet (equal to about 0, 3, 10 and 32 mg/kg body weight
per day, respectively). Survival, appearance, haematology, blood
biochemistry, urinalysis and organ weights were not changed. The
NOAEL was > 600 mg/kg diet (> 32 mg/kg body weight per day).
Slight growth retardation during part of the study was noted in the
high-dose males. The incidence of interstitial cell tumours in
testes showed a statistically significant increase (incidences:
0/50, 3/50, 1/50 and 6/50; historical control range: 3-7%)
(Bio/Dynamics Inc., 1981a). This finding, in itself constituting
some evidence for a carcinogenic effect in rats, should be judged in
the light of the absence of an effect at much higher dose levels in
the more recent 2-year study in rats (see below). This is also valid
for the slight growth retardation (i.e. no effect on growth at much
higher dose levels in the more recent study, see below). In the
recent 2-year study, rats (same strain) were fed 2000, 8000 or 20
000 mg technical glyphosate/kg diet (equal to about 100, 410 and
1060 mg/kg body weight per day) for 24 months. There was no effect
on survival or appearance. Growth was retarded in the high-dose
females. Haematology and blood biochemistry showed no effects. In
the high-dose males, the urine specific gravity (after 6 months
only) and urine pH were increased. A statistically significant
increased incidence of degenerative lens changes (basophilic
degeneration of the posterior subcapsular lens capsule or mature
cataracts) was found among the high-dose males (incidences 3/60,
4/60, 4/60 and 8/60 in the control, low-, medium- and high-dose
groups, respectively. However this finding was within the historical
control range of 0-33%. Liver weights were increased in the
high-dose males only. Histopathology showed an increased incidence
Table 12. Skin irritation tests on rabbits with glyphosate and its formulations
Product testeda Contact time (h) Draize scoreb Classificationc Reference
Glyposate
Glyphosate techn. 85% in water 4 0.8 slightly irritating Bio/Dynamics Inc. (1988b)
Glyphosate techn., moistened powder 4 0 not irritating FDRL (1988a)
Glyphosate techn., moistened powder 4 0 not irritating Inveresk Research Int. (1989e)
Glyphosate, IPA salt 65% in water 24 0.2 not irritating Monsanto (1981d)
Formulations
Roundup, undiluted 4 1.9 slightly irritating Bio/Dynamics Inc. (1988g)
"Compound No. 3607", undiluted 4 1.2 slightly irritating Inveresk Research Int. (1988c)
Roundup TX, undiluted 4 0.7 slightly irritating NOTOX (1987d)
Alphee, undiluted 4 1.0 slightly irritating Bio/Dynamics Inc. (1987c)
Sting, undiluted 4 1.3 slightly irritating Bio/Dynamics Inc. (1984c)
Sting TX, undiluted 4 3.6 moderately irritating NOTOX (1987i)
Roundup L&G, undiluted 4 1.0 slightly irritating Bio/Dynamics Inc. (1985c)
"Glyfosaat 360 g/litre, undiluted 4 0.3 not irritating NOTOX (1989d)
Agrichem Glyfosaat B, undiluted 4 1.7 slightly irritating NOTOX (1990d)
Agrichem Glyfosaat 2, undiluted 4 2.0 slightly irritating NOTOX (1990b)
Legend, undiluted 4 0.7 slightly irritating CIT (1991c)
a For glyphosate content of the tested formulations, see footnote a in Table 11.
b This score is the mean score per animal and was calculated using the data from
the original report. The standard Draize scoring system was used; in calculating
the mean response per animal, the maximum response observed for an animal was used.
c The classification is based on the mean Draize score per animal. Specification:
score 0-0.5 not irritating; 0.6-2.0 slightly irritating; 2.1-5.0 moderately
irritating; 5.1-8.0 severely irritating.
Table 13. Results of eye irritation tests in rabbits for glyphosate and its formulations.
Product testeda Irritation indexb Classificationc Reference
Glyphosate
Glyphosate techn. 85% in water, undiluted 45 strongly irritating Bio/Dynamics Inc. (1988d)
Glyphosate, IPA salt, 65% in water 0 not irritating Monsanto (1981e)
Glyphosate techn. (97.6%), undiluted 54 strongly irritating FDRL (1988c)
Glyphosate techn. (96-99%), undiluted 29 irritating Inveresk Research Int. (1989f)
Formulations
Roundup, undiluted 54 strongly irritating Bio/Dynamics Inc. (1990)
"Compound No. 3607", undiluted 13 irritating Inveresk Research Int. (1989g)
Roundup TX, undiluted 31 strongly irritating NOTOX (1987e)
Alphee, undiluted 6 slightly irritating Bio/Dynamics Inc. (1987d)
Sting, undiluted 104 extremely irritating Bio/Dynamics Inc. (1984d)
Sting TX, undiluted 19 irritating NOTOX (1987j)
Roundup L&G, undiluted 19 irritating Bio/Dynamics Inc. (1985d)
"Glyfosaat 360 g/litre", undiluted 33 strongly irritating NOTOX (1989a)
Agrichem Glyfosaat B, undiluted 58 strongly irritating NOTOX (1990c)
Agrichem Glyfosaat 2, undiluted 22 irritating NOTOX (1990a)
Legend, undiluted 21 irritating CIT (1991d)
Table 13 (continued)
a For glyphosate content of the tested formulations, see footnote a in Table 11.
b The irritation index represents a mean total score per animal for response in cornea, conjunctiva
and iris, using the standard Draize scoring system. The irritation index was calculated using the data
from the original report; in calculating the mean response per animal the maximum response observed
for an animal was used. The calculation procedure is as follows:
- score conjunctiva: A. chemosis: 0-4 Calculation partial
B. discharge: 0-3 index for conjunctiva:
C. erythema: 0-3 2 x (A + B + C) = 0-20
- score iris: 0-2 Calculation partial index for iris: 5 x (0-2) = 0-10
- score cornea: A. opacity: 0-4 Calculation partial index for cornea:
B. area: 0-4 5 x A x B = 0-80
The total irritation index is the sum of the partial indices (0-110).
c The classification is based on the following scheme: index 0-5 not irritating; 5.1-12 slightly
irritating; 12.1-30 irritating; 30.1-60 strongly irritating; 60.1-80 severely irritating; 80.1-110
extremely irritating.
of inflammation of the gastric squamous mucosa in the medium- and
high-dose groups (incidences in males: 2/58, 3/58, 5/59 and 7/59;
females: 0/59, 3/60, 9/60, and 6/59; historical range: 0-13.3%). The
incidence of pancreatic islet cell adenomas was increased
(statistically significant) among low- and high-dose males
(incidences: 1/58, 8/57, 5/60 and 7/59; historical control range of
test laboratory 1.8-8.5%). The incidence in the control group was
below the historical control range; the trend test for the observed
increase was negative. No pancreatic carcinomas were found. The
NOAEL in this study was 8000 mg/kg diet (410 mg/kg body weight per
day) (Monsanto, 1990b).
Glyphosate has been tested in the US National Toxicology
Program; pre-chronic studies have been completed (NTP, 1992).
7.4 Skin and eye irritation; sensitization
Many studies have been carried out with rabbits to examine the
potential of glyphosate and its formulations to produce skin and eye
irritation. The results of these studies are briefly summarized in
Tables 12 (skin irritation) and 13 (eye irritation). Glyphosate and
its formulations produce only mild skin irritation after undiluted
single application. The result of the 21-day dermal study in rabbits
with the formulation Roundup (Bio/Dynamics Inc., 1975; see
subsection 7.2.2) shows that repeated application of undiluted
formulation to the skin does lead to irritation. The eye-irritating
potential is considerable for undiluted glyphosate and, with a few
exceptions, also for the formulations. Single application generally
produces moderate to severe reactions.
Sensitization studies have been carried out in guinea-pigs with
glyphosate and its formulations. The results of these tests are
summarized in Table 14. Neither glyphosate itself nor the tested
formulations induced sensitization in any experiment.
The results of two dermal irritation studies and one dermal
sensitization study performed with human volunteers exposed to
Roundup are presented in subsection 8.1.
Table 14. Results of sensitization tests in guinea-pigs for glyphosate and its
formulations
Product testeda Method Result Reference
Glyphosate
Glyphosate Buehler negative Bio/Dynamics Inc.
(purity 99.7%) (1983c)
Glyphosate technical Magnusson & negative Safefarm Labs Inc.
Kligmanb (1991b)
Glyphosate techn. Magnusson & negative Inveresk Research
(96-99%) Kligman Int. (1989b)
Formulations
Roundup Buehler negative Bio/Dynamics Inc.
(1983b)
"Compound Magnusson & negative Inveresk Research
No. 3607" Kligman Int. (1988d)
Roundup L&G Buehler negative Bio/Dynamics Inc.
(1987e)
Legend Buehler negative Safefarm Labs Inc.
(1991a)
Sting Buehler negative Bio/Dynamics Inc.
(1986)
a For glyphosate content of the tested formulations, see footnote a
in Table 11.
b Method also called the maximization test.
7.5 Reproductive toxicity, embryotoxicity and teratogenicity
Technical glyphosate has been tested for teratogenicity in rats
and rabbits using the oral route. In addition, two oral
multi-generation reproduction studies in rats have been reported.
7.5.1 Teratogenicity studies
Glyphosate (technical) was given to pregnant Charles River COBS
CD rats by gavage at dose levels of 0, 300, 1000 and 3500 mg/kg body
weight per day on days 6-19 of gestation. At 3500 mg/kg the
following effects were observed: increased incidences of soft
stools, diarrhoea, breathing rattles, red nasal discharge and
reduced activity, increased mortality (6/25 dams dying before the
end of the treatment period), growth retardation, increased
incidence of early resorptions, decreases in total number of
implantations and the number of viable fetuses, increased number of
fetuses with reduced ossification of sternebrae. At the lower dose
levels these effects were absent. The NOAEL in this study was
1000 mg/kg body weight per day (IRDC, 1980b).
In Dutch belted rabbits technical glyphosate was tested at dose
levels of 0, 75, 175 and 350 mg/kg body weight per day
(administration by gavage) from days 6-27 of gestation. In dams the
incidences of diarrhoea and soft stools were increased in the
high-dose group and, to a slight degree, also in the medium-dose
group. The incidence of nasal discharge was increased in the
high-dose group only. In the medium- and high-dose groups 2 and 10
dams, respectively, died during the study from unknown causes. The
NOAEL in this study was 175 mg/kg body weight per day (IRDC, 1980c).
7.5.2 Reproduction studies
A three-generation feeding study in Charles River CD
(Sprague-Dawley) BR rats was conducted in 1980-1981 and a
two-generation study, using higher dose levels, was completed in
1990 with technical glyphosate. In the former study, dietary feeding
levels were continuously adjusted to achieve dose levels of 0, 3, 10
and 30 mg/kg body weight per day. The only effect noted was an
increased incidence of unilateral renal tubular dilation in the male
pups (randomly selected) of the F3b mating of the high-dose group
(incidence 6/10 versus 0/10 in controls, not determined in
intermediate groups, earlier litters not examined). The NOAEL in
this study was < 30 mg/kg body weight (Bio/Dynamics Inc., 1981b).
The more recent two-generation study had dose levels of 0, 2000,
10 000 and 30 000 mg technical glyphosate/kg diet (equivalent to 0,
100, 500 and 1500 mg/kg body weight per day). In the high-dose
group, the following effects were observed: soft stools and
decreased body weights in parent animals, slightly decreased litter
size and decreased pup weights (the latter seen at day 14 or 21 of
lactation). The decreased body weights of parents and pups were seen
to a slight degree in the medium-dose group also. No histological
effect on kidneys was present in the F2b male pups (15 and 23 pups
examined in control and high-dose groups, respectively; first
generation and F2a pups not examined). The NOAEL in this study was
10 000 mg/kg diet (500 mg/kg body weight per day) (Monsanto, 1990c).
With regard to these reproduction studies using technical
glyphosate, it should be noted that in both studies the number of
pups submitted to histopathological examination was limited. These
limitations make evaluation of the renal effect in pups, seen (at
30 mg/kg body weight) in the study of Bio/Dynamics Inc. (1981b),
difficult.
7.6 Mutagenicity and related end-points
The results of several studies are summarized in Table 15. The
results show that glyphosate is not mutagenic.
Table 15. Results of mutagenicity studies with glyphosate and its salts.
Test system Test compound and Resultc Reference
concentrations
Ames test, Salmonella technical glyphosate - Monsanto
typhimurium TA98, TA100, (98.4%); 0.1-1000 (1978c)
TA1535 & TA1537, with and µg/plate
without metabolic activation
Ames test, S. typhimurium technical glyphosate - IET (1978)
TA98, TA100, TA1535 & (98.4%); 10-5000
TA1537, with and without µg/plate
activation
Rec assay, Bacillus subtilis, technical glyphosate - IET (1978)
strain H17 (rec+) & M45 (98.4%); 20-2000
(rec-), without activation µg/disc
Reverse mutation assay in technical glyphosate - IET (1978)
Escherichia coli strain WP2hcr, (98.4%); 10-5000
with and without activation µg/plate
Forward mutation assay, technical glyphosate - Monsanto
CHO cells, in vitro (98.7%); 0-20 mg/ml (1983b)
(HGPRT-locus), with & (- activation) or
without activation 5-25 mg/ml (+ activation)
Table 15. (cont'd)
Test system Test compound and Resultc Reference
concentrations
Cytogenetic study technical glyphosate - Monsanto
(chromosome aberrations) (98.7%); 200-1000 mg/kg (1983f)
in rat bone marrow, body weight, i.p.b;
in vivo sampling after 6, 12
and 24 h
Micronucleus test in glyphosate (not - Benova
erythrocytes of mice, specified); ´ LD50, oral; et al.
in vivo sampling time not (1989)
specified
Dominant lethal test, mouse technical glyphosate - IRDC
in vivo (98.7%); 200-2000 mg/kg (1980a)
body weight, oral
Recessive sex-linked lethal glyphosate (not - Gopalan &
test, Drosophila melanogaster, specified); dose not Njagi
in vivo given (1981)
Unscheduled DNA repair assay technical glyphosate - Monsanto
rat hepatocytes, in vitro (98.7%); 0.0125-125 (1983c)
µg/ml
a No higher concentrations tested because these would result in
osmolalities much higher than physiological levels; these high
osmolalities can produce non-specific chromosomal aberrations or
sister chromatid exchange.
b In additional studies it was demonstrated that: (1) glyphosate
produced no effect on viability and mitotic index of bone marrow
cells of rats after i.p. doses of 200-1000 mg/kg body weight
(Monsanto, 1983g); and (2) after giving 14C-labelled glyphosate
i.p. significant concentrations of 14C reached the bone marrow
(peak levels reached after 0.5 h remaining virtually constant up to
10 h after dosing) (Monsanto, 1983h).
c - = negative result
8. EFFECTS ON HUMANS
Appraisal
The formulation Roundup containing glyphosate is acutely toxic
to humans when ingested intentionally or accidentally.
No controlled studies have been conducted, and therefore the
human NOAEL level cannot not be derived.
No data are available to show the impact on workers exposed
during the manufacture or formulation of glyphosate. No
compound-related effects were observed in a test group of five
applicators prior to and after exposure for one week.
The reported higher susceptibility of individuals older than
40 years to ingested Roundup intoxication is important and requires
further investigation.
8.1 Cases of intentional and accidental exposure
Many cases of acute intoxication with herbicides containing
glyphosate and surfactant (Roundup) have been reported; most of
these were suicide attempts. Talbot et al. (1991) reviewed 93 cases
of exposure to Roundup (Chinese names: lan-da, hao-ni-chun,
nian-nian-chun) in Taiwan. The classification of the severity of
acute poisoning with Roundup as given by these authors is presented
in Table 16. Severe effects occurred only in the cases of
intentional ingestion (80 of the 93 reported). Accidental exposures
led to only mild effects. The typical symptoms were erosion of the
gastrointestinal tract (66% of the self-poisonings), seen as sore
throat, dysphagia and gastrointestinal haemorrhage. Other organs
were affected less often (nonspecific leucocytosis 65%, lungs 23%,
liver 19%, cardiovascular system 18%, kidney 14% and CNS 12%). Death
(in 7/80 cases) occurred within hours after ingestion. The amount of
undiluted Roundup ingested (rough estimates) in the lethal cases
varied from 85 to 200 ml (corresponding to roughly 30 to 70 g
glyphosate acid); but much larger amounts (500 ml Roundup,
corresponding to 180 g glyphosate acid) were reported to have been
ingested by some patients with mild to moderate symptoms. Overall,
moderate symptoms were associated with estimated intakes of 20 to
500 ml, mild symptoms with 5 to 150 ml, no symptoms with 5 to 50 ml.
The authors pointed out that the patient's estimates of the amount
ingested, and the conversion ratio used in their paper may be
inaccurate (Talbot et al., 1991). Other reviews of cases of
intoxication with Roundup have reported similar findings (Sawada &
Nagai, 1987; Tominack et al., 1991). The data of Tominack et al.
(1991) suggested that people over 40 years of age who ingest amounts
greater than 150 ml Roundup are at greatest risk of a fatal outcome.
These authors also pointed out that the surfactant contained in
Roundup may be responsible for the clinical syndrome (as suggested
by Sawada & Nagai, 1987), but that the available evidence on this
point is, as yet, inconclusive.
Table 16. Classification of severity of acute poisoning with Roundupa
Classification Description
Asymptomatic no complaints and no abnormalities on physical or laboratory
examination.
Mild mainly gastrointestinal tract(GIT) symptoms (nausea, vomiting,
diarrhoea, abdominal pain, mouth and throat pain) that resolved
within 24 h. Vital signs were stable, and there was no renal,
pulmonary or cardiovascular involvement.
Moderate GIT symptoms lasting longer than 24 h, GIT haemorrhages, endoscopically
verified oesophagitis or gastritis, oral ulceration, hypotension
responsive to intravenous fluids, pulmonary dysfunction not
requiring intubation, acid-base disturbance, evidence of transient
hepatic or renal damage, or temporary oliguria.
Severe pulmonary dysfunction requiring intubation, renal failure requiring
dialysis, hypotension requiring treatment with pressor amines, cardiac
arrest, coma, repeated seizures, or death.
a From: Talbot et al. (1991)
Further clinical experiences with patients exposed to Roundup
either accidentally or through deliberate ingestion have been
reported by Temple & Smith (1992). Symptoms resulting from dermal
exposure incidental to the use of the product included periorbital
oedema and chemosis of the eye, cardiovascular effects (tachycardia
and elevated blood pressure), swelling and paraesthesia at the site
of dermal contact and prolonged skin irritation. Deliberate
ingestion resulted in more severe effects, including lethality from
apparent respiratory and cardiac arrest (Temple & Smith, 1992).
Two dermal irritation studies were carried out with volunteers.
Application of 0.9 ml of a 9:1 dilution of Roundup formulation in
water to the intact skin of the upper arm for 24 h produced no skin
changes (Shelanski, 1973). Maibach (1986) tested undiluted Roundup
(application of 0.1 ml to intact and abraded skin sites on the back
for 24 h) and found erythema in only 1/24 subjects (23/24 no
reaction) for the intact skin sites; for the abraded skin sites 4/24
subjects showed an equivocal reaction and 10/24 showed erythema
(10/24 no reaction). The same author reported very briefly the
absence of effect in a photoirritation study in humans using
undiluted Roundup as test compound (application to abraded skin of
upper arm for 24 h with irradiation with UVA light for 45 min)
(Maibach, 1986).
A sensitization study was performed in 204 human volunteers
with undiluted Roundup according to a modified Draize method. The
summary report (no detailed report available) stated that there was
no effect in any subject (Maibach, 1986). The same author reported
absence of photosensitization by Roundup in volunteers.
8.2 Occupational exposure
The results of several studies focused primarily on the
determination of the extent of exposure to glyphosate when the
compound is used as herbicide are presented in section 5.3. The
study of Jauhainen et al. (1991) included health examinations of a
test group of five workers prior to and after an exposure period of
1 week. These examinations included haematology, clinical chemistry,
ECG, pulmonary function tests, an interview for a health
questionnaire and a general clinical examination (including
recording of blood pressure, pulse rate and pressure craft of
hands). A control group consisted of five workers. No
compound-related effects were observed (Jauhainen et al., 1991). The
other studies described in section 5.3 did not include a health
evaluation of workers.
8.3 Subpopulations at special risk
The only information available on this point is some suggestive
evidence referring to oral intoxications with Roundup; Tominack
et al. (1991) suggested that people older than 40 years are at
greater-than-normal risk after ingestion of Roundup.
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND
FIELD
In this chapter, concentrations or doses of formulations with
glyphosate are always expressed as mg product per kg soil.
Therefore, these figures may have been recalculated from the
original data, in cases where the authors reported the data in, for
instance, mg a.i./litre instead of mg Roundup per litre. For
recalculation, the percentages of the active ingredient (free acid
or the salt), given in Table 2, were used.
9.1 Laboratory experiments
9.1.1 Microorganisms
9.1.1.1 Water
Appraisal
The prokaryotic cyanobacteria are generally more sensitive to
the effects of glyphosate than the eukaryotic true algae. Similar
enzyme systems are inhibited in microorganisms to those thought to
be responsible for the herbicidal properties of glyphosate in higher
plants. The single semi-field study suggests very variable results
but with no significant effect on populations or community
structure.
The acute and chronic toxicities of technical grade glyphosate
and its formulations to cyanobacteria, algae and diatoms are
summarized in Table 17. Technical grade glyphosate is slightly toxic
with 3- to 4-day EC50 values of 1.2-7.8 mg/litre, and 7-day NOEC
values of 0.3-34 mg/litre. Formulations of glyphosate may be more
toxic (3-day EC50 values of 1.0 to > 55 mg product/litre).
Toxicity to cyanobacteria and algae is dependent on the species
or strain tested. Wängberg & Blanck (1988) exposed 16 species in
pure cultures to Roundup for 14 days. The concentration at which
growth was inhibited completely was 16 mg Roundup/litre for the most
sensitive species (Raphidonema longiseta and Anabaena sp.) and
131 mg Roundup/litre for the least sensitive species (Selenastrum
capricornutum). The prokaryotic Cyanophyta were significantly more
affected by Roundup than the eukaryotic Chlorococcales.
In Pseudomonas chlororaphis Roundup severely inhibited
respiration at concentrations of > 2623 mg/litre, whereas in
Aeromonas hydrophila respiration was only slightly affected at
these concentrations (Chan & Leung, 1986). The bacteria were exposed
for 6 days.
Table 17. Aquatic microorganisms: acute and chronic toxicity of glyphosate and its formulations (EC50, NOEC)
Organism A Test Compound Test pH Hardness Temperature Experimental Parametera Concentration Reference
type water (mg CaCO3/ (°C) duration (mg/litre)
litre) (days)
Cyanobacteria
Anabaena A S Tgg a.m. 7.5 285 24 7 NOEC 9.7b,c Malcolm
flos-aquae Pirnie
Inc.
(1987a)
Green algae
Selenastrum - S Tgg a.m. 7.0 n.r. 26 3.5-4 EC50 7.8b,e Bozeman
capricornutum et al.
(1989)
S. capricornutum A S Tgg a.m. 7.5 285 24 7 NOEC 20b,c Malcolm
Pirnie
Inc.
(1987d)
S. capricornutum - S St a.m. 7.7-9.0 26 23 3 EC50 1.0b LISEC
(1989b)
S. capricornutum - S St a.m. 7.7-9.0 26 23 3 EC50 2.5d LISEC
(1989b)
S. capricornutum - S St a.m. 7.7-9.0 26 23 3 NOEC 0.2b LISEC
(1989b)
S. capricornutum - S Ru a.m. 7.7-10.0 26 24 3 EC50 2.1b LISEC
1989a)
S. capricornutum - S Ru a.m. 7.7-10.0 26 24 3 EC50 8.0d LISEC
(1989a)
S. capricornutum - S Ru a.m. 7.7-10.0 26 24 3 NOEC 0.7b LISEC
(1989a)
Table 17 (contd).
Organism A Test Compound Test pH Hardness Temperature Experimental Parametera Concentration Reference
type water (mg CaCO3/ (°C) duration (mg/litre)
litre) (days)
Chlorella - S Ru a.m. 7.5 n.r. 25 2.1-7 EC50 > 55b Hernando
pyrenoidosa et al.
(1989)
C. pyrenoidosa - S Ru a.m. 7.5 n.r. 25 2.1-7 NOEC < 55b Hernando
et al.
(1989)
Diatoms
Skeletonema - S Tgg a.m.h 8.2-8.5 n.r. 20 4 EC50 1.2g E G &
costatum Bionomics
(1978a)
S. costatum - S Tgg a.m.h 8.2-8.5 n.r. 20 4 EC50 1.3b E G &
Bionomics
(1978a)
S. costatum - S Tgg a.m.h 8.2-8.5 n.r. 20 4 NOEC < 0.6f E G &
Bionomics
(1978a)
S. costatum A S Tgg a.m.h 7.5 285 20 7 NOEC 0.3b,c Malcolm
Pirnie
Inc.
(1987b)
Navicula A S Tgg a.m. 7.5 285 20 7 NOEC 34b,c Malcolm
pelliculosa Pirnie
Inc.
(1987c)
Table 17 (continued)
a concentration of a formulation is expressed as mg of the formulation per litre
b based on biomass decrease
c based on actual concentrations
d based on inhibition of growth rate
e NOEC value not reported
f based on biomass and chlorophyll a decrease
g based on chlorophyll a decrease
h salinity was 30%
A = actual concentrations are measured; - = nominal concentrations; S = static system;
Tgg = technical grade glyphosate; Ru = Roundup;
St = Sting; a.m. = artificial medium; n.r. = not reported
Chan & Leung (1986) found that the activity of
5-enolpyruvyl-shikimic acid-3-phosphate synthase was inhibited
strongly in aquatic bacteria at the lowest tested concentration of
656 mg Roundup/litre. This enzyme takes part in the biosynthetic
sequence to phenylalanine, tryptophan, and tyrosine via the
conversion of shikimate to chorismate, and the conversion of
chorismate to anthralinate. In an in vitro experiment with
cell-free extracts of Aerobacter aerogenes, technical grade
glyphosate inhibited the conversion of shikimate to chorismate at
concentrations of > 0.2 mg a.i./litre (Amrhein et al., 1980).
In Chlorella pyrenoidosa Roundup affected the growth, the
greening process and the photosynthetic metabolism at the lowest
tested concentration of 55 mg/litre when exposed for 2.1 or 7 days
(Hernando et al., 1989). Synthesis of chlorophyll a and b and
carotenoids was then significantly inhibited. Roundup affected
synthesis of chlorophyll a to a greater extent than that of the
other pigments. In vivo and in vitro studies with isolated
chloroplasts showed inhibition of the photosystems PS I and PS II at
concentrations of > 55 mg Roundup/litre. Hernando et al. (1989)
suggested that glyphosate acted as an electron transport inhibitor
and that the stronger inhibition of PS II compared with PS I was due
to the surfactant polyoxyethyleneamine.
In periphytic algal communities that were collected in ponds of
boreal forests, Roundup decreased the carbon fixation rate by 50% at
concentrations of 243-479 mg/litre (Goldsborough & Brown, 1988). The
algae were exposed for 4 h. Austin et al. (1991) cultured periphyton
on glass plates suspended in artificial "stream-troughs" which were
supplied with flowing water pumped from natural streams in British
Columbia, Canada. The stream water was low in phosphorus and flowed
out of an oligotrophic lake. Glyphosate was added to give nominal
concentrations in the troughs of between 0.001 and 0.3 mg/litre. A
further series of treatments added nutrients to troughs. The
herbicide was not toxic to the periphyton. A transitory decrease in
growth was followed by a stimulation of biomass in the
glyphosate-treated troughs. Similar effects were seen with added
nutrient. The authors considered the effect to be the result of
algae using glyphosate as a phosphate source. Communities of
periphyton were similar in all treatments.
Various studies have indicated that glyphosate may affect
aromatic amino acid synthesis in microorganisms, in addition to the
greening process, respiration and photosynthesis (Amrhein et al.,
1980; Chan & Leung, 1986; Pipke & Amrhein, 1988).
9.1.1.2 Soil
Appraisal
Soil bacteria in culture have shown effects of glyphosate on
nitrogen fixation, denitrification and nitrification. However, field
studies after application of formulations have not shown significant
effects. Closely related species of bacteria have been shown to be
capable of degrading glyphosate (see chapter 4). The lack of
information on the bioavailability of glyphosate in soil makes it
difficult to relate the evaluation of effects in culture to the
actual exposure in the field.
Technical grade glyphosate inhibited the growth of bacteria
isolated from a sprayed garden soil of sandy loam to a lesser extent
than it did that of bacteria isolated from an unsprayed control
(Quinn et al., 1988). In a mineral salt medium the growth rate of
bacteria from the untreated site was reduced by 50% at 590 mg
a.i./litre at 50 h after application.
In various studies, glyphosate, applied at the recommended
rates, caused neither inhibition of processes that are part of the
nitrogen cycle nor inhibition of enzymes involved in microbial
activity.
Roundup did not affect dehydrogenase activity in sand and loamy
sand when applied at rates of 4.9 and 24 kg a.i./ha (NATEC, 1990).
In the same soils amended with lucerne (Medicago sativa), the same
application rates did not change significantly the amounts of
nitrate nitrogen, although in treated loamy sand there was a slight
increase. These experiments, with a duration of 28 days, indicated a
slight stimulation of nitrification in loamy sand. A stronger
stimulation of nitrification was found in silt loam, silty clay loam
and sandy loam up to 84 days after application of technical grade
glyphosate at rates of 5 and 25 mg a.i./kg (ABC Inc., 1978d). This
stimulation was not dose-related. In this experiment no
substance-related effects were found on nitrogen fixation and
nitrite formation.
Nitrogen fixation was not affected significantly at an
application rate of 13 mg a.i./kg dry weight whether the product
applied was technical grade glyphosate or Roundup, or whether
aerobic or anaerobic conditions were used (Carlisle & Trevors,
1986a). At concentrations > 127 mg a.i./kg dry weight, nitrogen
fixation was inhibited under anaerobic conditions, whereas this
could not be verified under aerobic conditions due to very low
acetylene reduction rates. In the same agricultural sandy loam,
denitrification under anaerobic conditions was stimulated at
concentrations of > 13 mg a.i./kg dry weight, whether technical
grade glyphosate or Roundup was applied. Denitrification was
stimulated more strongly, when, in addition to the test compound,
glucose was added. In the same soil, no substantial effects of
either test compound on nitrification were found at 77 mg a.i./kg
dry weight. Dose-related inhibition of nitrification was found at
concentrations > 230 mg a.i./kg dry weight for glyphosate and
Roundup with respect to nitrate and nitrite production,
respectively. For both substances the inhibition was transient in
these studies. The temperature used was not reported.
No substantial effects on nitrification or denitrification were
found in two agricultural soils from Southern Finland after
application of an unknown formulation at a rate of 2.6 kg a.i./ha
(Müller et al., 1981). A strong inhibition of nitrogenase activity
was possibly an indirect effect due to a changed C/N ratio after the
treatment. In this study lasting 28 days, pretreatment measurements
were used as a control.
Roundup did not inhibit nitrification in three agricultural
soils after treatment at a recommended application rate of 2.9 kg
a.i./ha in 25-day experiments (Stratton, 1990). In a sandy loam,
nitrification was stimulated after the application of 145 kg
a.i./ha, whereas nitrification rates were inhibited by 50% at rates
of 194-435 kg a.i./ha. Mineralization, expressed as the time-course
of mg N/g dry weight (N = ammonium or nitrate nitrogen), in two
agricultural sandy loam soils was stimulated significantly due to
the treatment with 100 mg a.i./kg dry weight during an experiment
lasting 70 days (Marsh et al., 1977).
Not only in agricultural soils but also in forest soils, the
effects of Roundup on the nitrogen cycle appear not to be
inhibitory. Preston & Trofymow (1989) found no significant effects
on nitrification or the immobilization of urea nitrogen in an
organic fir forest floor and its underlying mineral horizon due to
the application of 10 and 50 mg a.i./kg dry weight. This experiment
lasted for 40 days.
In two agricultural sandy loam soils exposed to 100 mg a.i./kg
dry weight for 210 days, transient slight effects on CO2 evolution
were observed by Marsh et al. (1977). These effects were both
stimulatory (first 20 days) and inhibitory (until approximately 130
days) in both the soil from a permanent grassland and the soil from
an arable field, but especially in the latter. Carlisle & Trevors
(1986b) observed a dose-related increase in both O2 consumption
and CO2 production in an agricultural sandy loam soil during a
test of 10 days, with both technical grade glyphosate and Roundup.
In general the increases were significant at concentrations greater
than or equal to 127 mg a.i./kg. At the lowest dose (13 mg a.i./kg
soil) no effects were observed except a significant increase in
oxygen consumption due to Roundup. Oxygen consumption at doses
greater than or equal to 127 mg a.i./kg was more strongly stimulated
by Roundup than by technical grade glyphosate, possibly due to the
isopropylamine or surfactant. Preston & Trofymow (1989) found no
significant effect on CO2 production in an organic fir forest
floor and its underlying mineral horizon after the application of 10
and 50 mg a.i./kg dry weight. This experiment lasted 40 days.
Aerobic H2 oxidation in an agricultural sandy loam was
significantly inhibited by both technical grade glyphosate and
Roundup, but only at the highest dose of 635 mg a.i./kg dry weight
(Carlisle & Trevors, 1986b). Inhibition was stronger under anaerobic
conditions, and was found at concentrations of > 127 mg a.i./kg
dry weight to be significant and dose related. Anaerobic inhibition
might have been due to increased H2 generation as a result of
stimulated fermentation.
No effects of glyphosate on the activities of 1,3-ß-glucanase
and urease in a silt loam were found after application of Roundup at
a rate of 12 mg a.i./kg dry weight (Lethbridge et al., 1981).
Preston & Trofymow (1989) found no significant effect on urea
hydrolysis in an organic fir forest floor and its underlying mineral
horizon amended with 200 mg urea nitrogen per kg dry weight, due to
treatment with Roundup at a rate of 50 mg a.i./kg dry weight.
The degradation of cellulose, starch, protein, or leaf litter
was not inhibited at concentrations of 5 and 25 mg a.i./kg soil in
three agricultural soils, with the exception of litter in silt loam
at the highest dose (ABC Inc., 1978e). In this experiment lasting 84
days, technical grade glyphosate was applied.
Bacterial growth of Rhizobium trifolii in sterile solutions
with Bergersen's broth was completely inhibited at solution
concentrations of 10 and 20 mg a.i./litre, applied as an unknown
formulation of glyphosate (Eberbach & Douglas, 1983). Only at 10 mg
a.i./litre did the bacterial growth recover within 4 days. At lower
concentrations no inhibition was found.
Mycelial growth of ectomycorrhizal fungi in pure cultures on
agar was inhibited by 50% or more at concentrations of > 1 mg
a.i./litre for Pisolithus tinctorius and of > 100 mg a.i./litre
for Cenococcum geophilum and Hebeloma longicaudum (Estok et al.,
1989). Ectomycorrhizal fungi commonly associated with pines (Pinus
sp) were significantly inhibited at concentrations of > 29 µg
Roundup/litre (Chakravarty & Chatarpaul, 1990a). The most sensitive
species were Cenococcum graniforme, Hebeloma crustuliniforme and
Laccaria laccata. Roundup increased the susceptibility of sandy
soil for Gaeumannomyces gramminis, a fungus causing "take-all
disease" in wheat crops (Mekwatanakarn & Sivasithamparam, 1987).
Application at a rate of 0.54 µg a.i./kg increased the survival and
pathogenicity of the fungus significantly after 140 days of
incubation at 25 °C. These authors concluded that Roundup affected
microbial antagonists of the fungus.
9.1.2 Aquatic organisms
9.1.2.1 Plants
Appraisal
There is conflicting information on the effects of sediment on
the phytotoxicity of glyphosate to aquatic plants; Lemna showed
reduced effects whilst Carthamus did not. Generally, glyphosate is
thought to be largely unavailable to plants when added to soil and
only effective as a herbicide when applied to foliage.
The chronic toxicity to aquatic macrophytes when exposed to
technical grade glyphosate or Roundup dissolved in water is
summarized in Tables 20 and 21. Glyphosate is slightly toxic with a
14-day NOEC value of 9 mg/litre. Roundup is also slightly toxic with
14-day NOEC values of 2.4-56 mg/litre. No data on acute toxicity for
plants were available.
When Roundup was sprayed at a rate of 0.8 kg a.i./ha, the
phytotoxicity to floating plants of the common duckweed (Lemna
minor) was dependent on the extent of washed-off deposit (Lockhart
et al., 1989). Phytotoxicity was highest when the sprayed deposits
were not washed off within 6 h after application. Suspensions of
50 mg/litre of inorganic bentonite clay reduced the phytotoxicity of
Roundup to common duckweed significantly (Hartman & Martin, 1984).
When exposed for 14 days, concentrations up to 24 mg Roundup/litre
had no effect on plant growth when sediment was added, whereas the
growth was reduced by 50% at 5 mg Roundup/litre when no sediment was
added.
Phytotoxicity of glyphosate to the safflower (Carthamus
tinctorius) was not significantly reduced when the test compound
was added to drainage water with suspended particles instead of
distilled water (Bowmer et al., 1986). In these bioassays the
inhibition of root elongation was measured when the plants were
exposed to concentrations in unfiltered water of approximately
0.1-3 mg a.i./litre. The maximum concentration of adsorbed
glyphosate was 2500 mg/kg.
Although it had inhibitory effects at high concentrations,
Roundup had a stimulatory effect on the growth of common duckweed
(Lemna minor) and tubers of sago pondweed (Potamogeton
pectinatus) at lower concentrations (Hartman & Martin, 1985;
Lockhart et al., 1989). Enhancement of growth of common duckweed and
sago pondweed was found at 7-56 and 3 mg Roundup/litre,
respectively. These stimulatory effects may refer to hormesis.
9.1.2.2 Invertebrates
Appraisal
The data from laboratory toxicity tests show that formulations
are often more toxic than technical glyphosate to aquatic
invertebrates. The surprising result that addition of clay particles
to Daphnia test systems increased the toxicity of glyphosate is
probably due to ingestion of herbicide bounds to the particles. Few
studies have been conducted in the presence of sediment; the
reported toxicity of glyphosate is, therefore, difficult to relate
to the field situation.
The acute and chronic toxicity of technical grade glyphosate
and its formulations to aquatic invertebrates are summarized in
Tables 18-21. Technical grade glyphosate is slightly to very
slightly toxic, with LC50 values of > 55 mg/litre and a 21-day
NOEC value of 100 mg/litre. Formulations of glyphosate are
moderately to very slightly toxic with 2-day EC50 values of
5.3-5600 mg product/litre and 21-day MATC values of 1.4-4.9 mg
product/litre. The higher toxicity of Roundup to crustaceans is
mainly due to the presence of surfactants (Servizi et al., 1987).
In a laboratory in vitro test with the gills of Mytilus
californianus, a marine water mollusc, the active uptake of glycine
was inhibited by 23 and 67%, respectively, when a mixture of
14C-labelled and unlabelled glyphosate was applied at rates of
0.2-1.7 mg/litre (Swinehart & Cheney, 1987). This inhibition might
be an indication for Mg2+-moderated binding of glycine to the gill
surface, whereas glyphosate can compete with glycine uptake by
forming a metal complex.
Water fleas (Daphnia magna) were less sensitive to Roundup,
when the water was aerated than when it was unaerated. The 2-day
LC50 value decreased from 37 (with aeration) to 24 mg (without
aeration) Roundup/litre (EG & G Bionomics, 1980f).
Addition of 50 mg/litre of inorganic bentonite clay decreased
the 2-day EC50 value for mature water fleas (Daphnia pulex) from
16 to 7 mg Roundup/litre (Hartman & Martin, 1984). This higher
toxicity might have been due to ingestion of particle-adsorbed
glyphosate. Addition of bentonite also increased the toxicity in a
chronic experiment with populations of Daphnia pulex. Immature
water fleas were more sensitive to glyphosate than the adults under
all conditions. Populations in all treatments had recovered within
14 days after the application (Hartman & Martin, 1984).
No avoidance of glyphosate was found when mayfly nymphs
(Ephemerella walkeri) were tested at concentrations of 0.2-2 mg
Roundup/litre, whereas avoidance was demonstrated at 24 mg/litre
(Folmar et al., 1979).
9.1.2.3 Vertebrates
Appraisal
The toxicity tests for fish are generally performed without
sediment. As the bioavailability of glyphosate itself will be
reduced under most conditions due to sorption onto sediment, no
toxic effects are expected. Toxic effects, however, can be expected
due to surfactants in some formulations. To a lesser extent,
life-stage, pH, water hardness, temperature, and the presence of
feed all influence toxicity. No adverse effects on the
osmoregulatory mechanism were found.
The acute and chronic toxicity of technical grade glyphosate
and its formulations to fish are summarized in Tables 18-21.
Technical grade glyphosate is moderately toxic with 4-day LC50
values of 10 to > 1000 mg/litre, a 21-day NOEC value of
52 mg/litre, and an MATC value of > 26 mg/litre. Formulations of
glyphosate have comparable toxicity with 4-day LC50 values of 2.4
to > 1000 mg product/litre, and 21-day NOEC values of 0.8-2.4 mg
product/litre. Toxicity may vary substantially, depending on the
species, the test compound and test conditions. In general,
technical grade glyphosate is less toxic than the formulations. This
difference is mainly due to a higher toxicity of surfactants in the
formulations (Folmar et al., 1979; Servizi et al., 1987; Mitchell
et al., 1987; Wan et al., 1989).
Table 18. Aquatic organisms: acute toxicity of technical grade glyphosate in a static test system
Organism A Test pH Hardness Temperature Experimental Parameter Concentration Reference
water (mg CaCO3/ (°C) duration (mg/litre)
litre) (days)
Molluscs
Crassostrea - n.w.a n.r. n.r. 25 2 EC50 > 10b Bionomics
virginica, eggs (1973a)
Echinodermata
Tripneustes - n.w.a 7.7-8.2 n.r. 20 4 EC50 > 1000c E G & Bionomics
esculentes (1978d)
Crustaceans
Daphnia magna, - w.w. 7.8-8.0 > 250 19 2 LC50 780 ABC Inc.
first instar (1978a)
Uca pugilator - a.m.a n.r. n.r. 21 4 LC50 934 Bionomics
(1973b)
Palaemonetes - a.m.a n.r. n.r. 21 4 LC50 281 Bionomics
vulgaris (1973b)
Mysidopsis bahia - n.w.a 6.4-8.3 n.r. 20 4 LC50 > 1000 E G &
Bionomics
(1978c)
Insects
Chironomus plumosus, - r.w. n.r. 40 22 2 EC50 55d Folmar et al.
fourth instar (1979)
Table 18 (contd).
Organism A Test pH Hardness Temperature Experimental Parameter Concentration Reference
water (mg CaCO3/ (°C) duration (mg/litre)
litre) (days)
Fish
Ictalurus - r.w. n.r. 40 22 4 LC50 130 Folmar et al.
punctatus (1979)
Salmo gairdnerii, - divers 6.3-8.2 5.3-148 n.r. 4 LC50 10-197 Wan et al.
0.4 cm, 0.5 g (1989)
Salmo gairdnerii, - r.w. 4.4-7.2 45 12 4 LC50 86 ABC Inc.
0.4 cm, 0.6 g (1978b)
Oncorhynchus keta, - divers 6.3-8.2 5.3-148 n.r. 4 LC50 10-148 Wan et al.
0.4 cm, 0.5 g (1989)
O. kisutch, A divers 6.3-8.2 5.3-148 n.r. 4 LC50 27-174 Wan et al.
0.4 cm, 0.5 g (1989)
O. tshawytsha, - divers 6.3-8.2 5.3-148 n.r. 4 LC50 19-211 Wan et al.
0.4 cm, 0.5 g (1989)
O. gorbusha, - divers 6.3-8.2 5.3-148 n.r. 4 LC50 14-190 Wan et al.
0.4 cm, 0.5 g (1989)
Lepomis - r.w. n.r. 40 22 4 LC50 140 Folmar et al.
macrochirus (1979)
L. macrochirus, - r.w. 6.6-7.0 45 21 4 LC50 120 ABC Inc.
0.3 cm, 1.0 g (1978c)
Pimephales - r.w. n.r. 40 22 4 LC50 97 Folmar et al.
promelas (1979)
Rasbora - r.w. n.r. 25 21 4 LC50 168 HRC (1977)
heteromorpha,
0.1-0.3 cm
Cyprinodon - n.w.e 7.6-8.3 n.r. 20 4 LC50 > 1000 E G & Bionomics
variegatus, (1978b)
0.7-1.0 cm
a salinity 20-35%
b based on abnormal development of oyster larvae
c based on immobility, drooping spines, and retracted podia
d based on immobilisation
e salinity 18%
A = actual concentrations are measured; - = nominal concentration; a.m. = artificial medium;
r.w. = reconstituted water; w.w. = well water; n.w. = natural surface water;
n.r. = not reported
Table 19. Aquatic organisms: acute toxicity of formulations with glyphosate
Organism A Test Compound Test pH Hardness Temperature Experimental Parameter Concentration Reference
type water (mg CaCO3/ (°C) duration (mg/litre)a
litre) (days)
Crustaceans
Daphnia - S Ru r.w. 8.2-8.3 175 22 2 EC50 5.3b E G & G
magna, first Bionomics
instar (1980e)
D. magna, - S Ru r.w. 7.7-8.1 175 22 2 EC50 24-37b E G & G
first instar Bionomics
(1980f)
D. magna, - S Ru r.w. n.r. 40 22 2 EC50 7.3b Folmar
first instar et al.
(1979)
D. magna, - S St w.w. 8.3-8.5 225-275 20 2 EC50 42b ABC Inc
first instar (1984c)
D. magna, - S RuD w.w. 7.9-8.6 255 20 2 EC50 930b ABC Inc
first instar (1981a)
D. pulex, - S Ru w.w. 7.6 282 15 2 EC50 19b Hartman &
mature Martin
(1984)
Gammarus A CF Ru w.w 7.9-8.3 255 17 2 EC50 42b,c ABC Inc
pseudolimnaeus (1982b)
Insects
Chironomus - S Rod r.w. 7.6-7.8 42-44 22 2 EC50 5600b Buhl &
riparius, Faerber
fourth instar (1989)
Table 19 (cont'd)(2)
Organism A Test Compound Test pH Hardness Temperature Experimental Parameter Concentration Reference
type water (mg CaCO3/ (°C) duration (mg/litre)a
litre) (days)
Chironomus - S Ru r.w. n.r. 40 22 2 EC50 44b Folmar
plumosus, et al.
fourth instar (1979)
Fish
Ictalurus - S Ru r.w. 6.3-7.2 24-40 22 4 LC50 52 E G & G
punctatus, Bionomics
0.7 cm, 3 g (1980a)
Ictalurus - S Ru r.w. n.r. 40 22 4 LC50 32 Folmar
punctatus et al.
(1979)
Salmo - S St r.w. 6.8-7.3 40-45 12 4 LC50 7.5 ABC Inc
gairdnerii, (1984a)
0.4 cm, 0.7 g
Salmo A CF Ru w.w. 8.0-8.2 255 12 4 LC50 8.2c ABC Inc
gairdnerii, (1982c)
0.5 cm, 2.4 g
Salmo - S Ru divers 6.3-8.2 5.3-148 n.r. 4 LC50 14-33 Wan et al.
gairdnerii, (1989)
0.4 cm, 0.5 g
Salmo - S Ru r.w. 6.6-7.6 40 12 4 LC50 36 E G & G
gairdnerii, Bionomics
0.3 cm, 0.3 g (1980c)
Salmo - S Ru w.w 6.4-7.3 26-26 12 4 LC50 22 E G & G
gairdnerii, Bionomics
0.4 cm, 0.7 g (1980g)
Table 19 (cont'd)(3)
Organism A Test Compound Test pH Hardness Temperature Experimental Parameter Concentration Reference
type water (mg CaCO3/ (°C) duration (mg/litre)a
litre) (days)
S. gairdnerii, A S Ru r.w 7.4-7.7 85 11 4 LC50 22 EVS
0.4 g Consultants
(1986a)
S. gairdnerii, A S Ru n.w 7.4-7.8 81 11 4 LC50 15 EVS
0.4 g Consultants
(1986a)
S. gairdnerii, A S Ru d.w 5.4-6.3 4.5 11 4 LC50 26 EVS
0.4 g Consultants
(1986a)
S. gairdnerii, - S Ru r.w approx. 40 12 4 LC50 3.2 Folmar et al.
1.0 g 7.2 (1979)
Salmo - S RuD w.w 4.6-7.1 45 12 4 LC50 > 1000 ABC Inc
gairdnerii, (1981c)
0.3 cm, 0.2 g
S. gairdnerii, - S Vis n.r. 6.0 9.6 12.3 4 LC50 34 Morgan &
9.5 cm Kiceniuk
(1992)
Lepomis - S St r.w. 6.8-7.5 40-45 22 4 LC50 4.5 ABC Inc
macrochirus, (1984b)
0.2 cm, 0.1 g
Lepomis - S RuD w.w. 4.9-7.1 45 22 4 LC50 > 1000 ABC Inc
macrochirus, (1981b)
0.2 cm, 0.1 g
Lepomis A CF Ru w.w. 8.0-8.2 255 22 4 LC50 5.8c ABC Inc
macrochirus, (1982a)
0.2 cm, 0.2 g
Table 19 (cont'd)(4)
Organism A Test Compound Test pH Hardness Temperature Experimental Parameter Concentration Reference
type water (mg CaCO3/ (°C) duration (mg/litre)a
litre) (days)
Lepomis - S Ru r.w. 6.4-7.5 40 22 4 LC50 46 E G & G
macrochirus, Bionomics
0.4 cm, 0.3 g (1980b)
Pimephales - S Ru w.w. 6.7-7.7 39-44 22 4 LC50 31 E G & G
promelas, Bionomics
0.4 cm, 0.6 g (1980d)
Pimephales - S Ru r.w. n.r. 40 22 4 LC50 5.6 Folmar
promelas et al.
(1979)
Cyprinus A S St w.w. 7.2-7.9 40-48 22-23 4 LC50 2.4 ABC Inc
carpio, (1990)
0.6 cm, 2.8 g
Oncorhynchus A S Ru divers 6.3-8.2 5.3-148 n.r. 4 LC50 13-33 Wan et al.
kisutch, (1989)
0.4 cm, 0.5 g
O. kisutch, A S Ru d.w. 5.5-6.4 4.5 11 4 LC50 22 EVS
11.8 g Consultants
(1986c)
O. keta, - S Ru divers 6.3-8.2 5.3-148 n.r. 4 LC50 11-20 Wan et al.
0.4 cm, 0.5 g (1989)
O. tshawytsha, - S Ru divers 6.3-8.2 5.3-148 n.r. 4 LC50 17-33 Wan et al.
0.4 cm, 0.5 g (1989)
O. tshawytsha, A S Ru d.w. 5.8-6.7 4.5 11 4 LC50 20 EVS
4.6 g Consultants
(1986b)
Table 19 (cont'd)(5)
Organism A Test Compound Test pH Hardness Temperature Experimental Parameter Concentration Reference
type water (mg CaCO3/ (°C) duration (mg/litre)a
litre) (days)
O. gorbusha, - S Ru divers 6.3-8.2 5.3-148 n.r. 4 LC50 14-33 Wan et al.
0.4 cm, 0.5 g (1989)
Oncorhynchus A S Ru n.w 7.7-8.0 84 4-5 4 LC50 27-29 Servizi
nerka, et al.
3-6.5 cm, 0.2-3.8 g (1987)
a All concentrations are expressed as mg of the formulation per litre
b Based on immobilisation
c Based on actual concentrations
A = actual concentrations are measured; - = nominal concentrations; CF = continous flow system;
S = static system; Tgg = technical grade glyphosate; Ru = Roundup; Rod = Rodeo; St = Sting;
RuD = Roundup D-Pak; d.w. = dechlorinated tap water; r.w. = reconstituted water;
w.w. = well water; n.w. = natural surface water; a.m. = artificial medium
Table 20. Aquatic organisms: chronic toxicity of glyphosate (NOEC/MATC)
Organism A Test Test Test pH Hardness Temperature Experimental Parameter Concentration Reference
type substance water (mg CaCO3/ (°C) duration (mg/litre)
litre) (days)
Macrophytes
Lemna gibba A S Tgg a.m. 7.5 285 25 14 NOEC 9a,b Malcolm
Pirnie
Inc.
(1987e)
Crustaceans
Daphnia magna, A SS Tgg w.w.c 6.8-8.2 160-180 20 21 NOEC 100a,d ABC Inc.
first instar (1989c)
Fish
Salmo A CF Tgg w.w. 5.9-7.8 40-48 14-15 21 NOEC 52a,e ABC Inc.
gairdnerii, (1989e)
0.5 cm, 1.3 g
Pimephales A CF Tgg w.w. 6.5-7.6 32-42 25 255 MATC > 26f E G & G
promelas, Bionomics
1.5 g (1975)
a Based on actual concentrations d Based on survival and reproduction
b Based on biomass decrease e Based on survival, behaviour, and coloration
c Mixed with natural surface water f Based on survival, growth and reproduction
A = actual concentrations are measured; CF = continous flow system; SS = semi-static system;
S = static system; Tgg = technical grade glyphosate; w.w. = well water; a.m. = artificial medium
Table 21. Aquatic organisms: chronic toxicity of formulations with glyphosate
Organism A Test Test Test pH Hardness Temperature Experimental Parameter Concentration Reference
type substance water (mg CaCO3/ (°C) duration (mg/litre)f
litre) (days)
Macrophytes
Lemna minor - S Ru a.m. n.r. n.r. 25 14 NOEC 56c,d Lockhart
et al.
(1989)
Lemna minor - S Ru a.m. n.r. n.r. 22 14 NOEC 2.4c,e Hartman &
Martin
(1984)
Potamogeton - S Ru a.m. n.r. n.r. 22 14 NOEC 33c,d Hartman &
pectinatus Martin
(tubers) (1985)
Crustaceans
Daphnia A SS St w.w.b 7.2-8.2 174 20 21 MATC 1.4g,i ABC Inc.
magna, (1989a)
first instar
Daphnia magna, A SS Ru w.w.b 7.6-8.3 160-180 20 21 MATC 4.9g,j ABC Inc.
first instar (1989b)
Fish
Salmo A CF St w.w. 7.3-7.8 40-48 14-16 21 NOEC 0.8a,h ABC Inc.
gairdnerii, (1989h)
0.5 cm,
1.3 g
Table 21. (cont'd)
Organism A Test Test Test pH Hardness Temperature Experimental Parameter Concentration Reference
type substance water (mg CaCO3/ (°C) duration (mg/litre)f
litre) (days)
Salmo A CF Ru w.w. 7.1-7.8 24-48 14-16 21 NOEC 2.4a,h ABC Inc.
gairdnerii, (1989d)
0.5 cm,
1.8 g
Salmo A CF Vis n.r. 6.0 9.6 12.3 approx. 60 NOEC > 0.04a,k Morgan &
gairdnerii, Kiceniuk
9.5 cm, 7.2 g (1992)
a Based on actual concentrations g Based on survival, reproduction and length of time to the first brood
b Mixed with natural surface water h Based on survival, behaviour and coloration
c Roundup dissolved in test water i NOEC is 1.0 mg Sting/litre
d Based on biomass decrease j NOEC is 3.2 mg Roundup/litre (actual concentration)
e Based on reduction of frond formation k Based on mortality and growth
f All concentrations are expressed as mg of the formulation per litre
A = actual concentrations are measured; - = nominal concentrations; CF = continous flow system;
SS = semi-static system; Ru = Roundup; St = Sting; Vis = Vision; w.w. = well water;
a.m. = artificial medium; n.r. = not reported.
In laboratory experiments the major factors influencing the
toxicity appear to be the tested species and its age, the presence
of surfactants, the hardness, pH, temperature and the availability
of ration. Wan et al. (1989) found that the toxicity of technical
grade glyphosate to salmonids increased when hardness and pH
decreased, whereas for Roundup and Accord CR the contrary was true,
due to the presence of a 75% tallow amine surfactant in the
formulations. This surfactant was most toxic in hard water with a
relatively high pH. A higher toxicity of Roundup to salmonids at
increasing hardness and pH was confirmed by Mitchell et al. (1987),
but only partially by Servizi et al. (1987). It was also confirmed
by Folmar et al. (1979), although they only investigated the effect
of pH in reconstituted water with a moderate hardness. These authors
demonstrated that the effects of a pH increase on the toxicity of
Roundup and technical grade glyphosate were not only seen in rainbow
trout (Salmo gairdnerii) but also in bluegill sunfish (Lepomis
macrochirus). For both species Roundup became more toxic as the pH
increased from 6.5 to 7.5, whereas technical grade glyphosate became
less toxic as the pH increased from 6.5 to 9.5. At pH values higher
than 7.5, the toxicity of Roundup remained constant. In the
investigations of Servizi et al. (1987), an antagonistic effect of
glyphosate on the toxic action of a surfactant was found.
Increased toxicity of formulations due to surfactants was not
only demonstrated for the tallow amine surfactant in Roundup but
also for ortho X-77 in Rodeo (Mitchell et al., 1987). However, even
in the presence of a surfactant, the acute toxicity of some
formulations may be very slight, as was found by ABC Inc. (1980a,b).
In these studies, 0.5% (v/v) of the surfactant X-77 was added to
Roundup D-Pak, resulting in a 4-day LC50 value of this mixture for
rainbow trout (Salmo gairdnerii) and for bluegill sunfish
(Lepomis macrochirus) of 240 and 830 mg/litre, respectively.
Folmar et al. (1979) performed acute toxicity tests with
various species in reconstituted water with a pH of 7.2 and a
hardness of 40 mg CaCO3/litre. In these tests it was demonstrated
for rainbow trout (Salmo gairdnerii) and channel catfish
(Ictalurus punctatus) that the sensitivity to Roundup increased in
the following order: eyed eggs, 2-g fingerlings, sac fry, swim-up
fry, and 1-g fingerlings. The 4-day LC50 values for the various
life-stages of rainbow trout decreased from 39 mg Roundup/litre for
eyed eggs to 3.2 mg/litre for small fingerlings. In an additional
test, a 4-h exposure to concentrations of > 12 mg Roundup/litre
affected the survival of sac fry and swim-up fry significantly.
Holdway & Dixon (1988) also demonstrated that toxicity is
dependent on the life-stage by applying technical grade glyphosate
to larvae of flagfish (Jordanella floridae). At concentrations up
to 30 mg a.i./litre, no 2- or 4-day-old larvae died, whereas 20% of
the 8-day-old larvae died at the top dose. The effect was even more
drastic when the larvae were not fed. This treatment killed 20% of
the oldest larvae even at 3 mg a.i./litre. According to the authors,
the effect of age might fit the idea of saltatory ontogeny, implying
critical periods for organs and tissues.
While investigating the effect of temperature on toxicity,
Folmar et al. (1979) demonstrated for rainbow trout (Salmo
gairdnerii) and bluegill sunfish (Lepomis macrochirus) that
toxicity increased with increasing temperatures. For the trout the
4-day LC50 values decreased from 34 mg Roundup/litre at 7 °C to
18 mg/litre at 17 °C. For the bluegill sunfish the 4-day LC50
values decreased from 18 mg/litre at 17 °C to 9.8 mg/litre at 27 °C.
Rainbow trout (Salmo gairdnerii) showed the same sensitivity
to Roundup, independent of whether the water was aerated or not
(EG & G Bionomics, 1980g). In this experiment the 4-day LC50 under
both conditions was 22 mg Roundup/litre.
The potential of coho salmon smolt (Oncorhynchus kisutch) to
adapt to changes in water salinity encountered during migration was
not influenced by the application of Roundup at actual
concentrations up to 2.8 mg/litre (EVS Consultants, 1986d). The
osmoregulatory mechanism, which is fully functional in smolts, was
unaffected as indicated by plasma Na+ concentrations, haematocrit
values and the condition of the fish. During the experiment in which
the fish were exposed for 10 days in fresh water and subsequently
allowed to recover in fresh or sea water, no abnormal behaviour was
observed.
No avoidance of glyphosate was found when rainbow trout were
tested at concentrations up to 24 mg Roundup/litre (Folmar et al.,
1979). Sublethal concentrations in acute toxicity tests with Roundup
may cause loss of motility, complete loss of equilibrium, darkened
pigmentation, or rapid respiration (EG & G Bionomics, 1980a,b,g; EVS
Consultants, 1986a,b,c).
Rainbow trout (Salmo gairdnerii) were exposed to glyphosate
(as Vision) at 0, 6.25, 25 and 100 µg Vision/litre in a continuous
flow system. There were no effects on growth or foraging behaviour,
and no histopathological liver effects. Two out of three types of
aggressive behaviour were also unaffected; the third, a warning
"wig-wag" increased in frequency at the top-dose 1 month after
treatment. After two months the frequency was equal to that of the
control (Morgan & Kiceniuk, 1992).
9.1.3 Terrestrial organisms
9.1.3.1 Plants
Nodulation of sub-clover (Trifolium subterraneum) was
inhibited by an unknown formulation with glyphosate in a
dose-related way at concentrations of 2-20 mg a.i./litre (Eberbach &
Douglas, 1989). In this experiment, 3-day-old seedlings were
inoculated with Rhizobium trifolii. The seedlings were cultured
for 56 days in soil-free systems with nutrient solutions. In an
additional experiment in which Rhizobium was exposed to the same
concentrations, repeated washing of the inoculi prior to nodule
initiation did not reduce the inhibition of the nodulation of the
sub-clover after inoculation. This indicated that the effect on
nodulation might be the result of damage to the bacteria rather than
to carry-over of glyphosate from the bacteria to the plant.
Seed germination of various forest species was not affected by
treatment with Roundup at concentrations up to 305 mg a.i. (free
acid)/kg dry weight. Seed germination was affected at the highest
tested dose of 1525 mg a.i. (free acid)/kg dry weight (Morash &
Freedman, 1988). The effect on seed germination was confirmed by
another experiment in which no differences were found between
sprayed and unsprayed plots with respect to seedling composition and
quantity. Morash & Freedman (1988) then incubated the soils of
clear-cuts in a greenhouse. The application of Roundup in the field
was at a rate of 2.3 kg a.i./ha.
Red pine seedlings (Pinus resinosa) were not affected by
treatment with Roundup, with the exception of a dose-related
decrease in root length (Chakravarty & Chatarpaul, 1990b).
Non-affected growth parameters were shoot height, shoot weight, root
weight, and mycorrhizal development. In this experiment lasting 60
days, Roundup was applied at rates of 0.54 and 3.2 kg a.i./ha. The
conifer seedlings were inoculated with an ectomycorrhizal fungus
(Paxillus involutus), 14 days after germination.
Glyphosate may affect various pathways of the secondary
metabolism in the plant, although the actual targets in plants have
not been located. The synthesis of aromatic amino acids, secondary
hydroxyphenolic compounds, chlorophyll and delta-amino-levulinic
acid were reported to be affected by glyphosate (Amrhein et al.,
1980; Duke & Hoagland, 1981; Kitchen et al., 1981a,b). Aromatic
amino acids are important for the synthesis of, for instance, some
alkaloids, the phytohormone indole-3-acetic acid and phenolic
compounds such as lignin and quinones.
With respect to the synthesis of aromatic amino acids, there
are indications that the actual target is not shikimate kinase or
anthranilate synthase, but probably
5-enolpyruvylshikimate-3-phosphate synthase or chorismate synthase
(Amrhein et al., 1980). When applied to hypocotyls of buckwheat
(Fagopyrum esculentum) and to cultured cells of smooth bedstraw
(Galium mollugo), technical grade glyphosate inhibited the
conversion of shikimate to chorismate. This inhibition in vivo and
in vitro was found at concentrations of > 10 mg a.i./litre. In
the cultured cells of Galium, the accumulation of shikimate was
concomitant with a decrease of anthraquinones (Amrhein et al.,
1980). Possibly the non-transportable carbon pool, to which carbon
was found to be diverted in sugar beets (Beta vulgaris) due to
glyphosate, was in fact shikimate (Gougler & Geiger, 1984). These
effects on carbon metabolism were found at application rates
equivalent to 5 kg a.i./ha leaves. Duke & Hoagland (1981) assumed
that possibly chelation of divalent ions such as Ca2+ and Mg2+
that are involved in many metabolic pathways was the main cause of
damage.
9.1.3.2 Invertebrates
Appraisal
Glyphosate has low toxicity for bees and earthworms.
Oral 2-day LD50 values of technical grade glyphosate and
Roundup for bees were 100 µg a.i./bee and > 100 µg Roundup/bee,
respectively. Contact 2-day LD50 values for these two substances
were likewise > 100 µg a.i./bee and > 100 µg Roundup/bee (HRC,
1972). The oral 2-day LD50 of Sting was also > 100 µg Sting/bee
(Altmann, 1984). In these experiments Apis mellifera was tested. The
LD50 values indicate a slightly acute toxicity of technical grade
glyphosate, Roundup, and Sting to honey-bees. Roundup was also
slightly toxic to green lacewings (Chrysoperla carnea) when they
were exposed by contact to 1 kg Roundup/ha (SFRSA, 1990). In this
experiment the average number of eggs per female and the larval and
pupal mortality were increased due to the treatment, resulting in an
overall reduction in beneficial capacity of 41%. The beneficial
capacity is a function of the larval and pupal mortality, and the
average number of eggs per treated and untreated female. No effects
on the food uptake and mortality of the beetle Poecilus cupreus
Bonelli were observed 15 days after application of 6 kg Sting/ha
(IFU, 1990).
When exposed to artificial soil contaminated with Roundup
D-pak, earthworms (Eisenia fetida) were soft and/or slack, in a
dose-related way, at concentrations > 500 mg Roundup D-pak/kg dry
weight (IBR, 1991a). No other adverse effects were found, indicating
a 14-day NOEC of 158 mg/kg. A comparable experiment with Roundup
also indicated slight toxicity for earthworms with a 14-day NOEC of
500 mg Roundup/kg dry weight (IBR, 1991b). At higher concentrations
thin, slack and lethargic worms with a dark skin were observed.
9.1.3.3 Vertebrates
Appraisal
Glyphosate has low toxicity to birds after acute oral or
short-term dietary exposure. Mammals tested showed effects (body
weight loss) only after high levels of dosing. Herbicide-treated
foliage was not avoided by deer in the single study reported.
The acute, subacute and chronic toxicity of glyphosate and its
formulations to birds is summarized in Table 22.
Male marsupials (Sminthopsis macroura) showed significant
body weight loss after exposure to feed contaminated with
concentrations of up to 5000 mg a.i/kg feed (Evans & Batty, 1986).
No other treatment-related effects were found in the male
marsupials. In female hopping-mice (Notomys alexis and Notomys
mitchelli) fed similar doses, no treatment-related effects were
found.
Glyphosate did not affect the daily chow consumption of
black-tailed deer (Odocoileus hemionus columbianus) when these
herbivores were exposed to browse treated with glyphosate at a rate
of 2.2 kg/ha (Sullivan & Sullivan, 1979). The deer did not avoid the
contaminated browse, and sometimes even preferred it. Irrespective
of whether they were given treated alfalfa (Medicago sativa),
treated alder (Alnus rubra) or untreated feed, the deer had the
same daily chow consumption.
9.2 Field observations
9.2.1 Microorganisms
Appraisal
Some effects on microorganisms have been reported in field
studies following application of glyphosate formulations. However,
these have been minor and reversible in most cases. It is not
possible to separate the direct toxic effects of the herbicide from
changes in the habitat caused by its intended herbicidal action.
Table 22. Birds: acute and chronic toxicity of glyphosate and its formulations
Species Compound Sex Age Route Experimental Parameter Concentration Reference
duration
(days)
Colinus virginianus Tgg n.r. n.r. diet 8 LC50 > 4640 mg/kg feed Hazleton Lab. Inc.
(1973a)
Colinus virginianus Tgg n.r. 14 days oral LD50 > 3851 mg/kg b.w. Wildlife Int Ltd.
(1978c)
Colinus virginianus Tgg M,F 5 months diet 119 NOEC > 1000 mg/kg feeda Wildlife Int Ltd.
(1978b)
Colinus virginianus Ru n.r. 10 days diet 8 LC50 > 5620 mg/kg feedb Wildlife Int Ltd.
(1990b)
Anas platyrhynchos Tgg n.r. n.r. diet 8 LC50 > 4640 mg/kg feed Hazleton Lab. Inc.
(1973b)
Anas platyrhynchos Tgg M,F 6 months diet 112 NOEC > 1000 mg/kg feeda Wildlife Int Ltd.
(1978a)
Anas platyrhynchos Ru n.r. 10 days diet 8 LC50 > 5620 mg/kg feedb Wildlife Int Ltd.
(1990a)
Poephilla guttata Ru M mature diet 7 LC50 < 16 393 mg/kg feedb Evans & Batty (1986)
Poephilla guttata Ru M mature diet 5 LC50 > 8197 mg/kg feedb Evans & Batty (1986)
a Based on reproduction impairment of one generation
b Concentrations expressed as mg of the formulation per kg body weight or feed
Tgg = technical grade glyphosate; Ru = Roundup; M = males; F = females; n.r. = not reported.
9.2.1.1 Water
In a pool located in Hong Kong, treatment with 656 mg
Roundup/litre caused a substantial decrease in bacteria within 14
days after treatment. The number of colony-forming units had
returned to the control level 30 days after treatment (Chan & Leung,
1986).
Diatom populations in the water and sediments of a pond and a
stream showed a significantly different density of some species when
aerially treated with Roundup at a rate of 2.2 kg a.i./ha (Sullivan
et al., 1981). The authors concluded that these differences were
probably due to seasonal and habitat variation, rather than to
treatment with the herbicide.
Roundup may have affected the increase in ash-free dry weight
and the chlorophyll a standing crop of periphyton in the first month
after spraying with Roundup at a rate of 2.2 kg a.i./ha (Holtby &
Baillie, 1989a). In this experiment lasting around 130 days, some
tributaries in a watershed in British Columbia (Canada) were
directly oversprayed.
9.2.1.2 Soil
When Roundup was applied to a sandy loam being prepared for
conifer forestation, no significant changes in the soil respiration
were found up to about 180 days after application of Roundup at a
rate of 0.54 kg a.i./ha (Chakravarty & Chatarpaul, 1990a).
Concomitantly the numbers of fungi and bacteria decreased
significantly during the first 2 months, but after about 180 days
the numbers had recovered. These results might indicate changes in
microbial populations due to application of Roundup at recommended
application rates.
Preston & Trofymow (1989) found no significant effects on the
number of bacteria, actinomycetes, and nitrogen fixers in
ferro-humic podsols covered with alder trees (Alnus rubra) in the
Carnation Creek watershed, Canada, due to treatment with Roundup.
The only consistent effect was a significant reduction of the number
of fungi at one of the two treated sites. In this site the fungi
appeared to have recovered at the end of the study. In this
experiment of about 180 days, Roundup was hand-sprayed at a rate of
2 kg a.i./ha. In an additional comparable intensive field trial of
one month, microflora populations appeared to have recovered from
treatment after one month, with the exception of the reduction of
fungi in the litter of one of the treated sites. Actinomycetes and
nitrogen fixers in the litter appeared to be reduced in numbers due
to the treatment but they subsequently recovered. This reduction was
not found in the underlying humus layer.
Stratton & Stewart (1992) studied microbial activity in forest
soil and litter following the application of Roundup at 4.7
litres/ha (equivalent to 1.7 kg a.i./ha) to a coniferous forest
previously clear-cut and replanted. Treated and untreated (covered
with plastic sheeting during application) areas of forest were used
to obtain soil and litter samples which were tested in situ over an
8 months period following spraying. Glyphosate had a generally
stimulatory effect on microbial biomass in litter (up to 80%
increase) but no significant effect in soil. There were no
significant effects on the numbers of bacteria, fungi or
actinomycetes in either soil or litter. Glyphosate generally
stimulated respiration in both soil and litter; the degree of
stimulation was very variable throughout the sampling period,
ranging from a few percent to 100% increases in CO2 evolution.
No substance- or dose-related effects on aerobic bacteria were
observed in a sandy soil in a semi-arid region of Argentina up to 96
days after the application of Roundup (Gómez & Sagardoy, 1985).
Doses of up to 2.8 kg a.i. (free acid)/ha were applied.
No substance-related effects on the growth of an
ectomycorrhizal fungus were found when Roundup was applied at
concentrations of up to 3.2 kg a.i./ha (Chakravarty & Chatarpaul,
1990b). In these experiments red pines (Pinus resinosa) were
inoculated with the fungus Paxillus involutus. In the field 74-86%
of the seedlings that were not inoculated were colonized by
indigenous mycorrhizal fungi within two months.
9.2.2 Aquatic organisms
Appraisal
Little effect has been reported on aquatic invertebrates or
fish exposed to glyphosate formulations sprayed in the field. Minor
mortality in a single study of young trout may reflect the greater
sensitivity of early life-stages.
9.2.2.1 Plants
No field data on toxicity to aquatic macrophytes are available.
9.2.2.2 Invertebrates
An increased drift of midge larvae (Chironomus plumosus) was
found in artificial outdoor streams treated with 4.9 mg
Roundup/litre (Folmar et al., 1979). No increased drift was found at
0.5 mg Roundup/litre. In streams in a coastal rainforest in British
Columbia, Canada, only the drift densities of an amphipod (Gammarus
sp) and mayflies (Paraleptophlebia sp) increased after treatment
with Roundup at a rate of 2 kg a.i./ha (Kreutzweiser et al., 1989).
Density peaks partly coincided with periods immediately following
rainfall, which might indicate an effect due to glyphosate run-off,
or an effect due to increased discharges.
During most streamflows, the abundance of benthic
macro-invertebrates in a stream and at sites in tributary swamps was
similar at untreated sites and at sites that had been treated with
Roundup at a rate of 2.2 kg a.i./ha (Scrivener & Carruthers, 1989).
However, after periods of frequent rainstorms leading to flooding,
the abundance at the treated sites was 40-50% lower.
Water-fleas (Daphnia magna) did not show any mortality in a
pond sprayed with Roundup at application rates of up to 220 kg
a.i./ha (Hildebrand et al., 1980). In this experiment the
water-fleas were exposed for 8 days in pens that were partly
immersed in the water.
9.2.2.3 Vertebrates
Fingerlings (2.1 g, 5.8 cm) of rainbow trout (Salmo
gairdnerii) that were exposed for 14 days to Roundup in
flow-through pens in shallow streams did not show any mortality or
substance-related effects at application rates of up to 220 kg a.i.
(free acid)/ha (Hildebrand et al., 1982). In this experiment Roundup
was sprayed manually on moderately flowing forest streams in British
Columbia, Canada. In the same area an aerial application of 2.2 kg
a.i. (free acid)/ha did not cause mortality or obvious signs of
stress in rainbow trout fingerlings in flow-through pens, which were
also exposed to Roundup for 14 days (Hildebrand et al., 1982). A
direct aerial application of 2.1 kg a.i./ha on a tributary of the
Carnation Creek watershed (British Columbia, Canada), however,
killed 2.6% of the 120 fingerlings of coho salmon (Oncorhynchus
kisutch) within 24 h after application, whereas no mortality
occurred at the unexposed sites (Holtby & Baillie, 1989b). Also some
stress of the caged fingerlings was indicated within the first 2 h
after application. Up to 2 years after application no consistent
effects of Roundup on over-winter mortality, probability of entering
and leaving the tributary, timing of spring emigration, and growth
rates were found.
In artificial outdoor streams in Colorado, USA, rainbow trout
(Salmo gairdnerii) were exposed to concentrations of up to 5 mg
Roundup/litre for 12 h. No adverse effects on fecundity and
gonadosomatic indices were found in this study by Folmar et al.
(1979).
Rainbow trout (Salmo gairdnerii) in pens that were immersed
in stream water avoided Roundup at concentrations of > 40 mg
Roundup/litre (Hildebrand et al., 1982).
9.2.3 Terrestrial organisms
Appraisal
Spray drift of herbicides will affect non-target plants.
Adequate buffer zones have been defined for some application
methods.
Changes in species diversity and population size and structure
have been reported for terrestrial invertebrates and vertebrates
following applications of glyphosate formulation in the field.
Modifications in available food plants, insect populations
associated with vegetation killed by the herbicide, and ground cover
following intended effects of the spray probably account for these
changes.
9.2.3.1 Plants
Red pine seedlings (Pinus resinosa) were not affected by
treatment with Roundup in a field study. There was no dose-related
decrease of the root length, as had been observed in a comparable
laboratory experiment with the same doses (Chakravarty & Chatarpaul,
1990b). In this experiment lasting 154 days Roundup was applied at
rates of up to 3.2 kg a.i./ha. The conifer seedlings were inoculated
with an ectomycorrhizal fungus (Paxillus involutus) at 14 days
after germination, and they were planted outdoor after being
cultured in a greenhouse for up to 70 days after germination.
No plants were found as indicator species for damage due to
drift of a formulation of glyphosate when this was applied with
hydraulic ground sprayers (Marrs et al., 1989). In this study,
native British species commonly found in nature reserves were
exposed to spray drift, at several distances downwind from a zone
sprayed with 0.5 and 2.2 kg a.i./ha. The effect of windspeed was
investigated by spraying at speeds of 2.5 and 3.5 m/second. Death
and severe growth suppression occurred at a distance of 2-6 m from
the sprayer. Sublethal damage also occurred, mostly near to the
sprayer, although for Prunella vulgaris damage occurred up to 20 m
away. Epinasty was the most frequent symptom of damage. Most of the
damaged plants recovered, however. Some of the species were
consistently more sensitive, i.e. Digitalis purpurea, Centaurea
nigra, Prunella vulgaris and Lychnis flos-cuculi. Marrs et al.
(1989) concluded that, when spraying with ground sprayers, buffer
zones around nature reserves should be 5-10 m.
9.2.3.2 Invertebrates
No significant and consistent effects on the number of
nematodes and springtails were found in the upper 3 cm of
ferro-humic podsols due to treatment with Roundup (Preston &
Trofymow, 1989). The soils were covered with alder trees (Alnus
rubra) and located in British Columbia, Canada. The only
consistent effect was a significant reduction in the number of both
oribatid and non-oribatid mites on one of the treated sites around
20 days after application. On this site the number of mites appeared
to have returned to normal by the end of the study. In this
experiment of around 180 days Roundup was hand-sprayed at a rate of
2 kg a.i./ha.
No substance- or dose-related effects on mites and springtails
were observed in a sandy soil in a semi-arid region of Argentina up
to 96 days after application of Roundup (Gómez & Sagardoy, 1985).
Applied doses were up to 2.8 kg a.i. (free acid)/ha.
The numbers of herbivorous insects and ground invertebrates
were significantly reduced up to 3 years after treatment with
Roundup in a 4- to 5-year-old clear-cut planted with spruce
seedlings (Picea sp) (Santillo et al., 1989b). No effects were
found on predatory insects. The clear-cut was located in Maine, USA,
and sprayed with 1.7 kg a.i./ha. During this experiment lasting 3
years, the vegetation did not recover completely, and, apparently,
the effects on invertebrates were mainly due to habitat change.
Unintentionally untreated areas in the sprayed site showed a much
lesser reduction of invertebrates. These areas may therefore be
considered as potential sources for recolonization.
9.2.3.3 Vertebrates
Treatment of 4- to 5-year old clear-cuts in Maine, USA, planted
with seedlings of spruce (Picea sp), with Roundup at a rate of
1.7 kg a.i./ha affected breeding bird populations up to 3 years
after treatment (Santillo et al., 1989a). Total bird densities
decreased with 36% due to reduced habitat complexity, as expressed
in regenerated hardwood, vegetation height and foliage height
diversity. The most sensitive species were the insectivorous common
yellowthroat (Geothlypis trichas), Lincoln's sparrows (Melospiza
lincolnii) and alder flycatchers (Empidonax alnorum). In less
than 7-year-old clear-cuts in the Oregon coast range, Canada,
planted with Douglas fir (Pseudotsuga menziesii), some breeding
bird populations were affected due to two closely spaced treatments
with Roundup at a rate of 0.8 kg a.i./ha each (Morrison & Meslow,
1984). Two years after the application, the densities of birds using
shrubs for nesting and foraging had recovered, concomitant with the
recovery of shrub vegetation. Sensitive species were the
rufous-sided towhee (Piplio erythrophthalmus) and MacGillyvray's
warbler (Oporornis tolmiei). On the other hand, the American
goldfinch (Carduelis tristis) increased one year after
application, apparently due to the treatment. Morrison & Meslow
(1986) stated that the effectiveness of the treatment, which was not
maximal in their study, is crucial to the degree to which bird
populations are affected.
Small mammals may be affected by treatment with glyphosate,
this depending mainly on the size of the treated area, the
vegetation type and the extent to which the vegetation is damaged.
Various experiments have been performed in and around clear-cuts
planted with conifers in locations in the USA and Canada.
Insectivorous shrews (Blarina brivicauda, Sorex cinereus and Sorex
hoyi) and herbivorous voles were less abundant due to a treatment
with Roundup at a rate of 1.7 kg a.i./ha (Santillo et al., 1989b).
This significant reduction of the number of shrews was maintained
for 3 years after application, whereas the population of voles
recovered. No effects on the omnivorous deer mice (Peromyscus
maniculatus) were observed. Deer mice also appeared to be
unaffected in an experiment in which Roundup was applied at a rate
of 3 kg a.i./ha (Sullivan, 1990). The population dynamics of both
deer mice and the herbivorous Oregon voles (Microtus oregoni)
appeared not to be influenced, although some partially significant
effects were observed that might have been due to the treatment. On
the sprayed site there was an increase in the number of recruits of
both species 3 years after application, and also an increased
fecundity of deer mice and a higher survival of female voles 1 and 3
years after application. Sullivan (1990) observed no physiological
changes that might have been due to ingestion. In a clear-cut
treated with Roundup at a rate of 1.1 kg a.i./ha, only the
population density of Southern Redbacked voles (Clethrionomys
gapperi) was reduced by about 80% in a 1-year experiment (D'Anieri
et al., 1987). In another clear-cut, no adverse effects on deer mice
populations were evident after treatment with Roundup at a rate of
2.2 kg a.i./ha (Sullivan & Sullivan, 1981). Contrary to the results
of Sullivan (1990), D'Anieri et al. (1987), Sullivan & Sullivan
(1981) and Santillo et al. (1989b), a significant reduction in the
population density of deer mice was observed by Ritchie et al.
(1987) on a sprayed clear-cut of 38 ha at around 11 months after
spraying with Roundup at a rate of 1.1-1.2 kg a.i./ha. However, no
adverse effects on fertility or fecundity were indicated. Probably
the effect on abundance was due to habitat change with respect to
feed provision and cover. Possibly the effects on deer mice observed
by Ritchie et al. (1987) were less confounded by immigration as the
sprayed area was larger than in the studies in which effects on deer
mice were lacking. However, when a site of 36 ha was sprayed twice
with Roundup at a rate of 0.8 kg a.i./ha on each occasion, deer mice
were not affected, possibly due to a relatively low effectiveness of
the treatment (Anthony & Morrison, 1985). In the treated area, even
an increase of Oregon voles was found after 1 year, concomitant with
an increase of grass and other plants. The results indicated that
small mammal populations were able to recover within 2 years after
application of glyphosate, dependent on the recovery of shrubs.
10. EVALUATION OF HUMAN HEALTH HAZARDS AND EFFECTS ON THE
ENVIRONMENT
10.1 Human health hazards
Results of direct measurements of glyphosate concentrations in
foodstuffs (as part of food surveillance), drinking-water or total
diets are not available.
Absorption from the gastrointestinal tract is limited to 36% or
less and percutaneous absorption is 5.5% or less. Glyphosate is
essentially not metabolized. Total body clearance is 99% in 7 days.
Residues in livestock animals and their products (including milk)
are minimal.
Summarized information on short- and long-term studies is given
in Table 23 and on teratogenicity and reproduction studies in Table
24.
In animals, glyphosate has very low acute toxicity by the oral
and dermal administration routes. The formulation Roundup is acutely
toxic to humans when ingested intentionally or accidentally. No
controlled studies are available and therefore the human NOAEL
cannot be derived.
Animal studies show that glyphosate is not carcinogenic,
mutagenic or teratogenic. Reproductive effects were only seen at
dose levels producing maternal toxicity.
In experimental animals (13-week studies in rats and mice), an
effect was observed in the parotid salivary glands, indicating that
glyphosate may be acting as a weak adrenergic agonist. In rats, this
occurred at feeding levels of > 205 mg/kg body weight per day.
The NOAEL in chronic feeding studies is > 410 mg/kg body weight
per day. A NOAEL of 175 mg/kg body weight per day observed in a
rabbit teratogenicity study was chosen as the appropriate basis for
toxicological evaluations in humans. Through application of a
suitable safety factor, safe levels for humans can be estimated for
use in the toxicological evaluation of any actual exposures. For
technical glyphosate a safety factor of 100 is considered
appropriate given the elaborate data sets available.
Glyphosate and its concentrated formulations produce moderate
to severe eye irritation, but only slight skin irritation. Neither
glyphosate nor tested formulations induce sensitization.
Table 23. Short-term and long-term studies on glyphosate
Species Test Dose levels Effects, dose level NOAEL
compound (mg/kg-1 diet) (mg/kg diet) [mg/kg diet]
unless otherwise mg kg-1 b.w. d-1
stated
Short-term studies
Mouse technical 5000, 10 000, decreased growth and increased [10 000]
glyphosate 50 000 weights in brain, heart, 1890 m,
kidneys (50 000) 2730 f
Mouse technical 3125, 6250, reduced weight gain (50 000) [3 125]
glyphosate 12 500, 25 000, lesions of salivary glands 507
50 000 (> 6250)
Rat technical 1000, 5000, no adverse effects [20 000]*
glyphosate 20 000 1267*
Rat technical 200 to 12 500 no adverse effects [12 500]*
glyphosate NG
Rat technical 3125, 6250, increased AP and ALAT (> 6250) [< 3 125]
glyphosate 12 500, 25 000, increased haematocrit and red < 205 m
50 000 cell parameters (> 12 500), < 213 f
increased bile acids,
decreased sperm counts
(> 25 000), histological
alterations in salivary glands
(> 3125), reduced weight
gain (> 25 000)
Dogs technical 20, 100, 500 no adverse effects [NG]
glyphosate mg kg-1 bw 500*
Cattle Roundup 400, 500, 630, decreased feed intake (> 630) [NG]
790 mg kg-1 bw d-1), diarrhoea (> 500) 400
increased blood parameters
(790)
Long-term studies
Mouse technical 1000, 5000, decreased growth (30 000), [5 000]
glyphosate 30 000 increased incidence of 814
hepatocyte hypertrophy and
necrosis (30 000), increased
incidence of urinary bladder
epithelial hyperplasia
(30 000)
Rat technical 2000, 8000, decreased growth (20 000), [8 000]
glyphosate 20 000 increased liver weights 410
(20 000), increased incidences
of degenerative lens changes
(20 000) and of gastric
inflammation (8000 and 20 000)
Rat technical 60, 200, 600 slightly decreased growth (600) a
glyphosate
m = males; f = females; * Highest dose tested; NG, not given;
a The slight effect at 600 mg/kg diet (32 mg/kg bw) is considered marginal in the light
of the absence of an effect on growth at higher dose levels (2000 and 8000 mg/kg diet)
in a more recent 2-year study in rats.
Available studies on exposures of workers involved in
appli-cation of the herbicide show that exposure is low when
protective clothing is worn. The following data illustrate this
point.
a) The highest estimated exposure (dermal and inhalation) of about
8000 µg/h, as reported in a study with spray applicators,
corrected for incomplete absorption, equals about 50 µg/kg body
weight per day (8-h working day for a 70-kg adult); between the
latter level and the NOAEL of 175 mg/kg body weight per day,
adjusted for incomplete absorption from the gastrointestinal
tract (30-60%), i.e. 52-63 mg/kg body weight per day, there is
a margin of safety of about 1100.
b) The highest exposure concentration found for forest brush saw
workers was 15.7 µg/m3; between this level and the NOAEL
expressed as glyphosate from the 4-week inhalation study with
Roundup of 16 mg/m3 there is a margin of safety of 1000 (this
is borne out by the absence of adverse findings in the workers'
health examination in the study.
Table 24. Summary of teratogenicity and reproduction studies on glyphosate
Species Test Dose levels Effects NOAELa
compound (mg/kg diet) (mg/kg
body
weight)
Rat technical 300, 1000, 3500 mortality, clinical signs and 1000
glyphosate mg/kg body decreased growth in dams,
weight, early resorptions, decreased
gestation days 6-19 numbers of implantations and
visible fetuses, decreased
ossification of fetal
sternebrae (all at 3500 only);
no fetal malformations
Rabbit technical 75, 175, 350 diarrhoea and soft stools (350, 175
glyphosate mg/kg body slight at 175), nasal discharge
weight, (350)
gestation days 6-27
Rat technical 3, 10, 30 mg/kg increased incidence of renal < 30b
glyphosate body weight tubular dilation in F3b male
given in diet, pups (30)
3 gens
Rat technical 2000, 10 000, soft stools of parents (30 000), 100b
glyphosate 30 000 mg/kg decreased litter size (30 000), (2000
diet, 2 gens decreased body weights of mg/kg
parents and pups (30 000 diet)
and 10 000)
a Based on all observed effects (both in dams and offspring)
b There is some discrepancy in the results, and in the NOAELs, of the two
reproduction studies carried out with technical glyphosate; the renal
effects in the 3-generation study were not reproduced in the more recent
2-generation study with higher dose levels (for details, see section 7.5.2.).
10.2 Evaluation of effects on the environment
Following application, glyphosate will selectively partition to
particulate matter suspended in surface water, aquatic sediment or
to the soil substrate. This partitioning is usually rapid. The
mechanism of sorption is only partially understood. There is little
reported information on desorption from soil; the information
available suggests "strong" binding. Mobility studies show little
leaching of glyphosate below the upper few centimetres of the soil
profile. The major metabolite, AMPA, also appears not to leach
through soil.
Given this environmental distribution, organisms living in
aquatic sediment or soil would be expected to come into closest
contact with residues of the herbicide.
There is very little information on the bioavailability of
sediment- or soil-bound glyphosate to either aquatic or terrestrial
organisms. Few bioaccumulation or ecotoxicity studies have been
performed with sediment present.
Comparison of exposure concentrations and toxic effects is,
therefore, difficult since the relevant organisms have not been
tested and exposure of tested organisms is not by a realistic route.
10.2.1 Exposure levels and toxic effects
Exposure concentrations have been calculated from experimental
application of Roundup in the field (see Table 7 in chapter 5). The
methodology is presented in Fig. 4. No monitoring results of
environmental concentrations following actual use in agriculture or
forestry are available.
The lowest LC(EC)50 and NOEC values for microorganisms,
invertebrates and fish have been taken from the toxicity tests
reported in chapter 9 (see Fig. 4). These all refer to organisms
living in the open water and are, therefore, of questionable
significance for a compound which partitions to sediment. There is
no information on species living in aquatic sediment and little
information available on soil-living organisms, with the exception
of microorganisms.
10.2.2 Hazard evaluation for aquatic organisms
Tables 25 and 26 compare the estimated mean and maximum
exposure concentrations, following aerial application of Roundup, to
the lowest reported LC(EC)50 and NOEC concentrations for acute and
chronic exposure of aquatic organisms. The ratio between exposure
and effect concentrations has been calculated. These tables are
meant as a guide to establishing possible hazard and are not
intended to estimate the degree of effect likely to be seen in the
field. The "possible hazard" classification is a simple one using
different classification phrases for order of magnitude segments of
the ratios.
The toxicity of formulations to aquatic organisms is greater
than for technical glyphosate in many cases. This increased toxicity
is due to surfactants present in the product. No account has been
taken of possible degradation of surfactants in the ratios
presented, since no data are available. The ratios, therefore, may
overestimate the toxicity of glyphosate. If the compound is bound to
sediment in the environment, this could also reduce its toxic
effect. Since no clear evidence is available to demonstrate this
reduced toxicity, no account has been taken of partitioning to
particulates. This will also tend to overestimate toxic effect of
glyphosate.
As can be seen from the tables, possible hazard for most
aquatic organisms is small or negligible. Fish and aquatic
invertebrates would not be affected by glyphosate use. Only
microorganisms, with both acute and chronic exposure, appear to be
susceptible to effects of the herbicide. The comparisons made in the
table do not allow estimates of the degree of toxic effects likely
to be seen in the field. From the field evidence available,
populations and communities of algae are not likely to be affected
after application of glyphosate formulation. Transitory changes in
number and functioning of aquatic microorganisms are possible after
use of the herbicide.
Since data are not available, evaluation of the hazard of bound
residues of glyphosate to sediment-living organisms is not possible.
Minimal bioaccumulation of glyphosate has been reported in both
laboratory experiments and in the field. The physicochemical
properties of the compound are consistent with this conclusion.
10.2.3 Hazard evaluation for terrestrial organisms
Limited test data show low toxicity of glyphosate and its
formulations to honey-bees, earthworms, birds and mammals. These
data suggest low risk for these organisms from use of the herbicide.
Field studies have been conducted and support the view that
glyphosate does not affect soil microorganisms in the long term.
Marked changes in populations of birds and small mammals have
been seen in field studies following application of glyphosate.
These seem to result from the changes in habitat, vegetation cover,
food organisms, etc., resulting from the intended herbicidal effect
of the compound.
Carlisle & Trevors (1986a) deduced from their experiments that
nitrate-reducing bacteria are involved in metabolizing glyphosate.
Involvement of nitrifying bacteria in the biodegradation of
glyphosate was also demonstrated by Murthy et al. (1989), when they
investigated the treatment of waste water from a Roundup formulating
factory.
Pseudomonas sp. may use glyphosate as a sole P or C source,
as demonstrated by Weidhase et al. (1990). Only slight growth of the
wild-type strain of the bacterium Pseudomonas fluorescens was
observed with glyphosate as sole carbon or nitrogen source. The
herbicide was metabolized to aminomethylphosphonate (Zboinska
et al., 1992). Murthy et al. (1989) isolated a denitrifying
bacterial species that was also able to use glyphosate as a C
source. This species was isolated from activated sludge in a
waste-water treatment plant. A mutant of Arthrobacter sp. strain
GLP-1 was able to utilize glyphosate as a sole N source, whereas
this was not possible for the normal strain (Pipke & Amrhrein,
1988), probably due to the uptake of inorganic P released during
biodegradation.
As the Biological Oxygen Demand and the Chemical Oxygen Demand
of glyphosate are < 2 mg/g and 0.53 g/g, respectively, glyphosate
cannot be considered as readily biodegradable (LISEC, 1990a,b). In
suitable systems, however, glyphosate is biodegradable, as shown by
Murthy et al. (1989), who investigated the biodegradation of
glyphosate in waste-water treatment plants under different
conditions in sequencing batch reactors on a laboratory scale. These
reactors were fed with waste water from a Roundup manufacturing
facility. Glyphosate was degraded completely within one cycle of
24 h, independent of whether there was an initial aerated or anoxic
phase of 4 h. However, more glyphosate could be processed with an
anoxic initial phase, probably due to better conditions for
denitrification. Not only denitrifiers but also ammonifiers and
nitrifiers appeared to be involved in the biodegradation of
glyphosate. Only at the very high concentration of approximately
5000 mg a.i./litre was biodegradation repressed by non-glyphosate
COD and inhibited by excess ammonia production.
Pseudomonas sp. strain LBr, Flavobacterium sp. and a
denitrifying bacterial species were isolated from activated sludge
as species with the ability to use glyphosate as a P source
(Balthazor & Hallas, 1986; Jacob et al., 1988; Murthy et al., 1989).
The denitrifier was also able to use glyphosate as a sole C source.
Flavobacterium sp. degraded glyphosate to AMPA in both the
presence and absence of PO43- (Balthazor & Hallas, 1986). In
this experiment the further degradation of AMPA appeared to be
hampered in the presence of PO43-.
Pseudomonas sp. strain LBr was capable of completely
eliminating amounts of glyphosate up to 3212 mg/litre from a growth
medium (Jacob et al., 1988).
Continuous exposure of an activated sludge treatment system in
a pilot plant increased the ability of the sludge to metabolize
glyphosate to AMPA (Hallas et al., 1992). In this trial an influent
concentration of 50 mg a.i./litre was reduced to less than 5 mg
a.i./litre under continuous-flow conditions with an average
residence time of 10 min. The sludge was inoculated with immobilized
bacteria capable of degrading glyphosate. The effectiveness of the
treatment was dependent on the presence of a nitrogen source and a
non-glyphosate carbon source, and required a pH range of 6.0 to 8.0.
No data are available on the amounts of glyphosate that can be
eliminated in conventional waste-water treatment plants under
practical conditions. In waste water from glyphosate-producing
plants, 28-45% is reported to be eliminated through biological
treatment (Task Force on Water Quality Guidelines, 1991).
No data are available on the biodegradability of the
surfactants in formulations. It is, however, probable that
polyoxyethylene amine is biodegraded fairly rapidly in view of the
biodegradability of structurally related compounds (Swisher, 1987).
4.4 Bioaccumulation
Appraisal
Glyphosate is not expected to bioaccumulate in view of its
high water solubility and its ionic character. This was confirmed by
several laboratory experiments with fish, crustaceans and molluscs
and by field experiments.
In a static test, channel catfish (Ictalurus punctatus) were
exposed to 0.94-0.99 mg 14C-labelled a.i./litre (actual
concentrations) for 10 days (ABC Inc, 1981d; Monsanto, 1981a). Of
the absorbed amount, 76% was recovered in the viscera. More than 90%
of the extractable residues in the viscera and the fillet was
identified as glyphosate, whereas less than 2% was identified as
AMPA. After 10 days of depuration 80% of the absorbed activity was
eliminated. For exposed channel catfish the calculated
bioconcentration factor based on the activity absorbed by the whole
fish was 0.27. For depurated channel catfish the calculated
bioconcentration factor was 0.052.
The marsh clam (Rangia cuneata) and crayfish (Procambarus
simulans) were exposed in static tests lasting 28 days to
synthetic uncontaminated sea water and a sandy loam sediment that
was incorporated with 3 mg 14C-labelled a.i./kg (ABC Inc.,
1982d,e). These experiments were set up to assess the degree of
bioconcentration of glyphosate when used in flooded rice levees and
tidal water. The calculated bioconcentration factor for the edible
parts of the clam increased during exposure up to 4.8, whereas for
the whole crayfish it increased up to 12. The highest concentrations
in the edible parts of the clam and the whole crayfish were 0.3 mg
14C-labelled residues/kg for both. After 28 days of depuration 48%
of the accumulated residues were eliminated from the edible parts of
the clam. The concentration in these parts was then 0.16 mg
14C-residues/kg. The crayfish finally had eliminated 91% after 14
days of depuration. The concentration in the whole crayfish was then
0.02 mg 14C-residues/kg. It must be stated that this test refers
to the accumulation of 14C and not glyphosate.
In a static test without sediment, in which rainbow trout
(Salmo gairdnerii) were exposed to 2 mg a.i./litre (nominal
concentration) for 12 h, the fillets of the fish contained 80 µg
a.i./kg (in the original article the erroneous figure of 80 mg/kg
was reported), and the eggs 60 µg a.i./kg (Folmar et al., 1979).
This indicates a bioconcentration factor of 0.04 for the edible
parts. Roundup was applied in this test.
In a flow-through test in which bluegill sunfish (Lepomis
macrochirus) were exposed to 11-13 mg 14C-labelled a.i./litre
(actual concentrations) for 35 days, calculated daily
bioconcentration factors based on the whole fish increased from <
0.1, 0.2 days after the start of the test, to 0.4-0.5 at the end
(ABC Inc., 1989f). Maximum concentrations in the whole fish, viscera
and fillet were 13, 7.6 and 4.8 mg 14C-residues/kg, respectively.
The time required to reach 90% of the steady state and the uptake
rate constant were calculated to be 120 days and 0.02 mg/kg fish x
(mg/litre water)-1 x day-1, respectively. During 21 days of
depuration, the half-life of depuration was calculated to be 35. A
slow decrease in tissue concentration during depuration was
indicated. After the period of depuration 2.2 mg 14C-residues/kg
whole fish was still present. In an additional study to characterize
the 14C-residues, 95-97% of the residues in the water was
glyphosate, whereas in the whole fish and tissues 28-91% of the
recovered activity was glyphosate (ABC Inc., 1989g). In a whole fish
sample 21 days after starting the test, 49% of the recovered
activity was found to be AMPA. By treating homogenates with
proteinase K it was indicated that a substantial amount of the
absorbed residues was tightly associated with, or incorporated into,
protein.
In a field experiment in a forest ecosystem in Oregon, USA,
neither glyphosate nor AMPA were recovered in salmon fingerlings
(Oncorhynchus kisutch) after aerial application of Roundup at a
rate of 3.3 kg a.i./ha (Newton et al., 1984). The fingerlings were
released at the downstream edge of the sprayed site and analysed up
to 55 days after treatment. Glyphosate was not recovered in carp
(Cyprinus carpio) in a field experiment in which ponds were
sprayed with Roundup at rates of 1.3-1.4 kg a.i./ha (Monsanto,
1980). In this experiment of approximately 90 days, AMPA was not
recovered until 30 days after application. It then increased up to
0.21 mg/kg whole fish, remained constant for another 30 days, and
then decreased to around the limit of determination (0.1 mg/kg) at
the end of the experiment.
In a forest ecosystem in Oregon, USA, Roundup was aerially
applied at a rate of 3.3 kg a.i./ha (Newton et al., 1984).
Concentrations in mammals were of the same order of magnitude as the
concentrations in litter and ground cover. The concentrations of
glyphosate in the viscera of herbivorous small mammals decreased
more slowly than in omnivorous and carnivorous small mammals, which
was probably due to a higher ingestion of contaminated litter. The
highest concentration was found in the viscera of omnivorous
deermice (Peromysces maniculatus) immediately after spraying: 5 mg
a.i./kg. Only small traces of AMPA were found in mammalian viscera.
4.5 Waste disposal
Small amounts of glyphosate can be disposed of by mixing with
alkali and soil prior to burial in a pit or trench, whereas large
amounts should be incinerated in units equipped with effluent gas
scrubbing (IRPTC, 1991).
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
Appraisal
The low toxicity, low volatility and low body absorption of
glyphosate makes its application by backpack sprayer safe under
field condition provided that the worker wears full protective
clothing.
5.1 Environmental levels
A synopsis of concentrations of glyphosate is tabulated in
Table 7. Measurements as part of regular monitoring programmes are
very scarce; measurements in field experiments with recommended
application rates simulating common agricultural practice are
therefore included in Table 7. Only maximum amounts are tabulated as
indicative values, since the rate at which they dissipate is not
included here (see sections 4.1 and 4.3). Data on the occurrence of
glyphosate and AMPA in sewage sludge are not available.
In biota the highest concentrations of glyphosate and AMPA were
found in fresh foliage and reindeer lichen (Cladonia rangiferina).
In abiota the highest concentrations of both compounds were found in
the soil (see Table 7). The occurrence of glyphosate in the
groundwater of Texas, USA, was reported by Hallberg (1989), but the
measured concentration and the year of measurement were not
specified.
Use of glyphosate as a herbicide may result in the presence of
residues in crops and animal tissues destined for human consumption.
Application as a herbicide may also be responsible for the presence
of glyphosate in drinking-water. Direct measurements of glyphosate
in foodstuffs (as part of food surveillance), drinking-water or
total diets have not been carried out. The only information
available comes from controlled residue studies. With technical
glyphosate formulated as the isopropylamine salt in aqueous
solution, numerous residue studies have been carried out in
vegetables, grasses, oil seeds, mammalian products, poultry products
and primary feed commodities. The results are summarized in the
various reports of the FAO/WHO Joint Meeting on Pesticide Residues
(FAO/WHO, 1986a, 1987, 1988). For detailed information on these
studies the reader is referred to these reports. The appraisals made
by the JMPR included the following more general statements.
Pre-harvest (5-14 days) application of glyphosate (isopropylamine
salt) in the cultivation of cereals results in significant residues
in the grain and plant materials. Studies are available to show the
fate of glyphosate in milling, baking and brewing. Residue levels in
white flour were approximately 10-20% of the levels in wheat, while
the bran residue levels were 2 to 4 times as high as those in the
wheat. Glyphosate residues were not lost during baking, but residue
levels decreased when bread was made from flour because of dilution.
Glyphosate residue levels in malt and beer derived from
field-treated barley were, respectively, about 25% and 4% of the
original level in the barley. Some glyphosate is lost during
washing, but most of the decrease can be attributed to dilution. The
levels in groats (processed oats) were about 50% of the levels in
the pre-harvest-treated oats. In all these cases, AMPA contributed
only a small proportion (average < 2.5%) of the total residues
(FAO/WHO, 1986a, 1987).
When administered to animals glyphosate is rapidly excreted
without degradation. Residues in cattle, pig and poultry meat, eggs
and milk were negligible after the animals were fed with a diet
containing 100 mg/kg glyphosate and aminoglyphosate acid. The
highest residues were in pig liver and kidney (up to 0.16 and
0.91 mg/kg, respectively) and cattle kidney (up to 1.4 mg/kg).
Residues in animal feeds arising from pre-harvest glyphosate
applications to cereals will result in only low residues in meat,
milk and eggs (FAO/WHO, 1986a).
On the basis of these residue studies the JMPR has estimated
the maximum residue levels that are likely to occur when glyphosate
(as isopropylamine salt) is used in practice, and recommended these
levels as Maximum Residue Limits (MRLs). These MRLs are presented in
FAO/WHO (1986a, 1987, 1988).
5.2 General population exposure
Apart from the controlled residue studies mentioned above, no
data are available.
Table 7. Maximum concentrations of glyphosate in environmental air, water, soil, sediment and biota
Sample Compound Location Yeara Concentration Reference
Netherlands
Surface water glyphosate Drentsche Aa 1988-1989 0.5-1 µg/litreb (Soppe, personal
Surface water AMPA Drentsche Aa 1988-1989 6 µg/litreb communication to the IPCS, 1991)
Finland
Loam soil (agric.) glyphosate Kettula 1978 17 mg/kg d.w. Müller et al. (1981)
Loam soil (agric.) AMPA Kettula 1978 3.2 mg/kg d.w. Müller et al. (1981)
Silt soil (agric.) glyphosate Kettula 1978 3.8 mg/kg d.w. Müller et al. (1981)
Silt soil (agric.) AMPA Kettula 1978 0.4 mg/kg d.w. Müller et al. (1981)
Wild berries glyphosate Laukaa, Konnevesi 1977 1.6-2.1 mg/kgc Siltanen et al. (1981)
Wild berries AMPA Laukaa, Konnevesi 1977 0.02-0.07 mg/kgc Siltanen et al. (1981)
Reindeer lichen glyphosate Laukaa, Konnevesi 1976 45 mg/kgc Siltanen et al. (1981)
Reindeer lichen AMPA Laukaa, Konnevesi 1976 2.1 mg/kgc Siltanen et al. (1981)
Canada
Wild berries glyphosate Harker, Lamplugh 1985 8-19 mg/kg f.w. Roy et al. (1989a)
Wild berries AMPA Harker, Lamplugh 1985 0.06-0.1 mg/kg f.w. Roy et al. (1989a)
Surface waterd glyphosate Carnation Creek 1984 162 µg/litre Feng et al. (1990)
Surface watere glyphosate Carnation Creek 1984 < 1 µg/litre Feng et al. (1990)
Surface waterd AMPA Carnation Creek 1984 approx 3 µg/litre Feng et al. (1990)
Pond water glyphosate Manitoba 1986 141 µg/litre Goldsborough & Beck (1989)
Pond water AMPA Manitoba 1986 2.2 µg/litre Goldsborough & Beck (1989)
Sedimentd glyphosate Carnation Creek 1984 6.8 mg/kg d.w. Feng et al. (1990)
Suspended sediment glyphosate Carnation Creek 1984 0.06 µg/litre Feng et al. (1990)
Soil glyphosate Carnation Creek 1984 40 mg/kg d.w. Feng & Thompson (1990)
Soil AMPA Carnation Creek 1984 9 mg/kg d.w. Feng & Thompson (1990)
Table 7. (cont'd)
Sample Compound Location Yeara Concentration Reference
Canada (cont'd)
Foliage (fresh) glyphosate Carnation Creek 1984 261-448 mg/kg d.w. Feng & Thompson (1990)
Foliage (fresh) AMPA Carnation Creek 1984 < 9 mg/kg d.w. Feng & Thompson (1990)
Topcrown foliage glyphosate Oregon Coast Range 1978 489 mg/kgc Newton et al. (1984)
Topcrown foliage AMPA Oregon Coast Range 1978 2.1 mg/kgc Newton et al. (1984)
Deermice (viscera) glyphosate Oregon Coast Range 1978 5.1 mg/kgc Newton et al. (1984)
USA
Pond water glyphosate Chassell, Corvallis, 1987 90-1700 µg/litre Monsanto (1990a)
Cuthbert
Pond water AMPA Chassell, Corvallis, 1987 2-35 µg/litre Monsanto (1990a)
Cuthbert
Stream water glyphosate Chassell, Corvallis, 1987 35-1237 µg/litre Monsanto (1990a)
Cuthbert
Stream water AMPA Chassell, Corvallis, 1987 < 1.0-10 µg/litre Monsanto (1990a)
Cuthbert
Pond sediment glyphosate Chassell, Corvallis, 1987 0.26-19 mg/kg d.w. Monsanto (1990a)
Cuthbert
Pond sediment AMPA Chassell, Corvallis, 1987 0.13-1.8 mg/kg d.w. Monsanto (1990a)
Cuthbert
Stream sediment glyphosate Chassell, Corvallis, 1987 0.11-0.69 mg/kg d.w. Monsanto (1990a)
Cuthbert
Stream sediment AMPA Chassell, Corvallis, 1987 < 0.05-0.38 mg/kg d.w. Monsanto (1990a)
Cuthbert
Soil (no litter on it) glyphosate Chassell, Corvallis, 1987 0.15-4.7 mg/kg d.w. Monsanto (1990a)
Cuthbert
Table 7. (cont'd)
Sample Compound Location Yeara Concentration Reference
USA (cont'd)
Soil (no litter on it) AMPA Chassell, Corvallis, 1987 0.18-0.51 mg/kg d.w. Monsanto (1990a)
Cuthbert
Soil (litter on it) glyphosate Chassell, Corvallis, 1987 0.07-1.4 mg/kg d.w. Monsanto (1990a)
Cuthbert
Soil (litter on it) AMPA Chassell, Corvallis, 1987 0.14-0.68 mg/kg d.w. Monsanto (1990a)
Cuthbert
Foliage (fresh) glyphosate Chassell, Corvallis, 1987 650-1300 mg/kgb Monsanto (1990a)
Cuthbert
Foliage (fresh) AMPA Chassell, Corvallis, 1987 1.7-2.6 mg/kgb Monsanto (1990a)
Cuthbert
a Sampling invariably took place during the autumn period.
b Analytical procedure was unvalidated; the water was sampled at an inlet point of
a drinking-water processing facility
c It was not reported whether values were based on dry or fresh weight
d Water unbuffered by vegetation
e Water buffered by vegetation
5.3 Occupational exposure during manufacture, formulation or use
In the study of Monsanto (1977), worker exposure to Roundup was
measured during herbicide mixing and application operations and upon
re-entry of treated fields. The formulation was applied on separate
plots using three application devices, i.e. a broadcast boom
sprayer, a handgun broadcast sprayer and a backpack/hand-gun
sprayer. Exposure time during mixing was < 5 min; during
application this was about 45 to 60 min. Inhalational exposure
during mixing and spraying was determined using a sampling device
placed close to the applicator's face; the total air volume sampled
was 15-20 times the daily ventilation volume. Dermal exposure during
mixing and spraying was monitored by determination of deposition on
gauze pads placed outside or inside the operator's clothing on
different parts of the body. Operator exposure (inhalation and skin,
determination method as above) upon re-entry was determined at 1, 3
and 7 days after application. In one treated plot, this was done by
having operators walk through the plot for 28 to 44 min; in two
other plots a dummy sampling device was used to determine exposure
upon re-entry. Using the measured glyphosate residues total worker
exposure was estimated. The results are presented in Table 8.
Table 8. Applicator/worker exposure to glyphosatea
Operation Average dermal Average inhalation Total exposure
exposure exposure (µg/h)
(µg/h) (µg/h)b
Tank filling 805.1 17.9 823.0
Boom spraying 271.4 0.19 271.59
Handgun spraying 7957.0 2.47 7959.47
Backpack spraying 3619.0 0.92 3619.92
Field re-entry:
1 day after treatment 2046.6 4.58 2051.18
3 days after treatment 2919.7 0.12 2919.82
7 days after treatment 15.9 0.12 16.02
a From: Monsanto (1977)
b Calculation based on an estimated breathing rate of 1.8 m3/h
Monsanto (1990) conducted another collaborative study at three
sites maintained by the USDA Forestry Service near Clayton, Georgia;
the Savanah River Plant, South Carolina; and Edgefield, South
Carolina. Glyphosate was being used to control vegetative growth
around pine seedlings planted in clear-cut forest areas. The workers
were biologically monitored by analysis of collected composite urine
specimens. Additionally, dermal/clothing deposition and simulated
inhalation exposure were monitored by passive dosimetry with cotton
cloth patches, hand rinses and air filters. At the three sites,
exposure of workers to glyphosate using backpack sprayers while
performing their duties under normal use conditions was monitored.
The analytical results indicated that the majority of urine
composite samples had unmeasurable residues of glyphosate.
Deposition on air filters, patches and hands from measurement of
washes, however, indicated a small amount of body exposure. It was
concluded that penetration of clothing did not exceed 3.84% and thus
clothing produced 96.2% protection. Body burden, as shown in urine
samples, was extremely low and in most cases below the detection
limit.
A new Monsanto study was conducted for the assessment of worker
exposure to glyphosate during mist blower application of Roundup
herbicide. Exposure was determined by a passive dosimetry technique
while workers sprayed weeds around palm trees in a plantation in
Malaysia. The workers were fitted with gauze patches at different
locations on their clothing. Air sampling was performed in the
breathing zone and the workers hands were washed at the end of the
day. The passive dosimetry body dose estimates were calculated for a
fully clothed worker with a long-sleeved shirt, long pants and
rubber boots. The hand exposure would account for bare hands during
the loading and spraying operations. Passive dosimetry estimates for
the four replicates, corrected for clothing and dermal penetration,
transport/storage/analytical recovery and normalized for body weight
and amount of chemical handled, averaged 1.88 µg/kg body weight per
kg a.i. This is little higher than the passive dosimetry estimates
of forestry workers who applied Roundup with knapsack sprayers,
which was 1.75 µg/kg body weight per kg a.i. Thus, it can be
concluded that workers applying Roundup herbicide with mist blowers,
experience some dermal exposure. In addition, inhalation exposure to
glyphosate may be higher during mist blower application. However,
Roundup demonstrated essentially no volatility. The only possible
route would be via air-borne particles. The actual amount of
glyphosate absorbed through inhalation would be much lower than the
estimated values because the measurement includes particles too
large to be inhaled (Monsanto, 1991).
Jauhiainen et al. (1991) examined the exposure of workers to
glyphosate during silvicultural clearing with brush saws equipped
with herbicide sprayers. Measurements of air concentrations during
spraying and urinary glyphosate levels both during and following
spraying were carried out. Most of the air samples had glyphosate
concentrations below 1.25 µg/m3; the highest value observed was
15.7 µg/m3. All urine samples taken had glyphosate concentrations
below the limit of detection of 0.1 mg/litre (Jauhiainen et al.,
1991).
Lavy et al. (1992) used two methods to monitor glyphosate
exposure of workers planting conifer seedlings. Firstly, they
estimated dermal exposure based on the rate of deposition on cotton
gauze patches, surface area exposed, and a dermal penetration rate
of 1.8%. This yielded dose estimates in the range of 0-875 or
0-1.7 µg/kg body weight per h. Secondly, they attempted to measure
urinary concentration levels but found no samples above the
detection limit of 0.01 mg/litre. Based on the negative results with
the urine samples, the authors concluded that the estimates based on
patch deposition overestimated exposure by at least a factor of 11
for the most highly exposed workers (Lavy et al., 1992).
It should be noted that a dermal penetration rate of 1.8% was
used in this calculation. In view of the discussion of the dermal
penetration studies (section 6.1), a value of 5.5% is to be
preferred. However, since the estimate based on 1.8% is already an
overestimation of exposure, it is not considered necessary to adjust
the estimate of Lavy et al. (1992) to 5.5%. In their final data
assessment, the authors estimated exposure from the biological
monitoring using postulated data, i.e. assuming a concentration in
urine of half the lower limit of method validation. This yielded
mean exposure values of 0.039 to 0.080 µg/kg body weight per h (Lavy
et al., 1992).
6. KINETICS AND METABOLISM IN LABORATORY
ANIMALS AND HUMANS
Appraisal
Absorption from the gastrointestinal tract after oral intake
is limited to 30-36% of the dose or less in various species, i.e.
rats, rabbits, laying hens and lactating goats. Percutaneous
absorption in Rhesus monkeys amounts to 5.5% only, and glyphosate is
very poorly absorbed through excised human abdominal skin.
Radiolabelled glyphosate distributes widely in the body, but
is primarily found in the bones where approximately 1% can be
detected after oral administration.
Glyphosate is essentially not metabolized. This validates
kinetic studies performed with radiolabelled compound.
After absorption, excretion of glyphosate occurs mainly in the
urine. Biliary excretion is limited and elimination through exhaled
air is very low. Total body clearance is 99% after 168 h.
6.1 Absorption
The absorption percentage in rats was reported to be 30-36%
after single oral dosage at 10 and 1000 mg/kg body weight;
calculations were based on excretion percentages in urine and
faeces, and on the fact that biliary elimination is probably a minor
route (Monsanto, 1988b; Brewster et al., 1991). From a similar study
carried out in 1973, total absorption percentages of approximately
20% (male rats) and 45% (female rats) can be derived (Monsanto,
1973a). Comparable results were obtained in a recent single dose
(5.6 or 56 mg/kg body weight) disposition study in F344/N rats (NTP,
1992), which indicated that 30% of the dose was absorbed.
In a 14-day oral study in rats with application of
14C-glyphosate via the diet (dose levels 1, 10 and 100 mg/kg
feed), the observed total excretion in urine was < 10% and in
faeces approximately 80-90%. Given the minor importance of the
biliary elimination route, these data indicate absorption levels of
about 15% or less (Monsanto, 1973c). The results of oral studies
with 14C-glyphosate in rabbits (Monsanto, 1973d), laying hens
(Hazleton Lab. Inc., 1988a) and lactating goats (Hazleton Lab. Inc.,
1988b) indicate gastrointestinal absorption percentages of
approximately 30% or less.
Percutaneous absorption has been studied in Rhesus monkeys and
in human tissue in vitro. After a single application of
14C-glyphosate (isoproylamine salt) as the undiluted Roundup
formulation to the shaven intact abdominal skin (contact time 24 h)
of Rhesus monkeys, absorption amounted to only 1.8% of the dose. In
this study, however, only 16% of the dose could be accounted for at
the end of the study (Maibach, 1983). This low recovery strongly
reduces the value of the study result (possibly skin absorption is
seriously underestimated in this study). From an identical study
with diluted Roundup formulation (1:29 with water), conducted by
Wester et al. (1991), also in Rhesus monkeys (contact time 12 h),
total absorption percentages of 3.7% (at low dose) or 5.5% (high
dose) can be derived. Using both undiluted and diluted Roundup
formulation, Wester et al. (1991) observed that percutaneous
absorption of 14C-glyphosate through human skin in vitro into
human plasma as receptor fluid was < 2% (contact-time up to
16 h). In another in vitro study with human skin, absorption of
14C-glyphosate from three undiluted formulations (i.e. MON 0139,
Roundup and Roundup spray mix) was studied (contact time 24 h); very
low absorption percentages of 0.028 to 0.152% were found (Franz,
1983). With regard to these in vitro results it should be pointed
out that this technique has not yet been fully validated and
therefore direct extrapolation to in vivo human skin absorption
should be undertaken cautiously.
The absorption after inhalational intake has not been
determined.
6.2 Distribution
Concentrations of 14C label in tissues were determined on day
7 after administration of a single oral dose (10 or 1000 mg/kg body
weight) of 14C-glyphosate to rats (Monsanto, 1988b). Although only
a small proportion was absorbed, the isotope was widely distributed
throughout the body, but was primarily found in bone. The principal
results are presented in Table 9.
In rats tissue concentrations of 14C label were determined on
several occasions throughout a treatment period of 14 days and a
post-dosing withdrawal period of 10 days (dietary administration of
14C-glyphosate at 1, 10 and 100 mg/kg diet). Maximum tissue levels
were reached after 10 days or less, with highest concentrations
(maximum 0.85 mg/kg at the 100 mg/kg dose level) in kidneys
(Monsanto, 1973c). It should be noted, however, that in this study
concentrations in bone or bone marrow were not measured. An increase
of 14C in excreta was observed during the withdrawal period after
an initial rapid decrease; this indicated mobilization from storage
in depot tissue (Monsanto, 1973c).
Table 9. Concentrations of 14C label (as mg glyphosate-equivalents/kg
fresh weight) in selected tissues of rats on day 7 after a single
oral dose (rounded values) (Monsanto, 1988b)
Dose: 10 mg/kg body weight Dose: 1000 mg/kg body weight
male female male female
Blood 0.0045 0.0027 0.33 0.17
Liver 0.030 0.014 1.9 1.3
Kidney 0.022 0.013 1.9 1.4
Spleen 0.012 0.0073 2.6 3.0
Lung 0.015 0.012 1.5 1.1
Thyroid 0.00080 0.00036 1.5 1.2
Nasal mucosa 0.0050 0.023 1.7 1.8
Stomach 0.0080 0.0037 2.4 2.4
Small intestines 0.022 0.018 1.9 1.6
Colon 0.034 0.016 11.0 9.2
Bone 0.55 0.31 30.6 19.7
Bone marrow 0.029 0.0064 4.1 12.5
In lactating goats concentrations of 14C label in milk were
measured after giving capsules containing a 9:1 mixture of
14C-glyphosate and 14C-aminomethylphosphonic acid (AMPA) to a
dose level equivalent to 120 mg/kg diet (expressed as free acid) for
5 days. Concentrations in milk (as mg equivalents glyphosate/kg
whole milk) ranged from 0.019 to 0.086 mg/kg during the test period;
at day 5 after the last dose the concentration was 0.006 mg/kg
(Hazleton Lab. Inc., 1988b; Monsanto, 1988d).
In a study on laying hens, carried out using a 9:1 mixture of
14C-glyphosate and 14C-AMPA, concentrations of radiolabel were
measured in eggs collected during a 7-day period of dietary
administration at 120 or 400 mg/kg diet. At 400 mg/kg, residues in
egg white were 0.010-0.032 mg/kg (expressed as
glyphosate-equivalents) and in egg yolk 0.096-0.753 mg/kg; at
120 mg/kg the corresponding concentration ranges were 0.003-0.017
and 0.002-0.24 mg/kg, respectively. At 120 mg/kg diet, no 14C was
detectable in egg white after 10 withdrawal days; in yolk
0.019 mg/kg was present at that time (at 400 mg/kg no withdrawal
test was conducted) (Hazleton Lab. Inc., 1988a).
6.3 Metabolic transformation
Biotransformation of glyphosate occurs to a very low degree
only. In rats it was shown that all of the 14C in urine and
faeces, after a single oral application of 14C-glyphosate, was
present as unchanged parent compound (Monsanto, 1973b). Also in
rats, > 97% of the 14C in excreta, after a single oral dose,
was shown to be unchanged compound. AMPA was the only metabolite,
covering only 0.2-0.3% of the applied 14C (Monsanto, 1988a). In
laying hens also, AMPA was the only metabolite, accounting for only
a minor part of the applied amount (Monsanto, 1988c).
6.4 Elimination and excretion
In the period of 0-5 days after a single oral application of
14C-glyphosate (6.7 mg/kg body weight) to rats the total excretion
in urine was 15% (males) and 35-43% (females) of the administered
dose; total excretion in faeces was 85% (males) or 50-55% (females).
Less than 1% of the radiolabel was expired as 14CO2 (Monsanto,
1973a). In a more recent study (Monsanto, 1988b), the very low level
of expiration as 14CO2 was confirmed but no significant
inter-sex difference in the level of 14C in excreta was observed.
The result of the latter study was that at both oral dose levels (10
and 1000 mg/kg body weight) elimination in faeces was 62-70% and
excretion in urine was 14-18% (1000 mg/kg body weight) or 22-29%
(10 mg/kg body weight); less than 0.2% of the dose was expired as
14CO2. After single intravenous application (dose 10 mg/kg body
weight) 75-79% appeared in urine and only 5-8% in faeces, a finding
that shows that biliary elimination occurs to a limited degree only
(Monsanto, 1988b).
Delayed excretion in rats during a 10-day post-dosing
withdrawal period was observed after daily oral administration via
the diet for 14 days (Monsanto, 1973c); this suggests that some
storage in tissue(s) occurs when uptake is prolonged. Tissue
equilibrium was attained by day 10 of the dosing period and
excretion equalled intake by day 6 of the dosing period.
In rabbits > 80% was eliminated in faeces (with additional
14C present in the gut) and 7-11% in urine within 5 days after
administration of a single oral dose (6.7 mg/kg body weight) of
14C-glyphosate. Less than 1% of the dose was expired as 14CO2
(Monsanto, 1973d). In one oral study in lactating goats lasting 5
days, total excretion in urine varied from 20 to 24% and in faeces
from 60 to 66% (Hazleton Lab. Inc., 1988b).
6.5 Retention and turnover
Total body clearance in the study of Monsanto (1973a) was
94-98% (males) or 82-84% (females) over a 48-h period after giving a
single oral dose of 14C-glyphosate; at 120 h post-dosing this was
99% (both sexes). In Monsanto (1988b), the kinetics of whole body
elimination were estimated using the radioactivity (14C) measured
in urine and faeces after a single oral dose of 14C-glyphosate (10
or 1000 mg/kg body weight). Because of the lack of biotransformation
of glyphosate it is valid to base kinetics on total radioactivity.
The elimination appeared to be biphasic. The half-life of the alpha
elimination phase at 10 mg/kg body weight was 5.87 h (males) or
6.22 h (females); at 1000 mg/kg body weight this was 5.26 h (males)
or 6.44 h (females). The half-life of the beta phase at 10 mg/kg
body weight was 79 h (males) or 106 h (females); at 1000 mg/kg body
weight this was 181 h (males) or 337 h (females). Pretreatment with
unlabelled compound for 14 days (carried out at the low dose level)
did not have an effect on whole body elimination. Seven days after
dosing, < 0.05% of the dose was present in organs and < 0.5% in
the remaining carcass. Highest concentrations were present in bone.
It was estimated that 0.2-0.6% of the oral dose was associated with
this site; after intravenous dosing this was approximately 1%
(Monsanto, 1988b). Brewster et al. (1991) reported that in rats
nearly all of the absorbed material had been eliminated from the
body 168 h after oral administration of 10 mg/kg body weight;
approximately 1% of the dose was still associated with the bone.
In a recent study on the disposition of glyphosate in F-344/N
rats, 1% of a single oral dose (5.6 or 56 mg/kg) was found in the
tissues 72 h after dosing; 20-30% of the administered radioactivity
was eliminated via urine and 70-80% via the faeces (NTP, 1992).
7. EFFECTS ON LABORATORY ANIMALS AND IN VITRO TEST SYSTEMS
Appraisal
Glyphosate, administered by oral and dermal routes, has a very
low acute toxicity. Both glyphosate and its concentrated
formulations produce moderate to severe eye irritation, but only
slight dermal irritation. Neither glyphosate nor tested formulations
induce sensitization.
Short-term feeding studies have been conducted in several
species. In CD-1 mice, increased liver, brain, heart and kidney
weights, and growth retardation were reported at 50 000 mg/kg diet.
At 10 000 mg/kg diet, an increase in relative liver weight was
reported; however, there were no differences in absolute liver
weights when this group was compared to controls. The relative
increase represented only a 9% increase over the liver weight
reported for controls and was not considered toxicologically
significant. Additionally, there were no gross or histopathological
changes in the liver at doses of 10 000 mg/kg or more. The Task
Group considered the NOAEL to be 50 000 mg/kg diet.
In a 13-week study conducted in Charles River CD
(Sprague-Dawley) BR rats, no treatment-related effects were observed
at doses up to 20 000 mg/kg diet. The NOAEL was greater than the
highest dose tested.
Two additional 13-week studies (one in rats and the other in
mice) were conducted by NTP in which lesions of the salivary glands
were observed in both species. The NOAEL in the rat study was
< 3125 mg/kg diet and that in the mouse study was 3125 mg/kg diet.
Other short-term and long-term studies conducted in different
strains and species did not reveal similar lesions. The lesions
indicate that glyphosate may be acting as a weak adrenergic agonist.
The toxicological significance of the salivary gland lesions
observed in the NTP studies is unknown.
In a 52-week study conducted in beagles, no compound-related
effects were reported. The NOAEL was 500 mg/kg body weight per day.
In a 7-day study with the Roundup formulation in female cattle, a
NOAEL of 400 mg Roundup/kg body weight was reported. At higher dose
levels, decreased feed intake and diarrhoea occurred.
In long-term feeding studies in both rats and mice, few toxic
effects were observed. These effects were present at relatively high
dose levels only. In mice, technical glyphosate produced growth
retardation, hepatocyte hypertrophy or necrosis at 30 000 mg/kg diet
only. At 5000 and 30 000 mg/kg diet an increase in epithelial
hyperplasia of the urinary bladder was reported. The increased
incidence of this lesion did not follow a dose-related trend and in
the highest dose tested the incidence was actually lower than that
reported at the medium dose level, in spite of a 6-fold increase in
glyphosate. The observation at the medium dose (5000 mg/kg) is not
considered a compound-related effect and the NOAEL is, therefore,
considered to be 5000 mg/kg diet (814 mg/kg body weight).
Long-term feeding studies in rats resulted in decreased
growth, increased liver weight and degenerative liver changes at
20 000 mg/kg diet only. At 8000 and 20 000 mg/kg diet, there was an
apparent increase in the incidence of inflammation of the gastric
mucosa in both sexes. The only statistically significant increase
was observed in the medium-dose females (15%). This value was also
outside the historical control range of 0-13%. This finding was not
considered to be a treatment-related effect. There was no
dose-related trend across all groups of treated females and there
was no statistically significant difference in any treated male
groups. The NOAEL was therefore 8000 mg/kg diet (410 mg/kg body
weight).
Studies in rats and rabbits indicated that technical
glyphosate is not teratogenic. Two multigeneration studies were
conducted with technical glyphosate. In the first study, the only
effect noted was an increased incidence of unilateral renal tubular
dilation in F3b male pups at 30 mg/kg body weight. In the second
study, decreased body weights were reported for parents and pups and
decreased litter size was associated with dose levels of
30 000 mg/kg diet. Decreased body weights reported for parents and
pups at 10 000 mg/kg diet were not toxicologically significant. In
parents, the decrease was only 2 to 4% below controls and for pups
the decrease was 5.6 to 6.6% lower than controls. The findings in
pups were also transient and did not occur consistently in all
litters. The NOAEL was 10 000 mg/kg diet. The absence of a renal
effect in pups at a higher dose level (1500 mg/kg body weight),
though not invalidating earlier findings of unilateral renal tubular
dilation in male F3b pups, indicates that the reproducibility of
this lesion and its toxicological significance are uncertain. It
should be noted that in no other toxicological study was an effect
on kidneys found.
Bioassays in mice and rats did not indicate that technical
glyphosate was carcinogenic.
Glyphosate has been shown to have no genotoxic potential in a
range of in vitro and in vivo studies.
7.1 Single exposure
Numerous acute toxicity studies have been performed to
determine LD50 values of glyphosate and of herbicide formulations
containing glyphosate as active ingredient. The results of these
studies are summarized in Tables 10 (results for glyphosate) and 11
(results for formulations). These data show that glyphosate and its
formulations have very low toxicity by the oral and dermal
administration routes. By the intraperitoneal route glyphosate is
markedly more toxic than by the other routes. General intoxication
symptoms include breathing difficulties, ataxia and convulsions.
The mechanism of the toxic action of glyphosate has been
studied in rats. Olorunsaga et al. (1979) observed dose-related
reduced respiratory control ratios and increased phosphatase
activity in mitochondria isolated from rat livers 5 h after single
intraperitoneal doses ranging from 15 to 120 mg/kg body weight. This
effect was also seen in rat liver mitochondria in vitro (Bababunmi
et al., 1979; Olorunsaga, 1982a,b). The authors suggest that acute
toxicity at lethal doses may occur as a result of the uncoupling of
oxidative phosphorylation.
The acute toxicity in rats of the surfactant
polyoxyethyleneamine, with which glyphosate is commonly formulated
in Roundup, was compared to that of glyphosate in a study by
Martinez et al. (1990). Both compounds exhibited pulmonary toxicity
following either oral or intratracheal administration. The toxicity
of the herbicide formulation was greater than can be accounted for
on the basis of the dose response data from either compound alone
(Martinez et al., 1990; Martinez & Brown, 1991).
A study was undertaken by Tai et al. (1990) to investigate the
effects of glyphosate, surfactant, and their combination in Roundup
on cardiovascular function in female beagles. They found that
glyphosate alone at plasma levels ranging from 923 to 3450 mg/litre,
which simulates the human ingestion situation, were shown to
increase the myocardial contractility. The surfactant alone
considerably reduced the cardiac output, the left ventricular stroke
work index and the mean arterial pressure. The joint effect of both
glyphosate and the surfactant in Roundup formulation resulted in
cardiac depression, which was mostly due to the surfactant since
glyphosate itself increased myocardial contractility. The authors
indicated that the probable cause of the observed increases in
pulmonary vascular resistance index and pulmonary artery pressure
was a direct vasoactive effect of glyphosate on the pulmonary
artery.
Table 10. Acute toxicity of glyphosate to experimental animals
Species (sex) Product tested LD50/LC50a Reference
Oral studies
Rat (m,f) glyphosate techn, > 5000 mg/kg bw FDRL (1988d)
purity 97.8%
Rat (m,f) glyphosate techn, > 5000 mg/kg bw Inveresk Research Int.
purity 96-99% (1989a)
Rat (m,f) glyphosate techn, > 5000 mg/kg bw NOTOX (1988)
purity 96-99%
Rat (m,f) 85.5% techn. > 5000 mg/kg bw Bio/Dynamics Inc.
glyphosate in (1988c)
water
Rat (m,f) glyphosate, IPA salt, > 5000 mg/kg bw Monsanto (1981b)
65% in water
Rat (m.f) glyphosate, EO saltb 2047 mg/kg bw Knapek et al. (1986)
Goat (f) glyphosate techn, 3500 mg/kg bw USDA (1987c)
purity 98.7%
Goat (f) glyphosate, IPA salt, 5700 mg/kg bw USDA (1987b)
65% in water
Dermal studies
Rat (m,f) glyphosate techn, > 2000 mg/kg bw Inveresk Research Int.
purity 96-99% (1989c)
Rabbit (m,f) glyphosate techn, > 5000 mg/kg bw FDRL (1988b)
purity 97.8%
Rabbit (m,f) 85.5% techn. > 5000 mg/kg bw Bio/Dynamics Inc.
glyphosate in water (1988a)
Rabbit (m,f) glyphosate, IPA salt, > 5000 mg/kg bw Monsanto (1981c)
65% in water
Intraperitoneal studies
Mouse (m) glyphosate (not 545 mg/kg bw
further specified)
Mouse (f) glyphosate (not 740 mg/kg bw FAO/WHO (1986b)
further specified)
Mouse (m,f) glyphosate (not 134 mg/kg bw FAO/WHO (1986b)
further specified)
Rat (m) glyphosate (not 281 mg/kg bw
further specified
Table 10. (cont'd)
Species (sex) Product tested LD50/LC50a Reference
Rat (f) glyphosate (not 467 mg/kg bw FAO/WHO (1986b)
further specified)
Rat (m,f) glyphosate (not 238 mg/kg bw FAO/WHO (1986b)
further specified)
a All values expressed as mg of product tested (as presented in "product tested"
column)
b EO is an abbreviation of 5-ethoxy-oleinamine salt.
Table 11. Acute toxicity of glyphosate formulations to experimental animals
Species (sex) Product testeda LD50/LC50b Reference
Oral studies
Mouse (m,f) Roundup >5000 mg/kg bw Mitsukaido Labs (1986)
Rat (m,f) Roundup 5000 mg/kg bw Bio/Dynamics Inc.
(1988e)
Rat (m,f) "Compound No. 3607" >5000 mg/kg bw Inveresk Research Int.
(1988a)
Rat (m,f) Roundup TX >5000 mg/kg bw NOTOX (1987a,b)
Rat (m,f) Alphee >5000 mg/kg bw Bio/Dynamics Inc.
(1987a)
Rat (m,f) Sting TX >5000 mg/kg bw NOTOX (1987f,g)
Rat (m,f) Sting 2510 mg/kg bw Younger Labs Inc.
(1984)
Rat (m,f) Sting 1950 mg/kg bw Bio/Dynamics Inc.
(1984b)
Rat (m,f) MON 8780 >5000 mg/kg bw Bio/Dynamics Inc.
(1985a)
Rat (m,f) Agrichem Glyfosaat B >5000 mg/kg bw NOTOX (1990e)
Rat (m,f) "Glyfosaat 360 g/litre" >2000 mg/kg bw NOTOX (1989c)
Rat (m,f) Legend >2000 mg/kg bw CIT (1991a)
Goat (f) Roundup 4860 mg/kg bw USDA (1983)
Table 11. (cont'd)
Species (sex) Product testeda LD50/LC50b Reference
Dermal studies
Rat (m,f) "Compound No. 3607" >2000 mg/kg bw Inveresk Research Int.
(1988b)
Rat (m,f) Roundup TX >4000 mg/kg bw NOTOX (1987c)
Rat (m,f) Sting TX >4000 mg/kg bw NOTOX (1987h)
Rat (m,f) MON 8780 >5000 mg/kg bw Bio/Dynamics Inc.
(1985b)
Rat (m,f) Agrichem Glyfosaat >4000 mg/kg bw NOTOX (1990f)
B or 2
Rat (m,f) "Glyfosaat 360 g/litre" >2000 mg/kg bw NOTOX (1989b)
Rat (m,f) Legend >2000 mg/kg bw CIT (1991b)
Rabbit (m,f) Roundup >5000 mg/kg bw Bio/Dynamics Inc.
(1988f)
Rabbit (m,f) Alphee >5000 mg/kg bw Bio/Dynamics Inc.
(1987b)
Rabbit (m,f) Sting >5000 mg/kg bw Bio/Dynamics Inc.
(1984a)
Inhalation studies
Rat (m,f) Roundup (aerosol) 3180 mg/m3 Monsanto (1983d)
Rat (m,f) "Compound No. 3607" >4860 mg/m3 Inveresk Research Int.
(1989d)
Rat (m,f) Alphee >8900 mg/m3 Monsanto (1987b)
a Composition of the formulations is given in Table 2, with the exceptions of
Agrichem Glyphosaat B (or 2), "Glyfosaat 360 g/litre" and "Compound No. 3607",
which all contain approximately 480 g/litre of the isopropylamine salt, and of MON
8780 (32.8% isopropylamine salt), and Legend (40% isopropylamine salt).
b All values given as mg formulation.
7.2 Short-term exposure
7.2.1 Oral studies
In CD-1 mice, a 13-week feeding study was conducted with
technical glyphosate (purity 98.7%) using dose levels of 5000,
10 000 and 50 000 mg/kg diet (equal to 940, 1890 and 9710 mg/kg body
weight per day in males and 1530, 2730 and 14 860 mg/kg body weight
per day in females). No effect on appearance or survival was
observed. Growth retardation and increased weights of brain, heart
and kidneys were observed at 50 000 mg/kg. Liver weights were
increased at 50 000 and 10 000 mg/kg. Limited histopathology showed
no adverse effects. The authors of the study concluded that the
NOAEL was 10 000 mg/kg diet (1890 mg/kg body weight per day)
(Bio/Dynamics Inc., 1979).
In a 13-week feeding study with technical glyphosate,
Sprague-Dawley rats received 1000, 5000 or 20 000 mg/kg diet (equal
to 63, 317 and 1267 mg/kg body weight per day in males and 84, 404
and 1623 mg/kg body weight per day in females). No effect on
appearance, survival or growth occurred. Haematology, blood
biochemistry and urinalysis, carried out at test end only, were also
unaffected. Organ weights (determined for liver, kidneys and testes
only) were not affected. Limited histopathology showed no adverse
effect in any tissue. The NOAEL in this study was 20 000 mg/kg diet
(1267 mg/kg body weight per day) (Monsanto, 1987a). Absence of
toxicity was also found in another 13-week feeding study on rats
using technical glyphosate and dose levels of 200 to 12 500 mg/kg
diet (Tauchi, 1979 as cited by FAO/WHO, 1986b).
Two further 13-week studies in rodents were conducted on behalf
of the US National Toxicology Program (1992). Both rats (F-344/N)
and mice (B6C3F1) were administered glyphosate (purity
approximately 99%) in feed at levels of 3125, 6250, 12 500, 25 000
and 50 000 mg/kg diet.
In rats, reduced weight gains were observed at 25 000 mg/kg
diet (males only) and at 50 000 mg/kg diet (males and females). No
changes in feed consumption were found. Minor increases in the
relative organ weight of liver, kidney and testes, and decreased
thymus weight were observed in males only at several dose levels;
these changes did not show a clear dose relation and therefore are
not considered to be compound-related effects. Small increases in
haematocrit and red cell blood counts were observed in male rats at
> 12 500 mg/kg diet. Clinical chemistry showed increased alkaline
phosphatase (AP) and alanine aminotransferase (ALAT) at >
6250 mg/kg diet in males and at > 12 500 mg/kg diet in females.
Bile acid levels in blood were increased at 25 000 mg/kg diet (males
only) and at 50 000 mg/kg diet (males and females). Decreases in
sperm count were observed in males at > 25 000 mg/kg diet. The
only histopathological lesions found were cytoplasmic alterations of
the parotid and submandibular salivary glands, consisting of
basophilic changes and hypertrophy of acinar cells. The parotid
gland was more severely affected. The magnitude of the effect was
dose-dependent, with focal lesions in less severe cases to diffuse
involvement at higher doses. Lesions of a similar nature and
magnitude were observed in both sexes. The sublingual gland was not
detectably altered. Effects on the salivary glands were observed
already at the lowest dose level tested of 3125 mg/kg diet (equal to
205 mg/kg body weight per day for males and 213 mg/kg body weight
per day for females). Thus, this study did not yield a NOAEL (NOAEL
< 3125 mg/kg diet) (NTP, 1992).
In mice, reduced weight gains were observed at 50 000 mg/kg
diet in both sexes. Increased organ weights were noted in heart,
kidney, liver, thymus, lung and testis; these changes did not show a
clear dose relation and therefore are not considered to be
compound-related effects. Feed consumption levels were not changed
significantly. Lesions in the parotid gland were observed, similar
to those observed in rats. The sublingual gland and the
submandibular salivary glands were not detectably altered. The
effects on the parotid gland were observed in mice at 6250 mg/kg
diet (equal to 1065 mg/kg body weight per day for males and
1411 mg/kg body weight per day for females), but were not seen at
the lowest dose level tested of 3125 mg/kg diet (equal to 507 mg/kg
body weight per day for males and 753 mg/kg body weight per day for
females). The NOAEL in this study was 3125 mg/kg diet (507 mg/kg
body weight per day) (NTP, 1992).
The salivary gland lesions could also be induced in rats by
14-day exposure at feed levels of 50 000 mg/kg diet. The salivary
glands lesions induced by glyphosate were similar to those which
could be induced by exposure to high subcutaneous doses of the
ß-adrenergic agonist isoproterenol and could be partially
ameliorated with the ß-adrenergic antagonist propanolol. This
indicates that glyphosate may induce the salivary gland lesions by
acting as a weak adrenergic agonist (NTP, 1992).
The short-term toxicity of technical glyphosate was also
studied in dogs. Beagle dogs received technical glyphosate in
gelatin capsules at dose levels of 0, 20, 100 or 500 mg/kg body
weight per day for 52 weeks. No effect occurred with respect to
clinical signs, body weight, feed consumption, ophthalmoscopy,
haematology, blood biochemistry, urinalysis, gross pathology and
histopathology. The only changes in treated groups relative to
controls were increased pituitary weights (absolute and relative) in
the medium- and high-dose males. Because there were no concomitant
histological changes present in pituitaries and given the absence of
an effect on this organ (and related organs) in all other studies,
the toxicological significance of the increased pituitary weights is
questionable. The NOAEL in this study was 500 mg/kg body weight per
day (Monsanto, 1985).
A 7-day oral study was carried out with Roundup in female
cattle weighing 160 to 272 kg. Brahman-cross heifers received 400,
500, 630 or 790 mg Roundup/kg body weight per day by nasogastric
tube. At 790 mg/kg, 1/3 animals died before test end, showing
laboured breathing and pneumonia caused by aspiration of rumen
contents. Decreased feed intake was seen at 630 and 790 mg/kg;
diarrhoea occurred at 500, 630 and 790 mg/kg body weight. Slight
increases in a number of blood parameters, occurring at 790 mg/kg
only, were probably due to extracellular fluid shifts and
haemoconcentration secondary to diarrhoea. The NOAEL in this study
was 400 mg Roundup/kg body weight per day (USDA, 1987a).
7.2.2 Dermal studies
Short-term dermal toxicity studies were carried out in rabbits
with technical glyphosate and the formulation Roundup.
Technical glyphosate, moistened with saline, was applied under
occlusion at dose levels of 100, 1000 and 5000 mg/kg body weight per
day to the shaven intact or abraded skin of rabbits for 6 h/day, 5
days/week for 3 weeks. No effect on survival and growth occurred.
Slight dermal irritation (barely perceptible erythema and oedema)
was observed at 5000 mg/kg body weight only. No evidence of systemic
toxicity was found (parameters: haematology and blood biochemistry
in five animals of each sex per group, organ weights, gross
pathology, limited histopathology) (IRDC, 1982). Absence of systemic
effects was also found in the somewhat more limited 21-day study in
rabbits (no haematology and blood biochemistry) with Roundup. Skin
effects were more severe in this study: at both dose levels (76 and
114 mg/kg body weight per day, undiluted formulation applied)
erythema and oedema were seen and, in addition, exudate and
fissuring occurred at the abraded skin sites. After a 4-week
recovery period the skin effects were no longer present
(Bio/Dynamics Inc., 1975).
7.2.3 Inhalational studies
A 4-week inhalation study was carried out on rats with a 1:3
dilution of Roundup formulation. Test concentrations of 50, 160 and
360 mg/m3 of the diluted formulation (equivalent to 17, 53 and
120 mg Roundup/m3) were administered as an aerosol spray for
6 h/day, 5 days/week. The mass median aerodynamic diameter of the
test material ranged from 1.8 to 2.7 µm with geometric standard
deviations between 1.7 and 2.0. An increased incidence of irritation
of the nasal turbinates (subacute inflammation), trachea
(mononuclear cell infiltration) and lungs (perivascular lymphoid
infiltrates/aggregates) was observed among the high-dose females
only. No signs of systemic toxicity were found (parameters:
survival, growth, limited haematology and blood biochemistry, organ
weights, limited histopathology) (Monsanto, 1983e).
7.3 Long-term toxicity and carcinogenicity
Only oral studies are available. Dietary studies using
technical glyphosate were performed in mice and rats.
In Charles River CD-1 mice technical glyphosate was fed in the
diet at concentrations of 0, 1000, 5000 or 30 000 mg/kg diet for 24
months (dose levels equal to 0, 157, 814 and 4841 mg/kg body weight
per day for males and 0, 190, 955 and 5874 mg/kg body weight per day
for females). No effect on survival or appearance was noted. Body
weights were decreased in the males of the high-dose group.
Haematology and organ weights showed no effects. Histopathology in
liver revealed an increased incidence of central lobular hepatocyte
hypertrophy among high-dose males (incidences: 9/49, 5/50, 3/50 and
17/50 in control, low-dose, medium-dose and high-dose males,
respectively) and an increased incidence of central lobular
hepatocyte necrosis also among high-dose males (incidences: 0/49,
2/50, 2/50 and 10/50). Increased incidences of epithelial
hyperplasia in the urinary bladder were present in the medium-dose
and high-dose males (incidences: 3/49, 3/50, 10/50 and 8/50). There
were no statistically significant increases in the frequency of
neoplastic lesions. The NOAEL in this study was 5000 mg/kg diet
(814 mg/kg body weight per day) (Bio/Dynamics Inc., 1983a).
Two chronic feeding studies on rats were conducted with
technical glyphosate, one in 1979-1981 and the other in 1988-1990.
The first study, carried out using Charles River CD (Sprague-Dawley)
BR rats, had dose levels of 0, 60, 200 and 600 mg technical
glyphosate/kg diet (equal to about 0, 3, 10 and 32 mg/kg body weight
per day, respectively). Survival, appearance, haematology, blood
biochemistry, urinalysis and organ weights were not changed. The
NOAEL was > 600 mg/kg diet (> 32 mg/kg body weight per day).
Slight growth retardation during part of the study was noted in the
high-dose males. The incidence of interstitial cell tumours in
testes showed a statistically significant increase (incidences:
0/50, 3/50, 1/50 and 6/50; historical control range: 3-7%)
(Bio/Dynamics Inc., 1981a). This finding, in itself constituting
some evidence for a carcinogenic effect in rats, should be judged in
the light of the absence of an effect at much higher dose levels in
the more recent 2-year study in rats (see below). This is also valid
for the slight growth retardation (i.e. no effect on growth at much
higher dose levels in the more recent study, see below). In the
recent 2-year study, rats (same strain) were fed 2000, 8000 or 20
000 mg technical glyphosate/kg diet (equal to about 100, 410 and
1060 mg/kg body weight per day) for 24 months. There was no effect
on survival or appearance. Growth was retarded in the high-dose
females. Haematology and blood biochemistry showed no effects. In
the high-dose males, the urine specific gravity (after 6 months
only) and urine pH were increased. A statistically significant
increased incidence of degenerative lens changes (basophilic
degeneration of the posterior subcapsular lens capsule or mature
cataracts) was found among the high-dose males (incidences 3/60,
4/60, 4/60 and 8/60 in the control, low-, medium- and high-dose
groups, respectively. However this finding was within the historical
control range of 0-33%. Liver weights were increased in the
high-dose males only. Histopathology showed an increased incidence
Table 12. Skin irritation tests on rabbits with glyphosate and its formulations
Product testeda Contact time (h) Draize scoreb Classificationc Reference
Glyposate
Glyphosate techn. 85% in water 4 0.8 slightly irritating Bio/Dynamics Inc. (1988b)
Glyphosate techn., moistened powder 4 0 not irritating FDRL (1988a)
Glyphosate techn., moistened powder 4 0 not irritating Inveresk Research Int. (1989e)
Glyphosate, IPA salt 65% in water 24 0.2 not irritating Monsanto (1981d)
Formulations
Roundup, undiluted 4 1.9 slightly irritating Bio/Dynamics Inc. (1988g)
"Compound No. 3607", undiluted 4 1.2 slightly irritating Inveresk Research Int. (1988c)
Roundup TX, undiluted 4 0.7 slightly irritating NOTOX (1987d)
Alphee, undiluted 4 1.0 slightly irritating Bio/Dynamics Inc. (1987c)
Sting, undiluted 4 1.3 slightly irritating Bio/Dynamics Inc. (1984c)
Sting TX, undiluted 4 3.6 moderately irritating NOTOX (1987i)
Roundup L&G, undiluted 4 1.0 slightly irritating Bio/Dynamics Inc. (1985c)
"Glyfosaat 360 g/litre, undiluted 4 0.3 not irritating NOTOX (1989d)
Agrichem Glyfosaat B, undiluted 4 1.7 slightly irritating NOTOX (1990d)
Agrichem Glyfosaat 2, undiluted 4 2.0 slightly irritating NOTOX (1990b)
Legend, undiluted 4 0.7 slightly irritating CIT (1991c)
a For glyphosate content of the tested formulations, see footnote a in Table 11.
b This score is the mean score per animal and was calculated using the data from
the original report. The standard Draize scoring system was used; in calculating
the mean response per animal, the maximum response observed for an animal was used.
c The classification is based on the mean Draize score per animal. Specification:
score 0-0.5 not irritating; 0.6-2.0 slightly irritating; 2.1-5.0 moderately
irritating; 5.1-8.0 severely irritating.
Table 13. Results of eye irritation tests in rabbits for glyphosate and its formulations.
Product testeda Irritation indexb Classificationc Reference
Glyphosate
Glyphosate techn. 85% in water, undiluted 45 strongly irritating Bio/Dynamics Inc. (1988d)
Glyphosate, IPA salt, 65% in water 0 not irritating Monsanto (1981e)
Glyphosate techn. (97.6%), undiluted 54 strongly irritating FDRL (1988c)
Glyphosate techn. (96-99%), undiluted 29 irritating Inveresk Research Int. (1989f)
Formulations
Roundup, undiluted 54 strongly irritating Bio/Dynamics Inc. (1990)
"Compound No. 3607", undiluted 13 irritating Inveresk Research Int. (1989g)
Roundup TX, undiluted 31 strongly irritating NOTOX (1987e)
Alphee, undiluted 6 slightly irritating Bio/Dynamics Inc. (1987d)
Sting, undiluted 104 extremely irritating Bio/Dynamics Inc. (1984d)
Sting TX, undiluted 19 irritating NOTOX (1987j)
Roundup L&G, undiluted 19 irritating Bio/Dynamics Inc. (1985d)
"Glyfosaat 360 g/litre", undiluted 33 strongly irritating NOTOX (1989a)
Agrichem Glyfosaat B, undiluted 58 strongly irritating NOTOX (1990c)
Agrichem Glyfosaat 2, undiluted 22 irritating NOTOX (1990a)
Legend, undiluted 21 irritating CIT (1991d)
Table 13 (continued)
a For glyphosate content of the tested formulations, see footnote a in Table 11.
b The irritation index represents a mean total score per animal for response in cornea, conjunctiva
and iris, using the standard Draize scoring system. The irritation index was calculated using the data
from the original report; in calculating the mean response per animal the maximum response observed
for an animal was used. The calculation procedure is as follows:
- score conjunctiva: A. chemosis: 0-4 Calculation partial
B. discharge: 0-3 index for conjunctiva:
C. erythema: 0-3 2 x (A + B + C) = 0-20
- score iris: 0-2 Calculation partial index for iris: 5 x (0-2) = 0-10
- score cornea: A. opacity: 0-4 Calculation partial index for cornea:
B. area: 0-4 5 x A x B = 0-80
The total irritation index is the sum of the partial indices (0-110).
c The classification is based on the following scheme: index 0-5 not irritating; 5.1-12 slightly
irritating; 12.1-30 irritating; 30.1-60 strongly irritating; 60.1-80 severely irritating; 80.1-110
extremely irritating.
of inflammation of the gastric squamous mucosa in the medium- and
high-dose groups (incidences in males: 2/58, 3/58, 5/59 and 7/59;
females: 0/59, 3/60, 9/60, and 6/59; historical range: 0-13.3%). The
incidence of pancreatic islet cell adenomas was increased
(statistically significant) among low- and high-dose males
(incidences: 1/58, 8/57, 5/60 and 7/59; historical control range of
test laboratory 1.8-8.5%). The incidence in the control group was
below the historical control range; the trend test for the observed
increase was negative. No pancreatic carcinomas were found. The
NOAEL in this study was 8000 mg/kg diet (410 mg/kg body weight per
day) (Monsanto, 1990b).
Glyphosate has been tested in the US National Toxicology
Program; pre-chronic studies have been completed (NTP, 1992).
7.4 Skin and eye irritation; sensitization
Many studies have been carried out with rabbits to examine the
potential of glyphosate and its formulations to produce skin and eye
irritation. The results of these studies are briefly summarized in
Tables 12 (skin irritation) and 13 (eye irritation). Glyphosate and
its formulations produce only mild skin irritation after undiluted
single application. The result of the 21-day dermal study in rabbits
with the formulation Roundup (Bio/Dynamics Inc., 1975; see
subsection 7.2.2) shows that repeated application of undiluted
formulation to the skin does lead to irritation. The eye-irritating
potential is considerable for undiluted glyphosate and, with a few
exceptions, also for the formulations. Single application generally
produces moderate to severe reactions.
Sensitization studies have been carried out in guinea-pigs with
glyphosate and its formulations. The results of these tests are
summarized in Table 14. Neither glyphosate itself nor the tested
formulations induced sensitization in any experiment.
The results of two dermal irritation studies and one dermal
sensitization study performed with human volunteers exposed to
Roundup are presented in subsection 8.1.
Table 14. Results of sensitization tests in guinea-pigs for glyphosate and its
formulations
Product testeda Method Result Reference
Glyphosate
Glyphosate Buehler negative Bio/Dynamics Inc.
(purity 99.7%) (1983c)
Glyphosate technical Magnusson & negative Safefarm Labs Inc.
Kligmanb (1991b)
Glyphosate techn. Magnusson & negative Inveresk Research
(96-99%) Kligman Int. (1989b)
Formulations
Roundup Buehler negative Bio/Dynamics Inc.
(1983b)
"Compound Magnusson & negative Inveresk Research
No. 3607" Kligman Int. (1988d)
Roundup L&G Buehler negative Bio/Dynamics Inc.
(1987e)
Legend Buehler negative Safefarm Labs Inc.
(1991a)
Sting Buehler negative Bio/Dynamics Inc.
(1986)
a For glyphosate content of the tested formulations, see footnote a
in Table 11.
b Method also called the maximization test.
7.5 Reproductive toxicity, embryotoxicity and teratogenicity
Technical glyphosate has been tested for teratogenicity in rats
and rabbits using the oral route. In addition, two oral
multi-generation reproduction studies in rats have been reported.
7.5.1 Teratogenicity studies
Glyphosate (technical) was given to pregnant Charles River COBS
CD rats by gavage at dose levels of 0, 300, 1000 and 3500 mg/kg body
weight per day on days 6-19 of gestation. At 3500 mg/kg the
following effects were observed: increased incidences of soft
stools, diarrhoea, breathing rattles, red nasal discharge and
reduced activity, increased mortality (6/25 dams dying before the
end of the treatment period), growth retardation, increased
incidence of early resorptions, decreases in total number of
implantations and the number of viable fetuses, increased number of
fetuses with reduced ossification of sternebrae. At the lower dose
levels these effects were absent. The NOAEL in this study was
1000 mg/kg body weight per day (IRDC, 1980b).
In Dutch belted rabbits technical glyphosate was tested at dose
levels of 0, 75, 175 and 350 mg/kg body weight per day
(administration by gavage) from days 6-27 of gestation. In dams the
incidences of diarrhoea and soft stools were increased in the
high-dose group and, to a slight degree, also in the medium-dose
group. The incidence of nasal discharge was increased in the
high-dose group only. In the medium- and high-dose groups 2 and 10
dams, respectively, died during the study from unknown causes. The
NOAEL in this study was 175 mg/kg body weight per day (IRDC, 1980c).
7.5.2 Reproduction studies
A three-generation feeding study in Charles River CD
(Sprague-Dawley) BR rats was conducted in 1980-1981 and a
two-generation study, using higher dose levels, was completed in
1990 with technical glyphosate. In the former study, dietary feeding
levels were continuously adjusted to achieve dose levels of 0, 3, 10
and 30 mg/kg body weight per day. The only effect noted was an
increased incidence of unilateral renal tubular dilation in the male
pups (randomly selected) of the F3b mating of the high-dose group
(incidence 6/10 versus 0/10 in controls, not determined in
intermediate groups, earlier litters not examined). The NOAEL in
this study was < 30 mg/kg body weight (Bio/Dynamics Inc., 1981b).
The more recent two-generation study had dose levels of 0, 2000,
10 000 and 30 000 mg technical glyphosate/kg diet (equivalent to 0,
100, 500 and 1500 mg/kg body weight per day). In the high-dose
group, the following effects were observed: soft stools and
decreased body weights in parent animals, slightly decreased litter
size and decreased pup weights (the latter seen at day 14 or 21 of
lactation). The decreased body weights of parents and pups were seen
to a slight degree in the medium-dose group also. No histological
effect on kidneys was present in the F2b male pups (15 and 23 pups
examined in control and high-dose groups, respectively; first
generation and F2a pups not examined). The NOAEL in this study was
10 000 mg/kg diet (500 mg/kg body weight per day) (Monsanto, 1990c).
With regard to these reproduction studies using technical
glyphosate, it should be noted that in both studies the number of
pups submitted to histopathological examination was limited. These
limitations make evaluation of the renal effect in pups, seen (at
30 mg/kg body weight) in the study of Bio/Dynamics Inc. (1981b),
difficult.
7.6 Mutagenicity and related end-points
The results of several studies are summarized in Table 15. The
results show that glyphosate is not mutagenic.
Table 15. Results of mutagenicity studies with glyphosate and its salts.
Test system Test compound and Resultc Reference
concentrations
Ames test, Salmonella technical glyphosate - Monsanto
typhimurium TA98, TA100, (98.4%); 0.1-1000 (1978c)
TA1535 & TA1537, with and µg/plate
without metabolic activation
Ames test, S. typhimurium technical glyphosate - IET (1978)
TA98, TA100, TA1535 & (98.4%); 10-5000
TA1537, with and without µg/plate
activation
Rec assay, Bacillus subtilis, technical glyphosate - IET (1978)
strain H17 (rec+) & M45 (98.4%); 20-2000
(rec-), without activation µg/disc
Reverse mutation assay in technical glyphosate - IET (1978)
Escherichia coli strain WP2hcr, (98.4%); 10-5000
with and without activation µg/plate
Forward mutation assay, technical glyphosate - Monsanto
CHO cells, in vitro (98.7%); 0-20 mg/ml (1983b)
(HGPRT-locus), with & (- activation) or
without activation 5-25 mg/ml (+ activation)
Table 15. (cont'd)
Test system Test compound and Resultc Reference
concentrations
Cytogenetic study technical glyphosate - Monsanto
(chromosome aberrations) (98.7%); 200-1000 mg/kg (1983f)
in rat bone marrow, body weight, i.p.b;
in vivo sampling after 6, 12
and 24 h
Micronucleus test in glyphosate (not - Benova
erythrocytes of mice, specified); ´ LD50, oral; et al.
in vivo sampling time not (1989)
specified
Dominant lethal test, mouse technical glyphosate - IRDC
in vivo (98.7%); 200-2000 mg/kg (1980a)
body weight, oral
Recessive sex-linked lethal glyphosate (not - Gopalan &
test, Drosophila melanogaster, specified); dose not Njagi
in vivo given (1981)
Unscheduled DNA repair assay technical glyphosate - Monsanto
rat hepatocytes, in vitro (98.7%); 0.0125-125 (1983c)
µg/ml
a No higher concentrations tested because these would result in
osmolalities much higher than physiological levels; these high
osmolalities can produce non-specific chromosomal aberrations or
sister chromatid exchange.
b In additional studies it was demonstrated that: (1) glyphosate
produced no effect on viability and mitotic index of bone marrow
cells of rats after i.p. doses of 200-1000 mg/kg body weight
(Monsanto, 1983g); and (2) after giving 14C-labelled glyphosate
i.p. significant concentrations of 14C reached the bone marrow
(peak levels reached after 0.5 h remaining virtually constant up to
10 h after dosing) (Monsanto, 1983h).
c - = negative result
8. EFFECTS ON HUMANS
Appraisal
The formulation Roundup containing glyphosate is acutely toxic
to humans when ingested intentionally or accidentally.
No controlled studies have been conducted, and therefore the
human NOAEL level cannot not be derived.
No data are available to show the impact on workers exposed
during the manufacture or formulation of glyphosate. No
compound-related effects were observed in a test group of five
applicators prior to and after exposure for one week.
The reported higher susceptibility of individuals older than
40 years to ingested Roundup intoxication is important and requires
further investigation.
8.1 Cases of intentional and accidental exposure
Many cases of acute intoxication with herbicides containing
glyphosate and surfactant (Roundup) have been reported; most of
these were suicide attempts. Talbot et al. (1991) reviewed 93 cases
of exposure to Roundup (Chinese names: lan-da, hao-ni-chun,
nian-nian-chun) in Taiwan. The classification of the severity of
acute poisoning with Roundup as given by these authors is presented
in Table 16. Severe effects occurred only in the cases of
intentional ingestion (80 of the 93 reported). Accidental exposures
led to only mild effects. The typical symptoms were erosion of the
gastrointestinal tract (66% of the self-poisonings), seen as sore
throat, dysphagia and gastrointestinal haemorrhage. Other organs
were affected less often (nonspecific leucocytosis 65%, lungs 23%,
liver 19%, cardiovascular system 18%, kidney 14% and CNS 12%). Death
(in 7/80 cases) occurred within hours after ingestion. The amount of
undiluted Roundup ingested (rough estimates) in the lethal cases
varied from 85 to 200 ml (corresponding to roughly 30 to 70 g
glyphosate acid); but much larger amounts (500 ml Roundup,
corresponding to 180 g glyphosate acid) were reported to have been
ingested by some patients with mild to moderate symptoms. Overall,
moderate symptoms were associated with estimated intakes of 20 to
500 ml, mild symptoms with 5 to 150 ml, no symptoms with 5 to 50 ml.
The authors pointed out that the patient's estimates of the amount
ingested, and the conversion ratio used in their paper may be
inaccurate (Talbot et al., 1991). Other reviews of cases of
intoxication with Roundup have reported similar findings (Sawada &
Nagai, 1987; Tominack et al., 1991). The data of Tominack et al.
(1991) suggested that people over 40 years of age who ingest amounts
greater than 150 ml Roundup are at greatest risk of a fatal outcome.
These authors also pointed out that the surfactant contained in
Roundup may be responsible for the clinical syndrome (as suggested
by Sawada & Nagai, 1987), but that the available evidence on this
point is, as yet, inconclusive.
Table 16. Classification of severity of acute poisoning with Roundupa
Classification Description
Asymptomatic no complaints and no abnormalities on physical or laboratory
examination.
Mild mainly gastrointestinal tract(GIT) symptoms (nausea, vomiting,
diarrhoea, abdominal pain, mouth and throat pain) that resolved
within 24 h. Vital signs were stable, and there was no renal,
pulmonary or cardiovascular involvement.
Moderate GIT symptoms lasting longer than 24 h, GIT haemorrhages, endoscopically
verified oesophagitis or gastritis, oral ulceration, hypotension
responsive to intravenous fluids, pulmonary dysfunction not
requiring intubation, acid-base disturbance, evidence of transient
hepatic or renal damage, or temporary oliguria.
Severe pulmonary dysfunction requiring intubation, renal failure requiring
dialysis, hypotension requiring treatment with pressor amines, cardiac
arrest, coma, repeated seizures, or death.
a From: Talbot et al. (1991)
Further clinical experiences with patients exposed to Roundup
either accidentally or through deliberate ingestion have been
reported by Temple & Smith (1992). Symptoms resulting from dermal
exposure incidental to the use of the product included periorbital
oedema and chemosis of the eye, cardiovascular effects (tachycardia
and elevated blood pressure), swelling and paraesthesia at the site
of dermal contact and prolonged skin irritation. Deliberate
ingestion resulted in more severe effects, including lethality from
apparent respiratory and cardiac arrest (Temple & Smith, 1992).
Two dermal irritation studies were carried out with volunteers.
Application of 0.9 ml of a 9:1 dilution of Roundup formulation in
water to the intact skin of the upper arm for 24 h produced no skin
changes (Shelanski, 1973). Maibach (1986) tested undiluted Roundup
(application of 0.1 ml to intact and abraded skin sites on the back
for 24 h) and found erythema in only 1/24 subjects (23/24 no
reaction) for the intact skin sites; for the abraded skin sites 4/24
subjects showed an equivocal reaction and 10/24 showed erythema
(10/24 no reaction). The same author reported very briefly the
absence of effect in a photoirritation study in humans using
undiluted Roundup as test compound (application to abraded skin of
upper arm for 24 h with irradiation with UVA light for 45 min)
(Maibach, 1986).
A sensitization study was performed in 204 human volunteers
with undiluted Roundup according to a modified Draize method. The
summary report (no detailed report available) stated that there was
no effect in any subject (Maibach, 1986). The same author reported
absence of photosensitization by Roundup in volunteers.
8.2 Occupational exposure
The results of several studies focused primarily on the
determination of the extent of exposure to glyphosate when the
compound is used as herbicide are presented in section 5.3. The
study of Jauhainen et al. (1991) included health examinations of a
test group of five workers prior to and after an exposure period of
1 week. These examinations included haematology, clinical chemistry,
ECG, pulmonary function tests, an interview for a health
questionnaire and a general clinical examination (including
recording of blood pressure, pulse rate and pressure craft of
hands). A control group consisted of five workers. No
compound-related effects were observed (Jauhainen et al., 1991). The
other studies described in section 5.3 did not include a health
evaluation of workers.
8.3 Subpopulations at special risk
The only information available on this point is some suggestive
evidence referring to oral intoxications with Roundup; Tominack
et al. (1991) suggested that people older than 40 years are at
greater-than-normal risk after ingestion of Roundup.
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND
FIELD
In this chapter, concentrations or doses of formulations with
glyphosate are always expressed as mg product per kg soil.
Therefore, these figures may have been recalculated from the
original data, in cases where the authors reported the data in, for
instance, mg a.i./litre instead of mg Roundup per litre. For
recalculation, the percentages of the active ingredient (free acid
or the salt), given in Table 2, were used.
9.1 Laboratory experiments
9.1.1 Microorganisms
9.1.1.1 Water
Appraisal
The prokaryotic cyanobacteria are generally more sensitive to
the effects of glyphosate than the eukaryotic true algae. Similar
enzyme systems are inhibited in microorganisms to those thought to
be responsible for the herbicidal properties of glyphosate in higher
plants. The single semi-field study suggests very variable results
but with no significant effect on populations or community
structure.
The acute and chronic toxicities of technical grade glyphosate
and its formulations to cyanobacteria, algae and diatoms are
summarized in Table 17. Technical grade glyphosate is slightly toxic
with 3- to 4-day EC50 values of 1.2-7.8 mg/litre, and 7-day NOEC
values of 0.3-34 mg/litre. Formulations of glyphosate may be more
toxic (3-day EC50 values of 1.0 to > 55 mg product/litre).
Toxicity to cyanobacteria and algae is dependent on the species
or strain tested. Wängberg & Blanck (1988) exposed 16 species in
pure cultures to Roundup for 14 days. The concentration at which
growth was inhibited completely was 16 mg Roundup/litre for the most
sensitive species (Raphidonema longiseta and Anabaena sp.) and
131 mg Roundup/litre for the least sensitive species (Selenastrum
capricornutum). The prokaryotic Cyanophyta were significantly more
affected by Roundup than the eukaryotic Chlorococcales.
In Pseudomonas chlororaphis Roundup severely inhibited
respiration at concentrations of > 2623 mg/litre, whereas in
Aeromonas hydrophila respiration was only slightly affected at
these concentrations (Chan & Leung, 1986). The bacteria were exposed
for 6 days.
Table 17. Aquatic microorganisms: acute and chronic toxicity of glyphosate and its formulations (EC50, NOEC)
Organism A Test Compound Test pH Hardness Temperature Experimental Parametera Concentration Reference
type water (mg CaCO3/ (°C) duration (mg/litre)
litre) (days)
Cyanobacteria
Anabaena A S Tgg a.m. 7.5 285 24 7 NOEC 9.7b,c Malcolm
flos-aquae Pirnie
Inc.
(1987a)
Green algae
Selenastrum - S Tgg a.m. 7.0 n.r. 26 3.5-4 EC50 7.8b,e Bozeman
capricornutum et al.
(1989)
S. capricornutum A S Tgg a.m. 7.5 285 24 7 NOEC 20b,c Malcolm
Pirnie
Inc.
(1987d)
S. capricornutum - S St a.m. 7.7-9.0 26 23 3 EC50 1.0b LISEC
(1989b)
S. capricornutum - S St a.m. 7.7-9.0 26 23 3 EC50 2.5d LISEC
(1989b)
S. capricornutum - S St a.m. 7.7-9.0 26 23 3 NOEC 0.2b LISEC
(1989b)
S. capricornutum - S Ru a.m. 7.7-10.0 26 24 3 EC50 2.1b LISEC
1989a)
S. capricornutum - S Ru a.m. 7.7-10.0 26 24 3 EC50 8.0d LISEC
(1989a)
S. capricornutum - S Ru a.m. 7.7-10.0 26 24 3 NOEC 0.7b LISEC
(1989a)
Table 17 (contd).
Organism A Test Compound Test pH Hardness Temperature Experimental Parametera Concentration Reference
type water (mg CaCO3/ (°C) duration (mg/litre)
litre) (days)
Chlorella - S Ru a.m. 7.5 n.r. 25 2.1-7 EC50 > 55b Hernando
pyrenoidosa et al.
(1989)
C. pyrenoidosa - S Ru a.m. 7.5 n.r. 25 2.1-7 NOEC < 55b Hernando
et al.
(1989)
Diatoms
Skeletonema - S Tgg a.m.h 8.2-8.5 n.r. 20 4 EC50 1.2g E G &
costatum Bionomics
(1978a)
S. costatum - S Tgg a.m.h 8.2-8.5 n.r. 20 4 EC50 1.3b E G &
Bionomics
(1978a)
S. costatum - S Tgg a.m.h 8.2-8.5 n.r. 20 4 NOEC < 0.6f E G &
Bionomics
(1978a)
S. costatum A S Tgg a.m.h 7.5 285 20 7 NOEC 0.3b,c Malcolm
Pirnie
Inc.
(1987b)
Navicula A S Tgg a.m. 7.5 285 20 7 NOEC 34b,c Malcolm
pelliculosa Pirnie
Inc.
(1987c)
Table 17 (continued)
a concentration of a formulation is expressed as mg of the formulation per litre
b based on biomass decrease
c based on actual concentrations
d based on inhibition of growth rate
e NOEC value not reported
f based on biomass and chlorophyll a decrease
g based on chlorophyll a decrease
h salinity was 30%
A = actual concentrations are measured; - = nominal concentrations; S = static system;
Tgg = technical grade glyphosate; Ru = Roundup;
St = Sting; a.m. = artificial medium; n.r. = not reported
Chan & Leung (1986) found that the activity of
5-enolpyruvyl-shikimic acid-3-phosphate synthase was inhibited
strongly in aquatic bacteria at the lowest tested concentration of
656 mg Roundup/litre. This enzyme takes part in the biosynthetic
sequence to phenylalanine, tryptophan, and tyrosine via the
conversion of shikimate to chorismate, and the conversion of
chorismate to anthralinate. In an in vitro experiment with
cell-free extracts of Aerobacter aerogenes, technical grade
glyphosate inhibited the conversion of shikimate to chorismate at
concentrations of > 0.2 mg a.i./litre (Amrhein et al., 1980).
In Chlorella pyrenoidosa Roundup affected the growth, the
greening process and the photosynthetic metabolism at the lowest
tested concentration of 55 mg/litre when exposed for 2.1 or 7 days
(Hernando et al., 1989). Synthesis of chlorophyll a and b and
carotenoids was then significantly inhibited. Roundup affected
synthesis of chlorophyll a to a greater extent than that of the
other pigments. In vivo and in vitro studies with isolated
chloroplasts showed inhibition of the photosystems PS I and PS II at
concentrations of > 55 mg Roundup/litre. Hernando et al. (1989)
suggested that glyphosate acted as an electron transport inhibitor
and that the stronger inhibition of PS II compared with PS I was due
to the surfactant polyoxyethyleneamine.
In periphytic algal communities that were collected in ponds of
boreal forests, Roundup decreased the carbon fixation rate by 50% at
concentrations of 243-479 mg/litre (Goldsborough & Brown, 1988). The
algae were exposed for 4 h. Austin et al. (1991) cultured periphyton
on glass plates suspended in artificial "stream-troughs" which were
supplied with flowing water pumped from natural streams in British
Columbia, Canada. The stream water was low in phosphorus and flowed
out of an oligotrophic lake. Glyphosate was added to give nominal
concentrations in the troughs of between 0.001 and 0.3 mg/litre. A
further series of treatments added nutrients to troughs. The
herbicide was not toxic to the periphyton. A transitory decrease in
growth was followed by a stimulation of biomass in the
glyphosate-treated troughs. Similar effects were seen with added
nutrient. The authors considered the effect to be the result of
algae using glyphosate as a phosphate source. Communities of
periphyton were similar in all treatments.
Various studies have indicated that glyphosate may affect
aromatic amino acid synthesis in microorganisms, in addition to the
greening process, respiration and photosynthesis (Amrhein et al.,
1980; Chan & Leung, 1986; Pipke & Amrhein, 1988).
9.1.1.2 Soil
Appraisal
Soil bacteria in culture have shown effects of glyphosate on
nitrogen fixation, denitrification and nitrification. However, field
studies after application of formulations have not shown significant
effects. Closely related species of bacteria have been shown to be
capable of degrading glyphosate (see chapter 4). The lack of
information on the bioavailability of glyphosate in soil makes it
difficult to relate the evaluation of effects in culture to the
actual exposure in the field.
Technical grade glyphosate inhibited the growth of bacteria
isolated from a sprayed garden soil of sandy loam to a lesser extent
than it did that of bacteria isolated from an unsprayed control
(Quinn et al., 1988). In a mineral salt medium the growth rate of
bacteria from the untreated site was reduced by 50% at 590 mg
a.i./litre at 50 h after application.
In various studies, glyphosate, applied at the recommended
rates, caused neither inhibition of processes that are part of the
nitrogen cycle nor inhibition of enzymes involved in microbial
activity.
Roundup did not affect dehydrogenase activity in sand and loamy
sand when applied at rates of 4.9 and 24 kg a.i./ha (NATEC, 1990).
In the same soils amended with lucerne (Medicago sativa), the same
application rates did not change significantly the amounts of
nitrate nitrogen, although in treated loamy sand there was a slight
increase. These experiments, with a duration of 28 days, indicated a
slight stimulation of nitrification in loamy sand. A stronger
stimulation of nitrification was found in silt loam, silty clay loam
and sandy loam up to 84 days after application of technical grade
glyphosate at rates of 5 and 25 mg a.i./kg (ABC Inc., 1978d). This
stimulation was not dose-related. In this experiment no
substance-related effects were found on nitrogen fixation and
nitrite formation.
Nitrogen fixation was not affected significantly at an
application rate of 13 mg a.i./kg dry weight whether the product
applied was technical grade glyphosate or Roundup, or whether
aerobic or anaerobic conditions were used (Carlisle & Trevors,
1986a). At concentrations > 127 mg a.i./kg dry weight, nitrogen
fixation was inhibited under anaerobic conditions, whereas this
could not be verified under aerobic conditions due to very low
acetylene reduction rates. In the same agricultural sandy loam,
denitrification under anaerobic conditions was stimulated at
concentrations of > 13 mg a.i./kg dry weight, whether technical
grade glyphosate or Roundup was applied. Denitrification was
stimulated more strongly, when, in addition to the test compound,
glucose was added. In the same soil, no substantial effects of
either test compound on nitrification were found at 77 mg a.i./kg
dry weight. Dose-related inhibition of nitrification was found at
concentrations > 230 mg a.i./kg dry weight for glyphosate and
Roundup with respect to nitrate and nitrite production,
respectively. For both substances the inhibition was transient in
these studies. The temperature used was not reported.
No substantial effects on nitrification or denitrification were
found in two agricultural soils from Southern Finland after
application of an unknown formulation at a rate of 2.6 kg a.i./ha
(Müller et al., 1981). A strong inhibition of nitrogenase activity
was possibly an indirect effect due to a changed C/N ratio after the
treatment. In this study lasting 28 days, pretreatment measurements
were used as a control.
Roundup did not inhibit nitrification in three agricultural
soils after treatment at a recommended application rate of 2.9 kg
a.i./ha in 25-day experiments (Stratton, 1990). In a sandy loam,
nitrification was stimulated after the application of 145 kg
a.i./ha, whereas nitrification rates were inhibited by 50% at rates
of 194-435 kg a.i./ha. Mineralization, expressed as the time-course
of mg N/g dry weight (N = ammonium or nitrate nitrogen), in two
agricultural sandy loam soils was stimulated significantly due to
the treatment with 100 mg a.i./kg dry weight during an experiment
lasting 70 days (Marsh et al., 1977).
Not only in agricultural soils but also in forest soils, the
effects of Roundup on the nitrogen cycle appear not to be
inhibitory. Preston & Trofymow (1989) found no significant effects
on nitrification or the immobilization of urea nitrogen in an
organic fir forest floor and its underlying mineral horizon due to
the application of 10 and 50 mg a.i./kg dry weight. This experiment
lasted for 40 days.
In two agricultural sandy loam soils exposed to 100 mg a.i./kg
dry weight for 210 days, transient slight effects on CO2 evolution
were observed by Marsh et al. (1977). These effects were both
stimulatory (first 20 days) and inhibitory (until approximately 130
days) in both the soil from a permanent grassland and the soil from
an arable field, but especially in the latter. Carlisle & Trevors
(1986b) observed a dose-related increase in both O2 consumption
and CO2 production in an agricultural sandy loam soil during a
test of 10 days, with both technical grade glyphosate and Roundup.
In general the increases were significant at concentrations greater
than or equal to 127 mg a.i./kg. At the lowest dose (13 mg a.i./kg
soil) no effects were observed except a significant increase in
oxygen consumption due to Roundup. Oxygen consumption at doses
greater than or equal to 127 mg a.i./kg was more strongly stimulated
by Roundup than by technical grade glyphosate, possibly due to the
isopropylamine or surfactant. Preston & Trofymow (1989) found no
significant effect on CO2 production in an organic fir forest
floor and its underlying mineral horizon after the application of 10
and 50 mg a.i./kg dry weight. This experiment lasted 40 days.
Aerobic H2 oxidation in an agricultural sandy loam was
significantly inhibited by both technical grade glyphosate and
Roundup, but only at the highest dose of 635 mg a.i./kg dry weight
(Carlisle & Trevors, 1986b). Inhibition was stronger under anaerobic
conditions, and was found at concentrations of > 127 mg a.i./kg
dry weight to be significant and dose related. Anaerobic inhibition
might have been due to increased H2 generation as a result of
stimulated fermentation.
No effects of glyphosate on the activities of 1,3-ß-glucanase
and urease in a silt loam were found after application of Roundup at
a rate of 12 mg a.i./kg dry weight (Lethbridge et al., 1981).
Preston & Trofymow (1989) found no significant effect on urea
hydrolysis in an organic fir forest floor and its underlying mineral
horizon amended with 200 mg urea nitrogen per kg dry weight, due to
treatment with Roundup at a rate of 50 mg a.i./kg dry weight.
The degradation of cellulose, starch, protein, or leaf litter
was not inhibited at concentrations of 5 and 25 mg a.i./kg soil in
three agricultural soils, with the exception of litter in silt loam
at the highest dose (ABC Inc., 1978e). In this experiment lasting 84
days, technical grade glyphosate was applied.
Bacterial growth of Rhizobium trifolii in sterile solutions
with Bergersen's broth was completely inhibited at solution
concentrations of 10 and 20 mg a.i./litre, applied as an unknown
formulation of glyphosate (Eberbach & Douglas, 1983). Only at 10 mg
a.i./litre did the bacterial growth recover within 4 days. At lower
concentrations no inhibition was found.
Mycelial growth of ectomycorrhizal fungi in pure cultures on
agar was inhibited by 50% or more at concentrations of > 1 mg
a.i./litre for Pisolithus tinctorius and of > 100 mg a.i./litre
for Cenococcum geophilum and Hebeloma longicaudum (Estok et al.,
1989). Ectomycorrhizal fungi commonly associated with pines (Pinus
sp) were significantly inhibited at concentrations of > 29 µg
Roundup/litre (Chakravarty & Chatarpaul, 1990a). The most sensitive
species were Cenococcum graniforme, Hebeloma crustuliniforme and
Laccaria laccata. Roundup increased the susceptibility of sandy
soil for Gaeumannomyces gramminis, a fungus causing "take-all
disease" in wheat crops (Mekwatanakarn & Sivasithamparam, 1987).
Application at a rate of 0.54 µg a.i./kg increased the survival and
pathogenicity of the fungus significantly after 140 days of
incubation at 25 °C. These authors concluded that Roundup affected
microbial antagonists of the fungus.
9.1.2 Aquatic organisms
9.1.2.1 Plants
Appraisal
There is conflicting information on the effects of sediment on
the phytotoxicity of glyphosate to aquatic plants; Lemna showed
reduced effects whilst Carthamus did not. Generally, glyphosate is
thought to be largely unavailable to plants when added to soil and
only effective as a herbicide when applied to foliage.
The chronic toxicity to aquatic macrophytes when exposed to
technical grade glyphosate or Roundup dissolved in water is
summarized in Tables 20 and 21. Glyphosate is slightly toxic with a
14-day NOEC value of 9 mg/litre. Roundup is also slightly toxic with
14-day NOEC values of 2.4-56 mg/litre. No data on acute toxicity for
plants were available.
When Roundup was sprayed at a rate of 0.8 kg a.i./ha, the
phytotoxicity to floating plants of the common duckweed (Lemna
minor) was dependent on the extent of washed-off deposit (Lockhart
et al., 1989). Phytotoxicity was highest when the sprayed deposits
were not washed off within 6 h after application. Suspensions of
50 mg/litre of inorganic bentonite clay reduced the phytotoxicity of
Roundup to common duckweed significantly (Hartman & Martin, 1984).
When exposed for 14 days, concentrations up to 24 mg Roundup/litre
had no effect on plant growth when sediment was added, whereas the
growth was reduced by 50% at 5 mg Roundup/litre when no sediment was
added.
Phytotoxicity of glyphosate to the safflower (Carthamus
tinctorius) was not significantly reduced when the test compound
was added to drainage water with suspended particles instead of
distilled water (Bowmer et al., 1986). In these bioassays the
inhibition of root elongation was measured when the plants were
exposed to concentrations in unfiltered water of approximately
0.1-3 mg a.i./litre. The maximum concentration of adsorbed
glyphosate was 2500 mg/kg.
Although it had inhibitory effects at high concentrations,
Roundup had a stimulatory effect on the growth of common duckweed
(Lemna minor) and tubers of sago pondweed (Potamogeton
pectinatus) at lower concentrations (Hartman & Martin, 1985;
Lockhart et al., 1989). Enhancement of growth of common duckweed and
sago pondweed was found at 7-56 and 3 mg Roundup/litre,
respectively. These stimulatory effects may refer to hormesis.
9.1.2.2 Invertebrates
Appraisal
The data from laboratory toxicity tests show that formulations
are often more toxic than technical glyphosate to aquatic
invertebrates. The surprising result that addition of clay particles
to Daphnia test systems increased the toxicity of glyphosate is
probably due to ingestion of herbicide bounds to the particles. Few
studies have been conducted in the presence of sediment; the
reported toxicity of glyphosate is, therefore, difficult to relate
to the field situation.
The acute and chronic toxicity of technical grade glyphosate
and its formulations to aquatic invertebrates are summarized in
Tables 18-21. Technical grade glyphosate is slightly to very
slightly toxic, with LC50 values of > 55 mg/litre and a 21-day
NOEC value of 100 mg/litre. Formulations of glyphosate are
moderately to very slightly toxic with 2-day EC50 values of
5.3-5600 mg product/litre and 21-day MATC values of 1.4-4.9 mg
product/litre. The higher toxicity of Roundup to crustaceans is
mainly due to the presence of surfactants (Servizi et al., 1987).
In a laboratory in vitro test with the gills of Mytilus
californianus, a marine water mollusc, the active uptake of glycine
was inhibited by 23 and 67%, respectively, when a mixture of
14C-labelled and unlabelled glyphosate was applied at rates of
0.2-1.7 mg/litre (Swinehart & Cheney, 1987). This inhibition might
be an indication for Mg2+-moderated binding of glycine to the gill
surface, whereas glyphosate can compete with glycine uptake by
forming a metal complex.
Water fleas (Daphnia magna) were less sensitive to Roundup,
when the water was aerated than when it was unaerated. The 2-day
LC50 value decreased from 37 (with aeration) to 24 mg (without
aeration) Roundup/litre (EG & G Bionomics, 1980f).
Addition of 50 mg/litre of inorganic bentonite clay decreased
the 2-day EC50 value for mature water fleas (Daphnia pulex) from
16 to 7 mg Roundup/litre (Hartman & Martin, 1984). This higher
toxicity might have been due to ingestion of particle-adsorbed
glyphosate. Addition of bentonite also increased the toxicity in a
chronic experiment with populations of Daphnia pulex. Immature
water fleas were more sensitive to glyphosate than the adults under
all conditions. Populations in all treatments had recovered within
14 days after the application (Hartman & Martin, 1984).
No avoidance of glyphosate was found when mayfly nymphs
(Ephemerella walkeri) were tested at concentrations of 0.2-2 mg
Roundup/litre, whereas avoidance was demonstrated at 24 mg/litre
(Folmar et al., 1979).
9.1.2.3 Vertebrates
Appraisal
The toxicity tests for fish are generally performed without
sediment. As the bioavailability of glyphosate itself will be
reduced under most conditions due to sorption onto sediment, no
toxic effects are expected. Toxic effects, however, can be expected
due to surfactants in some formulations. To a lesser extent,
life-stage, pH, water hardness, temperature, and the presence of
feed all influence toxicity. No adverse effects on the
osmoregulatory mechanism were found.
The acute and chronic toxicity of technical grade glyphosate
and its formulations to fish are summarized in Tables 18-21.
Technical grade glyphosate is moderately toxic with 4-day LC50
values of 10 to > 1000 mg/litre, a 21-day NOEC value of
52 mg/litre, and an MATC value of > 26 mg/litre. Formulations of
glyphosate have comparable toxicity with 4-day LC50 values of 2.4
to > 1000 mg product/litre, and 21-day NOEC values of 0.8-2.4 mg
product/litre. Toxicity may vary substantially, depending on the
species, the test compound and test conditions. In general,
technical grade glyphosate is less toxic than the formulations. This
difference is mainly due to a higher toxicity of surfactants in the
formulations (Folmar et al., 1979; Servizi et al., 1987; Mitchell
et al., 1987; Wan et al., 1989).
Table 18. Aquatic organisms: acute toxicity of technical grade glyphosate in a static test system
Organism A Test pH Hardness Temperature Experimental Parameter Concentration Reference
water (mg CaCO3/ (°C) duration (mg/litre)
litre) (days)
Molluscs
Crassostrea - n.w.a n.r. n.r. 25 2 EC50 > 10b Bionomics
virginica, eggs (1973a)
Echinodermata
Tripneustes - n.w.a 7.7-8.2 n.r. 20 4 EC50 > 1000c E G & Bionomics
esculentes (1978d)
Crustaceans
Daphnia magna, - w.w. 7.8-8.0 > 250 19 2 LC50 780 ABC Inc.
first instar (1978a)
Uca pugilator - a.m.a n.r. n.r. 21 4 LC50 934 Bionomics
(1973b)
Palaemonetes - a.m.a n.r. n.r. 21 4 LC50 281 Bionomics
vulgaris (1973b)
Mysidopsis bahia - n.w.a 6.4-8.3 n.r. 20 4 LC50 > 1000 E G &
Bionomics
(1978c)
Insects
Chironomus plumosus, - r.w. n.r. 40 22 2 EC50 55d Folmar et al.
fourth instar (1979)
Table 18 (contd).
Organism A Test pH Hardness Temperature Experimental Parameter Concentration Reference
water (mg CaCO3/ (°C) duration (mg/litre)
litre) (days)
Fish
Ictalurus - r.w. n.r. 40 22 4 LC50 130 Folmar et al.
punctatus (1979)
Salmo gairdnerii, - divers 6.3-8.2 5.3-148 n.r. 4 LC50 10-197 Wan et al.
0.4 cm, 0.5 g (1989)
Salmo gairdnerii, - r.w. 4.4-7.2 45 12 4 LC50 86 ABC Inc.
0.4 cm, 0.6 g (1978b)
Oncorhynchus keta, - divers 6.3-8.2 5.3-148 n.r. 4 LC50 10-148 Wan et al.
0.4 cm, 0.5 g (1989)
O. kisutch, A divers 6.3-8.2 5.3-148 n.r. 4 LC50 27-174 Wan et al.
0.4 cm, 0.5 g (1989)
O. tshawytsha, - divers 6.3-8.2 5.3-148 n.r. 4 LC50 19-211 Wan et al.
0.4 cm, 0.5 g (1989)
O. gorbusha, - divers 6.3-8.2 5.3-148 n.r. 4 LC50 14-190 Wan et al.
0.4 cm, 0.5 g (1989)
Lepomis - r.w. n.r. 40 22 4 LC50 140 Folmar et al.
macrochirus (1979)
L. macrochirus, - r.w. 6.6-7.0 45 21 4 LC50 120 ABC Inc.
0.3 cm, 1.0 g (1978c)
Pimephales - r.w. n.r. 40 22 4 LC50 97 Folmar et al.
promelas (1979)
Rasbora - r.w. n.r. 25 21 4 LC50 168 HRC (1977)
heteromorpha,
0.1-0.3 cm
Cyprinodon - n.w.e 7.6-8.3 n.r. 20 4 LC50 > 1000 E G & Bionomics
variegatus, (1978b)
0.7-1.0 cm
a salinity 20-35%
b based on abnormal development of oyster larvae
c based on immobility, drooping spines, and retracted podia
d based on immobilisation
e salinity 18%
A = actual concentrations are measured; - = nominal concentration; a.m. = artificial medium;
r.w. = reconstituted water; w.w. = well water; n.w. = natural surface water;
n.r. = not reported
Table 19. Aquatic organisms: acute toxicity of formulations with glyphosate
Organism A Test Compound Test pH Hardness Temperature Experimental Parameter Concentration Reference
type water (mg CaCO3/ (°C) duration (mg/litre)a
litre) (days)
Crustaceans
Daphnia - S Ru r.w. 8.2-8.3 175 22 2 EC50 5.3b E G & G
magna, first Bionomics
instar (1980e)
D. magna, - S Ru r.w. 7.7-8.1 175 22 2 EC50 24-37b E G & G
first instar Bionomics
(1980f)
D. magna, - S Ru r.w. n.r. 40 22 2 EC50 7.3b Folmar
first instar et al.
(1979)
D. magna, - S St w.w. 8.3-8.5 225-275 20 2 EC50 42b ABC Inc
first instar (1984c)
D. magna, - S RuD w.w. 7.9-8.6 255 20 2 EC50 930b ABC Inc
first instar (1981a)
D. pulex, - S Ru w.w. 7.6 282 15 2 EC50 19b Hartman &
mature Martin
(1984)
Gammarus A CF Ru w.w 7.9-8.3 255 17 2 EC50 42b,c ABC Inc
pseudolimnaeus (1982b)
Insects
Chironomus - S Rod r.w. 7.6-7.8 42-44 22 2 EC50 5600b Buhl &
riparius, Faerber
fourth instar (1989)
Table 19 (cont'd)(2)
Organism A Test Compound Test pH Hardness Temperature Experimental Parameter Concentration Reference
type water (mg CaCO3/ (°C) duration (mg/litre)a
litre) (days)
Chironomus - S Ru r.w. n.r. 40 22 2 EC50 44b Folmar
plumosus, et al.
fourth instar (1979)
Fish
Ictalurus - S Ru r.w. 6.3-7.2 24-40 22 4 LC50 52 E G & G
punctatus, Bionomics
0.7 cm, 3 g (1980a)
Ictalurus - S Ru r.w. n.r. 40 22 4 LC50 32 Folmar
punctatus et al.
(1979)
Salmo - S St r.w. 6.8-7.3 40-45 12 4 LC50 7.5 ABC Inc
gairdnerii, (1984a)
0.4 cm, 0.7 g
Salmo A CF Ru w.w. 8.0-8.2 255 12 4 LC50 8.2c ABC Inc
gairdnerii, (1982c)
0.5 cm, 2.4 g
Salmo - S Ru divers 6.3-8.2 5.3-148 n.r. 4 LC50 14-33 Wan et al.
gairdnerii, (1989)
0.4 cm, 0.5 g
Salmo - S Ru r.w. 6.6-7.6 40 12 4 LC50 36 E G & G
gairdnerii, Bionomics
0.3 cm, 0.3 g (1980c)
Salmo - S Ru w.w 6.4-7.3 26-26 12 4 LC50 22 E G & G
gairdnerii, Bionomics
0.4 cm, 0.7 g (1980g)
Table 19 (cont'd)(3)
Organism A Test Compound Test pH Hardness Temperature Experimental Parameter Concentration Reference
type water (mg CaCO3/ (°C) duration (mg/litre)a
litre) (days)
S. gairdnerii, A S Ru r.w 7.4-7.7 85 11 4 LC50 22 EVS
0.4 g Consultants
(1986a)
S. gairdnerii, A S Ru n.w 7.4-7.8 81 11 4 LC50 15 EVS
0.4 g Consultants
(1986a)
S. gairdnerii, A S Ru d.w 5.4-6.3 4.5 11 4 LC50 26 EVS
0.4 g Consultants
(1986a)
S. gairdnerii, - S Ru r.w approx. 40 12 4 LC50 3.2 Folmar et al.
1.0 g 7.2 (1979)
Salmo - S RuD w.w 4.6-7.1 45 12 4 LC50 > 1000 ABC Inc
gairdnerii, (1981c)
0.3 cm, 0.2 g
S. gairdnerii, - S Vis n.r. 6.0 9.6 12.3 4 LC50 34 Morgan &
9.5 cm Kiceniuk
(1992)
Lepomis - S St r.w. 6.8-7.5 40-45 22 4 LC50 4.5 ABC Inc
macrochirus, (1984b)
0.2 cm, 0.1 g
Lepomis - S RuD w.w. 4.9-7.1 45 22 4 LC50 > 1000 ABC Inc
macrochirus, (1981b)
0.2 cm, 0.1 g
Lepomis A CF Ru w.w. 8.0-8.2 255 22 4 LC50 5.8c ABC Inc
macrochirus, (1982a)
0.2 cm, 0.2 g
Table 19 (cont'd)(4)
Organism A Test Compound Test pH Hardness Temperature Experimental Parameter Concentration Reference
type water (mg CaCO3/ (°C) duration (mg/litre)a
litre) (days)
Lepomis - S Ru r.w. 6.4-7.5 40 22 4 LC50 46 E G & G
macrochirus, Bionomics
0.4 cm, 0.3 g (1980b)
Pimephales - S Ru w.w. 6.7-7.7 39-44 22 4 LC50 31 E G & G
promelas, Bionomics
0.4 cm, 0.6 g (1980d)
Pimephales - S Ru r.w. n.r. 40 22 4 LC50 5.6 Folmar
promelas et al.
(1979)
Cyprinus A S St w.w. 7.2-7.9 40-48 22-23 4 LC50 2.4 ABC Inc
carpio, (1990)
0.6 cm, 2.8 g
Oncorhynchus A S Ru divers 6.3-8.2 5.3-148 n.r. 4 LC50 13-33 Wan et al.
kisutch, (1989)
0.4 cm, 0.5 g
O. kisutch, A S Ru d.w. 5.5-6.4 4.5 11 4 LC50 22 EVS
11.8 g Consultants
(1986c)
O. keta, - S Ru divers 6.3-8.2 5.3-148 n.r. 4 LC50 11-20 Wan et al.
0.4 cm, 0.5 g (1989)
O. tshawytsha, - S Ru divers 6.3-8.2 5.3-148 n.r. 4 LC50 17-33 Wan et al.
0.4 cm, 0.5 g (1989)
O. tshawytsha, A S Ru d.w. 5.8-6.7 4.5 11 4 LC50 20 EVS
4.6 g Consultants
(1986b)
Table 19 (cont'd)(5)
Organism A Test Compound Test pH Hardness Temperature Experimental Parameter Concentration Reference
type water (mg CaCO3/ (°C) duration (mg/litre)a
litre) (days)
O. gorbusha, - S Ru divers 6.3-8.2 5.3-148 n.r. 4 LC50 14-33 Wan et al.
0.4 cm, 0.5 g (1989)
Oncorhynchus A S Ru n.w 7.7-8.0 84 4-5 4 LC50 27-29 Servizi
nerka, et al.
3-6.5 cm, 0.2-3.8 g (1987)
a All concentrations are expressed as mg of the formulation per litre
b Based on immobilisation
c Based on actual concentrations
A = actual concentrations are measured; - = nominal concentrations; CF = continous flow system;
S = static system; Tgg = technical grade glyphosate; Ru = Roundup; Rod = Rodeo; St = Sting;
RuD = Roundup D-Pak; d.w. = dechlorinated tap water; r.w. = reconstituted water;
w.w. = well water; n.w. = natural surface water; a.m. = artificial medium
Table 20. Aquatic organisms: chronic toxicity of glyphosate (NOEC/MATC)
Organism A Test Test Test pH Hardness Temperature Experimental Parameter Concentration Reference
type substance water (mg CaCO3/ (°C) duration (mg/litre)
litre) (days)
Macrophytes
Lemna gibba A S Tgg a.m. 7.5 285 25 14 NOEC 9a,b Malcolm
Pirnie
Inc.
(1987e)
Crustaceans
Daphnia magna, A SS Tgg w.w.c 6.8-8.2 160-180 20 21 NOEC 100a,d ABC Inc.
first instar (1989c)
Fish
Salmo A CF Tgg w.w. 5.9-7.8 40-48 14-15 21 NOEC 52a,e ABC Inc.
gairdnerii, (1989e)
0.5 cm, 1.3 g
Pimephales A CF Tgg w.w. 6.5-7.6 32-42 25 255 MATC > 26f E G & G
promelas, Bionomics
1.5 g (1975)
a Based on actual concentrations d Based on survival and reproduction
b Based on biomass decrease e Based on survival, behaviour, and coloration
c Mixed with natural surface water f Based on survival, growth and reproduction
A = actual concentrations are measured; CF = continous flow system; SS = semi-static system;
S = static system; Tgg = technical grade glyphosate; w.w. = well water; a.m. = artificial medium
Table 21. Aquatic organisms: chronic toxicity of formulations with glyphosate
Organism A Test Test Test pH Hardness Temperature Experimental Parameter Concentration Reference
type substance water (mg CaCO3/ (°C) duration (mg/litre)f
litre) (days)
Macrophytes
Lemna minor - S Ru a.m. n.r. n.r. 25 14 NOEC 56c,d Lockhart
et al.
(1989)
Lemna minor - S Ru a.m. n.r. n.r. 22 14 NOEC 2.4c,e Hartman &
Martin
(1984)
Potamogeton - S Ru a.m. n.r. n.r. 22 14 NOEC 33c,d Hartman &
pectinatus Martin
(tubers) (1985)
Crustaceans
Daphnia A SS St w.w.b 7.2-8.2 174 20 21 MATC 1.4g,i ABC Inc.
magna, (1989a)
first instar
Daphnia magna, A SS Ru w.w.b 7.6-8.3 160-180 20 21 MATC 4.9g,j ABC Inc.
first instar (1989b)
Fish
Salmo A CF St w.w. 7.3-7.8 40-48 14-16 21 NOEC 0.8a,h ABC Inc.
gairdnerii, (1989h)
0.5 cm,
1.3 g
Table 21. (cont'd)
Organism A Test Test Test pH Hardness Temperature Experimental Parameter Concentration Reference
type substance water (mg CaCO3/ (°C) duration (mg/litre)f
litre) (days)
Salmo A CF Ru w.w. 7.1-7.8 24-48 14-16 21 NOEC 2.4a,h ABC Inc.
gairdnerii, (1989d)
0.5 cm,
1.8 g
Salmo A CF Vis n.r. 6.0 9.6 12.3 approx. 60 NOEC > 0.04a,k Morgan &
gairdnerii, Kiceniuk
9.5 cm, 7.2 g (1992)
a Based on actual concentrations g Based on survival, reproduction and length of time to the first brood
b Mixed with natural surface water h Based on survival, behaviour and coloration
c Roundup dissolved in test water i NOEC is 1.0 mg Sting/litre
d Based on biomass decrease j NOEC is 3.2 mg Roundup/litre (actual concentration)
e Based on reduction of frond formation k Based on mortality and growth
f All concentrations are expressed as mg of the formulation per litre
A = actual concentrations are measured; - = nominal concentrations; CF = continous flow system;
SS = semi-static system; Ru = Roundup; St = Sting; Vis = Vision; w.w. = well water;
a.m. = artificial medium; n.r. = not reported.
In laboratory experiments the major factors influencing the
toxicity appear to be the tested species and its age, the presence
of surfactants, the hardness, pH, temperature and the availability
of ration. Wan et al. (1989) found that the toxicity of technical
grade glyphosate to salmonids increased when hardness and pH
decreased, whereas for Roundup and Accord CR the contrary was true,
due to the presence of a 75% tallow amine surfactant in the
formulations. This surfactant was most toxic in hard water with a
relatively high pH. A higher toxicity of Roundup to salmonids at
increasing hardness and pH was confirmed by Mitchell et al. (1987),
but only partially by Servizi et al. (1987). It was also confirmed
by Folmar et al. (1979), although they only investigated the effect
of pH in reconstituted water with a moderate hardness. These authors
demonstrated that the effects of a pH increase on the toxicity of
Roundup and technical grade glyphosate were not only seen in rainbow
trout (Salmo gairdnerii) but also in bluegill sunfish (Lepomis
macrochirus). For both species Roundup became more toxic as the pH
increased from 6.5 to 7.5, whereas technical grade glyphosate became
less toxic as the pH increased from 6.5 to 9.5. At pH values higher
than 7.5, the toxicity of Roundup remained constant. In the
investigations of Servizi et al. (1987), an antagonistic effect of
glyphosate on the toxic action of a surfactant was found.
Increased toxicity of formulations due to surfactants was not
only demonstrated for the tallow amine surfactant in Roundup but
also for ortho X-77 in Rodeo (Mitchell et al., 1987). However, even
in the presence of a surfactant, the acute toxicity of some
formulations may be very slight, as was found by ABC Inc. (1980a,b).
In these studies, 0.5% (v/v) of the surfactant X-77 was added to
Roundup D-Pak, resulting in a 4-day LC50 value of this mixture for
rainbow trout (Salmo gairdnerii) and for bluegill sunfish
(Lepomis macrochirus) of 240 and 830 mg/litre, respectively.
Folmar et al. (1979) performed acute toxicity tests with
various species in reconstituted water with a pH of 7.2 and a
hardness of 40 mg CaCO3/litre. In these tests it was demonstrated
for rainbow trout (Salmo gairdnerii) and channel catfish
(Ictalurus punctatus) that the sensitivity to Roundup increased in
the following order: eyed eggs, 2-g fingerlings, sac fry, swim-up
fry, and 1-g fingerlings. The 4-day LC50 values for the various
life-stages of rainbow trout decreased from 39 mg Roundup/litre for
eyed eggs to 3.2 mg/litre for small fingerlings. In an additional
test, a 4-h exposure to concentrations of > 12 mg Roundup/litre
affected the survival of sac fry and swim-up fry significantly.
Holdway & Dixon (1988) also demonstrated that toxicity is
dependent on the life-stage by applying technical grade glyphosate
to larvae of flagfish (Jordanella floridae). At concentrations up
to 30 mg a.i./litre, no 2- or 4-day-old larvae died, whereas 20% of
the 8-day-old larvae died at the top dose. The effect was even more
drastic when the larvae were not fed. This treatment killed 20% of
the oldest larvae even at 3 mg a.i./litre. According to the authors,
the effect of age might fit the idea of saltatory ontogeny, implying
critical periods for organs and tissues.
While investigating the effect of temperature on toxicity,
Folmar et al. (1979) demonstrated for rainbow trout (Salmo
gairdnerii) and bluegill sunfish (Lepomis macrochirus) that
toxicity increased with increasing temperatures. For the trout the
4-day LC50 values decreased from 34 mg Roundup/litre at 7 °C to
18 mg/litre at 17 °C. For the bluegill sunfish the 4-day LC50
values decreased from 18 mg/litre at 17 °C to 9.8 mg/litre at 27 °C.
Rainbow trout (Salmo gairdnerii) showed the same sensitivity
to Roundup, independent of whether the water was aerated or not
(EG & G Bionomics, 1980g). In this experiment the 4-day LC50 under
both conditions was 22 mg Roundup/litre.
The potential of coho salmon smolt (Oncorhynchus kisutch) to
adapt to changes in water salinity encountered during migration was
not influenced by the application of Roundup at actual
concentrations up to 2.8 mg/litre (EVS Consultants, 1986d). The
osmoregulatory mechanism, which is fully functional in smolts, was
unaffected as indicated by plasma Na+ concentrations, haematocrit
values and the condition of the fish. During the experiment in which
the fish were exposed for 10 days in fresh water and subsequently
allowed to recover in fresh or sea water, no abnormal behaviour was
observed.
No avoidance of glyphosate was found when rainbow trout were
tested at concentrations up to 24 mg Roundup/litre (Folmar et al.,
1979). Sublethal concentrations in acute toxicity tests with Roundup
may cause loss of motility, complete loss of equilibrium, darkened
pigmentation, or rapid respiration (EG & G Bionomics, 1980a,b,g; EVS
Consultants, 1986a,b,c).
Rainbow trout (Salmo gairdnerii) were exposed to glyphosate
(as Vision) at 0, 6.25, 25 and 100 µg Vision/litre in a continuous
flow system. There were no effects on growth or foraging behaviour,
and no histopathological liver effects. Two out of three types of
aggressive behaviour were also unaffected; the third, a warning
"wig-wag" increased in frequency at the top-dose 1 month after
treatment. After two months the frequency was equal to that of the
control (Morgan & Kiceniuk, 1992).
9.1.3 Terrestrial organisms
9.1.3.1 Plants
Nodulation of sub-clover (Trifolium subterraneum) was
inhibited by an unknown formulation with glyphosate in a
dose-related way at concentrations of 2-20 mg a.i./litre (Eberbach &
Douglas, 1989). In this experiment, 3-day-old seedlings were
inoculated with Rhizobium trifolii. The seedlings were cultured
for 56 days in soil-free systems with nutrient solutions. In an
additional experiment in which Rhizobium was exposed to the same
concentrations, repeated washing of the inoculi prior to nodule
initiation did not reduce the inhibition of the nodulation of the
sub-clover after inoculation. This indicated that the effect on
nodulation might be the result of damage to the bacteria rather than
to carry-over of glyphosate from the bacteria to the plant.
Seed germination of various forest species was not affected by
treatment with Roundup at concentrations up to 305 mg a.i. (free
acid)/kg dry weight. Seed germination was affected at the highest
tested dose of 1525 mg a.i. (free acid)/kg dry weight (Morash &
Freedman, 1988). The effect on seed germination was confirmed by
another experiment in which no differences were found between
sprayed and unsprayed plots with respect to seedling composition and
quantity. Morash & Freedman (1988) then incubated the soils of
clear-cuts in a greenhouse. The application of Roundup in the field
was at a rate of 2.3 kg a.i./ha.
Red pine seedlings (Pinus resinosa) were not affected by
treatment with Roundup, with the exception of a dose-related
decrease in root length (Chakravarty & Chatarpaul, 1990b).
Non-affected growth parameters were shoot height, shoot weight, root
weight, and mycorrhizal development. In this experiment lasting 60
days, Roundup was applied at rates of 0.54 and 3.2 kg a.i./ha. The
conifer seedlings were inoculated with an ectomycorrhizal fungus
(Paxillus involutus), 14 days after germination.
Glyphosate may affect various pathways of the secondary
metabolism in the plant, although the actual targets in plants have
not been located. The synthesis of aromatic amino acids, secondary
hydroxyphenolic compounds, chlorophyll and delta-amino-levulinic
acid were reported to be affected by glyphosate (Amrhein et al.,
1980; Duke & Hoagland, 1981; Kitchen et al., 1981a,b). Aromatic
amino acids are important for the synthesis of, for instance, some
alkaloids, the phytohormone indole-3-acetic acid and phenolic
compounds such as lignin and quinones.
With respect to the synthesis of aromatic amino acids, there
are indications that the actual target is not shikimate kinase or
anthranilate synthase, but probably
5-enolpyruvylshikimate-3-phosphate synthase or chorismate synthase
(Amrhein et al., 1980). When applied to hypocotyls of buckwheat
(Fagopyrum esculentum) and to cultured cells of smooth bedstraw
(Galium mollugo), technical grade glyphosate inhibited the
conversion of shikimate to chorismate. This inhibition in vivo and
in vitro was found at concentrations of > 10 mg a.i./litre. In
the cultured cells of Galium, the accumulation of shikimate was
concomitant with a decrease of anthraquinones (Amrhein et al.,
1980). Possibly the non-transportable carbon pool, to which carbon
was found to be diverted in sugar beets (Beta vulgaris) due to
glyphosate, was in fact shikimate (Gougler & Geiger, 1984). These
effects on carbon metabolism were found at application rates
equivalent to 5 kg a.i./ha leaves. Duke & Hoagland (1981) assumed
that possibly chelation of divalent ions such as Ca2+ and Mg2+
that are involved in many metabolic pathways was the main cause of
damage.
9.1.3.2 Invertebrates
Appraisal
Glyphosate has low toxicity for bees and earthworms.
Oral 2-day LD50 values of technical grade glyphosate and
Roundup for bees were 100 µg a.i./bee and > 100 µg Roundup/bee,
respectively. Contact 2-day LD50 values for these two substances
were likewise > 100 µg a.i./bee and > 100 µg Roundup/bee (HRC,
1972). The oral 2-day LD50 of Sting was also > 100 µg Sting/bee
(Altmann, 1984). In these experiments Apis mellifera was tested. The
LD50 values indicate a slightly acute toxicity of technical grade
glyphosate, Roundup, and Sting to honey-bees. Roundup was also
slightly toxic to green lacewings (Chrysoperla carnea) when they
were exposed by contact to 1 kg Roundup/ha (SFRSA, 1990). In this
experiment the average number of eggs per female and the larval and
pupal mortality were increased due to the treatment, resulting in an
overall reduction in beneficial capacity of 41%. The beneficial
capacity is a function of the larval and pupal mortality, and the
average number of eggs per treated and untreated female. No effects
on the food uptake and mortality of the beetle Poecilus cupreus
Bonelli were observed 15 days after application of 6 kg Sting/ha
(IFU, 1990).
When exposed to artificial soil contaminated with Roundup
D-pak, earthworms (Eisenia fetida) were soft and/or slack, in a
dose-related way, at concentrations > 500 mg Roundup D-pak/kg dry
weight (IBR, 1991a). No other adverse effects were found, indicating
a 14-day NOEC of 158 mg/kg. A comparable experiment with Roundup
also indicated slight toxicity for earthworms with a 14-day NOEC of
500 mg Roundup/kg dry weight (IBR, 1991b). At higher concentrations
thin, slack and lethargic worms with a dark skin were observed.
9.1.3.3 Vertebrates
Appraisal
Glyphosate has low toxicity to birds after acute oral or
short-term dietary exposure. Mammals tested showed effects (body
weight loss) only after high levels of dosing. Herbicide-treated
foliage was not avoided by deer in the single study reported.
The acute, subacute and chronic toxicity of glyphosate and its
formulations to birds is summarized in Table 22.
Male marsupials (Sminthopsis macroura) showed significant
body weight loss after exposure to feed contaminated with
concentrations of up to 5000 mg a.i/kg feed (Evans & Batty, 1986).
No other treatment-related effects were found in the male
marsupials. In female hopping-mice (Notomys alexis and Notomys
mitchelli) fed similar doses, no treatment-related effects were
found.
Glyphosate did not affect the daily chow consumption of
black-tailed deer (Odocoileus hemionus columbianus) when these
herbivores were exposed to browse treated with glyphosate at a rate
of 2.2 kg/ha (Sullivan & Sullivan, 1979). The deer did not avoid the
contaminated browse, and sometimes even preferred it. Irrespective
of whether they were given treated alfalfa (Medicago sativa),
treated alder (Alnus rubra) or untreated feed, the deer had the
same daily chow consumption.
9.2 Field observations
9.2.1 Microorganisms
Appraisal
Some effects on microorganisms have been reported in field
studies following application of glyphosate formulations. However,
these have been minor and reversible in most cases. It is not
possible to separate the direct toxic effects of the herbicide from
changes in the habitat caused by its intended herbicidal action.
Table 22. Birds: acute and chronic toxicity of glyphosate and its formulations
Species Compound Sex Age Route Experimental Parameter Concentration Reference
duration
(days)
Colinus virginianus Tgg n.r. n.r. diet 8 LC50 > 4640 mg/kg feed Hazleton Lab. Inc.
(1973a)
Colinus virginianus Tgg n.r. 14 days oral LD50 > 3851 mg/kg b.w. Wildlife Int Ltd.
(1978c)
Colinus virginianus Tgg M,F 5 months diet 119 NOEC > 1000 mg/kg feeda Wildlife Int Ltd.
(1978b)
Colinus virginianus Ru n.r. 10 days diet 8 LC50 > 5620 mg/kg feedb Wildlife Int Ltd.
(1990b)
Anas platyrhynchos Tgg n.r. n.r. diet 8 LC50 > 4640 mg/kg feed Hazleton Lab. Inc.
(1973b)
Anas platyrhynchos Tgg M,F 6 months diet 112 NOEC > 1000 mg/kg feeda Wildlife Int Ltd.
(1978a)
Anas platyrhynchos Ru n.r. 10 days diet 8 LC50 > 5620 mg/kg feedb Wildlife Int Ltd.
(1990a)
Poephilla guttata Ru M mature diet 7 LC50 < 16 393 mg/kg feedb Evans & Batty (1986)
Poephilla guttata Ru M mature diet 5 LC50 > 8197 mg/kg feedb Evans & Batty (1986)
a Based on reproduction impairment of one generation
b Concentrations expressed as mg of the formulation per kg body weight or feed
Tgg = technical grade glyphosate; Ru = Roundup; M = males; F = females; n.r. = not reported.
9.2.1.1 Water
In a pool located in Hong Kong, treatment with 656 mg
Roundup/litre caused a substantial decrease in bacteria within 14
days after treatment. The number of colony-forming units had
returned to the control level 30 days after treatment (Chan & Leung,
1986).
Diatom populations in the water and sediments of a pond and a
stream showed a significantly different density of some species when
aerially treated with Roundup at a rate of 2.2 kg a.i./ha (Sullivan
et al., 1981). The authors concluded that these differences were
probably due to seasonal and habitat variation, rather than to
treatment with the herbicide.
Roundup may have affected the increase in ash-free dry weight
and the chlorophyll a standing crop of periphyton in the first month
after spraying with Roundup at a rate of 2.2 kg a.i./ha (Holtby &
Baillie, 1989a). In this experiment lasting around 130 days, some
tributaries in a watershed in British Columbia (Canada) were
directly oversprayed.
9.2.1.2 Soil
When Roundup was applied to a sandy loam being prepared for
conifer forestation, no significant changes in the soil respiration
were found up to about 180 days after application of Roundup at a
rate of 0.54 kg a.i./ha (Chakravarty & Chatarpaul, 1990a).
Concomitantly the numbers of fungi and bacteria decreased
significantly during the first 2 months, but after about 180 days
the numbers had recovered. These results might indicate changes in
microbial populations due to application of Roundup at recommended
application rates.
Preston & Trofymow (1989) found no significant effects on the
number of bacteria, actinomycetes, and nitrogen fixers in
ferro-humic podsols covered with alder trees (Alnus rubra) in the
Carnation Creek watershed, Canada, due to treatment with Roundup.
The only consistent effect was a significant reduction of the number
of fungi at one of the two treated sites. In this site the fungi
appeared to have recovered at the end of the study. In this
experiment of about 180 days, Roundup was hand-sprayed at a rate of
2 kg a.i./ha. In an additional comparable intensive field trial of
one month, microflora populations appeared to have recovered from
treatment after one month, with the exception of the reduction of
fungi in the litter of one of the treated sites. Actinomycetes and
nitrogen fixers in the litter appeared to be reduced in numbers due
to the treatment but they subsequently recovered. This reduction was
not found in the underlying humus layer.
Stratton & Stewart (1992) studied microbial activity in forest
soil and litter following the application of Roundup at 4.7
litres/ha (equivalent to 1.7 kg a.i./ha) to a coniferous forest
previously clear-cut and replanted. Treated and untreated (covered
with plastic sheeting during application) areas of forest were used
to obtain soil and litter samples which were tested in situ over an
8 months period following spraying. Glyphosate had a generally
stimulatory effect on microbial biomass in litter (up to 80%
increase) but no significant effect in soil. There were no
significant effects on the numbers of bacteria, fungi or
actinomycetes in either soil or litter. Glyphosate generally
stimulated respiration in both soil and litter; the degree of
stimulation was very variable throughout the sampling period,
ranging from a few percent to 100% increases in CO2 evolution.
No substance- or dose-related effects on aerobic bacteria were
observed in a sandy soil in a semi-arid region of Argentina up to 96
days after the application of Roundup (Gómez & Sagardoy, 1985).
Doses of up to 2.8 kg a.i. (free acid)/ha were applied.
No substance-related effects on the growth of an
ectomycorrhizal fungus were found when Roundup was applied at
concentrations of up to 3.2 kg a.i./ha (Chakravarty & Chatarpaul,
1990b). In these experiments red pines (Pinus resinosa) were
inoculated with the fungus Paxillus involutus. In the field 74-86%
of the seedlings that were not inoculated were colonized by
indigenous mycorrhizal fungi within two months.
9.2.2 Aquatic organisms
Appraisal
Little effect has been reported on aquatic invertebrates or
fish exposed to glyphosate formulations sprayed in the field. Minor
mortality in a single study of young trout may reflect the greater
sensitivity of early life-stages.
9.2.2.1 Plants
No field data on toxicity to aquatic macrophytes are available.
9.2.2.2 Invertebrates
An increased drift of midge larvae (Chironomus plumosus) was
found in artificial outdoor streams treated with 4.9 mg
Roundup/litre (Folmar et al., 1979). No increased drift was found at
0.5 mg Roundup/litre. In streams in a coastal rainforest in British
Columbia, Canada, only the drift densities of an amphipod (Gammarus
sp) and mayflies (Paraleptophlebia sp) increased after treatment
with Roundup at a rate of 2 kg a.i./ha (Kreutzweiser et al., 1989).
Density peaks partly coincided with periods immediately following
rainfall, which might indicate an effect due to glyphosate run-off,
or an effect due to increased discharges.
During most streamflows, the abundance of benthic
macro-invertebrates in a stream and at sites in tributary swamps was
similar at untreated sites and at sites that had been treated with
Roundup at a rate of 2.2 kg a.i./ha (Scrivener & Carruthers, 1989).
However, after periods of frequent rainstorms leading to flooding,
the abundance at the treated sites was 40-50% lower.
Water-fleas (Daphnia magna) did not show any mortality in a
pond sprayed with Roundup at application rates of up to 220 kg
a.i./ha (Hildebrand et al., 1980). In this experiment the
water-fleas were exposed for 8 days in pens that were partly
immersed in the water.
9.2.2.3 Vertebrates
Fingerlings (2.1 g, 5.8 cm) of rainbow trout (Salmo
gairdnerii) that were exposed for 14 days to Roundup in
flow-through pens in shallow streams did not show any mortality or
substance-related effects at application rates of up to 220 kg a.i.
(free acid)/ha (Hildebrand et al., 1982). In this experiment Roundup
was sprayed manually on moderately flowing forest streams in British
Columbia, Canada. In the same area an aerial application of 2.2 kg
a.i. (free acid)/ha did not cause mortality or obvious signs of
stress in rainbow trout fingerlings in flow-through pens, which were
also exposed to Roundup for 14 days (Hildebrand et al., 1982). A
direct aerial application of 2.1 kg a.i./ha on a tributary of the
Carnation Creek watershed (British Columbia, Canada), however,
killed 2.6% of the 120 fingerlings of coho salmon (Oncorhynchus
kisutch) within 24 h after application, whereas no mortality
occurred at the unexposed sites (Holtby & Baillie, 1989b). Also some
stress of the caged fingerlings was indicated within the first 2 h
after application. Up to 2 years after application no consistent
effects of Roundup on over-winter mortality, probability of entering
and leaving the tributary, timing of spring emigration, and growth
rates were found.
In artificial outdoor streams in Colorado, USA, rainbow trout
(Salmo gairdnerii) were exposed to concentrations of up to 5 mg
Roundup/litre for 12 h. No adverse effects on fecundity and
gonadosomatic indices were found in this study by Folmar et al.
(1979).
Rainbow trout (Salmo gairdnerii) in pens that were immersed
in stream water avoided Roundup at concentrations of > 40 mg
Roundup/litre (Hildebrand et al., 1982).
9.2.3 Terrestrial organisms
Appraisal
Spray drift of herbicides will affect non-target plants.
Adequate buffer zones have been defined for some application
methods.
Changes in species diversity and population size and structure
have been reported for terrestrial invertebrates and vertebrates
following applications of glyphosate formulation in the field.
Modifications in available food plants, insect populations
associated with vegetation killed by the herbicide, and ground cover
following intended effects of the spray probably account for these
changes.
9.2.3.1 Plants
Red pine seedlings (Pinus resinosa) were not affected by
treatment with Roundup in a field study. There was no dose-related
decrease of the root length, as had been observed in a comparable
laboratory experiment with the same doses (Chakravarty & Chatarpaul,
1990b). In this experiment lasting 154 days Roundup was applied at
rates of up to 3.2 kg a.i./ha. The conifer seedlings were inoculated
with an ectomycorrhizal fungus (Paxillus involutus) at 14 days
after germination, and they were planted outdoor after being
cultured in a greenhouse for up to 70 days after germination.
No plants were found as indicator species for damage due to
drift of a formulation of glyphosate when this was applied with
hydraulic ground sprayers (Marrs et al., 1989). In this study,
native British species commonly found in nature reserves were
exposed to spray drift, at several distances downwind from a zone
sprayed with 0.5 and 2.2 kg a.i./ha. The effect of windspeed was
investigated by spraying at speeds of 2.5 and 3.5 m/second. Death
and severe growth suppression occurred at a distance of 2-6 m from
the sprayer. Sublethal damage also occurred, mostly near to the
sprayer, although for Prunella vulgaris damage occurred up to 20 m
away. Epinasty was the most frequent symptom of damage. Most of the
damaged plants recovered, however. Some of the species were
consistently more sensitive, i.e. Digitalis purpurea, Centaurea
nigra, Prunella vulgaris and Lychnis flos-cuculi. Marrs et al.
(1989) concluded that, when spraying with ground sprayers, buffer
zones around nature reserves should be 5-10 m.
9.2.3.2 Invertebrates
No significant and consistent effects on the number of
nematodes and springtails were found in the upper 3 cm of
ferro-humic podsols due to treatment with Roundup (Preston &
Trofymow, 1989). The soils were covered with alder trees (Alnus
rubra) and located in British Columbia, Canada. The only
consistent effect was a significant reduction in the number of both
oribatid and non-oribatid mites on one of the treated sites around
20 days after application. On this site the number of mites appeared
to have returned to normal by the end of the study. In this
experiment of around 180 days Roundup was hand-sprayed at a rate of
2 kg a.i./ha.
No substance- or dose-related effects on mites and springtails
were observed in a sandy soil in a semi-arid region of Argentina up
to 96 days after application of Roundup (Gómez & Sagardoy, 1985).
Applied doses were up to 2.8 kg a.i. (free acid)/ha.
The numbers of herbivorous insects and ground invertebrates
were significantly reduced up to 3 years after treatment with
Roundup in a 4- to 5-year-old clear-cut planted with spruce
seedlings (Picea sp) (Santillo et al., 1989b). No effects were
found on predatory insects. The clear-cut was located in Maine, USA,
and sprayed with 1.7 kg a.i./ha. During this experiment lasting 3
years, the vegetation did not recover completely, and, apparently,
the effects on invertebrates were mainly due to habitat change.
Unintentionally untreated areas in the sprayed site showed a much
lesser reduction of invertebrates. These areas may therefore be
considered as potential sources for recolonization.
9.2.3.3 Vertebrates
Treatment of 4- to 5-year old clear-cuts in Maine, USA, planted
with seedlings of spruce (Picea sp), with Roundup at a rate of
1.7 kg a.i./ha affected breeding bird populations up to 3 years
after treatment (Santillo et al., 1989a). Total bird densities
decreased with 36% due to reduced habitat complexity, as expressed
in regenerated hardwood, vegetation height and foliage height
diversity. The most sensitive species were the insectivorous common
yellowthroat (Geothlypis trichas), Lincoln's sparrows (Melospiza
lincolnii) and alder flycatchers (Empidonax alnorum). In less
than 7-year-old clear-cuts in the Oregon coast range, Canada,
planted with Douglas fir (Pseudotsuga menziesii), some breeding
bird populations were affected due to two closely spaced treatments
with Roundup at a rate of 0.8 kg a.i./ha each (Morrison & Meslow,
1984). Two years after the application, the densities of birds using
shrubs for nesting and foraging had recovered, concomitant with the
recovery of shrub vegetation. Sensitive species were the
rufous-sided towhee (Piplio erythrophthalmus) and MacGillyvray's
warbler (Oporornis tolmiei). On the other hand, the American
goldfinch (Carduelis tristis) increased one year after
application, apparently due to the treatment. Morrison & Meslow
(1986) stated that the effectiveness of the treatment, which was not
maximal in their study, is crucial to the degree to which bird
populations are affected.
Small mammals may be affected by treatment with glyphosate,
this depending mainly on the size of the treated area, the
vegetation type and the extent to which the vegetation is damaged.
Various experiments have been performed in and around clear-cuts
planted with conifers in locations in the USA and Canada.
Insectivorous shrews (Blarina brivicauda, Sorex cinereus and Sorex
hoyi) and herbivorous voles were less abundant due to a treatment
with Roundup at a rate of 1.7 kg a.i./ha (Santillo et al., 1989b).
This significant reduction of the number of shrews was maintained
for 3 years after application, whereas the population of voles
recovered. No effects on the omnivorous deer mice (Peromyscus
maniculatus) were observed. Deer mice also appeared to be
unaffected in an experiment in which Roundup was applied at a rate
of 3 kg a.i./ha (Sullivan, 1990). The population dynamics of both
deer mice and the herbivorous Oregon voles (Microtus oregoni)
appeared not to be influenced, although some partially significant
effects were observed that might have been due to the treatment. On
the sprayed site there was an increase in the number of recruits of
both species 3 years after application, and also an increased
fecundity of deer mice and a higher survival of female voles 1 and 3
years after application. Sullivan (1990) observed no physiological
changes that might have been due to ingestion. In a clear-cut
treated with Roundup at a rate of 1.1 kg a.i./ha, only the
population density of Southern Redbacked voles (Clethrionomys
gapperi) was reduced by about 80% in a 1-year experiment (D'Anieri
et al., 1987). In another clear-cut, no adverse effects on deer mice
populations were evident after treatment with Roundup at a rate of
2.2 kg a.i./ha (Sullivan & Sullivan, 1981). Contrary to the results
of Sullivan (1990), D'Anieri et al. (1987), Sullivan & Sullivan
(1981) and Santillo et al. (1989b), a significant reduction in the
population density of deer mice was observed by Ritchie et al.
(1987) on a sprayed clear-cut of 38 ha at around 11 months after
spraying with Roundup at a rate of 1.1-1.2 kg a.i./ha. However, no
adverse effects on fertility or fecundity were indicated. Probably
the effect on abundance was due to habitat change with respect to
feed provision and cover. Possibly the effects on deer mice observed
by Ritchie et al. (1987) were less confounded by immigration as the
sprayed area was larger than in the studies in which effects on deer
mice were lacking. However, when a site of 36 ha was sprayed twice
with Roundup at a rate of 0.8 kg a.i./ha on each occasion, deer mice
were not affected, possibly due to a relatively low effectiveness of
the treatment (Anthony & Morrison, 1985). In the treated area, even
an increase of Oregon voles was found after 1 year, concomitant with
an increase of grass and other plants. The results indicated that
small mammal populations were able to recover within 2 years after
application of glyphosate, dependent on the recovery of shrubs.
10. EVALUATION OF HUMAN HEALTH HAZARDS AND EFFECTS ON THE
ENVIRONMENT
10.1 Human health hazards
Results of direct measurements of glyphosate concentrations in
foodstuffs (as part of food surveillance), drinking-water or total
diets are not available.
Absorption from the gastrointestinal tract is limited to 36% or
less and percutaneous absorption is 5.5% or less. Glyphosate is
essentially not metabolized. Total body clearance is 99% in 7 days.
Residues in livestock animals and their products (including milk)
are minimal.
Summarized information on short- and long-term studies is given
in Table 23 and on teratogenicity and reproduction studies in Table
24.
In animals, glyphosate has very low acute toxicity by the oral
and dermal administration routes. The formulation Roundup is acutely
toxic to humans when ingested intentionally or accidentally. No
controlled studies are available and therefore the human NOAEL
cannot be derived.
Animal studies show that glyphosate is not carcinogenic,
mutagenic or teratogenic. Reproductive effects were only seen at
dose levels producing maternal toxicity.
In experimental animals (13-week studies in rats and mice), an
effect was observed in the parotid salivary glands, indicating that
glyphosate may be acting as a weak adrenergic agonist. In rats, this
occurred at feeding levels of > 205 mg/kg body weight per day.
The NOAEL in chronic feeding studies is > 410 mg/kg body weight
per day. A NOAEL of 175 mg/kg body weight per day observed in a
rabbit teratogenicity study was chosen as the appropriate basis for
toxicological evaluations in humans. Through application of a
suitable safety factor, safe levels for humans can be estimated for
use in the toxicological evaluation of any actual exposures. For
technical glyphosate a safety factor of 100 is considered
appropriate given the elaborate data sets available.
Glyphosate and its concentrated formulations produce moderate
to severe eye irritation, but only slight skin irritation. Neither
glyphosate nor tested formulations induce sensitization.
Table 23. Short-term and long-term studies on glyphosate
Species Test Dose levels Effects, dose level NOAEL
compound (mg/kg-1 diet) (mg/kg diet) [mg/kg diet]
unless otherwise mg kg-1 b.w. d-1
stated
Short-term studies
Mouse technical 5000, 10 000, decreased growth and increased [10 000]
glyphosate 50 000 weights in brain, heart, 1890 m,
kidneys (50 000) 2730 f
Mouse technical 3125, 6250, reduced weight gain (50 000) [3 125]
glyphosate 12 500, 25 000, lesions of salivary glands 507
50 000 (> 6250)
Rat technical 1000, 5000, no adverse effects [20 000]*
glyphosate 20 000 1267*
Rat technical 200 to 12 500 no adverse effects [12 500]*
glyphosate NG
Rat technical 3125, 6250, increased AP and ALAT (> 6250) [< 3 125]
glyphosate 12 500, 25 000, increased haematocrit and red < 205 m
50 000 cell parameters (> 12 500), < 213 f
increased bile acids,
decreased sperm counts
(> 25 000), histological
alterations in salivary glands
(> 3125), reduced weight
gain (> 25 000)
Dogs technical 20, 100, 500 no adverse effects [NG]
glyphosate mg kg-1 bw 500*
Cattle Roundup 400, 500, 630, decreased feed intake (> 630) [NG]
790 mg kg-1 bw d-1), diarrhoea (> 500) 400
increased blood parameters
(790)
Long-term studies
Mouse technical 1000, 5000, decreased growth (30 000), [5 000]
glyphosate 30 000 increased incidence of 814
hepatocyte hypertrophy and
necrosis (30 000), increased
incidence of urinary bladder
epithelial hyperplasia
(30 000)
Rat technical 2000, 8000, decreased growth (20 000), [8 000]
glyphosate 20 000 increased liver weights 410
(20 000), increased incidences
of degenerative lens changes
(20 000) and of gastric
inflammation (8000 and 20 000)
Rat technical 60, 200, 600 slightly decreased growth (600) a
glyphosate
m = males; f = females; * Highest dose tested; NG, not given;
a The slight effect at 600 mg/kg diet (32 mg/kg bw) is considered marginal in the light
of the absence of an effect on growth at higher dose levels (2000 and 8000 mg/kg diet)
in a more recent 2-year study in rats.
Available studies on exposures of workers involved in
appli-cation of the herbicide show that exposure is low when
protective clothing is worn. The following data illustrate this
point.
a) The highest estimated exposure (dermal and inhalation) of about
8000 µg/h, as reported in a study with spray applicators,
corrected for incomplete absorption, equals about 50 µg/kg body
weight per day (8-h working day for a 70-kg adult); between the
latter level and the NOAEL of 175 mg/kg body weight per day,
adjusted for incomplete absorption from the gastrointestinal
tract (30-60%), i.e. 52-63 mg/kg body weight per day, there is
a margin of safety of about 1100.
b) The highest exposure concentration found for forest brush saw
workers was 15.7 µg/m3; between this level and the NOAEL
expressed as glyphosate from the 4-week inhalation study with
Roundup of 16 mg/m3 there is a margin of safety of 1000 (this
is borne out by the absence of adverse findings in the workers'
health examination in the study.
Table 24. Summary of teratogenicity and reproduction studies on glyphosate
Species Test Dose levels Effects NOAELa
compound (mg/kg diet) (mg/kg
body
weight)
Rat technical 300, 1000, 3500 mortality, clinical signs and 1000
glyphosate mg/kg body decreased growth in dams,
weight, early resorptions, decreased
gestation days 6-19 numbers of implantations and
visible fetuses, decreased
ossification of fetal
sternebrae (all at 3500 only);
no fetal malformations
Rabbit technical 75, 175, 350 diarrhoea and soft stools (350, 175
glyphosate mg/kg body slight at 175), nasal discharge
weight, (350)
gestation days 6-27
Rat technical 3, 10, 30 mg/kg increased incidence of renal < 30b
glyphosate body weight tubular dilation in F3b male
given in diet, pups (30)
3 gens
Rat technical 2000, 10 000, soft stools of parents (30 000), 100b
glyphosate 30 000 mg/kg decreased litter size (30 000), (2000
diet, 2 gens decreased body weights of mg/kg
parents and pups (30 000 diet)
and 10 000)
a Based on all observed effects (both in dams and offspring)
b There is some discrepancy in the results, and in the NOAELs, of the two
reproduction studies carried out with technical glyphosate; the renal
effects in the 3-generation study were not reproduced in the more recent
2-generation study with higher dose levels (for details, see section 7.5.2.).
10.2 Evaluation of effects on the environment
Following application, glyphosate will selectively partition to
particulate matter suspended in surface water, aquatic sediment or
to the soil substrate. This partitioning is usually rapid. The
mechanism of sorption is only partially understood. There is little
reported information on desorption from soil; the information
available suggests "strong" binding. Mobility studies show little
leaching of glyphosate below the upper few centimetres of the soil
profile. The major metabolite, AMPA, also appears not to leach
through soil.
Given this environmental distribution, organisms living in
aquatic sediment or soil would be expected to come into closest
contact with residues of the herbicide.
There is very little information on the bioavailability of
sediment- or soil-bound glyphosate to either aquatic or terrestrial
organisms. Few bioaccumulation or ecotoxicity studies have been
performed with sediment present.
Comparison of exposure concentrations and toxic effects is,
therefore, difficult since the relevant organisms have not been
tested and exposure of tested organisms is not by a realistic route.
10.2.1 Exposure levels and toxic effects
Exposure concentrations have been calculated from experimental
application of Roundup in the field (see Table 7 in chapter 5). The
methodology is presented in Fig. 4. No monitoring results of
environmental concentrations following actual use in agriculture or
forestry are available.
The lowest LC(EC)50 and NOEC values for microorganisms,
invertebrates and fish have been taken from the toxicity tests
reported in chapter 9 (see Fig. 4). These all refer to organisms
living in the open water and are, therefore, of questionable
significance for a compound which partitions to sediment. There is
no information on species living in aquatic sediment and little
information available on soil-living organisms, with the exception
of microorganisms.
10.2.2 Hazard evaluation for aquatic organisms
Tables 25 and 26 compare the estimated mean and maximum
exposure concentrations, following aerial application of Roundup, to
the lowest reported LC(EC)50 and NOEC concentrations for acute and
chronic exposure of aquatic organisms. The ratio between exposure
and effect concentrations has been calculated. These tables are
meant as a guide to establishing possible hazard and are not
intended to estimate the degree of effect likely to be seen in the
field. The "possible hazard" classification is a simple one using
different classification phrases for order of magnitude segments of
the ratios.
The toxicity of formulations to aquatic organisms is greater
than for technical glyphosate in many cases. This increased toxicity
is due to surfactants present in the product. No account has been
taken of possible degradation of surfactants in the ratios
presented, since no data are available. The ratios, therefore, may
overestimate the toxicity of glyphosate. If the compound is bound to
sediment in the environment, this could also reduce its toxic
effect. Since no clear evidence is available to demonstrate this
reduced toxicity, no account has been taken of partitioning to
particulates. This will also tend to overestimate toxic effect of
glyphosate.
As can be seen from the tables, possible hazard for most
aquatic organisms is small or negligible. Fish and aquatic
invertebrates would not be affected by glyphosate use. Only
microorganisms, with both acute and chronic exposure, appear to be
susceptible to effects of the herbicide. The comparisons made in the
table do not allow estimates of the degree of toxic effects likely
to be seen in the field. From the field evidence available,
populations and communities of algae are not likely to be affected
after application of glyphosate formulation. Transitory changes in
number and functioning of aquatic microorganisms are possible after
use of the herbicide.
Since data are not available, evaluation of the hazard of bound
residues of glyphosate to sediment-living organisms is not possible.
Minimal bioaccumulation of glyphosate has been reported in both
laboratory experiments and in the field. The physicochemical
properties of the compound are consistent with this conclusion.
10.2.3 Hazard evaluation for terrestrial organisms
Limited test data show low toxicity of glyphosate and its
formulations to honey-bees, earthworms, birds and mammals. These
data suggest low risk for these organisms from use of the herbicide.
Field studies have been conducted and support the view that
glyphosate does not affect soil microorganisms in the long term.
Marked changes in populations of birds and small mammals have
been seen in field studies following application of glyphosate.
These seem to result from the changes in habitat, vegetation cover,
food organisms, etc., resulting from the intended herbicidal effect
of the compound.
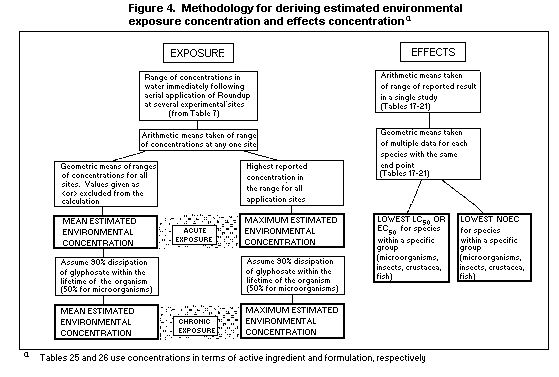 Table 25. Indications of environmental hazard for aquatic organisms by technical grade glyphosate
Effect Organisms Estimated exposure Toxicity data End-point Ratio of exposure Possible
concentration (mg a.i./litre) to toxicity hazard
(mg a.i./litre) concentrations
Mean estimated exposure concentration
Acute microorganisms 0.1 EC50 = 1.2 mortality 0.082 small
Acute insects 0.1 EC50 = 55 mortality 0.0018 negligible
Acute crustaceans 0.1 EC50 = 281 mortality 0.00035 negligible
Acute fish 0.1 LC50 = 94 mortality 0.0010 negligible
Chronic algae 0.05 NOEC = 0.3 growth 0.17 present
Chronic crustaceans 0.01 NOEC = 100 reproduction 0.00010 negligible
Chronic fish 0.01 NOEC = 52 behaviour 0.00019 negligible
Maximum estimated exposure concentration
Acute microorganisms 1.7 EC50 = 1.2 mortality 1.4 large
Acute insects 1.7 EC50 = 55 mortality 0.031 small
Acute crustaceans 1.7 EC50 = 281 mortality 0.0060 negligible
Acute fish 1.7 LC50 = 94 mortality 0.018 small
Chronic algae 1.7 NOEC = 0.3 growth 5.7 large
Chronic crustaceans 0.17 NOEC = 100 reproduction 0.0017 negligible
Chronic fish 0.17 NOEC = 52 behaviour 0.003 negligible
Table 26. Indications of environmental hazards for aquatic organisms by Roundup
Effect Organisms Estimated exposure Toxicity data End-point Ratio of exposure Possible
concentration (mg Roundup/litre) to toxicity hazard
(mg Roundup/litre) concentrations
Mean estimated exposure concentration
Acute microorganisms 0.32 EC50 = 2.1 mortality 0.15 present
Acute crustaceans 0.32 EC50 = 10 mortality 0.032 small
Acute insects 0.32 EC50 = 44 mortality 0.0073 negligible
Acute fish 0.32 LC50 = 13 mortality 0.025 small
Chronic microorganisms 0.16 NOEC = 0.7 growth 0.23 present
Chronic crustaceans 0.032 NOEC = 3.5 reproduction 0.0091 negligible
Chronic fish 0.032 NOEC = 2.4 behaviour 0.013 negligible
Maximum estimated exposure concentration
Acute microorganisms 5.6 EC50 = 2.1 mortality 2.7 large
Acute crustaceans 5.6 EC50 = 10 mortality 0.56 present
Acute insects 5.6 EC50 = 44 mortality 0.13 present
Acute fish 5.6 LC50 = 13 mortality 0.43 present
Chronic microorganisms 5.6 NOEC = 0.7 growth 8.0 large
Chronic crustaceans 0.56 NOEC = 3.5 reproduction 0.16 small
Chronic fish 0.56 NOEC = 2.4 behaviour 0.23 small
11. RECOMMENDATIONS FOR PROTECTION OF HUMAN HEALTH
a) Protective clothing is necessary to ensure the safety of
herbicide applicators.
b) A market-basket survey would be useful to determine the
possible exposure of the general population.
12. FURTHER RESEARCH
a) Further research is required to determine whether ß-adrenergic
effects observed in rodents have any implications for human
health.
b) The role of adjuvants in the toxicity of glyphosate
formulations needs to be investigated further in laboratory
mammals and organisms in the environment.
c) A controlled study on exposure of agricultural workers is
needed.
d) The bioavailability of sediment- and soil-bound glyphosate in
the environment should be studied.
e) Studies on the environmental behaviour and fate of adjuvants
are required.
f) Further toxicity studies of sediment-living organisms are
needed.
g) The effects of phosphate fertilizers on the binding of
glyphosate to soils should be investigated.
h) Analytical techniques that are less costly but still adequate
should be developed.
REFERENCES
ABC Inc. (1978a) Acute toxicity of technical glyphosate (AB-78-201)
to Daphnia magna. Columbia, Missouri, Analytical Biochemistry
Laboratories, Inc. (Unpublished report submitted by Monsanto Ltd).
ABC Inc. (1978b) Acute toxicity of technical glyphosate (AB-78-165)
to rainbow trout (Salmo gairdnerii). Columbia, Missouri,
Analytical Biochemistry Laboratories, Inc. (Unpublished report
submitted by Monsanto Ltd).
ABC Inc. (1978c) Acute toxicity of technical glyphosate to bluegill
sunfish (Lepomis macrochirus). Columbia, Missouri, Analytical
Biochemistry Laboratories, Inc. (Unpublished report submitted by
Monsanto Ltd).
ABC Inc. (1978d) The effect of glyphosate on nitrogen fixation and
nitrification in soil with time. Columbia, Missouri, Analytical
Biochemistry Laboratories, Inc. (Unpublished report submitted by
Monsanto Ltd).
ABC Inc. (1978e) The effect of glyphosate on the degradation of
cellulose, starch, protein, and leaf litter on soil. Columbia,
Missouri, Analytical Biochemistry Laboratories, Inc. (Unpublished
report submitted by Monsanto Ltd).
ABC Inc. (1980a) Acute toxicity of MON-0139-X-77 (AB-80-262) to
rainbow trout (Salmo gairdnerii). Columbia, Missouri, Analytical
Biochemistry Laboratories, Inc. (Unpublished report submitted by
Monsanto Ltd).
ABC Inc. (1980b) Acute toxicity of MON-0139-X-77 (AB-80-263) to
bluegill sunfish (Lepomis macrochirus). Columbia, Missouri,
Analytical Biochemistry Laboratories, Inc. (Unpublished report
submitted by Monsanto Ltd).
ABC Inc. (1981a) Acute toxicity of MON 0139 (Lot LURT 12011)
(AB-81-074) to Daphnia magna. Columbia, Missouri, Analytical
Biochemistry Laboratories, Inc. (Unpublished report submitted by
Monsanto Ltd).
ABC Inc. (1981b) Acute toxicity of MON 0139 (Lot LURT 12011)
(AB-81-073) to Lepomis macrochirus. Columbia, Missouri, Analytical
Biochemistry Laboratories, Inc. (Unpublished report submitted by
Monsanto Ltd).
ABC Inc. (1981c) Acute toxicity of MON 0139 (Lot LURT 12011)
(AB-81-072) to rainbow trout (Salmo gairdnerii). Columbia,
Missouri, Analytical Biochemistry Laboratories, Inc. (Unpublished
report submitted by Monsanto Ltd).
ABC Inc. (1981d) A short-term bioconcentration study of
14C-glyphosate with channel catfish (Ictalurus punctatus) in a
static system. Columbia, Missouri, Analytical Biochemistry
Laboratories, Inc. (Unpublished report submitted by Monsanto Ltd).
ABC Inc. (1982a) Dynamic 96-hour acute toxicity of Roundup
(AB-82-33) to bluegill sunfish (Lepomis macrochirus). Columbia,
Missouri, Analytical Biochemistry Laboratories, Inc. (Unpublished
report submitted by Monsanto Ltd).
ABC Inc. (1982b) Dynamic 48-hour acute toxicity of Roundup
(AB-82-035) to Gammarus pseudolimnaeus. Columbia, Missouri,
Analytical Biochemistry Laboratories, Inc. (Unpublished report
submitted by Monsanto Ltd).
ABC Inc. (1982c) Dynamic 96-hour acute toxicity of Roundup
(AB-82-34) to rainbow trout (Salmo gairdnerii). Columbia,
Missouri, Analytical Biochemistry Laboratories, Inc. (Unpublished
report submitted by Monsanto Ltd).
ABC Inc. (1982d) Residue accumulation study in molluscs (Rangia
cuneata) with 14C-glyphosate under static conditions. Columbia,
Missouri, Analytical Biochemistry Laboratories, Inc. (Unpublished
report submitted by Monsanto Ltd).
ABC Inc. (1982e) Residue accumulation study in crayfish
(Procambarus simulans) with 14C-glyphosate under static
conditions. Columbia, Missouri, Analytical Biochemistry
Laboratories, Inc. (Unpublished report submitted by Monsanto Ltd).
ABC Inc. (1984a) Acute toxicity of LLN 8306 to rainbow trout (Salmo
gairdnerii). Columbia, Missouri, Analytical Biochemistry
Laboratories, Inc. (Unpublished report submitted by Monsanto Ltd).
ABC Inc. (1984b) Acute toxicity of LLN 8306 to bluegill sunfish
(Lepomis macrochirus). Columbia, Missouri, Analytical Biochemistry
Laboratories, Inc. (Unpublished report submitted by Monsanto Ltd).
ABC Inc. (1984c) Acute toxicity of LLN 8306 to Daphnia magna.
Columbia, Missouri, Analytical Biochemistry Laboratories, Inc.
(Unpublished report submitted by Monsanto Ltd).
ABC Inc. (1989a) 21-day prolonged static renewal toxicity of MON
8755 to Daphnia magna. Columbia, Missouri, Analytical Biochemistry
Laboratories, Inc. (Unpublished report No. 37933 submitted by
Monsanto Ltd).
ABC Inc. (1989b) 21-day prolonged static renewal toxicity of Roundup
to Daphnia magna. Columbia, Missouri, Analytical Biochemistry
Laboratories, Inc. (Unpublished report No. 37934 submitted by
Monsanto Ltd).
ABC Inc. (1989c) 21-day prolonged static renewal toxicity of
glyphosate technical to Daphnia magna. Columbia, Missouri,
Analytical Biochemistry Laboratories, Inc. (Unpublished report No.
37757 submitted by Monsanto Ltd).
ABC Inc. (1989d) Flow-through toxicity of Roundup to rainbow trout
(Salmo gairdnerii) for a 21-day exposure period. Columbia,
Missouri, Analytical Biochemistry Laboratories, Inc. (Unpublished
report No. 37694 submitted by Monsanto Ltd).
ABC Inc. (1989e) Flow-through toxicity of glyphosate to rainbow
trout (Salmo gairdnerii) for a 21-day duration period. Columbia,
Missouri, Analytical Biochemistry Laboratories, Inc. (Unpublished
report No. 37695 submitted by Monsanto Ltd).
ABC Inc. (1989f) Uptake, depuration, and bioconcentration of
14C glyphosate to bluegill sunfish (Lepomis macrochirus). Part
I: MSL-9304. Columbia, Missouri, Analytical Biochemistry
Laboratories, Inc. (Unpublished report submitted by Monsanto Ltd).
ABC Inc. (1989g) Uptake, depuration, and bioconcentration of
14C glyphosate to bluegill sunfish (Lepomis macrochirus).
Characterization and quantitation of glyphosate and its metabolites.
Part II: MSL-9303. Columbia, Missouri, Analytical Biochemistry
Laboratories, Inc. (Unpublished report submitted by Monsanto Ltd).
ABC Inc. (1989h) Flow-through toxicity of MON 8755 to rainbow trout
(Salmo gairdnerii) for a 21-day duration period. Columbia,
Missouri, Analytical Biochemistry Laboratories, Inc. (Unpublished
report No. 37759 submitted by Monsanto Ltd).
ABC Inc. (1990) Acute toxicity of MON 8755 to common carp (Cyprinus
carpio). Columbia, Missouri, Analytical Biochemistry Laboratories,
Inc. (Unpublished report No. 38256 submitted by Monsanto Ltd).
Ackurst P (1989) Introduction: issues and concerns relating to the
use of herbicides. In: Reynolds P ed. Proceedings of the Carnation
Creek Workshop, Nanaimo, 7-10 December 1987. Victoria, British
Columbia, Forestry Canada/British Columbia Ministry of Forests, pp
7-11.
Altmann G (1984) Laboratory testing of your preparation LLN 83/06
for danger to bees. Unpublished data of University of Saarland,
Saarbrücken, Department of Zoology, provided by Monsanto.
Amrhein N, Deus B, Gehrke P, & Steinrücken HC (1980) The site of the
inhibition of the shikimate pathway by glyphosate. Plant Physiol,
66: 830-834.
Anthony RG & Morrison ML (1985) Influence of glyphosate herbicide on
small-mammal populations in western Oregon. Northwest Sci, 59(3):
159-168.
Austin AP, Harris GE, & Lucey WP (1991) Impact of an organophosphate
herbicide (glyphosate) on periphyton communities developed in
experimental streams. Bull Environ Contam Toxicol, 47: 29-35.
Bababunmi EA, Olorunsogo OO, & Bassir O (1979) The uncoupling effect
of N-(phosphonomethyl)glycine on isolated rat liver mitochondria.
Biochem Pharmacol, 18: 925-927.
Balthazor TM & Hallas LE (1986) Glyphosate-degrading microorganisms
from industrial activated sludge. Appl Environ Microbiol, 51(2):
432-434.
Benova EK, Roupova I, Yagova A, Vuglenov A, & Bineva M (1989)
Mutagenicity studies on six pesticides in mice. Environ Mol Mutagen,
14(Suppl 15): 19 (Abstract).
Bio/Dynamics Inc. (1975) A twenty-one day dermal toxicity study of
MON 2139 in male rabbits (Project No. 75-1245). East Millstone, New
Jersey, Bio/Dynamics Inc., Division of Biology and Safety Evaluation
(Unpublished report submitted by Monsanto Ltd).
Bio/Dynamics Inc. (1979) A three month feeding study of glyphosate
(Roundup technical) in mice (Project No. 77-211) - Final report.
East Millstone, New Jersey, Bio/Dynamics Inc., Division of Biology
and Safety Evaluation (Unpublished report submitted by Monsanto
Ltd).
Bio/Dynamics Inc. (1981a) A lifetime feeding study of glyphosate
(Roundup technical) in rats (Project No. 410/77 [BDN-77-416]). East
Millstone, New Jersey, Bio/Dynamics Inc., Division of Biology and
Safety Evaluation (Unpublished report submitted by Monsanto Ltd).
Bio/Dynamics Inc. (1981b) A three-generation reproduction study in
rats with glyphosate (Project No. 77-2063 [BDN-77-147]) - Final
report. East Millstone, New Jersey, Bio/Dynamics Inc., Division of
Biology and Safety Evaluation (Unpublished report submitted by
Monsanto Ltd).
Bio/Dynamics Inc. (1983a) A chronic feeding study of glyphosate
(Roundup technical) in mice (Project No. 77-2061 [BDN-77-420]). East
Millstone, New Jersey, Bio/Dynamics Inc., Division of Biology and
Safety Evaluation (Unpublished report submitted by Monsanto Ltd).
Bio/Dynamics Inc. (1983b) A dermal sensitization study in guinea
pigs - Test material: Roundup formulation (Project No. 4234-83).
East Millstone, New Jersey, Bio/Dynamics Inc., Division of Biology
and Safety Evaluation (Unpublished report submitted by Monsanto Ltd,
Reference No. BD-83-007).
Bio/Dynamics Inc. (1983c) A dermal sensitization study in guinea
pigs - Test material: glyphosate (Project No. 4235-82). East
Millstone, New Jersey, Bio/Dynamics Inc., Division of Biology and
Safety Evaluation (Unpublished report submitted by Monsanto Ltd,
Reference No. BD-83-008).
Bio/Dynamics Inc. (1984a) Acute dermal toxicity study in rabbits -
Test material: LLN-83-06 (Project No. 4885-83). East Millstone, New
Jersey, Bio/Dynamics Inc., Department of Toxicology (Unpublished
report submitted by Monsanto Ltd, Reference No. BD-83-380).
Bio/Dynamics Inc. (1984b) Acute oral toxicity study in rats - Test
material: LLN-83-06 (Project No. 4884-83). East Millstone, New
Jersey, Bio/Dynamics Inc., Department of Toxicology (Unpublished
report submitted by Monsanto Ltd, Reference No. BD-83-380).
Bio/Dynamics Inc. (1984c) Primary dermal irritation study in rabbits
(4-hour exposure) - Test material: LLN-83-06 (Project No. 4886-83).
East Millstone, New Jersey, Bio/Dynamics Inc., Department of
Toxicology (Unpublished report submitted by Monsanto Ltd, Reference
No. BD-83-380).
Bio/Dynamics Inc. (1984d) Eye irritation study in rabbits - Test
material: LLN-83-06 (Project No. 4887-83). East Millstone, New
Jersey, Bio/Dynamics Inc., Department of Toxicology (Unpublished
report submitted by Monsanto Ltd, Reference No. BD-83-380).
Bio/Dynamics Inc. (1985a) Acute oral toxicity study in rats - Test
material: MON 8780 (Project No. 5301-84). East Millstone, New
Jersey, Bio/Dynamics Inc., Department of Toxicology (Unpublished
report submitted by Monsanto Ltd, Reference No. BD-84-343).
Bio/Dynamics Inc. (1985b) Acute dermal toxicity study in rabbits -
Test material: MON 8780 (Project No. 5302-84). East Millstone, New
Jersey, Bio/Dynamics Inc., Department of Toxicology (Unpublished
report submitted by Monsanto Ltd, Reference No. BD-84-343).
Bio/Dynamics Inc. (1985c) Primary dermal irritation study in rabbits
(4-hour exposure) - Test material: MON 8780 (Project No. 5303-84).
East Millstone, New Jersey, Bio/Dynamics Inc., Department of
Toxicology (Unpublished report submitted by Monsanto Ltd).
Bio/Dynamics Inc. (1985d) Eye irritation study in rabbits - Test
material: MON 8780 (Project No. 5304-84). East Millstone, New
Jersey, Bio/Dynamics Inc., Department of Toxicology (Unpublished
report submitted by Monsanto Ltd, Reference No. BD-84-343).
Bio/Dynamics Inc. (1986) A closed-patch repeated insult dermal
sensitization study in guinea pigs (Buehler method) - Test material:
MON 8756 (Project No. 6210-85). East Millstone, New Jersey,
Bio/Dynamics Inc., Department of Toxicology (Unpublished report
submitted by Monsanto Ltd, Reference No. BD-86-5).
Bio/Dynamics Inc. (1987a) Acute oral toxicity study in rats - Test
material: Roundup L&G Ready-to-use (Project No. 4033-86). East
Millstone, New Jersey, Bio/Dynamics Inc., Department of Toxicology
(Unpublished report submitted by Monsanto Ltd, Reference No.
BD-87-4).
Bio/Dynamics Inc. (1987b) Acute dermal toxicity study in rabbits -
Test material: Roundup L&G Ready-to-use (Project No. 4034-86). East
Millstone, New Jersey, Bio/Dynamics Inc., Department of Toxicology
(Unpublished report submitted by Monsanto Ltd, Reference
No. BD-87-3).
Bio/Dynamics Inc. (1987c) Primary dermal irritation study in rabbits
(4-hour exposure/semi-occlusive covering) - Test material: Roundup
L&G Ready-to-Use (Project No. 4035-86). East Millstone, New Jersey,
Bio/Dynamics Inc., Department of Toxicology (Unpublished report
submitted by Monsanto Ltd, Reference No. BD-87-4).
Bio/Dynamics Inc. (1987d) Eye irritation study in rabbits - Test
material: Roundup L&G Ready-to-Use (Project No. 4036-86). East
Millstone, New Jersey, Bio/Dynamics Inc., Department of Toxicology
(Unpublished report submitted by Monsanto Ltd, Reference
No. BD-87-4).
Bio/Dynamics Inc. (1987e) A closed-patch repeated insult dermal
sensitization study in guinea pigs (Project No. 6945-86). East
Millstone, New Jersey, Bio/Dynamics Inc., Division of Biology and
Safety Evaluation (Unpublished report submitted by Monsanto Ltd,
Reference No. BD-86-442).
Bio/Dynamics Inc. (1988a) Acute dermal toxicity study in rabbits -
Test material: Glyphosate Wet Cake (Project No. 4886-88). East
Millstone, New Jersey, Bio/Dynamics Inc., Department of Toxicology
(Unpublished report submitted by Monsanto Ltd, Reference No.
BD-88-114).
Bio/Dynamics Inc. (1988b) Primary dermal irritation study in rabbits
(4-hour exposure/semi-occlusive covering) - Test material:
Glyphosate Wet Cake (Project No. 4887-88). East Millstone, New
Jersey, Bio/Dynamics Inc., Department of Toxicology (Unpublished
report submitted by Monsanto Ltd, Reference No. BD-88-114).
Bio/Dynamics Inc. (1988c) Acute oral toxicity study in rats - Test
material: Glyphosate Wet Cake (Project No. 4885-88). East Millstone,
New Jersey, Bio/Dynamics Inc., Department of Toxicology (Unpublished
report submitted by Monsanto Ltd, Reference No. BD-88-114).
Bio/Dynamics Inc. (1988d) Eye irritation study in rabbits - Test
material: Glyphosate Wet Cake (Project No. 4888-88). East Millstone,
New Jersey, Bio/Dynamics Inc., Department of Toxicology (Unpublished
report submitted by Montsanto Ltd, Reference No. BD-88-114).
Bio/Dynamics Inc. (1988e) Acute oral toxicity study in rats - Test
material: Roundup (Project No. 4546-87). East Millstone, New Jersey,
Bio/Dynamics Inc., Department of Toxicology (Unpublished report
submitted by Monsanto Ltd, Reference No. BD-87-283).
Bio/Dynamics Inc. (1988f) Acute dermal toxicity study in rabbits -
Test material: Roundup (Project No. 4547-87). East Millstone, New
Jersey, Bio/Dynamics Inc., Department of Toxicology (Unpublished
report submitted by Monsanto Ltd, Reference No. BD-87-283).
Bio/Dynamics Inc. (1988g) Primary dermal irritation study in rabbits
(4-hour exposure/semi-occlusive covering) - Test material: Roundup
(Project No. 4548-87). East Millstone, New Jersey, Bio/Dynamics
Inc., Department of Toxicology (Unpublished report submitted by
Monsanto Ltd, Reference No. BD-87-283).
Bio/Dynamics Inc. (1990) Eye irritation study in rabbits - Test
material: MON 2139 (Project No. 5833-90). East Millstone, New
Jersey, Bio/Dynamics Inc., Department of Toxicology (Unpublished
report submitted by Monsanto, Reference No. BD-90-246).
Bionomics (1973a) Acute toxicity of Roundup (technical) to atlantic
oyster (Crassostrea virginica). Wareham, Massachusetts, Bionomics
(Unpublished report submitted by Monsanto Ltd).
Bionomics (1973b) Acute toxicity of Roundup (technical) to grass
shrimp (Palaemonetes vulgaris) and fiddler crab (Uca pugilator).
Wareham, Massachusetts, Bionomics (Unpublished report submitted by
Monsanto Ltd).
Bowmer KH, Boulton PM, Short DL, & Higgins ML (1986)
Glyphosate-sediment interactions and phytotoxicity in turbid water.
Pestic Sci, 17(2): 79-88.
Bozeman J, Koopman B, & Bitton G (1989) Toxicity testing using
immobilized algae. Aquat Toxicol, 14(4): 345-352.
Buhl KJ & Faerber NL (1989) Acute toxicity of selected herbicides
and surfactants to larvae of the midge Chironomus riparius. Arch
Environ Contam Toxicol, 18(4): 530-536.
Bunyatyan YA & Gevorgyan AA (1984) [Determination of the active
ingredient of Roundup and its metabolite in the environment.] Gig i
Sanit, 5: 43-44 (in Russian).
Burns AJ (1983) Liquid chromatographic determination of glyphosate
technical and its formulation: collaborative study. J Assoc Off Anal
Chem, 66(5): 1214-1219.
Carlisle SM & Trevors JT (1986a) Effect of the herbicide glyphosate
on nitrification, denitrification, and acetylene reduction in soil.
Water Air Soil Pollut, 29(2): 189-203.
Carlisle SM & Trevors JT (1986b) Effect of the herbicide glyphosate
on respiration and hydrogen consumption in soil. Water Air Soil
Pollut, 27(3/4): 391-401.
Chakravarty P & Chatarpaul L (1990a) Non-target effects of
herbicides: I. Effect of glyphosate and hexazinone on soil microbial
activity, microbial population, and in vitro growth of
ectomycorrhizal fungi. Pestic Sci, 28(3): 233-242.
Chakravarty P & Chatarpaul L (1990b) Non-target effect of
herbicides: II. The influence of glyphosate on ectomycorrhizal
symbiosis of red pine (Pinus resinosa) under greenhouse and field
conditions. Pestic Sci, 28(3): 243-248.
Chan KY & Leung SC (1986) Effects of paraquat and glyphosate on
growth, respiration, and enzyme activity of aquatic bacteria. Bull
Environ Contam Toxicol, 36(1): 52-59.
CIT (1991a) EXP 30578 - Acute oral toxicity in rats (Study No. 7076
TAR). Miserey, France, Centre International de Toxicologie (CIT)
(Unpublished report submitted by Rhône Poulenc, Lyon, France).
CIT (1991b) EXP 30578 - Acute dermal toxicity in rats (Study No.
7077 TAR). Miserey, France, Centre International de Toxicologie
(CIT) (Unpublished report submitted by Rhône Poulenc, Lyon, France).
CIT (1991c) EXP 30578 - Acute dermal irritation in rabbits (Study
No. 7079 TAL). Miserey, France, Centre International de Toxicologie
(CIT) (Unpublished report submitted by Rhône Poulenc, Lyon, France).
CIT (1991d) EXP 30578 - Acute eye irritation in rabbits (Study No.
7078 TAL). Miserey, France, Centre International de Toxicologie
(CIT) (Unpublished report submitted by Rhône Poulenc, Lyon, France).
Cowell JE, Kunstman JL, Nord PJ, Steinmetz JR, & Wilson GR (1986)
Validation of an analytical residue method for analysis of
glyphosate and metabolite: an interlaboratory study. J Agric Food
Chem, 34(6): 955-960.
Deyrup CL, Chang SM, Weintraub RA, & Moye HA (1985) Simultaneous
esterification and acylation of pesticides for analysis by gas
chromatography. 1. Derivatization of glyphosate and
(aminomethyl)phosphonic acid with fluorinated
alcohols-perfluorinated anhydrides. J Agric Food Chem, 33(5):
944-947.
Dickson SJ, Meinhold RH, Beer ID, & Koelmeyer TD (1988) Rapid
determination of glyphosate in postmortem specimens using 31P NMR. J
Anal Toxicol, 12(5): 284-286.
D'Anieri P, Leslie DM, & McCormack ML (1987) Small mammals in
glyphosate-treated clearcuts in Northern Maine. Can Field-Nat,
101(4): 547-550.
Dubelman S (1988) Glyphosate. Anal Methods Pestic Plant Growth
Regul, 16: 69-82.
Duke SO & Hoagland RE (1981) Effects of glyphosate on the metabolism
of phenolic compounds: VII. Root-fed amino acids and glyphosate
toxicity in soybean (Glycine max) seedlings. Weed Sci, 29(3):
297-302.
Eberbach PL & Douglas LA (1983) Persistence of glyphosate in a sandy
loam. Soil Biol Biochem, 15(4): 485-487.
Eberbach PL & Douglas LA (1989) Herbicide effects on the growth and
nodulation potential of Rhizobium trifolii with Trifolium
subterraneum L. Plant Soil, 119: 15-23.
Edwards WM, Triplett GB, & Kramer RM (1980) A watershed study of
glyphosate transport in runoff. J Environ Qual, 9(4): 661-664.
EG & G Bionomics (1975) Chronic toxicity of glyphosate to the
fathead minnow (Pimephales promelas Rafinesque). Aquatic
Toxicology Laboratory. Wareham, Massachusetts, EG & G Bionomics
(Unpublished report submitted by Monsanto Ltd).
EG & G Bionomics (1978a) Toxicity of seven test materials to the
marine alga, Skeletonema costatum. Marine Research Laboratory,
Pensacola, Florida, EG & G Bionomics (Unpublished report No.
BP-78-4-031 submitted by Monsanto Ltd).
EG & G Bionomics (1978b) Toxicity of seven test materials to
sheepshead minnows, Cyprinodon variegatus. Marine Research
Laboratory, Pensacola, Florida, EG & G Bionomics (Unpublished report
No. BP-78-4-029 submitted by Monsanto Ltd).
EG & G Bionomics (1978c) Toxicity of seven test materials to mysid
shrimp, Mysidopsis bahia. Marine Research Laboratory, Pensacola,
Florida, EG & G Bionomics (Unpublished report No. BP-78-4-032
submitted by Monsanto Ltd).
EG & G Bionomics (1978d) Toxicity of seven test materials to the
white sea urchin, Tripneustes esculentus. Marine Research
Laboratory, Pensacola, Florida, EG & G Bionomics (Unpublished report
No. BP-78-4-030 submitted by Monsanto Ltd).
EG & G Bionomics (1980a) Acute toxicity of Roundup to channel
catfish (Ictalurus punctatus). Aquatic Toxicology Laboratory.
Wareham, Massachusetts, EG & G Bionomics (Unpublished report No.
BW-80-4-641 submitted by Monsanto Ltd).
EG & G Bionomics (1980b) Acute toxicity of Roundup to bluegill
(Lepomis macrochirus). Aquatic Toxicology Laboratory. Wareham,
Massachusetts, EG & G Bionomics (Unpublished report No. BW-80-4-634
submitted by Monsanto Ltd).
EG & G Bionomics (1980c) Acute toxicity of Roundup to the rainbow
trout (Salmo gairdnerii). Aquatic Toxicology Laboratory. Wareham,
Massachusetts, EG & G Bionomics (Unpublished report No. BW-80-4-635
submitted by Monsanto Ltd).
EG & G Bionomics (1980d) Acute toxicity of Roundup to the fathead
minnow (Pimephales promelas). Aquatic Toxicology Laboratory.
Wareham, Massachusetts, EG & G Bionomics (Unpublished report No.
BW-80-4-653 submitted by Monsanto Ltd).
EG & G Bionomics (1980e) Acute toxicity of Roundup to the water flea
(Daphnia magna). Aquatic Toxicology Laboratory. Wareham,
Massachusetts, EG & G Bionomics (Unpublished report No. BW-80-4-636
submitted by Monsanto Ltd).
EG & G Bionomics (1980f) Acute toxicity of Roundup to the water flea
(Daphnia magna) with and without continuous aeration. Aquatic
Toxicology Laboratory. Wareham, Massachusetts, EG & G Bionomics
(Unpublished report No. BW-80-6-690 submitted by Monsanto Ltd).
EG & G Bionomics (1980g) Acute toxicity of Roundup to the rainbow
trout (Salmo gairdnerii) with continuous aeration and without
aeration. Aquatic Toxicology Laboratory. Wareham, Massachusetts,
EG & G Bionomics (Unpublished report No. BW-80-6-686 submitted by
Monsanto Ltd).
Estok D, Freedman B, & Boyle D (1989) Effects of the herbicides
2,4-D, glyphosate, hexazinone, and triclopyr on the growth of three
species of ectomycorrhizal fungi. Bull Environ Contam Toxicol,
42(6): 835-839.
Evans DD & Batty MJ (1986) Effects of high dietary concentrations of
glyphosate (Roundup) on a species of bird, marsupial and rodent
indigenous to Australia. Environ Toxicol Chem, 5: 399-401.
EVS Consultants (1986a) Acute toxicity of Roundup herbicide to
rainbow trout. Seattle, Washington, EVS Consultants (Unpublished
report submitted by Monsanto Ltd).
EVS Consultants (1986b) Acute toxicity of Roundup herbicide to
chinook salmon. Seattle, Washington, EVS Consultants (Unpublished
report submitted by Monsanto Ltd).
EVS Consultants (1986c) Acute toxicity of Roundup herbicide to coho
salmon. Seattle, Washington, EVS Consultants (Unpublished report
submitted by Monsanto Ltd). EVS Consultants (1986d) Seawater
challenge testing of coho salmon smolts following Roundupr herbicide
exposure. Seattle, Washington, EVS Consultants (Unpublished report
submitted by Monsanto Ltd).
FAO/WHO (1986a) Pesticide residues in food - Evaluations 1986. Part
I - Residues. Joint Meeting of the FAO Panel of Experts Residues in
Food and the Environment and the WHO Expert Group on Pesticide
Residues, Rome, 29 September-8 October 1986. Rome, Food and
Agriculture Organization of the United Nations (FAO Plant Production
and Protection Paper 78/1).
FAO/WHO (1986b) Pesticide residues in food - Evaluations 1986. Part
II - Toxicology. Joint Meeting of the FAO Panel of Experts Residues
in Food and the Environment and the WHO Expert Group on Pesticide
Residues, Rome, 29 September-8 October 1986. Rome, Food and
Agriculture Organization of the United Nations (FAO Plant Production
and Protection Paper 78/2).
FAO/WHO (1987) Pesticide residues in food - Evaluations 1987. Part I
- Residues. Joint Meeting of the FAO Panel of Experts Residues in
Food and the Environment and the WHO Expert Group on Pesticide
Residues, Geneva, 21-30 September 1987. Rome, Food and Agriculture
Organization of the United Nations (FAO Plant Production and
Protection Paper 86/1).
FAO/WHO (1988) Pesticide residues in food - Evaluations 1988. Part I
- Residues. Joint Meeting of the FAO Panel of Experts Residues in
Food and the Environment and the WHO Expert Group on Pesticide
Residues, Rome, 19-28 September 1988. Rome, Food and Agriculture
Organization of the United Nations (FAO Plant Production and
Protection Paper 93/1).
FDRL (1988a) Primary dermal irritation study of glyphosate
batch/lot/nbr No. XLI-55 in New Zealand White rabbits (Study No.
88.2053.010). Waverly, New York, Food & Drug Research Laboratories
(Unpublished report submitted by Monsanto Ltd - Monsanto study No.
FD-88-29).
FDRL (1988b) Acute dermal toxicity study of glyphosate batch/lot/nbr
No. XLI-55 in New Zealand White rabbits (Study No. 88.2053.008).
Waverly, New York, Food & Drug Research Laboratories (Unpublished
report submitted by Monsanto Ltd - Monsanto study No. FD-88-29).
FDRL (1988c) Primary eye irritation study of glyphosate
batch/lot/nbr No. XLI-55 in New Zealand White rabbits (Study No.
88.2053.009). Waverly, New York, Food & Drug Research Laboratories
(Unpublished report submitted by Monsanto Ltd - Monsanto study No.
FD-88-29).
FDRL (1988d) Acute oral toxicity study of glyphosate batch/lot/nbr
No. XLI-55 in Sprague-Dawley rats (Study No. 88.2053.007). Waverly,
New York, Food & Drug Research Laboratories (Unpublished report
submitted by Monsanto Ltd - Monsanto study No. FD-88-29).
Feller MC (1989) Effects of forest herbicide applications on
streamwater chemistry in southwestern British Columbia. Water Resour
Bull, 25(3): 607-616.
Feng JC & Thompson DG (1990) Fate of glyphosate in a Canadian forest
watershed. 2. Persistence in foliage and soils. J Agric Food Chem,
38(4): 1118-1125.
Feng JC, Thompson DG, & Reynolds PE (1990) Fate of glyphosate in a
Canadian forest watershed. 1. Aquatic residues and off-target
deposit assessment. J Agric Food Chem, 38(4): 1110-1118.
Folmar LC, Sanders HO, & Julin AM (1979) Toxicity of the herbicide
glyphosate and several of its formulations to fish and aquatic
invertebrates. Arch Environ Contam Toxicol, 8: 269-278.
Franz TJ (1983) Evaluation of the percutaneous absorption of Roundup
formulations in man using an in vitro technique (Study No.
UW-81-346). Seattle, Washington, University of Washington, School of
Medicine (Unpublished report submitted by Monsanto Ltd).
Friestad HO & Bronstad JO (1985) Improved polarographic method for
determination of glyphosate herbicide in crops, soils, and water. J
Assoc Off Anal Chem, 68(1): 76-79.
Gauch R, Leuenberger U, & Müller U (1989) [Determination of
glyphosate herbicide and its main metabolite aminomethylphosphonic
acid (AMPA) in drinking water by HPLC.] Z Lebensm.unters Forsch,
188(1): 36-38 (in German).
Glass RL (1981) Colorimetric determination of glyphosate in water
after oxidation to orthophosphate. Anal Chem, 53(6): 921-923.
Glass RL (1983) Liquid chromatographic determination of glyphosate
in fortified soil and water. J Agric Food Chem, 31(2): 280-282.
Glass RL (1987) Adsorption of glyphosate by soils and clay minerals.
J Agric Food Chem, 35: 497-500.
Goldsborough LG & Beck AE (1989) Rapid dissipation of glyphosate in
small forest ponds. Arch Environ Contam Toxicol, 18(4): 537-544.
Goldsborough LG & Brown DJ (1988) Effect of glyphosate (Roundup
formulation) on periphytic algal photosynthesis. Bull Environ Contam
Toxicol, 41(2): 253-260.
Gómez MA & Sagardoy MA (1985) [Influence of glyphosate herbicide on
the microflora and mesofauna of a sandy soil in a semi-arid region.]
Rev Latinoam Microbiol, 27: 351-357 (in Spanish).
Gopalan HNB & Njagi GDE (1981) Mutagenicity testing of pesticides.
III. Drosophila: recessive sex-linked lethals. Genetics,
97(Suppl): 544 (Abstract).
Gougler JA & Geiger DR (1981) Uptake and distribution of
N-phosphonomethylglycine in sugar beet plants. Plant Physiol, 68:
668-672.
Gougler JA & Geiger DR (1984) Carbon partitioning and herbicide
transport in glyphosate-treated sugarbeet (Beta vulgaris). Weed
Sci, 32(4): 546-551.
Guinivan RA, Thompson NP, & Wheeler WB (1982) Derivatization and
cleanup improvements in determination of residues of glyphosate and
aminomethylphosphonic acid in blueberries. J Assoc Off Anal Chem,
65(1): 35-39.
Haag WR & Yao CCD (1992) Rate constants for reaction of hydroxyl
radicals with several drinking water contaminants. Environ Sci
Technol, 26: 1005-1013.
Hallas LE, Adams WJ, & Heitkamp MA (1992) Glyphosate degradation by
immobilized bacteria: field studies with industrial wastewater
effluent. Appl Environ Microbiol, 51: 432-434.
Hallberg GL (1989) Pesticide pollution of groundwater in the humid
United States. Agric Ecosyst Environ, 26: 299-367.
Hance RJ (1976) Adsorption of glyphosate by soils. Pestic Sci, 7:
363-366.
Hartman WA & Martin DB (1984) Effect of suspended bentonite clay on
the acute toxicity of glyphosate to Daphnia pulex and Lemna
minor. Bull Environ Contam Toxicol, 33(3): 355-361.
Hartman WA & Martin DB (1985) Effects of four agricultural
pesticides on Daphnia pulex, Lemna minor, and Potamogeton
pectinatus. Bull Environ Contam Toxicol, 35(5): 646-651.
Hazleton Laboratories, Inc. (1973a) Eight-day dietary LC50 -
bobwhite quail. Final report. Vienna, Virginia, Hazleton
Laboratories, Inc. (Unpublished report submitted by Monsanto Ltd).
Hazleton Laboratories, Inc. (1973b) Eight-day dietary LC50 -
mallard ducks. Final report. Vienna, Virginia, Hazleton
Laboratories, Inc. (Unpublished report submitted by Monsanto Ltd).
Hazleton Lab. Inc. (1988a) Metabolism study of synthetic
13C/14C-labeled glyphosate and aminomethylphosphonic acid in
laying hens, Part I. Hazleton Laboratories Inc. Project No. HLA
6103-112. Madison, Wisconsin, Monsanto Agricultural Company, Report
No. MSL-7591. Unpublished report supplied by Monsanto Ltd.
Hazleton Lab. Inc. (1988b) Metabolism study of synthetic
13C/14C-labeled glyphosate and aminomethylphosphonic acid in
lactating goats, Part I. Hazleton Laboratories Inc. Project No. HLA
6103-113. Madison, Wisconsin, Monsanto Agricultural Company, Report
No. MSL-7586. Unpublished report supplied by Monsanto Ltd.
Heinonen-Tanski H (1989) The effect of temperature and liming on the
degradation of glyphosate in two arctic forest soils. Soil Biol
Biochem, 21(2): 313-317.
Heinonen-Tanski H, Rosenberg C, Siltanen H, Kilpi S, & Simojoki P
(1985) The effect of the annual use of pesticides on soil
microorganisms, pesticide residues in the soil and barley yields.
Pestic Sci, 16(4): 341-348.
Hensley DL, Beuerman DSN, & Carpenter PL (1978) The inactivation of
glyphosate by various soils and metal salts. Weed Res, 18: 287-291.
Hernando F, Royuela M, Muñoz-Rueda A, & Gonzalez-Murua C (1989)
Effect of glyphosate on the greening process and photosynthetic
metabolism in Chlorella pyrenoidosa. J Plant Physiol, 136: 26-31.
Hildebrand LD, Sullivan DS, & Sullivan TP (1980) Effects of Roundup
herbicide on populations of Daphnia magna in a forest pond. Bull
Environ Contam Toxicol, 25(3): 353-357.
Hildebrand LD, Sullivan DS, & Sullivan TP (1982) Experimental
studies of rainbow trout populations exposed to field applications
of Roundup herbicide. Arch Environ Contam Toxicol, 11(1): 93-98.
Hoffman DJ & Albers PH (1984) Evaluation of potential embryotoxicity
and teratogenicity of 42 herbicides, insecticides, and petroleum
contaminants to mallard eggs. Arch Environ Contam Toxicol, 13(1):
15-27.
Holdway DA & Dixon DG (1988) Acute toxicity of permethrin or
glyphosate pulse exposure to larval white sucker (Catostomus
commersoni) and juvenile flagfish (Jordanella floridae) as
modified by age and ration level. Environ Toxicol Chem, 7(1): 63-68.
Holtby LB & Baillie SJ (1989a) Effects of the herbicide Roundup
(glyphosate) on periphyton in Carnation Creek, British Columbia. In:
Reynolds P ed. Proceedings of the Carnation Creek Workshop, Nanaimo,
7-10 December 1987. Victoria, British Columbia, Forestry
Canada/British Columbia Ministry of Forests, pp 224-231.
Holtby LB & Baillie SJ (1989b) Effects of the herbicide Roundup on
coho salmon fingerlings in an over-sprayed tributary of Carnation
Creek, British Columbia. In: Reynolds P ed. Proceedings of the
Carnation Creek Workshop, Nanaimo, 7-10 December 1987. Victoria,
British Columbia, Forestry Canada/British Columbia Ministry of
Forests, pp 273-285.
HRC (1972) The acute contact and oral toxicities of CP67573 and MON
2139 to worker honey bees. Huntingdon, UK, Huntingdon Research
Centre (Unpublished report submitted by Monsanto Ltd).
HRC (1977) The acute toxicity of glyphosate to harlequin fish
(Rasbora heteromorpha). Huntingdon, UK, Huntingdon Research Centre
(Unpublished report submitted by Monsanto Ltd).
HRC (1988) The acute oral toxicity of ICIA0224 to the bobwhite
quail. Huntingdon, UK, Huntingdon Research Centre (Unpublished
report No. ISN 177/881062 submitted by Monsanto Ltd).
IBR (1991a) Final report - Acute toxicity in earthworms according to
OECD 207-test article: "Technical isopropylamin salt of glyphosate =
MON 0139" (Project No. 80-91-2078-00-90). Hannover, International
Bioresearch (Unpublished report submitted by Monsanto Ltd).
IBR (1991b) Final report - Acute toxicity in earthworms according to
OECD 207-test article: "Roundup" (Project No. 80-91-0599-00-90).
Hannover, International Bioresearch (Unpublished report submitted by
Monsanto Ltd).
IET (1978) Microbial mutagenicity testing on CP67573 (glyphosate).
Mitsukaido, Japan, Institute of Environmental Toxicology (IET)
(Study sponsor Monsanto Ltd - Monsanto unpublished report No.
ET-78-241).
IFU (1990) [Side-effects of Swing on the ground-beetle Poecilus
cupreus Bonnelli under laboratory conditions (Project No.
110/01-Pc).] Unpublished report of Institute for Environmental
Analysis and Biotechnology, Niefern-Öschelbron, supplied by Monsanto
Ltd (in German).
Inveresk Research Int. (1988a) Compound No. 3607: Acute oral
toxicity (limit) test in rats (IRI project No. 241496). Musselburgh,
Scotland, Inveresk Research International (Unpublished report No.
5585 submitted by Cheminova A/S, Denmark).
Inveresk Research Int. (1988b) Compound No. 3607: Acute dermal
toxicity (limit) test in rats (IRI project No.241496). Musselburgh,
Scotland, Inveresk Research International (Unpublished report No.
5586 submitted by Cheminova A/S, Denmark).
Inveresk Research Int. (1988c) Compound 3707: Primary skin
irritation in rabbits (IRI project No. 241496). Musselburgh,
Scotland, Inveresk Research International (Unpublished report No.
5587 submitted by Cheminova A/S, Denmark).
Inveresk Research Int. (1988d) Compound No. 3607: Magnusson-Kligman
Maximisation test in guinea pigs (IRI project No. 241496).
Musselburgh, Scotland, Inveresk Research International (Unpublished
report No. 5589 submitted by Cheminova A/S, Denmark).
Inveresk Research Int. (1989a) Glyphosate technical: acute oral
toxicity (limit) test in rats (IRI project No. 243268). Musselburgh,
Scotland, Inveresk Research International (Unpublished report No.
5883 submitted by Cheminova A/S, Denmark).
Inveresk Research Int. (1989b) Glyphosate technical:
Magnusson-Kligman Maximisation test in guinea pigs (IRI project No.
243268). Musselburgh, Scotland, Inveresk Research International
(Unpublished report No. 5587 submitted by Cheminova A/S, Denmark).
Inveresk Research Int. (1989c) Glyphosate technical: acute dermal
toxicity (limit) test in rats (IRI project No. 243268). Musselburgh,
Scotland, Inveresk Research International (Unpublished report No.
5884 submitted by Cheminova A/S, Denmark).
Inveresk Research Int. (1989d) Glyphosate soluble liquid formulation
(code No. 3607) - Acute inhalation toxicity study in rats (limit
test) (IRI project No. 640023). Musselburgh, Scotland, Inveresk
Research International (Unpublished report No. 5603 submitted by
Cheminova A/S, Denmark).
Inveresk Research Int. (1989e) Glyphosate technical: Primary skin
irritation in rabbits (IRI project No. 243268). Musselburgh,
Scotland, Inveresk Research International (Unpublished report No.
5885 submitted by Cheminova A/S, Denmark).
Inveresk Research Int. (1989f) Glyphosate technical: Primary eye
irritation in rabbits (IRI project No. 243268). Musselburgh,
Scotland, Inveresk Research International (Unpublished report No.
5886 submitted by Cheminova A/S, Denmark).
Inveresk Research Int. (1989g) Compound No. 3607: Primary eye
irritation in rabbits (IRI project No. 241496). Musselburgh,
Scotland, Inveresk Research International (Unpublished report No.
5588 submitted by Cheminova A/S, Denmark).
IRDC (1980a) Test article - Technical glyphosate: Dominant lethal
study in mice (Study No. 401-064). Mattawan, Michigan, International
Research and Development Corporation (Unpublished report submitted
Monsanto Ltd, Reference No. IR-79-014).
IRDC (1980b) Test article - Technical glyphosate: Teratology study
in rats (Study No. 401-054). Mattawan, Michigan, International
Research and Development Corporation (Unpublished report submitted
by Monsanto Ltd, Reference No. IR-79-016).
IRDC (1980c) Test article - Technical glyphosate: Teratology study
in rabbits (Study No. 401-056). Mattawan, Michigan, International
Research and Development Corporation (Unpublished report submitted
by Monsanto Ltd, Reference No. IR-79-018).
IRDC (1982) Test article - Glyphosate technical: 21-day dermal
toxicity study in rabbits (Study No. 401-168). Mattawan, Michigan,
International Research and Development Corporation (Unpublished
report submitted by Monsanto Ltd, Reference No. IR-81-195).
IRPTC (1991) Data profile on glyphosate. Geneva, International
Register for Potentially Toxic Chemicals, United Nations Environment
Programme.
Jacob GS, Garbow JR, Hallas LE, Kimack NM, Kishore GM, & Schaefer J
(1988) Metabolism of glyphosate in Pseudomonas sp. strain LBr.
Appl Environ Microbiol, 54(12): 2953-2958.
Jauhiainen A, Räsänen K, Sarantila R, Nuutinen J, & Kangas J (1991)
Occupational exposure of forest workers to glyphosate during brush
saw spraying work. Am Ind Hyg Assoc J, 52(2): 61-64.
Kishore GM & Jacob GS (1987) Degradation of glyphosate by
Pseudomonas sp. PG2982 via a sarcosine intermediate. J Biol Chem,
262(25): 12164-12168.
Kitchen LM, Witt WW, & Rieck CE (1981a) Inhibition of chlorophyll
accumulation by glyphosate. Weed Sci, 29(4): 513-516.
Kitchen LM, Witt WW, & Rieck CE (1981b) Inhibition of
delta-aminolevulinic acid synthesis by glyphosate. Weed Sci, 29(5):
571-577.
Knapek R, Kobes S, & Kita I (1986) [Toxicological evaluation of
N-phosphono-methylglycine.] Z gesamte Hyg, 32(9): 537-539 (in
German).
Konar SK & Roy DN (1990) Method for the determination of residues of
the herbicide glyphosate and its principal metabolite,
aminomethylphosphonic acid, in plant materials by nitrogen-selective
gas chromatography. Anal Chim Acta, 229(2): 277-280.
Kreutzweiser DP, Kingsbury PD, & Feng JC (1989) Drift response of
stream invertebrates to aerial applications of glyphosate. Bull
Environ Contam Toxicol, 42(3): 331-338.
Lavy TL, Cowell JE, Steinmetz JR, & Massey JH (1992) Conifer
seedling nursery worker exposure to glyphosate. Arch Environ Contam
Toxicol, 22: 6-13.
Lethbridge G, Bull AT, & Burns RG (1981) Effects of pesticides on
1,3-ß-glucanase and urease activities in soil in the presence and
absence of fertilisers, lime and organic materials. Pestic Sci,
12(2): 147-155.
LISEC (1989a) Alga, growth inhibition test. Effect of MON 2139 on
the growth of Selenastrum capricornutum. Bokrijk, Belgium, LISEC,
Study Centre for Ecology and Forestry (Unpublished report submitted
by Monsanto Ltd).
LISEC (1989b) Alga, growth inhibition test. Effect of MON 8755 on
the growth of Selenastrum capricornutum. Bokrijk, Belgium, LISEC,
Study Centre for Ecology and Forestry (Unpublished report submitted
by Monsanto Ltd).
LISEC (1990a) Degradation: Biological oxygen demand. Bokrijk,
Belgium, LISEC, Study Centre for Ecology and Forestry (Unpublished
report submitted by Monsanto Ltd).
LISEC (1990b) Degradation: Chemical oxygen demand. Bokrijk, Belgium,
LISEC, Study Centre for Ecology and Forestry (Unpublished report
submitted by Monsanto Ltd). Liu C-M, McLean PA, Sookdeo CC, & Cannon
FC (1991) Degradation of the herbicide glyphosate by members of the
family Rhizobiaceae. Appl Environ Microbiol, 57: 1799-1804.
Lockhart WL, Billeck BN, & Baron CL (1989) Bioassays with a floating
aquatic plant (Lemna minor) for effects of sprayed and dissolved
glyphosate. Hydrobiologia, 188/189: 353-359.
Lundgren LN (1986) A new method for the determination of glyphosate
and (aminomethyl)phosphonic acid residues in soils. J Agric Food
Chem, 34(3): 535-538.
Lund-Hoie K & Friestad HO (1986) Photodegradation of the herbicide
glyphosate in water. Bull Environ Contam Toxicol, 36(5): 723-729.
Madhun YA, Young JL, & Freed VH (1986) Binding of herbicides by
water-soluble organic materials from soil. J Environ Qual, 15(1):
64-68.
Maibach HI (1983) (a) Elimination of 14C-glyphosate in Rhesus
monkeys following a single dose. (b) Percutaneous absorption in
Roundup formulation in Rhesus monkeys following a single oral dose
(Study No. MA-81-349). San Francisco, California, California School
of Medicine (Unpublished report submitted by Monsanto Ltd).
Maibach HI (1986) Irritation, sensitive, photoirritation and
photosensitization assays with a glyphosate herbicide. Contact
Dermatitis, 15: 152-156.
Malcolm Pirnie Inc. (1987a) The toxicity of glyphosate technical to
Anabaena flos-aquae. White Plains, New York, Malcolm Pirnie Inc.
(Unpublished report submitted by Monsanto Ltd).
Malcolm Pirnie Inc. (1987b) The toxicity of glyphosate technical to
Skeletonema costatum. White Plains, New York, Malcolm Pirnie Inc.
(Unpublished report submitted by Monsanto Ltd).
Malcolm Pirnie Inc. (1987c) The toxicity of glyphosate technical to
Navicula pelliculosa. White Plains, New York, Malcolm Pirnie Inc.
(Unpublished report submitted by Monsanto Ltd).
Malcolm Pirnie Inc. (1987d) The toxicity of glyphosate technical to
Selenastrum capricornutum. White Plains, New York, Malcolm Pirnie
Inc. (Unpublished report submitted by Monsanto Ltd).
Malcolm Pirnie Inc. (1987e) The toxicity of glyphosate technical to
Lemna gibba. White Plains, New York, Malcolm Pirnie Inc.
(Unpublished report submitted by Monsanto Ltd).
Marcotte AL, Bradley M, & Hughley DH ed. (1977) Pesticide analytical
manual. Washington, DC, Food and Drug Administration.
Marrs RH, Williams CT, Frost AJ, & Plant RA (1989) Assessment of the
effects of herbicide spray drift on a range of plant species of
conservation interest. Environ Pollut, 59(1): 71-86.
Marsh JAP (1985) Effects of herbicide-fertilizer interactions on
nitrogen and phosphorus transformations and herbicide persistence in
soil. Pestic Sci, 16(1): 93-100.
Marsh JAP, Davies HA, & Grossbard E (1977) The effect of herbicides
on respiration and transformation of nitrogen in two soils I.
Metribuzin and glyphosate. Weed Res, 17: 77-82.
Martinez TT & Brown K (1991) Oral and pulmonary toxicology of the
surfactant used in Roundup herbicide. Proc West Pharmacol Soc, 34:
43-46.
Martinez TT, Long WC, & Hiller R (1990) Comparison of the toxicology
of the herbicide Roundup by oral and pulmonary routes of exposure.
Proc West Pharmacol Soc, 33: 193-197.
Mekwatanakarn P & Sivasithamparam K (1987) Effect of certain
herbicides on saprophytic survival and biological suppression of the
take-all fungus. New Phytol, 106: 153-159.
Miles CJ & Moye HA (1988a) Postcolumn photolysis of pesticides for
fluorometric determination by high-performance liquid
chromatography. Anal Chem, 60(3): 220-226.
Miles CJ & Moye HA (1988b) Extraction of glyphosate herbicide from
soil and clay minerals and determination of residues in soils. J
Agric Food Chem, 36(3): 486-491.
Miles CJ, Wallace LR, & Moye HA (1986) Determination of glyphosate
herbicide and (aminomethyl)phosphonic acid in natural waters by
liquid chromatography using pre-column fluorogenic labeling with
9-fluorenylmethyl chloroformate. J Assoc Off Anal Chem, 69(3):
458-461.
Mitchell DG, Chapman PM, & Long TJ (1987) Acute toxicity of Roundup
and Rodeo herbicides to rainbow trout, chinook, and coho salmon.
Bull Environ Contam Toxicol, 39(6): 1028-1035.
Mitsukaido Laboratories (1986) Roundup herbicide: Acute oral
toxicity study in mice - Final report. Tokyo, Mitsukaido
Laboratories, Institute of Environmental Toxicology (Unpublished
report No. ET-85-377 submitted by Monsanto Ltd).
Monsanto (1972a) The degradation of MON-0573 in river and lake
bottom sediments and surface water. St. Louis, Missouri, Monsanto
Ltd (Unpublished report No. 276).
Monsanto (1972b) The rate of dissipation of MON-0573 in soil. St.
Louis, Missouri, Monsanto Ltd (Unpublished report No. 271).
Monsanto (1972c) The photolysis, run-off, and leaching of MON-0573
on or in soil. St. Louis, Missouri, Monsanto Ltd (Unpublished report
No. 258).
Monsanto (1972d) The degradation and metabolism of MON-0573 in soil.
St. Louis, Missouri, Monsanto Ltd (Unpublished report No. 269).
Monsanto (1973a) CP 67573 Residue and metabolism - The gross
metabolism of N-phos-phonomethylglycine-14C (CP 67573-14C) in
the laboratory rat following a single dose. St. Louis, Missouri,
Monsanto Commercial Products Company, Agricultural Division Research
Department (Unpublished report No. 297 submitted by Monsanto Ltd).
Monsanto (1973b) CP 67573 Residue and metabolism - The isolation and
identification of the metabolites of CP 67573=13C excreted by the
laboratory rat. St. Louis, Missouri, Monsanto Commercial Products
Company, Agricultural Division Research Department (Unpublished
report No. 306 submitted by Monsanto Ltd).
Monsanto (1973c) CP 67573 Residue and metabolism - The dynamics of
accumulation and depletion of orally ingested
N-phosphonomethylglycine-14C. St. Louis, Missouri, Monsanto
Commercial Products Company, Agricultural Division Research
Department (Unpublished report No. 309 submitted by Monsanto Ltd).
Monsanto (1973d) CP 67573 Residue and metabolism - The gross
distribution of N-phosphonomethylglycine-14C (CP 67573-14C) in
the rabbit. St. Louis, Missouri, Monsanto Commercial Products
Company, Agricultural Division Research Department (Unpublished
report No. 298 submitted by Monsanto Ltd).
Monsanto (1978a) Photodegradation and anaerobic aquatic metabolism
of glyphosate, N-phosphonomethylglycine. St. Louis, Missouri,
Monsanto Ltd (Unpublished report No. MSL-0598).
Monsanto (1978b) Solubility, volatility, adsorption, and partition
coefficients, leaching, and aquatic metabolism of MON 0573 and MON
0101. St. Louis, Missouri, Monsanto Ltd (Unpublished report No.
MSL-0207).
Monsanto (1978c) Final report on Salmonella mutagenicity assay of
glyphosate (sample no. 04) (Test No. LF-78-161). St. Louis,
Missouri, Monsanto Environmental Health Laboratory (Unpublished
report submitted by Monsanto Ltd).
Monsanto (1980) Results of a pond test with Roundup for a period of
three months. Burgwedel, Germany, Monsanto Ltd (Unpublished report
submitted by Ökolimna to Monsanto Ltd).
Monsanto (1981a) A reinvestigation of the static exposure of channel
catfish to 14C-labelled glyphosate, N-(phosphonomethyl)glycine.
St. Louis, Missouri, Monsanto Ltd (Unpublished report No. MSL-2056).
Monsanto (1981b) Acute oral toxicity of MON 0139 to rats (Study No.
800257). St. Louis, Missouri, Monsanto Environmental Health
Laboratory (Unpublished report submitted by Monsanto Ltd).
Monsanto (1981c) Acute dermal toxicity of MON 0139 to rabbits (Study
No. 800258). St. Louis, Missouri, Monsanto Environmental Health
Laboratory (Unpublished report submitted by Monsanto Ltd).
Monsanto (1981d) Primary skin irritation of MON 0139 to rabbits
(Study No. 800259). St. Louis, Missouri, Monsanto Environmental
Health Laboratory (Unpublished report submitted by Monsanto Ltd).
Monsanto (1981e) Primary eye irritation of MON 0139 to rabbits
(Study No. 800260). St. Louis, Missouri, Monsanto Environmental
Health Laboratory (Unpublished report submitted by Monsanto Ltd).
Monsanto (1983a) Dissipation of glyphosate in U.S. field soils
following direct application of Roundupr herbicide. St. Louis,
Missouri, Monsanto Ltd (Unpublished report No. MSL-3210).
Monsanto (1983b) CHO/HGPRT Gene mutation assay with glyphosate
(Study No. ML-83-155). St. Louis, Missouri, Monsanto Environmental
Health Laboratory (Unpublished report submitted by Monsanto Ltd).
Monsanto (1983c) The hepatocyte primary culture/DNA repair assay on
compound JJN-1020 (glyphosate) using rat hepatocytes in cultures
(Study No. AH-83-181). St. Louis, Missouri, Monsanto Environmental
Health Laboratory (Unpublished report submitted by Monsanto Ltd).
Monsanto (1983d) Acute inhalation toxicity of Roundup formulation to
male and female Sprague-Dawley rats (Study No. 810093; DMEH project
No. ML-81-2019. St. Louis, Missouri, Monsanto Environmental Health
Laboratory (Unpublished report submitted by Monsanto Ltd).
Monsanto (1983e) Four-week study of 33-1/33% use-dilution of Roundup
in water administered to male and female Sprague/Dawley rats by
inhalation (Project No. ML-83-015/EHL 830025). St. Louis, Missouri,
Monsanto Environmental Health Laboratory (Unpublished report
submitted by Monsanto Ltd).
Monsanto (1983f) in vivo bone marrow cytogenetics study of
glyphosate in Sprague-Dawley rats (Project No. ML-83-236). St.
Louis, Missouri, Monsanto Environmental Health Laboratory
(Unpublished report submitted by Monsanto Ltd).
Monsanto (1983g) Effects of glyphosate on rat bone marrow cells
(Study No. ML-83-160; EHL study No. 830082). St. Louis, Missouri,
Monsanto Environmental Health Laboratory (Unpublished report
submitted by Monsanto Ltd).
Monsanto (1983h) A study of the plasma and bone marrow levels of
glyphosate following intraperitoneal administration in the rat
(Study No. ML-83-218; EHL study No. 830109). St. Louis, Missouri,
Monsanto Environmental Health Laboratory (Unpublished report
submitted by Monsanto Ltd).
Monsanto (1985) Twelve month study of glyphosate administered by
gelatine capsule to Beagle dogs (Project No. ML-83-137). St. Louis,
Missouri, Monsanto Environmental Health Laboratory (Unpublished
report No. MSL 5069 submitted by Monsanto Ltd).
Monsanto (1987a) 90 day study of glyphosate administered in feed to
Sprague/Dawley rats (Project No. ML-86-351/EHL 86128). St. Louis,
Missouri, Monsanto Environmental Health Laboratory (Unpublished
report No. MSL-7575 submitted by Monsanto Ltd).
Monsanto (1987b) Acute inhalation study of Roundup L&G Ready-to-Use
(Study No. 86148; DMEH project No. ML-87-6). St. Louis, Missouri,
Monsanto Environmental Health Laboratory (Unpublished report
submitted by Monsanto Ltd).
Monsanto (1988a) Metabolism of glyphosate in Sprague-Dawley rats.
Part II. Identification, characterization, and quantitation of
glyphosate and its metabolites after intravenous and oral
administration. St. Louis, Missouri, Monsanto Ltd (Unpublished
report No. MSL-7206).
Monsanto (1988b) The metabolism of glyphosate in Sprague Dawley rats
- Part I. Excretion and tissue distribution of glyphosate and its
metabolites following intravenous and oral administration. St.
Louis, Missouri, Monsanto Environmental Health Laboratory/Monsanto
Life Sciences Research Center (Unpublished report No. MSL-7215
submitted by Monsanto Ltd).
Monsanto (1988c) Metabolism of 13C/14C-labeled glyphosate and
aminomethylphosphonic acid in laying hens, Part II. St. Louis,
Missouri, Monsanto Life Sciences Research Center (Unpublished report
No. MSL-7420 submitted by Monsanto Ltd). Monsanto (1988d) Metabolism
study of synthetic 13C/14C-labeled glyphosate and
aminomethylphosphonic acid in lactating goats, Part II. St. Louis,
Missouri, Monsanto Life Sciences Research Center (Unpublished report
No. MSL-7458 submitted by Monsanto Ltd).
Monsanto (1990a) Dissipation of glyphosate and aminomethylphosphonic
acid in forestry sites. St. Louis, Missouri, Monsanto Ltd
(Unpublished report No. MSL-9940).
Monsanto (1990b) Chronic study of glyphosate administered in feed to
albino rats (Project No. MSL-10495). St. Louis, Missouri, Monsanto
Environmental Health Laboratory (Unpublished report submitted by
Monsanto Ltd).
Monsanto (1990c) Two generation reproduction feeding study with
glyphosate in Sprague-Dawley rats (Study No. MSL-10387). St. Louis,
Missouri, Monsanto Environmental Health Laboratory (Unpublished
report submitted by Monsanto Ltd).
Morash R & Freedman B (1989) The effects of several herbicides on
the germination of seeds in the forest floor. Can J For Res, 19(3):
347-350.
Morgan MJ & Kiceniuk JW (1992) Response of rainbow trout to a two
month exposure to Vision, a glyphosate herbicide. Bull Environ
Contam Toxicol, 48: 772-780.
Morrison ML & Meslow EC (1984) Effects of the herbicide glyphosate
on bird community structure, Western Oregon. For Sci, 30(1): 95-106.
Moye HA & Deyrup CL (1984) A simple single-step derivatization
method for the gas chromatographic analysis of the herbicide
glyphosate and its metabolite. J Agric Food Chem, 32(2): 192-195.
Moye HA, Miles CJ, & Scherer SJ (1983) A simplified high-performance
liquid chromatographic residue procedure for the determination of
glyphosate herbicide and (aminomethyl)phosphonic acid in fruits and
vegetables employing postcolumn fluorogenic labeling. J Agric Food
Chem, 31(1): 69-72.
Müller MM, Rosenberg C, Siltanen H, & Wartiovaara T (1981) Fate of
glyphosate and its influence on nitrogen-cycling in two Finnish
agriculture soils. Bull Environ Contam Toxicol, 27(5): 724-730.
Murthy DVS, Irvine RL, & Hallas LE (1989) Principles of organism
selection for the degradation of glyphosate in a sequencing batch
reactor. In: 43rd Purdue Industrial Waste Conference Proceedings.
Chelsea, Lewis Publishers, Inc., pp 267-274.
NATEC (1990) Effect of the herbicide Roundup on the activity of the
microflora of soil (Project No. NA 90 9151). Unpublished report of
Institute for Technical Scientific Services GmbH, Hamburg, supplied
by Monsanto Ltd.
Newton M, Howard KM, Kelpsas BR, Danhaus R, Lottman CM, & Dubelman S
(1984) Fate of glyphosate in an Oregon forest ecosystem. J Agric
Food Chem, 32(5): 1144-1151.
NOTOX (1987a) Evaluation of the acute oral toxicity of MON 14478 in
the rat (Monsanto project No. NO-87-290). 's-Hertogenbosch, The
Netherlands, NOTOX - Toxicological Research & Consultancy
(Unpublished report No. NOTOX 0646/824 submitted by Monsanto Ltd).
NOTOX (1987b) Evaluation of the acute oral toxicity of MON 14478 in
the rat (Monsanto project No. NO-87-347). 's-Hertogenbosch, the
Netherlands, NOTOX - Toxicological Research & Consultancy
(Unpublished report No. NOTOX 0646/901 submitted by Monsanto Ltd).
NOTOX (1987c) Evaluation of the acute dermal toxicity of MON 14478
in the rat (Monsanto project No. NO-87-290). 's-Hertogenbosch, The
Netherlands, NOTOX - Toxicological Research & Consultancy
(Unpublished report No. NOTOX 0646/826 submitted by Monsanto Ltd).
NOTOX (1987d) Assessment of primary skin irritation/corrosion by MON
14478 in the rabbit (Monsanto project No. NO-87-290).
's-Hertogenbosch, The Netherlands, NOTOX - Toxicological Research &
Consultancy (Unpublished report No. NOTOX 0646/820 submitted by
Monsanto Ltd).
NOTOX (1987e) Assessment of acute eye irritation by MON 14478 in the
rabbit (Monsanto project No. NO-87-290). 's-Hertogenbosch, The
Netherlands, NOTOX - Toxicological Research & Consultancy
(Unpublished report No. NOTOX 0646/822 submitted by Monsanto Ltd).
NOTOX (1987f) Evaluation of the acute oral toxicity of MON 14477 in
the rat (Monsanto project No. NO-87-288). 's-Hertogenbosch, The
Netherlands, NOTOX - Toxicological Research & Consultancy
(Unpublished report No. NOTOX 0645/823 submitted by Monsanto Ltd).
NOTOX (1987g) Evaluation of the acute oral toxicity of MON 14477 in
the rat (Monsanto project No. NO-87-346). 's-Hertogenbosch, The
Netherlands, NOTOX - Toxicological Research & Consultancy
(Unpublished report No. NOTOX 0645/900 submitted by Monsanto Ltd).
NOTOX (1987h) Evaluation of the acute dermal toxicity of MON 14477
in the rat (Monsanto project No. NO-87-288). 's-Hertogenbosch, The
Netherlands, NOTOX - Toxicological Research & Consultancy
(Unpublished report No. NOTOX 0645/825 submitted by Monsanto Ltd).
NOTOX (1987i) Assessment of primary skin irritation by MON 14477 in
the rabbit (Monsanto project No. NO-87-288). 's-Hertogenbosch, The
Netherlands, NOTOX - Toxicological Research & Consultancy
(Unpublished report No. NOTOX 0645/819 submitted by Monsanto Ltd).
NOTOX (1987j) Assessment of acute eye irritation by MON 14477 in the
rabbit (Monsanto project No. NO-87-288). 's-Hertogenbosch, The
Netherlands, NOTOX - Toxicological Research & Consultancy
(Unpublished report No. NOTOX 0645/821 submitted by Monsanto Ltd).
NOTOX (1988) Acute oral toxicity of glyphosate in the rat.
's-Hertogenbosch, The Netherlands, NOTOX - Toxicological Research &
Consultancy (Unpublished report No. RCC NOTOX 1111/1428 submitted by
Agrichem B.V., The Netherlands).
NOTOX (1989a) Acute eye irritation/corrosion study with Glyposaat
360 g/l in rabbits. 's-Hertogenbosch, The Netherlands, NOTOX -
Toxicological Research & Consultancy (Unpublished report No. RCC
NOTOX 1268/171 (002082) submitted by Luxan B.V., The Netherlands).
NOTOX (1989b) Assessment of acute dermal toxicity with Glyposaat
360 g/l in the rat. 's-Hertogenbosch, The Netherlands, NOTOX -
Toxicological Research & Consultancy (Unpublished report No. RCC
NOTOX 1268/1718 (002071) submitted by Luxan B.V., The Netherlands).
NOTOX (1989c) Assessment of acute oral toxicity with Glyposaat
360 g/l in the rat. 's-Hertogenbosch, The Netherlands, NOTOX -
Toxicological Research & Consultancy (Unpublished report No. RCC
NOTOX 1268/1715 (002069) submitted by Luxan B.V., The Netherlands).
NOTOX (1989d) Primary skin irritation/corrosion study with Glyposaat
360 g/l in rabbits (4-hour semi-occlusive application).
's-Hertogenbosch, The Netherlands, NOTOX - Toxicological Research &
Consultancy (Unpublished report No. RCC NOTOX 1268/1716 submitted by
Luxan B.V., The Netherlands).
NOTOX (1990a) Acute eye irritation/corrosion study with Agrichem
glyposaat 2 (RCC NOTOX substance 11934) in the rabbit.
's-Hertogenbosch, The Netherlands, NOTOX - Toxicological Research &
Consultancy (Unpublished report No. RCC NOTOX 042492 submitted by
Agrichem B.V., The Netherlands).
NOTOX (1990b) Primary skin irritation/corrosion study with Agrichem
glyposaat 2 (RCC NOTOX substance 11961) in rabbits (4-hour
semi-occlusive application). 's-Hertogenbosch, The Netherlands,
NOTOX - Toxicological Research & Consultancy (Unpublished report No.
RCC NOTOX 042525 submitted by Agrichem B.V., The Netherlands).
NOTOX (1990c) Acute eye irritation/corrosion study with Agrichem
glyposaat B (RCC NOTOX substance 4175) in the rabbit.
's-Hertogenbosch, The Netherlands, NOTOX - Toxicological Research &
Consultancy (Unpublished report No. RCC NOTOX 027934 submitted by
Agrichem B.V., The Netherlands).
NOTOX (1990d) Primary skin irritation/corrosion study with Glyposaat
B (RCC NOTOX substance 4175) in rabbits (4-hour semi-occlusive
application). 's-Hertogenbosch, The Netherlands, NOTOX -
Toxicological Research & Consultancy (Unpublished report No. RCC
NOTOX 027923 submitted by Agrichem B.V., The Netherlands).
NOTOX (1990e) Assessment of acute oral toxicity with Glyposaat B
(RCC NOTOX substance 4175) in the rat. 's-Hertogenbosch, the
Netherlands, NOTOX - Toxicological Research & Consultancy
(Unpublished report No. RCC NOTOX 027901 submitted by Agrichem B.V.,
The Netherlands).
NOTOX (1990f) Assessment of acute dermal toxicity with Glyposaat B
(RCC NOTOX substance 4175) in the rat. 's-Hertogenbosch, The
Netherlands, NOTOX - Toxicological Research & Consultancy
(Unpublished No. RCC NOTOX 027912 submitted by Agrichem B.V., The
Netherlands).
NTP (1992) NTP technical report on toxicity studies of glyphosate
(CAS No. 1071-83-6). Research Triangle Park, North Carolina,
National Toxicology Program (Toxicity Report Series No. 16).
OECD (1991) Report of the OECD Workshop on the Extrapolation of
Laboratory Aquatic Data to the Real Environment. Paris, Organisation
for Economic Co-operation and Development (Environment monograph No.
59).
Olorunsaga OO (1982a) Defective nicotinamide nucleotide
transhydrogenase reaction in hepatic mitochondria of
N-(phosphonomethyl)glycine treated rats. Biochem Pharmacol, 31:
2191-2192.
Olorunsaga OO (1982b) Inhibition of energy-dependent
transhydrogenase reaction by N-(phosphonomethyl)glycine in isolated
rat mitochondria. Toxicol. Lett. 10: 91-95.
Olorunsaga OO, Bababunmi EA, & Bassir O (1979) Effect of glyphosate
on rat liver mitochondria in vivo. Bull Environ Contam Toxicol, 22:
257-364.
Payne N (1992) Off-target glyphosate from aerial silvicultural
applications, and buffer zones required around sensitive areas.
Pestic Sci, 34: 1-8.
Payne N & Thompson DG (1992) Off-target glyphosate deposits from
aerial silvicultural applications under various meteorological
conditions. Pestic Sci, 34: 53-59.
Payne N, Feng J, & Reynolds P (1989) Off-target deposit measurements
and buffer zones required around water for various aerial
applications of glyphosate. In: Reynolds P ed. Proceedings of the
Carnation Creek Workshop, Nanaimo, 7-10 December 1987. Victoria,
British Columbia, Forestry Canada/British Columbia Ministry of
Forests, pp 88-109.
Piccolo A, Celano G, & Pietramellara G (1992) Adsorption of the
herbicide glyphosate on a metal-humic acid complex. Sci Total
Environ, 123/124: 77-82.
Pipke R & Amrhein N (1988) Isolation and characterization of a
mutant of Arthrobacter sp. strain GLP-1 which utilizes the herbicide
glyphosate as its sole source of phosphorus and nitrogen. Appl
Environ Microbiol, 54(11): 2868-2870.
Powell HA, Kerby NW, & Rowell P (1990) High-performance liquid
chromatographic determination of the herbicide glyphosate and its
metabolite (aminomethyl)phosphonic acid and their extraction from
cyanobacteria. J Chromatogr, 502(1): 201-207.
Preston CM & Trofymow JA (1989) Effects of glyphosate (Roundup) on
biological activity of forest soils. In: Reynolds P ed. Proceedings
of the Carnation Creek Workshop, Nanaimo, 7-10 December 1987.
Victoria, British Columbia, Forestry Canada/British Columbia
Ministry of Forests, pp 122-140.
PTRL East Inc. (1990a) Aerobic aquatic metabolism of [14C]
glyphosate. Richmond, Kentucky, Pharmacology and Toxicology Research
Laboratory East, Inc. (Unpublished Report No. 1300 submitted by
Monsanto Ltd).
PTRL East Inc. (1990b) Anaerobic aquatic metabolism of [14C]
glyphosate. Richmond, Kentucky, Pharmacology and Toxicology Research
Laboratory East, Inc. (Unpublished report No. 1304 submitted by
Monsanto Ltd.
PTRL East Inc. (1991) Aerobic metabolism of [14C] glyphosate in
sandy loam and silt loam soils with biometer flask. Richmond,
Kentucky, Pharmacology and Toxicology Research Laboratory East, Inc.
(Unpublished report No. 1301 submitted by Monsanto Ltd).
PTRL Inc. (1989) Photodegradation of [14C] glyphosate in/on soil
by natural sunlight (Project No. 153W). Richmond, Kentucky,
Pharmacology and Toxicology Research Laboratory, Inc (Unpublished
report submitted by Monsanto Ltd).
PTRL Inc. (1990) Degradation study: photodegradation of [14C]
glyphosate in a buffered aqueous solution at pH 5, 7, and 9 by
natural sunlight. Richmond, Kentucky, Pharmacology and Toxicology
Research Laboratory, Inc. (Unpublished report No. 233W-1 submitted
by Monsanto Ltd).
Quinn JP, Pedden JMM, & Dick RE (1988) Glyphosate tolerance and
utilization by the microflora of soils treated with the herbicide.
Appl Microbiol Biotechnol, 29(5): 511-516.
Ragab MTH (1978) Thin-layer chromatographic detection of glyphosate
herbicide (N-phosphonomethyl glycine) and its aminomethyl phosphonic
acid metabolite. Chemosphere, 2: 143-153.
Riley CM, Wiesner CJ, & Sexsmith WA (1991) Estimating off-target
spray deposition on the ground following the aerial application of
glyphosate for conifer release in New Brunswick. J Environ Sci
Health, B26: 185-208.
Ritchie DC, Harestad AS, & Archibald R (1987) Glyphosate treatment
and deer mice in clearcut and forest. Northwest Sci, 61(3): 199-202.
RIVM (1991) Catch-up operation old pesticides: an integration.
Bilthoven, The Netherlands, National Institute of Public Health and
Environmental Protection (Report no. 678801002).
Roseboom H & Berkhoff CJ (1982) Determination of the herbicide
glyphosate and its major metabolite aminomethylphosphonic acid by
high-performance liquid chromatography after fluorescence labelling.
Anal Chim Acta, 135(2): 373-377.
Roy DN & Konar SK (1989) Development of an analytical method for the
determination of glyphosate and (aminomethyl)phosphonic acid
residues in soils by nitrogen-selective gas chromatography. J Agric
Food Chem, 37(2): 441-443.
Roy DN, Konar SK, Banerjee S, Charles DA, Thompson DG, & Prasad R
(1989a) Uptake and persistence of the herbicide glyphosate (Vision)
in fruit of wild blueberry and red raspberry. Can J For Res, 19(7):
842-847.
Roy DN, Konar SK, Banerjee S, Charles DA, Thompson DG, & Prasad R
(1989b) Persistence, movement, and degradation of glyphosate in
selected Canadian boreal forest soils. J Agric Food Chem, 37(2):
437-440.
Rueppel ML, Brightwell BB, Schaefer J, & Marvel JT (1977) Metabolism
and degradation of glyphosate in soil and water. J Agric Food Chem,
25(3): 517-528.
Safefarm Labs Inc. (1991a) EXP 30578 - Modified nine-induction
Buehler delayed contact hypersensitivity study in the guinea pig
(Project No. 282/1219. Derby, UK, Safefarm Laboratories Inc.
(Unpublished report submitted by Rhône Poulenc, Lyon, France).
Safefarm Labs Inc. (1991b) Luxan glyphosate techn. - Magnusson &
Kligman maximisation study in the guinea pig (Project No. 349/11).
Derby, UK, Safefarm Laboratories Inc. (Unpublished report submitted
by Luxan B.V., The Netherlands).
Santillo DJ, Brown PW, & Leslie DM (1989a) Response of songbirds to
glyphosate-induced habitat changes on clearcuts. J Wildl Manage,
53(1): 64-71.
Santillo DJ, Leslie DM, & Brown PW (1989b) Response of small mammals
to glyphosate application on clearcuts. J Wildl Manage, 53(1):
164-172.
Sawada Y & Nadai Y (1987) [Roundup poisoning - its clinical
observation - possible involvement of surfactant.] J Clin Exp Med,
143(1): 25-27 (in Japanese).
Scrivener JC (1989) Comparative changes in concentration of
dissolved ions in the stream following logging, slash burning, and
herbicide application (glyphosate) at Carnation Creek, British
Columbia. In: Reynolds P ed. Proceedings of the Carnation Creek
Workshop, Nanaimo, 7-10 December 1987. Victoria, British Columbia,
Forestry Canada/British Columbia Ministry of Forests, pp 197-211.
Scrivener JC & Carruthers S (1989) Changes in the invertebrate
populations of the main stream and back channels of Carnation Creek,
British Columbia, following spraying with the herbicide Roundup
(glyphosate). In: Reynolds P ed. Proceedings of the Carnation Creek
Workshop, Nanaimo, 7-10 December 1987. Victoria, British Columbia,
Forestry Canada/British Columbia Ministry of Forests, pp 263-272.
Servizi JA, Gordon RW, & Martens DW (1987) Acute toxicity of Garlon
4 and Roundup herbicides to salmon, Daphnia, and trout. Bull Environ
Contam Toxicol, 39(1): 15-22.
SFRSA (1990) Effect of Roundup (glyphosate) on Chrysoperla carnea
(Test No. 121). Zurich, Swiss Federal Research Station for Agronomy
(Unpublished report submitted by Monsanto Ltd).
Shelanski MV (1973) Evaluation of potential hazards by dermal
contact. - Test material: MON 2139. Shelanski Holding Company, PA,
USA (Client: Monsanto Company) (Project No. Sh-72-19). Conshohocken,
Pennsylvania, Shelanski Holding Company (Unpublished report
submitted by Monsanto Ltd).
Siltanen H, Rosenberg C, Raatikainen M, & Raatikainen T (1981)
Triclopyr, glyphosate and phenoxyherbicide residues in cowberries,
bilberries and lichen. Bull Environ Contam Toxicol, 27(5): 731-737.
Smid D & Hiller LK (1981) Phytotoxicity and translocation of
glyphosate in the potato (Solanum tuberosum) prior to tuber
initiation. Weed Sci, 29(2): 218-223.
Sprankle P, Meggitt WF, & Penner D (1975) Adsorption, mobility, and
microbial degradation of glyphosate in the soil. Weed Sci, 23(3):
229-234.
Stark J (1983) Persistence of herbicides in forest soils. Weeds Weed
Control, 24(1): 275-286.
Stratton GW (1990) Effects of the herbicide glyphosate on
nitrification in four soils from Atlantic Canada. Water Air Soil
Pollut, 51(3/4): 373-383.
Stratton GW & Stewart KE (1992) Glyphosate effects on microbial
biomass in a coniferous forest soil. Environ Toxicol Water Qual, 7:
223-236.
Subramaniam V & Hoggard PE (1988) Metal complexes of glyphosate. J
Agric Food Chem, 36(6): 1326-1329.
Sullivan TP (1990) Influence of forest herbicide on deer mouse and
oregon vole population dynamics. J Wildl Manage, 54(4): 566-576.
Sullivan TP & Sullivan DS (1979) The effects of glyphosate herbicide
on food preference and consumption in black-tailed deer. Can J Zool,
57: 1406-1412.
Sullivan TP & Sullivan DS (1981) Responses of a deer mouse
population to a forest herbicide application: reproduction, growth,
and survival. Can J Zool, 59: 1148-1154.
Sullivan DS, Sullivan TP & Bisalputra T (1981) Effects of Roundup
herbicide on diatom populations in the aquatic environment of a
coastal forest. Bull Environ Contam Toxicol, 26(1): 91-96.
Sundaram A (1990) Effect of a Nalco-Trol II on bioavailability of
glyphosate in laboratory trials. J Environ Sci Health, B25(3):
309-332.
Swinehart JH & Cheney MA (1987) Interactions of organic pollutants
with gills of the bivalve molluscs Anodonta californiensis and
Mytilus californianus: uptake and effect on membrane fluxes II.
Comp Biochem, 88C(2): 293-299.
Swisher RD (1987) Surfactant biodegradation, New York, Basel, Marcel
Dekker, Inc.
Talbot AR, Shiaw M-H, Huang J-S, Yang S-F, Goo T-S, Wang S-H, Chen
C-L, & Sanford TR (1991) Acute poisoning with a
glyphosate-surfactant herbicide (Roundup): A review of 93 cases. Hum
Exp Toxicol, 10: 1-8.
Task Force on Water Quality Guidelines (1991) V.3 Glyphosate. In:
Canadian Water Quality Guidelines updates. Canadian Council of
Resource and Environment Ministers, Ottawa, V.8-V.16.
Tauchi K (1979) Sub-acute toxicity study of CP67573
(N-phosphonomethylglycine) to rats in dietary administration for 90
days. Institute for Animal Reproduction (Unpublished report No.
79/112 submitted to FAO/WHO-JMPR by Monsanto Europe S.A.).
Temple WA & Smith NA (1992) Glyphosate herbicide poisoning
experience in New-Zealand. N Z Med J, 105: 173-174.
Thompson DG, Cowell JE, Daniels RJ, Staznik B, & Macdonald LM (1989)
Liquid chromatographic method for quantitation of glyphosate and
metabolite residues in organic and mineral soils, stream sediments,
and hardwood foliage. J Assoc Off Anal Chem, 72(2): 355-360.
Tominack R L, Yang G-Y, Tsai W-J, Chung H-M, & Deng J-F (1991)
Taiwan national poison center survey of glyphosate-surfactant
herbicide ingestions. Clin Toxicol, 29(1): 91-109.
Tomita M, Okuyama T, Watanabe S, Uno B, & Kawai S (1991) High
performance liquid chromatographic determination of glyphosate and
(aminomethyl)phosphonic acid in human serum after conversion into
P-toluenesulphonyl derivatives. J Chromatogr, 566: 239-243.
Torstensson L & Stark J (1981) Decomposition of 14C-labelled
glyphosate in Swedish forest soils. In: Proceedings of the EWRS
Symposium on Theory and Practice of the Use of soil Applied
Herbicides. Uppsala, Swedish University of Agricultural Science,
Department of Microbiology, pp 72-79.
Torstensson L & Stenström J (1986) "Basic" respiration rate as a
tool for prediction of pesticide persistence in soil. Toxic Assess,
1(1): 57-72. Torstensson NT, Lundgren LN, & Stenström J (1989)
Influence of climatic and edaphic factors on persistence of
glyphosate and 2,4-D in forest soils. Ecotoxicol Environ Saf, 18(2):
230-239.
Tuinstra LGMT & Kienhuis PGM (1987) Automated two-dimensional HPLC
residue procedure for glyphosate on cereals and vegetables with
postcolumn fluoregenic labelling. Chromatographia, 24: 696-700.
USDA (1983) The acute oral toxicity of Roundup formulation in female
goats (Study No. 80004). US Department of Agriculture, College
Station, Texas, Veterinary Toxicology and Entomology Research
Laboratory, Veterinary Research Unit (Unpublished report supplied by
Monsanto Ltd - Monsanto study No. VT-80-452).
USDA (1987a) The subacute toxicity of Roundup herbicide (MON-2139)
in female cattle (Study no. 82001). US Department of Agriculture,
College Station, Texas, Veterinary Toxicology and Entomology
Research Laboratory, Veterinary Research Unit (Unpublished report
submitted by Monsanto Ltd - Monsanto study No. VT-82-001).
USDA (1987b) The acute oral toxicity of the isopropylamine salt of
glyphosate (MON 0139) in female goats (Study No. 80007). US
Department of Agriculture, College Station, Texas, Veterinary
Toxicology and Entomology Research Laboratory, Veterinary Research
Unit (Unpublished Report submitted by Monsanto Ltd - Monsanto study
No. VT-80-451).
USDA (1987c) The acute toxicity of glyphosate in female goats (Study
No. 80006). US Department of Agriculture, College Station, Texas,
Veterinary Toxicology and Entomology Research Laboratory, Veterinary
Research Unit (Unpublished report submitted by Monsanto Ltd -
Monsanto study No. VT-80-450).
Wan MT, Watts RG, & Moul DJ (1989) Effects of different dilution
water types on the acute toxicity to juvenile pacific salmonids and
rainbow trout of glyphosate and its formulated products. Bull
Environ Contam Toxicol, 43(3): 378-385.
Wängberg SA & Blanck H (1988) Multivariate patterns of algal
sensitivity to chemicals in relation to phylogeny. Ecotoxicol
Environ Saf, 16(1): 72-82.
Weidhase R, Albrecht B, Stock M, & Weidhase RA (1990) [Utilization
of glyphosate by Pseudomonas sp. GS.] Zent.bl Mikrobiol, 145(6):
433-438 (in German).
Wester RC, Melendres JC, Sarason R, McMaster J, & Maibach H I (1991)
Glyphosate skin binding, absorption, residual tissue distribution,
and skin decontamination. Fundam Appl Toxicol, 16: 725-732.
Wigfield YY & Lanouette M (1990) Simplified liquid chromatographic
determination of glyphosate and metabolite residues in environmental
water using post-column fluorogenic labelling. Anal Chim Acta,
233(2): 311-314.
Wildlife Int. Ltd (1978a) One-generation reproduction study-mallard
duck-glyphosate technical. Final report. Easton, Maryland, Wildlife
International Ltd (Unpublished report submitted by Monsanto Ltd).
Wildlife Int. Ltd (1978b) One-generation reproduction study-bobwhite
quail-glyphosate technical. Final report. Easton, Maryland, Wildlife
International Ltd (Unpublished report submitted by Monsanto Ltd).
Wildlife Int. Ltd (1978c) Acute oral LD50 of technical glyphosate
in the bobwhite (Project No. 139-140). Easton, Maryland, Wildlife
International Ltd (Unpublished report submitted by Monsanto Ltd).
Wildlife Int. Ltd (1990a) Roundup herbicide: a dietary LC50 study
with the mallard (Project No. 139-260). Easton, Maryland, Wildlife
International Ltd (Unpublished report submitted by Monsanto Ltd).
Wildlife Int. Ltd (1990b) Roundup herbicide: a dietary LC50 study
with the bobwhite (Project No. 139-259). Easton, Maryland, Wildlife
International Ltd (Unpublished report submitted by Monsanto Ltd).
WSSA (1983) Herbicide handbook of the Weed Science Society of
America, 5th ed. Champaign-Urbana, Illinois, Weed Science Society of
America.
Yao CCD & Haag WR (1991) Rate constants for direct reactions of
ozone with several drinking water contaminants. Water Res, 25:
761-773.
Young JC, Khan SU & Marriage PB (1977) Fluorescence detection and
determination of glyphosate via its N-nitroso derivative by
thin-layer chromatography. J Agric Food Chem, 25: 918-922.
Younger Labs Inc. (1984) Acute oral toxicity in albino rats - Test
material: LLN-83-06 (Project No. YO-84-024). St. Louis, Missouri,
Younger Laboratories, Inc. (Unpublished report submitted by Monsanto
Ltd).
Zboinska E, Lejczak B, & Lafarski P (1992) Organophosphonate
utilization by the wild-type strain of Pseudomonas fluorescens.
Appl Environ Microbiol, 58: 2993-2999.
RESUME
1. Identité, propriétés physiques et chimiques et méthodes d'analyse
Le glyphosate, ou N-(phosphonométhyl)glycine, est un acide
organique faible. Sa formule brute est C3H8NO5P. Il est
généralement présenté sous la forme du sel de l'acide correspondant
déprotoné et d'un cation comme l'isopropyl-ammonium ou le
triméthylsulfonium. La pureté du glyphosate de qualité technique est
généralement supérieure à 90%. Le glyphosate de qualité technique se
présente sous la forme d'une poudre cristalline blanche inodore dont
la densité est de 1,704. Sa tension de vapeur est très faible et il
est très soluble dans l'eau. Le coefficient de partage octanol-eau
(log Kow) est égal à -2,8. Le glyphosate est amphotère et il peut
exister sous différentes formes ioniques, selon le pH du milieu.
Le dosage du glyphosate est généralement une opération
laborieuse, complexe et coûteuse. La méthode la plus courante
consiste à en préparer un dérivé avec une substance fluorigène,
avant ou après passage sur colonne. Le dosage s'effectue en général
par chromatographie liquide à haute performance ou chromatographie
gaz-liquide. Les limites de détection dans l'eau, les plantes, le
sol et l'urine humaine sont respectivement de 0,02-3,2 µg/litre,
0,01-0,3 mg/kg, 0,05-1 mg/kg et 0,1 mg/litre.
2. Sources d'exposition humaine et environnementale
Le glyphosate est un herbicide non sélectif utilisé en
traitement endothérapique après l'émergence; il est utilisé partout
dans le monde sur des terrains agricoles ou non. On l'épand en
plusieurs formulations commerciales sur de nombreuses récoltes. La
plus courante est le Roundup qui consiste en un sel
d'isopropylammonium. La dose d'emploi recommandée ne dépasse 5,8 kg
de matière active par hectare et dépend de l'usage auquel on le
destine. L'environnement peut être contaminé par suite du dépôt
d'embruns ou de la libération accidentelle du produit.
3. Transport, distribution et transformation dans l'environnement
Les principaux processus de dissipation qui interviennent après
l'épandage de cet herbicide sont les suivants: formation de
complexes avec certains ions présents dans l'eau comme Ca2+ et
Mg2+, sorption aux sédiments ainsi qu'aux particules en suspension
dans l'eau et le sol, photodécomposition dans l'eau, fixation par
les plantes et biodégradation.
Le glyphosate disparaît de l'eau avec des valeurs du TD50
(temps de dissipation) qui vont de quelques jours à plus de 91
jours. Le principal milieu récepteur est constitué par les sédiments
ou les particules en suspension.
En laboratoire, les coefficients d'adsorption (Ks/l) du
glyphosate varient de 8 à 377 dm3/kg pour différents sols et
substances argileuses. On ne dispose d'aucune donnée sur la sorption
de l'acide aminométhylphosphonique (AMPA) qui en est le principal
métabolite, dans les conditions du laboratoire.
La valeur du Rf ne dépasse pas 0,2, selon certaines mesures par
chromatographie sur couche mince de terre. Dans des conditions de
lessivage reproduisant des précipitations extrêmement fortes, on
récupère dans l'éluat d'une colonne de terre, entre 0,1 et 11% de
l'activité appliquée initialement. L'expérimentation sur le terrain
montre qu'il n'y a probablement pas lessivage de l'AMPA.
L'expérimentation sur le terrain montre qu'en ce qui concerne
la dissipation du glyphosate dans le sol, les valeurs du TD50
varient de 3 à 174 jours, principalement en fonction des conditions
édaphiques et climatiques. Certaines expériences sur le terrain ont
montré que le ruissellement pouvait entraîner jusqu'à 1,8% de la
dose appliquée sur la sol.
Au laboratoire jusqu'à 45% de l'activité appliquée peut être
absorbée par le feuillage après traitement, après quoi il y a une
migration importante dans la plante.
L'hydrolyse du glyphosate en tampon stérile est très lente, les
valeurs du TD50 étant >> à 35 jours. En ce qui concerne la
photodécomposition dans l'eau dans les conditions naturelles, les
valeurs du TD50 sont < à 28 jours. Lors d'une étude qui s'est
prolongée pendant 31 jours, on n'a pas enregistré de
photodécomposition notable dans le sol.
Le temps nécessaire à la biodégradation de 50% d'une quantité
donnée de glyphosate dans l'ensemble d'un système d'épreuve en
présence d'eau et de sédiments était < à 14 jours en aérobiose et
compris entre 14 et 22 jours en anaérobiose. Dans le sol, le temps
de demi-biodégradation du glyphosate est de 2 à 3 jours en
aérobiose.
Le principal métabolite qui se forme dans le sol et dans l'eau
est l'AMPA. La quantité maximale d'AMPA présente dans le sol est
d'environ 20% de l'activité appliquée en aérobiose et de 0,5% de
cette activité en anaérobiose. Ce chiffre atteint 25% dans les
sédiments dans les deux types de conditions.
Les épreuves de laboratoire montrent que chez les invertébrés
et les poissons, le facteur de bioconcentration est faible. Lors
d'une épreuve en aquarium à écoulement continu, on a constaté que
chez Lépomis macrochirus le temps de demi-épuration était de 35
jours après une exposition de même durée. Après exposition continue
à du glyphosate, on retrouve de l'AMPA chez ce même poisson pendant
des périodes allant jusqu'à 21 jours. Des mesures sur le terrain
n'ont pas permis de déceler la présence de glyphosate chez les
poissons vivant dans des eaux sur lesquelles cet herbicide avait été
directement pulvérisé. Lors d'une expérience, on a décelé de l'AMPA
chez les carpes jusqu'à 90 jours après l'épandage. Une autre
expérience menée sur le terrain a montré qu'il n'y avait pas de
bioamplification du glyphosate dans les portées de petits mammifères
herbivores ou omnivores vivant en brousse. On a notamment mesuré des
concentrations allant jusqu'à 5 mg de matière active par kg chez des
souris du genre Peromyscus, immédiatement après l'épandage.
Plusieurs souches de bactéries peuvent décomposer le
glyphosate. On a identifié des bactéries qui sont capables
d'utiliser de composé comme seule source de phosphore, de carbone ou
d'azote. La croissance est alors plus lente que lorsque elles
utilisent des sources inorganiques de P, de C et de N. On est fondé
à penser, d'après les observations effectuées sur le terrain, que
certaines populations bactériennes se sont adaptées à la
métabolisation du glyphosate. La présence de phosphate inorganique
inhibe la décomposition du glyphosate par certaines bactéries mais
pas toutes. La biodécomposition du glyphosate peut comporter un
co-métabolisme avec d'autres sources d'énergie.
4. Concentrations dans l'environnement et exposition humaine
Il n'existe que de très rares données provenant de programmes
de surveillance systématique et concernant la présence de glyphosate
dans la faune et la flore ainsi que dans le milieu abiotique. Pour
avoir une idée des concentrations maximales dans l'environnement, on
fait appel aux données fournies par des essais sur le terrain au
cours desquels on simule des épandages à usage agricole; ces
concentrations sont les suivantes: < 1-1700 µg/litre dans les eaux
de surface, 0,07-40 mg/kg de poids sec dans le sol, < 0,05-19 mg/kg
de poids sec dans les sédiments, 261-1300 mg/kg dans les feuilles,
5 mg/kg dans les viscères des souris du genre Peromyscus,
1,6-19 mg/kg dans les baies sauvages et 45 mg/kg dans les lichens.
Les concentrations maximales correspondantes d'AMPA sont les
suivantes: < 1-35 µg/litre (eaux de surface), 0,1-9 mg/kg de poids
sec (sol), < 0,05-1,8 mg/kg de poids sec (sédiments), 1,7-<
9 mg/kg (feuilles), 0,02-0,1 mg/kg (baies sauvages) et 2,1 mg/kg
(lichens). Les concentrations ci-dessus de glyphosate sont celles
que l'on observe en général immédiatement après l'épandage. En ce
qui concerne les lichens, la concentration mentionnée a été observée
270 jours après l'épandage.
On ne dispose pas de mesures de la dose journalière ingérée par
l'homme avec les aliments et l'eau de boisson (études de rations
totales). Les quelques données disponibles au sujet de l'exposition
professionnelle indiquent que celle-ci est faible pour les ouvriers
qui épandent du glyphosate comme désherbant sous forme de Roundup.
5. Cinétique et métabolisme chez les animaux de laboratoire et
l'homme
Le glyphosate technique n'est que partiellement résorbé au
niveau des voies digestives. Lors d'études effectuées sur du
glyphosate marqué au carbone-14, on a observé des pourcentages
d'absorption de 30 à 36% chez plusieurs espèces. L'absorption par
voie percutanée est faible. Dans le cas de l'herbicide Roundup, le
glyphosate qu'il contient est absorbé dans une proportion < à
5,5% à travers la peau (durée de contact environ 24 heures). En ce
qui concerne les tissus de l'organisme, la concentration maximale,
correspondant à environ 1% de la dose ingérée, se retrouve dans les
eaux. Après administration d'une seule dose par voie orale, le
produit est éliminé à hauteur de 62 à 69% dans les matières fécales
sans absorption. Après absorption, 14 à 29% de la dose passe dans
l'urine et 0,2% au maximum dans l'air expiré. Après administration
par voie intraveineuse, le taux d'excrétion dans les voies biliaires
n'a été que de 5 à 8%. Chez des chèvres en lactation, on a montré
que le glyphosate n'était excrété dans le lait qu'en faible
proportion (concentration < à 0,1 mg/kg de lait entier pour une
dose ingérée de 120 mg/kg de nourriture). Le glyphosate n'est
métabolisé que dans une très faible proportion. Son seul métabolite,
l'AMPA, correspond à 0,3% de la dose ou même moins; le reste
correspond au produit initial. Il faut environ 168 heures pour que
le glyphosate soit éliminé en totalité de l'organisme (99% d'une
dose orale).
6. Effets sur les mammifères de laboratoire et les systèmes
d'épreuve in vitro
Chez l'animal de laboratoire, le glyphosate technique ne
présente qu'une très faible toxicité aiguë lorsqu'il est administré
par la voie orale ou percutanée; il est nettement plus toxique par
la voie intrapéritonéale que par les autres voies d'administration.
Des études d'alimentation de brève durée ont été effectuées sur
plusieurs espèces, mais la plupart de ces épreuves n'ont guère
révélé d'effets. Lors d'une épreuve de 13 semaines sur des souris au
cours de laquelle on a utilisé du glyphosate technique, on a
constaté une augmentation du poids de plusieurs organes ainsi qu'un
retard de croissance à la dose de 50 000 mg/kg de nourriture. Lors
d'une étude de même durée sur le rat, on n'a pas observé d'effet
(les doses de glyphosate technique utilisées allaient jusqu'à
20 000 mg/kg de nourriture). Lors d'une autre étude de 13 semaines,
on a observé des lésions au niveau des glandes salivaires chez des
rats et des souris. Chez les souris, la dose sans effet létal
observable était de 3125 mg/kg de nourriture; chez le rat, elle
était < à 3125 mg/kg de nourriture. Aucun de ces effets n'a été
observé lors d'études à court ou à long terme effectuées sur
diverses souches et espèces. Les lésions au niveau des glandes
salivaires incitent à penser que le glyphosate pourrait se comporter
comme un agoniste adrénérgique de faible activité.
La toxicité à long terme a été étudiée sur des souris et des
rats. Peu d'effets ont été observés et dans presque tous les cas,
uniquement à des doses relativement élevées. Chez les souris, le
glyphosate technique a produit un retard de croissance, une
hypertrophie ou une nécrose des hépatocytes ainsi qu'une hyperplasie
de l'épithélium vésical à la dose de 30 000 mg/kg. Chez les rats, le
même composé a entraîné une réduction de la croissance, une
augmentation du poids du foie, une dégénérescence du cristallin et
une inflammation de la muqueuse gastrique à la dose de 20 000 mg/kg
de nourriture.
Les études dont on connaît les résultats ne concluent pas à
l'existence d'un pouvoir mutagène, cancérogène ou tératogène du
glyphosate technique. Deux études ont été effectuées sur plusieurs
générations de rats. Les principaux effets du glyphosate technique
consistaient en une réduction du poids corporel des géniteurs et des
ratons ainsi qu'une diminution de la taille des portées, à la dose
de 30 000 mg/kg de nourriture. Dans une étude portant sur la
reproduction, on a constaté une augmentation dans l'incidence de la
dilatation unilatérale des tubules rénaux chez les ratons mâles de
la génération F3b, à la dose de 30 mg/kg de poids corporel.
Toutefois la reproductibilité de cette lésion reste incertaine du
fait qu'elle n'a pas été observée chez les ratons soumis à une dose
plus élevée, dans la deuxième de ces études.
7. Effets sur l'homme
Les études contrôlées dont on dispose se limitent à trois
études sur l'irritation et la sensibilisation provoquées par le
glyphosate chez des volontaires humains, et qui ont toutes donné des
résultats négatifs. Plusieurs cas d'intoxication (la plupart du
temps volontaires) avec un herbicide composé de glyphosate
technique, le Roundup, ont été signalés. Une étude, consacrée à des
travailleurs qui épandaient du Roundup, n'a pas révélé d'effets
indésirables. Les données disponibles sur l'exposition
professionnelle d'ouvriers appliquant du Roundup montrent que le
niveau d'exposition est très inférieur à la dose sans effet létal
observable qui ressort de l'expérimentation animale.
8. Effets sur les êtres vivants dans leur milieu naturel
Le glyphosate de qualité technique est légèrement à modérément
toxique pour les microorganismes aquatiques avec une CE50 (3 à 4
jours) allant de 1,2 à 7,8 mg/litre et une concentration sans effets
observables à 7 jours allant de 0,3 à 34 mg/litre. Sous ses
différentes formulations, le glyphosate est légèrement à fortement
toxique pour les microorganismes aquatiques avec des valeurs de la
CE50 à 3 jours allant de 1,0 à plus de 55 mg de produit par litre.
Les cyanophycées (algues bleues) sont plus sensibles au Roundup que
les algues proprement dites. Les processus physiologiques affectés
sont notamment le verdissement, la respiration, la photosynthèse et
la synthèse des acides aminés aromatiques.
Sur les bactéries terricoles en culture, le glyphosate agit au
niveau de la fixation de l'azote, de la dénitrification et de la
nitrification. Cependant des observations effectuées sur le terrain
après épandage de diverses formulations de glyphosate n'ont pas
révélé la présence d'effets sensibles. Des bactéries appartenant à
des espèces étroitement apparentées aux bactéries précitées se sont
révélées capables de dégrader le glyphosate.
Chez les champignons ectomycorhiziens, la croissance du
mycélium en culture pure est inhibée par des concentrations > à
29 mg de Roundup par litre. Les genres sensibles à cette inhibition
sont Cenococcum, Hebeloma et Laccaria.
Le glyphosate est légèrement toxique pour les macrophytes
aquatiques avec une valeur de la concentration sans effets
observables à 14 jours de 9 mg/litre, en solution dans l'eau. le
Roundup est également légèrement toxique avec, pour cette
concentration, des valeurs allant de 2,4 à 56 mg/litre, également en
solution dans l'eau. On ne dispose d'aucune donnée sur la toxicité
aiguë. La phytotoxicité est beaucoup plus importante en l'absence de
lessivage des dépôts d'herbicide.
Le glyphosate de qualité technique est très légèrement à
légèrement toxique pour les invertébrés aquatiques avec des valeurs
de la CL50 ou de la CE50 à 2-4 jours > 55 mg/litre et une
valeur de la concentration sans effets observables à 21 jours de
100 mg/litre. Les diverses formulations de glyphosate sont très
légèrement à modérément toxiques pour les invertébrés aquatiques
pour des valeurs de la CE50 à 2 jours s'étageant entre 5,3 et
5600 mg de produit par litre et des valeurs de la MATC à 21 jours
allant de 1,4 à 4,9 mg de produit par litre. La toxicité plus forte
du Roundup est essentiellement due à la présence d'agents
tensioactifs.
Le glyphosate de qualité technique est très légèrement à
modérément toxique pour les poissons avec des valeurs de la CL50 à
quatre jours allant de 10 à > 1000 mg/litre, une valeur de la
concentration sans effets observables à 21 jours de 52 mg/litre et
une valeur de la MATC de > 26 mg/litre. Les diverses formulations
du glyphosate sont également très légèrement à modérément toxiques
pour les poissons avec des valeurs de la CL50 à quatre jours de
2,4 à > 1000 mg de produit par litre et des valeurs de la
concentration sans effets observables à 21 jours allant de 0,8 à
2,4 mg de produit par litre. C'est la carpe qui s'est révélée être
l'espèce la plus sensible, après exposition à une formulation de
glyphosate appelée Sting. Sur le terrain, on n'a pas constaté
d'effets sur les poissons qui soient attribuables au traitement par
le Roundup, à l'exception d'un stress constaté immédiatement après
l'épandage du produit à la dose recommandée en évitant que celle-ci
ne dépasse 40 mg de Roundup par litre.
On constate que le glyphosate inhibe, dans une proportion qui
dépend de la dose, la formation de nodosités par le trèfle
souterrain inoculé par du Rhizobium, en culture hors-sol et en
présence de solutions nutritives contenant une concentration de
matière active > 2 mg/litre. La germination de diverses espèces
forestières n'est pas affectée par la présence de glyphosate aux
doses d'emploi recommandées. Avec des doses d'emploi > 0,54 kg de
matière active par hectare, on constate au laboratoire qu'il y a
réduction, proportionnée à la dose, de la longueur des racines des
jeunes pousses de pins sylvestres. Cette diminution n'a pas été
confirmée lors d'une étude du même genre sur le terrain.
Le glyphosate de qualité technique et le Roundup sont
légèrement toxiques pour les abeilles en application orale ou
topique. Les valeurs de la DL50 à deux jours sont > 100 µg (de
matière active ou de produit) par abeille. La DL50 par voie orale
à deux jours du Sting pour les abeilles est > 100 µg/abeille. Le
Roundup et le Roundup D-pack sont légèrement toxiques pour les
lombrics avec des valeurs de la concentration sans effets
observables à 14 jours respectivement égales à 500 et 158 mg de
produit par kg de poids sec. Aucun effet nocif, attribuable au
Roundup, n'a été observé sur la fécondité et la fertilité d'un
certain nombre d'insectes appartenant au groupe des névroptères et
le Sting n'a pas non plus produit d'effets sur la consommation de
nourriture ou la mortalité des insectes du genre Poecilus.
Le glyphosate de qualité technique est légèrement toxique pour
les oiseaux avec une DL50 > 3851 mg/kg de poids corporel, une
CL50 à huit jours > 4640 mg/kg et des valeurs de la concentration
sans effets observables à 112-119 jours, > 1000 mg/kg de
nourriture. On a constaté que le Roundup et une autre formulation
dont le nom n'est pas connu était également toxique pour les
oiseaux, avec un DL50 > 2686 mg de produit par kg de poids
corporel et une CL50 à huit jours > à 5620 mg de produit par kg
de nourriture. Généralement, on ne constate, sur les mammifères de
laboratoire, aucun effet qui soit attribuable au traitement par le
glyphosate de qualité technique ou le Roundup. Les effets attribués
au traitement par cet herbicide et constatés chez les oiseaux et les
mammifères dans leur milieu naturel, semblent être dus
principalement aux modifications du biotope consécutives au
traitement herbicide.
RESUMEN
1. Identidad, propiedades físicas y químicas y métodos analíticos
El glifosato es un ácido orgánico débil formado por una
molécula de glicina y otra de fosfonometilo. La fórmula empírica es
C3H8NO5P. Normalmente se formula como una sal del ácido del
glifosato en el que se ha sustituido un protón por un catión, por
ejemplo la isopropilamina o el trimetilsulfonio. La pureza del
glifosato de calidad técnica suele ser superior al 90%. Este es un
polvo cristalino blanco e inodoro con un peso específico de 1,704,
una presión de vapor muy baja y una solubilidad en agua alta. El
coeficiente de reparto octanol/agua (log Kow) es -2,8. El
glifosato es anfótero y se puede encontrar formando compuestos
iónicos diversos, en función del Ph del medio.
Su determinación es en general laboriosa, compleja y costosa.
El método más habitual es la transformación con sustancias
fluorogénicas en derivados más fácilmente detectables y se puede
utilizar antes o después de la columna. La determinación se suele
llevar a cabo mediante cromatografía líquida de alto rendimiento o
cromatografía gas-líquido. Los límites de determinación del
glifosato en el agua, las plantas, el suelo y la orina humana son de
0,02-3,2 µg/litro, 0,01-0,3 mg/kg, 0,05-1 mg/kg y 0,1 mg/litro,
respectivamente.
2. Fuentes de exposición humana y ambiental
El glifosato es un herbicida que actúa después del brote de
manera sistémica y no selectiva, y se utiliza en zonas agrícolas y
no agrícolas de todo el mundo. Se aplica a numerosos cultivos con
formulaciones comerciales diferentes. La más importante es el
Roundup, en el que el glifosato aparece en forma de la sal de
isopropilamina. Las dosis de aplicación recomendadas no superan los
5,8 kg de a.i./ha y dependen del tipo de uso. Se puede producir
exposición ambiental como consecuencia de la deposición debida a
corrientes o escapes accidentales.
3. Transporte, distribución y transformación en el medio ambiente
Las más importantes vías de desaparición del glifosato tras su
aplicación son la formación en el agua de complejos con iones, por
ejemplo con el Ca2+ y el Mg2+, la sorción al sedimento, las
partículas suspendidas en el agua y el suelo, la fotodegradación en
el agua, la fijación en las plantas y la biodegradación.
El glifosato desaparece del agua con unos valores de TD50 que
oscilan entre varios días y más de 91 días. Se ha comprobado que se
deposita sobre todo en las partículas del sedimento o suspendidas.
Los coeficientes de adsorción (Ks/l) del glifosato en
experimentos de laboratorio varían entre 8 y 377 dm3/kg para
diferentes suelos y minerales arcillosos. No se dispone de datos, en
condiciones de laboratorio, sobre la sorción del ácido
aminometilfosfónico (AAMF), su principal metabolito.
En los experimentos de cromatografía en capa fina, los valores
Rf del glifosato no son superiores a 0,2 en el suelo. En el eluato
de columnas de suelo obtenido en condiciones de lixiviación
simulando una precipitación muy intensa se recupera una cantidad que
oscila entre menos del 0,1% y el 11% de la dosis aplicada. De los
estudios sobre el terreno se desprende que no es probable la
lixiviación del AAMF.
En los experimentos sobre el terreno el glifosato desaparece
del suelo con un TD50 que varía entre 3 y 174 días, principalmente
en función de las condiciones edáficas o climáticas. En algunos
experimentos sobre el terreno desaparecía del suelo, debido a la
escorrentía, hasta el 1,8% de la dosis aplicada.
En condiciones de laboratorio, las hojas tratadas podrían
absorber hasta el 45% de la cantidad aplicada, produciéndose a
continuación un importante desplazamiento.
La hidrólisis del glifosato en tampones estériles es muy baja,
con valores de TD50 >> 35 días. En condiciones naturales, la
fotodegradación en agua se produce con valores de TD50 < 28 días.
En el curso de un estudio de 31 días no se registró una
fotodegradación importante en el suelo.
El tiempo necesario para la biodegradación del 50% del
glifosato en el sistema completo de una prueba con agua y sedimento
es < 14 días en condiciones aerobias y de 14 a 22 días en
condiciones anaerobias de laboratorio. El tiempo necesario para la
biodegradación del 50% del glifosato en el suelo es de 2-3 días en
condiciones aerobias.
El metabolito principal en el suelo y el agua es el AAMF. Las
cantidades máximas de AAMF en el suelo son de aproximadamente el 20%
de la dosis aplicada en condiciones aerobias, y del 0,5% en
condiciones anaerobias. Las cantidades máximas de AAMF en el
sedimento son del 25%, tanto en condiciones aerobias como
anaerobias.
De las pruebas de laboratorio se desprende que los factores de
bioconcentración en invertebrados y peces son bajos. Tras una
exposición al glifosato de 35 días, Lepomis macrochirus mostró en
una prueba en corriente un periodo de semidepuración de 35 días. Se
recuperó AAMF en Lepomis macrochirus hasta 21 días después de una
exposición continuada. No se detectó glifosato en peces que vivían
en agua directamente rociada en experimentos sobre el terreno. En un
experimento se detectó AAMF en carpas hasta 90 días después de la
aplicación. En otro experimento sobre el terreno no se observó
bioampliación del glifosato en el lecho de pequeños mamíferos
herbívoros y omnívoros de un ecosistema de matorral boscoso. En este
mismo experimento, inmediatamente después del rociado se
determinaron concentraciones de hasta 5 mg de a.i./kg en ratones de
pies blancos (Peromyscus leucopus).
Existen diversas bacterias que pueden degradar el glifosato. Se
han identificado cepas capaces de utilizar este compuesto como única
fuente de fósforo, de carbono o de nitrógeno. El crecimiento es
lento si se compara con el obtenido de fuentes inorgánicas de P, C y
N. Hay pruebas en el medio ambiente de la existencia de poblaciones
bacterianas que se han adaptado para metabolizar el glifosato. La
presencia de fosfato inorgánico inhibe la degradación de este
compuesto por algunas bacterias, pero no por todas. La
biodegradación del glifosato podría tener un metabolismo común con
el de otras fuentes de energía.
4. Niveles ambientales y exposición humana
Los datos sobre la presencia de glifosato en la biota y la
abiota del medio ambiente como parte de programas de vigilancia
regular son muy escasos. Se utilizan datos obtenidos en experimentos
sobre el terreno en los que se simula la práctica agrícola normal
para indicar las concentraciones máximas en el medio ambiente: <
1-1700 µg/litro de agua superficial, 0,07-40 mg/kg de peso seco de
suelo, < 0,05-19 mg/kg de peso seco de sedimento, 261-1300 mg/kg de
follaje, 5 mg/kg de vísceras de ratón de pies blancos, 1,6-19 mg/kg
de bayas silvestres y 45 mg/kg de líquenes. Las concentraciones
máximas correspondientes de AAMF son las siguientes: <
1-35 µg/litro (agua superficial), 0,1-9 mg/kg de peso seco (suelo),
< 0,05-1,8 mg/kg de peso seco (sedimento), 1,7-< 9 mg/kg
(follaje), 0,02-0,1 mg/kg (bayas silvestres) y 2,1 mg/kg (líquenes).
Las concentraciones de glifosato mencionadas más arriba se suelen
encontrar inmediatamente después de la aplicación. La concentración
en los líquenes se determinó 270 días después de dicha aplicación.
No se dispone de mediciones de la ingestión humana diaria de
glifosato a través de los alimentos y el agua de bebida (estudios
completos de alimentación). Los escasos datos disponibles sobre la
exposición ocupacional ponen de manifiesto que los niveles de
exposición para los trabajadores que aplican el glifosato en la
formulación del herbicida Roundup son bajos.
5. Cinética y metabolismo en animales de laboratorio y en el ser
humano
La absorción del glifosato de calidad técnica en el tracto
intestinal es sólo parcial. En estudios con glifosato marcado con
14C, se encontró en varias especies un porcentaje de absorción del
30% al 36%. La absorción cutánea es baja. De la formulación del
herbicida Roundup, a través de la piel sólo se absorbe < 5,5% del
glifosato presente (tiempo de contacto de unas 24 horas). En los
tejidos del organismo, la concentración más alta, aproximadamente el
1% de la dosis oral, se encuentran en los huesos. Tras una dosis
oral única, se eliminó en las heces sin absorción el 62-69%. Del
glifosato absorbido, un 14-29% se excretó en la orina y el 0,2% o
menos en el aire expirado. La excreción biliar posterior a la
administración intravenosa fue sólo del 5-8%. Se observó que la
excreción en la leche de cabras lactantes se producía sólo en escasa
proporción (concentración < 0,1 mg/kg de leche entera a un nivel
de dosis de 120 mg/kg de alimentos). La biotransformación del
glifosato se da únicamente en un grado muy bajo. El único
metabolito, el AAMF, representa el 0,3% de la dosis o menos; el
resto es glifosato inalterado. La eliminación de todo el organismo
(99% de una dosis oral) se produce aproximadamente en 168 horas.
6. Efectos en animales de laboratorio y en sistemas de prueba
in vitro
El glifosato de calidad técnica administrado por vía oral y
cutánea a animales de experimentación tiene una toxicidad aguda muy
baja; por vía intraperitoneal es notablemente más tóxico que por
cualquier otra. Aunque se han realizado estudios de alimentación de
corta duración en varias especies, en la mayor parte de estas
pruebas se han observado pocos efectos. En un estudio de 13 semanas
realizado en ratones con glifosato de calidad técnica, a una
concentración de 50 000 mg/kg de alimento, se observó aumento de
peso de varios órganos y un retraso del crecimiento. En un estudio
de 13 semanas en ratas no se advirtieron efectos (con dosis de
glifosato de calidad técnica de hasta 20 000 mg/kg de alimento). En
otro estudio de 13 semanas se detectaron lesiones en las glándulas
salivales de ratas y ratones. En ratones, el NOAEL fue de 3125 mg/kg
de alimento; en ratas fue < 3125 mg/kg de alimento. Estos
resultados no se obtuvieron en ningún otro estudio de corta o larga
duración realizado en diferentes razas y especies. Las lesiones de
las glándulas salivales parecen indicar que el glifosato puede
actuar como agonista adrenérgico débil.
Se estudió la toxicidad a largo plazo en ratones y ratas. Se
observaron escasos efectos y, en la mayor parte de los casos, sólo a
dosis relativamente altas. Con dosis de 30 000 mg/kg de glifosato de
calidad técnica se produjo en los ratones retraso del crecimiento,
hipertrofia o necrosis de los hepatocitos e hiperplasia epitelial de
la vejiga urinaria. La misma prueba en ratas con dosis de
20 000 mg/kg de alimento provocó una disminución del crecimiento,
aumento del peso del hígado, cambios degenerativos del cristalino e
inflamación gástrica.
De los estudios disponibles no se desprende que el glifosato de
calidad técnica tenga actividad mutagénica, carcinogénica o
teratogénica. Se realizaron con este compuesto dos estudios en
varias generaciones de ratas. Los principales efectos del glifosato
de calidad técnica con dosis de 30 000 mg/kg de alimento fueron una
disminución del peso corporal de los padres y las crías y la
reducción del tamaño de la camada. Se ha informado que en un estudio
de reproducción con dosis de 30 mg/kg de peso corporal se produjo un
aumento del número de casos de dilatación tubular renal unilateral
en crías macho de la F3b. La ausencia de efectos renales en las
crías con dosis más elevadas en el otro estudio de reproducción pone
de manifiesto que la reproducibilidad de la lesión es incierta.
7. Efectos en el ser humano
Sólo se dispone de tres estudios controlados sobre
irritación/sensibilización en voluntarios, cuyos resultados indican
la ausencia de efectos. Se ha informado de varios casos de
intoxicación (la mayor parte intencionados) con la formulación
Roundup de herbicida a base de glifosato de calidad técnica. No se
detectaron efectos adversos tras realizar un estudio para determinar
el estado de salud de los trabajadores que aplican la formulación
del herbicida Roundup. Los datos disponibles sobre exposición en el
trabajo de quienes aplican el Roundup indican que los niveles de
exposición están muy por debajo del NOAEL obtenido en los
experimentos correspondientes con animales.
8. Efectos sobre otros organismos en el laboratorio y en el medio
ambiente
El glifosato de calidad técnica tiene una toxicidad de moderada
a ligera para los microorganismos acuáticos, con valores de CE50
(3-4 días) de 1,2-7,8 mg/litro y valores de NOEC (7 días) de
0,3-34 mg/litro. Las formulaciones de glifosato son entre
ligeramente tóxicas y muy tóxicas para los microorganismos
acuáticos, con valores de CE50 en tres días de 1,0 a > 55 mg de
producto por litro. Las cianofíceas (algas verdeazuladas) son más
sensibles al Roundup que las algas verdaderas. Afecta a diversos
procesos fisiológicos, entre ellos la formación del color verde, la
respiración, la fotosíntesis y la síntesis de aminoácidos
aromáticos.
En cultivos de bacterias del suelo se ha comprobado la
influencia del glifosato sobre la fijación del nitrógeno, la
desnitrificación y la nitrificación. Sin embargo, en estudios sobre
el terreno no se han observado efectos significativos tras la
aplicación de varias formulaciones. Diversas bacterias estrechamente
relacionadas han demostrado que son capaces de degradar el
glifosato.
A concentraciones > 29 µg de Roundup/litro se inhibe el
crecimiento de los micelios de las ectomicorrizas en cultivos puros.
Son géneros sensibles Cenococcum, Hebeloma y Laccaria.
Cuando se disuelve en agua, el glifosato es ligeramente tóxico
para las macrofitas acuáticas, con un valor de NOEC en 14 días de
9 mg/litro. El Roundup disuelto en agua es también ligeramente
tóxico, con valores de NOEC en 14 días de 2,4-56 mg de
Roundup/litro. No se dispone de datos acerca de su toxicidad aguda.
La fitotoxicidad es mucho más elevada cuando el agua no arrastra los
depósitos del rociado.
La toxicidad del glifosato de calidad técnica para los
invertebrados acuáticos varía entre ligera y muy ligera, con unos
valores de la CL50 o la CE50 en 2 a 4 días de > 55 mg/litro,
y un valor de NOEC en 21 días de 100 mg/litro. Las formulaciones de
glifosato tienen una toxicidad entre moderada y muy ligera para los
invertebrados acuáticos, con valores de CE50 en 2 días de
5,3-5600 mg de producto/litro y valores de MATC en 21 días de
1,4-4,9 mg de producto por litro. La toxicidad más elevada del
Roundup se debe fundamentalmente a la presencia de surfactantes.
La toxicidad del glifosato de calidad técnica para los peces es
entre moderada y muy ligera, con valores de CL50 en 4 días de 10 a
> 1000 mg/litro, una NOEC en 21 días de 52 mg/litro, y un valor
MATC de > 26 mg/litro. Las formulaciones del glifosato tienen
también una toxicidad entre moderada y muy ligera para los peces,
con valores de CL50 en 4 días de 2,4 a > 1000 mg de producto por
litro y valores de NOEC en 21 días de 0,8-2,4 mg de producto/litro.
La especie más sensible es la carpa, cuando se la expone a la
formulación Sting. No se han observado en los peces efectos
relacionados con el tratamiento de Roundup en el medio ambiente,
salvo cierta tensión inmediatamente después de la aplicación de una
dosis recomendada y evitando concentraciones > 40 mg de
Roundup/litro.
En sistemas de cultivo sin suelo con soluciones nutrientes en
concentraciones > 2 mg de i.a./litro se produce una inhibición
dependiente de la dosis de la nodulación del trébol subterráneo
inoculado con Rhizobium. El glifosato en las dosis de aplicación
recomendadas no afecta a la germinación de las semillas. La longitud
de las raíces de los plantones de pino rojo disminuye en condiciones
de laboratorio en función de la dosis con unas concentraciones de
aplicación > 0,54 kg de i.a./ha. Esta reducción no se confirmó en
un experimento comparable sobre el terreno.
El glifosato de calidad técnica y el Roundup son ligeramente
tóxicos para las abejas cuando se aplican por vía oral o tópica. Los
valores de la DL50 en 2 días son > 100 µg (i.a. o producto) por
abeja. La DL50 en 2 días por vía oral de Sting para las abejas es
> 100 µg/abeja. Roundup y Roundup D-pak son ligeramente tóxicos
para las lombrices de tierra, con valores NOEC en 14 días de 500 y
158 mg de producto por kg de peso seco, respectivamente. No se
observaron efectos adversos del Roundup sobre la fecundidad y
fertilidad de especies de la familia Chrysopidae, y tampoco se
detectaron efectos de Sting en la ingestión de alimentos y la
mortalidad del escarabajo Poecilus.
El glifosato de calidad técnica es ligeramente tóxico para las
aves, con una DL50 > 3851 mg/kg de peso corporal, una CL50 en 8
días de > 4640 mg/kg de alimento, y valores NOEC en 112-119 días de
> 1000 mg/kg de alimento. Roundup y una formulación desconocida
son también ligeramente tóxicos para las aves, con una DL50 de >
2686 mg de producto/kg de peso corporal y una CL50 en 8 días de >
5620 mg de producto/kg de alimento. En condiciones de laboratorio no
se han observado en general sobre los mamíferos efectos relacionados
con el tratamiento de glifosato de calidad técnica o Roundup, salvo
con dosis de aplicación muy elevadas. Los efectos relacionados con
el tratamiento en las aves y mamíferos del medio ambiente parecen
deberse fundamentalmente a cambios de hábitat después del
tratamiento con Roundup.
Table 25. Indications of environmental hazard for aquatic organisms by technical grade glyphosate
Effect Organisms Estimated exposure Toxicity data End-point Ratio of exposure Possible
concentration (mg a.i./litre) to toxicity hazard
(mg a.i./litre) concentrations
Mean estimated exposure concentration
Acute microorganisms 0.1 EC50 = 1.2 mortality 0.082 small
Acute insects 0.1 EC50 = 55 mortality 0.0018 negligible
Acute crustaceans 0.1 EC50 = 281 mortality 0.00035 negligible
Acute fish 0.1 LC50 = 94 mortality 0.0010 negligible
Chronic algae 0.05 NOEC = 0.3 growth 0.17 present
Chronic crustaceans 0.01 NOEC = 100 reproduction 0.00010 negligible
Chronic fish 0.01 NOEC = 52 behaviour 0.00019 negligible
Maximum estimated exposure concentration
Acute microorganisms 1.7 EC50 = 1.2 mortality 1.4 large
Acute insects 1.7 EC50 = 55 mortality 0.031 small
Acute crustaceans 1.7 EC50 = 281 mortality 0.0060 negligible
Acute fish 1.7 LC50 = 94 mortality 0.018 small
Chronic algae 1.7 NOEC = 0.3 growth 5.7 large
Chronic crustaceans 0.17 NOEC = 100 reproduction 0.0017 negligible
Chronic fish 0.17 NOEC = 52 behaviour 0.003 negligible
Table 26. Indications of environmental hazards for aquatic organisms by Roundup
Effect Organisms Estimated exposure Toxicity data End-point Ratio of exposure Possible
concentration (mg Roundup/litre) to toxicity hazard
(mg Roundup/litre) concentrations
Mean estimated exposure concentration
Acute microorganisms 0.32 EC50 = 2.1 mortality 0.15 present
Acute crustaceans 0.32 EC50 = 10 mortality 0.032 small
Acute insects 0.32 EC50 = 44 mortality 0.0073 negligible
Acute fish 0.32 LC50 = 13 mortality 0.025 small
Chronic microorganisms 0.16 NOEC = 0.7 growth 0.23 present
Chronic crustaceans 0.032 NOEC = 3.5 reproduction 0.0091 negligible
Chronic fish 0.032 NOEC = 2.4 behaviour 0.013 negligible
Maximum estimated exposure concentration
Acute microorganisms 5.6 EC50 = 2.1 mortality 2.7 large
Acute crustaceans 5.6 EC50 = 10 mortality 0.56 present
Acute insects 5.6 EC50 = 44 mortality 0.13 present
Acute fish 5.6 LC50 = 13 mortality 0.43 present
Chronic microorganisms 5.6 NOEC = 0.7 growth 8.0 large
Chronic crustaceans 0.56 NOEC = 3.5 reproduction 0.16 small
Chronic fish 0.56 NOEC = 2.4 behaviour 0.23 small
11. RECOMMENDATIONS FOR PROTECTION OF HUMAN HEALTH
a) Protective clothing is necessary to ensure the safety of
herbicide applicators.
b) A market-basket survey would be useful to determine the
possible exposure of the general population.
12. FURTHER RESEARCH
a) Further research is required to determine whether ß-adrenergic
effects observed in rodents have any implications for human
health.
b) The role of adjuvants in the toxicity of glyphosate
formulations needs to be investigated further in laboratory
mammals and organisms in the environment.
c) A controlled study on exposure of agricultural workers is
needed.
d) The bioavailability of sediment- and soil-bound glyphosate in
the environment should be studied.
e) Studies on the environmental behaviour and fate of adjuvants
are required.
f) Further toxicity studies of sediment-living organisms are
needed.
g) The effects of phosphate fertilizers on the binding of
glyphosate to soils should be investigated.
h) Analytical techniques that are less costly but still adequate
should be developed.
REFERENCES
ABC Inc. (1978a) Acute toxicity of technical glyphosate (AB-78-201)
to Daphnia magna. Columbia, Missouri, Analytical Biochemistry
Laboratories, Inc. (Unpublished report submitted by Monsanto Ltd).
ABC Inc. (1978b) Acute toxicity of technical glyphosate (AB-78-165)
to rainbow trout (Salmo gairdnerii). Columbia, Missouri,
Analytical Biochemistry Laboratories, Inc. (Unpublished report
submitted by Monsanto Ltd).
ABC Inc. (1978c) Acute toxicity of technical glyphosate to bluegill
sunfish (Lepomis macrochirus). Columbia, Missouri, Analytical
Biochemistry Laboratories, Inc. (Unpublished report submitted by
Monsanto Ltd).
ABC Inc. (1978d) The effect of glyphosate on nitrogen fixation and
nitrification in soil with time. Columbia, Missouri, Analytical
Biochemistry Laboratories, Inc. (Unpublished report submitted by
Monsanto Ltd).
ABC Inc. (1978e) The effect of glyphosate on the degradation of
cellulose, starch, protein, and leaf litter on soil. Columbia,
Missouri, Analytical Biochemistry Laboratories, Inc. (Unpublished
report submitted by Monsanto Ltd).
ABC Inc. (1980a) Acute toxicity of MON-0139-X-77 (AB-80-262) to
rainbow trout (Salmo gairdnerii). Columbia, Missouri, Analytical
Biochemistry Laboratories, Inc. (Unpublished report submitted by
Monsanto Ltd).
ABC Inc. (1980b) Acute toxicity of MON-0139-X-77 (AB-80-263) to
bluegill sunfish (Lepomis macrochirus). Columbia, Missouri,
Analytical Biochemistry Laboratories, Inc. (Unpublished report
submitted by Monsanto Ltd).
ABC Inc. (1981a) Acute toxicity of MON 0139 (Lot LURT 12011)
(AB-81-074) to Daphnia magna. Columbia, Missouri, Analytical
Biochemistry Laboratories, Inc. (Unpublished report submitted by
Monsanto Ltd).
ABC Inc. (1981b) Acute toxicity of MON 0139 (Lot LURT 12011)
(AB-81-073) to Lepomis macrochirus. Columbia, Missouri, Analytical
Biochemistry Laboratories, Inc. (Unpublished report submitted by
Monsanto Ltd).
ABC Inc. (1981c) Acute toxicity of MON 0139 (Lot LURT 12011)
(AB-81-072) to rainbow trout (Salmo gairdnerii). Columbia,
Missouri, Analytical Biochemistry Laboratories, Inc. (Unpublished
report submitted by Monsanto Ltd).
ABC Inc. (1981d) A short-term bioconcentration study of
14C-glyphosate with channel catfish (Ictalurus punctatus) in a
static system. Columbia, Missouri, Analytical Biochemistry
Laboratories, Inc. (Unpublished report submitted by Monsanto Ltd).
ABC Inc. (1982a) Dynamic 96-hour acute toxicity of Roundup
(AB-82-33) to bluegill sunfish (Lepomis macrochirus). Columbia,
Missouri, Analytical Biochemistry Laboratories, Inc. (Unpublished
report submitted by Monsanto Ltd).
ABC Inc. (1982b) Dynamic 48-hour acute toxicity of Roundup
(AB-82-035) to Gammarus pseudolimnaeus. Columbia, Missouri,
Analytical Biochemistry Laboratories, Inc. (Unpublished report
submitted by Monsanto Ltd).
ABC Inc. (1982c) Dynamic 96-hour acute toxicity of Roundup
(AB-82-34) to rainbow trout (Salmo gairdnerii). Columbia,
Missouri, Analytical Biochemistry Laboratories, Inc. (Unpublished
report submitted by Monsanto Ltd).
ABC Inc. (1982d) Residue accumulation study in molluscs (Rangia
cuneata) with 14C-glyphosate under static conditions. Columbia,
Missouri, Analytical Biochemistry Laboratories, Inc. (Unpublished
report submitted by Monsanto Ltd).
ABC Inc. (1982e) Residue accumulation study in crayfish
(Procambarus simulans) with 14C-glyphosate under static
conditions. Columbia, Missouri, Analytical Biochemistry
Laboratories, Inc. (Unpublished report submitted by Monsanto Ltd).
ABC Inc. (1984a) Acute toxicity of LLN 8306 to rainbow trout (Salmo
gairdnerii). Columbia, Missouri, Analytical Biochemistry
Laboratories, Inc. (Unpublished report submitted by Monsanto Ltd).
ABC Inc. (1984b) Acute toxicity of LLN 8306 to bluegill sunfish
(Lepomis macrochirus). Columbia, Missouri, Analytical Biochemistry
Laboratories, Inc. (Unpublished report submitted by Monsanto Ltd).
ABC Inc. (1984c) Acute toxicity of LLN 8306 to Daphnia magna.
Columbia, Missouri, Analytical Biochemistry Laboratories, Inc.
(Unpublished report submitted by Monsanto Ltd).
ABC Inc. (1989a) 21-day prolonged static renewal toxicity of MON
8755 to Daphnia magna. Columbia, Missouri, Analytical Biochemistry
Laboratories, Inc. (Unpublished report No. 37933 submitted by
Monsanto Ltd).
ABC Inc. (1989b) 21-day prolonged static renewal toxicity of Roundup
to Daphnia magna. Columbia, Missouri, Analytical Biochemistry
Laboratories, Inc. (Unpublished report No. 37934 submitted by
Monsanto Ltd).
ABC Inc. (1989c) 21-day prolonged static renewal toxicity of
glyphosate technical to Daphnia magna. Columbia, Missouri,
Analytical Biochemistry Laboratories, Inc. (Unpublished report No.
37757 submitted by Monsanto Ltd).
ABC Inc. (1989d) Flow-through toxicity of Roundup to rainbow trout
(Salmo gairdnerii) for a 21-day exposure period. Columbia,
Missouri, Analytical Biochemistry Laboratories, Inc. (Unpublished
report No. 37694 submitted by Monsanto Ltd).
ABC Inc. (1989e) Flow-through toxicity of glyphosate to rainbow
trout (Salmo gairdnerii) for a 21-day duration period. Columbia,
Missouri, Analytical Biochemistry Laboratories, Inc. (Unpublished
report No. 37695 submitted by Monsanto Ltd).
ABC Inc. (1989f) Uptake, depuration, and bioconcentration of
14C glyphosate to bluegill sunfish (Lepomis macrochirus). Part
I: MSL-9304. Columbia, Missouri, Analytical Biochemistry
Laboratories, Inc. (Unpublished report submitted by Monsanto Ltd).
ABC Inc. (1989g) Uptake, depuration, and bioconcentration of
14C glyphosate to bluegill sunfish (Lepomis macrochirus).
Characterization and quantitation of glyphosate and its metabolites.
Part II: MSL-9303. Columbia, Missouri, Analytical Biochemistry
Laboratories, Inc. (Unpublished report submitted by Monsanto Ltd).
ABC Inc. (1989h) Flow-through toxicity of MON 8755 to rainbow trout
(Salmo gairdnerii) for a 21-day duration period. Columbia,
Missouri, Analytical Biochemistry Laboratories, Inc. (Unpublished
report No. 37759 submitted by Monsanto Ltd).
ABC Inc. (1990) Acute toxicity of MON 8755 to common carp (Cyprinus
carpio). Columbia, Missouri, Analytical Biochemistry Laboratories,
Inc. (Unpublished report No. 38256 submitted by Monsanto Ltd).
Ackurst P (1989) Introduction: issues and concerns relating to the
use of herbicides. In: Reynolds P ed. Proceedings of the Carnation
Creek Workshop, Nanaimo, 7-10 December 1987. Victoria, British
Columbia, Forestry Canada/British Columbia Ministry of Forests, pp
7-11.
Altmann G (1984) Laboratory testing of your preparation LLN 83/06
for danger to bees. Unpublished data of University of Saarland,
Saarbrücken, Department of Zoology, provided by Monsanto.
Amrhein N, Deus B, Gehrke P, & Steinrücken HC (1980) The site of the
inhibition of the shikimate pathway by glyphosate. Plant Physiol,
66: 830-834.
Anthony RG & Morrison ML (1985) Influence of glyphosate herbicide on
small-mammal populations in western Oregon. Northwest Sci, 59(3):
159-168.
Austin AP, Harris GE, & Lucey WP (1991) Impact of an organophosphate
herbicide (glyphosate) on periphyton communities developed in
experimental streams. Bull Environ Contam Toxicol, 47: 29-35.
Bababunmi EA, Olorunsogo OO, & Bassir O (1979) The uncoupling effect
of N-(phosphonomethyl)glycine on isolated rat liver mitochondria.
Biochem Pharmacol, 18: 925-927.
Balthazor TM & Hallas LE (1986) Glyphosate-degrading microorganisms
from industrial activated sludge. Appl Environ Microbiol, 51(2):
432-434.
Benova EK, Roupova I, Yagova A, Vuglenov A, & Bineva M (1989)
Mutagenicity studies on six pesticides in mice. Environ Mol Mutagen,
14(Suppl 15): 19 (Abstract).
Bio/Dynamics Inc. (1975) A twenty-one day dermal toxicity study of
MON 2139 in male rabbits (Project No. 75-1245). East Millstone, New
Jersey, Bio/Dynamics Inc., Division of Biology and Safety Evaluation
(Unpublished report submitted by Monsanto Ltd).
Bio/Dynamics Inc. (1979) A three month feeding study of glyphosate
(Roundup technical) in mice (Project No. 77-211) - Final report.
East Millstone, New Jersey, Bio/Dynamics Inc., Division of Biology
and Safety Evaluation (Unpublished report submitted by Monsanto
Ltd).
Bio/Dynamics Inc. (1981a) A lifetime feeding study of glyphosate
(Roundup technical) in rats (Project No. 410/77 [BDN-77-416]). East
Millstone, New Jersey, Bio/Dynamics Inc., Division of Biology and
Safety Evaluation (Unpublished report submitted by Monsanto Ltd).
Bio/Dynamics Inc. (1981b) A three-generation reproduction study in
rats with glyphosate (Project No. 77-2063 [BDN-77-147]) - Final
report. East Millstone, New Jersey, Bio/Dynamics Inc., Division of
Biology and Safety Evaluation (Unpublished report submitted by
Monsanto Ltd).
Bio/Dynamics Inc. (1983a) A chronic feeding study of glyphosate
(Roundup technical) in mice (Project No. 77-2061 [BDN-77-420]). East
Millstone, New Jersey, Bio/Dynamics Inc., Division of Biology and
Safety Evaluation (Unpublished report submitted by Monsanto Ltd).
Bio/Dynamics Inc. (1983b) A dermal sensitization study in guinea
pigs - Test material: Roundup formulation (Project No. 4234-83).
East Millstone, New Jersey, Bio/Dynamics Inc., Division of Biology
and Safety Evaluation (Unpublished report submitted by Monsanto Ltd,
Reference No. BD-83-007).
Bio/Dynamics Inc. (1983c) A dermal sensitization study in guinea
pigs - Test material: glyphosate (Project No. 4235-82). East
Millstone, New Jersey, Bio/Dynamics Inc., Division of Biology and
Safety Evaluation (Unpublished report submitted by Monsanto Ltd,
Reference No. BD-83-008).
Bio/Dynamics Inc. (1984a) Acute dermal toxicity study in rabbits -
Test material: LLN-83-06 (Project No. 4885-83). East Millstone, New
Jersey, Bio/Dynamics Inc., Department of Toxicology (Unpublished
report submitted by Monsanto Ltd, Reference No. BD-83-380).
Bio/Dynamics Inc. (1984b) Acute oral toxicity study in rats - Test
material: LLN-83-06 (Project No. 4884-83). East Millstone, New
Jersey, Bio/Dynamics Inc., Department of Toxicology (Unpublished
report submitted by Monsanto Ltd, Reference No. BD-83-380).
Bio/Dynamics Inc. (1984c) Primary dermal irritation study in rabbits
(4-hour exposure) - Test material: LLN-83-06 (Project No. 4886-83).
East Millstone, New Jersey, Bio/Dynamics Inc., Department of
Toxicology (Unpublished report submitted by Monsanto Ltd, Reference
No. BD-83-380).
Bio/Dynamics Inc. (1984d) Eye irritation study in rabbits - Test
material: LLN-83-06 (Project No. 4887-83). East Millstone, New
Jersey, Bio/Dynamics Inc., Department of Toxicology (Unpublished
report submitted by Monsanto Ltd, Reference No. BD-83-380).
Bio/Dynamics Inc. (1985a) Acute oral toxicity study in rats - Test
material: MON 8780 (Project No. 5301-84). East Millstone, New
Jersey, Bio/Dynamics Inc., Department of Toxicology (Unpublished
report submitted by Monsanto Ltd, Reference No. BD-84-343).
Bio/Dynamics Inc. (1985b) Acute dermal toxicity study in rabbits -
Test material: MON 8780 (Project No. 5302-84). East Millstone, New
Jersey, Bio/Dynamics Inc., Department of Toxicology (Unpublished
report submitted by Monsanto Ltd, Reference No. BD-84-343).
Bio/Dynamics Inc. (1985c) Primary dermal irritation study in rabbits
(4-hour exposure) - Test material: MON 8780 (Project No. 5303-84).
East Millstone, New Jersey, Bio/Dynamics Inc., Department of
Toxicology (Unpublished report submitted by Monsanto Ltd).
Bio/Dynamics Inc. (1985d) Eye irritation study in rabbits - Test
material: MON 8780 (Project No. 5304-84). East Millstone, New
Jersey, Bio/Dynamics Inc., Department of Toxicology (Unpublished
report submitted by Monsanto Ltd, Reference No. BD-84-343).
Bio/Dynamics Inc. (1986) A closed-patch repeated insult dermal
sensitization study in guinea pigs (Buehler method) - Test material:
MON 8756 (Project No. 6210-85). East Millstone, New Jersey,
Bio/Dynamics Inc., Department of Toxicology (Unpublished report
submitted by Monsanto Ltd, Reference No. BD-86-5).
Bio/Dynamics Inc. (1987a) Acute oral toxicity study in rats - Test
material: Roundup L&G Ready-to-use (Project No. 4033-86). East
Millstone, New Jersey, Bio/Dynamics Inc., Department of Toxicology
(Unpublished report submitted by Monsanto Ltd, Reference No.
BD-87-4).
Bio/Dynamics Inc. (1987b) Acute dermal toxicity study in rabbits -
Test material: Roundup L&G Ready-to-use (Project No. 4034-86). East
Millstone, New Jersey, Bio/Dynamics Inc., Department of Toxicology
(Unpublished report submitted by Monsanto Ltd, Reference
No. BD-87-3).
Bio/Dynamics Inc. (1987c) Primary dermal irritation study in rabbits
(4-hour exposure/semi-occlusive covering) - Test material: Roundup
L&G Ready-to-Use (Project No. 4035-86). East Millstone, New Jersey,
Bio/Dynamics Inc., Department of Toxicology (Unpublished report
submitted by Monsanto Ltd, Reference No. BD-87-4).
Bio/Dynamics Inc. (1987d) Eye irritation study in rabbits - Test
material: Roundup L&G Ready-to-Use (Project No. 4036-86). East
Millstone, New Jersey, Bio/Dynamics Inc., Department of Toxicology
(Unpublished report submitted by Monsanto Ltd, Reference
No. BD-87-4).
Bio/Dynamics Inc. (1987e) A closed-patch repeated insult dermal
sensitization study in guinea pigs (Project No. 6945-86). East
Millstone, New Jersey, Bio/Dynamics Inc., Division of Biology and
Safety Evaluation (Unpublished report submitted by Monsanto Ltd,
Reference No. BD-86-442).
Bio/Dynamics Inc. (1988a) Acute dermal toxicity study in rabbits -
Test material: Glyphosate Wet Cake (Project No. 4886-88). East
Millstone, New Jersey, Bio/Dynamics Inc., Department of Toxicology
(Unpublished report submitted by Monsanto Ltd, Reference No.
BD-88-114).
Bio/Dynamics Inc. (1988b) Primary dermal irritation study in rabbits
(4-hour exposure/semi-occlusive covering) - Test material:
Glyphosate Wet Cake (Project No. 4887-88). East Millstone, New
Jersey, Bio/Dynamics Inc., Department of Toxicology (Unpublished
report submitted by Monsanto Ltd, Reference No. BD-88-114).
Bio/Dynamics Inc. (1988c) Acute oral toxicity study in rats - Test
material: Glyphosate Wet Cake (Project No. 4885-88). East Millstone,
New Jersey, Bio/Dynamics Inc., Department of Toxicology (Unpublished
report submitted by Monsanto Ltd, Reference No. BD-88-114).
Bio/Dynamics Inc. (1988d) Eye irritation study in rabbits - Test
material: Glyphosate Wet Cake (Project No. 4888-88). East Millstone,
New Jersey, Bio/Dynamics Inc., Department of Toxicology (Unpublished
report submitted by Montsanto Ltd, Reference No. BD-88-114).
Bio/Dynamics Inc. (1988e) Acute oral toxicity study in rats - Test
material: Roundup (Project No. 4546-87). East Millstone, New Jersey,
Bio/Dynamics Inc., Department of Toxicology (Unpublished report
submitted by Monsanto Ltd, Reference No. BD-87-283).
Bio/Dynamics Inc. (1988f) Acute dermal toxicity study in rabbits -
Test material: Roundup (Project No. 4547-87). East Millstone, New
Jersey, Bio/Dynamics Inc., Department of Toxicology (Unpublished
report submitted by Monsanto Ltd, Reference No. BD-87-283).
Bio/Dynamics Inc. (1988g) Primary dermal irritation study in rabbits
(4-hour exposure/semi-occlusive covering) - Test material: Roundup
(Project No. 4548-87). East Millstone, New Jersey, Bio/Dynamics
Inc., Department of Toxicology (Unpublished report submitted by
Monsanto Ltd, Reference No. BD-87-283).
Bio/Dynamics Inc. (1990) Eye irritation study in rabbits - Test
material: MON 2139 (Project No. 5833-90). East Millstone, New
Jersey, Bio/Dynamics Inc., Department of Toxicology (Unpublished
report submitted by Monsanto, Reference No. BD-90-246).
Bionomics (1973a) Acute toxicity of Roundup (technical) to atlantic
oyster (Crassostrea virginica). Wareham, Massachusetts, Bionomics
(Unpublished report submitted by Monsanto Ltd).
Bionomics (1973b) Acute toxicity of Roundup (technical) to grass
shrimp (Palaemonetes vulgaris) and fiddler crab (Uca pugilator).
Wareham, Massachusetts, Bionomics (Unpublished report submitted by
Monsanto Ltd).
Bowmer KH, Boulton PM, Short DL, & Higgins ML (1986)
Glyphosate-sediment interactions and phytotoxicity in turbid water.
Pestic Sci, 17(2): 79-88.
Bozeman J, Koopman B, & Bitton G (1989) Toxicity testing using
immobilized algae. Aquat Toxicol, 14(4): 345-352.
Buhl KJ & Faerber NL (1989) Acute toxicity of selected herbicides
and surfactants to larvae of the midge Chironomus riparius. Arch
Environ Contam Toxicol, 18(4): 530-536.
Bunyatyan YA & Gevorgyan AA (1984) [Determination of the active
ingredient of Roundup and its metabolite in the environment.] Gig i
Sanit, 5: 43-44 (in Russian).
Burns AJ (1983) Liquid chromatographic determination of glyphosate
technical and its formulation: collaborative study. J Assoc Off Anal
Chem, 66(5): 1214-1219.
Carlisle SM & Trevors JT (1986a) Effect of the herbicide glyphosate
on nitrification, denitrification, and acetylene reduction in soil.
Water Air Soil Pollut, 29(2): 189-203.
Carlisle SM & Trevors JT (1986b) Effect of the herbicide glyphosate
on respiration and hydrogen consumption in soil. Water Air Soil
Pollut, 27(3/4): 391-401.
Chakravarty P & Chatarpaul L (1990a) Non-target effects of
herbicides: I. Effect of glyphosate and hexazinone on soil microbial
activity, microbial population, and in vitro growth of
ectomycorrhizal fungi. Pestic Sci, 28(3): 233-242.
Chakravarty P & Chatarpaul L (1990b) Non-target effect of
herbicides: II. The influence of glyphosate on ectomycorrhizal
symbiosis of red pine (Pinus resinosa) under greenhouse and field
conditions. Pestic Sci, 28(3): 243-248.
Chan KY & Leung SC (1986) Effects of paraquat and glyphosate on
growth, respiration, and enzyme activity of aquatic bacteria. Bull
Environ Contam Toxicol, 36(1): 52-59.
CIT (1991a) EXP 30578 - Acute oral toxicity in rats (Study No. 7076
TAR). Miserey, France, Centre International de Toxicologie (CIT)
(Unpublished report submitted by Rhône Poulenc, Lyon, France).
CIT (1991b) EXP 30578 - Acute dermal toxicity in rats (Study No.
7077 TAR). Miserey, France, Centre International de Toxicologie
(CIT) (Unpublished report submitted by Rhône Poulenc, Lyon, France).
CIT (1991c) EXP 30578 - Acute dermal irritation in rabbits (Study
No. 7079 TAL). Miserey, France, Centre International de Toxicologie
(CIT) (Unpublished report submitted by Rhône Poulenc, Lyon, France).
CIT (1991d) EXP 30578 - Acute eye irritation in rabbits (Study No.
7078 TAL). Miserey, France, Centre International de Toxicologie
(CIT) (Unpublished report submitted by Rhône Poulenc, Lyon, France).
Cowell JE, Kunstman JL, Nord PJ, Steinmetz JR, & Wilson GR (1986)
Validation of an analytical residue method for analysis of
glyphosate and metabolite: an interlaboratory study. J Agric Food
Chem, 34(6): 955-960.
Deyrup CL, Chang SM, Weintraub RA, & Moye HA (1985) Simultaneous
esterification and acylation of pesticides for analysis by gas
chromatography. 1. Derivatization of glyphosate and
(aminomethyl)phosphonic acid with fluorinated
alcohols-perfluorinated anhydrides. J Agric Food Chem, 33(5):
944-947.
Dickson SJ, Meinhold RH, Beer ID, & Koelmeyer TD (1988) Rapid
determination of glyphosate in postmortem specimens using 31P NMR. J
Anal Toxicol, 12(5): 284-286.
D'Anieri P, Leslie DM, & McCormack ML (1987) Small mammals in
glyphosate-treated clearcuts in Northern Maine. Can Field-Nat,
101(4): 547-550.
Dubelman S (1988) Glyphosate. Anal Methods Pestic Plant Growth
Regul, 16: 69-82.
Duke SO & Hoagland RE (1981) Effects of glyphosate on the metabolism
of phenolic compounds: VII. Root-fed amino acids and glyphosate
toxicity in soybean (Glycine max) seedlings. Weed Sci, 29(3):
297-302.
Eberbach PL & Douglas LA (1983) Persistence of glyphosate in a sandy
loam. Soil Biol Biochem, 15(4): 485-487.
Eberbach PL & Douglas LA (1989) Herbicide effects on the growth and
nodulation potential of Rhizobium trifolii with Trifolium
subterraneum L. Plant Soil, 119: 15-23.
Edwards WM, Triplett GB, & Kramer RM (1980) A watershed study of
glyphosate transport in runoff. J Environ Qual, 9(4): 661-664.
EG & G Bionomics (1975) Chronic toxicity of glyphosate to the
fathead minnow (Pimephales promelas Rafinesque). Aquatic
Toxicology Laboratory. Wareham, Massachusetts, EG & G Bionomics
(Unpublished report submitted by Monsanto Ltd).
EG & G Bionomics (1978a) Toxicity of seven test materials to the
marine alga, Skeletonema costatum. Marine Research Laboratory,
Pensacola, Florida, EG & G Bionomics (Unpublished report No.
BP-78-4-031 submitted by Monsanto Ltd).
EG & G Bionomics (1978b) Toxicity of seven test materials to
sheepshead minnows, Cyprinodon variegatus. Marine Research
Laboratory, Pensacola, Florida, EG & G Bionomics (Unpublished report
No. BP-78-4-029 submitted by Monsanto Ltd).
EG & G Bionomics (1978c) Toxicity of seven test materials to mysid
shrimp, Mysidopsis bahia. Marine Research Laboratory, Pensacola,
Florida, EG & G Bionomics (Unpublished report No. BP-78-4-032
submitted by Monsanto Ltd).
EG & G Bionomics (1978d) Toxicity of seven test materials to the
white sea urchin, Tripneustes esculentus. Marine Research
Laboratory, Pensacola, Florida, EG & G Bionomics (Unpublished report
No. BP-78-4-030 submitted by Monsanto Ltd).
EG & G Bionomics (1980a) Acute toxicity of Roundup to channel
catfish (Ictalurus punctatus). Aquatic Toxicology Laboratory.
Wareham, Massachusetts, EG & G Bionomics (Unpublished report No.
BW-80-4-641 submitted by Monsanto Ltd).
EG & G Bionomics (1980b) Acute toxicity of Roundup to bluegill
(Lepomis macrochirus). Aquatic Toxicology Laboratory. Wareham,
Massachusetts, EG & G Bionomics (Unpublished report No. BW-80-4-634
submitted by Monsanto Ltd).
EG & G Bionomics (1980c) Acute toxicity of Roundup to the rainbow
trout (Salmo gairdnerii). Aquatic Toxicology Laboratory. Wareham,
Massachusetts, EG & G Bionomics (Unpublished report No. BW-80-4-635
submitted by Monsanto Ltd).
EG & G Bionomics (1980d) Acute toxicity of Roundup to the fathead
minnow (Pimephales promelas). Aquatic Toxicology Laboratory.
Wareham, Massachusetts, EG & G Bionomics (Unpublished report No.
BW-80-4-653 submitted by Monsanto Ltd).
EG & G Bionomics (1980e) Acute toxicity of Roundup to the water flea
(Daphnia magna). Aquatic Toxicology Laboratory. Wareham,
Massachusetts, EG & G Bionomics (Unpublished report No. BW-80-4-636
submitted by Monsanto Ltd).
EG & G Bionomics (1980f) Acute toxicity of Roundup to the water flea
(Daphnia magna) with and without continuous aeration. Aquatic
Toxicology Laboratory. Wareham, Massachusetts, EG & G Bionomics
(Unpublished report No. BW-80-6-690 submitted by Monsanto Ltd).
EG & G Bionomics (1980g) Acute toxicity of Roundup to the rainbow
trout (Salmo gairdnerii) with continuous aeration and without
aeration. Aquatic Toxicology Laboratory. Wareham, Massachusetts,
EG & G Bionomics (Unpublished report No. BW-80-6-686 submitted by
Monsanto Ltd).
Estok D, Freedman B, & Boyle D (1989) Effects of the herbicides
2,4-D, glyphosate, hexazinone, and triclopyr on the growth of three
species of ectomycorrhizal fungi. Bull Environ Contam Toxicol,
42(6): 835-839.
Evans DD & Batty MJ (1986) Effects of high dietary concentrations of
glyphosate (Roundup) on a species of bird, marsupial and rodent
indigenous to Australia. Environ Toxicol Chem, 5: 399-401.
EVS Consultants (1986a) Acute toxicity of Roundup herbicide to
rainbow trout. Seattle, Washington, EVS Consultants (Unpublished
report submitted by Monsanto Ltd).
EVS Consultants (1986b) Acute toxicity of Roundup herbicide to
chinook salmon. Seattle, Washington, EVS Consultants (Unpublished
report submitted by Monsanto Ltd).
EVS Consultants (1986c) Acute toxicity of Roundup herbicide to coho
salmon. Seattle, Washington, EVS Consultants (Unpublished report
submitted by Monsanto Ltd). EVS Consultants (1986d) Seawater
challenge testing of coho salmon smolts following Roundupr herbicide
exposure. Seattle, Washington, EVS Consultants (Unpublished report
submitted by Monsanto Ltd).
FAO/WHO (1986a) Pesticide residues in food - Evaluations 1986. Part
I - Residues. Joint Meeting of the FAO Panel of Experts Residues in
Food and the Environment and the WHO Expert Group on Pesticide
Residues, Rome, 29 September-8 October 1986. Rome, Food and
Agriculture Organization of the United Nations (FAO Plant Production
and Protection Paper 78/1).
FAO/WHO (1986b) Pesticide residues in food - Evaluations 1986. Part
II - Toxicology. Joint Meeting of the FAO Panel of Experts Residues
in Food and the Environment and the WHO Expert Group on Pesticide
Residues, Rome, 29 September-8 October 1986. Rome, Food and
Agriculture Organization of the United Nations (FAO Plant Production
and Protection Paper 78/2).
FAO/WHO (1987) Pesticide residues in food - Evaluations 1987. Part I
- Residues. Joint Meeting of the FAO Panel of Experts Residues in
Food and the Environment and the WHO Expert Group on Pesticide
Residues, Geneva, 21-30 September 1987. Rome, Food and Agriculture
Organization of the United Nations (FAO Plant Production and
Protection Paper 86/1).
FAO/WHO (1988) Pesticide residues in food - Evaluations 1988. Part I
- Residues. Joint Meeting of the FAO Panel of Experts Residues in
Food and the Environment and the WHO Expert Group on Pesticide
Residues, Rome, 19-28 September 1988. Rome, Food and Agriculture
Organization of the United Nations (FAO Plant Production and
Protection Paper 93/1).
FDRL (1988a) Primary dermal irritation study of glyphosate
batch/lot/nbr No. XLI-55 in New Zealand White rabbits (Study No.
88.2053.010). Waverly, New York, Food & Drug Research Laboratories
(Unpublished report submitted by Monsanto Ltd - Monsanto study No.
FD-88-29).
FDRL (1988b) Acute dermal toxicity study of glyphosate batch/lot/nbr
No. XLI-55 in New Zealand White rabbits (Study No. 88.2053.008).
Waverly, New York, Food & Drug Research Laboratories (Unpublished
report submitted by Monsanto Ltd - Monsanto study No. FD-88-29).
FDRL (1988c) Primary eye irritation study of glyphosate
batch/lot/nbr No. XLI-55 in New Zealand White rabbits (Study No.
88.2053.009). Waverly, New York, Food & Drug Research Laboratories
(Unpublished report submitted by Monsanto Ltd - Monsanto study No.
FD-88-29).
FDRL (1988d) Acute oral toxicity study of glyphosate batch/lot/nbr
No. XLI-55 in Sprague-Dawley rats (Study No. 88.2053.007). Waverly,
New York, Food & Drug Research Laboratories (Unpublished report
submitted by Monsanto Ltd - Monsanto study No. FD-88-29).
Feller MC (1989) Effects of forest herbicide applications on
streamwater chemistry in southwestern British Columbia. Water Resour
Bull, 25(3): 607-616.
Feng JC & Thompson DG (1990) Fate of glyphosate in a Canadian forest
watershed. 2. Persistence in foliage and soils. J Agric Food Chem,
38(4): 1118-1125.
Feng JC, Thompson DG, & Reynolds PE (1990) Fate of glyphosate in a
Canadian forest watershed. 1. Aquatic residues and off-target
deposit assessment. J Agric Food Chem, 38(4): 1110-1118.
Folmar LC, Sanders HO, & Julin AM (1979) Toxicity of the herbicide
glyphosate and several of its formulations to fish and aquatic
invertebrates. Arch Environ Contam Toxicol, 8: 269-278.
Franz TJ (1983) Evaluation of the percutaneous absorption of Roundup
formulations in man using an in vitro technique (Study No.
UW-81-346). Seattle, Washington, University of Washington, School of
Medicine (Unpublished report submitted by Monsanto Ltd).
Friestad HO & Bronstad JO (1985) Improved polarographic method for
determination of glyphosate herbicide in crops, soils, and water. J
Assoc Off Anal Chem, 68(1): 76-79.
Gauch R, Leuenberger U, & Müller U (1989) [Determination of
glyphosate herbicide and its main metabolite aminomethylphosphonic
acid (AMPA) in drinking water by HPLC.] Z Lebensm.unters Forsch,
188(1): 36-38 (in German).
Glass RL (1981) Colorimetric determination of glyphosate in water
after oxidation to orthophosphate. Anal Chem, 53(6): 921-923.
Glass RL (1983) Liquid chromatographic determination of glyphosate
in fortified soil and water. J Agric Food Chem, 31(2): 280-282.
Glass RL (1987) Adsorption of glyphosate by soils and clay minerals.
J Agric Food Chem, 35: 497-500.
Goldsborough LG & Beck AE (1989) Rapid dissipation of glyphosate in
small forest ponds. Arch Environ Contam Toxicol, 18(4): 537-544.
Goldsborough LG & Brown DJ (1988) Effect of glyphosate (Roundup
formulation) on periphytic algal photosynthesis. Bull Environ Contam
Toxicol, 41(2): 253-260.
Gómez MA & Sagardoy MA (1985) [Influence of glyphosate herbicide on
the microflora and mesofauna of a sandy soil in a semi-arid region.]
Rev Latinoam Microbiol, 27: 351-357 (in Spanish).
Gopalan HNB & Njagi GDE (1981) Mutagenicity testing of pesticides.
III. Drosophila: recessive sex-linked lethals. Genetics,
97(Suppl): 544 (Abstract).
Gougler JA & Geiger DR (1981) Uptake and distribution of
N-phosphonomethylglycine in sugar beet plants. Plant Physiol, 68:
668-672.
Gougler JA & Geiger DR (1984) Carbon partitioning and herbicide
transport in glyphosate-treated sugarbeet (Beta vulgaris). Weed
Sci, 32(4): 546-551.
Guinivan RA, Thompson NP, & Wheeler WB (1982) Derivatization and
cleanup improvements in determination of residues of glyphosate and
aminomethylphosphonic acid in blueberries. J Assoc Off Anal Chem,
65(1): 35-39.
Haag WR & Yao CCD (1992) Rate constants for reaction of hydroxyl
radicals with several drinking water contaminants. Environ Sci
Technol, 26: 1005-1013.
Hallas LE, Adams WJ, & Heitkamp MA (1992) Glyphosate degradation by
immobilized bacteria: field studies with industrial wastewater
effluent. Appl Environ Microbiol, 51: 432-434.
Hallberg GL (1989) Pesticide pollution of groundwater in the humid
United States. Agric Ecosyst Environ, 26: 299-367.
Hance RJ (1976) Adsorption of glyphosate by soils. Pestic Sci, 7:
363-366.
Hartman WA & Martin DB (1984) Effect of suspended bentonite clay on
the acute toxicity of glyphosate to Daphnia pulex and Lemna
minor. Bull Environ Contam Toxicol, 33(3): 355-361.
Hartman WA & Martin DB (1985) Effects of four agricultural
pesticides on Daphnia pulex, Lemna minor, and Potamogeton
pectinatus. Bull Environ Contam Toxicol, 35(5): 646-651.
Hazleton Laboratories, Inc. (1973a) Eight-day dietary LC50 -
bobwhite quail. Final report. Vienna, Virginia, Hazleton
Laboratories, Inc. (Unpublished report submitted by Monsanto Ltd).
Hazleton Laboratories, Inc. (1973b) Eight-day dietary LC50 -
mallard ducks. Final report. Vienna, Virginia, Hazleton
Laboratories, Inc. (Unpublished report submitted by Monsanto Ltd).
Hazleton Lab. Inc. (1988a) Metabolism study of synthetic
13C/14C-labeled glyphosate and aminomethylphosphonic acid in
laying hens, Part I. Hazleton Laboratories Inc. Project No. HLA
6103-112. Madison, Wisconsin, Monsanto Agricultural Company, Report
No. MSL-7591. Unpublished report supplied by Monsanto Ltd.
Hazleton Lab. Inc. (1988b) Metabolism study of synthetic
13C/14C-labeled glyphosate and aminomethylphosphonic acid in
lactating goats, Part I. Hazleton Laboratories Inc. Project No. HLA
6103-113. Madison, Wisconsin, Monsanto Agricultural Company, Report
No. MSL-7586. Unpublished report supplied by Monsanto Ltd.
Heinonen-Tanski H (1989) The effect of temperature and liming on the
degradation of glyphosate in two arctic forest soils. Soil Biol
Biochem, 21(2): 313-317.
Heinonen-Tanski H, Rosenberg C, Siltanen H, Kilpi S, & Simojoki P
(1985) The effect of the annual use of pesticides on soil
microorganisms, pesticide residues in the soil and barley yields.
Pestic Sci, 16(4): 341-348.
Hensley DL, Beuerman DSN, & Carpenter PL (1978) The inactivation of
glyphosate by various soils and metal salts. Weed Res, 18: 287-291.
Hernando F, Royuela M, Muñoz-Rueda A, & Gonzalez-Murua C (1989)
Effect of glyphosate on the greening process and photosynthetic
metabolism in Chlorella pyrenoidosa. J Plant Physiol, 136: 26-31.
Hildebrand LD, Sullivan DS, & Sullivan TP (1980) Effects of Roundup
herbicide on populations of Daphnia magna in a forest pond. Bull
Environ Contam Toxicol, 25(3): 353-357.
Hildebrand LD, Sullivan DS, & Sullivan TP (1982) Experimental
studies of rainbow trout populations exposed to field applications
of Roundup herbicide. Arch Environ Contam Toxicol, 11(1): 93-98.
Hoffman DJ & Albers PH (1984) Evaluation of potential embryotoxicity
and teratogenicity of 42 herbicides, insecticides, and petroleum
contaminants to mallard eggs. Arch Environ Contam Toxicol, 13(1):
15-27.
Holdway DA & Dixon DG (1988) Acute toxicity of permethrin or
glyphosate pulse exposure to larval white sucker (Catostomus
commersoni) and juvenile flagfish (Jordanella floridae) as
modified by age and ration level. Environ Toxicol Chem, 7(1): 63-68.
Holtby LB & Baillie SJ (1989a) Effects of the herbicide Roundup
(glyphosate) on periphyton in Carnation Creek, British Columbia. In:
Reynolds P ed. Proceedings of the Carnation Creek Workshop, Nanaimo,
7-10 December 1987. Victoria, British Columbia, Forestry
Canada/British Columbia Ministry of Forests, pp 224-231.
Holtby LB & Baillie SJ (1989b) Effects of the herbicide Roundup on
coho salmon fingerlings in an over-sprayed tributary of Carnation
Creek, British Columbia. In: Reynolds P ed. Proceedings of the
Carnation Creek Workshop, Nanaimo, 7-10 December 1987. Victoria,
British Columbia, Forestry Canada/British Columbia Ministry of
Forests, pp 273-285.
HRC (1972) The acute contact and oral toxicities of CP67573 and MON
2139 to worker honey bees. Huntingdon, UK, Huntingdon Research
Centre (Unpublished report submitted by Monsanto Ltd).
HRC (1977) The acute toxicity of glyphosate to harlequin fish
(Rasbora heteromorpha). Huntingdon, UK, Huntingdon Research Centre
(Unpublished report submitted by Monsanto Ltd).
HRC (1988) The acute oral toxicity of ICIA0224 to the bobwhite
quail. Huntingdon, UK, Huntingdon Research Centre (Unpublished
report No. ISN 177/881062 submitted by Monsanto Ltd).
IBR (1991a) Final report - Acute toxicity in earthworms according to
OECD 207-test article: "Technical isopropylamin salt of glyphosate =
MON 0139" (Project No. 80-91-2078-00-90). Hannover, International
Bioresearch (Unpublished report submitted by Monsanto Ltd).
IBR (1991b) Final report - Acute toxicity in earthworms according to
OECD 207-test article: "Roundup" (Project No. 80-91-0599-00-90).
Hannover, International Bioresearch (Unpublished report submitted by
Monsanto Ltd).
IET (1978) Microbial mutagenicity testing on CP67573 (glyphosate).
Mitsukaido, Japan, Institute of Environmental Toxicology (IET)
(Study sponsor Monsanto Ltd - Monsanto unpublished report No.
ET-78-241).
IFU (1990) [Side-effects of Swing on the ground-beetle Poecilus
cupreus Bonnelli under laboratory conditions (Project No.
110/01-Pc).] Unpublished report of Institute for Environmental
Analysis and Biotechnology, Niefern-Öschelbron, supplied by Monsanto
Ltd (in German).
Inveresk Research Int. (1988a) Compound No. 3607: Acute oral
toxicity (limit) test in rats (IRI project No. 241496). Musselburgh,
Scotland, Inveresk Research International (Unpublished report No.
5585 submitted by Cheminova A/S, Denmark).
Inveresk Research Int. (1988b) Compound No. 3607: Acute dermal
toxicity (limit) test in rats (IRI project No.241496). Musselburgh,
Scotland, Inveresk Research International (Unpublished report No.
5586 submitted by Cheminova A/S, Denmark).
Inveresk Research Int. (1988c) Compound 3707: Primary skin
irritation in rabbits (IRI project No. 241496). Musselburgh,
Scotland, Inveresk Research International (Unpublished report No.
5587 submitted by Cheminova A/S, Denmark).
Inveresk Research Int. (1988d) Compound No. 3607: Magnusson-Kligman
Maximisation test in guinea pigs (IRI project No. 241496).
Musselburgh, Scotland, Inveresk Research International (Unpublished
report No. 5589 submitted by Cheminova A/S, Denmark).
Inveresk Research Int. (1989a) Glyphosate technical: acute oral
toxicity (limit) test in rats (IRI project No. 243268). Musselburgh,
Scotland, Inveresk Research International (Unpublished report No.
5883 submitted by Cheminova A/S, Denmark).
Inveresk Research Int. (1989b) Glyphosate technical:
Magnusson-Kligman Maximisation test in guinea pigs (IRI project No.
243268). Musselburgh, Scotland, Inveresk Research International
(Unpublished report No. 5587 submitted by Cheminova A/S, Denmark).
Inveresk Research Int. (1989c) Glyphosate technical: acute dermal
toxicity (limit) test in rats (IRI project No. 243268). Musselburgh,
Scotland, Inveresk Research International (Unpublished report No.
5884 submitted by Cheminova A/S, Denmark).
Inveresk Research Int. (1989d) Glyphosate soluble liquid formulation
(code No. 3607) - Acute inhalation toxicity study in rats (limit
test) (IRI project No. 640023). Musselburgh, Scotland, Inveresk
Research International (Unpublished report No. 5603 submitted by
Cheminova A/S, Denmark).
Inveresk Research Int. (1989e) Glyphosate technical: Primary skin
irritation in rabbits (IRI project No. 243268). Musselburgh,
Scotland, Inveresk Research International (Unpublished report No.
5885 submitted by Cheminova A/S, Denmark).
Inveresk Research Int. (1989f) Glyphosate technical: Primary eye
irritation in rabbits (IRI project No. 243268). Musselburgh,
Scotland, Inveresk Research International (Unpublished report No.
5886 submitted by Cheminova A/S, Denmark).
Inveresk Research Int. (1989g) Compound No. 3607: Primary eye
irritation in rabbits (IRI project No. 241496). Musselburgh,
Scotland, Inveresk Research International (Unpublished report No.
5588 submitted by Cheminova A/S, Denmark).
IRDC (1980a) Test article - Technical glyphosate: Dominant lethal
study in mice (Study No. 401-064). Mattawan, Michigan, International
Research and Development Corporation (Unpublished report submitted
Monsanto Ltd, Reference No. IR-79-014).
IRDC (1980b) Test article - Technical glyphosate: Teratology study
in rats (Study No. 401-054). Mattawan, Michigan, International
Research and Development Corporation (Unpublished report submitted
by Monsanto Ltd, Reference No. IR-79-016).
IRDC (1980c) Test article - Technical glyphosate: Teratology study
in rabbits (Study No. 401-056). Mattawan, Michigan, International
Research and Development Corporation (Unpublished report submitted
by Monsanto Ltd, Reference No. IR-79-018).
IRDC (1982) Test article - Glyphosate technical: 21-day dermal
toxicity study in rabbits (Study No. 401-168). Mattawan, Michigan,
International Research and Development Corporation (Unpublished
report submitted by Monsanto Ltd, Reference No. IR-81-195).
IRPTC (1991) Data profile on glyphosate. Geneva, International
Register for Potentially Toxic Chemicals, United Nations Environment
Programme.
Jacob GS, Garbow JR, Hallas LE, Kimack NM, Kishore GM, & Schaefer J
(1988) Metabolism of glyphosate in Pseudomonas sp. strain LBr.
Appl Environ Microbiol, 54(12): 2953-2958.
Jauhiainen A, Räsänen K, Sarantila R, Nuutinen J, & Kangas J (1991)
Occupational exposure of forest workers to glyphosate during brush
saw spraying work. Am Ind Hyg Assoc J, 52(2): 61-64.
Kishore GM & Jacob GS (1987) Degradation of glyphosate by
Pseudomonas sp. PG2982 via a sarcosine intermediate. J Biol Chem,
262(25): 12164-12168.
Kitchen LM, Witt WW, & Rieck CE (1981a) Inhibition of chlorophyll
accumulation by glyphosate. Weed Sci, 29(4): 513-516.
Kitchen LM, Witt WW, & Rieck CE (1981b) Inhibition of
delta-aminolevulinic acid synthesis by glyphosate. Weed Sci, 29(5):
571-577.
Knapek R, Kobes S, & Kita I (1986) [Toxicological evaluation of
N-phosphono-methylglycine.] Z gesamte Hyg, 32(9): 537-539 (in
German).
Konar SK & Roy DN (1990) Method for the determination of residues of
the herbicide glyphosate and its principal metabolite,
aminomethylphosphonic acid, in plant materials by nitrogen-selective
gas chromatography. Anal Chim Acta, 229(2): 277-280.
Kreutzweiser DP, Kingsbury PD, & Feng JC (1989) Drift response of
stream invertebrates to aerial applications of glyphosate. Bull
Environ Contam Toxicol, 42(3): 331-338.
Lavy TL, Cowell JE, Steinmetz JR, & Massey JH (1992) Conifer
seedling nursery worker exposure to glyphosate. Arch Environ Contam
Toxicol, 22: 6-13.
Lethbridge G, Bull AT, & Burns RG (1981) Effects of pesticides on
1,3-ß-glucanase and urease activities in soil in the presence and
absence of fertilisers, lime and organic materials. Pestic Sci,
12(2): 147-155.
LISEC (1989a) Alga, growth inhibition test. Effect of MON 2139 on
the growth of Selenastrum capricornutum. Bokrijk, Belgium, LISEC,
Study Centre for Ecology and Forestry (Unpublished report submitted
by Monsanto Ltd).
LISEC (1989b) Alga, growth inhibition test. Effect of MON 8755 on
the growth of Selenastrum capricornutum. Bokrijk, Belgium, LISEC,
Study Centre for Ecology and Forestry (Unpublished report submitted
by Monsanto Ltd).
LISEC (1990a) Degradation: Biological oxygen demand. Bokrijk,
Belgium, LISEC, Study Centre for Ecology and Forestry (Unpublished
report submitted by Monsanto Ltd).
LISEC (1990b) Degradation: Chemical oxygen demand. Bokrijk, Belgium,
LISEC, Study Centre for Ecology and Forestry (Unpublished report
submitted by Monsanto Ltd). Liu C-M, McLean PA, Sookdeo CC, & Cannon
FC (1991) Degradation of the herbicide glyphosate by members of the
family Rhizobiaceae. Appl Environ Microbiol, 57: 1799-1804.
Lockhart WL, Billeck BN, & Baron CL (1989) Bioassays with a floating
aquatic plant (Lemna minor) for effects of sprayed and dissolved
glyphosate. Hydrobiologia, 188/189: 353-359.
Lundgren LN (1986) A new method for the determination of glyphosate
and (aminomethyl)phosphonic acid residues in soils. J Agric Food
Chem, 34(3): 535-538.
Lund-Hoie K & Friestad HO (1986) Photodegradation of the herbicide
glyphosate in water. Bull Environ Contam Toxicol, 36(5): 723-729.
Madhun YA, Young JL, & Freed VH (1986) Binding of herbicides by
water-soluble organic materials from soil. J Environ Qual, 15(1):
64-68.
Maibach HI (1983) (a) Elimination of 14C-glyphosate in Rhesus
monkeys following a single dose. (b) Percutaneous absorption in
Roundup formulation in Rhesus monkeys following a single oral dose
(Study No. MA-81-349). San Francisco, California, California School
of Medicine (Unpublished report submitted by Monsanto Ltd).
Maibach HI (1986) Irritation, sensitive, photoirritation and
photosensitization assays with a glyphosate herbicide. Contact
Dermatitis, 15: 152-156.
Malcolm Pirnie Inc. (1987a) The toxicity of glyphosate technical to
Anabaena flos-aquae. White Plains, New York, Malcolm Pirnie Inc.
(Unpublished report submitted by Monsanto Ltd).
Malcolm Pirnie Inc. (1987b) The toxicity of glyphosate technical to
Skeletonema costatum. White Plains, New York, Malcolm Pirnie Inc.
(Unpublished report submitted by Monsanto Ltd).
Malcolm Pirnie Inc. (1987c) The toxicity of glyphosate technical to
Navicula pelliculosa. White Plains, New York, Malcolm Pirnie Inc.
(Unpublished report submitted by Monsanto Ltd).
Malcolm Pirnie Inc. (1987d) The toxicity of glyphosate technical to
Selenastrum capricornutum. White Plains, New York, Malcolm Pirnie
Inc. (Unpublished report submitted by Monsanto Ltd).
Malcolm Pirnie Inc. (1987e) The toxicity of glyphosate technical to
Lemna gibba. White Plains, New York, Malcolm Pirnie Inc.
(Unpublished report submitted by Monsanto Ltd).
Marcotte AL, Bradley M, & Hughley DH ed. (1977) Pesticide analytical
manual. Washington, DC, Food and Drug Administration.
Marrs RH, Williams CT, Frost AJ, & Plant RA (1989) Assessment of the
effects of herbicide spray drift on a range of plant species of
conservation interest. Environ Pollut, 59(1): 71-86.
Marsh JAP (1985) Effects of herbicide-fertilizer interactions on
nitrogen and phosphorus transformations and herbicide persistence in
soil. Pestic Sci, 16(1): 93-100.
Marsh JAP, Davies HA, & Grossbard E (1977) The effect of herbicides
on respiration and transformation of nitrogen in two soils I.
Metribuzin and glyphosate. Weed Res, 17: 77-82.
Martinez TT & Brown K (1991) Oral and pulmonary toxicology of the
surfactant used in Roundup herbicide. Proc West Pharmacol Soc, 34:
43-46.
Martinez TT, Long WC, & Hiller R (1990) Comparison of the toxicology
of the herbicide Roundup by oral and pulmonary routes of exposure.
Proc West Pharmacol Soc, 33: 193-197.
Mekwatanakarn P & Sivasithamparam K (1987) Effect of certain
herbicides on saprophytic survival and biological suppression of the
take-all fungus. New Phytol, 106: 153-159.
Miles CJ & Moye HA (1988a) Postcolumn photolysis of pesticides for
fluorometric determination by high-performance liquid
chromatography. Anal Chem, 60(3): 220-226.
Miles CJ & Moye HA (1988b) Extraction of glyphosate herbicide from
soil and clay minerals and determination of residues in soils. J
Agric Food Chem, 36(3): 486-491.
Miles CJ, Wallace LR, & Moye HA (1986) Determination of glyphosate
herbicide and (aminomethyl)phosphonic acid in natural waters by
liquid chromatography using pre-column fluorogenic labeling with
9-fluorenylmethyl chloroformate. J Assoc Off Anal Chem, 69(3):
458-461.
Mitchell DG, Chapman PM, & Long TJ (1987) Acute toxicity of Roundup
and Rodeo herbicides to rainbow trout, chinook, and coho salmon.
Bull Environ Contam Toxicol, 39(6): 1028-1035.
Mitsukaido Laboratories (1986) Roundup herbicide: Acute oral
toxicity study in mice - Final report. Tokyo, Mitsukaido
Laboratories, Institute of Environmental Toxicology (Unpublished
report No. ET-85-377 submitted by Monsanto Ltd).
Monsanto (1972a) The degradation of MON-0573 in river and lake
bottom sediments and surface water. St. Louis, Missouri, Monsanto
Ltd (Unpublished report No. 276).
Monsanto (1972b) The rate of dissipation of MON-0573 in soil. St.
Louis, Missouri, Monsanto Ltd (Unpublished report No. 271).
Monsanto (1972c) The photolysis, run-off, and leaching of MON-0573
on or in soil. St. Louis, Missouri, Monsanto Ltd (Unpublished report
No. 258).
Monsanto (1972d) The degradation and metabolism of MON-0573 in soil.
St. Louis, Missouri, Monsanto Ltd (Unpublished report No. 269).
Monsanto (1973a) CP 67573 Residue and metabolism - The gross
metabolism of N-phos-phonomethylglycine-14C (CP 67573-14C) in
the laboratory rat following a single dose. St. Louis, Missouri,
Monsanto Commercial Products Company, Agricultural Division Research
Department (Unpublished report No. 297 submitted by Monsanto Ltd).
Monsanto (1973b) CP 67573 Residue and metabolism - The isolation and
identification of the metabolites of CP 67573=13C excreted by the
laboratory rat. St. Louis, Missouri, Monsanto Commercial Products
Company, Agricultural Division Research Department (Unpublished
report No. 306 submitted by Monsanto Ltd).
Monsanto (1973c) CP 67573 Residue and metabolism - The dynamics of
accumulation and depletion of orally ingested
N-phosphonomethylglycine-14C. St. Louis, Missouri, Monsanto
Commercial Products Company, Agricultural Division Research
Department (Unpublished report No. 309 submitted by Monsanto Ltd).
Monsanto (1973d) CP 67573 Residue and metabolism - The gross
distribution of N-phosphonomethylglycine-14C (CP 67573-14C) in
the rabbit. St. Louis, Missouri, Monsanto Commercial Products
Company, Agricultural Division Research Department (Unpublished
report No. 298 submitted by Monsanto Ltd).
Monsanto (1978a) Photodegradation and anaerobic aquatic metabolism
of glyphosate, N-phosphonomethylglycine. St. Louis, Missouri,
Monsanto Ltd (Unpublished report No. MSL-0598).
Monsanto (1978b) Solubility, volatility, adsorption, and partition
coefficients, leaching, and aquatic metabolism of MON 0573 and MON
0101. St. Louis, Missouri, Monsanto Ltd (Unpublished report No.
MSL-0207).
Monsanto (1978c) Final report on Salmonella mutagenicity assay of
glyphosate (sample no. 04) (Test No. LF-78-161). St. Louis,
Missouri, Monsanto Environmental Health Laboratory (Unpublished
report submitted by Monsanto Ltd).
Monsanto (1980) Results of a pond test with Roundup for a period of
three months. Burgwedel, Germany, Monsanto Ltd (Unpublished report
submitted by Ökolimna to Monsanto Ltd).
Monsanto (1981a) A reinvestigation of the static exposure of channel
catfish to 14C-labelled glyphosate, N-(phosphonomethyl)glycine.
St. Louis, Missouri, Monsanto Ltd (Unpublished report No. MSL-2056).
Monsanto (1981b) Acute oral toxicity of MON 0139 to rats (Study No.
800257). St. Louis, Missouri, Monsanto Environmental Health
Laboratory (Unpublished report submitted by Monsanto Ltd).
Monsanto (1981c) Acute dermal toxicity of MON 0139 to rabbits (Study
No. 800258). St. Louis, Missouri, Monsanto Environmental Health
Laboratory (Unpublished report submitted by Monsanto Ltd).
Monsanto (1981d) Primary skin irritation of MON 0139 to rabbits
(Study No. 800259). St. Louis, Missouri, Monsanto Environmental
Health Laboratory (Unpublished report submitted by Monsanto Ltd).
Monsanto (1981e) Primary eye irritation of MON 0139 to rabbits
(Study No. 800260). St. Louis, Missouri, Monsanto Environmental
Health Laboratory (Unpublished report submitted by Monsanto Ltd).
Monsanto (1983a) Dissipation of glyphosate in U.S. field soils
following direct application of Roundupr herbicide. St. Louis,
Missouri, Monsanto Ltd (Unpublished report No. MSL-3210).
Monsanto (1983b) CHO/HGPRT Gene mutation assay with glyphosate
(Study No. ML-83-155). St. Louis, Missouri, Monsanto Environmental
Health Laboratory (Unpublished report submitted by Monsanto Ltd).
Monsanto (1983c) The hepatocyte primary culture/DNA repair assay on
compound JJN-1020 (glyphosate) using rat hepatocytes in cultures
(Study No. AH-83-181). St. Louis, Missouri, Monsanto Environmental
Health Laboratory (Unpublished report submitted by Monsanto Ltd).
Monsanto (1983d) Acute inhalation toxicity of Roundup formulation to
male and female Sprague-Dawley rats (Study No. 810093; DMEH project
No. ML-81-2019. St. Louis, Missouri, Monsanto Environmental Health
Laboratory (Unpublished report submitted by Monsanto Ltd).
Monsanto (1983e) Four-week study of 33-1/33% use-dilution of Roundup
in water administered to male and female Sprague/Dawley rats by
inhalation (Project No. ML-83-015/EHL 830025). St. Louis, Missouri,
Monsanto Environmental Health Laboratory (Unpublished report
submitted by Monsanto Ltd).
Monsanto (1983f) in vivo bone marrow cytogenetics study of
glyphosate in Sprague-Dawley rats (Project No. ML-83-236). St.
Louis, Missouri, Monsanto Environmental Health Laboratory
(Unpublished report submitted by Monsanto Ltd).
Monsanto (1983g) Effects of glyphosate on rat bone marrow cells
(Study No. ML-83-160; EHL study No. 830082). St. Louis, Missouri,
Monsanto Environmental Health Laboratory (Unpublished report
submitted by Monsanto Ltd).
Monsanto (1983h) A study of the plasma and bone marrow levels of
glyphosate following intraperitoneal administration in the rat
(Study No. ML-83-218; EHL study No. 830109). St. Louis, Missouri,
Monsanto Environmental Health Laboratory (Unpublished report
submitted by Monsanto Ltd).
Monsanto (1985) Twelve month study of glyphosate administered by
gelatine capsule to Beagle dogs (Project No. ML-83-137). St. Louis,
Missouri, Monsanto Environmental Health Laboratory (Unpublished
report No. MSL 5069 submitted by Monsanto Ltd).
Monsanto (1987a) 90 day study of glyphosate administered in feed to
Sprague/Dawley rats (Project No. ML-86-351/EHL 86128). St. Louis,
Missouri, Monsanto Environmental Health Laboratory (Unpublished
report No. MSL-7575 submitted by Monsanto Ltd).
Monsanto (1987b) Acute inhalation study of Roundup L&G Ready-to-Use
(Study No. 86148; DMEH project No. ML-87-6). St. Louis, Missouri,
Monsanto Environmental Health Laboratory (Unpublished report
submitted by Monsanto Ltd).
Monsanto (1988a) Metabolism of glyphosate in Sprague-Dawley rats.
Part II. Identification, characterization, and quantitation of
glyphosate and its metabolites after intravenous and oral
administration. St. Louis, Missouri, Monsanto Ltd (Unpublished
report No. MSL-7206).
Monsanto (1988b) The metabolism of glyphosate in Sprague Dawley rats
- Part I. Excretion and tissue distribution of glyphosate and its
metabolites following intravenous and oral administration. St.
Louis, Missouri, Monsanto Environmental Health Laboratory/Monsanto
Life Sciences Research Center (Unpublished report No. MSL-7215
submitted by Monsanto Ltd).
Monsanto (1988c) Metabolism of 13C/14C-labeled glyphosate and
aminomethylphosphonic acid in laying hens, Part II. St. Louis,
Missouri, Monsanto Life Sciences Research Center (Unpublished report
No. MSL-7420 submitted by Monsanto Ltd). Monsanto (1988d) Metabolism
study of synthetic 13C/14C-labeled glyphosate and
aminomethylphosphonic acid in lactating goats, Part II. St. Louis,
Missouri, Monsanto Life Sciences Research Center (Unpublished report
No. MSL-7458 submitted by Monsanto Ltd).
Monsanto (1990a) Dissipation of glyphosate and aminomethylphosphonic
acid in forestry sites. St. Louis, Missouri, Monsanto Ltd
(Unpublished report No. MSL-9940).
Monsanto (1990b) Chronic study of glyphosate administered in feed to
albino rats (Project No. MSL-10495). St. Louis, Missouri, Monsanto
Environmental Health Laboratory (Unpublished report submitted by
Monsanto Ltd).
Monsanto (1990c) Two generation reproduction feeding study with
glyphosate in Sprague-Dawley rats (Study No. MSL-10387). St. Louis,
Missouri, Monsanto Environmental Health Laboratory (Unpublished
report submitted by Monsanto Ltd).
Morash R & Freedman B (1989) The effects of several herbicides on
the germination of seeds in the forest floor. Can J For Res, 19(3):
347-350.
Morgan MJ & Kiceniuk JW (1992) Response of rainbow trout to a two
month exposure to Vision, a glyphosate herbicide. Bull Environ
Contam Toxicol, 48: 772-780.
Morrison ML & Meslow EC (1984) Effects of the herbicide glyphosate
on bird community structure, Western Oregon. For Sci, 30(1): 95-106.
Moye HA & Deyrup CL (1984) A simple single-step derivatization
method for the gas chromatographic analysis of the herbicide
glyphosate and its metabolite. J Agric Food Chem, 32(2): 192-195.
Moye HA, Miles CJ, & Scherer SJ (1983) A simplified high-performance
liquid chromatographic residue procedure for the determination of
glyphosate herbicide and (aminomethyl)phosphonic acid in fruits and
vegetables employing postcolumn fluorogenic labeling. J Agric Food
Chem, 31(1): 69-72.
Müller MM, Rosenberg C, Siltanen H, & Wartiovaara T (1981) Fate of
glyphosate and its influence on nitrogen-cycling in two Finnish
agriculture soils. Bull Environ Contam Toxicol, 27(5): 724-730.
Murthy DVS, Irvine RL, & Hallas LE (1989) Principles of organism
selection for the degradation of glyphosate in a sequencing batch
reactor. In: 43rd Purdue Industrial Waste Conference Proceedings.
Chelsea, Lewis Publishers, Inc., pp 267-274.
NATEC (1990) Effect of the herbicide Roundup on the activity of the
microflora of soil (Project No. NA 90 9151). Unpublished report of
Institute for Technical Scientific Services GmbH, Hamburg, supplied
by Monsanto Ltd.
Newton M, Howard KM, Kelpsas BR, Danhaus R, Lottman CM, & Dubelman S
(1984) Fate of glyphosate in an Oregon forest ecosystem. J Agric
Food Chem, 32(5): 1144-1151.
NOTOX (1987a) Evaluation of the acute oral toxicity of MON 14478 in
the rat (Monsanto project No. NO-87-290). 's-Hertogenbosch, The
Netherlands, NOTOX - Toxicological Research & Consultancy
(Unpublished report No. NOTOX 0646/824 submitted by Monsanto Ltd).
NOTOX (1987b) Evaluation of the acute oral toxicity of MON 14478 in
the rat (Monsanto project No. NO-87-347). 's-Hertogenbosch, the
Netherlands, NOTOX - Toxicological Research & Consultancy
(Unpublished report No. NOTOX 0646/901 submitted by Monsanto Ltd).
NOTOX (1987c) Evaluation of the acute dermal toxicity of MON 14478
in the rat (Monsanto project No. NO-87-290). 's-Hertogenbosch, The
Netherlands, NOTOX - Toxicological Research & Consultancy
(Unpublished report No. NOTOX 0646/826 submitted by Monsanto Ltd).
NOTOX (1987d) Assessment of primary skin irritation/corrosion by MON
14478 in the rabbit (Monsanto project No. NO-87-290).
's-Hertogenbosch, The Netherlands, NOTOX - Toxicological Research &
Consultancy (Unpublished report No. NOTOX 0646/820 submitted by
Monsanto Ltd).
NOTOX (1987e) Assessment of acute eye irritation by MON 14478 in the
rabbit (Monsanto project No. NO-87-290). 's-Hertogenbosch, The
Netherlands, NOTOX - Toxicological Research & Consultancy
(Unpublished report No. NOTOX 0646/822 submitted by Monsanto Ltd).
NOTOX (1987f) Evaluation of the acute oral toxicity of MON 14477 in
the rat (Monsanto project No. NO-87-288). 's-Hertogenbosch, The
Netherlands, NOTOX - Toxicological Research & Consultancy
(Unpublished report No. NOTOX 0645/823 submitted by Monsanto Ltd).
NOTOX (1987g) Evaluation of the acute oral toxicity of MON 14477 in
the rat (Monsanto project No. NO-87-346). 's-Hertogenbosch, The
Netherlands, NOTOX - Toxicological Research & Consultancy
(Unpublished report No. NOTOX 0645/900 submitted by Monsanto Ltd).
NOTOX (1987h) Evaluation of the acute dermal toxicity of MON 14477
in the rat (Monsanto project No. NO-87-288). 's-Hertogenbosch, The
Netherlands, NOTOX - Toxicological Research & Consultancy
(Unpublished report No. NOTOX 0645/825 submitted by Monsanto Ltd).
NOTOX (1987i) Assessment of primary skin irritation by MON 14477 in
the rabbit (Monsanto project No. NO-87-288). 's-Hertogenbosch, The
Netherlands, NOTOX - Toxicological Research & Consultancy
(Unpublished report No. NOTOX 0645/819 submitted by Monsanto Ltd).
NOTOX (1987j) Assessment of acute eye irritation by MON 14477 in the
rabbit (Monsanto project No. NO-87-288). 's-Hertogenbosch, The
Netherlands, NOTOX - Toxicological Research & Consultancy
(Unpublished report No. NOTOX 0645/821 submitted by Monsanto Ltd).
NOTOX (1988) Acute oral toxicity of glyphosate in the rat.
's-Hertogenbosch, The Netherlands, NOTOX - Toxicological Research &
Consultancy (Unpublished report No. RCC NOTOX 1111/1428 submitted by
Agrichem B.V., The Netherlands).
NOTOX (1989a) Acute eye irritation/corrosion study with Glyposaat
360 g/l in rabbits. 's-Hertogenbosch, The Netherlands, NOTOX -
Toxicological Research & Consultancy (Unpublished report No. RCC
NOTOX 1268/171 (002082) submitted by Luxan B.V., The Netherlands).
NOTOX (1989b) Assessment of acute dermal toxicity with Glyposaat
360 g/l in the rat. 's-Hertogenbosch, The Netherlands, NOTOX -
Toxicological Research & Consultancy (Unpublished report No. RCC
NOTOX 1268/1718 (002071) submitted by Luxan B.V., The Netherlands).
NOTOX (1989c) Assessment of acute oral toxicity with Glyposaat
360 g/l in the rat. 's-Hertogenbosch, The Netherlands, NOTOX -
Toxicological Research & Consultancy (Unpublished report No. RCC
NOTOX 1268/1715 (002069) submitted by Luxan B.V., The Netherlands).
NOTOX (1989d) Primary skin irritation/corrosion study with Glyposaat
360 g/l in rabbits (4-hour semi-occlusive application).
's-Hertogenbosch, The Netherlands, NOTOX - Toxicological Research &
Consultancy (Unpublished report No. RCC NOTOX 1268/1716 submitted by
Luxan B.V., The Netherlands).
NOTOX (1990a) Acute eye irritation/corrosion study with Agrichem
glyposaat 2 (RCC NOTOX substance 11934) in the rabbit.
's-Hertogenbosch, The Netherlands, NOTOX - Toxicological Research &
Consultancy (Unpublished report No. RCC NOTOX 042492 submitted by
Agrichem B.V., The Netherlands).
NOTOX (1990b) Primary skin irritation/corrosion study with Agrichem
glyposaat 2 (RCC NOTOX substance 11961) in rabbits (4-hour
semi-occlusive application). 's-Hertogenbosch, The Netherlands,
NOTOX - Toxicological Research & Consultancy (Unpublished report No.
RCC NOTOX 042525 submitted by Agrichem B.V., The Netherlands).
NOTOX (1990c) Acute eye irritation/corrosion study with Agrichem
glyposaat B (RCC NOTOX substance 4175) in the rabbit.
's-Hertogenbosch, The Netherlands, NOTOX - Toxicological Research &
Consultancy (Unpublished report No. RCC NOTOX 027934 submitted by
Agrichem B.V., The Netherlands).
NOTOX (1990d) Primary skin irritation/corrosion study with Glyposaat
B (RCC NOTOX substance 4175) in rabbits (4-hour semi-occlusive
application). 's-Hertogenbosch, The Netherlands, NOTOX -
Toxicological Research & Consultancy (Unpublished report No. RCC
NOTOX 027923 submitted by Agrichem B.V., The Netherlands).
NOTOX (1990e) Assessment of acute oral toxicity with Glyposaat B
(RCC NOTOX substance 4175) in the rat. 's-Hertogenbosch, the
Netherlands, NOTOX - Toxicological Research & Consultancy
(Unpublished report No. RCC NOTOX 027901 submitted by Agrichem B.V.,
The Netherlands).
NOTOX (1990f) Assessment of acute dermal toxicity with Glyposaat B
(RCC NOTOX substance 4175) in the rat. 's-Hertogenbosch, The
Netherlands, NOTOX - Toxicological Research & Consultancy
(Unpublished No. RCC NOTOX 027912 submitted by Agrichem B.V., The
Netherlands).
NTP (1992) NTP technical report on toxicity studies of glyphosate
(CAS No. 1071-83-6). Research Triangle Park, North Carolina,
National Toxicology Program (Toxicity Report Series No. 16).
OECD (1991) Report of the OECD Workshop on the Extrapolation of
Laboratory Aquatic Data to the Real Environment. Paris, Organisation
for Economic Co-operation and Development (Environment monograph No.
59).
Olorunsaga OO (1982a) Defective nicotinamide nucleotide
transhydrogenase reaction in hepatic mitochondria of
N-(phosphonomethyl)glycine treated rats. Biochem Pharmacol, 31:
2191-2192.
Olorunsaga OO (1982b) Inhibition of energy-dependent
transhydrogenase reaction by N-(phosphonomethyl)glycine in isolated
rat mitochondria. Toxicol. Lett. 10: 91-95.
Olorunsaga OO, Bababunmi EA, & Bassir O (1979) Effect of glyphosate
on rat liver mitochondria in vivo. Bull Environ Contam Toxicol, 22:
257-364.
Payne N (1992) Off-target glyphosate from aerial silvicultural
applications, and buffer zones required around sensitive areas.
Pestic Sci, 34: 1-8.
Payne N & Thompson DG (1992) Off-target glyphosate deposits from
aerial silvicultural applications under various meteorological
conditions. Pestic Sci, 34: 53-59.
Payne N, Feng J, & Reynolds P (1989) Off-target deposit measurements
and buffer zones required around water for various aerial
applications of glyphosate. In: Reynolds P ed. Proceedings of the
Carnation Creek Workshop, Nanaimo, 7-10 December 1987. Victoria,
British Columbia, Forestry Canada/British Columbia Ministry of
Forests, pp 88-109.
Piccolo A, Celano G, & Pietramellara G (1992) Adsorption of the
herbicide glyphosate on a metal-humic acid complex. Sci Total
Environ, 123/124: 77-82.
Pipke R & Amrhein N (1988) Isolation and characterization of a
mutant of Arthrobacter sp. strain GLP-1 which utilizes the herbicide
glyphosate as its sole source of phosphorus and nitrogen. Appl
Environ Microbiol, 54(11): 2868-2870.
Powell HA, Kerby NW, & Rowell P (1990) High-performance liquid
chromatographic determination of the herbicide glyphosate and its
metabolite (aminomethyl)phosphonic acid and their extraction from
cyanobacteria. J Chromatogr, 502(1): 201-207.
Preston CM & Trofymow JA (1989) Effects of glyphosate (Roundup) on
biological activity of forest soils. In: Reynolds P ed. Proceedings
of the Carnation Creek Workshop, Nanaimo, 7-10 December 1987.
Victoria, British Columbia, Forestry Canada/British Columbia
Ministry of Forests, pp 122-140.
PTRL East Inc. (1990a) Aerobic aquatic metabolism of [14C]
glyphosate. Richmond, Kentucky, Pharmacology and Toxicology Research
Laboratory East, Inc. (Unpublished Report No. 1300 submitted by
Monsanto Ltd).
PTRL East Inc. (1990b) Anaerobic aquatic metabolism of [14C]
glyphosate. Richmond, Kentucky, Pharmacology and Toxicology Research
Laboratory East, Inc. (Unpublished report No. 1304 submitted by
Monsanto Ltd.
PTRL East Inc. (1991) Aerobic metabolism of [14C] glyphosate in
sandy loam and silt loam soils with biometer flask. Richmond,
Kentucky, Pharmacology and Toxicology Research Laboratory East, Inc.
(Unpublished report No. 1301 submitted by Monsanto Ltd).
PTRL Inc. (1989) Photodegradation of [14C] glyphosate in/on soil
by natural sunlight (Project No. 153W). Richmond, Kentucky,
Pharmacology and Toxicology Research Laboratory, Inc (Unpublished
report submitted by Monsanto Ltd).
PTRL Inc. (1990) Degradation study: photodegradation of [14C]
glyphosate in a buffered aqueous solution at pH 5, 7, and 9 by
natural sunlight. Richmond, Kentucky, Pharmacology and Toxicology
Research Laboratory, Inc. (Unpublished report No. 233W-1 submitted
by Monsanto Ltd).
Quinn JP, Pedden JMM, & Dick RE (1988) Glyphosate tolerance and
utilization by the microflora of soils treated with the herbicide.
Appl Microbiol Biotechnol, 29(5): 511-516.
Ragab MTH (1978) Thin-layer chromatographic detection of glyphosate
herbicide (N-phosphonomethyl glycine) and its aminomethyl phosphonic
acid metabolite. Chemosphere, 2: 143-153.
Riley CM, Wiesner CJ, & Sexsmith WA (1991) Estimating off-target
spray deposition on the ground following the aerial application of
glyphosate for conifer release in New Brunswick. J Environ Sci
Health, B26: 185-208.
Ritchie DC, Harestad AS, & Archibald R (1987) Glyphosate treatment
and deer mice in clearcut and forest. Northwest Sci, 61(3): 199-202.
RIVM (1991) Catch-up operation old pesticides: an integration.
Bilthoven, The Netherlands, National Institute of Public Health and
Environmental Protection (Report no. 678801002).
Roseboom H & Berkhoff CJ (1982) Determination of the herbicide
glyphosate and its major metabolite aminomethylphosphonic acid by
high-performance liquid chromatography after fluorescence labelling.
Anal Chim Acta, 135(2): 373-377.
Roy DN & Konar SK (1989) Development of an analytical method for the
determination of glyphosate and (aminomethyl)phosphonic acid
residues in soils by nitrogen-selective gas chromatography. J Agric
Food Chem, 37(2): 441-443.
Roy DN, Konar SK, Banerjee S, Charles DA, Thompson DG, & Prasad R
(1989a) Uptake and persistence of the herbicide glyphosate (Vision)
in fruit of wild blueberry and red raspberry. Can J For Res, 19(7):
842-847.
Roy DN, Konar SK, Banerjee S, Charles DA, Thompson DG, & Prasad R
(1989b) Persistence, movement, and degradation of glyphosate in
selected Canadian boreal forest soils. J Agric Food Chem, 37(2):
437-440.
Rueppel ML, Brightwell BB, Schaefer J, & Marvel JT (1977) Metabolism
and degradation of glyphosate in soil and water. J Agric Food Chem,
25(3): 517-528.
Safefarm Labs Inc. (1991a) EXP 30578 - Modified nine-induction
Buehler delayed contact hypersensitivity study in the guinea pig
(Project No. 282/1219. Derby, UK, Safefarm Laboratories Inc.
(Unpublished report submitted by Rhône Poulenc, Lyon, France).
Safefarm Labs Inc. (1991b) Luxan glyphosate techn. - Magnusson &
Kligman maximisation study in the guinea pig (Project No. 349/11).
Derby, UK, Safefarm Laboratories Inc. (Unpublished report submitted
by Luxan B.V., The Netherlands).
Santillo DJ, Brown PW, & Leslie DM (1989a) Response of songbirds to
glyphosate-induced habitat changes on clearcuts. J Wildl Manage,
53(1): 64-71.
Santillo DJ, Leslie DM, & Brown PW (1989b) Response of small mammals
to glyphosate application on clearcuts. J Wildl Manage, 53(1):
164-172.
Sawada Y & Nadai Y (1987) [Roundup poisoning - its clinical
observation - possible involvement of surfactant.] J Clin Exp Med,
143(1): 25-27 (in Japanese).
Scrivener JC (1989) Comparative changes in concentration of
dissolved ions in the stream following logging, slash burning, and
herbicide application (glyphosate) at Carnation Creek, British
Columbia. In: Reynolds P ed. Proceedings of the Carnation Creek
Workshop, Nanaimo, 7-10 December 1987. Victoria, British Columbia,
Forestry Canada/British Columbia Ministry of Forests, pp 197-211.
Scrivener JC & Carruthers S (1989) Changes in the invertebrate
populations of the main stream and back channels of Carnation Creek,
British Columbia, following spraying with the herbicide Roundup
(glyphosate). In: Reynolds P ed. Proceedings of the Carnation Creek
Workshop, Nanaimo, 7-10 December 1987. Victoria, British Columbia,
Forestry Canada/British Columbia Ministry of Forests, pp 263-272.
Servizi JA, Gordon RW, & Martens DW (1987) Acute toxicity of Garlon
4 and Roundup herbicides to salmon, Daphnia, and trout. Bull Environ
Contam Toxicol, 39(1): 15-22.
SFRSA (1990) Effect of Roundup (glyphosate) on Chrysoperla carnea
(Test No. 121). Zurich, Swiss Federal Research Station for Agronomy
(Unpublished report submitted by Monsanto Ltd).
Shelanski MV (1973) Evaluation of potential hazards by dermal
contact. - Test material: MON 2139. Shelanski Holding Company, PA,
USA (Client: Monsanto Company) (Project No. Sh-72-19). Conshohocken,
Pennsylvania, Shelanski Holding Company (Unpublished report
submitted by Monsanto Ltd).
Siltanen H, Rosenberg C, Raatikainen M, & Raatikainen T (1981)
Triclopyr, glyphosate and phenoxyherbicide residues in cowberries,
bilberries and lichen. Bull Environ Contam Toxicol, 27(5): 731-737.
Smid D & Hiller LK (1981) Phytotoxicity and translocation of
glyphosate in the potato (Solanum tuberosum) prior to tuber
initiation. Weed Sci, 29(2): 218-223.
Sprankle P, Meggitt WF, & Penner D (1975) Adsorption, mobility, and
microbial degradation of glyphosate in the soil. Weed Sci, 23(3):
229-234.
Stark J (1983) Persistence of herbicides in forest soils. Weeds Weed
Control, 24(1): 275-286.
Stratton GW (1990) Effects of the herbicide glyphosate on
nitrification in four soils from Atlantic Canada. Water Air Soil
Pollut, 51(3/4): 373-383.
Stratton GW & Stewart KE (1992) Glyphosate effects on microbial
biomass in a coniferous forest soil. Environ Toxicol Water Qual, 7:
223-236.
Subramaniam V & Hoggard PE (1988) Metal complexes of glyphosate. J
Agric Food Chem, 36(6): 1326-1329.
Sullivan TP (1990) Influence of forest herbicide on deer mouse and
oregon vole population dynamics. J Wildl Manage, 54(4): 566-576.
Sullivan TP & Sullivan DS (1979) The effects of glyphosate herbicide
on food preference and consumption in black-tailed deer. Can J Zool,
57: 1406-1412.
Sullivan TP & Sullivan DS (1981) Responses of a deer mouse
population to a forest herbicide application: reproduction, growth,
and survival. Can J Zool, 59: 1148-1154.
Sullivan DS, Sullivan TP & Bisalputra T (1981) Effects of Roundup
herbicide on diatom populations in the aquatic environment of a
coastal forest. Bull Environ Contam Toxicol, 26(1): 91-96.
Sundaram A (1990) Effect of a Nalco-Trol II on bioavailability of
glyphosate in laboratory trials. J Environ Sci Health, B25(3):
309-332.
Swinehart JH & Cheney MA (1987) Interactions of organic pollutants
with gills of the bivalve molluscs Anodonta californiensis and
Mytilus californianus: uptake and effect on membrane fluxes II.
Comp Biochem, 88C(2): 293-299.
Swisher RD (1987) Surfactant biodegradation, New York, Basel, Marcel
Dekker, Inc.
Talbot AR, Shiaw M-H, Huang J-S, Yang S-F, Goo T-S, Wang S-H, Chen
C-L, & Sanford TR (1991) Acute poisoning with a
glyphosate-surfactant herbicide (Roundup): A review of 93 cases. Hum
Exp Toxicol, 10: 1-8.
Task Force on Water Quality Guidelines (1991) V.3 Glyphosate. In:
Canadian Water Quality Guidelines updates. Canadian Council of
Resource and Environment Ministers, Ottawa, V.8-V.16.
Tauchi K (1979) Sub-acute toxicity study of CP67573
(N-phosphonomethylglycine) to rats in dietary administration for 90
days. Institute for Animal Reproduction (Unpublished report No.
79/112 submitted to FAO/WHO-JMPR by Monsanto Europe S.A.).
Temple WA & Smith NA (1992) Glyphosate herbicide poisoning
experience in New-Zealand. N Z Med J, 105: 173-174.
Thompson DG, Cowell JE, Daniels RJ, Staznik B, & Macdonald LM (1989)
Liquid chromatographic method for quantitation of glyphosate and
metabolite residues in organic and mineral soils, stream sediments,
and hardwood foliage. J Assoc Off Anal Chem, 72(2): 355-360.
Tominack R L, Yang G-Y, Tsai W-J, Chung H-M, & Deng J-F (1991)
Taiwan national poison center survey of glyphosate-surfactant
herbicide ingestions. Clin Toxicol, 29(1): 91-109.
Tomita M, Okuyama T, Watanabe S, Uno B, & Kawai S (1991) High
performance liquid chromatographic determination of glyphosate and
(aminomethyl)phosphonic acid in human serum after conversion into
P-toluenesulphonyl derivatives. J Chromatogr, 566: 239-243.
Torstensson L & Stark J (1981) Decomposition of 14C-labelled
glyphosate in Swedish forest soils. In: Proceedings of the EWRS
Symposium on Theory and Practice of the Use of soil Applied
Herbicides. Uppsala, Swedish University of Agricultural Science,
Department of Microbiology, pp 72-79.
Torstensson L & Stenström J (1986) "Basic" respiration rate as a
tool for prediction of pesticide persistence in soil. Toxic Assess,
1(1): 57-72. Torstensson NT, Lundgren LN, & Stenström J (1989)
Influence of climatic and edaphic factors on persistence of
glyphosate and 2,4-D in forest soils. Ecotoxicol Environ Saf, 18(2):
230-239.
Tuinstra LGMT & Kienhuis PGM (1987) Automated two-dimensional HPLC
residue procedure for glyphosate on cereals and vegetables with
postcolumn fluoregenic labelling. Chromatographia, 24: 696-700.
USDA (1983) The acute oral toxicity of Roundup formulation in female
goats (Study No. 80004). US Department of Agriculture, College
Station, Texas, Veterinary Toxicology and Entomology Research
Laboratory, Veterinary Research Unit (Unpublished report supplied by
Monsanto Ltd - Monsanto study No. VT-80-452).
USDA (1987a) The subacute toxicity of Roundup herbicide (MON-2139)
in female cattle (Study no. 82001). US Department of Agriculture,
College Station, Texas, Veterinary Toxicology and Entomology
Research Laboratory, Veterinary Research Unit (Unpublished report
submitted by Monsanto Ltd - Monsanto study No. VT-82-001).
USDA (1987b) The acute oral toxicity of the isopropylamine salt of
glyphosate (MON 0139) in female goats (Study No. 80007). US
Department of Agriculture, College Station, Texas, Veterinary
Toxicology and Entomology Research Laboratory, Veterinary Research
Unit (Unpublished Report submitted by Monsanto Ltd - Monsanto study
No. VT-80-451).
USDA (1987c) The acute toxicity of glyphosate in female goats (Study
No. 80006). US Department of Agriculture, College Station, Texas,
Veterinary Toxicology and Entomology Research Laboratory, Veterinary
Research Unit (Unpublished report submitted by Monsanto Ltd -
Monsanto study No. VT-80-450).
Wan MT, Watts RG, & Moul DJ (1989) Effects of different dilution
water types on the acute toxicity to juvenile pacific salmonids and
rainbow trout of glyphosate and its formulated products. Bull
Environ Contam Toxicol, 43(3): 378-385.
Wängberg SA & Blanck H (1988) Multivariate patterns of algal
sensitivity to chemicals in relation to phylogeny. Ecotoxicol
Environ Saf, 16(1): 72-82.
Weidhase R, Albrecht B, Stock M, & Weidhase RA (1990) [Utilization
of glyphosate by Pseudomonas sp. GS.] Zent.bl Mikrobiol, 145(6):
433-438 (in German).
Wester RC, Melendres JC, Sarason R, McMaster J, & Maibach H I (1991)
Glyphosate skin binding, absorption, residual tissue distribution,
and skin decontamination. Fundam Appl Toxicol, 16: 725-732.
Wigfield YY & Lanouette M (1990) Simplified liquid chromatographic
determination of glyphosate and metabolite residues in environmental
water using post-column fluorogenic labelling. Anal Chim Acta,
233(2): 311-314.
Wildlife Int. Ltd (1978a) One-generation reproduction study-mallard
duck-glyphosate technical. Final report. Easton, Maryland, Wildlife
International Ltd (Unpublished report submitted by Monsanto Ltd).
Wildlife Int. Ltd (1978b) One-generation reproduction study-bobwhite
quail-glyphosate technical. Final report. Easton, Maryland, Wildlife
International Ltd (Unpublished report submitted by Monsanto Ltd).
Wildlife Int. Ltd (1978c) Acute oral LD50 of technical glyphosate
in the bobwhite (Project No. 139-140). Easton, Maryland, Wildlife
International Ltd (Unpublished report submitted by Monsanto Ltd).
Wildlife Int. Ltd (1990a) Roundup herbicide: a dietary LC50 study
with the mallard (Project No. 139-260). Easton, Maryland, Wildlife
International Ltd (Unpublished report submitted by Monsanto Ltd).
Wildlife Int. Ltd (1990b) Roundup herbicide: a dietary LC50 study
with the bobwhite (Project No. 139-259). Easton, Maryland, Wildlife
International Ltd (Unpublished report submitted by Monsanto Ltd).
WSSA (1983) Herbicide handbook of the Weed Science Society of
America, 5th ed. Champaign-Urbana, Illinois, Weed Science Society of
America.
Yao CCD & Haag WR (1991) Rate constants for direct reactions of
ozone with several drinking water contaminants. Water Res, 25:
761-773.
Young JC, Khan SU & Marriage PB (1977) Fluorescence detection and
determination of glyphosate via its N-nitroso derivative by
thin-layer chromatography. J Agric Food Chem, 25: 918-922.
Younger Labs Inc. (1984) Acute oral toxicity in albino rats - Test
material: LLN-83-06 (Project No. YO-84-024). St. Louis, Missouri,
Younger Laboratories, Inc. (Unpublished report submitted by Monsanto
Ltd).
Zboinska E, Lejczak B, & Lafarski P (1992) Organophosphonate
utilization by the wild-type strain of Pseudomonas fluorescens.
Appl Environ Microbiol, 58: 2993-2999.
RESUME
1. Identité, propriétés physiques et chimiques et méthodes d'analyse
Le glyphosate, ou N-(phosphonométhyl)glycine, est un acide
organique faible. Sa formule brute est C3H8NO5P. Il est
généralement présenté sous la forme du sel de l'acide correspondant
déprotoné et d'un cation comme l'isopropyl-ammonium ou le
triméthylsulfonium. La pureté du glyphosate de qualité technique est
généralement supérieure à 90%. Le glyphosate de qualité technique se
présente sous la forme d'une poudre cristalline blanche inodore dont
la densité est de 1,704. Sa tension de vapeur est très faible et il
est très soluble dans l'eau. Le coefficient de partage octanol-eau
(log Kow) est égal à -2,8. Le glyphosate est amphotère et il peut
exister sous différentes formes ioniques, selon le pH du milieu.
Le dosage du glyphosate est généralement une opération
laborieuse, complexe et coûteuse. La méthode la plus courante
consiste à en préparer un dérivé avec une substance fluorigène,
avant ou après passage sur colonne. Le dosage s'effectue en général
par chromatographie liquide à haute performance ou chromatographie
gaz-liquide. Les limites de détection dans l'eau, les plantes, le
sol et l'urine humaine sont respectivement de 0,02-3,2 µg/litre,
0,01-0,3 mg/kg, 0,05-1 mg/kg et 0,1 mg/litre.
2. Sources d'exposition humaine et environnementale
Le glyphosate est un herbicide non sélectif utilisé en
traitement endothérapique après l'émergence; il est utilisé partout
dans le monde sur des terrains agricoles ou non. On l'épand en
plusieurs formulations commerciales sur de nombreuses récoltes. La
plus courante est le Roundup qui consiste en un sel
d'isopropylammonium. La dose d'emploi recommandée ne dépasse 5,8 kg
de matière active par hectare et dépend de l'usage auquel on le
destine. L'environnement peut être contaminé par suite du dépôt
d'embruns ou de la libération accidentelle du produit.
3. Transport, distribution et transformation dans l'environnement
Les principaux processus de dissipation qui interviennent après
l'épandage de cet herbicide sont les suivants: formation de
complexes avec certains ions présents dans l'eau comme Ca2+ et
Mg2+, sorption aux sédiments ainsi qu'aux particules en suspension
dans l'eau et le sol, photodécomposition dans l'eau, fixation par
les plantes et biodégradation.
Le glyphosate disparaît de l'eau avec des valeurs du TD50
(temps de dissipation) qui vont de quelques jours à plus de 91
jours. Le principal milieu récepteur est constitué par les sédiments
ou les particules en suspension.
En laboratoire, les coefficients d'adsorption (Ks/l) du
glyphosate varient de 8 à 377 dm3/kg pour différents sols et
substances argileuses. On ne dispose d'aucune donnée sur la sorption
de l'acide aminométhylphosphonique (AMPA) qui en est le principal
métabolite, dans les conditions du laboratoire.
La valeur du Rf ne dépasse pas 0,2, selon certaines mesures par
chromatographie sur couche mince de terre. Dans des conditions de
lessivage reproduisant des précipitations extrêmement fortes, on
récupère dans l'éluat d'une colonne de terre, entre 0,1 et 11% de
l'activité appliquée initialement. L'expérimentation sur le terrain
montre qu'il n'y a probablement pas lessivage de l'AMPA.
L'expérimentation sur le terrain montre qu'en ce qui concerne
la dissipation du glyphosate dans le sol, les valeurs du TD50
varient de 3 à 174 jours, principalement en fonction des conditions
édaphiques et climatiques. Certaines expériences sur le terrain ont
montré que le ruissellement pouvait entraîner jusqu'à 1,8% de la
dose appliquée sur la sol.
Au laboratoire jusqu'à 45% de l'activité appliquée peut être
absorbée par le feuillage après traitement, après quoi il y a une
migration importante dans la plante.
L'hydrolyse du glyphosate en tampon stérile est très lente, les
valeurs du TD50 étant >> à 35 jours. En ce qui concerne la
photodécomposition dans l'eau dans les conditions naturelles, les
valeurs du TD50 sont < à 28 jours. Lors d'une étude qui s'est
prolongée pendant 31 jours, on n'a pas enregistré de
photodécomposition notable dans le sol.
Le temps nécessaire à la biodégradation de 50% d'une quantité
donnée de glyphosate dans l'ensemble d'un système d'épreuve en
présence d'eau et de sédiments était < à 14 jours en aérobiose et
compris entre 14 et 22 jours en anaérobiose. Dans le sol, le temps
de demi-biodégradation du glyphosate est de 2 à 3 jours en
aérobiose.
Le principal métabolite qui se forme dans le sol et dans l'eau
est l'AMPA. La quantité maximale d'AMPA présente dans le sol est
d'environ 20% de l'activité appliquée en aérobiose et de 0,5% de
cette activité en anaérobiose. Ce chiffre atteint 25% dans les
sédiments dans les deux types de conditions.
Les épreuves de laboratoire montrent que chez les invertébrés
et les poissons, le facteur de bioconcentration est faible. Lors
d'une épreuve en aquarium à écoulement continu, on a constaté que
chez Lépomis macrochirus le temps de demi-épuration était de 35
jours après une exposition de même durée. Après exposition continue
à du glyphosate, on retrouve de l'AMPA chez ce même poisson pendant
des périodes allant jusqu'à 21 jours. Des mesures sur le terrain
n'ont pas permis de déceler la présence de glyphosate chez les
poissons vivant dans des eaux sur lesquelles cet herbicide avait été
directement pulvérisé. Lors d'une expérience, on a décelé de l'AMPA
chez les carpes jusqu'à 90 jours après l'épandage. Une autre
expérience menée sur le terrain a montré qu'il n'y avait pas de
bioamplification du glyphosate dans les portées de petits mammifères
herbivores ou omnivores vivant en brousse. On a notamment mesuré des
concentrations allant jusqu'à 5 mg de matière active par kg chez des
souris du genre Peromyscus, immédiatement après l'épandage.
Plusieurs souches de bactéries peuvent décomposer le
glyphosate. On a identifié des bactéries qui sont capables
d'utiliser de composé comme seule source de phosphore, de carbone ou
d'azote. La croissance est alors plus lente que lorsque elles
utilisent des sources inorganiques de P, de C et de N. On est fondé
à penser, d'après les observations effectuées sur le terrain, que
certaines populations bactériennes se sont adaptées à la
métabolisation du glyphosate. La présence de phosphate inorganique
inhibe la décomposition du glyphosate par certaines bactéries mais
pas toutes. La biodécomposition du glyphosate peut comporter un
co-métabolisme avec d'autres sources d'énergie.
4. Concentrations dans l'environnement et exposition humaine
Il n'existe que de très rares données provenant de programmes
de surveillance systématique et concernant la présence de glyphosate
dans la faune et la flore ainsi que dans le milieu abiotique. Pour
avoir une idée des concentrations maximales dans l'environnement, on
fait appel aux données fournies par des essais sur le terrain au
cours desquels on simule des épandages à usage agricole; ces
concentrations sont les suivantes: < 1-1700 µg/litre dans les eaux
de surface, 0,07-40 mg/kg de poids sec dans le sol, < 0,05-19 mg/kg
de poids sec dans les sédiments, 261-1300 mg/kg dans les feuilles,
5 mg/kg dans les viscères des souris du genre Peromyscus,
1,6-19 mg/kg dans les baies sauvages et 45 mg/kg dans les lichens.
Les concentrations maximales correspondantes d'AMPA sont les
suivantes: < 1-35 µg/litre (eaux de surface), 0,1-9 mg/kg de poids
sec (sol), < 0,05-1,8 mg/kg de poids sec (sédiments), 1,7-<
9 mg/kg (feuilles), 0,02-0,1 mg/kg (baies sauvages) et 2,1 mg/kg
(lichens). Les concentrations ci-dessus de glyphosate sont celles
que l'on observe en général immédiatement après l'épandage. En ce
qui concerne les lichens, la concentration mentionnée a été observée
270 jours après l'épandage.
On ne dispose pas de mesures de la dose journalière ingérée par
l'homme avec les aliments et l'eau de boisson (études de rations
totales). Les quelques données disponibles au sujet de l'exposition
professionnelle indiquent que celle-ci est faible pour les ouvriers
qui épandent du glyphosate comme désherbant sous forme de Roundup.
5. Cinétique et métabolisme chez les animaux de laboratoire et
l'homme
Le glyphosate technique n'est que partiellement résorbé au
niveau des voies digestives. Lors d'études effectuées sur du
glyphosate marqué au carbone-14, on a observé des pourcentages
d'absorption de 30 à 36% chez plusieurs espèces. L'absorption par
voie percutanée est faible. Dans le cas de l'herbicide Roundup, le
glyphosate qu'il contient est absorbé dans une proportion < à
5,5% à travers la peau (durée de contact environ 24 heures). En ce
qui concerne les tissus de l'organisme, la concentration maximale,
correspondant à environ 1% de la dose ingérée, se retrouve dans les
eaux. Après administration d'une seule dose par voie orale, le
produit est éliminé à hauteur de 62 à 69% dans les matières fécales
sans absorption. Après absorption, 14 à 29% de la dose passe dans
l'urine et 0,2% au maximum dans l'air expiré. Après administration
par voie intraveineuse, le taux d'excrétion dans les voies biliaires
n'a été que de 5 à 8%. Chez des chèvres en lactation, on a montré
que le glyphosate n'était excrété dans le lait qu'en faible
proportion (concentration < à 0,1 mg/kg de lait entier pour une
dose ingérée de 120 mg/kg de nourriture). Le glyphosate n'est
métabolisé que dans une très faible proportion. Son seul métabolite,
l'AMPA, correspond à 0,3% de la dose ou même moins; le reste
correspond au produit initial. Il faut environ 168 heures pour que
le glyphosate soit éliminé en totalité de l'organisme (99% d'une
dose orale).
6. Effets sur les mammifères de laboratoire et les systèmes
d'épreuve in vitro
Chez l'animal de laboratoire, le glyphosate technique ne
présente qu'une très faible toxicité aiguë lorsqu'il est administré
par la voie orale ou percutanée; il est nettement plus toxique par
la voie intrapéritonéale que par les autres voies d'administration.
Des études d'alimentation de brève durée ont été effectuées sur
plusieurs espèces, mais la plupart de ces épreuves n'ont guère
révélé d'effets. Lors d'une épreuve de 13 semaines sur des souris au
cours de laquelle on a utilisé du glyphosate technique, on a
constaté une augmentation du poids de plusieurs organes ainsi qu'un
retard de croissance à la dose de 50 000 mg/kg de nourriture. Lors
d'une étude de même durée sur le rat, on n'a pas observé d'effet
(les doses de glyphosate technique utilisées allaient jusqu'à
20 000 mg/kg de nourriture). Lors d'une autre étude de 13 semaines,
on a observé des lésions au niveau des glandes salivaires chez des
rats et des souris. Chez les souris, la dose sans effet létal
observable était de 3125 mg/kg de nourriture; chez le rat, elle
était < à 3125 mg/kg de nourriture. Aucun de ces effets n'a été
observé lors d'études à court ou à long terme effectuées sur
diverses souches et espèces. Les lésions au niveau des glandes
salivaires incitent à penser que le glyphosate pourrait se comporter
comme un agoniste adrénérgique de faible activité.
La toxicité à long terme a été étudiée sur des souris et des
rats. Peu d'effets ont été observés et dans presque tous les cas,
uniquement à des doses relativement élevées. Chez les souris, le
glyphosate technique a produit un retard de croissance, une
hypertrophie ou une nécrose des hépatocytes ainsi qu'une hyperplasie
de l'épithélium vésical à la dose de 30 000 mg/kg. Chez les rats, le
même composé a entraîné une réduction de la croissance, une
augmentation du poids du foie, une dégénérescence du cristallin et
une inflammation de la muqueuse gastrique à la dose de 20 000 mg/kg
de nourriture.
Les études dont on connaît les résultats ne concluent pas à
l'existence d'un pouvoir mutagène, cancérogène ou tératogène du
glyphosate technique. Deux études ont été effectuées sur plusieurs
générations de rats. Les principaux effets du glyphosate technique
consistaient en une réduction du poids corporel des géniteurs et des
ratons ainsi qu'une diminution de la taille des portées, à la dose
de 30 000 mg/kg de nourriture. Dans une étude portant sur la
reproduction, on a constaté une augmentation dans l'incidence de la
dilatation unilatérale des tubules rénaux chez les ratons mâles de
la génération F3b, à la dose de 30 mg/kg de poids corporel.
Toutefois la reproductibilité de cette lésion reste incertaine du
fait qu'elle n'a pas été observée chez les ratons soumis à une dose
plus élevée, dans la deuxième de ces études.
7. Effets sur l'homme
Les études contrôlées dont on dispose se limitent à trois
études sur l'irritation et la sensibilisation provoquées par le
glyphosate chez des volontaires humains, et qui ont toutes donné des
résultats négatifs. Plusieurs cas d'intoxication (la plupart du
temps volontaires) avec un herbicide composé de glyphosate
technique, le Roundup, ont été signalés. Une étude, consacrée à des
travailleurs qui épandaient du Roundup, n'a pas révélé d'effets
indésirables. Les données disponibles sur l'exposition
professionnelle d'ouvriers appliquant du Roundup montrent que le
niveau d'exposition est très inférieur à la dose sans effet létal
observable qui ressort de l'expérimentation animale.
8. Effets sur les êtres vivants dans leur milieu naturel
Le glyphosate de qualité technique est légèrement à modérément
toxique pour les microorganismes aquatiques avec une CE50 (3 à 4
jours) allant de 1,2 à 7,8 mg/litre et une concentration sans effets
observables à 7 jours allant de 0,3 à 34 mg/litre. Sous ses
différentes formulations, le glyphosate est légèrement à fortement
toxique pour les microorganismes aquatiques avec des valeurs de la
CE50 à 3 jours allant de 1,0 à plus de 55 mg de produit par litre.
Les cyanophycées (algues bleues) sont plus sensibles au Roundup que
les algues proprement dites. Les processus physiologiques affectés
sont notamment le verdissement, la respiration, la photosynthèse et
la synthèse des acides aminés aromatiques.
Sur les bactéries terricoles en culture, le glyphosate agit au
niveau de la fixation de l'azote, de la dénitrification et de la
nitrification. Cependant des observations effectuées sur le terrain
après épandage de diverses formulations de glyphosate n'ont pas
révélé la présence d'effets sensibles. Des bactéries appartenant à
des espèces étroitement apparentées aux bactéries précitées se sont
révélées capables de dégrader le glyphosate.
Chez les champignons ectomycorhiziens, la croissance du
mycélium en culture pure est inhibée par des concentrations > à
29 mg de Roundup par litre. Les genres sensibles à cette inhibition
sont Cenococcum, Hebeloma et Laccaria.
Le glyphosate est légèrement toxique pour les macrophytes
aquatiques avec une valeur de la concentration sans effets
observables à 14 jours de 9 mg/litre, en solution dans l'eau. le
Roundup est également légèrement toxique avec, pour cette
concentration, des valeurs allant de 2,4 à 56 mg/litre, également en
solution dans l'eau. On ne dispose d'aucune donnée sur la toxicité
aiguë. La phytotoxicité est beaucoup plus importante en l'absence de
lessivage des dépôts d'herbicide.
Le glyphosate de qualité technique est très légèrement à
légèrement toxique pour les invertébrés aquatiques avec des valeurs
de la CL50 ou de la CE50 à 2-4 jours > 55 mg/litre et une
valeur de la concentration sans effets observables à 21 jours de
100 mg/litre. Les diverses formulations de glyphosate sont très
légèrement à modérément toxiques pour les invertébrés aquatiques
pour des valeurs de la CE50 à 2 jours s'étageant entre 5,3 et
5600 mg de produit par litre et des valeurs de la MATC à 21 jours
allant de 1,4 à 4,9 mg de produit par litre. La toxicité plus forte
du Roundup est essentiellement due à la présence d'agents
tensioactifs.
Le glyphosate de qualité technique est très légèrement à
modérément toxique pour les poissons avec des valeurs de la CL50 à
quatre jours allant de 10 à > 1000 mg/litre, une valeur de la
concentration sans effets observables à 21 jours de 52 mg/litre et
une valeur de la MATC de > 26 mg/litre. Les diverses formulations
du glyphosate sont également très légèrement à modérément toxiques
pour les poissons avec des valeurs de la CL50 à quatre jours de
2,4 à > 1000 mg de produit par litre et des valeurs de la
concentration sans effets observables à 21 jours allant de 0,8 à
2,4 mg de produit par litre. C'est la carpe qui s'est révélée être
l'espèce la plus sensible, après exposition à une formulation de
glyphosate appelée Sting. Sur le terrain, on n'a pas constaté
d'effets sur les poissons qui soient attribuables au traitement par
le Roundup, à l'exception d'un stress constaté immédiatement après
l'épandage du produit à la dose recommandée en évitant que celle-ci
ne dépasse 40 mg de Roundup par litre.
On constate que le glyphosate inhibe, dans une proportion qui
dépend de la dose, la formation de nodosités par le trèfle
souterrain inoculé par du Rhizobium, en culture hors-sol et en
présence de solutions nutritives contenant une concentration de
matière active > 2 mg/litre. La germination de diverses espèces
forestières n'est pas affectée par la présence de glyphosate aux
doses d'emploi recommandées. Avec des doses d'emploi > 0,54 kg de
matière active par hectare, on constate au laboratoire qu'il y a
réduction, proportionnée à la dose, de la longueur des racines des
jeunes pousses de pins sylvestres. Cette diminution n'a pas été
confirmée lors d'une étude du même genre sur le terrain.
Le glyphosate de qualité technique et le Roundup sont
légèrement toxiques pour les abeilles en application orale ou
topique. Les valeurs de la DL50 à deux jours sont > 100 µg (de
matière active ou de produit) par abeille. La DL50 par voie orale
à deux jours du Sting pour les abeilles est > 100 µg/abeille. Le
Roundup et le Roundup D-pack sont légèrement toxiques pour les
lombrics avec des valeurs de la concentration sans effets
observables à 14 jours respectivement égales à 500 et 158 mg de
produit par kg de poids sec. Aucun effet nocif, attribuable au
Roundup, n'a été observé sur la fécondité et la fertilité d'un
certain nombre d'insectes appartenant au groupe des névroptères et
le Sting n'a pas non plus produit d'effets sur la consommation de
nourriture ou la mortalité des insectes du genre Poecilus.
Le glyphosate de qualité technique est légèrement toxique pour
les oiseaux avec une DL50 > 3851 mg/kg de poids corporel, une
CL50 à huit jours > 4640 mg/kg et des valeurs de la concentration
sans effets observables à 112-119 jours, > 1000 mg/kg de
nourriture. On a constaté que le Roundup et une autre formulation
dont le nom n'est pas connu était également toxique pour les
oiseaux, avec un DL50 > 2686 mg de produit par kg de poids
corporel et une CL50 à huit jours > à 5620 mg de produit par kg
de nourriture. Généralement, on ne constate, sur les mammifères de
laboratoire, aucun effet qui soit attribuable au traitement par le
glyphosate de qualité technique ou le Roundup. Les effets attribués
au traitement par cet herbicide et constatés chez les oiseaux et les
mammifères dans leur milieu naturel, semblent être dus
principalement aux modifications du biotope consécutives au
traitement herbicide.
RESUMEN
1. Identidad, propiedades físicas y químicas y métodos analíticos
El glifosato es un ácido orgánico débil formado por una
molécula de glicina y otra de fosfonometilo. La fórmula empírica es
C3H8NO5P. Normalmente se formula como una sal del ácido del
glifosato en el que se ha sustituido un protón por un catión, por
ejemplo la isopropilamina o el trimetilsulfonio. La pureza del
glifosato de calidad técnica suele ser superior al 90%. Este es un
polvo cristalino blanco e inodoro con un peso específico de 1,704,
una presión de vapor muy baja y una solubilidad en agua alta. El
coeficiente de reparto octanol/agua (log Kow) es -2,8. El
glifosato es anfótero y se puede encontrar formando compuestos
iónicos diversos, en función del Ph del medio.
Su determinación es en general laboriosa, compleja y costosa.
El método más habitual es la transformación con sustancias
fluorogénicas en derivados más fácilmente detectables y se puede
utilizar antes o después de la columna. La determinación se suele
llevar a cabo mediante cromatografía líquida de alto rendimiento o
cromatografía gas-líquido. Los límites de determinación del
glifosato en el agua, las plantas, el suelo y la orina humana son de
0,02-3,2 µg/litro, 0,01-0,3 mg/kg, 0,05-1 mg/kg y 0,1 mg/litro,
respectivamente.
2. Fuentes de exposición humana y ambiental
El glifosato es un herbicida que actúa después del brote de
manera sistémica y no selectiva, y se utiliza en zonas agrícolas y
no agrícolas de todo el mundo. Se aplica a numerosos cultivos con
formulaciones comerciales diferentes. La más importante es el
Roundup, en el que el glifosato aparece en forma de la sal de
isopropilamina. Las dosis de aplicación recomendadas no superan los
5,8 kg de a.i./ha y dependen del tipo de uso. Se puede producir
exposición ambiental como consecuencia de la deposición debida a
corrientes o escapes accidentales.
3. Transporte, distribución y transformación en el medio ambiente
Las más importantes vías de desaparición del glifosato tras su
aplicación son la formación en el agua de complejos con iones, por
ejemplo con el Ca2+ y el Mg2+, la sorción al sedimento, las
partículas suspendidas en el agua y el suelo, la fotodegradación en
el agua, la fijación en las plantas y la biodegradación.
El glifosato desaparece del agua con unos valores de TD50 que
oscilan entre varios días y más de 91 días. Se ha comprobado que se
deposita sobre todo en las partículas del sedimento o suspendidas.
Los coeficientes de adsorción (Ks/l) del glifosato en
experimentos de laboratorio varían entre 8 y 377 dm3/kg para
diferentes suelos y minerales arcillosos. No se dispone de datos, en
condiciones de laboratorio, sobre la sorción del ácido
aminometilfosfónico (AAMF), su principal metabolito.
En los experimentos de cromatografía en capa fina, los valores
Rf del glifosato no son superiores a 0,2 en el suelo. En el eluato
de columnas de suelo obtenido en condiciones de lixiviación
simulando una precipitación muy intensa se recupera una cantidad que
oscila entre menos del 0,1% y el 11% de la dosis aplicada. De los
estudios sobre el terreno se desprende que no es probable la
lixiviación del AAMF.
En los experimentos sobre el terreno el glifosato desaparece
del suelo con un TD50 que varía entre 3 y 174 días, principalmente
en función de las condiciones edáficas o climáticas. En algunos
experimentos sobre el terreno desaparecía del suelo, debido a la
escorrentía, hasta el 1,8% de la dosis aplicada.
En condiciones de laboratorio, las hojas tratadas podrían
absorber hasta el 45% de la cantidad aplicada, produciéndose a
continuación un importante desplazamiento.
La hidrólisis del glifosato en tampones estériles es muy baja,
con valores de TD50 >> 35 días. En condiciones naturales, la
fotodegradación en agua se produce con valores de TD50 < 28 días.
En el curso de un estudio de 31 días no se registró una
fotodegradación importante en el suelo.
El tiempo necesario para la biodegradación del 50% del
glifosato en el sistema completo de una prueba con agua y sedimento
es < 14 días en condiciones aerobias y de 14 a 22 días en
condiciones anaerobias de laboratorio. El tiempo necesario para la
biodegradación del 50% del glifosato en el suelo es de 2-3 días en
condiciones aerobias.
El metabolito principal en el suelo y el agua es el AAMF. Las
cantidades máximas de AAMF en el suelo son de aproximadamente el 20%
de la dosis aplicada en condiciones aerobias, y del 0,5% en
condiciones anaerobias. Las cantidades máximas de AAMF en el
sedimento son del 25%, tanto en condiciones aerobias como
anaerobias.
De las pruebas de laboratorio se desprende que los factores de
bioconcentración en invertebrados y peces son bajos. Tras una
exposición al glifosato de 35 días, Lepomis macrochirus mostró en
una prueba en corriente un periodo de semidepuración de 35 días. Se
recuperó AAMF en Lepomis macrochirus hasta 21 días después de una
exposición continuada. No se detectó glifosato en peces que vivían
en agua directamente rociada en experimentos sobre el terreno. En un
experimento se detectó AAMF en carpas hasta 90 días después de la
aplicación. En otro experimento sobre el terreno no se observó
bioampliación del glifosato en el lecho de pequeños mamíferos
herbívoros y omnívoros de un ecosistema de matorral boscoso. En este
mismo experimento, inmediatamente después del rociado se
determinaron concentraciones de hasta 5 mg de a.i./kg en ratones de
pies blancos (Peromyscus leucopus).
Existen diversas bacterias que pueden degradar el glifosato. Se
han identificado cepas capaces de utilizar este compuesto como única
fuente de fósforo, de carbono o de nitrógeno. El crecimiento es
lento si se compara con el obtenido de fuentes inorgánicas de P, C y
N. Hay pruebas en el medio ambiente de la existencia de poblaciones
bacterianas que se han adaptado para metabolizar el glifosato. La
presencia de fosfato inorgánico inhibe la degradación de este
compuesto por algunas bacterias, pero no por todas. La
biodegradación del glifosato podría tener un metabolismo común con
el de otras fuentes de energía.
4. Niveles ambientales y exposición humana
Los datos sobre la presencia de glifosato en la biota y la
abiota del medio ambiente como parte de programas de vigilancia
regular son muy escasos. Se utilizan datos obtenidos en experimentos
sobre el terreno en los que se simula la práctica agrícola normal
para indicar las concentraciones máximas en el medio ambiente: <
1-1700 µg/litro de agua superficial, 0,07-40 mg/kg de peso seco de
suelo, < 0,05-19 mg/kg de peso seco de sedimento, 261-1300 mg/kg de
follaje, 5 mg/kg de vísceras de ratón de pies blancos, 1,6-19 mg/kg
de bayas silvestres y 45 mg/kg de líquenes. Las concentraciones
máximas correspondientes de AAMF son las siguientes: <
1-35 µg/litro (agua superficial), 0,1-9 mg/kg de peso seco (suelo),
< 0,05-1,8 mg/kg de peso seco (sedimento), 1,7-< 9 mg/kg
(follaje), 0,02-0,1 mg/kg (bayas silvestres) y 2,1 mg/kg (líquenes).
Las concentraciones de glifosato mencionadas más arriba se suelen
encontrar inmediatamente después de la aplicación. La concentración
en los líquenes se determinó 270 días después de dicha aplicación.
No se dispone de mediciones de la ingestión humana diaria de
glifosato a través de los alimentos y el agua de bebida (estudios
completos de alimentación). Los escasos datos disponibles sobre la
exposición ocupacional ponen de manifiesto que los niveles de
exposición para los trabajadores que aplican el glifosato en la
formulación del herbicida Roundup son bajos.
5. Cinética y metabolismo en animales de laboratorio y en el ser
humano
La absorción del glifosato de calidad técnica en el tracto
intestinal es sólo parcial. En estudios con glifosato marcado con
14C, se encontró en varias especies un porcentaje de absorción del
30% al 36%. La absorción cutánea es baja. De la formulación del
herbicida Roundup, a través de la piel sólo se absorbe < 5,5% del
glifosato presente (tiempo de contacto de unas 24 horas). En los
tejidos del organismo, la concentración más alta, aproximadamente el
1% de la dosis oral, se encuentran en los huesos. Tras una dosis
oral única, se eliminó en las heces sin absorción el 62-69%. Del
glifosato absorbido, un 14-29% se excretó en la orina y el 0,2% o
menos en el aire expirado. La excreción biliar posterior a la
administración intravenosa fue sólo del 5-8%. Se observó que la
excreción en la leche de cabras lactantes se producía sólo en escasa
proporción (concentración < 0,1 mg/kg de leche entera a un nivel
de dosis de 120 mg/kg de alimentos). La biotransformación del
glifosato se da únicamente en un grado muy bajo. El único
metabolito, el AAMF, representa el 0,3% de la dosis o menos; el
resto es glifosato inalterado. La eliminación de todo el organismo
(99% de una dosis oral) se produce aproximadamente en 168 horas.
6. Efectos en animales de laboratorio y en sistemas de prueba
in vitro
El glifosato de calidad técnica administrado por vía oral y
cutánea a animales de experimentación tiene una toxicidad aguda muy
baja; por vía intraperitoneal es notablemente más tóxico que por
cualquier otra. Aunque se han realizado estudios de alimentación de
corta duración en varias especies, en la mayor parte de estas
pruebas se han observado pocos efectos. En un estudio de 13 semanas
realizado en ratones con glifosato de calidad técnica, a una
concentración de 50 000 mg/kg de alimento, se observó aumento de
peso de varios órganos y un retraso del crecimiento. En un estudio
de 13 semanas en ratas no se advirtieron efectos (con dosis de
glifosato de calidad técnica de hasta 20 000 mg/kg de alimento). En
otro estudio de 13 semanas se detectaron lesiones en las glándulas
salivales de ratas y ratones. En ratones, el NOAEL fue de 3125 mg/kg
de alimento; en ratas fue < 3125 mg/kg de alimento. Estos
resultados no se obtuvieron en ningún otro estudio de corta o larga
duración realizado en diferentes razas y especies. Las lesiones de
las glándulas salivales parecen indicar que el glifosato puede
actuar como agonista adrenérgico débil.
Se estudió la toxicidad a largo plazo en ratones y ratas. Se
observaron escasos efectos y, en la mayor parte de los casos, sólo a
dosis relativamente altas. Con dosis de 30 000 mg/kg de glifosato de
calidad técnica se produjo en los ratones retraso del crecimiento,
hipertrofia o necrosis de los hepatocitos e hiperplasia epitelial de
la vejiga urinaria. La misma prueba en ratas con dosis de
20 000 mg/kg de alimento provocó una disminución del crecimiento,
aumento del peso del hígado, cambios degenerativos del cristalino e
inflamación gástrica.
De los estudios disponibles no se desprende que el glifosato de
calidad técnica tenga actividad mutagénica, carcinogénica o
teratogénica. Se realizaron con este compuesto dos estudios en
varias generaciones de ratas. Los principales efectos del glifosato
de calidad técnica con dosis de 30 000 mg/kg de alimento fueron una
disminución del peso corporal de los padres y las crías y la
reducción del tamaño de la camada. Se ha informado que en un estudio
de reproducción con dosis de 30 mg/kg de peso corporal se produjo un
aumento del número de casos de dilatación tubular renal unilateral
en crías macho de la F3b. La ausencia de efectos renales en las
crías con dosis más elevadas en el otro estudio de reproducción pone
de manifiesto que la reproducibilidad de la lesión es incierta.
7. Efectos en el ser humano
Sólo se dispone de tres estudios controlados sobre
irritación/sensibilización en voluntarios, cuyos resultados indican
la ausencia de efectos. Se ha informado de varios casos de
intoxicación (la mayor parte intencionados) con la formulación
Roundup de herbicida a base de glifosato de calidad técnica. No se
detectaron efectos adversos tras realizar un estudio para determinar
el estado de salud de los trabajadores que aplican la formulación
del herbicida Roundup. Los datos disponibles sobre exposición en el
trabajo de quienes aplican el Roundup indican que los niveles de
exposición están muy por debajo del NOAEL obtenido en los
experimentos correspondientes con animales.
8. Efectos sobre otros organismos en el laboratorio y en el medio
ambiente
El glifosato de calidad técnica tiene una toxicidad de moderada
a ligera para los microorganismos acuáticos, con valores de CE50
(3-4 días) de 1,2-7,8 mg/litro y valores de NOEC (7 días) de
0,3-34 mg/litro. Las formulaciones de glifosato son entre
ligeramente tóxicas y muy tóxicas para los microorganismos
acuáticos, con valores de CE50 en tres días de 1,0 a > 55 mg de
producto por litro. Las cianofíceas (algas verdeazuladas) son más
sensibles al Roundup que las algas verdaderas. Afecta a diversos
procesos fisiológicos, entre ellos la formación del color verde, la
respiración, la fotosíntesis y la síntesis de aminoácidos
aromáticos.
En cultivos de bacterias del suelo se ha comprobado la
influencia del glifosato sobre la fijación del nitrógeno, la
desnitrificación y la nitrificación. Sin embargo, en estudios sobre
el terreno no se han observado efectos significativos tras la
aplicación de varias formulaciones. Diversas bacterias estrechamente
relacionadas han demostrado que son capaces de degradar el
glifosato.
A concentraciones > 29 µg de Roundup/litro se inhibe el
crecimiento de los micelios de las ectomicorrizas en cultivos puros.
Son géneros sensibles Cenococcum, Hebeloma y Laccaria.
Cuando se disuelve en agua, el glifosato es ligeramente tóxico
para las macrofitas acuáticas, con un valor de NOEC en 14 días de
9 mg/litro. El Roundup disuelto en agua es también ligeramente
tóxico, con valores de NOEC en 14 días de 2,4-56 mg de
Roundup/litro. No se dispone de datos acerca de su toxicidad aguda.
La fitotoxicidad es mucho más elevada cuando el agua no arrastra los
depósitos del rociado.
La toxicidad del glifosato de calidad técnica para los
invertebrados acuáticos varía entre ligera y muy ligera, con unos
valores de la CL50 o la CE50 en 2 a 4 días de > 55 mg/litro,
y un valor de NOEC en 21 días de 100 mg/litro. Las formulaciones de
glifosato tienen una toxicidad entre moderada y muy ligera para los
invertebrados acuáticos, con valores de CE50 en 2 días de
5,3-5600 mg de producto/litro y valores de MATC en 21 días de
1,4-4,9 mg de producto por litro. La toxicidad más elevada del
Roundup se debe fundamentalmente a la presencia de surfactantes.
La toxicidad del glifosato de calidad técnica para los peces es
entre moderada y muy ligera, con valores de CL50 en 4 días de 10 a
> 1000 mg/litro, una NOEC en 21 días de 52 mg/litro, y un valor
MATC de > 26 mg/litro. Las formulaciones del glifosato tienen
también una toxicidad entre moderada y muy ligera para los peces,
con valores de CL50 en 4 días de 2,4 a > 1000 mg de producto por
litro y valores de NOEC en 21 días de 0,8-2,4 mg de producto/litro.
La especie más sensible es la carpa, cuando se la expone a la
formulación Sting. No se han observado en los peces efectos
relacionados con el tratamiento de Roundup en el medio ambiente,
salvo cierta tensión inmediatamente después de la aplicación de una
dosis recomendada y evitando concentraciones > 40 mg de
Roundup/litro.
En sistemas de cultivo sin suelo con soluciones nutrientes en
concentraciones > 2 mg de i.a./litro se produce una inhibición
dependiente de la dosis de la nodulación del trébol subterráneo
inoculado con Rhizobium. El glifosato en las dosis de aplicación
recomendadas no afecta a la germinación de las semillas. La longitud
de las raíces de los plantones de pino rojo disminuye en condiciones
de laboratorio en función de la dosis con unas concentraciones de
aplicación > 0,54 kg de i.a./ha. Esta reducción no se confirmó en
un experimento comparable sobre el terreno.
El glifosato de calidad técnica y el Roundup son ligeramente
tóxicos para las abejas cuando se aplican por vía oral o tópica. Los
valores de la DL50 en 2 días son > 100 µg (i.a. o producto) por
abeja. La DL50 en 2 días por vía oral de Sting para las abejas es
> 100 µg/abeja. Roundup y Roundup D-pak son ligeramente tóxicos
para las lombrices de tierra, con valores NOEC en 14 días de 500 y
158 mg de producto por kg de peso seco, respectivamente. No se
observaron efectos adversos del Roundup sobre la fecundidad y
fertilidad de especies de la familia Chrysopidae, y tampoco se
detectaron efectos de Sting en la ingestión de alimentos y la
mortalidad del escarabajo Poecilus.
El glifosato de calidad técnica es ligeramente tóxico para las
aves, con una DL50 > 3851 mg/kg de peso corporal, una CL50 en 8
días de > 4640 mg/kg de alimento, y valores NOEC en 112-119 días de
> 1000 mg/kg de alimento. Roundup y una formulación desconocida
son también ligeramente tóxicos para las aves, con una DL50 de >
2686 mg de producto/kg de peso corporal y una CL50 en 8 días de >
5620 mg de producto/kg de alimento. En condiciones de laboratorio no
se han observado en general sobre los mamíferos efectos relacionados
con el tratamiento de glifosato de calidad técnica o Roundup, salvo
con dosis de aplicación muy elevadas. Los efectos relacionados con
el tratamiento en las aves y mamíferos del medio ambiente parecen
deberse fundamentalmente a cambios de hábitat después del
tratamiento con Roundup.
 The relative molecular mass of glyphosate is 169.07. Technical
grade glyphosate has a purity of > 80%, but the purity generally
exceeds 90%. Glyphosate usually is formulated as a salt of the
deprotonated acid of glyphosate and a cation, e.g., isopropylamine.
The CAS registry number of the salt of glyphosate and
isopropyl-amine is
The relative molecular mass of glyphosate is 169.07. Technical
grade glyphosate has a purity of > 80%, but the purity generally
exceeds 90%. Glyphosate usually is formulated as a salt of the
deprotonated acid of glyphosate and a cation, e.g., isopropylamine.
The CAS registry number of the salt of glyphosate and
isopropyl-amine is  Table 2. Composition of various commercial formulations with glyphosatea
Name Synonyms Concentration Country
a.i. (%)
Roundup Spasor, 48.0 (w/v); Most countries
Sting Vision, 41.0 (w/w)b
Swing, 21.7 (w/w) Belgium, Cameroon, France,
Arcade, Holland, Kenya, Malawi,
Tomahawk Portugal, South Africa,
United Kingdom
Armada Frontier 16.6 (w/w) Belgium, Cameroon, Ivory
Coast, Gabon, Greece, Zaire
Dardo Ricochetg, 12.2 (w/w) Cameroon, Egypt, France,
Rival, Greece, Israel, Italy,
Ultrasonic Portugal, Spain, United
Kingdom
Squadron 20.2 (w/w) Argentina, Australia, Columbia
Stirrup Nomix, Expedite 18.3 (w/w) France, United Kingdom
Wallop 20.8 (w/w) Malaysia
Deploy Dryc 94.0 (w/w) USA
Quotamakerd 75.0 (w/w) USA
Landmaster IIe 13.3 (w/w) USA
Landmaster BW,
Campaignf 12.9 (w/w) USA
Roundup D-Pak 62.0 (w/w) USA
Rodeo 53.8 (w/w) USA
Ranger 28.6 (w/w) USA
Roundup Lawn and
Garden Conc. 18.0 (w/w) USA
Roundup-Ready-
To-Use 0.96 (w/w) USA
Fusta 22.5 (w/w) Spain
Table 2 (continued)
a all formulations produced by Monsanto Ltd; data provided by Monsanto Ltd
b based on the isopropylamine salt; equivalent to 36.0% (w/v) and 30.5% (w/w)
of the free acid
c dry formulation of the monoammonium salt
d dry formulation of the sodium sesqui salt
e also contains 11.1% 2,4-D (isopropylamine salt)
f also contains 20.6% 2,4-D (isopropylamine salt)
g also contains simazine
Formulations may contain other active ingredients, e.g.,
simazine in Ricochet, 2,4-D in Landmaster, and MCPA in Fusta.
2.4 Conversion factors
1 ppm = 6.91 mg/m3 at 25 °C and 101.3 kPa
1 mg/m3 = 0.145 ppm
2.5 Analytical methods
2.5.1 Sample handling and preparation
The first preparative step before detection and measurement of
glyphosate is generally extraction. As both glyphosate and its main
metabolite aminomethylphosphonic acid (AMPA, see Fig. 2) show high
polarity, and are therefore highly water soluble, they are difficult
to extract with organic solvents. However, various methods have been
developed. Some recently developed extraction methods for different
media are summarized in Table 3.
Table 2. Composition of various commercial formulations with glyphosatea
Name Synonyms Concentration Country
a.i. (%)
Roundup Spasor, 48.0 (w/v); Most countries
Sting Vision, 41.0 (w/w)b
Swing, 21.7 (w/w) Belgium, Cameroon, France,
Arcade, Holland, Kenya, Malawi,
Tomahawk Portugal, South Africa,
United Kingdom
Armada Frontier 16.6 (w/w) Belgium, Cameroon, Ivory
Coast, Gabon, Greece, Zaire
Dardo Ricochetg, 12.2 (w/w) Cameroon, Egypt, France,
Rival, Greece, Israel, Italy,
Ultrasonic Portugal, Spain, United
Kingdom
Squadron 20.2 (w/w) Argentina, Australia, Columbia
Stirrup Nomix, Expedite 18.3 (w/w) France, United Kingdom
Wallop 20.8 (w/w) Malaysia
Deploy Dryc 94.0 (w/w) USA
Quotamakerd 75.0 (w/w) USA
Landmaster IIe 13.3 (w/w) USA
Landmaster BW,
Campaignf 12.9 (w/w) USA
Roundup D-Pak 62.0 (w/w) USA
Rodeo 53.8 (w/w) USA
Ranger 28.6 (w/w) USA
Roundup Lawn and
Garden Conc. 18.0 (w/w) USA
Roundup-Ready-
To-Use 0.96 (w/w) USA
Fusta 22.5 (w/w) Spain
Table 2 (continued)
a all formulations produced by Monsanto Ltd; data provided by Monsanto Ltd
b based on the isopropylamine salt; equivalent to 36.0% (w/v) and 30.5% (w/w)
of the free acid
c dry formulation of the monoammonium salt
d dry formulation of the sodium sesqui salt
e also contains 11.1% 2,4-D (isopropylamine salt)
f also contains 20.6% 2,4-D (isopropylamine salt)
g also contains simazine
Formulations may contain other active ingredients, e.g.,
simazine in Ricochet, 2,4-D in Landmaster, and MCPA in Fusta.
2.4 Conversion factors
1 ppm = 6.91 mg/m3 at 25 °C and 101.3 kPa
1 mg/m3 = 0.145 ppm
2.5 Analytical methods
2.5.1 Sample handling and preparation
The first preparative step before detection and measurement of
glyphosate is generally extraction. As both glyphosate and its main
metabolite aminomethylphosphonic acid (AMPA, see Fig. 2) show high
polarity, and are therefore highly water soluble, they are difficult
to extract with organic solvents. However, various methods have been
developed. Some recently developed extraction methods for different
media are summarized in Table 3.
 Table 3. Sampling, preparation, and analysis of glyphosate
Medium Sampling Preparations Derivatization Analytical Limit of Recovery Reference
volume or reagent method determinationa
weight
Air n.r. collected onto an trifluoroacetic GC-MS and approx. 0.3 94% Jauhiainen
absorption liquid; anhydride and GC-EC µg/m3 et al. (1991)
evaporation to trifluoroethanol
dryness
Cyano-bacteria 100 ml dry, resuspend in PITC HPLC with a n.r.b 78% Powell et al.
methanol/sodium - radically (1990)
acetate/ column compressed
triethylamine
Plants 5 g extraction with trifluoroacetic GC-NPD 0.03 mg/kg 72-92% Konar & Roy,
water/chloroform; anhydride and (1990)
preconcentration trifluoroethanol
and clean-up on
cation-exchange
resin
Plants 25-50 g extraction with TLC with 0.01 mg/kg n.r. Bunyathyan &
water/chloroform; ninhydrin Gevorgyan
preconcentration detection (1984)
and clean-up on
anion-exchange
and cation-exchange
resin; evaporation
to dryness
Table 3. cont'd (2)
Medium Sampling Preparations Derivatization Analytical Limit of Recovery Reference
volume or reagent method determinationa
weight
Water 250 ml extraction with o-phthalaldehyde LC 3.2 µg/litre 89% Wigfield &
dichloromethane; Lanouette
adsorption on (1990)
anion-exchange
resin
Water 25 ml extraction with FMOCCl HPLC and TLC 0.02 µg/litre 80% Gauch et al.
dichloromethane/ (1989)
2-propanol;
acidification
with H2SO4;
evaporation
to dryness
Water 1-1.5 litre no extraction; TLC with - 0.05 mg/litre n.r. Bunyathyan &
preconcentration ninhydrin Gevorgyan
and clean-up detectionc (1984)
with anion-exchange
and cation-exchange
resin
Soil 5 g extraction with trifluoroacetic GC-NPD 0.05 mg/kg 75% Roy & Konar
deionized water/ anhydride/ (1989)
H3PO4; addition trifluoroethanol
of Darco
charcoal
Table 3. cont'd (3)
Medium Sampling Preparations Derivatization Analytical Limit of Recovery Reference
volume or reagent method determinationa
weight
Soil 2 g (sandy extraction with FMOCCl HPLC 1 mg/kg 80-119% Miles & Moye,
soil); 25 g KH2PO4 (sandy soil), (1988b)
(clayish KOH (clayish soil);
soil) no clean-up
Soil, 5 g (soil); extraction with NH4OH; ninhydrin LC 0.05-0.1 73-79% Thompson
sediment, 20 g (sed); adsorption on mg/kg (soil); (soil) et al. (1989)
foliage 5 g (fol) anion-exchange resin; 0.1 mg/kg 65-84%
further clean-up with (sed); 0.3 (sed)
Dowex cation-exchange mg/kg (fol)d 81-84%
resin (fol)
Urine and 5-6 g extraction with H2O HPLC (ion n.r. 81-99% Monsanto
faeces of (only faeces); protein pair, strong (1988a)
the rat precipitation and anion and
lyophilization cation-exchange),
(only urine); LSC, 1H NMR, 31P
clean-up with C18 NMR, GC/MS
column
Urine n.r. adsorption on trifluoroacetic GC-MS and 0.1 mg/litre n.r. Jauhiainen
(human anion-exchange resin anhydride/ GC-EC et al. (1991)
male) (SAX); elution of the trifluoroethanol
resin with HCl;
evaporation to
dryness
Table 3. cont'd (4)
Medium Sampling Preparations Derivatization Analytical Limit of Recovery Reference
volume or reagent method determinationa
weight
Serum 0.5 ml extraction with p-toluene HPLC with UV n.r.e n.r Tomita et
(human) trichloroacetic sulfonyl chloride detection al. (1991)
acid; adsorption
on anion-exchange
resin; elution
with HCl; evaporation
to dryness
a In no study with a non-liquid medium was it reported whether the limit of determination was based on dry or fresh weight,
except in the study of Thompson et al. (1989).
b The order of magnitude was reported to be picomol.
c The use of TLC with ninhydrin, copper nitrate and rhodamine B detection is reported for glyphosate in distilled water in
Ragab (1978).
d The limits of determination in soil, sediment, and foliage are expressed per kg dry weight.
e Only the limit of detection was reported: 0.3 mg/litre (approximately 75% recovery).
PITC = phenylisothiocyanate; FMOCCl = 9-fluorenyl-methyl chloroformate; GC =gas chromatography;
(HP)LC = (high-performance) liquid chromatography; TLC = thin layer chromatography; MS = mass spectroscopy;
EC = electron capture detector; NPD = nitrogen-phosphorus detector; n.r. = not reported;
LSC = liquid scintillation counting; NMR = nuclear magnetic resonance; sed = sediment; fol = foliage
The second preparative step is the clean-up, which may include
extraction, preconcentration by evaporation, ion-exchange
chromatography or gel chromatography. Clean-up procedures may
involve different combinations of chromatographic techniques. In a
validation study in which plant tissues and water were analysed, a
Chelex column was combined with anion-exchange clean-up (Cowell
et al., 1986). No chromatography was included in the clean-up
procedures for analysing glyphosate and AMPA in natural waters
(Miles et al., 1986). In this procedure samples were successively
filtrated, supplied with phosphate buffer, concentrated by
evaporation, and filtrated, prior to derivatization.
Samples with urine and faeces of the rat were subjected to clean-up
with a C18 column (Monsanto, 1988a). Prior to this extraction,
proteins were precipitated and the samples were lyophilized; samples
of faeces were, however, only extracted with water.
The third preparative step is derivatization. Derivatization
with a fluorogenic reagent is common. Burns (1983), however,
developed a preparation technique without derivatization.
Derivatization prior to detection and measurement with HPLC can be
pre-column (Miles et al., 1986; Lundgren, 1986; Miles & Moye,
1988a) or post-column (Moye et al., 1983; Tuinstra & Kienhuis,
1987). 9-Fluorenylmethyl chloroformate, phenylisothiocyanate and
1-fluoro-2,4-dinitrobenzene may be used as pre-column reagents,
whereas ortho-phthalaldehyde-mercaptoethanol and ninhydrin may be
used as post-column fluorogenic reagents. With post-column
techniques, derivatives can be formed on-line, but it requires more
equipment and experience. On the other hand, pre-column techniques
are often more rapid and require less equipment and experience. In
general the facilities required for derivatization with fluorogenic
substances are very specific, and therefore not available in many
laboratories (Konar & Roy, 1990). These authors proposed
derivatization with a mixture of trifluoroacetic anhydride and
trifluoroethanol prior to analysis with gas chromatography as a
simpler, less laborious and more economical method. This proposal
referred to the determination of glyphosate and AMPA in plant
tissues. This and other recently developed techniques of clean-up
and derivatization are summarized in Table 2. These techniques are
intended to simplify and improve preparative techniques, which in
general used to be complex and costly (Marcotte et al., 1977;
Guinivan et al., 1982; Roseboom & Berkhoff, 1982; Moye et al.,
1983; Moye & Deyrup, 1984; Deyrup et al., 1985; Miles et al.,
1986; Lundgren, 1986; Miles & Moye, 1988b).
Sample preparation and derivatization, as developed by Powell
et al. (1990) for cyanobacteria without deproteinization (see
Table 3), should also be usable for plant and animal tissue. In this
case, a simple maceration step prior to ethanol extraction should be
included. Bunyathyan & Gevorgyan (1984) developed preparative
techniques for different media prior to analysis with TLC. Only
their procedures for plants and water are summarized in Table 3. The
preparative technique for soil samples was comparable with that of
Thompson et al. (1989), although samples of 25-50 g were required.
Bunyathyan & Gevorgyan (1984) also developed a method for preparing
20-litre air samples prior to TLC. They extracted the residues
collected on a filter with water before clean-up on a
cation-exchange resin.
2.5.2 Analytical methods
Various analytical methods for the determination of glyphosate
have been described, including thin-layer chromatography (Young
et al., 1977; Ragab, 1978; Bunyathyan & Gevorgyan, 1984),
colorimetry (Glass, 1981), differential pulse polarography (Friestad
& Bronstad, 1985), gas chromatography (Guinivan et al., 1982; Moye
& Deyrup, 1984; Deyrup et al., 1985), high-performance liquid
chromatography (Miles & Moye, 1988a; Powell et al., 1990), and
31P NMR (Dickson et al., 1988). Some of these techniques, their
analytical recoveries and limits of determination are listed in
Table 3. The corresponding determination limits for AMPA, i.e.
analysed with the same techniques, are listed in Table 4. Recoveries
in the different media appear to be higher for glyphosate than for
AMPA. This is probably due to optimization of the systems for
glyphosate, as was done by Thompson et al.(1989).
Table 4. Limits of determination of AMPA
Medium Limit of Recovery Reference
determination
Plants 0.01 mg/kg 61-73% Konar & Roy (1990)
Water 1.2 µg/litre 86% Wigfield & Lanouette (1990)
Soil 0.01 mg/kg 66% Roy & Konar (1989)
Soil 0.03-0.05 mg/kg 58-68% Thompson et al. (1989)
Sediment 0.03 mg/kg 54-67% Thompson et al. (1989)
Foliage 0.008 mg/kg 55-70% Thompson et al. (1989)
Urine (human) 0.05 mg/litre n.r. Jauhiainen et al. (1991)
Serum (human) n.r.a n.r. Tomita et al. (1991)
a n.r. = not reported; only the limit of detection was reported: 0.2 mg/litre
(approximately 88% recovery)
TLC techniques are generally based on silica gel or cellulose
plates; cellulose plates give a better separation (Dubelman, 1988).
Ninhydrin and phosphate sensitive reagents may be used for
detection, although interference from co-extractives may occur.
According to Dubelman (1988), fluorogenic reagents may be preferable
in case of interference.
Fluorogenic derivatives can be determined in HPLC analysis with
fluorescence detectors (Wigfield & Lanouette, 1990) and also with a
spectrophotometer (Powell et al., 1990). In a GC analysis a
nitrogen-phosphorus, electron capture or a flame photometric
detector can be used.
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1 Anthropogenic sources
3.1.1 Production levels and processes
No data on the world production of glyphosate and its
formulations are available. In addition, no data on losses to the
environment during normal production and formulation or accidental
losses have been reported.
The first phase of the production of glyphosate consists of
refluxing a mixture of glycine (50 parts), chloromethylphosphonic
acid (92 parts), an aqueous solution with 50% sodium hydroxide (150
parts), and water (100 parts) in a suitable reaction vessel. Another
50 parts of an aqueous solution with 50% sodium hydroxide are added
to maintain the pH between 10 and 12, whereafter the reaction
mixture is refluxed for another 20 h. The mixture is then cooled to
room temperature and filtered. After adding 160 parts of
concentrated hydrochloric acid, this mixture is again filtered.
Glyphosate will slowly precipitate in the filtrate (IRPTC, 1991).
3.1.2 Uses
Glyphosate is a post-emergent, systemic and non-selective
herbicide intended for use against deep-rooted perennial species,
and also biennial and annual broad-leaved, grass and sedge species
(WSSA, 1983; Monsanto, personal communication to the IPCS, 1991).
Glyphosate is used in both agriculture and forestry. Fields of
agricultural use include grassland renovation, horticulture,
fructiculture, arable cultivation, and rice cultivation. Use in
forestry includes the killing of fast growing competitors in conifer
plantations or conservation areas, and the treatment of tree stumps.
Glyphosate may also be used for weed killing in non-agricultural
areas such as water systems, including irrigation and temporarily
drained waters, parks, road verges and gardens.
The uses of glyphosate indicate that it can be applied in
various crops for specific purposes. The major formulation Roundup
may, for instance, be used in pre-plant treatments for seed bed
preparations, and also against bracken infestations in forestry,
against couchgrass (Elytrigia repens) infestations on pastures, in
direct treatments between rows of crops, or by direct wiping of the
leaves of the weed, assuming the weeds are taller than the existing
crop.
Glyphosate is used worldwide. In 1987, 35 160 ha of the area in
British Columbia where vegetation management activities took place
had been treated with Roundup. This was 94% of the total area where
there were such activities (Ackurst, 1989).
The application rates of glyphosate are dependent on the
formulation and type of use. In the Netherlands, recommended rates
for the application of Roundup are 0.3-2.9 kg a.i./ha. In Canada the
recommended application rates of Roundup are 1.1-1.7 kg a.i./ha for
annual weeds and 1.2-5.8 kg a.i./ha for perennial weeds. The
recommended application rates for Vision in Canadian forestry are
1.1-2.1 kg a.i./ha (Task Force on Water Quality Guidelines, 1991).
Glyphosate is generally applied as a 0.5-5% solution in water by
spraying, and as a 10-50% solution in water by wiping with, for
instance, a rope-wick (Monsanto, personal communication to the IPCS,
1991).
The timing of application is dependent on the use. Application
in late summer or autumn is recommended for use in forestry in
Canada (Hildebrand et al., 1982). Application in agriculture may
be pre- or post-harvest. In the Netherlands, for instance,
glyphosate may be applied to cereals, potatoes and asparagus
immediately (up to 7 days) before harvest, but only when the
ripening is complete. Treatment of immature crops would result in
higher residue levels, early crop desiccation and reduced yields.
Glyphosate may be applied in different ways. For large-scale
treatments aerial application can be appropriate, small-scale
treatments can be done with spraying equipment on the back or behind
vehicles, or by wiping equipment.
Aerial applications will lead to losses due to wind-drift.
Exposure of flora and fauna due to off-target deposits may take
place. These downwind deposits depend on the meteorological
conditions, the plant canopy structure and the application method,
including the release height (Payne et al., 1989; Feng et al.,
1990; Payne, 1992; Payne & Thompson, 1992). The non-volatile
tank-mix fraction and the speed of the aircraft may influence the
drop-size spectrum, and it can be expected that dispersal systems
causing relatively small droplets and having a relatively low
non-volatile fraction will cause the highest off-target deposits.
Payne (1992) assumed that the large differences in deposits in two
comparable experiments were due more to different aircraft airspeeds
than to different wind speeds. In these experiments the maximum
deposits at a downwind distance of 50 m were 19 and 3 mg a.i./m2
at aircraft airspeeds of 45 and 11-20 m/second, respectively. The
application rate in both experiments was 2.1 kg a.i./ha. In other
experiments with the same application rate, Payne & Thompson (1992)
found that the meteorological conditions had a considerable impact
on the off-target deposition up to 400 m downwind when spraying at
different wind speeds (2.2-5.7 m/second) and turbulences. The
deposits at a downwind distance of 400 m varied between 0.001 and
0.06 mg a.i./m2, whereas they varied between 0.6 and 4 mg
a.i./m2 at a downwind distance of 50 m. Remarkably, the deposition
was highest with an intermediate wind speed and intensity of
turbulence. Payne et al.(1989) investigated the deposits for
aerial applications of Roundup with different dispersal systems.
When 2.1 kg a.i./ha was applied with a helicopter in a single
crosswind swath over 100 ha, up to 13.4 mg a.i./m2 was deposited
on a downwind distance of 50 m. This maximum deposition was caused
by a D8-46 hydraulic nozzle, whereas the highest depositions with a
Thru Valve Boom and a Microfoil Boom were 2 and 0.4 mg a.i./m2,
respectively. These depositions were also found at a downwind
distance of 50 m. At the time of application the windspeed 13 m
above ground level was 0.4-0.5 m/second. Riley et al.(1991)
modelled spray deposition of glyphosate using results from
helicopter applications under semi-operational conditions. The study
was designed to test the appropriateness of a New Brunswick "buffer
zone" of 65 m to minimize the effects of spray drift. At a distance
of 65 m, it was estimated that between 3.7% and 5.6% of the nominal
spray rate was deposited.
3.1.3 Drinking-water
Appraisal
The low mobility of glyphosate in soil would indicate a
minimal potential for the contamination of drinking-water from
groundwater aquifers. The only possible source of drinking-water
contamination is, therefore, surface waters. There have been no
reported incidences of drinking-water contamination with
glyphosate.
Conventional plants for processing of drinking-water would not
remove glyphosate, but this could be achieved by coprecipitation
after adding iron salts (AMA van der Linden, personal communication
to the IPCS, 1991). Ozone, increasingly used as an alternative to
chlorine in drinking-water treatment, does effectively remove
glyphosate through the hydroxyl radical (HO. ) chain processes that
occur in most ozonated waters (Yao & Haag, 1991; Haag & Yao, 1992).
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION AND TRANSFORMATION
4.1 Transport and distribution between media
Appraisal
Following application, glyphosate selectively partitions to
particulate matter suspended in surface water or to the soil
substrate. This partitioning is usually rapid and occurred within 14
days in reported studies. The mechanism of sorption to soil is only
partially understood. Glyphosate can adsorb to soils through
phosphate binding sites. Competition with inorganic phosphate has
been demonstrated in the laboratory but not measured in the field.
Specific ions (Fe2+, Fe3+ and Al3+ ) complex glyphosate; metal
complexes with humic acids in soil may be a main binding mechanism
for glyphosate in soil. There is little reported information on
desorption from soil; the data available suggest "strong" binding.
This is supported by mobility studies which show little leaching of
glyphosate below the upper few centimetres of the soil profile. The
major metabolite, AMPA, is also retained in the upper soil layers.
There is very little information on the bioavailability of
sediment-bound glyphosate to either aquatic or terrestrial
organisms. Bioaccumulation and ecotoxicity studies have not,
generally, been performed with added sediment.
Applied glyphosate can be translocated in plants. Glyphosate
in plant foliage or leaf litter does not seem to represent a source
of contamination of aquatic systems. Animals can ingest the
herbicide residues in or on plants.
Dissipation of glyphosate from soil has been widely studied
with very variable results (DT50 between 3 and 174 days).
Biodegradation appears to be the major source of dissipation.
Run-off was minimal in experimental studies, but field results
suggest that aquatic systems may be receiving glyphosate bound to
soil particles following rainfall.
In this chapter the terms biodegradation and dissipation are
used to distinguish between the decrease of the concentration in,
for instance, the soil that is due to microbes transforming the
molecule to a smaller size (biodegradation) and the decrease of the
concentration that might be due to microbial activity but also to
other processes, e.g., sorption, leaching and run-off (dissipation).
4.1.1 Water
Glyphosate dissipates from the water with 50% dissipation times
ranging from a few days to 2 weeks (Newton et al., 1984; Monsanto
1990a; see also Table 5). These DT50 values were deduced from both
laboratory and field experiments in which sediment or suspended
particles were shown to be the major sink.
In water with a near-neutral pH, the formation of an insoluble
complex of Ca2+ with glyphosate was demonstrated in a laboratory
experiment (Subramaniam & Hoggard, 1988). It was confirmed with
X-ray powder diffraction and infrared spectra that this complex was
not an ionic salt. At a near-neutral pH, the dianionic species of
glyphosate is dominant. Insoluble complexes have also been found
with Mg2+, Fe3+ and Cu2+.
In a field experiment in a temperate coastal rainforest in
British Columbia, Canada, the highest concentration of glyphosate in
water was 162 µg/litre (Feng et al., 1990). This maximum was found
in a directly sprayed tributary 2 h after an aerial application of
Roundup at a rate of 2 kg a.i./ha. Concentrations in oversprayed
tributaries without a high cover of overhanging riparian vegetation
increased after the first rainfall. In oversprayed tributaries with
a high cover of riparian vegetation almost no residues were found.
Within 96 h after application the residues in all waters had
declined below detection limits, indicating rapid dissipation. After
rainstorms, peak concentrations of glyphosate were found in the
sediments and on suspended particles of the oversprayed tributaries,
with maximum concentrations of 7 mg a.i./kg dry weight and 0.06 µg
a.i./litre unfiltered water, respectively. The amounts in the
sediments of these waters were variable but declined over time. As
0.1-2 mg residue/kg dry weight sediment was found between 196 and
364 days after application, the residues appear to be persistent in
sediments of oversprayed waters. Feng et al.(1990) concluded,
therefore, that after rainstorms sediments appear to be the major
sink.
In another field experiment in the same forest ecosystem,
glyphosate dissipated rapidly from a small perennial, very slow
flowing stream, in a site of 8 ha aerially sprayed with Roundup at a
rate of 3.3 kg a.i./ha (Newton et al., 1984). In water, 50% of the
initial concentration had dissipated in 2 days. In sediment, maximum
concentrations of approximately 0.6 mg a.i./kg were found 14 days
after application. These were reduced to approximately 0.3 mg
a.i./kg in 28 days, and to < 0.2 mg a.i./kg in 55 days after
application. A comparable rapid dissipation from the water column
was found for small forest ponds in a boreal forest in Manitoba,
Canada, after applying Roundup at a rate of 0.9 kg a.i./ha
(Goldsborough & Beck, 1989). The highest concentration in filtered
water was 141 µg a.i./litre, within 6 h after application. The main
mechanism of dissipation was probably sorption to the sediment. This
was confirmed by additional experiments with polyethylene basins
filled with unfiltered water and sediment that were placed in the
spray zone. Without sediment, only a very small amount of the dose
actually applied had dissipated after 30 days, whereas with sediment
the initial concentrations in the water had decreased by 50%,
approximately 6 days after application.
Comparable dissipation patterns were found in a field
experiment (Monsanto, 1990a) in which Accord (30.5% a.i. w/w) was
applied at a rate of 4.2 kg Accord/ha on three forestry sites with
non-flowing pond water and flowing water. Concentrations of up to
1700 µg/litre filtered water were found in the pond water
immediately after spraying. The initial concentrations in both pond
and flowing water were reduced by 50% within 7 days. Concomitantly
initial AMPA concentrations (maximally 35 µg/litre) were reduced by
50% within the same period. In flowing water the dissipation of both
glyphosate and AMPA was even more rapid. Concentrations of
glyphosate increased up to 19 mg/kg dry weight in the sediment of
one pond 28 days after application. Concentrations of up to 1 mg/kg
of both compounds were measured in the sediments of non-flowing
ponds up to 400 days after application.
In field experiments in turbid Australian irrigation water,
glyphosate adsorbed to suspended particles at different rates,
apparently mainly depending on the application rate (Bowmer et al.,
1986). At an initial concentration of 5 mg a.i./litre, 10-16% of the
load adsorbed to suspended matter, whereas at an initial
concentration of 0.05 mg a.i./litre, 53-71% adsorbed. In more saline
water the degree of sorption was less, probably due to rapid
flocculation. Maximum adsorbed amounts were approximately 7000 mg
a.i./kg in less saline supply water, and approximately 2500 mg
a.i./kg in more saline drainage water. When a supply channel was
emptied before spraying with 3.6 kg a.i./ha for control of aquatic
weeds, and filled again with water 4 days after the treatment, the
amount in the unfiltered water used for irrigation was 7% of the
applied dose.
4.1.2 Soil sorption
Glyphosate is readily bound to many soils and clay minerals
(Sprankle et al., 1975; Hance, 1976; Glass, 1987; Miles & Moye,
1988b). In laboratory experiments in which glyphosate was added to
aqueous soil suspensions, the adsorption coefficient Ks/l was
18-377 dm3/kg in nine soils ranging from sandy loam to peat
(Hance, 1976), and 33-76 dm3/kg in three soils ranging from sandy
loam to clay loam (Glass, 1987). These Ks/l values indicate strong
sorption. In both experiments the sorption could be described by the
Freundlich equation. Glass (1987) found Ks/l values for the clay
minerals montmorillonite, illite and kaolinite of 138, 115 and 8
dm3/kg, respectively.
Table 5. Biodegradationa of technical grade glyphosate in water and sediments in the laboratory
Water type Sediment Test Sediment Organic Temperature pH of Experimental Parameter Time Reference
type type (%) matter in (°C) water time (days)
sediment (days)
(%)
Pond water silty A 17 0.9 23-25 5.9-7.0 30 DT50 14b PTRL East
clay Inc. (1990a)
loam
Pond water silty An 16 0.9 20-27 5.7-6.5 365 DT50 14c,e PTRL East
clay Inc. (1990b)
loam
Surface waterd n.r. A 9 n.r. 30 8.2-8.6 14 DT50 < 14 Monsanto
(1972a)
Lake water sandy An 33 1.4 30 6.6 42 DT50 22e Monsanto
clay (1978a)
loam
a Biodegradation in the whole system
b The biodegradation stopped after approximately 15 days
c The biodegradation stopped after approximately 150 days
d Three rivers and one lake in the USA
e Approximate value derived from data of the author(s)
A = aerobic; An = anaerobic; n.r. = not reported.
The mechanism of sorption of glyphosate to soil is only
partially understood. Several factors may be involved. The
phosphonic moiety adsorbs weakly to unoccupied phosphate binding
sites and can be displaced by phosphate (Hance, 1976). In laboratory
experiments with nine soils the author showed that sorption was
positively correlated with the unoccupied phosphate sorption
capacity, and not correlated with the total phosphate sorption
capacity, organic matter, clay, iron or aluminium content. No data
are available that confirm competition of glyphosate and phosphate
under field conditions, e.g., after application of artificial
fertiliser. Miles & Moye (1988b) suggested that the main mechanism
was probably by H-bonding and ion-exchange, as the degree of
sorption in their experiments was not correlated with cation
exchange capacity (CEC) values or surface areas. Contrary to the
results of Miles & Moye (1988b) and of Hance (1976), sorption
appeared to be correlated with CEC values and clay content in a
sorption study with clay loam, silt loam and sandy loam (Glass,
1987).
The binding is also influenced by the presence of specific
cations. Hensley et al.(1978) demonstrated that Fe2+, Fe3+
and Al3+ inactivated glyphosate much more than Ca2+, K+ and
Na+. This was confirmed by Glass (1987) and Sprankle et al.
(1975). Glass (1987) suggested that glyphosate was complexed by
cations, released from cation-saturated clays via a cation-exchange
with solution protons.
According to Heinonen-Tanski (1989), most of the soil-bound
residues of glyphosate were recovered in the fulvic acid fraction
(21-33%). Sorption of glyphosate to fulvic acids was also reported
by Madhun et al.(1986), who added 14C-glyphosate to an aqueous
soil extract (ASE) of peat. In this study sorption was mainly on ASE
fractions with a relative molecular mass ¾ 1000. Piccolo et al.
(1992) studied the interaction of glyphosate with a pure iron-humic
acid complex. Maximum adsorption values indicated that adsorption to
the complex occurred to as great an extent as to whole soils. This
suggested that humic acid complexes with polyvalent cations might
represent a main binding substrate for glyphosate in soils. There
was no desorption of bound residues of glyphosate following shaking
with 0.01 mol CaCl2/litre solution for 48 h, the maximum shaking
time for the adsorption studies.
Desorption of glyphosate with ionized water from
montmorillonite and illite needed three days before reaching an
equilibrium in a study of Miles & Moye (1988b).
It can be concluded that sorption of glyphosate can be expected
in the presence of available phosphate binding sites, the presence
of iron and aluminium (oxides or hydroxides), and appropriate
combinations of clay and organic matter.
4.1.3 Mobility in soils
In view of its Ks/l, glyphosate can be expected to be
immobile or slightly mobile in many soils. This was confirmed by
several experiments, both in the laboratory and in the field. In
thin-layer chromatography studies with sandy loam, clay loam and
sandy clay loam, the Rf values of 14C-glyphosate were 0.14-0.20
(Sprankle et al., 1975). In comparable studies with silt loam,
silty clay loam, and sandy loam Rf values were < 0.2 (Monsanto,
1972c). In a leaching study with columns of 30 cm and a high water
flux of 51 cm over less than 2 days, < 0.1-6.6% of the applied
activity was leached (Monsanto, 1978b). This experiment was
performed with eight soils, ranging from sandy loam (organic matter
content 0.7%) to volcanic ash (organic matter content 9.5%). More
than 90% of the applied activity was recovered in the upper 0-14 cm
layer.
Only one leaching study under laboratory conditions with
respect to the mobility of AMPA has been reported. In this
experiment with 30-day-old residues, < 0.1-1.6% of the applied
activity was leached over 45 days (Monsanto, 1978b). The columns
were 30 cm and the water flux over 45 days was low (17 cm). The
amount of AMPA that was recovered after 45 days in the upper 0-2 cm
layer was low (0.5-12% of the applied activity), due to a high rate
of mineralisation.
4.1.4 Dissipation from the soil in the field
Many field experiments on the dissipation of glyphosate from
the soil have been performed. Some relevant studies are summarized
in Table 6. They indicate DT50 values based on dissipation that
range from 3 to 174 days depending on edaphic and climatic
conditions. In a forest brush ecosystem in Oregon, USA, the DT50
value in loam was 29 days with and 40 days without litter (Newton
et al., 1984). In field experiments in Sweden, Roundup was sprayed
over reforestated sites (Torstensson et al., 1989). In the soils
of these sites the DT50 values were < 50 days, apparently
depending on the soil respiration rate. The dissipation consisted of
a fast first, and a much slower second phase, especially in sites in
northern Sweden, which was possibly due to a longer frost period. In
these sites 1-2% of the actually applied dose was recovered 1080
days after application. A comparable dissipation pattern was found
in a field experiment on Finnish agricultural soils (Heinonen-Tanski
et al., 1985). In this experiment 25% of the concentration in a
sandy loam 2 days after the treatment was recovered one year after
application. The application rate was 1.4 kg a.i./ha.
A study in a temperate coastal rain forest in British Columbia,
Canada, showed that, 360 days after application, 6-18% of the
initial levels was recovered (Feng et al., 1990). In this
experiment Roundup was applied at a rate of 2 kg a.i./ha. The soils
were alluvial sandy loam or sandy clay loam with highly organic
surface horizons. Some of these soils were well drained, others were
seasonally flooded. At each sampling time more than 90% of the
recovered residues was in the upper 0-15 cm layer. Under all
conditions the amount of glyphosate declined over time, whereas
there was a transient increase of AMPA.
In other field experiments on boreal forest soils, comparable
dissipation patterns were found. Stark (1983) reported DT90 values
of 30-720 days, and Roy et al.(1989b) found a DT50 value of
approximately 20 days on a sandy soil planted with jackpines (Pinus
banksiana). In the field experiments of Roy et al.(1989b),
glyphosate was detectable up to 335 days after application; almost
all residues in the sandy soil were recovered in the organic top
layer. In field experiments of Monsanto (1990a) in three forest
locations in the USA, the concentration course of glyphosate
appeared to be rather irregular, especially during the first four
months. However, 50% of the initial concentrations in the soil had
mostly dissipated within 120 days. One clear exception was a site in
Corvallis in which glyphosate increased up to 0.15 mg/kg dry weight,
180 days after application. On the same site AMPA increased up to
0.32 mg/kg, 346 days after application. The application rate in
these experiments was 4.2 kg Accord/ha.
On a clay soil of a clear-cut boreal forest area, Roy et al.
(1989b) found no dissipation of glyphosate due to run-off on a 8°
slope. In a field experiment on agricultural soils without
conventional tillage, the dissipation of glyphosate due to run-off
on 6-16° slopes was < 1% of the applied dose when 1.1-3.4 kg
a.i./ha was applied (Edwards et al., 1980). However, when 9.0 kg
a.i./ha was applied, 1.8% of the applied dose dissipated due to
run-off, mainly because of a rainstorm shortly after application.
4.1.5 Uptake and dissipation from plants
Uptake of 14C-glyphosate by leaves of trembling aspen
seedlings (Populus tremuloides) was initially rapid, after which
it slowed down (Sundaram, 1990). The seedlings were exposed to
Roundup that was dripped with a micro-applicator on some central
leaves. The application rate was 0.35 kg a.i./ha leaf surface area.
Most activity was washable from the leaves (61-77%), and 22-28% was
recovered in the treated leaves within 48 h. As only 1-10% was
recovered in the other parts of the seedlings, this indicated a
rather low translocation after absorption. A rapid uptake of
14C-glyphosate within a few hours was indicated for sugar beets
(Beta vulgaris), when applied to a mature leaf (Gougler & Geiger,
1981). 14C-glyphosate probably entered the phloem in a
non-facilitated way. The subsequent transport through the phloem
Table 6. Biodegradation and dissipation of glyphosate in soils
Soil type Compound Test Moisture Temperature pH Organic Experimental DT50 Reference
type content (°C) matter duration (days)
(%) (%) (days)
Biodegradation
Sandy loam Tgg L,A 14-16 25 7.3 2.8 360 2b PTRL East Inc. (1991)
Silt loam Tgg L,A 12-14 25 7.5 1.0 360 2b PTRL East Inc. (1991)
Dissipation
Sandy loam Tgg G 11 32 5.7 1.0 112 130b Monsanto (1972b); Rueppel
et al. (1977)
Silt loam Tgg G 11 32 6.5 1.0 112 3b Monsanto (1972b); Rueppel
et al. (1977)
Silty clay loam Tgg G 11 32 7.0 6.0 112 25-27b Monsanto (1972b); Rueppel
et al. (1977)
Sand Ru F n.r. n.r. 3.5-3.7 40 762 approx Roy et al. (1989b)
(humoferric 20a
podsol)
Sandy loam, Ru F n.r. n.r. 4.2-4.9 15-31 360 45-60b Feng & Thompson (1990)
sandy clay loam
Loam Ru F n.r. n.r. 4.0-4.7 3.8-5.2 55 29-40b Newton et al. (1984)
Loamy sand Ru F n.r. n.r. n.r. 0.8 370 3-4b Monsanto (1983a)
Sandy clay loam Ru F n.r. n.r. n.r. 7.0 370 122-174b Monsanto (1983a)
a Based on data of the author(s) b Data reported by the author(s)
L = laboratory study; F = field study; G = greenhouse study; A = aerobic; An = anaerobic;
Tgg = technical grade glyphosate; Ru = Roundup; n.r. = not reported
appeared to be according to an "intermediate permeability
mechanism". When exposed for a longer time, plants may show
substantial translocation of absorbed 14C-glyphosate, as was shown
for potatoes (Solanum tuberosum) by Smid & Hiller (1981). In the
treated leaves of the potatoes 45% of the absorbed activity was
recovered, whereas the rest was mainly translocated to the apical
meristem and the roots. Up to 5% was recovered in the mother tuber.
The degree of translocation was age-dependent, as older plants
showed less translocation than younger plants.
The uptake of glyphosate by red raspberries (Rubus strigosus)
was 9% of the amount that was deposited on the leaves after spraying
Roundup at a rate of 2 kg a.i./ha (Roy et al., 1989a). In the same
field experiment the uptake was 14% by wild blueberries (Vaccinium
myrilloides). Most glyphosate was recovered in the washings, which
was also found under laboratory conditions. The initial absorbed
amounts were 0.92-2.0 mg a.i./kg dry weight. The absorbed and
washable amounts together were reduced by 50% within 13 days in the
raspberries and within 20 days in the blueberries. AMPA was
detectable up to 33 days after application. Metabolism occurred to
only a minor extent as AMPA concentrations were less than 1.5% of
the concurrent concentrations of glyphosate (similar results were
reported by FAO/WHO, 1986b). In a field experiment by Feng &
Thompson (1990) in a temperate coastal rainforest in British
Columbia, Canada, the main target species for treatment with Roundup
were red alder (Alnus rubra) and salmonberry (Rubus spectabilis).
Immediately after spraying, the concentrations in leaf tissue were
up to 448 mg a.i./kg dry weight. Glyphosate dissipated rapidly from
the leaf litter with a DT50 value of 8-9 days. The leaf litter
included leaves directly exposed on the trees and existing leaf
litter from natural defoliation before treatment with Roundup. The
authors assumed that leaf litter of these major brush species is an
insignificant source of glyphosate input into streams or onto forest
floor, because of the fast dissipation. A rapid dissipation of
glyphosate from fresh foliage was also found in a field study
(Monsanto, 1990a) in which initial concentrations of up to 1300 mg
a.i./kg and 2.6 mg AMPA/kg decreased rapidly. A transient
accumulation of glyphosate and AMPA was found in the leaf litter on
some sites, but these amounts were reduced by approximately 90%
within 100 days.
Glyphosate dissipated completely from wild berries (Vaccinium
vitis-idaea, Vaccinium myrtilus) within one year in a field
experiment in Finland in which Roundup was applied at a rate of
0.25-2.2 kg a.i./ha with a knapsack sprayer (Siltanen et al.,
1981). Contrary to this dissipation pattern was that of glyphosate
in reindeer lichens (Cladonia rangiferina) that were sampled in
the same experimental plots. Around 270 days after application,
dose-related concentrations of glyphosate and AMPA were recovered in
lichens with maxima of 45 and 2.1 mg/kg for glyphosate and AMPA,
respectively. Approximately 390 days after application of 0.8 kg
a.i./ha, 6.4 and 0.3 mg/kg of glyphosate and AMPA were still
detectable.
4.1.6 Ingestion by animals
As the concentration in the foliage may increase up to high
amounts immediately after application, this implies the possibility
of entry into the food chain through ingestion by herbivorous or
omnivorous animals. This was confirmed by Sullivan & Sullivan (1979)
who investigated the effects of glyphosate on the feed preference
and daily chow consumption of black-tailed deer (Odocoileus
hemionus columbianus). These herbivores did not avoid eating
browse of alder (Alnus rubra) and alfalfa (Medicago sativa) that
was treated with glyphosate at a rate of 2.2 kg/ha. Sometimes the
treated alder browse was even preferred. Reindeer may be exposed to
glyphosate, since reindeer lichens, which are an important food
source, can take up a substantial amount of glyphosate (see above).
4.2 Abiotic degradation
Appraisal
Hydrolysis of glyphosate is very slow. Photodegradation in the
field may occur.
4.2.1 Hydrolytic cleavage
Hydrolysis of glyphosate in sterile buffers is very slow. After
32 days < 6.3% of the applied activity was recovered as AMPA,
after applying 14C-glyphosate at rates of 25 and 250 mg/litre to
aqueous buffer solutions of pH 3, 6 and 9 (Monsanto, 1978b). These
tests were performed at both 5 and 35 °C.
4.2.2 Photodegradation
Photochemical degradation in water may occur under both
laboratory and field conditions, mainly depending on the type of
light source. In sterile aqueous buffers of pH 5, 7, and 9, less
than 1% of the applied dose was degraded (photodecomposition of
14C-phosphonomethyl-labelled glyphosate) during 29-31 days, when
exposed to sunlight (PTRL Inc., 1990).
Lund-Hoie & Friestad (1986) exposed Roundup to several light
sources under different conditions. When exposed to UV light (lambda
= 254 nm) under laboratory conditions, concentrations of 1 and
2000 mg a.i./litre in deionized water showed DT50 values of 4 and 14
days, respectively. When exposed to sunlight under field conditions
1 mg a.i./litre in polluted water without sediment showed a much
slower decomposition (DT50 > 63 days), possibly due to pollution
preventing adequate UV penetration in the water. Polluted water with
sediments showed a rapid dissipation from water, probably due to
adsorption onto the sediments. In another field experiment 2 and
100 mg a.i./litre in deionized or polluted water without sediment
showed DT50 values of < 28 days, when exposed to sunlight. At
the low concentration the dissipation in polluted water was more
rapid than in deionized water. In the dark no dissipation occurred.
In laboratory experiments 1 mg/litre of glyphosate in
sterilized natural and deionized water showed DT50 values of 4 to >
14 days when exposed to artificial light (350-450 nm) in
photoreactors without sediment (Monsanto, 1978a). In these
experiments Ca2+ acted as a photosensitizing agent.
Photodegradation by sunlight of glyphosate applied to a soil
appeared to be an insignificant route of dissipation (PTRL Inc.,
1989). In this study, 14C-glyphosate mixed with unlabelled
glyphosate was exposed for 31 days to natural sunlight, after
application to a sandy loam at a rate of 4.5 kg a.i./ha.
Extrapolated DT50 values that were based on first-order kinetics
were 90 days in the sunlight and 96 days in the dark, indicating no
substantial degradation due to photolysis. The temperature of the
soil surface was 22-23 °C. Under unnatural light conditions
glyphosate appeared not to be photodegraded substantially (Monsanto,
1972c; Rueppel et al., 1977; Monsanto, 1978a).
4.3 Biodegradation
Appraisal
Selected studies of the biodegradation of glyphosate have been
considered; selection was on the basis of test conditions and modern
methodologies. There is considerable variation in rate of breakdown
in water, aquatic sediment and soil. Degradation occurs more rapidly
in aerobic than anaerobic conditions. Half-times for biodegradation
in the three media under laboratory conditions range between a few
days and approximately 20 days. No data on biodegradation under
anaerobic conditions are available.
The main route of biodegradation of glyphosate appears to be
by splitting the C-N bond to produce AMPA. However, a second route
with splitting of the C-P bond can also occur.
A range of bacterial strains can degrade glyphosate. Bacteria
capable of using the compound as sole phosphorus, sole carbon or
sole nitrogen source have been identified. Growth is slow compared
to growth on inorganic sources of P, C or N. There is evidence from
the field that bacterial populations adapt to the metabolism of
glyphosate. Presence of inorganic phosphate inhibits degradation of
glyphosate with some, but not all, bacteria. Biodegradation of
glyphosate may involve co-metabolism.
The most relevant laboratory experiments in which the
biodegradation in systems with water and sediment have been studied
are summarized in Table 5. These studies indicate that the rate of
biodegradation may vary substantially, depending on experimental
conditions, e.g., the availability of oxygen, temperature and type
of sediment. The time needed for 50% biodegradation of glyphosate in
the whole system of a test with water and sediment is < 14 days
under aerobic and 14-22 days under anaerobic conditions in the
laboratory.
In the experiments of PTRL East Inc. (1990a,b), less then 10%
of the applied activity was recovered in the pond water over a
period of 30 days under aerobic condition and 365 days under
anaerobic conditions. During all experiments more than 50% of the
applied activity was recovered in the sediment.
In experiments with water and their associated sediments the
amount of a.i. declines over time with a generally transient
increase of 14C-AMPA, an increase of 14CO2, and an increase of
sediment-bound residues. An exception to this pattern of
biodegradation can be observed in some aerobic and anaerobic
experiments that were performed with pond water and a silty clay
loam sediment (PTRL East Inc, 1990a,b). In this water/sediment
system the biodegradation stopped after approximately 15 days under
aerobic conditions and after approximately 150 days under anaerobic
conditions. The glyphosate residues (a.i. plus AMPA) at both time
points remained approximately 40% of the applied dose, which
indicated substantial persistence in spite of the rapid initial
degradation.
AMPA is the main metabolite of glyphosate found in both the
water column and the sediment. Maximum amounts of AMPA under both
aerobic and anaerobic conditions in the sediment were 25% of the
applied activity (PTRL East Inc., 1990a,b). These maxima were found
at 7-20 days after application. In the same experiments maximum
amounts of sediment-bound residue were 9% of the applied activity
under aerobic conditions and 4% under anaerobic conditions. These
maxima were found at the end of the experiments. The amounts of
evolved 14CO2 in these studies gradually increased in most cases
up to 24 and 35% of the applied activity after 30 days (aerobic),
and 365 days (anaerobic), respectively. This indicates substantial
differences in the mineralization rate. These differences are partly
due to the availability of oxygen, since under anaerobic conditions
the mineralization rate was slower than under aerobic conditions.
This was also found by Monsanto (1972a, 1978a). In the aerobic
experiments of Monsanto (1972a), four sediments that differed by up
to two orders of magnitude in the total number of micro-organisms
did not show substantial differences in mineralization rate.
Biodegradation studies with glyphosate in the soil under
conditions where unequivocal interpretation is justified are scarce.
Table 6 summarizes some relevant studies, indicating that the
biodegradation rate may differ substantially, depending on the
experimental conditions. The laboratory and greenhouse experiments
in Table 6 were performed with moisture contents (> 75% of the
field capacity) that were adequate for optimal biodegradation.
In most laboratory experiments the biodegradation rate of
glyphosate in soils appears to be rapid (see Table 6). Mostly
biodegradation can be described with linear first-order kinetics.
Sometimes a non-linear first-order model taking into account
spatial variability better describes the results observed (PTRL East
Inc., 1991):
C = C0 (1 + ßt)-alpha
C in this equation is the concentration in the soil at time t,
C0 the initial concentration, and alpha and ß are rate constants
reflecting spatial variability.
The main metabolite under aerobic conditions of glyphosate in
soil is AMPA. In aerobic laboratory experiments the maximum amounts
in sandy loam and silt loam were 27 and 29%, respectively, of the
applied activity. These maxima were reached 14 days after
application (PTRL East Inc., 1991). From the data of PTRL East Inc.
(1991), DT50 values for AMPA of approximately 50 days in sandy and
silty loam can be derived. That AMPA is more persistent than
glyphosate was also shown in a laboratory experiment with sandy loam
(Monsanto, 1972b). The amounts of AMPA after 111 days were 10-17% of
the applied activity. In this study, the temperature (32 °C) was
higher than in the other studies discussed above.
Some minor unidentified metabolites were quantified in an
aerobic laboratory experiment lasting 364 days with sandy loam and
silt loam (PTRL East Inc., 1991). Two unknown metabolites did not
exceed 3.5% of the applied activity, whereas some other unknown
metabolites did not exceed 1.5% each. Rueppel et al.(1977)
quantified some minor metabolites that did not exceed 1% of the
applied activity. These metabolites were
N-methylamino-methylphosphonic acid, glycine,
N,N-dimethylaminomethylphos-phonic acid, hydroxymethylphosphonic
acid, and two unknown metabolites.
In aerobic laboratory experiments, the amounts of soil-bound
residues immediately after application were 9-35% of the applied
dose, after which they showed an irregular time-course during these
experiments of approximately 112 days (Monsanto, 1972b). In general,
the initial amounts were also the maximum amounts. In other
laboratory experiments however, maximum amounts of soil-bound
residues appeared to be reached after 14 days, whereafter they
remained more or less constant or even decreased (PTRL East Inc.,
1991). These maximum amounts were 7-9% of the applied activity, and
were probably lower compared with other studies due to better
extraction procedures.
Mineralization in the soil occurs under both aerobic and
anaerobic conditions in the laboratory, although the rates may
differ greatly, apparently mainly depending on the soil respiration
rate and the temperature. When 14C-phosphonomethyl-labelled
glyphosate was applied to sandy loam and silt loam, 70-78% 14CO2
evolved during an aerobic laboratory experiment of 360 days (PTRL
East Inc., 1991). In this study the application rate was 4 mg
a.i./kg dry weight. In an aerobic laboratory study with 15 Swedish
forest soils, DT50 values based on 14CO2 evolution varied
between 6 and 200 days. Mineralization was highly correlated with
the soil respiration rate, but not with pH or organic matter content
(Torstensson & Stark, 1981). This was confirmed by Torstensson &
Stenström (1986) and Heinonen-Tanski (1989). Torstensson & Stenström
(1986) reported that glyphosate was co-metabolized. In this case,
co-metabolizing microorganisms are not supplied with energy by
biodegrading glyphosate.
Establishing the correlation between soil respiration and
mineralization requires both a standardized measurement of the
respiration rate and an accurate measurement of the actual dose that
reaches the soil (Torstensson & Stenström, 1986). In a laboratory
experiment simulating temperatures under arctic conditions in forest
soils, 51-71% of the applied activity was recovered as 14CO2 217
days after application of 14C-glyphosate. In this study the
mineralization rate was reduced 10-15 times during a temperature
decrease of 10 °C over the first part of the study. The rate
increased only 3.7-4 times with a temperature increase of 10 °C
during the second part (Heinonen-Tanski, 1989).
Glyphosate in the soil appears to be degradable by
micro-organisms in two ways (Jacob et al., 1988), as shown in
Fig. 3. One route is via the formation of AMPA and a C2 fragment
which might be glyoxylate. This scheme for degradation was proposed
by many researchers (Monsanto, 1972b; PTRL East Inc., 1991). In this
route the splitting of the C-N bond is the first step. There is,
however, another route of biodegradation via sarcosine
(N-methyl-glycine) and orthophosphate, after which sarcosine is
degraded to glycine and a one-carbon unit that eventually might form
CO2 via formaldehyde (Kishore & Jacob, 1987; Jacob et al., 1988).
In this route the splitting of the C-P bond is the first step. In
experiments with 14C-glyphosate, isolated cultures of Pseudomonas
sp. strain LBr were able to degrade glyphosate according to both
routes (Jacob et al., 1988). Approximately 5% of the applied
14C-glyphosate was not degraded via AMPA, but via sarcosine.
The growth rate of bacteria isolated from a sandy loam garden
soil that was sprayed with Tumbleweed (a garden product) was less
inhibited by technical grade glyphosate than the growth rate of
bacteria from an unsprayed control (Quin et al., 1988). This
indicated adaptation of the bacterial populations of the sprayed
site. As addition of aromatic amino acids prevented growth
inhibition in the population of the unsprayed site to a greater
extent than in the population of the sprayed site, different
mechanisms of biochemical interference were indicated. The
composition of the bacterial population on the unsprayed site was
also different from the sprayed one. Pseudomonas sp. and
lactose-fermenting bacteria could be identified in an inoculum from
the sprayed soil able to use glyphosate as a sole source of
phosphorus (Quinn et al., 1988). A different regulatory mechanism
for biodegradation in unsprayed and sprayed sites was assumed: in
the latter the aromatic amino acid pathway might be regulated by
direct control of 3-deoxy-D-arabino-heptulosonate-7-phosphate (DAHP)
by the end-products, whereas in the unsprayed site DAHP synthase
might be indirectly regulated by prephenate. Also in other
experiments bacteria were shown to use glyphosate as a sole P source
(Kishore & Jacob, 1987; Pipke & Amrhein, 1988; Weidhase et al.,
1990), thereby primarily degrading glyphosate to orthophosphate and
sarcosine, by splitting the C-P bond. In the study of Weidhase et
al. (1990), 18.2% of the applied activity was recovered as sarcosine
8 h after application of 14C-1-methyl-labelled glyphosate to a
pure culture of Pseudomonas sp. GS. This biodegradation route of
glyphosate via sarcosine was also demonstrated by Kishore & Jacob
(1987). In their experiments with glyphosate as sole P source for
Pseudomonas sp. PG2982, one hour after application of
14C-labelled glyphosate, glycine, phosphate, and a one-carbon
unit, possibly formaldehyde, were identified as metabolites. After
one hour, the 14CO2 evolution when the phosphonomethyl moiety
was labelled was substantially higher, as compared with the 1- or
2-glycine-labelled moieties. The authors suggested that the
so-called phosphate-starvation-inducible proteins, as identified by
others, might be responsible for cleaving the C-P bond. In an
experiment with pure cultures of a mutant of Arthrobacter sp.
GLP-1 able to use glyphosate as a sole P source, 90% of the applied
activity was released as orthophosphate at 240 h after application
of 14C-1-methyl-labelled glyphosate (Pipke & Amrhein, 1988).
Orthophosphate inhibited further biodegradation of glyphosate.
Flavobacterium sp. was found by Balthazor & Hallas (1986) to be
able to degrade glyphosate in spite of the presence of
orthophosphate. Liu et al. (1991) showed that 12 strains of bacteria
from the family Rhizobiaceae could degrade glyphosate present in the
medium as the sole phosphorus source; although growth of the
bacteria was slower than with inorganic phosphate. Sarcosine was the
intermediate breakdown product, indicating initial cleavage of the
C-P bond, in Rhizobium meliloti, the strain used for detailed
metabolic studies.
Table 3. Sampling, preparation, and analysis of glyphosate
Medium Sampling Preparations Derivatization Analytical Limit of Recovery Reference
volume or reagent method determinationa
weight
Air n.r. collected onto an trifluoroacetic GC-MS and approx. 0.3 94% Jauhiainen
absorption liquid; anhydride and GC-EC µg/m3 et al. (1991)
evaporation to trifluoroethanol
dryness
Cyano-bacteria 100 ml dry, resuspend in PITC HPLC with a n.r.b 78% Powell et al.
methanol/sodium - radically (1990)
acetate/ column compressed
triethylamine
Plants 5 g extraction with trifluoroacetic GC-NPD 0.03 mg/kg 72-92% Konar & Roy,
water/chloroform; anhydride and (1990)
preconcentration trifluoroethanol
and clean-up on
cation-exchange
resin
Plants 25-50 g extraction with TLC with 0.01 mg/kg n.r. Bunyathyan &
water/chloroform; ninhydrin Gevorgyan
preconcentration detection (1984)
and clean-up on
anion-exchange
and cation-exchange
resin; evaporation
to dryness
Table 3. cont'd (2)
Medium Sampling Preparations Derivatization Analytical Limit of Recovery Reference
volume or reagent method determinationa
weight
Water 250 ml extraction with o-phthalaldehyde LC 3.2 µg/litre 89% Wigfield &
dichloromethane; Lanouette
adsorption on (1990)
anion-exchange
resin
Water 25 ml extraction with FMOCCl HPLC and TLC 0.02 µg/litre 80% Gauch et al.
dichloromethane/ (1989)
2-propanol;
acidification
with H2SO4;
evaporation
to dryness
Water 1-1.5 litre no extraction; TLC with - 0.05 mg/litre n.r. Bunyathyan &
preconcentration ninhydrin Gevorgyan
and clean-up detectionc (1984)
with anion-exchange
and cation-exchange
resin
Soil 5 g extraction with trifluoroacetic GC-NPD 0.05 mg/kg 75% Roy & Konar
deionized water/ anhydride/ (1989)
H3PO4; addition trifluoroethanol
of Darco
charcoal
Table 3. cont'd (3)
Medium Sampling Preparations Derivatization Analytical Limit of Recovery Reference
volume or reagent method determinationa
weight
Soil 2 g (sandy extraction with FMOCCl HPLC 1 mg/kg 80-119% Miles & Moye,
soil); 25 g KH2PO4 (sandy soil), (1988b)
(clayish KOH (clayish soil);
soil) no clean-up
Soil, 5 g (soil); extraction with NH4OH; ninhydrin LC 0.05-0.1 73-79% Thompson
sediment, 20 g (sed); adsorption on mg/kg (soil); (soil) et al. (1989)
foliage 5 g (fol) anion-exchange resin; 0.1 mg/kg 65-84%
further clean-up with (sed); 0.3 (sed)
Dowex cation-exchange mg/kg (fol)d 81-84%
resin (fol)
Urine and 5-6 g extraction with H2O HPLC (ion n.r. 81-99% Monsanto
faeces of (only faeces); protein pair, strong (1988a)
the rat precipitation and anion and
lyophilization cation-exchange),
(only urine); LSC, 1H NMR, 31P
clean-up with C18 NMR, GC/MS
column
Urine n.r. adsorption on trifluoroacetic GC-MS and 0.1 mg/litre n.r. Jauhiainen
(human anion-exchange resin anhydride/ GC-EC et al. (1991)
male) (SAX); elution of the trifluoroethanol
resin with HCl;
evaporation to
dryness
Table 3. cont'd (4)
Medium Sampling Preparations Derivatization Analytical Limit of Recovery Reference
volume or reagent method determinationa
weight
Serum 0.5 ml extraction with p-toluene HPLC with UV n.r.e n.r Tomita et
(human) trichloroacetic sulfonyl chloride detection al. (1991)
acid; adsorption
on anion-exchange
resin; elution
with HCl; evaporation
to dryness
a In no study with a non-liquid medium was it reported whether the limit of determination was based on dry or fresh weight,
except in the study of Thompson et al. (1989).
b The order of magnitude was reported to be picomol.
c The use of TLC with ninhydrin, copper nitrate and rhodamine B detection is reported for glyphosate in distilled water in
Ragab (1978).
d The limits of determination in soil, sediment, and foliage are expressed per kg dry weight.
e Only the limit of detection was reported: 0.3 mg/litre (approximately 75% recovery).
PITC = phenylisothiocyanate; FMOCCl = 9-fluorenyl-methyl chloroformate; GC =gas chromatography;
(HP)LC = (high-performance) liquid chromatography; TLC = thin layer chromatography; MS = mass spectroscopy;
EC = electron capture detector; NPD = nitrogen-phosphorus detector; n.r. = not reported;
LSC = liquid scintillation counting; NMR = nuclear magnetic resonance; sed = sediment; fol = foliage
The second preparative step is the clean-up, which may include
extraction, preconcentration by evaporation, ion-exchange
chromatography or gel chromatography. Clean-up procedures may
involve different combinations of chromatographic techniques. In a
validation study in which plant tissues and water were analysed, a
Chelex column was combined with anion-exchange clean-up (Cowell
et al., 1986). No chromatography was included in the clean-up
procedures for analysing glyphosate and AMPA in natural waters
(Miles et al., 1986). In this procedure samples were successively
filtrated, supplied with phosphate buffer, concentrated by
evaporation, and filtrated, prior to derivatization.
Samples with urine and faeces of the rat were subjected to clean-up
with a C18 column (Monsanto, 1988a). Prior to this extraction,
proteins were precipitated and the samples were lyophilized; samples
of faeces were, however, only extracted with water.
The third preparative step is derivatization. Derivatization
with a fluorogenic reagent is common. Burns (1983), however,
developed a preparation technique without derivatization.
Derivatization prior to detection and measurement with HPLC can be
pre-column (Miles et al., 1986; Lundgren, 1986; Miles & Moye,
1988a) or post-column (Moye et al., 1983; Tuinstra & Kienhuis,
1987). 9-Fluorenylmethyl chloroformate, phenylisothiocyanate and
1-fluoro-2,4-dinitrobenzene may be used as pre-column reagents,
whereas ortho-phthalaldehyde-mercaptoethanol and ninhydrin may be
used as post-column fluorogenic reagents. With post-column
techniques, derivatives can be formed on-line, but it requires more
equipment and experience. On the other hand, pre-column techniques
are often more rapid and require less equipment and experience. In
general the facilities required for derivatization with fluorogenic
substances are very specific, and therefore not available in many
laboratories (Konar & Roy, 1990). These authors proposed
derivatization with a mixture of trifluoroacetic anhydride and
trifluoroethanol prior to analysis with gas chromatography as a
simpler, less laborious and more economical method. This proposal
referred to the determination of glyphosate and AMPA in plant
tissues. This and other recently developed techniques of clean-up
and derivatization are summarized in Table 2. These techniques are
intended to simplify and improve preparative techniques, which in
general used to be complex and costly (Marcotte et al., 1977;
Guinivan et al., 1982; Roseboom & Berkhoff, 1982; Moye et al.,
1983; Moye & Deyrup, 1984; Deyrup et al., 1985; Miles et al.,
1986; Lundgren, 1986; Miles & Moye, 1988b).
Sample preparation and derivatization, as developed by Powell
et al. (1990) for cyanobacteria without deproteinization (see
Table 3), should also be usable for plant and animal tissue. In this
case, a simple maceration step prior to ethanol extraction should be
included. Bunyathyan & Gevorgyan (1984) developed preparative
techniques for different media prior to analysis with TLC. Only
their procedures for plants and water are summarized in Table 3. The
preparative technique for soil samples was comparable with that of
Thompson et al. (1989), although samples of 25-50 g were required.
Bunyathyan & Gevorgyan (1984) also developed a method for preparing
20-litre air samples prior to TLC. They extracted the residues
collected on a filter with water before clean-up on a
cation-exchange resin.
2.5.2 Analytical methods
Various analytical methods for the determination of glyphosate
have been described, including thin-layer chromatography (Young
et al., 1977; Ragab, 1978; Bunyathyan & Gevorgyan, 1984),
colorimetry (Glass, 1981), differential pulse polarography (Friestad
& Bronstad, 1985), gas chromatography (Guinivan et al., 1982; Moye
& Deyrup, 1984; Deyrup et al., 1985), high-performance liquid
chromatography (Miles & Moye, 1988a; Powell et al., 1990), and
31P NMR (Dickson et al., 1988). Some of these techniques, their
analytical recoveries and limits of determination are listed in
Table 3. The corresponding determination limits for AMPA, i.e.
analysed with the same techniques, are listed in Table 4. Recoveries
in the different media appear to be higher for glyphosate than for
AMPA. This is probably due to optimization of the systems for
glyphosate, as was done by Thompson et al.(1989).
Table 4. Limits of determination of AMPA
Medium Limit of Recovery Reference
determination
Plants 0.01 mg/kg 61-73% Konar & Roy (1990)
Water 1.2 µg/litre 86% Wigfield & Lanouette (1990)
Soil 0.01 mg/kg 66% Roy & Konar (1989)
Soil 0.03-0.05 mg/kg 58-68% Thompson et al. (1989)
Sediment 0.03 mg/kg 54-67% Thompson et al. (1989)
Foliage 0.008 mg/kg 55-70% Thompson et al. (1989)
Urine (human) 0.05 mg/litre n.r. Jauhiainen et al. (1991)
Serum (human) n.r.a n.r. Tomita et al. (1991)
a n.r. = not reported; only the limit of detection was reported: 0.2 mg/litre
(approximately 88% recovery)
TLC techniques are generally based on silica gel or cellulose
plates; cellulose plates give a better separation (Dubelman, 1988).
Ninhydrin and phosphate sensitive reagents may be used for
detection, although interference from co-extractives may occur.
According to Dubelman (1988), fluorogenic reagents may be preferable
in case of interference.
Fluorogenic derivatives can be determined in HPLC analysis with
fluorescence detectors (Wigfield & Lanouette, 1990) and also with a
spectrophotometer (Powell et al., 1990). In a GC analysis a
nitrogen-phosphorus, electron capture or a flame photometric
detector can be used.
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1 Anthropogenic sources
3.1.1 Production levels and processes
No data on the world production of glyphosate and its
formulations are available. In addition, no data on losses to the
environment during normal production and formulation or accidental
losses have been reported.
The first phase of the production of glyphosate consists of
refluxing a mixture of glycine (50 parts), chloromethylphosphonic
acid (92 parts), an aqueous solution with 50% sodium hydroxide (150
parts), and water (100 parts) in a suitable reaction vessel. Another
50 parts of an aqueous solution with 50% sodium hydroxide are added
to maintain the pH between 10 and 12, whereafter the reaction
mixture is refluxed for another 20 h. The mixture is then cooled to
room temperature and filtered. After adding 160 parts of
concentrated hydrochloric acid, this mixture is again filtered.
Glyphosate will slowly precipitate in the filtrate (IRPTC, 1991).
3.1.2 Uses
Glyphosate is a post-emergent, systemic and non-selective
herbicide intended for use against deep-rooted perennial species,
and also biennial and annual broad-leaved, grass and sedge species
(WSSA, 1983; Monsanto, personal communication to the IPCS, 1991).
Glyphosate is used in both agriculture and forestry. Fields of
agricultural use include grassland renovation, horticulture,
fructiculture, arable cultivation, and rice cultivation. Use in
forestry includes the killing of fast growing competitors in conifer
plantations or conservation areas, and the treatment of tree stumps.
Glyphosate may also be used for weed killing in non-agricultural
areas such as water systems, including irrigation and temporarily
drained waters, parks, road verges and gardens.
The uses of glyphosate indicate that it can be applied in
various crops for specific purposes. The major formulation Roundup
may, for instance, be used in pre-plant treatments for seed bed
preparations, and also against bracken infestations in forestry,
against couchgrass (Elytrigia repens) infestations on pastures, in
direct treatments between rows of crops, or by direct wiping of the
leaves of the weed, assuming the weeds are taller than the existing
crop.
Glyphosate is used worldwide. In 1987, 35 160 ha of the area in
British Columbia where vegetation management activities took place
had been treated with Roundup. This was 94% of the total area where
there were such activities (Ackurst, 1989).
The application rates of glyphosate are dependent on the
formulation and type of use. In the Netherlands, recommended rates
for the application of Roundup are 0.3-2.9 kg a.i./ha. In Canada the
recommended application rates of Roundup are 1.1-1.7 kg a.i./ha for
annual weeds and 1.2-5.8 kg a.i./ha for perennial weeds. The
recommended application rates for Vision in Canadian forestry are
1.1-2.1 kg a.i./ha (Task Force on Water Quality Guidelines, 1991).
Glyphosate is generally applied as a 0.5-5% solution in water by
spraying, and as a 10-50% solution in water by wiping with, for
instance, a rope-wick (Monsanto, personal communication to the IPCS,
1991).
The timing of application is dependent on the use. Application
in late summer or autumn is recommended for use in forestry in
Canada (Hildebrand et al., 1982). Application in agriculture may
be pre- or post-harvest. In the Netherlands, for instance,
glyphosate may be applied to cereals, potatoes and asparagus
immediately (up to 7 days) before harvest, but only when the
ripening is complete. Treatment of immature crops would result in
higher residue levels, early crop desiccation and reduced yields.
Glyphosate may be applied in different ways. For large-scale
treatments aerial application can be appropriate, small-scale
treatments can be done with spraying equipment on the back or behind
vehicles, or by wiping equipment.
Aerial applications will lead to losses due to wind-drift.
Exposure of flora and fauna due to off-target deposits may take
place. These downwind deposits depend on the meteorological
conditions, the plant canopy structure and the application method,
including the release height (Payne et al., 1989; Feng et al.,
1990; Payne, 1992; Payne & Thompson, 1992). The non-volatile
tank-mix fraction and the speed of the aircraft may influence the
drop-size spectrum, and it can be expected that dispersal systems
causing relatively small droplets and having a relatively low
non-volatile fraction will cause the highest off-target deposits.
Payne (1992) assumed that the large differences in deposits in two
comparable experiments were due more to different aircraft airspeeds
than to different wind speeds. In these experiments the maximum
deposits at a downwind distance of 50 m were 19 and 3 mg a.i./m2
at aircraft airspeeds of 45 and 11-20 m/second, respectively. The
application rate in both experiments was 2.1 kg a.i./ha. In other
experiments with the same application rate, Payne & Thompson (1992)
found that the meteorological conditions had a considerable impact
on the off-target deposition up to 400 m downwind when spraying at
different wind speeds (2.2-5.7 m/second) and turbulences. The
deposits at a downwind distance of 400 m varied between 0.001 and
0.06 mg a.i./m2, whereas they varied between 0.6 and 4 mg
a.i./m2 at a downwind distance of 50 m. Remarkably, the deposition
was highest with an intermediate wind speed and intensity of
turbulence. Payne et al.(1989) investigated the deposits for
aerial applications of Roundup with different dispersal systems.
When 2.1 kg a.i./ha was applied with a helicopter in a single
crosswind swath over 100 ha, up to 13.4 mg a.i./m2 was deposited
on a downwind distance of 50 m. This maximum deposition was caused
by a D8-46 hydraulic nozzle, whereas the highest depositions with a
Thru Valve Boom and a Microfoil Boom were 2 and 0.4 mg a.i./m2,
respectively. These depositions were also found at a downwind
distance of 50 m. At the time of application the windspeed 13 m
above ground level was 0.4-0.5 m/second. Riley et al.(1991)
modelled spray deposition of glyphosate using results from
helicopter applications under semi-operational conditions. The study
was designed to test the appropriateness of a New Brunswick "buffer
zone" of 65 m to minimize the effects of spray drift. At a distance
of 65 m, it was estimated that between 3.7% and 5.6% of the nominal
spray rate was deposited.
3.1.3 Drinking-water
Appraisal
The low mobility of glyphosate in soil would indicate a
minimal potential for the contamination of drinking-water from
groundwater aquifers. The only possible source of drinking-water
contamination is, therefore, surface waters. There have been no
reported incidences of drinking-water contamination with
glyphosate.
Conventional plants for processing of drinking-water would not
remove glyphosate, but this could be achieved by coprecipitation
after adding iron salts (AMA van der Linden, personal communication
to the IPCS, 1991). Ozone, increasingly used as an alternative to
chlorine in drinking-water treatment, does effectively remove
glyphosate through the hydroxyl radical (HO. ) chain processes that
occur in most ozonated waters (Yao & Haag, 1991; Haag & Yao, 1992).
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION AND TRANSFORMATION
4.1 Transport and distribution between media
Appraisal
Following application, glyphosate selectively partitions to
particulate matter suspended in surface water or to the soil
substrate. This partitioning is usually rapid and occurred within 14
days in reported studies. The mechanism of sorption to soil is only
partially understood. Glyphosate can adsorb to soils through
phosphate binding sites. Competition with inorganic phosphate has
been demonstrated in the laboratory but not measured in the field.
Specific ions (Fe2+, Fe3+ and Al3+ ) complex glyphosate; metal
complexes with humic acids in soil may be a main binding mechanism
for glyphosate in soil. There is little reported information on
desorption from soil; the data available suggest "strong" binding.
This is supported by mobility studies which show little leaching of
glyphosate below the upper few centimetres of the soil profile. The
major metabolite, AMPA, is also retained in the upper soil layers.
There is very little information on the bioavailability of
sediment-bound glyphosate to either aquatic or terrestrial
organisms. Bioaccumulation and ecotoxicity studies have not,
generally, been performed with added sediment.
Applied glyphosate can be translocated in plants. Glyphosate
in plant foliage or leaf litter does not seem to represent a source
of contamination of aquatic systems. Animals can ingest the
herbicide residues in or on plants.
Dissipation of glyphosate from soil has been widely studied
with very variable results (DT50 between 3 and 174 days).
Biodegradation appears to be the major source of dissipation.
Run-off was minimal in experimental studies, but field results
suggest that aquatic systems may be receiving glyphosate bound to
soil particles following rainfall.
In this chapter the terms biodegradation and dissipation are
used to distinguish between the decrease of the concentration in,
for instance, the soil that is due to microbes transforming the
molecule to a smaller size (biodegradation) and the decrease of the
concentration that might be due to microbial activity but also to
other processes, e.g., sorption, leaching and run-off (dissipation).
4.1.1 Water
Glyphosate dissipates from the water with 50% dissipation times
ranging from a few days to 2 weeks (Newton et al., 1984; Monsanto
1990a; see also Table 5). These DT50 values were deduced from both
laboratory and field experiments in which sediment or suspended
particles were shown to be the major sink.
In water with a near-neutral pH, the formation of an insoluble
complex of Ca2+ with glyphosate was demonstrated in a laboratory
experiment (Subramaniam & Hoggard, 1988). It was confirmed with
X-ray powder diffraction and infrared spectra that this complex was
not an ionic salt. At a near-neutral pH, the dianionic species of
glyphosate is dominant. Insoluble complexes have also been found
with Mg2+, Fe3+ and Cu2+.
In a field experiment in a temperate coastal rainforest in
British Columbia, Canada, the highest concentration of glyphosate in
water was 162 µg/litre (Feng et al., 1990). This maximum was found
in a directly sprayed tributary 2 h after an aerial application of
Roundup at a rate of 2 kg a.i./ha. Concentrations in oversprayed
tributaries without a high cover of overhanging riparian vegetation
increased after the first rainfall. In oversprayed tributaries with
a high cover of riparian vegetation almost no residues were found.
Within 96 h after application the residues in all waters had
declined below detection limits, indicating rapid dissipation. After
rainstorms, peak concentrations of glyphosate were found in the
sediments and on suspended particles of the oversprayed tributaries,
with maximum concentrations of 7 mg a.i./kg dry weight and 0.06 µg
a.i./litre unfiltered water, respectively. The amounts in the
sediments of these waters were variable but declined over time. As
0.1-2 mg residue/kg dry weight sediment was found between 196 and
364 days after application, the residues appear to be persistent in
sediments of oversprayed waters. Feng et al.(1990) concluded,
therefore, that after rainstorms sediments appear to be the major
sink.
In another field experiment in the same forest ecosystem,
glyphosate dissipated rapidly from a small perennial, very slow
flowing stream, in a site of 8 ha aerially sprayed with Roundup at a
rate of 3.3 kg a.i./ha (Newton et al., 1984). In water, 50% of the
initial concentration had dissipated in 2 days. In sediment, maximum
concentrations of approximately 0.6 mg a.i./kg were found 14 days
after application. These were reduced to approximately 0.3 mg
a.i./kg in 28 days, and to < 0.2 mg a.i./kg in 55 days after
application. A comparable rapid dissipation from the water column
was found for small forest ponds in a boreal forest in Manitoba,
Canada, after applying Roundup at a rate of 0.9 kg a.i./ha
(Goldsborough & Beck, 1989). The highest concentration in filtered
water was 141 µg a.i./litre, within 6 h after application. The main
mechanism of dissipation was probably sorption to the sediment. This
was confirmed by additional experiments with polyethylene basins
filled with unfiltered water and sediment that were placed in the
spray zone. Without sediment, only a very small amount of the dose
actually applied had dissipated after 30 days, whereas with sediment
the initial concentrations in the water had decreased by 50%,
approximately 6 days after application.
Comparable dissipation patterns were found in a field
experiment (Monsanto, 1990a) in which Accord (30.5% a.i. w/w) was
applied at a rate of 4.2 kg Accord/ha on three forestry sites with
non-flowing pond water and flowing water. Concentrations of up to
1700 µg/litre filtered water were found in the pond water
immediately after spraying. The initial concentrations in both pond
and flowing water were reduced by 50% within 7 days. Concomitantly
initial AMPA concentrations (maximally 35 µg/litre) were reduced by
50% within the same period. In flowing water the dissipation of both
glyphosate and AMPA was even more rapid. Concentrations of
glyphosate increased up to 19 mg/kg dry weight in the sediment of
one pond 28 days after application. Concentrations of up to 1 mg/kg
of both compounds were measured in the sediments of non-flowing
ponds up to 400 days after application.
In field experiments in turbid Australian irrigation water,
glyphosate adsorbed to suspended particles at different rates,
apparently mainly depending on the application rate (Bowmer et al.,
1986). At an initial concentration of 5 mg a.i./litre, 10-16% of the
load adsorbed to suspended matter, whereas at an initial
concentration of 0.05 mg a.i./litre, 53-71% adsorbed. In more saline
water the degree of sorption was less, probably due to rapid
flocculation. Maximum adsorbed amounts were approximately 7000 mg
a.i./kg in less saline supply water, and approximately 2500 mg
a.i./kg in more saline drainage water. When a supply channel was
emptied before spraying with 3.6 kg a.i./ha for control of aquatic
weeds, and filled again with water 4 days after the treatment, the
amount in the unfiltered water used for irrigation was 7% of the
applied dose.
4.1.2 Soil sorption
Glyphosate is readily bound to many soils and clay minerals
(Sprankle et al., 1975; Hance, 1976; Glass, 1987; Miles & Moye,
1988b). In laboratory experiments in which glyphosate was added to
aqueous soil suspensions, the adsorption coefficient Ks/l was
18-377 dm3/kg in nine soils ranging from sandy loam to peat
(Hance, 1976), and 33-76 dm3/kg in three soils ranging from sandy
loam to clay loam (Glass, 1987). These Ks/l values indicate strong
sorption. In both experiments the sorption could be described by the
Freundlich equation. Glass (1987) found Ks/l values for the clay
minerals montmorillonite, illite and kaolinite of 138, 115 and 8
dm3/kg, respectively.
Table 5. Biodegradationa of technical grade glyphosate in water and sediments in the laboratory
Water type Sediment Test Sediment Organic Temperature pH of Experimental Parameter Time Reference
type type (%) matter in (°C) water time (days)
sediment (days)
(%)
Pond water silty A 17 0.9 23-25 5.9-7.0 30 DT50 14b PTRL East
clay Inc. (1990a)
loam
Pond water silty An 16 0.9 20-27 5.7-6.5 365 DT50 14c,e PTRL East
clay Inc. (1990b)
loam
Surface waterd n.r. A 9 n.r. 30 8.2-8.6 14 DT50 < 14 Monsanto
(1972a)
Lake water sandy An 33 1.4 30 6.6 42 DT50 22e Monsanto
clay (1978a)
loam
a Biodegradation in the whole system
b The biodegradation stopped after approximately 15 days
c The biodegradation stopped after approximately 150 days
d Three rivers and one lake in the USA
e Approximate value derived from data of the author(s)
A = aerobic; An = anaerobic; n.r. = not reported.
The mechanism of sorption of glyphosate to soil is only
partially understood. Several factors may be involved. The
phosphonic moiety adsorbs weakly to unoccupied phosphate binding
sites and can be displaced by phosphate (Hance, 1976). In laboratory
experiments with nine soils the author showed that sorption was
positively correlated with the unoccupied phosphate sorption
capacity, and not correlated with the total phosphate sorption
capacity, organic matter, clay, iron or aluminium content. No data
are available that confirm competition of glyphosate and phosphate
under field conditions, e.g., after application of artificial
fertiliser. Miles & Moye (1988b) suggested that the main mechanism
was probably by H-bonding and ion-exchange, as the degree of
sorption in their experiments was not correlated with cation
exchange capacity (CEC) values or surface areas. Contrary to the
results of Miles & Moye (1988b) and of Hance (1976), sorption
appeared to be correlated with CEC values and clay content in a
sorption study with clay loam, silt loam and sandy loam (Glass,
1987).
The binding is also influenced by the presence of specific
cations. Hensley et al.(1978) demonstrated that Fe2+, Fe3+
and Al3+ inactivated glyphosate much more than Ca2+, K+ and
Na+. This was confirmed by Glass (1987) and Sprankle et al.
(1975). Glass (1987) suggested that glyphosate was complexed by
cations, released from cation-saturated clays via a cation-exchange
with solution protons.
According to Heinonen-Tanski (1989), most of the soil-bound
residues of glyphosate were recovered in the fulvic acid fraction
(21-33%). Sorption of glyphosate to fulvic acids was also reported
by Madhun et al.(1986), who added 14C-glyphosate to an aqueous
soil extract (ASE) of peat. In this study sorption was mainly on ASE
fractions with a relative molecular mass ¾ 1000. Piccolo et al.
(1992) studied the interaction of glyphosate with a pure iron-humic
acid complex. Maximum adsorption values indicated that adsorption to
the complex occurred to as great an extent as to whole soils. This
suggested that humic acid complexes with polyvalent cations might
represent a main binding substrate for glyphosate in soils. There
was no desorption of bound residues of glyphosate following shaking
with 0.01 mol CaCl2/litre solution for 48 h, the maximum shaking
time for the adsorption studies.
Desorption of glyphosate with ionized water from
montmorillonite and illite needed three days before reaching an
equilibrium in a study of Miles & Moye (1988b).
It can be concluded that sorption of glyphosate can be expected
in the presence of available phosphate binding sites, the presence
of iron and aluminium (oxides or hydroxides), and appropriate
combinations of clay and organic matter.
4.1.3 Mobility in soils
In view of its Ks/l, glyphosate can be expected to be
immobile or slightly mobile in many soils. This was confirmed by
several experiments, both in the laboratory and in the field. In
thin-layer chromatography studies with sandy loam, clay loam and
sandy clay loam, the Rf values of 14C-glyphosate were 0.14-0.20
(Sprankle et al., 1975). In comparable studies with silt loam,
silty clay loam, and sandy loam Rf values were < 0.2 (Monsanto,
1972c). In a leaching study with columns of 30 cm and a high water
flux of 51 cm over less than 2 days, < 0.1-6.6% of the applied
activity was leached (Monsanto, 1978b). This experiment was
performed with eight soils, ranging from sandy loam (organic matter
content 0.7%) to volcanic ash (organic matter content 9.5%). More
than 90% of the applied activity was recovered in the upper 0-14 cm
layer.
Only one leaching study under laboratory conditions with
respect to the mobility of AMPA has been reported. In this
experiment with 30-day-old residues, < 0.1-1.6% of the applied
activity was leached over 45 days (Monsanto, 1978b). The columns
were 30 cm and the water flux over 45 days was low (17 cm). The
amount of AMPA that was recovered after 45 days in the upper 0-2 cm
layer was low (0.5-12% of the applied activity), due to a high rate
of mineralisation.
4.1.4 Dissipation from the soil in the field
Many field experiments on the dissipation of glyphosate from
the soil have been performed. Some relevant studies are summarized
in Table 6. They indicate DT50 values based on dissipation that
range from 3 to 174 days depending on edaphic and climatic
conditions. In a forest brush ecosystem in Oregon, USA, the DT50
value in loam was 29 days with and 40 days without litter (Newton
et al., 1984). In field experiments in Sweden, Roundup was sprayed
over reforestated sites (Torstensson et al., 1989). In the soils
of these sites the DT50 values were < 50 days, apparently
depending on the soil respiration rate. The dissipation consisted of
a fast first, and a much slower second phase, especially in sites in
northern Sweden, which was possibly due to a longer frost period. In
these sites 1-2% of the actually applied dose was recovered 1080
days after application. A comparable dissipation pattern was found
in a field experiment on Finnish agricultural soils (Heinonen-Tanski
et al., 1985). In this experiment 25% of the concentration in a
sandy loam 2 days after the treatment was recovered one year after
application. The application rate was 1.4 kg a.i./ha.
A study in a temperate coastal rain forest in British Columbia,
Canada, showed that, 360 days after application, 6-18% of the
initial levels was recovered (Feng et al., 1990). In this
experiment Roundup was applied at a rate of 2 kg a.i./ha. The soils
were alluvial sandy loam or sandy clay loam with highly organic
surface horizons. Some of these soils were well drained, others were
seasonally flooded. At each sampling time more than 90% of the
recovered residues was in the upper 0-15 cm layer. Under all
conditions the amount of glyphosate declined over time, whereas
there was a transient increase of AMPA.
In other field experiments on boreal forest soils, comparable
dissipation patterns were found. Stark (1983) reported DT90 values
of 30-720 days, and Roy et al.(1989b) found a DT50 value of
approximately 20 days on a sandy soil planted with jackpines (Pinus
banksiana). In the field experiments of Roy et al.(1989b),
glyphosate was detectable up to 335 days after application; almost
all residues in the sandy soil were recovered in the organic top
layer. In field experiments of Monsanto (1990a) in three forest
locations in the USA, the concentration course of glyphosate
appeared to be rather irregular, especially during the first four
months. However, 50% of the initial concentrations in the soil had
mostly dissipated within 120 days. One clear exception was a site in
Corvallis in which glyphosate increased up to 0.15 mg/kg dry weight,
180 days after application. On the same site AMPA increased up to
0.32 mg/kg, 346 days after application. The application rate in
these experiments was 4.2 kg Accord/ha.
On a clay soil of a clear-cut boreal forest area, Roy et al.
(1989b) found no dissipation of glyphosate due to run-off on a 8°
slope. In a field experiment on agricultural soils without
conventional tillage, the dissipation of glyphosate due to run-off
on 6-16° slopes was < 1% of the applied dose when 1.1-3.4 kg
a.i./ha was applied (Edwards et al., 1980). However, when 9.0 kg
a.i./ha was applied, 1.8% of the applied dose dissipated due to
run-off, mainly because of a rainstorm shortly after application.
4.1.5 Uptake and dissipation from plants
Uptake of 14C-glyphosate by leaves of trembling aspen
seedlings (Populus tremuloides) was initially rapid, after which
it slowed down (Sundaram, 1990). The seedlings were exposed to
Roundup that was dripped with a micro-applicator on some central
leaves. The application rate was 0.35 kg a.i./ha leaf surface area.
Most activity was washable from the leaves (61-77%), and 22-28% was
recovered in the treated leaves within 48 h. As only 1-10% was
recovered in the other parts of the seedlings, this indicated a
rather low translocation after absorption. A rapid uptake of
14C-glyphosate within a few hours was indicated for sugar beets
(Beta vulgaris), when applied to a mature leaf (Gougler & Geiger,
1981). 14C-glyphosate probably entered the phloem in a
non-facilitated way. The subsequent transport through the phloem
Table 6. Biodegradation and dissipation of glyphosate in soils
Soil type Compound Test Moisture Temperature pH Organic Experimental DT50 Reference
type content (°C) matter duration (days)
(%) (%) (days)
Biodegradation
Sandy loam Tgg L,A 14-16 25 7.3 2.8 360 2b PTRL East Inc. (1991)
Silt loam Tgg L,A 12-14 25 7.5 1.0 360 2b PTRL East Inc. (1991)
Dissipation
Sandy loam Tgg G 11 32 5.7 1.0 112 130b Monsanto (1972b); Rueppel
et al. (1977)
Silt loam Tgg G 11 32 6.5 1.0 112 3b Monsanto (1972b); Rueppel
et al. (1977)
Silty clay loam Tgg G 11 32 7.0 6.0 112 25-27b Monsanto (1972b); Rueppel
et al. (1977)
Sand Ru F n.r. n.r. 3.5-3.7 40 762 approx Roy et al. (1989b)
(humoferric 20a
podsol)
Sandy loam, Ru F n.r. n.r. 4.2-4.9 15-31 360 45-60b Feng & Thompson (1990)
sandy clay loam
Loam Ru F n.r. n.r. 4.0-4.7 3.8-5.2 55 29-40b Newton et al. (1984)
Loamy sand Ru F n.r. n.r. n.r. 0.8 370 3-4b Monsanto (1983a)
Sandy clay loam Ru F n.r. n.r. n.r. 7.0 370 122-174b Monsanto (1983a)
a Based on data of the author(s) b Data reported by the author(s)
L = laboratory study; F = field study; G = greenhouse study; A = aerobic; An = anaerobic;
Tgg = technical grade glyphosate; Ru = Roundup; n.r. = not reported
appeared to be according to an "intermediate permeability
mechanism". When exposed for a longer time, plants may show
substantial translocation of absorbed 14C-glyphosate, as was shown
for potatoes (Solanum tuberosum) by Smid & Hiller (1981). In the
treated leaves of the potatoes 45% of the absorbed activity was
recovered, whereas the rest was mainly translocated to the apical
meristem and the roots. Up to 5% was recovered in the mother tuber.
The degree of translocation was age-dependent, as older plants
showed less translocation than younger plants.
The uptake of glyphosate by red raspberries (Rubus strigosus)
was 9% of the amount that was deposited on the leaves after spraying
Roundup at a rate of 2 kg a.i./ha (Roy et al., 1989a). In the same
field experiment the uptake was 14% by wild blueberries (Vaccinium
myrilloides). Most glyphosate was recovered in the washings, which
was also found under laboratory conditions. The initial absorbed
amounts were 0.92-2.0 mg a.i./kg dry weight. The absorbed and
washable amounts together were reduced by 50% within 13 days in the
raspberries and within 20 days in the blueberries. AMPA was
detectable up to 33 days after application. Metabolism occurred to
only a minor extent as AMPA concentrations were less than 1.5% of
the concurrent concentrations of glyphosate (similar results were
reported by FAO/WHO, 1986b). In a field experiment by Feng &
Thompson (1990) in a temperate coastal rainforest in British
Columbia, Canada, the main target species for treatment with Roundup
were red alder (Alnus rubra) and salmonberry (Rubus spectabilis).
Immediately after spraying, the concentrations in leaf tissue were
up to 448 mg a.i./kg dry weight. Glyphosate dissipated rapidly from
the leaf litter with a DT50 value of 8-9 days. The leaf litter
included leaves directly exposed on the trees and existing leaf
litter from natural defoliation before treatment with Roundup. The
authors assumed that leaf litter of these major brush species is an
insignificant source of glyphosate input into streams or onto forest
floor, because of the fast dissipation. A rapid dissipation of
glyphosate from fresh foliage was also found in a field study
(Monsanto, 1990a) in which initial concentrations of up to 1300 mg
a.i./kg and 2.6 mg AMPA/kg decreased rapidly. A transient
accumulation of glyphosate and AMPA was found in the leaf litter on
some sites, but these amounts were reduced by approximately 90%
within 100 days.
Glyphosate dissipated completely from wild berries (Vaccinium
vitis-idaea, Vaccinium myrtilus) within one year in a field
experiment in Finland in which Roundup was applied at a rate of
0.25-2.2 kg a.i./ha with a knapsack sprayer (Siltanen et al.,
1981). Contrary to this dissipation pattern was that of glyphosate
in reindeer lichens (Cladonia rangiferina) that were sampled in
the same experimental plots. Around 270 days after application,
dose-related concentrations of glyphosate and AMPA were recovered in
lichens with maxima of 45 and 2.1 mg/kg for glyphosate and AMPA,
respectively. Approximately 390 days after application of 0.8 kg
a.i./ha, 6.4 and 0.3 mg/kg of glyphosate and AMPA were still
detectable.
4.1.6 Ingestion by animals
As the concentration in the foliage may increase up to high
amounts immediately after application, this implies the possibility
of entry into the food chain through ingestion by herbivorous or
omnivorous animals. This was confirmed by Sullivan & Sullivan (1979)
who investigated the effects of glyphosate on the feed preference
and daily chow consumption of black-tailed deer (Odocoileus
hemionus columbianus). These herbivores did not avoid eating
browse of alder (Alnus rubra) and alfalfa (Medicago sativa) that
was treated with glyphosate at a rate of 2.2 kg/ha. Sometimes the
treated alder browse was even preferred. Reindeer may be exposed to
glyphosate, since reindeer lichens, which are an important food
source, can take up a substantial amount of glyphosate (see above).
4.2 Abiotic degradation
Appraisal
Hydrolysis of glyphosate is very slow. Photodegradation in the
field may occur.
4.2.1 Hydrolytic cleavage
Hydrolysis of glyphosate in sterile buffers is very slow. After
32 days < 6.3% of the applied activity was recovered as AMPA,
after applying 14C-glyphosate at rates of 25 and 250 mg/litre to
aqueous buffer solutions of pH 3, 6 and 9 (Monsanto, 1978b). These
tests were performed at both 5 and 35 °C.
4.2.2 Photodegradation
Photochemical degradation in water may occur under both
laboratory and field conditions, mainly depending on the type of
light source. In sterile aqueous buffers of pH 5, 7, and 9, less
than 1% of the applied dose was degraded (photodecomposition of
14C-phosphonomethyl-labelled glyphosate) during 29-31 days, when
exposed to sunlight (PTRL Inc., 1990).
Lund-Hoie & Friestad (1986) exposed Roundup to several light
sources under different conditions. When exposed to UV light (lambda
= 254 nm) under laboratory conditions, concentrations of 1 and
2000 mg a.i./litre in deionized water showed DT50 values of 4 and 14
days, respectively. When exposed to sunlight under field conditions
1 mg a.i./litre in polluted water without sediment showed a much
slower decomposition (DT50 > 63 days), possibly due to pollution
preventing adequate UV penetration in the water. Polluted water with
sediments showed a rapid dissipation from water, probably due to
adsorption onto the sediments. In another field experiment 2 and
100 mg a.i./litre in deionized or polluted water without sediment
showed DT50 values of < 28 days, when exposed to sunlight. At
the low concentration the dissipation in polluted water was more
rapid than in deionized water. In the dark no dissipation occurred.
In laboratory experiments 1 mg/litre of glyphosate in
sterilized natural and deionized water showed DT50 values of 4 to >
14 days when exposed to artificial light (350-450 nm) in
photoreactors without sediment (Monsanto, 1978a). In these
experiments Ca2+ acted as a photosensitizing agent.
Photodegradation by sunlight of glyphosate applied to a soil
appeared to be an insignificant route of dissipation (PTRL Inc.,
1989). In this study, 14C-glyphosate mixed with unlabelled
glyphosate was exposed for 31 days to natural sunlight, after
application to a sandy loam at a rate of 4.5 kg a.i./ha.
Extrapolated DT50 values that were based on first-order kinetics
were 90 days in the sunlight and 96 days in the dark, indicating no
substantial degradation due to photolysis. The temperature of the
soil surface was 22-23 °C. Under unnatural light conditions
glyphosate appeared not to be photodegraded substantially (Monsanto,
1972c; Rueppel et al., 1977; Monsanto, 1978a).
4.3 Biodegradation
Appraisal
Selected studies of the biodegradation of glyphosate have been
considered; selection was on the basis of test conditions and modern
methodologies. There is considerable variation in rate of breakdown
in water, aquatic sediment and soil. Degradation occurs more rapidly
in aerobic than anaerobic conditions. Half-times for biodegradation
in the three media under laboratory conditions range between a few
days and approximately 20 days. No data on biodegradation under
anaerobic conditions are available.
The main route of biodegradation of glyphosate appears to be
by splitting the C-N bond to produce AMPA. However, a second route
with splitting of the C-P bond can also occur.
A range of bacterial strains can degrade glyphosate. Bacteria
capable of using the compound as sole phosphorus, sole carbon or
sole nitrogen source have been identified. Growth is slow compared
to growth on inorganic sources of P, C or N. There is evidence from
the field that bacterial populations adapt to the metabolism of
glyphosate. Presence of inorganic phosphate inhibits degradation of
glyphosate with some, but not all, bacteria. Biodegradation of
glyphosate may involve co-metabolism.
The most relevant laboratory experiments in which the
biodegradation in systems with water and sediment have been studied
are summarized in Table 5. These studies indicate that the rate of
biodegradation may vary substantially, depending on experimental
conditions, e.g., the availability of oxygen, temperature and type
of sediment. The time needed for 50% biodegradation of glyphosate in
the whole system of a test with water and sediment is < 14 days
under aerobic and 14-22 days under anaerobic conditions in the
laboratory.
In the experiments of PTRL East Inc. (1990a,b), less then 10%
of the applied activity was recovered in the pond water over a
period of 30 days under aerobic condition and 365 days under
anaerobic conditions. During all experiments more than 50% of the
applied activity was recovered in the sediment.
In experiments with water and their associated sediments the
amount of a.i. declines over time with a generally transient
increase of 14C-AMPA, an increase of 14CO2, and an increase of
sediment-bound residues. An exception to this pattern of
biodegradation can be observed in some aerobic and anaerobic
experiments that were performed with pond water and a silty clay
loam sediment (PTRL East Inc, 1990a,b). In this water/sediment
system the biodegradation stopped after approximately 15 days under
aerobic conditions and after approximately 150 days under anaerobic
conditions. The glyphosate residues (a.i. plus AMPA) at both time
points remained approximately 40% of the applied dose, which
indicated substantial persistence in spite of the rapid initial
degradation.
AMPA is the main metabolite of glyphosate found in both the
water column and the sediment. Maximum amounts of AMPA under both
aerobic and anaerobic conditions in the sediment were 25% of the
applied activity (PTRL East Inc., 1990a,b). These maxima were found
at 7-20 days after application. In the same experiments maximum
amounts of sediment-bound residue were 9% of the applied activity
under aerobic conditions and 4% under anaerobic conditions. These
maxima were found at the end of the experiments. The amounts of
evolved 14CO2 in these studies gradually increased in most cases
up to 24 and 35% of the applied activity after 30 days (aerobic),
and 365 days (anaerobic), respectively. This indicates substantial
differences in the mineralization rate. These differences are partly
due to the availability of oxygen, since under anaerobic conditions
the mineralization rate was slower than under aerobic conditions.
This was also found by Monsanto (1972a, 1978a). In the aerobic
experiments of Monsanto (1972a), four sediments that differed by up
to two orders of magnitude in the total number of micro-organisms
did not show substantial differences in mineralization rate.
Biodegradation studies with glyphosate in the soil under
conditions where unequivocal interpretation is justified are scarce.
Table 6 summarizes some relevant studies, indicating that the
biodegradation rate may differ substantially, depending on the
experimental conditions. The laboratory and greenhouse experiments
in Table 6 were performed with moisture contents (> 75% of the
field capacity) that were adequate for optimal biodegradation.
In most laboratory experiments the biodegradation rate of
glyphosate in soils appears to be rapid (see Table 6). Mostly
biodegradation can be described with linear first-order kinetics.
Sometimes a non-linear first-order model taking into account
spatial variability better describes the results observed (PTRL East
Inc., 1991):
C = C0 (1 + ßt)-alpha
C in this equation is the concentration in the soil at time t,
C0 the initial concentration, and alpha and ß are rate constants
reflecting spatial variability.
The main metabolite under aerobic conditions of glyphosate in
soil is AMPA. In aerobic laboratory experiments the maximum amounts
in sandy loam and silt loam were 27 and 29%, respectively, of the
applied activity. These maxima were reached 14 days after
application (PTRL East Inc., 1991). From the data of PTRL East Inc.
(1991), DT50 values for AMPA of approximately 50 days in sandy and
silty loam can be derived. That AMPA is more persistent than
glyphosate was also shown in a laboratory experiment with sandy loam
(Monsanto, 1972b). The amounts of AMPA after 111 days were 10-17% of
the applied activity. In this study, the temperature (32 °C) was
higher than in the other studies discussed above.
Some minor unidentified metabolites were quantified in an
aerobic laboratory experiment lasting 364 days with sandy loam and
silt loam (PTRL East Inc., 1991). Two unknown metabolites did not
exceed 3.5% of the applied activity, whereas some other unknown
metabolites did not exceed 1.5% each. Rueppel et al.(1977)
quantified some minor metabolites that did not exceed 1% of the
applied activity. These metabolites were
N-methylamino-methylphosphonic acid, glycine,
N,N-dimethylaminomethylphos-phonic acid, hydroxymethylphosphonic
acid, and two unknown metabolites.
In aerobic laboratory experiments, the amounts of soil-bound
residues immediately after application were 9-35% of the applied
dose, after which they showed an irregular time-course during these
experiments of approximately 112 days (Monsanto, 1972b). In general,
the initial amounts were also the maximum amounts. In other
laboratory experiments however, maximum amounts of soil-bound
residues appeared to be reached after 14 days, whereafter they
remained more or less constant or even decreased (PTRL East Inc.,
1991). These maximum amounts were 7-9% of the applied activity, and
were probably lower compared with other studies due to better
extraction procedures.
Mineralization in the soil occurs under both aerobic and
anaerobic conditions in the laboratory, although the rates may
differ greatly, apparently mainly depending on the soil respiration
rate and the temperature. When 14C-phosphonomethyl-labelled
glyphosate was applied to sandy loam and silt loam, 70-78% 14CO2
evolved during an aerobic laboratory experiment of 360 days (PTRL
East Inc., 1991). In this study the application rate was 4 mg
a.i./kg dry weight. In an aerobic laboratory study with 15 Swedish
forest soils, DT50 values based on 14CO2 evolution varied
between 6 and 200 days. Mineralization was highly correlated with
the soil respiration rate, but not with pH or organic matter content
(Torstensson & Stark, 1981). This was confirmed by Torstensson &
Stenström (1986) and Heinonen-Tanski (1989). Torstensson & Stenström
(1986) reported that glyphosate was co-metabolized. In this case,
co-metabolizing microorganisms are not supplied with energy by
biodegrading glyphosate.
Establishing the correlation between soil respiration and
mineralization requires both a standardized measurement of the
respiration rate and an accurate measurement of the actual dose that
reaches the soil (Torstensson & Stenström, 1986). In a laboratory
experiment simulating temperatures under arctic conditions in forest
soils, 51-71% of the applied activity was recovered as 14CO2 217
days after application of 14C-glyphosate. In this study the
mineralization rate was reduced 10-15 times during a temperature
decrease of 10 °C over the first part of the study. The rate
increased only 3.7-4 times with a temperature increase of 10 °C
during the second part (Heinonen-Tanski, 1989).
Glyphosate in the soil appears to be degradable by
micro-organisms in two ways (Jacob et al., 1988), as shown in
Fig. 3. One route is via the formation of AMPA and a C2 fragment
which might be glyoxylate. This scheme for degradation was proposed
by many researchers (Monsanto, 1972b; PTRL East Inc., 1991). In this
route the splitting of the C-N bond is the first step. There is,
however, another route of biodegradation via sarcosine
(N-methyl-glycine) and orthophosphate, after which sarcosine is
degraded to glycine and a one-carbon unit that eventually might form
CO2 via formaldehyde (Kishore & Jacob, 1987; Jacob et al., 1988).
In this route the splitting of the C-P bond is the first step. In
experiments with 14C-glyphosate, isolated cultures of Pseudomonas
sp. strain LBr were able to degrade glyphosate according to both
routes (Jacob et al., 1988). Approximately 5% of the applied
14C-glyphosate was not degraded via AMPA, but via sarcosine.
The growth rate of bacteria isolated from a sandy loam garden
soil that was sprayed with Tumbleweed (a garden product) was less
inhibited by technical grade glyphosate than the growth rate of
bacteria from an unsprayed control (Quin et al., 1988). This
indicated adaptation of the bacterial populations of the sprayed
site. As addition of aromatic amino acids prevented growth
inhibition in the population of the unsprayed site to a greater
extent than in the population of the sprayed site, different
mechanisms of biochemical interference were indicated. The
composition of the bacterial population on the unsprayed site was
also different from the sprayed one. Pseudomonas sp. and
lactose-fermenting bacteria could be identified in an inoculum from
the sprayed soil able to use glyphosate as a sole source of
phosphorus (Quinn et al., 1988). A different regulatory mechanism
for biodegradation in unsprayed and sprayed sites was assumed: in
the latter the aromatic amino acid pathway might be regulated by
direct control of 3-deoxy-D-arabino-heptulosonate-7-phosphate (DAHP)
by the end-products, whereas in the unsprayed site DAHP synthase
might be indirectly regulated by prephenate. Also in other
experiments bacteria were shown to use glyphosate as a sole P source
(Kishore & Jacob, 1987; Pipke & Amrhein, 1988; Weidhase et al.,
1990), thereby primarily degrading glyphosate to orthophosphate and
sarcosine, by splitting the C-P bond. In the study of Weidhase et
al. (1990), 18.2% of the applied activity was recovered as sarcosine
8 h after application of 14C-1-methyl-labelled glyphosate to a
pure culture of Pseudomonas sp. GS. This biodegradation route of
glyphosate via sarcosine was also demonstrated by Kishore & Jacob
(1987). In their experiments with glyphosate as sole P source for
Pseudomonas sp. PG2982, one hour after application of
14C-labelled glyphosate, glycine, phosphate, and a one-carbon
unit, possibly formaldehyde, were identified as metabolites. After
one hour, the 14CO2 evolution when the phosphonomethyl moiety
was labelled was substantially higher, as compared with the 1- or
2-glycine-labelled moieties. The authors suggested that the
so-called phosphate-starvation-inducible proteins, as identified by
others, might be responsible for cleaving the C-P bond. In an
experiment with pure cultures of a mutant of Arthrobacter sp.
GLP-1 able to use glyphosate as a sole P source, 90% of the applied
activity was released as orthophosphate at 240 h after application
of 14C-1-methyl-labelled glyphosate (Pipke & Amrhein, 1988).
Orthophosphate inhibited further biodegradation of glyphosate.
Flavobacterium sp. was found by Balthazor & Hallas (1986) to be
able to degrade glyphosate in spite of the presence of
orthophosphate. Liu et al. (1991) showed that 12 strains of bacteria
from the family Rhizobiaceae could degrade glyphosate present in the
medium as the sole phosphorus source; although growth of the
bacteria was slower than with inorganic phosphate. Sarcosine was the
intermediate breakdown product, indicating initial cleavage of the
C-P bond, in Rhizobium meliloti, the strain used for detailed
metabolic studies.
 Carlisle & Trevors (1986a) deduced from their experiments that
nitrate-reducing bacteria are involved in metabolizing glyphosate.
Involvement of nitrifying bacteria in the biodegradation of
glyphosate was also demonstrated by Murthy et al. (1989), when they
investigated the treatment of waste water from a Roundup formulating
factory.
Pseudomonas sp. may use glyphosate as a sole P or C source,
as demonstrated by Weidhase et al. (1990). Only slight growth of the
wild-type strain of the bacterium Pseudomonas fluorescens was
observed with glyphosate as sole carbon or nitrogen source. The
herbicide was metabolized to aminomethylphosphonate (Zboinska
et al., 1992). Murthy et al. (1989) isolated a denitrifying
bacterial species that was also able to use glyphosate as a C
source. This species was isolated from activated sludge in a
waste-water treatment plant. A mutant of Arthrobacter sp. strain
GLP-1 was able to utilize glyphosate as a sole N source, whereas
this was not possible for the normal strain (Pipke & Amrhrein,
1988), probably due to the uptake of inorganic P released during
biodegradation.
As the Biological Oxygen Demand and the Chemical Oxygen Demand
of glyphosate are < 2 mg/g and 0.53 g/g, respectively, glyphosate
cannot be considered as readily biodegradable (LISEC, 1990a,b). In
suitable systems, however, glyphosate is biodegradable, as shown by
Murthy et al. (1989), who investigated the biodegradation of
glyphosate in waste-water treatment plants under different
conditions in sequencing batch reactors on a laboratory scale. These
reactors were fed with waste water from a Roundup manufacturing
facility. Glyphosate was degraded completely within one cycle of
24 h, independent of whether there was an initial aerated or anoxic
phase of 4 h. However, more glyphosate could be processed with an
anoxic initial phase, probably due to better conditions for
denitrification. Not only denitrifiers but also ammonifiers and
nitrifiers appeared to be involved in the biodegradation of
glyphosate. Only at the very high concentration of approximately
5000 mg a.i./litre was biodegradation repressed by non-glyphosate
COD and inhibited by excess ammonia production.
Pseudomonas sp. strain LBr, Flavobacterium sp. and a
denitrifying bacterial species were isolated from activated sludge
as species with the ability to use glyphosate as a P source
(Balthazor & Hallas, 1986; Jacob et al., 1988; Murthy et al., 1989).
The denitrifier was also able to use glyphosate as a sole C source.
Flavobacterium sp. degraded glyphosate to AMPA in both the
presence and absence of PO43- (Balthazor & Hallas, 1986). In
this experiment the further degradation of AMPA appeared to be
hampered in the presence of PO43-.
Pseudomonas sp. strain LBr was capable of completely
eliminating amounts of glyphosate up to 3212 mg/litre from a growth
medium (Jacob et al., 1988).
Continuous exposure of an activated sludge treatment system in
a pilot plant increased the ability of the sludge to metabolize
glyphosate to AMPA (Hallas et al., 1992). In this trial an influent
concentration of 50 mg a.i./litre was reduced to less than 5 mg
a.i./litre under continuous-flow conditions with an average
residence time of 10 min. The sludge was inoculated with immobilized
bacteria capable of degrading glyphosate. The effectiveness of the
treatment was dependent on the presence of a nitrogen source and a
non-glyphosate carbon source, and required a pH range of 6.0 to 8.0.
No data are available on the amounts of glyphosate that can be
eliminated in conventional waste-water treatment plants under
practical conditions. In waste water from glyphosate-producing
plants, 28-45% is reported to be eliminated through biological
treatment (Task Force on Water Quality Guidelines, 1991).
No data are available on the biodegradability of the
surfactants in formulations. It is, however, probable that
polyoxyethylene amine is biodegraded fairly rapidly in view of the
biodegradability of structurally related compounds (Swisher, 1987).
4.4 Bioaccumulation
Appraisal
Glyphosate is not expected to bioaccumulate in view of its
high water solubility and its ionic character. This was confirmed by
several laboratory experiments with fish, crustaceans and molluscs
and by field experiments.
In a static test, channel catfish (Ictalurus punctatus) were
exposed to 0.94-0.99 mg 14C-labelled a.i./litre (actual
concentrations) for 10 days (ABC Inc, 1981d; Monsanto, 1981a). Of
the absorbed amount, 76% was recovered in the viscera. More than 90%
of the extractable residues in the viscera and the fillet was
identified as glyphosate, whereas less than 2% was identified as
AMPA. After 10 days of depuration 80% of the absorbed activity was
eliminated. For exposed channel catfish the calculated
bioconcentration factor based on the activity absorbed by the whole
fish was 0.27. For depurated channel catfish the calculated
bioconcentration factor was 0.052.
The marsh clam (Rangia cuneata) and crayfish (Procambarus
simulans) were exposed in static tests lasting 28 days to
synthetic uncontaminated sea water and a sandy loam sediment that
was incorporated with 3 mg 14C-labelled a.i./kg (ABC Inc.,
1982d,e). These experiments were set up to assess the degree of
bioconcentration of glyphosate when used in flooded rice levees and
tidal water. The calculated bioconcentration factor for the edible
parts of the clam increased during exposure up to 4.8, whereas for
the whole crayfish it increased up to 12. The highest concentrations
in the edible parts of the clam and the whole crayfish were 0.3 mg
14C-labelled residues/kg for both. After 28 days of depuration 48%
of the accumulated residues were eliminated from the edible parts of
the clam. The concentration in these parts was then 0.16 mg
14C-residues/kg. The crayfish finally had eliminated 91% after 14
days of depuration. The concentration in the whole crayfish was then
0.02 mg 14C-residues/kg. It must be stated that this test refers
to the accumulation of 14C and not glyphosate.
In a static test without sediment, in which rainbow trout
(Salmo gairdnerii) were exposed to 2 mg a.i./litre (nominal
concentration) for 12 h, the fillets of the fish contained 80 µg
a.i./kg (in the original article the erroneous figure of 80 mg/kg
was reported), and the eggs 60 µg a.i./kg (Folmar et al., 1979).
This indicates a bioconcentration factor of 0.04 for the edible
parts. Roundup was applied in this test.
In a flow-through test in which bluegill sunfish (Lepomis
macrochirus) were exposed to 11-13 mg 14C-labelled a.i./litre
(actual concentrations) for 35 days, calculated daily
bioconcentration factors based on the whole fish increased from <
0.1, 0.2 days after the start of the test, to 0.4-0.5 at the end
(ABC Inc., 1989f). Maximum concentrations in the whole fish, viscera
and fillet were 13, 7.6 and 4.8 mg 14C-residues/kg, respectively.
The time required to reach 90% of the steady state and the uptake
rate constant were calculated to be 120 days and 0.02 mg/kg fish x
(mg/litre water)-1 x day-1, respectively. During 21 days of
depuration, the half-life of depuration was calculated to be 35. A
slow decrease in tissue concentration during depuration was
indicated. After the period of depuration 2.2 mg 14C-residues/kg
whole fish was still present. In an additional study to characterize
the 14C-residues, 95-97% of the residues in the water was
glyphosate, whereas in the whole fish and tissues 28-91% of the
recovered activity was glyphosate (ABC Inc., 1989g). In a whole fish
sample 21 days after starting the test, 49% of the recovered
activity was found to be AMPA. By treating homogenates with
proteinase K it was indicated that a substantial amount of the
absorbed residues was tightly associated with, or incorporated into,
protein.
In a field experiment in a forest ecosystem in Oregon, USA,
neither glyphosate nor AMPA were recovered in salmon fingerlings
(Oncorhynchus kisutch) after aerial application of Roundup at a
rate of 3.3 kg a.i./ha (Newton et al., 1984). The fingerlings were
released at the downstream edge of the sprayed site and analysed up
to 55 days after treatment. Glyphosate was not recovered in carp
(Cyprinus carpio) in a field experiment in which ponds were
sprayed with Roundup at rates of 1.3-1.4 kg a.i./ha (Monsanto,
1980). In this experiment of approximately 90 days, AMPA was not
recovered until 30 days after application. It then increased up to
0.21 mg/kg whole fish, remained constant for another 30 days, and
then decreased to around the limit of determination (0.1 mg/kg) at
the end of the experiment.
In a forest ecosystem in Oregon, USA, Roundup was aerially
applied at a rate of 3.3 kg a.i./ha (Newton et al., 1984).
Concentrations in mammals were of the same order of magnitude as the
concentrations in litter and ground cover. The concentrations of
glyphosate in the viscera of herbivorous small mammals decreased
more slowly than in omnivorous and carnivorous small mammals, which
was probably due to a higher ingestion of contaminated litter. The
highest concentration was found in the viscera of omnivorous
deermice (Peromysces maniculatus) immediately after spraying: 5 mg
a.i./kg. Only small traces of AMPA were found in mammalian viscera.
4.5 Waste disposal
Small amounts of glyphosate can be disposed of by mixing with
alkali and soil prior to burial in a pit or trench, whereas large
amounts should be incinerated in units equipped with effluent gas
scrubbing (IRPTC, 1991).
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
Appraisal
The low toxicity, low volatility and low body absorption of
glyphosate makes its application by backpack sprayer safe under
field condition provided that the worker wears full protective
clothing.
5.1 Environmental levels
A synopsis of concentrations of glyphosate is tabulated in
Table 7. Measurements as part of regular monitoring programmes are
very scarce; measurements in field experiments with recommended
application rates simulating common agricultural practice are
therefore included in Table 7. Only maximum amounts are tabulated as
indicative values, since the rate at which they dissipate is not
included here (see sections 4.1 and 4.3). Data on the occurrence of
glyphosate and AMPA in sewage sludge are not available.
In biota the highest concentrations of glyphosate and AMPA were
found in fresh foliage and reindeer lichen (Cladonia rangiferina).
In abiota the highest concentrations of both compounds were found in
the soil (see Table 7). The occurrence of glyphosate in the
groundwater of Texas, USA, was reported by Hallberg (1989), but the
measured concentration and the year of measurement were not
specified.
Use of glyphosate as a herbicide may result in the presence of
residues in crops and animal tissues destined for human consumption.
Application as a herbicide may also be responsible for the presence
of glyphosate in drinking-water. Direct measurements of glyphosate
in foodstuffs (as part of food surveillance), drinking-water or
total diets have not been carried out. The only information
available comes from controlled residue studies. With technical
glyphosate formulated as the isopropylamine salt in aqueous
solution, numerous residue studies have been carried out in
vegetables, grasses, oil seeds, mammalian products, poultry products
and primary feed commodities. The results are summarized in the
various reports of the FAO/WHO Joint Meeting on Pesticide Residues
(FAO/WHO, 1986a, 1987, 1988). For detailed information on these
studies the reader is referred to these reports. The appraisals made
by the JMPR included the following more general statements.
Pre-harvest (5-14 days) application of glyphosate (isopropylamine
salt) in the cultivation of cereals results in significant residues
in the grain and plant materials. Studies are available to show the
fate of glyphosate in milling, baking and brewing. Residue levels in
white flour were approximately 10-20% of the levels in wheat, while
the bran residue levels were 2 to 4 times as high as those in the
wheat. Glyphosate residues were not lost during baking, but residue
levels decreased when bread was made from flour because of dilution.
Glyphosate residue levels in malt and beer derived from
field-treated barley were, respectively, about 25% and 4% of the
original level in the barley. Some glyphosate is lost during
washing, but most of the decrease can be attributed to dilution. The
levels in groats (processed oats) were about 50% of the levels in
the pre-harvest-treated oats. In all these cases, AMPA contributed
only a small proportion (average < 2.5%) of the total residues
(FAO/WHO, 1986a, 1987).
When administered to animals glyphosate is rapidly excreted
without degradation. Residues in cattle, pig and poultry meat, eggs
and milk were negligible after the animals were fed with a diet
containing 100 mg/kg glyphosate and aminoglyphosate acid. The
highest residues were in pig liver and kidney (up to 0.16 and
0.91 mg/kg, respectively) and cattle kidney (up to 1.4 mg/kg).
Residues in animal feeds arising from pre-harvest glyphosate
applications to cereals will result in only low residues in meat,
milk and eggs (FAO/WHO, 1986a).
On the basis of these residue studies the JMPR has estimated
the maximum residue levels that are likely to occur when glyphosate
(as isopropylamine salt) is used in practice, and recommended these
levels as Maximum Residue Limits (MRLs). These MRLs are presented in
FAO/WHO (1986a, 1987, 1988).
5.2 General population exposure
Apart from the controlled residue studies mentioned above, no
data are available.
Table 7. Maximum concentrations of glyphosate in environmental air, water, soil, sediment and biota
Sample Compound Location Yeara Concentration Reference
Netherlands
Surface water glyphosate Drentsche Aa 1988-1989 0.5-1 µg/litreb (Soppe, personal
Surface water AMPA Drentsche Aa 1988-1989 6 µg/litreb communication to the IPCS, 1991)
Finland
Loam soil (agric.) glyphosate Kettula 1978 17 mg/kg d.w. Müller et al. (1981)
Loam soil (agric.) AMPA Kettula 1978 3.2 mg/kg d.w. Müller et al. (1981)
Silt soil (agric.) glyphosate Kettula 1978 3.8 mg/kg d.w. Müller et al. (1981)
Silt soil (agric.) AMPA Kettula 1978 0.4 mg/kg d.w. Müller et al. (1981)
Wild berries glyphosate Laukaa, Konnevesi 1977 1.6-2.1 mg/kgc Siltanen et al. (1981)
Wild berries AMPA Laukaa, Konnevesi 1977 0.02-0.07 mg/kgc Siltanen et al. (1981)
Reindeer lichen glyphosate Laukaa, Konnevesi 1976 45 mg/kgc Siltanen et al. (1981)
Reindeer lichen AMPA Laukaa, Konnevesi 1976 2.1 mg/kgc Siltanen et al. (1981)
Canada
Wild berries glyphosate Harker, Lamplugh 1985 8-19 mg/kg f.w. Roy et al. (1989a)
Wild berries AMPA Harker, Lamplugh 1985 0.06-0.1 mg/kg f.w. Roy et al. (1989a)
Surface waterd glyphosate Carnation Creek 1984 162 µg/litre Feng et al. (1990)
Surface watere glyphosate Carnation Creek 1984 < 1 µg/litre Feng et al. (1990)
Surface waterd AMPA Carnation Creek 1984 approx 3 µg/litre Feng et al. (1990)
Pond water glyphosate Manitoba 1986 141 µg/litre Goldsborough & Beck (1989)
Pond water AMPA Manitoba 1986 2.2 µg/litre Goldsborough & Beck (1989)
Sedimentd glyphosate Carnation Creek 1984 6.8 mg/kg d.w. Feng et al. (1990)
Suspended sediment glyphosate Carnation Creek 1984 0.06 µg/litre Feng et al. (1990)
Soil glyphosate Carnation Creek 1984 40 mg/kg d.w. Feng & Thompson (1990)
Soil AMPA Carnation Creek 1984 9 mg/kg d.w. Feng & Thompson (1990)
Table 7. (cont'd)
Sample Compound Location Yeara Concentration Reference
Canada (cont'd)
Foliage (fresh) glyphosate Carnation Creek 1984 261-448 mg/kg d.w. Feng & Thompson (1990)
Foliage (fresh) AMPA Carnation Creek 1984 < 9 mg/kg d.w. Feng & Thompson (1990)
Topcrown foliage glyphosate Oregon Coast Range 1978 489 mg/kgc Newton et al. (1984)
Topcrown foliage AMPA Oregon Coast Range 1978 2.1 mg/kgc Newton et al. (1984)
Deermice (viscera) glyphosate Oregon Coast Range 1978 5.1 mg/kgc Newton et al. (1984)
USA
Pond water glyphosate Chassell, Corvallis, 1987 90-1700 µg/litre Monsanto (1990a)
Cuthbert
Pond water AMPA Chassell, Corvallis, 1987 2-35 µg/litre Monsanto (1990a)
Cuthbert
Stream water glyphosate Chassell, Corvallis, 1987 35-1237 µg/litre Monsanto (1990a)
Cuthbert
Stream water AMPA Chassell, Corvallis, 1987 < 1.0-10 µg/litre Monsanto (1990a)
Cuthbert
Pond sediment glyphosate Chassell, Corvallis, 1987 0.26-19 mg/kg d.w. Monsanto (1990a)
Cuthbert
Pond sediment AMPA Chassell, Corvallis, 1987 0.13-1.8 mg/kg d.w. Monsanto (1990a)
Cuthbert
Stream sediment glyphosate Chassell, Corvallis, 1987 0.11-0.69 mg/kg d.w. Monsanto (1990a)
Cuthbert
Stream sediment AMPA Chassell, Corvallis, 1987 < 0.05-0.38 mg/kg d.w. Monsanto (1990a)
Cuthbert
Soil (no litter on it) glyphosate Chassell, Corvallis, 1987 0.15-4.7 mg/kg d.w. Monsanto (1990a)
Cuthbert
Table 7. (cont'd)
Sample Compound Location Yeara Concentration Reference
USA (cont'd)
Soil (no litter on it) AMPA Chassell, Corvallis, 1987 0.18-0.51 mg/kg d.w. Monsanto (1990a)
Cuthbert
Soil (litter on it) glyphosate Chassell, Corvallis, 1987 0.07-1.4 mg/kg d.w. Monsanto (1990a)
Cuthbert
Soil (litter on it) AMPA Chassell, Corvallis, 1987 0.14-0.68 mg/kg d.w. Monsanto (1990a)
Cuthbert
Foliage (fresh) glyphosate Chassell, Corvallis, 1987 650-1300 mg/kgb Monsanto (1990a)
Cuthbert
Foliage (fresh) AMPA Chassell, Corvallis, 1987 1.7-2.6 mg/kgb Monsanto (1990a)
Cuthbert
a Sampling invariably took place during the autumn period.
b Analytical procedure was unvalidated; the water was sampled at an inlet point of
a drinking-water processing facility
c It was not reported whether values were based on dry or fresh weight
d Water unbuffered by vegetation
e Water buffered by vegetation
5.3 Occupational exposure during manufacture, formulation or use
In the study of Monsanto (1977), worker exposure to Roundup was
measured during herbicide mixing and application operations and upon
re-entry of treated fields. The formulation was applied on separate
plots using three application devices, i.e. a broadcast boom
sprayer, a handgun broadcast sprayer and a backpack/hand-gun
sprayer. Exposure time during mixing was < 5 min; during
application this was about 45 to 60 min. Inhalational exposure
during mixing and spraying was determined using a sampling device
placed close to the applicator's face; the total air volume sampled
was 15-20 times the daily ventilation volume. Dermal exposure during
mixing and spraying was monitored by determination of deposition on
gauze pads placed outside or inside the operator's clothing on
different parts of the body. Operator exposure (inhalation and skin,
determination method as above) upon re-entry was determined at 1, 3
and 7 days after application. In one treated plot, this was done by
having operators walk through the plot for 28 to 44 min; in two
other plots a dummy sampling device was used to determine exposure
upon re-entry. Using the measured glyphosate residues total worker
exposure was estimated. The results are presented in Table 8.
Table 8. Applicator/worker exposure to glyphosatea
Operation Average dermal Average inhalation Total exposure
exposure exposure (µg/h)
(µg/h) (µg/h)b
Tank filling 805.1 17.9 823.0
Boom spraying 271.4 0.19 271.59
Handgun spraying 7957.0 2.47 7959.47
Backpack spraying 3619.0 0.92 3619.92
Field re-entry:
1 day after treatment 2046.6 4.58 2051.18
3 days after treatment 2919.7 0.12 2919.82
7 days after treatment 15.9 0.12 16.02
a From: Monsanto (1977)
b Calculation based on an estimated breathing rate of 1.8 m3/h
Monsanto (1990) conducted another collaborative study at three
sites maintained by the USDA Forestry Service near Clayton, Georgia;
the Savanah River Plant, South Carolina; and Edgefield, South
Carolina. Glyphosate was being used to control vegetative growth
around pine seedlings planted in clear-cut forest areas. The workers
were biologically monitored by analysis of collected composite urine
specimens. Additionally, dermal/clothing deposition and simulated
inhalation exposure were monitored by passive dosimetry with cotton
cloth patches, hand rinses and air filters. At the three sites,
exposure of workers to glyphosate using backpack sprayers while
performing their duties under normal use conditions was monitored.
The analytical results indicated that the majority of urine
composite samples had unmeasurable residues of glyphosate.
Deposition on air filters, patches and hands from measurement of
washes, however, indicated a small amount of body exposure. It was
concluded that penetration of clothing did not exceed 3.84% and thus
clothing produced 96.2% protection. Body burden, as shown in urine
samples, was extremely low and in most cases below the detection
limit.
A new Monsanto study was conducted for the assessment of worker
exposure to glyphosate during mist blower application of Roundup
herbicide. Exposure was determined by a passive dosimetry technique
while workers sprayed weeds around palm trees in a plantation in
Malaysia. The workers were fitted with gauze patches at different
locations on their clothing. Air sampling was performed in the
breathing zone and the workers hands were washed at the end of the
day. The passive dosimetry body dose estimates were calculated for a
fully clothed worker with a long-sleeved shirt, long pants and
rubber boots. The hand exposure would account for bare hands during
the loading and spraying operations. Passive dosimetry estimates for
the four replicates, corrected for clothing and dermal penetration,
transport/storage/analytical recovery and normalized for body weight
and amount of chemical handled, averaged 1.88 µg/kg body weight per
kg a.i. This is little higher than the passive dosimetry estimates
of forestry workers who applied Roundup with knapsack sprayers,
which was 1.75 µg/kg body weight per kg a.i. Thus, it can be
concluded that workers applying Roundup herbicide with mist blowers,
experience some dermal exposure. In addition, inhalation exposure to
glyphosate may be higher during mist blower application. However,
Roundup demonstrated essentially no volatility. The only possible
route would be via air-borne particles. The actual amount of
glyphosate absorbed through inhalation would be much lower than the
estimated values because the measurement includes particles too
large to be inhaled (Monsanto, 1991).
Jauhiainen et al. (1991) examined the exposure of workers to
glyphosate during silvicultural clearing with brush saws equipped
with herbicide sprayers. Measurements of air concentrations during
spraying and urinary glyphosate levels both during and following
spraying were carried out. Most of the air samples had glyphosate
concentrations below 1.25 µg/m3; the highest value observed was
15.7 µg/m3. All urine samples taken had glyphosate concentrations
below the limit of detection of 0.1 mg/litre (Jauhiainen et al.,
1991).
Lavy et al. (1992) used two methods to monitor glyphosate
exposure of workers planting conifer seedlings. Firstly, they
estimated dermal exposure based on the rate of deposition on cotton
gauze patches, surface area exposed, and a dermal penetration rate
of 1.8%. This yielded dose estimates in the range of 0-875 or
0-1.7 µg/kg body weight per h. Secondly, they attempted to measure
urinary concentration levels but found no samples above the
detection limit of 0.01 mg/litre. Based on the negative results with
the urine samples, the authors concluded that the estimates based on
patch deposition overestimated exposure by at least a factor of 11
for the most highly exposed workers (Lavy et al., 1992).
It should be noted that a dermal penetration rate of 1.8% was
used in this calculation. In view of the discussion of the dermal
penetration studies (section 6.1), a value of 5.5% is to be
preferred. However, since the estimate based on 1.8% is already an
overestimation of exposure, it is not considered necessary to adjust
the estimate of Lavy et al. (1992) to 5.5%. In their final data
assessment, the authors estimated exposure from the biological
monitoring using postulated data, i.e. assuming a concentration in
urine of half the lower limit of method validation. This yielded
mean exposure values of 0.039 to 0.080 µg/kg body weight per h (Lavy
et al., 1992).
6. KINETICS AND METABOLISM IN LABORATORY
ANIMALS AND HUMANS
Appraisal
Absorption from the gastrointestinal tract after oral intake
is limited to 30-36% of the dose or less in various species, i.e.
rats, rabbits, laying hens and lactating goats. Percutaneous
absorption in Rhesus monkeys amounts to 5.5% only, and glyphosate is
very poorly absorbed through excised human abdominal skin.
Radiolabelled glyphosate distributes widely in the body, but
is primarily found in the bones where approximately 1% can be
detected after oral administration.
Glyphosate is essentially not metabolized. This validates
kinetic studies performed with radiolabelled compound.
After absorption, excretion of glyphosate occurs mainly in the
urine. Biliary excretion is limited and elimination through exhaled
air is very low. Total body clearance is 99% after 168 h.
6.1 Absorption
The absorption percentage in rats was reported to be 30-36%
after single oral dosage at 10 and 1000 mg/kg body weight;
calculations were based on excretion percentages in urine and
faeces, and on the fact that biliary elimination is probably a minor
route (Monsanto, 1988b; Brewster et al., 1991). From a similar study
carried out in 1973, total absorption percentages of approximately
20% (male rats) and 45% (female rats) can be derived (Monsanto,
1973a). Comparable results were obtained in a recent single dose
(5.6 or 56 mg/kg body weight) disposition study in F344/N rats (NTP,
1992), which indicated that 30% of the dose was absorbed.
In a 14-day oral study in rats with application of
14C-glyphosate via the diet (dose levels 1, 10 and 100 mg/kg
feed), the observed total excretion in urine was < 10% and in
faeces approximately 80-90%. Given the minor importance of the
biliary elimination route, these data indicate absorption levels of
about 15% or less (Monsanto, 1973c). The results of oral studies
with 14C-glyphosate in rabbits (Monsanto, 1973d), laying hens
(Hazleton Lab. Inc., 1988a) and lactating goats (Hazleton Lab. Inc.,
1988b) indicate gastrointestinal absorption percentages of
approximately 30% or less.
Percutaneous absorption has been studied in Rhesus monkeys and
in human tissue in vitro. After a single application of
14C-glyphosate (isoproylamine salt) as the undiluted Roundup
formulation to the shaven intact abdominal skin (contact time 24 h)
of Rhesus monkeys, absorption amounted to only 1.8% of the dose. In
this study, however, only 16% of the dose could be accounted for at
the end of the study (Maibach, 1983). This low recovery strongly
reduces the value of the study result (possibly skin absorption is
seriously underestimated in this study). From an identical study
with diluted Roundup formulation (1:29 with water), conducted by
Wester et al. (1991), also in Rhesus monkeys (contact time 12 h),
total absorption percentages of 3.7% (at low dose) or 5.5% (high
dose) can be derived. Using both undiluted and diluted Roundup
formulation, Wester et al. (1991) observed that percutaneous
absorption of 14C-glyphosate through human skin in vitro into
human plasma as receptor fluid was < 2% (contact-time up to
16 h). In another in vitro study with human skin, absorption of
14C-glyphosate from three undiluted formulations (i.e. MON 0139,
Roundup and Roundup spray mix) was studied (contact time 24 h); very
low absorption percentages of 0.028 to 0.152% were found (Franz,
1983). With regard to these in vitro results it should be pointed
out that this technique has not yet been fully validated and
therefore direct extrapolation to in vivo human skin absorption
should be undertaken cautiously.
The absorption after inhalational intake has not been
determined.
6.2 Distribution
Concentrations of 14C label in tissues were determined on day
7 after administration of a single oral dose (10 or 1000 mg/kg body
weight) of 14C-glyphosate to rats (Monsanto, 1988b). Although only
a small proportion was absorbed, the isotope was widely distributed
throughout the body, but was primarily found in bone. The principal
results are presented in Table 9.
In rats tissue concentrations of 14C label were determined on
several occasions throughout a treatment period of 14 days and a
post-dosing withdrawal period of 10 days (dietary administration of
14C-glyphosate at 1, 10 and 100 mg/kg diet). Maximum tissue levels
were reached after 10 days or less, with highest concentrations
(maximum 0.85 mg/kg at the 100 mg/kg dose level) in kidneys
(Monsanto, 1973c). It should be noted, however, that in this study
concentrations in bone or bone marrow were not measured. An increase
of 14C in excreta was observed during the withdrawal period after
an initial rapid decrease; this indicated mobilization from storage
in depot tissue (Monsanto, 1973c).
Table 9. Concentrations of 14C label (as mg glyphosate-equivalents/kg
fresh weight) in selected tissues of rats on day 7 after a single
oral dose (rounded values) (Monsanto, 1988b)
Dose: 10 mg/kg body weight Dose: 1000 mg/kg body weight
male female male female
Blood 0.0045 0.0027 0.33 0.17
Liver 0.030 0.014 1.9 1.3
Kidney 0.022 0.013 1.9 1.4
Spleen 0.012 0.0073 2.6 3.0
Lung 0.015 0.012 1.5 1.1
Thyroid 0.00080 0.00036 1.5 1.2
Nasal mucosa 0.0050 0.023 1.7 1.8
Stomach 0.0080 0.0037 2.4 2.4
Small intestines 0.022 0.018 1.9 1.6
Colon 0.034 0.016 11.0 9.2
Bone 0.55 0.31 30.6 19.7
Bone marrow 0.029 0.0064 4.1 12.5
In lactating goats concentrations of 14C label in milk were
measured after giving capsules containing a 9:1 mixture of
14C-glyphosate and 14C-aminomethylphosphonic acid (AMPA) to a
dose level equivalent to 120 mg/kg diet (expressed as free acid) for
5 days. Concentrations in milk (as mg equivalents glyphosate/kg
whole milk) ranged from 0.019 to 0.086 mg/kg during the test period;
at day 5 after the last dose the concentration was 0.006 mg/kg
(Hazleton Lab. Inc., 1988b; Monsanto, 1988d).
In a study on laying hens, carried out using a 9:1 mixture of
14C-glyphosate and 14C-AMPA, concentrations of radiolabel were
measured in eggs collected during a 7-day period of dietary
administration at 120 or 400 mg/kg diet. At 400 mg/kg, residues in
egg white were 0.010-0.032 mg/kg (expressed as
glyphosate-equivalents) and in egg yolk 0.096-0.753 mg/kg; at
120 mg/kg the corresponding concentration ranges were 0.003-0.017
and 0.002-0.24 mg/kg, respectively. At 120 mg/kg diet, no 14C was
detectable in egg white after 10 withdrawal days; in yolk
0.019 mg/kg was present at that time (at 400 mg/kg no withdrawal
test was conducted) (Hazleton Lab. Inc., 1988a).
6.3 Metabolic transformation
Biotransformation of glyphosate occurs to a very low degree
only. In rats it was shown that all of the 14C in urine and
faeces, after a single oral application of 14C-glyphosate, was
present as unchanged parent compound (Monsanto, 1973b). Also in
rats, > 97% of the 14C in excreta, after a single oral dose,
was shown to be unchanged compound. AMPA was the only metabolite,
covering only 0.2-0.3% of the applied 14C (Monsanto, 1988a). In
laying hens also, AMPA was the only metabolite, accounting for only
a minor part of the applied amount (Monsanto, 1988c).
6.4 Elimination and excretion
In the period of 0-5 days after a single oral application of
14C-glyphosate (6.7 mg/kg body weight) to rats the total excretion
in urine was 15% (males) and 35-43% (females) of the administered
dose; total excretion in faeces was 85% (males) or 50-55% (females).
Less than 1% of the radiolabel was expired as 14CO2 (Monsanto,
1973a). In a more recent study (Monsanto, 1988b), the very low level
of expiration as 14CO2 was confirmed but no significant
inter-sex difference in the level of 14C in excreta was observed.
The result of the latter study was that at both oral dose levels (10
and 1000 mg/kg body weight) elimination in faeces was 62-70% and
excretion in urine was 14-18% (1000 mg/kg body weight) or 22-29%
(10 mg/kg body weight); less than 0.2% of the dose was expired as
14CO2. After single intravenous application (dose 10 mg/kg body
weight) 75-79% appeared in urine and only 5-8% in faeces, a finding
that shows that biliary elimination occurs to a limited degree only
(Monsanto, 1988b).
Delayed excretion in rats during a 10-day post-dosing
withdrawal period was observed after daily oral administration via
the diet for 14 days (Monsanto, 1973c); this suggests that some
storage in tissue(s) occurs when uptake is prolonged. Tissue
equilibrium was attained by day 10 of the dosing period and
excretion equalled intake by day 6 of the dosing period.
In rabbits > 80% was eliminated in faeces (with additional
14C present in the gut) and 7-11% in urine within 5 days after
administration of a single oral dose (6.7 mg/kg body weight) of
14C-glyphosate. Less than 1% of the dose was expired as 14CO2
(Monsanto, 1973d). In one oral study in lactating goats lasting 5
days, total excretion in urine varied from 20 to 24% and in faeces
from 60 to 66% (Hazleton Lab. Inc., 1988b).
6.5 Retention and turnover
Total body clearance in the study of Monsanto (1973a) was
94-98% (males) or 82-84% (females) over a 48-h period after giving a
single oral dose of 14C-glyphosate; at 120 h post-dosing this was
99% (both sexes). In Monsanto (1988b), the kinetics of whole body
elimination were estimated using the radioactivity (14C) measured
in urine and faeces after a single oral dose of 14C-glyphosate (10
or 1000 mg/kg body weight). Because of the lack of biotransformation
of glyphosate it is valid to base kinetics on total radioactivity.
The elimination appeared to be biphasic. The half-life of the alpha
elimination phase at 10 mg/kg body weight was 5.87 h (males) or
6.22 h (females); at 1000 mg/kg body weight this was 5.26 h (males)
or 6.44 h (females). The half-life of the beta phase at 10 mg/kg
body weight was 79 h (males) or 106 h (females); at 1000 mg/kg body
weight this was 181 h (males) or 337 h (females). Pretreatment with
unlabelled compound for 14 days (carried out at the low dose level)
did not have an effect on whole body elimination. Seven days after
dosing, < 0.05% of the dose was present in organs and < 0.5% in
the remaining carcass. Highest concentrations were present in bone.
It was estimated that 0.2-0.6% of the oral dose was associated with
this site; after intravenous dosing this was approximately 1%
(Monsanto, 1988b). Brewster et al. (1991) reported that in rats
nearly all of the absorbed material had been eliminated from the
body 168 h after oral administration of 10 mg/kg body weight;
approximately 1% of the dose was still associated with the bone.
In a recent study on the disposition of glyphosate in F-344/N
rats, 1% of a single oral dose (5.6 or 56 mg/kg) was found in the
tissues 72 h after dosing; 20-30% of the administered radioactivity
was eliminated via urine and 70-80% via the faeces (NTP, 1992).
7. EFFECTS ON LABORATORY ANIMALS AND IN VITRO TEST SYSTEMS
Appraisal
Glyphosate, administered by oral and dermal routes, has a very
low acute toxicity. Both glyphosate and its concentrated
formulations produce moderate to severe eye irritation, but only
slight dermal irritation. Neither glyphosate nor tested formulations
induce sensitization.
Short-term feeding studies have been conducted in several
species. In CD-1 mice, increased liver, brain, heart and kidney
weights, and growth retardation were reported at 50 000 mg/kg diet.
At 10 000 mg/kg diet, an increase in relative liver weight was
reported; however, there were no differences in absolute liver
weights when this group was compared to controls. The relative
increase represented only a 9% increase over the liver weight
reported for controls and was not considered toxicologically
significant. Additionally, there were no gross or histopathological
changes in the liver at doses of 10 000 mg/kg or more. The Task
Group considered the NOAEL to be 50 000 mg/kg diet.
In a 13-week study conducted in Charles River CD
(Sprague-Dawley) BR rats, no treatment-related effects were observed
at doses up to 20 000 mg/kg diet. The NOAEL was greater than the
highest dose tested.
Two additional 13-week studies (one in rats and the other in
mice) were conducted by NTP in which lesions of the salivary glands
were observed in both species. The NOAEL in the rat study was
< 3125 mg/kg diet and that in the mouse study was 3125 mg/kg diet.
Other short-term and long-term studies conducted in different
strains and species did not reveal similar lesions. The lesions
indicate that glyphosate may be acting as a weak adrenergic agonist.
The toxicological significance of the salivary gland lesions
observed in the NTP studies is unknown.
In a 52-week study conducted in beagles, no compound-related
effects were reported. The NOAEL was 500 mg/kg body weight per day.
In a 7-day study with the Roundup formulation in female cattle, a
NOAEL of 400 mg Roundup/kg body weight was reported. At higher dose
levels, decreased feed intake and diarrhoea occurred.
In long-term feeding studies in both rats and mice, few toxic
effects were observed. These effects were present at relatively high
dose levels only. In mice, technical glyphosate produced growth
retardation, hepatocyte hypertrophy or necrosis at 30 000 mg/kg diet
only. At 5000 and 30 000 mg/kg diet an increase in epithelial
hyperplasia of the urinary bladder was reported. The increased
incidence of this lesion did not follow a dose-related trend and in
the highest dose tested the incidence was actually lower than that
reported at the medium dose level, in spite of a 6-fold increase in
glyphosate. The observation at the medium dose (5000 mg/kg) is not
considered a compound-related effect and the NOAEL is, therefore,
considered to be 5000 mg/kg diet (814 mg/kg body weight).
Long-term feeding studies in rats resulted in decreased
growth, increased liver weight and degenerative liver changes at
20 000 mg/kg diet only. At 8000 and 20 000 mg/kg diet, there was an
apparent increase in the incidence of inflammation of the gastric
mucosa in both sexes. The only statistically significant increase
was observed in the medium-dose females (15%). This value was also
outside the historical control range of 0-13%. This finding was not
considered to be a treatment-related effect. There was no
dose-related trend across all groups of treated females and there
was no statistically significant difference in any treated male
groups. The NOAEL was therefore 8000 mg/kg diet (410 mg/kg body
weight).
Studies in rats and rabbits indicated that technical
glyphosate is not teratogenic. Two multigeneration studies were
conducted with technical glyphosate. In the first study, the only
effect noted was an increased incidence of unilateral renal tubular
dilation in F3b male pups at 30 mg/kg body weight. In the second
study, decreased body weights were reported for parents and pups and
decreased litter size was associated with dose levels of
30 000 mg/kg diet. Decreased body weights reported for parents and
pups at 10 000 mg/kg diet were not toxicologically significant. In
parents, the decrease was only 2 to 4% below controls and for pups
the decrease was 5.6 to 6.6% lower than controls. The findings in
pups were also transient and did not occur consistently in all
litters. The NOAEL was 10 000 mg/kg diet. The absence of a renal
effect in pups at a higher dose level (1500 mg/kg body weight),
though not invalidating earlier findings of unilateral renal tubular
dilation in male F3b pups, indicates that the reproducibility of
this lesion and its toxicological significance are uncertain. It
should be noted that in no other toxicological study was an effect
on kidneys found.
Bioassays in mice and rats did not indicate that technical
glyphosate was carcinogenic.
Glyphosate has been shown to have no genotoxic potential in a
range of in vitro and in vivo studies.
7.1 Single exposure
Numerous acute toxicity studies have been performed to
determine LD50 values of glyphosate and of herbicide formulations
containing glyphosate as active ingredient. The results of these
studies are summarized in Tables 10 (results for glyphosate) and 11
(results for formulations). These data show that glyphosate and its
formulations have very low toxicity by the oral and dermal
administration routes. By the intraperitoneal route glyphosate is
markedly more toxic than by the other routes. General intoxication
symptoms include breathing difficulties, ataxia and convulsions.
The mechanism of the toxic action of glyphosate has been
studied in rats. Olorunsaga et al. (1979) observed dose-related
reduced respiratory control ratios and increased phosphatase
activity in mitochondria isolated from rat livers 5 h after single
intraperitoneal doses ranging from 15 to 120 mg/kg body weight. This
effect was also seen in rat liver mitochondria in vitro (Bababunmi
et al., 1979; Olorunsaga, 1982a,b). The authors suggest that acute
toxicity at lethal doses may occur as a result of the uncoupling of
oxidative phosphorylation.
The acute toxicity in rats of the surfactant
polyoxyethyleneamine, with which glyphosate is commonly formulated
in Roundup, was compared to that of glyphosate in a study by
Martinez et al. (1990). Both compounds exhibited pulmonary toxicity
following either oral or intratracheal administration. The toxicity
of the herbicide formulation was greater than can be accounted for
on the basis of the dose response data from either compound alone
(Martinez et al., 1990; Martinez & Brown, 1991).
A study was undertaken by Tai et al. (1990) to investigate the
effects of glyphosate, surfactant, and their combination in Roundup
on cardiovascular function in female beagles. They found that
glyphosate alone at plasma levels ranging from 923 to 3450 mg/litre,
which simulates the human ingestion situation, were shown to
increase the myocardial contractility. The surfactant alone
considerably reduced the cardiac output, the left ventricular stroke
work index and the mean arterial pressure. The joint effect of both
glyphosate and the surfactant in Roundup formulation resulted in
cardiac depression, which was mostly due to the surfactant since
glyphosate itself increased myocardial contractility. The authors
indicated that the probable cause of the observed increases in
pulmonary vascular resistance index and pulmonary artery pressure
was a direct vasoactive effect of glyphosate on the pulmonary
artery.
Table 10. Acute toxicity of glyphosate to experimental animals
Species (sex) Product tested LD50/LC50a Reference
Oral studies
Rat (m,f) glyphosate techn, > 5000 mg/kg bw FDRL (1988d)
purity 97.8%
Rat (m,f) glyphosate techn, > 5000 mg/kg bw Inveresk Research Int.
purity 96-99% (1989a)
Rat (m,f) glyphosate techn, > 5000 mg/kg bw NOTOX (1988)
purity 96-99%
Rat (m,f) 85.5% techn. > 5000 mg/kg bw Bio/Dynamics Inc.
glyphosate in (1988c)
water
Rat (m,f) glyphosate, IPA salt, > 5000 mg/kg bw Monsanto (1981b)
65% in water
Rat (m.f) glyphosate, EO saltb 2047 mg/kg bw Knapek et al. (1986)
Goat (f) glyphosate techn, 3500 mg/kg bw USDA (1987c)
purity 98.7%
Goat (f) glyphosate, IPA salt, 5700 mg/kg bw USDA (1987b)
65% in water
Dermal studies
Rat (m,f) glyphosate techn, > 2000 mg/kg bw Inveresk Research Int.
purity 96-99% (1989c)
Rabbit (m,f) glyphosate techn, > 5000 mg/kg bw FDRL (1988b)
purity 97.8%
Rabbit (m,f) 85.5% techn. > 5000 mg/kg bw Bio/Dynamics Inc.
glyphosate in water (1988a)
Rabbit (m,f) glyphosate, IPA salt, > 5000 mg/kg bw Monsanto (1981c)
65% in water
Intraperitoneal studies
Mouse (m) glyphosate (not 545 mg/kg bw
further specified)
Mouse (f) glyphosate (not 740 mg/kg bw FAO/WHO (1986b)
further specified)
Mouse (m,f) glyphosate (not 134 mg/kg bw FAO/WHO (1986b)
further specified)
Rat (m) glyphosate (not 281 mg/kg bw
further specified
Table 10. (cont'd)
Species (sex) Product tested LD50/LC50a Reference
Rat (f) glyphosate (not 467 mg/kg bw FAO/WHO (1986b)
further specified)
Rat (m,f) glyphosate (not 238 mg/kg bw FAO/WHO (1986b)
further specified)
a All values expressed as mg of product tested (as presented in "product tested"
column)
b EO is an abbreviation of 5-ethoxy-oleinamine salt.
Table 11. Acute toxicity of glyphosate formulations to experimental animals
Species (sex) Product testeda LD50/LC50b Reference
Oral studies
Mouse (m,f) Roundup >5000 mg/kg bw Mitsukaido Labs (1986)
Rat (m,f) Roundup 5000 mg/kg bw Bio/Dynamics Inc.
(1988e)
Rat (m,f) "Compound No. 3607" >5000 mg/kg bw Inveresk Research Int.
(1988a)
Rat (m,f) Roundup TX >5000 mg/kg bw NOTOX (1987a,b)
Rat (m,f) Alphee >5000 mg/kg bw Bio/Dynamics Inc.
(1987a)
Rat (m,f) Sting TX >5000 mg/kg bw NOTOX (1987f,g)
Rat (m,f) Sting 2510 mg/kg bw Younger Labs Inc.
(1984)
Rat (m,f) Sting 1950 mg/kg bw Bio/Dynamics Inc.
(1984b)
Rat (m,f) MON 8780 >5000 mg/kg bw Bio/Dynamics Inc.
(1985a)
Rat (m,f) Agrichem Glyfosaat B >5000 mg/kg bw NOTOX (1990e)
Rat (m,f) "Glyfosaat 360 g/litre" >2000 mg/kg bw NOTOX (1989c)
Rat (m,f) Legend >2000 mg/kg bw CIT (1991a)
Goat (f) Roundup 4860 mg/kg bw USDA (1983)
Table 11. (cont'd)
Species (sex) Product testeda LD50/LC50b Reference
Dermal studies
Rat (m,f) "Compound No. 3607" >2000 mg/kg bw Inveresk Research Int.
(1988b)
Rat (m,f) Roundup TX >4000 mg/kg bw NOTOX (1987c)
Rat (m,f) Sting TX >4000 mg/kg bw NOTOX (1987h)
Rat (m,f) MON 8780 >5000 mg/kg bw Bio/Dynamics Inc.
(1985b)
Rat (m,f) Agrichem Glyfosaat >4000 mg/kg bw NOTOX (1990f)
B or 2
Rat (m,f) "Glyfosaat 360 g/litre" >2000 mg/kg bw NOTOX (1989b)
Rat (m,f) Legend >2000 mg/kg bw CIT (1991b)
Rabbit (m,f) Roundup >5000 mg/kg bw Bio/Dynamics Inc.
(1988f)
Rabbit (m,f) Alphee >5000 mg/kg bw Bio/Dynamics Inc.
(1987b)
Rabbit (m,f) Sting >5000 mg/kg bw Bio/Dynamics Inc.
(1984a)
Inhalation studies
Rat (m,f) Roundup (aerosol) 3180 mg/m3 Monsanto (1983d)
Rat (m,f) "Compound No. 3607" >4860 mg/m3 Inveresk Research Int.
(1989d)
Rat (m,f) Alphee >8900 mg/m3 Monsanto (1987b)
a Composition of the formulations is given in Table 2, with the exceptions of
Agrichem Glyphosaat B (or 2), "Glyfosaat 360 g/litre" and "Compound No. 3607",
which all contain approximately 480 g/litre of the isopropylamine salt, and of MON
8780 (32.8% isopropylamine salt), and Legend (40% isopropylamine salt).
b All values given as mg formulation.
7.2 Short-term exposure
7.2.1 Oral studies
In CD-1 mice, a 13-week feeding study was conducted with
technical glyphosate (purity 98.7%) using dose levels of 5000,
10 000 and 50 000 mg/kg diet (equal to 940, 1890 and 9710 mg/kg body
weight per day in males and 1530, 2730 and 14 860 mg/kg body weight
per day in females). No effect on appearance or survival was
observed. Growth retardation and increased weights of brain, heart
and kidneys were observed at 50 000 mg/kg. Liver weights were
increased at 50 000 and 10 000 mg/kg. Limited histopathology showed
no adverse effects. The authors of the study concluded that the
NOAEL was 10 000 mg/kg diet (1890 mg/kg body weight per day)
(Bio/Dynamics Inc., 1979).
In a 13-week feeding study with technical glyphosate,
Sprague-Dawley rats received 1000, 5000 or 20 000 mg/kg diet (equal
to 63, 317 and 1267 mg/kg body weight per day in males and 84, 404
and 1623 mg/kg body weight per day in females). No effect on
appearance, survival or growth occurred. Haematology, blood
biochemistry and urinalysis, carried out at test end only, were also
unaffected. Organ weights (determined for liver, kidneys and testes
only) were not affected. Limited histopathology showed no adverse
effect in any tissue. The NOAEL in this study was 20 000 mg/kg diet
(1267 mg/kg body weight per day) (Monsanto, 1987a). Absence of
toxicity was also found in another 13-week feeding study on rats
using technical glyphosate and dose levels of 200 to 12 500 mg/kg
diet (Tauchi, 1979 as cited by FAO/WHO, 1986b).
Two further 13-week studies in rodents were conducted on behalf
of the US National Toxicology Program (1992). Both rats (F-344/N)
and mice (B6C3F1) were administered glyphosate (purity
approximately 99%) in feed at levels of 3125, 6250, 12 500, 25 000
and 50 000 mg/kg diet.
In rats, reduced weight gains were observed at 25 000 mg/kg
diet (males only) and at 50 000 mg/kg diet (males and females). No
changes in feed consumption were found. Minor increases in the
relative organ weight of liver, kidney and testes, and decreased
thymus weight were observed in males only at several dose levels;
these changes did not show a clear dose relation and therefore are
not considered to be compound-related effects. Small increases in
haematocrit and red cell blood counts were observed in male rats at
> 12 500 mg/kg diet. Clinical chemistry showed increased alkaline
phosphatase (AP) and alanine aminotransferase (ALAT) at >
6250 mg/kg diet in males and at > 12 500 mg/kg diet in females.
Bile acid levels in blood were increased at 25 000 mg/kg diet (males
only) and at 50 000 mg/kg diet (males and females). Decreases in
sperm count were observed in males at > 25 000 mg/kg diet. The
only histopathological lesions found were cytoplasmic alterations of
the parotid and submandibular salivary glands, consisting of
basophilic changes and hypertrophy of acinar cells. The parotid
gland was more severely affected. The magnitude of the effect was
dose-dependent, with focal lesions in less severe cases to diffuse
involvement at higher doses. Lesions of a similar nature and
magnitude were observed in both sexes. The sublingual gland was not
detectably altered. Effects on the salivary glands were observed
already at the lowest dose level tested of 3125 mg/kg diet (equal to
205 mg/kg body weight per day for males and 213 mg/kg body weight
per day for females). Thus, this study did not yield a NOAEL (NOAEL
< 3125 mg/kg diet) (NTP, 1992).
In mice, reduced weight gains were observed at 50 000 mg/kg
diet in both sexes. Increased organ weights were noted in heart,
kidney, liver, thymus, lung and testis; these changes did not show a
clear dose relation and therefore are not considered to be
compound-related effects. Feed consumption levels were not changed
significantly. Lesions in the parotid gland were observed, similar
to those observed in rats. The sublingual gland and the
submandibular salivary glands were not detectably altered. The
effects on the parotid gland were observed in mice at 6250 mg/kg
diet (equal to 1065 mg/kg body weight per day for males and
1411 mg/kg body weight per day for females), but were not seen at
the lowest dose level tested of 3125 mg/kg diet (equal to 507 mg/kg
body weight per day for males and 753 mg/kg body weight per day for
females). The NOAEL in this study was 3125 mg/kg diet (507 mg/kg
body weight per day) (NTP, 1992).
The salivary gland lesions could also be induced in rats by
14-day exposure at feed levels of 50 000 mg/kg diet. The salivary
glands lesions induced by glyphosate were similar to those which
could be induced by exposure to high subcutaneous doses of the
ß-adrenergic agonist isoproterenol and could be partially
ameliorated with the ß-adrenergic antagonist propanolol. This
indicates that glyphosate may induce the salivary gland lesions by
acting as a weak adrenergic agonist (NTP, 1992).
The short-term toxicity of technical glyphosate was also
studied in dogs. Beagle dogs received technical glyphosate in
gelatin capsules at dose levels of 0, 20, 100 or 500 mg/kg body
weight per day for 52 weeks. No effect occurred with respect to
clinical signs, body weight, feed consumption, ophthalmoscopy,
haematology, blood biochemistry, urinalysis, gross pathology and
histopathology. The only changes in treated groups relative to
controls were increased pituitary weights (absolute and relative) in
the medium- and high-dose males. Because there were no concomitant
histological changes present in pituitaries and given the absence of
an effect on this organ (and related organs) in all other studies,
the toxicological significance of the increased pituitary weights is
questionable. The NOAEL in this study was 500 mg/kg body weight per
day (Monsanto, 1985).
A 7-day oral study was carried out with Roundup in female
cattle weighing 160 to 272 kg. Brahman-cross heifers received 400,
500, 630 or 790 mg Roundup/kg body weight per day by nasogastric
tube. At 790 mg/kg, 1/3 animals died before test end, showing
laboured breathing and pneumonia caused by aspiration of rumen
contents. Decreased feed intake was seen at 630 and 790 mg/kg;
diarrhoea occurred at 500, 630 and 790 mg/kg body weight. Slight
increases in a number of blood parameters, occurring at 790 mg/kg
only, were probably due to extracellular fluid shifts and
haemoconcentration secondary to diarrhoea. The NOAEL in this study
was 400 mg Roundup/kg body weight per day (USDA, 1987a).
7.2.2 Dermal studies
Short-term dermal toxicity studies were carried out in rabbits
with technical glyphosate and the formulation Roundup.
Technical glyphosate, moistened with saline, was applied under
occlusion at dose levels of 100, 1000 and 5000 mg/kg body weight per
day to the shaven intact or abraded skin of rabbits for 6 h/day, 5
days/week for 3 weeks. No effect on survival and growth occurred.
Slight dermal irritation (barely perceptible erythema and oedema)
was observed at 5000 mg/kg body weight only. No evidence of systemic
toxicity was found (parameters: haematology and blood biochemistry
in five animals of each sex per group, organ weights, gross
pathology, limited histopathology) (IRDC, 1982). Absence of systemic
effects was also found in the somewhat more limited 21-day study in
rabbits (no haematology and blood biochemistry) with Roundup. Skin
effects were more severe in this study: at both dose levels (76 and
114 mg/kg body weight per day, undiluted formulation applied)
erythema and oedema were seen and, in addition, exudate and
fissuring occurred at the abraded skin sites. After a 4-week
recovery period the skin effects were no longer present
(Bio/Dynamics Inc., 1975).
7.2.3 Inhalational studies
A 4-week inhalation study was carried out on rats with a 1:3
dilution of Roundup formulation. Test concentrations of 50, 160 and
360 mg/m3 of the diluted formulation (equivalent to 17, 53 and
120 mg Roundup/m3) were administered as an aerosol spray for
6 h/day, 5 days/week. The mass median aerodynamic diameter of the
test material ranged from 1.8 to 2.7 µm with geometric standard
deviations between 1.7 and 2.0. An increased incidence of irritation
of the nasal turbinates (subacute inflammation), trachea
(mononuclear cell infiltration) and lungs (perivascular lymphoid
infiltrates/aggregates) was observed among the high-dose females
only. No signs of systemic toxicity were found (parameters:
survival, growth, limited haematology and blood biochemistry, organ
weights, limited histopathology) (Monsanto, 1983e).
7.3 Long-term toxicity and carcinogenicity
Only oral studies are available. Dietary studies using
technical glyphosate were performed in mice and rats.
In Charles River CD-1 mice technical glyphosate was fed in the
diet at concentrations of 0, 1000, 5000 or 30 000 mg/kg diet for 24
months (dose levels equal to 0, 157, 814 and 4841 mg/kg body weight
per day for males and 0, 190, 955 and 5874 mg/kg body weight per day
for females). No effect on survival or appearance was noted. Body
weights were decreased in the males of the high-dose group.
Haematology and organ weights showed no effects. Histopathology in
liver revealed an increased incidence of central lobular hepatocyte
hypertrophy among high-dose males (incidences: 9/49, 5/50, 3/50 and
17/50 in control, low-dose, medium-dose and high-dose males,
respectively) and an increased incidence of central lobular
hepatocyte necrosis also among high-dose males (incidences: 0/49,
2/50, 2/50 and 10/50). Increased incidences of epithelial
hyperplasia in the urinary bladder were present in the medium-dose
and high-dose males (incidences: 3/49, 3/50, 10/50 and 8/50). There
were no statistically significant increases in the frequency of
neoplastic lesions. The NOAEL in this study was 5000 mg/kg diet
(814 mg/kg body weight per day) (Bio/Dynamics Inc., 1983a).
Two chronic feeding studies on rats were conducted with
technical glyphosate, one in 1979-1981 and the other in 1988-1990.
The first study, carried out using Charles River CD (Sprague-Dawley)
BR rats, had dose levels of 0, 60, 200 and 600 mg technical
glyphosate/kg diet (equal to about 0, 3, 10 and 32 mg/kg body weight
per day, respectively). Survival, appearance, haematology, blood
biochemistry, urinalysis and organ weights were not changed. The
NOAEL was > 600 mg/kg diet (> 32 mg/kg body weight per day).
Slight growth retardation during part of the study was noted in the
high-dose males. The incidence of interstitial cell tumours in
testes showed a statistically significant increase (incidences:
0/50, 3/50, 1/50 and 6/50; historical control range: 3-7%)
(Bio/Dynamics Inc., 1981a). This finding, in itself constituting
some evidence for a carcinogenic effect in rats, should be judged in
the light of the absence of an effect at much higher dose levels in
the more recent 2-year study in rats (see below). This is also valid
for the slight growth retardation (i.e. no effect on growth at much
higher dose levels in the more recent study, see below). In the
recent 2-year study, rats (same strain) were fed 2000, 8000 or 20
000 mg technical glyphosate/kg diet (equal to about 100, 410 and
1060 mg/kg body weight per day) for 24 months. There was no effect
on survival or appearance. Growth was retarded in the high-dose
females. Haematology and blood biochemistry showed no effects. In
the high-dose males, the urine specific gravity (after 6 months
only) and urine pH were increased. A statistically significant
increased incidence of degenerative lens changes (basophilic
degeneration of the posterior subcapsular lens capsule or mature
cataracts) was found among the high-dose males (incidences 3/60,
4/60, 4/60 and 8/60 in the control, low-, medium- and high-dose
groups, respectively. However this finding was within the historical
control range of 0-33%. Liver weights were increased in the
high-dose males only. Histopathology showed an increased incidence
Table 12. Skin irritation tests on rabbits with glyphosate and its formulations
Product testeda Contact time (h) Draize scoreb Classificationc Reference
Glyposate
Glyphosate techn. 85% in water 4 0.8 slightly irritating Bio/Dynamics Inc. (1988b)
Glyphosate techn., moistened powder 4 0 not irritating FDRL (1988a)
Glyphosate techn., moistened powder 4 0 not irritating Inveresk Research Int. (1989e)
Glyphosate, IPA salt 65% in water 24 0.2 not irritating Monsanto (1981d)
Formulations
Roundup, undiluted 4 1.9 slightly irritating Bio/Dynamics Inc. (1988g)
"Compound No. 3607", undiluted 4 1.2 slightly irritating Inveresk Research Int. (1988c)
Roundup TX, undiluted 4 0.7 slightly irritating NOTOX (1987d)
Alphee, undiluted 4 1.0 slightly irritating Bio/Dynamics Inc. (1987c)
Sting, undiluted 4 1.3 slightly irritating Bio/Dynamics Inc. (1984c)
Sting TX, undiluted 4 3.6 moderately irritating NOTOX (1987i)
Roundup L&G, undiluted 4 1.0 slightly irritating Bio/Dynamics Inc. (1985c)
"Glyfosaat 360 g/litre, undiluted 4 0.3 not irritating NOTOX (1989d)
Agrichem Glyfosaat B, undiluted 4 1.7 slightly irritating NOTOX (1990d)
Agrichem Glyfosaat 2, undiluted 4 2.0 slightly irritating NOTOX (1990b)
Legend, undiluted 4 0.7 slightly irritating CIT (1991c)
a For glyphosate content of the tested formulations, see footnote a in Table 11.
b This score is the mean score per animal and was calculated using the data from
the original report. The standard Draize scoring system was used; in calculating
the mean response per animal, the maximum response observed for an animal was used.
c The classification is based on the mean Draize score per animal. Specification:
score 0-0.5 not irritating; 0.6-2.0 slightly irritating; 2.1-5.0 moderately
irritating; 5.1-8.0 severely irritating.
Table 13. Results of eye irritation tests in rabbits for glyphosate and its formulations.
Product testeda Irritation indexb Classificationc Reference
Glyphosate
Glyphosate techn. 85% in water, undiluted 45 strongly irritating Bio/Dynamics Inc. (1988d)
Glyphosate, IPA salt, 65% in water 0 not irritating Monsanto (1981e)
Glyphosate techn. (97.6%), undiluted 54 strongly irritating FDRL (1988c)
Glyphosate techn. (96-99%), undiluted 29 irritating Inveresk Research Int. (1989f)
Formulations
Roundup, undiluted 54 strongly irritating Bio/Dynamics Inc. (1990)
"Compound No. 3607", undiluted 13 irritating Inveresk Research Int. (1989g)
Roundup TX, undiluted 31 strongly irritating NOTOX (1987e)
Alphee, undiluted 6 slightly irritating Bio/Dynamics Inc. (1987d)
Sting, undiluted 104 extremely irritating Bio/Dynamics Inc. (1984d)
Sting TX, undiluted 19 irritating NOTOX (1987j)
Roundup L&G, undiluted 19 irritating Bio/Dynamics Inc. (1985d)
"Glyfosaat 360 g/litre", undiluted 33 strongly irritating NOTOX (1989a)
Agrichem Glyfosaat B, undiluted 58 strongly irritating NOTOX (1990c)
Agrichem Glyfosaat 2, undiluted 22 irritating NOTOX (1990a)
Legend, undiluted 21 irritating CIT (1991d)
Table 13 (continued)
a For glyphosate content of the tested formulations, see footnote a in Table 11.
b The irritation index represents a mean total score per animal for response in cornea, conjunctiva
and iris, using the standard Draize scoring system. The irritation index was calculated using the data
from the original report; in calculating the mean response per animal the maximum response observed
for an animal was used. The calculation procedure is as follows:
- score conjunctiva: A. chemosis: 0-4 Calculation partial
B. discharge: 0-3 index for conjunctiva:
C. erythema: 0-3 2 x (A + B + C) = 0-20
- score iris: 0-2 Calculation partial index for iris: 5 x (0-2) = 0-10
- score cornea: A. opacity: 0-4 Calculation partial index for cornea:
B. area: 0-4 5 x A x B = 0-80
The total irritation index is the sum of the partial indices (0-110).
c The classification is based on the following scheme: index 0-5 not irritating; 5.1-12 slightly
irritating; 12.1-30 irritating; 30.1-60 strongly irritating; 60.1-80 severely irritating; 80.1-110
extremely irritating.
of inflammation of the gastric squamous mucosa in the medium- and
high-dose groups (incidences in males: 2/58, 3/58, 5/59 and 7/59;
females: 0/59, 3/60, 9/60, and 6/59; historical range: 0-13.3%). The
incidence of pancreatic islet cell adenomas was increased
(statistically significant) among low- and high-dose males
(incidences: 1/58, 8/57, 5/60 and 7/59; historical control range of
test laboratory 1.8-8.5%). The incidence in the control group was
below the historical control range; the trend test for the observed
increase was negative. No pancreatic carcinomas were found. The
NOAEL in this study was 8000 mg/kg diet (410 mg/kg body weight per
day) (Monsanto, 1990b).
Glyphosate has been tested in the US National Toxicology
Program; pre-chronic studies have been completed (NTP, 1992).
7.4 Skin and eye irritation; sensitization
Many studies have been carried out with rabbits to examine the
potential of glyphosate and its formulations to produce skin and eye
irritation. The results of these studies are briefly summarized in
Tables 12 (skin irritation) and 13 (eye irritation). Glyphosate and
its formulations produce only mild skin irritation after undiluted
single application. The result of the 21-day dermal study in rabbits
with the formulation Roundup (Bio/Dynamics Inc., 1975; see
subsection 7.2.2) shows that repeated application of undiluted
formulation to the skin does lead to irritation. The eye-irritating
potential is considerable for undiluted glyphosate and, with a few
exceptions, also for the formulations. Single application generally
produces moderate to severe reactions.
Sensitization studies have been carried out in guinea-pigs with
glyphosate and its formulations. The results of these tests are
summarized in Table 14. Neither glyphosate itself nor the tested
formulations induced sensitization in any experiment.
The results of two dermal irritation studies and one dermal
sensitization study performed with human volunteers exposed to
Roundup are presented in subsection 8.1.
Table 14. Results of sensitization tests in guinea-pigs for glyphosate and its
formulations
Product testeda Method Result Reference
Glyphosate
Glyphosate Buehler negative Bio/Dynamics Inc.
(purity 99.7%) (1983c)
Glyphosate technical Magnusson & negative Safefarm Labs Inc.
Kligmanb (1991b)
Glyphosate techn. Magnusson & negative Inveresk Research
(96-99%) Kligman Int. (1989b)
Formulations
Roundup Buehler negative Bio/Dynamics Inc.
(1983b)
"Compound Magnusson & negative Inveresk Research
No. 3607" Kligman Int. (1988d)
Roundup L&G Buehler negative Bio/Dynamics Inc.
(1987e)
Legend Buehler negative Safefarm Labs Inc.
(1991a)
Sting Buehler negative Bio/Dynamics Inc.
(1986)
a For glyphosate content of the tested formulations, see footnote a
in Table 11.
b Method also called the maximization test.
7.5 Reproductive toxicity, embryotoxicity and teratogenicity
Technical glyphosate has been tested for teratogenicity in rats
and rabbits using the oral route. In addition, two oral
multi-generation reproduction studies in rats have been reported.
7.5.1 Teratogenicity studies
Glyphosate (technical) was given to pregnant Charles River COBS
CD rats by gavage at dose levels of 0, 300, 1000 and 3500 mg/kg body
weight per day on days 6-19 of gestation. At 3500 mg/kg the
following effects were observed: increased incidences of soft
stools, diarrhoea, breathing rattles, red nasal discharge and
reduced activity, increased mortality (6/25 dams dying before the
end of the treatment period), growth retardation, increased
incidence of early resorptions, decreases in total number of
implantations and the number of viable fetuses, increased number of
fetuses with reduced ossification of sternebrae. At the lower dose
levels these effects were absent. The NOAEL in this study was
1000 mg/kg body weight per day (IRDC, 1980b).
In Dutch belted rabbits technical glyphosate was tested at dose
levels of 0, 75, 175 and 350 mg/kg body weight per day
(administration by gavage) from days 6-27 of gestation. In dams the
incidences of diarrhoea and soft stools were increased in the
high-dose group and, to a slight degree, also in the medium-dose
group. The incidence of nasal discharge was increased in the
high-dose group only. In the medium- and high-dose groups 2 and 10
dams, respectively, died during the study from unknown causes. The
NOAEL in this study was 175 mg/kg body weight per day (IRDC, 1980c).
7.5.2 Reproduction studies
A three-generation feeding study in Charles River CD
(Sprague-Dawley) BR rats was conducted in 1980-1981 and a
two-generation study, using higher dose levels, was completed in
1990 with technical glyphosate. In the former study, dietary feeding
levels were continuously adjusted to achieve dose levels of 0, 3, 10
and 30 mg/kg body weight per day. The only effect noted was an
increased incidence of unilateral renal tubular dilation in the male
pups (randomly selected) of the F3b mating of the high-dose group
(incidence 6/10 versus 0/10 in controls, not determined in
intermediate groups, earlier litters not examined). The NOAEL in
this study was < 30 mg/kg body weight (Bio/Dynamics Inc., 1981b).
The more recent two-generation study had dose levels of 0, 2000,
10 000 and 30 000 mg technical glyphosate/kg diet (equivalent to 0,
100, 500 and 1500 mg/kg body weight per day). In the high-dose
group, the following effects were observed: soft stools and
decreased body weights in parent animals, slightly decreased litter
size and decreased pup weights (the latter seen at day 14 or 21 of
lactation). The decreased body weights of parents and pups were seen
to a slight degree in the medium-dose group also. No histological
effect on kidneys was present in the F2b male pups (15 and 23 pups
examined in control and high-dose groups, respectively; first
generation and F2a pups not examined). The NOAEL in this study was
10 000 mg/kg diet (500 mg/kg body weight per day) (Monsanto, 1990c).
With regard to these reproduction studies using technical
glyphosate, it should be noted that in both studies the number of
pups submitted to histopathological examination was limited. These
limitations make evaluation of the renal effect in pups, seen (at
30 mg/kg body weight) in the study of Bio/Dynamics Inc. (1981b),
difficult.
7.6 Mutagenicity and related end-points
The results of several studies are summarized in Table 15. The
results show that glyphosate is not mutagenic.
Table 15. Results of mutagenicity studies with glyphosate and its salts.
Test system Test compound and Resultc Reference
concentrations
Ames test, Salmonella technical glyphosate - Monsanto
typhimurium TA98, TA100, (98.4%); 0.1-1000 (1978c)
TA1535 & TA1537, with and µg/plate
without metabolic activation
Ames test, S. typhimurium technical glyphosate - IET (1978)
TA98, TA100, TA1535 & (98.4%); 10-5000
TA1537, with and without µg/plate
activation
Rec assay, Bacillus subtilis, technical glyphosate - IET (1978)
strain H17 (rec+) & M45 (98.4%); 20-2000
(rec-), without activation µg/disc
Reverse mutation assay in technical glyphosate - IET (1978)
Escherichia coli strain WP2hcr, (98.4%); 10-5000
with and without activation µg/plate
Forward mutation assay, technical glyphosate - Monsanto
CHO cells, in vitro (98.7%); 0-20 mg/ml (1983b)
(HGPRT-locus), with & (- activation) or
without activation 5-25 mg/ml (+ activation)
Table 15. (cont'd)
Test system Test compound and Resultc Reference
concentrations
Cytogenetic study technical glyphosate - Monsanto
(chromosome aberrations) (98.7%); 200-1000 mg/kg (1983f)
in rat bone marrow, body weight, i.p.b;
in vivo sampling after 6, 12
and 24 h
Micronucleus test in glyphosate (not - Benova
erythrocytes of mice, specified); ´ LD50, oral; et al.
in vivo sampling time not (1989)
specified
Dominant lethal test, mouse technical glyphosate - IRDC
in vivo (98.7%); 200-2000 mg/kg (1980a)
body weight, oral
Recessive sex-linked lethal glyphosate (not - Gopalan &
test, Drosophila melanogaster, specified); dose not Njagi
in vivo given (1981)
Unscheduled DNA repair assay technical glyphosate - Monsanto
rat hepatocytes, in vitro (98.7%); 0.0125-125 (1983c)
µg/ml
a No higher concentrations tested because these would result in
osmolalities much higher than physiological levels; these high
osmolalities can produce non-specific chromosomal aberrations or
sister chromatid exchange.
b In additional studies it was demonstrated that: (1) glyphosate
produced no effect on viability and mitotic index of bone marrow
cells of rats after i.p. doses of 200-1000 mg/kg body weight
(Monsanto, 1983g); and (2) after giving 14C-labelled glyphosate
i.p. significant concentrations of 14C reached the bone marrow
(peak levels reached after 0.5 h remaining virtually constant up to
10 h after dosing) (Monsanto, 1983h).
c - = negative result
8. EFFECTS ON HUMANS
Appraisal
The formulation Roundup containing glyphosate is acutely toxic
to humans when ingested intentionally or accidentally.
No controlled studies have been conducted, and therefore the
human NOAEL level cannot not be derived.
No data are available to show the impact on workers exposed
during the manufacture or formulation of glyphosate. No
compound-related effects were observed in a test group of five
applicators prior to and after exposure for one week.
The reported higher susceptibility of individuals older than
40 years to ingested Roundup intoxication is important and requires
further investigation.
8.1 Cases of intentional and accidental exposure
Many cases of acute intoxication with herbicides containing
glyphosate and surfactant (Roundup) have been reported; most of
these were suicide attempts. Talbot et al. (1991) reviewed 93 cases
of exposure to Roundup (Chinese names: lan-da, hao-ni-chun,
nian-nian-chun) in Taiwan. The classification of the severity of
acute poisoning with Roundup as given by these authors is presented
in Table 16. Severe effects occurred only in the cases of
intentional ingestion (80 of the 93 reported). Accidental exposures
led to only mild effects. The typical symptoms were erosion of the
gastrointestinal tract (66% of the self-poisonings), seen as sore
throat, dysphagia and gastrointestinal haemorrhage. Other organs
were affected less often (nonspecific leucocytosis 65%, lungs 23%,
liver 19%, cardiovascular system 18%, kidney 14% and CNS 12%). Death
(in 7/80 cases) occurred within hours after ingestion. The amount of
undiluted Roundup ingested (rough estimates) in the lethal cases
varied from 85 to 200 ml (corresponding to roughly 30 to 70 g
glyphosate acid); but much larger amounts (500 ml Roundup,
corresponding to 180 g glyphosate acid) were reported to have been
ingested by some patients with mild to moderate symptoms. Overall,
moderate symptoms were associated with estimated intakes of 20 to
500 ml, mild symptoms with 5 to 150 ml, no symptoms with 5 to 50 ml.
The authors pointed out that the patient's estimates of the amount
ingested, and the conversion ratio used in their paper may be
inaccurate (Talbot et al., 1991). Other reviews of cases of
intoxication with Roundup have reported similar findings (Sawada &
Nagai, 1987; Tominack et al., 1991). The data of Tominack et al.
(1991) suggested that people over 40 years of age who ingest amounts
greater than 150 ml Roundup are at greatest risk of a fatal outcome.
These authors also pointed out that the surfactant contained in
Roundup may be responsible for the clinical syndrome (as suggested
by Sawada & Nagai, 1987), but that the available evidence on this
point is, as yet, inconclusive.
Table 16. Classification of severity of acute poisoning with Roundupa
Classification Description
Asymptomatic no complaints and no abnormalities on physical or laboratory
examination.
Mild mainly gastrointestinal tract(GIT) symptoms (nausea, vomiting,
diarrhoea, abdominal pain, mouth and throat pain) that resolved
within 24 h. Vital signs were stable, and there was no renal,
pulmonary or cardiovascular involvement.
Moderate GIT symptoms lasting longer than 24 h, GIT haemorrhages, endoscopically
verified oesophagitis or gastritis, oral ulceration, hypotension
responsive to intravenous fluids, pulmonary dysfunction not
requiring intubation, acid-base disturbance, evidence of transient
hepatic or renal damage, or temporary oliguria.
Severe pulmonary dysfunction requiring intubation, renal failure requiring
dialysis, hypotension requiring treatment with pressor amines, cardiac
arrest, coma, repeated seizures, or death.
a From: Talbot et al. (1991)
Further clinical experiences with patients exposed to Roundup
either accidentally or through deliberate ingestion have been
reported by Temple & Smith (1992). Symptoms resulting from dermal
exposure incidental to the use of the product included periorbital
oedema and chemosis of the eye, cardiovascular effects (tachycardia
and elevated blood pressure), swelling and paraesthesia at the site
of dermal contact and prolonged skin irritation. Deliberate
ingestion resulted in more severe effects, including lethality from
apparent respiratory and cardiac arrest (Temple & Smith, 1992).
Two dermal irritation studies were carried out with volunteers.
Application of 0.9 ml of a 9:1 dilution of Roundup formulation in
water to the intact skin of the upper arm for 24 h produced no skin
changes (Shelanski, 1973). Maibach (1986) tested undiluted Roundup
(application of 0.1 ml to intact and abraded skin sites on the back
for 24 h) and found erythema in only 1/24 subjects (23/24 no
reaction) for the intact skin sites; for the abraded skin sites 4/24
subjects showed an equivocal reaction and 10/24 showed erythema
(10/24 no reaction). The same author reported very briefly the
absence of effect in a photoirritation study in humans using
undiluted Roundup as test compound (application to abraded skin of
upper arm for 24 h with irradiation with UVA light for 45 min)
(Maibach, 1986).
A sensitization study was performed in 204 human volunteers
with undiluted Roundup according to a modified Draize method. The
summary report (no detailed report available) stated that there was
no effect in any subject (Maibach, 1986). The same author reported
absence of photosensitization by Roundup in volunteers.
8.2 Occupational exposure
The results of several studies focused primarily on the
determination of the extent of exposure to glyphosate when the
compound is used as herbicide are presented in section 5.3. The
study of Jauhainen et al. (1991) included health examinations of a
test group of five workers prior to and after an exposure period of
1 week. These examinations included haematology, clinical chemistry,
ECG, pulmonary function tests, an interview for a health
questionnaire and a general clinical examination (including
recording of blood pressure, pulse rate and pressure craft of
hands). A control group consisted of five workers. No
compound-related effects were observed (Jauhainen et al., 1991). The
other studies described in section 5.3 did not include a health
evaluation of workers.
8.3 Subpopulations at special risk
The only information available on this point is some suggestive
evidence referring to oral intoxications with Roundup; Tominack
et al. (1991) suggested that people older than 40 years are at
greater-than-normal risk after ingestion of Roundup.
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND
FIELD
In this chapter, concentrations or doses of formulations with
glyphosate are always expressed as mg product per kg soil.
Therefore, these figures may have been recalculated from the
original data, in cases where the authors reported the data in, for
instance, mg a.i./litre instead of mg Roundup per litre. For
recalculation, the percentages of the active ingredient (free acid
or the salt), given in Table 2, were used.
9.1 Laboratory experiments
9.1.1 Microorganisms
9.1.1.1 Water
Appraisal
The prokaryotic cyanobacteria are generally more sensitive to
the effects of glyphosate than the eukaryotic true algae. Similar
enzyme systems are inhibited in microorganisms to those thought to
be responsible for the herbicidal properties of glyphosate in higher
plants. The single semi-field study suggests very variable results
but with no significant effect on populations or community
structure.
The acute and chronic toxicities of technical grade glyphosate
and its formulations to cyanobacteria, algae and diatoms are
summarized in Table 17. Technical grade glyphosate is slightly toxic
with 3- to 4-day EC50 values of 1.2-7.8 mg/litre, and 7-day NOEC
values of 0.3-34 mg/litre. Formulations of glyphosate may be more
toxic (3-day EC50 values of 1.0 to > 55 mg product/litre).
Toxicity to cyanobacteria and algae is dependent on the species
or strain tested. Wängberg & Blanck (1988) exposed 16 species in
pure cultures to Roundup for 14 days. The concentration at which
growth was inhibited completely was 16 mg Roundup/litre for the most
sensitive species (Raphidonema longiseta and Anabaena sp.) and
131 mg Roundup/litre for the least sensitive species (Selenastrum
capricornutum). The prokaryotic Cyanophyta were significantly more
affected by Roundup than the eukaryotic Chlorococcales.
In Pseudomonas chlororaphis Roundup severely inhibited
respiration at concentrations of > 2623 mg/litre, whereas in
Aeromonas hydrophila respiration was only slightly affected at
these concentrations (Chan & Leung, 1986). The bacteria were exposed
for 6 days.
Table 17. Aquatic microorganisms: acute and chronic toxicity of glyphosate and its formulations (EC50, NOEC)
Organism A Test Compound Test pH Hardness Temperature Experimental Parametera Concentration Reference
type water (mg CaCO3/ (°C) duration (mg/litre)
litre) (days)
Cyanobacteria
Anabaena A S Tgg a.m. 7.5 285 24 7 NOEC 9.7b,c Malcolm
flos-aquae Pirnie
Inc.
(1987a)
Green algae
Selenastrum - S Tgg a.m. 7.0 n.r. 26 3.5-4 EC50 7.8b,e Bozeman
capricornutum et al.
(1989)
S. capricornutum A S Tgg a.m. 7.5 285 24 7 NOEC 20b,c Malcolm
Pirnie
Inc.
(1987d)
S. capricornutum - S St a.m. 7.7-9.0 26 23 3 EC50 1.0b LISEC
(1989b)
S. capricornutum - S St a.m. 7.7-9.0 26 23 3 EC50 2.5d LISEC
(1989b)
S. capricornutum - S St a.m. 7.7-9.0 26 23 3 NOEC 0.2b LISEC
(1989b)
S. capricornutum - S Ru a.m. 7.7-10.0 26 24 3 EC50 2.1b LISEC
1989a)
S. capricornutum - S Ru a.m. 7.7-10.0 26 24 3 EC50 8.0d LISEC
(1989a)
S. capricornutum - S Ru a.m. 7.7-10.0 26 24 3 NOEC 0.7b LISEC
(1989a)
Table 17 (contd).
Organism A Test Compound Test pH Hardness Temperature Experimental Parametera Concentration Reference
type water (mg CaCO3/ (°C) duration (mg/litre)
litre) (days)
Chlorella - S Ru a.m. 7.5 n.r. 25 2.1-7 EC50 > 55b Hernando
pyrenoidosa et al.
(1989)
C. pyrenoidosa - S Ru a.m. 7.5 n.r. 25 2.1-7 NOEC < 55b Hernando
et al.
(1989)
Diatoms
Skeletonema - S Tgg a.m.h 8.2-8.5 n.r. 20 4 EC50 1.2g E G &
costatum Bionomics
(1978a)
S. costatum - S Tgg a.m.h 8.2-8.5 n.r. 20 4 EC50 1.3b E G &
Bionomics
(1978a)
S. costatum - S Tgg a.m.h 8.2-8.5 n.r. 20 4 NOEC < 0.6f E G &
Bionomics
(1978a)
S. costatum A S Tgg a.m.h 7.5 285 20 7 NOEC 0.3b,c Malcolm
Pirnie
Inc.
(1987b)
Navicula A S Tgg a.m. 7.5 285 20 7 NOEC 34b,c Malcolm
pelliculosa Pirnie
Inc.
(1987c)
Table 17 (continued)
a concentration of a formulation is expressed as mg of the formulation per litre
b based on biomass decrease
c based on actual concentrations
d based on inhibition of growth rate
e NOEC value not reported
f based on biomass and chlorophyll a decrease
g based on chlorophyll a decrease
h salinity was 30%
A = actual concentrations are measured; - = nominal concentrations; S = static system;
Tgg = technical grade glyphosate; Ru = Roundup;
St = Sting; a.m. = artificial medium; n.r. = not reported
Chan & Leung (1986) found that the activity of
5-enolpyruvyl-shikimic acid-3-phosphate synthase was inhibited
strongly in aquatic bacteria at the lowest tested concentration of
656 mg Roundup/litre. This enzyme takes part in the biosynthetic
sequence to phenylalanine, tryptophan, and tyrosine via the
conversion of shikimate to chorismate, and the conversion of
chorismate to anthralinate. In an in vitro experiment with
cell-free extracts of Aerobacter aerogenes, technical grade
glyphosate inhibited the conversion of shikimate to chorismate at
concentrations of > 0.2 mg a.i./litre (Amrhein et al., 1980).
In Chlorella pyrenoidosa Roundup affected the growth, the
greening process and the photosynthetic metabolism at the lowest
tested concentration of 55 mg/litre when exposed for 2.1 or 7 days
(Hernando et al., 1989). Synthesis of chlorophyll a and b and
carotenoids was then significantly inhibited. Roundup affected
synthesis of chlorophyll a to a greater extent than that of the
other pigments. In vivo and in vitro studies with isolated
chloroplasts showed inhibition of the photosystems PS I and PS II at
concentrations of > 55 mg Roundup/litre. Hernando et al. (1989)
suggested that glyphosate acted as an electron transport inhibitor
and that the stronger inhibition of PS II compared with PS I was due
to the surfactant polyoxyethyleneamine.
In periphytic algal communities that were collected in ponds of
boreal forests, Roundup decreased the carbon fixation rate by 50% at
concentrations of 243-479 mg/litre (Goldsborough & Brown, 1988). The
algae were exposed for 4 h. Austin et al. (1991) cultured periphyton
on glass plates suspended in artificial "stream-troughs" which were
supplied with flowing water pumped from natural streams in British
Columbia, Canada. The stream water was low in phosphorus and flowed
out of an oligotrophic lake. Glyphosate was added to give nominal
concentrations in the troughs of between 0.001 and 0.3 mg/litre. A
further series of treatments added nutrients to troughs. The
herbicide was not toxic to the periphyton. A transitory decrease in
growth was followed by a stimulation of biomass in the
glyphosate-treated troughs. Similar effects were seen with added
nutrient. The authors considered the effect to be the result of
algae using glyphosate as a phosphate source. Communities of
periphyton were similar in all treatments.
Various studies have indicated that glyphosate may affect
aromatic amino acid synthesis in microorganisms, in addition to the
greening process, respiration and photosynthesis (Amrhein et al.,
1980; Chan & Leung, 1986; Pipke & Amrhein, 1988).
9.1.1.2 Soil
Appraisal
Soil bacteria in culture have shown effects of glyphosate on
nitrogen fixation, denitrification and nitrification. However, field
studies after application of formulations have not shown significant
effects. Closely related species of bacteria have been shown to be
capable of degrading glyphosate (see chapter 4). The lack of
information on the bioavailability of glyphosate in soil makes it
difficult to relate the evaluation of effects in culture to the
actual exposure in the field.
Technical grade glyphosate inhibited the growth of bacteria
isolated from a sprayed garden soil of sandy loam to a lesser extent
than it did that of bacteria isolated from an unsprayed control
(Quinn et al., 1988). In a mineral salt medium the growth rate of
bacteria from the untreated site was reduced by 50% at 590 mg
a.i./litre at 50 h after application.
In various studies, glyphosate, applied at the recommended
rates, caused neither inhibition of processes that are part of the
nitrogen cycle nor inhibition of enzymes involved in microbial
activity.
Roundup did not affect dehydrogenase activity in sand and loamy
sand when applied at rates of 4.9 and 24 kg a.i./ha (NATEC, 1990).
In the same soils amended with lucerne (Medicago sativa), the same
application rates did not change significantly the amounts of
nitrate nitrogen, although in treated loamy sand there was a slight
increase. These experiments, with a duration of 28 days, indicated a
slight stimulation of nitrification in loamy sand. A stronger
stimulation of nitrification was found in silt loam, silty clay loam
and sandy loam up to 84 days after application of technical grade
glyphosate at rates of 5 and 25 mg a.i./kg (ABC Inc., 1978d). This
stimulation was not dose-related. In this experiment no
substance-related effects were found on nitrogen fixation and
nitrite formation.
Nitrogen fixation was not affected significantly at an
application rate of 13 mg a.i./kg dry weight whether the product
applied was technical grade glyphosate or Roundup, or whether
aerobic or anaerobic conditions were used (Carlisle & Trevors,
1986a). At concentrations > 127 mg a.i./kg dry weight, nitrogen
fixation was inhibited under anaerobic conditions, whereas this
could not be verified under aerobic conditions due to very low
acetylene reduction rates. In the same agricultural sandy loam,
denitrification under anaerobic conditions was stimulated at
concentrations of > 13 mg a.i./kg dry weight, whether technical
grade glyphosate or Roundup was applied. Denitrification was
stimulated more strongly, when, in addition to the test compound,
glucose was added. In the same soil, no substantial effects of
either test compound on nitrification were found at 77 mg a.i./kg
dry weight. Dose-related inhibition of nitrification was found at
concentrations > 230 mg a.i./kg dry weight for glyphosate and
Roundup with respect to nitrate and nitrite production,
respectively. For both substances the inhibition was transient in
these studies. The temperature used was not reported.
No substantial effects on nitrification or denitrification were
found in two agricultural soils from Southern Finland after
application of an unknown formulation at a rate of 2.6 kg a.i./ha
(Müller et al., 1981). A strong inhibition of nitrogenase activity
was possibly an indirect effect due to a changed C/N ratio after the
treatment. In this study lasting 28 days, pretreatment measurements
were used as a control.
Roundup did not inhibit nitrification in three agricultural
soils after treatment at a recommended application rate of 2.9 kg
a.i./ha in 25-day experiments (Stratton, 1990). In a sandy loam,
nitrification was stimulated after the application of 145 kg
a.i./ha, whereas nitrification rates were inhibited by 50% at rates
of 194-435 kg a.i./ha. Mineralization, expressed as the time-course
of mg N/g dry weight (N = ammonium or nitrate nitrogen), in two
agricultural sandy loam soils was stimulated significantly due to
the treatment with 100 mg a.i./kg dry weight during an experiment
lasting 70 days (Marsh et al., 1977).
Not only in agricultural soils but also in forest soils, the
effects of Roundup on the nitrogen cycle appear not to be
inhibitory. Preston & Trofymow (1989) found no significant effects
on nitrification or the immobilization of urea nitrogen in an
organic fir forest floor and its underlying mineral horizon due to
the application of 10 and 50 mg a.i./kg dry weight. This experiment
lasted for 40 days.
In two agricultural sandy loam soils exposed to 100 mg a.i./kg
dry weight for 210 days, transient slight effects on CO2 evolution
were observed by Marsh et al. (1977). These effects were both
stimulatory (first 20 days) and inhibitory (until approximately 130
days) in both the soil from a permanent grassland and the soil from
an arable field, but especially in the latter. Carlisle & Trevors
(1986b) observed a dose-related increase in both O2 consumption
and CO2 production in an agricultural sandy loam soil during a
test of 10 days, with both technical grade glyphosate and Roundup.
In general the increases were significant at concentrations greater
than or equal to 127 mg a.i./kg. At the lowest dose (13 mg a.i./kg
soil) no effects were observed except a significant increase in
oxygen consumption due to Roundup. Oxygen consumption at doses
greater than or equal to 127 mg a.i./kg was more strongly stimulated
by Roundup than by technical grade glyphosate, possibly due to the
isopropylamine or surfactant. Preston & Trofymow (1989) found no
significant effect on CO2 production in an organic fir forest
floor and its underlying mineral horizon after the application of 10
and 50 mg a.i./kg dry weight. This experiment lasted 40 days.
Aerobic H2 oxidation in an agricultural sandy loam was
significantly inhibited by both technical grade glyphosate and
Roundup, but only at the highest dose of 635 mg a.i./kg dry weight
(Carlisle & Trevors, 1986b). Inhibition was stronger under anaerobic
conditions, and was found at concentrations of > 127 mg a.i./kg
dry weight to be significant and dose related. Anaerobic inhibition
might have been due to increased H2 generation as a result of
stimulated fermentation.
No effects of glyphosate on the activities of 1,3-ß-glucanase
and urease in a silt loam were found after application of Roundup at
a rate of 12 mg a.i./kg dry weight (Lethbridge et al., 1981).
Preston & Trofymow (1989) found no significant effect on urea
hydrolysis in an organic fir forest floor and its underlying mineral
horizon amended with 200 mg urea nitrogen per kg dry weight, due to
treatment with Roundup at a rate of 50 mg a.i./kg dry weight.
The degradation of cellulose, starch, protein, or leaf litter
was not inhibited at concentrations of 5 and 25 mg a.i./kg soil in
three agricultural soils, with the exception of litter in silt loam
at the highest dose (ABC Inc., 1978e). In this experiment lasting 84
days, technical grade glyphosate was applied.
Bacterial growth of Rhizobium trifolii in sterile solutions
with Bergersen's broth was completely inhibited at solution
concentrations of 10 and 20 mg a.i./litre, applied as an unknown
formulation of glyphosate (Eberbach & Douglas, 1983). Only at 10 mg
a.i./litre did the bacterial growth recover within 4 days. At lower
concentrations no inhibition was found.
Mycelial growth of ectomycorrhizal fungi in pure cultures on
agar was inhibited by 50% or more at concentrations of > 1 mg
a.i./litre for Pisolithus tinctorius and of > 100 mg a.i./litre
for Cenococcum geophilum and Hebeloma longicaudum (Estok et al.,
1989). Ectomycorrhizal fungi commonly associated with pines (Pinus
sp) were significantly inhibited at concentrations of > 29 µg
Roundup/litre (Chakravarty & Chatarpaul, 1990a). The most sensitive
species were Cenococcum graniforme, Hebeloma crustuliniforme and
Laccaria laccata. Roundup increased the susceptibility of sandy
soil for Gaeumannomyces gramminis, a fungus causing "take-all
disease" in wheat crops (Mekwatanakarn & Sivasithamparam, 1987).
Application at a rate of 0.54 µg a.i./kg increased the survival and
pathogenicity of the fungus significantly after 140 days of
incubation at 25 °C. These authors concluded that Roundup affected
microbial antagonists of the fungus.
9.1.2 Aquatic organisms
9.1.2.1 Plants
Appraisal
There is conflicting information on the effects of sediment on
the phytotoxicity of glyphosate to aquatic plants; Lemna showed
reduced effects whilst Carthamus did not. Generally, glyphosate is
thought to be largely unavailable to plants when added to soil and
only effective as a herbicide when applied to foliage.
The chronic toxicity to aquatic macrophytes when exposed to
technical grade glyphosate or Roundup dissolved in water is
summarized in Tables 20 and 21. Glyphosate is slightly toxic with a
14-day NOEC value of 9 mg/litre. Roundup is also slightly toxic with
14-day NOEC values of 2.4-56 mg/litre. No data on acute toxicity for
plants were available.
When Roundup was sprayed at a rate of 0.8 kg a.i./ha, the
phytotoxicity to floating plants of the common duckweed (Lemna
minor) was dependent on the extent of washed-off deposit (Lockhart
et al., 1989). Phytotoxicity was highest when the sprayed deposits
were not washed off within 6 h after application. Suspensions of
50 mg/litre of inorganic bentonite clay reduced the phytotoxicity of
Roundup to common duckweed significantly (Hartman & Martin, 1984).
When exposed for 14 days, concentrations up to 24 mg Roundup/litre
had no effect on plant growth when sediment was added, whereas the
growth was reduced by 50% at 5 mg Roundup/litre when no sediment was
added.
Phytotoxicity of glyphosate to the safflower (Carthamus
tinctorius) was not significantly reduced when the test compound
was added to drainage water with suspended particles instead of
distilled water (Bowmer et al., 1986). In these bioassays the
inhibition of root elongation was measured when the plants were
exposed to concentrations in unfiltered water of approximately
0.1-3 mg a.i./litre. The maximum concentration of adsorbed
glyphosate was 2500 mg/kg.
Although it had inhibitory effects at high concentrations,
Roundup had a stimulatory effect on the growth of common duckweed
(Lemna minor) and tubers of sago pondweed (Potamogeton
pectinatus) at lower concentrations (Hartman & Martin, 1985;
Lockhart et al., 1989). Enhancement of growth of common duckweed and
sago pondweed was found at 7-56 and 3 mg Roundup/litre,
respectively. These stimulatory effects may refer to hormesis.
9.1.2.2 Invertebrates
Appraisal
The data from laboratory toxicity tests show that formulations
are often more toxic than technical glyphosate to aquatic
invertebrates. The surprising result that addition of clay particles
to Daphnia test systems increased the toxicity of glyphosate is
probably due to ingestion of herbicide bounds to the particles. Few
studies have been conducted in the presence of sediment; the
reported toxicity of glyphosate is, therefore, difficult to relate
to the field situation.
The acute and chronic toxicity of technical grade glyphosate
and its formulations to aquatic invertebrates are summarized in
Tables 18-21. Technical grade glyphosate is slightly to very
slightly toxic, with LC50 values of > 55 mg/litre and a 21-day
NOEC value of 100 mg/litre. Formulations of glyphosate are
moderately to very slightly toxic with 2-day EC50 values of
5.3-5600 mg product/litre and 21-day MATC values of 1.4-4.9 mg
product/litre. The higher toxicity of Roundup to crustaceans is
mainly due to the presence of surfactants (Servizi et al., 1987).
In a laboratory in vitro test with the gills of Mytilus
californianus, a marine water mollusc, the active uptake of glycine
was inhibited by 23 and 67%, respectively, when a mixture of
14C-labelled and unlabelled glyphosate was applied at rates of
0.2-1.7 mg/litre (Swinehart & Cheney, 1987). This inhibition might
be an indication for Mg2+-moderated binding of glycine to the gill
surface, whereas glyphosate can compete with glycine uptake by
forming a metal complex.
Water fleas (Daphnia magna) were less sensitive to Roundup,
when the water was aerated than when it was unaerated. The 2-day
LC50 value decreased from 37 (with aeration) to 24 mg (without
aeration) Roundup/litre (EG & G Bionomics, 1980f).
Addition of 50 mg/litre of inorganic bentonite clay decreased
the 2-day EC50 value for mature water fleas (Daphnia pulex) from
16 to 7 mg Roundup/litre (Hartman & Martin, 1984). This higher
toxicity might have been due to ingestion of particle-adsorbed
glyphosate. Addition of bentonite also increased the toxicity in a
chronic experiment with populations of Daphnia pulex. Immature
water fleas were more sensitive to glyphosate than the adults under
all conditions. Populations in all treatments had recovered within
14 days after the application (Hartman & Martin, 1984).
No avoidance of glyphosate was found when mayfly nymphs
(Ephemerella walkeri) were tested at concentrations of 0.2-2 mg
Roundup/litre, whereas avoidance was demonstrated at 24 mg/litre
(Folmar et al., 1979).
9.1.2.3 Vertebrates
Appraisal
The toxicity tests for fish are generally performed without
sediment. As the bioavailability of glyphosate itself will be
reduced under most conditions due to sorption onto sediment, no
toxic effects are expected. Toxic effects, however, can be expected
due to surfactants in some formulations. To a lesser extent,
life-stage, pH, water hardness, temperature, and the presence of
feed all influence toxicity. No adverse effects on the
osmoregulatory mechanism were found.
The acute and chronic toxicity of technical grade glyphosate
and its formulations to fish are summarized in Tables 18-21.
Technical grade glyphosate is moderately toxic with 4-day LC50
values of 10 to > 1000 mg/litre, a 21-day NOEC value of
52 mg/litre, and an MATC value of > 26 mg/litre. Formulations of
glyphosate have comparable toxicity with 4-day LC50 values of 2.4
to > 1000 mg product/litre, and 21-day NOEC values of 0.8-2.4 mg
product/litre. Toxicity may vary substantially, depending on the
species, the test compound and test conditions. In general,
technical grade glyphosate is less toxic than the formulations. This
difference is mainly due to a higher toxicity of surfactants in the
formulations (Folmar et al., 1979; Servizi et al., 1987; Mitchell
et al., 1987; Wan et al., 1989).
Table 18. Aquatic organisms: acute toxicity of technical grade glyphosate in a static test system
Organism A Test pH Hardness Temperature Experimental Parameter Concentration Reference
water (mg CaCO3/ (°C) duration (mg/litre)
litre) (days)
Molluscs
Crassostrea - n.w.a n.r. n.r. 25 2 EC50 > 10b Bionomics
virginica, eggs (1973a)
Echinodermata
Tripneustes - n.w.a 7.7-8.2 n.r. 20 4 EC50 > 1000c E G & Bionomics
esculentes (1978d)
Crustaceans
Daphnia magna, - w.w. 7.8-8.0 > 250 19 2 LC50 780 ABC Inc.
first instar (1978a)
Uca pugilator - a.m.a n.r. n.r. 21 4 LC50 934 Bionomics
(1973b)
Palaemonetes - a.m.a n.r. n.r. 21 4 LC50 281 Bionomics
vulgaris (1973b)
Mysidopsis bahia - n.w.a 6.4-8.3 n.r. 20 4 LC50 > 1000 E G &
Bionomics
(1978c)
Insects
Chironomus plumosus, - r.w. n.r. 40 22 2 EC50 55d Folmar et al.
fourth instar (1979)
Table 18 (contd).
Organism A Test pH Hardness Temperature Experimental Parameter Concentration Reference
water (mg CaCO3/ (°C) duration (mg/litre)
litre) (days)
Fish
Ictalurus - r.w. n.r. 40 22 4 LC50 130 Folmar et al.
punctatus (1979)
Salmo gairdnerii, - divers 6.3-8.2 5.3-148 n.r. 4 LC50 10-197 Wan et al.
0.4 cm, 0.5 g (1989)
Salmo gairdnerii, - r.w. 4.4-7.2 45 12 4 LC50 86 ABC Inc.
0.4 cm, 0.6 g (1978b)
Oncorhynchus keta, - divers 6.3-8.2 5.3-148 n.r. 4 LC50 10-148 Wan et al.
0.4 cm, 0.5 g (1989)
O. kisutch, A divers 6.3-8.2 5.3-148 n.r. 4 LC50 27-174 Wan et al.
0.4 cm, 0.5 g (1989)
O. tshawytsha, - divers 6.3-8.2 5.3-148 n.r. 4 LC50 19-211 Wan et al.
0.4 cm, 0.5 g (1989)
O. gorbusha, - divers 6.3-8.2 5.3-148 n.r. 4 LC50 14-190 Wan et al.
0.4 cm, 0.5 g (1989)
Lepomis - r.w. n.r. 40 22 4 LC50 140 Folmar et al.
macrochirus (1979)
L. macrochirus, - r.w. 6.6-7.0 45 21 4 LC50 120 ABC Inc.
0.3 cm, 1.0 g (1978c)
Pimephales - r.w. n.r. 40 22 4 LC50 97 Folmar et al.
promelas (1979)
Rasbora - r.w. n.r. 25 21 4 LC50 168 HRC (1977)
heteromorpha,
0.1-0.3 cm
Cyprinodon - n.w.e 7.6-8.3 n.r. 20 4 LC50 > 1000 E G & Bionomics
variegatus, (1978b)
0.7-1.0 cm
a salinity 20-35%
b based on abnormal development of oyster larvae
c based on immobility, drooping spines, and retracted podia
d based on immobilisation
e salinity 18%
A = actual concentrations are measured; - = nominal concentration; a.m. = artificial medium;
r.w. = reconstituted water; w.w. = well water; n.w. = natural surface water;
n.r. = not reported
Table 19. Aquatic organisms: acute toxicity of formulations with glyphosate
Organism A Test Compound Test pH Hardness Temperature Experimental Parameter Concentration Reference
type water (mg CaCO3/ (°C) duration (mg/litre)a
litre) (days)
Crustaceans
Daphnia - S Ru r.w. 8.2-8.3 175 22 2 EC50 5.3b E G & G
magna, first Bionomics
instar (1980e)
D. magna, - S Ru r.w. 7.7-8.1 175 22 2 EC50 24-37b E G & G
first instar Bionomics
(1980f)
D. magna, - S Ru r.w. n.r. 40 22 2 EC50 7.3b Folmar
first instar et al.
(1979)
D. magna, - S St w.w. 8.3-8.5 225-275 20 2 EC50 42b ABC Inc
first instar (1984c)
D. magna, - S RuD w.w. 7.9-8.6 255 20 2 EC50 930b ABC Inc
first instar (1981a)
D. pulex, - S Ru w.w. 7.6 282 15 2 EC50 19b Hartman &
mature Martin
(1984)
Gammarus A CF Ru w.w 7.9-8.3 255 17 2 EC50 42b,c ABC Inc
pseudolimnaeus (1982b)
Insects
Chironomus - S Rod r.w. 7.6-7.8 42-44 22 2 EC50 5600b Buhl &
riparius, Faerber
fourth instar (1989)
Table 19 (cont'd)(2)
Organism A Test Compound Test pH Hardness Temperature Experimental Parameter Concentration Reference
type water (mg CaCO3/ (°C) duration (mg/litre)a
litre) (days)
Chironomus - S Ru r.w. n.r. 40 22 2 EC50 44b Folmar
plumosus, et al.
fourth instar (1979)
Fish
Ictalurus - S Ru r.w. 6.3-7.2 24-40 22 4 LC50 52 E G & G
punctatus, Bionomics
0.7 cm, 3 g (1980a)
Ictalurus - S Ru r.w. n.r. 40 22 4 LC50 32 Folmar
punctatus et al.
(1979)
Salmo - S St r.w. 6.8-7.3 40-45 12 4 LC50 7.5 ABC Inc
gairdnerii, (1984a)
0.4 cm, 0.7 g
Salmo A CF Ru w.w. 8.0-8.2 255 12 4 LC50 8.2c ABC Inc
gairdnerii, (1982c)
0.5 cm, 2.4 g
Salmo - S Ru divers 6.3-8.2 5.3-148 n.r. 4 LC50 14-33 Wan et al.
gairdnerii, (1989)
0.4 cm, 0.5 g
Salmo - S Ru r.w. 6.6-7.6 40 12 4 LC50 36 E G & G
gairdnerii, Bionomics
0.3 cm, 0.3 g (1980c)
Salmo - S Ru w.w 6.4-7.3 26-26 12 4 LC50 22 E G & G
gairdnerii, Bionomics
0.4 cm, 0.7 g (1980g)
Table 19 (cont'd)(3)
Organism A Test Compound Test pH Hardness Temperature Experimental Parameter Concentration Reference
type water (mg CaCO3/ (°C) duration (mg/litre)a
litre) (days)
S. gairdnerii, A S Ru r.w 7.4-7.7 85 11 4 LC50 22 EVS
0.4 g Consultants
(1986a)
S. gairdnerii, A S Ru n.w 7.4-7.8 81 11 4 LC50 15 EVS
0.4 g Consultants
(1986a)
S. gairdnerii, A S Ru d.w 5.4-6.3 4.5 11 4 LC50 26 EVS
0.4 g Consultants
(1986a)
S. gairdnerii, - S Ru r.w approx. 40 12 4 LC50 3.2 Folmar et al.
1.0 g 7.2 (1979)
Salmo - S RuD w.w 4.6-7.1 45 12 4 LC50 > 1000 ABC Inc
gairdnerii, (1981c)
0.3 cm, 0.2 g
S. gairdnerii, - S Vis n.r. 6.0 9.6 12.3 4 LC50 34 Morgan &
9.5 cm Kiceniuk
(1992)
Lepomis - S St r.w. 6.8-7.5 40-45 22 4 LC50 4.5 ABC Inc
macrochirus, (1984b)
0.2 cm, 0.1 g
Lepomis - S RuD w.w. 4.9-7.1 45 22 4 LC50 > 1000 ABC Inc
macrochirus, (1981b)
0.2 cm, 0.1 g
Lepomis A CF Ru w.w. 8.0-8.2 255 22 4 LC50 5.8c ABC Inc
macrochirus, (1982a)
0.2 cm, 0.2 g
Table 19 (cont'd)(4)
Organism A Test Compound Test pH Hardness Temperature Experimental Parameter Concentration Reference
type water (mg CaCO3/ (°C) duration (mg/litre)a
litre) (days)
Lepomis - S Ru r.w. 6.4-7.5 40 22 4 LC50 46 E G & G
macrochirus, Bionomics
0.4 cm, 0.3 g (1980b)
Pimephales - S Ru w.w. 6.7-7.7 39-44 22 4 LC50 31 E G & G
promelas, Bionomics
0.4 cm, 0.6 g (1980d)
Pimephales - S Ru r.w. n.r. 40 22 4 LC50 5.6 Folmar
promelas et al.
(1979)
Cyprinus A S St w.w. 7.2-7.9 40-48 22-23 4 LC50 2.4 ABC Inc
carpio, (1990)
0.6 cm, 2.8 g
Oncorhynchus A S Ru divers 6.3-8.2 5.3-148 n.r. 4 LC50 13-33 Wan et al.
kisutch, (1989)
0.4 cm, 0.5 g
O. kisutch, A S Ru d.w. 5.5-6.4 4.5 11 4 LC50 22 EVS
11.8 g Consultants
(1986c)
O. keta, - S Ru divers 6.3-8.2 5.3-148 n.r. 4 LC50 11-20 Wan et al.
0.4 cm, 0.5 g (1989)
O. tshawytsha, - S Ru divers 6.3-8.2 5.3-148 n.r. 4 LC50 17-33 Wan et al.
0.4 cm, 0.5 g (1989)
O. tshawytsha, A S Ru d.w. 5.8-6.7 4.5 11 4 LC50 20 EVS
4.6 g Consultants
(1986b)
Table 19 (cont'd)(5)
Organism A Test Compound Test pH Hardness Temperature Experimental Parameter Concentration Reference
type water (mg CaCO3/ (°C) duration (mg/litre)a
litre) (days)
O. gorbusha, - S Ru divers 6.3-8.2 5.3-148 n.r. 4 LC50 14-33 Wan et al.
0.4 cm, 0.5 g (1989)
Oncorhynchus A S Ru n.w 7.7-8.0 84 4-5 4 LC50 27-29 Servizi
nerka, et al.
3-6.5 cm, 0.2-3.8 g (1987)
a All concentrations are expressed as mg of the formulation per litre
b Based on immobilisation
c Based on actual concentrations
A = actual concentrations are measured; - = nominal concentrations; CF = continous flow system;
S = static system; Tgg = technical grade glyphosate; Ru = Roundup; Rod = Rodeo; St = Sting;
RuD = Roundup D-Pak; d.w. = dechlorinated tap water; r.w. = reconstituted water;
w.w. = well water; n.w. = natural surface water; a.m. = artificial medium
Table 20. Aquatic organisms: chronic toxicity of glyphosate (NOEC/MATC)
Organism A Test Test Test pH Hardness Temperature Experimental Parameter Concentration Reference
type substance water (mg CaCO3/ (°C) duration (mg/litre)
litre) (days)
Macrophytes
Lemna gibba A S Tgg a.m. 7.5 285 25 14 NOEC 9a,b Malcolm
Pirnie
Inc.
(1987e)
Crustaceans
Daphnia magna, A SS Tgg w.w.c 6.8-8.2 160-180 20 21 NOEC 100a,d ABC Inc.
first instar (1989c)
Fish
Salmo A CF Tgg w.w. 5.9-7.8 40-48 14-15 21 NOEC 52a,e ABC Inc.
gairdnerii, (1989e)
0.5 cm, 1.3 g
Pimephales A CF Tgg w.w. 6.5-7.6 32-42 25 255 MATC > 26f E G & G
promelas, Bionomics
1.5 g (1975)
a Based on actual concentrations d Based on survival and reproduction
b Based on biomass decrease e Based on survival, behaviour, and coloration
c Mixed with natural surface water f Based on survival, growth and reproduction
A = actual concentrations are measured; CF = continous flow system; SS = semi-static system;
S = static system; Tgg = technical grade glyphosate; w.w. = well water; a.m. = artificial medium
Table 21. Aquatic organisms: chronic toxicity of formulations with glyphosate
Organism A Test Test Test pH Hardness Temperature Experimental Parameter Concentration Reference
type substance water (mg CaCO3/ (°C) duration (mg/litre)f
litre) (days)
Macrophytes
Lemna minor - S Ru a.m. n.r. n.r. 25 14 NOEC 56c,d Lockhart
et al.
(1989)
Lemna minor - S Ru a.m. n.r. n.r. 22 14 NOEC 2.4c,e Hartman &
Martin
(1984)
Potamogeton - S Ru a.m. n.r. n.r. 22 14 NOEC 33c,d Hartman &
pectinatus Martin
(tubers) (1985)
Crustaceans
Daphnia A SS St w.w.b 7.2-8.2 174 20 21 MATC 1.4g,i ABC Inc.
magna, (1989a)
first instar
Daphnia magna, A SS Ru w.w.b 7.6-8.3 160-180 20 21 MATC 4.9g,j ABC Inc.
first instar (1989b)
Fish
Salmo A CF St w.w. 7.3-7.8 40-48 14-16 21 NOEC 0.8a,h ABC Inc.
gairdnerii, (1989h)
0.5 cm,
1.3 g
Table 21. (cont'd)
Organism A Test Test Test pH Hardness Temperature Experimental Parameter Concentration Reference
type substance water (mg CaCO3/ (°C) duration (mg/litre)f
litre) (days)
Salmo A CF Ru w.w. 7.1-7.8 24-48 14-16 21 NOEC 2.4a,h ABC Inc.
gairdnerii, (1989d)
0.5 cm,
1.8 g
Salmo A CF Vis n.r. 6.0 9.6 12.3 approx. 60 NOEC > 0.04a,k Morgan &
gairdnerii, Kiceniuk
9.5 cm, 7.2 g (1992)
a Based on actual concentrations g Based on survival, reproduction and length of time to the first brood
b Mixed with natural surface water h Based on survival, behaviour and coloration
c Roundup dissolved in test water i NOEC is 1.0 mg Sting/litre
d Based on biomass decrease j NOEC is 3.2 mg Roundup/litre (actual concentration)
e Based on reduction of frond formation k Based on mortality and growth
f All concentrations are expressed as mg of the formulation per litre
A = actual concentrations are measured; - = nominal concentrations; CF = continous flow system;
SS = semi-static system; Ru = Roundup; St = Sting; Vis = Vision; w.w. = well water;
a.m. = artificial medium; n.r. = not reported.
In laboratory experiments the major factors influencing the
toxicity appear to be the tested species and its age, the presence
of surfactants, the hardness, pH, temperature and the availability
of ration. Wan et al. (1989) found that the toxicity of technical
grade glyphosate to salmonids increased when hardness and pH
decreased, whereas for Roundup and Accord CR the contrary was true,
due to the presence of a 75% tallow amine surfactant in the
formulations. This surfactant was most toxic in hard water with a
relatively high pH. A higher toxicity of Roundup to salmonids at
increasing hardness and pH was confirmed by Mitchell et al. (1987),
but only partially by Servizi et al. (1987). It was also confirmed
by Folmar et al. (1979), although they only investigated the effect
of pH in reconstituted water with a moderate hardness. These authors
demonstrated that the effects of a pH increase on the toxicity of
Roundup and technical grade glyphosate were not only seen in rainbow
trout (Salmo gairdnerii) but also in bluegill sunfish (Lepomis
macrochirus). For both species Roundup became more toxic as the pH
increased from 6.5 to 7.5, whereas technical grade glyphosate became
less toxic as the pH increased from 6.5 to 9.5. At pH values higher
than 7.5, the toxicity of Roundup remained constant. In the
investigations of Servizi et al. (1987), an antagonistic effect of
glyphosate on the toxic action of a surfactant was found.
Increased toxicity of formulations due to surfactants was not
only demonstrated for the tallow amine surfactant in Roundup but
also for ortho X-77 in Rodeo (Mitchell et al., 1987). However, even
in the presence of a surfactant, the acute toxicity of some
formulations may be very slight, as was found by ABC Inc. (1980a,b).
In these studies, 0.5% (v/v) of the surfactant X-77 was added to
Roundup D-Pak, resulting in a 4-day LC50 value of this mixture for
rainbow trout (Salmo gairdnerii) and for bluegill sunfish
(Lepomis macrochirus) of 240 and 830 mg/litre, respectively.
Folmar et al. (1979) performed acute toxicity tests with
various species in reconstituted water with a pH of 7.2 and a
hardness of 40 mg CaCO3/litre. In these tests it was demonstrated
for rainbow trout (Salmo gairdnerii) and channel catfish
(Ictalurus punctatus) that the sensitivity to Roundup increased in
the following order: eyed eggs, 2-g fingerlings, sac fry, swim-up
fry, and 1-g fingerlings. The 4-day LC50 values for the various
life-stages of rainbow trout decreased from 39 mg Roundup/litre for
eyed eggs to 3.2 mg/litre for small fingerlings. In an additional
test, a 4-h exposure to concentrations of > 12 mg Roundup/litre
affected the survival of sac fry and swim-up fry significantly.
Holdway & Dixon (1988) also demonstrated that toxicity is
dependent on the life-stage by applying technical grade glyphosate
to larvae of flagfish (Jordanella floridae). At concentrations up
to 30 mg a.i./litre, no 2- or 4-day-old larvae died, whereas 20% of
the 8-day-old larvae died at the top dose. The effect was even more
drastic when the larvae were not fed. This treatment killed 20% of
the oldest larvae even at 3 mg a.i./litre. According to the authors,
the effect of age might fit the idea of saltatory ontogeny, implying
critical periods for organs and tissues.
While investigating the effect of temperature on toxicity,
Folmar et al. (1979) demonstrated for rainbow trout (Salmo
gairdnerii) and bluegill sunfish (Lepomis macrochirus) that
toxicity increased with increasing temperatures. For the trout the
4-day LC50 values decreased from 34 mg Roundup/litre at 7 °C to
18 mg/litre at 17 °C. For the bluegill sunfish the 4-day LC50
values decreased from 18 mg/litre at 17 °C to 9.8 mg/litre at 27 °C.
Rainbow trout (Salmo gairdnerii) showed the same sensitivity
to Roundup, independent of whether the water was aerated or not
(EG & G Bionomics, 1980g). In this experiment the 4-day LC50 under
both conditions was 22 mg Roundup/litre.
The potential of coho salmon smolt (Oncorhynchus kisutch) to
adapt to changes in water salinity encountered during migration was
not influenced by the application of Roundup at actual
concentrations up to 2.8 mg/litre (EVS Consultants, 1986d). The
osmoregulatory mechanism, which is fully functional in smolts, was
unaffected as indicated by plasma Na+ concentrations, haematocrit
values and the condition of the fish. During the experiment in which
the fish were exposed for 10 days in fresh water and subsequently
allowed to recover in fresh or sea water, no abnormal behaviour was
observed.
No avoidance of glyphosate was found when rainbow trout were
tested at concentrations up to 24 mg Roundup/litre (Folmar et al.,
1979). Sublethal concentrations in acute toxicity tests with Roundup
may cause loss of motility, complete loss of equilibrium, darkened
pigmentation, or rapid respiration (EG & G Bionomics, 1980a,b,g; EVS
Consultants, 1986a,b,c).
Rainbow trout (Salmo gairdnerii) were exposed to glyphosate
(as Vision) at 0, 6.25, 25 and 100 µg Vision/litre in a continuous
flow system. There were no effects on growth or foraging behaviour,
and no histopathological liver effects. Two out of three types of
aggressive behaviour were also unaffected; the third, a warning
"wig-wag" increased in frequency at the top-dose 1 month after
treatment. After two months the frequency was equal to that of the
control (Morgan & Kiceniuk, 1992).
9.1.3 Terrestrial organisms
9.1.3.1 Plants
Nodulation of sub-clover (Trifolium subterraneum) was
inhibited by an unknown formulation with glyphosate in a
dose-related way at concentrations of 2-20 mg a.i./litre (Eberbach &
Douglas, 1989). In this experiment, 3-day-old seedlings were
inoculated with Rhizobium trifolii. The seedlings were cultured
for 56 days in soil-free systems with nutrient solutions. In an
additional experiment in which Rhizobium was exposed to the same
concentrations, repeated washing of the inoculi prior to nodule
initiation did not reduce the inhibition of the nodulation of the
sub-clover after inoculation. This indicated that the effect on
nodulation might be the result of damage to the bacteria rather than
to carry-over of glyphosate from the bacteria to the plant.
Seed germination of various forest species was not affected by
treatment with Roundup at concentrations up to 305 mg a.i. (free
acid)/kg dry weight. Seed germination was affected at the highest
tested dose of 1525 mg a.i. (free acid)/kg dry weight (Morash &
Freedman, 1988). The effect on seed germination was confirmed by
another experiment in which no differences were found between
sprayed and unsprayed plots with respect to seedling composition and
quantity. Morash & Freedman (1988) then incubated the soils of
clear-cuts in a greenhouse. The application of Roundup in the field
was at a rate of 2.3 kg a.i./ha.
Red pine seedlings (Pinus resinosa) were not affected by
treatment with Roundup, with the exception of a dose-related
decrease in root length (Chakravarty & Chatarpaul, 1990b).
Non-affected growth parameters were shoot height, shoot weight, root
weight, and mycorrhizal development. In this experiment lasting 60
days, Roundup was applied at rates of 0.54 and 3.2 kg a.i./ha. The
conifer seedlings were inoculated with an ectomycorrhizal fungus
(Paxillus involutus), 14 days after germination.
Glyphosate may affect various pathways of the secondary
metabolism in the plant, although the actual targets in plants have
not been located. The synthesis of aromatic amino acids, secondary
hydroxyphenolic compounds, chlorophyll and delta-amino-levulinic
acid were reported to be affected by glyphosate (Amrhein et al.,
1980; Duke & Hoagland, 1981; Kitchen et al., 1981a,b). Aromatic
amino acids are important for the synthesis of, for instance, some
alkaloids, the phytohormone indole-3-acetic acid and phenolic
compounds such as lignin and quinones.
With respect to the synthesis of aromatic amino acids, there
are indications that the actual target is not shikimate kinase or
anthranilate synthase, but probably
5-enolpyruvylshikimate-3-phosphate synthase or chorismate synthase
(Amrhein et al., 1980). When applied to hypocotyls of buckwheat
(Fagopyrum esculentum) and to cultured cells of smooth bedstraw
(Galium mollugo), technical grade glyphosate inhibited the
conversion of shikimate to chorismate. This inhibition in vivo and
in vitro was found at concentrations of > 10 mg a.i./litre. In
the cultured cells of Galium, the accumulation of shikimate was
concomitant with a decrease of anthraquinones (Amrhein et al.,
1980). Possibly the non-transportable carbon pool, to which carbon
was found to be diverted in sugar beets (Beta vulgaris) due to
glyphosate, was in fact shikimate (Gougler & Geiger, 1984). These
effects on carbon metabolism were found at application rates
equivalent to 5 kg a.i./ha leaves. Duke & Hoagland (1981) assumed
that possibly chelation of divalent ions such as Ca2+ and Mg2+
that are involved in many metabolic pathways was the main cause of
damage.
9.1.3.2 Invertebrates
Appraisal
Glyphosate has low toxicity for bees and earthworms.
Oral 2-day LD50 values of technical grade glyphosate and
Roundup for bees were 100 µg a.i./bee and > 100 µg Roundup/bee,
respectively. Contact 2-day LD50 values for these two substances
were likewise > 100 µg a.i./bee and > 100 µg Roundup/bee (HRC,
1972). The oral 2-day LD50 of Sting was also > 100 µg Sting/bee
(Altmann, 1984). In these experiments Apis mellifera was tested. The
LD50 values indicate a slightly acute toxicity of technical grade
glyphosate, Roundup, and Sting to honey-bees. Roundup was also
slightly toxic to green lacewings (Chrysoperla carnea) when they
were exposed by contact to 1 kg Roundup/ha (SFRSA, 1990). In this
experiment the average number of eggs per female and the larval and
pupal mortality were increased due to the treatment, resulting in an
overall reduction in beneficial capacity of 41%. The beneficial
capacity is a function of the larval and pupal mortality, and the
average number of eggs per treated and untreated female. No effects
on the food uptake and mortality of the beetle Poecilus cupreus
Bonelli were observed 15 days after application of 6 kg Sting/ha
(IFU, 1990).
When exposed to artificial soil contaminated with Roundup
D-pak, earthworms (Eisenia fetida) were soft and/or slack, in a
dose-related way, at concentrations > 500 mg Roundup D-pak/kg dry
weight (IBR, 1991a). No other adverse effects were found, indicating
a 14-day NOEC of 158 mg/kg. A comparable experiment with Roundup
also indicated slight toxicity for earthworms with a 14-day NOEC of
500 mg Roundup/kg dry weight (IBR, 1991b). At higher concentrations
thin, slack and lethargic worms with a dark skin were observed.
9.1.3.3 Vertebrates
Appraisal
Glyphosate has low toxicity to birds after acute oral or
short-term dietary exposure. Mammals tested showed effects (body
weight loss) only after high levels of dosing. Herbicide-treated
foliage was not avoided by deer in the single study reported.
The acute, subacute and chronic toxicity of glyphosate and its
formulations to birds is summarized in Table 22.
Male marsupials (Sminthopsis macroura) showed significant
body weight loss after exposure to feed contaminated with
concentrations of up to 5000 mg a.i/kg feed (Evans & Batty, 1986).
No other treatment-related effects were found in the male
marsupials. In female hopping-mice (Notomys alexis and Notomys
mitchelli) fed similar doses, no treatment-related effects were
found.
Glyphosate did not affect the daily chow consumption of
black-tailed deer (Odocoileus hemionus columbianus) when these
herbivores were exposed to browse treated with glyphosate at a rate
of 2.2 kg/ha (Sullivan & Sullivan, 1979). The deer did not avoid the
contaminated browse, and sometimes even preferred it. Irrespective
of whether they were given treated alfalfa (Medicago sativa),
treated alder (Alnus rubra) or untreated feed, the deer had the
same daily chow consumption.
9.2 Field observations
9.2.1 Microorganisms
Appraisal
Some effects on microorganisms have been reported in field
studies following application of glyphosate formulations. However,
these have been minor and reversible in most cases. It is not
possible to separate the direct toxic effects of the herbicide from
changes in the habitat caused by its intended herbicidal action.
Table 22. Birds: acute and chronic toxicity of glyphosate and its formulations
Species Compound Sex Age Route Experimental Parameter Concentration Reference
duration
(days)
Colinus virginianus Tgg n.r. n.r. diet 8 LC50 > 4640 mg/kg feed Hazleton Lab. Inc.
(1973a)
Colinus virginianus Tgg n.r. 14 days oral LD50 > 3851 mg/kg b.w. Wildlife Int Ltd.
(1978c)
Colinus virginianus Tgg M,F 5 months diet 119 NOEC > 1000 mg/kg feeda Wildlife Int Ltd.
(1978b)
Colinus virginianus Ru n.r. 10 days diet 8 LC50 > 5620 mg/kg feedb Wildlife Int Ltd.
(1990b)
Anas platyrhynchos Tgg n.r. n.r. diet 8 LC50 > 4640 mg/kg feed Hazleton Lab. Inc.
(1973b)
Anas platyrhynchos Tgg M,F 6 months diet 112 NOEC > 1000 mg/kg feeda Wildlife Int Ltd.
(1978a)
Anas platyrhynchos Ru n.r. 10 days diet 8 LC50 > 5620 mg/kg feedb Wildlife Int Ltd.
(1990a)
Poephilla guttata Ru M mature diet 7 LC50 < 16 393 mg/kg feedb Evans & Batty (1986)
Poephilla guttata Ru M mature diet 5 LC50 > 8197 mg/kg feedb Evans & Batty (1986)
a Based on reproduction impairment of one generation
b Concentrations expressed as mg of the formulation per kg body weight or feed
Tgg = technical grade glyphosate; Ru = Roundup; M = males; F = females; n.r. = not reported.
9.2.1.1 Water
In a pool located in Hong Kong, treatment with 656 mg
Roundup/litre caused a substantial decrease in bacteria within 14
days after treatment. The number of colony-forming units had
returned to the control level 30 days after treatment (Chan & Leung,
1986).
Diatom populations in the water and sediments of a pond and a
stream showed a significantly different density of some species when
aerially treated with Roundup at a rate of 2.2 kg a.i./ha (Sullivan
et al., 1981). The authors concluded that these differences were
probably due to seasonal and habitat variation, rather than to
treatment with the herbicide.
Roundup may have affected the increase in ash-free dry weight
and the chlorophyll a standing crop of periphyton in the first month
after spraying with Roundup at a rate of 2.2 kg a.i./ha (Holtby &
Baillie, 1989a). In this experiment lasting around 130 days, some
tributaries in a watershed in British Columbia (Canada) were
directly oversprayed.
9.2.1.2 Soil
When Roundup was applied to a sandy loam being prepared for
conifer forestation, no significant changes in the soil respiration
were found up to about 180 days after application of Roundup at a
rate of 0.54 kg a.i./ha (Chakravarty & Chatarpaul, 1990a).
Concomitantly the numbers of fungi and bacteria decreased
significantly during the first 2 months, but after about 180 days
the numbers had recovered. These results might indicate changes in
microbial populations due to application of Roundup at recommended
application rates.
Preston & Trofymow (1989) found no significant effects on the
number of bacteria, actinomycetes, and nitrogen fixers in
ferro-humic podsols covered with alder trees (Alnus rubra) in the
Carnation Creek watershed, Canada, due to treatment with Roundup.
The only consistent effect was a significant reduction of the number
of fungi at one of the two treated sites. In this site the fungi
appeared to have recovered at the end of the study. In this
experiment of about 180 days, Roundup was hand-sprayed at a rate of
2 kg a.i./ha. In an additional comparable intensive field trial of
one month, microflora populations appeared to have recovered from
treatment after one month, with the exception of the reduction of
fungi in the litter of one of the treated sites. Actinomycetes and
nitrogen fixers in the litter appeared to be reduced in numbers due
to the treatment but they subsequently recovered. This reduction was
not found in the underlying humus layer.
Stratton & Stewart (1992) studied microbial activity in forest
soil and litter following the application of Roundup at 4.7
litres/ha (equivalent to 1.7 kg a.i./ha) to a coniferous forest
previously clear-cut and replanted. Treated and untreated (covered
with plastic sheeting during application) areas of forest were used
to obtain soil and litter samples which were tested in situ over an
8 months period following spraying. Glyphosate had a generally
stimulatory effect on microbial biomass in litter (up to 80%
increase) but no significant effect in soil. There were no
significant effects on the numbers of bacteria, fungi or
actinomycetes in either soil or litter. Glyphosate generally
stimulated respiration in both soil and litter; the degree of
stimulation was very variable throughout the sampling period,
ranging from a few percent to 100% increases in CO2 evolution.
No substance- or dose-related effects on aerobic bacteria were
observed in a sandy soil in a semi-arid region of Argentina up to 96
days after the application of Roundup (Gómez & Sagardoy, 1985).
Doses of up to 2.8 kg a.i. (free acid)/ha were applied.
No substance-related effects on the growth of an
ectomycorrhizal fungus were found when Roundup was applied at
concentrations of up to 3.2 kg a.i./ha (Chakravarty & Chatarpaul,
1990b). In these experiments red pines (Pinus resinosa) were
inoculated with the fungus Paxillus involutus. In the field 74-86%
of the seedlings that were not inoculated were colonized by
indigenous mycorrhizal fungi within two months.
9.2.2 Aquatic organisms
Appraisal
Little effect has been reported on aquatic invertebrates or
fish exposed to glyphosate formulations sprayed in the field. Minor
mortality in a single study of young trout may reflect the greater
sensitivity of early life-stages.
9.2.2.1 Plants
No field data on toxicity to aquatic macrophytes are available.
9.2.2.2 Invertebrates
An increased drift of midge larvae (Chironomus plumosus) was
found in artificial outdoor streams treated with 4.9 mg
Roundup/litre (Folmar et al., 1979). No increased drift was found at
0.5 mg Roundup/litre. In streams in a coastal rainforest in British
Columbia, Canada, only the drift densities of an amphipod (Gammarus
sp) and mayflies (Paraleptophlebia sp) increased after treatment
with Roundup at a rate of 2 kg a.i./ha (Kreutzweiser et al., 1989).
Density peaks partly coincided with periods immediately following
rainfall, which might indicate an effect due to glyphosate run-off,
or an effect due to increased discharges.
During most streamflows, the abundance of benthic
macro-invertebrates in a stream and at sites in tributary swamps was
similar at untreated sites and at sites that had been treated with
Roundup at a rate of 2.2 kg a.i./ha (Scrivener & Carruthers, 1989).
However, after periods of frequent rainstorms leading to flooding,
the abundance at the treated sites was 40-50% lower.
Water-fleas (Daphnia magna) did not show any mortality in a
pond sprayed with Roundup at application rates of up to 220 kg
a.i./ha (Hildebrand et al., 1980). In this experiment the
water-fleas were exposed for 8 days in pens that were partly
immersed in the water.
9.2.2.3 Vertebrates
Fingerlings (2.1 g, 5.8 cm) of rainbow trout (Salmo
gairdnerii) that were exposed for 14 days to Roundup in
flow-through pens in shallow streams did not show any mortality or
substance-related effects at application rates of up to 220 kg a.i.
(free acid)/ha (Hildebrand et al., 1982). In this experiment Roundup
was sprayed manually on moderately flowing forest streams in British
Columbia, Canada. In the same area an aerial application of 2.2 kg
a.i. (free acid)/ha did not cause mortality or obvious signs of
stress in rainbow trout fingerlings in flow-through pens, which were
also exposed to Roundup for 14 days (Hildebrand et al., 1982). A
direct aerial application of 2.1 kg a.i./ha on a tributary of the
Carnation Creek watershed (British Columbia, Canada), however,
killed 2.6% of the 120 fingerlings of coho salmon (Oncorhynchus
kisutch) within 24 h after application, whereas no mortality
occurred at the unexposed sites (Holtby & Baillie, 1989b). Also some
stress of the caged fingerlings was indicated within the first 2 h
after application. Up to 2 years after application no consistent
effects of Roundup on over-winter mortality, probability of entering
and leaving the tributary, timing of spring emigration, and growth
rates were found.
In artificial outdoor streams in Colorado, USA, rainbow trout
(Salmo gairdnerii) were exposed to concentrations of up to 5 mg
Roundup/litre for 12 h. No adverse effects on fecundity and
gonadosomatic indices were found in this study by Folmar et al.
(1979).
Rainbow trout (Salmo gairdnerii) in pens that were immersed
in stream water avoided Roundup at concentrations of > 40 mg
Roundup/litre (Hildebrand et al., 1982).
9.2.3 Terrestrial organisms
Appraisal
Spray drift of herbicides will affect non-target plants.
Adequate buffer zones have been defined for some application
methods.
Changes in species diversity and population size and structure
have been reported for terrestrial invertebrates and vertebrates
following applications of glyphosate formulation in the field.
Modifications in available food plants, insect populations
associated with vegetation killed by the herbicide, and ground cover
following intended effects of the spray probably account for these
changes.
9.2.3.1 Plants
Red pine seedlings (Pinus resinosa) were not affected by
treatment with Roundup in a field study. There was no dose-related
decrease of the root length, as had been observed in a comparable
laboratory experiment with the same doses (Chakravarty & Chatarpaul,
1990b). In this experiment lasting 154 days Roundup was applied at
rates of up to 3.2 kg a.i./ha. The conifer seedlings were inoculated
with an ectomycorrhizal fungus (Paxillus involutus) at 14 days
after germination, and they were planted outdoor after being
cultured in a greenhouse for up to 70 days after germination.
No plants were found as indicator species for damage due to
drift of a formulation of glyphosate when this was applied with
hydraulic ground sprayers (Marrs et al., 1989). In this study,
native British species commonly found in nature reserves were
exposed to spray drift, at several distances downwind from a zone
sprayed with 0.5 and 2.2 kg a.i./ha. The effect of windspeed was
investigated by spraying at speeds of 2.5 and 3.5 m/second. Death
and severe growth suppression occurred at a distance of 2-6 m from
the sprayer. Sublethal damage also occurred, mostly near to the
sprayer, although for Prunella vulgaris damage occurred up to 20 m
away. Epinasty was the most frequent symptom of damage. Most of the
damaged plants recovered, however. Some of the species were
consistently more sensitive, i.e. Digitalis purpurea, Centaurea
nigra, Prunella vulgaris and Lychnis flos-cuculi. Marrs et al.
(1989) concluded that, when spraying with ground sprayers, buffer
zones around nature reserves should be 5-10 m.
9.2.3.2 Invertebrates
No significant and consistent effects on the number of
nematodes and springtails were found in the upper 3 cm of
ferro-humic podsols due to treatment with Roundup (Preston &
Trofymow, 1989). The soils were covered with alder trees (Alnus
rubra) and located in British Columbia, Canada. The only
consistent effect was a significant reduction in the number of both
oribatid and non-oribatid mites on one of the treated sites around
20 days after application. On this site the number of mites appeared
to have returned to normal by the end of the study. In this
experiment of around 180 days Roundup was hand-sprayed at a rate of
2 kg a.i./ha.
No substance- or dose-related effects on mites and springtails
were observed in a sandy soil in a semi-arid region of Argentina up
to 96 days after application of Roundup (Gómez & Sagardoy, 1985).
Applied doses were up to 2.8 kg a.i. (free acid)/ha.
The numbers of herbivorous insects and ground invertebrates
were significantly reduced up to 3 years after treatment with
Roundup in a 4- to 5-year-old clear-cut planted with spruce
seedlings (Picea sp) (Santillo et al., 1989b). No effects were
found on predatory insects. The clear-cut was located in Maine, USA,
and sprayed with 1.7 kg a.i./ha. During this experiment lasting 3
years, the vegetation did not recover completely, and, apparently,
the effects on invertebrates were mainly due to habitat change.
Unintentionally untreated areas in the sprayed site showed a much
lesser reduction of invertebrates. These areas may therefore be
considered as potential sources for recolonization.
9.2.3.3 Vertebrates
Treatment of 4- to 5-year old clear-cuts in Maine, USA, planted
with seedlings of spruce (Picea sp), with Roundup at a rate of
1.7 kg a.i./ha affected breeding bird populations up to 3 years
after treatment (Santillo et al., 1989a). Total bird densities
decreased with 36% due to reduced habitat complexity, as expressed
in regenerated hardwood, vegetation height and foliage height
diversity. The most sensitive species were the insectivorous common
yellowthroat (Geothlypis trichas), Lincoln's sparrows (Melospiza
lincolnii) and alder flycatchers (Empidonax alnorum). In less
than 7-year-old clear-cuts in the Oregon coast range, Canada,
planted with Douglas fir (Pseudotsuga menziesii), some breeding
bird populations were affected due to two closely spaced treatments
with Roundup at a rate of 0.8 kg a.i./ha each (Morrison & Meslow,
1984). Two years after the application, the densities of birds using
shrubs for nesting and foraging had recovered, concomitant with the
recovery of shrub vegetation. Sensitive species were the
rufous-sided towhee (Piplio erythrophthalmus) and MacGillyvray's
warbler (Oporornis tolmiei). On the other hand, the American
goldfinch (Carduelis tristis) increased one year after
application, apparently due to the treatment. Morrison & Meslow
(1986) stated that the effectiveness of the treatment, which was not
maximal in their study, is crucial to the degree to which bird
populations are affected.
Small mammals may be affected by treatment with glyphosate,
this depending mainly on the size of the treated area, the
vegetation type and the extent to which the vegetation is damaged.
Various experiments have been performed in and around clear-cuts
planted with conifers in locations in the USA and Canada.
Insectivorous shrews (Blarina brivicauda, Sorex cinereus and Sorex
hoyi) and herbivorous voles were less abundant due to a treatment
with Roundup at a rate of 1.7 kg a.i./ha (Santillo et al., 1989b).
This significant reduction of the number of shrews was maintained
for 3 years after application, whereas the population of voles
recovered. No effects on the omnivorous deer mice (Peromyscus
maniculatus) were observed. Deer mice also appeared to be
unaffected in an experiment in which Roundup was applied at a rate
of 3 kg a.i./ha (Sullivan, 1990). The population dynamics of both
deer mice and the herbivorous Oregon voles (Microtus oregoni)
appeared not to be influenced, although some partially significant
effects were observed that might have been due to the treatment. On
the sprayed site there was an increase in the number of recruits of
both species 3 years after application, and also an increased
fecundity of deer mice and a higher survival of female voles 1 and 3
years after application. Sullivan (1990) observed no physiological
changes that might have been due to ingestion. In a clear-cut
treated with Roundup at a rate of 1.1 kg a.i./ha, only the
population density of Southern Redbacked voles (Clethrionomys
gapperi) was reduced by about 80% in a 1-year experiment (D'Anieri
et al., 1987). In another clear-cut, no adverse effects on deer mice
populations were evident after treatment with Roundup at a rate of
2.2 kg a.i./ha (Sullivan & Sullivan, 1981). Contrary to the results
of Sullivan (1990), D'Anieri et al. (1987), Sullivan & Sullivan
(1981) and Santillo et al. (1989b), a significant reduction in the
population density of deer mice was observed by Ritchie et al.
(1987) on a sprayed clear-cut of 38 ha at around 11 months after
spraying with Roundup at a rate of 1.1-1.2 kg a.i./ha. However, no
adverse effects on fertility or fecundity were indicated. Probably
the effect on abundance was due to habitat change with respect to
feed provision and cover. Possibly the effects on deer mice observed
by Ritchie et al. (1987) were less confounded by immigration as the
sprayed area was larger than in the studies in which effects on deer
mice were lacking. However, when a site of 36 ha was sprayed twice
with Roundup at a rate of 0.8 kg a.i./ha on each occasion, deer mice
were not affected, possibly due to a relatively low effectiveness of
the treatment (Anthony & Morrison, 1985). In the treated area, even
an increase of Oregon voles was found after 1 year, concomitant with
an increase of grass and other plants. The results indicated that
small mammal populations were able to recover within 2 years after
application of glyphosate, dependent on the recovery of shrubs.
10. EVALUATION OF HUMAN HEALTH HAZARDS AND EFFECTS ON THE
ENVIRONMENT
10.1 Human health hazards
Results of direct measurements of glyphosate concentrations in
foodstuffs (as part of food surveillance), drinking-water or total
diets are not available.
Absorption from the gastrointestinal tract is limited to 36% or
less and percutaneous absorption is 5.5% or less. Glyphosate is
essentially not metabolized. Total body clearance is 99% in 7 days.
Residues in livestock animals and their products (including milk)
are minimal.
Summarized information on short- and long-term studies is given
in Table 23 and on teratogenicity and reproduction studies in Table
24.
In animals, glyphosate has very low acute toxicity by the oral
and dermal administration routes. The formulation Roundup is acutely
toxic to humans when ingested intentionally or accidentally. No
controlled studies are available and therefore the human NOAEL
cannot be derived.
Animal studies show that glyphosate is not carcinogenic,
mutagenic or teratogenic. Reproductive effects were only seen at
dose levels producing maternal toxicity.
In experimental animals (13-week studies in rats and mice), an
effect was observed in the parotid salivary glands, indicating that
glyphosate may be acting as a weak adrenergic agonist. In rats, this
occurred at feeding levels of > 205 mg/kg body weight per day.
The NOAEL in chronic feeding studies is > 410 mg/kg body weight
per day. A NOAEL of 175 mg/kg body weight per day observed in a
rabbit teratogenicity study was chosen as the appropriate basis for
toxicological evaluations in humans. Through application of a
suitable safety factor, safe levels for humans can be estimated for
use in the toxicological evaluation of any actual exposures. For
technical glyphosate a safety factor of 100 is considered
appropriate given the elaborate data sets available.
Glyphosate and its concentrated formulations produce moderate
to severe eye irritation, but only slight skin irritation. Neither
glyphosate nor tested formulations induce sensitization.
Table 23. Short-term and long-term studies on glyphosate
Species Test Dose levels Effects, dose level NOAEL
compound (mg/kg-1 diet) (mg/kg diet) [mg/kg diet]
unless otherwise mg kg-1 b.w. d-1
stated
Short-term studies
Mouse technical 5000, 10 000, decreased growth and increased [10 000]
glyphosate 50 000 weights in brain, heart, 1890 m,
kidneys (50 000) 2730 f
Mouse technical 3125, 6250, reduced weight gain (50 000) [3 125]
glyphosate 12 500, 25 000, lesions of salivary glands 507
50 000 (> 6250)
Rat technical 1000, 5000, no adverse effects [20 000]*
glyphosate 20 000 1267*
Rat technical 200 to 12 500 no adverse effects [12 500]*
glyphosate NG
Rat technical 3125, 6250, increased AP and ALAT (> 6250) [< 3 125]
glyphosate 12 500, 25 000, increased haematocrit and red < 205 m
50 000 cell parameters (> 12 500), < 213 f
increased bile acids,
decreased sperm counts
(> 25 000), histological
alterations in salivary glands
(> 3125), reduced weight
gain (> 25 000)
Dogs technical 20, 100, 500 no adverse effects [NG]
glyphosate mg kg-1 bw 500*
Cattle Roundup 400, 500, 630, decreased feed intake (> 630) [NG]
790 mg kg-1 bw d-1), diarrhoea (> 500) 400
increased blood parameters
(790)
Long-term studies
Mouse technical 1000, 5000, decreased growth (30 000), [5 000]
glyphosate 30 000 increased incidence of 814
hepatocyte hypertrophy and
necrosis (30 000), increased
incidence of urinary bladder
epithelial hyperplasia
(30 000)
Rat technical 2000, 8000, decreased growth (20 000), [8 000]
glyphosate 20 000 increased liver weights 410
(20 000), increased incidences
of degenerative lens changes
(20 000) and of gastric
inflammation (8000 and 20 000)
Rat technical 60, 200, 600 slightly decreased growth (600) a
glyphosate
m = males; f = females; * Highest dose tested; NG, not given;
a The slight effect at 600 mg/kg diet (32 mg/kg bw) is considered marginal in the light
of the absence of an effect on growth at higher dose levels (2000 and 8000 mg/kg diet)
in a more recent 2-year study in rats.
Available studies on exposures of workers involved in
appli-cation of the herbicide show that exposure is low when
protective clothing is worn. The following data illustrate this
point.
a) The highest estimated exposure (dermal and inhalation) of about
8000 µg/h, as reported in a study with spray applicators,
corrected for incomplete absorption, equals about 50 µg/kg body
weight per day (8-h working day for a 70-kg adult); between the
latter level and the NOAEL of 175 mg/kg body weight per day,
adjusted for incomplete absorption from the gastrointestinal
tract (30-60%), i.e. 52-63 mg/kg body weight per day, there is
a margin of safety of about 1100.
b) The highest exposure concentration found for forest brush saw
workers was 15.7 µg/m3; between this level and the NOAEL
expressed as glyphosate from the 4-week inhalation study with
Roundup of 16 mg/m3 there is a margin of safety of 1000 (this
is borne out by the absence of adverse findings in the workers'
health examination in the study.
Table 24. Summary of teratogenicity and reproduction studies on glyphosate
Species Test Dose levels Effects NOAELa
compound (mg/kg diet) (mg/kg
body
weight)
Rat technical 300, 1000, 3500 mortality, clinical signs and 1000
glyphosate mg/kg body decreased growth in dams,
weight, early resorptions, decreased
gestation days 6-19 numbers of implantations and
visible fetuses, decreased
ossification of fetal
sternebrae (all at 3500 only);
no fetal malformations
Rabbit technical 75, 175, 350 diarrhoea and soft stools (350, 175
glyphosate mg/kg body slight at 175), nasal discharge
weight, (350)
gestation days 6-27
Rat technical 3, 10, 30 mg/kg increased incidence of renal < 30b
glyphosate body weight tubular dilation in F3b male
given in diet, pups (30)
3 gens
Rat technical 2000, 10 000, soft stools of parents (30 000), 100b
glyphosate 30 000 mg/kg decreased litter size (30 000), (2000
diet, 2 gens decreased body weights of mg/kg
parents and pups (30 000 diet)
and 10 000)
a Based on all observed effects (both in dams and offspring)
b There is some discrepancy in the results, and in the NOAELs, of the two
reproduction studies carried out with technical glyphosate; the renal
effects in the 3-generation study were not reproduced in the more recent
2-generation study with higher dose levels (for details, see section 7.5.2.).
10.2 Evaluation of effects on the environment
Following application, glyphosate will selectively partition to
particulate matter suspended in surface water, aquatic sediment or
to the soil substrate. This partitioning is usually rapid. The
mechanism of sorption is only partially understood. There is little
reported information on desorption from soil; the information
available suggests "strong" binding. Mobility studies show little
leaching of glyphosate below the upper few centimetres of the soil
profile. The major metabolite, AMPA, also appears not to leach
through soil.
Given this environmental distribution, organisms living in
aquatic sediment or soil would be expected to come into closest
contact with residues of the herbicide.
There is very little information on the bioavailability of
sediment- or soil-bound glyphosate to either aquatic or terrestrial
organisms. Few bioaccumulation or ecotoxicity studies have been
performed with sediment present.
Comparison of exposure concentrations and toxic effects is,
therefore, difficult since the relevant organisms have not been
tested and exposure of tested organisms is not by a realistic route.
10.2.1 Exposure levels and toxic effects
Exposure concentrations have been calculated from experimental
application of Roundup in the field (see Table 7 in chapter 5). The
methodology is presented in Fig. 4. No monitoring results of
environmental concentrations following actual use in agriculture or
forestry are available.
The lowest LC(EC)50 and NOEC values for microorganisms,
invertebrates and fish have been taken from the toxicity tests
reported in chapter 9 (see Fig. 4). These all refer to organisms
living in the open water and are, therefore, of questionable
significance for a compound which partitions to sediment. There is
no information on species living in aquatic sediment and little
information available on soil-living organisms, with the exception
of microorganisms.
10.2.2 Hazard evaluation for aquatic organisms
Tables 25 and 26 compare the estimated mean and maximum
exposure concentrations, following aerial application of Roundup, to
the lowest reported LC(EC)50 and NOEC concentrations for acute and
chronic exposure of aquatic organisms. The ratio between exposure
and effect concentrations has been calculated. These tables are
meant as a guide to establishing possible hazard and are not
intended to estimate the degree of effect likely to be seen in the
field. The "possible hazard" classification is a simple one using
different classification phrases for order of magnitude segments of
the ratios.
The toxicity of formulations to aquatic organisms is greater
than for technical glyphosate in many cases. This increased toxicity
is due to surfactants present in the product. No account has been
taken of possible degradation of surfactants in the ratios
presented, since no data are available. The ratios, therefore, may
overestimate the toxicity of glyphosate. If the compound is bound to
sediment in the environment, this could also reduce its toxic
effect. Since no clear evidence is available to demonstrate this
reduced toxicity, no account has been taken of partitioning to
particulates. This will also tend to overestimate toxic effect of
glyphosate.
As can be seen from the tables, possible hazard for most
aquatic organisms is small or negligible. Fish and aquatic
invertebrates would not be affected by glyphosate use. Only
microorganisms, with both acute and chronic exposure, appear to be
susceptible to effects of the herbicide. The comparisons made in the
table do not allow estimates of the degree of toxic effects likely
to be seen in the field. From the field evidence available,
populations and communities of algae are not likely to be affected
after application of glyphosate formulation. Transitory changes in
number and functioning of aquatic microorganisms are possible after
use of the herbicide.
Since data are not available, evaluation of the hazard of bound
residues of glyphosate to sediment-living organisms is not possible.
Minimal bioaccumulation of glyphosate has been reported in both
laboratory experiments and in the field. The physicochemical
properties of the compound are consistent with this conclusion.
10.2.3 Hazard evaluation for terrestrial organisms
Limited test data show low toxicity of glyphosate and its
formulations to honey-bees, earthworms, birds and mammals. These
data suggest low risk for these organisms from use of the herbicide.
Field studies have been conducted and support the view that
glyphosate does not affect soil microorganisms in the long term.
Marked changes in populations of birds and small mammals have
been seen in field studies following application of glyphosate.
These seem to result from the changes in habitat, vegetation cover,
food organisms, etc., resulting from the intended herbicidal effect
of the compound.
Carlisle & Trevors (1986a) deduced from their experiments that
nitrate-reducing bacteria are involved in metabolizing glyphosate.
Involvement of nitrifying bacteria in the biodegradation of
glyphosate was also demonstrated by Murthy et al. (1989), when they
investigated the treatment of waste water from a Roundup formulating
factory.
Pseudomonas sp. may use glyphosate as a sole P or C source,
as demonstrated by Weidhase et al. (1990). Only slight growth of the
wild-type strain of the bacterium Pseudomonas fluorescens was
observed with glyphosate as sole carbon or nitrogen source. The
herbicide was metabolized to aminomethylphosphonate (Zboinska
et al., 1992). Murthy et al. (1989) isolated a denitrifying
bacterial species that was also able to use glyphosate as a C
source. This species was isolated from activated sludge in a
waste-water treatment plant. A mutant of Arthrobacter sp. strain
GLP-1 was able to utilize glyphosate as a sole N source, whereas
this was not possible for the normal strain (Pipke & Amrhrein,
1988), probably due to the uptake of inorganic P released during
biodegradation.
As the Biological Oxygen Demand and the Chemical Oxygen Demand
of glyphosate are < 2 mg/g and 0.53 g/g, respectively, glyphosate
cannot be considered as readily biodegradable (LISEC, 1990a,b). In
suitable systems, however, glyphosate is biodegradable, as shown by
Murthy et al. (1989), who investigated the biodegradation of
glyphosate in waste-water treatment plants under different
conditions in sequencing batch reactors on a laboratory scale. These
reactors were fed with waste water from a Roundup manufacturing
facility. Glyphosate was degraded completely within one cycle of
24 h, independent of whether there was an initial aerated or anoxic
phase of 4 h. However, more glyphosate could be processed with an
anoxic initial phase, probably due to better conditions for
denitrification. Not only denitrifiers but also ammonifiers and
nitrifiers appeared to be involved in the biodegradation of
glyphosate. Only at the very high concentration of approximately
5000 mg a.i./litre was biodegradation repressed by non-glyphosate
COD and inhibited by excess ammonia production.
Pseudomonas sp. strain LBr, Flavobacterium sp. and a
denitrifying bacterial species were isolated from activated sludge
as species with the ability to use glyphosate as a P source
(Balthazor & Hallas, 1986; Jacob et al., 1988; Murthy et al., 1989).
The denitrifier was also able to use glyphosate as a sole C source.
Flavobacterium sp. degraded glyphosate to AMPA in both the
presence and absence of PO43- (Balthazor & Hallas, 1986). In
this experiment the further degradation of AMPA appeared to be
hampered in the presence of PO43-.
Pseudomonas sp. strain LBr was capable of completely
eliminating amounts of glyphosate up to 3212 mg/litre from a growth
medium (Jacob et al., 1988).
Continuous exposure of an activated sludge treatment system in
a pilot plant increased the ability of the sludge to metabolize
glyphosate to AMPA (Hallas et al., 1992). In this trial an influent
concentration of 50 mg a.i./litre was reduced to less than 5 mg
a.i./litre under continuous-flow conditions with an average
residence time of 10 min. The sludge was inoculated with immobilized
bacteria capable of degrading glyphosate. The effectiveness of the
treatment was dependent on the presence of a nitrogen source and a
non-glyphosate carbon source, and required a pH range of 6.0 to 8.0.
No data are available on the amounts of glyphosate that can be
eliminated in conventional waste-water treatment plants under
practical conditions. In waste water from glyphosate-producing
plants, 28-45% is reported to be eliminated through biological
treatment (Task Force on Water Quality Guidelines, 1991).
No data are available on the biodegradability of the
surfactants in formulations. It is, however, probable that
polyoxyethylene amine is biodegraded fairly rapidly in view of the
biodegradability of structurally related compounds (Swisher, 1987).
4.4 Bioaccumulation
Appraisal
Glyphosate is not expected to bioaccumulate in view of its
high water solubility and its ionic character. This was confirmed by
several laboratory experiments with fish, crustaceans and molluscs
and by field experiments.
In a static test, channel catfish (Ictalurus punctatus) were
exposed to 0.94-0.99 mg 14C-labelled a.i./litre (actual
concentrations) for 10 days (ABC Inc, 1981d; Monsanto, 1981a). Of
the absorbed amount, 76% was recovered in the viscera. More than 90%
of the extractable residues in the viscera and the fillet was
identified as glyphosate, whereas less than 2% was identified as
AMPA. After 10 days of depuration 80% of the absorbed activity was
eliminated. For exposed channel catfish the calculated
bioconcentration factor based on the activity absorbed by the whole
fish was 0.27. For depurated channel catfish the calculated
bioconcentration factor was 0.052.
The marsh clam (Rangia cuneata) and crayfish (Procambarus
simulans) were exposed in static tests lasting 28 days to
synthetic uncontaminated sea water and a sandy loam sediment that
was incorporated with 3 mg 14C-labelled a.i./kg (ABC Inc.,
1982d,e). These experiments were set up to assess the degree of
bioconcentration of glyphosate when used in flooded rice levees and
tidal water. The calculated bioconcentration factor for the edible
parts of the clam increased during exposure up to 4.8, whereas for
the whole crayfish it increased up to 12. The highest concentrations
in the edible parts of the clam and the whole crayfish were 0.3 mg
14C-labelled residues/kg for both. After 28 days of depuration 48%
of the accumulated residues were eliminated from the edible parts of
the clam. The concentration in these parts was then 0.16 mg
14C-residues/kg. The crayfish finally had eliminated 91% after 14
days of depuration. The concentration in the whole crayfish was then
0.02 mg 14C-residues/kg. It must be stated that this test refers
to the accumulation of 14C and not glyphosate.
In a static test without sediment, in which rainbow trout
(Salmo gairdnerii) were exposed to 2 mg a.i./litre (nominal
concentration) for 12 h, the fillets of the fish contained 80 µg
a.i./kg (in the original article the erroneous figure of 80 mg/kg
was reported), and the eggs 60 µg a.i./kg (Folmar et al., 1979).
This indicates a bioconcentration factor of 0.04 for the edible
parts. Roundup was applied in this test.
In a flow-through test in which bluegill sunfish (Lepomis
macrochirus) were exposed to 11-13 mg 14C-labelled a.i./litre
(actual concentrations) for 35 days, calculated daily
bioconcentration factors based on the whole fish increased from <
0.1, 0.2 days after the start of the test, to 0.4-0.5 at the end
(ABC Inc., 1989f). Maximum concentrations in the whole fish, viscera
and fillet were 13, 7.6 and 4.8 mg 14C-residues/kg, respectively.
The time required to reach 90% of the steady state and the uptake
rate constant were calculated to be 120 days and 0.02 mg/kg fish x
(mg/litre water)-1 x day-1, respectively. During 21 days of
depuration, the half-life of depuration was calculated to be 35. A
slow decrease in tissue concentration during depuration was
indicated. After the period of depuration 2.2 mg 14C-residues/kg
whole fish was still present. In an additional study to characterize
the 14C-residues, 95-97% of the residues in the water was
glyphosate, whereas in the whole fish and tissues 28-91% of the
recovered activity was glyphosate (ABC Inc., 1989g). In a whole fish
sample 21 days after starting the test, 49% of the recovered
activity was found to be AMPA. By treating homogenates with
proteinase K it was indicated that a substantial amount of the
absorbed residues was tightly associated with, or incorporated into,
protein.
In a field experiment in a forest ecosystem in Oregon, USA,
neither glyphosate nor AMPA were recovered in salmon fingerlings
(Oncorhynchus kisutch) after aerial application of Roundup at a
rate of 3.3 kg a.i./ha (Newton et al., 1984). The fingerlings were
released at the downstream edge of the sprayed site and analysed up
to 55 days after treatment. Glyphosate was not recovered in carp
(Cyprinus carpio) in a field experiment in which ponds were
sprayed with Roundup at rates of 1.3-1.4 kg a.i./ha (Monsanto,
1980). In this experiment of approximately 90 days, AMPA was not
recovered until 30 days after application. It then increased up to
0.21 mg/kg whole fish, remained constant for another 30 days, and
then decreased to around the limit of determination (0.1 mg/kg) at
the end of the experiment.
In a forest ecosystem in Oregon, USA, Roundup was aerially
applied at a rate of 3.3 kg a.i./ha (Newton et al., 1984).
Concentrations in mammals were of the same order of magnitude as the
concentrations in litter and ground cover. The concentrations of
glyphosate in the viscera of herbivorous small mammals decreased
more slowly than in omnivorous and carnivorous small mammals, which
was probably due to a higher ingestion of contaminated litter. The
highest concentration was found in the viscera of omnivorous
deermice (Peromysces maniculatus) immediately after spraying: 5 mg
a.i./kg. Only small traces of AMPA were found in mammalian viscera.
4.5 Waste disposal
Small amounts of glyphosate can be disposed of by mixing with
alkali and soil prior to burial in a pit or trench, whereas large
amounts should be incinerated in units equipped with effluent gas
scrubbing (IRPTC, 1991).
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
Appraisal
The low toxicity, low volatility and low body absorption of
glyphosate makes its application by backpack sprayer safe under
field condition provided that the worker wears full protective
clothing.
5.1 Environmental levels
A synopsis of concentrations of glyphosate is tabulated in
Table 7. Measurements as part of regular monitoring programmes are
very scarce; measurements in field experiments with recommended
application rates simulating common agricultural practice are
therefore included in Table 7. Only maximum amounts are tabulated as
indicative values, since the rate at which they dissipate is not
included here (see sections 4.1 and 4.3). Data on the occurrence of
glyphosate and AMPA in sewage sludge are not available.
In biota the highest concentrations of glyphosate and AMPA were
found in fresh foliage and reindeer lichen (Cladonia rangiferina).
In abiota the highest concentrations of both compounds were found in
the soil (see Table 7). The occurrence of glyphosate in the
groundwater of Texas, USA, was reported by Hallberg (1989), but the
measured concentration and the year of measurement were not
specified.
Use of glyphosate as a herbicide may result in the presence of
residues in crops and animal tissues destined for human consumption.
Application as a herbicide may also be responsible for the presence
of glyphosate in drinking-water. Direct measurements of glyphosate
in foodstuffs (as part of food surveillance), drinking-water or
total diets have not been carried out. The only information
available comes from controlled residue studies. With technical
glyphosate formulated as the isopropylamine salt in aqueous
solution, numerous residue studies have been carried out in
vegetables, grasses, oil seeds, mammalian products, poultry products
and primary feed commodities. The results are summarized in the
various reports of the FAO/WHO Joint Meeting on Pesticide Residues
(FAO/WHO, 1986a, 1987, 1988). For detailed information on these
studies the reader is referred to these reports. The appraisals made
by the JMPR included the following more general statements.
Pre-harvest (5-14 days) application of glyphosate (isopropylamine
salt) in the cultivation of cereals results in significant residues
in the grain and plant materials. Studies are available to show the
fate of glyphosate in milling, baking and brewing. Residue levels in
white flour were approximately 10-20% of the levels in wheat, while
the bran residue levels were 2 to 4 times as high as those in the
wheat. Glyphosate residues were not lost during baking, but residue
levels decreased when bread was made from flour because of dilution.
Glyphosate residue levels in malt and beer derived from
field-treated barley were, respectively, about 25% and 4% of the
original level in the barley. Some glyphosate is lost during
washing, but most of the decrease can be attributed to dilution. The
levels in groats (processed oats) were about 50% of the levels in
the pre-harvest-treated oats. In all these cases, AMPA contributed
only a small proportion (average < 2.5%) of the total residues
(FAO/WHO, 1986a, 1987).
When administered to animals glyphosate is rapidly excreted
without degradation. Residues in cattle, pig and poultry meat, eggs
and milk were negligible after the animals were fed with a diet
containing 100 mg/kg glyphosate and aminoglyphosate acid. The
highest residues were in pig liver and kidney (up to 0.16 and
0.91 mg/kg, respectively) and cattle kidney (up to 1.4 mg/kg).
Residues in animal feeds arising from pre-harvest glyphosate
applications to cereals will result in only low residues in meat,
milk and eggs (FAO/WHO, 1986a).
On the basis of these residue studies the JMPR has estimated
the maximum residue levels that are likely to occur when glyphosate
(as isopropylamine salt) is used in practice, and recommended these
levels as Maximum Residue Limits (MRLs). These MRLs are presented in
FAO/WHO (1986a, 1987, 1988).
5.2 General population exposure
Apart from the controlled residue studies mentioned above, no
data are available.
Table 7. Maximum concentrations of glyphosate in environmental air, water, soil, sediment and biota
Sample Compound Location Yeara Concentration Reference
Netherlands
Surface water glyphosate Drentsche Aa 1988-1989 0.5-1 µg/litreb (Soppe, personal
Surface water AMPA Drentsche Aa 1988-1989 6 µg/litreb communication to the IPCS, 1991)
Finland
Loam soil (agric.) glyphosate Kettula 1978 17 mg/kg d.w. Müller et al. (1981)
Loam soil (agric.) AMPA Kettula 1978 3.2 mg/kg d.w. Müller et al. (1981)
Silt soil (agric.) glyphosate Kettula 1978 3.8 mg/kg d.w. Müller et al. (1981)
Silt soil (agric.) AMPA Kettula 1978 0.4 mg/kg d.w. Müller et al. (1981)
Wild berries glyphosate Laukaa, Konnevesi 1977 1.6-2.1 mg/kgc Siltanen et al. (1981)
Wild berries AMPA Laukaa, Konnevesi 1977 0.02-0.07 mg/kgc Siltanen et al. (1981)
Reindeer lichen glyphosate Laukaa, Konnevesi 1976 45 mg/kgc Siltanen et al. (1981)
Reindeer lichen AMPA Laukaa, Konnevesi 1976 2.1 mg/kgc Siltanen et al. (1981)
Canada
Wild berries glyphosate Harker, Lamplugh 1985 8-19 mg/kg f.w. Roy et al. (1989a)
Wild berries AMPA Harker, Lamplugh 1985 0.06-0.1 mg/kg f.w. Roy et al. (1989a)
Surface waterd glyphosate Carnation Creek 1984 162 µg/litre Feng et al. (1990)
Surface watere glyphosate Carnation Creek 1984 < 1 µg/litre Feng et al. (1990)
Surface waterd AMPA Carnation Creek 1984 approx 3 µg/litre Feng et al. (1990)
Pond water glyphosate Manitoba 1986 141 µg/litre Goldsborough & Beck (1989)
Pond water AMPA Manitoba 1986 2.2 µg/litre Goldsborough & Beck (1989)
Sedimentd glyphosate Carnation Creek 1984 6.8 mg/kg d.w. Feng et al. (1990)
Suspended sediment glyphosate Carnation Creek 1984 0.06 µg/litre Feng et al. (1990)
Soil glyphosate Carnation Creek 1984 40 mg/kg d.w. Feng & Thompson (1990)
Soil AMPA Carnation Creek 1984 9 mg/kg d.w. Feng & Thompson (1990)
Table 7. (cont'd)
Sample Compound Location Yeara Concentration Reference
Canada (cont'd)
Foliage (fresh) glyphosate Carnation Creek 1984 261-448 mg/kg d.w. Feng & Thompson (1990)
Foliage (fresh) AMPA Carnation Creek 1984 < 9 mg/kg d.w. Feng & Thompson (1990)
Topcrown foliage glyphosate Oregon Coast Range 1978 489 mg/kgc Newton et al. (1984)
Topcrown foliage AMPA Oregon Coast Range 1978 2.1 mg/kgc Newton et al. (1984)
Deermice (viscera) glyphosate Oregon Coast Range 1978 5.1 mg/kgc Newton et al. (1984)
USA
Pond water glyphosate Chassell, Corvallis, 1987 90-1700 µg/litre Monsanto (1990a)
Cuthbert
Pond water AMPA Chassell, Corvallis, 1987 2-35 µg/litre Monsanto (1990a)
Cuthbert
Stream water glyphosate Chassell, Corvallis, 1987 35-1237 µg/litre Monsanto (1990a)
Cuthbert
Stream water AMPA Chassell, Corvallis, 1987 < 1.0-10 µg/litre Monsanto (1990a)
Cuthbert
Pond sediment glyphosate Chassell, Corvallis, 1987 0.26-19 mg/kg d.w. Monsanto (1990a)
Cuthbert
Pond sediment AMPA Chassell, Corvallis, 1987 0.13-1.8 mg/kg d.w. Monsanto (1990a)
Cuthbert
Stream sediment glyphosate Chassell, Corvallis, 1987 0.11-0.69 mg/kg d.w. Monsanto (1990a)
Cuthbert
Stream sediment AMPA Chassell, Corvallis, 1987 < 0.05-0.38 mg/kg d.w. Monsanto (1990a)
Cuthbert
Soil (no litter on it) glyphosate Chassell, Corvallis, 1987 0.15-4.7 mg/kg d.w. Monsanto (1990a)
Cuthbert
Table 7. (cont'd)
Sample Compound Location Yeara Concentration Reference
USA (cont'd)
Soil (no litter on it) AMPA Chassell, Corvallis, 1987 0.18-0.51 mg/kg d.w. Monsanto (1990a)
Cuthbert
Soil (litter on it) glyphosate Chassell, Corvallis, 1987 0.07-1.4 mg/kg d.w. Monsanto (1990a)
Cuthbert
Soil (litter on it) AMPA Chassell, Corvallis, 1987 0.14-0.68 mg/kg d.w. Monsanto (1990a)
Cuthbert
Foliage (fresh) glyphosate Chassell, Corvallis, 1987 650-1300 mg/kgb Monsanto (1990a)
Cuthbert
Foliage (fresh) AMPA Chassell, Corvallis, 1987 1.7-2.6 mg/kgb Monsanto (1990a)
Cuthbert
a Sampling invariably took place during the autumn period.
b Analytical procedure was unvalidated; the water was sampled at an inlet point of
a drinking-water processing facility
c It was not reported whether values were based on dry or fresh weight
d Water unbuffered by vegetation
e Water buffered by vegetation
5.3 Occupational exposure during manufacture, formulation or use
In the study of Monsanto (1977), worker exposure to Roundup was
measured during herbicide mixing and application operations and upon
re-entry of treated fields. The formulation was applied on separate
plots using three application devices, i.e. a broadcast boom
sprayer, a handgun broadcast sprayer and a backpack/hand-gun
sprayer. Exposure time during mixing was < 5 min; during
application this was about 45 to 60 min. Inhalational exposure
during mixing and spraying was determined using a sampling device
placed close to the applicator's face; the total air volume sampled
was 15-20 times the daily ventilation volume. Dermal exposure during
mixing and spraying was monitored by determination of deposition on
gauze pads placed outside or inside the operator's clothing on
different parts of the body. Operator exposure (inhalation and skin,
determination method as above) upon re-entry was determined at 1, 3
and 7 days after application. In one treated plot, this was done by
having operators walk through the plot for 28 to 44 min; in two
other plots a dummy sampling device was used to determine exposure
upon re-entry. Using the measured glyphosate residues total worker
exposure was estimated. The results are presented in Table 8.
Table 8. Applicator/worker exposure to glyphosatea
Operation Average dermal Average inhalation Total exposure
exposure exposure (µg/h)
(µg/h) (µg/h)b
Tank filling 805.1 17.9 823.0
Boom spraying 271.4 0.19 271.59
Handgun spraying 7957.0 2.47 7959.47
Backpack spraying 3619.0 0.92 3619.92
Field re-entry:
1 day after treatment 2046.6 4.58 2051.18
3 days after treatment 2919.7 0.12 2919.82
7 days after treatment 15.9 0.12 16.02
a From: Monsanto (1977)
b Calculation based on an estimated breathing rate of 1.8 m3/h
Monsanto (1990) conducted another collaborative study at three
sites maintained by the USDA Forestry Service near Clayton, Georgia;
the Savanah River Plant, South Carolina; and Edgefield, South
Carolina. Glyphosate was being used to control vegetative growth
around pine seedlings planted in clear-cut forest areas. The workers
were biologically monitored by analysis of collected composite urine
specimens. Additionally, dermal/clothing deposition and simulated
inhalation exposure were monitored by passive dosimetry with cotton
cloth patches, hand rinses and air filters. At the three sites,
exposure of workers to glyphosate using backpack sprayers while
performing their duties under normal use conditions was monitored.
The analytical results indicated that the majority of urine
composite samples had unmeasurable residues of glyphosate.
Deposition on air filters, patches and hands from measurement of
washes, however, indicated a small amount of body exposure. It was
concluded that penetration of clothing did not exceed 3.84% and thus
clothing produced 96.2% protection. Body burden, as shown in urine
samples, was extremely low and in most cases below the detection
limit.
A new Monsanto study was conducted for the assessment of worker
exposure to glyphosate during mist blower application of Roundup
herbicide. Exposure was determined by a passive dosimetry technique
while workers sprayed weeds around palm trees in a plantation in
Malaysia. The workers were fitted with gauze patches at different
locations on their clothing. Air sampling was performed in the
breathing zone and the workers hands were washed at the end of the
day. The passive dosimetry body dose estimates were calculated for a
fully clothed worker with a long-sleeved shirt, long pants and
rubber boots. The hand exposure would account for bare hands during
the loading and spraying operations. Passive dosimetry estimates for
the four replicates, corrected for clothing and dermal penetration,
transport/storage/analytical recovery and normalized for body weight
and amount of chemical handled, averaged 1.88 µg/kg body weight per
kg a.i. This is little higher than the passive dosimetry estimates
of forestry workers who applied Roundup with knapsack sprayers,
which was 1.75 µg/kg body weight per kg a.i. Thus, it can be
concluded that workers applying Roundup herbicide with mist blowers,
experience some dermal exposure. In addition, inhalation exposure to
glyphosate may be higher during mist blower application. However,
Roundup demonstrated essentially no volatility. The only possible
route would be via air-borne particles. The actual amount of
glyphosate absorbed through inhalation would be much lower than the
estimated values because the measurement includes particles too
large to be inhaled (Monsanto, 1991).
Jauhiainen et al. (1991) examined the exposure of workers to
glyphosate during silvicultural clearing with brush saws equipped
with herbicide sprayers. Measurements of air concentrations during
spraying and urinary glyphosate levels both during and following
spraying were carried out. Most of the air samples had glyphosate
concentrations below 1.25 µg/m3; the highest value observed was
15.7 µg/m3. All urine samples taken had glyphosate concentrations
below the limit of detection of 0.1 mg/litre (Jauhiainen et al.,
1991).
Lavy et al. (1992) used two methods to monitor glyphosate
exposure of workers planting conifer seedlings. Firstly, they
estimated dermal exposure based on the rate of deposition on cotton
gauze patches, surface area exposed, and a dermal penetration rate
of 1.8%. This yielded dose estimates in the range of 0-875 or
0-1.7 µg/kg body weight per h. Secondly, they attempted to measure
urinary concentration levels but found no samples above the
detection limit of 0.01 mg/litre. Based on the negative results with
the urine samples, the authors concluded that the estimates based on
patch deposition overestimated exposure by at least a factor of 11
for the most highly exposed workers (Lavy et al., 1992).
It should be noted that a dermal penetration rate of 1.8% was
used in this calculation. In view of the discussion of the dermal
penetration studies (section 6.1), a value of 5.5% is to be
preferred. However, since the estimate based on 1.8% is already an
overestimation of exposure, it is not considered necessary to adjust
the estimate of Lavy et al. (1992) to 5.5%. In their final data
assessment, the authors estimated exposure from the biological
monitoring using postulated data, i.e. assuming a concentration in
urine of half the lower limit of method validation. This yielded
mean exposure values of 0.039 to 0.080 µg/kg body weight per h (Lavy
et al., 1992).
6. KINETICS AND METABOLISM IN LABORATORY
ANIMALS AND HUMANS
Appraisal
Absorption from the gastrointestinal tract after oral intake
is limited to 30-36% of the dose or less in various species, i.e.
rats, rabbits, laying hens and lactating goats. Percutaneous
absorption in Rhesus monkeys amounts to 5.5% only, and glyphosate is
very poorly absorbed through excised human abdominal skin.
Radiolabelled glyphosate distributes widely in the body, but
is primarily found in the bones where approximately 1% can be
detected after oral administration.
Glyphosate is essentially not metabolized. This validates
kinetic studies performed with radiolabelled compound.
After absorption, excretion of glyphosate occurs mainly in the
urine. Biliary excretion is limited and elimination through exhaled
air is very low. Total body clearance is 99% after 168 h.
6.1 Absorption
The absorption percentage in rats was reported to be 30-36%
after single oral dosage at 10 and 1000 mg/kg body weight;
calculations were based on excretion percentages in urine and
faeces, and on the fact that biliary elimination is probably a minor
route (Monsanto, 1988b; Brewster et al., 1991). From a similar study
carried out in 1973, total absorption percentages of approximately
20% (male rats) and 45% (female rats) can be derived (Monsanto,
1973a). Comparable results were obtained in a recent single dose
(5.6 or 56 mg/kg body weight) disposition study in F344/N rats (NTP,
1992), which indicated that 30% of the dose was absorbed.
In a 14-day oral study in rats with application of
14C-glyphosate via the diet (dose levels 1, 10 and 100 mg/kg
feed), the observed total excretion in urine was < 10% and in
faeces approximately 80-90%. Given the minor importance of the
biliary elimination route, these data indicate absorption levels of
about 15% or less (Monsanto, 1973c). The results of oral studies
with 14C-glyphosate in rabbits (Monsanto, 1973d), laying hens
(Hazleton Lab. Inc., 1988a) and lactating goats (Hazleton Lab. Inc.,
1988b) indicate gastrointestinal absorption percentages of
approximately 30% or less.
Percutaneous absorption has been studied in Rhesus monkeys and
in human tissue in vitro. After a single application of
14C-glyphosate (isoproylamine salt) as the undiluted Roundup
formulation to the shaven intact abdominal skin (contact time 24 h)
of Rhesus monkeys, absorption amounted to only 1.8% of the dose. In
this study, however, only 16% of the dose could be accounted for at
the end of the study (Maibach, 1983). This low recovery strongly
reduces the value of the study result (possibly skin absorption is
seriously underestimated in this study). From an identical study
with diluted Roundup formulation (1:29 with water), conducted by
Wester et al. (1991), also in Rhesus monkeys (contact time 12 h),
total absorption percentages of 3.7% (at low dose) or 5.5% (high
dose) can be derived. Using both undiluted and diluted Roundup
formulation, Wester et al. (1991) observed that percutaneous
absorption of 14C-glyphosate through human skin in vitro into
human plasma as receptor fluid was < 2% (contact-time up to
16 h). In another in vitro study with human skin, absorption of
14C-glyphosate from three undiluted formulations (i.e. MON 0139,
Roundup and Roundup spray mix) was studied (contact time 24 h); very
low absorption percentages of 0.028 to 0.152% were found (Franz,
1983). With regard to these in vitro results it should be pointed
out that this technique has not yet been fully validated and
therefore direct extrapolation to in vivo human skin absorption
should be undertaken cautiously.
The absorption after inhalational intake has not been
determined.
6.2 Distribution
Concentrations of 14C label in tissues were determined on day
7 after administration of a single oral dose (10 or 1000 mg/kg body
weight) of 14C-glyphosate to rats (Monsanto, 1988b). Although only
a small proportion was absorbed, the isotope was widely distributed
throughout the body, but was primarily found in bone. The principal
results are presented in Table 9.
In rats tissue concentrations of 14C label were determined on
several occasions throughout a treatment period of 14 days and a
post-dosing withdrawal period of 10 days (dietary administration of
14C-glyphosate at 1, 10 and 100 mg/kg diet). Maximum tissue levels
were reached after 10 days or less, with highest concentrations
(maximum 0.85 mg/kg at the 100 mg/kg dose level) in kidneys
(Monsanto, 1973c). It should be noted, however, that in this study
concentrations in bone or bone marrow were not measured. An increase
of 14C in excreta was observed during the withdrawal period after
an initial rapid decrease; this indicated mobilization from storage
in depot tissue (Monsanto, 1973c).
Table 9. Concentrations of 14C label (as mg glyphosate-equivalents/kg
fresh weight) in selected tissues of rats on day 7 after a single
oral dose (rounded values) (Monsanto, 1988b)
Dose: 10 mg/kg body weight Dose: 1000 mg/kg body weight
male female male female
Blood 0.0045 0.0027 0.33 0.17
Liver 0.030 0.014 1.9 1.3
Kidney 0.022 0.013 1.9 1.4
Spleen 0.012 0.0073 2.6 3.0
Lung 0.015 0.012 1.5 1.1
Thyroid 0.00080 0.00036 1.5 1.2
Nasal mucosa 0.0050 0.023 1.7 1.8
Stomach 0.0080 0.0037 2.4 2.4
Small intestines 0.022 0.018 1.9 1.6
Colon 0.034 0.016 11.0 9.2
Bone 0.55 0.31 30.6 19.7
Bone marrow 0.029 0.0064 4.1 12.5
In lactating goats concentrations of 14C label in milk were
measured after giving capsules containing a 9:1 mixture of
14C-glyphosate and 14C-aminomethylphosphonic acid (AMPA) to a
dose level equivalent to 120 mg/kg diet (expressed as free acid) for
5 days. Concentrations in milk (as mg equivalents glyphosate/kg
whole milk) ranged from 0.019 to 0.086 mg/kg during the test period;
at day 5 after the last dose the concentration was 0.006 mg/kg
(Hazleton Lab. Inc., 1988b; Monsanto, 1988d).
In a study on laying hens, carried out using a 9:1 mixture of
14C-glyphosate and 14C-AMPA, concentrations of radiolabel were
measured in eggs collected during a 7-day period of dietary
administration at 120 or 400 mg/kg diet. At 400 mg/kg, residues in
egg white were 0.010-0.032 mg/kg (expressed as
glyphosate-equivalents) and in egg yolk 0.096-0.753 mg/kg; at
120 mg/kg the corresponding concentration ranges were 0.003-0.017
and 0.002-0.24 mg/kg, respectively. At 120 mg/kg diet, no 14C was
detectable in egg white after 10 withdrawal days; in yolk
0.019 mg/kg was present at that time (at 400 mg/kg no withdrawal
test was conducted) (Hazleton Lab. Inc., 1988a).
6.3 Metabolic transformation
Biotransformation of glyphosate occurs to a very low degree
only. In rats it was shown that all of the 14C in urine and
faeces, after a single oral application of 14C-glyphosate, was
present as unchanged parent compound (Monsanto, 1973b). Also in
rats, > 97% of the 14C in excreta, after a single oral dose,
was shown to be unchanged compound. AMPA was the only metabolite,
covering only 0.2-0.3% of the applied 14C (Monsanto, 1988a). In
laying hens also, AMPA was the only metabolite, accounting for only
a minor part of the applied amount (Monsanto, 1988c).
6.4 Elimination and excretion
In the period of 0-5 days after a single oral application of
14C-glyphosate (6.7 mg/kg body weight) to rats the total excretion
in urine was 15% (males) and 35-43% (females) of the administered
dose; total excretion in faeces was 85% (males) or 50-55% (females).
Less than 1% of the radiolabel was expired as 14CO2 (Monsanto,
1973a). In a more recent study (Monsanto, 1988b), the very low level
of expiration as 14CO2 was confirmed but no significant
inter-sex difference in the level of 14C in excreta was observed.
The result of the latter study was that at both oral dose levels (10
and 1000 mg/kg body weight) elimination in faeces was 62-70% and
excretion in urine was 14-18% (1000 mg/kg body weight) or 22-29%
(10 mg/kg body weight); less than 0.2% of the dose was expired as
14CO2. After single intravenous application (dose 10 mg/kg body
weight) 75-79% appeared in urine and only 5-8% in faeces, a finding
that shows that biliary elimination occurs to a limited degree only
(Monsanto, 1988b).
Delayed excretion in rats during a 10-day post-dosing
withdrawal period was observed after daily oral administration via
the diet for 14 days (Monsanto, 1973c); this suggests that some
storage in tissue(s) occurs when uptake is prolonged. Tissue
equilibrium was attained by day 10 of the dosing period and
excretion equalled intake by day 6 of the dosing period.
In rabbits > 80% was eliminated in faeces (with additional
14C present in the gut) and 7-11% in urine within 5 days after
administration of a single oral dose (6.7 mg/kg body weight) of
14C-glyphosate. Less than 1% of the dose was expired as 14CO2
(Monsanto, 1973d). In one oral study in lactating goats lasting 5
days, total excretion in urine varied from 20 to 24% and in faeces
from 60 to 66% (Hazleton Lab. Inc., 1988b).
6.5 Retention and turnover
Total body clearance in the study of Monsanto (1973a) was
94-98% (males) or 82-84% (females) over a 48-h period after giving a
single oral dose of 14C-glyphosate; at 120 h post-dosing this was
99% (both sexes). In Monsanto (1988b), the kinetics of whole body
elimination were estimated using the radioactivity (14C) measured
in urine and faeces after a single oral dose of 14C-glyphosate (10
or 1000 mg/kg body weight). Because of the lack of biotransformation
of glyphosate it is valid to base kinetics on total radioactivity.
The elimination appeared to be biphasic. The half-life of the alpha
elimination phase at 10 mg/kg body weight was 5.87 h (males) or
6.22 h (females); at 1000 mg/kg body weight this was 5.26 h (males)
or 6.44 h (females). The half-life of the beta phase at 10 mg/kg
body weight was 79 h (males) or 106 h (females); at 1000 mg/kg body
weight this was 181 h (males) or 337 h (females). Pretreatment with
unlabelled compound for 14 days (carried out at the low dose level)
did not have an effect on whole body elimination. Seven days after
dosing, < 0.05% of the dose was present in organs and < 0.5% in
the remaining carcass. Highest concentrations were present in bone.
It was estimated that 0.2-0.6% of the oral dose was associated with
this site; after intravenous dosing this was approximately 1%
(Monsanto, 1988b). Brewster et al. (1991) reported that in rats
nearly all of the absorbed material had been eliminated from the
body 168 h after oral administration of 10 mg/kg body weight;
approximately 1% of the dose was still associated with the bone.
In a recent study on the disposition of glyphosate in F-344/N
rats, 1% of a single oral dose (5.6 or 56 mg/kg) was found in the
tissues 72 h after dosing; 20-30% of the administered radioactivity
was eliminated via urine and 70-80% via the faeces (NTP, 1992).
7. EFFECTS ON LABORATORY ANIMALS AND IN VITRO TEST SYSTEMS
Appraisal
Glyphosate, administered by oral and dermal routes, has a very
low acute toxicity. Both glyphosate and its concentrated
formulations produce moderate to severe eye irritation, but only
slight dermal irritation. Neither glyphosate nor tested formulations
induce sensitization.
Short-term feeding studies have been conducted in several
species. In CD-1 mice, increased liver, brain, heart and kidney
weights, and growth retardation were reported at 50 000 mg/kg diet.
At 10 000 mg/kg diet, an increase in relative liver weight was
reported; however, there were no differences in absolute liver
weights when this group was compared to controls. The relative
increase represented only a 9% increase over the liver weight
reported for controls and was not considered toxicologically
significant. Additionally, there were no gross or histopathological
changes in the liver at doses of 10 000 mg/kg or more. The Task
Group considered the NOAEL to be 50 000 mg/kg diet.
In a 13-week study conducted in Charles River CD
(Sprague-Dawley) BR rats, no treatment-related effects were observed
at doses up to 20 000 mg/kg diet. The NOAEL was greater than the
highest dose tested.
Two additional 13-week studies (one in rats and the other in
mice) were conducted by NTP in which lesions of the salivary glands
were observed in both species. The NOAEL in the rat study was
< 3125 mg/kg diet and that in the mouse study was 3125 mg/kg diet.
Other short-term and long-term studies conducted in different
strains and species did not reveal similar lesions. The lesions
indicate that glyphosate may be acting as a weak adrenergic agonist.
The toxicological significance of the salivary gland lesions
observed in the NTP studies is unknown.
In a 52-week study conducted in beagles, no compound-related
effects were reported. The NOAEL was 500 mg/kg body weight per day.
In a 7-day study with the Roundup formulation in female cattle, a
NOAEL of 400 mg Roundup/kg body weight was reported. At higher dose
levels, decreased feed intake and diarrhoea occurred.
In long-term feeding studies in both rats and mice, few toxic
effects were observed. These effects were present at relatively high
dose levels only. In mice, technical glyphosate produced growth
retardation, hepatocyte hypertrophy or necrosis at 30 000 mg/kg diet
only. At 5000 and 30 000 mg/kg diet an increase in epithelial
hyperplasia of the urinary bladder was reported. The increased
incidence of this lesion did not follow a dose-related trend and in
the highest dose tested the incidence was actually lower than that
reported at the medium dose level, in spite of a 6-fold increase in
glyphosate. The observation at the medium dose (5000 mg/kg) is not
considered a compound-related effect and the NOAEL is, therefore,
considered to be 5000 mg/kg diet (814 mg/kg body weight).
Long-term feeding studies in rats resulted in decreased
growth, increased liver weight and degenerative liver changes at
20 000 mg/kg diet only. At 8000 and 20 000 mg/kg diet, there was an
apparent increase in the incidence of inflammation of the gastric
mucosa in both sexes. The only statistically significant increase
was observed in the medium-dose females (15%). This value was also
outside the historical control range of 0-13%. This finding was not
considered to be a treatment-related effect. There was no
dose-related trend across all groups of treated females and there
was no statistically significant difference in any treated male
groups. The NOAEL was therefore 8000 mg/kg diet (410 mg/kg body
weight).
Studies in rats and rabbits indicated that technical
glyphosate is not teratogenic. Two multigeneration studies were
conducted with technical glyphosate. In the first study, the only
effect noted was an increased incidence of unilateral renal tubular
dilation in F3b male pups at 30 mg/kg body weight. In the second
study, decreased body weights were reported for parents and pups and
decreased litter size was associated with dose levels of
30 000 mg/kg diet. Decreased body weights reported for parents and
pups at 10 000 mg/kg diet were not toxicologically significant. In
parents, the decrease was only 2 to 4% below controls and for pups
the decrease was 5.6 to 6.6% lower than controls. The findings in
pups were also transient and did not occur consistently in all
litters. The NOAEL was 10 000 mg/kg diet. The absence of a renal
effect in pups at a higher dose level (1500 mg/kg body weight),
though not invalidating earlier findings of unilateral renal tubular
dilation in male F3b pups, indicates that the reproducibility of
this lesion and its toxicological significance are uncertain. It
should be noted that in no other toxicological study was an effect
on kidneys found.
Bioassays in mice and rats did not indicate that technical
glyphosate was carcinogenic.
Glyphosate has been shown to have no genotoxic potential in a
range of in vitro and in vivo studies.
7.1 Single exposure
Numerous acute toxicity studies have been performed to
determine LD50 values of glyphosate and of herbicide formulations
containing glyphosate as active ingredient. The results of these
studies are summarized in Tables 10 (results for glyphosate) and 11
(results for formulations). These data show that glyphosate and its
formulations have very low toxicity by the oral and dermal
administration routes. By the intraperitoneal route glyphosate is
markedly more toxic than by the other routes. General intoxication
symptoms include breathing difficulties, ataxia and convulsions.
The mechanism of the toxic action of glyphosate has been
studied in rats. Olorunsaga et al. (1979) observed dose-related
reduced respiratory control ratios and increased phosphatase
activity in mitochondria isolated from rat livers 5 h after single
intraperitoneal doses ranging from 15 to 120 mg/kg body weight. This
effect was also seen in rat liver mitochondria in vitro (Bababunmi
et al., 1979; Olorunsaga, 1982a,b). The authors suggest that acute
toxicity at lethal doses may occur as a result of the uncoupling of
oxidative phosphorylation.
The acute toxicity in rats of the surfactant
polyoxyethyleneamine, with which glyphosate is commonly formulated
in Roundup, was compared to that of glyphosate in a study by
Martinez et al. (1990). Both compounds exhibited pulmonary toxicity
following either oral or intratracheal administration. The toxicity
of the herbicide formulation was greater than can be accounted for
on the basis of the dose response data from either compound alone
(Martinez et al., 1990; Martinez & Brown, 1991).
A study was undertaken by Tai et al. (1990) to investigate the
effects of glyphosate, surfactant, and their combination in Roundup
on cardiovascular function in female beagles. They found that
glyphosate alone at plasma levels ranging from 923 to 3450 mg/litre,
which simulates the human ingestion situation, were shown to
increase the myocardial contractility. The surfactant alone
considerably reduced the cardiac output, the left ventricular stroke
work index and the mean arterial pressure. The joint effect of both
glyphosate and the surfactant in Roundup formulation resulted in
cardiac depression, which was mostly due to the surfactant since
glyphosate itself increased myocardial contractility. The authors
indicated that the probable cause of the observed increases in
pulmonary vascular resistance index and pulmonary artery pressure
was a direct vasoactive effect of glyphosate on the pulmonary
artery.
Table 10. Acute toxicity of glyphosate to experimental animals
Species (sex) Product tested LD50/LC50a Reference
Oral studies
Rat (m,f) glyphosate techn, > 5000 mg/kg bw FDRL (1988d)
purity 97.8%
Rat (m,f) glyphosate techn, > 5000 mg/kg bw Inveresk Research Int.
purity 96-99% (1989a)
Rat (m,f) glyphosate techn, > 5000 mg/kg bw NOTOX (1988)
purity 96-99%
Rat (m,f) 85.5% techn. > 5000 mg/kg bw Bio/Dynamics Inc.
glyphosate in (1988c)
water
Rat (m,f) glyphosate, IPA salt, > 5000 mg/kg bw Monsanto (1981b)
65% in water
Rat (m.f) glyphosate, EO saltb 2047 mg/kg bw Knapek et al. (1986)
Goat (f) glyphosate techn, 3500 mg/kg bw USDA (1987c)
purity 98.7%
Goat (f) glyphosate, IPA salt, 5700 mg/kg bw USDA (1987b)
65% in water
Dermal studies
Rat (m,f) glyphosate techn, > 2000 mg/kg bw Inveresk Research Int.
purity 96-99% (1989c)
Rabbit (m,f) glyphosate techn, > 5000 mg/kg bw FDRL (1988b)
purity 97.8%
Rabbit (m,f) 85.5% techn. > 5000 mg/kg bw Bio/Dynamics Inc.
glyphosate in water (1988a)
Rabbit (m,f) glyphosate, IPA salt, > 5000 mg/kg bw Monsanto (1981c)
65% in water
Intraperitoneal studies
Mouse (m) glyphosate (not 545 mg/kg bw
further specified)
Mouse (f) glyphosate (not 740 mg/kg bw FAO/WHO (1986b)
further specified)
Mouse (m,f) glyphosate (not 134 mg/kg bw FAO/WHO (1986b)
further specified)
Rat (m) glyphosate (not 281 mg/kg bw
further specified
Table 10. (cont'd)
Species (sex) Product tested LD50/LC50a Reference
Rat (f) glyphosate (not 467 mg/kg bw FAO/WHO (1986b)
further specified)
Rat (m,f) glyphosate (not 238 mg/kg bw FAO/WHO (1986b)
further specified)
a All values expressed as mg of product tested (as presented in "product tested"
column)
b EO is an abbreviation of 5-ethoxy-oleinamine salt.
Table 11. Acute toxicity of glyphosate formulations to experimental animals
Species (sex) Product testeda LD50/LC50b Reference
Oral studies
Mouse (m,f) Roundup >5000 mg/kg bw Mitsukaido Labs (1986)
Rat (m,f) Roundup 5000 mg/kg bw Bio/Dynamics Inc.
(1988e)
Rat (m,f) "Compound No. 3607" >5000 mg/kg bw Inveresk Research Int.
(1988a)
Rat (m,f) Roundup TX >5000 mg/kg bw NOTOX (1987a,b)
Rat (m,f) Alphee >5000 mg/kg bw Bio/Dynamics Inc.
(1987a)
Rat (m,f) Sting TX >5000 mg/kg bw NOTOX (1987f,g)
Rat (m,f) Sting 2510 mg/kg bw Younger Labs Inc.
(1984)
Rat (m,f) Sting 1950 mg/kg bw Bio/Dynamics Inc.
(1984b)
Rat (m,f) MON 8780 >5000 mg/kg bw Bio/Dynamics Inc.
(1985a)
Rat (m,f) Agrichem Glyfosaat B >5000 mg/kg bw NOTOX (1990e)
Rat (m,f) "Glyfosaat 360 g/litre" >2000 mg/kg bw NOTOX (1989c)
Rat (m,f) Legend >2000 mg/kg bw CIT (1991a)
Goat (f) Roundup 4860 mg/kg bw USDA (1983)
Table 11. (cont'd)
Species (sex) Product testeda LD50/LC50b Reference
Dermal studies
Rat (m,f) "Compound No. 3607" >2000 mg/kg bw Inveresk Research Int.
(1988b)
Rat (m,f) Roundup TX >4000 mg/kg bw NOTOX (1987c)
Rat (m,f) Sting TX >4000 mg/kg bw NOTOX (1987h)
Rat (m,f) MON 8780 >5000 mg/kg bw Bio/Dynamics Inc.
(1985b)
Rat (m,f) Agrichem Glyfosaat >4000 mg/kg bw NOTOX (1990f)
B or 2
Rat (m,f) "Glyfosaat 360 g/litre" >2000 mg/kg bw NOTOX (1989b)
Rat (m,f) Legend >2000 mg/kg bw CIT (1991b)
Rabbit (m,f) Roundup >5000 mg/kg bw Bio/Dynamics Inc.
(1988f)
Rabbit (m,f) Alphee >5000 mg/kg bw Bio/Dynamics Inc.
(1987b)
Rabbit (m,f) Sting >5000 mg/kg bw Bio/Dynamics Inc.
(1984a)
Inhalation studies
Rat (m,f) Roundup (aerosol) 3180 mg/m3 Monsanto (1983d)
Rat (m,f) "Compound No. 3607" >4860 mg/m3 Inveresk Research Int.
(1989d)
Rat (m,f) Alphee >8900 mg/m3 Monsanto (1987b)
a Composition of the formulations is given in Table 2, with the exceptions of
Agrichem Glyphosaat B (or 2), "Glyfosaat 360 g/litre" and "Compound No. 3607",
which all contain approximately 480 g/litre of the isopropylamine salt, and of MON
8780 (32.8% isopropylamine salt), and Legend (40% isopropylamine salt).
b All values given as mg formulation.
7.2 Short-term exposure
7.2.1 Oral studies
In CD-1 mice, a 13-week feeding study was conducted with
technical glyphosate (purity 98.7%) using dose levels of 5000,
10 000 and 50 000 mg/kg diet (equal to 940, 1890 and 9710 mg/kg body
weight per day in males and 1530, 2730 and 14 860 mg/kg body weight
per day in females). No effect on appearance or survival was
observed. Growth retardation and increased weights of brain, heart
and kidneys were observed at 50 000 mg/kg. Liver weights were
increased at 50 000 and 10 000 mg/kg. Limited histopathology showed
no adverse effects. The authors of the study concluded that the
NOAEL was 10 000 mg/kg diet (1890 mg/kg body weight per day)
(Bio/Dynamics Inc., 1979).
In a 13-week feeding study with technical glyphosate,
Sprague-Dawley rats received 1000, 5000 or 20 000 mg/kg diet (equal
to 63, 317 and 1267 mg/kg body weight per day in males and 84, 404
and 1623 mg/kg body weight per day in females). No effect on
appearance, survival or growth occurred. Haematology, blood
biochemistry and urinalysis, carried out at test end only, were also
unaffected. Organ weights (determined for liver, kidneys and testes
only) were not affected. Limited histopathology showed no adverse
effect in any tissue. The NOAEL in this study was 20 000 mg/kg diet
(1267 mg/kg body weight per day) (Monsanto, 1987a). Absence of
toxicity was also found in another 13-week feeding study on rats
using technical glyphosate and dose levels of 200 to 12 500 mg/kg
diet (Tauchi, 1979 as cited by FAO/WHO, 1986b).
Two further 13-week studies in rodents were conducted on behalf
of the US National Toxicology Program (1992). Both rats (F-344/N)
and mice (B6C3F1) were administered glyphosate (purity
approximately 99%) in feed at levels of 3125, 6250, 12 500, 25 000
and 50 000 mg/kg diet.
In rats, reduced weight gains were observed at 25 000 mg/kg
diet (males only) and at 50 000 mg/kg diet (males and females). No
changes in feed consumption were found. Minor increases in the
relative organ weight of liver, kidney and testes, and decreased
thymus weight were observed in males only at several dose levels;
these changes did not show a clear dose relation and therefore are
not considered to be compound-related effects. Small increases in
haematocrit and red cell blood counts were observed in male rats at
> 12 500 mg/kg diet. Clinical chemistry showed increased alkaline
phosphatase (AP) and alanine aminotransferase (ALAT) at >
6250 mg/kg diet in males and at > 12 500 mg/kg diet in females.
Bile acid levels in blood were increased at 25 000 mg/kg diet (males
only) and at 50 000 mg/kg diet (males and females). Decreases in
sperm count were observed in males at > 25 000 mg/kg diet. The
only histopathological lesions found were cytoplasmic alterations of
the parotid and submandibular salivary glands, consisting of
basophilic changes and hypertrophy of acinar cells. The parotid
gland was more severely affected. The magnitude of the effect was
dose-dependent, with focal lesions in less severe cases to diffuse
involvement at higher doses. Lesions of a similar nature and
magnitude were observed in both sexes. The sublingual gland was not
detectably altered. Effects on the salivary glands were observed
already at the lowest dose level tested of 3125 mg/kg diet (equal to
205 mg/kg body weight per day for males and 213 mg/kg body weight
per day for females). Thus, this study did not yield a NOAEL (NOAEL
< 3125 mg/kg diet) (NTP, 1992).
In mice, reduced weight gains were observed at 50 000 mg/kg
diet in both sexes. Increased organ weights were noted in heart,
kidney, liver, thymus, lung and testis; these changes did not show a
clear dose relation and therefore are not considered to be
compound-related effects. Feed consumption levels were not changed
significantly. Lesions in the parotid gland were observed, similar
to those observed in rats. The sublingual gland and the
submandibular salivary glands were not detectably altered. The
effects on the parotid gland were observed in mice at 6250 mg/kg
diet (equal to 1065 mg/kg body weight per day for males and
1411 mg/kg body weight per day for females), but were not seen at
the lowest dose level tested of 3125 mg/kg diet (equal to 507 mg/kg
body weight per day for males and 753 mg/kg body weight per day for
females). The NOAEL in this study was 3125 mg/kg diet (507 mg/kg
body weight per day) (NTP, 1992).
The salivary gland lesions could also be induced in rats by
14-day exposure at feed levels of 50 000 mg/kg diet. The salivary
glands lesions induced by glyphosate were similar to those which
could be induced by exposure to high subcutaneous doses of the
ß-adrenergic agonist isoproterenol and could be partially
ameliorated with the ß-adrenergic antagonist propanolol. This
indicates that glyphosate may induce the salivary gland lesions by
acting as a weak adrenergic agonist (NTP, 1992).
The short-term toxicity of technical glyphosate was also
studied in dogs. Beagle dogs received technical glyphosate in
gelatin capsules at dose levels of 0, 20, 100 or 500 mg/kg body
weight per day for 52 weeks. No effect occurred with respect to
clinical signs, body weight, feed consumption, ophthalmoscopy,
haematology, blood biochemistry, urinalysis, gross pathology and
histopathology. The only changes in treated groups relative to
controls were increased pituitary weights (absolute and relative) in
the medium- and high-dose males. Because there were no concomitant
histological changes present in pituitaries and given the absence of
an effect on this organ (and related organs) in all other studies,
the toxicological significance of the increased pituitary weights is
questionable. The NOAEL in this study was 500 mg/kg body weight per
day (Monsanto, 1985).
A 7-day oral study was carried out with Roundup in female
cattle weighing 160 to 272 kg. Brahman-cross heifers received 400,
500, 630 or 790 mg Roundup/kg body weight per day by nasogastric
tube. At 790 mg/kg, 1/3 animals died before test end, showing
laboured breathing and pneumonia caused by aspiration of rumen
contents. Decreased feed intake was seen at 630 and 790 mg/kg;
diarrhoea occurred at 500, 630 and 790 mg/kg body weight. Slight
increases in a number of blood parameters, occurring at 790 mg/kg
only, were probably due to extracellular fluid shifts and
haemoconcentration secondary to diarrhoea. The NOAEL in this study
was 400 mg Roundup/kg body weight per day (USDA, 1987a).
7.2.2 Dermal studies
Short-term dermal toxicity studies were carried out in rabbits
with technical glyphosate and the formulation Roundup.
Technical glyphosate, moistened with saline, was applied under
occlusion at dose levels of 100, 1000 and 5000 mg/kg body weight per
day to the shaven intact or abraded skin of rabbits for 6 h/day, 5
days/week for 3 weeks. No effect on survival and growth occurred.
Slight dermal irritation (barely perceptible erythema and oedema)
was observed at 5000 mg/kg body weight only. No evidence of systemic
toxicity was found (parameters: haematology and blood biochemistry
in five animals of each sex per group, organ weights, gross
pathology, limited histopathology) (IRDC, 1982). Absence of systemic
effects was also found in the somewhat more limited 21-day study in
rabbits (no haematology and blood biochemistry) with Roundup. Skin
effects were more severe in this study: at both dose levels (76 and
114 mg/kg body weight per day, undiluted formulation applied)
erythema and oedema were seen and, in addition, exudate and
fissuring occurred at the abraded skin sites. After a 4-week
recovery period the skin effects were no longer present
(Bio/Dynamics Inc., 1975).
7.2.3 Inhalational studies
A 4-week inhalation study was carried out on rats with a 1:3
dilution of Roundup formulation. Test concentrations of 50, 160 and
360 mg/m3 of the diluted formulation (equivalent to 17, 53 and
120 mg Roundup/m3) were administered as an aerosol spray for
6 h/day, 5 days/week. The mass median aerodynamic diameter of the
test material ranged from 1.8 to 2.7 µm with geometric standard
deviations between 1.7 and 2.0. An increased incidence of irritation
of the nasal turbinates (subacute inflammation), trachea
(mononuclear cell infiltration) and lungs (perivascular lymphoid
infiltrates/aggregates) was observed among the high-dose females
only. No signs of systemic toxicity were found (parameters:
survival, growth, limited haematology and blood biochemistry, organ
weights, limited histopathology) (Monsanto, 1983e).
7.3 Long-term toxicity and carcinogenicity
Only oral studies are available. Dietary studies using
technical glyphosate were performed in mice and rats.
In Charles River CD-1 mice technical glyphosate was fed in the
diet at concentrations of 0, 1000, 5000 or 30 000 mg/kg diet for 24
months (dose levels equal to 0, 157, 814 and 4841 mg/kg body weight
per day for males and 0, 190, 955 and 5874 mg/kg body weight per day
for females). No effect on survival or appearance was noted. Body
weights were decreased in the males of the high-dose group.
Haematology and organ weights showed no effects. Histopathology in
liver revealed an increased incidence of central lobular hepatocyte
hypertrophy among high-dose males (incidences: 9/49, 5/50, 3/50 and
17/50 in control, low-dose, medium-dose and high-dose males,
respectively) and an increased incidence of central lobular
hepatocyte necrosis also among high-dose males (incidences: 0/49,
2/50, 2/50 and 10/50). Increased incidences of epithelial
hyperplasia in the urinary bladder were present in the medium-dose
and high-dose males (incidences: 3/49, 3/50, 10/50 and 8/50). There
were no statistically significant increases in the frequency of
neoplastic lesions. The NOAEL in this study was 5000 mg/kg diet
(814 mg/kg body weight per day) (Bio/Dynamics Inc., 1983a).
Two chronic feeding studies on rats were conducted with
technical glyphosate, one in 1979-1981 and the other in 1988-1990.
The first study, carried out using Charles River CD (Sprague-Dawley)
BR rats, had dose levels of 0, 60, 200 and 600 mg technical
glyphosate/kg diet (equal to about 0, 3, 10 and 32 mg/kg body weight
per day, respectively). Survival, appearance, haematology, blood
biochemistry, urinalysis and organ weights were not changed. The
NOAEL was > 600 mg/kg diet (> 32 mg/kg body weight per day).
Slight growth retardation during part of the study was noted in the
high-dose males. The incidence of interstitial cell tumours in
testes showed a statistically significant increase (incidences:
0/50, 3/50, 1/50 and 6/50; historical control range: 3-7%)
(Bio/Dynamics Inc., 1981a). This finding, in itself constituting
some evidence for a carcinogenic effect in rats, should be judged in
the light of the absence of an effect at much higher dose levels in
the more recent 2-year study in rats (see below). This is also valid
for the slight growth retardation (i.e. no effect on growth at much
higher dose levels in the more recent study, see below). In the
recent 2-year study, rats (same strain) were fed 2000, 8000 or 20
000 mg technical glyphosate/kg diet (equal to about 100, 410 and
1060 mg/kg body weight per day) for 24 months. There was no effect
on survival or appearance. Growth was retarded in the high-dose
females. Haematology and blood biochemistry showed no effects. In
the high-dose males, the urine specific gravity (after 6 months
only) and urine pH were increased. A statistically significant
increased incidence of degenerative lens changes (basophilic
degeneration of the posterior subcapsular lens capsule or mature
cataracts) was found among the high-dose males (incidences 3/60,
4/60, 4/60 and 8/60 in the control, low-, medium- and high-dose
groups, respectively. However this finding was within the historical
control range of 0-33%. Liver weights were increased in the
high-dose males only. Histopathology showed an increased incidence
Table 12. Skin irritation tests on rabbits with glyphosate and its formulations
Product testeda Contact time (h) Draize scoreb Classificationc Reference
Glyposate
Glyphosate techn. 85% in water 4 0.8 slightly irritating Bio/Dynamics Inc. (1988b)
Glyphosate techn., moistened powder 4 0 not irritating FDRL (1988a)
Glyphosate techn., moistened powder 4 0 not irritating Inveresk Research Int. (1989e)
Glyphosate, IPA salt 65% in water 24 0.2 not irritating Monsanto (1981d)
Formulations
Roundup, undiluted 4 1.9 slightly irritating Bio/Dynamics Inc. (1988g)
"Compound No. 3607", undiluted 4 1.2 slightly irritating Inveresk Research Int. (1988c)
Roundup TX, undiluted 4 0.7 slightly irritating NOTOX (1987d)
Alphee, undiluted 4 1.0 slightly irritating Bio/Dynamics Inc. (1987c)
Sting, undiluted 4 1.3 slightly irritating Bio/Dynamics Inc. (1984c)
Sting TX, undiluted 4 3.6 moderately irritating NOTOX (1987i)
Roundup L&G, undiluted 4 1.0 slightly irritating Bio/Dynamics Inc. (1985c)
"Glyfosaat 360 g/litre, undiluted 4 0.3 not irritating NOTOX (1989d)
Agrichem Glyfosaat B, undiluted 4 1.7 slightly irritating NOTOX (1990d)
Agrichem Glyfosaat 2, undiluted 4 2.0 slightly irritating NOTOX (1990b)
Legend, undiluted 4 0.7 slightly irritating CIT (1991c)
a For glyphosate content of the tested formulations, see footnote a in Table 11.
b This score is the mean score per animal and was calculated using the data from
the original report. The standard Draize scoring system was used; in calculating
the mean response per animal, the maximum response observed for an animal was used.
c The classification is based on the mean Draize score per animal. Specification:
score 0-0.5 not irritating; 0.6-2.0 slightly irritating; 2.1-5.0 moderately
irritating; 5.1-8.0 severely irritating.
Table 13. Results of eye irritation tests in rabbits for glyphosate and its formulations.
Product testeda Irritation indexb Classificationc Reference
Glyphosate
Glyphosate techn. 85% in water, undiluted 45 strongly irritating Bio/Dynamics Inc. (1988d)
Glyphosate, IPA salt, 65% in water 0 not irritating Monsanto (1981e)
Glyphosate techn. (97.6%), undiluted 54 strongly irritating FDRL (1988c)
Glyphosate techn. (96-99%), undiluted 29 irritating Inveresk Research Int. (1989f)
Formulations
Roundup, undiluted 54 strongly irritating Bio/Dynamics Inc. (1990)
"Compound No. 3607", undiluted 13 irritating Inveresk Research Int. (1989g)
Roundup TX, undiluted 31 strongly irritating NOTOX (1987e)
Alphee, undiluted 6 slightly irritating Bio/Dynamics Inc. (1987d)
Sting, undiluted 104 extremely irritating Bio/Dynamics Inc. (1984d)
Sting TX, undiluted 19 irritating NOTOX (1987j)
Roundup L&G, undiluted 19 irritating Bio/Dynamics Inc. (1985d)
"Glyfosaat 360 g/litre", undiluted 33 strongly irritating NOTOX (1989a)
Agrichem Glyfosaat B, undiluted 58 strongly irritating NOTOX (1990c)
Agrichem Glyfosaat 2, undiluted 22 irritating NOTOX (1990a)
Legend, undiluted 21 irritating CIT (1991d)
Table 13 (continued)
a For glyphosate content of the tested formulations, see footnote a in Table 11.
b The irritation index represents a mean total score per animal for response in cornea, conjunctiva
and iris, using the standard Draize scoring system. The irritation index was calculated using the data
from the original report; in calculating the mean response per animal the maximum response observed
for an animal was used. The calculation procedure is as follows:
- score conjunctiva: A. chemosis: 0-4 Calculation partial
B. discharge: 0-3 index for conjunctiva:
C. erythema: 0-3 2 x (A + B + C) = 0-20
- score iris: 0-2 Calculation partial index for iris: 5 x (0-2) = 0-10
- score cornea: A. opacity: 0-4 Calculation partial index for cornea:
B. area: 0-4 5 x A x B = 0-80
The total irritation index is the sum of the partial indices (0-110).
c The classification is based on the following scheme: index 0-5 not irritating; 5.1-12 slightly
irritating; 12.1-30 irritating; 30.1-60 strongly irritating; 60.1-80 severely irritating; 80.1-110
extremely irritating.
of inflammation of the gastric squamous mucosa in the medium- and
high-dose groups (incidences in males: 2/58, 3/58, 5/59 and 7/59;
females: 0/59, 3/60, 9/60, and 6/59; historical range: 0-13.3%). The
incidence of pancreatic islet cell adenomas was increased
(statistically significant) among low- and high-dose males
(incidences: 1/58, 8/57, 5/60 and 7/59; historical control range of
test laboratory 1.8-8.5%). The incidence in the control group was
below the historical control range; the trend test for the observed
increase was negative. No pancreatic carcinomas were found. The
NOAEL in this study was 8000 mg/kg diet (410 mg/kg body weight per
day) (Monsanto, 1990b).
Glyphosate has been tested in the US National Toxicology
Program; pre-chronic studies have been completed (NTP, 1992).
7.4 Skin and eye irritation; sensitization
Many studies have been carried out with rabbits to examine the
potential of glyphosate and its formulations to produce skin and eye
irritation. The results of these studies are briefly summarized in
Tables 12 (skin irritation) and 13 (eye irritation). Glyphosate and
its formulations produce only mild skin irritation after undiluted
single application. The result of the 21-day dermal study in rabbits
with the formulation Roundup (Bio/Dynamics Inc., 1975; see
subsection 7.2.2) shows that repeated application of undiluted
formulation to the skin does lead to irritation. The eye-irritating
potential is considerable for undiluted glyphosate and, with a few
exceptions, also for the formulations. Single application generally
produces moderate to severe reactions.
Sensitization studies have been carried out in guinea-pigs with
glyphosate and its formulations. The results of these tests are
summarized in Table 14. Neither glyphosate itself nor the tested
formulations induced sensitization in any experiment.
The results of two dermal irritation studies and one dermal
sensitization study performed with human volunteers exposed to
Roundup are presented in subsection 8.1.
Table 14. Results of sensitization tests in guinea-pigs for glyphosate and its
formulations
Product testeda Method Result Reference
Glyphosate
Glyphosate Buehler negative Bio/Dynamics Inc.
(purity 99.7%) (1983c)
Glyphosate technical Magnusson & negative Safefarm Labs Inc.
Kligmanb (1991b)
Glyphosate techn. Magnusson & negative Inveresk Research
(96-99%) Kligman Int. (1989b)
Formulations
Roundup Buehler negative Bio/Dynamics Inc.
(1983b)
"Compound Magnusson & negative Inveresk Research
No. 3607" Kligman Int. (1988d)
Roundup L&G Buehler negative Bio/Dynamics Inc.
(1987e)
Legend Buehler negative Safefarm Labs Inc.
(1991a)
Sting Buehler negative Bio/Dynamics Inc.
(1986)
a For glyphosate content of the tested formulations, see footnote a
in Table 11.
b Method also called the maximization test.
7.5 Reproductive toxicity, embryotoxicity and teratogenicity
Technical glyphosate has been tested for teratogenicity in rats
and rabbits using the oral route. In addition, two oral
multi-generation reproduction studies in rats have been reported.
7.5.1 Teratogenicity studies
Glyphosate (technical) was given to pregnant Charles River COBS
CD rats by gavage at dose levels of 0, 300, 1000 and 3500 mg/kg body
weight per day on days 6-19 of gestation. At 3500 mg/kg the
following effects were observed: increased incidences of soft
stools, diarrhoea, breathing rattles, red nasal discharge and
reduced activity, increased mortality (6/25 dams dying before the
end of the treatment period), growth retardation, increased
incidence of early resorptions, decreases in total number of
implantations and the number of viable fetuses, increased number of
fetuses with reduced ossification of sternebrae. At the lower dose
levels these effects were absent. The NOAEL in this study was
1000 mg/kg body weight per day (IRDC, 1980b).
In Dutch belted rabbits technical glyphosate was tested at dose
levels of 0, 75, 175 and 350 mg/kg body weight per day
(administration by gavage) from days 6-27 of gestation. In dams the
incidences of diarrhoea and soft stools were increased in the
high-dose group and, to a slight degree, also in the medium-dose
group. The incidence of nasal discharge was increased in the
high-dose group only. In the medium- and high-dose groups 2 and 10
dams, respectively, died during the study from unknown causes. The
NOAEL in this study was 175 mg/kg body weight per day (IRDC, 1980c).
7.5.2 Reproduction studies
A three-generation feeding study in Charles River CD
(Sprague-Dawley) BR rats was conducted in 1980-1981 and a
two-generation study, using higher dose levels, was completed in
1990 with technical glyphosate. In the former study, dietary feeding
levels were continuously adjusted to achieve dose levels of 0, 3, 10
and 30 mg/kg body weight per day. The only effect noted was an
increased incidence of unilateral renal tubular dilation in the male
pups (randomly selected) of the F3b mating of the high-dose group
(incidence 6/10 versus 0/10 in controls, not determined in
intermediate groups, earlier litters not examined). The NOAEL in
this study was < 30 mg/kg body weight (Bio/Dynamics Inc., 1981b).
The more recent two-generation study had dose levels of 0, 2000,
10 000 and 30 000 mg technical glyphosate/kg diet (equivalent to 0,
100, 500 and 1500 mg/kg body weight per day). In the high-dose
group, the following effects were observed: soft stools and
decreased body weights in parent animals, slightly decreased litter
size and decreased pup weights (the latter seen at day 14 or 21 of
lactation). The decreased body weights of parents and pups were seen
to a slight degree in the medium-dose group also. No histological
effect on kidneys was present in the F2b male pups (15 and 23 pups
examined in control and high-dose groups, respectively; first
generation and F2a pups not examined). The NOAEL in this study was
10 000 mg/kg diet (500 mg/kg body weight per day) (Monsanto, 1990c).
With regard to these reproduction studies using technical
glyphosate, it should be noted that in both studies the number of
pups submitted to histopathological examination was limited. These
limitations make evaluation of the renal effect in pups, seen (at
30 mg/kg body weight) in the study of Bio/Dynamics Inc. (1981b),
difficult.
7.6 Mutagenicity and related end-points
The results of several studies are summarized in Table 15. The
results show that glyphosate is not mutagenic.
Table 15. Results of mutagenicity studies with glyphosate and its salts.
Test system Test compound and Resultc Reference
concentrations
Ames test, Salmonella technical glyphosate - Monsanto
typhimurium TA98, TA100, (98.4%); 0.1-1000 (1978c)
TA1535 & TA1537, with and µg/plate
without metabolic activation
Ames test, S. typhimurium technical glyphosate - IET (1978)
TA98, TA100, TA1535 & (98.4%); 10-5000
TA1537, with and without µg/plate
activation
Rec assay, Bacillus subtilis, technical glyphosate - IET (1978)
strain H17 (rec+) & M45 (98.4%); 20-2000
(rec-), without activation µg/disc
Reverse mutation assay in technical glyphosate - IET (1978)
Escherichia coli strain WP2hcr, (98.4%); 10-5000
with and without activation µg/plate
Forward mutation assay, technical glyphosate - Monsanto
CHO cells, in vitro (98.7%); 0-20 mg/ml (1983b)
(HGPRT-locus), with & (- activation) or
without activation 5-25 mg/ml (+ activation)
Table 15. (cont'd)
Test system Test compound and Resultc Reference
concentrations
Cytogenetic study technical glyphosate - Monsanto
(chromosome aberrations) (98.7%); 200-1000 mg/kg (1983f)
in rat bone marrow, body weight, i.p.b;
in vivo sampling after 6, 12
and 24 h
Micronucleus test in glyphosate (not - Benova
erythrocytes of mice, specified); ´ LD50, oral; et al.
in vivo sampling time not (1989)
specified
Dominant lethal test, mouse technical glyphosate - IRDC
in vivo (98.7%); 200-2000 mg/kg (1980a)
body weight, oral
Recessive sex-linked lethal glyphosate (not - Gopalan &
test, Drosophila melanogaster, specified); dose not Njagi
in vivo given (1981)
Unscheduled DNA repair assay technical glyphosate - Monsanto
rat hepatocytes, in vitro (98.7%); 0.0125-125 (1983c)
µg/ml
a No higher concentrations tested because these would result in
osmolalities much higher than physiological levels; these high
osmolalities can produce non-specific chromosomal aberrations or
sister chromatid exchange.
b In additional studies it was demonstrated that: (1) glyphosate
produced no effect on viability and mitotic index of bone marrow
cells of rats after i.p. doses of 200-1000 mg/kg body weight
(Monsanto, 1983g); and (2) after giving 14C-labelled glyphosate
i.p. significant concentrations of 14C reached the bone marrow
(peak levels reached after 0.5 h remaining virtually constant up to
10 h after dosing) (Monsanto, 1983h).
c - = negative result
8. EFFECTS ON HUMANS
Appraisal
The formulation Roundup containing glyphosate is acutely toxic
to humans when ingested intentionally or accidentally.
No controlled studies have been conducted, and therefore the
human NOAEL level cannot not be derived.
No data are available to show the impact on workers exposed
during the manufacture or formulation of glyphosate. No
compound-related effects were observed in a test group of five
applicators prior to and after exposure for one week.
The reported higher susceptibility of individuals older than
40 years to ingested Roundup intoxication is important and requires
further investigation.
8.1 Cases of intentional and accidental exposure
Many cases of acute intoxication with herbicides containing
glyphosate and surfactant (Roundup) have been reported; most of
these were suicide attempts. Talbot et al. (1991) reviewed 93 cases
of exposure to Roundup (Chinese names: lan-da, hao-ni-chun,
nian-nian-chun) in Taiwan. The classification of the severity of
acute poisoning with Roundup as given by these authors is presented
in Table 16. Severe effects occurred only in the cases of
intentional ingestion (80 of the 93 reported). Accidental exposures
led to only mild effects. The typical symptoms were erosion of the
gastrointestinal tract (66% of the self-poisonings), seen as sore
throat, dysphagia and gastrointestinal haemorrhage. Other organs
were affected less often (nonspecific leucocytosis 65%, lungs 23%,
liver 19%, cardiovascular system 18%, kidney 14% and CNS 12%). Death
(in 7/80 cases) occurred within hours after ingestion. The amount of
undiluted Roundup ingested (rough estimates) in the lethal cases
varied from 85 to 200 ml (corresponding to roughly 30 to 70 g
glyphosate acid); but much larger amounts (500 ml Roundup,
corresponding to 180 g glyphosate acid) were reported to have been
ingested by some patients with mild to moderate symptoms. Overall,
moderate symptoms were associated with estimated intakes of 20 to
500 ml, mild symptoms with 5 to 150 ml, no symptoms with 5 to 50 ml.
The authors pointed out that the patient's estimates of the amount
ingested, and the conversion ratio used in their paper may be
inaccurate (Talbot et al., 1991). Other reviews of cases of
intoxication with Roundup have reported similar findings (Sawada &
Nagai, 1987; Tominack et al., 1991). The data of Tominack et al.
(1991) suggested that people over 40 years of age who ingest amounts
greater than 150 ml Roundup are at greatest risk of a fatal outcome.
These authors also pointed out that the surfactant contained in
Roundup may be responsible for the clinical syndrome (as suggested
by Sawada & Nagai, 1987), but that the available evidence on this
point is, as yet, inconclusive.
Table 16. Classification of severity of acute poisoning with Roundupa
Classification Description
Asymptomatic no complaints and no abnormalities on physical or laboratory
examination.
Mild mainly gastrointestinal tract(GIT) symptoms (nausea, vomiting,
diarrhoea, abdominal pain, mouth and throat pain) that resolved
within 24 h. Vital signs were stable, and there was no renal,
pulmonary or cardiovascular involvement.
Moderate GIT symptoms lasting longer than 24 h, GIT haemorrhages, endoscopically
verified oesophagitis or gastritis, oral ulceration, hypotension
responsive to intravenous fluids, pulmonary dysfunction not
requiring intubation, acid-base disturbance, evidence of transient
hepatic or renal damage, or temporary oliguria.
Severe pulmonary dysfunction requiring intubation, renal failure requiring
dialysis, hypotension requiring treatment with pressor amines, cardiac
arrest, coma, repeated seizures, or death.
a From: Talbot et al. (1991)
Further clinical experiences with patients exposed to Roundup
either accidentally or through deliberate ingestion have been
reported by Temple & Smith (1992). Symptoms resulting from dermal
exposure incidental to the use of the product included periorbital
oedema and chemosis of the eye, cardiovascular effects (tachycardia
and elevated blood pressure), swelling and paraesthesia at the site
of dermal contact and prolonged skin irritation. Deliberate
ingestion resulted in more severe effects, including lethality from
apparent respiratory and cardiac arrest (Temple & Smith, 1992).
Two dermal irritation studies were carried out with volunteers.
Application of 0.9 ml of a 9:1 dilution of Roundup formulation in
water to the intact skin of the upper arm for 24 h produced no skin
changes (Shelanski, 1973). Maibach (1986) tested undiluted Roundup
(application of 0.1 ml to intact and abraded skin sites on the back
for 24 h) and found erythema in only 1/24 subjects (23/24 no
reaction) for the intact skin sites; for the abraded skin sites 4/24
subjects showed an equivocal reaction and 10/24 showed erythema
(10/24 no reaction). The same author reported very briefly the
absence of effect in a photoirritation study in humans using
undiluted Roundup as test compound (application to abraded skin of
upper arm for 24 h with irradiation with UVA light for 45 min)
(Maibach, 1986).
A sensitization study was performed in 204 human volunteers
with undiluted Roundup according to a modified Draize method. The
summary report (no detailed report available) stated that there was
no effect in any subject (Maibach, 1986). The same author reported
absence of photosensitization by Roundup in volunteers.
8.2 Occupational exposure
The results of several studies focused primarily on the
determination of the extent of exposure to glyphosate when the
compound is used as herbicide are presented in section 5.3. The
study of Jauhainen et al. (1991) included health examinations of a
test group of five workers prior to and after an exposure period of
1 week. These examinations included haematology, clinical chemistry,
ECG, pulmonary function tests, an interview for a health
questionnaire and a general clinical examination (including
recording of blood pressure, pulse rate and pressure craft of
hands). A control group consisted of five workers. No
compound-related effects were observed (Jauhainen et al., 1991). The
other studies described in section 5.3 did not include a health
evaluation of workers.
8.3 Subpopulations at special risk
The only information available on this point is some suggestive
evidence referring to oral intoxications with Roundup; Tominack
et al. (1991) suggested that people older than 40 years are at
greater-than-normal risk after ingestion of Roundup.
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND
FIELD
In this chapter, concentrations or doses of formulations with
glyphosate are always expressed as mg product per kg soil.
Therefore, these figures may have been recalculated from the
original data, in cases where the authors reported the data in, for
instance, mg a.i./litre instead of mg Roundup per litre. For
recalculation, the percentages of the active ingredient (free acid
or the salt), given in Table 2, were used.
9.1 Laboratory experiments
9.1.1 Microorganisms
9.1.1.1 Water
Appraisal
The prokaryotic cyanobacteria are generally more sensitive to
the effects of glyphosate than the eukaryotic true algae. Similar
enzyme systems are inhibited in microorganisms to those thought to
be responsible for the herbicidal properties of glyphosate in higher
plants. The single semi-field study suggests very variable results
but with no significant effect on populations or community
structure.
The acute and chronic toxicities of technical grade glyphosate
and its formulations to cyanobacteria, algae and diatoms are
summarized in Table 17. Technical grade glyphosate is slightly toxic
with 3- to 4-day EC50 values of 1.2-7.8 mg/litre, and 7-day NOEC
values of 0.3-34 mg/litre. Formulations of glyphosate may be more
toxic (3-day EC50 values of 1.0 to > 55 mg product/litre).
Toxicity to cyanobacteria and algae is dependent on the species
or strain tested. Wängberg & Blanck (1988) exposed 16 species in
pure cultures to Roundup for 14 days. The concentration at which
growth was inhibited completely was 16 mg Roundup/litre for the most
sensitive species (Raphidonema longiseta and Anabaena sp.) and
131 mg Roundup/litre for the least sensitive species (Selenastrum
capricornutum). The prokaryotic Cyanophyta were significantly more
affected by Roundup than the eukaryotic Chlorococcales.
In Pseudomonas chlororaphis Roundup severely inhibited
respiration at concentrations of > 2623 mg/litre, whereas in
Aeromonas hydrophila respiration was only slightly affected at
these concentrations (Chan & Leung, 1986). The bacteria were exposed
for 6 days.
Table 17. Aquatic microorganisms: acute and chronic toxicity of glyphosate and its formulations (EC50, NOEC)
Organism A Test Compound Test pH Hardness Temperature Experimental Parametera Concentration Reference
type water (mg CaCO3/ (°C) duration (mg/litre)
litre) (days)
Cyanobacteria
Anabaena A S Tgg a.m. 7.5 285 24 7 NOEC 9.7b,c Malcolm
flos-aquae Pirnie
Inc.
(1987a)
Green algae
Selenastrum - S Tgg a.m. 7.0 n.r. 26 3.5-4 EC50 7.8b,e Bozeman
capricornutum et al.
(1989)
S. capricornutum A S Tgg a.m. 7.5 285 24 7 NOEC 20b,c Malcolm
Pirnie
Inc.
(1987d)
S. capricornutum - S St a.m. 7.7-9.0 26 23 3 EC50 1.0b LISEC
(1989b)
S. capricornutum - S St a.m. 7.7-9.0 26 23 3 EC50 2.5d LISEC
(1989b)
S. capricornutum - S St a.m. 7.7-9.0 26 23 3 NOEC 0.2b LISEC
(1989b)
S. capricornutum - S Ru a.m. 7.7-10.0 26 24 3 EC50 2.1b LISEC
1989a)
S. capricornutum - S Ru a.m. 7.7-10.0 26 24 3 EC50 8.0d LISEC
(1989a)
S. capricornutum - S Ru a.m. 7.7-10.0 26 24 3 NOEC 0.7b LISEC
(1989a)
Table 17 (contd).
Organism A Test Compound Test pH Hardness Temperature Experimental Parametera Concentration Reference
type water (mg CaCO3/ (°C) duration (mg/litre)
litre) (days)
Chlorella - S Ru a.m. 7.5 n.r. 25 2.1-7 EC50 > 55b Hernando
pyrenoidosa et al.
(1989)
C. pyrenoidosa - S Ru a.m. 7.5 n.r. 25 2.1-7 NOEC < 55b Hernando
et al.
(1989)
Diatoms
Skeletonema - S Tgg a.m.h 8.2-8.5 n.r. 20 4 EC50 1.2g E G &
costatum Bionomics
(1978a)
S. costatum - S Tgg a.m.h 8.2-8.5 n.r. 20 4 EC50 1.3b E G &
Bionomics
(1978a)
S. costatum - S Tgg a.m.h 8.2-8.5 n.r. 20 4 NOEC < 0.6f E G &
Bionomics
(1978a)
S. costatum A S Tgg a.m.h 7.5 285 20 7 NOEC 0.3b,c Malcolm
Pirnie
Inc.
(1987b)
Navicula A S Tgg a.m. 7.5 285 20 7 NOEC 34b,c Malcolm
pelliculosa Pirnie
Inc.
(1987c)
Table 17 (continued)
a concentration of a formulation is expressed as mg of the formulation per litre
b based on biomass decrease
c based on actual concentrations
d based on inhibition of growth rate
e NOEC value not reported
f based on biomass and chlorophyll a decrease
g based on chlorophyll a decrease
h salinity was 30%
A = actual concentrations are measured; - = nominal concentrations; S = static system;
Tgg = technical grade glyphosate; Ru = Roundup;
St = Sting; a.m. = artificial medium; n.r. = not reported
Chan & Leung (1986) found that the activity of
5-enolpyruvyl-shikimic acid-3-phosphate synthase was inhibited
strongly in aquatic bacteria at the lowest tested concentration of
656 mg Roundup/litre. This enzyme takes part in the biosynthetic
sequence to phenylalanine, tryptophan, and tyrosine via the
conversion of shikimate to chorismate, and the conversion of
chorismate to anthralinate. In an in vitro experiment with
cell-free extracts of Aerobacter aerogenes, technical grade
glyphosate inhibited the conversion of shikimate to chorismate at
concentrations of > 0.2 mg a.i./litre (Amrhein et al., 1980).
In Chlorella pyrenoidosa Roundup affected the growth, the
greening process and the photosynthetic metabolism at the lowest
tested concentration of 55 mg/litre when exposed for 2.1 or 7 days
(Hernando et al., 1989). Synthesis of chlorophyll a and b and
carotenoids was then significantly inhibited. Roundup affected
synthesis of chlorophyll a to a greater extent than that of the
other pigments. In vivo and in vitro studies with isolated
chloroplasts showed inhibition of the photosystems PS I and PS II at
concentrations of > 55 mg Roundup/litre. Hernando et al. (1989)
suggested that glyphosate acted as an electron transport inhibitor
and that the stronger inhibition of PS II compared with PS I was due
to the surfactant polyoxyethyleneamine.
In periphytic algal communities that were collected in ponds of
boreal forests, Roundup decreased the carbon fixation rate by 50% at
concentrations of 243-479 mg/litre (Goldsborough & Brown, 1988). The
algae were exposed for 4 h. Austin et al. (1991) cultured periphyton
on glass plates suspended in artificial "stream-troughs" which were
supplied with flowing water pumped from natural streams in British
Columbia, Canada. The stream water was low in phosphorus and flowed
out of an oligotrophic lake. Glyphosate was added to give nominal
concentrations in the troughs of between 0.001 and 0.3 mg/litre. A
further series of treatments added nutrients to troughs. The
herbicide was not toxic to the periphyton. A transitory decrease in
growth was followed by a stimulation of biomass in the
glyphosate-treated troughs. Similar effects were seen with added
nutrient. The authors considered the effect to be the result of
algae using glyphosate as a phosphate source. Communities of
periphyton were similar in all treatments.
Various studies have indicated that glyphosate may affect
aromatic amino acid synthesis in microorganisms, in addition to the
greening process, respiration and photosynthesis (Amrhein et al.,
1980; Chan & Leung, 1986; Pipke & Amrhein, 1988).
9.1.1.2 Soil
Appraisal
Soil bacteria in culture have shown effects of glyphosate on
nitrogen fixation, denitrification and nitrification. However, field
studies after application of formulations have not shown significant
effects. Closely related species of bacteria have been shown to be
capable of degrading glyphosate (see chapter 4). The lack of
information on the bioavailability of glyphosate in soil makes it
difficult to relate the evaluation of effects in culture to the
actual exposure in the field.
Technical grade glyphosate inhibited the growth of bacteria
isolated from a sprayed garden soil of sandy loam to a lesser extent
than it did that of bacteria isolated from an unsprayed control
(Quinn et al., 1988). In a mineral salt medium the growth rate of
bacteria from the untreated site was reduced by 50% at 590 mg
a.i./litre at 50 h after application.
In various studies, glyphosate, applied at the recommended
rates, caused neither inhibition of processes that are part of the
nitrogen cycle nor inhibition of enzymes involved in microbial
activity.
Roundup did not affect dehydrogenase activity in sand and loamy
sand when applied at rates of 4.9 and 24 kg a.i./ha (NATEC, 1990).
In the same soils amended with lucerne (Medicago sativa), the same
application rates did not change significantly the amounts of
nitrate nitrogen, although in treated loamy sand there was a slight
increase. These experiments, with a duration of 28 days, indicated a
slight stimulation of nitrification in loamy sand. A stronger
stimulation of nitrification was found in silt loam, silty clay loam
and sandy loam up to 84 days after application of technical grade
glyphosate at rates of 5 and 25 mg a.i./kg (ABC Inc., 1978d). This
stimulation was not dose-related. In this experiment no
substance-related effects were found on nitrogen fixation and
nitrite formation.
Nitrogen fixation was not affected significantly at an
application rate of 13 mg a.i./kg dry weight whether the product
applied was technical grade glyphosate or Roundup, or whether
aerobic or anaerobic conditions were used (Carlisle & Trevors,
1986a). At concentrations > 127 mg a.i./kg dry weight, nitrogen
fixation was inhibited under anaerobic conditions, whereas this
could not be verified under aerobic conditions due to very low
acetylene reduction rates. In the same agricultural sandy loam,
denitrification under anaerobic conditions was stimulated at
concentrations of > 13 mg a.i./kg dry weight, whether technical
grade glyphosate or Roundup was applied. Denitrification was
stimulated more strongly, when, in addition to the test compound,
glucose was added. In the same soil, no substantial effects of
either test compound on nitrification were found at 77 mg a.i./kg
dry weight. Dose-related inhibition of nitrification was found at
concentrations > 230 mg a.i./kg dry weight for glyphosate and
Roundup with respect to nitrate and nitrite production,
respectively. For both substances the inhibition was transient in
these studies. The temperature used was not reported.
No substantial effects on nitrification or denitrification were
found in two agricultural soils from Southern Finland after
application of an unknown formulation at a rate of 2.6 kg a.i./ha
(Müller et al., 1981). A strong inhibition of nitrogenase activity
was possibly an indirect effect due to a changed C/N ratio after the
treatment. In this study lasting 28 days, pretreatment measurements
were used as a control.
Roundup did not inhibit nitrification in three agricultural
soils after treatment at a recommended application rate of 2.9 kg
a.i./ha in 25-day experiments (Stratton, 1990). In a sandy loam,
nitrification was stimulated after the application of 145 kg
a.i./ha, whereas nitrification rates were inhibited by 50% at rates
of 194-435 kg a.i./ha. Mineralization, expressed as the time-course
of mg N/g dry weight (N = ammonium or nitrate nitrogen), in two
agricultural sandy loam soils was stimulated significantly due to
the treatment with 100 mg a.i./kg dry weight during an experiment
lasting 70 days (Marsh et al., 1977).
Not only in agricultural soils but also in forest soils, the
effects of Roundup on the nitrogen cycle appear not to be
inhibitory. Preston & Trofymow (1989) found no significant effects
on nitrification or the immobilization of urea nitrogen in an
organic fir forest floor and its underlying mineral horizon due to
the application of 10 and 50 mg a.i./kg dry weight. This experiment
lasted for 40 days.
In two agricultural sandy loam soils exposed to 100 mg a.i./kg
dry weight for 210 days, transient slight effects on CO2 evolution
were observed by Marsh et al. (1977). These effects were both
stimulatory (first 20 days) and inhibitory (until approximately 130
days) in both the soil from a permanent grassland and the soil from
an arable field, but especially in the latter. Carlisle & Trevors
(1986b) observed a dose-related increase in both O2 consumption
and CO2 production in an agricultural sandy loam soil during a
test of 10 days, with both technical grade glyphosate and Roundup.
In general the increases were significant at concentrations greater
than or equal to 127 mg a.i./kg. At the lowest dose (13 mg a.i./kg
soil) no effects were observed except a significant increase in
oxygen consumption due to Roundup. Oxygen consumption at doses
greater than or equal to 127 mg a.i./kg was more strongly stimulated
by Roundup than by technical grade glyphosate, possibly due to the
isopropylamine or surfactant. Preston & Trofymow (1989) found no
significant effect on CO2 production in an organic fir forest
floor and its underlying mineral horizon after the application of 10
and 50 mg a.i./kg dry weight. This experiment lasted 40 days.
Aerobic H2 oxidation in an agricultural sandy loam was
significantly inhibited by both technical grade glyphosate and
Roundup, but only at the highest dose of 635 mg a.i./kg dry weight
(Carlisle & Trevors, 1986b). Inhibition was stronger under anaerobic
conditions, and was found at concentrations of > 127 mg a.i./kg
dry weight to be significant and dose related. Anaerobic inhibition
might have been due to increased H2 generation as a result of
stimulated fermentation.
No effects of glyphosate on the activities of 1,3-ß-glucanase
and urease in a silt loam were found after application of Roundup at
a rate of 12 mg a.i./kg dry weight (Lethbridge et al., 1981).
Preston & Trofymow (1989) found no significant effect on urea
hydrolysis in an organic fir forest floor and its underlying mineral
horizon amended with 200 mg urea nitrogen per kg dry weight, due to
treatment with Roundup at a rate of 50 mg a.i./kg dry weight.
The degradation of cellulose, starch, protein, or leaf litter
was not inhibited at concentrations of 5 and 25 mg a.i./kg soil in
three agricultural soils, with the exception of litter in silt loam
at the highest dose (ABC Inc., 1978e). In this experiment lasting 84
days, technical grade glyphosate was applied.
Bacterial growth of Rhizobium trifolii in sterile solutions
with Bergersen's broth was completely inhibited at solution
concentrations of 10 and 20 mg a.i./litre, applied as an unknown
formulation of glyphosate (Eberbach & Douglas, 1983). Only at 10 mg
a.i./litre did the bacterial growth recover within 4 days. At lower
concentrations no inhibition was found.
Mycelial growth of ectomycorrhizal fungi in pure cultures on
agar was inhibited by 50% or more at concentrations of > 1 mg
a.i./litre for Pisolithus tinctorius and of > 100 mg a.i./litre
for Cenococcum geophilum and Hebeloma longicaudum (Estok et al.,
1989). Ectomycorrhizal fungi commonly associated with pines (Pinus
sp) were significantly inhibited at concentrations of > 29 µg
Roundup/litre (Chakravarty & Chatarpaul, 1990a). The most sensitive
species were Cenococcum graniforme, Hebeloma crustuliniforme and
Laccaria laccata. Roundup increased the susceptibility of sandy
soil for Gaeumannomyces gramminis, a fungus causing "take-all
disease" in wheat crops (Mekwatanakarn & Sivasithamparam, 1987).
Application at a rate of 0.54 µg a.i./kg increased the survival and
pathogenicity of the fungus significantly after 140 days of
incubation at 25 °C. These authors concluded that Roundup affected
microbial antagonists of the fungus.
9.1.2 Aquatic organisms
9.1.2.1 Plants
Appraisal
There is conflicting information on the effects of sediment on
the phytotoxicity of glyphosate to aquatic plants; Lemna showed
reduced effects whilst Carthamus did not. Generally, glyphosate is
thought to be largely unavailable to plants when added to soil and
only effective as a herbicide when applied to foliage.
The chronic toxicity to aquatic macrophytes when exposed to
technical grade glyphosate or Roundup dissolved in water is
summarized in Tables 20 and 21. Glyphosate is slightly toxic with a
14-day NOEC value of 9 mg/litre. Roundup is also slightly toxic with
14-day NOEC values of 2.4-56 mg/litre. No data on acute toxicity for
plants were available.
When Roundup was sprayed at a rate of 0.8 kg a.i./ha, the
phytotoxicity to floating plants of the common duckweed (Lemna
minor) was dependent on the extent of washed-off deposit (Lockhart
et al., 1989). Phytotoxicity was highest when the sprayed deposits
were not washed off within 6 h after application. Suspensions of
50 mg/litre of inorganic bentonite clay reduced the phytotoxicity of
Roundup to common duckweed significantly (Hartman & Martin, 1984).
When exposed for 14 days, concentrations up to 24 mg Roundup/litre
had no effect on plant growth when sediment was added, whereas the
growth was reduced by 50% at 5 mg Roundup/litre when no sediment was
added.
Phytotoxicity of glyphosate to the safflower (Carthamus
tinctorius) was not significantly reduced when the test compound
was added to drainage water with suspended particles instead of
distilled water (Bowmer et al., 1986). In these bioassays the
inhibition of root elongation was measured when the plants were
exposed to concentrations in unfiltered water of approximately
0.1-3 mg a.i./litre. The maximum concentration of adsorbed
glyphosate was 2500 mg/kg.
Although it had inhibitory effects at high concentrations,
Roundup had a stimulatory effect on the growth of common duckweed
(Lemna minor) and tubers of sago pondweed (Potamogeton
pectinatus) at lower concentrations (Hartman & Martin, 1985;
Lockhart et al., 1989). Enhancement of growth of common duckweed and
sago pondweed was found at 7-56 and 3 mg Roundup/litre,
respectively. These stimulatory effects may refer to hormesis.
9.1.2.2 Invertebrates
Appraisal
The data from laboratory toxicity tests show that formulations
are often more toxic than technical glyphosate to aquatic
invertebrates. The surprising result that addition of clay particles
to Daphnia test systems increased the toxicity of glyphosate is
probably due to ingestion of herbicide bounds to the particles. Few
studies have been conducted in the presence of sediment; the
reported toxicity of glyphosate is, therefore, difficult to relate
to the field situation.
The acute and chronic toxicity of technical grade glyphosate
and its formulations to aquatic invertebrates are summarized in
Tables 18-21. Technical grade glyphosate is slightly to very
slightly toxic, with LC50 values of > 55 mg/litre and a 21-day
NOEC value of 100 mg/litre. Formulations of glyphosate are
moderately to very slightly toxic with 2-day EC50 values of
5.3-5600 mg product/litre and 21-day MATC values of 1.4-4.9 mg
product/litre. The higher toxicity of Roundup to crustaceans is
mainly due to the presence of surfactants (Servizi et al., 1987).
In a laboratory in vitro test with the gills of Mytilus
californianus, a marine water mollusc, the active uptake of glycine
was inhibited by 23 and 67%, respectively, when a mixture of
14C-labelled and unlabelled glyphosate was applied at rates of
0.2-1.7 mg/litre (Swinehart & Cheney, 1987). This inhibition might
be an indication for Mg2+-moderated binding of glycine to the gill
surface, whereas glyphosate can compete with glycine uptake by
forming a metal complex.
Water fleas (Daphnia magna) were less sensitive to Roundup,
when the water was aerated than when it was unaerated. The 2-day
LC50 value decreased from 37 (with aeration) to 24 mg (without
aeration) Roundup/litre (EG & G Bionomics, 1980f).
Addition of 50 mg/litre of inorganic bentonite clay decreased
the 2-day EC50 value for mature water fleas (Daphnia pulex) from
16 to 7 mg Roundup/litre (Hartman & Martin, 1984). This higher
toxicity might have been due to ingestion of particle-adsorbed
glyphosate. Addition of bentonite also increased the toxicity in a
chronic experiment with populations of Daphnia pulex. Immature
water fleas were more sensitive to glyphosate than the adults under
all conditions. Populations in all treatments had recovered within
14 days after the application (Hartman & Martin, 1984).
No avoidance of glyphosate was found when mayfly nymphs
(Ephemerella walkeri) were tested at concentrations of 0.2-2 mg
Roundup/litre, whereas avoidance was demonstrated at 24 mg/litre
(Folmar et al., 1979).
9.1.2.3 Vertebrates
Appraisal
The toxicity tests for fish are generally performed without
sediment. As the bioavailability of glyphosate itself will be
reduced under most conditions due to sorption onto sediment, no
toxic effects are expected. Toxic effects, however, can be expected
due to surfactants in some formulations. To a lesser extent,
life-stage, pH, water hardness, temperature, and the presence of
feed all influence toxicity. No adverse effects on the
osmoregulatory mechanism were found.
The acute and chronic toxicity of technical grade glyphosate
and its formulations to fish are summarized in Tables 18-21.
Technical grade glyphosate is moderately toxic with 4-day LC50
values of 10 to > 1000 mg/litre, a 21-day NOEC value of
52 mg/litre, and an MATC value of > 26 mg/litre. Formulations of
glyphosate have comparable toxicity with 4-day LC50 values of 2.4
to > 1000 mg product/litre, and 21-day NOEC values of 0.8-2.4 mg
product/litre. Toxicity may vary substantially, depending on the
species, the test compound and test conditions. In general,
technical grade glyphosate is less toxic than the formulations. This
difference is mainly due to a higher toxicity of surfactants in the
formulations (Folmar et al., 1979; Servizi et al., 1987; Mitchell
et al., 1987; Wan et al., 1989).
Table 18. Aquatic organisms: acute toxicity of technical grade glyphosate in a static test system
Organism A Test pH Hardness Temperature Experimental Parameter Concentration Reference
water (mg CaCO3/ (°C) duration (mg/litre)
litre) (days)
Molluscs
Crassostrea - n.w.a n.r. n.r. 25 2 EC50 > 10b Bionomics
virginica, eggs (1973a)
Echinodermata
Tripneustes - n.w.a 7.7-8.2 n.r. 20 4 EC50 > 1000c E G & Bionomics
esculentes (1978d)
Crustaceans
Daphnia magna, - w.w. 7.8-8.0 > 250 19 2 LC50 780 ABC Inc.
first instar (1978a)
Uca pugilator - a.m.a n.r. n.r. 21 4 LC50 934 Bionomics
(1973b)
Palaemonetes - a.m.a n.r. n.r. 21 4 LC50 281 Bionomics
vulgaris (1973b)
Mysidopsis bahia - n.w.a 6.4-8.3 n.r. 20 4 LC50 > 1000 E G &
Bionomics
(1978c)
Insects
Chironomus plumosus, - r.w. n.r. 40 22 2 EC50 55d Folmar et al.
fourth instar (1979)
Table 18 (contd).
Organism A Test pH Hardness Temperature Experimental Parameter Concentration Reference
water (mg CaCO3/ (°C) duration (mg/litre)
litre) (days)
Fish
Ictalurus - r.w. n.r. 40 22 4 LC50 130 Folmar et al.
punctatus (1979)
Salmo gairdnerii, - divers 6.3-8.2 5.3-148 n.r. 4 LC50 10-197 Wan et al.
0.4 cm, 0.5 g (1989)
Salmo gairdnerii, - r.w. 4.4-7.2 45 12 4 LC50 86 ABC Inc.
0.4 cm, 0.6 g (1978b)
Oncorhynchus keta, - divers 6.3-8.2 5.3-148 n.r. 4 LC50 10-148 Wan et al.
0.4 cm, 0.5 g (1989)
O. kisutch, A divers 6.3-8.2 5.3-148 n.r. 4 LC50 27-174 Wan et al.
0.4 cm, 0.5 g (1989)
O. tshawytsha, - divers 6.3-8.2 5.3-148 n.r. 4 LC50 19-211 Wan et al.
0.4 cm, 0.5 g (1989)
O. gorbusha, - divers 6.3-8.2 5.3-148 n.r. 4 LC50 14-190 Wan et al.
0.4 cm, 0.5 g (1989)
Lepomis - r.w. n.r. 40 22 4 LC50 140 Folmar et al.
macrochirus (1979)
L. macrochirus, - r.w. 6.6-7.0 45 21 4 LC50 120 ABC Inc.
0.3 cm, 1.0 g (1978c)
Pimephales - r.w. n.r. 40 22 4 LC50 97 Folmar et al.
promelas (1979)
Rasbora - r.w. n.r. 25 21 4 LC50 168 HRC (1977)
heteromorpha,
0.1-0.3 cm
Cyprinodon - n.w.e 7.6-8.3 n.r. 20 4 LC50 > 1000 E G & Bionomics
variegatus, (1978b)
0.7-1.0 cm
a salinity 20-35%
b based on abnormal development of oyster larvae
c based on immobility, drooping spines, and retracted podia
d based on immobilisation
e salinity 18%
A = actual concentrations are measured; - = nominal concentration; a.m. = artificial medium;
r.w. = reconstituted water; w.w. = well water; n.w. = natural surface water;
n.r. = not reported
Table 19. Aquatic organisms: acute toxicity of formulations with glyphosate
Organism A Test Compound Test pH Hardness Temperature Experimental Parameter Concentration Reference
type water (mg CaCO3/ (°C) duration (mg/litre)a
litre) (days)
Crustaceans
Daphnia - S Ru r.w. 8.2-8.3 175 22 2 EC50 5.3b E G & G
magna, first Bionomics
instar (1980e)
D. magna, - S Ru r.w. 7.7-8.1 175 22 2 EC50 24-37b E G & G
first instar Bionomics
(1980f)
D. magna, - S Ru r.w. n.r. 40 22 2 EC50 7.3b Folmar
first instar et al.
(1979)
D. magna, - S St w.w. 8.3-8.5 225-275 20 2 EC50 42b ABC Inc
first instar (1984c)
D. magna, - S RuD w.w. 7.9-8.6 255 20 2 EC50 930b ABC Inc
first instar (1981a)
D. pulex, - S Ru w.w. 7.6 282 15 2 EC50 19b Hartman &
mature Martin
(1984)
Gammarus A CF Ru w.w 7.9-8.3 255 17 2 EC50 42b,c ABC Inc
pseudolimnaeus (1982b)
Insects
Chironomus - S Rod r.w. 7.6-7.8 42-44 22 2 EC50 5600b Buhl &
riparius, Faerber
fourth instar (1989)
Table 19 (cont'd)(2)
Organism A Test Compound Test pH Hardness Temperature Experimental Parameter Concentration Reference
type water (mg CaCO3/ (°C) duration (mg/litre)a
litre) (days)
Chironomus - S Ru r.w. n.r. 40 22 2 EC50 44b Folmar
plumosus, et al.
fourth instar (1979)
Fish
Ictalurus - S Ru r.w. 6.3-7.2 24-40 22 4 LC50 52 E G & G
punctatus, Bionomics
0.7 cm, 3 g (1980a)
Ictalurus - S Ru r.w. n.r. 40 22 4 LC50 32 Folmar
punctatus et al.
(1979)
Salmo - S St r.w. 6.8-7.3 40-45 12 4 LC50 7.5 ABC Inc
gairdnerii, (1984a)
0.4 cm, 0.7 g
Salmo A CF Ru w.w. 8.0-8.2 255 12 4 LC50 8.2c ABC Inc
gairdnerii, (1982c)
0.5 cm, 2.4 g
Salmo - S Ru divers 6.3-8.2 5.3-148 n.r. 4 LC50 14-33 Wan et al.
gairdnerii, (1989)
0.4 cm, 0.5 g
Salmo - S Ru r.w. 6.6-7.6 40 12 4 LC50 36 E G & G
gairdnerii, Bionomics
0.3 cm, 0.3 g (1980c)
Salmo - S Ru w.w 6.4-7.3 26-26 12 4 LC50 22 E G & G
gairdnerii, Bionomics
0.4 cm, 0.7 g (1980g)
Table 19 (cont'd)(3)
Organism A Test Compound Test pH Hardness Temperature Experimental Parameter Concentration Reference
type water (mg CaCO3/ (°C) duration (mg/litre)a
litre) (days)
S. gairdnerii, A S Ru r.w 7.4-7.7 85 11 4 LC50 22 EVS
0.4 g Consultants
(1986a)
S. gairdnerii, A S Ru n.w 7.4-7.8 81 11 4 LC50 15 EVS
0.4 g Consultants
(1986a)
S. gairdnerii, A S Ru d.w 5.4-6.3 4.5 11 4 LC50 26 EVS
0.4 g Consultants
(1986a)
S. gairdnerii, - S Ru r.w approx. 40 12 4 LC50 3.2 Folmar et al.
1.0 g 7.2 (1979)
Salmo - S RuD w.w 4.6-7.1 45 12 4 LC50 > 1000 ABC Inc
gairdnerii, (1981c)
0.3 cm, 0.2 g
S. gairdnerii, - S Vis n.r. 6.0 9.6 12.3 4 LC50 34 Morgan &
9.5 cm Kiceniuk
(1992)
Lepomis - S St r.w. 6.8-7.5 40-45 22 4 LC50 4.5 ABC Inc
macrochirus, (1984b)
0.2 cm, 0.1 g
Lepomis - S RuD w.w. 4.9-7.1 45 22 4 LC50 > 1000 ABC Inc
macrochirus, (1981b)
0.2 cm, 0.1 g
Lepomis A CF Ru w.w. 8.0-8.2 255 22 4 LC50 5.8c ABC Inc
macrochirus, (1982a)
0.2 cm, 0.2 g
Table 19 (cont'd)(4)
Organism A Test Compound Test pH Hardness Temperature Experimental Parameter Concentration Reference
type water (mg CaCO3/ (°C) duration (mg/litre)a
litre) (days)
Lepomis - S Ru r.w. 6.4-7.5 40 22 4 LC50 46 E G & G
macrochirus, Bionomics
0.4 cm, 0.3 g (1980b)
Pimephales - S Ru w.w. 6.7-7.7 39-44 22 4 LC50 31 E G & G
promelas, Bionomics
0.4 cm, 0.6 g (1980d)
Pimephales - S Ru r.w. n.r. 40 22 4 LC50 5.6 Folmar
promelas et al.
(1979)
Cyprinus A S St w.w. 7.2-7.9 40-48 22-23 4 LC50 2.4 ABC Inc
carpio, (1990)
0.6 cm, 2.8 g
Oncorhynchus A S Ru divers 6.3-8.2 5.3-148 n.r. 4 LC50 13-33 Wan et al.
kisutch, (1989)
0.4 cm, 0.5 g
O. kisutch, A S Ru d.w. 5.5-6.4 4.5 11 4 LC50 22 EVS
11.8 g Consultants
(1986c)
O. keta, - S Ru divers 6.3-8.2 5.3-148 n.r. 4 LC50 11-20 Wan et al.
0.4 cm, 0.5 g (1989)
O. tshawytsha, - S Ru divers 6.3-8.2 5.3-148 n.r. 4 LC50 17-33 Wan et al.
0.4 cm, 0.5 g (1989)
O. tshawytsha, A S Ru d.w. 5.8-6.7 4.5 11 4 LC50 20 EVS
4.6 g Consultants
(1986b)
Table 19 (cont'd)(5)
Organism A Test Compound Test pH Hardness Temperature Experimental Parameter Concentration Reference
type water (mg CaCO3/ (°C) duration (mg/litre)a
litre) (days)
O. gorbusha, - S Ru divers 6.3-8.2 5.3-148 n.r. 4 LC50 14-33 Wan et al.
0.4 cm, 0.5 g (1989)
Oncorhynchus A S Ru n.w 7.7-8.0 84 4-5 4 LC50 27-29 Servizi
nerka, et al.
3-6.5 cm, 0.2-3.8 g (1987)
a All concentrations are expressed as mg of the formulation per litre
b Based on immobilisation
c Based on actual concentrations
A = actual concentrations are measured; - = nominal concentrations; CF = continous flow system;
S = static system; Tgg = technical grade glyphosate; Ru = Roundup; Rod = Rodeo; St = Sting;
RuD = Roundup D-Pak; d.w. = dechlorinated tap water; r.w. = reconstituted water;
w.w. = well water; n.w. = natural surface water; a.m. = artificial medium
Table 20. Aquatic organisms: chronic toxicity of glyphosate (NOEC/MATC)
Organism A Test Test Test pH Hardness Temperature Experimental Parameter Concentration Reference
type substance water (mg CaCO3/ (°C) duration (mg/litre)
litre) (days)
Macrophytes
Lemna gibba A S Tgg a.m. 7.5 285 25 14 NOEC 9a,b Malcolm
Pirnie
Inc.
(1987e)
Crustaceans
Daphnia magna, A SS Tgg w.w.c 6.8-8.2 160-180 20 21 NOEC 100a,d ABC Inc.
first instar (1989c)
Fish
Salmo A CF Tgg w.w. 5.9-7.8 40-48 14-15 21 NOEC 52a,e ABC Inc.
gairdnerii, (1989e)
0.5 cm, 1.3 g
Pimephales A CF Tgg w.w. 6.5-7.6 32-42 25 255 MATC > 26f E G & G
promelas, Bionomics
1.5 g (1975)
a Based on actual concentrations d Based on survival and reproduction
b Based on biomass decrease e Based on survival, behaviour, and coloration
c Mixed with natural surface water f Based on survival, growth and reproduction
A = actual concentrations are measured; CF = continous flow system; SS = semi-static system;
S = static system; Tgg = technical grade glyphosate; w.w. = well water; a.m. = artificial medium
Table 21. Aquatic organisms: chronic toxicity of formulations with glyphosate
Organism A Test Test Test pH Hardness Temperature Experimental Parameter Concentration Reference
type substance water (mg CaCO3/ (°C) duration (mg/litre)f
litre) (days)
Macrophytes
Lemna minor - S Ru a.m. n.r. n.r. 25 14 NOEC 56c,d Lockhart
et al.
(1989)
Lemna minor - S Ru a.m. n.r. n.r. 22 14 NOEC 2.4c,e Hartman &
Martin
(1984)
Potamogeton - S Ru a.m. n.r. n.r. 22 14 NOEC 33c,d Hartman &
pectinatus Martin
(tubers) (1985)
Crustaceans
Daphnia A SS St w.w.b 7.2-8.2 174 20 21 MATC 1.4g,i ABC Inc.
magna, (1989a)
first instar
Daphnia magna, A SS Ru w.w.b 7.6-8.3 160-180 20 21 MATC 4.9g,j ABC Inc.
first instar (1989b)
Fish
Salmo A CF St w.w. 7.3-7.8 40-48 14-16 21 NOEC 0.8a,h ABC Inc.
gairdnerii, (1989h)
0.5 cm,
1.3 g
Table 21. (cont'd)
Organism A Test Test Test pH Hardness Temperature Experimental Parameter Concentration Reference
type substance water (mg CaCO3/ (°C) duration (mg/litre)f
litre) (days)
Salmo A CF Ru w.w. 7.1-7.8 24-48 14-16 21 NOEC 2.4a,h ABC Inc.
gairdnerii, (1989d)
0.5 cm,
1.8 g
Salmo A CF Vis n.r. 6.0 9.6 12.3 approx. 60 NOEC > 0.04a,k Morgan &
gairdnerii, Kiceniuk
9.5 cm, 7.2 g (1992)
a Based on actual concentrations g Based on survival, reproduction and length of time to the first brood
b Mixed with natural surface water h Based on survival, behaviour and coloration
c Roundup dissolved in test water i NOEC is 1.0 mg Sting/litre
d Based on biomass decrease j NOEC is 3.2 mg Roundup/litre (actual concentration)
e Based on reduction of frond formation k Based on mortality and growth
f All concentrations are expressed as mg of the formulation per litre
A = actual concentrations are measured; - = nominal concentrations; CF = continous flow system;
SS = semi-static system; Ru = Roundup; St = Sting; Vis = Vision; w.w. = well water;
a.m. = artificial medium; n.r. = not reported.
In laboratory experiments the major factors influencing the
toxicity appear to be the tested species and its age, the presence
of surfactants, the hardness, pH, temperature and the availability
of ration. Wan et al. (1989) found that the toxicity of technical
grade glyphosate to salmonids increased when hardness and pH
decreased, whereas for Roundup and Accord CR the contrary was true,
due to the presence of a 75% tallow amine surfactant in the
formulations. This surfactant was most toxic in hard water with a
relatively high pH. A higher toxicity of Roundup to salmonids at
increasing hardness and pH was confirmed by Mitchell et al. (1987),
but only partially by Servizi et al. (1987). It was also confirmed
by Folmar et al. (1979), although they only investigated the effect
of pH in reconstituted water with a moderate hardness. These authors
demonstrated that the effects of a pH increase on the toxicity of
Roundup and technical grade glyphosate were not only seen in rainbow
trout (Salmo gairdnerii) but also in bluegill sunfish (Lepomis
macrochirus). For both species Roundup became more toxic as the pH
increased from 6.5 to 7.5, whereas technical grade glyphosate became
less toxic as the pH increased from 6.5 to 9.5. At pH values higher
than 7.5, the toxicity of Roundup remained constant. In the
investigations of Servizi et al. (1987), an antagonistic effect of
glyphosate on the toxic action of a surfactant was found.
Increased toxicity of formulations due to surfactants was not
only demonstrated for the tallow amine surfactant in Roundup but
also for ortho X-77 in Rodeo (Mitchell et al., 1987). However, even
in the presence of a surfactant, the acute toxicity of some
formulations may be very slight, as was found by ABC Inc. (1980a,b).
In these studies, 0.5% (v/v) of the surfactant X-77 was added to
Roundup D-Pak, resulting in a 4-day LC50 value of this mixture for
rainbow trout (Salmo gairdnerii) and for bluegill sunfish
(Lepomis macrochirus) of 240 and 830 mg/litre, respectively.
Folmar et al. (1979) performed acute toxicity tests with
various species in reconstituted water with a pH of 7.2 and a
hardness of 40 mg CaCO3/litre. In these tests it was demonstrated
for rainbow trout (Salmo gairdnerii) and channel catfish
(Ictalurus punctatus) that the sensitivity to Roundup increased in
the following order: eyed eggs, 2-g fingerlings, sac fry, swim-up
fry, and 1-g fingerlings. The 4-day LC50 values for the various
life-stages of rainbow trout decreased from 39 mg Roundup/litre for
eyed eggs to 3.2 mg/litre for small fingerlings. In an additional
test, a 4-h exposure to concentrations of > 12 mg Roundup/litre
affected the survival of sac fry and swim-up fry significantly.
Holdway & Dixon (1988) also demonstrated that toxicity is
dependent on the life-stage by applying technical grade glyphosate
to larvae of flagfish (Jordanella floridae). At concentrations up
to 30 mg a.i./litre, no 2- or 4-day-old larvae died, whereas 20% of
the 8-day-old larvae died at the top dose. The effect was even more
drastic when the larvae were not fed. This treatment killed 20% of
the oldest larvae even at 3 mg a.i./litre. According to the authors,
the effect of age might fit the idea of saltatory ontogeny, implying
critical periods for organs and tissues.
While investigating the effect of temperature on toxicity,
Folmar et al. (1979) demonstrated for rainbow trout (Salmo
gairdnerii) and bluegill sunfish (Lepomis macrochirus) that
toxicity increased with increasing temperatures. For the trout the
4-day LC50 values decreased from 34 mg Roundup/litre at 7 °C to
18 mg/litre at 17 °C. For the bluegill sunfish the 4-day LC50
values decreased from 18 mg/litre at 17 °C to 9.8 mg/litre at 27 °C.
Rainbow trout (Salmo gairdnerii) showed the same sensitivity
to Roundup, independent of whether the water was aerated or not
(EG & G Bionomics, 1980g). In this experiment the 4-day LC50 under
both conditions was 22 mg Roundup/litre.
The potential of coho salmon smolt (Oncorhynchus kisutch) to
adapt to changes in water salinity encountered during migration was
not influenced by the application of Roundup at actual
concentrations up to 2.8 mg/litre (EVS Consultants, 1986d). The
osmoregulatory mechanism, which is fully functional in smolts, was
unaffected as indicated by plasma Na+ concentrations, haematocrit
values and the condition of the fish. During the experiment in which
the fish were exposed for 10 days in fresh water and subsequently
allowed to recover in fresh or sea water, no abnormal behaviour was
observed.
No avoidance of glyphosate was found when rainbow trout were
tested at concentrations up to 24 mg Roundup/litre (Folmar et al.,
1979). Sublethal concentrations in acute toxicity tests with Roundup
may cause loss of motility, complete loss of equilibrium, darkened
pigmentation, or rapid respiration (EG & G Bionomics, 1980a,b,g; EVS
Consultants, 1986a,b,c).
Rainbow trout (Salmo gairdnerii) were exposed to glyphosate
(as Vision) at 0, 6.25, 25 and 100 µg Vision/litre in a continuous
flow system. There were no effects on growth or foraging behaviour,
and no histopathological liver effects. Two out of three types of
aggressive behaviour were also unaffected; the third, a warning
"wig-wag" increased in frequency at the top-dose 1 month after
treatment. After two months the frequency was equal to that of the
control (Morgan & Kiceniuk, 1992).
9.1.3 Terrestrial organisms
9.1.3.1 Plants
Nodulation of sub-clover (Trifolium subterraneum) was
inhibited by an unknown formulation with glyphosate in a
dose-related way at concentrations of 2-20 mg a.i./litre (Eberbach &
Douglas, 1989). In this experiment, 3-day-old seedlings were
inoculated with Rhizobium trifolii. The seedlings were cultured
for 56 days in soil-free systems with nutrient solutions. In an
additional experiment in which Rhizobium was exposed to the same
concentrations, repeated washing of the inoculi prior to nodule
initiation did not reduce the inhibition of the nodulation of the
sub-clover after inoculation. This indicated that the effect on
nodulation might be the result of damage to the bacteria rather than
to carry-over of glyphosate from the bacteria to the plant.
Seed germination of various forest species was not affected by
treatment with Roundup at concentrations up to 305 mg a.i. (free
acid)/kg dry weight. Seed germination was affected at the highest
tested dose of 1525 mg a.i. (free acid)/kg dry weight (Morash &
Freedman, 1988). The effect on seed germination was confirmed by
another experiment in which no differences were found between
sprayed and unsprayed plots with respect to seedling composition and
quantity. Morash & Freedman (1988) then incubated the soils of
clear-cuts in a greenhouse. The application of Roundup in the field
was at a rate of 2.3 kg a.i./ha.
Red pine seedlings (Pinus resinosa) were not affected by
treatment with Roundup, with the exception of a dose-related
decrease in root length (Chakravarty & Chatarpaul, 1990b).
Non-affected growth parameters were shoot height, shoot weight, root
weight, and mycorrhizal development. In this experiment lasting 60
days, Roundup was applied at rates of 0.54 and 3.2 kg a.i./ha. The
conifer seedlings were inoculated with an ectomycorrhizal fungus
(Paxillus involutus), 14 days after germination.
Glyphosate may affect various pathways of the secondary
metabolism in the plant, although the actual targets in plants have
not been located. The synthesis of aromatic amino acids, secondary
hydroxyphenolic compounds, chlorophyll and delta-amino-levulinic
acid were reported to be affected by glyphosate (Amrhein et al.,
1980; Duke & Hoagland, 1981; Kitchen et al., 1981a,b). Aromatic
amino acids are important for the synthesis of, for instance, some
alkaloids, the phytohormone indole-3-acetic acid and phenolic
compounds such as lignin and quinones.
With respect to the synthesis of aromatic amino acids, there
are indications that the actual target is not shikimate kinase or
anthranilate synthase, but probably
5-enolpyruvylshikimate-3-phosphate synthase or chorismate synthase
(Amrhein et al., 1980). When applied to hypocotyls of buckwheat
(Fagopyrum esculentum) and to cultured cells of smooth bedstraw
(Galium mollugo), technical grade glyphosate inhibited the
conversion of shikimate to chorismate. This inhibition in vivo and
in vitro was found at concentrations of > 10 mg a.i./litre. In
the cultured cells of Galium, the accumulation of shikimate was
concomitant with a decrease of anthraquinones (Amrhein et al.,
1980). Possibly the non-transportable carbon pool, to which carbon
was found to be diverted in sugar beets (Beta vulgaris) due to
glyphosate, was in fact shikimate (Gougler & Geiger, 1984). These
effects on carbon metabolism were found at application rates
equivalent to 5 kg a.i./ha leaves. Duke & Hoagland (1981) assumed
that possibly chelation of divalent ions such as Ca2+ and Mg2+
that are involved in many metabolic pathways was the main cause of
damage.
9.1.3.2 Invertebrates
Appraisal
Glyphosate has low toxicity for bees and earthworms.
Oral 2-day LD50 values of technical grade glyphosate and
Roundup for bees were 100 µg a.i./bee and > 100 µg Roundup/bee,
respectively. Contact 2-day LD50 values for these two substances
were likewise > 100 µg a.i./bee and > 100 µg Roundup/bee (HRC,
1972). The oral 2-day LD50 of Sting was also > 100 µg Sting/bee
(Altmann, 1984). In these experiments Apis mellifera was tested. The
LD50 values indicate a slightly acute toxicity of technical grade
glyphosate, Roundup, and Sting to honey-bees. Roundup was also
slightly toxic to green lacewings (Chrysoperla carnea) when they
were exposed by contact to 1 kg Roundup/ha (SFRSA, 1990). In this
experiment the average number of eggs per female and the larval and
pupal mortality were increased due to the treatment, resulting in an
overall reduction in beneficial capacity of 41%. The beneficial
capacity is a function of the larval and pupal mortality, and the
average number of eggs per treated and untreated female. No effects
on the food uptake and mortality of the beetle Poecilus cupreus
Bonelli were observed 15 days after application of 6 kg Sting/ha
(IFU, 1990).
When exposed to artificial soil contaminated with Roundup
D-pak, earthworms (Eisenia fetida) were soft and/or slack, in a
dose-related way, at concentrations > 500 mg Roundup D-pak/kg dry
weight (IBR, 1991a). No other adverse effects were found, indicating
a 14-day NOEC of 158 mg/kg. A comparable experiment with Roundup
also indicated slight toxicity for earthworms with a 14-day NOEC of
500 mg Roundup/kg dry weight (IBR, 1991b). At higher concentrations
thin, slack and lethargic worms with a dark skin were observed.
9.1.3.3 Vertebrates
Appraisal
Glyphosate has low toxicity to birds after acute oral or
short-term dietary exposure. Mammals tested showed effects (body
weight loss) only after high levels of dosing. Herbicide-treated
foliage was not avoided by deer in the single study reported.
The acute, subacute and chronic toxicity of glyphosate and its
formulations to birds is summarized in Table 22.
Male marsupials (Sminthopsis macroura) showed significant
body weight loss after exposure to feed contaminated with
concentrations of up to 5000 mg a.i/kg feed (Evans & Batty, 1986).
No other treatment-related effects were found in the male
marsupials. In female hopping-mice (Notomys alexis and Notomys
mitchelli) fed similar doses, no treatment-related effects were
found.
Glyphosate did not affect the daily chow consumption of
black-tailed deer (Odocoileus hemionus columbianus) when these
herbivores were exposed to browse treated with glyphosate at a rate
of 2.2 kg/ha (Sullivan & Sullivan, 1979). The deer did not avoid the
contaminated browse, and sometimes even preferred it. Irrespective
of whether they were given treated alfalfa (Medicago sativa),
treated alder (Alnus rubra) or untreated feed, the deer had the
same daily chow consumption.
9.2 Field observations
9.2.1 Microorganisms
Appraisal
Some effects on microorganisms have been reported in field
studies following application of glyphosate formulations. However,
these have been minor and reversible in most cases. It is not
possible to separate the direct toxic effects of the herbicide from
changes in the habitat caused by its intended herbicidal action.
Table 22. Birds: acute and chronic toxicity of glyphosate and its formulations
Species Compound Sex Age Route Experimental Parameter Concentration Reference
duration
(days)
Colinus virginianus Tgg n.r. n.r. diet 8 LC50 > 4640 mg/kg feed Hazleton Lab. Inc.
(1973a)
Colinus virginianus Tgg n.r. 14 days oral LD50 > 3851 mg/kg b.w. Wildlife Int Ltd.
(1978c)
Colinus virginianus Tgg M,F 5 months diet 119 NOEC > 1000 mg/kg feeda Wildlife Int Ltd.
(1978b)
Colinus virginianus Ru n.r. 10 days diet 8 LC50 > 5620 mg/kg feedb Wildlife Int Ltd.
(1990b)
Anas platyrhynchos Tgg n.r. n.r. diet 8 LC50 > 4640 mg/kg feed Hazleton Lab. Inc.
(1973b)
Anas platyrhynchos Tgg M,F 6 months diet 112 NOEC > 1000 mg/kg feeda Wildlife Int Ltd.
(1978a)
Anas platyrhynchos Ru n.r. 10 days diet 8 LC50 > 5620 mg/kg feedb Wildlife Int Ltd.
(1990a)
Poephilla guttata Ru M mature diet 7 LC50 < 16 393 mg/kg feedb Evans & Batty (1986)
Poephilla guttata Ru M mature diet 5 LC50 > 8197 mg/kg feedb Evans & Batty (1986)
a Based on reproduction impairment of one generation
b Concentrations expressed as mg of the formulation per kg body weight or feed
Tgg = technical grade glyphosate; Ru = Roundup; M = males; F = females; n.r. = not reported.
9.2.1.1 Water
In a pool located in Hong Kong, treatment with 656 mg
Roundup/litre caused a substantial decrease in bacteria within 14
days after treatment. The number of colony-forming units had
returned to the control level 30 days after treatment (Chan & Leung,
1986).
Diatom populations in the water and sediments of a pond and a
stream showed a significantly different density of some species when
aerially treated with Roundup at a rate of 2.2 kg a.i./ha (Sullivan
et al., 1981). The authors concluded that these differences were
probably due to seasonal and habitat variation, rather than to
treatment with the herbicide.
Roundup may have affected the increase in ash-free dry weight
and the chlorophyll a standing crop of periphyton in the first month
after spraying with Roundup at a rate of 2.2 kg a.i./ha (Holtby &
Baillie, 1989a). In this experiment lasting around 130 days, some
tributaries in a watershed in British Columbia (Canada) were
directly oversprayed.
9.2.1.2 Soil
When Roundup was applied to a sandy loam being prepared for
conifer forestation, no significant changes in the soil respiration
were found up to about 180 days after application of Roundup at a
rate of 0.54 kg a.i./ha (Chakravarty & Chatarpaul, 1990a).
Concomitantly the numbers of fungi and bacteria decreased
significantly during the first 2 months, but after about 180 days
the numbers had recovered. These results might indicate changes in
microbial populations due to application of Roundup at recommended
application rates.
Preston & Trofymow (1989) found no significant effects on the
number of bacteria, actinomycetes, and nitrogen fixers in
ferro-humic podsols covered with alder trees (Alnus rubra) in the
Carnation Creek watershed, Canada, due to treatment with Roundup.
The only consistent effect was a significant reduction of the number
of fungi at one of the two treated sites. In this site the fungi
appeared to have recovered at the end of the study. In this
experiment of about 180 days, Roundup was hand-sprayed at a rate of
2 kg a.i./ha. In an additional comparable intensive field trial of
one month, microflora populations appeared to have recovered from
treatment after one month, with the exception of the reduction of
fungi in the litter of one of the treated sites. Actinomycetes and
nitrogen fixers in the litter appeared to be reduced in numbers due
to the treatment but they subsequently recovered. This reduction was
not found in the underlying humus layer.
Stratton & Stewart (1992) studied microbial activity in forest
soil and litter following the application of Roundup at 4.7
litres/ha (equivalent to 1.7 kg a.i./ha) to a coniferous forest
previously clear-cut and replanted. Treated and untreated (covered
with plastic sheeting during application) areas of forest were used
to obtain soil and litter samples which were tested in situ over an
8 months period following spraying. Glyphosate had a generally
stimulatory effect on microbial biomass in litter (up to 80%
increase) but no significant effect in soil. There were no
significant effects on the numbers of bacteria, fungi or
actinomycetes in either soil or litter. Glyphosate generally
stimulated respiration in both soil and litter; the degree of
stimulation was very variable throughout the sampling period,
ranging from a few percent to 100% increases in CO2 evolution.
No substance- or dose-related effects on aerobic bacteria were
observed in a sandy soil in a semi-arid region of Argentina up to 96
days after the application of Roundup (Gómez & Sagardoy, 1985).
Doses of up to 2.8 kg a.i. (free acid)/ha were applied.
No substance-related effects on the growth of an
ectomycorrhizal fungus were found when Roundup was applied at
concentrations of up to 3.2 kg a.i./ha (Chakravarty & Chatarpaul,
1990b). In these experiments red pines (Pinus resinosa) were
inoculated with the fungus Paxillus involutus. In the field 74-86%
of the seedlings that were not inoculated were colonized by
indigenous mycorrhizal fungi within two months.
9.2.2 Aquatic organisms
Appraisal
Little effect has been reported on aquatic invertebrates or
fish exposed to glyphosate formulations sprayed in the field. Minor
mortality in a single study of young trout may reflect the greater
sensitivity of early life-stages.
9.2.2.1 Plants
No field data on toxicity to aquatic macrophytes are available.
9.2.2.2 Invertebrates
An increased drift of midge larvae (Chironomus plumosus) was
found in artificial outdoor streams treated with 4.9 mg
Roundup/litre (Folmar et al., 1979). No increased drift was found at
0.5 mg Roundup/litre. In streams in a coastal rainforest in British
Columbia, Canada, only the drift densities of an amphipod (Gammarus
sp) and mayflies (Paraleptophlebia sp) increased after treatment
with Roundup at a rate of 2 kg a.i./ha (Kreutzweiser et al., 1989).
Density peaks partly coincided with periods immediately following
rainfall, which might indicate an effect due to glyphosate run-off,
or an effect due to increased discharges.
During most streamflows, the abundance of benthic
macro-invertebrates in a stream and at sites in tributary swamps was
similar at untreated sites and at sites that had been treated with
Roundup at a rate of 2.2 kg a.i./ha (Scrivener & Carruthers, 1989).
However, after periods of frequent rainstorms leading to flooding,
the abundance at the treated sites was 40-50% lower.
Water-fleas (Daphnia magna) did not show any mortality in a
pond sprayed with Roundup at application rates of up to 220 kg
a.i./ha (Hildebrand et al., 1980). In this experiment the
water-fleas were exposed for 8 days in pens that were partly
immersed in the water.
9.2.2.3 Vertebrates
Fingerlings (2.1 g, 5.8 cm) of rainbow trout (Salmo
gairdnerii) that were exposed for 14 days to Roundup in
flow-through pens in shallow streams did not show any mortality or
substance-related effects at application rates of up to 220 kg a.i.
(free acid)/ha (Hildebrand et al., 1982). In this experiment Roundup
was sprayed manually on moderately flowing forest streams in British
Columbia, Canada. In the same area an aerial application of 2.2 kg
a.i. (free acid)/ha did not cause mortality or obvious signs of
stress in rainbow trout fingerlings in flow-through pens, which were
also exposed to Roundup for 14 days (Hildebrand et al., 1982). A
direct aerial application of 2.1 kg a.i./ha on a tributary of the
Carnation Creek watershed (British Columbia, Canada), however,
killed 2.6% of the 120 fingerlings of coho salmon (Oncorhynchus
kisutch) within 24 h after application, whereas no mortality
occurred at the unexposed sites (Holtby & Baillie, 1989b). Also some
stress of the caged fingerlings was indicated within the first 2 h
after application. Up to 2 years after application no consistent
effects of Roundup on over-winter mortality, probability of entering
and leaving the tributary, timing of spring emigration, and growth
rates were found.
In artificial outdoor streams in Colorado, USA, rainbow trout
(Salmo gairdnerii) were exposed to concentrations of up to 5 mg
Roundup/litre for 12 h. No adverse effects on fecundity and
gonadosomatic indices were found in this study by Folmar et al.
(1979).
Rainbow trout (Salmo gairdnerii) in pens that were immersed
in stream water avoided Roundup at concentrations of > 40 mg
Roundup/litre (Hildebrand et al., 1982).
9.2.3 Terrestrial organisms
Appraisal
Spray drift of herbicides will affect non-target plants.
Adequate buffer zones have been defined for some application
methods.
Changes in species diversity and population size and structure
have been reported for terrestrial invertebrates and vertebrates
following applications of glyphosate formulation in the field.
Modifications in available food plants, insect populations
associated with vegetation killed by the herbicide, and ground cover
following intended effects of the spray probably account for these
changes.
9.2.3.1 Plants
Red pine seedlings (Pinus resinosa) were not affected by
treatment with Roundup in a field study. There was no dose-related
decrease of the root length, as had been observed in a comparable
laboratory experiment with the same doses (Chakravarty & Chatarpaul,
1990b). In this experiment lasting 154 days Roundup was applied at
rates of up to 3.2 kg a.i./ha. The conifer seedlings were inoculated
with an ectomycorrhizal fungus (Paxillus involutus) at 14 days
after germination, and they were planted outdoor after being
cultured in a greenhouse for up to 70 days after germination.
No plants were found as indicator species for damage due to
drift of a formulation of glyphosate when this was applied with
hydraulic ground sprayers (Marrs et al., 1989). In this study,
native British species commonly found in nature reserves were
exposed to spray drift, at several distances downwind from a zone
sprayed with 0.5 and 2.2 kg a.i./ha. The effect of windspeed was
investigated by spraying at speeds of 2.5 and 3.5 m/second. Death
and severe growth suppression occurred at a distance of 2-6 m from
the sprayer. Sublethal damage also occurred, mostly near to the
sprayer, although for Prunella vulgaris damage occurred up to 20 m
away. Epinasty was the most frequent symptom of damage. Most of the
damaged plants recovered, however. Some of the species were
consistently more sensitive, i.e. Digitalis purpurea, Centaurea
nigra, Prunella vulgaris and Lychnis flos-cuculi. Marrs et al.
(1989) concluded that, when spraying with ground sprayers, buffer
zones around nature reserves should be 5-10 m.
9.2.3.2 Invertebrates
No significant and consistent effects on the number of
nematodes and springtails were found in the upper 3 cm of
ferro-humic podsols due to treatment with Roundup (Preston &
Trofymow, 1989). The soils were covered with alder trees (Alnus
rubra) and located in British Columbia, Canada. The only
consistent effect was a significant reduction in the number of both
oribatid and non-oribatid mites on one of the treated sites around
20 days after application. On this site the number of mites appeared
to have returned to normal by the end of the study. In this
experiment of around 180 days Roundup was hand-sprayed at a rate of
2 kg a.i./ha.
No substance- or dose-related effects on mites and springtails
were observed in a sandy soil in a semi-arid region of Argentina up
to 96 days after application of Roundup (Gómez & Sagardoy, 1985).
Applied doses were up to 2.8 kg a.i. (free acid)/ha.
The numbers of herbivorous insects and ground invertebrates
were significantly reduced up to 3 years after treatment with
Roundup in a 4- to 5-year-old clear-cut planted with spruce
seedlings (Picea sp) (Santillo et al., 1989b). No effects were
found on predatory insects. The clear-cut was located in Maine, USA,
and sprayed with 1.7 kg a.i./ha. During this experiment lasting 3
years, the vegetation did not recover completely, and, apparently,
the effects on invertebrates were mainly due to habitat change.
Unintentionally untreated areas in the sprayed site showed a much
lesser reduction of invertebrates. These areas may therefore be
considered as potential sources for recolonization.
9.2.3.3 Vertebrates
Treatment of 4- to 5-year old clear-cuts in Maine, USA, planted
with seedlings of spruce (Picea sp), with Roundup at a rate of
1.7 kg a.i./ha affected breeding bird populations up to 3 years
after treatment (Santillo et al., 1989a). Total bird densities
decreased with 36% due to reduced habitat complexity, as expressed
in regenerated hardwood, vegetation height and foliage height
diversity. The most sensitive species were the insectivorous common
yellowthroat (Geothlypis trichas), Lincoln's sparrows (Melospiza
lincolnii) and alder flycatchers (Empidonax alnorum). In less
than 7-year-old clear-cuts in the Oregon coast range, Canada,
planted with Douglas fir (Pseudotsuga menziesii), some breeding
bird populations were affected due to two closely spaced treatments
with Roundup at a rate of 0.8 kg a.i./ha each (Morrison & Meslow,
1984). Two years after the application, the densities of birds using
shrubs for nesting and foraging had recovered, concomitant with the
recovery of shrub vegetation. Sensitive species were the
rufous-sided towhee (Piplio erythrophthalmus) and MacGillyvray's
warbler (Oporornis tolmiei). On the other hand, the American
goldfinch (Carduelis tristis) increased one year after
application, apparently due to the treatment. Morrison & Meslow
(1986) stated that the effectiveness of the treatment, which was not
maximal in their study, is crucial to the degree to which bird
populations are affected.
Small mammals may be affected by treatment with glyphosate,
this depending mainly on the size of the treated area, the
vegetation type and the extent to which the vegetation is damaged.
Various experiments have been performed in and around clear-cuts
planted with conifers in locations in the USA and Canada.
Insectivorous shrews (Blarina brivicauda, Sorex cinereus and Sorex
hoyi) and herbivorous voles were less abundant due to a treatment
with Roundup at a rate of 1.7 kg a.i./ha (Santillo et al., 1989b).
This significant reduction of the number of shrews was maintained
for 3 years after application, whereas the population of voles
recovered. No effects on the omnivorous deer mice (Peromyscus
maniculatus) were observed. Deer mice also appeared to be
unaffected in an experiment in which Roundup was applied at a rate
of 3 kg a.i./ha (Sullivan, 1990). The population dynamics of both
deer mice and the herbivorous Oregon voles (Microtus oregoni)
appeared not to be influenced, although some partially significant
effects were observed that might have been due to the treatment. On
the sprayed site there was an increase in the number of recruits of
both species 3 years after application, and also an increased
fecundity of deer mice and a higher survival of female voles 1 and 3
years after application. Sullivan (1990) observed no physiological
changes that might have been due to ingestion. In a clear-cut
treated with Roundup at a rate of 1.1 kg a.i./ha, only the
population density of Southern Redbacked voles (Clethrionomys
gapperi) was reduced by about 80% in a 1-year experiment (D'Anieri
et al., 1987). In another clear-cut, no adverse effects on deer mice
populations were evident after treatment with Roundup at a rate of
2.2 kg a.i./ha (Sullivan & Sullivan, 1981). Contrary to the results
of Sullivan (1990), D'Anieri et al. (1987), Sullivan & Sullivan
(1981) and Santillo et al. (1989b), a significant reduction in the
population density of deer mice was observed by Ritchie et al.
(1987) on a sprayed clear-cut of 38 ha at around 11 months after
spraying with Roundup at a rate of 1.1-1.2 kg a.i./ha. However, no
adverse effects on fertility or fecundity were indicated. Probably
the effect on abundance was due to habitat change with respect to
feed provision and cover. Possibly the effects on deer mice observed
by Ritchie et al. (1987) were less confounded by immigration as the
sprayed area was larger than in the studies in which effects on deer
mice were lacking. However, when a site of 36 ha was sprayed twice
with Roundup at a rate of 0.8 kg a.i./ha on each occasion, deer mice
were not affected, possibly due to a relatively low effectiveness of
the treatment (Anthony & Morrison, 1985). In the treated area, even
an increase of Oregon voles was found after 1 year, concomitant with
an increase of grass and other plants. The results indicated that
small mammal populations were able to recover within 2 years after
application of glyphosate, dependent on the recovery of shrubs.
10. EVALUATION OF HUMAN HEALTH HAZARDS AND EFFECTS ON THE
ENVIRONMENT
10.1 Human health hazards
Results of direct measurements of glyphosate concentrations in
foodstuffs (as part of food surveillance), drinking-water or total
diets are not available.
Absorption from the gastrointestinal tract is limited to 36% or
less and percutaneous absorption is 5.5% or less. Glyphosate is
essentially not metabolized. Total body clearance is 99% in 7 days.
Residues in livestock animals and their products (including milk)
are minimal.
Summarized information on short- and long-term studies is given
in Table 23 and on teratogenicity and reproduction studies in Table
24.
In animals, glyphosate has very low acute toxicity by the oral
and dermal administration routes. The formulation Roundup is acutely
toxic to humans when ingested intentionally or accidentally. No
controlled studies are available and therefore the human NOAEL
cannot be derived.
Animal studies show that glyphosate is not carcinogenic,
mutagenic or teratogenic. Reproductive effects were only seen at
dose levels producing maternal toxicity.
In experimental animals (13-week studies in rats and mice), an
effect was observed in the parotid salivary glands, indicating that
glyphosate may be acting as a weak adrenergic agonist. In rats, this
occurred at feeding levels of > 205 mg/kg body weight per day.
The NOAEL in chronic feeding studies is > 410 mg/kg body weight
per day. A NOAEL of 175 mg/kg body weight per day observed in a
rabbit teratogenicity study was chosen as the appropriate basis for
toxicological evaluations in humans. Through application of a
suitable safety factor, safe levels for humans can be estimated for
use in the toxicological evaluation of any actual exposures. For
technical glyphosate a safety factor of 100 is considered
appropriate given the elaborate data sets available.
Glyphosate and its concentrated formulations produce moderate
to severe eye irritation, but only slight skin irritation. Neither
glyphosate nor tested formulations induce sensitization.
Table 23. Short-term and long-term studies on glyphosate
Species Test Dose levels Effects, dose level NOAEL
compound (mg/kg-1 diet) (mg/kg diet) [mg/kg diet]
unless otherwise mg kg-1 b.w. d-1
stated
Short-term studies
Mouse technical 5000, 10 000, decreased growth and increased [10 000]
glyphosate 50 000 weights in brain, heart, 1890 m,
kidneys (50 000) 2730 f
Mouse technical 3125, 6250, reduced weight gain (50 000) [3 125]
glyphosate 12 500, 25 000, lesions of salivary glands 507
50 000 (> 6250)
Rat technical 1000, 5000, no adverse effects [20 000]*
glyphosate 20 000 1267*
Rat technical 200 to 12 500 no adverse effects [12 500]*
glyphosate NG
Rat technical 3125, 6250, increased AP and ALAT (> 6250) [< 3 125]
glyphosate 12 500, 25 000, increased haematocrit and red < 205 m
50 000 cell parameters (> 12 500), < 213 f
increased bile acids,
decreased sperm counts
(> 25 000), histological
alterations in salivary glands
(> 3125), reduced weight
gain (> 25 000)
Dogs technical 20, 100, 500 no adverse effects [NG]
glyphosate mg kg-1 bw 500*
Cattle Roundup 400, 500, 630, decreased feed intake (> 630) [NG]
790 mg kg-1 bw d-1), diarrhoea (> 500) 400
increased blood parameters
(790)
Long-term studies
Mouse technical 1000, 5000, decreased growth (30 000), [5 000]
glyphosate 30 000 increased incidence of 814
hepatocyte hypertrophy and
necrosis (30 000), increased
incidence of urinary bladder
epithelial hyperplasia
(30 000)
Rat technical 2000, 8000, decreased growth (20 000), [8 000]
glyphosate 20 000 increased liver weights 410
(20 000), increased incidences
of degenerative lens changes
(20 000) and of gastric
inflammation (8000 and 20 000)
Rat technical 60, 200, 600 slightly decreased growth (600) a
glyphosate
m = males; f = females; * Highest dose tested; NG, not given;
a The slight effect at 600 mg/kg diet (32 mg/kg bw) is considered marginal in the light
of the absence of an effect on growth at higher dose levels (2000 and 8000 mg/kg diet)
in a more recent 2-year study in rats.
Available studies on exposures of workers involved in
appli-cation of the herbicide show that exposure is low when
protective clothing is worn. The following data illustrate this
point.
a) The highest estimated exposure (dermal and inhalation) of about
8000 µg/h, as reported in a study with spray applicators,
corrected for incomplete absorption, equals about 50 µg/kg body
weight per day (8-h working day for a 70-kg adult); between the
latter level and the NOAEL of 175 mg/kg body weight per day,
adjusted for incomplete absorption from the gastrointestinal
tract (30-60%), i.e. 52-63 mg/kg body weight per day, there is
a margin of safety of about 1100.
b) The highest exposure concentration found for forest brush saw
workers was 15.7 µg/m3; between this level and the NOAEL
expressed as glyphosate from the 4-week inhalation study with
Roundup of 16 mg/m3 there is a margin of safety of 1000 (this
is borne out by the absence of adverse findings in the workers'
health examination in the study.
Table 24. Summary of teratogenicity and reproduction studies on glyphosate
Species Test Dose levels Effects NOAELa
compound (mg/kg diet) (mg/kg
body
weight)
Rat technical 300, 1000, 3500 mortality, clinical signs and 1000
glyphosate mg/kg body decreased growth in dams,
weight, early resorptions, decreased
gestation days 6-19 numbers of implantations and
visible fetuses, decreased
ossification of fetal
sternebrae (all at 3500 only);
no fetal malformations
Rabbit technical 75, 175, 350 diarrhoea and soft stools (350, 175
glyphosate mg/kg body slight at 175), nasal discharge
weight, (350)
gestation days 6-27
Rat technical 3, 10, 30 mg/kg increased incidence of renal < 30b
glyphosate body weight tubular dilation in F3b male
given in diet, pups (30)
3 gens
Rat technical 2000, 10 000, soft stools of parents (30 000), 100b
glyphosate 30 000 mg/kg decreased litter size (30 000), (2000
diet, 2 gens decreased body weights of mg/kg
parents and pups (30 000 diet)
and 10 000)
a Based on all observed effects (both in dams and offspring)
b There is some discrepancy in the results, and in the NOAELs, of the two
reproduction studies carried out with technical glyphosate; the renal
effects in the 3-generation study were not reproduced in the more recent
2-generation study with higher dose levels (for details, see section 7.5.2.).
10.2 Evaluation of effects on the environment
Following application, glyphosate will selectively partition to
particulate matter suspended in surface water, aquatic sediment or
to the soil substrate. This partitioning is usually rapid. The
mechanism of sorption is only partially understood. There is little
reported information on desorption from soil; the information
available suggests "strong" binding. Mobility studies show little
leaching of glyphosate below the upper few centimetres of the soil
profile. The major metabolite, AMPA, also appears not to leach
through soil.
Given this environmental distribution, organisms living in
aquatic sediment or soil would be expected to come into closest
contact with residues of the herbicide.
There is very little information on the bioavailability of
sediment- or soil-bound glyphosate to either aquatic or terrestrial
organisms. Few bioaccumulation or ecotoxicity studies have been
performed with sediment present.
Comparison of exposure concentrations and toxic effects is,
therefore, difficult since the relevant organisms have not been
tested and exposure of tested organisms is not by a realistic route.
10.2.1 Exposure levels and toxic effects
Exposure concentrations have been calculated from experimental
application of Roundup in the field (see Table 7 in chapter 5). The
methodology is presented in Fig. 4. No monitoring results of
environmental concentrations following actual use in agriculture or
forestry are available.
The lowest LC(EC)50 and NOEC values for microorganisms,
invertebrates and fish have been taken from the toxicity tests
reported in chapter 9 (see Fig. 4). These all refer to organisms
living in the open water and are, therefore, of questionable
significance for a compound which partitions to sediment. There is
no information on species living in aquatic sediment and little
information available on soil-living organisms, with the exception
of microorganisms.
10.2.2 Hazard evaluation for aquatic organisms
Tables 25 and 26 compare the estimated mean and maximum
exposure concentrations, following aerial application of Roundup, to
the lowest reported LC(EC)50 and NOEC concentrations for acute and
chronic exposure of aquatic organisms. The ratio between exposure
and effect concentrations has been calculated. These tables are
meant as a guide to establishing possible hazard and are not
intended to estimate the degree of effect likely to be seen in the
field. The "possible hazard" classification is a simple one using
different classification phrases for order of magnitude segments of
the ratios.
The toxicity of formulations to aquatic organisms is greater
than for technical glyphosate in many cases. This increased toxicity
is due to surfactants present in the product. No account has been
taken of possible degradation of surfactants in the ratios
presented, since no data are available. The ratios, therefore, may
overestimate the toxicity of glyphosate. If the compound is bound to
sediment in the environment, this could also reduce its toxic
effect. Since no clear evidence is available to demonstrate this
reduced toxicity, no account has been taken of partitioning to
particulates. This will also tend to overestimate toxic effect of
glyphosate.
As can be seen from the tables, possible hazard for most
aquatic organisms is small or negligible. Fish and aquatic
invertebrates would not be affected by glyphosate use. Only
microorganisms, with both acute and chronic exposure, appear to be
susceptible to effects of the herbicide. The comparisons made in the
table do not allow estimates of the degree of toxic effects likely
to be seen in the field. From the field evidence available,
populations and communities of algae are not likely to be affected
after application of glyphosate formulation. Transitory changes in
number and functioning of aquatic microorganisms are possible after
use of the herbicide.
Since data are not available, evaluation of the hazard of bound
residues of glyphosate to sediment-living organisms is not possible.
Minimal bioaccumulation of glyphosate has been reported in both
laboratory experiments and in the field. The physicochemical
properties of the compound are consistent with this conclusion.
10.2.3 Hazard evaluation for terrestrial organisms
Limited test data show low toxicity of glyphosate and its
formulations to honey-bees, earthworms, birds and mammals. These
data suggest low risk for these organisms from use of the herbicide.
Field studies have been conducted and support the view that
glyphosate does not affect soil microorganisms in the long term.
Marked changes in populations of birds and small mammals have
been seen in field studies following application of glyphosate.
These seem to result from the changes in habitat, vegetation cover,
food organisms, etc., resulting from the intended herbicidal effect
of the compound.
 Table 25. Indications of environmental hazard for aquatic organisms by technical grade glyphosate
Effect Organisms Estimated exposure Toxicity data End-point Ratio of exposure Possible
concentration (mg a.i./litre) to toxicity hazard
(mg a.i./litre) concentrations
Mean estimated exposure concentration
Acute microorganisms 0.1 EC50 = 1.2 mortality 0.082 small
Acute insects 0.1 EC50 = 55 mortality 0.0018 negligible
Acute crustaceans 0.1 EC50 = 281 mortality 0.00035 negligible
Acute fish 0.1 LC50 = 94 mortality 0.0010 negligible
Chronic algae 0.05 NOEC = 0.3 growth 0.17 present
Chronic crustaceans 0.01 NOEC = 100 reproduction 0.00010 negligible
Chronic fish 0.01 NOEC = 52 behaviour 0.00019 negligible
Maximum estimated exposure concentration
Acute microorganisms 1.7 EC50 = 1.2 mortality 1.4 large
Acute insects 1.7 EC50 = 55 mortality 0.031 small
Acute crustaceans 1.7 EC50 = 281 mortality 0.0060 negligible
Acute fish 1.7 LC50 = 94 mortality 0.018 small
Chronic algae 1.7 NOEC = 0.3 growth 5.7 large
Chronic crustaceans 0.17 NOEC = 100 reproduction 0.0017 negligible
Chronic fish 0.17 NOEC = 52 behaviour 0.003 negligible
Table 26. Indications of environmental hazards for aquatic organisms by Roundup
Effect Organisms Estimated exposure Toxicity data End-point Ratio of exposure Possible
concentration (mg Roundup/litre) to toxicity hazard
(mg Roundup/litre) concentrations
Mean estimated exposure concentration
Acute microorganisms 0.32 EC50 = 2.1 mortality 0.15 present
Acute crustaceans 0.32 EC50 = 10 mortality 0.032 small
Acute insects 0.32 EC50 = 44 mortality 0.0073 negligible
Acute fish 0.32 LC50 = 13 mortality 0.025 small
Chronic microorganisms 0.16 NOEC = 0.7 growth 0.23 present
Chronic crustaceans 0.032 NOEC = 3.5 reproduction 0.0091 negligible
Chronic fish 0.032 NOEC = 2.4 behaviour 0.013 negligible
Maximum estimated exposure concentration
Acute microorganisms 5.6 EC50 = 2.1 mortality 2.7 large
Acute crustaceans 5.6 EC50 = 10 mortality 0.56 present
Acute insects 5.6 EC50 = 44 mortality 0.13 present
Acute fish 5.6 LC50 = 13 mortality 0.43 present
Chronic microorganisms 5.6 NOEC = 0.7 growth 8.0 large
Chronic crustaceans 0.56 NOEC = 3.5 reproduction 0.16 small
Chronic fish 0.56 NOEC = 2.4 behaviour 0.23 small
11. RECOMMENDATIONS FOR PROTECTION OF HUMAN HEALTH
a) Protective clothing is necessary to ensure the safety of
herbicide applicators.
b) A market-basket survey would be useful to determine the
possible exposure of the general population.
12. FURTHER RESEARCH
a) Further research is required to determine whether ß-adrenergic
effects observed in rodents have any implications for human
health.
b) The role of adjuvants in the toxicity of glyphosate
formulations needs to be investigated further in laboratory
mammals and organisms in the environment.
c) A controlled study on exposure of agricultural workers is
needed.
d) The bioavailability of sediment- and soil-bound glyphosate in
the environment should be studied.
e) Studies on the environmental behaviour and fate of adjuvants
are required.
f) Further toxicity studies of sediment-living organisms are
needed.
g) The effects of phosphate fertilizers on the binding of
glyphosate to soils should be investigated.
h) Analytical techniques that are less costly but still adequate
should be developed.
REFERENCES
ABC Inc. (1978a) Acute toxicity of technical glyphosate (AB-78-201)
to Daphnia magna. Columbia, Missouri, Analytical Biochemistry
Laboratories, Inc. (Unpublished report submitted by Monsanto Ltd).
ABC Inc. (1978b) Acute toxicity of technical glyphosate (AB-78-165)
to rainbow trout (Salmo gairdnerii). Columbia, Missouri,
Analytical Biochemistry Laboratories, Inc. (Unpublished report
submitted by Monsanto Ltd).
ABC Inc. (1978c) Acute toxicity of technical glyphosate to bluegill
sunfish (Lepomis macrochirus). Columbia, Missouri, Analytical
Biochemistry Laboratories, Inc. (Unpublished report submitted by
Monsanto Ltd).
ABC Inc. (1978d) The effect of glyphosate on nitrogen fixation and
nitrification in soil with time. Columbia, Missouri, Analytical
Biochemistry Laboratories, Inc. (Unpublished report submitted by
Monsanto Ltd).
ABC Inc. (1978e) The effect of glyphosate on the degradation of
cellulose, starch, protein, and leaf litter on soil. Columbia,
Missouri, Analytical Biochemistry Laboratories, Inc. (Unpublished
report submitted by Monsanto Ltd).
ABC Inc. (1980a) Acute toxicity of MON-0139-X-77 (AB-80-262) to
rainbow trout (Salmo gairdnerii). Columbia, Missouri, Analytical
Biochemistry Laboratories, Inc. (Unpublished report submitted by
Monsanto Ltd).
ABC Inc. (1980b) Acute toxicity of MON-0139-X-77 (AB-80-263) to
bluegill sunfish (Lepomis macrochirus). Columbia, Missouri,
Analytical Biochemistry Laboratories, Inc. (Unpublished report
submitted by Monsanto Ltd).
ABC Inc. (1981a) Acute toxicity of MON 0139 (Lot LURT 12011)
(AB-81-074) to Daphnia magna. Columbia, Missouri, Analytical
Biochemistry Laboratories, Inc. (Unpublished report submitted by
Monsanto Ltd).
ABC Inc. (1981b) Acute toxicity of MON 0139 (Lot LURT 12011)
(AB-81-073) to Lepomis macrochirus. Columbia, Missouri, Analytical
Biochemistry Laboratories, Inc. (Unpublished report submitted by
Monsanto Ltd).
ABC Inc. (1981c) Acute toxicity of MON 0139 (Lot LURT 12011)
(AB-81-072) to rainbow trout (Salmo gairdnerii). Columbia,
Missouri, Analytical Biochemistry Laboratories, Inc. (Unpublished
report submitted by Monsanto Ltd).
ABC Inc. (1981d) A short-term bioconcentration study of
14C-glyphosate with channel catfish (Ictalurus punctatus) in a
static system. Columbia, Missouri, Analytical Biochemistry
Laboratories, Inc. (Unpublished report submitted by Monsanto Ltd).
ABC Inc. (1982a) Dynamic 96-hour acute toxicity of Roundup
(AB-82-33) to bluegill sunfish (Lepomis macrochirus). Columbia,
Missouri, Analytical Biochemistry Laboratories, Inc. (Unpublished
report submitted by Monsanto Ltd).
ABC Inc. (1982b) Dynamic 48-hour acute toxicity of Roundup
(AB-82-035) to Gammarus pseudolimnaeus. Columbia, Missouri,
Analytical Biochemistry Laboratories, Inc. (Unpublished report
submitted by Monsanto Ltd).
ABC Inc. (1982c) Dynamic 96-hour acute toxicity of Roundup
(AB-82-34) to rainbow trout (Salmo gairdnerii). Columbia,
Missouri, Analytical Biochemistry Laboratories, Inc. (Unpublished
report submitted by Monsanto Ltd).
ABC Inc. (1982d) Residue accumulation study in molluscs (Rangia
cuneata) with 14C-glyphosate under static conditions. Columbia,
Missouri, Analytical Biochemistry Laboratories, Inc. (Unpublished
report submitted by Monsanto Ltd).
ABC Inc. (1982e) Residue accumulation study in crayfish
(Procambarus simulans) with 14C-glyphosate under static
conditions. Columbia, Missouri, Analytical Biochemistry
Laboratories, Inc. (Unpublished report submitted by Monsanto Ltd).
ABC Inc. (1984a) Acute toxicity of LLN 8306 to rainbow trout (Salmo
gairdnerii). Columbia, Missouri, Analytical Biochemistry
Laboratories, Inc. (Unpublished report submitted by Monsanto Ltd).
ABC Inc. (1984b) Acute toxicity of LLN 8306 to bluegill sunfish
(Lepomis macrochirus). Columbia, Missouri, Analytical Biochemistry
Laboratories, Inc. (Unpublished report submitted by Monsanto Ltd).
ABC Inc. (1984c) Acute toxicity of LLN 8306 to Daphnia magna.
Columbia, Missouri, Analytical Biochemistry Laboratories, Inc.
(Unpublished report submitted by Monsanto Ltd).
ABC Inc. (1989a) 21-day prolonged static renewal toxicity of MON
8755 to Daphnia magna. Columbia, Missouri, Analytical Biochemistry
Laboratories, Inc. (Unpublished report No. 37933 submitted by
Monsanto Ltd).
ABC Inc. (1989b) 21-day prolonged static renewal toxicity of Roundup
to Daphnia magna. Columbia, Missouri, Analytical Biochemistry
Laboratories, Inc. (Unpublished report No. 37934 submitted by
Monsanto Ltd).
ABC Inc. (1989c) 21-day prolonged static renewal toxicity of
glyphosate technical to Daphnia magna. Columbia, Missouri,
Analytical Biochemistry Laboratories, Inc. (Unpublished report No.
37757 submitted by Monsanto Ltd).
ABC Inc. (1989d) Flow-through toxicity of Roundup to rainbow trout
(Salmo gairdnerii) for a 21-day exposure period. Columbia,
Missouri, Analytical Biochemistry Laboratories, Inc. (Unpublished
report No. 37694 submitted by Monsanto Ltd).
ABC Inc. (1989e) Flow-through toxicity of glyphosate to rainbow
trout (Salmo gairdnerii) for a 21-day duration period. Columbia,
Missouri, Analytical Biochemistry Laboratories, Inc. (Unpublished
report No. 37695 submitted by Monsanto Ltd).
ABC Inc. (1989f) Uptake, depuration, and bioconcentration of
14C glyphosate to bluegill sunfish (Lepomis macrochirus). Part
I: MSL-9304. Columbia, Missouri, Analytical Biochemistry
Laboratories, Inc. (Unpublished report submitted by Monsanto Ltd).
ABC Inc. (1989g) Uptake, depuration, and bioconcentration of
14C glyphosate to bluegill sunfish (Lepomis macrochirus).
Characterization and quantitation of glyphosate and its metabolites.
Part II: MSL-9303. Columbia, Missouri, Analytical Biochemistry
Laboratories, Inc. (Unpublished report submitted by Monsanto Ltd).
ABC Inc. (1989h) Flow-through toxicity of MON 8755 to rainbow trout
(Salmo gairdnerii) for a 21-day duration period. Columbia,
Missouri, Analytical Biochemistry Laboratories, Inc. (Unpublished
report No. 37759 submitted by Monsanto Ltd).
ABC Inc. (1990) Acute toxicity of MON 8755 to common carp (Cyprinus
carpio). Columbia, Missouri, Analytical Biochemistry Laboratories,
Inc. (Unpublished report No. 38256 submitted by Monsanto Ltd).
Ackurst P (1989) Introduction: issues and concerns relating to the
use of herbicides. In: Reynolds P ed. Proceedings of the Carnation
Creek Workshop, Nanaimo, 7-10 December 1987. Victoria, British
Columbia, Forestry Canada/British Columbia Ministry of Forests, pp
7-11.
Altmann G (1984) Laboratory testing of your preparation LLN 83/06
for danger to bees. Unpublished data of University of Saarland,
Saarbrücken, Department of Zoology, provided by Monsanto.
Amrhein N, Deus B, Gehrke P, & Steinrücken HC (1980) The site of the
inhibition of the shikimate pathway by glyphosate. Plant Physiol,
66: 830-834.
Anthony RG & Morrison ML (1985) Influence of glyphosate herbicide on
small-mammal populations in western Oregon. Northwest Sci, 59(3):
159-168.
Austin AP, Harris GE, & Lucey WP (1991) Impact of an organophosphate
herbicide (glyphosate) on periphyton communities developed in
experimental streams. Bull Environ Contam Toxicol, 47: 29-35.
Bababunmi EA, Olorunsogo OO, & Bassir O (1979) The uncoupling effect
of N-(phosphonomethyl)glycine on isolated rat liver mitochondria.
Biochem Pharmacol, 18: 925-927.
Balthazor TM & Hallas LE (1986) Glyphosate-degrading microorganisms
from industrial activated sludge. Appl Environ Microbiol, 51(2):
432-434.
Benova EK, Roupova I, Yagova A, Vuglenov A, & Bineva M (1989)
Mutagenicity studies on six pesticides in mice. Environ Mol Mutagen,
14(Suppl 15): 19 (Abstract).
Bio/Dynamics Inc. (1975) A twenty-one day dermal toxicity study of
MON 2139 in male rabbits (Project No. 75-1245). East Millstone, New
Jersey, Bio/Dynamics Inc., Division of Biology and Safety Evaluation
(Unpublished report submitted by Monsanto Ltd).
Bio/Dynamics Inc. (1979) A three month feeding study of glyphosate
(Roundup technical) in mice (Project No. 77-211) - Final report.
East Millstone, New Jersey, Bio/Dynamics Inc., Division of Biology
and Safety Evaluation (Unpublished report submitted by Monsanto
Ltd).
Bio/Dynamics Inc. (1981a) A lifetime feeding study of glyphosate
(Roundup technical) in rats (Project No. 410/77 [BDN-77-416]). East
Millstone, New Jersey, Bio/Dynamics Inc., Division of Biology and
Safety Evaluation (Unpublished report submitted by Monsanto Ltd).
Bio/Dynamics Inc. (1981b) A three-generation reproduction study in
rats with glyphosate (Project No. 77-2063 [BDN-77-147]) - Final
report. East Millstone, New Jersey, Bio/Dynamics Inc., Division of
Biology and Safety Evaluation (Unpublished report submitted by
Monsanto Ltd).
Bio/Dynamics Inc. (1983a) A chronic feeding study of glyphosate
(Roundup technical) in mice (Project No. 77-2061 [BDN-77-420]). East
Millstone, New Jersey, Bio/Dynamics Inc., Division of Biology and
Safety Evaluation (Unpublished report submitted by Monsanto Ltd).
Bio/Dynamics Inc. (1983b) A dermal sensitization study in guinea
pigs - Test material: Roundup formulation (Project No. 4234-83).
East Millstone, New Jersey, Bio/Dynamics Inc., Division of Biology
and Safety Evaluation (Unpublished report submitted by Monsanto Ltd,
Reference No. BD-83-007).
Bio/Dynamics Inc. (1983c) A dermal sensitization study in guinea
pigs - Test material: glyphosate (Project No. 4235-82). East
Millstone, New Jersey, Bio/Dynamics Inc., Division of Biology and
Safety Evaluation (Unpublished report submitted by Monsanto Ltd,
Reference No. BD-83-008).
Bio/Dynamics Inc. (1984a) Acute dermal toxicity study in rabbits -
Test material: LLN-83-06 (Project No. 4885-83). East Millstone, New
Jersey, Bio/Dynamics Inc., Department of Toxicology (Unpublished
report submitted by Monsanto Ltd, Reference No. BD-83-380).
Bio/Dynamics Inc. (1984b) Acute oral toxicity study in rats - Test
material: LLN-83-06 (Project No. 4884-83). East Millstone, New
Jersey, Bio/Dynamics Inc., Department of Toxicology (Unpublished
report submitted by Monsanto Ltd, Reference No. BD-83-380).
Bio/Dynamics Inc. (1984c) Primary dermal irritation study in rabbits
(4-hour exposure) - Test material: LLN-83-06 (Project No. 4886-83).
East Millstone, New Jersey, Bio/Dynamics Inc., Department of
Toxicology (Unpublished report submitted by Monsanto Ltd, Reference
No. BD-83-380).
Bio/Dynamics Inc. (1984d) Eye irritation study in rabbits - Test
material: LLN-83-06 (Project No. 4887-83). East Millstone, New
Jersey, Bio/Dynamics Inc., Department of Toxicology (Unpublished
report submitted by Monsanto Ltd, Reference No. BD-83-380).
Bio/Dynamics Inc. (1985a) Acute oral toxicity study in rats - Test
material: MON 8780 (Project No. 5301-84). East Millstone, New
Jersey, Bio/Dynamics Inc., Department of Toxicology (Unpublished
report submitted by Monsanto Ltd, Reference No. BD-84-343).
Bio/Dynamics Inc. (1985b) Acute dermal toxicity study in rabbits -
Test material: MON 8780 (Project No. 5302-84). East Millstone, New
Jersey, Bio/Dynamics Inc., Department of Toxicology (Unpublished
report submitted by Monsanto Ltd, Reference No. BD-84-343).
Bio/Dynamics Inc. (1985c) Primary dermal irritation study in rabbits
(4-hour exposure) - Test material: MON 8780 (Project No. 5303-84).
East Millstone, New Jersey, Bio/Dynamics Inc., Department of
Toxicology (Unpublished report submitted by Monsanto Ltd).
Bio/Dynamics Inc. (1985d) Eye irritation study in rabbits - Test
material: MON 8780 (Project No. 5304-84). East Millstone, New
Jersey, Bio/Dynamics Inc., Department of Toxicology (Unpublished
report submitted by Monsanto Ltd, Reference No. BD-84-343).
Bio/Dynamics Inc. (1986) A closed-patch repeated insult dermal
sensitization study in guinea pigs (Buehler method) - Test material:
MON 8756 (Project No. 6210-85). East Millstone, New Jersey,
Bio/Dynamics Inc., Department of Toxicology (Unpublished report
submitted by Monsanto Ltd, Reference No. BD-86-5).
Bio/Dynamics Inc. (1987a) Acute oral toxicity study in rats - Test
material: Roundup L&G Ready-to-use (Project No. 4033-86). East
Millstone, New Jersey, Bio/Dynamics Inc., Department of Toxicology
(Unpublished report submitted by Monsanto Ltd, Reference No.
BD-87-4).
Bio/Dynamics Inc. (1987b) Acute dermal toxicity study in rabbits -
Test material: Roundup L&G Ready-to-use (Project No. 4034-86). East
Millstone, New Jersey, Bio/Dynamics Inc., Department of Toxicology
(Unpublished report submitted by Monsanto Ltd, Reference
No. BD-87-3).
Bio/Dynamics Inc. (1987c) Primary dermal irritation study in rabbits
(4-hour exposure/semi-occlusive covering) - Test material: Roundup
L&G Ready-to-Use (Project No. 4035-86). East Millstone, New Jersey,
Bio/Dynamics Inc., Department of Toxicology (Unpublished report
submitted by Monsanto Ltd, Reference No. BD-87-4).
Bio/Dynamics Inc. (1987d) Eye irritation study in rabbits - Test
material: Roundup L&G Ready-to-Use (Project No. 4036-86). East
Millstone, New Jersey, Bio/Dynamics Inc., Department of Toxicology
(Unpublished report submitted by Monsanto Ltd, Reference
No. BD-87-4).
Bio/Dynamics Inc. (1987e) A closed-patch repeated insult dermal
sensitization study in guinea pigs (Project No. 6945-86). East
Millstone, New Jersey, Bio/Dynamics Inc., Division of Biology and
Safety Evaluation (Unpublished report submitted by Monsanto Ltd,
Reference No. BD-86-442).
Bio/Dynamics Inc. (1988a) Acute dermal toxicity study in rabbits -
Test material: Glyphosate Wet Cake (Project No. 4886-88). East
Millstone, New Jersey, Bio/Dynamics Inc., Department of Toxicology
(Unpublished report submitted by Monsanto Ltd, Reference No.
BD-88-114).
Bio/Dynamics Inc. (1988b) Primary dermal irritation study in rabbits
(4-hour exposure/semi-occlusive covering) - Test material:
Glyphosate Wet Cake (Project No. 4887-88). East Millstone, New
Jersey, Bio/Dynamics Inc., Department of Toxicology (Unpublished
report submitted by Monsanto Ltd, Reference No. BD-88-114).
Bio/Dynamics Inc. (1988c) Acute oral toxicity study in rats - Test
material: Glyphosate Wet Cake (Project No. 4885-88). East Millstone,
New Jersey, Bio/Dynamics Inc., Department of Toxicology (Unpublished
report submitted by Monsanto Ltd, Reference No. BD-88-114).
Bio/Dynamics Inc. (1988d) Eye irritation study in rabbits - Test
material: Glyphosate Wet Cake (Project No. 4888-88). East Millstone,
New Jersey, Bio/Dynamics Inc., Department of Toxicology (Unpublished
report submitted by Montsanto Ltd, Reference No. BD-88-114).
Bio/Dynamics Inc. (1988e) Acute oral toxicity study in rats - Test
material: Roundup (Project No. 4546-87). East Millstone, New Jersey,
Bio/Dynamics Inc., Department of Toxicology (Unpublished report
submitted by Monsanto Ltd, Reference No. BD-87-283).
Bio/Dynamics Inc. (1988f) Acute dermal toxicity study in rabbits -
Test material: Roundup (Project No. 4547-87). East Millstone, New
Jersey, Bio/Dynamics Inc., Department of Toxicology (Unpublished
report submitted by Monsanto Ltd, Reference No. BD-87-283).
Bio/Dynamics Inc. (1988g) Primary dermal irritation study in rabbits
(4-hour exposure/semi-occlusive covering) - Test material: Roundup
(Project No. 4548-87). East Millstone, New Jersey, Bio/Dynamics
Inc., Department of Toxicology (Unpublished report submitted by
Monsanto Ltd, Reference No. BD-87-283).
Bio/Dynamics Inc. (1990) Eye irritation study in rabbits - Test
material: MON 2139 (Project No. 5833-90). East Millstone, New
Jersey, Bio/Dynamics Inc., Department of Toxicology (Unpublished
report submitted by Monsanto, Reference No. BD-90-246).
Bionomics (1973a) Acute toxicity of Roundup (technical) to atlantic
oyster (Crassostrea virginica). Wareham, Massachusetts, Bionomics
(Unpublished report submitted by Monsanto Ltd).
Bionomics (1973b) Acute toxicity of Roundup (technical) to grass
shrimp (Palaemonetes vulgaris) and fiddler crab (Uca pugilator).
Wareham, Massachusetts, Bionomics (Unpublished report submitted by
Monsanto Ltd).
Bowmer KH, Boulton PM, Short DL, & Higgins ML (1986)
Glyphosate-sediment interactions and phytotoxicity in turbid water.
Pestic Sci, 17(2): 79-88.
Bozeman J, Koopman B, & Bitton G (1989) Toxicity testing using
immobilized algae. Aquat Toxicol, 14(4): 345-352.
Buhl KJ & Faerber NL (1989) Acute toxicity of selected herbicides
and surfactants to larvae of the midge Chironomus riparius. Arch
Environ Contam Toxicol, 18(4): 530-536.
Bunyatyan YA & Gevorgyan AA (1984) [Determination of the active
ingredient of Roundup and its metabolite in the environment.] Gig i
Sanit, 5: 43-44 (in Russian).
Burns AJ (1983) Liquid chromatographic determination of glyphosate
technical and its formulation: collaborative study. J Assoc Off Anal
Chem, 66(5): 1214-1219.
Carlisle SM & Trevors JT (1986a) Effect of the herbicide glyphosate
on nitrification, denitrification, and acetylene reduction in soil.
Water Air Soil Pollut, 29(2): 189-203.
Carlisle SM & Trevors JT (1986b) Effect of the herbicide glyphosate
on respiration and hydrogen consumption in soil. Water Air Soil
Pollut, 27(3/4): 391-401.
Chakravarty P & Chatarpaul L (1990a) Non-target effects of
herbicides: I. Effect of glyphosate and hexazinone on soil microbial
activity, microbial population, and in vitro growth of
ectomycorrhizal fungi. Pestic Sci, 28(3): 233-242.
Chakravarty P & Chatarpaul L (1990b) Non-target effect of
herbicides: II. The influence of glyphosate on ectomycorrhizal
symbiosis of red pine (Pinus resinosa) under greenhouse and field
conditions. Pestic Sci, 28(3): 243-248.
Chan KY & Leung SC (1986) Effects of paraquat and glyphosate on
growth, respiration, and enzyme activity of aquatic bacteria. Bull
Environ Contam Toxicol, 36(1): 52-59.
CIT (1991a) EXP 30578 - Acute oral toxicity in rats (Study No. 7076
TAR). Miserey, France, Centre International de Toxicologie (CIT)
(Unpublished report submitted by Rhône Poulenc, Lyon, France).
CIT (1991b) EXP 30578 - Acute dermal toxicity in rats (Study No.
7077 TAR). Miserey, France, Centre International de Toxicologie
(CIT) (Unpublished report submitted by Rhône Poulenc, Lyon, France).
CIT (1991c) EXP 30578 - Acute dermal irritation in rabbits (Study
No. 7079 TAL). Miserey, France, Centre International de Toxicologie
(CIT) (Unpublished report submitted by Rhône Poulenc, Lyon, France).
CIT (1991d) EXP 30578 - Acute eye irritation in rabbits (Study No.
7078 TAL). Miserey, France, Centre International de Toxicologie
(CIT) (Unpublished report submitted by Rhône Poulenc, Lyon, France).
Cowell JE, Kunstman JL, Nord PJ, Steinmetz JR, & Wilson GR (1986)
Validation of an analytical residue method for analysis of
glyphosate and metabolite: an interlaboratory study. J Agric Food
Chem, 34(6): 955-960.
Deyrup CL, Chang SM, Weintraub RA, & Moye HA (1985) Simultaneous
esterification and acylation of pesticides for analysis by gas
chromatography. 1. Derivatization of glyphosate and
(aminomethyl)phosphonic acid with fluorinated
alcohols-perfluorinated anhydrides. J Agric Food Chem, 33(5):
944-947.
Dickson SJ, Meinhold RH, Beer ID, & Koelmeyer TD (1988) Rapid
determination of glyphosate in postmortem specimens using 31P NMR. J
Anal Toxicol, 12(5): 284-286.
D'Anieri P, Leslie DM, & McCormack ML (1987) Small mammals in
glyphosate-treated clearcuts in Northern Maine. Can Field-Nat,
101(4): 547-550.
Dubelman S (1988) Glyphosate. Anal Methods Pestic Plant Growth
Regul, 16: 69-82.
Duke SO & Hoagland RE (1981) Effects of glyphosate on the metabolism
of phenolic compounds: VII. Root-fed amino acids and glyphosate
toxicity in soybean (Glycine max) seedlings. Weed Sci, 29(3):
297-302.
Eberbach PL & Douglas LA (1983) Persistence of glyphosate in a sandy
loam. Soil Biol Biochem, 15(4): 485-487.
Eberbach PL & Douglas LA (1989) Herbicide effects on the growth and
nodulation potential of Rhizobium trifolii with Trifolium
subterraneum L. Plant Soil, 119: 15-23.
Edwards WM, Triplett GB, & Kramer RM (1980) A watershed study of
glyphosate transport in runoff. J Environ Qual, 9(4): 661-664.
EG & G Bionomics (1975) Chronic toxicity of glyphosate to the
fathead minnow (Pimephales promelas Rafinesque). Aquatic
Toxicology Laboratory. Wareham, Massachusetts, EG & G Bionomics
(Unpublished report submitted by Monsanto Ltd).
EG & G Bionomics (1978a) Toxicity of seven test materials to the
marine alga, Skeletonema costatum. Marine Research Laboratory,
Pensacola, Florida, EG & G Bionomics (Unpublished report No.
BP-78-4-031 submitted by Monsanto Ltd).
EG & G Bionomics (1978b) Toxicity of seven test materials to
sheepshead minnows, Cyprinodon variegatus. Marine Research
Laboratory, Pensacola, Florida, EG & G Bionomics (Unpublished report
No. BP-78-4-029 submitted by Monsanto Ltd).
EG & G Bionomics (1978c) Toxicity of seven test materials to mysid
shrimp, Mysidopsis bahia. Marine Research Laboratory, Pensacola,
Florida, EG & G Bionomics (Unpublished report No. BP-78-4-032
submitted by Monsanto Ltd).
EG & G Bionomics (1978d) Toxicity of seven test materials to the
white sea urchin, Tripneustes esculentus. Marine Research
Laboratory, Pensacola, Florida, EG & G Bionomics (Unpublished report
No. BP-78-4-030 submitted by Monsanto Ltd).
EG & G Bionomics (1980a) Acute toxicity of Roundup to channel
catfish (Ictalurus punctatus). Aquatic Toxicology Laboratory.
Wareham, Massachusetts, EG & G Bionomics (Unpublished report No.
BW-80-4-641 submitted by Monsanto Ltd).
EG & G Bionomics (1980b) Acute toxicity of Roundup to bluegill
(Lepomis macrochirus). Aquatic Toxicology Laboratory. Wareham,
Massachusetts, EG & G Bionomics (Unpublished report No. BW-80-4-634
submitted by Monsanto Ltd).
EG & G Bionomics (1980c) Acute toxicity of Roundup to the rainbow
trout (Salmo gairdnerii). Aquatic Toxicology Laboratory. Wareham,
Massachusetts, EG & G Bionomics (Unpublished report No. BW-80-4-635
submitted by Monsanto Ltd).
EG & G Bionomics (1980d) Acute toxicity of Roundup to the fathead
minnow (Pimephales promelas). Aquatic Toxicology Laboratory.
Wareham, Massachusetts, EG & G Bionomics (Unpublished report No.
BW-80-4-653 submitted by Monsanto Ltd).
EG & G Bionomics (1980e) Acute toxicity of Roundup to the water flea
(Daphnia magna). Aquatic Toxicology Laboratory. Wareham,
Massachusetts, EG & G Bionomics (Unpublished report No. BW-80-4-636
submitted by Monsanto Ltd).
EG & G Bionomics (1980f) Acute toxicity of Roundup to the water flea
(Daphnia magna) with and without continuous aeration. Aquatic
Toxicology Laboratory. Wareham, Massachusetts, EG & G Bionomics
(Unpublished report No. BW-80-6-690 submitted by Monsanto Ltd).
EG & G Bionomics (1980g) Acute toxicity of Roundup to the rainbow
trout (Salmo gairdnerii) with continuous aeration and without
aeration. Aquatic Toxicology Laboratory. Wareham, Massachusetts,
EG & G Bionomics (Unpublished report No. BW-80-6-686 submitted by
Monsanto Ltd).
Estok D, Freedman B, & Boyle D (1989) Effects of the herbicides
2,4-D, glyphosate, hexazinone, and triclopyr on the growth of three
species of ectomycorrhizal fungi. Bull Environ Contam Toxicol,
42(6): 835-839.
Evans DD & Batty MJ (1986) Effects of high dietary concentrations of
glyphosate (Roundup) on a species of bird, marsupial and rodent
indigenous to Australia. Environ Toxicol Chem, 5: 399-401.
EVS Consultants (1986a) Acute toxicity of Roundup herbicide to
rainbow trout. Seattle, Washington, EVS Consultants (Unpublished
report submitted by Monsanto Ltd).
EVS Consultants (1986b) Acute toxicity of Roundup herbicide to
chinook salmon. Seattle, Washington, EVS Consultants (Unpublished
report submitted by Monsanto Ltd).
EVS Consultants (1986c) Acute toxicity of Roundup herbicide to coho
salmon. Seattle, Washington, EVS Consultants (Unpublished report
submitted by Monsanto Ltd). EVS Consultants (1986d) Seawater
challenge testing of coho salmon smolts following Roundupr herbicide
exposure. Seattle, Washington, EVS Consultants (Unpublished report
submitted by Monsanto Ltd).
FAO/WHO (1986a) Pesticide residues in food - Evaluations 1986. Part
I - Residues. Joint Meeting of the FAO Panel of Experts Residues in
Food and the Environment and the WHO Expert Group on Pesticide
Residues, Rome, 29 September-8 October 1986. Rome, Food and
Agriculture Organization of the United Nations (FAO Plant Production
and Protection Paper 78/1).
FAO/WHO (1986b) Pesticide residues in food - Evaluations 1986. Part
II - Toxicology. Joint Meeting of the FAO Panel of Experts Residues
in Food and the Environment and the WHO Expert Group on Pesticide
Residues, Rome, 29 September-8 October 1986. Rome, Food and
Agriculture Organization of the United Nations (FAO Plant Production
and Protection Paper 78/2).
FAO/WHO (1987) Pesticide residues in food - Evaluations 1987. Part I
- Residues. Joint Meeting of the FAO Panel of Experts Residues in
Food and the Environment and the WHO Expert Group on Pesticide
Residues, Geneva, 21-30 September 1987. Rome, Food and Agriculture
Organization of the United Nations (FAO Plant Production and
Protection Paper 86/1).
FAO/WHO (1988) Pesticide residues in food - Evaluations 1988. Part I
- Residues. Joint Meeting of the FAO Panel of Experts Residues in
Food and the Environment and the WHO Expert Group on Pesticide
Residues, Rome, 19-28 September 1988. Rome, Food and Agriculture
Organization of the United Nations (FAO Plant Production and
Protection Paper 93/1).
FDRL (1988a) Primary dermal irritation study of glyphosate
batch/lot/nbr No. XLI-55 in New Zealand White rabbits (Study No.
88.2053.010). Waverly, New York, Food & Drug Research Laboratories
(Unpublished report submitted by Monsanto Ltd - Monsanto study No.
FD-88-29).
FDRL (1988b) Acute dermal toxicity study of glyphosate batch/lot/nbr
No. XLI-55 in New Zealand White rabbits (Study No. 88.2053.008).
Waverly, New York, Food & Drug Research Laboratories (Unpublished
report submitted by Monsanto Ltd - Monsanto study No. FD-88-29).
FDRL (1988c) Primary eye irritation study of glyphosate
batch/lot/nbr No. XLI-55 in New Zealand White rabbits (Study No.
88.2053.009). Waverly, New York, Food & Drug Research Laboratories
(Unpublished report submitted by Monsanto Ltd - Monsanto study No.
FD-88-29).
FDRL (1988d) Acute oral toxicity study of glyphosate batch/lot/nbr
No. XLI-55 in Sprague-Dawley rats (Study No. 88.2053.007). Waverly,
New York, Food & Drug Research Laboratories (Unpublished report
submitted by Monsanto Ltd - Monsanto study No. FD-88-29).
Feller MC (1989) Effects of forest herbicide applications on
streamwater chemistry in southwestern British Columbia. Water Resour
Bull, 25(3): 607-616.
Feng JC & Thompson DG (1990) Fate of glyphosate in a Canadian forest
watershed. 2. Persistence in foliage and soils. J Agric Food Chem,
38(4): 1118-1125.
Feng JC, Thompson DG, & Reynolds PE (1990) Fate of glyphosate in a
Canadian forest watershed. 1. Aquatic residues and off-target
deposit assessment. J Agric Food Chem, 38(4): 1110-1118.
Folmar LC, Sanders HO, & Julin AM (1979) Toxicity of the herbicide
glyphosate and several of its formulations to fish and aquatic
invertebrates. Arch Environ Contam Toxicol, 8: 269-278.
Franz TJ (1983) Evaluation of the percutaneous absorption of Roundup
formulations in man using an in vitro technique (Study No.
UW-81-346). Seattle, Washington, University of Washington, School of
Medicine (Unpublished report submitted by Monsanto Ltd).
Friestad HO & Bronstad JO (1985) Improved polarographic method for
determination of glyphosate herbicide in crops, soils, and water. J
Assoc Off Anal Chem, 68(1): 76-79.
Gauch R, Leuenberger U, & Müller U (1989) [Determination of
glyphosate herbicide and its main metabolite aminomethylphosphonic
acid (AMPA) in drinking water by HPLC.] Z Lebensm.unters Forsch,
188(1): 36-38 (in German).
Glass RL (1981) Colorimetric determination of glyphosate in water
after oxidation to orthophosphate. Anal Chem, 53(6): 921-923.
Glass RL (1983) Liquid chromatographic determination of glyphosate
in fortified soil and water. J Agric Food Chem, 31(2): 280-282.
Glass RL (1987) Adsorption of glyphosate by soils and clay minerals.
J Agric Food Chem, 35: 497-500.
Goldsborough LG & Beck AE (1989) Rapid dissipation of glyphosate in
small forest ponds. Arch Environ Contam Toxicol, 18(4): 537-544.
Goldsborough LG & Brown DJ (1988) Effect of glyphosate (Roundup
formulation) on periphytic algal photosynthesis. Bull Environ Contam
Toxicol, 41(2): 253-260.
Gómez MA & Sagardoy MA (1985) [Influence of glyphosate herbicide on
the microflora and mesofauna of a sandy soil in a semi-arid region.]
Rev Latinoam Microbiol, 27: 351-357 (in Spanish).
Gopalan HNB & Njagi GDE (1981) Mutagenicity testing of pesticides.
III. Drosophila: recessive sex-linked lethals. Genetics,
97(Suppl): 544 (Abstract).
Gougler JA & Geiger DR (1981) Uptake and distribution of
N-phosphonomethylglycine in sugar beet plants. Plant Physiol, 68:
668-672.
Gougler JA & Geiger DR (1984) Carbon partitioning and herbicide
transport in glyphosate-treated sugarbeet (Beta vulgaris). Weed
Sci, 32(4): 546-551.
Guinivan RA, Thompson NP, & Wheeler WB (1982) Derivatization and
cleanup improvements in determination of residues of glyphosate and
aminomethylphosphonic acid in blueberries. J Assoc Off Anal Chem,
65(1): 35-39.
Haag WR & Yao CCD (1992) Rate constants for reaction of hydroxyl
radicals with several drinking water contaminants. Environ Sci
Technol, 26: 1005-1013.
Hallas LE, Adams WJ, & Heitkamp MA (1992) Glyphosate degradation by
immobilized bacteria: field studies with industrial wastewater
effluent. Appl Environ Microbiol, 51: 432-434.
Hallberg GL (1989) Pesticide pollution of groundwater in the humid
United States. Agric Ecosyst Environ, 26: 299-367.
Hance RJ (1976) Adsorption of glyphosate by soils. Pestic Sci, 7:
363-366.
Hartman WA & Martin DB (1984) Effect of suspended bentonite clay on
the acute toxicity of glyphosate to Daphnia pulex and Lemna
minor. Bull Environ Contam Toxicol, 33(3): 355-361.
Hartman WA & Martin DB (1985) Effects of four agricultural
pesticides on Daphnia pulex, Lemna minor, and Potamogeton
pectinatus. Bull Environ Contam Toxicol, 35(5): 646-651.
Hazleton Laboratories, Inc. (1973a) Eight-day dietary LC50 -
bobwhite quail. Final report. Vienna, Virginia, Hazleton
Laboratories, Inc. (Unpublished report submitted by Monsanto Ltd).
Hazleton Laboratories, Inc. (1973b) Eight-day dietary LC50 -
mallard ducks. Final report. Vienna, Virginia, Hazleton
Laboratories, Inc. (Unpublished report submitted by Monsanto Ltd).
Hazleton Lab. Inc. (1988a) Metabolism study of synthetic
13C/14C-labeled glyphosate and aminomethylphosphonic acid in
laying hens, Part I. Hazleton Laboratories Inc. Project No. HLA
6103-112. Madison, Wisconsin, Monsanto Agricultural Company, Report
No. MSL-7591. Unpublished report supplied by Monsanto Ltd.
Hazleton Lab. Inc. (1988b) Metabolism study of synthetic
13C/14C-labeled glyphosate and aminomethylphosphonic acid in
lactating goats, Part I. Hazleton Laboratories Inc. Project No. HLA
6103-113. Madison, Wisconsin, Monsanto Agricultural Company, Report
No. MSL-7586. Unpublished report supplied by Monsanto Ltd.
Heinonen-Tanski H (1989) The effect of temperature and liming on the
degradation of glyphosate in two arctic forest soils. Soil Biol
Biochem, 21(2): 313-317.
Heinonen-Tanski H, Rosenberg C, Siltanen H, Kilpi S, & Simojoki P
(1985) The effect of the annual use of pesticides on soil
microorganisms, pesticide residues in the soil and barley yields.
Pestic Sci, 16(4): 341-348.
Hensley DL, Beuerman DSN, & Carpenter PL (1978) The inactivation of
glyphosate by various soils and metal salts. Weed Res, 18: 287-291.
Hernando F, Royuela M, Muñoz-Rueda A, & Gonzalez-Murua C (1989)
Effect of glyphosate on the greening process and photosynthetic
metabolism in Chlorella pyrenoidosa. J Plant Physiol, 136: 26-31.
Hildebrand LD, Sullivan DS, & Sullivan TP (1980) Effects of Roundup
herbicide on populations of Daphnia magna in a forest pond. Bull
Environ Contam Toxicol, 25(3): 353-357.
Hildebrand LD, Sullivan DS, & Sullivan TP (1982) Experimental
studies of rainbow trout populations exposed to field applications
of Roundup herbicide. Arch Environ Contam Toxicol, 11(1): 93-98.
Hoffman DJ & Albers PH (1984) Evaluation of potential embryotoxicity
and teratogenicity of 42 herbicides, insecticides, and petroleum
contaminants to mallard eggs. Arch Environ Contam Toxicol, 13(1):
15-27.
Holdway DA & Dixon DG (1988) Acute toxicity of permethrin or
glyphosate pulse exposure to larval white sucker (Catostomus
commersoni) and juvenile flagfish (Jordanella floridae) as
modified by age and ration level. Environ Toxicol Chem, 7(1): 63-68.
Holtby LB & Baillie SJ (1989a) Effects of the herbicide Roundup
(glyphosate) on periphyton in Carnation Creek, British Columbia. In:
Reynolds P ed. Proceedings of the Carnation Creek Workshop, Nanaimo,
7-10 December 1987. Victoria, British Columbia, Forestry
Canada/British Columbia Ministry of Forests, pp 224-231.
Holtby LB & Baillie SJ (1989b) Effects of the herbicide Roundup on
coho salmon fingerlings in an over-sprayed tributary of Carnation
Creek, British Columbia. In: Reynolds P ed. Proceedings of the
Carnation Creek Workshop, Nanaimo, 7-10 December 1987. Victoria,
British Columbia, Forestry Canada/British Columbia Ministry of
Forests, pp 273-285.
HRC (1972) The acute contact and oral toxicities of CP67573 and MON
2139 to worker honey bees. Huntingdon, UK, Huntingdon Research
Centre (Unpublished report submitted by Monsanto Ltd).
HRC (1977) The acute toxicity of glyphosate to harlequin fish
(Rasbora heteromorpha). Huntingdon, UK, Huntingdon Research Centre
(Unpublished report submitted by Monsanto Ltd).
HRC (1988) The acute oral toxicity of ICIA0224 to the bobwhite
quail. Huntingdon, UK, Huntingdon Research Centre (Unpublished
report No. ISN 177/881062 submitted by Monsanto Ltd).
IBR (1991a) Final report - Acute toxicity in earthworms according to
OECD 207-test article: "Technical isopropylamin salt of glyphosate =
MON 0139" (Project No. 80-91-2078-00-90). Hannover, International
Bioresearch (Unpublished report submitted by Monsanto Ltd).
IBR (1991b) Final report - Acute toxicity in earthworms according to
OECD 207-test article: "Roundup" (Project No. 80-91-0599-00-90).
Hannover, International Bioresearch (Unpublished report submitted by
Monsanto Ltd).
IET (1978) Microbial mutagenicity testing on CP67573 (glyphosate).
Mitsukaido, Japan, Institute of Environmental Toxicology (IET)
(Study sponsor Monsanto Ltd - Monsanto unpublished report No.
ET-78-241).
IFU (1990) [Side-effects of Swing on the ground-beetle Poecilus
cupreus Bonnelli under laboratory conditions (Project No.
110/01-Pc).] Unpublished report of Institute for Environmental
Analysis and Biotechnology, Niefern-Öschelbron, supplied by Monsanto
Ltd (in German).
Inveresk Research Int. (1988a) Compound No. 3607: Acute oral
toxicity (limit) test in rats (IRI project No. 241496). Musselburgh,
Scotland, Inveresk Research International (Unpublished report No.
5585 submitted by Cheminova A/S, Denmark).
Inveresk Research Int. (1988b) Compound No. 3607: Acute dermal
toxicity (limit) test in rats (IRI project No.241496). Musselburgh,
Scotland, Inveresk Research International (Unpublished report No.
5586 submitted by Cheminova A/S, Denmark).
Inveresk Research Int. (1988c) Compound 3707: Primary skin
irritation in rabbits (IRI project No. 241496). Musselburgh,
Scotland, Inveresk Research International (Unpublished report No.
5587 submitted by Cheminova A/S, Denmark).
Inveresk Research Int. (1988d) Compound No. 3607: Magnusson-Kligman
Maximisation test in guinea pigs (IRI project No. 241496).
Musselburgh, Scotland, Inveresk Research International (Unpublished
report No. 5589 submitted by Cheminova A/S, Denmark).
Inveresk Research Int. (1989a) Glyphosate technical: acute oral
toxicity (limit) test in rats (IRI project No. 243268). Musselburgh,
Scotland, Inveresk Research International (Unpublished report No.
5883 submitted by Cheminova A/S, Denmark).
Inveresk Research Int. (1989b) Glyphosate technical:
Magnusson-Kligman Maximisation test in guinea pigs (IRI project No.
243268). Musselburgh, Scotland, Inveresk Research International
(Unpublished report No. 5587 submitted by Cheminova A/S, Denmark).
Inveresk Research Int. (1989c) Glyphosate technical: acute dermal
toxicity (limit) test in rats (IRI project No. 243268). Musselburgh,
Scotland, Inveresk Research International (Unpublished report No.
5884 submitted by Cheminova A/S, Denmark).
Inveresk Research Int. (1989d) Glyphosate soluble liquid formulation
(code No. 3607) - Acute inhalation toxicity study in rats (limit
test) (IRI project No. 640023). Musselburgh, Scotland, Inveresk
Research International (Unpublished report No. 5603 submitted by
Cheminova A/S, Denmark).
Inveresk Research Int. (1989e) Glyphosate technical: Primary skin
irritation in rabbits (IRI project No. 243268). Musselburgh,
Scotland, Inveresk Research International (Unpublished report No.
5885 submitted by Cheminova A/S, Denmark).
Inveresk Research Int. (1989f) Glyphosate technical: Primary eye
irritation in rabbits (IRI project No. 243268). Musselburgh,
Scotland, Inveresk Research International (Unpublished report No.
5886 submitted by Cheminova A/S, Denmark).
Inveresk Research Int. (1989g) Compound No. 3607: Primary eye
irritation in rabbits (IRI project No. 241496). Musselburgh,
Scotland, Inveresk Research International (Unpublished report No.
5588 submitted by Cheminova A/S, Denmark).
IRDC (1980a) Test article - Technical glyphosate: Dominant lethal
study in mice (Study No. 401-064). Mattawan, Michigan, International
Research and Development Corporation (Unpublished report submitted
Monsanto Ltd, Reference No. IR-79-014).
IRDC (1980b) Test article - Technical glyphosate: Teratology study
in rats (Study No. 401-054). Mattawan, Michigan, International
Research and Development Corporation (Unpublished report submitted
by Monsanto Ltd, Reference No. IR-79-016).
IRDC (1980c) Test article - Technical glyphosate: Teratology study
in rabbits (Study No. 401-056). Mattawan, Michigan, International
Research and Development Corporation (Unpublished report submitted
by Monsanto Ltd, Reference No. IR-79-018).
IRDC (1982) Test article - Glyphosate technical: 21-day dermal
toxicity study in rabbits (Study No. 401-168). Mattawan, Michigan,
International Research and Development Corporation (Unpublished
report submitted by Monsanto Ltd, Reference No. IR-81-195).
IRPTC (1991) Data profile on glyphosate. Geneva, International
Register for Potentially Toxic Chemicals, United Nations Environment
Programme.
Jacob GS, Garbow JR, Hallas LE, Kimack NM, Kishore GM, & Schaefer J
(1988) Metabolism of glyphosate in Pseudomonas sp. strain LBr.
Appl Environ Microbiol, 54(12): 2953-2958.
Jauhiainen A, Räsänen K, Sarantila R, Nuutinen J, & Kangas J (1991)
Occupational exposure of forest workers to glyphosate during brush
saw spraying work. Am Ind Hyg Assoc J, 52(2): 61-64.
Kishore GM & Jacob GS (1987) Degradation of glyphosate by
Pseudomonas sp. PG2982 via a sarcosine intermediate. J Biol Chem,
262(25): 12164-12168.
Kitchen LM, Witt WW, & Rieck CE (1981a) Inhibition of chlorophyll
accumulation by glyphosate. Weed Sci, 29(4): 513-516.
Kitchen LM, Witt WW, & Rieck CE (1981b) Inhibition of
delta-aminolevulinic acid synthesis by glyphosate. Weed Sci, 29(5):
571-577.
Knapek R, Kobes S, & Kita I (1986) [Toxicological evaluation of
N-phosphono-methylglycine.] Z gesamte Hyg, 32(9): 537-539 (in
German).
Konar SK & Roy DN (1990) Method for the determination of residues of
the herbicide glyphosate and its principal metabolite,
aminomethylphosphonic acid, in plant materials by nitrogen-selective
gas chromatography. Anal Chim Acta, 229(2): 277-280.
Kreutzweiser DP, Kingsbury PD, & Feng JC (1989) Drift response of
stream invertebrates to aerial applications of glyphosate. Bull
Environ Contam Toxicol, 42(3): 331-338.
Lavy TL, Cowell JE, Steinmetz JR, & Massey JH (1992) Conifer
seedling nursery worker exposure to glyphosate. Arch Environ Contam
Toxicol, 22: 6-13.
Lethbridge G, Bull AT, & Burns RG (1981) Effects of pesticides on
1,3-ß-glucanase and urease activities in soil in the presence and
absence of fertilisers, lime and organic materials. Pestic Sci,
12(2): 147-155.
LISEC (1989a) Alga, growth inhibition test. Effect of MON 2139 on
the growth of Selenastrum capricornutum. Bokrijk, Belgium, LISEC,
Study Centre for Ecology and Forestry (Unpublished report submitted
by Monsanto Ltd).
LISEC (1989b) Alga, growth inhibition test. Effect of MON 8755 on
the growth of Selenastrum capricornutum. Bokrijk, Belgium, LISEC,
Study Centre for Ecology and Forestry (Unpublished report submitted
by Monsanto Ltd).
LISEC (1990a) Degradation: Biological oxygen demand. Bokrijk,
Belgium, LISEC, Study Centre for Ecology and Forestry (Unpublished
report submitted by Monsanto Ltd).
LISEC (1990b) Degradation: Chemical oxygen demand. Bokrijk, Belgium,
LISEC, Study Centre for Ecology and Forestry (Unpublished report
submitted by Monsanto Ltd). Liu C-M, McLean PA, Sookdeo CC, & Cannon
FC (1991) Degradation of the herbicide glyphosate by members of the
family Rhizobiaceae. Appl Environ Microbiol, 57: 1799-1804.
Lockhart WL, Billeck BN, & Baron CL (1989) Bioassays with a floating
aquatic plant (Lemna minor) for effects of sprayed and dissolved
glyphosate. Hydrobiologia, 188/189: 353-359.
Lundgren LN (1986) A new method for the determination of glyphosate
and (aminomethyl)phosphonic acid residues in soils. J Agric Food
Chem, 34(3): 535-538.
Lund-Hoie K & Friestad HO (1986) Photodegradation of the herbicide
glyphosate in water. Bull Environ Contam Toxicol, 36(5): 723-729.
Madhun YA, Young JL, & Freed VH (1986) Binding of herbicides by
water-soluble organic materials from soil. J Environ Qual, 15(1):
64-68.
Maibach HI (1983) (a) Elimination of 14C-glyphosate in Rhesus
monkeys following a single dose. (b) Percutaneous absorption in
Roundup formulation in Rhesus monkeys following a single oral dose
(Study No. MA-81-349). San Francisco, California, California School
of Medicine (Unpublished report submitted by Monsanto Ltd).
Maibach HI (1986) Irritation, sensitive, photoirritation and
photosensitization assays with a glyphosate herbicide. Contact
Dermatitis, 15: 152-156.
Malcolm Pirnie Inc. (1987a) The toxicity of glyphosate technical to
Anabaena flos-aquae. White Plains, New York, Malcolm Pirnie Inc.
(Unpublished report submitted by Monsanto Ltd).
Malcolm Pirnie Inc. (1987b) The toxicity of glyphosate technical to
Skeletonema costatum. White Plains, New York, Malcolm Pirnie Inc.
(Unpublished report submitted by Monsanto Ltd).
Malcolm Pirnie Inc. (1987c) The toxicity of glyphosate technical to
Navicula pelliculosa. White Plains, New York, Malcolm Pirnie Inc.
(Unpublished report submitted by Monsanto Ltd).
Malcolm Pirnie Inc. (1987d) The toxicity of glyphosate technical to
Selenastrum capricornutum. White Plains, New York, Malcolm Pirnie
Inc. (Unpublished report submitted by Monsanto Ltd).
Malcolm Pirnie Inc. (1987e) The toxicity of glyphosate technical to
Lemna gibba. White Plains, New York, Malcolm Pirnie Inc.
(Unpublished report submitted by Monsanto Ltd).
Marcotte AL, Bradley M, & Hughley DH ed. (1977) Pesticide analytical
manual. Washington, DC, Food and Drug Administration.
Marrs RH, Williams CT, Frost AJ, & Plant RA (1989) Assessment of the
effects of herbicide spray drift on a range of plant species of
conservation interest. Environ Pollut, 59(1): 71-86.
Marsh JAP (1985) Effects of herbicide-fertilizer interactions on
nitrogen and phosphorus transformations and herbicide persistence in
soil. Pestic Sci, 16(1): 93-100.
Marsh JAP, Davies HA, & Grossbard E (1977) The effect of herbicides
on respiration and transformation of nitrogen in two soils I.
Metribuzin and glyphosate. Weed Res, 17: 77-82.
Martinez TT & Brown K (1991) Oral and pulmonary toxicology of the
surfactant used in Roundup herbicide. Proc West Pharmacol Soc, 34:
43-46.
Martinez TT, Long WC, & Hiller R (1990) Comparison of the toxicology
of the herbicide Roundup by oral and pulmonary routes of exposure.
Proc West Pharmacol Soc, 33: 193-197.
Mekwatanakarn P & Sivasithamparam K (1987) Effect of certain
herbicides on saprophytic survival and biological suppression of the
take-all fungus. New Phytol, 106: 153-159.
Miles CJ & Moye HA (1988a) Postcolumn photolysis of pesticides for
fluorometric determination by high-performance liquid
chromatography. Anal Chem, 60(3): 220-226.
Miles CJ & Moye HA (1988b) Extraction of glyphosate herbicide from
soil and clay minerals and determination of residues in soils. J
Agric Food Chem, 36(3): 486-491.
Miles CJ, Wallace LR, & Moye HA (1986) Determination of glyphosate
herbicide and (aminomethyl)phosphonic acid in natural waters by
liquid chromatography using pre-column fluorogenic labeling with
9-fluorenylmethyl chloroformate. J Assoc Off Anal Chem, 69(3):
458-461.
Mitchell DG, Chapman PM, & Long TJ (1987) Acute toxicity of Roundup
and Rodeo herbicides to rainbow trout, chinook, and coho salmon.
Bull Environ Contam Toxicol, 39(6): 1028-1035.
Mitsukaido Laboratories (1986) Roundup herbicide: Acute oral
toxicity study in mice - Final report. Tokyo, Mitsukaido
Laboratories, Institute of Environmental Toxicology (Unpublished
report No. ET-85-377 submitted by Monsanto Ltd).
Monsanto (1972a) The degradation of MON-0573 in river and lake
bottom sediments and surface water. St. Louis, Missouri, Monsanto
Ltd (Unpublished report No. 276).
Monsanto (1972b) The rate of dissipation of MON-0573 in soil. St.
Louis, Missouri, Monsanto Ltd (Unpublished report No. 271).
Monsanto (1972c) The photolysis, run-off, and leaching of MON-0573
on or in soil. St. Louis, Missouri, Monsanto Ltd (Unpublished report
No. 258).
Monsanto (1972d) The degradation and metabolism of MON-0573 in soil.
St. Louis, Missouri, Monsanto Ltd (Unpublished report No. 269).
Monsanto (1973a) CP 67573 Residue and metabolism - The gross
metabolism of N-phos-phonomethylglycine-14C (CP 67573-14C) in
the laboratory rat following a single dose. St. Louis, Missouri,
Monsanto Commercial Products Company, Agricultural Division Research
Department (Unpublished report No. 297 submitted by Monsanto Ltd).
Monsanto (1973b) CP 67573 Residue and metabolism - The isolation and
identification of the metabolites of CP 67573=13C excreted by the
laboratory rat. St. Louis, Missouri, Monsanto Commercial Products
Company, Agricultural Division Research Department (Unpublished
report No. 306 submitted by Monsanto Ltd).
Monsanto (1973c) CP 67573 Residue and metabolism - The dynamics of
accumulation and depletion of orally ingested
N-phosphonomethylglycine-14C. St. Louis, Missouri, Monsanto
Commercial Products Company, Agricultural Division Research
Department (Unpublished report No. 309 submitted by Monsanto Ltd).
Monsanto (1973d) CP 67573 Residue and metabolism - The gross
distribution of N-phosphonomethylglycine-14C (CP 67573-14C) in
the rabbit. St. Louis, Missouri, Monsanto Commercial Products
Company, Agricultural Division Research Department (Unpublished
report No. 298 submitted by Monsanto Ltd).
Monsanto (1978a) Photodegradation and anaerobic aquatic metabolism
of glyphosate, N-phosphonomethylglycine. St. Louis, Missouri,
Monsanto Ltd (Unpublished report No. MSL-0598).
Monsanto (1978b) Solubility, volatility, adsorption, and partition
coefficients, leaching, and aquatic metabolism of MON 0573 and MON
0101. St. Louis, Missouri, Monsanto Ltd (Unpublished report No.
MSL-0207).
Monsanto (1978c) Final report on Salmonella mutagenicity assay of
glyphosate (sample no. 04) (Test No. LF-78-161). St. Louis,
Missouri, Monsanto Environmental Health Laboratory (Unpublished
report submitted by Monsanto Ltd).
Monsanto (1980) Results of a pond test with Roundup for a period of
three months. Burgwedel, Germany, Monsanto Ltd (Unpublished report
submitted by Ökolimna to Monsanto Ltd).
Monsanto (1981a) A reinvestigation of the static exposure of channel
catfish to 14C-labelled glyphosate, N-(phosphonomethyl)glycine.
St. Louis, Missouri, Monsanto Ltd (Unpublished report No. MSL-2056).
Monsanto (1981b) Acute oral toxicity of MON 0139 to rats (Study No.
800257). St. Louis, Missouri, Monsanto Environmental Health
Laboratory (Unpublished report submitted by Monsanto Ltd).
Monsanto (1981c) Acute dermal toxicity of MON 0139 to rabbits (Study
No. 800258). St. Louis, Missouri, Monsanto Environmental Health
Laboratory (Unpublished report submitted by Monsanto Ltd).
Monsanto (1981d) Primary skin irritation of MON 0139 to rabbits
(Study No. 800259). St. Louis, Missouri, Monsanto Environmental
Health Laboratory (Unpublished report submitted by Monsanto Ltd).
Monsanto (1981e) Primary eye irritation of MON 0139 to rabbits
(Study No. 800260). St. Louis, Missouri, Monsanto Environmental
Health Laboratory (Unpublished report submitted by Monsanto Ltd).
Monsanto (1983a) Dissipation of glyphosate in U.S. field soils
following direct application of Roundupr herbicide. St. Louis,
Missouri, Monsanto Ltd (Unpublished report No. MSL-3210).
Monsanto (1983b) CHO/HGPRT Gene mutation assay with glyphosate
(Study No. ML-83-155). St. Louis, Missouri, Monsanto Environmental
Health Laboratory (Unpublished report submitted by Monsanto Ltd).
Monsanto (1983c) The hepatocyte primary culture/DNA repair assay on
compound JJN-1020 (glyphosate) using rat hepatocytes in cultures
(Study No. AH-83-181). St. Louis, Missouri, Monsanto Environmental
Health Laboratory (Unpublished report submitted by Monsanto Ltd).
Monsanto (1983d) Acute inhalation toxicity of Roundup formulation to
male and female Sprague-Dawley rats (Study No. 810093; DMEH project
No. ML-81-2019. St. Louis, Missouri, Monsanto Environmental Health
Laboratory (Unpublished report submitted by Monsanto Ltd).
Monsanto (1983e) Four-week study of 33-1/33% use-dilution of Roundup
in water administered to male and female Sprague/Dawley rats by
inhalation (Project No. ML-83-015/EHL 830025). St. Louis, Missouri,
Monsanto Environmental Health Laboratory (Unpublished report
submitted by Monsanto Ltd).
Monsanto (1983f) in vivo bone marrow cytogenetics study of
glyphosate in Sprague-Dawley rats (Project No. ML-83-236). St.
Louis, Missouri, Monsanto Environmental Health Laboratory
(Unpublished report submitted by Monsanto Ltd).
Monsanto (1983g) Effects of glyphosate on rat bone marrow cells
(Study No. ML-83-160; EHL study No. 830082). St. Louis, Missouri,
Monsanto Environmental Health Laboratory (Unpublished report
submitted by Monsanto Ltd).
Monsanto (1983h) A study of the plasma and bone marrow levels of
glyphosate following intraperitoneal administration in the rat
(Study No. ML-83-218; EHL study No. 830109). St. Louis, Missouri,
Monsanto Environmental Health Laboratory (Unpublished report
submitted by Monsanto Ltd).
Monsanto (1985) Twelve month study of glyphosate administered by
gelatine capsule to Beagle dogs (Project No. ML-83-137). St. Louis,
Missouri, Monsanto Environmental Health Laboratory (Unpublished
report No. MSL 5069 submitted by Monsanto Ltd).
Monsanto (1987a) 90 day study of glyphosate administered in feed to
Sprague/Dawley rats (Project No. ML-86-351/EHL 86128). St. Louis,
Missouri, Monsanto Environmental Health Laboratory (Unpublished
report No. MSL-7575 submitted by Monsanto Ltd).
Monsanto (1987b) Acute inhalation study of Roundup L&G Ready-to-Use
(Study No. 86148; DMEH project No. ML-87-6). St. Louis, Missouri,
Monsanto Environmental Health Laboratory (Unpublished report
submitted by Monsanto Ltd).
Monsanto (1988a) Metabolism of glyphosate in Sprague-Dawley rats.
Part II. Identification, characterization, and quantitation of
glyphosate and its metabolites after intravenous and oral
administration. St. Louis, Missouri, Monsanto Ltd (Unpublished
report No. MSL-7206).
Monsanto (1988b) The metabolism of glyphosate in Sprague Dawley rats
- Part I. Excretion and tissue distribution of glyphosate and its
metabolites following intravenous and oral administration. St.
Louis, Missouri, Monsanto Environmental Health Laboratory/Monsanto
Life Sciences Research Center (Unpublished report No. MSL-7215
submitted by Monsanto Ltd).
Monsanto (1988c) Metabolism of 13C/14C-labeled glyphosate and
aminomethylphosphonic acid in laying hens, Part II. St. Louis,
Missouri, Monsanto Life Sciences Research Center (Unpublished report
No. MSL-7420 submitted by Monsanto Ltd). Monsanto (1988d) Metabolism
study of synthetic 13C/14C-labeled glyphosate and
aminomethylphosphonic acid in lactating goats, Part II. St. Louis,
Missouri, Monsanto Life Sciences Research Center (Unpublished report
No. MSL-7458 submitted by Monsanto Ltd).
Monsanto (1990a) Dissipation of glyphosate and aminomethylphosphonic
acid in forestry sites. St. Louis, Missouri, Monsanto Ltd
(Unpublished report No. MSL-9940).
Monsanto (1990b) Chronic study of glyphosate administered in feed to
albino rats (Project No. MSL-10495). St. Louis, Missouri, Monsanto
Environmental Health Laboratory (Unpublished report submitted by
Monsanto Ltd).
Monsanto (1990c) Two generation reproduction feeding study with
glyphosate in Sprague-Dawley rats (Study No. MSL-10387). St. Louis,
Missouri, Monsanto Environmental Health Laboratory (Unpublished
report submitted by Monsanto Ltd).
Morash R & Freedman B (1989) The effects of several herbicides on
the germination of seeds in the forest floor. Can J For Res, 19(3):
347-350.
Morgan MJ & Kiceniuk JW (1992) Response of rainbow trout to a two
month exposure to Vision, a glyphosate herbicide. Bull Environ
Contam Toxicol, 48: 772-780.
Morrison ML & Meslow EC (1984) Effects of the herbicide glyphosate
on bird community structure, Western Oregon. For Sci, 30(1): 95-106.
Moye HA & Deyrup CL (1984) A simple single-step derivatization
method for the gas chromatographic analysis of the herbicide
glyphosate and its metabolite. J Agric Food Chem, 32(2): 192-195.
Moye HA, Miles CJ, & Scherer SJ (1983) A simplified high-performance
liquid chromatographic residue procedure for the determination of
glyphosate herbicide and (aminomethyl)phosphonic acid in fruits and
vegetables employing postcolumn fluorogenic labeling. J Agric Food
Chem, 31(1): 69-72.
Müller MM, Rosenberg C, Siltanen H, & Wartiovaara T (1981) Fate of
glyphosate and its influence on nitrogen-cycling in two Finnish
agriculture soils. Bull Environ Contam Toxicol, 27(5): 724-730.
Murthy DVS, Irvine RL, & Hallas LE (1989) Principles of organism
selection for the degradation of glyphosate in a sequencing batch
reactor. In: 43rd Purdue Industrial Waste Conference Proceedings.
Chelsea, Lewis Publishers, Inc., pp 267-274.
NATEC (1990) Effect of the herbicide Roundup on the activity of the
microflora of soil (Project No. NA 90 9151). Unpublished report of
Institute for Technical Scientific Services GmbH, Hamburg, supplied
by Monsanto Ltd.
Newton M, Howard KM, Kelpsas BR, Danhaus R, Lottman CM, & Dubelman S
(1984) Fate of glyphosate in an Oregon forest ecosystem. J Agric
Food Chem, 32(5): 1144-1151.
NOTOX (1987a) Evaluation of the acute oral toxicity of MON 14478 in
the rat (Monsanto project No. NO-87-290). 's-Hertogenbosch, The
Netherlands, NOTOX - Toxicological Research & Consultancy
(Unpublished report No. NOTOX 0646/824 submitted by Monsanto Ltd).
NOTOX (1987b) Evaluation of the acute oral toxicity of MON 14478 in
the rat (Monsanto project No. NO-87-347). 's-Hertogenbosch, the
Netherlands, NOTOX - Toxicological Research & Consultancy
(Unpublished report No. NOTOX 0646/901 submitted by Monsanto Ltd).
NOTOX (1987c) Evaluation of the acute dermal toxicity of MON 14478
in the rat (Monsanto project No. NO-87-290). 's-Hertogenbosch, The
Netherlands, NOTOX - Toxicological Research & Consultancy
(Unpublished report No. NOTOX 0646/826 submitted by Monsanto Ltd).
NOTOX (1987d) Assessment of primary skin irritation/corrosion by MON
14478 in the rabbit (Monsanto project No. NO-87-290).
's-Hertogenbosch, The Netherlands, NOTOX - Toxicological Research &
Consultancy (Unpublished report No. NOTOX 0646/820 submitted by
Monsanto Ltd).
NOTOX (1987e) Assessment of acute eye irritation by MON 14478 in the
rabbit (Monsanto project No. NO-87-290). 's-Hertogenbosch, The
Netherlands, NOTOX - Toxicological Research & Consultancy
(Unpublished report No. NOTOX 0646/822 submitted by Monsanto Ltd).
NOTOX (1987f) Evaluation of the acute oral toxicity of MON 14477 in
the rat (Monsanto project No. NO-87-288). 's-Hertogenbosch, The
Netherlands, NOTOX - Toxicological Research & Consultancy
(Unpublished report No. NOTOX 0645/823 submitted by Monsanto Ltd).
NOTOX (1987g) Evaluation of the acute oral toxicity of MON 14477 in
the rat (Monsanto project No. NO-87-346). 's-Hertogenbosch, The
Netherlands, NOTOX - Toxicological Research & Consultancy
(Unpublished report No. NOTOX 0645/900 submitted by Monsanto Ltd).
NOTOX (1987h) Evaluation of the acute dermal toxicity of MON 14477
in the rat (Monsanto project No. NO-87-288). 's-Hertogenbosch, The
Netherlands, NOTOX - Toxicological Research & Consultancy
(Unpublished report No. NOTOX 0645/825 submitted by Monsanto Ltd).
NOTOX (1987i) Assessment of primary skin irritation by MON 14477 in
the rabbit (Monsanto project No. NO-87-288). 's-Hertogenbosch, The
Netherlands, NOTOX - Toxicological Research & Consultancy
(Unpublished report No. NOTOX 0645/819 submitted by Monsanto Ltd).
NOTOX (1987j) Assessment of acute eye irritation by MON 14477 in the
rabbit (Monsanto project No. NO-87-288). 's-Hertogenbosch, The
Netherlands, NOTOX - Toxicological Research & Consultancy
(Unpublished report No. NOTOX 0645/821 submitted by Monsanto Ltd).
NOTOX (1988) Acute oral toxicity of glyphosate in the rat.
's-Hertogenbosch, The Netherlands, NOTOX - Toxicological Research &
Consultancy (Unpublished report No. RCC NOTOX 1111/1428 submitted by
Agrichem B.V., The Netherlands).
NOTOX (1989a) Acute eye irritation/corrosion study with Glyposaat
360 g/l in rabbits. 's-Hertogenbosch, The Netherlands, NOTOX -
Toxicological Research & Consultancy (Unpublished report No. RCC
NOTOX 1268/171 (002082) submitted by Luxan B.V., The Netherlands).
NOTOX (1989b) Assessment of acute dermal toxicity with Glyposaat
360 g/l in the rat. 's-Hertogenbosch, The Netherlands, NOTOX -
Toxicological Research & Consultancy (Unpublished report No. RCC
NOTOX 1268/1718 (002071) submitted by Luxan B.V., The Netherlands).
NOTOX (1989c) Assessment of acute oral toxicity with Glyposaat
360 g/l in the rat. 's-Hertogenbosch, The Netherlands, NOTOX -
Toxicological Research & Consultancy (Unpublished report No. RCC
NOTOX 1268/1715 (002069) submitted by Luxan B.V., The Netherlands).
NOTOX (1989d) Primary skin irritation/corrosion study with Glyposaat
360 g/l in rabbits (4-hour semi-occlusive application).
's-Hertogenbosch, The Netherlands, NOTOX - Toxicological Research &
Consultancy (Unpublished report No. RCC NOTOX 1268/1716 submitted by
Luxan B.V., The Netherlands).
NOTOX (1990a) Acute eye irritation/corrosion study with Agrichem
glyposaat 2 (RCC NOTOX substance 11934) in the rabbit.
's-Hertogenbosch, The Netherlands, NOTOX - Toxicological Research &
Consultancy (Unpublished report No. RCC NOTOX 042492 submitted by
Agrichem B.V., The Netherlands).
NOTOX (1990b) Primary skin irritation/corrosion study with Agrichem
glyposaat 2 (RCC NOTOX substance 11961) in rabbits (4-hour
semi-occlusive application). 's-Hertogenbosch, The Netherlands,
NOTOX - Toxicological Research & Consultancy (Unpublished report No.
RCC NOTOX 042525 submitted by Agrichem B.V., The Netherlands).
NOTOX (1990c) Acute eye irritation/corrosion study with Agrichem
glyposaat B (RCC NOTOX substance 4175) in the rabbit.
's-Hertogenbosch, The Netherlands, NOTOX - Toxicological Research &
Consultancy (Unpublished report No. RCC NOTOX 027934 submitted by
Agrichem B.V., The Netherlands).
NOTOX (1990d) Primary skin irritation/corrosion study with Glyposaat
B (RCC NOTOX substance 4175) in rabbits (4-hour semi-occlusive
application). 's-Hertogenbosch, The Netherlands, NOTOX -
Toxicological Research & Consultancy (Unpublished report No. RCC
NOTOX 027923 submitted by Agrichem B.V., The Netherlands).
NOTOX (1990e) Assessment of acute oral toxicity with Glyposaat B
(RCC NOTOX substance 4175) in the rat. 's-Hertogenbosch, the
Netherlands, NOTOX - Toxicological Research & Consultancy
(Unpublished report No. RCC NOTOX 027901 submitted by Agrichem B.V.,
The Netherlands).
NOTOX (1990f) Assessment of acute dermal toxicity with Glyposaat B
(RCC NOTOX substance 4175) in the rat. 's-Hertogenbosch, The
Netherlands, NOTOX - Toxicological Research & Consultancy
(Unpublished No. RCC NOTOX 027912 submitted by Agrichem B.V., The
Netherlands).
NTP (1992) NTP technical report on toxicity studies of glyphosate
(CAS No.
Table 25. Indications of environmental hazard for aquatic organisms by technical grade glyphosate
Effect Organisms Estimated exposure Toxicity data End-point Ratio of exposure Possible
concentration (mg a.i./litre) to toxicity hazard
(mg a.i./litre) concentrations
Mean estimated exposure concentration
Acute microorganisms 0.1 EC50 = 1.2 mortality 0.082 small
Acute insects 0.1 EC50 = 55 mortality 0.0018 negligible
Acute crustaceans 0.1 EC50 = 281 mortality 0.00035 negligible
Acute fish 0.1 LC50 = 94 mortality 0.0010 negligible
Chronic algae 0.05 NOEC = 0.3 growth 0.17 present
Chronic crustaceans 0.01 NOEC = 100 reproduction 0.00010 negligible
Chronic fish 0.01 NOEC = 52 behaviour 0.00019 negligible
Maximum estimated exposure concentration
Acute microorganisms 1.7 EC50 = 1.2 mortality 1.4 large
Acute insects 1.7 EC50 = 55 mortality 0.031 small
Acute crustaceans 1.7 EC50 = 281 mortality 0.0060 negligible
Acute fish 1.7 LC50 = 94 mortality 0.018 small
Chronic algae 1.7 NOEC = 0.3 growth 5.7 large
Chronic crustaceans 0.17 NOEC = 100 reproduction 0.0017 negligible
Chronic fish 0.17 NOEC = 52 behaviour 0.003 negligible
Table 26. Indications of environmental hazards for aquatic organisms by Roundup
Effect Organisms Estimated exposure Toxicity data End-point Ratio of exposure Possible
concentration (mg Roundup/litre) to toxicity hazard
(mg Roundup/litre) concentrations
Mean estimated exposure concentration
Acute microorganisms 0.32 EC50 = 2.1 mortality 0.15 present
Acute crustaceans 0.32 EC50 = 10 mortality 0.032 small
Acute insects 0.32 EC50 = 44 mortality 0.0073 negligible
Acute fish 0.32 LC50 = 13 mortality 0.025 small
Chronic microorganisms 0.16 NOEC = 0.7 growth 0.23 present
Chronic crustaceans 0.032 NOEC = 3.5 reproduction 0.0091 negligible
Chronic fish 0.032 NOEC = 2.4 behaviour 0.013 negligible
Maximum estimated exposure concentration
Acute microorganisms 5.6 EC50 = 2.1 mortality 2.7 large
Acute crustaceans 5.6 EC50 = 10 mortality 0.56 present
Acute insects 5.6 EC50 = 44 mortality 0.13 present
Acute fish 5.6 LC50 = 13 mortality 0.43 present
Chronic microorganisms 5.6 NOEC = 0.7 growth 8.0 large
Chronic crustaceans 0.56 NOEC = 3.5 reproduction 0.16 small
Chronic fish 0.56 NOEC = 2.4 behaviour 0.23 small
11. RECOMMENDATIONS FOR PROTECTION OF HUMAN HEALTH
a) Protective clothing is necessary to ensure the safety of
herbicide applicators.
b) A market-basket survey would be useful to determine the
possible exposure of the general population.
12. FURTHER RESEARCH
a) Further research is required to determine whether ß-adrenergic
effects observed in rodents have any implications for human
health.
b) The role of adjuvants in the toxicity of glyphosate
formulations needs to be investigated further in laboratory
mammals and organisms in the environment.
c) A controlled study on exposure of agricultural workers is
needed.
d) The bioavailability of sediment- and soil-bound glyphosate in
the environment should be studied.
e) Studies on the environmental behaviour and fate of adjuvants
are required.
f) Further toxicity studies of sediment-living organisms are
needed.
g) The effects of phosphate fertilizers on the binding of
glyphosate to soils should be investigated.
h) Analytical techniques that are less costly but still adequate
should be developed.
REFERENCES
ABC Inc. (1978a) Acute toxicity of technical glyphosate (AB-78-201)
to Daphnia magna. Columbia, Missouri, Analytical Biochemistry
Laboratories, Inc. (Unpublished report submitted by Monsanto Ltd).
ABC Inc. (1978b) Acute toxicity of technical glyphosate (AB-78-165)
to rainbow trout (Salmo gairdnerii). Columbia, Missouri,
Analytical Biochemistry Laboratories, Inc. (Unpublished report
submitted by Monsanto Ltd).
ABC Inc. (1978c) Acute toxicity of technical glyphosate to bluegill
sunfish (Lepomis macrochirus). Columbia, Missouri, Analytical
Biochemistry Laboratories, Inc. (Unpublished report submitted by
Monsanto Ltd).
ABC Inc. (1978d) The effect of glyphosate on nitrogen fixation and
nitrification in soil with time. Columbia, Missouri, Analytical
Biochemistry Laboratories, Inc. (Unpublished report submitted by
Monsanto Ltd).
ABC Inc. (1978e) The effect of glyphosate on the degradation of
cellulose, starch, protein, and leaf litter on soil. Columbia,
Missouri, Analytical Biochemistry Laboratories, Inc. (Unpublished
report submitted by Monsanto Ltd).
ABC Inc. (1980a) Acute toxicity of MON-0139-X-77 (AB-80-262) to
rainbow trout (Salmo gairdnerii). Columbia, Missouri, Analytical
Biochemistry Laboratories, Inc. (Unpublished report submitted by
Monsanto Ltd).
ABC Inc. (1980b) Acute toxicity of MON-0139-X-77 (AB-80-263) to
bluegill sunfish (Lepomis macrochirus). Columbia, Missouri,
Analytical Biochemistry Laboratories, Inc. (Unpublished report
submitted by Monsanto Ltd).
ABC Inc. (1981a) Acute toxicity of MON 0139 (Lot LURT 12011)
(AB-81-074) to Daphnia magna. Columbia, Missouri, Analytical
Biochemistry Laboratories, Inc. (Unpublished report submitted by
Monsanto Ltd).
ABC Inc. (1981b) Acute toxicity of MON 0139 (Lot LURT 12011)
(AB-81-073) to Lepomis macrochirus. Columbia, Missouri, Analytical
Biochemistry Laboratories, Inc. (Unpublished report submitted by
Monsanto Ltd).
ABC Inc. (1981c) Acute toxicity of MON 0139 (Lot LURT 12011)
(AB-81-072) to rainbow trout (Salmo gairdnerii). Columbia,
Missouri, Analytical Biochemistry Laboratories, Inc. (Unpublished
report submitted by Monsanto Ltd).
ABC Inc. (1981d) A short-term bioconcentration study of
14C-glyphosate with channel catfish (Ictalurus punctatus) in a
static system. Columbia, Missouri, Analytical Biochemistry
Laboratories, Inc. (Unpublished report submitted by Monsanto Ltd).
ABC Inc. (1982a) Dynamic 96-hour acute toxicity of Roundup
(AB-82-33) to bluegill sunfish (Lepomis macrochirus). Columbia,
Missouri, Analytical Biochemistry Laboratories, Inc. (Unpublished
report submitted by Monsanto Ltd).
ABC Inc. (1982b) Dynamic 48-hour acute toxicity of Roundup
(AB-82-035) to Gammarus pseudolimnaeus. Columbia, Missouri,
Analytical Biochemistry Laboratories, Inc. (Unpublished report
submitted by Monsanto Ltd).
ABC Inc. (1982c) Dynamic 96-hour acute toxicity of Roundup
(AB-82-34) to rainbow trout (Salmo gairdnerii). Columbia,
Missouri, Analytical Biochemistry Laboratories, Inc. (Unpublished
report submitted by Monsanto Ltd).
ABC Inc. (1982d) Residue accumulation study in molluscs (Rangia
cuneata) with 14C-glyphosate under static conditions. Columbia,
Missouri, Analytical Biochemistry Laboratories, Inc. (Unpublished
report submitted by Monsanto Ltd).
ABC Inc. (1982e) Residue accumulation study in crayfish
(Procambarus simulans) with 14C-glyphosate under static
conditions. Columbia, Missouri, Analytical Biochemistry
Laboratories, Inc. (Unpublished report submitted by Monsanto Ltd).
ABC Inc. (1984a) Acute toxicity of LLN 8306 to rainbow trout (Salmo
gairdnerii). Columbia, Missouri, Analytical Biochemistry
Laboratories, Inc. (Unpublished report submitted by Monsanto Ltd).
ABC Inc. (1984b) Acute toxicity of LLN 8306 to bluegill sunfish
(Lepomis macrochirus). Columbia, Missouri, Analytical Biochemistry
Laboratories, Inc. (Unpublished report submitted by Monsanto Ltd).
ABC Inc. (1984c) Acute toxicity of LLN 8306 to Daphnia magna.
Columbia, Missouri, Analytical Biochemistry Laboratories, Inc.
(Unpublished report submitted by Monsanto Ltd).
ABC Inc. (1989a) 21-day prolonged static renewal toxicity of MON
8755 to Daphnia magna. Columbia, Missouri, Analytical Biochemistry
Laboratories, Inc. (Unpublished report No. 37933 submitted by
Monsanto Ltd).
ABC Inc. (1989b) 21-day prolonged static renewal toxicity of Roundup
to Daphnia magna. Columbia, Missouri, Analytical Biochemistry
Laboratories, Inc. (Unpublished report No. 37934 submitted by
Monsanto Ltd).
ABC Inc. (1989c) 21-day prolonged static renewal toxicity of
glyphosate technical to Daphnia magna. Columbia, Missouri,
Analytical Biochemistry Laboratories, Inc. (Unpublished report No.
37757 submitted by Monsanto Ltd).
ABC Inc. (1989d) Flow-through toxicity of Roundup to rainbow trout
(Salmo gairdnerii) for a 21-day exposure period. Columbia,
Missouri, Analytical Biochemistry Laboratories, Inc. (Unpublished
report No. 37694 submitted by Monsanto Ltd).
ABC Inc. (1989e) Flow-through toxicity of glyphosate to rainbow
trout (Salmo gairdnerii) for a 21-day duration period. Columbia,
Missouri, Analytical Biochemistry Laboratories, Inc. (Unpublished
report No. 37695 submitted by Monsanto Ltd).
ABC Inc. (1989f) Uptake, depuration, and bioconcentration of
14C glyphosate to bluegill sunfish (Lepomis macrochirus). Part
I: MSL-9304. Columbia, Missouri, Analytical Biochemistry
Laboratories, Inc. (Unpublished report submitted by Monsanto Ltd).
ABC Inc. (1989g) Uptake, depuration, and bioconcentration of
14C glyphosate to bluegill sunfish (Lepomis macrochirus).
Characterization and quantitation of glyphosate and its metabolites.
Part II: MSL-9303. Columbia, Missouri, Analytical Biochemistry
Laboratories, Inc. (Unpublished report submitted by Monsanto Ltd).
ABC Inc. (1989h) Flow-through toxicity of MON 8755 to rainbow trout
(Salmo gairdnerii) for a 21-day duration period. Columbia,
Missouri, Analytical Biochemistry Laboratories, Inc. (Unpublished
report No. 37759 submitted by Monsanto Ltd).
ABC Inc. (1990) Acute toxicity of MON 8755 to common carp (Cyprinus
carpio). Columbia, Missouri, Analytical Biochemistry Laboratories,
Inc. (Unpublished report No. 38256 submitted by Monsanto Ltd).
Ackurst P (1989) Introduction: issues and concerns relating to the
use of herbicides. In: Reynolds P ed. Proceedings of the Carnation
Creek Workshop, Nanaimo, 7-10 December 1987. Victoria, British
Columbia, Forestry Canada/British Columbia Ministry of Forests, pp
7-11.
Altmann G (1984) Laboratory testing of your preparation LLN 83/06
for danger to bees. Unpublished data of University of Saarland,
Saarbrücken, Department of Zoology, provided by Monsanto.
Amrhein N, Deus B, Gehrke P, & Steinrücken HC (1980) The site of the
inhibition of the shikimate pathway by glyphosate. Plant Physiol,
66: 830-834.
Anthony RG & Morrison ML (1985) Influence of glyphosate herbicide on
small-mammal populations in western Oregon. Northwest Sci, 59(3):
159-168.
Austin AP, Harris GE, & Lucey WP (1991) Impact of an organophosphate
herbicide (glyphosate) on periphyton communities developed in
experimental streams. Bull Environ Contam Toxicol, 47: 29-35.
Bababunmi EA, Olorunsogo OO, & Bassir O (1979) The uncoupling effect
of N-(phosphonomethyl)glycine on isolated rat liver mitochondria.
Biochem Pharmacol, 18: 925-927.
Balthazor TM & Hallas LE (1986) Glyphosate-degrading microorganisms
from industrial activated sludge. Appl Environ Microbiol, 51(2):
432-434.
Benova EK, Roupova I, Yagova A, Vuglenov A, & Bineva M (1989)
Mutagenicity studies on six pesticides in mice. Environ Mol Mutagen,
14(Suppl 15): 19 (Abstract).
Bio/Dynamics Inc. (1975) A twenty-one day dermal toxicity study of
MON 2139 in male rabbits (Project No. 75-1245). East Millstone, New
Jersey, Bio/Dynamics Inc., Division of Biology and Safety Evaluation
(Unpublished report submitted by Monsanto Ltd).
Bio/Dynamics Inc. (1979) A three month feeding study of glyphosate
(Roundup technical) in mice (Project No. 77-211) - Final report.
East Millstone, New Jersey, Bio/Dynamics Inc., Division of Biology
and Safety Evaluation (Unpublished report submitted by Monsanto
Ltd).
Bio/Dynamics Inc. (1981a) A lifetime feeding study of glyphosate
(Roundup technical) in rats (Project No. 410/77 [BDN-77-416]). East
Millstone, New Jersey, Bio/Dynamics Inc., Division of Biology and
Safety Evaluation (Unpublished report submitted by Monsanto Ltd).
Bio/Dynamics Inc. (1981b) A three-generation reproduction study in
rats with glyphosate (Project No. 77-2063 [BDN-77-147]) - Final
report. East Millstone, New Jersey, Bio/Dynamics Inc., Division of
Biology and Safety Evaluation (Unpublished report submitted by
Monsanto Ltd).
Bio/Dynamics Inc. (1983a) A chronic feeding study of glyphosate
(Roundup technical) in mice (Project No. 77-2061 [BDN-77-420]). East
Millstone, New Jersey, Bio/Dynamics Inc., Division of Biology and
Safety Evaluation (Unpublished report submitted by Monsanto Ltd).
Bio/Dynamics Inc. (1983b) A dermal sensitization study in guinea
pigs - Test material: Roundup formulation (Project No. 4234-83).
East Millstone, New Jersey, Bio/Dynamics Inc., Division of Biology
and Safety Evaluation (Unpublished report submitted by Monsanto Ltd,
Reference No. BD-83-007).
Bio/Dynamics Inc. (1983c) A dermal sensitization study in guinea
pigs - Test material: glyphosate (Project No. 4235-82). East
Millstone, New Jersey, Bio/Dynamics Inc., Division of Biology and
Safety Evaluation (Unpublished report submitted by Monsanto Ltd,
Reference No. BD-83-008).
Bio/Dynamics Inc. (1984a) Acute dermal toxicity study in rabbits -
Test material: LLN-83-06 (Project No. 4885-83). East Millstone, New
Jersey, Bio/Dynamics Inc., Department of Toxicology (Unpublished
report submitted by Monsanto Ltd, Reference No. BD-83-380).
Bio/Dynamics Inc. (1984b) Acute oral toxicity study in rats - Test
material: LLN-83-06 (Project No. 4884-83). East Millstone, New
Jersey, Bio/Dynamics Inc., Department of Toxicology (Unpublished
report submitted by Monsanto Ltd, Reference No. BD-83-380).
Bio/Dynamics Inc. (1984c) Primary dermal irritation study in rabbits
(4-hour exposure) - Test material: LLN-83-06 (Project No. 4886-83).
East Millstone, New Jersey, Bio/Dynamics Inc., Department of
Toxicology (Unpublished report submitted by Monsanto Ltd, Reference
No. BD-83-380).
Bio/Dynamics Inc. (1984d) Eye irritation study in rabbits - Test
material: LLN-83-06 (Project No. 4887-83). East Millstone, New
Jersey, Bio/Dynamics Inc., Department of Toxicology (Unpublished
report submitted by Monsanto Ltd, Reference No. BD-83-380).
Bio/Dynamics Inc. (1985a) Acute oral toxicity study in rats - Test
material: MON 8780 (Project No. 5301-84). East Millstone, New
Jersey, Bio/Dynamics Inc., Department of Toxicology (Unpublished
report submitted by Monsanto Ltd, Reference No. BD-84-343).
Bio/Dynamics Inc. (1985b) Acute dermal toxicity study in rabbits -
Test material: MON 8780 (Project No. 5302-84). East Millstone, New
Jersey, Bio/Dynamics Inc., Department of Toxicology (Unpublished
report submitted by Monsanto Ltd, Reference No. BD-84-343).
Bio/Dynamics Inc. (1985c) Primary dermal irritation study in rabbits
(4-hour exposure) - Test material: MON 8780 (Project No. 5303-84).
East Millstone, New Jersey, Bio/Dynamics Inc., Department of
Toxicology (Unpublished report submitted by Monsanto Ltd).
Bio/Dynamics Inc. (1985d) Eye irritation study in rabbits - Test
material: MON 8780 (Project No. 5304-84). East Millstone, New
Jersey, Bio/Dynamics Inc., Department of Toxicology (Unpublished
report submitted by Monsanto Ltd, Reference No. BD-84-343).
Bio/Dynamics Inc. (1986) A closed-patch repeated insult dermal
sensitization study in guinea pigs (Buehler method) - Test material:
MON 8756 (Project No. 6210-85). East Millstone, New Jersey,
Bio/Dynamics Inc., Department of Toxicology (Unpublished report
submitted by Monsanto Ltd, Reference No. BD-86-5).
Bio/Dynamics Inc. (1987a) Acute oral toxicity study in rats - Test
material: Roundup L&G Ready-to-use (Project No. 4033-86). East
Millstone, New Jersey, Bio/Dynamics Inc., Department of Toxicology
(Unpublished report submitted by Monsanto Ltd, Reference No.
BD-87-4).
Bio/Dynamics Inc. (1987b) Acute dermal toxicity study in rabbits -
Test material: Roundup L&G Ready-to-use (Project No. 4034-86). East
Millstone, New Jersey, Bio/Dynamics Inc., Department of Toxicology
(Unpublished report submitted by Monsanto Ltd, Reference
No. BD-87-3).
Bio/Dynamics Inc. (1987c) Primary dermal irritation study in rabbits
(4-hour exposure/semi-occlusive covering) - Test material: Roundup
L&G Ready-to-Use (Project No. 4035-86). East Millstone, New Jersey,
Bio/Dynamics Inc., Department of Toxicology (Unpublished report
submitted by Monsanto Ltd, Reference No. BD-87-4).
Bio/Dynamics Inc. (1987d) Eye irritation study in rabbits - Test
material: Roundup L&G Ready-to-Use (Project No. 4036-86). East
Millstone, New Jersey, Bio/Dynamics Inc., Department of Toxicology
(Unpublished report submitted by Monsanto Ltd, Reference
No. BD-87-4).
Bio/Dynamics Inc. (1987e) A closed-patch repeated insult dermal
sensitization study in guinea pigs (Project No. 6945-86). East
Millstone, New Jersey, Bio/Dynamics Inc., Division of Biology and
Safety Evaluation (Unpublished report submitted by Monsanto Ltd,
Reference No. BD-86-442).
Bio/Dynamics Inc. (1988a) Acute dermal toxicity study in rabbits -
Test material: Glyphosate Wet Cake (Project No. 4886-88). East
Millstone, New Jersey, Bio/Dynamics Inc., Department of Toxicology
(Unpublished report submitted by Monsanto Ltd, Reference No.
BD-88-114).
Bio/Dynamics Inc. (1988b) Primary dermal irritation study in rabbits
(4-hour exposure/semi-occlusive covering) - Test material:
Glyphosate Wet Cake (Project No. 4887-88). East Millstone, New
Jersey, Bio/Dynamics Inc., Department of Toxicology (Unpublished
report submitted by Monsanto Ltd, Reference No. BD-88-114).
Bio/Dynamics Inc. (1988c) Acute oral toxicity study in rats - Test
material: Glyphosate Wet Cake (Project No. 4885-88). East Millstone,
New Jersey, Bio/Dynamics Inc., Department of Toxicology (Unpublished
report submitted by Monsanto Ltd, Reference No. BD-88-114).
Bio/Dynamics Inc. (1988d) Eye irritation study in rabbits - Test
material: Glyphosate Wet Cake (Project No. 4888-88). East Millstone,
New Jersey, Bio/Dynamics Inc., Department of Toxicology (Unpublished
report submitted by Montsanto Ltd, Reference No. BD-88-114).
Bio/Dynamics Inc. (1988e) Acute oral toxicity study in rats - Test
material: Roundup (Project No. 4546-87). East Millstone, New Jersey,
Bio/Dynamics Inc., Department of Toxicology (Unpublished report
submitted by Monsanto Ltd, Reference No. BD-87-283).
Bio/Dynamics Inc. (1988f) Acute dermal toxicity study in rabbits -
Test material: Roundup (Project No. 4547-87). East Millstone, New
Jersey, Bio/Dynamics Inc., Department of Toxicology (Unpublished
report submitted by Monsanto Ltd, Reference No. BD-87-283).
Bio/Dynamics Inc. (1988g) Primary dermal irritation study in rabbits
(4-hour exposure/semi-occlusive covering) - Test material: Roundup
(Project No. 4548-87). East Millstone, New Jersey, Bio/Dynamics
Inc., Department of Toxicology (Unpublished report submitted by
Monsanto Ltd, Reference No. BD-87-283).
Bio/Dynamics Inc. (1990) Eye irritation study in rabbits - Test
material: MON 2139 (Project No. 5833-90). East Millstone, New
Jersey, Bio/Dynamics Inc., Department of Toxicology (Unpublished
report submitted by Monsanto, Reference No. BD-90-246).
Bionomics (1973a) Acute toxicity of Roundup (technical) to atlantic
oyster (Crassostrea virginica). Wareham, Massachusetts, Bionomics
(Unpublished report submitted by Monsanto Ltd).
Bionomics (1973b) Acute toxicity of Roundup (technical) to grass
shrimp (Palaemonetes vulgaris) and fiddler crab (Uca pugilator).
Wareham, Massachusetts, Bionomics (Unpublished report submitted by
Monsanto Ltd).
Bowmer KH, Boulton PM, Short DL, & Higgins ML (1986)
Glyphosate-sediment interactions and phytotoxicity in turbid water.
Pestic Sci, 17(2): 79-88.
Bozeman J, Koopman B, & Bitton G (1989) Toxicity testing using
immobilized algae. Aquat Toxicol, 14(4): 345-352.
Buhl KJ & Faerber NL (1989) Acute toxicity of selected herbicides
and surfactants to larvae of the midge Chironomus riparius. Arch
Environ Contam Toxicol, 18(4): 530-536.
Bunyatyan YA & Gevorgyan AA (1984) [Determination of the active
ingredient of Roundup and its metabolite in the environment.] Gig i
Sanit, 5: 43-44 (in Russian).
Burns AJ (1983) Liquid chromatographic determination of glyphosate
technical and its formulation: collaborative study. J Assoc Off Anal
Chem, 66(5): 1214-1219.
Carlisle SM & Trevors JT (1986a) Effect of the herbicide glyphosate
on nitrification, denitrification, and acetylene reduction in soil.
Water Air Soil Pollut, 29(2): 189-203.
Carlisle SM & Trevors JT (1986b) Effect of the herbicide glyphosate
on respiration and hydrogen consumption in soil. Water Air Soil
Pollut, 27(3/4): 391-401.
Chakravarty P & Chatarpaul L (1990a) Non-target effects of
herbicides: I. Effect of glyphosate and hexazinone on soil microbial
activity, microbial population, and in vitro growth of
ectomycorrhizal fungi. Pestic Sci, 28(3): 233-242.
Chakravarty P & Chatarpaul L (1990b) Non-target effect of
herbicides: II. The influence of glyphosate on ectomycorrhizal
symbiosis of red pine (Pinus resinosa) under greenhouse and field
conditions. Pestic Sci, 28(3): 243-248.
Chan KY & Leung SC (1986) Effects of paraquat and glyphosate on
growth, respiration, and enzyme activity of aquatic bacteria. Bull
Environ Contam Toxicol, 36(1): 52-59.
CIT (1991a) EXP 30578 - Acute oral toxicity in rats (Study No. 7076
TAR). Miserey, France, Centre International de Toxicologie (CIT)
(Unpublished report submitted by Rhône Poulenc, Lyon, France).
CIT (1991b) EXP 30578 - Acute dermal toxicity in rats (Study No.
7077 TAR). Miserey, France, Centre International de Toxicologie
(CIT) (Unpublished report submitted by Rhône Poulenc, Lyon, France).
CIT (1991c) EXP 30578 - Acute dermal irritation in rabbits (Study
No. 7079 TAL). Miserey, France, Centre International de Toxicologie
(CIT) (Unpublished report submitted by Rhône Poulenc, Lyon, France).
CIT (1991d) EXP 30578 - Acute eye irritation in rabbits (Study No.
7078 TAL). Miserey, France, Centre International de Toxicologie
(CIT) (Unpublished report submitted by Rhône Poulenc, Lyon, France).
Cowell JE, Kunstman JL, Nord PJ, Steinmetz JR, & Wilson GR (1986)
Validation of an analytical residue method for analysis of
glyphosate and metabolite: an interlaboratory study. J Agric Food
Chem, 34(6): 955-960.
Deyrup CL, Chang SM, Weintraub RA, & Moye HA (1985) Simultaneous
esterification and acylation of pesticides for analysis by gas
chromatography. 1. Derivatization of glyphosate and
(aminomethyl)phosphonic acid with fluorinated
alcohols-perfluorinated anhydrides. J Agric Food Chem, 33(5):
944-947.
Dickson SJ, Meinhold RH, Beer ID, & Koelmeyer TD (1988) Rapid
determination of glyphosate in postmortem specimens using 31P NMR. J
Anal Toxicol, 12(5): 284-286.
D'Anieri P, Leslie DM, & McCormack ML (1987) Small mammals in
glyphosate-treated clearcuts in Northern Maine. Can Field-Nat,
101(4): 547-550.
Dubelman S (1988) Glyphosate. Anal Methods Pestic Plant Growth
Regul, 16: 69-82.
Duke SO & Hoagland RE (1981) Effects of glyphosate on the metabolism
of phenolic compounds: VII. Root-fed amino acids and glyphosate
toxicity in soybean (Glycine max) seedlings. Weed Sci, 29(3):
297-302.
Eberbach PL & Douglas LA (1983) Persistence of glyphosate in a sandy
loam. Soil Biol Biochem, 15(4): 485-487.
Eberbach PL & Douglas LA (1989) Herbicide effects on the growth and
nodulation potential of Rhizobium trifolii with Trifolium
subterraneum L. Plant Soil, 119: 15-23.
Edwards WM, Triplett GB, & Kramer RM (1980) A watershed study of
glyphosate transport in runoff. J Environ Qual, 9(4): 661-664.
EG & G Bionomics (1975) Chronic toxicity of glyphosate to the
fathead minnow (Pimephales promelas Rafinesque). Aquatic
Toxicology Laboratory. Wareham, Massachusetts, EG & G Bionomics
(Unpublished report submitted by Monsanto Ltd).
EG & G Bionomics (1978a) Toxicity of seven test materials to the
marine alga, Skeletonema costatum. Marine Research Laboratory,
Pensacola, Florida, EG & G Bionomics (Unpublished report No.
BP-78-4-031 submitted by Monsanto Ltd).
EG & G Bionomics (1978b) Toxicity of seven test materials to
sheepshead minnows, Cyprinodon variegatus. Marine Research
Laboratory, Pensacola, Florida, EG & G Bionomics (Unpublished report
No. BP-78-4-029 submitted by Monsanto Ltd).
EG & G Bionomics (1978c) Toxicity of seven test materials to mysid
shrimp, Mysidopsis bahia. Marine Research Laboratory, Pensacola,
Florida, EG & G Bionomics (Unpublished report No. BP-78-4-032
submitted by Monsanto Ltd).
EG & G Bionomics (1978d) Toxicity of seven test materials to the
white sea urchin, Tripneustes esculentus. Marine Research
Laboratory, Pensacola, Florida, EG & G Bionomics (Unpublished report
No. BP-78-4-030 submitted by Monsanto Ltd).
EG & G Bionomics (1980a) Acute toxicity of Roundup to channel
catfish (Ictalurus punctatus). Aquatic Toxicology Laboratory.
Wareham, Massachusetts, EG & G Bionomics (Unpublished report No.
BW-80-4-641 submitted by Monsanto Ltd).
EG & G Bionomics (1980b) Acute toxicity of Roundup to bluegill
(Lepomis macrochirus). Aquatic Toxicology Laboratory. Wareham,
Massachusetts, EG & G Bionomics (Unpublished report No. BW-80-4-634
submitted by Monsanto Ltd).
EG & G Bionomics (1980c) Acute toxicity of Roundup to the rainbow
trout (Salmo gairdnerii). Aquatic Toxicology Laboratory. Wareham,
Massachusetts, EG & G Bionomics (Unpublished report No. BW-80-4-635
submitted by Monsanto Ltd).
EG & G Bionomics (1980d) Acute toxicity of Roundup to the fathead
minnow (Pimephales promelas). Aquatic Toxicology Laboratory.
Wareham, Massachusetts, EG & G Bionomics (Unpublished report No.
BW-80-4-653 submitted by Monsanto Ltd).
EG & G Bionomics (1980e) Acute toxicity of Roundup to the water flea
(Daphnia magna). Aquatic Toxicology Laboratory. Wareham,
Massachusetts, EG & G Bionomics (Unpublished report No. BW-80-4-636
submitted by Monsanto Ltd).
EG & G Bionomics (1980f) Acute toxicity of Roundup to the water flea
(Daphnia magna) with and without continuous aeration. Aquatic
Toxicology Laboratory. Wareham, Massachusetts, EG & G Bionomics
(Unpublished report No. BW-80-6-690 submitted by Monsanto Ltd).
EG & G Bionomics (1980g) Acute toxicity of Roundup to the rainbow
trout (Salmo gairdnerii) with continuous aeration and without
aeration. Aquatic Toxicology Laboratory. Wareham, Massachusetts,
EG & G Bionomics (Unpublished report No. BW-80-6-686 submitted by
Monsanto Ltd).
Estok D, Freedman B, & Boyle D (1989) Effects of the herbicides
2,4-D, glyphosate, hexazinone, and triclopyr on the growth of three
species of ectomycorrhizal fungi. Bull Environ Contam Toxicol,
42(6): 835-839.
Evans DD & Batty MJ (1986) Effects of high dietary concentrations of
glyphosate (Roundup) on a species of bird, marsupial and rodent
indigenous to Australia. Environ Toxicol Chem, 5: 399-401.
EVS Consultants (1986a) Acute toxicity of Roundup herbicide to
rainbow trout. Seattle, Washington, EVS Consultants (Unpublished
report submitted by Monsanto Ltd).
EVS Consultants (1986b) Acute toxicity of Roundup herbicide to
chinook salmon. Seattle, Washington, EVS Consultants (Unpublished
report submitted by Monsanto Ltd).
EVS Consultants (1986c) Acute toxicity of Roundup herbicide to coho
salmon. Seattle, Washington, EVS Consultants (Unpublished report
submitted by Monsanto Ltd). EVS Consultants (1986d) Seawater
challenge testing of coho salmon smolts following Roundupr herbicide
exposure. Seattle, Washington, EVS Consultants (Unpublished report
submitted by Monsanto Ltd).
FAO/WHO (1986a) Pesticide residues in food - Evaluations 1986. Part
I - Residues. Joint Meeting of the FAO Panel of Experts Residues in
Food and the Environment and the WHO Expert Group on Pesticide
Residues, Rome, 29 September-8 October 1986. Rome, Food and
Agriculture Organization of the United Nations (FAO Plant Production
and Protection Paper 78/1).
FAO/WHO (1986b) Pesticide residues in food - Evaluations 1986. Part
II - Toxicology. Joint Meeting of the FAO Panel of Experts Residues
in Food and the Environment and the WHO Expert Group on Pesticide
Residues, Rome, 29 September-8 October 1986. Rome, Food and
Agriculture Organization of the United Nations (FAO Plant Production
and Protection Paper 78/2).
FAO/WHO (1987) Pesticide residues in food - Evaluations 1987. Part I
- Residues. Joint Meeting of the FAO Panel of Experts Residues in
Food and the Environment and the WHO Expert Group on Pesticide
Residues, Geneva, 21-30 September 1987. Rome, Food and Agriculture
Organization of the United Nations (FAO Plant Production and
Protection Paper 86/1).
FAO/WHO (1988) Pesticide residues in food - Evaluations 1988. Part I
- Residues. Joint Meeting of the FAO Panel of Experts Residues in
Food and the Environment and the WHO Expert Group on Pesticide
Residues, Rome, 19-28 September 1988. Rome, Food and Agriculture
Organization of the United Nations (FAO Plant Production and
Protection Paper 93/1).
FDRL (1988a) Primary dermal irritation study of glyphosate
batch/lot/nbr No. XLI-55 in New Zealand White rabbits (Study No.
88.2053.010). Waverly, New York, Food & Drug Research Laboratories
(Unpublished report submitted by Monsanto Ltd - Monsanto study No.
FD-88-29).
FDRL (1988b) Acute dermal toxicity study of glyphosate batch/lot/nbr
No. XLI-55 in New Zealand White rabbits (Study No. 88.2053.008).
Waverly, New York, Food & Drug Research Laboratories (Unpublished
report submitted by Monsanto Ltd - Monsanto study No. FD-88-29).
FDRL (1988c) Primary eye irritation study of glyphosate
batch/lot/nbr No. XLI-55 in New Zealand White rabbits (Study No.
88.2053.009). Waverly, New York, Food & Drug Research Laboratories
(Unpublished report submitted by Monsanto Ltd - Monsanto study No.
FD-88-29).
FDRL (1988d) Acute oral toxicity study of glyphosate batch/lot/nbr
No. XLI-55 in Sprague-Dawley rats (Study No. 88.2053.007). Waverly,
New York, Food & Drug Research Laboratories (Unpublished report
submitted by Monsanto Ltd - Monsanto study No. FD-88-29).
Feller MC (1989) Effects of forest herbicide applications on
streamwater chemistry in southwestern British Columbia. Water Resour
Bull, 25(3): 607-616.
Feng JC & Thompson DG (1990) Fate of glyphosate in a Canadian forest
watershed. 2. Persistence in foliage and soils. J Agric Food Chem,
38(4): 1118-1125.
Feng JC, Thompson DG, & Reynolds PE (1990) Fate of glyphosate in a
Canadian forest watershed. 1. Aquatic residues and off-target
deposit assessment. J Agric Food Chem, 38(4): 1110-1118.
Folmar LC, Sanders HO, & Julin AM (1979) Toxicity of the herbicide
glyphosate and several of its formulations to fish and aquatic
invertebrates. Arch Environ Contam Toxicol, 8: 269-278.
Franz TJ (1983) Evaluation of the percutaneous absorption of Roundup
formulations in man using an in vitro technique (Study No.
UW-81-346). Seattle, Washington, University of Washington, School of
Medicine (Unpublished report submitted by Monsanto Ltd).
Friestad HO & Bronstad JO (1985) Improved polarographic method for
determination of glyphosate herbicide in crops, soils, and water. J
Assoc Off Anal Chem, 68(1): 76-79.
Gauch R, Leuenberger U, & Müller U (1989) [Determination of
glyphosate herbicide and its main metabolite aminomethylphosphonic
acid (AMPA) in drinking water by HPLC.] Z Lebensm.unters Forsch,
188(1): 36-38 (in German).
Glass RL (1981) Colorimetric determination of glyphosate in water
after oxidation to orthophosphate. Anal Chem, 53(6): 921-923.
Glass RL (1983) Liquid chromatographic determination of glyphosate
in fortified soil and water. J Agric Food Chem, 31(2): 280-282.
Glass RL (1987) Adsorption of glyphosate by soils and clay minerals.
J Agric Food Chem, 35: 497-500.
Goldsborough LG & Beck AE (1989) Rapid dissipation of glyphosate in
small forest ponds. Arch Environ Contam Toxicol, 18(4): 537-544.
Goldsborough LG & Brown DJ (1988) Effect of glyphosate (Roundup
formulation) on periphytic algal photosynthesis. Bull Environ Contam
Toxicol, 41(2): 253-260.
Gómez MA & Sagardoy MA (1985) [Influence of glyphosate herbicide on
the microflora and mesofauna of a sandy soil in a semi-arid region.]
Rev Latinoam Microbiol, 27: 351-357 (in Spanish).
Gopalan HNB & Njagi GDE (1981) Mutagenicity testing of pesticides.
III. Drosophila: recessive sex-linked lethals. Genetics,
97(Suppl): 544 (Abstract).
Gougler JA & Geiger DR (1981) Uptake and distribution of
N-phosphonomethylglycine in sugar beet plants. Plant Physiol, 68:
668-672.
Gougler JA & Geiger DR (1984) Carbon partitioning and herbicide
transport in glyphosate-treated sugarbeet (Beta vulgaris). Weed
Sci, 32(4): 546-551.
Guinivan RA, Thompson NP, & Wheeler WB (1982) Derivatization and
cleanup improvements in determination of residues of glyphosate and
aminomethylphosphonic acid in blueberries. J Assoc Off Anal Chem,
65(1): 35-39.
Haag WR & Yao CCD (1992) Rate constants for reaction of hydroxyl
radicals with several drinking water contaminants. Environ Sci
Technol, 26: 1005-1013.
Hallas LE, Adams WJ, & Heitkamp MA (1992) Glyphosate degradation by
immobilized bacteria: field studies with industrial wastewater
effluent. Appl Environ Microbiol, 51: 432-434.
Hallberg GL (1989) Pesticide pollution of groundwater in the humid
United States. Agric Ecosyst Environ, 26: 299-367.
Hance RJ (1976) Adsorption of glyphosate by soils. Pestic Sci, 7:
363-366.
Hartman WA & Martin DB (1984) Effect of suspended bentonite clay on
the acute toxicity of glyphosate to Daphnia pulex and Lemna
minor. Bull Environ Contam Toxicol, 33(3): 355-361.
Hartman WA & Martin DB (1985) Effects of four agricultural
pesticides on Daphnia pulex, Lemna minor, and Potamogeton
pectinatus. Bull Environ Contam Toxicol, 35(5): 646-651.
Hazleton Laboratories, Inc. (1973a) Eight-day dietary LC50 -
bobwhite quail. Final report. Vienna, Virginia, Hazleton
Laboratories, Inc. (Unpublished report submitted by Monsanto Ltd).
Hazleton Laboratories, Inc. (1973b) Eight-day dietary LC50 -
mallard ducks. Final report. Vienna, Virginia, Hazleton
Laboratories, Inc. (Unpublished report submitted by Monsanto Ltd).
Hazleton Lab. Inc. (1988a) Metabolism study of synthetic
13C/14C-labeled glyphosate and aminomethylphosphonic acid in
laying hens, Part I. Hazleton Laboratories Inc. Project No. HLA
6103-112. Madison, Wisconsin, Monsanto Agricultural Company, Report
No. MSL-7591. Unpublished report supplied by Monsanto Ltd.
Hazleton Lab. Inc. (1988b) Metabolism study of synthetic
13C/14C-labeled glyphosate and aminomethylphosphonic acid in
lactating goats, Part I. Hazleton Laboratories Inc. Project No. HLA
6103-113. Madison, Wisconsin, Monsanto Agricultural Company, Report
No. MSL-7586. Unpublished report supplied by Monsanto Ltd.
Heinonen-Tanski H (1989) The effect of temperature and liming on the
degradation of glyphosate in two arctic forest soils. Soil Biol
Biochem, 21(2): 313-317.
Heinonen-Tanski H, Rosenberg C, Siltanen H, Kilpi S, & Simojoki P
(1985) The effect of the annual use of pesticides on soil
microorganisms, pesticide residues in the soil and barley yields.
Pestic Sci, 16(4): 341-348.
Hensley DL, Beuerman DSN, & Carpenter PL (1978) The inactivation of
glyphosate by various soils and metal salts. Weed Res, 18: 287-291.
Hernando F, Royuela M, Muñoz-Rueda A, & Gonzalez-Murua C (1989)
Effect of glyphosate on the greening process and photosynthetic
metabolism in Chlorella pyrenoidosa. J Plant Physiol, 136: 26-31.
Hildebrand LD, Sullivan DS, & Sullivan TP (1980) Effects of Roundup
herbicide on populations of Daphnia magna in a forest pond. Bull
Environ Contam Toxicol, 25(3): 353-357.
Hildebrand LD, Sullivan DS, & Sullivan TP (1982) Experimental
studies of rainbow trout populations exposed to field applications
of Roundup herbicide. Arch Environ Contam Toxicol, 11(1): 93-98.
Hoffman DJ & Albers PH (1984) Evaluation of potential embryotoxicity
and teratogenicity of 42 herbicides, insecticides, and petroleum
contaminants to mallard eggs. Arch Environ Contam Toxicol, 13(1):
15-27.
Holdway DA & Dixon DG (1988) Acute toxicity of permethrin or
glyphosate pulse exposure to larval white sucker (Catostomus
commersoni) and juvenile flagfish (Jordanella floridae) as
modified by age and ration level. Environ Toxicol Chem, 7(1): 63-68.
Holtby LB & Baillie SJ (1989a) Effects of the herbicide Roundup
(glyphosate) on periphyton in Carnation Creek, British Columbia. In:
Reynolds P ed. Proceedings of the Carnation Creek Workshop, Nanaimo,
7-10 December 1987. Victoria, British Columbia, Forestry
Canada/British Columbia Ministry of Forests, pp 224-231.
Holtby LB & Baillie SJ (1989b) Effects of the herbicide Roundup on
coho salmon fingerlings in an over-sprayed tributary of Carnation
Creek, British Columbia. In: Reynolds P ed. Proceedings of the
Carnation Creek Workshop, Nanaimo, 7-10 December 1987. Victoria,
British Columbia, Forestry Canada/British Columbia Ministry of
Forests, pp 273-285.
HRC (1972) The acute contact and oral toxicities of CP67573 and MON
2139 to worker honey bees. Huntingdon, UK, Huntingdon Research
Centre (Unpublished report submitted by Monsanto Ltd).
HRC (1977) The acute toxicity of glyphosate to harlequin fish
(Rasbora heteromorpha). Huntingdon, UK, Huntingdon Research Centre
(Unpublished report submitted by Monsanto Ltd).
HRC (1988) The acute oral toxicity of ICIA0224 to the bobwhite
quail. Huntingdon, UK, Huntingdon Research Centre (Unpublished
report No. ISN 177/881062 submitted by Monsanto Ltd).
IBR (1991a) Final report - Acute toxicity in earthworms according to
OECD 207-test article: "Technical isopropylamin salt of glyphosate =
MON 0139" (Project No. 80-91-2078-00-90). Hannover, International
Bioresearch (Unpublished report submitted by Monsanto Ltd).
IBR (1991b) Final report - Acute toxicity in earthworms according to
OECD 207-test article: "Roundup" (Project No. 80-91-0599-00-90).
Hannover, International Bioresearch (Unpublished report submitted by
Monsanto Ltd).
IET (1978) Microbial mutagenicity testing on CP67573 (glyphosate).
Mitsukaido, Japan, Institute of Environmental Toxicology (IET)
(Study sponsor Monsanto Ltd - Monsanto unpublished report No.
ET-78-241).
IFU (1990) [Side-effects of Swing on the ground-beetle Poecilus
cupreus Bonnelli under laboratory conditions (Project No.
110/01-Pc).] Unpublished report of Institute for Environmental
Analysis and Biotechnology, Niefern-Öschelbron, supplied by Monsanto
Ltd (in German).
Inveresk Research Int. (1988a) Compound No. 3607: Acute oral
toxicity (limit) test in rats (IRI project No. 241496). Musselburgh,
Scotland, Inveresk Research International (Unpublished report No.
5585 submitted by Cheminova A/S, Denmark).
Inveresk Research Int. (1988b) Compound No. 3607: Acute dermal
toxicity (limit) test in rats (IRI project No.241496). Musselburgh,
Scotland, Inveresk Research International (Unpublished report No.
5586 submitted by Cheminova A/S, Denmark).
Inveresk Research Int. (1988c) Compound 3707: Primary skin
irritation in rabbits (IRI project No. 241496). Musselburgh,
Scotland, Inveresk Research International (Unpublished report No.
5587 submitted by Cheminova A/S, Denmark).
Inveresk Research Int. (1988d) Compound No. 3607: Magnusson-Kligman
Maximisation test in guinea pigs (IRI project No. 241496).
Musselburgh, Scotland, Inveresk Research International (Unpublished
report No. 5589 submitted by Cheminova A/S, Denmark).
Inveresk Research Int. (1989a) Glyphosate technical: acute oral
toxicity (limit) test in rats (IRI project No. 243268). Musselburgh,
Scotland, Inveresk Research International (Unpublished report No.
5883 submitted by Cheminova A/S, Denmark).
Inveresk Research Int. (1989b) Glyphosate technical:
Magnusson-Kligman Maximisation test in guinea pigs (IRI project No.
243268). Musselburgh, Scotland, Inveresk Research International
(Unpublished report No. 5587 submitted by Cheminova A/S, Denmark).
Inveresk Research Int. (1989c) Glyphosate technical: acute dermal
toxicity (limit) test in rats (IRI project No. 243268). Musselburgh,
Scotland, Inveresk Research International (Unpublished report No.
5884 submitted by Cheminova A/S, Denmark).
Inveresk Research Int. (1989d) Glyphosate soluble liquid formulation
(code No. 3607) - Acute inhalation toxicity study in rats (limit
test) (IRI project No. 640023). Musselburgh, Scotland, Inveresk
Research International (Unpublished report No. 5603 submitted by
Cheminova A/S, Denmark).
Inveresk Research Int. (1989e) Glyphosate technical: Primary skin
irritation in rabbits (IRI project No. 243268). Musselburgh,
Scotland, Inveresk Research International (Unpublished report No.
5885 submitted by Cheminova A/S, Denmark).
Inveresk Research Int. (1989f) Glyphosate technical: Primary eye
irritation in rabbits (IRI project No. 243268). Musselburgh,
Scotland, Inveresk Research International (Unpublished report No.
5886 submitted by Cheminova A/S, Denmark).
Inveresk Research Int. (1989g) Compound No. 3607: Primary eye
irritation in rabbits (IRI project No. 241496). Musselburgh,
Scotland, Inveresk Research International (Unpublished report No.
5588 submitted by Cheminova A/S, Denmark).
IRDC (1980a) Test article - Technical glyphosate: Dominant lethal
study in mice (Study No. 401-064). Mattawan, Michigan, International
Research and Development Corporation (Unpublished report submitted
Monsanto Ltd, Reference No. IR-79-014).
IRDC (1980b) Test article - Technical glyphosate: Teratology study
in rats (Study No. 401-054). Mattawan, Michigan, International
Research and Development Corporation (Unpublished report submitted
by Monsanto Ltd, Reference No. IR-79-016).
IRDC (1980c) Test article - Technical glyphosate: Teratology study
in rabbits (Study No. 401-056). Mattawan, Michigan, International
Research and Development Corporation (Unpublished report submitted
by Monsanto Ltd, Reference No. IR-79-018).
IRDC (1982) Test article - Glyphosate technical: 21-day dermal
toxicity study in rabbits (Study No. 401-168). Mattawan, Michigan,
International Research and Development Corporation (Unpublished
report submitted by Monsanto Ltd, Reference No. IR-81-195).
IRPTC (1991) Data profile on glyphosate. Geneva, International
Register for Potentially Toxic Chemicals, United Nations Environment
Programme.
Jacob GS, Garbow JR, Hallas LE, Kimack NM, Kishore GM, & Schaefer J
(1988) Metabolism of glyphosate in Pseudomonas sp. strain LBr.
Appl Environ Microbiol, 54(12): 2953-2958.
Jauhiainen A, Räsänen K, Sarantila R, Nuutinen J, & Kangas J (1991)
Occupational exposure of forest workers to glyphosate during brush
saw spraying work. Am Ind Hyg Assoc J, 52(2): 61-64.
Kishore GM & Jacob GS (1987) Degradation of glyphosate by
Pseudomonas sp. PG2982 via a sarcosine intermediate. J Biol Chem,
262(25): 12164-12168.
Kitchen LM, Witt WW, & Rieck CE (1981a) Inhibition of chlorophyll
accumulation by glyphosate. Weed Sci, 29(4): 513-516.
Kitchen LM, Witt WW, & Rieck CE (1981b) Inhibition of
delta-aminolevulinic acid synthesis by glyphosate. Weed Sci, 29(5):
571-577.
Knapek R, Kobes S, & Kita I (1986) [Toxicological evaluation of
N-phosphono-methylglycine.] Z gesamte Hyg, 32(9): 537-539 (in
German).
Konar SK & Roy DN (1990) Method for the determination of residues of
the herbicide glyphosate and its principal metabolite,
aminomethylphosphonic acid, in plant materials by nitrogen-selective
gas chromatography. Anal Chim Acta, 229(2): 277-280.
Kreutzweiser DP, Kingsbury PD, & Feng JC (1989) Drift response of
stream invertebrates to aerial applications of glyphosate. Bull
Environ Contam Toxicol, 42(3): 331-338.
Lavy TL, Cowell JE, Steinmetz JR, & Massey JH (1992) Conifer
seedling nursery worker exposure to glyphosate. Arch Environ Contam
Toxicol, 22: 6-13.
Lethbridge G, Bull AT, & Burns RG (1981) Effects of pesticides on
1,3-ß-glucanase and urease activities in soil in the presence and
absence of fertilisers, lime and organic materials. Pestic Sci,
12(2): 147-155.
LISEC (1989a) Alga, growth inhibition test. Effect of MON 2139 on
the growth of Selenastrum capricornutum. Bokrijk, Belgium, LISEC,
Study Centre for Ecology and Forestry (Unpublished report submitted
by Monsanto Ltd).
LISEC (1989b) Alga, growth inhibition test. Effect of MON 8755 on
the growth of Selenastrum capricornutum. Bokrijk, Belgium, LISEC,
Study Centre for Ecology and Forestry (Unpublished report submitted
by Monsanto Ltd).
LISEC (1990a) Degradation: Biological oxygen demand. Bokrijk,
Belgium, LISEC, Study Centre for Ecology and Forestry (Unpublished
report submitted by Monsanto Ltd).
LISEC (1990b) Degradation: Chemical oxygen demand. Bokrijk, Belgium,
LISEC, Study Centre for Ecology and Forestry (Unpublished report
submitted by Monsanto Ltd). Liu C-M, McLean PA, Sookdeo CC, & Cannon
FC (1991) Degradation of the herbicide glyphosate by members of the
family Rhizobiaceae. Appl Environ Microbiol, 57: 1799-1804.
Lockhart WL, Billeck BN, & Baron CL (1989) Bioassays with a floating
aquatic plant (Lemna minor) for effects of sprayed and dissolved
glyphosate. Hydrobiologia, 188/189: 353-359.
Lundgren LN (1986) A new method for the determination of glyphosate
and (aminomethyl)phosphonic acid residues in soils. J Agric Food
Chem, 34(3): 535-538.
Lund-Hoie K & Friestad HO (1986) Photodegradation of the herbicide
glyphosate in water. Bull Environ Contam Toxicol, 36(5): 723-729.
Madhun YA, Young JL, & Freed VH (1986) Binding of herbicides by
water-soluble organic materials from soil. J Environ Qual, 15(1):
64-68.
Maibach HI (1983) (a) Elimination of 14C-glyphosate in Rhesus
monkeys following a single dose. (b) Percutaneous absorption in
Roundup formulation in Rhesus monkeys following a single oral dose
(Study No. MA-81-349). San Francisco, California, California School
of Medicine (Unpublished report submitted by Monsanto Ltd).
Maibach HI (1986) Irritation, sensitive, photoirritation and
photosensitization assays with a glyphosate herbicide. Contact
Dermatitis, 15: 152-156.
Malcolm Pirnie Inc. (1987a) The toxicity of glyphosate technical to
Anabaena flos-aquae. White Plains, New York, Malcolm Pirnie Inc.
(Unpublished report submitted by Monsanto Ltd).
Malcolm Pirnie Inc. (1987b) The toxicity of glyphosate technical to
Skeletonema costatum. White Plains, New York, Malcolm Pirnie Inc.
(Unpublished report submitted by Monsanto Ltd).
Malcolm Pirnie Inc. (1987c) The toxicity of glyphosate technical to
Navicula pelliculosa. White Plains, New York, Malcolm Pirnie Inc.
(Unpublished report submitted by Monsanto Ltd).
Malcolm Pirnie Inc. (1987d) The toxicity of glyphosate technical to
Selenastrum capricornutum. White Plains, New York, Malcolm Pirnie
Inc. (Unpublished report submitted by Monsanto Ltd).
Malcolm Pirnie Inc. (1987e) The toxicity of glyphosate technical to
Lemna gibba. White Plains, New York, Malcolm Pirnie Inc.
(Unpublished report submitted by Monsanto Ltd).
Marcotte AL, Bradley M, & Hughley DH ed. (1977) Pesticide analytical
manual. Washington, DC, Food and Drug Administration.
Marrs RH, Williams CT, Frost AJ, & Plant RA (1989) Assessment of the
effects of herbicide spray drift on a range of plant species of
conservation interest. Environ Pollut, 59(1): 71-86.
Marsh JAP (1985) Effects of herbicide-fertilizer interactions on
nitrogen and phosphorus transformations and herbicide persistence in
soil. Pestic Sci, 16(1): 93-100.
Marsh JAP, Davies HA, & Grossbard E (1977) The effect of herbicides
on respiration and transformation of nitrogen in two soils I.
Metribuzin and glyphosate. Weed Res, 17: 77-82.
Martinez TT & Brown K (1991) Oral and pulmonary toxicology of the
surfactant used in Roundup herbicide. Proc West Pharmacol Soc, 34:
43-46.
Martinez TT, Long WC, & Hiller R (1990) Comparison of the toxicology
of the herbicide Roundup by oral and pulmonary routes of exposure.
Proc West Pharmacol Soc, 33: 193-197.
Mekwatanakarn P & Sivasithamparam K (1987) Effect of certain
herbicides on saprophytic survival and biological suppression of the
take-all fungus. New Phytol, 106: 153-159.
Miles CJ & Moye HA (1988a) Postcolumn photolysis of pesticides for
fluorometric determination by high-performance liquid
chromatography. Anal Chem, 60(3): 220-226.
Miles CJ & Moye HA (1988b) Extraction of glyphosate herbicide from
soil and clay minerals and determination of residues in soils. J
Agric Food Chem, 36(3): 486-491.
Miles CJ, Wallace LR, & Moye HA (1986) Determination of glyphosate
herbicide and (aminomethyl)phosphonic acid in natural waters by
liquid chromatography using pre-column fluorogenic labeling with
9-fluorenylmethyl chloroformate. J Assoc Off Anal Chem, 69(3):
458-461.
Mitchell DG, Chapman PM, & Long TJ (1987) Acute toxicity of Roundup
and Rodeo herbicides to rainbow trout, chinook, and coho salmon.
Bull Environ Contam Toxicol, 39(6): 1028-1035.
Mitsukaido Laboratories (1986) Roundup herbicide: Acute oral
toxicity study in mice - Final report. Tokyo, Mitsukaido
Laboratories, Institute of Environmental Toxicology (Unpublished
report No. ET-85-377 submitted by Monsanto Ltd).
Monsanto (1972a) The degradation of MON-0573 in river and lake
bottom sediments and surface water. St. Louis, Missouri, Monsanto
Ltd (Unpublished report No. 276).
Monsanto (1972b) The rate of dissipation of MON-0573 in soil. St.
Louis, Missouri, Monsanto Ltd (Unpublished report No. 271).
Monsanto (1972c) The photolysis, run-off, and leaching of MON-0573
on or in soil. St. Louis, Missouri, Monsanto Ltd (Unpublished report
No. 258).
Monsanto (1972d) The degradation and metabolism of MON-0573 in soil.
St. Louis, Missouri, Monsanto Ltd (Unpublished report No. 269).
Monsanto (1973a) CP 67573 Residue and metabolism - The gross
metabolism of N-phos-phonomethylglycine-14C (CP 67573-14C) in
the laboratory rat following a single dose. St. Louis, Missouri,
Monsanto Commercial Products Company, Agricultural Division Research
Department (Unpublished report No. 297 submitted by Monsanto Ltd).
Monsanto (1973b) CP 67573 Residue and metabolism - The isolation and
identification of the metabolites of CP 67573=13C excreted by the
laboratory rat. St. Louis, Missouri, Monsanto Commercial Products
Company, Agricultural Division Research Department (Unpublished
report No. 306 submitted by Monsanto Ltd).
Monsanto (1973c) CP 67573 Residue and metabolism - The dynamics of
accumulation and depletion of orally ingested
N-phosphonomethylglycine-14C. St. Louis, Missouri, Monsanto
Commercial Products Company, Agricultural Division Research
Department (Unpublished report No. 309 submitted by Monsanto Ltd).
Monsanto (1973d) CP 67573 Residue and metabolism - The gross
distribution of N-phosphonomethylglycine-14C (CP 67573-14C) in
the rabbit. St. Louis, Missouri, Monsanto Commercial Products
Company, Agricultural Division Research Department (Unpublished
report No. 298 submitted by Monsanto Ltd).
Monsanto (1978a) Photodegradation and anaerobic aquatic metabolism
of glyphosate, N-phosphonomethylglycine. St. Louis, Missouri,
Monsanto Ltd (Unpublished report No. MSL-0598).
Monsanto (1978b) Solubility, volatility, adsorption, and partition
coefficients, leaching, and aquatic metabolism of MON 0573 and MON
0101. St. Louis, Missouri, Monsanto Ltd (Unpublished report No.
MSL-0207).
Monsanto (1978c) Final report on Salmonella mutagenicity assay of
glyphosate (sample no. 04) (Test No. LF-78-161). St. Louis,
Missouri, Monsanto Environmental Health Laboratory (Unpublished
report submitted by Monsanto Ltd).
Monsanto (1980) Results of a pond test with Roundup for a period of
three months. Burgwedel, Germany, Monsanto Ltd (Unpublished report
submitted by Ökolimna to Monsanto Ltd).
Monsanto (1981a) A reinvestigation of the static exposure of channel
catfish to 14C-labelled glyphosate, N-(phosphonomethyl)glycine.
St. Louis, Missouri, Monsanto Ltd (Unpublished report No. MSL-2056).
Monsanto (1981b) Acute oral toxicity of MON 0139 to rats (Study No.
800257). St. Louis, Missouri, Monsanto Environmental Health
Laboratory (Unpublished report submitted by Monsanto Ltd).
Monsanto (1981c) Acute dermal toxicity of MON 0139 to rabbits (Study
No. 800258). St. Louis, Missouri, Monsanto Environmental Health
Laboratory (Unpublished report submitted by Monsanto Ltd).
Monsanto (1981d) Primary skin irritation of MON 0139 to rabbits
(Study No. 800259). St. Louis, Missouri, Monsanto Environmental
Health Laboratory (Unpublished report submitted by Monsanto Ltd).
Monsanto (1981e) Primary eye irritation of MON 0139 to rabbits
(Study No. 800260). St. Louis, Missouri, Monsanto Environmental
Health Laboratory (Unpublished report submitted by Monsanto Ltd).
Monsanto (1983a) Dissipation of glyphosate in U.S. field soils
following direct application of Roundupr herbicide. St. Louis,
Missouri, Monsanto Ltd (Unpublished report No. MSL-3210).
Monsanto (1983b) CHO/HGPRT Gene mutation assay with glyphosate
(Study No. ML-83-155). St. Louis, Missouri, Monsanto Environmental
Health Laboratory (Unpublished report submitted by Monsanto Ltd).
Monsanto (1983c) The hepatocyte primary culture/DNA repair assay on
compound JJN-1020 (glyphosate) using rat hepatocytes in cultures
(Study No. AH-83-181). St. Louis, Missouri, Monsanto Environmental
Health Laboratory (Unpublished report submitted by Monsanto Ltd).
Monsanto (1983d) Acute inhalation toxicity of Roundup formulation to
male and female Sprague-Dawley rats (Study No. 810093; DMEH project
No. ML-81-2019. St. Louis, Missouri, Monsanto Environmental Health
Laboratory (Unpublished report submitted by Monsanto Ltd).
Monsanto (1983e) Four-week study of 33-1/33% use-dilution of Roundup
in water administered to male and female Sprague/Dawley rats by
inhalation (Project No. ML-83-015/EHL 830025). St. Louis, Missouri,
Monsanto Environmental Health Laboratory (Unpublished report
submitted by Monsanto Ltd).
Monsanto (1983f) in vivo bone marrow cytogenetics study of
glyphosate in Sprague-Dawley rats (Project No. ML-83-236). St.
Louis, Missouri, Monsanto Environmental Health Laboratory
(Unpublished report submitted by Monsanto Ltd).
Monsanto (1983g) Effects of glyphosate on rat bone marrow cells
(Study No. ML-83-160; EHL study No. 830082). St. Louis, Missouri,
Monsanto Environmental Health Laboratory (Unpublished report
submitted by Monsanto Ltd).
Monsanto (1983h) A study of the plasma and bone marrow levels of
glyphosate following intraperitoneal administration in the rat
(Study No. ML-83-218; EHL study No. 830109). St. Louis, Missouri,
Monsanto Environmental Health Laboratory (Unpublished report
submitted by Monsanto Ltd).
Monsanto (1985) Twelve month study of glyphosate administered by
gelatine capsule to Beagle dogs (Project No. ML-83-137). St. Louis,
Missouri, Monsanto Environmental Health Laboratory (Unpublished
report No. MSL 5069 submitted by Monsanto Ltd).
Monsanto (1987a) 90 day study of glyphosate administered in feed to
Sprague/Dawley rats (Project No. ML-86-351/EHL 86128). St. Louis,
Missouri, Monsanto Environmental Health Laboratory (Unpublished
report No. MSL-7575 submitted by Monsanto Ltd).
Monsanto (1987b) Acute inhalation study of Roundup L&G Ready-to-Use
(Study No. 86148; DMEH project No. ML-87-6). St. Louis, Missouri,
Monsanto Environmental Health Laboratory (Unpublished report
submitted by Monsanto Ltd).
Monsanto (1988a) Metabolism of glyphosate in Sprague-Dawley rats.
Part II. Identification, characterization, and quantitation of
glyphosate and its metabolites after intravenous and oral
administration. St. Louis, Missouri, Monsanto Ltd (Unpublished
report No. MSL-7206).
Monsanto (1988b) The metabolism of glyphosate in Sprague Dawley rats
- Part I. Excretion and tissue distribution of glyphosate and its
metabolites following intravenous and oral administration. St.
Louis, Missouri, Monsanto Environmental Health Laboratory/Monsanto
Life Sciences Research Center (Unpublished report No. MSL-7215
submitted by Monsanto Ltd).
Monsanto (1988c) Metabolism of 13C/14C-labeled glyphosate and
aminomethylphosphonic acid in laying hens, Part II. St. Louis,
Missouri, Monsanto Life Sciences Research Center (Unpublished report
No. MSL-7420 submitted by Monsanto Ltd). Monsanto (1988d) Metabolism
study of synthetic 13C/14C-labeled glyphosate and
aminomethylphosphonic acid in lactating goats, Part II. St. Louis,
Missouri, Monsanto Life Sciences Research Center (Unpublished report
No. MSL-7458 submitted by Monsanto Ltd).
Monsanto (1990a) Dissipation of glyphosate and aminomethylphosphonic
acid in forestry sites. St. Louis, Missouri, Monsanto Ltd
(Unpublished report No. MSL-9940).
Monsanto (1990b) Chronic study of glyphosate administered in feed to
albino rats (Project No. MSL-10495). St. Louis, Missouri, Monsanto
Environmental Health Laboratory (Unpublished report submitted by
Monsanto Ltd).
Monsanto (1990c) Two generation reproduction feeding study with
glyphosate in Sprague-Dawley rats (Study No. MSL-10387). St. Louis,
Missouri, Monsanto Environmental Health Laboratory (Unpublished
report submitted by Monsanto Ltd).
Morash R & Freedman B (1989) The effects of several herbicides on
the germination of seeds in the forest floor. Can J For Res, 19(3):
347-350.
Morgan MJ & Kiceniuk JW (1992) Response of rainbow trout to a two
month exposure to Vision, a glyphosate herbicide. Bull Environ
Contam Toxicol, 48: 772-780.
Morrison ML & Meslow EC (1984) Effects of the herbicide glyphosate
on bird community structure, Western Oregon. For Sci, 30(1): 95-106.
Moye HA & Deyrup CL (1984) A simple single-step derivatization
method for the gas chromatographic analysis of the herbicide
glyphosate and its metabolite. J Agric Food Chem, 32(2): 192-195.
Moye HA, Miles CJ, & Scherer SJ (1983) A simplified high-performance
liquid chromatographic residue procedure for the determination of
glyphosate herbicide and (aminomethyl)phosphonic acid in fruits and
vegetables employing postcolumn fluorogenic labeling. J Agric Food
Chem, 31(1): 69-72.
Müller MM, Rosenberg C, Siltanen H, & Wartiovaara T (1981) Fate of
glyphosate and its influence on nitrogen-cycling in two Finnish
agriculture soils. Bull Environ Contam Toxicol, 27(5): 724-730.
Murthy DVS, Irvine RL, & Hallas LE (1989) Principles of organism
selection for the degradation of glyphosate in a sequencing batch
reactor. In: 43rd Purdue Industrial Waste Conference Proceedings.
Chelsea, Lewis Publishers, Inc., pp 267-274.
NATEC (1990) Effect of the herbicide Roundup on the activity of the
microflora of soil (Project No. NA 90 9151). Unpublished report of
Institute for Technical Scientific Services GmbH, Hamburg, supplied
by Monsanto Ltd.
Newton M, Howard KM, Kelpsas BR, Danhaus R, Lottman CM, & Dubelman S
(1984) Fate of glyphosate in an Oregon forest ecosystem. J Agric
Food Chem, 32(5): 1144-1151.
NOTOX (1987a) Evaluation of the acute oral toxicity of MON 14478 in
the rat (Monsanto project No. NO-87-290). 's-Hertogenbosch, The
Netherlands, NOTOX - Toxicological Research & Consultancy
(Unpublished report No. NOTOX 0646/824 submitted by Monsanto Ltd).
NOTOX (1987b) Evaluation of the acute oral toxicity of MON 14478 in
the rat (Monsanto project No. NO-87-347). 's-Hertogenbosch, the
Netherlands, NOTOX - Toxicological Research & Consultancy
(Unpublished report No. NOTOX 0646/901 submitted by Monsanto Ltd).
NOTOX (1987c) Evaluation of the acute dermal toxicity of MON 14478
in the rat (Monsanto project No. NO-87-290). 's-Hertogenbosch, The
Netherlands, NOTOX - Toxicological Research & Consultancy
(Unpublished report No. NOTOX 0646/826 submitted by Monsanto Ltd).
NOTOX (1987d) Assessment of primary skin irritation/corrosion by MON
14478 in the rabbit (Monsanto project No. NO-87-290).
's-Hertogenbosch, The Netherlands, NOTOX - Toxicological Research &
Consultancy (Unpublished report No. NOTOX 0646/820 submitted by
Monsanto Ltd).
NOTOX (1987e) Assessment of acute eye irritation by MON 14478 in the
rabbit (Monsanto project No. NO-87-290). 's-Hertogenbosch, The
Netherlands, NOTOX - Toxicological Research & Consultancy
(Unpublished report No. NOTOX 0646/822 submitted by Monsanto Ltd).
NOTOX (1987f) Evaluation of the acute oral toxicity of MON 14477 in
the rat (Monsanto project No. NO-87-288). 's-Hertogenbosch, The
Netherlands, NOTOX - Toxicological Research & Consultancy
(Unpublished report No. NOTOX 0645/823 submitted by Monsanto Ltd).
NOTOX (1987g) Evaluation of the acute oral toxicity of MON 14477 in
the rat (Monsanto project No. NO-87-346). 's-Hertogenbosch, The
Netherlands, NOTOX - Toxicological Research & Consultancy
(Unpublished report No. NOTOX 0645/900 submitted by Monsanto Ltd).
NOTOX (1987h) Evaluation of the acute dermal toxicity of MON 14477
in the rat (Monsanto project No. NO-87-288). 's-Hertogenbosch, The
Netherlands, NOTOX - Toxicological Research & Consultancy
(Unpublished report No. NOTOX 0645/825 submitted by Monsanto Ltd).
NOTOX (1987i) Assessment of primary skin irritation by MON 14477 in
the rabbit (Monsanto project No. NO-87-288). 's-Hertogenbosch, The
Netherlands, NOTOX - Toxicological Research & Consultancy
(Unpublished report No. NOTOX 0645/819 submitted by Monsanto Ltd).
NOTOX (1987j) Assessment of acute eye irritation by MON 14477 in the
rabbit (Monsanto project No. NO-87-288). 's-Hertogenbosch, The
Netherlands, NOTOX - Toxicological Research & Consultancy
(Unpublished report No. NOTOX 0645/821 submitted by Monsanto Ltd).
NOTOX (1988) Acute oral toxicity of glyphosate in the rat.
's-Hertogenbosch, The Netherlands, NOTOX - Toxicological Research &
Consultancy (Unpublished report No. RCC NOTOX 1111/1428 submitted by
Agrichem B.V., The Netherlands).
NOTOX (1989a) Acute eye irritation/corrosion study with Glyposaat
360 g/l in rabbits. 's-Hertogenbosch, The Netherlands, NOTOX -
Toxicological Research & Consultancy (Unpublished report No. RCC
NOTOX 1268/171 (002082) submitted by Luxan B.V., The Netherlands).
NOTOX (1989b) Assessment of acute dermal toxicity with Glyposaat
360 g/l in the rat. 's-Hertogenbosch, The Netherlands, NOTOX -
Toxicological Research & Consultancy (Unpublished report No. RCC
NOTOX 1268/1718 (002071) submitted by Luxan B.V., The Netherlands).
NOTOX (1989c) Assessment of acute oral toxicity with Glyposaat
360 g/l in the rat. 's-Hertogenbosch, The Netherlands, NOTOX -
Toxicological Research & Consultancy (Unpublished report No. RCC
NOTOX 1268/1715 (002069) submitted by Luxan B.V., The Netherlands).
NOTOX (1989d) Primary skin irritation/corrosion study with Glyposaat
360 g/l in rabbits (4-hour semi-occlusive application).
's-Hertogenbosch, The Netherlands, NOTOX - Toxicological Research &
Consultancy (Unpublished report No. RCC NOTOX 1268/1716 submitted by
Luxan B.V., The Netherlands).
NOTOX (1990a) Acute eye irritation/corrosion study with Agrichem
glyposaat 2 (RCC NOTOX substance 11934) in the rabbit.
's-Hertogenbosch, The Netherlands, NOTOX - Toxicological Research &
Consultancy (Unpublished report No. RCC NOTOX 042492 submitted by
Agrichem B.V., The Netherlands).
NOTOX (1990b) Primary skin irritation/corrosion study with Agrichem
glyposaat 2 (RCC NOTOX substance 11961) in rabbits (4-hour
semi-occlusive application). 's-Hertogenbosch, The Netherlands,
NOTOX - Toxicological Research & Consultancy (Unpublished report No.
RCC NOTOX 042525 submitted by Agrichem B.V., The Netherlands).
NOTOX (1990c) Acute eye irritation/corrosion study with Agrichem
glyposaat B (RCC NOTOX substance 4175) in the rabbit.
's-Hertogenbosch, The Netherlands, NOTOX - Toxicological Research &
Consultancy (Unpublished report No. RCC NOTOX 027934 submitted by
Agrichem B.V., The Netherlands).
NOTOX (1990d) Primary skin irritation/corrosion study with Glyposaat
B (RCC NOTOX substance 4175) in rabbits (4-hour semi-occlusive
application). 's-Hertogenbosch, The Netherlands, NOTOX -
Toxicological Research & Consultancy (Unpublished report No. RCC
NOTOX 027923 submitted by Agrichem B.V., The Netherlands).
NOTOX (1990e) Assessment of acute oral toxicity with Glyposaat B
(RCC NOTOX substance 4175) in the rat. 's-Hertogenbosch, the
Netherlands, NOTOX - Toxicological Research & Consultancy
(Unpublished report No. RCC NOTOX 027901 submitted by Agrichem B.V.,
The Netherlands).
NOTOX (1990f) Assessment of acute dermal toxicity with Glyposaat B
(RCC NOTOX substance 4175) in the rat. 's-Hertogenbosch, The
Netherlands, NOTOX - Toxicological Research & Consultancy
(Unpublished No. RCC NOTOX 027912 submitted by Agrichem B.V., The
Netherlands).
NTP (1992) NTP technical report on toxicity studies of glyphosate
(CAS No. 